User login
VIDEO: Ivermectin shows promise for treating papulopustular rosacea
DENVER – A limited number of safe and effective topical medications are currently available to treat chronic papulopustular (inflammatory) rosacea. In a video interview at the annual meeting of the American Academy of Dermatology, Dr. Linda Stein Gold highlighted results of two pivotal phase 3 trials of ivermectin 1%, an investigational drug being evaluated for the treatment of the disorder. Both studies of ivermectin 1% cream met their co-primary efficacy endpoints of treatment success as defined by the Investigator’s Global Assessment (IGA) rating of clear skin and change in inflammatory lesion count.
DENVER – A limited number of safe and effective topical medications are currently available to treat chronic papulopustular (inflammatory) rosacea. In a video interview at the annual meeting of the American Academy of Dermatology, Dr. Linda Stein Gold highlighted results of two pivotal phase 3 trials of ivermectin 1%, an investigational drug being evaluated for the treatment of the disorder. Both studies of ivermectin 1% cream met their co-primary efficacy endpoints of treatment success as defined by the Investigator’s Global Assessment (IGA) rating of clear skin and change in inflammatory lesion count.
DENVER – A limited number of safe and effective topical medications are currently available to treat chronic papulopustular (inflammatory) rosacea. In a video interview at the annual meeting of the American Academy of Dermatology, Dr. Linda Stein Gold highlighted results of two pivotal phase 3 trials of ivermectin 1%, an investigational drug being evaluated for the treatment of the disorder. Both studies of ivermectin 1% cream met their co-primary efficacy endpoints of treatment success as defined by the Investigator’s Global Assessment (IGA) rating of clear skin and change in inflammatory lesion count.
AT THE AAD ANNUAL MEETING
VIDEO: Don’t be afraid to treat acne in pregnant patients
DENVER – When it comes to treating pregnant women with acne, Dr. Jonette Keri’s goal is to prevent scarring. Her main message: there are several safe treatment options for women before, during, and after pregnancy, so don’t be afraid to use them.
Dr. Keri, associate professor of dermatology at the University of Miami Miller School of Medicine and chief of the dermatology service at Miami VA Hospital, shares her practice pearls in this video interview.
On Twitter @naseemmiller
DENVER – When it comes to treating pregnant women with acne, Dr. Jonette Keri’s goal is to prevent scarring. Her main message: there are several safe treatment options for women before, during, and after pregnancy, so don’t be afraid to use them.
Dr. Keri, associate professor of dermatology at the University of Miami Miller School of Medicine and chief of the dermatology service at Miami VA Hospital, shares her practice pearls in this video interview.
On Twitter @naseemmiller
DENVER – When it comes to treating pregnant women with acne, Dr. Jonette Keri’s goal is to prevent scarring. Her main message: there are several safe treatment options for women before, during, and after pregnancy, so don’t be afraid to use them.
Dr. Keri, associate professor of dermatology at the University of Miami Miller School of Medicine and chief of the dermatology service at Miami VA Hospital, shares her practice pearls in this video interview.
On Twitter @naseemmiller
AT THE AAD ANNUAL MEETING
Plugging the practice gaps in pediatric acne therapy
WAIKOLOA, HAWAII – A major impetus behind the first-ever evidence-based guidelines on the management of pediatric acne was to close significant practice gaps identified between what the acne experts recommend and what many clinicians do, according to the lead author of the guidelines.
"One of the points we wanted both dermatologists and primary care physicians to be aware of is that when you see acne in a 7- to 10-year-old, it’s predictive of much worse acne years later. That has been underappreciated," Dr. Lawrence F. Eichenfield said at the Hawaii Dermatology Seminar sponsored by Global Academy for Medical Education/Skin Disease Education Foundation (SDEF).
"It used to be said that you didn’t need to start worrying about acne until age 12, but the data shows that acne is now incredibly common in 8-, 9-, and 10-year-olds. There is clear evidence that puberty is occurring earlier, and acne is, too. Acne can be the first sign of normal puberty, starting as young as 7 years of age," he observed.
Another point the guidelines panel sought to publicize: While the traditional view has been that scarring acne comes from deep nodular and nodulocystic lesions, new data suggest that’s not the case. Plenty of patients with acne scars never had nodulocystic acne, nor did they pick at their lesions, Dr. Eichenfield said. Serial imaging studies demonstrate that acne scars don’t necessarily even come from inflammatory acne lesions. Instead, many such scars arise from erythematous red spots, simple papules, or even closed comedones. And scarring isn’t always an adult process; it can occur in pediatric patients.
"If you see evidence of scarring, that may move you to be much more aggressive in your acne therapy. It’s still easier for us to prevent acne scarring than it is to fix it. Prevention is clearly the way to go," observed Dr. Eichenfield, chief of pediatric and adolescent dermatology at Rady Children’s Hospital and professor of clinical pediatrics and medicine at the University of California, San Diego.
He noted that one significant practice gap in acne therapy highlighted in a recent national study involves underutilization of topical retinoids. Investigators analyzed data from the National Ambulatory Medical Care Survey for 2005-2010 and determined that a topical retinoid was prescribed in 41% of acne-related physician visits. Moreover, patients who saw a pediatrician or family physician were 77% less likely to get a prescription for a topical retinoid than those who visited a dermatologist. Older age, male gender, and being on Medicaid were other factors associated with a significantly lower likelihood of receiving a prescription for a topical retinoid (J. Dermatol. Treat. 2014; 25:110-4).
That 41% rate for topical retinoids is much too low, according to Dr. Eichenfield. The recent guidelines emphasize that topical retinoids are useful as monotherapy or in fixed-combination products in treating acne of all types and severities in children and adolescents of all ages.
Another recent study that focused on national treatment patterns for acne in 7- to 12-year-olds, found striking differences according to physician specialty. The top three medications prescribed by dermatologists in these preadolescent patients were the topical retinoid adapalene in 35.9%, benzoyl peroxide in 16.9%, and the topical retinoid tretinoin in 16.1%. In contrast, the top three prescribed by pediatricians and family physicians were minocycline in 13.4%, oral clindamycin in 10.5%, and tretinoin in 10.5% (Pediatr. Dermatol. 2013;30:689-94). The fairly common use of oral clindamycin for acne documented in this study is disturbing; acne experts almost never use that drug because there are other oral antibiotic options with much better safety profiles, Dr. Eichenfield said.
The guidelines (Pediatrics 2013;131:S163-86), which were developed by the American Acne and Rosacea Society and endorsed by the American Academy of Pediatrics, contain detailed acne treatment algorithms. Among the key points:
• Topical antibiotics are not recommended as monotherapy for acne for longer than a few weeks because of the risk of inducing bacterial resistance. Beyond several weeks, topical benzoyl peroxide should be added, because it has a potent antimicrobial effect across a broad range of organisms and it does not promote bacterial resistance.
• The guidelines recommend oral antibiotics as appropriate for moderate to severe acne at any age, but not as monotherapy because of the growing threat posed by global antibiotic resistance. Instead, oral antibiotics should always be used with topical retinoids and usually in conjunction with benzoyl peroxide. The patient should be using the retinoid when starting the oral antibiotic so that when the oral antibiotic is discontinued, usually after a few months, the topical agent can assume a key role in maintaining remission.
• The second-generation oral tetracyclines, doxycycline and minocycline, are sometimes preferable to tetracycline because of their greater ease of use, better absorption, and less frequent dosing. But none of these drugs should be used in children under age 8, for whom the options are erythromycin, azithromycin, and trimethoprim/sulfamethoxazole.
• Combined oral contraceptives are useful as second-line therapy in pubertal girls with moderate to severe acne after assessment for tobacco use and family history of thromboembolic events. Because of concerns about possible deleterious effects on growth and bone density, however, it’s worth considering withholding oral contraceptives for acne until 1 year after onset of menstruation.
• First-line therapy for severe acne is an oral antibiotic in combination with a topical retinoid, benzoyl peroxide, and sometimes a topical antibiotic as well.
"I know there’s a practice gap there because some dermatologists like to go straight to oral isotretinoin. But many acne experts feel that the use of an oral cycline-based antibiotic is highly useful prior to initiating isotretinoin, because the anti-inflammatory effect helps minimize the severe flares we sometimes get with initiation of isotretinoin," Dr. Eichenfield explained.
SDEF and this news organization are owned by the same parent company.
Dr. Eichenfield reported having served as a clinical investigator and/or consultant to Allergan, Galderma, GlaxoSmithKline (Stiefel), and Medicis/Valeant.
WAIKOLOA, HAWAII – A major impetus behind the first-ever evidence-based guidelines on the management of pediatric acne was to close significant practice gaps identified between what the acne experts recommend and what many clinicians do, according to the lead author of the guidelines.
"One of the points we wanted both dermatologists and primary care physicians to be aware of is that when you see acne in a 7- to 10-year-old, it’s predictive of much worse acne years later. That has been underappreciated," Dr. Lawrence F. Eichenfield said at the Hawaii Dermatology Seminar sponsored by Global Academy for Medical Education/Skin Disease Education Foundation (SDEF).
"It used to be said that you didn’t need to start worrying about acne until age 12, but the data shows that acne is now incredibly common in 8-, 9-, and 10-year-olds. There is clear evidence that puberty is occurring earlier, and acne is, too. Acne can be the first sign of normal puberty, starting as young as 7 years of age," he observed.
Another point the guidelines panel sought to publicize: While the traditional view has been that scarring acne comes from deep nodular and nodulocystic lesions, new data suggest that’s not the case. Plenty of patients with acne scars never had nodulocystic acne, nor did they pick at their lesions, Dr. Eichenfield said. Serial imaging studies demonstrate that acne scars don’t necessarily even come from inflammatory acne lesions. Instead, many such scars arise from erythematous red spots, simple papules, or even closed comedones. And scarring isn’t always an adult process; it can occur in pediatric patients.
"If you see evidence of scarring, that may move you to be much more aggressive in your acne therapy. It’s still easier for us to prevent acne scarring than it is to fix it. Prevention is clearly the way to go," observed Dr. Eichenfield, chief of pediatric and adolescent dermatology at Rady Children’s Hospital and professor of clinical pediatrics and medicine at the University of California, San Diego.
He noted that one significant practice gap in acne therapy highlighted in a recent national study involves underutilization of topical retinoids. Investigators analyzed data from the National Ambulatory Medical Care Survey for 2005-2010 and determined that a topical retinoid was prescribed in 41% of acne-related physician visits. Moreover, patients who saw a pediatrician or family physician were 77% less likely to get a prescription for a topical retinoid than those who visited a dermatologist. Older age, male gender, and being on Medicaid were other factors associated with a significantly lower likelihood of receiving a prescription for a topical retinoid (J. Dermatol. Treat. 2014; 25:110-4).
That 41% rate for topical retinoids is much too low, according to Dr. Eichenfield. The recent guidelines emphasize that topical retinoids are useful as monotherapy or in fixed-combination products in treating acne of all types and severities in children and adolescents of all ages.
Another recent study that focused on national treatment patterns for acne in 7- to 12-year-olds, found striking differences according to physician specialty. The top three medications prescribed by dermatologists in these preadolescent patients were the topical retinoid adapalene in 35.9%, benzoyl peroxide in 16.9%, and the topical retinoid tretinoin in 16.1%. In contrast, the top three prescribed by pediatricians and family physicians were minocycline in 13.4%, oral clindamycin in 10.5%, and tretinoin in 10.5% (Pediatr. Dermatol. 2013;30:689-94). The fairly common use of oral clindamycin for acne documented in this study is disturbing; acne experts almost never use that drug because there are other oral antibiotic options with much better safety profiles, Dr. Eichenfield said.
The guidelines (Pediatrics 2013;131:S163-86), which were developed by the American Acne and Rosacea Society and endorsed by the American Academy of Pediatrics, contain detailed acne treatment algorithms. Among the key points:
• Topical antibiotics are not recommended as monotherapy for acne for longer than a few weeks because of the risk of inducing bacterial resistance. Beyond several weeks, topical benzoyl peroxide should be added, because it has a potent antimicrobial effect across a broad range of organisms and it does not promote bacterial resistance.
• The guidelines recommend oral antibiotics as appropriate for moderate to severe acne at any age, but not as monotherapy because of the growing threat posed by global antibiotic resistance. Instead, oral antibiotics should always be used with topical retinoids and usually in conjunction with benzoyl peroxide. The patient should be using the retinoid when starting the oral antibiotic so that when the oral antibiotic is discontinued, usually after a few months, the topical agent can assume a key role in maintaining remission.
• The second-generation oral tetracyclines, doxycycline and minocycline, are sometimes preferable to tetracycline because of their greater ease of use, better absorption, and less frequent dosing. But none of these drugs should be used in children under age 8, for whom the options are erythromycin, azithromycin, and trimethoprim/sulfamethoxazole.
• Combined oral contraceptives are useful as second-line therapy in pubertal girls with moderate to severe acne after assessment for tobacco use and family history of thromboembolic events. Because of concerns about possible deleterious effects on growth and bone density, however, it’s worth considering withholding oral contraceptives for acne until 1 year after onset of menstruation.
• First-line therapy for severe acne is an oral antibiotic in combination with a topical retinoid, benzoyl peroxide, and sometimes a topical antibiotic as well.
"I know there’s a practice gap there because some dermatologists like to go straight to oral isotretinoin. But many acne experts feel that the use of an oral cycline-based antibiotic is highly useful prior to initiating isotretinoin, because the anti-inflammatory effect helps minimize the severe flares we sometimes get with initiation of isotretinoin," Dr. Eichenfield explained.
SDEF and this news organization are owned by the same parent company.
Dr. Eichenfield reported having served as a clinical investigator and/or consultant to Allergan, Galderma, GlaxoSmithKline (Stiefel), and Medicis/Valeant.
WAIKOLOA, HAWAII – A major impetus behind the first-ever evidence-based guidelines on the management of pediatric acne was to close significant practice gaps identified between what the acne experts recommend and what many clinicians do, according to the lead author of the guidelines.
"One of the points we wanted both dermatologists and primary care physicians to be aware of is that when you see acne in a 7- to 10-year-old, it’s predictive of much worse acne years later. That has been underappreciated," Dr. Lawrence F. Eichenfield said at the Hawaii Dermatology Seminar sponsored by Global Academy for Medical Education/Skin Disease Education Foundation (SDEF).
"It used to be said that you didn’t need to start worrying about acne until age 12, but the data shows that acne is now incredibly common in 8-, 9-, and 10-year-olds. There is clear evidence that puberty is occurring earlier, and acne is, too. Acne can be the first sign of normal puberty, starting as young as 7 years of age," he observed.
Another point the guidelines panel sought to publicize: While the traditional view has been that scarring acne comes from deep nodular and nodulocystic lesions, new data suggest that’s not the case. Plenty of patients with acne scars never had nodulocystic acne, nor did they pick at their lesions, Dr. Eichenfield said. Serial imaging studies demonstrate that acne scars don’t necessarily even come from inflammatory acne lesions. Instead, many such scars arise from erythematous red spots, simple papules, or even closed comedones. And scarring isn’t always an adult process; it can occur in pediatric patients.
"If you see evidence of scarring, that may move you to be much more aggressive in your acne therapy. It’s still easier for us to prevent acne scarring than it is to fix it. Prevention is clearly the way to go," observed Dr. Eichenfield, chief of pediatric and adolescent dermatology at Rady Children’s Hospital and professor of clinical pediatrics and medicine at the University of California, San Diego.
He noted that one significant practice gap in acne therapy highlighted in a recent national study involves underutilization of topical retinoids. Investigators analyzed data from the National Ambulatory Medical Care Survey for 2005-2010 and determined that a topical retinoid was prescribed in 41% of acne-related physician visits. Moreover, patients who saw a pediatrician or family physician were 77% less likely to get a prescription for a topical retinoid than those who visited a dermatologist. Older age, male gender, and being on Medicaid were other factors associated with a significantly lower likelihood of receiving a prescription for a topical retinoid (J. Dermatol. Treat. 2014; 25:110-4).
That 41% rate for topical retinoids is much too low, according to Dr. Eichenfield. The recent guidelines emphasize that topical retinoids are useful as monotherapy or in fixed-combination products in treating acne of all types and severities in children and adolescents of all ages.
Another recent study that focused on national treatment patterns for acne in 7- to 12-year-olds, found striking differences according to physician specialty. The top three medications prescribed by dermatologists in these preadolescent patients were the topical retinoid adapalene in 35.9%, benzoyl peroxide in 16.9%, and the topical retinoid tretinoin in 16.1%. In contrast, the top three prescribed by pediatricians and family physicians were minocycline in 13.4%, oral clindamycin in 10.5%, and tretinoin in 10.5% (Pediatr. Dermatol. 2013;30:689-94). The fairly common use of oral clindamycin for acne documented in this study is disturbing; acne experts almost never use that drug because there are other oral antibiotic options with much better safety profiles, Dr. Eichenfield said.
The guidelines (Pediatrics 2013;131:S163-86), which were developed by the American Acne and Rosacea Society and endorsed by the American Academy of Pediatrics, contain detailed acne treatment algorithms. Among the key points:
• Topical antibiotics are not recommended as monotherapy for acne for longer than a few weeks because of the risk of inducing bacterial resistance. Beyond several weeks, topical benzoyl peroxide should be added, because it has a potent antimicrobial effect across a broad range of organisms and it does not promote bacterial resistance.
• The guidelines recommend oral antibiotics as appropriate for moderate to severe acne at any age, but not as monotherapy because of the growing threat posed by global antibiotic resistance. Instead, oral antibiotics should always be used with topical retinoids and usually in conjunction with benzoyl peroxide. The patient should be using the retinoid when starting the oral antibiotic so that when the oral antibiotic is discontinued, usually after a few months, the topical agent can assume a key role in maintaining remission.
• The second-generation oral tetracyclines, doxycycline and minocycline, are sometimes preferable to tetracycline because of their greater ease of use, better absorption, and less frequent dosing. But none of these drugs should be used in children under age 8, for whom the options are erythromycin, azithromycin, and trimethoprim/sulfamethoxazole.
• Combined oral contraceptives are useful as second-line therapy in pubertal girls with moderate to severe acne after assessment for tobacco use and family history of thromboembolic events. Because of concerns about possible deleterious effects on growth and bone density, however, it’s worth considering withholding oral contraceptives for acne until 1 year after onset of menstruation.
• First-line therapy for severe acne is an oral antibiotic in combination with a topical retinoid, benzoyl peroxide, and sometimes a topical antibiotic as well.
"I know there’s a practice gap there because some dermatologists like to go straight to oral isotretinoin. But many acne experts feel that the use of an oral cycline-based antibiotic is highly useful prior to initiating isotretinoin, because the anti-inflammatory effect helps minimize the severe flares we sometimes get with initiation of isotretinoin," Dr. Eichenfield explained.
SDEF and this news organization are owned by the same parent company.
Dr. Eichenfield reported having served as a clinical investigator and/or consultant to Allergan, Galderma, GlaxoSmithKline (Stiefel), and Medicis/Valeant.
EXPERT ANALYSIS FROM SDEF HAWAII DERMATOLOGY SEMINAR
Consensus Recommendations From the American Acne & Rosacea Society on the Management of Rosacea, Part 5: A Guide on the Management of Rosacea
VIDEO: Practice pearls improve acne treatment
PALM BEACH, ARUBA – Which combination of retinoids and antibiotics delivers the best acne outcomes? And what dietary advice maximizes isotretinoin’s effectiveness? Dr. Guy Webster of the Wilmington (Del.) Veterans Affairs Medical Center discusses the right combination and timing of treatment with antibiotics and retinoids, offers tips for taking isotretinoin correctly, explains his approach to topical treatment of truncal acne, and outlines what to do when isotretinoin use triggers acne fulminans.
PALM BEACH, ARUBA – Which combination of retinoids and antibiotics delivers the best acne outcomes? And what dietary advice maximizes isotretinoin’s effectiveness? Dr. Guy Webster of the Wilmington (Del.) Veterans Affairs Medical Center discusses the right combination and timing of treatment with antibiotics and retinoids, offers tips for taking isotretinoin correctly, explains his approach to topical treatment of truncal acne, and outlines what to do when isotretinoin use triggers acne fulminans.
PALM BEACH, ARUBA – Which combination of retinoids and antibiotics delivers the best acne outcomes? And what dietary advice maximizes isotretinoin’s effectiveness? Dr. Guy Webster of the Wilmington (Del.) Veterans Affairs Medical Center discusses the right combination and timing of treatment with antibiotics and retinoids, offers tips for taking isotretinoin correctly, explains his approach to topical treatment of truncal acne, and outlines what to do when isotretinoin use triggers acne fulminans.
ANALYSIS FROM THE CARIBBEAN DERMATOLOGY SYMPOSIUM
VIDEO: A closer look at new pediatric acne guidelines
WAIKOLOA, HAWAII – Dr. Lawrence Eichenfield of the University of California, San Diego, speaks about the recent publication of comprehensive guidelines for the management of pediatric acne and outlines the treatment gaps that persist.
Dr. Eichenfield made his remarks during a video interview at the Hawaii Dermatology Seminar.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
WAIKOLOA, HAWAII – Dr. Lawrence Eichenfield of the University of California, San Diego, speaks about the recent publication of comprehensive guidelines for the management of pediatric acne and outlines the treatment gaps that persist.
Dr. Eichenfield made his remarks during a video interview at the Hawaii Dermatology Seminar.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
WAIKOLOA, HAWAII – Dr. Lawrence Eichenfield of the University of California, San Diego, speaks about the recent publication of comprehensive guidelines for the management of pediatric acne and outlines the treatment gaps that persist.
Dr. Eichenfield made his remarks during a video interview at the Hawaii Dermatology Seminar.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
EXPERT ANALYSIS FROM SDEF HAWAII DERMATOLOGY SEMINAR
VIDEO: Tips for treating acne in breastfeeding women
WAIKOLOA, HAWAII – Acne treatment during pregnancy gets lots of attention, but no one talks about acne treatment for breastfeeding women, according to Dr. Hilary Baldwin.
She spoke with us in a video interview at the Hawaii Dermatology Seminar about how breastfeeding women can safely treat their acne.
WAIKOLOA, HAWAII – Acne treatment during pregnancy gets lots of attention, but no one talks about acne treatment for breastfeeding women, according to Dr. Hilary Baldwin.
She spoke with us in a video interview at the Hawaii Dermatology Seminar about how breastfeeding women can safely treat their acne.
WAIKOLOA, HAWAII – Acne treatment during pregnancy gets lots of attention, but no one talks about acne treatment for breastfeeding women, according to Dr. Hilary Baldwin.
She spoke with us in a video interview at the Hawaii Dermatology Seminar about how breastfeeding women can safely treat their acne.
ANALYSIS FROM SDEF HAWAII DERMATOLOGY SEMINAR
Don’t overlook venerable benzoyl peroxide for acne
WAIKOLOA, HAWAII – Benzoyl peroxide has stood the test of time amid a diverse field of acne therapies, according to Dr. Linda Stein Gold. She spoke with us in a video interview about why benzoyl peroxide "should still play a central role in the treatment of acne."
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
WAIKOLOA, HAWAII – Benzoyl peroxide has stood the test of time amid a diverse field of acne therapies, according to Dr. Linda Stein Gold. She spoke with us in a video interview about why benzoyl peroxide "should still play a central role in the treatment of acne."
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
WAIKOLOA, HAWAII – Benzoyl peroxide has stood the test of time amid a diverse field of acne therapies, according to Dr. Linda Stein Gold. She spoke with us in a video interview about why benzoyl peroxide "should still play a central role in the treatment of acne."
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
EXPERT ANALYSIS FROM SDEF HAWAII DERMATOLOGY SYMPOSIUM
Novel topical rosacea drug sails through phase III
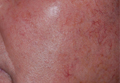
WAIKOLOA, HAWAII – Ivermectin 1% cream for the treatment of papulopustular rosacea convincingly hit all of its primary efficacy and safety endpoints in two pivotal phase III clinical trials.
The studies included 1,371 subjects randomized in a double-blind fashion 2:1 to 3 months of once-daily topical invermectin 1% or vehicle. These were patients with significant papulopustular rosacea (PPR): In one trial, 75% of patients were rated at baseline as having moderate disease and 25% severe, while in the other study the percentages were 80% and 20%, respectively, Dr. Hilary E. Baldwin reported at the SDEF Hawaii Dermatology Seminar sponsored by the Global Academy for Medical Education/Skin Disease Education Foundation.
The two prespecified coprimary endpoints were treatment success as defined by an Investigator Global Assessment rating of clear or almost clear at 12 weeks, along with reduction in inflammatory lesions from baseline through 12 weeks.
Roughly 40% of topical ivermectin–treated patients met the clear or almost-clear treatment success standard, a rate two- to fourfold greater than in controls in the two trials. The difference between the active treatment group and controls became statistically significant as early as week 4.
In addition, the inflammatory lesion count dropped by an average of more than 20 lesions at 12 weeks in the topical ivermectin–treated patients, an effect more than one-third larger than documented in controls. Here again, the benefit was significant by week 4, according to Dr. Baldwin, a dermatologist at SUNY Downstate Medical Center, Brooklyn.
The incidence of treatment-related dermatologic adverse events was 2.5% in patients on topical ivermectin, compared with 6.3% in controls.
Moreover, in a related single-blind, 9-month safety study involving 910 patients on ivermectin 1% cream once daily and 481 on topical azelaic acid 15% twice daily, the rate of treatment-related dermatologic adverse events was 1.3% in the ivermectin arm, compared with 5.3% with azelaic acid.
Stepping back from these compelling clinical trial data, Dr. Baldwin observed that the exact mechanism of benefit for topical invermectin in PPR has yet to be determined. The medication is certainly acaricidal and is known to kill the demodex mites that reside in the pilosebaceous units of patients with PPR. But it’s tough to think of demodex mites as causative in rosacea because they are also present in the pilosebaceous units of individuals without rosacea.
The latest thinking regarding the pathogenesis of rosacea is that this common chronic inflammatory skin disease is not caused by demodex mites, Propionibacterium acnes, or any other pathogen, she explained. In the current concept, cathelicidin proteins that are present in the epidermis as part of the vanguard of the innate immune system play a key role. When these proteins detect a foreign invader on the skin – bacterial, viral, or fungal – they release toxic enzymes, including cathelicidin LL-37, which kill the offending organism. High levels of LL-37 are proinflammatory, angiogenic, and activate the acquired immune system, effects that would explain the chronic skin redness and telangiectasias of rosacea. The trouble is, demodex is not a foreign invader.
"The innate immune system is not supposed to be triggered by demodex or P. acnes. They’re supposed to be in the follicle. They live there," Dr. Baldwin said.
Why the innate immune system of patients with PPR is apparently alerted by demodex, part of the normal fauna, requires further study, she added.
She anticipates that the full results of the recently completed pivotal phase III trials, sponsored by Galderma, will be published later this year.
SDEF and this news organization are owned by the same parent company.
Dr. Baldwin is on the advisory boards and/or speakers bureaus of Galderma and 10 other pharmaceutical and cosmetics companies.
WAIKOLOA, HAWAII – Ivermectin 1% cream for the treatment of papulopustular rosacea convincingly hit all of its primary efficacy and safety endpoints in two pivotal phase III clinical trials.
The studies included 1,371 subjects randomized in a double-blind fashion 2:1 to 3 months of once-daily topical invermectin 1% or vehicle. These were patients with significant papulopustular rosacea (PPR): In one trial, 75% of patients were rated at baseline as having moderate disease and 25% severe, while in the other study the percentages were 80% and 20%, respectively, Dr. Hilary E. Baldwin reported at the SDEF Hawaii Dermatology Seminar sponsored by the Global Academy for Medical Education/Skin Disease Education Foundation.
The two prespecified coprimary endpoints were treatment success as defined by an Investigator Global Assessment rating of clear or almost clear at 12 weeks, along with reduction in inflammatory lesions from baseline through 12 weeks.
Roughly 40% of topical ivermectin–treated patients met the clear or almost-clear treatment success standard, a rate two- to fourfold greater than in controls in the two trials. The difference between the active treatment group and controls became statistically significant as early as week 4.
In addition, the inflammatory lesion count dropped by an average of more than 20 lesions at 12 weeks in the topical ivermectin–treated patients, an effect more than one-third larger than documented in controls. Here again, the benefit was significant by week 4, according to Dr. Baldwin, a dermatologist at SUNY Downstate Medical Center, Brooklyn.
The incidence of treatment-related dermatologic adverse events was 2.5% in patients on topical ivermectin, compared with 6.3% in controls.
Moreover, in a related single-blind, 9-month safety study involving 910 patients on ivermectin 1% cream once daily and 481 on topical azelaic acid 15% twice daily, the rate of treatment-related dermatologic adverse events was 1.3% in the ivermectin arm, compared with 5.3% with azelaic acid.
Stepping back from these compelling clinical trial data, Dr. Baldwin observed that the exact mechanism of benefit for topical invermectin in PPR has yet to be determined. The medication is certainly acaricidal and is known to kill the demodex mites that reside in the pilosebaceous units of patients with PPR. But it’s tough to think of demodex mites as causative in rosacea because they are also present in the pilosebaceous units of individuals without rosacea.
The latest thinking regarding the pathogenesis of rosacea is that this common chronic inflammatory skin disease is not caused by demodex mites, Propionibacterium acnes, or any other pathogen, she explained. In the current concept, cathelicidin proteins that are present in the epidermis as part of the vanguard of the innate immune system play a key role. When these proteins detect a foreign invader on the skin – bacterial, viral, or fungal – they release toxic enzymes, including cathelicidin LL-37, which kill the offending organism. High levels of LL-37 are proinflammatory, angiogenic, and activate the acquired immune system, effects that would explain the chronic skin redness and telangiectasias of rosacea. The trouble is, demodex is not a foreign invader.
"The innate immune system is not supposed to be triggered by demodex or P. acnes. They’re supposed to be in the follicle. They live there," Dr. Baldwin said.
Why the innate immune system of patients with PPR is apparently alerted by demodex, part of the normal fauna, requires further study, she added.
She anticipates that the full results of the recently completed pivotal phase III trials, sponsored by Galderma, will be published later this year.
SDEF and this news organization are owned by the same parent company.
Dr. Baldwin is on the advisory boards and/or speakers bureaus of Galderma and 10 other pharmaceutical and cosmetics companies.
WAIKOLOA, HAWAII – Ivermectin 1% cream for the treatment of papulopustular rosacea convincingly hit all of its primary efficacy and safety endpoints in two pivotal phase III clinical trials.
The studies included 1,371 subjects randomized in a double-blind fashion 2:1 to 3 months of once-daily topical invermectin 1% or vehicle. These were patients with significant papulopustular rosacea (PPR): In one trial, 75% of patients were rated at baseline as having moderate disease and 25% severe, while in the other study the percentages were 80% and 20%, respectively, Dr. Hilary E. Baldwin reported at the SDEF Hawaii Dermatology Seminar sponsored by the Global Academy for Medical Education/Skin Disease Education Foundation.
The two prespecified coprimary endpoints were treatment success as defined by an Investigator Global Assessment rating of clear or almost clear at 12 weeks, along with reduction in inflammatory lesions from baseline through 12 weeks.
Roughly 40% of topical ivermectin–treated patients met the clear or almost-clear treatment success standard, a rate two- to fourfold greater than in controls in the two trials. The difference between the active treatment group and controls became statistically significant as early as week 4.
In addition, the inflammatory lesion count dropped by an average of more than 20 lesions at 12 weeks in the topical ivermectin–treated patients, an effect more than one-third larger than documented in controls. Here again, the benefit was significant by week 4, according to Dr. Baldwin, a dermatologist at SUNY Downstate Medical Center, Brooklyn.
The incidence of treatment-related dermatologic adverse events was 2.5% in patients on topical ivermectin, compared with 6.3% in controls.
Moreover, in a related single-blind, 9-month safety study involving 910 patients on ivermectin 1% cream once daily and 481 on topical azelaic acid 15% twice daily, the rate of treatment-related dermatologic adverse events was 1.3% in the ivermectin arm, compared with 5.3% with azelaic acid.
Stepping back from these compelling clinical trial data, Dr. Baldwin observed that the exact mechanism of benefit for topical invermectin in PPR has yet to be determined. The medication is certainly acaricidal and is known to kill the demodex mites that reside in the pilosebaceous units of patients with PPR. But it’s tough to think of demodex mites as causative in rosacea because they are also present in the pilosebaceous units of individuals without rosacea.
The latest thinking regarding the pathogenesis of rosacea is that this common chronic inflammatory skin disease is not caused by demodex mites, Propionibacterium acnes, or any other pathogen, she explained. In the current concept, cathelicidin proteins that are present in the epidermis as part of the vanguard of the innate immune system play a key role. When these proteins detect a foreign invader on the skin – bacterial, viral, or fungal – they release toxic enzymes, including cathelicidin LL-37, which kill the offending organism. High levels of LL-37 are proinflammatory, angiogenic, and activate the acquired immune system, effects that would explain the chronic skin redness and telangiectasias of rosacea. The trouble is, demodex is not a foreign invader.
"The innate immune system is not supposed to be triggered by demodex or P. acnes. They’re supposed to be in the follicle. They live there," Dr. Baldwin said.
Why the innate immune system of patients with PPR is apparently alerted by demodex, part of the normal fauna, requires further study, she added.
She anticipates that the full results of the recently completed pivotal phase III trials, sponsored by Galderma, will be published later this year.
SDEF and this news organization are owned by the same parent company.
Dr. Baldwin is on the advisory boards and/or speakers bureaus of Galderma and 10 other pharmaceutical and cosmetics companies.
EXPERT ANALYSIS FROM SDEF HAWAII DERMATOLOGY SEMINAR