User login
Triple-combination for severe acne avoids isotretinoin
MAUI, HAWAII – Combination therapy for severe acne, with a trio of familiar, well tolerated agents, knocked down the skin disease severity in a phase IV study such that 80% of patients deemed candidates for isotretinoin at baseline no longer qualified for the powerful oral retinoid 12 weeks later, Dr. Guy F. Webster reported at the Hawaii Dermatology Seminar sponsored by Global Academy for Medical Education/Skin Disease Education Foundation.
These data provide welcome news for patients who can’t take isotretinoin or don’t want to, as well as for the many physicians reluctant to prescribe the drug because of the considerable regulatory hassles and potentially serious side effects, including teratogenicity.
The treatment regimen in this open-label multicenter study consisted of an oral antibiotic, a topical antibiotic/retinoid agent, and benzoyl peroxide. More specifically, the 97 study participants aged 12-29 years, all with grade 3-4 moderate to severe facial acne by Investigator’s Global Assessment (IGA), were placed on once-daily minocycline HCL extended release at about 1 mg/kg, clindamycin phosphate 1.2%/tretinoin 0.025% gel, and 6% benzoyl peroxide foaming cloths. Patients were evaluated at weeks 0, 2, 4, 8, and 12.
At week 2, 44% of subjects already had at least a 1-grade improvement in IGA; by week 12, 89% did. Moreover, 56% of patients had at least a 2-grade improvement in IGA.
At least a 1-grade improvement on the Global Aesthetic Improvement Scale was documented in 83% of subjects at week 2 and 96% at week 12.
"With this therapy, you can get patients with really bad acne from bad to really mild without resorting to big-time drugs," observed Dr. Webster, professor of dermatology and internal medicine at Thomas Jefferson University, Philadelphia.
Week 12 mean facial inflammatory lesion counts fell by 62%, and noninflammatory lesion counts decreased by 49% from baselines of 33 and 44 lesions, respectively.
At baseline, 69 patients were judged by three blinded assessors of clinical photos to have acne sufficiently severe for them to be candidates for isotretinoin therapy. By week 12, this number had dwindled to 14 patients. In other words, 80% of patients were no longer deemed to be candidates for isotretinoin.
Eight patients experienced treatment-related adverse events consisting of transient mild to moderate irritation and/or redness, burning, stinging, and dry skin.
The results of this Phase-4 study are consistent with studies of other multidrug regimens for acne, albeit mostly conducted in less severely affected patients.
"The general paradigm is that mixed therapies are useful because other than isotretinoin and maybe spironolactone, no one drug is strong enough to stop acne effectively. If you just hit the [Propionibacterium acnes] hard, you can’t get it down to where there’s no P. acnes. If you blunt the immune response, you’re still just blunting it, not turning it off. And if you’re addressing the plug in the follicle, it’s not a complete or rapid response," the dermatologist explained.
In clinical practice, Dr. Webster said he typically stops the oral antibiotic cold at about 12 weeks to avoid pigmentary changes and other side effects of long-term antibiotic therapy. At least 75% of patients can maintain their gains with topical therapy alone.
Compliance is often an issue with combination therapy. Patients need to understand that if they don’t use all of the medications consistently from day 1 they won’t get better.
"It’s tough with kids because kids expect to get better overnight. They see it on the Proactiv commercials and wonder why in the world they’re not better in 2 days," the dermatologist observed.
In this phase IV study, however, patient compliance was consistently excellent, perhaps because of the high disease severity. The treatment compliance rate was 91% at week 2 and 86% at week 12.
Dr. Webster is a consultant for several pharmaceutical companies, including Valeant, whose subsidiary Medicis sponsored the phase IV study.
SDEF and this news organization are owned by the same parent company.
*This story was updated March 1, 2013.
MAUI, HAWAII – Combination therapy for severe acne, with a trio of familiar, well tolerated agents, knocked down the skin disease severity in a phase IV study such that 80% of patients deemed candidates for isotretinoin at baseline no longer qualified for the powerful oral retinoid 12 weeks later, Dr. Guy F. Webster reported at the Hawaii Dermatology Seminar sponsored by Global Academy for Medical Education/Skin Disease Education Foundation.
These data provide welcome news for patients who can’t take isotretinoin or don’t want to, as well as for the many physicians reluctant to prescribe the drug because of the considerable regulatory hassles and potentially serious side effects, including teratogenicity.
The treatment regimen in this open-label multicenter study consisted of an oral antibiotic, a topical antibiotic/retinoid agent, and benzoyl peroxide. More specifically, the 97 study participants aged 12-29 years, all with grade 3-4 moderate to severe facial acne by Investigator’s Global Assessment (IGA), were placed on once-daily minocycline HCL extended release at about 1 mg/kg, clindamycin phosphate 1.2%/tretinoin 0.025% gel, and 6% benzoyl peroxide foaming cloths. Patients were evaluated at weeks 0, 2, 4, 8, and 12.
At week 2, 44% of subjects already had at least a 1-grade improvement in IGA; by week 12, 89% did. Moreover, 56% of patients had at least a 2-grade improvement in IGA.
At least a 1-grade improvement on the Global Aesthetic Improvement Scale was documented in 83% of subjects at week 2 and 96% at week 12.
"With this therapy, you can get patients with really bad acne from bad to really mild without resorting to big-time drugs," observed Dr. Webster, professor of dermatology and internal medicine at Thomas Jefferson University, Philadelphia.
Week 12 mean facial inflammatory lesion counts fell by 62%, and noninflammatory lesion counts decreased by 49% from baselines of 33 and 44 lesions, respectively.
At baseline, 69 patients were judged by three blinded assessors of clinical photos to have acne sufficiently severe for them to be candidates for isotretinoin therapy. By week 12, this number had dwindled to 14 patients. In other words, 80% of patients were no longer deemed to be candidates for isotretinoin.
Eight patients experienced treatment-related adverse events consisting of transient mild to moderate irritation and/or redness, burning, stinging, and dry skin.
The results of this Phase-4 study are consistent with studies of other multidrug regimens for acne, albeit mostly conducted in less severely affected patients.
"The general paradigm is that mixed therapies are useful because other than isotretinoin and maybe spironolactone, no one drug is strong enough to stop acne effectively. If you just hit the [Propionibacterium acnes] hard, you can’t get it down to where there’s no P. acnes. If you blunt the immune response, you’re still just blunting it, not turning it off. And if you’re addressing the plug in the follicle, it’s not a complete or rapid response," the dermatologist explained.
In clinical practice, Dr. Webster said he typically stops the oral antibiotic cold at about 12 weeks to avoid pigmentary changes and other side effects of long-term antibiotic therapy. At least 75% of patients can maintain their gains with topical therapy alone.
Compliance is often an issue with combination therapy. Patients need to understand that if they don’t use all of the medications consistently from day 1 they won’t get better.
"It’s tough with kids because kids expect to get better overnight. They see it on the Proactiv commercials and wonder why in the world they’re not better in 2 days," the dermatologist observed.
In this phase IV study, however, patient compliance was consistently excellent, perhaps because of the high disease severity. The treatment compliance rate was 91% at week 2 and 86% at week 12.
Dr. Webster is a consultant for several pharmaceutical companies, including Valeant, whose subsidiary Medicis sponsored the phase IV study.
SDEF and this news organization are owned by the same parent company.
*This story was updated March 1, 2013.
MAUI, HAWAII – Combination therapy for severe acne, with a trio of familiar, well tolerated agents, knocked down the skin disease severity in a phase IV study such that 80% of patients deemed candidates for isotretinoin at baseline no longer qualified for the powerful oral retinoid 12 weeks later, Dr. Guy F. Webster reported at the Hawaii Dermatology Seminar sponsored by Global Academy for Medical Education/Skin Disease Education Foundation.
These data provide welcome news for patients who can’t take isotretinoin or don’t want to, as well as for the many physicians reluctant to prescribe the drug because of the considerable regulatory hassles and potentially serious side effects, including teratogenicity.
The treatment regimen in this open-label multicenter study consisted of an oral antibiotic, a topical antibiotic/retinoid agent, and benzoyl peroxide. More specifically, the 97 study participants aged 12-29 years, all with grade 3-4 moderate to severe facial acne by Investigator’s Global Assessment (IGA), were placed on once-daily minocycline HCL extended release at about 1 mg/kg, clindamycin phosphate 1.2%/tretinoin 0.025% gel, and 6% benzoyl peroxide foaming cloths. Patients were evaluated at weeks 0, 2, 4, 8, and 12.
At week 2, 44% of subjects already had at least a 1-grade improvement in IGA; by week 12, 89% did. Moreover, 56% of patients had at least a 2-grade improvement in IGA.
At least a 1-grade improvement on the Global Aesthetic Improvement Scale was documented in 83% of subjects at week 2 and 96% at week 12.
"With this therapy, you can get patients with really bad acne from bad to really mild without resorting to big-time drugs," observed Dr. Webster, professor of dermatology and internal medicine at Thomas Jefferson University, Philadelphia.
Week 12 mean facial inflammatory lesion counts fell by 62%, and noninflammatory lesion counts decreased by 49% from baselines of 33 and 44 lesions, respectively.
At baseline, 69 patients were judged by three blinded assessors of clinical photos to have acne sufficiently severe for them to be candidates for isotretinoin therapy. By week 12, this number had dwindled to 14 patients. In other words, 80% of patients were no longer deemed to be candidates for isotretinoin.
Eight patients experienced treatment-related adverse events consisting of transient mild to moderate irritation and/or redness, burning, stinging, and dry skin.
The results of this Phase-4 study are consistent with studies of other multidrug regimens for acne, albeit mostly conducted in less severely affected patients.
"The general paradigm is that mixed therapies are useful because other than isotretinoin and maybe spironolactone, no one drug is strong enough to stop acne effectively. If you just hit the [Propionibacterium acnes] hard, you can’t get it down to where there’s no P. acnes. If you blunt the immune response, you’re still just blunting it, not turning it off. And if you’re addressing the plug in the follicle, it’s not a complete or rapid response," the dermatologist explained.
In clinical practice, Dr. Webster said he typically stops the oral antibiotic cold at about 12 weeks to avoid pigmentary changes and other side effects of long-term antibiotic therapy. At least 75% of patients can maintain their gains with topical therapy alone.
Compliance is often an issue with combination therapy. Patients need to understand that if they don’t use all of the medications consistently from day 1 they won’t get better.
"It’s tough with kids because kids expect to get better overnight. They see it on the Proactiv commercials and wonder why in the world they’re not better in 2 days," the dermatologist observed.
In this phase IV study, however, patient compliance was consistently excellent, perhaps because of the high disease severity. The treatment compliance rate was 91% at week 2 and 86% at week 12.
Dr. Webster is a consultant for several pharmaceutical companies, including Valeant, whose subsidiary Medicis sponsored the phase IV study.
SDEF and this news organization are owned by the same parent company.
*This story was updated March 1, 2013.
AT THE HAWAII DERMATOLOGY SEMINAR SPONSORED BY SKIN DISEASE EDUCATION FOUNDATION (SDEF)
Major Finding: Eighty percent of patients with acne sufficiently severe that blinded evaluators judged them to be candidates for isotretinoin at baseline no longer qualified for the potent oral retinoid after 12 weeks on triple therapy with an oral antibiotic, benzoyl peroxide, and a topical antibiotic/retinoid.
Data Source: An open-label, multicenter, phase IV study involving 97 patients with moderate to severe acne.
Disclosures: The study was sponsored by Medicis. The presenter is a consultant to the company.
The puzzling relationship between diet and acne
The relationship between acne and diet has been an ongoing debate. There are no meta-analyses, randomized controlled clinical studies, or well-designed scientific trials that follow evidence-based guidelines to elucidate a cause-effect relationship. However, for decades anecdotal evidence has shown that acne and insulin resistance, such as that seen in patients with polycystic ovarian syndrome (PCOS), are highly linked. Now the literature points to the growing relationship between nutrition and the prevalence of acne, especially to glycemic index and the consumption of dairy.
Glycemic index is a ranking system based on the quality and quantity of consumed carbohydrates and its ability to raise blood sugar levels. Foods with high glycemic indices such as potatoes, bread, chips, and pasta, require more insulin to maintain blood glucose levels within the normal range. High-glycemic diets that are prevalent in the United States not only lead to insulin resistance, diabetes, obesity, and heart disease but also to acne.
Several studies have looked at the glycemic load, insulin sensitivity, and hormonal mediators correlating to acne (Am. J. Clin. Nutr. 2007; 86:107-15; J. Dermatol. Sci. 2008;50:41-52). Foods with a high-glycemic index may contribute to acne by elevating serum insulin concentrations (which can stimulate sebocyte proliferation and sebum production), suppress sex hormone-binding globulin (SHBG) concentrations, and raise androgen concentrations. On the contrary, low-glycemic-index foods increase SHBG and reduce androgen levels; this is of great importance because higher SHBG levels are associated with lower acne severity. Consumption of fat and carbohydrates increases sebum production and affects sebum composition, ultimately encouraging acne production (Br. J. Dermatol. 1967;79:119-21).
A new study by Anna Di Landro et al. published in the December 2012 found a link between acne and the consumption of milk, particularly in those drinking skim milk and more than three servings of milk per week (J. Am. Acad. Dermatol. 2012;67:1129-35).
Dr. Di Landro et al. also found that the consumption of fish had a protective effect on acne. This interesting finding points to the larger issue of acne developing in ethnic populations that immigrate to the United States. Population studies have shown that non-Western diets have a reduced incidence of acne. Western diets are deficient in long-chain omega-3 fatty acids. The ratio of omega-6 to omega-3 fatty acids in our Western diet is 10:1 to 20:1, vs. 3:1 to 2:1 in a non-Western diet. Omega-6 fatty acids in increased concentrations induce proinflammatory mediators and have been associated with the development of inflammatory acne. Western diets with high consumption of seafood have high levels of omega-3 fatty acids and have shown to decrease inflammatory mediators in the skin (Arch. Dermatol. 2003;139:941-2).
In my clinic, the ethnic populations that immigrate to the United States often develop acne to a greater extent than they had in their native countries. Although factors including stress, hormonal differences in foods, and pollution can be confounding factors, we must not ignore the Western diet that these populations adapt to is higher in refined sugars and carbohydrates and lower in vegetables and lean protein. Every acne patient in my clinic is asked to complete a nutritional questionnaire discussing the intake of fast food, carbohydrates, juice, sodas, and processed sugar. We have noticed that acne improves clinically and is more responsive to traditional acne medications when patients reduce their consumption of processed sugars and dairy and increase their intake of lean protein. Similarly, our PCOS patients who are treated with medications such as metformin, which improves the body’s ability to regulate blood glucose levels, have improvements in their acne. So, is acne a marker for early insulin resistance?
The underlying etiology of acne is multifactorial, although now we can appreciate diet as one of the causative factors. Although there is no direct correlation between obesity or insulin resistance and the prevalence of acne, a low glycemic index diet in combination with topical and systemic acne medications can be a powerful method of treating acne. Nutritional counseling is an adjunct educational service we should provide to our patients in addition to skin care advice and medical treatments for acne.
No single food directly causes acne, but a balanced diet can alter its severity. Encouraging our patients to eat a variety of fruits and vegetables, lean protein, and healthy fats can prevent the inflammation seen with acne and also can protect against cardiovascular disease, type II diabetes, and even obesity.
It is unfortunate that the medical education system in the United States has no formal nutrition education. Nearly every field of medicine including internal medicine, cardiology, endocrinology, allergy, pediatrics, obstetrics and gynecology, surgery, and not the least, dermatology, is influenced in some realm by nutrition. As the population diversifies, so will the importance of dietary guidance. We need to educate ourselves and our residents-in-training to better appreciate the symbiotic relationship between diet and skin health and to provide this guidance to our patients.
Dr. Talakoub is in private practice in McLean, Va.
Do you have questions about treating patients with dark skin? If so, send them to [email protected].
The relationship between acne and diet has been an ongoing debate. There are no meta-analyses, randomized controlled clinical studies, or well-designed scientific trials that follow evidence-based guidelines to elucidate a cause-effect relationship. However, for decades anecdotal evidence has shown that acne and insulin resistance, such as that seen in patients with polycystic ovarian syndrome (PCOS), are highly linked. Now the literature points to the growing relationship between nutrition and the prevalence of acne, especially to glycemic index and the consumption of dairy.
Glycemic index is a ranking system based on the quality and quantity of consumed carbohydrates and its ability to raise blood sugar levels. Foods with high glycemic indices such as potatoes, bread, chips, and pasta, require more insulin to maintain blood glucose levels within the normal range. High-glycemic diets that are prevalent in the United States not only lead to insulin resistance, diabetes, obesity, and heart disease but also to acne.
Several studies have looked at the glycemic load, insulin sensitivity, and hormonal mediators correlating to acne (Am. J. Clin. Nutr. 2007; 86:107-15; J. Dermatol. Sci. 2008;50:41-52). Foods with a high-glycemic index may contribute to acne by elevating serum insulin concentrations (which can stimulate sebocyte proliferation and sebum production), suppress sex hormone-binding globulin (SHBG) concentrations, and raise androgen concentrations. On the contrary, low-glycemic-index foods increase SHBG and reduce androgen levels; this is of great importance because higher SHBG levels are associated with lower acne severity. Consumption of fat and carbohydrates increases sebum production and affects sebum composition, ultimately encouraging acne production (Br. J. Dermatol. 1967;79:119-21).
A new study by Anna Di Landro et al. published in the December 2012 found a link between acne and the consumption of milk, particularly in those drinking skim milk and more than three servings of milk per week (J. Am. Acad. Dermatol. 2012;67:1129-35).
Dr. Di Landro et al. also found that the consumption of fish had a protective effect on acne. This interesting finding points to the larger issue of acne developing in ethnic populations that immigrate to the United States. Population studies have shown that non-Western diets have a reduced incidence of acne. Western diets are deficient in long-chain omega-3 fatty acids. The ratio of omega-6 to omega-3 fatty acids in our Western diet is 10:1 to 20:1, vs. 3:1 to 2:1 in a non-Western diet. Omega-6 fatty acids in increased concentrations induce proinflammatory mediators and have been associated with the development of inflammatory acne. Western diets with high consumption of seafood have high levels of omega-3 fatty acids and have shown to decrease inflammatory mediators in the skin (Arch. Dermatol. 2003;139:941-2).
In my clinic, the ethnic populations that immigrate to the United States often develop acne to a greater extent than they had in their native countries. Although factors including stress, hormonal differences in foods, and pollution can be confounding factors, we must not ignore the Western diet that these populations adapt to is higher in refined sugars and carbohydrates and lower in vegetables and lean protein. Every acne patient in my clinic is asked to complete a nutritional questionnaire discussing the intake of fast food, carbohydrates, juice, sodas, and processed sugar. We have noticed that acne improves clinically and is more responsive to traditional acne medications when patients reduce their consumption of processed sugars and dairy and increase their intake of lean protein. Similarly, our PCOS patients who are treated with medications such as metformin, which improves the body’s ability to regulate blood glucose levels, have improvements in their acne. So, is acne a marker for early insulin resistance?
The underlying etiology of acne is multifactorial, although now we can appreciate diet as one of the causative factors. Although there is no direct correlation between obesity or insulin resistance and the prevalence of acne, a low glycemic index diet in combination with topical and systemic acne medications can be a powerful method of treating acne. Nutritional counseling is an adjunct educational service we should provide to our patients in addition to skin care advice and medical treatments for acne.
No single food directly causes acne, but a balanced diet can alter its severity. Encouraging our patients to eat a variety of fruits and vegetables, lean protein, and healthy fats can prevent the inflammation seen with acne and also can protect against cardiovascular disease, type II diabetes, and even obesity.
It is unfortunate that the medical education system in the United States has no formal nutrition education. Nearly every field of medicine including internal medicine, cardiology, endocrinology, allergy, pediatrics, obstetrics and gynecology, surgery, and not the least, dermatology, is influenced in some realm by nutrition. As the population diversifies, so will the importance of dietary guidance. We need to educate ourselves and our residents-in-training to better appreciate the symbiotic relationship between diet and skin health and to provide this guidance to our patients.
Dr. Talakoub is in private practice in McLean, Va.
Do you have questions about treating patients with dark skin? If so, send them to [email protected].
The relationship between acne and diet has been an ongoing debate. There are no meta-analyses, randomized controlled clinical studies, or well-designed scientific trials that follow evidence-based guidelines to elucidate a cause-effect relationship. However, for decades anecdotal evidence has shown that acne and insulin resistance, such as that seen in patients with polycystic ovarian syndrome (PCOS), are highly linked. Now the literature points to the growing relationship between nutrition and the prevalence of acne, especially to glycemic index and the consumption of dairy.
Glycemic index is a ranking system based on the quality and quantity of consumed carbohydrates and its ability to raise blood sugar levels. Foods with high glycemic indices such as potatoes, bread, chips, and pasta, require more insulin to maintain blood glucose levels within the normal range. High-glycemic diets that are prevalent in the United States not only lead to insulin resistance, diabetes, obesity, and heart disease but also to acne.
Several studies have looked at the glycemic load, insulin sensitivity, and hormonal mediators correlating to acne (Am. J. Clin. Nutr. 2007; 86:107-15; J. Dermatol. Sci. 2008;50:41-52). Foods with a high-glycemic index may contribute to acne by elevating serum insulin concentrations (which can stimulate sebocyte proliferation and sebum production), suppress sex hormone-binding globulin (SHBG) concentrations, and raise androgen concentrations. On the contrary, low-glycemic-index foods increase SHBG and reduce androgen levels; this is of great importance because higher SHBG levels are associated with lower acne severity. Consumption of fat and carbohydrates increases sebum production and affects sebum composition, ultimately encouraging acne production (Br. J. Dermatol. 1967;79:119-21).
A new study by Anna Di Landro et al. published in the December 2012 found a link between acne and the consumption of milk, particularly in those drinking skim milk and more than three servings of milk per week (J. Am. Acad. Dermatol. 2012;67:1129-35).
Dr. Di Landro et al. also found that the consumption of fish had a protective effect on acne. This interesting finding points to the larger issue of acne developing in ethnic populations that immigrate to the United States. Population studies have shown that non-Western diets have a reduced incidence of acne. Western diets are deficient in long-chain omega-3 fatty acids. The ratio of omega-6 to omega-3 fatty acids in our Western diet is 10:1 to 20:1, vs. 3:1 to 2:1 in a non-Western diet. Omega-6 fatty acids in increased concentrations induce proinflammatory mediators and have been associated with the development of inflammatory acne. Western diets with high consumption of seafood have high levels of omega-3 fatty acids and have shown to decrease inflammatory mediators in the skin (Arch. Dermatol. 2003;139:941-2).
In my clinic, the ethnic populations that immigrate to the United States often develop acne to a greater extent than they had in their native countries. Although factors including stress, hormonal differences in foods, and pollution can be confounding factors, we must not ignore the Western diet that these populations adapt to is higher in refined sugars and carbohydrates and lower in vegetables and lean protein. Every acne patient in my clinic is asked to complete a nutritional questionnaire discussing the intake of fast food, carbohydrates, juice, sodas, and processed sugar. We have noticed that acne improves clinically and is more responsive to traditional acne medications when patients reduce their consumption of processed sugars and dairy and increase their intake of lean protein. Similarly, our PCOS patients who are treated with medications such as metformin, which improves the body’s ability to regulate blood glucose levels, have improvements in their acne. So, is acne a marker for early insulin resistance?
The underlying etiology of acne is multifactorial, although now we can appreciate diet as one of the causative factors. Although there is no direct correlation between obesity or insulin resistance and the prevalence of acne, a low glycemic index diet in combination with topical and systemic acne medications can be a powerful method of treating acne. Nutritional counseling is an adjunct educational service we should provide to our patients in addition to skin care advice and medical treatments for acne.
No single food directly causes acne, but a balanced diet can alter its severity. Encouraging our patients to eat a variety of fruits and vegetables, lean protein, and healthy fats can prevent the inflammation seen with acne and also can protect against cardiovascular disease, type II diabetes, and even obesity.
It is unfortunate that the medical education system in the United States has no formal nutrition education. Nearly every field of medicine including internal medicine, cardiology, endocrinology, allergy, pediatrics, obstetrics and gynecology, surgery, and not the least, dermatology, is influenced in some realm by nutrition. As the population diversifies, so will the importance of dietary guidance. We need to educate ourselves and our residents-in-training to better appreciate the symbiotic relationship between diet and skin health and to provide this guidance to our patients.
Dr. Talakoub is in private practice in McLean, Va.
Do you have questions about treating patients with dark skin? If so, send them to [email protected].
Nanoparticles take aim at acne
Nanotechnology stands to make a big difference in the way acne is treated.
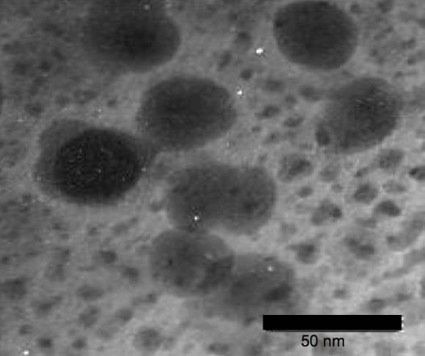
A 0.1% concentration of benzoyl peroxide encapsulated in chitosan-alginate nanoparticles showed more antimicrobial activity against acne bacteria than typical benzoyl peroxide or the nanoparticles alone in a recently published in vitro study (J. Invest. Dermatol. 2012 Nov. 29 [doi:10.1038/jid.2012.399]).
Chitosan-alginate has both antimicrobial and anti-inflammatory impact on Propionibacterium acnes, wrote Dr. Adam Friedman of Albert Einstein College of Medicine, New York, and his colleagues. The nanoparticle structure preserves the antimicrobial and immunological properties of chitosan while alginate lends stability.
Benzoyl peroxide is, of course, already a first-line therapy for acne, but encapsulating benzoyl peroxide in the nanoparticles stands to reduce the irritating side effects common with topical benzoyl peroxide use, the researchers noted. Less irritation could greatly improve patient compliance.
In the study, scanning electron microscopy and transmission electron microscopy showed "severe disruption, destruction, and ‘peeling’ of the cell wall," the researchers wrote. "These images suggest that the cell damage is due to an osmotic disturbance" created by the encapsulated benzoyl peroxide.
Nanoparticle encapsulation "provides previously undescribed therapeutic opportunities, including delivery of multidrug regimens to combat resistant microbes and inflammatory disease states," the researchers said.
Nanotechnology stands to make a big difference in the way acne is treated.
A 0.1% concentration of benzoyl peroxide encapsulated in chitosan-alginate nanoparticles showed more antimicrobial activity against acne bacteria than typical benzoyl peroxide or the nanoparticles alone in a recently published in vitro study (J. Invest. Dermatol. 2012 Nov. 29 [doi:10.1038/jid.2012.399]).
Chitosan-alginate has both antimicrobial and anti-inflammatory impact on Propionibacterium acnes, wrote Dr. Adam Friedman of Albert Einstein College of Medicine, New York, and his colleagues. The nanoparticle structure preserves the antimicrobial and immunological properties of chitosan while alginate lends stability.
Benzoyl peroxide is, of course, already a first-line therapy for acne, but encapsulating benzoyl peroxide in the nanoparticles stands to reduce the irritating side effects common with topical benzoyl peroxide use, the researchers noted. Less irritation could greatly improve patient compliance.
In the study, scanning electron microscopy and transmission electron microscopy showed "severe disruption, destruction, and ‘peeling’ of the cell wall," the researchers wrote. "These images suggest that the cell damage is due to an osmotic disturbance" created by the encapsulated benzoyl peroxide.
Nanoparticle encapsulation "provides previously undescribed therapeutic opportunities, including delivery of multidrug regimens to combat resistant microbes and inflammatory disease states," the researchers said.
Nanotechnology stands to make a big difference in the way acne is treated.
A 0.1% concentration of benzoyl peroxide encapsulated in chitosan-alginate nanoparticles showed more antimicrobial activity against acne bacteria than typical benzoyl peroxide or the nanoparticles alone in a recently published in vitro study (J. Invest. Dermatol. 2012 Nov. 29 [doi:10.1038/jid.2012.399]).
Chitosan-alginate has both antimicrobial and anti-inflammatory impact on Propionibacterium acnes, wrote Dr. Adam Friedman of Albert Einstein College of Medicine, New York, and his colleagues. The nanoparticle structure preserves the antimicrobial and immunological properties of chitosan while alginate lends stability.
Benzoyl peroxide is, of course, already a first-line therapy for acne, but encapsulating benzoyl peroxide in the nanoparticles stands to reduce the irritating side effects common with topical benzoyl peroxide use, the researchers noted. Less irritation could greatly improve patient compliance.
In the study, scanning electron microscopy and transmission electron microscopy showed "severe disruption, destruction, and ‘peeling’ of the cell wall," the researchers wrote. "These images suggest that the cell damage is due to an osmotic disturbance" created by the encapsulated benzoyl peroxide.
Nanoparticle encapsulation "provides previously undescribed therapeutic opportunities, including delivery of multidrug regimens to combat resistant microbes and inflammatory disease states," the researchers said.
Acne 101: Educate Patients Before Topical Therapy
LAS VEGAS – Acne patients need to know it’s a bad idea to spot-treat comedones with topical retinoids, according to Dr. Linda F. Stein Gold.
"We have to educate our patients that if they spot treat, they’re going to have acne indefinitely until their body decides it’s done having acne," she said. "You have to educate them that they have to treat the entire acne-prone area and [keep treating it] to maintain remission. Their skin may look clear, but they are not cured."
Topical retinoids remain the gold standard for acne treatment. They clear and prevent comedones, help clindamycin and other antibiotics penetrate the skin, and calm inflammation, which is probably the initial step in acne’s development, noted Dr. Stein Gold, director of dermatology research at Henry Ford Hospital, Detroit.
Microsphere and micronized tretinoin gel formulations are less irritating than generic topical tretinoin, and they’re less apt to be deactivated by benzoyl peroxide and ultraviolet light, she said at SDEF Las Vegas Dermatology Seminar.
One of the newer topicals combines benzoyl peroxide and a retinoid stable in its presence, adapalene. One study found about a 70% reduction in lesions after 4 months of use (J. Drugs Dermatol. 2007;6:899-905). With that topical combination and other acne treatments, patients should be told that it may take a while to see maximal improvements, she said.
With any retinoid treatment, patients should also expect flare-ups of redness, irritation, and dryness in the first 2 weeks. "If I’m concerned about irritation, I’ll ask them to go every other night for the first 2 weeks until they adjust to the medication, and then titrate up to every night," Dr. Stein Gold noted. "I also have them use a moisturizer and general cleanser." But she tells them not to use facial scrubs, because scrubbing does "more harm than good."
Benzoil peroxide also remains important, either alone or in combination, because Propionibacterium acnes bacteria do not develop resistance to it, and it helps prevent resistance when used with antibiotics.
"You get a nice reduction both in inflammatory and noninflammatory lesions with benzoil peroxide," she said, but patients should be warned about possible skin bleaching.
The concentration of benzoyl peroxide isn’t that important, Dr. Stein Gold explained. "We know that 2.5% and 10% gels have fairly similar efficacy," she added (Int. J. Dermatol. 1986;25:664-7).
Benzoil peroxide gels are known to work well, although foams and cleansers are available for patients who find them too irritating. Cleansers appear most effective at reducing P. acnes on the face, as long as patients wait 20 seconds before rinsing. One study found foams effective on the back when massaged into dry skin for 20 seconds and patients waited 2 minutes before showering (J. Drugs Dermatol. 2012;11:830-3).
Whatever the treatment, Dr. Stein Gold noted, "stress compliance. My first question is, ‘Did you get a chance to fill your medicine?’ and then, ‘How many times do you think you got a chance to use it?’ "
She cautioned physicians to "have no expectations" – that way, patients won’t be afraid to admit that they only used it once or twice. Whatever their usage, "you say, ‘Great. Good for you. Keep on going.’ "
In addition, "the simpler you make the regimen, the more likely it is your patients are going to" stick with it, Dr. Stein Gold explained.
Dr. Stein Gold is a consultant or researcher for Galderma, Leo, Medicis, Novartis, and Stiefel. SDEF and this news organization are owned by Frontline Medical Communications.
LAS VEGAS – Acne patients need to know it’s a bad idea to spot-treat comedones with topical retinoids, according to Dr. Linda F. Stein Gold.
"We have to educate our patients that if they spot treat, they’re going to have acne indefinitely until their body decides it’s done having acne," she said. "You have to educate them that they have to treat the entire acne-prone area and [keep treating it] to maintain remission. Their skin may look clear, but they are not cured."
Topical retinoids remain the gold standard for acne treatment. They clear and prevent comedones, help clindamycin and other antibiotics penetrate the skin, and calm inflammation, which is probably the initial step in acne’s development, noted Dr. Stein Gold, director of dermatology research at Henry Ford Hospital, Detroit.
Microsphere and micronized tretinoin gel formulations are less irritating than generic topical tretinoin, and they’re less apt to be deactivated by benzoyl peroxide and ultraviolet light, she said at SDEF Las Vegas Dermatology Seminar.
One of the newer topicals combines benzoyl peroxide and a retinoid stable in its presence, adapalene. One study found about a 70% reduction in lesions after 4 months of use (J. Drugs Dermatol. 2007;6:899-905). With that topical combination and other acne treatments, patients should be told that it may take a while to see maximal improvements, she said.
With any retinoid treatment, patients should also expect flare-ups of redness, irritation, and dryness in the first 2 weeks. "If I’m concerned about irritation, I’ll ask them to go every other night for the first 2 weeks until they adjust to the medication, and then titrate up to every night," Dr. Stein Gold noted. "I also have them use a moisturizer and general cleanser." But she tells them not to use facial scrubs, because scrubbing does "more harm than good."
Benzoil peroxide also remains important, either alone or in combination, because Propionibacterium acnes bacteria do not develop resistance to it, and it helps prevent resistance when used with antibiotics.
"You get a nice reduction both in inflammatory and noninflammatory lesions with benzoil peroxide," she said, but patients should be warned about possible skin bleaching.
The concentration of benzoyl peroxide isn’t that important, Dr. Stein Gold explained. "We know that 2.5% and 10% gels have fairly similar efficacy," she added (Int. J. Dermatol. 1986;25:664-7).
Benzoil peroxide gels are known to work well, although foams and cleansers are available for patients who find them too irritating. Cleansers appear most effective at reducing P. acnes on the face, as long as patients wait 20 seconds before rinsing. One study found foams effective on the back when massaged into dry skin for 20 seconds and patients waited 2 minutes before showering (J. Drugs Dermatol. 2012;11:830-3).
Whatever the treatment, Dr. Stein Gold noted, "stress compliance. My first question is, ‘Did you get a chance to fill your medicine?’ and then, ‘How many times do you think you got a chance to use it?’ "
She cautioned physicians to "have no expectations" – that way, patients won’t be afraid to admit that they only used it once or twice. Whatever their usage, "you say, ‘Great. Good for you. Keep on going.’ "
In addition, "the simpler you make the regimen, the more likely it is your patients are going to" stick with it, Dr. Stein Gold explained.
Dr. Stein Gold is a consultant or researcher for Galderma, Leo, Medicis, Novartis, and Stiefel. SDEF and this news organization are owned by Frontline Medical Communications.
LAS VEGAS – Acne patients need to know it’s a bad idea to spot-treat comedones with topical retinoids, according to Dr. Linda F. Stein Gold.
"We have to educate our patients that if they spot treat, they’re going to have acne indefinitely until their body decides it’s done having acne," she said. "You have to educate them that they have to treat the entire acne-prone area and [keep treating it] to maintain remission. Their skin may look clear, but they are not cured."
Topical retinoids remain the gold standard for acne treatment. They clear and prevent comedones, help clindamycin and other antibiotics penetrate the skin, and calm inflammation, which is probably the initial step in acne’s development, noted Dr. Stein Gold, director of dermatology research at Henry Ford Hospital, Detroit.
Microsphere and micronized tretinoin gel formulations are less irritating than generic topical tretinoin, and they’re less apt to be deactivated by benzoyl peroxide and ultraviolet light, she said at SDEF Las Vegas Dermatology Seminar.
One of the newer topicals combines benzoyl peroxide and a retinoid stable in its presence, adapalene. One study found about a 70% reduction in lesions after 4 months of use (J. Drugs Dermatol. 2007;6:899-905). With that topical combination and other acne treatments, patients should be told that it may take a while to see maximal improvements, she said.
With any retinoid treatment, patients should also expect flare-ups of redness, irritation, and dryness in the first 2 weeks. "If I’m concerned about irritation, I’ll ask them to go every other night for the first 2 weeks until they adjust to the medication, and then titrate up to every night," Dr. Stein Gold noted. "I also have them use a moisturizer and general cleanser." But she tells them not to use facial scrubs, because scrubbing does "more harm than good."
Benzoil peroxide also remains important, either alone or in combination, because Propionibacterium acnes bacteria do not develop resistance to it, and it helps prevent resistance when used with antibiotics.
"You get a nice reduction both in inflammatory and noninflammatory lesions with benzoil peroxide," she said, but patients should be warned about possible skin bleaching.
The concentration of benzoyl peroxide isn’t that important, Dr. Stein Gold explained. "We know that 2.5% and 10% gels have fairly similar efficacy," she added (Int. J. Dermatol. 1986;25:664-7).
Benzoil peroxide gels are known to work well, although foams and cleansers are available for patients who find them too irritating. Cleansers appear most effective at reducing P. acnes on the face, as long as patients wait 20 seconds before rinsing. One study found foams effective on the back when massaged into dry skin for 20 seconds and patients waited 2 minutes before showering (J. Drugs Dermatol. 2012;11:830-3).
Whatever the treatment, Dr. Stein Gold noted, "stress compliance. My first question is, ‘Did you get a chance to fill your medicine?’ and then, ‘How many times do you think you got a chance to use it?’ "
She cautioned physicians to "have no expectations" – that way, patients won’t be afraid to admit that they only used it once or twice. Whatever their usage, "you say, ‘Great. Good for you. Keep on going.’ "
In addition, "the simpler you make the regimen, the more likely it is your patients are going to" stick with it, Dr. Stein Gold explained.
Dr. Stein Gold is a consultant or researcher for Galderma, Leo, Medicis, Novartis, and Stiefel. SDEF and this news organization are owned by Frontline Medical Communications.
EXPERT ANALYSIS FROM SDEF LAS VEGAS DERMATOLOGY SEMINAR
Limit Oral Acne Antibiotics to 3 Months
LAS VEGAS – Acne patients treated with oral antibiotics don’t necessarily need to stay on them for more than 3 months, according to Dr. Joshua Zeichner.
"Even if you are initially treating them with an oral antibiotic for severe acne, you can maintain them after about 12 weeks just on a topical therapy like tazarotene, explained Dr. Zeichner, director of cosmetic and clinical research in the dermatology department at Mt. Sinai Medical Center in New York. "You don’t necessarily need to keep your patients on oral antibiotics for long periods of time."
To prevent resistance and other problems, "try to limit use to about 3 months, and think about maintenance using just a topical therapy," he said at the Las Vegas Dermatology seminar, sponsored by the Skin Disease Education Foundation.
Several studies prove the point. In one, 189 patients with severe acne received 0.1% tazarotene gel each evening and 100 mg minocycline twice daily for 12 weeks. The 110 (58%) with at least a 75% global improvement were then assigned to 12 weeks of maintenance with tazarotene gel, minocycline, or tazarotene plus minocycline.
Keeping the antibiotic onboard past 12 weeks made no difference. All three groups – including the tazarotene-only group – did equally well. At week 24, more than 80% of the patients had at least a 50% global improvement from baseline, and 50% had at least a 75% improvement (Arch. Dermatol. 2006;142:605-12).
In his own practice, Dr. Zeichner said he often puts patients on oral antibiotics with topical therapies while they wait a month for their oral isotretinoin prescriptions to come through. "There are a lot of cases where patients don’t even end up going on isotretinoin because they are doing well," he said.
The approach also offers an alternative for severe acne patients who, for whatever reason, can’t take isotretinoin.
Another combination that’s been shown to work is minocycline extended release (Solodyn) about 1 mg/kg daily, daily clindamycin phosphate 1.2%/tretinoin 0.025% gel, and benzoil peroxide 6% foaming cloths. There’s an excellent chance isotretinoin candidates will no longer be candidates after 12 weeks of treatment, Dr. Zeichner said.
Some patients will need to stay on oral antibiotics a bit longer than 3 months. Even so, "try to get them off the antibiotic as early as possible. If they flare up again, [you can always] give them another course," he said.
Although the antibiotic may be stopped, Dr. Zeichner cautioned, it’s important to continue topical treatment to keep acne from coming back.
"I’d much rather see women on hormonal-type therapies than on oral antibiotics. I feel it gets a little more to the root of the issue," he noted.
Dr. Zeichner is a consultant, an investigator, or an advisory board member for several pharmaceutical companies, including Allergan, Beiersdorf, Galderma, Medicis, and Valeant. The SDEF and this publication are owned by Frontline Medical Communications.
LAS VEGAS – Acne patients treated with oral antibiotics don’t necessarily need to stay on them for more than 3 months, according to Dr. Joshua Zeichner.
"Even if you are initially treating them with an oral antibiotic for severe acne, you can maintain them after about 12 weeks just on a topical therapy like tazarotene, explained Dr. Zeichner, director of cosmetic and clinical research in the dermatology department at Mt. Sinai Medical Center in New York. "You don’t necessarily need to keep your patients on oral antibiotics for long periods of time."
To prevent resistance and other problems, "try to limit use to about 3 months, and think about maintenance using just a topical therapy," he said at the Las Vegas Dermatology seminar, sponsored by the Skin Disease Education Foundation.
Several studies prove the point. In one, 189 patients with severe acne received 0.1% tazarotene gel each evening and 100 mg minocycline twice daily for 12 weeks. The 110 (58%) with at least a 75% global improvement were then assigned to 12 weeks of maintenance with tazarotene gel, minocycline, or tazarotene plus minocycline.
Keeping the antibiotic onboard past 12 weeks made no difference. All three groups – including the tazarotene-only group – did equally well. At week 24, more than 80% of the patients had at least a 50% global improvement from baseline, and 50% had at least a 75% improvement (Arch. Dermatol. 2006;142:605-12).
In his own practice, Dr. Zeichner said he often puts patients on oral antibiotics with topical therapies while they wait a month for their oral isotretinoin prescriptions to come through. "There are a lot of cases where patients don’t even end up going on isotretinoin because they are doing well," he said.
The approach also offers an alternative for severe acne patients who, for whatever reason, can’t take isotretinoin.
Another combination that’s been shown to work is minocycline extended release (Solodyn) about 1 mg/kg daily, daily clindamycin phosphate 1.2%/tretinoin 0.025% gel, and benzoil peroxide 6% foaming cloths. There’s an excellent chance isotretinoin candidates will no longer be candidates after 12 weeks of treatment, Dr. Zeichner said.
Some patients will need to stay on oral antibiotics a bit longer than 3 months. Even so, "try to get them off the antibiotic as early as possible. If they flare up again, [you can always] give them another course," he said.
Although the antibiotic may be stopped, Dr. Zeichner cautioned, it’s important to continue topical treatment to keep acne from coming back.
"I’d much rather see women on hormonal-type therapies than on oral antibiotics. I feel it gets a little more to the root of the issue," he noted.
Dr. Zeichner is a consultant, an investigator, or an advisory board member for several pharmaceutical companies, including Allergan, Beiersdorf, Galderma, Medicis, and Valeant. The SDEF and this publication are owned by Frontline Medical Communications.
LAS VEGAS – Acne patients treated with oral antibiotics don’t necessarily need to stay on them for more than 3 months, according to Dr. Joshua Zeichner.
"Even if you are initially treating them with an oral antibiotic for severe acne, you can maintain them after about 12 weeks just on a topical therapy like tazarotene, explained Dr. Zeichner, director of cosmetic and clinical research in the dermatology department at Mt. Sinai Medical Center in New York. "You don’t necessarily need to keep your patients on oral antibiotics for long periods of time."
To prevent resistance and other problems, "try to limit use to about 3 months, and think about maintenance using just a topical therapy," he said at the Las Vegas Dermatology seminar, sponsored by the Skin Disease Education Foundation.
Several studies prove the point. In one, 189 patients with severe acne received 0.1% tazarotene gel each evening and 100 mg minocycline twice daily for 12 weeks. The 110 (58%) with at least a 75% global improvement were then assigned to 12 weeks of maintenance with tazarotene gel, minocycline, or tazarotene plus minocycline.
Keeping the antibiotic onboard past 12 weeks made no difference. All three groups – including the tazarotene-only group – did equally well. At week 24, more than 80% of the patients had at least a 50% global improvement from baseline, and 50% had at least a 75% improvement (Arch. Dermatol. 2006;142:605-12).
In his own practice, Dr. Zeichner said he often puts patients on oral antibiotics with topical therapies while they wait a month for their oral isotretinoin prescriptions to come through. "There are a lot of cases where patients don’t even end up going on isotretinoin because they are doing well," he said.
The approach also offers an alternative for severe acne patients who, for whatever reason, can’t take isotretinoin.
Another combination that’s been shown to work is minocycline extended release (Solodyn) about 1 mg/kg daily, daily clindamycin phosphate 1.2%/tretinoin 0.025% gel, and benzoil peroxide 6% foaming cloths. There’s an excellent chance isotretinoin candidates will no longer be candidates after 12 weeks of treatment, Dr. Zeichner said.
Some patients will need to stay on oral antibiotics a bit longer than 3 months. Even so, "try to get them off the antibiotic as early as possible. If they flare up again, [you can always] give them another course," he said.
Although the antibiotic may be stopped, Dr. Zeichner cautioned, it’s important to continue topical treatment to keep acne from coming back.
"I’d much rather see women on hormonal-type therapies than on oral antibiotics. I feel it gets a little more to the root of the issue," he noted.
Dr. Zeichner is a consultant, an investigator, or an advisory board member for several pharmaceutical companies, including Allergan, Beiersdorf, Galderma, Medicis, and Valeant. The SDEF and this publication are owned by Frontline Medical Communications.
EXPERT ANALYSIS FROM THE LAS VEGAS DERMATOLOGY SEMINAR
New Acne Treatment to Hit U.S. Market
A coming, branded formulation of isotretinoin will offer compliance and efficacy advantages not previously available to U.S. dermatologists.
Absorica is a novel, patented brand formulation of isotretinoin marketed in the United States by Ranbaxy Pharmaceuticals Inc. *It will be available by the end of 2012 for the treatment of severe recalcitrant nodular acne.
The new drug, approved by the Food and Drug Administration in May, isn’t just another therapeutic option for dermatologists looking to treat acne – it’s a potential game changer in terms of improving efficacy and preventing relapse, according to Dr. Eric "Billy" Baum of the University of Alabama, Birmingham, who is a member of the speakers’ bureau for Ranbaxy.
Absorica is not rated as A-B equivalent to Accutane and the other isotretinoins, Dr. Baum said.
"I think that this is going to be a great advantage to dermatologists today," he said in an interview. "They’ll be able to have a type of isotretinoin that they know they are going to see really good results with. They are going to get the high blood levels that they need in order to get the maximum improvement, maximum absorption, and maximum efficacy."
Absorption is the key difference between how Absorica works and how other isotretinoins work, Dr. Baum explained at the meeting sponsored by Skin Disease Education Foundation.
Isotretinoin is highly lipophilic and is a poorly solubilized molecule. As a result, patients don’t get the maximum benefit of the drug unless they take it with a high-fat meal, ideally about 50 grams of fat, according to Dr. Baum. That’s the equivalent of a breakfast of two fried eggs in butter, two strips of bacon, two slices of toast with butter, 4 ounces of hash brown potatoes, and 8 ounces of whole milk, he said. Since isotretinoin is taken twice daily, patients should also eat another high-fat meal at dinnertime, he said.
But most adolescent patients taking isotretinoin don’t eat that type of high-fat diet – in fact, about a third of adolescents don’t eat breakfast at all, he said.
With the traditional form of isotretinoin, only about 40% of the drug was absorbed when patients were fasting. In contrast, with Absorica nearly 70% is absorbed, Dr. Baum said. "There is now a greater likelihood for patients to achieve cumulative targeted doses of isotretinoin in the 20 weeks of treatment, thereby increasing success of treatment and lowering the risk of relapse."
The better absorption with Absorica is due to a patented drug delivery system, which delivers isotretinoin along with fatty molecules, optimizing absorption in the small intestine, Dr. Baum explained.
A phase III trial with more than 900 patients showed no statistical difference in efficacy at 20 weeks between Absorica and Accutane when patients have eaten a high-fat meal. "You get the results that were achieved with the original Accutane under fed conditions with less concern about what patients will actually eat," Dr. Baum said.
There was also no notable difference in psychiatric, gastrointestinal, vascular, cardiac, or ophthalmic side effects between the two formulations, Dr. Baum said, adding that like all isotretinoins, Absorica patients and prescribers are obligated to participate in the iPLEDGE risk management program.
"This is a medication that’s been very highly studied, the largest clinical study on isotretinoin ever completed," he said.
The better rate of absorption is this formulation’s big clinical advantage, which should result in fewer relapses, Dr. Baum said. That could also make the drug more cost effective, because it would cut down on subsequent treatments in the event of relapse.
"It is important to treat isotretinoin patients most effectively in one cycle, if possible," Dr. Baum said. "This is the time where the patient is most motivated and has the most family support to comply with the complicated instructions of isotretinoin and iPLEDGE."
SDEF and this news organization are owned by Frontline Medical Communications.
*CORRECTION (11/07/12): A previous version of this story incorrectly reported when Absorica will be available in the U.S. This version has been updated.
A coming, branded formulation of isotretinoin will offer compliance and efficacy advantages not previously available to U.S. dermatologists.
Absorica is a novel, patented brand formulation of isotretinoin marketed in the United States by Ranbaxy Pharmaceuticals Inc. *It will be available by the end of 2012 for the treatment of severe recalcitrant nodular acne.
The new drug, approved by the Food and Drug Administration in May, isn’t just another therapeutic option for dermatologists looking to treat acne – it’s a potential game changer in terms of improving efficacy and preventing relapse, according to Dr. Eric "Billy" Baum of the University of Alabama, Birmingham, who is a member of the speakers’ bureau for Ranbaxy.
Absorica is not rated as A-B equivalent to Accutane and the other isotretinoins, Dr. Baum said.
"I think that this is going to be a great advantage to dermatologists today," he said in an interview. "They’ll be able to have a type of isotretinoin that they know they are going to see really good results with. They are going to get the high blood levels that they need in order to get the maximum improvement, maximum absorption, and maximum efficacy."
Absorption is the key difference between how Absorica works and how other isotretinoins work, Dr. Baum explained at the meeting sponsored by Skin Disease Education Foundation.
Isotretinoin is highly lipophilic and is a poorly solubilized molecule. As a result, patients don’t get the maximum benefit of the drug unless they take it with a high-fat meal, ideally about 50 grams of fat, according to Dr. Baum. That’s the equivalent of a breakfast of two fried eggs in butter, two strips of bacon, two slices of toast with butter, 4 ounces of hash brown potatoes, and 8 ounces of whole milk, he said. Since isotretinoin is taken twice daily, patients should also eat another high-fat meal at dinnertime, he said.
But most adolescent patients taking isotretinoin don’t eat that type of high-fat diet – in fact, about a third of adolescents don’t eat breakfast at all, he said.
With the traditional form of isotretinoin, only about 40% of the drug was absorbed when patients were fasting. In contrast, with Absorica nearly 70% is absorbed, Dr. Baum said. "There is now a greater likelihood for patients to achieve cumulative targeted doses of isotretinoin in the 20 weeks of treatment, thereby increasing success of treatment and lowering the risk of relapse."
The better absorption with Absorica is due to a patented drug delivery system, which delivers isotretinoin along with fatty molecules, optimizing absorption in the small intestine, Dr. Baum explained.
A phase III trial with more than 900 patients showed no statistical difference in efficacy at 20 weeks between Absorica and Accutane when patients have eaten a high-fat meal. "You get the results that were achieved with the original Accutane under fed conditions with less concern about what patients will actually eat," Dr. Baum said.
There was also no notable difference in psychiatric, gastrointestinal, vascular, cardiac, or ophthalmic side effects between the two formulations, Dr. Baum said, adding that like all isotretinoins, Absorica patients and prescribers are obligated to participate in the iPLEDGE risk management program.
"This is a medication that’s been very highly studied, the largest clinical study on isotretinoin ever completed," he said.
The better rate of absorption is this formulation’s big clinical advantage, which should result in fewer relapses, Dr. Baum said. That could also make the drug more cost effective, because it would cut down on subsequent treatments in the event of relapse.
"It is important to treat isotretinoin patients most effectively in one cycle, if possible," Dr. Baum said. "This is the time where the patient is most motivated and has the most family support to comply with the complicated instructions of isotretinoin and iPLEDGE."
SDEF and this news organization are owned by Frontline Medical Communications.
*CORRECTION (11/07/12): A previous version of this story incorrectly reported when Absorica will be available in the U.S. This version has been updated.
A coming, branded formulation of isotretinoin will offer compliance and efficacy advantages not previously available to U.S. dermatologists.
Absorica is a novel, patented brand formulation of isotretinoin marketed in the United States by Ranbaxy Pharmaceuticals Inc. *It will be available by the end of 2012 for the treatment of severe recalcitrant nodular acne.
The new drug, approved by the Food and Drug Administration in May, isn’t just another therapeutic option for dermatologists looking to treat acne – it’s a potential game changer in terms of improving efficacy and preventing relapse, according to Dr. Eric "Billy" Baum of the University of Alabama, Birmingham, who is a member of the speakers’ bureau for Ranbaxy.
Absorica is not rated as A-B equivalent to Accutane and the other isotretinoins, Dr. Baum said.
"I think that this is going to be a great advantage to dermatologists today," he said in an interview. "They’ll be able to have a type of isotretinoin that they know they are going to see really good results with. They are going to get the high blood levels that they need in order to get the maximum improvement, maximum absorption, and maximum efficacy."
Absorption is the key difference between how Absorica works and how other isotretinoins work, Dr. Baum explained at the meeting sponsored by Skin Disease Education Foundation.
Isotretinoin is highly lipophilic and is a poorly solubilized molecule. As a result, patients don’t get the maximum benefit of the drug unless they take it with a high-fat meal, ideally about 50 grams of fat, according to Dr. Baum. That’s the equivalent of a breakfast of two fried eggs in butter, two strips of bacon, two slices of toast with butter, 4 ounces of hash brown potatoes, and 8 ounces of whole milk, he said. Since isotretinoin is taken twice daily, patients should also eat another high-fat meal at dinnertime, he said.
But most adolescent patients taking isotretinoin don’t eat that type of high-fat diet – in fact, about a third of adolescents don’t eat breakfast at all, he said.
With the traditional form of isotretinoin, only about 40% of the drug was absorbed when patients were fasting. In contrast, with Absorica nearly 70% is absorbed, Dr. Baum said. "There is now a greater likelihood for patients to achieve cumulative targeted doses of isotretinoin in the 20 weeks of treatment, thereby increasing success of treatment and lowering the risk of relapse."
The better absorption with Absorica is due to a patented drug delivery system, which delivers isotretinoin along with fatty molecules, optimizing absorption in the small intestine, Dr. Baum explained.
A phase III trial with more than 900 patients showed no statistical difference in efficacy at 20 weeks between Absorica and Accutane when patients have eaten a high-fat meal. "You get the results that were achieved with the original Accutane under fed conditions with less concern about what patients will actually eat," Dr. Baum said.
There was also no notable difference in psychiatric, gastrointestinal, vascular, cardiac, or ophthalmic side effects between the two formulations, Dr. Baum said, adding that like all isotretinoins, Absorica patients and prescribers are obligated to participate in the iPLEDGE risk management program.
"This is a medication that’s been very highly studied, the largest clinical study on isotretinoin ever completed," he said.
The better rate of absorption is this formulation’s big clinical advantage, which should result in fewer relapses, Dr. Baum said. That could also make the drug more cost effective, because it would cut down on subsequent treatments in the event of relapse.
"It is important to treat isotretinoin patients most effectively in one cycle, if possible," Dr. Baum said. "This is the time where the patient is most motivated and has the most family support to comply with the complicated instructions of isotretinoin and iPLEDGE."
SDEF and this news organization are owned by Frontline Medical Communications.
*CORRECTION (11/07/12): A previous version of this story incorrectly reported when Absorica will be available in the U.S. This version has been updated.
EXPERT ANALYSIS FROM THE SDEF LAS VEGAS DERMATOLOGY SEMINAR
Novel Rosacea Gel Scores in Phase III Trial
PRAGUE – Once-daily brimonidine tartrate gel 0.5% for the treatment of moderate to severe facial erythema of rosacea hit its efficacy and safety end points in a pivotal phase III clinical trial.
The randomized, double-blind, vehicle-controlled, multicenter study totaled 260 patients with persistent moderate to severe facial redness from rosacea. The patients were randomized to once-daily brimonidine tartrate gel 0.5% or a vehicle gel for 4 weeks, with 4 weeks of subsequent off-label follow-up.
The proprietary brimonidine tartrate gel proved faster acting and significantly more effective than the vehicle. Thirty minutes after the first application on study day 1, 28% of patients in the active treatment arm showed at least a 1-grade improvement on both Patient Self-Assessment and Clinician’s Erythema Assessment, compared with 7% of controls, Dr. Y. May Ma reported at the annual congress of the European Academy of Dermatology and Venereology.
The other efficacy end point was maintenance of a 2-grade improvement on both study measures for 12 hours after application on day 29. This was achieved in 23% of patients in the brimonidine tartrate gel cohort and 8% of controls.
No tachyphylaxis or rebound worsening of erythema occurred during the off-treatment follow-up, nor did the brimonidine tartrate group experience aggravation of telangiectasias or inflammatory lesions during that phase of the study, according to Dr. Ma of Galderma Laboratories, Sophia Antipolis, France.
The adverse events were generally mild, transient, and local. The most frequent events in the brimonidine tartrate group were worsening erythema and/or flushing in seven patients, itching in four, and skin irritation in three. No changes in blood pressure or heart rate were noted.
At present no medical therapies are approved for the treatment of persistent facial redness of rosacea. While brimonidine tartrate gel doesn’t tackle the underlying cause of rosacea, the topical vasoconstrictor does improve the appearance of rosacea patients who have persistent erythema. Galderma is preparing to file for U.S. and European marketing approval of the topical agent.
The trial was sponsored by Galderma Laboratories and presented by Dr. Ma, a company employee.
PRAGUE – Once-daily brimonidine tartrate gel 0.5% for the treatment of moderate to severe facial erythema of rosacea hit its efficacy and safety end points in a pivotal phase III clinical trial.
The randomized, double-blind, vehicle-controlled, multicenter study totaled 260 patients with persistent moderate to severe facial redness from rosacea. The patients were randomized to once-daily brimonidine tartrate gel 0.5% or a vehicle gel for 4 weeks, with 4 weeks of subsequent off-label follow-up.
The proprietary brimonidine tartrate gel proved faster acting and significantly more effective than the vehicle. Thirty minutes after the first application on study day 1, 28% of patients in the active treatment arm showed at least a 1-grade improvement on both Patient Self-Assessment and Clinician’s Erythema Assessment, compared with 7% of controls, Dr. Y. May Ma reported at the annual congress of the European Academy of Dermatology and Venereology.
The other efficacy end point was maintenance of a 2-grade improvement on both study measures for 12 hours after application on day 29. This was achieved in 23% of patients in the brimonidine tartrate gel cohort and 8% of controls.
No tachyphylaxis or rebound worsening of erythema occurred during the off-treatment follow-up, nor did the brimonidine tartrate group experience aggravation of telangiectasias or inflammatory lesions during that phase of the study, according to Dr. Ma of Galderma Laboratories, Sophia Antipolis, France.
The adverse events were generally mild, transient, and local. The most frequent events in the brimonidine tartrate group were worsening erythema and/or flushing in seven patients, itching in four, and skin irritation in three. No changes in blood pressure or heart rate were noted.
At present no medical therapies are approved for the treatment of persistent facial redness of rosacea. While brimonidine tartrate gel doesn’t tackle the underlying cause of rosacea, the topical vasoconstrictor does improve the appearance of rosacea patients who have persistent erythema. Galderma is preparing to file for U.S. and European marketing approval of the topical agent.
The trial was sponsored by Galderma Laboratories and presented by Dr. Ma, a company employee.
PRAGUE – Once-daily brimonidine tartrate gel 0.5% for the treatment of moderate to severe facial erythema of rosacea hit its efficacy and safety end points in a pivotal phase III clinical trial.
The randomized, double-blind, vehicle-controlled, multicenter study totaled 260 patients with persistent moderate to severe facial redness from rosacea. The patients were randomized to once-daily brimonidine tartrate gel 0.5% or a vehicle gel for 4 weeks, with 4 weeks of subsequent off-label follow-up.
The proprietary brimonidine tartrate gel proved faster acting and significantly more effective than the vehicle. Thirty minutes after the first application on study day 1, 28% of patients in the active treatment arm showed at least a 1-grade improvement on both Patient Self-Assessment and Clinician’s Erythema Assessment, compared with 7% of controls, Dr. Y. May Ma reported at the annual congress of the European Academy of Dermatology and Venereology.
The other efficacy end point was maintenance of a 2-grade improvement on both study measures for 12 hours after application on day 29. This was achieved in 23% of patients in the brimonidine tartrate gel cohort and 8% of controls.
No tachyphylaxis or rebound worsening of erythema occurred during the off-treatment follow-up, nor did the brimonidine tartrate group experience aggravation of telangiectasias or inflammatory lesions during that phase of the study, according to Dr. Ma of Galderma Laboratories, Sophia Antipolis, France.
The adverse events were generally mild, transient, and local. The most frequent events in the brimonidine tartrate group were worsening erythema and/or flushing in seven patients, itching in four, and skin irritation in three. No changes in blood pressure or heart rate were noted.
At present no medical therapies are approved for the treatment of persistent facial redness of rosacea. While brimonidine tartrate gel doesn’t tackle the underlying cause of rosacea, the topical vasoconstrictor does improve the appearance of rosacea patients who have persistent erythema. Galderma is preparing to file for U.S. and European marketing approval of the topical agent.
The trial was sponsored by Galderma Laboratories and presented by Dr. Ma, a company employee.
AT THE ANNUAL CONGRESS OF THE EUROPEAN ACADEMY OF DERMATOLOGY AND VENEREOLOGY
Major Finding: Thirty minutes after the first application on study day 1, 28% of patients in the active treatment arm showed at least a 1-grade improvement on both Patient Self-Assessment and Clinician’s Erythema Assessment, compared with 7% of controls
Data Source: The data come from a phase III, randomized, double-blind, multicenter, vehicle-controlled clinical trial of 260 patients with moderate to severe facial erythema of rosacea.
Disclosures: The trial was sponsored by Galderma Laboratories and presented by Dr. Ma, a company employee.
Low-Dose Isotretinoin Tames Adult Acne
PRAGUE – Oral isotretinoin dosed at 5 mg per day proved to be highly effective, fast acting, and well tolerated for persistent, low-grade, adult acne in a randomized, double-blind clinical trial.
Results of this study provide physicians with evidence supporting the use of low-dose isotretinoin in the management of adult acne, Dr. Marius Rademaker said at the annual congress of the European Academy of Dermatology and Venereology.
"There’s a high degree of dissatisfaction with treatment among adult acne sufferers because of their usual slow response to the traditional acne therapies, the poor clearance, and the very high relapse rate when you stop treatment. The standards of a woman of 35 with adult acne are quite different from those of a 15-year-old. I think people no longer want 70% improvement, they want 100% clearance," said Dr. Rademaker, a dermatologist at Waikato Hospital in Hamilton, New Zealand.
There have been few randomized, controlled studies focusing on treatment of adult acne, he reported, adding that he could find no studies involving systemic antibiotics for acne in adults. He found a few studies on topical retinoid trials, but they only included a minority of adults. And, he found no studies assessing the effectiveness of low dose isotretinoin for adults.
Therefore, he conducted a randomized trial of isotretinoin at 5 mg/day to determine if a lower dose would be as effective and would have fewer adverse events than the standard dose of 0.5-1.0 mg/kg per day. Avoiding relapse upon discontinuation of isotretinoin appears to be more a function of the duration of sebaceous gland suppression – longer is better – than of cumulative dose, he added.
He reported on 58 adults aged 25-55 with low-grade, indolent acne that had persisted since adolescence. Nearly 90% were women. Participants were randomized double-blind to 16 weeks of isotretinoin 5 mg/day or placebo, followed by an additional 16 weeks of open-label isotretinoin in both study arms. The primary end point was the change in the number of facial acne lesions between baseline and week 16.
The acne lesion count in the isotretinoin group was reduced by half within the first 4 weeks of the study, from a mean baseline of 10.6 lesions. By week 16, the group’s mean acne lesion count had dropped to 3.2. After a further 16 weeks of open-label therapy, it had fallen to 1.3.
In contrast, the mean acne lesion count in the control group didn’t change significantly over the first 16 weeks from a baseline of 9.7 lesions. After a subsequent 16 weeks of open-label isotretinoin, acne count dropped to 3.9.
A secondary outcome measure was change in Dermatology Life Quality Index (DLQI) scores. From a mean baseline score of 4.8, indicative of moderate skin disease–related disability, the score fell to 1.3 after 16 weeks of double-blind isotretinoin and to 1.2 after another 16 weeks of open-label therapy. The mean DLQI score of 4.9 was unchanged in the control group after 16 weeks of placebo, but dropped to 2.3 after 16 weeks of open-label isotretinoin.
In an interview, Dr. Neil S. Goldberg, a dermatologist in Bronxville, N.Y, questioned the blindness of any study involving isotretinoin. "No isotretinoin study can be blinded. The side effects of isotretinoin, even low dose, are just too obvious."
The most common side effect in the study was dry lips, which nearly two-thirds of patients reported while on isotretinoin. Dry skin, musculoskeletal aches and pains, dry eyes, and fatigue were less frequently reported. One patient withdrew from the study because of anxiety and mood changes that may or may not have been treatment related, Dr. Rademaker said.
He added that in his experience, after a year of treatment at 5 mg/day, virtually all patients with persistent low-grade adult acne no longer have any acne. He is pursing the possibilities of conducting a long-term, follow-up study.
"Isotretinoin seems to me to be just as valuable a treatment in adults as in teenagers," said Dr. Steven R. Feldman, professor of dermatology at Wake Forest Baptist Medical Center in Winston-Salem, N.C. "My standard approach is to use it in the same way, though using it in lower doses for longer periods of time is reasonable and often effective with fewer side effects." Caution is warranted, however, when prescribing isotretinoin to women of childbearing potential because of the drug’s known teratogenicity.
In response to audience questions about restrictions placed upon isotretinoin prescribing in New Zealand, Dr. Rademaker replied that his country’s pregnancy prevention plan requires patient education but not mandatory pregnancy tests. "And our pregnancy rates [for females on isotretinoin] are lower than in Europe or the U.S.," he said.
"iPledge makes everything harder. It inhibits flexibility. In Australia – where my two nieces and nephew live – when they started isotretinoin, the doctor just gave them 6 months of isotretinoin and sent them on their way," said Dr. Goldberg.
Clinical acne is present in 45% of women aged 21-30 years, 26% of women aged 31-40, and 12% of women aged 41-50, according to the results of a recent cross-sectional study of 2,895 U.S. females aged 10-70 years (J. Womens Health [Larchmt] 2012;21:223-30).
Dr. Rademaker’s investigator-initiated study was sponsored by Douglas Pharmaceuticals (an isotretinoin manufacturer in New Zealand). He had no other conflicts of interest. Dr. Feldman reported significant financial relationships with several pharmaceutical companies. Dr. Goldberg had no financial conflicts to report.
In the United States, 5 mg of isotretinoin is not available, so dermatologists usually prescribe 10 mg per day as a low dose, according to Dr. Hilary E. Baldwin. She noted that she prescribes low-dose isotretinoin for adults, but not for getting patients from 70% to 100% clear.

|
|
She added that she often prescribes isotretinoin for women who tend to get one to two large acne lesions per week. "Topical products are ineffective on this type of lesion and it is hard to justify long-term use of antibiotics for 1-2 lesions per week. Antibiotic resistance is of crucial importance in these patients. Alternatives to long term/low dose isotretinoin include oral contraceptives and spironolactone. However, some adult patients exceed the safe-age contraindications for OCPs," she said.
Dr. Baldwin is vice chair of dermatology at the State University of New York, Brooklyn. She has received research funds from and/or serves as a consultant to Allergan, Coria, Galderma, GlaxoSmithKline, Graceway Pharmaceuticals, L’Oreal, Ortho Dermatologics, Medicis, and Sanofi-Aventis.
In the United States, 5 mg of isotretinoin is not available, so dermatologists usually prescribe 10 mg per day as a low dose, according to Dr. Hilary E. Baldwin. She noted that she prescribes low-dose isotretinoin for adults, but not for getting patients from 70% to 100% clear.

|
|
She added that she often prescribes isotretinoin for women who tend to get one to two large acne lesions per week. "Topical products are ineffective on this type of lesion and it is hard to justify long-term use of antibiotics for 1-2 lesions per week. Antibiotic resistance is of crucial importance in these patients. Alternatives to long term/low dose isotretinoin include oral contraceptives and spironolactone. However, some adult patients exceed the safe-age contraindications for OCPs," she said.
Dr. Baldwin is vice chair of dermatology at the State University of New York, Brooklyn. She has received research funds from and/or serves as a consultant to Allergan, Coria, Galderma, GlaxoSmithKline, Graceway Pharmaceuticals, L’Oreal, Ortho Dermatologics, Medicis, and Sanofi-Aventis.
In the United States, 5 mg of isotretinoin is not available, so dermatologists usually prescribe 10 mg per day as a low dose, according to Dr. Hilary E. Baldwin. She noted that she prescribes low-dose isotretinoin for adults, but not for getting patients from 70% to 100% clear.

|
|
She added that she often prescribes isotretinoin for women who tend to get one to two large acne lesions per week. "Topical products are ineffective on this type of lesion and it is hard to justify long-term use of antibiotics for 1-2 lesions per week. Antibiotic resistance is of crucial importance in these patients. Alternatives to long term/low dose isotretinoin include oral contraceptives and spironolactone. However, some adult patients exceed the safe-age contraindications for OCPs," she said.
Dr. Baldwin is vice chair of dermatology at the State University of New York, Brooklyn. She has received research funds from and/or serves as a consultant to Allergan, Coria, Galderma, GlaxoSmithKline, Graceway Pharmaceuticals, L’Oreal, Ortho Dermatologics, Medicis, and Sanofi-Aventis.
PRAGUE – Oral isotretinoin dosed at 5 mg per day proved to be highly effective, fast acting, and well tolerated for persistent, low-grade, adult acne in a randomized, double-blind clinical trial.
Results of this study provide physicians with evidence supporting the use of low-dose isotretinoin in the management of adult acne, Dr. Marius Rademaker said at the annual congress of the European Academy of Dermatology and Venereology.
"There’s a high degree of dissatisfaction with treatment among adult acne sufferers because of their usual slow response to the traditional acne therapies, the poor clearance, and the very high relapse rate when you stop treatment. The standards of a woman of 35 with adult acne are quite different from those of a 15-year-old. I think people no longer want 70% improvement, they want 100% clearance," said Dr. Rademaker, a dermatologist at Waikato Hospital in Hamilton, New Zealand.
There have been few randomized, controlled studies focusing on treatment of adult acne, he reported, adding that he could find no studies involving systemic antibiotics for acne in adults. He found a few studies on topical retinoid trials, but they only included a minority of adults. And, he found no studies assessing the effectiveness of low dose isotretinoin for adults.
Therefore, he conducted a randomized trial of isotretinoin at 5 mg/day to determine if a lower dose would be as effective and would have fewer adverse events than the standard dose of 0.5-1.0 mg/kg per day. Avoiding relapse upon discontinuation of isotretinoin appears to be more a function of the duration of sebaceous gland suppression – longer is better – than of cumulative dose, he added.
He reported on 58 adults aged 25-55 with low-grade, indolent acne that had persisted since adolescence. Nearly 90% were women. Participants were randomized double-blind to 16 weeks of isotretinoin 5 mg/day or placebo, followed by an additional 16 weeks of open-label isotretinoin in both study arms. The primary end point was the change in the number of facial acne lesions between baseline and week 16.
The acne lesion count in the isotretinoin group was reduced by half within the first 4 weeks of the study, from a mean baseline of 10.6 lesions. By week 16, the group’s mean acne lesion count had dropped to 3.2. After a further 16 weeks of open-label therapy, it had fallen to 1.3.
In contrast, the mean acne lesion count in the control group didn’t change significantly over the first 16 weeks from a baseline of 9.7 lesions. After a subsequent 16 weeks of open-label isotretinoin, acne count dropped to 3.9.
A secondary outcome measure was change in Dermatology Life Quality Index (DLQI) scores. From a mean baseline score of 4.8, indicative of moderate skin disease–related disability, the score fell to 1.3 after 16 weeks of double-blind isotretinoin and to 1.2 after another 16 weeks of open-label therapy. The mean DLQI score of 4.9 was unchanged in the control group after 16 weeks of placebo, but dropped to 2.3 after 16 weeks of open-label isotretinoin.
In an interview, Dr. Neil S. Goldberg, a dermatologist in Bronxville, N.Y, questioned the blindness of any study involving isotretinoin. "No isotretinoin study can be blinded. The side effects of isotretinoin, even low dose, are just too obvious."
The most common side effect in the study was dry lips, which nearly two-thirds of patients reported while on isotretinoin. Dry skin, musculoskeletal aches and pains, dry eyes, and fatigue were less frequently reported. One patient withdrew from the study because of anxiety and mood changes that may or may not have been treatment related, Dr. Rademaker said.
He added that in his experience, after a year of treatment at 5 mg/day, virtually all patients with persistent low-grade adult acne no longer have any acne. He is pursing the possibilities of conducting a long-term, follow-up study.
"Isotretinoin seems to me to be just as valuable a treatment in adults as in teenagers," said Dr. Steven R. Feldman, professor of dermatology at Wake Forest Baptist Medical Center in Winston-Salem, N.C. "My standard approach is to use it in the same way, though using it in lower doses for longer periods of time is reasonable and often effective with fewer side effects." Caution is warranted, however, when prescribing isotretinoin to women of childbearing potential because of the drug’s known teratogenicity.
In response to audience questions about restrictions placed upon isotretinoin prescribing in New Zealand, Dr. Rademaker replied that his country’s pregnancy prevention plan requires patient education but not mandatory pregnancy tests. "And our pregnancy rates [for females on isotretinoin] are lower than in Europe or the U.S.," he said.
"iPledge makes everything harder. It inhibits flexibility. In Australia – where my two nieces and nephew live – when they started isotretinoin, the doctor just gave them 6 months of isotretinoin and sent them on their way," said Dr. Goldberg.
Clinical acne is present in 45% of women aged 21-30 years, 26% of women aged 31-40, and 12% of women aged 41-50, according to the results of a recent cross-sectional study of 2,895 U.S. females aged 10-70 years (J. Womens Health [Larchmt] 2012;21:223-30).
Dr. Rademaker’s investigator-initiated study was sponsored by Douglas Pharmaceuticals (an isotretinoin manufacturer in New Zealand). He had no other conflicts of interest. Dr. Feldman reported significant financial relationships with several pharmaceutical companies. Dr. Goldberg had no financial conflicts to report.
PRAGUE – Oral isotretinoin dosed at 5 mg per day proved to be highly effective, fast acting, and well tolerated for persistent, low-grade, adult acne in a randomized, double-blind clinical trial.
Results of this study provide physicians with evidence supporting the use of low-dose isotretinoin in the management of adult acne, Dr. Marius Rademaker said at the annual congress of the European Academy of Dermatology and Venereology.
"There’s a high degree of dissatisfaction with treatment among adult acne sufferers because of their usual slow response to the traditional acne therapies, the poor clearance, and the very high relapse rate when you stop treatment. The standards of a woman of 35 with adult acne are quite different from those of a 15-year-old. I think people no longer want 70% improvement, they want 100% clearance," said Dr. Rademaker, a dermatologist at Waikato Hospital in Hamilton, New Zealand.
There have been few randomized, controlled studies focusing on treatment of adult acne, he reported, adding that he could find no studies involving systemic antibiotics for acne in adults. He found a few studies on topical retinoid trials, but they only included a minority of adults. And, he found no studies assessing the effectiveness of low dose isotretinoin for adults.
Therefore, he conducted a randomized trial of isotretinoin at 5 mg/day to determine if a lower dose would be as effective and would have fewer adverse events than the standard dose of 0.5-1.0 mg/kg per day. Avoiding relapse upon discontinuation of isotretinoin appears to be more a function of the duration of sebaceous gland suppression – longer is better – than of cumulative dose, he added.
He reported on 58 adults aged 25-55 with low-grade, indolent acne that had persisted since adolescence. Nearly 90% were women. Participants were randomized double-blind to 16 weeks of isotretinoin 5 mg/day or placebo, followed by an additional 16 weeks of open-label isotretinoin in both study arms. The primary end point was the change in the number of facial acne lesions between baseline and week 16.
The acne lesion count in the isotretinoin group was reduced by half within the first 4 weeks of the study, from a mean baseline of 10.6 lesions. By week 16, the group’s mean acne lesion count had dropped to 3.2. After a further 16 weeks of open-label therapy, it had fallen to 1.3.
In contrast, the mean acne lesion count in the control group didn’t change significantly over the first 16 weeks from a baseline of 9.7 lesions. After a subsequent 16 weeks of open-label isotretinoin, acne count dropped to 3.9.
A secondary outcome measure was change in Dermatology Life Quality Index (DLQI) scores. From a mean baseline score of 4.8, indicative of moderate skin disease–related disability, the score fell to 1.3 after 16 weeks of double-blind isotretinoin and to 1.2 after another 16 weeks of open-label therapy. The mean DLQI score of 4.9 was unchanged in the control group after 16 weeks of placebo, but dropped to 2.3 after 16 weeks of open-label isotretinoin.
In an interview, Dr. Neil S. Goldberg, a dermatologist in Bronxville, N.Y, questioned the blindness of any study involving isotretinoin. "No isotretinoin study can be blinded. The side effects of isotretinoin, even low dose, are just too obvious."
The most common side effect in the study was dry lips, which nearly two-thirds of patients reported while on isotretinoin. Dry skin, musculoskeletal aches and pains, dry eyes, and fatigue were less frequently reported. One patient withdrew from the study because of anxiety and mood changes that may or may not have been treatment related, Dr. Rademaker said.
He added that in his experience, after a year of treatment at 5 mg/day, virtually all patients with persistent low-grade adult acne no longer have any acne. He is pursing the possibilities of conducting a long-term, follow-up study.
"Isotretinoin seems to me to be just as valuable a treatment in adults as in teenagers," said Dr. Steven R. Feldman, professor of dermatology at Wake Forest Baptist Medical Center in Winston-Salem, N.C. "My standard approach is to use it in the same way, though using it in lower doses for longer periods of time is reasonable and often effective with fewer side effects." Caution is warranted, however, when prescribing isotretinoin to women of childbearing potential because of the drug’s known teratogenicity.
In response to audience questions about restrictions placed upon isotretinoin prescribing in New Zealand, Dr. Rademaker replied that his country’s pregnancy prevention plan requires patient education but not mandatory pregnancy tests. "And our pregnancy rates [for females on isotretinoin] are lower than in Europe or the U.S.," he said.
"iPledge makes everything harder. It inhibits flexibility. In Australia – where my two nieces and nephew live – when they started isotretinoin, the doctor just gave them 6 months of isotretinoin and sent them on their way," said Dr. Goldberg.
Clinical acne is present in 45% of women aged 21-30 years, 26% of women aged 31-40, and 12% of women aged 41-50, according to the results of a recent cross-sectional study of 2,895 U.S. females aged 10-70 years (J. Womens Health [Larchmt] 2012;21:223-30).
Dr. Rademaker’s investigator-initiated study was sponsored by Douglas Pharmaceuticals (an isotretinoin manufacturer in New Zealand). He had no other conflicts of interest. Dr. Feldman reported significant financial relationships with several pharmaceutical companies. Dr. Goldberg had no financial conflicts to report.
AT THE ANNUAL CONGRESS OF THE EUROPEAN ACADEMY OF DERMATOLOGY AND VENEREOLOGY
Major Finding: Sixteen weeks of low-dose isotretinoin at 5 mg/day in patients with persistent, low-grade adult acne resulted in a reduction in mean facial lesion count from 10.6 at baseline to 3.2, with a further 16 weeks of open-label isotretinoin dropping the lesion count to 1.3.
Data Source: This was a randomized, double-blind, placebo-controlled trial involving 58 adults aged 25-55 with persistent, low-grade acne since adolescence.
Disclosures: Dr. Rademaker’s investigator-initiated study was sponsored by Douglas Pharmaceuticals (an isotretinoin manufacturer in New Zealand). He had no other conflicts of interest.
Adult Acne Woes: The Skinny Podcast
In this month's program, Dermatologist Toby Maurer, who founded the HIV dermatology clinic at San Francisco General Hospital, talks about the toll Kaposi's sarcoma is taking on HIV patients.
We review the role of online dermatology videos in the education of medical students.
Then, reporter Mary Ellen Schneider highlights the top read stories on www.skinandallergynews.com, and Dr. Lily Talakoub talks about a condition that continues to plague adult patients - acne.
Lastly, Dr. Alan Rockoff relays a tale about children and warts.
In this month's program, Dermatologist Toby Maurer, who founded the HIV dermatology clinic at San Francisco General Hospital, talks about the toll Kaposi's sarcoma is taking on HIV patients.
We review the role of online dermatology videos in the education of medical students.
Then, reporter Mary Ellen Schneider highlights the top read stories on www.skinandallergynews.com, and Dr. Lily Talakoub talks about a condition that continues to plague adult patients - acne.
Lastly, Dr. Alan Rockoff relays a tale about children and warts.
In this month's program, Dermatologist Toby Maurer, who founded the HIV dermatology clinic at San Francisco General Hospital, talks about the toll Kaposi's sarcoma is taking on HIV patients.
We review the role of online dermatology videos in the education of medical students.
Then, reporter Mary Ellen Schneider highlights the top read stories on www.skinandallergynews.com, and Dr. Lily Talakoub talks about a condition that continues to plague adult patients - acne.
Lastly, Dr. Alan Rockoff relays a tale about children and warts.
Acne Prescriptions for Topical Combination Therapy Rising
BOSTON – Although there are proven clinical advantages to prescribing topical combination therapies for acne, the benefits must be weighed against the higher cost and loss of flexibility of the products, according to Dr. Laura F. Sandoval.
The use of "combination products allows physicians to adhere to current acne treatment guidelines," which can be complex, as they direct the use of multiple agents for optimal treatment, noted Dr. Sandoval and her colleagues. Combination products with benzoyl peroxide (BPO), however, help decrease the incidence of antibiotic resistance, which is a growing concern in the treatment of acne.
On the heels of a recent meta-analysis of studies comparing combination topical therapy to retinoid monotherapy for acne vulgaris, in which combination treatment was more effective than monotherapy in eight of the nine studies evaluated (J. Drugs Dermatol. 2011;10:636-44), Dr. Sandoval and her colleagues at the Center for Dermatology Research at Wake Forest University, Winston-Salem, N.C., sought to assess practice trends regarding the use of topical combination products in specialty and general practices.
The investigators used the National Ambulatory Medical Care Survey (NAMCS) database to identify acne visits from 1989 to 2009 and to compare prescribing practices between dermatologists and primary care physicians.
From 1989 to 2009, dermatologists and primary care physicians prescribed combination products to 11.5% and 12.6% of their acne patients, respectively, representing frequency increases during the same time period of 0.66% and 1.26%, Dr. Sandoval and her colleagues reported at the American Academy of Dermatology’s Summer Academy Meeting.
From 2007 to 2009, the respective prescription rates of combination products were 20% (dermatology) and 33.8% (primary care). The findings may be a reflection of clinicians’ desire to streamline patient management and improve treatment adherence – both of which can be compromised by the complexity of juggling topical drugs from multiple classes, Dr. Sandoval said in an interview.
During the 20-year period, topical retinoids were the most common treatment for acne and tretinoin was the top retinoid, accounting for 19.2% of prescriptions for acne from dermatologists and 17.9% from primary care physicians, Dr. Sandoval reported in a poster at the meeting. Two combination products – clindamycin/BPO and erythromycin/BPO – were among the top 10 products used by both dermatologists (4.7% and 6.3% of prescriptions, respectively) and primary care physicians (3.4% and 7.9%, respectively).
From 2007 to 2009, dermatologists prescribed clindamycin/BPO for 16.4% of acne visits and clindamycin/tretinoin for 3.9%, while primary care physicians prescribed clindamycin/BPO for 15.6% of acne visits, erythromycin/BPO for 14.6% of acne visits, and the oral contraceptive combination norgestimate/ethinyl estradiol for 8.6%, according to the analysis.
The Center for Dermatology Research at Wake Forest is supported by an educational grant from Galderma. The principal investigator, Dr. Steven R. Feldman, reported significant financial relationships with several pharmaceutical companies, including Galderma. Dr. Sandoval and her other coauthors reported no conflicts of interest.
). The findings may be a reflection of clinicians’ desire to streamline patient management and improve treatment adherence – both of which can be compromised by the complexity of juggling topical drugs from multiple classes, Dr. Sandoval said in an interview.
During the 20-year period, topical retinoids were the most common treatment for acne and tretinoin was the top retinoid, accounting for 19.2% of prescriptions for acne from dermatologists and 17.9% from primary care physicians, Dr. Sandoval reported in a poster at the meeting. Two combination products – clindamycin/BPO and erythromycin/BPO – were among the
BOSTON – Although there are proven clinical advantages to prescribing topical combination therapies for acne, the benefits must be weighed against the higher cost and loss of flexibility of the products, according to Dr. Laura F. Sandoval.
The use of "combination products allows physicians to adhere to current acne treatment guidelines," which can be complex, as they direct the use of multiple agents for optimal treatment, noted Dr. Sandoval and her colleagues. Combination products with benzoyl peroxide (BPO), however, help decrease the incidence of antibiotic resistance, which is a growing concern in the treatment of acne.
On the heels of a recent meta-analysis of studies comparing combination topical therapy to retinoid monotherapy for acne vulgaris, in which combination treatment was more effective than monotherapy in eight of the nine studies evaluated (J. Drugs Dermatol. 2011;10:636-44), Dr. Sandoval and her colleagues at the Center for Dermatology Research at Wake Forest University, Winston-Salem, N.C., sought to assess practice trends regarding the use of topical combination products in specialty and general practices.
The investigators used the National Ambulatory Medical Care Survey (NAMCS) database to identify acne visits from 1989 to 2009 and to compare prescribing practices between dermatologists and primary care physicians.
From 1989 to 2009, dermatologists and primary care physicians prescribed combination products to 11.5% and 12.6% of their acne patients, respectively, representing frequency increases during the same time period of 0.66% and 1.26%, Dr. Sandoval and her colleagues reported at the American Academy of Dermatology’s Summer Academy Meeting.
From 2007 to 2009, the respective prescription rates of combination products were 20% (dermatology) and 33.8% (primary care). The findings may be a reflection of clinicians’ desire to streamline patient management and improve treatment adherence – both of which can be compromised by the complexity of juggling topical drugs from multiple classes, Dr. Sandoval said in an interview.
During the 20-year period, topical retinoids were the most common treatment for acne and tretinoin was the top retinoid, accounting for 19.2% of prescriptions for acne from dermatologists and 17.9% from primary care physicians, Dr. Sandoval reported in a poster at the meeting. Two combination products – clindamycin/BPO and erythromycin/BPO – were among the top 10 products used by both dermatologists (4.7% and 6.3% of prescriptions, respectively) and primary care physicians (3.4% and 7.9%, respectively).
From 2007 to 2009, dermatologists prescribed clindamycin/BPO for 16.4% of acne visits and clindamycin/tretinoin for 3.9%, while primary care physicians prescribed clindamycin/BPO for 15.6% of acne visits, erythromycin/BPO for 14.6% of acne visits, and the oral contraceptive combination norgestimate/ethinyl estradiol for 8.6%, according to the analysis.
The Center for Dermatology Research at Wake Forest is supported by an educational grant from Galderma. The principal investigator, Dr. Steven R. Feldman, reported significant financial relationships with several pharmaceutical companies, including Galderma. Dr. Sandoval and her other coauthors reported no conflicts of interest.
BOSTON – Although there are proven clinical advantages to prescribing topical combination therapies for acne, the benefits must be weighed against the higher cost and loss of flexibility of the products, according to Dr. Laura F. Sandoval.
The use of "combination products allows physicians to adhere to current acne treatment guidelines," which can be complex, as they direct the use of multiple agents for optimal treatment, noted Dr. Sandoval and her colleagues. Combination products with benzoyl peroxide (BPO), however, help decrease the incidence of antibiotic resistance, which is a growing concern in the treatment of acne.
On the heels of a recent meta-analysis of studies comparing combination topical therapy to retinoid monotherapy for acne vulgaris, in which combination treatment was more effective than monotherapy in eight of the nine studies evaluated (J. Drugs Dermatol. 2011;10:636-44), Dr. Sandoval and her colleagues at the Center for Dermatology Research at Wake Forest University, Winston-Salem, N.C., sought to assess practice trends regarding the use of topical combination products in specialty and general practices.
The investigators used the National Ambulatory Medical Care Survey (NAMCS) database to identify acne visits from 1989 to 2009 and to compare prescribing practices between dermatologists and primary care physicians.
From 1989 to 2009, dermatologists and primary care physicians prescribed combination products to 11.5% and 12.6% of their acne patients, respectively, representing frequency increases during the same time period of 0.66% and 1.26%, Dr. Sandoval and her colleagues reported at the American Academy of Dermatology’s Summer Academy Meeting.
From 2007 to 2009, the respective prescription rates of combination products were 20% (dermatology) and 33.8% (primary care). The findings may be a reflection of clinicians’ desire to streamline patient management and improve treatment adherence – both of which can be compromised by the complexity of juggling topical drugs from multiple classes, Dr. Sandoval said in an interview.
During the 20-year period, topical retinoids were the most common treatment for acne and tretinoin was the top retinoid, accounting for 19.2% of prescriptions for acne from dermatologists and 17.9% from primary care physicians, Dr. Sandoval reported in a poster at the meeting. Two combination products – clindamycin/BPO and erythromycin/BPO – were among the top 10 products used by both dermatologists (4.7% and 6.3% of prescriptions, respectively) and primary care physicians (3.4% and 7.9%, respectively).
From 2007 to 2009, dermatologists prescribed clindamycin/BPO for 16.4% of acne visits and clindamycin/tretinoin for 3.9%, while primary care physicians prescribed clindamycin/BPO for 15.6% of acne visits, erythromycin/BPO for 14.6% of acne visits, and the oral contraceptive combination norgestimate/ethinyl estradiol for 8.6%, according to the analysis.
The Center for Dermatology Research at Wake Forest is supported by an educational grant from Galderma. The principal investigator, Dr. Steven R. Feldman, reported significant financial relationships with several pharmaceutical companies, including Galderma. Dr. Sandoval and her other coauthors reported no conflicts of interest.
). The findings may be a reflection of clinicians’ desire to streamline patient management and improve treatment adherence – both of which can be compromised by the complexity of juggling topical drugs from multiple classes, Dr. Sandoval said in an interview.
During the 20-year period, topical retinoids were the most common treatment for acne and tretinoin was the top retinoid, accounting for 19.2% of prescriptions for acne from dermatologists and 17.9% from primary care physicians, Dr. Sandoval reported in a poster at the meeting. Two combination products – clindamycin/BPO and erythromycin/BPO – were among the
). The findings may be a reflection of clinicians’ desire to streamline patient management and improve treatment adherence – both of which can be compromised by the complexity of juggling topical drugs from multiple classes, Dr. Sandoval said in an interview.
During the 20-year period, topical retinoids were the most common treatment for acne and tretinoin was the top retinoid, accounting for 19.2% of prescriptions for acne from dermatologists and 17.9% from primary care physicians, Dr. Sandoval reported in a poster at the meeting. Two combination products – clindamycin/BPO and erythromycin/BPO – were among the
AT THE AMERICAN ACADEMY OF DERMATOLOGY'S SUMMER ACADEMY MEETING
Major Finding: From 2007 to 2009, dermatologists and primary care physicians prescribed topical fixed combination treatment for 20% and 33.8% of their acne vulgaris patients, respectively.
Data Source: Analysis of data from the National Ambulatory Medical Care Survey on prescribing trends for the treatment of acne vulgaris for 1989-2009.
Disclosures: The Center for Dermatology Research at Wake Forest is supported by an educational grant from Galderma. The principal investigator, Dr. Steven R. Feldman, reported significant financial relationships with several pharmaceutical companies, including Galderma. Dr. Sandoval and her other coauthors reported no conflicts of interest.