User login
Acne Severity Grading Scale in the Works
A group of experts has identified what they believe to be essential clinical components of an ideal severity grading scale for acne vulgaris.
Although more than 25 systems are in existence for acne grading, there is neither a gold standard nor a standardized system consistently used in research or clinical practice. "The reasons for this are multiple, including differing needs of the clinical versus research paradigms, the persistence of simpler tools with inadequate accuracy, the inefficiency of research methods such as lesion counting, and a previous lack of consensus building in this area," lead author Dr. Jerry Tan, a dermatologist at the University of Western Ontario in London, said in an interview.
The panel of 12 acne experts determined that an ideal scale should include the clinical components of primary acne lesions; their quantity, extent, and facial and extrafacial sites of involvement; and features of clinimetric properties, categorization, efficiency, and acceptance.
This consensus is considered a first step toward the development of a new acne severity grading scale. In the meantime, "this information can best be used by practicing dermatologists as an initial phase in further identifying and developing a standard for acne severity grading in the future," said Dr. Tan.
The panel arrived at this consensus via the "Delphi method," in which each member responded to a three-phase, online, anonymous survey. In the first Delphi round, they were asked open-ended questions about what components and features would be essential to the scale, and whether any current scales included the components the member deemed essential and the features deemed important (J. Am. Acad. Dermatol. 2012;67:187-93 [doi: 10.1016/j.jaad.2011.09.005]).
In the first round, the group identified primary acne lesions (evaluation of inflammatory or noninflammatory lesions together or separately), secondary lesions (such as scarring or pigmentary changes), quantity of lesions, extrafacial sites of involvement, extent of involvement, and patient experiences as being essential clinical components. Features deemed important included clinimetric properties (such as validity and reproducibility), efficiency/ease of use, categorization of severity (i.e., based on descriptive text and/or photographic examples), and acceptance (by physicians, patients, and other stakeholders).
In the next round, panel members were asked to grade each component and feature on a seven-point scale, and to provide subcategories for inclusion.
In the final consensus, the group agreed that the scale should include separate evaluation of inflammatory and noninflammatory primary lesions; determination of the quantity of lesions by counting and numerical range; grading of extrafacial sites including the chest, back, neck, and shoulders; and determination of extent of involvement using proportion descriptors such as "one third or less."
The panel also came to a consensus on excluding patient experiences, while a slight majority also opted for excluding secondary lesions. In addition, a consensus was achieved for inclusion of the clinimetric properties (validity, reproducibility, discriminatory capacity, and responsivity), efficiency, acceptability, and categorization of severity.
The agreement to exclude patient experiences was a bit of a surprise, according to Dr. Tan. The finding may reflect the focus of the group on expert-determined severity, as well as the availability of quality-of-life scales that are particular to patient experience with acne and are routinely used in conjunction with clinician-based global acne severity assessments in clinical trials.
The group also agreed that while several current acne severity grading scales contain some of these elements, none contain all. For example, the eight-point severity grade scale by Allen and Smith includes type and quantity of lesions and proportion of facial involvement, but it is limited to the face (Arch. Dermatol. 1982;118:23-5). The ECLA (Echelle de Cotation des Lésions d’Acné) scale comprises numerical ranges of primary acne lesions, and includes extrafacial sites, but it does not include proportion descriptors of anatomical sites, and the scale has not been validated (Ann. Derm. Venereol. 1999;126:136-41), they wrote.
"The next steps are to identify current systems which meet at least some of the identified clinical components and features from the Delphi process. This may then facilitate development of the ideal acne grading tool for future clinical practice and research," Dr. Tan said in the interview.
Dr. Tan is an advisory board member, speaker, consultant, and/or investigator for Bayer, Cipher, and other companies. All but two of the other panel members also reported conflicts of interest.
A group of experts has identified what they believe to be essential clinical components of an ideal severity grading scale for acne vulgaris.
Although more than 25 systems are in existence for acne grading, there is neither a gold standard nor a standardized system consistently used in research or clinical practice. "The reasons for this are multiple, including differing needs of the clinical versus research paradigms, the persistence of simpler tools with inadequate accuracy, the inefficiency of research methods such as lesion counting, and a previous lack of consensus building in this area," lead author Dr. Jerry Tan, a dermatologist at the University of Western Ontario in London, said in an interview.
The panel of 12 acne experts determined that an ideal scale should include the clinical components of primary acne lesions; their quantity, extent, and facial and extrafacial sites of involvement; and features of clinimetric properties, categorization, efficiency, and acceptance.
This consensus is considered a first step toward the development of a new acne severity grading scale. In the meantime, "this information can best be used by practicing dermatologists as an initial phase in further identifying and developing a standard for acne severity grading in the future," said Dr. Tan.
The panel arrived at this consensus via the "Delphi method," in which each member responded to a three-phase, online, anonymous survey. In the first Delphi round, they were asked open-ended questions about what components and features would be essential to the scale, and whether any current scales included the components the member deemed essential and the features deemed important (J. Am. Acad. Dermatol. 2012;67:187-93 [doi: 10.1016/j.jaad.2011.09.005]).
In the first round, the group identified primary acne lesions (evaluation of inflammatory or noninflammatory lesions together or separately), secondary lesions (such as scarring or pigmentary changes), quantity of lesions, extrafacial sites of involvement, extent of involvement, and patient experiences as being essential clinical components. Features deemed important included clinimetric properties (such as validity and reproducibility), efficiency/ease of use, categorization of severity (i.e., based on descriptive text and/or photographic examples), and acceptance (by physicians, patients, and other stakeholders).
In the next round, panel members were asked to grade each component and feature on a seven-point scale, and to provide subcategories for inclusion.
In the final consensus, the group agreed that the scale should include separate evaluation of inflammatory and noninflammatory primary lesions; determination of the quantity of lesions by counting and numerical range; grading of extrafacial sites including the chest, back, neck, and shoulders; and determination of extent of involvement using proportion descriptors such as "one third or less."
The panel also came to a consensus on excluding patient experiences, while a slight majority also opted for excluding secondary lesions. In addition, a consensus was achieved for inclusion of the clinimetric properties (validity, reproducibility, discriminatory capacity, and responsivity), efficiency, acceptability, and categorization of severity.
The agreement to exclude patient experiences was a bit of a surprise, according to Dr. Tan. The finding may reflect the focus of the group on expert-determined severity, as well as the availability of quality-of-life scales that are particular to patient experience with acne and are routinely used in conjunction with clinician-based global acne severity assessments in clinical trials.
The group also agreed that while several current acne severity grading scales contain some of these elements, none contain all. For example, the eight-point severity grade scale by Allen and Smith includes type and quantity of lesions and proportion of facial involvement, but it is limited to the face (Arch. Dermatol. 1982;118:23-5). The ECLA (Echelle de Cotation des Lésions d’Acné) scale comprises numerical ranges of primary acne lesions, and includes extrafacial sites, but it does not include proportion descriptors of anatomical sites, and the scale has not been validated (Ann. Derm. Venereol. 1999;126:136-41), they wrote.
"The next steps are to identify current systems which meet at least some of the identified clinical components and features from the Delphi process. This may then facilitate development of the ideal acne grading tool for future clinical practice and research," Dr. Tan said in the interview.
Dr. Tan is an advisory board member, speaker, consultant, and/or investigator for Bayer, Cipher, and other companies. All but two of the other panel members also reported conflicts of interest.
A group of experts has identified what they believe to be essential clinical components of an ideal severity grading scale for acne vulgaris.
Although more than 25 systems are in existence for acne grading, there is neither a gold standard nor a standardized system consistently used in research or clinical practice. "The reasons for this are multiple, including differing needs of the clinical versus research paradigms, the persistence of simpler tools with inadequate accuracy, the inefficiency of research methods such as lesion counting, and a previous lack of consensus building in this area," lead author Dr. Jerry Tan, a dermatologist at the University of Western Ontario in London, said in an interview.
The panel of 12 acne experts determined that an ideal scale should include the clinical components of primary acne lesions; their quantity, extent, and facial and extrafacial sites of involvement; and features of clinimetric properties, categorization, efficiency, and acceptance.
This consensus is considered a first step toward the development of a new acne severity grading scale. In the meantime, "this information can best be used by practicing dermatologists as an initial phase in further identifying and developing a standard for acne severity grading in the future," said Dr. Tan.
The panel arrived at this consensus via the "Delphi method," in which each member responded to a three-phase, online, anonymous survey. In the first Delphi round, they were asked open-ended questions about what components and features would be essential to the scale, and whether any current scales included the components the member deemed essential and the features deemed important (J. Am. Acad. Dermatol. 2012;67:187-93 [doi: 10.1016/j.jaad.2011.09.005]).
In the first round, the group identified primary acne lesions (evaluation of inflammatory or noninflammatory lesions together or separately), secondary lesions (such as scarring or pigmentary changes), quantity of lesions, extrafacial sites of involvement, extent of involvement, and patient experiences as being essential clinical components. Features deemed important included clinimetric properties (such as validity and reproducibility), efficiency/ease of use, categorization of severity (i.e., based on descriptive text and/or photographic examples), and acceptance (by physicians, patients, and other stakeholders).
In the next round, panel members were asked to grade each component and feature on a seven-point scale, and to provide subcategories for inclusion.
In the final consensus, the group agreed that the scale should include separate evaluation of inflammatory and noninflammatory primary lesions; determination of the quantity of lesions by counting and numerical range; grading of extrafacial sites including the chest, back, neck, and shoulders; and determination of extent of involvement using proportion descriptors such as "one third or less."
The panel also came to a consensus on excluding patient experiences, while a slight majority also opted for excluding secondary lesions. In addition, a consensus was achieved for inclusion of the clinimetric properties (validity, reproducibility, discriminatory capacity, and responsivity), efficiency, acceptability, and categorization of severity.
The agreement to exclude patient experiences was a bit of a surprise, according to Dr. Tan. The finding may reflect the focus of the group on expert-determined severity, as well as the availability of quality-of-life scales that are particular to patient experience with acne and are routinely used in conjunction with clinician-based global acne severity assessments in clinical trials.
The group also agreed that while several current acne severity grading scales contain some of these elements, none contain all. For example, the eight-point severity grade scale by Allen and Smith includes type and quantity of lesions and proportion of facial involvement, but it is limited to the face (Arch. Dermatol. 1982;118:23-5). The ECLA (Echelle de Cotation des Lésions d’Acné) scale comprises numerical ranges of primary acne lesions, and includes extrafacial sites, but it does not include proportion descriptors of anatomical sites, and the scale has not been validated (Ann. Derm. Venereol. 1999;126:136-41), they wrote.
"The next steps are to identify current systems which meet at least some of the identified clinical components and features from the Delphi process. This may then facilitate development of the ideal acne grading tool for future clinical practice and research," Dr. Tan said in the interview.
Dr. Tan is an advisory board member, speaker, consultant, and/or investigator for Bayer, Cipher, and other companies. All but two of the other panel members also reported conflicts of interest.
FROM THE JOURNAL OF THE AMERICAN ACADEMY OF DERMATOLOGY
Derm Research Unsettles Ped's Treatment of Acne
BOSTON – Pediatricians treat preadolescent acne nearly as often as dermatologists but appear to be less comfortable with the use of topical retinoids, according to the results of a study of almost 55 million pediatric acne visits.
"This study identifies a significant knowledge gap among pediatricians, in terms of treatment of acne based on age of the patient. This is especially important in the preadolescent age group, since pediatricians treat acne in this population nearly as much as dermatologists," Dr. Laura F. Sandoval wrote in a poster presented at the American Academy of Dermatology’s Summer Academy Meeting.
Treatment by physicians in different specialties differed markedly. The younger the child, the more likely a pediatrician treated the acne; most (75.6%) neonatal or infantile acne was managed by a pediatrician. However, the older the child, the more likely a dermatologist treated the acne; slightly more than two-thirds (67.1%) of adolescent acne was managed by a dermatologist. Dermatologists and pediatricians almost equally managed preadolescent acne – 38.4% and 34.2%, respectively.
NAMCS (National Ambulatory Medical Care Survey) data were collected for outpatient visits of children receiving a diagnosis of acne vulgaris during 1993-2009. Patient visits were stratified by age groups: younger than 1 year (neonatal or infantile acne), 1-6 years (mid-childhood acne), 7-11 years (preadolescent acne), and 12-18 years (adolescent acne). Medications prescribed for each age group were compared across physician specialties.
There were almost 55 million estimated visits for patients aged 18 years and younger with a diagnosis of acne. Adolescent acne accounted for most of these visits (91.4%), followed by preadolescent visits (4.8%), mid-childhood visits (0.9%) and neonatal or infantile acne visits (3.0%).
Treatment of preadolescent and adolescent acne differed substantially between dermatologists and pediatricians/primary care physicians (PCPs), with prescribing differences being most pronounced in the preadolescent population. Topical retinoids were prescribed mainly by dermatologists in this age group, while oral antibiotics were preferred by pediatricians/PCPs.
"Comedonal acne is the most common type of acne in preadolescents and thus warrants the use of topical retinoids. Most PCPs have minimal dermatologic education and may be unaware of the benefits of retinoids," wrote Dr. Sandoval of the Center for Dermatology Research at Wake Forest University in Winston-Salem, N.C., and her coauthors.
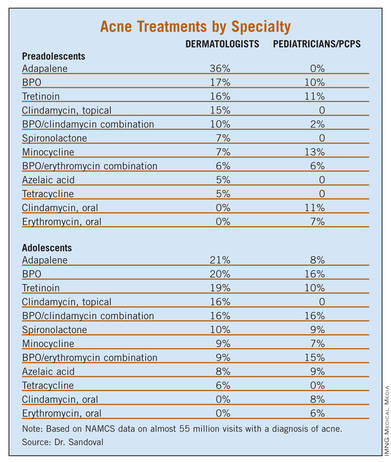
The most common treatment for preadolescent acne across all specialties was adapalene (14.4%), followed by benzoyl peroxide [BPO] (12.8%), tretinoin (12.5%), minocycline (10.4%), and a combination of BPO/erythromycin (8.1%). The most common treatment for adolescent acne was tretinoin (19.5%), followed by isotretinoin (18.1%), minocycline (16.9%), BPO (16.1%) and adapalene (14.1%).
Isotretinoin was the only medication commonly prescribed in adolescents but not in preadolescents, by both dermatologists and pediatricians/PCPs.
This could be because severe acne is typically rare in young children, according to Dr. Sandoval and her colleagues. And, severity is not recorded by the NAMCS, making it difficult to determine whether preadolescent children had severe enough acne to warrant the use of isotretinoin.
However, when topicals, BPO, and antibiotics fail, isotretinoin should be considered, the researchers noted. Also, isotretinoin should be considered in preadolescents when scarring is a concern.
While dermatologists prescribed isotretinoin and topical retinoids more frequently for adolescent acne than did pediatricians/PCPs, "Hesitancy to prescribe isotretinoin by PCPs may be due to strict requirements of federal monitoring programs, the need for monitoring blood work, and/or safety concerns," the authors noted.
Although tetracycline is the only Food and Drug Administration–approved drug for use in children aged 8 years and older, the data showed it was prescribed to an estimated 120,000 patients younger than 8 years, including children as young as 3 years. This practice was seen among both dermatologists and pediatricians. In all cases, it was used in conjunction with tretinoin, BPO, and/or topical clindamycin.
Minocycline is only FDA approved for use in patients 12 years and older. However, it was prescribed more often in younger patients than are doxycycline and tetracycline by both dermatologists and pediatricians/PCPs.
"All specialties recognize that off-label prescribing is necessary given the very limited range of treatment that is currently FDA-approved for preadolescent patients," the researchers wrote. "PCPs may have outdated concerns in regards to the efficacy and tolerability of retinoids, which is not supported by newer retinoid formulations."
The Center for Dermatology Research at Wake Forest is supported by an educational grant from Galderma. Principal investigator, Dr. Steven R. Feldman, reported significant financial relationships with several pharmaceutical companies, including Galderma. Dr. Sandoval and her other coauthors reported no conflicts of interest.
BOSTON – Pediatricians treat preadolescent acne nearly as often as dermatologists but appear to be less comfortable with the use of topical retinoids, according to the results of a study of almost 55 million pediatric acne visits.
"This study identifies a significant knowledge gap among pediatricians, in terms of treatment of acne based on age of the patient. This is especially important in the preadolescent age group, since pediatricians treat acne in this population nearly as much as dermatologists," Dr. Laura F. Sandoval wrote in a poster presented at the American Academy of Dermatology’s Summer Academy Meeting.
Treatment by physicians in different specialties differed markedly. The younger the child, the more likely a pediatrician treated the acne; most (75.6%) neonatal or infantile acne was managed by a pediatrician. However, the older the child, the more likely a dermatologist treated the acne; slightly more than two-thirds (67.1%) of adolescent acne was managed by a dermatologist. Dermatologists and pediatricians almost equally managed preadolescent acne – 38.4% and 34.2%, respectively.
NAMCS (National Ambulatory Medical Care Survey) data were collected for outpatient visits of children receiving a diagnosis of acne vulgaris during 1993-2009. Patient visits were stratified by age groups: younger than 1 year (neonatal or infantile acne), 1-6 years (mid-childhood acne), 7-11 years (preadolescent acne), and 12-18 years (adolescent acne). Medications prescribed for each age group were compared across physician specialties.
There were almost 55 million estimated visits for patients aged 18 years and younger with a diagnosis of acne. Adolescent acne accounted for most of these visits (91.4%), followed by preadolescent visits (4.8%), mid-childhood visits (0.9%) and neonatal or infantile acne visits (3.0%).
Treatment of preadolescent and adolescent acne differed substantially between dermatologists and pediatricians/primary care physicians (PCPs), with prescribing differences being most pronounced in the preadolescent population. Topical retinoids were prescribed mainly by dermatologists in this age group, while oral antibiotics were preferred by pediatricians/PCPs.
"Comedonal acne is the most common type of acne in preadolescents and thus warrants the use of topical retinoids. Most PCPs have minimal dermatologic education and may be unaware of the benefits of retinoids," wrote Dr. Sandoval of the Center for Dermatology Research at Wake Forest University in Winston-Salem, N.C., and her coauthors.
The most common treatment for preadolescent acne across all specialties was adapalene (14.4%), followed by benzoyl peroxide [BPO] (12.8%), tretinoin (12.5%), minocycline (10.4%), and a combination of BPO/erythromycin (8.1%). The most common treatment for adolescent acne was tretinoin (19.5%), followed by isotretinoin (18.1%), minocycline (16.9%), BPO (16.1%) and adapalene (14.1%).
Isotretinoin was the only medication commonly prescribed in adolescents but not in preadolescents, by both dermatologists and pediatricians/PCPs.

This could be because severe acne is typically rare in young children, according to Dr. Sandoval and her colleagues. And, severity is not recorded by the NAMCS, making it difficult to determine whether preadolescent children had severe enough acne to warrant the use of isotretinoin.
However, when topicals, BPO, and antibiotics fail, isotretinoin should be considered, the researchers noted. Also, isotretinoin should be considered in preadolescents when scarring is a concern.
While dermatologists prescribed isotretinoin and topical retinoids more frequently for adolescent acne than did pediatricians/PCPs, "Hesitancy to prescribe isotretinoin by PCPs may be due to strict requirements of federal monitoring programs, the need for monitoring blood work, and/or safety concerns," the authors noted.
Although tetracycline is the only Food and Drug Administration–approved drug for use in children aged 8 years and older, the data showed it was prescribed to an estimated 120,000 patients younger than 8 years, including children as young as 3 years. This practice was seen among both dermatologists and pediatricians. In all cases, it was used in conjunction with tretinoin, BPO, and/or topical clindamycin.
Minocycline is only FDA approved for use in patients 12 years and older. However, it was prescribed more often in younger patients than are doxycycline and tetracycline by both dermatologists and pediatricians/PCPs.
"All specialties recognize that off-label prescribing is necessary given the very limited range of treatment that is currently FDA-approved for preadolescent patients," the researchers wrote. "PCPs may have outdated concerns in regards to the efficacy and tolerability of retinoids, which is not supported by newer retinoid formulations."
The Center for Dermatology Research at Wake Forest is supported by an educational grant from Galderma. Principal investigator, Dr. Steven R. Feldman, reported significant financial relationships with several pharmaceutical companies, including Galderma. Dr. Sandoval and her other coauthors reported no conflicts of interest.
BOSTON – Pediatricians treat preadolescent acne nearly as often as dermatologists but appear to be less comfortable with the use of topical retinoids, according to the results of a study of almost 55 million pediatric acne visits.
"This study identifies a significant knowledge gap among pediatricians, in terms of treatment of acne based on age of the patient. This is especially important in the preadolescent age group, since pediatricians treat acne in this population nearly as much as dermatologists," Dr. Laura F. Sandoval wrote in a poster presented at the American Academy of Dermatology’s Summer Academy Meeting.
Treatment by physicians in different specialties differed markedly. The younger the child, the more likely a pediatrician treated the acne; most (75.6%) neonatal or infantile acne was managed by a pediatrician. However, the older the child, the more likely a dermatologist treated the acne; slightly more than two-thirds (67.1%) of adolescent acne was managed by a dermatologist. Dermatologists and pediatricians almost equally managed preadolescent acne – 38.4% and 34.2%, respectively.
NAMCS (National Ambulatory Medical Care Survey) data were collected for outpatient visits of children receiving a diagnosis of acne vulgaris during 1993-2009. Patient visits were stratified by age groups: younger than 1 year (neonatal or infantile acne), 1-6 years (mid-childhood acne), 7-11 years (preadolescent acne), and 12-18 years (adolescent acne). Medications prescribed for each age group were compared across physician specialties.
There were almost 55 million estimated visits for patients aged 18 years and younger with a diagnosis of acne. Adolescent acne accounted for most of these visits (91.4%), followed by preadolescent visits (4.8%), mid-childhood visits (0.9%) and neonatal or infantile acne visits (3.0%).
Treatment of preadolescent and adolescent acne differed substantially between dermatologists and pediatricians/primary care physicians (PCPs), with prescribing differences being most pronounced in the preadolescent population. Topical retinoids were prescribed mainly by dermatologists in this age group, while oral antibiotics were preferred by pediatricians/PCPs.
"Comedonal acne is the most common type of acne in preadolescents and thus warrants the use of topical retinoids. Most PCPs have minimal dermatologic education and may be unaware of the benefits of retinoids," wrote Dr. Sandoval of the Center for Dermatology Research at Wake Forest University in Winston-Salem, N.C., and her coauthors.
The most common treatment for preadolescent acne across all specialties was adapalene (14.4%), followed by benzoyl peroxide [BPO] (12.8%), tretinoin (12.5%), minocycline (10.4%), and a combination of BPO/erythromycin (8.1%). The most common treatment for adolescent acne was tretinoin (19.5%), followed by isotretinoin (18.1%), minocycline (16.9%), BPO (16.1%) and adapalene (14.1%).
Isotretinoin was the only medication commonly prescribed in adolescents but not in preadolescents, by both dermatologists and pediatricians/PCPs.

This could be because severe acne is typically rare in young children, according to Dr. Sandoval and her colleagues. And, severity is not recorded by the NAMCS, making it difficult to determine whether preadolescent children had severe enough acne to warrant the use of isotretinoin.
However, when topicals, BPO, and antibiotics fail, isotretinoin should be considered, the researchers noted. Also, isotretinoin should be considered in preadolescents when scarring is a concern.
While dermatologists prescribed isotretinoin and topical retinoids more frequently for adolescent acne than did pediatricians/PCPs, "Hesitancy to prescribe isotretinoin by PCPs may be due to strict requirements of federal monitoring programs, the need for monitoring blood work, and/or safety concerns," the authors noted.
Although tetracycline is the only Food and Drug Administration–approved drug for use in children aged 8 years and older, the data showed it was prescribed to an estimated 120,000 patients younger than 8 years, including children as young as 3 years. This practice was seen among both dermatologists and pediatricians. In all cases, it was used in conjunction with tretinoin, BPO, and/or topical clindamycin.
Minocycline is only FDA approved for use in patients 12 years and older. However, it was prescribed more often in younger patients than are doxycycline and tetracycline by both dermatologists and pediatricians/PCPs.
"All specialties recognize that off-label prescribing is necessary given the very limited range of treatment that is currently FDA-approved for preadolescent patients," the researchers wrote. "PCPs may have outdated concerns in regards to the efficacy and tolerability of retinoids, which is not supported by newer retinoid formulations."
The Center for Dermatology Research at Wake Forest is supported by an educational grant from Galderma. Principal investigator, Dr. Steven R. Feldman, reported significant financial relationships with several pharmaceutical companies, including Galderma. Dr. Sandoval and her other coauthors reported no conflicts of interest.
AT THE AMERICAN ACADEMY OF DERMATOLOGY'S SUMMER ACADEMY MEETING
Major Finding: Most (75.6%) neonatal and infantile acne was managed by a pediatrician, while most (67.1%) adolescent acne was managed by a dermatologist.
Data Source: NAMCS data was collected for outpatient visits by children receiving a diagnosis of acne vulgaris from 1993 to 2009.
Disclosures: The Center for Dermatology Research at Wake Forest is supported by an educational grant from Galderma. Principal investigator, Dr. Steven R. Feldman, reported significant financial relationships with several pharmaceutical companies, including Galderma. Dr. Sandoval and her other coauthors reported no conflicts of interest.
Is it Acne or Is it Rosacea? An Important Distinction
What Is Your Diagnosis? Demodex Folliculitis
Drug Samples Found to Sway Acne Prescribing
RALEIGH, N.C. – Offering free drug samples to newly diagnosed acne patients was found to increase the likelihood of prescribing more expensive medications, according to a new study.
"The benefits of free sample distribution in dermatology clinics must be weighed against the significant negative impact that free samples have on prescribing and prescription costs. Clinical trials comparing the efficacy of new branded generic drugs with existing alternatives should increasingly be used to justify their increased retail cost," said study investigator Michael Hurley, a Stanford (Calif.) University medical student.
The investigators analyzed all prescriptions written for newly diagnosed acne patients in 2010 at two dermatology clinics, one in an academic medical center that does not allow samples, and the second at an affiliated neighborhood clinic that does.
At the no-samples clinic, 17% of prescriptions written by office-based dermatologists for acne patients at their initial office visit were for branded or branded-generic drugs, compared with a 74% rate at the neighborhood clinic allowing free samples.
The average prescription costs were also higher at the clinic that uses free samples. The average retail cost of the top 20 prescribed acne medications at each site (which collectively accounted for roughly 70% of all acne prescriptions) was $204 at the neighborhood clinic, compared with $70.49 at the clinic with no free samples. After the average number of prescriptions written per visit was taken into account, this amounted to a cost difference of about $260 per office visit, reported Mr. Hurley.
In a multivariate regression analysis accounting for patient characteristics and other potential confounding factors, dermatologists who provided free samples of acne drugs were 3.4-fold more likely to prescribe a branded or branded generic drug than a less expensive generic.
After identifying the marked difference in dermatologists’ acne drug prescribing patterns at the two clinics in Northern California, Mr. Hurley and his coinvestigators analyzed national data to establish trends.
Data from the National Disease and Therapeutic Index – a national survey of physician self-reported office visits, diagnoses, and treatments conducted by IMS Health – were assessed. The data showed that in 2010, 79% of all prescriptions written by office-based dermatologists for acne patients at their initial visit were for branded or branded-generic drugs.
The data also found that the proportion of acne prescriptions written with a free sample increased from 38% to 51% in the last decade. Prescriptions for branded generic medications rose similarly, most likely because of the increased use of free samples of those drugs, said Mr. Hurley. Meanwhile the percentage of acne prescriptions for generic drugs has remained flat, and in absolute numbers has actually decreased, he said.
Upon close scrutiny of dermatologists’ acne prescribing nationally in 2010, 2005, and 2001, Mr. Hurley concluded that although dermatologists’ drug preferences do change over time as new medications enter the market, their prescribing consistently remains closely related to what’s available as a free sample.
For example, during 2010 the top five acne medications prescribed by dermatologists were (in descending order) Epiduo, doxycycline hyclate, Metrogel, Solodyn, and Differin. The top five prescribed with a free sample were Epiduo, Metrogel, Solodyn, Ziana, and Oracea.
The top five agents prescribed overall in 2005 were Differin, Benzaclin, Duac, Retin-A-Micro, and doxycycline hyclate, whereas the top five prescribed with a free sample during that year were Differin, Duac, Benzaclin, Retin-A-Micro, and Metrogel.
Mr. Hurley reported having no financial conflicts.
RALEIGH, N.C. – Offering free drug samples to newly diagnosed acne patients was found to increase the likelihood of prescribing more expensive medications, according to a new study.
"The benefits of free sample distribution in dermatology clinics must be weighed against the significant negative impact that free samples have on prescribing and prescription costs. Clinical trials comparing the efficacy of new branded generic drugs with existing alternatives should increasingly be used to justify their increased retail cost," said study investigator Michael Hurley, a Stanford (Calif.) University medical student.
The investigators analyzed all prescriptions written for newly diagnosed acne patients in 2010 at two dermatology clinics, one in an academic medical center that does not allow samples, and the second at an affiliated neighborhood clinic that does.
At the no-samples clinic, 17% of prescriptions written by office-based dermatologists for acne patients at their initial office visit were for branded or branded-generic drugs, compared with a 74% rate at the neighborhood clinic allowing free samples.
The average prescription costs were also higher at the clinic that uses free samples. The average retail cost of the top 20 prescribed acne medications at each site (which collectively accounted for roughly 70% of all acne prescriptions) was $204 at the neighborhood clinic, compared with $70.49 at the clinic with no free samples. After the average number of prescriptions written per visit was taken into account, this amounted to a cost difference of about $260 per office visit, reported Mr. Hurley.
In a multivariate regression analysis accounting for patient characteristics and other potential confounding factors, dermatologists who provided free samples of acne drugs were 3.4-fold more likely to prescribe a branded or branded generic drug than a less expensive generic.
After identifying the marked difference in dermatologists’ acne drug prescribing patterns at the two clinics in Northern California, Mr. Hurley and his coinvestigators analyzed national data to establish trends.
Data from the National Disease and Therapeutic Index – a national survey of physician self-reported office visits, diagnoses, and treatments conducted by IMS Health – were assessed. The data showed that in 2010, 79% of all prescriptions written by office-based dermatologists for acne patients at their initial visit were for branded or branded-generic drugs.
The data also found that the proportion of acne prescriptions written with a free sample increased from 38% to 51% in the last decade. Prescriptions for branded generic medications rose similarly, most likely because of the increased use of free samples of those drugs, said Mr. Hurley. Meanwhile the percentage of acne prescriptions for generic drugs has remained flat, and in absolute numbers has actually decreased, he said.
Upon close scrutiny of dermatologists’ acne prescribing nationally in 2010, 2005, and 2001, Mr. Hurley concluded that although dermatologists’ drug preferences do change over time as new medications enter the market, their prescribing consistently remains closely related to what’s available as a free sample.
For example, during 2010 the top five acne medications prescribed by dermatologists were (in descending order) Epiduo, doxycycline hyclate, Metrogel, Solodyn, and Differin. The top five prescribed with a free sample were Epiduo, Metrogel, Solodyn, Ziana, and Oracea.
The top five agents prescribed overall in 2005 were Differin, Benzaclin, Duac, Retin-A-Micro, and doxycycline hyclate, whereas the top five prescribed with a free sample during that year were Differin, Duac, Benzaclin, Retin-A-Micro, and Metrogel.
Mr. Hurley reported having no financial conflicts.
RALEIGH, N.C. – Offering free drug samples to newly diagnosed acne patients was found to increase the likelihood of prescribing more expensive medications, according to a new study.
"The benefits of free sample distribution in dermatology clinics must be weighed against the significant negative impact that free samples have on prescribing and prescription costs. Clinical trials comparing the efficacy of new branded generic drugs with existing alternatives should increasingly be used to justify their increased retail cost," said study investigator Michael Hurley, a Stanford (Calif.) University medical student.
The investigators analyzed all prescriptions written for newly diagnosed acne patients in 2010 at two dermatology clinics, one in an academic medical center that does not allow samples, and the second at an affiliated neighborhood clinic that does.
At the no-samples clinic, 17% of prescriptions written by office-based dermatologists for acne patients at their initial office visit were for branded or branded-generic drugs, compared with a 74% rate at the neighborhood clinic allowing free samples.
The average prescription costs were also higher at the clinic that uses free samples. The average retail cost of the top 20 prescribed acne medications at each site (which collectively accounted for roughly 70% of all acne prescriptions) was $204 at the neighborhood clinic, compared with $70.49 at the clinic with no free samples. After the average number of prescriptions written per visit was taken into account, this amounted to a cost difference of about $260 per office visit, reported Mr. Hurley.
In a multivariate regression analysis accounting for patient characteristics and other potential confounding factors, dermatologists who provided free samples of acne drugs were 3.4-fold more likely to prescribe a branded or branded generic drug than a less expensive generic.
After identifying the marked difference in dermatologists’ acne drug prescribing patterns at the two clinics in Northern California, Mr. Hurley and his coinvestigators analyzed national data to establish trends.
Data from the National Disease and Therapeutic Index – a national survey of physician self-reported office visits, diagnoses, and treatments conducted by IMS Health – were assessed. The data showed that in 2010, 79% of all prescriptions written by office-based dermatologists for acne patients at their initial visit were for branded or branded-generic drugs.
The data also found that the proportion of acne prescriptions written with a free sample increased from 38% to 51% in the last decade. Prescriptions for branded generic medications rose similarly, most likely because of the increased use of free samples of those drugs, said Mr. Hurley. Meanwhile the percentage of acne prescriptions for generic drugs has remained flat, and in absolute numbers has actually decreased, he said.
Upon close scrutiny of dermatologists’ acne prescribing nationally in 2010, 2005, and 2001, Mr. Hurley concluded that although dermatologists’ drug preferences do change over time as new medications enter the market, their prescribing consistently remains closely related to what’s available as a free sample.
For example, during 2010 the top five acne medications prescribed by dermatologists were (in descending order) Epiduo, doxycycline hyclate, Metrogel, Solodyn, and Differin. The top five prescribed with a free sample were Epiduo, Metrogel, Solodyn, Ziana, and Oracea.
The top five agents prescribed overall in 2005 were Differin, Benzaclin, Duac, Retin-A-Micro, and doxycycline hyclate, whereas the top five prescribed with a free sample during that year were Differin, Duac, Benzaclin, Retin-A-Micro, and Metrogel.
Mr. Hurley reported having no financial conflicts.
AT THE ANNUAL MEETING OF THE SOCIETY FOR INVESTIGATIVE DERMATOLOGY
Major Finding: At the no-samples clinic, 17% of prescriptions written by office-based dermatologists for acne patients at their initial office visit were for branded or branded-generic drugs, compared with a 74% rate at the neighborhood clinic allowing free samples.
Data Source: All prescriptions written for newly diagnosed acne patients in 2010 at two dermatology clinics were analyzed, one in an academic medical center that does not allow samples, and the second at an affiliated neighborhood clinic that does.
Disclosures: Mr. Hurley reported having no financial conflicts.
Acne and Rosacea: Epidemiology, Diagnosis, and Treatment [book review]
Ziana Proves Less Irritating Than Epiduo for Acne
RALEIGH, N.C. - Topical clindamycin phosphate 1.2%/tretinoin 0.025% gel was found to be significantly less irritating than benzoyl peroxide 2.5%/adapalene 0.1% gel was during the first 3 weeks of acne therapy, according to a double-blind, randomized, split-face comparative trial.
However, it should be noted that at all times, study participants scored their irritation as moderate or less with both medications. “Both products are pretty well tolerated. Nevertheless, looking at patient outcomes, clindamycin/tretinoin [Ziana]does seem to be less irritating than benzoyl peroxide/adapalene [Epiduo], which could be of clinical significance because it might [affect] patient compliance,” noted Dr. Renato Goreshi of the department of dermatology at the Oregon Health and Science University, Portland.
He presented a double-blind study involving 24 patients with mild to moderate facial acne at the annual meeting of the Society for Investigative Dermatology. All were white, and most were in their mid-20s.
Participants applied clindamycin/tretinoin to one side of their face and benzoyl peroxide/adapalene to the other side once daily for 3 weeks. They kept a daily record scoring burning/stinging and itching on a 0-3 scale. In addition, investigators measured transepidermal water loss and conducted Investigator’s Global Assessments of dryness/scaling and erythema at baseline and weekly thereafter.
The primary study outcome was transepidermal water loss, a reliable and validated measure of epidermal barrier disruption. Transepidermal water loss was significantly greater at weeks 1, 2, and 3 on the benzoyl peroxide/adapalene–treated side of the face.
The benzoyl peroxide/adapalene–treated side also scored threefold higher on the patients’ self-assessed rating of burning/stinging, and twice as high on itching.
“This isn’t entirely surprising, since in studies in atopic dermatitis we also see that there’s a correlation between higher transepidermal water-loss scores and increased pruritus, which is what we saw here,” Dr. Goreshi said.
The difference in composite irritancy scores was larger in the first half of the study than in the second half, when scores headed toward convergence.
Investigators found no significant difference between the two products in erythema or dryness/scaling.
There was also no significant difference between the two topical agents in terms of reduction of acne lesion counts. However, one wouldn’t expect to see significant differences during this short of a study, which was designed to look at irritancy, not efficacy, Dr. Goreshi noted.
The study was funded by Medicis, which markets Ziana.
RALEIGH, N.C. - Topical clindamycin phosphate 1.2%/tretinoin 0.025% gel was found to be significantly less irritating than benzoyl peroxide 2.5%/adapalene 0.1% gel was during the first 3 weeks of acne therapy, according to a double-blind, randomized, split-face comparative trial.
However, it should be noted that at all times, study participants scored their irritation as moderate or less with both medications. “Both products are pretty well tolerated. Nevertheless, looking at patient outcomes, clindamycin/tretinoin [Ziana]does seem to be less irritating than benzoyl peroxide/adapalene [Epiduo], which could be of clinical significance because it might [affect] patient compliance,” noted Dr. Renato Goreshi of the department of dermatology at the Oregon Health and Science University, Portland.
He presented a double-blind study involving 24 patients with mild to moderate facial acne at the annual meeting of the Society for Investigative Dermatology. All were white, and most were in their mid-20s.
Participants applied clindamycin/tretinoin to one side of their face and benzoyl peroxide/adapalene to the other side once daily for 3 weeks. They kept a daily record scoring burning/stinging and itching on a 0-3 scale. In addition, investigators measured transepidermal water loss and conducted Investigator’s Global Assessments of dryness/scaling and erythema at baseline and weekly thereafter.
The primary study outcome was transepidermal water loss, a reliable and validated measure of epidermal barrier disruption. Transepidermal water loss was significantly greater at weeks 1, 2, and 3 on the benzoyl peroxide/adapalene–treated side of the face.
The benzoyl peroxide/adapalene–treated side also scored threefold higher on the patients’ self-assessed rating of burning/stinging, and twice as high on itching.
“This isn’t entirely surprising, since in studies in atopic dermatitis we also see that there’s a correlation between higher transepidermal water-loss scores and increased pruritus, which is what we saw here,” Dr. Goreshi said.
The difference in composite irritancy scores was larger in the first half of the study than in the second half, when scores headed toward convergence.
Investigators found no significant difference between the two products in erythema or dryness/scaling.
There was also no significant difference between the two topical agents in terms of reduction of acne lesion counts. However, one wouldn’t expect to see significant differences during this short of a study, which was designed to look at irritancy, not efficacy, Dr. Goreshi noted.
The study was funded by Medicis, which markets Ziana.
RALEIGH, N.C. - Topical clindamycin phosphate 1.2%/tretinoin 0.025% gel was found to be significantly less irritating than benzoyl peroxide 2.5%/adapalene 0.1% gel was during the first 3 weeks of acne therapy, according to a double-blind, randomized, split-face comparative trial.
However, it should be noted that at all times, study participants scored their irritation as moderate or less with both medications. “Both products are pretty well tolerated. Nevertheless, looking at patient outcomes, clindamycin/tretinoin [Ziana]does seem to be less irritating than benzoyl peroxide/adapalene [Epiduo], which could be of clinical significance because it might [affect] patient compliance,” noted Dr. Renato Goreshi of the department of dermatology at the Oregon Health and Science University, Portland.
He presented a double-blind study involving 24 patients with mild to moderate facial acne at the annual meeting of the Society for Investigative Dermatology. All were white, and most were in their mid-20s.
Participants applied clindamycin/tretinoin to one side of their face and benzoyl peroxide/adapalene to the other side once daily for 3 weeks. They kept a daily record scoring burning/stinging and itching on a 0-3 scale. In addition, investigators measured transepidermal water loss and conducted Investigator’s Global Assessments of dryness/scaling and erythema at baseline and weekly thereafter.
The primary study outcome was transepidermal water loss, a reliable and validated measure of epidermal barrier disruption. Transepidermal water loss was significantly greater at weeks 1, 2, and 3 on the benzoyl peroxide/adapalene–treated side of the face.
The benzoyl peroxide/adapalene–treated side also scored threefold higher on the patients’ self-assessed rating of burning/stinging, and twice as high on itching.
“This isn’t entirely surprising, since in studies in atopic dermatitis we also see that there’s a correlation between higher transepidermal water-loss scores and increased pruritus, which is what we saw here,” Dr. Goreshi said.
The difference in composite irritancy scores was larger in the first half of the study than in the second half, when scores headed toward convergence.
Investigators found no significant difference between the two products in erythema or dryness/scaling.
There was also no significant difference between the two topical agents in terms of reduction of acne lesion counts. However, one wouldn’t expect to see significant differences during this short of a study, which was designed to look at irritancy, not efficacy, Dr. Goreshi noted.
The study was funded by Medicis, which markets Ziana.
FROM THE ANNUAL MEETING OF THE SOCIETY FOR INVESTIGATIVE DERMATOLOGY
Major Finding: Transepidermal water loss was significantly greater at weeks 1, 2, and 3 on the benzoyl peroxide/adapalene–treated side of the face.
Data Source: Results were taken from a double-blind, randomized, split-face comparative trial involving 24 patients with mild to moderate facial acne.
Disclosures: The study was sponsored by Medicis.
Red Light for Acne
Several light devices based on red light–emitting diodes (LEDs) have made their way to the market in recent years. Although red light is not truly a cosmeceutical, it is a significant emerging adjuvant therapy that, like blue light, has been studied in comparison to and in conjunction with topical options primarily to treat acne. Of course, acne is the most common skin disorder prompting visits to the dermatologist, with an estimated 85% of adolescents affected, many into adulthood (J. Am. Acad. Dermatol. 2008;58:56-9).
This discussion will consider red light when used alone and when used in combination with blue light. Blue light is the most effective light to target Propionibacterium acnes (specifically at wavelengths of 407-420 nm). However, many devices are utilizing red light because it has a purported anti-inflammatory effect and penetrates deeper into the skin (Dermatol. Ther. 2005;18:253-66).
Erythrasma
Darras-Vercambre et al. evaluated the effects of red light for the treatment of erythrasma (a superficial skin infection provoked by Corynebacterium minutissimum) in 13 patients. One treatment (80 J/cm2) by red light (broadband, peak at 635 nm) without exogenous photosensitizing molecules was administered to each subject. Therapy was effective and well tolerated, with three patients experiencing complete recovery, and significant reduction of lesions in most other cases. The authors noted that the key to their study, given the absence of an exogenous photosensitizing agent, was capitalizing on the presence of porphyrins in the lesions. They concluded that the use of red light alone for this localized infection is easy and inexpensive, but that an optimal method has not yet been established (Photodermatol. Photoimmunol. Photomed. 2006;22:153-6).
Acne
In 2007, Na and Suh evaluated the efficacy of red light phototherapy with a portable device in 28 volunteers with mild to moderate acne in a split-face randomized trial. Phototherapy was performed twice daily for 15 minutes for a total of 8 weeks to one side of the face. The investigators concluded that red light phototherapy alone is an effective therapeutic option for acne, as they noted significant reductions in noninflammatory and inflammatory lesion counts on the treated side versus the untreated side, a drop from 3.9 to 1.9 in the visual analog scale on the treatment side, and significant disparities between the treatment and control sides after 8 weeks (Dermatol. Surg. 2007;33:1228-33, discussion 1233).
A 2006 article in the British Journal of Dermatology reported good clinical results from acne treatment with photodynamic therapy (PDT) using methyl aminolevulinate (MAL) and red light, but there were adverse side effects that prompted 7 of 19 subjects to discontinue the study (Br. J. Dermatol. 2006;154:969-76). In response to the study, Mavilia et al. wrote a letter to the journal acknowledging their more effective combination therapy using a lower concentration of MAL and low doses of red light. All 16 patients completed the study, in which the count of inflammatory lesions fell an average of 66% with mild but tolerable side effects, including a subtle sensation of heat, then minimal erythema during the procedure and slight scaling that began 3 days after treatment (Br. J. Dermatol. 2007;157:810-1).
In a small study of patients with moderate facial acne, Zane et al. exposed 15 women to 20 J/cm2 of broadband red light (600-750 nm) twice weekly for 4 weeks. They also measured skin sebum, pH, hydration, and transepidermal water loss (TEWL). Untreated lesions of the trunk served as controls. The investigators found the treatment safe, well tolerated, and effective, with significant improvement in acne lesions and reduction of sebum excretion and TEWL after 4 weeks of therapy and at the 3-month follow-up visit. They speculated that the improvement was due to the decreased colonization of P. acnes, decimated by photoactivated endogenous porphyrin, and concluded that this inexpensive therapy warrants inclusion among treatment options for moderate acne (Photodermatol. Photoimmunol. Photomed. 2008;24:244-8).
In a 2009 study of 19 patients with moderate to severe facial acne who received a single treatment of low-dose, red-light PDT on the left cheek and MAL 3 hours before red light on the right cheek, both therapies yielded significant reductions in acne score. Red light was found to be as effective as MAL-PDT (Acta Derm. Venereol. 2009;89:372-8).
Combined Blue and Red Light Phototherapy
In 2006, Goldberg and Russell evaluated the combination of blue (415 nm) and red (633 nm) LED phototherapy in 24 patients with Fitzpatrick skin types II-V and mild to severe symmetric facial acne. Twenty-two patients completed the trial, which included two sessions per week (separated by 3 days) alternating between blue and red light for a total of eight sessions. Mild microdermabrasion was used at the start of each session. The mean decrease in lesion count was significant after 4 weeks (46%) and 12 weeks (81%). Inflammatory lesions responded better than did noninflammatory ones, and severe acne responded slightly better than mild acne. The investigators concluded that the combination of blue and red LED phototherapy is free of side effects and pain, and exhibits great potential for the treatment of mild to severe acne (J. Cosmet. Laser Ther. 2006;8:71-5).
In 2007, Lee et al. set out to examine the efficacy of combining blue and red LED phototherapy for acne in a study of 24 patients with mild to moderately severe facial acne. Twice weekly for 4 weeks, patients were treated with quasi-monochromatic LED devices, alternating blue (415 nm) and red (633 nm) light. Fourteen patients self-reported improvements in skin tone and texture. Improvements in noninflammatory and inflammatory lesions were substantial (34.28% and 77.93%, respectively). The researchers concluded that combined blue and red LED phototherapy is a safe and effective option, especially for papulopustular acne (Lasers Surg. Med. 2007;39:180-8).
In 2009, Sadick evaluated the efficacy of the combination of blue (415 nm) and near-infrared (830 nm) LED therapy for moderate acne in 13 females and 4 males ranging in skin type from II to VI and in Burton acne grade at baseline from 1 to 5. Twice-weekly 20-minute sessions were conducted for 4 weeks, alternating between blue and near-infrared light. Eleven patients exhibited improvement ranging from 0% to 83.3%, and 6 patients discontinued the study. A decreasing trend was observed in the Burton grade. Noninflammatory lesion counts improved in seven patients but increased in four. Sadick noted that these results paled in comparison to the effectiveness of the blue and red combination at lowering inflammatory lesions seen previously, but encouraged the study of the combination phototherapy in a much larger population (J. Cosmet. Laser Ther. 2009;11:125-8).
Several recent reviews have found that red light–activated MAL-PDT, the combination of blue and red light, and aminolevulinic acid as a photosensitizing agent before treatment with blue light, red light, or the 595-nm pulsed dye laser are among the most promising evidence-based laser- and light-based therapies for acne (Semin. Cutan. Med. Surg. 2008;27:207-11; J. Eur. Acad. Dermatol. Venereol. 2008;22:267-78; Dermatol. Surg. 2007;33:1005-26).
In a systematic literature review of randomized controlled trials of light and laser therapies for acne vulgaris (using the Cochrane Central Register of Controlled Trials, MEDLINE, Embase, CINAHL, PsycINFO, LILACS, ISI Science Citation Index, and Dissertation Abstracts International), Hamilton et al. found that trials of blue light, blue-red light, and infrared radiation were more successful, especially when multiple treatments were used. Notably, blue-red light demonstrated better short-term effectiveness than did topical 5% benzoyl peroxide cream (Br. J. Dermatol. 2009;160:1273-85).
Kim and Armstrong have noted that blue light has been demonstrated to photoinactivate P. acnes, but it does not penetrate deeply into the skin. It is believed to work synergistically, however, with red light, which is less effective than blue light at exciting porphyrins but can reach deeper sebaceous glands and may impart an anti-inflammatory effect by inciting cytokine release from macrophages (Dermatol. Surg. 2007;33:1005-26). Indeed, Kim and Armstrong found that combined blue-red light therapy was more effective at lowering the number of inflammatory acne lesions than were benzoyl peroxide monotherapy and blue light monotherapy (Lasers Surg. Med. 1989;9:497-505).
Conclusions
A lengthy review of the literature and personal experience treating patients have convinced me that blue light is an effective treatment for acne. P. acnes is most susceptible to the blue light wavelengths of 407-420 nm. Addition of red light may help speed resolution of inflammatory lesions through an anti-inflammatory effect. Blue and red light devices are efficacious when used in the office if the devices deliver enough joules.
Many at-home devices and iPhone apps have hit the market. These are a great alternative to irritating topicals and antibiotics, and they may help increase compliance. However, many at-home light devices are too weak (do not emit enough joules), or emit a broad range of light (rather than 407-420 nm). The manufacturers of some of these products claim that the heat produced by the devices improves acne, but there is a paucity of research proving this point. In my opinion, using an at-home device twice a day that delivers 407-420 nm (with or without the addition of red light), and delivers enough joules (at least 25 J/cm2), is an effective method of treating acne. For comparison purposes, the in-office Omnilux delivers around 49 J/cm2 but is used only two or three times per week. Know your wavelengths and joules when trying to decide which device to sell in your practice or recommend to patients.
Several light devices based on red light–emitting diodes (LEDs) have made their way to the market in recent years. Although red light is not truly a cosmeceutical, it is a significant emerging adjuvant therapy that, like blue light, has been studied in comparison to and in conjunction with topical options primarily to treat acne. Of course, acne is the most common skin disorder prompting visits to the dermatologist, with an estimated 85% of adolescents affected, many into adulthood (J. Am. Acad. Dermatol. 2008;58:56-9).
This discussion will consider red light when used alone and when used in combination with blue light. Blue light is the most effective light to target Propionibacterium acnes (specifically at wavelengths of 407-420 nm). However, many devices are utilizing red light because it has a purported anti-inflammatory effect and penetrates deeper into the skin (Dermatol. Ther. 2005;18:253-66).
Erythrasma
Darras-Vercambre et al. evaluated the effects of red light for the treatment of erythrasma (a superficial skin infection provoked by Corynebacterium minutissimum) in 13 patients. One treatment (80 J/cm2) by red light (broadband, peak at 635 nm) without exogenous photosensitizing molecules was administered to each subject. Therapy was effective and well tolerated, with three patients experiencing complete recovery, and significant reduction of lesions in most other cases. The authors noted that the key to their study, given the absence of an exogenous photosensitizing agent, was capitalizing on the presence of porphyrins in the lesions. They concluded that the use of red light alone for this localized infection is easy and inexpensive, but that an optimal method has not yet been established (Photodermatol. Photoimmunol. Photomed. 2006;22:153-6).
Acne
In 2007, Na and Suh evaluated the efficacy of red light phototherapy with a portable device in 28 volunteers with mild to moderate acne in a split-face randomized trial. Phototherapy was performed twice daily for 15 minutes for a total of 8 weeks to one side of the face. The investigators concluded that red light phototherapy alone is an effective therapeutic option for acne, as they noted significant reductions in noninflammatory and inflammatory lesion counts on the treated side versus the untreated side, a drop from 3.9 to 1.9 in the visual analog scale on the treatment side, and significant disparities between the treatment and control sides after 8 weeks (Dermatol. Surg. 2007;33:1228-33, discussion 1233).
A 2006 article in the British Journal of Dermatology reported good clinical results from acne treatment with photodynamic therapy (PDT) using methyl aminolevulinate (MAL) and red light, but there were adverse side effects that prompted 7 of 19 subjects to discontinue the study (Br. J. Dermatol. 2006;154:969-76). In response to the study, Mavilia et al. wrote a letter to the journal acknowledging their more effective combination therapy using a lower concentration of MAL and low doses of red light. All 16 patients completed the study, in which the count of inflammatory lesions fell an average of 66% with mild but tolerable side effects, including a subtle sensation of heat, then minimal erythema during the procedure and slight scaling that began 3 days after treatment (Br. J. Dermatol. 2007;157:810-1).
In a small study of patients with moderate facial acne, Zane et al. exposed 15 women to 20 J/cm2 of broadband red light (600-750 nm) twice weekly for 4 weeks. They also measured skin sebum, pH, hydration, and transepidermal water loss (TEWL). Untreated lesions of the trunk served as controls. The investigators found the treatment safe, well tolerated, and effective, with significant improvement in acne lesions and reduction of sebum excretion and TEWL after 4 weeks of therapy and at the 3-month follow-up visit. They speculated that the improvement was due to the decreased colonization of P. acnes, decimated by photoactivated endogenous porphyrin, and concluded that this inexpensive therapy warrants inclusion among treatment options for moderate acne (Photodermatol. Photoimmunol. Photomed. 2008;24:244-8).
In a 2009 study of 19 patients with moderate to severe facial acne who received a single treatment of low-dose, red-light PDT on the left cheek and MAL 3 hours before red light on the right cheek, both therapies yielded significant reductions in acne score. Red light was found to be as effective as MAL-PDT (Acta Derm. Venereol. 2009;89:372-8).
Combined Blue and Red Light Phototherapy
In 2006, Goldberg and Russell evaluated the combination of blue (415 nm) and red (633 nm) LED phototherapy in 24 patients with Fitzpatrick skin types II-V and mild to severe symmetric facial acne. Twenty-two patients completed the trial, which included two sessions per week (separated by 3 days) alternating between blue and red light for a total of eight sessions. Mild microdermabrasion was used at the start of each session. The mean decrease in lesion count was significant after 4 weeks (46%) and 12 weeks (81%). Inflammatory lesions responded better than did noninflammatory ones, and severe acne responded slightly better than mild acne. The investigators concluded that the combination of blue and red LED phototherapy is free of side effects and pain, and exhibits great potential for the treatment of mild to severe acne (J. Cosmet. Laser Ther. 2006;8:71-5).
In 2007, Lee et al. set out to examine the efficacy of combining blue and red LED phototherapy for acne in a study of 24 patients with mild to moderately severe facial acne. Twice weekly for 4 weeks, patients were treated with quasi-monochromatic LED devices, alternating blue (415 nm) and red (633 nm) light. Fourteen patients self-reported improvements in skin tone and texture. Improvements in noninflammatory and inflammatory lesions were substantial (34.28% and 77.93%, respectively). The researchers concluded that combined blue and red LED phototherapy is a safe and effective option, especially for papulopustular acne (Lasers Surg. Med. 2007;39:180-8).
In 2009, Sadick evaluated the efficacy of the combination of blue (415 nm) and near-infrared (830 nm) LED therapy for moderate acne in 13 females and 4 males ranging in skin type from II to VI and in Burton acne grade at baseline from 1 to 5. Twice-weekly 20-minute sessions were conducted for 4 weeks, alternating between blue and near-infrared light. Eleven patients exhibited improvement ranging from 0% to 83.3%, and 6 patients discontinued the study. A decreasing trend was observed in the Burton grade. Noninflammatory lesion counts improved in seven patients but increased in four. Sadick noted that these results paled in comparison to the effectiveness of the blue and red combination at lowering inflammatory lesions seen previously, but encouraged the study of the combination phototherapy in a much larger population (J. Cosmet. Laser Ther. 2009;11:125-8).
Several recent reviews have found that red light–activated MAL-PDT, the combination of blue and red light, and aminolevulinic acid as a photosensitizing agent before treatment with blue light, red light, or the 595-nm pulsed dye laser are among the most promising evidence-based laser- and light-based therapies for acne (Semin. Cutan. Med. Surg. 2008;27:207-11; J. Eur. Acad. Dermatol. Venereol. 2008;22:267-78; Dermatol. Surg. 2007;33:1005-26).
In a systematic literature review of randomized controlled trials of light and laser therapies for acne vulgaris (using the Cochrane Central Register of Controlled Trials, MEDLINE, Embase, CINAHL, PsycINFO, LILACS, ISI Science Citation Index, and Dissertation Abstracts International), Hamilton et al. found that trials of blue light, blue-red light, and infrared radiation were more successful, especially when multiple treatments were used. Notably, blue-red light demonstrated better short-term effectiveness than did topical 5% benzoyl peroxide cream (Br. J. Dermatol. 2009;160:1273-85).
Kim and Armstrong have noted that blue light has been demonstrated to photoinactivate P. acnes, but it does not penetrate deeply into the skin. It is believed to work synergistically, however, with red light, which is less effective than blue light at exciting porphyrins but can reach deeper sebaceous glands and may impart an anti-inflammatory effect by inciting cytokine release from macrophages (Dermatol. Surg. 2007;33:1005-26). Indeed, Kim and Armstrong found that combined blue-red light therapy was more effective at lowering the number of inflammatory acne lesions than were benzoyl peroxide monotherapy and blue light monotherapy (Lasers Surg. Med. 1989;9:497-505).
Conclusions
A lengthy review of the literature and personal experience treating patients have convinced me that blue light is an effective treatment for acne. P. acnes is most susceptible to the blue light wavelengths of 407-420 nm. Addition of red light may help speed resolution of inflammatory lesions through an anti-inflammatory effect. Blue and red light devices are efficacious when used in the office if the devices deliver enough joules.
Many at-home devices and iPhone apps have hit the market. These are a great alternative to irritating topicals and antibiotics, and they may help increase compliance. However, many at-home light devices are too weak (do not emit enough joules), or emit a broad range of light (rather than 407-420 nm). The manufacturers of some of these products claim that the heat produced by the devices improves acne, but there is a paucity of research proving this point. In my opinion, using an at-home device twice a day that delivers 407-420 nm (with or without the addition of red light), and delivers enough joules (at least 25 J/cm2), is an effective method of treating acne. For comparison purposes, the in-office Omnilux delivers around 49 J/cm2 but is used only two or three times per week. Know your wavelengths and joules when trying to decide which device to sell in your practice or recommend to patients.
Several light devices based on red light–emitting diodes (LEDs) have made their way to the market in recent years. Although red light is not truly a cosmeceutical, it is a significant emerging adjuvant therapy that, like blue light, has been studied in comparison to and in conjunction with topical options primarily to treat acne. Of course, acne is the most common skin disorder prompting visits to the dermatologist, with an estimated 85% of adolescents affected, many into adulthood (J. Am. Acad. Dermatol. 2008;58:56-9).
This discussion will consider red light when used alone and when used in combination with blue light. Blue light is the most effective light to target Propionibacterium acnes (specifically at wavelengths of 407-420 nm). However, many devices are utilizing red light because it has a purported anti-inflammatory effect and penetrates deeper into the skin (Dermatol. Ther. 2005;18:253-66).
Erythrasma
Darras-Vercambre et al. evaluated the effects of red light for the treatment of erythrasma (a superficial skin infection provoked by Corynebacterium minutissimum) in 13 patients. One treatment (80 J/cm2) by red light (broadband, peak at 635 nm) without exogenous photosensitizing molecules was administered to each subject. Therapy was effective and well tolerated, with three patients experiencing complete recovery, and significant reduction of lesions in most other cases. The authors noted that the key to their study, given the absence of an exogenous photosensitizing agent, was capitalizing on the presence of porphyrins in the lesions. They concluded that the use of red light alone for this localized infection is easy and inexpensive, but that an optimal method has not yet been established (Photodermatol. Photoimmunol. Photomed. 2006;22:153-6).
Acne
In 2007, Na and Suh evaluated the efficacy of red light phototherapy with a portable device in 28 volunteers with mild to moderate acne in a split-face randomized trial. Phototherapy was performed twice daily for 15 minutes for a total of 8 weeks to one side of the face. The investigators concluded that red light phototherapy alone is an effective therapeutic option for acne, as they noted significant reductions in noninflammatory and inflammatory lesion counts on the treated side versus the untreated side, a drop from 3.9 to 1.9 in the visual analog scale on the treatment side, and significant disparities between the treatment and control sides after 8 weeks (Dermatol. Surg. 2007;33:1228-33, discussion 1233).
A 2006 article in the British Journal of Dermatology reported good clinical results from acne treatment with photodynamic therapy (PDT) using methyl aminolevulinate (MAL) and red light, but there were adverse side effects that prompted 7 of 19 subjects to discontinue the study (Br. J. Dermatol. 2006;154:969-76). In response to the study, Mavilia et al. wrote a letter to the journal acknowledging their more effective combination therapy using a lower concentration of MAL and low doses of red light. All 16 patients completed the study, in which the count of inflammatory lesions fell an average of 66% with mild but tolerable side effects, including a subtle sensation of heat, then minimal erythema during the procedure and slight scaling that began 3 days after treatment (Br. J. Dermatol. 2007;157:810-1).
In a small study of patients with moderate facial acne, Zane et al. exposed 15 women to 20 J/cm2 of broadband red light (600-750 nm) twice weekly for 4 weeks. They also measured skin sebum, pH, hydration, and transepidermal water loss (TEWL). Untreated lesions of the trunk served as controls. The investigators found the treatment safe, well tolerated, and effective, with significant improvement in acne lesions and reduction of sebum excretion and TEWL after 4 weeks of therapy and at the 3-month follow-up visit. They speculated that the improvement was due to the decreased colonization of P. acnes, decimated by photoactivated endogenous porphyrin, and concluded that this inexpensive therapy warrants inclusion among treatment options for moderate acne (Photodermatol. Photoimmunol. Photomed. 2008;24:244-8).
In a 2009 study of 19 patients with moderate to severe facial acne who received a single treatment of low-dose, red-light PDT on the left cheek and MAL 3 hours before red light on the right cheek, both therapies yielded significant reductions in acne score. Red light was found to be as effective as MAL-PDT (Acta Derm. Venereol. 2009;89:372-8).
Combined Blue and Red Light Phototherapy
In 2006, Goldberg and Russell evaluated the combination of blue (415 nm) and red (633 nm) LED phototherapy in 24 patients with Fitzpatrick skin types II-V and mild to severe symmetric facial acne. Twenty-two patients completed the trial, which included two sessions per week (separated by 3 days) alternating between blue and red light for a total of eight sessions. Mild microdermabrasion was used at the start of each session. The mean decrease in lesion count was significant after 4 weeks (46%) and 12 weeks (81%). Inflammatory lesions responded better than did noninflammatory ones, and severe acne responded slightly better than mild acne. The investigators concluded that the combination of blue and red LED phototherapy is free of side effects and pain, and exhibits great potential for the treatment of mild to severe acne (J. Cosmet. Laser Ther. 2006;8:71-5).
In 2007, Lee et al. set out to examine the efficacy of combining blue and red LED phototherapy for acne in a study of 24 patients with mild to moderately severe facial acne. Twice weekly for 4 weeks, patients were treated with quasi-monochromatic LED devices, alternating blue (415 nm) and red (633 nm) light. Fourteen patients self-reported improvements in skin tone and texture. Improvements in noninflammatory and inflammatory lesions were substantial (34.28% and 77.93%, respectively). The researchers concluded that combined blue and red LED phototherapy is a safe and effective option, especially for papulopustular acne (Lasers Surg. Med. 2007;39:180-8).
In 2009, Sadick evaluated the efficacy of the combination of blue (415 nm) and near-infrared (830 nm) LED therapy for moderate acne in 13 females and 4 males ranging in skin type from II to VI and in Burton acne grade at baseline from 1 to 5. Twice-weekly 20-minute sessions were conducted for 4 weeks, alternating between blue and near-infrared light. Eleven patients exhibited improvement ranging from 0% to 83.3%, and 6 patients discontinued the study. A decreasing trend was observed in the Burton grade. Noninflammatory lesion counts improved in seven patients but increased in four. Sadick noted that these results paled in comparison to the effectiveness of the blue and red combination at lowering inflammatory lesions seen previously, but encouraged the study of the combination phototherapy in a much larger population (J. Cosmet. Laser Ther. 2009;11:125-8).
Several recent reviews have found that red light–activated MAL-PDT, the combination of blue and red light, and aminolevulinic acid as a photosensitizing agent before treatment with blue light, red light, or the 595-nm pulsed dye laser are among the most promising evidence-based laser- and light-based therapies for acne (Semin. Cutan. Med. Surg. 2008;27:207-11; J. Eur. Acad. Dermatol. Venereol. 2008;22:267-78; Dermatol. Surg. 2007;33:1005-26).
In a systematic literature review of randomized controlled trials of light and laser therapies for acne vulgaris (using the Cochrane Central Register of Controlled Trials, MEDLINE, Embase, CINAHL, PsycINFO, LILACS, ISI Science Citation Index, and Dissertation Abstracts International), Hamilton et al. found that trials of blue light, blue-red light, and infrared radiation were more successful, especially when multiple treatments were used. Notably, blue-red light demonstrated better short-term effectiveness than did topical 5% benzoyl peroxide cream (Br. J. Dermatol. 2009;160:1273-85).
Kim and Armstrong have noted that blue light has been demonstrated to photoinactivate P. acnes, but it does not penetrate deeply into the skin. It is believed to work synergistically, however, with red light, which is less effective than blue light at exciting porphyrins but can reach deeper sebaceous glands and may impart an anti-inflammatory effect by inciting cytokine release from macrophages (Dermatol. Surg. 2007;33:1005-26). Indeed, Kim and Armstrong found that combined blue-red light therapy was more effective at lowering the number of inflammatory acne lesions than were benzoyl peroxide monotherapy and blue light monotherapy (Lasers Surg. Med. 1989;9:497-505).
Conclusions
A lengthy review of the literature and personal experience treating patients have convinced me that blue light is an effective treatment for acne. P. acnes is most susceptible to the blue light wavelengths of 407-420 nm. Addition of red light may help speed resolution of inflammatory lesions through an anti-inflammatory effect. Blue and red light devices are efficacious when used in the office if the devices deliver enough joules.
Many at-home devices and iPhone apps have hit the market. These are a great alternative to irritating topicals and antibiotics, and they may help increase compliance. However, many at-home light devices are too weak (do not emit enough joules), or emit a broad range of light (rather than 407-420 nm). The manufacturers of some of these products claim that the heat produced by the devices improves acne, but there is a paucity of research proving this point. In my opinion, using an at-home device twice a day that delivers 407-420 nm (with or without the addition of red light), and delivers enough joules (at least 25 J/cm2), is an effective method of treating acne. For comparison purposes, the in-office Omnilux delivers around 49 J/cm2 but is used only two or three times per week. Know your wavelengths and joules when trying to decide which device to sell in your practice or recommend to patients.
When to Consider PCOS in Female Acne Patients
SAN DIEGO – Correctly diagnosed and adequately treated polycystic ovary syndrome can appreciably improve acne in a select group of female patients, according to Dr. Anne W. Lucky.
Dr. Lucky offered tips on evaluating and treating acne and PCOS at the annual meeting of the American Academy of Dermatology. She said that PCOS should be considered in female patients with early onset acne, acne that is refractory to conventional therapy, relapse that occurs after treatment with isotretinoin, persistence of acne beyond adolescence, or late onset of acne, she said.
Several laboratory tests are typically used to diagnose PCOS but agreement on which tests to use is limited. "This is somewhat controversial," said Dr. Lucky, a professor of dermatology at the University of Cincinnati. She recommended the following tests: free testosterone, DHEAS (dehydroepiandrosterone sulfate), and the ratio of LH (luteinizing hormone) to FSH (follicle-stimulating hormone). "If I have a good suspicion or find abnormalities – [I include] fasting glucose and insulin and a fasting lipid profile," she said.
Treatment options include combination oral contraceptives, glucocorticoids (for specific adrenal abnormalities), and anti-androgens among others.
"By and large, oral contraceptives are the No. 1 choice. I mentioned combination contraceptives because it’s the estrogen that’s helpful. Progesterone-only contraceptives can actually worsen acne," Dr. Lucky said. "We don’t treat girls before menarche because they don’t have a risk for pregnancy and we might influence bone growth if we treat too early. You can suspect PCOS very early but you may have to suspend your treatment until they get a little older."
Using contraceptives – especially in girls or adolescents -- usually involves discussions with a gynecologist, a parent, and the child. "Our biggest hurdle is that the teenage population has heard the word on the street that the pill is going to make them fat and they don’t want to take it. Actually [the gynecologists that I work with] tell me that the studies show that some children gain weight, some lose weight, but most stay the same weight with the combination pills," she said.
In the United States, spironolactone is the only antiandrogen treatment option. It works by being a competitive inhibitor of androgen receptors. The drug does not lower serum androgen levels, but it prevents the action of androgen – increasing estrogenicity. However, the use of spironolactone for PCOS is considered off label. The drug is indicated for the treatment of hypertension and the label carries a warning that it should only be used for this indication, Dr. Lucky noted.
Dr. Lucky reported that she has financial relationships with Amgen, Galderma, and Johnson & Johnson.
SAN DIEGO – Correctly diagnosed and adequately treated polycystic ovary syndrome can appreciably improve acne in a select group of female patients, according to Dr. Anne W. Lucky.
Dr. Lucky offered tips on evaluating and treating acne and PCOS at the annual meeting of the American Academy of Dermatology. She said that PCOS should be considered in female patients with early onset acne, acne that is refractory to conventional therapy, relapse that occurs after treatment with isotretinoin, persistence of acne beyond adolescence, or late onset of acne, she said.
Several laboratory tests are typically used to diagnose PCOS but agreement on which tests to use is limited. "This is somewhat controversial," said Dr. Lucky, a professor of dermatology at the University of Cincinnati. She recommended the following tests: free testosterone, DHEAS (dehydroepiandrosterone sulfate), and the ratio of LH (luteinizing hormone) to FSH (follicle-stimulating hormone). "If I have a good suspicion or find abnormalities – [I include] fasting glucose and insulin and a fasting lipid profile," she said.
Treatment options include combination oral contraceptives, glucocorticoids (for specific adrenal abnormalities), and anti-androgens among others.
"By and large, oral contraceptives are the No. 1 choice. I mentioned combination contraceptives because it’s the estrogen that’s helpful. Progesterone-only contraceptives can actually worsen acne," Dr. Lucky said. "We don’t treat girls before menarche because they don’t have a risk for pregnancy and we might influence bone growth if we treat too early. You can suspect PCOS very early but you may have to suspend your treatment until they get a little older."
Using contraceptives – especially in girls or adolescents -- usually involves discussions with a gynecologist, a parent, and the child. "Our biggest hurdle is that the teenage population has heard the word on the street that the pill is going to make them fat and they don’t want to take it. Actually [the gynecologists that I work with] tell me that the studies show that some children gain weight, some lose weight, but most stay the same weight with the combination pills," she said.
In the United States, spironolactone is the only antiandrogen treatment option. It works by being a competitive inhibitor of androgen receptors. The drug does not lower serum androgen levels, but it prevents the action of androgen – increasing estrogenicity. However, the use of spironolactone for PCOS is considered off label. The drug is indicated for the treatment of hypertension and the label carries a warning that it should only be used for this indication, Dr. Lucky noted.
Dr. Lucky reported that she has financial relationships with Amgen, Galderma, and Johnson & Johnson.
SAN DIEGO – Correctly diagnosed and adequately treated polycystic ovary syndrome can appreciably improve acne in a select group of female patients, according to Dr. Anne W. Lucky.
Dr. Lucky offered tips on evaluating and treating acne and PCOS at the annual meeting of the American Academy of Dermatology. She said that PCOS should be considered in female patients with early onset acne, acne that is refractory to conventional therapy, relapse that occurs after treatment with isotretinoin, persistence of acne beyond adolescence, or late onset of acne, she said.
Several laboratory tests are typically used to diagnose PCOS but agreement on which tests to use is limited. "This is somewhat controversial," said Dr. Lucky, a professor of dermatology at the University of Cincinnati. She recommended the following tests: free testosterone, DHEAS (dehydroepiandrosterone sulfate), and the ratio of LH (luteinizing hormone) to FSH (follicle-stimulating hormone). "If I have a good suspicion or find abnormalities – [I include] fasting glucose and insulin and a fasting lipid profile," she said.
Treatment options include combination oral contraceptives, glucocorticoids (for specific adrenal abnormalities), and anti-androgens among others.
"By and large, oral contraceptives are the No. 1 choice. I mentioned combination contraceptives because it’s the estrogen that’s helpful. Progesterone-only contraceptives can actually worsen acne," Dr. Lucky said. "We don’t treat girls before menarche because they don’t have a risk for pregnancy and we might influence bone growth if we treat too early. You can suspect PCOS very early but you may have to suspend your treatment until they get a little older."
Using contraceptives – especially in girls or adolescents -- usually involves discussions with a gynecologist, a parent, and the child. "Our biggest hurdle is that the teenage population has heard the word on the street that the pill is going to make them fat and they don’t want to take it. Actually [the gynecologists that I work with] tell me that the studies show that some children gain weight, some lose weight, but most stay the same weight with the combination pills," she said.
In the United States, spironolactone is the only antiandrogen treatment option. It works by being a competitive inhibitor of androgen receptors. The drug does not lower serum androgen levels, but it prevents the action of androgen – increasing estrogenicity. However, the use of spironolactone for PCOS is considered off label. The drug is indicated for the treatment of hypertension and the label carries a warning that it should only be used for this indication, Dr. Lucky noted.
Dr. Lucky reported that she has financial relationships with Amgen, Galderma, and Johnson & Johnson.
EXPERT ANALYSIS FROM THE ANNUAL MEETING OF THE AMERICAN ACADEMY OF DERMATOLOGY
Expert: Treat Rosacea Presentation, Not Subtype
MIAMI BEACH – Rather than working to identify a particular rosacea subtype in your patient, adopt a simpler strategy and treat based on presenting signs, Dr. James Q. Del Rosso said at the South Beach Symposium.
"Why not evaluate and treat a patient with rosacea based on the clinical features they present with?" Dr. Del Rosso asked. For example, the common feature in the vast majority of patients is central facial diffuse erythema. "We are talking about 80% or 90% of the patients we see."
Rosacea is an umbrella term. "We talk a lot about subtypes, but that doesn’t necessarily answer all the questions," Dr. Del Rosso said. "I want to suggest we narrow the definition a bit so we focus on the more common clinical situations in practice."
In other words, rather than distinguishing papulopustular rosacea from erythematotelangiectatic or phymatous subtypes, consider whether a patient has intermittent or persistent rosacea. Intermittent rosacea features inflammatory lesions, perilesional erythema, and background erythema. In contrast, persistent rosacea will present as background erythema (more prominently), as well as telangiectasias and phymatous changes to the skin.
This classification reflects more "real world" presentations of rosacea, said Dr. Del Rosso, a dermatologist in group practice in Las Vegas.
Treatment
The clinical features of rosacea go beyond diagnosis classification and can guide selection of therapy as well. In terms of topical therapies, metronidazole 1% and 0.75% and azelaic acid 15% gel are approved by the Food and Drug Administration to treat papulopustular rosacea, which is characterized by inflammatory lesions, erythema, and telangiectasias.
"There is a mechanism supporting why topical or oral metronidazole is effective," Dr. Del Rosso said. Metronidazole modifies the augmented, innate immune response implicated early in the development of rosacea. In terms of azelaic acid, data about mechanism of action are only available in mice.
This immune response occurs very early in the pathogenesis of rosacea and during flares, Dr. Del Rosso said. Researchers have identified another important pathway, a neurogenic vascular dysregulation that could explain the vasodilation and diffuse erythema commonly seen in many patients. Although it is unclear which mechanism arises first, Dr. Del Rosso said, "I’m going to roll the dice and I will [guess that] innate immunity happens first."
Conventional oral agents can treat rosacea effectively as well. Tetracyclines, for example, work through a variety of anti-inflammatory effects. "Current evidence does not support the need to eradicate or suppress a bacterium," Dr. Del Rosso said. For this reason, multiple studies support the efficacy of subantimicrobial doxycycline for its anti-inflammatory actions (Am. J. Clin. Dermatol. 2010;11:217-22; Cutis 2010;86:16-25).
Recent evidence suggests lower-dose doxycycline (40-mg extended-release doxycycline, for example) is sufficient to inhibit matrix metalloproteinase (MMP) enzymes that break down connective tissue proteins (Pharmacol. Res. 2011;63:130-45). MMPs are upregulated in rosacea and other conditions that feature dermal destruction.
"All of the tetracyclines seem to work on blocking MMP activity, but doxycycline works the best," Dr. David E. Cohen said in a separate presentation at the meeting. Doxycycline can inhibit multiple pathways involved in rosacea, he said. Recent findings "suggest a previously unknown mechanism of action of subantimicrobial doxycycline."
Cathelicidin Pathways
Another new finding is that the MMP pathway and the cathelicidin pathways are no longer thought to be distinct, said Dr. Cohen of the department of dermatology at New York University Langone Medical Center in New York City.
"Last year I said these two things were independently elevated [in rosacea]," Dr. Cohen said. "Now we know they can trigger each other. It’s a vicious cycle, a vortex."
Cathelicidins are protective peptides in the skin. These endogenous players cleave and kill virus and fungi and recruit a host immune response, Dr. Cohen said. "They are inactive normally. So the cathelicidins are there for a good reason, but they are always ‘on’ in rosacea." When these peptides are overexpressed, as they are in rosacea, they can trigger inflammation and angiogenesis.
A specific cathelicidin garnering a lot of attention is LL-37, a vasoactive and inflammatory host-defense peptide also overexpressed in rosacea. "This is very important," Dr. Del Rosso said. "LL-37 just keeps popping up in the pathogenesis of skin disorders." Various levels of LL-37 are implicated in atopic dermatitis and cutaneous lupus, for example. In healthy skin, LL-37 helps to maintain homeostatic immunity and fights bacteria and viruses in the skin.
Dr. Del Rosso disclosed that he is a consultant, researcher, and member of the speakers bureaus for Allergan and Galderma. Dr. Cohen disclosed that he is a consultant for, receives honoraria from, and is on the advisory board for Galderma.
MIAMI BEACH – Rather than working to identify a particular rosacea subtype in your patient, adopt a simpler strategy and treat based on presenting signs, Dr. James Q. Del Rosso said at the South Beach Symposium.
"Why not evaluate and treat a patient with rosacea based on the clinical features they present with?" Dr. Del Rosso asked. For example, the common feature in the vast majority of patients is central facial diffuse erythema. "We are talking about 80% or 90% of the patients we see."
Rosacea is an umbrella term. "We talk a lot about subtypes, but that doesn’t necessarily answer all the questions," Dr. Del Rosso said. "I want to suggest we narrow the definition a bit so we focus on the more common clinical situations in practice."
In other words, rather than distinguishing papulopustular rosacea from erythematotelangiectatic or phymatous subtypes, consider whether a patient has intermittent or persistent rosacea. Intermittent rosacea features inflammatory lesions, perilesional erythema, and background erythema. In contrast, persistent rosacea will present as background erythema (more prominently), as well as telangiectasias and phymatous changes to the skin.
This classification reflects more "real world" presentations of rosacea, said Dr. Del Rosso, a dermatologist in group practice in Las Vegas.
Treatment
The clinical features of rosacea go beyond diagnosis classification and can guide selection of therapy as well. In terms of topical therapies, metronidazole 1% and 0.75% and azelaic acid 15% gel are approved by the Food and Drug Administration to treat papulopustular rosacea, which is characterized by inflammatory lesions, erythema, and telangiectasias.
"There is a mechanism supporting why topical or oral metronidazole is effective," Dr. Del Rosso said. Metronidazole modifies the augmented, innate immune response implicated early in the development of rosacea. In terms of azelaic acid, data about mechanism of action are only available in mice.
This immune response occurs very early in the pathogenesis of rosacea and during flares, Dr. Del Rosso said. Researchers have identified another important pathway, a neurogenic vascular dysregulation that could explain the vasodilation and diffuse erythema commonly seen in many patients. Although it is unclear which mechanism arises first, Dr. Del Rosso said, "I’m going to roll the dice and I will [guess that] innate immunity happens first."
Conventional oral agents can treat rosacea effectively as well. Tetracyclines, for example, work through a variety of anti-inflammatory effects. "Current evidence does not support the need to eradicate or suppress a bacterium," Dr. Del Rosso said. For this reason, multiple studies support the efficacy of subantimicrobial doxycycline for its anti-inflammatory actions (Am. J. Clin. Dermatol. 2010;11:217-22; Cutis 2010;86:16-25).
Recent evidence suggests lower-dose doxycycline (40-mg extended-release doxycycline, for example) is sufficient to inhibit matrix metalloproteinase (MMP) enzymes that break down connective tissue proteins (Pharmacol. Res. 2011;63:130-45). MMPs are upregulated in rosacea and other conditions that feature dermal destruction.
"All of the tetracyclines seem to work on blocking MMP activity, but doxycycline works the best," Dr. David E. Cohen said in a separate presentation at the meeting. Doxycycline can inhibit multiple pathways involved in rosacea, he said. Recent findings "suggest a previously unknown mechanism of action of subantimicrobial doxycycline."
Cathelicidin Pathways
Another new finding is that the MMP pathway and the cathelicidin pathways are no longer thought to be distinct, said Dr. Cohen of the department of dermatology at New York University Langone Medical Center in New York City.
"Last year I said these two things were independently elevated [in rosacea]," Dr. Cohen said. "Now we know they can trigger each other. It’s a vicious cycle, a vortex."
Cathelicidins are protective peptides in the skin. These endogenous players cleave and kill virus and fungi and recruit a host immune response, Dr. Cohen said. "They are inactive normally. So the cathelicidins are there for a good reason, but they are always ‘on’ in rosacea." When these peptides are overexpressed, as they are in rosacea, they can trigger inflammation and angiogenesis.
A specific cathelicidin garnering a lot of attention is LL-37, a vasoactive and inflammatory host-defense peptide also overexpressed in rosacea. "This is very important," Dr. Del Rosso said. "LL-37 just keeps popping up in the pathogenesis of skin disorders." Various levels of LL-37 are implicated in atopic dermatitis and cutaneous lupus, for example. In healthy skin, LL-37 helps to maintain homeostatic immunity and fights bacteria and viruses in the skin.
Dr. Del Rosso disclosed that he is a consultant, researcher, and member of the speakers bureaus for Allergan and Galderma. Dr. Cohen disclosed that he is a consultant for, receives honoraria from, and is on the advisory board for Galderma.
MIAMI BEACH – Rather than working to identify a particular rosacea subtype in your patient, adopt a simpler strategy and treat based on presenting signs, Dr. James Q. Del Rosso said at the South Beach Symposium.
"Why not evaluate and treat a patient with rosacea based on the clinical features they present with?" Dr. Del Rosso asked. For example, the common feature in the vast majority of patients is central facial diffuse erythema. "We are talking about 80% or 90% of the patients we see."
Rosacea is an umbrella term. "We talk a lot about subtypes, but that doesn’t necessarily answer all the questions," Dr. Del Rosso said. "I want to suggest we narrow the definition a bit so we focus on the more common clinical situations in practice."
In other words, rather than distinguishing papulopustular rosacea from erythematotelangiectatic or phymatous subtypes, consider whether a patient has intermittent or persistent rosacea. Intermittent rosacea features inflammatory lesions, perilesional erythema, and background erythema. In contrast, persistent rosacea will present as background erythema (more prominently), as well as telangiectasias and phymatous changes to the skin.
This classification reflects more "real world" presentations of rosacea, said Dr. Del Rosso, a dermatologist in group practice in Las Vegas.
Treatment
The clinical features of rosacea go beyond diagnosis classification and can guide selection of therapy as well. In terms of topical therapies, metronidazole 1% and 0.75% and azelaic acid 15% gel are approved by the Food and Drug Administration to treat papulopustular rosacea, which is characterized by inflammatory lesions, erythema, and telangiectasias.
"There is a mechanism supporting why topical or oral metronidazole is effective," Dr. Del Rosso said. Metronidazole modifies the augmented, innate immune response implicated early in the development of rosacea. In terms of azelaic acid, data about mechanism of action are only available in mice.
This immune response occurs very early in the pathogenesis of rosacea and during flares, Dr. Del Rosso said. Researchers have identified another important pathway, a neurogenic vascular dysregulation that could explain the vasodilation and diffuse erythema commonly seen in many patients. Although it is unclear which mechanism arises first, Dr. Del Rosso said, "I’m going to roll the dice and I will [guess that] innate immunity happens first."
Conventional oral agents can treat rosacea effectively as well. Tetracyclines, for example, work through a variety of anti-inflammatory effects. "Current evidence does not support the need to eradicate or suppress a bacterium," Dr. Del Rosso said. For this reason, multiple studies support the efficacy of subantimicrobial doxycycline for its anti-inflammatory actions (Am. J. Clin. Dermatol. 2010;11:217-22; Cutis 2010;86:16-25).
Recent evidence suggests lower-dose doxycycline (40-mg extended-release doxycycline, for example) is sufficient to inhibit matrix metalloproteinase (MMP) enzymes that break down connective tissue proteins (Pharmacol. Res. 2011;63:130-45). MMPs are upregulated in rosacea and other conditions that feature dermal destruction.
"All of the tetracyclines seem to work on blocking MMP activity, but doxycycline works the best," Dr. David E. Cohen said in a separate presentation at the meeting. Doxycycline can inhibit multiple pathways involved in rosacea, he said. Recent findings "suggest a previously unknown mechanism of action of subantimicrobial doxycycline."
Cathelicidin Pathways
Another new finding is that the MMP pathway and the cathelicidin pathways are no longer thought to be distinct, said Dr. Cohen of the department of dermatology at New York University Langone Medical Center in New York City.
"Last year I said these two things were independently elevated [in rosacea]," Dr. Cohen said. "Now we know they can trigger each other. It’s a vicious cycle, a vortex."
Cathelicidins are protective peptides in the skin. These endogenous players cleave and kill virus and fungi and recruit a host immune response, Dr. Cohen said. "They are inactive normally. So the cathelicidins are there for a good reason, but they are always ‘on’ in rosacea." When these peptides are overexpressed, as they are in rosacea, they can trigger inflammation and angiogenesis.
A specific cathelicidin garnering a lot of attention is LL-37, a vasoactive and inflammatory host-defense peptide also overexpressed in rosacea. "This is very important," Dr. Del Rosso said. "LL-37 just keeps popping up in the pathogenesis of skin disorders." Various levels of LL-37 are implicated in atopic dermatitis and cutaneous lupus, for example. In healthy skin, LL-37 helps to maintain homeostatic immunity and fights bacteria and viruses in the skin.
Dr. Del Rosso disclosed that he is a consultant, researcher, and member of the speakers bureaus for Allergan and Galderma. Dr. Cohen disclosed that he is a consultant for, receives honoraria from, and is on the advisory board for Galderma.
EXPERT ANALYSIS FROM THE SOUTH BEACH SYMPOSIUM