User login
Is hepatitis C an STI?
A 32-year-old woman had sex with a man she met while on vacation 6 weeks ago. She was intoxicated at the time and does not know much about the person. She recalls having engaged in vaginal intercourse without a condom. She does not have any symptoms.
She previously received baseline lab testing per Centers for Disease Control and Prevention guidelines 2 years ago with a negative HIV test and negative hepatitis C test. She asks for testing for STIs. What would you recommend?
A. HIV, hepatitis C, gonorrhea, chlamydia, and human papillomavirus
B. HIV, hepatitis C, gonorrhea, chlamydia, and herpes simplex virus
C. HIV, hepatitis C, gonorrhea, and chlamydia
D. HIV, gonorrhea, and chlamydia
E. Gonorrhea and chlamydia
HIV risk estimate
The most practical answer is E, check for gonorrhea and chlamydia. Many protocols in place for evaluating people for STIs will test for hepatitis C as well as HIV with single exposures. In this column, we will look at the lack of evidence of heterosexual sexual transmission of hepatitis C.
In regards to HIV risk, the estimated risk of transmission male to female from an HIV-infected individual is 0.08% per sexual encounter.1 The prevalence in the United States – where HIV occurs in about 0.5% of the adult population – was used to estimate the risk of a person with unknown HIV status acquiring HIV. The calculated risk from one sexual encounter would be 0.0004 (1 in 250,000).
Studies of hepatitis C transmission
Tahan and colleagues did a prospective study of 600 heterosexual couples where one partner had hepatitis C and the other didn’t. Over a mean of 3 years of follow-up, none of the seronegative spouses developed hepatitis C.2
Terrault and colleagues completed a cross-sectional study of hepatitis C virus (HCV)–positive individuals and their monogamous heterosexual partners to evaluate risk of sexual transmission of HCV.3 Based on 8,377 person-years of follow-up, the estimated maximum transmission rate was 0.07%/year, which was about 1/190,000 sexual contacts. No specific sexual practices were associated with transmission. The authors of this study concurred with CDC recommendations that persons with HCV infection in long-term monogamous relationships need not change their sexual practices.4
Vandelli and colleagues followed 776 heterosexual partners of HCV-infected individuals over 10 years.5 None of the couples reported condom use. Over the follow up period, three HCV infections occurred, but based on discordance of the typing of viral isolates, sexual transmission was excluded.
Jin and colleagues completed a systematic review of studies looking at possible sexual transmission of HCV in gay and bisexual men.6 HIV-positive men had a HCV incidence of 6.4 per 1,000 person-years, compared with 0.4 per 1000 person-years in HIV-negative men. The authors discussed several possible causes for increased transmission risk in HIV-infected individuals including coexisting STIs and higher HCV viral load in semen of HIV-infected individuals, as well as lower immunity.
Summary
In hepatitis C–discordant heterosexual couples, hepatitis C does not appear to be sexually transmitted.
The risk of sexual transmission of hepatitis C to non–HIV-infected individuals appears to be exceedingly low.
Many thanks to Hunter Handsfield, MD, for suggesting this topic and sharing supporting articles.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and he serves as third-year medical student clerkship director at the University of Washington. He is a member of the editorial advisory board of Internal Medicine News. Dr. Paauw has no conflicts to disclose. Contact him at [email protected].
1. Boily MC et al. Lancet Infect Dis. 2009 Feb;9(2):118-29.
2. Tahan V et al. Am J Gastroenterol. 2005;100:821-4.
3. Terrault NA et al. Hepatology. 2013;57:881-9
4. Centers for Disease Control and Prevention. MMWR Recomm Rep. 1998;47:1-38.
5. Vandelli C et al. Am J Gastroenterol. 2004;99:855-9.
6. Jin F et al. Sexual Health.2017;14:28-41.
A 32-year-old woman had sex with a man she met while on vacation 6 weeks ago. She was intoxicated at the time and does not know much about the person. She recalls having engaged in vaginal intercourse without a condom. She does not have any symptoms.
She previously received baseline lab testing per Centers for Disease Control and Prevention guidelines 2 years ago with a negative HIV test and negative hepatitis C test. She asks for testing for STIs. What would you recommend?
A. HIV, hepatitis C, gonorrhea, chlamydia, and human papillomavirus
B. HIV, hepatitis C, gonorrhea, chlamydia, and herpes simplex virus
C. HIV, hepatitis C, gonorrhea, and chlamydia
D. HIV, gonorrhea, and chlamydia
E. Gonorrhea and chlamydia
HIV risk estimate
The most practical answer is E, check for gonorrhea and chlamydia. Many protocols in place for evaluating people for STIs will test for hepatitis C as well as HIV with single exposures. In this column, we will look at the lack of evidence of heterosexual sexual transmission of hepatitis C.
In regards to HIV risk, the estimated risk of transmission male to female from an HIV-infected individual is 0.08% per sexual encounter.1 The prevalence in the United States – where HIV occurs in about 0.5% of the adult population – was used to estimate the risk of a person with unknown HIV status acquiring HIV. The calculated risk from one sexual encounter would be 0.0004 (1 in 250,000).
Studies of hepatitis C transmission
Tahan and colleagues did a prospective study of 600 heterosexual couples where one partner had hepatitis C and the other didn’t. Over a mean of 3 years of follow-up, none of the seronegative spouses developed hepatitis C.2
Terrault and colleagues completed a cross-sectional study of hepatitis C virus (HCV)–positive individuals and their monogamous heterosexual partners to evaluate risk of sexual transmission of HCV.3 Based on 8,377 person-years of follow-up, the estimated maximum transmission rate was 0.07%/year, which was about 1/190,000 sexual contacts. No specific sexual practices were associated with transmission. The authors of this study concurred with CDC recommendations that persons with HCV infection in long-term monogamous relationships need not change their sexual practices.4
Vandelli and colleagues followed 776 heterosexual partners of HCV-infected individuals over 10 years.5 None of the couples reported condom use. Over the follow up period, three HCV infections occurred, but based on discordance of the typing of viral isolates, sexual transmission was excluded.
Jin and colleagues completed a systematic review of studies looking at possible sexual transmission of HCV in gay and bisexual men.6 HIV-positive men had a HCV incidence of 6.4 per 1,000 person-years, compared with 0.4 per 1000 person-years in HIV-negative men. The authors discussed several possible causes for increased transmission risk in HIV-infected individuals including coexisting STIs and higher HCV viral load in semen of HIV-infected individuals, as well as lower immunity.
Summary
In hepatitis C–discordant heterosexual couples, hepatitis C does not appear to be sexually transmitted.
The risk of sexual transmission of hepatitis C to non–HIV-infected individuals appears to be exceedingly low.
Many thanks to Hunter Handsfield, MD, for suggesting this topic and sharing supporting articles.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and he serves as third-year medical student clerkship director at the University of Washington. He is a member of the editorial advisory board of Internal Medicine News. Dr. Paauw has no conflicts to disclose. Contact him at [email protected].
1. Boily MC et al. Lancet Infect Dis. 2009 Feb;9(2):118-29.
2. Tahan V et al. Am J Gastroenterol. 2005;100:821-4.
3. Terrault NA et al. Hepatology. 2013;57:881-9
4. Centers for Disease Control and Prevention. MMWR Recomm Rep. 1998;47:1-38.
5. Vandelli C et al. Am J Gastroenterol. 2004;99:855-9.
6. Jin F et al. Sexual Health.2017;14:28-41.
A 32-year-old woman had sex with a man she met while on vacation 6 weeks ago. She was intoxicated at the time and does not know much about the person. She recalls having engaged in vaginal intercourse without a condom. She does not have any symptoms.
She previously received baseline lab testing per Centers for Disease Control and Prevention guidelines 2 years ago with a negative HIV test and negative hepatitis C test. She asks for testing for STIs. What would you recommend?
A. HIV, hepatitis C, gonorrhea, chlamydia, and human papillomavirus
B. HIV, hepatitis C, gonorrhea, chlamydia, and herpes simplex virus
C. HIV, hepatitis C, gonorrhea, and chlamydia
D. HIV, gonorrhea, and chlamydia
E. Gonorrhea and chlamydia
HIV risk estimate
The most practical answer is E, check for gonorrhea and chlamydia. Many protocols in place for evaluating people for STIs will test for hepatitis C as well as HIV with single exposures. In this column, we will look at the lack of evidence of heterosexual sexual transmission of hepatitis C.
In regards to HIV risk, the estimated risk of transmission male to female from an HIV-infected individual is 0.08% per sexual encounter.1 The prevalence in the United States – where HIV occurs in about 0.5% of the adult population – was used to estimate the risk of a person with unknown HIV status acquiring HIV. The calculated risk from one sexual encounter would be 0.0004 (1 in 250,000).
Studies of hepatitis C transmission
Tahan and colleagues did a prospective study of 600 heterosexual couples where one partner had hepatitis C and the other didn’t. Over a mean of 3 years of follow-up, none of the seronegative spouses developed hepatitis C.2
Terrault and colleagues completed a cross-sectional study of hepatitis C virus (HCV)–positive individuals and their monogamous heterosexual partners to evaluate risk of sexual transmission of HCV.3 Based on 8,377 person-years of follow-up, the estimated maximum transmission rate was 0.07%/year, which was about 1/190,000 sexual contacts. No specific sexual practices were associated with transmission. The authors of this study concurred with CDC recommendations that persons with HCV infection in long-term monogamous relationships need not change their sexual practices.4
Vandelli and colleagues followed 776 heterosexual partners of HCV-infected individuals over 10 years.5 None of the couples reported condom use. Over the follow up period, three HCV infections occurred, but based on discordance of the typing of viral isolates, sexual transmission was excluded.
Jin and colleagues completed a systematic review of studies looking at possible sexual transmission of HCV in gay and bisexual men.6 HIV-positive men had a HCV incidence of 6.4 per 1,000 person-years, compared with 0.4 per 1000 person-years in HIV-negative men. The authors discussed several possible causes for increased transmission risk in HIV-infected individuals including coexisting STIs and higher HCV viral load in semen of HIV-infected individuals, as well as lower immunity.
Summary
In hepatitis C–discordant heterosexual couples, hepatitis C does not appear to be sexually transmitted.
The risk of sexual transmission of hepatitis C to non–HIV-infected individuals appears to be exceedingly low.
Many thanks to Hunter Handsfield, MD, for suggesting this topic and sharing supporting articles.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and he serves as third-year medical student clerkship director at the University of Washington. He is a member of the editorial advisory board of Internal Medicine News. Dr. Paauw has no conflicts to disclose. Contact him at [email protected].
1. Boily MC et al. Lancet Infect Dis. 2009 Feb;9(2):118-29.
2. Tahan V et al. Am J Gastroenterol. 2005;100:821-4.
3. Terrault NA et al. Hepatology. 2013;57:881-9
4. Centers for Disease Control and Prevention. MMWR Recomm Rep. 1998;47:1-38.
5. Vandelli C et al. Am J Gastroenterol. 2004;99:855-9.
6. Jin F et al. Sexual Health.2017;14:28-41.
Tuberculosis: The disease that changed world history
Almost forgotten today, tuberculosis is still one of the deadliest infectious diseases in the world. In an interview with Coliquio, Ronald D. Gerste, MD, PhD, an ophthalmologist and historian, looked back on this disease’s eventful history, which encompasses outstanding discoveries and catastrophic failures in diagnosis and treatment from the Middle Ages to the present day.
Under different names, TB has affected mankind for millennia. One of these names was the “aesthetic disease,” because it led to weight loss and pallor in the younger patients that it often affected. This was considered the ideal of beauty in the Victorian era. Many celebrities suffered from the disease, including poets and artists such as Friedrich Schiller, Lord Byron, and the Bronte family. As recently as the early 1990s, the disease almost changed world history, because Nelson Mandela became ill before the negotiations that led to the end of apartheid in South Africa.
Today, the global community is still not on track to meet its self-imposed targets for controlling the infectious disease, as reported by the World Health Organization on World TB Day in late March. Children and young people are the leading victims. In 2020 alone, 1.1 million children and adolescents under age 15 years were infected with TB, and 226,000 died of the disease, according to the WHO.
Q: Nelson Mandela was ill with tuberculosis during his imprisonment. How did the disease manifest itself in the future Nobel Peace Prize winner, and what is known about the treatment?
Ronald D. Gerste: Nelson Mandela contracted tuberculosis in 1988. At that time, he was 70 years old and had been in prison for 26 years. The disease presented in him with the almost classic symptom: He was coughing up blood and was also increasingly fatigued and losing weight. After doctors initially suspected a viral infection, but then TB was proven, he was treated with medication, and fluid was also drained from his lungs. [Mr.] Mandela was hospitalized for six weeks at Tygerberg Hospital in Cape Town, the second largest hospital in South Africa. The therapy worked well, but [Mr.] Mandela’s lungs remained damaged. He was subsequently prone to pneumonia and was repeatedly hospitalized for pneumonia in 2012 and 2013.
Q: Mandela was lucky that the treatment worked for him. A few years later, the first antibiotic-resistant pathogen strains developed. How did medical research respond to this development?
Gerste: The emergence of multidrug resistant (MDR) strains of the pathogen prompted the WHO to declare a “global health emergency” in 1993. Three years later, World TB Day was proclaimed to raise awareness of the threat posed by this disease, which has been known since ancient times. It always takes place on March 24, the day in 1882 when Robert Koch gave his famous lecture in Berlin in which he announced the discovery of the pathogen Mycobacterium tuberculosis.
Medical research has introduced new drugs into TB therapy, such as bedaquiline and delamanid. But MDR tuberculosis therapy remains a global challenge and has diminished hopes of eradicating tuberculosis, as we did with smallpox some 40 years ago. Today, only 56% of all MDR-TB patients worldwide are successfully treated.
Q: As already mentioned, the TB pathogen was discovered by Robert Koch. How did this come about?
Gerste: Along with cholera, TB was a great epidemic of the 19th century. For an ambitious researcher like Robert Koch, who had made a name for himself with the discovery of anthrax in 1876, there was no more rewarding goal than to find the cause of this infectious disease, which claimed the lives of many famous people such as Kafka, Dostoevsky, and Schiller, as well as many whose names are forgotten today.
[Dr.] Koch worked with his cultures for several years; the method of staining with methylene blue that was developed by the young Paul Ehrlich represented a breakthrough. To this method, [Dr.] Koch added a second, brownish dye. After countless experiments, this allowed slightly curved bacilli to be identified in tuberculous material under the microscope.
On the evening of March 24, 1882, [Dr.] Koch gave a lecture at the Institute of Physiology in Berlin with the title “Etiology of TB,” which sounded less than sensational on the invitations. One or two dozen participants had been expected, but more than one hundred came; numerous listeners had to make do with standing room behind the rows of chairs in the lecture hall. After a rather dry presentation ([Dr.] Koch was not a great orator nor a self-promoter), he presented his results to those present.
His assistants had set up a series of microscopes in the lecture hall through which everyone could get a glimpse of this enemy of humanity: the tubercle bacillus. When [Dr.] Koch had finished his remarks, there was silence in the hall. There was no burst of applause; the audience was too deeply aware that they had witnessed a historic moment. Paul Ehrlich later said that this evening had been the most significant scientific experience of his life. Over the next few weeks, the newspapers made a national hero out of Robert Koch, and the Emperor appointed him a Privy Councilor of the Government. The country doctor from Pomerania was now the figurehead of science in the young German Empire.
Q: Shortly after his discovery, [Dr.] Koch advertised a vaccination against TB with the active ingredient tuberculin. Was he able to convince with that too?
Gerste: No, this was the big flop, almost the disaster of a remarkable scientific career. The preparation of attenuated tubercle bacilli with water and glycerin not only did not prevent infection at all, it proved fatal for numerous users. However, tuberculin has survived in a modified form: as a tuberculin test, in which a characteristic skin rash indicates that a tested person has already had contact with the Mycobacterium.
Q: How have diagnostic options and treatment of the disease evolved since Robert Koch’s lifetime?
Gerste: A very decisive advance was made in diagnostics. With the rather accidental discovery of the rays soon named after him by Wilhelm Conrad Röntgen in the last days of 1895, it became possible to visualize the lung changes that tuberculosis caused in an unexpected way on living patients; the serial examinations for TB by X-rays were the logical consequence. Both scientists received Nobel Prizes, which were still new at the time, within a few years of each other: [Dr.] Röntgen in 1901 for physics, and [Dr]. Koch in 1905 for medicine and physiology.
Effective drugs were practically unavailable toward the end of the 19th century. For those who could afford it, however, a whole new world of (hoped-for or perceived) healing from “consumption” opened up: the sanatorium, located high in the mountains, surrounded by “fresh air.” The most famous of these climatic health resorts is probably Davos. It is no disrespect to the Swiss Confederation, which I hold in high esteem, to point out that Switzerland owes its high status as a tourist destination and thus its prosperity in part to TB.
Q: Things were quite different in earlier times. Until 250 years ago, the hopes of many patients rested on the medieval healing method of the “royal touch.” What’s that all about?
Gerste: In the Middle Ages, a “healing method” emerged from which not only lepers and other seriously ill people but also those suffering from consumption expected to be saved: the “royal touch,” which was first described by the Frankish king Clovis in 496. This ceremony was based on the idea that the king or queen, anointed by God, could improve or even cure the ailment of a sick person through a brief touch.
With the transition from the Middle Ages to the early modern period, this act, during which thousands often gathered in front of the ruler’s residence, was practiced on a large scale. The sufferers passed by the anointed ruler as if in a procession and were briefly touched by him or her. The extremely few “successes” were of course exploited by royal propaganda to proclaim the blessing that the reign of the king or queen meant for the country. But on those who nevertheless fell victim to TB or another ailment, the chroniclers remained silent.
Charles II of England, who ruled from 1660 to 1685 during the Restoration after the English Civil War, is said to have touched 92,102 sick people during this period, according to contemporary counts. The record for a single day’s performance is probably held by Louis XVI of France, who is said to have touched a total of 2,400 sufferers on June 14, 1775. Some of them may have stood and cheered in the Paris crowd 18 years later as the king climbed the steps to the guillotine.
Q: Another invention associated with TB diagnosis is the stethoscope. How did it come about?
Gerste: A young physician named René-Théophile-Hyacinthe Laënnec had already experienced the importance of diagnosing TB in his student years. His teacher in Paris was Xavier Bichat, considered the founder of histology, who died of TB in [Dr.] Laënnec’s second year at the age of only 30. [Dr.] Laënnec was a devotee of auscultation and made it work with a massively overweight patient by rolling up a sheet of paper, then placing this on the woman’s thorax to listen to her heart sounds. He developed the idea further and built a hollow wooden tube with a metal earpiece. In 1818, he presented the device at the meeting of the Academy of Sciences in Paris; he called it a stethoscope. He used his new instrument primarily to auscultate the lungs of patients with TB and distinguished the sounds of TB cavities from those of other lung diseases such as pneumonia and emphysema.
Q: Back to the present day: The WHO wants to eradicate TB once and for all. What are the hopes and fears in the fight against this disease?
Gerste: There is no doubt that we are currently taking a step backwards in these efforts, and this is not only due to multiresistant pathogens. Especially in poorer countries particularly affected by TB, treatment and screening programs have been disrupted by lockdown measures targeting COVID-19. The WHO suspects that in the first pandemic year, 2020, about half a million additional people may have died from TB because they never received a diagnosis.
Dr. Gerste, born in 1957, is a physician and historian. Dr. Gerste has lived for many years as a correspondent and book author in Washington, D.C., where he writes primarily for the New Journal of Zürich, the FAS, Back Then, the German Medical Journal, and other academic journals.
This article was translated from Coliquio.
Almost forgotten today, tuberculosis is still one of the deadliest infectious diseases in the world. In an interview with Coliquio, Ronald D. Gerste, MD, PhD, an ophthalmologist and historian, looked back on this disease’s eventful history, which encompasses outstanding discoveries and catastrophic failures in diagnosis and treatment from the Middle Ages to the present day.
Under different names, TB has affected mankind for millennia. One of these names was the “aesthetic disease,” because it led to weight loss and pallor in the younger patients that it often affected. This was considered the ideal of beauty in the Victorian era. Many celebrities suffered from the disease, including poets and artists such as Friedrich Schiller, Lord Byron, and the Bronte family. As recently as the early 1990s, the disease almost changed world history, because Nelson Mandela became ill before the negotiations that led to the end of apartheid in South Africa.
Today, the global community is still not on track to meet its self-imposed targets for controlling the infectious disease, as reported by the World Health Organization on World TB Day in late March. Children and young people are the leading victims. In 2020 alone, 1.1 million children and adolescents under age 15 years were infected with TB, and 226,000 died of the disease, according to the WHO.
Q: Nelson Mandela was ill with tuberculosis during his imprisonment. How did the disease manifest itself in the future Nobel Peace Prize winner, and what is known about the treatment?
Ronald D. Gerste: Nelson Mandela contracted tuberculosis in 1988. At that time, he was 70 years old and had been in prison for 26 years. The disease presented in him with the almost classic symptom: He was coughing up blood and was also increasingly fatigued and losing weight. After doctors initially suspected a viral infection, but then TB was proven, he was treated with medication, and fluid was also drained from his lungs. [Mr.] Mandela was hospitalized for six weeks at Tygerberg Hospital in Cape Town, the second largest hospital in South Africa. The therapy worked well, but [Mr.] Mandela’s lungs remained damaged. He was subsequently prone to pneumonia and was repeatedly hospitalized for pneumonia in 2012 and 2013.
Q: Mandela was lucky that the treatment worked for him. A few years later, the first antibiotic-resistant pathogen strains developed. How did medical research respond to this development?
Gerste: The emergence of multidrug resistant (MDR) strains of the pathogen prompted the WHO to declare a “global health emergency” in 1993. Three years later, World TB Day was proclaimed to raise awareness of the threat posed by this disease, which has been known since ancient times. It always takes place on March 24, the day in 1882 when Robert Koch gave his famous lecture in Berlin in which he announced the discovery of the pathogen Mycobacterium tuberculosis.
Medical research has introduced new drugs into TB therapy, such as bedaquiline and delamanid. But MDR tuberculosis therapy remains a global challenge and has diminished hopes of eradicating tuberculosis, as we did with smallpox some 40 years ago. Today, only 56% of all MDR-TB patients worldwide are successfully treated.
Q: As already mentioned, the TB pathogen was discovered by Robert Koch. How did this come about?
Gerste: Along with cholera, TB was a great epidemic of the 19th century. For an ambitious researcher like Robert Koch, who had made a name for himself with the discovery of anthrax in 1876, there was no more rewarding goal than to find the cause of this infectious disease, which claimed the lives of many famous people such as Kafka, Dostoevsky, and Schiller, as well as many whose names are forgotten today.
[Dr.] Koch worked with his cultures for several years; the method of staining with methylene blue that was developed by the young Paul Ehrlich represented a breakthrough. To this method, [Dr.] Koch added a second, brownish dye. After countless experiments, this allowed slightly curved bacilli to be identified in tuberculous material under the microscope.
On the evening of March 24, 1882, [Dr.] Koch gave a lecture at the Institute of Physiology in Berlin with the title “Etiology of TB,” which sounded less than sensational on the invitations. One or two dozen participants had been expected, but more than one hundred came; numerous listeners had to make do with standing room behind the rows of chairs in the lecture hall. After a rather dry presentation ([Dr.] Koch was not a great orator nor a self-promoter), he presented his results to those present.
His assistants had set up a series of microscopes in the lecture hall through which everyone could get a glimpse of this enemy of humanity: the tubercle bacillus. When [Dr.] Koch had finished his remarks, there was silence in the hall. There was no burst of applause; the audience was too deeply aware that they had witnessed a historic moment. Paul Ehrlich later said that this evening had been the most significant scientific experience of his life. Over the next few weeks, the newspapers made a national hero out of Robert Koch, and the Emperor appointed him a Privy Councilor of the Government. The country doctor from Pomerania was now the figurehead of science in the young German Empire.
Q: Shortly after his discovery, [Dr.] Koch advertised a vaccination against TB with the active ingredient tuberculin. Was he able to convince with that too?
Gerste: No, this was the big flop, almost the disaster of a remarkable scientific career. The preparation of attenuated tubercle bacilli with water and glycerin not only did not prevent infection at all, it proved fatal for numerous users. However, tuberculin has survived in a modified form: as a tuberculin test, in which a characteristic skin rash indicates that a tested person has already had contact with the Mycobacterium.
Q: How have diagnostic options and treatment of the disease evolved since Robert Koch’s lifetime?
Gerste: A very decisive advance was made in diagnostics. With the rather accidental discovery of the rays soon named after him by Wilhelm Conrad Röntgen in the last days of 1895, it became possible to visualize the lung changes that tuberculosis caused in an unexpected way on living patients; the serial examinations for TB by X-rays were the logical consequence. Both scientists received Nobel Prizes, which were still new at the time, within a few years of each other: [Dr.] Röntgen in 1901 for physics, and [Dr]. Koch in 1905 for medicine and physiology.
Effective drugs were practically unavailable toward the end of the 19th century. For those who could afford it, however, a whole new world of (hoped-for or perceived) healing from “consumption” opened up: the sanatorium, located high in the mountains, surrounded by “fresh air.” The most famous of these climatic health resorts is probably Davos. It is no disrespect to the Swiss Confederation, which I hold in high esteem, to point out that Switzerland owes its high status as a tourist destination and thus its prosperity in part to TB.
Q: Things were quite different in earlier times. Until 250 years ago, the hopes of many patients rested on the medieval healing method of the “royal touch.” What’s that all about?
Gerste: In the Middle Ages, a “healing method” emerged from which not only lepers and other seriously ill people but also those suffering from consumption expected to be saved: the “royal touch,” which was first described by the Frankish king Clovis in 496. This ceremony was based on the idea that the king or queen, anointed by God, could improve or even cure the ailment of a sick person through a brief touch.
With the transition from the Middle Ages to the early modern period, this act, during which thousands often gathered in front of the ruler’s residence, was practiced on a large scale. The sufferers passed by the anointed ruler as if in a procession and were briefly touched by him or her. The extremely few “successes” were of course exploited by royal propaganda to proclaim the blessing that the reign of the king or queen meant for the country. But on those who nevertheless fell victim to TB or another ailment, the chroniclers remained silent.
Charles II of England, who ruled from 1660 to 1685 during the Restoration after the English Civil War, is said to have touched 92,102 sick people during this period, according to contemporary counts. The record for a single day’s performance is probably held by Louis XVI of France, who is said to have touched a total of 2,400 sufferers on June 14, 1775. Some of them may have stood and cheered in the Paris crowd 18 years later as the king climbed the steps to the guillotine.
Q: Another invention associated with TB diagnosis is the stethoscope. How did it come about?
Gerste: A young physician named René-Théophile-Hyacinthe Laënnec had already experienced the importance of diagnosing TB in his student years. His teacher in Paris was Xavier Bichat, considered the founder of histology, who died of TB in [Dr.] Laënnec’s second year at the age of only 30. [Dr.] Laënnec was a devotee of auscultation and made it work with a massively overweight patient by rolling up a sheet of paper, then placing this on the woman’s thorax to listen to her heart sounds. He developed the idea further and built a hollow wooden tube with a metal earpiece. In 1818, he presented the device at the meeting of the Academy of Sciences in Paris; he called it a stethoscope. He used his new instrument primarily to auscultate the lungs of patients with TB and distinguished the sounds of TB cavities from those of other lung diseases such as pneumonia and emphysema.
Q: Back to the present day: The WHO wants to eradicate TB once and for all. What are the hopes and fears in the fight against this disease?
Gerste: There is no doubt that we are currently taking a step backwards in these efforts, and this is not only due to multiresistant pathogens. Especially in poorer countries particularly affected by TB, treatment and screening programs have been disrupted by lockdown measures targeting COVID-19. The WHO suspects that in the first pandemic year, 2020, about half a million additional people may have died from TB because they never received a diagnosis.
Dr. Gerste, born in 1957, is a physician and historian. Dr. Gerste has lived for many years as a correspondent and book author in Washington, D.C., where he writes primarily for the New Journal of Zürich, the FAS, Back Then, the German Medical Journal, and other academic journals.
This article was translated from Coliquio.
Almost forgotten today, tuberculosis is still one of the deadliest infectious diseases in the world. In an interview with Coliquio, Ronald D. Gerste, MD, PhD, an ophthalmologist and historian, looked back on this disease’s eventful history, which encompasses outstanding discoveries and catastrophic failures in diagnosis and treatment from the Middle Ages to the present day.
Under different names, TB has affected mankind for millennia. One of these names was the “aesthetic disease,” because it led to weight loss and pallor in the younger patients that it often affected. This was considered the ideal of beauty in the Victorian era. Many celebrities suffered from the disease, including poets and artists such as Friedrich Schiller, Lord Byron, and the Bronte family. As recently as the early 1990s, the disease almost changed world history, because Nelson Mandela became ill before the negotiations that led to the end of apartheid in South Africa.
Today, the global community is still not on track to meet its self-imposed targets for controlling the infectious disease, as reported by the World Health Organization on World TB Day in late March. Children and young people are the leading victims. In 2020 alone, 1.1 million children and adolescents under age 15 years were infected with TB, and 226,000 died of the disease, according to the WHO.
Q: Nelson Mandela was ill with tuberculosis during his imprisonment. How did the disease manifest itself in the future Nobel Peace Prize winner, and what is known about the treatment?
Ronald D. Gerste: Nelson Mandela contracted tuberculosis in 1988. At that time, he was 70 years old and had been in prison for 26 years. The disease presented in him with the almost classic symptom: He was coughing up blood and was also increasingly fatigued and losing weight. After doctors initially suspected a viral infection, but then TB was proven, he was treated with medication, and fluid was also drained from his lungs. [Mr.] Mandela was hospitalized for six weeks at Tygerberg Hospital in Cape Town, the second largest hospital in South Africa. The therapy worked well, but [Mr.] Mandela’s lungs remained damaged. He was subsequently prone to pneumonia and was repeatedly hospitalized for pneumonia in 2012 and 2013.
Q: Mandela was lucky that the treatment worked for him. A few years later, the first antibiotic-resistant pathogen strains developed. How did medical research respond to this development?
Gerste: The emergence of multidrug resistant (MDR) strains of the pathogen prompted the WHO to declare a “global health emergency” in 1993. Three years later, World TB Day was proclaimed to raise awareness of the threat posed by this disease, which has been known since ancient times. It always takes place on March 24, the day in 1882 when Robert Koch gave his famous lecture in Berlin in which he announced the discovery of the pathogen Mycobacterium tuberculosis.
Medical research has introduced new drugs into TB therapy, such as bedaquiline and delamanid. But MDR tuberculosis therapy remains a global challenge and has diminished hopes of eradicating tuberculosis, as we did with smallpox some 40 years ago. Today, only 56% of all MDR-TB patients worldwide are successfully treated.
Q: As already mentioned, the TB pathogen was discovered by Robert Koch. How did this come about?
Gerste: Along with cholera, TB was a great epidemic of the 19th century. For an ambitious researcher like Robert Koch, who had made a name for himself with the discovery of anthrax in 1876, there was no more rewarding goal than to find the cause of this infectious disease, which claimed the lives of many famous people such as Kafka, Dostoevsky, and Schiller, as well as many whose names are forgotten today.
[Dr.] Koch worked with his cultures for several years; the method of staining with methylene blue that was developed by the young Paul Ehrlich represented a breakthrough. To this method, [Dr.] Koch added a second, brownish dye. After countless experiments, this allowed slightly curved bacilli to be identified in tuberculous material under the microscope.
On the evening of March 24, 1882, [Dr.] Koch gave a lecture at the Institute of Physiology in Berlin with the title “Etiology of TB,” which sounded less than sensational on the invitations. One or two dozen participants had been expected, but more than one hundred came; numerous listeners had to make do with standing room behind the rows of chairs in the lecture hall. After a rather dry presentation ([Dr.] Koch was not a great orator nor a self-promoter), he presented his results to those present.
His assistants had set up a series of microscopes in the lecture hall through which everyone could get a glimpse of this enemy of humanity: the tubercle bacillus. When [Dr.] Koch had finished his remarks, there was silence in the hall. There was no burst of applause; the audience was too deeply aware that they had witnessed a historic moment. Paul Ehrlich later said that this evening had been the most significant scientific experience of his life. Over the next few weeks, the newspapers made a national hero out of Robert Koch, and the Emperor appointed him a Privy Councilor of the Government. The country doctor from Pomerania was now the figurehead of science in the young German Empire.
Q: Shortly after his discovery, [Dr.] Koch advertised a vaccination against TB with the active ingredient tuberculin. Was he able to convince with that too?
Gerste: No, this was the big flop, almost the disaster of a remarkable scientific career. The preparation of attenuated tubercle bacilli with water and glycerin not only did not prevent infection at all, it proved fatal for numerous users. However, tuberculin has survived in a modified form: as a tuberculin test, in which a characteristic skin rash indicates that a tested person has already had contact with the Mycobacterium.
Q: How have diagnostic options and treatment of the disease evolved since Robert Koch’s lifetime?
Gerste: A very decisive advance was made in diagnostics. With the rather accidental discovery of the rays soon named after him by Wilhelm Conrad Röntgen in the last days of 1895, it became possible to visualize the lung changes that tuberculosis caused in an unexpected way on living patients; the serial examinations for TB by X-rays were the logical consequence. Both scientists received Nobel Prizes, which were still new at the time, within a few years of each other: [Dr.] Röntgen in 1901 for physics, and [Dr]. Koch in 1905 for medicine and physiology.
Effective drugs were practically unavailable toward the end of the 19th century. For those who could afford it, however, a whole new world of (hoped-for or perceived) healing from “consumption” opened up: the sanatorium, located high in the mountains, surrounded by “fresh air.” The most famous of these climatic health resorts is probably Davos. It is no disrespect to the Swiss Confederation, which I hold in high esteem, to point out that Switzerland owes its high status as a tourist destination and thus its prosperity in part to TB.
Q: Things were quite different in earlier times. Until 250 years ago, the hopes of many patients rested on the medieval healing method of the “royal touch.” What’s that all about?
Gerste: In the Middle Ages, a “healing method” emerged from which not only lepers and other seriously ill people but also those suffering from consumption expected to be saved: the “royal touch,” which was first described by the Frankish king Clovis in 496. This ceremony was based on the idea that the king or queen, anointed by God, could improve or even cure the ailment of a sick person through a brief touch.
With the transition from the Middle Ages to the early modern period, this act, during which thousands often gathered in front of the ruler’s residence, was practiced on a large scale. The sufferers passed by the anointed ruler as if in a procession and were briefly touched by him or her. The extremely few “successes” were of course exploited by royal propaganda to proclaim the blessing that the reign of the king or queen meant for the country. But on those who nevertheless fell victim to TB or another ailment, the chroniclers remained silent.
Charles II of England, who ruled from 1660 to 1685 during the Restoration after the English Civil War, is said to have touched 92,102 sick people during this period, according to contemporary counts. The record for a single day’s performance is probably held by Louis XVI of France, who is said to have touched a total of 2,400 sufferers on June 14, 1775. Some of them may have stood and cheered in the Paris crowd 18 years later as the king climbed the steps to the guillotine.
Q: Another invention associated with TB diagnosis is the stethoscope. How did it come about?
Gerste: A young physician named René-Théophile-Hyacinthe Laënnec had already experienced the importance of diagnosing TB in his student years. His teacher in Paris was Xavier Bichat, considered the founder of histology, who died of TB in [Dr.] Laënnec’s second year at the age of only 30. [Dr.] Laënnec was a devotee of auscultation and made it work with a massively overweight patient by rolling up a sheet of paper, then placing this on the woman’s thorax to listen to her heart sounds. He developed the idea further and built a hollow wooden tube with a metal earpiece. In 1818, he presented the device at the meeting of the Academy of Sciences in Paris; he called it a stethoscope. He used his new instrument primarily to auscultate the lungs of patients with TB and distinguished the sounds of TB cavities from those of other lung diseases such as pneumonia and emphysema.
Q: Back to the present day: The WHO wants to eradicate TB once and for all. What are the hopes and fears in the fight against this disease?
Gerste: There is no doubt that we are currently taking a step backwards in these efforts, and this is not only due to multiresistant pathogens. Especially in poorer countries particularly affected by TB, treatment and screening programs have been disrupted by lockdown measures targeting COVID-19. The WHO suspects that in the first pandemic year, 2020, about half a million additional people may have died from TB because they never received a diagnosis.
Dr. Gerste, born in 1957, is a physician and historian. Dr. Gerste has lived for many years as a correspondent and book author in Washington, D.C., where he writes primarily for the New Journal of Zürich, the FAS, Back Then, the German Medical Journal, and other academic journals.
This article was translated from Coliquio.
Neuropsychiatric risks of COVID-19: New data
The neuropsychiatric ramifications of severe COVID-19 infection appear to be no different than for other severe acute respiratory infections (SARI).
This suggests that disease severity, rather than pathogen, is the most relevant factor in new-onset neuropsychiatric illness, the investigators note.
The risk of new-onset neuropsychological illness after severe COVID-19 infection are “substantial, but similar to those after other severe respiratory infections,” study investigator Peter Watkinson, MD, Nuffield Department of Clinical Neurosciences, University of Oxford, and John Radcliffe Hospital, Oxford, England, told this news organization.
The study was published online in JAMA Psychiatry.
Significant mental health burden
Research has shown a significant burden of neuropsychological illness after severe COVID-19 infection. However, it’s unclear how this risk compares to SARI.
To investigate, Dr. Watkinson and colleagues evaluated electronic health record data on more than 8.3 million adults, including 16,679 (0.02%) who survived a hospital admission for SARI and 32,525 (0.03%) who survived a hospital stay for COVID-19.
Compared with the remaining population, risks of new anxiety disorder, dementia, psychotic disorder, depression, and bipolar disorder diagnoses were significantly and similarly increased in adults surviving hospitalization for either COVID-19 or SARI.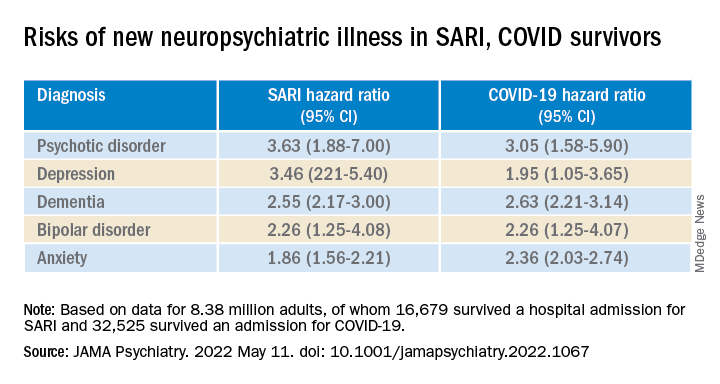
Compared with the wider population, survivors of severe SARI or COVID-19 were also at increased risk of starting treatment with antidepressants, hypnotics/anxiolytics, or antipsychotics.
When comparing survivors of SARI hospitalization to survivors of COVID-19 hospitalization, no significant differences were observed in the postdischarge rates of new-onset anxiety disorder, dementia, depression, or bipolar affective disorder.
The SARI and COVID groups also did not differ in terms of their postdischarge risks of antidepressant or hypnotic/anxiolytic use, but the COVID survivors had a 20% lower risk of starting an antipsychotic.
“In this cohort study, SARI were found to be associated with significant postacute neuropsychiatric morbidity, for which COVID-19 is not distinctly different,” Dr. Watkinson and colleagues write.
“These results may help refine our understanding of the post–severe COVID-19 phenotype and may inform post-discharge support for patients requiring hospital-based and intensive care for SARI regardless of causative pathogen,” they write.
Caveats, cautionary notes
Kevin McConway, PhD, emeritus professor of applied statistics at the Open University in Milton Keynes, England, described the study as “impressive.” However, he pointed out that the study’s observational design is a limitation.
“One can never be absolutely certain about the interpretation of findings of an observational study. What the research can’t tell us is what caused the increased psychiatric risks for people hospitalized with COVID-19 or some other serious respiratory disease,” Dr. McConway said.
“It can’t tell us what might happen in the future, when, we all hope, many fewer are being hospitalized with COVID-19 than was the case in those first two waves, and the current backlog of provision of some health services has decreased,” he added.
“So we can’t just say that, in general, serious COVID-19 has much the same neuropsychiatric consequences as other very serious respiratory illness. Maybe it does, maybe it doesn’t,” Dr. McConway cautioned.
Max Taquet, PhD, with the University of Oxford, noted that the study is limited to hospitalized adult patients, leaving open the question of risk in nonhospitalized individuals – which is the overwhelming majority of patients with COVID-19 – or in children.
Whether the neuropsychiatric risks have remained the same since the emergence of the Omicron variant also remains “an open question since all patients in this study were diagnosed before July 2021,” Dr. Taquet said in statement.
The study was funded by the Wellcome Trust, the John Fell Oxford University Press Research Fund, the Oxford Wellcome Institutional Strategic Support Fund and Cancer Research UK, through the Cancer Research UK Oxford Centre. Dr. Watkinson disclosed grants from the National Institute for Health Research and Sensyne Health outside the submitted work; and serving as chief medical officer for Sensyne Health prior to this work, as well as holding shares in the company. Dr. McConway is a trustee of the UK Science Media Centre and a member of its advisory committee. His comments were provided in his capacity as an independent professional statistician. Dr. Taquet has worked on similar studies trying to identify, quantify, and specify the neurological and psychiatric consequences of COVID-19.
A version of this article first appeared on Medscape.com.
The neuropsychiatric ramifications of severe COVID-19 infection appear to be no different than for other severe acute respiratory infections (SARI).
This suggests that disease severity, rather than pathogen, is the most relevant factor in new-onset neuropsychiatric illness, the investigators note.
The risk of new-onset neuropsychological illness after severe COVID-19 infection are “substantial, but similar to those after other severe respiratory infections,” study investigator Peter Watkinson, MD, Nuffield Department of Clinical Neurosciences, University of Oxford, and John Radcliffe Hospital, Oxford, England, told this news organization.
The study was published online in JAMA Psychiatry.
Significant mental health burden
Research has shown a significant burden of neuropsychological illness after severe COVID-19 infection. However, it’s unclear how this risk compares to SARI.
To investigate, Dr. Watkinson and colleagues evaluated electronic health record data on more than 8.3 million adults, including 16,679 (0.02%) who survived a hospital admission for SARI and 32,525 (0.03%) who survived a hospital stay for COVID-19.
Compared with the remaining population, risks of new anxiety disorder, dementia, psychotic disorder, depression, and bipolar disorder diagnoses were significantly and similarly increased in adults surviving hospitalization for either COVID-19 or SARI.
Compared with the wider population, survivors of severe SARI or COVID-19 were also at increased risk of starting treatment with antidepressants, hypnotics/anxiolytics, or antipsychotics.
When comparing survivors of SARI hospitalization to survivors of COVID-19 hospitalization, no significant differences were observed in the postdischarge rates of new-onset anxiety disorder, dementia, depression, or bipolar affective disorder.
The SARI and COVID groups also did not differ in terms of their postdischarge risks of antidepressant or hypnotic/anxiolytic use, but the COVID survivors had a 20% lower risk of starting an antipsychotic.
“In this cohort study, SARI were found to be associated with significant postacute neuropsychiatric morbidity, for which COVID-19 is not distinctly different,” Dr. Watkinson and colleagues write.
“These results may help refine our understanding of the post–severe COVID-19 phenotype and may inform post-discharge support for patients requiring hospital-based and intensive care for SARI regardless of causative pathogen,” they write.
Caveats, cautionary notes
Kevin McConway, PhD, emeritus professor of applied statistics at the Open University in Milton Keynes, England, described the study as “impressive.” However, he pointed out that the study’s observational design is a limitation.
“One can never be absolutely certain about the interpretation of findings of an observational study. What the research can’t tell us is what caused the increased psychiatric risks for people hospitalized with COVID-19 or some other serious respiratory disease,” Dr. McConway said.
“It can’t tell us what might happen in the future, when, we all hope, many fewer are being hospitalized with COVID-19 than was the case in those first two waves, and the current backlog of provision of some health services has decreased,” he added.
“So we can’t just say that, in general, serious COVID-19 has much the same neuropsychiatric consequences as other very serious respiratory illness. Maybe it does, maybe it doesn’t,” Dr. McConway cautioned.
Max Taquet, PhD, with the University of Oxford, noted that the study is limited to hospitalized adult patients, leaving open the question of risk in nonhospitalized individuals – which is the overwhelming majority of patients with COVID-19 – or in children.
Whether the neuropsychiatric risks have remained the same since the emergence of the Omicron variant also remains “an open question since all patients in this study were diagnosed before July 2021,” Dr. Taquet said in statement.
The study was funded by the Wellcome Trust, the John Fell Oxford University Press Research Fund, the Oxford Wellcome Institutional Strategic Support Fund and Cancer Research UK, through the Cancer Research UK Oxford Centre. Dr. Watkinson disclosed grants from the National Institute for Health Research and Sensyne Health outside the submitted work; and serving as chief medical officer for Sensyne Health prior to this work, as well as holding shares in the company. Dr. McConway is a trustee of the UK Science Media Centre and a member of its advisory committee. His comments were provided in his capacity as an independent professional statistician. Dr. Taquet has worked on similar studies trying to identify, quantify, and specify the neurological and psychiatric consequences of COVID-19.
A version of this article first appeared on Medscape.com.
The neuropsychiatric ramifications of severe COVID-19 infection appear to be no different than for other severe acute respiratory infections (SARI).
This suggests that disease severity, rather than pathogen, is the most relevant factor in new-onset neuropsychiatric illness, the investigators note.
The risk of new-onset neuropsychological illness after severe COVID-19 infection are “substantial, but similar to those after other severe respiratory infections,” study investigator Peter Watkinson, MD, Nuffield Department of Clinical Neurosciences, University of Oxford, and John Radcliffe Hospital, Oxford, England, told this news organization.
The study was published online in JAMA Psychiatry.
Significant mental health burden
Research has shown a significant burden of neuropsychological illness after severe COVID-19 infection. However, it’s unclear how this risk compares to SARI.
To investigate, Dr. Watkinson and colleagues evaluated electronic health record data on more than 8.3 million adults, including 16,679 (0.02%) who survived a hospital admission for SARI and 32,525 (0.03%) who survived a hospital stay for COVID-19.
Compared with the remaining population, risks of new anxiety disorder, dementia, psychotic disorder, depression, and bipolar disorder diagnoses were significantly and similarly increased in adults surviving hospitalization for either COVID-19 or SARI.
Compared with the wider population, survivors of severe SARI or COVID-19 were also at increased risk of starting treatment with antidepressants, hypnotics/anxiolytics, or antipsychotics.
When comparing survivors of SARI hospitalization to survivors of COVID-19 hospitalization, no significant differences were observed in the postdischarge rates of new-onset anxiety disorder, dementia, depression, or bipolar affective disorder.
The SARI and COVID groups also did not differ in terms of their postdischarge risks of antidepressant or hypnotic/anxiolytic use, but the COVID survivors had a 20% lower risk of starting an antipsychotic.
“In this cohort study, SARI were found to be associated with significant postacute neuropsychiatric morbidity, for which COVID-19 is not distinctly different,” Dr. Watkinson and colleagues write.
“These results may help refine our understanding of the post–severe COVID-19 phenotype and may inform post-discharge support for patients requiring hospital-based and intensive care for SARI regardless of causative pathogen,” they write.
Caveats, cautionary notes
Kevin McConway, PhD, emeritus professor of applied statistics at the Open University in Milton Keynes, England, described the study as “impressive.” However, he pointed out that the study’s observational design is a limitation.
“One can never be absolutely certain about the interpretation of findings of an observational study. What the research can’t tell us is what caused the increased psychiatric risks for people hospitalized with COVID-19 or some other serious respiratory disease,” Dr. McConway said.
“It can’t tell us what might happen in the future, when, we all hope, many fewer are being hospitalized with COVID-19 than was the case in those first two waves, and the current backlog of provision of some health services has decreased,” he added.
“So we can’t just say that, in general, serious COVID-19 has much the same neuropsychiatric consequences as other very serious respiratory illness. Maybe it does, maybe it doesn’t,” Dr. McConway cautioned.
Max Taquet, PhD, with the University of Oxford, noted that the study is limited to hospitalized adult patients, leaving open the question of risk in nonhospitalized individuals – which is the overwhelming majority of patients with COVID-19 – or in children.
Whether the neuropsychiatric risks have remained the same since the emergence of the Omicron variant also remains “an open question since all patients in this study were diagnosed before July 2021,” Dr. Taquet said in statement.
The study was funded by the Wellcome Trust, the John Fell Oxford University Press Research Fund, the Oxford Wellcome Institutional Strategic Support Fund and Cancer Research UK, through the Cancer Research UK Oxford Centre. Dr. Watkinson disclosed grants from the National Institute for Health Research and Sensyne Health outside the submitted work; and serving as chief medical officer for Sensyne Health prior to this work, as well as holding shares in the company. Dr. McConway is a trustee of the UK Science Media Centre and a member of its advisory committee. His comments were provided in his capacity as an independent professional statistician. Dr. Taquet has worked on similar studies trying to identify, quantify, and specify the neurological and psychiatric consequences of COVID-19.
A version of this article first appeared on Medscape.com.
Most COVID-19 survivors return to work within 2 years
The burden of persistent COVID-19 symptoms appeared to improve over time, but a higher percentage of former patients reported poor health, compared with the general population. This suggests that some patients need more time to completely recover from COVID-19, wrote the authors of the new study, which was published in The Lancet Respiratory Medicine. Previous research has shown that the health effects of COVID-19 last for up to a year, but data from longer-term studies are limited, said Lixue Huang, MD, of Capital Medical University, Beijing, one of the study authors, and colleagues.
Methods and results
In the new study, the researchers reviewed data from 1,192 adult patients who were discharged from the hospital after surviving COVID-19 between Jan. 7, 2020, and May 29, 2020. The researchers measured the participants’ health outcomes at 6 months, 12 months, and 2 years after their onset of symptoms. A community-based dataset of 3,383 adults with no history of COVID-19 served as controls to measure the recovery of the COVID-19 patients. The median age of the patients at the time of hospital discharge was 57 years, and 46% were women. The median follow-up time after the onset of symptoms was 185 days, 349 days, and 685 days for the 6-month, 12-month, and 2-year visits, respectively. The researchers measured health outcomes using a 6-min walking distance (6MWD) test, laboratory tests, and questionnaires about symptoms, mental health, health-related quality of life, returning to work, and health care use since leaving the hospital.
Overall, the proportion of COVID-19 survivors with at least one symptom decreased from 68% at 6 months to 55% at 2 years (P < .0001). The most frequent symptoms were fatigue and muscle weakness, reported by approximately one-third of the patients (31%); sleep problems also were reported by 31% of the patients.
The proportion of individuals with poor results on the 6MWD decreased continuously over time, not only in COVID-19 survivors overall, but also in three subgroups of varying initial disease severity. Of the 494 survivors who reported working before becoming ill, 438 (89%) had returned to their original jobs 2 years later. The most common reasons for not returning to work were decreased physical function, unwillingness to return, and unemployment, the researchers noted.
However, at 2 years, COVID-19 survivors reported more pain and discomfort, as well as more anxiety and depression, compared with the controls (23% vs. 5% and 12% vs. 5%, respectively).
In addition, significantly more survivors who needed high levels of respiratory support while hospitalized had lung diffusion impairment (65%), reduced residual volume (62%), and total lung capacity (39%), compared with matched controls (36%, 20%, and 6%, respectively) at 2 years.
Long-COVID concerns
Approximately half of the survivors had symptoms of long COVID at 2 years. These individuals were more likely to report pain or discomfort or anxiety or depression, as well as mobility problems, compared to survivors without long COVID. Participants with long-COVID symptoms were more than twice as likely to have an outpatient clinic visit (odds ratio, 2.82), and not quite twice as likely to be rehospitalized (OR, 1.64).
“We found that [health-related quality of life], exercise capacity, and mental health continued to improve throughout the 2 years regardless of initial disease severity, but about half still had symptomatic sequelae at 2 years,” the researchers wrote in their paper.
Findings can inform doctor-patient discussions
“We are increasingly recognizing that the health effects of COVID-19 may persist beyond acute illness, therefore this is a timely study to assess the long-term impact of COVID-19 with a long follow-up period,” said Suman Pal, MD, an internal medicine physician at the University of New Mexico, Albuquerque, in an interview.
The findings are consistent with the existing literature, said Dr. Pal, who was not involved in the study. The data from the study “can help clinicians have discussions regarding expected recovery and long-term prognosis for patients with COVID-19,” he noted.
What patients should know is that “studies such as this can help COVID-19 survivors understand and monitor persistent symptoms they may experience, and bring them to the attention of their clinicians,” said Dr. Pal.
However, “As a single-center study with high attrition of subjects during the study period, the findings may not be generalizable,” Dr. Pal emphasized. “Larger-scale studies and patient registries distributed over different geographical areas and time periods will help obtain a better understanding of the nature and prevalence of long COVID,” he said.
The study findings were limited by several factors, including the lack of formerly hospitalized controls with respiratory infections other than COVID-19 to determine which outcomes are COVID-19 specific, the researchers noted. Other limitations included the use of data from only patients at a single center, and from the early stages of the pandemic, as well as the use of self-reports for comorbidities and health outcomes, they said.
However, the results represent the longest-known published longitudinal follow-up of patients who recovered from acute COVID-19, the researchers emphasized. Study strengths included the large sample size, longitudinal design, and long-term follow-up with non-COVID controls to determine outcomes. The researchers noted their plans to conduct annual follow-ups in the current study population. They added that more research is needed to explore rehabilitation programs to promote recovery for COVID-19 survivors and to reduce the effects of long COVID.
The study was supported by the Chinese Academy of Medical Sciences, National Natural Science Foundation of China, National Key Research and Development Program of China, National Administration of Traditional Chinese Medicine, Major Projects of National Science and Technology on New Drug Creation and Development of Pulmonary Tuberculosis, China Evergrande Group, Jack Ma Foundation, Sino Biopharmaceutical, Ping An Insurance (Group), and New Sunshine Charity Foundation. The researchers and Dr. Pal had no financial conflicts to disclose.
This article was updated on 5/16/2022.
The burden of persistent COVID-19 symptoms appeared to improve over time, but a higher percentage of former patients reported poor health, compared with the general population. This suggests that some patients need more time to completely recover from COVID-19, wrote the authors of the new study, which was published in The Lancet Respiratory Medicine. Previous research has shown that the health effects of COVID-19 last for up to a year, but data from longer-term studies are limited, said Lixue Huang, MD, of Capital Medical University, Beijing, one of the study authors, and colleagues.
Methods and results
In the new study, the researchers reviewed data from 1,192 adult patients who were discharged from the hospital after surviving COVID-19 between Jan. 7, 2020, and May 29, 2020. The researchers measured the participants’ health outcomes at 6 months, 12 months, and 2 years after their onset of symptoms. A community-based dataset of 3,383 adults with no history of COVID-19 served as controls to measure the recovery of the COVID-19 patients. The median age of the patients at the time of hospital discharge was 57 years, and 46% were women. The median follow-up time after the onset of symptoms was 185 days, 349 days, and 685 days for the 6-month, 12-month, and 2-year visits, respectively. The researchers measured health outcomes using a 6-min walking distance (6MWD) test, laboratory tests, and questionnaires about symptoms, mental health, health-related quality of life, returning to work, and health care use since leaving the hospital.
Overall, the proportion of COVID-19 survivors with at least one symptom decreased from 68% at 6 months to 55% at 2 years (P < .0001). The most frequent symptoms were fatigue and muscle weakness, reported by approximately one-third of the patients (31%); sleep problems also were reported by 31% of the patients.
The proportion of individuals with poor results on the 6MWD decreased continuously over time, not only in COVID-19 survivors overall, but also in three subgroups of varying initial disease severity. Of the 494 survivors who reported working before becoming ill, 438 (89%) had returned to their original jobs 2 years later. The most common reasons for not returning to work were decreased physical function, unwillingness to return, and unemployment, the researchers noted.
However, at 2 years, COVID-19 survivors reported more pain and discomfort, as well as more anxiety and depression, compared with the controls (23% vs. 5% and 12% vs. 5%, respectively).
In addition, significantly more survivors who needed high levels of respiratory support while hospitalized had lung diffusion impairment (65%), reduced residual volume (62%), and total lung capacity (39%), compared with matched controls (36%, 20%, and 6%, respectively) at 2 years.
Long-COVID concerns
Approximately half of the survivors had symptoms of long COVID at 2 years. These individuals were more likely to report pain or discomfort or anxiety or depression, as well as mobility problems, compared to survivors without long COVID. Participants with long-COVID symptoms were more than twice as likely to have an outpatient clinic visit (odds ratio, 2.82), and not quite twice as likely to be rehospitalized (OR, 1.64).
“We found that [health-related quality of life], exercise capacity, and mental health continued to improve throughout the 2 years regardless of initial disease severity, but about half still had symptomatic sequelae at 2 years,” the researchers wrote in their paper.
Findings can inform doctor-patient discussions
“We are increasingly recognizing that the health effects of COVID-19 may persist beyond acute illness, therefore this is a timely study to assess the long-term impact of COVID-19 with a long follow-up period,” said Suman Pal, MD, an internal medicine physician at the University of New Mexico, Albuquerque, in an interview.
The findings are consistent with the existing literature, said Dr. Pal, who was not involved in the study. The data from the study “can help clinicians have discussions regarding expected recovery and long-term prognosis for patients with COVID-19,” he noted.
What patients should know is that “studies such as this can help COVID-19 survivors understand and monitor persistent symptoms they may experience, and bring them to the attention of their clinicians,” said Dr. Pal.
However, “As a single-center study with high attrition of subjects during the study period, the findings may not be generalizable,” Dr. Pal emphasized. “Larger-scale studies and patient registries distributed over different geographical areas and time periods will help obtain a better understanding of the nature and prevalence of long COVID,” he said.
The study findings were limited by several factors, including the lack of formerly hospitalized controls with respiratory infections other than COVID-19 to determine which outcomes are COVID-19 specific, the researchers noted. Other limitations included the use of data from only patients at a single center, and from the early stages of the pandemic, as well as the use of self-reports for comorbidities and health outcomes, they said.
However, the results represent the longest-known published longitudinal follow-up of patients who recovered from acute COVID-19, the researchers emphasized. Study strengths included the large sample size, longitudinal design, and long-term follow-up with non-COVID controls to determine outcomes. The researchers noted their plans to conduct annual follow-ups in the current study population. They added that more research is needed to explore rehabilitation programs to promote recovery for COVID-19 survivors and to reduce the effects of long COVID.
The study was supported by the Chinese Academy of Medical Sciences, National Natural Science Foundation of China, National Key Research and Development Program of China, National Administration of Traditional Chinese Medicine, Major Projects of National Science and Technology on New Drug Creation and Development of Pulmonary Tuberculosis, China Evergrande Group, Jack Ma Foundation, Sino Biopharmaceutical, Ping An Insurance (Group), and New Sunshine Charity Foundation. The researchers and Dr. Pal had no financial conflicts to disclose.
This article was updated on 5/16/2022.
The burden of persistent COVID-19 symptoms appeared to improve over time, but a higher percentage of former patients reported poor health, compared with the general population. This suggests that some patients need more time to completely recover from COVID-19, wrote the authors of the new study, which was published in The Lancet Respiratory Medicine. Previous research has shown that the health effects of COVID-19 last for up to a year, but data from longer-term studies are limited, said Lixue Huang, MD, of Capital Medical University, Beijing, one of the study authors, and colleagues.
Methods and results
In the new study, the researchers reviewed data from 1,192 adult patients who were discharged from the hospital after surviving COVID-19 between Jan. 7, 2020, and May 29, 2020. The researchers measured the participants’ health outcomes at 6 months, 12 months, and 2 years after their onset of symptoms. A community-based dataset of 3,383 adults with no history of COVID-19 served as controls to measure the recovery of the COVID-19 patients. The median age of the patients at the time of hospital discharge was 57 years, and 46% were women. The median follow-up time after the onset of symptoms was 185 days, 349 days, and 685 days for the 6-month, 12-month, and 2-year visits, respectively. The researchers measured health outcomes using a 6-min walking distance (6MWD) test, laboratory tests, and questionnaires about symptoms, mental health, health-related quality of life, returning to work, and health care use since leaving the hospital.
Overall, the proportion of COVID-19 survivors with at least one symptom decreased from 68% at 6 months to 55% at 2 years (P < .0001). The most frequent symptoms were fatigue and muscle weakness, reported by approximately one-third of the patients (31%); sleep problems also were reported by 31% of the patients.
The proportion of individuals with poor results on the 6MWD decreased continuously over time, not only in COVID-19 survivors overall, but also in three subgroups of varying initial disease severity. Of the 494 survivors who reported working before becoming ill, 438 (89%) had returned to their original jobs 2 years later. The most common reasons for not returning to work were decreased physical function, unwillingness to return, and unemployment, the researchers noted.
However, at 2 years, COVID-19 survivors reported more pain and discomfort, as well as more anxiety and depression, compared with the controls (23% vs. 5% and 12% vs. 5%, respectively).
In addition, significantly more survivors who needed high levels of respiratory support while hospitalized had lung diffusion impairment (65%), reduced residual volume (62%), and total lung capacity (39%), compared with matched controls (36%, 20%, and 6%, respectively) at 2 years.
Long-COVID concerns
Approximately half of the survivors had symptoms of long COVID at 2 years. These individuals were more likely to report pain or discomfort or anxiety or depression, as well as mobility problems, compared to survivors without long COVID. Participants with long-COVID symptoms were more than twice as likely to have an outpatient clinic visit (odds ratio, 2.82), and not quite twice as likely to be rehospitalized (OR, 1.64).
“We found that [health-related quality of life], exercise capacity, and mental health continued to improve throughout the 2 years regardless of initial disease severity, but about half still had symptomatic sequelae at 2 years,” the researchers wrote in their paper.
Findings can inform doctor-patient discussions
“We are increasingly recognizing that the health effects of COVID-19 may persist beyond acute illness, therefore this is a timely study to assess the long-term impact of COVID-19 with a long follow-up period,” said Suman Pal, MD, an internal medicine physician at the University of New Mexico, Albuquerque, in an interview.
The findings are consistent with the existing literature, said Dr. Pal, who was not involved in the study. The data from the study “can help clinicians have discussions regarding expected recovery and long-term prognosis for patients with COVID-19,” he noted.
What patients should know is that “studies such as this can help COVID-19 survivors understand and monitor persistent symptoms they may experience, and bring them to the attention of their clinicians,” said Dr. Pal.
However, “As a single-center study with high attrition of subjects during the study period, the findings may not be generalizable,” Dr. Pal emphasized. “Larger-scale studies and patient registries distributed over different geographical areas and time periods will help obtain a better understanding of the nature and prevalence of long COVID,” he said.
The study findings were limited by several factors, including the lack of formerly hospitalized controls with respiratory infections other than COVID-19 to determine which outcomes are COVID-19 specific, the researchers noted. Other limitations included the use of data from only patients at a single center, and from the early stages of the pandemic, as well as the use of self-reports for comorbidities and health outcomes, they said.
However, the results represent the longest-known published longitudinal follow-up of patients who recovered from acute COVID-19, the researchers emphasized. Study strengths included the large sample size, longitudinal design, and long-term follow-up with non-COVID controls to determine outcomes. The researchers noted their plans to conduct annual follow-ups in the current study population. They added that more research is needed to explore rehabilitation programs to promote recovery for COVID-19 survivors and to reduce the effects of long COVID.
The study was supported by the Chinese Academy of Medical Sciences, National Natural Science Foundation of China, National Key Research and Development Program of China, National Administration of Traditional Chinese Medicine, Major Projects of National Science and Technology on New Drug Creation and Development of Pulmonary Tuberculosis, China Evergrande Group, Jack Ma Foundation, Sino Biopharmaceutical, Ping An Insurance (Group), and New Sunshine Charity Foundation. The researchers and Dr. Pal had no financial conflicts to disclose.
This article was updated on 5/16/2022.
FROM THE LANCET RESPIRATORY MEDICINE
Nontuberculous mycobacterial lung disease can be challenging to treat
Living in coastal areas of Florida and California has great appeal for many, with the warm, sunny climate and nearby fresh water and salt water.
But, unknown to many, those balmy coasts also carry the risk of infection from nontuberculous (atypical) mycobacteria (NTM). Unlike its relative, tuberculosis, NTM is not transmitted from person to person, with one exception: patients with cystic fibrosis.
It is estimated that there were 181,000 people with NTM lung disease in the U.S. in 2015, and according to one study, the incidence is increasing by 8.2% annually among those aged 65 years and older. But NTM doesn’t only affect the elderly; it’s estimated that 31% of all NTM patients are younger than 65 years.
With the warm, moist soil and water, NTM is most commonly found in Florida, California, Hawaii, and the Gulf Coast states. The incidence is somewhat lower in states along the Great Lakes. Other states are not without risk – but NTM is perhaps even more likely to be overlooked in these states by physicians because of a lack of awareness of the disease.
Rebecca Prevots, PhD, MPH, chief of the epidemiology and population studies unit of the Division of Intramural Research at the National Institute of Allergy and Infectious Diseases, told this news organization that “why NTM is increasing is one of the most common questions” she gets, followed by whether it is due to climate change. “The short answer is, we don’t know.”
She suggests that the increase in diagnoses is due to a combination of increased awareness, host susceptibility, and perhaps environmental changes. One problem is that NTM is not a reportable disease. Also, public health resources have been decimated, both through funding cuts and loss of personnel. Dr. Prevots said, “It’s not just NTM surveillance that is important, but you can’t just make a certain condition reportable and expect to have good data without putting resources to it. ... Diseases are made reportable at the state level. There’s no mandated reporting up to CDC. So CDC is piloting reporting events through their emerging infectious program.”
Anthony Cannella, MD, assistant professor of infectious diseases at the University of South Florida (USF), is in the midst of NTM. He told this news organization that “there’s a huge circle with big old dots right over the center of the state.” He is adamant that “a soil-water survey has to occur. We need to know what the devil is happening.”
Florida legislators agreed to allocate $519,000 for NTM testing and surveillance in 2019. But Florida Governor Ron DeSantis vetoed that line item in the budget. WUSF (a National Public Radio affiliate on the USF campus) was unable to get a response to their query about this from the governor’s office.
Who gets NTM?
Mycobacterium avium complex primarily causes lung disease, which presents as two clinical syndromes.
“These infections don’t affect everyone,” Kenneth Olivier, MD, MPH, chief of pulmonary clinical medicine, Cardiovascular Pulmonary Branch of the National Heart, Lung, and Blood Institute, said in an interview. They affect “patients that have underlying genetic conditions that cause abnormalities in the airway clearance mechanisms, particularly cystic fibrosis and primary ciliary dyskinesia [and], to some extent, patients with COPD.”
The second group is “comprised mainly of postmenopausal women, many of whom have had no predisposing medical problems prior to onset of generally frequent throat clearing or chronic cough, which is what brings them to medical attention.” Dr. Olivier added that “many of these patients have a fairly unique appearance. They tend to have a high prevalence of curvature of the spine, scoliosis, indentation of the chest wall (pectus excavatum), and physical characteristics that overlap heritable connective tissue disorders like Marfan syndrome or Ehlers-Danlos syndrome.”
Dr. Olivier pointed out a major problem in NTM diagnosis and treatment: “The guidelines-based approach to chronic cough generally calls for treating postnasal drip, airway reactivity, asthma type symptoms first empirically, before doing different diagnostic studies. That generally causes a delay in obtaining things like CT scan, where you can see the characteristic changes.”
Dr. Cannella added, “People are starting to become more aware of it. It’s kind of like pneumocystis back in the 80s. ... We’ve had patients who have had long periods of febrile neutropenia, and NTM wasn’t on the radar. Now we’ve picked up at least seven or eight.”
In addition to pulmonary infections, nosocomial outbreaks have occurred, owing to contaminated heater-cooler units, catheter infections, nail salons, or to medical tourism. These more commonly involve rapidly growing species, such as M abscessus, M chelonae, and M fortuitum. Clinicians should also be aware of skin infections from M marinum, which come from wounds from aquariums, fish, or shellfish. Incubation can occur over months, highlighting the importance of a detailed history and special cultures.
Diagnostics
The diagnosis of NTM is delayed for several reasons. One is the lack of awareness among clinicians about NTM and its risk factors, including hobbies such as gardening or working in places where dirt is aerosolized, such as on road crews, or even from hot tubs. A thorough history is critical.
Another is not recognizing the need for an acid-fast bacilli (AFB) culture, which requires specialized media. Fortunately, NTM can be picked up on fungal cultures, Dr. Cannella noted. Clinicians are sometimes discouraged from culturing AFB because doing so may not be cost-effective. And many hospital laboratories are increasingly sending cultures to outside labs, and it can take days – sometimes even more than a week – to receive a report of results.
Charles Daley, MD, chief of the Division of Mycobacterial and Respiratory Infections at National Jewish Health, expressed his frustration about labs in an interview, saying diagnostics is “an important hole in the U.S., as our laboratories do not provide clinicians with the results that they need to make good decisions. Most laboratories in the U.S. just don’t speciate the organisms or subspeciate in the setting of abscesses. They don’t tell the clinician enough about the susceptibility, particularly whether there’s inducible resistance. As a clinician, you just don’t have the information to make the right decisions. ... We need to improve diagnostics in NTM. Everything is there and available. They just don’t want to do it because it increases the costs.”
Men tend to have fibrocavitary disease, which shows on ordinary chest x-rays, but CT scans are essential for women because women tend to have either nodular disease or bronchiectasis, which does not show on a plain film.
Treatment
A standard treatment for NTM lung disease includes three or four medications – clarithromycin or azithromycin, rifampin or rifabutin, ethambutol, and streptomycin or amikacin. In vitro resistance is important in predicting the clinical response to a macrolide or amikacin.
For bronchiectatic disease, National Jewish Hospital recommends treatment three times per week rather than daily therapy, as it is better tolerated. Azithromycin is preferred over clarithromycin. Amikacin should be added if there is cavitary or severe disease, and the macrolide is then given daily.
Dr. Olivier suggested that physicians stagger the initiation of those drugs to improve the tolerability of the difficult regimen. Generally, treatment is for 18 months – a year after sputum cultures become negative.
If therapy fails – that is, sputum is persistently positive at 6 months – amikacin liposomal inhalation solution (Arikayce) is likely to be added. Patients should be monitored with monthly safety labs, sputum cultures, and an audiogram (if receiving amikacin). Every 3 months, vestibular tests, eye exams, and spirometry should be conducted, and every 6 months, physicians should order a CT, an audiogram, and an electrocardiogram.
Despite completing such a rigorous regimen, about half of patients experience reinfection because of their underlying host susceptibility. Genomic sequencing shows that these are new infections, not relapses, Dr. Prevots said. She also noted that gastroesophageal reflux disease is a significant risk factor because of chronic aspiration.
Dr. Daley outlined the newer treatments being studied. They include Arikayce, omadocycline, and bedaquiline. He added, “There’s a neutrophil elastase inhibitor trial that’s ongoing, a huge trial. There’s another one looking at basically eosinophilic inflammation.”
Other trials are in the offing, he said, all focusing on the inflammatory response – a development he described as exciting, because for the longest time, there were few if any NTM trials.
Dr. Cannella is also buoyed by the potential synergy of dual beta-lactam therapy with ceftaroline and a carbapenem for M abscessus infections, which are notoriously difficult to treat.
There are unique problems facing drug development for NTM because, for approval, the U.S. Food and Drug Administration requires the drug to “improve how a patient feels, functions, or survives.” NTM is associated with low mortality, so that “is off the table,” Dr. Daley explained. It’s hard to quantify improvement in function. The top two symptoms to measure are coughing and fatigue, he said. But both are difficult to measure, and some of the medicines worsen cough. Some research groups are now trying to validate patient-reported outcome instruments to satisfy the FDA’s requirements.
Tips for patients and physicians
The experts this news organization spoke to had very consistent recommendations for patients:
- NTM is resistant to chlorine and bromine, so tap water is a major source of infection. Patients should consider to greater than 130° F and using metal showerheads or bathing rather than showering.
- Good bathroom ventilation helps.
- Patients should consider using a water filter that filters entities less than 5 mcm in size – but not carbon filters, which concentrate the organisms.
- Humidifiers and hot tubs should be avoided.
- A good face mask, such as an N95, should be worn when gardening or repotting plants.
Dr. Olivier stressed that clinicians should familiarize themselves with the guidelines for diagnosing and treating NTM. In particular, clinicians should be aware that using azithromycin for bronchitis might cause resistance in NTM. “Macrolide resistance turns what may be a slowly progressive or bothersome infection into a lethal infection with a 1-year mortality of 35%.”
He concluded, “I would just urge that if the patient’s on their second or third Z-Pak within a year, it’s probably time to look for other causes of what might be happening.”
Dr. Cannella, Dr. Prevots, and Dr. Olivier reported no relevant financial relationships. Dr. Cannella adds, “My views are not those of my employers, the U.S. Dept of VA, or the University of South Florida Morsani College of Medicine.” Dr. Daley reports research grants/contracts with AN2, Beyond Air, Bugworks, Insmed, and Paratek and service on advisory boards or as a consultant for AN2, AstraZeneca, Genentech, Insmed, Matinas, Paratek, Pfizer, and Spero.
A version of this article first appeared on Medscape.com.
Living in coastal areas of Florida and California has great appeal for many, with the warm, sunny climate and nearby fresh water and salt water.
But, unknown to many, those balmy coasts also carry the risk of infection from nontuberculous (atypical) mycobacteria (NTM). Unlike its relative, tuberculosis, NTM is not transmitted from person to person, with one exception: patients with cystic fibrosis.
It is estimated that there were 181,000 people with NTM lung disease in the U.S. in 2015, and according to one study, the incidence is increasing by 8.2% annually among those aged 65 years and older. But NTM doesn’t only affect the elderly; it’s estimated that 31% of all NTM patients are younger than 65 years.
With the warm, moist soil and water, NTM is most commonly found in Florida, California, Hawaii, and the Gulf Coast states. The incidence is somewhat lower in states along the Great Lakes. Other states are not without risk – but NTM is perhaps even more likely to be overlooked in these states by physicians because of a lack of awareness of the disease.
Rebecca Prevots, PhD, MPH, chief of the epidemiology and population studies unit of the Division of Intramural Research at the National Institute of Allergy and Infectious Diseases, told this news organization that “why NTM is increasing is one of the most common questions” she gets, followed by whether it is due to climate change. “The short answer is, we don’t know.”
She suggests that the increase in diagnoses is due to a combination of increased awareness, host susceptibility, and perhaps environmental changes. One problem is that NTM is not a reportable disease. Also, public health resources have been decimated, both through funding cuts and loss of personnel. Dr. Prevots said, “It’s not just NTM surveillance that is important, but you can’t just make a certain condition reportable and expect to have good data without putting resources to it. ... Diseases are made reportable at the state level. There’s no mandated reporting up to CDC. So CDC is piloting reporting events through their emerging infectious program.”
Anthony Cannella, MD, assistant professor of infectious diseases at the University of South Florida (USF), is in the midst of NTM. He told this news organization that “there’s a huge circle with big old dots right over the center of the state.” He is adamant that “a soil-water survey has to occur. We need to know what the devil is happening.”
Florida legislators agreed to allocate $519,000 for NTM testing and surveillance in 2019. But Florida Governor Ron DeSantis vetoed that line item in the budget. WUSF (a National Public Radio affiliate on the USF campus) was unable to get a response to their query about this from the governor’s office.
Who gets NTM?
Mycobacterium avium complex primarily causes lung disease, which presents as two clinical syndromes.
“These infections don’t affect everyone,” Kenneth Olivier, MD, MPH, chief of pulmonary clinical medicine, Cardiovascular Pulmonary Branch of the National Heart, Lung, and Blood Institute, said in an interview. They affect “patients that have underlying genetic conditions that cause abnormalities in the airway clearance mechanisms, particularly cystic fibrosis and primary ciliary dyskinesia [and], to some extent, patients with COPD.”
The second group is “comprised mainly of postmenopausal women, many of whom have had no predisposing medical problems prior to onset of generally frequent throat clearing or chronic cough, which is what brings them to medical attention.” Dr. Olivier added that “many of these patients have a fairly unique appearance. They tend to have a high prevalence of curvature of the spine, scoliosis, indentation of the chest wall (pectus excavatum), and physical characteristics that overlap heritable connective tissue disorders like Marfan syndrome or Ehlers-Danlos syndrome.”
Dr. Olivier pointed out a major problem in NTM diagnosis and treatment: “The guidelines-based approach to chronic cough generally calls for treating postnasal drip, airway reactivity, asthma type symptoms first empirically, before doing different diagnostic studies. That generally causes a delay in obtaining things like CT scan, where you can see the characteristic changes.”
Dr. Cannella added, “People are starting to become more aware of it. It’s kind of like pneumocystis back in the 80s. ... We’ve had patients who have had long periods of febrile neutropenia, and NTM wasn’t on the radar. Now we’ve picked up at least seven or eight.”
In addition to pulmonary infections, nosocomial outbreaks have occurred, owing to contaminated heater-cooler units, catheter infections, nail salons, or to medical tourism. These more commonly involve rapidly growing species, such as M abscessus, M chelonae, and M fortuitum. Clinicians should also be aware of skin infections from M marinum, which come from wounds from aquariums, fish, or shellfish. Incubation can occur over months, highlighting the importance of a detailed history and special cultures.
Diagnostics
The diagnosis of NTM is delayed for several reasons. One is the lack of awareness among clinicians about NTM and its risk factors, including hobbies such as gardening or working in places where dirt is aerosolized, such as on road crews, or even from hot tubs. A thorough history is critical.
Another is not recognizing the need for an acid-fast bacilli (AFB) culture, which requires specialized media. Fortunately, NTM can be picked up on fungal cultures, Dr. Cannella noted. Clinicians are sometimes discouraged from culturing AFB because doing so may not be cost-effective. And many hospital laboratories are increasingly sending cultures to outside labs, and it can take days – sometimes even more than a week – to receive a report of results.
Charles Daley, MD, chief of the Division of Mycobacterial and Respiratory Infections at National Jewish Health, expressed his frustration about labs in an interview, saying diagnostics is “an important hole in the U.S., as our laboratories do not provide clinicians with the results that they need to make good decisions. Most laboratories in the U.S. just don’t speciate the organisms or subspeciate in the setting of abscesses. They don’t tell the clinician enough about the susceptibility, particularly whether there’s inducible resistance. As a clinician, you just don’t have the information to make the right decisions. ... We need to improve diagnostics in NTM. Everything is there and available. They just don’t want to do it because it increases the costs.”
Men tend to have fibrocavitary disease, which shows on ordinary chest x-rays, but CT scans are essential for women because women tend to have either nodular disease or bronchiectasis, which does not show on a plain film.
Treatment
A standard treatment for NTM lung disease includes three or four medications – clarithromycin or azithromycin, rifampin or rifabutin, ethambutol, and streptomycin or amikacin. In vitro resistance is important in predicting the clinical response to a macrolide or amikacin.
For bronchiectatic disease, National Jewish Hospital recommends treatment three times per week rather than daily therapy, as it is better tolerated. Azithromycin is preferred over clarithromycin. Amikacin should be added if there is cavitary or severe disease, and the macrolide is then given daily.
Dr. Olivier suggested that physicians stagger the initiation of those drugs to improve the tolerability of the difficult regimen. Generally, treatment is for 18 months – a year after sputum cultures become negative.
If therapy fails – that is, sputum is persistently positive at 6 months – amikacin liposomal inhalation solution (Arikayce) is likely to be added. Patients should be monitored with monthly safety labs, sputum cultures, and an audiogram (if receiving amikacin). Every 3 months, vestibular tests, eye exams, and spirometry should be conducted, and every 6 months, physicians should order a CT, an audiogram, and an electrocardiogram.
Despite completing such a rigorous regimen, about half of patients experience reinfection because of their underlying host susceptibility. Genomic sequencing shows that these are new infections, not relapses, Dr. Prevots said. She also noted that gastroesophageal reflux disease is a significant risk factor because of chronic aspiration.
Dr. Daley outlined the newer treatments being studied. They include Arikayce, omadocycline, and bedaquiline. He added, “There’s a neutrophil elastase inhibitor trial that’s ongoing, a huge trial. There’s another one looking at basically eosinophilic inflammation.”
Other trials are in the offing, he said, all focusing on the inflammatory response – a development he described as exciting, because for the longest time, there were few if any NTM trials.
Dr. Cannella is also buoyed by the potential synergy of dual beta-lactam therapy with ceftaroline and a carbapenem for M abscessus infections, which are notoriously difficult to treat.
There are unique problems facing drug development for NTM because, for approval, the U.S. Food and Drug Administration requires the drug to “improve how a patient feels, functions, or survives.” NTM is associated with low mortality, so that “is off the table,” Dr. Daley explained. It’s hard to quantify improvement in function. The top two symptoms to measure are coughing and fatigue, he said. But both are difficult to measure, and some of the medicines worsen cough. Some research groups are now trying to validate patient-reported outcome instruments to satisfy the FDA’s requirements.
Tips for patients and physicians
The experts this news organization spoke to had very consistent recommendations for patients:
- NTM is resistant to chlorine and bromine, so tap water is a major source of infection. Patients should consider to greater than 130° F and using metal showerheads or bathing rather than showering.
- Good bathroom ventilation helps.
- Patients should consider using a water filter that filters entities less than 5 mcm in size – but not carbon filters, which concentrate the organisms.
- Humidifiers and hot tubs should be avoided.
- A good face mask, such as an N95, should be worn when gardening or repotting plants.
Dr. Olivier stressed that clinicians should familiarize themselves with the guidelines for diagnosing and treating NTM. In particular, clinicians should be aware that using azithromycin for bronchitis might cause resistance in NTM. “Macrolide resistance turns what may be a slowly progressive or bothersome infection into a lethal infection with a 1-year mortality of 35%.”
He concluded, “I would just urge that if the patient’s on their second or third Z-Pak within a year, it’s probably time to look for other causes of what might be happening.”
Dr. Cannella, Dr. Prevots, and Dr. Olivier reported no relevant financial relationships. Dr. Cannella adds, “My views are not those of my employers, the U.S. Dept of VA, or the University of South Florida Morsani College of Medicine.” Dr. Daley reports research grants/contracts with AN2, Beyond Air, Bugworks, Insmed, and Paratek and service on advisory boards or as a consultant for AN2, AstraZeneca, Genentech, Insmed, Matinas, Paratek, Pfizer, and Spero.
A version of this article first appeared on Medscape.com.
Living in coastal areas of Florida and California has great appeal for many, with the warm, sunny climate and nearby fresh water and salt water.
But, unknown to many, those balmy coasts also carry the risk of infection from nontuberculous (atypical) mycobacteria (NTM). Unlike its relative, tuberculosis, NTM is not transmitted from person to person, with one exception: patients with cystic fibrosis.
It is estimated that there were 181,000 people with NTM lung disease in the U.S. in 2015, and according to one study, the incidence is increasing by 8.2% annually among those aged 65 years and older. But NTM doesn’t only affect the elderly; it’s estimated that 31% of all NTM patients are younger than 65 years.
With the warm, moist soil and water, NTM is most commonly found in Florida, California, Hawaii, and the Gulf Coast states. The incidence is somewhat lower in states along the Great Lakes. Other states are not without risk – but NTM is perhaps even more likely to be overlooked in these states by physicians because of a lack of awareness of the disease.
Rebecca Prevots, PhD, MPH, chief of the epidemiology and population studies unit of the Division of Intramural Research at the National Institute of Allergy and Infectious Diseases, told this news organization that “why NTM is increasing is one of the most common questions” she gets, followed by whether it is due to climate change. “The short answer is, we don’t know.”
She suggests that the increase in diagnoses is due to a combination of increased awareness, host susceptibility, and perhaps environmental changes. One problem is that NTM is not a reportable disease. Also, public health resources have been decimated, both through funding cuts and loss of personnel. Dr. Prevots said, “It’s not just NTM surveillance that is important, but you can’t just make a certain condition reportable and expect to have good data without putting resources to it. ... Diseases are made reportable at the state level. There’s no mandated reporting up to CDC. So CDC is piloting reporting events through their emerging infectious program.”
Anthony Cannella, MD, assistant professor of infectious diseases at the University of South Florida (USF), is in the midst of NTM. He told this news organization that “there’s a huge circle with big old dots right over the center of the state.” He is adamant that “a soil-water survey has to occur. We need to know what the devil is happening.”
Florida legislators agreed to allocate $519,000 for NTM testing and surveillance in 2019. But Florida Governor Ron DeSantis vetoed that line item in the budget. WUSF (a National Public Radio affiliate on the USF campus) was unable to get a response to their query about this from the governor’s office.
Who gets NTM?
Mycobacterium avium complex primarily causes lung disease, which presents as two clinical syndromes.
“These infections don’t affect everyone,” Kenneth Olivier, MD, MPH, chief of pulmonary clinical medicine, Cardiovascular Pulmonary Branch of the National Heart, Lung, and Blood Institute, said in an interview. They affect “patients that have underlying genetic conditions that cause abnormalities in the airway clearance mechanisms, particularly cystic fibrosis and primary ciliary dyskinesia [and], to some extent, patients with COPD.”
The second group is “comprised mainly of postmenopausal women, many of whom have had no predisposing medical problems prior to onset of generally frequent throat clearing or chronic cough, which is what brings them to medical attention.” Dr. Olivier added that “many of these patients have a fairly unique appearance. They tend to have a high prevalence of curvature of the spine, scoliosis, indentation of the chest wall (pectus excavatum), and physical characteristics that overlap heritable connective tissue disorders like Marfan syndrome or Ehlers-Danlos syndrome.”
Dr. Olivier pointed out a major problem in NTM diagnosis and treatment: “The guidelines-based approach to chronic cough generally calls for treating postnasal drip, airway reactivity, asthma type symptoms first empirically, before doing different diagnostic studies. That generally causes a delay in obtaining things like CT scan, where you can see the characteristic changes.”
Dr. Cannella added, “People are starting to become more aware of it. It’s kind of like pneumocystis back in the 80s. ... We’ve had patients who have had long periods of febrile neutropenia, and NTM wasn’t on the radar. Now we’ve picked up at least seven or eight.”
In addition to pulmonary infections, nosocomial outbreaks have occurred, owing to contaminated heater-cooler units, catheter infections, nail salons, or to medical tourism. These more commonly involve rapidly growing species, such as M abscessus, M chelonae, and M fortuitum. Clinicians should also be aware of skin infections from M marinum, which come from wounds from aquariums, fish, or shellfish. Incubation can occur over months, highlighting the importance of a detailed history and special cultures.
Diagnostics
The diagnosis of NTM is delayed for several reasons. One is the lack of awareness among clinicians about NTM and its risk factors, including hobbies such as gardening or working in places where dirt is aerosolized, such as on road crews, or even from hot tubs. A thorough history is critical.
Another is not recognizing the need for an acid-fast bacilli (AFB) culture, which requires specialized media. Fortunately, NTM can be picked up on fungal cultures, Dr. Cannella noted. Clinicians are sometimes discouraged from culturing AFB because doing so may not be cost-effective. And many hospital laboratories are increasingly sending cultures to outside labs, and it can take days – sometimes even more than a week – to receive a report of results.
Charles Daley, MD, chief of the Division of Mycobacterial and Respiratory Infections at National Jewish Health, expressed his frustration about labs in an interview, saying diagnostics is “an important hole in the U.S., as our laboratories do not provide clinicians with the results that they need to make good decisions. Most laboratories in the U.S. just don’t speciate the organisms or subspeciate in the setting of abscesses. They don’t tell the clinician enough about the susceptibility, particularly whether there’s inducible resistance. As a clinician, you just don’t have the information to make the right decisions. ... We need to improve diagnostics in NTM. Everything is there and available. They just don’t want to do it because it increases the costs.”
Men tend to have fibrocavitary disease, which shows on ordinary chest x-rays, but CT scans are essential for women because women tend to have either nodular disease or bronchiectasis, which does not show on a plain film.
Treatment
A standard treatment for NTM lung disease includes three or four medications – clarithromycin or azithromycin, rifampin or rifabutin, ethambutol, and streptomycin or amikacin. In vitro resistance is important in predicting the clinical response to a macrolide or amikacin.
For bronchiectatic disease, National Jewish Hospital recommends treatment three times per week rather than daily therapy, as it is better tolerated. Azithromycin is preferred over clarithromycin. Amikacin should be added if there is cavitary or severe disease, and the macrolide is then given daily.
Dr. Olivier suggested that physicians stagger the initiation of those drugs to improve the tolerability of the difficult regimen. Generally, treatment is for 18 months – a year after sputum cultures become negative.
If therapy fails – that is, sputum is persistently positive at 6 months – amikacin liposomal inhalation solution (Arikayce) is likely to be added. Patients should be monitored with monthly safety labs, sputum cultures, and an audiogram (if receiving amikacin). Every 3 months, vestibular tests, eye exams, and spirometry should be conducted, and every 6 months, physicians should order a CT, an audiogram, and an electrocardiogram.
Despite completing such a rigorous regimen, about half of patients experience reinfection because of their underlying host susceptibility. Genomic sequencing shows that these are new infections, not relapses, Dr. Prevots said. She also noted that gastroesophageal reflux disease is a significant risk factor because of chronic aspiration.
Dr. Daley outlined the newer treatments being studied. They include Arikayce, omadocycline, and bedaquiline. He added, “There’s a neutrophil elastase inhibitor trial that’s ongoing, a huge trial. There’s another one looking at basically eosinophilic inflammation.”
Other trials are in the offing, he said, all focusing on the inflammatory response – a development he described as exciting, because for the longest time, there were few if any NTM trials.
Dr. Cannella is also buoyed by the potential synergy of dual beta-lactam therapy with ceftaroline and a carbapenem for M abscessus infections, which are notoriously difficult to treat.
There are unique problems facing drug development for NTM because, for approval, the U.S. Food and Drug Administration requires the drug to “improve how a patient feels, functions, or survives.” NTM is associated with low mortality, so that “is off the table,” Dr. Daley explained. It’s hard to quantify improvement in function. The top two symptoms to measure are coughing and fatigue, he said. But both are difficult to measure, and some of the medicines worsen cough. Some research groups are now trying to validate patient-reported outcome instruments to satisfy the FDA’s requirements.
Tips for patients and physicians
The experts this news organization spoke to had very consistent recommendations for patients:
- NTM is resistant to chlorine and bromine, so tap water is a major source of infection. Patients should consider to greater than 130° F and using metal showerheads or bathing rather than showering.
- Good bathroom ventilation helps.
- Patients should consider using a water filter that filters entities less than 5 mcm in size – but not carbon filters, which concentrate the organisms.
- Humidifiers and hot tubs should be avoided.
- A good face mask, such as an N95, should be worn when gardening or repotting plants.
Dr. Olivier stressed that clinicians should familiarize themselves with the guidelines for diagnosing and treating NTM. In particular, clinicians should be aware that using azithromycin for bronchitis might cause resistance in NTM. “Macrolide resistance turns what may be a slowly progressive or bothersome infection into a lethal infection with a 1-year mortality of 35%.”
He concluded, “I would just urge that if the patient’s on their second or third Z-Pak within a year, it’s probably time to look for other causes of what might be happening.”
Dr. Cannella, Dr. Prevots, and Dr. Olivier reported no relevant financial relationships. Dr. Cannella adds, “My views are not those of my employers, the U.S. Dept of VA, or the University of South Florida Morsani College of Medicine.” Dr. Daley reports research grants/contracts with AN2, Beyond Air, Bugworks, Insmed, and Paratek and service on advisory boards or as a consultant for AN2, AstraZeneca, Genentech, Insmed, Matinas, Paratek, Pfizer, and Spero.
A version of this article first appeared on Medscape.com.
Can gram stains guide antibiotics for pneumonia in critical care?
Similar outcomes in patients with ventilator-associated pneumonia (VAP) suggest that antibiotics selected by Gram staining were noninferior to those based on guidelines and also significantly decreased the use of broad-spectrum antibiotics in this patient population.
The findings were published in JAMA Network Open. The multicenter, open-label, noninferiority, randomized trial, Gram Stain-Guided Antibiotics Choice for VAP (GRACE-VAP), was conducted for 2 years in intensive care units (ICUs) of a dozen tertiary referral hospitals in Japan, from April 1, 2018, through May 31, 2020.
The authors noted in their paper that the 2016 clinical practice guidelines for VAP published by the Infectious Diseases Society of America (IDSA) and the American Thoracic Society recommend antibiotic agents active against both methicillin-resistant Staphylococcus aureus (MRSA) and Pseudomonas aeruginosa as an empirical treatment. Adherence to these guidelines may lead to overuse of broad-spectrum antibiotic agents and could be associated with the accelerated emergence of antimicrobial-resistant organisms, the authors postulated.
The study sought to answer the question: Can Gram staining be used as an alternative to established guidelines to direct antibiotic use – thereby curbing the use of broad-spectrum antibiotics – without compromising patient safety and clinical outcomes?
A total of 206 patients, with a mean age of 69, took part in the study. The same number of patients were assigned to each arm. Patients aged 15 years or older with a VAP diagnosis and a modified Clinical Pulmonary Infection Score of 5 or higher were included.
Investigators reported that 79 patients (76.7%) responded to antibiotics in the Gram stain-guided group and 74 (71.8%) responded in the guideline-based group (risk difference, 0.05; 95% confidence interval, –0.07 to 0.17; P < .001, for noninferiority).
There was a decrease in antipseudomonal agent use comparing the Gram stain-guided group with the guideline-based group (30.1%; 95% CI, 21.5% to 39.9%; P < .001). There also was a decrease in anti-MRSA agents in the Gram stain-guided group, compared with the guideline-based group (38.8%; 95% CI, 29.4% to 48.9%; P < .001).
The 28-day cumulative incidence of mortality was 13.6% (n = 14) in the Gram stain-guided group versus 17.5% (n = 18) in the guideline-based group. Escalation of antibiotics according to culture results was performed in seven patients (6.8%) in the Gram stain-guided group and in one patient (1.0%) in the guideline-based group. No significant differences in study arms were observed on other measures, such as ICU-free days, ventilator-free days, and adverse events.
The authors concluded that their findings support the use of Gram staining as a strategy to manage infectious diseases and contain the development of multidrug resistant organisms (MDROs) in the setting of critical care.
“In the GRACE-VAP trial, we used the time-honored Gram stain technique as part of the daily management of infectious diseases. We believe that the trial results are acceptable and have the potential to change the strategy of antibiotic choice worldwide,” the authors wrote.
Benjamin D. Galvan MLS(ASCP), CIC, an infection preventionist with a professional background in clinical microbiology, noted that Gram staining is more accessible and significantly less costly than the rapid polymerase chain reaction testing certain institutions use to rapidly identify MDROs to help tailor therapy.
But one of the pitfalls with relying on Gram stain collection to guide antibiotic use is that it is operator dependent and subject to extrinsic factors, like prior antibiotic use, he pointed out.
“If it is not collected, set up, and read properly, the Gram stain is not going to necessarily be reliable” said Mr. Galvan, also a member of the national communications committee for the Association for Professionals in Infection Control and Epidemiology. He added that the sample in the study was not representative of institutions dealing with elevated rates of multidrug resistance.
“Even from their own results, they were looking at hospitals that have a low rate of multidrug resistance,” he said. “It was not clear if MRSA or just Staphylococcus aureus was identified in significant quantities upon review, and they recognized a lower-than-expected number of isolates of Pseudomonas aeruginosa.”
Establishing antibiotic treatment from the results of Gram-stain collection may not be sufficiently comprehensive, he said.
“Generally speaking, basing it (antibiotic therapy) solely off of a Gram stain is not looking at the whole picture,” said Mr. Galvan, noting that the 2016 IDSA guidelines call for an evaluation of the clinical status, including risk, of the individual patient, as well as locally available antibiotic resistance data.
Moreover, the evidence-based IDSA guidelines are in place to help address the issue of antimicrobial resistance trends, already recommending tailoring empiric antibiotic therapy based upon the levels of resistance in the local population, according to Galvan.
While the study suggests that this Gram-stain-driven tailoring of empiric antibiotic therapy may be noninferior to current guidelines in health care settings with low MDRO rates, its utility may not be suitable in hospitals that are already dealing with high rates of MDROs, such as Pseudomonas aeruginosa and Acinetobacter baumannii, or severe clinical cases of VAP, Mr. Galvan explained.
The researchers and Mr. Galvan disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Similar outcomes in patients with ventilator-associated pneumonia (VAP) suggest that antibiotics selected by Gram staining were noninferior to those based on guidelines and also significantly decreased the use of broad-spectrum antibiotics in this patient population.
The findings were published in JAMA Network Open. The multicenter, open-label, noninferiority, randomized trial, Gram Stain-Guided Antibiotics Choice for VAP (GRACE-VAP), was conducted for 2 years in intensive care units (ICUs) of a dozen tertiary referral hospitals in Japan, from April 1, 2018, through May 31, 2020.
The authors noted in their paper that the 2016 clinical practice guidelines for VAP published by the Infectious Diseases Society of America (IDSA) and the American Thoracic Society recommend antibiotic agents active against both methicillin-resistant Staphylococcus aureus (MRSA) and Pseudomonas aeruginosa as an empirical treatment. Adherence to these guidelines may lead to overuse of broad-spectrum antibiotic agents and could be associated with the accelerated emergence of antimicrobial-resistant organisms, the authors postulated.
The study sought to answer the question: Can Gram staining be used as an alternative to established guidelines to direct antibiotic use – thereby curbing the use of broad-spectrum antibiotics – without compromising patient safety and clinical outcomes?
A total of 206 patients, with a mean age of 69, took part in the study. The same number of patients were assigned to each arm. Patients aged 15 years or older with a VAP diagnosis and a modified Clinical Pulmonary Infection Score of 5 or higher were included.
Investigators reported that 79 patients (76.7%) responded to antibiotics in the Gram stain-guided group and 74 (71.8%) responded in the guideline-based group (risk difference, 0.05; 95% confidence interval, –0.07 to 0.17; P < .001, for noninferiority).
There was a decrease in antipseudomonal agent use comparing the Gram stain-guided group with the guideline-based group (30.1%; 95% CI, 21.5% to 39.9%; P < .001). There also was a decrease in anti-MRSA agents in the Gram stain-guided group, compared with the guideline-based group (38.8%; 95% CI, 29.4% to 48.9%; P < .001).
The 28-day cumulative incidence of mortality was 13.6% (n = 14) in the Gram stain-guided group versus 17.5% (n = 18) in the guideline-based group. Escalation of antibiotics according to culture results was performed in seven patients (6.8%) in the Gram stain-guided group and in one patient (1.0%) in the guideline-based group. No significant differences in study arms were observed on other measures, such as ICU-free days, ventilator-free days, and adverse events.
The authors concluded that their findings support the use of Gram staining as a strategy to manage infectious diseases and contain the development of multidrug resistant organisms (MDROs) in the setting of critical care.
“In the GRACE-VAP trial, we used the time-honored Gram stain technique as part of the daily management of infectious diseases. We believe that the trial results are acceptable and have the potential to change the strategy of antibiotic choice worldwide,” the authors wrote.
Benjamin D. Galvan MLS(ASCP), CIC, an infection preventionist with a professional background in clinical microbiology, noted that Gram staining is more accessible and significantly less costly than the rapid polymerase chain reaction testing certain institutions use to rapidly identify MDROs to help tailor therapy.
But one of the pitfalls with relying on Gram stain collection to guide antibiotic use is that it is operator dependent and subject to extrinsic factors, like prior antibiotic use, he pointed out.
“If it is not collected, set up, and read properly, the Gram stain is not going to necessarily be reliable” said Mr. Galvan, also a member of the national communications committee for the Association for Professionals in Infection Control and Epidemiology. He added that the sample in the study was not representative of institutions dealing with elevated rates of multidrug resistance.
“Even from their own results, they were looking at hospitals that have a low rate of multidrug resistance,” he said. “It was not clear if MRSA or just Staphylococcus aureus was identified in significant quantities upon review, and they recognized a lower-than-expected number of isolates of Pseudomonas aeruginosa.”
Establishing antibiotic treatment from the results of Gram-stain collection may not be sufficiently comprehensive, he said.
“Generally speaking, basing it (antibiotic therapy) solely off of a Gram stain is not looking at the whole picture,” said Mr. Galvan, noting that the 2016 IDSA guidelines call for an evaluation of the clinical status, including risk, of the individual patient, as well as locally available antibiotic resistance data.
Moreover, the evidence-based IDSA guidelines are in place to help address the issue of antimicrobial resistance trends, already recommending tailoring empiric antibiotic therapy based upon the levels of resistance in the local population, according to Galvan.
While the study suggests that this Gram-stain-driven tailoring of empiric antibiotic therapy may be noninferior to current guidelines in health care settings with low MDRO rates, its utility may not be suitable in hospitals that are already dealing with high rates of MDROs, such as Pseudomonas aeruginosa and Acinetobacter baumannii, or severe clinical cases of VAP, Mr. Galvan explained.
The researchers and Mr. Galvan disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Similar outcomes in patients with ventilator-associated pneumonia (VAP) suggest that antibiotics selected by Gram staining were noninferior to those based on guidelines and also significantly decreased the use of broad-spectrum antibiotics in this patient population.
The findings were published in JAMA Network Open. The multicenter, open-label, noninferiority, randomized trial, Gram Stain-Guided Antibiotics Choice for VAP (GRACE-VAP), was conducted for 2 years in intensive care units (ICUs) of a dozen tertiary referral hospitals in Japan, from April 1, 2018, through May 31, 2020.
The authors noted in their paper that the 2016 clinical practice guidelines for VAP published by the Infectious Diseases Society of America (IDSA) and the American Thoracic Society recommend antibiotic agents active against both methicillin-resistant Staphylococcus aureus (MRSA) and Pseudomonas aeruginosa as an empirical treatment. Adherence to these guidelines may lead to overuse of broad-spectrum antibiotic agents and could be associated with the accelerated emergence of antimicrobial-resistant organisms, the authors postulated.
The study sought to answer the question: Can Gram staining be used as an alternative to established guidelines to direct antibiotic use – thereby curbing the use of broad-spectrum antibiotics – without compromising patient safety and clinical outcomes?
A total of 206 patients, with a mean age of 69, took part in the study. The same number of patients were assigned to each arm. Patients aged 15 years or older with a VAP diagnosis and a modified Clinical Pulmonary Infection Score of 5 or higher were included.
Investigators reported that 79 patients (76.7%) responded to antibiotics in the Gram stain-guided group and 74 (71.8%) responded in the guideline-based group (risk difference, 0.05; 95% confidence interval, –0.07 to 0.17; P < .001, for noninferiority).
There was a decrease in antipseudomonal agent use comparing the Gram stain-guided group with the guideline-based group (30.1%; 95% CI, 21.5% to 39.9%; P < .001). There also was a decrease in anti-MRSA agents in the Gram stain-guided group, compared with the guideline-based group (38.8%; 95% CI, 29.4% to 48.9%; P < .001).
The 28-day cumulative incidence of mortality was 13.6% (n = 14) in the Gram stain-guided group versus 17.5% (n = 18) in the guideline-based group. Escalation of antibiotics according to culture results was performed in seven patients (6.8%) in the Gram stain-guided group and in one patient (1.0%) in the guideline-based group. No significant differences in study arms were observed on other measures, such as ICU-free days, ventilator-free days, and adverse events.
The authors concluded that their findings support the use of Gram staining as a strategy to manage infectious diseases and contain the development of multidrug resistant organisms (MDROs) in the setting of critical care.
“In the GRACE-VAP trial, we used the time-honored Gram stain technique as part of the daily management of infectious diseases. We believe that the trial results are acceptable and have the potential to change the strategy of antibiotic choice worldwide,” the authors wrote.
Benjamin D. Galvan MLS(ASCP), CIC, an infection preventionist with a professional background in clinical microbiology, noted that Gram staining is more accessible and significantly less costly than the rapid polymerase chain reaction testing certain institutions use to rapidly identify MDROs to help tailor therapy.
But one of the pitfalls with relying on Gram stain collection to guide antibiotic use is that it is operator dependent and subject to extrinsic factors, like prior antibiotic use, he pointed out.
“If it is not collected, set up, and read properly, the Gram stain is not going to necessarily be reliable” said Mr. Galvan, also a member of the national communications committee for the Association for Professionals in Infection Control and Epidemiology. He added that the sample in the study was not representative of institutions dealing with elevated rates of multidrug resistance.
“Even from their own results, they were looking at hospitals that have a low rate of multidrug resistance,” he said. “It was not clear if MRSA or just Staphylococcus aureus was identified in significant quantities upon review, and they recognized a lower-than-expected number of isolates of Pseudomonas aeruginosa.”
Establishing antibiotic treatment from the results of Gram-stain collection may not be sufficiently comprehensive, he said.
“Generally speaking, basing it (antibiotic therapy) solely off of a Gram stain is not looking at the whole picture,” said Mr. Galvan, noting that the 2016 IDSA guidelines call for an evaluation of the clinical status, including risk, of the individual patient, as well as locally available antibiotic resistance data.
Moreover, the evidence-based IDSA guidelines are in place to help address the issue of antimicrobial resistance trends, already recommending tailoring empiric antibiotic therapy based upon the levels of resistance in the local population, according to Galvan.
While the study suggests that this Gram-stain-driven tailoring of empiric antibiotic therapy may be noninferior to current guidelines in health care settings with low MDRO rates, its utility may not be suitable in hospitals that are already dealing with high rates of MDROs, such as Pseudomonas aeruginosa and Acinetobacter baumannii, or severe clinical cases of VAP, Mr. Galvan explained.
The researchers and Mr. Galvan disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Pneumonia shows strong connection to chronic otitis media
Individuals with a prior diagnosis of pneumonia were significantly more likely to develop chronic otitis media (COM) than were those without a history of pneumonia, based on data from a nationwide cohort study of more than 100,000 patients.
“Recently, middle ear diseases, including COM, have been recognized as respiratory tract diseases beyond the pathophysiological concepts of ventilation dysfunction, with recurrent infection that occurs from anatomically adjacent structures such as the middle ear, mastoid cavity, and eustachian tube,” but the potential link between pneumonia and chronic otitis media and adults in particular has not been examined, wrote Sung Kyun Kim, MD, of Hallym University, Dongtan, South Korea, and colleagues.
In a study recently published in the International Journal of Infectious Diseases, the researchers identified 23,436 adults with COM and 93,744 controls aged 40 years and older from a Korean health insurance database between 2002 and 2015.
The overall incidence of pneumonia in the study population was significantly higher in the COM group compared with controls (9.3% vs. 7.2%, P <.001). The odds ratios of pneumonia were significantly higher in the COM group compared with controls, and a history of pneumonia increased the odds of COM regardless of sex and across all ages.
Pneumonia was defined as when a patient had a diagnosis of pneumonia based on ICD-10 codes and underwent a chest x-ray or chest CT scan. Chronic otitis media was defined as when a patient had a diagnosis based on ICD-10 codes at least two times with one of the following conditions: chronic serous otitis media, chronic mucoid otitis media, other chronic nonsuppurative otitis media, unspecified nonsuppurative otitis media, chronic tubotympanic suppurative otitis media, chronic atticoantral suppurative otitis media, other chronic suppurative otitis media, or unspecified suppurative otitis media.
Age groups were divided into 5-year intervals, and patients were classified into income groups and rural vs. urban residence.
In a further sensitivity analysis, individuals who were diagnosed with pneumonia five or more times before the index date had a significantly higher odds ratio for COM compared with those with less than five diagnoses of pneumonia (adjusted odds ratio, 1.34; P < .001).
Microbiome dysbiosis may explain part of the connection between pneumonia and COM, the researchers wrote in their discussion. Pathogens in the lungs can prompt changes in the microbiome dynamics, as might the use of antibiotics, they said. In addition, “Mucus plugging in the airway caused by pneumonia induces hypoxic conditions and leads to the expression of inflammatory markers in the eustachian tube and middle ear mucosa,” they noted.
The study findings were limited by several factors, including the retrospective design and lack of data on microbiological cultures for antibiotic susceptibility, radiologic findings on the severity of pneumonia, results of pulmonary function tests, and hearing thresholds, the researchers noted. Other limitations were the exclusion of the frequency of upper respiratory infections and antibiotic use due to lack of data, they said.
However, the results show an association between pneumonia diagnoses and increased incidence of COM, which suggests a novel perspective that “infection of the lower respiratory tract may affect the function of the eustachian tube and the middle ear to later cause COM,” they concluded.
The study received no outside funding. The researchers have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Individuals with a prior diagnosis of pneumonia were significantly more likely to develop chronic otitis media (COM) than were those without a history of pneumonia, based on data from a nationwide cohort study of more than 100,000 patients.
“Recently, middle ear diseases, including COM, have been recognized as respiratory tract diseases beyond the pathophysiological concepts of ventilation dysfunction, with recurrent infection that occurs from anatomically adjacent structures such as the middle ear, mastoid cavity, and eustachian tube,” but the potential link between pneumonia and chronic otitis media and adults in particular has not been examined, wrote Sung Kyun Kim, MD, of Hallym University, Dongtan, South Korea, and colleagues.
In a study recently published in the International Journal of Infectious Diseases, the researchers identified 23,436 adults with COM and 93,744 controls aged 40 years and older from a Korean health insurance database between 2002 and 2015.
The overall incidence of pneumonia in the study population was significantly higher in the COM group compared with controls (9.3% vs. 7.2%, P <.001). The odds ratios of pneumonia were significantly higher in the COM group compared with controls, and a history of pneumonia increased the odds of COM regardless of sex and across all ages.
Pneumonia was defined as when a patient had a diagnosis of pneumonia based on ICD-10 codes and underwent a chest x-ray or chest CT scan. Chronic otitis media was defined as when a patient had a diagnosis based on ICD-10 codes at least two times with one of the following conditions: chronic serous otitis media, chronic mucoid otitis media, other chronic nonsuppurative otitis media, unspecified nonsuppurative otitis media, chronic tubotympanic suppurative otitis media, chronic atticoantral suppurative otitis media, other chronic suppurative otitis media, or unspecified suppurative otitis media.
Age groups were divided into 5-year intervals, and patients were classified into income groups and rural vs. urban residence.
In a further sensitivity analysis, individuals who were diagnosed with pneumonia five or more times before the index date had a significantly higher odds ratio for COM compared with those with less than five diagnoses of pneumonia (adjusted odds ratio, 1.34; P < .001).
Microbiome dysbiosis may explain part of the connection between pneumonia and COM, the researchers wrote in their discussion. Pathogens in the lungs can prompt changes in the microbiome dynamics, as might the use of antibiotics, they said. In addition, “Mucus plugging in the airway caused by pneumonia induces hypoxic conditions and leads to the expression of inflammatory markers in the eustachian tube and middle ear mucosa,” they noted.
The study findings were limited by several factors, including the retrospective design and lack of data on microbiological cultures for antibiotic susceptibility, radiologic findings on the severity of pneumonia, results of pulmonary function tests, and hearing thresholds, the researchers noted. Other limitations were the exclusion of the frequency of upper respiratory infections and antibiotic use due to lack of data, they said.
However, the results show an association between pneumonia diagnoses and increased incidence of COM, which suggests a novel perspective that “infection of the lower respiratory tract may affect the function of the eustachian tube and the middle ear to later cause COM,” they concluded.
The study received no outside funding. The researchers have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Individuals with a prior diagnosis of pneumonia were significantly more likely to develop chronic otitis media (COM) than were those without a history of pneumonia, based on data from a nationwide cohort study of more than 100,000 patients.
“Recently, middle ear diseases, including COM, have been recognized as respiratory tract diseases beyond the pathophysiological concepts of ventilation dysfunction, with recurrent infection that occurs from anatomically adjacent structures such as the middle ear, mastoid cavity, and eustachian tube,” but the potential link between pneumonia and chronic otitis media and adults in particular has not been examined, wrote Sung Kyun Kim, MD, of Hallym University, Dongtan, South Korea, and colleagues.
In a study recently published in the International Journal of Infectious Diseases, the researchers identified 23,436 adults with COM and 93,744 controls aged 40 years and older from a Korean health insurance database between 2002 and 2015.
The overall incidence of pneumonia in the study population was significantly higher in the COM group compared with controls (9.3% vs. 7.2%, P <.001). The odds ratios of pneumonia were significantly higher in the COM group compared with controls, and a history of pneumonia increased the odds of COM regardless of sex and across all ages.
Pneumonia was defined as when a patient had a diagnosis of pneumonia based on ICD-10 codes and underwent a chest x-ray or chest CT scan. Chronic otitis media was defined as when a patient had a diagnosis based on ICD-10 codes at least two times with one of the following conditions: chronic serous otitis media, chronic mucoid otitis media, other chronic nonsuppurative otitis media, unspecified nonsuppurative otitis media, chronic tubotympanic suppurative otitis media, chronic atticoantral suppurative otitis media, other chronic suppurative otitis media, or unspecified suppurative otitis media.
Age groups were divided into 5-year intervals, and patients were classified into income groups and rural vs. urban residence.
In a further sensitivity analysis, individuals who were diagnosed with pneumonia five or more times before the index date had a significantly higher odds ratio for COM compared with those with less than five diagnoses of pneumonia (adjusted odds ratio, 1.34; P < .001).
Microbiome dysbiosis may explain part of the connection between pneumonia and COM, the researchers wrote in their discussion. Pathogens in the lungs can prompt changes in the microbiome dynamics, as might the use of antibiotics, they said. In addition, “Mucus plugging in the airway caused by pneumonia induces hypoxic conditions and leads to the expression of inflammatory markers in the eustachian tube and middle ear mucosa,” they noted.
The study findings were limited by several factors, including the retrospective design and lack of data on microbiological cultures for antibiotic susceptibility, radiologic findings on the severity of pneumonia, results of pulmonary function tests, and hearing thresholds, the researchers noted. Other limitations were the exclusion of the frequency of upper respiratory infections and antibiotic use due to lack of data, they said.
However, the results show an association between pneumonia diagnoses and increased incidence of COM, which suggests a novel perspective that “infection of the lower respiratory tract may affect the function of the eustachian tube and the middle ear to later cause COM,” they concluded.
The study received no outside funding. The researchers have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE INTERNATIONAL JOURNAL OF INFECTIOUS DISEASES
Pneumococcal pneumonia outcomes worse than those of Legionnaires disease
Outcomes for patients with bacteremic Streptococcus pneumoniae were significantly worse than those for patients with Legionnaires disease (LD), based on data from 106 individuals.
Reported cases of LD in the United States have increased in recent decades, but they are likely under-reported, wrote Sima Salahie, MD, of Wayne State University School of Medicine, Detroit, and Central Michigan University College of Medicine, Grosse Pointe Woods, and colleagues.
Clinical presentations may be similar for both conditions, but different antimicrobial therapies are needed; therefore, identifying distinguishing factors can promote better management of hospitalized patients, they reported.
In a retrospective case companion study published in the American Journal of the Medical Sciences, the researchers reviewed data from 51 adults with LD and 55 with bacteremic S. pneumoniae pneumonia (SP) who were hospitalized at a single center between 2013 and 2018. Diagnoses were confirmed by laboratory and radiology results. In addition, data were collected on clinical features including body mass index, systolic and diastolic blood pressure, pulse, respiratory rate, and temperature.
Overall, patients with SP were significantly more likely than those with LD to require mechanical ventilation (P = .04), intensive care unit stay (P = .004), and to die (P = .002). Patients with SP also had higher rates of septic shock compared to LD patients, although this difference fell short of statistical significance (49.1% vs. 30.4%; P = .06).
In a multivariate analysis, male sex, diarrhea, higher body mass index, hyponatremia, and lower Charleston Weighted Index of Comorbidity (CWIC) score were significant independent predictors of LD, with odds ratios of 21.6, 4.5, 1.13, 5.6, and 0.61, respectively.
The incidence of LD peaked in summer, while the incidence of SP peaked in the winter, the researchers noted. “Seasonality is a variable that has not always been included in previous scoring systems but should be considered in future modeling,” they said.
“Noteworthy is that LD represented almost as many cases as documented bacteremic pneumococcal pneumonia,” the researchers wrote in their discussion. “This occurred at a time when there was no outbreak of L. pneumophila in our community, and as these were all community acquired, there was no evidence of a nosocomial outbreak in our institution,” they said.
The study findings were limited by several factors, including the possible underestimation of SP because of the requirement for positive blood cultures and the lack of other methods of diagnosing SP, the researchers noted.
“However, the data suggest variables to distinguish LD from SP,” they said. “Establishing reliable clinical and laboratory parameters embedded in a simple diagnostic score that can accurately identify patients with LD may be helpful in aiding physicians’ early diagnosis in distinguishing LD from SP but will need to be defined.”
The study received no outside funding. The researchers disclosed no financial conflicts.
A version of this article first appeared on Medscape.com.
Outcomes for patients with bacteremic Streptococcus pneumoniae were significantly worse than those for patients with Legionnaires disease (LD), based on data from 106 individuals.
Reported cases of LD in the United States have increased in recent decades, but they are likely under-reported, wrote Sima Salahie, MD, of Wayne State University School of Medicine, Detroit, and Central Michigan University College of Medicine, Grosse Pointe Woods, and colleagues.
Clinical presentations may be similar for both conditions, but different antimicrobial therapies are needed; therefore, identifying distinguishing factors can promote better management of hospitalized patients, they reported.
In a retrospective case companion study published in the American Journal of the Medical Sciences, the researchers reviewed data from 51 adults with LD and 55 with bacteremic S. pneumoniae pneumonia (SP) who were hospitalized at a single center between 2013 and 2018. Diagnoses were confirmed by laboratory and radiology results. In addition, data were collected on clinical features including body mass index, systolic and diastolic blood pressure, pulse, respiratory rate, and temperature.
Overall, patients with SP were significantly more likely than those with LD to require mechanical ventilation (P = .04), intensive care unit stay (P = .004), and to die (P = .002). Patients with SP also had higher rates of septic shock compared to LD patients, although this difference fell short of statistical significance (49.1% vs. 30.4%; P = .06).
In a multivariate analysis, male sex, diarrhea, higher body mass index, hyponatremia, and lower Charleston Weighted Index of Comorbidity (CWIC) score were significant independent predictors of LD, with odds ratios of 21.6, 4.5, 1.13, 5.6, and 0.61, respectively.
The incidence of LD peaked in summer, while the incidence of SP peaked in the winter, the researchers noted. “Seasonality is a variable that has not always been included in previous scoring systems but should be considered in future modeling,” they said.
“Noteworthy is that LD represented almost as many cases as documented bacteremic pneumococcal pneumonia,” the researchers wrote in their discussion. “This occurred at a time when there was no outbreak of L. pneumophila in our community, and as these were all community acquired, there was no evidence of a nosocomial outbreak in our institution,” they said.
The study findings were limited by several factors, including the possible underestimation of SP because of the requirement for positive blood cultures and the lack of other methods of diagnosing SP, the researchers noted.
“However, the data suggest variables to distinguish LD from SP,” they said. “Establishing reliable clinical and laboratory parameters embedded in a simple diagnostic score that can accurately identify patients with LD may be helpful in aiding physicians’ early diagnosis in distinguishing LD from SP but will need to be defined.”
The study received no outside funding. The researchers disclosed no financial conflicts.
A version of this article first appeared on Medscape.com.
Outcomes for patients with bacteremic Streptococcus pneumoniae were significantly worse than those for patients with Legionnaires disease (LD), based on data from 106 individuals.
Reported cases of LD in the United States have increased in recent decades, but they are likely under-reported, wrote Sima Salahie, MD, of Wayne State University School of Medicine, Detroit, and Central Michigan University College of Medicine, Grosse Pointe Woods, and colleagues.
Clinical presentations may be similar for both conditions, but different antimicrobial therapies are needed; therefore, identifying distinguishing factors can promote better management of hospitalized patients, they reported.
In a retrospective case companion study published in the American Journal of the Medical Sciences, the researchers reviewed data from 51 adults with LD and 55 with bacteremic S. pneumoniae pneumonia (SP) who were hospitalized at a single center between 2013 and 2018. Diagnoses were confirmed by laboratory and radiology results. In addition, data were collected on clinical features including body mass index, systolic and diastolic blood pressure, pulse, respiratory rate, and temperature.
Overall, patients with SP were significantly more likely than those with LD to require mechanical ventilation (P = .04), intensive care unit stay (P = .004), and to die (P = .002). Patients with SP also had higher rates of septic shock compared to LD patients, although this difference fell short of statistical significance (49.1% vs. 30.4%; P = .06).
In a multivariate analysis, male sex, diarrhea, higher body mass index, hyponatremia, and lower Charleston Weighted Index of Comorbidity (CWIC) score were significant independent predictors of LD, with odds ratios of 21.6, 4.5, 1.13, 5.6, and 0.61, respectively.
The incidence of LD peaked in summer, while the incidence of SP peaked in the winter, the researchers noted. “Seasonality is a variable that has not always been included in previous scoring systems but should be considered in future modeling,” they said.
“Noteworthy is that LD represented almost as many cases as documented bacteremic pneumococcal pneumonia,” the researchers wrote in their discussion. “This occurred at a time when there was no outbreak of L. pneumophila in our community, and as these were all community acquired, there was no evidence of a nosocomial outbreak in our institution,” they said.
The study findings were limited by several factors, including the possible underestimation of SP because of the requirement for positive blood cultures and the lack of other methods of diagnosing SP, the researchers noted.
“However, the data suggest variables to distinguish LD from SP,” they said. “Establishing reliable clinical and laboratory parameters embedded in a simple diagnostic score that can accurately identify patients with LD may be helpful in aiding physicians’ early diagnosis in distinguishing LD from SP but will need to be defined.”
The study received no outside funding. The researchers disclosed no financial conflicts.
A version of this article first appeared on Medscape.com.
Babies of pregnant women who get RSV vaccine likely to be prescribed fewer antimicrobials
Babies born to moms who were vaccinated against respiratory syncytial virus (RSV) while pregnant appear to need fewer antimicrobial prescriptions than babies of unvaccinated moms, according to authors of a recent study.
To fight antimicrobial resistance, we need to use fewer antimicrobial drugs, the authors write in Proceedings of the National Academy of Sciences.
“In this study, an RSV vaccine was administered to pregnant women to prevent infection in their infants by the transfer of protective antibody to the infant,” Kathryn M. Edwards, MD, a professor of pediatrics and the scientific director of the Vanderbilt vaccine research program at Vanderbilt University in Nashville, Tenn., told this news organization. Dr. Edwards was not involved in the study.
“The authors investigated the impact of the vaccine on the use of antibiotics in infants during the first 90 days of life,” Dr. Edwards added in an email. “They found that the use of antibiotics was less in infants born to mothers who received the RSV vaccine than in infants born to mothers who received placebo. … They suggest that reducing RSV infection in infants will reduce respiratory infections that trigger antibiotic use.”
Senior author Ramanan Laxminarayan, PhD, MPH, director and senior fellow at the Center for Disease Dynamics, Economics & Policy in Washington and his colleagues conducted a secondary analysis of a double-blind, randomized controlled trial at 87 sites in 11 countries on several continents.
In the original study, which was conducted between December 2015 and May 2018, 3,005 maternal participants and 2,978 infant participants received the experimental RSV F vaccine, and 1,573 maternal participants and 1,546 infants received a placebo shot. Baseline characteristics of mothers and infants were well balanced, according to the authors.
In the current study, infants born to mothers who received the RSV vaccine were found to be 12.9% (95% confidence interval, 1.3%-23.1%) less likely to be prescribed antimicrobials during their first 3 months of life, compared with infants whose mothers received placebo. Vaccine efficacy against antimicrobial prescriptions for acute lower respiratory tract infections was 16.9% (95% CI, 1.4%-29.4%).
During the first 3 months of life, for every 100 infants born, maternal vaccination prevented 3.6 courses of antimicrobials in high-income countries (20.2% of all antimicrobial prescribing), and 5.1 courses in low- and middle-income countries (10.9% of all antimicrobial prescribing).
In addition to finding that lower respiratory tract infections accounted for 69%-73% of all antimicrobial prescribing prevented by maternal vaccination, the researchers found marked vaccine efficacy (71.3% [95% CI, 28.1%-88.6%]) against acute otitis media–associated antimicrobial prescription in infants in high-income countries.
RSV vaccine is ‘one of our best investments’
RSV, the authors explain, is a major cause of upper and lower respiratory tract infections that develop as a single agent or along with bacterial pathogens.
“With decreases in bacterial pneumonia following the introduction of the pneumococcal conjugate vaccine, a vaccine against RSV represents one of our best investments to lower the burden of respiratory infections in children,” Dr. Laxminarayan said in a press release.
“These findings are not unexpected because viral infections can trigger bacterial infections such as otitis, and reducing viral infections will reduce bacterial infections,” Dr. Edwards said. “Also, viral infections are often treated with antibiotics because the provider cannot rule out a bacterial infection.”
She acknowledged the value of investigating multiple outcomes but added that “the study was underpowered to assess the full impact of the antibiotics.”
“If a more effective RSV vaccine can be designed, the impact on reducing antibiotic use will likely be even greater,” Dr. Edwards advised. “Also, the vaccine was not highly effective in preventing RSV pneumonia. If it had been more effective, the antibiotic impact would likely have been greater.”
The authors acknowledged the study’s limitations. “Results of this post hoc secondary analysis should be viewed as hypothesis generating, as the trial was not powered for determination of effects against antimicrobial prescribing, and our analyses were not adjusted for multiplicity,” they write, and they joined Dr. Edwards in recommending further related research.
First author Joseph A. Lewnard, PhD, declares financial support from Pfizer unrelated to this research, three authors are employees of Novavax, and Dr. Laxminarayan has disclosed no relevant financial relationships. Dr. Edwards reports funding from the National Institutes of Health and the Centers for Disease Control and Prevention; consultancy to BioNEt and IBM; membership on data safety and monitoring boards for Pfizer, Sanofi, GSK, Merck, X-4 Pharma, Roche, and Seqirus. The Bill & Melinda Gates Foundation supported the study.
A version of this article first appeared on Medscape.com.
Babies born to moms who were vaccinated against respiratory syncytial virus (RSV) while pregnant appear to need fewer antimicrobial prescriptions than babies of unvaccinated moms, according to authors of a recent study.
To fight antimicrobial resistance, we need to use fewer antimicrobial drugs, the authors write in Proceedings of the National Academy of Sciences.
“In this study, an RSV vaccine was administered to pregnant women to prevent infection in their infants by the transfer of protective antibody to the infant,” Kathryn M. Edwards, MD, a professor of pediatrics and the scientific director of the Vanderbilt vaccine research program at Vanderbilt University in Nashville, Tenn., told this news organization. Dr. Edwards was not involved in the study.
“The authors investigated the impact of the vaccine on the use of antibiotics in infants during the first 90 days of life,” Dr. Edwards added in an email. “They found that the use of antibiotics was less in infants born to mothers who received the RSV vaccine than in infants born to mothers who received placebo. … They suggest that reducing RSV infection in infants will reduce respiratory infections that trigger antibiotic use.”
Senior author Ramanan Laxminarayan, PhD, MPH, director and senior fellow at the Center for Disease Dynamics, Economics & Policy in Washington and his colleagues conducted a secondary analysis of a double-blind, randomized controlled trial at 87 sites in 11 countries on several continents.
In the original study, which was conducted between December 2015 and May 2018, 3,005 maternal participants and 2,978 infant participants received the experimental RSV F vaccine, and 1,573 maternal participants and 1,546 infants received a placebo shot. Baseline characteristics of mothers and infants were well balanced, according to the authors.
In the current study, infants born to mothers who received the RSV vaccine were found to be 12.9% (95% confidence interval, 1.3%-23.1%) less likely to be prescribed antimicrobials during their first 3 months of life, compared with infants whose mothers received placebo. Vaccine efficacy against antimicrobial prescriptions for acute lower respiratory tract infections was 16.9% (95% CI, 1.4%-29.4%).
During the first 3 months of life, for every 100 infants born, maternal vaccination prevented 3.6 courses of antimicrobials in high-income countries (20.2% of all antimicrobial prescribing), and 5.1 courses in low- and middle-income countries (10.9% of all antimicrobial prescribing).
In addition to finding that lower respiratory tract infections accounted for 69%-73% of all antimicrobial prescribing prevented by maternal vaccination, the researchers found marked vaccine efficacy (71.3% [95% CI, 28.1%-88.6%]) against acute otitis media–associated antimicrobial prescription in infants in high-income countries.
RSV vaccine is ‘one of our best investments’
RSV, the authors explain, is a major cause of upper and lower respiratory tract infections that develop as a single agent or along with bacterial pathogens.
“With decreases in bacterial pneumonia following the introduction of the pneumococcal conjugate vaccine, a vaccine against RSV represents one of our best investments to lower the burden of respiratory infections in children,” Dr. Laxminarayan said in a press release.
“These findings are not unexpected because viral infections can trigger bacterial infections such as otitis, and reducing viral infections will reduce bacterial infections,” Dr. Edwards said. “Also, viral infections are often treated with antibiotics because the provider cannot rule out a bacterial infection.”
She acknowledged the value of investigating multiple outcomes but added that “the study was underpowered to assess the full impact of the antibiotics.”
“If a more effective RSV vaccine can be designed, the impact on reducing antibiotic use will likely be even greater,” Dr. Edwards advised. “Also, the vaccine was not highly effective in preventing RSV pneumonia. If it had been more effective, the antibiotic impact would likely have been greater.”
The authors acknowledged the study’s limitations. “Results of this post hoc secondary analysis should be viewed as hypothesis generating, as the trial was not powered for determination of effects against antimicrobial prescribing, and our analyses were not adjusted for multiplicity,” they write, and they joined Dr. Edwards in recommending further related research.
First author Joseph A. Lewnard, PhD, declares financial support from Pfizer unrelated to this research, three authors are employees of Novavax, and Dr. Laxminarayan has disclosed no relevant financial relationships. Dr. Edwards reports funding from the National Institutes of Health and the Centers for Disease Control and Prevention; consultancy to BioNEt and IBM; membership on data safety and monitoring boards for Pfizer, Sanofi, GSK, Merck, X-4 Pharma, Roche, and Seqirus. The Bill & Melinda Gates Foundation supported the study.
A version of this article first appeared on Medscape.com.
Babies born to moms who were vaccinated against respiratory syncytial virus (RSV) while pregnant appear to need fewer antimicrobial prescriptions than babies of unvaccinated moms, according to authors of a recent study.
To fight antimicrobial resistance, we need to use fewer antimicrobial drugs, the authors write in Proceedings of the National Academy of Sciences.
“In this study, an RSV vaccine was administered to pregnant women to prevent infection in their infants by the transfer of protective antibody to the infant,” Kathryn M. Edwards, MD, a professor of pediatrics and the scientific director of the Vanderbilt vaccine research program at Vanderbilt University in Nashville, Tenn., told this news organization. Dr. Edwards was not involved in the study.
“The authors investigated the impact of the vaccine on the use of antibiotics in infants during the first 90 days of life,” Dr. Edwards added in an email. “They found that the use of antibiotics was less in infants born to mothers who received the RSV vaccine than in infants born to mothers who received placebo. … They suggest that reducing RSV infection in infants will reduce respiratory infections that trigger antibiotic use.”
Senior author Ramanan Laxminarayan, PhD, MPH, director and senior fellow at the Center for Disease Dynamics, Economics & Policy in Washington and his colleagues conducted a secondary analysis of a double-blind, randomized controlled trial at 87 sites in 11 countries on several continents.
In the original study, which was conducted between December 2015 and May 2018, 3,005 maternal participants and 2,978 infant participants received the experimental RSV F vaccine, and 1,573 maternal participants and 1,546 infants received a placebo shot. Baseline characteristics of mothers and infants were well balanced, according to the authors.
In the current study, infants born to mothers who received the RSV vaccine were found to be 12.9% (95% confidence interval, 1.3%-23.1%) less likely to be prescribed antimicrobials during their first 3 months of life, compared with infants whose mothers received placebo. Vaccine efficacy against antimicrobial prescriptions for acute lower respiratory tract infections was 16.9% (95% CI, 1.4%-29.4%).
During the first 3 months of life, for every 100 infants born, maternal vaccination prevented 3.6 courses of antimicrobials in high-income countries (20.2% of all antimicrobial prescribing), and 5.1 courses in low- and middle-income countries (10.9% of all antimicrobial prescribing).
In addition to finding that lower respiratory tract infections accounted for 69%-73% of all antimicrobial prescribing prevented by maternal vaccination, the researchers found marked vaccine efficacy (71.3% [95% CI, 28.1%-88.6%]) against acute otitis media–associated antimicrobial prescription in infants in high-income countries.
RSV vaccine is ‘one of our best investments’
RSV, the authors explain, is a major cause of upper and lower respiratory tract infections that develop as a single agent or along with bacterial pathogens.
“With decreases in bacterial pneumonia following the introduction of the pneumococcal conjugate vaccine, a vaccine against RSV represents one of our best investments to lower the burden of respiratory infections in children,” Dr. Laxminarayan said in a press release.
“These findings are not unexpected because viral infections can trigger bacterial infections such as otitis, and reducing viral infections will reduce bacterial infections,” Dr. Edwards said. “Also, viral infections are often treated with antibiotics because the provider cannot rule out a bacterial infection.”
She acknowledged the value of investigating multiple outcomes but added that “the study was underpowered to assess the full impact of the antibiotics.”
“If a more effective RSV vaccine can be designed, the impact on reducing antibiotic use will likely be even greater,” Dr. Edwards advised. “Also, the vaccine was not highly effective in preventing RSV pneumonia. If it had been more effective, the antibiotic impact would likely have been greater.”
The authors acknowledged the study’s limitations. “Results of this post hoc secondary analysis should be viewed as hypothesis generating, as the trial was not powered for determination of effects against antimicrobial prescribing, and our analyses were not adjusted for multiplicity,” they write, and they joined Dr. Edwards in recommending further related research.
First author Joseph A. Lewnard, PhD, declares financial support from Pfizer unrelated to this research, three authors are employees of Novavax, and Dr. Laxminarayan has disclosed no relevant financial relationships. Dr. Edwards reports funding from the National Institutes of Health and the Centers for Disease Control and Prevention; consultancy to BioNEt and IBM; membership on data safety and monitoring boards for Pfizer, Sanofi, GSK, Merck, X-4 Pharma, Roche, and Seqirus. The Bill & Melinda Gates Foundation supported the study.
A version of this article first appeared on Medscape.com.
FROM PROCEEDINGS OF THE NATIONAL ACADEMY OF SCIENCES
TB treatment can be shortened for most children: study
The World Health Organization is expected to recommend truncating treatment of children with mild tuberculosis by 2 months – from 6 months to 4 – after a randomized trial found similar outcomes with the shorter regimen.
An international team of investigators found the abbreviated course of antibiotics was no less effective or safe than conventional treatment and saved an average of $17.34 per child – money that could be used to mitigate the toll of TB, which is estimated to sicken 1.1 million children worldwide each year.
The findings come as deaths from TB are rising as a result of the COVID-19 pandemic, which has hindered efforts to find and treat patients. In 2020, according to the WHO, an estimated 1.5 million people died from TB, the first year-over-year increase in such deaths since 2005.
Nearly a quarter of children with TB die, primarily because they go undiagnosed, according to the researchers, who published the study in the New England Journal of Medicine. Shorter treatment “translates into very large cost savings that could be used to improve screening and diagnosis to address the current case detection gap,” first author Anna Turkova, MD, of University College London, told this news organization.
The standard TB regimen is based on trials in adults with severe respiratory disease. However, about two-thirds of children have nonsevere infections.
For the study, Dr. Turkova and colleagues assigned 1,204 children with TB in four countries – Uganda, Zambia, South Africa, and India – to either a 4- or 6-month regimen with first-line medications rifampin, isoniazid, pyrazinamide, and ethambutol. Participants were aged 2 months to 15 years and had symptomatic nonsevere lung or lymph node infections with a negative test on a sputum smear microscopy. Eleven percent also had HIV.
After 18 months, 16 participants in the group that received the shortened treatment and 18 in the standard treatment group had experienced an unfavorable outcome – defined as treatment failure, recurrence of TB, loss to follow-up, or death (adjusted difference, -0.4 percentage points; 95% confidence interval, -2.2 to 1.5).
Similar numbers – 47 in the 4-month group and 48 in the 6-month group – experienced severe or life-threatening adverse events, most commonly chest infections, such as pneumonia, and liver problems, during treatment or up to 30 days after the last dose.
New guidelines coming soon
The WHO plans to issue new guidelines and a handbook for TB management in children and adolescents on March 24, World Tuberculosis Day, a spokesman for the agency told Medscape.
Anna Mandalakas, MD, PhD, director of the Global Tuberculosis Program at Baylor College of Medicine, department of pediatrics, Houston, said the shorter regimen should enable more children to successfully complete TB treatment.
“It can be challenging to convince young children to take medications on a regular basis for 6 months,” Dr. Mandalakas, a member of a WHO guidelines development group that reviewed the study, told this news organization. “Despite best intentions, parents often become fatigued and give up the medicine battle.”
Leo Martinez, PhD, an epidemiologist at Boston University School of Public Health who studies pediatric TB, noted that study’s cost-effectiveness analysis applies only to health care costs. Families often suffer financially through lost wages, transportation to health care facilities, and lost employment, fueling a cycle of poverty and disease in low-income countries, he said.
A WHO statement noted that long treatment regimens can add toxicity and risk of drug interactions for children with HIV.
Separate efforts have been underway to hasten TB treatment in different groups of patients. A study published in NEJM showed that 4 months of the potent antibiotic rifapentine, along with another antibiotic, moxifloxacin, was non-inferior to the standard 6-month regimen in patients aged 12 and older. According to the editorial accompanying that study, the research illustrated the potential for shorter treatment courses that would be cheaper and less cumbersome, although that particular combination poses hurdles such as adherence issues and potential bacterial resistance.
Experts agreed that improved diagnostic procedures are critical to significantly reducing TB pediatric deaths – an issue that Dr. Turkova said will be addressed in WHO’s forthcoming handbook.
Because no gold-standard test exists for TB, and symptoms often overlap with other infections, widespread screening of children in households where adults have been diagnosed with TB has been found to improve detection of the disease. “Training of health care workers, easy-to-implement diagnostic algorithms, and widely accessible training materials on chest radiography in childhood TB should also improve case finding and treatment initiation,” she said.
The trial was supported by U.K. government and charitable research funders. Dr. Turkova and Dr. Martinez reported no financial disclosures. Dr. Mandalakas reported honoraria from WHO to support the preparation of diagnostics and treatment chapters in the operational handbook, for providing lectures for Medscape, and for serving on a data safety monitoring board for Janssen Pharmaceuticals.
A version of this article first appeared on Medscape.com.
The World Health Organization is expected to recommend truncating treatment of children with mild tuberculosis by 2 months – from 6 months to 4 – after a randomized trial found similar outcomes with the shorter regimen.
An international team of investigators found the abbreviated course of antibiotics was no less effective or safe than conventional treatment and saved an average of $17.34 per child – money that could be used to mitigate the toll of TB, which is estimated to sicken 1.1 million children worldwide each year.
The findings come as deaths from TB are rising as a result of the COVID-19 pandemic, which has hindered efforts to find and treat patients. In 2020, according to the WHO, an estimated 1.5 million people died from TB, the first year-over-year increase in such deaths since 2005.
Nearly a quarter of children with TB die, primarily because they go undiagnosed, according to the researchers, who published the study in the New England Journal of Medicine. Shorter treatment “translates into very large cost savings that could be used to improve screening and diagnosis to address the current case detection gap,” first author Anna Turkova, MD, of University College London, told this news organization.
The standard TB regimen is based on trials in adults with severe respiratory disease. However, about two-thirds of children have nonsevere infections.
For the study, Dr. Turkova and colleagues assigned 1,204 children with TB in four countries – Uganda, Zambia, South Africa, and India – to either a 4- or 6-month regimen with first-line medications rifampin, isoniazid, pyrazinamide, and ethambutol. Participants were aged 2 months to 15 years and had symptomatic nonsevere lung or lymph node infections with a negative test on a sputum smear microscopy. Eleven percent also had HIV.
After 18 months, 16 participants in the group that received the shortened treatment and 18 in the standard treatment group had experienced an unfavorable outcome – defined as treatment failure, recurrence of TB, loss to follow-up, or death (adjusted difference, -0.4 percentage points; 95% confidence interval, -2.2 to 1.5).
Similar numbers – 47 in the 4-month group and 48 in the 6-month group – experienced severe or life-threatening adverse events, most commonly chest infections, such as pneumonia, and liver problems, during treatment or up to 30 days after the last dose.
New guidelines coming soon
The WHO plans to issue new guidelines and a handbook for TB management in children and adolescents on March 24, World Tuberculosis Day, a spokesman for the agency told Medscape.
Anna Mandalakas, MD, PhD, director of the Global Tuberculosis Program at Baylor College of Medicine, department of pediatrics, Houston, said the shorter regimen should enable more children to successfully complete TB treatment.
“It can be challenging to convince young children to take medications on a regular basis for 6 months,” Dr. Mandalakas, a member of a WHO guidelines development group that reviewed the study, told this news organization. “Despite best intentions, parents often become fatigued and give up the medicine battle.”
Leo Martinez, PhD, an epidemiologist at Boston University School of Public Health who studies pediatric TB, noted that study’s cost-effectiveness analysis applies only to health care costs. Families often suffer financially through lost wages, transportation to health care facilities, and lost employment, fueling a cycle of poverty and disease in low-income countries, he said.
A WHO statement noted that long treatment regimens can add toxicity and risk of drug interactions for children with HIV.
Separate efforts have been underway to hasten TB treatment in different groups of patients. A study published in NEJM showed that 4 months of the potent antibiotic rifapentine, along with another antibiotic, moxifloxacin, was non-inferior to the standard 6-month regimen in patients aged 12 and older. According to the editorial accompanying that study, the research illustrated the potential for shorter treatment courses that would be cheaper and less cumbersome, although that particular combination poses hurdles such as adherence issues and potential bacterial resistance.
Experts agreed that improved diagnostic procedures are critical to significantly reducing TB pediatric deaths – an issue that Dr. Turkova said will be addressed in WHO’s forthcoming handbook.
Because no gold-standard test exists for TB, and symptoms often overlap with other infections, widespread screening of children in households where adults have been diagnosed with TB has been found to improve detection of the disease. “Training of health care workers, easy-to-implement diagnostic algorithms, and widely accessible training materials on chest radiography in childhood TB should also improve case finding and treatment initiation,” she said.
The trial was supported by U.K. government and charitable research funders. Dr. Turkova and Dr. Martinez reported no financial disclosures. Dr. Mandalakas reported honoraria from WHO to support the preparation of diagnostics and treatment chapters in the operational handbook, for providing lectures for Medscape, and for serving on a data safety monitoring board for Janssen Pharmaceuticals.
A version of this article first appeared on Medscape.com.
The World Health Organization is expected to recommend truncating treatment of children with mild tuberculosis by 2 months – from 6 months to 4 – after a randomized trial found similar outcomes with the shorter regimen.
An international team of investigators found the abbreviated course of antibiotics was no less effective or safe than conventional treatment and saved an average of $17.34 per child – money that could be used to mitigate the toll of TB, which is estimated to sicken 1.1 million children worldwide each year.
The findings come as deaths from TB are rising as a result of the COVID-19 pandemic, which has hindered efforts to find and treat patients. In 2020, according to the WHO, an estimated 1.5 million people died from TB, the first year-over-year increase in such deaths since 2005.
Nearly a quarter of children with TB die, primarily because they go undiagnosed, according to the researchers, who published the study in the New England Journal of Medicine. Shorter treatment “translates into very large cost savings that could be used to improve screening and diagnosis to address the current case detection gap,” first author Anna Turkova, MD, of University College London, told this news organization.
The standard TB regimen is based on trials in adults with severe respiratory disease. However, about two-thirds of children have nonsevere infections.
For the study, Dr. Turkova and colleagues assigned 1,204 children with TB in four countries – Uganda, Zambia, South Africa, and India – to either a 4- or 6-month regimen with first-line medications rifampin, isoniazid, pyrazinamide, and ethambutol. Participants were aged 2 months to 15 years and had symptomatic nonsevere lung or lymph node infections with a negative test on a sputum smear microscopy. Eleven percent also had HIV.
After 18 months, 16 participants in the group that received the shortened treatment and 18 in the standard treatment group had experienced an unfavorable outcome – defined as treatment failure, recurrence of TB, loss to follow-up, or death (adjusted difference, -0.4 percentage points; 95% confidence interval, -2.2 to 1.5).
Similar numbers – 47 in the 4-month group and 48 in the 6-month group – experienced severe or life-threatening adverse events, most commonly chest infections, such as pneumonia, and liver problems, during treatment or up to 30 days after the last dose.
New guidelines coming soon
The WHO plans to issue new guidelines and a handbook for TB management in children and adolescents on March 24, World Tuberculosis Day, a spokesman for the agency told Medscape.
Anna Mandalakas, MD, PhD, director of the Global Tuberculosis Program at Baylor College of Medicine, department of pediatrics, Houston, said the shorter regimen should enable more children to successfully complete TB treatment.
“It can be challenging to convince young children to take medications on a regular basis for 6 months,” Dr. Mandalakas, a member of a WHO guidelines development group that reviewed the study, told this news organization. “Despite best intentions, parents often become fatigued and give up the medicine battle.”
Leo Martinez, PhD, an epidemiologist at Boston University School of Public Health who studies pediatric TB, noted that study’s cost-effectiveness analysis applies only to health care costs. Families often suffer financially through lost wages, transportation to health care facilities, and lost employment, fueling a cycle of poverty and disease in low-income countries, he said.
A WHO statement noted that long treatment regimens can add toxicity and risk of drug interactions for children with HIV.
Separate efforts have been underway to hasten TB treatment in different groups of patients. A study published in NEJM showed that 4 months of the potent antibiotic rifapentine, along with another antibiotic, moxifloxacin, was non-inferior to the standard 6-month regimen in patients aged 12 and older. According to the editorial accompanying that study, the research illustrated the potential for shorter treatment courses that would be cheaper and less cumbersome, although that particular combination poses hurdles such as adherence issues and potential bacterial resistance.
Experts agreed that improved diagnostic procedures are critical to significantly reducing TB pediatric deaths – an issue that Dr. Turkova said will be addressed in WHO’s forthcoming handbook.
Because no gold-standard test exists for TB, and symptoms often overlap with other infections, widespread screening of children in households where adults have been diagnosed with TB has been found to improve detection of the disease. “Training of health care workers, easy-to-implement diagnostic algorithms, and widely accessible training materials on chest radiography in childhood TB should also improve case finding and treatment initiation,” she said.
The trial was supported by U.K. government and charitable research funders. Dr. Turkova and Dr. Martinez reported no financial disclosures. Dr. Mandalakas reported honoraria from WHO to support the preparation of diagnostics and treatment chapters in the operational handbook, for providing lectures for Medscape, and for serving on a data safety monitoring board for Janssen Pharmaceuticals.
A version of this article first appeared on Medscape.com.



