User login
New Agents for the Treatment of Erythematotelangiectatic Rosacea
Rosacea is a common chronic disorder that predominantly manifests as facial erythema, telangiectasia, and flushing.1 Other primary clinical features include inflammatory lesions (eg, papules, pustules) and occasionally edema and rhinophyma. Ocular involvement also may occur. Rosacea patients routinely report sensitive skin with symptoms such as stinging, burning, and intolerance to topical agents (eg, medications, moisturizers, cosmetics).2,3
Rosacea affects facial appearance and can impair a patient’s emotional well-being. It may limit social and professional activities. Many patients and nonpatients alike presume the appearance of facial redness suggests alcohol overuse or emotional distress.4-6 A reduction in facial erythema as well as improvements in other clinical signs of rosacea have been shown to reduce patient self-consciousness and lead to increased socialization.7 Facial erythema is a major factor that adversely affects quality of life in rosacea patients.8
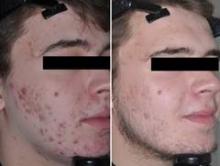
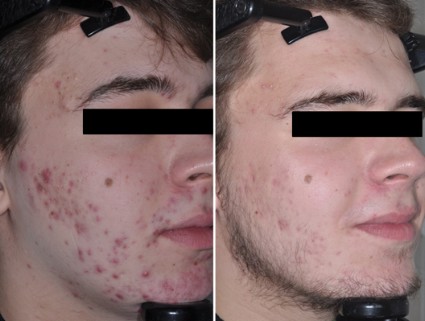
Although a number of options are available to successfully treat inflammatory lesions of rosacea, facial erythema has been the most difficult manifestation of the disease to medically treat.2,9 Chronic erythema and episodic flushing may be at least partially related to cutaneous vasomotor responses causing both transient and persistent dilation of facial blood vessels.10-12 The agents used for treating the inflammatory components of rosacea reduce erythema associated with papules and pustules but have little effect on the background erythema that is so noticeable. Oxymetazoline and brimonidine tartrate have been evaluated as potential rapid treatments of facial erythema. Daily application of oxymetazoline solution 0.05% has been shown to reduce facial erythema in rosacea patients.13
Brimonidine tartrate is a highly selective α2-adrenergic receptor agonist with potent vasoconstrictor activity.14 It has been used to treat open-angle glaucoma for more than 15 years with well-documented safety and efficacy.15 Phase 2 and 3 studies for once-daily application of brimonidine tartrate gel 0.5% have confirmed its efficacy and safety for the treatment of facial erythema in rosacea patients,9,12 and it was approved by the US Food and Drug Administration (brimonidine gel 0.33%) for persistent (nontransient) facial erythema of rosacea in late 2013. The effect was shown to occur in some patients as soon as 30 minutes following product application and usually persisted for approximately 9 to 12 hours. Erythema gradually returned as the effect waned, returning to baseline by 24 hours after application.12
Clinical efficacy of brimonidine gel was evaluated by a 2-grade improvement (primary end point) or 1-grade improvement (secondary end point) based on patient and clinician assessments using the patient self-assessment (PSA) and clinical erythema assessment (CEA), respectively, over the course of the 4-week studies. Patients also evaluated their satisfaction with the overall appearance of their facial skin.12 A 2-grade improvement on both PSA and CEA has been noted as a stringent criterion in evaluating the effectiveness of treatment of facial erythema.14 A 1-grade improvement on both PSA and CEA has been recognized as clinically relevant as a measure of effect that is noticeable to both patients and clinicians.12 Patients who achieved either a 1- or 2-grade improvement in both PSA and CEA were substantially more likely to report overall satisfaction with their appearance. A 2-grade improvement in both PSA and CEA scales was accompanied with 80% of patients rating their skin appearance as satisfactory or better. Conversely, few patients (ie, <10%) who did not achieve at least 1-grade composite success were satisfied with their appearance. Adverse effects were uncommon, with no evidence of tachyphylaxis and rare occurrence of rebound erythema.12
In summary, although there are a number of options available to reduce or eliminate inflammatory lesions of rosacea, effective medical treatment of the erythematous component of rosacea, which can be the most distressing aspect of the disease for patients, has been unsatisfactory. With the availability of topical brimonidine tartrate, dermatologists have a proven medication to improve facial redness in rosacea patients. Other options such as oxymetazoline may become available in the future.
- Crawford GH, Pelle MT, James WD. Rosacea: I. etiology, pathogenesis, and subtype classification. J Am Acad Dermatol. 2004;51:327-341.
- Wilkin J, Dahl M, Detmar M, et al. Standard grading system for rosacea: report of the National Rosacea Society expert committee on the classification and staging of rosacea. J Am Acad Dermatol. 2004;50:907-912.
- Odom R, Dahl M, Dover J, et al. Standard management options for rosacea, part I: overview and broad spectrum of care. Cutis. 2009;84:43-47.
- Baldwin HE. Systemic therapy for rosacea. Skin Therapy Lett. 2007;12:1-5.
- Drake L. Rosacea patients feel effects of their condition in social settings. Rosacea Review. Published Fall 2012. http://www.rosacea.org/rr/2012/fall/article_3.php. Accessed May 29, 2014.
- Aksoy B, Altaykan-Hapa A, Egemen D, et al. The impact of rosacea on quality of life: effects of demographic and clinical characteristics and various treatment modalities. Br J Dermatol. 2010;163:719-725.
- Wolf JE Jr, Del Rosso JQ. The CLEAR trial: results of a large community-based study of metronidazole gel in rosacea. Cutis. 2007;79:73-80.
- Nicholson K, Abramova L, Chren MM, et al. A pilot quality-of-life instrument for acne rosacea. J Am Acad Dermatol. 2007;57:213-221.
- Fowler J, Jarratt M, Moore A, et al. Once-daily topical brimonidine tartrate gel 0.5% is a novel treatment for moderate to severe facial erythema of rosacea: results of two multicentre, randomized and vehicle-controlled studies. Br J Dermatol. 2012;166:633-641.
- Steinhoff M, Buddenkotte J, Aubert J, et al. Clinical, cellular and molecular aspects in the pathophysiology of rosacea. J Investig Dermatol Symp Proc. 2011;15:2-11.
- Wilkin JK. Rosacea: pathophysiology and treatment. Arch Dermatol. 1994;130:359-362.
- Fowler J, Jackson JM, Moore A, et al. Efficacy and safety of once-daily topical brimonidine tartrate gel 0.5% for the treatment of moderate to severe facial erythema of rosacea: results of two randomized, double-blind, vehicle-controlled pivotal studies. J Drugs Dermatol. 2013;12:650-656.
- Del Rosso JQ. The central role, evaluation, and medical management of diffuse and persistent facial erythema of rosacea. J Clin Aesthet Dermatol. 2012;5:26-36.
- Rahman MQ, Ramaesh K, Montgomery DNI. Brimonidine for glaucoma. Expert Opin Drug Saf. 2010;9:483-491.
- Toris C, Camras C, Yablonski M. Acute versus chronic effects of brimonidine on aqueous humor dynamics in ocular hypertensive patients. Am J Ophthalmol. 1999;128:8-14.
Rosacea is a common chronic disorder that predominantly manifests as facial erythema, telangiectasia, and flushing.1 Other primary clinical features include inflammatory lesions (eg, papules, pustules) and occasionally edema and rhinophyma. Ocular involvement also may occur. Rosacea patients routinely report sensitive skin with symptoms such as stinging, burning, and intolerance to topical agents (eg, medications, moisturizers, cosmetics).2,3
Rosacea affects facial appearance and can impair a patient’s emotional well-being. It may limit social and professional activities. Many patients and nonpatients alike presume the appearance of facial redness suggests alcohol overuse or emotional distress.4-6 A reduction in facial erythema as well as improvements in other clinical signs of rosacea have been shown to reduce patient self-consciousness and lead to increased socialization.7 Facial erythema is a major factor that adversely affects quality of life in rosacea patients.8
Although a number of options are available to successfully treat inflammatory lesions of rosacea, facial erythema has been the most difficult manifestation of the disease to medically treat.2,9 Chronic erythema and episodic flushing may be at least partially related to cutaneous vasomotor responses causing both transient and persistent dilation of facial blood vessels.10-12 The agents used for treating the inflammatory components of rosacea reduce erythema associated with papules and pustules but have little effect on the background erythema that is so noticeable. Oxymetazoline and brimonidine tartrate have been evaluated as potential rapid treatments of facial erythema. Daily application of oxymetazoline solution 0.05% has been shown to reduce facial erythema in rosacea patients.13
Brimonidine tartrate is a highly selective α2-adrenergic receptor agonist with potent vasoconstrictor activity.14 It has been used to treat open-angle glaucoma for more than 15 years with well-documented safety and efficacy.15 Phase 2 and 3 studies for once-daily application of brimonidine tartrate gel 0.5% have confirmed its efficacy and safety for the treatment of facial erythema in rosacea patients,9,12 and it was approved by the US Food and Drug Administration (brimonidine gel 0.33%) for persistent (nontransient) facial erythema of rosacea in late 2013. The effect was shown to occur in some patients as soon as 30 minutes following product application and usually persisted for approximately 9 to 12 hours. Erythema gradually returned as the effect waned, returning to baseline by 24 hours after application.12
Clinical efficacy of brimonidine gel was evaluated by a 2-grade improvement (primary end point) or 1-grade improvement (secondary end point) based on patient and clinician assessments using the patient self-assessment (PSA) and clinical erythema assessment (CEA), respectively, over the course of the 4-week studies. Patients also evaluated their satisfaction with the overall appearance of their facial skin.12 A 2-grade improvement on both PSA and CEA has been noted as a stringent criterion in evaluating the effectiveness of treatment of facial erythema.14 A 1-grade improvement on both PSA and CEA has been recognized as clinically relevant as a measure of effect that is noticeable to both patients and clinicians.12 Patients who achieved either a 1- or 2-grade improvement in both PSA and CEA were substantially more likely to report overall satisfaction with their appearance. A 2-grade improvement in both PSA and CEA scales was accompanied with 80% of patients rating their skin appearance as satisfactory or better. Conversely, few patients (ie, <10%) who did not achieve at least 1-grade composite success were satisfied with their appearance. Adverse effects were uncommon, with no evidence of tachyphylaxis and rare occurrence of rebound erythema.12
In summary, although there are a number of options available to reduce or eliminate inflammatory lesions of rosacea, effective medical treatment of the erythematous component of rosacea, which can be the most distressing aspect of the disease for patients, has been unsatisfactory. With the availability of topical brimonidine tartrate, dermatologists have a proven medication to improve facial redness in rosacea patients. Other options such as oxymetazoline may become available in the future.
Rosacea is a common chronic disorder that predominantly manifests as facial erythema, telangiectasia, and flushing.1 Other primary clinical features include inflammatory lesions (eg, papules, pustules) and occasionally edema and rhinophyma. Ocular involvement also may occur. Rosacea patients routinely report sensitive skin with symptoms such as stinging, burning, and intolerance to topical agents (eg, medications, moisturizers, cosmetics).2,3
Rosacea affects facial appearance and can impair a patient’s emotional well-being. It may limit social and professional activities. Many patients and nonpatients alike presume the appearance of facial redness suggests alcohol overuse or emotional distress.4-6 A reduction in facial erythema as well as improvements in other clinical signs of rosacea have been shown to reduce patient self-consciousness and lead to increased socialization.7 Facial erythema is a major factor that adversely affects quality of life in rosacea patients.8
Although a number of options are available to successfully treat inflammatory lesions of rosacea, facial erythema has been the most difficult manifestation of the disease to medically treat.2,9 Chronic erythema and episodic flushing may be at least partially related to cutaneous vasomotor responses causing both transient and persistent dilation of facial blood vessels.10-12 The agents used for treating the inflammatory components of rosacea reduce erythema associated with papules and pustules but have little effect on the background erythema that is so noticeable. Oxymetazoline and brimonidine tartrate have been evaluated as potential rapid treatments of facial erythema. Daily application of oxymetazoline solution 0.05% has been shown to reduce facial erythema in rosacea patients.13
Brimonidine tartrate is a highly selective α2-adrenergic receptor agonist with potent vasoconstrictor activity.14 It has been used to treat open-angle glaucoma for more than 15 years with well-documented safety and efficacy.15 Phase 2 and 3 studies for once-daily application of brimonidine tartrate gel 0.5% have confirmed its efficacy and safety for the treatment of facial erythema in rosacea patients,9,12 and it was approved by the US Food and Drug Administration (brimonidine gel 0.33%) for persistent (nontransient) facial erythema of rosacea in late 2013. The effect was shown to occur in some patients as soon as 30 minutes following product application and usually persisted for approximately 9 to 12 hours. Erythema gradually returned as the effect waned, returning to baseline by 24 hours after application.12
Clinical efficacy of brimonidine gel was evaluated by a 2-grade improvement (primary end point) or 1-grade improvement (secondary end point) based on patient and clinician assessments using the patient self-assessment (PSA) and clinical erythema assessment (CEA), respectively, over the course of the 4-week studies. Patients also evaluated their satisfaction with the overall appearance of their facial skin.12 A 2-grade improvement on both PSA and CEA has been noted as a stringent criterion in evaluating the effectiveness of treatment of facial erythema.14 A 1-grade improvement on both PSA and CEA has been recognized as clinically relevant as a measure of effect that is noticeable to both patients and clinicians.12 Patients who achieved either a 1- or 2-grade improvement in both PSA and CEA were substantially more likely to report overall satisfaction with their appearance. A 2-grade improvement in both PSA and CEA scales was accompanied with 80% of patients rating their skin appearance as satisfactory or better. Conversely, few patients (ie, <10%) who did not achieve at least 1-grade composite success were satisfied with their appearance. Adverse effects were uncommon, with no evidence of tachyphylaxis and rare occurrence of rebound erythema.12
In summary, although there are a number of options available to reduce or eliminate inflammatory lesions of rosacea, effective medical treatment of the erythematous component of rosacea, which can be the most distressing aspect of the disease for patients, has been unsatisfactory. With the availability of topical brimonidine tartrate, dermatologists have a proven medication to improve facial redness in rosacea patients. Other options such as oxymetazoline may become available in the future.
- Crawford GH, Pelle MT, James WD. Rosacea: I. etiology, pathogenesis, and subtype classification. J Am Acad Dermatol. 2004;51:327-341.
- Wilkin J, Dahl M, Detmar M, et al. Standard grading system for rosacea: report of the National Rosacea Society expert committee on the classification and staging of rosacea. J Am Acad Dermatol. 2004;50:907-912.
- Odom R, Dahl M, Dover J, et al. Standard management options for rosacea, part I: overview and broad spectrum of care. Cutis. 2009;84:43-47.
- Baldwin HE. Systemic therapy for rosacea. Skin Therapy Lett. 2007;12:1-5.
- Drake L. Rosacea patients feel effects of their condition in social settings. Rosacea Review. Published Fall 2012. http://www.rosacea.org/rr/2012/fall/article_3.php. Accessed May 29, 2014.
- Aksoy B, Altaykan-Hapa A, Egemen D, et al. The impact of rosacea on quality of life: effects of demographic and clinical characteristics and various treatment modalities. Br J Dermatol. 2010;163:719-725.
- Wolf JE Jr, Del Rosso JQ. The CLEAR trial: results of a large community-based study of metronidazole gel in rosacea. Cutis. 2007;79:73-80.
- Nicholson K, Abramova L, Chren MM, et al. A pilot quality-of-life instrument for acne rosacea. J Am Acad Dermatol. 2007;57:213-221.
- Fowler J, Jarratt M, Moore A, et al. Once-daily topical brimonidine tartrate gel 0.5% is a novel treatment for moderate to severe facial erythema of rosacea: results of two multicentre, randomized and vehicle-controlled studies. Br J Dermatol. 2012;166:633-641.
- Steinhoff M, Buddenkotte J, Aubert J, et al. Clinical, cellular and molecular aspects in the pathophysiology of rosacea. J Investig Dermatol Symp Proc. 2011;15:2-11.
- Wilkin JK. Rosacea: pathophysiology and treatment. Arch Dermatol. 1994;130:359-362.
- Fowler J, Jackson JM, Moore A, et al. Efficacy and safety of once-daily topical brimonidine tartrate gel 0.5% for the treatment of moderate to severe facial erythema of rosacea: results of two randomized, double-blind, vehicle-controlled pivotal studies. J Drugs Dermatol. 2013;12:650-656.
- Del Rosso JQ. The central role, evaluation, and medical management of diffuse and persistent facial erythema of rosacea. J Clin Aesthet Dermatol. 2012;5:26-36.
- Rahman MQ, Ramaesh K, Montgomery DNI. Brimonidine for glaucoma. Expert Opin Drug Saf. 2010;9:483-491.
- Toris C, Camras C, Yablonski M. Acute versus chronic effects of brimonidine on aqueous humor dynamics in ocular hypertensive patients. Am J Ophthalmol. 1999;128:8-14.
- Crawford GH, Pelle MT, James WD. Rosacea: I. etiology, pathogenesis, and subtype classification. J Am Acad Dermatol. 2004;51:327-341.
- Wilkin J, Dahl M, Detmar M, et al. Standard grading system for rosacea: report of the National Rosacea Society expert committee on the classification and staging of rosacea. J Am Acad Dermatol. 2004;50:907-912.
- Odom R, Dahl M, Dover J, et al. Standard management options for rosacea, part I: overview and broad spectrum of care. Cutis. 2009;84:43-47.
- Baldwin HE. Systemic therapy for rosacea. Skin Therapy Lett. 2007;12:1-5.
- Drake L. Rosacea patients feel effects of their condition in social settings. Rosacea Review. Published Fall 2012. http://www.rosacea.org/rr/2012/fall/article_3.php. Accessed May 29, 2014.
- Aksoy B, Altaykan-Hapa A, Egemen D, et al. The impact of rosacea on quality of life: effects of demographic and clinical characteristics and various treatment modalities. Br J Dermatol. 2010;163:719-725.
- Wolf JE Jr, Del Rosso JQ. The CLEAR trial: results of a large community-based study of metronidazole gel in rosacea. Cutis. 2007;79:73-80.
- Nicholson K, Abramova L, Chren MM, et al. A pilot quality-of-life instrument for acne rosacea. J Am Acad Dermatol. 2007;57:213-221.
- Fowler J, Jarratt M, Moore A, et al. Once-daily topical brimonidine tartrate gel 0.5% is a novel treatment for moderate to severe facial erythema of rosacea: results of two multicentre, randomized and vehicle-controlled studies. Br J Dermatol. 2012;166:633-641.
- Steinhoff M, Buddenkotte J, Aubert J, et al. Clinical, cellular and molecular aspects in the pathophysiology of rosacea. J Investig Dermatol Symp Proc. 2011;15:2-11.
- Wilkin JK. Rosacea: pathophysiology and treatment. Arch Dermatol. 1994;130:359-362.
- Fowler J, Jackson JM, Moore A, et al. Efficacy and safety of once-daily topical brimonidine tartrate gel 0.5% for the treatment of moderate to severe facial erythema of rosacea: results of two randomized, double-blind, vehicle-controlled pivotal studies. J Drugs Dermatol. 2013;12:650-656.
- Del Rosso JQ. The central role, evaluation, and medical management of diffuse and persistent facial erythema of rosacea. J Clin Aesthet Dermatol. 2012;5:26-36.
- Rahman MQ, Ramaesh K, Montgomery DNI. Brimonidine for glaucoma. Expert Opin Drug Saf. 2010;9:483-491.
- Toris C, Camras C, Yablonski M. Acute versus chronic effects of brimonidine on aqueous humor dynamics in ocular hypertensive patients. Am J Ophthalmol. 1999;128:8-14.
OTC topical acne meds can cause severe reactions
The Food and Drug Administration says that some over-the-counter acne products can cause severe hypersensitivity or allergic reactions and is warning consumers to discontinue use immediately and seek emergency medical attention if they experience such symptoms.
The reactions seem to be on the upswing, according to the agency’s review of reports from 1969 to early 2013.
The agency said in safety announcement that it has not determined whether the reactions are being triggered by the active ingredients in the products – benzoyl peroxide or salicylic acid – or by the inactive ingredients or a combination of the inactive and active ingredients.
The products include Proactiv, Neutrogena, MaxClarity, Oxy, Ambi, Aveeno, Clean & Clear, and private label store brands, and are sold as gels, lotions, face washes, solutions, cleansing pads, toners, and face scrubs, among other products, according to the agency.
The FDA has received reports stating that – within minutes to a day or longer of use – consumers have had hypersensitivity reactions that include throat tightness, difficulty breathing, or swelling of the eyes, face, lips, or tongue. Hives and itching are also indications of an allergic reaction.
From 1969 to January 2013, the agency identified 131 cases of hypersensitivity reactions, with the majority reported in 2012-2013. A total of 86% (113) of the cases were in women, and the reactions were reported in people aged 11-78 years.
There were no deaths, but 58 of the consumers were hospitalized. A total of 38 of the 131 cases (29%) were categorized as anaphylactic reactions, and the remainder were categorized as nonanaphylactic hypersensitivity. Almost half of the consumers said they had discontinued use after the reaction, the majority of whom reported some degree of recovery after discontinuing product use, with final outcomes unavailable for the rest. Four of the patients who used the product again reported a recurrence of the reaction.
The agency is encouraging manufacturers to add directions on testing for hypersensitivity to the labels of all OTC topical acne products. Some labels already include those instructions, which direct users to apply a small amount to one or two small affected areas of the skin for 3 days. If there is no reaction – topical or otherwise – then the product can be used according to the directions.
Consumers are also being urged to avoid using a product if they have previously experienced a hypersensitivity reaction with its use.
The FDA says that physicians should be aware that some topical prescription acne drug products also contain warnings about allergic reactions, including anaphylaxis.
Physicians can report adverse events involving OTC topical acne products to the FDA MedWatch program.
On Twitter @aliciaault
The Food and Drug Administration says that some over-the-counter acne products can cause severe hypersensitivity or allergic reactions and is warning consumers to discontinue use immediately and seek emergency medical attention if they experience such symptoms.
The reactions seem to be on the upswing, according to the agency’s review of reports from 1969 to early 2013.
The agency said in safety announcement that it has not determined whether the reactions are being triggered by the active ingredients in the products – benzoyl peroxide or salicylic acid – or by the inactive ingredients or a combination of the inactive and active ingredients.
The products include Proactiv, Neutrogena, MaxClarity, Oxy, Ambi, Aveeno, Clean & Clear, and private label store brands, and are sold as gels, lotions, face washes, solutions, cleansing pads, toners, and face scrubs, among other products, according to the agency.
The FDA has received reports stating that – within minutes to a day or longer of use – consumers have had hypersensitivity reactions that include throat tightness, difficulty breathing, or swelling of the eyes, face, lips, or tongue. Hives and itching are also indications of an allergic reaction.
From 1969 to January 2013, the agency identified 131 cases of hypersensitivity reactions, with the majority reported in 2012-2013. A total of 86% (113) of the cases were in women, and the reactions were reported in people aged 11-78 years.
There were no deaths, but 58 of the consumers were hospitalized. A total of 38 of the 131 cases (29%) were categorized as anaphylactic reactions, and the remainder were categorized as nonanaphylactic hypersensitivity. Almost half of the consumers said they had discontinued use after the reaction, the majority of whom reported some degree of recovery after discontinuing product use, with final outcomes unavailable for the rest. Four of the patients who used the product again reported a recurrence of the reaction.
The agency is encouraging manufacturers to add directions on testing for hypersensitivity to the labels of all OTC topical acne products. Some labels already include those instructions, which direct users to apply a small amount to one or two small affected areas of the skin for 3 days. If there is no reaction – topical or otherwise – then the product can be used according to the directions.
Consumers are also being urged to avoid using a product if they have previously experienced a hypersensitivity reaction with its use.
The FDA says that physicians should be aware that some topical prescription acne drug products also contain warnings about allergic reactions, including anaphylaxis.
Physicians can report adverse events involving OTC topical acne products to the FDA MedWatch program.
On Twitter @aliciaault
The Food and Drug Administration says that some over-the-counter acne products can cause severe hypersensitivity or allergic reactions and is warning consumers to discontinue use immediately and seek emergency medical attention if they experience such symptoms.
The reactions seem to be on the upswing, according to the agency’s review of reports from 1969 to early 2013.
The agency said in safety announcement that it has not determined whether the reactions are being triggered by the active ingredients in the products – benzoyl peroxide or salicylic acid – or by the inactive ingredients or a combination of the inactive and active ingredients.
The products include Proactiv, Neutrogena, MaxClarity, Oxy, Ambi, Aveeno, Clean & Clear, and private label store brands, and are sold as gels, lotions, face washes, solutions, cleansing pads, toners, and face scrubs, among other products, according to the agency.
The FDA has received reports stating that – within minutes to a day or longer of use – consumers have had hypersensitivity reactions that include throat tightness, difficulty breathing, or swelling of the eyes, face, lips, or tongue. Hives and itching are also indications of an allergic reaction.
From 1969 to January 2013, the agency identified 131 cases of hypersensitivity reactions, with the majority reported in 2012-2013. A total of 86% (113) of the cases were in women, and the reactions were reported in people aged 11-78 years.
There were no deaths, but 58 of the consumers were hospitalized. A total of 38 of the 131 cases (29%) were categorized as anaphylactic reactions, and the remainder were categorized as nonanaphylactic hypersensitivity. Almost half of the consumers said they had discontinued use after the reaction, the majority of whom reported some degree of recovery after discontinuing product use, with final outcomes unavailable for the rest. Four of the patients who used the product again reported a recurrence of the reaction.
The agency is encouraging manufacturers to add directions on testing for hypersensitivity to the labels of all OTC topical acne products. Some labels already include those instructions, which direct users to apply a small amount to one or two small affected areas of the skin for 3 days. If there is no reaction – topical or otherwise – then the product can be used according to the directions.
Consumers are also being urged to avoid using a product if they have previously experienced a hypersensitivity reaction with its use.
The FDA says that physicians should be aware that some topical prescription acne drug products also contain warnings about allergic reactions, including anaphylaxis.
Physicians can report adverse events involving OTC topical acne products to the FDA MedWatch program.
On Twitter @aliciaault
Adapalene/benzoyl peroxide gel halves acne lesion counts in 1 month
WAIKOLOA, HAWAII – The fixed-dose combination of adapalene/benzoyl peroxide gel 0.1%/2.5% typically results in reductions of 40%-50% in inflammatory and 30%-40% in noninflammatory acne lesion counts during the first 4 weeks of therapy.
In a pooled analysis of 14 studies totaling 2,358 acne patients aged 9-61 years, the proportion with an Investigator’s Global Assessment (IGA) of moderate or severe acne dropped from 92% at baseline to 51% at 4 weeks, according to Dr. Linda Stein Gold.
The pooled analysis was carried out to provide physicians and patients with information as to what to realistically expect in the first 4 weeks of therapy. The analysis is particularly timely in light of the Food and Drug Administration’s recent expansion of the indication for adapalene/benzoyl peroxide gel 0.1%/2.5% (Epiduo) to include acne patients as young as 9 years of age, noted Dr. Stein Gold, director of clinical research in the department of dermatology at Henry Ford Hospital, Detroit.
Mild skin irritation was common, especially in the first 2 weeks of use.
"It’s a fiction that retinoids are too harsh for younger patients to use," Dr. Stein Gold said. "I tell all my acne patients, especially the younger ones, no matter what topical retinoid they’re using, to use it every other night for the first 2 weeks, make sure their skin is completely dry, and use a gentle cleanser and a good moisturizer," she said at the Hawaii Dermatology Seminar sponsored by Global Academy for Medical Education/Skin Disease Education Foundation. "If they get through those first 2 weeks, they’ll see that the tolerability really improves."
Dr. Stein Gold said she likes the fixed combination of adapalene/benzoyl peroxide gel 0.1%/2.5% because it combines several key elements of cutting-edge acne treatment. Topical retinoids are not mere comedone busters, as formerly thought, but are also effective agents for papules and pustular lesions. While they do not kill Propionibacterium acnes, they down-regulate Toll-like receptor 2, which is produced by the bacterium and induces proinflammatory cytokines. Topical retinoids are a key part of maintenance-of-remission strategies. And adapalene is unique among topical retinoids in that it is inherently stable with benzoyl peroxide (BPO) and is stable in daylight.
BPO is unique in that it has potent antibacterial activity, but never causes P. acnes resistance, even after many years of treatment. BPO is quite effective for inflammatory lesions and moderately effective for comedonal acne lesions, Dr. Stein Gold noted. Also, it is well established that BPO at a concentration of 2.5% is as efficacious as 5% or 10%, and much better tolerated than at the higher concentrations. The molecule size and the use of a vehicle with good penetration into the hair follicle are much more important factors in treatment effectiveness than is the BPO concentration, she added.
In one study of acne patients with antibiotic-resistant P. acnes, including clindamycin-, doxycycline-, erythromycin-, and minocycline-resistant microorganisms, 88% of the antibiotic-resistant P. acnes bacteria were killed after 2 weeks of treatment with adapalene/BPO gel 0.1%/2.5%. After 4 weeks of treatment, 97% of the antibiotic-resistant organisms were dead. That’s testimony to the P. acnes–killing power of BPO, said Dr. Stein Gold.
"There’s a sense among many that with all the newer medications we have for acne, benzoyl peroxide is really your grandfather’s treatment, with no place in today’s modern world. This is totally false. I really feel that benzoyl peroxide should play a central role in all of our acne patients’ treatment regimens, unless of course they’re allergic to it, which occurs in only a small percentage of our patients," she said.
The pooled analysis was funded by Galderma. Dr. Stein Gold is a consultant to Galderma, Stiefel, Medicis, and Warner Chilcott.
SDEF and this news organization are owned by the same parent company.
WAIKOLOA, HAWAII – The fixed-dose combination of adapalene/benzoyl peroxide gel 0.1%/2.5% typically results in reductions of 40%-50% in inflammatory and 30%-40% in noninflammatory acne lesion counts during the first 4 weeks of therapy.
In a pooled analysis of 14 studies totaling 2,358 acne patients aged 9-61 years, the proportion with an Investigator’s Global Assessment (IGA) of moderate or severe acne dropped from 92% at baseline to 51% at 4 weeks, according to Dr. Linda Stein Gold.
The pooled analysis was carried out to provide physicians and patients with information as to what to realistically expect in the first 4 weeks of therapy. The analysis is particularly timely in light of the Food and Drug Administration’s recent expansion of the indication for adapalene/benzoyl peroxide gel 0.1%/2.5% (Epiduo) to include acne patients as young as 9 years of age, noted Dr. Stein Gold, director of clinical research in the department of dermatology at Henry Ford Hospital, Detroit.
Mild skin irritation was common, especially in the first 2 weeks of use.
"It’s a fiction that retinoids are too harsh for younger patients to use," Dr. Stein Gold said. "I tell all my acne patients, especially the younger ones, no matter what topical retinoid they’re using, to use it every other night for the first 2 weeks, make sure their skin is completely dry, and use a gentle cleanser and a good moisturizer," she said at the Hawaii Dermatology Seminar sponsored by Global Academy for Medical Education/Skin Disease Education Foundation. "If they get through those first 2 weeks, they’ll see that the tolerability really improves."
Dr. Stein Gold said she likes the fixed combination of adapalene/benzoyl peroxide gel 0.1%/2.5% because it combines several key elements of cutting-edge acne treatment. Topical retinoids are not mere comedone busters, as formerly thought, but are also effective agents for papules and pustular lesions. While they do not kill Propionibacterium acnes, they down-regulate Toll-like receptor 2, which is produced by the bacterium and induces proinflammatory cytokines. Topical retinoids are a key part of maintenance-of-remission strategies. And adapalene is unique among topical retinoids in that it is inherently stable with benzoyl peroxide (BPO) and is stable in daylight.
BPO is unique in that it has potent antibacterial activity, but never causes P. acnes resistance, even after many years of treatment. BPO is quite effective for inflammatory lesions and moderately effective for comedonal acne lesions, Dr. Stein Gold noted. Also, it is well established that BPO at a concentration of 2.5% is as efficacious as 5% or 10%, and much better tolerated than at the higher concentrations. The molecule size and the use of a vehicle with good penetration into the hair follicle are much more important factors in treatment effectiveness than is the BPO concentration, she added.
In one study of acne patients with antibiotic-resistant P. acnes, including clindamycin-, doxycycline-, erythromycin-, and minocycline-resistant microorganisms, 88% of the antibiotic-resistant P. acnes bacteria were killed after 2 weeks of treatment with adapalene/BPO gel 0.1%/2.5%. After 4 weeks of treatment, 97% of the antibiotic-resistant organisms were dead. That’s testimony to the P. acnes–killing power of BPO, said Dr. Stein Gold.
"There’s a sense among many that with all the newer medications we have for acne, benzoyl peroxide is really your grandfather’s treatment, with no place in today’s modern world. This is totally false. I really feel that benzoyl peroxide should play a central role in all of our acne patients’ treatment regimens, unless of course they’re allergic to it, which occurs in only a small percentage of our patients," she said.
The pooled analysis was funded by Galderma. Dr. Stein Gold is a consultant to Galderma, Stiefel, Medicis, and Warner Chilcott.
SDEF and this news organization are owned by the same parent company.
WAIKOLOA, HAWAII – The fixed-dose combination of adapalene/benzoyl peroxide gel 0.1%/2.5% typically results in reductions of 40%-50% in inflammatory and 30%-40% in noninflammatory acne lesion counts during the first 4 weeks of therapy.
In a pooled analysis of 14 studies totaling 2,358 acne patients aged 9-61 years, the proportion with an Investigator’s Global Assessment (IGA) of moderate or severe acne dropped from 92% at baseline to 51% at 4 weeks, according to Dr. Linda Stein Gold.
The pooled analysis was carried out to provide physicians and patients with information as to what to realistically expect in the first 4 weeks of therapy. The analysis is particularly timely in light of the Food and Drug Administration’s recent expansion of the indication for adapalene/benzoyl peroxide gel 0.1%/2.5% (Epiduo) to include acne patients as young as 9 years of age, noted Dr. Stein Gold, director of clinical research in the department of dermatology at Henry Ford Hospital, Detroit.
Mild skin irritation was common, especially in the first 2 weeks of use.
"It’s a fiction that retinoids are too harsh for younger patients to use," Dr. Stein Gold said. "I tell all my acne patients, especially the younger ones, no matter what topical retinoid they’re using, to use it every other night for the first 2 weeks, make sure their skin is completely dry, and use a gentle cleanser and a good moisturizer," she said at the Hawaii Dermatology Seminar sponsored by Global Academy for Medical Education/Skin Disease Education Foundation. "If they get through those first 2 weeks, they’ll see that the tolerability really improves."
Dr. Stein Gold said she likes the fixed combination of adapalene/benzoyl peroxide gel 0.1%/2.5% because it combines several key elements of cutting-edge acne treatment. Topical retinoids are not mere comedone busters, as formerly thought, but are also effective agents for papules and pustular lesions. While they do not kill Propionibacterium acnes, they down-regulate Toll-like receptor 2, which is produced by the bacterium and induces proinflammatory cytokines. Topical retinoids are a key part of maintenance-of-remission strategies. And adapalene is unique among topical retinoids in that it is inherently stable with benzoyl peroxide (BPO) and is stable in daylight.
BPO is unique in that it has potent antibacterial activity, but never causes P. acnes resistance, even after many years of treatment. BPO is quite effective for inflammatory lesions and moderately effective for comedonal acne lesions, Dr. Stein Gold noted. Also, it is well established that BPO at a concentration of 2.5% is as efficacious as 5% or 10%, and much better tolerated than at the higher concentrations. The molecule size and the use of a vehicle with good penetration into the hair follicle are much more important factors in treatment effectiveness than is the BPO concentration, she added.
In one study of acne patients with antibiotic-resistant P. acnes, including clindamycin-, doxycycline-, erythromycin-, and minocycline-resistant microorganisms, 88% of the antibiotic-resistant P. acnes bacteria were killed after 2 weeks of treatment with adapalene/BPO gel 0.1%/2.5%. After 4 weeks of treatment, 97% of the antibiotic-resistant organisms were dead. That’s testimony to the P. acnes–killing power of BPO, said Dr. Stein Gold.
"There’s a sense among many that with all the newer medications we have for acne, benzoyl peroxide is really your grandfather’s treatment, with no place in today’s modern world. This is totally false. I really feel that benzoyl peroxide should play a central role in all of our acne patients’ treatment regimens, unless of course they’re allergic to it, which occurs in only a small percentage of our patients," she said.
The pooled analysis was funded by Galderma. Dr. Stein Gold is a consultant to Galderma, Stiefel, Medicis, and Warner Chilcott.
SDEF and this news organization are owned by the same parent company.
EXPERT ANALYSIS FROM SDEF HAWAII DERMATOLOGY SEMINAR
Gold-coated microparticles aid acne treatment
PHOENIX – Application of gold-coated microparticles to the skin may enhance laser photothermolysis of sebaceous glands to treat acne, according to three small, preliminary studies.
Inadequate contrast in sebaceous glands has limited the effectiveness of selective photothermolysis to treat acne. Specially engineered 0.150-mcm microparticles of inert gold coating a silica core were designed for surface plasmon resonance at near-infrared wavelengths to optimize contrast and high absorption of light in the sebaceous glands during laser treatment.
In a randomized, controlled crossover study, 48 patients with acne vulgaris received either immediate or delayed treatment for 12 weeks, after which the delayed-treatment group also began receiving the treatment. Immediate treatment included three treatments at 2-week intervals. The treatment consisted of a microparticle suspension gently massaged into facial skin for about 8 minutes, superficial skin cleansing, and pulsed irradiation with two passes of an 800-nm laser. Patients in the delayed-treatment group used an over-the-counter face wash (2% salicylic acid) twice daily.
The mean number of inflammatory lesions decreased by 34% at 12 weeks after treatment started, compared with a 16% reduction in the control group, a statistically significant difference, Dilip Paithankar, Ph.D., reported at the annual meeting of the American Society for Laser Medicine and Surgery.
At 28 weeks after the crossover to treatment for all subjects (40 weeks after the start of the study), the mean number of inflammatory lesions decreased by 61%, reported Dr. Paithankar, chief technology officer at Sebacia, Duluth, Ga., which is developing the gold-coated microparticles treatment.
In another randomized, sham-controlled study, 49 patients with acne vulgaris underwent three laser photothermolysis treatments at weekly intervals using either the gold-coated microparticles or a control vehicle without the particles. Mean inflammatory lesion counts decreased significantly more in the treatment group than in the control group at 8, 12, and 16 weeks of follow-up. In the treatment group, mean inflammatory lesion counts were 44% lower at 8 weeks, 49% lower at 12 weeks, and 53% lower at 16 weeks, compared with baseline. In the control group, mean inflammatory lesion counts were 14% lower, 22% lower, and 31% lower at those time points, respectively, compared with baseline.
Inflammatory lesions were clear or almost clear by the end of the study in 24% in the treatment group and in none of the control patients.
Data from a separate histologic study suggest that using ultrasound to assist delivery of the gold-coated microparticles may improve selective destruction from laser treatment, Dr. Girish Munavalli reported at the meeting. Ultrasound has been used in other settings to enhance delivery of small molecules through the stratum corneum.
The study used an ultrasound horn vibrating in a microparticle suspension placed in an enclosure above preauricular skin in 37 subjects, followed by 800-nm laser treatment. Biopsies taken within 15 minutes showed localized thermal injury to infundibuli and sebaceous glands with no collateral damage to surrounding tissue or to the epidermis, reported Dr. Munavalli, a dermatologist in group practice in Charlotte, N.C. The extent of thermal damage correlated with the duration of the ultrasound.
The level of damage could be expected to improve acne, but that needs confirmation in clinical trials, he said.
In each of the studies, the treatments were well tolerated, with minimal side effects including mild to moderate pain (using no anesthetic) and short-lived erythema and edema.
Dr. Paithankar works for Sebacia. Dr. Munavalli reported having no disclosures; some of his coinvestigators work for Sebacia.
On Twitter @sherryboschert
PHOENIX – Application of gold-coated microparticles to the skin may enhance laser photothermolysis of sebaceous glands to treat acne, according to three small, preliminary studies.
Inadequate contrast in sebaceous glands has limited the effectiveness of selective photothermolysis to treat acne. Specially engineered 0.150-mcm microparticles of inert gold coating a silica core were designed for surface plasmon resonance at near-infrared wavelengths to optimize contrast and high absorption of light in the sebaceous glands during laser treatment.
In a randomized, controlled crossover study, 48 patients with acne vulgaris received either immediate or delayed treatment for 12 weeks, after which the delayed-treatment group also began receiving the treatment. Immediate treatment included three treatments at 2-week intervals. The treatment consisted of a microparticle suspension gently massaged into facial skin for about 8 minutes, superficial skin cleansing, and pulsed irradiation with two passes of an 800-nm laser. Patients in the delayed-treatment group used an over-the-counter face wash (2% salicylic acid) twice daily.
The mean number of inflammatory lesions decreased by 34% at 12 weeks after treatment started, compared with a 16% reduction in the control group, a statistically significant difference, Dilip Paithankar, Ph.D., reported at the annual meeting of the American Society for Laser Medicine and Surgery.
At 28 weeks after the crossover to treatment for all subjects (40 weeks after the start of the study), the mean number of inflammatory lesions decreased by 61%, reported Dr. Paithankar, chief technology officer at Sebacia, Duluth, Ga., which is developing the gold-coated microparticles treatment.
In another randomized, sham-controlled study, 49 patients with acne vulgaris underwent three laser photothermolysis treatments at weekly intervals using either the gold-coated microparticles or a control vehicle without the particles. Mean inflammatory lesion counts decreased significantly more in the treatment group than in the control group at 8, 12, and 16 weeks of follow-up. In the treatment group, mean inflammatory lesion counts were 44% lower at 8 weeks, 49% lower at 12 weeks, and 53% lower at 16 weeks, compared with baseline. In the control group, mean inflammatory lesion counts were 14% lower, 22% lower, and 31% lower at those time points, respectively, compared with baseline.
Inflammatory lesions were clear or almost clear by the end of the study in 24% in the treatment group and in none of the control patients.
Data from a separate histologic study suggest that using ultrasound to assist delivery of the gold-coated microparticles may improve selective destruction from laser treatment, Dr. Girish Munavalli reported at the meeting. Ultrasound has been used in other settings to enhance delivery of small molecules through the stratum corneum.
The study used an ultrasound horn vibrating in a microparticle suspension placed in an enclosure above preauricular skin in 37 subjects, followed by 800-nm laser treatment. Biopsies taken within 15 minutes showed localized thermal injury to infundibuli and sebaceous glands with no collateral damage to surrounding tissue or to the epidermis, reported Dr. Munavalli, a dermatologist in group practice in Charlotte, N.C. The extent of thermal damage correlated with the duration of the ultrasound.
The level of damage could be expected to improve acne, but that needs confirmation in clinical trials, he said.
In each of the studies, the treatments were well tolerated, with minimal side effects including mild to moderate pain (using no anesthetic) and short-lived erythema and edema.
Dr. Paithankar works for Sebacia. Dr. Munavalli reported having no disclosures; some of his coinvestigators work for Sebacia.
On Twitter @sherryboschert
PHOENIX – Application of gold-coated microparticles to the skin may enhance laser photothermolysis of sebaceous glands to treat acne, according to three small, preliminary studies.
Inadequate contrast in sebaceous glands has limited the effectiveness of selective photothermolysis to treat acne. Specially engineered 0.150-mcm microparticles of inert gold coating a silica core were designed for surface plasmon resonance at near-infrared wavelengths to optimize contrast and high absorption of light in the sebaceous glands during laser treatment.
In a randomized, controlled crossover study, 48 patients with acne vulgaris received either immediate or delayed treatment for 12 weeks, after which the delayed-treatment group also began receiving the treatment. Immediate treatment included three treatments at 2-week intervals. The treatment consisted of a microparticle suspension gently massaged into facial skin for about 8 minutes, superficial skin cleansing, and pulsed irradiation with two passes of an 800-nm laser. Patients in the delayed-treatment group used an over-the-counter face wash (2% salicylic acid) twice daily.
The mean number of inflammatory lesions decreased by 34% at 12 weeks after treatment started, compared with a 16% reduction in the control group, a statistically significant difference, Dilip Paithankar, Ph.D., reported at the annual meeting of the American Society for Laser Medicine and Surgery.
At 28 weeks after the crossover to treatment for all subjects (40 weeks after the start of the study), the mean number of inflammatory lesions decreased by 61%, reported Dr. Paithankar, chief technology officer at Sebacia, Duluth, Ga., which is developing the gold-coated microparticles treatment.
In another randomized, sham-controlled study, 49 patients with acne vulgaris underwent three laser photothermolysis treatments at weekly intervals using either the gold-coated microparticles or a control vehicle without the particles. Mean inflammatory lesion counts decreased significantly more in the treatment group than in the control group at 8, 12, and 16 weeks of follow-up. In the treatment group, mean inflammatory lesion counts were 44% lower at 8 weeks, 49% lower at 12 weeks, and 53% lower at 16 weeks, compared with baseline. In the control group, mean inflammatory lesion counts were 14% lower, 22% lower, and 31% lower at those time points, respectively, compared with baseline.
Inflammatory lesions were clear or almost clear by the end of the study in 24% in the treatment group and in none of the control patients.
Data from a separate histologic study suggest that using ultrasound to assist delivery of the gold-coated microparticles may improve selective destruction from laser treatment, Dr. Girish Munavalli reported at the meeting. Ultrasound has been used in other settings to enhance delivery of small molecules through the stratum corneum.
The study used an ultrasound horn vibrating in a microparticle suspension placed in an enclosure above preauricular skin in 37 subjects, followed by 800-nm laser treatment. Biopsies taken within 15 minutes showed localized thermal injury to infundibuli and sebaceous glands with no collateral damage to surrounding tissue or to the epidermis, reported Dr. Munavalli, a dermatologist in group practice in Charlotte, N.C. The extent of thermal damage correlated with the duration of the ultrasound.
The level of damage could be expected to improve acne, but that needs confirmation in clinical trials, he said.
In each of the studies, the treatments were well tolerated, with minimal side effects including mild to moderate pain (using no anesthetic) and short-lived erythema and edema.
Dr. Paithankar works for Sebacia. Dr. Munavalli reported having no disclosures; some of his coinvestigators work for Sebacia.
On Twitter @sherryboschert
AT LASER 2014
Key clinical point: Inadequate contrast has limited the use of laser targeting of the sebaceous glands; specially designed gold-coated microparticles are designed to improve contrast and promote light absorption.
Major finding: Laser treatment of acne vulgaris preceded by application of gold-coated microparticles decreased mean inflammatory lesion counts by 34% and 49% at 12 weeks in two separate studies, compared with reductions of 16% and 22% in control groups.
Data source: Two randomized, controlled trials of 48 patients and 49 patients, respectively, with acne vulgaris, and a separate histologic study of 37 subjects.
Disclosures: Dr. Paithankar works for Sebacia, which developed the gold-coated microparticles treatment. Dr. Munavalli reported having no disclosures; some of his coinvestigators work for Sebacia.
Acne scar improvements maintained after laser treatment
PHOENIX – Improvements in acne scarring were maintained 3 months after six laser treatments with a diffractive lens array and a picosecond 755-nm alexandrite laser. Changes seen on histology suggest the improvements stem from more than collagen remodeling alone.
Twenty adults with facial acne scars and Fitzpatrick skin types I-V underwent six laser treatments performed every 4-8 weeks at a single center using a 755-nm wavelength, diffractive lens array, pulse duration of 750-850 picoseconds, and repetition rate of 5 Hz, with a fixed spot size of 6 mm and fluence of 0.71 J/cm2.
Three physicians who were blinded to the results evaluated two- and three-dimensional images taken at baseline and at follow-up visits. Analysis of two-dimensional photos produced acne scar improvement scores averaging 1.5 at the 1-month follow-up visit and 1.4 at the 3-month visit on a 4-point scale (with 0 indicating 0-25% improvement and 3 indicating greater than 75% improvement), Dr. Jeremy Brauer and his associates reported.
Scar volume decreased by an average of 24% by 1 month post treatment and 27% by 3 months post treatment, according to analyses of the three-dimensional images, Dr. Brauer said at the annual meeting of the American Society for Laser Medicine and Surgery.
Histology of biopsy specimens taken at baseline and at 3 months after the last treatment showed elongation and increased density of elastic fibers, increased dermal collagen, and increased dermal mucin after treatment. These findings suggest that the improvements in scarring are caused by dermal changes beyond remodeling of collagen alone, said Dr. Brauer of the Laser & Skin Surgery Center in New York.
Dr. Brauer and his associates previously reported that the picosecond laser and diffractive lens array can improve acne scarring with minimal preparation and down time, "in many cases with no anesthesia at all," he said. The investigators offered topical anesthesia and antiviral prophylaxis to patients, but most declined.
The diffractive lens array delivers varying levels of heat at a fixed spot size and fluence. High-energy pulses that are 500 mcm apart deliver 70% of the fluence, with a lower level of heating surrounding these higher-level pulses. In total, approximately 10% of the tissue is exposed to the high-fluence regions.
The 15 women and 5 men in the study ranged in age from 27 to 62 years.
Previous methods of treating acne scarring with lasers, intense pulsed light, or other energy devices typically cause thermal injury that produces collagen synthesis and remodeling. Those procedures usually require anesthesia and can cause collateral damage that may take a long time to heal, he said.
The investigators chose the picosecond 755-nm alexandrite laser because it has been used successfully to remove tattoos or treat pigmented lesions while causing fewer side effects and coupled it with the specialized diffractive lens array.
The study excluded patients with hypersensitivity to light; localized or systemic infection, prior laser treatment in the past 3 months; and a history of skin cancer, keloidal scarring, immunosuppression, or immune deficiency disorder. Also excluded were patients who used isotretinoin in the past year and women who were pregnant, breastfeeding, or contemplating pregnancy.
Dr. Brauer and a coinvestigator reported financial associations with Cynosure, which also loaned equipment for the study.
On Twitter @sherryboschert
PHOENIX – Improvements in acne scarring were maintained 3 months after six laser treatments with a diffractive lens array and a picosecond 755-nm alexandrite laser. Changes seen on histology suggest the improvements stem from more than collagen remodeling alone.
Twenty adults with facial acne scars and Fitzpatrick skin types I-V underwent six laser treatments performed every 4-8 weeks at a single center using a 755-nm wavelength, diffractive lens array, pulse duration of 750-850 picoseconds, and repetition rate of 5 Hz, with a fixed spot size of 6 mm and fluence of 0.71 J/cm2.
Three physicians who were blinded to the results evaluated two- and three-dimensional images taken at baseline and at follow-up visits. Analysis of two-dimensional photos produced acne scar improvement scores averaging 1.5 at the 1-month follow-up visit and 1.4 at the 3-month visit on a 4-point scale (with 0 indicating 0-25% improvement and 3 indicating greater than 75% improvement), Dr. Jeremy Brauer and his associates reported.
Scar volume decreased by an average of 24% by 1 month post treatment and 27% by 3 months post treatment, according to analyses of the three-dimensional images, Dr. Brauer said at the annual meeting of the American Society for Laser Medicine and Surgery.
Histology of biopsy specimens taken at baseline and at 3 months after the last treatment showed elongation and increased density of elastic fibers, increased dermal collagen, and increased dermal mucin after treatment. These findings suggest that the improvements in scarring are caused by dermal changes beyond remodeling of collagen alone, said Dr. Brauer of the Laser & Skin Surgery Center in New York.
Dr. Brauer and his associates previously reported that the picosecond laser and diffractive lens array can improve acne scarring with minimal preparation and down time, "in many cases with no anesthesia at all," he said. The investigators offered topical anesthesia and antiviral prophylaxis to patients, but most declined.
The diffractive lens array delivers varying levels of heat at a fixed spot size and fluence. High-energy pulses that are 500 mcm apart deliver 70% of the fluence, with a lower level of heating surrounding these higher-level pulses. In total, approximately 10% of the tissue is exposed to the high-fluence regions.
The 15 women and 5 men in the study ranged in age from 27 to 62 years.
Previous methods of treating acne scarring with lasers, intense pulsed light, or other energy devices typically cause thermal injury that produces collagen synthesis and remodeling. Those procedures usually require anesthesia and can cause collateral damage that may take a long time to heal, he said.
The investigators chose the picosecond 755-nm alexandrite laser because it has been used successfully to remove tattoos or treat pigmented lesions while causing fewer side effects and coupled it with the specialized diffractive lens array.
The study excluded patients with hypersensitivity to light; localized or systemic infection, prior laser treatment in the past 3 months; and a history of skin cancer, keloidal scarring, immunosuppression, or immune deficiency disorder. Also excluded were patients who used isotretinoin in the past year and women who were pregnant, breastfeeding, or contemplating pregnancy.
Dr. Brauer and a coinvestigator reported financial associations with Cynosure, which also loaned equipment for the study.
On Twitter @sherryboschert
PHOENIX – Improvements in acne scarring were maintained 3 months after six laser treatments with a diffractive lens array and a picosecond 755-nm alexandrite laser. Changes seen on histology suggest the improvements stem from more than collagen remodeling alone.
Twenty adults with facial acne scars and Fitzpatrick skin types I-V underwent six laser treatments performed every 4-8 weeks at a single center using a 755-nm wavelength, diffractive lens array, pulse duration of 750-850 picoseconds, and repetition rate of 5 Hz, with a fixed spot size of 6 mm and fluence of 0.71 J/cm2.
Three physicians who were blinded to the results evaluated two- and three-dimensional images taken at baseline and at follow-up visits. Analysis of two-dimensional photos produced acne scar improvement scores averaging 1.5 at the 1-month follow-up visit and 1.4 at the 3-month visit on a 4-point scale (with 0 indicating 0-25% improvement and 3 indicating greater than 75% improvement), Dr. Jeremy Brauer and his associates reported.
Scar volume decreased by an average of 24% by 1 month post treatment and 27% by 3 months post treatment, according to analyses of the three-dimensional images, Dr. Brauer said at the annual meeting of the American Society for Laser Medicine and Surgery.
Histology of biopsy specimens taken at baseline and at 3 months after the last treatment showed elongation and increased density of elastic fibers, increased dermal collagen, and increased dermal mucin after treatment. These findings suggest that the improvements in scarring are caused by dermal changes beyond remodeling of collagen alone, said Dr. Brauer of the Laser & Skin Surgery Center in New York.
Dr. Brauer and his associates previously reported that the picosecond laser and diffractive lens array can improve acne scarring with minimal preparation and down time, "in many cases with no anesthesia at all," he said. The investigators offered topical anesthesia and antiviral prophylaxis to patients, but most declined.
The diffractive lens array delivers varying levels of heat at a fixed spot size and fluence. High-energy pulses that are 500 mcm apart deliver 70% of the fluence, with a lower level of heating surrounding these higher-level pulses. In total, approximately 10% of the tissue is exposed to the high-fluence regions.
The 15 women and 5 men in the study ranged in age from 27 to 62 years.
Previous methods of treating acne scarring with lasers, intense pulsed light, or other energy devices typically cause thermal injury that produces collagen synthesis and remodeling. Those procedures usually require anesthesia and can cause collateral damage that may take a long time to heal, he said.
The investigators chose the picosecond 755-nm alexandrite laser because it has been used successfully to remove tattoos or treat pigmented lesions while causing fewer side effects and coupled it with the specialized diffractive lens array.
The study excluded patients with hypersensitivity to light; localized or systemic infection, prior laser treatment in the past 3 months; and a history of skin cancer, keloidal scarring, immunosuppression, or immune deficiency disorder. Also excluded were patients who used isotretinoin in the past year and women who were pregnant, breastfeeding, or contemplating pregnancy.
Dr. Brauer and a coinvestigator reported financial associations with Cynosure, which also loaned equipment for the study.
On Twitter @sherryboschert
AT LASER 2014
Major finding: Acne scar volume decreased 24% by 1 month after treatment ended and 27% by 3 months post treatment, compared with baseline.
Key clinical point: Histologic data suggest that using a diffractive lens array and a picosecond 755-nm laser to treat acne scars caused dermal changes beyond skin remodeling alone.
Data source: Single-center study of 20 adults and photographic assessments by three physicians who were blinded to the results.
Disclosures: Dr. Brauer and a coinvestigator reported financial associations with Cynosure, which also loaned equipment for the study.
VIDEO: Laser innovations target tattoos, acne, body fat
PHOENIX – Advances in laser technology allow dermatologists to remove tattoos in half the time but at twice the cost. Cold therapy for sebaceous glands theoretically could cure acne. Nonsurgical body sculpting and contouring techniques are making it easier to take away love handles and other unwanted body fat.
These are some of the highlights from the annual meeting of the American Society for Laser Medicine and Surgery. Dr. Jeffrey S. Dover, immediate past president of the ASLMS, explains the details of these developments and how to make the most of them in practice. Dr. Dover is a dermatologist practicing in Chestnut Hill, Mass.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @sherryboschert
PHOENIX – Advances in laser technology allow dermatologists to remove tattoos in half the time but at twice the cost. Cold therapy for sebaceous glands theoretically could cure acne. Nonsurgical body sculpting and contouring techniques are making it easier to take away love handles and other unwanted body fat.
These are some of the highlights from the annual meeting of the American Society for Laser Medicine and Surgery. Dr. Jeffrey S. Dover, immediate past president of the ASLMS, explains the details of these developments and how to make the most of them in practice. Dr. Dover is a dermatologist practicing in Chestnut Hill, Mass.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @sherryboschert
PHOENIX – Advances in laser technology allow dermatologists to remove tattoos in half the time but at twice the cost. Cold therapy for sebaceous glands theoretically could cure acne. Nonsurgical body sculpting and contouring techniques are making it easier to take away love handles and other unwanted body fat.
These are some of the highlights from the annual meeting of the American Society for Laser Medicine and Surgery. Dr. Jeffrey S. Dover, immediate past president of the ASLMS, explains the details of these developments and how to make the most of them in practice. Dr. Dover is a dermatologist practicing in Chestnut Hill, Mass.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @sherryboschert
AT LASER 2014
Investigational acne treatment targets the sebaceous gland
DENVER – In the not-so-distant future, a treatment option for patients with moderate to severe acne may involve destruction of the sebaceous gland, according to Dr. R. Rox Anderson, director of the Wellman Center for Photomedicine at Massachusetts General Hospital, Boston.
"If you look at the existing devices that are available for treating acne, none of them are really targeting the sebaceous gland itself," said Dr. Anderson. "They’re having secondary effects by eating skin or changing inflammatory reactions."
At the annual meeting of the American Academy of Dermatology, Dr. Anderson presented findings from a randomized phase I study of an investigational technique to target the sebaceous gland that consists of externally delivered plasmonic microparticles followed by pulsed laser irradiation, much like laser hair removal.
From a physiologic standpoint, Dr. Anderson likened the sebaceous gland to a small version of a lung. "It has an outflow tract that puts sebum out, but it doesn’t know how to inhale; it only exhales sebum," he explained. "The question is, can we make [the sebaceous gland] inhale and drive material into the gland itself? The strategy is to come up with a light-absorbing material, force it into the gland, then once the gland is made to absorb light, do selective photothermolysis – the same principles that were developed for hair removal."
Dr. Anderson and his associates randomized 48 adult patients with moderate to severe acne to treatment using a technology being developed by Duluth, Ga.–based company Sebacia, or to a control treatment. The 23 patients in the treatment arm received massage of 3 mL of a topical gold nanoparticle suspension and 800-nm laser irradiation without anesthesia for 12 weeks. In the control group, 25 patients used an over-the-counter face wash that contained salicylic acid, followed by laser treatment for 12 weeks, and then were crossed over to treatment with gold nanoparticles. The laser treatments occurred three times, two weeks apart, and consisted of two passes on a 9-mm square spot delivered at a fluence of 25-35 J/cm3 with a 30-second pulse duration. The study was conducted at two sites in Poland.
At 12 weeks, patients in the nanoparticle group had a 34% reduction in inflammatory lesion counts, compared with 16% in the salicylic acid group, a difference that reached significance (P = .02). At 7 months, patients in the treatment group had a 61% reduction in inflammatory lesion counts, compared with a 50% reduction among patients in the crossover group. "What was interesting to me is the lack of side effects," Dr. Anderson said. The treatment "was very well tolerated, very much like laser hair removal. You don’t have the pustulation and side effects of photodynamic therapy."
The gold nanoparticles used in the study were 150-300 nanometers in diameter and "are basically like a tattoo," Dr. Anderson said. "Once they get into the glands, do they stay there? Or do they come out?" To answer this question, the researchers measured the number of nanograms of material inserted, and observed that the sebaceus gland "constantly spits these particles out," even after laser exposure, he said.
Dr. Anderson characterized selective photothermolysis of the sebaceous gland as a technology "at the beginning, but we’ll see how this goes. It’s not available clinically yet, but maybe we’ll see it in our hands someday."
Dr. Anderson disclosed that he is a consultant for numerous pharmaceutical and device companies, including Sebacia.
DENVER – In the not-so-distant future, a treatment option for patients with moderate to severe acne may involve destruction of the sebaceous gland, according to Dr. R. Rox Anderson, director of the Wellman Center for Photomedicine at Massachusetts General Hospital, Boston.
"If you look at the existing devices that are available for treating acne, none of them are really targeting the sebaceous gland itself," said Dr. Anderson. "They’re having secondary effects by eating skin or changing inflammatory reactions."
At the annual meeting of the American Academy of Dermatology, Dr. Anderson presented findings from a randomized phase I study of an investigational technique to target the sebaceous gland that consists of externally delivered plasmonic microparticles followed by pulsed laser irradiation, much like laser hair removal.
From a physiologic standpoint, Dr. Anderson likened the sebaceous gland to a small version of a lung. "It has an outflow tract that puts sebum out, but it doesn’t know how to inhale; it only exhales sebum," he explained. "The question is, can we make [the sebaceous gland] inhale and drive material into the gland itself? The strategy is to come up with a light-absorbing material, force it into the gland, then once the gland is made to absorb light, do selective photothermolysis – the same principles that were developed for hair removal."
Dr. Anderson and his associates randomized 48 adult patients with moderate to severe acne to treatment using a technology being developed by Duluth, Ga.–based company Sebacia, or to a control treatment. The 23 patients in the treatment arm received massage of 3 mL of a topical gold nanoparticle suspension and 800-nm laser irradiation without anesthesia for 12 weeks. In the control group, 25 patients used an over-the-counter face wash that contained salicylic acid, followed by laser treatment for 12 weeks, and then were crossed over to treatment with gold nanoparticles. The laser treatments occurred three times, two weeks apart, and consisted of two passes on a 9-mm square spot delivered at a fluence of 25-35 J/cm3 with a 30-second pulse duration. The study was conducted at two sites in Poland.
At 12 weeks, patients in the nanoparticle group had a 34% reduction in inflammatory lesion counts, compared with 16% in the salicylic acid group, a difference that reached significance (P = .02). At 7 months, patients in the treatment group had a 61% reduction in inflammatory lesion counts, compared with a 50% reduction among patients in the crossover group. "What was interesting to me is the lack of side effects," Dr. Anderson said. The treatment "was very well tolerated, very much like laser hair removal. You don’t have the pustulation and side effects of photodynamic therapy."
The gold nanoparticles used in the study were 150-300 nanometers in diameter and "are basically like a tattoo," Dr. Anderson said. "Once they get into the glands, do they stay there? Or do they come out?" To answer this question, the researchers measured the number of nanograms of material inserted, and observed that the sebaceus gland "constantly spits these particles out," even after laser exposure, he said.
Dr. Anderson characterized selective photothermolysis of the sebaceous gland as a technology "at the beginning, but we’ll see how this goes. It’s not available clinically yet, but maybe we’ll see it in our hands someday."
Dr. Anderson disclosed that he is a consultant for numerous pharmaceutical and device companies, including Sebacia.
DENVER – In the not-so-distant future, a treatment option for patients with moderate to severe acne may involve destruction of the sebaceous gland, according to Dr. R. Rox Anderson, director of the Wellman Center for Photomedicine at Massachusetts General Hospital, Boston.
"If you look at the existing devices that are available for treating acne, none of them are really targeting the sebaceous gland itself," said Dr. Anderson. "They’re having secondary effects by eating skin or changing inflammatory reactions."
At the annual meeting of the American Academy of Dermatology, Dr. Anderson presented findings from a randomized phase I study of an investigational technique to target the sebaceous gland that consists of externally delivered plasmonic microparticles followed by pulsed laser irradiation, much like laser hair removal.
From a physiologic standpoint, Dr. Anderson likened the sebaceous gland to a small version of a lung. "It has an outflow tract that puts sebum out, but it doesn’t know how to inhale; it only exhales sebum," he explained. "The question is, can we make [the sebaceous gland] inhale and drive material into the gland itself? The strategy is to come up with a light-absorbing material, force it into the gland, then once the gland is made to absorb light, do selective photothermolysis – the same principles that were developed for hair removal."
Dr. Anderson and his associates randomized 48 adult patients with moderate to severe acne to treatment using a technology being developed by Duluth, Ga.–based company Sebacia, or to a control treatment. The 23 patients in the treatment arm received massage of 3 mL of a topical gold nanoparticle suspension and 800-nm laser irradiation without anesthesia for 12 weeks. In the control group, 25 patients used an over-the-counter face wash that contained salicylic acid, followed by laser treatment for 12 weeks, and then were crossed over to treatment with gold nanoparticles. The laser treatments occurred three times, two weeks apart, and consisted of two passes on a 9-mm square spot delivered at a fluence of 25-35 J/cm3 with a 30-second pulse duration. The study was conducted at two sites in Poland.
At 12 weeks, patients in the nanoparticle group had a 34% reduction in inflammatory lesion counts, compared with 16% in the salicylic acid group, a difference that reached significance (P = .02). At 7 months, patients in the treatment group had a 61% reduction in inflammatory lesion counts, compared with a 50% reduction among patients in the crossover group. "What was interesting to me is the lack of side effects," Dr. Anderson said. The treatment "was very well tolerated, very much like laser hair removal. You don’t have the pustulation and side effects of photodynamic therapy."
The gold nanoparticles used in the study were 150-300 nanometers in diameter and "are basically like a tattoo," Dr. Anderson said. "Once they get into the glands, do they stay there? Or do they come out?" To answer this question, the researchers measured the number of nanograms of material inserted, and observed that the sebaceus gland "constantly spits these particles out," even after laser exposure, he said.
Dr. Anderson characterized selective photothermolysis of the sebaceous gland as a technology "at the beginning, but we’ll see how this goes. It’s not available clinically yet, but maybe we’ll see it in our hands someday."
Dr. Anderson disclosed that he is a consultant for numerous pharmaceutical and device companies, including Sebacia.
EXPERT ANALYSIS FROM THE AAD ANNUAL MEETING
Major Finding: At 12 weeks, patients in the treatment group had a 34% reduction in inflammatory lesion counts, compared with 16% in the salicylic acid group, a significant difference.
Data Source: A randomized study of 48 patients with moderate to severe acne.
Disclosures: Dr. Anderson disclosed that he is a consultant for numerous pharmaceutical and device companies, including Sebacia.
Red Is Wrong; In the Red Also Is Wrong
A recent study in the Journal of Drugs in Dermatology (2014;13:56-61) featured long-term data regarding use of brimonidine gel, which was approved by the US Food and Drug Administration last year as the only topical agent on the market to treat erythema associated with rosacea. Compared to the initial safety and efficacy in phase 2 and 3 trials with 4-week use, this study spanned 12 months and assessed the erythema and adverse events associated with the drug. The decrease in erythema with once-daily application was observed after first use and was durable until the end of the study with no tachyphylaxis. The side-effect profile was mild and included flushing and irritancy events that typically improved over time.
What’s the issue?
The manufacturers of this new product tout that “Red is Wrong” (http://www.rediswrong.com). Although this strong statement virtually forces people to sprint to the Web site to see how they could improve their facial erythema and my patients have reported quite favorably on its efficacy and tolerability, obtaining the medication has been problematic. Similar to many of the coupon cards and assistance provided by pharmaceutical companies, the true segment of the population for which they serve is more limited than it appears, as health insurance plans with prescription deductibles, Medicare, Medicaid (and any spin-offs of the sort in the last few months involving US health care cost-management gymnastics) typically are excluded from discounts, and the out-of-pocket cost can be well over $200 per month. In an era when we have heroically developed a new and effective drug for a common and problematic indication, how will we practically provide it to the masses?
A recent study in the Journal of Drugs in Dermatology (2014;13:56-61) featured long-term data regarding use of brimonidine gel, which was approved by the US Food and Drug Administration last year as the only topical agent on the market to treat erythema associated with rosacea. Compared to the initial safety and efficacy in phase 2 and 3 trials with 4-week use, this study spanned 12 months and assessed the erythema and adverse events associated with the drug. The decrease in erythema with once-daily application was observed after first use and was durable until the end of the study with no tachyphylaxis. The side-effect profile was mild and included flushing and irritancy events that typically improved over time.
What’s the issue?
The manufacturers of this new product tout that “Red is Wrong” (http://www.rediswrong.com). Although this strong statement virtually forces people to sprint to the Web site to see how they could improve their facial erythema and my patients have reported quite favorably on its efficacy and tolerability, obtaining the medication has been problematic. Similar to many of the coupon cards and assistance provided by pharmaceutical companies, the true segment of the population for which they serve is more limited than it appears, as health insurance plans with prescription deductibles, Medicare, Medicaid (and any spin-offs of the sort in the last few months involving US health care cost-management gymnastics) typically are excluded from discounts, and the out-of-pocket cost can be well over $200 per month. In an era when we have heroically developed a new and effective drug for a common and problematic indication, how will we practically provide it to the masses?
A recent study in the Journal of Drugs in Dermatology (2014;13:56-61) featured long-term data regarding use of brimonidine gel, which was approved by the US Food and Drug Administration last year as the only topical agent on the market to treat erythema associated with rosacea. Compared to the initial safety and efficacy in phase 2 and 3 trials with 4-week use, this study spanned 12 months and assessed the erythema and adverse events associated with the drug. The decrease in erythema with once-daily application was observed after first use and was durable until the end of the study with no tachyphylaxis. The side-effect profile was mild and included flushing and irritancy events that typically improved over time.
What’s the issue?
The manufacturers of this new product tout that “Red is Wrong” (http://www.rediswrong.com). Although this strong statement virtually forces people to sprint to the Web site to see how they could improve their facial erythema and my patients have reported quite favorably on its efficacy and tolerability, obtaining the medication has been problematic. Similar to many of the coupon cards and assistance provided by pharmaceutical companies, the true segment of the population for which they serve is more limited than it appears, as health insurance plans with prescription deductibles, Medicare, Medicaid (and any spin-offs of the sort in the last few months involving US health care cost-management gymnastics) typically are excluded from discounts, and the out-of-pocket cost can be well over $200 per month. In an era when we have heroically developed a new and effective drug for a common and problematic indication, how will we practically provide it to the masses?
Childhood rosacea may wear acne mask
WAIKOLOA, HAWAII – Rosacea is generally thought of as a common adult disorder with onset typically at age 30-50 years. But recent evidence indicates it can occur during early childhood, too.
Idiopathic facial aseptic granuloma – an uncommon condition sometimes mistaken for unusually early acne – is actually often an expression of childhood rosacea.
"If you see these atypical skin lesions that look like acne cysts in young children who also have facial flushing, erythema, and perhaps pustules without comedones, be sure to look carefully at the eyes. Many times, they will have ocular rosacea with recurrent chalazions, conjunctival hyperemia, or keratitis. And if you think it’s ocular rosacea, you may want to make a referral to ophthalmology for assistance with ocular rosacea management," Dr. Lawrence F. Eichenfield said at the Hawaii Dermatology Seminar sponsored by Global Academy for Medical Education/Skin Disease Education Foundation.
"I find that many times oral antibiotics are highly useful in this subset," added Dr. Eichenfield, chief of pediatric and adolescent dermatology at Rady Children’s Hospital and professor of clinical pediatrics and medicine at the University of California, San Diego.
The pathogenesis of idiopathic facial aseptic granuloma (IFAG) is poorly understood. French dermatologists were first to identify the link between IFAG and rosacea. In a multicenter study involving 20 girls and 18 boys with IFAG, the investigators determined that 16 of the children, or 42%, met two or more criteria for rosacea (Pediatr. Dermatol. 2013;30:429-32).
Median age at diagnosis of IFAG was 43 months, and the children were subsequently followed for a median of 3.9 years before their evaluation for possible rosacea. Eleven of the 32 (34%) children with a single IFAG lesion met criteria for childhood rosacea, as did 5 of the 6 (83%) children with multiple skin lesions.
"So be aware: Although rosacea is very uncommon in our preteens and teens, it can occur," Dr. Eichenfield observed.
He reported having served as a clinical investigator and/or consultant to Allergan, Galderma, GSK-Stiefel, and Medicis/Valeant.
The SDEF and this news organization are owned by the same parent company.
WAIKOLOA, HAWAII – Rosacea is generally thought of as a common adult disorder with onset typically at age 30-50 years. But recent evidence indicates it can occur during early childhood, too.
Idiopathic facial aseptic granuloma – an uncommon condition sometimes mistaken for unusually early acne – is actually often an expression of childhood rosacea.
"If you see these atypical skin lesions that look like acne cysts in young children who also have facial flushing, erythema, and perhaps pustules without comedones, be sure to look carefully at the eyes. Many times, they will have ocular rosacea with recurrent chalazions, conjunctival hyperemia, or keratitis. And if you think it’s ocular rosacea, you may want to make a referral to ophthalmology for assistance with ocular rosacea management," Dr. Lawrence F. Eichenfield said at the Hawaii Dermatology Seminar sponsored by Global Academy for Medical Education/Skin Disease Education Foundation.
"I find that many times oral antibiotics are highly useful in this subset," added Dr. Eichenfield, chief of pediatric and adolescent dermatology at Rady Children’s Hospital and professor of clinical pediatrics and medicine at the University of California, San Diego.
The pathogenesis of idiopathic facial aseptic granuloma (IFAG) is poorly understood. French dermatologists were first to identify the link between IFAG and rosacea. In a multicenter study involving 20 girls and 18 boys with IFAG, the investigators determined that 16 of the children, or 42%, met two or more criteria for rosacea (Pediatr. Dermatol. 2013;30:429-32).
Median age at diagnosis of IFAG was 43 months, and the children were subsequently followed for a median of 3.9 years before their evaluation for possible rosacea. Eleven of the 32 (34%) children with a single IFAG lesion met criteria for childhood rosacea, as did 5 of the 6 (83%) children with multiple skin lesions.
"So be aware: Although rosacea is very uncommon in our preteens and teens, it can occur," Dr. Eichenfield observed.
He reported having served as a clinical investigator and/or consultant to Allergan, Galderma, GSK-Stiefel, and Medicis/Valeant.
The SDEF and this news organization are owned by the same parent company.
WAIKOLOA, HAWAII – Rosacea is generally thought of as a common adult disorder with onset typically at age 30-50 years. But recent evidence indicates it can occur during early childhood, too.
Idiopathic facial aseptic granuloma – an uncommon condition sometimes mistaken for unusually early acne – is actually often an expression of childhood rosacea.
"If you see these atypical skin lesions that look like acne cysts in young children who also have facial flushing, erythema, and perhaps pustules without comedones, be sure to look carefully at the eyes. Many times, they will have ocular rosacea with recurrent chalazions, conjunctival hyperemia, or keratitis. And if you think it’s ocular rosacea, you may want to make a referral to ophthalmology for assistance with ocular rosacea management," Dr. Lawrence F. Eichenfield said at the Hawaii Dermatology Seminar sponsored by Global Academy for Medical Education/Skin Disease Education Foundation.
"I find that many times oral antibiotics are highly useful in this subset," added Dr. Eichenfield, chief of pediatric and adolescent dermatology at Rady Children’s Hospital and professor of clinical pediatrics and medicine at the University of California, San Diego.
The pathogenesis of idiopathic facial aseptic granuloma (IFAG) is poorly understood. French dermatologists were first to identify the link between IFAG and rosacea. In a multicenter study involving 20 girls and 18 boys with IFAG, the investigators determined that 16 of the children, or 42%, met two or more criteria for rosacea (Pediatr. Dermatol. 2013;30:429-32).
Median age at diagnosis of IFAG was 43 months, and the children were subsequently followed for a median of 3.9 years before their evaluation for possible rosacea. Eleven of the 32 (34%) children with a single IFAG lesion met criteria for childhood rosacea, as did 5 of the 6 (83%) children with multiple skin lesions.
"So be aware: Although rosacea is very uncommon in our preteens and teens, it can occur," Dr. Eichenfield observed.
He reported having served as a clinical investigator and/or consultant to Allergan, Galderma, GSK-Stiefel, and Medicis/Valeant.
The SDEF and this news organization are owned by the same parent company.
EXPERT ANALYSIS FROM SDEF HAWAII DERMATOLOGY SEMINAR
Novel agent for papulopustular rosacea found safe, effective in phase III trials
DENVER – Ivermectin 1% cream applied once daily was effective and safe in treating patients with moderate to severe papulopustular rosacea, based on data from a pair of phase III studies.
Ivermectin 1% cream, a novel agent being developed by Galderma Laboratories, is of interest to rosacea researchers because it possesses unique anti-inflammatory and anti-parasitic properties, Dr. Linda Stein Gold said at the annual meeting of the American Academy of Dermatology.
Dr. Stein Gold of the department of dermatology at Henry Ford Medical Center, Detroit, explained that Demodex folliculorum, which is typically found on the human face, may trigger an immune response in rosacea patients. Ivermectin 1% is anti-parasitic against Demodex, providing an "innovative treatment" option in this patient population, she said. The investigational agent has not yet been approved by the Food and Drug Administration.
Dr. Stein Gold and her associates set out to assess the efficacy and safety of 1% ivermectin cream vs. a control vehicle cream in patients with moderate to severe papulopustular rosacea. The design included two identical, double-blind, parallel group, 12-week studies.
In both studies, a total of 910 patients were randomized 2:1 to receive 1% ivermectin cream or a control cream once daily. There were two co-primary endpoints: the percentage of patients who achieved a "clear" or "almost clear" score on the Investigator’s Global Assessment (IGA) scale at week 12, and the change in inflammatory lesion counts from baseline to week 12. The secondary efficacy endpoint assessment was the percentage change in inflammatory lesion count from baseline to week 12. The researchers also assessed safety endpoints by examining adverse events.
The mean age of patients was 50 years, 96% were white, and 67% were women. Patients had about 30 lesions each; 76-82% were classified as having moderate rosacea based on IGA score, and the rest had severe disease. More than 90% of patients in each arm completed the study through week 12.
At week 12 in both studies, a significantly higher percentage of patients in the treatment group achieved treatment success compared with those in the control group (38-40% vs. 12-19%, respectively; P less than .001). That difference was seen as early as week four.
Both studies also demonstrated that treatment with ivermectin 1% was significantly superior to the control vehicle in reducing lesion counts, with significance seen as early as week two and continuing for the duration of the study. Patients in the treatment groups in both studies showed an average reduction of more than 20 lesions, while controls in the two studies had reductions of 12 and 13.4 lesions, respectively.
Fewer treatment-related adverse events were reported in the ivermectin group, compared with the control group (3.4% vs. 7.2%, respectively). The most common treatment-related adverse event in the first study was sensation of skin burning (1.8% in the ivermectin group vs. 2.6% in controls) while the most common adverse event in the second study were pruritus and dry skin (0.7% and 0.9%, respectively). "This drug was exceptionally well tolerated, very safe as well as efficacious," Dr. Stein Gold said.
In an interview, she acknowledged certain limitations of the study, including the fact that "we did not look at maintenance of efficacy when a patient stops the drug. However, that is being looked at in other studies."
The study was funded by Galderma R&D. Dr. Stein Gold disclosed that she is a consultant for Galderma.
DENVER – Ivermectin 1% cream applied once daily was effective and safe in treating patients with moderate to severe papulopustular rosacea, based on data from a pair of phase III studies.
Ivermectin 1% cream, a novel agent being developed by Galderma Laboratories, is of interest to rosacea researchers because it possesses unique anti-inflammatory and anti-parasitic properties, Dr. Linda Stein Gold said at the annual meeting of the American Academy of Dermatology.
Dr. Stein Gold of the department of dermatology at Henry Ford Medical Center, Detroit, explained that Demodex folliculorum, which is typically found on the human face, may trigger an immune response in rosacea patients. Ivermectin 1% is anti-parasitic against Demodex, providing an "innovative treatment" option in this patient population, she said. The investigational agent has not yet been approved by the Food and Drug Administration.
Dr. Stein Gold and her associates set out to assess the efficacy and safety of 1% ivermectin cream vs. a control vehicle cream in patients with moderate to severe papulopustular rosacea. The design included two identical, double-blind, parallel group, 12-week studies.
In both studies, a total of 910 patients were randomized 2:1 to receive 1% ivermectin cream or a control cream once daily. There were two co-primary endpoints: the percentage of patients who achieved a "clear" or "almost clear" score on the Investigator’s Global Assessment (IGA) scale at week 12, and the change in inflammatory lesion counts from baseline to week 12. The secondary efficacy endpoint assessment was the percentage change in inflammatory lesion count from baseline to week 12. The researchers also assessed safety endpoints by examining adverse events.
The mean age of patients was 50 years, 96% were white, and 67% were women. Patients had about 30 lesions each; 76-82% were classified as having moderate rosacea based on IGA score, and the rest had severe disease. More than 90% of patients in each arm completed the study through week 12.
At week 12 in both studies, a significantly higher percentage of patients in the treatment group achieved treatment success compared with those in the control group (38-40% vs. 12-19%, respectively; P less than .001). That difference was seen as early as week four.
Both studies also demonstrated that treatment with ivermectin 1% was significantly superior to the control vehicle in reducing lesion counts, with significance seen as early as week two and continuing for the duration of the study. Patients in the treatment groups in both studies showed an average reduction of more than 20 lesions, while controls in the two studies had reductions of 12 and 13.4 lesions, respectively.
Fewer treatment-related adverse events were reported in the ivermectin group, compared with the control group (3.4% vs. 7.2%, respectively). The most common treatment-related adverse event in the first study was sensation of skin burning (1.8% in the ivermectin group vs. 2.6% in controls) while the most common adverse event in the second study were pruritus and dry skin (0.7% and 0.9%, respectively). "This drug was exceptionally well tolerated, very safe as well as efficacious," Dr. Stein Gold said.
In an interview, she acknowledged certain limitations of the study, including the fact that "we did not look at maintenance of efficacy when a patient stops the drug. However, that is being looked at in other studies."
The study was funded by Galderma R&D. Dr. Stein Gold disclosed that she is a consultant for Galderma.
DENVER – Ivermectin 1% cream applied once daily was effective and safe in treating patients with moderate to severe papulopustular rosacea, based on data from a pair of phase III studies.
Ivermectin 1% cream, a novel agent being developed by Galderma Laboratories, is of interest to rosacea researchers because it possesses unique anti-inflammatory and anti-parasitic properties, Dr. Linda Stein Gold said at the annual meeting of the American Academy of Dermatology.
Dr. Stein Gold of the department of dermatology at Henry Ford Medical Center, Detroit, explained that Demodex folliculorum, which is typically found on the human face, may trigger an immune response in rosacea patients. Ivermectin 1% is anti-parasitic against Demodex, providing an "innovative treatment" option in this patient population, she said. The investigational agent has not yet been approved by the Food and Drug Administration.
Dr. Stein Gold and her associates set out to assess the efficacy and safety of 1% ivermectin cream vs. a control vehicle cream in patients with moderate to severe papulopustular rosacea. The design included two identical, double-blind, parallel group, 12-week studies.
In both studies, a total of 910 patients were randomized 2:1 to receive 1% ivermectin cream or a control cream once daily. There were two co-primary endpoints: the percentage of patients who achieved a "clear" or "almost clear" score on the Investigator’s Global Assessment (IGA) scale at week 12, and the change in inflammatory lesion counts from baseline to week 12. The secondary efficacy endpoint assessment was the percentage change in inflammatory lesion count from baseline to week 12. The researchers also assessed safety endpoints by examining adverse events.
The mean age of patients was 50 years, 96% were white, and 67% were women. Patients had about 30 lesions each; 76-82% were classified as having moderate rosacea based on IGA score, and the rest had severe disease. More than 90% of patients in each arm completed the study through week 12.
At week 12 in both studies, a significantly higher percentage of patients in the treatment group achieved treatment success compared with those in the control group (38-40% vs. 12-19%, respectively; P less than .001). That difference was seen as early as week four.
Both studies also demonstrated that treatment with ivermectin 1% was significantly superior to the control vehicle in reducing lesion counts, with significance seen as early as week two and continuing for the duration of the study. Patients in the treatment groups in both studies showed an average reduction of more than 20 lesions, while controls in the two studies had reductions of 12 and 13.4 lesions, respectively.
Fewer treatment-related adverse events were reported in the ivermectin group, compared with the control group (3.4% vs. 7.2%, respectively). The most common treatment-related adverse event in the first study was sensation of skin burning (1.8% in the ivermectin group vs. 2.6% in controls) while the most common adverse event in the second study were pruritus and dry skin (0.7% and 0.9%, respectively). "This drug was exceptionally well tolerated, very safe as well as efficacious," Dr. Stein Gold said.
In an interview, she acknowledged certain limitations of the study, including the fact that "we did not look at maintenance of efficacy when a patient stops the drug. However, that is being looked at in other studies."
The study was funded by Galderma R&D. Dr. Stein Gold disclosed that she is a consultant for Galderma.
AT THE AAD ANNUAL MEETING
Major Finding: At week 12, a significantly higher percentage of rosacea patients in the ivermectin 1% cream treatment group achieved scores of "clear" or "almost clear" on the Investigator’s Global Assessment compared with those in the control group (38-40% vs. 12-19%, respectively; P less than .001).
Data Source: Two pivotal phase 3 studies in which 910 patients with moderate to severe papulopustular rosacea were randomized 2:1 to receive 1% ivermectin cream or a vehicle cream (control) once daily for 12 weeks.
Disclosures: The study was funded by Galderma R&D. Dr. Stein Gold disclosed that she is a consultant for Galderma.