User login
‘Alarming’ new data on disordered sleep after COVID-19
Such disturbances are especially common among Black people, new research shows.
The “high” prevalence of moderate to severe sleep disturbances is “alarming,” study investigator Cinthya Pena Orbea, MD, sleep specialist at the Cleveland Clinic, said in an interview.
The findings were presented at the annual meeting of the Associated Professional Sleep Societies.
Dr. Pena and colleagues analyzed data on 962 patients with PASC seen at the Cleveland Clinic ReCOVer Clinic between February 2021 and April 2022.
More than two-thirds of patients (67.2%) reported at least moderate fatigue, while 21.8% reported severe fatigue, Dr. Pena reported.
In addition, 41.3% reported at least moderate sleep disturbances, while 8% of patients reported severe sleep disturbances, including insomnia, “which may impair quality of life,” Dr. Pena said.
Obesity, mood disorders, and Black race emerged as contributors to problems with sleep and fatigue after COVID.
Notably, after adjusting for demographics, Black race conferred threefold higher odds of moderate to severe sleep disturbances.
“We don’t know why this is, and one of our next steps is to better understand race-specific determinants of sleep disturbances after COVID and create targeted interventions,” Dr. Pena said.
How long after COVID the fatigue and sleep problems last “remains uncertain,” Dr. Pena acknowledged. However, in her clinical experience with therapy, patients’ sleep and fatigue may improve after 6 or 8 months.
Ruth Benca, MD, PhD, cochair of the Alliance for Sleep, is not surprised by the Cleveland Clinic findings.
“Sleep disturbances and fatigue are part of the sequelae of COVID,” Dr. Benca, who was not involved in the study, said in an interview.
“We know that people who have had COVID have more trouble sleeping afterwards. There is the COVID insomnia created in all of us just out of our worries, fears, isolation, and stress. And then there’s an actual impact of having the infection itself on worsening sleep,” said Dr. Benca, with Wake Forest University and Atrium Health Wake Forest Baptist, both in Winston-Salem, N.C.
The study had no specific funding. The authors have disclosed no relevant financial relationships. Dr. Benca is a consultant for Idorsia Pharmaceuticals.
A version of this article first appeared on Medscape.com.
Such disturbances are especially common among Black people, new research shows.
The “high” prevalence of moderate to severe sleep disturbances is “alarming,” study investigator Cinthya Pena Orbea, MD, sleep specialist at the Cleveland Clinic, said in an interview.
The findings were presented at the annual meeting of the Associated Professional Sleep Societies.
Dr. Pena and colleagues analyzed data on 962 patients with PASC seen at the Cleveland Clinic ReCOVer Clinic between February 2021 and April 2022.
More than two-thirds of patients (67.2%) reported at least moderate fatigue, while 21.8% reported severe fatigue, Dr. Pena reported.
In addition, 41.3% reported at least moderate sleep disturbances, while 8% of patients reported severe sleep disturbances, including insomnia, “which may impair quality of life,” Dr. Pena said.
Obesity, mood disorders, and Black race emerged as contributors to problems with sleep and fatigue after COVID.
Notably, after adjusting for demographics, Black race conferred threefold higher odds of moderate to severe sleep disturbances.
“We don’t know why this is, and one of our next steps is to better understand race-specific determinants of sleep disturbances after COVID and create targeted interventions,” Dr. Pena said.
How long after COVID the fatigue and sleep problems last “remains uncertain,” Dr. Pena acknowledged. However, in her clinical experience with therapy, patients’ sleep and fatigue may improve after 6 or 8 months.
Ruth Benca, MD, PhD, cochair of the Alliance for Sleep, is not surprised by the Cleveland Clinic findings.
“Sleep disturbances and fatigue are part of the sequelae of COVID,” Dr. Benca, who was not involved in the study, said in an interview.
“We know that people who have had COVID have more trouble sleeping afterwards. There is the COVID insomnia created in all of us just out of our worries, fears, isolation, and stress. And then there’s an actual impact of having the infection itself on worsening sleep,” said Dr. Benca, with Wake Forest University and Atrium Health Wake Forest Baptist, both in Winston-Salem, N.C.
The study had no specific funding. The authors have disclosed no relevant financial relationships. Dr. Benca is a consultant for Idorsia Pharmaceuticals.
A version of this article first appeared on Medscape.com.
Such disturbances are especially common among Black people, new research shows.
The “high” prevalence of moderate to severe sleep disturbances is “alarming,” study investigator Cinthya Pena Orbea, MD, sleep specialist at the Cleveland Clinic, said in an interview.
The findings were presented at the annual meeting of the Associated Professional Sleep Societies.
Dr. Pena and colleagues analyzed data on 962 patients with PASC seen at the Cleveland Clinic ReCOVer Clinic between February 2021 and April 2022.
More than two-thirds of patients (67.2%) reported at least moderate fatigue, while 21.8% reported severe fatigue, Dr. Pena reported.
In addition, 41.3% reported at least moderate sleep disturbances, while 8% of patients reported severe sleep disturbances, including insomnia, “which may impair quality of life,” Dr. Pena said.
Obesity, mood disorders, and Black race emerged as contributors to problems with sleep and fatigue after COVID.
Notably, after adjusting for demographics, Black race conferred threefold higher odds of moderate to severe sleep disturbances.
“We don’t know why this is, and one of our next steps is to better understand race-specific determinants of sleep disturbances after COVID and create targeted interventions,” Dr. Pena said.
How long after COVID the fatigue and sleep problems last “remains uncertain,” Dr. Pena acknowledged. However, in her clinical experience with therapy, patients’ sleep and fatigue may improve after 6 or 8 months.
Ruth Benca, MD, PhD, cochair of the Alliance for Sleep, is not surprised by the Cleveland Clinic findings.
“Sleep disturbances and fatigue are part of the sequelae of COVID,” Dr. Benca, who was not involved in the study, said in an interview.
“We know that people who have had COVID have more trouble sleeping afterwards. There is the COVID insomnia created in all of us just out of our worries, fears, isolation, and stress. And then there’s an actual impact of having the infection itself on worsening sleep,” said Dr. Benca, with Wake Forest University and Atrium Health Wake Forest Baptist, both in Winston-Salem, N.C.
The study had no specific funding. The authors have disclosed no relevant financial relationships. Dr. Benca is a consultant for Idorsia Pharmaceuticals.
A version of this article first appeared on Medscape.com.
FROM SLEEP 2022
The power of napping
As a physician who has had a career-long obsession with the underappreciated value of sleep, a recent study published in the journal Child Development caught my eye. The findings presented by a group of Australian-based psychologists and educators suggest a positive association between napping and learning by preschool children. While the study itself relied on a very small sample and may not prove to be repeatable, the authors included in their introduction an excellent discussion of a large collection of recent studies supporting the educational benefit of sleep in general and napping in particular.
Although sleep seems to finally be receiving some of the attention it deserves, I am still concerned that as a profession we are failing to give it the appropriate weight at our health maintenance visits. This is particularly true of napping. Understandably, napping doesn’t feel urgent to parents in those turbulent first 4 or 5 months of night wakings and erratic settling. However, as a child approaches the 6-month milestone, napping is a topic ripe for well-considered anticipatory guidance.
When the recurrent cycles of awake-eat-sleep begin to develop into a somewhat predictable pattern and solid food is introduced, it’s time to suggest to parents a strategy that will encourage a napping pattern that will hopefully habituate into toddlerhood and beyond.
It can begin simply as a matter of defining the feeding in the middle of the day as lunch and then programming the period immediately following that meal as a siesta – a segment of the day completely reserved for rest. Many warm-weather countries have been using this strategy for centuries. Try to go to the pharmacy to pick up a prescription at 2 o’clock in the afternoon in rural Spain. It just ain’t gonna happen.
Most adults and children I know seem to be sleepy during this midday postprandial period. It makes more than a little sense to harness this natural drowsiness into creating a napping habit. However, the challenge for many young families is controlling their schedule to create a period of time when nothing else is going on in the child’s environment, leaving sleep as the only option. For some parents this requires the discipline to pause their own lives long enough so that the children realize that they aren’t missing out on something fun. This means no TV, no phone conversations, no visitors. Obviously, it also means not scheduling any appointments during this siesta period. Skilled day care providers have been doing this for years. But the message hasn’t seeped into the general population and sadly I occasionally see mothers with toddlers in the grocery store at 1 in the afternoon.
Once the nap/siesta is firmly welded to lunch, this gives the parent the ability to make minor adjustments that reflect the child’s stamina. If the child seems to be tiring/getting grumpy, serve up lunch a bit early and the restorative nap follows. As the child gets older and his or her stamina improves he or she may not be sleepy but the siesta remains as a quiet time. Some days it may be a nap, some days just a rest for an hour. By counseling parents to define the period after lunch as a siesta you will be helping them avoid that dreaded transition period called “giving up the nap.”
You may already be including this strategy in your anticipatory guidance. It may help to add to your advice the accumulating evidence that napping may play an important role in the child’s development and education.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
As a physician who has had a career-long obsession with the underappreciated value of sleep, a recent study published in the journal Child Development caught my eye. The findings presented by a group of Australian-based psychologists and educators suggest a positive association between napping and learning by preschool children. While the study itself relied on a very small sample and may not prove to be repeatable, the authors included in their introduction an excellent discussion of a large collection of recent studies supporting the educational benefit of sleep in general and napping in particular.
Although sleep seems to finally be receiving some of the attention it deserves, I am still concerned that as a profession we are failing to give it the appropriate weight at our health maintenance visits. This is particularly true of napping. Understandably, napping doesn’t feel urgent to parents in those turbulent first 4 or 5 months of night wakings and erratic settling. However, as a child approaches the 6-month milestone, napping is a topic ripe for well-considered anticipatory guidance.
When the recurrent cycles of awake-eat-sleep begin to develop into a somewhat predictable pattern and solid food is introduced, it’s time to suggest to parents a strategy that will encourage a napping pattern that will hopefully habituate into toddlerhood and beyond.
It can begin simply as a matter of defining the feeding in the middle of the day as lunch and then programming the period immediately following that meal as a siesta – a segment of the day completely reserved for rest. Many warm-weather countries have been using this strategy for centuries. Try to go to the pharmacy to pick up a prescription at 2 o’clock in the afternoon in rural Spain. It just ain’t gonna happen.
Most adults and children I know seem to be sleepy during this midday postprandial period. It makes more than a little sense to harness this natural drowsiness into creating a napping habit. However, the challenge for many young families is controlling their schedule to create a period of time when nothing else is going on in the child’s environment, leaving sleep as the only option. For some parents this requires the discipline to pause their own lives long enough so that the children realize that they aren’t missing out on something fun. This means no TV, no phone conversations, no visitors. Obviously, it also means not scheduling any appointments during this siesta period. Skilled day care providers have been doing this for years. But the message hasn’t seeped into the general population and sadly I occasionally see mothers with toddlers in the grocery store at 1 in the afternoon.
Once the nap/siesta is firmly welded to lunch, this gives the parent the ability to make minor adjustments that reflect the child’s stamina. If the child seems to be tiring/getting grumpy, serve up lunch a bit early and the restorative nap follows. As the child gets older and his or her stamina improves he or she may not be sleepy but the siesta remains as a quiet time. Some days it may be a nap, some days just a rest for an hour. By counseling parents to define the period after lunch as a siesta you will be helping them avoid that dreaded transition period called “giving up the nap.”
You may already be including this strategy in your anticipatory guidance. It may help to add to your advice the accumulating evidence that napping may play an important role in the child’s development and education.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
As a physician who has had a career-long obsession with the underappreciated value of sleep, a recent study published in the journal Child Development caught my eye. The findings presented by a group of Australian-based psychologists and educators suggest a positive association between napping and learning by preschool children. While the study itself relied on a very small sample and may not prove to be repeatable, the authors included in their introduction an excellent discussion of a large collection of recent studies supporting the educational benefit of sleep in general and napping in particular.
Although sleep seems to finally be receiving some of the attention it deserves, I am still concerned that as a profession we are failing to give it the appropriate weight at our health maintenance visits. This is particularly true of napping. Understandably, napping doesn’t feel urgent to parents in those turbulent first 4 or 5 months of night wakings and erratic settling. However, as a child approaches the 6-month milestone, napping is a topic ripe for well-considered anticipatory guidance.
When the recurrent cycles of awake-eat-sleep begin to develop into a somewhat predictable pattern and solid food is introduced, it’s time to suggest to parents a strategy that will encourage a napping pattern that will hopefully habituate into toddlerhood and beyond.
It can begin simply as a matter of defining the feeding in the middle of the day as lunch and then programming the period immediately following that meal as a siesta – a segment of the day completely reserved for rest. Many warm-weather countries have been using this strategy for centuries. Try to go to the pharmacy to pick up a prescription at 2 o’clock in the afternoon in rural Spain. It just ain’t gonna happen.
Most adults and children I know seem to be sleepy during this midday postprandial period. It makes more than a little sense to harness this natural drowsiness into creating a napping habit. However, the challenge for many young families is controlling their schedule to create a period of time when nothing else is going on in the child’s environment, leaving sleep as the only option. For some parents this requires the discipline to pause their own lives long enough so that the children realize that they aren’t missing out on something fun. This means no TV, no phone conversations, no visitors. Obviously, it also means not scheduling any appointments during this siesta period. Skilled day care providers have been doing this for years. But the message hasn’t seeped into the general population and sadly I occasionally see mothers with toddlers in the grocery store at 1 in the afternoon.
Once the nap/siesta is firmly welded to lunch, this gives the parent the ability to make minor adjustments that reflect the child’s stamina. If the child seems to be tiring/getting grumpy, serve up lunch a bit early and the restorative nap follows. As the child gets older and his or her stamina improves he or she may not be sleepy but the siesta remains as a quiet time. Some days it may be a nap, some days just a rest for an hour. By counseling parents to define the period after lunch as a siesta you will be helping them avoid that dreaded transition period called “giving up the nap.”
You may already be including this strategy in your anticipatory guidance. It may help to add to your advice the accumulating evidence that napping may play an important role in the child’s development and education.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
Parents fall short on infant sleep safety
Less than 10% of parents followed recommended safe sleep practices for their infants aged 12 months and younger at both sleep onset and after nighttime waking, based on data from a survey of 1,500 parents published in Pediatrics.
Sleep-related death remains a major cause of infant mortality in the United States despite the early success of public health campaigns for safe sleep practices, such as “Back to Sleep,” and many parents persist in unsafe practices such as prone positioning and bed-sharing, Mersine A. Bryan, MD, of the University of Washington, Seattle, and colleagues wrote. “Though nighttime waking is common for infants, less attention has been paid to the safety of second-sleep practices.”
To examine the prevalence and safety of infant second-sleep practices, the researchers used a cross-sectional online survey to collect information on sleep practices from parents of infants aged 12 months and younger; 74% of the respondents were female, 65% were White, 12% were Black, and 17% were Hispanic. The mean age of the infants was 6.6 months, and 24% were aged 3 months and younger.
The survey included parent reports of three safe sleep practices based on the American Academy of Pediatrics 2016 Safe Infant Sleep Guidelines: supine infant sleep position, use of a separate sleep space (vs. bed sharing), and use of an approved surface/safe location (such as a bassinet, crib, cradle, or play yard vs. an adult bed).
Parents were asked to report sleep practices at sleep onset and at nighttime waking, and the researchers used a composite score to determine safe practices were met at each of these two time points.
Of the 1,500 participants, 581 (39%), reported any second-sleep practice. Of the 482 who reported on all three sleep practices at both time points, 29% met all three safe sleep criteria at sleep onset and 9% met all three safe sleep criteria at sleep onset and nighttime waking.
Of the parents who reported second sleep practices, 39% reported changes in practice after nighttime waking from sleep onset. Significantly more parents who switched practices between sleep onset and nighttime waking shifted from a safer to a less safe practice, the researchers noted.
For positioning, 67% of respondents overall reported placing infants on their backs at sleep onset. Among the 564 who reported a second sleep position, 42% placed infants on their backs again; 13% switched from supine to nonsupine positions and 7% changed from nonsupine to supine.
For sleep spaces, 72% of participants overall reported a separate sleep space for infants at sleep onset. Of the 508 who reported on second-sleep spaces, 54% kept infants in a separate space after nighttime waking, 18% shifted to a shared space after nighttime waking. Of those in shared spaces at sleep onset, 8% shifted to separate spaces after nighttime waking.
For sleep location, 71% of respondents overall used an approved sleep surface at sleep onset. Of the 560 who reported sleep location at both time points, 42% remained in a safe location after nighttime waking, while 30% were moved from a safe to an unsafe location, and 10% of those in an unsafe location were moved from an unsafe to a safe location.
In a multivariate analysis, the researchers examined the demographics associated with changes in sleep practice after nighttime waking. Parents younger than 25 years, first-time parents, those who identified as Black non-Hispanic or Hispanic, smokers, and those with preterm infants (less than 37 weeks’ gestation) were more likely to change sleep practices after nighttime waking. However, parents who reported a safe sleep practice at sleep onset were more likely to do so after nighttime waking.
“We hypothesize that expansion of existing strategies to promote infant safe sleep practices to include sleep practices after nighttime waking can have a positive impact on infant safe sleep,” the researchers wrote.
The study findings were limited by several factors including the use of an online survey, which limited the study population to those with internet and computer access, and the reliance on self-reports and only two time points, the researchers noted. Other limitations included the inclusion of only three of the AAP sleep recommendations, and the inclusion of only English speakers.
However, the results were strengthened by the large, diverse, and geographically representative sample of parents.
“When advising families about infant sleep, pediatricians should discuss nighttime wakings with parents because they are common and reinforce the need for safe sleep practices every time,” the researchers noted.
Increase opportunities for education
The current study is important because infants continue to die or experience life-long catastrophic health outcomes as a result of not following safe sleep practices, Cathy Haut, DNP, CPNP-AC, CPNP-PC, a pediatric nurse practitioner in Rehoboth Beach, Del., said in an interview.
“I am not surprised by the study findings,” said Dr. Haut, who was not involved in the study. “As a pediatric nurse practitioner for over 35 years, I see infant sleep as a continuing challenge for families. In today’s fast-paced world, multiple priorities leave parents few resources for managing their own well-being, with adequate sleep being one health requirement that is often not met for them.”
To improve safe sleep practices, “it is imperative for health care providers in any setting to address safe sleep practices for infants and children,” said Dr. Haut. “In addition to safety, opportunity for adequate hours of sleep is also important.” She acknowledged that, “in the office setting, time is a huge barrier to completing comprehensive anticipatory guidance. When parents ask questions about sleep, they are often doing everything they can to physically make it through the night with a crying infant. Enforcing safe practices at this point is extremely difficult.”
However, some opportunities for safe sleep education include the prenatal period when parents can take time to listen and plan, not just for feeding preferences but for safe infant sleep practices, Dr. Haut noted.
“When sleep is a problem, families can be invited back to the office for additional counseling and education, which allows more time than within a scheduled health visit,” Dr. Haut emphasized. “Finally, enhanced public awareness is an aspect of learning. In my career I have seen the devastating results of suffocation while cosleeping as well as injuries from falling from a bed or inappropriate sleeping space, and other poor outcomes from inadequate support for safe sleep habits.”
As for additional research, studies are needed to include larger populations and “to further quantify positive outcomes of following safe sleeping practices,” said Dr. Haut. The results of these studies should be made available to the general public, not only to health care professionals.
The study was supported by Seattle Children’s Research Institute. The researchers had no financial conflicts to disclose. Dr. Haut had no financial conflicts to disclose and serves on the editorial advisory board of Pediatric News.
Less than 10% of parents followed recommended safe sleep practices for their infants aged 12 months and younger at both sleep onset and after nighttime waking, based on data from a survey of 1,500 parents published in Pediatrics.
Sleep-related death remains a major cause of infant mortality in the United States despite the early success of public health campaigns for safe sleep practices, such as “Back to Sleep,” and many parents persist in unsafe practices such as prone positioning and bed-sharing, Mersine A. Bryan, MD, of the University of Washington, Seattle, and colleagues wrote. “Though nighttime waking is common for infants, less attention has been paid to the safety of second-sleep practices.”
To examine the prevalence and safety of infant second-sleep practices, the researchers used a cross-sectional online survey to collect information on sleep practices from parents of infants aged 12 months and younger; 74% of the respondents were female, 65% were White, 12% were Black, and 17% were Hispanic. The mean age of the infants was 6.6 months, and 24% were aged 3 months and younger.
The survey included parent reports of three safe sleep practices based on the American Academy of Pediatrics 2016 Safe Infant Sleep Guidelines: supine infant sleep position, use of a separate sleep space (vs. bed sharing), and use of an approved surface/safe location (such as a bassinet, crib, cradle, or play yard vs. an adult bed).
Parents were asked to report sleep practices at sleep onset and at nighttime waking, and the researchers used a composite score to determine safe practices were met at each of these two time points.
Of the 1,500 participants, 581 (39%), reported any second-sleep practice. Of the 482 who reported on all three sleep practices at both time points, 29% met all three safe sleep criteria at sleep onset and 9% met all three safe sleep criteria at sleep onset and nighttime waking.
Of the parents who reported second sleep practices, 39% reported changes in practice after nighttime waking from sleep onset. Significantly more parents who switched practices between sleep onset and nighttime waking shifted from a safer to a less safe practice, the researchers noted.
For positioning, 67% of respondents overall reported placing infants on their backs at sleep onset. Among the 564 who reported a second sleep position, 42% placed infants on their backs again; 13% switched from supine to nonsupine positions and 7% changed from nonsupine to supine.
For sleep spaces, 72% of participants overall reported a separate sleep space for infants at sleep onset. Of the 508 who reported on second-sleep spaces, 54% kept infants in a separate space after nighttime waking, 18% shifted to a shared space after nighttime waking. Of those in shared spaces at sleep onset, 8% shifted to separate spaces after nighttime waking.
For sleep location, 71% of respondents overall used an approved sleep surface at sleep onset. Of the 560 who reported sleep location at both time points, 42% remained in a safe location after nighttime waking, while 30% were moved from a safe to an unsafe location, and 10% of those in an unsafe location were moved from an unsafe to a safe location.
In a multivariate analysis, the researchers examined the demographics associated with changes in sleep practice after nighttime waking. Parents younger than 25 years, first-time parents, those who identified as Black non-Hispanic or Hispanic, smokers, and those with preterm infants (less than 37 weeks’ gestation) were more likely to change sleep practices after nighttime waking. However, parents who reported a safe sleep practice at sleep onset were more likely to do so after nighttime waking.
“We hypothesize that expansion of existing strategies to promote infant safe sleep practices to include sleep practices after nighttime waking can have a positive impact on infant safe sleep,” the researchers wrote.
The study findings were limited by several factors including the use of an online survey, which limited the study population to those with internet and computer access, and the reliance on self-reports and only two time points, the researchers noted. Other limitations included the inclusion of only three of the AAP sleep recommendations, and the inclusion of only English speakers.
However, the results were strengthened by the large, diverse, and geographically representative sample of parents.
“When advising families about infant sleep, pediatricians should discuss nighttime wakings with parents because they are common and reinforce the need for safe sleep practices every time,” the researchers noted.
Increase opportunities for education
The current study is important because infants continue to die or experience life-long catastrophic health outcomes as a result of not following safe sleep practices, Cathy Haut, DNP, CPNP-AC, CPNP-PC, a pediatric nurse practitioner in Rehoboth Beach, Del., said in an interview.
“I am not surprised by the study findings,” said Dr. Haut, who was not involved in the study. “As a pediatric nurse practitioner for over 35 years, I see infant sleep as a continuing challenge for families. In today’s fast-paced world, multiple priorities leave parents few resources for managing their own well-being, with adequate sleep being one health requirement that is often not met for them.”
To improve safe sleep practices, “it is imperative for health care providers in any setting to address safe sleep practices for infants and children,” said Dr. Haut. “In addition to safety, opportunity for adequate hours of sleep is also important.” She acknowledged that, “in the office setting, time is a huge barrier to completing comprehensive anticipatory guidance. When parents ask questions about sleep, they are often doing everything they can to physically make it through the night with a crying infant. Enforcing safe practices at this point is extremely difficult.”
However, some opportunities for safe sleep education include the prenatal period when parents can take time to listen and plan, not just for feeding preferences but for safe infant sleep practices, Dr. Haut noted.
“When sleep is a problem, families can be invited back to the office for additional counseling and education, which allows more time than within a scheduled health visit,” Dr. Haut emphasized. “Finally, enhanced public awareness is an aspect of learning. In my career I have seen the devastating results of suffocation while cosleeping as well as injuries from falling from a bed or inappropriate sleeping space, and other poor outcomes from inadequate support for safe sleep habits.”
As for additional research, studies are needed to include larger populations and “to further quantify positive outcomes of following safe sleeping practices,” said Dr. Haut. The results of these studies should be made available to the general public, not only to health care professionals.
The study was supported by Seattle Children’s Research Institute. The researchers had no financial conflicts to disclose. Dr. Haut had no financial conflicts to disclose and serves on the editorial advisory board of Pediatric News.
Less than 10% of parents followed recommended safe sleep practices for their infants aged 12 months and younger at both sleep onset and after nighttime waking, based on data from a survey of 1,500 parents published in Pediatrics.
Sleep-related death remains a major cause of infant mortality in the United States despite the early success of public health campaigns for safe sleep practices, such as “Back to Sleep,” and many parents persist in unsafe practices such as prone positioning and bed-sharing, Mersine A. Bryan, MD, of the University of Washington, Seattle, and colleagues wrote. “Though nighttime waking is common for infants, less attention has been paid to the safety of second-sleep practices.”
To examine the prevalence and safety of infant second-sleep practices, the researchers used a cross-sectional online survey to collect information on sleep practices from parents of infants aged 12 months and younger; 74% of the respondents were female, 65% were White, 12% were Black, and 17% were Hispanic. The mean age of the infants was 6.6 months, and 24% were aged 3 months and younger.
The survey included parent reports of three safe sleep practices based on the American Academy of Pediatrics 2016 Safe Infant Sleep Guidelines: supine infant sleep position, use of a separate sleep space (vs. bed sharing), and use of an approved surface/safe location (such as a bassinet, crib, cradle, or play yard vs. an adult bed).
Parents were asked to report sleep practices at sleep onset and at nighttime waking, and the researchers used a composite score to determine safe practices were met at each of these two time points.
Of the 1,500 participants, 581 (39%), reported any second-sleep practice. Of the 482 who reported on all three sleep practices at both time points, 29% met all three safe sleep criteria at sleep onset and 9% met all three safe sleep criteria at sleep onset and nighttime waking.
Of the parents who reported second sleep practices, 39% reported changes in practice after nighttime waking from sleep onset. Significantly more parents who switched practices between sleep onset and nighttime waking shifted from a safer to a less safe practice, the researchers noted.
For positioning, 67% of respondents overall reported placing infants on their backs at sleep onset. Among the 564 who reported a second sleep position, 42% placed infants on their backs again; 13% switched from supine to nonsupine positions and 7% changed from nonsupine to supine.
For sleep spaces, 72% of participants overall reported a separate sleep space for infants at sleep onset. Of the 508 who reported on second-sleep spaces, 54% kept infants in a separate space after nighttime waking, 18% shifted to a shared space after nighttime waking. Of those in shared spaces at sleep onset, 8% shifted to separate spaces after nighttime waking.
For sleep location, 71% of respondents overall used an approved sleep surface at sleep onset. Of the 560 who reported sleep location at both time points, 42% remained in a safe location after nighttime waking, while 30% were moved from a safe to an unsafe location, and 10% of those in an unsafe location were moved from an unsafe to a safe location.
In a multivariate analysis, the researchers examined the demographics associated with changes in sleep practice after nighttime waking. Parents younger than 25 years, first-time parents, those who identified as Black non-Hispanic or Hispanic, smokers, and those with preterm infants (less than 37 weeks’ gestation) were more likely to change sleep practices after nighttime waking. However, parents who reported a safe sleep practice at sleep onset were more likely to do so after nighttime waking.
“We hypothesize that expansion of existing strategies to promote infant safe sleep practices to include sleep practices after nighttime waking can have a positive impact on infant safe sleep,” the researchers wrote.
The study findings were limited by several factors including the use of an online survey, which limited the study population to those with internet and computer access, and the reliance on self-reports and only two time points, the researchers noted. Other limitations included the inclusion of only three of the AAP sleep recommendations, and the inclusion of only English speakers.
However, the results were strengthened by the large, diverse, and geographically representative sample of parents.
“When advising families about infant sleep, pediatricians should discuss nighttime wakings with parents because they are common and reinforce the need for safe sleep practices every time,” the researchers noted.
Increase opportunities for education
The current study is important because infants continue to die or experience life-long catastrophic health outcomes as a result of not following safe sleep practices, Cathy Haut, DNP, CPNP-AC, CPNP-PC, a pediatric nurse practitioner in Rehoboth Beach, Del., said in an interview.
“I am not surprised by the study findings,” said Dr. Haut, who was not involved in the study. “As a pediatric nurse practitioner for over 35 years, I see infant sleep as a continuing challenge for families. In today’s fast-paced world, multiple priorities leave parents few resources for managing their own well-being, with adequate sleep being one health requirement that is often not met for them.”
To improve safe sleep practices, “it is imperative for health care providers in any setting to address safe sleep practices for infants and children,” said Dr. Haut. “In addition to safety, opportunity for adequate hours of sleep is also important.” She acknowledged that, “in the office setting, time is a huge barrier to completing comprehensive anticipatory guidance. When parents ask questions about sleep, they are often doing everything they can to physically make it through the night with a crying infant. Enforcing safe practices at this point is extremely difficult.”
However, some opportunities for safe sleep education include the prenatal period when parents can take time to listen and plan, not just for feeding preferences but for safe infant sleep practices, Dr. Haut noted.
“When sleep is a problem, families can be invited back to the office for additional counseling and education, which allows more time than within a scheduled health visit,” Dr. Haut emphasized. “Finally, enhanced public awareness is an aspect of learning. In my career I have seen the devastating results of suffocation while cosleeping as well as injuries from falling from a bed or inappropriate sleeping space, and other poor outcomes from inadequate support for safe sleep habits.”
As for additional research, studies are needed to include larger populations and “to further quantify positive outcomes of following safe sleeping practices,” said Dr. Haut. The results of these studies should be made available to the general public, not only to health care professionals.
The study was supported by Seattle Children’s Research Institute. The researchers had no financial conflicts to disclose. Dr. Haut had no financial conflicts to disclose and serves on the editorial advisory board of Pediatric News.
FROM PEDIATRICS
New law bans infant sleep products linked to 200 deaths
A new law will ban certain infant sleep products blamed for the deaths of more than 200 babies in the United States.
On May 16, President Joe Biden signed legislation that prohibits the manufacture and sale of crib bumpers or inclined sleepers for infants, due to the risk of suffocation, according to CBS News.
H.R. 3182, or the Safe Sleep for Babies Act of 2021, notes that sleepers and bumpers will be considered “banned hazardous products” under the Consumer Product Safety Act. It gives manufacturers and retailers 180 days to comply with the new rule.
“The dangers posed to babies have been apparent for years,” Teresa Murray, who directs the consumer watchdog office for the U.S. PIRG Education Fund, said in a statement.
“It’s unfortunate that this law could take months to take effect,” she said. “Parents and caregivers need to recognize the dangers of these products and get them out of their homes now.”
H.R. 3182 defines inclined sleepers as products that have a sleep surface slanted greater than 10 degrees and are intended for babies up to 1 year old. Crib bumpers include any material that is designed to cover the sides of a crib, which includes padding or vinyl bumper guards but not non-padded mesh crib liners.
The U.S. Consumer Product Safety Commission has received reports of more than 113 deaths involving crib bumpers between 1990 and 2019, as well as 113 nonfatal incidents between 2008 and 2019, according to a report from the commission.
More than 100 babies have died from infant-inclined sleep products, according to the commission, which has recalled numerous versions in recent years. But older models are still in circulation, CBS News reported.
Last year, the commission approved a federal safety rule that bans several types of sleep products for babies under 5 months old. Set to take effect next month, the rule requires products marketed for infants to meet the same federal safety standards as required for cribs and similar products.
Parents and advocates have called for a ban on these products for decades, according to CBS, since they can lead to suffocation when an infant’s nose and mouth are covered by a bumper or become stuck between a bumper and crib mattress.
Sudden unexpected infant death, or SUID – which includes sudden infant death syndrome, or SIDS – is the leading cause of injury death in infancy, according to the American Academy of Pediatrics. The group’s recommendations for safe sleep advise that infants should sleep on their back on a firm, flat surface without any extra padding, pillows, blankets, stuffed toys, bumpers, or other soft items in the sleep space.
A version of this article first appeared on WebMD.com.
A new law will ban certain infant sleep products blamed for the deaths of more than 200 babies in the United States.
On May 16, President Joe Biden signed legislation that prohibits the manufacture and sale of crib bumpers or inclined sleepers for infants, due to the risk of suffocation, according to CBS News.
H.R. 3182, or the Safe Sleep for Babies Act of 2021, notes that sleepers and bumpers will be considered “banned hazardous products” under the Consumer Product Safety Act. It gives manufacturers and retailers 180 days to comply with the new rule.
“The dangers posed to babies have been apparent for years,” Teresa Murray, who directs the consumer watchdog office for the U.S. PIRG Education Fund, said in a statement.
“It’s unfortunate that this law could take months to take effect,” she said. “Parents and caregivers need to recognize the dangers of these products and get them out of their homes now.”
H.R. 3182 defines inclined sleepers as products that have a sleep surface slanted greater than 10 degrees and are intended for babies up to 1 year old. Crib bumpers include any material that is designed to cover the sides of a crib, which includes padding or vinyl bumper guards but not non-padded mesh crib liners.
The U.S. Consumer Product Safety Commission has received reports of more than 113 deaths involving crib bumpers between 1990 and 2019, as well as 113 nonfatal incidents between 2008 and 2019, according to a report from the commission.
More than 100 babies have died from infant-inclined sleep products, according to the commission, which has recalled numerous versions in recent years. But older models are still in circulation, CBS News reported.
Last year, the commission approved a federal safety rule that bans several types of sleep products for babies under 5 months old. Set to take effect next month, the rule requires products marketed for infants to meet the same federal safety standards as required for cribs and similar products.
Parents and advocates have called for a ban on these products for decades, according to CBS, since they can lead to suffocation when an infant’s nose and mouth are covered by a bumper or become stuck between a bumper and crib mattress.
Sudden unexpected infant death, or SUID – which includes sudden infant death syndrome, or SIDS – is the leading cause of injury death in infancy, according to the American Academy of Pediatrics. The group’s recommendations for safe sleep advise that infants should sleep on their back on a firm, flat surface without any extra padding, pillows, blankets, stuffed toys, bumpers, or other soft items in the sleep space.
A version of this article first appeared on WebMD.com.
A new law will ban certain infant sleep products blamed for the deaths of more than 200 babies in the United States.
On May 16, President Joe Biden signed legislation that prohibits the manufacture and sale of crib bumpers or inclined sleepers for infants, due to the risk of suffocation, according to CBS News.
H.R. 3182, or the Safe Sleep for Babies Act of 2021, notes that sleepers and bumpers will be considered “banned hazardous products” under the Consumer Product Safety Act. It gives manufacturers and retailers 180 days to comply with the new rule.
“The dangers posed to babies have been apparent for years,” Teresa Murray, who directs the consumer watchdog office for the U.S. PIRG Education Fund, said in a statement.
“It’s unfortunate that this law could take months to take effect,” she said. “Parents and caregivers need to recognize the dangers of these products and get them out of their homes now.”
H.R. 3182 defines inclined sleepers as products that have a sleep surface slanted greater than 10 degrees and are intended for babies up to 1 year old. Crib bumpers include any material that is designed to cover the sides of a crib, which includes padding or vinyl bumper guards but not non-padded mesh crib liners.
The U.S. Consumer Product Safety Commission has received reports of more than 113 deaths involving crib bumpers between 1990 and 2019, as well as 113 nonfatal incidents between 2008 and 2019, according to a report from the commission.
More than 100 babies have died from infant-inclined sleep products, according to the commission, which has recalled numerous versions in recent years. But older models are still in circulation, CBS News reported.
Last year, the commission approved a federal safety rule that bans several types of sleep products for babies under 5 months old. Set to take effect next month, the rule requires products marketed for infants to meet the same federal safety standards as required for cribs and similar products.
Parents and advocates have called for a ban on these products for decades, according to CBS, since they can lead to suffocation when an infant’s nose and mouth are covered by a bumper or become stuck between a bumper and crib mattress.
Sudden unexpected infant death, or SUID – which includes sudden infant death syndrome, or SIDS – is the leading cause of injury death in infancy, according to the American Academy of Pediatrics. The group’s recommendations for safe sleep advise that infants should sleep on their back on a firm, flat surface without any extra padding, pillows, blankets, stuffed toys, bumpers, or other soft items in the sleep space.
A version of this article first appeared on WebMD.com.
Low butyrylcholinesterase: A possible biomarker of SIDS risk?
Reduced levels of the cholinergic-system enzyme butyrylcholinesterase (BChE) may provide another piece of the puzzle for sudden infant death syndrome (SIDS), preliminary data from Australian researchers suggested.
A small case-control study led by Carmel T. Harrington, PhD,* a sleep medicine expert and honorary research fellow at the Children’s Hospital at Westmead (Australia), found that measurements in 722 dried blood spots taken during neonatal screening 2 or 3 days after birth were lower in babies who subsequently died of SIDS, compared with those of matched surviving controls and other babies who died of non-SIDS causes.
In groups in which cases were reported as SIDS death (n = 26) there was strong evidence that lower BChE-specific activity was associated with death (odds ratio, 0.73 per U/mg; 95% confidence interval, 0.60-0.89, P = .0014). In groups with a non-SIDS death (n = 41), there was no evidence of a linear association between BChE activity and death (OR, 1.001 per U/mg; 95% CI, 0.89-1.13, P = .99). A cohort of 655 age- and sex-matched controls served as a reference group.
Writing online in eBioMedicine, the researchers concluded that a previously unidentified cholinergic deficit, identifiable by abnormal BChE-specific activity, is present at birth in SIDS babies and represents a measurable, specific vulnerability prior to their death. “The finding presents the possibility of identifying infants at future risk for SIDS and it provides a specific avenue for future research into interventions prior to death.”
They hypothesized that the association is evidence of an altered cholinergic homeostasis and claim theirs is the first study to identify a measurable biochemical marker in babies who succumbed to SIDS. The marker “could plausibly produce functional alterations to an infant’s autonomic and arousal responses to an exogenous stressor leaving them vulnerable to sudden death.”
Commenting in a press release, Dr. Harrington said that “babies have a very powerful mechanism to let us know when they are not happy. Usually, if a baby is confronted with a life-threatening situation, such as difficulty breathing during sleep because they are on their tummies, they will arouse and cry out. What this research shows is that some babies don’t have this same robust arousal response.” Despite the sparse data, she believes that BChE is likely involved.
Providing a U.S. perspective on the study but not involved in it, Fern R. Hauck, MD, MS, a professor of family medicine and public health at the University of Virginia, Charlottesville, said that “the media coverage presenting this as the ‘cause of SIDS,’ for which we may find a cure within 5 years, is very disturbing and very misleading. The data are very preliminary and results are based on only 26 SIDS cases.” In addition, the blood samples were more than 2 years old.
This research needs to be repeated in other labs in larger and diverse SIDS populations, she added. “Furthermore, we are not provided any racial-ethnic information about the SIDS cases in this study. In the U.S., the infants who are at greatest risk of dying from SIDS are most commonly African American and Native American/Alaska Native, and thus, these studies would need to be repeated in U.S. populations.”
Dr. Hauck added that, while the differences in blood levels of this enzyme were statistically different, even if this is confirmed by larger studies, there was enough overlap in the blood levels between cases and controls that it could not be used as a blood test at this point with any reasonable predictive value.
As the authors pointed out, she said, the leading theory of SIDS causation is that multiple factors interact. “While everyone would be happy to find one single explanation, it is not so simple. This research does, however, bring into focus the issues of arousal in SIDS and work on biomarkers. The arousal issue is one researchers have been working on for a long time.”
The SIDS research community has long been interested in biomarkers, Dr. Hauck continued. “Dr. Hannah Kinney’s first autoradiography study reported decreased muscarinic cholinergic receptor binding in the arcuate nucleus in SIDS, which the butyrylcholinesterase work further elaborates. More recently, Dr. Kinney reported abnormal cholinergic binding in the mesopontine reticular formation that is related to arousal and REM.”
Moreover, Robin Haynes and colleagues reported in 2017 that differences in serotonin can similarly be found in newborns on a newborn blood test, she said. “Like the butyrylcholinesterase research, there is a lot of work to do before understanding how specifically it can identify risk. The problem with using it prematurely is that it will unnecessarily alarm parents that their baby will die, and, to make it worse, be inaccurate in our warning.”
She also expressed concern that with the focus on a biomarker, parents will forget that SIDS and other sleep-related infant deaths have come down considerably in the United States thanks to greater emphasis on promoting safe infant sleep behaviors.
The research was supported by a crowdfunding campaign and by NSW Health Pathology. The authors disclosed no conflicts of interest. Dr. Hauck disclosed no conflicts of interest.
* This story was corrected on 5/20/2022.
Reduced levels of the cholinergic-system enzyme butyrylcholinesterase (BChE) may provide another piece of the puzzle for sudden infant death syndrome (SIDS), preliminary data from Australian researchers suggested.
A small case-control study led by Carmel T. Harrington, PhD,* a sleep medicine expert and honorary research fellow at the Children’s Hospital at Westmead (Australia), found that measurements in 722 dried blood spots taken during neonatal screening 2 or 3 days after birth were lower in babies who subsequently died of SIDS, compared with those of matched surviving controls and other babies who died of non-SIDS causes.
In groups in which cases were reported as SIDS death (n = 26) there was strong evidence that lower BChE-specific activity was associated with death (odds ratio, 0.73 per U/mg; 95% confidence interval, 0.60-0.89, P = .0014). In groups with a non-SIDS death (n = 41), there was no evidence of a linear association between BChE activity and death (OR, 1.001 per U/mg; 95% CI, 0.89-1.13, P = .99). A cohort of 655 age- and sex-matched controls served as a reference group.
Writing online in eBioMedicine, the researchers concluded that a previously unidentified cholinergic deficit, identifiable by abnormal BChE-specific activity, is present at birth in SIDS babies and represents a measurable, specific vulnerability prior to their death. “The finding presents the possibility of identifying infants at future risk for SIDS and it provides a specific avenue for future research into interventions prior to death.”
They hypothesized that the association is evidence of an altered cholinergic homeostasis and claim theirs is the first study to identify a measurable biochemical marker in babies who succumbed to SIDS. The marker “could plausibly produce functional alterations to an infant’s autonomic and arousal responses to an exogenous stressor leaving them vulnerable to sudden death.”
Commenting in a press release, Dr. Harrington said that “babies have a very powerful mechanism to let us know when they are not happy. Usually, if a baby is confronted with a life-threatening situation, such as difficulty breathing during sleep because they are on their tummies, they will arouse and cry out. What this research shows is that some babies don’t have this same robust arousal response.” Despite the sparse data, she believes that BChE is likely involved.
Providing a U.S. perspective on the study but not involved in it, Fern R. Hauck, MD, MS, a professor of family medicine and public health at the University of Virginia, Charlottesville, said that “the media coverage presenting this as the ‘cause of SIDS,’ for which we may find a cure within 5 years, is very disturbing and very misleading. The data are very preliminary and results are based on only 26 SIDS cases.” In addition, the blood samples were more than 2 years old.
This research needs to be repeated in other labs in larger and diverse SIDS populations, she added. “Furthermore, we are not provided any racial-ethnic information about the SIDS cases in this study. In the U.S., the infants who are at greatest risk of dying from SIDS are most commonly African American and Native American/Alaska Native, and thus, these studies would need to be repeated in U.S. populations.”
Dr. Hauck added that, while the differences in blood levels of this enzyme were statistically different, even if this is confirmed by larger studies, there was enough overlap in the blood levels between cases and controls that it could not be used as a blood test at this point with any reasonable predictive value.
As the authors pointed out, she said, the leading theory of SIDS causation is that multiple factors interact. “While everyone would be happy to find one single explanation, it is not so simple. This research does, however, bring into focus the issues of arousal in SIDS and work on biomarkers. The arousal issue is one researchers have been working on for a long time.”
The SIDS research community has long been interested in biomarkers, Dr. Hauck continued. “Dr. Hannah Kinney’s first autoradiography study reported decreased muscarinic cholinergic receptor binding in the arcuate nucleus in SIDS, which the butyrylcholinesterase work further elaborates. More recently, Dr. Kinney reported abnormal cholinergic binding in the mesopontine reticular formation that is related to arousal and REM.”
Moreover, Robin Haynes and colleagues reported in 2017 that differences in serotonin can similarly be found in newborns on a newborn blood test, she said. “Like the butyrylcholinesterase research, there is a lot of work to do before understanding how specifically it can identify risk. The problem with using it prematurely is that it will unnecessarily alarm parents that their baby will die, and, to make it worse, be inaccurate in our warning.”
She also expressed concern that with the focus on a biomarker, parents will forget that SIDS and other sleep-related infant deaths have come down considerably in the United States thanks to greater emphasis on promoting safe infant sleep behaviors.
The research was supported by a crowdfunding campaign and by NSW Health Pathology. The authors disclosed no conflicts of interest. Dr. Hauck disclosed no conflicts of interest.
* This story was corrected on 5/20/2022.
Reduced levels of the cholinergic-system enzyme butyrylcholinesterase (BChE) may provide another piece of the puzzle for sudden infant death syndrome (SIDS), preliminary data from Australian researchers suggested.
A small case-control study led by Carmel T. Harrington, PhD,* a sleep medicine expert and honorary research fellow at the Children’s Hospital at Westmead (Australia), found that measurements in 722 dried blood spots taken during neonatal screening 2 or 3 days after birth were lower in babies who subsequently died of SIDS, compared with those of matched surviving controls and other babies who died of non-SIDS causes.
In groups in which cases were reported as SIDS death (n = 26) there was strong evidence that lower BChE-specific activity was associated with death (odds ratio, 0.73 per U/mg; 95% confidence interval, 0.60-0.89, P = .0014). In groups with a non-SIDS death (n = 41), there was no evidence of a linear association between BChE activity and death (OR, 1.001 per U/mg; 95% CI, 0.89-1.13, P = .99). A cohort of 655 age- and sex-matched controls served as a reference group.
Writing online in eBioMedicine, the researchers concluded that a previously unidentified cholinergic deficit, identifiable by abnormal BChE-specific activity, is present at birth in SIDS babies and represents a measurable, specific vulnerability prior to their death. “The finding presents the possibility of identifying infants at future risk for SIDS and it provides a specific avenue for future research into interventions prior to death.”
They hypothesized that the association is evidence of an altered cholinergic homeostasis and claim theirs is the first study to identify a measurable biochemical marker in babies who succumbed to SIDS. The marker “could plausibly produce functional alterations to an infant’s autonomic and arousal responses to an exogenous stressor leaving them vulnerable to sudden death.”
Commenting in a press release, Dr. Harrington said that “babies have a very powerful mechanism to let us know when they are not happy. Usually, if a baby is confronted with a life-threatening situation, such as difficulty breathing during sleep because they are on their tummies, they will arouse and cry out. What this research shows is that some babies don’t have this same robust arousal response.” Despite the sparse data, she believes that BChE is likely involved.
Providing a U.S. perspective on the study but not involved in it, Fern R. Hauck, MD, MS, a professor of family medicine and public health at the University of Virginia, Charlottesville, said that “the media coverage presenting this as the ‘cause of SIDS,’ for which we may find a cure within 5 years, is very disturbing and very misleading. The data are very preliminary and results are based on only 26 SIDS cases.” In addition, the blood samples were more than 2 years old.
This research needs to be repeated in other labs in larger and diverse SIDS populations, she added. “Furthermore, we are not provided any racial-ethnic information about the SIDS cases in this study. In the U.S., the infants who are at greatest risk of dying from SIDS are most commonly African American and Native American/Alaska Native, and thus, these studies would need to be repeated in U.S. populations.”
Dr. Hauck added that, while the differences in blood levels of this enzyme were statistically different, even if this is confirmed by larger studies, there was enough overlap in the blood levels between cases and controls that it could not be used as a blood test at this point with any reasonable predictive value.
As the authors pointed out, she said, the leading theory of SIDS causation is that multiple factors interact. “While everyone would be happy to find one single explanation, it is not so simple. This research does, however, bring into focus the issues of arousal in SIDS and work on biomarkers. The arousal issue is one researchers have been working on for a long time.”
The SIDS research community has long been interested in biomarkers, Dr. Hauck continued. “Dr. Hannah Kinney’s first autoradiography study reported decreased muscarinic cholinergic receptor binding in the arcuate nucleus in SIDS, which the butyrylcholinesterase work further elaborates. More recently, Dr. Kinney reported abnormal cholinergic binding in the mesopontine reticular formation that is related to arousal and REM.”
Moreover, Robin Haynes and colleagues reported in 2017 that differences in serotonin can similarly be found in newborns on a newborn blood test, she said. “Like the butyrylcholinesterase research, there is a lot of work to do before understanding how specifically it can identify risk. The problem with using it prematurely is that it will unnecessarily alarm parents that their baby will die, and, to make it worse, be inaccurate in our warning.”
She also expressed concern that with the focus on a biomarker, parents will forget that SIDS and other sleep-related infant deaths have come down considerably in the United States thanks to greater emphasis on promoting safe infant sleep behaviors.
The research was supported by a crowdfunding campaign and by NSW Health Pathology. The authors disclosed no conflicts of interest. Dr. Hauck disclosed no conflicts of interest.
* This story was corrected on 5/20/2022.
FROM EBIOMEDICINE
The quest for a good night’s sleep: An update on pharmacologic therapy for insomnia
Insomnia is one of the most common complaints in medicine, driving millions of clinic visits each year (Table 1). It is estimated that approximately 30% of individuals report at least short-term insomnia symptoms and 10% report chronic insomnia. These rates are even higher in groups that may be more susceptible to insomnia, including women, the elderly, and those of disadvantaged socioeconomic status (Ohayon MM. Sleep Med Rev. 2002;[2]:97-111). While most patients with insomnia find their sleep difficulties self-resolve within 3 months, a substantial number of patients will find their insomnia to persist for longer and require intervention (Sateia MJ et al. J Clin Sleep Med. 2017;13[2]:307-49).
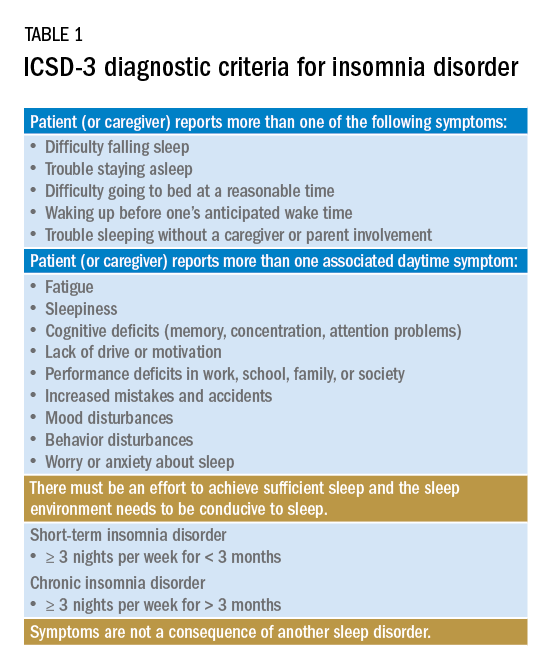
For individuals requiring treatment, cognitive behavioral therapy for insomnia (CBT-I) is considered first-line therapy by the American Academy of Sleep Medicine for both acute and chronic insomnia. Unfortunately, obtaining CBT-I for a patient is often a challenge as the number of trained therapists offering this service is limited, resulting in long wait times or, in some cases, a complete lack of access to this treatment option. Judicious use of sedative-hypnotic medications may be a reasonable alternative for patients with insomnia who are unable to undergo CBT-I, who are still symptomatic despite undergoing CBT-I, or, in some cases, as a temporary treatment (Sateia MJ et al. J Clin Sleep Med. 2017;13[2]:307-49).
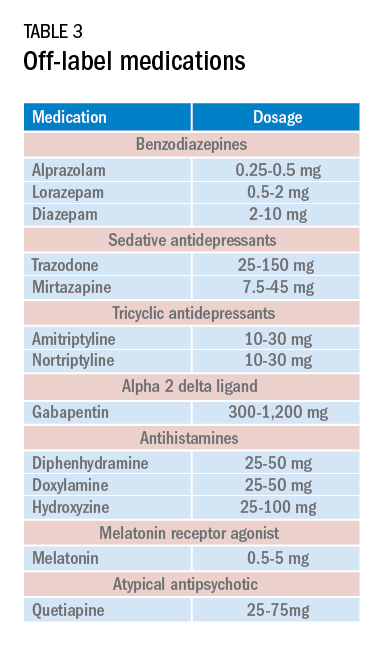
Current medications used to treat insomnia are listed in Tables 2 and 3, some of which carry an FDA approval to be used as a hypnotic, while others are used in an off-label manner.
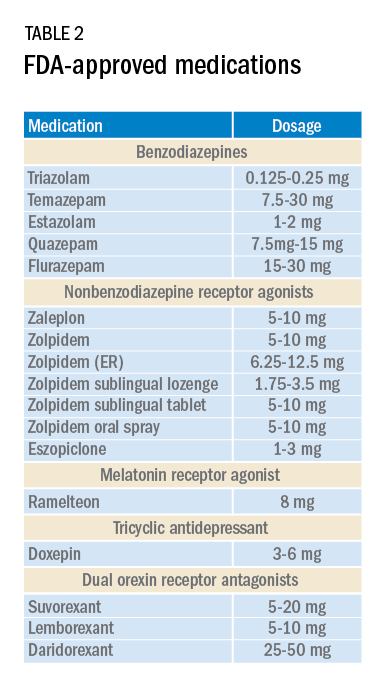
Cautions abound with use of many of these medications. Common concerns include safety, particularly for elderly patients and long-term use, and the potential for developing tolerance and dependence.
Most medications that have been used for insomnia have been available for decades, but, in recent years, a new class of hypnotics has emerged. Dual orexin receptor antagonists (DORAs) are the newest class of FDA-approved medications (Table 4).
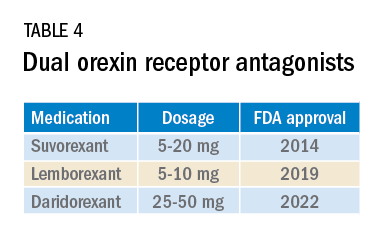
Orexin is a neuropeptide found primarily in the lateral hypothalamus and binds to the orexin 1 and orexin 2 receptors leading to a number of downstream effects, including stimulating wakefulness. Loss of orexin-generating neurons has been implicated as the cause of type 1 narcolepsy, and antagonism of their effects can facilitate sleep by suppressing wakefulness. The first medication in the DORA class to be FDA-approved was suvorexant in 2014, followed by lemborexant’s FDA approval in 2019. These are both indicated for treating sleep onset and sleep maintenance insomnia and have been shown to improve both subjective and objective measures of sleep. The most common side effects reported for both suvorexant and lemborexant are headache and somnolence, with morning-after sleepiness being a frequent complaint.
In January 2022, a new medication in the DORA class named daridorexant was approved by the FDA (Table 5).
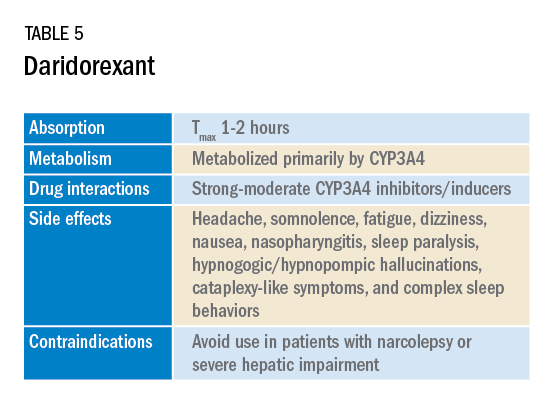
Daridorexant, like its DORA counterparts, has been shown to have efficacy in improving subjective and objective markers of insomnia. This has included polysomnographic measures of wake after sleep onset and latency to persistent sleep, as well as subjective total sleep time. Importantly, in addition to positive sleep outcomes, improvements with daytime function have also been observed with this medication (Mignot E et al. Lancet Neurol. 2022;21[2]:125-39). Daridorexant’s half-life of approximately 8 hours is shorter than that of the other available DORAs, leading to fewer day-after effects. The combination of effectiveness for sleep initiation and maintenance without daytime impairment distinguishes daridorexant from the other DORAs and even other classes of sleep medication.
Safety, especially in patients of age 65 and older, is an important concern with sleep medication, particularly with respect to polypharmacy, over-sedation, increased fall risk, and cognitive impairment, but daridorexant’s available safety data suggest a favorable safety profile (Zammit G et al. Neurology. 2020;94[21]:e2222-32).
Daridorexant at the highest dose available, 50 mg, did not worsen respiratory function, in terms of the apnea-hypopnea index and oxygen saturation in individuals with mild-moderate obstructive sleep apnea regardless of sleep stage (Boof ML et al. Sleep. 2021;44[6]:zsaa275). However, more safety and longitudinal data are needed to have a fuller understanding of any potential limitations of this medication.
While we continue to recommend CBT-I as the first-line treatment whenever possible for patients with insomnia, not all patients have access to this treatment and not all patients will respond satisfactorily to it. Thus, pharmacologic treatment can continue to play an important role in the management of some patients’ insomnia. Each class of medications used for treating insomnia features a unique constellation of advantages and limitations, meaning that the more available options, the greater the chances of finding an option that will be both effective and safe for a particular patient. The growing DORA class, especially its newest available entrant, daridorexant, represents a continued expansion of the armamentarium of options against insomnia.
Dr. Pelekanos and Dr. Sum-Ping are with the Division of Sleep Medicine, Department of Psychiatry & Behavioral Sciences, Stanford University, Stanford, California.
Insomnia is one of the most common complaints in medicine, driving millions of clinic visits each year (Table 1). It is estimated that approximately 30% of individuals report at least short-term insomnia symptoms and 10% report chronic insomnia. These rates are even higher in groups that may be more susceptible to insomnia, including women, the elderly, and those of disadvantaged socioeconomic status (Ohayon MM. Sleep Med Rev. 2002;[2]:97-111). While most patients with insomnia find their sleep difficulties self-resolve within 3 months, a substantial number of patients will find their insomnia to persist for longer and require intervention (Sateia MJ et al. J Clin Sleep Med. 2017;13[2]:307-49).

For individuals requiring treatment, cognitive behavioral therapy for insomnia (CBT-I) is considered first-line therapy by the American Academy of Sleep Medicine for both acute and chronic insomnia. Unfortunately, obtaining CBT-I for a patient is often a challenge as the number of trained therapists offering this service is limited, resulting in long wait times or, in some cases, a complete lack of access to this treatment option. Judicious use of sedative-hypnotic medications may be a reasonable alternative for patients with insomnia who are unable to undergo CBT-I, who are still symptomatic despite undergoing CBT-I, or, in some cases, as a temporary treatment (Sateia MJ et al. J Clin Sleep Med. 2017;13[2]:307-49).
Current medications used to treat insomnia are listed in Tables 2 and 3, some of which carry an FDA approval to be used as a hypnotic, while others are used in an off-label manner.

Cautions abound with use of many of these medications. Common concerns include safety, particularly for elderly patients and long-term use, and the potential for developing tolerance and dependence.

Most medications that have been used for insomnia have been available for decades, but, in recent years, a new class of hypnotics has emerged. Dual orexin receptor antagonists (DORAs) are the newest class of FDA-approved medications (Table 4).

Orexin is a neuropeptide found primarily in the lateral hypothalamus and binds to the orexin 1 and orexin 2 receptors leading to a number of downstream effects, including stimulating wakefulness. Loss of orexin-generating neurons has been implicated as the cause of type 1 narcolepsy, and antagonism of their effects can facilitate sleep by suppressing wakefulness. The first medication in the DORA class to be FDA-approved was suvorexant in 2014, followed by lemborexant’s FDA approval in 2019. These are both indicated for treating sleep onset and sleep maintenance insomnia and have been shown to improve both subjective and objective measures of sleep. The most common side effects reported for both suvorexant and lemborexant are headache and somnolence, with morning-after sleepiness being a frequent complaint.
In January 2022, a new medication in the DORA class named daridorexant was approved by the FDA (Table 5).

Daridorexant, like its DORA counterparts, has been shown to have efficacy in improving subjective and objective markers of insomnia. This has included polysomnographic measures of wake after sleep onset and latency to persistent sleep, as well as subjective total sleep time. Importantly, in addition to positive sleep outcomes, improvements with daytime function have also been observed with this medication (Mignot E et al. Lancet Neurol. 2022;21[2]:125-39). Daridorexant’s half-life of approximately 8 hours is shorter than that of the other available DORAs, leading to fewer day-after effects. The combination of effectiveness for sleep initiation and maintenance without daytime impairment distinguishes daridorexant from the other DORAs and even other classes of sleep medication.
Safety, especially in patients of age 65 and older, is an important concern with sleep medication, particularly with respect to polypharmacy, over-sedation, increased fall risk, and cognitive impairment, but daridorexant’s available safety data suggest a favorable safety profile (Zammit G et al. Neurology. 2020;94[21]:e2222-32).
Daridorexant at the highest dose available, 50 mg, did not worsen respiratory function, in terms of the apnea-hypopnea index and oxygen saturation in individuals with mild-moderate obstructive sleep apnea regardless of sleep stage (Boof ML et al. Sleep. 2021;44[6]:zsaa275). However, more safety and longitudinal data are needed to have a fuller understanding of any potential limitations of this medication.
While we continue to recommend CBT-I as the first-line treatment whenever possible for patients with insomnia, not all patients have access to this treatment and not all patients will respond satisfactorily to it. Thus, pharmacologic treatment can continue to play an important role in the management of some patients’ insomnia. Each class of medications used for treating insomnia features a unique constellation of advantages and limitations, meaning that the more available options, the greater the chances of finding an option that will be both effective and safe for a particular patient. The growing DORA class, especially its newest available entrant, daridorexant, represents a continued expansion of the armamentarium of options against insomnia.
Dr. Pelekanos and Dr. Sum-Ping are with the Division of Sleep Medicine, Department of Psychiatry & Behavioral Sciences, Stanford University, Stanford, California.
Insomnia is one of the most common complaints in medicine, driving millions of clinic visits each year (Table 1). It is estimated that approximately 30% of individuals report at least short-term insomnia symptoms and 10% report chronic insomnia. These rates are even higher in groups that may be more susceptible to insomnia, including women, the elderly, and those of disadvantaged socioeconomic status (Ohayon MM. Sleep Med Rev. 2002;[2]:97-111). While most patients with insomnia find their sleep difficulties self-resolve within 3 months, a substantial number of patients will find their insomnia to persist for longer and require intervention (Sateia MJ et al. J Clin Sleep Med. 2017;13[2]:307-49).

For individuals requiring treatment, cognitive behavioral therapy for insomnia (CBT-I) is considered first-line therapy by the American Academy of Sleep Medicine for both acute and chronic insomnia. Unfortunately, obtaining CBT-I for a patient is often a challenge as the number of trained therapists offering this service is limited, resulting in long wait times or, in some cases, a complete lack of access to this treatment option. Judicious use of sedative-hypnotic medications may be a reasonable alternative for patients with insomnia who are unable to undergo CBT-I, who are still symptomatic despite undergoing CBT-I, or, in some cases, as a temporary treatment (Sateia MJ et al. J Clin Sleep Med. 2017;13[2]:307-49).
Current medications used to treat insomnia are listed in Tables 2 and 3, some of which carry an FDA approval to be used as a hypnotic, while others are used in an off-label manner.

Cautions abound with use of many of these medications. Common concerns include safety, particularly for elderly patients and long-term use, and the potential for developing tolerance and dependence.

Most medications that have been used for insomnia have been available for decades, but, in recent years, a new class of hypnotics has emerged. Dual orexin receptor antagonists (DORAs) are the newest class of FDA-approved medications (Table 4).

Orexin is a neuropeptide found primarily in the lateral hypothalamus and binds to the orexin 1 and orexin 2 receptors leading to a number of downstream effects, including stimulating wakefulness. Loss of orexin-generating neurons has been implicated as the cause of type 1 narcolepsy, and antagonism of their effects can facilitate sleep by suppressing wakefulness. The first medication in the DORA class to be FDA-approved was suvorexant in 2014, followed by lemborexant’s FDA approval in 2019. These are both indicated for treating sleep onset and sleep maintenance insomnia and have been shown to improve both subjective and objective measures of sleep. The most common side effects reported for both suvorexant and lemborexant are headache and somnolence, with morning-after sleepiness being a frequent complaint.
In January 2022, a new medication in the DORA class named daridorexant was approved by the FDA (Table 5).

Daridorexant, like its DORA counterparts, has been shown to have efficacy in improving subjective and objective markers of insomnia. This has included polysomnographic measures of wake after sleep onset and latency to persistent sleep, as well as subjective total sleep time. Importantly, in addition to positive sleep outcomes, improvements with daytime function have also been observed with this medication (Mignot E et al. Lancet Neurol. 2022;21[2]:125-39). Daridorexant’s half-life of approximately 8 hours is shorter than that of the other available DORAs, leading to fewer day-after effects. The combination of effectiveness for sleep initiation and maintenance without daytime impairment distinguishes daridorexant from the other DORAs and even other classes of sleep medication.
Safety, especially in patients of age 65 and older, is an important concern with sleep medication, particularly with respect to polypharmacy, over-sedation, increased fall risk, and cognitive impairment, but daridorexant’s available safety data suggest a favorable safety profile (Zammit G et al. Neurology. 2020;94[21]:e2222-32).
Daridorexant at the highest dose available, 50 mg, did not worsen respiratory function, in terms of the apnea-hypopnea index and oxygen saturation in individuals with mild-moderate obstructive sleep apnea regardless of sleep stage (Boof ML et al. Sleep. 2021;44[6]:zsaa275). However, more safety and longitudinal data are needed to have a fuller understanding of any potential limitations of this medication.
While we continue to recommend CBT-I as the first-line treatment whenever possible for patients with insomnia, not all patients have access to this treatment and not all patients will respond satisfactorily to it. Thus, pharmacologic treatment can continue to play an important role in the management of some patients’ insomnia. Each class of medications used for treating insomnia features a unique constellation of advantages and limitations, meaning that the more available options, the greater the chances of finding an option that will be both effective and safe for a particular patient. The growing DORA class, especially its newest available entrant, daridorexant, represents a continued expansion of the armamentarium of options against insomnia.
Dr. Pelekanos and Dr. Sum-Ping are with the Division of Sleep Medicine, Department of Psychiatry & Behavioral Sciences, Stanford University, Stanford, California.
Seven hours of sleep is ideal for middle aged and older
Sleep disturbances are common in older age, and previous studies have shown associations between too much or too little sleep and increased risk of cognitive decline, but the ideal amount of sleep for preserving mental health has not been well described, according to the authors of the new paper.
In the study published in Nature Aging, the team of researchers from China and the United Kingdom reviewed data from the UK Biobank, a national database of individuals in the United Kingdom that includes cognitive assessments, mental health questionnaires, and brain imaging data, as well as genetic information.
Sleep is important for physical and psychological health, and also serves a neuroprotective function by clearing waste products from the brain, lead author Yuzhu Li of Fudan University, Shanghai, China, and colleagues wrote.
The study population included 498,277 participants, aged 38-73 years, who completed touchscreen questionnaires about sleep duration between 2006 and 2010. The average age at baseline was 56.5 years, 54% were female, and the mean sleep duration was 7.15 hours.
The researchers also reviewed brain imaging data and genetic data from 39,692 participants in 2014 to examine the relationships between sleep duration and brain structure and between sleep duration and genetic risk. In addition, 156,884 participants completed an online follow-up mental health questionnaire in 2016-2017 to assess the longitudinal impact of sleep on mental health.
Both excessive and insufficient sleep was associated with impaired cognitive performance, evidenced by the U-shaped curve found by the researchers in their data analysis, which used quadratic associations.
Specific cognitive functions including pair matching, trail making, prospective memory, and reaction time were significantly impaired with too much or too little sleep, the researchers said. “This demonstrated the positive association of both insufficient and excessive sleep duration with inferior performance on cognitive tasks.”
When the researchers analyzed the association between sleep duration and mental health, sleep duration also showed a U-shaped association with symptoms of anxiety, depression, mental distress, mania, and self-harm, while well-being showed an inverted U-shape. All associations between sleep duration and mental health were statistically significant after controlling for confounding variables (P < .001).
On further analysis (using two-line tests), the researchers determined that consistent sleep duration of approximately 7 hours per night was optimal for cognitive performance and for good mental health.
The researchers also used neuroimaging data to examine the relationship between sleep duration and brain structure. Overall, greater changes were seen in the regions of the brain involved in cognitive processing and memory.
“The most significant cortical volumes nonlinearly associated with sleep duration included the precentral cortex, the superior frontal gyrus, the lateral orbitofrontal cortex, the pars orbitalis, the frontal pole, and the middle temporal cortex,” the researchers wrote (P < .05 for all).
The association between sleep duration and cognitive function diminished among individuals older than 65 years, compared with those aged approximately 40 years, which suggests that optimal sleep duration may be more beneficial in middle age, the researchers noted. However, no similar impact of age was seen for mental health. For brain structure, the nonlinear relationship between sleep duration and cortical volumes was greatest in those aged 44-59 years, and gradually flattened with older age.
Research supports sleep discussions with patients
“Primary care physicians can use this study in their discussions with middle-aged and older patients to recommend optimal sleep duration and measures to achieve this sleep target,” Noel Deep, MD, a general internist in group practice in Antigo, Wisc., who was not involved in the study, said in an interview.
“This study is important because it demonstrated that both inadequate and excessive sleep patterns were associated with cognitive and mental health changes,” said Dr. Deep. “It supported previous observations of cognitive decline and mental health disorders being linked to disturbed sleep. But this study was unique because it provides data supporting an optimal sleep duration of 7 hours and the ill effects of both insufficient and excessive sleep duration.
“The usual thought process has been to assume that older individuals may not require as much sleep as the younger individuals, but this study supports an optimal time duration of sleep of 7 hours that benefits the older individuals. It was also interesting to note the mental health effects caused by the inadequate and excessive sleep durations,” he added.
As for additional research, “I would like to look into the quality of the sleep, in addition to the duration of sleep,” said Dr. Deep. For example, whether the excessive sleep was caused by poor quality sleep or fragmented sleep leading to the structural and subsequent cognitive decline.
Study limitations
“The current study relied on self-reporting of the sleep duration and was not observed and recorded data,” Dr. Deep noted. “It would also be beneficial to not only rely on healthy volunteers reporting the sleep duration, but also obtain sleep data from individuals with known brain disorders.”
The study findings were limited by several other factors, including the use of total sleep duration only, without other measures of sleep hygiene, the researchers noted. More research is needed to investigate the mechanisms driving the association between too much and not enough sleep and poor mental health and cognitive function.
The study was supported by the National Key R&D Program of China, the Shanghai Municipal Science and Technology Major Project, the Shanghai Center for Brain Science and Brain-Inspired Technology, the 111 Project, the National Natural Sciences Foundation of China and the Shanghai Rising Star Program.
The researchers had no financial conflicts to disclose. Dr. Deep had no financial conflicts to disclose, but serves on the editorial advisory board of Internal Medicine News.
Sleep disturbances are common in older age, and previous studies have shown associations between too much or too little sleep and increased risk of cognitive decline, but the ideal amount of sleep for preserving mental health has not been well described, according to the authors of the new paper.
In the study published in Nature Aging, the team of researchers from China and the United Kingdom reviewed data from the UK Biobank, a national database of individuals in the United Kingdom that includes cognitive assessments, mental health questionnaires, and brain imaging data, as well as genetic information.
Sleep is important for physical and psychological health, and also serves a neuroprotective function by clearing waste products from the brain, lead author Yuzhu Li of Fudan University, Shanghai, China, and colleagues wrote.
The study population included 498,277 participants, aged 38-73 years, who completed touchscreen questionnaires about sleep duration between 2006 and 2010. The average age at baseline was 56.5 years, 54% were female, and the mean sleep duration was 7.15 hours.
The researchers also reviewed brain imaging data and genetic data from 39,692 participants in 2014 to examine the relationships between sleep duration and brain structure and between sleep duration and genetic risk. In addition, 156,884 participants completed an online follow-up mental health questionnaire in 2016-2017 to assess the longitudinal impact of sleep on mental health.
Both excessive and insufficient sleep was associated with impaired cognitive performance, evidenced by the U-shaped curve found by the researchers in their data analysis, which used quadratic associations.
Specific cognitive functions including pair matching, trail making, prospective memory, and reaction time were significantly impaired with too much or too little sleep, the researchers said. “This demonstrated the positive association of both insufficient and excessive sleep duration with inferior performance on cognitive tasks.”
When the researchers analyzed the association between sleep duration and mental health, sleep duration also showed a U-shaped association with symptoms of anxiety, depression, mental distress, mania, and self-harm, while well-being showed an inverted U-shape. All associations between sleep duration and mental health were statistically significant after controlling for confounding variables (P < .001).
On further analysis (using two-line tests), the researchers determined that consistent sleep duration of approximately 7 hours per night was optimal for cognitive performance and for good mental health.
The researchers also used neuroimaging data to examine the relationship between sleep duration and brain structure. Overall, greater changes were seen in the regions of the brain involved in cognitive processing and memory.
“The most significant cortical volumes nonlinearly associated with sleep duration included the precentral cortex, the superior frontal gyrus, the lateral orbitofrontal cortex, the pars orbitalis, the frontal pole, and the middle temporal cortex,” the researchers wrote (P < .05 for all).
The association between sleep duration and cognitive function diminished among individuals older than 65 years, compared with those aged approximately 40 years, which suggests that optimal sleep duration may be more beneficial in middle age, the researchers noted. However, no similar impact of age was seen for mental health. For brain structure, the nonlinear relationship between sleep duration and cortical volumes was greatest in those aged 44-59 years, and gradually flattened with older age.
Research supports sleep discussions with patients
“Primary care physicians can use this study in their discussions with middle-aged and older patients to recommend optimal sleep duration and measures to achieve this sleep target,” Noel Deep, MD, a general internist in group practice in Antigo, Wisc., who was not involved in the study, said in an interview.
“This study is important because it demonstrated that both inadequate and excessive sleep patterns were associated with cognitive and mental health changes,” said Dr. Deep. “It supported previous observations of cognitive decline and mental health disorders being linked to disturbed sleep. But this study was unique because it provides data supporting an optimal sleep duration of 7 hours and the ill effects of both insufficient and excessive sleep duration.
“The usual thought process has been to assume that older individuals may not require as much sleep as the younger individuals, but this study supports an optimal time duration of sleep of 7 hours that benefits the older individuals. It was also interesting to note the mental health effects caused by the inadequate and excessive sleep durations,” he added.
As for additional research, “I would like to look into the quality of the sleep, in addition to the duration of sleep,” said Dr. Deep. For example, whether the excessive sleep was caused by poor quality sleep or fragmented sleep leading to the structural and subsequent cognitive decline.
Study limitations
“The current study relied on self-reporting of the sleep duration and was not observed and recorded data,” Dr. Deep noted. “It would also be beneficial to not only rely on healthy volunteers reporting the sleep duration, but also obtain sleep data from individuals with known brain disorders.”
The study findings were limited by several other factors, including the use of total sleep duration only, without other measures of sleep hygiene, the researchers noted. More research is needed to investigate the mechanisms driving the association between too much and not enough sleep and poor mental health and cognitive function.
The study was supported by the National Key R&D Program of China, the Shanghai Municipal Science and Technology Major Project, the Shanghai Center for Brain Science and Brain-Inspired Technology, the 111 Project, the National Natural Sciences Foundation of China and the Shanghai Rising Star Program.
The researchers had no financial conflicts to disclose. Dr. Deep had no financial conflicts to disclose, but serves on the editorial advisory board of Internal Medicine News.
Sleep disturbances are common in older age, and previous studies have shown associations between too much or too little sleep and increased risk of cognitive decline, but the ideal amount of sleep for preserving mental health has not been well described, according to the authors of the new paper.
In the study published in Nature Aging, the team of researchers from China and the United Kingdom reviewed data from the UK Biobank, a national database of individuals in the United Kingdom that includes cognitive assessments, mental health questionnaires, and brain imaging data, as well as genetic information.
Sleep is important for physical and psychological health, and also serves a neuroprotective function by clearing waste products from the brain, lead author Yuzhu Li of Fudan University, Shanghai, China, and colleagues wrote.
The study population included 498,277 participants, aged 38-73 years, who completed touchscreen questionnaires about sleep duration between 2006 and 2010. The average age at baseline was 56.5 years, 54% were female, and the mean sleep duration was 7.15 hours.
The researchers also reviewed brain imaging data and genetic data from 39,692 participants in 2014 to examine the relationships between sleep duration and brain structure and between sleep duration and genetic risk. In addition, 156,884 participants completed an online follow-up mental health questionnaire in 2016-2017 to assess the longitudinal impact of sleep on mental health.
Both excessive and insufficient sleep was associated with impaired cognitive performance, evidenced by the U-shaped curve found by the researchers in their data analysis, which used quadratic associations.
Specific cognitive functions including pair matching, trail making, prospective memory, and reaction time were significantly impaired with too much or too little sleep, the researchers said. “This demonstrated the positive association of both insufficient and excessive sleep duration with inferior performance on cognitive tasks.”
When the researchers analyzed the association between sleep duration and mental health, sleep duration also showed a U-shaped association with symptoms of anxiety, depression, mental distress, mania, and self-harm, while well-being showed an inverted U-shape. All associations between sleep duration and mental health were statistically significant after controlling for confounding variables (P < .001).
On further analysis (using two-line tests), the researchers determined that consistent sleep duration of approximately 7 hours per night was optimal for cognitive performance and for good mental health.
The researchers also used neuroimaging data to examine the relationship between sleep duration and brain structure. Overall, greater changes were seen in the regions of the brain involved in cognitive processing and memory.
“The most significant cortical volumes nonlinearly associated with sleep duration included the precentral cortex, the superior frontal gyrus, the lateral orbitofrontal cortex, the pars orbitalis, the frontal pole, and the middle temporal cortex,” the researchers wrote (P < .05 for all).
The association between sleep duration and cognitive function diminished among individuals older than 65 years, compared with those aged approximately 40 years, which suggests that optimal sleep duration may be more beneficial in middle age, the researchers noted. However, no similar impact of age was seen for mental health. For brain structure, the nonlinear relationship between sleep duration and cortical volumes was greatest in those aged 44-59 years, and gradually flattened with older age.
Research supports sleep discussions with patients
“Primary care physicians can use this study in their discussions with middle-aged and older patients to recommend optimal sleep duration and measures to achieve this sleep target,” Noel Deep, MD, a general internist in group practice in Antigo, Wisc., who was not involved in the study, said in an interview.
“This study is important because it demonstrated that both inadequate and excessive sleep patterns were associated with cognitive and mental health changes,” said Dr. Deep. “It supported previous observations of cognitive decline and mental health disorders being linked to disturbed sleep. But this study was unique because it provides data supporting an optimal sleep duration of 7 hours and the ill effects of both insufficient and excessive sleep duration.
“The usual thought process has been to assume that older individuals may not require as much sleep as the younger individuals, but this study supports an optimal time duration of sleep of 7 hours that benefits the older individuals. It was also interesting to note the mental health effects caused by the inadequate and excessive sleep durations,” he added.
As for additional research, “I would like to look into the quality of the sleep, in addition to the duration of sleep,” said Dr. Deep. For example, whether the excessive sleep was caused by poor quality sleep or fragmented sleep leading to the structural and subsequent cognitive decline.
Study limitations
“The current study relied on self-reporting of the sleep duration and was not observed and recorded data,” Dr. Deep noted. “It would also be beneficial to not only rely on healthy volunteers reporting the sleep duration, but also obtain sleep data from individuals with known brain disorders.”
The study findings were limited by several other factors, including the use of total sleep duration only, without other measures of sleep hygiene, the researchers noted. More research is needed to investigate the mechanisms driving the association between too much and not enough sleep and poor mental health and cognitive function.
The study was supported by the National Key R&D Program of China, the Shanghai Municipal Science and Technology Major Project, the Shanghai Center for Brain Science and Brain-Inspired Technology, the 111 Project, the National Natural Sciences Foundation of China and the Shanghai Rising Star Program.
The researchers had no financial conflicts to disclose. Dr. Deep had no financial conflicts to disclose, but serves on the editorial advisory board of Internal Medicine News.
FROM NATURE AGING
Severe COVID-19 adds 20 years of cognitive aging: Study
adding that the impairment is “equivalent to losing 10 IQ points.”
In their study, published in eClinicalMedicine, a team of scientists from the University of Cambridge and Imperial College London said there is growing evidence that COVID-19 can cause lasting cognitive and mental health problems. Patients report fatigue, “brain fog,” problems recalling words, sleep disturbances, anxiety, and even posttraumatic stress disorder months after infection.
The researchers analyzed data from 46 individuals who received critical care for COVID-19 at Addenbrooke’s Hospital between March and July 2020 (27 females, 19 males, mean age 51 years, 16 of whom had mechanical ventilation) and were recruited to the NIHR COVID-19 BioResource project.
At an average of 6 months after acute COVID-19 illness, the study participants underwent detailed computerized cognitive tests via the Cognitron platform, comprising eight tasks deployed on an iPad measuring mental function such as memory, attention, and reasoning. Also assessed were anxiety, depression, and posttraumatic stress disorder via standard mood, anxiety, and posttraumatic stress scales – specifically the Generalized Anxiety Disorder 7 (GAD-7), the Patient Health Questionnaire 9 (PHQ-9), and the PTSD Checklist for Diagnostic and Statistical Manual of Mental Disorders 5 (PCL-5). Their data were compared against 460 controls – matched for age, sex, education, and first language – and the pattern of deficits across tasks was qualitatively compared with normal age-related decline and early-stage dementia.
Less accurate and slower response times
The authors highlighted how this was the first time a “rigorous assessment and comparison” had been carried out in relation to the after-effects of severe COVID-19.
“Cognitive impairment is common to a wide range of neurological disorders, including dementia, and even routine aging, but the patterns we saw – the cognitive ‘fingerprint’ of COVID-19 – was distinct from all of these,” said David Menon, MD, division of anesthesia at the University of Cambridge, England, and the study’s senior author.
The scientists found that COVID-19 survivors were less accurate and had slower response times than the control population, and added that survivors scored particularly poorly on verbal analogical reasoning and showed slower processing speeds.
Critically, the scale of the cognitive deficits correlated with acute illness severity, but not fatigue or mental health status at the time of cognitive assessment, said the authors.
Recovery ‘at best gradual’
The effects were strongest for those with more severe acute illness, and who required mechanical ventilation, said the authors, who found that acute illness severity was “better at predicting the cognitive deficits.”
The authors pointed out how these deficits were still detectable when patients were followed up 6 months later, and that, although patients’ scores and reaction times began to improve over time, any recovery was “at best gradual” and likely to be influenced by factors such as illness severity and its neurological or psychological impacts.
“We followed some patients up as late as 10 months after their acute infection, so were able to see a very slow improvement,” Dr. Menon said. He explained how, while this improvement was not statistically significant, it was “at least heading in the right direction.”
However, he warned it is very possible that some of these individuals “will never fully recover.”
The cognitive deficits observed may be due to several factors in combination, said the authors, including inadequate oxygen or blood supply to the brain, blockage of large or small blood vessels due to clotting, and microscopic bleeds. They highlighted how the most important mechanism, however, may be “damage caused by the body’s own inflammatory response and immune system.”
Adam Hampshire, PhD, of the department of brain sciences at Imperial College London, one of the study’s authors, described how around 40,000 people have been through intensive care with COVID-19 in England alone, with many more despite having been very sick not admitted to hospital. This means there is a “large number of people out there still experiencing problems with cognition many months later,” he said. “We urgently need to look at what can be done to help these people.”
A version of this article first appeared on Univadis.
adding that the impairment is “equivalent to losing 10 IQ points.”
In their study, published in eClinicalMedicine, a team of scientists from the University of Cambridge and Imperial College London said there is growing evidence that COVID-19 can cause lasting cognitive and mental health problems. Patients report fatigue, “brain fog,” problems recalling words, sleep disturbances, anxiety, and even posttraumatic stress disorder months after infection.
The researchers analyzed data from 46 individuals who received critical care for COVID-19 at Addenbrooke’s Hospital between March and July 2020 (27 females, 19 males, mean age 51 years, 16 of whom had mechanical ventilation) and were recruited to the NIHR COVID-19 BioResource project.
At an average of 6 months after acute COVID-19 illness, the study participants underwent detailed computerized cognitive tests via the Cognitron platform, comprising eight tasks deployed on an iPad measuring mental function such as memory, attention, and reasoning. Also assessed were anxiety, depression, and posttraumatic stress disorder via standard mood, anxiety, and posttraumatic stress scales – specifically the Generalized Anxiety Disorder 7 (GAD-7), the Patient Health Questionnaire 9 (PHQ-9), and the PTSD Checklist for Diagnostic and Statistical Manual of Mental Disorders 5 (PCL-5). Their data were compared against 460 controls – matched for age, sex, education, and first language – and the pattern of deficits across tasks was qualitatively compared with normal age-related decline and early-stage dementia.
Less accurate and slower response times
The authors highlighted how this was the first time a “rigorous assessment and comparison” had been carried out in relation to the after-effects of severe COVID-19.
“Cognitive impairment is common to a wide range of neurological disorders, including dementia, and even routine aging, but the patterns we saw – the cognitive ‘fingerprint’ of COVID-19 – was distinct from all of these,” said David Menon, MD, division of anesthesia at the University of Cambridge, England, and the study’s senior author.
The scientists found that COVID-19 survivors were less accurate and had slower response times than the control population, and added that survivors scored particularly poorly on verbal analogical reasoning and showed slower processing speeds.
Critically, the scale of the cognitive deficits correlated with acute illness severity, but not fatigue or mental health status at the time of cognitive assessment, said the authors.
Recovery ‘at best gradual’
The effects were strongest for those with more severe acute illness, and who required mechanical ventilation, said the authors, who found that acute illness severity was “better at predicting the cognitive deficits.”
The authors pointed out how these deficits were still detectable when patients were followed up 6 months later, and that, although patients’ scores and reaction times began to improve over time, any recovery was “at best gradual” and likely to be influenced by factors such as illness severity and its neurological or psychological impacts.
“We followed some patients up as late as 10 months after their acute infection, so were able to see a very slow improvement,” Dr. Menon said. He explained how, while this improvement was not statistically significant, it was “at least heading in the right direction.”
However, he warned it is very possible that some of these individuals “will never fully recover.”
The cognitive deficits observed may be due to several factors in combination, said the authors, including inadequate oxygen or blood supply to the brain, blockage of large or small blood vessels due to clotting, and microscopic bleeds. They highlighted how the most important mechanism, however, may be “damage caused by the body’s own inflammatory response and immune system.”
Adam Hampshire, PhD, of the department of brain sciences at Imperial College London, one of the study’s authors, described how around 40,000 people have been through intensive care with COVID-19 in England alone, with many more despite having been very sick not admitted to hospital. This means there is a “large number of people out there still experiencing problems with cognition many months later,” he said. “We urgently need to look at what can be done to help these people.”
A version of this article first appeared on Univadis.
adding that the impairment is “equivalent to losing 10 IQ points.”
In their study, published in eClinicalMedicine, a team of scientists from the University of Cambridge and Imperial College London said there is growing evidence that COVID-19 can cause lasting cognitive and mental health problems. Patients report fatigue, “brain fog,” problems recalling words, sleep disturbances, anxiety, and even posttraumatic stress disorder months after infection.
The researchers analyzed data from 46 individuals who received critical care for COVID-19 at Addenbrooke’s Hospital between March and July 2020 (27 females, 19 males, mean age 51 years, 16 of whom had mechanical ventilation) and were recruited to the NIHR COVID-19 BioResource project.
At an average of 6 months after acute COVID-19 illness, the study participants underwent detailed computerized cognitive tests via the Cognitron platform, comprising eight tasks deployed on an iPad measuring mental function such as memory, attention, and reasoning. Also assessed were anxiety, depression, and posttraumatic stress disorder via standard mood, anxiety, and posttraumatic stress scales – specifically the Generalized Anxiety Disorder 7 (GAD-7), the Patient Health Questionnaire 9 (PHQ-9), and the PTSD Checklist for Diagnostic and Statistical Manual of Mental Disorders 5 (PCL-5). Their data were compared against 460 controls – matched for age, sex, education, and first language – and the pattern of deficits across tasks was qualitatively compared with normal age-related decline and early-stage dementia.
Less accurate and slower response times
The authors highlighted how this was the first time a “rigorous assessment and comparison” had been carried out in relation to the after-effects of severe COVID-19.
“Cognitive impairment is common to a wide range of neurological disorders, including dementia, and even routine aging, but the patterns we saw – the cognitive ‘fingerprint’ of COVID-19 – was distinct from all of these,” said David Menon, MD, division of anesthesia at the University of Cambridge, England, and the study’s senior author.
The scientists found that COVID-19 survivors were less accurate and had slower response times than the control population, and added that survivors scored particularly poorly on verbal analogical reasoning and showed slower processing speeds.
Critically, the scale of the cognitive deficits correlated with acute illness severity, but not fatigue or mental health status at the time of cognitive assessment, said the authors.
Recovery ‘at best gradual’
The effects were strongest for those with more severe acute illness, and who required mechanical ventilation, said the authors, who found that acute illness severity was “better at predicting the cognitive deficits.”
The authors pointed out how these deficits were still detectable when patients were followed up 6 months later, and that, although patients’ scores and reaction times began to improve over time, any recovery was “at best gradual” and likely to be influenced by factors such as illness severity and its neurological or psychological impacts.
“We followed some patients up as late as 10 months after their acute infection, so were able to see a very slow improvement,” Dr. Menon said. He explained how, while this improvement was not statistically significant, it was “at least heading in the right direction.”
However, he warned it is very possible that some of these individuals “will never fully recover.”
The cognitive deficits observed may be due to several factors in combination, said the authors, including inadequate oxygen or blood supply to the brain, blockage of large or small blood vessels due to clotting, and microscopic bleeds. They highlighted how the most important mechanism, however, may be “damage caused by the body’s own inflammatory response and immune system.”
Adam Hampshire, PhD, of the department of brain sciences at Imperial College London, one of the study’s authors, described how around 40,000 people have been through intensive care with COVID-19 in England alone, with many more despite having been very sick not admitted to hospital. This means there is a “large number of people out there still experiencing problems with cognition many months later,” he said. “We urgently need to look at what can be done to help these people.”
A version of this article first appeared on Univadis.
FROM ECLINICAL MEDICINE
Implant may alleviate sleep apnea in teens with Down syndrome
Upper airway hypoglossal nerve stimulation is safe and effective in adolescents with Down syndrome and severe persistent obstructive sleep apnea (OSA) occurring after adenotonsillectomy and who couldn’t tolerate positive airway pressure, early research suggests.
In a phase I study, 42 adolescents received a surgically implanted device that moves the tongue forward during sleep. Results at 1-year follow-up showed 66% “responded well” to treatment and showed a drop in apnea-hypopnea index (AHI) of at least 50%.
“Parents came back to us and said not only is the sleep better but my child seems to be doing better during the day,” lead investigator Christopher Hartnick, MD, director of the Division of Pediatric Otolaryngology and the Pediatric Airway, Voice, and Swallowing Center at Massachusetts Eye and Ear, Boston, told this news organization.
The findings were published online in JAMA Otolaryngology – Head and Neck Surgery.
Limited options
Upper airway simulation has been shown previously to be effective for adults with OSA, but up until now, the process has not been evaluated in children.
The device used in the current study “stimulates the hypoglossal nerve to protrude the tongue and open the airway on inspiration during sleep,” the investigators note.
“Hypoglossal nerve stimulation may be a particularly suitable therapy for patients with Down syndrome because it can augment neuromuscular airway tone and reduce anatomical obstruction at the base of the tongue, a common site of residual obstruction in children with Down syndrome,” they add.
“This study was born out of the frustration of not having an effective treatment option for children with Down syndrome who struggle with sleep apnea,” Dr. Hartnick said in a news release.
A total of 42 adolescents (67% male; mean age, 15 years) with Down syndrome and persistent severe OSA after adenotonsillectomy were implanted with the hypoglossal nerve stimulator. All were followed for 12 months.
The surgery was safe, with the most common adverse event being temporary tongue discomfort in five patients (12%). This typically resolved in weeks, the researchers note.
High response, adherence rates
Results showed response rates and adherence to therapy was high. The mean duration of nightly therapy was 9 hours, with 40 children (95.2%) using the device at least 4 hours every night.
The implant was also effective, with a mean decrease in AHI of 12.9 events per hour (95% confidence interval, –17.0 to –8.7 events per hour).
Nearly two-thirds of the children had at least a 50% reduction in their AHI, while roughly three-fourths had a 12-month follow-up AHI of less than 10 events per hour.
There were also significant improvements in polysomnographic and parent-reported quality of life outcomes 12 months after the implant, including improvement in sleep and daily functioning, behavior, and language.
“Sleep apnea remains one of the most common conditions that I grapple with working with patients with Down syndrome and their families,” co-investigator Brian Skotko, MD, Emma Campbell endowed chair on Down syndrome at Massachusetts General Hospital, Boston, said in the release.
“Until now, so many of our patients had run out of treatment options, and their health and well-being were declining. Now, with the hypoglossal nerve stimulator treatment, we may have an effective and safe way to treat apnea and maximize brain health for people with Down syndrome,” Dr. Skotko added.
Dr. Hartnick and Dr. Skotko have received a $4 million, 5-year grant from the National Institutes of Health to assess whether upper airway stimulation might help cognition in children with Down syndrome.
Landmark investigation
Co-authors of an invited commentary said they “applaud” the researchers for their “landmark” investigation, which demonstrated a response to upper airway stimulation in children with Down syndrome and OSA that is on par with what has been achieved in adults with OSA.
“They have established the safety of the procedure; however, future research is necessary to optimize the results of implant,” write Norman Friedman, MD, and Katherine Green, MD, both from the department of otolaryngology – head and neck surgery, University of Colorado School of Medicine, Aurora.
“Further assessment regarding patient selection and the systematic preoperative identification of potential barriers that might affect successful use of therapy will be beneficial to improve longitudinal outcomes and success in this population that is uniquely different from the adult cohorts that have received implants to date,” they add.
The study was funded by Inspire Medical Systems, which provided eight devices for the study but otherwise did not have a role in its design and conduct. The LuMind IDSC Down Syndrome Foundation also provided funding for the study. Dr. Hartnick and the editorialists have disclosed no relevant financial relationships. A complete list of disclosures for the other investigators is available in the original article.
A version of this article first appeared on Medscape.com.
Upper airway hypoglossal nerve stimulation is safe and effective in adolescents with Down syndrome and severe persistent obstructive sleep apnea (OSA) occurring after adenotonsillectomy and who couldn’t tolerate positive airway pressure, early research suggests.
In a phase I study, 42 adolescents received a surgically implanted device that moves the tongue forward during sleep. Results at 1-year follow-up showed 66% “responded well” to treatment and showed a drop in apnea-hypopnea index (AHI) of at least 50%.
“Parents came back to us and said not only is the sleep better but my child seems to be doing better during the day,” lead investigator Christopher Hartnick, MD, director of the Division of Pediatric Otolaryngology and the Pediatric Airway, Voice, and Swallowing Center at Massachusetts Eye and Ear, Boston, told this news organization.
The findings were published online in JAMA Otolaryngology – Head and Neck Surgery.
Limited options
Upper airway simulation has been shown previously to be effective for adults with OSA, but up until now, the process has not been evaluated in children.
The device used in the current study “stimulates the hypoglossal nerve to protrude the tongue and open the airway on inspiration during sleep,” the investigators note.
“Hypoglossal nerve stimulation may be a particularly suitable therapy for patients with Down syndrome because it can augment neuromuscular airway tone and reduce anatomical obstruction at the base of the tongue, a common site of residual obstruction in children with Down syndrome,” they add.
“This study was born out of the frustration of not having an effective treatment option for children with Down syndrome who struggle with sleep apnea,” Dr. Hartnick said in a news release.
A total of 42 adolescents (67% male; mean age, 15 years) with Down syndrome and persistent severe OSA after adenotonsillectomy were implanted with the hypoglossal nerve stimulator. All were followed for 12 months.
The surgery was safe, with the most common adverse event being temporary tongue discomfort in five patients (12%). This typically resolved in weeks, the researchers note.
High response, adherence rates
Results showed response rates and adherence to therapy was high. The mean duration of nightly therapy was 9 hours, with 40 children (95.2%) using the device at least 4 hours every night.
The implant was also effective, with a mean decrease in AHI of 12.9 events per hour (95% confidence interval, –17.0 to –8.7 events per hour).
Nearly two-thirds of the children had at least a 50% reduction in their AHI, while roughly three-fourths had a 12-month follow-up AHI of less than 10 events per hour.
There were also significant improvements in polysomnographic and parent-reported quality of life outcomes 12 months after the implant, including improvement in sleep and daily functioning, behavior, and language.
“Sleep apnea remains one of the most common conditions that I grapple with working with patients with Down syndrome and their families,” co-investigator Brian Skotko, MD, Emma Campbell endowed chair on Down syndrome at Massachusetts General Hospital, Boston, said in the release.
“Until now, so many of our patients had run out of treatment options, and their health and well-being were declining. Now, with the hypoglossal nerve stimulator treatment, we may have an effective and safe way to treat apnea and maximize brain health for people with Down syndrome,” Dr. Skotko added.
Dr. Hartnick and Dr. Skotko have received a $4 million, 5-year grant from the National Institutes of Health to assess whether upper airway stimulation might help cognition in children with Down syndrome.
Landmark investigation
Co-authors of an invited commentary said they “applaud” the researchers for their “landmark” investigation, which demonstrated a response to upper airway stimulation in children with Down syndrome and OSA that is on par with what has been achieved in adults with OSA.
“They have established the safety of the procedure; however, future research is necessary to optimize the results of implant,” write Norman Friedman, MD, and Katherine Green, MD, both from the department of otolaryngology – head and neck surgery, University of Colorado School of Medicine, Aurora.
“Further assessment regarding patient selection and the systematic preoperative identification of potential barriers that might affect successful use of therapy will be beneficial to improve longitudinal outcomes and success in this population that is uniquely different from the adult cohorts that have received implants to date,” they add.
The study was funded by Inspire Medical Systems, which provided eight devices for the study but otherwise did not have a role in its design and conduct. The LuMind IDSC Down Syndrome Foundation also provided funding for the study. Dr. Hartnick and the editorialists have disclosed no relevant financial relationships. A complete list of disclosures for the other investigators is available in the original article.
A version of this article first appeared on Medscape.com.
Upper airway hypoglossal nerve stimulation is safe and effective in adolescents with Down syndrome and severe persistent obstructive sleep apnea (OSA) occurring after adenotonsillectomy and who couldn’t tolerate positive airway pressure, early research suggests.
In a phase I study, 42 adolescents received a surgically implanted device that moves the tongue forward during sleep. Results at 1-year follow-up showed 66% “responded well” to treatment and showed a drop in apnea-hypopnea index (AHI) of at least 50%.
“Parents came back to us and said not only is the sleep better but my child seems to be doing better during the day,” lead investigator Christopher Hartnick, MD, director of the Division of Pediatric Otolaryngology and the Pediatric Airway, Voice, and Swallowing Center at Massachusetts Eye and Ear, Boston, told this news organization.
The findings were published online in JAMA Otolaryngology – Head and Neck Surgery.
Limited options
Upper airway simulation has been shown previously to be effective for adults with OSA, but up until now, the process has not been evaluated in children.
The device used in the current study “stimulates the hypoglossal nerve to protrude the tongue and open the airway on inspiration during sleep,” the investigators note.
“Hypoglossal nerve stimulation may be a particularly suitable therapy for patients with Down syndrome because it can augment neuromuscular airway tone and reduce anatomical obstruction at the base of the tongue, a common site of residual obstruction in children with Down syndrome,” they add.
“This study was born out of the frustration of not having an effective treatment option for children with Down syndrome who struggle with sleep apnea,” Dr. Hartnick said in a news release.
A total of 42 adolescents (67% male; mean age, 15 years) with Down syndrome and persistent severe OSA after adenotonsillectomy were implanted with the hypoglossal nerve stimulator. All were followed for 12 months.
The surgery was safe, with the most common adverse event being temporary tongue discomfort in five patients (12%). This typically resolved in weeks, the researchers note.
High response, adherence rates
Results showed response rates and adherence to therapy was high. The mean duration of nightly therapy was 9 hours, with 40 children (95.2%) using the device at least 4 hours every night.
The implant was also effective, with a mean decrease in AHI of 12.9 events per hour (95% confidence interval, –17.0 to –8.7 events per hour).
Nearly two-thirds of the children had at least a 50% reduction in their AHI, while roughly three-fourths had a 12-month follow-up AHI of less than 10 events per hour.
There were also significant improvements in polysomnographic and parent-reported quality of life outcomes 12 months after the implant, including improvement in sleep and daily functioning, behavior, and language.
“Sleep apnea remains one of the most common conditions that I grapple with working with patients with Down syndrome and their families,” co-investigator Brian Skotko, MD, Emma Campbell endowed chair on Down syndrome at Massachusetts General Hospital, Boston, said in the release.
“Until now, so many of our patients had run out of treatment options, and their health and well-being were declining. Now, with the hypoglossal nerve stimulator treatment, we may have an effective and safe way to treat apnea and maximize brain health for people with Down syndrome,” Dr. Skotko added.
Dr. Hartnick and Dr. Skotko have received a $4 million, 5-year grant from the National Institutes of Health to assess whether upper airway stimulation might help cognition in children with Down syndrome.
Landmark investigation
Co-authors of an invited commentary said they “applaud” the researchers for their “landmark” investigation, which demonstrated a response to upper airway stimulation in children with Down syndrome and OSA that is on par with what has been achieved in adults with OSA.
“They have established the safety of the procedure; however, future research is necessary to optimize the results of implant,” write Norman Friedman, MD, and Katherine Green, MD, both from the department of otolaryngology – head and neck surgery, University of Colorado School of Medicine, Aurora.
“Further assessment regarding patient selection and the systematic preoperative identification of potential barriers that might affect successful use of therapy will be beneficial to improve longitudinal outcomes and success in this population that is uniquely different from the adult cohorts that have received implants to date,” they add.
The study was funded by Inspire Medical Systems, which provided eight devices for the study but otherwise did not have a role in its design and conduct. The LuMind IDSC Down Syndrome Foundation also provided funding for the study. Dr. Hartnick and the editorialists have disclosed no relevant financial relationships. A complete list of disclosures for the other investigators is available in the original article.
A version of this article first appeared on Medscape.com.
Internet intervention improved insomnia in Black women
Data from previous studies suggest that women are up to 40% more likely to experience insomnia disorder compared with men, Eric S. Zhou, PhD, of Harvard Medical School, Boston, and colleagues wrote. The risk is even higher among Black women, but research on tailored treatments for this particular population has been limited.
In their study, published in JAMA Psychiatry, the researchers recruited women with elevated insomnia symptoms who were enrolled in the Black Women’s Health Study, an ongoing national, longitudinal research cohort in the United States. Participants were recruited between October 2019 and June 2020.The participants were randomized to an Internet-delivered behavior intervention (108 women), a stakeholder-informed Internet intervention tailored to Black women (110 women), or non-Internet patient education about sleep (115 women).
The Internet intervention, known as Sleep Healthy Using the Internet (SHUTi), was a 6-session program lasting 45-60 minutes per session and delivered over 6-9 weeks. The program included core elements of cognitive behavioral therapy and took into account information provided by patients about their baseline sleep function, treatment adherence, and sleep progress.
The tailored version of SHUTi for Black women (SHUTi-BWHS) was similar, but included Black actors for video vignettes and the inclusion of content about the cultural and social contexts in which insomnia often occurs for Black women, such while managing neighborhood noise and or living in crowded environments.
A third group received standard patient education material about sleep through a noninteractive website, and served as the control group.
The primary outcome of insomnia severity was measured using the Insomnia Severity Index (ISI), a 0- to 28-point scale. Scores for the ISI are based on responses to seven questions, including some that ask participants to rate the severity of their insomnia symptoms.
Clinically significant improvement in insomnia was defined as a reduction in score of more than 7 points. Patients were assessed at baseline, at 9 weeks, and again at approximately 6 months.
Significantly greater reductions in insomnia severity seen in intervention groups vs. control group
Overall, women randomized to SHUTi or SHUTi-BWHS) reported a significantly greater reduction in insomnia symptoms from baseline to 6 months, compared with the control group (P < .001), with ISI score decreases of 10.0, 9.3, and 3.6, respectively. No statistically significant differences in ISI score changes appeared between the between the SHUTi-BWHS and SHUTi groups.
Also, significantly more women in the SHUTi-BWHS group than in the SHUTi group completed the intervention (78.2% vs. 64.8%).
Treatment response was similar between the SHUTI-BWHS and SHUTi groups; 47.3% and 46.3%, respectively, had a decrease in ISI score of more than 7 points. In addition, 37% of women in the SHUTi-BWHS and 38% of women in the SHUTi groups reached ISI scores of less than 8 points, defined as full resolution of insomnia, by the last follow-up visit.
Both the SHUTi and SHUTi-BWHS interventions had dramatic effects on insomnia, but the increased number of women who completed the intervention in the SHUTi-BWHS group supports the value of tailored intervention, the researchers noted. “Similar to prior SHUTi trials, there was a direct association between the participant’s level of intervention engagement and their improvement in sleep.”
The average age of the participants was 60 years, 62% were single, and 44% had a graduate degree or higher. Approximately 5% were being actively treated for sleep apnea.
The study findings were limited by several factors including the relatively high socioeconomic status of the study participants, lack of data on medical mistrust, and inability to detect smaller differences between SHUTi and SHUTi-BWHS, the researchers noted.
Choose Internet-based CBT first for insomnia
“This was an excellent paper that sought to see the relative efficacy of standard version of Internet-delivered CBT-I [cognitive-behavioral therapy for insomnia] versus a culturally tailored version for Black women,” said Neil Skolnik, MD, professor of family and community medicine at Thomas Jefferson University, Philadelphia, in an interview. “The trial confirmed that, compared with sleep education, which was used as the control, Internet-delivered CBT-I is effective in the treatment of insomnia.”
“These results demonstrate two important things,” said Dr. Skolnik. “The most important is that Internet-delivered CBT-I works, and since it is both safe and effective, should be the first-line therapy for patients who want treatment for insomnia.”
Secondly, “the fact that more people completed culturally tailored versions suggests that, when culturally tailored versions are available, their use is preferable, as it might facilitate a higher proportion of patients being successful in their insomnia treatment,” he added.
The study was supported by the Patient-Centered Outcomes Research Institute. The Black Women’s Health Study is supported by the National Cancer Institute. Dr. Zhou disclosed support from both PCORI and the NCI during the study. Dr. Skolnik, who was not involved in the study, disclosed serving on the advisory board for Idorsia Pharmaceuticals. He is also a member of the editorial advisory board of Family Practice News.
Data from previous studies suggest that women are up to 40% more likely to experience insomnia disorder compared with men, Eric S. Zhou, PhD, of Harvard Medical School, Boston, and colleagues wrote. The risk is even higher among Black women, but research on tailored treatments for this particular population has been limited.
In their study, published in JAMA Psychiatry, the researchers recruited women with elevated insomnia symptoms who were enrolled in the Black Women’s Health Study, an ongoing national, longitudinal research cohort in the United States. Participants were recruited between October 2019 and June 2020.The participants were randomized to an Internet-delivered behavior intervention (108 women), a stakeholder-informed Internet intervention tailored to Black women (110 women), or non-Internet patient education about sleep (115 women).
The Internet intervention, known as Sleep Healthy Using the Internet (SHUTi), was a 6-session program lasting 45-60 minutes per session and delivered over 6-9 weeks. The program included core elements of cognitive behavioral therapy and took into account information provided by patients about their baseline sleep function, treatment adherence, and sleep progress.
The tailored version of SHUTi for Black women (SHUTi-BWHS) was similar, but included Black actors for video vignettes and the inclusion of content about the cultural and social contexts in which insomnia often occurs for Black women, such while managing neighborhood noise and or living in crowded environments.
A third group received standard patient education material about sleep through a noninteractive website, and served as the control group.
The primary outcome of insomnia severity was measured using the Insomnia Severity Index (ISI), a 0- to 28-point scale. Scores for the ISI are based on responses to seven questions, including some that ask participants to rate the severity of their insomnia symptoms.
Clinically significant improvement in insomnia was defined as a reduction in score of more than 7 points. Patients were assessed at baseline, at 9 weeks, and again at approximately 6 months.
Significantly greater reductions in insomnia severity seen in intervention groups vs. control group
Overall, women randomized to SHUTi or SHUTi-BWHS) reported a significantly greater reduction in insomnia symptoms from baseline to 6 months, compared with the control group (P < .001), with ISI score decreases of 10.0, 9.3, and 3.6, respectively. No statistically significant differences in ISI score changes appeared between the between the SHUTi-BWHS and SHUTi groups.
Also, significantly more women in the SHUTi-BWHS group than in the SHUTi group completed the intervention (78.2% vs. 64.8%).
Treatment response was similar between the SHUTI-BWHS and SHUTi groups; 47.3% and 46.3%, respectively, had a decrease in ISI score of more than 7 points. In addition, 37% of women in the SHUTi-BWHS and 38% of women in the SHUTi groups reached ISI scores of less than 8 points, defined as full resolution of insomnia, by the last follow-up visit.
Both the SHUTi and SHUTi-BWHS interventions had dramatic effects on insomnia, but the increased number of women who completed the intervention in the SHUTi-BWHS group supports the value of tailored intervention, the researchers noted. “Similar to prior SHUTi trials, there was a direct association between the participant’s level of intervention engagement and their improvement in sleep.”
The average age of the participants was 60 years, 62% were single, and 44% had a graduate degree or higher. Approximately 5% were being actively treated for sleep apnea.
The study findings were limited by several factors including the relatively high socioeconomic status of the study participants, lack of data on medical mistrust, and inability to detect smaller differences between SHUTi and SHUTi-BWHS, the researchers noted.
Choose Internet-based CBT first for insomnia
“This was an excellent paper that sought to see the relative efficacy of standard version of Internet-delivered CBT-I [cognitive-behavioral therapy for insomnia] versus a culturally tailored version for Black women,” said Neil Skolnik, MD, professor of family and community medicine at Thomas Jefferson University, Philadelphia, in an interview. “The trial confirmed that, compared with sleep education, which was used as the control, Internet-delivered CBT-I is effective in the treatment of insomnia.”
“These results demonstrate two important things,” said Dr. Skolnik. “The most important is that Internet-delivered CBT-I works, and since it is both safe and effective, should be the first-line therapy for patients who want treatment for insomnia.”
Secondly, “the fact that more people completed culturally tailored versions suggests that, when culturally tailored versions are available, their use is preferable, as it might facilitate a higher proportion of patients being successful in their insomnia treatment,” he added.
The study was supported by the Patient-Centered Outcomes Research Institute. The Black Women’s Health Study is supported by the National Cancer Institute. Dr. Zhou disclosed support from both PCORI and the NCI during the study. Dr. Skolnik, who was not involved in the study, disclosed serving on the advisory board for Idorsia Pharmaceuticals. He is also a member of the editorial advisory board of Family Practice News.
Data from previous studies suggest that women are up to 40% more likely to experience insomnia disorder compared with men, Eric S. Zhou, PhD, of Harvard Medical School, Boston, and colleagues wrote. The risk is even higher among Black women, but research on tailored treatments for this particular population has been limited.
In their study, published in JAMA Psychiatry, the researchers recruited women with elevated insomnia symptoms who were enrolled in the Black Women’s Health Study, an ongoing national, longitudinal research cohort in the United States. Participants were recruited between October 2019 and June 2020.The participants were randomized to an Internet-delivered behavior intervention (108 women), a stakeholder-informed Internet intervention tailored to Black women (110 women), or non-Internet patient education about sleep (115 women).
The Internet intervention, known as Sleep Healthy Using the Internet (SHUTi), was a 6-session program lasting 45-60 minutes per session and delivered over 6-9 weeks. The program included core elements of cognitive behavioral therapy and took into account information provided by patients about their baseline sleep function, treatment adherence, and sleep progress.
The tailored version of SHUTi for Black women (SHUTi-BWHS) was similar, but included Black actors for video vignettes and the inclusion of content about the cultural and social contexts in which insomnia often occurs for Black women, such while managing neighborhood noise and or living in crowded environments.
A third group received standard patient education material about sleep through a noninteractive website, and served as the control group.
The primary outcome of insomnia severity was measured using the Insomnia Severity Index (ISI), a 0- to 28-point scale. Scores for the ISI are based on responses to seven questions, including some that ask participants to rate the severity of their insomnia symptoms.
Clinically significant improvement in insomnia was defined as a reduction in score of more than 7 points. Patients were assessed at baseline, at 9 weeks, and again at approximately 6 months.
Significantly greater reductions in insomnia severity seen in intervention groups vs. control group
Overall, women randomized to SHUTi or SHUTi-BWHS) reported a significantly greater reduction in insomnia symptoms from baseline to 6 months, compared with the control group (P < .001), with ISI score decreases of 10.0, 9.3, and 3.6, respectively. No statistically significant differences in ISI score changes appeared between the between the SHUTi-BWHS and SHUTi groups.
Also, significantly more women in the SHUTi-BWHS group than in the SHUTi group completed the intervention (78.2% vs. 64.8%).
Treatment response was similar between the SHUTI-BWHS and SHUTi groups; 47.3% and 46.3%, respectively, had a decrease in ISI score of more than 7 points. In addition, 37% of women in the SHUTi-BWHS and 38% of women in the SHUTi groups reached ISI scores of less than 8 points, defined as full resolution of insomnia, by the last follow-up visit.
Both the SHUTi and SHUTi-BWHS interventions had dramatic effects on insomnia, but the increased number of women who completed the intervention in the SHUTi-BWHS group supports the value of tailored intervention, the researchers noted. “Similar to prior SHUTi trials, there was a direct association between the participant’s level of intervention engagement and their improvement in sleep.”
The average age of the participants was 60 years, 62% were single, and 44% had a graduate degree or higher. Approximately 5% were being actively treated for sleep apnea.
The study findings were limited by several factors including the relatively high socioeconomic status of the study participants, lack of data on medical mistrust, and inability to detect smaller differences between SHUTi and SHUTi-BWHS, the researchers noted.
Choose Internet-based CBT first for insomnia
“This was an excellent paper that sought to see the relative efficacy of standard version of Internet-delivered CBT-I [cognitive-behavioral therapy for insomnia] versus a culturally tailored version for Black women,” said Neil Skolnik, MD, professor of family and community medicine at Thomas Jefferson University, Philadelphia, in an interview. “The trial confirmed that, compared with sleep education, which was used as the control, Internet-delivered CBT-I is effective in the treatment of insomnia.”
“These results demonstrate two important things,” said Dr. Skolnik. “The most important is that Internet-delivered CBT-I works, and since it is both safe and effective, should be the first-line therapy for patients who want treatment for insomnia.”
Secondly, “the fact that more people completed culturally tailored versions suggests that, when culturally tailored versions are available, their use is preferable, as it might facilitate a higher proportion of patients being successful in their insomnia treatment,” he added.
The study was supported by the Patient-Centered Outcomes Research Institute. The Black Women’s Health Study is supported by the National Cancer Institute. Dr. Zhou disclosed support from both PCORI and the NCI during the study. Dr. Skolnik, who was not involved in the study, disclosed serving on the advisory board for Idorsia Pharmaceuticals. He is also a member of the editorial advisory board of Family Practice News.
FROM JAMA PSYCHIATRY











