User login
Distinguish ‘sleepiness’ from ‘fatigue’ to help diagnose hypersomnia
, according to Ruth M. Benca, MD, PhD.
Fatigue, feeling tired, and lack of energy are common complaints that accompany insomnia and psychiatric disorders, but these patients do not fall asleep quickly in a restful setting and will have normal multiple sleep latency test (MSLT) in a laboratory. In contrast, excessive sleepiness, or hypersomnia, occurs when patients sleep more than 11 hours in a 24-hour period.
Patients with hypersomnia fall asleep in low stimulus situations and devote more energy to staying awake during situations. This excessive sleepiness can be dangerous in the context of activities such as driving, Dr. Benca said. These patients will also have low sleep latencies (< 8 minutes) when tested through MSLT in a laboratory, she added. Patients with hypersomnia may be irritable, have reduced attention or concentration, and have poor memory.
The primary cause of hypersomnia is sleep deprivation, but “both hypersomnia and fatigue are common complaints in psychiatric patients, including depression, bipolar disorder, seasonal affective disorder, [and] psychosis,” Dr. Benca explained. Other causes of hypersomnia include sleep disorders such as sleep apnea, circadian rhythm disorders and periodic limb movements, neurologic or degenerative disorders, mental disorders, and effects of medication. Idiopathic hypersomnia and narcolepsy are uncommon causes of hypersomnia and usually diagnosed in a sleep laboratory setting, she said.
In patients with depression, hypersomnia looks like patients having “nonimperative sleepiness,” Dr. Benca said. “They may spend a lot of time in bed; they may report long and nonrefreshing naps or long sleep time.”
There also is an issue with sleep inertia in patients with depression and hypersomnia, and with patients taking a long time to wake up and begin their day. In these patients, “when we put them in the sleep laboratory, the objective studies generally do not show that they are excessively sleepy, despite their reports of subjectively being sleepy,” she said.
There is not much objective MSLT or subjective measure data for hypersomnia in patients with schizophrenia despite these patients reporting daytime sleepiness or hypersomnolence, Dr. Benca admitted. Hypersomnia in patients with schizophrenia may be related to drug effects, poor sleep hygiene, circadian rhythm abnormalities, or comorbid sleep disorders. “Excessive sleepiness may also be related to the schizophrenia itself,” she said.
Treatments for hypersomnia
The first priority for patients with hypersomnia is to avoid sleep deprivation and practice good sleep hygiene – factors that are important both in insomnia and hypersomnia. “Make sure that patients are having adequate time in bed and having regular hours of sleep,” Dr. Benca said.
For patients with comorbid psychiatric, medical and sleep disorders, focus on getting rid of medications that may cause sleepiness, including sedating medications and antidepressants, and consider using stimulants if appropriate. While there are Food and Drug Administration–approved medications for narcolepsy and some are approved for hypersomnia in patients with obstructive sleep apnea (OSA), none are officially approved to treat hypersomnia in psychiatric patients.
“Whenever we use these drugs for those reasons, we’re using them off label,” Dr. Benca said.
Modafinil/armodafinil, approved for narcolepsy, shift-work disorder, and OSA in Ehlers-Danlos syndrome, is one off-label option for patients with hypersomnia. “They are lower potency and less addictive than the amphetamines, [with] fewer side effects,” Dr. Benca explained, but should be prescribed with caution in some women because of potential reduced efficacy of oral contraceptives. Side effects of modafinil include headache, nausea, eosinophilia, diarrhea, dry mouth, and anorexia.
Methylphenidate is another option for hypersomnia, available in racemic mixture, pure D-isomer, and time-release formulations.
Patients taking methylphenidate may experience nervousness, insomnia, anorexia, nausea, dizziness, hypertension, hypotension, hypersensitivity reactions, tachycardia, and headache as side effects.
For patients with central nervous system hypersomnias, amphetamines can be used, with methamphetamines having a “very similar profile” and similar side effects, including insomnia, restlessness, tachycardia, dizziness, diarrhea, constipation, hypertension, impotence, and rare cases of psychotic episodes.
Practice parameters released by the American Academy of Sleep Medicine in 2007 suggest that modafinil may have efficacy in idiopathic hypersomnia, Parkinson’s disease, myotonic dystrophy, and multiple sclerosis. The practice parameters also suggest hypersomnias of central origin can be treated with modafinil, amphetamine, methamphetamine, dextroamphetamine, and methylphenidate based on evidence or “long history of use” (Sleep. 2007;30:1705-11).
“Interestingly, there is no mention of psychiatric disorders in these practice parameters, and they report that there are mixed results using stimulants off label for sleepiness and fatigue in traumatic brain injury and poststroke fatigue,” Dr. Benca said.
Dr. Benca reported that she is a consultant to Eisai, Idorsia, Jazz, Merck, and Sunovion. Global Academy and this news organization are owned by the same parent company.
, according to Ruth M. Benca, MD, PhD.
Fatigue, feeling tired, and lack of energy are common complaints that accompany insomnia and psychiatric disorders, but these patients do not fall asleep quickly in a restful setting and will have normal multiple sleep latency test (MSLT) in a laboratory. In contrast, excessive sleepiness, or hypersomnia, occurs when patients sleep more than 11 hours in a 24-hour period.
Patients with hypersomnia fall asleep in low stimulus situations and devote more energy to staying awake during situations. This excessive sleepiness can be dangerous in the context of activities such as driving, Dr. Benca said. These patients will also have low sleep latencies (< 8 minutes) when tested through MSLT in a laboratory, she added. Patients with hypersomnia may be irritable, have reduced attention or concentration, and have poor memory.
The primary cause of hypersomnia is sleep deprivation, but “both hypersomnia and fatigue are common complaints in psychiatric patients, including depression, bipolar disorder, seasonal affective disorder, [and] psychosis,” Dr. Benca explained. Other causes of hypersomnia include sleep disorders such as sleep apnea, circadian rhythm disorders and periodic limb movements, neurologic or degenerative disorders, mental disorders, and effects of medication. Idiopathic hypersomnia and narcolepsy are uncommon causes of hypersomnia and usually diagnosed in a sleep laboratory setting, she said.
In patients with depression, hypersomnia looks like patients having “nonimperative sleepiness,” Dr. Benca said. “They may spend a lot of time in bed; they may report long and nonrefreshing naps or long sleep time.”
There also is an issue with sleep inertia in patients with depression and hypersomnia, and with patients taking a long time to wake up and begin their day. In these patients, “when we put them in the sleep laboratory, the objective studies generally do not show that they are excessively sleepy, despite their reports of subjectively being sleepy,” she said.
There is not much objective MSLT or subjective measure data for hypersomnia in patients with schizophrenia despite these patients reporting daytime sleepiness or hypersomnolence, Dr. Benca admitted. Hypersomnia in patients with schizophrenia may be related to drug effects, poor sleep hygiene, circadian rhythm abnormalities, or comorbid sleep disorders. “Excessive sleepiness may also be related to the schizophrenia itself,” she said.
Treatments for hypersomnia
The first priority for patients with hypersomnia is to avoid sleep deprivation and practice good sleep hygiene – factors that are important both in insomnia and hypersomnia. “Make sure that patients are having adequate time in bed and having regular hours of sleep,” Dr. Benca said.
For patients with comorbid psychiatric, medical and sleep disorders, focus on getting rid of medications that may cause sleepiness, including sedating medications and antidepressants, and consider using stimulants if appropriate. While there are Food and Drug Administration–approved medications for narcolepsy and some are approved for hypersomnia in patients with obstructive sleep apnea (OSA), none are officially approved to treat hypersomnia in psychiatric patients.
“Whenever we use these drugs for those reasons, we’re using them off label,” Dr. Benca said.
Modafinil/armodafinil, approved for narcolepsy, shift-work disorder, and OSA in Ehlers-Danlos syndrome, is one off-label option for patients with hypersomnia. “They are lower potency and less addictive than the amphetamines, [with] fewer side effects,” Dr. Benca explained, but should be prescribed with caution in some women because of potential reduced efficacy of oral contraceptives. Side effects of modafinil include headache, nausea, eosinophilia, diarrhea, dry mouth, and anorexia.
Methylphenidate is another option for hypersomnia, available in racemic mixture, pure D-isomer, and time-release formulations.
Patients taking methylphenidate may experience nervousness, insomnia, anorexia, nausea, dizziness, hypertension, hypotension, hypersensitivity reactions, tachycardia, and headache as side effects.
For patients with central nervous system hypersomnias, amphetamines can be used, with methamphetamines having a “very similar profile” and similar side effects, including insomnia, restlessness, tachycardia, dizziness, diarrhea, constipation, hypertension, impotence, and rare cases of psychotic episodes.
Practice parameters released by the American Academy of Sleep Medicine in 2007 suggest that modafinil may have efficacy in idiopathic hypersomnia, Parkinson’s disease, myotonic dystrophy, and multiple sclerosis. The practice parameters also suggest hypersomnias of central origin can be treated with modafinil, amphetamine, methamphetamine, dextroamphetamine, and methylphenidate based on evidence or “long history of use” (Sleep. 2007;30:1705-11).
“Interestingly, there is no mention of psychiatric disorders in these practice parameters, and they report that there are mixed results using stimulants off label for sleepiness and fatigue in traumatic brain injury and poststroke fatigue,” Dr. Benca said.
Dr. Benca reported that she is a consultant to Eisai, Idorsia, Jazz, Merck, and Sunovion. Global Academy and this news organization are owned by the same parent company.
, according to Ruth M. Benca, MD, PhD.
Fatigue, feeling tired, and lack of energy are common complaints that accompany insomnia and psychiatric disorders, but these patients do not fall asleep quickly in a restful setting and will have normal multiple sleep latency test (MSLT) in a laboratory. In contrast, excessive sleepiness, or hypersomnia, occurs when patients sleep more than 11 hours in a 24-hour period.
Patients with hypersomnia fall asleep in low stimulus situations and devote more energy to staying awake during situations. This excessive sleepiness can be dangerous in the context of activities such as driving, Dr. Benca said. These patients will also have low sleep latencies (< 8 minutes) when tested through MSLT in a laboratory, she added. Patients with hypersomnia may be irritable, have reduced attention or concentration, and have poor memory.
The primary cause of hypersomnia is sleep deprivation, but “both hypersomnia and fatigue are common complaints in psychiatric patients, including depression, bipolar disorder, seasonal affective disorder, [and] psychosis,” Dr. Benca explained. Other causes of hypersomnia include sleep disorders such as sleep apnea, circadian rhythm disorders and periodic limb movements, neurologic or degenerative disorders, mental disorders, and effects of medication. Idiopathic hypersomnia and narcolepsy are uncommon causes of hypersomnia and usually diagnosed in a sleep laboratory setting, she said.
In patients with depression, hypersomnia looks like patients having “nonimperative sleepiness,” Dr. Benca said. “They may spend a lot of time in bed; they may report long and nonrefreshing naps or long sleep time.”
There also is an issue with sleep inertia in patients with depression and hypersomnia, and with patients taking a long time to wake up and begin their day. In these patients, “when we put them in the sleep laboratory, the objective studies generally do not show that they are excessively sleepy, despite their reports of subjectively being sleepy,” she said.
There is not much objective MSLT or subjective measure data for hypersomnia in patients with schizophrenia despite these patients reporting daytime sleepiness or hypersomnolence, Dr. Benca admitted. Hypersomnia in patients with schizophrenia may be related to drug effects, poor sleep hygiene, circadian rhythm abnormalities, or comorbid sleep disorders. “Excessive sleepiness may also be related to the schizophrenia itself,” she said.
Treatments for hypersomnia
The first priority for patients with hypersomnia is to avoid sleep deprivation and practice good sleep hygiene – factors that are important both in insomnia and hypersomnia. “Make sure that patients are having adequate time in bed and having regular hours of sleep,” Dr. Benca said.
For patients with comorbid psychiatric, medical and sleep disorders, focus on getting rid of medications that may cause sleepiness, including sedating medications and antidepressants, and consider using stimulants if appropriate. While there are Food and Drug Administration–approved medications for narcolepsy and some are approved for hypersomnia in patients with obstructive sleep apnea (OSA), none are officially approved to treat hypersomnia in psychiatric patients.
“Whenever we use these drugs for those reasons, we’re using them off label,” Dr. Benca said.
Modafinil/armodafinil, approved for narcolepsy, shift-work disorder, and OSA in Ehlers-Danlos syndrome, is one off-label option for patients with hypersomnia. “They are lower potency and less addictive than the amphetamines, [with] fewer side effects,” Dr. Benca explained, but should be prescribed with caution in some women because of potential reduced efficacy of oral contraceptives. Side effects of modafinil include headache, nausea, eosinophilia, diarrhea, dry mouth, and anorexia.
Methylphenidate is another option for hypersomnia, available in racemic mixture, pure D-isomer, and time-release formulations.
Patients taking methylphenidate may experience nervousness, insomnia, anorexia, nausea, dizziness, hypertension, hypotension, hypersensitivity reactions, tachycardia, and headache as side effects.
For patients with central nervous system hypersomnias, amphetamines can be used, with methamphetamines having a “very similar profile” and similar side effects, including insomnia, restlessness, tachycardia, dizziness, diarrhea, constipation, hypertension, impotence, and rare cases of psychotic episodes.
Practice parameters released by the American Academy of Sleep Medicine in 2007 suggest that modafinil may have efficacy in idiopathic hypersomnia, Parkinson’s disease, myotonic dystrophy, and multiple sclerosis. The practice parameters also suggest hypersomnias of central origin can be treated with modafinil, amphetamine, methamphetamine, dextroamphetamine, and methylphenidate based on evidence or “long history of use” (Sleep. 2007;30:1705-11).
“Interestingly, there is no mention of psychiatric disorders in these practice parameters, and they report that there are mixed results using stimulants off label for sleepiness and fatigue in traumatic brain injury and poststroke fatigue,” Dr. Benca said.
Dr. Benca reported that she is a consultant to Eisai, Idorsia, Jazz, Merck, and Sunovion. Global Academy and this news organization are owned by the same parent company.
FROM PSYCHOPHARMACOLOGY UPDATE
Biometric changes on fitness trackers, smartwatches detect COVID-19
A smartphone app that combines passively collected physiologic data from wearable devices, such as fitness trackers, and self-reported symptoms can discriminate between COVID-19–positive and –negative individuals among those who report symptoms, new data suggest.
After analyzing data from more than 30,000 participants, researchers from the Digital Engagement and Tracking for Early Control and Treatment (DETECT) study concluded that adding individual changes in sensor data improves models based on symptoms alone for differentiating symptomatic persons who are COVID-19 positive and symptomatic persons who are COVID-19 negative.
The combination can potentially identify infection clusters before wider community spread occurs, Giorgio Quer, PhD, and colleagues report in an article published online Oct. 29 in Nature Medicine. DETECT investigators note that marrying participant-reported symptoms with personal sensor data, such as deviation from normal sleep duration and resting heart rate, resulted in an area under the curve (AUC) of 0.80 (interquartile range [IQR], 0.73-0.86) for differentiating between symptomatic individuals who were positive and those who were negative for COVID-19.
“By better characterizing each individual’s unique baseline, you can then identify changes that may indicate that someone has a viral illness,” said Dr. Quer, director of artificial intelligence at Scripps Research Translational Institute in La Jolla, Calif. “In previous research, we found that the proportion of individuals with elevated resting heart rate and sleep duration compared with their normal could significantly improve real-time detection of influenza-like illness rates at the state level,” he said in an interview.
Thus, continuous passively captured data may be a useful adjunct to bricks-and-mortar site testing, which is generally a one-off or infrequent sampling assay and is not always easily accessible, he added. Furthermore, traditional screening with temperature and symptom reporting is inadequate. An elevation in temperature is not as common as frequently believed for people who test positive for COVID-19, Dr. Quer continued. “Early identification via sensor variables of those who are presymptomatic or even asymptomatic would be especially valuable, as people may potentially be infectious during this period, and early detection is the ultimate goal,” Dr. Quer said.
According to his group, adding these physiologic changes from baseline values significantly outperformed detection (P < .01) using a British model described in an earlier study by by Cristina Menni, PhD, and associates. That method, in which symptoms were considered alone, yielded an AUC of 0.71 (IQR, 0.63-0.79).
According to Dr. Quer, one in five Americans currently wear an electronic device. “If we could enroll even a small percentage of these individuals, we’d be able to potentially identify clusters before they have the opportunity to spread,” he said.
DETECT study details
During the period March 15 to June 7, 2020, the study enrolled 30,529 participants from all 50 states. They ranged in age from younger than 35 years (23.1%) to older than 65 years (12.8%); the majority (63.5%) were aged 35-65 years, and 62% were women. Sensor devices in use by the cohort included Fitbit activity trackers (78.4%) and Apple HealthKit (31.2%).
Participants downloaded an app called MyDataHelps, which collects smartwatch and activity tracker information, including self-reported symptoms and diagnostic testing results. The app also monitors changes from baseline in resting heart rate, sleep duration, and physical activity, as measured by steps.
Overall, 3,811 participants reported having at least one symptom of some kind (e.g., fatigue, cough, dyspnea, loss of taste or smell). Of these, 54 reported testing positive for COVID-19, and 279 reported testing negative.
Sleep and activity were significantly different for the positive and negative groups, with an AUC of 0.68 (IQR, 0.57-0.79) for the sleep metric and 0.69 (IQR, 0.61-0.77) for the activity metric, suggesting that these parameters were more affected in COVID-19–positive participants.
When the investigators combined resting heart rate, sleep, and activity into a single metric, predictive performance improved to an AUC of 0.72 (IQR, 0.64-0.80).
The next step, Dr. Quer said, is to include an alert to notify users of possible infection.
Alerting users to possible COVID-19 infection
In a similar study, an alert feature was already incorporated. The study, led by Michael P. Snyder, PhD, director of the Center for Genomics and Personalized Medicine at Stanford (Calif.) University, will soon be published online in Nature Biomedical Engineering. In that study, presymptomatic detection of COVID-19 was achieved in more than 80% of participants using resting heart rate.
“The median is 4 days prior to symptom formation,” Dr. Snyder said in an interview. “We have an alarm system to notify people when their heart rate is elevated. So a positive signal from a smartwatch can be used to follow up by polymerase chain reaction [testing].”
Dr. Snyder said these approaches offer a roadmap to containing widespread infections. “Public health authorities need to be open to these technologies and begin incorporating them into their tracking,” he said. “Right now, people do temperature checks, which are of limited value. Resting heart rate is much better information.”
Although the DETECT researchers have not yet received feedback on their results, they believe public health authorities could recommend the use of such apps. “These are devices that people routinely wear for tracking their fitness and sleep, so it would be relatively easy to use the data for viral illness tracking,” said co–lead author Jennifer Radin, PhD, an epidemiologist at Scripps. “Testing resources are still limited and don’t allow for routine serial testing of individuals who may be asymptomatic or presymptomatic. Wearables can offer a different way to routinely monitor and screen people for changes in their data that may indicate COVID-19.”
The marshaling of data through consumer digital platforms to fight the coronavirus is gaining ground. New York State and New Jersey are already embracing smartphone apps to alert individuals to possible exposure to the virus.
More than 710,000 New Yorkers have downloaded the COVID NY Alert app, launched in October to help protect individuals and communities from COVID-19 by sending alerts without compromising privacy or personal information. “Upon receiving a notification about a potential exposure, users are then able to self-quarantine, get tested, and reduce the potential exposure risk to family, friends, coworkers, and others,” Jonah Bruno, a spokesperson for the New York State Department of Health, said in an interview.
And recently the Mayo Clinic and Safe Health Systems launched a platform to store COVID-19 testing and vaccination data.
Both the Scripps and Stanford platforms are part of a global technologic response to the COVID-19 pandemic. Prospective studies, led by device manufacturers and academic institutions, allow individuals to voluntarily share sensor and clinical data to address the crisis. Similar approaches have been used to track COVID-19 in large populations in Germany via the Corona Data Donation app.
The study by Dr. Quer and colleagues was funded by a grant from the National Center for Advancing Translational Sciences at the National Institutes of Health. One coauthor reported grants from Janssen and personal fees from Otsuka and Livongo outside of the submitted work. The other authors have disclosed no relevant financial relationships. Dr. Snyder has ties to Personalis, Qbio, January, SensOmics, Protos, Mirvie, and Oralome.
A version of this article originally appeared on Medscape.com.
A smartphone app that combines passively collected physiologic data from wearable devices, such as fitness trackers, and self-reported symptoms can discriminate between COVID-19–positive and –negative individuals among those who report symptoms, new data suggest.
After analyzing data from more than 30,000 participants, researchers from the Digital Engagement and Tracking for Early Control and Treatment (DETECT) study concluded that adding individual changes in sensor data improves models based on symptoms alone for differentiating symptomatic persons who are COVID-19 positive and symptomatic persons who are COVID-19 negative.
The combination can potentially identify infection clusters before wider community spread occurs, Giorgio Quer, PhD, and colleagues report in an article published online Oct. 29 in Nature Medicine. DETECT investigators note that marrying participant-reported symptoms with personal sensor data, such as deviation from normal sleep duration and resting heart rate, resulted in an area under the curve (AUC) of 0.80 (interquartile range [IQR], 0.73-0.86) for differentiating between symptomatic individuals who were positive and those who were negative for COVID-19.
“By better characterizing each individual’s unique baseline, you can then identify changes that may indicate that someone has a viral illness,” said Dr. Quer, director of artificial intelligence at Scripps Research Translational Institute in La Jolla, Calif. “In previous research, we found that the proportion of individuals with elevated resting heart rate and sleep duration compared with their normal could significantly improve real-time detection of influenza-like illness rates at the state level,” he said in an interview.
Thus, continuous passively captured data may be a useful adjunct to bricks-and-mortar site testing, which is generally a one-off or infrequent sampling assay and is not always easily accessible, he added. Furthermore, traditional screening with temperature and symptom reporting is inadequate. An elevation in temperature is not as common as frequently believed for people who test positive for COVID-19, Dr. Quer continued. “Early identification via sensor variables of those who are presymptomatic or even asymptomatic would be especially valuable, as people may potentially be infectious during this period, and early detection is the ultimate goal,” Dr. Quer said.
According to his group, adding these physiologic changes from baseline values significantly outperformed detection (P < .01) using a British model described in an earlier study by by Cristina Menni, PhD, and associates. That method, in which symptoms were considered alone, yielded an AUC of 0.71 (IQR, 0.63-0.79).
According to Dr. Quer, one in five Americans currently wear an electronic device. “If we could enroll even a small percentage of these individuals, we’d be able to potentially identify clusters before they have the opportunity to spread,” he said.
DETECT study details
During the period March 15 to June 7, 2020, the study enrolled 30,529 participants from all 50 states. They ranged in age from younger than 35 years (23.1%) to older than 65 years (12.8%); the majority (63.5%) were aged 35-65 years, and 62% were women. Sensor devices in use by the cohort included Fitbit activity trackers (78.4%) and Apple HealthKit (31.2%).
Participants downloaded an app called MyDataHelps, which collects smartwatch and activity tracker information, including self-reported symptoms and diagnostic testing results. The app also monitors changes from baseline in resting heart rate, sleep duration, and physical activity, as measured by steps.
Overall, 3,811 participants reported having at least one symptom of some kind (e.g., fatigue, cough, dyspnea, loss of taste or smell). Of these, 54 reported testing positive for COVID-19, and 279 reported testing negative.
Sleep and activity were significantly different for the positive and negative groups, with an AUC of 0.68 (IQR, 0.57-0.79) for the sleep metric and 0.69 (IQR, 0.61-0.77) for the activity metric, suggesting that these parameters were more affected in COVID-19–positive participants.
When the investigators combined resting heart rate, sleep, and activity into a single metric, predictive performance improved to an AUC of 0.72 (IQR, 0.64-0.80).
The next step, Dr. Quer said, is to include an alert to notify users of possible infection.
Alerting users to possible COVID-19 infection
In a similar study, an alert feature was already incorporated. The study, led by Michael P. Snyder, PhD, director of the Center for Genomics and Personalized Medicine at Stanford (Calif.) University, will soon be published online in Nature Biomedical Engineering. In that study, presymptomatic detection of COVID-19 was achieved in more than 80% of participants using resting heart rate.
“The median is 4 days prior to symptom formation,” Dr. Snyder said in an interview. “We have an alarm system to notify people when their heart rate is elevated. So a positive signal from a smartwatch can be used to follow up by polymerase chain reaction [testing].”
Dr. Snyder said these approaches offer a roadmap to containing widespread infections. “Public health authorities need to be open to these technologies and begin incorporating them into their tracking,” he said. “Right now, people do temperature checks, which are of limited value. Resting heart rate is much better information.”
Although the DETECT researchers have not yet received feedback on their results, they believe public health authorities could recommend the use of such apps. “These are devices that people routinely wear for tracking their fitness and sleep, so it would be relatively easy to use the data for viral illness tracking,” said co–lead author Jennifer Radin, PhD, an epidemiologist at Scripps. “Testing resources are still limited and don’t allow for routine serial testing of individuals who may be asymptomatic or presymptomatic. Wearables can offer a different way to routinely monitor and screen people for changes in their data that may indicate COVID-19.”
The marshaling of data through consumer digital platforms to fight the coronavirus is gaining ground. New York State and New Jersey are already embracing smartphone apps to alert individuals to possible exposure to the virus.
More than 710,000 New Yorkers have downloaded the COVID NY Alert app, launched in October to help protect individuals and communities from COVID-19 by sending alerts without compromising privacy or personal information. “Upon receiving a notification about a potential exposure, users are then able to self-quarantine, get tested, and reduce the potential exposure risk to family, friends, coworkers, and others,” Jonah Bruno, a spokesperson for the New York State Department of Health, said in an interview.
And recently the Mayo Clinic and Safe Health Systems launched a platform to store COVID-19 testing and vaccination data.
Both the Scripps and Stanford platforms are part of a global technologic response to the COVID-19 pandemic. Prospective studies, led by device manufacturers and academic institutions, allow individuals to voluntarily share sensor and clinical data to address the crisis. Similar approaches have been used to track COVID-19 in large populations in Germany via the Corona Data Donation app.
The study by Dr. Quer and colleagues was funded by a grant from the National Center for Advancing Translational Sciences at the National Institutes of Health. One coauthor reported grants from Janssen and personal fees from Otsuka and Livongo outside of the submitted work. The other authors have disclosed no relevant financial relationships. Dr. Snyder has ties to Personalis, Qbio, January, SensOmics, Protos, Mirvie, and Oralome.
A version of this article originally appeared on Medscape.com.
A smartphone app that combines passively collected physiologic data from wearable devices, such as fitness trackers, and self-reported symptoms can discriminate between COVID-19–positive and –negative individuals among those who report symptoms, new data suggest.
After analyzing data from more than 30,000 participants, researchers from the Digital Engagement and Tracking for Early Control and Treatment (DETECT) study concluded that adding individual changes in sensor data improves models based on symptoms alone for differentiating symptomatic persons who are COVID-19 positive and symptomatic persons who are COVID-19 negative.
The combination can potentially identify infection clusters before wider community spread occurs, Giorgio Quer, PhD, and colleagues report in an article published online Oct. 29 in Nature Medicine. DETECT investigators note that marrying participant-reported symptoms with personal sensor data, such as deviation from normal sleep duration and resting heart rate, resulted in an area under the curve (AUC) of 0.80 (interquartile range [IQR], 0.73-0.86) for differentiating between symptomatic individuals who were positive and those who were negative for COVID-19.
“By better characterizing each individual’s unique baseline, you can then identify changes that may indicate that someone has a viral illness,” said Dr. Quer, director of artificial intelligence at Scripps Research Translational Institute in La Jolla, Calif. “In previous research, we found that the proportion of individuals with elevated resting heart rate and sleep duration compared with their normal could significantly improve real-time detection of influenza-like illness rates at the state level,” he said in an interview.
Thus, continuous passively captured data may be a useful adjunct to bricks-and-mortar site testing, which is generally a one-off or infrequent sampling assay and is not always easily accessible, he added. Furthermore, traditional screening with temperature and symptom reporting is inadequate. An elevation in temperature is not as common as frequently believed for people who test positive for COVID-19, Dr. Quer continued. “Early identification via sensor variables of those who are presymptomatic or even asymptomatic would be especially valuable, as people may potentially be infectious during this period, and early detection is the ultimate goal,” Dr. Quer said.
According to his group, adding these physiologic changes from baseline values significantly outperformed detection (P < .01) using a British model described in an earlier study by by Cristina Menni, PhD, and associates. That method, in which symptoms were considered alone, yielded an AUC of 0.71 (IQR, 0.63-0.79).
According to Dr. Quer, one in five Americans currently wear an electronic device. “If we could enroll even a small percentage of these individuals, we’d be able to potentially identify clusters before they have the opportunity to spread,” he said.
DETECT study details
During the period March 15 to June 7, 2020, the study enrolled 30,529 participants from all 50 states. They ranged in age from younger than 35 years (23.1%) to older than 65 years (12.8%); the majority (63.5%) were aged 35-65 years, and 62% were women. Sensor devices in use by the cohort included Fitbit activity trackers (78.4%) and Apple HealthKit (31.2%).
Participants downloaded an app called MyDataHelps, which collects smartwatch and activity tracker information, including self-reported symptoms and diagnostic testing results. The app also monitors changes from baseline in resting heart rate, sleep duration, and physical activity, as measured by steps.
Overall, 3,811 participants reported having at least one symptom of some kind (e.g., fatigue, cough, dyspnea, loss of taste or smell). Of these, 54 reported testing positive for COVID-19, and 279 reported testing negative.
Sleep and activity were significantly different for the positive and negative groups, with an AUC of 0.68 (IQR, 0.57-0.79) for the sleep metric and 0.69 (IQR, 0.61-0.77) for the activity metric, suggesting that these parameters were more affected in COVID-19–positive participants.
When the investigators combined resting heart rate, sleep, and activity into a single metric, predictive performance improved to an AUC of 0.72 (IQR, 0.64-0.80).
The next step, Dr. Quer said, is to include an alert to notify users of possible infection.
Alerting users to possible COVID-19 infection
In a similar study, an alert feature was already incorporated. The study, led by Michael P. Snyder, PhD, director of the Center for Genomics and Personalized Medicine at Stanford (Calif.) University, will soon be published online in Nature Biomedical Engineering. In that study, presymptomatic detection of COVID-19 was achieved in more than 80% of participants using resting heart rate.
“The median is 4 days prior to symptom formation,” Dr. Snyder said in an interview. “We have an alarm system to notify people when their heart rate is elevated. So a positive signal from a smartwatch can be used to follow up by polymerase chain reaction [testing].”
Dr. Snyder said these approaches offer a roadmap to containing widespread infections. “Public health authorities need to be open to these technologies and begin incorporating them into their tracking,” he said. “Right now, people do temperature checks, which are of limited value. Resting heart rate is much better information.”
Although the DETECT researchers have not yet received feedback on their results, they believe public health authorities could recommend the use of such apps. “These are devices that people routinely wear for tracking their fitness and sleep, so it would be relatively easy to use the data for viral illness tracking,” said co–lead author Jennifer Radin, PhD, an epidemiologist at Scripps. “Testing resources are still limited and don’t allow for routine serial testing of individuals who may be asymptomatic or presymptomatic. Wearables can offer a different way to routinely monitor and screen people for changes in their data that may indicate COVID-19.”
The marshaling of data through consumer digital platforms to fight the coronavirus is gaining ground. New York State and New Jersey are already embracing smartphone apps to alert individuals to possible exposure to the virus.
More than 710,000 New Yorkers have downloaded the COVID NY Alert app, launched in October to help protect individuals and communities from COVID-19 by sending alerts without compromising privacy or personal information. “Upon receiving a notification about a potential exposure, users are then able to self-quarantine, get tested, and reduce the potential exposure risk to family, friends, coworkers, and others,” Jonah Bruno, a spokesperson for the New York State Department of Health, said in an interview.
And recently the Mayo Clinic and Safe Health Systems launched a platform to store COVID-19 testing and vaccination data.
Both the Scripps and Stanford platforms are part of a global technologic response to the COVID-19 pandemic. Prospective studies, led by device manufacturers and academic institutions, allow individuals to voluntarily share sensor and clinical data to address the crisis. Similar approaches have been used to track COVID-19 in large populations in Germany via the Corona Data Donation app.
The study by Dr. Quer and colleagues was funded by a grant from the National Center for Advancing Translational Sciences at the National Institutes of Health. One coauthor reported grants from Janssen and personal fees from Otsuka and Livongo outside of the submitted work. The other authors have disclosed no relevant financial relationships. Dr. Snyder has ties to Personalis, Qbio, January, SensOmics, Protos, Mirvie, and Oralome.
A version of this article originally appeared on Medscape.com.
Lemborexant for insomnia
Lemborexant, FDA-approved for the treatment of insomnia, has demonstrated efficacy in improving both sleep onset and sleep maintenance.1 This novel compound is now the second approved insomnia medication classed as a dual orexin receptor antagonist (Table 1). This targeted mechanism of action aims to enhance sleep while limiting the adverse effects associated with traditional hypnotics.
Clinical implications
Insomnia symptoms affect approximately one-third of the general population at least occasionally. Approximately 10% of individuals meet DSM-5 criteria for insomnia disorder, which require nighttime sleep difficulty and daytime consequences persisting for a minimum of 3 months.2 The prevalence is considerably higher in patients with chronic medical disorders and comorbid psychiatric conditions, especially mood, anxiety, substance use, and stress- and trauma-related disorders. Clinical guidelines for treating insomnia disorder typically recommend cognitive-behavioral therapy for insomnia as a first choice and FDA-approved insomnia medications as secondary options.3
Currently approved insomnia medications fall into 4 distinct pharmacodynamics categories.4 Benzodiazepine receptor agonist hypnotics include 5 medications with classic benzodiazepine structures (estazolam, flurazepam, quazepam, temazepam, and triazolam) and 3 compounds (eszopiclone, zaleplon, and zolpidem) with alternate structures but similar mechanisms of action. There is 1 melatonin receptor agonist (ramelteon) and 1 histamine receptor antagonist (low-dose doxepin). Joining suvorexant (approved in 2014), lemborexant is the second dual orexin receptor antagonist.
The orexin (also called hypocretin) system was first described in 1998 and its fundamental role in promoting and coordinating wakefulness was quickly established.5 A relatively small number of hypothalamic neurons located in the lateral and perifornical regions produce 2 similar orexin neuropeptides (orexin A and orexin B) with widespread distributions, notably reinforcing the wake-promoting activity of histamine, acetylcholine, dopamine, serotonin, and norepinephrine. Consistent with the typical sleep-wake cycle, orexin release is limited during the nighttime. The orexin neuropeptides interact with 2 G-protein-coupled orexin receptors (OX1R, OX2R).
Animal studies showed that impairment in orexin system activity was associated with symptoms characteristic of narcolepsy, including cataplexy and excessive sleep episodes. Soon after, it was found that humans diagnosed with narcolepsy with cataplexy had markedly low CSF orexin levels.6 This recognition that excessively sleepy people with narcolepsy had a profound decrease in orexin production led to the hypothesis that pharmacologically decreasing orexin activity might be sleep-enhancing for insomnia patients, who presumably are excessively aroused. Numerous compounds soon were evaluated for their potential as orexin receptor antagonists. The efficacy of treating insomnia with a dual orexin receptor antagonist in humans was first reported in 2007 with almorexant, a compound that remains investigational.7 Research continues to investigate both single and dual orexin antagonist molecules for insomnia and other potential indications.
How it works
Unlike most hypnotics, which have widespread CNS depressant effects, lemborexant has a more targeted action in promoting sleep by suppressing the wake drive supported by the orexin system.8 Lemborexant is highly selective for the OX1R and OX2R orexin receptors, where it functions as a competitive antagonist. It is hypothesized that by modulating orexin activity with a receptor antagonist, excessive arousal associated with insomnia can be reduced, thus improving nighttime sleep. The pharmacokinetic properties allow benefits for both sleep onset and maintenance.
Pharmacokinetics
Lemborexant is available in immediate-release tablets with a peak concentration time (Tmax) of approximately 1 to 3 hours after ingestion. When taken after a high-fat and high-calorie meal, there is a delay in the Tmax, a decrease in the maximum plasma concentration (Cmax), and an increase in the concentration area under the curve (AUC0-inf).1
Continue to: Metabolism is primarily through...
Metabolism is primarily through the cytochrome P450 (CYP) 3A4 pathway, and to a lesser extent through CYP3A5. Concomitant use with moderate or strong CYP3A inhibitors or inducers should be avoided, while use with weak CYP3A inhibitors should be limited to the 5-mg dose of lemborexant.
Lemborexant has the potential to induce the metabolism of CYP2B6 substrates, such as bupropion and methadone, possibly leading to reduced efficacy for these medications. Accordingly, the clinical responses to any CYP2B6 substrates should be monitored and dosage adjustments considered.
Concomitant use of lemborexant with alcohol should be avoided because there may be increased impairment in postural stability and memory, in part due to increases in the medication’s Cmax and AUC, as well as the direct effects of alcohol.
Efficacy
In randomized, placebo-controlled trials, lemborexant demonstrated both objective and subjective evidence of clinically significant benefits for sleep onset and sleep maintenance in patients diagnosed with insomnia disorder.1 The 2 pivotal efficacy studies were:
- Sunrise 1, a 4-week trial with older adults that included laboratory polysomnography (PSG) studies (objective) and patient-reported sleep measures (subjective) on selected nights9
- Sunrise 2, a 6-month trial assessing patient-reported sleep characteristics in adults and older adults.10
Sunrise 1 was performed with older adults with insomnia who were randomized to groups with nightly use of lemborexant, 5 mg (n = 266), lemborexant, 10 mg (n = 269), zolpidem extended-release, 6.25 mg, as an active comparator (n = 263), or placebo (n = 208).9 The age range was 55 to 88 years with a median age of 63 years. Most patients (86.4%) were women. Because this study focused on the assessment of efficacy for treating sleep maintenance difficulty, the inclusion criteria required a subjective report of experiencing a wake time after sleep onset (sWASO) of at least 60 minutes for 3 or more nights per week over the previous 4 weeks. The zolpidem extended-release 6.25 mg comparison was chosen because it has an indication for sleep maintenance insomnia with this recommended dose for older adults.
Continue to: Laboratory PSG monitoring...
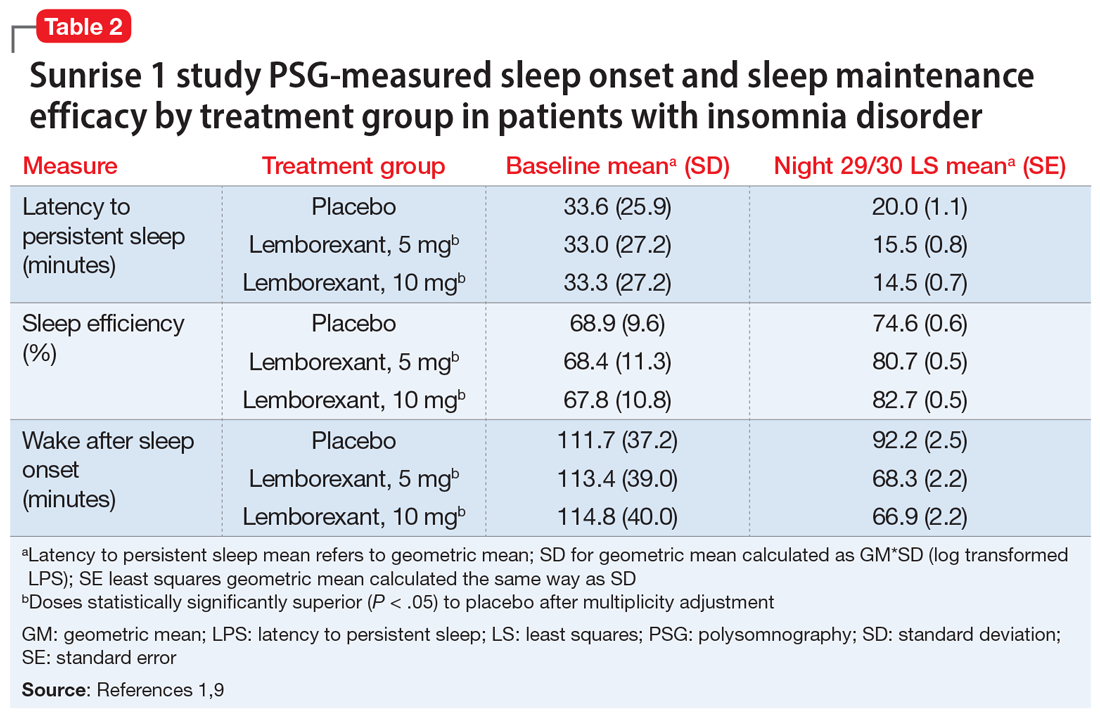
Laboratory PSG monitoring was performed for 2 consecutive nights at baseline (before treatment), the first 2 treatment nights, and the final 2 treatment nights (Nights 29 and 30). The primary study endpoint was the change in latency to persistent sleep (LPS) from baseline to the final 2 nights for the lemborexant doses compared with placebo. Additional PSG-based endpoints were similar comparisons for sleep efficiency (percent time asleep during the 8-hour laboratory recording period) and objective wake after sleep onset (WASO) compared with placebo, and WASO during the second half of the night (WASO2H) compared with zolpidem. Patients completed Insomnia Severity Index (ISI) questionnaires at baseline and the end of the treatment to compare disease severity. Subjective assessments were done daily with electronic diary entries that included sleep onset latency (sSOL), sWASO, and subjective sleep efficiency.
In comparison with placebo, both lemborexant doses were associated with significantly improved PSG measures of LPS, WASO, and sleep efficiency during nights 1 and 2 that were maintained through Nights 29 and 30 (Table 21,9). The lemborexant doses also demonstrated significant improvements in WASO2H compared with zolpidem and placebo on the first 2 and final 2 treatment nights. Analyses of the subjective assessments (sSOL, sWASO, and sleep efficiency) compared the baseline with means for the first and the last treatment weeks. At both lemborexant doses, the sSOL was significantly reduced during the first and last weeks compared with placebo and zolpidem. Subjective sleep efficiency was significantly improved at both time points for the lemborexant doses, though these were not significantly different from the zolpidem values. The sWASO values were significantly decreased for both lemborexant doses at both time points compared with placebo. During the first treatment week, both lemborexant doses did not differ significantly from zolpidem in the sWASO change from baseline; however, at the final treatment week, the zolpidem value was significantly improved compared with lemborexant, 5 mg, but not significantly different from lemborexant, 10 mg. The ISI change from baseline to the end of the treatment period showed significant improvement for the lemborexant doses and zolpidem extended-release compared with placebo.
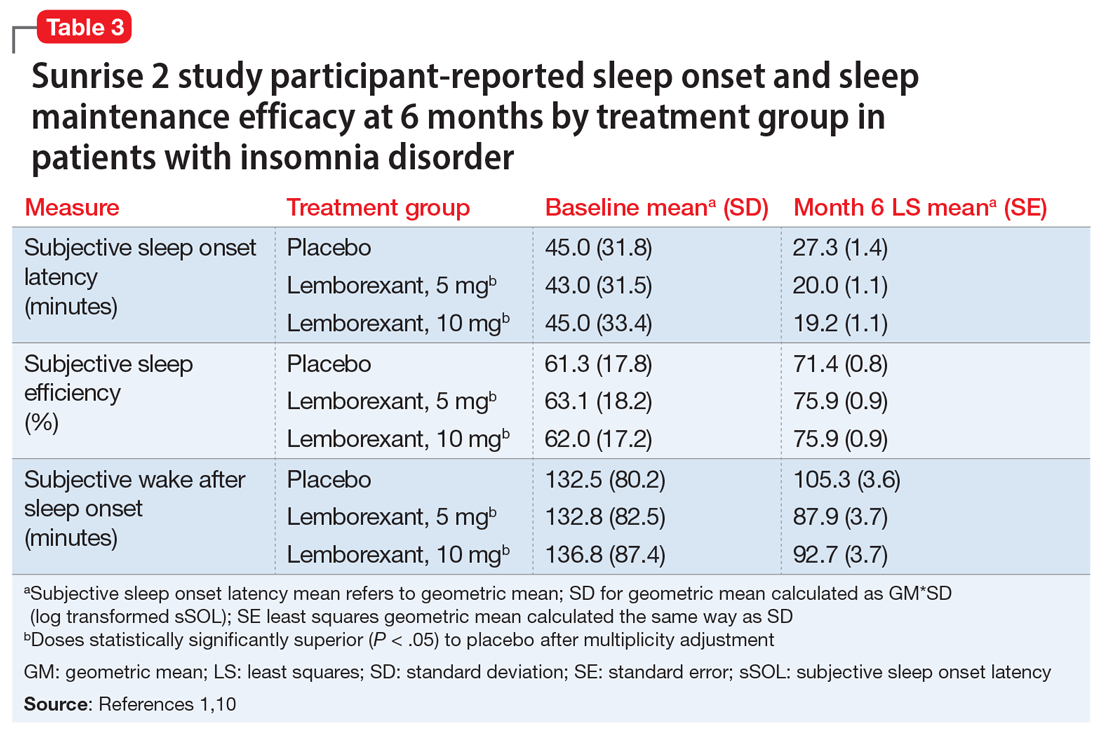
In the Sunrise 2 study, patients who met the criteria for insomnia disorder (age range 18 to 88, mean 55; 68% female) were randomized to groups taking nightly doses of lemborexant, 5 mg (n = 323), lemborexant, 10 mg (n = 323), or placebo (n = 325) for 6 months.10 Inclusion criteria required an sSOL of at least 30 minutes and/or a sWASO of at least 60 minutes 3 times a week or more during the previous 4 weeks. Efficacy was assessed with daily electronic diary entries, with analyses of change from baseline for sSOL (primary endpoint, baseline to end of 6-month study period), sWASO, and patient-reported sleep efficiency (sSEF). Subjective total sleep time (sTST) represented the estimated time asleep during the time in bed. Additional diary assessments related to sleep quality and morning alertness. All of these subjective assessments were compared as 7-day means for the first week of treatment and the last week of each treatment month.
The superiority of lemborexant, 5 mg and 10 mg, compared with placebo was demonstrated by significant improvements in sSOL, sSEF, sWASO, and sTST during the initial week of the treatment period that remained significant at the end of the 6-month placebo-controlled period (Table 31,10). At the end of 6 months, there were significantly more sleep-onset responders and sleep-maintenance responders among patients taking lemborexant compared with those taking placebo. Sleep-onset responders were patients with a baseline sSOL >30 minutes and a mean sSOL ≤20 minutes at the end of the study. Sleep-maintenance responders were participants with a baseline sWASO >60 minutes who at the end of the study had a mean sWASO ≤60 minutes that included a reduction of at least 10 minutes.
Following the 6-month placebo-controlled treatment period, the Sunrise 2 study continued for an additional 6 months of nightly active treatment for continued safety and efficacy assessment. Patients assigned to lemborexant, 5 mg or 10 mg, during the initial period continued on those doses. Those in the placebo group were randomized to either of the 2 lemborexant doses.
Continue to: Safety studies and adverse reactions
Safety studies and adverse reactions
Potential medication effects on middle-of-the-night and next-morning postural stability (body sway measured with an ataxiameter) and cognitive performance, as well as middle-of-the-night auditory awakening threshold, were assessed in a randomized, 4-way crossover study of 56 healthy older adults (women age ≥55 [77.8%], men age ≥65) given a single bedtime dose of placebo, lemborexant, 5 mg, lemborexant, 10 mg, and zolpidem extended-release, 6.25 mg, on separate nights.11 The results were compared with data from a baseline night with the same measures performed prior to the randomization. The middle-of-the-night assessments were done approximately 4 hours after the dose and the next-morning measures were done after 8 hours in bed. The auditory threshold analysis showed no significant differences among the 4 study nights. Compared with placebo, the middle-of-the-night postural stability was significantly worse for both lemborexant doses and zolpidem; however, the zolpidem effect was significantly worse than with either lemborexant dose. The next-morning postural stability measures showed no significant difference from placebo for the lemborexant doses, but zolpidem continued to show a significantly worsened result. The cognitive performance assessment battery provided 4 domain factor scores (power of attention, continuity of attention, quality of memory, and speed of memory retrieval). The middle-of-the-night battery showed no significant difference between lemborexant, 5 mg, and placebo in any domain, while both lemborexant, 10 mg, and zolpidem showed worse performance on some of the attention and/or memory tests. The next-morning cognitive assessment revealed no significant differences from placebo for the treatments.
Respiratory safety was examined in a placebo-controlled, 2-period crossover study of 38 patients diagnosed with mild obstructive sleep apnea who received lemborexant, 10 mg, or placebo nightly during each 8-day period.12 Neither the apnea-hypopnea index nor the mean oxygen saturation during the lemborexant nights were significantly different from the placebo nights.
The most common adverse reaction during the month-long Sunrise 1 study and the first 30 days of the Sunrise 2 study was somnolence or fatigue, which occurred in 1% receiving placebo, 7% receiving lemborexant, 5 mg, and 10% receiving lemborexant, 10 mg. Headache was reported by 3.5% receiving placebo, 5.9% receiving lemborexant, 5 mg, and 4.5% receiving lemborexant, 10 mg. Nightmare or abnormal dreams occurred with 0.9% receiving placebo, 0.9% receiving lemborexant, 5 mg, and 2.2% receiving lemborexant, 10 mg.1
Unique clinical issues
Because investigations of individuals who abused sedatives for recreational purposes showed lemborexant had a likeability rating similar to zolpidem and significantly greater than placebo, the US Drug Enforcement Agency has categorized lemborexant as a Schedule IV controlled substance. Research has not shown evidence of physical dependence or withdrawal signs or symptoms upon discontinuation of lemborexant.1
Contraindications
Narcolepsy is the only contraindication to the use of lemborexant.1 Narcolepsy is associated with a decrease in the orexin-producing neurons in the hypothalamus, presumably causing the excessive sleepiness, sleep paralysis, hypnagogic hallucinations, and cataplexy characteristic of the disorder. Hypothetically, an orexin antagonist medication could exacerbate these symptoms.
Continue to: Dosing
Dosing
Lemborexant should be taken no more than once per night immediately before going to bed and with at least 7 hours remaining before the planned time of awakening.1 The recommended starting dose is 5 mg. The dosage may be increased to a maximum of 10 mg if the initial dose is well tolerated but insufficiently effective. Patients with moderate hepatic impairment or who are concomitantly taking weak CYP3A inhibitors should receive a maximum of 5 mg once nightly. Lemborexant should be avoided in patients with severe hepatic impairment and in those taking moderate or strong CYP3A inhibitors or inducers.
Orexin receptor antagonists do not share cross-tolerance with other hypnotics; this should be taken into consideration when switching to lemborexant. Abruptly stopping a benzodiazepine receptor agonist hypnotic may lead to rebound insomnia and thus may confound the interpretation of the clinical response when starting lemborexant.
Patients prescribed lemborexant should be educated about possible impairment in alertness and motor coordination, especially with the 10-mg dose, which may affect next-morning driving in sensitive individuals.13 Caution is advised with doses >5 mg in patients age ≥65 due to possible somnolence and a higher risk of falls.1
Bottom Line
Lemborexant is a dual orexin receptor antagonist indicated for the treatment of insomnia characterized by difficulties with sleep onset and/or sleep maintenance. It promotes sleep by suppressing the wake drive supported by the orexin system. In randomized, placebo-controlled trials, lemborexant demonstrated objective and subjective evidence of clinically significant benefits for sleep onset and sleep maintenance.
Related Resource
- Sateia MJ, Buysse DJ, Krystal AD, et al. Clinical practice guideline for the pharmacologic treatment of chronic insomnia in adults: an American Academy of Sleep Medicine clinical practice guideline. J Clin Sleep Med. 2017;13(2):307-349.
Drug Brand Names
Bupropion • Wellbutrin
Doxepin • Silenor
Eszopiclone • Lunesta
Lemborexant • Dayvigo
Methadone • Methadose, Dolophine
Quazepam • Doral
Ramelteon • Rozerem
Suvorexant • Belsomra
Temazepam • Restoril
Triazolam • Halcion
Zaleplon • Sonata
Zolpidem • Ambien, Intermezzo
1. Dayvigo [package insert]. Woodcliff Lake, NJ: Eisai Inc.; 2020.
2. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
3. Qaseem A, Kansagara D, Forciea MA, et al; Clinical Guidelines Committee of the American College of Physicians. Management of chronic insomnia disorder in adults: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2016;165(2):125-133.
4. Neubauer DN, Pandi-Perumal SR, Spence DW, et al. Pharmacotherapy of insomnia. J Cent Nerv Syst Dis. 2018;10:1179573518770672. doi: 10.1177/1179573518770672.
5. Saper CB, Chou TC, Scammell TE. The sleep switch: hypothalamic control of sleep and wakefulness. Trends Neurosci. 2001;24(12):726-731.
6. Mignot E. Sleep, sleep disorders and hypocretin (orexin). Sleep Med. 2004;5(suppl 1):S2-S8.
7. Boss C, Brisbare-Roch C, Jenck F, et al. Orexin receptor antagonism: a new principle in neuroscience. Chimia. 2008;62:974-979.
8. Landry I, Nakai K, Ferry J, et al. Pharmacokinetics, pharmacodynamics, and safety of the dual orexin receptor antagonist lemborexant: findings from single-dose and multiple-ascending-dose phase 1 studies in healthy adults. Clin Pharmacol Drug Dev. 2020. doi: 10.1002/cpdd.817.
9. Rosenberg R, Murphy P, Zammit G, et al. Comparison of lemborexant with placebo and zolpidem tartrate extended release for the treatment of older adults with insomnia disorder: a phase 3 randomized clinical trial. JAMA Netw Open. 2019;2(12):e1918254. doi: 10.1001/jamanetworkopen.2019.18254.
10. Karppa M, Yardley J, Pinner K, et al. Long-term efficacy and tolerability of lemborexant compared with placebo in adults with insomnia disorder: results from the phase 3 randomized clinical trial SUNRISE 2. Sleep. 2020;43(9):zsaa123. doi: 10.1093/sleep/zsaa123.
11. Murphy P, Kumar D, Zammit G, et al. Safety of lemborexant versus placebo and zolpidem: effects on auditory awakening threshold, postural stability, and cognitive performance in healthy older participants in the middle of the night and upon morning awakening. J Clin Sleep Med. 2020;16(5):765-773.
12. Cheng JY, Filippov G, Moline M, et al. Respiratory safety of lemborexant in healthy adult and elderly subjects with mild obstructive sleep apnea: a randomized, double-blind, placebo-controlled, crossover study. J Sleep Res. 2020:e13021. doi: 10.1111/jsr.13021.
13. Vermeeren A, Jongen S, Murphy P, et al. On-the-road driving performance the morning after bedtime administration of lemborexant in healthy adult and elderly volunteers. Sleep. 2019;42(4):10.1093/sleep/zsy260. doi: 10.1093/sleep/zsy260.
Lemborexant, FDA-approved for the treatment of insomnia, has demonstrated efficacy in improving both sleep onset and sleep maintenance.1 This novel compound is now the second approved insomnia medication classed as a dual orexin receptor antagonist (Table 1). This targeted mechanism of action aims to enhance sleep while limiting the adverse effects associated with traditional hypnotics.
Clinical implications
Insomnia symptoms affect approximately one-third of the general population at least occasionally. Approximately 10% of individuals meet DSM-5 criteria for insomnia disorder, which require nighttime sleep difficulty and daytime consequences persisting for a minimum of 3 months.2 The prevalence is considerably higher in patients with chronic medical disorders and comorbid psychiatric conditions, especially mood, anxiety, substance use, and stress- and trauma-related disorders. Clinical guidelines for treating insomnia disorder typically recommend cognitive-behavioral therapy for insomnia as a first choice and FDA-approved insomnia medications as secondary options.3
Currently approved insomnia medications fall into 4 distinct pharmacodynamics categories.4 Benzodiazepine receptor agonist hypnotics include 5 medications with classic benzodiazepine structures (estazolam, flurazepam, quazepam, temazepam, and triazolam) and 3 compounds (eszopiclone, zaleplon, and zolpidem) with alternate structures but similar mechanisms of action. There is 1 melatonin receptor agonist (ramelteon) and 1 histamine receptor antagonist (low-dose doxepin). Joining suvorexant (approved in 2014), lemborexant is the second dual orexin receptor antagonist.
The orexin (also called hypocretin) system was first described in 1998 and its fundamental role in promoting and coordinating wakefulness was quickly established.5 A relatively small number of hypothalamic neurons located in the lateral and perifornical regions produce 2 similar orexin neuropeptides (orexin A and orexin B) with widespread distributions, notably reinforcing the wake-promoting activity of histamine, acetylcholine, dopamine, serotonin, and norepinephrine. Consistent with the typical sleep-wake cycle, orexin release is limited during the nighttime. The orexin neuropeptides interact with 2 G-protein-coupled orexin receptors (OX1R, OX2R).
Animal studies showed that impairment in orexin system activity was associated with symptoms characteristic of narcolepsy, including cataplexy and excessive sleep episodes. Soon after, it was found that humans diagnosed with narcolepsy with cataplexy had markedly low CSF orexin levels.6 This recognition that excessively sleepy people with narcolepsy had a profound decrease in orexin production led to the hypothesis that pharmacologically decreasing orexin activity might be sleep-enhancing for insomnia patients, who presumably are excessively aroused. Numerous compounds soon were evaluated for their potential as orexin receptor antagonists. The efficacy of treating insomnia with a dual orexin receptor antagonist in humans was first reported in 2007 with almorexant, a compound that remains investigational.7 Research continues to investigate both single and dual orexin antagonist molecules for insomnia and other potential indications.
How it works
Unlike most hypnotics, which have widespread CNS depressant effects, lemborexant has a more targeted action in promoting sleep by suppressing the wake drive supported by the orexin system.8 Lemborexant is highly selective for the OX1R and OX2R orexin receptors, where it functions as a competitive antagonist. It is hypothesized that by modulating orexin activity with a receptor antagonist, excessive arousal associated with insomnia can be reduced, thus improving nighttime sleep. The pharmacokinetic properties allow benefits for both sleep onset and maintenance.
Pharmacokinetics
Lemborexant is available in immediate-release tablets with a peak concentration time (Tmax) of approximately 1 to 3 hours after ingestion. When taken after a high-fat and high-calorie meal, there is a delay in the Tmax, a decrease in the maximum plasma concentration (Cmax), and an increase in the concentration area under the curve (AUC0-inf).1
Continue to: Metabolism is primarily through...
Metabolism is primarily through the cytochrome P450 (CYP) 3A4 pathway, and to a lesser extent through CYP3A5. Concomitant use with moderate or strong CYP3A inhibitors or inducers should be avoided, while use with weak CYP3A inhibitors should be limited to the 5-mg dose of lemborexant.
Lemborexant has the potential to induce the metabolism of CYP2B6 substrates, such as bupropion and methadone, possibly leading to reduced efficacy for these medications. Accordingly, the clinical responses to any CYP2B6 substrates should be monitored and dosage adjustments considered.
Concomitant use of lemborexant with alcohol should be avoided because there may be increased impairment in postural stability and memory, in part due to increases in the medication’s Cmax and AUC, as well as the direct effects of alcohol.
Efficacy
In randomized, placebo-controlled trials, lemborexant demonstrated both objective and subjective evidence of clinically significant benefits for sleep onset and sleep maintenance in patients diagnosed with insomnia disorder.1 The 2 pivotal efficacy studies were:
- Sunrise 1, a 4-week trial with older adults that included laboratory polysomnography (PSG) studies (objective) and patient-reported sleep measures (subjective) on selected nights9
- Sunrise 2, a 6-month trial assessing patient-reported sleep characteristics in adults and older adults.10
Sunrise 1 was performed with older adults with insomnia who were randomized to groups with nightly use of lemborexant, 5 mg (n = 266), lemborexant, 10 mg (n = 269), zolpidem extended-release, 6.25 mg, as an active comparator (n = 263), or placebo (n = 208).9 The age range was 55 to 88 years with a median age of 63 years. Most patients (86.4%) were women. Because this study focused on the assessment of efficacy for treating sleep maintenance difficulty, the inclusion criteria required a subjective report of experiencing a wake time after sleep onset (sWASO) of at least 60 minutes for 3 or more nights per week over the previous 4 weeks. The zolpidem extended-release 6.25 mg comparison was chosen because it has an indication for sleep maintenance insomnia with this recommended dose for older adults.
Continue to: Laboratory PSG monitoring...
Laboratory PSG monitoring was performed for 2 consecutive nights at baseline (before treatment), the first 2 treatment nights, and the final 2 treatment nights (Nights 29 and 30). The primary study endpoint was the change in latency to persistent sleep (LPS) from baseline to the final 2 nights for the lemborexant doses compared with placebo. Additional PSG-based endpoints were similar comparisons for sleep efficiency (percent time asleep during the 8-hour laboratory recording period) and objective wake after sleep onset (WASO) compared with placebo, and WASO during the second half of the night (WASO2H) compared with zolpidem. Patients completed Insomnia Severity Index (ISI) questionnaires at baseline and the end of the treatment to compare disease severity. Subjective assessments were done daily with electronic diary entries that included sleep onset latency (sSOL), sWASO, and subjective sleep efficiency.
In comparison with placebo, both lemborexant doses were associated with significantly improved PSG measures of LPS, WASO, and sleep efficiency during nights 1 and 2 that were maintained through Nights 29 and 30 (Table 21,9). The lemborexant doses also demonstrated significant improvements in WASO2H compared with zolpidem and placebo on the first 2 and final 2 treatment nights. Analyses of the subjective assessments (sSOL, sWASO, and sleep efficiency) compared the baseline with means for the first and the last treatment weeks. At both lemborexant doses, the sSOL was significantly reduced during the first and last weeks compared with placebo and zolpidem. Subjective sleep efficiency was significantly improved at both time points for the lemborexant doses, though these were not significantly different from the zolpidem values. The sWASO values were significantly decreased for both lemborexant doses at both time points compared with placebo. During the first treatment week, both lemborexant doses did not differ significantly from zolpidem in the sWASO change from baseline; however, at the final treatment week, the zolpidem value was significantly improved compared with lemborexant, 5 mg, but not significantly different from lemborexant, 10 mg. The ISI change from baseline to the end of the treatment period showed significant improvement for the lemborexant doses and zolpidem extended-release compared with placebo.
In the Sunrise 2 study, patients who met the criteria for insomnia disorder (age range 18 to 88, mean 55; 68% female) were randomized to groups taking nightly doses of lemborexant, 5 mg (n = 323), lemborexant, 10 mg (n = 323), or placebo (n = 325) for 6 months.10 Inclusion criteria required an sSOL of at least 30 minutes and/or a sWASO of at least 60 minutes 3 times a week or more during the previous 4 weeks. Efficacy was assessed with daily electronic diary entries, with analyses of change from baseline for sSOL (primary endpoint, baseline to end of 6-month study period), sWASO, and patient-reported sleep efficiency (sSEF). Subjective total sleep time (sTST) represented the estimated time asleep during the time in bed. Additional diary assessments related to sleep quality and morning alertness. All of these subjective assessments were compared as 7-day means for the first week of treatment and the last week of each treatment month.
The superiority of lemborexant, 5 mg and 10 mg, compared with placebo was demonstrated by significant improvements in sSOL, sSEF, sWASO, and sTST during the initial week of the treatment period that remained significant at the end of the 6-month placebo-controlled period (Table 31,10). At the end of 6 months, there were significantly more sleep-onset responders and sleep-maintenance responders among patients taking lemborexant compared with those taking placebo. Sleep-onset responders were patients with a baseline sSOL >30 minutes and a mean sSOL ≤20 minutes at the end of the study. Sleep-maintenance responders were participants with a baseline sWASO >60 minutes who at the end of the study had a mean sWASO ≤60 minutes that included a reduction of at least 10 minutes.
Following the 6-month placebo-controlled treatment period, the Sunrise 2 study continued for an additional 6 months of nightly active treatment for continued safety and efficacy assessment. Patients assigned to lemborexant, 5 mg or 10 mg, during the initial period continued on those doses. Those in the placebo group were randomized to either of the 2 lemborexant doses.
Continue to: Safety studies and adverse reactions
Safety studies and adverse reactions
Potential medication effects on middle-of-the-night and next-morning postural stability (body sway measured with an ataxiameter) and cognitive performance, as well as middle-of-the-night auditory awakening threshold, were assessed in a randomized, 4-way crossover study of 56 healthy older adults (women age ≥55 [77.8%], men age ≥65) given a single bedtime dose of placebo, lemborexant, 5 mg, lemborexant, 10 mg, and zolpidem extended-release, 6.25 mg, on separate nights.11 The results were compared with data from a baseline night with the same measures performed prior to the randomization. The middle-of-the-night assessments were done approximately 4 hours after the dose and the next-morning measures were done after 8 hours in bed. The auditory threshold analysis showed no significant differences among the 4 study nights. Compared with placebo, the middle-of-the-night postural stability was significantly worse for both lemborexant doses and zolpidem; however, the zolpidem effect was significantly worse than with either lemborexant dose. The next-morning postural stability measures showed no significant difference from placebo for the lemborexant doses, but zolpidem continued to show a significantly worsened result. The cognitive performance assessment battery provided 4 domain factor scores (power of attention, continuity of attention, quality of memory, and speed of memory retrieval). The middle-of-the-night battery showed no significant difference between lemborexant, 5 mg, and placebo in any domain, while both lemborexant, 10 mg, and zolpidem showed worse performance on some of the attention and/or memory tests. The next-morning cognitive assessment revealed no significant differences from placebo for the treatments.
Respiratory safety was examined in a placebo-controlled, 2-period crossover study of 38 patients diagnosed with mild obstructive sleep apnea who received lemborexant, 10 mg, or placebo nightly during each 8-day period.12 Neither the apnea-hypopnea index nor the mean oxygen saturation during the lemborexant nights were significantly different from the placebo nights.
The most common adverse reaction during the month-long Sunrise 1 study and the first 30 days of the Sunrise 2 study was somnolence or fatigue, which occurred in 1% receiving placebo, 7% receiving lemborexant, 5 mg, and 10% receiving lemborexant, 10 mg. Headache was reported by 3.5% receiving placebo, 5.9% receiving lemborexant, 5 mg, and 4.5% receiving lemborexant, 10 mg. Nightmare or abnormal dreams occurred with 0.9% receiving placebo, 0.9% receiving lemborexant, 5 mg, and 2.2% receiving lemborexant, 10 mg.1
Unique clinical issues
Because investigations of individuals who abused sedatives for recreational purposes showed lemborexant had a likeability rating similar to zolpidem and significantly greater than placebo, the US Drug Enforcement Agency has categorized lemborexant as a Schedule IV controlled substance. Research has not shown evidence of physical dependence or withdrawal signs or symptoms upon discontinuation of lemborexant.1
Contraindications
Narcolepsy is the only contraindication to the use of lemborexant.1 Narcolepsy is associated with a decrease in the orexin-producing neurons in the hypothalamus, presumably causing the excessive sleepiness, sleep paralysis, hypnagogic hallucinations, and cataplexy characteristic of the disorder. Hypothetically, an orexin antagonist medication could exacerbate these symptoms.
Continue to: Dosing
Dosing
Lemborexant should be taken no more than once per night immediately before going to bed and with at least 7 hours remaining before the planned time of awakening.1 The recommended starting dose is 5 mg. The dosage may be increased to a maximum of 10 mg if the initial dose is well tolerated but insufficiently effective. Patients with moderate hepatic impairment or who are concomitantly taking weak CYP3A inhibitors should receive a maximum of 5 mg once nightly. Lemborexant should be avoided in patients with severe hepatic impairment and in those taking moderate or strong CYP3A inhibitors or inducers.
Orexin receptor antagonists do not share cross-tolerance with other hypnotics; this should be taken into consideration when switching to lemborexant. Abruptly stopping a benzodiazepine receptor agonist hypnotic may lead to rebound insomnia and thus may confound the interpretation of the clinical response when starting lemborexant.
Patients prescribed lemborexant should be educated about possible impairment in alertness and motor coordination, especially with the 10-mg dose, which may affect next-morning driving in sensitive individuals.13 Caution is advised with doses >5 mg in patients age ≥65 due to possible somnolence and a higher risk of falls.1
Bottom Line
Lemborexant is a dual orexin receptor antagonist indicated for the treatment of insomnia characterized by difficulties with sleep onset and/or sleep maintenance. It promotes sleep by suppressing the wake drive supported by the orexin system. In randomized, placebo-controlled trials, lemborexant demonstrated objective and subjective evidence of clinically significant benefits for sleep onset and sleep maintenance.
Related Resource
- Sateia MJ, Buysse DJ, Krystal AD, et al. Clinical practice guideline for the pharmacologic treatment of chronic insomnia in adults: an American Academy of Sleep Medicine clinical practice guideline. J Clin Sleep Med. 2017;13(2):307-349.
Drug Brand Names
Bupropion • Wellbutrin
Doxepin • Silenor
Eszopiclone • Lunesta
Lemborexant • Dayvigo
Methadone • Methadose, Dolophine
Quazepam • Doral
Ramelteon • Rozerem
Suvorexant • Belsomra
Temazepam • Restoril
Triazolam • Halcion
Zaleplon • Sonata
Zolpidem • Ambien, Intermezzo
Lemborexant, FDA-approved for the treatment of insomnia, has demonstrated efficacy in improving both sleep onset and sleep maintenance.1 This novel compound is now the second approved insomnia medication classed as a dual orexin receptor antagonist (Table 1). This targeted mechanism of action aims to enhance sleep while limiting the adverse effects associated with traditional hypnotics.
Clinical implications
Insomnia symptoms affect approximately one-third of the general population at least occasionally. Approximately 10% of individuals meet DSM-5 criteria for insomnia disorder, which require nighttime sleep difficulty and daytime consequences persisting for a minimum of 3 months.2 The prevalence is considerably higher in patients with chronic medical disorders and comorbid psychiatric conditions, especially mood, anxiety, substance use, and stress- and trauma-related disorders. Clinical guidelines for treating insomnia disorder typically recommend cognitive-behavioral therapy for insomnia as a first choice and FDA-approved insomnia medications as secondary options.3
Currently approved insomnia medications fall into 4 distinct pharmacodynamics categories.4 Benzodiazepine receptor agonist hypnotics include 5 medications with classic benzodiazepine structures (estazolam, flurazepam, quazepam, temazepam, and triazolam) and 3 compounds (eszopiclone, zaleplon, and zolpidem) with alternate structures but similar mechanisms of action. There is 1 melatonin receptor agonist (ramelteon) and 1 histamine receptor antagonist (low-dose doxepin). Joining suvorexant (approved in 2014), lemborexant is the second dual orexin receptor antagonist.
The orexin (also called hypocretin) system was first described in 1998 and its fundamental role in promoting and coordinating wakefulness was quickly established.5 A relatively small number of hypothalamic neurons located in the lateral and perifornical regions produce 2 similar orexin neuropeptides (orexin A and orexin B) with widespread distributions, notably reinforcing the wake-promoting activity of histamine, acetylcholine, dopamine, serotonin, and norepinephrine. Consistent with the typical sleep-wake cycle, orexin release is limited during the nighttime. The orexin neuropeptides interact with 2 G-protein-coupled orexin receptors (OX1R, OX2R).
Animal studies showed that impairment in orexin system activity was associated with symptoms characteristic of narcolepsy, including cataplexy and excessive sleep episodes. Soon after, it was found that humans diagnosed with narcolepsy with cataplexy had markedly low CSF orexin levels.6 This recognition that excessively sleepy people with narcolepsy had a profound decrease in orexin production led to the hypothesis that pharmacologically decreasing orexin activity might be sleep-enhancing for insomnia patients, who presumably are excessively aroused. Numerous compounds soon were evaluated for their potential as orexin receptor antagonists. The efficacy of treating insomnia with a dual orexin receptor antagonist in humans was first reported in 2007 with almorexant, a compound that remains investigational.7 Research continues to investigate both single and dual orexin antagonist molecules for insomnia and other potential indications.
How it works
Unlike most hypnotics, which have widespread CNS depressant effects, lemborexant has a more targeted action in promoting sleep by suppressing the wake drive supported by the orexin system.8 Lemborexant is highly selective for the OX1R and OX2R orexin receptors, where it functions as a competitive antagonist. It is hypothesized that by modulating orexin activity with a receptor antagonist, excessive arousal associated with insomnia can be reduced, thus improving nighttime sleep. The pharmacokinetic properties allow benefits for both sleep onset and maintenance.
Pharmacokinetics
Lemborexant is available in immediate-release tablets with a peak concentration time (Tmax) of approximately 1 to 3 hours after ingestion. When taken after a high-fat and high-calorie meal, there is a delay in the Tmax, a decrease in the maximum plasma concentration (Cmax), and an increase in the concentration area under the curve (AUC0-inf).1
Continue to: Metabolism is primarily through...
Metabolism is primarily through the cytochrome P450 (CYP) 3A4 pathway, and to a lesser extent through CYP3A5. Concomitant use with moderate or strong CYP3A inhibitors or inducers should be avoided, while use with weak CYP3A inhibitors should be limited to the 5-mg dose of lemborexant.
Lemborexant has the potential to induce the metabolism of CYP2B6 substrates, such as bupropion and methadone, possibly leading to reduced efficacy for these medications. Accordingly, the clinical responses to any CYP2B6 substrates should be monitored and dosage adjustments considered.
Concomitant use of lemborexant with alcohol should be avoided because there may be increased impairment in postural stability and memory, in part due to increases in the medication’s Cmax and AUC, as well as the direct effects of alcohol.
Efficacy
In randomized, placebo-controlled trials, lemborexant demonstrated both objective and subjective evidence of clinically significant benefits for sleep onset and sleep maintenance in patients diagnosed with insomnia disorder.1 The 2 pivotal efficacy studies were:
- Sunrise 1, a 4-week trial with older adults that included laboratory polysomnography (PSG) studies (objective) and patient-reported sleep measures (subjective) on selected nights9
- Sunrise 2, a 6-month trial assessing patient-reported sleep characteristics in adults and older adults.10
Sunrise 1 was performed with older adults with insomnia who were randomized to groups with nightly use of lemborexant, 5 mg (n = 266), lemborexant, 10 mg (n = 269), zolpidem extended-release, 6.25 mg, as an active comparator (n = 263), or placebo (n = 208).9 The age range was 55 to 88 years with a median age of 63 years. Most patients (86.4%) were women. Because this study focused on the assessment of efficacy for treating sleep maintenance difficulty, the inclusion criteria required a subjective report of experiencing a wake time after sleep onset (sWASO) of at least 60 minutes for 3 or more nights per week over the previous 4 weeks. The zolpidem extended-release 6.25 mg comparison was chosen because it has an indication for sleep maintenance insomnia with this recommended dose for older adults.
Continue to: Laboratory PSG monitoring...
Laboratory PSG monitoring was performed for 2 consecutive nights at baseline (before treatment), the first 2 treatment nights, and the final 2 treatment nights (Nights 29 and 30). The primary study endpoint was the change in latency to persistent sleep (LPS) from baseline to the final 2 nights for the lemborexant doses compared with placebo. Additional PSG-based endpoints were similar comparisons for sleep efficiency (percent time asleep during the 8-hour laboratory recording period) and objective wake after sleep onset (WASO) compared with placebo, and WASO during the second half of the night (WASO2H) compared with zolpidem. Patients completed Insomnia Severity Index (ISI) questionnaires at baseline and the end of the treatment to compare disease severity. Subjective assessments were done daily with electronic diary entries that included sleep onset latency (sSOL), sWASO, and subjective sleep efficiency.
In comparison with placebo, both lemborexant doses were associated with significantly improved PSG measures of LPS, WASO, and sleep efficiency during nights 1 and 2 that were maintained through Nights 29 and 30 (Table 21,9). The lemborexant doses also demonstrated significant improvements in WASO2H compared with zolpidem and placebo on the first 2 and final 2 treatment nights. Analyses of the subjective assessments (sSOL, sWASO, and sleep efficiency) compared the baseline with means for the first and the last treatment weeks. At both lemborexant doses, the sSOL was significantly reduced during the first and last weeks compared with placebo and zolpidem. Subjective sleep efficiency was significantly improved at both time points for the lemborexant doses, though these were not significantly different from the zolpidem values. The sWASO values were significantly decreased for both lemborexant doses at both time points compared with placebo. During the first treatment week, both lemborexant doses did not differ significantly from zolpidem in the sWASO change from baseline; however, at the final treatment week, the zolpidem value was significantly improved compared with lemborexant, 5 mg, but not significantly different from lemborexant, 10 mg. The ISI change from baseline to the end of the treatment period showed significant improvement for the lemborexant doses and zolpidem extended-release compared with placebo.
In the Sunrise 2 study, patients who met the criteria for insomnia disorder (age range 18 to 88, mean 55; 68% female) were randomized to groups taking nightly doses of lemborexant, 5 mg (n = 323), lemborexant, 10 mg (n = 323), or placebo (n = 325) for 6 months.10 Inclusion criteria required an sSOL of at least 30 minutes and/or a sWASO of at least 60 minutes 3 times a week or more during the previous 4 weeks. Efficacy was assessed with daily electronic diary entries, with analyses of change from baseline for sSOL (primary endpoint, baseline to end of 6-month study period), sWASO, and patient-reported sleep efficiency (sSEF). Subjective total sleep time (sTST) represented the estimated time asleep during the time in bed. Additional diary assessments related to sleep quality and morning alertness. All of these subjective assessments were compared as 7-day means for the first week of treatment and the last week of each treatment month.
The superiority of lemborexant, 5 mg and 10 mg, compared with placebo was demonstrated by significant improvements in sSOL, sSEF, sWASO, and sTST during the initial week of the treatment period that remained significant at the end of the 6-month placebo-controlled period (Table 31,10). At the end of 6 months, there were significantly more sleep-onset responders and sleep-maintenance responders among patients taking lemborexant compared with those taking placebo. Sleep-onset responders were patients with a baseline sSOL >30 minutes and a mean sSOL ≤20 minutes at the end of the study. Sleep-maintenance responders were participants with a baseline sWASO >60 minutes who at the end of the study had a mean sWASO ≤60 minutes that included a reduction of at least 10 minutes.
Following the 6-month placebo-controlled treatment period, the Sunrise 2 study continued for an additional 6 months of nightly active treatment for continued safety and efficacy assessment. Patients assigned to lemborexant, 5 mg or 10 mg, during the initial period continued on those doses. Those in the placebo group were randomized to either of the 2 lemborexant doses.
Continue to: Safety studies and adverse reactions
Safety studies and adverse reactions
Potential medication effects on middle-of-the-night and next-morning postural stability (body sway measured with an ataxiameter) and cognitive performance, as well as middle-of-the-night auditory awakening threshold, were assessed in a randomized, 4-way crossover study of 56 healthy older adults (women age ≥55 [77.8%], men age ≥65) given a single bedtime dose of placebo, lemborexant, 5 mg, lemborexant, 10 mg, and zolpidem extended-release, 6.25 mg, on separate nights.11 The results were compared with data from a baseline night with the same measures performed prior to the randomization. The middle-of-the-night assessments were done approximately 4 hours after the dose and the next-morning measures were done after 8 hours in bed. The auditory threshold analysis showed no significant differences among the 4 study nights. Compared with placebo, the middle-of-the-night postural stability was significantly worse for both lemborexant doses and zolpidem; however, the zolpidem effect was significantly worse than with either lemborexant dose. The next-morning postural stability measures showed no significant difference from placebo for the lemborexant doses, but zolpidem continued to show a significantly worsened result. The cognitive performance assessment battery provided 4 domain factor scores (power of attention, continuity of attention, quality of memory, and speed of memory retrieval). The middle-of-the-night battery showed no significant difference between lemborexant, 5 mg, and placebo in any domain, while both lemborexant, 10 mg, and zolpidem showed worse performance on some of the attention and/or memory tests. The next-morning cognitive assessment revealed no significant differences from placebo for the treatments.
Respiratory safety was examined in a placebo-controlled, 2-period crossover study of 38 patients diagnosed with mild obstructive sleep apnea who received lemborexant, 10 mg, or placebo nightly during each 8-day period.12 Neither the apnea-hypopnea index nor the mean oxygen saturation during the lemborexant nights were significantly different from the placebo nights.
The most common adverse reaction during the month-long Sunrise 1 study and the first 30 days of the Sunrise 2 study was somnolence or fatigue, which occurred in 1% receiving placebo, 7% receiving lemborexant, 5 mg, and 10% receiving lemborexant, 10 mg. Headache was reported by 3.5% receiving placebo, 5.9% receiving lemborexant, 5 mg, and 4.5% receiving lemborexant, 10 mg. Nightmare or abnormal dreams occurred with 0.9% receiving placebo, 0.9% receiving lemborexant, 5 mg, and 2.2% receiving lemborexant, 10 mg.1
Unique clinical issues
Because investigations of individuals who abused sedatives for recreational purposes showed lemborexant had a likeability rating similar to zolpidem and significantly greater than placebo, the US Drug Enforcement Agency has categorized lemborexant as a Schedule IV controlled substance. Research has not shown evidence of physical dependence or withdrawal signs or symptoms upon discontinuation of lemborexant.1
Contraindications
Narcolepsy is the only contraindication to the use of lemborexant.1 Narcolepsy is associated with a decrease in the orexin-producing neurons in the hypothalamus, presumably causing the excessive sleepiness, sleep paralysis, hypnagogic hallucinations, and cataplexy characteristic of the disorder. Hypothetically, an orexin antagonist medication could exacerbate these symptoms.
Continue to: Dosing
Dosing
Lemborexant should be taken no more than once per night immediately before going to bed and with at least 7 hours remaining before the planned time of awakening.1 The recommended starting dose is 5 mg. The dosage may be increased to a maximum of 10 mg if the initial dose is well tolerated but insufficiently effective. Patients with moderate hepatic impairment or who are concomitantly taking weak CYP3A inhibitors should receive a maximum of 5 mg once nightly. Lemborexant should be avoided in patients with severe hepatic impairment and in those taking moderate or strong CYP3A inhibitors or inducers.
Orexin receptor antagonists do not share cross-tolerance with other hypnotics; this should be taken into consideration when switching to lemborexant. Abruptly stopping a benzodiazepine receptor agonist hypnotic may lead to rebound insomnia and thus may confound the interpretation of the clinical response when starting lemborexant.
Patients prescribed lemborexant should be educated about possible impairment in alertness and motor coordination, especially with the 10-mg dose, which may affect next-morning driving in sensitive individuals.13 Caution is advised with doses >5 mg in patients age ≥65 due to possible somnolence and a higher risk of falls.1
Bottom Line
Lemborexant is a dual orexin receptor antagonist indicated for the treatment of insomnia characterized by difficulties with sleep onset and/or sleep maintenance. It promotes sleep by suppressing the wake drive supported by the orexin system. In randomized, placebo-controlled trials, lemborexant demonstrated objective and subjective evidence of clinically significant benefits for sleep onset and sleep maintenance.
Related Resource
- Sateia MJ, Buysse DJ, Krystal AD, et al. Clinical practice guideline for the pharmacologic treatment of chronic insomnia in adults: an American Academy of Sleep Medicine clinical practice guideline. J Clin Sleep Med. 2017;13(2):307-349.
Drug Brand Names
Bupropion • Wellbutrin
Doxepin • Silenor
Eszopiclone • Lunesta
Lemborexant • Dayvigo
Methadone • Methadose, Dolophine
Quazepam • Doral
Ramelteon • Rozerem
Suvorexant • Belsomra
Temazepam • Restoril
Triazolam • Halcion
Zaleplon • Sonata
Zolpidem • Ambien, Intermezzo
1. Dayvigo [package insert]. Woodcliff Lake, NJ: Eisai Inc.; 2020.
2. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
3. Qaseem A, Kansagara D, Forciea MA, et al; Clinical Guidelines Committee of the American College of Physicians. Management of chronic insomnia disorder in adults: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2016;165(2):125-133.
4. Neubauer DN, Pandi-Perumal SR, Spence DW, et al. Pharmacotherapy of insomnia. J Cent Nerv Syst Dis. 2018;10:1179573518770672. doi: 10.1177/1179573518770672.
5. Saper CB, Chou TC, Scammell TE. The sleep switch: hypothalamic control of sleep and wakefulness. Trends Neurosci. 2001;24(12):726-731.
6. Mignot E. Sleep, sleep disorders and hypocretin (orexin). Sleep Med. 2004;5(suppl 1):S2-S8.
7. Boss C, Brisbare-Roch C, Jenck F, et al. Orexin receptor antagonism: a new principle in neuroscience. Chimia. 2008;62:974-979.
8. Landry I, Nakai K, Ferry J, et al. Pharmacokinetics, pharmacodynamics, and safety of the dual orexin receptor antagonist lemborexant: findings from single-dose and multiple-ascending-dose phase 1 studies in healthy adults. Clin Pharmacol Drug Dev. 2020. doi: 10.1002/cpdd.817.
9. Rosenberg R, Murphy P, Zammit G, et al. Comparison of lemborexant with placebo and zolpidem tartrate extended release for the treatment of older adults with insomnia disorder: a phase 3 randomized clinical trial. JAMA Netw Open. 2019;2(12):e1918254. doi: 10.1001/jamanetworkopen.2019.18254.
10. Karppa M, Yardley J, Pinner K, et al. Long-term efficacy and tolerability of lemborexant compared with placebo in adults with insomnia disorder: results from the phase 3 randomized clinical trial SUNRISE 2. Sleep. 2020;43(9):zsaa123. doi: 10.1093/sleep/zsaa123.
11. Murphy P, Kumar D, Zammit G, et al. Safety of lemborexant versus placebo and zolpidem: effects on auditory awakening threshold, postural stability, and cognitive performance in healthy older participants in the middle of the night and upon morning awakening. J Clin Sleep Med. 2020;16(5):765-773.
12. Cheng JY, Filippov G, Moline M, et al. Respiratory safety of lemborexant in healthy adult and elderly subjects with mild obstructive sleep apnea: a randomized, double-blind, placebo-controlled, crossover study. J Sleep Res. 2020:e13021. doi: 10.1111/jsr.13021.
13. Vermeeren A, Jongen S, Murphy P, et al. On-the-road driving performance the morning after bedtime administration of lemborexant in healthy adult and elderly volunteers. Sleep. 2019;42(4):10.1093/sleep/zsy260. doi: 10.1093/sleep/zsy260.
1. Dayvigo [package insert]. Woodcliff Lake, NJ: Eisai Inc.; 2020.
2. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
3. Qaseem A, Kansagara D, Forciea MA, et al; Clinical Guidelines Committee of the American College of Physicians. Management of chronic insomnia disorder in adults: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2016;165(2):125-133.
4. Neubauer DN, Pandi-Perumal SR, Spence DW, et al. Pharmacotherapy of insomnia. J Cent Nerv Syst Dis. 2018;10:1179573518770672. doi: 10.1177/1179573518770672.
5. Saper CB, Chou TC, Scammell TE. The sleep switch: hypothalamic control of sleep and wakefulness. Trends Neurosci. 2001;24(12):726-731.
6. Mignot E. Sleep, sleep disorders and hypocretin (orexin). Sleep Med. 2004;5(suppl 1):S2-S8.
7. Boss C, Brisbare-Roch C, Jenck F, et al. Orexin receptor antagonism: a new principle in neuroscience. Chimia. 2008;62:974-979.
8. Landry I, Nakai K, Ferry J, et al. Pharmacokinetics, pharmacodynamics, and safety of the dual orexin receptor antagonist lemborexant: findings from single-dose and multiple-ascending-dose phase 1 studies in healthy adults. Clin Pharmacol Drug Dev. 2020. doi: 10.1002/cpdd.817.
9. Rosenberg R, Murphy P, Zammit G, et al. Comparison of lemborexant with placebo and zolpidem tartrate extended release for the treatment of older adults with insomnia disorder: a phase 3 randomized clinical trial. JAMA Netw Open. 2019;2(12):e1918254. doi: 10.1001/jamanetworkopen.2019.18254.
10. Karppa M, Yardley J, Pinner K, et al. Long-term efficacy and tolerability of lemborexant compared with placebo in adults with insomnia disorder: results from the phase 3 randomized clinical trial SUNRISE 2. Sleep. 2020;43(9):zsaa123. doi: 10.1093/sleep/zsaa123.
11. Murphy P, Kumar D, Zammit G, et al. Safety of lemborexant versus placebo and zolpidem: effects on auditory awakening threshold, postural stability, and cognitive performance in healthy older participants in the middle of the night and upon morning awakening. J Clin Sleep Med. 2020;16(5):765-773.
12. Cheng JY, Filippov G, Moline M, et al. Respiratory safety of lemborexant in healthy adult and elderly subjects with mild obstructive sleep apnea: a randomized, double-blind, placebo-controlled, crossover study. J Sleep Res. 2020:e13021. doi: 10.1111/jsr.13021.
13. Vermeeren A, Jongen S, Murphy P, et al. On-the-road driving performance the morning after bedtime administration of lemborexant in healthy adult and elderly volunteers. Sleep. 2019;42(4):10.1093/sleep/zsy260. doi: 10.1093/sleep/zsy260.
Sleep apnea found to impact pain severity in younger adults
Sleep specialists might want to take a closer look at the connections between obstructive sleep apnea, chronic pain, and reported pain intensity in younger patients. Young adults with a diagnosis of obstructive sleep apnea (OSA) are more likely to report moderate to severe pain intensity, compared with their peers who do not have the diagnosis, results from a large cross-sectional analysis of veterans showed.
“Because of the high burden of chronic pain conditions in younger adults, this study highlights the need to understand the impact of OSA diagnosis and treatment on pain intensity,” researchers led by Wardah Athar, a graduate student at Yale University, New Haven, Conn., and Lori A. Bastian, MD, MPH, a professor of internal medicine at Yale, wrote in an article published in the Annals of the American Thoracic Society. “This understanding would then help inform the development of interventions to promote screening for OSA among young adults with chronic pain and pain management among those with diagnosed OSA.”
In an effort to assess whether young adults with diagnosed OSA are more likely to report higher pain intensity, compared with those without OSA, the researchers drew from a sample of 858,226 veterans from Operation Enduring Freedom, Operation Iraqi Freedom, and Operation New Dawn who had at least one visit to a VA clinic between 2001 and 2014. They used ICD-9 codes to identify OSA and assessed self-reported responses to pain measures on a 0-10 numeric scale which were recorded in each veteran’s EMR. Next, they averaged pain intensity responses over a 12-month period and categorized them as none (0), mild (1-3), and moderate/severe (4–10). Covariates included age, sex, education, race, mental health diagnoses, headache diagnoses, pain diagnoses, hypertension, diabetes, body mass index, and smoking status. The researchers used multivariate logistic regression models and multiple imputation to generate values for missing variables.
The mean age of the patients was 30 years, 64% were White, 17% were Black, 12% were Hispanic, and remainder were other/unknown race/ethnicity. Ninety percent were male, and 20% had greater than a high school education. Of the 858,226 patients, 91,244 (11%) had a diagnosis of OSA. Compared with patients who had no diagnosis of OSA, the unadjusted odds of reporting moderate/severe pain was 48% higher among those with OSA (odds ratio 1.48; P < .0001). After the researchers adjusted for all covariates in the model, the association between OSA and moderate/severe pain remained significant though attenuated, with an adjusted odds ratio of 1.09 (P < .0001).
Several characteristics were different between those who had a diagnosis of OSA and those who did not, including age (a mean of 36 vs. 26 years, respectively) and having the following diagnoses: pain (36% vs. 16%), headache (28% vs. 14%), diabetes (12% vs. 2%), hypertension (40% vs. 12%), and a body mass index of 30 kg/m2 or greater (69% vs. 35%). Certain psychiatric disorders were also common among patients with OSA, including major depressive disorder (20% vs. 10%), posttraumatic stress disorder (50% vs. 30%), and substance use disorder (26% vs. 17%). Patients with OSA were also more likely to have been prescribed benzodiazepines or opioids within 90 days of their OSA diagnosis. Although men were more likely to have a diagnosis of OSA, no differences related to sex in the association of OSA and pain were observed in sex-based stratified analyses.
“Based on these results, we suggest more thorough and more frequent pain intensity screening in patients with OSA, particularly in those patients who are younger than 60 years old without significant comorbid illness,” the researchers concluded. “Furthermore, we also recommend increased OSA screening for patients with moderate/severe pain intensity and pain diagnoses.” One tool they recommend is the STOP-Bang (Snoring, Tiredness, Observed Apnea, Blood Pressure, Body Mass Index, Age, Neck Circumference, and Gender) questionnaire, which has been validated in multiple settings.
Commenting on the findings of this study, Krishna M. Sundar, MD, FCCP, medical director of the Sleep-Wake Center at the University of Utah, Salt Lake City, commended the study design. “One of the problems with sleep apnea studies is that there are always confounding effects, especially from BMI. This is a population that has a significant medical burden of disease, but I think this is a well-done study to look at the relationship between pain and OSA in a younger population. The authors tried to adjust for all these confounders and they still found a significant association. This indicates that sleep affects one’s pain threshold. And sleep apnea, by mechanisms still yet to be defined, also alters that pain threshold. It may also affect the expression of pain or management of pain, making treatment more problematic in this population,” he said in an interview.
A key limitation of the study, he continued, was the fact it evaluated only one aspect of sleep: OSA. “They didn’t look at duration of sleep, comorbid insomnia, or fragmentation of sleep from apnea or from other causes,” Dr. Sundar said. “We have multiple ways of treating sleep apnea. Clearly, we need studies of treating sleep apnea with [continuous positive airway pressure] and how that affects the occurrence of pain. The relevant practical aspect of this is that there are pain clinics all over the country that should screen for sleep apnea. Along the same lines, sleep practitioners should be aware that pain has an important association with sleep apnea.”
The study was supported by the Health Services Research & Development in the Department of Veterans Affairs of the Veterans Health Administration, the Yale School of Medicine Medical Student Fellowship, and the U.S. National Institutes of Health.
SOURCE: Athar W et al. Ann Am Thorac Soc. 2020;17(10):1273-48.
Correction, 10/28/20: An earlier version of this article misstated Wardah Athar's name in the photo caption.
Sleep specialists might want to take a closer look at the connections between obstructive sleep apnea, chronic pain, and reported pain intensity in younger patients. Young adults with a diagnosis of obstructive sleep apnea (OSA) are more likely to report moderate to severe pain intensity, compared with their peers who do not have the diagnosis, results from a large cross-sectional analysis of veterans showed.
“Because of the high burden of chronic pain conditions in younger adults, this study highlights the need to understand the impact of OSA diagnosis and treatment on pain intensity,” researchers led by Wardah Athar, a graduate student at Yale University, New Haven, Conn., and Lori A. Bastian, MD, MPH, a professor of internal medicine at Yale, wrote in an article published in the Annals of the American Thoracic Society. “This understanding would then help inform the development of interventions to promote screening for OSA among young adults with chronic pain and pain management among those with diagnosed OSA.”
In an effort to assess whether young adults with diagnosed OSA are more likely to report higher pain intensity, compared with those without OSA, the researchers drew from a sample of 858,226 veterans from Operation Enduring Freedom, Operation Iraqi Freedom, and Operation New Dawn who had at least one visit to a VA clinic between 2001 and 2014. They used ICD-9 codes to identify OSA and assessed self-reported responses to pain measures on a 0-10 numeric scale which were recorded in each veteran’s EMR. Next, they averaged pain intensity responses over a 12-month period and categorized them as none (0), mild (1-3), and moderate/severe (4–10). Covariates included age, sex, education, race, mental health diagnoses, headache diagnoses, pain diagnoses, hypertension, diabetes, body mass index, and smoking status. The researchers used multivariate logistic regression models and multiple imputation to generate values for missing variables.
The mean age of the patients was 30 years, 64% were White, 17% were Black, 12% were Hispanic, and remainder were other/unknown race/ethnicity. Ninety percent were male, and 20% had greater than a high school education. Of the 858,226 patients, 91,244 (11%) had a diagnosis of OSA. Compared with patients who had no diagnosis of OSA, the unadjusted odds of reporting moderate/severe pain was 48% higher among those with OSA (odds ratio 1.48; P < .0001). After the researchers adjusted for all covariates in the model, the association between OSA and moderate/severe pain remained significant though attenuated, with an adjusted odds ratio of 1.09 (P < .0001).
Several characteristics were different between those who had a diagnosis of OSA and those who did not, including age (a mean of 36 vs. 26 years, respectively) and having the following diagnoses: pain (36% vs. 16%), headache (28% vs. 14%), diabetes (12% vs. 2%), hypertension (40% vs. 12%), and a body mass index of 30 kg/m2 or greater (69% vs. 35%). Certain psychiatric disorders were also common among patients with OSA, including major depressive disorder (20% vs. 10%), posttraumatic stress disorder (50% vs. 30%), and substance use disorder (26% vs. 17%). Patients with OSA were also more likely to have been prescribed benzodiazepines or opioids within 90 days of their OSA diagnosis. Although men were more likely to have a diagnosis of OSA, no differences related to sex in the association of OSA and pain were observed in sex-based stratified analyses.
“Based on these results, we suggest more thorough and more frequent pain intensity screening in patients with OSA, particularly in those patients who are younger than 60 years old without significant comorbid illness,” the researchers concluded. “Furthermore, we also recommend increased OSA screening for patients with moderate/severe pain intensity and pain diagnoses.” One tool they recommend is the STOP-Bang (Snoring, Tiredness, Observed Apnea, Blood Pressure, Body Mass Index, Age, Neck Circumference, and Gender) questionnaire, which has been validated in multiple settings.
Commenting on the findings of this study, Krishna M. Sundar, MD, FCCP, medical director of the Sleep-Wake Center at the University of Utah, Salt Lake City, commended the study design. “One of the problems with sleep apnea studies is that there are always confounding effects, especially from BMI. This is a population that has a significant medical burden of disease, but I think this is a well-done study to look at the relationship between pain and OSA in a younger population. The authors tried to adjust for all these confounders and they still found a significant association. This indicates that sleep affects one’s pain threshold. And sleep apnea, by mechanisms still yet to be defined, also alters that pain threshold. It may also affect the expression of pain or management of pain, making treatment more problematic in this population,” he said in an interview.
A key limitation of the study, he continued, was the fact it evaluated only one aspect of sleep: OSA. “They didn’t look at duration of sleep, comorbid insomnia, or fragmentation of sleep from apnea or from other causes,” Dr. Sundar said. “We have multiple ways of treating sleep apnea. Clearly, we need studies of treating sleep apnea with [continuous positive airway pressure] and how that affects the occurrence of pain. The relevant practical aspect of this is that there are pain clinics all over the country that should screen for sleep apnea. Along the same lines, sleep practitioners should be aware that pain has an important association with sleep apnea.”
The study was supported by the Health Services Research & Development in the Department of Veterans Affairs of the Veterans Health Administration, the Yale School of Medicine Medical Student Fellowship, and the U.S. National Institutes of Health.
SOURCE: Athar W et al. Ann Am Thorac Soc. 2020;17(10):1273-48.
Correction, 10/28/20: An earlier version of this article misstated Wardah Athar's name in the photo caption.
Sleep specialists might want to take a closer look at the connections between obstructive sleep apnea, chronic pain, and reported pain intensity in younger patients. Young adults with a diagnosis of obstructive sleep apnea (OSA) are more likely to report moderate to severe pain intensity, compared with their peers who do not have the diagnosis, results from a large cross-sectional analysis of veterans showed.
“Because of the high burden of chronic pain conditions in younger adults, this study highlights the need to understand the impact of OSA diagnosis and treatment on pain intensity,” researchers led by Wardah Athar, a graduate student at Yale University, New Haven, Conn., and Lori A. Bastian, MD, MPH, a professor of internal medicine at Yale, wrote in an article published in the Annals of the American Thoracic Society. “This understanding would then help inform the development of interventions to promote screening for OSA among young adults with chronic pain and pain management among those with diagnosed OSA.”
In an effort to assess whether young adults with diagnosed OSA are more likely to report higher pain intensity, compared with those without OSA, the researchers drew from a sample of 858,226 veterans from Operation Enduring Freedom, Operation Iraqi Freedom, and Operation New Dawn who had at least one visit to a VA clinic between 2001 and 2014. They used ICD-9 codes to identify OSA and assessed self-reported responses to pain measures on a 0-10 numeric scale which were recorded in each veteran’s EMR. Next, they averaged pain intensity responses over a 12-month period and categorized them as none (0), mild (1-3), and moderate/severe (4–10). Covariates included age, sex, education, race, mental health diagnoses, headache diagnoses, pain diagnoses, hypertension, diabetes, body mass index, and smoking status. The researchers used multivariate logistic regression models and multiple imputation to generate values for missing variables.
The mean age of the patients was 30 years, 64% were White, 17% were Black, 12% were Hispanic, and remainder were other/unknown race/ethnicity. Ninety percent were male, and 20% had greater than a high school education. Of the 858,226 patients, 91,244 (11%) had a diagnosis of OSA. Compared with patients who had no diagnosis of OSA, the unadjusted odds of reporting moderate/severe pain was 48% higher among those with OSA (odds ratio 1.48; P < .0001). After the researchers adjusted for all covariates in the model, the association between OSA and moderate/severe pain remained significant though attenuated, with an adjusted odds ratio of 1.09 (P < .0001).
Several characteristics were different between those who had a diagnosis of OSA and those who did not, including age (a mean of 36 vs. 26 years, respectively) and having the following diagnoses: pain (36% vs. 16%), headache (28% vs. 14%), diabetes (12% vs. 2%), hypertension (40% vs. 12%), and a body mass index of 30 kg/m2 or greater (69% vs. 35%). Certain psychiatric disorders were also common among patients with OSA, including major depressive disorder (20% vs. 10%), posttraumatic stress disorder (50% vs. 30%), and substance use disorder (26% vs. 17%). Patients with OSA were also more likely to have been prescribed benzodiazepines or opioids within 90 days of their OSA diagnosis. Although men were more likely to have a diagnosis of OSA, no differences related to sex in the association of OSA and pain were observed in sex-based stratified analyses.
“Based on these results, we suggest more thorough and more frequent pain intensity screening in patients with OSA, particularly in those patients who are younger than 60 years old without significant comorbid illness,” the researchers concluded. “Furthermore, we also recommend increased OSA screening for patients with moderate/severe pain intensity and pain diagnoses.” One tool they recommend is the STOP-Bang (Snoring, Tiredness, Observed Apnea, Blood Pressure, Body Mass Index, Age, Neck Circumference, and Gender) questionnaire, which has been validated in multiple settings.
Commenting on the findings of this study, Krishna M. Sundar, MD, FCCP, medical director of the Sleep-Wake Center at the University of Utah, Salt Lake City, commended the study design. “One of the problems with sleep apnea studies is that there are always confounding effects, especially from BMI. This is a population that has a significant medical burden of disease, but I think this is a well-done study to look at the relationship between pain and OSA in a younger population. The authors tried to adjust for all these confounders and they still found a significant association. This indicates that sleep affects one’s pain threshold. And sleep apnea, by mechanisms still yet to be defined, also alters that pain threshold. It may also affect the expression of pain or management of pain, making treatment more problematic in this population,” he said in an interview.
A key limitation of the study, he continued, was the fact it evaluated only one aspect of sleep: OSA. “They didn’t look at duration of sleep, comorbid insomnia, or fragmentation of sleep from apnea or from other causes,” Dr. Sundar said. “We have multiple ways of treating sleep apnea. Clearly, we need studies of treating sleep apnea with [continuous positive airway pressure] and how that affects the occurrence of pain. The relevant practical aspect of this is that there are pain clinics all over the country that should screen for sleep apnea. Along the same lines, sleep practitioners should be aware that pain has an important association with sleep apnea.”
The study was supported by the Health Services Research & Development in the Department of Veterans Affairs of the Veterans Health Administration, the Yale School of Medicine Medical Student Fellowship, and the U.S. National Institutes of Health.
SOURCE: Athar W et al. Ann Am Thorac Soc. 2020;17(10):1273-48.
Correction, 10/28/20: An earlier version of this article misstated Wardah Athar's name in the photo caption.
FROM ANNALS OF THE AMERICAN THORACIC SOCIETY
Sleep-disordered breathing in neuromuscular disease
Sleep-disordered breathing (SDB) is a common sleep disturbance in neuromuscular disease (NMD) affecting 36% to 53% of diagnosed adults (Arens R, et al. Paediatr Respir Rev. 2010;11[1]:24). Disturbances in sleep may serve as the earliest sign of muscle weakness in these patients, at times being detected before their underlying neuromuscular disease is diagnosed. This is of paramount importance to sleep medicine and pulmonary physicians who may be among the first specialists to evaluate these patients and can play a vital role in the recognition and diagnosis of neuromuscular disease. Herein, we will provide a guide to aid the reader in recognizing the early signs and symptoms of NMD as it pertains to sleep, as earlier diagnosis may lead to improved quality of life or possibly even survival, in some cases.
Pathophysiology
To begin, it is important to understand the pathophysiology of NMD and how it is altered during the sleep state. Sleep-related physiologic changes in healthy humans include reduction in upper airway muscle tone, blunting of chemoreceptors associated with pharyngeal dilator augmentation, and sleep stage-specific changes in skeletal muscle tone. In patients with NMD, these changes may not be adequately compensated for, leading to sleep-disordered breathing that can present as sleep apnea, hypoventilation, or hypoxia (Govindarajan R, et al. Sleep Issues in Neuromuscular Disorders: A Clinical Guide. Springer International Publishing AG, Springer Nature 2018).
Central respiratory control
The respiratory centers in the pons and medulla are generally spared from the primary effects of most NMD; however, over time, they may be affected secondarily. Similar to obesity hypoventilation syndrome (OHS), untreated chronic sleep-related hypoventilation from NMD can impair the sensitivity of respiratory chemoreceptors leading to worsening hypoventilation.
Upper airway resistance
Pharyngeal muscle tone is key to maintaining a patent airway during sleep. In some NMD, bulbar muscle weakness with pharyngeal dilator muscle hypotonia leads to increased upper airway resistance, especially during REM sleep, which can result in obstructive sleep apnea (OSA). In addition to weakness affecting the upper airway musculature, anatomical changes may also contribute to sleep-disordered breathing. In Pompe disease, for example, macroglossia and fibro-fatty replacement of tongue muscles may occur, leading to the development of OSA.
Diaphragm weakness
In NMD that affects the diaphragm, there is an increased reliance on the skeletal muscles of respiration to maintain adequate ventilation as the underlying disease progresses. Generally, weakness of the diaphragm will cause disturbances in REM sleep first as, during REM, ventilation predominately depends on the diaphragm and patients lose the assistance of their skeletal muscles. However, over time, the progressive weakening of the diaphragm will progress to involve NREM sleep as well, clinically manifesting with frank sleep apnea, hypoventilation, and, ultimately, chronic hypercapnic respiratory failure.
Inspiratory muscle weakness
As noted above, there are many other muscles used in inspiration in addition to the diaphragm. Other primary muscles include the intercostal and scalene muscles, and accessory muscles include the sternocleidomastoid, pectoralis, latissimus dorsi, erector spinae, and trapezius muscles. While sleep and breathing problems may begin early in the course of a neuromuscular disease, the complex restrictive lung disease pattern that we see in these patients may not develop until the respiratory muscles of the chest wall are involved. This restriction, which corresponds to lower lung volumes, leads to a fall in the caudal traction force of the airways which can lead to reduction in the pharyngeal airway cross section. Because these issues are worsened in the supine position, their pathophysiologic effects on respiration are most notable during sleep, putting patients at higher risk of OSA.
Cardiac abnormalities
Lastly, it should be noted that diseases such as the muscular dystrophies, myotonic dystrophy, mitochondriopathies, and nemaline myopathy can be associated with a cardiomyopathy ,which can lead to central sleep apnea in the form of Cheyne-Stokes breathing.
Sleep-disordered breathing in specific NMDs
In amyotrophic lateral sclerosis (ALS), up to 75% of patients may have SDB, the majority of which is central sleep apnea (CSA) and hypoventilation although they still have a higher prevalence of obstructive sleep apnea (OSA) than the general population. Whether the diaphragm or the pharyngeal muscles are predominantly affected may have something to do with the type of apnea a patient experiences; however, studies have shown that even in bulbar ALS, CSA is most common. It should be noted, that this is not Cheyne-Stokes CSA, but rather lack of chest wall and abdominal movement due to weakness. (David WS, et al. J Neurol Sci. 1997;152[suppl 1]:S29-35).
In myasthenia gravis (MG), about 40% to 60% of patients have SDB, and about 30% develop overt respiratory weakness, generally late in the course of their disease. Many of these patients report excessive daytime sleepiness, often attributed to myasthenic fatigue requiring treatment with corticosteroids. It is important to evaluate for sleep apnea, given that if diagnosed and treated, their generalized fatigue may improve and the need for steroids may be reduced or eliminated altogether. It is also important to note that the respiratory and sleep issues MG patients face may not correlate with the severity of their overall disease, such that patients well-controlled on medications from a generalized weakness standpoint may still require home noninvasive ventilation (NIV) for chronic respiratory failure due to weakness of the respiratory system muscles.
Duchenne muscular dystrophy (DMD), an X-linked disease associated with dysfunction of dystrophin synthesis, is often diagnosed in early childhood and gradually progresses over years. Their initial sleep and respiratory symptoms can be subtle and may start with increased nighttime awakenings and daytime somnolence. Generally, these patients will develop OSA in the first decade of life and progress to hypoventilation in their second decade and beyond. These patients are especially important to recognize, as studies have shown appropriate NIV therapy may significantly prolong their life (Finder JD, et al; American Thoracic Society. Am J Respir Crit Care Med. 2004(Aug 15);170[4]:456-465).
In addition to the well-known motor neuron and neuromuscular diseases mentioned above, neuropathic diseases can lead to sleep disturbances, as well. In Charcot-Marie-Tooth (CMT), pharyngeal and laryngeal neuropathy, as well as hypoglossal nerve dysfunction, lead to OSA. Similar to ALS and MG, there is a significant amount of CSA and hypoventilation, likely related to phrenic neuropathy. In contrast to MG, in CMT, the severity of neuropathic disease does correlate to the severity of sleep apnea.
Testing
Testing can range from overnight oximetry to polysomnogram (PSG) with CO2 monitoring. Generally, all patients with a rapidly progressive neuromuscular disease should get pulmonary function testing (PFT) (upright and supine) to evaluate forced vital capacity (FVC) every 3 to 6 months to monitor for respiratory failure. Laboratory studies that can be helpful in assessing for SDB are the PaCO2 (> 45 mm Hg) measured on an arterial blood gas and serum bicarbonate levels (>27 mmol/L or a base excess >4 mmol/L). Patients can qualify for NIV with an overnight SaO2 less than or equal to 88% for greater than or equal to 5 minutes in a 2-hour recording period, PaCO2 greater than or equal to 45 mm Hg, forced vital capacity (FVC) < 50% of predicted, or maximal inspiratory pressure (MIP) <60 cm H2O. For ALS specifically, sniff nasal pressure < 40 cm H2O and orthopnea are additional criteria that can be used. It is worth noting that a PSG is not required for NIV qualification in neuromuscular respiratory insufficiency. However, PSG is beneficial in patients with preserved PFTs but suspected of having early nocturnal respiratory impairment.
Therapy
NIV is the mainstay of therapy for SDB in patients with NMD and has been associated with a slower decline in FVC and improved survival in some cases, as demonstrated in studies of patients with DMD or ALS. Generally, a bi-level PAP mode is preferred; the expiratory positive airway pressure prevents micro-atelectasis and improves V/Q matching and the inspiratory positive airway pressure reduces inspiratory muscle load and optimizes ventilation. As weakness progresses, patients may have difficulty creating enough negative force to initiate a spontaneous breath, thus a mode with a set respiratory rate is preferred that can be implemented in bi-level PAP or more advanced modes such as volume-assured pressure support (VAPS) modality. For patients who are unable to tolerate NIV, particularly those with severe bulbar disease and difficult to manage respiratory secretions, tracheostomy with mechanical ventilation may ultimately be needed. This decision should be made as part of a multidisciplinary shared decision-making conversation with the patient, their family, and their team of providers.
Summary
Sleep is a particularly vulnerable state for patients with NMD, and in many patients, disturbances in sleep may be the first clue to their ultimate diagnosis. It is important that sleep medicine and pulmonary specialists understand the pathophysiology and management of NMD as they can play a vital role in the interdisciplinary care of these patients.
Dr. Greer is a Sleep Medicine Fellow, Division of Pulmonary, Allergy, Critical Care, and Sleep Medicine; Dr. Collop is Professor of Medicine and Neurology, Director, Emory Sleep Center; Emory University, Atlanta, Georgia.
Sleep-disordered breathing (SDB) is a common sleep disturbance in neuromuscular disease (NMD) affecting 36% to 53% of diagnosed adults (Arens R, et al. Paediatr Respir Rev. 2010;11[1]:24). Disturbances in sleep may serve as the earliest sign of muscle weakness in these patients, at times being detected before their underlying neuromuscular disease is diagnosed. This is of paramount importance to sleep medicine and pulmonary physicians who may be among the first specialists to evaluate these patients and can play a vital role in the recognition and diagnosis of neuromuscular disease. Herein, we will provide a guide to aid the reader in recognizing the early signs and symptoms of NMD as it pertains to sleep, as earlier diagnosis may lead to improved quality of life or possibly even survival, in some cases.
Pathophysiology
To begin, it is important to understand the pathophysiology of NMD and how it is altered during the sleep state. Sleep-related physiologic changes in healthy humans include reduction in upper airway muscle tone, blunting of chemoreceptors associated with pharyngeal dilator augmentation, and sleep stage-specific changes in skeletal muscle tone. In patients with NMD, these changes may not be adequately compensated for, leading to sleep-disordered breathing that can present as sleep apnea, hypoventilation, or hypoxia (Govindarajan R, et al. Sleep Issues in Neuromuscular Disorders: A Clinical Guide. Springer International Publishing AG, Springer Nature 2018).
Central respiratory control
The respiratory centers in the pons and medulla are generally spared from the primary effects of most NMD; however, over time, they may be affected secondarily. Similar to obesity hypoventilation syndrome (OHS), untreated chronic sleep-related hypoventilation from NMD can impair the sensitivity of respiratory chemoreceptors leading to worsening hypoventilation.
Upper airway resistance
Pharyngeal muscle tone is key to maintaining a patent airway during sleep. In some NMD, bulbar muscle weakness with pharyngeal dilator muscle hypotonia leads to increased upper airway resistance, especially during REM sleep, which can result in obstructive sleep apnea (OSA). In addition to weakness affecting the upper airway musculature, anatomical changes may also contribute to sleep-disordered breathing. In Pompe disease, for example, macroglossia and fibro-fatty replacement of tongue muscles may occur, leading to the development of OSA.
Diaphragm weakness
In NMD that affects the diaphragm, there is an increased reliance on the skeletal muscles of respiration to maintain adequate ventilation as the underlying disease progresses. Generally, weakness of the diaphragm will cause disturbances in REM sleep first as, during REM, ventilation predominately depends on the diaphragm and patients lose the assistance of their skeletal muscles. However, over time, the progressive weakening of the diaphragm will progress to involve NREM sleep as well, clinically manifesting with frank sleep apnea, hypoventilation, and, ultimately, chronic hypercapnic respiratory failure.
Inspiratory muscle weakness
As noted above, there are many other muscles used in inspiration in addition to the diaphragm. Other primary muscles include the intercostal and scalene muscles, and accessory muscles include the sternocleidomastoid, pectoralis, latissimus dorsi, erector spinae, and trapezius muscles. While sleep and breathing problems may begin early in the course of a neuromuscular disease, the complex restrictive lung disease pattern that we see in these patients may not develop until the respiratory muscles of the chest wall are involved. This restriction, which corresponds to lower lung volumes, leads to a fall in the caudal traction force of the airways which can lead to reduction in the pharyngeal airway cross section. Because these issues are worsened in the supine position, their pathophysiologic effects on respiration are most notable during sleep, putting patients at higher risk of OSA.
Cardiac abnormalities
Lastly, it should be noted that diseases such as the muscular dystrophies, myotonic dystrophy, mitochondriopathies, and nemaline myopathy can be associated with a cardiomyopathy ,which can lead to central sleep apnea in the form of Cheyne-Stokes breathing.
Sleep-disordered breathing in specific NMDs
In amyotrophic lateral sclerosis (ALS), up to 75% of patients may have SDB, the majority of which is central sleep apnea (CSA) and hypoventilation although they still have a higher prevalence of obstructive sleep apnea (OSA) than the general population. Whether the diaphragm or the pharyngeal muscles are predominantly affected may have something to do with the type of apnea a patient experiences; however, studies have shown that even in bulbar ALS, CSA is most common. It should be noted, that this is not Cheyne-Stokes CSA, but rather lack of chest wall and abdominal movement due to weakness. (David WS, et al. J Neurol Sci. 1997;152[suppl 1]:S29-35).
In myasthenia gravis (MG), about 40% to 60% of patients have SDB, and about 30% develop overt respiratory weakness, generally late in the course of their disease. Many of these patients report excessive daytime sleepiness, often attributed to myasthenic fatigue requiring treatment with corticosteroids. It is important to evaluate for sleep apnea, given that if diagnosed and treated, their generalized fatigue may improve and the need for steroids may be reduced or eliminated altogether. It is also important to note that the respiratory and sleep issues MG patients face may not correlate with the severity of their overall disease, such that patients well-controlled on medications from a generalized weakness standpoint may still require home noninvasive ventilation (NIV) for chronic respiratory failure due to weakness of the respiratory system muscles.
Duchenne muscular dystrophy (DMD), an X-linked disease associated with dysfunction of dystrophin synthesis, is often diagnosed in early childhood and gradually progresses over years. Their initial sleep and respiratory symptoms can be subtle and may start with increased nighttime awakenings and daytime somnolence. Generally, these patients will develop OSA in the first decade of life and progress to hypoventilation in their second decade and beyond. These patients are especially important to recognize, as studies have shown appropriate NIV therapy may significantly prolong their life (Finder JD, et al; American Thoracic Society. Am J Respir Crit Care Med. 2004(Aug 15);170[4]:456-465).
In addition to the well-known motor neuron and neuromuscular diseases mentioned above, neuropathic diseases can lead to sleep disturbances, as well. In Charcot-Marie-Tooth (CMT), pharyngeal and laryngeal neuropathy, as well as hypoglossal nerve dysfunction, lead to OSA. Similar to ALS and MG, there is a significant amount of CSA and hypoventilation, likely related to phrenic neuropathy. In contrast to MG, in CMT, the severity of neuropathic disease does correlate to the severity of sleep apnea.
Testing
Testing can range from overnight oximetry to polysomnogram (PSG) with CO2 monitoring. Generally, all patients with a rapidly progressive neuromuscular disease should get pulmonary function testing (PFT) (upright and supine) to evaluate forced vital capacity (FVC) every 3 to 6 months to monitor for respiratory failure. Laboratory studies that can be helpful in assessing for SDB are the PaCO2 (> 45 mm Hg) measured on an arterial blood gas and serum bicarbonate levels (>27 mmol/L or a base excess >4 mmol/L). Patients can qualify for NIV with an overnight SaO2 less than or equal to 88% for greater than or equal to 5 minutes in a 2-hour recording period, PaCO2 greater than or equal to 45 mm Hg, forced vital capacity (FVC) < 50% of predicted, or maximal inspiratory pressure (MIP) <60 cm H2O. For ALS specifically, sniff nasal pressure < 40 cm H2O and orthopnea are additional criteria that can be used. It is worth noting that a PSG is not required for NIV qualification in neuromuscular respiratory insufficiency. However, PSG is beneficial in patients with preserved PFTs but suspected of having early nocturnal respiratory impairment.
Therapy
NIV is the mainstay of therapy for SDB in patients with NMD and has been associated with a slower decline in FVC and improved survival in some cases, as demonstrated in studies of patients with DMD or ALS. Generally, a bi-level PAP mode is preferred; the expiratory positive airway pressure prevents micro-atelectasis and improves V/Q matching and the inspiratory positive airway pressure reduces inspiratory muscle load and optimizes ventilation. As weakness progresses, patients may have difficulty creating enough negative force to initiate a spontaneous breath, thus a mode with a set respiratory rate is preferred that can be implemented in bi-level PAP or more advanced modes such as volume-assured pressure support (VAPS) modality. For patients who are unable to tolerate NIV, particularly those with severe bulbar disease and difficult to manage respiratory secretions, tracheostomy with mechanical ventilation may ultimately be needed. This decision should be made as part of a multidisciplinary shared decision-making conversation with the patient, their family, and their team of providers.
Summary
Sleep is a particularly vulnerable state for patients with NMD, and in many patients, disturbances in sleep may be the first clue to their ultimate diagnosis. It is important that sleep medicine and pulmonary specialists understand the pathophysiology and management of NMD as they can play a vital role in the interdisciplinary care of these patients.
Dr. Greer is a Sleep Medicine Fellow, Division of Pulmonary, Allergy, Critical Care, and Sleep Medicine; Dr. Collop is Professor of Medicine and Neurology, Director, Emory Sleep Center; Emory University, Atlanta, Georgia.
Sleep-disordered breathing (SDB) is a common sleep disturbance in neuromuscular disease (NMD) affecting 36% to 53% of diagnosed adults (Arens R, et al. Paediatr Respir Rev. 2010;11[1]:24). Disturbances in sleep may serve as the earliest sign of muscle weakness in these patients, at times being detected before their underlying neuromuscular disease is diagnosed. This is of paramount importance to sleep medicine and pulmonary physicians who may be among the first specialists to evaluate these patients and can play a vital role in the recognition and diagnosis of neuromuscular disease. Herein, we will provide a guide to aid the reader in recognizing the early signs and symptoms of NMD as it pertains to sleep, as earlier diagnosis may lead to improved quality of life or possibly even survival, in some cases.
Pathophysiology
To begin, it is important to understand the pathophysiology of NMD and how it is altered during the sleep state. Sleep-related physiologic changes in healthy humans include reduction in upper airway muscle tone, blunting of chemoreceptors associated with pharyngeal dilator augmentation, and sleep stage-specific changes in skeletal muscle tone. In patients with NMD, these changes may not be adequately compensated for, leading to sleep-disordered breathing that can present as sleep apnea, hypoventilation, or hypoxia (Govindarajan R, et al. Sleep Issues in Neuromuscular Disorders: A Clinical Guide. Springer International Publishing AG, Springer Nature 2018).
Central respiratory control
The respiratory centers in the pons and medulla are generally spared from the primary effects of most NMD; however, over time, they may be affected secondarily. Similar to obesity hypoventilation syndrome (OHS), untreated chronic sleep-related hypoventilation from NMD can impair the sensitivity of respiratory chemoreceptors leading to worsening hypoventilation.
Upper airway resistance
Pharyngeal muscle tone is key to maintaining a patent airway during sleep. In some NMD, bulbar muscle weakness with pharyngeal dilator muscle hypotonia leads to increased upper airway resistance, especially during REM sleep, which can result in obstructive sleep apnea (OSA). In addition to weakness affecting the upper airway musculature, anatomical changes may also contribute to sleep-disordered breathing. In Pompe disease, for example, macroglossia and fibro-fatty replacement of tongue muscles may occur, leading to the development of OSA.
Diaphragm weakness
In NMD that affects the diaphragm, there is an increased reliance on the skeletal muscles of respiration to maintain adequate ventilation as the underlying disease progresses. Generally, weakness of the diaphragm will cause disturbances in REM sleep first as, during REM, ventilation predominately depends on the diaphragm and patients lose the assistance of their skeletal muscles. However, over time, the progressive weakening of the diaphragm will progress to involve NREM sleep as well, clinically manifesting with frank sleep apnea, hypoventilation, and, ultimately, chronic hypercapnic respiratory failure.
Inspiratory muscle weakness
As noted above, there are many other muscles used in inspiration in addition to the diaphragm. Other primary muscles include the intercostal and scalene muscles, and accessory muscles include the sternocleidomastoid, pectoralis, latissimus dorsi, erector spinae, and trapezius muscles. While sleep and breathing problems may begin early in the course of a neuromuscular disease, the complex restrictive lung disease pattern that we see in these patients may not develop until the respiratory muscles of the chest wall are involved. This restriction, which corresponds to lower lung volumes, leads to a fall in the caudal traction force of the airways which can lead to reduction in the pharyngeal airway cross section. Because these issues are worsened in the supine position, their pathophysiologic effects on respiration are most notable during sleep, putting patients at higher risk of OSA.
Cardiac abnormalities
Lastly, it should be noted that diseases such as the muscular dystrophies, myotonic dystrophy, mitochondriopathies, and nemaline myopathy can be associated with a cardiomyopathy ,which can lead to central sleep apnea in the form of Cheyne-Stokes breathing.
Sleep-disordered breathing in specific NMDs
In amyotrophic lateral sclerosis (ALS), up to 75% of patients may have SDB, the majority of which is central sleep apnea (CSA) and hypoventilation although they still have a higher prevalence of obstructive sleep apnea (OSA) than the general population. Whether the diaphragm or the pharyngeal muscles are predominantly affected may have something to do with the type of apnea a patient experiences; however, studies have shown that even in bulbar ALS, CSA is most common. It should be noted, that this is not Cheyne-Stokes CSA, but rather lack of chest wall and abdominal movement due to weakness. (David WS, et al. J Neurol Sci. 1997;152[suppl 1]:S29-35).
In myasthenia gravis (MG), about 40% to 60% of patients have SDB, and about 30% develop overt respiratory weakness, generally late in the course of their disease. Many of these patients report excessive daytime sleepiness, often attributed to myasthenic fatigue requiring treatment with corticosteroids. It is important to evaluate for sleep apnea, given that if diagnosed and treated, their generalized fatigue may improve and the need for steroids may be reduced or eliminated altogether. It is also important to note that the respiratory and sleep issues MG patients face may not correlate with the severity of their overall disease, such that patients well-controlled on medications from a generalized weakness standpoint may still require home noninvasive ventilation (NIV) for chronic respiratory failure due to weakness of the respiratory system muscles.
Duchenne muscular dystrophy (DMD), an X-linked disease associated with dysfunction of dystrophin synthesis, is often diagnosed in early childhood and gradually progresses over years. Their initial sleep and respiratory symptoms can be subtle and may start with increased nighttime awakenings and daytime somnolence. Generally, these patients will develop OSA in the first decade of life and progress to hypoventilation in their second decade and beyond. These patients are especially important to recognize, as studies have shown appropriate NIV therapy may significantly prolong their life (Finder JD, et al; American Thoracic Society. Am J Respir Crit Care Med. 2004(Aug 15);170[4]:456-465).
In addition to the well-known motor neuron and neuromuscular diseases mentioned above, neuropathic diseases can lead to sleep disturbances, as well. In Charcot-Marie-Tooth (CMT), pharyngeal and laryngeal neuropathy, as well as hypoglossal nerve dysfunction, lead to OSA. Similar to ALS and MG, there is a significant amount of CSA and hypoventilation, likely related to phrenic neuropathy. In contrast to MG, in CMT, the severity of neuropathic disease does correlate to the severity of sleep apnea.
Testing
Testing can range from overnight oximetry to polysomnogram (PSG) with CO2 monitoring. Generally, all patients with a rapidly progressive neuromuscular disease should get pulmonary function testing (PFT) (upright and supine) to evaluate forced vital capacity (FVC) every 3 to 6 months to monitor for respiratory failure. Laboratory studies that can be helpful in assessing for SDB are the PaCO2 (> 45 mm Hg) measured on an arterial blood gas and serum bicarbonate levels (>27 mmol/L or a base excess >4 mmol/L). Patients can qualify for NIV with an overnight SaO2 less than or equal to 88% for greater than or equal to 5 minutes in a 2-hour recording period, PaCO2 greater than or equal to 45 mm Hg, forced vital capacity (FVC) < 50% of predicted, or maximal inspiratory pressure (MIP) <60 cm H2O. For ALS specifically, sniff nasal pressure < 40 cm H2O and orthopnea are additional criteria that can be used. It is worth noting that a PSG is not required for NIV qualification in neuromuscular respiratory insufficiency. However, PSG is beneficial in patients with preserved PFTs but suspected of having early nocturnal respiratory impairment.
Therapy
NIV is the mainstay of therapy for SDB in patients with NMD and has been associated with a slower decline in FVC and improved survival in some cases, as demonstrated in studies of patients with DMD or ALS. Generally, a bi-level PAP mode is preferred; the expiratory positive airway pressure prevents micro-atelectasis and improves V/Q matching and the inspiratory positive airway pressure reduces inspiratory muscle load and optimizes ventilation. As weakness progresses, patients may have difficulty creating enough negative force to initiate a spontaneous breath, thus a mode with a set respiratory rate is preferred that can be implemented in bi-level PAP or more advanced modes such as volume-assured pressure support (VAPS) modality. For patients who are unable to tolerate NIV, particularly those with severe bulbar disease and difficult to manage respiratory secretions, tracheostomy with mechanical ventilation may ultimately be needed. This decision should be made as part of a multidisciplinary shared decision-making conversation with the patient, their family, and their team of providers.
Summary
Sleep is a particularly vulnerable state for patients with NMD, and in many patients, disturbances in sleep may be the first clue to their ultimate diagnosis. It is important that sleep medicine and pulmonary specialists understand the pathophysiology and management of NMD as they can play a vital role in the interdisciplinary care of these patients.
Dr. Greer is a Sleep Medicine Fellow, Division of Pulmonary, Allergy, Critical Care, and Sleep Medicine; Dr. Collop is Professor of Medicine and Neurology, Director, Emory Sleep Center; Emory University, Atlanta, Georgia.
OSA diagnoses not carried forward to the inpatient setting
Obstructive sleep apnea diagnoses may not be carried over to the inpatient setting, with potentially negative consequences for clinical outcomes, quality of life, and health care costs, an investigator said at the virtual meeting of the American College of Chest Physicians.
In a retrospective, single-center study, nearly 40% of patients with obstructive sleep apnea (OSA) diagnosed in the outpatient setting did not have a corresponding diagnosis during hospitalization, according to researcher Nitasa Sahu, MD.*
The missed OSA diagnoses could have especially negative implications for patients who don’t continue on positive airway pressure (PAP) therapy during the hospital stay, said Dr. Sahu, a fellow in pulmonary/critical care at St. Luke’s University Health Network in Bethlehem, Pa.
The finding indicates a large-magnitude opportunity to improve health care through better communication and optimized care, according to the researcher.
“Obstructive sleep apnea is underrecognized, it’s underdiagnosed, and it has a lot of implications for a patient’s hospitalization,” she said in interview
Clinical pathways should be set up to ensure that patients with OSA are properly identified and use their prescribed treatment, according to Dr. Sahu.
“I think that should, and would, reduce overall health care costs, with better outcomes as well,” she said.
Pulmonologist Saadia A. Faiz, MD, FCCP, said she hoped this study, presented at a late-breaking abstract at the virtual meeting, would highlight the importance of OSA screening and call attention to barriers to screening that may be in place in the inpatient setting.
That’s especially important because, after admission, the focus is often on the cause of admission rather than underlying comorbidities such as OSA, said Dr. Faiz, professor in the department of pulmonary medicine at the University of Texas MD Anderson Cancer Center in Houston.
“Working in a cancer hospital, the focus is always on the cancer, so sometimes even the patient will dismiss issues with their sleep,” Dr. Faiz said of her own experience in an interview.
“Often with sleep apnea, for people in the general population, the reason they seek medical attention is because their spouse notices that they’re snoring, so it is something that is not as emphasized,” added Dr. Faiz, who was not involved in the study.
In their study, Dr. Sahu and coauthors reviewed electronic health record data for adults hospitalized on the general internal medicine service at Penn State Hershey Medical Center from January 2017 through 2018. They restricted their search to first admissions.
The researchers looked for ICD-9 codes indicating an OSA diagnosis during their inpatient admission. They looked for the same codes in the preceding 5 years to see if the patients had a prior outpatient OSA diagnosis.
The inpatient cohort included 13,067 patients, of whom 53% were male, 87% were White, and 77% were over 50 years of age. Comorbidities included hypertension in 42%, atrial fibrillation in 21%, type 2 diabetes mellitus in 14%, congestive heart failure in 15%, and prior stroke in 0.5%.
A total of 991 individuals in the inpatient cohort had a prior outpatient OSA diagnosis. Of that group, 376 patients (38%) did not have an inpatient OSA diagnosis on inpatient record, according to the reported study data.
That large proportion of discordant diagnoses suggests a lot of missed opportunities to provide OSA therapy in the inpatient setting and to reinforce chronic disease state management, according to Dr. Sahu and colleagues.
How those discordant OSA diagnoses impact length of stay, cost of care, and readmissions are unanswered questions that deserve further study, Dr. Sahu said.
Among patients who did not have outpatient OSA diagnoses, another 804 patients, or about 6%, ended up with an inpatient diagnosis during their hospitalization, the researchers also reported.
While a number of those inpatient OSA diagnoses could have been coded in error, it’s also possible that they were indeed cases of OSA that went unrecognized until the individuals were hospitalized, Dr. Sahu said.
Dr. Sahu had no relevant relationships to report related to the study. One of four study coauthors reported relationships with Boehringer-Ingelheim, Nitto Denko, and Galapagos.
SOURCE: Sahu N. CHEST 2020. Abstract.
*Correction, 11/3/20: An earlier version of this article misstated the name of Nitasa Sahu, MD.
Obstructive sleep apnea diagnoses may not be carried over to the inpatient setting, with potentially negative consequences for clinical outcomes, quality of life, and health care costs, an investigator said at the virtual meeting of the American College of Chest Physicians.
In a retrospective, single-center study, nearly 40% of patients with obstructive sleep apnea (OSA) diagnosed in the outpatient setting did not have a corresponding diagnosis during hospitalization, according to researcher Nitasa Sahu, MD.*
The missed OSA diagnoses could have especially negative implications for patients who don’t continue on positive airway pressure (PAP) therapy during the hospital stay, said Dr. Sahu, a fellow in pulmonary/critical care at St. Luke’s University Health Network in Bethlehem, Pa.
The finding indicates a large-magnitude opportunity to improve health care through better communication and optimized care, according to the researcher.
“Obstructive sleep apnea is underrecognized, it’s underdiagnosed, and it has a lot of implications for a patient’s hospitalization,” she said in interview
Clinical pathways should be set up to ensure that patients with OSA are properly identified and use their prescribed treatment, according to Dr. Sahu.
“I think that should, and would, reduce overall health care costs, with better outcomes as well,” she said.
Pulmonologist Saadia A. Faiz, MD, FCCP, said she hoped this study, presented at a late-breaking abstract at the virtual meeting, would highlight the importance of OSA screening and call attention to barriers to screening that may be in place in the inpatient setting.
That’s especially important because, after admission, the focus is often on the cause of admission rather than underlying comorbidities such as OSA, said Dr. Faiz, professor in the department of pulmonary medicine at the University of Texas MD Anderson Cancer Center in Houston.
“Working in a cancer hospital, the focus is always on the cancer, so sometimes even the patient will dismiss issues with their sleep,” Dr. Faiz said of her own experience in an interview.
“Often with sleep apnea, for people in the general population, the reason they seek medical attention is because their spouse notices that they’re snoring, so it is something that is not as emphasized,” added Dr. Faiz, who was not involved in the study.
In their study, Dr. Sahu and coauthors reviewed electronic health record data for adults hospitalized on the general internal medicine service at Penn State Hershey Medical Center from January 2017 through 2018. They restricted their search to first admissions.
The researchers looked for ICD-9 codes indicating an OSA diagnosis during their inpatient admission. They looked for the same codes in the preceding 5 years to see if the patients had a prior outpatient OSA diagnosis.
The inpatient cohort included 13,067 patients, of whom 53% were male, 87% were White, and 77% were over 50 years of age. Comorbidities included hypertension in 42%, atrial fibrillation in 21%, type 2 diabetes mellitus in 14%, congestive heart failure in 15%, and prior stroke in 0.5%.
A total of 991 individuals in the inpatient cohort had a prior outpatient OSA diagnosis. Of that group, 376 patients (38%) did not have an inpatient OSA diagnosis on inpatient record, according to the reported study data.
That large proportion of discordant diagnoses suggests a lot of missed opportunities to provide OSA therapy in the inpatient setting and to reinforce chronic disease state management, according to Dr. Sahu and colleagues.
How those discordant OSA diagnoses impact length of stay, cost of care, and readmissions are unanswered questions that deserve further study, Dr. Sahu said.
Among patients who did not have outpatient OSA diagnoses, another 804 patients, or about 6%, ended up with an inpatient diagnosis during their hospitalization, the researchers also reported.
While a number of those inpatient OSA diagnoses could have been coded in error, it’s also possible that they were indeed cases of OSA that went unrecognized until the individuals were hospitalized, Dr. Sahu said.
Dr. Sahu had no relevant relationships to report related to the study. One of four study coauthors reported relationships with Boehringer-Ingelheim, Nitto Denko, and Galapagos.
SOURCE: Sahu N. CHEST 2020. Abstract.
*Correction, 11/3/20: An earlier version of this article misstated the name of Nitasa Sahu, MD.
Obstructive sleep apnea diagnoses may not be carried over to the inpatient setting, with potentially negative consequences for clinical outcomes, quality of life, and health care costs, an investigator said at the virtual meeting of the American College of Chest Physicians.
In a retrospective, single-center study, nearly 40% of patients with obstructive sleep apnea (OSA) diagnosed in the outpatient setting did not have a corresponding diagnosis during hospitalization, according to researcher Nitasa Sahu, MD.*
The missed OSA diagnoses could have especially negative implications for patients who don’t continue on positive airway pressure (PAP) therapy during the hospital stay, said Dr. Sahu, a fellow in pulmonary/critical care at St. Luke’s University Health Network in Bethlehem, Pa.
The finding indicates a large-magnitude opportunity to improve health care through better communication and optimized care, according to the researcher.
“Obstructive sleep apnea is underrecognized, it’s underdiagnosed, and it has a lot of implications for a patient’s hospitalization,” she said in interview
Clinical pathways should be set up to ensure that patients with OSA are properly identified and use their prescribed treatment, according to Dr. Sahu.
“I think that should, and would, reduce overall health care costs, with better outcomes as well,” she said.
Pulmonologist Saadia A. Faiz, MD, FCCP, said she hoped this study, presented at a late-breaking abstract at the virtual meeting, would highlight the importance of OSA screening and call attention to barriers to screening that may be in place in the inpatient setting.
That’s especially important because, after admission, the focus is often on the cause of admission rather than underlying comorbidities such as OSA, said Dr. Faiz, professor in the department of pulmonary medicine at the University of Texas MD Anderson Cancer Center in Houston.
“Working in a cancer hospital, the focus is always on the cancer, so sometimes even the patient will dismiss issues with their sleep,” Dr. Faiz said of her own experience in an interview.
“Often with sleep apnea, for people in the general population, the reason they seek medical attention is because their spouse notices that they’re snoring, so it is something that is not as emphasized,” added Dr. Faiz, who was not involved in the study.
In their study, Dr. Sahu and coauthors reviewed electronic health record data for adults hospitalized on the general internal medicine service at Penn State Hershey Medical Center from January 2017 through 2018. They restricted their search to first admissions.
The researchers looked for ICD-9 codes indicating an OSA diagnosis during their inpatient admission. They looked for the same codes in the preceding 5 years to see if the patients had a prior outpatient OSA diagnosis.
The inpatient cohort included 13,067 patients, of whom 53% were male, 87% were White, and 77% were over 50 years of age. Comorbidities included hypertension in 42%, atrial fibrillation in 21%, type 2 diabetes mellitus in 14%, congestive heart failure in 15%, and prior stroke in 0.5%.
A total of 991 individuals in the inpatient cohort had a prior outpatient OSA diagnosis. Of that group, 376 patients (38%) did not have an inpatient OSA diagnosis on inpatient record, according to the reported study data.
That large proportion of discordant diagnoses suggests a lot of missed opportunities to provide OSA therapy in the inpatient setting and to reinforce chronic disease state management, according to Dr. Sahu and colleagues.
How those discordant OSA diagnoses impact length of stay, cost of care, and readmissions are unanswered questions that deserve further study, Dr. Sahu said.
Among patients who did not have outpatient OSA diagnoses, another 804 patients, or about 6%, ended up with an inpatient diagnosis during their hospitalization, the researchers also reported.
While a number of those inpatient OSA diagnoses could have been coded in error, it’s also possible that they were indeed cases of OSA that went unrecognized until the individuals were hospitalized, Dr. Sahu said.
Dr. Sahu had no relevant relationships to report related to the study. One of four study coauthors reported relationships with Boehringer-Ingelheim, Nitto Denko, and Galapagos.
SOURCE: Sahu N. CHEST 2020. Abstract.
*Correction, 11/3/20: An earlier version of this article misstated the name of Nitasa Sahu, MD.
FROM CHEST 2020
Use of e-cigarettes may be linked to sleep deprivation
compared with those who have never used e-cigarettes, according to the first study to evaluate the association in a large, nationally representative population of young adults.
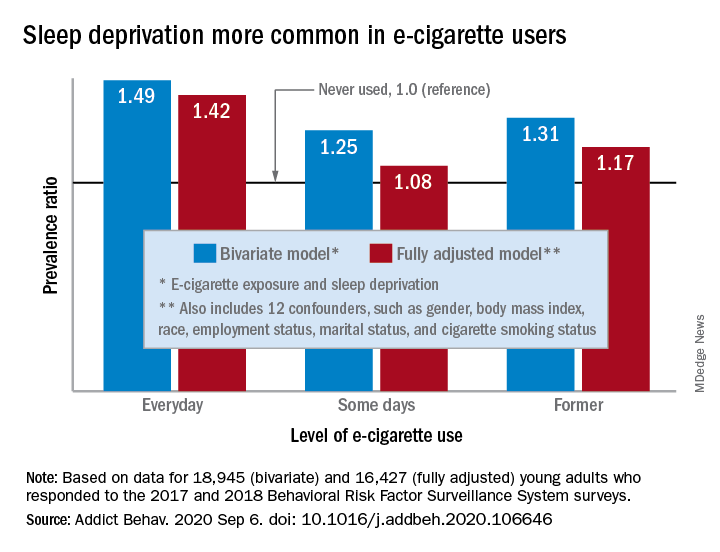
“The e-cigarette use and sleep deprivation association seems to have a dose-response nature as the point estimate of the association increased with increased exposure to e-cigarette,” Sina Kianersi, DVM, and associates at Indiana University, Bloomington, said in Addictive Behaviors.
Sleep deprivation was 49% more prevalent among everyday users of e-cigarettes, compared with nonusers. Prevalence ratios for former users (1.31) and occasional users (1.25) also showed significantly higher sleep deprivation, compared with nonusers, they reported based on a bivariate analysis of data from young adults aged 18-24 years who participated in the 2017 and 2018 Behavioral Risk Factor Surveillance System surveys.
After adjustment for multiple confounders, young adults who currently used e-cigarettes every day were 42% more likely to report sleep deprivation than those who never used e-cigarettes, a difference that was statistically significant. The prevalence of sleep deprivation among those who used e-cigarettes on some days was not significantly higher (prevalence ratio, 1.08), but the ratio between former users and never users was a significant 1.17, the investigators said.
“The nicotine in the inhaled e-cigarette aerosols may have negative effects on sleep architecture and disturb the neurotransmitters that regulate sleep cycle,” they suggested, and since higher doses of nicotine produce greater reductions in sleep duration, “those who use e-cigarette on a daily basis might consume higher doses of nicotine, compared to some days, former, and never users, and therefore get fewer hours of sleep.”
Nicotine withdrawal, on the other hand, has been found to increase sleep duration in a dose-dependent manner, which “could explain the smaller [prevalence ratios] observed for the association between e-cigarette use and sleep deprivation among former and some days e-cigarette users,” Dr. Kianersi and associates added.
The bivariate analysis involved 18,945 survey respondents, of whom 16,427 were included in the fully adjusted model using 12 confounding factors.
SOURCE: Kianersi S et al. Addict Behav. 2020 Sep 6. doi: 10.1016/j.addbeh.2020.106646.
compared with those who have never used e-cigarettes, according to the first study to evaluate the association in a large, nationally representative population of young adults.

“The e-cigarette use and sleep deprivation association seems to have a dose-response nature as the point estimate of the association increased with increased exposure to e-cigarette,” Sina Kianersi, DVM, and associates at Indiana University, Bloomington, said in Addictive Behaviors.
Sleep deprivation was 49% more prevalent among everyday users of e-cigarettes, compared with nonusers. Prevalence ratios for former users (1.31) and occasional users (1.25) also showed significantly higher sleep deprivation, compared with nonusers, they reported based on a bivariate analysis of data from young adults aged 18-24 years who participated in the 2017 and 2018 Behavioral Risk Factor Surveillance System surveys.
After adjustment for multiple confounders, young adults who currently used e-cigarettes every day were 42% more likely to report sleep deprivation than those who never used e-cigarettes, a difference that was statistically significant. The prevalence of sleep deprivation among those who used e-cigarettes on some days was not significantly higher (prevalence ratio, 1.08), but the ratio between former users and never users was a significant 1.17, the investigators said.
“The nicotine in the inhaled e-cigarette aerosols may have negative effects on sleep architecture and disturb the neurotransmitters that regulate sleep cycle,” they suggested, and since higher doses of nicotine produce greater reductions in sleep duration, “those who use e-cigarette on a daily basis might consume higher doses of nicotine, compared to some days, former, and never users, and therefore get fewer hours of sleep.”
Nicotine withdrawal, on the other hand, has been found to increase sleep duration in a dose-dependent manner, which “could explain the smaller [prevalence ratios] observed for the association between e-cigarette use and sleep deprivation among former and some days e-cigarette users,” Dr. Kianersi and associates added.
The bivariate analysis involved 18,945 survey respondents, of whom 16,427 were included in the fully adjusted model using 12 confounding factors.
SOURCE: Kianersi S et al. Addict Behav. 2020 Sep 6. doi: 10.1016/j.addbeh.2020.106646.
compared with those who have never used e-cigarettes, according to the first study to evaluate the association in a large, nationally representative population of young adults.

“The e-cigarette use and sleep deprivation association seems to have a dose-response nature as the point estimate of the association increased with increased exposure to e-cigarette,” Sina Kianersi, DVM, and associates at Indiana University, Bloomington, said in Addictive Behaviors.
Sleep deprivation was 49% more prevalent among everyday users of e-cigarettes, compared with nonusers. Prevalence ratios for former users (1.31) and occasional users (1.25) also showed significantly higher sleep deprivation, compared with nonusers, they reported based on a bivariate analysis of data from young adults aged 18-24 years who participated in the 2017 and 2018 Behavioral Risk Factor Surveillance System surveys.
After adjustment for multiple confounders, young adults who currently used e-cigarettes every day were 42% more likely to report sleep deprivation than those who never used e-cigarettes, a difference that was statistically significant. The prevalence of sleep deprivation among those who used e-cigarettes on some days was not significantly higher (prevalence ratio, 1.08), but the ratio between former users and never users was a significant 1.17, the investigators said.
“The nicotine in the inhaled e-cigarette aerosols may have negative effects on sleep architecture and disturb the neurotransmitters that regulate sleep cycle,” they suggested, and since higher doses of nicotine produce greater reductions in sleep duration, “those who use e-cigarette on a daily basis might consume higher doses of nicotine, compared to some days, former, and never users, and therefore get fewer hours of sleep.”
Nicotine withdrawal, on the other hand, has been found to increase sleep duration in a dose-dependent manner, which “could explain the smaller [prevalence ratios] observed for the association between e-cigarette use and sleep deprivation among former and some days e-cigarette users,” Dr. Kianersi and associates added.
The bivariate analysis involved 18,945 survey respondents, of whom 16,427 were included in the fully adjusted model using 12 confounding factors.
SOURCE: Kianersi S et al. Addict Behav. 2020 Sep 6. doi: 10.1016/j.addbeh.2020.106646.
FROM ADDICTIVE BEHAVIORS
Screening algorithm safely selects patients for OSA treatment before bariatric surgery
A novel algorithm for selecting patients who require treatment for obstructive sleep apnea (OSA) before undergoing bariatric surgery proved safe in a prospective cohort study of 1,103 patients.
Screening for OSA is recommended before bariatric surgery. OSA has been associated in several meta-analyses with increased risk for postoperative complications – not limited to bariatric surgery – and some studies have suggested that this increased risk may be limited to severe OSA, said Frédéric Series, MD, of Université Laval, Quebec City, at the virtual annual meeting of the Associated Sleep Societies.
The preoperative screening algorithm, which utilizes the results of nocturnal home oximetry and morning capillary gas measurements, effectively stratified patients for the risk of postoperative adverse events and “safely selected patients who don’t need [continuous positive airway pressure] before bariatric surgery,” he said. “The risk of postoperative adverse events following bariatric surgery was not increased in untreated OSA patients with low or moderate risk of severe OSA and hypoventilation.”
The study also demonstrated, he said, that patients with severe OSA with or without hypoventilation, even when correctly treated, remain at higher risk for complications.
The algorithm utilizes an oxygen desaturation index (ODI) corresponding to 3% drops in SaO2 and the percent of the total recording time with an SaO2 below 90%, as well as capillary gas measurements (PCO2). Treatment was initiated for those with severe OSA (ODI ≥ 25/hr, < 10% of recording time with a SaO2 below 90%) or OSA with hypoventilation (PCO2 ≥ 45).
“When the ODI was less than 25 per hour, and when the total recording time spent below 90% SaO2 was less than 10%, with PCO2 < 45 mmHg, we expected no need for CPAP treatment,” Dr. Series said. For analysis, the investigators considered part of the untreated group – those with an ODI < 10/hr (no or mild OSA) – as a control group.
Treated patients underwent CPAP/BiPAP for a mean duration of 1.5 months. Good treatment compliance was mandatory for surgery, and treatment was continued immediately after extubation, in the recovery room, in nearly all patients, Dr. Series reported.
The analysis covered 1,103 patients: 447 controls (40.8%), 358 untreated (32.7%), 289 treated for OSA (26.4%) and 9 (0.8%) treated for OSA + hypoventilation. Patients with OSA, particularly those with severe OSA and those with hypoventilation, were older and heavier and significantly more likely to have hypertension and diabetes than controls.
There were no differences between the four groups in 10-day reoperation or 30-day readmission occurrence, and postoperative complications were “particularly infrequent in the control and OSA-untreated groups, with no differences between these two groups,” Dr. Series said.
Cardiac arrhythmia (mainly atrial fibrillation) occurred more frequently in the OSA-treated group (2.4%) and the OSA/hypoventilation patients (11%) than in the other groups (0.5%-0.6%).
Respiratory failure occurred in about one-third of patients with hypoventilation, and admission to the ICU was “dramatically higher” in patients with hypoventilation (67%), because of respiratory failure, arrhythmia, or other unstable medical conditions, Dr. Series said.
There were no differences between the groups in the duration of surgery or the amount of anesthetic used, but the length of stay in the recovery room was significantly longer in the OSA-treated and hypoventilation groups. The length of hospital stay was also longer in these groups. Sleeve gastrectomy was the most frequent bariatric surgical procedure across all groups, including 100% of patients with hypoventilation, he noted.
Asked to comment on the study, Octavian C. Ioachimescu, MD, PhD, of Emory University in Atlanta and the Atlanta Veterans Affairs Medical Center in Decatur, said the algorithm “clearly deserves further validation in other clinical-based cohorts and longer-term outcome assessment.”
Dr. Series reported that he has no relevant disclosures. Dr. Ioachimescu also said he has no relevant disclosures.
A novel algorithm for selecting patients who require treatment for obstructive sleep apnea (OSA) before undergoing bariatric surgery proved safe in a prospective cohort study of 1,103 patients.
Screening for OSA is recommended before bariatric surgery. OSA has been associated in several meta-analyses with increased risk for postoperative complications – not limited to bariatric surgery – and some studies have suggested that this increased risk may be limited to severe OSA, said Frédéric Series, MD, of Université Laval, Quebec City, at the virtual annual meeting of the Associated Sleep Societies.
The preoperative screening algorithm, which utilizes the results of nocturnal home oximetry and morning capillary gas measurements, effectively stratified patients for the risk of postoperative adverse events and “safely selected patients who don’t need [continuous positive airway pressure] before bariatric surgery,” he said. “The risk of postoperative adverse events following bariatric surgery was not increased in untreated OSA patients with low or moderate risk of severe OSA and hypoventilation.”
The study also demonstrated, he said, that patients with severe OSA with or without hypoventilation, even when correctly treated, remain at higher risk for complications.
The algorithm utilizes an oxygen desaturation index (ODI) corresponding to 3% drops in SaO2 and the percent of the total recording time with an SaO2 below 90%, as well as capillary gas measurements (PCO2). Treatment was initiated for those with severe OSA (ODI ≥ 25/hr, < 10% of recording time with a SaO2 below 90%) or OSA with hypoventilation (PCO2 ≥ 45).
“When the ODI was less than 25 per hour, and when the total recording time spent below 90% SaO2 was less than 10%, with PCO2 < 45 mmHg, we expected no need for CPAP treatment,” Dr. Series said. For analysis, the investigators considered part of the untreated group – those with an ODI < 10/hr (no or mild OSA) – as a control group.
Treated patients underwent CPAP/BiPAP for a mean duration of 1.5 months. Good treatment compliance was mandatory for surgery, and treatment was continued immediately after extubation, in the recovery room, in nearly all patients, Dr. Series reported.
The analysis covered 1,103 patients: 447 controls (40.8%), 358 untreated (32.7%), 289 treated for OSA (26.4%) and 9 (0.8%) treated for OSA + hypoventilation. Patients with OSA, particularly those with severe OSA and those with hypoventilation, were older and heavier and significantly more likely to have hypertension and diabetes than controls.
There were no differences between the four groups in 10-day reoperation or 30-day readmission occurrence, and postoperative complications were “particularly infrequent in the control and OSA-untreated groups, with no differences between these two groups,” Dr. Series said.
Cardiac arrhythmia (mainly atrial fibrillation) occurred more frequently in the OSA-treated group (2.4%) and the OSA/hypoventilation patients (11%) than in the other groups (0.5%-0.6%).
Respiratory failure occurred in about one-third of patients with hypoventilation, and admission to the ICU was “dramatically higher” in patients with hypoventilation (67%), because of respiratory failure, arrhythmia, or other unstable medical conditions, Dr. Series said.
There were no differences between the groups in the duration of surgery or the amount of anesthetic used, but the length of stay in the recovery room was significantly longer in the OSA-treated and hypoventilation groups. The length of hospital stay was also longer in these groups. Sleeve gastrectomy was the most frequent bariatric surgical procedure across all groups, including 100% of patients with hypoventilation, he noted.
Asked to comment on the study, Octavian C. Ioachimescu, MD, PhD, of Emory University in Atlanta and the Atlanta Veterans Affairs Medical Center in Decatur, said the algorithm “clearly deserves further validation in other clinical-based cohorts and longer-term outcome assessment.”
Dr. Series reported that he has no relevant disclosures. Dr. Ioachimescu also said he has no relevant disclosures.
A novel algorithm for selecting patients who require treatment for obstructive sleep apnea (OSA) before undergoing bariatric surgery proved safe in a prospective cohort study of 1,103 patients.
Screening for OSA is recommended before bariatric surgery. OSA has been associated in several meta-analyses with increased risk for postoperative complications – not limited to bariatric surgery – and some studies have suggested that this increased risk may be limited to severe OSA, said Frédéric Series, MD, of Université Laval, Quebec City, at the virtual annual meeting of the Associated Sleep Societies.
The preoperative screening algorithm, which utilizes the results of nocturnal home oximetry and morning capillary gas measurements, effectively stratified patients for the risk of postoperative adverse events and “safely selected patients who don’t need [continuous positive airway pressure] before bariatric surgery,” he said. “The risk of postoperative adverse events following bariatric surgery was not increased in untreated OSA patients with low or moderate risk of severe OSA and hypoventilation.”
The study also demonstrated, he said, that patients with severe OSA with or without hypoventilation, even when correctly treated, remain at higher risk for complications.
The algorithm utilizes an oxygen desaturation index (ODI) corresponding to 3% drops in SaO2 and the percent of the total recording time with an SaO2 below 90%, as well as capillary gas measurements (PCO2). Treatment was initiated for those with severe OSA (ODI ≥ 25/hr, < 10% of recording time with a SaO2 below 90%) or OSA with hypoventilation (PCO2 ≥ 45).
“When the ODI was less than 25 per hour, and when the total recording time spent below 90% SaO2 was less than 10%, with PCO2 < 45 mmHg, we expected no need for CPAP treatment,” Dr. Series said. For analysis, the investigators considered part of the untreated group – those with an ODI < 10/hr (no or mild OSA) – as a control group.
Treated patients underwent CPAP/BiPAP for a mean duration of 1.5 months. Good treatment compliance was mandatory for surgery, and treatment was continued immediately after extubation, in the recovery room, in nearly all patients, Dr. Series reported.
The analysis covered 1,103 patients: 447 controls (40.8%), 358 untreated (32.7%), 289 treated for OSA (26.4%) and 9 (0.8%) treated for OSA + hypoventilation. Patients with OSA, particularly those with severe OSA and those with hypoventilation, were older and heavier and significantly more likely to have hypertension and diabetes than controls.
There were no differences between the four groups in 10-day reoperation or 30-day readmission occurrence, and postoperative complications were “particularly infrequent in the control and OSA-untreated groups, with no differences between these two groups,” Dr. Series said.
Cardiac arrhythmia (mainly atrial fibrillation) occurred more frequently in the OSA-treated group (2.4%) and the OSA/hypoventilation patients (11%) than in the other groups (0.5%-0.6%).
Respiratory failure occurred in about one-third of patients with hypoventilation, and admission to the ICU was “dramatically higher” in patients with hypoventilation (67%), because of respiratory failure, arrhythmia, or other unstable medical conditions, Dr. Series said.
There were no differences between the groups in the duration of surgery or the amount of anesthetic used, but the length of stay in the recovery room was significantly longer in the OSA-treated and hypoventilation groups. The length of hospital stay was also longer in these groups. Sleeve gastrectomy was the most frequent bariatric surgical procedure across all groups, including 100% of patients with hypoventilation, he noted.
Asked to comment on the study, Octavian C. Ioachimescu, MD, PhD, of Emory University in Atlanta and the Atlanta Veterans Affairs Medical Center in Decatur, said the algorithm “clearly deserves further validation in other clinical-based cohorts and longer-term outcome assessment.”
Dr. Series reported that he has no relevant disclosures. Dr. Ioachimescu also said he has no relevant disclosures.
REPORTING FROM SLEEP 2020
FDA orders stronger warnings on benzodiazepines
The Food and Drug Administration wants updated boxed warnings on benzodiazepines to reflect the “serious” risks of abuse, misuse, addiction, physical dependence, and withdrawal reactions associated with these medications.
“The current prescribing information for benzodiazepines does not provide adequate warnings about these serious risks and harms associated with these medicines so they may be prescribed and used inappropriately,” the FDA said in a safety communication.
The FDA also wants revisions to the patient medication guides for benzodiazepines to help educate patients and caregivers about these risks.
“While benzodiazepines are important therapies for many Americans, they are also commonly abused and misused, often together with opioid pain relievers and other medicines, alcohol, and illicit drugs,” FDA Commissioner Stephen M. Hahn, MD, said in a statement.
“We are taking measures and requiring new labeling information to help health care professionals and patients better understand that, while benzodiazepines have many treatment benefits, they also carry with them an increased risk of abuse, misuse, addiction, and dependence,” said Dr. Hahn.
Ninety-two million prescriptions in 2019
Benzodiazepines are widely used to treat anxiety, insomnia, seizures, and other conditions, often for extended periods of time.
According to the FDA, in 2019, an estimated 92 million benzodiazepine prescriptions were dispensed from U.S. outpatient pharmacies, most commonly alprazolam, clonazepam, and lorazepam.
Data from 2018 show that roughly 5.4 million people in the United States 12 years and older abused or misused benzodiazepines in the previous year.
Although the precise risk of benzodiazepine addiction remains unclear, population data “clearly indicate that both primary benzodiazepine use disorders and polysubstance addiction involving benzodiazepines do occur,” the FDA said.
Data from the National Survey on Drug Use and Health from 2015-2016 suggest that half million community-dwelling U.S. adults were estimated to have a benzodiazepine use disorder.
Jump in overdose deaths
Overdose deaths involving benzodiazepines jumped from 1,298 in 2010 to 11,537 in 2017 – an increase of more 780%. Most of these deaths involved benzodiazepines taken with prescription opioids.
the FDA said.
The agency urged particular caution when prescribing benzodiazepines with opioids and other central nervous system depressants, which has resulted in serious adverse events including severe respiratory depression and death.
The FDA also says patients and caregivers should be warned about the risks of abuse, misuse, addiction, dependence, and withdrawal with benzodiazepines and the associated signs and symptoms.
Physicians are encouraged to report adverse events involving benzodiazepines or other medicines to the FDA’s MedWatch program.
A version of this article originally appeared on Medscape.com.
The Food and Drug Administration wants updated boxed warnings on benzodiazepines to reflect the “serious” risks of abuse, misuse, addiction, physical dependence, and withdrawal reactions associated with these medications.
“The current prescribing information for benzodiazepines does not provide adequate warnings about these serious risks and harms associated with these medicines so they may be prescribed and used inappropriately,” the FDA said in a safety communication.
The FDA also wants revisions to the patient medication guides for benzodiazepines to help educate patients and caregivers about these risks.
“While benzodiazepines are important therapies for many Americans, they are also commonly abused and misused, often together with opioid pain relievers and other medicines, alcohol, and illicit drugs,” FDA Commissioner Stephen M. Hahn, MD, said in a statement.
“We are taking measures and requiring new labeling information to help health care professionals and patients better understand that, while benzodiazepines have many treatment benefits, they also carry with them an increased risk of abuse, misuse, addiction, and dependence,” said Dr. Hahn.
Ninety-two million prescriptions in 2019
Benzodiazepines are widely used to treat anxiety, insomnia, seizures, and other conditions, often for extended periods of time.
According to the FDA, in 2019, an estimated 92 million benzodiazepine prescriptions were dispensed from U.S. outpatient pharmacies, most commonly alprazolam, clonazepam, and lorazepam.
Data from 2018 show that roughly 5.4 million people in the United States 12 years and older abused or misused benzodiazepines in the previous year.
Although the precise risk of benzodiazepine addiction remains unclear, population data “clearly indicate that both primary benzodiazepine use disorders and polysubstance addiction involving benzodiazepines do occur,” the FDA said.
Data from the National Survey on Drug Use and Health from 2015-2016 suggest that half million community-dwelling U.S. adults were estimated to have a benzodiazepine use disorder.
Jump in overdose deaths
Overdose deaths involving benzodiazepines jumped from 1,298 in 2010 to 11,537 in 2017 – an increase of more 780%. Most of these deaths involved benzodiazepines taken with prescription opioids.
the FDA said.
The agency urged particular caution when prescribing benzodiazepines with opioids and other central nervous system depressants, which has resulted in serious adverse events including severe respiratory depression and death.
The FDA also says patients and caregivers should be warned about the risks of abuse, misuse, addiction, dependence, and withdrawal with benzodiazepines and the associated signs and symptoms.
Physicians are encouraged to report adverse events involving benzodiazepines or other medicines to the FDA’s MedWatch program.
A version of this article originally appeared on Medscape.com.
The Food and Drug Administration wants updated boxed warnings on benzodiazepines to reflect the “serious” risks of abuse, misuse, addiction, physical dependence, and withdrawal reactions associated with these medications.
“The current prescribing information for benzodiazepines does not provide adequate warnings about these serious risks and harms associated with these medicines so they may be prescribed and used inappropriately,” the FDA said in a safety communication.
The FDA also wants revisions to the patient medication guides for benzodiazepines to help educate patients and caregivers about these risks.
“While benzodiazepines are important therapies for many Americans, they are also commonly abused and misused, often together with opioid pain relievers and other medicines, alcohol, and illicit drugs,” FDA Commissioner Stephen M. Hahn, MD, said in a statement.
“We are taking measures and requiring new labeling information to help health care professionals and patients better understand that, while benzodiazepines have many treatment benefits, they also carry with them an increased risk of abuse, misuse, addiction, and dependence,” said Dr. Hahn.
Ninety-two million prescriptions in 2019
Benzodiazepines are widely used to treat anxiety, insomnia, seizures, and other conditions, often for extended periods of time.
According to the FDA, in 2019, an estimated 92 million benzodiazepine prescriptions were dispensed from U.S. outpatient pharmacies, most commonly alprazolam, clonazepam, and lorazepam.
Data from 2018 show that roughly 5.4 million people in the United States 12 years and older abused or misused benzodiazepines in the previous year.
Although the precise risk of benzodiazepine addiction remains unclear, population data “clearly indicate that both primary benzodiazepine use disorders and polysubstance addiction involving benzodiazepines do occur,” the FDA said.
Data from the National Survey on Drug Use and Health from 2015-2016 suggest that half million community-dwelling U.S. adults were estimated to have a benzodiazepine use disorder.
Jump in overdose deaths
Overdose deaths involving benzodiazepines jumped from 1,298 in 2010 to 11,537 in 2017 – an increase of more 780%. Most of these deaths involved benzodiazepines taken with prescription opioids.
the FDA said.
The agency urged particular caution when prescribing benzodiazepines with opioids and other central nervous system depressants, which has resulted in serious adverse events including severe respiratory depression and death.
The FDA also says patients and caregivers should be warned about the risks of abuse, misuse, addiction, dependence, and withdrawal with benzodiazepines and the associated signs and symptoms.
Physicians are encouraged to report adverse events involving benzodiazepines or other medicines to the FDA’s MedWatch program.
A version of this article originally appeared on Medscape.com.
Study validates OSA phenotypes in Latinos
Three previously described clinical phenotypes of obstructive sleep apnea (OSA) have been validated in a large and diverse Hispanic/Latino community-based population for the first time, according to findings presented at the virtual annual meeting of the Associated Professional Sleep Societies.
The three OSA symptom profiles present in this population – labeled “minimally symptomatic,” “disturbed sleep,” and “daytime sleepiness” – are consistent with recent findings from the Sleep Apnea Global Interdisciplinary Consortium, which were published in Sleep, but there are notable differences in the prevalence of these clusters, with the minimally symptomatic cluster much more prevalent than in prior research, reported Kevin Gonzalez, of the University of California, San Diego.
“Other biopsychosocial factors may be contributing to OSA phenotypes among Hispanics and Latinos,” Mr. Gonzalez said in his presentation. Prior research to characterize the heterogeneity of sleep apnea has not included a diverse Latino population, he emphasized.
The adults studied were aged 18-74 years and participants in the multisite Hispanic Community Health Study/Study of Latinos (HCHS/SOL), a comprehensive study of Hispanic/Latino health and disease in the United States. Their respiratory events were measured overnight in HCHS/SOL sleep reading centers with an ARES Unicorder 5.2, B-Alert. Sleep patterns and risk factors were assessed using the Sleep Heart Health Study Sleep Habits Questionnaire and the Epworth Sleepiness Scale.
Participants meeting the criteria for moderate to severe OSA (with an Apnea Hypopnea Index of 15 or above) were included in the analysis (n = 1,623). Their average age was 52.4 ± 13.9 years, and 34.1% were female.
To identify phenotype clusters, investigators performed a latent class analysis using 15 common OSA symptoms and a survey weighted to adjust for selection bias. The three clusters offering the “best” fit for the data aligned with the previously reported phenotypes and identified daytime sleepiness in 15.3%, disturbed sleep (insomnia-like symptoms) in 37.7%, and minimally symptomatic (a low symptom profile) in 46.9%.
These phenotypes were reported in the European Respiratory Journal in 2014 in a cluster analysis of data from a sleep apnea cohort in Iceland and later replicated in the analysis of data from the Sleep Apnea Global Interdisciplinary Consortium published in Sleep in 2018. The consortium study also added two additional phenotypes, labeled “upper airway symptoms dominant” and “sleepiness dominant.”
The prevalence of a “minimally symptomatic group” in the new analysis of the Hispanics/Latinos in the United States is much higher than reported in these prior studies, at least partly, the investigators believed, because the “prior studies were clinical samples, and the people who were minimally symptomatic didn’t get to the sleep centers,” Mr. Gonzalez said in an interview after the meeting.
Patients with a phenotype of daytime sleepiness – the most common phenotype in prior research – constituted only a minority in the Hispanic/Latino population, he said.
Alberto Ramos, MD, of the University of Miami and the principal investigator, said in an interview that the research team is currently analyzing “if and how these different [phenotypic] clusters could affect the incidence of comorbidities” recorded in the HCHS/SOL study, such as hypertension, diabetes, cardiovascular disease, and cognitive decline.
For now, he said, the findings suggest that OSA may be especially underrecognized in Hispanics and Latinos and that there is more research to be done to better identify and stratify patients with varying symptomatology for more personalized treatment and for clinical trial selection. “Maybe we should expand our criteria ... broaden our [recognition] of the presentation of sleep apnea and the symptoms associated with it, not only in Hispanics but maybe in the general population,” Dr. Ramos said.
In commenting on the study, Krishna M. Sundar, MD, FCCP, director of the Sleep-Wake Center at the University of Utah, Salt Lake City, said that insomnia and daytime sleepiness are “key associations with obstructive sleep apnea and may predict different outcomes with untreated OSA.” Such heterogeneity is “only beginning to be appreciated,” he said. “The expression of OSA with these symptoms points to how OSA impacts quality of life” and how symptomatology in addition to Apnea Hypopnea Index “may be an important determinant of treatment benefit and compliance.”
The investigators reported no relevant disclosures. Dr. Sundar said that he is cofounder of Hypnoscure, software for population management of sleep apnea, but with no monies received.
Three previously described clinical phenotypes of obstructive sleep apnea (OSA) have been validated in a large and diverse Hispanic/Latino community-based population for the first time, according to findings presented at the virtual annual meeting of the Associated Professional Sleep Societies.
The three OSA symptom profiles present in this population – labeled “minimally symptomatic,” “disturbed sleep,” and “daytime sleepiness” – are consistent with recent findings from the Sleep Apnea Global Interdisciplinary Consortium, which were published in Sleep, but there are notable differences in the prevalence of these clusters, with the minimally symptomatic cluster much more prevalent than in prior research, reported Kevin Gonzalez, of the University of California, San Diego.
“Other biopsychosocial factors may be contributing to OSA phenotypes among Hispanics and Latinos,” Mr. Gonzalez said in his presentation. Prior research to characterize the heterogeneity of sleep apnea has not included a diverse Latino population, he emphasized.
The adults studied were aged 18-74 years and participants in the multisite Hispanic Community Health Study/Study of Latinos (HCHS/SOL), a comprehensive study of Hispanic/Latino health and disease in the United States. Their respiratory events were measured overnight in HCHS/SOL sleep reading centers with an ARES Unicorder 5.2, B-Alert. Sleep patterns and risk factors were assessed using the Sleep Heart Health Study Sleep Habits Questionnaire and the Epworth Sleepiness Scale.
Participants meeting the criteria for moderate to severe OSA (with an Apnea Hypopnea Index of 15 or above) were included in the analysis (n = 1,623). Their average age was 52.4 ± 13.9 years, and 34.1% were female.
To identify phenotype clusters, investigators performed a latent class analysis using 15 common OSA symptoms and a survey weighted to adjust for selection bias. The three clusters offering the “best” fit for the data aligned with the previously reported phenotypes and identified daytime sleepiness in 15.3%, disturbed sleep (insomnia-like symptoms) in 37.7%, and minimally symptomatic (a low symptom profile) in 46.9%.
These phenotypes were reported in the European Respiratory Journal in 2014 in a cluster analysis of data from a sleep apnea cohort in Iceland and later replicated in the analysis of data from the Sleep Apnea Global Interdisciplinary Consortium published in Sleep in 2018. The consortium study also added two additional phenotypes, labeled “upper airway symptoms dominant” and “sleepiness dominant.”
The prevalence of a “minimally symptomatic group” in the new analysis of the Hispanics/Latinos in the United States is much higher than reported in these prior studies, at least partly, the investigators believed, because the “prior studies were clinical samples, and the people who were minimally symptomatic didn’t get to the sleep centers,” Mr. Gonzalez said in an interview after the meeting.
Patients with a phenotype of daytime sleepiness – the most common phenotype in prior research – constituted only a minority in the Hispanic/Latino population, he said.
Alberto Ramos, MD, of the University of Miami and the principal investigator, said in an interview that the research team is currently analyzing “if and how these different [phenotypic] clusters could affect the incidence of comorbidities” recorded in the HCHS/SOL study, such as hypertension, diabetes, cardiovascular disease, and cognitive decline.
For now, he said, the findings suggest that OSA may be especially underrecognized in Hispanics and Latinos and that there is more research to be done to better identify and stratify patients with varying symptomatology for more personalized treatment and for clinical trial selection. “Maybe we should expand our criteria ... broaden our [recognition] of the presentation of sleep apnea and the symptoms associated with it, not only in Hispanics but maybe in the general population,” Dr. Ramos said.
In commenting on the study, Krishna M. Sundar, MD, FCCP, director of the Sleep-Wake Center at the University of Utah, Salt Lake City, said that insomnia and daytime sleepiness are “key associations with obstructive sleep apnea and may predict different outcomes with untreated OSA.” Such heterogeneity is “only beginning to be appreciated,” he said. “The expression of OSA with these symptoms points to how OSA impacts quality of life” and how symptomatology in addition to Apnea Hypopnea Index “may be an important determinant of treatment benefit and compliance.”
The investigators reported no relevant disclosures. Dr. Sundar said that he is cofounder of Hypnoscure, software for population management of sleep apnea, but with no monies received.
Three previously described clinical phenotypes of obstructive sleep apnea (OSA) have been validated in a large and diverse Hispanic/Latino community-based population for the first time, according to findings presented at the virtual annual meeting of the Associated Professional Sleep Societies.
The three OSA symptom profiles present in this population – labeled “minimally symptomatic,” “disturbed sleep,” and “daytime sleepiness” – are consistent with recent findings from the Sleep Apnea Global Interdisciplinary Consortium, which were published in Sleep, but there are notable differences in the prevalence of these clusters, with the minimally symptomatic cluster much more prevalent than in prior research, reported Kevin Gonzalez, of the University of California, San Diego.
“Other biopsychosocial factors may be contributing to OSA phenotypes among Hispanics and Latinos,” Mr. Gonzalez said in his presentation. Prior research to characterize the heterogeneity of sleep apnea has not included a diverse Latino population, he emphasized.
The adults studied were aged 18-74 years and participants in the multisite Hispanic Community Health Study/Study of Latinos (HCHS/SOL), a comprehensive study of Hispanic/Latino health and disease in the United States. Their respiratory events were measured overnight in HCHS/SOL sleep reading centers with an ARES Unicorder 5.2, B-Alert. Sleep patterns and risk factors were assessed using the Sleep Heart Health Study Sleep Habits Questionnaire and the Epworth Sleepiness Scale.
Participants meeting the criteria for moderate to severe OSA (with an Apnea Hypopnea Index of 15 or above) were included in the analysis (n = 1,623). Their average age was 52.4 ± 13.9 years, and 34.1% were female.
To identify phenotype clusters, investigators performed a latent class analysis using 15 common OSA symptoms and a survey weighted to adjust for selection bias. The three clusters offering the “best” fit for the data aligned with the previously reported phenotypes and identified daytime sleepiness in 15.3%, disturbed sleep (insomnia-like symptoms) in 37.7%, and minimally symptomatic (a low symptom profile) in 46.9%.
These phenotypes were reported in the European Respiratory Journal in 2014 in a cluster analysis of data from a sleep apnea cohort in Iceland and later replicated in the analysis of data from the Sleep Apnea Global Interdisciplinary Consortium published in Sleep in 2018. The consortium study also added two additional phenotypes, labeled “upper airway symptoms dominant” and “sleepiness dominant.”
The prevalence of a “minimally symptomatic group” in the new analysis of the Hispanics/Latinos in the United States is much higher than reported in these prior studies, at least partly, the investigators believed, because the “prior studies were clinical samples, and the people who were minimally symptomatic didn’t get to the sleep centers,” Mr. Gonzalez said in an interview after the meeting.
Patients with a phenotype of daytime sleepiness – the most common phenotype in prior research – constituted only a minority in the Hispanic/Latino population, he said.
Alberto Ramos, MD, of the University of Miami and the principal investigator, said in an interview that the research team is currently analyzing “if and how these different [phenotypic] clusters could affect the incidence of comorbidities” recorded in the HCHS/SOL study, such as hypertension, diabetes, cardiovascular disease, and cognitive decline.
For now, he said, the findings suggest that OSA may be especially underrecognized in Hispanics and Latinos and that there is more research to be done to better identify and stratify patients with varying symptomatology for more personalized treatment and for clinical trial selection. “Maybe we should expand our criteria ... broaden our [recognition] of the presentation of sleep apnea and the symptoms associated with it, not only in Hispanics but maybe in the general population,” Dr. Ramos said.
In commenting on the study, Krishna M. Sundar, MD, FCCP, director of the Sleep-Wake Center at the University of Utah, Salt Lake City, said that insomnia and daytime sleepiness are “key associations with obstructive sleep apnea and may predict different outcomes with untreated OSA.” Such heterogeneity is “only beginning to be appreciated,” he said. “The expression of OSA with these symptoms points to how OSA impacts quality of life” and how symptomatology in addition to Apnea Hypopnea Index “may be an important determinant of treatment benefit and compliance.”
The investigators reported no relevant disclosures. Dr. Sundar said that he is cofounder of Hypnoscure, software for population management of sleep apnea, but with no monies received.
REPORTING FROM SLEEP 2020