User login
China’s health authorities release large coronavirus case series
The Chinese Center for Disease Control and Prevention has released the largest case series to date for novel coronavirus 2019 (COVID-19), and a summary of key findings appears in JAMA.
- The virus, which spread from a single city to a whole country in only 30 days, has so far has caused over 72,314 cases as of Feb. 11, 2020, and 1,023 fatalities (2.3%) overall.
- The age distribution shows that most of the cases (87%) occurred in patients aged 30-79 years, while 10% were in patients 29 years and younger and 3% at 80 years and older.
- Following the SARS outbreak in 2002-2003, the Chinese government adjusted its epidemic response protocol. For example, according to the summary, while there were 300 cases and 5 deaths with SARS before the Chinese government reported it to the World Health Organization, there were only 27 cases and no deaths with COVID-19 before it was reported to that agency.
- A major goal, the authors wrote, is to buy enough time for scientific research, hopefully before the disease has become too widespread.
The summary argues that, while some measures the Chinese government has taken could be seen as extreme, the overall benefits and lives saved outweigh the potential infringement on civil liberties. It also suggests that countries need to work together in situations like this because disease pathogens do not respect geopolitical borders.
SOURCE: Wu Z, McGoogan JM. JAMA. 2020 Feb 24. doi: 10.1001/jama.2020.2648.
The Chinese Center for Disease Control and Prevention has released the largest case series to date for novel coronavirus 2019 (COVID-19), and a summary of key findings appears in JAMA.
- The virus, which spread from a single city to a whole country in only 30 days, has so far has caused over 72,314 cases as of Feb. 11, 2020, and 1,023 fatalities (2.3%) overall.
- The age distribution shows that most of the cases (87%) occurred in patients aged 30-79 years, while 10% were in patients 29 years and younger and 3% at 80 years and older.
- Following the SARS outbreak in 2002-2003, the Chinese government adjusted its epidemic response protocol. For example, according to the summary, while there were 300 cases and 5 deaths with SARS before the Chinese government reported it to the World Health Organization, there were only 27 cases and no deaths with COVID-19 before it was reported to that agency.
- A major goal, the authors wrote, is to buy enough time for scientific research, hopefully before the disease has become too widespread.
The summary argues that, while some measures the Chinese government has taken could be seen as extreme, the overall benefits and lives saved outweigh the potential infringement on civil liberties. It also suggests that countries need to work together in situations like this because disease pathogens do not respect geopolitical borders.
SOURCE: Wu Z, McGoogan JM. JAMA. 2020 Feb 24. doi: 10.1001/jama.2020.2648.
The Chinese Center for Disease Control and Prevention has released the largest case series to date for novel coronavirus 2019 (COVID-19), and a summary of key findings appears in JAMA.
- The virus, which spread from a single city to a whole country in only 30 days, has so far has caused over 72,314 cases as of Feb. 11, 2020, and 1,023 fatalities (2.3%) overall.
- The age distribution shows that most of the cases (87%) occurred in patients aged 30-79 years, while 10% were in patients 29 years and younger and 3% at 80 years and older.
- Following the SARS outbreak in 2002-2003, the Chinese government adjusted its epidemic response protocol. For example, according to the summary, while there were 300 cases and 5 deaths with SARS before the Chinese government reported it to the World Health Organization, there were only 27 cases and no deaths with COVID-19 before it was reported to that agency.
- A major goal, the authors wrote, is to buy enough time for scientific research, hopefully before the disease has become too widespread.
The summary argues that, while some measures the Chinese government has taken could be seen as extreme, the overall benefits and lives saved outweigh the potential infringement on civil liberties. It also suggests that countries need to work together in situations like this because disease pathogens do not respect geopolitical borders.
SOURCE: Wu Z, McGoogan JM. JAMA. 2020 Feb 24. doi: 10.1001/jama.2020.2648.
FROM JAMA
Study implicates gut bacteria in PAH
Model finds microbiota highly predictive
A unique collection of bacteria in the gut may have a strong association with pulmonary arterial hypertension and could be highly predictive of the disease in undiagnosed patients, according to a study published in the journal Hypertension.
This is the first study to show that people with PAH have a common specific gut microbiota profile, wrote lead study author Mohan Raizada, PhD, distinguished professor in the department of physiology and functional genomics at the University of Florida, Gainesville.
The findings have the potential to change how cardiologists diagnose and treat PAH, he added. “While current PAH treatments focus on the lungs, looking at the lung/gut axis could open the door to new therapies centered in the digestive system,” Dr. Raizada said.
The researchers developed a model that found the specific microbiota profile was 83% accurate in predicting the presence or absence of PAH. If a larger study can validate the findings, the researchers wrote, this could lead to a new test for diagnosing PAH that’s less invasive than cardiac catheterization. It could also lead to new treatments that target the gut microbiome.
Study investigators collected stool samples from 18 PAH patients and 12 people without a history of cardiopulmonary disease. The microbiota DNA from the stool samples were isolated and sequenced. The analysis revealed that PAH patients had reduced richness and evenness of the gut bacteria, known as alpha diversity. They had increased levels of bacteria associated with atherosclerosis, and healthy patients had increased levels of bacteria that produced short-chain fatty acids.
Although recent studies have begun to show potential associations between the gut microbiome and cardiovascular diseases, this research is in its infancy, Mariell Jessup, MD, commented. “Even though the study by Dr. Raizada and colleagues predicted pulmonary arterial hypertension based on an individual’s microbiome with some accuracy, it is an observational study, so it does not prove cause and effect. Many other factors, especially diet, affect the gut microbiome,” added Dr. Jessup, Chief Science and Medical Officer for the American Heart Association.
She stressed that, “In addition, even if studies confirm an association between the gut microbiome and cardiovascular diseases such as PAH, more research is needed to determine if improving gut microbiota could directly impact PAH or other cardiovascular diseases. The findings of this study will not impact clinical practice.”
Dr. Raizada and his coinvestigators offered two possible mechanisms through which the gut microbiome influences pulmonary physiology. One is that lower levels of bacteria that produce the short-chain fatty acid butyrate, such as Coprococcus, Butyrivibrio, Lachnospiraceae, and Eubacterium, along with Clostridia in the gut of PAH patients, may increase gut permeability. Reduced butyrate weakens gut barrier function and can induce inflammation and leakage. This can allow microbial metabolites to enter the circulatory system, disrupting metabolism and immunity and affecting pulmonary vessels.
The second potential mechanism is that increased Collinsella in the PAH cohort may be the culprit that increases gut permeability, resulting in the ensuing gut barrier dysfunction and inflammation. The study noted Collinsella contributed most of the increased genes for the biosynthesis on the amino acid proline in these patients, and that a previous study implicated Collinsella and its parent, Cariobacteriales, in trimethylamine/trimethylamine N-oxide production (TMA/TMAO) in atherosclerosis (Cell. 2015;163[7]:1585-95). The non-PAH patients had higher levels of bacteria that had a low correlation with TMA/TMAO.
“We were very surprised to see such an association within a small group of study subjects,” wrote Dr. Raizada and associates. “It usually requires hundreds of patients to achieve such significance.”
More research is needed to determine if the specific microbiota associated with PAH causes the disease or is a result of it, they concluded.
The study was funded by grants from the National Institutes of Health, the NIH National Center for Research Resources, and the U.S. Department of Defense. Dr. Raizada and coauthors reported no relevant financial relationships.
SOURCE: Raizada MK et al. Hypertension. 2020. doi: 10.1161/HYPERTENSIONAHA.119.14294.
Model finds microbiota highly predictive
Model finds microbiota highly predictive
A unique collection of bacteria in the gut may have a strong association with pulmonary arterial hypertension and could be highly predictive of the disease in undiagnosed patients, according to a study published in the journal Hypertension.
This is the first study to show that people with PAH have a common specific gut microbiota profile, wrote lead study author Mohan Raizada, PhD, distinguished professor in the department of physiology and functional genomics at the University of Florida, Gainesville.
The findings have the potential to change how cardiologists diagnose and treat PAH, he added. “While current PAH treatments focus on the lungs, looking at the lung/gut axis could open the door to new therapies centered in the digestive system,” Dr. Raizada said.
The researchers developed a model that found the specific microbiota profile was 83% accurate in predicting the presence or absence of PAH. If a larger study can validate the findings, the researchers wrote, this could lead to a new test for diagnosing PAH that’s less invasive than cardiac catheterization. It could also lead to new treatments that target the gut microbiome.
Study investigators collected stool samples from 18 PAH patients and 12 people without a history of cardiopulmonary disease. The microbiota DNA from the stool samples were isolated and sequenced. The analysis revealed that PAH patients had reduced richness and evenness of the gut bacteria, known as alpha diversity. They had increased levels of bacteria associated with atherosclerosis, and healthy patients had increased levels of bacteria that produced short-chain fatty acids.
Although recent studies have begun to show potential associations between the gut microbiome and cardiovascular diseases, this research is in its infancy, Mariell Jessup, MD, commented. “Even though the study by Dr. Raizada and colleagues predicted pulmonary arterial hypertension based on an individual’s microbiome with some accuracy, it is an observational study, so it does not prove cause and effect. Many other factors, especially diet, affect the gut microbiome,” added Dr. Jessup, Chief Science and Medical Officer for the American Heart Association.
She stressed that, “In addition, even if studies confirm an association between the gut microbiome and cardiovascular diseases such as PAH, more research is needed to determine if improving gut microbiota could directly impact PAH or other cardiovascular diseases. The findings of this study will not impact clinical practice.”
Dr. Raizada and his coinvestigators offered two possible mechanisms through which the gut microbiome influences pulmonary physiology. One is that lower levels of bacteria that produce the short-chain fatty acid butyrate, such as Coprococcus, Butyrivibrio, Lachnospiraceae, and Eubacterium, along with Clostridia in the gut of PAH patients, may increase gut permeability. Reduced butyrate weakens gut barrier function and can induce inflammation and leakage. This can allow microbial metabolites to enter the circulatory system, disrupting metabolism and immunity and affecting pulmonary vessels.
The second potential mechanism is that increased Collinsella in the PAH cohort may be the culprit that increases gut permeability, resulting in the ensuing gut barrier dysfunction and inflammation. The study noted Collinsella contributed most of the increased genes for the biosynthesis on the amino acid proline in these patients, and that a previous study implicated Collinsella and its parent, Cariobacteriales, in trimethylamine/trimethylamine N-oxide production (TMA/TMAO) in atherosclerosis (Cell. 2015;163[7]:1585-95). The non-PAH patients had higher levels of bacteria that had a low correlation with TMA/TMAO.
“We were very surprised to see such an association within a small group of study subjects,” wrote Dr. Raizada and associates. “It usually requires hundreds of patients to achieve such significance.”
More research is needed to determine if the specific microbiota associated with PAH causes the disease or is a result of it, they concluded.
The study was funded by grants from the National Institutes of Health, the NIH National Center for Research Resources, and the U.S. Department of Defense. Dr. Raizada and coauthors reported no relevant financial relationships.
SOURCE: Raizada MK et al. Hypertension. 2020. doi: 10.1161/HYPERTENSIONAHA.119.14294.
A unique collection of bacteria in the gut may have a strong association with pulmonary arterial hypertension and could be highly predictive of the disease in undiagnosed patients, according to a study published in the journal Hypertension.
This is the first study to show that people with PAH have a common specific gut microbiota profile, wrote lead study author Mohan Raizada, PhD, distinguished professor in the department of physiology and functional genomics at the University of Florida, Gainesville.
The findings have the potential to change how cardiologists diagnose and treat PAH, he added. “While current PAH treatments focus on the lungs, looking at the lung/gut axis could open the door to new therapies centered in the digestive system,” Dr. Raizada said.
The researchers developed a model that found the specific microbiota profile was 83% accurate in predicting the presence or absence of PAH. If a larger study can validate the findings, the researchers wrote, this could lead to a new test for diagnosing PAH that’s less invasive than cardiac catheterization. It could also lead to new treatments that target the gut microbiome.
Study investigators collected stool samples from 18 PAH patients and 12 people without a history of cardiopulmonary disease. The microbiota DNA from the stool samples were isolated and sequenced. The analysis revealed that PAH patients had reduced richness and evenness of the gut bacteria, known as alpha diversity. They had increased levels of bacteria associated with atherosclerosis, and healthy patients had increased levels of bacteria that produced short-chain fatty acids.
Although recent studies have begun to show potential associations between the gut microbiome and cardiovascular diseases, this research is in its infancy, Mariell Jessup, MD, commented. “Even though the study by Dr. Raizada and colleagues predicted pulmonary arterial hypertension based on an individual’s microbiome with some accuracy, it is an observational study, so it does not prove cause and effect. Many other factors, especially diet, affect the gut microbiome,” added Dr. Jessup, Chief Science and Medical Officer for the American Heart Association.
She stressed that, “In addition, even if studies confirm an association between the gut microbiome and cardiovascular diseases such as PAH, more research is needed to determine if improving gut microbiota could directly impact PAH or other cardiovascular diseases. The findings of this study will not impact clinical practice.”
Dr. Raizada and his coinvestigators offered two possible mechanisms through which the gut microbiome influences pulmonary physiology. One is that lower levels of bacteria that produce the short-chain fatty acid butyrate, such as Coprococcus, Butyrivibrio, Lachnospiraceae, and Eubacterium, along with Clostridia in the gut of PAH patients, may increase gut permeability. Reduced butyrate weakens gut barrier function and can induce inflammation and leakage. This can allow microbial metabolites to enter the circulatory system, disrupting metabolism and immunity and affecting pulmonary vessels.
The second potential mechanism is that increased Collinsella in the PAH cohort may be the culprit that increases gut permeability, resulting in the ensuing gut barrier dysfunction and inflammation. The study noted Collinsella contributed most of the increased genes for the biosynthesis on the amino acid proline in these patients, and that a previous study implicated Collinsella and its parent, Cariobacteriales, in trimethylamine/trimethylamine N-oxide production (TMA/TMAO) in atherosclerosis (Cell. 2015;163[7]:1585-95). The non-PAH patients had higher levels of bacteria that had a low correlation with TMA/TMAO.
“We were very surprised to see such an association within a small group of study subjects,” wrote Dr. Raizada and associates. “It usually requires hundreds of patients to achieve such significance.”
More research is needed to determine if the specific microbiota associated with PAH causes the disease or is a result of it, they concluded.
The study was funded by grants from the National Institutes of Health, the NIH National Center for Research Resources, and the U.S. Department of Defense. Dr. Raizada and coauthors reported no relevant financial relationships.
SOURCE: Raizada MK et al. Hypertension. 2020. doi: 10.1161/HYPERTENSIONAHA.119.14294.
FROM HYPERTENSION
Guidance defines vaping-related respiratory syndrome
ORLANDO – Knowledge of vaping devices, familiarity with terminology, and the ability to quickly pinpoint individuals at risk of lung injury are just a few skills that can help critical care professionals confronted with patients who may have vaping-associated lung disease, according to a new guidance document.
The guidance offers a risk-stratification system that classifies patients into groups based on exposure, symptoms, and imaging results, and provides specific evaluation needs and management strategies for each. The guidance is designed to help critical care professionals efficiently identify those at high risk of respiratory failure.
Physicians also need to communicate with patients to identify what substances are being vaped and develop effective methods to encourage abstinence, according to the authors, led by Craig M. Lilly, MD, FCCP, professor of medicine, anesthesiology, and surgery at the University of Massachusetts, Worcester.
“I would encourage every intensivist, when they leave their intensive care unit at night, [to ask], ‘have I advised against vaping today?’ ” Dr. Lilly said at the Critical Care Congress sponsored by the Society of Critical Care Medicine.
The guidelines, concurrently published as a review article in Critical Care Explorations, propose the term vaping-associated respiratory distress syndrome (VARDS), which the authors say constitutes an acute and progressive respiratory syndrome marked by pathologic changes of lung injury and potentially life-threatening hypoxemic respiratory failure.
They also introduce the three-group Worcester classification system, which is intended to triage vaping-exposed individuals for risk of VARDS based on the presence or absence of vaping-related symptoms and infiltrates, and normal or abnormal oxygen saturation.
“It’s very simple,” said Dr. Lilly, who added that the risk stratification model was developed at the request of Massachusetts public health officials.
Patients with vaping exposure but no symptoms attributable to vaping, such as cough, chest pain, or weight loss, are classified as Worcester Low Risk and testing is not recommended, he said.
By contrast, individuals are considered Worcester Medium Risk if they have vaping exposure, symptoms, and a vaping-associated abnormal pattern on imaging, but no hypoxemia; the presence of hypoxemia would tip the scale toward Worcester High Risk.
“Most patients that have died from vaping have been sent out of emergency rooms when they were noted to be hypoxic,” Dr. Lilly told meeting attendees.
Louella B. Amos, MD, a pediatric pulmonologist at Children’s Hospital of Wisconsin in Milwaukee, said she expects the guidance and risk stratification system will be useful not only for critical care specialists, but for other health care providers as well.
“It’s important to make decisions relatively quickly, depending on the severity of symptoms, and I think this is nice and simple,” Dr. Amos said in an interview.
“We always triage when we see patients, either at the door or in our clinic, or behind that, even in the hospital,” she said. “So I think this can be a great tool for everybody, not only the intensivist, but people who are triaging at the front.”
Management of individuals at low risk of VARDS begins with encouragement of abstinence. “We think that every vaping patient should be advised to quit vaping,” Dr. Lilly said. Patients who are interested in quitting who have not yet worked with someone in their health care team whom they trust can be referred to their primary care physicians for counseling, he added, while those struggling with addiction, unable to quit, and unable to partner with a primary care physician can be referred to an addiction medicine specialist.
For moderate-risk patients, vaping cessation is “absolutely mandatory,” said Dr. Lilly, who recommended monitoring of vaping abstinence, outpatient evaluation based on imaging studies, and adequate follow-up to ensure symptoms resolve, tests normalize, and daily activities bounce back to baseline levels.
The guidance offers more extensive recommendations for the VARDS high-risk group, including supervised vaping abstinence, continuous pulse oximetry, and early intervention with noninvasive ventilation, and mechanical ventilation if required, Dr. Lilly said.
Judging vaping exposure is challenging, requiring clinicians to have a familiarity with the many different devices that are available.
Beyond device type, he added, it’s important to know the various terms for devices and lingo that patients may use to describe them, what solutions are vaped, whether those solutions are commercially prepared or off the street, the dose the device delivers, and a number of other factors, he said.
Clinical evaluation typically comes down to unexplained cough, chest pain, weight loss, fatigue, or dyspnea, though one other clue is whether there are gastrointestinal symptoms: “The same way that aerosols can go down to the lungs, they also go into the GI tract, and when nausea, vomiting, or cramping abdominal pain is tightly associated with vaping exposure, one should assume that the patient has been toxin exposed,” he explained.
Dr. Lilly said he had no financial relationships to disclose.
ORLANDO – Knowledge of vaping devices, familiarity with terminology, and the ability to quickly pinpoint individuals at risk of lung injury are just a few skills that can help critical care professionals confronted with patients who may have vaping-associated lung disease, according to a new guidance document.
The guidance offers a risk-stratification system that classifies patients into groups based on exposure, symptoms, and imaging results, and provides specific evaluation needs and management strategies for each. The guidance is designed to help critical care professionals efficiently identify those at high risk of respiratory failure.
Physicians also need to communicate with patients to identify what substances are being vaped and develop effective methods to encourage abstinence, according to the authors, led by Craig M. Lilly, MD, FCCP, professor of medicine, anesthesiology, and surgery at the University of Massachusetts, Worcester.
“I would encourage every intensivist, when they leave their intensive care unit at night, [to ask], ‘have I advised against vaping today?’ ” Dr. Lilly said at the Critical Care Congress sponsored by the Society of Critical Care Medicine.
The guidelines, concurrently published as a review article in Critical Care Explorations, propose the term vaping-associated respiratory distress syndrome (VARDS), which the authors say constitutes an acute and progressive respiratory syndrome marked by pathologic changes of lung injury and potentially life-threatening hypoxemic respiratory failure.
They also introduce the three-group Worcester classification system, which is intended to triage vaping-exposed individuals for risk of VARDS based on the presence or absence of vaping-related symptoms and infiltrates, and normal or abnormal oxygen saturation.
“It’s very simple,” said Dr. Lilly, who added that the risk stratification model was developed at the request of Massachusetts public health officials.
Patients with vaping exposure but no symptoms attributable to vaping, such as cough, chest pain, or weight loss, are classified as Worcester Low Risk and testing is not recommended, he said.
By contrast, individuals are considered Worcester Medium Risk if they have vaping exposure, symptoms, and a vaping-associated abnormal pattern on imaging, but no hypoxemia; the presence of hypoxemia would tip the scale toward Worcester High Risk.
“Most patients that have died from vaping have been sent out of emergency rooms when they were noted to be hypoxic,” Dr. Lilly told meeting attendees.
Louella B. Amos, MD, a pediatric pulmonologist at Children’s Hospital of Wisconsin in Milwaukee, said she expects the guidance and risk stratification system will be useful not only for critical care specialists, but for other health care providers as well.
“It’s important to make decisions relatively quickly, depending on the severity of symptoms, and I think this is nice and simple,” Dr. Amos said in an interview.
“We always triage when we see patients, either at the door or in our clinic, or behind that, even in the hospital,” she said. “So I think this can be a great tool for everybody, not only the intensivist, but people who are triaging at the front.”
Management of individuals at low risk of VARDS begins with encouragement of abstinence. “We think that every vaping patient should be advised to quit vaping,” Dr. Lilly said. Patients who are interested in quitting who have not yet worked with someone in their health care team whom they trust can be referred to their primary care physicians for counseling, he added, while those struggling with addiction, unable to quit, and unable to partner with a primary care physician can be referred to an addiction medicine specialist.
For moderate-risk patients, vaping cessation is “absolutely mandatory,” said Dr. Lilly, who recommended monitoring of vaping abstinence, outpatient evaluation based on imaging studies, and adequate follow-up to ensure symptoms resolve, tests normalize, and daily activities bounce back to baseline levels.
The guidance offers more extensive recommendations for the VARDS high-risk group, including supervised vaping abstinence, continuous pulse oximetry, and early intervention with noninvasive ventilation, and mechanical ventilation if required, Dr. Lilly said.
Judging vaping exposure is challenging, requiring clinicians to have a familiarity with the many different devices that are available.
Beyond device type, he added, it’s important to know the various terms for devices and lingo that patients may use to describe them, what solutions are vaped, whether those solutions are commercially prepared or off the street, the dose the device delivers, and a number of other factors, he said.
Clinical evaluation typically comes down to unexplained cough, chest pain, weight loss, fatigue, or dyspnea, though one other clue is whether there are gastrointestinal symptoms: “The same way that aerosols can go down to the lungs, they also go into the GI tract, and when nausea, vomiting, or cramping abdominal pain is tightly associated with vaping exposure, one should assume that the patient has been toxin exposed,” he explained.
Dr. Lilly said he had no financial relationships to disclose.
ORLANDO – Knowledge of vaping devices, familiarity with terminology, and the ability to quickly pinpoint individuals at risk of lung injury are just a few skills that can help critical care professionals confronted with patients who may have vaping-associated lung disease, according to a new guidance document.
The guidance offers a risk-stratification system that classifies patients into groups based on exposure, symptoms, and imaging results, and provides specific evaluation needs and management strategies for each. The guidance is designed to help critical care professionals efficiently identify those at high risk of respiratory failure.
Physicians also need to communicate with patients to identify what substances are being vaped and develop effective methods to encourage abstinence, according to the authors, led by Craig M. Lilly, MD, FCCP, professor of medicine, anesthesiology, and surgery at the University of Massachusetts, Worcester.
“I would encourage every intensivist, when they leave their intensive care unit at night, [to ask], ‘have I advised against vaping today?’ ” Dr. Lilly said at the Critical Care Congress sponsored by the Society of Critical Care Medicine.
The guidelines, concurrently published as a review article in Critical Care Explorations, propose the term vaping-associated respiratory distress syndrome (VARDS), which the authors say constitutes an acute and progressive respiratory syndrome marked by pathologic changes of lung injury and potentially life-threatening hypoxemic respiratory failure.
They also introduce the three-group Worcester classification system, which is intended to triage vaping-exposed individuals for risk of VARDS based on the presence or absence of vaping-related symptoms and infiltrates, and normal or abnormal oxygen saturation.
“It’s very simple,” said Dr. Lilly, who added that the risk stratification model was developed at the request of Massachusetts public health officials.
Patients with vaping exposure but no symptoms attributable to vaping, such as cough, chest pain, or weight loss, are classified as Worcester Low Risk and testing is not recommended, he said.
By contrast, individuals are considered Worcester Medium Risk if they have vaping exposure, symptoms, and a vaping-associated abnormal pattern on imaging, but no hypoxemia; the presence of hypoxemia would tip the scale toward Worcester High Risk.
“Most patients that have died from vaping have been sent out of emergency rooms when they were noted to be hypoxic,” Dr. Lilly told meeting attendees.
Louella B. Amos, MD, a pediatric pulmonologist at Children’s Hospital of Wisconsin in Milwaukee, said she expects the guidance and risk stratification system will be useful not only for critical care specialists, but for other health care providers as well.
“It’s important to make decisions relatively quickly, depending on the severity of symptoms, and I think this is nice and simple,” Dr. Amos said in an interview.
“We always triage when we see patients, either at the door or in our clinic, or behind that, even in the hospital,” she said. “So I think this can be a great tool for everybody, not only the intensivist, but people who are triaging at the front.”
Management of individuals at low risk of VARDS begins with encouragement of abstinence. “We think that every vaping patient should be advised to quit vaping,” Dr. Lilly said. Patients who are interested in quitting who have not yet worked with someone in their health care team whom they trust can be referred to their primary care physicians for counseling, he added, while those struggling with addiction, unable to quit, and unable to partner with a primary care physician can be referred to an addiction medicine specialist.
For moderate-risk patients, vaping cessation is “absolutely mandatory,” said Dr. Lilly, who recommended monitoring of vaping abstinence, outpatient evaluation based on imaging studies, and adequate follow-up to ensure symptoms resolve, tests normalize, and daily activities bounce back to baseline levels.
The guidance offers more extensive recommendations for the VARDS high-risk group, including supervised vaping abstinence, continuous pulse oximetry, and early intervention with noninvasive ventilation, and mechanical ventilation if required, Dr. Lilly said.
Judging vaping exposure is challenging, requiring clinicians to have a familiarity with the many different devices that are available.
Beyond device type, he added, it’s important to know the various terms for devices and lingo that patients may use to describe them, what solutions are vaped, whether those solutions are commercially prepared or off the street, the dose the device delivers, and a number of other factors, he said.
Clinical evaluation typically comes down to unexplained cough, chest pain, weight loss, fatigue, or dyspnea, though one other clue is whether there are gastrointestinal symptoms: “The same way that aerosols can go down to the lungs, they also go into the GI tract, and when nausea, vomiting, or cramping abdominal pain is tightly associated with vaping exposure, one should assume that the patient has been toxin exposed,” he explained.
Dr. Lilly said he had no financial relationships to disclose.
REPORTING FROM CCC49
Vitamin E acetate found in more vapers’ lung fluid
Analysis of additional lung fluid samples confirms the presence of vitamin E acetate in patients with electronic-cigarette, or vaping, product use–associated lung injury, according to a report on 51 patients in 16 states.
The average age of the patients was 23 years; 69% were male.
The report extends previous work by the Centers for Disease Control and Prevention to test for harmful substances in bronchoalveolar-lavage (BAL) fluid obtained from patients with electronic-cigarette, or vaping, product use–associated lung injury (EVALI) as part of a strategy to understand and manage the recent outbreak of EVALI cases in the United States, wrote Benjamin C. Blount, PhD, of the Division of Laboratory Sciences at the CDC’s National Center for Environmental Health, and colleagues.
“CDC was addressing a serious outbreak of lung injury that was sometimes lethal; but after the first 10 weeks of the outbreak investigation, the cause was still unknown,” Dr. Blount said in an interview. “Possible theories could not be evaluated unless the laboratory could develop tests that could confidently connect exposure to lung injury. Detection of toxicants in bronchoalveolar-lavage fluid from patients with EVALI can provide direct information on exposure within the lung.”
In a study published in the New England Journal of Medicine, the researchers examined the BAL of 51 cases of EVALI from 16 states. They analyzed the samples for multiple toxicants, including vitamin E acetate, plant oils, medium-chain triglyceride oil, coconut oil, petroleum distillates, and diluent terpenes.
Overall, 77% of the patients reported using products containing THC, 67% reported using products containing nicotine, and 51% reported using both types.
Researchers found vitamin E acetate in 48 of the 51 patients (94%); no vitamin E acetate was found in the BAL of healthy controls. Coconut oil and limonene were found in one patient each, but none of the other toxicants was found in the samples from the patients or controls.
In addition, 47 of the 50 patients for whom data were available either had detectable tetrahydrocannabinol (THC) or its metabolites in their BAL fluid samples, or they reported vaping THC products within 90 days before they became ill. Nicotine or its metabolites were found in 30 of 47 patients (64%).
The study findings were limited by several factors, including the potential role of vitamin E acetate as a marker for exposure to other toxicants, the uncertainty of the role of aerosolized constituents formed when vitamin E acetate is heated, and the lack of data on the timing and burden of toxicant exposure, the investigators noted.
As for the next steps in research, “additional studies are needed to examine the respiratory effects of inhaling aerosolized vitamin E acetate and provide information on whether vitamin E acetate in isolation causes lung injury,” Dr. Blount explained. Analysis of the aerosol and gases generated by case-associated product fluids is ongoing.
“When CDC developed the BAL study for this response, we considered several possible toxicants in this investigation to find a possible cause of the outbreak,” Dr. Blount noted. “To accomplish the study, CDC’s Environmental Health Laboratory developed 12 analytical methods and validated them in less than 3 weeks because of the urgent nature of the emergency.”
Dr. Blount said he would advise clinicians to “continue to reference CDC guidance on treating suspected or EVALI patients.” In December, the CDC published updated guidance for clinicians on hospitalized EVALI patients. “Following this guidance and other recommendations could reduce EVALI-associated morbidity and mortality,” Dr. Blount said.
The study was supported in part by the National Cancer Institute, the FDA Center for Tobacco Products, and Ohio State University Pelotonia Intramural Research. The researchers had no financial conflicts to disclose.
SOURCE: Blount BC et al. N Engl J Med. 2020 Feb 20. doi: 10.1056/NEJMoa1916433.
Analysis of additional lung fluid samples confirms the presence of vitamin E acetate in patients with electronic-cigarette, or vaping, product use–associated lung injury, according to a report on 51 patients in 16 states.
The average age of the patients was 23 years; 69% were male.
The report extends previous work by the Centers for Disease Control and Prevention to test for harmful substances in bronchoalveolar-lavage (BAL) fluid obtained from patients with electronic-cigarette, or vaping, product use–associated lung injury (EVALI) as part of a strategy to understand and manage the recent outbreak of EVALI cases in the United States, wrote Benjamin C. Blount, PhD, of the Division of Laboratory Sciences at the CDC’s National Center for Environmental Health, and colleagues.
“CDC was addressing a serious outbreak of lung injury that was sometimes lethal; but after the first 10 weeks of the outbreak investigation, the cause was still unknown,” Dr. Blount said in an interview. “Possible theories could not be evaluated unless the laboratory could develop tests that could confidently connect exposure to lung injury. Detection of toxicants in bronchoalveolar-lavage fluid from patients with EVALI can provide direct information on exposure within the lung.”
In a study published in the New England Journal of Medicine, the researchers examined the BAL of 51 cases of EVALI from 16 states. They analyzed the samples for multiple toxicants, including vitamin E acetate, plant oils, medium-chain triglyceride oil, coconut oil, petroleum distillates, and diluent terpenes.
Overall, 77% of the patients reported using products containing THC, 67% reported using products containing nicotine, and 51% reported using both types.
Researchers found vitamin E acetate in 48 of the 51 patients (94%); no vitamin E acetate was found in the BAL of healthy controls. Coconut oil and limonene were found in one patient each, but none of the other toxicants was found in the samples from the patients or controls.
In addition, 47 of the 50 patients for whom data were available either had detectable tetrahydrocannabinol (THC) or its metabolites in their BAL fluid samples, or they reported vaping THC products within 90 days before they became ill. Nicotine or its metabolites were found in 30 of 47 patients (64%).
The study findings were limited by several factors, including the potential role of vitamin E acetate as a marker for exposure to other toxicants, the uncertainty of the role of aerosolized constituents formed when vitamin E acetate is heated, and the lack of data on the timing and burden of toxicant exposure, the investigators noted.
As for the next steps in research, “additional studies are needed to examine the respiratory effects of inhaling aerosolized vitamin E acetate and provide information on whether vitamin E acetate in isolation causes lung injury,” Dr. Blount explained. Analysis of the aerosol and gases generated by case-associated product fluids is ongoing.
“When CDC developed the BAL study for this response, we considered several possible toxicants in this investigation to find a possible cause of the outbreak,” Dr. Blount noted. “To accomplish the study, CDC’s Environmental Health Laboratory developed 12 analytical methods and validated them in less than 3 weeks because of the urgent nature of the emergency.”
Dr. Blount said he would advise clinicians to “continue to reference CDC guidance on treating suspected or EVALI patients.” In December, the CDC published updated guidance for clinicians on hospitalized EVALI patients. “Following this guidance and other recommendations could reduce EVALI-associated morbidity and mortality,” Dr. Blount said.
The study was supported in part by the National Cancer Institute, the FDA Center for Tobacco Products, and Ohio State University Pelotonia Intramural Research. The researchers had no financial conflicts to disclose.
SOURCE: Blount BC et al. N Engl J Med. 2020 Feb 20. doi: 10.1056/NEJMoa1916433.
Analysis of additional lung fluid samples confirms the presence of vitamin E acetate in patients with electronic-cigarette, or vaping, product use–associated lung injury, according to a report on 51 patients in 16 states.
The average age of the patients was 23 years; 69% were male.
The report extends previous work by the Centers for Disease Control and Prevention to test for harmful substances in bronchoalveolar-lavage (BAL) fluid obtained from patients with electronic-cigarette, or vaping, product use–associated lung injury (EVALI) as part of a strategy to understand and manage the recent outbreak of EVALI cases in the United States, wrote Benjamin C. Blount, PhD, of the Division of Laboratory Sciences at the CDC’s National Center for Environmental Health, and colleagues.
“CDC was addressing a serious outbreak of lung injury that was sometimes lethal; but after the first 10 weeks of the outbreak investigation, the cause was still unknown,” Dr. Blount said in an interview. “Possible theories could not be evaluated unless the laboratory could develop tests that could confidently connect exposure to lung injury. Detection of toxicants in bronchoalveolar-lavage fluid from patients with EVALI can provide direct information on exposure within the lung.”
In a study published in the New England Journal of Medicine, the researchers examined the BAL of 51 cases of EVALI from 16 states. They analyzed the samples for multiple toxicants, including vitamin E acetate, plant oils, medium-chain triglyceride oil, coconut oil, petroleum distillates, and diluent terpenes.
Overall, 77% of the patients reported using products containing THC, 67% reported using products containing nicotine, and 51% reported using both types.
Researchers found vitamin E acetate in 48 of the 51 patients (94%); no vitamin E acetate was found in the BAL of healthy controls. Coconut oil and limonene were found in one patient each, but none of the other toxicants was found in the samples from the patients or controls.
In addition, 47 of the 50 patients for whom data were available either had detectable tetrahydrocannabinol (THC) or its metabolites in their BAL fluid samples, or they reported vaping THC products within 90 days before they became ill. Nicotine or its metabolites were found in 30 of 47 patients (64%).
The study findings were limited by several factors, including the potential role of vitamin E acetate as a marker for exposure to other toxicants, the uncertainty of the role of aerosolized constituents formed when vitamin E acetate is heated, and the lack of data on the timing and burden of toxicant exposure, the investigators noted.
As for the next steps in research, “additional studies are needed to examine the respiratory effects of inhaling aerosolized vitamin E acetate and provide information on whether vitamin E acetate in isolation causes lung injury,” Dr. Blount explained. Analysis of the aerosol and gases generated by case-associated product fluids is ongoing.
“When CDC developed the BAL study for this response, we considered several possible toxicants in this investigation to find a possible cause of the outbreak,” Dr. Blount noted. “To accomplish the study, CDC’s Environmental Health Laboratory developed 12 analytical methods and validated them in less than 3 weeks because of the urgent nature of the emergency.”
Dr. Blount said he would advise clinicians to “continue to reference CDC guidance on treating suspected or EVALI patients.” In December, the CDC published updated guidance for clinicians on hospitalized EVALI patients. “Following this guidance and other recommendations could reduce EVALI-associated morbidity and mortality,” Dr. Blount said.
The study was supported in part by the National Cancer Institute, the FDA Center for Tobacco Products, and Ohio State University Pelotonia Intramural Research. The researchers had no financial conflicts to disclose.
SOURCE: Blount BC et al. N Engl J Med. 2020 Feb 20. doi: 10.1056/NEJMoa1916433.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Drop in flu activity suggests season may have peaked
Influenza activity dropped during the week ending Feb. 15, according to the Centers for Disease Control and Prevention. That decline, along with revised data from the 2 previous weeks, suggests that the 2019-2020 season has peaked for the second time. The rate of outpatient visits for influenza-like illness (ILI) came in at 6.1% for the week ending Feb. 15, after two straight weeks at 6.7%, the CDC’s influenza division reported Feb. 21.
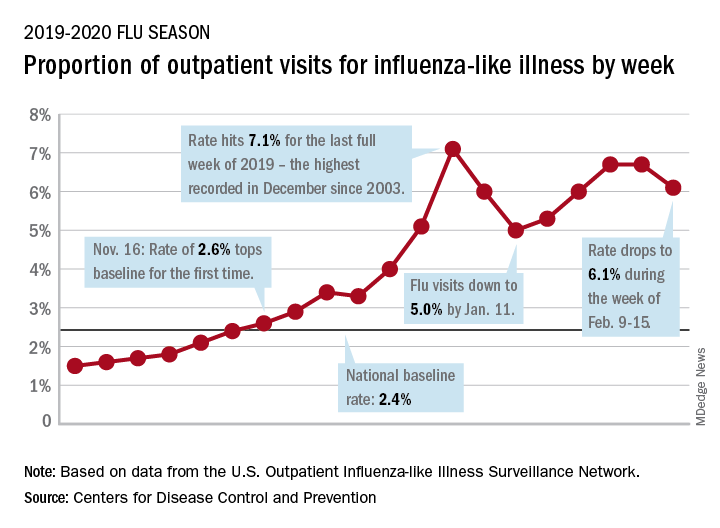
The rates for those 2 earlier weeks had previously been reported at 6.8% (Feb. 8) and 6.6% (Feb. 1), which means that there have now been 2 consecutive weeks without an increase in national ILI activity.
State-level activity was down slightly as well. For the week ending Feb. 15, there were 39 states and Puerto Rico at the highest level of activity on the CDC’s 1-10 scale, compared with 41 states and Puerto Rico the week before. The number of states in the “high” range, which includes levels 8 and 9, went from 44 to 45, however, CDC data show.
Laboratory measures also dropped a bit. For the week, 29.6% of respiratory specimens tested positive for influenza, compared with 30.3% the previous week. The predominance of influenza A continued to increase, as type A went from 59.4% to 63.5% of positive specimens and type B dropped from 40.6% to 36.5%, the influenza division said.
In a separate report, the CDC announced interim flu vaccine effectiveness estimates.For the 2019-2020 season so far, “flu vaccines are reducing doctor’s visits for flu illness by almost half (45%). This is consistent with estimates of flu vaccine effectiveness (VE) from previous flu seasons that ranged from 40% to 60% when flu vaccine viruses were similar to circulating influenza viruses,” the CDC said.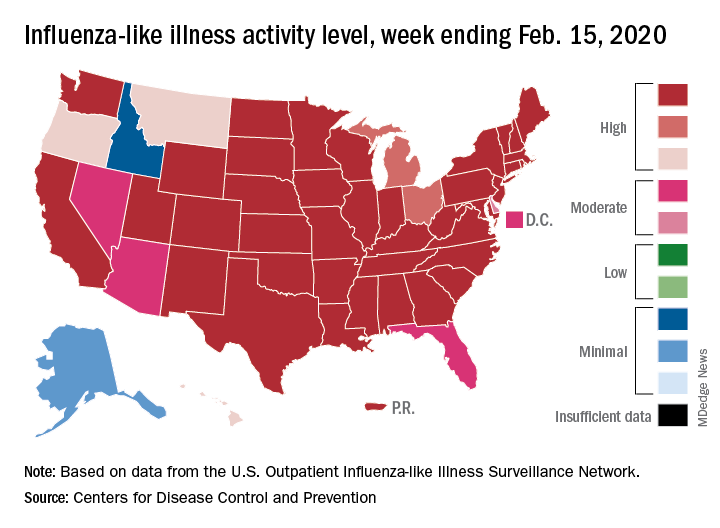
Although VE among children aged 6 months to 17 years is even higher, at 55%, this season “has been especially bad for children. Flu hospitalization rates among children are higher than at this time in other recent seasons, including the 2017-18 season,” the CDC noted.
The number of pediatric flu deaths for 2019-2020 – now up to 105 – is “higher for the same time period than in every season since reporting began in 2004-05, with the exception of the 2009 pandemic,” the CDC added.
Interim VE estimates for other age groups are 25% for adults aged 18-49 and 43% for those 50 years and older. “The lower VE point estimates observed among adults 18-49 years appear to be associated with a trend suggesting lower VE in this age group against A(H1N1)pdm09 viruses,” the CDC said.
Influenza activity dropped during the week ending Feb. 15, according to the Centers for Disease Control and Prevention. That decline, along with revised data from the 2 previous weeks, suggests that the 2019-2020 season has peaked for the second time. The rate of outpatient visits for influenza-like illness (ILI) came in at 6.1% for the week ending Feb. 15, after two straight weeks at 6.7%, the CDC’s influenza division reported Feb. 21.

The rates for those 2 earlier weeks had previously been reported at 6.8% (Feb. 8) and 6.6% (Feb. 1), which means that there have now been 2 consecutive weeks without an increase in national ILI activity.
State-level activity was down slightly as well. For the week ending Feb. 15, there were 39 states and Puerto Rico at the highest level of activity on the CDC’s 1-10 scale, compared with 41 states and Puerto Rico the week before. The number of states in the “high” range, which includes levels 8 and 9, went from 44 to 45, however, CDC data show.
Laboratory measures also dropped a bit. For the week, 29.6% of respiratory specimens tested positive for influenza, compared with 30.3% the previous week. The predominance of influenza A continued to increase, as type A went from 59.4% to 63.5% of positive specimens and type B dropped from 40.6% to 36.5%, the influenza division said.
In a separate report, the CDC announced interim flu vaccine effectiveness estimates.For the 2019-2020 season so far, “flu vaccines are reducing doctor’s visits for flu illness by almost half (45%). This is consistent with estimates of flu vaccine effectiveness (VE) from previous flu seasons that ranged from 40% to 60% when flu vaccine viruses were similar to circulating influenza viruses,” the CDC said.
Although VE among children aged 6 months to 17 years is even higher, at 55%, this season “has been especially bad for children. Flu hospitalization rates among children are higher than at this time in other recent seasons, including the 2017-18 season,” the CDC noted.
The number of pediatric flu deaths for 2019-2020 – now up to 105 – is “higher for the same time period than in every season since reporting began in 2004-05, with the exception of the 2009 pandemic,” the CDC added.
Interim VE estimates for other age groups are 25% for adults aged 18-49 and 43% for those 50 years and older. “The lower VE point estimates observed among adults 18-49 years appear to be associated with a trend suggesting lower VE in this age group against A(H1N1)pdm09 viruses,” the CDC said.
Influenza activity dropped during the week ending Feb. 15, according to the Centers for Disease Control and Prevention. That decline, along with revised data from the 2 previous weeks, suggests that the 2019-2020 season has peaked for the second time. The rate of outpatient visits for influenza-like illness (ILI) came in at 6.1% for the week ending Feb. 15, after two straight weeks at 6.7%, the CDC’s influenza division reported Feb. 21.

The rates for those 2 earlier weeks had previously been reported at 6.8% (Feb. 8) and 6.6% (Feb. 1), which means that there have now been 2 consecutive weeks without an increase in national ILI activity.
State-level activity was down slightly as well. For the week ending Feb. 15, there were 39 states and Puerto Rico at the highest level of activity on the CDC’s 1-10 scale, compared with 41 states and Puerto Rico the week before. The number of states in the “high” range, which includes levels 8 and 9, went from 44 to 45, however, CDC data show.
Laboratory measures also dropped a bit. For the week, 29.6% of respiratory specimens tested positive for influenza, compared with 30.3% the previous week. The predominance of influenza A continued to increase, as type A went from 59.4% to 63.5% of positive specimens and type B dropped from 40.6% to 36.5%, the influenza division said.
In a separate report, the CDC announced interim flu vaccine effectiveness estimates.For the 2019-2020 season so far, “flu vaccines are reducing doctor’s visits for flu illness by almost half (45%). This is consistent with estimates of flu vaccine effectiveness (VE) from previous flu seasons that ranged from 40% to 60% when flu vaccine viruses were similar to circulating influenza viruses,” the CDC said.
Although VE among children aged 6 months to 17 years is even higher, at 55%, this season “has been especially bad for children. Flu hospitalization rates among children are higher than at this time in other recent seasons, including the 2017-18 season,” the CDC noted.
The number of pediatric flu deaths for 2019-2020 – now up to 105 – is “higher for the same time period than in every season since reporting began in 2004-05, with the exception of the 2009 pandemic,” the CDC added.
Interim VE estimates for other age groups are 25% for adults aged 18-49 and 43% for those 50 years and older. “The lower VE point estimates observed among adults 18-49 years appear to be associated with a trend suggesting lower VE in this age group against A(H1N1)pdm09 viruses,” the CDC said.
FROM THE CDC
Risk factors found for respiratory AEs in children following OSA surgery
Underlying cardiac disease, airway anomalies, and younger age each independently boosted the risk of severe perioperative respiratory adverse events (PRAE) in children undergoing adenotonsillectomy to treat obstructive sleep apnea, in a review of 374 patients treated at a single Canadian tertiary-referral center.
In contrast, the analysis failed to show independent, significant effects from any assessed polysomnography or oximetry parameters on the rate of postoperative respiratory complications. The utility of preoperative polysomnography or oximetry for risk stratification is questionable for pediatric patients scheduled to adenotonsillectomy to treat obstructive sleep apnea, wrote Sherri L. Katz, MD, of the University of Ottawa, and associates in a recent report published in the Journal of Clinical Sleep Medicine, although they also added that making these assessments may be “unavoidable” because of their need for diagnosing obstructive sleep apnea and determining the need for surgery.
Despite this caveat, “overall our study results highlight the need to better define the complex interaction between comorbidities, age, nocturnal respiratory events, and gas exchange abnormalities in predicting risk for PRAE” after adenotonsillectomy, the researchers wrote. These findings “are consistent with existing clinical care guidelines,” and “cardiac and craniofacial conditions have been associated with risk of postoperative complications in other studies.”
The analysis used data collected from all children aged 0-18 years who underwent polysomnography assessment followed by adenotonsillectomy at one Canadian tertiary-referral center, Children’s Hospital of Eastern Ontario in Ottawa, during 2010-2016. Their median age was just over 6 years, and 39 patients (10%) were younger than 3 years at the time of their surgery. More than three-quarters of the patients, 286, had at least one identified comorbidity, and nearly half had at least two comorbidities. Polysomnography identified sleep-disordered breathing in 344 of the children (92%), and diagnosed obstructive sleep apnea in 256 (68%), including 148 (43% of the full cohort) with a severe apnea-hypopnea index.
Sixty-six of the children (18%) had at least one severe PRAE that required intervention. Specifically these were either oxygen desaturations requiring intervention or need for airway or ventilatory support with interventions such as jaw thrust, oral or nasal airway placement, bag and mask ventilation, or endotracheal intubation.
A multivariate regression analysis of the measured comorbidity, polysomnography, and oximetry parameters, as well as age, identified three factors that independently linked with a statistically significant increase in the rate of severe PRAE: airway anomaly, underlying cardiac disease, and young age. Patients with an airway anomaly had a 219% increased rate of PRAE, compared with those with no anomaly; patients with underlying cardiac disease had a 109% increased rate, compared with those without cardiac disease; and patients aged younger than 3 years had a 310% higher rate of PRAE, compared with the children aged 6 years or older, while children aged 3-5 years had a 121% higher rate of PRAE, compared with older children.
The study received no commercial funding. Dr. Katz has received honoraria for speaking from Biogen that had no relevance to the study.
SOURCE: Katz SL et al. J Clin Sleep Med. 2020 Jan 15;16(1):41-8.
This well-conducted, retrospective, chart-review study adds important information to the published literature about risk stratification for children in a tertiary-referral population undergoing adenotonsillectomy. Their findings indicate that younger children remain at higher risk as well as those children with complex comorbid medical disease. They also show that children with severe sleep apnea or significant oxyhemoglobin desaturation are likewise at higher risk of postoperative respiratory compromise – emphasizing the need for preoperative polysomnography – particularly in a tertiary setting where many patients have medical comorbidities.
Despite the strengths of this study in assessing perioperative risk for respiratory compromise in a referral population with highly prevalent medical comorbidities, this study does not provide significant insight into the management of otherwise healthy children in a community setting who are undergoing adenotonsillectomy. This is important because a large number of adenotonsillectomies are performed outside of a tertiary-referral center and many of these children may not have undergone preoperative polysomnography to stratify risk. The utility of preoperative polysomnography in the evaluation of all children undergoing adenotonsillectomy remains controversial, with diverging recommendations from two major U.S. medical groups.
This study does not address the utility of polysomnography in community-based populations of otherwise healthy children. It is imperative to accurately ascertain risk so perioperative planning can ensure the safety of children at higher risk following adenotonsillectomy; however, there remains a paucity of studies assessing the cost-effectiveness as well as the positive and negative predictive value of polysomnographic findings. This study highlights the need for community-based studies of otherwise healthy children undergoing adenotonsillectomy to ensure that children at risk receive appropriate monitoring in an inpatient setting whereas those at lesser risk are not unnecessarily hospitalized postoperatively.
Heidi V. Connolly, MD, and Laura E. Tomaselli, MD, are pediatric sleep medicine physicians, and Margo K. McKenna Benoit, MD, is an otolaryngologist at the University of Rochester (N.Y.). They made these comments in a commentary that accompanied the published report ( J Clin Sleep Med. 2020 Jan 15;16[1]:3-4 ). They had no disclosures.
This well-conducted, retrospective, chart-review study adds important information to the published literature about risk stratification for children in a tertiary-referral population undergoing adenotonsillectomy. Their findings indicate that younger children remain at higher risk as well as those children with complex comorbid medical disease. They also show that children with severe sleep apnea or significant oxyhemoglobin desaturation are likewise at higher risk of postoperative respiratory compromise – emphasizing the need for preoperative polysomnography – particularly in a tertiary setting where many patients have medical comorbidities.
Despite the strengths of this study in assessing perioperative risk for respiratory compromise in a referral population with highly prevalent medical comorbidities, this study does not provide significant insight into the management of otherwise healthy children in a community setting who are undergoing adenotonsillectomy. This is important because a large number of adenotonsillectomies are performed outside of a tertiary-referral center and many of these children may not have undergone preoperative polysomnography to stratify risk. The utility of preoperative polysomnography in the evaluation of all children undergoing adenotonsillectomy remains controversial, with diverging recommendations from two major U.S. medical groups.
This study does not address the utility of polysomnography in community-based populations of otherwise healthy children. It is imperative to accurately ascertain risk so perioperative planning can ensure the safety of children at higher risk following adenotonsillectomy; however, there remains a paucity of studies assessing the cost-effectiveness as well as the positive and negative predictive value of polysomnographic findings. This study highlights the need for community-based studies of otherwise healthy children undergoing adenotonsillectomy to ensure that children at risk receive appropriate monitoring in an inpatient setting whereas those at lesser risk are not unnecessarily hospitalized postoperatively.
Heidi V. Connolly, MD, and Laura E. Tomaselli, MD, are pediatric sleep medicine physicians, and Margo K. McKenna Benoit, MD, is an otolaryngologist at the University of Rochester (N.Y.). They made these comments in a commentary that accompanied the published report ( J Clin Sleep Med. 2020 Jan 15;16[1]:3-4 ). They had no disclosures.
This well-conducted, retrospective, chart-review study adds important information to the published literature about risk stratification for children in a tertiary-referral population undergoing adenotonsillectomy. Their findings indicate that younger children remain at higher risk as well as those children with complex comorbid medical disease. They also show that children with severe sleep apnea or significant oxyhemoglobin desaturation are likewise at higher risk of postoperative respiratory compromise – emphasizing the need for preoperative polysomnography – particularly in a tertiary setting where many patients have medical comorbidities.
Despite the strengths of this study in assessing perioperative risk for respiratory compromise in a referral population with highly prevalent medical comorbidities, this study does not provide significant insight into the management of otherwise healthy children in a community setting who are undergoing adenotonsillectomy. This is important because a large number of adenotonsillectomies are performed outside of a tertiary-referral center and many of these children may not have undergone preoperative polysomnography to stratify risk. The utility of preoperative polysomnography in the evaluation of all children undergoing adenotonsillectomy remains controversial, with diverging recommendations from two major U.S. medical groups.
This study does not address the utility of polysomnography in community-based populations of otherwise healthy children. It is imperative to accurately ascertain risk so perioperative planning can ensure the safety of children at higher risk following adenotonsillectomy; however, there remains a paucity of studies assessing the cost-effectiveness as well as the positive and negative predictive value of polysomnographic findings. This study highlights the need for community-based studies of otherwise healthy children undergoing adenotonsillectomy to ensure that children at risk receive appropriate monitoring in an inpatient setting whereas those at lesser risk are not unnecessarily hospitalized postoperatively.
Heidi V. Connolly, MD, and Laura E. Tomaselli, MD, are pediatric sleep medicine physicians, and Margo K. McKenna Benoit, MD, is an otolaryngologist at the University of Rochester (N.Y.). They made these comments in a commentary that accompanied the published report ( J Clin Sleep Med. 2020 Jan 15;16[1]:3-4 ). They had no disclosures.
Underlying cardiac disease, airway anomalies, and younger age each independently boosted the risk of severe perioperative respiratory adverse events (PRAE) in children undergoing adenotonsillectomy to treat obstructive sleep apnea, in a review of 374 patients treated at a single Canadian tertiary-referral center.
In contrast, the analysis failed to show independent, significant effects from any assessed polysomnography or oximetry parameters on the rate of postoperative respiratory complications. The utility of preoperative polysomnography or oximetry for risk stratification is questionable for pediatric patients scheduled to adenotonsillectomy to treat obstructive sleep apnea, wrote Sherri L. Katz, MD, of the University of Ottawa, and associates in a recent report published in the Journal of Clinical Sleep Medicine, although they also added that making these assessments may be “unavoidable” because of their need for diagnosing obstructive sleep apnea and determining the need for surgery.
Despite this caveat, “overall our study results highlight the need to better define the complex interaction between comorbidities, age, nocturnal respiratory events, and gas exchange abnormalities in predicting risk for PRAE” after adenotonsillectomy, the researchers wrote. These findings “are consistent with existing clinical care guidelines,” and “cardiac and craniofacial conditions have been associated with risk of postoperative complications in other studies.”
The analysis used data collected from all children aged 0-18 years who underwent polysomnography assessment followed by adenotonsillectomy at one Canadian tertiary-referral center, Children’s Hospital of Eastern Ontario in Ottawa, during 2010-2016. Their median age was just over 6 years, and 39 patients (10%) were younger than 3 years at the time of their surgery. More than three-quarters of the patients, 286, had at least one identified comorbidity, and nearly half had at least two comorbidities. Polysomnography identified sleep-disordered breathing in 344 of the children (92%), and diagnosed obstructive sleep apnea in 256 (68%), including 148 (43% of the full cohort) with a severe apnea-hypopnea index.
Sixty-six of the children (18%) had at least one severe PRAE that required intervention. Specifically these were either oxygen desaturations requiring intervention or need for airway or ventilatory support with interventions such as jaw thrust, oral or nasal airway placement, bag and mask ventilation, or endotracheal intubation.
A multivariate regression analysis of the measured comorbidity, polysomnography, and oximetry parameters, as well as age, identified three factors that independently linked with a statistically significant increase in the rate of severe PRAE: airway anomaly, underlying cardiac disease, and young age. Patients with an airway anomaly had a 219% increased rate of PRAE, compared with those with no anomaly; patients with underlying cardiac disease had a 109% increased rate, compared with those without cardiac disease; and patients aged younger than 3 years had a 310% higher rate of PRAE, compared with the children aged 6 years or older, while children aged 3-5 years had a 121% higher rate of PRAE, compared with older children.
The study received no commercial funding. Dr. Katz has received honoraria for speaking from Biogen that had no relevance to the study.
SOURCE: Katz SL et al. J Clin Sleep Med. 2020 Jan 15;16(1):41-8.
Underlying cardiac disease, airway anomalies, and younger age each independently boosted the risk of severe perioperative respiratory adverse events (PRAE) in children undergoing adenotonsillectomy to treat obstructive sleep apnea, in a review of 374 patients treated at a single Canadian tertiary-referral center.
In contrast, the analysis failed to show independent, significant effects from any assessed polysomnography or oximetry parameters on the rate of postoperative respiratory complications. The utility of preoperative polysomnography or oximetry for risk stratification is questionable for pediatric patients scheduled to adenotonsillectomy to treat obstructive sleep apnea, wrote Sherri L. Katz, MD, of the University of Ottawa, and associates in a recent report published in the Journal of Clinical Sleep Medicine, although they also added that making these assessments may be “unavoidable” because of their need for diagnosing obstructive sleep apnea and determining the need for surgery.
Despite this caveat, “overall our study results highlight the need to better define the complex interaction between comorbidities, age, nocturnal respiratory events, and gas exchange abnormalities in predicting risk for PRAE” after adenotonsillectomy, the researchers wrote. These findings “are consistent with existing clinical care guidelines,” and “cardiac and craniofacial conditions have been associated with risk of postoperative complications in other studies.”
The analysis used data collected from all children aged 0-18 years who underwent polysomnography assessment followed by adenotonsillectomy at one Canadian tertiary-referral center, Children’s Hospital of Eastern Ontario in Ottawa, during 2010-2016. Their median age was just over 6 years, and 39 patients (10%) were younger than 3 years at the time of their surgery. More than three-quarters of the patients, 286, had at least one identified comorbidity, and nearly half had at least two comorbidities. Polysomnography identified sleep-disordered breathing in 344 of the children (92%), and diagnosed obstructive sleep apnea in 256 (68%), including 148 (43% of the full cohort) with a severe apnea-hypopnea index.
Sixty-six of the children (18%) had at least one severe PRAE that required intervention. Specifically these were either oxygen desaturations requiring intervention or need for airway or ventilatory support with interventions such as jaw thrust, oral or nasal airway placement, bag and mask ventilation, or endotracheal intubation.
A multivariate regression analysis of the measured comorbidity, polysomnography, and oximetry parameters, as well as age, identified three factors that independently linked with a statistically significant increase in the rate of severe PRAE: airway anomaly, underlying cardiac disease, and young age. Patients with an airway anomaly had a 219% increased rate of PRAE, compared with those with no anomaly; patients with underlying cardiac disease had a 109% increased rate, compared with those without cardiac disease; and patients aged younger than 3 years had a 310% higher rate of PRAE, compared with the children aged 6 years or older, while children aged 3-5 years had a 121% higher rate of PRAE, compared with older children.
The study received no commercial funding. Dr. Katz has received honoraria for speaking from Biogen that had no relevance to the study.
SOURCE: Katz SL et al. J Clin Sleep Med. 2020 Jan 15;16(1):41-8.
FROM THE JOURNAL OF CLINICAL SLEEP MEDICINE
Doctors look to existing drugs in coronavirus fight
COVID-19, the infection caused by the newly identified coronavirus, is a currently a disease with no pharmaceutical weapons against it. There’s no vaccine to prevent it, and no drugs can treat it.
But researchers are racing to change that. A vaccine could be ready to test as soon as April. More than two dozen studies have already been registered on ClinicalTrials.gov, a website that tracks research. These studies aim to test everything from traditional Chinese medicine to vitamin C, stem cells, steroids, and medications that fight other viruses, like the flu and HIV. The hope is that something about how these repurposed remedies work will help patients who are desperately ill with no other prospects.
Anthony Fauci, MD, director of the National Institute of Allergy and Infectious Diseases, says this is all part of the playbook for brand-new diseases. “There’s a lot of empiric guessing,” he says. “They’re going to propose a whole lot of drugs that already exist. They’re going to say, here’s the data that shows it blocks the virus” in a test tube. But test tubes aren’t people, and many drugs that seem to work in a lab won’t end up helping patients.
Coronaviruses are especially hard to stop once they invade the body. Unlike many other kinds of viruses, they have a fail-safe against tampering – a “proofreader” that constantly inspects their code, looking for errors, including the potentially life-saving errors that drugs could introduce.
Dr. Fauci said that researchers will be able to make better guesses about how to help people when they can try drugs in animals. “We don’t have an animal model yet of the new coronavirus. When we do get an animal model, that will be a big boon to drugs because then, you can clearly test them in a physiological way, whether they work,” he says.
Looking to drugs for HIV and flu
One of the drugs already under study is the combination of two HIV medications: lopinavir and ritonavir (Kaletra). Kaletra stops viruses by interfering with the enzymes they need to infect cells, called proteases.
One study being done at the Guangzhou Eighth People’s Hospital in China is testing Kaletra against Arbidol, an antiviral drug approved in China and Russia to treat the flu. Two groups of patients will take the medications along with standard care. A third group in the study will receive only standard care, typically supportive therapy with oxygen and IV fluids that are meant to support the body so the immune system can fight off a virus on its own.
An Ebola drug gets a second look
One repurposed drug generating a lot of buzz is an experimental infusion called remdesivir (Xembify). It was originally tested against the Ebola virus. While it didn’t work for that infection, it has been shown to shut down the new coronavirus, at least in test tubes. It’s been given to a small number of COVID-19 patients already, including one in Washington state.
In order to have better evidence of how well it may work in people, two studies in Beijing are comparing remdesivir to a dummy pill to see if the drug can help patients with both mild and severe symptoms recover from their illnesses. Viruses work by infecting cells, taking over their machinery, and getting them to crank out more copies of the virus, which then goes on to infect more cells. Remdesivir is a mimic that fools a virus into replacing one of its four building blocks with a chemical fake. Once in the virus’s blueprints, the imposter acts like a stop sign that keeps the virus from copying itself.
Other kinds of drugs in the same class – called nucleotide analogs – are used to attack cancer and other infectious viruses like hepatitis.
Last week, Chinese scientists published study showing remdesivir was effective against the new coronavirus, 2019-nCoV. Out of seven drugs tested, only remdesivir and an older drug called chloroquine (Aralen), which is used to treat malaria, worked, at least in test tubes. “It functions like a knife that just cuts off the RNA strand,” says Mark Denison, MD, a pediatric infectious disease specialist at Vanderbilt University in Nashville. “They can’t replicate any more. It stops them from doing that.” Dr. Denison is part of a team of researchers in Tennessee and North Carolina that discovered remdesivir could stop coronaviruses, like severe acute respiratory syndrome (SARS) and Middle East respiratory syndrome (MERS), in test tubes and animals. He has studied coronaviruses in his lab for 30 years. He knew they would pose a threat again. “We’re shocked, but not surprised, that this has happened again,” he says of the China-based outbreak of 2019-nCoV.
After the SARS outbreak, which infected more than 8,000 people in 26 countries during 2002-2003, and MERS, which has infected nearly 2,500 people in 27 countries since 2012, researchers knew they had to start looking for treatments that would work against coronaviruses. Dr. Denison reached out to Gilead Sciences, a company best known for its antiviral medications that treat HIV and hepatitis C, and asked it to send drug candidates for him to test on coronaviruses. “The idea was that we didn’t want a drug that would just work against SARS or MERS,” he says. “We wanted drugs that worked against every coronavirus.”
Many of the agents he tried didn’t work until Dr. Denison and his team knocked out the virus’s pesky proofreader. Remdesivir seems to be able to defeat the proofreader, though Dr. Denison admits that he does not know how the drug gets around a virus’s defenses. He has a grant from the National Institutes of Health to study that. Gilead has been giving remdesivir to “a small number” of coronavirus patients in the United States and Europe on a compassionate basis.
One of those patients was a 35-year-old man in Everett, Wash., who had gotten pneumonia after being infected with the new coronavirus during a trip to see family in Wuhan, China, the epicenter of the outbreak. His doctors started IV remdesivir on the evening of his 7th day in the hospital. On the 8th day, he improved. He was well enough to stop using oxygen. Signs of pneumonia were gone. He got his appetite back. His case was recently published in the New England Journal of Medicine, igniting a firestorm of interest in the therapy.
Unfortunately, though, even Dr. Denison says a single person’s case isn’t enough proof that the medication can treat the new coronavirus. The patient, who has not been identified, was getting expert care. He may have improved on his own, despite getting the drug. He said the challenge in people will be to find out two things: whether the medication can block the spread of virus in the body and whether it can reverse the disease. “You can remove the source of injury, but you still have the injury,” he said. Other important questions include how soon the drug may need to be given after infection for it work and whether it may cause significant side effects.
A promising pill
Another drug, a nucleoside analog, that appears to be able to defeat the coronavirus proofreader, EIDD-2801, was developed by Emory University in Atlanta. It was originally intended to treat the flu but has shown some effectiveness against coronaviruses like SARS and MERS.
The FDA recently reached out to Emory asking if it had any drug candidates that might work against the new coronavirus. “It’s a good shot on goal here,” says George Painter, PhD, CEO of Drug Innovation Ventures at Emory. EIDD-2801 can be taken as a pill, which makes it easier to use outside of a hospital setting.
“The capsules for the trial are being made at the end of this month. So we’re close,” Painter says. “We’re right on the edge.”
While these early tests are just getting started, and it will be months until researchers have results, the World Health Organization has sounded a note of caution.
In new guidelines for the clinical management of COVID-19, the WHO reminded doctors and patients that there’s not enough evidence to recommend any specific treatment for infected patients.
Right now, the guidelines recommend that doctors offer supportive care to help the body fight off an infection on its own.
The organization says unlicensed treatments should be given only in the context of clinical trials that have been ethically reviewed or with strict clinical monitoring in emergencies.
This article first appeared on WebMD.com.
COVID-19, the infection caused by the newly identified coronavirus, is a currently a disease with no pharmaceutical weapons against it. There’s no vaccine to prevent it, and no drugs can treat it.
But researchers are racing to change that. A vaccine could be ready to test as soon as April. More than two dozen studies have already been registered on ClinicalTrials.gov, a website that tracks research. These studies aim to test everything from traditional Chinese medicine to vitamin C, stem cells, steroids, and medications that fight other viruses, like the flu and HIV. The hope is that something about how these repurposed remedies work will help patients who are desperately ill with no other prospects.
Anthony Fauci, MD, director of the National Institute of Allergy and Infectious Diseases, says this is all part of the playbook for brand-new diseases. “There’s a lot of empiric guessing,” he says. “They’re going to propose a whole lot of drugs that already exist. They’re going to say, here’s the data that shows it blocks the virus” in a test tube. But test tubes aren’t people, and many drugs that seem to work in a lab won’t end up helping patients.
Coronaviruses are especially hard to stop once they invade the body. Unlike many other kinds of viruses, they have a fail-safe against tampering – a “proofreader” that constantly inspects their code, looking for errors, including the potentially life-saving errors that drugs could introduce.
Dr. Fauci said that researchers will be able to make better guesses about how to help people when they can try drugs in animals. “We don’t have an animal model yet of the new coronavirus. When we do get an animal model, that will be a big boon to drugs because then, you can clearly test them in a physiological way, whether they work,” he says.
Looking to drugs for HIV and flu
One of the drugs already under study is the combination of two HIV medications: lopinavir and ritonavir (Kaletra). Kaletra stops viruses by interfering with the enzymes they need to infect cells, called proteases.
One study being done at the Guangzhou Eighth People’s Hospital in China is testing Kaletra against Arbidol, an antiviral drug approved in China and Russia to treat the flu. Two groups of patients will take the medications along with standard care. A third group in the study will receive only standard care, typically supportive therapy with oxygen and IV fluids that are meant to support the body so the immune system can fight off a virus on its own.
An Ebola drug gets a second look
One repurposed drug generating a lot of buzz is an experimental infusion called remdesivir (Xembify). It was originally tested against the Ebola virus. While it didn’t work for that infection, it has been shown to shut down the new coronavirus, at least in test tubes. It’s been given to a small number of COVID-19 patients already, including one in Washington state.
In order to have better evidence of how well it may work in people, two studies in Beijing are comparing remdesivir to a dummy pill to see if the drug can help patients with both mild and severe symptoms recover from their illnesses. Viruses work by infecting cells, taking over their machinery, and getting them to crank out more copies of the virus, which then goes on to infect more cells. Remdesivir is a mimic that fools a virus into replacing one of its four building blocks with a chemical fake. Once in the virus’s blueprints, the imposter acts like a stop sign that keeps the virus from copying itself.
Other kinds of drugs in the same class – called nucleotide analogs – are used to attack cancer and other infectious viruses like hepatitis.
Last week, Chinese scientists published study showing remdesivir was effective against the new coronavirus, 2019-nCoV. Out of seven drugs tested, only remdesivir and an older drug called chloroquine (Aralen), which is used to treat malaria, worked, at least in test tubes. “It functions like a knife that just cuts off the RNA strand,” says Mark Denison, MD, a pediatric infectious disease specialist at Vanderbilt University in Nashville. “They can’t replicate any more. It stops them from doing that.” Dr. Denison is part of a team of researchers in Tennessee and North Carolina that discovered remdesivir could stop coronaviruses, like severe acute respiratory syndrome (SARS) and Middle East respiratory syndrome (MERS), in test tubes and animals. He has studied coronaviruses in his lab for 30 years. He knew they would pose a threat again. “We’re shocked, but not surprised, that this has happened again,” he says of the China-based outbreak of 2019-nCoV.
After the SARS outbreak, which infected more than 8,000 people in 26 countries during 2002-2003, and MERS, which has infected nearly 2,500 people in 27 countries since 2012, researchers knew they had to start looking for treatments that would work against coronaviruses. Dr. Denison reached out to Gilead Sciences, a company best known for its antiviral medications that treat HIV and hepatitis C, and asked it to send drug candidates for him to test on coronaviruses. “The idea was that we didn’t want a drug that would just work against SARS or MERS,” he says. “We wanted drugs that worked against every coronavirus.”
Many of the agents he tried didn’t work until Dr. Denison and his team knocked out the virus’s pesky proofreader. Remdesivir seems to be able to defeat the proofreader, though Dr. Denison admits that he does not know how the drug gets around a virus’s defenses. He has a grant from the National Institutes of Health to study that. Gilead has been giving remdesivir to “a small number” of coronavirus patients in the United States and Europe on a compassionate basis.
One of those patients was a 35-year-old man in Everett, Wash., who had gotten pneumonia after being infected with the new coronavirus during a trip to see family in Wuhan, China, the epicenter of the outbreak. His doctors started IV remdesivir on the evening of his 7th day in the hospital. On the 8th day, he improved. He was well enough to stop using oxygen. Signs of pneumonia were gone. He got his appetite back. His case was recently published in the New England Journal of Medicine, igniting a firestorm of interest in the therapy.
Unfortunately, though, even Dr. Denison says a single person’s case isn’t enough proof that the medication can treat the new coronavirus. The patient, who has not been identified, was getting expert care. He may have improved on his own, despite getting the drug. He said the challenge in people will be to find out two things: whether the medication can block the spread of virus in the body and whether it can reverse the disease. “You can remove the source of injury, but you still have the injury,” he said. Other important questions include how soon the drug may need to be given after infection for it work and whether it may cause significant side effects.
A promising pill
Another drug, a nucleoside analog, that appears to be able to defeat the coronavirus proofreader, EIDD-2801, was developed by Emory University in Atlanta. It was originally intended to treat the flu but has shown some effectiveness against coronaviruses like SARS and MERS.
The FDA recently reached out to Emory asking if it had any drug candidates that might work against the new coronavirus. “It’s a good shot on goal here,” says George Painter, PhD, CEO of Drug Innovation Ventures at Emory. EIDD-2801 can be taken as a pill, which makes it easier to use outside of a hospital setting.
“The capsules for the trial are being made at the end of this month. So we’re close,” Painter says. “We’re right on the edge.”
While these early tests are just getting started, and it will be months until researchers have results, the World Health Organization has sounded a note of caution.
In new guidelines for the clinical management of COVID-19, the WHO reminded doctors and patients that there’s not enough evidence to recommend any specific treatment for infected patients.
Right now, the guidelines recommend that doctors offer supportive care to help the body fight off an infection on its own.
The organization says unlicensed treatments should be given only in the context of clinical trials that have been ethically reviewed or with strict clinical monitoring in emergencies.
This article first appeared on WebMD.com.
COVID-19, the infection caused by the newly identified coronavirus, is a currently a disease with no pharmaceutical weapons against it. There’s no vaccine to prevent it, and no drugs can treat it.
But researchers are racing to change that. A vaccine could be ready to test as soon as April. More than two dozen studies have already been registered on ClinicalTrials.gov, a website that tracks research. These studies aim to test everything from traditional Chinese medicine to vitamin C, stem cells, steroids, and medications that fight other viruses, like the flu and HIV. The hope is that something about how these repurposed remedies work will help patients who are desperately ill with no other prospects.
Anthony Fauci, MD, director of the National Institute of Allergy and Infectious Diseases, says this is all part of the playbook for brand-new diseases. “There’s a lot of empiric guessing,” he says. “They’re going to propose a whole lot of drugs that already exist. They’re going to say, here’s the data that shows it blocks the virus” in a test tube. But test tubes aren’t people, and many drugs that seem to work in a lab won’t end up helping patients.
Coronaviruses are especially hard to stop once they invade the body. Unlike many other kinds of viruses, they have a fail-safe against tampering – a “proofreader” that constantly inspects their code, looking for errors, including the potentially life-saving errors that drugs could introduce.
Dr. Fauci said that researchers will be able to make better guesses about how to help people when they can try drugs in animals. “We don’t have an animal model yet of the new coronavirus. When we do get an animal model, that will be a big boon to drugs because then, you can clearly test them in a physiological way, whether they work,” he says.
Looking to drugs for HIV and flu
One of the drugs already under study is the combination of two HIV medications: lopinavir and ritonavir (Kaletra). Kaletra stops viruses by interfering with the enzymes they need to infect cells, called proteases.
One study being done at the Guangzhou Eighth People’s Hospital in China is testing Kaletra against Arbidol, an antiviral drug approved in China and Russia to treat the flu. Two groups of patients will take the medications along with standard care. A third group in the study will receive only standard care, typically supportive therapy with oxygen and IV fluids that are meant to support the body so the immune system can fight off a virus on its own.
An Ebola drug gets a second look
One repurposed drug generating a lot of buzz is an experimental infusion called remdesivir (Xembify). It was originally tested against the Ebola virus. While it didn’t work for that infection, it has been shown to shut down the new coronavirus, at least in test tubes. It’s been given to a small number of COVID-19 patients already, including one in Washington state.
In order to have better evidence of how well it may work in people, two studies in Beijing are comparing remdesivir to a dummy pill to see if the drug can help patients with both mild and severe symptoms recover from their illnesses. Viruses work by infecting cells, taking over their machinery, and getting them to crank out more copies of the virus, which then goes on to infect more cells. Remdesivir is a mimic that fools a virus into replacing one of its four building blocks with a chemical fake. Once in the virus’s blueprints, the imposter acts like a stop sign that keeps the virus from copying itself.
Other kinds of drugs in the same class – called nucleotide analogs – are used to attack cancer and other infectious viruses like hepatitis.
Last week, Chinese scientists published study showing remdesivir was effective against the new coronavirus, 2019-nCoV. Out of seven drugs tested, only remdesivir and an older drug called chloroquine (Aralen), which is used to treat malaria, worked, at least in test tubes. “It functions like a knife that just cuts off the RNA strand,” says Mark Denison, MD, a pediatric infectious disease specialist at Vanderbilt University in Nashville. “They can’t replicate any more. It stops them from doing that.” Dr. Denison is part of a team of researchers in Tennessee and North Carolina that discovered remdesivir could stop coronaviruses, like severe acute respiratory syndrome (SARS) and Middle East respiratory syndrome (MERS), in test tubes and animals. He has studied coronaviruses in his lab for 30 years. He knew they would pose a threat again. “We’re shocked, but not surprised, that this has happened again,” he says of the China-based outbreak of 2019-nCoV.
After the SARS outbreak, which infected more than 8,000 people in 26 countries during 2002-2003, and MERS, which has infected nearly 2,500 people in 27 countries since 2012, researchers knew they had to start looking for treatments that would work against coronaviruses. Dr. Denison reached out to Gilead Sciences, a company best known for its antiviral medications that treat HIV and hepatitis C, and asked it to send drug candidates for him to test on coronaviruses. “The idea was that we didn’t want a drug that would just work against SARS or MERS,” he says. “We wanted drugs that worked against every coronavirus.”
Many of the agents he tried didn’t work until Dr. Denison and his team knocked out the virus’s pesky proofreader. Remdesivir seems to be able to defeat the proofreader, though Dr. Denison admits that he does not know how the drug gets around a virus’s defenses. He has a grant from the National Institutes of Health to study that. Gilead has been giving remdesivir to “a small number” of coronavirus patients in the United States and Europe on a compassionate basis.
One of those patients was a 35-year-old man in Everett, Wash., who had gotten pneumonia after being infected with the new coronavirus during a trip to see family in Wuhan, China, the epicenter of the outbreak. His doctors started IV remdesivir on the evening of his 7th day in the hospital. On the 8th day, he improved. He was well enough to stop using oxygen. Signs of pneumonia were gone. He got his appetite back. His case was recently published in the New England Journal of Medicine, igniting a firestorm of interest in the therapy.
Unfortunately, though, even Dr. Denison says a single person’s case isn’t enough proof that the medication can treat the new coronavirus. The patient, who has not been identified, was getting expert care. He may have improved on his own, despite getting the drug. He said the challenge in people will be to find out two things: whether the medication can block the spread of virus in the body and whether it can reverse the disease. “You can remove the source of injury, but you still have the injury,” he said. Other important questions include how soon the drug may need to be given after infection for it work and whether it may cause significant side effects.
A promising pill
Another drug, a nucleoside analog, that appears to be able to defeat the coronavirus proofreader, EIDD-2801, was developed by Emory University in Atlanta. It was originally intended to treat the flu but has shown some effectiveness against coronaviruses like SARS and MERS.
The FDA recently reached out to Emory asking if it had any drug candidates that might work against the new coronavirus. “It’s a good shot on goal here,” says George Painter, PhD, CEO of Drug Innovation Ventures at Emory. EIDD-2801 can be taken as a pill, which makes it easier to use outside of a hospital setting.
“The capsules for the trial are being made at the end of this month. So we’re close,” Painter says. “We’re right on the edge.”
While these early tests are just getting started, and it will be months until researchers have results, the World Health Organization has sounded a note of caution.
In new guidelines for the clinical management of COVID-19, the WHO reminded doctors and patients that there’s not enough evidence to recommend any specific treatment for infected patients.
Right now, the guidelines recommend that doctors offer supportive care to help the body fight off an infection on its own.
The organization says unlicensed treatments should be given only in the context of clinical trials that have been ethically reviewed or with strict clinical monitoring in emergencies.
This article first appeared on WebMD.com.
Critical care admissions up for pediatric opioid poisonings
ORLANDO – The proportion of children and adolescents admitted to critical care for serious poisonings has increased in recent years, according to authors of a study of more than 750,000 reported opioid exposures.
Critical care units were involved in 10% of pediatric opioid poisoning cases registered in 2015-2018, up from 7% in 2005-2009, reported Megan E. Land, MD, of Emory University, Atlanta, and coinvestigators.
Attempted suicide has represented an increasingly large proportion of pediatric opioid poisonings from 2005 to 2018, according to the researchers, based on retrospective analysis of cases reported to U.S. poison centers.
Mortality related to these pediatric poisonings increased over time, and among children and adolescents admitted to a pediatric ICU, CPR and naloxone use also increased over time, Dr. Land and associates noted.
These said Dr. Land, who presented the findings at the Critical Care Congress sponsored by the Society of Critical Care Medicine.
“I think that this really requires a two-pronged approach,” she explained. “One is that we need to increase mental health resources for kids to address adolescent suicidality, and secondly, we need to decrease access to opioids in the hands of pediatric patients by decreasing prescribing and then also getting those that are unused out of the homes.”
Jeffrey Zimmerman, MD, past president of SCCM, said these findings on pediatric opioid poisonings represent the “iceberg tip” of a much larger societal issue that has impacts well beyond critical care.
“I think acutely, we’re well equipped to deal with the situation in terms of interventions,” Dr. Zimmerman said in an interview. “The bigger issue is dealing with what happens afterward, when the patient leaves the ICU in the hospital.”
When the issue is chronic opioid use among adolescents or children, critical care specialists can help by initiating opioid tapering in the hospital setting, rather than allowing the complete weaning process to play out at home, he said.
All clinicians can help prevent future injury by asking questions of the child and family to ensure that any opiates and other prescription medications at home are locked up, he added.
“These aren’t very glamorous things, but they’re common sense, and there’s more need for this common sense now than there ever has been,” Dr. Zimmerman concluded.
The study by Dr. Land and colleagues included data on primary opioid ingestions registered at 55 poison control centers in the United States. They assessed trends over three time periods: 2005-2009, 2010-2014, and 2015-2018.
They found that children under 19 years of age accounted for 28% of the 753,592 opioid poisonings reported over that time period.
The overall number of reported opioid poisonings among children declined somewhat since about 2010. However, the proportion admitted to a critical care unit increased from 7% in the 2005-2009 period to 10% in the 2015-2018 period, said Dr. Land, who added that the probability of a moderate or major effect increased by 0.55% and 0.11% per year, respectively, over the 14 years studied.
Mortality – 0.21% overall – increased from 0.18% in the earliest era to 0.28% in the most recent era, according to the investigators.
Suicidal intent increased from 14% in the earliest era to 21% in the most recent era, and was linked to near tenfold odds of undergoing a pediatric ICU procedure, Dr. Land and colleagues reported.
Among those children admitted to a pediatric ICU, use of CPR increased from 1% to 3% in the earliest and latest time periods, respectively; likewise, naloxone administration increased from 42% to 51% over those two time periods. By contrast, there was no change in use of mechanical ventilation (12%) or vasopressors (3%) over time, they added.
The opioids most commonly linked to pediatric ICU procedures were fentanyl (odds ratio, 12), heroin (OR, 11), and methadone (OR, 15).
Some funding for the study came from the Georgia Poison Center. Dr. Land had no disclosures relevant to the research.
SOURCE: Land M et al. Crit Care Med. 2020 doi: 10.1097/01.ccm.0000618708.38414.ea.
ORLANDO – The proportion of children and adolescents admitted to critical care for serious poisonings has increased in recent years, according to authors of a study of more than 750,000 reported opioid exposures.
Critical care units were involved in 10% of pediatric opioid poisoning cases registered in 2015-2018, up from 7% in 2005-2009, reported Megan E. Land, MD, of Emory University, Atlanta, and coinvestigators.
Attempted suicide has represented an increasingly large proportion of pediatric opioid poisonings from 2005 to 2018, according to the researchers, based on retrospective analysis of cases reported to U.S. poison centers.
Mortality related to these pediatric poisonings increased over time, and among children and adolescents admitted to a pediatric ICU, CPR and naloxone use also increased over time, Dr. Land and associates noted.
These said Dr. Land, who presented the findings at the Critical Care Congress sponsored by the Society of Critical Care Medicine.
“I think that this really requires a two-pronged approach,” she explained. “One is that we need to increase mental health resources for kids to address adolescent suicidality, and secondly, we need to decrease access to opioids in the hands of pediatric patients by decreasing prescribing and then also getting those that are unused out of the homes.”
Jeffrey Zimmerman, MD, past president of SCCM, said these findings on pediatric opioid poisonings represent the “iceberg tip” of a much larger societal issue that has impacts well beyond critical care.
“I think acutely, we’re well equipped to deal with the situation in terms of interventions,” Dr. Zimmerman said in an interview. “The bigger issue is dealing with what happens afterward, when the patient leaves the ICU in the hospital.”
When the issue is chronic opioid use among adolescents or children, critical care specialists can help by initiating opioid tapering in the hospital setting, rather than allowing the complete weaning process to play out at home, he said.
All clinicians can help prevent future injury by asking questions of the child and family to ensure that any opiates and other prescription medications at home are locked up, he added.
“These aren’t very glamorous things, but they’re common sense, and there’s more need for this common sense now than there ever has been,” Dr. Zimmerman concluded.
The study by Dr. Land and colleagues included data on primary opioid ingestions registered at 55 poison control centers in the United States. They assessed trends over three time periods: 2005-2009, 2010-2014, and 2015-2018.
They found that children under 19 years of age accounted for 28% of the 753,592 opioid poisonings reported over that time period.
The overall number of reported opioid poisonings among children declined somewhat since about 2010. However, the proportion admitted to a critical care unit increased from 7% in the 2005-2009 period to 10% in the 2015-2018 period, said Dr. Land, who added that the probability of a moderate or major effect increased by 0.55% and 0.11% per year, respectively, over the 14 years studied.
Mortality – 0.21% overall – increased from 0.18% in the earliest era to 0.28% in the most recent era, according to the investigators.
Suicidal intent increased from 14% in the earliest era to 21% in the most recent era, and was linked to near tenfold odds of undergoing a pediatric ICU procedure, Dr. Land and colleagues reported.
Among those children admitted to a pediatric ICU, use of CPR increased from 1% to 3% in the earliest and latest time periods, respectively; likewise, naloxone administration increased from 42% to 51% over those two time periods. By contrast, there was no change in use of mechanical ventilation (12%) or vasopressors (3%) over time, they added.
The opioids most commonly linked to pediatric ICU procedures were fentanyl (odds ratio, 12), heroin (OR, 11), and methadone (OR, 15).
Some funding for the study came from the Georgia Poison Center. Dr. Land had no disclosures relevant to the research.
SOURCE: Land M et al. Crit Care Med. 2020 doi: 10.1097/01.ccm.0000618708.38414.ea.
ORLANDO – The proportion of children and adolescents admitted to critical care for serious poisonings has increased in recent years, according to authors of a study of more than 750,000 reported opioid exposures.
Critical care units were involved in 10% of pediatric opioid poisoning cases registered in 2015-2018, up from 7% in 2005-2009, reported Megan E. Land, MD, of Emory University, Atlanta, and coinvestigators.
Attempted suicide has represented an increasingly large proportion of pediatric opioid poisonings from 2005 to 2018, according to the researchers, based on retrospective analysis of cases reported to U.S. poison centers.
Mortality related to these pediatric poisonings increased over time, and among children and adolescents admitted to a pediatric ICU, CPR and naloxone use also increased over time, Dr. Land and associates noted.
These said Dr. Land, who presented the findings at the Critical Care Congress sponsored by the Society of Critical Care Medicine.
“I think that this really requires a two-pronged approach,” she explained. “One is that we need to increase mental health resources for kids to address adolescent suicidality, and secondly, we need to decrease access to opioids in the hands of pediatric patients by decreasing prescribing and then also getting those that are unused out of the homes.”
Jeffrey Zimmerman, MD, past president of SCCM, said these findings on pediatric opioid poisonings represent the “iceberg tip” of a much larger societal issue that has impacts well beyond critical care.
“I think acutely, we’re well equipped to deal with the situation in terms of interventions,” Dr. Zimmerman said in an interview. “The bigger issue is dealing with what happens afterward, when the patient leaves the ICU in the hospital.”
When the issue is chronic opioid use among adolescents or children, critical care specialists can help by initiating opioid tapering in the hospital setting, rather than allowing the complete weaning process to play out at home, he said.
All clinicians can help prevent future injury by asking questions of the child and family to ensure that any opiates and other prescription medications at home are locked up, he added.
“These aren’t very glamorous things, but they’re common sense, and there’s more need for this common sense now than there ever has been,” Dr. Zimmerman concluded.
The study by Dr. Land and colleagues included data on primary opioid ingestions registered at 55 poison control centers in the United States. They assessed trends over three time periods: 2005-2009, 2010-2014, and 2015-2018.
They found that children under 19 years of age accounted for 28% of the 753,592 opioid poisonings reported over that time period.
The overall number of reported opioid poisonings among children declined somewhat since about 2010. However, the proportion admitted to a critical care unit increased from 7% in the 2005-2009 period to 10% in the 2015-2018 period, said Dr. Land, who added that the probability of a moderate or major effect increased by 0.55% and 0.11% per year, respectively, over the 14 years studied.
Mortality – 0.21% overall – increased from 0.18% in the earliest era to 0.28% in the most recent era, according to the investigators.
Suicidal intent increased from 14% in the earliest era to 21% in the most recent era, and was linked to near tenfold odds of undergoing a pediatric ICU procedure, Dr. Land and colleagues reported.
Among those children admitted to a pediatric ICU, use of CPR increased from 1% to 3% in the earliest and latest time periods, respectively; likewise, naloxone administration increased from 42% to 51% over those two time periods. By contrast, there was no change in use of mechanical ventilation (12%) or vasopressors (3%) over time, they added.
The opioids most commonly linked to pediatric ICU procedures were fentanyl (odds ratio, 12), heroin (OR, 11), and methadone (OR, 15).
Some funding for the study came from the Georgia Poison Center. Dr. Land had no disclosures relevant to the research.
SOURCE: Land M et al. Crit Care Med. 2020 doi: 10.1097/01.ccm.0000618708.38414.ea.
REPORTING FROM CCC49
Infection with 2019 novel coronavirus extends to infants
between Dec. 8, 2019, and Feb. 6, 2020, based on data from the Chinese central government and local health departments.
“As of February 6, 2020, China reported 31,211 confirmed cases of COVID-19 and 637 fatalities,” wrote Min Wei, MD, of Wuhan University, China, and colleagues. However, “few infections in children have been reported.”
In a research letter published in JAMA, the investigators reviewed data from nine infants aged 28 days to 1 year who were hospitalized with a diagnosis of COVID-19 between Dec. 8, 2019, and Feb. 6, 2020. The ages of the infants ranged from 1 month to 11 months, and seven were female. The patients included two children from Beijing, two from Hainan, and one each from the areas of Guangdong, Anhui, Shanghai, Zhejiang, and Guizhou.
All infected infants had at least one infected family member, and the infants’ infections occurred after the family members’ infections; seven infants lived in Wuhan or had family members who had visited Wuhan.
One of the infants had no symptoms but tested positive for the 2019 novel coronavirus, and two others had a diagnosis but missing information on any symptoms. Fever occurred in four patients, and mild upper respiratory tract symptoms occurred in two patients.
None of the infants died, and none reported severe complications or the need for intensive care or mechanical ventilation, the investigators said. The fact that most of the infants were female might suggest that they are more susceptible to the virus than males, although overall COVID-19 viral infections have been more common in adult men, especially those with chronic comorbidities, Dr. Wei and associates noted.
The study findings were limited by the small sample size and lack of symptom data for some patients, the researchers said. However, the results confirm that the COVID-19 virus is transmissible to infants younger than 1 year, and adult caregivers should exercise protective measures including wearing masks, washing hands before contact with infants, and routinely sterilizing toys and tableware, they emphasized.
The study was supported by the National Natural Science Foundation of China and the Fundamental Research Funds for the Central Universities. The researchers had no financial conflicts to disclose.
SOURCE: Wei M et al. JAMA. 2020 Feb 14. doi:10.1001/jama.2020.2131.
between Dec. 8, 2019, and Feb. 6, 2020, based on data from the Chinese central government and local health departments.
“As of February 6, 2020, China reported 31,211 confirmed cases of COVID-19 and 637 fatalities,” wrote Min Wei, MD, of Wuhan University, China, and colleagues. However, “few infections in children have been reported.”
In a research letter published in JAMA, the investigators reviewed data from nine infants aged 28 days to 1 year who were hospitalized with a diagnosis of COVID-19 between Dec. 8, 2019, and Feb. 6, 2020. The ages of the infants ranged from 1 month to 11 months, and seven were female. The patients included two children from Beijing, two from Hainan, and one each from the areas of Guangdong, Anhui, Shanghai, Zhejiang, and Guizhou.
All infected infants had at least one infected family member, and the infants’ infections occurred after the family members’ infections; seven infants lived in Wuhan or had family members who had visited Wuhan.
One of the infants had no symptoms but tested positive for the 2019 novel coronavirus, and two others had a diagnosis but missing information on any symptoms. Fever occurred in four patients, and mild upper respiratory tract symptoms occurred in two patients.
None of the infants died, and none reported severe complications or the need for intensive care or mechanical ventilation, the investigators said. The fact that most of the infants were female might suggest that they are more susceptible to the virus than males, although overall COVID-19 viral infections have been more common in adult men, especially those with chronic comorbidities, Dr. Wei and associates noted.
The study findings were limited by the small sample size and lack of symptom data for some patients, the researchers said. However, the results confirm that the COVID-19 virus is transmissible to infants younger than 1 year, and adult caregivers should exercise protective measures including wearing masks, washing hands before contact with infants, and routinely sterilizing toys and tableware, they emphasized.
The study was supported by the National Natural Science Foundation of China and the Fundamental Research Funds for the Central Universities. The researchers had no financial conflicts to disclose.
SOURCE: Wei M et al. JAMA. 2020 Feb 14. doi:10.1001/jama.2020.2131.
between Dec. 8, 2019, and Feb. 6, 2020, based on data from the Chinese central government and local health departments.
“As of February 6, 2020, China reported 31,211 confirmed cases of COVID-19 and 637 fatalities,” wrote Min Wei, MD, of Wuhan University, China, and colleagues. However, “few infections in children have been reported.”
In a research letter published in JAMA, the investigators reviewed data from nine infants aged 28 days to 1 year who were hospitalized with a diagnosis of COVID-19 between Dec. 8, 2019, and Feb. 6, 2020. The ages of the infants ranged from 1 month to 11 months, and seven were female. The patients included two children from Beijing, two from Hainan, and one each from the areas of Guangdong, Anhui, Shanghai, Zhejiang, and Guizhou.
All infected infants had at least one infected family member, and the infants’ infections occurred after the family members’ infections; seven infants lived in Wuhan or had family members who had visited Wuhan.
One of the infants had no symptoms but tested positive for the 2019 novel coronavirus, and two others had a diagnosis but missing information on any symptoms. Fever occurred in four patients, and mild upper respiratory tract symptoms occurred in two patients.
None of the infants died, and none reported severe complications or the need for intensive care or mechanical ventilation, the investigators said. The fact that most of the infants were female might suggest that they are more susceptible to the virus than males, although overall COVID-19 viral infections have been more common in adult men, especially those with chronic comorbidities, Dr. Wei and associates noted.
The study findings were limited by the small sample size and lack of symptom data for some patients, the researchers said. However, the results confirm that the COVID-19 virus is transmissible to infants younger than 1 year, and adult caregivers should exercise protective measures including wearing masks, washing hands before contact with infants, and routinely sterilizing toys and tableware, they emphasized.
The study was supported by the National Natural Science Foundation of China and the Fundamental Research Funds for the Central Universities. The researchers had no financial conflicts to disclose.
SOURCE: Wei M et al. JAMA. 2020 Feb 14. doi:10.1001/jama.2020.2131.
FROM JAMA
Survey queries pulmonologists' happiness at work
Only 26% of pulmonologists report that they are happy at work, with about twice as many happy outside of work, according to Medscape’s Pulmonologist Lifestyle, Happiness & Burnout Report 2020. Dermatologists are the happiest at work, at 41%, and neurologists are the least happy, at 18%. 
According to the report, which surveyed more than 15,000 physicians from various specialties, 29% of pulmonologists report feeling burned out, with 5% reporting feeling depressed and 12% both depressed and burned out. An overabundance of bureaucratic tasks is the lead contributor to burnout (52%), according to pulmonologists, followed by lack of respect from administrators, employers, colleagues, and staff (38%) and spending too many hours at work (35%).
Pulmonologists report that exercise is the biggest way they cope with burnout (47%), compared with neurologists, for example, who ranked it third at 40%. Other ways they deal with burnout include isolating themselves from others (43%) and playing or listening to music (38%).
Among depressed or burned-out pulmonologists, 70% reported not planning to seek professional help or seeking it in the past, while 12% reported currently seeking professional help. Furthermore, almost half of pulmonologists (48%) say they’re unlikely to participate in workplace programs.
When asked for reasons they wouldn’t seek professional help, 60% said they deal with it without professional help and 49% didn’t think their symptoms were severe enough, while 31% were simply too busy.
The slideshow of the full report is available on Medscape.com.
Only 26% of pulmonologists report that they are happy at work, with about twice as many happy outside of work, according to Medscape’s Pulmonologist Lifestyle, Happiness & Burnout Report 2020. Dermatologists are the happiest at work, at 41%, and neurologists are the least happy, at 18%. 
According to the report, which surveyed more than 15,000 physicians from various specialties, 29% of pulmonologists report feeling burned out, with 5% reporting feeling depressed and 12% both depressed and burned out. An overabundance of bureaucratic tasks is the lead contributor to burnout (52%), according to pulmonologists, followed by lack of respect from administrators, employers, colleagues, and staff (38%) and spending too many hours at work (35%).
Pulmonologists report that exercise is the biggest way they cope with burnout (47%), compared with neurologists, for example, who ranked it third at 40%. Other ways they deal with burnout include isolating themselves from others (43%) and playing or listening to music (38%).
Among depressed or burned-out pulmonologists, 70% reported not planning to seek professional help or seeking it in the past, while 12% reported currently seeking professional help. Furthermore, almost half of pulmonologists (48%) say they’re unlikely to participate in workplace programs.
When asked for reasons they wouldn’t seek professional help, 60% said they deal with it without professional help and 49% didn’t think their symptoms were severe enough, while 31% were simply too busy.
The slideshow of the full report is available on Medscape.com.
Only 26% of pulmonologists report that they are happy at work, with about twice as many happy outside of work, according to Medscape’s Pulmonologist Lifestyle, Happiness & Burnout Report 2020. Dermatologists are the happiest at work, at 41%, and neurologists are the least happy, at 18%. 
According to the report, which surveyed more than 15,000 physicians from various specialties, 29% of pulmonologists report feeling burned out, with 5% reporting feeling depressed and 12% both depressed and burned out. An overabundance of bureaucratic tasks is the lead contributor to burnout (52%), according to pulmonologists, followed by lack of respect from administrators, employers, colleagues, and staff (38%) and spending too many hours at work (35%).
Pulmonologists report that exercise is the biggest way they cope with burnout (47%), compared with neurologists, for example, who ranked it third at 40%. Other ways they deal with burnout include isolating themselves from others (43%) and playing or listening to music (38%).
Among depressed or burned-out pulmonologists, 70% reported not planning to seek professional help or seeking it in the past, while 12% reported currently seeking professional help. Furthermore, almost half of pulmonologists (48%) say they’re unlikely to participate in workplace programs.
When asked for reasons they wouldn’t seek professional help, 60% said they deal with it without professional help and 49% didn’t think their symptoms were severe enough, while 31% were simply too busy.
The slideshow of the full report is available on Medscape.com.








