User login
It’s tough to get a good night’s sleep in outer space
Shorter sleep duration, more wakefulness, and changes in the sleep cycle brought on by microgravity make it tough for astronauts to get a good night’s sleep while they’re in outer space, a new study shows. In research that has implications for earthlings as well as astronauts, scientists found that the “
“Our results support other studies indicating that sleep architecture can adapt to different environments. Also, the sleep deficits that our subjects were facing while working around the clock in a high-pressure environment provide further evidence for the danger of stress and shift-work schedules for humans anywhere,” study investigator Oliver Piltch, of Harvard University, Cambridge, Mass., said in a release.
The findings were presented at the virtual annual meeting of the Associated Professional Sleep Societies.
Sleep architecture affected
The researchers studied sleep architecture in four cosmonauts and one astronaut before, during, and after missions to the Mir space station. Using the NightCap sleep monitor, they recorded a total of 324 nights of sleep – 112 preflight nights, 83 in-flight nights, and 61 postflight nights.
Despite having the same “sleep opportunity” in space as on earth, the astronauts were on average sleeping an hour less each night during the space mission compared with when on earth before or after their mission (5.7 vs. 6.7 hours; P < .0001). In space, the astronauts also spent significantly more time awake in bed, leading to a 17.7% reduction in sleep efficiency.
Sleep architecture was also affected by spaceflight. In space, the time in non–rapid eye movement (non–REM) and REM sleep decreased by 14.1% and 25.8%, respectively. On average, it took about 90 minutes after falling asleep for astronauts to reach their first episode of REM sleep in space – nearly 1.5 times longer than on earth. “There were marked shifts in sleep architecture compared to baseline, and some of these evolved over the course of the mission,” said Mr. Piltch.
“Our findings were consistent with previous studies that focus on the issue of sleep continuity. We found significant decreases in sleep efficiency during spaceflight despite similar times in bed,” he noted.
Mr. Piltch said it’s important to understand how sleep is affected by spaceflight in order to better equip astronauts for success on long-duration flights, such as a trip to Mars or the Moon. He also pointed to a recent study in the Lancet Neurology that showed that 78% of the international space station crew take hypnotics on 52% of nights in space. “So it doesn’t look like they sleep very well in space,” he said.
High-stakes environment
Reached for comment, Camilo A. Ruiz, DO, medical director, Choice Physicians Sleep Center, Fort Lauderdale, Fla., said the findings add to the “limited” data currently available on sleep in space and microgravity. “To a certain point, the results of this study could have been expected since sleep continuity and sleep architecture disruption is present during stressful periods of human life or in changes to the sleep rituals we hold dear, such as our beds and quiet bedrooms,” said Dr. Ruiz, who was not involved in the study.
“The potential harm to astronauts from their sleep continuity and deranged sleep architecture is that the decreased alertness, performance, vigilance, and psychomotor skills they exhibit in that high-stakes environment such as space flight can lead to serious accidents that can jeopardize the safety of the crew and vessel,” Dr. Ruiz noted.
“These research areas are on the forefront of space medicine that will allow mankind to lead successful interplanetary missions and colonization of these planets with long-term resident astronauts,” he added.
The study was supported by funding from the Mary Gordon Roberts Fellowship, the National Academy of Sciences, the National Institute of Mental Health, the MacArthur Foundation Mind-Body Network, and Healthdyne Technologies. Mr. Piltch and Dr. Ruiz have no disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Shorter sleep duration, more wakefulness, and changes in the sleep cycle brought on by microgravity make it tough for astronauts to get a good night’s sleep while they’re in outer space, a new study shows. In research that has implications for earthlings as well as astronauts, scientists found that the “
“Our results support other studies indicating that sleep architecture can adapt to different environments. Also, the sleep deficits that our subjects were facing while working around the clock in a high-pressure environment provide further evidence for the danger of stress and shift-work schedules for humans anywhere,” study investigator Oliver Piltch, of Harvard University, Cambridge, Mass., said in a release.
The findings were presented at the virtual annual meeting of the Associated Professional Sleep Societies.
Sleep architecture affected
The researchers studied sleep architecture in four cosmonauts and one astronaut before, during, and after missions to the Mir space station. Using the NightCap sleep monitor, they recorded a total of 324 nights of sleep – 112 preflight nights, 83 in-flight nights, and 61 postflight nights.
Despite having the same “sleep opportunity” in space as on earth, the astronauts were on average sleeping an hour less each night during the space mission compared with when on earth before or after their mission (5.7 vs. 6.7 hours; P < .0001). In space, the astronauts also spent significantly more time awake in bed, leading to a 17.7% reduction in sleep efficiency.
Sleep architecture was also affected by spaceflight. In space, the time in non–rapid eye movement (non–REM) and REM sleep decreased by 14.1% and 25.8%, respectively. On average, it took about 90 minutes after falling asleep for astronauts to reach their first episode of REM sleep in space – nearly 1.5 times longer than on earth. “There were marked shifts in sleep architecture compared to baseline, and some of these evolved over the course of the mission,” said Mr. Piltch.
“Our findings were consistent with previous studies that focus on the issue of sleep continuity. We found significant decreases in sleep efficiency during spaceflight despite similar times in bed,” he noted.
Mr. Piltch said it’s important to understand how sleep is affected by spaceflight in order to better equip astronauts for success on long-duration flights, such as a trip to Mars or the Moon. He also pointed to a recent study in the Lancet Neurology that showed that 78% of the international space station crew take hypnotics on 52% of nights in space. “So it doesn’t look like they sleep very well in space,” he said.
High-stakes environment
Reached for comment, Camilo A. Ruiz, DO, medical director, Choice Physicians Sleep Center, Fort Lauderdale, Fla., said the findings add to the “limited” data currently available on sleep in space and microgravity. “To a certain point, the results of this study could have been expected since sleep continuity and sleep architecture disruption is present during stressful periods of human life or in changes to the sleep rituals we hold dear, such as our beds and quiet bedrooms,” said Dr. Ruiz, who was not involved in the study.
“The potential harm to astronauts from their sleep continuity and deranged sleep architecture is that the decreased alertness, performance, vigilance, and psychomotor skills they exhibit in that high-stakes environment such as space flight can lead to serious accidents that can jeopardize the safety of the crew and vessel,” Dr. Ruiz noted.
“These research areas are on the forefront of space medicine that will allow mankind to lead successful interplanetary missions and colonization of these planets with long-term resident astronauts,” he added.
The study was supported by funding from the Mary Gordon Roberts Fellowship, the National Academy of Sciences, the National Institute of Mental Health, the MacArthur Foundation Mind-Body Network, and Healthdyne Technologies. Mr. Piltch and Dr. Ruiz have no disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Shorter sleep duration, more wakefulness, and changes in the sleep cycle brought on by microgravity make it tough for astronauts to get a good night’s sleep while they’re in outer space, a new study shows. In research that has implications for earthlings as well as astronauts, scientists found that the “
“Our results support other studies indicating that sleep architecture can adapt to different environments. Also, the sleep deficits that our subjects were facing while working around the clock in a high-pressure environment provide further evidence for the danger of stress and shift-work schedules for humans anywhere,” study investigator Oliver Piltch, of Harvard University, Cambridge, Mass., said in a release.
The findings were presented at the virtual annual meeting of the Associated Professional Sleep Societies.
Sleep architecture affected
The researchers studied sleep architecture in four cosmonauts and one astronaut before, during, and after missions to the Mir space station. Using the NightCap sleep monitor, they recorded a total of 324 nights of sleep – 112 preflight nights, 83 in-flight nights, and 61 postflight nights.
Despite having the same “sleep opportunity” in space as on earth, the astronauts were on average sleeping an hour less each night during the space mission compared with when on earth before or after their mission (5.7 vs. 6.7 hours; P < .0001). In space, the astronauts also spent significantly more time awake in bed, leading to a 17.7% reduction in sleep efficiency.
Sleep architecture was also affected by spaceflight. In space, the time in non–rapid eye movement (non–REM) and REM sleep decreased by 14.1% and 25.8%, respectively. On average, it took about 90 minutes after falling asleep for astronauts to reach their first episode of REM sleep in space – nearly 1.5 times longer than on earth. “There were marked shifts in sleep architecture compared to baseline, and some of these evolved over the course of the mission,” said Mr. Piltch.
“Our findings were consistent with previous studies that focus on the issue of sleep continuity. We found significant decreases in sleep efficiency during spaceflight despite similar times in bed,” he noted.
Mr. Piltch said it’s important to understand how sleep is affected by spaceflight in order to better equip astronauts for success on long-duration flights, such as a trip to Mars or the Moon. He also pointed to a recent study in the Lancet Neurology that showed that 78% of the international space station crew take hypnotics on 52% of nights in space. “So it doesn’t look like they sleep very well in space,” he said.
High-stakes environment
Reached for comment, Camilo A. Ruiz, DO, medical director, Choice Physicians Sleep Center, Fort Lauderdale, Fla., said the findings add to the “limited” data currently available on sleep in space and microgravity. “To a certain point, the results of this study could have been expected since sleep continuity and sleep architecture disruption is present during stressful periods of human life or in changes to the sleep rituals we hold dear, such as our beds and quiet bedrooms,” said Dr. Ruiz, who was not involved in the study.
“The potential harm to astronauts from their sleep continuity and deranged sleep architecture is that the decreased alertness, performance, vigilance, and psychomotor skills they exhibit in that high-stakes environment such as space flight can lead to serious accidents that can jeopardize the safety of the crew and vessel,” Dr. Ruiz noted.
“These research areas are on the forefront of space medicine that will allow mankind to lead successful interplanetary missions and colonization of these planets with long-term resident astronauts,” he added.
The study was supported by funding from the Mary Gordon Roberts Fellowship, the National Academy of Sciences, the National Institute of Mental Health, the MacArthur Foundation Mind-Body Network, and Healthdyne Technologies. Mr. Piltch and Dr. Ruiz have no disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
FROM SLEEP 2020
Nightmares: An independent risk factor for heart disease?
, new research shows. In what researchers describe as “surprising” findings, results from a large study of relatively young military veterans showed those who had nightmares two or more times per week had significantly increased risks for hypertension, myocardial infarction, or other heart problems.
“A diagnosis of PTSD incorporates sleep disturbance as a symptom. Thus, we were surprised to find that nightmares continued to be associated with CVD after controlling not only for PTSD and demographic factors, but also smoking and depression diagnosis,” said Christi Ulmer, PhD, of the department of psychiatry and behavioral sciences, Duke University Medical Center, Durham, N.C.
The findings were presented at the virtual annual meeting of the Associated Professional Sleep Societies.
Unclear mechanism
The study included 3,468 veterans (77% male) with a mean age of 38 years who had served one or two tours of duty since Sept. 11, 2001. Nearly one-third (31%) met criteria for PTSD, and 33% self-reported having at least one cardiovascular condition, such as heart problems, hypertension, stroke, and MI.
Nightmare frequency and severity was assessed using the Davidson Trauma Scale. Nightmares were considered frequent if they occurred two or more times per week and moderate to severe if they were at least moderately distressing. About 31% of veterans reported having frequent nightmares, and 35% reported moderately distressing nightmares over the past week.
After adjusting for age, race, and sex, frequent nightmares were associated with hypertension (odds ratio, 1.51; 95% confidence interval, 1.28-1.78), heart problems (OR, 1.50; 95% CI, 1.11-2.02), and MI (OR, 2.32; 95% CI, 1.18-4.54).
Associations between frequent nightmares and hypertension (OR, 1.43; 95% CI, 1.17-1.73) and heart problems (OR, 1.43; 95% CI, 1.00-2.05) remained significant after further adjusting for smoking, depression, and PTSD.
“Our cross-sectional findings set the stage for future research examining the possibility that nightmares may confer cardiovascular disease risks beyond those conferred by PTSD diagnosis alone,” Dr. Ulmer said in a news release.
Dr. Ulmer also said that, because the study was based on self-reported data, the findings are “very preliminary.” Before doctors adjust clinical practices, it’s important that our findings be replicated using longitudinal studies, clinically diagnosed medical conditions, and objectively assessed sleep,” she said.
She added that more research is needed to uncover mechanisms explaining these associations and determine if reducing the frequency and severity of nightmares can lead to improved cardiovascular health.
Timely research
Reached for comment, Rajkumar (Raj) Dasgupta, MD, of the University of Southern California, Los Angeles, noted “the correlation between nightmares and heart disease is a timely topic right now with COVID-19 as more people may be having nightmares.”
“If a patient mentions nightmares, I do think it’s important not to just glaze over it, but to talk more about it and document it in the patient record, especially in patients with cardiovascular disease, atrial fibrillation, diabetes, and hypertension,” said Dr. Dasgupta, who wasn’t involved in the study.
The research was supported by the Veterans Integrated Service Network 6 Mental Illness Research, Education and Clinical Center and the Department of Veterans Affairs HSR&D ADAPT Center at the Durham VA Health Care System. Dr. Ulmer and Dr. Dasgupta have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
, new research shows. In what researchers describe as “surprising” findings, results from a large study of relatively young military veterans showed those who had nightmares two or more times per week had significantly increased risks for hypertension, myocardial infarction, or other heart problems.
“A diagnosis of PTSD incorporates sleep disturbance as a symptom. Thus, we were surprised to find that nightmares continued to be associated with CVD after controlling not only for PTSD and demographic factors, but also smoking and depression diagnosis,” said Christi Ulmer, PhD, of the department of psychiatry and behavioral sciences, Duke University Medical Center, Durham, N.C.
The findings were presented at the virtual annual meeting of the Associated Professional Sleep Societies.
Unclear mechanism
The study included 3,468 veterans (77% male) with a mean age of 38 years who had served one or two tours of duty since Sept. 11, 2001. Nearly one-third (31%) met criteria for PTSD, and 33% self-reported having at least one cardiovascular condition, such as heart problems, hypertension, stroke, and MI.
Nightmare frequency and severity was assessed using the Davidson Trauma Scale. Nightmares were considered frequent if they occurred two or more times per week and moderate to severe if they were at least moderately distressing. About 31% of veterans reported having frequent nightmares, and 35% reported moderately distressing nightmares over the past week.
After adjusting for age, race, and sex, frequent nightmares were associated with hypertension (odds ratio, 1.51; 95% confidence interval, 1.28-1.78), heart problems (OR, 1.50; 95% CI, 1.11-2.02), and MI (OR, 2.32; 95% CI, 1.18-4.54).
Associations between frequent nightmares and hypertension (OR, 1.43; 95% CI, 1.17-1.73) and heart problems (OR, 1.43; 95% CI, 1.00-2.05) remained significant after further adjusting for smoking, depression, and PTSD.
“Our cross-sectional findings set the stage for future research examining the possibility that nightmares may confer cardiovascular disease risks beyond those conferred by PTSD diagnosis alone,” Dr. Ulmer said in a news release.
Dr. Ulmer also said that, because the study was based on self-reported data, the findings are “very preliminary.” Before doctors adjust clinical practices, it’s important that our findings be replicated using longitudinal studies, clinically diagnosed medical conditions, and objectively assessed sleep,” she said.
She added that more research is needed to uncover mechanisms explaining these associations and determine if reducing the frequency and severity of nightmares can lead to improved cardiovascular health.
Timely research
Reached for comment, Rajkumar (Raj) Dasgupta, MD, of the University of Southern California, Los Angeles, noted “the correlation between nightmares and heart disease is a timely topic right now with COVID-19 as more people may be having nightmares.”
“If a patient mentions nightmares, I do think it’s important not to just glaze over it, but to talk more about it and document it in the patient record, especially in patients with cardiovascular disease, atrial fibrillation, diabetes, and hypertension,” said Dr. Dasgupta, who wasn’t involved in the study.
The research was supported by the Veterans Integrated Service Network 6 Mental Illness Research, Education and Clinical Center and the Department of Veterans Affairs HSR&D ADAPT Center at the Durham VA Health Care System. Dr. Ulmer and Dr. Dasgupta have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
, new research shows. In what researchers describe as “surprising” findings, results from a large study of relatively young military veterans showed those who had nightmares two or more times per week had significantly increased risks for hypertension, myocardial infarction, or other heart problems.
“A diagnosis of PTSD incorporates sleep disturbance as a symptom. Thus, we were surprised to find that nightmares continued to be associated with CVD after controlling not only for PTSD and demographic factors, but also smoking and depression diagnosis,” said Christi Ulmer, PhD, of the department of psychiatry and behavioral sciences, Duke University Medical Center, Durham, N.C.
The findings were presented at the virtual annual meeting of the Associated Professional Sleep Societies.
Unclear mechanism
The study included 3,468 veterans (77% male) with a mean age of 38 years who had served one or two tours of duty since Sept. 11, 2001. Nearly one-third (31%) met criteria for PTSD, and 33% self-reported having at least one cardiovascular condition, such as heart problems, hypertension, stroke, and MI.
Nightmare frequency and severity was assessed using the Davidson Trauma Scale. Nightmares were considered frequent if they occurred two or more times per week and moderate to severe if they were at least moderately distressing. About 31% of veterans reported having frequent nightmares, and 35% reported moderately distressing nightmares over the past week.
After adjusting for age, race, and sex, frequent nightmares were associated with hypertension (odds ratio, 1.51; 95% confidence interval, 1.28-1.78), heart problems (OR, 1.50; 95% CI, 1.11-2.02), and MI (OR, 2.32; 95% CI, 1.18-4.54).
Associations between frequent nightmares and hypertension (OR, 1.43; 95% CI, 1.17-1.73) and heart problems (OR, 1.43; 95% CI, 1.00-2.05) remained significant after further adjusting for smoking, depression, and PTSD.
“Our cross-sectional findings set the stage for future research examining the possibility that nightmares may confer cardiovascular disease risks beyond those conferred by PTSD diagnosis alone,” Dr. Ulmer said in a news release.
Dr. Ulmer also said that, because the study was based on self-reported data, the findings are “very preliminary.” Before doctors adjust clinical practices, it’s important that our findings be replicated using longitudinal studies, clinically diagnosed medical conditions, and objectively assessed sleep,” she said.
She added that more research is needed to uncover mechanisms explaining these associations and determine if reducing the frequency and severity of nightmares can lead to improved cardiovascular health.
Timely research
Reached for comment, Rajkumar (Raj) Dasgupta, MD, of the University of Southern California, Los Angeles, noted “the correlation between nightmares and heart disease is a timely topic right now with COVID-19 as more people may be having nightmares.”
“If a patient mentions nightmares, I do think it’s important not to just glaze over it, but to talk more about it and document it in the patient record, especially in patients with cardiovascular disease, atrial fibrillation, diabetes, and hypertension,” said Dr. Dasgupta, who wasn’t involved in the study.
The research was supported by the Veterans Integrated Service Network 6 Mental Illness Research, Education and Clinical Center and the Department of Veterans Affairs HSR&D ADAPT Center at the Durham VA Health Care System. Dr. Ulmer and Dr. Dasgupta have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
FROM SLEEP 2020
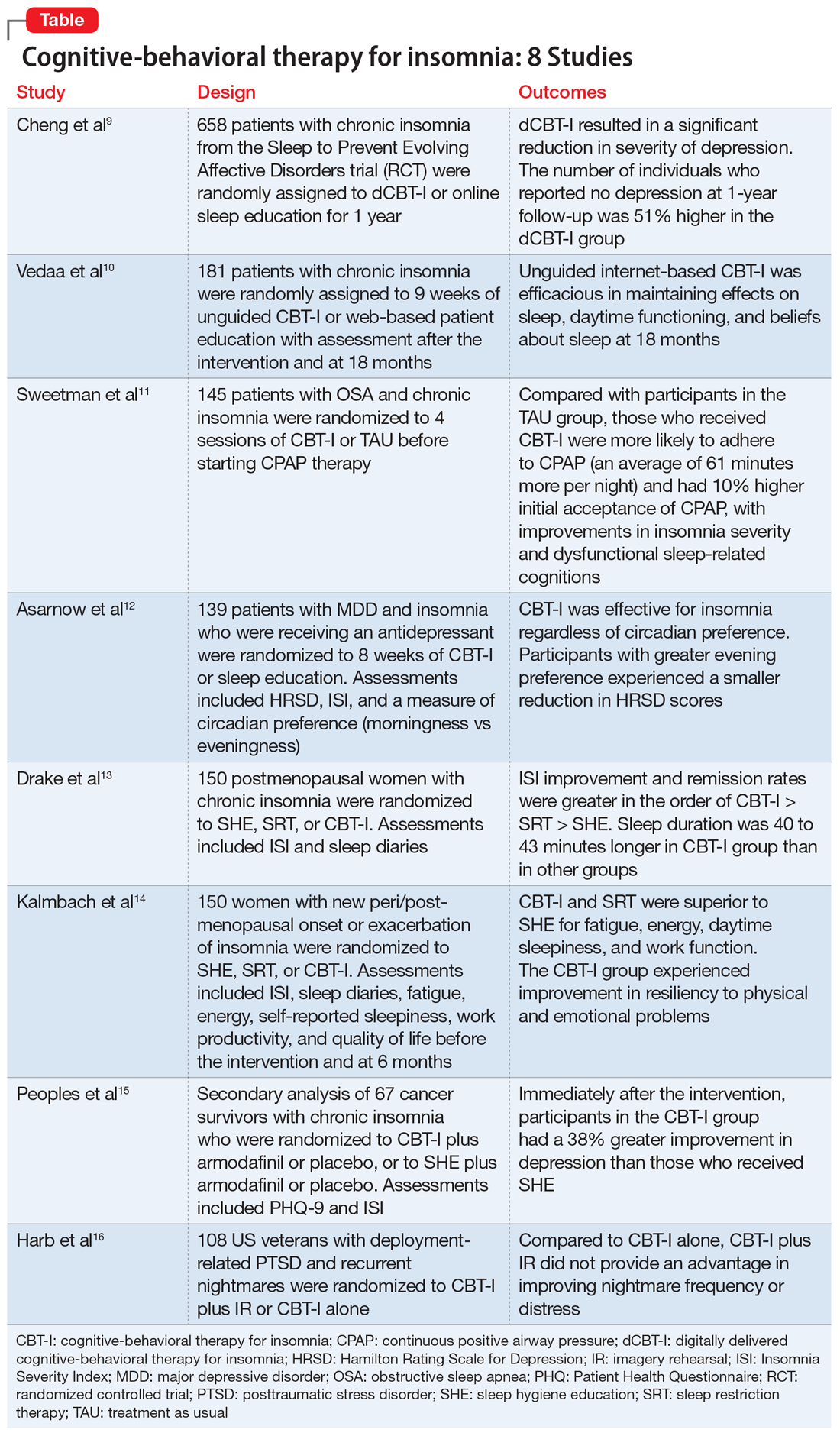
Cognitive-behavioral therapy for insomnia: A review of 8 studies
The prevalence of insomnia in the general population is approximately 6% to 10%.1 In addition, an estimated 30% of the general population may have symptoms of insomnia without meeting the diagnostic criteria.2 As a disorder, insomnia is characterized by a persistent difficulty initiating or maintaining sleep, or early morning awakening with inability to return to sleep, that has been present for at least 3 months. Additionally, the sleep difficulties must occur at least 3 nights a week, result in impaired daytime functioning, and cause significant distress.1
Cognitive-behavioral therapy for insomnia (CBT-I) is an effective treatment, supported by several systematic reviews and meta-analyses.3-5 In the short term, CBT-I is as effective as pharmacotherapy.6 However, CBT-I is the preferred treatment for chronic insomnia, according to recommendations in European and American guidelines.7,8
Here we review 8 recent studies that examined CBT-I. These studies are summarized in the Table.9-16
1. Cheng P, Kalmbach DA, Tallent G, et al. Depression prevention via digital cognitive behavioral therapy for insomnia: a randomized controlled trial. Sleep. 2019;42(10):zsz150. doi: 10.1093/sleep/zsz150.
Although CBT-I is a first-line treatment for chronic insomnia, it is underutilized in clinical practice primarily due to limited availability. Because few clinicians are certified in CBT-I, it has become necessary to develop alternative modes of delivery for CBT-I, such as fully automated, internet-delivered approaches to reach more patients with insomnia. Digital CBT-I (dCBT-I) is comparable to in-person CBT-I in improving insomnia symptoms and reducing concurrent depressive symptoms with insomnia. It is unclear if unguided, internet-delivered CBT-I is effective for achieving remission from depression or preventing depression in the long term. Chen et al9 examined the efficacy of dCBT-I in reducing and preventing depression over a 1-year follow-up.
Study design
- Participants from various centers in Southeastern Michigan were recruited between 2016 and 2017. Data was obtained from the Sleep to Prevent Evolving Affective Disorders (SPREAD) trial.
- Participants who met DSM-5 criteria for chronic insomnia disorder were randomized to dCBT-I (n = 358) using the Sleepio digital CBT program via the internet or to online sleep education (n = 300).
- The primary outcome was depression, measured using the Quick Inventory of Depressive Symptomatology-Self Report (QIDS-SR-16) at 1-year follow-up. Depression incidence was also tested against insomnia treatment response.
Outcomes
- The severity of depression was significantly lower at 1-year follow-up in the dCBT-I group compared with the control group.
- The dCBT-I group showed a 51% higher remission rate than the control group at 1-year follow-up.
- The incidence of moderate to severe depression in individuals with minimal to no depression at baseline was halved at 1 year after receiving dCBT-I treatment compared with the control group.
Continue to: Conclusion
Conclusion
- dCBT-I can improve depression and insomnia and has a sustained antidepressant effect.
- dCBT-I is effective for preventing depression. In other words, the risk of developing depression is decreased when dCBT-I is used to treat insomnia in individuals with minimal to no depression at baseline.
2. Vedaa Ø, Hagatun S, Kallestad H, et al. Long-term effects of an unguided online cognitive behavioral therapy for chronic insomnia. J Clin Sleep Med. 2019;15(1):101-110.
dCBT-I is effective for treating insomnia in the short term; however, little is known about the long-term effectiveness of dCBT-I on sleep and daytime symptoms. Vedaa et al10 evaluated the efficacy of dCBT-I at 18 months after the intervention.
Study design
- In this randomized controlled trial (RCT), the efficacy of unguided, internet-delivered CBT-I (n = 95) was compared with web-based patient education (n = 86) for patients with chronic insomnia.
- Participants were assessed at baseline, after a 9-week intervention period, and at 6-month follow-up. Participants in the internet CBT-I group were reassessed at 18 months after the intervention using online questionnaires, including the Insomnia Severity Index (ISI), Bergen Insomnia Scale (BIS), Brief Dysfunctional Beliefs and Attitudes Scale, Hospital Anxiety and Depression Scale, Chalder Fatigue Questionnaire, and sleep diaries.
Outcomes
- At 18 months, significant improvements were noted from baseline ISI and BIS scores and in levels of daytime fatigue, as well as psychological distress and beliefs about sleep.
- Sleep diary variables—including sleep onset latency, time awake during the night (wake time after sleep onset), early morning awakening, total sleep time, and sleep efficiency—showed significant improvement from baseline to 18-month follow-up (at least moderate effect size).
- Improvements were maintained from the completion of the 9-week intervention to follow-up at 18 months.
Continue to: Conclusion
Conclusion
- Fully-automated, internet-based CBT-I is efficacious in maintaining positive effects on sleep and daytime functioning up to 18 months after completing treatment.
3. Sweetman A, Lack L, Catcheside PG, et al. Cognitive and behavioral therapy for insomnia increases the use of continuous positive airway pressure therapy in obstructive sleep apnea participants with comorbid insomnia: a randomized clinical trial. Sleep. 2019;42(12):zsz178. doi: 10.1093/sleep/zsz178.
Comorbid insomnia and sleep apnea (COMISA) can affect a patient’s ability to accept and comply with continuous positive airway pressure (CPAP) therapy. Providing adequate treatment for these patients can be challenging.
Sweetman et al11 evaluated the acceptance and use of CPAP in patients with obstructive sleep apnea and chronic insomnia following initial treatment with CBT-I compared with treatment as usual (TAU).
Study design
- In this RCT, 145 participants with COMISA were randomized to 4 sessions of CBT-I or TAU before starting CPAP therapy until 6 months after randomization.
- Primary outcomes were objective CPAP adherence and objective sleep efficiency at the end of 6 months.
- Secondary outcomes were CPAP acceptance/rejection, changes in sleep parameters, global insomnia severity, and daytime impairments at 6 months.
Continue to: Outcomes
Outcomes
- The CBT-I group had higher initial CPAP acceptance and greater average nightly adherence to CPAP (61 minutes more) than the TAU group.
- Significant improvements were noted in global insomnia severity, nighttime insomnia complaints, and dysfunctional sleep-related cognitions at 6 months in the CBT-I group compared with TAU.
- No differences between the 2 groups were noted in sleep diary parameters or daytime impairments at 6 months.
Conclusions
- Patients with COMISA can benefit from receiving CBT-I before starting CPAP therapy because CBT-I can improve immediate acceptance of CPAP and may help to maintain adherence to CPAP over time.
- Patients with sleep apnea should be evaluated for comorbid insomnia, and CBT-I should be considered before starting CPAP treatment.
4. Asarnow LD, Bei B, Krystal A, et al. Circadian preference as a moderator of depression outcome following cognitive behavioral therapy for insomnia plus antidepressant medications: a report from the TRIAD study. J Clin Sleep Med. 2019;15(4):573-580.
The Treatment of Insomnia and Depression (TRIAD) study reported the effects of combining antidepressants with CBT-I in patients with major depressive disorder (MDD) and insomnia. Asarnow et al12 examined the moderation of circadian preference in the reduction of depression and insomnia symptoms severity during the same trial.
Study design
- In this RCT, 139 participants with MDD and insomnia were treated with an antidepressant (escitalopram, sertraline, or desvenlafaxine) and randomized to 8 weeks of CBT-I or control therapy (sleep education).
- Measurements used were Composite Scale of Morningness for circadian preference (morningness vs eveningness), depression severity with the Hamilton Rating Scale for Depression, and insomnia severity using the ISI.
Continue to: Outcomes
Outcomes
- CBT-I was effective for insomnia regardless of circadian preference.
- A smaller reduction in depression scores was noted in participants with greater evening preference.
- Depression outcomes were better among participants with evening preference if they were assigned to CBT-I vs control therapy.
- The control therapy (sleep education) was particularly ineffective in reducing depression symptoms in participants with evening preference.
Conclusion
- Individuals with MDD and insomnia and an evening preference are at an increased risk for poor response to antidepressants alone.
- Outcomes for both depression and insomnia improve if CBT-I is combined with antidepressants.
- Offering sleep education alone is not sufficient.
5. Drake CL, Kalmbach DA, Arnedt JT, et al. Treating chronic insomnia in postmenopausal women: a randomized clinical trial comparing cognitive-behavioral therapy for insomnia, sleep restriction therapy, and sleep hygiene education. Sleep. 2019;42(2):zsy217. doi: 10.1093/sleep/zsy217.
Postmenopausal women with sleep disturbances experience higher medical and psychiatric comorbidities, and have higher alcohol consumption and stress levels than postmenopausal women with good sleep. Nonpharmacologic insomnia treatments with durable effects are imperative for postmenopausal women because they are safer than pharmacologic approaches. Although CBT-I is the recommended first-line treatment for chronic insomnia, its application in menopause-related insomnia is limited. Drake et al13 evaluated the efficacy of CBT-I in menopause-related insomnia compared with sleep restriction therapy (SRT) and sleep hygiene education (SHE).
Study design
- This RCT was conducted at a health system with 6 hospitals in Michigan.
- Postmenopausal women who met DSM-5 criteria for chronic insomnia disorder (n = 150) were randomized into 1 of 3 groups: SHE, SRT, or CBT-I.
- Primary outcome measures were ISI scores and sleep diaries that documented multiple sleep parameters, including sleep onset latency, wake time after sleep onset, number of awakenings in the middle of the night, time in bed, total sleep time, and sleep efficiency. These were measured at baseline, after completion of treatment, and 6 months after treatment.
Continue to: Outcomes
Outcomes
- Both CBT-I and SRT outperformed SHE on the ISI and for most of the sleep parameters on sleep diaries immediately after treatment completion and at 6 months after treatment.
- Total sleep time was 40 to 43 minutes longer in the CBT-I group than in the SRT and SHE groups at 6-month follow-up.
- Remission rates (sleep onset latency ≤30 minutes, wake time after sleep onset ≤30 minutes, sleep efficiency ≥85%) were significantly higher in CBT-I group (CBT-I > SRT > SHE).
Conclusion
- Sleep hygiene education as a standalone treatment is not useful for treating chronic insomnia.
- Both CBT-I and SRT are efficacious for menopause-related insomnia.
- CBT-I may be a better option than SRT because it produces higher remission rates and better long-term outcomes.
6. Kalmbach DA, Cheng P, Arnedt JT, et al. Improving daytime functioning, work performance, and quality of life in postmenopausal women with insomnia: comparing cognitive behavioral therapy for insomnia, sleep restriction therapy, and sleep hygiene education. J Clin Sleep Med. 2019;15(7):999-1010.
CBT-I has shown efficacy in the treatment of insomnia in postmenopausal women. In this study, Kalmbach et al14 compared 3 nonpharmacologic modalities—CBT-I, SRT, and SHE—for the treatment of menopause-related insomnia and daytime impairment.
Study design
- In this RCT, 150 participants with new peri- and post-menopausal onset or exacerbation of insomnia were randomized to 1 of 3 groups: SHE, SRT, or CBT-I.
- Participants were assessed at baseline, after treatment completion, and at 6-month follow-up using the ISI, sleep diaries, Fatigue Severity Scale, Epworth Sleepiness Scale, Work Productivity and Activity Impairment Questionnaire, and 36-item Medical Outcomes Study Short Form Health Survey.
Continue to: Outcomes
Outcomes
- In both the CBT-I and SRT groups, significant improvements were noted in fatigue, energy, daytime sleepiness, and work function after treatment completion and at 6-month follow-up.
- Improvements were noted in emotional well-being and resiliency to physical and emotional problems in the CBT-I group at 6 months.
- Improvements in overall general health and social functioning, less pain, and fewer hot flashes were reported by postmenopausal women who remitted from insomnia; however, these benefits were not directly related to any specific treatment modality.
Conclusion
- CBT-I and SRT are superior to SHE for improving daytime functioning, and some aspects of life quality and work productivity, in postmenopausal women with insomnia.
- CBT-I may be superior to SRT in producing larger improvements in fatigue, energy level, and daytime sleep propensity.
- CBT-I can improve emotional well-being and resilience to emotional problems in postmenopausal women with insomnia.
7. Peoples AR, Garland SN, Pigeon WR, et al. Cognitive behavioral therapy for insomnia reduces depression in cancer survivors. J Clin Sleep Med. 2019;15(1):129-137.
Depression is common in patients with cancer and is usually associated with comorbid insomnia. Depression has significant effect on treatment compliance, coping with illness, and quality of life. Peoples et al15 examined the effects of CBT-I on depression in cancer survivors.
Study design
- This was a secondary analysis of a multicenter, randomized, placebo-controlled trial that evaluated interventions for cancer survivors with chronic insomnia in which the primary outcome measure was insomnia severity.
- Cancer survivors (n = 67) were randomized to CBT-I plus armodafinil or placebo or to SHE plus armodafinil or placebo.
- The Patient Health Questionnaire-9 (PHQ-9) and ISI were used to measure depression and insomnia at baseline, after 7-weeks of intervention, and at 3 months postintervention.
Continue to: Outcomes
Outcomes
- Immediately after completing the intervention, cancer survivors treated with CBT-I had significantly less depression (38% greater improvement in depression) compared with those who received SHE (control group).
- In the CBT-I group, 23% of cancer survivors achieved a clinically important reduction (5-point reduction on PHQ-9 total score) in depression at postintervention compared with 6% of those in the control group.
- At 3 months after the intervention, only 14% of cancer survivors in CBT-I group reported depression (PHQ-9 score >4), whereas 47% of those in the control group (SHE) reported depression.
Conclusion
- CBT-I improves both depression and insomnia in cancer survivors, and the improvements are sustained at 3 months after completing treatment.
- Improvement in insomnia severity appears to mediate the positive effects of CBT-I on depression.
8. Harb GC, Cook JM, Phelps AJ, et al. Randomized controlled trial of imagery rehearsal for posttraumatic nightmares in combat veterans. J Clin Sleep Med. 2019;15(5):757-767.
The American Academy of Sleep Medicine recommends imagery rehearsal (IR) therapy, which incorporates some components of CBT-I, for the treatment of recurrent posttraumatic stress disorder (PTSD)–related nightmares. In this study, Harb et al16 compared CBT-I plus IR to CBT-I alone for the treatment of nightmares in veterans with combat-related PTSD.
Study design
- This RCT included male and female US veterans (n = 108) deployed to Iraq and Afghanistan with current PTSD and recurrent nightmares related to deployment.
- Participants were randomized to 6 sessions of CBT-I plus IR or CBT-I alone.
- Primary outcome measures included frequency of nightmares and distress associated with nightmares.
Continue to: Outcomes
Outcomes
- A significant improvement in nightmares was noted in both groups (29% of participants showed a clinically-significant reduction in nightmare frequency and 22% of participants achieved remission).
- CBT-I plus IR was not superior to CBT-I only at postintervention and at 6-month follow-up.
Conclusion
- Both IR and CBT-I demonstrated efficacy for decreasing nightmare frequency and distress.
- Combining IR and CBT-I may not provide a synergistic advantage over CBT-I alone for treating PTSD-related nightmares in veterans.
1. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
2. Morin CM, LeBlanc M, Daley M, et al. Epidemiology of insomnia: prevalence, self-help treatments, consultations, and determinants of help-seeking behaviors. Sleep Med. 2006;7(2):123-130.
3. Trauer JM, Qian MY, Doyle JS, et al. Cognitive behavioral therapy for chronic insomnia: a systematic review and meta-analysis. Ann Intern Med. 2015;163(3):191-204.
4. Wu JQ, Appleman ER, Salazar RD, et al. Cognitive behavioral therapy for insomnia comorbid with psychiatric and medical conditions: a meta-analysis. JAMA Intern Med. 2015;175(9):1461-1472.
5. van Straten A, van der Zweerde T, Kleiboer A, et al. Cognitive and behavioral therapies in the treatment of insomnia: a meta-analysis. Sleep Med Rev. 2018;38:3-16.
6. Smith MT, Perlis ML, Park A, et al. Comparative meta-analysis of pharmacotherapy and behavior therapy for persistent insomnia. Am J Psychiatry. 2002;159(1):5-11.
7. Qaseem A, Kansagara D, Forciea MA, et al. Management of chronic insomnia disorder in adults: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2016;165(2):125-133.
8. Riemann D, Baglioni C, Bassetti C, et al. European guideline for the diagnosis and treatment of insomnia. J Sleep Res. 2017;26(6):675-700.
9. Cheng P, Kalmbach DA, Tallent G, et al. Depression prevention via digital cognitive behavioral therapy for insomnia: a randomized controlled trial. Sleep. 2019;42(10):zsz150. doi: 10.1093/sleep/zsz150.
10. Vedaa Ø, Hagatun S, Kallestad H, et al. Long-term effects of an unguided online cognitive behavioral therapy for chronic insomnia. J Clin Sleep Med. 2019;15(1):101-110.
11. Sweetman A, Lack L, Catcheside PG, et al. Cognitive and behavioral therapy for insomnia increases the use of continuous positive airway pressure therapy in obstructive sleep apnea participants with comorbid insomnia: a randomized clinical trial. Sleep. 2019;42(12):zsz178. doi: 10.1093/sleep/zsz178.
12. Asarnow LD, Bei B, Krystal A, et al. Circadian preference as a moderator of depression outcome following cognitive behavioral therapy for insomnia plus antidepressant medications: a report from the TRIAD study. J Clin Sleep Med. 2019;15(4):573-580.
13. Drake CL, Kalmbach DA, Arnedt JT, et al. Treating chronic insomnia in postmenopausal women: a randomized clinical trial comparing cognitive-behavioral therapy for insomnia, sleep restriction therapy, and sleep hygiene education. Sleep. 2019;42(2):zsy217. doi: 10.1093/sleep/zsy217.
14. Kalmbach DA, Cheng P, Arnedt JT, et al. Improving daytime functioning, work performance, and quality of life in postmenopausal women with insomnia: comparing cognitive behavioral therapy for insomnia, sleep restriction therapy, and sleep hygiene education. J Clin Sleep Med. 2019;15(7):999-1010.
15. Peoples AR, Garland SN, Pigeon WR, et al. Cognitive behavioral therapy for insomnia reduces depression in cancer survivors. J Clin Sleep Med. 2019;15(1):129-137.
16. Harb GC, Cook JM, Phelps AJ, et al. Randomized controlled trial of imagery rehearsal for posttraumatic nightmares in combat veterans. J Clin Sleep Med. 2019;15(5):757-767.
The prevalence of insomnia in the general population is approximately 6% to 10%.1 In addition, an estimated 30% of the general population may have symptoms of insomnia without meeting the diagnostic criteria.2 As a disorder, insomnia is characterized by a persistent difficulty initiating or maintaining sleep, or early morning awakening with inability to return to sleep, that has been present for at least 3 months. Additionally, the sleep difficulties must occur at least 3 nights a week, result in impaired daytime functioning, and cause significant distress.1
Cognitive-behavioral therapy for insomnia (CBT-I) is an effective treatment, supported by several systematic reviews and meta-analyses.3-5 In the short term, CBT-I is as effective as pharmacotherapy.6 However, CBT-I is the preferred treatment for chronic insomnia, according to recommendations in European and American guidelines.7,8
Here we review 8 recent studies that examined CBT-I. These studies are summarized in the Table.9-16

1. Cheng P, Kalmbach DA, Tallent G, et al. Depression prevention via digital cognitive behavioral therapy for insomnia: a randomized controlled trial. Sleep. 2019;42(10):zsz150. doi: 10.1093/sleep/zsz150.
Although CBT-I is a first-line treatment for chronic insomnia, it is underutilized in clinical practice primarily due to limited availability. Because few clinicians are certified in CBT-I, it has become necessary to develop alternative modes of delivery for CBT-I, such as fully automated, internet-delivered approaches to reach more patients with insomnia. Digital CBT-I (dCBT-I) is comparable to in-person CBT-I in improving insomnia symptoms and reducing concurrent depressive symptoms with insomnia. It is unclear if unguided, internet-delivered CBT-I is effective for achieving remission from depression or preventing depression in the long term. Chen et al9 examined the efficacy of dCBT-I in reducing and preventing depression over a 1-year follow-up.
Study design
- Participants from various centers in Southeastern Michigan were recruited between 2016 and 2017. Data was obtained from the Sleep to Prevent Evolving Affective Disorders (SPREAD) trial.
- Participants who met DSM-5 criteria for chronic insomnia disorder were randomized to dCBT-I (n = 358) using the Sleepio digital CBT program via the internet or to online sleep education (n = 300).
- The primary outcome was depression, measured using the Quick Inventory of Depressive Symptomatology-Self Report (QIDS-SR-16) at 1-year follow-up. Depression incidence was also tested against insomnia treatment response.
Outcomes
- The severity of depression was significantly lower at 1-year follow-up in the dCBT-I group compared with the control group.
- The dCBT-I group showed a 51% higher remission rate than the control group at 1-year follow-up.
- The incidence of moderate to severe depression in individuals with minimal to no depression at baseline was halved at 1 year after receiving dCBT-I treatment compared with the control group.
Continue to: Conclusion
Conclusion
- dCBT-I can improve depression and insomnia and has a sustained antidepressant effect.
- dCBT-I is effective for preventing depression. In other words, the risk of developing depression is decreased when dCBT-I is used to treat insomnia in individuals with minimal to no depression at baseline.
2. Vedaa Ø, Hagatun S, Kallestad H, et al. Long-term effects of an unguided online cognitive behavioral therapy for chronic insomnia. J Clin Sleep Med. 2019;15(1):101-110.
dCBT-I is effective for treating insomnia in the short term; however, little is known about the long-term effectiveness of dCBT-I on sleep and daytime symptoms. Vedaa et al10 evaluated the efficacy of dCBT-I at 18 months after the intervention.
Study design
- In this randomized controlled trial (RCT), the efficacy of unguided, internet-delivered CBT-I (n = 95) was compared with web-based patient education (n = 86) for patients with chronic insomnia.
- Participants were assessed at baseline, after a 9-week intervention period, and at 6-month follow-up. Participants in the internet CBT-I group were reassessed at 18 months after the intervention using online questionnaires, including the Insomnia Severity Index (ISI), Bergen Insomnia Scale (BIS), Brief Dysfunctional Beliefs and Attitudes Scale, Hospital Anxiety and Depression Scale, Chalder Fatigue Questionnaire, and sleep diaries.
Outcomes
- At 18 months, significant improvements were noted from baseline ISI and BIS scores and in levels of daytime fatigue, as well as psychological distress and beliefs about sleep.
- Sleep diary variables—including sleep onset latency, time awake during the night (wake time after sleep onset), early morning awakening, total sleep time, and sleep efficiency—showed significant improvement from baseline to 18-month follow-up (at least moderate effect size).
- Improvements were maintained from the completion of the 9-week intervention to follow-up at 18 months.
Continue to: Conclusion
Conclusion
- Fully-automated, internet-based CBT-I is efficacious in maintaining positive effects on sleep and daytime functioning up to 18 months after completing treatment.
3. Sweetman A, Lack L, Catcheside PG, et al. Cognitive and behavioral therapy for insomnia increases the use of continuous positive airway pressure therapy in obstructive sleep apnea participants with comorbid insomnia: a randomized clinical trial. Sleep. 2019;42(12):zsz178. doi: 10.1093/sleep/zsz178.
Comorbid insomnia and sleep apnea (COMISA) can affect a patient’s ability to accept and comply with continuous positive airway pressure (CPAP) therapy. Providing adequate treatment for these patients can be challenging.
Sweetman et al11 evaluated the acceptance and use of CPAP in patients with obstructive sleep apnea and chronic insomnia following initial treatment with CBT-I compared with treatment as usual (TAU).
Study design
- In this RCT, 145 participants with COMISA were randomized to 4 sessions of CBT-I or TAU before starting CPAP therapy until 6 months after randomization.
- Primary outcomes were objective CPAP adherence and objective sleep efficiency at the end of 6 months.
- Secondary outcomes were CPAP acceptance/rejection, changes in sleep parameters, global insomnia severity, and daytime impairments at 6 months.
Continue to: Outcomes
Outcomes
- The CBT-I group had higher initial CPAP acceptance and greater average nightly adherence to CPAP (61 minutes more) than the TAU group.
- Significant improvements were noted in global insomnia severity, nighttime insomnia complaints, and dysfunctional sleep-related cognitions at 6 months in the CBT-I group compared with TAU.
- No differences between the 2 groups were noted in sleep diary parameters or daytime impairments at 6 months.
Conclusions
- Patients with COMISA can benefit from receiving CBT-I before starting CPAP therapy because CBT-I can improve immediate acceptance of CPAP and may help to maintain adherence to CPAP over time.
- Patients with sleep apnea should be evaluated for comorbid insomnia, and CBT-I should be considered before starting CPAP treatment.
4. Asarnow LD, Bei B, Krystal A, et al. Circadian preference as a moderator of depression outcome following cognitive behavioral therapy for insomnia plus antidepressant medications: a report from the TRIAD study. J Clin Sleep Med. 2019;15(4):573-580.
The Treatment of Insomnia and Depression (TRIAD) study reported the effects of combining antidepressants with CBT-I in patients with major depressive disorder (MDD) and insomnia. Asarnow et al12 examined the moderation of circadian preference in the reduction of depression and insomnia symptoms severity during the same trial.
Study design
- In this RCT, 139 participants with MDD and insomnia were treated with an antidepressant (escitalopram, sertraline, or desvenlafaxine) and randomized to 8 weeks of CBT-I or control therapy (sleep education).
- Measurements used were Composite Scale of Morningness for circadian preference (morningness vs eveningness), depression severity with the Hamilton Rating Scale for Depression, and insomnia severity using the ISI.
Continue to: Outcomes
Outcomes
- CBT-I was effective for insomnia regardless of circadian preference.
- A smaller reduction in depression scores was noted in participants with greater evening preference.
- Depression outcomes were better among participants with evening preference if they were assigned to CBT-I vs control therapy.
- The control therapy (sleep education) was particularly ineffective in reducing depression symptoms in participants with evening preference.
Conclusion
- Individuals with MDD and insomnia and an evening preference are at an increased risk for poor response to antidepressants alone.
- Outcomes for both depression and insomnia improve if CBT-I is combined with antidepressants.
- Offering sleep education alone is not sufficient.
5. Drake CL, Kalmbach DA, Arnedt JT, et al. Treating chronic insomnia in postmenopausal women: a randomized clinical trial comparing cognitive-behavioral therapy for insomnia, sleep restriction therapy, and sleep hygiene education. Sleep. 2019;42(2):zsy217. doi: 10.1093/sleep/zsy217.
Postmenopausal women with sleep disturbances experience higher medical and psychiatric comorbidities, and have higher alcohol consumption and stress levels than postmenopausal women with good sleep. Nonpharmacologic insomnia treatments with durable effects are imperative for postmenopausal women because they are safer than pharmacologic approaches. Although CBT-I is the recommended first-line treatment for chronic insomnia, its application in menopause-related insomnia is limited. Drake et al13 evaluated the efficacy of CBT-I in menopause-related insomnia compared with sleep restriction therapy (SRT) and sleep hygiene education (SHE).
Study design
- This RCT was conducted at a health system with 6 hospitals in Michigan.
- Postmenopausal women who met DSM-5 criteria for chronic insomnia disorder (n = 150) were randomized into 1 of 3 groups: SHE, SRT, or CBT-I.
- Primary outcome measures were ISI scores and sleep diaries that documented multiple sleep parameters, including sleep onset latency, wake time after sleep onset, number of awakenings in the middle of the night, time in bed, total sleep time, and sleep efficiency. These were measured at baseline, after completion of treatment, and 6 months after treatment.
Continue to: Outcomes
Outcomes
- Both CBT-I and SRT outperformed SHE on the ISI and for most of the sleep parameters on sleep diaries immediately after treatment completion and at 6 months after treatment.
- Total sleep time was 40 to 43 minutes longer in the CBT-I group than in the SRT and SHE groups at 6-month follow-up.
- Remission rates (sleep onset latency ≤30 minutes, wake time after sleep onset ≤30 minutes, sleep efficiency ≥85%) were significantly higher in CBT-I group (CBT-I > SRT > SHE).
Conclusion
- Sleep hygiene education as a standalone treatment is not useful for treating chronic insomnia.
- Both CBT-I and SRT are efficacious for menopause-related insomnia.
- CBT-I may be a better option than SRT because it produces higher remission rates and better long-term outcomes.
6. Kalmbach DA, Cheng P, Arnedt JT, et al. Improving daytime functioning, work performance, and quality of life in postmenopausal women with insomnia: comparing cognitive behavioral therapy for insomnia, sleep restriction therapy, and sleep hygiene education. J Clin Sleep Med. 2019;15(7):999-1010.
CBT-I has shown efficacy in the treatment of insomnia in postmenopausal women. In this study, Kalmbach et al14 compared 3 nonpharmacologic modalities—CBT-I, SRT, and SHE—for the treatment of menopause-related insomnia and daytime impairment.
Study design
- In this RCT, 150 participants with new peri- and post-menopausal onset or exacerbation of insomnia were randomized to 1 of 3 groups: SHE, SRT, or CBT-I.
- Participants were assessed at baseline, after treatment completion, and at 6-month follow-up using the ISI, sleep diaries, Fatigue Severity Scale, Epworth Sleepiness Scale, Work Productivity and Activity Impairment Questionnaire, and 36-item Medical Outcomes Study Short Form Health Survey.
Continue to: Outcomes
Outcomes
- In both the CBT-I and SRT groups, significant improvements were noted in fatigue, energy, daytime sleepiness, and work function after treatment completion and at 6-month follow-up.
- Improvements were noted in emotional well-being and resiliency to physical and emotional problems in the CBT-I group at 6 months.
- Improvements in overall general health and social functioning, less pain, and fewer hot flashes were reported by postmenopausal women who remitted from insomnia; however, these benefits were not directly related to any specific treatment modality.
Conclusion
- CBT-I and SRT are superior to SHE for improving daytime functioning, and some aspects of life quality and work productivity, in postmenopausal women with insomnia.
- CBT-I may be superior to SRT in producing larger improvements in fatigue, energy level, and daytime sleep propensity.
- CBT-I can improve emotional well-being and resilience to emotional problems in postmenopausal women with insomnia.
7. Peoples AR, Garland SN, Pigeon WR, et al. Cognitive behavioral therapy for insomnia reduces depression in cancer survivors. J Clin Sleep Med. 2019;15(1):129-137.
Depression is common in patients with cancer and is usually associated with comorbid insomnia. Depression has significant effect on treatment compliance, coping with illness, and quality of life. Peoples et al15 examined the effects of CBT-I on depression in cancer survivors.
Study design
- This was a secondary analysis of a multicenter, randomized, placebo-controlled trial that evaluated interventions for cancer survivors with chronic insomnia in which the primary outcome measure was insomnia severity.
- Cancer survivors (n = 67) were randomized to CBT-I plus armodafinil or placebo or to SHE plus armodafinil or placebo.
- The Patient Health Questionnaire-9 (PHQ-9) and ISI were used to measure depression and insomnia at baseline, after 7-weeks of intervention, and at 3 months postintervention.
Continue to: Outcomes
Outcomes
- Immediately after completing the intervention, cancer survivors treated with CBT-I had significantly less depression (38% greater improvement in depression) compared with those who received SHE (control group).
- In the CBT-I group, 23% of cancer survivors achieved a clinically important reduction (5-point reduction on PHQ-9 total score) in depression at postintervention compared with 6% of those in the control group.
- At 3 months after the intervention, only 14% of cancer survivors in CBT-I group reported depression (PHQ-9 score >4), whereas 47% of those in the control group (SHE) reported depression.
Conclusion
- CBT-I improves both depression and insomnia in cancer survivors, and the improvements are sustained at 3 months after completing treatment.
- Improvement in insomnia severity appears to mediate the positive effects of CBT-I on depression.
8. Harb GC, Cook JM, Phelps AJ, et al. Randomized controlled trial of imagery rehearsal for posttraumatic nightmares in combat veterans. J Clin Sleep Med. 2019;15(5):757-767.
The American Academy of Sleep Medicine recommends imagery rehearsal (IR) therapy, which incorporates some components of CBT-I, for the treatment of recurrent posttraumatic stress disorder (PTSD)–related nightmares. In this study, Harb et al16 compared CBT-I plus IR to CBT-I alone for the treatment of nightmares in veterans with combat-related PTSD.
Study design
- This RCT included male and female US veterans (n = 108) deployed to Iraq and Afghanistan with current PTSD and recurrent nightmares related to deployment.
- Participants were randomized to 6 sessions of CBT-I plus IR or CBT-I alone.
- Primary outcome measures included frequency of nightmares and distress associated with nightmares.
Continue to: Outcomes
Outcomes
- A significant improvement in nightmares was noted in both groups (29% of participants showed a clinically-significant reduction in nightmare frequency and 22% of participants achieved remission).
- CBT-I plus IR was not superior to CBT-I only at postintervention and at 6-month follow-up.
Conclusion
- Both IR and CBT-I demonstrated efficacy for decreasing nightmare frequency and distress.
- Combining IR and CBT-I may not provide a synergistic advantage over CBT-I alone for treating PTSD-related nightmares in veterans.
The prevalence of insomnia in the general population is approximately 6% to 10%.1 In addition, an estimated 30% of the general population may have symptoms of insomnia without meeting the diagnostic criteria.2 As a disorder, insomnia is characterized by a persistent difficulty initiating or maintaining sleep, or early morning awakening with inability to return to sleep, that has been present for at least 3 months. Additionally, the sleep difficulties must occur at least 3 nights a week, result in impaired daytime functioning, and cause significant distress.1
Cognitive-behavioral therapy for insomnia (CBT-I) is an effective treatment, supported by several systematic reviews and meta-analyses.3-5 In the short term, CBT-I is as effective as pharmacotherapy.6 However, CBT-I is the preferred treatment for chronic insomnia, according to recommendations in European and American guidelines.7,8
Here we review 8 recent studies that examined CBT-I. These studies are summarized in the Table.9-16

1. Cheng P, Kalmbach DA, Tallent G, et al. Depression prevention via digital cognitive behavioral therapy for insomnia: a randomized controlled trial. Sleep. 2019;42(10):zsz150. doi: 10.1093/sleep/zsz150.
Although CBT-I is a first-line treatment for chronic insomnia, it is underutilized in clinical practice primarily due to limited availability. Because few clinicians are certified in CBT-I, it has become necessary to develop alternative modes of delivery for CBT-I, such as fully automated, internet-delivered approaches to reach more patients with insomnia. Digital CBT-I (dCBT-I) is comparable to in-person CBT-I in improving insomnia symptoms and reducing concurrent depressive symptoms with insomnia. It is unclear if unguided, internet-delivered CBT-I is effective for achieving remission from depression or preventing depression in the long term. Chen et al9 examined the efficacy of dCBT-I in reducing and preventing depression over a 1-year follow-up.
Study design
- Participants from various centers in Southeastern Michigan were recruited between 2016 and 2017. Data was obtained from the Sleep to Prevent Evolving Affective Disorders (SPREAD) trial.
- Participants who met DSM-5 criteria for chronic insomnia disorder were randomized to dCBT-I (n = 358) using the Sleepio digital CBT program via the internet or to online sleep education (n = 300).
- The primary outcome was depression, measured using the Quick Inventory of Depressive Symptomatology-Self Report (QIDS-SR-16) at 1-year follow-up. Depression incidence was also tested against insomnia treatment response.
Outcomes
- The severity of depression was significantly lower at 1-year follow-up in the dCBT-I group compared with the control group.
- The dCBT-I group showed a 51% higher remission rate than the control group at 1-year follow-up.
- The incidence of moderate to severe depression in individuals with minimal to no depression at baseline was halved at 1 year after receiving dCBT-I treatment compared with the control group.
Continue to: Conclusion
Conclusion
- dCBT-I can improve depression and insomnia and has a sustained antidepressant effect.
- dCBT-I is effective for preventing depression. In other words, the risk of developing depression is decreased when dCBT-I is used to treat insomnia in individuals with minimal to no depression at baseline.
2. Vedaa Ø, Hagatun S, Kallestad H, et al. Long-term effects of an unguided online cognitive behavioral therapy for chronic insomnia. J Clin Sleep Med. 2019;15(1):101-110.
dCBT-I is effective for treating insomnia in the short term; however, little is known about the long-term effectiveness of dCBT-I on sleep and daytime symptoms. Vedaa et al10 evaluated the efficacy of dCBT-I at 18 months after the intervention.
Study design
- In this randomized controlled trial (RCT), the efficacy of unguided, internet-delivered CBT-I (n = 95) was compared with web-based patient education (n = 86) for patients with chronic insomnia.
- Participants were assessed at baseline, after a 9-week intervention period, and at 6-month follow-up. Participants in the internet CBT-I group were reassessed at 18 months after the intervention using online questionnaires, including the Insomnia Severity Index (ISI), Bergen Insomnia Scale (BIS), Brief Dysfunctional Beliefs and Attitudes Scale, Hospital Anxiety and Depression Scale, Chalder Fatigue Questionnaire, and sleep diaries.
Outcomes
- At 18 months, significant improvements were noted from baseline ISI and BIS scores and in levels of daytime fatigue, as well as psychological distress and beliefs about sleep.
- Sleep diary variables—including sleep onset latency, time awake during the night (wake time after sleep onset), early morning awakening, total sleep time, and sleep efficiency—showed significant improvement from baseline to 18-month follow-up (at least moderate effect size).
- Improvements were maintained from the completion of the 9-week intervention to follow-up at 18 months.
Continue to: Conclusion
Conclusion
- Fully-automated, internet-based CBT-I is efficacious in maintaining positive effects on sleep and daytime functioning up to 18 months after completing treatment.
3. Sweetman A, Lack L, Catcheside PG, et al. Cognitive and behavioral therapy for insomnia increases the use of continuous positive airway pressure therapy in obstructive sleep apnea participants with comorbid insomnia: a randomized clinical trial. Sleep. 2019;42(12):zsz178. doi: 10.1093/sleep/zsz178.
Comorbid insomnia and sleep apnea (COMISA) can affect a patient’s ability to accept and comply with continuous positive airway pressure (CPAP) therapy. Providing adequate treatment for these patients can be challenging.
Sweetman et al11 evaluated the acceptance and use of CPAP in patients with obstructive sleep apnea and chronic insomnia following initial treatment with CBT-I compared with treatment as usual (TAU).
Study design
- In this RCT, 145 participants with COMISA were randomized to 4 sessions of CBT-I or TAU before starting CPAP therapy until 6 months after randomization.
- Primary outcomes were objective CPAP adherence and objective sleep efficiency at the end of 6 months.
- Secondary outcomes were CPAP acceptance/rejection, changes in sleep parameters, global insomnia severity, and daytime impairments at 6 months.
Continue to: Outcomes
Outcomes
- The CBT-I group had higher initial CPAP acceptance and greater average nightly adherence to CPAP (61 minutes more) than the TAU group.
- Significant improvements were noted in global insomnia severity, nighttime insomnia complaints, and dysfunctional sleep-related cognitions at 6 months in the CBT-I group compared with TAU.
- No differences between the 2 groups were noted in sleep diary parameters or daytime impairments at 6 months.
Conclusions
- Patients with COMISA can benefit from receiving CBT-I before starting CPAP therapy because CBT-I can improve immediate acceptance of CPAP and may help to maintain adherence to CPAP over time.
- Patients with sleep apnea should be evaluated for comorbid insomnia, and CBT-I should be considered before starting CPAP treatment.
4. Asarnow LD, Bei B, Krystal A, et al. Circadian preference as a moderator of depression outcome following cognitive behavioral therapy for insomnia plus antidepressant medications: a report from the TRIAD study. J Clin Sleep Med. 2019;15(4):573-580.
The Treatment of Insomnia and Depression (TRIAD) study reported the effects of combining antidepressants with CBT-I in patients with major depressive disorder (MDD) and insomnia. Asarnow et al12 examined the moderation of circadian preference in the reduction of depression and insomnia symptoms severity during the same trial.
Study design
- In this RCT, 139 participants with MDD and insomnia were treated with an antidepressant (escitalopram, sertraline, or desvenlafaxine) and randomized to 8 weeks of CBT-I or control therapy (sleep education).
- Measurements used were Composite Scale of Morningness for circadian preference (morningness vs eveningness), depression severity with the Hamilton Rating Scale for Depression, and insomnia severity using the ISI.
Continue to: Outcomes
Outcomes
- CBT-I was effective for insomnia regardless of circadian preference.
- A smaller reduction in depression scores was noted in participants with greater evening preference.
- Depression outcomes were better among participants with evening preference if they were assigned to CBT-I vs control therapy.
- The control therapy (sleep education) was particularly ineffective in reducing depression symptoms in participants with evening preference.
Conclusion
- Individuals with MDD and insomnia and an evening preference are at an increased risk for poor response to antidepressants alone.
- Outcomes for both depression and insomnia improve if CBT-I is combined with antidepressants.
- Offering sleep education alone is not sufficient.
5. Drake CL, Kalmbach DA, Arnedt JT, et al. Treating chronic insomnia in postmenopausal women: a randomized clinical trial comparing cognitive-behavioral therapy for insomnia, sleep restriction therapy, and sleep hygiene education. Sleep. 2019;42(2):zsy217. doi: 10.1093/sleep/zsy217.
Postmenopausal women with sleep disturbances experience higher medical and psychiatric comorbidities, and have higher alcohol consumption and stress levels than postmenopausal women with good sleep. Nonpharmacologic insomnia treatments with durable effects are imperative for postmenopausal women because they are safer than pharmacologic approaches. Although CBT-I is the recommended first-line treatment for chronic insomnia, its application in menopause-related insomnia is limited. Drake et al13 evaluated the efficacy of CBT-I in menopause-related insomnia compared with sleep restriction therapy (SRT) and sleep hygiene education (SHE).
Study design
- This RCT was conducted at a health system with 6 hospitals in Michigan.
- Postmenopausal women who met DSM-5 criteria for chronic insomnia disorder (n = 150) were randomized into 1 of 3 groups: SHE, SRT, or CBT-I.
- Primary outcome measures were ISI scores and sleep diaries that documented multiple sleep parameters, including sleep onset latency, wake time after sleep onset, number of awakenings in the middle of the night, time in bed, total sleep time, and sleep efficiency. These were measured at baseline, after completion of treatment, and 6 months after treatment.
Continue to: Outcomes
Outcomes
- Both CBT-I and SRT outperformed SHE on the ISI and for most of the sleep parameters on sleep diaries immediately after treatment completion and at 6 months after treatment.
- Total sleep time was 40 to 43 minutes longer in the CBT-I group than in the SRT and SHE groups at 6-month follow-up.
- Remission rates (sleep onset latency ≤30 minutes, wake time after sleep onset ≤30 minutes, sleep efficiency ≥85%) were significantly higher in CBT-I group (CBT-I > SRT > SHE).
Conclusion
- Sleep hygiene education as a standalone treatment is not useful for treating chronic insomnia.
- Both CBT-I and SRT are efficacious for menopause-related insomnia.
- CBT-I may be a better option than SRT because it produces higher remission rates and better long-term outcomes.
6. Kalmbach DA, Cheng P, Arnedt JT, et al. Improving daytime functioning, work performance, and quality of life in postmenopausal women with insomnia: comparing cognitive behavioral therapy for insomnia, sleep restriction therapy, and sleep hygiene education. J Clin Sleep Med. 2019;15(7):999-1010.
CBT-I has shown efficacy in the treatment of insomnia in postmenopausal women. In this study, Kalmbach et al14 compared 3 nonpharmacologic modalities—CBT-I, SRT, and SHE—for the treatment of menopause-related insomnia and daytime impairment.
Study design
- In this RCT, 150 participants with new peri- and post-menopausal onset or exacerbation of insomnia were randomized to 1 of 3 groups: SHE, SRT, or CBT-I.
- Participants were assessed at baseline, after treatment completion, and at 6-month follow-up using the ISI, sleep diaries, Fatigue Severity Scale, Epworth Sleepiness Scale, Work Productivity and Activity Impairment Questionnaire, and 36-item Medical Outcomes Study Short Form Health Survey.
Continue to: Outcomes
Outcomes
- In both the CBT-I and SRT groups, significant improvements were noted in fatigue, energy, daytime sleepiness, and work function after treatment completion and at 6-month follow-up.
- Improvements were noted in emotional well-being and resiliency to physical and emotional problems in the CBT-I group at 6 months.
- Improvements in overall general health and social functioning, less pain, and fewer hot flashes were reported by postmenopausal women who remitted from insomnia; however, these benefits were not directly related to any specific treatment modality.
Conclusion
- CBT-I and SRT are superior to SHE for improving daytime functioning, and some aspects of life quality and work productivity, in postmenopausal women with insomnia.
- CBT-I may be superior to SRT in producing larger improvements in fatigue, energy level, and daytime sleep propensity.
- CBT-I can improve emotional well-being and resilience to emotional problems in postmenopausal women with insomnia.
7. Peoples AR, Garland SN, Pigeon WR, et al. Cognitive behavioral therapy for insomnia reduces depression in cancer survivors. J Clin Sleep Med. 2019;15(1):129-137.
Depression is common in patients with cancer and is usually associated with comorbid insomnia. Depression has significant effect on treatment compliance, coping with illness, and quality of life. Peoples et al15 examined the effects of CBT-I on depression in cancer survivors.
Study design
- This was a secondary analysis of a multicenter, randomized, placebo-controlled trial that evaluated interventions for cancer survivors with chronic insomnia in which the primary outcome measure was insomnia severity.
- Cancer survivors (n = 67) were randomized to CBT-I plus armodafinil or placebo or to SHE plus armodafinil or placebo.
- The Patient Health Questionnaire-9 (PHQ-9) and ISI were used to measure depression and insomnia at baseline, after 7-weeks of intervention, and at 3 months postintervention.
Continue to: Outcomes
Outcomes
- Immediately after completing the intervention, cancer survivors treated with CBT-I had significantly less depression (38% greater improvement in depression) compared with those who received SHE (control group).
- In the CBT-I group, 23% of cancer survivors achieved a clinically important reduction (5-point reduction on PHQ-9 total score) in depression at postintervention compared with 6% of those in the control group.
- At 3 months after the intervention, only 14% of cancer survivors in CBT-I group reported depression (PHQ-9 score >4), whereas 47% of those in the control group (SHE) reported depression.
Conclusion
- CBT-I improves both depression and insomnia in cancer survivors, and the improvements are sustained at 3 months after completing treatment.
- Improvement in insomnia severity appears to mediate the positive effects of CBT-I on depression.
8. Harb GC, Cook JM, Phelps AJ, et al. Randomized controlled trial of imagery rehearsal for posttraumatic nightmares in combat veterans. J Clin Sleep Med. 2019;15(5):757-767.
The American Academy of Sleep Medicine recommends imagery rehearsal (IR) therapy, which incorporates some components of CBT-I, for the treatment of recurrent posttraumatic stress disorder (PTSD)–related nightmares. In this study, Harb et al16 compared CBT-I plus IR to CBT-I alone for the treatment of nightmares in veterans with combat-related PTSD.
Study design
- This RCT included male and female US veterans (n = 108) deployed to Iraq and Afghanistan with current PTSD and recurrent nightmares related to deployment.
- Participants were randomized to 6 sessions of CBT-I plus IR or CBT-I alone.
- Primary outcome measures included frequency of nightmares and distress associated with nightmares.
Continue to: Outcomes
Outcomes
- A significant improvement in nightmares was noted in both groups (29% of participants showed a clinically-significant reduction in nightmare frequency and 22% of participants achieved remission).
- CBT-I plus IR was not superior to CBT-I only at postintervention and at 6-month follow-up.
Conclusion
- Both IR and CBT-I demonstrated efficacy for decreasing nightmare frequency and distress.
- Combining IR and CBT-I may not provide a synergistic advantage over CBT-I alone for treating PTSD-related nightmares in veterans.
1. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
2. Morin CM, LeBlanc M, Daley M, et al. Epidemiology of insomnia: prevalence, self-help treatments, consultations, and determinants of help-seeking behaviors. Sleep Med. 2006;7(2):123-130.
3. Trauer JM, Qian MY, Doyle JS, et al. Cognitive behavioral therapy for chronic insomnia: a systematic review and meta-analysis. Ann Intern Med. 2015;163(3):191-204.
4. Wu JQ, Appleman ER, Salazar RD, et al. Cognitive behavioral therapy for insomnia comorbid with psychiatric and medical conditions: a meta-analysis. JAMA Intern Med. 2015;175(9):1461-1472.
5. van Straten A, van der Zweerde T, Kleiboer A, et al. Cognitive and behavioral therapies in the treatment of insomnia: a meta-analysis. Sleep Med Rev. 2018;38:3-16.
6. Smith MT, Perlis ML, Park A, et al. Comparative meta-analysis of pharmacotherapy and behavior therapy for persistent insomnia. Am J Psychiatry. 2002;159(1):5-11.
7. Qaseem A, Kansagara D, Forciea MA, et al. Management of chronic insomnia disorder in adults: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2016;165(2):125-133.
8. Riemann D, Baglioni C, Bassetti C, et al. European guideline for the diagnosis and treatment of insomnia. J Sleep Res. 2017;26(6):675-700.
9. Cheng P, Kalmbach DA, Tallent G, et al. Depression prevention via digital cognitive behavioral therapy for insomnia: a randomized controlled trial. Sleep. 2019;42(10):zsz150. doi: 10.1093/sleep/zsz150.
10. Vedaa Ø, Hagatun S, Kallestad H, et al. Long-term effects of an unguided online cognitive behavioral therapy for chronic insomnia. J Clin Sleep Med. 2019;15(1):101-110.
11. Sweetman A, Lack L, Catcheside PG, et al. Cognitive and behavioral therapy for insomnia increases the use of continuous positive airway pressure therapy in obstructive sleep apnea participants with comorbid insomnia: a randomized clinical trial. Sleep. 2019;42(12):zsz178. doi: 10.1093/sleep/zsz178.
12. Asarnow LD, Bei B, Krystal A, et al. Circadian preference as a moderator of depression outcome following cognitive behavioral therapy for insomnia plus antidepressant medications: a report from the TRIAD study. J Clin Sleep Med. 2019;15(4):573-580.
13. Drake CL, Kalmbach DA, Arnedt JT, et al. Treating chronic insomnia in postmenopausal women: a randomized clinical trial comparing cognitive-behavioral therapy for insomnia, sleep restriction therapy, and sleep hygiene education. Sleep. 2019;42(2):zsy217. doi: 10.1093/sleep/zsy217.
14. Kalmbach DA, Cheng P, Arnedt JT, et al. Improving daytime functioning, work performance, and quality of life in postmenopausal women with insomnia: comparing cognitive behavioral therapy for insomnia, sleep restriction therapy, and sleep hygiene education. J Clin Sleep Med. 2019;15(7):999-1010.
15. Peoples AR, Garland SN, Pigeon WR, et al. Cognitive behavioral therapy for insomnia reduces depression in cancer survivors. J Clin Sleep Med. 2019;15(1):129-137.
16. Harb GC, Cook JM, Phelps AJ, et al. Randomized controlled trial of imagery rehearsal for posttraumatic nightmares in combat veterans. J Clin Sleep Med. 2019;15(5):757-767.
1. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
2. Morin CM, LeBlanc M, Daley M, et al. Epidemiology of insomnia: prevalence, self-help treatments, consultations, and determinants of help-seeking behaviors. Sleep Med. 2006;7(2):123-130.
3. Trauer JM, Qian MY, Doyle JS, et al. Cognitive behavioral therapy for chronic insomnia: a systematic review and meta-analysis. Ann Intern Med. 2015;163(3):191-204.
4. Wu JQ, Appleman ER, Salazar RD, et al. Cognitive behavioral therapy for insomnia comorbid with psychiatric and medical conditions: a meta-analysis. JAMA Intern Med. 2015;175(9):1461-1472.
5. van Straten A, van der Zweerde T, Kleiboer A, et al. Cognitive and behavioral therapies in the treatment of insomnia: a meta-analysis. Sleep Med Rev. 2018;38:3-16.
6. Smith MT, Perlis ML, Park A, et al. Comparative meta-analysis of pharmacotherapy and behavior therapy for persistent insomnia. Am J Psychiatry. 2002;159(1):5-11.
7. Qaseem A, Kansagara D, Forciea MA, et al. Management of chronic insomnia disorder in adults: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2016;165(2):125-133.
8. Riemann D, Baglioni C, Bassetti C, et al. European guideline for the diagnosis and treatment of insomnia. J Sleep Res. 2017;26(6):675-700.
9. Cheng P, Kalmbach DA, Tallent G, et al. Depression prevention via digital cognitive behavioral therapy for insomnia: a randomized controlled trial. Sleep. 2019;42(10):zsz150. doi: 10.1093/sleep/zsz150.
10. Vedaa Ø, Hagatun S, Kallestad H, et al. Long-term effects of an unguided online cognitive behavioral therapy for chronic insomnia. J Clin Sleep Med. 2019;15(1):101-110.
11. Sweetman A, Lack L, Catcheside PG, et al. Cognitive and behavioral therapy for insomnia increases the use of continuous positive airway pressure therapy in obstructive sleep apnea participants with comorbid insomnia: a randomized clinical trial. Sleep. 2019;42(12):zsz178. doi: 10.1093/sleep/zsz178.
12. Asarnow LD, Bei B, Krystal A, et al. Circadian preference as a moderator of depression outcome following cognitive behavioral therapy for insomnia plus antidepressant medications: a report from the TRIAD study. J Clin Sleep Med. 2019;15(4):573-580.
13. Drake CL, Kalmbach DA, Arnedt JT, et al. Treating chronic insomnia in postmenopausal women: a randomized clinical trial comparing cognitive-behavioral therapy for insomnia, sleep restriction therapy, and sleep hygiene education. Sleep. 2019;42(2):zsy217. doi: 10.1093/sleep/zsy217.
14. Kalmbach DA, Cheng P, Arnedt JT, et al. Improving daytime functioning, work performance, and quality of life in postmenopausal women with insomnia: comparing cognitive behavioral therapy for insomnia, sleep restriction therapy, and sleep hygiene education. J Clin Sleep Med. 2019;15(7):999-1010.
15. Peoples AR, Garland SN, Pigeon WR, et al. Cognitive behavioral therapy for insomnia reduces depression in cancer survivors. J Clin Sleep Med. 2019;15(1):129-137.
16. Harb GC, Cook JM, Phelps AJ, et al. Randomized controlled trial of imagery rehearsal for posttraumatic nightmares in combat veterans. J Clin Sleep Med. 2019;15(5):757-767.
An update on the pharmacologic treatment of hypersomnia
The hypersomnias are an etiologically diverse group of disorders of wakefulness and sleep, characterized principally by excessive daytime sleepiness (EDS), often despite sufficient or even long total sleep durations. Hypersomnolence may be severely disabling and isolating for patients, is associated with decreased quality of life and economic disadvantage, and, in some cases, may pose a personal and public health danger through drowsy driving. Though historically, management of these patients has been principally supportive and aimed at reducing daytime functional impairment, new and evolving treatments are quickly changing management paradigms in this population. This brief review highlights some of the newest pharmacotherapeutic advances in this dynamic field.
Hypersomnolence is a common presenting concern primary care and sleep clinics, with an estimated prevalence of EDS in the general adult population of as high as 6%.1 The initial diagnosis of hypersomnia is, broadly, a clinical one, with careful consideration to the patient’s report of daytime sleepiness and functional impairment, sleep/wake cycle, and any medical comorbidities. The primary hypersomnias include narcolepsy type 1 (narcolepsy with cataplexy, NT1) and narcolepsy type 2 (without cataplexy, NT2), Kleine-Levin Syndrome (KLS), and idiopathic hypersomnia. Secondary hypersomnia disorders are more commonly encountered in clinical practice and include hypersomnia attributable to another medical condition (including psychiatric and neurologic disorders), hypersomnia related to medication effects, and EDS related to behaviorally insufficient sleep. Distinguishing primary and secondary etiologies, when possible, is important as treatment pathways may vary considerably between hypersomnias.
Generally, overnight in-lab polysomnography is warranted to exclude untreated or sub-optimally treated sleep-disordered breathing or movement disorders which may undermine sleep quality. In the absence of any such findings, this is usually followed by daytime multiple sleep latency testing (MSLT). The MSLT is comprised of four to five scheduled daytime naps in the sleep lab and is designed to quantify a patient’s propensity to sleep during the day and to identify architectural sleep abnormalities which indicate narcolepsy. Specifically, narcolepsy is identified by MSLT when a patient exhibits a sleep onset latency of ≤ 8 minutes and at least two sleep-onset REM periods (SOREMPs), or, one SOREMP on MSLT with a second noted on the preceding night’s PSG. Actigraphy or sleep logs may be helpful in quantifying a patient’s total sleep time in their home environment. Adjunctive laboratory tests for narcolepsy including HLA typing and CSF hypocretin testing may sometimes be indicated.
General hypersomnia management usually consists of the use of wakefulness promoting agents, such as stimulants (eg, dexmethylphenidate) and dopamine-modulating agents (eg, modafinil, armodafinil), and adjunctive supportive strategies, including planned daytime naps and elimination of modifiable secondary causes. In those patients with hypersomnolence associated with depression or anxiety, the use of antidepressants, including SSRI, SNRI, and DNRIs, is often effective, and these medications can also improve cataplexy symptoms in narcoleptics. KLS may respond to treatment with lithium, shortening the duration of the striking hypersomnolent episodes characteristic of this rare syndrome, and there is some indication that ketamine may also be a helpful adjunctive in some cases. In treatment-refractory cases of hypersomnolence associated with GABA-A receptor potentiation, drugs such as flumazenil and clarithromycin appear to improve subjective measures of hypersomnolence.2,3 In patients with narcolepsy, sodium oxybate (available as Xyrem and, more recently, as a generic medication) has proven to be clinically very useful, reducing EDS and the frequency and severity of cataplexy and sleep disturbance associated with this condition. In July 2020, the FDA approved a new, low-sodium formulation of sodium oxybate (Xywav) for patients 7 years of age and older with a diagnosis of narcolepsy, a helpful option in those patients with cardiovascular and renal disease.
Despite this broadening armamentarium, in many cases daytime sleepiness and functional impairment is refractory to typical pharmacotherapy. In this context, we would like to highlight two newer pharmacotherapeutic options, solriamfetol and pitolisant.
Solriamfetol
Solriamfetol (Sunosi) is a Schedule IV FDA-approved medication indicated for treatment of EDS in adults with narcolepsy or obstructive sleep apnea. The precise mechanism of action is unknown, but this medication is believed to inhibit both dopamine and norepinerphrine reuptake in the brain, similar to the widely-prescribed NDRI buproprion. In a 12-week RCT study on its effects on narcolepsy in adults, solriamfetol improved important measures of wakefulness and sleepiness, without associated polysomnographic evidence of significant sleep disruption.4 In another 12-week RCT study of solriamfetol in adult patients with EDS related to OSA, there was a dose-dependent improvement in measures of wakefulness.5 Some notable side-effects seen with this medication include anxiety and elevated mood, as well as increases in blood pressure. A subsequent study of this medication found that it was efficacious at maintenance of improvements at 6 months.6 Given the theorized mechanism of action as an NDRI, future observation and studies could provide insights on its effect on depression, as well.
Pitolisant
Histaminergic neurons within the CNS play an important role in the promotion of wakefulness. Pitolisant (Wakix) is an interesting wakefulness-promoting agent for adult patients with narcolepsy. It acts as an inverse agonist and antagonist of histamine H3 receptors, resulting in a reduction of the usual feedback inhibition effected through the H3 receptor, thereby enhancing CNS release of histamine and other neurotransmitters. This medication was approved by the FDA in August 2019 and is currently indicated for adult patients with narcolepsy. The HARMONY I trial comparing pitolisant with both placebo and modafinil in adults with narcolepsy and EDS demonstrated improvement in measures of sleepiness and maintenance of wakefulness over placebo, and noninferiority to modafinil.7 In addition, pitolisant had a favorable side-effect profile compared with modafinil. Subsequent studies have reaffirmed the safety profile of pitolisant, including its minimal abuse potential. In one recent placebo-controlled trial of the use of pitolisant in a population of 268 adults with positive airway pressure (PAP) non-adherence, pitolisant was found to improve measures of EDS and related patient-reported measurements in patients with OSA who were CPAP nonadherent.8 Though generally well-tolerated by patients, in initial clinical trials pitolisant was associated with increased headache, insomnia, and nausea relative to placebo, among other less commonly reported adverse effects. Pitolisant is QT interval-prolonging, so caution must be taken when using this medication in combination other medications which may induce QT interval prolongation, including SSRIs.
Future directions
Greater awareness of the hypersomnias and their management has led to improved outcomes and access to care for these patients, yet these disorders remain burdensome and the treatments imperfect. Looking forward, novel pharmacotherapies that target underlying mechanisms rather than symptom palliation will allow for more precise treatments. Ongoing investigations of hypocretin receptor agonists seek to target one critical central mediator of wakefulness. Recent studies have highlighted the association of dysautonomia with hypersomnia, offering interesting insight into possible future targets to improve the function and quality of life of these patients.9 Similarly, understanding of the interplay between psychiatric disorders and primary and secondary hypersomnias may offer new therapeutic pathways.
As treatment plans targeting hypersomnia become more comprehensive and holistic, with an increased emphasis on self-care, sleep hygiene, and mental health awareness, in addition to mechanism-specific treatments, we hope they will ultimately provide improved symptom and burden relief for our patients.
Dr. Shih Yee-Marie Tan Gipson is a psychiatrist and Dr. Kevin Gipson is a sleep medicine specialist, both with Massachusetts General Hospital, Boston.
References
1 Dauvilliers, et al. Hypersomnia. Dialogues Clin Neurosci. 2005;7(4):347-356.
2 Trotti, et al. Clarithromycin in gamma-aminobutyric acid-related hypersomnolence: A randomized, crossover trial. Ann Neurol. 2015;78(3):454-465. doi: 10.1002/ana.24459.
3 Trotti, et al. Flumazenil for the treatment of refractory hypersomnolence: Clinical experience with 153 patients. J Clin Sleep Med. 2016;12(10):1389-1394. doi: 10.5664/jcsm.6196.
4 Thorpy, et al. A randomized study of solriamfetol for excessive sleepiness in narcolepsy. Ann Neurol. 2019; 85(3):359-370. doi: 10.1002/ana.25423.
5 Schweitzer, et al. Solriamfetol for excessive sleepiness in obstructive sleep apnea (TONES 3): A randomized controlled trial. Am J Respir Crit Care Med. 2019;199(11):1421-1431. doi: 10.1164/rccm.201806-1100OC.
6 Malhotra, et al. Long-term study of the safety and maintenance of efficacy of solriamfetol (JZP-110) in the treatment of excessive sleepiness in participants with narcolepsy or obstructive sleep apnea. Sleep. 2020; 43(2): doi: 10.1093/sleep/zsz220.
7 Dauvilliers, et al. Pitolisant versus placebo or modafinil in patients with narcolepsy: a double-blind, randomised trial. Lancet Neurol. 2013;12(11):1068-1075. doi: 10.1016/S1474-4422(13)70225-4.
8 Dauvilliers, et al. Pitolisant for daytime sleepiness in obstructive sleep apnea patients refusing CPAP: A randomized trial. Am J Respir Crit Care Med. 2020. doi: 10.1164/rccm.201907-1284OC.
9 Miglis, et al. Frequency and severity of autonomic symptoms in idiopathic hypersomnia. J Clin Sleep Med. 2020; 16(5):749-756. doi: 10.5664/jcsm.8344.
The hypersomnias are an etiologically diverse group of disorders of wakefulness and sleep, characterized principally by excessive daytime sleepiness (EDS), often despite sufficient or even long total sleep durations. Hypersomnolence may be severely disabling and isolating for patients, is associated with decreased quality of life and economic disadvantage, and, in some cases, may pose a personal and public health danger through drowsy driving. Though historically, management of these patients has been principally supportive and aimed at reducing daytime functional impairment, new and evolving treatments are quickly changing management paradigms in this population. This brief review highlights some of the newest pharmacotherapeutic advances in this dynamic field.
Hypersomnolence is a common presenting concern primary care and sleep clinics, with an estimated prevalence of EDS in the general adult population of as high as 6%.1 The initial diagnosis of hypersomnia is, broadly, a clinical one, with careful consideration to the patient’s report of daytime sleepiness and functional impairment, sleep/wake cycle, and any medical comorbidities. The primary hypersomnias include narcolepsy type 1 (narcolepsy with cataplexy, NT1) and narcolepsy type 2 (without cataplexy, NT2), Kleine-Levin Syndrome (KLS), and idiopathic hypersomnia. Secondary hypersomnia disorders are more commonly encountered in clinical practice and include hypersomnia attributable to another medical condition (including psychiatric and neurologic disorders), hypersomnia related to medication effects, and EDS related to behaviorally insufficient sleep. Distinguishing primary and secondary etiologies, when possible, is important as treatment pathways may vary considerably between hypersomnias.
Generally, overnight in-lab polysomnography is warranted to exclude untreated or sub-optimally treated sleep-disordered breathing or movement disorders which may undermine sleep quality. In the absence of any such findings, this is usually followed by daytime multiple sleep latency testing (MSLT). The MSLT is comprised of four to five scheduled daytime naps in the sleep lab and is designed to quantify a patient’s propensity to sleep during the day and to identify architectural sleep abnormalities which indicate narcolepsy. Specifically, narcolepsy is identified by MSLT when a patient exhibits a sleep onset latency of ≤ 8 minutes and at least two sleep-onset REM periods (SOREMPs), or, one SOREMP on MSLT with a second noted on the preceding night’s PSG. Actigraphy or sleep logs may be helpful in quantifying a patient’s total sleep time in their home environment. Adjunctive laboratory tests for narcolepsy including HLA typing and CSF hypocretin testing may sometimes be indicated.
General hypersomnia management usually consists of the use of wakefulness promoting agents, such as stimulants (eg, dexmethylphenidate) and dopamine-modulating agents (eg, modafinil, armodafinil), and adjunctive supportive strategies, including planned daytime naps and elimination of modifiable secondary causes. In those patients with hypersomnolence associated with depression or anxiety, the use of antidepressants, including SSRI, SNRI, and DNRIs, is often effective, and these medications can also improve cataplexy symptoms in narcoleptics. KLS may respond to treatment with lithium, shortening the duration of the striking hypersomnolent episodes characteristic of this rare syndrome, and there is some indication that ketamine may also be a helpful adjunctive in some cases. In treatment-refractory cases of hypersomnolence associated with GABA-A receptor potentiation, drugs such as flumazenil and clarithromycin appear to improve subjective measures of hypersomnolence.2,3 In patients with narcolepsy, sodium oxybate (available as Xyrem and, more recently, as a generic medication) has proven to be clinically very useful, reducing EDS and the frequency and severity of cataplexy and sleep disturbance associated with this condition. In July 2020, the FDA approved a new, low-sodium formulation of sodium oxybate (Xywav) for patients 7 years of age and older with a diagnosis of narcolepsy, a helpful option in those patients with cardiovascular and renal disease.
Despite this broadening armamentarium, in many cases daytime sleepiness and functional impairment is refractory to typical pharmacotherapy. In this context, we would like to highlight two newer pharmacotherapeutic options, solriamfetol and pitolisant.
Solriamfetol
Solriamfetol (Sunosi) is a Schedule IV FDA-approved medication indicated for treatment of EDS in adults with narcolepsy or obstructive sleep apnea. The precise mechanism of action is unknown, but this medication is believed to inhibit both dopamine and norepinerphrine reuptake in the brain, similar to the widely-prescribed NDRI buproprion. In a 12-week RCT study on its effects on narcolepsy in adults, solriamfetol improved important measures of wakefulness and sleepiness, without associated polysomnographic evidence of significant sleep disruption.4 In another 12-week RCT study of solriamfetol in adult patients with EDS related to OSA, there was a dose-dependent improvement in measures of wakefulness.5 Some notable side-effects seen with this medication include anxiety and elevated mood, as well as increases in blood pressure. A subsequent study of this medication found that it was efficacious at maintenance of improvements at 6 months.6 Given the theorized mechanism of action as an NDRI, future observation and studies could provide insights on its effect on depression, as well.
Pitolisant
Histaminergic neurons within the CNS play an important role in the promotion of wakefulness. Pitolisant (Wakix) is an interesting wakefulness-promoting agent for adult patients with narcolepsy. It acts as an inverse agonist and antagonist of histamine H3 receptors, resulting in a reduction of the usual feedback inhibition effected through the H3 receptor, thereby enhancing CNS release of histamine and other neurotransmitters. This medication was approved by the FDA in August 2019 and is currently indicated for adult patients with narcolepsy. The HARMONY I trial comparing pitolisant with both placebo and modafinil in adults with narcolepsy and EDS demonstrated improvement in measures of sleepiness and maintenance of wakefulness over placebo, and noninferiority to modafinil.7 In addition, pitolisant had a favorable side-effect profile compared with modafinil. Subsequent studies have reaffirmed the safety profile of pitolisant, including its minimal abuse potential. In one recent placebo-controlled trial of the use of pitolisant in a population of 268 adults with positive airway pressure (PAP) non-adherence, pitolisant was found to improve measures of EDS and related patient-reported measurements in patients with OSA who were CPAP nonadherent.8 Though generally well-tolerated by patients, in initial clinical trials pitolisant was associated with increased headache, insomnia, and nausea relative to placebo, among other less commonly reported adverse effects. Pitolisant is QT interval-prolonging, so caution must be taken when using this medication in combination other medications which may induce QT interval prolongation, including SSRIs.
Future directions
Greater awareness of the hypersomnias and their management has led to improved outcomes and access to care for these patients, yet these disorders remain burdensome and the treatments imperfect. Looking forward, novel pharmacotherapies that target underlying mechanisms rather than symptom palliation will allow for more precise treatments. Ongoing investigations of hypocretin receptor agonists seek to target one critical central mediator of wakefulness. Recent studies have highlighted the association of dysautonomia with hypersomnia, offering interesting insight into possible future targets to improve the function and quality of life of these patients.9 Similarly, understanding of the interplay between psychiatric disorders and primary and secondary hypersomnias may offer new therapeutic pathways.
As treatment plans targeting hypersomnia become more comprehensive and holistic, with an increased emphasis on self-care, sleep hygiene, and mental health awareness, in addition to mechanism-specific treatments, we hope they will ultimately provide improved symptom and burden relief for our patients.
Dr. Shih Yee-Marie Tan Gipson is a psychiatrist and Dr. Kevin Gipson is a sleep medicine specialist, both with Massachusetts General Hospital, Boston.
References
1 Dauvilliers, et al. Hypersomnia. Dialogues Clin Neurosci. 2005;7(4):347-356.
2 Trotti, et al. Clarithromycin in gamma-aminobutyric acid-related hypersomnolence: A randomized, crossover trial. Ann Neurol. 2015;78(3):454-465. doi: 10.1002/ana.24459.
3 Trotti, et al. Flumazenil for the treatment of refractory hypersomnolence: Clinical experience with 153 patients. J Clin Sleep Med. 2016;12(10):1389-1394. doi: 10.5664/jcsm.6196.
4 Thorpy, et al. A randomized study of solriamfetol for excessive sleepiness in narcolepsy. Ann Neurol. 2019; 85(3):359-370. doi: 10.1002/ana.25423.
5 Schweitzer, et al. Solriamfetol for excessive sleepiness in obstructive sleep apnea (TONES 3): A randomized controlled trial. Am J Respir Crit Care Med. 2019;199(11):1421-1431. doi: 10.1164/rccm.201806-1100OC.
6 Malhotra, et al. Long-term study of the safety and maintenance of efficacy of solriamfetol (JZP-110) in the treatment of excessive sleepiness in participants with narcolepsy or obstructive sleep apnea. Sleep. 2020; 43(2): doi: 10.1093/sleep/zsz220.
7 Dauvilliers, et al. Pitolisant versus placebo or modafinil in patients with narcolepsy: a double-blind, randomised trial. Lancet Neurol. 2013;12(11):1068-1075. doi: 10.1016/S1474-4422(13)70225-4.
8 Dauvilliers, et al. Pitolisant for daytime sleepiness in obstructive sleep apnea patients refusing CPAP: A randomized trial. Am J Respir Crit Care Med. 2020. doi: 10.1164/rccm.201907-1284OC.
9 Miglis, et al. Frequency and severity of autonomic symptoms in idiopathic hypersomnia. J Clin Sleep Med. 2020; 16(5):749-756. doi: 10.5664/jcsm.8344.
The hypersomnias are an etiologically diverse group of disorders of wakefulness and sleep, characterized principally by excessive daytime sleepiness (EDS), often despite sufficient or even long total sleep durations. Hypersomnolence may be severely disabling and isolating for patients, is associated with decreased quality of life and economic disadvantage, and, in some cases, may pose a personal and public health danger through drowsy driving. Though historically, management of these patients has been principally supportive and aimed at reducing daytime functional impairment, new and evolving treatments are quickly changing management paradigms in this population. This brief review highlights some of the newest pharmacotherapeutic advances in this dynamic field.
Hypersomnolence is a common presenting concern primary care and sleep clinics, with an estimated prevalence of EDS in the general adult population of as high as 6%.1 The initial diagnosis of hypersomnia is, broadly, a clinical one, with careful consideration to the patient’s report of daytime sleepiness and functional impairment, sleep/wake cycle, and any medical comorbidities. The primary hypersomnias include narcolepsy type 1 (narcolepsy with cataplexy, NT1) and narcolepsy type 2 (without cataplexy, NT2), Kleine-Levin Syndrome (KLS), and idiopathic hypersomnia. Secondary hypersomnia disorders are more commonly encountered in clinical practice and include hypersomnia attributable to another medical condition (including psychiatric and neurologic disorders), hypersomnia related to medication effects, and EDS related to behaviorally insufficient sleep. Distinguishing primary and secondary etiologies, when possible, is important as treatment pathways may vary considerably between hypersomnias.
Generally, overnight in-lab polysomnography is warranted to exclude untreated or sub-optimally treated sleep-disordered breathing or movement disorders which may undermine sleep quality. In the absence of any such findings, this is usually followed by daytime multiple sleep latency testing (MSLT). The MSLT is comprised of four to five scheduled daytime naps in the sleep lab and is designed to quantify a patient’s propensity to sleep during the day and to identify architectural sleep abnormalities which indicate narcolepsy. Specifically, narcolepsy is identified by MSLT when a patient exhibits a sleep onset latency of ≤ 8 minutes and at least two sleep-onset REM periods (SOREMPs), or, one SOREMP on MSLT with a second noted on the preceding night’s PSG. Actigraphy or sleep logs may be helpful in quantifying a patient’s total sleep time in their home environment. Adjunctive laboratory tests for narcolepsy including HLA typing and CSF hypocretin testing may sometimes be indicated.
General hypersomnia management usually consists of the use of wakefulness promoting agents, such as stimulants (eg, dexmethylphenidate) and dopamine-modulating agents (eg, modafinil, armodafinil), and adjunctive supportive strategies, including planned daytime naps and elimination of modifiable secondary causes. In those patients with hypersomnolence associated with depression or anxiety, the use of antidepressants, including SSRI, SNRI, and DNRIs, is often effective, and these medications can also improve cataplexy symptoms in narcoleptics. KLS may respond to treatment with lithium, shortening the duration of the striking hypersomnolent episodes characteristic of this rare syndrome, and there is some indication that ketamine may also be a helpful adjunctive in some cases. In treatment-refractory cases of hypersomnolence associated with GABA-A receptor potentiation, drugs such as flumazenil and clarithromycin appear to improve subjective measures of hypersomnolence.2,3 In patients with narcolepsy, sodium oxybate (available as Xyrem and, more recently, as a generic medication) has proven to be clinically very useful, reducing EDS and the frequency and severity of cataplexy and sleep disturbance associated with this condition. In July 2020, the FDA approved a new, low-sodium formulation of sodium oxybate (Xywav) for patients 7 years of age and older with a diagnosis of narcolepsy, a helpful option in those patients with cardiovascular and renal disease.
Despite this broadening armamentarium, in many cases daytime sleepiness and functional impairment is refractory to typical pharmacotherapy. In this context, we would like to highlight two newer pharmacotherapeutic options, solriamfetol and pitolisant.
Solriamfetol
Solriamfetol (Sunosi) is a Schedule IV FDA-approved medication indicated for treatment of EDS in adults with narcolepsy or obstructive sleep apnea. The precise mechanism of action is unknown, but this medication is believed to inhibit both dopamine and norepinerphrine reuptake in the brain, similar to the widely-prescribed NDRI buproprion. In a 12-week RCT study on its effects on narcolepsy in adults, solriamfetol improved important measures of wakefulness and sleepiness, without associated polysomnographic evidence of significant sleep disruption.4 In another 12-week RCT study of solriamfetol in adult patients with EDS related to OSA, there was a dose-dependent improvement in measures of wakefulness.5 Some notable side-effects seen with this medication include anxiety and elevated mood, as well as increases in blood pressure. A subsequent study of this medication found that it was efficacious at maintenance of improvements at 6 months.6 Given the theorized mechanism of action as an NDRI, future observation and studies could provide insights on its effect on depression, as well.
Pitolisant
Histaminergic neurons within the CNS play an important role in the promotion of wakefulness. Pitolisant (Wakix) is an interesting wakefulness-promoting agent for adult patients with narcolepsy. It acts as an inverse agonist and antagonist of histamine H3 receptors, resulting in a reduction of the usual feedback inhibition effected through the H3 receptor, thereby enhancing CNS release of histamine and other neurotransmitters. This medication was approved by the FDA in August 2019 and is currently indicated for adult patients with narcolepsy. The HARMONY I trial comparing pitolisant with both placebo and modafinil in adults with narcolepsy and EDS demonstrated improvement in measures of sleepiness and maintenance of wakefulness over placebo, and noninferiority to modafinil.7 In addition, pitolisant had a favorable side-effect profile compared with modafinil. Subsequent studies have reaffirmed the safety profile of pitolisant, including its minimal abuse potential. In one recent placebo-controlled trial of the use of pitolisant in a population of 268 adults with positive airway pressure (PAP) non-adherence, pitolisant was found to improve measures of EDS and related patient-reported measurements in patients with OSA who were CPAP nonadherent.8 Though generally well-tolerated by patients, in initial clinical trials pitolisant was associated with increased headache, insomnia, and nausea relative to placebo, among other less commonly reported adverse effects. Pitolisant is QT interval-prolonging, so caution must be taken when using this medication in combination other medications which may induce QT interval prolongation, including SSRIs.
Future directions
Greater awareness of the hypersomnias and their management has led to improved outcomes and access to care for these patients, yet these disorders remain burdensome and the treatments imperfect. Looking forward, novel pharmacotherapies that target underlying mechanisms rather than symptom palliation will allow for more precise treatments. Ongoing investigations of hypocretin receptor agonists seek to target one critical central mediator of wakefulness. Recent studies have highlighted the association of dysautonomia with hypersomnia, offering interesting insight into possible future targets to improve the function and quality of life of these patients.9 Similarly, understanding of the interplay between psychiatric disorders and primary and secondary hypersomnias may offer new therapeutic pathways.
As treatment plans targeting hypersomnia become more comprehensive and holistic, with an increased emphasis on self-care, sleep hygiene, and mental health awareness, in addition to mechanism-specific treatments, we hope they will ultimately provide improved symptom and burden relief for our patients.
Dr. Shih Yee-Marie Tan Gipson is a psychiatrist and Dr. Kevin Gipson is a sleep medicine specialist, both with Massachusetts General Hospital, Boston.
References
1 Dauvilliers, et al. Hypersomnia. Dialogues Clin Neurosci. 2005;7(4):347-356.
2 Trotti, et al. Clarithromycin in gamma-aminobutyric acid-related hypersomnolence: A randomized, crossover trial. Ann Neurol. 2015;78(3):454-465. doi: 10.1002/ana.24459.
3 Trotti, et al. Flumazenil for the treatment of refractory hypersomnolence: Clinical experience with 153 patients. J Clin Sleep Med. 2016;12(10):1389-1394. doi: 10.5664/jcsm.6196.
4 Thorpy, et al. A randomized study of solriamfetol for excessive sleepiness in narcolepsy. Ann Neurol. 2019; 85(3):359-370. doi: 10.1002/ana.25423.
5 Schweitzer, et al. Solriamfetol for excessive sleepiness in obstructive sleep apnea (TONES 3): A randomized controlled trial. Am J Respir Crit Care Med. 2019;199(11):1421-1431. doi: 10.1164/rccm.201806-1100OC.
6 Malhotra, et al. Long-term study of the safety and maintenance of efficacy of solriamfetol (JZP-110) in the treatment of excessive sleepiness in participants with narcolepsy or obstructive sleep apnea. Sleep. 2020; 43(2): doi: 10.1093/sleep/zsz220.
7 Dauvilliers, et al. Pitolisant versus placebo or modafinil in patients with narcolepsy: a double-blind, randomised trial. Lancet Neurol. 2013;12(11):1068-1075. doi: 10.1016/S1474-4422(13)70225-4.
8 Dauvilliers, et al. Pitolisant for daytime sleepiness in obstructive sleep apnea patients refusing CPAP: A randomized trial. Am J Respir Crit Care Med. 2020. doi: 10.1164/rccm.201907-1284OC.
9 Miglis, et al. Frequency and severity of autonomic symptoms in idiopathic hypersomnia. J Clin Sleep Med. 2020; 16(5):749-756. doi: 10.5664/jcsm.8344.
Alzheimer’s disease may affect sleep patterns
new research suggests.
The causal association between disturbed sleep and Alzheimer’s disease that has been observed in previous studies may have resulted from reverse causation, the researchers noted. The current Mendelian randomization analysis also failed to find a causal relationship between Alzheimer’s disease and major depressive disorder. Future studies should examine the genetic heterogeneity of depression syndromes to test for causal relationships between subtypes of depression with distinct causes and Alzheimer’s disease.
Mendelian randomization compares individuals who have different genetic profiles for a given exposure. “Given that genetic variants are inherited at random, these two groups are comparable, and any differences are not likely to be due to other associated factors,” such as confounding bias, said corresponding author Abbas Dehghan, PhD, reader in cardiometabolic disease epidemiology at Imperial College London. “Moreover, given that genetic information is constant over the lifetime, the chances for reverse causation are small.”
The findings were published online August 19 in Neurology.
Causal questions
Many patients with late-life neurodegenerative disorders such as Alzheimer’s disease have comorbid depression, but whether these two disorders have a causal relationship or common risk factors has been unclear, the investigators noted. Abnormal sleep patterns are symptoms of both depression and Alzheimer’s disease. Abnormal sleep is also associated with cognitive decline and anxiety.
The researchers hypothesized that sleep causally affects major depressive disorder and Alzheimer’s disease but that there is no causal relationship between major depressive disorder and Alzheimer’s disease. They conducted a bidirectional, two-sample Mendelian randomization study to test these hypotheses.
The investigators conducted genomewide association studies (GWASs) using data from the prospective, population-based U.K. Biobank. Sleep phenotypes were measured by self-report or accelerometer. Genetic associations were derived from 403,195 patients for chronotype, 237,627 patients for insomnia, 446,118 people for sleep duration, and 85,670 people for accelerometer-derived phenotypes.
Two binary variables from sleep duration were derived: short sleep (duration of less than 7 hours) and long sleep (duration of 9 or more hours). A sleep episode was defined as a period of at least 5 minutes with a change on the dorsal-ventral axis of less than 5 degrees. The durations of all sleep episodes were added to calculate total sleep duration.
Major depressive disorder was diagnosed clinically in accordance with DSM-IV criteria. Genetic associations were derived from 9,240 case patients and 9,519 control participants. Alzheimer’s disease was diagnosed on the basis of physician examination or autopsy results. Genetic associations were obtained from a meta-analysis of GWAS on participants of European ancestry in the International Genomics of Alzheimer’s Project, which included 21,982 case patients and 41,944 control participants.
More risk factor research needed
Results showed no causal relationships between sleep-related phenotypes and major depressive disorder in either direction. Causal relationships between major depressive disorder and Alzheimer’s disease were found in both directions, but neither was statistically significant.
A genetically higher risk for Alzheimer’s disease was associated with being a “morning person,” being at decreased risk for insomnia, having shorter sleep duration on self-report and accelerometer, having decreased likelihood of reporting long sleep, having an earlier timing of the least active 5 hours, and having a smaller number of sleep episodes. However, no analysis supported a causal effect of sleep-related phenotypes on risk for Alzheimer’s disease.
Because APOE4 can influence disease processes that may contribute to Alzheimer’s disease risk, the investigators also conducted a sensitivity analysis that excluded APOE single-nucleotide polymorphisms. In this analysis, the causal associations of Alzheimer’s disease with self-reported and accelerometer-based sleep duration were not significant. The sensitivity analysis did support the other causal associations between Alzheimer’s disease and sleep phenotypes, however.
The causal associations between major depressive disorder and Alzheimer’s disease observed in other studies may have been the result of confounding, and the participants may have had other associated characteristics that put them at risk for the disease, said Dr. Dehghan. Furthermore, the previous studies considered various sleep phenotypes together, whereas in the current study, the investigators examined them separately.
The results suggest that preclinical and clinical Alzheimer’s disease may affect sleep phenotypes differently. Sleep management thus could be an important approach to improving quality of life for patients with Alzheimer’s disease, the researchers wrote.
“Our study indicates that depression and sleep disorders are not likely to be a causal factor for Alzheimer’s disease,” Dr. Dehghan said. “We need to search for other risk factors for the prevention of Alzheimer’s disease.”
Several strengths, lacks details
Walter A. Kukull, PhD, professor of epidemiology and director of the National Alzheimer’s Coordinating Center at the University of Washington, Seattle, noted that the investigators appear to have implemented their chosen methods of causal association analysis well. “They attempted to examine the direction of the causal arrow for risk factors … and that is a step usually not well examined in other studies.”
He added that the collection of objective measures, such as of sleep, is another strength of the study.
However, “the common weakness of the basic GWAS sample is that clinical symptomatology determined Alzheimer’s disease diagnosis. Thus, asymptomatic or very mildly symptomatic persons with Alzheimer’s disease pathology in their brains were likely included among normal controls,” said Dr. Kukull, who was not involved with the research.
Because of an apparent lack of biomarker data, patients who had been diagnosed with Alzheimer’s disease may in fact have had a different form of dementia. Given the nature of their data, the investigators could have done little to compensate for these possibilities, Dr. Kukull added. In addition, the article lacks details that would improve the interpretation of the results.
“Timing is everything with regard to potential associations between risk factor and outcome,” Dr. Kukull said. “With the exceptions of genes, it would be nice to know more about the timing of risk factors’ onset and Alzheimer’s disease onset.”
Still, the results indicate potential areas of future study, he noted. “Primarily, further research must address the question of pathological onset of disease and misclassification of diagnosis in both cases and controls due to lack of biomarker-confirmed diagnosis. Then research can also struggle with the timing of potential risk factors with respect to disease.”
The study was funded by the U.K. Dementia Research Institute. Dr. Dehghan and Dr. Kukull reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
new research suggests.
The causal association between disturbed sleep and Alzheimer’s disease that has been observed in previous studies may have resulted from reverse causation, the researchers noted. The current Mendelian randomization analysis also failed to find a causal relationship between Alzheimer’s disease and major depressive disorder. Future studies should examine the genetic heterogeneity of depression syndromes to test for causal relationships between subtypes of depression with distinct causes and Alzheimer’s disease.
Mendelian randomization compares individuals who have different genetic profiles for a given exposure. “Given that genetic variants are inherited at random, these two groups are comparable, and any differences are not likely to be due to other associated factors,” such as confounding bias, said corresponding author Abbas Dehghan, PhD, reader in cardiometabolic disease epidemiology at Imperial College London. “Moreover, given that genetic information is constant over the lifetime, the chances for reverse causation are small.”
The findings were published online August 19 in Neurology.
Causal questions
Many patients with late-life neurodegenerative disorders such as Alzheimer’s disease have comorbid depression, but whether these two disorders have a causal relationship or common risk factors has been unclear, the investigators noted. Abnormal sleep patterns are symptoms of both depression and Alzheimer’s disease. Abnormal sleep is also associated with cognitive decline and anxiety.
The researchers hypothesized that sleep causally affects major depressive disorder and Alzheimer’s disease but that there is no causal relationship between major depressive disorder and Alzheimer’s disease. They conducted a bidirectional, two-sample Mendelian randomization study to test these hypotheses.
The investigators conducted genomewide association studies (GWASs) using data from the prospective, population-based U.K. Biobank. Sleep phenotypes were measured by self-report or accelerometer. Genetic associations were derived from 403,195 patients for chronotype, 237,627 patients for insomnia, 446,118 people for sleep duration, and 85,670 people for accelerometer-derived phenotypes.
Two binary variables from sleep duration were derived: short sleep (duration of less than 7 hours) and long sleep (duration of 9 or more hours). A sleep episode was defined as a period of at least 5 minutes with a change on the dorsal-ventral axis of less than 5 degrees. The durations of all sleep episodes were added to calculate total sleep duration.
Major depressive disorder was diagnosed clinically in accordance with DSM-IV criteria. Genetic associations were derived from 9,240 case patients and 9,519 control participants. Alzheimer’s disease was diagnosed on the basis of physician examination or autopsy results. Genetic associations were obtained from a meta-analysis of GWAS on participants of European ancestry in the International Genomics of Alzheimer’s Project, which included 21,982 case patients and 41,944 control participants.
More risk factor research needed
Results showed no causal relationships between sleep-related phenotypes and major depressive disorder in either direction. Causal relationships between major depressive disorder and Alzheimer’s disease were found in both directions, but neither was statistically significant.
A genetically higher risk for Alzheimer’s disease was associated with being a “morning person,” being at decreased risk for insomnia, having shorter sleep duration on self-report and accelerometer, having decreased likelihood of reporting long sleep, having an earlier timing of the least active 5 hours, and having a smaller number of sleep episodes. However, no analysis supported a causal effect of sleep-related phenotypes on risk for Alzheimer’s disease.
Because APOE4 can influence disease processes that may contribute to Alzheimer’s disease risk, the investigators also conducted a sensitivity analysis that excluded APOE single-nucleotide polymorphisms. In this analysis, the causal associations of Alzheimer’s disease with self-reported and accelerometer-based sleep duration were not significant. The sensitivity analysis did support the other causal associations between Alzheimer’s disease and sleep phenotypes, however.
The causal associations between major depressive disorder and Alzheimer’s disease observed in other studies may have been the result of confounding, and the participants may have had other associated characteristics that put them at risk for the disease, said Dr. Dehghan. Furthermore, the previous studies considered various sleep phenotypes together, whereas in the current study, the investigators examined them separately.
The results suggest that preclinical and clinical Alzheimer’s disease may affect sleep phenotypes differently. Sleep management thus could be an important approach to improving quality of life for patients with Alzheimer’s disease, the researchers wrote.
“Our study indicates that depression and sleep disorders are not likely to be a causal factor for Alzheimer’s disease,” Dr. Dehghan said. “We need to search for other risk factors for the prevention of Alzheimer’s disease.”
Several strengths, lacks details
Walter A. Kukull, PhD, professor of epidemiology and director of the National Alzheimer’s Coordinating Center at the University of Washington, Seattle, noted that the investigators appear to have implemented their chosen methods of causal association analysis well. “They attempted to examine the direction of the causal arrow for risk factors … and that is a step usually not well examined in other studies.”
He added that the collection of objective measures, such as of sleep, is another strength of the study.
However, “the common weakness of the basic GWAS sample is that clinical symptomatology determined Alzheimer’s disease diagnosis. Thus, asymptomatic or very mildly symptomatic persons with Alzheimer’s disease pathology in their brains were likely included among normal controls,” said Dr. Kukull, who was not involved with the research.
Because of an apparent lack of biomarker data, patients who had been diagnosed with Alzheimer’s disease may in fact have had a different form of dementia. Given the nature of their data, the investigators could have done little to compensate for these possibilities, Dr. Kukull added. In addition, the article lacks details that would improve the interpretation of the results.
“Timing is everything with regard to potential associations between risk factor and outcome,” Dr. Kukull said. “With the exceptions of genes, it would be nice to know more about the timing of risk factors’ onset and Alzheimer’s disease onset.”
Still, the results indicate potential areas of future study, he noted. “Primarily, further research must address the question of pathological onset of disease and misclassification of diagnosis in both cases and controls due to lack of biomarker-confirmed diagnosis. Then research can also struggle with the timing of potential risk factors with respect to disease.”
The study was funded by the U.K. Dementia Research Institute. Dr. Dehghan and Dr. Kukull reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
new research suggests.
The causal association between disturbed sleep and Alzheimer’s disease that has been observed in previous studies may have resulted from reverse causation, the researchers noted. The current Mendelian randomization analysis also failed to find a causal relationship between Alzheimer’s disease and major depressive disorder. Future studies should examine the genetic heterogeneity of depression syndromes to test for causal relationships between subtypes of depression with distinct causes and Alzheimer’s disease.
Mendelian randomization compares individuals who have different genetic profiles for a given exposure. “Given that genetic variants are inherited at random, these two groups are comparable, and any differences are not likely to be due to other associated factors,” such as confounding bias, said corresponding author Abbas Dehghan, PhD, reader in cardiometabolic disease epidemiology at Imperial College London. “Moreover, given that genetic information is constant over the lifetime, the chances for reverse causation are small.”
The findings were published online August 19 in Neurology.
Causal questions
Many patients with late-life neurodegenerative disorders such as Alzheimer’s disease have comorbid depression, but whether these two disorders have a causal relationship or common risk factors has been unclear, the investigators noted. Abnormal sleep patterns are symptoms of both depression and Alzheimer’s disease. Abnormal sleep is also associated with cognitive decline and anxiety.
The researchers hypothesized that sleep causally affects major depressive disorder and Alzheimer’s disease but that there is no causal relationship between major depressive disorder and Alzheimer’s disease. They conducted a bidirectional, two-sample Mendelian randomization study to test these hypotheses.
The investigators conducted genomewide association studies (GWASs) using data from the prospective, population-based U.K. Biobank. Sleep phenotypes were measured by self-report or accelerometer. Genetic associations were derived from 403,195 patients for chronotype, 237,627 patients for insomnia, 446,118 people for sleep duration, and 85,670 people for accelerometer-derived phenotypes.
Two binary variables from sleep duration were derived: short sleep (duration of less than 7 hours) and long sleep (duration of 9 or more hours). A sleep episode was defined as a period of at least 5 minutes with a change on the dorsal-ventral axis of less than 5 degrees. The durations of all sleep episodes were added to calculate total sleep duration.
Major depressive disorder was diagnosed clinically in accordance with DSM-IV criteria. Genetic associations were derived from 9,240 case patients and 9,519 control participants. Alzheimer’s disease was diagnosed on the basis of physician examination or autopsy results. Genetic associations were obtained from a meta-analysis of GWAS on participants of European ancestry in the International Genomics of Alzheimer’s Project, which included 21,982 case patients and 41,944 control participants.
More risk factor research needed
Results showed no causal relationships between sleep-related phenotypes and major depressive disorder in either direction. Causal relationships between major depressive disorder and Alzheimer’s disease were found in both directions, but neither was statistically significant.
A genetically higher risk for Alzheimer’s disease was associated with being a “morning person,” being at decreased risk for insomnia, having shorter sleep duration on self-report and accelerometer, having decreased likelihood of reporting long sleep, having an earlier timing of the least active 5 hours, and having a smaller number of sleep episodes. However, no analysis supported a causal effect of sleep-related phenotypes on risk for Alzheimer’s disease.
Because APOE4 can influence disease processes that may contribute to Alzheimer’s disease risk, the investigators also conducted a sensitivity analysis that excluded APOE single-nucleotide polymorphisms. In this analysis, the causal associations of Alzheimer’s disease with self-reported and accelerometer-based sleep duration were not significant. The sensitivity analysis did support the other causal associations between Alzheimer’s disease and sleep phenotypes, however.
The causal associations between major depressive disorder and Alzheimer’s disease observed in other studies may have been the result of confounding, and the participants may have had other associated characteristics that put them at risk for the disease, said Dr. Dehghan. Furthermore, the previous studies considered various sleep phenotypes together, whereas in the current study, the investigators examined them separately.
The results suggest that preclinical and clinical Alzheimer’s disease may affect sleep phenotypes differently. Sleep management thus could be an important approach to improving quality of life for patients with Alzheimer’s disease, the researchers wrote.
“Our study indicates that depression and sleep disorders are not likely to be a causal factor for Alzheimer’s disease,” Dr. Dehghan said. “We need to search for other risk factors for the prevention of Alzheimer’s disease.”
Several strengths, lacks details
Walter A. Kukull, PhD, professor of epidemiology and director of the National Alzheimer’s Coordinating Center at the University of Washington, Seattle, noted that the investigators appear to have implemented their chosen methods of causal association analysis well. “They attempted to examine the direction of the causal arrow for risk factors … and that is a step usually not well examined in other studies.”
He added that the collection of objective measures, such as of sleep, is another strength of the study.
However, “the common weakness of the basic GWAS sample is that clinical symptomatology determined Alzheimer’s disease diagnosis. Thus, asymptomatic or very mildly symptomatic persons with Alzheimer’s disease pathology in their brains were likely included among normal controls,” said Dr. Kukull, who was not involved with the research.
Because of an apparent lack of biomarker data, patients who had been diagnosed with Alzheimer’s disease may in fact have had a different form of dementia. Given the nature of their data, the investigators could have done little to compensate for these possibilities, Dr. Kukull added. In addition, the article lacks details that would improve the interpretation of the results.
“Timing is everything with regard to potential associations between risk factor and outcome,” Dr. Kukull said. “With the exceptions of genes, it would be nice to know more about the timing of risk factors’ onset and Alzheimer’s disease onset.”
Still, the results indicate potential areas of future study, he noted. “Primarily, further research must address the question of pathological onset of disease and misclassification of diagnosis in both cases and controls due to lack of biomarker-confirmed diagnosis. Then research can also struggle with the timing of potential risk factors with respect to disease.”
The study was funded by the U.K. Dementia Research Institute. Dr. Dehghan and Dr. Kukull reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Clinical pearls for administering cognitive exams during the pandemic
Patients have often been labeled as “poor historians” if they are not able to recollect their own medical history, whether through illness or difficulties in communication. But Fred Ovsiew, MD, speaking at Focus on Neuropsychiatry presented by Current Psychiatry and the American Academy of Clinical Psychiatrists, sees that label as an excuse on the part of the clinician.
“I strongly advise you to drop that phrase from your vocabulary if you do use it, because the patient is not the historian. The doctor, the clinician is the historian,” Dr. Ovsiew said at the meeting, presented by Global Academy for Medical Education. “It is the clinician’s job to put the story together using the account by the patient as one source, but [also] interviewing a collateral informant and/or reviewing records, which is necessary in almost every case of a neuropsychiatric illness.”
Rather, clinicians taking history at the bedside should focus on why the patients cannot give a narrative account of their illness. Patients can have narrative incapacity on a psychogenic basis, such as in patients with conversion or somatoform disorder, he explained. “I think this is a result of the narrative incapacity that develops in people who have had trauma or adverse experiences in childhood and insecure attachment. This is shown on the adult attachment interview as a disorganized account of their childhoods.”
Other patients might not be able to recount their medical history because they are amnestic, which leaves their account vague because of a lack of access to information. “It may be frozen in time in the sense that, up to a certain point in their life, they can recount the history,” Dr. Ovsiew said. “But in recent years, their account becomes vague.”
Patients with right hemisphere lesions might not know that their account has incongruity and is implausible, while patients with dorsolateral prefrontal lesions might be aspontaneous, use few words to describe their situation, and have poor insight. Those with ventromedial prefrontal lesions can be impulsive and have poor insight, not considering alternative possibilities, Dr. Ovsiew noted.
Asking open-ended questions of the patient is the first step to identifying any potential narrative incapacity, followed by a detailed medical history by the clinician. When taking a medical history, try avoiding what Dr. Ovsiew calls the “anything like that?” problem, where a clinician asks a question about a cluster of symptoms that would make sense to a doctor, but not a patient. For example, a doctor might ask whether a patient is experiencing “chest pain or leg swelling – anything like that?” because he or she knows what those symptoms have in common, but the patient might not know the relationship between those symptoms. “You can’t count on the patient to tell you all the relevant information,” he said. “You have to know what to ask about.”
“Patients with brain disease have subtle personality changes, sometimes more obvious personality changes. These need to be inquired about,” Dr. Ovsiew said. “The patient with apathy has reduced negative as well as positive emotions. The patient with depression has reduced positive emotions, but often tells you very clearly about the negative emotions of sadness, guilt. The patient with depression has diurnal variation in mood, a very telling symptom, especially when it’s disclosed spontaneously,” Dr. Ovsiew explained. “The point is, you need to know to ask about it.”
When taking a sleep history, clinicians should be aware of sleep disturbances apart from insomnia and early waking. REM sleep behavior disorder is a condition that should be inquired about. Obstructive sleep apnea is a condition that might not be immediately apparent to the patient, but a bed partner can identify whether a patient has problems breathing throughout the night.
“This is an important condition to uncover for the neuropsychiatrist because it contributes to treatment resistance and depression, and it contributes to cognitive impairment,” Dr. Ovsiew said. “These patients commonly have mild difficulties with attention and concentration.”
Always ask about head injury in every history, which can be relevant to later onset depression, PTSD, and cognitive impairment. Every head injury follows a trajectory of retrograde amnesia and altered state of consciousness (including coma), followed by a period of posttraumatic amnesia. Duration of these states can be used to assess the severity of brain injury, but the 15-point Glasgow Coma Scale is another way to assess injury severity, Dr. Ovsiew explained.
However, the two do not always overlap, he noted. “Someone may have a Glasgow Coma Scale score that is 9-12, predicting moderate brain injury, but they may have a short duration of amnesia. These don’t always follow the same path. There are many different ways of classifying how severe the brain injury is.”
Keep probes brief, straightforward
Cognitive exams of patients with suspected psychiatric disorders should be simple, easy to administer and focused on a single domain of cognition. “Probes should be brief. They should not require specialized equipment. The Purdue Pegboard Test might be a great neuropsychological instrument, but very few of us carry a pegboard around in our medical bags,” Dr. Ovsiew said.
The probe administered should also be accessible to the patient. The serial sevens clinical test, where a patient is asked to repeatedly subtract 7 from 100, is only effective at testing concentration if the patient is capable of completing the test. “There are going to be patients who can’t do the task, but it’s not because of concentration failure, it’s because of subtraction failure,” he said.
When assessing attention, effective tasks include having the patient perform the digit span test forward and backward, count backward from 20 to 1, listing the months of the year in reverse, and performing the Mental Alternation Test. However, Dr. Ovsiew explained there may be some barriers for patients in completing these tasks. “The person may be aphasic and not know the alphabet. The person may have English as a second language and not be skilled at giving the alphabet in English. In some cases, you may want to check and not assume that the patient can count and does know the alphabet.”
In assessing language, listen for aphasic abnormalities. “The patient, of course, is speaking throughout the interview, but you need to take a moment to listen for prosody, to listen to rate of speech, to listen for paraphasic errors or word-finding problems,” Dr. Ovsiew said. Any abnormalities should be probed further through confrontation naming tasks, which can be done in person and with some success through video, but not by phone. Naming to definition (“What do you call the part of a shirt that covers the arm?”) is one way of administering the test over the phone.
Visuospatial function can be assessed by clock drawing but also carries problems. Patients who do not plan their clock before beginning to draw, for example, may have an executive function problem instead of a visuospatial problem, Dr. Ovsiew noted. Patients in whom a clinician suspects hemineglect should be given a visual search task or line by section task. “I like doing clock drawing. It’s a nice screening test. It’s becoming, I think, less useful as people count on digital clocks and have trouble even imagining what an analog clock looks like.”
An approach that is better suited to in-person assessment, but also works by video, is the Poppelreuter figure visual perceptual function test, which is a prompt for the patient that involves common household items overlaying one another “in atypical positions and atypical configurations” where the patient is instructed to describe the items they see on the card. Another approach that works over video is the interlocking finger test, where the patient is asked to copy the hand positions made by the clinician.
Dr. Ovsiew admitted that visuospatial function is nearly impossible to assess over the phone. Asking topographical questions (“If you’re driving from Chicago to Los Angeles, is the Pacific Ocean in front of you, behind you, to your left, or to your right?”) may help judge visuospatial function, but this relies on the patient having the topographic knowledge to answer the questions. Some patients who are topographically disoriented can’t do them at all,” Dr. Ovsiew said.
Bedside neuropsychiatry assesses encoding of a memory, its retention and its retrieval as well as verbal and visual cues. Each one of these aspects of memory can be impaired on its own and should be explored separately, Dr. Ovsiew explained. “Neuropsychiatric clinicians have a rough-and-ready, seat-of-the-pants way of approaching this that wouldn’t pass muster if you’re a psychologist, but is the best we can do at the bedside.”
To test retrieval and retention, the Three Words–Three Shapes test works well in person, with some difficulty by video, and is not possible to administer over the phone. In lieu of that test, giving the patient a simple word list and asking them to repeat the list in order. Using the word list, “these different stages of memory function can be parsed out pretty well at the bedside or chairside, and even by the phone. Figuring out where the memory failure is diagnostically important,” Dr. Ovsiew said.
Executive function, which involves activation, planning, sequencing, maintaining, self-monitoring, and flexible employment of action and attention, is “complicated to evaluate because there are multiple aspects of executive function, multiple deficits that can be seen with executive dysfunction, and they don’t all correlate with each other.”
Within executive function evaluation, the Mental Alternation Test can assess working memory, motor sequencing can be assessed through the ring/fist, fist/edge/palm, alternating fist, and rampart tests. The Go/No-Go test can be used to assess response inhibition. For effortful retrieval evaluation, spontaneous word-list generation – such as thinking of all the items one can buy at a supermarket– can test category fluency, while a task to name all the words starting with a certain letter can assess letter stimulus.
Executive function “is of crucial importance in the neuropsychiatric evaluation because it’s strongly correlated with how well the person functions outside the office,” Dr. Ovsiew said.
Global Academy and this news organization are owned by the same parent company. Dr. Ovsiew reported relationships with Wolters Kluwer Health in the form of consulting, receiving royalty payments, and related activities.
Patients have often been labeled as “poor historians” if they are not able to recollect their own medical history, whether through illness or difficulties in communication. But Fred Ovsiew, MD, speaking at Focus on Neuropsychiatry presented by Current Psychiatry and the American Academy of Clinical Psychiatrists, sees that label as an excuse on the part of the clinician.
“I strongly advise you to drop that phrase from your vocabulary if you do use it, because the patient is not the historian. The doctor, the clinician is the historian,” Dr. Ovsiew said at the meeting, presented by Global Academy for Medical Education. “It is the clinician’s job to put the story together using the account by the patient as one source, but [also] interviewing a collateral informant and/or reviewing records, which is necessary in almost every case of a neuropsychiatric illness.”
Rather, clinicians taking history at the bedside should focus on why the patients cannot give a narrative account of their illness. Patients can have narrative incapacity on a psychogenic basis, such as in patients with conversion or somatoform disorder, he explained. “I think this is a result of the narrative incapacity that develops in people who have had trauma or adverse experiences in childhood and insecure attachment. This is shown on the adult attachment interview as a disorganized account of their childhoods.”
Other patients might not be able to recount their medical history because they are amnestic, which leaves their account vague because of a lack of access to information. “It may be frozen in time in the sense that, up to a certain point in their life, they can recount the history,” Dr. Ovsiew said. “But in recent years, their account becomes vague.”
Patients with right hemisphere lesions might not know that their account has incongruity and is implausible, while patients with dorsolateral prefrontal lesions might be aspontaneous, use few words to describe their situation, and have poor insight. Those with ventromedial prefrontal lesions can be impulsive and have poor insight, not considering alternative possibilities, Dr. Ovsiew noted.
Asking open-ended questions of the patient is the first step to identifying any potential narrative incapacity, followed by a detailed medical history by the clinician. When taking a medical history, try avoiding what Dr. Ovsiew calls the “anything like that?” problem, where a clinician asks a question about a cluster of symptoms that would make sense to a doctor, but not a patient. For example, a doctor might ask whether a patient is experiencing “chest pain or leg swelling – anything like that?” because he or she knows what those symptoms have in common, but the patient might not know the relationship between those symptoms. “You can’t count on the patient to tell you all the relevant information,” he said. “You have to know what to ask about.”
“Patients with brain disease have subtle personality changes, sometimes more obvious personality changes. These need to be inquired about,” Dr. Ovsiew said. “The patient with apathy has reduced negative as well as positive emotions. The patient with depression has reduced positive emotions, but often tells you very clearly about the negative emotions of sadness, guilt. The patient with depression has diurnal variation in mood, a very telling symptom, especially when it’s disclosed spontaneously,” Dr. Ovsiew explained. “The point is, you need to know to ask about it.”
When taking a sleep history, clinicians should be aware of sleep disturbances apart from insomnia and early waking. REM sleep behavior disorder is a condition that should be inquired about. Obstructive sleep apnea is a condition that might not be immediately apparent to the patient, but a bed partner can identify whether a patient has problems breathing throughout the night.
“This is an important condition to uncover for the neuropsychiatrist because it contributes to treatment resistance and depression, and it contributes to cognitive impairment,” Dr. Ovsiew said. “These patients commonly have mild difficulties with attention and concentration.”
Always ask about head injury in every history, which can be relevant to later onset depression, PTSD, and cognitive impairment. Every head injury follows a trajectory of retrograde amnesia and altered state of consciousness (including coma), followed by a period of posttraumatic amnesia. Duration of these states can be used to assess the severity of brain injury, but the 15-point Glasgow Coma Scale is another way to assess injury severity, Dr. Ovsiew explained.
However, the two do not always overlap, he noted. “Someone may have a Glasgow Coma Scale score that is 9-12, predicting moderate brain injury, but they may have a short duration of amnesia. These don’t always follow the same path. There are many different ways of classifying how severe the brain injury is.”
Keep probes brief, straightforward
Cognitive exams of patients with suspected psychiatric disorders should be simple, easy to administer and focused on a single domain of cognition. “Probes should be brief. They should not require specialized equipment. The Purdue Pegboard Test might be a great neuropsychological instrument, but very few of us carry a pegboard around in our medical bags,” Dr. Ovsiew said.
The probe administered should also be accessible to the patient. The serial sevens clinical test, where a patient is asked to repeatedly subtract 7 from 100, is only effective at testing concentration if the patient is capable of completing the test. “There are going to be patients who can’t do the task, but it’s not because of concentration failure, it’s because of subtraction failure,” he said.
When assessing attention, effective tasks include having the patient perform the digit span test forward and backward, count backward from 20 to 1, listing the months of the year in reverse, and performing the Mental Alternation Test. However, Dr. Ovsiew explained there may be some barriers for patients in completing these tasks. “The person may be aphasic and not know the alphabet. The person may have English as a second language and not be skilled at giving the alphabet in English. In some cases, you may want to check and not assume that the patient can count and does know the alphabet.”
In assessing language, listen for aphasic abnormalities. “The patient, of course, is speaking throughout the interview, but you need to take a moment to listen for prosody, to listen to rate of speech, to listen for paraphasic errors or word-finding problems,” Dr. Ovsiew said. Any abnormalities should be probed further through confrontation naming tasks, which can be done in person and with some success through video, but not by phone. Naming to definition (“What do you call the part of a shirt that covers the arm?”) is one way of administering the test over the phone.
Visuospatial function can be assessed by clock drawing but also carries problems. Patients who do not plan their clock before beginning to draw, for example, may have an executive function problem instead of a visuospatial problem, Dr. Ovsiew noted. Patients in whom a clinician suspects hemineglect should be given a visual search task or line by section task. “I like doing clock drawing. It’s a nice screening test. It’s becoming, I think, less useful as people count on digital clocks and have trouble even imagining what an analog clock looks like.”
An approach that is better suited to in-person assessment, but also works by video, is the Poppelreuter figure visual perceptual function test, which is a prompt for the patient that involves common household items overlaying one another “in atypical positions and atypical configurations” where the patient is instructed to describe the items they see on the card. Another approach that works over video is the interlocking finger test, where the patient is asked to copy the hand positions made by the clinician.
Dr. Ovsiew admitted that visuospatial function is nearly impossible to assess over the phone. Asking topographical questions (“If you’re driving from Chicago to Los Angeles, is the Pacific Ocean in front of you, behind you, to your left, or to your right?”) may help judge visuospatial function, but this relies on the patient having the topographic knowledge to answer the questions. Some patients who are topographically disoriented can’t do them at all,” Dr. Ovsiew said.
Bedside neuropsychiatry assesses encoding of a memory, its retention and its retrieval as well as verbal and visual cues. Each one of these aspects of memory can be impaired on its own and should be explored separately, Dr. Ovsiew explained. “Neuropsychiatric clinicians have a rough-and-ready, seat-of-the-pants way of approaching this that wouldn’t pass muster if you’re a psychologist, but is the best we can do at the bedside.”
To test retrieval and retention, the Three Words–Three Shapes test works well in person, with some difficulty by video, and is not possible to administer over the phone. In lieu of that test, giving the patient a simple word list and asking them to repeat the list in order. Using the word list, “these different stages of memory function can be parsed out pretty well at the bedside or chairside, and even by the phone. Figuring out where the memory failure is diagnostically important,” Dr. Ovsiew said.
Executive function, which involves activation, planning, sequencing, maintaining, self-monitoring, and flexible employment of action and attention, is “complicated to evaluate because there are multiple aspects of executive function, multiple deficits that can be seen with executive dysfunction, and they don’t all correlate with each other.”
Within executive function evaluation, the Mental Alternation Test can assess working memory, motor sequencing can be assessed through the ring/fist, fist/edge/palm, alternating fist, and rampart tests. The Go/No-Go test can be used to assess response inhibition. For effortful retrieval evaluation, spontaneous word-list generation – such as thinking of all the items one can buy at a supermarket– can test category fluency, while a task to name all the words starting with a certain letter can assess letter stimulus.
Executive function “is of crucial importance in the neuropsychiatric evaluation because it’s strongly correlated with how well the person functions outside the office,” Dr. Ovsiew said.
Global Academy and this news organization are owned by the same parent company. Dr. Ovsiew reported relationships with Wolters Kluwer Health in the form of consulting, receiving royalty payments, and related activities.
Patients have often been labeled as “poor historians” if they are not able to recollect their own medical history, whether through illness or difficulties in communication. But Fred Ovsiew, MD, speaking at Focus on Neuropsychiatry presented by Current Psychiatry and the American Academy of Clinical Psychiatrists, sees that label as an excuse on the part of the clinician.
“I strongly advise you to drop that phrase from your vocabulary if you do use it, because the patient is not the historian. The doctor, the clinician is the historian,” Dr. Ovsiew said at the meeting, presented by Global Academy for Medical Education. “It is the clinician’s job to put the story together using the account by the patient as one source, but [also] interviewing a collateral informant and/or reviewing records, which is necessary in almost every case of a neuropsychiatric illness.”
Rather, clinicians taking history at the bedside should focus on why the patients cannot give a narrative account of their illness. Patients can have narrative incapacity on a psychogenic basis, such as in patients with conversion or somatoform disorder, he explained. “I think this is a result of the narrative incapacity that develops in people who have had trauma or adverse experiences in childhood and insecure attachment. This is shown on the adult attachment interview as a disorganized account of their childhoods.”
Other patients might not be able to recount their medical history because they are amnestic, which leaves their account vague because of a lack of access to information. “It may be frozen in time in the sense that, up to a certain point in their life, they can recount the history,” Dr. Ovsiew said. “But in recent years, their account becomes vague.”
Patients with right hemisphere lesions might not know that their account has incongruity and is implausible, while patients with dorsolateral prefrontal lesions might be aspontaneous, use few words to describe their situation, and have poor insight. Those with ventromedial prefrontal lesions can be impulsive and have poor insight, not considering alternative possibilities, Dr. Ovsiew noted.
Asking open-ended questions of the patient is the first step to identifying any potential narrative incapacity, followed by a detailed medical history by the clinician. When taking a medical history, try avoiding what Dr. Ovsiew calls the “anything like that?” problem, where a clinician asks a question about a cluster of symptoms that would make sense to a doctor, but not a patient. For example, a doctor might ask whether a patient is experiencing “chest pain or leg swelling – anything like that?” because he or she knows what those symptoms have in common, but the patient might not know the relationship between those symptoms. “You can’t count on the patient to tell you all the relevant information,” he said. “You have to know what to ask about.”
“Patients with brain disease have subtle personality changes, sometimes more obvious personality changes. These need to be inquired about,” Dr. Ovsiew said. “The patient with apathy has reduced negative as well as positive emotions. The patient with depression has reduced positive emotions, but often tells you very clearly about the negative emotions of sadness, guilt. The patient with depression has diurnal variation in mood, a very telling symptom, especially when it’s disclosed spontaneously,” Dr. Ovsiew explained. “The point is, you need to know to ask about it.”
When taking a sleep history, clinicians should be aware of sleep disturbances apart from insomnia and early waking. REM sleep behavior disorder is a condition that should be inquired about. Obstructive sleep apnea is a condition that might not be immediately apparent to the patient, but a bed partner can identify whether a patient has problems breathing throughout the night.
“This is an important condition to uncover for the neuropsychiatrist because it contributes to treatment resistance and depression, and it contributes to cognitive impairment,” Dr. Ovsiew said. “These patients commonly have mild difficulties with attention and concentration.”
Always ask about head injury in every history, which can be relevant to later onset depression, PTSD, and cognitive impairment. Every head injury follows a trajectory of retrograde amnesia and altered state of consciousness (including coma), followed by a period of posttraumatic amnesia. Duration of these states can be used to assess the severity of brain injury, but the 15-point Glasgow Coma Scale is another way to assess injury severity, Dr. Ovsiew explained.
However, the two do not always overlap, he noted. “Someone may have a Glasgow Coma Scale score that is 9-12, predicting moderate brain injury, but they may have a short duration of amnesia. These don’t always follow the same path. There are many different ways of classifying how severe the brain injury is.”
Keep probes brief, straightforward
Cognitive exams of patients with suspected psychiatric disorders should be simple, easy to administer and focused on a single domain of cognition. “Probes should be brief. They should not require specialized equipment. The Purdue Pegboard Test might be a great neuropsychological instrument, but very few of us carry a pegboard around in our medical bags,” Dr. Ovsiew said.
The probe administered should also be accessible to the patient. The serial sevens clinical test, where a patient is asked to repeatedly subtract 7 from 100, is only effective at testing concentration if the patient is capable of completing the test. “There are going to be patients who can’t do the task, but it’s not because of concentration failure, it’s because of subtraction failure,” he said.
When assessing attention, effective tasks include having the patient perform the digit span test forward and backward, count backward from 20 to 1, listing the months of the year in reverse, and performing the Mental Alternation Test. However, Dr. Ovsiew explained there may be some barriers for patients in completing these tasks. “The person may be aphasic and not know the alphabet. The person may have English as a second language and not be skilled at giving the alphabet in English. In some cases, you may want to check and not assume that the patient can count and does know the alphabet.”
In assessing language, listen for aphasic abnormalities. “The patient, of course, is speaking throughout the interview, but you need to take a moment to listen for prosody, to listen to rate of speech, to listen for paraphasic errors or word-finding problems,” Dr. Ovsiew said. Any abnormalities should be probed further through confrontation naming tasks, which can be done in person and with some success through video, but not by phone. Naming to definition (“What do you call the part of a shirt that covers the arm?”) is one way of administering the test over the phone.
Visuospatial function can be assessed by clock drawing but also carries problems. Patients who do not plan their clock before beginning to draw, for example, may have an executive function problem instead of a visuospatial problem, Dr. Ovsiew noted. Patients in whom a clinician suspects hemineglect should be given a visual search task or line by section task. “I like doing clock drawing. It’s a nice screening test. It’s becoming, I think, less useful as people count on digital clocks and have trouble even imagining what an analog clock looks like.”
An approach that is better suited to in-person assessment, but also works by video, is the Poppelreuter figure visual perceptual function test, which is a prompt for the patient that involves common household items overlaying one another “in atypical positions and atypical configurations” where the patient is instructed to describe the items they see on the card. Another approach that works over video is the interlocking finger test, where the patient is asked to copy the hand positions made by the clinician.
Dr. Ovsiew admitted that visuospatial function is nearly impossible to assess over the phone. Asking topographical questions (“If you’re driving from Chicago to Los Angeles, is the Pacific Ocean in front of you, behind you, to your left, or to your right?”) may help judge visuospatial function, but this relies on the patient having the topographic knowledge to answer the questions. Some patients who are topographically disoriented can’t do them at all,” Dr. Ovsiew said.
Bedside neuropsychiatry assesses encoding of a memory, its retention and its retrieval as well as verbal and visual cues. Each one of these aspects of memory can be impaired on its own and should be explored separately, Dr. Ovsiew explained. “Neuropsychiatric clinicians have a rough-and-ready, seat-of-the-pants way of approaching this that wouldn’t pass muster if you’re a psychologist, but is the best we can do at the bedside.”
To test retrieval and retention, the Three Words–Three Shapes test works well in person, with some difficulty by video, and is not possible to administer over the phone. In lieu of that test, giving the patient a simple word list and asking them to repeat the list in order. Using the word list, “these different stages of memory function can be parsed out pretty well at the bedside or chairside, and even by the phone. Figuring out where the memory failure is diagnostically important,” Dr. Ovsiew said.
Executive function, which involves activation, planning, sequencing, maintaining, self-monitoring, and flexible employment of action and attention, is “complicated to evaluate because there are multiple aspects of executive function, multiple deficits that can be seen with executive dysfunction, and they don’t all correlate with each other.”
Within executive function evaluation, the Mental Alternation Test can assess working memory, motor sequencing can be assessed through the ring/fist, fist/edge/palm, alternating fist, and rampart tests. The Go/No-Go test can be used to assess response inhibition. For effortful retrieval evaluation, spontaneous word-list generation – such as thinking of all the items one can buy at a supermarket– can test category fluency, while a task to name all the words starting with a certain letter can assess letter stimulus.
Executive function “is of crucial importance in the neuropsychiatric evaluation because it’s strongly correlated with how well the person functions outside the office,” Dr. Ovsiew said.
Global Academy and this news organization are owned by the same parent company. Dr. Ovsiew reported relationships with Wolters Kluwer Health in the form of consulting, receiving royalty payments, and related activities.
FROM FOCUS ON NEUROPSYCHIATRY 2020
Effects of Computer-Based Documentation Procedures on Health Care Workload Assessment and Resource Allocation: An Example From VA Sleep Medicine Programs
Health care systems are faced with the challenge of meeting increasing patient care demands with finite resources.1 Advocating for additional capital—specifically, human resources—requires compelling data that accurately capture workload credit. When workload is not captured accurately, clinicians may be tasked with providing care to a high volume of patients without appropriate resource allocation. This understaffing can delay care delivery and increase the risk of diagnostic and treatment errors.2 Furthermore, workers in understaffed medical facilities are more likely to experience burnout, which leads to high workforce turnover.
Computer based documentation (CBD) is used often in medical practices to track patient care and clinical workload. However, improperly designed and implemented CBD systems can contribute to cumbersome documentation tasks and inaccurate or incomplete data capture.3 Conversely, CBD can be a useful tool to capture workload credit and can subsequently facilitate justification for medical staff allocation to meet patient care demands. This article uses our experience with US Department of Veterans Affairs (VA) national sleep medicine programs to illustrate the impact of CBD procedures on health care workload assessment and allocation. Specifically, we examine how appropriate workload capture facilitates growth and improves the efficiency of health care programs.
The VA is the largest integrated health care system in the US, serving 9 million veterans at 1,255 facilities, including 170 VA Medical Centers (VAMCs).4 As veterans’ demands for VA medical services have outpaced available resources, there have been several media reports of lapses in timely care delivery.5-7 These lapses have been due, in part, to insufficient workforce resource allocation within the Veterans Health Administration (VHA) facilities. A 2012 audit of physician staffing levels conducted by the VA Inspector General concluded that the VA did not have an effective staffing methodology to ensure appropriate staffing levels for specialty care services.8 The lack of staffing plans and productivity standards limits the ability of medical facility officials to make informed business decisions regarding the appropriate number of specialty physicians required to meet patient care needs.8 In 2017, the Government Accountability Office (GAO) issued a report to Congress that stated the “VA’s productivity metrics and efficiency models do not provide complete and accurate information, they may misrepresent the true level of productivity and efficiency across VAMCs and limit the VA’s ability to determine the extent to which its resources are being used effectively.”9 To understand how and why many VA medical facilities remain understaffed, and therefore struggle to provide health care to veterans in a timely fashion, a description of VA CBD procedures is provided.
Background
VA Directive 1082 on Patient Care Data requires the capture of all outpatient and inpatient billable encounter data.10 Accurate capture of workload informs budget allocation models and is necessary for health care provider (HCP) productivity metrics. These data points help identify staff shortages relative to the generated workload. The Veterans Equitable Resource Allocation (VERA) model is used to allocate general purpose funds to the Veterans Integrated Service Networks (VISNs) regional network of VHA facilities. The underlying data components of the VERA model rely on comprehensive data systems that track and analyze the many management information systems used in VHA. Historically, at least 90% of the funds allocated by the VERA model have been attributed directly to patient care. All workload that is appropriately documented is accounted for in the VERA patient classification process, which is the official data source for funding patient care in VHA.
VA medical facilities use Stop Codes (formerly known as Decision Support System Identifiers) to identify workload for all outpatient encounters and inpatient professional services. Each code is composed of a 6-character descriptor that includes a primary Stop Code and a credit (secondary) Stop Code. Primary Stop Codes—the first 3 numbers in the sequence—designate the main clinical group responsible for patient care, such as sleep medicine or neurology. Secondary Stop Codes—the last 3 numbers in the sequence—further define the primary workgroup, such as the type of services provided (eg, telehealth) or the type of HCP (eg, nurse practitioner). These codes help ensure that workload and generated revenue are allocated or credited to the proper specialty care service.11 An example of how changes or inaccuracies in Stop Code reporting can affect VHA clinical workload assessment and resource allocation is provided by the VHA sleep medicine program.
The prevalence of sleep disorders—particularly apnea and insomnia—among US military service members and veterans has increased dramatically over the past 2 decades and continues to rise.12-14 Consequently, demand for sleep care services at VHA facilities also has increased substantially (Figure 1). Unfortunately, this demand has outpaced the VHA’s staffing models, sometimes resulting in long wait times for appointments.15 In fact, sleep medicine remains one of the most backlogged services in the VHA, despite significant improvements in program efficiency achieved by incorporating telehealth modalities.16 Untreated sleep disorders are associated with increased risk of depression, anxiety, impaired neurocognitive functions, cardiovascular disease, motor vehicle accidents, and premature death.17-23
A major contributor to understaffing of VHA sleep medicine programs is the CBD system’s historical inability to accurately track sleep resources and demand for sleep care services. For many years, Stop Codes attributed sleep workload credit primarily to pulmonary medicine, neurology, and internal medicine workgroups. Within these workgroups, few individuals contributed to sleep care, but the entire workgroup received credit for these services, masking the workload of sleep care providers. Additional barriers to accurate sleep medicine workload capture within the VHA included (1) inability to centrally identify personnel, including physicians, as providers of sleep care; (2) limited and variable understanding among VA sleep physicians of the importance of proper encounter form completion (the mechanism by which the cost of a service is calculated); and (3) a lack of awareness that encounter closure is directly linked to productivity measures such as relative value units (RVUs) that support sleep medicine programs and the salaries of those who provide care.
Methods
The critical role of accurate CBD in health care administration is illustrated by the proper use of Stop Codes as a foundational step in tracking services provided to justify adequate resource allocation within VA. A complete redesign of tracking sleep service documentation was initiated in 2014 and resulted in national changes to sleep medicine Stop Codes. The Stop Code initiative was the first step of several to improve CBD for VA sleep services.
Primary Stop Code 349 designates sleep medicine encounters in VA facilities (Table). However, before changes were implemented in fiscal year (FY) 2015, Stop Codes for VHA sleep care did not differentiate between specific services provided, such as laboratory-based sleep testing, at-home sleep testing, education/training sessions, follow-up appointments, equipment consults, telephone or video consults, or administrative tasks. In early FY 2015, several changes were made to Stop Codes used for VHA sleep medicine services nationwide to capture the breadth of services that were being provided; services that had previously been performed but were not documented. A new standardized coding methodology was established for continuous positive airway pressure (CPAP) clinics (349/116 or 349/117); telephone consults for sleep care (324/349); and store and forward sleep telehealth encounters (349/694, 349/695, or 349/696).
In the VA, store-and-forward telehealth refers to asynchronous telemedicine involving the acquisition and storing of clinical information (eg, data, image, sound, or video) that another site or clinician reviews later for evaluation and interpretation. In sleep medicine, data uploaded from home sleep apnea test units or CPAP devices are examples of this asynchronous telehealth model. The goal of these changes in VA Stop Codes was to accurately assess the volume of sleep care delivered and the demand for sleep care (consult volumes); enable planning for resource allocation and utilization appropriately; provide veterans with consistent access to sleep services across the country; and facilitate reductions in wait times for sleep care appointments. Results of these changes were immediate and dramatic in terms of data capture and reporting.
Results
Figure 1 illustrates an increase in patient encounters in VA sleep clinics by 24,197 (19.6%) in the first quarter of Stop Code change implementation (FY 2015, quarter 2) compared with those of the previous quarter. VHA sleep clinic patient encounters increased in subsequent quarters of FY 2015 by 29,910 (20.2%) and 11,206 (6.3%) respectively. By the end of FY 2015, reported sleep clinic encounters increased by 190,803 compared with the those at the end of FY 2014, an increase of 42.7%.
Figures 2, 3, and 4 show the additional effects of sleep Stop Code changes that were implemented in FY 2015 for CPAP clinics, telephone encounters, and store-and-forward telehealth encounters, respectively. The large increases in reported sleep patient encounters between FY 2014 and FY 2016 reflect changes in CBD and are not entirely due to actual changes in clinical workloads. These results indicate that workloads in many VHA sleep medicine clinics were grossly underreported or misallocated to other specialty services prior to the changes implemented in FY 2015. This discrepancy in care delivery vs workload capture is a contributing factor to the understaffing that continues to challenge VHA sleep programs. However, the improved accuracy of workload reporting that resulted from Stop Code modifications has resulted in only a small proportional increase in VHA clinical resources allocated to provide adequate services and care for veterans with sleep disorders.
In response to the substantial and increasing demand for sleep services by veterans, the VA Office of Rural Health (ORH) funded an enterprise-wide initiative (EWI) to develop and implement a national TeleSleep Program.16 The goal of this program is to improve the health and well-being of rural veterans by increasing their access to sleep care and services.
Discussion
Inaccuracies in CBD procedures can adversely affect health care workload assessment and allocation, contributing to ongoing challenges faced by sleep medicine clinics and other VHA programs that have limited staff yet strive to provide timely and high-quality care to veterans. “Not only does inaccurate coding contribute to miscalculations in staffing and resource allocation, it can also contribute to inaccuracies in overall measures of VA healthcare efficiency,” the GAO reported to Congress.9 The GAO went on to recommend that the VA should ensure the accuracy of underlying staffing and workload data. VHA sleep medicine programs have made efforts to educate HCPs and administrators on the importance of accurate CBD as a tool for accurate data capture that is necessary to facilitate improvements in health care availability and delivery.
In 2018, the VA Sleep Program Office released an updated set of Stop Code changes, including expansion of telehealth codes and improved designation of laboratory and home sleep testing services. These changes are anticipated to result in accurate documentation of VA sleep clinic workload and services, especially as the VA TeleSleep EWI to reach rural veterans expands.16 In light of the improved accuracy of reporting of delivered sleep services due to changes in Stop Codes over the past 4 years, VHA sleep medicine providers continue to advocate for allocation of resources commensurate with their clinical workload. An appropriate administrative response to the significant clinical workload performed by disproportionately few providers should include the authorization of increased resources and personnel for sleep medicine as well as providing the tools needed to further streamline workflow efficiency (eg, artificial intelligence, machine learning, and population health management).
Conclusions
Despite the barriers faced by many large integrated health care systems, VHA sleep medicine leadership continues to implement changes in CBD protocols that improve the accuracy of clinical workload tracking and reporting. Ultimately, these changes will support proposals for increased resources necessary to improve the quality and availability of sleep care for veterans. This example from VA illustrates the importance of accurate workload capture and its role in informing administrators of health care systems as they strive to meet the needs of patients. Although some VA sleep medicine programs continue to face challenges imposed by systemwide limitations, the ORH TeleSleep Program is a major initiative that improves veterans’ access to care by disseminating and implementing effective telehealth technologies and strategies.16
Acknowledgments
This work was supported by a VA Office of Rural Health Enterprise-Wide Initiative.
1. World Health Organization. Workload indicators of staffing need (WISN). https://www.who.int/hrh/resources/WISN_Eng_UsersManual.pdf?ua=1. Published December 2015. Accessed June 24, 2020.
2. American Association for Respiratory Care. Position statement: best practices in respiratory care productivity and staffing. https://www.aarc.org/wp-content/uploads/2017/03/statement-of-best-practices_productivity-and-staffing.pdf. Revised July 2015. Accessed June 24, 2020.
3. Wu DTY, Smart N, Ciemins EL, Lanham HJ, Lindberg C, Zheng K. Using EHR audit trail logs to analyze clinical workflow: a case study from community-based ambulatory clinics. AMIA Annu Symp Proc. 2018;2017:1820-1827. Published 2018 Apr 16.
4. US Department of Veterans Affairs, Veterans Health Administration. https://www.va.gov/health.
5. Cohen T. VA crisis: solutions exist, but haven’t happened, panel hears. https://www.cnn.com/2014/06/12/politics/va-reforms/index.html. Published June 12, 2014. Accessed June 24, 2020.
6. Richardson B. IG probes uncover more problems at VA hospitals. https://thehill.com/policy/defense/258652-ig-probes-uncover-more-problems-at-va-hospitals. Published October 30, 2015. Accessed June 24, 2020.
7. Slack D. Inaccurate VA wait times prelude thousands of vets from getting outside care, probe finds. USA Today. March 3, 2017. https://www.usatoday.com/story/news/politics/2017/03/03/veterans-affairs-inspector-general-widespread-inaccuracies-wait-times/98693856. Accessed June 24, 2020.
8. US Department of Veterans Affairs, Office of the Inspector General. Veterans Health Administration: audit of physician staffing levels for specialty care services. https://www.va.gov/oig/pubs/VAOIG-11-01827-36.pdf. Published December 27, 2012. Accessed June 24, 2020.
9. Government Accountability Office. VA health care: improvements needed in data and monitoring of clinical productivity and efficiency. https://www.gao.gov/assets/690/684869.pdf. Published May 2017. Accessed June 24, 2020.
10. US Department of Veterans Affairs, Veterans Health Administration. VHA Directive 1082. Patient care data capture. https://www.va.gov/vhapublications/ViewPublication.asp?pub_ID=3091. Published March 24, 2015. Accessed June 24, 2020.
11. US Department of Veterans Affairs, Veterans Health Administration. VHA Handbook 1006.02. VHA site classifications and definitions. https://www.va.gov/vhapublications/ViewPublication.asp?pub_ID=2970. Published December 30, 2013. Accessed June 24, 2020.
12. Alexander M, Ray MA, Hébert JR, et al. The National Veteran Sleep Disorder Study: Descriptive Epidemiology and Secular Trends, 2000-2010. Sleep. 2016;39(7):1399-1410. Published 2016 Jul 1. doi:10.5665/sleep.5972.
13. A Caldwell J, Knapik JJ, Lieberman HR. Trends and factors associated with insomnia and sleep apnea in all United States military service members from 2005 to 2014. J Sleep Res. 2017;26(5):665-670. doi:10.1111/jsr.12543
14. Klingaman EA, Brownlow JA, Boland EM, Mosti C, Gehrman PR. Prevalence, predictors and correlates of insomnia in US army soldiers. J Sleep Res. 2018;27(3):e12612. doi:10.1111/jsr.12612
15. Sharafkhaneh A, Richardson P, Hirshkowitz M. Sleep apnea in a high risk population: a study of Veterans Health Administration beneficiaries. Sleep Med. 2004;5(4):345-350. doi:10.1016/j.sleep.2004.01.019.
16. Sarmiento KF, Folmer RL, Stepnowsky CJ, et al. National Expansion of Sleep Telemedicine for Veterans: The TeleSleep Program. J Clin Sleep Med. 2019;15(9):1355-1364. doi:10.5664/jcsm.7934
17. Van Dongen HP, Maislin G, Mullington JM, Dinges DF. The cumulative cost of additional wakefulness: dose-response effects on neurobehavioral functions and sleep physiology from chronic sleep restriction and total sleep deprivation [published correction appears in Sleep. 2004 Jun 15;27(4):600]. Sleep. 2003;26(2):117-126. doi:10.1093/sleep/26.2.117
18. Johnson EO, Roth T, Breslau N. The association of insomnia with anxiety disorders and depression: exploration of the direction of risk. J Psychiatr Res. 2006;40(8):700-708. doi:10.1016/j.jpsychires.2006.07.008
19. Léger D, Bayon V, Ohayon MM, et al. Insomnia and accidents: cross-sectional study (EQUINOX) on sleep-related home, work and car accidents in 5293 subjects with insomnia from 10 countries. J Sleep Res. 2014;23(2):143-152. doi:10.1111/jsr.12104
20. Franklin KA, Lindberg E. Obstructive sleep apnea is a common disorder in the population-a review on the epidemiology of sleep apnea. J Thorac Dis. 2015;7(8):1311-1322. doi:10.3978/j.issn.2072-1439.2015.06.11
21. Javaheri S, Redline S. Insomnia and Risk of Cardiovascular Disease. Chest. 2017;152(2):435-444. doi:10.1016/j.chest.2017.01.026
22. Linz D, McEvoy RD, Cowie MR, et al. Associations of obstructivesSleepaApnea with atrial fibrillation and continuous positive airway pressure treatment: a review. JAMA Cardiol. 2018;3(6):532-540. doi:10.1001/jamacardio.2018.0095
23. Ogilvie RP, Lakshminarayan K, Iber C, Patel SR, Lutsey PL. Joint effects of OSA and self-reported sleepiness on incident CHD and stroke. Sleep Med. 2018;44:32-37. doi:10.1016/j.sleep.2018.01.004
Health care systems are faced with the challenge of meeting increasing patient care demands with finite resources.1 Advocating for additional capital—specifically, human resources—requires compelling data that accurately capture workload credit. When workload is not captured accurately, clinicians may be tasked with providing care to a high volume of patients without appropriate resource allocation. This understaffing can delay care delivery and increase the risk of diagnostic and treatment errors.2 Furthermore, workers in understaffed medical facilities are more likely to experience burnout, which leads to high workforce turnover.
Computer based documentation (CBD) is used often in medical practices to track patient care and clinical workload. However, improperly designed and implemented CBD systems can contribute to cumbersome documentation tasks and inaccurate or incomplete data capture.3 Conversely, CBD can be a useful tool to capture workload credit and can subsequently facilitate justification for medical staff allocation to meet patient care demands. This article uses our experience with US Department of Veterans Affairs (VA) national sleep medicine programs to illustrate the impact of CBD procedures on health care workload assessment and allocation. Specifically, we examine how appropriate workload capture facilitates growth and improves the efficiency of health care programs.
The VA is the largest integrated health care system in the US, serving 9 million veterans at 1,255 facilities, including 170 VA Medical Centers (VAMCs).4 As veterans’ demands for VA medical services have outpaced available resources, there have been several media reports of lapses in timely care delivery.5-7 These lapses have been due, in part, to insufficient workforce resource allocation within the Veterans Health Administration (VHA) facilities. A 2012 audit of physician staffing levels conducted by the VA Inspector General concluded that the VA did not have an effective staffing methodology to ensure appropriate staffing levels for specialty care services.8 The lack of staffing plans and productivity standards limits the ability of medical facility officials to make informed business decisions regarding the appropriate number of specialty physicians required to meet patient care needs.8 In 2017, the Government Accountability Office (GAO) issued a report to Congress that stated the “VA’s productivity metrics and efficiency models do not provide complete and accurate information, they may misrepresent the true level of productivity and efficiency across VAMCs and limit the VA’s ability to determine the extent to which its resources are being used effectively.”9 To understand how and why many VA medical facilities remain understaffed, and therefore struggle to provide health care to veterans in a timely fashion, a description of VA CBD procedures is provided.
Background
VA Directive 1082 on Patient Care Data requires the capture of all outpatient and inpatient billable encounter data.10 Accurate capture of workload informs budget allocation models and is necessary for health care provider (HCP) productivity metrics. These data points help identify staff shortages relative to the generated workload. The Veterans Equitable Resource Allocation (VERA) model is used to allocate general purpose funds to the Veterans Integrated Service Networks (VISNs) regional network of VHA facilities. The underlying data components of the VERA model rely on comprehensive data systems that track and analyze the many management information systems used in VHA. Historically, at least 90% of the funds allocated by the VERA model have been attributed directly to patient care. All workload that is appropriately documented is accounted for in the VERA patient classification process, which is the official data source for funding patient care in VHA.
VA medical facilities use Stop Codes (formerly known as Decision Support System Identifiers) to identify workload for all outpatient encounters and inpatient professional services. Each code is composed of a 6-character descriptor that includes a primary Stop Code and a credit (secondary) Stop Code. Primary Stop Codes—the first 3 numbers in the sequence—designate the main clinical group responsible for patient care, such as sleep medicine or neurology. Secondary Stop Codes—the last 3 numbers in the sequence—further define the primary workgroup, such as the type of services provided (eg, telehealth) or the type of HCP (eg, nurse practitioner). These codes help ensure that workload and generated revenue are allocated or credited to the proper specialty care service.11 An example of how changes or inaccuracies in Stop Code reporting can affect VHA clinical workload assessment and resource allocation is provided by the VHA sleep medicine program.
The prevalence of sleep disorders—particularly apnea and insomnia—among US military service members and veterans has increased dramatically over the past 2 decades and continues to rise.12-14 Consequently, demand for sleep care services at VHA facilities also has increased substantially (Figure 1). Unfortunately, this demand has outpaced the VHA’s staffing models, sometimes resulting in long wait times for appointments.15 In fact, sleep medicine remains one of the most backlogged services in the VHA, despite significant improvements in program efficiency achieved by incorporating telehealth modalities.16 Untreated sleep disorders are associated with increased risk of depression, anxiety, impaired neurocognitive functions, cardiovascular disease, motor vehicle accidents, and premature death.17-23
A major contributor to understaffing of VHA sleep medicine programs is the CBD system’s historical inability to accurately track sleep resources and demand for sleep care services. For many years, Stop Codes attributed sleep workload credit primarily to pulmonary medicine, neurology, and internal medicine workgroups. Within these workgroups, few individuals contributed to sleep care, but the entire workgroup received credit for these services, masking the workload of sleep care providers. Additional barriers to accurate sleep medicine workload capture within the VHA included (1) inability to centrally identify personnel, including physicians, as providers of sleep care; (2) limited and variable understanding among VA sleep physicians of the importance of proper encounter form completion (the mechanism by which the cost of a service is calculated); and (3) a lack of awareness that encounter closure is directly linked to productivity measures such as relative value units (RVUs) that support sleep medicine programs and the salaries of those who provide care.
Methods
The critical role of accurate CBD in health care administration is illustrated by the proper use of Stop Codes as a foundational step in tracking services provided to justify adequate resource allocation within VA. A complete redesign of tracking sleep service documentation was initiated in 2014 and resulted in national changes to sleep medicine Stop Codes. The Stop Code initiative was the first step of several to improve CBD for VA sleep services.
Primary Stop Code 349 designates sleep medicine encounters in VA facilities (Table). However, before changes were implemented in fiscal year (FY) 2015, Stop Codes for VHA sleep care did not differentiate between specific services provided, such as laboratory-based sleep testing, at-home sleep testing, education/training sessions, follow-up appointments, equipment consults, telephone or video consults, or administrative tasks. In early FY 2015, several changes were made to Stop Codes used for VHA sleep medicine services nationwide to capture the breadth of services that were being provided; services that had previously been performed but were not documented. A new standardized coding methodology was established for continuous positive airway pressure (CPAP) clinics (349/116 or 349/117); telephone consults for sleep care (324/349); and store and forward sleep telehealth encounters (349/694, 349/695, or 349/696).
In the VA, store-and-forward telehealth refers to asynchronous telemedicine involving the acquisition and storing of clinical information (eg, data, image, sound, or video) that another site or clinician reviews later for evaluation and interpretation. In sleep medicine, data uploaded from home sleep apnea test units or CPAP devices are examples of this asynchronous telehealth model. The goal of these changes in VA Stop Codes was to accurately assess the volume of sleep care delivered and the demand for sleep care (consult volumes); enable planning for resource allocation and utilization appropriately; provide veterans with consistent access to sleep services across the country; and facilitate reductions in wait times for sleep care appointments. Results of these changes were immediate and dramatic in terms of data capture and reporting.
Results
Figure 1 illustrates an increase in patient encounters in VA sleep clinics by 24,197 (19.6%) in the first quarter of Stop Code change implementation (FY 2015, quarter 2) compared with those of the previous quarter. VHA sleep clinic patient encounters increased in subsequent quarters of FY 2015 by 29,910 (20.2%) and 11,206 (6.3%) respectively. By the end of FY 2015, reported sleep clinic encounters increased by 190,803 compared with the those at the end of FY 2014, an increase of 42.7%.
Figures 2, 3, and 4 show the additional effects of sleep Stop Code changes that were implemented in FY 2015 for CPAP clinics, telephone encounters, and store-and-forward telehealth encounters, respectively. The large increases in reported sleep patient encounters between FY 2014 and FY 2016 reflect changes in CBD and are not entirely due to actual changes in clinical workloads. These results indicate that workloads in many VHA sleep medicine clinics were grossly underreported or misallocated to other specialty services prior to the changes implemented in FY 2015. This discrepancy in care delivery vs workload capture is a contributing factor to the understaffing that continues to challenge VHA sleep programs. However, the improved accuracy of workload reporting that resulted from Stop Code modifications has resulted in only a small proportional increase in VHA clinical resources allocated to provide adequate services and care for veterans with sleep disorders.
In response to the substantial and increasing demand for sleep services by veterans, the VA Office of Rural Health (ORH) funded an enterprise-wide initiative (EWI) to develop and implement a national TeleSleep Program.16 The goal of this program is to improve the health and well-being of rural veterans by increasing their access to sleep care and services.
Discussion
Inaccuracies in CBD procedures can adversely affect health care workload assessment and allocation, contributing to ongoing challenges faced by sleep medicine clinics and other VHA programs that have limited staff yet strive to provide timely and high-quality care to veterans. “Not only does inaccurate coding contribute to miscalculations in staffing and resource allocation, it can also contribute to inaccuracies in overall measures of VA healthcare efficiency,” the GAO reported to Congress.9 The GAO went on to recommend that the VA should ensure the accuracy of underlying staffing and workload data. VHA sleep medicine programs have made efforts to educate HCPs and administrators on the importance of accurate CBD as a tool for accurate data capture that is necessary to facilitate improvements in health care availability and delivery.
In 2018, the VA Sleep Program Office released an updated set of Stop Code changes, including expansion of telehealth codes and improved designation of laboratory and home sleep testing services. These changes are anticipated to result in accurate documentation of VA sleep clinic workload and services, especially as the VA TeleSleep EWI to reach rural veterans expands.16 In light of the improved accuracy of reporting of delivered sleep services due to changes in Stop Codes over the past 4 years, VHA sleep medicine providers continue to advocate for allocation of resources commensurate with their clinical workload. An appropriate administrative response to the significant clinical workload performed by disproportionately few providers should include the authorization of increased resources and personnel for sleep medicine as well as providing the tools needed to further streamline workflow efficiency (eg, artificial intelligence, machine learning, and population health management).
Conclusions
Despite the barriers faced by many large integrated health care systems, VHA sleep medicine leadership continues to implement changes in CBD protocols that improve the accuracy of clinical workload tracking and reporting. Ultimately, these changes will support proposals for increased resources necessary to improve the quality and availability of sleep care for veterans. This example from VA illustrates the importance of accurate workload capture and its role in informing administrators of health care systems as they strive to meet the needs of patients. Although some VA sleep medicine programs continue to face challenges imposed by systemwide limitations, the ORH TeleSleep Program is a major initiative that improves veterans’ access to care by disseminating and implementing effective telehealth technologies and strategies.16
Acknowledgments
This work was supported by a VA Office of Rural Health Enterprise-Wide Initiative.
Health care systems are faced with the challenge of meeting increasing patient care demands with finite resources.1 Advocating for additional capital—specifically, human resources—requires compelling data that accurately capture workload credit. When workload is not captured accurately, clinicians may be tasked with providing care to a high volume of patients without appropriate resource allocation. This understaffing can delay care delivery and increase the risk of diagnostic and treatment errors.2 Furthermore, workers in understaffed medical facilities are more likely to experience burnout, which leads to high workforce turnover.
Computer based documentation (CBD) is used often in medical practices to track patient care and clinical workload. However, improperly designed and implemented CBD systems can contribute to cumbersome documentation tasks and inaccurate or incomplete data capture.3 Conversely, CBD can be a useful tool to capture workload credit and can subsequently facilitate justification for medical staff allocation to meet patient care demands. This article uses our experience with US Department of Veterans Affairs (VA) national sleep medicine programs to illustrate the impact of CBD procedures on health care workload assessment and allocation. Specifically, we examine how appropriate workload capture facilitates growth and improves the efficiency of health care programs.
The VA is the largest integrated health care system in the US, serving 9 million veterans at 1,255 facilities, including 170 VA Medical Centers (VAMCs).4 As veterans’ demands for VA medical services have outpaced available resources, there have been several media reports of lapses in timely care delivery.5-7 These lapses have been due, in part, to insufficient workforce resource allocation within the Veterans Health Administration (VHA) facilities. A 2012 audit of physician staffing levels conducted by the VA Inspector General concluded that the VA did not have an effective staffing methodology to ensure appropriate staffing levels for specialty care services.8 The lack of staffing plans and productivity standards limits the ability of medical facility officials to make informed business decisions regarding the appropriate number of specialty physicians required to meet patient care needs.8 In 2017, the Government Accountability Office (GAO) issued a report to Congress that stated the “VA’s productivity metrics and efficiency models do not provide complete and accurate information, they may misrepresent the true level of productivity and efficiency across VAMCs and limit the VA’s ability to determine the extent to which its resources are being used effectively.”9 To understand how and why many VA medical facilities remain understaffed, and therefore struggle to provide health care to veterans in a timely fashion, a description of VA CBD procedures is provided.
Background
VA Directive 1082 on Patient Care Data requires the capture of all outpatient and inpatient billable encounter data.10 Accurate capture of workload informs budget allocation models and is necessary for health care provider (HCP) productivity metrics. These data points help identify staff shortages relative to the generated workload. The Veterans Equitable Resource Allocation (VERA) model is used to allocate general purpose funds to the Veterans Integrated Service Networks (VISNs) regional network of VHA facilities. The underlying data components of the VERA model rely on comprehensive data systems that track and analyze the many management information systems used in VHA. Historically, at least 90% of the funds allocated by the VERA model have been attributed directly to patient care. All workload that is appropriately documented is accounted for in the VERA patient classification process, which is the official data source for funding patient care in VHA.
VA medical facilities use Stop Codes (formerly known as Decision Support System Identifiers) to identify workload for all outpatient encounters and inpatient professional services. Each code is composed of a 6-character descriptor that includes a primary Stop Code and a credit (secondary) Stop Code. Primary Stop Codes—the first 3 numbers in the sequence—designate the main clinical group responsible for patient care, such as sleep medicine or neurology. Secondary Stop Codes—the last 3 numbers in the sequence—further define the primary workgroup, such as the type of services provided (eg, telehealth) or the type of HCP (eg, nurse practitioner). These codes help ensure that workload and generated revenue are allocated or credited to the proper specialty care service.11 An example of how changes or inaccuracies in Stop Code reporting can affect VHA clinical workload assessment and resource allocation is provided by the VHA sleep medicine program.
The prevalence of sleep disorders—particularly apnea and insomnia—among US military service members and veterans has increased dramatically over the past 2 decades and continues to rise.12-14 Consequently, demand for sleep care services at VHA facilities also has increased substantially (Figure 1). Unfortunately, this demand has outpaced the VHA’s staffing models, sometimes resulting in long wait times for appointments.15 In fact, sleep medicine remains one of the most backlogged services in the VHA, despite significant improvements in program efficiency achieved by incorporating telehealth modalities.16 Untreated sleep disorders are associated with increased risk of depression, anxiety, impaired neurocognitive functions, cardiovascular disease, motor vehicle accidents, and premature death.17-23
A major contributor to understaffing of VHA sleep medicine programs is the CBD system’s historical inability to accurately track sleep resources and demand for sleep care services. For many years, Stop Codes attributed sleep workload credit primarily to pulmonary medicine, neurology, and internal medicine workgroups. Within these workgroups, few individuals contributed to sleep care, but the entire workgroup received credit for these services, masking the workload of sleep care providers. Additional barriers to accurate sleep medicine workload capture within the VHA included (1) inability to centrally identify personnel, including physicians, as providers of sleep care; (2) limited and variable understanding among VA sleep physicians of the importance of proper encounter form completion (the mechanism by which the cost of a service is calculated); and (3) a lack of awareness that encounter closure is directly linked to productivity measures such as relative value units (RVUs) that support sleep medicine programs and the salaries of those who provide care.
Methods
The critical role of accurate CBD in health care administration is illustrated by the proper use of Stop Codes as a foundational step in tracking services provided to justify adequate resource allocation within VA. A complete redesign of tracking sleep service documentation was initiated in 2014 and resulted in national changes to sleep medicine Stop Codes. The Stop Code initiative was the first step of several to improve CBD for VA sleep services.
Primary Stop Code 349 designates sleep medicine encounters in VA facilities (Table). However, before changes were implemented in fiscal year (FY) 2015, Stop Codes for VHA sleep care did not differentiate between specific services provided, such as laboratory-based sleep testing, at-home sleep testing, education/training sessions, follow-up appointments, equipment consults, telephone or video consults, or administrative tasks. In early FY 2015, several changes were made to Stop Codes used for VHA sleep medicine services nationwide to capture the breadth of services that were being provided; services that had previously been performed but were not documented. A new standardized coding methodology was established for continuous positive airway pressure (CPAP) clinics (349/116 or 349/117); telephone consults for sleep care (324/349); and store and forward sleep telehealth encounters (349/694, 349/695, or 349/696).
In the VA, store-and-forward telehealth refers to asynchronous telemedicine involving the acquisition and storing of clinical information (eg, data, image, sound, or video) that another site or clinician reviews later for evaluation and interpretation. In sleep medicine, data uploaded from home sleep apnea test units or CPAP devices are examples of this asynchronous telehealth model. The goal of these changes in VA Stop Codes was to accurately assess the volume of sleep care delivered and the demand for sleep care (consult volumes); enable planning for resource allocation and utilization appropriately; provide veterans with consistent access to sleep services across the country; and facilitate reductions in wait times for sleep care appointments. Results of these changes were immediate and dramatic in terms of data capture and reporting.
Results
Figure 1 illustrates an increase in patient encounters in VA sleep clinics by 24,197 (19.6%) in the first quarter of Stop Code change implementation (FY 2015, quarter 2) compared with those of the previous quarter. VHA sleep clinic patient encounters increased in subsequent quarters of FY 2015 by 29,910 (20.2%) and 11,206 (6.3%) respectively. By the end of FY 2015, reported sleep clinic encounters increased by 190,803 compared with the those at the end of FY 2014, an increase of 42.7%.
Figures 2, 3, and 4 show the additional effects of sleep Stop Code changes that were implemented in FY 2015 for CPAP clinics, telephone encounters, and store-and-forward telehealth encounters, respectively. The large increases in reported sleep patient encounters between FY 2014 and FY 2016 reflect changes in CBD and are not entirely due to actual changes in clinical workloads. These results indicate that workloads in many VHA sleep medicine clinics were grossly underreported or misallocated to other specialty services prior to the changes implemented in FY 2015. This discrepancy in care delivery vs workload capture is a contributing factor to the understaffing that continues to challenge VHA sleep programs. However, the improved accuracy of workload reporting that resulted from Stop Code modifications has resulted in only a small proportional increase in VHA clinical resources allocated to provide adequate services and care for veterans with sleep disorders.
In response to the substantial and increasing demand for sleep services by veterans, the VA Office of Rural Health (ORH) funded an enterprise-wide initiative (EWI) to develop and implement a national TeleSleep Program.16 The goal of this program is to improve the health and well-being of rural veterans by increasing their access to sleep care and services.
Discussion
Inaccuracies in CBD procedures can adversely affect health care workload assessment and allocation, contributing to ongoing challenges faced by sleep medicine clinics and other VHA programs that have limited staff yet strive to provide timely and high-quality care to veterans. “Not only does inaccurate coding contribute to miscalculations in staffing and resource allocation, it can also contribute to inaccuracies in overall measures of VA healthcare efficiency,” the GAO reported to Congress.9 The GAO went on to recommend that the VA should ensure the accuracy of underlying staffing and workload data. VHA sleep medicine programs have made efforts to educate HCPs and administrators on the importance of accurate CBD as a tool for accurate data capture that is necessary to facilitate improvements in health care availability and delivery.
In 2018, the VA Sleep Program Office released an updated set of Stop Code changes, including expansion of telehealth codes and improved designation of laboratory and home sleep testing services. These changes are anticipated to result in accurate documentation of VA sleep clinic workload and services, especially as the VA TeleSleep EWI to reach rural veterans expands.16 In light of the improved accuracy of reporting of delivered sleep services due to changes in Stop Codes over the past 4 years, VHA sleep medicine providers continue to advocate for allocation of resources commensurate with their clinical workload. An appropriate administrative response to the significant clinical workload performed by disproportionately few providers should include the authorization of increased resources and personnel for sleep medicine as well as providing the tools needed to further streamline workflow efficiency (eg, artificial intelligence, machine learning, and population health management).
Conclusions
Despite the barriers faced by many large integrated health care systems, VHA sleep medicine leadership continues to implement changes in CBD protocols that improve the accuracy of clinical workload tracking and reporting. Ultimately, these changes will support proposals for increased resources necessary to improve the quality and availability of sleep care for veterans. This example from VA illustrates the importance of accurate workload capture and its role in informing administrators of health care systems as they strive to meet the needs of patients. Although some VA sleep medicine programs continue to face challenges imposed by systemwide limitations, the ORH TeleSleep Program is a major initiative that improves veterans’ access to care by disseminating and implementing effective telehealth technologies and strategies.16
Acknowledgments
This work was supported by a VA Office of Rural Health Enterprise-Wide Initiative.
1. World Health Organization. Workload indicators of staffing need (WISN). https://www.who.int/hrh/resources/WISN_Eng_UsersManual.pdf?ua=1. Published December 2015. Accessed June 24, 2020.
2. American Association for Respiratory Care. Position statement: best practices in respiratory care productivity and staffing. https://www.aarc.org/wp-content/uploads/2017/03/statement-of-best-practices_productivity-and-staffing.pdf. Revised July 2015. Accessed June 24, 2020.
3. Wu DTY, Smart N, Ciemins EL, Lanham HJ, Lindberg C, Zheng K. Using EHR audit trail logs to analyze clinical workflow: a case study from community-based ambulatory clinics. AMIA Annu Symp Proc. 2018;2017:1820-1827. Published 2018 Apr 16.
4. US Department of Veterans Affairs, Veterans Health Administration. https://www.va.gov/health.
5. Cohen T. VA crisis: solutions exist, but haven’t happened, panel hears. https://www.cnn.com/2014/06/12/politics/va-reforms/index.html. Published June 12, 2014. Accessed June 24, 2020.
6. Richardson B. IG probes uncover more problems at VA hospitals. https://thehill.com/policy/defense/258652-ig-probes-uncover-more-problems-at-va-hospitals. Published October 30, 2015. Accessed June 24, 2020.
7. Slack D. Inaccurate VA wait times prelude thousands of vets from getting outside care, probe finds. USA Today. March 3, 2017. https://www.usatoday.com/story/news/politics/2017/03/03/veterans-affairs-inspector-general-widespread-inaccuracies-wait-times/98693856. Accessed June 24, 2020.
8. US Department of Veterans Affairs, Office of the Inspector General. Veterans Health Administration: audit of physician staffing levels for specialty care services. https://www.va.gov/oig/pubs/VAOIG-11-01827-36.pdf. Published December 27, 2012. Accessed June 24, 2020.
9. Government Accountability Office. VA health care: improvements needed in data and monitoring of clinical productivity and efficiency. https://www.gao.gov/assets/690/684869.pdf. Published May 2017. Accessed June 24, 2020.
10. US Department of Veterans Affairs, Veterans Health Administration. VHA Directive 1082. Patient care data capture. https://www.va.gov/vhapublications/ViewPublication.asp?pub_ID=3091. Published March 24, 2015. Accessed June 24, 2020.
11. US Department of Veterans Affairs, Veterans Health Administration. VHA Handbook 1006.02. VHA site classifications and definitions. https://www.va.gov/vhapublications/ViewPublication.asp?pub_ID=2970. Published December 30, 2013. Accessed June 24, 2020.
12. Alexander M, Ray MA, Hébert JR, et al. The National Veteran Sleep Disorder Study: Descriptive Epidemiology and Secular Trends, 2000-2010. Sleep. 2016;39(7):1399-1410. Published 2016 Jul 1. doi:10.5665/sleep.5972.
13. A Caldwell J, Knapik JJ, Lieberman HR. Trends and factors associated with insomnia and sleep apnea in all United States military service members from 2005 to 2014. J Sleep Res. 2017;26(5):665-670. doi:10.1111/jsr.12543
14. Klingaman EA, Brownlow JA, Boland EM, Mosti C, Gehrman PR. Prevalence, predictors and correlates of insomnia in US army soldiers. J Sleep Res. 2018;27(3):e12612. doi:10.1111/jsr.12612
15. Sharafkhaneh A, Richardson P, Hirshkowitz M. Sleep apnea in a high risk population: a study of Veterans Health Administration beneficiaries. Sleep Med. 2004;5(4):345-350. doi:10.1016/j.sleep.2004.01.019.
16. Sarmiento KF, Folmer RL, Stepnowsky CJ, et al. National Expansion of Sleep Telemedicine for Veterans: The TeleSleep Program. J Clin Sleep Med. 2019;15(9):1355-1364. doi:10.5664/jcsm.7934
17. Van Dongen HP, Maislin G, Mullington JM, Dinges DF. The cumulative cost of additional wakefulness: dose-response effects on neurobehavioral functions and sleep physiology from chronic sleep restriction and total sleep deprivation [published correction appears in Sleep. 2004 Jun 15;27(4):600]. Sleep. 2003;26(2):117-126. doi:10.1093/sleep/26.2.117
18. Johnson EO, Roth T, Breslau N. The association of insomnia with anxiety disorders and depression: exploration of the direction of risk. J Psychiatr Res. 2006;40(8):700-708. doi:10.1016/j.jpsychires.2006.07.008
19. Léger D, Bayon V, Ohayon MM, et al. Insomnia and accidents: cross-sectional study (EQUINOX) on sleep-related home, work and car accidents in 5293 subjects with insomnia from 10 countries. J Sleep Res. 2014;23(2):143-152. doi:10.1111/jsr.12104
20. Franklin KA, Lindberg E. Obstructive sleep apnea is a common disorder in the population-a review on the epidemiology of sleep apnea. J Thorac Dis. 2015;7(8):1311-1322. doi:10.3978/j.issn.2072-1439.2015.06.11
21. Javaheri S, Redline S. Insomnia and Risk of Cardiovascular Disease. Chest. 2017;152(2):435-444. doi:10.1016/j.chest.2017.01.026
22. Linz D, McEvoy RD, Cowie MR, et al. Associations of obstructivesSleepaApnea with atrial fibrillation and continuous positive airway pressure treatment: a review. JAMA Cardiol. 2018;3(6):532-540. doi:10.1001/jamacardio.2018.0095
23. Ogilvie RP, Lakshminarayan K, Iber C, Patel SR, Lutsey PL. Joint effects of OSA and self-reported sleepiness on incident CHD and stroke. Sleep Med. 2018;44:32-37. doi:10.1016/j.sleep.2018.01.004
1. World Health Organization. Workload indicators of staffing need (WISN). https://www.who.int/hrh/resources/WISN_Eng_UsersManual.pdf?ua=1. Published December 2015. Accessed June 24, 2020.
2. American Association for Respiratory Care. Position statement: best practices in respiratory care productivity and staffing. https://www.aarc.org/wp-content/uploads/2017/03/statement-of-best-practices_productivity-and-staffing.pdf. Revised July 2015. Accessed June 24, 2020.
3. Wu DTY, Smart N, Ciemins EL, Lanham HJ, Lindberg C, Zheng K. Using EHR audit trail logs to analyze clinical workflow: a case study from community-based ambulatory clinics. AMIA Annu Symp Proc. 2018;2017:1820-1827. Published 2018 Apr 16.
4. US Department of Veterans Affairs, Veterans Health Administration. https://www.va.gov/health.
5. Cohen T. VA crisis: solutions exist, but haven’t happened, panel hears. https://www.cnn.com/2014/06/12/politics/va-reforms/index.html. Published June 12, 2014. Accessed June 24, 2020.
6. Richardson B. IG probes uncover more problems at VA hospitals. https://thehill.com/policy/defense/258652-ig-probes-uncover-more-problems-at-va-hospitals. Published October 30, 2015. Accessed June 24, 2020.
7. Slack D. Inaccurate VA wait times prelude thousands of vets from getting outside care, probe finds. USA Today. March 3, 2017. https://www.usatoday.com/story/news/politics/2017/03/03/veterans-affairs-inspector-general-widespread-inaccuracies-wait-times/98693856. Accessed June 24, 2020.
8. US Department of Veterans Affairs, Office of the Inspector General. Veterans Health Administration: audit of physician staffing levels for specialty care services. https://www.va.gov/oig/pubs/VAOIG-11-01827-36.pdf. Published December 27, 2012. Accessed June 24, 2020.
9. Government Accountability Office. VA health care: improvements needed in data and monitoring of clinical productivity and efficiency. https://www.gao.gov/assets/690/684869.pdf. Published May 2017. Accessed June 24, 2020.
10. US Department of Veterans Affairs, Veterans Health Administration. VHA Directive 1082. Patient care data capture. https://www.va.gov/vhapublications/ViewPublication.asp?pub_ID=3091. Published March 24, 2015. Accessed June 24, 2020.
11. US Department of Veterans Affairs, Veterans Health Administration. VHA Handbook 1006.02. VHA site classifications and definitions. https://www.va.gov/vhapublications/ViewPublication.asp?pub_ID=2970. Published December 30, 2013. Accessed June 24, 2020.
12. Alexander M, Ray MA, Hébert JR, et al. The National Veteran Sleep Disorder Study: Descriptive Epidemiology and Secular Trends, 2000-2010. Sleep. 2016;39(7):1399-1410. Published 2016 Jul 1. doi:10.5665/sleep.5972.
13. A Caldwell J, Knapik JJ, Lieberman HR. Trends and factors associated with insomnia and sleep apnea in all United States military service members from 2005 to 2014. J Sleep Res. 2017;26(5):665-670. doi:10.1111/jsr.12543
14. Klingaman EA, Brownlow JA, Boland EM, Mosti C, Gehrman PR. Prevalence, predictors and correlates of insomnia in US army soldiers. J Sleep Res. 2018;27(3):e12612. doi:10.1111/jsr.12612
15. Sharafkhaneh A, Richardson P, Hirshkowitz M. Sleep apnea in a high risk population: a study of Veterans Health Administration beneficiaries. Sleep Med. 2004;5(4):345-350. doi:10.1016/j.sleep.2004.01.019.
16. Sarmiento KF, Folmer RL, Stepnowsky CJ, et al. National Expansion of Sleep Telemedicine for Veterans: The TeleSleep Program. J Clin Sleep Med. 2019;15(9):1355-1364. doi:10.5664/jcsm.7934
17. Van Dongen HP, Maislin G, Mullington JM, Dinges DF. The cumulative cost of additional wakefulness: dose-response effects on neurobehavioral functions and sleep physiology from chronic sleep restriction and total sleep deprivation [published correction appears in Sleep. 2004 Jun 15;27(4):600]. Sleep. 2003;26(2):117-126. doi:10.1093/sleep/26.2.117
18. Johnson EO, Roth T, Breslau N. The association of insomnia with anxiety disorders and depression: exploration of the direction of risk. J Psychiatr Res. 2006;40(8):700-708. doi:10.1016/j.jpsychires.2006.07.008
19. Léger D, Bayon V, Ohayon MM, et al. Insomnia and accidents: cross-sectional study (EQUINOX) on sleep-related home, work and car accidents in 5293 subjects with insomnia from 10 countries. J Sleep Res. 2014;23(2):143-152. doi:10.1111/jsr.12104
20. Franklin KA, Lindberg E. Obstructive sleep apnea is a common disorder in the population-a review on the epidemiology of sleep apnea. J Thorac Dis. 2015;7(8):1311-1322. doi:10.3978/j.issn.2072-1439.2015.06.11
21. Javaheri S, Redline S. Insomnia and Risk of Cardiovascular Disease. Chest. 2017;152(2):435-444. doi:10.1016/j.chest.2017.01.026
22. Linz D, McEvoy RD, Cowie MR, et al. Associations of obstructivesSleepaApnea with atrial fibrillation and continuous positive airway pressure treatment: a review. JAMA Cardiol. 2018;3(6):532-540. doi:10.1001/jamacardio.2018.0095
23. Ogilvie RP, Lakshminarayan K, Iber C, Patel SR, Lutsey PL. Joint effects of OSA and self-reported sleepiness on incident CHD and stroke. Sleep Med. 2018;44:32-37. doi:10.1016/j.sleep.2018.01.004
Sleep problems in young children linked to lower QOL in later years
Sleep problems in children from birth to middle childhood may lead to decreased emotional well-being and quality of life by the time a child is 10-11 years old, a recent longitudinal study has found.
The effects of these impairments increased over time and included internalizing and externalizing concerns, self-control, and quality of life, but did not appear to significantly affect cognitive or academic skills, according to Ariel A. Williamson, PhD, DBSM, of Children’s Hospital of Philadelphia, and colleagues. While children with consistent sleep problems experienced the worse outcomes, mild sleep problems also were associated with impairment, the researchers said.
“The range of impairments across academic and psychosocial domains in middle childhood indicate that it is important to screen for sleep problems consistently over the course of a child’s development, especially to target children who experience persistent sleep problems over time,” said Dr. Williamson in a press release.
The researchers examined data from 5,107 children in the Longitudinal Study of Australian Children – Birth Cohort, where sleep problems and well-being outcomes were measured at multiple time points. Behaviors such as difficulty getting off to sleep at night, not happy to sleep alone, and waking during the night were defined as sleep problems. The investigators found five main domains of sleep issues: children who had persistent sleep problems through middle childhood (7.7%), limited sleep problems as an infant or during preschool (9.0%), mild sleep problems over time (14.4%), increased sleep problems during middle childhood (17.0%), and a group that did not experience sleep problems (51.9%).
Caregivers reported sleep issues in the cohort, while well-being outcomes were reported by caregivers and teachers, and tasks were completed by the children at 10-11 years of age. Dr. Williamson and colleagues examined well-being in terms of emotional and behavioral functioning, health-related quality of life, cognitive skills, and academic achievement.
Different reports from teacher and caregivers
Teacher and caregivers reported different effects in children with persistent sleep problems. Teachers reported moderate internalizing (effect size, –0.65; 95% confidence interval [CI],–0.87 to –0.43; P < .001) and externalizing concerns (ES, –0.40; 95% CI, –0.58 to –0.21; P less than .001), compared with children who did not have sleep problems, whereas caregivers reported large internalizing (ES, –0.75; 95% CI, –0.92 to –0.57; P less than .001) and externalizing concerns (ES, –0.70; 95% CI, –0.86 to –0.53; P < .001). Children with persistent sleep problems had moderate impairment of self-control as reported by caregivers, compared with children with no sleep problems (ES, –0.37; 95% CI, –0.52 to –0.21; P < .001). Psychosocial and health-related quality of life reported by caregivers were worse in children with persistent sleep problems, compared with children who did not have sleep problems (ES range, –0.78 to –0.90; 95% CI, –1.06 to –0.56; P < .001).
For children who exhibited increased sleep problems in middle childhood, caregivers (ES for both, –0.61; 95% CI, –0.76 to –0.46; P < .001) and teachers (ES range, –0.29 to –0.39; 95% CI, –0.53 to –0.15; P < .001) reported greater rates of internalizing and externalizing symptoms, compared with children who had no sleep issues.
Small impairments in internalizing internal or externalizing symptoms were seen in children who had limited sleep problems as an infant or in preschool (ES, –0.12; 95% CI, –0.23 to –0.01; P < .05) as reported by teachers, and in children with mild sleep problems over time (ES, –0.19; 95% CI, –0.30 to –0.08; P < .001) as reported by caregivers. There were no significant impairments in self-control for children in either the infant or preschool impairment group or in the group of children with mild sleep problems.
Across all groups, sleep problems did not significantly impair nonverbal reasoning, and most areas of academic competencies were not significantly impaired among groups except in language and literacy, and mathematical thinking for children with persistent sleep problems (ES, –0.41 for both; 95% CI, –0.60 to –0.23; P < .001). Children with increased sleep problems during middle childhood “had few academic and cognitive impairments,” and academic impairments among children with mild sleep problems were not significant.
Expert opinion
Brandon M. Seay MD, FAAP, pediatric pulmonologist and sleep specialist at Children’s Healthcare of Atlanta, said in an interview that the study is one of the first to offer longitudinal data for impairment in children with sleep problems. He said the paper emphasizes the need for recognizing when children are demonstrating sleep problems. “It just shows that problems that aren’t dealt with earlier on definitely have bigger impacts on sleep as you go through life,” he said.
Although primary care physicians and pediatricians should be already asking questions about sleep through anticipatory guidance, he said, intervening earlier for sleep problems is important. He noted children who exhibit sleep problems over time are more likely to have issues in handling their emotions and eventually may develop cognitive issues. “[W]e know that if these problems continue to go through, this paper’s showing us that they have worse effects down the road,” he said.
Impact of the COVID-19 crisis
These problems may also be worsened by the COVID-19 pandemic. Dr. Seay noted that with many parents working from home, sleep schedules can be affected and parents may also be co-sleeping with their children, which can cause chronic insomnia and early waking. To help address sleep issues, especially ones that may have arisen during COVID-19, parents should make sure their children show up for primary care visits to report problems, and clinicians should make a sleep routine a focus of conversations around sleep problems.
Prior to the pandemic, “we already were hitting upon that in sleep clinic, making sure [they] get the same schedule every day,” said Dr. Seay. For parents with children who have “issues with insomnia or waking up during the night, having that routine in place does help to mitigate that a little bit, so if that routine is not there, it can actually exacerbate the issues.”
This study was funded by the Australian federal government. The authors report no relevant conflicts of interest. Dr. Seay reports no relevant conflicts of interest.
SOURCE: Williamson AA et al. J Child Psychol Psychiatry. 2020 Jul 26. doi:10.1111/jcpp.13303.
Sleep problems in children from birth to middle childhood may lead to decreased emotional well-being and quality of life by the time a child is 10-11 years old, a recent longitudinal study has found.
The effects of these impairments increased over time and included internalizing and externalizing concerns, self-control, and quality of life, but did not appear to significantly affect cognitive or academic skills, according to Ariel A. Williamson, PhD, DBSM, of Children’s Hospital of Philadelphia, and colleagues. While children with consistent sleep problems experienced the worse outcomes, mild sleep problems also were associated with impairment, the researchers said.
“The range of impairments across academic and psychosocial domains in middle childhood indicate that it is important to screen for sleep problems consistently over the course of a child’s development, especially to target children who experience persistent sleep problems over time,” said Dr. Williamson in a press release.
The researchers examined data from 5,107 children in the Longitudinal Study of Australian Children – Birth Cohort, where sleep problems and well-being outcomes were measured at multiple time points. Behaviors such as difficulty getting off to sleep at night, not happy to sleep alone, and waking during the night were defined as sleep problems. The investigators found five main domains of sleep issues: children who had persistent sleep problems through middle childhood (7.7%), limited sleep problems as an infant or during preschool (9.0%), mild sleep problems over time (14.4%), increased sleep problems during middle childhood (17.0%), and a group that did not experience sleep problems (51.9%).
Caregivers reported sleep issues in the cohort, while well-being outcomes were reported by caregivers and teachers, and tasks were completed by the children at 10-11 years of age. Dr. Williamson and colleagues examined well-being in terms of emotional and behavioral functioning, health-related quality of life, cognitive skills, and academic achievement.
Different reports from teacher and caregivers
Teacher and caregivers reported different effects in children with persistent sleep problems. Teachers reported moderate internalizing (effect size, –0.65; 95% confidence interval [CI],–0.87 to –0.43; P < .001) and externalizing concerns (ES, –0.40; 95% CI, –0.58 to –0.21; P less than .001), compared with children who did not have sleep problems, whereas caregivers reported large internalizing (ES, –0.75; 95% CI, –0.92 to –0.57; P less than .001) and externalizing concerns (ES, –0.70; 95% CI, –0.86 to –0.53; P < .001). Children with persistent sleep problems had moderate impairment of self-control as reported by caregivers, compared with children with no sleep problems (ES, –0.37; 95% CI, –0.52 to –0.21; P < .001). Psychosocial and health-related quality of life reported by caregivers were worse in children with persistent sleep problems, compared with children who did not have sleep problems (ES range, –0.78 to –0.90; 95% CI, –1.06 to –0.56; P < .001).
For children who exhibited increased sleep problems in middle childhood, caregivers (ES for both, –0.61; 95% CI, –0.76 to –0.46; P < .001) and teachers (ES range, –0.29 to –0.39; 95% CI, –0.53 to –0.15; P < .001) reported greater rates of internalizing and externalizing symptoms, compared with children who had no sleep issues.
Small impairments in internalizing internal or externalizing symptoms were seen in children who had limited sleep problems as an infant or in preschool (ES, –0.12; 95% CI, –0.23 to –0.01; P < .05) as reported by teachers, and in children with mild sleep problems over time (ES, –0.19; 95% CI, –0.30 to –0.08; P < .001) as reported by caregivers. There were no significant impairments in self-control for children in either the infant or preschool impairment group or in the group of children with mild sleep problems.
Across all groups, sleep problems did not significantly impair nonverbal reasoning, and most areas of academic competencies were not significantly impaired among groups except in language and literacy, and mathematical thinking for children with persistent sleep problems (ES, –0.41 for both; 95% CI, –0.60 to –0.23; P < .001). Children with increased sleep problems during middle childhood “had few academic and cognitive impairments,” and academic impairments among children with mild sleep problems were not significant.
Expert opinion
Brandon M. Seay MD, FAAP, pediatric pulmonologist and sleep specialist at Children’s Healthcare of Atlanta, said in an interview that the study is one of the first to offer longitudinal data for impairment in children with sleep problems. He said the paper emphasizes the need for recognizing when children are demonstrating sleep problems. “It just shows that problems that aren’t dealt with earlier on definitely have bigger impacts on sleep as you go through life,” he said.
Although primary care physicians and pediatricians should be already asking questions about sleep through anticipatory guidance, he said, intervening earlier for sleep problems is important. He noted children who exhibit sleep problems over time are more likely to have issues in handling their emotions and eventually may develop cognitive issues. “[W]e know that if these problems continue to go through, this paper’s showing us that they have worse effects down the road,” he said.
Impact of the COVID-19 crisis
These problems may also be worsened by the COVID-19 pandemic. Dr. Seay noted that with many parents working from home, sleep schedules can be affected and parents may also be co-sleeping with their children, which can cause chronic insomnia and early waking. To help address sleep issues, especially ones that may have arisen during COVID-19, parents should make sure their children show up for primary care visits to report problems, and clinicians should make a sleep routine a focus of conversations around sleep problems.
Prior to the pandemic, “we already were hitting upon that in sleep clinic, making sure [they] get the same schedule every day,” said Dr. Seay. For parents with children who have “issues with insomnia or waking up during the night, having that routine in place does help to mitigate that a little bit, so if that routine is not there, it can actually exacerbate the issues.”
This study was funded by the Australian federal government. The authors report no relevant conflicts of interest. Dr. Seay reports no relevant conflicts of interest.
SOURCE: Williamson AA et al. J Child Psychol Psychiatry. 2020 Jul 26. doi:10.1111/jcpp.13303.
Sleep problems in children from birth to middle childhood may lead to decreased emotional well-being and quality of life by the time a child is 10-11 years old, a recent longitudinal study has found.
The effects of these impairments increased over time and included internalizing and externalizing concerns, self-control, and quality of life, but did not appear to significantly affect cognitive or academic skills, according to Ariel A. Williamson, PhD, DBSM, of Children’s Hospital of Philadelphia, and colleagues. While children with consistent sleep problems experienced the worse outcomes, mild sleep problems also were associated with impairment, the researchers said.
“The range of impairments across academic and psychosocial domains in middle childhood indicate that it is important to screen for sleep problems consistently over the course of a child’s development, especially to target children who experience persistent sleep problems over time,” said Dr. Williamson in a press release.
The researchers examined data from 5,107 children in the Longitudinal Study of Australian Children – Birth Cohort, where sleep problems and well-being outcomes were measured at multiple time points. Behaviors such as difficulty getting off to sleep at night, not happy to sleep alone, and waking during the night were defined as sleep problems. The investigators found five main domains of sleep issues: children who had persistent sleep problems through middle childhood (7.7%), limited sleep problems as an infant or during preschool (9.0%), mild sleep problems over time (14.4%), increased sleep problems during middle childhood (17.0%), and a group that did not experience sleep problems (51.9%).
Caregivers reported sleep issues in the cohort, while well-being outcomes were reported by caregivers and teachers, and tasks were completed by the children at 10-11 years of age. Dr. Williamson and colleagues examined well-being in terms of emotional and behavioral functioning, health-related quality of life, cognitive skills, and academic achievement.
Different reports from teacher and caregivers
Teacher and caregivers reported different effects in children with persistent sleep problems. Teachers reported moderate internalizing (effect size, –0.65; 95% confidence interval [CI],–0.87 to –0.43; P < .001) and externalizing concerns (ES, –0.40; 95% CI, –0.58 to –0.21; P less than .001), compared with children who did not have sleep problems, whereas caregivers reported large internalizing (ES, –0.75; 95% CI, –0.92 to –0.57; P less than .001) and externalizing concerns (ES, –0.70; 95% CI, –0.86 to –0.53; P < .001). Children with persistent sleep problems had moderate impairment of self-control as reported by caregivers, compared with children with no sleep problems (ES, –0.37; 95% CI, –0.52 to –0.21; P < .001). Psychosocial and health-related quality of life reported by caregivers were worse in children with persistent sleep problems, compared with children who did not have sleep problems (ES range, –0.78 to –0.90; 95% CI, –1.06 to –0.56; P < .001).
For children who exhibited increased sleep problems in middle childhood, caregivers (ES for both, –0.61; 95% CI, –0.76 to –0.46; P < .001) and teachers (ES range, –0.29 to –0.39; 95% CI, –0.53 to –0.15; P < .001) reported greater rates of internalizing and externalizing symptoms, compared with children who had no sleep issues.
Small impairments in internalizing internal or externalizing symptoms were seen in children who had limited sleep problems as an infant or in preschool (ES, –0.12; 95% CI, –0.23 to –0.01; P < .05) as reported by teachers, and in children with mild sleep problems over time (ES, –0.19; 95% CI, –0.30 to –0.08; P < .001) as reported by caregivers. There were no significant impairments in self-control for children in either the infant or preschool impairment group or in the group of children with mild sleep problems.
Across all groups, sleep problems did not significantly impair nonverbal reasoning, and most areas of academic competencies were not significantly impaired among groups except in language and literacy, and mathematical thinking for children with persistent sleep problems (ES, –0.41 for both; 95% CI, –0.60 to –0.23; P < .001). Children with increased sleep problems during middle childhood “had few academic and cognitive impairments,” and academic impairments among children with mild sleep problems were not significant.
Expert opinion
Brandon M. Seay MD, FAAP, pediatric pulmonologist and sleep specialist at Children’s Healthcare of Atlanta, said in an interview that the study is one of the first to offer longitudinal data for impairment in children with sleep problems. He said the paper emphasizes the need for recognizing when children are demonstrating sleep problems. “It just shows that problems that aren’t dealt with earlier on definitely have bigger impacts on sleep as you go through life,” he said.
Although primary care physicians and pediatricians should be already asking questions about sleep through anticipatory guidance, he said, intervening earlier for sleep problems is important. He noted children who exhibit sleep problems over time are more likely to have issues in handling their emotions and eventually may develop cognitive issues. “[W]e know that if these problems continue to go through, this paper’s showing us that they have worse effects down the road,” he said.
Impact of the COVID-19 crisis
These problems may also be worsened by the COVID-19 pandemic. Dr. Seay noted that with many parents working from home, sleep schedules can be affected and parents may also be co-sleeping with their children, which can cause chronic insomnia and early waking. To help address sleep issues, especially ones that may have arisen during COVID-19, parents should make sure their children show up for primary care visits to report problems, and clinicians should make a sleep routine a focus of conversations around sleep problems.
Prior to the pandemic, “we already were hitting upon that in sleep clinic, making sure [they] get the same schedule every day,” said Dr. Seay. For parents with children who have “issues with insomnia or waking up during the night, having that routine in place does help to mitigate that a little bit, so if that routine is not there, it can actually exacerbate the issues.”
This study was funded by the Australian federal government. The authors report no relevant conflicts of interest. Dr. Seay reports no relevant conflicts of interest.
SOURCE: Williamson AA et al. J Child Psychol Psychiatry. 2020 Jul 26. doi:10.1111/jcpp.13303.
FROM JOURNAL OF CHILD PSYCHOLOGY AND PSYCHIATRY
‘Long sleep’ or apnea in middle age double risk for Alzheimer’s disease
new research suggests. A U.K. Biobank study of more than 500,000 individuals also showed that excessive daytime sleepiness was associated with increased risk for Alzheimer’s disease.
“Addressing sleep problems in middle-age may play a role in improving brain health,” said lead author Lei Gao, MD, assistant professor of anesthesia at Harvard Medical School and associate scientist in the division of sleep and circadian disorders at Brigham and Women’s Hospital, both in Boston.
The findings were presented at the virtual annual meeting of the Alzheimer’s Association International Conference 2020.
Intricately linked
Sleep disturbances are common and on the rise around the world. In recent years, researchers have become increasingly aware of the intricate link between sleep health and brain health, Dr. Gao noted.
The current study included 502,538 individuals from the U.K. Biobank (mean age, 57 years) who were free from Alzheimer’s disease at baseline. They were followed for up to 12 years. The participants self-reported sleep traits, including hours of nighttime sleep, daytime sleepiness, sleep apnea diagnosis, snoring, and napping. Researchers determined Alzheimer’s disease diagnoses from hospital admissions and from death registries.
In addition to adjusting for age, sex, education, and ethnicity, the full model adjusted for socioeconomic status, body mass index, physical activity, smoking and alcohol use, cardiovascular diseases and risk factors, neurological diseases, respiratory diseases, depression/anxiety, and medication use. Over the course of a mean follow-up of 6.4 years, 932 participants developed Alzheimer’s disease.
Complex disorder
Compared with those who got an average of 6-9 hours of sleep per night, those getting more than 9 hours had a higher risk for Alzheimer’s disease (hazard ratio, 2.04; 95% confidence interval, 1.56-2.67; P < .001). Having sleep apnea also raised the risk significantly (HR, 2.05; 95% CI, 1.23-3.42; P = .006), as did daytime sleepiness (HR, 1.56; 95% CI, 1.18-2.03; P = .001).
Dr. Gao noted that daytime sleepiness and sleep apnea remained predictive after controlling for sleep duration. “In fact, all three sleep traits remained associated with Alzheimer’s disease within the same model, suggesting some degree of independence.”
Interestingly, snoring, which is a common symptom of sleep apnea, was not linked to Alzheimer’s disease risk. The “vast majority” of people who snore don’t meet criteria for a diagnosis of sleep apnea, which was particularly true for this large cohort of relatively healthy study participants, Dr. Gao noted.
“Sleep apnea is a complex, multisystemic sleep disorder associated with obesity, high blood pressure, and often other heart problems,” he said.
He added that, as an anesthesiologist, he is particularly wary if patients have this condition, “given their increased risk for airway difficulties, adverse cardiac events, postoperative respiratory complications, and confusion or delirium, which is also associated with higher risk for eventual Alzheimer’s disease and death.”
These multisystemic factors may be driving the link to Alzheimer’s disease. “We certainly need to address this better as the population ages and obesity rates rise,” Dr. Gao said.
No association with napping
Unlike another of Dr. Gao’s studies that was conducted in a much older population, napping was not a risk factor for Alzheimer’s disease in the current study’s younger participants. It could be that the impacts of different sleep traits on health outcome change with age, Dr. Gao said, or this could represent a limitation of using self-reported sleep measures as opposed to objective and/or quantitative measures, such as actigraphy. The reasons for napping, which differ around the world with the habit being common in certain parts, may also help explain differences in observed associations.
Although the investigators tried to control for comorbidities and medication use, there “most certainly” could be a reverse causation at work. For example, sleeping too much could be both a cause and a symptom of dementia. Dr. Gao noted that sleep disturbances often become more prevalent with dementia, and sleeping too much or complaining of daytime sleepiness may be a result of preclinical Alzheimer’s disease. Even if there is a reverse causation, however, the average time to Alzheimer’s disease diagnosis was over 6 years in this study. “This may be a significant window of time to intervene,” he said.
To improve sleep health, he recommends going to bed and waking at similar times every day, avoiding caffeine or alcohol close to bedtime, limiting screen time before bed, dimming lights, and reducing noise.
It’s also important to have sleep apnea treated. “While more studies are needed, it’s generally believed that addressing the pauses in breathing, the apnea episodes, will help reduce cardiovascular health risks such as obesity, high blood pressure and heart failure. All are known to be strongly linked to dementia risk,” Dr. Gao said.
Results from an assessment of 100,000 actigraphy records from a subset of the same population are expected soon and will add objective confirmation of these self-reported results, he added.
Unique, powerful
Commenting on the findings, Alberto Ramos, MD, associate professor of clinical neurology and research director of the sleep medicine program at the University of Miami, called the study “unique” and “powerful” because of its prospective design and large sample size.
“Another strength of the study was that it included a population-based sample as opposed to one from a memory or sleep clinic where people already have symptoms or are already sick,” said Dr. Ramos, who was not involved with the research.
In addition, while most studies that have linked sleep disturbances with dementia risk have been in older adults, this study’s population was middle-aged to start out, he noted.
Dr. Gao and Dr. Ramos reported no relevant financial relationships. Although Dr. Gao’s lab receives funding from the National Institutes of Health, the BrightFocus Foundation, the University of Manchester, the Medical Biodynamics Program, Brigham and Women’s Hospital, and the Broad Institute, the study itself does not have its own specific funding.
A version of this article originally appeared on Medscape.com.
new research suggests. A U.K. Biobank study of more than 500,000 individuals also showed that excessive daytime sleepiness was associated with increased risk for Alzheimer’s disease.
“Addressing sleep problems in middle-age may play a role in improving brain health,” said lead author Lei Gao, MD, assistant professor of anesthesia at Harvard Medical School and associate scientist in the division of sleep and circadian disorders at Brigham and Women’s Hospital, both in Boston.
The findings were presented at the virtual annual meeting of the Alzheimer’s Association International Conference 2020.
Intricately linked
Sleep disturbances are common and on the rise around the world. In recent years, researchers have become increasingly aware of the intricate link between sleep health and brain health, Dr. Gao noted.
The current study included 502,538 individuals from the U.K. Biobank (mean age, 57 years) who were free from Alzheimer’s disease at baseline. They were followed for up to 12 years. The participants self-reported sleep traits, including hours of nighttime sleep, daytime sleepiness, sleep apnea diagnosis, snoring, and napping. Researchers determined Alzheimer’s disease diagnoses from hospital admissions and from death registries.
In addition to adjusting for age, sex, education, and ethnicity, the full model adjusted for socioeconomic status, body mass index, physical activity, smoking and alcohol use, cardiovascular diseases and risk factors, neurological diseases, respiratory diseases, depression/anxiety, and medication use. Over the course of a mean follow-up of 6.4 years, 932 participants developed Alzheimer’s disease.
Complex disorder
Compared with those who got an average of 6-9 hours of sleep per night, those getting more than 9 hours had a higher risk for Alzheimer’s disease (hazard ratio, 2.04; 95% confidence interval, 1.56-2.67; P < .001). Having sleep apnea also raised the risk significantly (HR, 2.05; 95% CI, 1.23-3.42; P = .006), as did daytime sleepiness (HR, 1.56; 95% CI, 1.18-2.03; P = .001).
Dr. Gao noted that daytime sleepiness and sleep apnea remained predictive after controlling for sleep duration. “In fact, all three sleep traits remained associated with Alzheimer’s disease within the same model, suggesting some degree of independence.”
Interestingly, snoring, which is a common symptom of sleep apnea, was not linked to Alzheimer’s disease risk. The “vast majority” of people who snore don’t meet criteria for a diagnosis of sleep apnea, which was particularly true for this large cohort of relatively healthy study participants, Dr. Gao noted.
“Sleep apnea is a complex, multisystemic sleep disorder associated with obesity, high blood pressure, and often other heart problems,” he said.
He added that, as an anesthesiologist, he is particularly wary if patients have this condition, “given their increased risk for airway difficulties, adverse cardiac events, postoperative respiratory complications, and confusion or delirium, which is also associated with higher risk for eventual Alzheimer’s disease and death.”
These multisystemic factors may be driving the link to Alzheimer’s disease. “We certainly need to address this better as the population ages and obesity rates rise,” Dr. Gao said.
No association with napping
Unlike another of Dr. Gao’s studies that was conducted in a much older population, napping was not a risk factor for Alzheimer’s disease in the current study’s younger participants. It could be that the impacts of different sleep traits on health outcome change with age, Dr. Gao said, or this could represent a limitation of using self-reported sleep measures as opposed to objective and/or quantitative measures, such as actigraphy. The reasons for napping, which differ around the world with the habit being common in certain parts, may also help explain differences in observed associations.
Although the investigators tried to control for comorbidities and medication use, there “most certainly” could be a reverse causation at work. For example, sleeping too much could be both a cause and a symptom of dementia. Dr. Gao noted that sleep disturbances often become more prevalent with dementia, and sleeping too much or complaining of daytime sleepiness may be a result of preclinical Alzheimer’s disease. Even if there is a reverse causation, however, the average time to Alzheimer’s disease diagnosis was over 6 years in this study. “This may be a significant window of time to intervene,” he said.
To improve sleep health, he recommends going to bed and waking at similar times every day, avoiding caffeine or alcohol close to bedtime, limiting screen time before bed, dimming lights, and reducing noise.
It’s also important to have sleep apnea treated. “While more studies are needed, it’s generally believed that addressing the pauses in breathing, the apnea episodes, will help reduce cardiovascular health risks such as obesity, high blood pressure and heart failure. All are known to be strongly linked to dementia risk,” Dr. Gao said.
Results from an assessment of 100,000 actigraphy records from a subset of the same population are expected soon and will add objective confirmation of these self-reported results, he added.
Unique, powerful
Commenting on the findings, Alberto Ramos, MD, associate professor of clinical neurology and research director of the sleep medicine program at the University of Miami, called the study “unique” and “powerful” because of its prospective design and large sample size.
“Another strength of the study was that it included a population-based sample as opposed to one from a memory or sleep clinic where people already have symptoms or are already sick,” said Dr. Ramos, who was not involved with the research.
In addition, while most studies that have linked sleep disturbances with dementia risk have been in older adults, this study’s population was middle-aged to start out, he noted.
Dr. Gao and Dr. Ramos reported no relevant financial relationships. Although Dr. Gao’s lab receives funding from the National Institutes of Health, the BrightFocus Foundation, the University of Manchester, the Medical Biodynamics Program, Brigham and Women’s Hospital, and the Broad Institute, the study itself does not have its own specific funding.
A version of this article originally appeared on Medscape.com.
new research suggests. A U.K. Biobank study of more than 500,000 individuals also showed that excessive daytime sleepiness was associated with increased risk for Alzheimer’s disease.
“Addressing sleep problems in middle-age may play a role in improving brain health,” said lead author Lei Gao, MD, assistant professor of anesthesia at Harvard Medical School and associate scientist in the division of sleep and circadian disorders at Brigham and Women’s Hospital, both in Boston.
The findings were presented at the virtual annual meeting of the Alzheimer’s Association International Conference 2020.
Intricately linked
Sleep disturbances are common and on the rise around the world. In recent years, researchers have become increasingly aware of the intricate link between sleep health and brain health, Dr. Gao noted.
The current study included 502,538 individuals from the U.K. Biobank (mean age, 57 years) who were free from Alzheimer’s disease at baseline. They were followed for up to 12 years. The participants self-reported sleep traits, including hours of nighttime sleep, daytime sleepiness, sleep apnea diagnosis, snoring, and napping. Researchers determined Alzheimer’s disease diagnoses from hospital admissions and from death registries.
In addition to adjusting for age, sex, education, and ethnicity, the full model adjusted for socioeconomic status, body mass index, physical activity, smoking and alcohol use, cardiovascular diseases and risk factors, neurological diseases, respiratory diseases, depression/anxiety, and medication use. Over the course of a mean follow-up of 6.4 years, 932 participants developed Alzheimer’s disease.
Complex disorder
Compared with those who got an average of 6-9 hours of sleep per night, those getting more than 9 hours had a higher risk for Alzheimer’s disease (hazard ratio, 2.04; 95% confidence interval, 1.56-2.67; P < .001). Having sleep apnea also raised the risk significantly (HR, 2.05; 95% CI, 1.23-3.42; P = .006), as did daytime sleepiness (HR, 1.56; 95% CI, 1.18-2.03; P = .001).
Dr. Gao noted that daytime sleepiness and sleep apnea remained predictive after controlling for sleep duration. “In fact, all three sleep traits remained associated with Alzheimer’s disease within the same model, suggesting some degree of independence.”
Interestingly, snoring, which is a common symptom of sleep apnea, was not linked to Alzheimer’s disease risk. The “vast majority” of people who snore don’t meet criteria for a diagnosis of sleep apnea, which was particularly true for this large cohort of relatively healthy study participants, Dr. Gao noted.
“Sleep apnea is a complex, multisystemic sleep disorder associated with obesity, high blood pressure, and often other heart problems,” he said.
He added that, as an anesthesiologist, he is particularly wary if patients have this condition, “given their increased risk for airway difficulties, adverse cardiac events, postoperative respiratory complications, and confusion or delirium, which is also associated with higher risk for eventual Alzheimer’s disease and death.”
These multisystemic factors may be driving the link to Alzheimer’s disease. “We certainly need to address this better as the population ages and obesity rates rise,” Dr. Gao said.
No association with napping
Unlike another of Dr. Gao’s studies that was conducted in a much older population, napping was not a risk factor for Alzheimer’s disease in the current study’s younger participants. It could be that the impacts of different sleep traits on health outcome change with age, Dr. Gao said, or this could represent a limitation of using self-reported sleep measures as opposed to objective and/or quantitative measures, such as actigraphy. The reasons for napping, which differ around the world with the habit being common in certain parts, may also help explain differences in observed associations.
Although the investigators tried to control for comorbidities and medication use, there “most certainly” could be a reverse causation at work. For example, sleeping too much could be both a cause and a symptom of dementia. Dr. Gao noted that sleep disturbances often become more prevalent with dementia, and sleeping too much or complaining of daytime sleepiness may be a result of preclinical Alzheimer’s disease. Even if there is a reverse causation, however, the average time to Alzheimer’s disease diagnosis was over 6 years in this study. “This may be a significant window of time to intervene,” he said.
To improve sleep health, he recommends going to bed and waking at similar times every day, avoiding caffeine or alcohol close to bedtime, limiting screen time before bed, dimming lights, and reducing noise.
It’s also important to have sleep apnea treated. “While more studies are needed, it’s generally believed that addressing the pauses in breathing, the apnea episodes, will help reduce cardiovascular health risks such as obesity, high blood pressure and heart failure. All are known to be strongly linked to dementia risk,” Dr. Gao said.
Results from an assessment of 100,000 actigraphy records from a subset of the same population are expected soon and will add objective confirmation of these self-reported results, he added.
Unique, powerful
Commenting on the findings, Alberto Ramos, MD, associate professor of clinical neurology and research director of the sleep medicine program at the University of Miami, called the study “unique” and “powerful” because of its prospective design and large sample size.
“Another strength of the study was that it included a population-based sample as opposed to one from a memory or sleep clinic where people already have symptoms or are already sick,” said Dr. Ramos, who was not involved with the research.
In addition, while most studies that have linked sleep disturbances with dementia risk have been in older adults, this study’s population was middle-aged to start out, he noted.
Dr. Gao and Dr. Ramos reported no relevant financial relationships. Although Dr. Gao’s lab receives funding from the National Institutes of Health, the BrightFocus Foundation, the University of Manchester, the Medical Biodynamics Program, Brigham and Women’s Hospital, and the Broad Institute, the study itself does not have its own specific funding.
A version of this article originally appeared on Medscape.com.
FROM AAIC 2020
Patients with COPD plus sleep problems should be screened for mood disorders
A study has shown a strong link between sleeping disturbances and depression in patients with chronic obstructive pulmonary disease.
Adults with clinically stable COPD who reported sleep problems were significantly more likely to report depression or anxiety, poor self-efficacy, and poor health-related quality of life, compared with those not reporting sleep problems, according to the findings from a study of 245 patients.
Sleep problems are common in patients with COPD and have been associated with poor COPD-related outcomes, wrote Sang Hee Lee, MD, of Wonkwang University Sanbon Hospital, Gunpo-si, South Korea, and colleagues.
“However, there is a lack of research on factors associated with sleep disturbance in patients with COPD,” they wrote.
In a prospective, multicenter, cross-sectional study published in the Clinical Respiratory Journal, the researchers enrolled 245 adults with COPD who completed the COPD and Asthma Impact Scale (CASIS) to determine sleep impairment. The CASIS was developed to measure sleep-related problems associated with respiratory disease, and scored on a scale of 1-100, with higher scores indicating greater sleep impairment. The average CASIS score was 40.9. The average age of the patients was 67 years, and 92% were men.
Patients’ health-related quality of life, anxiety/depression, and self-efficacy were assessed using the St. George’s Respiratory Questionnaire (SGRQ), the 36-item Short-Form Health Survey (SF-36), Hospital Anxiety and Depression Scale (HADS), and the COPD Self-Efficacy Scale (CSES). The average scores on these measures were 36.0 for the SGRQ; 48.1 and 50.6, respectively, for the physical and mental components of the SF-36; 3.8 and 6.4, respectively, for the HADS-A and HADS-D measures of anxiety and depression; and 3.3 on the CSES.
Worse sleep in these patients was associated with worse scores on measures of mood. In a multivariate analysis, higher scores on all four measures of health-related quality of life were significantly associated with higher CASIS scores (P = .006 for SGRQ; P = .037 for SF-36, P < .001 for HADS, and P = .010 for CSES).
Although the CASIS did not allow for measurement of symptom severity and did not include many items related to breathing problems, the test “shows good internal consistency, test-retest reproducibility, and construct validity according to previous studies,” the researchers wrote. “The CASIS may be a good tool for evaluating sleep disturbances in COPD patients, and further study is needed,” they added.
The study findings were limited by several factors including the cross-sectional study design, lack of data on obstructive sleep apnea, and lack of information on specific treatments such as at-home oxygen use or high-dose steroid use, the researchers noted. However, the results were strengthened by the use of a disease-specific sleep measure, and the study is the first known to include self-efficacy in relation to sleep quality in COPD patients, they reported.
The results highlight the association between depression, poor quality of life, and self-efficacy in relation to poor sleep, and suggest that “Sleep quality could be improved by enhancing HRQL and self-efficacy,” the researchers said. “Screening for mood disorder in patients with COPD is also needed,” they concluded.
The study was supported by the Basic Science Research Program of the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology. The researchers had no financial conflicts to disclose.
SOURCE: Lee SH et al. Clin Respir J. 2020 Jul 24. doi: 10.1111/crj.13235.
A study has shown a strong link between sleeping disturbances and depression in patients with chronic obstructive pulmonary disease.
Adults with clinically stable COPD who reported sleep problems were significantly more likely to report depression or anxiety, poor self-efficacy, and poor health-related quality of life, compared with those not reporting sleep problems, according to the findings from a study of 245 patients.
Sleep problems are common in patients with COPD and have been associated with poor COPD-related outcomes, wrote Sang Hee Lee, MD, of Wonkwang University Sanbon Hospital, Gunpo-si, South Korea, and colleagues.
“However, there is a lack of research on factors associated with sleep disturbance in patients with COPD,” they wrote.
In a prospective, multicenter, cross-sectional study published in the Clinical Respiratory Journal, the researchers enrolled 245 adults with COPD who completed the COPD and Asthma Impact Scale (CASIS) to determine sleep impairment. The CASIS was developed to measure sleep-related problems associated with respiratory disease, and scored on a scale of 1-100, with higher scores indicating greater sleep impairment. The average CASIS score was 40.9. The average age of the patients was 67 years, and 92% were men.
Patients’ health-related quality of life, anxiety/depression, and self-efficacy were assessed using the St. George’s Respiratory Questionnaire (SGRQ), the 36-item Short-Form Health Survey (SF-36), Hospital Anxiety and Depression Scale (HADS), and the COPD Self-Efficacy Scale (CSES). The average scores on these measures were 36.0 for the SGRQ; 48.1 and 50.6, respectively, for the physical and mental components of the SF-36; 3.8 and 6.4, respectively, for the HADS-A and HADS-D measures of anxiety and depression; and 3.3 on the CSES.
Worse sleep in these patients was associated with worse scores on measures of mood. In a multivariate analysis, higher scores on all four measures of health-related quality of life were significantly associated with higher CASIS scores (P = .006 for SGRQ; P = .037 for SF-36, P < .001 for HADS, and P = .010 for CSES).
Although the CASIS did not allow for measurement of symptom severity and did not include many items related to breathing problems, the test “shows good internal consistency, test-retest reproducibility, and construct validity according to previous studies,” the researchers wrote. “The CASIS may be a good tool for evaluating sleep disturbances in COPD patients, and further study is needed,” they added.
The study findings were limited by several factors including the cross-sectional study design, lack of data on obstructive sleep apnea, and lack of information on specific treatments such as at-home oxygen use or high-dose steroid use, the researchers noted. However, the results were strengthened by the use of a disease-specific sleep measure, and the study is the first known to include self-efficacy in relation to sleep quality in COPD patients, they reported.
The results highlight the association between depression, poor quality of life, and self-efficacy in relation to poor sleep, and suggest that “Sleep quality could be improved by enhancing HRQL and self-efficacy,” the researchers said. “Screening for mood disorder in patients with COPD is also needed,” they concluded.
The study was supported by the Basic Science Research Program of the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology. The researchers had no financial conflicts to disclose.
SOURCE: Lee SH et al. Clin Respir J. 2020 Jul 24. doi: 10.1111/crj.13235.
A study has shown a strong link between sleeping disturbances and depression in patients with chronic obstructive pulmonary disease.
Adults with clinically stable COPD who reported sleep problems were significantly more likely to report depression or anxiety, poor self-efficacy, and poor health-related quality of life, compared with those not reporting sleep problems, according to the findings from a study of 245 patients.
Sleep problems are common in patients with COPD and have been associated with poor COPD-related outcomes, wrote Sang Hee Lee, MD, of Wonkwang University Sanbon Hospital, Gunpo-si, South Korea, and colleagues.
“However, there is a lack of research on factors associated with sleep disturbance in patients with COPD,” they wrote.
In a prospective, multicenter, cross-sectional study published in the Clinical Respiratory Journal, the researchers enrolled 245 adults with COPD who completed the COPD and Asthma Impact Scale (CASIS) to determine sleep impairment. The CASIS was developed to measure sleep-related problems associated with respiratory disease, and scored on a scale of 1-100, with higher scores indicating greater sleep impairment. The average CASIS score was 40.9. The average age of the patients was 67 years, and 92% were men.
Patients’ health-related quality of life, anxiety/depression, and self-efficacy were assessed using the St. George’s Respiratory Questionnaire (SGRQ), the 36-item Short-Form Health Survey (SF-36), Hospital Anxiety and Depression Scale (HADS), and the COPD Self-Efficacy Scale (CSES). The average scores on these measures were 36.0 for the SGRQ; 48.1 and 50.6, respectively, for the physical and mental components of the SF-36; 3.8 and 6.4, respectively, for the HADS-A and HADS-D measures of anxiety and depression; and 3.3 on the CSES.
Worse sleep in these patients was associated with worse scores on measures of mood. In a multivariate analysis, higher scores on all four measures of health-related quality of life were significantly associated with higher CASIS scores (P = .006 for SGRQ; P = .037 for SF-36, P < .001 for HADS, and P = .010 for CSES).
Although the CASIS did not allow for measurement of symptom severity and did not include many items related to breathing problems, the test “shows good internal consistency, test-retest reproducibility, and construct validity according to previous studies,” the researchers wrote. “The CASIS may be a good tool for evaluating sleep disturbances in COPD patients, and further study is needed,” they added.
The study findings were limited by several factors including the cross-sectional study design, lack of data on obstructive sleep apnea, and lack of information on specific treatments such as at-home oxygen use or high-dose steroid use, the researchers noted. However, the results were strengthened by the use of a disease-specific sleep measure, and the study is the first known to include self-efficacy in relation to sleep quality in COPD patients, they reported.
The results highlight the association between depression, poor quality of life, and self-efficacy in relation to poor sleep, and suggest that “Sleep quality could be improved by enhancing HRQL and self-efficacy,” the researchers said. “Screening for mood disorder in patients with COPD is also needed,” they concluded.
The study was supported by the Basic Science Research Program of the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology. The researchers had no financial conflicts to disclose.
SOURCE: Lee SH et al. Clin Respir J. 2020 Jul 24. doi: 10.1111/crj.13235.
FROM THE CLINICAL RESPIRATORY JOURNAL