User login
Sleepless in the pandemic
Sleep difficulties during the COVID-19 crisis may be exacerbated by media overexposure and other factors causing fear and stress, according to findings from a large survey of French individuals.
“Physicians usually recommend coping with sleep disorders by exercising, going outside, avoiding screen time, and having a regular schedule – all recommendations difficult to apply during lockdown. Being forced to stay home and the ensuing boredom and loneliness may have led to increased [media exposure], especially among disadvantaged people and overexposure to media COVID-19 content may have contributed to fright and emotional distress,” Damien Leger of the Centre du Sommeil et de la Vigilance, Hôtel Dieu APHP, Université de Paris, and his colleagues wrote in the journal Sleep.
The investigators analyzed data from survey respondents about their sleep problems since the COVID-19 lockdown and other topics such as employment, daily activities, and sleep medications. The survey was part of a large research project, COCONEL, that has been developed to study the French population on a variety of behaviors and comprises 750,000 permanent panelists who respond to surveys. The survey was sent to random sample of panelists with no topic label to avoid selection bias. Of the 25,800 surveys sent, 1,005 responses were recorded.
Respondents were classified as having severe sleep problems if they reported that their daytime activities were affected or if their sleeping medications had increased since the lockdown. While 73% of respondents reported poor sleep in the 8 previous days, 25% reported severe sleep problems, and 54% reported that their sleep problems had worsened during the COVID-19 lockdown.
A media exposure score was created with a Likert scale (strongly agree, agree, disagree, strongly disagree) about media exposures of different types. The investigators also queried respondents about the degree to which they found media coverage of the pandemic provoked a fear response. Overall, 68% of respondents agreed that media images and stories about COVD-19 were frightening.
The researchers found a strong association between severe sleeping problems and a high media exposure score (risk ratio, 1.49; 95% confidence interval, 1.10-2.01; P < .05).
In addition, trepidation and fear from media exposure to COVID-19 news were also associated with severe sleep problems (RR, 1.27; 95% CI, 0.92-1.75; P < .05). “Suffering from sleep problems may have increased media use at night, and thus increased stress and/or psychological distress and reinforced sleeping problems,” the investigators wrote.
Not surprisingly, respondents with financial difficulties due to the pandemic also reported severe sleeping difficulties (RR, 1.99; 95% CI, 1.49-2.65; P < .05).
For individuals who have been treated for sleep problems, the COVID-19 pandemic may ratchet up their sleep challenges. The strongest association with severe sleep problems was found in those respondents who were already taking sleeping medications before the pandemic (RR, 2.72; 95% CI, 2.04-3.61; P < .05).
The COCONEL survey has been funded by the French and National Agency for Research, the Fondation de France, and the National Research Institute for Sustainable Development.
SOURCE: Leger D et al. Sleep. 2020, Jul 25. doi: 10.1093/sleep/zsaa125.
Sleep difficulties during the COVID-19 crisis may be exacerbated by media overexposure and other factors causing fear and stress, according to findings from a large survey of French individuals.
“Physicians usually recommend coping with sleep disorders by exercising, going outside, avoiding screen time, and having a regular schedule – all recommendations difficult to apply during lockdown. Being forced to stay home and the ensuing boredom and loneliness may have led to increased [media exposure], especially among disadvantaged people and overexposure to media COVID-19 content may have contributed to fright and emotional distress,” Damien Leger of the Centre du Sommeil et de la Vigilance, Hôtel Dieu APHP, Université de Paris, and his colleagues wrote in the journal Sleep.
The investigators analyzed data from survey respondents about their sleep problems since the COVID-19 lockdown and other topics such as employment, daily activities, and sleep medications. The survey was part of a large research project, COCONEL, that has been developed to study the French population on a variety of behaviors and comprises 750,000 permanent panelists who respond to surveys. The survey was sent to random sample of panelists with no topic label to avoid selection bias. Of the 25,800 surveys sent, 1,005 responses were recorded.
Respondents were classified as having severe sleep problems if they reported that their daytime activities were affected or if their sleeping medications had increased since the lockdown. While 73% of respondents reported poor sleep in the 8 previous days, 25% reported severe sleep problems, and 54% reported that their sleep problems had worsened during the COVID-19 lockdown.
A media exposure score was created with a Likert scale (strongly agree, agree, disagree, strongly disagree) about media exposures of different types. The investigators also queried respondents about the degree to which they found media coverage of the pandemic provoked a fear response. Overall, 68% of respondents agreed that media images and stories about COVD-19 were frightening.
The researchers found a strong association between severe sleeping problems and a high media exposure score (risk ratio, 1.49; 95% confidence interval, 1.10-2.01; P < .05).
In addition, trepidation and fear from media exposure to COVID-19 news were also associated with severe sleep problems (RR, 1.27; 95% CI, 0.92-1.75; P < .05). “Suffering from sleep problems may have increased media use at night, and thus increased stress and/or psychological distress and reinforced sleeping problems,” the investigators wrote.
Not surprisingly, respondents with financial difficulties due to the pandemic also reported severe sleeping difficulties (RR, 1.99; 95% CI, 1.49-2.65; P < .05).
For individuals who have been treated for sleep problems, the COVID-19 pandemic may ratchet up their sleep challenges. The strongest association with severe sleep problems was found in those respondents who were already taking sleeping medications before the pandemic (RR, 2.72; 95% CI, 2.04-3.61; P < .05).
The COCONEL survey has been funded by the French and National Agency for Research, the Fondation de France, and the National Research Institute for Sustainable Development.
SOURCE: Leger D et al. Sleep. 2020, Jul 25. doi: 10.1093/sleep/zsaa125.
Sleep difficulties during the COVID-19 crisis may be exacerbated by media overexposure and other factors causing fear and stress, according to findings from a large survey of French individuals.
“Physicians usually recommend coping with sleep disorders by exercising, going outside, avoiding screen time, and having a regular schedule – all recommendations difficult to apply during lockdown. Being forced to stay home and the ensuing boredom and loneliness may have led to increased [media exposure], especially among disadvantaged people and overexposure to media COVID-19 content may have contributed to fright and emotional distress,” Damien Leger of the Centre du Sommeil et de la Vigilance, Hôtel Dieu APHP, Université de Paris, and his colleagues wrote in the journal Sleep.
The investigators analyzed data from survey respondents about their sleep problems since the COVID-19 lockdown and other topics such as employment, daily activities, and sleep medications. The survey was part of a large research project, COCONEL, that has been developed to study the French population on a variety of behaviors and comprises 750,000 permanent panelists who respond to surveys. The survey was sent to random sample of panelists with no topic label to avoid selection bias. Of the 25,800 surveys sent, 1,005 responses were recorded.
Respondents were classified as having severe sleep problems if they reported that their daytime activities were affected or if their sleeping medications had increased since the lockdown. While 73% of respondents reported poor sleep in the 8 previous days, 25% reported severe sleep problems, and 54% reported that their sleep problems had worsened during the COVID-19 lockdown.
A media exposure score was created with a Likert scale (strongly agree, agree, disagree, strongly disagree) about media exposures of different types. The investigators also queried respondents about the degree to which they found media coverage of the pandemic provoked a fear response. Overall, 68% of respondents agreed that media images and stories about COVD-19 were frightening.
The researchers found a strong association between severe sleeping problems and a high media exposure score (risk ratio, 1.49; 95% confidence interval, 1.10-2.01; P < .05).
In addition, trepidation and fear from media exposure to COVID-19 news were also associated with severe sleep problems (RR, 1.27; 95% CI, 0.92-1.75; P < .05). “Suffering from sleep problems may have increased media use at night, and thus increased stress and/or psychological distress and reinforced sleeping problems,” the investigators wrote.
Not surprisingly, respondents with financial difficulties due to the pandemic also reported severe sleeping difficulties (RR, 1.99; 95% CI, 1.49-2.65; P < .05).
For individuals who have been treated for sleep problems, the COVID-19 pandemic may ratchet up their sleep challenges. The strongest association with severe sleep problems was found in those respondents who were already taking sleeping medications before the pandemic (RR, 2.72; 95% CI, 2.04-3.61; P < .05).
The COCONEL survey has been funded by the French and National Agency for Research, the Fondation de France, and the National Research Institute for Sustainable Development.
SOURCE: Leger D et al. Sleep. 2020, Jul 25. doi: 10.1093/sleep/zsaa125.
FROM SLEEP
Pandemic-related stress causing health issues in many Americans
Over the last 2 months, more than half of Americans have experienced some sort of adverse effect caused by stress related to the COVID-19 pandemic, according to a survey from the Kaiser Family Foundation (KFF).
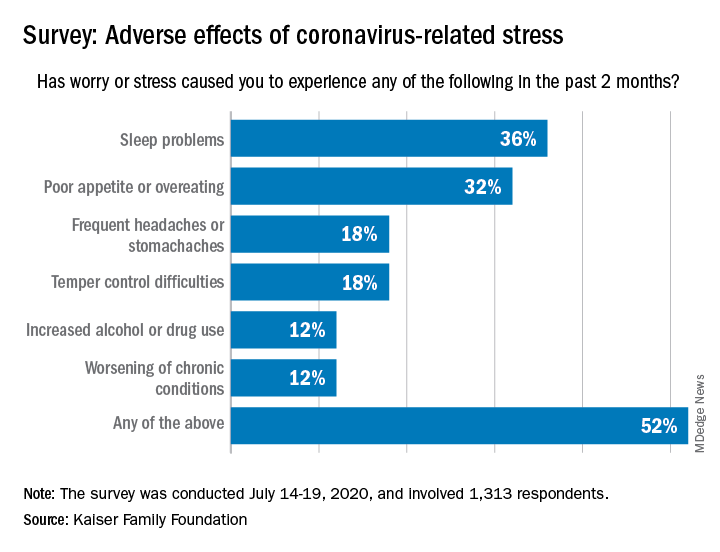
More than a third (36%) of the 1,313 respondents said they either had difficulty sleeping, falling asleep, or sleeping too much, KFF said in its latest Health Tracking Poll, conducted July 14-19, 2020. That was followed by poor appetite or overeating, which was mentioned by 32% of those surveyed.
Other adverse effects included frequent headaches or stomachaches (18%), temper-control issues (18%), increased drug or alcohol use (12%), and worsening of chronic conditions such as diabetes or hypertension (12%). Altogether, 52% of Americans have had at least one of these issues in the past 2 months, Liz Hamel and associates at KFF reported.
breaking down to 26% reporting a major impact and 28% reporting a minor impact (figures have been rounded), they said.
“As life with the coronavirus pandemic wears on, Americans increasingly say it is taking a negative toll on their mental health,” the investigators wrote. Earlier polls showed that pandemic-related stress was having an impact on mental health for 39% of respondents in May, compared with 45% in early April and 32% in March.
In the July poll, Black adults were much more likely to report a negative mental health impact (68%) than were Hispanics or Whites, who were both at 51%. Age was also a factor: The youngest group of respondents (ages 18-29 years) had the highest negative-impact rate (62%), and the oldest group (65 years and older) had the lowest (47%), they said.
When it came to reporting the adverse effects of stress or worry, however, the situation was somewhat different. Hispanics had the highest rate of such effects at 63%, while Blacks had a rate of 57% and 47% of Whites reported issues with sleep, eating, temper, and other problems, Ms. Hamel and associates reported.
Over the last 2 months, more than half of Americans have experienced some sort of adverse effect caused by stress related to the COVID-19 pandemic, according to a survey from the Kaiser Family Foundation (KFF).

More than a third (36%) of the 1,313 respondents said they either had difficulty sleeping, falling asleep, or sleeping too much, KFF said in its latest Health Tracking Poll, conducted July 14-19, 2020. That was followed by poor appetite or overeating, which was mentioned by 32% of those surveyed.
Other adverse effects included frequent headaches or stomachaches (18%), temper-control issues (18%), increased drug or alcohol use (12%), and worsening of chronic conditions such as diabetes or hypertension (12%). Altogether, 52% of Americans have had at least one of these issues in the past 2 months, Liz Hamel and associates at KFF reported.
breaking down to 26% reporting a major impact and 28% reporting a minor impact (figures have been rounded), they said.
“As life with the coronavirus pandemic wears on, Americans increasingly say it is taking a negative toll on their mental health,” the investigators wrote. Earlier polls showed that pandemic-related stress was having an impact on mental health for 39% of respondents in May, compared with 45% in early April and 32% in March.
In the July poll, Black adults were much more likely to report a negative mental health impact (68%) than were Hispanics or Whites, who were both at 51%. Age was also a factor: The youngest group of respondents (ages 18-29 years) had the highest negative-impact rate (62%), and the oldest group (65 years and older) had the lowest (47%), they said.
When it came to reporting the adverse effects of stress or worry, however, the situation was somewhat different. Hispanics had the highest rate of such effects at 63%, while Blacks had a rate of 57% and 47% of Whites reported issues with sleep, eating, temper, and other problems, Ms. Hamel and associates reported.
Over the last 2 months, more than half of Americans have experienced some sort of adverse effect caused by stress related to the COVID-19 pandemic, according to a survey from the Kaiser Family Foundation (KFF).

More than a third (36%) of the 1,313 respondents said they either had difficulty sleeping, falling asleep, or sleeping too much, KFF said in its latest Health Tracking Poll, conducted July 14-19, 2020. That was followed by poor appetite or overeating, which was mentioned by 32% of those surveyed.
Other adverse effects included frequent headaches or stomachaches (18%), temper-control issues (18%), increased drug or alcohol use (12%), and worsening of chronic conditions such as diabetes or hypertension (12%). Altogether, 52% of Americans have had at least one of these issues in the past 2 months, Liz Hamel and associates at KFF reported.
breaking down to 26% reporting a major impact and 28% reporting a minor impact (figures have been rounded), they said.
“As life with the coronavirus pandemic wears on, Americans increasingly say it is taking a negative toll on their mental health,” the investigators wrote. Earlier polls showed that pandemic-related stress was having an impact on mental health for 39% of respondents in May, compared with 45% in early April and 32% in March.
In the July poll, Black adults were much more likely to report a negative mental health impact (68%) than were Hispanics or Whites, who were both at 51%. Age was also a factor: The youngest group of respondents (ages 18-29 years) had the highest negative-impact rate (62%), and the oldest group (65 years and older) had the lowest (47%), they said.
When it came to reporting the adverse effects of stress or worry, however, the situation was somewhat different. Hispanics had the highest rate of such effects at 63%, while Blacks had a rate of 57% and 47% of Whites reported issues with sleep, eating, temper, and other problems, Ms. Hamel and associates reported.
FDA approves low-sodium treatment option for narcolepsy
Xywav is a novel oxybate product with a unique composition of cations, resulting in 92% less sodium than sodium oxybate (Xyrem, Jazz Pharmaceuticals) at the recommended dosage range of 6 to 9 grams, the company said in a news release.
The FDA approved the drug based on a phase 3 trial involving 201 patients who had narcolepsy with cataplexy.
As reported by Medscape Medical News from the World Sleep 2019 meeting, Xywav demonstrated highly statistically significant differences (P < .0001) in weekly number of cataplexy attacks (primary efficacy endpoint) and Epworth Sleepiness Scale scores (key secondary outcome) vs placebo.
“Based on the efficacy demonstrated in the clinical program, the approval of Xywav is important for people living with cataplexy or EDS associated with narcolepsy,” lead investigator Richard K. Bogan, MD, said in the company’s news release.
He noted that the average American consumes too much sodium. “Excess sodium intake has been linked with increases in blood pressure, hypertension, stroke, and other cardiovascular disease,” said Dr. Bogan, associate clinical professor at the University of South Carolina School of Medicine, Columbia.
“Xywav makes it possible for patients to have a lower-sodium oxybate treatment option. This may help patients taking sodium oxybate better align with daily sodium intake recommendations, including those by the American Heart Association,” he added.
The overall safety profile of Xywav is in line with sodium oxybate, the company said. The most common adverse reactions in adults, occurring in at least 5% of participants, were headache, nausea, dizziness, decreased appetite, parasomnia, diarrhea, hyperhidrosis (excessive sweating), anxiety, and vomiting.
Xywav has a boxed warning as a CNS depressant and for its potential for abuse and misuse. As a result, the drug is only available through a Risk Evaluation and Mitigation Strategy (REMS) program.
The US Drug Enforcement Agency has designated Xywav as a schedule III drug, meaning it has a moderate to low potential for physical and psychological dependence.
The company plans to launch Xywav by the end of the year. Full prescribing information and a medication guide are available online.
This article first appeared on Medscape.com.
Xywav is a novel oxybate product with a unique composition of cations, resulting in 92% less sodium than sodium oxybate (Xyrem, Jazz Pharmaceuticals) at the recommended dosage range of 6 to 9 grams, the company said in a news release.
The FDA approved the drug based on a phase 3 trial involving 201 patients who had narcolepsy with cataplexy.
As reported by Medscape Medical News from the World Sleep 2019 meeting, Xywav demonstrated highly statistically significant differences (P < .0001) in weekly number of cataplexy attacks (primary efficacy endpoint) and Epworth Sleepiness Scale scores (key secondary outcome) vs placebo.
“Based on the efficacy demonstrated in the clinical program, the approval of Xywav is important for people living with cataplexy or EDS associated with narcolepsy,” lead investigator Richard K. Bogan, MD, said in the company’s news release.
He noted that the average American consumes too much sodium. “Excess sodium intake has been linked with increases in blood pressure, hypertension, stroke, and other cardiovascular disease,” said Dr. Bogan, associate clinical professor at the University of South Carolina School of Medicine, Columbia.
“Xywav makes it possible for patients to have a lower-sodium oxybate treatment option. This may help patients taking sodium oxybate better align with daily sodium intake recommendations, including those by the American Heart Association,” he added.
The overall safety profile of Xywav is in line with sodium oxybate, the company said. The most common adverse reactions in adults, occurring in at least 5% of participants, were headache, nausea, dizziness, decreased appetite, parasomnia, diarrhea, hyperhidrosis (excessive sweating), anxiety, and vomiting.
Xywav has a boxed warning as a CNS depressant and for its potential for abuse and misuse. As a result, the drug is only available through a Risk Evaluation and Mitigation Strategy (REMS) program.
The US Drug Enforcement Agency has designated Xywav as a schedule III drug, meaning it has a moderate to low potential for physical and psychological dependence.
The company plans to launch Xywav by the end of the year. Full prescribing information and a medication guide are available online.
This article first appeared on Medscape.com.
Xywav is a novel oxybate product with a unique composition of cations, resulting in 92% less sodium than sodium oxybate (Xyrem, Jazz Pharmaceuticals) at the recommended dosage range of 6 to 9 grams, the company said in a news release.
The FDA approved the drug based on a phase 3 trial involving 201 patients who had narcolepsy with cataplexy.
As reported by Medscape Medical News from the World Sleep 2019 meeting, Xywav demonstrated highly statistically significant differences (P < .0001) in weekly number of cataplexy attacks (primary efficacy endpoint) and Epworth Sleepiness Scale scores (key secondary outcome) vs placebo.
“Based on the efficacy demonstrated in the clinical program, the approval of Xywav is important for people living with cataplexy or EDS associated with narcolepsy,” lead investigator Richard K. Bogan, MD, said in the company’s news release.
He noted that the average American consumes too much sodium. “Excess sodium intake has been linked with increases in blood pressure, hypertension, stroke, and other cardiovascular disease,” said Dr. Bogan, associate clinical professor at the University of South Carolina School of Medicine, Columbia.
“Xywav makes it possible for patients to have a lower-sodium oxybate treatment option. This may help patients taking sodium oxybate better align with daily sodium intake recommendations, including those by the American Heart Association,” he added.
The overall safety profile of Xywav is in line with sodium oxybate, the company said. The most common adverse reactions in adults, occurring in at least 5% of participants, were headache, nausea, dizziness, decreased appetite, parasomnia, diarrhea, hyperhidrosis (excessive sweating), anxiety, and vomiting.
Xywav has a boxed warning as a CNS depressant and for its potential for abuse and misuse. As a result, the drug is only available through a Risk Evaluation and Mitigation Strategy (REMS) program.
The US Drug Enforcement Agency has designated Xywav as a schedule III drug, meaning it has a moderate to low potential for physical and psychological dependence.
The company plans to launch Xywav by the end of the year. Full prescribing information and a medication guide are available online.
This article first appeared on Medscape.com.
Socioeconomic status key factor in CPAP adherence in older adults
The benefits of continuous positive airway pressure therapy for patients with obstructive sleep apnea are well documented, but it only works if patients can adhere to the therapy.
A large national study of older Medicare patients with obstructive sleep apnea (OSA) has identified lower socioeconomic status and comorbidities as independent risk factors for nonadherence to continuous positive airway pressure (CPAP) therapy.
“[The] present results represent the largest study to date of rates and predictors of CPAP adherence among older adults in the United States. In our national sample of Medicare beneficiaries, adherence rates were generally lower than previously reported in smaller, clinic-based studies,” Emerson M. Wickwire, PhD, of the Sleep Disorders Center and division of pulmonary and critical care medicine at the University of Maryland, Baltimore, and colleagues wrote in Sleep.
Dr. Wickwire and colleagues estimated CPAP machine adherence using a 5% sample of Medicare claims data, identifying 3,229 Medicare beneficiaries with OSA who began CPAP therapy between 2009 and 2011. Individuals in the sample were aged at least 65 years with a new diagnosis of OSA, 88.1% of beneficiaries were white, and 52.3% were male.
The researchers applied objective adherence criteria set by the Centers for Medicare & Medicaid Services, which defines CPAP adherence as a patient using CPAP for at least 4 hours on 70% of nights, or CPAP use for 21 of 30 consecutive days within 90 days after beginning therapy.
Using CPAP machine charges as a measure of who adhered to therapy, they found 1,420 of 3,229 individuals (44%) achieved adherence under these criteria, which included making 13 monthly payments during their CPAP machine’s “rent-to-own” period. Partial adherence was found in 997 individuals (30.9%) who made between 4-12 payments on their CPAP machine, while 812 individuals (25.2%) made 4 payments or fewer on their CPAP machines, which the researchers classified as nonadherence. Nonadherers tended to be slightly younger (mean, 72.5 years vs. 79.2 years; P < .001) and had a higher number of comorbidities (35.2% vs. 30.4%; P = .002), compared with individuals with high adherence. Anxiety (odds ratio, 1.34; 95% confidence interval, 1.12-1.61), anemia (OR, 1.16; 95% CI, 1.02-1.32), fibromyalgia (OR, 1.19; 95% CI, 1.03-1.38), traumatic brain injury (OR, 1.58; 95% CI, 1.21-2.07), and Medicaid eligibility (OR, 1.48; 95% CI, 1.24-1.75) were all independently associated with lower CPAP adherence. Medicaid eligibility was considered an indicator of lower socioeconomic status.
Krishna M. Sundar, MD, FCCP, director at the Sleep-Wake Center in the University of Utah pulmonary division in Salt Lake City and CHEST Physician editorial board member, said in an interview that studies have shown early signs of adherence within the first few weeks are an important indicator of overall adherence to CPAP therapy. However, the use of CPAP machine payments in the study by Dr. Wickwire and colleagues was a novel way to track adherence.
Some of the issues with nonadherence may be related to challenges in using the technology, but it is the clinician’s role to communicate with patients about the effectiveness of CPAP and identifying reasons for nonadherence while also attempting to tease out the subtle socioeconomic factors related to nonadherence, Dr. Sundar noted. “We need to alter our practice to make sure that we communicate with these patients and better understand what are the social factors in getting the CPAP or utilizing CPAP, and also following these patients more closely, especially in the first month of starting CPAP therapy.
“Just because somebody has severe sleep apnea and other comorbid conditions does not mean that they’re going to wear the CPAP,” he said. “So, the fact that socioeconomic factors play an equal if not more important role in terms of predicting CPAP adherence. That is an important takeaway.”
Octavian C. Ioachimescu, MD, FCCP, of Emory University, Atlanta, and the Atlanta Veteran Affairs Administration and CHEST Physician editorial board member, said in an interview that the study raises a major question of what is next. “What can we offer to these patients, and what is the real-world compliance to that ‘next-best’ modality?” Dr. Ioachimescu said. “What are the outcomes of these individuals in the point-of-care environment, or ‘real world?’ ”
The analysis by the authors adds the perspective of a “real-world depiction of clinical care for patients with OSA,” Dr. Ioachimescu said. “One major lesson of such an analysis is that the health care goal setting that is referential to initial, randomized, well-controlled studies on highly selected patient populations need to be reassessed periodically from the point of view of actual results in the clinics.”
Clinicians may need to borrow ideas from other therapeutic fields to help improve patient adherence, he said. “[W]e may be able to develop and implement in the future peer involvement, behavioral and cognitive approaches, motivational enhancement interventions, as well as elements of acceptance and commitment techniques, all in the larger context of more integrated and in the same time individualized approaches to therapy.”
The investigators concluded that, “relative to Medicare-only beneficiaries, those eligible for both Medicare and Medicaid were significantly less likely to adhere to CPAP. Future research should seek to develop a deeper understanding of the mechanisms through which [socioeconomic status] and other social determinants impact patient experience throughout the OSA diagnostic and treatment process, including receiving, acclimating, and adhering to CPAP therapy.”
Dr. Sundar concurred with this assessment and said more research is needed on factors impacting adherence such as poverty, homelessness, and home support systems. “It’s not just coordinating with the patient. Clearly, more work is needed in understanding the social aspects of CPAP adherence.”
This study was funded in part by an investigator-initiated grant provided by ResMed to Dr. Wickmire’s institution, the University of Maryland, Baltimore. Dr. Wickmire reported being a scientific consultant to DayZz, Eisai, Merck, and Purdue and holds shares in WellTap. Dr. Oldstone is a ResMed employee and shareholder. Dr. Sundar reported being a cofounder of Hypnoscure, which creates software for population management of sleep apnea, and an investigator in trials where ResMed and Respironics devices were used. Dr. Ioachimescu reported no relevant financial disclosures.
SOURCE: Wickwire EM et al. Sleep. 2020 Jun 23. doi: 10.1093/sleep/zsaa122.
The benefits of continuous positive airway pressure therapy for patients with obstructive sleep apnea are well documented, but it only works if patients can adhere to the therapy.
A large national study of older Medicare patients with obstructive sleep apnea (OSA) has identified lower socioeconomic status and comorbidities as independent risk factors for nonadherence to continuous positive airway pressure (CPAP) therapy.
“[The] present results represent the largest study to date of rates and predictors of CPAP adherence among older adults in the United States. In our national sample of Medicare beneficiaries, adherence rates were generally lower than previously reported in smaller, clinic-based studies,” Emerson M. Wickwire, PhD, of the Sleep Disorders Center and division of pulmonary and critical care medicine at the University of Maryland, Baltimore, and colleagues wrote in Sleep.
Dr. Wickwire and colleagues estimated CPAP machine adherence using a 5% sample of Medicare claims data, identifying 3,229 Medicare beneficiaries with OSA who began CPAP therapy between 2009 and 2011. Individuals in the sample were aged at least 65 years with a new diagnosis of OSA, 88.1% of beneficiaries were white, and 52.3% were male.
The researchers applied objective adherence criteria set by the Centers for Medicare & Medicaid Services, which defines CPAP adherence as a patient using CPAP for at least 4 hours on 70% of nights, or CPAP use for 21 of 30 consecutive days within 90 days after beginning therapy.
Using CPAP machine charges as a measure of who adhered to therapy, they found 1,420 of 3,229 individuals (44%) achieved adherence under these criteria, which included making 13 monthly payments during their CPAP machine’s “rent-to-own” period. Partial adherence was found in 997 individuals (30.9%) who made between 4-12 payments on their CPAP machine, while 812 individuals (25.2%) made 4 payments or fewer on their CPAP machines, which the researchers classified as nonadherence. Nonadherers tended to be slightly younger (mean, 72.5 years vs. 79.2 years; P < .001) and had a higher number of comorbidities (35.2% vs. 30.4%; P = .002), compared with individuals with high adherence. Anxiety (odds ratio, 1.34; 95% confidence interval, 1.12-1.61), anemia (OR, 1.16; 95% CI, 1.02-1.32), fibromyalgia (OR, 1.19; 95% CI, 1.03-1.38), traumatic brain injury (OR, 1.58; 95% CI, 1.21-2.07), and Medicaid eligibility (OR, 1.48; 95% CI, 1.24-1.75) were all independently associated with lower CPAP adherence. Medicaid eligibility was considered an indicator of lower socioeconomic status.
Krishna M. Sundar, MD, FCCP, director at the Sleep-Wake Center in the University of Utah pulmonary division in Salt Lake City and CHEST Physician editorial board member, said in an interview that studies have shown early signs of adherence within the first few weeks are an important indicator of overall adherence to CPAP therapy. However, the use of CPAP machine payments in the study by Dr. Wickwire and colleagues was a novel way to track adherence.
Some of the issues with nonadherence may be related to challenges in using the technology, but it is the clinician’s role to communicate with patients about the effectiveness of CPAP and identifying reasons for nonadherence while also attempting to tease out the subtle socioeconomic factors related to nonadherence, Dr. Sundar noted. “We need to alter our practice to make sure that we communicate with these patients and better understand what are the social factors in getting the CPAP or utilizing CPAP, and also following these patients more closely, especially in the first month of starting CPAP therapy.
“Just because somebody has severe sleep apnea and other comorbid conditions does not mean that they’re going to wear the CPAP,” he said. “So, the fact that socioeconomic factors play an equal if not more important role in terms of predicting CPAP adherence. That is an important takeaway.”
Octavian C. Ioachimescu, MD, FCCP, of Emory University, Atlanta, and the Atlanta Veteran Affairs Administration and CHEST Physician editorial board member, said in an interview that the study raises a major question of what is next. “What can we offer to these patients, and what is the real-world compliance to that ‘next-best’ modality?” Dr. Ioachimescu said. “What are the outcomes of these individuals in the point-of-care environment, or ‘real world?’ ”
The analysis by the authors adds the perspective of a “real-world depiction of clinical care for patients with OSA,” Dr. Ioachimescu said. “One major lesson of such an analysis is that the health care goal setting that is referential to initial, randomized, well-controlled studies on highly selected patient populations need to be reassessed periodically from the point of view of actual results in the clinics.”
Clinicians may need to borrow ideas from other therapeutic fields to help improve patient adherence, he said. “[W]e may be able to develop and implement in the future peer involvement, behavioral and cognitive approaches, motivational enhancement interventions, as well as elements of acceptance and commitment techniques, all in the larger context of more integrated and in the same time individualized approaches to therapy.”
The investigators concluded that, “relative to Medicare-only beneficiaries, those eligible for both Medicare and Medicaid were significantly less likely to adhere to CPAP. Future research should seek to develop a deeper understanding of the mechanisms through which [socioeconomic status] and other social determinants impact patient experience throughout the OSA diagnostic and treatment process, including receiving, acclimating, and adhering to CPAP therapy.”
Dr. Sundar concurred with this assessment and said more research is needed on factors impacting adherence such as poverty, homelessness, and home support systems. “It’s not just coordinating with the patient. Clearly, more work is needed in understanding the social aspects of CPAP adherence.”
This study was funded in part by an investigator-initiated grant provided by ResMed to Dr. Wickmire’s institution, the University of Maryland, Baltimore. Dr. Wickmire reported being a scientific consultant to DayZz, Eisai, Merck, and Purdue and holds shares in WellTap. Dr. Oldstone is a ResMed employee and shareholder. Dr. Sundar reported being a cofounder of Hypnoscure, which creates software for population management of sleep apnea, and an investigator in trials where ResMed and Respironics devices were used. Dr. Ioachimescu reported no relevant financial disclosures.
SOURCE: Wickwire EM et al. Sleep. 2020 Jun 23. doi: 10.1093/sleep/zsaa122.
The benefits of continuous positive airway pressure therapy for patients with obstructive sleep apnea are well documented, but it only works if patients can adhere to the therapy.
A large national study of older Medicare patients with obstructive sleep apnea (OSA) has identified lower socioeconomic status and comorbidities as independent risk factors for nonadherence to continuous positive airway pressure (CPAP) therapy.
“[The] present results represent the largest study to date of rates and predictors of CPAP adherence among older adults in the United States. In our national sample of Medicare beneficiaries, adherence rates were generally lower than previously reported in smaller, clinic-based studies,” Emerson M. Wickwire, PhD, of the Sleep Disorders Center and division of pulmonary and critical care medicine at the University of Maryland, Baltimore, and colleagues wrote in Sleep.
Dr. Wickwire and colleagues estimated CPAP machine adherence using a 5% sample of Medicare claims data, identifying 3,229 Medicare beneficiaries with OSA who began CPAP therapy between 2009 and 2011. Individuals in the sample were aged at least 65 years with a new diagnosis of OSA, 88.1% of beneficiaries were white, and 52.3% were male.
The researchers applied objective adherence criteria set by the Centers for Medicare & Medicaid Services, which defines CPAP adherence as a patient using CPAP for at least 4 hours on 70% of nights, or CPAP use for 21 of 30 consecutive days within 90 days after beginning therapy.
Using CPAP machine charges as a measure of who adhered to therapy, they found 1,420 of 3,229 individuals (44%) achieved adherence under these criteria, which included making 13 monthly payments during their CPAP machine’s “rent-to-own” period. Partial adherence was found in 997 individuals (30.9%) who made between 4-12 payments on their CPAP machine, while 812 individuals (25.2%) made 4 payments or fewer on their CPAP machines, which the researchers classified as nonadherence. Nonadherers tended to be slightly younger (mean, 72.5 years vs. 79.2 years; P < .001) and had a higher number of comorbidities (35.2% vs. 30.4%; P = .002), compared with individuals with high adherence. Anxiety (odds ratio, 1.34; 95% confidence interval, 1.12-1.61), anemia (OR, 1.16; 95% CI, 1.02-1.32), fibromyalgia (OR, 1.19; 95% CI, 1.03-1.38), traumatic brain injury (OR, 1.58; 95% CI, 1.21-2.07), and Medicaid eligibility (OR, 1.48; 95% CI, 1.24-1.75) were all independently associated with lower CPAP adherence. Medicaid eligibility was considered an indicator of lower socioeconomic status.
Krishna M. Sundar, MD, FCCP, director at the Sleep-Wake Center in the University of Utah pulmonary division in Salt Lake City and CHEST Physician editorial board member, said in an interview that studies have shown early signs of adherence within the first few weeks are an important indicator of overall adherence to CPAP therapy. However, the use of CPAP machine payments in the study by Dr. Wickwire and colleagues was a novel way to track adherence.
Some of the issues with nonadherence may be related to challenges in using the technology, but it is the clinician’s role to communicate with patients about the effectiveness of CPAP and identifying reasons for nonadherence while also attempting to tease out the subtle socioeconomic factors related to nonadherence, Dr. Sundar noted. “We need to alter our practice to make sure that we communicate with these patients and better understand what are the social factors in getting the CPAP or utilizing CPAP, and also following these patients more closely, especially in the first month of starting CPAP therapy.
“Just because somebody has severe sleep apnea and other comorbid conditions does not mean that they’re going to wear the CPAP,” he said. “So, the fact that socioeconomic factors play an equal if not more important role in terms of predicting CPAP adherence. That is an important takeaway.”
Octavian C. Ioachimescu, MD, FCCP, of Emory University, Atlanta, and the Atlanta Veteran Affairs Administration and CHEST Physician editorial board member, said in an interview that the study raises a major question of what is next. “What can we offer to these patients, and what is the real-world compliance to that ‘next-best’ modality?” Dr. Ioachimescu said. “What are the outcomes of these individuals in the point-of-care environment, or ‘real world?’ ”
The analysis by the authors adds the perspective of a “real-world depiction of clinical care for patients with OSA,” Dr. Ioachimescu said. “One major lesson of such an analysis is that the health care goal setting that is referential to initial, randomized, well-controlled studies on highly selected patient populations need to be reassessed periodically from the point of view of actual results in the clinics.”
Clinicians may need to borrow ideas from other therapeutic fields to help improve patient adherence, he said. “[W]e may be able to develop and implement in the future peer involvement, behavioral and cognitive approaches, motivational enhancement interventions, as well as elements of acceptance and commitment techniques, all in the larger context of more integrated and in the same time individualized approaches to therapy.”
The investigators concluded that, “relative to Medicare-only beneficiaries, those eligible for both Medicare and Medicaid were significantly less likely to adhere to CPAP. Future research should seek to develop a deeper understanding of the mechanisms through which [socioeconomic status] and other social determinants impact patient experience throughout the OSA diagnostic and treatment process, including receiving, acclimating, and adhering to CPAP therapy.”
Dr. Sundar concurred with this assessment and said more research is needed on factors impacting adherence such as poverty, homelessness, and home support systems. “It’s not just coordinating with the patient. Clearly, more work is needed in understanding the social aspects of CPAP adherence.”
This study was funded in part by an investigator-initiated grant provided by ResMed to Dr. Wickmire’s institution, the University of Maryland, Baltimore. Dr. Wickmire reported being a scientific consultant to DayZz, Eisai, Merck, and Purdue and holds shares in WellTap. Dr. Oldstone is a ResMed employee and shareholder. Dr. Sundar reported being a cofounder of Hypnoscure, which creates software for population management of sleep apnea, and an investigator in trials where ResMed and Respironics devices were used. Dr. Ioachimescu reported no relevant financial disclosures.
SOURCE: Wickwire EM et al. Sleep. 2020 Jun 23. doi: 10.1093/sleep/zsaa122.
FROM SLEEP
Less REM sleep tied to higher mortality
Less rapid eye movement (REM) sleep is associated with an increased risk for death in middle-aged and older adults, new research suggests.
Investigators at the University of California, San Diego, found that, over a 12-year period, each 5% reduction in REM sleep was associated with a 13% increase in mortality rate. However, the investigators noted that this is only an association and does not indicate cause and effect.
“Determining causality can be difficult,” study investigator Sonia Ancoli-Israel, PhD, professor emeritus of psychiatry at the University of California, San Diego, said in an interview.
“It is therefore important that physicians and the public understand that our findings suggest an increased risk, but that does not mean that reduced REM will always result in shorter survival. With all the self-monitoring sleep gadgets available to the public, I would caution against any panic if one notices reduced REM. But mentioning it to a physician may be a clue to examine what else might be going on with that patient that could more easily be targeted,” Dr. Ancoli-Israel added.
The research was published online July 6 in JAMA Neurology.
Negative consequences
Approximately 50-70 million Americans have problems with sleep. Such problems have a multitude of consequences for health, including cardiovascular disease; metabolic, psychiatric, and cognitive disorders; lower quality of life; and increased mortality.
The investigators noted that the aspects of sleep that may be driving this association remain unclear. Because decreased REM sleep has been associated with poor mental and physical health outcomes, the researchers hypothesized that decreased REM sleep may be associated with an increased risk for death.
To test this hypothesis, they conducted a multicenter, population-based, cross-sectional investigation using data from independent cohorts – the Outcomes of Sleep Disorders in Older Men (MrOS) Sleep Study and the Wisconsin Sleep Cohort (WSC). The MrOS cohort included 2,675 men (mean age, 76.3 years) who were recruited from December 2003 to March 2005 at six U.S. centers and were followed for a median of 12.1 years. The WSC cohort included 1,386 individuals (54.3% men; mean age, 51.5 years) and had a median follow-up of 20.8 years. Data from this study were used to replicate the findings from the MrOS study.
Primary outcome measures included all-cause and cause-specific mortality, which were confirmed using death certificates.
Participants in both cohorts underwent polysomnography and evaluation with the Epworth Sleepiness Scale. For MrOS participants, investigators calculated the total number of minutes per night spent in REM sleep and the corresponding percentage of total sleep time.
Less sleep, more death
Self-report sleep measures in MrOS participants were collected using the Pittsburgh Sleep Quality Index and the Functional Outcomes of Sleep Questionnaire
The investigators contacted participants in MrOS every 4 months to determine vital status. Cause of death was categorized by the ICD-9 as cardiovascular, cancer, and other. In WSC, the researchers identified deaths by matching participants’ social security numbers with national and state registries. The cause of death was categorized in the same manner as in the MrOS cohort.
Approximately half (53%) of the MrOS cohort died during follow-up. For each mortality category, the highest percentage of deaths occurred among those in the lowest quartile percentage of REM sleep. Adjusted analyses revealed that the MrOS participants had a 13% higher mortality rate for every 5% reduction in REM sleep (hazard ratio, 1.13; 95% confidence interval, 1.08-1.19). These findings were similar for cardiovascular and other causes of death but were not significant for cancer-related mortality. For all mortality categories, the mortality rate was higher for participants who had less than 15% REM sleep per night in comparison with individuals who had 15% or more.
The findings were similar in the WSC cohort despite its younger age, the inclusion of women, and longer follow-up (HR, 1.13; 95% CI, 1.08-1.19). Compared with MrOS participants, WSC participants were more likely to be obese and to use more antidepressants or sedatives. Overall, the mean percentage of REM sleep was 19.2%. Participants in the lowest quartile of REM sleep generally were older, had higher rates of antidepressant use, hypertension, heart attack, and transient ischemic attack, as well as engaging in less physical activity.
Ask about sleep
When the data were stratified by sex, the association between decreased REM sleep and mortality was significant for women but not for men.
“Obtaining a sleep study, representative of the patient’s usual sleep, that shows reduced REM time should alert the neurologist to look for reasons for low REM,” the study’s coinvestigator, Susan Redline, MD, MPH, Peter C. Farrell Professor of Sleep Medicine at Harvard Medical School in Boston, said in an interview.
Dr. Redline added that measures to promote sleep health, such as encouraging regular, sufficient nightly sleep; offering guidance on avoiding alcohol before bedtime and on other healthy sleep practices; and treating sleep disorders may be beneficial.
Low REM time, especially interpreted with other relevant clinical information, may alert the neurologist that a patient may have risk factors for poorer health, she added.
Sleep studies are expensive and are in high demand, so “the most realistic approach is for the neurologist to be asking each and every patient about their sleep,” said Ancoli-Israel.
“By asking a few more questions in every intake, the neurologist is more likely to determine if there are any occult sleep disorders that need to be addressed. By improving sleep in general, one is more likely to also improve any REM abnormalities,” she said.
Disease indicator?
In an accompanying editorial, Michael S. Jaffee, MD, vice chair of neurology at the University of Florida in Gainesville, and colleagues noted that the study raises the question of whether REM sleep “could serve as a biomarker for general health.”
“Since the known roles of REM sleep do not easily suggest a causal link with mortality ... it seems more likely that REM sleep reduction is either a crude marker of health or specific disease states that decrease REM sleep may play an important role in contributing to mortality,” they wrote.
Neurologists should remember that certain medications affect sleep architecture, the editorialists advised. They note that serotonin reuptake inhibitors, selective serotonin and norepinephrine reuptake inhibitors, and tricyclic antidepressants reduce REM sleep, and that gabapentin, prazosin, and bupropion, on the other hand, increase REM sleep. However, data regarding whether these medications have an effect on mortality are insufficient.
The editorialists wrote that the study findings are a “welcome addition to the literature and demonstrate definitively that the association between sleep and mortality extends beyond the simple measure of total sleep time.”
Funding for the MrOS and WSC studies was provided by the National Institutes of Health and the National Institute on Aging. Dr. Ancoli-Israel consults for Eisai and Merck on matters unrelated to the study. Dr. Redline has received grants and personal fees from Jazz Pharmaceuticals, consulting fees from Respicardia, and personal fees from Eisai unrelated to the study. Dr. Jaffee served on a data and safety monitoring board for Helius Medical Technologies and consulted for the National Collegiate Athletic Association and the Department of Defense.
A version of this article originally appeared on Medscape.com.
Less rapid eye movement (REM) sleep is associated with an increased risk for death in middle-aged and older adults, new research suggests.
Investigators at the University of California, San Diego, found that, over a 12-year period, each 5% reduction in REM sleep was associated with a 13% increase in mortality rate. However, the investigators noted that this is only an association and does not indicate cause and effect.
“Determining causality can be difficult,” study investigator Sonia Ancoli-Israel, PhD, professor emeritus of psychiatry at the University of California, San Diego, said in an interview.
“It is therefore important that physicians and the public understand that our findings suggest an increased risk, but that does not mean that reduced REM will always result in shorter survival. With all the self-monitoring sleep gadgets available to the public, I would caution against any panic if one notices reduced REM. But mentioning it to a physician may be a clue to examine what else might be going on with that patient that could more easily be targeted,” Dr. Ancoli-Israel added.
The research was published online July 6 in JAMA Neurology.
Negative consequences
Approximately 50-70 million Americans have problems with sleep. Such problems have a multitude of consequences for health, including cardiovascular disease; metabolic, psychiatric, and cognitive disorders; lower quality of life; and increased mortality.
The investigators noted that the aspects of sleep that may be driving this association remain unclear. Because decreased REM sleep has been associated with poor mental and physical health outcomes, the researchers hypothesized that decreased REM sleep may be associated with an increased risk for death.
To test this hypothesis, they conducted a multicenter, population-based, cross-sectional investigation using data from independent cohorts – the Outcomes of Sleep Disorders in Older Men (MrOS) Sleep Study and the Wisconsin Sleep Cohort (WSC). The MrOS cohort included 2,675 men (mean age, 76.3 years) who were recruited from December 2003 to March 2005 at six U.S. centers and were followed for a median of 12.1 years. The WSC cohort included 1,386 individuals (54.3% men; mean age, 51.5 years) and had a median follow-up of 20.8 years. Data from this study were used to replicate the findings from the MrOS study.
Primary outcome measures included all-cause and cause-specific mortality, which were confirmed using death certificates.
Participants in both cohorts underwent polysomnography and evaluation with the Epworth Sleepiness Scale. For MrOS participants, investigators calculated the total number of minutes per night spent in REM sleep and the corresponding percentage of total sleep time.
Less sleep, more death
Self-report sleep measures in MrOS participants were collected using the Pittsburgh Sleep Quality Index and the Functional Outcomes of Sleep Questionnaire
The investigators contacted participants in MrOS every 4 months to determine vital status. Cause of death was categorized by the ICD-9 as cardiovascular, cancer, and other. In WSC, the researchers identified deaths by matching participants’ social security numbers with national and state registries. The cause of death was categorized in the same manner as in the MrOS cohort.
Approximately half (53%) of the MrOS cohort died during follow-up. For each mortality category, the highest percentage of deaths occurred among those in the lowest quartile percentage of REM sleep. Adjusted analyses revealed that the MrOS participants had a 13% higher mortality rate for every 5% reduction in REM sleep (hazard ratio, 1.13; 95% confidence interval, 1.08-1.19). These findings were similar for cardiovascular and other causes of death but were not significant for cancer-related mortality. For all mortality categories, the mortality rate was higher for participants who had less than 15% REM sleep per night in comparison with individuals who had 15% or more.
The findings were similar in the WSC cohort despite its younger age, the inclusion of women, and longer follow-up (HR, 1.13; 95% CI, 1.08-1.19). Compared with MrOS participants, WSC participants were more likely to be obese and to use more antidepressants or sedatives. Overall, the mean percentage of REM sleep was 19.2%. Participants in the lowest quartile of REM sleep generally were older, had higher rates of antidepressant use, hypertension, heart attack, and transient ischemic attack, as well as engaging in less physical activity.
Ask about sleep
When the data were stratified by sex, the association between decreased REM sleep and mortality was significant for women but not for men.
“Obtaining a sleep study, representative of the patient’s usual sleep, that shows reduced REM time should alert the neurologist to look for reasons for low REM,” the study’s coinvestigator, Susan Redline, MD, MPH, Peter C. Farrell Professor of Sleep Medicine at Harvard Medical School in Boston, said in an interview.
Dr. Redline added that measures to promote sleep health, such as encouraging regular, sufficient nightly sleep; offering guidance on avoiding alcohol before bedtime and on other healthy sleep practices; and treating sleep disorders may be beneficial.
Low REM time, especially interpreted with other relevant clinical information, may alert the neurologist that a patient may have risk factors for poorer health, she added.
Sleep studies are expensive and are in high demand, so “the most realistic approach is for the neurologist to be asking each and every patient about their sleep,” said Ancoli-Israel.
“By asking a few more questions in every intake, the neurologist is more likely to determine if there are any occult sleep disorders that need to be addressed. By improving sleep in general, one is more likely to also improve any REM abnormalities,” she said.
Disease indicator?
In an accompanying editorial, Michael S. Jaffee, MD, vice chair of neurology at the University of Florida in Gainesville, and colleagues noted that the study raises the question of whether REM sleep “could serve as a biomarker for general health.”
“Since the known roles of REM sleep do not easily suggest a causal link with mortality ... it seems more likely that REM sleep reduction is either a crude marker of health or specific disease states that decrease REM sleep may play an important role in contributing to mortality,” they wrote.
Neurologists should remember that certain medications affect sleep architecture, the editorialists advised. They note that serotonin reuptake inhibitors, selective serotonin and norepinephrine reuptake inhibitors, and tricyclic antidepressants reduce REM sleep, and that gabapentin, prazosin, and bupropion, on the other hand, increase REM sleep. However, data regarding whether these medications have an effect on mortality are insufficient.
The editorialists wrote that the study findings are a “welcome addition to the literature and demonstrate definitively that the association between sleep and mortality extends beyond the simple measure of total sleep time.”
Funding for the MrOS and WSC studies was provided by the National Institutes of Health and the National Institute on Aging. Dr. Ancoli-Israel consults for Eisai and Merck on matters unrelated to the study. Dr. Redline has received grants and personal fees from Jazz Pharmaceuticals, consulting fees from Respicardia, and personal fees from Eisai unrelated to the study. Dr. Jaffee served on a data and safety monitoring board for Helius Medical Technologies and consulted for the National Collegiate Athletic Association and the Department of Defense.
A version of this article originally appeared on Medscape.com.
Less rapid eye movement (REM) sleep is associated with an increased risk for death in middle-aged and older adults, new research suggests.
Investigators at the University of California, San Diego, found that, over a 12-year period, each 5% reduction in REM sleep was associated with a 13% increase in mortality rate. However, the investigators noted that this is only an association and does not indicate cause and effect.
“Determining causality can be difficult,” study investigator Sonia Ancoli-Israel, PhD, professor emeritus of psychiatry at the University of California, San Diego, said in an interview.
“It is therefore important that physicians and the public understand that our findings suggest an increased risk, but that does not mean that reduced REM will always result in shorter survival. With all the self-monitoring sleep gadgets available to the public, I would caution against any panic if one notices reduced REM. But mentioning it to a physician may be a clue to examine what else might be going on with that patient that could more easily be targeted,” Dr. Ancoli-Israel added.
The research was published online July 6 in JAMA Neurology.
Negative consequences
Approximately 50-70 million Americans have problems with sleep. Such problems have a multitude of consequences for health, including cardiovascular disease; metabolic, psychiatric, and cognitive disorders; lower quality of life; and increased mortality.
The investigators noted that the aspects of sleep that may be driving this association remain unclear. Because decreased REM sleep has been associated with poor mental and physical health outcomes, the researchers hypothesized that decreased REM sleep may be associated with an increased risk for death.
To test this hypothesis, they conducted a multicenter, population-based, cross-sectional investigation using data from independent cohorts – the Outcomes of Sleep Disorders in Older Men (MrOS) Sleep Study and the Wisconsin Sleep Cohort (WSC). The MrOS cohort included 2,675 men (mean age, 76.3 years) who were recruited from December 2003 to March 2005 at six U.S. centers and were followed for a median of 12.1 years. The WSC cohort included 1,386 individuals (54.3% men; mean age, 51.5 years) and had a median follow-up of 20.8 years. Data from this study were used to replicate the findings from the MrOS study.
Primary outcome measures included all-cause and cause-specific mortality, which were confirmed using death certificates.
Participants in both cohorts underwent polysomnography and evaluation with the Epworth Sleepiness Scale. For MrOS participants, investigators calculated the total number of minutes per night spent in REM sleep and the corresponding percentage of total sleep time.
Less sleep, more death
Self-report sleep measures in MrOS participants were collected using the Pittsburgh Sleep Quality Index and the Functional Outcomes of Sleep Questionnaire
The investigators contacted participants in MrOS every 4 months to determine vital status. Cause of death was categorized by the ICD-9 as cardiovascular, cancer, and other. In WSC, the researchers identified deaths by matching participants’ social security numbers with national and state registries. The cause of death was categorized in the same manner as in the MrOS cohort.
Approximately half (53%) of the MrOS cohort died during follow-up. For each mortality category, the highest percentage of deaths occurred among those in the lowest quartile percentage of REM sleep. Adjusted analyses revealed that the MrOS participants had a 13% higher mortality rate for every 5% reduction in REM sleep (hazard ratio, 1.13; 95% confidence interval, 1.08-1.19). These findings were similar for cardiovascular and other causes of death but were not significant for cancer-related mortality. For all mortality categories, the mortality rate was higher for participants who had less than 15% REM sleep per night in comparison with individuals who had 15% or more.
The findings were similar in the WSC cohort despite its younger age, the inclusion of women, and longer follow-up (HR, 1.13; 95% CI, 1.08-1.19). Compared with MrOS participants, WSC participants were more likely to be obese and to use more antidepressants or sedatives. Overall, the mean percentage of REM sleep was 19.2%. Participants in the lowest quartile of REM sleep generally were older, had higher rates of antidepressant use, hypertension, heart attack, and transient ischemic attack, as well as engaging in less physical activity.
Ask about sleep
When the data were stratified by sex, the association between decreased REM sleep and mortality was significant for women but not for men.
“Obtaining a sleep study, representative of the patient’s usual sleep, that shows reduced REM time should alert the neurologist to look for reasons for low REM,” the study’s coinvestigator, Susan Redline, MD, MPH, Peter C. Farrell Professor of Sleep Medicine at Harvard Medical School in Boston, said in an interview.
Dr. Redline added that measures to promote sleep health, such as encouraging regular, sufficient nightly sleep; offering guidance on avoiding alcohol before bedtime and on other healthy sleep practices; and treating sleep disorders may be beneficial.
Low REM time, especially interpreted with other relevant clinical information, may alert the neurologist that a patient may have risk factors for poorer health, she added.
Sleep studies are expensive and are in high demand, so “the most realistic approach is for the neurologist to be asking each and every patient about their sleep,” said Ancoli-Israel.
“By asking a few more questions in every intake, the neurologist is more likely to determine if there are any occult sleep disorders that need to be addressed. By improving sleep in general, one is more likely to also improve any REM abnormalities,” she said.
Disease indicator?
In an accompanying editorial, Michael S. Jaffee, MD, vice chair of neurology at the University of Florida in Gainesville, and colleagues noted that the study raises the question of whether REM sleep “could serve as a biomarker for general health.”
“Since the known roles of REM sleep do not easily suggest a causal link with mortality ... it seems more likely that REM sleep reduction is either a crude marker of health or specific disease states that decrease REM sleep may play an important role in contributing to mortality,” they wrote.
Neurologists should remember that certain medications affect sleep architecture, the editorialists advised. They note that serotonin reuptake inhibitors, selective serotonin and norepinephrine reuptake inhibitors, and tricyclic antidepressants reduce REM sleep, and that gabapentin, prazosin, and bupropion, on the other hand, increase REM sleep. However, data regarding whether these medications have an effect on mortality are insufficient.
The editorialists wrote that the study findings are a “welcome addition to the literature and demonstrate definitively that the association between sleep and mortality extends beyond the simple measure of total sleep time.”
Funding for the MrOS and WSC studies was provided by the National Institutes of Health and the National Institute on Aging. Dr. Ancoli-Israel consults for Eisai and Merck on matters unrelated to the study. Dr. Redline has received grants and personal fees from Jazz Pharmaceuticals, consulting fees from Respicardia, and personal fees from Eisai unrelated to the study. Dr. Jaffee served on a data and safety monitoring board for Helius Medical Technologies and consulted for the National Collegiate Athletic Association and the Department of Defense.
A version of this article originally appeared on Medscape.com.
COVID-19 and the future of telehealth for sleep medicine
On March 18, 2020, the doors to our sleep center were physically closed. Two potential exposures to COVID-19 within a few hours, the palpable anxiety of our team, and a poor grasp of the virus and the growing pandemic moved us to make this decision. Up to that point, we could not help but feel we were playing “catch up” with our evolving set of safety measures to the escalating risk. Like so many other sleep centers around the country, a complete transition to virtual care was needed to ensure the safety of our patients and our team. It was perhaps that moment that we felt the emotional impact that our world had changed, altering both our personal lives and sleep medicine practice as we knew it. This event, while unfortunate, also provided a transformative opportunity to reimagine our identity, accelerating the efforts to bring the future of sleep medicine into the present.
Our team’s clinical evolution and innovation efforts have been guided by efforts to reconsider sleep medicine paradigms. Innovation progress was deliberate with incremental implementations that typically required repeat business cases with multiple approving parties and budgetary access. Those barriers largely dissolved once COVID-19 intensified, and a large portion of the strategies on our roadmap were put into production. In a matter of a couple weeks, our services completely transitioned to remote and virtual care, while most of the team of 55 persons were moved to “work-from-home.” A suite of technologies (automated questionnaires, automated and two-way text messaging templates, consumer wearable technologies, and population management dashboards) were put on the table (Somnoware, Inc.), and each of our longitudinal care teams (eg, adult obstructive sleep apnea, pediatrics, chronic respiratory failure, commercial driver, insomnia programs, etc) worked to embed them into new care pathways. This effort further consolidated technology as the backbone of our work and the enabler of remote virtual collaboration between sleep center personnel (respiratory case managers, medical assistants and nursing team, and physician and leadership personnel) to enhance our team-based approach. Moreover, we felt this point in time was ripe to swallow the proverbial “red pill” and approach patient care with shifted paradigms. We discuss three areas of active effort to leverage technology in this COVID-19 environment to accelerate a transition toward how we envision the future of sleep medicine.
Reimagined sleep diagnostics
Our virtual obstructive sleep apnea (OSA) diagnostic process includes utilizing a disposable home sleep apnea test (HSAT) device with wireless data transfer (WatchPAT ONE, Itamar Medical) while HSAT and PAP (positive airway pressure) setups are supported by information sheets, online videos (YouTube), automated interactive platforms (Emmi Solutions; Hwang D. Am J Respir Crit Care Med. 2018 Jan 1;197[1]:117), and synchronous provider video visits. Our more radical shift, however, is in approaching OSA diagnosis based principally on symptoms and secondarily supported by physiologic measurements and response to therapy. This “clinical diagnosis” approach reduces our reliance on traditional sleep testing and allows patient wearables to provide supportive physiologic data (eg, oximetry) to help determine OSA severity and phenotype. Its immediate impact is in limiting the need to send and retrieve potentially contaminated equipment. Broader clinical advantages include overcoming the imprecise nature of the apnea-hypopnea index (which often has dramatic night-to-night variability) through data collection over extended durations, improving disease assessment due to availability of complementary sleep/activity data in the person’s usual setting, and tracking changes after therapy initiation.
Our post-COVID-19 re-opening of polysomnography (PSG) services, after a temporary shutdown, introduces home PSG (Type II) for approximately half our patients without suspected complex breathing conditions while reserving attended PSG (Type I) for those who may require noninvasive ventilation. The immediate incentive is in reducing viral exposure by limiting patient traffic and risk of PAP trial aerosolization while also improving access to accommodate the backlog of patients requiring PSG. This approach furthers the paradigm shift to emphasizing care in the home setting. Testing in the patient’s usual environment and enabling multiple night/day testing may be clinically advantageous.
Shift in emphasis to care management
The emphasis of sleep medicine has traditionally focused on diagnostics through performing PSG and HSAT. Our field has invested tremendous effort in developing guidelines for processing sleep studies, but the scoring and interpretation of those studies is extremely labor intensive. Reimagining the diagnostic approach reduces the need to manually process studies—wearable data are produced automatically, HSAT can be auto-scored, and artificial intelligence platforms can score PSGs (Goldstein CA. J Clin Sleep Med. 2020 Apr 15;16[4]:609), which allows a shift in resources and emphasis to follow-up care. A comprehensive discussion of technology-based tools to enhance care management is beyond the purview of this editorial. However, an overview of our current efforts includes: (1) utilizing population management dashboards to automatically risk stratify different cohorts of patients (eg, adult OSA, pediatrics, commercial drivers, chronic respiratory failure, etc) to identify patients “at-risk” (eg, based on OSA severity, symptoms, co-morbidities, and PAP adherence); (2) applying enhanced patient-provider interchange tools that include automated and “intelligent” electronic questionnaires, automated personalized text messaging/emails, and two-way messaging to deliver care; (3) utilizing remote patient monitoring to enhance holistic, personalized management, such as with remote activity/sleep trackers, blood pressure monitors, glucometers, and weight scales. We are engaged with efforts to validate the impact of these data to provide more personalized feedback, directly impact clinical outcomes, facilitate interdisciplinary collaboration, and identify acutely ill patients. Furthermore, a holistic approach beyond a narrow focus on PAP may create a positive collateral effect on adherence by targeting engagement with broader areas of health; and (4) implementing machine learning tools to directly support providers and patients (examples discussed in the next section.) Each of our teams has created workflows embedding these strategies throughout new care pathways. 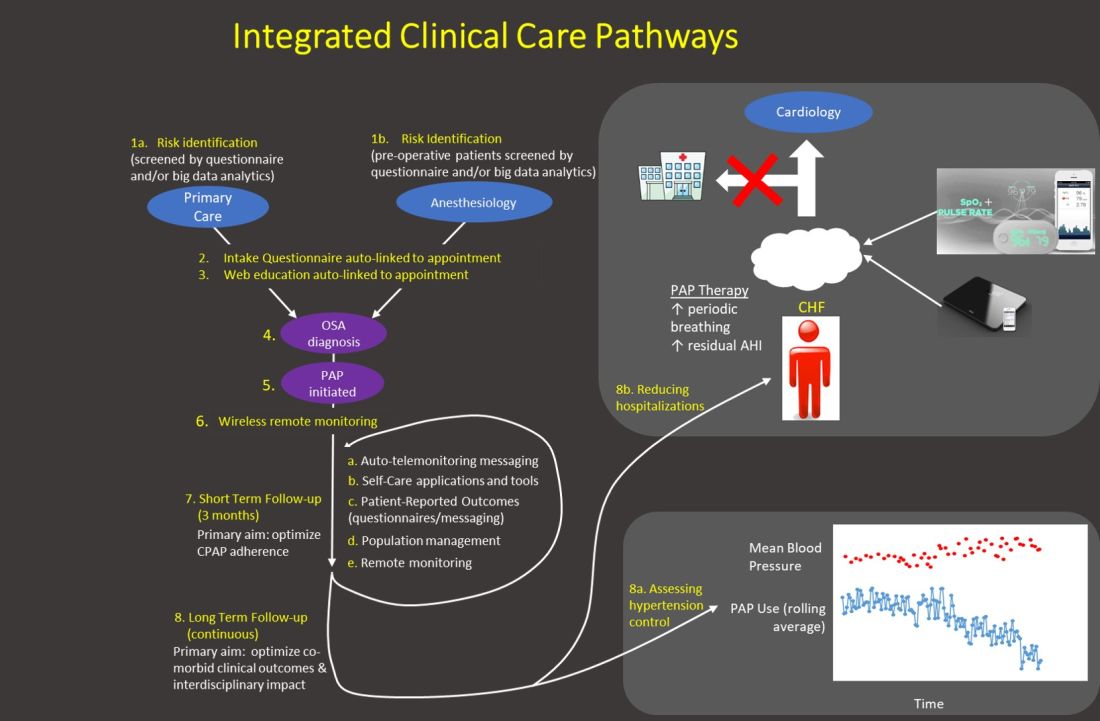
Generally, our emphasis during the first 3 months after PAP initiation focuses on achieving therapy adherence, and the post-3-month period broadens the efforts to target clinical outcomes. Recent trials with low PAP usage that failed to confirm the benefit of PAP on cardiovascular outcomes (McEvoy DR, et al. N Engl J Med. 2016;375:919) strongly suggest greater investment in cost-effective long-term strategies is imperative to increase our field’s relevance.
Application of artificial intelligence
We describe current efforts to apply artificial intelligence (AI) into clinical care: (1) We are implementing machine learning (ML) PSG scoring, which can potentially improve both the consistency and efficiency of scoring, further enabling greater investment in follow-up care. The future of sleep study processing, however, will likely depend on computer vision to “view” details inaccessible to the human eye and produce novel metrics that better inform clinical phenotypes (eg, cardiovascular risk, response to alternative therapies, etc). For example, “brain age” has been derived from EEG tracings that could reflect the degree of impact of sleep disorders on neurocognitive function (Fernandez C, unpublished data); (2) Machine learning clinical decision tools are in development to predict PAP adherence and timing of discontinuation, predict timing of cardiovascular disease onset and hospitalization, personalizing adherence targets, automating triaging of patients to home or PSG testing, and innumerable other predictions at clinical decision inflection points. Prediction outputs may be presented as risk profiles embedded in each patient’s “chart,” as personalized alerts, and in gamification strategies. For example, machine learning personalized cardiovascular risk scores can be regularly updated based on degree of PAP use to incentivize adherence; (3) Artificial providers may provide consistent, personalized, and holistic supplementary care. Many people rely on AI-bots for social support and cognitive-behavioral therapy (CBT) for depression. A sleep wellness bot, currently in planning stages, is intended to be the primary interface for many of the strategies described above that enhance engagement with PAP and therapies for comorbid conditions, provide CBT and lifestyle accountability, and collect patient reported data. This artificial provider would be a constant companion providing interactive, personalized, and continuous management to complement traditional intermittent live-person care.
The current health-care environment embodies the principle to “never let a serious crisis go to waste.” COVID-19 has accelerated the progression into the future by fostering an opening to embrace novel application of technologies to support changes in paradigms. Furthermore, health-care infrastructures that typically progress deliberately changed seemingly in a single moment. The Center for Medicare Services issued broad authorization to reimburse for telemedicine in response to COVID-19. Continued evolution in infrastructures will dictate progress with innovation, and a greater transition to outcomes-based incentives may be necessary to accommodate many of the strategies described above that rely on nonsynchronous care. But, we may be experiencing the moment when health care starts to catch up with the world in its embrace of technology. Sleep and pulmonary medicine can be a leader by providing a successful template for other specialties in optimizing chronic disease management.
Dr. Hwang is Medical Director, Kaiser Permanente SBC Sleep Center, and co-chair, Sleep Medicine, Kaiser Permanente Southern California.
On March 18, 2020, the doors to our sleep center were physically closed. Two potential exposures to COVID-19 within a few hours, the palpable anxiety of our team, and a poor grasp of the virus and the growing pandemic moved us to make this decision. Up to that point, we could not help but feel we were playing “catch up” with our evolving set of safety measures to the escalating risk. Like so many other sleep centers around the country, a complete transition to virtual care was needed to ensure the safety of our patients and our team. It was perhaps that moment that we felt the emotional impact that our world had changed, altering both our personal lives and sleep medicine practice as we knew it. This event, while unfortunate, also provided a transformative opportunity to reimagine our identity, accelerating the efforts to bring the future of sleep medicine into the present.
Our team’s clinical evolution and innovation efforts have been guided by efforts to reconsider sleep medicine paradigms. Innovation progress was deliberate with incremental implementations that typically required repeat business cases with multiple approving parties and budgetary access. Those barriers largely dissolved once COVID-19 intensified, and a large portion of the strategies on our roadmap were put into production. In a matter of a couple weeks, our services completely transitioned to remote and virtual care, while most of the team of 55 persons were moved to “work-from-home.” A suite of technologies (automated questionnaires, automated and two-way text messaging templates, consumer wearable technologies, and population management dashboards) were put on the table (Somnoware, Inc.), and each of our longitudinal care teams (eg, adult obstructive sleep apnea, pediatrics, chronic respiratory failure, commercial driver, insomnia programs, etc) worked to embed them into new care pathways. This effort further consolidated technology as the backbone of our work and the enabler of remote virtual collaboration between sleep center personnel (respiratory case managers, medical assistants and nursing team, and physician and leadership personnel) to enhance our team-based approach. Moreover, we felt this point in time was ripe to swallow the proverbial “red pill” and approach patient care with shifted paradigms. We discuss three areas of active effort to leverage technology in this COVID-19 environment to accelerate a transition toward how we envision the future of sleep medicine.
Reimagined sleep diagnostics
Our virtual obstructive sleep apnea (OSA) diagnostic process includes utilizing a disposable home sleep apnea test (HSAT) device with wireless data transfer (WatchPAT ONE, Itamar Medical) while HSAT and PAP (positive airway pressure) setups are supported by information sheets, online videos (YouTube), automated interactive platforms (Emmi Solutions; Hwang D. Am J Respir Crit Care Med. 2018 Jan 1;197[1]:117), and synchronous provider video visits. Our more radical shift, however, is in approaching OSA diagnosis based principally on symptoms and secondarily supported by physiologic measurements and response to therapy. This “clinical diagnosis” approach reduces our reliance on traditional sleep testing and allows patient wearables to provide supportive physiologic data (eg, oximetry) to help determine OSA severity and phenotype. Its immediate impact is in limiting the need to send and retrieve potentially contaminated equipment. Broader clinical advantages include overcoming the imprecise nature of the apnea-hypopnea index (which often has dramatic night-to-night variability) through data collection over extended durations, improving disease assessment due to availability of complementary sleep/activity data in the person’s usual setting, and tracking changes after therapy initiation.
Our post-COVID-19 re-opening of polysomnography (PSG) services, after a temporary shutdown, introduces home PSG (Type II) for approximately half our patients without suspected complex breathing conditions while reserving attended PSG (Type I) for those who may require noninvasive ventilation. The immediate incentive is in reducing viral exposure by limiting patient traffic and risk of PAP trial aerosolization while also improving access to accommodate the backlog of patients requiring PSG. This approach furthers the paradigm shift to emphasizing care in the home setting. Testing in the patient’s usual environment and enabling multiple night/day testing may be clinically advantageous.
Shift in emphasis to care management
The emphasis of sleep medicine has traditionally focused on diagnostics through performing PSG and HSAT. Our field has invested tremendous effort in developing guidelines for processing sleep studies, but the scoring and interpretation of those studies is extremely labor intensive. Reimagining the diagnostic approach reduces the need to manually process studies—wearable data are produced automatically, HSAT can be auto-scored, and artificial intelligence platforms can score PSGs (Goldstein CA. J Clin Sleep Med. 2020 Apr 15;16[4]:609), which allows a shift in resources and emphasis to follow-up care. A comprehensive discussion of technology-based tools to enhance care management is beyond the purview of this editorial. However, an overview of our current efforts includes: (1) utilizing population management dashboards to automatically risk stratify different cohorts of patients (eg, adult OSA, pediatrics, commercial drivers, chronic respiratory failure, etc) to identify patients “at-risk” (eg, based on OSA severity, symptoms, co-morbidities, and PAP adherence); (2) applying enhanced patient-provider interchange tools that include automated and “intelligent” electronic questionnaires, automated personalized text messaging/emails, and two-way messaging to deliver care; (3) utilizing remote patient monitoring to enhance holistic, personalized management, such as with remote activity/sleep trackers, blood pressure monitors, glucometers, and weight scales. We are engaged with efforts to validate the impact of these data to provide more personalized feedback, directly impact clinical outcomes, facilitate interdisciplinary collaboration, and identify acutely ill patients. Furthermore, a holistic approach beyond a narrow focus on PAP may create a positive collateral effect on adherence by targeting engagement with broader areas of health; and (4) implementing machine learning tools to directly support providers and patients (examples discussed in the next section.) Each of our teams has created workflows embedding these strategies throughout new care pathways. 
Generally, our emphasis during the first 3 months after PAP initiation focuses on achieving therapy adherence, and the post-3-month period broadens the efforts to target clinical outcomes. Recent trials with low PAP usage that failed to confirm the benefit of PAP on cardiovascular outcomes (McEvoy DR, et al. N Engl J Med. 2016;375:919) strongly suggest greater investment in cost-effective long-term strategies is imperative to increase our field’s relevance.
Application of artificial intelligence
We describe current efforts to apply artificial intelligence (AI) into clinical care: (1) We are implementing machine learning (ML) PSG scoring, which can potentially improve both the consistency and efficiency of scoring, further enabling greater investment in follow-up care. The future of sleep study processing, however, will likely depend on computer vision to “view” details inaccessible to the human eye and produce novel metrics that better inform clinical phenotypes (eg, cardiovascular risk, response to alternative therapies, etc). For example, “brain age” has been derived from EEG tracings that could reflect the degree of impact of sleep disorders on neurocognitive function (Fernandez C, unpublished data); (2) Machine learning clinical decision tools are in development to predict PAP adherence and timing of discontinuation, predict timing of cardiovascular disease onset and hospitalization, personalizing adherence targets, automating triaging of patients to home or PSG testing, and innumerable other predictions at clinical decision inflection points. Prediction outputs may be presented as risk profiles embedded in each patient’s “chart,” as personalized alerts, and in gamification strategies. For example, machine learning personalized cardiovascular risk scores can be regularly updated based on degree of PAP use to incentivize adherence; (3) Artificial providers may provide consistent, personalized, and holistic supplementary care. Many people rely on AI-bots for social support and cognitive-behavioral therapy (CBT) for depression. A sleep wellness bot, currently in planning stages, is intended to be the primary interface for many of the strategies described above that enhance engagement with PAP and therapies for comorbid conditions, provide CBT and lifestyle accountability, and collect patient reported data. This artificial provider would be a constant companion providing interactive, personalized, and continuous management to complement traditional intermittent live-person care.
The current health-care environment embodies the principle to “never let a serious crisis go to waste.” COVID-19 has accelerated the progression into the future by fostering an opening to embrace novel application of technologies to support changes in paradigms. Furthermore, health-care infrastructures that typically progress deliberately changed seemingly in a single moment. The Center for Medicare Services issued broad authorization to reimburse for telemedicine in response to COVID-19. Continued evolution in infrastructures will dictate progress with innovation, and a greater transition to outcomes-based incentives may be necessary to accommodate many of the strategies described above that rely on nonsynchronous care. But, we may be experiencing the moment when health care starts to catch up with the world in its embrace of technology. Sleep and pulmonary medicine can be a leader by providing a successful template for other specialties in optimizing chronic disease management.
Dr. Hwang is Medical Director, Kaiser Permanente SBC Sleep Center, and co-chair, Sleep Medicine, Kaiser Permanente Southern California.
On March 18, 2020, the doors to our sleep center were physically closed. Two potential exposures to COVID-19 within a few hours, the palpable anxiety of our team, and a poor grasp of the virus and the growing pandemic moved us to make this decision. Up to that point, we could not help but feel we were playing “catch up” with our evolving set of safety measures to the escalating risk. Like so many other sleep centers around the country, a complete transition to virtual care was needed to ensure the safety of our patients and our team. It was perhaps that moment that we felt the emotional impact that our world had changed, altering both our personal lives and sleep medicine practice as we knew it. This event, while unfortunate, also provided a transformative opportunity to reimagine our identity, accelerating the efforts to bring the future of sleep medicine into the present.
Our team’s clinical evolution and innovation efforts have been guided by efforts to reconsider sleep medicine paradigms. Innovation progress was deliberate with incremental implementations that typically required repeat business cases with multiple approving parties and budgetary access. Those barriers largely dissolved once COVID-19 intensified, and a large portion of the strategies on our roadmap were put into production. In a matter of a couple weeks, our services completely transitioned to remote and virtual care, while most of the team of 55 persons were moved to “work-from-home.” A suite of technologies (automated questionnaires, automated and two-way text messaging templates, consumer wearable technologies, and population management dashboards) were put on the table (Somnoware, Inc.), and each of our longitudinal care teams (eg, adult obstructive sleep apnea, pediatrics, chronic respiratory failure, commercial driver, insomnia programs, etc) worked to embed them into new care pathways. This effort further consolidated technology as the backbone of our work and the enabler of remote virtual collaboration between sleep center personnel (respiratory case managers, medical assistants and nursing team, and physician and leadership personnel) to enhance our team-based approach. Moreover, we felt this point in time was ripe to swallow the proverbial “red pill” and approach patient care with shifted paradigms. We discuss three areas of active effort to leverage technology in this COVID-19 environment to accelerate a transition toward how we envision the future of sleep medicine.
Reimagined sleep diagnostics
Our virtual obstructive sleep apnea (OSA) diagnostic process includes utilizing a disposable home sleep apnea test (HSAT) device with wireless data transfer (WatchPAT ONE, Itamar Medical) while HSAT and PAP (positive airway pressure) setups are supported by information sheets, online videos (YouTube), automated interactive platforms (Emmi Solutions; Hwang D. Am J Respir Crit Care Med. 2018 Jan 1;197[1]:117), and synchronous provider video visits. Our more radical shift, however, is in approaching OSA diagnosis based principally on symptoms and secondarily supported by physiologic measurements and response to therapy. This “clinical diagnosis” approach reduces our reliance on traditional sleep testing and allows patient wearables to provide supportive physiologic data (eg, oximetry) to help determine OSA severity and phenotype. Its immediate impact is in limiting the need to send and retrieve potentially contaminated equipment. Broader clinical advantages include overcoming the imprecise nature of the apnea-hypopnea index (which often has dramatic night-to-night variability) through data collection over extended durations, improving disease assessment due to availability of complementary sleep/activity data in the person’s usual setting, and tracking changes after therapy initiation.
Our post-COVID-19 re-opening of polysomnography (PSG) services, after a temporary shutdown, introduces home PSG (Type II) for approximately half our patients without suspected complex breathing conditions while reserving attended PSG (Type I) for those who may require noninvasive ventilation. The immediate incentive is in reducing viral exposure by limiting patient traffic and risk of PAP trial aerosolization while also improving access to accommodate the backlog of patients requiring PSG. This approach furthers the paradigm shift to emphasizing care in the home setting. Testing in the patient’s usual environment and enabling multiple night/day testing may be clinically advantageous.
Shift in emphasis to care management
The emphasis of sleep medicine has traditionally focused on diagnostics through performing PSG and HSAT. Our field has invested tremendous effort in developing guidelines for processing sleep studies, but the scoring and interpretation of those studies is extremely labor intensive. Reimagining the diagnostic approach reduces the need to manually process studies—wearable data are produced automatically, HSAT can be auto-scored, and artificial intelligence platforms can score PSGs (Goldstein CA. J Clin Sleep Med. 2020 Apr 15;16[4]:609), which allows a shift in resources and emphasis to follow-up care. A comprehensive discussion of technology-based tools to enhance care management is beyond the purview of this editorial. However, an overview of our current efforts includes: (1) utilizing population management dashboards to automatically risk stratify different cohorts of patients (eg, adult OSA, pediatrics, commercial drivers, chronic respiratory failure, etc) to identify patients “at-risk” (eg, based on OSA severity, symptoms, co-morbidities, and PAP adherence); (2) applying enhanced patient-provider interchange tools that include automated and “intelligent” electronic questionnaires, automated personalized text messaging/emails, and two-way messaging to deliver care; (3) utilizing remote patient monitoring to enhance holistic, personalized management, such as with remote activity/sleep trackers, blood pressure monitors, glucometers, and weight scales. We are engaged with efforts to validate the impact of these data to provide more personalized feedback, directly impact clinical outcomes, facilitate interdisciplinary collaboration, and identify acutely ill patients. Furthermore, a holistic approach beyond a narrow focus on PAP may create a positive collateral effect on adherence by targeting engagement with broader areas of health; and (4) implementing machine learning tools to directly support providers and patients (examples discussed in the next section.) Each of our teams has created workflows embedding these strategies throughout new care pathways. 
Generally, our emphasis during the first 3 months after PAP initiation focuses on achieving therapy adherence, and the post-3-month period broadens the efforts to target clinical outcomes. Recent trials with low PAP usage that failed to confirm the benefit of PAP on cardiovascular outcomes (McEvoy DR, et al. N Engl J Med. 2016;375:919) strongly suggest greater investment in cost-effective long-term strategies is imperative to increase our field’s relevance.
Application of artificial intelligence
We describe current efforts to apply artificial intelligence (AI) into clinical care: (1) We are implementing machine learning (ML) PSG scoring, which can potentially improve both the consistency and efficiency of scoring, further enabling greater investment in follow-up care. The future of sleep study processing, however, will likely depend on computer vision to “view” details inaccessible to the human eye and produce novel metrics that better inform clinical phenotypes (eg, cardiovascular risk, response to alternative therapies, etc). For example, “brain age” has been derived from EEG tracings that could reflect the degree of impact of sleep disorders on neurocognitive function (Fernandez C, unpublished data); (2) Machine learning clinical decision tools are in development to predict PAP adherence and timing of discontinuation, predict timing of cardiovascular disease onset and hospitalization, personalizing adherence targets, automating triaging of patients to home or PSG testing, and innumerable other predictions at clinical decision inflection points. Prediction outputs may be presented as risk profiles embedded in each patient’s “chart,” as personalized alerts, and in gamification strategies. For example, machine learning personalized cardiovascular risk scores can be regularly updated based on degree of PAP use to incentivize adherence; (3) Artificial providers may provide consistent, personalized, and holistic supplementary care. Many people rely on AI-bots for social support and cognitive-behavioral therapy (CBT) for depression. A sleep wellness bot, currently in planning stages, is intended to be the primary interface for many of the strategies described above that enhance engagement with PAP and therapies for comorbid conditions, provide CBT and lifestyle accountability, and collect patient reported data. This artificial provider would be a constant companion providing interactive, personalized, and continuous management to complement traditional intermittent live-person care.
The current health-care environment embodies the principle to “never let a serious crisis go to waste.” COVID-19 has accelerated the progression into the future by fostering an opening to embrace novel application of technologies to support changes in paradigms. Furthermore, health-care infrastructures that typically progress deliberately changed seemingly in a single moment. The Center for Medicare Services issued broad authorization to reimburse for telemedicine in response to COVID-19. Continued evolution in infrastructures will dictate progress with innovation, and a greater transition to outcomes-based incentives may be necessary to accommodate many of the strategies described above that rely on nonsynchronous care. But, we may be experiencing the moment when health care starts to catch up with the world in its embrace of technology. Sleep and pulmonary medicine can be a leader by providing a successful template for other specialties in optimizing chronic disease management.
Dr. Hwang is Medical Director, Kaiser Permanente SBC Sleep Center, and co-chair, Sleep Medicine, Kaiser Permanente Southern California.
Children with cystic fibrosis and their caregivers face sleep difficulties
, according to results from a new study.
Children aged 6-12 years had more sleep issues compared with preschoolers or teenagers, researchers also found, and the quality of sleep among caregivers was seen strongly linked to that of their children with CF.
For research published in the Journal of Cystic Fibrosis, Kelly C. Byars, PsyD, and colleagues at Cincinnati Children’s Medical Center and the University of Cincinnati surveyed parents of 91 medically stable patients with cystic fibrosis aged 18 and younger at a single CF treatment center between 2016 and 2017.
Fifty-four percent of the children in the study were female, the mean age was 9 years, and 90% of the caregivers were mothers. In addition to the sleep questionnaires, the researchers looked at the children’s available lung function data from around the time of the survey. Forced expiratory volume in one second (FEV1) measures showed the vast majority had no obstructive lung disease (73% of the cohort) or only mild symptoms (18%) at the time their caregivers were surveyed.
Overall, some 40% of caregivers said they had concerns about their own sleep, while 29% said they were concerned for their children’s sleep. Parents reported night waking, daytime sleepiness, and difficulty falling asleep as their main problems, and difficulty falling asleep as the top issue for their children, along with daytime sleepiness, night waking, and mouth breathing.
Sleep issues were most pronounced for children aged 6-12 and their caregivers, a group for which 44% of caregivers said they were concerned for their children’s sleep and 55% for their own sleep. For this same group only 8% of parents reported their children having nocturnal cough, and just 5% reported gastrointestinal problems at night.
Overall, the caregivers in the study reported inadequate sleep, with more than half saying they got less than 7 hours per night. Similarly, more than half of the school-age and adolescent patients with CF were getting less than the nightly minimum recommended by the American Academy of Sleep Medicine.
The researchers noted “large effects for parent and child associations for insomnia symptoms that may be amenable to treatment,” especially trouble returning to sleep and daytime sleepiness.
The study “is the first to examine parent reported sleep disturbances and sleep duration in both parents and their children with CF spanning a broad age range and including patients who were medically stable and predominantly free of lung dysfunction,” Dr. Byars and colleagues wrote in their analysis, adding that sleep health should be integrated into care protocols for CF patients and their families, and families of children with other chronic illnesses.
In a comment on Dr. Byars and colleagues’ study, Hovig Artinian, MD, a pediatric pulmonary and sleep medicine specialist at Helen DeVos Children’s Hospital in Grand Rapids, Mich., said the findings “highlight for all of us that we must regularly assess and address sleep disturbances in our children with CF specifically, but also in all children with chronic conditions.”
Children with CF “carry a heavy burden,” Dr. Artinian said, “balancing living their lives with daily interruptions to their typical day to complete multiple treatments. As a result, sleep can be impacted even when there are no other clinical or objective signs of illness, so that was not an entirely surprising finding.” Difficulties with sleep onset and maintenance can be prevalent in the absence of changes in children’s daytime behavior or any other psychological signs, Dr. Artinian said, noting that in his practice he routinely asks families whether children snore (something recommended by the American Academy of Pediatrics for all well-child checks) and whether they have any other concerns about their sleep.
“Even if the answer is ‘no’ the first time, the act of asking plants a seed in their minds to keep an eye open and to know they can discuss it with us at a future visit if concerns come up,” Dr. Artinian said.
Dr. Byars and colleagues noted several limitations to their study including its cross-sectional, single-center design, potential participant selection bias, reliance on parent reports of child sleep, and use of a novel, nonvalidated survey instrument.
The researchers received funding from the Boomer Esiason Foundation for their study and disclosed no financial conflicts of interest. Dr. Artinian had no relevant disclosures.
SOURCE: Byars K et al. J Cyst Fibros. 2020 May. doi: 10.1016/j.jcf.2020.04.003.
, according to results from a new study.
Children aged 6-12 years had more sleep issues compared with preschoolers or teenagers, researchers also found, and the quality of sleep among caregivers was seen strongly linked to that of their children with CF.
For research published in the Journal of Cystic Fibrosis, Kelly C. Byars, PsyD, and colleagues at Cincinnati Children’s Medical Center and the University of Cincinnati surveyed parents of 91 medically stable patients with cystic fibrosis aged 18 and younger at a single CF treatment center between 2016 and 2017.
Fifty-four percent of the children in the study were female, the mean age was 9 years, and 90% of the caregivers were mothers. In addition to the sleep questionnaires, the researchers looked at the children’s available lung function data from around the time of the survey. Forced expiratory volume in one second (FEV1) measures showed the vast majority had no obstructive lung disease (73% of the cohort) or only mild symptoms (18%) at the time their caregivers were surveyed.
Overall, some 40% of caregivers said they had concerns about their own sleep, while 29% said they were concerned for their children’s sleep. Parents reported night waking, daytime sleepiness, and difficulty falling asleep as their main problems, and difficulty falling asleep as the top issue for their children, along with daytime sleepiness, night waking, and mouth breathing.
Sleep issues were most pronounced for children aged 6-12 and their caregivers, a group for which 44% of caregivers said they were concerned for their children’s sleep and 55% for their own sleep. For this same group only 8% of parents reported their children having nocturnal cough, and just 5% reported gastrointestinal problems at night.
Overall, the caregivers in the study reported inadequate sleep, with more than half saying they got less than 7 hours per night. Similarly, more than half of the school-age and adolescent patients with CF were getting less than the nightly minimum recommended by the American Academy of Sleep Medicine.
The researchers noted “large effects for parent and child associations for insomnia symptoms that may be amenable to treatment,” especially trouble returning to sleep and daytime sleepiness.
The study “is the first to examine parent reported sleep disturbances and sleep duration in both parents and their children with CF spanning a broad age range and including patients who were medically stable and predominantly free of lung dysfunction,” Dr. Byars and colleagues wrote in their analysis, adding that sleep health should be integrated into care protocols for CF patients and their families, and families of children with other chronic illnesses.
In a comment on Dr. Byars and colleagues’ study, Hovig Artinian, MD, a pediatric pulmonary and sleep medicine specialist at Helen DeVos Children’s Hospital in Grand Rapids, Mich., said the findings “highlight for all of us that we must regularly assess and address sleep disturbances in our children with CF specifically, but also in all children with chronic conditions.”
Children with CF “carry a heavy burden,” Dr. Artinian said, “balancing living their lives with daily interruptions to their typical day to complete multiple treatments. As a result, sleep can be impacted even when there are no other clinical or objective signs of illness, so that was not an entirely surprising finding.” Difficulties with sleep onset and maintenance can be prevalent in the absence of changes in children’s daytime behavior or any other psychological signs, Dr. Artinian said, noting that in his practice he routinely asks families whether children snore (something recommended by the American Academy of Pediatrics for all well-child checks) and whether they have any other concerns about their sleep.
“Even if the answer is ‘no’ the first time, the act of asking plants a seed in their minds to keep an eye open and to know they can discuss it with us at a future visit if concerns come up,” Dr. Artinian said.
Dr. Byars and colleagues noted several limitations to their study including its cross-sectional, single-center design, potential participant selection bias, reliance on parent reports of child sleep, and use of a novel, nonvalidated survey instrument.
The researchers received funding from the Boomer Esiason Foundation for their study and disclosed no financial conflicts of interest. Dr. Artinian had no relevant disclosures.
SOURCE: Byars K et al. J Cyst Fibros. 2020 May. doi: 10.1016/j.jcf.2020.04.003.
, according to results from a new study.
Children aged 6-12 years had more sleep issues compared with preschoolers or teenagers, researchers also found, and the quality of sleep among caregivers was seen strongly linked to that of their children with CF.
For research published in the Journal of Cystic Fibrosis, Kelly C. Byars, PsyD, and colleagues at Cincinnati Children’s Medical Center and the University of Cincinnati surveyed parents of 91 medically stable patients with cystic fibrosis aged 18 and younger at a single CF treatment center between 2016 and 2017.
Fifty-four percent of the children in the study were female, the mean age was 9 years, and 90% of the caregivers were mothers. In addition to the sleep questionnaires, the researchers looked at the children’s available lung function data from around the time of the survey. Forced expiratory volume in one second (FEV1) measures showed the vast majority had no obstructive lung disease (73% of the cohort) or only mild symptoms (18%) at the time their caregivers were surveyed.
Overall, some 40% of caregivers said they had concerns about their own sleep, while 29% said they were concerned for their children’s sleep. Parents reported night waking, daytime sleepiness, and difficulty falling asleep as their main problems, and difficulty falling asleep as the top issue for their children, along with daytime sleepiness, night waking, and mouth breathing.
Sleep issues were most pronounced for children aged 6-12 and their caregivers, a group for which 44% of caregivers said they were concerned for their children’s sleep and 55% for their own sleep. For this same group only 8% of parents reported their children having nocturnal cough, and just 5% reported gastrointestinal problems at night.
Overall, the caregivers in the study reported inadequate sleep, with more than half saying they got less than 7 hours per night. Similarly, more than half of the school-age and adolescent patients with CF were getting less than the nightly minimum recommended by the American Academy of Sleep Medicine.
The researchers noted “large effects for parent and child associations for insomnia symptoms that may be amenable to treatment,” especially trouble returning to sleep and daytime sleepiness.
The study “is the first to examine parent reported sleep disturbances and sleep duration in both parents and their children with CF spanning a broad age range and including patients who were medically stable and predominantly free of lung dysfunction,” Dr. Byars and colleagues wrote in their analysis, adding that sleep health should be integrated into care protocols for CF patients and their families, and families of children with other chronic illnesses.
In a comment on Dr. Byars and colleagues’ study, Hovig Artinian, MD, a pediatric pulmonary and sleep medicine specialist at Helen DeVos Children’s Hospital in Grand Rapids, Mich., said the findings “highlight for all of us that we must regularly assess and address sleep disturbances in our children with CF specifically, but also in all children with chronic conditions.”
Children with CF “carry a heavy burden,” Dr. Artinian said, “balancing living their lives with daily interruptions to their typical day to complete multiple treatments. As a result, sleep can be impacted even when there are no other clinical or objective signs of illness, so that was not an entirely surprising finding.” Difficulties with sleep onset and maintenance can be prevalent in the absence of changes in children’s daytime behavior or any other psychological signs, Dr. Artinian said, noting that in his practice he routinely asks families whether children snore (something recommended by the American Academy of Pediatrics for all well-child checks) and whether they have any other concerns about their sleep.
“Even if the answer is ‘no’ the first time, the act of asking plants a seed in their minds to keep an eye open and to know they can discuss it with us at a future visit if concerns come up,” Dr. Artinian said.
Dr. Byars and colleagues noted several limitations to their study including its cross-sectional, single-center design, potential participant selection bias, reliance on parent reports of child sleep, and use of a novel, nonvalidated survey instrument.
The researchers received funding from the Boomer Esiason Foundation for their study and disclosed no financial conflicts of interest. Dr. Artinian had no relevant disclosures.
SOURCE: Byars K et al. J Cyst Fibros. 2020 May. doi: 10.1016/j.jcf.2020.04.003.
FROM THE JOURNAL OF CYSTIC FIBROSIS
Steroid-Induced Sleep Disturbance and Delirium: A Focused Review for Critically Ill Patients
Sleep disturbance in the critically ill has received much attention over recent years as this is a common result of intensive care unit (ICU) admission. Disruptions in sleep not only can, at a minimum, cause distress and lower patient satisfaction, but also inhibit recovery from illness and increase morbidity.1,2 Several studies have been conducted highlighting the altered sleep patterns of critically ill patients; although total sleep time may seem normal (7-9 hours), patients can experience multiple awakenings per hour, more time in light sleep (stages 1 and 2), and less time in restorative sleep (stages 3 and 4, [REM]rapid eye movement).2-5
There are several hypothesized physiologic detriments that contribute to slower ICU recovery with sleep deprivation. Research in noncritically ill subjects suggests that sleep deprivation contributes to hypoventilation and potentially prolonged time on the ventilator.6-9 Cardiovascular morbidity may be adversely affected by inflammatory cytokine release seen in sleep disruption.10,11 Studies of noncritically ill patients also suggest that immune response is impaired, potentially protracting infection recovery.12,13 Finally, although not directly investigated, sleep deprivation may contribute to ICU delirium, an independent adverse effect (AE) associated with increased mortality and worse long-term outcomes.14-16
The Society of Critical Care Medicine (SCCM) recently updated its consensus guidelines for the management of pain, agitation/sedation, delirium, immobility, and sleep disruption (PADIS) in adult patients.17 These guidelines offer limited interventions to promote sleep in ICU patients based on available evidence and steer the clinician toward minimizing exacerbating factors. Although factors that affect sleep patterns are multifactorial, such as noise levels, pain, mechanical ventilation, and inflammatory mediators, medication therapy is a known modifiable risk factor for sleep disturbance in critically ill patients.2 This focused review will specifically evaluate the effects of steroids on sleep deprivation, psychosis, delirium, and what is known about these effects in a critically ill population.
To include articles relevant to a critically ill population, a systematic search of MEDLINE and PubMed from 1966 to 2019 was performed using the following Medical Subject Headings (MeSH) terms: delirium/etiology, psychoses, substance-induced/etiology, sleep-wake disorders/chemically induced, neurocognitive disorders/chemically induced, dyssomnias/drug effects plus glucocorticoids/adverse effects, adrenal cortex hormones/adverse effects, prednisone/adverse effects, methylprednisolone/adverse effects, and hydrocortisone/adverse effects. The initial search produced 285 articles. Case reports, reviews, letters, and articles pertaining to primary care or palliative populations were excluded, leaving 8 relevant articles for inclusion (Table 1).18-25
ICU Steroid Use
Steroids are commonly used in the ICU and affect nearly every critically ill population. Common indications for steroids in the ICU include anaphylaxis, airway edema, septic shock, asthma and COPD exacerbations, pneumocystis pneumonia, adrenal crisis, antiemetic treatment, elevated intracranial pressure from tumors, autoimmune disorders, and stress doses needed for chronic steroid users before invasive procedures.26 Whether divided into glucocorticoid or mineralocorticoid subgroups, corticosteroids offer therapeutic benefit from their pharmacologic similarity to endogenously produced cortisol, which includes anti-inflammatory, immunosuppressive, antiproliferative, and vasoconstrictive effects.
Steroid receptors are present in most human tissue, and in varying degrees of binding affinity produce a wide variety of effects. After passive diffusion across cell membranes, steroid-receptor activation binds to various DNA sites, called glucocorticoid regulatory elements, which either stimulates or inhibits transcription of multiple nearby genes.
At the cellular level, corticosteroids inhibit the release of arachidonic acid through upstream production of lipocortin peptides and antagonism of phospholipase A2. This action decreases subsequent inflammatory mediators, including kinins, histamine, liposomal enzymes, and prostaglandins. Steroids also inhibit NF-κB, which further decreases expression of proinflammatory genes while promoting interleukin-10 and its anti-inflammatory properties. Antiproliferative effects of steroids are seen by triggering cell apoptosis and inhibition of fibroblast proliferation.27,28
By binding to mineralocorticoid receptors, steroids cause sodium retention coupled with hydrogen and potassium excretion in the distal renal tubule. Steroids also promote vasoconstriction by upregulating the production and sensitivity of β receptors in the endothelium while suppressing the production of vasodilators. Although rarely used for these physiologic effects, steroids also are involved in a number of metabolic pathways, including calcium regulation, gluconeogenesis, protein metabolism, and fat distribution. Given the similar structure to cortisol, exogenous steroids depress the hypothalamic-pituitary axis (HPA) and decrease the release of adrenocorticotropic hormone (ACTH). Tapering doses of steroid regimens is often required to allow natural androgen and cortisol synthesis and prevent steroid withdrawal.27,28
The potency of various exogenous steroids closely parallels their ability to retain sodium (Table 2). Prolonged activation of steroid receptors can have numerous systemic AEs, including unwanted neurocognitive effects (Table 3). Insomnia and psychosis are commonly described in corticosteroid clinical trials, and in one meta-analysis, both are associated with high costs per episode per year.29
Steroid-Induced Sleep Disruption and Psychosis
Sleep disruption caused by exogenous administration of steroids is thought to trigger other psychostimulant effects, such as mood swings, nervousness, psychoses, and delirium.30 Similarly, the SCCM PADIS guidelines included an ungraded statement: “although an association between sleep quality and delirium occurrence exists in critically ill adults, a cause-effect relationship has not been established.”17 For this review, these AEs will be discussed as related events.
The medical literature proposes 3 pathways primarily responsible for neurocognitive AEs of steroids: behavior changes through modification of the HPA axis, changes in natural sleep-wake cycles, and hyperarousal caused by modification in neuroinhibitory pathways (Figure).
HPA Axis Modification
Under either physical or psychological stress, neural circuits in the brain release corticotropin-releasing hormone (CRH), dehydroepiandrosterone (DHEA), and arginine vasopressin, which go on to activate the sympathetic nervous system and the HPA axis. CRH from the hypothalamus goes on to stimulate ACTH release from the pituitary. ACTH then stimulates cortisol secretion from the adrenal glands. Circulating cortisol feeds into several structures of the brain, including the pituitary, hippocampus, and amygdala. Steroid-receptor complexes alter gene transcription in the central nervous system (CNS), affecting the production of neurotransmitters (eg, dopamine, serotonin) and neuropeptides (eg, somatostatin, β-endorphin). Feedback inhibition ensues, with downregulation of the HPA axis, which prevents depletion of endogenous production of steroids.31 DHEA has protective effects against excessive cortisol activity, but DHEA secretion declines with prolonged cortisol exposure. Exogenous steroids may have different effects than endogenous steroids, and neurocognitive sequelae stem from disruption and imbalance of these physiologic mechanisms.32,33
Steroid receptors are densely located in behavior centers in the brain: the amygdala, septum, and hippocampus. Pharmacologic changes in gene expression alter norepinephrine and serotonin levels in the brain as well as their receptors.32 Prolonged exposure to exogenous steroids has been shown to decrease amygdala and hippocampal volumes.34,35 Furthermore, prolonged corticosteroid exposure has been shown to decrease the number of steroid receptors in the hippocampus, pituitary gland, and amygdala.36 In a somewhat paradoxical finding, the production of CNS proinflammatory cytokines like interleuken-1β and tumor necrosis factor α has been seen after steroid administration, suggesting alternate gene signaling in the CNS.37 Although not proven conclusively, it is felt that these physiologic changes and hyperactivity of the HPA axis are predominantly responsible for changes in behavior, mood, memory, and eventually psychosis in steroid-treated patients.33,38
Finally, alterations in cognition and behavior may be related to steroid-induced changes in CNS carbohydrate, protein, and lipid metabolism with subsequent cellular neurotoxicity.32,38 Glucose uptake into the hippocampus is decreased with steroid exposure. Additionally, breakdown of metabolic compounds to produce energy can be destructive if left unchecked for prolonged periods. DHEA, growth hormone, and testosterone work to repair catabolic damage produced by cortisol, known as anabolic balance. A low anabolic balance (low DHEA levels to high cortisol levels) leads to a cascade of dysregulation in brain activity.39
Changes in Natural Sleep-Wake Cycles
Natural sleep pathways are also affected by steroids. The sleep-wake cycle is primarily regulated in the hypothalamus with circadian release of melatonin from the pineal gland. Melatonin release is highest at night, where it promotes sleep onset and continuity. Upstream, tryptophan is an amino acid that serves as a precursor to serotonin and melatonin.40 Both endogenous and exogenous corticosteroids decrease serum melatonin levels with a markedly diminished circadian rhythm secretion.41,42Demish and colleagues found a significant decrease in mean (SD) nocturnal melatonin plasma levels after the evening administration of oral dexamethasone 1 mg in 11 healthy volunteers: 127 (42) pg/mL before vs 73 (38) pg/mL after; P < .01.42 This result is likely due to decreased cellular metabolism and melatonin synthesis in the pineal gland. Of note, melatonin has neuroprotective affects, and the administration of melatonin has been shown to reverse some steroid-induced neurotoxicities in animal models.43
Steroids also reduce the uptake of tryptophan into the brain.33 Additionally, in animal models, dexamethasone administration caused a significant decrease in the gene expression of tryptophan hydroxylase, which is part of the multistep pathway in synthesizing serotonin from L-tryptophan. These effects upstream could inhibit the biosynthetic capacity of both melatonin and serotonin.44
A third pathway investigated in sleep regulation are the orexin neuropeptides. Orexins are produced in the hypothalamus and stimulate daytime wake activity in monoaminergic and cholinergic neurons. Subsequently, orexin receptor antagonists are a newer class of drugs aimed at mitigating nighttime hyperarousal and sleep disruption. Orexin overexpression may be a causal factor in steroid-induced sleep disturbance. However, this effect was specifically evaluated in a recent study in children with acute lymphoblastic leukemia, which showed that cerebral spinal fluid orexin levels (SD) were not significantly different from baseline after dexamethasone administration: 574 (26.6) pg/mL vs 580 (126.1) pg/mL; P = .8.45
Hyperarousal State
Finally, a hyperarousal state is thought to be produced by nongenomic changes to natural neuroinhibitory regulation seen with nonclassical steroid production called neurosteroids. Animal studies revealed that high levels of steroids were found in the CNS long after adrenalectomy, suggesting CNS de novo synthesis.46 In addition to altering gene expression at classic intercellular steroid receptors, neurosteroids can alter neurotransmission by direct interaction on ion-gated membranes and other receptors on the cell surface. Restlessness and insomnia could be due to γ-aminobutyric acid type A (GABAA) receptor modulation in the CNS where neuroactive steroids slow the rate of recovery of GABAA and potentially inhibit postsynaptic GABAergic transmission. It also is hypothesized that neuroactive steroids have excitatory action at nicotinic acetylcholine, 5HT3 receptors, and through increasing the fractional open time of the N-methyl-D-aspartate -activated channels.47 Allopregnanolone and DHEA are neurosteroids that act as GABAA agonists and have neuroprotective effects with anxiolytic, antidepressant, and antiaggressive properties.
Neurosteroids are synthesized from cholesterol in the hippocampus. Neurosteroids are upregulated in response to stress by CNS cortisol effects on various enzyme expressions.47 Whether exogenous steroid administration affects this biosynthesis vs the stress response in the HPA axis itself is not fully elucidated. Monteleone and colleagues found that dexamethasone 1 mg given orally significantly reduced cortisol and DHEA and allopregnanolone levels in both healthy volunteers and anorexia nervosa patients.48 Similarly, Genazzani and colleagues demonstrated that oral dexamethasone administration (0.5 mg every 6 hours) caused significant reductions in both serum allopregnanolone and DHEA levels.49
Outcomes Studies
The majority of reported data in steroid-induced insomnia and psychosis is in noncritically ill populations. In a randomized, prospective crossover study of healthy volunteers, dexamethasone administration (3 mg every 8 hours for 48 hours) resulted in significant changes in sleep patterns measured with polysomnography. Compared with placebo, steroid treatment showed significantly longer percentage (SD) of stage 0/awake times (11.7% [11.4] vs 2.9% [1.8]; P < .05); longer percentage (SD) of REM sleep latency (363.8 [74.5] minutes vs 202.8 [79.6] minutes; P < .01), and a reduced number (SD) of REM periods (3.8 [2.6] vs 9.7 [3.6]; P < .01).50 Insomnia was one of the most commonly self-reported AEs (> 60%) in a survey of 2,446 chronic steroid users, and the incidence increased as steroid doses increased.51
A prospective, open-label study of 240 patients with cancer demonstrated significant sleep disruptions using the Pittsburgh Sleep Quality Index with the use of high-dose steroids in chemotherapy.52 Naber and colleagues evaluated 50 previously healthy patients taking methylprednisolone 119 mg (41 mg/d) for retinitis and uveitis.53 They reported 26% to 34% of subjects experienced hypomanic syndrome based on a semistructured interview examination. Symptoms developed within 3 days and persisted for the 8-day course of therapy. Brown and colleagues prospectively evaluated 32 asthmatic patients prescribed bursts of prednisone > 40 mg daily. They observed significantly increased scores in the Young Mania Rating Scale within 3 to 7 days of starting therapy, which dissipated to baseline after stopping therapy.54
Despite a high reported incidence of neurologic AEs, outcomes in critically ill populations are mixed. Study methods are varied, and many were largely observational. No prospective, randomized studies exist to date specifically aimed and powered to evaluate the effects of steroids on sleep disturbances or delirium in a critically ill population. Furthermore, sleep quality is difficult to measure in this population, and self-reporting often is not an option. In critical care trials, if AEs such as insomnia, delirium, or psychosis are recorded at all, there is heterogeneity in the definitions, and these AEs are generally poorly defined (eg, psychiatric or neurologic disorder not otherwise specified), making pooled analysis of this outcome difficult.55
One of the largest observational studies in hospitalized patients was through the Boston Collaborative Drug Surveillance Program. A total of 718 consecutively enrolled inpatients who received prednisone were monitored for acute reactions. Psychiatric AEs were rare (1.3%) with low doses (< 40 mg/d), more prevalent (4.6%) with higher doses (41-80 mg/d), and most prevalent (18.4%) with the highest doses (> 80 mg/d), suggesting CNS AEs are dose dependent.18 A single-center, retrospective review of 755 psychiatric consults in hospitalized patients revealed that 54% of manic patients were due to corticosteroid administration.19 In a prospective observational study of 206 consecutive ICU admissions, steroid administration was an independent risk factor for development of ICU delirium, using the Confusion Assessment Method-ICU (CAM-ICU) at a single center (odds ratio [OR], 2.8; 95% CI, 1.05-7.28).25
Two studies in hospitalized oncology patients found conflicting results using the Nursing Delirium Screening Scale (Nu-DESC). One did not find a significant association between delirium and dexamethasone equivalent doses > 15 mg, while the second found an increased hazard ratio (HR) for a positive Nu-DESC score (HR, 2.67; 95% CI, 1.18-6.03).20,21 Similarly, conflicting results were found in 2 studies using first-order Markov models. In one prospective cohort study, 520 consecutive mechanically ventilated patients in 13 ICUs were monitored for the transition to delirium (CAM-ICU positive) from nondelirium states. Steroid administration was significantly associated with transitioning to delirium (OR, 1.52; 95% CI, 1.05-2.21).22 This conflicts with a similar study by Wolters and colleagues, which monitored 1,112 ICU patients who were given a median prednisone equivalent of 50 mg (interquartile range, 25-75 mg). Steroid administration was not significantly associated with the transition to delirium from an awake without delirium state (OR, 1.08; 95% CI, 0.89-1.32; adjusted OR, 1.00; 95% CI, 0.99-1.01 per 10-mg increase in prednisone equivalent).23
Mitigating Effects
Although steroid therapy often cannot be altered in the critically ill population, research showed that steroid overuse is common in ICUs.56,57 Minimizing dosage and duration are important ways clinicians can mitigate unwanted effects. CNS AEs seen with steroids often can be reversed once therapy is discontinued. Avoiding split-dose administration has been proposed given the natural diurnal production of cortisol.58 A review by Flaherty discusses the importance of avoiding pharmacologic agents in hospitalized older patients if possible due to known risks (falls, dependency, hip fractures, rebound insomnia, and risk of delirium) and provides a HELP ME SLEEP nomogram for nonpharmacologic interventions in hospitalized patients (Table 4).59
Historically, lithium has been recommended for steroid-induced mania with chronic steroid use; however, given the large volume and electrolyte shifts seen in critically ill patients, this may not be a viable option. Antidepressants, especially tricyclics, should generally be avoided in steroid-induced psychosis as these may exacerbate symptoms. If symptoms are severe, either typical (haloperidol) or atypical (olanzapine, quetiapine, risperidone) antipsychotics have been used with success.60 Given the known depletion of serum melatonin levels, melatonin supplements are an attractive and relatively safe option for steroid-induced insomnia; however, there are no robust studies specifically aimed at this intervention for this population.
Conclusions
With known, multimodal foci driving sleep impairment in ICU patients, PADIS guidelines recommend myriad interventions for improvement. Recommendations include noise and light reduction with earplugs and/or eyeshades to improve sleep quality. Nocturnal assist-control ventilation may improve sleep quality in ventilated patients. Finally, the development of institutional protocols for promoting sleep quality in ICU patients is recommended.17
1. Simini B. Patients’ perceptions of intensive care. Lancet. 1999;354(9178):571-572. doi: 10.1016/S0140-6736(99)02728-2
2. Delaney LJ, Van Haren F, Lopez V. Sleeping on a problem: the impact of sleep disturbance on intensive care patients—a clinical review. Ann Intensive Care. 2015;15:3. doi: 10.1186/s13613-015-0043-2
3. Friese RS, Diaz-Arrastia R, McBride D, Frankel H, Gentilello LM. Quality and quantity of sleep in the surgical intensive care unit; are our patients sleeping? J Trauma. 2007;63(6):1210-1214. doi: 10.1097/TA.0b013e31815b83d7
4. Elliott R, McKinley S, Cistulli P, Fien M. Characterisation of sleep in intensive care using 24-hour polysomnography: an observational study. Crit Care 2013;17(2):R46.
5. Aurell J, Elmqvist D. Sleep in the surgical intensive care unit: continuous polygraphic recording of sleep in patients receiving postoperative care. BJM (Clin Res Ed). 1985;290(6474)1029-1032. doi: 10.1136/bmj.290.6474.1029
6. White DP, Douglas NJ, Pickett CK, Zwillich CW, Weil JV. Sleep deprivation and the control of ventilation. Am Rev Respir Dis. 1983;128(6):984-986. doi: 10.1164/arrd.1983.128.6.984
7. Series F, Roy N, Marc I. Effects of sleep deprivation and sleep fragmentation on upper airway collapsibility in normal subjects. Am J Respir Crit Care Med. 1994;150(2):481-485. doi: 10.1164/ajrccm.150.2.8049833
8. Tadjalli A, Peever J. Sleep loss reduces respiratory motor plasticity. Adv Exp Med Biol. 2010;669:289-292.
doi: 10.1007/978-1-4419-5692-7_59
9. Roche Campo F, Drouot X, Thille AW, et al. Poor sleep quality is associated with late noninvasive ventilation failure in patients with acute hypercapnic respiratory failure. Crit Care Med. 2010;38(2):447-485. doi: 10.1097/CCM.0b013e3181bc8243
10. Sauvet F, Leftheriotis G, Gomez-Merino D, et al. Effect of acute sleep deprivation on vascular function in healthy subjects. J Appl Physiol (1985). 2010;108(1):68-75. doi: 10.1152/japplphysiol.00851.2009
11. Frey DJ, Fleshner M, Wright KP Jr. The effects of 40 hours of total sleep deprivation on inflammatory markers in healthy young adults. Brain Behav Immun. 2007;21(8):1050-1057. doi: 10.1016/j.bbi.2007.04.003
12. Spiegel K, Sheridan JF, Van Cauter E. Effect of sleep deprivation on response to immunization. JAMA 2002;288(12):1471-1472. doi: 10.1001/jama.288.12.1471-a
13. Dinges DF, Douglas SD, Zuagg L, et al. Leukocytosis and natural killer cell function parallel neurobehavioral fatigue induced by 64 hours of sleep deprivation. J Clin Invest. 1994;93(5):1930-1939. doi: 10.1172/JCI117184
14. Weinhouse GL, Schwab RJ, Watson PL, et al. Bench-to-bedside review: delirium in ICU patients— importance of sleep deprivation. Crit Care. 2009;13(6):234. doi: 10.1186/cc8131
15. Ely EW, Shintani A, Truman B, et al. Delirium as a predictor of mortality in mechanically ventilated patients in the intensive care unit. JAMA. 2004;291(14):1753-1762. doi: 10.1001/jama.291.14.1753
16. Girard TD, Jackson JC, Pandharipande PP, et al. Delirium as a predictor of long-term cognitive impairment in survivors of critical illness. Crit Care Med. 2010;38(7):1513-1520. doi: 10.1097/CCM.0b013e3181e47be1
17. Devlin JW, Skrobik Y, Gelinas C, et al. Clinical practice guidelines for the prevention and management of pain, agitation/sedation, delirium, immobility, and sleep disruption in adult patients in the ICU. Crit Care Med. 2018;46(9):e825-e873
18. The Boston Collaborative Drug Surveillance Program. Acute adverse reactions to prednisone in relation to dosage. Clin Pharmacol Ther. 1972;13(5):694-698. doi: 10.1002/cpt1972135part1694
19. Rundell JR, Wise MG. Causes of organic mood disorder. J Neuropsychiatry Clin Neurosci. 1989;1(4):398-400. doi: 10.1176/jnp.1.4.398
20. Gaudreau JD, Gagnon P, Harel F, Roy MA, Tremblay A. Psychoactive medications and risk of delirium in hospitalized cancer patients. J Clin Oncol. 2005;23(27):6712-6718. doi: 10.1200/JCO.2005.05.140
21. Gaudreau JD, Gagnon P, Roy MA, Harel F, Tremblay A. Opioid medications and longitudinal risk of delirium in hospitalized cancer patients. Cancer. 2007;109(11):2365-2373.
doi: 10.1002/cncr.22665
22. Schreiber MP, Colantuoni E, Bienvenu OJ, et al. Corticosteroids and transition to delirium in patients with acute lung injury. Crit Care Med. 2014;42(6):1480-1486. doi: 10.1097/CCM.0000000000000247
23. Wolters AE, Veldhuijzen DS, Zaal IJ, et al. Systemic corticosteroids and transition to delirium in critically ill patients. Crit Care Med. 2015;43(12):e585-e588. doi: 10.1097/CCM.0000000000001302
24. Matschke J, Muller-Beissenhirtz H, Novotny J, et al. A randomized trial of daily prednisone versus pulsed dexamethasone in treatment-naïve adult patients with immune thrombocytopenia: EIS 2002 study. Acta Haematol. 2016;136(2):101-107. doi: 10.1159/000445420
25. Tilouche N, Hassen M, Ali HBS, Jaoued AHO, Gharbi R, Atrous SS. Delirium in the intensive care unit: incidence, risk factors, and impact on outcome. Indian J Crit Care Med. 2018;22:144-149. doi: 10.4103/ijccm.IJCCM_244_17
26. Young A, Marsh S. Steroid use in critical care. BJA Education. 2018;18(5):129-134. doi: 10.1016/j.bjae.2018.01.005
27. DiPiro J, Talbert R, Yee G, Matzke GR, Wells BG, Posey M. Pharmacotherapy: A Pathophysiologic Approach. 4th ed. New York: McGraw-Hill; 1999:1277-1278.
28. Schimmer
29. Sarnes E, Crofford L, Watson M, Dennis G, Kan H, Bass D. Incidence of US costs of corticosteroid-associated adverse events: a systematic literature review. Clin Ther. 2011;33(10):1413-1432.
30. Idzikowsi C, Shapiro CM. ABC of sleep disorders, non-psychotropic drugs and sleep. BMJ. 1993;306(6885):1118-1120. doi: 10.1136/bmj.306.6885.1118

31. Tasker JG, Herman JP. Mechanisms of rapid glucocorticoid feedback inhibition of the hypothalamic-pituitary-adrenal axis. Stress. 2011;14(4):398-406.
doi: 10.3109/10253890.2011.586446
32. Wolkowitz OM, Reus VI, Weingartner H, et al. Cognitive effects of corticosteroids. Am J Psychiatry 1990;147(10):1297-1303. doi: 10.1176/ajp.147.10.1297
33. McEwen BS, Davis PG, Parsons B, Pfaff DW. The brain as a target for steroid hormone action. Ann Rev Neurosci. 1979;2:65-112. doi: 10.1146/annurev.ne.02.030179.000433
34. Brown ES, Woolston DJ, Frol AM. Amygdala volume in patients receiving chronic corticosteroid therapy. Biol Psychiatry. 2008;63(7):705-709.
doi: 10.1016/j.biopsych.2007.09.014
35. Brown ES, Woolston D, Frol A, et al. Hippocampal volume, spectroscopy, cognition, and mood in patients receiving corticosteroid. Biol Psychiatry. 2004;55(5):538-545.
36. Sapolsky RM, McEwen BS. Down-regulation of neural corticosterone receptors by corticosterone and dexamethasone. Brain Res. 1985;339(1):161-165.
doi: 10.1016/0006-8993(85)90638-9
37. Sorrells SF, Caso JR, Munhoz CD, Spolsky RM. The stressed CNS: when glucocorticoids aggravate inflammation. Neuron. 2009;64(1):33-39.
doi: 10.1016/j.neuron.2009.09.032
38. Wolkowitz OM, Burke H, Epel ES, Reus VI. Glucocorticoids: mood, memory, and mechanisms. Ann NY Acad Sci. 2009;1179:19-40. doi: 10.1111/j.1749-6632.2009.04980.x
39. Wolkowitz OM, Epel ES, Reus VI. Stress hormone-related psychopathology: pathophysiological and treatment implications. World J Biol Psychiatry. 2001;2(3):115-143. doi: 10.3109/15622970109026799
40. Paredes S, Barriga C, Reiter R, Rodrigues A. Assessment of the potential role of tryptophan as the precursor of serotonin and melatonin for the aged sleep-wake cycle and immune function: Streptopelia Risoria as a model. Int J Tryptophan Res. 2009;2:23-36. doi: 10.4137/ijtr.s1129
41. Soszyński P, Stowińska-Srzednicka J, Kasperlik-Zatuska A, Zgliczyński S. Decreased melatonin concentration in Cushing’s Syndrome. Horm Metab Res. 1989;21(12):673-674. doi: 10.1055/s-2007-1009317
42. Demish L, Demish K, Neckelsen T. Influence of dexamethasone on nocturnal melatonin production in healthy adult subjects. J Pineal Res. 1988;5(3):317-321. doi: 10.1111/j.1600-079x.1988.tb00657.x
43. Assaf N, Shalby AB, Khalil WK, Ahmed HH. Biochemical and genetic alterations of oxidant/antioxidant status of the brain in rats treated with dexamethasone: protective roles of melatonin and acetyl-L-carnitine. J Physiol Biochem. 2012;68(1):77-90. doi: 10.1007/s13105-011-0121-3
44. Clark MS, Russo AF. Tissue-specific glucocorticoid regulation of tryptophan hydroxylase mRNA levels. Brain Res Mol Brain Res. 1997;48(2):346-54. doi: 10.1016/s0169-328x(97)00106-x
45. Kram DE, Krasnow SM, Levasseur PR, Zhu X, Stork LC, Marks DL. Dexamethasone chemotherapy does not disrupt orexin signaling. PLoS One. 2016;11(12):e0168731. doi: 10.1371/journal.pone.0168731
46. Mellon S. Neurosteroids: biochemistry, modes of action, and clinical relevance. J Clin Endocrinol Metab. 1994;78(5):1003-1008. doi: 10.1210/jcem.78.5.8175951
47. Zorumski C, Paul SM, Izumi Y, Covey DF, Mennerick S . Neurosteroids, stress and depression: potential therapeutic opportunities. Neurosci Biobehav Rev. 2013;37(1):109-122. doi: 10.1016/j.neubiorev.2012.10.005
48. Monteleone P, Luisi M, Martiadis V, et al. Impaired reduction of enhanced levels of dehydroepiandrosterone by oral dexamethasone in anorexia nervosa. Psychoneuroendocrinology. 2006;31(4):537-542. doi: 10.1016/j.psyneuen.2005.08.015
49. Genazzani AR, Petraglia F, Bernardi F, et al. Circulating levels of allopregnanolone in humans: gender, age, and endocrine influences. J Clin Endocrinol Metab. 1998;83(6):2099-3103. doi: 10.1210/jcem.83.6.4905
50. Moser NJ, Phillips BA, Guthrie G, Barnett G. Effects of dexamethasone on sleep. Pharmacol Toxicol. 1996;79(2):100-102. doi: 10.1111/j.1600-0773.1996.tb00249.x
51. Curtis J, Westfall A, Allison J, et al. Population-based assessment of adverse events associated with long-term glucocorticoid use. Arthritis Rheum. 2006;55(3):420-426. doi: 10.1002/art.21984
52. Zhao J, Dai YH, Xi QS, Yu SY. A clinical study on insomnia in patients with cancer during chemotherapy containing high-dose glucocorticoids. Pharmazie. 2013;68(6):421-427
53. Naber D, Sand P, Heigl B. Psychopathological and neuropsychological effects of 8-days corticosteroid treatment. A prospective study. Psychoneuroendocrinology. 1996;21(1):25-31. doi: 10.1016/0306-4530(95)00031-3
54. Brown ES, Suppes T, Khan DA, Carmody TJ 3rd. Mood changes during prednisone bursts in outpatients with asthma. J Clin Psychopharmacol. 2002;22(1):55-61.
doi: 10.1097/00004714-200202000-00009
55. Warrington TP, Bostwick JM. Psychiatric adverse effects of corticosteroids. Mayo Clin Proc. 2006;81(10):1361-1367. doi: 10.4065/81.10.1361
56. Britt RC, Devine A, Swallen KC et al. Corticosteroid use in the intensive care unit: at what cost? Arch Surg. 2006;141(2):145-159. doi:10.1001/archsurg.141.2.145
57. Kiser TH, Allen RR, Valuck RJ, Moss M, Vanivier RW. Outcomes associated with corticosteroid dosage in critically ill patients in acute exacerbations of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2014;189(9):1052-1064. doi: 10.1164/rccm.201401-0058OC
58. Bourne RS, Mills GH. Sleep disruption in critically ill patients—pharmacological considerations. Anaesthesia. 2004;59(4):374-384. doi: 10.1111/j. 1365-2044.2004.03664.x
59. Flaherty JH. Insomnia among hospitalized older persons. Clin Geriatr Med. 2008;24(1):51-67. doi: 10.1016/j.cger.2007.08.012
60. Sirios F. Steroid psychosis: a review. Gen Hosp Psychiatry. 2003;25(1):27-33. doi: 10.1016/s0163-8343(02)00241-4
Sleep disturbance in the critically ill has received much attention over recent years as this is a common result of intensive care unit (ICU) admission. Disruptions in sleep not only can, at a minimum, cause distress and lower patient satisfaction, but also inhibit recovery from illness and increase morbidity.1,2 Several studies have been conducted highlighting the altered sleep patterns of critically ill patients; although total sleep time may seem normal (7-9 hours), patients can experience multiple awakenings per hour, more time in light sleep (stages 1 and 2), and less time in restorative sleep (stages 3 and 4, [REM]rapid eye movement).2-5
There are several hypothesized physiologic detriments that contribute to slower ICU recovery with sleep deprivation. Research in noncritically ill subjects suggests that sleep deprivation contributes to hypoventilation and potentially prolonged time on the ventilator.6-9 Cardiovascular morbidity may be adversely affected by inflammatory cytokine release seen in sleep disruption.10,11 Studies of noncritically ill patients also suggest that immune response is impaired, potentially protracting infection recovery.12,13 Finally, although not directly investigated, sleep deprivation may contribute to ICU delirium, an independent adverse effect (AE) associated with increased mortality and worse long-term outcomes.14-16
The Society of Critical Care Medicine (SCCM) recently updated its consensus guidelines for the management of pain, agitation/sedation, delirium, immobility, and sleep disruption (PADIS) in adult patients.17 These guidelines offer limited interventions to promote sleep in ICU patients based on available evidence and steer the clinician toward minimizing exacerbating factors. Although factors that affect sleep patterns are multifactorial, such as noise levels, pain, mechanical ventilation, and inflammatory mediators, medication therapy is a known modifiable risk factor for sleep disturbance in critically ill patients.2 This focused review will specifically evaluate the effects of steroids on sleep deprivation, psychosis, delirium, and what is known about these effects in a critically ill population.
To include articles relevant to a critically ill population, a systematic search of MEDLINE and PubMed from 1966 to 2019 was performed using the following Medical Subject Headings (MeSH) terms: delirium/etiology, psychoses, substance-induced/etiology, sleep-wake disorders/chemically induced, neurocognitive disorders/chemically induced, dyssomnias/drug effects plus glucocorticoids/adverse effects, adrenal cortex hormones/adverse effects, prednisone/adverse effects, methylprednisolone/adverse effects, and hydrocortisone/adverse effects. The initial search produced 285 articles. Case reports, reviews, letters, and articles pertaining to primary care or palliative populations were excluded, leaving 8 relevant articles for inclusion (Table 1).18-25
ICU Steroid Use
Steroids are commonly used in the ICU and affect nearly every critically ill population. Common indications for steroids in the ICU include anaphylaxis, airway edema, septic shock, asthma and COPD exacerbations, pneumocystis pneumonia, adrenal crisis, antiemetic treatment, elevated intracranial pressure from tumors, autoimmune disorders, and stress doses needed for chronic steroid users before invasive procedures.26 Whether divided into glucocorticoid or mineralocorticoid subgroups, corticosteroids offer therapeutic benefit from their pharmacologic similarity to endogenously produced cortisol, which includes anti-inflammatory, immunosuppressive, antiproliferative, and vasoconstrictive effects.
Steroid receptors are present in most human tissue, and in varying degrees of binding affinity produce a wide variety of effects. After passive diffusion across cell membranes, steroid-receptor activation binds to various DNA sites, called glucocorticoid regulatory elements, which either stimulates or inhibits transcription of multiple nearby genes.
At the cellular level, corticosteroids inhibit the release of arachidonic acid through upstream production of lipocortin peptides and antagonism of phospholipase A2. This action decreases subsequent inflammatory mediators, including kinins, histamine, liposomal enzymes, and prostaglandins. Steroids also inhibit NF-κB, which further decreases expression of proinflammatory genes while promoting interleukin-10 and its anti-inflammatory properties. Antiproliferative effects of steroids are seen by triggering cell apoptosis and inhibition of fibroblast proliferation.27,28
By binding to mineralocorticoid receptors, steroids cause sodium retention coupled with hydrogen and potassium excretion in the distal renal tubule. Steroids also promote vasoconstriction by upregulating the production and sensitivity of β receptors in the endothelium while suppressing the production of vasodilators. Although rarely used for these physiologic effects, steroids also are involved in a number of metabolic pathways, including calcium regulation, gluconeogenesis, protein metabolism, and fat distribution. Given the similar structure to cortisol, exogenous steroids depress the hypothalamic-pituitary axis (HPA) and decrease the release of adrenocorticotropic hormone (ACTH). Tapering doses of steroid regimens is often required to allow natural androgen and cortisol synthesis and prevent steroid withdrawal.27,28
The potency of various exogenous steroids closely parallels their ability to retain sodium (Table 2). Prolonged activation of steroid receptors can have numerous systemic AEs, including unwanted neurocognitive effects (Table 3). Insomnia and psychosis are commonly described in corticosteroid clinical trials, and in one meta-analysis, both are associated with high costs per episode per year.29
Steroid-Induced Sleep Disruption and Psychosis
Sleep disruption caused by exogenous administration of steroids is thought to trigger other psychostimulant effects, such as mood swings, nervousness, psychoses, and delirium.30 Similarly, the SCCM PADIS guidelines included an ungraded statement: “although an association between sleep quality and delirium occurrence exists in critically ill adults, a cause-effect relationship has not been established.”17 For this review, these AEs will be discussed as related events.
The medical literature proposes 3 pathways primarily responsible for neurocognitive AEs of steroids: behavior changes through modification of the HPA axis, changes in natural sleep-wake cycles, and hyperarousal caused by modification in neuroinhibitory pathways (Figure).
HPA Axis Modification
Under either physical or psychological stress, neural circuits in the brain release corticotropin-releasing hormone (CRH), dehydroepiandrosterone (DHEA), and arginine vasopressin, which go on to activate the sympathetic nervous system and the HPA axis. CRH from the hypothalamus goes on to stimulate ACTH release from the pituitary. ACTH then stimulates cortisol secretion from the adrenal glands. Circulating cortisol feeds into several structures of the brain, including the pituitary, hippocampus, and amygdala. Steroid-receptor complexes alter gene transcription in the central nervous system (CNS), affecting the production of neurotransmitters (eg, dopamine, serotonin) and neuropeptides (eg, somatostatin, β-endorphin). Feedback inhibition ensues, with downregulation of the HPA axis, which prevents depletion of endogenous production of steroids.31 DHEA has protective effects against excessive cortisol activity, but DHEA secretion declines with prolonged cortisol exposure. Exogenous steroids may have different effects than endogenous steroids, and neurocognitive sequelae stem from disruption and imbalance of these physiologic mechanisms.32,33
Steroid receptors are densely located in behavior centers in the brain: the amygdala, septum, and hippocampus. Pharmacologic changes in gene expression alter norepinephrine and serotonin levels in the brain as well as their receptors.32 Prolonged exposure to exogenous steroids has been shown to decrease amygdala and hippocampal volumes.34,35 Furthermore, prolonged corticosteroid exposure has been shown to decrease the number of steroid receptors in the hippocampus, pituitary gland, and amygdala.36 In a somewhat paradoxical finding, the production of CNS proinflammatory cytokines like interleuken-1β and tumor necrosis factor α has been seen after steroid administration, suggesting alternate gene signaling in the CNS.37 Although not proven conclusively, it is felt that these physiologic changes and hyperactivity of the HPA axis are predominantly responsible for changes in behavior, mood, memory, and eventually psychosis in steroid-treated patients.33,38
Finally, alterations in cognition and behavior may be related to steroid-induced changes in CNS carbohydrate, protein, and lipid metabolism with subsequent cellular neurotoxicity.32,38 Glucose uptake into the hippocampus is decreased with steroid exposure. Additionally, breakdown of metabolic compounds to produce energy can be destructive if left unchecked for prolonged periods. DHEA, growth hormone, and testosterone work to repair catabolic damage produced by cortisol, known as anabolic balance. A low anabolic balance (low DHEA levels to high cortisol levels) leads to a cascade of dysregulation in brain activity.39
Changes in Natural Sleep-Wake Cycles
Natural sleep pathways are also affected by steroids. The sleep-wake cycle is primarily regulated in the hypothalamus with circadian release of melatonin from the pineal gland. Melatonin release is highest at night, where it promotes sleep onset and continuity. Upstream, tryptophan is an amino acid that serves as a precursor to serotonin and melatonin.40 Both endogenous and exogenous corticosteroids decrease serum melatonin levels with a markedly diminished circadian rhythm secretion.41,42Demish and colleagues found a significant decrease in mean (SD) nocturnal melatonin plasma levels after the evening administration of oral dexamethasone 1 mg in 11 healthy volunteers: 127 (42) pg/mL before vs 73 (38) pg/mL after; P < .01.42 This result is likely due to decreased cellular metabolism and melatonin synthesis in the pineal gland. Of note, melatonin has neuroprotective affects, and the administration of melatonin has been shown to reverse some steroid-induced neurotoxicities in animal models.43
Steroids also reduce the uptake of tryptophan into the brain.33 Additionally, in animal models, dexamethasone administration caused a significant decrease in the gene expression of tryptophan hydroxylase, which is part of the multistep pathway in synthesizing serotonin from L-tryptophan. These effects upstream could inhibit the biosynthetic capacity of both melatonin and serotonin.44
A third pathway investigated in sleep regulation are the orexin neuropeptides. Orexins are produced in the hypothalamus and stimulate daytime wake activity in monoaminergic and cholinergic neurons. Subsequently, orexin receptor antagonists are a newer class of drugs aimed at mitigating nighttime hyperarousal and sleep disruption. Orexin overexpression may be a causal factor in steroid-induced sleep disturbance. However, this effect was specifically evaluated in a recent study in children with acute lymphoblastic leukemia, which showed that cerebral spinal fluid orexin levels (SD) were not significantly different from baseline after dexamethasone administration: 574 (26.6) pg/mL vs 580 (126.1) pg/mL; P = .8.45
Hyperarousal State
Finally, a hyperarousal state is thought to be produced by nongenomic changes to natural neuroinhibitory regulation seen with nonclassical steroid production called neurosteroids. Animal studies revealed that high levels of steroids were found in the CNS long after adrenalectomy, suggesting CNS de novo synthesis.46 In addition to altering gene expression at classic intercellular steroid receptors, neurosteroids can alter neurotransmission by direct interaction on ion-gated membranes and other receptors on the cell surface. Restlessness and insomnia could be due to γ-aminobutyric acid type A (GABAA) receptor modulation in the CNS where neuroactive steroids slow the rate of recovery of GABAA and potentially inhibit postsynaptic GABAergic transmission. It also is hypothesized that neuroactive steroids have excitatory action at nicotinic acetylcholine, 5HT3 receptors, and through increasing the fractional open time of the N-methyl-D-aspartate -activated channels.47 Allopregnanolone and DHEA are neurosteroids that act as GABAA agonists and have neuroprotective effects with anxiolytic, antidepressant, and antiaggressive properties.
Neurosteroids are synthesized from cholesterol in the hippocampus. Neurosteroids are upregulated in response to stress by CNS cortisol effects on various enzyme expressions.47 Whether exogenous steroid administration affects this biosynthesis vs the stress response in the HPA axis itself is not fully elucidated. Monteleone and colleagues found that dexamethasone 1 mg given orally significantly reduced cortisol and DHEA and allopregnanolone levels in both healthy volunteers and anorexia nervosa patients.48 Similarly, Genazzani and colleagues demonstrated that oral dexamethasone administration (0.5 mg every 6 hours) caused significant reductions in both serum allopregnanolone and DHEA levels.49
Outcomes Studies
The majority of reported data in steroid-induced insomnia and psychosis is in noncritically ill populations. In a randomized, prospective crossover study of healthy volunteers, dexamethasone administration (3 mg every 8 hours for 48 hours) resulted in significant changes in sleep patterns measured with polysomnography. Compared with placebo, steroid treatment showed significantly longer percentage (SD) of stage 0/awake times (11.7% [11.4] vs 2.9% [1.8]; P < .05); longer percentage (SD) of REM sleep latency (363.8 [74.5] minutes vs 202.8 [79.6] minutes; P < .01), and a reduced number (SD) of REM periods (3.8 [2.6] vs 9.7 [3.6]; P < .01).50 Insomnia was one of the most commonly self-reported AEs (> 60%) in a survey of 2,446 chronic steroid users, and the incidence increased as steroid doses increased.51
A prospective, open-label study of 240 patients with cancer demonstrated significant sleep disruptions using the Pittsburgh Sleep Quality Index with the use of high-dose steroids in chemotherapy.52 Naber and colleagues evaluated 50 previously healthy patients taking methylprednisolone 119 mg (41 mg/d) for retinitis and uveitis.53 They reported 26% to 34% of subjects experienced hypomanic syndrome based on a semistructured interview examination. Symptoms developed within 3 days and persisted for the 8-day course of therapy. Brown and colleagues prospectively evaluated 32 asthmatic patients prescribed bursts of prednisone > 40 mg daily. They observed significantly increased scores in the Young Mania Rating Scale within 3 to 7 days of starting therapy, which dissipated to baseline after stopping therapy.54
Despite a high reported incidence of neurologic AEs, outcomes in critically ill populations are mixed. Study methods are varied, and many were largely observational. No prospective, randomized studies exist to date specifically aimed and powered to evaluate the effects of steroids on sleep disturbances or delirium in a critically ill population. Furthermore, sleep quality is difficult to measure in this population, and self-reporting often is not an option. In critical care trials, if AEs such as insomnia, delirium, or psychosis are recorded at all, there is heterogeneity in the definitions, and these AEs are generally poorly defined (eg, psychiatric or neurologic disorder not otherwise specified), making pooled analysis of this outcome difficult.55
One of the largest observational studies in hospitalized patients was through the Boston Collaborative Drug Surveillance Program. A total of 718 consecutively enrolled inpatients who received prednisone were monitored for acute reactions. Psychiatric AEs were rare (1.3%) with low doses (< 40 mg/d), more prevalent (4.6%) with higher doses (41-80 mg/d), and most prevalent (18.4%) with the highest doses (> 80 mg/d), suggesting CNS AEs are dose dependent.18 A single-center, retrospective review of 755 psychiatric consults in hospitalized patients revealed that 54% of manic patients were due to corticosteroid administration.19 In a prospective observational study of 206 consecutive ICU admissions, steroid administration was an independent risk factor for development of ICU delirium, using the Confusion Assessment Method-ICU (CAM-ICU) at a single center (odds ratio [OR], 2.8; 95% CI, 1.05-7.28).25
Two studies in hospitalized oncology patients found conflicting results using the Nursing Delirium Screening Scale (Nu-DESC). One did not find a significant association between delirium and dexamethasone equivalent doses > 15 mg, while the second found an increased hazard ratio (HR) for a positive Nu-DESC score (HR, 2.67; 95% CI, 1.18-6.03).20,21 Similarly, conflicting results were found in 2 studies using first-order Markov models. In one prospective cohort study, 520 consecutive mechanically ventilated patients in 13 ICUs were monitored for the transition to delirium (CAM-ICU positive) from nondelirium states. Steroid administration was significantly associated with transitioning to delirium (OR, 1.52; 95% CI, 1.05-2.21).22 This conflicts with a similar study by Wolters and colleagues, which monitored 1,112 ICU patients who were given a median prednisone equivalent of 50 mg (interquartile range, 25-75 mg). Steroid administration was not significantly associated with the transition to delirium from an awake without delirium state (OR, 1.08; 95% CI, 0.89-1.32; adjusted OR, 1.00; 95% CI, 0.99-1.01 per 10-mg increase in prednisone equivalent).23
Mitigating Effects
Although steroid therapy often cannot be altered in the critically ill population, research showed that steroid overuse is common in ICUs.56,57 Minimizing dosage and duration are important ways clinicians can mitigate unwanted effects. CNS AEs seen with steroids often can be reversed once therapy is discontinued. Avoiding split-dose administration has been proposed given the natural diurnal production of cortisol.58 A review by Flaherty discusses the importance of avoiding pharmacologic agents in hospitalized older patients if possible due to known risks (falls, dependency, hip fractures, rebound insomnia, and risk of delirium) and provides a HELP ME SLEEP nomogram for nonpharmacologic interventions in hospitalized patients (Table 4).59
Historically, lithium has been recommended for steroid-induced mania with chronic steroid use; however, given the large volume and electrolyte shifts seen in critically ill patients, this may not be a viable option. Antidepressants, especially tricyclics, should generally be avoided in steroid-induced psychosis as these may exacerbate symptoms. If symptoms are severe, either typical (haloperidol) or atypical (olanzapine, quetiapine, risperidone) antipsychotics have been used with success.60 Given the known depletion of serum melatonin levels, melatonin supplements are an attractive and relatively safe option for steroid-induced insomnia; however, there are no robust studies specifically aimed at this intervention for this population.
Conclusions
With known, multimodal foci driving sleep impairment in ICU patients, PADIS guidelines recommend myriad interventions for improvement. Recommendations include noise and light reduction with earplugs and/or eyeshades to improve sleep quality. Nocturnal assist-control ventilation may improve sleep quality in ventilated patients. Finally, the development of institutional protocols for promoting sleep quality in ICU patients is recommended.17
Sleep disturbance in the critically ill has received much attention over recent years as this is a common result of intensive care unit (ICU) admission. Disruptions in sleep not only can, at a minimum, cause distress and lower patient satisfaction, but also inhibit recovery from illness and increase morbidity.1,2 Several studies have been conducted highlighting the altered sleep patterns of critically ill patients; although total sleep time may seem normal (7-9 hours), patients can experience multiple awakenings per hour, more time in light sleep (stages 1 and 2), and less time in restorative sleep (stages 3 and 4, [REM]rapid eye movement).2-5
There are several hypothesized physiologic detriments that contribute to slower ICU recovery with sleep deprivation. Research in noncritically ill subjects suggests that sleep deprivation contributes to hypoventilation and potentially prolonged time on the ventilator.6-9 Cardiovascular morbidity may be adversely affected by inflammatory cytokine release seen in sleep disruption.10,11 Studies of noncritically ill patients also suggest that immune response is impaired, potentially protracting infection recovery.12,13 Finally, although not directly investigated, sleep deprivation may contribute to ICU delirium, an independent adverse effect (AE) associated with increased mortality and worse long-term outcomes.14-16
The Society of Critical Care Medicine (SCCM) recently updated its consensus guidelines for the management of pain, agitation/sedation, delirium, immobility, and sleep disruption (PADIS) in adult patients.17 These guidelines offer limited interventions to promote sleep in ICU patients based on available evidence and steer the clinician toward minimizing exacerbating factors. Although factors that affect sleep patterns are multifactorial, such as noise levels, pain, mechanical ventilation, and inflammatory mediators, medication therapy is a known modifiable risk factor for sleep disturbance in critically ill patients.2 This focused review will specifically evaluate the effects of steroids on sleep deprivation, psychosis, delirium, and what is known about these effects in a critically ill population.
To include articles relevant to a critically ill population, a systematic search of MEDLINE and PubMed from 1966 to 2019 was performed using the following Medical Subject Headings (MeSH) terms: delirium/etiology, psychoses, substance-induced/etiology, sleep-wake disorders/chemically induced, neurocognitive disorders/chemically induced, dyssomnias/drug effects plus glucocorticoids/adverse effects, adrenal cortex hormones/adverse effects, prednisone/adverse effects, methylprednisolone/adverse effects, and hydrocortisone/adverse effects. The initial search produced 285 articles. Case reports, reviews, letters, and articles pertaining to primary care or palliative populations were excluded, leaving 8 relevant articles for inclusion (Table 1).18-25
ICU Steroid Use
Steroids are commonly used in the ICU and affect nearly every critically ill population. Common indications for steroids in the ICU include anaphylaxis, airway edema, septic shock, asthma and COPD exacerbations, pneumocystis pneumonia, adrenal crisis, antiemetic treatment, elevated intracranial pressure from tumors, autoimmune disorders, and stress doses needed for chronic steroid users before invasive procedures.26 Whether divided into glucocorticoid or mineralocorticoid subgroups, corticosteroids offer therapeutic benefit from their pharmacologic similarity to endogenously produced cortisol, which includes anti-inflammatory, immunosuppressive, antiproliferative, and vasoconstrictive effects.
Steroid receptors are present in most human tissue, and in varying degrees of binding affinity produce a wide variety of effects. After passive diffusion across cell membranes, steroid-receptor activation binds to various DNA sites, called glucocorticoid regulatory elements, which either stimulates or inhibits transcription of multiple nearby genes.
At the cellular level, corticosteroids inhibit the release of arachidonic acid through upstream production of lipocortin peptides and antagonism of phospholipase A2. This action decreases subsequent inflammatory mediators, including kinins, histamine, liposomal enzymes, and prostaglandins. Steroids also inhibit NF-κB, which further decreases expression of proinflammatory genes while promoting interleukin-10 and its anti-inflammatory properties. Antiproliferative effects of steroids are seen by triggering cell apoptosis and inhibition of fibroblast proliferation.27,28
By binding to mineralocorticoid receptors, steroids cause sodium retention coupled with hydrogen and potassium excretion in the distal renal tubule. Steroids also promote vasoconstriction by upregulating the production and sensitivity of β receptors in the endothelium while suppressing the production of vasodilators. Although rarely used for these physiologic effects, steroids also are involved in a number of metabolic pathways, including calcium regulation, gluconeogenesis, protein metabolism, and fat distribution. Given the similar structure to cortisol, exogenous steroids depress the hypothalamic-pituitary axis (HPA) and decrease the release of adrenocorticotropic hormone (ACTH). Tapering doses of steroid regimens is often required to allow natural androgen and cortisol synthesis and prevent steroid withdrawal.27,28
The potency of various exogenous steroids closely parallels their ability to retain sodium (Table 2). Prolonged activation of steroid receptors can have numerous systemic AEs, including unwanted neurocognitive effects (Table 3). Insomnia and psychosis are commonly described in corticosteroid clinical trials, and in one meta-analysis, both are associated with high costs per episode per year.29
Steroid-Induced Sleep Disruption and Psychosis
Sleep disruption caused by exogenous administration of steroids is thought to trigger other psychostimulant effects, such as mood swings, nervousness, psychoses, and delirium.30 Similarly, the SCCM PADIS guidelines included an ungraded statement: “although an association between sleep quality and delirium occurrence exists in critically ill adults, a cause-effect relationship has not been established.”17 For this review, these AEs will be discussed as related events.
The medical literature proposes 3 pathways primarily responsible for neurocognitive AEs of steroids: behavior changes through modification of the HPA axis, changes in natural sleep-wake cycles, and hyperarousal caused by modification in neuroinhibitory pathways (Figure).
HPA Axis Modification
Under either physical or psychological stress, neural circuits in the brain release corticotropin-releasing hormone (CRH), dehydroepiandrosterone (DHEA), and arginine vasopressin, which go on to activate the sympathetic nervous system and the HPA axis. CRH from the hypothalamus goes on to stimulate ACTH release from the pituitary. ACTH then stimulates cortisol secretion from the adrenal glands. Circulating cortisol feeds into several structures of the brain, including the pituitary, hippocampus, and amygdala. Steroid-receptor complexes alter gene transcription in the central nervous system (CNS), affecting the production of neurotransmitters (eg, dopamine, serotonin) and neuropeptides (eg, somatostatin, β-endorphin). Feedback inhibition ensues, with downregulation of the HPA axis, which prevents depletion of endogenous production of steroids.31 DHEA has protective effects against excessive cortisol activity, but DHEA secretion declines with prolonged cortisol exposure. Exogenous steroids may have different effects than endogenous steroids, and neurocognitive sequelae stem from disruption and imbalance of these physiologic mechanisms.32,33
Steroid receptors are densely located in behavior centers in the brain: the amygdala, septum, and hippocampus. Pharmacologic changes in gene expression alter norepinephrine and serotonin levels in the brain as well as their receptors.32 Prolonged exposure to exogenous steroids has been shown to decrease amygdala and hippocampal volumes.34,35 Furthermore, prolonged corticosteroid exposure has been shown to decrease the number of steroid receptors in the hippocampus, pituitary gland, and amygdala.36 In a somewhat paradoxical finding, the production of CNS proinflammatory cytokines like interleuken-1β and tumor necrosis factor α has been seen after steroid administration, suggesting alternate gene signaling in the CNS.37 Although not proven conclusively, it is felt that these physiologic changes and hyperactivity of the HPA axis are predominantly responsible for changes in behavior, mood, memory, and eventually psychosis in steroid-treated patients.33,38
Finally, alterations in cognition and behavior may be related to steroid-induced changes in CNS carbohydrate, protein, and lipid metabolism with subsequent cellular neurotoxicity.32,38 Glucose uptake into the hippocampus is decreased with steroid exposure. Additionally, breakdown of metabolic compounds to produce energy can be destructive if left unchecked for prolonged periods. DHEA, growth hormone, and testosterone work to repair catabolic damage produced by cortisol, known as anabolic balance. A low anabolic balance (low DHEA levels to high cortisol levels) leads to a cascade of dysregulation in brain activity.39
Changes in Natural Sleep-Wake Cycles
Natural sleep pathways are also affected by steroids. The sleep-wake cycle is primarily regulated in the hypothalamus with circadian release of melatonin from the pineal gland. Melatonin release is highest at night, where it promotes sleep onset and continuity. Upstream, tryptophan is an amino acid that serves as a precursor to serotonin and melatonin.40 Both endogenous and exogenous corticosteroids decrease serum melatonin levels with a markedly diminished circadian rhythm secretion.41,42Demish and colleagues found a significant decrease in mean (SD) nocturnal melatonin plasma levels after the evening administration of oral dexamethasone 1 mg in 11 healthy volunteers: 127 (42) pg/mL before vs 73 (38) pg/mL after; P < .01.42 This result is likely due to decreased cellular metabolism and melatonin synthesis in the pineal gland. Of note, melatonin has neuroprotective affects, and the administration of melatonin has been shown to reverse some steroid-induced neurotoxicities in animal models.43
Steroids also reduce the uptake of tryptophan into the brain.33 Additionally, in animal models, dexamethasone administration caused a significant decrease in the gene expression of tryptophan hydroxylase, which is part of the multistep pathway in synthesizing serotonin from L-tryptophan. These effects upstream could inhibit the biosynthetic capacity of both melatonin and serotonin.44
A third pathway investigated in sleep regulation are the orexin neuropeptides. Orexins are produced in the hypothalamus and stimulate daytime wake activity in monoaminergic and cholinergic neurons. Subsequently, orexin receptor antagonists are a newer class of drugs aimed at mitigating nighttime hyperarousal and sleep disruption. Orexin overexpression may be a causal factor in steroid-induced sleep disturbance. However, this effect was specifically evaluated in a recent study in children with acute lymphoblastic leukemia, which showed that cerebral spinal fluid orexin levels (SD) were not significantly different from baseline after dexamethasone administration: 574 (26.6) pg/mL vs 580 (126.1) pg/mL; P = .8.45
Hyperarousal State
Finally, a hyperarousal state is thought to be produced by nongenomic changes to natural neuroinhibitory regulation seen with nonclassical steroid production called neurosteroids. Animal studies revealed that high levels of steroids were found in the CNS long after adrenalectomy, suggesting CNS de novo synthesis.46 In addition to altering gene expression at classic intercellular steroid receptors, neurosteroids can alter neurotransmission by direct interaction on ion-gated membranes and other receptors on the cell surface. Restlessness and insomnia could be due to γ-aminobutyric acid type A (GABAA) receptor modulation in the CNS where neuroactive steroids slow the rate of recovery of GABAA and potentially inhibit postsynaptic GABAergic transmission. It also is hypothesized that neuroactive steroids have excitatory action at nicotinic acetylcholine, 5HT3 receptors, and through increasing the fractional open time of the N-methyl-D-aspartate -activated channels.47 Allopregnanolone and DHEA are neurosteroids that act as GABAA agonists and have neuroprotective effects with anxiolytic, antidepressant, and antiaggressive properties.
Neurosteroids are synthesized from cholesterol in the hippocampus. Neurosteroids are upregulated in response to stress by CNS cortisol effects on various enzyme expressions.47 Whether exogenous steroid administration affects this biosynthesis vs the stress response in the HPA axis itself is not fully elucidated. Monteleone and colleagues found that dexamethasone 1 mg given orally significantly reduced cortisol and DHEA and allopregnanolone levels in both healthy volunteers and anorexia nervosa patients.48 Similarly, Genazzani and colleagues demonstrated that oral dexamethasone administration (0.5 mg every 6 hours) caused significant reductions in both serum allopregnanolone and DHEA levels.49
Outcomes Studies
The majority of reported data in steroid-induced insomnia and psychosis is in noncritically ill populations. In a randomized, prospective crossover study of healthy volunteers, dexamethasone administration (3 mg every 8 hours for 48 hours) resulted in significant changes in sleep patterns measured with polysomnography. Compared with placebo, steroid treatment showed significantly longer percentage (SD) of stage 0/awake times (11.7% [11.4] vs 2.9% [1.8]; P < .05); longer percentage (SD) of REM sleep latency (363.8 [74.5] minutes vs 202.8 [79.6] minutes; P < .01), and a reduced number (SD) of REM periods (3.8 [2.6] vs 9.7 [3.6]; P < .01).50 Insomnia was one of the most commonly self-reported AEs (> 60%) in a survey of 2,446 chronic steroid users, and the incidence increased as steroid doses increased.51
A prospective, open-label study of 240 patients with cancer demonstrated significant sleep disruptions using the Pittsburgh Sleep Quality Index with the use of high-dose steroids in chemotherapy.52 Naber and colleagues evaluated 50 previously healthy patients taking methylprednisolone 119 mg (41 mg/d) for retinitis and uveitis.53 They reported 26% to 34% of subjects experienced hypomanic syndrome based on a semistructured interview examination. Symptoms developed within 3 days and persisted for the 8-day course of therapy. Brown and colleagues prospectively evaluated 32 asthmatic patients prescribed bursts of prednisone > 40 mg daily. They observed significantly increased scores in the Young Mania Rating Scale within 3 to 7 days of starting therapy, which dissipated to baseline after stopping therapy.54
Despite a high reported incidence of neurologic AEs, outcomes in critically ill populations are mixed. Study methods are varied, and many were largely observational. No prospective, randomized studies exist to date specifically aimed and powered to evaluate the effects of steroids on sleep disturbances or delirium in a critically ill population. Furthermore, sleep quality is difficult to measure in this population, and self-reporting often is not an option. In critical care trials, if AEs such as insomnia, delirium, or psychosis are recorded at all, there is heterogeneity in the definitions, and these AEs are generally poorly defined (eg, psychiatric or neurologic disorder not otherwise specified), making pooled analysis of this outcome difficult.55
One of the largest observational studies in hospitalized patients was through the Boston Collaborative Drug Surveillance Program. A total of 718 consecutively enrolled inpatients who received prednisone were monitored for acute reactions. Psychiatric AEs were rare (1.3%) with low doses (< 40 mg/d), more prevalent (4.6%) with higher doses (41-80 mg/d), and most prevalent (18.4%) with the highest doses (> 80 mg/d), suggesting CNS AEs are dose dependent.18 A single-center, retrospective review of 755 psychiatric consults in hospitalized patients revealed that 54% of manic patients were due to corticosteroid administration.19 In a prospective observational study of 206 consecutive ICU admissions, steroid administration was an independent risk factor for development of ICU delirium, using the Confusion Assessment Method-ICU (CAM-ICU) at a single center (odds ratio [OR], 2.8; 95% CI, 1.05-7.28).25
Two studies in hospitalized oncology patients found conflicting results using the Nursing Delirium Screening Scale (Nu-DESC). One did not find a significant association between delirium and dexamethasone equivalent doses > 15 mg, while the second found an increased hazard ratio (HR) for a positive Nu-DESC score (HR, 2.67; 95% CI, 1.18-6.03).20,21 Similarly, conflicting results were found in 2 studies using first-order Markov models. In one prospective cohort study, 520 consecutive mechanically ventilated patients in 13 ICUs were monitored for the transition to delirium (CAM-ICU positive) from nondelirium states. Steroid administration was significantly associated with transitioning to delirium (OR, 1.52; 95% CI, 1.05-2.21).22 This conflicts with a similar study by Wolters and colleagues, which monitored 1,112 ICU patients who were given a median prednisone equivalent of 50 mg (interquartile range, 25-75 mg). Steroid administration was not significantly associated with the transition to delirium from an awake without delirium state (OR, 1.08; 95% CI, 0.89-1.32; adjusted OR, 1.00; 95% CI, 0.99-1.01 per 10-mg increase in prednisone equivalent).23
Mitigating Effects
Although steroid therapy often cannot be altered in the critically ill population, research showed that steroid overuse is common in ICUs.56,57 Minimizing dosage and duration are important ways clinicians can mitigate unwanted effects. CNS AEs seen with steroids often can be reversed once therapy is discontinued. Avoiding split-dose administration has been proposed given the natural diurnal production of cortisol.58 A review by Flaherty discusses the importance of avoiding pharmacologic agents in hospitalized older patients if possible due to known risks (falls, dependency, hip fractures, rebound insomnia, and risk of delirium) and provides a HELP ME SLEEP nomogram for nonpharmacologic interventions in hospitalized patients (Table 4).59
Historically, lithium has been recommended for steroid-induced mania with chronic steroid use; however, given the large volume and electrolyte shifts seen in critically ill patients, this may not be a viable option. Antidepressants, especially tricyclics, should generally be avoided in steroid-induced psychosis as these may exacerbate symptoms. If symptoms are severe, either typical (haloperidol) or atypical (olanzapine, quetiapine, risperidone) antipsychotics have been used with success.60 Given the known depletion of serum melatonin levels, melatonin supplements are an attractive and relatively safe option for steroid-induced insomnia; however, there are no robust studies specifically aimed at this intervention for this population.
Conclusions
With known, multimodal foci driving sleep impairment in ICU patients, PADIS guidelines recommend myriad interventions for improvement. Recommendations include noise and light reduction with earplugs and/or eyeshades to improve sleep quality. Nocturnal assist-control ventilation may improve sleep quality in ventilated patients. Finally, the development of institutional protocols for promoting sleep quality in ICU patients is recommended.17
1. Simini B. Patients’ perceptions of intensive care. Lancet. 1999;354(9178):571-572. doi: 10.1016/S0140-6736(99)02728-2
2. Delaney LJ, Van Haren F, Lopez V. Sleeping on a problem: the impact of sleep disturbance on intensive care patients—a clinical review. Ann Intensive Care. 2015;15:3. doi: 10.1186/s13613-015-0043-2
3. Friese RS, Diaz-Arrastia R, McBride D, Frankel H, Gentilello LM. Quality and quantity of sleep in the surgical intensive care unit; are our patients sleeping? J Trauma. 2007;63(6):1210-1214. doi: 10.1097/TA.0b013e31815b83d7
4. Elliott R, McKinley S, Cistulli P, Fien M. Characterisation of sleep in intensive care using 24-hour polysomnography: an observational study. Crit Care 2013;17(2):R46.
5. Aurell J, Elmqvist D. Sleep in the surgical intensive care unit: continuous polygraphic recording of sleep in patients receiving postoperative care. BJM (Clin Res Ed). 1985;290(6474)1029-1032. doi: 10.1136/bmj.290.6474.1029
6. White DP, Douglas NJ, Pickett CK, Zwillich CW, Weil JV. Sleep deprivation and the control of ventilation. Am Rev Respir Dis. 1983;128(6):984-986. doi: 10.1164/arrd.1983.128.6.984
7. Series F, Roy N, Marc I. Effects of sleep deprivation and sleep fragmentation on upper airway collapsibility in normal subjects. Am J Respir Crit Care Med. 1994;150(2):481-485. doi: 10.1164/ajrccm.150.2.8049833
8. Tadjalli A, Peever J. Sleep loss reduces respiratory motor plasticity. Adv Exp Med Biol. 2010;669:289-292.
doi: 10.1007/978-1-4419-5692-7_59
9. Roche Campo F, Drouot X, Thille AW, et al. Poor sleep quality is associated with late noninvasive ventilation failure in patients with acute hypercapnic respiratory failure. Crit Care Med. 2010;38(2):447-485. doi: 10.1097/CCM.0b013e3181bc8243
10. Sauvet F, Leftheriotis G, Gomez-Merino D, et al. Effect of acute sleep deprivation on vascular function in healthy subjects. J Appl Physiol (1985). 2010;108(1):68-75. doi: 10.1152/japplphysiol.00851.2009
11. Frey DJ, Fleshner M, Wright KP Jr. The effects of 40 hours of total sleep deprivation on inflammatory markers in healthy young adults. Brain Behav Immun. 2007;21(8):1050-1057. doi: 10.1016/j.bbi.2007.04.003
12. Spiegel K, Sheridan JF, Van Cauter E. Effect of sleep deprivation on response to immunization. JAMA 2002;288(12):1471-1472. doi: 10.1001/jama.288.12.1471-a
13. Dinges DF, Douglas SD, Zuagg L, et al. Leukocytosis and natural killer cell function parallel neurobehavioral fatigue induced by 64 hours of sleep deprivation. J Clin Invest. 1994;93(5):1930-1939. doi: 10.1172/JCI117184
14. Weinhouse GL, Schwab RJ, Watson PL, et al. Bench-to-bedside review: delirium in ICU patients— importance of sleep deprivation. Crit Care. 2009;13(6):234. doi: 10.1186/cc8131
15. Ely EW, Shintani A, Truman B, et al. Delirium as a predictor of mortality in mechanically ventilated patients in the intensive care unit. JAMA. 2004;291(14):1753-1762. doi: 10.1001/jama.291.14.1753
16. Girard TD, Jackson JC, Pandharipande PP, et al. Delirium as a predictor of long-term cognitive impairment in survivors of critical illness. Crit Care Med. 2010;38(7):1513-1520. doi: 10.1097/CCM.0b013e3181e47be1
17. Devlin JW, Skrobik Y, Gelinas C, et al. Clinical practice guidelines for the prevention and management of pain, agitation/sedation, delirium, immobility, and sleep disruption in adult patients in the ICU. Crit Care Med. 2018;46(9):e825-e873
18. The Boston Collaborative Drug Surveillance Program. Acute adverse reactions to prednisone in relation to dosage. Clin Pharmacol Ther. 1972;13(5):694-698. doi: 10.1002/cpt1972135part1694
19. Rundell JR, Wise MG. Causes of organic mood disorder. J Neuropsychiatry Clin Neurosci. 1989;1(4):398-400. doi: 10.1176/jnp.1.4.398
20. Gaudreau JD, Gagnon P, Harel F, Roy MA, Tremblay A. Psychoactive medications and risk of delirium in hospitalized cancer patients. J Clin Oncol. 2005;23(27):6712-6718. doi: 10.1200/JCO.2005.05.140
21. Gaudreau JD, Gagnon P, Roy MA, Harel F, Tremblay A. Opioid medications and longitudinal risk of delirium in hospitalized cancer patients. Cancer. 2007;109(11):2365-2373.
doi: 10.1002/cncr.22665
22. Schreiber MP, Colantuoni E, Bienvenu OJ, et al. Corticosteroids and transition to delirium in patients with acute lung injury. Crit Care Med. 2014;42(6):1480-1486. doi: 10.1097/CCM.0000000000000247
23. Wolters AE, Veldhuijzen DS, Zaal IJ, et al. Systemic corticosteroids and transition to delirium in critically ill patients. Crit Care Med. 2015;43(12):e585-e588. doi: 10.1097/CCM.0000000000001302
24. Matschke J, Muller-Beissenhirtz H, Novotny J, et al. A randomized trial of daily prednisone versus pulsed dexamethasone in treatment-naïve adult patients with immune thrombocytopenia: EIS 2002 study. Acta Haematol. 2016;136(2):101-107. doi: 10.1159/000445420
25. Tilouche N, Hassen M, Ali HBS, Jaoued AHO, Gharbi R, Atrous SS. Delirium in the intensive care unit: incidence, risk factors, and impact on outcome. Indian J Crit Care Med. 2018;22:144-149. doi: 10.4103/ijccm.IJCCM_244_17
26. Young A, Marsh S. Steroid use in critical care. BJA Education. 2018;18(5):129-134. doi: 10.1016/j.bjae.2018.01.005
27. DiPiro J, Talbert R, Yee G, Matzke GR, Wells BG, Posey M. Pharmacotherapy: A Pathophysiologic Approach. 4th ed. New York: McGraw-Hill; 1999:1277-1278.
28. Schimmer
29. Sarnes E, Crofford L, Watson M, Dennis G, Kan H, Bass D. Incidence of US costs of corticosteroid-associated adverse events: a systematic literature review. Clin Ther. 2011;33(10):1413-1432.
30. Idzikowsi C, Shapiro CM. ABC of sleep disorders, non-psychotropic drugs and sleep. BMJ. 1993;306(6885):1118-1120. doi: 10.1136/bmj.306.6885.1118

31. Tasker JG, Herman JP. Mechanisms of rapid glucocorticoid feedback inhibition of the hypothalamic-pituitary-adrenal axis. Stress. 2011;14(4):398-406.
doi: 10.3109/10253890.2011.586446
32. Wolkowitz OM, Reus VI, Weingartner H, et al. Cognitive effects of corticosteroids. Am J Psychiatry 1990;147(10):1297-1303. doi: 10.1176/ajp.147.10.1297
33. McEwen BS, Davis PG, Parsons B, Pfaff DW. The brain as a target for steroid hormone action. Ann Rev Neurosci. 1979;2:65-112. doi: 10.1146/annurev.ne.02.030179.000433
34. Brown ES, Woolston DJ, Frol AM. Amygdala volume in patients receiving chronic corticosteroid therapy. Biol Psychiatry. 2008;63(7):705-709.
doi: 10.1016/j.biopsych.2007.09.014
35. Brown ES, Woolston D, Frol A, et al. Hippocampal volume, spectroscopy, cognition, and mood in patients receiving corticosteroid. Biol Psychiatry. 2004;55(5):538-545.
36. Sapolsky RM, McEwen BS. Down-regulation of neural corticosterone receptors by corticosterone and dexamethasone. Brain Res. 1985;339(1):161-165.
doi: 10.1016/0006-8993(85)90638-9
37. Sorrells SF, Caso JR, Munhoz CD, Spolsky RM. The stressed CNS: when glucocorticoids aggravate inflammation. Neuron. 2009;64(1):33-39.
doi: 10.1016/j.neuron.2009.09.032
38. Wolkowitz OM, Burke H, Epel ES, Reus VI. Glucocorticoids: mood, memory, and mechanisms. Ann NY Acad Sci. 2009;1179:19-40. doi: 10.1111/j.1749-6632.2009.04980.x
39. Wolkowitz OM, Epel ES, Reus VI. Stress hormone-related psychopathology: pathophysiological and treatment implications. World J Biol Psychiatry. 2001;2(3):115-143. doi: 10.3109/15622970109026799
40. Paredes S, Barriga C, Reiter R, Rodrigues A. Assessment of the potential role of tryptophan as the precursor of serotonin and melatonin for the aged sleep-wake cycle and immune function: Streptopelia Risoria as a model. Int J Tryptophan Res. 2009;2:23-36. doi: 10.4137/ijtr.s1129
41. Soszyński P, Stowińska-Srzednicka J, Kasperlik-Zatuska A, Zgliczyński S. Decreased melatonin concentration in Cushing’s Syndrome. Horm Metab Res. 1989;21(12):673-674. doi: 10.1055/s-2007-1009317
42. Demish L, Demish K, Neckelsen T. Influence of dexamethasone on nocturnal melatonin production in healthy adult subjects. J Pineal Res. 1988;5(3):317-321. doi: 10.1111/j.1600-079x.1988.tb00657.x
43. Assaf N, Shalby AB, Khalil WK, Ahmed HH. Biochemical and genetic alterations of oxidant/antioxidant status of the brain in rats treated with dexamethasone: protective roles of melatonin and acetyl-L-carnitine. J Physiol Biochem. 2012;68(1):77-90. doi: 10.1007/s13105-011-0121-3
44. Clark MS, Russo AF. Tissue-specific glucocorticoid regulation of tryptophan hydroxylase mRNA levels. Brain Res Mol Brain Res. 1997;48(2):346-54. doi: 10.1016/s0169-328x(97)00106-x
45. Kram DE, Krasnow SM, Levasseur PR, Zhu X, Stork LC, Marks DL. Dexamethasone chemotherapy does not disrupt orexin signaling. PLoS One. 2016;11(12):e0168731. doi: 10.1371/journal.pone.0168731
46. Mellon S. Neurosteroids: biochemistry, modes of action, and clinical relevance. J Clin Endocrinol Metab. 1994;78(5):1003-1008. doi: 10.1210/jcem.78.5.8175951
47. Zorumski C, Paul SM, Izumi Y, Covey DF, Mennerick S . Neurosteroids, stress and depression: potential therapeutic opportunities. Neurosci Biobehav Rev. 2013;37(1):109-122. doi: 10.1016/j.neubiorev.2012.10.005
48. Monteleone P, Luisi M, Martiadis V, et al. Impaired reduction of enhanced levels of dehydroepiandrosterone by oral dexamethasone in anorexia nervosa. Psychoneuroendocrinology. 2006;31(4):537-542. doi: 10.1016/j.psyneuen.2005.08.015
49. Genazzani AR, Petraglia F, Bernardi F, et al. Circulating levels of allopregnanolone in humans: gender, age, and endocrine influences. J Clin Endocrinol Metab. 1998;83(6):2099-3103. doi: 10.1210/jcem.83.6.4905
50. Moser NJ, Phillips BA, Guthrie G, Barnett G. Effects of dexamethasone on sleep. Pharmacol Toxicol. 1996;79(2):100-102. doi: 10.1111/j.1600-0773.1996.tb00249.x
51. Curtis J, Westfall A, Allison J, et al. Population-based assessment of adverse events associated with long-term glucocorticoid use. Arthritis Rheum. 2006;55(3):420-426. doi: 10.1002/art.21984
52. Zhao J, Dai YH, Xi QS, Yu SY. A clinical study on insomnia in patients with cancer during chemotherapy containing high-dose glucocorticoids. Pharmazie. 2013;68(6):421-427
53. Naber D, Sand P, Heigl B. Psychopathological and neuropsychological effects of 8-days corticosteroid treatment. A prospective study. Psychoneuroendocrinology. 1996;21(1):25-31. doi: 10.1016/0306-4530(95)00031-3
54. Brown ES, Suppes T, Khan DA, Carmody TJ 3rd. Mood changes during prednisone bursts in outpatients with asthma. J Clin Psychopharmacol. 2002;22(1):55-61.
doi: 10.1097/00004714-200202000-00009
55. Warrington TP, Bostwick JM. Psychiatric adverse effects of corticosteroids. Mayo Clin Proc. 2006;81(10):1361-1367. doi: 10.4065/81.10.1361
56. Britt RC, Devine A, Swallen KC et al. Corticosteroid use in the intensive care unit: at what cost? Arch Surg. 2006;141(2):145-159. doi:10.1001/archsurg.141.2.145
57. Kiser TH, Allen RR, Valuck RJ, Moss M, Vanivier RW. Outcomes associated with corticosteroid dosage in critically ill patients in acute exacerbations of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2014;189(9):1052-1064. doi: 10.1164/rccm.201401-0058OC
58. Bourne RS, Mills GH. Sleep disruption in critically ill patients—pharmacological considerations. Anaesthesia. 2004;59(4):374-384. doi: 10.1111/j. 1365-2044.2004.03664.x
59. Flaherty JH. Insomnia among hospitalized older persons. Clin Geriatr Med. 2008;24(1):51-67. doi: 10.1016/j.cger.2007.08.012
60. Sirios F. Steroid psychosis: a review. Gen Hosp Psychiatry. 2003;25(1):27-33. doi: 10.1016/s0163-8343(02)00241-4
1. Simini B. Patients’ perceptions of intensive care. Lancet. 1999;354(9178):571-572. doi: 10.1016/S0140-6736(99)02728-2
2. Delaney LJ, Van Haren F, Lopez V. Sleeping on a problem: the impact of sleep disturbance on intensive care patients—a clinical review. Ann Intensive Care. 2015;15:3. doi: 10.1186/s13613-015-0043-2
3. Friese RS, Diaz-Arrastia R, McBride D, Frankel H, Gentilello LM. Quality and quantity of sleep in the surgical intensive care unit; are our patients sleeping? J Trauma. 2007;63(6):1210-1214. doi: 10.1097/TA.0b013e31815b83d7
4. Elliott R, McKinley S, Cistulli P, Fien M. Characterisation of sleep in intensive care using 24-hour polysomnography: an observational study. Crit Care 2013;17(2):R46.
5. Aurell J, Elmqvist D. Sleep in the surgical intensive care unit: continuous polygraphic recording of sleep in patients receiving postoperative care. BJM (Clin Res Ed). 1985;290(6474)1029-1032. doi: 10.1136/bmj.290.6474.1029
6. White DP, Douglas NJ, Pickett CK, Zwillich CW, Weil JV. Sleep deprivation and the control of ventilation. Am Rev Respir Dis. 1983;128(6):984-986. doi: 10.1164/arrd.1983.128.6.984
7. Series F, Roy N, Marc I. Effects of sleep deprivation and sleep fragmentation on upper airway collapsibility in normal subjects. Am J Respir Crit Care Med. 1994;150(2):481-485. doi: 10.1164/ajrccm.150.2.8049833
8. Tadjalli A, Peever J. Sleep loss reduces respiratory motor plasticity. Adv Exp Med Biol. 2010;669:289-292.
doi: 10.1007/978-1-4419-5692-7_59
9. Roche Campo F, Drouot X, Thille AW, et al. Poor sleep quality is associated with late noninvasive ventilation failure in patients with acute hypercapnic respiratory failure. Crit Care Med. 2010;38(2):447-485. doi: 10.1097/CCM.0b013e3181bc8243
10. Sauvet F, Leftheriotis G, Gomez-Merino D, et al. Effect of acute sleep deprivation on vascular function in healthy subjects. J Appl Physiol (1985). 2010;108(1):68-75. doi: 10.1152/japplphysiol.00851.2009
11. Frey DJ, Fleshner M, Wright KP Jr. The effects of 40 hours of total sleep deprivation on inflammatory markers in healthy young adults. Brain Behav Immun. 2007;21(8):1050-1057. doi: 10.1016/j.bbi.2007.04.003
12. Spiegel K, Sheridan JF, Van Cauter E. Effect of sleep deprivation on response to immunization. JAMA 2002;288(12):1471-1472. doi: 10.1001/jama.288.12.1471-a
13. Dinges DF, Douglas SD, Zuagg L, et al. Leukocytosis and natural killer cell function parallel neurobehavioral fatigue induced by 64 hours of sleep deprivation. J Clin Invest. 1994;93(5):1930-1939. doi: 10.1172/JCI117184
14. Weinhouse GL, Schwab RJ, Watson PL, et al. Bench-to-bedside review: delirium in ICU patients— importance of sleep deprivation. Crit Care. 2009;13(6):234. doi: 10.1186/cc8131
15. Ely EW, Shintani A, Truman B, et al. Delirium as a predictor of mortality in mechanically ventilated patients in the intensive care unit. JAMA. 2004;291(14):1753-1762. doi: 10.1001/jama.291.14.1753
16. Girard TD, Jackson JC, Pandharipande PP, et al. Delirium as a predictor of long-term cognitive impairment in survivors of critical illness. Crit Care Med. 2010;38(7):1513-1520. doi: 10.1097/CCM.0b013e3181e47be1
17. Devlin JW, Skrobik Y, Gelinas C, et al. Clinical practice guidelines for the prevention and management of pain, agitation/sedation, delirium, immobility, and sleep disruption in adult patients in the ICU. Crit Care Med. 2018;46(9):e825-e873
18. The Boston Collaborative Drug Surveillance Program. Acute adverse reactions to prednisone in relation to dosage. Clin Pharmacol Ther. 1972;13(5):694-698. doi: 10.1002/cpt1972135part1694
19. Rundell JR, Wise MG. Causes of organic mood disorder. J Neuropsychiatry Clin Neurosci. 1989;1(4):398-400. doi: 10.1176/jnp.1.4.398
20. Gaudreau JD, Gagnon P, Harel F, Roy MA, Tremblay A. Psychoactive medications and risk of delirium in hospitalized cancer patients. J Clin Oncol. 2005;23(27):6712-6718. doi: 10.1200/JCO.2005.05.140
21. Gaudreau JD, Gagnon P, Roy MA, Harel F, Tremblay A. Opioid medications and longitudinal risk of delirium in hospitalized cancer patients. Cancer. 2007;109(11):2365-2373.
doi: 10.1002/cncr.22665
22. Schreiber MP, Colantuoni E, Bienvenu OJ, et al. Corticosteroids and transition to delirium in patients with acute lung injury. Crit Care Med. 2014;42(6):1480-1486. doi: 10.1097/CCM.0000000000000247
23. Wolters AE, Veldhuijzen DS, Zaal IJ, et al. Systemic corticosteroids and transition to delirium in critically ill patients. Crit Care Med. 2015;43(12):e585-e588. doi: 10.1097/CCM.0000000000001302
24. Matschke J, Muller-Beissenhirtz H, Novotny J, et al. A randomized trial of daily prednisone versus pulsed dexamethasone in treatment-naïve adult patients with immune thrombocytopenia: EIS 2002 study. Acta Haematol. 2016;136(2):101-107. doi: 10.1159/000445420
25. Tilouche N, Hassen M, Ali HBS, Jaoued AHO, Gharbi R, Atrous SS. Delirium in the intensive care unit: incidence, risk factors, and impact on outcome. Indian J Crit Care Med. 2018;22:144-149. doi: 10.4103/ijccm.IJCCM_244_17
26. Young A, Marsh S. Steroid use in critical care. BJA Education. 2018;18(5):129-134. doi: 10.1016/j.bjae.2018.01.005
27. DiPiro J, Talbert R, Yee G, Matzke GR, Wells BG, Posey M. Pharmacotherapy: A Pathophysiologic Approach. 4th ed. New York: McGraw-Hill; 1999:1277-1278.
28. Schimmer
29. Sarnes E, Crofford L, Watson M, Dennis G, Kan H, Bass D. Incidence of US costs of corticosteroid-associated adverse events: a systematic literature review. Clin Ther. 2011;33(10):1413-1432.
30. Idzikowsi C, Shapiro CM. ABC of sleep disorders, non-psychotropic drugs and sleep. BMJ. 1993;306(6885):1118-1120. doi: 10.1136/bmj.306.6885.1118

31. Tasker JG, Herman JP. Mechanisms of rapid glucocorticoid feedback inhibition of the hypothalamic-pituitary-adrenal axis. Stress. 2011;14(4):398-406.
doi: 10.3109/10253890.2011.586446
32. Wolkowitz OM, Reus VI, Weingartner H, et al. Cognitive effects of corticosteroids. Am J Psychiatry 1990;147(10):1297-1303. doi: 10.1176/ajp.147.10.1297
33. McEwen BS, Davis PG, Parsons B, Pfaff DW. The brain as a target for steroid hormone action. Ann Rev Neurosci. 1979;2:65-112. doi: 10.1146/annurev.ne.02.030179.000433
34. Brown ES, Woolston DJ, Frol AM. Amygdala volume in patients receiving chronic corticosteroid therapy. Biol Psychiatry. 2008;63(7):705-709.
doi: 10.1016/j.biopsych.2007.09.014
35. Brown ES, Woolston D, Frol A, et al. Hippocampal volume, spectroscopy, cognition, and mood in patients receiving corticosteroid. Biol Psychiatry. 2004;55(5):538-545.
36. Sapolsky RM, McEwen BS. Down-regulation of neural corticosterone receptors by corticosterone and dexamethasone. Brain Res. 1985;339(1):161-165.
doi: 10.1016/0006-8993(85)90638-9
37. Sorrells SF, Caso JR, Munhoz CD, Spolsky RM. The stressed CNS: when glucocorticoids aggravate inflammation. Neuron. 2009;64(1):33-39.
doi: 10.1016/j.neuron.2009.09.032
38. Wolkowitz OM, Burke H, Epel ES, Reus VI. Glucocorticoids: mood, memory, and mechanisms. Ann NY Acad Sci. 2009;1179:19-40. doi: 10.1111/j.1749-6632.2009.04980.x
39. Wolkowitz OM, Epel ES, Reus VI. Stress hormone-related psychopathology: pathophysiological and treatment implications. World J Biol Psychiatry. 2001;2(3):115-143. doi: 10.3109/15622970109026799
40. Paredes S, Barriga C, Reiter R, Rodrigues A. Assessment of the potential role of tryptophan as the precursor of serotonin and melatonin for the aged sleep-wake cycle and immune function: Streptopelia Risoria as a model. Int J Tryptophan Res. 2009;2:23-36. doi: 10.4137/ijtr.s1129
41. Soszyński P, Stowińska-Srzednicka J, Kasperlik-Zatuska A, Zgliczyński S. Decreased melatonin concentration in Cushing’s Syndrome. Horm Metab Res. 1989;21(12):673-674. doi: 10.1055/s-2007-1009317
42. Demish L, Demish K, Neckelsen T. Influence of dexamethasone on nocturnal melatonin production in healthy adult subjects. J Pineal Res. 1988;5(3):317-321. doi: 10.1111/j.1600-079x.1988.tb00657.x
43. Assaf N, Shalby AB, Khalil WK, Ahmed HH. Biochemical and genetic alterations of oxidant/antioxidant status of the brain in rats treated with dexamethasone: protective roles of melatonin and acetyl-L-carnitine. J Physiol Biochem. 2012;68(1):77-90. doi: 10.1007/s13105-011-0121-3
44. Clark MS, Russo AF. Tissue-specific glucocorticoid regulation of tryptophan hydroxylase mRNA levels. Brain Res Mol Brain Res. 1997;48(2):346-54. doi: 10.1016/s0169-328x(97)00106-x
45. Kram DE, Krasnow SM, Levasseur PR, Zhu X, Stork LC, Marks DL. Dexamethasone chemotherapy does not disrupt orexin signaling. PLoS One. 2016;11(12):e0168731. doi: 10.1371/journal.pone.0168731
46. Mellon S. Neurosteroids: biochemistry, modes of action, and clinical relevance. J Clin Endocrinol Metab. 1994;78(5):1003-1008. doi: 10.1210/jcem.78.5.8175951
47. Zorumski C, Paul SM, Izumi Y, Covey DF, Mennerick S . Neurosteroids, stress and depression: potential therapeutic opportunities. Neurosci Biobehav Rev. 2013;37(1):109-122. doi: 10.1016/j.neubiorev.2012.10.005
48. Monteleone P, Luisi M, Martiadis V, et al. Impaired reduction of enhanced levels of dehydroepiandrosterone by oral dexamethasone in anorexia nervosa. Psychoneuroendocrinology. 2006;31(4):537-542. doi: 10.1016/j.psyneuen.2005.08.015
49. Genazzani AR, Petraglia F, Bernardi F, et al. Circulating levels of allopregnanolone in humans: gender, age, and endocrine influences. J Clin Endocrinol Metab. 1998;83(6):2099-3103. doi: 10.1210/jcem.83.6.4905
50. Moser NJ, Phillips BA, Guthrie G, Barnett G. Effects of dexamethasone on sleep. Pharmacol Toxicol. 1996;79(2):100-102. doi: 10.1111/j.1600-0773.1996.tb00249.x
51. Curtis J, Westfall A, Allison J, et al. Population-based assessment of adverse events associated with long-term glucocorticoid use. Arthritis Rheum. 2006;55(3):420-426. doi: 10.1002/art.21984
52. Zhao J, Dai YH, Xi QS, Yu SY. A clinical study on insomnia in patients with cancer during chemotherapy containing high-dose glucocorticoids. Pharmazie. 2013;68(6):421-427
53. Naber D, Sand P, Heigl B. Psychopathological and neuropsychological effects of 8-days corticosteroid treatment. A prospective study. Psychoneuroendocrinology. 1996;21(1):25-31. doi: 10.1016/0306-4530(95)00031-3
54. Brown ES, Suppes T, Khan DA, Carmody TJ 3rd. Mood changes during prednisone bursts in outpatients with asthma. J Clin Psychopharmacol. 2002;22(1):55-61.
doi: 10.1097/00004714-200202000-00009
55. Warrington TP, Bostwick JM. Psychiatric adverse effects of corticosteroids. Mayo Clin Proc. 2006;81(10):1361-1367. doi: 10.4065/81.10.1361
56. Britt RC, Devine A, Swallen KC et al. Corticosteroid use in the intensive care unit: at what cost? Arch Surg. 2006;141(2):145-159. doi:10.1001/archsurg.141.2.145
57. Kiser TH, Allen RR, Valuck RJ, Moss M, Vanivier RW. Outcomes associated with corticosteroid dosage in critically ill patients in acute exacerbations of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2014;189(9):1052-1064. doi: 10.1164/rccm.201401-0058OC
58. Bourne RS, Mills GH. Sleep disruption in critically ill patients—pharmacological considerations. Anaesthesia. 2004;59(4):374-384. doi: 10.1111/j. 1365-2044.2004.03664.x
59. Flaherty JH. Insomnia among hospitalized older persons. Clin Geriatr Med. 2008;24(1):51-67. doi: 10.1016/j.cger.2007.08.012
60. Sirios F. Steroid psychosis: a review. Gen Hosp Psychiatry. 2003;25(1):27-33. doi: 10.1016/s0163-8343(02)00241-4
Sleep burden index predicts recurrent stroke
A sleep burden index that considers multiple sleep-wake disturbances (SWDs) predicts subsequent cardiocerebrovascular events during the 2 years after a stroke, preliminary results on an ongoing study suggest.
The index, which combines sleep duration, sleep disordered breathing, restless leg syndrome (RLS), insomnia, and sleep duration, is a better predictor of new events than a single sleep disorder alone.
With further evidence of its usefulness, “the sleep burden index could be integrated into clinical routine,” Simone B. Duss, PhD, of the department of neurology at Bern (Switzerland) University Hospital, told a press briefing.
The findings were presented online at the Congress of the European Academy of Neurology 2020, which transitioned to a virtual meeting because of the COVID-19 pandemic.
Sleep-wake disorders are very common in stroke patients and may preexist or appear de novo as a consequence of brain damage, said Dr. Duss. “They may also be a result of medical, psychological, or environmental challenges these patients face after a stroke.”
Clear Evidence
There’s “clear evidence” that sleep disordered breathing is a risk factor for stroke, and negatively affects stroke outcome if left untreated, said Dr. Duss.
But for other SWDs, such as insomnia, RLS, and long and short sleep duration, “the evidence is less compelling,” she said. “However, some studies still suggest they influence stroke risk and outcome.”
Experts believe that sleep disturbances after a stroke lead to sleep fragmentation, as well as decreased slow wave sleep and REM sleep.
“This negatively affects inflammatory neuroprotective and synaptic plasticity processes during the recovery process of a stroke,” said Dr. Duss. “In the end, this results in worse outcomes with regard to recurrent events but also in activities of daily living and mood.”
The new analysis aimed to assess the impact of sleep-wake disturbances on recurrent events and outcomes following a stroke or transient ischemic attack (TIA). It included 438 patients with acute stroke (85%) or TIA (15%). The mean age of the study population was 65 years, and 64% were male.
Researchers used the National Institutes of Health Stroke Scale (NIHSS) to assess stroke severity. At admission, the mean NIHSS score was 4. Most strokes (77.2%) were supratentorial.
About one-fifth of stroke patients and one-third of TIA patients had experienced a previous event.
Researchers used functional outcome scores to assess the clinical course of the stroke or TIA. In addition, they regularly asked patients about recurrence of cardiocerebrovascular events.
Investigators assessed sleep disordered breathing during the acute phase of stroke, so within the first few days, using respirography. They collected information on the presence of other sleep-wake disturbances from questionnaires and clinical interviews at 1 month, 3 months, 1 year, and 2 years after the event.
About 26% of subjects showed severe sleep disordered breathing, “meaning that they had more than 20 apnea-hypopnea events per hour,” said Dr. Duss.
More than a quarter of patients reported subclinical symptoms of insomnia (measured using the Insomnia Severity Index), and up to 10% reported severe insomnia symptoms corresponding to the clinical diagnosis of insomnia, she said.
About 9% of patients in the acute phase of stroke, and 6% in the more chronic phase, fulfilled the diagnostic criteria of RLS.
More ‘skewed’
The results for sleep duration were relatively “skewed,” said Dr. Duss. More patients reported longer sleep duration (more than 9 hours) at 1 month than at month 3, and more reported shorter sleep duration (4.0 hours or less) at month 3 than at month 1.
The researchers built a sleep burden index for the combined impact of the various sleep-wake disturbances.
They used this index as a predictor of subsequent cardiocerebrovascular events within 3 months after an event. They used a composite outcome that included recurrent stroke or TIA, MI, heart failure, and urgent revascularization, as well as new cardiocerebrovascular events, from 3 to 24 months.
The analysis showed that the mean sleep burden index was higher for stroke patients with a recurrent event, compared with stroke or TIA patients without a recurrent event (P = .0002).
A multiple logistical regression model with the presence or absence of a recurrent event as an outcome showed that the sleep burden index is a significant predictor of recurrent events (odds ratio, 2.10; P = .001). This was true even after controlling for age, gender, and baseline stroke severity.
The baseline apnea/hypopnea index and sleep duration were also significant predictors of new events. Importantly, though, the sleep burden index remained a significant predictor of recurrent events even after excluding the apnea/hypopnea index component, said Dr. Duss. “So the predictive power of the sleep burden index is not only driven by the apnea-hypopnea index at the beginning of a stroke.”
Sleep-wake disturbances “should be more carefully assessed and considered in comprehensive treatment approaches,” not only in stroke patients, but in neurologic patients in general, said Dr. Duss
She noted that these are preliminary observations from an ongoing study. The results need to be confirmed and should be when the study is finalized, she said.
Researchers are also analyzing MRI data to assess whether certain brain lesions are associated with sleep disturbances.
Jesse Dawson, MD, professor of stroke medicine at the University of Glasgow, said the clinical scoring system the study included “will be a big help in design and conduct of clinical trials.”
Although he and other stroke experts are aware of the high prevalence of sleep disorders after stroke, “we don’t routinely look for them as we’re uncertain whether intervention is of benefit,” said Dr. Dawson.
This new study “suggests there is an association with adverse outcome,” he said.
The research was supported by grants from the Swiss National Science Foundation and the Swiss Heart Foundation. Dr. Duss and Dr. Dawson disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
A sleep burden index that considers multiple sleep-wake disturbances (SWDs) predicts subsequent cardiocerebrovascular events during the 2 years after a stroke, preliminary results on an ongoing study suggest.
The index, which combines sleep duration, sleep disordered breathing, restless leg syndrome (RLS), insomnia, and sleep duration, is a better predictor of new events than a single sleep disorder alone.
With further evidence of its usefulness, “the sleep burden index could be integrated into clinical routine,” Simone B. Duss, PhD, of the department of neurology at Bern (Switzerland) University Hospital, told a press briefing.
The findings were presented online at the Congress of the European Academy of Neurology 2020, which transitioned to a virtual meeting because of the COVID-19 pandemic.
Sleep-wake disorders are very common in stroke patients and may preexist or appear de novo as a consequence of brain damage, said Dr. Duss. “They may also be a result of medical, psychological, or environmental challenges these patients face after a stroke.”
Clear Evidence
There’s “clear evidence” that sleep disordered breathing is a risk factor for stroke, and negatively affects stroke outcome if left untreated, said Dr. Duss.
But for other SWDs, such as insomnia, RLS, and long and short sleep duration, “the evidence is less compelling,” she said. “However, some studies still suggest they influence stroke risk and outcome.”
Experts believe that sleep disturbances after a stroke lead to sleep fragmentation, as well as decreased slow wave sleep and REM sleep.
“This negatively affects inflammatory neuroprotective and synaptic plasticity processes during the recovery process of a stroke,” said Dr. Duss. “In the end, this results in worse outcomes with regard to recurrent events but also in activities of daily living and mood.”
The new analysis aimed to assess the impact of sleep-wake disturbances on recurrent events and outcomes following a stroke or transient ischemic attack (TIA). It included 438 patients with acute stroke (85%) or TIA (15%). The mean age of the study population was 65 years, and 64% were male.
Researchers used the National Institutes of Health Stroke Scale (NIHSS) to assess stroke severity. At admission, the mean NIHSS score was 4. Most strokes (77.2%) were supratentorial.
About one-fifth of stroke patients and one-third of TIA patients had experienced a previous event.
Researchers used functional outcome scores to assess the clinical course of the stroke or TIA. In addition, they regularly asked patients about recurrence of cardiocerebrovascular events.
Investigators assessed sleep disordered breathing during the acute phase of stroke, so within the first few days, using respirography. They collected information on the presence of other sleep-wake disturbances from questionnaires and clinical interviews at 1 month, 3 months, 1 year, and 2 years after the event.
About 26% of subjects showed severe sleep disordered breathing, “meaning that they had more than 20 apnea-hypopnea events per hour,” said Dr. Duss.
More than a quarter of patients reported subclinical symptoms of insomnia (measured using the Insomnia Severity Index), and up to 10% reported severe insomnia symptoms corresponding to the clinical diagnosis of insomnia, she said.
About 9% of patients in the acute phase of stroke, and 6% in the more chronic phase, fulfilled the diagnostic criteria of RLS.
More ‘skewed’
The results for sleep duration were relatively “skewed,” said Dr. Duss. More patients reported longer sleep duration (more than 9 hours) at 1 month than at month 3, and more reported shorter sleep duration (4.0 hours or less) at month 3 than at month 1.
The researchers built a sleep burden index for the combined impact of the various sleep-wake disturbances.
They used this index as a predictor of subsequent cardiocerebrovascular events within 3 months after an event. They used a composite outcome that included recurrent stroke or TIA, MI, heart failure, and urgent revascularization, as well as new cardiocerebrovascular events, from 3 to 24 months.
The analysis showed that the mean sleep burden index was higher for stroke patients with a recurrent event, compared with stroke or TIA patients without a recurrent event (P = .0002).
A multiple logistical regression model with the presence or absence of a recurrent event as an outcome showed that the sleep burden index is a significant predictor of recurrent events (odds ratio, 2.10; P = .001). This was true even after controlling for age, gender, and baseline stroke severity.
The baseline apnea/hypopnea index and sleep duration were also significant predictors of new events. Importantly, though, the sleep burden index remained a significant predictor of recurrent events even after excluding the apnea/hypopnea index component, said Dr. Duss. “So the predictive power of the sleep burden index is not only driven by the apnea-hypopnea index at the beginning of a stroke.”
Sleep-wake disturbances “should be more carefully assessed and considered in comprehensive treatment approaches,” not only in stroke patients, but in neurologic patients in general, said Dr. Duss
She noted that these are preliminary observations from an ongoing study. The results need to be confirmed and should be when the study is finalized, she said.
Researchers are also analyzing MRI data to assess whether certain brain lesions are associated with sleep disturbances.
Jesse Dawson, MD, professor of stroke medicine at the University of Glasgow, said the clinical scoring system the study included “will be a big help in design and conduct of clinical trials.”
Although he and other stroke experts are aware of the high prevalence of sleep disorders after stroke, “we don’t routinely look for them as we’re uncertain whether intervention is of benefit,” said Dr. Dawson.
This new study “suggests there is an association with adverse outcome,” he said.
The research was supported by grants from the Swiss National Science Foundation and the Swiss Heart Foundation. Dr. Duss and Dr. Dawson disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
A sleep burden index that considers multiple sleep-wake disturbances (SWDs) predicts subsequent cardiocerebrovascular events during the 2 years after a stroke, preliminary results on an ongoing study suggest.
The index, which combines sleep duration, sleep disordered breathing, restless leg syndrome (RLS), insomnia, and sleep duration, is a better predictor of new events than a single sleep disorder alone.
With further evidence of its usefulness, “the sleep burden index could be integrated into clinical routine,” Simone B. Duss, PhD, of the department of neurology at Bern (Switzerland) University Hospital, told a press briefing.
The findings were presented online at the Congress of the European Academy of Neurology 2020, which transitioned to a virtual meeting because of the COVID-19 pandemic.
Sleep-wake disorders are very common in stroke patients and may preexist or appear de novo as a consequence of brain damage, said Dr. Duss. “They may also be a result of medical, psychological, or environmental challenges these patients face after a stroke.”
Clear Evidence
There’s “clear evidence” that sleep disordered breathing is a risk factor for stroke, and negatively affects stroke outcome if left untreated, said Dr. Duss.
But for other SWDs, such as insomnia, RLS, and long and short sleep duration, “the evidence is less compelling,” she said. “However, some studies still suggest they influence stroke risk and outcome.”
Experts believe that sleep disturbances after a stroke lead to sleep fragmentation, as well as decreased slow wave sleep and REM sleep.
“This negatively affects inflammatory neuroprotective and synaptic plasticity processes during the recovery process of a stroke,” said Dr. Duss. “In the end, this results in worse outcomes with regard to recurrent events but also in activities of daily living and mood.”
The new analysis aimed to assess the impact of sleep-wake disturbances on recurrent events and outcomes following a stroke or transient ischemic attack (TIA). It included 438 patients with acute stroke (85%) or TIA (15%). The mean age of the study population was 65 years, and 64% were male.
Researchers used the National Institutes of Health Stroke Scale (NIHSS) to assess stroke severity. At admission, the mean NIHSS score was 4. Most strokes (77.2%) were supratentorial.
About one-fifth of stroke patients and one-third of TIA patients had experienced a previous event.
Researchers used functional outcome scores to assess the clinical course of the stroke or TIA. In addition, they regularly asked patients about recurrence of cardiocerebrovascular events.
Investigators assessed sleep disordered breathing during the acute phase of stroke, so within the first few days, using respirography. They collected information on the presence of other sleep-wake disturbances from questionnaires and clinical interviews at 1 month, 3 months, 1 year, and 2 years after the event.
About 26% of subjects showed severe sleep disordered breathing, “meaning that they had more than 20 apnea-hypopnea events per hour,” said Dr. Duss.
More than a quarter of patients reported subclinical symptoms of insomnia (measured using the Insomnia Severity Index), and up to 10% reported severe insomnia symptoms corresponding to the clinical diagnosis of insomnia, she said.
About 9% of patients in the acute phase of stroke, and 6% in the more chronic phase, fulfilled the diagnostic criteria of RLS.
More ‘skewed’
The results for sleep duration were relatively “skewed,” said Dr. Duss. More patients reported longer sleep duration (more than 9 hours) at 1 month than at month 3, and more reported shorter sleep duration (4.0 hours or less) at month 3 than at month 1.
The researchers built a sleep burden index for the combined impact of the various sleep-wake disturbances.
They used this index as a predictor of subsequent cardiocerebrovascular events within 3 months after an event. They used a composite outcome that included recurrent stroke or TIA, MI, heart failure, and urgent revascularization, as well as new cardiocerebrovascular events, from 3 to 24 months.
The analysis showed that the mean sleep burden index was higher for stroke patients with a recurrent event, compared with stroke or TIA patients without a recurrent event (P = .0002).
A multiple logistical regression model with the presence or absence of a recurrent event as an outcome showed that the sleep burden index is a significant predictor of recurrent events (odds ratio, 2.10; P = .001). This was true even after controlling for age, gender, and baseline stroke severity.
The baseline apnea/hypopnea index and sleep duration were also significant predictors of new events. Importantly, though, the sleep burden index remained a significant predictor of recurrent events even after excluding the apnea/hypopnea index component, said Dr. Duss. “So the predictive power of the sleep burden index is not only driven by the apnea-hypopnea index at the beginning of a stroke.”
Sleep-wake disturbances “should be more carefully assessed and considered in comprehensive treatment approaches,” not only in stroke patients, but in neurologic patients in general, said Dr. Duss
She noted that these are preliminary observations from an ongoing study. The results need to be confirmed and should be when the study is finalized, she said.
Researchers are also analyzing MRI data to assess whether certain brain lesions are associated with sleep disturbances.
Jesse Dawson, MD, professor of stroke medicine at the University of Glasgow, said the clinical scoring system the study included “will be a big help in design and conduct of clinical trials.”
Although he and other stroke experts are aware of the high prevalence of sleep disorders after stroke, “we don’t routinely look for them as we’re uncertain whether intervention is of benefit,” said Dr. Dawson.
This new study “suggests there is an association with adverse outcome,” he said.
The research was supported by grants from the Swiss National Science Foundation and the Swiss Heart Foundation. Dr. Duss and Dr. Dawson disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
COVID-19: Delirium first, depression, anxiety, insomnia later?
Severe COVID-19 may cause delirium in the acute stage of illness, followed by the possibility of depression, anxiety, fatigue, insomnia, and posttraumatic stress disorder (PTSD) over the longer term, new research suggests.
Results from “the first systematic review and meta-analysis of the psychiatric consequences of coronavirus infection” showed that previous coronavirus epidemics were associated with a significant psychiatric burden in both the acute and post-illness stages.
“Most people with COVID-19 will not develop any mental health problems, even among those with severe cases requiring hospitalization, but given the huge numbers of people getting sick, the global impact on mental health could be considerable,” co–lead investigator Jonathan Rogers, MRCPsych, Department of Psychiatry, University College London, United Kingdom, said in a news release.
The study was published online May 18 in Lancet Psychiatry.
Need for Monitoring, Support
The researchers analyzed 65 peer-reviewed studies and seven preprint articles with data on acute and post-illness psychiatric and neuropsychiatric features of patients who had been hospitalized with COVID-19, as well as two other diseases caused by coronaviruses – severe acute respiratory syndrome (SARS), in 2002–2004, and Middle East respiratory syndrome (MERS), in 2012.
“Our main findings are that signs suggestive of delirium are common in the acute stage of SARS, MERS, and COVID-19; there is evidence of depression, anxiety, fatigue, and post-traumatic stress disorder in the post-illness stage of previous coronavirus epidemics, but there are few data yet on COVID-19,” the investigators write.
The data show that among patients acutely ill with SARS and MERS, 28% experienced confusion, 33% had depressed mood, 36% had anxiety, 34% suffered from impaired memory, and 42% had insomnia.
After recovery from SARS and MERS, sleep disorder, frequent recall of traumatic memories, emotional lability, impaired concentration, fatigue, and impaired memory were reported in more than 15% of patients during a follow-up period that ranged from 6 weeks to 39 months.
In a meta-analysis, the point prevalence in the post-illness stage was 32% for PTSD and about 15% for depression and anxiety.
In patients acutely ill with severe COVID-19, available data suggest that 65% experience delirium, 69% have agitation after withdrawal of sedation, and 21% have altered consciousness.
In one study, 33% of patients had a dysexecutive syndrome at discharge, characterized by symptoms such as inattention, disorientation, or poorly organized movements in response to command. Currently, data are very limited regarding patients who have recovered from COVID-19, the investigators caution.
“, and monitored after they recover to ensure they do not develop mental illnesses, and are able to access treatment if needed,” senior author Anthony David, FMedSci, from UCL Institute of Mental Health, said in a news release.
“While most people with COVID-19 will recover without experiencing mental illness, we need to research which factors may contribute to enduring mental health problems, and develop interventions to prevent and treat them,” he added.
Be Prepared
The coauthors of a linked commentary say it makes sense, from a biological perspective, to merge data on these three coronavirus diseases, given the degree to which they resemble each other.
They caution, however, that treatment of COVID-19 seems to be different from treatment of SARS and MERS. In addition, the social and economic situation of COVID-19 survivors’ return is completely different from that of SARS and MERS survivors.
Findings from previous coronavirus outbreaks are “useful, but might not be exact predictors of prevalences of psychiatric complications for patients with COVID-19,” write Iris Sommer, MD, PhD, from University Medical Center Groningen, the Netherlands, and P. Roberto Bakker, MD, PhD, from Maastricht University Medical Center, the Netherlands.
“The warning from [this study] that we should prepare to treat large numbers of patients with COVID-19 who go on to develop delirium, post-traumatic stress disorder, anxiety, and depression is an important message for the psychiatric community,” they add.
Sommer and Bakker also say the reported estimates of prevalence in this study should be interpreted with caution, “as true numbers of both acute and long-term psychiatric disorders for patients with COVID-19 might be considerably higher.”
Funding for the study was provided by the Wellcome Trust, the UK National Institute for Health Research (NIHR), the UK Medical Research Council, the NIHR Biomedical Research Center at the University College London Hospitals NHS Foundation Trust, and the University College London. The authors of the study and the commentary have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Severe COVID-19 may cause delirium in the acute stage of illness, followed by the possibility of depression, anxiety, fatigue, insomnia, and posttraumatic stress disorder (PTSD) over the longer term, new research suggests.
Results from “the first systematic review and meta-analysis of the psychiatric consequences of coronavirus infection” showed that previous coronavirus epidemics were associated with a significant psychiatric burden in both the acute and post-illness stages.
“Most people with COVID-19 will not develop any mental health problems, even among those with severe cases requiring hospitalization, but given the huge numbers of people getting sick, the global impact on mental health could be considerable,” co–lead investigator Jonathan Rogers, MRCPsych, Department of Psychiatry, University College London, United Kingdom, said in a news release.
The study was published online May 18 in Lancet Psychiatry.
Need for Monitoring, Support
The researchers analyzed 65 peer-reviewed studies and seven preprint articles with data on acute and post-illness psychiatric and neuropsychiatric features of patients who had been hospitalized with COVID-19, as well as two other diseases caused by coronaviruses – severe acute respiratory syndrome (SARS), in 2002–2004, and Middle East respiratory syndrome (MERS), in 2012.
“Our main findings are that signs suggestive of delirium are common in the acute stage of SARS, MERS, and COVID-19; there is evidence of depression, anxiety, fatigue, and post-traumatic stress disorder in the post-illness stage of previous coronavirus epidemics, but there are few data yet on COVID-19,” the investigators write.
The data show that among patients acutely ill with SARS and MERS, 28% experienced confusion, 33% had depressed mood, 36% had anxiety, 34% suffered from impaired memory, and 42% had insomnia.
After recovery from SARS and MERS, sleep disorder, frequent recall of traumatic memories, emotional lability, impaired concentration, fatigue, and impaired memory were reported in more than 15% of patients during a follow-up period that ranged from 6 weeks to 39 months.
In a meta-analysis, the point prevalence in the post-illness stage was 32% for PTSD and about 15% for depression and anxiety.
In patients acutely ill with severe COVID-19, available data suggest that 65% experience delirium, 69% have agitation after withdrawal of sedation, and 21% have altered consciousness.
In one study, 33% of patients had a dysexecutive syndrome at discharge, characterized by symptoms such as inattention, disorientation, or poorly organized movements in response to command. Currently, data are very limited regarding patients who have recovered from COVID-19, the investigators caution.
“, and monitored after they recover to ensure they do not develop mental illnesses, and are able to access treatment if needed,” senior author Anthony David, FMedSci, from UCL Institute of Mental Health, said in a news release.
“While most people with COVID-19 will recover without experiencing mental illness, we need to research which factors may contribute to enduring mental health problems, and develop interventions to prevent and treat them,” he added.
Be Prepared
The coauthors of a linked commentary say it makes sense, from a biological perspective, to merge data on these three coronavirus diseases, given the degree to which they resemble each other.
They caution, however, that treatment of COVID-19 seems to be different from treatment of SARS and MERS. In addition, the social and economic situation of COVID-19 survivors’ return is completely different from that of SARS and MERS survivors.
Findings from previous coronavirus outbreaks are “useful, but might not be exact predictors of prevalences of psychiatric complications for patients with COVID-19,” write Iris Sommer, MD, PhD, from University Medical Center Groningen, the Netherlands, and P. Roberto Bakker, MD, PhD, from Maastricht University Medical Center, the Netherlands.
“The warning from [this study] that we should prepare to treat large numbers of patients with COVID-19 who go on to develop delirium, post-traumatic stress disorder, anxiety, and depression is an important message for the psychiatric community,” they add.
Sommer and Bakker also say the reported estimates of prevalence in this study should be interpreted with caution, “as true numbers of both acute and long-term psychiatric disorders for patients with COVID-19 might be considerably higher.”
Funding for the study was provided by the Wellcome Trust, the UK National Institute for Health Research (NIHR), the UK Medical Research Council, the NIHR Biomedical Research Center at the University College London Hospitals NHS Foundation Trust, and the University College London. The authors of the study and the commentary have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Severe COVID-19 may cause delirium in the acute stage of illness, followed by the possibility of depression, anxiety, fatigue, insomnia, and posttraumatic stress disorder (PTSD) over the longer term, new research suggests.
Results from “the first systematic review and meta-analysis of the psychiatric consequences of coronavirus infection” showed that previous coronavirus epidemics were associated with a significant psychiatric burden in both the acute and post-illness stages.
“Most people with COVID-19 will not develop any mental health problems, even among those with severe cases requiring hospitalization, but given the huge numbers of people getting sick, the global impact on mental health could be considerable,” co–lead investigator Jonathan Rogers, MRCPsych, Department of Psychiatry, University College London, United Kingdom, said in a news release.
The study was published online May 18 in Lancet Psychiatry.
Need for Monitoring, Support
The researchers analyzed 65 peer-reviewed studies and seven preprint articles with data on acute and post-illness psychiatric and neuropsychiatric features of patients who had been hospitalized with COVID-19, as well as two other diseases caused by coronaviruses – severe acute respiratory syndrome (SARS), in 2002–2004, and Middle East respiratory syndrome (MERS), in 2012.
“Our main findings are that signs suggestive of delirium are common in the acute stage of SARS, MERS, and COVID-19; there is evidence of depression, anxiety, fatigue, and post-traumatic stress disorder in the post-illness stage of previous coronavirus epidemics, but there are few data yet on COVID-19,” the investigators write.
The data show that among patients acutely ill with SARS and MERS, 28% experienced confusion, 33% had depressed mood, 36% had anxiety, 34% suffered from impaired memory, and 42% had insomnia.
After recovery from SARS and MERS, sleep disorder, frequent recall of traumatic memories, emotional lability, impaired concentration, fatigue, and impaired memory were reported in more than 15% of patients during a follow-up period that ranged from 6 weeks to 39 months.
In a meta-analysis, the point prevalence in the post-illness stage was 32% for PTSD and about 15% for depression and anxiety.
In patients acutely ill with severe COVID-19, available data suggest that 65% experience delirium, 69% have agitation after withdrawal of sedation, and 21% have altered consciousness.
In one study, 33% of patients had a dysexecutive syndrome at discharge, characterized by symptoms such as inattention, disorientation, or poorly organized movements in response to command. Currently, data are very limited regarding patients who have recovered from COVID-19, the investigators caution.
“, and monitored after they recover to ensure they do not develop mental illnesses, and are able to access treatment if needed,” senior author Anthony David, FMedSci, from UCL Institute of Mental Health, said in a news release.
“While most people with COVID-19 will recover without experiencing mental illness, we need to research which factors may contribute to enduring mental health problems, and develop interventions to prevent and treat them,” he added.
Be Prepared
The coauthors of a linked commentary say it makes sense, from a biological perspective, to merge data on these three coronavirus diseases, given the degree to which they resemble each other.
They caution, however, that treatment of COVID-19 seems to be different from treatment of SARS and MERS. In addition, the social and economic situation of COVID-19 survivors’ return is completely different from that of SARS and MERS survivors.
Findings from previous coronavirus outbreaks are “useful, but might not be exact predictors of prevalences of psychiatric complications for patients with COVID-19,” write Iris Sommer, MD, PhD, from University Medical Center Groningen, the Netherlands, and P. Roberto Bakker, MD, PhD, from Maastricht University Medical Center, the Netherlands.
“The warning from [this study] that we should prepare to treat large numbers of patients with COVID-19 who go on to develop delirium, post-traumatic stress disorder, anxiety, and depression is an important message for the psychiatric community,” they add.
Sommer and Bakker also say the reported estimates of prevalence in this study should be interpreted with caution, “as true numbers of both acute and long-term psychiatric disorders for patients with COVID-19 might be considerably higher.”
Funding for the study was provided by the Wellcome Trust, the UK National Institute for Health Research (NIHR), the UK Medical Research Council, the NIHR Biomedical Research Center at the University College London Hospitals NHS Foundation Trust, and the University College London. The authors of the study and the commentary have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.