User login
High-dose antipsychotics show some benefit in treatment-resistant cases
NEW ORLEANS – Patients with severe schizophrenia who fail to respond to treatment with standard doses of second-generation antipsychotics show significant improvement with – and tolerance of – higher maintenance doses of the drugs, new research shows.
“The use of [higher doses of] long-acting injectable second-generation antipsychotics shows improvement not only in treatment adherence, but also in diminished relapses and suicide attempts compared with other previous treatment options used with these severely ill patients,” lead author Juan Jose Fernandez-Miranda, MD, said in an interview.
Dr. Fernandez-Miranda, of the Mental Health Service of the Principality of Asturias, in Gijón, Spain, underscored the tolerability of the novel approach of high doses: “No important side effects were found, and less than occurred with previous treatments,” he said.
While higher doses of second-generation antipsychotics for patients with treatment refractory schizophrenia are sometimes considered necessary, particularly with acute psychosis, evidence of benefits of the approach is lacking, and there are concerns about adverse events such as extrapyramidal symptoms and hyperprolactinemia.
To investigate the effects, the authors evaluated patients in a community-based, case managed program with severe, (CGI-S = 5), resistant schizophrenia.
All had been treated in the previous 3 years with at least two different antipsychotics, including clozapine in a few cases, with poor outcomes when receiving standard doses, and eligibility included being at risk of medication noncompliance, and/or experiencing a lack of effectiveness or adverse effects with previous antipsychotics.
For the second 3 years of the observational study, they were treated with doses of at least 75 mg of risperidone long-acting injectable (n = 60), 175 mg or more of monthly paliperidone palmitate (n = 60), or 600 mg or higher of aripiprazole once monthly (n = 30).
During the study, the average antipsychotic doses were: risperidone 111.2 mg/14 days; paliperidone palmitate 231.2 mg. eq./28 days; and aripiprazole 780 mg/28 days. In addition to the intensive pharmacological intervention, patients received psychosocial integrated intervention, as in the previous 3 years.
Over the 3 years with the higher maintenance doses, significant improvements were observed with all of the injectable treatment groups in terms of decreases on the Clinical Global Impression Scale – Severity score (CGI-S; P < .01) and in the four areas of the World Health Organization Disability Schedule (WHO-DAS), including in self-care, occupational, family, and social measures (P < .01 through P < .001).
Scores on the Medication Adherence Rating Scale (MARS), increased with all of the long-acting injectables (P < .01), particularly with paliperidone palmitate and aripiprazole.
Patients had significant decreases in hospital admissions at the end of the 36-month treatments and reductions in suicide attempts (both P < .001), compared with the previous 3 years, without any differences across the three injectables.
Importantly, tolerability was good for all of the long-acting antidepressants, with reductions in side effects as well as biological parameters compared with previous treatments, notably in the aripiprazole group.
While reductions in weight and prolactin levels were observed in all long-acting treatments, the differences were statistically significant only among patients treated with aripiprazole (P < .05), as was expected.
Two patients treated with aripiprazole discontinued treatment because of side effects from treatment, and the rate was five with paliperidone palmitate and nine with risperidone.
One person in the aripiprazole group discontinued because of a lack of effectiveness, while two discontinued in the paliperidone palmitate group and four with risperidone.
Dr. Fernandez-Miranda noted that “both the intensive case-managed multicomponent treatment and use of high doses of long-acting antipsychotics were in all probability linked to the high adherence and positive clinical outcomes.”
The results provide evidence that “long-acting second-generation antipsychotics are a remarkable option for patients with severe schizophrenia and a background of treatment discontinuation or intolerable adverse effects with other antipsychotics,” Dr. Fernandez-Miranda added.
“We suggest that, in some illness critical conditions, high doses of long-acting second-generation antipsychotics could represent an alternative to clozapine,” he added.
Some hesitation warranted
Commenting on the study, T. Scott Stroup, MD, MPH, professor of psychiatry at Columbia University, New York, noted the key limitations of a lack of randomization and comparison group of clozapine or typical-dose long-acting injectables.
“In addition, pre-post or mirror-image designs may be affected by expectation bias and regression to the mean,” he said in an interview.
“I don’t doubt that some patients do well on relatively high doses of long-acting injectable medications and that some tolerate these doses,” he noted “Most adverse effects are dose related, but without a typical-dose comparison group we cannot assess this.”
Ultimately, Dr. Stroup recommends sticking with standard recommendations – at least to start.
“My take-home message is that clozapine remains the treatment of choice for treatment-resistant schizophrenia, and in most cases clozapine should be tried before considering high-dose long-acting injectables,” he said.
“If there is uncertainty about whether someone is taking a prescribed oral antipsychotic medication, then a trial of a typical dose of a long-acting injectable is a good option to rule out pseudo-treatment resistance.”
Furthermore, “this study doesn’t affect the recommendation that people who need antipsychotic medications should receive the lowest effective dose,” he said.
The authors and Dr. Stroup had no disclosures to report.
NEW ORLEANS – Patients with severe schizophrenia who fail to respond to treatment with standard doses of second-generation antipsychotics show significant improvement with – and tolerance of – higher maintenance doses of the drugs, new research shows.
“The use of [higher doses of] long-acting injectable second-generation antipsychotics shows improvement not only in treatment adherence, but also in diminished relapses and suicide attempts compared with other previous treatment options used with these severely ill patients,” lead author Juan Jose Fernandez-Miranda, MD, said in an interview.
Dr. Fernandez-Miranda, of the Mental Health Service of the Principality of Asturias, in Gijón, Spain, underscored the tolerability of the novel approach of high doses: “No important side effects were found, and less than occurred with previous treatments,” he said.
While higher doses of second-generation antipsychotics for patients with treatment refractory schizophrenia are sometimes considered necessary, particularly with acute psychosis, evidence of benefits of the approach is lacking, and there are concerns about adverse events such as extrapyramidal symptoms and hyperprolactinemia.
To investigate the effects, the authors evaluated patients in a community-based, case managed program with severe, (CGI-S = 5), resistant schizophrenia.
All had been treated in the previous 3 years with at least two different antipsychotics, including clozapine in a few cases, with poor outcomes when receiving standard doses, and eligibility included being at risk of medication noncompliance, and/or experiencing a lack of effectiveness or adverse effects with previous antipsychotics.
For the second 3 years of the observational study, they were treated with doses of at least 75 mg of risperidone long-acting injectable (n = 60), 175 mg or more of monthly paliperidone palmitate (n = 60), or 600 mg or higher of aripiprazole once monthly (n = 30).
During the study, the average antipsychotic doses were: risperidone 111.2 mg/14 days; paliperidone palmitate 231.2 mg. eq./28 days; and aripiprazole 780 mg/28 days. In addition to the intensive pharmacological intervention, patients received psychosocial integrated intervention, as in the previous 3 years.
Over the 3 years with the higher maintenance doses, significant improvements were observed with all of the injectable treatment groups in terms of decreases on the Clinical Global Impression Scale – Severity score (CGI-S; P < .01) and in the four areas of the World Health Organization Disability Schedule (WHO-DAS), including in self-care, occupational, family, and social measures (P < .01 through P < .001).
Scores on the Medication Adherence Rating Scale (MARS), increased with all of the long-acting injectables (P < .01), particularly with paliperidone palmitate and aripiprazole.
Patients had significant decreases in hospital admissions at the end of the 36-month treatments and reductions in suicide attempts (both P < .001), compared with the previous 3 years, without any differences across the three injectables.
Importantly, tolerability was good for all of the long-acting antidepressants, with reductions in side effects as well as biological parameters compared with previous treatments, notably in the aripiprazole group.
While reductions in weight and prolactin levels were observed in all long-acting treatments, the differences were statistically significant only among patients treated with aripiprazole (P < .05), as was expected.
Two patients treated with aripiprazole discontinued treatment because of side effects from treatment, and the rate was five with paliperidone palmitate and nine with risperidone.
One person in the aripiprazole group discontinued because of a lack of effectiveness, while two discontinued in the paliperidone palmitate group and four with risperidone.
Dr. Fernandez-Miranda noted that “both the intensive case-managed multicomponent treatment and use of high doses of long-acting antipsychotics were in all probability linked to the high adherence and positive clinical outcomes.”
The results provide evidence that “long-acting second-generation antipsychotics are a remarkable option for patients with severe schizophrenia and a background of treatment discontinuation or intolerable adverse effects with other antipsychotics,” Dr. Fernandez-Miranda added.
“We suggest that, in some illness critical conditions, high doses of long-acting second-generation antipsychotics could represent an alternative to clozapine,” he added.
Some hesitation warranted
Commenting on the study, T. Scott Stroup, MD, MPH, professor of psychiatry at Columbia University, New York, noted the key limitations of a lack of randomization and comparison group of clozapine or typical-dose long-acting injectables.
“In addition, pre-post or mirror-image designs may be affected by expectation bias and regression to the mean,” he said in an interview.
“I don’t doubt that some patients do well on relatively high doses of long-acting injectable medications and that some tolerate these doses,” he noted “Most adverse effects are dose related, but without a typical-dose comparison group we cannot assess this.”
Ultimately, Dr. Stroup recommends sticking with standard recommendations – at least to start.
“My take-home message is that clozapine remains the treatment of choice for treatment-resistant schizophrenia, and in most cases clozapine should be tried before considering high-dose long-acting injectables,” he said.
“If there is uncertainty about whether someone is taking a prescribed oral antipsychotic medication, then a trial of a typical dose of a long-acting injectable is a good option to rule out pseudo-treatment resistance.”
Furthermore, “this study doesn’t affect the recommendation that people who need antipsychotic medications should receive the lowest effective dose,” he said.
The authors and Dr. Stroup had no disclosures to report.
NEW ORLEANS – Patients with severe schizophrenia who fail to respond to treatment with standard doses of second-generation antipsychotics show significant improvement with – and tolerance of – higher maintenance doses of the drugs, new research shows.
“The use of [higher doses of] long-acting injectable second-generation antipsychotics shows improvement not only in treatment adherence, but also in diminished relapses and suicide attempts compared with other previous treatment options used with these severely ill patients,” lead author Juan Jose Fernandez-Miranda, MD, said in an interview.
Dr. Fernandez-Miranda, of the Mental Health Service of the Principality of Asturias, in Gijón, Spain, underscored the tolerability of the novel approach of high doses: “No important side effects were found, and less than occurred with previous treatments,” he said.
While higher doses of second-generation antipsychotics for patients with treatment refractory schizophrenia are sometimes considered necessary, particularly with acute psychosis, evidence of benefits of the approach is lacking, and there are concerns about adverse events such as extrapyramidal symptoms and hyperprolactinemia.
To investigate the effects, the authors evaluated patients in a community-based, case managed program with severe, (CGI-S = 5), resistant schizophrenia.
All had been treated in the previous 3 years with at least two different antipsychotics, including clozapine in a few cases, with poor outcomes when receiving standard doses, and eligibility included being at risk of medication noncompliance, and/or experiencing a lack of effectiveness or adverse effects with previous antipsychotics.
For the second 3 years of the observational study, they were treated with doses of at least 75 mg of risperidone long-acting injectable (n = 60), 175 mg or more of monthly paliperidone palmitate (n = 60), or 600 mg or higher of aripiprazole once monthly (n = 30).
During the study, the average antipsychotic doses were: risperidone 111.2 mg/14 days; paliperidone palmitate 231.2 mg. eq./28 days; and aripiprazole 780 mg/28 days. In addition to the intensive pharmacological intervention, patients received psychosocial integrated intervention, as in the previous 3 years.
Over the 3 years with the higher maintenance doses, significant improvements were observed with all of the injectable treatment groups in terms of decreases on the Clinical Global Impression Scale – Severity score (CGI-S; P < .01) and in the four areas of the World Health Organization Disability Schedule (WHO-DAS), including in self-care, occupational, family, and social measures (P < .01 through P < .001).
Scores on the Medication Adherence Rating Scale (MARS), increased with all of the long-acting injectables (P < .01), particularly with paliperidone palmitate and aripiprazole.
Patients had significant decreases in hospital admissions at the end of the 36-month treatments and reductions in suicide attempts (both P < .001), compared with the previous 3 years, without any differences across the three injectables.
Importantly, tolerability was good for all of the long-acting antidepressants, with reductions in side effects as well as biological parameters compared with previous treatments, notably in the aripiprazole group.
While reductions in weight and prolactin levels were observed in all long-acting treatments, the differences were statistically significant only among patients treated with aripiprazole (P < .05), as was expected.
Two patients treated with aripiprazole discontinued treatment because of side effects from treatment, and the rate was five with paliperidone palmitate and nine with risperidone.
One person in the aripiprazole group discontinued because of a lack of effectiveness, while two discontinued in the paliperidone palmitate group and four with risperidone.
Dr. Fernandez-Miranda noted that “both the intensive case-managed multicomponent treatment and use of high doses of long-acting antipsychotics were in all probability linked to the high adherence and positive clinical outcomes.”
The results provide evidence that “long-acting second-generation antipsychotics are a remarkable option for patients with severe schizophrenia and a background of treatment discontinuation or intolerable adverse effects with other antipsychotics,” Dr. Fernandez-Miranda added.
“We suggest that, in some illness critical conditions, high doses of long-acting second-generation antipsychotics could represent an alternative to clozapine,” he added.
Some hesitation warranted
Commenting on the study, T. Scott Stroup, MD, MPH, professor of psychiatry at Columbia University, New York, noted the key limitations of a lack of randomization and comparison group of clozapine or typical-dose long-acting injectables.
“In addition, pre-post or mirror-image designs may be affected by expectation bias and regression to the mean,” he said in an interview.
“I don’t doubt that some patients do well on relatively high doses of long-acting injectable medications and that some tolerate these doses,” he noted “Most adverse effects are dose related, but without a typical-dose comparison group we cannot assess this.”
Ultimately, Dr. Stroup recommends sticking with standard recommendations – at least to start.
“My take-home message is that clozapine remains the treatment of choice for treatment-resistant schizophrenia, and in most cases clozapine should be tried before considering high-dose long-acting injectables,” he said.
“If there is uncertainty about whether someone is taking a prescribed oral antipsychotic medication, then a trial of a typical dose of a long-acting injectable is a good option to rule out pseudo-treatment resistance.”
Furthermore, “this study doesn’t affect the recommendation that people who need antipsychotic medications should receive the lowest effective dose,” he said.
The authors and Dr. Stroup had no disclosures to report.
AT APA 2022
Early metformin minimizes antipsychotic-induced weight gain
MAR DEL PLATA, ARGENTINA – , according to a new evidence-based Irish guideline for the management of this common complication in adults with psychoses who are taking medications.
The document was discussed during one of the sessions of the XXXV Argentine Congress of Psychiatry of the Association of Argentine Psychiatrists. The document also was presented by one of its authors at the European Congress on Obesity 2022.
The guideline encourages psychiatrists not to underestimate the adverse metabolic effects of their treatments and encourages them to contemplate and carry out this prevention and management strategy, commented María Delia Michat, PhD, professor of clinical psychiatry and psychopharmacology at the APSA Postgraduate Training Institute, Buenos Aires.
“Although it is always good to work as a team, it is usually we psychiatrists who coordinate the pharmacological treatment of our patients, and we have to know how to manage drugs that can prevent cardiovascular disease,” Dr. Michat said in an interview.
“The new guideline is helpful because it protocolizes the use of metformin, which is the cheapest drug and has the most evidence for antipsychotic-induced weight gain,” she added.
Avoiding metabolic syndrome
In patients with schizophrenia, obesity rates are 40% higher than in the general population, and 80% of patients develop weight gain after their first treatment, noted Dr. Michat. “Right away, weight gain is seen in the first month. And it is a serious problem, because patients with schizophrenia, major depression, or bipolar disorder already have an increased risk of premature mortality, especially from cardiovascular diseases, and they have an increased risk of metabolic syndrome. And we sometimes give drugs that further increase that risk,” she said.
Being overweight is a major criterion for defining metabolic syndrome. Dr. Michat noted that, among the antipsychotic drugs that increase weight the most are clozapine, olanzapine, chlorpromazine, quetiapine, and risperidone, in addition to other psychoactive drugs, such as valproic acid, lithium, mirtazapine, and tricyclic antidepressants.
Several clinical trials, such as a pioneering Chinese study from 2008, have shown the potential of metformin to mitigate the weight gain induced by this type of drug.
However, Dr. Michat noted that so far the major guidelines (for example, the Canadian Network for Mood and Anxiety Treatments [CANMAT]/International Society for Bipolar Disorders [ISBD] for bipolar disorder and the American Psychiatric Association [APA] for schizophrenia) “say very little” on how to address this complication. They propose what she defined as a “problematic” order of action in which the initial emphasis is on promoting lifestyle changes, which are difficult for these patients to carry out, as well as general proposals for changing medication (which is not simple to implement when the patient’s condition is stabilized) and eventual consultation with a clinician to start therapy with metformin or other drugs, such as liraglutide, semaglutide, and topiramate.
The new clinical practice guideline, which was published in Evidence-Based Mental Health (of the BMJ journal group), was written by a multidisciplinary team of pharmacists, psychiatrists, and mental health nurses from Ireland. It aims to fill that gap. The investigators reviewed 1,270 scientific articles and analyzed 26 of them in depth, including seven randomized clinical trials and a 2016 systematic review and meta-analysis. The authors made a “strong” recommendation, for which there was moderate-quality evidence, that for patients for whom a lifestyle intervention is unacceptable or inappropriate the use of metformin is an “alternative first-line intervention” for antipsychotic drug–induced weight gain.
Likewise, as a strong recommendation with moderate-quality evidence, the guidance encourages the use of metformin when nonpharmacologic intervention does not seem to be effective.
The guideline also says it is preferable to start metformin early for patients who gain more than 7% of their baseline weight within the first month of antipsychotic treatment. It also endorses metformin when weight gain is established.
Other recommendations include evaluating baseline kidney function before starting metformin treatment and suggest a dose adjustment when the estimated glomerular filtration rate (eGFR) is < 60 mL/min/1.73 m2. The guidance says the use of metformin is contraindicated for patients in whom eGFR is <30 mL/min per 1.73 m2. The proposed starting dosage is 500 mg twice per day with meals, with increments of 500 mg every 1-2 weeks until reaching a target dose of 2,000 mg/day. The guidance recommends that consideration always be given to individual tolerability and efficacy.
Treatment goals should be personalized and agreed upon with patients. In the case of early intervention, the guideline proposes initially stabilizing the weight gained or, if possible, reverse excess weight. When weight gain is established, the goal would be to lose at least 5% of the weight within the next 6 months.
The authors also recommend monitoring kidney function annually, as well as vitamin B12 levels and individual tolerability and compliance. Gastrointestinal adverse effects can be managed by dose reduction or slower dose titration. The risk of lactic acidosis, which affects 4.3 per 100,000 person-years among those taking metformin, can be attenuated by adjusting the dose according to kidney function or avoiding prescribing it to patients who have a history of alcohol abuse or who are receiving treatment that may interact with the drug.
Validating pharmacologic management
The lead author of the new guideline, Ita Fitzgerald, a teacher in clinical pharmacy and senior pharmacist at St. Patrick’s Mental Health Services in Dublin, pointed out that there is a bias toward not using drugs for weight management and shifting the responsibility onto the patients themselves, something that is very often out of their control.
“The purpose of the guideline was to decide on a range of criteria to maximize the use of metformin, to recognize that for many people, pharmacological management is a valid and important option that could and should be more widely used and to provide precise and practical guidance to physicians to facilitate a more widespread use,” Ms. Fitzgerald said in an interview.
According to Fitzgerald, who is pursuing her doctorate at University College Cork (Ireland), one of the most outstanding results of the work is that it highlights that the main benefit of metformin is to flatten rather than reverse antipsychotic-induced weight gain and that indicating it late can nullify that effect.
“In all the recommendations, we try very hard to shift the focus from metformin’s role as a weight reversal agent to one as a weight management agent that should be used early in treatment, which is when most weight gain occurs. If metformin succeeds in flattening that increase, that’s a huge potential benefit for an inexpensive and easily accessible drug. When people have already established weight gain, metformin may not be enough and alternative treatments should be used,” she said.
In addition to its effects on weight, metformin has many other potential health benefits. Of particular importance is that it reduces hyperphagia-mediated antipsychotic-induced weight gain, Ms. Fitzgerald pointed out.
“This is subjectively very important for patients and provides a more positive experience when taking antipsychotics. Antipsychotic-induced weight gain is one of the main reasons for premature discontinuation or incomplete adherence to these drugs and therefore needs to be addressed proactively,” she concluded.
Ms. Fitzgerald and Dr. Michat have disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com. This article was translated from the Medscape Spanish edition.
MAR DEL PLATA, ARGENTINA – , according to a new evidence-based Irish guideline for the management of this common complication in adults with psychoses who are taking medications.
The document was discussed during one of the sessions of the XXXV Argentine Congress of Psychiatry of the Association of Argentine Psychiatrists. The document also was presented by one of its authors at the European Congress on Obesity 2022.
The guideline encourages psychiatrists not to underestimate the adverse metabolic effects of their treatments and encourages them to contemplate and carry out this prevention and management strategy, commented María Delia Michat, PhD, professor of clinical psychiatry and psychopharmacology at the APSA Postgraduate Training Institute, Buenos Aires.
“Although it is always good to work as a team, it is usually we psychiatrists who coordinate the pharmacological treatment of our patients, and we have to know how to manage drugs that can prevent cardiovascular disease,” Dr. Michat said in an interview.
“The new guideline is helpful because it protocolizes the use of metformin, which is the cheapest drug and has the most evidence for antipsychotic-induced weight gain,” she added.
Avoiding metabolic syndrome
In patients with schizophrenia, obesity rates are 40% higher than in the general population, and 80% of patients develop weight gain after their first treatment, noted Dr. Michat. “Right away, weight gain is seen in the first month. And it is a serious problem, because patients with schizophrenia, major depression, or bipolar disorder already have an increased risk of premature mortality, especially from cardiovascular diseases, and they have an increased risk of metabolic syndrome. And we sometimes give drugs that further increase that risk,” she said.
Being overweight is a major criterion for defining metabolic syndrome. Dr. Michat noted that, among the antipsychotic drugs that increase weight the most are clozapine, olanzapine, chlorpromazine, quetiapine, and risperidone, in addition to other psychoactive drugs, such as valproic acid, lithium, mirtazapine, and tricyclic antidepressants.
Several clinical trials, such as a pioneering Chinese study from 2008, have shown the potential of metformin to mitigate the weight gain induced by this type of drug.
However, Dr. Michat noted that so far the major guidelines (for example, the Canadian Network for Mood and Anxiety Treatments [CANMAT]/International Society for Bipolar Disorders [ISBD] for bipolar disorder and the American Psychiatric Association [APA] for schizophrenia) “say very little” on how to address this complication. They propose what she defined as a “problematic” order of action in which the initial emphasis is on promoting lifestyle changes, which are difficult for these patients to carry out, as well as general proposals for changing medication (which is not simple to implement when the patient’s condition is stabilized) and eventual consultation with a clinician to start therapy with metformin or other drugs, such as liraglutide, semaglutide, and topiramate.
The new clinical practice guideline, which was published in Evidence-Based Mental Health (of the BMJ journal group), was written by a multidisciplinary team of pharmacists, psychiatrists, and mental health nurses from Ireland. It aims to fill that gap. The investigators reviewed 1,270 scientific articles and analyzed 26 of them in depth, including seven randomized clinical trials and a 2016 systematic review and meta-analysis. The authors made a “strong” recommendation, for which there was moderate-quality evidence, that for patients for whom a lifestyle intervention is unacceptable or inappropriate the use of metformin is an “alternative first-line intervention” for antipsychotic drug–induced weight gain.
Likewise, as a strong recommendation with moderate-quality evidence, the guidance encourages the use of metformin when nonpharmacologic intervention does not seem to be effective.
The guideline also says it is preferable to start metformin early for patients who gain more than 7% of their baseline weight within the first month of antipsychotic treatment. It also endorses metformin when weight gain is established.
Other recommendations include evaluating baseline kidney function before starting metformin treatment and suggest a dose adjustment when the estimated glomerular filtration rate (eGFR) is < 60 mL/min/1.73 m2. The guidance says the use of metformin is contraindicated for patients in whom eGFR is <30 mL/min per 1.73 m2. The proposed starting dosage is 500 mg twice per day with meals, with increments of 500 mg every 1-2 weeks until reaching a target dose of 2,000 mg/day. The guidance recommends that consideration always be given to individual tolerability and efficacy.
Treatment goals should be personalized and agreed upon with patients. In the case of early intervention, the guideline proposes initially stabilizing the weight gained or, if possible, reverse excess weight. When weight gain is established, the goal would be to lose at least 5% of the weight within the next 6 months.
The authors also recommend monitoring kidney function annually, as well as vitamin B12 levels and individual tolerability and compliance. Gastrointestinal adverse effects can be managed by dose reduction or slower dose titration. The risk of lactic acidosis, which affects 4.3 per 100,000 person-years among those taking metformin, can be attenuated by adjusting the dose according to kidney function or avoiding prescribing it to patients who have a history of alcohol abuse or who are receiving treatment that may interact with the drug.
Validating pharmacologic management
The lead author of the new guideline, Ita Fitzgerald, a teacher in clinical pharmacy and senior pharmacist at St. Patrick’s Mental Health Services in Dublin, pointed out that there is a bias toward not using drugs for weight management and shifting the responsibility onto the patients themselves, something that is very often out of their control.
“The purpose of the guideline was to decide on a range of criteria to maximize the use of metformin, to recognize that for many people, pharmacological management is a valid and important option that could and should be more widely used and to provide precise and practical guidance to physicians to facilitate a more widespread use,” Ms. Fitzgerald said in an interview.
According to Fitzgerald, who is pursuing her doctorate at University College Cork (Ireland), one of the most outstanding results of the work is that it highlights that the main benefit of metformin is to flatten rather than reverse antipsychotic-induced weight gain and that indicating it late can nullify that effect.
“In all the recommendations, we try very hard to shift the focus from metformin’s role as a weight reversal agent to one as a weight management agent that should be used early in treatment, which is when most weight gain occurs. If metformin succeeds in flattening that increase, that’s a huge potential benefit for an inexpensive and easily accessible drug. When people have already established weight gain, metformin may not be enough and alternative treatments should be used,” she said.
In addition to its effects on weight, metformin has many other potential health benefits. Of particular importance is that it reduces hyperphagia-mediated antipsychotic-induced weight gain, Ms. Fitzgerald pointed out.
“This is subjectively very important for patients and provides a more positive experience when taking antipsychotics. Antipsychotic-induced weight gain is one of the main reasons for premature discontinuation or incomplete adherence to these drugs and therefore needs to be addressed proactively,” she concluded.
Ms. Fitzgerald and Dr. Michat have disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com. This article was translated from the Medscape Spanish edition.
MAR DEL PLATA, ARGENTINA – , according to a new evidence-based Irish guideline for the management of this common complication in adults with psychoses who are taking medications.
The document was discussed during one of the sessions of the XXXV Argentine Congress of Psychiatry of the Association of Argentine Psychiatrists. The document also was presented by one of its authors at the European Congress on Obesity 2022.
The guideline encourages psychiatrists not to underestimate the adverse metabolic effects of their treatments and encourages them to contemplate and carry out this prevention and management strategy, commented María Delia Michat, PhD, professor of clinical psychiatry and psychopharmacology at the APSA Postgraduate Training Institute, Buenos Aires.
“Although it is always good to work as a team, it is usually we psychiatrists who coordinate the pharmacological treatment of our patients, and we have to know how to manage drugs that can prevent cardiovascular disease,” Dr. Michat said in an interview.
“The new guideline is helpful because it protocolizes the use of metformin, which is the cheapest drug and has the most evidence for antipsychotic-induced weight gain,” she added.
Avoiding metabolic syndrome
In patients with schizophrenia, obesity rates are 40% higher than in the general population, and 80% of patients develop weight gain after their first treatment, noted Dr. Michat. “Right away, weight gain is seen in the first month. And it is a serious problem, because patients with schizophrenia, major depression, or bipolar disorder already have an increased risk of premature mortality, especially from cardiovascular diseases, and they have an increased risk of metabolic syndrome. And we sometimes give drugs that further increase that risk,” she said.
Being overweight is a major criterion for defining metabolic syndrome. Dr. Michat noted that, among the antipsychotic drugs that increase weight the most are clozapine, olanzapine, chlorpromazine, quetiapine, and risperidone, in addition to other psychoactive drugs, such as valproic acid, lithium, mirtazapine, and tricyclic antidepressants.
Several clinical trials, such as a pioneering Chinese study from 2008, have shown the potential of metformin to mitigate the weight gain induced by this type of drug.
However, Dr. Michat noted that so far the major guidelines (for example, the Canadian Network for Mood and Anxiety Treatments [CANMAT]/International Society for Bipolar Disorders [ISBD] for bipolar disorder and the American Psychiatric Association [APA] for schizophrenia) “say very little” on how to address this complication. They propose what she defined as a “problematic” order of action in which the initial emphasis is on promoting lifestyle changes, which are difficult for these patients to carry out, as well as general proposals for changing medication (which is not simple to implement when the patient’s condition is stabilized) and eventual consultation with a clinician to start therapy with metformin or other drugs, such as liraglutide, semaglutide, and topiramate.
The new clinical practice guideline, which was published in Evidence-Based Mental Health (of the BMJ journal group), was written by a multidisciplinary team of pharmacists, psychiatrists, and mental health nurses from Ireland. It aims to fill that gap. The investigators reviewed 1,270 scientific articles and analyzed 26 of them in depth, including seven randomized clinical trials and a 2016 systematic review and meta-analysis. The authors made a “strong” recommendation, for which there was moderate-quality evidence, that for patients for whom a lifestyle intervention is unacceptable or inappropriate the use of metformin is an “alternative first-line intervention” for antipsychotic drug–induced weight gain.
Likewise, as a strong recommendation with moderate-quality evidence, the guidance encourages the use of metformin when nonpharmacologic intervention does not seem to be effective.
The guideline also says it is preferable to start metformin early for patients who gain more than 7% of their baseline weight within the first month of antipsychotic treatment. It also endorses metformin when weight gain is established.
Other recommendations include evaluating baseline kidney function before starting metformin treatment and suggest a dose adjustment when the estimated glomerular filtration rate (eGFR) is < 60 mL/min/1.73 m2. The guidance says the use of metformin is contraindicated for patients in whom eGFR is <30 mL/min per 1.73 m2. The proposed starting dosage is 500 mg twice per day with meals, with increments of 500 mg every 1-2 weeks until reaching a target dose of 2,000 mg/day. The guidance recommends that consideration always be given to individual tolerability and efficacy.
Treatment goals should be personalized and agreed upon with patients. In the case of early intervention, the guideline proposes initially stabilizing the weight gained or, if possible, reverse excess weight. When weight gain is established, the goal would be to lose at least 5% of the weight within the next 6 months.
The authors also recommend monitoring kidney function annually, as well as vitamin B12 levels and individual tolerability and compliance. Gastrointestinal adverse effects can be managed by dose reduction or slower dose titration. The risk of lactic acidosis, which affects 4.3 per 100,000 person-years among those taking metformin, can be attenuated by adjusting the dose according to kidney function or avoiding prescribing it to patients who have a history of alcohol abuse or who are receiving treatment that may interact with the drug.
Validating pharmacologic management
The lead author of the new guideline, Ita Fitzgerald, a teacher in clinical pharmacy and senior pharmacist at St. Patrick’s Mental Health Services in Dublin, pointed out that there is a bias toward not using drugs for weight management and shifting the responsibility onto the patients themselves, something that is very often out of their control.
“The purpose of the guideline was to decide on a range of criteria to maximize the use of metformin, to recognize that for many people, pharmacological management is a valid and important option that could and should be more widely used and to provide precise and practical guidance to physicians to facilitate a more widespread use,” Ms. Fitzgerald said in an interview.
According to Fitzgerald, who is pursuing her doctorate at University College Cork (Ireland), one of the most outstanding results of the work is that it highlights that the main benefit of metformin is to flatten rather than reverse antipsychotic-induced weight gain and that indicating it late can nullify that effect.
“In all the recommendations, we try very hard to shift the focus from metformin’s role as a weight reversal agent to one as a weight management agent that should be used early in treatment, which is when most weight gain occurs. If metformin succeeds in flattening that increase, that’s a huge potential benefit for an inexpensive and easily accessible drug. When people have already established weight gain, metformin may not be enough and alternative treatments should be used,” she said.
In addition to its effects on weight, metformin has many other potential health benefits. Of particular importance is that it reduces hyperphagia-mediated antipsychotic-induced weight gain, Ms. Fitzgerald pointed out.
“This is subjectively very important for patients and provides a more positive experience when taking antipsychotics. Antipsychotic-induced weight gain is one of the main reasons for premature discontinuation or incomplete adherence to these drugs and therefore needs to be addressed proactively,” she concluded.
Ms. Fitzgerald and Dr. Michat have disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com. This article was translated from the Medscape Spanish edition.
Dexmedetomidine sublingual film for agitation
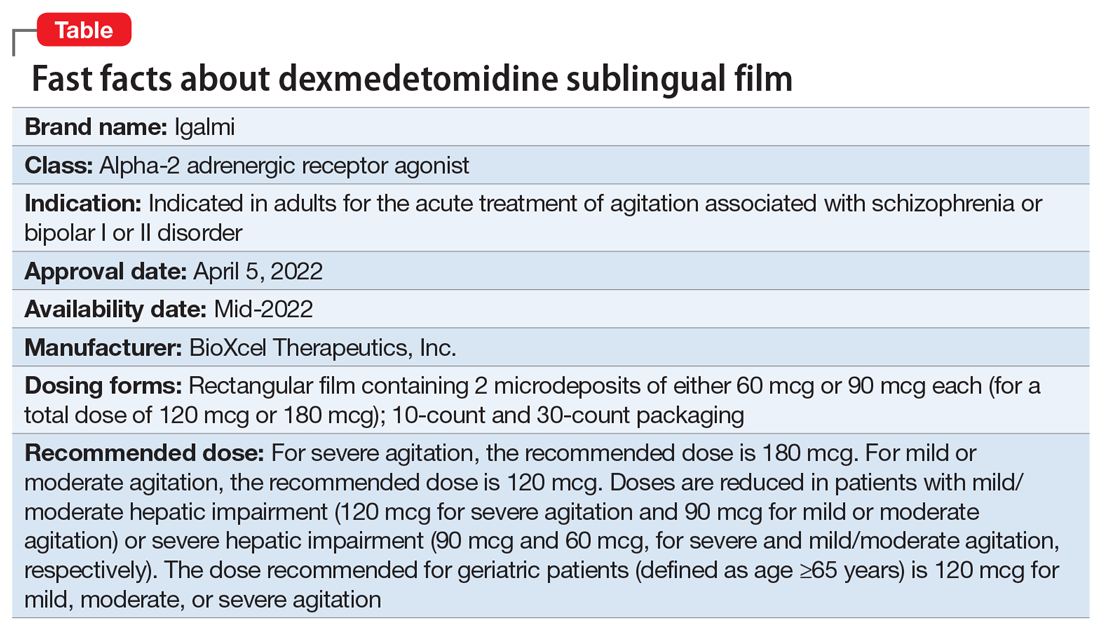
Approved by the FDA on April 5, 2022, dexmedetomidine sublingual film (Igalmi, manufactured and distributed by BioXcel Therapeutics, Inc., New Haven, CT USA) is indicated in adults for the acute treatment of agitation associated with schizophrenia or bipolar I or II disorder (Table).1,2 It is administered sublingually or buccally under the supervision of a health care provider. After administration, patients should have their vital signs and alertness assessed but there is no FDA Risk Evaluation and Mitigation Strategy (REMS) required for use. A limitation of use is that the safety and effectiveness of dexmedetomidine sublingual film has not been established beyond 24 hours from the first dose.2 There are no contraindications for use.2
Dexmedetomidine is a well-known efficacious alpha-2 adrenergic receptor agonist available since 1999 in an IV formulation indicated for sedation of initially intubated and mechanically ventilated patients in an ICU setting, and sedation of nonintubated patients prior to and/or during surgical and other procedures.3,4 The reformulation of dexmedetomidine as a sublingual film allows the broader use of this agent in psychiatric settings when managing agitation in patients with schizophrenia or bipolar disorder, and thus potentially avoiding the use of IM administration of antipsychotics and/or benzodiazepines. Noninvasive formulations, although requiring cooperation from patients, have the potential to improve overall patient experience, thereby improving future cooperation between patients and health care professionals.5
Dosing
Dexmedetomidine sublingual film is distributed commercially in the following strengths: 180 mcg and 120 mcg. It consists of a lightly mint-flavored, rectangular film containing 2 microdeposits of dexmedetomidine hydrochloride. Dosage strengths of 90 mcg and 60 mcg are available by cutting the 180 mcg or 120 mcg film in half
If agitation persists after the initial dose, up to 2 additional doses (90 mcg if the initial dose was 180 mcg, otherwise 60 mcg if the initial dose was 120, 90, or 60 mcg) may be given at least 2 hours apart. Assessment of vital signs, including orthostatic measurements, is required prior to the administration of any subsequent doses. Due to risk of hypotension, additional doses are not recommended in patients with systolic blood pressure <90 mm Hg, diastolic blood pressure <60 mm Hg, heart rate <60 beats per minute, or postural decrease in systolic blood pressure ≥20 mm Hg or in diastolic blood pressure ≥10 mm Hg.
Mechanism of action and pharmacodynamics
Dexmedetomidine is an alpha-2 adrenergic receptor agonist and the mechanism of action in the acute treatment of agitation is thought to be due to activation of presynaptic alpha-2 adrenergic receptors.2 Binding affinities (Ki values) are 4 to 6 nM at the alpha-2 adrenergic receptor subtypes.2
Dexmedetomidine exhibits concentration-dependent QT prolongation, with mean QTc increases from baseline from 6 msec (120 mcg single dose) to 11 msec (180 mcg plus 2 additional doses of 90 mcg 2 hours apart for a total of 3 doses).2 Placing the observation about QTc prolongation into clinical context, studies of IM administration of ziprasidone 20 mg and 30 mg and haloperidol 7.5 mg and 10 mg resulted in changes of the QTc interval of 4.6 msec and 6.0 msec, respectively, after 1 dose.6 After a second injection, these values were 12.8 msec and 14.7 msec, respectively.6
Clinical pharmacokinetics
The sublingual film formulation is absorbed orally, bypassing first-pass metabolism, and achieving higher dexmedetomidine bioavailability than ingested formulations.7 Exposure is dose-dependent, with dexmedetomidine being quantifiable in plasma after 5 to 20 minutes post dosing, and with a plasma half-life of 2 to 3 hours.2,8 Mean time for the film to dissolve in the mouth was approximately 6 to 8 minutes following sublingual administration, and 18 minutes following buccal administration.2 Absolute bioavailability was approximately 72% and 82% following sublingual and buccal administration, respectively.2 Mean maximal plasma concentrations of dexmedetomidine were reached approximately 2 hours after sublingual or buccal administration.2 Compared to drinking water at 2 hours post administration, early water intake (as early as 15 minutes post-dose) had minimal effects on the rate or extent of sublingual absorption but was not assessed after buccal administration.2 The average protein binding was 94% and was constant across the different plasma concentrations evaluated and similar in males and females, but significantly decreased in participants with hepatic impairment compared to healthy individuals.2 In contrast, the pharmacokinetic profile of dexmedetomidine is not significantly different in patients with creatinine clearance <30 mL/minute compared to those with normal renal function.2 Dexmedetomidine undergoes almost complete biotransformation to inactive metabolites via direct glucuronidation as well as cytochrome P450 (CYP) (primarily CYP2A6)–mediated metabolism.2 There is no evidence of any CYP–mediated drug interactions that are likely to be of clinical relevance.2
Continue to: Efficacy
Efficacy
The efficacy and tolerability of 120 mcg and 180 mcg doses of dexmedetomidine sublingual film was evaluated in 2 similarly designed, randomized, double-blind, placebo-controlled, Phase 3 trials in the treatment of acute agitation associated with schizophrenia, schizoaffective, or schizophreniform disorder9 and bipolar I or II disorder.10 These studies included a total of 758 adult patients age range 18 to 71 (mean age approximately 46.5), with about 59% male participants.2 In contrast to other agents approved by the FDA for treatment of agitation associated with bipolar disorder, dexmedetomidine sublingual film was assessed in patients regardless of polarity (manic, mixed features, or depressed).5 The primary efficacy measure for the dexmedetomidine sublingual film studies was the investigator-administered Positive and Negative Syndrome Scale-Excited Component (PANSS-EC), consisting of the following 5 items: excitement, tension, hostility, uncooperativeness, and poor impulse control.11 The items from the PANSS-EC are rated from 1 (not present) to 7 (extremely severe) and thus the total scores range from 5 to 35. For enrollment in the studies, patients had to be judged to be clinically agitated with a total PANSS-EC score ≥14, with at least 1 individual item score ≥4.2
After study medication administration, the PANSS-EC was assessed from 10 minutes through 24 hours, with the primary endpoint being at 2 hours post-dose. Patients with schizophrenia or bipolar disorder who were treated with dexmedetomidine sublingual film 120 mcg or 180 mcg had superior symptomatic improvements from baseline to 2 hours post-dose compared to placebo, with treatment effects beginning as early as 20 to 30 minutes post-dose (for patients with schizophrenia, dexmedetomidine was statistically significantly superior to placebo beginning at 20 minutes following dosing with the 180 mcg dose and 30 minutes after the 120 mcg dose; for patients with bipolar disorder, differences from placebo were statistically significant beginning at 20 minutes after treatment with both the 120 mcg and 180 mcg doses).2 Evaluation of effect size for dexmedetomidine vs placebo for PANSS-EC response at 2 hours (defined as ≥40% improvement from baseline) resulted in a number needed to treat (NNT) of 3 when combining both studies and both doses,12 comparing favorably with the NNT values observed for IM formulations of aripiprazole, haloperidol, lorazepam, olanzapine, and ziprasidone,13 and inhaled loxapine.14
Overall tolerability and safety
The highlights of the prescribing information contain warnings and precautions regarding hypotension/orthostatic hypotension/bradycardia, QT interval prolongation, and somnolence.2 Advice is provided to ensure that patients are alert and not experiencing orthostatic or symptomatic hypotension prior to resuming ambulation, a concern commonly raised when assessing potential treatments for agitation.15 Dexmedetomidine sublingual film should be avoided in patients with risk factors for prolonged QT interval, a precaution that was evident for the use of ziprasidone16 and where an effect is also noted with haloperidol.6 As per the prescribing information, the most common adverse reactions (incidence ≥5% and at least twice the rate of placebo) are somnolence, oral paresthesia or oral hypoesthesia, dizziness, dry mouth, hypotension, and orthostatic hypotension. Rates of adverse reactions of somnolence (including fatigue and sluggishness) with dexmedetomidine 120 mcg or 180 mcg are almost the same (22% and 23%, respectively), and higher than the 6% observed with placebo.2 Other adverse reactions are substantially lower in frequency. These include oral paresthesia or oral hypoesthesia (6%, 7%, and 1%, for dexmedetomidine 120 mcg, 180 mcg, or placebo, respectively), dizziness (4%, 6%, 1%), hypotension (5%, 5%, 0%), orthostatic hypotension (3%, 5%, <1%), dry mouth (7%, 4%, 1%), nausea (2%, 3%, 2%), bradycardia (2%, 2%, 0%), and abdominal discomfort (0%, 2%, 1%).2
Regarding dose-dependent changes in blood pressure during the studies, 16%, 18%, and 9% of patients treated with 120 mcg, 180 mcg, and placebo, respectively, experienced orthostatic hypotension at 2 hours post dose. However, at 24 hours, none of the patients in the 180-mcg group experienced a systolic blood pressure ≤90 mm Hg with a decrease ≥20 mm Hg, compared with one patient (<1%) in the 120-mcg group and none in the placebo group.2
The prescribing information advises that concomitant use of dexmedetomidine sublingual film with anesthetics, sedatives, hypnotics, or opioids is likely to lead to enhanced CNS depressant effects, and that the prescriber should consider a reduction in dosage of dexmedetomidine or the concomitant anesthetic, sedative, hypnotic, or opioid.2
Summary
Dexmedetomidine sublingual film is an oral medication indicated in adults for the acute treatment of agitation associated with schizophrenia or bipolar I or II disorder. The recommended dose depends on severity of agitation, age, and the presence of hepatic impairment. A dose of 180 mcg is recommended for severe agitation and a dose of 120 mcg is recommended for mild or moderate agitation, with doses adjusted lower in the presence of hepatic impairment. There are no contraindications but there are warnings and precautions regarding hypotension/orthostatic hypotension/bradycardia, QT interval prolongation, and somnolence. Clinicians should monitor vital signs and alertness after administration to prevent falls and syncope; however, there is no FDA REMS required for use. The clinical trial evidence supporting the use of dexmedetomidine is robust, with evidence of a treatment effect as early as 20 minutes after administration. Noninvasive formulations, although requiring cooperation from patients, have the potential to improve overall patient experience, thereby improving future cooperation between patients and health care professionals.
Bottom Line
Dexmedetomidine sublingual film provides an opportunity to rethink the approach to the management of agitation and avoid the potentially unnecessary use of IM injections. Dexmedetomidine sublingual film acts rapidly and is simple to use.
Related Resources
- Dexmedetomidine sublingual film (Iglami) prescribing information. https://www.igalmihcp.com/igalmi-pi.pdf
Drug Brand Names
Aripiprazole • Abilify
Dexmedetomidine • Igalmi, Precedex
Haloperidol • Haldol
Lorazepam • Ativan
Loxapine inhaled • Adasuve
Olanzapine • Zyprexa
Ziprasidone • Geodon
1. US Food and Drug Administration. NDA 215390 Approval Letter. Accessed April 5, 2022. https://www.accessdata.fda.gov/drugsatfda_docs/appletter/2022/215390Orig1s000ltr.pdf
2. Igalmi [package insert]. BioXcel Therapeutics, Inc; 2022.
3. Weerink MAS, Struys MMRF, Hannivoort LN, et al. Clinical pharmacokinetics and pharmacodynamics of dexmedetomidine. Clin Pharmacokinet. 2017;56(8):893-913. doi:10.1007/s40262-017-0507-7
4. Precedex [package insert]. Hospira, Inc; 2021.
5. Zeller SL, Citrome L. Managing agitation associated with schizophrenia and bipolar disorder in the emergency setting. West J Emerg Med. 2016;17(2):165-172. doi:10.5811/westjem.2015.12.28763
6. Miceli JJ, Tensfeldt TG, Shiovitz T, et al. Effects of high-dose ziprasidone and haloperidol on the QTc interval after intramuscular administration: a randomized, single-blind, parallel-group study in patients with schizophrenia or schizoaffective disorder. Clin Ther. 2010;32(3):472-491. doi:10.1016/j.clinthera.2010.03.003
7. Yocca F, DeVivo M, Seth S, et al. Dexmedetomidine—highly favorable pharmacokinetic and pharmacological features for a CNS therapeutic drug. Poster presented at: 58th Annual Meeting of the American College of Neuropsychopharmacology; December 8-11, 2019; Orlando, FL.
8. Adedoyin A, Preskorn S, Lathia CD. Pharmacokinetics of dexmedetomidine after a single sublingual dose of BXCL501 in patients with agitation associated with schizophrenia. Poster presented at: 23rd Annual Conference of the International Society for Bipolar Disorders; May 13-15, 2021. Virtual. Session 17.
9. Citrome LL, Lauriello J, Risinger R, et al. A novel rapidly effective treatment of agitation for schizophrenia with the oral dissolving film BXCL501. Poster presented at: American Psychiatric Association Annual Meeting; May 1-3, 2021. Virtual. Accessed November 11, 2021. https://www.psychiatry.org/File%20Library/Psychiatrists/Meetings/Annual-Meeting/2021/2021-APA-Annual-Meeting-Poster-Proceedings.pdf
10. Preskorn SH, Zeller S, Citrome L, et al. Effect of sublingual dexmedetomidine vs placebo on acute agitation associated with bipolar disorder: a randomized clinical trial. JAMA. 2022;327(8):727-736. doi:10.1001/jama.2022.0799
11. Montoya A, Valladares A, Lizán L, et al. Validation of the Excited Component of the Positive and Negative Syndrome Scale (PANSS-EC) in a naturalistic sample of 278 patients with acute psychosis and agitation in a psychiatric emergency room. Health Qual Life Outcomes. 2011;9:18. doi:10.1186/1477-7525-9-18
12. Citrome L, Palko L, Hokett S, et al. Number needed to treat and number needed to harm from two phase 3 studies of BXCL501 for treating acute agitation in patients with schizophrenia and bipolar disorder. Poster presented at: Academy of Managed Care Pharmacy Nexus 2021; October 18-21, 2021; Denver, CO.
13. Citrome L. Comparison of intramuscular ziprasidone, olanzapine, or aripiprazole for agitation: a quantitative review of efficacy and safety. J Clin Psychiatry. 2007;68(12):1876-1885. doi:10.4088/jcp.v68n1207
14. Citrome L. Inhaled loxapine for agitation revisited: focus on effect sizes from 2 Phase III randomised controlled trials in persons with schizophrenia or bipolar disorder. Int J Clin Pract. 2012;66(3):318-325. doi:10.1111/j.1742-1241.2011.02890.x
15. Wilson MP, Pepper D, Currier GW, et al. The psychopharmacology of agitation: consensus statement of the American Association for Emergency Psychiatry project Beta psychopharmacology workgroup. West J Emerg Med. 2012;13(1):26-34. doi:10.5811/westjem.2011.9.6866
16. Zimbroff DL, Allen MH, Battaglia J, et al. Best clinical practice with ziprasidone IM: update after 2 years of experience. CNS Spectr. 2005;10(9):1-15. doi:10.1017/s1092852900025487
Approved by the FDA on April 5, 2022, dexmedetomidine sublingual film (Igalmi, manufactured and distributed by BioXcel Therapeutics, Inc., New Haven, CT USA) is indicated in adults for the acute treatment of agitation associated with schizophrenia or bipolar I or II disorder (Table).1,2 It is administered sublingually or buccally under the supervision of a health care provider. After administration, patients should have their vital signs and alertness assessed but there is no FDA Risk Evaluation and Mitigation Strategy (REMS) required for use. A limitation of use is that the safety and effectiveness of dexmedetomidine sublingual film has not been established beyond 24 hours from the first dose.2 There are no contraindications for use.2

Dexmedetomidine is a well-known efficacious alpha-2 adrenergic receptor agonist available since 1999 in an IV formulation indicated for sedation of initially intubated and mechanically ventilated patients in an ICU setting, and sedation of nonintubated patients prior to and/or during surgical and other procedures.3,4 The reformulation of dexmedetomidine as a sublingual film allows the broader use of this agent in psychiatric settings when managing agitation in patients with schizophrenia or bipolar disorder, and thus potentially avoiding the use of IM administration of antipsychotics and/or benzodiazepines. Noninvasive formulations, although requiring cooperation from patients, have the potential to improve overall patient experience, thereby improving future cooperation between patients and health care professionals.5
Dosing
Dexmedetomidine sublingual film is distributed commercially in the following strengths: 180 mcg and 120 mcg. It consists of a lightly mint-flavored, rectangular film containing 2 microdeposits of dexmedetomidine hydrochloride. Dosage strengths of 90 mcg and 60 mcg are available by cutting the 180 mcg or 120 mcg film in half
If agitation persists after the initial dose, up to 2 additional doses (90 mcg if the initial dose was 180 mcg, otherwise 60 mcg if the initial dose was 120, 90, or 60 mcg) may be given at least 2 hours apart. Assessment of vital signs, including orthostatic measurements, is required prior to the administration of any subsequent doses. Due to risk of hypotension, additional doses are not recommended in patients with systolic blood pressure <90 mm Hg, diastolic blood pressure <60 mm Hg, heart rate <60 beats per minute, or postural decrease in systolic blood pressure ≥20 mm Hg or in diastolic blood pressure ≥10 mm Hg.
Mechanism of action and pharmacodynamics
Dexmedetomidine is an alpha-2 adrenergic receptor agonist and the mechanism of action in the acute treatment of agitation is thought to be due to activation of presynaptic alpha-2 adrenergic receptors.2 Binding affinities (Ki values) are 4 to 6 nM at the alpha-2 adrenergic receptor subtypes.2
Dexmedetomidine exhibits concentration-dependent QT prolongation, with mean QTc increases from baseline from 6 msec (120 mcg single dose) to 11 msec (180 mcg plus 2 additional doses of 90 mcg 2 hours apart for a total of 3 doses).2 Placing the observation about QTc prolongation into clinical context, studies of IM administration of ziprasidone 20 mg and 30 mg and haloperidol 7.5 mg and 10 mg resulted in changes of the QTc interval of 4.6 msec and 6.0 msec, respectively, after 1 dose.6 After a second injection, these values were 12.8 msec and 14.7 msec, respectively.6
Clinical pharmacokinetics
The sublingual film formulation is absorbed orally, bypassing first-pass metabolism, and achieving higher dexmedetomidine bioavailability than ingested formulations.7 Exposure is dose-dependent, with dexmedetomidine being quantifiable in plasma after 5 to 20 minutes post dosing, and with a plasma half-life of 2 to 3 hours.2,8 Mean time for the film to dissolve in the mouth was approximately 6 to 8 minutes following sublingual administration, and 18 minutes following buccal administration.2 Absolute bioavailability was approximately 72% and 82% following sublingual and buccal administration, respectively.2 Mean maximal plasma concentrations of dexmedetomidine were reached approximately 2 hours after sublingual or buccal administration.2 Compared to drinking water at 2 hours post administration, early water intake (as early as 15 minutes post-dose) had minimal effects on the rate or extent of sublingual absorption but was not assessed after buccal administration.2 The average protein binding was 94% and was constant across the different plasma concentrations evaluated and similar in males and females, but significantly decreased in participants with hepatic impairment compared to healthy individuals.2 In contrast, the pharmacokinetic profile of dexmedetomidine is not significantly different in patients with creatinine clearance <30 mL/minute compared to those with normal renal function.2 Dexmedetomidine undergoes almost complete biotransformation to inactive metabolites via direct glucuronidation as well as cytochrome P450 (CYP) (primarily CYP2A6)–mediated metabolism.2 There is no evidence of any CYP–mediated drug interactions that are likely to be of clinical relevance.2
Continue to: Efficacy
Efficacy
The efficacy and tolerability of 120 mcg and 180 mcg doses of dexmedetomidine sublingual film was evaluated in 2 similarly designed, randomized, double-blind, placebo-controlled, Phase 3 trials in the treatment of acute agitation associated with schizophrenia, schizoaffective, or schizophreniform disorder9 and bipolar I or II disorder.10 These studies included a total of 758 adult patients age range 18 to 71 (mean age approximately 46.5), with about 59% male participants.2 In contrast to other agents approved by the FDA for treatment of agitation associated with bipolar disorder, dexmedetomidine sublingual film was assessed in patients regardless of polarity (manic, mixed features, or depressed).5 The primary efficacy measure for the dexmedetomidine sublingual film studies was the investigator-administered Positive and Negative Syndrome Scale-Excited Component (PANSS-EC), consisting of the following 5 items: excitement, tension, hostility, uncooperativeness, and poor impulse control.11 The items from the PANSS-EC are rated from 1 (not present) to 7 (extremely severe) and thus the total scores range from 5 to 35. For enrollment in the studies, patients had to be judged to be clinically agitated with a total PANSS-EC score ≥14, with at least 1 individual item score ≥4.2
After study medication administration, the PANSS-EC was assessed from 10 minutes through 24 hours, with the primary endpoint being at 2 hours post-dose. Patients with schizophrenia or bipolar disorder who were treated with dexmedetomidine sublingual film 120 mcg or 180 mcg had superior symptomatic improvements from baseline to 2 hours post-dose compared to placebo, with treatment effects beginning as early as 20 to 30 minutes post-dose (for patients with schizophrenia, dexmedetomidine was statistically significantly superior to placebo beginning at 20 minutes following dosing with the 180 mcg dose and 30 minutes after the 120 mcg dose; for patients with bipolar disorder, differences from placebo were statistically significant beginning at 20 minutes after treatment with both the 120 mcg and 180 mcg doses).2 Evaluation of effect size for dexmedetomidine vs placebo for PANSS-EC response at 2 hours (defined as ≥40% improvement from baseline) resulted in a number needed to treat (NNT) of 3 when combining both studies and both doses,12 comparing favorably with the NNT values observed for IM formulations of aripiprazole, haloperidol, lorazepam, olanzapine, and ziprasidone,13 and inhaled loxapine.14
Overall tolerability and safety
The highlights of the prescribing information contain warnings and precautions regarding hypotension/orthostatic hypotension/bradycardia, QT interval prolongation, and somnolence.2 Advice is provided to ensure that patients are alert and not experiencing orthostatic or symptomatic hypotension prior to resuming ambulation, a concern commonly raised when assessing potential treatments for agitation.15 Dexmedetomidine sublingual film should be avoided in patients with risk factors for prolonged QT interval, a precaution that was evident for the use of ziprasidone16 and where an effect is also noted with haloperidol.6 As per the prescribing information, the most common adverse reactions (incidence ≥5% and at least twice the rate of placebo) are somnolence, oral paresthesia or oral hypoesthesia, dizziness, dry mouth, hypotension, and orthostatic hypotension. Rates of adverse reactions of somnolence (including fatigue and sluggishness) with dexmedetomidine 120 mcg or 180 mcg are almost the same (22% and 23%, respectively), and higher than the 6% observed with placebo.2 Other adverse reactions are substantially lower in frequency. These include oral paresthesia or oral hypoesthesia (6%, 7%, and 1%, for dexmedetomidine 120 mcg, 180 mcg, or placebo, respectively), dizziness (4%, 6%, 1%), hypotension (5%, 5%, 0%), orthostatic hypotension (3%, 5%, <1%), dry mouth (7%, 4%, 1%), nausea (2%, 3%, 2%), bradycardia (2%, 2%, 0%), and abdominal discomfort (0%, 2%, 1%).2
Regarding dose-dependent changes in blood pressure during the studies, 16%, 18%, and 9% of patients treated with 120 mcg, 180 mcg, and placebo, respectively, experienced orthostatic hypotension at 2 hours post dose. However, at 24 hours, none of the patients in the 180-mcg group experienced a systolic blood pressure ≤90 mm Hg with a decrease ≥20 mm Hg, compared with one patient (<1%) in the 120-mcg group and none in the placebo group.2
The prescribing information advises that concomitant use of dexmedetomidine sublingual film with anesthetics, sedatives, hypnotics, or opioids is likely to lead to enhanced CNS depressant effects, and that the prescriber should consider a reduction in dosage of dexmedetomidine or the concomitant anesthetic, sedative, hypnotic, or opioid.2
Summary
Dexmedetomidine sublingual film is an oral medication indicated in adults for the acute treatment of agitation associated with schizophrenia or bipolar I or II disorder. The recommended dose depends on severity of agitation, age, and the presence of hepatic impairment. A dose of 180 mcg is recommended for severe agitation and a dose of 120 mcg is recommended for mild or moderate agitation, with doses adjusted lower in the presence of hepatic impairment. There are no contraindications but there are warnings and precautions regarding hypotension/orthostatic hypotension/bradycardia, QT interval prolongation, and somnolence. Clinicians should monitor vital signs and alertness after administration to prevent falls and syncope; however, there is no FDA REMS required for use. The clinical trial evidence supporting the use of dexmedetomidine is robust, with evidence of a treatment effect as early as 20 minutes after administration. Noninvasive formulations, although requiring cooperation from patients, have the potential to improve overall patient experience, thereby improving future cooperation between patients and health care professionals.
Bottom Line
Dexmedetomidine sublingual film provides an opportunity to rethink the approach to the management of agitation and avoid the potentially unnecessary use of IM injections. Dexmedetomidine sublingual film acts rapidly and is simple to use.
Related Resources
- Dexmedetomidine sublingual film (Iglami) prescribing information. https://www.igalmihcp.com/igalmi-pi.pdf
Drug Brand Names
Aripiprazole • Abilify
Dexmedetomidine • Igalmi, Precedex
Haloperidol • Haldol
Lorazepam • Ativan
Loxapine inhaled • Adasuve
Olanzapine • Zyprexa
Ziprasidone • Geodon
Approved by the FDA on April 5, 2022, dexmedetomidine sublingual film (Igalmi, manufactured and distributed by BioXcel Therapeutics, Inc., New Haven, CT USA) is indicated in adults for the acute treatment of agitation associated with schizophrenia or bipolar I or II disorder (Table).1,2 It is administered sublingually or buccally under the supervision of a health care provider. After administration, patients should have their vital signs and alertness assessed but there is no FDA Risk Evaluation and Mitigation Strategy (REMS) required for use. A limitation of use is that the safety and effectiveness of dexmedetomidine sublingual film has not been established beyond 24 hours from the first dose.2 There are no contraindications for use.2

Dexmedetomidine is a well-known efficacious alpha-2 adrenergic receptor agonist available since 1999 in an IV formulation indicated for sedation of initially intubated and mechanically ventilated patients in an ICU setting, and sedation of nonintubated patients prior to and/or during surgical and other procedures.3,4 The reformulation of dexmedetomidine as a sublingual film allows the broader use of this agent in psychiatric settings when managing agitation in patients with schizophrenia or bipolar disorder, and thus potentially avoiding the use of IM administration of antipsychotics and/or benzodiazepines. Noninvasive formulations, although requiring cooperation from patients, have the potential to improve overall patient experience, thereby improving future cooperation between patients and health care professionals.5
Dosing
Dexmedetomidine sublingual film is distributed commercially in the following strengths: 180 mcg and 120 mcg. It consists of a lightly mint-flavored, rectangular film containing 2 microdeposits of dexmedetomidine hydrochloride. Dosage strengths of 90 mcg and 60 mcg are available by cutting the 180 mcg or 120 mcg film in half
If agitation persists after the initial dose, up to 2 additional doses (90 mcg if the initial dose was 180 mcg, otherwise 60 mcg if the initial dose was 120, 90, or 60 mcg) may be given at least 2 hours apart. Assessment of vital signs, including orthostatic measurements, is required prior to the administration of any subsequent doses. Due to risk of hypotension, additional doses are not recommended in patients with systolic blood pressure <90 mm Hg, diastolic blood pressure <60 mm Hg, heart rate <60 beats per minute, or postural decrease in systolic blood pressure ≥20 mm Hg or in diastolic blood pressure ≥10 mm Hg.
Mechanism of action and pharmacodynamics
Dexmedetomidine is an alpha-2 adrenergic receptor agonist and the mechanism of action in the acute treatment of agitation is thought to be due to activation of presynaptic alpha-2 adrenergic receptors.2 Binding affinities (Ki values) are 4 to 6 nM at the alpha-2 adrenergic receptor subtypes.2
Dexmedetomidine exhibits concentration-dependent QT prolongation, with mean QTc increases from baseline from 6 msec (120 mcg single dose) to 11 msec (180 mcg plus 2 additional doses of 90 mcg 2 hours apart for a total of 3 doses).2 Placing the observation about QTc prolongation into clinical context, studies of IM administration of ziprasidone 20 mg and 30 mg and haloperidol 7.5 mg and 10 mg resulted in changes of the QTc interval of 4.6 msec and 6.0 msec, respectively, after 1 dose.6 After a second injection, these values were 12.8 msec and 14.7 msec, respectively.6
Clinical pharmacokinetics
The sublingual film formulation is absorbed orally, bypassing first-pass metabolism, and achieving higher dexmedetomidine bioavailability than ingested formulations.7 Exposure is dose-dependent, with dexmedetomidine being quantifiable in plasma after 5 to 20 minutes post dosing, and with a plasma half-life of 2 to 3 hours.2,8 Mean time for the film to dissolve in the mouth was approximately 6 to 8 minutes following sublingual administration, and 18 minutes following buccal administration.2 Absolute bioavailability was approximately 72% and 82% following sublingual and buccal administration, respectively.2 Mean maximal plasma concentrations of dexmedetomidine were reached approximately 2 hours after sublingual or buccal administration.2 Compared to drinking water at 2 hours post administration, early water intake (as early as 15 minutes post-dose) had minimal effects on the rate or extent of sublingual absorption but was not assessed after buccal administration.2 The average protein binding was 94% and was constant across the different plasma concentrations evaluated and similar in males and females, but significantly decreased in participants with hepatic impairment compared to healthy individuals.2 In contrast, the pharmacokinetic profile of dexmedetomidine is not significantly different in patients with creatinine clearance <30 mL/minute compared to those with normal renal function.2 Dexmedetomidine undergoes almost complete biotransformation to inactive metabolites via direct glucuronidation as well as cytochrome P450 (CYP) (primarily CYP2A6)–mediated metabolism.2 There is no evidence of any CYP–mediated drug interactions that are likely to be of clinical relevance.2
Continue to: Efficacy
Efficacy
The efficacy and tolerability of 120 mcg and 180 mcg doses of dexmedetomidine sublingual film was evaluated in 2 similarly designed, randomized, double-blind, placebo-controlled, Phase 3 trials in the treatment of acute agitation associated with schizophrenia, schizoaffective, or schizophreniform disorder9 and bipolar I or II disorder.10 These studies included a total of 758 adult patients age range 18 to 71 (mean age approximately 46.5), with about 59% male participants.2 In contrast to other agents approved by the FDA for treatment of agitation associated with bipolar disorder, dexmedetomidine sublingual film was assessed in patients regardless of polarity (manic, mixed features, or depressed).5 The primary efficacy measure for the dexmedetomidine sublingual film studies was the investigator-administered Positive and Negative Syndrome Scale-Excited Component (PANSS-EC), consisting of the following 5 items: excitement, tension, hostility, uncooperativeness, and poor impulse control.11 The items from the PANSS-EC are rated from 1 (not present) to 7 (extremely severe) and thus the total scores range from 5 to 35. For enrollment in the studies, patients had to be judged to be clinically agitated with a total PANSS-EC score ≥14, with at least 1 individual item score ≥4.2
After study medication administration, the PANSS-EC was assessed from 10 minutes through 24 hours, with the primary endpoint being at 2 hours post-dose. Patients with schizophrenia or bipolar disorder who were treated with dexmedetomidine sublingual film 120 mcg or 180 mcg had superior symptomatic improvements from baseline to 2 hours post-dose compared to placebo, with treatment effects beginning as early as 20 to 30 minutes post-dose (for patients with schizophrenia, dexmedetomidine was statistically significantly superior to placebo beginning at 20 minutes following dosing with the 180 mcg dose and 30 minutes after the 120 mcg dose; for patients with bipolar disorder, differences from placebo were statistically significant beginning at 20 minutes after treatment with both the 120 mcg and 180 mcg doses).2 Evaluation of effect size for dexmedetomidine vs placebo for PANSS-EC response at 2 hours (defined as ≥40% improvement from baseline) resulted in a number needed to treat (NNT) of 3 when combining both studies and both doses,12 comparing favorably with the NNT values observed for IM formulations of aripiprazole, haloperidol, lorazepam, olanzapine, and ziprasidone,13 and inhaled loxapine.14
Overall tolerability and safety
The highlights of the prescribing information contain warnings and precautions regarding hypotension/orthostatic hypotension/bradycardia, QT interval prolongation, and somnolence.2 Advice is provided to ensure that patients are alert and not experiencing orthostatic or symptomatic hypotension prior to resuming ambulation, a concern commonly raised when assessing potential treatments for agitation.15 Dexmedetomidine sublingual film should be avoided in patients with risk factors for prolonged QT interval, a precaution that was evident for the use of ziprasidone16 and where an effect is also noted with haloperidol.6 As per the prescribing information, the most common adverse reactions (incidence ≥5% and at least twice the rate of placebo) are somnolence, oral paresthesia or oral hypoesthesia, dizziness, dry mouth, hypotension, and orthostatic hypotension. Rates of adverse reactions of somnolence (including fatigue and sluggishness) with dexmedetomidine 120 mcg or 180 mcg are almost the same (22% and 23%, respectively), and higher than the 6% observed with placebo.2 Other adverse reactions are substantially lower in frequency. These include oral paresthesia or oral hypoesthesia (6%, 7%, and 1%, for dexmedetomidine 120 mcg, 180 mcg, or placebo, respectively), dizziness (4%, 6%, 1%), hypotension (5%, 5%, 0%), orthostatic hypotension (3%, 5%, <1%), dry mouth (7%, 4%, 1%), nausea (2%, 3%, 2%), bradycardia (2%, 2%, 0%), and abdominal discomfort (0%, 2%, 1%).2
Regarding dose-dependent changes in blood pressure during the studies, 16%, 18%, and 9% of patients treated with 120 mcg, 180 mcg, and placebo, respectively, experienced orthostatic hypotension at 2 hours post dose. However, at 24 hours, none of the patients in the 180-mcg group experienced a systolic blood pressure ≤90 mm Hg with a decrease ≥20 mm Hg, compared with one patient (<1%) in the 120-mcg group and none in the placebo group.2
The prescribing information advises that concomitant use of dexmedetomidine sublingual film with anesthetics, sedatives, hypnotics, or opioids is likely to lead to enhanced CNS depressant effects, and that the prescriber should consider a reduction in dosage of dexmedetomidine or the concomitant anesthetic, sedative, hypnotic, or opioid.2
Summary
Dexmedetomidine sublingual film is an oral medication indicated in adults for the acute treatment of agitation associated with schizophrenia or bipolar I or II disorder. The recommended dose depends on severity of agitation, age, and the presence of hepatic impairment. A dose of 180 mcg is recommended for severe agitation and a dose of 120 mcg is recommended for mild or moderate agitation, with doses adjusted lower in the presence of hepatic impairment. There are no contraindications but there are warnings and precautions regarding hypotension/orthostatic hypotension/bradycardia, QT interval prolongation, and somnolence. Clinicians should monitor vital signs and alertness after administration to prevent falls and syncope; however, there is no FDA REMS required for use. The clinical trial evidence supporting the use of dexmedetomidine is robust, with evidence of a treatment effect as early as 20 minutes after administration. Noninvasive formulations, although requiring cooperation from patients, have the potential to improve overall patient experience, thereby improving future cooperation between patients and health care professionals.
Bottom Line
Dexmedetomidine sublingual film provides an opportunity to rethink the approach to the management of agitation and avoid the potentially unnecessary use of IM injections. Dexmedetomidine sublingual film acts rapidly and is simple to use.
Related Resources
- Dexmedetomidine sublingual film (Iglami) prescribing information. https://www.igalmihcp.com/igalmi-pi.pdf
Drug Brand Names
Aripiprazole • Abilify
Dexmedetomidine • Igalmi, Precedex
Haloperidol • Haldol
Lorazepam • Ativan
Loxapine inhaled • Adasuve
Olanzapine • Zyprexa
Ziprasidone • Geodon
1. US Food and Drug Administration. NDA 215390 Approval Letter. Accessed April 5, 2022. https://www.accessdata.fda.gov/drugsatfda_docs/appletter/2022/215390Orig1s000ltr.pdf
2. Igalmi [package insert]. BioXcel Therapeutics, Inc; 2022.
3. Weerink MAS, Struys MMRF, Hannivoort LN, et al. Clinical pharmacokinetics and pharmacodynamics of dexmedetomidine. Clin Pharmacokinet. 2017;56(8):893-913. doi:10.1007/s40262-017-0507-7
4. Precedex [package insert]. Hospira, Inc; 2021.
5. Zeller SL, Citrome L. Managing agitation associated with schizophrenia and bipolar disorder in the emergency setting. West J Emerg Med. 2016;17(2):165-172. doi:10.5811/westjem.2015.12.28763
6. Miceli JJ, Tensfeldt TG, Shiovitz T, et al. Effects of high-dose ziprasidone and haloperidol on the QTc interval after intramuscular administration: a randomized, single-blind, parallel-group study in patients with schizophrenia or schizoaffective disorder. Clin Ther. 2010;32(3):472-491. doi:10.1016/j.clinthera.2010.03.003
7. Yocca F, DeVivo M, Seth S, et al. Dexmedetomidine—highly favorable pharmacokinetic and pharmacological features for a CNS therapeutic drug. Poster presented at: 58th Annual Meeting of the American College of Neuropsychopharmacology; December 8-11, 2019; Orlando, FL.
8. Adedoyin A, Preskorn S, Lathia CD. Pharmacokinetics of dexmedetomidine after a single sublingual dose of BXCL501 in patients with agitation associated with schizophrenia. Poster presented at: 23rd Annual Conference of the International Society for Bipolar Disorders; May 13-15, 2021. Virtual. Session 17.
9. Citrome LL, Lauriello J, Risinger R, et al. A novel rapidly effective treatment of agitation for schizophrenia with the oral dissolving film BXCL501. Poster presented at: American Psychiatric Association Annual Meeting; May 1-3, 2021. Virtual. Accessed November 11, 2021. https://www.psychiatry.org/File%20Library/Psychiatrists/Meetings/Annual-Meeting/2021/2021-APA-Annual-Meeting-Poster-Proceedings.pdf
10. Preskorn SH, Zeller S, Citrome L, et al. Effect of sublingual dexmedetomidine vs placebo on acute agitation associated with bipolar disorder: a randomized clinical trial. JAMA. 2022;327(8):727-736. doi:10.1001/jama.2022.0799
11. Montoya A, Valladares A, Lizán L, et al. Validation of the Excited Component of the Positive and Negative Syndrome Scale (PANSS-EC) in a naturalistic sample of 278 patients with acute psychosis and agitation in a psychiatric emergency room. Health Qual Life Outcomes. 2011;9:18. doi:10.1186/1477-7525-9-18
12. Citrome L, Palko L, Hokett S, et al. Number needed to treat and number needed to harm from two phase 3 studies of BXCL501 for treating acute agitation in patients with schizophrenia and bipolar disorder. Poster presented at: Academy of Managed Care Pharmacy Nexus 2021; October 18-21, 2021; Denver, CO.
13. Citrome L. Comparison of intramuscular ziprasidone, olanzapine, or aripiprazole for agitation: a quantitative review of efficacy and safety. J Clin Psychiatry. 2007;68(12):1876-1885. doi:10.4088/jcp.v68n1207
14. Citrome L. Inhaled loxapine for agitation revisited: focus on effect sizes from 2 Phase III randomised controlled trials in persons with schizophrenia or bipolar disorder. Int J Clin Pract. 2012;66(3):318-325. doi:10.1111/j.1742-1241.2011.02890.x
15. Wilson MP, Pepper D, Currier GW, et al. The psychopharmacology of agitation: consensus statement of the American Association for Emergency Psychiatry project Beta psychopharmacology workgroup. West J Emerg Med. 2012;13(1):26-34. doi:10.5811/westjem.2011.9.6866
16. Zimbroff DL, Allen MH, Battaglia J, et al. Best clinical practice with ziprasidone IM: update after 2 years of experience. CNS Spectr. 2005;10(9):1-15. doi:10.1017/s1092852900025487
1. US Food and Drug Administration. NDA 215390 Approval Letter. Accessed April 5, 2022. https://www.accessdata.fda.gov/drugsatfda_docs/appletter/2022/215390Orig1s000ltr.pdf
2. Igalmi [package insert]. BioXcel Therapeutics, Inc; 2022.
3. Weerink MAS, Struys MMRF, Hannivoort LN, et al. Clinical pharmacokinetics and pharmacodynamics of dexmedetomidine. Clin Pharmacokinet. 2017;56(8):893-913. doi:10.1007/s40262-017-0507-7
4. Precedex [package insert]. Hospira, Inc; 2021.
5. Zeller SL, Citrome L. Managing agitation associated with schizophrenia and bipolar disorder in the emergency setting. West J Emerg Med. 2016;17(2):165-172. doi:10.5811/westjem.2015.12.28763
6. Miceli JJ, Tensfeldt TG, Shiovitz T, et al. Effects of high-dose ziprasidone and haloperidol on the QTc interval after intramuscular administration: a randomized, single-blind, parallel-group study in patients with schizophrenia or schizoaffective disorder. Clin Ther. 2010;32(3):472-491. doi:10.1016/j.clinthera.2010.03.003
7. Yocca F, DeVivo M, Seth S, et al. Dexmedetomidine—highly favorable pharmacokinetic and pharmacological features for a CNS therapeutic drug. Poster presented at: 58th Annual Meeting of the American College of Neuropsychopharmacology; December 8-11, 2019; Orlando, FL.
8. Adedoyin A, Preskorn S, Lathia CD. Pharmacokinetics of dexmedetomidine after a single sublingual dose of BXCL501 in patients with agitation associated with schizophrenia. Poster presented at: 23rd Annual Conference of the International Society for Bipolar Disorders; May 13-15, 2021. Virtual. Session 17.
9. Citrome LL, Lauriello J, Risinger R, et al. A novel rapidly effective treatment of agitation for schizophrenia with the oral dissolving film BXCL501. Poster presented at: American Psychiatric Association Annual Meeting; May 1-3, 2021. Virtual. Accessed November 11, 2021. https://www.psychiatry.org/File%20Library/Psychiatrists/Meetings/Annual-Meeting/2021/2021-APA-Annual-Meeting-Poster-Proceedings.pdf
10. Preskorn SH, Zeller S, Citrome L, et al. Effect of sublingual dexmedetomidine vs placebo on acute agitation associated with bipolar disorder: a randomized clinical trial. JAMA. 2022;327(8):727-736. doi:10.1001/jama.2022.0799
11. Montoya A, Valladares A, Lizán L, et al. Validation of the Excited Component of the Positive and Negative Syndrome Scale (PANSS-EC) in a naturalistic sample of 278 patients with acute psychosis and agitation in a psychiatric emergency room. Health Qual Life Outcomes. 2011;9:18. doi:10.1186/1477-7525-9-18
12. Citrome L, Palko L, Hokett S, et al. Number needed to treat and number needed to harm from two phase 3 studies of BXCL501 for treating acute agitation in patients with schizophrenia and bipolar disorder. Poster presented at: Academy of Managed Care Pharmacy Nexus 2021; October 18-21, 2021; Denver, CO.
13. Citrome L. Comparison of intramuscular ziprasidone, olanzapine, or aripiprazole for agitation: a quantitative review of efficacy and safety. J Clin Psychiatry. 2007;68(12):1876-1885. doi:10.4088/jcp.v68n1207
14. Citrome L. Inhaled loxapine for agitation revisited: focus on effect sizes from 2 Phase III randomised controlled trials in persons with schizophrenia or bipolar disorder. Int J Clin Pract. 2012;66(3):318-325. doi:10.1111/j.1742-1241.2011.02890.x
15. Wilson MP, Pepper D, Currier GW, et al. The psychopharmacology of agitation: consensus statement of the American Association for Emergency Psychiatry project Beta psychopharmacology workgroup. West J Emerg Med. 2012;13(1):26-34. doi:10.5811/westjem.2011.9.6866
16. Zimbroff DL, Allen MH, Battaglia J, et al. Best clinical practice with ziprasidone IM: update after 2 years of experience. CNS Spectr. 2005;10(9):1-15. doi:10.1017/s1092852900025487
Should clozapine be discontinued in a patient receiving chemotherapy?
CASE Schizophrenia, leukemia, and chemotherapy
Mr. A, age 30, has schizophrenia but has been stable on clozapine 600 mg/d. He presents to the emergency department with generalized pain that started in his right scapula, arm, elbow, and back. Laboratory tests and a diagnostic examination reveal severe leukocytosis, thrombocytopenia, and anemia, and clinicians diagnose Mr. A with B-cell acute lymphocytic leukemia (B-ALL). Upon admission, Mr. A is neutropenic with an absolute neutrophil count (ANC) of 1,420 µL (reference range 2,500 to 6,000 µL). The hematology team recommends chemotherapy. The treating clinicians also consult the psychiatry team for recommendations on how to best manage Mr. A’s schizophrenia during chemotherapy, including whether clozapine should be discontinued.
HISTORY Stable on clozapine for >10 years
Mr. A was diagnosed with schizophrenia at age 15 after developing paranoia and auditory hallucinations of people talking to him and to each other. He had been hospitalized multiple times for worsened auditory hallucinations and paranoia that led to significant agitation and violence. Previous treatment with multiple antipsychotics, including haloperidol, quetiapine, aripiprazole, olanzapine, risperidone, and ziprasidone, was not successful. Mr. A began clozapine >10 years ago, and his symptoms have been stable since, without any further psychiatric hospitalizations. Mr. A takes clozapine 600 mg/d and divalproex sodium 1,500 mg/d, which he tolerates well and without significant adverse effects. Though he continues to have intermittent auditory hallucinations, they are mild and manageable. Mr. A lives with his mother, who reports he occasionally talks to himself but when he does not take clozapine, the auditory hallucinations worsen and cause him to become paranoid and aggressive. His ANC is monitored monthly and had been normal for several years until he was diagnosed with B-ALL.
[polldaddy:11125941]
The authors’ observations
The decision to continue clozapine during chemotherapy is challenging and should weigh the risk of agranulocytosis against that of psychiatric destabilization. Because clozapine and chemotherapy are both associated with agranulocytosis, there is concern that concurrent treatment could increase this risk in an additive or synergistic manner. To the best of our knowledge, there are currently no controlled studies investigating the interactions between clozapine and chemotherapeutic agents. Evidence on the hematopoietic consequences of concurrent clozapine and chemotherapy treatment has been limited to case reports because the topic does not lend itself well to randomized controlled trials.
A recent systematic review found no adverse outcomes among the 27 published cases in which clozapine was continued during myelosuppressive chemotherapy.1 The most notable finding was an association between clozapine discontinuation and psychiatric decompensation, which was reported in 12 of 13 cases in which clozapine was prophylactically discontinued to minimize the risk of agranulocytosis.
Patient-specific factors must also be considered, such as the likelihood that psychotic symptoms will recur or worsen if clozapine is discontinued, as well as the extent to which symptom recurrence would interfere with cancer treatment. Clinicians should evaluate the feasibility of switching to another antipsychotic by obtaining a thorough history of the patient’s previous antipsychotics, doses, treatment duration, and response. However, many patients are treated with clozapine because their psychotic symptoms did not improve with other treatments. The character and severity of the patient’s psychotic symptoms when untreated or prior to clozapine treatment can provide a clearer understanding of how a recurrence of symptoms may interfere with cancer treatment. To formulate an accurate assessment of risks and benefits, it is necessary to consider both available evidence and patient-specific factors. The significant agitation and paranoia that Mr. A experienced when not taking clozapine was likely to disrupt chemotherapy. Thus, the adverse consequences of discontinuing clozapine were both severe and likely.
TREATMENT Continuing clozapine
After an extensive discussion of risks, benefits, and alternative treatments with the hematology and psychiatry teams, Mr. A and his family decide to continue clozapine with increased ANC monitoring during chemotherapy. Concurrent treatment was pursued with close collaboration among the patient, the patient’s family, and the hematology and pharmacy teams, and in careful consideration of the clozapine risk evaluation and mitigation strategy. Mr. A’s ANC was monitored daily during chemotherapy treatments and weekly in the intervals between treatments.
As expected, chemotherapy resulted in bone marrow suppression and pancytopenia. Mr. A’s ANC steadily decreased during the next 10 days until it reached 0 µL. This was consistent with the predicted ANC nadir between Day 10 and Day 14, after which recovery was expected. However, Mr. A’s ANC remained at 0 µL on Day 15.
[polldaddy:11125947]
Continue to: The authors' observations
The authors’ observations
Temporary decreases in ANC are expected during chemotherapy, and the timing of onset and recovery is often well characterized. Prior to Day 15, the observed progressive marrow suppression was solely due to chemotherapy. However, because Mr. A’s ANC remained 0 µL longer than anticipated, reevaluation of clozapine’s effects was warranted.
Timing, clinical course, and comprehensive hematologic monitoring can provide important clues as to whether clozapine may be responsible for prolonged neutropenia. Though a prolonged ANC of 0 µL raised concern for clozapine-induced agranulocytosis (CIAG), comprehensive monitoring of hematologic cell lines was reassuring because CIAG selectively targets granulocytic cells (neutrophils).2 In contrast, chemotherapy can affect other cell lineages, including lymphocytes, red blood cells, and platelets, which causes pancytopenia.3 For Mr. A, though the clinical presentation of pancytopenia was significant and concerning, it was inconsistent with CIAG.
Additionally, the patient’s baseline risk of CIAG should be considered. After 18 weeks of clozapine treatment, the risk of CIAG decreases to a level similar to that associated with other antipsychotics.4,5 Therefore, CIAG would be unlikely in a patient treated with clozapine for more than 1 year and who did not have a history of neutropenia, as was the case with Mr. A.
While bone marrow biopsy can help differentiate between the causes of agranulocytosis,6 it is highly invasive and may not be necessary if laboratory evidence is sufficient. However, if a treatment team is strongly considering discontinuing clozapine and there are no suitable alternatives, a biopsy may provide additional clarification.
TREATMENT CAR T-cell therapy and cancer remission
Clozapine is continued with daily monitoring. On Day 19, Mr. A’s ANC increases, reaching 2,600 µL by discharge on Day 40. Mr. A remains psychiatrically stable throughout his hospitalization and does not experience any complications associated with neutropenia, despite its prolonged duration.
Continue to: Unfortunately, multiple cycles of...
Unfortunately, multiple cycles of chemotherapy fail to induce remission. Mr. A is referred for CD19/CD22 chimeric antigen receptor (CAR) T-cell therapy, which helps achieve remission. Allogeneic hematopoietic stem cell transplant (HSCT) is recommended to maximize the likelihood of sustained remission.7 As with chemotherapy, Mr. A and his family agree with the multidisciplinary treatment recommendation to continue clozapine during both CAR T-cell therapy and HSCT, because the risks associated with psychiatric decompensation were greater than a potential increased risk of agranulocytosis. Clozapine treatment is continued throughout both therapies without issue.
Four months after HSCT, Mr. A is admitted for neutropenic fever and left face cellulitis. Upon admission, his ANC is 30 µL and subsequently decreases to 0 µL. In addition to neutropenia, Mr. A is also anemic and thrombocytopenic. He undergoes a bone marrow biopsy.
[polldaddy:11125950]
The authors’ observations
While no published cases have examined the bone marrow of patients experiencing CIAG, 2 retrospective studies have characterized 2 classes of bone marrow findings associated with drug-induced agranulocytosis resulting from nonchemotherapeutic agents (Table).8,9 Type I marrow appears hypercellular with adequate neutrophil precursors but an arrested neutrophil maturation, with few or no mature forms of neutrophils beyond myelocytes.8,9 Type II demonstrates a severe reduction or complete absence of granulocytic precursors with normal or increased erythropoiesis and megakaryocytes.8,9 These findings have been used to accurately differentiate between chemotherapy and nonchemotherapy drug-induced agranulocytosis,6 resulting in successful identification and discontinuation of the responsible agent.
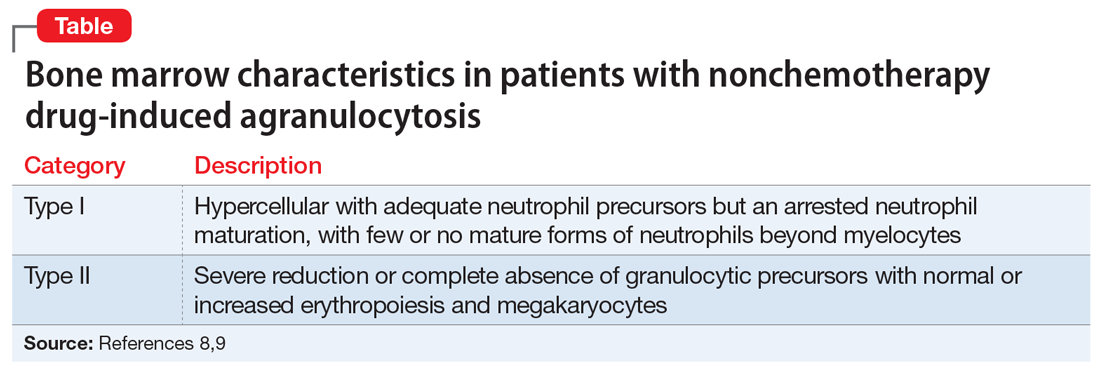
Mr. A’s bone marrow biopsy showed severe pancytopenia with profound neutropenia and normocytic anemia, without evidence of residual leukemia, inconsistent with Type I or Type II. Findings were suggestive of a myelodysplastic syndrome, consistent with secondary graft failure. Symptoms resolved after treatment with antibiotics, granulocyte colony-stimulating factor, epoetin alfa, and thrombopoietin. Mr. A’s ANC remained 0 µL for 22 days before returning to normal (>1,500 µL) by Day 29. He had no secondary complications resulting from neutropenia. As the clinical evidence suggested, Mr. A’s neutropenia was unlikely to be due to clozapine. Clozapine was continued throughout his cancer treatment, and he remained psychiatrically stable.
Clozapine, cancer treatments, and agranulocytosis
This case demonstrates that clozapine can be safely continued during a variety of cancer treatments (ie, chemotherapy, CAR T-cell therapy, HSCT), even with the development of agranulocytosis and prolonged neutropenia. Evidence to guide psychiatric clinicians to evaluate the likelihood that agranulocytosis is clozapine-induced is limited.
Continue to: We offer an algorithm...
We offer an algorithm to assist clinicians faced with this challenging clinical dilemma (Figure). Based on our experience and limited current evidence, we recommend continuing clozapine during cancer treatment unless there is clear evidence to suggest otherwise. Presently, no evidence in published literature suggests worsened outcomes in patients treated concurrently with clozapine and cancer therapies.
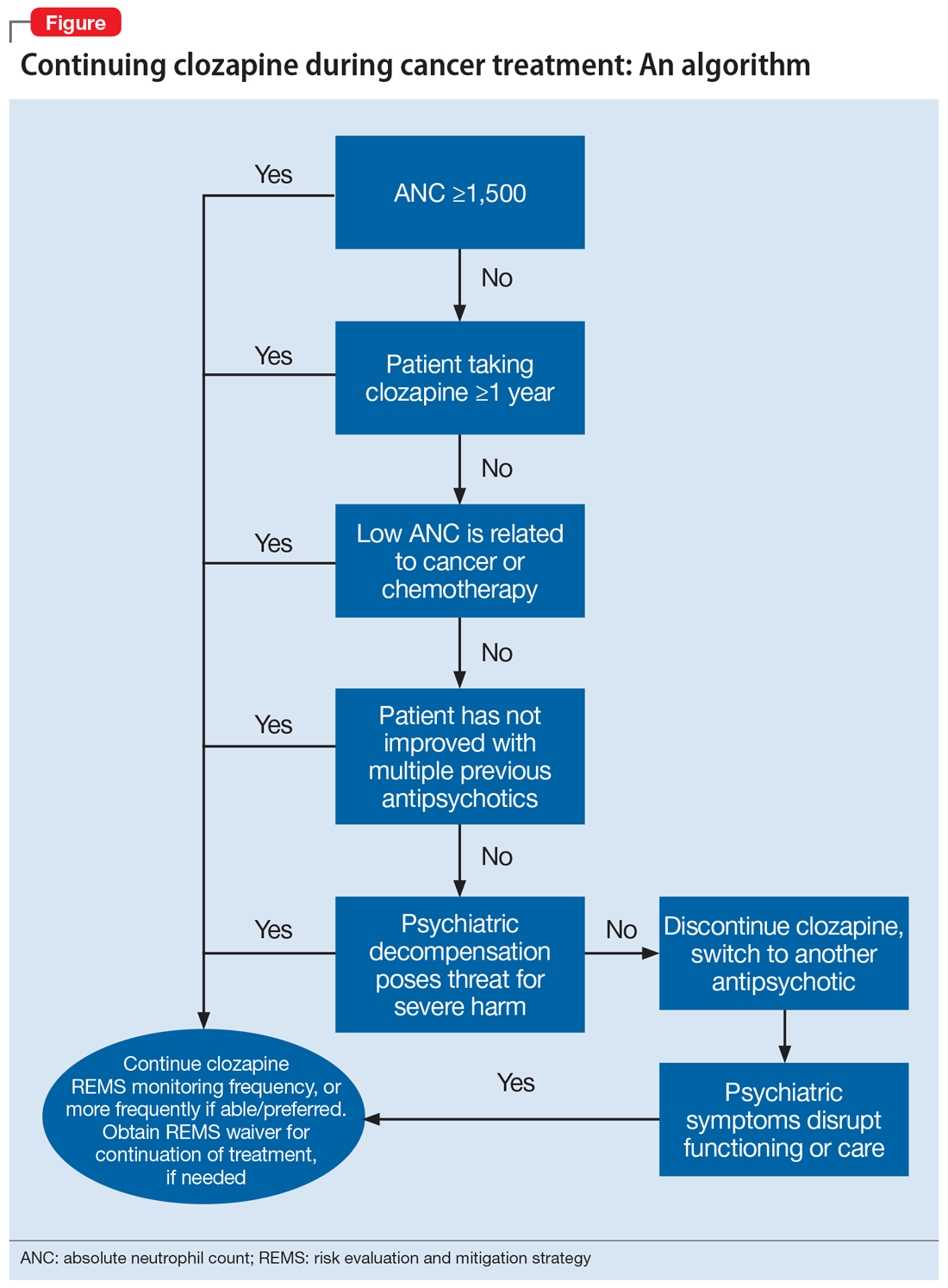
OUTCOME Cancer-free and psychiatrically stable
Mr. A continues clozapine therapy throughout all phases of treatment, without interruption. No adverse effects are determined to be secondary to clozapine. He remains psychiatrically stable throughout treatment, and able to participate and engage in his oncologic therapy. Mr. A is now more than 1 year in remission with no recurrence of graft failure, and his psychiatric symptoms continue to be well controlled with clozapine.
Bottom Line
Clozapine can be safely continued during a variety of cancer treatments (ie, chemotherapy, CAR T-cell therapy, HSCT), even in patients who develop agranulocytosis and prolonged neutropenia. Based on our experience and limited evidence, we offer an algorithm to assist clinicians faced with this challenging clinical dilemma.
Related Resources
- Grainger BT, Arcasoy MO, Kenedi CA. Feasibility of myelosuppressive chemotherapy in psychiatric patients on clozapine: a systematic review of the literature. Eur J Haematol. 2019;103(4):277-286. doi:10.1111/ejh.13285
- Daniel JS, Gross T. Managing clozapine-induced neutropenia and agranulocytosis. Current Psychiatry. 2016;15(12):51-53.
Drug Brand Names
Aripiprazole • Abilify
Clozapine • Clozaril
Divalproex sodium • Depakote
Epoetin alfa • Epogen
Haloperidol • Haldol
Olanzapine • Zyprexa
Quetiapine • Seroquel
Risperidone • Risperdal
Ziprasidone • Geodon
1. Grainger BT, Arcasoy MO, Kenedi CA. Feasibility of myelosuppressive chemotherapy in psychiatric patients on clozapine: a systematic review of the literature. Eur J Haematol. 2019;103(4):277-286.
2. Pick AM, Nystrom KK. Nonchemotherapy drug-induced neutropenia and agranulocytosis: could medications be the culprit? J Pharm Pract. 2014:27(5):447-452.
3. Epstein RS, Aapro MS, Basu Roy UK, et al. Patient burden and real-world management of chemotherapy-induced myelosuppression: results from an online survey of patients with solid tumors. Adv Ther. 2020;37(8):3606-3618.
4. Alvir JM, Lieberman JA, Safferman AZ, et al. Clozapine-induced agranulocytosis. Incidence and risk factors in the United States. N Engl J Med. 1993;329(3):162-167.
5. Atkin K, Kendall F, Gould D, et al. Neutropenia and agranulocytosis in patients receiving clozapine in the UK and Ireland. Br J Psychiatry. 1996;169(4):483-488.
6. Azadeh N, Kelemen K, Fonseca R. Amitriptyline-induced agranulocytosis with bone marrow confirmation. Clin Lymphoma Myeloma Leuk. 2014;14(5):e183-e185.
7. Liu J, Zhang X, Zhong JF, et al. CAR-T cells and allogeneic hematopoietic stem cell transplantation for relapsed/refractory B-cell acute lymphoblastic leukemia. Immunotherapy. 2017;9(13):1115-1125.
8. Apinantriyo B, Lekhakula A, Rujirojindakul P. Incidence, etiology and bone marrow characteristics of non-chemotherapy-induced agranulocytosis. Hematology. 2011;16(1):50-53.
9. Yang J, Zhong J, Xiao XH, et al. The relationship between bone marrow characteristics and the clinical prognosis of antithyroid drug-induced agranulocytosis. Endocr J. 2013;60(2):185-189.
CASE Schizophrenia, leukemia, and chemotherapy
Mr. A, age 30, has schizophrenia but has been stable on clozapine 600 mg/d. He presents to the emergency department with generalized pain that started in his right scapula, arm, elbow, and back. Laboratory tests and a diagnostic examination reveal severe leukocytosis, thrombocytopenia, and anemia, and clinicians diagnose Mr. A with B-cell acute lymphocytic leukemia (B-ALL). Upon admission, Mr. A is neutropenic with an absolute neutrophil count (ANC) of 1,420 µL (reference range 2,500 to 6,000 µL). The hematology team recommends chemotherapy. The treating clinicians also consult the psychiatry team for recommendations on how to best manage Mr. A’s schizophrenia during chemotherapy, including whether clozapine should be discontinued.
HISTORY Stable on clozapine for >10 years
Mr. A was diagnosed with schizophrenia at age 15 after developing paranoia and auditory hallucinations of people talking to him and to each other. He had been hospitalized multiple times for worsened auditory hallucinations and paranoia that led to significant agitation and violence. Previous treatment with multiple antipsychotics, including haloperidol, quetiapine, aripiprazole, olanzapine, risperidone, and ziprasidone, was not successful. Mr. A began clozapine >10 years ago, and his symptoms have been stable since, without any further psychiatric hospitalizations. Mr. A takes clozapine 600 mg/d and divalproex sodium 1,500 mg/d, which he tolerates well and without significant adverse effects. Though he continues to have intermittent auditory hallucinations, they are mild and manageable. Mr. A lives with his mother, who reports he occasionally talks to himself but when he does not take clozapine, the auditory hallucinations worsen and cause him to become paranoid and aggressive. His ANC is monitored monthly and had been normal for several years until he was diagnosed with B-ALL.
[polldaddy:11125941]
The authors’ observations
The decision to continue clozapine during chemotherapy is challenging and should weigh the risk of agranulocytosis against that of psychiatric destabilization. Because clozapine and chemotherapy are both associated with agranulocytosis, there is concern that concurrent treatment could increase this risk in an additive or synergistic manner. To the best of our knowledge, there are currently no controlled studies investigating the interactions between clozapine and chemotherapeutic agents. Evidence on the hematopoietic consequences of concurrent clozapine and chemotherapy treatment has been limited to case reports because the topic does not lend itself well to randomized controlled trials.
A recent systematic review found no adverse outcomes among the 27 published cases in which clozapine was continued during myelosuppressive chemotherapy.1 The most notable finding was an association between clozapine discontinuation and psychiatric decompensation, which was reported in 12 of 13 cases in which clozapine was prophylactically discontinued to minimize the risk of agranulocytosis.
Patient-specific factors must also be considered, such as the likelihood that psychotic symptoms will recur or worsen if clozapine is discontinued, as well as the extent to which symptom recurrence would interfere with cancer treatment. Clinicians should evaluate the feasibility of switching to another antipsychotic by obtaining a thorough history of the patient’s previous antipsychotics, doses, treatment duration, and response. However, many patients are treated with clozapine because their psychotic symptoms did not improve with other treatments. The character and severity of the patient’s psychotic symptoms when untreated or prior to clozapine treatment can provide a clearer understanding of how a recurrence of symptoms may interfere with cancer treatment. To formulate an accurate assessment of risks and benefits, it is necessary to consider both available evidence and patient-specific factors. The significant agitation and paranoia that Mr. A experienced when not taking clozapine was likely to disrupt chemotherapy. Thus, the adverse consequences of discontinuing clozapine were both severe and likely.
TREATMENT Continuing clozapine
After an extensive discussion of risks, benefits, and alternative treatments with the hematology and psychiatry teams, Mr. A and his family decide to continue clozapine with increased ANC monitoring during chemotherapy. Concurrent treatment was pursued with close collaboration among the patient, the patient’s family, and the hematology and pharmacy teams, and in careful consideration of the clozapine risk evaluation and mitigation strategy. Mr. A’s ANC was monitored daily during chemotherapy treatments and weekly in the intervals between treatments.
As expected, chemotherapy resulted in bone marrow suppression and pancytopenia. Mr. A’s ANC steadily decreased during the next 10 days until it reached 0 µL. This was consistent with the predicted ANC nadir between Day 10 and Day 14, after which recovery was expected. However, Mr. A’s ANC remained at 0 µL on Day 15.
[polldaddy:11125947]
Continue to: The authors' observations
The authors’ observations
Temporary decreases in ANC are expected during chemotherapy, and the timing of onset and recovery is often well characterized. Prior to Day 15, the observed progressive marrow suppression was solely due to chemotherapy. However, because Mr. A’s ANC remained 0 µL longer than anticipated, reevaluation of clozapine’s effects was warranted.
Timing, clinical course, and comprehensive hematologic monitoring can provide important clues as to whether clozapine may be responsible for prolonged neutropenia. Though a prolonged ANC of 0 µL raised concern for clozapine-induced agranulocytosis (CIAG), comprehensive monitoring of hematologic cell lines was reassuring because CIAG selectively targets granulocytic cells (neutrophils).2 In contrast, chemotherapy can affect other cell lineages, including lymphocytes, red blood cells, and platelets, which causes pancytopenia.3 For Mr. A, though the clinical presentation of pancytopenia was significant and concerning, it was inconsistent with CIAG.
Additionally, the patient’s baseline risk of CIAG should be considered. After 18 weeks of clozapine treatment, the risk of CIAG decreases to a level similar to that associated with other antipsychotics.4,5 Therefore, CIAG would be unlikely in a patient treated with clozapine for more than 1 year and who did not have a history of neutropenia, as was the case with Mr. A.
While bone marrow biopsy can help differentiate between the causes of agranulocytosis,6 it is highly invasive and may not be necessary if laboratory evidence is sufficient. However, if a treatment team is strongly considering discontinuing clozapine and there are no suitable alternatives, a biopsy may provide additional clarification.
TREATMENT CAR T-cell therapy and cancer remission
Clozapine is continued with daily monitoring. On Day 19, Mr. A’s ANC increases, reaching 2,600 µL by discharge on Day 40. Mr. A remains psychiatrically stable throughout his hospitalization and does not experience any complications associated with neutropenia, despite its prolonged duration.
Continue to: Unfortunately, multiple cycles of...
Unfortunately, multiple cycles of chemotherapy fail to induce remission. Mr. A is referred for CD19/CD22 chimeric antigen receptor (CAR) T-cell therapy, which helps achieve remission. Allogeneic hematopoietic stem cell transplant (HSCT) is recommended to maximize the likelihood of sustained remission.7 As with chemotherapy, Mr. A and his family agree with the multidisciplinary treatment recommendation to continue clozapine during both CAR T-cell therapy and HSCT, because the risks associated with psychiatric decompensation were greater than a potential increased risk of agranulocytosis. Clozapine treatment is continued throughout both therapies without issue.
Four months after HSCT, Mr. A is admitted for neutropenic fever and left face cellulitis. Upon admission, his ANC is 30 µL and subsequently decreases to 0 µL. In addition to neutropenia, Mr. A is also anemic and thrombocytopenic. He undergoes a bone marrow biopsy.
[polldaddy:11125950]
The authors’ observations
While no published cases have examined the bone marrow of patients experiencing CIAG, 2 retrospective studies have characterized 2 classes of bone marrow findings associated with drug-induced agranulocytosis resulting from nonchemotherapeutic agents (Table).8,9 Type I marrow appears hypercellular with adequate neutrophil precursors but an arrested neutrophil maturation, with few or no mature forms of neutrophils beyond myelocytes.8,9 Type II demonstrates a severe reduction or complete absence of granulocytic precursors with normal or increased erythropoiesis and megakaryocytes.8,9 These findings have been used to accurately differentiate between chemotherapy and nonchemotherapy drug-induced agranulocytosis,6 resulting in successful identification and discontinuation of the responsible agent.

Mr. A’s bone marrow biopsy showed severe pancytopenia with profound neutropenia and normocytic anemia, without evidence of residual leukemia, inconsistent with Type I or Type II. Findings were suggestive of a myelodysplastic syndrome, consistent with secondary graft failure. Symptoms resolved after treatment with antibiotics, granulocyte colony-stimulating factor, epoetin alfa, and thrombopoietin. Mr. A’s ANC remained 0 µL for 22 days before returning to normal (>1,500 µL) by Day 29. He had no secondary complications resulting from neutropenia. As the clinical evidence suggested, Mr. A’s neutropenia was unlikely to be due to clozapine. Clozapine was continued throughout his cancer treatment, and he remained psychiatrically stable.
Clozapine, cancer treatments, and agranulocytosis
This case demonstrates that clozapine can be safely continued during a variety of cancer treatments (ie, chemotherapy, CAR T-cell therapy, HSCT), even with the development of agranulocytosis and prolonged neutropenia. Evidence to guide psychiatric clinicians to evaluate the likelihood that agranulocytosis is clozapine-induced is limited.
Continue to: We offer an algorithm...
We offer an algorithm to assist clinicians faced with this challenging clinical dilemma (Figure). Based on our experience and limited current evidence, we recommend continuing clozapine during cancer treatment unless there is clear evidence to suggest otherwise. Presently, no evidence in published literature suggests worsened outcomes in patients treated concurrently with clozapine and cancer therapies.

OUTCOME Cancer-free and psychiatrically stable
Mr. A continues clozapine therapy throughout all phases of treatment, without interruption. No adverse effects are determined to be secondary to clozapine. He remains psychiatrically stable throughout treatment, and able to participate and engage in his oncologic therapy. Mr. A is now more than 1 year in remission with no recurrence of graft failure, and his psychiatric symptoms continue to be well controlled with clozapine.
Bottom Line
Clozapine can be safely continued during a variety of cancer treatments (ie, chemotherapy, CAR T-cell therapy, HSCT), even in patients who develop agranulocytosis and prolonged neutropenia. Based on our experience and limited evidence, we offer an algorithm to assist clinicians faced with this challenging clinical dilemma.
Related Resources
- Grainger BT, Arcasoy MO, Kenedi CA. Feasibility of myelosuppressive chemotherapy in psychiatric patients on clozapine: a systematic review of the literature. Eur J Haematol. 2019;103(4):277-286. doi:10.1111/ejh.13285
- Daniel JS, Gross T. Managing clozapine-induced neutropenia and agranulocytosis. Current Psychiatry. 2016;15(12):51-53.
Drug Brand Names
Aripiprazole • Abilify
Clozapine • Clozaril
Divalproex sodium • Depakote
Epoetin alfa • Epogen
Haloperidol • Haldol
Olanzapine • Zyprexa
Quetiapine • Seroquel
Risperidone • Risperdal
Ziprasidone • Geodon
CASE Schizophrenia, leukemia, and chemotherapy
Mr. A, age 30, has schizophrenia but has been stable on clozapine 600 mg/d. He presents to the emergency department with generalized pain that started in his right scapula, arm, elbow, and back. Laboratory tests and a diagnostic examination reveal severe leukocytosis, thrombocytopenia, and anemia, and clinicians diagnose Mr. A with B-cell acute lymphocytic leukemia (B-ALL). Upon admission, Mr. A is neutropenic with an absolute neutrophil count (ANC) of 1,420 µL (reference range 2,500 to 6,000 µL). The hematology team recommends chemotherapy. The treating clinicians also consult the psychiatry team for recommendations on how to best manage Mr. A’s schizophrenia during chemotherapy, including whether clozapine should be discontinued.
HISTORY Stable on clozapine for >10 years
Mr. A was diagnosed with schizophrenia at age 15 after developing paranoia and auditory hallucinations of people talking to him and to each other. He had been hospitalized multiple times for worsened auditory hallucinations and paranoia that led to significant agitation and violence. Previous treatment with multiple antipsychotics, including haloperidol, quetiapine, aripiprazole, olanzapine, risperidone, and ziprasidone, was not successful. Mr. A began clozapine >10 years ago, and his symptoms have been stable since, without any further psychiatric hospitalizations. Mr. A takes clozapine 600 mg/d and divalproex sodium 1,500 mg/d, which he tolerates well and without significant adverse effects. Though he continues to have intermittent auditory hallucinations, they are mild and manageable. Mr. A lives with his mother, who reports he occasionally talks to himself but when he does not take clozapine, the auditory hallucinations worsen and cause him to become paranoid and aggressive. His ANC is monitored monthly and had been normal for several years until he was diagnosed with B-ALL.
[polldaddy:11125941]
The authors’ observations
The decision to continue clozapine during chemotherapy is challenging and should weigh the risk of agranulocytosis against that of psychiatric destabilization. Because clozapine and chemotherapy are both associated with agranulocytosis, there is concern that concurrent treatment could increase this risk in an additive or synergistic manner. To the best of our knowledge, there are currently no controlled studies investigating the interactions between clozapine and chemotherapeutic agents. Evidence on the hematopoietic consequences of concurrent clozapine and chemotherapy treatment has been limited to case reports because the topic does not lend itself well to randomized controlled trials.
A recent systematic review found no adverse outcomes among the 27 published cases in which clozapine was continued during myelosuppressive chemotherapy.1 The most notable finding was an association between clozapine discontinuation and psychiatric decompensation, which was reported in 12 of 13 cases in which clozapine was prophylactically discontinued to minimize the risk of agranulocytosis.
Patient-specific factors must also be considered, such as the likelihood that psychotic symptoms will recur or worsen if clozapine is discontinued, as well as the extent to which symptom recurrence would interfere with cancer treatment. Clinicians should evaluate the feasibility of switching to another antipsychotic by obtaining a thorough history of the patient’s previous antipsychotics, doses, treatment duration, and response. However, many patients are treated with clozapine because their psychotic symptoms did not improve with other treatments. The character and severity of the patient’s psychotic symptoms when untreated or prior to clozapine treatment can provide a clearer understanding of how a recurrence of symptoms may interfere with cancer treatment. To formulate an accurate assessment of risks and benefits, it is necessary to consider both available evidence and patient-specific factors. The significant agitation and paranoia that Mr. A experienced when not taking clozapine was likely to disrupt chemotherapy. Thus, the adverse consequences of discontinuing clozapine were both severe and likely.
TREATMENT Continuing clozapine
After an extensive discussion of risks, benefits, and alternative treatments with the hematology and psychiatry teams, Mr. A and his family decide to continue clozapine with increased ANC monitoring during chemotherapy. Concurrent treatment was pursued with close collaboration among the patient, the patient’s family, and the hematology and pharmacy teams, and in careful consideration of the clozapine risk evaluation and mitigation strategy. Mr. A’s ANC was monitored daily during chemotherapy treatments and weekly in the intervals between treatments.
As expected, chemotherapy resulted in bone marrow suppression and pancytopenia. Mr. A’s ANC steadily decreased during the next 10 days until it reached 0 µL. This was consistent with the predicted ANC nadir between Day 10 and Day 14, after which recovery was expected. However, Mr. A’s ANC remained at 0 µL on Day 15.
[polldaddy:11125947]
Continue to: The authors' observations
The authors’ observations
Temporary decreases in ANC are expected during chemotherapy, and the timing of onset and recovery is often well characterized. Prior to Day 15, the observed progressive marrow suppression was solely due to chemotherapy. However, because Mr. A’s ANC remained 0 µL longer than anticipated, reevaluation of clozapine’s effects was warranted.
Timing, clinical course, and comprehensive hematologic monitoring can provide important clues as to whether clozapine may be responsible for prolonged neutropenia. Though a prolonged ANC of 0 µL raised concern for clozapine-induced agranulocytosis (CIAG), comprehensive monitoring of hematologic cell lines was reassuring because CIAG selectively targets granulocytic cells (neutrophils).2 In contrast, chemotherapy can affect other cell lineages, including lymphocytes, red blood cells, and platelets, which causes pancytopenia.3 For Mr. A, though the clinical presentation of pancytopenia was significant and concerning, it was inconsistent with CIAG.
Additionally, the patient’s baseline risk of CIAG should be considered. After 18 weeks of clozapine treatment, the risk of CIAG decreases to a level similar to that associated with other antipsychotics.4,5 Therefore, CIAG would be unlikely in a patient treated with clozapine for more than 1 year and who did not have a history of neutropenia, as was the case with Mr. A.
While bone marrow biopsy can help differentiate between the causes of agranulocytosis,6 it is highly invasive and may not be necessary if laboratory evidence is sufficient. However, if a treatment team is strongly considering discontinuing clozapine and there are no suitable alternatives, a biopsy may provide additional clarification.
TREATMENT CAR T-cell therapy and cancer remission
Clozapine is continued with daily monitoring. On Day 19, Mr. A’s ANC increases, reaching 2,600 µL by discharge on Day 40. Mr. A remains psychiatrically stable throughout his hospitalization and does not experience any complications associated with neutropenia, despite its prolonged duration.
Continue to: Unfortunately, multiple cycles of...
Unfortunately, multiple cycles of chemotherapy fail to induce remission. Mr. A is referred for CD19/CD22 chimeric antigen receptor (CAR) T-cell therapy, which helps achieve remission. Allogeneic hematopoietic stem cell transplant (HSCT) is recommended to maximize the likelihood of sustained remission.7 As with chemotherapy, Mr. A and his family agree with the multidisciplinary treatment recommendation to continue clozapine during both CAR T-cell therapy and HSCT, because the risks associated with psychiatric decompensation were greater than a potential increased risk of agranulocytosis. Clozapine treatment is continued throughout both therapies without issue.
Four months after HSCT, Mr. A is admitted for neutropenic fever and left face cellulitis. Upon admission, his ANC is 30 µL and subsequently decreases to 0 µL. In addition to neutropenia, Mr. A is also anemic and thrombocytopenic. He undergoes a bone marrow biopsy.
[polldaddy:11125950]
The authors’ observations
While no published cases have examined the bone marrow of patients experiencing CIAG, 2 retrospective studies have characterized 2 classes of bone marrow findings associated with drug-induced agranulocytosis resulting from nonchemotherapeutic agents (Table).8,9 Type I marrow appears hypercellular with adequate neutrophil precursors but an arrested neutrophil maturation, with few or no mature forms of neutrophils beyond myelocytes.8,9 Type II demonstrates a severe reduction or complete absence of granulocytic precursors with normal or increased erythropoiesis and megakaryocytes.8,9 These findings have been used to accurately differentiate between chemotherapy and nonchemotherapy drug-induced agranulocytosis,6 resulting in successful identification and discontinuation of the responsible agent.

Mr. A’s bone marrow biopsy showed severe pancytopenia with profound neutropenia and normocytic anemia, without evidence of residual leukemia, inconsistent with Type I or Type II. Findings were suggestive of a myelodysplastic syndrome, consistent with secondary graft failure. Symptoms resolved after treatment with antibiotics, granulocyte colony-stimulating factor, epoetin alfa, and thrombopoietin. Mr. A’s ANC remained 0 µL for 22 days before returning to normal (>1,500 µL) by Day 29. He had no secondary complications resulting from neutropenia. As the clinical evidence suggested, Mr. A’s neutropenia was unlikely to be due to clozapine. Clozapine was continued throughout his cancer treatment, and he remained psychiatrically stable.
Clozapine, cancer treatments, and agranulocytosis
This case demonstrates that clozapine can be safely continued during a variety of cancer treatments (ie, chemotherapy, CAR T-cell therapy, HSCT), even with the development of agranulocytosis and prolonged neutropenia. Evidence to guide psychiatric clinicians to evaluate the likelihood that agranulocytosis is clozapine-induced is limited.
Continue to: We offer an algorithm...
We offer an algorithm to assist clinicians faced with this challenging clinical dilemma (Figure). Based on our experience and limited current evidence, we recommend continuing clozapine during cancer treatment unless there is clear evidence to suggest otherwise. Presently, no evidence in published literature suggests worsened outcomes in patients treated concurrently with clozapine and cancer therapies.

OUTCOME Cancer-free and psychiatrically stable
Mr. A continues clozapine therapy throughout all phases of treatment, without interruption. No adverse effects are determined to be secondary to clozapine. He remains psychiatrically stable throughout treatment, and able to participate and engage in his oncologic therapy. Mr. A is now more than 1 year in remission with no recurrence of graft failure, and his psychiatric symptoms continue to be well controlled with clozapine.
Bottom Line
Clozapine can be safely continued during a variety of cancer treatments (ie, chemotherapy, CAR T-cell therapy, HSCT), even in patients who develop agranulocytosis and prolonged neutropenia. Based on our experience and limited evidence, we offer an algorithm to assist clinicians faced with this challenging clinical dilemma.
Related Resources
- Grainger BT, Arcasoy MO, Kenedi CA. Feasibility of myelosuppressive chemotherapy in psychiatric patients on clozapine: a systematic review of the literature. Eur J Haematol. 2019;103(4):277-286. doi:10.1111/ejh.13285
- Daniel JS, Gross T. Managing clozapine-induced neutropenia and agranulocytosis. Current Psychiatry. 2016;15(12):51-53.
Drug Brand Names
Aripiprazole • Abilify
Clozapine • Clozaril
Divalproex sodium • Depakote
Epoetin alfa • Epogen
Haloperidol • Haldol
Olanzapine • Zyprexa
Quetiapine • Seroquel
Risperidone • Risperdal
Ziprasidone • Geodon
1. Grainger BT, Arcasoy MO, Kenedi CA. Feasibility of myelosuppressive chemotherapy in psychiatric patients on clozapine: a systematic review of the literature. Eur J Haematol. 2019;103(4):277-286.
2. Pick AM, Nystrom KK. Nonchemotherapy drug-induced neutropenia and agranulocytosis: could medications be the culprit? J Pharm Pract. 2014:27(5):447-452.
3. Epstein RS, Aapro MS, Basu Roy UK, et al. Patient burden and real-world management of chemotherapy-induced myelosuppression: results from an online survey of patients with solid tumors. Adv Ther. 2020;37(8):3606-3618.
4. Alvir JM, Lieberman JA, Safferman AZ, et al. Clozapine-induced agranulocytosis. Incidence and risk factors in the United States. N Engl J Med. 1993;329(3):162-167.
5. Atkin K, Kendall F, Gould D, et al. Neutropenia and agranulocytosis in patients receiving clozapine in the UK and Ireland. Br J Psychiatry. 1996;169(4):483-488.
6. Azadeh N, Kelemen K, Fonseca R. Amitriptyline-induced agranulocytosis with bone marrow confirmation. Clin Lymphoma Myeloma Leuk. 2014;14(5):e183-e185.
7. Liu J, Zhang X, Zhong JF, et al. CAR-T cells and allogeneic hematopoietic stem cell transplantation for relapsed/refractory B-cell acute lymphoblastic leukemia. Immunotherapy. 2017;9(13):1115-1125.
8. Apinantriyo B, Lekhakula A, Rujirojindakul P. Incidence, etiology and bone marrow characteristics of non-chemotherapy-induced agranulocytosis. Hematology. 2011;16(1):50-53.
9. Yang J, Zhong J, Xiao XH, et al. The relationship between bone marrow characteristics and the clinical prognosis of antithyroid drug-induced agranulocytosis. Endocr J. 2013;60(2):185-189.
1. Grainger BT, Arcasoy MO, Kenedi CA. Feasibility of myelosuppressive chemotherapy in psychiatric patients on clozapine: a systematic review of the literature. Eur J Haematol. 2019;103(4):277-286.
2. Pick AM, Nystrom KK. Nonchemotherapy drug-induced neutropenia and agranulocytosis: could medications be the culprit? J Pharm Pract. 2014:27(5):447-452.
3. Epstein RS, Aapro MS, Basu Roy UK, et al. Patient burden and real-world management of chemotherapy-induced myelosuppression: results from an online survey of patients with solid tumors. Adv Ther. 2020;37(8):3606-3618.
4. Alvir JM, Lieberman JA, Safferman AZ, et al. Clozapine-induced agranulocytosis. Incidence and risk factors in the United States. N Engl J Med. 1993;329(3):162-167.
5. Atkin K, Kendall F, Gould D, et al. Neutropenia and agranulocytosis in patients receiving clozapine in the UK and Ireland. Br J Psychiatry. 1996;169(4):483-488.
6. Azadeh N, Kelemen K, Fonseca R. Amitriptyline-induced agranulocytosis with bone marrow confirmation. Clin Lymphoma Myeloma Leuk. 2014;14(5):e183-e185.
7. Liu J, Zhang X, Zhong JF, et al. CAR-T cells and allogeneic hematopoietic stem cell transplantation for relapsed/refractory B-cell acute lymphoblastic leukemia. Immunotherapy. 2017;9(13):1115-1125.
8. Apinantriyo B, Lekhakula A, Rujirojindakul P. Incidence, etiology and bone marrow characteristics of non-chemotherapy-induced agranulocytosis. Hematology. 2011;16(1):50-53.
9. Yang J, Zhong J, Xiao XH, et al. The relationship between bone marrow characteristics and the clinical prognosis of antithyroid drug-induced agranulocytosis. Endocr J. 2013;60(2):185-189.
Telepsychiatry helped maintain standard of schizophrenia care during COVID
, new survey data show.
“Mental health centers rose to the challenge and did what they needed to do for their patients,” study investigator Dawn Velligan, PhD, University of Texas Health Science Center at San Antonio, told this news organization.
“Some decided to put patients on longer-acting injectable formulations. Some centers gave injections outside to make people feel safer,” Dr. Velligan said.
She added that other patients who might not have had transportation, or were too afraid to come in, were switched to oral medications. However, “switching to orals isn’t something that should be done lightly. I would only want patients to switch to orals as a last resort, but you do what you have to do,” Dr. Velligan said.
The findings were presented at the annual meeting of the American Psychiatric Association.
No going back?
When COVID hit, many mental health clinics closed for in-person visits. “This was unprecedented and we wanted to understand how clinics adapted their services and clinical management of patients with schizophrenia” on LAIs, Dr. Velligan said.
She and her colleagues surveyed 35 mental health clinics, with one respondent at each clinic, between October and November 2020.
All 35 clinics reported using telepsychiatry; 15 had been using telepsychiatry before the pandemic, while 20 (57%) began using it after COVID hit.
Across outpatient visit types, telepsychiatry use for noninjection visits rose from 12%-15% before the pandemic to 45%-69% after the pandemic.
In addition, patients were more apt to keep their telehealth visit. The frequency of appointment “no shows” and/or cancellations for telepsychiatry visits decreased by roughly one-third after the pandemic, compared with before the pandemic.
For patients with schizophrenia treated with LAIs, the frequency of telepsychiatry visits increased in 46% of the clinics during the pandemic.
For these patients, management options included switching patients from LAIs to oral antipsychotics in 34% of clinics and switching patients to LAIs with longer injection intervals in 31% of clinics.
Chief barriers to telepsychiatry visits were low reimbursement rate and lack of access to technology/reliable Internet.
Nearly all respondents reported being satisfied with the use of telepsychiatry to support patients with schizophrenia, whether treated with LAIs (94%) or with oral antipsychotics (97%).
Sixty percent of respondents reported no change in medication adherence for patients treated with LAIs since the start of the pandemic, while less than half (43%) reported no change in adherence to oral antipsychotics.
Most respondents (69%) felt that telepsychiatry visits would very likely continue to be used in combination with in-person office visits after the pandemic.
“Telemedicine is here to stay,” Dr. Velligan said.
Moving to a ‘hybrid universe’
Hector Colon-Rivera, MD, University of Pittsburgh Medical Center and president of the APA’s Hispanic Caucus, agrees.
Commenting on the findings, he noted that, because of shifts in care brought on by COVID, psychiatrists had to adopt telemedicine practices. As a result, many “now feel more comfortable” with telehealth visits for medication management and psychotherapy, said Dr. Colon-Rivera, who was not involved with the research.
He added this study is important because it shows that even patients with severe mental illness can be successfully managed with telepsychiatry, and with good adherence.
“Especially for patients with schizophrenia who have access issues, telepsychiatry is really helpful,” Dr. Colon-Rivera said.
“Telepsychiatry is becoming standard. Most clinics are moving to the hybrid universe now by having a telemedicine component and also seeing patients in person. Even places like emergency rooms and psychiatrists who do consults on medical floors are using telepsychiatry as an option,” he added.
Study funding was provided by Alkermes. Dr. Velligan has reported financial relationships with Alkermes, Otsuka, Janssen, and Lyndra. Dr. Colon-Rivera has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, new survey data show.
“Mental health centers rose to the challenge and did what they needed to do for their patients,” study investigator Dawn Velligan, PhD, University of Texas Health Science Center at San Antonio, told this news organization.
“Some decided to put patients on longer-acting injectable formulations. Some centers gave injections outside to make people feel safer,” Dr. Velligan said.
She added that other patients who might not have had transportation, or were too afraid to come in, were switched to oral medications. However, “switching to orals isn’t something that should be done lightly. I would only want patients to switch to orals as a last resort, but you do what you have to do,” Dr. Velligan said.
The findings were presented at the annual meeting of the American Psychiatric Association.
No going back?
When COVID hit, many mental health clinics closed for in-person visits. “This was unprecedented and we wanted to understand how clinics adapted their services and clinical management of patients with schizophrenia” on LAIs, Dr. Velligan said.
She and her colleagues surveyed 35 mental health clinics, with one respondent at each clinic, between October and November 2020.
All 35 clinics reported using telepsychiatry; 15 had been using telepsychiatry before the pandemic, while 20 (57%) began using it after COVID hit.
Across outpatient visit types, telepsychiatry use for noninjection visits rose from 12%-15% before the pandemic to 45%-69% after the pandemic.
In addition, patients were more apt to keep their telehealth visit. The frequency of appointment “no shows” and/or cancellations for telepsychiatry visits decreased by roughly one-third after the pandemic, compared with before the pandemic.
For patients with schizophrenia treated with LAIs, the frequency of telepsychiatry visits increased in 46% of the clinics during the pandemic.
For these patients, management options included switching patients from LAIs to oral antipsychotics in 34% of clinics and switching patients to LAIs with longer injection intervals in 31% of clinics.
Chief barriers to telepsychiatry visits were low reimbursement rate and lack of access to technology/reliable Internet.
Nearly all respondents reported being satisfied with the use of telepsychiatry to support patients with schizophrenia, whether treated with LAIs (94%) or with oral antipsychotics (97%).
Sixty percent of respondents reported no change in medication adherence for patients treated with LAIs since the start of the pandemic, while less than half (43%) reported no change in adherence to oral antipsychotics.
Most respondents (69%) felt that telepsychiatry visits would very likely continue to be used in combination with in-person office visits after the pandemic.
“Telemedicine is here to stay,” Dr. Velligan said.
Moving to a ‘hybrid universe’
Hector Colon-Rivera, MD, University of Pittsburgh Medical Center and president of the APA’s Hispanic Caucus, agrees.
Commenting on the findings, he noted that, because of shifts in care brought on by COVID, psychiatrists had to adopt telemedicine practices. As a result, many “now feel more comfortable” with telehealth visits for medication management and psychotherapy, said Dr. Colon-Rivera, who was not involved with the research.
He added this study is important because it shows that even patients with severe mental illness can be successfully managed with telepsychiatry, and with good adherence.
“Especially for patients with schizophrenia who have access issues, telepsychiatry is really helpful,” Dr. Colon-Rivera said.
“Telepsychiatry is becoming standard. Most clinics are moving to the hybrid universe now by having a telemedicine component and also seeing patients in person. Even places like emergency rooms and psychiatrists who do consults on medical floors are using telepsychiatry as an option,” he added.
Study funding was provided by Alkermes. Dr. Velligan has reported financial relationships with Alkermes, Otsuka, Janssen, and Lyndra. Dr. Colon-Rivera has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, new survey data show.
“Mental health centers rose to the challenge and did what they needed to do for their patients,” study investigator Dawn Velligan, PhD, University of Texas Health Science Center at San Antonio, told this news organization.
“Some decided to put patients on longer-acting injectable formulations. Some centers gave injections outside to make people feel safer,” Dr. Velligan said.
She added that other patients who might not have had transportation, or were too afraid to come in, were switched to oral medications. However, “switching to orals isn’t something that should be done lightly. I would only want patients to switch to orals as a last resort, but you do what you have to do,” Dr. Velligan said.
The findings were presented at the annual meeting of the American Psychiatric Association.
No going back?
When COVID hit, many mental health clinics closed for in-person visits. “This was unprecedented and we wanted to understand how clinics adapted their services and clinical management of patients with schizophrenia” on LAIs, Dr. Velligan said.
She and her colleagues surveyed 35 mental health clinics, with one respondent at each clinic, between October and November 2020.
All 35 clinics reported using telepsychiatry; 15 had been using telepsychiatry before the pandemic, while 20 (57%) began using it after COVID hit.
Across outpatient visit types, telepsychiatry use for noninjection visits rose from 12%-15% before the pandemic to 45%-69% after the pandemic.
In addition, patients were more apt to keep their telehealth visit. The frequency of appointment “no shows” and/or cancellations for telepsychiatry visits decreased by roughly one-third after the pandemic, compared with before the pandemic.
For patients with schizophrenia treated with LAIs, the frequency of telepsychiatry visits increased in 46% of the clinics during the pandemic.
For these patients, management options included switching patients from LAIs to oral antipsychotics in 34% of clinics and switching patients to LAIs with longer injection intervals in 31% of clinics.
Chief barriers to telepsychiatry visits were low reimbursement rate and lack of access to technology/reliable Internet.
Nearly all respondents reported being satisfied with the use of telepsychiatry to support patients with schizophrenia, whether treated with LAIs (94%) or with oral antipsychotics (97%).
Sixty percent of respondents reported no change in medication adherence for patients treated with LAIs since the start of the pandemic, while less than half (43%) reported no change in adherence to oral antipsychotics.
Most respondents (69%) felt that telepsychiatry visits would very likely continue to be used in combination with in-person office visits after the pandemic.
“Telemedicine is here to stay,” Dr. Velligan said.
Moving to a ‘hybrid universe’
Hector Colon-Rivera, MD, University of Pittsburgh Medical Center and president of the APA’s Hispanic Caucus, agrees.
Commenting on the findings, he noted that, because of shifts in care brought on by COVID, psychiatrists had to adopt telemedicine practices. As a result, many “now feel more comfortable” with telehealth visits for medication management and psychotherapy, said Dr. Colon-Rivera, who was not involved with the research.
He added this study is important because it shows that even patients with severe mental illness can be successfully managed with telepsychiatry, and with good adherence.
“Especially for patients with schizophrenia who have access issues, telepsychiatry is really helpful,” Dr. Colon-Rivera said.
“Telepsychiatry is becoming standard. Most clinics are moving to the hybrid universe now by having a telemedicine component and also seeing patients in person. Even places like emergency rooms and psychiatrists who do consults on medical floors are using telepsychiatry as an option,” he added.
Study funding was provided by Alkermes. Dr. Velligan has reported financial relationships with Alkermes, Otsuka, Janssen, and Lyndra. Dr. Colon-Rivera has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM APA 2022
Antipsychotic safe, effective for resistant depression in phase 3 trial
, new results from a phase 3 study show.
Already approved by the U.S. Food and Drug Administration to treat adults with schizophrenia and manic, mixed, or depressive episodes of bipolar I disorder, cariprazine is under investigation as an add-on therapy for MDD.
“Even patients who appear to be nonresponsive to standard antidepressant drugs have a very good chance of responding” to cariprazine, lead study author Gary Sachs, MD, associate clinical professor of psychiatry at Massachusetts General Hospital, Boston, told this news organization.
He noted that cariprazine, which is a partial agonist at D2 and D3, as well as 5-HT1A, “is an entirely different class” of drugs.
“It’s worth understanding how to use drugs like cariprazine and expanding our nomenclature; instead of referring to these drugs as atypical antipsychotics, perhaps referring to them as atypical antidepressants makes more sense,” Dr. Sachs said.
The findings were presented at the annual meeting of the American Psychiatric Association.
More options critical
MDD is among the most common psychiatric disorders in the United States. In 2020, an estimated 21 million adults had at least one major depressive episode.
Previous research has shown almost half of patients with MDD do not experience satisfactory results from their current treatment regimen. Therefore, research on more options for patients is critical, Dr. Sachs said.
Results from a previously published placebo-controlled study showed adjunctive treatment with cariprazine at 2-mg to 4.5-mg per day doses was more effective than placebo in improving depressive symptoms in adults with MDD.
The new analysis included patients with MDD and an inadequate response to antidepressant therapy, including selective serotonin reuptake inhibitors, serotonin and norepinephrine reuptake inhibitors (SNRIs), or tricyclic antidepressants. They were recruited from 116 centers in the United States and Europe.
Dr. Sachs noted that a nonresponse to an adequate dose of an antidepressant typically means having less than a 50% improvement over 6 weeks or more.
Researchers randomly assigned the patients to oral cariprazine 1.5 mg/day, cariprazine 3 mg/day, or placebo. All continued to take their antidepressant monotherapy.
The analysis included 757 mostly White participants (mean age, 44.8 years; 73.4% women). All had experienced depression for a “huge” part of their life (average, about 14 years), “not to mention their adult life,” said Dr. Sachs.
In addition, at the start of the study, the participants had been depressed for almost 8 months on average.
The primary endpoint was change at week 6 in Montgomery-Åsberg Depression Rating Scale (MADRS) total score. The mean baseline MADRS total score was 32.5.
Less is sometimes more
Results showed a significantly greater mean reduction in MADRS total score for cariprazine 1.5 mg/day vs. placebo at week 6 (P = .005). Significant differences from placebo were observed as early as week 2 and were maintained at week 4, as well as week 6.
“I can say with great confidence that the 1.5-mg dose met all the standards for efficacy,” Dr. Sachs said.
However, this was not the case for the 3-mg/day dose. Although there was a numerically greater reduction in MADRS total score for this dosage of the drug vs. placebo at week 6, the difference was not statistically significant (P = .07).
At week 6, more patients taking the active drug at 1.5 mg/day than placebo responded to treatment, defined as 50% or greater reduction in MADRS total score (44% vs. 34.9%, respectively; P < .05).
Researchers also assessed scores on the Clinical Global Impressions, finding significantly greater score improvement for both the 1.5-mg/day (P = .0026) and 3-mg/day (P =.0076) groups vs. the placebo group.
Improvement at week 6 in mean total score on the Hamilton Depression Rating Scale (HAM-17) reached nominal significance for cariprazine 1.5 mg/day vs. placebo – but not for 3 mg/day.
The results of this “high-quality” double-blind, randomized, controlled, parallel group study provide “what I regard as proven efficacy,” Dr. Sachs said.
He added that the investigational drug was also relatively safe. “The vast majority of patients tolerated it quite well,” he stressed. In addition, the drop-out rate because of adverse events was “quite low overall.”
The only adverse events (AEs) that occurred with the active treatment at a frequency of 5% or more and double that of placebo were akathisia and nausea. Changes in weight were relatively small, at less than 1 kg, in all treatment groups.
There was one serious AE in each active drug group, one of which was a kidney infection. There were two serious AEs reported in the placebo group, including one patient with multiple sclerosis. There were no deaths.
Dr. Sachs noted an advantage of cariprazine is its long half-life, which makes it more user-friendly because “it forgives you if you miss a dose or two.”
Drug manufacturer AbbVie’s supplemental New Drug Application for cariprazine is currently under review by the FDA for expanded use as adjunctive treatment of MDD. A decision by the agency is expected by the end of this year.
Another potential treatment option
Commenting on the findings, James Murrough, MD, PhD, associate professor of psychiatry and of neuroscience and director of the Depression and Anxiety Center for Discovery and Treatment at the Icahn School of Medicine at Mount Sinai, New York, said he welcomes research into additional treatments for MDD.
“Each medicine in a particular class has a unique pharmacology, so a larger number of medication options may help the clinician find a good match for a particular patient,” said Dr. Murrough, who was not involved with the research.
He noted cariprazine is “somewhat unique” among the dopamine modulators in “preferring interactions with the D3 receptor, one of many types of dopamine receptors.”
Although the study results showed cariprazine was effective in MDD, it “does not entirely break new ground” because previous research has already established the drug’s efficacy as adjunctive therapy for patients with depression not responding to a standard antidepressant, said Dr. Murrough.
He also noted that the lower dose, but not the higher dose, of the drug was found to be significantly beneficial for patients, compared with placebo.
“This is a good reminder that higher doses of a medication are not always better,” Dr. Murrough said.
The study was funded by AbbVie. Dr. Sachs is a full-time employee of Signant Health, which conducted the training and quality control for this study. Dr. Murrough has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, new results from a phase 3 study show.
Already approved by the U.S. Food and Drug Administration to treat adults with schizophrenia and manic, mixed, or depressive episodes of bipolar I disorder, cariprazine is under investigation as an add-on therapy for MDD.
“Even patients who appear to be nonresponsive to standard antidepressant drugs have a very good chance of responding” to cariprazine, lead study author Gary Sachs, MD, associate clinical professor of psychiatry at Massachusetts General Hospital, Boston, told this news organization.
He noted that cariprazine, which is a partial agonist at D2 and D3, as well as 5-HT1A, “is an entirely different class” of drugs.
“It’s worth understanding how to use drugs like cariprazine and expanding our nomenclature; instead of referring to these drugs as atypical antipsychotics, perhaps referring to them as atypical antidepressants makes more sense,” Dr. Sachs said.
The findings were presented at the annual meeting of the American Psychiatric Association.
More options critical
MDD is among the most common psychiatric disorders in the United States. In 2020, an estimated 21 million adults had at least one major depressive episode.
Previous research has shown almost half of patients with MDD do not experience satisfactory results from their current treatment regimen. Therefore, research on more options for patients is critical, Dr. Sachs said.
Results from a previously published placebo-controlled study showed adjunctive treatment with cariprazine at 2-mg to 4.5-mg per day doses was more effective than placebo in improving depressive symptoms in adults with MDD.
The new analysis included patients with MDD and an inadequate response to antidepressant therapy, including selective serotonin reuptake inhibitors, serotonin and norepinephrine reuptake inhibitors (SNRIs), or tricyclic antidepressants. They were recruited from 116 centers in the United States and Europe.
Dr. Sachs noted that a nonresponse to an adequate dose of an antidepressant typically means having less than a 50% improvement over 6 weeks or more.
Researchers randomly assigned the patients to oral cariprazine 1.5 mg/day, cariprazine 3 mg/day, or placebo. All continued to take their antidepressant monotherapy.
The analysis included 757 mostly White participants (mean age, 44.8 years; 73.4% women). All had experienced depression for a “huge” part of their life (average, about 14 years), “not to mention their adult life,” said Dr. Sachs.
In addition, at the start of the study, the participants had been depressed for almost 8 months on average.
The primary endpoint was change at week 6 in Montgomery-Åsberg Depression Rating Scale (MADRS) total score. The mean baseline MADRS total score was 32.5.
Less is sometimes more
Results showed a significantly greater mean reduction in MADRS total score for cariprazine 1.5 mg/day vs. placebo at week 6 (P = .005). Significant differences from placebo were observed as early as week 2 and were maintained at week 4, as well as week 6.
“I can say with great confidence that the 1.5-mg dose met all the standards for efficacy,” Dr. Sachs said.
However, this was not the case for the 3-mg/day dose. Although there was a numerically greater reduction in MADRS total score for this dosage of the drug vs. placebo at week 6, the difference was not statistically significant (P = .07).
At week 6, more patients taking the active drug at 1.5 mg/day than placebo responded to treatment, defined as 50% or greater reduction in MADRS total score (44% vs. 34.9%, respectively; P < .05).
Researchers also assessed scores on the Clinical Global Impressions, finding significantly greater score improvement for both the 1.5-mg/day (P = .0026) and 3-mg/day (P =.0076) groups vs. the placebo group.
Improvement at week 6 in mean total score on the Hamilton Depression Rating Scale (HAM-17) reached nominal significance for cariprazine 1.5 mg/day vs. placebo – but not for 3 mg/day.
The results of this “high-quality” double-blind, randomized, controlled, parallel group study provide “what I regard as proven efficacy,” Dr. Sachs said.
He added that the investigational drug was also relatively safe. “The vast majority of patients tolerated it quite well,” he stressed. In addition, the drop-out rate because of adverse events was “quite low overall.”
The only adverse events (AEs) that occurred with the active treatment at a frequency of 5% or more and double that of placebo were akathisia and nausea. Changes in weight were relatively small, at less than 1 kg, in all treatment groups.
There was one serious AE in each active drug group, one of which was a kidney infection. There were two serious AEs reported in the placebo group, including one patient with multiple sclerosis. There were no deaths.
Dr. Sachs noted an advantage of cariprazine is its long half-life, which makes it more user-friendly because “it forgives you if you miss a dose or two.”
Drug manufacturer AbbVie’s supplemental New Drug Application for cariprazine is currently under review by the FDA for expanded use as adjunctive treatment of MDD. A decision by the agency is expected by the end of this year.
Another potential treatment option
Commenting on the findings, James Murrough, MD, PhD, associate professor of psychiatry and of neuroscience and director of the Depression and Anxiety Center for Discovery and Treatment at the Icahn School of Medicine at Mount Sinai, New York, said he welcomes research into additional treatments for MDD.
“Each medicine in a particular class has a unique pharmacology, so a larger number of medication options may help the clinician find a good match for a particular patient,” said Dr. Murrough, who was not involved with the research.
He noted cariprazine is “somewhat unique” among the dopamine modulators in “preferring interactions with the D3 receptor, one of many types of dopamine receptors.”
Although the study results showed cariprazine was effective in MDD, it “does not entirely break new ground” because previous research has already established the drug’s efficacy as adjunctive therapy for patients with depression not responding to a standard antidepressant, said Dr. Murrough.
He also noted that the lower dose, but not the higher dose, of the drug was found to be significantly beneficial for patients, compared with placebo.
“This is a good reminder that higher doses of a medication are not always better,” Dr. Murrough said.
The study was funded by AbbVie. Dr. Sachs is a full-time employee of Signant Health, which conducted the training and quality control for this study. Dr. Murrough has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, new results from a phase 3 study show.
Already approved by the U.S. Food and Drug Administration to treat adults with schizophrenia and manic, mixed, or depressive episodes of bipolar I disorder, cariprazine is under investigation as an add-on therapy for MDD.
“Even patients who appear to be nonresponsive to standard antidepressant drugs have a very good chance of responding” to cariprazine, lead study author Gary Sachs, MD, associate clinical professor of psychiatry at Massachusetts General Hospital, Boston, told this news organization.
He noted that cariprazine, which is a partial agonist at D2 and D3, as well as 5-HT1A, “is an entirely different class” of drugs.
“It’s worth understanding how to use drugs like cariprazine and expanding our nomenclature; instead of referring to these drugs as atypical antipsychotics, perhaps referring to them as atypical antidepressants makes more sense,” Dr. Sachs said.
The findings were presented at the annual meeting of the American Psychiatric Association.
More options critical
MDD is among the most common psychiatric disorders in the United States. In 2020, an estimated 21 million adults had at least one major depressive episode.
Previous research has shown almost half of patients with MDD do not experience satisfactory results from their current treatment regimen. Therefore, research on more options for patients is critical, Dr. Sachs said.
Results from a previously published placebo-controlled study showed adjunctive treatment with cariprazine at 2-mg to 4.5-mg per day doses was more effective than placebo in improving depressive symptoms in adults with MDD.
The new analysis included patients with MDD and an inadequate response to antidepressant therapy, including selective serotonin reuptake inhibitors, serotonin and norepinephrine reuptake inhibitors (SNRIs), or tricyclic antidepressants. They were recruited from 116 centers in the United States and Europe.
Dr. Sachs noted that a nonresponse to an adequate dose of an antidepressant typically means having less than a 50% improvement over 6 weeks or more.
Researchers randomly assigned the patients to oral cariprazine 1.5 mg/day, cariprazine 3 mg/day, or placebo. All continued to take their antidepressant monotherapy.
The analysis included 757 mostly White participants (mean age, 44.8 years; 73.4% women). All had experienced depression for a “huge” part of their life (average, about 14 years), “not to mention their adult life,” said Dr. Sachs.
In addition, at the start of the study, the participants had been depressed for almost 8 months on average.
The primary endpoint was change at week 6 in Montgomery-Åsberg Depression Rating Scale (MADRS) total score. The mean baseline MADRS total score was 32.5.
Less is sometimes more
Results showed a significantly greater mean reduction in MADRS total score for cariprazine 1.5 mg/day vs. placebo at week 6 (P = .005). Significant differences from placebo were observed as early as week 2 and were maintained at week 4, as well as week 6.
“I can say with great confidence that the 1.5-mg dose met all the standards for efficacy,” Dr. Sachs said.
However, this was not the case for the 3-mg/day dose. Although there was a numerically greater reduction in MADRS total score for this dosage of the drug vs. placebo at week 6, the difference was not statistically significant (P = .07).
At week 6, more patients taking the active drug at 1.5 mg/day than placebo responded to treatment, defined as 50% or greater reduction in MADRS total score (44% vs. 34.9%, respectively; P < .05).
Researchers also assessed scores on the Clinical Global Impressions, finding significantly greater score improvement for both the 1.5-mg/day (P = .0026) and 3-mg/day (P =.0076) groups vs. the placebo group.
Improvement at week 6 in mean total score on the Hamilton Depression Rating Scale (HAM-17) reached nominal significance for cariprazine 1.5 mg/day vs. placebo – but not for 3 mg/day.
The results of this “high-quality” double-blind, randomized, controlled, parallel group study provide “what I regard as proven efficacy,” Dr. Sachs said.
He added that the investigational drug was also relatively safe. “The vast majority of patients tolerated it quite well,” he stressed. In addition, the drop-out rate because of adverse events was “quite low overall.”
The only adverse events (AEs) that occurred with the active treatment at a frequency of 5% or more and double that of placebo were akathisia and nausea. Changes in weight were relatively small, at less than 1 kg, in all treatment groups.
There was one serious AE in each active drug group, one of which was a kidney infection. There were two serious AEs reported in the placebo group, including one patient with multiple sclerosis. There were no deaths.
Dr. Sachs noted an advantage of cariprazine is its long half-life, which makes it more user-friendly because “it forgives you if you miss a dose or two.”
Drug manufacturer AbbVie’s supplemental New Drug Application for cariprazine is currently under review by the FDA for expanded use as adjunctive treatment of MDD. A decision by the agency is expected by the end of this year.
Another potential treatment option
Commenting on the findings, James Murrough, MD, PhD, associate professor of psychiatry and of neuroscience and director of the Depression and Anxiety Center for Discovery and Treatment at the Icahn School of Medicine at Mount Sinai, New York, said he welcomes research into additional treatments for MDD.
“Each medicine in a particular class has a unique pharmacology, so a larger number of medication options may help the clinician find a good match for a particular patient,” said Dr. Murrough, who was not involved with the research.
He noted cariprazine is “somewhat unique” among the dopamine modulators in “preferring interactions with the D3 receptor, one of many types of dopamine receptors.”
Although the study results showed cariprazine was effective in MDD, it “does not entirely break new ground” because previous research has already established the drug’s efficacy as adjunctive therapy for patients with depression not responding to a standard antidepressant, said Dr. Murrough.
He also noted that the lower dose, but not the higher dose, of the drug was found to be significantly beneficial for patients, compared with placebo.
“This is a good reminder that higher doses of a medication are not always better,” Dr. Murrough said.
The study was funded by AbbVie. Dr. Sachs is a full-time employee of Signant Health, which conducted the training and quality control for this study. Dr. Murrough has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM APA 2022
Multiple mental health woes? Blame it on genetics
Investigators conducted a genetic analysis of 11 major psychiatric disorders, including schizophrenia and bipolar disorder.
“Our findings confirm that high comorbidity across some disorders in part reflects overlapping pathways of genetic risk,” lead author Andrew Grotzinger, PhD, department of psychology and neuroscience, University of Colorado at Boulder, said in a press release.
The results could lead to the development of treatments that address multiple psychiatric disorders at once and help reshape the way diagnoses are established, the researchers note.
The findings were published online in Nature Genetics.
Common genetic patterns
Using the massive UK Biobank and the Psychiatric Genomics Consortium, the researchers applied novel statistical genetic methods to identify common patterns across 11 major psychiatric disorders: schizophrenia, bipolar disorder, major depressive disorder, anxiety disorder, anorexia nervosa, obsessive-compulsive disorder (OCD), Tourette syndrome, post traumatic stress disorder, problematic alcohol use, attention deficit hyperactive disorder, and autism.
The average total sample size per disorder was 156,771 participants, with a range of 9,725 to 802,939 participants.
In all, the investigators identified 152 genetic variants shared across multiple disorders, including those already known to influence certain types of brain cells.
For example, they found that 70% of the genetic signal associated with schizophrenia was also associated with bipolar disorder.
Results also showed that anorexia nervosa and OCD have a strong, shared genetic architecture and that individuals with a genetic predisposition to low body mass index also tend to have a genetic predisposition to these two disorders.
Not surprisingly, the researchers note, there was a large genetic overlap between anxiety disorder and major depressive disorder.
They also observed that psychiatric disorders that tend to cluster together also tend to share genes that influence how and when individuals are physically active during the day.
For example, patients with internalizing disorders such as anxiety and depression tend to have a genetic architecture associated with low movement throughout the day. On the other hand, those with OCD and anorexia tend to have genes associated with higher movement throughout the day.
“When you think about it, it makes sense,” said Dr. Grotzinger. Depressed individuals often experience fatigue or low energy while those with compulsive disorders may have a tough time sitting still, he noted.
One treatment for multiple disorders?
“Collectively, these results offer key insights into the shared and disorder-specific mechanisms of genetic risk for psychiatric disease,” the investigators write.
Their research is also a first step toward developing therapies that can address multiple disorders with one treatment, they add.
“People are more likely today to be prescribed multiple medications intended to treat multiple diagnoses, and in some instances those medicines can have side effects,” Dr. Grotzinger said.
“By identifying what is shared across these issues, we can hopefully come up with ways to target them in a different way that doesn’t require four separate pills or four separate psychotherapy interventions,” he added.
Dr. Grotzinger noted that, for now, the knowledge that genetics are underlying their disorders may provide comfort to some patients.
“It’s important for people to know that they didn’t just get a terrible roll of the dice in life – that they are not facing multiple different issues but rather one set of risk factors bleeding into them all,” he said.
This research had no commercial funding. Dr. Grotzinger reported no relevant disclosures.
A version of this article first appeared on Medscape.com.
Investigators conducted a genetic analysis of 11 major psychiatric disorders, including schizophrenia and bipolar disorder.
“Our findings confirm that high comorbidity across some disorders in part reflects overlapping pathways of genetic risk,” lead author Andrew Grotzinger, PhD, department of psychology and neuroscience, University of Colorado at Boulder, said in a press release.
The results could lead to the development of treatments that address multiple psychiatric disorders at once and help reshape the way diagnoses are established, the researchers note.
The findings were published online in Nature Genetics.
Common genetic patterns
Using the massive UK Biobank and the Psychiatric Genomics Consortium, the researchers applied novel statistical genetic methods to identify common patterns across 11 major psychiatric disorders: schizophrenia, bipolar disorder, major depressive disorder, anxiety disorder, anorexia nervosa, obsessive-compulsive disorder (OCD), Tourette syndrome, post traumatic stress disorder, problematic alcohol use, attention deficit hyperactive disorder, and autism.
The average total sample size per disorder was 156,771 participants, with a range of 9,725 to 802,939 participants.
In all, the investigators identified 152 genetic variants shared across multiple disorders, including those already known to influence certain types of brain cells.
For example, they found that 70% of the genetic signal associated with schizophrenia was also associated with bipolar disorder.
Results also showed that anorexia nervosa and OCD have a strong, shared genetic architecture and that individuals with a genetic predisposition to low body mass index also tend to have a genetic predisposition to these two disorders.
Not surprisingly, the researchers note, there was a large genetic overlap between anxiety disorder and major depressive disorder.
They also observed that psychiatric disorders that tend to cluster together also tend to share genes that influence how and when individuals are physically active during the day.
For example, patients with internalizing disorders such as anxiety and depression tend to have a genetic architecture associated with low movement throughout the day. On the other hand, those with OCD and anorexia tend to have genes associated with higher movement throughout the day.
“When you think about it, it makes sense,” said Dr. Grotzinger. Depressed individuals often experience fatigue or low energy while those with compulsive disorders may have a tough time sitting still, he noted.
One treatment for multiple disorders?
“Collectively, these results offer key insights into the shared and disorder-specific mechanisms of genetic risk for psychiatric disease,” the investigators write.
Their research is also a first step toward developing therapies that can address multiple disorders with one treatment, they add.
“People are more likely today to be prescribed multiple medications intended to treat multiple diagnoses, and in some instances those medicines can have side effects,” Dr. Grotzinger said.
“By identifying what is shared across these issues, we can hopefully come up with ways to target them in a different way that doesn’t require four separate pills or four separate psychotherapy interventions,” he added.
Dr. Grotzinger noted that, for now, the knowledge that genetics are underlying their disorders may provide comfort to some patients.
“It’s important for people to know that they didn’t just get a terrible roll of the dice in life – that they are not facing multiple different issues but rather one set of risk factors bleeding into them all,” he said.
This research had no commercial funding. Dr. Grotzinger reported no relevant disclosures.
A version of this article first appeared on Medscape.com.
Investigators conducted a genetic analysis of 11 major psychiatric disorders, including schizophrenia and bipolar disorder.
“Our findings confirm that high comorbidity across some disorders in part reflects overlapping pathways of genetic risk,” lead author Andrew Grotzinger, PhD, department of psychology and neuroscience, University of Colorado at Boulder, said in a press release.
The results could lead to the development of treatments that address multiple psychiatric disorders at once and help reshape the way diagnoses are established, the researchers note.
The findings were published online in Nature Genetics.
Common genetic patterns
Using the massive UK Biobank and the Psychiatric Genomics Consortium, the researchers applied novel statistical genetic methods to identify common patterns across 11 major psychiatric disorders: schizophrenia, bipolar disorder, major depressive disorder, anxiety disorder, anorexia nervosa, obsessive-compulsive disorder (OCD), Tourette syndrome, post traumatic stress disorder, problematic alcohol use, attention deficit hyperactive disorder, and autism.
The average total sample size per disorder was 156,771 participants, with a range of 9,725 to 802,939 participants.
In all, the investigators identified 152 genetic variants shared across multiple disorders, including those already known to influence certain types of brain cells.
For example, they found that 70% of the genetic signal associated with schizophrenia was also associated with bipolar disorder.
Results also showed that anorexia nervosa and OCD have a strong, shared genetic architecture and that individuals with a genetic predisposition to low body mass index also tend to have a genetic predisposition to these two disorders.
Not surprisingly, the researchers note, there was a large genetic overlap between anxiety disorder and major depressive disorder.
They also observed that psychiatric disorders that tend to cluster together also tend to share genes that influence how and when individuals are physically active during the day.
For example, patients with internalizing disorders such as anxiety and depression tend to have a genetic architecture associated with low movement throughout the day. On the other hand, those with OCD and anorexia tend to have genes associated with higher movement throughout the day.
“When you think about it, it makes sense,” said Dr. Grotzinger. Depressed individuals often experience fatigue or low energy while those with compulsive disorders may have a tough time sitting still, he noted.
One treatment for multiple disorders?
“Collectively, these results offer key insights into the shared and disorder-specific mechanisms of genetic risk for psychiatric disease,” the investigators write.
Their research is also a first step toward developing therapies that can address multiple disorders with one treatment, they add.
“People are more likely today to be prescribed multiple medications intended to treat multiple diagnoses, and in some instances those medicines can have side effects,” Dr. Grotzinger said.
“By identifying what is shared across these issues, we can hopefully come up with ways to target them in a different way that doesn’t require four separate pills or four separate psychotherapy interventions,” he added.
Dr. Grotzinger noted that, for now, the knowledge that genetics are underlying their disorders may provide comfort to some patients.
“It’s important for people to know that they didn’t just get a terrible roll of the dice in life – that they are not facing multiple different issues but rather one set of risk factors bleeding into them all,” he said.
This research had no commercial funding. Dr. Grotzinger reported no relevant disclosures.
A version of this article first appeared on Medscape.com.
FROM NATURE GENETICS
The psychopathic brain: New insight
Using MRI, researchers found that the striatum was about 10% larger on average in adults with psychopathic traits than in matched control persons and that this relationship was mediated by stimulation seeking and impulsivity.
The striatum is a subcortical region of the forebrain involved in the cognitive processing of reward-related information and motivational aspects of behavior.
“Our study’s results help advance our knowledge about what underlies antisocial behavior such as psychopathy,” co-author and neurocriminologist Olivia Choy, PhD, with Nanyang Technological University, Singapore, said in a news release.
“In addition to social environmental influences, it is important to consider that there can be differences in biology – in this case, the size of brain structures – between antisocial and non-antisocial individuals,” Dr. Choy added.
The study was published online in the Journal of Psychiatric Research.
Antisocial, egocentric
Individuals with psychopathic traits typically have an egocentric and antisocial personality. They generally lack remorse for their actions or empathy for others and often have criminal tendencies.
Some prior research suggests links between psychopathy and an overactive striatum, but it was unclear what role striatal volume plays in this behavior.
For the study, investigators assessed striatal volume using MRI in 120 adults living in the community, and they assessed psychopathy using the Psychopathy Checklist – Revised.
Correlational analyses showed that increased striatal volumes were associated with more psychopathic traits (P = .001) in both men and women.
Volumetric increases were found for all subregions of the striatum in psychopathic individuals, after controlling for age, substance dependence, substance abuse, antisocial personality disorder, attention-deficit/hyperactivity disorder, social adversity, and total brain volume.
An analysis of 18 psychopathic individuals showed that striatal volumes were increased 9.4%, compared with 18 propensity-matched control persons (P = .01).
Abnormal reward processing
Stimulation seeking and impulsivity partly mediated the striatal-psychopathy relationship, accounting for 49.4% of this association.
These findings “replicate and build on initial studies indicating striatal enlargement in adults with psychopathy, yielding an updated effect size of d = 0.48,” the researchers note.
The results are “consistent with the notion that striatal abnormalities in individuals with psychopathy partly reflect increased sensation-seeking and impulsivity and support the hypothesis of abnormal reward processing in psychopathy,” they add.
“We have always known that psychopaths go to extreme lengths to seek out rewards, including criminal activities that involve property, sex, and drugs,” co-author Adrian Raine, DPhil, department of criminology, psychiatry, and psychology, University of Pennsylvania, Philadelphia, said in a news release.
“We are now finding out a neurobiological underpinning of this impulsive and stimulating behavior in the form of enlargement to the striatum, a key brain area involved in rewards,” Dr. Raine added.
What causes striatal enlargement in individuals with psychopathy still needs to be determined.
In human development, the striatum typically becomes smaller as a child matures, suggesting that psychopathy is associated with differences in brain development, the researchers suggest.
“Because biological traits, such as the size of one’s striatum, can be inherited to child from parent, these findings give added support to neurodevelopmental perspectives of psychopathy – that the brains of these offenders do not develop normally throughout childhood and adolescence,” said Dr. Raine.
Larger studies needed
Commenting on the findings for this news organization, Terrie E. Moffitt, PhD, professor of psychology, Duke University, Durham, N.C., noted that there is “general consensus among brain-imaging researchers that testing brain-behavior relations requires very large samples in the thousands and also samples of research participants who represent the full extent of variation in the population as well as possible – from rich to poor, from well to unwell, from high IQ to low IQ, from strong mental health to mental illness, etc.
“It would be grand to see this study’s provocative finding replicated in a large, representative sampling design,” Dr. Moffitt said.
The study was supported in part by the National Institutes of Health. Dr. Choy, Dr. Raine, and Dr. Moffitt have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Using MRI, researchers found that the striatum was about 10% larger on average in adults with psychopathic traits than in matched control persons and that this relationship was mediated by stimulation seeking and impulsivity.
The striatum is a subcortical region of the forebrain involved in the cognitive processing of reward-related information and motivational aspects of behavior.
“Our study’s results help advance our knowledge about what underlies antisocial behavior such as psychopathy,” co-author and neurocriminologist Olivia Choy, PhD, with Nanyang Technological University, Singapore, said in a news release.
“In addition to social environmental influences, it is important to consider that there can be differences in biology – in this case, the size of brain structures – between antisocial and non-antisocial individuals,” Dr. Choy added.
The study was published online in the Journal of Psychiatric Research.
Antisocial, egocentric
Individuals with psychopathic traits typically have an egocentric and antisocial personality. They generally lack remorse for their actions or empathy for others and often have criminal tendencies.
Some prior research suggests links between psychopathy and an overactive striatum, but it was unclear what role striatal volume plays in this behavior.
For the study, investigators assessed striatal volume using MRI in 120 adults living in the community, and they assessed psychopathy using the Psychopathy Checklist – Revised.
Correlational analyses showed that increased striatal volumes were associated with more psychopathic traits (P = .001) in both men and women.
Volumetric increases were found for all subregions of the striatum in psychopathic individuals, after controlling for age, substance dependence, substance abuse, antisocial personality disorder, attention-deficit/hyperactivity disorder, social adversity, and total brain volume.
An analysis of 18 psychopathic individuals showed that striatal volumes were increased 9.4%, compared with 18 propensity-matched control persons (P = .01).
Abnormal reward processing
Stimulation seeking and impulsivity partly mediated the striatal-psychopathy relationship, accounting for 49.4% of this association.
These findings “replicate and build on initial studies indicating striatal enlargement in adults with psychopathy, yielding an updated effect size of d = 0.48,” the researchers note.
The results are “consistent with the notion that striatal abnormalities in individuals with psychopathy partly reflect increased sensation-seeking and impulsivity and support the hypothesis of abnormal reward processing in psychopathy,” they add.
“We have always known that psychopaths go to extreme lengths to seek out rewards, including criminal activities that involve property, sex, and drugs,” co-author Adrian Raine, DPhil, department of criminology, psychiatry, and psychology, University of Pennsylvania, Philadelphia, said in a news release.
“We are now finding out a neurobiological underpinning of this impulsive and stimulating behavior in the form of enlargement to the striatum, a key brain area involved in rewards,” Dr. Raine added.
What causes striatal enlargement in individuals with psychopathy still needs to be determined.
In human development, the striatum typically becomes smaller as a child matures, suggesting that psychopathy is associated with differences in brain development, the researchers suggest.
“Because biological traits, such as the size of one’s striatum, can be inherited to child from parent, these findings give added support to neurodevelopmental perspectives of psychopathy – that the brains of these offenders do not develop normally throughout childhood and adolescence,” said Dr. Raine.
Larger studies needed
Commenting on the findings for this news organization, Terrie E. Moffitt, PhD, professor of psychology, Duke University, Durham, N.C., noted that there is “general consensus among brain-imaging researchers that testing brain-behavior relations requires very large samples in the thousands and also samples of research participants who represent the full extent of variation in the population as well as possible – from rich to poor, from well to unwell, from high IQ to low IQ, from strong mental health to mental illness, etc.
“It would be grand to see this study’s provocative finding replicated in a large, representative sampling design,” Dr. Moffitt said.
The study was supported in part by the National Institutes of Health. Dr. Choy, Dr. Raine, and Dr. Moffitt have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Using MRI, researchers found that the striatum was about 10% larger on average in adults with psychopathic traits than in matched control persons and that this relationship was mediated by stimulation seeking and impulsivity.
The striatum is a subcortical region of the forebrain involved in the cognitive processing of reward-related information and motivational aspects of behavior.
“Our study’s results help advance our knowledge about what underlies antisocial behavior such as psychopathy,” co-author and neurocriminologist Olivia Choy, PhD, with Nanyang Technological University, Singapore, said in a news release.
“In addition to social environmental influences, it is important to consider that there can be differences in biology – in this case, the size of brain structures – between antisocial and non-antisocial individuals,” Dr. Choy added.
The study was published online in the Journal of Psychiatric Research.
Antisocial, egocentric
Individuals with psychopathic traits typically have an egocentric and antisocial personality. They generally lack remorse for their actions or empathy for others and often have criminal tendencies.
Some prior research suggests links between psychopathy and an overactive striatum, but it was unclear what role striatal volume plays in this behavior.
For the study, investigators assessed striatal volume using MRI in 120 adults living in the community, and they assessed psychopathy using the Psychopathy Checklist – Revised.
Correlational analyses showed that increased striatal volumes were associated with more psychopathic traits (P = .001) in both men and women.
Volumetric increases were found for all subregions of the striatum in psychopathic individuals, after controlling for age, substance dependence, substance abuse, antisocial personality disorder, attention-deficit/hyperactivity disorder, social adversity, and total brain volume.
An analysis of 18 psychopathic individuals showed that striatal volumes were increased 9.4%, compared with 18 propensity-matched control persons (P = .01).
Abnormal reward processing
Stimulation seeking and impulsivity partly mediated the striatal-psychopathy relationship, accounting for 49.4% of this association.
These findings “replicate and build on initial studies indicating striatal enlargement in adults with psychopathy, yielding an updated effect size of d = 0.48,” the researchers note.
The results are “consistent with the notion that striatal abnormalities in individuals with psychopathy partly reflect increased sensation-seeking and impulsivity and support the hypothesis of abnormal reward processing in psychopathy,” they add.
“We have always known that psychopaths go to extreme lengths to seek out rewards, including criminal activities that involve property, sex, and drugs,” co-author Adrian Raine, DPhil, department of criminology, psychiatry, and psychology, University of Pennsylvania, Philadelphia, said in a news release.
“We are now finding out a neurobiological underpinning of this impulsive and stimulating behavior in the form of enlargement to the striatum, a key brain area involved in rewards,” Dr. Raine added.
What causes striatal enlargement in individuals with psychopathy still needs to be determined.
In human development, the striatum typically becomes smaller as a child matures, suggesting that psychopathy is associated with differences in brain development, the researchers suggest.
“Because biological traits, such as the size of one’s striatum, can be inherited to child from parent, these findings give added support to neurodevelopmental perspectives of psychopathy – that the brains of these offenders do not develop normally throughout childhood and adolescence,” said Dr. Raine.
Larger studies needed
Commenting on the findings for this news organization, Terrie E. Moffitt, PhD, professor of psychology, Duke University, Durham, N.C., noted that there is “general consensus among brain-imaging researchers that testing brain-behavior relations requires very large samples in the thousands and also samples of research participants who represent the full extent of variation in the population as well as possible – from rich to poor, from well to unwell, from high IQ to low IQ, from strong mental health to mental illness, etc.
“It would be grand to see this study’s provocative finding replicated in a large, representative sampling design,” Dr. Moffitt said.
The study was supported in part by the National Institutes of Health. Dr. Choy, Dr. Raine, and Dr. Moffitt have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Neuropsychiatric risks of COVID-19: New data
The neuropsychiatric ramifications of severe COVID-19 infection appear to be no different than for other severe acute respiratory infections (SARI).
This suggests that disease severity, rather than pathogen, is the most relevant factor in new-onset neuropsychiatric illness, the investigators note.
The risk of new-onset neuropsychological illness after severe COVID-19 infection are “substantial, but similar to those after other severe respiratory infections,” study investigator Peter Watkinson, MD, Nuffield Department of Clinical Neurosciences, University of Oxford, and John Radcliffe Hospital, Oxford, England, told this news organization.
The study was published online in JAMA Psychiatry.
Significant mental health burden
Research has shown a significant burden of neuropsychological illness after severe COVID-19 infection. However, it’s unclear how this risk compares to SARI.
To investigate, Dr. Watkinson and colleagues evaluated electronic health record data on more than 8.3 million adults, including 16,679 (0.02%) who survived a hospital admission for SARI and 32,525 (0.03%) who survived a hospital stay for COVID-19.
Compared with the remaining population, risks of new anxiety disorder, dementia, psychotic disorder, depression, and bipolar disorder diagnoses were significantly and similarly increased in adults surviving hospitalization for either COVID-19 or SARI.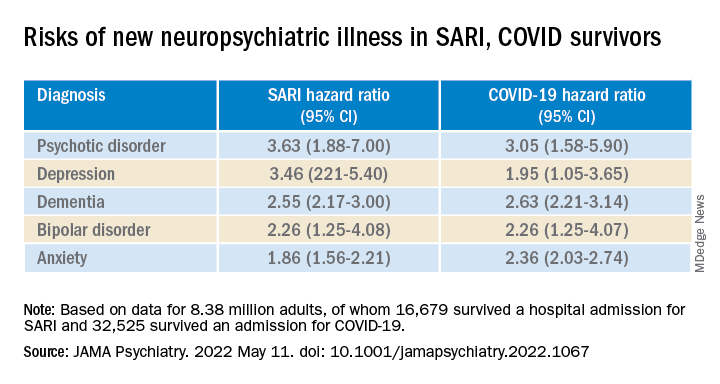
Compared with the wider population, survivors of severe SARI or COVID-19 were also at increased risk of starting treatment with antidepressants, hypnotics/anxiolytics, or antipsychotics.
When comparing survivors of SARI hospitalization to survivors of COVID-19 hospitalization, no significant differences were observed in the postdischarge rates of new-onset anxiety disorder, dementia, depression, or bipolar affective disorder.
The SARI and COVID groups also did not differ in terms of their postdischarge risks of antidepressant or hypnotic/anxiolytic use, but the COVID survivors had a 20% lower risk of starting an antipsychotic.
“In this cohort study, SARI were found to be associated with significant postacute neuropsychiatric morbidity, for which COVID-19 is not distinctly different,” Dr. Watkinson and colleagues write.
“These results may help refine our understanding of the post–severe COVID-19 phenotype and may inform post-discharge support for patients requiring hospital-based and intensive care for SARI regardless of causative pathogen,” they write.
Caveats, cautionary notes
Kevin McConway, PhD, emeritus professor of applied statistics at the Open University in Milton Keynes, England, described the study as “impressive.” However, he pointed out that the study’s observational design is a limitation.
“One can never be absolutely certain about the interpretation of findings of an observational study. What the research can’t tell us is what caused the increased psychiatric risks for people hospitalized with COVID-19 or some other serious respiratory disease,” Dr. McConway said.
“It can’t tell us what might happen in the future, when, we all hope, many fewer are being hospitalized with COVID-19 than was the case in those first two waves, and the current backlog of provision of some health services has decreased,” he added.
“So we can’t just say that, in general, serious COVID-19 has much the same neuropsychiatric consequences as other very serious respiratory illness. Maybe it does, maybe it doesn’t,” Dr. McConway cautioned.
Max Taquet, PhD, with the University of Oxford, noted that the study is limited to hospitalized adult patients, leaving open the question of risk in nonhospitalized individuals – which is the overwhelming majority of patients with COVID-19 – or in children.
Whether the neuropsychiatric risks have remained the same since the emergence of the Omicron variant also remains “an open question since all patients in this study were diagnosed before July 2021,” Dr. Taquet said in statement.
The study was funded by the Wellcome Trust, the John Fell Oxford University Press Research Fund, the Oxford Wellcome Institutional Strategic Support Fund and Cancer Research UK, through the Cancer Research UK Oxford Centre. Dr. Watkinson disclosed grants from the National Institute for Health Research and Sensyne Health outside the submitted work; and serving as chief medical officer for Sensyne Health prior to this work, as well as holding shares in the company. Dr. McConway is a trustee of the UK Science Media Centre and a member of its advisory committee. His comments were provided in his capacity as an independent professional statistician. Dr. Taquet has worked on similar studies trying to identify, quantify, and specify the neurological and psychiatric consequences of COVID-19.
A version of this article first appeared on Medscape.com.
The neuropsychiatric ramifications of severe COVID-19 infection appear to be no different than for other severe acute respiratory infections (SARI).
This suggests that disease severity, rather than pathogen, is the most relevant factor in new-onset neuropsychiatric illness, the investigators note.
The risk of new-onset neuropsychological illness after severe COVID-19 infection are “substantial, but similar to those after other severe respiratory infections,” study investigator Peter Watkinson, MD, Nuffield Department of Clinical Neurosciences, University of Oxford, and John Radcliffe Hospital, Oxford, England, told this news organization.
The study was published online in JAMA Psychiatry.
Significant mental health burden
Research has shown a significant burden of neuropsychological illness after severe COVID-19 infection. However, it’s unclear how this risk compares to SARI.
To investigate, Dr. Watkinson and colleagues evaluated electronic health record data on more than 8.3 million adults, including 16,679 (0.02%) who survived a hospital admission for SARI and 32,525 (0.03%) who survived a hospital stay for COVID-19.
Compared with the remaining population, risks of new anxiety disorder, dementia, psychotic disorder, depression, and bipolar disorder diagnoses were significantly and similarly increased in adults surviving hospitalization for either COVID-19 or SARI.
Compared with the wider population, survivors of severe SARI or COVID-19 were also at increased risk of starting treatment with antidepressants, hypnotics/anxiolytics, or antipsychotics.
When comparing survivors of SARI hospitalization to survivors of COVID-19 hospitalization, no significant differences were observed in the postdischarge rates of new-onset anxiety disorder, dementia, depression, or bipolar affective disorder.
The SARI and COVID groups also did not differ in terms of their postdischarge risks of antidepressant or hypnotic/anxiolytic use, but the COVID survivors had a 20% lower risk of starting an antipsychotic.
“In this cohort study, SARI were found to be associated with significant postacute neuropsychiatric morbidity, for which COVID-19 is not distinctly different,” Dr. Watkinson and colleagues write.
“These results may help refine our understanding of the post–severe COVID-19 phenotype and may inform post-discharge support for patients requiring hospital-based and intensive care for SARI regardless of causative pathogen,” they write.
Caveats, cautionary notes
Kevin McConway, PhD, emeritus professor of applied statistics at the Open University in Milton Keynes, England, described the study as “impressive.” However, he pointed out that the study’s observational design is a limitation.
“One can never be absolutely certain about the interpretation of findings of an observational study. What the research can’t tell us is what caused the increased psychiatric risks for people hospitalized with COVID-19 or some other serious respiratory disease,” Dr. McConway said.
“It can’t tell us what might happen in the future, when, we all hope, many fewer are being hospitalized with COVID-19 than was the case in those first two waves, and the current backlog of provision of some health services has decreased,” he added.
“So we can’t just say that, in general, serious COVID-19 has much the same neuropsychiatric consequences as other very serious respiratory illness. Maybe it does, maybe it doesn’t,” Dr. McConway cautioned.
Max Taquet, PhD, with the University of Oxford, noted that the study is limited to hospitalized adult patients, leaving open the question of risk in nonhospitalized individuals – which is the overwhelming majority of patients with COVID-19 – or in children.
Whether the neuropsychiatric risks have remained the same since the emergence of the Omicron variant also remains “an open question since all patients in this study were diagnosed before July 2021,” Dr. Taquet said in statement.
The study was funded by the Wellcome Trust, the John Fell Oxford University Press Research Fund, the Oxford Wellcome Institutional Strategic Support Fund and Cancer Research UK, through the Cancer Research UK Oxford Centre. Dr. Watkinson disclosed grants from the National Institute for Health Research and Sensyne Health outside the submitted work; and serving as chief medical officer for Sensyne Health prior to this work, as well as holding shares in the company. Dr. McConway is a trustee of the UK Science Media Centre and a member of its advisory committee. His comments were provided in his capacity as an independent professional statistician. Dr. Taquet has worked on similar studies trying to identify, quantify, and specify the neurological and psychiatric consequences of COVID-19.
A version of this article first appeared on Medscape.com.
The neuropsychiatric ramifications of severe COVID-19 infection appear to be no different than for other severe acute respiratory infections (SARI).
This suggests that disease severity, rather than pathogen, is the most relevant factor in new-onset neuropsychiatric illness, the investigators note.
The risk of new-onset neuropsychological illness after severe COVID-19 infection are “substantial, but similar to those after other severe respiratory infections,” study investigator Peter Watkinson, MD, Nuffield Department of Clinical Neurosciences, University of Oxford, and John Radcliffe Hospital, Oxford, England, told this news organization.
The study was published online in JAMA Psychiatry.
Significant mental health burden
Research has shown a significant burden of neuropsychological illness after severe COVID-19 infection. However, it’s unclear how this risk compares to SARI.
To investigate, Dr. Watkinson and colleagues evaluated electronic health record data on more than 8.3 million adults, including 16,679 (0.02%) who survived a hospital admission for SARI and 32,525 (0.03%) who survived a hospital stay for COVID-19.
Compared with the remaining population, risks of new anxiety disorder, dementia, psychotic disorder, depression, and bipolar disorder diagnoses were significantly and similarly increased in adults surviving hospitalization for either COVID-19 or SARI.
Compared with the wider population, survivors of severe SARI or COVID-19 were also at increased risk of starting treatment with antidepressants, hypnotics/anxiolytics, or antipsychotics.
When comparing survivors of SARI hospitalization to survivors of COVID-19 hospitalization, no significant differences were observed in the postdischarge rates of new-onset anxiety disorder, dementia, depression, or bipolar affective disorder.
The SARI and COVID groups also did not differ in terms of their postdischarge risks of antidepressant or hypnotic/anxiolytic use, but the COVID survivors had a 20% lower risk of starting an antipsychotic.
“In this cohort study, SARI were found to be associated with significant postacute neuropsychiatric morbidity, for which COVID-19 is not distinctly different,” Dr. Watkinson and colleagues write.
“These results may help refine our understanding of the post–severe COVID-19 phenotype and may inform post-discharge support for patients requiring hospital-based and intensive care for SARI regardless of causative pathogen,” they write.
Caveats, cautionary notes
Kevin McConway, PhD, emeritus professor of applied statistics at the Open University in Milton Keynes, England, described the study as “impressive.” However, he pointed out that the study’s observational design is a limitation.
“One can never be absolutely certain about the interpretation of findings of an observational study. What the research can’t tell us is what caused the increased psychiatric risks for people hospitalized with COVID-19 or some other serious respiratory disease,” Dr. McConway said.
“It can’t tell us what might happen in the future, when, we all hope, many fewer are being hospitalized with COVID-19 than was the case in those first two waves, and the current backlog of provision of some health services has decreased,” he added.
“So we can’t just say that, in general, serious COVID-19 has much the same neuropsychiatric consequences as other very serious respiratory illness. Maybe it does, maybe it doesn’t,” Dr. McConway cautioned.
Max Taquet, PhD, with the University of Oxford, noted that the study is limited to hospitalized adult patients, leaving open the question of risk in nonhospitalized individuals – which is the overwhelming majority of patients with COVID-19 – or in children.
Whether the neuropsychiatric risks have remained the same since the emergence of the Omicron variant also remains “an open question since all patients in this study were diagnosed before July 2021,” Dr. Taquet said in statement.
The study was funded by the Wellcome Trust, the John Fell Oxford University Press Research Fund, the Oxford Wellcome Institutional Strategic Support Fund and Cancer Research UK, through the Cancer Research UK Oxford Centre. Dr. Watkinson disclosed grants from the National Institute for Health Research and Sensyne Health outside the submitted work; and serving as chief medical officer for Sensyne Health prior to this work, as well as holding shares in the company. Dr. McConway is a trustee of the UK Science Media Centre and a member of its advisory committee. His comments were provided in his capacity as an independent professional statistician. Dr. Taquet has worked on similar studies trying to identify, quantify, and specify the neurological and psychiatric consequences of COVID-19.
A version of this article first appeared on Medscape.com.
Clozapine and cancer risk in schizophrenia patients: New data
Long-term treatment with clozapine is associated with a small but significant risk of hematological malignancies in individuals with schizophrenia, new research shows.
The study was published online in The Lancet Psychiatry.
An unresolved issue
Clozapine is more effective than other antipsychotics for managing symptoms and suicidal behavior in schizophrenia, with the lowest mortality, compared with other antipsychotics, but its use is restricted in many countries, the researchers note.
Reports of nine deaths associated with clozapine use – eight due to agranulocytosis and one due to leukemia – in southwestern Finland in 1975 resulted in worldwide withdrawal of the drug. In 1990, clozapine was relaunched with stipulations for strict blood count control. The cumulative incidence of clozapine-induced agranulocytosis or severe neutropenia is estimated at about 0.9%.
Several small studies from Australia, Denmark, and the United States, and a large pharmacovigilance study, suggest that clozapine treatment might be associated with an increased risk of hematological malignancies.
“Previous studies have suggested a possible risk of hematological malignancies associated with clozapine, but due to methodological issues, the question had remained unsettled,” said Dr. Tiihonen.
Finland has among the highest rates of clozapine use in the world, where 20% of schizophrenia cases are treated with the drug. In most other countries, clozapine use is less than half of that, in Finland largely because of agranulocytosis concerns.
To examine the risk of hematological malignancies associated with long-term use of clozapine and other antipsychotics, the investigators conducted a large prospective case-control and cohort study that used data from Finnish national registers and included all patients with schizophrenia.
“Unlike previous studies, we employed prospectively gathered data from a nationwide cohort [including all patients with schizophrenia], had a long follow-up time, and studied the dose-response of the risk of hematological malignancies,” Dr. Tiihonen noted.
The nested case-control study was constructed by individually matching cases of lymphoid and hematopoietic tissue malignancy and pairing them with up to 10 matched controls with schizophrenia but without cancer.
Inclusion criteria were restricted to malignancies diagnosed on a histological basis. Individuals outside the ages of 18-85 years were excluded, as were those with a previous malignancy. Analyses were done using conditional logistic regression adjusted for comorbid conditions.
Patient education, vigilant monitoring
The case-control analysis was based on 516 patients with a first-time diagnosis of lymphoid and hematopoietic tissue malignancy from 2000-2017 and diagnosed after first diagnosis of schizophrenia.
Of these, 102 patients were excluded because of a diagnosis with no histological basis, five were excluded because of age, and 34 for a previous malignancy, resulting in 375 patients with malignancies matched with 10 controls for a total of 3,743 study participants.
Of the 375 patients with hematological malignancies (305 had lymphoma, 42 leukemia, 22 myeloma, six unspecified) in 2000-2017, 208 (55%) were men and 167 (45%) were women. Ethnicity data were not available.
Compared with non-use of clozapine, clozapine use was associated with increased odds of hematological malignancies in a dose-response manner (adjusted odds ratio, 3.35; 95% confidence interval, 2.22-5.05] for ≥ 5,000 defined daily dose cumulative exposure (P < .0001).
Exposure to other antipsychotic medications was not associated with increased odds of hematological malignancies. A complementary analysis showed that the clozapine-related risk increase was specific to hematological malignancies only.
Over 17 years follow-up of the base cohort, 37 deaths occurred due to hematological malignancy among patients exposed to clozapine in 26 patients with ongoing use at the time they were diagnosed with malignancy and in 11 patients who did not use clozapine at the exact time of their cancer diagnosis. Only three deaths occurred due to agranulocytosis, the investigators report.
The use of a nationwide registry for the study makes it “unlikely” that there were any undiagnosed/unreported malignancies, the researchers note. This, plus the “robust dose-response finding, and additional analysis showing no substantial difference in odds of other cancers between users of clozapine versus other antipsychotics suggest the association is causal, and not attributable to surveillance bias,” they write.
These findings, the investigators note, suggest patients taking clozapine and their caregivers need to be educated about the signs of hematological malignancies. Furthermore, they call for mental health providers to be “vigilant” in monitoring for potential signs and symptoms of hematological malignancy in patients taking the drug.
A ‘vital’ medication
Commenting on the findings, Stephen Marder, MD, professor of psychiatry and biobehavioral sciences and vice chair of the department of psychiatry at UCLA, noted the link between clozapine and agranulocytosis.
“Clozapine has been previously associated with agranulocytosis. Over the years that seemed to be the main concern of clinicians. The monitoring system for agranulocytosis has been a burden on the system and for patients, but not really a significant cause for concern with the safety of the drug,” said Dr. Marder, who is also director of the VISN 22 Mental Illness Research, Education and Clinical Center for the Department of Veterans Affairs and director of the section on psychosis at the UCLA Neuropsychiatric Institute.
In fact, he noted recent research, including studies from this group that used large databases from Finland, which showed that clozapine was actually associated with a lower mortality risk than other antipsychotics.
The fact that the study showed prolonged use of clozapine at high doses was associated with a “very small” risk of hematological abnormalities does not undermine its standing as “the most effective antipsychotic [that is] associated with a lower risk of death,” said Dr. Marder.
“On the other hand,” he added, “it does suggest that clinicians should tell patients about it and, when they review the blood monitoring, they look at things beyond the neutrophil count” that may suggest malignancy.
“Clozapine has a vital role as the most effective antipsychotic drug and the only drug that has an indication for treatment-resistant schizophrenia and schizophrenia associated with suicidality,” said Dr. Marder.
The study was funded by the Finnish Ministry of Social Affairs and Health through the developmental fund for Niuvanniemi Hospital and by the Academy of Finland. Dr. Tiihonen and Dr. Marder have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Long-term treatment with clozapine is associated with a small but significant risk of hematological malignancies in individuals with schizophrenia, new research shows.
The study was published online in The Lancet Psychiatry.
An unresolved issue
Clozapine is more effective than other antipsychotics for managing symptoms and suicidal behavior in schizophrenia, with the lowest mortality, compared with other antipsychotics, but its use is restricted in many countries, the researchers note.
Reports of nine deaths associated with clozapine use – eight due to agranulocytosis and one due to leukemia – in southwestern Finland in 1975 resulted in worldwide withdrawal of the drug. In 1990, clozapine was relaunched with stipulations for strict blood count control. The cumulative incidence of clozapine-induced agranulocytosis or severe neutropenia is estimated at about 0.9%.
Several small studies from Australia, Denmark, and the United States, and a large pharmacovigilance study, suggest that clozapine treatment might be associated with an increased risk of hematological malignancies.
“Previous studies have suggested a possible risk of hematological malignancies associated with clozapine, but due to methodological issues, the question had remained unsettled,” said Dr. Tiihonen.
Finland has among the highest rates of clozapine use in the world, where 20% of schizophrenia cases are treated with the drug. In most other countries, clozapine use is less than half of that, in Finland largely because of agranulocytosis concerns.
To examine the risk of hematological malignancies associated with long-term use of clozapine and other antipsychotics, the investigators conducted a large prospective case-control and cohort study that used data from Finnish national registers and included all patients with schizophrenia.
“Unlike previous studies, we employed prospectively gathered data from a nationwide cohort [including all patients with schizophrenia], had a long follow-up time, and studied the dose-response of the risk of hematological malignancies,” Dr. Tiihonen noted.
The nested case-control study was constructed by individually matching cases of lymphoid and hematopoietic tissue malignancy and pairing them with up to 10 matched controls with schizophrenia but without cancer.
Inclusion criteria were restricted to malignancies diagnosed on a histological basis. Individuals outside the ages of 18-85 years were excluded, as were those with a previous malignancy. Analyses were done using conditional logistic regression adjusted for comorbid conditions.
Patient education, vigilant monitoring
The case-control analysis was based on 516 patients with a first-time diagnosis of lymphoid and hematopoietic tissue malignancy from 2000-2017 and diagnosed after first diagnosis of schizophrenia.
Of these, 102 patients were excluded because of a diagnosis with no histological basis, five were excluded because of age, and 34 for a previous malignancy, resulting in 375 patients with malignancies matched with 10 controls for a total of 3,743 study participants.
Of the 375 patients with hematological malignancies (305 had lymphoma, 42 leukemia, 22 myeloma, six unspecified) in 2000-2017, 208 (55%) were men and 167 (45%) were women. Ethnicity data were not available.
Compared with non-use of clozapine, clozapine use was associated with increased odds of hematological malignancies in a dose-response manner (adjusted odds ratio, 3.35; 95% confidence interval, 2.22-5.05] for ≥ 5,000 defined daily dose cumulative exposure (P < .0001).
Exposure to other antipsychotic medications was not associated with increased odds of hematological malignancies. A complementary analysis showed that the clozapine-related risk increase was specific to hematological malignancies only.
Over 17 years follow-up of the base cohort, 37 deaths occurred due to hematological malignancy among patients exposed to clozapine in 26 patients with ongoing use at the time they were diagnosed with malignancy and in 11 patients who did not use clozapine at the exact time of their cancer diagnosis. Only three deaths occurred due to agranulocytosis, the investigators report.
The use of a nationwide registry for the study makes it “unlikely” that there were any undiagnosed/unreported malignancies, the researchers note. This, plus the “robust dose-response finding, and additional analysis showing no substantial difference in odds of other cancers between users of clozapine versus other antipsychotics suggest the association is causal, and not attributable to surveillance bias,” they write.
These findings, the investigators note, suggest patients taking clozapine and their caregivers need to be educated about the signs of hematological malignancies. Furthermore, they call for mental health providers to be “vigilant” in monitoring for potential signs and symptoms of hematological malignancy in patients taking the drug.
A ‘vital’ medication
Commenting on the findings, Stephen Marder, MD, professor of psychiatry and biobehavioral sciences and vice chair of the department of psychiatry at UCLA, noted the link between clozapine and agranulocytosis.
“Clozapine has been previously associated with agranulocytosis. Over the years that seemed to be the main concern of clinicians. The monitoring system for agranulocytosis has been a burden on the system and for patients, but not really a significant cause for concern with the safety of the drug,” said Dr. Marder, who is also director of the VISN 22 Mental Illness Research, Education and Clinical Center for the Department of Veterans Affairs and director of the section on psychosis at the UCLA Neuropsychiatric Institute.
In fact, he noted recent research, including studies from this group that used large databases from Finland, which showed that clozapine was actually associated with a lower mortality risk than other antipsychotics.
The fact that the study showed prolonged use of clozapine at high doses was associated with a “very small” risk of hematological abnormalities does not undermine its standing as “the most effective antipsychotic [that is] associated with a lower risk of death,” said Dr. Marder.
“On the other hand,” he added, “it does suggest that clinicians should tell patients about it and, when they review the blood monitoring, they look at things beyond the neutrophil count” that may suggest malignancy.
“Clozapine has a vital role as the most effective antipsychotic drug and the only drug that has an indication for treatment-resistant schizophrenia and schizophrenia associated with suicidality,” said Dr. Marder.
The study was funded by the Finnish Ministry of Social Affairs and Health through the developmental fund for Niuvanniemi Hospital and by the Academy of Finland. Dr. Tiihonen and Dr. Marder have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Long-term treatment with clozapine is associated with a small but significant risk of hematological malignancies in individuals with schizophrenia, new research shows.
The study was published online in The Lancet Psychiatry.
An unresolved issue
Clozapine is more effective than other antipsychotics for managing symptoms and suicidal behavior in schizophrenia, with the lowest mortality, compared with other antipsychotics, but its use is restricted in many countries, the researchers note.
Reports of nine deaths associated with clozapine use – eight due to agranulocytosis and one due to leukemia – in southwestern Finland in 1975 resulted in worldwide withdrawal of the drug. In 1990, clozapine was relaunched with stipulations for strict blood count control. The cumulative incidence of clozapine-induced agranulocytosis or severe neutropenia is estimated at about 0.9%.
Several small studies from Australia, Denmark, and the United States, and a large pharmacovigilance study, suggest that clozapine treatment might be associated with an increased risk of hematological malignancies.
“Previous studies have suggested a possible risk of hematological malignancies associated with clozapine, but due to methodological issues, the question had remained unsettled,” said Dr. Tiihonen.
Finland has among the highest rates of clozapine use in the world, where 20% of schizophrenia cases are treated with the drug. In most other countries, clozapine use is less than half of that, in Finland largely because of agranulocytosis concerns.
To examine the risk of hematological malignancies associated with long-term use of clozapine and other antipsychotics, the investigators conducted a large prospective case-control and cohort study that used data from Finnish national registers and included all patients with schizophrenia.
“Unlike previous studies, we employed prospectively gathered data from a nationwide cohort [including all patients with schizophrenia], had a long follow-up time, and studied the dose-response of the risk of hematological malignancies,” Dr. Tiihonen noted.
The nested case-control study was constructed by individually matching cases of lymphoid and hematopoietic tissue malignancy and pairing them with up to 10 matched controls with schizophrenia but without cancer.
Inclusion criteria were restricted to malignancies diagnosed on a histological basis. Individuals outside the ages of 18-85 years were excluded, as were those with a previous malignancy. Analyses were done using conditional logistic regression adjusted for comorbid conditions.
Patient education, vigilant monitoring
The case-control analysis was based on 516 patients with a first-time diagnosis of lymphoid and hematopoietic tissue malignancy from 2000-2017 and diagnosed after first diagnosis of schizophrenia.
Of these, 102 patients were excluded because of a diagnosis with no histological basis, five were excluded because of age, and 34 for a previous malignancy, resulting in 375 patients with malignancies matched with 10 controls for a total of 3,743 study participants.
Of the 375 patients with hematological malignancies (305 had lymphoma, 42 leukemia, 22 myeloma, six unspecified) in 2000-2017, 208 (55%) were men and 167 (45%) were women. Ethnicity data were not available.
Compared with non-use of clozapine, clozapine use was associated with increased odds of hematological malignancies in a dose-response manner (adjusted odds ratio, 3.35; 95% confidence interval, 2.22-5.05] for ≥ 5,000 defined daily dose cumulative exposure (P < .0001).
Exposure to other antipsychotic medications was not associated with increased odds of hematological malignancies. A complementary analysis showed that the clozapine-related risk increase was specific to hematological malignancies only.
Over 17 years follow-up of the base cohort, 37 deaths occurred due to hematological malignancy among patients exposed to clozapine in 26 patients with ongoing use at the time they were diagnosed with malignancy and in 11 patients who did not use clozapine at the exact time of their cancer diagnosis. Only three deaths occurred due to agranulocytosis, the investigators report.
The use of a nationwide registry for the study makes it “unlikely” that there were any undiagnosed/unreported malignancies, the researchers note. This, plus the “robust dose-response finding, and additional analysis showing no substantial difference in odds of other cancers between users of clozapine versus other antipsychotics suggest the association is causal, and not attributable to surveillance bias,” they write.
These findings, the investigators note, suggest patients taking clozapine and their caregivers need to be educated about the signs of hematological malignancies. Furthermore, they call for mental health providers to be “vigilant” in monitoring for potential signs and symptoms of hematological malignancy in patients taking the drug.
A ‘vital’ medication
Commenting on the findings, Stephen Marder, MD, professor of psychiatry and biobehavioral sciences and vice chair of the department of psychiatry at UCLA, noted the link between clozapine and agranulocytosis.
“Clozapine has been previously associated with agranulocytosis. Over the years that seemed to be the main concern of clinicians. The monitoring system for agranulocytosis has been a burden on the system and for patients, but not really a significant cause for concern with the safety of the drug,” said Dr. Marder, who is also director of the VISN 22 Mental Illness Research, Education and Clinical Center for the Department of Veterans Affairs and director of the section on psychosis at the UCLA Neuropsychiatric Institute.
In fact, he noted recent research, including studies from this group that used large databases from Finland, which showed that clozapine was actually associated with a lower mortality risk than other antipsychotics.
The fact that the study showed prolonged use of clozapine at high doses was associated with a “very small” risk of hematological abnormalities does not undermine its standing as “the most effective antipsychotic [that is] associated with a lower risk of death,” said Dr. Marder.
“On the other hand,” he added, “it does suggest that clinicians should tell patients about it and, when they review the blood monitoring, they look at things beyond the neutrophil count” that may suggest malignancy.
“Clozapine has a vital role as the most effective antipsychotic drug and the only drug that has an indication for treatment-resistant schizophrenia and schizophrenia associated with suicidality,” said Dr. Marder.
The study was funded by the Finnish Ministry of Social Affairs and Health through the developmental fund for Niuvanniemi Hospital and by the Academy of Finland. Dr. Tiihonen and Dr. Marder have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE LANCET PSYCHIATRY