User login
FDA approves Dayvigo for insomnia
Lemborexant will be available in 5-mg and 10-mg tablets after the Drug Enforcement Administration schedules the drug, which is expected to occur within 90 days, according to a statement from Eisai.
Lemborexant is an orexin receptor antagonist. Its approval is based on two phase 3 studies, SUNRISE 1 and SUNRISE 2, that included approximately 2,000 adults with insomnia. Investigators assessed lemborexant versus active comparators for as long as 1 month and versus placebo for 6 months.
In these studies, lemborexant significantly improved objective and subjective measures of sleep onset and sleep maintenance, compared with placebo. The medication was not associated with rebound insomnia or withdrawal effects after treatment discontinuation.
In the phase 3 trials, somnolence was the most common adverse reaction that occurred in at least 5% of patients who received lemborexant and at twice the rate in patients who received placebo (lemborexant 10 mg, 10%; lemborexant 5 mg, 7%; placebo, 1%).
In a middle-of-the-night safety study, lemborexant was associated with dose-dependent worsening on measures of attention and memory, compared with placebo. Treatment did not meaningfully affect ability to awaken to sound, however.
Previously reported data showed that lemborexant was effective in male and female patients and was well tolerated by both sexes and that the medication did not impair postural stability and driving performance.
Lemborexant will be available in 5-mg and 10-mg tablets after the Drug Enforcement Administration schedules the drug, which is expected to occur within 90 days, according to a statement from Eisai.
Lemborexant is an orexin receptor antagonist. Its approval is based on two phase 3 studies, SUNRISE 1 and SUNRISE 2, that included approximately 2,000 adults with insomnia. Investigators assessed lemborexant versus active comparators for as long as 1 month and versus placebo for 6 months.
In these studies, lemborexant significantly improved objective and subjective measures of sleep onset and sleep maintenance, compared with placebo. The medication was not associated with rebound insomnia or withdrawal effects after treatment discontinuation.
In the phase 3 trials, somnolence was the most common adverse reaction that occurred in at least 5% of patients who received lemborexant and at twice the rate in patients who received placebo (lemborexant 10 mg, 10%; lemborexant 5 mg, 7%; placebo, 1%).
In a middle-of-the-night safety study, lemborexant was associated with dose-dependent worsening on measures of attention and memory, compared with placebo. Treatment did not meaningfully affect ability to awaken to sound, however.
Previously reported data showed that lemborexant was effective in male and female patients and was well tolerated by both sexes and that the medication did not impair postural stability and driving performance.
Lemborexant will be available in 5-mg and 10-mg tablets after the Drug Enforcement Administration schedules the drug, which is expected to occur within 90 days, according to a statement from Eisai.
Lemborexant is an orexin receptor antagonist. Its approval is based on two phase 3 studies, SUNRISE 1 and SUNRISE 2, that included approximately 2,000 adults with insomnia. Investigators assessed lemborexant versus active comparators for as long as 1 month and versus placebo for 6 months.
In these studies, lemborexant significantly improved objective and subjective measures of sleep onset and sleep maintenance, compared with placebo. The medication was not associated with rebound insomnia or withdrawal effects after treatment discontinuation.
In the phase 3 trials, somnolence was the most common adverse reaction that occurred in at least 5% of patients who received lemborexant and at twice the rate in patients who received placebo (lemborexant 10 mg, 10%; lemborexant 5 mg, 7%; placebo, 1%).
In a middle-of-the-night safety study, lemborexant was associated with dose-dependent worsening on measures of attention and memory, compared with placebo. Treatment did not meaningfully affect ability to awaken to sound, however.
Previously reported data showed that lemborexant was effective in male and female patients and was well tolerated by both sexes and that the medication did not impair postural stability and driving performance.
Novel analysis links insomnia to first-onset major depressive disorder
a prospective study of 768 adults with a history of depression suggests.
Insomnia has been identified as a risk factor for depression, but the impact of lifetime depression history and the role of insomnia in major depressive disorder (MDD) remains unclear, wrote Tessa Blanken, MSc, of the Netherlands Institute for Neuroscience, Amsterdam, and colleagues. Studies of this relationship have been hampered by the difficulty of isolating the impact of insomnia as an independent predictor of MDD from depression and other disorders.
In a study published in Sleep, the researchers reviewed data from 768 adults aged 18-65 years who were participants in the Netherlands Study of Depression and Anxiety, a multicenter, longitudinal study that included four assessments over 6 years. The participants had no current or prior diagnosis of MDD. The average age of the participants was 41 years, and 63% were women.
The investigators used Network Outcome Analysis to study the link between insomnia and MDD. The investigators wrote, “Network modeling techniques provide a unique framework to study the interactions among symptoms and their role in the development and maintenance of psychiatric disorders. Using network analysis we can estimate the unique association between pairs of symptoms, while controlling for the state and associations of all other symptoms.”
Over 6-years’ follow-up, 141 participants (18%) were diagnosed with first-onset MDD. Overall, insomnia severity was a significant predictor of first-onset MDD (hazard ratio 1.11, 95% confidence interval). The analysis showed that the predictive effect of insomnia on first-onset MDD was driven solely by the item “Did you have trouble falling asleep” (hazard ratio, 1.33; 95% confidence interval, 1.12-1.57; observed range, 0-4). Those individuals who had trouble falling asleep 3-4 times or more than 4 times a week were 2.3 or 3.2 times, respectively, more likely to develop first-onset MDD. None of the other sleep complaints, such as nocturnal and early morning awakening, significantly increased the risk of first-onset MDD.
The study findings were limited by several factors including the full impact of short sleep duration and lack of chronotype assessment, the researchers noted. However, “the identification of ‘difficulty initiating sleep’ as a risk factor is particularly promising because a recent meta-analysis showed that cognitive behavioural therapy, the treatment of choice for insomnia, is highly effective,” the researchers wrote. The results suggest that treating problems in sleep initiation could contribute to preventing first-onset depression and reducing the overall burden of MDD, they concluded.
The study was supported by the European Research Council. The researchers had no financial conflicts to disclose.
SOURCE: Blanken TF et al. Sleep. 2019 Dec 2. doi: 10.1093/sleep/zsz288.
a prospective study of 768 adults with a history of depression suggests.
Insomnia has been identified as a risk factor for depression, but the impact of lifetime depression history and the role of insomnia in major depressive disorder (MDD) remains unclear, wrote Tessa Blanken, MSc, of the Netherlands Institute for Neuroscience, Amsterdam, and colleagues. Studies of this relationship have been hampered by the difficulty of isolating the impact of insomnia as an independent predictor of MDD from depression and other disorders.
In a study published in Sleep, the researchers reviewed data from 768 adults aged 18-65 years who were participants in the Netherlands Study of Depression and Anxiety, a multicenter, longitudinal study that included four assessments over 6 years. The participants had no current or prior diagnosis of MDD. The average age of the participants was 41 years, and 63% were women.
The investigators used Network Outcome Analysis to study the link between insomnia and MDD. The investigators wrote, “Network modeling techniques provide a unique framework to study the interactions among symptoms and their role in the development and maintenance of psychiatric disorders. Using network analysis we can estimate the unique association between pairs of symptoms, while controlling for the state and associations of all other symptoms.”
Over 6-years’ follow-up, 141 participants (18%) were diagnosed with first-onset MDD. Overall, insomnia severity was a significant predictor of first-onset MDD (hazard ratio 1.11, 95% confidence interval). The analysis showed that the predictive effect of insomnia on first-onset MDD was driven solely by the item “Did you have trouble falling asleep” (hazard ratio, 1.33; 95% confidence interval, 1.12-1.57; observed range, 0-4). Those individuals who had trouble falling asleep 3-4 times or more than 4 times a week were 2.3 or 3.2 times, respectively, more likely to develop first-onset MDD. None of the other sleep complaints, such as nocturnal and early morning awakening, significantly increased the risk of first-onset MDD.
The study findings were limited by several factors including the full impact of short sleep duration and lack of chronotype assessment, the researchers noted. However, “the identification of ‘difficulty initiating sleep’ as a risk factor is particularly promising because a recent meta-analysis showed that cognitive behavioural therapy, the treatment of choice for insomnia, is highly effective,” the researchers wrote. The results suggest that treating problems in sleep initiation could contribute to preventing first-onset depression and reducing the overall burden of MDD, they concluded.
The study was supported by the European Research Council. The researchers had no financial conflicts to disclose.
SOURCE: Blanken TF et al. Sleep. 2019 Dec 2. doi: 10.1093/sleep/zsz288.
a prospective study of 768 adults with a history of depression suggests.
Insomnia has been identified as a risk factor for depression, but the impact of lifetime depression history and the role of insomnia in major depressive disorder (MDD) remains unclear, wrote Tessa Blanken, MSc, of the Netherlands Institute for Neuroscience, Amsterdam, and colleagues. Studies of this relationship have been hampered by the difficulty of isolating the impact of insomnia as an independent predictor of MDD from depression and other disorders.
In a study published in Sleep, the researchers reviewed data from 768 adults aged 18-65 years who were participants in the Netherlands Study of Depression and Anxiety, a multicenter, longitudinal study that included four assessments over 6 years. The participants had no current or prior diagnosis of MDD. The average age of the participants was 41 years, and 63% were women.
The investigators used Network Outcome Analysis to study the link between insomnia and MDD. The investigators wrote, “Network modeling techniques provide a unique framework to study the interactions among symptoms and their role in the development and maintenance of psychiatric disorders. Using network analysis we can estimate the unique association between pairs of symptoms, while controlling for the state and associations of all other symptoms.”
Over 6-years’ follow-up, 141 participants (18%) were diagnosed with first-onset MDD. Overall, insomnia severity was a significant predictor of first-onset MDD (hazard ratio 1.11, 95% confidence interval). The analysis showed that the predictive effect of insomnia on first-onset MDD was driven solely by the item “Did you have trouble falling asleep” (hazard ratio, 1.33; 95% confidence interval, 1.12-1.57; observed range, 0-4). Those individuals who had trouble falling asleep 3-4 times or more than 4 times a week were 2.3 or 3.2 times, respectively, more likely to develop first-onset MDD. None of the other sleep complaints, such as nocturnal and early morning awakening, significantly increased the risk of first-onset MDD.
The study findings were limited by several factors including the full impact of short sleep duration and lack of chronotype assessment, the researchers noted. However, “the identification of ‘difficulty initiating sleep’ as a risk factor is particularly promising because a recent meta-analysis showed that cognitive behavioural therapy, the treatment of choice for insomnia, is highly effective,” the researchers wrote. The results suggest that treating problems in sleep initiation could contribute to preventing first-onset depression and reducing the overall burden of MDD, they concluded.
The study was supported by the European Research Council. The researchers had no financial conflicts to disclose.
SOURCE: Blanken TF et al. Sleep. 2019 Dec 2. doi: 10.1093/sleep/zsz288.
FROM SLEEP
Heart rate changes during sleep may be diagnostic tool for depression
A heart rate–profiling algorithm shows promise at distinguishing differences in heart rate patterns during sleep between people with depression and healthy controls, research shows.
The algorithm was modeled using machine learning based on 1,203 polysomnograms from either people with depression or healthy controls, according to Mysa Saad, of the sleep research unit of the Royal’s Institute of Mental Health Research in Ottawa, and associates. That final algorithm was then tested on a new sample of 174 individuals (87 controls, 87 with depression) to categorize each person as either depressed or not depressed. This result was compared with medical record diagnoses. The study was published in BMC Psychiatry.
Compared with the control group, and in overall time. The algorithm incorrectly identified 15 patients with depression as being in the control group, and incorrectly identified 20 controls as having depression. The overall accuracy was 79.9%, with a sensitivity of 82.8% and a specificity of 77%.
“In addition to providing an improved biological underpinning for the diagnosis of depression, this [tool] could possibly offer supplemental information to psychiatric clinical assessment, and objective measures for early screening. Moreover, the use of distinct physiological variables as biomarkers of depression may help emphasize the interactions between mental and physical health. This may contribute to reducing the stigma associated with depression, lifting some social barriers to accessing psychiatric treatment, and allowing for more holistic patient care,” the investigators concluded.
Medibio provided partial funding for the salaries of research assistants; no other conflicts of interest were reported.
SOURCE: Saad M et al. BMC Psychiatry. 2019 Jun 7. doi: 10.1186/s12888-019-2152-1.
A heart rate–profiling algorithm shows promise at distinguishing differences in heart rate patterns during sleep between people with depression and healthy controls, research shows.
The algorithm was modeled using machine learning based on 1,203 polysomnograms from either people with depression or healthy controls, according to Mysa Saad, of the sleep research unit of the Royal’s Institute of Mental Health Research in Ottawa, and associates. That final algorithm was then tested on a new sample of 174 individuals (87 controls, 87 with depression) to categorize each person as either depressed or not depressed. This result was compared with medical record diagnoses. The study was published in BMC Psychiatry.
Compared with the control group, and in overall time. The algorithm incorrectly identified 15 patients with depression as being in the control group, and incorrectly identified 20 controls as having depression. The overall accuracy was 79.9%, with a sensitivity of 82.8% and a specificity of 77%.
“In addition to providing an improved biological underpinning for the diagnosis of depression, this [tool] could possibly offer supplemental information to psychiatric clinical assessment, and objective measures for early screening. Moreover, the use of distinct physiological variables as biomarkers of depression may help emphasize the interactions between mental and physical health. This may contribute to reducing the stigma associated with depression, lifting some social barriers to accessing psychiatric treatment, and allowing for more holistic patient care,” the investigators concluded.
Medibio provided partial funding for the salaries of research assistants; no other conflicts of interest were reported.
SOURCE: Saad M et al. BMC Psychiatry. 2019 Jun 7. doi: 10.1186/s12888-019-2152-1.
A heart rate–profiling algorithm shows promise at distinguishing differences in heart rate patterns during sleep between people with depression and healthy controls, research shows.
The algorithm was modeled using machine learning based on 1,203 polysomnograms from either people with depression or healthy controls, according to Mysa Saad, of the sleep research unit of the Royal’s Institute of Mental Health Research in Ottawa, and associates. That final algorithm was then tested on a new sample of 174 individuals (87 controls, 87 with depression) to categorize each person as either depressed or not depressed. This result was compared with medical record diagnoses. The study was published in BMC Psychiatry.
Compared with the control group, and in overall time. The algorithm incorrectly identified 15 patients with depression as being in the control group, and incorrectly identified 20 controls as having depression. The overall accuracy was 79.9%, with a sensitivity of 82.8% and a specificity of 77%.
“In addition to providing an improved biological underpinning for the diagnosis of depression, this [tool] could possibly offer supplemental information to psychiatric clinical assessment, and objective measures for early screening. Moreover, the use of distinct physiological variables as biomarkers of depression may help emphasize the interactions between mental and physical health. This may contribute to reducing the stigma associated with depression, lifting some social barriers to accessing psychiatric treatment, and allowing for more holistic patient care,” the investigators concluded.
Medibio provided partial funding for the salaries of research assistants; no other conflicts of interest were reported.
SOURCE: Saad M et al. BMC Psychiatry. 2019 Jun 7. doi: 10.1186/s12888-019-2152-1.
FROM BMC PSYCHIATRY
Poor sleep due to ADHD or ADHD due to poor sleep?
The day wouldn’t be so bad if he would just go to sleep at night! How many times have you heard this plea from parents of your patients with ADHD? Sleep is important for everyone, but getting enough is both more important and more difficult for children with ADHD. About three-quarters of children with ADHD have significant problems with sleep, most even before any medication treatment. And inadequate sleep can exacerbate or even cause ADHD symptoms!
Solving sleep problems for children with ADHD is not always simple. The kinds of sleep issues that are more common in children (and adults) with ADHD, compared with typical children, include behavioral bedtime resistance, circadian rhythm sleep disorder (CRSD), insomnia, morning sleepiness, night waking, periodic limb movement disorder (PLMD), restless leg syndrome (RLS), and sleep disordered breathing (SDB). Such a broad differential means a careful history and sometimes even lab studies may be needed.
Both initial and follow-up visits for ADHD should include a sleep history or, ideally, a tool such as BEARS sleep screening tool or Children’s Sleep Habits Questionnaire and a 2-week sleep diary (http://www.sleepfoundation.org/). These are good ways to collect signs of allergies or apnea (for SDB), limb movements or limb pain (for RLS or PLMD), mouth breathing, night waking, and snoring.
You also need to ask about alcohol, drugs, caffeine, and nicotine; asthma; comorbid conditions such as mental health disorders or their treatments; and enuresis (alone or part of nocturnal seizures).
Do I need to remind you to find out about electronics activating the child before bedtime – hidden under the covers, or signaling messages from friends in the middle of the night – and to encourage limits on these? Some sleep disorders warrant polysomnography in a sleep lab or from MyZeo.com (for PLMD and some SDB) or ferritin less than 50 mg/L (for RLS) for diagnosis and to guide treatment. Nasal steroids, antihistamines, or montelukast may help SDB when there are enlarged tonsils or adenoids, but adenotonsillectomy is usually curative.
The first line and most effective treatment for sleep problems in children with or without ADHD is improving sleep hygiene. The key component is establishing habits for the entire sleep cycle: a steady pattern of reduced stimulation in the hour before bedtime (sans electronics); a friendly rather than irritated bedtime routine; and the same bedtime and wake up time, ideally 7 days per week. Bedtime stories read to the child can soothe at any age, not just toddlers! Of course, both children and families want fun and special occasions. For most, varying bedtime by up to 1 hour won’t mess up their biological clock, but for some even this should be avoided. Sleeping alone in a cool, dark, quiet room, nightly in the same bed (not used for other activities), is considered the ideal. Earplugs, white noise generators, and eye masks may be helpful. If sleeping with siblings is a necessity, bedtimes can be staggered to put the child to bed earlier or after others are asleep.
Struggles postponing bedtime may be part of a pattern of oppositionality common in ADHD, but the child may not be tired due to being off schedule (from CRSD), napping on the bus or after school, sleeping in mornings, or unrealistic parent expectations for sleep duration. Parents may want their hyperactive children to give them a break and go to bed at 8 p.m., but children aged 6-10 years need only 10-11 hours and those aged 10-17 years need 8.5-9.25 hours of sleep.
Not tired may instead be “wired” from lingering stimulant effects or even lack of such medication leaving the child overactive or rebounding from earlier medications. Lower afternoon doses or shorter-acting medication may solve lasting medication issues, but sometimes an additional low dose of stimulants actually will help a child with ADHD settle at bedtime. All stimulant medications can prolong sleep onset, often by 30 minutes, but this varies by individual and tends to resolve on its own a few weeks after a new or changed medicine. Switching medication category may allow a child to fall asleep faster. Atomoxetine and alpha agonists are less likely to delay sleep than methylphenidate (MPH).
What if sleep hygiene, behavioral methods, and adjusting ADHD medications is not enough? If sleep issues are causing significant problems, medication for sleep is worth a try. Controlled-release melatonin 1-2 hours before bedtime has data for effectiveness. There is no defined dose, so the lowest effective dose should be used, but 3-6 mg may be needed. Because many families with a child with ADHD are not organized enough to give medicine on this schedule, sublingual melatonin that acts in 15-20 minutes is a good alternative or even first choice. Clonidine 0.05-0.2 mg 1 hour before bedtime speeds sleep onset, lasts 3 hours, and does not carry over to sedation the next day. Stronger psychopharmaceuticals can assist sleep onset, including low dose mirtazapine or trazodone, but have the side effect of daytime sleepiness.
Management of waking in the middle of the night can be more difficult to treat as sleep drive has been dissipated. First, consider whether trips out of bed reflect a sleep association that has not been extinguished. Daytime atomoxetine or, better yet, MPH may improve night waking, and sometimes even a low-dose evening, long-acting medication, such as osmotic release oral system (OROS) extended release methylphenidate HCL (OROS MPH), helps. Short-acting clonidine or melatonin in the middle of the night or bedtime mirtazapine or trazodone also may be worth a try.
When dealing with sleep, keep in mind that 50% or more of children with ADHD have a coexisting mental health disorder. Anxiety, separation anxiety, depression, and dysthymia all often affect sleep onset, night waking, and sometimes early morning waking. The child or teen may need extra reassurance or company at bedtime (siblings or pets may suffice). Reading positive stories or playing soft music may be better at setting a positive mood and sense of safety for sleep, certainly more so than social media, which should be avoided.
Keep in mind that substance use is more common in ADHD as well as with those other mental health conditions and can interfere with restful sleep and make RLS worse. Bipolar disorder can be mistaken for ADHD as it often presents with hyperactivity but also can be comorbid. Sleep problems are increased sixfold when both are present. Prolonged periods awake at night and diminished need for sleep are signs that help differentiate bipolar from ADHD. Medication management for the bipolar disorder with atypicals can reduce sleep latency and reduce REM sleep, but also causes morning fatigue. Medications to treat other mental health problems can help sleep onset (for example, anticonvulsants, atypicals), or prolong it (SSRIs), change REM states (atypicals), and even exacerbate RLS (SSRIs). You can make changes or work with the child’s mental health specialist if medications are causing significant sleep problems.
When we help improve sleep for children with ADHD, it can lessen not only ADHD symptoms but also some symptoms of other mental health disorders, improve learning and behavior, and greatly improve family quality of life!
Dr. Howard is assistant professor of pediatrics at Johns Hopkins University, Baltimore, and creator of CHADIS (www.CHADIS.com). She had no other relevant disclosures. Dr. Howard’s contribution to this publication was as a paid expert to MDedge News. E-mail her at [email protected].
The day wouldn’t be so bad if he would just go to sleep at night! How many times have you heard this plea from parents of your patients with ADHD? Sleep is important for everyone, but getting enough is both more important and more difficult for children with ADHD. About three-quarters of children with ADHD have significant problems with sleep, most even before any medication treatment. And inadequate sleep can exacerbate or even cause ADHD symptoms!
Solving sleep problems for children with ADHD is not always simple. The kinds of sleep issues that are more common in children (and adults) with ADHD, compared with typical children, include behavioral bedtime resistance, circadian rhythm sleep disorder (CRSD), insomnia, morning sleepiness, night waking, periodic limb movement disorder (PLMD), restless leg syndrome (RLS), and sleep disordered breathing (SDB). Such a broad differential means a careful history and sometimes even lab studies may be needed.
Both initial and follow-up visits for ADHD should include a sleep history or, ideally, a tool such as BEARS sleep screening tool or Children’s Sleep Habits Questionnaire and a 2-week sleep diary (http://www.sleepfoundation.org/). These are good ways to collect signs of allergies or apnea (for SDB), limb movements or limb pain (for RLS or PLMD), mouth breathing, night waking, and snoring.
You also need to ask about alcohol, drugs, caffeine, and nicotine; asthma; comorbid conditions such as mental health disorders or their treatments; and enuresis (alone or part of nocturnal seizures).
Do I need to remind you to find out about electronics activating the child before bedtime – hidden under the covers, or signaling messages from friends in the middle of the night – and to encourage limits on these? Some sleep disorders warrant polysomnography in a sleep lab or from MyZeo.com (for PLMD and some SDB) or ferritin less than 50 mg/L (for RLS) for diagnosis and to guide treatment. Nasal steroids, antihistamines, or montelukast may help SDB when there are enlarged tonsils or adenoids, but adenotonsillectomy is usually curative.
The first line and most effective treatment for sleep problems in children with or without ADHD is improving sleep hygiene. The key component is establishing habits for the entire sleep cycle: a steady pattern of reduced stimulation in the hour before bedtime (sans electronics); a friendly rather than irritated bedtime routine; and the same bedtime and wake up time, ideally 7 days per week. Bedtime stories read to the child can soothe at any age, not just toddlers! Of course, both children and families want fun and special occasions. For most, varying bedtime by up to 1 hour won’t mess up their biological clock, but for some even this should be avoided. Sleeping alone in a cool, dark, quiet room, nightly in the same bed (not used for other activities), is considered the ideal. Earplugs, white noise generators, and eye masks may be helpful. If sleeping with siblings is a necessity, bedtimes can be staggered to put the child to bed earlier or after others are asleep.
Struggles postponing bedtime may be part of a pattern of oppositionality common in ADHD, but the child may not be tired due to being off schedule (from CRSD), napping on the bus or after school, sleeping in mornings, or unrealistic parent expectations for sleep duration. Parents may want their hyperactive children to give them a break and go to bed at 8 p.m., but children aged 6-10 years need only 10-11 hours and those aged 10-17 years need 8.5-9.25 hours of sleep.
Not tired may instead be “wired” from lingering stimulant effects or even lack of such medication leaving the child overactive or rebounding from earlier medications. Lower afternoon doses or shorter-acting medication may solve lasting medication issues, but sometimes an additional low dose of stimulants actually will help a child with ADHD settle at bedtime. All stimulant medications can prolong sleep onset, often by 30 minutes, but this varies by individual and tends to resolve on its own a few weeks after a new or changed medicine. Switching medication category may allow a child to fall asleep faster. Atomoxetine and alpha agonists are less likely to delay sleep than methylphenidate (MPH).
What if sleep hygiene, behavioral methods, and adjusting ADHD medications is not enough? If sleep issues are causing significant problems, medication for sleep is worth a try. Controlled-release melatonin 1-2 hours before bedtime has data for effectiveness. There is no defined dose, so the lowest effective dose should be used, but 3-6 mg may be needed. Because many families with a child with ADHD are not organized enough to give medicine on this schedule, sublingual melatonin that acts in 15-20 minutes is a good alternative or even first choice. Clonidine 0.05-0.2 mg 1 hour before bedtime speeds sleep onset, lasts 3 hours, and does not carry over to sedation the next day. Stronger psychopharmaceuticals can assist sleep onset, including low dose mirtazapine or trazodone, but have the side effect of daytime sleepiness.
Management of waking in the middle of the night can be more difficult to treat as sleep drive has been dissipated. First, consider whether trips out of bed reflect a sleep association that has not been extinguished. Daytime atomoxetine or, better yet, MPH may improve night waking, and sometimes even a low-dose evening, long-acting medication, such as osmotic release oral system (OROS) extended release methylphenidate HCL (OROS MPH), helps. Short-acting clonidine or melatonin in the middle of the night or bedtime mirtazapine or trazodone also may be worth a try.
When dealing with sleep, keep in mind that 50% or more of children with ADHD have a coexisting mental health disorder. Anxiety, separation anxiety, depression, and dysthymia all often affect sleep onset, night waking, and sometimes early morning waking. The child or teen may need extra reassurance or company at bedtime (siblings or pets may suffice). Reading positive stories or playing soft music may be better at setting a positive mood and sense of safety for sleep, certainly more so than social media, which should be avoided.
Keep in mind that substance use is more common in ADHD as well as with those other mental health conditions and can interfere with restful sleep and make RLS worse. Bipolar disorder can be mistaken for ADHD as it often presents with hyperactivity but also can be comorbid. Sleep problems are increased sixfold when both are present. Prolonged periods awake at night and diminished need for sleep are signs that help differentiate bipolar from ADHD. Medication management for the bipolar disorder with atypicals can reduce sleep latency and reduce REM sleep, but also causes morning fatigue. Medications to treat other mental health problems can help sleep onset (for example, anticonvulsants, atypicals), or prolong it (SSRIs), change REM states (atypicals), and even exacerbate RLS (SSRIs). You can make changes or work with the child’s mental health specialist if medications are causing significant sleep problems.
When we help improve sleep for children with ADHD, it can lessen not only ADHD symptoms but also some symptoms of other mental health disorders, improve learning and behavior, and greatly improve family quality of life!
Dr. Howard is assistant professor of pediatrics at Johns Hopkins University, Baltimore, and creator of CHADIS (www.CHADIS.com). She had no other relevant disclosures. Dr. Howard’s contribution to this publication was as a paid expert to MDedge News. E-mail her at [email protected].
The day wouldn’t be so bad if he would just go to sleep at night! How many times have you heard this plea from parents of your patients with ADHD? Sleep is important for everyone, but getting enough is both more important and more difficult for children with ADHD. About three-quarters of children with ADHD have significant problems with sleep, most even before any medication treatment. And inadequate sleep can exacerbate or even cause ADHD symptoms!
Solving sleep problems for children with ADHD is not always simple. The kinds of sleep issues that are more common in children (and adults) with ADHD, compared with typical children, include behavioral bedtime resistance, circadian rhythm sleep disorder (CRSD), insomnia, morning sleepiness, night waking, periodic limb movement disorder (PLMD), restless leg syndrome (RLS), and sleep disordered breathing (SDB). Such a broad differential means a careful history and sometimes even lab studies may be needed.
Both initial and follow-up visits for ADHD should include a sleep history or, ideally, a tool such as BEARS sleep screening tool or Children’s Sleep Habits Questionnaire and a 2-week sleep diary (http://www.sleepfoundation.org/). These are good ways to collect signs of allergies or apnea (for SDB), limb movements or limb pain (for RLS or PLMD), mouth breathing, night waking, and snoring.
You also need to ask about alcohol, drugs, caffeine, and nicotine; asthma; comorbid conditions such as mental health disorders or their treatments; and enuresis (alone or part of nocturnal seizures).
Do I need to remind you to find out about electronics activating the child before bedtime – hidden under the covers, or signaling messages from friends in the middle of the night – and to encourage limits on these? Some sleep disorders warrant polysomnography in a sleep lab or from MyZeo.com (for PLMD and some SDB) or ferritin less than 50 mg/L (for RLS) for diagnosis and to guide treatment. Nasal steroids, antihistamines, or montelukast may help SDB when there are enlarged tonsils or adenoids, but adenotonsillectomy is usually curative.
The first line and most effective treatment for sleep problems in children with or without ADHD is improving sleep hygiene. The key component is establishing habits for the entire sleep cycle: a steady pattern of reduced stimulation in the hour before bedtime (sans electronics); a friendly rather than irritated bedtime routine; and the same bedtime and wake up time, ideally 7 days per week. Bedtime stories read to the child can soothe at any age, not just toddlers! Of course, both children and families want fun and special occasions. For most, varying bedtime by up to 1 hour won’t mess up their biological clock, but for some even this should be avoided. Sleeping alone in a cool, dark, quiet room, nightly in the same bed (not used for other activities), is considered the ideal. Earplugs, white noise generators, and eye masks may be helpful. If sleeping with siblings is a necessity, bedtimes can be staggered to put the child to bed earlier or after others are asleep.
Struggles postponing bedtime may be part of a pattern of oppositionality common in ADHD, but the child may not be tired due to being off schedule (from CRSD), napping on the bus or after school, sleeping in mornings, or unrealistic parent expectations for sleep duration. Parents may want their hyperactive children to give them a break and go to bed at 8 p.m., but children aged 6-10 years need only 10-11 hours and those aged 10-17 years need 8.5-9.25 hours of sleep.
Not tired may instead be “wired” from lingering stimulant effects or even lack of such medication leaving the child overactive or rebounding from earlier medications. Lower afternoon doses or shorter-acting medication may solve lasting medication issues, but sometimes an additional low dose of stimulants actually will help a child with ADHD settle at bedtime. All stimulant medications can prolong sleep onset, often by 30 minutes, but this varies by individual and tends to resolve on its own a few weeks after a new or changed medicine. Switching medication category may allow a child to fall asleep faster. Atomoxetine and alpha agonists are less likely to delay sleep than methylphenidate (MPH).
What if sleep hygiene, behavioral methods, and adjusting ADHD medications is not enough? If sleep issues are causing significant problems, medication for sleep is worth a try. Controlled-release melatonin 1-2 hours before bedtime has data for effectiveness. There is no defined dose, so the lowest effective dose should be used, but 3-6 mg may be needed. Because many families with a child with ADHD are not organized enough to give medicine on this schedule, sublingual melatonin that acts in 15-20 minutes is a good alternative or even first choice. Clonidine 0.05-0.2 mg 1 hour before bedtime speeds sleep onset, lasts 3 hours, and does not carry over to sedation the next day. Stronger psychopharmaceuticals can assist sleep onset, including low dose mirtazapine or trazodone, but have the side effect of daytime sleepiness.
Management of waking in the middle of the night can be more difficult to treat as sleep drive has been dissipated. First, consider whether trips out of bed reflect a sleep association that has not been extinguished. Daytime atomoxetine or, better yet, MPH may improve night waking, and sometimes even a low-dose evening, long-acting medication, such as osmotic release oral system (OROS) extended release methylphenidate HCL (OROS MPH), helps. Short-acting clonidine or melatonin in the middle of the night or bedtime mirtazapine or trazodone also may be worth a try.
When dealing with sleep, keep in mind that 50% or more of children with ADHD have a coexisting mental health disorder. Anxiety, separation anxiety, depression, and dysthymia all often affect sleep onset, night waking, and sometimes early morning waking. The child or teen may need extra reassurance or company at bedtime (siblings or pets may suffice). Reading positive stories or playing soft music may be better at setting a positive mood and sense of safety for sleep, certainly more so than social media, which should be avoided.
Keep in mind that substance use is more common in ADHD as well as with those other mental health conditions and can interfere with restful sleep and make RLS worse. Bipolar disorder can be mistaken for ADHD as it often presents with hyperactivity but also can be comorbid. Sleep problems are increased sixfold when both are present. Prolonged periods awake at night and diminished need for sleep are signs that help differentiate bipolar from ADHD. Medication management for the bipolar disorder with atypicals can reduce sleep latency and reduce REM sleep, but also causes morning fatigue. Medications to treat other mental health problems can help sleep onset (for example, anticonvulsants, atypicals), or prolong it (SSRIs), change REM states (atypicals), and even exacerbate RLS (SSRIs). You can make changes or work with the child’s mental health specialist if medications are causing significant sleep problems.
When we help improve sleep for children with ADHD, it can lessen not only ADHD symptoms but also some symptoms of other mental health disorders, improve learning and behavior, and greatly improve family quality of life!
Dr. Howard is assistant professor of pediatrics at Johns Hopkins University, Baltimore, and creator of CHADIS (www.CHADIS.com). She had no other relevant disclosures. Dr. Howard’s contribution to this publication was as a paid expert to MDedge News. E-mail her at [email protected].
What to do when the evidence is not conclusive
Family physicians try to base treatment decisions on the very best available evidence from randomized trials and other high-quality studies. Very often, however, the evidence is not conclusive. Family physicians are confronted with questions about a wide variety of treatments that may or may not be effective. The classic example for me is the use of chondroitin sulfate/glucosamine for knee osteoarthritis. The preponderance of evidence tells us it is not effective, but one long-term clinical trial did find some benefit.1 And some patients swear by it!
In this issue of JFP, we have 2 articles that fall into this category: 1 by Hahn about the treatment of asthma with macrolides and the other by Sorsby et al about use of positive airway pressure (PAP) for obstructive sleep apnea (OSA).
The article by Hahn is an extensive literature review regarding the effectiveness of macrolides for asthma. Despite 2 meta-analyses and many clinical trials, the results are not conclusive; but they are highly suggestive that macrolides may benefit patients with new-onset asthma and severe asthma that does not respond completely to mainstream treatments. Why don't we have conclusive evidence? Because the right studies have not been done. Most studies of macrolides for asthma have not focused on these 2 groups, so any treatment effect may have been diluted by including patients not likely to respond.
The issue with PAP, also known as CPAP (or continuous positive airway pressure), for the treatment of OSA is different. In this case, the question is: What conditions and outcomes are improved by use of PAP? Studies strongly support that PAP is effective in reducing daytime sleepiness and motor vehicle accidents associated with OSA. Most of us had high hopes that PAP also would reduce the adverse cardiovascular outcomes associated with OSA. But the results of large randomized trials have not found a protective effective.
Enthusiasts argue that the studies have not been of sufficient duration and that the participants did not use their PAP devices long enough each night. Some follow-up studies have suggested a protective effective when the device is used for many years, but those studies have the major flaw of volunteer bias, meaning those who adhere to any treatment have better health outcomes than those who do not adhere.
What should you do when there is uncertainty regarding effectiveness? Use shared decision making: What does the patient want to do after you have explained the possible benefits and harms?
1. Reginster JY, Deroisy R, Rovati LC, et. al. Long-term effects of glucosamine sulphate on osteoarthritis progression: a randomised, placebo-controlled clinical trial. Lancet. 2001;357:251–256.
Family physicians try to base treatment decisions on the very best available evidence from randomized trials and other high-quality studies. Very often, however, the evidence is not conclusive. Family physicians are confronted with questions about a wide variety of treatments that may or may not be effective. The classic example for me is the use of chondroitin sulfate/glucosamine for knee osteoarthritis. The preponderance of evidence tells us it is not effective, but one long-term clinical trial did find some benefit.1 And some patients swear by it!
In this issue of JFP, we have 2 articles that fall into this category: 1 by Hahn about the treatment of asthma with macrolides and the other by Sorsby et al about use of positive airway pressure (PAP) for obstructive sleep apnea (OSA).
The article by Hahn is an extensive literature review regarding the effectiveness of macrolides for asthma. Despite 2 meta-analyses and many clinical trials, the results are not conclusive; but they are highly suggestive that macrolides may benefit patients with new-onset asthma and severe asthma that does not respond completely to mainstream treatments. Why don't we have conclusive evidence? Because the right studies have not been done. Most studies of macrolides for asthma have not focused on these 2 groups, so any treatment effect may have been diluted by including patients not likely to respond.
The issue with PAP, also known as CPAP (or continuous positive airway pressure), for the treatment of OSA is different. In this case, the question is: What conditions and outcomes are improved by use of PAP? Studies strongly support that PAP is effective in reducing daytime sleepiness and motor vehicle accidents associated with OSA. Most of us had high hopes that PAP also would reduce the adverse cardiovascular outcomes associated with OSA. But the results of large randomized trials have not found a protective effective.
Enthusiasts argue that the studies have not been of sufficient duration and that the participants did not use their PAP devices long enough each night. Some follow-up studies have suggested a protective effective when the device is used for many years, but those studies have the major flaw of volunteer bias, meaning those who adhere to any treatment have better health outcomes than those who do not adhere.
What should you do when there is uncertainty regarding effectiveness? Use shared decision making: What does the patient want to do after you have explained the possible benefits and harms?
Family physicians try to base treatment decisions on the very best available evidence from randomized trials and other high-quality studies. Very often, however, the evidence is not conclusive. Family physicians are confronted with questions about a wide variety of treatments that may or may not be effective. The classic example for me is the use of chondroitin sulfate/glucosamine for knee osteoarthritis. The preponderance of evidence tells us it is not effective, but one long-term clinical trial did find some benefit.1 And some patients swear by it!
In this issue of JFP, we have 2 articles that fall into this category: 1 by Hahn about the treatment of asthma with macrolides and the other by Sorsby et al about use of positive airway pressure (PAP) for obstructive sleep apnea (OSA).
The article by Hahn is an extensive literature review regarding the effectiveness of macrolides for asthma. Despite 2 meta-analyses and many clinical trials, the results are not conclusive; but they are highly suggestive that macrolides may benefit patients with new-onset asthma and severe asthma that does not respond completely to mainstream treatments. Why don't we have conclusive evidence? Because the right studies have not been done. Most studies of macrolides for asthma have not focused on these 2 groups, so any treatment effect may have been diluted by including patients not likely to respond.
The issue with PAP, also known as CPAP (or continuous positive airway pressure), for the treatment of OSA is different. In this case, the question is: What conditions and outcomes are improved by use of PAP? Studies strongly support that PAP is effective in reducing daytime sleepiness and motor vehicle accidents associated with OSA. Most of us had high hopes that PAP also would reduce the adverse cardiovascular outcomes associated with OSA. But the results of large randomized trials have not found a protective effective.
Enthusiasts argue that the studies have not been of sufficient duration and that the participants did not use their PAP devices long enough each night. Some follow-up studies have suggested a protective effective when the device is used for many years, but those studies have the major flaw of volunteer bias, meaning those who adhere to any treatment have better health outcomes than those who do not adhere.
What should you do when there is uncertainty regarding effectiveness? Use shared decision making: What does the patient want to do after you have explained the possible benefits and harms?
1. Reginster JY, Deroisy R, Rovati LC, et. al. Long-term effects of glucosamine sulphate on osteoarthritis progression: a randomised, placebo-controlled clinical trial. Lancet. 2001;357:251–256.
1. Reginster JY, Deroisy R, Rovati LC, et. al. Long-term effects of glucosamine sulphate on osteoarthritis progression: a randomised, placebo-controlled clinical trial. Lancet. 2001;357:251–256.
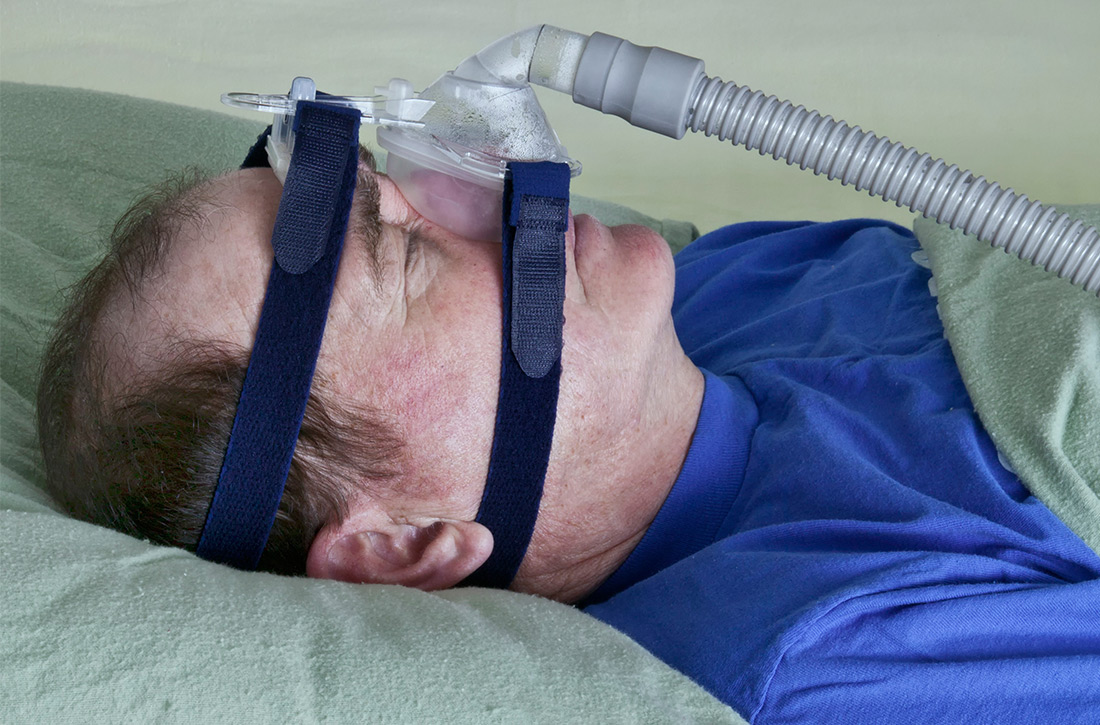
Treatment of OSA: What (else) can it accomplish?
Obstructive sleep apnea (OSA) is a common cause of daytime sleepiness, and severe OSA is a risk factor for hypertension, cardiovascular events, atrial fibrillation (AF), insulin resistance, cognitive impairment, motor vehicle crashes, adverse pregnancy outcomes, and overall mortality.1-8 The hazard ratio for mortality for patients with severe OSA may be as high as 3.8.5
OSA is diagnosed by the apnea-hypopnea index (AHI), defined as the number of apnea or hypopnea events per hour as determined by polysomnography. An AHI score ≤ 5 is considered normal; > 5 to ≤ 15 is mild; > 15 to < 30 is moderate; and ≥ 30 is severe. Most studies of OSA treatment use reduction of AHI as the measure of treatment effectiveness, and several types of treatment improve AHI.
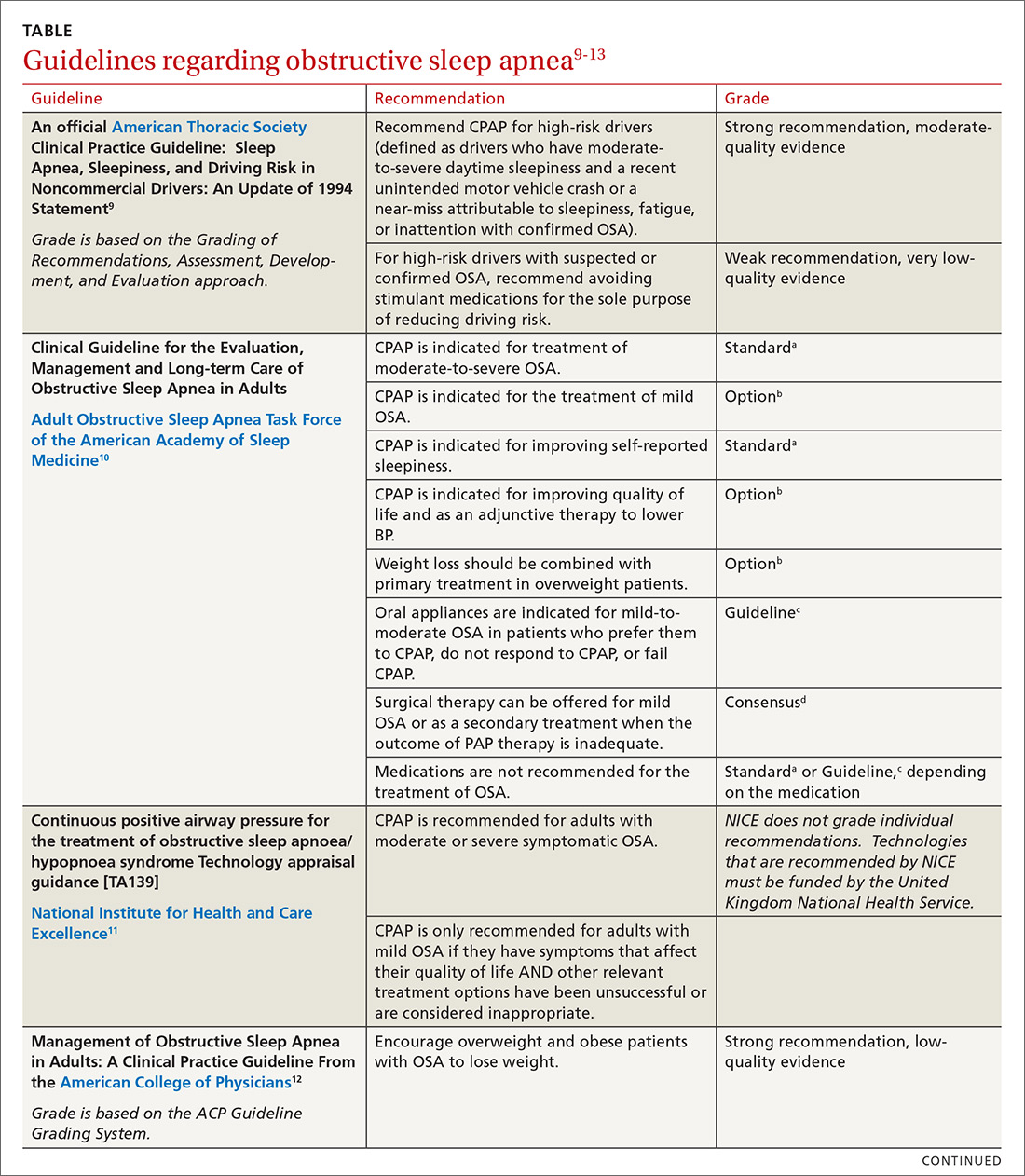
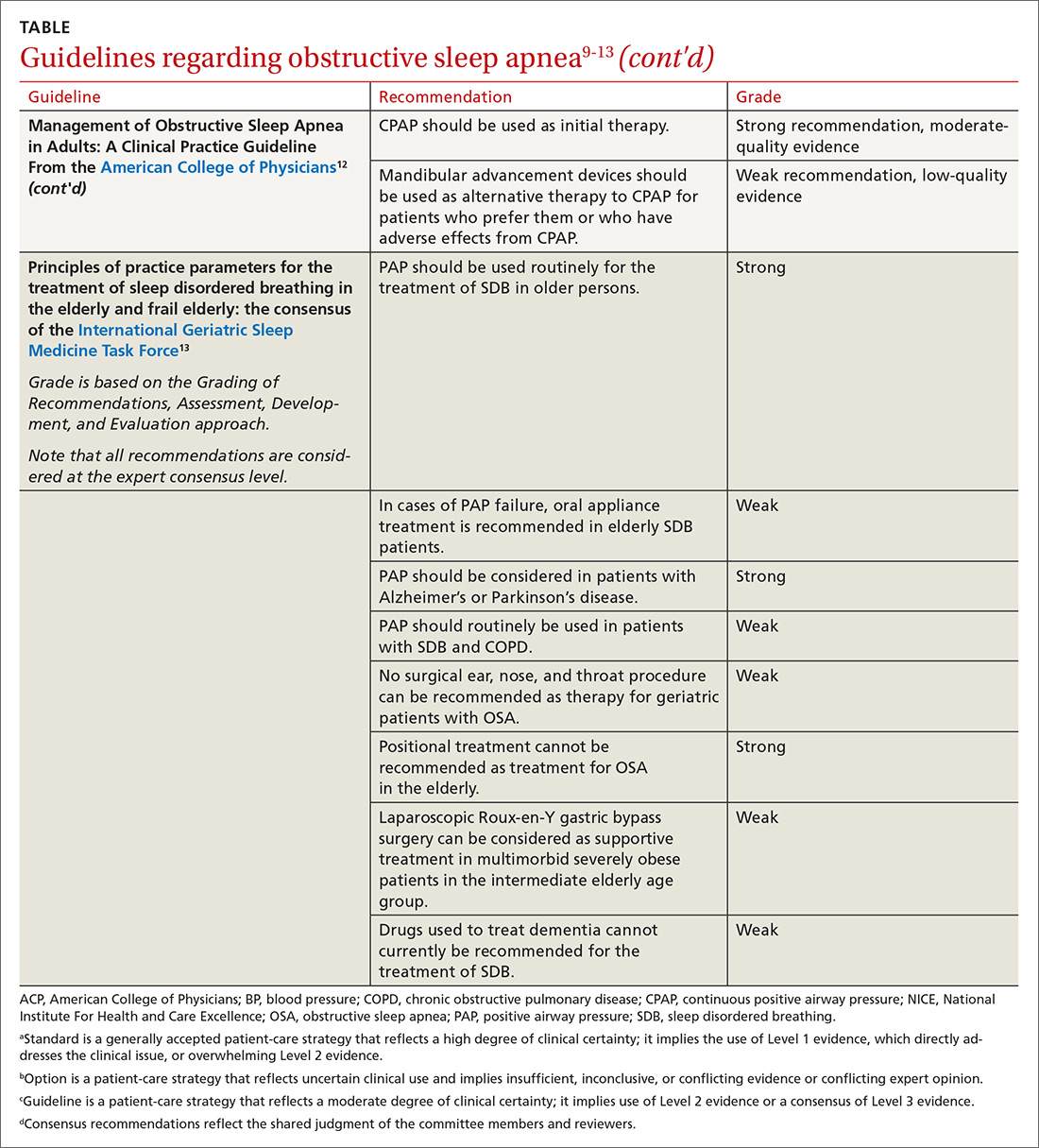
In family medicine, we generally want to know whether treatment of OSA will improve outcomes of significance to patients. A recent systematic review of evidence for the US Preventive Services Task Force found that it was unclear whether OSA treatment improved most health outcomes, including mortality, cardiovascular events, or motor vehicle crashes.6 Several other organizations have published guidelines regarding OSA treatment; these guidelines are reviewed in the TABLE.9-13
This article summarizes the current evidence surrounding the effect of treatment of OSA on outcomes of significance to patients. While multiple treatments have been advocated for patients with OSA, positive airway pressure (PAP) is the most widely used and studied and is recommended as standard treatment by most guidelines.9-13 Most available evidence about patient-oriented outcomes involves treatment with PAP; where there is evidence about the effect of other OSA treatments on a particular outcome, that evidence is also summarized.
Benefits of OSA treatment
Patients with OSA who have excessive daytime sleepiness can gain substantial symptomatic benefit from treatment of their OSA with PAP or oral appliances (OAs), and might benefit from hypoglossal nerve stimulation or other surgical treatment. PAP is probably more effective than OAs in patients who use it ≥ 4 hours/night, but it is more difficult to comply with PAP.14
Evidence that treatment of asymptomatic OSA benefits other medical conditions is often conflicting. Given the low risk of treatment, it is reasonable to consider offering a trial of treatment, preferably with PAP, to asymptomatic patients with moderate-to-severe OSA and certain comorbidities, including obesity, resistant hypertension, high cardiovascular risk, congestive heart failure (CHF), AF, diabetes that is difficult to control, and pregnancy. Such patients should be strongly encouraged to use PAP ≥ 4 hours/night, and should be advised that benefits may not be immediately apparent.
Treatment of OSA improves daytime sleepiness
Daytime sleepiness is typically measured with the Epworth Sleepiness Scale (ESS), a self-administered questionnaire assessing a person’s level of drowsiness and propensity to fall asleep in 8 different daytime situations. Each situation is scored between 0 (would never doze) and 3 (high chance of dozing), with the scores then totaled to provide an overall score between 0 and 24. A score > 10 is considered abnormal.
Continue to: Treament of OSA...
Treatment of OSA with either PAP or OAs significantly improves ESS scores, with PAP being more effective.13 The difference appears to widen in patients with greater daytime sleepiness; in other words, patients with greater daytime sleepiness will gain even greater benefit from PAP, both overall and when compared with OAs.15
One randomized trial of an intensive lifestyle modification program for patients with OSA failed to show improvement in the ESS in the intention-to-treat analysis, but did demonstrate a 2.4-point greater reduction in ESS scores in those patients who successfully followed the program (achieving weight loss).16 Surgical treatments for OSA, such as uvulopalatopharyngoplasty or maxillary advancement, have been shown in some (but not all) studies to improve ESS scores; the different types of surgical treatment and the heterogeneity of studies prevents estimation of effect size.17 A meta-analysis of case series studies of hypoglossal nerve stimulation reported a mean improvement of 4.5 points on the ESS;18 comparison with other interventions is lacking.
Improved quality of life
Both PAP and OAs have been shown to improve sleep-related quality of life in patients with OSA. However, while the improvement is statistically significant, the effect size is small.14
That could be said of a study by Lewis et al.19 These researchers randomized patients with moderate-to-severe OSA and known coronary artery disease (CAD) or at least 3 risk factors for CAD to receive PAP, nocturnal oxygen, or lifestyle education.19 The patients randomized to receive PAP improved vitality scores by only 3.6 points on a 100-point scale; this was significantly better statistically than the improvement achieved by those randomized to lifestyle education. Smaller improvements were noted in depression, social function, and general health. Patients who had more daytime sleepiness at baseline had greater improvements in function.19
Cognitive function findings are mixed
In a systematic review published in 2004, Aloia et al4 found measurable impairments on neuropsychological tests of global cognitive functioning, attention/vigilance, executive functioning, memory, psychomotor function, and constructional abilities in patients with OSA. The results of treatment studies (all but 1 using PAP) were mixed. No studies showed improvement in psychomotor speed or language, and studies disagreed on whether treatment produced benefits in global cognition, attention, or executive functions.4
Continue to: Findings of more recent studies...
Findings of more recent studies remain mixed. A 3-month Spanish trial of PAP in older adults with severe OSA showed improvement in 2 of 4 neuropsychological tests of cognitive function; this was a secondary outcome measure.20 The PREDICT trial in the United Kingdom demonstrated a reduction in daytime sleepiness but no improvement in cognitive function in PAP-treated older adults with OSA but without dementia over a 1-year period.21
In contrast, a French long-term study of adults ages ≥ 65 years with severe (but not necessarily symptomatic) OSA showed better maintenance of memory performance; these results must be interpreted with caution, however, because the study was not randomized, controlled, or blinded, and the results were not adjusted for potential confounders.22 The severity of OSA may influence the impact of PAP treatment on cognitive function.
The prevalence of OSA in patients with dementia is high, and more severe dementia is associated with more severe OSA.23 Although it is intuitive that disrupted sleep may worsen cognitive function, and that treatment could improve it, minimal benefit on cognitive function was shown by neuropsychological testing in patients with Alzheimer’s disease and OSA treated with continuous positive airway pressure (CPAP) vs sham CPAP in 1 small short-term randomized trial.23
In another study of patients with Alzheimer’s disease, this time an observational (nonrandomized, non-controlled, single-blind) study of patients who also had severe symptomatic OSA, researchers followed the patients for 3 years and found a significant delay in median annual cognitive decline of 1.5 points per year on the Mini-Mental Status Examination in patients treated with PAP compared with those who did not receive PAP treatment.24
Hypertension: Small but positive results
A meta-analysis of PAP use in patients with OSA and resistant hypertension (defined as inadequate control while taking at least 3 antihypertensive agents or control requiring at least 4 agents) documented significant blood pressure (BP) lowering, with a pooled estimate of -7.21 mm Hg systolic and -4.99 mm Hg diastolic.25 The decrease in BP was demonstrated in both sleepy and non-sleepy subjects.
Continue to: Multiple studies have...
Multiple studies have shown a small reduction in BP readings (generally about 2 mm Hg) with PAP treatment in nonresistant hypertensive patients with OSA who are sleepy.26 Conversely, the literature is mixed on whether treatment of non-sleepy patients with OSA reduces BP. One long-term study demonstrated a small (1.89 mm Hg systolic, 2.19 mm Hg diastolic) BP reduction effect of PAP in non-sleepy subjects with OSA.27 Similarly, research has shown mandibular advancement devices to lower BP in patients with OSA, in a range similar to that achieved with PAP.28 Whether very small reductions in BP improve important clinical outcomes such as stroke or heart disease is unknown.
CV risk: Again, findings are mixed
The SAVE study is the largest randomized investigation of the effect of treatment of OSA with PAP for secondary prevention of cardiovascular events.29 The trial involved 2717 adults with cardiovascular disease, moderate-to-severe OSA, and minimal sleepiness, and had as its primary composite endpoint death from cardiovascular causes, myocardial infarction (MI), stroke, hospitalization for unstable angina, heart failure, or transient ischemic attack. Patients with severe daytime sleepiness or severe hypoxemia were excluded. The study found no difference between PAP and usual care in the primary outcome, despite a significant reduction in the AHI from a mean of 29 at baseline to 3.7 with PAP treatment.
Similarly, a randomized controlled trial (RCT) of 725 patients with non-sleepy OSA failed to show a reduction in cardiovascular events or in the development of hypertension.30 Peker et al31 randomized 244 adults with recently revascularized coronary artery disease and OSA without daytime sleepiness to auto-titrating CPAP or usual care and did not find a statistically significant difference in revascularization, MI, stroke, or cardiovascular mortality; however, those patients who were compliant with CPAP for ≥ 4 hours/night did have a statistically significant reduction in the combined endpoint.
In contrast, a trial of patients with first-ever stroke and moderate-to-severe OSA who were randomized to early nasal CPAP or usual care demonstrated better 5-year cardiovascular survival for the patients in the CPAP group, and a trend toward better cardiovascular event-free survival.32 Degree of daytime sleepiness was not stated in this study.
A recent meta-analysis of RCTs failed to find a reduction in major adverse cardiovascular events (MACE) in patients with moderate-to-severe OSA treated with PAP.33 In this study, subgroup analysis documented benefit in patients who were adherent with PAP for ≥ 4 hours/night. A larger meta-analysis, however, did not find a reduction in MACE even in the adherent subgroup.34
Continue to: AF and OSA
AF and OSA: An interesting relationship
OSA is an independent risk factor for AF, approximately doubling the risk.35 A review of 10,132 patients with AF (1841 with OSA) in a large observational study demonstrated no difference in outcomes of all-cause mortality, first hospitalization, major bleeding, or major cardiovascular events in OSA patients who were or were not treated with PAP. The PAP-treated patients did have a slightly lower (16% vs 18%) risk of worsening of AF over 2 years.36 Overall, AF patients with OSA had more symptoms and higher admission rates, but no difference in overall mortality or MACE. Observational studies have suggested that PAP treatment of OSA facilitates maintenance of normal sinus rhythm after cardioversion and after ablation.37
CHF: Results look promising
In one small study, 24 patients with heart failure with reduced ejection fraction who were optimally medically treated were randomized to receive PAP or sham PAP for 1 month.38 The treatment group demonstrated reduced systolic BP, reduced end systolic dimension, and significant improvement in ejection fraction from 25 ± 2.8% to 33.8 ± 2.4%.
OSA Tx improves insulin sensitivity
OSA is associated with impaired glucose tolerance, and PAP treatment of OSA has been documented to improve insulin sensitivity.39,40 An efficacy study utilizing PAP in a laboratory setting for 8 hours/night demonstrated significant reduction in fasting blood sugar and a reduction in the dawn phenomenon (an increase in early morning fasting glucose as a result of rebound from hypoglycemia during sleep).39 A 2015 meta-analysis of short-term studies also showed improvement in insulin sensitivity in OSA patients treated with PAP, but failed to find any reduction in A1C or in body mass index.40
All-cause mortality: Difference in findings between short- and long-term studies
Yu et al’s34 meta-analysis of 10 RCTs involving 7266 participants found no difference in mortality in treated (vs no treatment or sham treatment) OSA patients. This was true even in the more adherent subgroup. These studies were relatively short-term, with the longest mean follow-up being 68 months.
However, several longer-term population-based studies have suggested that OSA treatment improves all-cause mortality. An 18-year follow-up of a Wisconsin cohort documented dramatically increased mortality in patients with severe sleep apnea; mortality was even higher when patients treated with PAP were removed from the analysis, suggesting that PAP treatment was protective, mainly for cardiovascular death.5
Continue to: A Danish registry...
A Danish registry documented that patients treated with CPAP had higher rates of comorbidities before and during treatment; when these comorbidities were controlled, men ages ≥ 60 years had improved survival when treated with CPAP. There was no survival benefit in women.41
A recent analysis—the Sleep Heart Health Study—followed patients with obesity and severe OSA for a mean of 11.1 years and calculated a hazard ratio for all-cause mortality associated with prescribed PAP therapy of 0.58 (95% confidence interval [CI], 0.35-0.96) after propensity matching.42 The difference in mortality appeared 6 to 7 years after PAP therapy was prescribed. This delay may explain the failure of shorter-term studies to demonstrate evidence of benefit.
OSA Tx reduces motor vehicle crashes
Drowsy driving is widely accepted as a risk for motor vehicle crashes. Successful treatment of OSA with PAP has been shown to improve driving performance on a driving simulator.43 An analysis of 15 studies similarly demonstrated a significant reduction in driving accidents (incident rate ratio [IRR] = 0.45) and in near-miss accidents (IRR = 0.23) in patients with OSA treated with CPAP.44
Pulmonary hypertension: OSA Tx lowers pulmonary arterial pressure
Patients with OSA have higher than expected rates of pulmonary arterial hypertension—as high as 22%—documented by pulmonary artery catheterization findings.45 A meta-analysis of studies that examined the effect of PAP in patients with OSA and coexisting pulmonary hypertension but without other overt pulmonary or cardiac disease found significant reductions in pulmonary artery pressure.46 Whether this finding translates into improved patient-oriented outcomes is unknown.
OSA and pregnancy outcomes
A national cohort study demonstrated that OSA is an independent risk factor for multiple adverse pregnancy outcomes, including gestational diabetes, hypertensive disorders in pregnancy, intrauterine growth retardation, and stillbirth.7 OSA was also associated with the rare serious adverse outcomes of congestive heart failure, cardiomyopathy, and pulmonary embolism.7 There is little evidence to date with which to determine whether treatment of OSA improves outcomes, but PAP treatment is documented to be safe in pregnant women.8
CORRESPONDENCE
Stephen C. Sorsby, MD, MHA, University of Arkansas for Medical Sciences, 4301 West Markham, Slot 530, Little Rock, AR 72205; [email protected].
1. Peppard PE, Young T, Palta M, et al. Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med. 2000;342:1378-1384.
2. Marin JM, Carrizo SJ, Vicente E, et al. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet. 2005;365:1046-1053.
3. Iftikhar IH, Hoyos CM, Phillips CL, et al. Meta-analyses of the association of sleep apnea with insulin resistance, and the effects of CPAP on HOMA-IR, adiponectin, and visceral adipose fat. J Clin Sleep Med. 2015;11:475-485.
4. Aloia MS, Arnedt JT, Davis JD, et al. Neuropsychological sequelae of obstructive sleep apnea-hypopnea syndrome: a critical review. J Int Neuropsychol Soc. 2004;10:772-785.
5. Young T, Finn L, Peppard PE, et al. Sleep disordered breathing and mortality: eighteen-year follow-up of the Wisconsin sleep cohort. Sleep. 2008;31:1071-1078.
6. Jonas DE, Amick HR, Feltner C, et al. Screening for obstructive sleep apnea in adults: evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2017;317:415-433.
7. Bourjeily G, Danilack VA, Bublitz MA, et al. Obstructive sleep apnea in pregnancy is associated with adverse maternal outcomes: a national cohort. Sleep Med. 2017;35:50-57.
8. Booth JM, Tonidandel AM. Peripartum management of obstructive sleep apnea. Clin Obstet Gyn. 2017;60:405-417.
9. Strohl KP, Brown DB, Collop N, et al. An official American Thoracic Society Clinical Practice Guideline: sleep apnea, sleepiness, and driving risk in noncommercial drivers. An update of a 1994 Statement. Am J Respir Crit Care Med. 2013;187:1259-1266.
10. Epstein LJ, Kristo D, Strollo PJ Jr, et al. Clinical guideline for the evaluation, management and long-term care of obstructive sleep apnea in adults. J Clin Sleep Med. 2009;5:263-276.
11. National Institute for Health and Care Excellence. Continuous positive airway pressure for the treatment of obstructive sleep apnoea/hypopnoea syndrome. Technology appraisal guidance [TA139]. https://www.nice.org.uk/guidance/ta139. Revised February 2012. Accessed October 28, 2019.
12. Qaseem A, Holty JE, Owens DK, et al. Management of obstructive sleep apnea in adults: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2013;159:471-483.
13. Netzer NC, Ancoli-Israel S, Bliwise DL, et al. Principles of practice parameters for the treatment of sleep disordered breathing in the elderly and frail elderly: the consensus of the International Geriatric Sleep Medicine Task Force. Eur Respir J. 2016;48:992-1018.
14. Phillips CL, Grunstein RR, Darendeliler MA, et al. Health outcomes of continuous positive airway pressure versus oral appliance treatment for obstructive sleep apnea: a randomized controlled trial. Am J Respir Crit Care Med. 2013;187:879-887.
15. Bratton DJ, Gaisl T, Schlatzer C, et al. Comparison of the effects of continuous positive airway pressure and mandibular advancement devices on sleepiness in patients with obstructive sleep apnoea: a network meta-analysis. Lancet Respir Med. 2015;3:869-878.
16. Ng SSS, Chan RSM, Woo J, et al. A randomized controlled study to examine the effect of a lifestyle modification program in OSA. Chest. 2015;148:1193-1203.
17. Sundaram S, Bridgman SA, Lim J, et al. Surgery for obstructive sleep apnoea. Cochrane Database Syst Rev. 2005;4:CD001004.
18. Certal VF, Zaghi S, Riaz M, et al. Hypoglossal nerve stimulation in the treatment of obstructive sleep apnea: a systematic review and meta-analysis. Laryngoscope. 2015; 125:1254-1264.
19. Lewis EF, Rui W, Punjabi N, et al. Impact of continuous positive airway pressure and oxygen on health status in patients with coronary heart disease, cardiovascular risk factors, and obstructive sleep apnea: A Heart Biomarker Evaluation in Apnea Treatment (HEARTBEAT) analysis. Am Heart J. 2017;189:59-67.
20. Martinez-Garcia MA, Chiner E, Hernandez L, et al. Obstructive sleep apnoea in the elderly: role of continuous positive airway pressure treatment. Eur Respir J. 2015;46:142-151.
21. McMillan A, Bratton DJ, Faria R, et al. Continuous positive airway pressure in older people with obstructive sleep apnoea syndrome (PREDICT): a 12-month, multicentre, randomised trial. Lancet Respir Med. 2014;2:804-812.
22. Crawford-Achour E, Dauphinot V, Martin MS, et al. Protective effect of long-term CPAP therapy on cognitive performance in elderly patients with severe OSA: the PROOF study. J Clin Sleep Med. 2015;11:519-524.
23. Ancoli-Israel S, Palmer BW, Cooke JR, et al. Cognitive effects of treating obstructive sleep apnea in Alzheimer’s disease: a randomized controlled study. J Am Geriatr Soc. 2008;56:2076-2081.
24. Troussière AC, Charley CM, Salleron J, et al. Treatment of sleep apnoea syndrome decreases cognitive decline in patients with Alzheimer’s disease. J Neurol Neurosurg Psychiatry. 2014;85:1405-1408.
25. Haentjens P, Van Meerhaeghe A, Moscariello A, et al. The impact of continuous positive airway pressure on blood pressure in patients with obstructive sleep apnea syndrome: evidence from a meta-analysis of placebo-controlled randomized trials. Arch Intern Med. 2007;167:757-764.
26. Montesi SB, Edwards BA, Malhotra A, et al. The effect of continuous positive airway pressure treatment on blood pressure: a systematic review and meta-analysis of randomized controlled trials. J Clin Sleep Med. 2012;8:587-596.
27. Barbé F, Durán-Cantolla J, Capote F, et al. Long-term effect of continuous positive airway pressure in hypertensive patients with sleep apnea. Am J Respir Crit Care Med. 2010;181:718-726.
28. Bratton DJ, Gaisl T, Wons AM, et al. CPAP vs mandibular advancement devices and blood pressure in patients with obstructive sleep apnea: a systematic review and meta-analysis. JAMA. 2015;314:2280-2293.
29. McEvoy RD, Antic NA, Heeley E, et al. CPAP for prevention of cardiovascular events in obstructive sleep apnea. N Engl J Med. 2016;375:919-931.
30. Barbé F, Durán-Cantolla J, Sánchez-de-la-Torre M, et al. Effect of continuous positive airway pressure on the incidence of hypertension and cardiovascular events in nonsleepy patients with obstructive sleep apnea: a randomized controlled trial. JAMA. 2012;307:2161-2168.
31. Peker Y, Glantz H, Eulenburg C, et al. Effect of positive airway pressure on cardiovascular outcomes in coronary artery disease patients with nonsleepy obstructive sleep apnea. The RICCADSA Randomized Controlled Trial. Am J Respir Crit Care Med. 2016;194:613-620.
32. Parra O, Sánchez-Armengol Á, Capote F, et al. Efficacy of continuous positive airway pressure treatment on 5-year survival in patients with ischaemic stroke and obstructive sleep apnea: a randomized controlled trial. J Sleep Res. 2015;24:47-53.
33. Abuzaid AS, Al Ashray HS, Elbadaway A, et al. Meta-analysis of cardiovascular outcomes with continuous positive airway pressure in patients with obstructive sleep apnea. Am J Card. 2017;120:693-699.
34. Yu J, Zhou Z, McEvoy D, et al. Association of positive airway pressure with cardiovascular events and death in adults with sleep apnea: a systematic review and meta-analysis. JAMA. 2017;318:156-166.
35. Gami AS, Hodge DO, Herges RM, et al. Obstructive sleep apnea, obesity, and the incident risk of atrial fibrillation. J Amer Coll of Card. 2007;49:565-571.
36. Holmqvist F, Guan N, Zhu Z, et al. Impact of obstructive sleep apnea and continuous positive airway pressure therapy on outcomes in patients with atrial fibrillation—results from the Outcomes Registry for Better Informed Treatment of Atrial Fibrillation (ORBIT-AF). Am Heart J. 2015;169:647-654.e2.
37. Nalliah CJ, Sanders P, Kalman JM. Obstructive sleep apnea treatment and atrial fibrillation: a need for definitive evidence. J Cardiovasc Electrophysiol. 2016;27:1001-1010.
38. Kaneko Y, Floras JS, Usui K, et al. Cardiovascular effects of continuous positive airway pressure in patients with heart failure and obstructive sleep apnea. N Engl J Med. 2003;348:1233-1241
39. Pamidi S, Wroblewski K, Stepien M, et al. Eight hours of nightly continuous positive airway pressure treatment of obstructive sleep apnea improves glucose metabolism in patients with prediabetes: a randomized controlled trial. Am J Respir Crit Care Med. 2015;192:96-105.
40. Feng Y, Zhang Z, Dong ZZ. Effects of continuous positive airway pressure therapy on glycaemic control, insulin sensitivity and body mass index in patients with obstructive sleep apnoea and type 2 diabetes: a systematic review and meta-analysis. NPJ Prim Care Respir Med. 2015;25:15005.
41. Jennum P, Tonnesen P, Ibsen R, et al. Obstructive sleep apnea: effect of comorbidities and positive airway pressure on all-cause mortality. Sleep Med. 2017;36:62-66.
42. Lisan Q, Van Sloten T, Marques Vidal P, et al. Association of positive airway pressure prescription with mortality in patients with obesity and severe obstructive sleep apnea: the sleep heart health study. JAMA Otolaryngol Head Neck Surg. 2019;145:509-515.
43. Mazza S, Pépin JL, Naëgelé B, et al. Driving ability in sleep apnoea patients before and after CPAP treatment: evaluation on a road safety platform. Eur Respir J. 2006;28:1020-1028.
44. Antonopoulos CN, Sergentanis TN, Daskalopoulou SS, et al. Nasal continuous positive airway pressure (nCPAP) treatment for obstructive sleep apnea, road traffic accidents and driving simulator performance: a meta-analysis. Sleep Med Rev. 2011;15:301-310.
45. Minai OA, Ricaurte B, Kaw R, et al. Frequency and impact of pulmonary hypertension in patients with obstructive sleep apnea syndrome. Am J Cardiol. 2009;104:1300-1306.
46. Imran TF, Ghazipura M, Liu S, et al. Effect of continuous positive airway pressure treatment on pulmonary artery pressure in patients with isolated obstructive sleep apnea: a meta-analysis. Heart Fail Rev. 2016;21:591-598.
Obstructive sleep apnea (OSA) is a common cause of daytime sleepiness, and severe OSA is a risk factor for hypertension, cardiovascular events, atrial fibrillation (AF), insulin resistance, cognitive impairment, motor vehicle crashes, adverse pregnancy outcomes, and overall mortality.1-8 The hazard ratio for mortality for patients with severe OSA may be as high as 3.8.5
OSA is diagnosed by the apnea-hypopnea index (AHI), defined as the number of apnea or hypopnea events per hour as determined by polysomnography. An AHI score ≤ 5 is considered normal; > 5 to ≤ 15 is mild; > 15 to < 30 is moderate; and ≥ 30 is severe. Most studies of OSA treatment use reduction of AHI as the measure of treatment effectiveness, and several types of treatment improve AHI.
In family medicine, we generally want to know whether treatment of OSA will improve outcomes of significance to patients. A recent systematic review of evidence for the US Preventive Services Task Force found that it was unclear whether OSA treatment improved most health outcomes, including mortality, cardiovascular events, or motor vehicle crashes.6 Several other organizations have published guidelines regarding OSA treatment; these guidelines are reviewed in the TABLE.9-13

This article summarizes the current evidence surrounding the effect of treatment of OSA on outcomes of significance to patients. While multiple treatments have been advocated for patients with OSA, positive airway pressure (PAP) is the most widely used and studied and is recommended as standard treatment by most guidelines.9-13 Most available evidence about patient-oriented outcomes involves treatment with PAP; where there is evidence about the effect of other OSA treatments on a particular outcome, that evidence is also summarized.

Benefits of OSA treatment
Patients with OSA who have excessive daytime sleepiness can gain substantial symptomatic benefit from treatment of their OSA with PAP or oral appliances (OAs), and might benefit from hypoglossal nerve stimulation or other surgical treatment. PAP is probably more effective than OAs in patients who use it ≥ 4 hours/night, but it is more difficult to comply with PAP.14
Evidence that treatment of asymptomatic OSA benefits other medical conditions is often conflicting. Given the low risk of treatment, it is reasonable to consider offering a trial of treatment, preferably with PAP, to asymptomatic patients with moderate-to-severe OSA and certain comorbidities, including obesity, resistant hypertension, high cardiovascular risk, congestive heart failure (CHF), AF, diabetes that is difficult to control, and pregnancy. Such patients should be strongly encouraged to use PAP ≥ 4 hours/night, and should be advised that benefits may not be immediately apparent.
Treatment of OSA improves daytime sleepiness
Daytime sleepiness is typically measured with the Epworth Sleepiness Scale (ESS), a self-administered questionnaire assessing a person’s level of drowsiness and propensity to fall asleep in 8 different daytime situations. Each situation is scored between 0 (would never doze) and 3 (high chance of dozing), with the scores then totaled to provide an overall score between 0 and 24. A score > 10 is considered abnormal.
Continue to: Treament of OSA...
Treatment of OSA with either PAP or OAs significantly improves ESS scores, with PAP being more effective.13 The difference appears to widen in patients with greater daytime sleepiness; in other words, patients with greater daytime sleepiness will gain even greater benefit from PAP, both overall and when compared with OAs.15
One randomized trial of an intensive lifestyle modification program for patients with OSA failed to show improvement in the ESS in the intention-to-treat analysis, but did demonstrate a 2.4-point greater reduction in ESS scores in those patients who successfully followed the program (achieving weight loss).16 Surgical treatments for OSA, such as uvulopalatopharyngoplasty or maxillary advancement, have been shown in some (but not all) studies to improve ESS scores; the different types of surgical treatment and the heterogeneity of studies prevents estimation of effect size.17 A meta-analysis of case series studies of hypoglossal nerve stimulation reported a mean improvement of 4.5 points on the ESS;18 comparison with other interventions is lacking.
Improved quality of life
Both PAP and OAs have been shown to improve sleep-related quality of life in patients with OSA. However, while the improvement is statistically significant, the effect size is small.14
That could be said of a study by Lewis et al.19 These researchers randomized patients with moderate-to-severe OSA and known coronary artery disease (CAD) or at least 3 risk factors for CAD to receive PAP, nocturnal oxygen, or lifestyle education.19 The patients randomized to receive PAP improved vitality scores by only 3.6 points on a 100-point scale; this was significantly better statistically than the improvement achieved by those randomized to lifestyle education. Smaller improvements were noted in depression, social function, and general health. Patients who had more daytime sleepiness at baseline had greater improvements in function.19
Cognitive function findings are mixed
In a systematic review published in 2004, Aloia et al4 found measurable impairments on neuropsychological tests of global cognitive functioning, attention/vigilance, executive functioning, memory, psychomotor function, and constructional abilities in patients with OSA. The results of treatment studies (all but 1 using PAP) were mixed. No studies showed improvement in psychomotor speed or language, and studies disagreed on whether treatment produced benefits in global cognition, attention, or executive functions.4
Continue to: Findings of more recent studies...
Findings of more recent studies remain mixed. A 3-month Spanish trial of PAP in older adults with severe OSA showed improvement in 2 of 4 neuropsychological tests of cognitive function; this was a secondary outcome measure.20 The PREDICT trial in the United Kingdom demonstrated a reduction in daytime sleepiness but no improvement in cognitive function in PAP-treated older adults with OSA but without dementia over a 1-year period.21
In contrast, a French long-term study of adults ages ≥ 65 years with severe (but not necessarily symptomatic) OSA showed better maintenance of memory performance; these results must be interpreted with caution, however, because the study was not randomized, controlled, or blinded, and the results were not adjusted for potential confounders.22 The severity of OSA may influence the impact of PAP treatment on cognitive function.
The prevalence of OSA in patients with dementia is high, and more severe dementia is associated with more severe OSA.23 Although it is intuitive that disrupted sleep may worsen cognitive function, and that treatment could improve it, minimal benefit on cognitive function was shown by neuropsychological testing in patients with Alzheimer’s disease and OSA treated with continuous positive airway pressure (CPAP) vs sham CPAP in 1 small short-term randomized trial.23
In another study of patients with Alzheimer’s disease, this time an observational (nonrandomized, non-controlled, single-blind) study of patients who also had severe symptomatic OSA, researchers followed the patients for 3 years and found a significant delay in median annual cognitive decline of 1.5 points per year on the Mini-Mental Status Examination in patients treated with PAP compared with those who did not receive PAP treatment.24
Hypertension: Small but positive results
A meta-analysis of PAP use in patients with OSA and resistant hypertension (defined as inadequate control while taking at least 3 antihypertensive agents or control requiring at least 4 agents) documented significant blood pressure (BP) lowering, with a pooled estimate of -7.21 mm Hg systolic and -4.99 mm Hg diastolic.25 The decrease in BP was demonstrated in both sleepy and non-sleepy subjects.
Continue to: Multiple studies have...
Multiple studies have shown a small reduction in BP readings (generally about 2 mm Hg) with PAP treatment in nonresistant hypertensive patients with OSA who are sleepy.26 Conversely, the literature is mixed on whether treatment of non-sleepy patients with OSA reduces BP. One long-term study demonstrated a small (1.89 mm Hg systolic, 2.19 mm Hg diastolic) BP reduction effect of PAP in non-sleepy subjects with OSA.27 Similarly, research has shown mandibular advancement devices to lower BP in patients with OSA, in a range similar to that achieved with PAP.28 Whether very small reductions in BP improve important clinical outcomes such as stroke or heart disease is unknown.
CV risk: Again, findings are mixed
The SAVE study is the largest randomized investigation of the effect of treatment of OSA with PAP for secondary prevention of cardiovascular events.29 The trial involved 2717 adults with cardiovascular disease, moderate-to-severe OSA, and minimal sleepiness, and had as its primary composite endpoint death from cardiovascular causes, myocardial infarction (MI), stroke, hospitalization for unstable angina, heart failure, or transient ischemic attack. Patients with severe daytime sleepiness or severe hypoxemia were excluded. The study found no difference between PAP and usual care in the primary outcome, despite a significant reduction in the AHI from a mean of 29 at baseline to 3.7 with PAP treatment.
Similarly, a randomized controlled trial (RCT) of 725 patients with non-sleepy OSA failed to show a reduction in cardiovascular events or in the development of hypertension.30 Peker et al31 randomized 244 adults with recently revascularized coronary artery disease and OSA without daytime sleepiness to auto-titrating CPAP or usual care and did not find a statistically significant difference in revascularization, MI, stroke, or cardiovascular mortality; however, those patients who were compliant with CPAP for ≥ 4 hours/night did have a statistically significant reduction in the combined endpoint.
In contrast, a trial of patients with first-ever stroke and moderate-to-severe OSA who were randomized to early nasal CPAP or usual care demonstrated better 5-year cardiovascular survival for the patients in the CPAP group, and a trend toward better cardiovascular event-free survival.32 Degree of daytime sleepiness was not stated in this study.
A recent meta-analysis of RCTs failed to find a reduction in major adverse cardiovascular events (MACE) in patients with moderate-to-severe OSA treated with PAP.33 In this study, subgroup analysis documented benefit in patients who were adherent with PAP for ≥ 4 hours/night. A larger meta-analysis, however, did not find a reduction in MACE even in the adherent subgroup.34
Continue to: AF and OSA
AF and OSA: An interesting relationship
OSA is an independent risk factor for AF, approximately doubling the risk.35 A review of 10,132 patients with AF (1841 with OSA) in a large observational study demonstrated no difference in outcomes of all-cause mortality, first hospitalization, major bleeding, or major cardiovascular events in OSA patients who were or were not treated with PAP. The PAP-treated patients did have a slightly lower (16% vs 18%) risk of worsening of AF over 2 years.36 Overall, AF patients with OSA had more symptoms and higher admission rates, but no difference in overall mortality or MACE. Observational studies have suggested that PAP treatment of OSA facilitates maintenance of normal sinus rhythm after cardioversion and after ablation.37
CHF: Results look promising
In one small study, 24 patients with heart failure with reduced ejection fraction who were optimally medically treated were randomized to receive PAP or sham PAP for 1 month.38 The treatment group demonstrated reduced systolic BP, reduced end systolic dimension, and significant improvement in ejection fraction from 25 ± 2.8% to 33.8 ± 2.4%.
OSA Tx improves insulin sensitivity
OSA is associated with impaired glucose tolerance, and PAP treatment of OSA has been documented to improve insulin sensitivity.39,40 An efficacy study utilizing PAP in a laboratory setting for 8 hours/night demonstrated significant reduction in fasting blood sugar and a reduction in the dawn phenomenon (an increase in early morning fasting glucose as a result of rebound from hypoglycemia during sleep).39 A 2015 meta-analysis of short-term studies also showed improvement in insulin sensitivity in OSA patients treated with PAP, but failed to find any reduction in A1C or in body mass index.40
All-cause mortality: Difference in findings between short- and long-term studies
Yu et al’s34 meta-analysis of 10 RCTs involving 7266 participants found no difference in mortality in treated (vs no treatment or sham treatment) OSA patients. This was true even in the more adherent subgroup. These studies were relatively short-term, with the longest mean follow-up being 68 months.
However, several longer-term population-based studies have suggested that OSA treatment improves all-cause mortality. An 18-year follow-up of a Wisconsin cohort documented dramatically increased mortality in patients with severe sleep apnea; mortality was even higher when patients treated with PAP were removed from the analysis, suggesting that PAP treatment was protective, mainly for cardiovascular death.5
Continue to: A Danish registry...
A Danish registry documented that patients treated with CPAP had higher rates of comorbidities before and during treatment; when these comorbidities were controlled, men ages ≥ 60 years had improved survival when treated with CPAP. There was no survival benefit in women.41
A recent analysis—the Sleep Heart Health Study—followed patients with obesity and severe OSA for a mean of 11.1 years and calculated a hazard ratio for all-cause mortality associated with prescribed PAP therapy of 0.58 (95% confidence interval [CI], 0.35-0.96) after propensity matching.42 The difference in mortality appeared 6 to 7 years after PAP therapy was prescribed. This delay may explain the failure of shorter-term studies to demonstrate evidence of benefit.
OSA Tx reduces motor vehicle crashes
Drowsy driving is widely accepted as a risk for motor vehicle crashes. Successful treatment of OSA with PAP has been shown to improve driving performance on a driving simulator.43 An analysis of 15 studies similarly demonstrated a significant reduction in driving accidents (incident rate ratio [IRR] = 0.45) and in near-miss accidents (IRR = 0.23) in patients with OSA treated with CPAP.44
Pulmonary hypertension: OSA Tx lowers pulmonary arterial pressure
Patients with OSA have higher than expected rates of pulmonary arterial hypertension—as high as 22%—documented by pulmonary artery catheterization findings.45 A meta-analysis of studies that examined the effect of PAP in patients with OSA and coexisting pulmonary hypertension but without other overt pulmonary or cardiac disease found significant reductions in pulmonary artery pressure.46 Whether this finding translates into improved patient-oriented outcomes is unknown.
OSA and pregnancy outcomes
A national cohort study demonstrated that OSA is an independent risk factor for multiple adverse pregnancy outcomes, including gestational diabetes, hypertensive disorders in pregnancy, intrauterine growth retardation, and stillbirth.7 OSA was also associated with the rare serious adverse outcomes of congestive heart failure, cardiomyopathy, and pulmonary embolism.7 There is little evidence to date with which to determine whether treatment of OSA improves outcomes, but PAP treatment is documented to be safe in pregnant women.8
CORRESPONDENCE
Stephen C. Sorsby, MD, MHA, University of Arkansas for Medical Sciences, 4301 West Markham, Slot 530, Little Rock, AR 72205; [email protected].
Obstructive sleep apnea (OSA) is a common cause of daytime sleepiness, and severe OSA is a risk factor for hypertension, cardiovascular events, atrial fibrillation (AF), insulin resistance, cognitive impairment, motor vehicle crashes, adverse pregnancy outcomes, and overall mortality.1-8 The hazard ratio for mortality for patients with severe OSA may be as high as 3.8.5
OSA is diagnosed by the apnea-hypopnea index (AHI), defined as the number of apnea or hypopnea events per hour as determined by polysomnography. An AHI score ≤ 5 is considered normal; > 5 to ≤ 15 is mild; > 15 to < 30 is moderate; and ≥ 30 is severe. Most studies of OSA treatment use reduction of AHI as the measure of treatment effectiveness, and several types of treatment improve AHI.
In family medicine, we generally want to know whether treatment of OSA will improve outcomes of significance to patients. A recent systematic review of evidence for the US Preventive Services Task Force found that it was unclear whether OSA treatment improved most health outcomes, including mortality, cardiovascular events, or motor vehicle crashes.6 Several other organizations have published guidelines regarding OSA treatment; these guidelines are reviewed in the TABLE.9-13

This article summarizes the current evidence surrounding the effect of treatment of OSA on outcomes of significance to patients. While multiple treatments have been advocated for patients with OSA, positive airway pressure (PAP) is the most widely used and studied and is recommended as standard treatment by most guidelines.9-13 Most available evidence about patient-oriented outcomes involves treatment with PAP; where there is evidence about the effect of other OSA treatments on a particular outcome, that evidence is also summarized.

Benefits of OSA treatment
Patients with OSA who have excessive daytime sleepiness can gain substantial symptomatic benefit from treatment of their OSA with PAP or oral appliances (OAs), and might benefit from hypoglossal nerve stimulation or other surgical treatment. PAP is probably more effective than OAs in patients who use it ≥ 4 hours/night, but it is more difficult to comply with PAP.14
Evidence that treatment of asymptomatic OSA benefits other medical conditions is often conflicting. Given the low risk of treatment, it is reasonable to consider offering a trial of treatment, preferably with PAP, to asymptomatic patients with moderate-to-severe OSA and certain comorbidities, including obesity, resistant hypertension, high cardiovascular risk, congestive heart failure (CHF), AF, diabetes that is difficult to control, and pregnancy. Such patients should be strongly encouraged to use PAP ≥ 4 hours/night, and should be advised that benefits may not be immediately apparent.
Treatment of OSA improves daytime sleepiness
Daytime sleepiness is typically measured with the Epworth Sleepiness Scale (ESS), a self-administered questionnaire assessing a person’s level of drowsiness and propensity to fall asleep in 8 different daytime situations. Each situation is scored between 0 (would never doze) and 3 (high chance of dozing), with the scores then totaled to provide an overall score between 0 and 24. A score > 10 is considered abnormal.
Continue to: Treament of OSA...
Treatment of OSA with either PAP or OAs significantly improves ESS scores, with PAP being more effective.13 The difference appears to widen in patients with greater daytime sleepiness; in other words, patients with greater daytime sleepiness will gain even greater benefit from PAP, both overall and when compared with OAs.15
One randomized trial of an intensive lifestyle modification program for patients with OSA failed to show improvement in the ESS in the intention-to-treat analysis, but did demonstrate a 2.4-point greater reduction in ESS scores in those patients who successfully followed the program (achieving weight loss).16 Surgical treatments for OSA, such as uvulopalatopharyngoplasty or maxillary advancement, have been shown in some (but not all) studies to improve ESS scores; the different types of surgical treatment and the heterogeneity of studies prevents estimation of effect size.17 A meta-analysis of case series studies of hypoglossal nerve stimulation reported a mean improvement of 4.5 points on the ESS;18 comparison with other interventions is lacking.
Improved quality of life
Both PAP and OAs have been shown to improve sleep-related quality of life in patients with OSA. However, while the improvement is statistically significant, the effect size is small.14
That could be said of a study by Lewis et al.19 These researchers randomized patients with moderate-to-severe OSA and known coronary artery disease (CAD) or at least 3 risk factors for CAD to receive PAP, nocturnal oxygen, or lifestyle education.19 The patients randomized to receive PAP improved vitality scores by only 3.6 points on a 100-point scale; this was significantly better statistically than the improvement achieved by those randomized to lifestyle education. Smaller improvements were noted in depression, social function, and general health. Patients who had more daytime sleepiness at baseline had greater improvements in function.19
Cognitive function findings are mixed
In a systematic review published in 2004, Aloia et al4 found measurable impairments on neuropsychological tests of global cognitive functioning, attention/vigilance, executive functioning, memory, psychomotor function, and constructional abilities in patients with OSA. The results of treatment studies (all but 1 using PAP) were mixed. No studies showed improvement in psychomotor speed or language, and studies disagreed on whether treatment produced benefits in global cognition, attention, or executive functions.4
Continue to: Findings of more recent studies...
Findings of more recent studies remain mixed. A 3-month Spanish trial of PAP in older adults with severe OSA showed improvement in 2 of 4 neuropsychological tests of cognitive function; this was a secondary outcome measure.20 The PREDICT trial in the United Kingdom demonstrated a reduction in daytime sleepiness but no improvement in cognitive function in PAP-treated older adults with OSA but without dementia over a 1-year period.21
In contrast, a French long-term study of adults ages ≥ 65 years with severe (but not necessarily symptomatic) OSA showed better maintenance of memory performance; these results must be interpreted with caution, however, because the study was not randomized, controlled, or blinded, and the results were not adjusted for potential confounders.22 The severity of OSA may influence the impact of PAP treatment on cognitive function.
The prevalence of OSA in patients with dementia is high, and more severe dementia is associated with more severe OSA.23 Although it is intuitive that disrupted sleep may worsen cognitive function, and that treatment could improve it, minimal benefit on cognitive function was shown by neuropsychological testing in patients with Alzheimer’s disease and OSA treated with continuous positive airway pressure (CPAP) vs sham CPAP in 1 small short-term randomized trial.23
In another study of patients with Alzheimer’s disease, this time an observational (nonrandomized, non-controlled, single-blind) study of patients who also had severe symptomatic OSA, researchers followed the patients for 3 years and found a significant delay in median annual cognitive decline of 1.5 points per year on the Mini-Mental Status Examination in patients treated with PAP compared with those who did not receive PAP treatment.24
Hypertension: Small but positive results
A meta-analysis of PAP use in patients with OSA and resistant hypertension (defined as inadequate control while taking at least 3 antihypertensive agents or control requiring at least 4 agents) documented significant blood pressure (BP) lowering, with a pooled estimate of -7.21 mm Hg systolic and -4.99 mm Hg diastolic.25 The decrease in BP was demonstrated in both sleepy and non-sleepy subjects.
Continue to: Multiple studies have...
Multiple studies have shown a small reduction in BP readings (generally about 2 mm Hg) with PAP treatment in nonresistant hypertensive patients with OSA who are sleepy.26 Conversely, the literature is mixed on whether treatment of non-sleepy patients with OSA reduces BP. One long-term study demonstrated a small (1.89 mm Hg systolic, 2.19 mm Hg diastolic) BP reduction effect of PAP in non-sleepy subjects with OSA.27 Similarly, research has shown mandibular advancement devices to lower BP in patients with OSA, in a range similar to that achieved with PAP.28 Whether very small reductions in BP improve important clinical outcomes such as stroke or heart disease is unknown.
CV risk: Again, findings are mixed
The SAVE study is the largest randomized investigation of the effect of treatment of OSA with PAP for secondary prevention of cardiovascular events.29 The trial involved 2717 adults with cardiovascular disease, moderate-to-severe OSA, and minimal sleepiness, and had as its primary composite endpoint death from cardiovascular causes, myocardial infarction (MI), stroke, hospitalization for unstable angina, heart failure, or transient ischemic attack. Patients with severe daytime sleepiness or severe hypoxemia were excluded. The study found no difference between PAP and usual care in the primary outcome, despite a significant reduction in the AHI from a mean of 29 at baseline to 3.7 with PAP treatment.
Similarly, a randomized controlled trial (RCT) of 725 patients with non-sleepy OSA failed to show a reduction in cardiovascular events or in the development of hypertension.30 Peker et al31 randomized 244 adults with recently revascularized coronary artery disease and OSA without daytime sleepiness to auto-titrating CPAP or usual care and did not find a statistically significant difference in revascularization, MI, stroke, or cardiovascular mortality; however, those patients who were compliant with CPAP for ≥ 4 hours/night did have a statistically significant reduction in the combined endpoint.
In contrast, a trial of patients with first-ever stroke and moderate-to-severe OSA who were randomized to early nasal CPAP or usual care demonstrated better 5-year cardiovascular survival for the patients in the CPAP group, and a trend toward better cardiovascular event-free survival.32 Degree of daytime sleepiness was not stated in this study.
A recent meta-analysis of RCTs failed to find a reduction in major adverse cardiovascular events (MACE) in patients with moderate-to-severe OSA treated with PAP.33 In this study, subgroup analysis documented benefit in patients who were adherent with PAP for ≥ 4 hours/night. A larger meta-analysis, however, did not find a reduction in MACE even in the adherent subgroup.34
Continue to: AF and OSA
AF and OSA: An interesting relationship
OSA is an independent risk factor for AF, approximately doubling the risk.35 A review of 10,132 patients with AF (1841 with OSA) in a large observational study demonstrated no difference in outcomes of all-cause mortality, first hospitalization, major bleeding, or major cardiovascular events in OSA patients who were or were not treated with PAP. The PAP-treated patients did have a slightly lower (16% vs 18%) risk of worsening of AF over 2 years.36 Overall, AF patients with OSA had more symptoms and higher admission rates, but no difference in overall mortality or MACE. Observational studies have suggested that PAP treatment of OSA facilitates maintenance of normal sinus rhythm after cardioversion and after ablation.37
CHF: Results look promising
In one small study, 24 patients with heart failure with reduced ejection fraction who were optimally medically treated were randomized to receive PAP or sham PAP for 1 month.38 The treatment group demonstrated reduced systolic BP, reduced end systolic dimension, and significant improvement in ejection fraction from 25 ± 2.8% to 33.8 ± 2.4%.
OSA Tx improves insulin sensitivity
OSA is associated with impaired glucose tolerance, and PAP treatment of OSA has been documented to improve insulin sensitivity.39,40 An efficacy study utilizing PAP in a laboratory setting for 8 hours/night demonstrated significant reduction in fasting blood sugar and a reduction in the dawn phenomenon (an increase in early morning fasting glucose as a result of rebound from hypoglycemia during sleep).39 A 2015 meta-analysis of short-term studies also showed improvement in insulin sensitivity in OSA patients treated with PAP, but failed to find any reduction in A1C or in body mass index.40
All-cause mortality: Difference in findings between short- and long-term studies
Yu et al’s34 meta-analysis of 10 RCTs involving 7266 participants found no difference in mortality in treated (vs no treatment or sham treatment) OSA patients. This was true even in the more adherent subgroup. These studies were relatively short-term, with the longest mean follow-up being 68 months.
However, several longer-term population-based studies have suggested that OSA treatment improves all-cause mortality. An 18-year follow-up of a Wisconsin cohort documented dramatically increased mortality in patients with severe sleep apnea; mortality was even higher when patients treated with PAP were removed from the analysis, suggesting that PAP treatment was protective, mainly for cardiovascular death.5
Continue to: A Danish registry...
A Danish registry documented that patients treated with CPAP had higher rates of comorbidities before and during treatment; when these comorbidities were controlled, men ages ≥ 60 years had improved survival when treated with CPAP. There was no survival benefit in women.41
A recent analysis—the Sleep Heart Health Study—followed patients with obesity and severe OSA for a mean of 11.1 years and calculated a hazard ratio for all-cause mortality associated with prescribed PAP therapy of 0.58 (95% confidence interval [CI], 0.35-0.96) after propensity matching.42 The difference in mortality appeared 6 to 7 years after PAP therapy was prescribed. This delay may explain the failure of shorter-term studies to demonstrate evidence of benefit.
OSA Tx reduces motor vehicle crashes
Drowsy driving is widely accepted as a risk for motor vehicle crashes. Successful treatment of OSA with PAP has been shown to improve driving performance on a driving simulator.43 An analysis of 15 studies similarly demonstrated a significant reduction in driving accidents (incident rate ratio [IRR] = 0.45) and in near-miss accidents (IRR = 0.23) in patients with OSA treated with CPAP.44
Pulmonary hypertension: OSA Tx lowers pulmonary arterial pressure
Patients with OSA have higher than expected rates of pulmonary arterial hypertension—as high as 22%—documented by pulmonary artery catheterization findings.45 A meta-analysis of studies that examined the effect of PAP in patients with OSA and coexisting pulmonary hypertension but without other overt pulmonary or cardiac disease found significant reductions in pulmonary artery pressure.46 Whether this finding translates into improved patient-oriented outcomes is unknown.
OSA and pregnancy outcomes
A national cohort study demonstrated that OSA is an independent risk factor for multiple adverse pregnancy outcomes, including gestational diabetes, hypertensive disorders in pregnancy, intrauterine growth retardation, and stillbirth.7 OSA was also associated with the rare serious adverse outcomes of congestive heart failure, cardiomyopathy, and pulmonary embolism.7 There is little evidence to date with which to determine whether treatment of OSA improves outcomes, but PAP treatment is documented to be safe in pregnant women.8
CORRESPONDENCE
Stephen C. Sorsby, MD, MHA, University of Arkansas for Medical Sciences, 4301 West Markham, Slot 530, Little Rock, AR 72205; [email protected].
1. Peppard PE, Young T, Palta M, et al. Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med. 2000;342:1378-1384.
2. Marin JM, Carrizo SJ, Vicente E, et al. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet. 2005;365:1046-1053.
3. Iftikhar IH, Hoyos CM, Phillips CL, et al. Meta-analyses of the association of sleep apnea with insulin resistance, and the effects of CPAP on HOMA-IR, adiponectin, and visceral adipose fat. J Clin Sleep Med. 2015;11:475-485.
4. Aloia MS, Arnedt JT, Davis JD, et al. Neuropsychological sequelae of obstructive sleep apnea-hypopnea syndrome: a critical review. J Int Neuropsychol Soc. 2004;10:772-785.
5. Young T, Finn L, Peppard PE, et al. Sleep disordered breathing and mortality: eighteen-year follow-up of the Wisconsin sleep cohort. Sleep. 2008;31:1071-1078.
6. Jonas DE, Amick HR, Feltner C, et al. Screening for obstructive sleep apnea in adults: evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2017;317:415-433.
7. Bourjeily G, Danilack VA, Bublitz MA, et al. Obstructive sleep apnea in pregnancy is associated with adverse maternal outcomes: a national cohort. Sleep Med. 2017;35:50-57.
8. Booth JM, Tonidandel AM. Peripartum management of obstructive sleep apnea. Clin Obstet Gyn. 2017;60:405-417.
9. Strohl KP, Brown DB, Collop N, et al. An official American Thoracic Society Clinical Practice Guideline: sleep apnea, sleepiness, and driving risk in noncommercial drivers. An update of a 1994 Statement. Am J Respir Crit Care Med. 2013;187:1259-1266.
10. Epstein LJ, Kristo D, Strollo PJ Jr, et al. Clinical guideline for the evaluation, management and long-term care of obstructive sleep apnea in adults. J Clin Sleep Med. 2009;5:263-276.
11. National Institute for Health and Care Excellence. Continuous positive airway pressure for the treatment of obstructive sleep apnoea/hypopnoea syndrome. Technology appraisal guidance [TA139]. https://www.nice.org.uk/guidance/ta139. Revised February 2012. Accessed October 28, 2019.
12. Qaseem A, Holty JE, Owens DK, et al. Management of obstructive sleep apnea in adults: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2013;159:471-483.
13. Netzer NC, Ancoli-Israel S, Bliwise DL, et al. Principles of practice parameters for the treatment of sleep disordered breathing in the elderly and frail elderly: the consensus of the International Geriatric Sleep Medicine Task Force. Eur Respir J. 2016;48:992-1018.
14. Phillips CL, Grunstein RR, Darendeliler MA, et al. Health outcomes of continuous positive airway pressure versus oral appliance treatment for obstructive sleep apnea: a randomized controlled trial. Am J Respir Crit Care Med. 2013;187:879-887.
15. Bratton DJ, Gaisl T, Schlatzer C, et al. Comparison of the effects of continuous positive airway pressure and mandibular advancement devices on sleepiness in patients with obstructive sleep apnoea: a network meta-analysis. Lancet Respir Med. 2015;3:869-878.
16. Ng SSS, Chan RSM, Woo J, et al. A randomized controlled study to examine the effect of a lifestyle modification program in OSA. Chest. 2015;148:1193-1203.
17. Sundaram S, Bridgman SA, Lim J, et al. Surgery for obstructive sleep apnoea. Cochrane Database Syst Rev. 2005;4:CD001004.
18. Certal VF, Zaghi S, Riaz M, et al. Hypoglossal nerve stimulation in the treatment of obstructive sleep apnea: a systematic review and meta-analysis. Laryngoscope. 2015; 125:1254-1264.
19. Lewis EF, Rui W, Punjabi N, et al. Impact of continuous positive airway pressure and oxygen on health status in patients with coronary heart disease, cardiovascular risk factors, and obstructive sleep apnea: A Heart Biomarker Evaluation in Apnea Treatment (HEARTBEAT) analysis. Am Heart J. 2017;189:59-67.
20. Martinez-Garcia MA, Chiner E, Hernandez L, et al. Obstructive sleep apnoea in the elderly: role of continuous positive airway pressure treatment. Eur Respir J. 2015;46:142-151.
21. McMillan A, Bratton DJ, Faria R, et al. Continuous positive airway pressure in older people with obstructive sleep apnoea syndrome (PREDICT): a 12-month, multicentre, randomised trial. Lancet Respir Med. 2014;2:804-812.
22. Crawford-Achour E, Dauphinot V, Martin MS, et al. Protective effect of long-term CPAP therapy on cognitive performance in elderly patients with severe OSA: the PROOF study. J Clin Sleep Med. 2015;11:519-524.
23. Ancoli-Israel S, Palmer BW, Cooke JR, et al. Cognitive effects of treating obstructive sleep apnea in Alzheimer’s disease: a randomized controlled study. J Am Geriatr Soc. 2008;56:2076-2081.
24. Troussière AC, Charley CM, Salleron J, et al. Treatment of sleep apnoea syndrome decreases cognitive decline in patients with Alzheimer’s disease. J Neurol Neurosurg Psychiatry. 2014;85:1405-1408.
25. Haentjens P, Van Meerhaeghe A, Moscariello A, et al. The impact of continuous positive airway pressure on blood pressure in patients with obstructive sleep apnea syndrome: evidence from a meta-analysis of placebo-controlled randomized trials. Arch Intern Med. 2007;167:757-764.
26. Montesi SB, Edwards BA, Malhotra A, et al. The effect of continuous positive airway pressure treatment on blood pressure: a systematic review and meta-analysis of randomized controlled trials. J Clin Sleep Med. 2012;8:587-596.
27. Barbé F, Durán-Cantolla J, Capote F, et al. Long-term effect of continuous positive airway pressure in hypertensive patients with sleep apnea. Am J Respir Crit Care Med. 2010;181:718-726.
28. Bratton DJ, Gaisl T, Wons AM, et al. CPAP vs mandibular advancement devices and blood pressure in patients with obstructive sleep apnea: a systematic review and meta-analysis. JAMA. 2015;314:2280-2293.
29. McEvoy RD, Antic NA, Heeley E, et al. CPAP for prevention of cardiovascular events in obstructive sleep apnea. N Engl J Med. 2016;375:919-931.
30. Barbé F, Durán-Cantolla J, Sánchez-de-la-Torre M, et al. Effect of continuous positive airway pressure on the incidence of hypertension and cardiovascular events in nonsleepy patients with obstructive sleep apnea: a randomized controlled trial. JAMA. 2012;307:2161-2168.
31. Peker Y, Glantz H, Eulenburg C, et al. Effect of positive airway pressure on cardiovascular outcomes in coronary artery disease patients with nonsleepy obstructive sleep apnea. The RICCADSA Randomized Controlled Trial. Am J Respir Crit Care Med. 2016;194:613-620.
32. Parra O, Sánchez-Armengol Á, Capote F, et al. Efficacy of continuous positive airway pressure treatment on 5-year survival in patients with ischaemic stroke and obstructive sleep apnea: a randomized controlled trial. J Sleep Res. 2015;24:47-53.
33. Abuzaid AS, Al Ashray HS, Elbadaway A, et al. Meta-analysis of cardiovascular outcomes with continuous positive airway pressure in patients with obstructive sleep apnea. Am J Card. 2017;120:693-699.
34. Yu J, Zhou Z, McEvoy D, et al. Association of positive airway pressure with cardiovascular events and death in adults with sleep apnea: a systematic review and meta-analysis. JAMA. 2017;318:156-166.
35. Gami AS, Hodge DO, Herges RM, et al. Obstructive sleep apnea, obesity, and the incident risk of atrial fibrillation. J Amer Coll of Card. 2007;49:565-571.
36. Holmqvist F, Guan N, Zhu Z, et al. Impact of obstructive sleep apnea and continuous positive airway pressure therapy on outcomes in patients with atrial fibrillation—results from the Outcomes Registry for Better Informed Treatment of Atrial Fibrillation (ORBIT-AF). Am Heart J. 2015;169:647-654.e2.
37. Nalliah CJ, Sanders P, Kalman JM. Obstructive sleep apnea treatment and atrial fibrillation: a need for definitive evidence. J Cardiovasc Electrophysiol. 2016;27:1001-1010.
38. Kaneko Y, Floras JS, Usui K, et al. Cardiovascular effects of continuous positive airway pressure in patients with heart failure and obstructive sleep apnea. N Engl J Med. 2003;348:1233-1241
39. Pamidi S, Wroblewski K, Stepien M, et al. Eight hours of nightly continuous positive airway pressure treatment of obstructive sleep apnea improves glucose metabolism in patients with prediabetes: a randomized controlled trial. Am J Respir Crit Care Med. 2015;192:96-105.
40. Feng Y, Zhang Z, Dong ZZ. Effects of continuous positive airway pressure therapy on glycaemic control, insulin sensitivity and body mass index in patients with obstructive sleep apnoea and type 2 diabetes: a systematic review and meta-analysis. NPJ Prim Care Respir Med. 2015;25:15005.
41. Jennum P, Tonnesen P, Ibsen R, et al. Obstructive sleep apnea: effect of comorbidities and positive airway pressure on all-cause mortality. Sleep Med. 2017;36:62-66.
42. Lisan Q, Van Sloten T, Marques Vidal P, et al. Association of positive airway pressure prescription with mortality in patients with obesity and severe obstructive sleep apnea: the sleep heart health study. JAMA Otolaryngol Head Neck Surg. 2019;145:509-515.
43. Mazza S, Pépin JL, Naëgelé B, et al. Driving ability in sleep apnoea patients before and after CPAP treatment: evaluation on a road safety platform. Eur Respir J. 2006;28:1020-1028.
44. Antonopoulos CN, Sergentanis TN, Daskalopoulou SS, et al. Nasal continuous positive airway pressure (nCPAP) treatment for obstructive sleep apnea, road traffic accidents and driving simulator performance: a meta-analysis. Sleep Med Rev. 2011;15:301-310.
45. Minai OA, Ricaurte B, Kaw R, et al. Frequency and impact of pulmonary hypertension in patients with obstructive sleep apnea syndrome. Am J Cardiol. 2009;104:1300-1306.
46. Imran TF, Ghazipura M, Liu S, et al. Effect of continuous positive airway pressure treatment on pulmonary artery pressure in patients with isolated obstructive sleep apnea: a meta-analysis. Heart Fail Rev. 2016;21:591-598.
1. Peppard PE, Young T, Palta M, et al. Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med. 2000;342:1378-1384.
2. Marin JM, Carrizo SJ, Vicente E, et al. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet. 2005;365:1046-1053.
3. Iftikhar IH, Hoyos CM, Phillips CL, et al. Meta-analyses of the association of sleep apnea with insulin resistance, and the effects of CPAP on HOMA-IR, adiponectin, and visceral adipose fat. J Clin Sleep Med. 2015;11:475-485.
4. Aloia MS, Arnedt JT, Davis JD, et al. Neuropsychological sequelae of obstructive sleep apnea-hypopnea syndrome: a critical review. J Int Neuropsychol Soc. 2004;10:772-785.
5. Young T, Finn L, Peppard PE, et al. Sleep disordered breathing and mortality: eighteen-year follow-up of the Wisconsin sleep cohort. Sleep. 2008;31:1071-1078.
6. Jonas DE, Amick HR, Feltner C, et al. Screening for obstructive sleep apnea in adults: evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2017;317:415-433.
7. Bourjeily G, Danilack VA, Bublitz MA, et al. Obstructive sleep apnea in pregnancy is associated with adverse maternal outcomes: a national cohort. Sleep Med. 2017;35:50-57.
8. Booth JM, Tonidandel AM. Peripartum management of obstructive sleep apnea. Clin Obstet Gyn. 2017;60:405-417.
9. Strohl KP, Brown DB, Collop N, et al. An official American Thoracic Society Clinical Practice Guideline: sleep apnea, sleepiness, and driving risk in noncommercial drivers. An update of a 1994 Statement. Am J Respir Crit Care Med. 2013;187:1259-1266.
10. Epstein LJ, Kristo D, Strollo PJ Jr, et al. Clinical guideline for the evaluation, management and long-term care of obstructive sleep apnea in adults. J Clin Sleep Med. 2009;5:263-276.
11. National Institute for Health and Care Excellence. Continuous positive airway pressure for the treatment of obstructive sleep apnoea/hypopnoea syndrome. Technology appraisal guidance [TA139]. https://www.nice.org.uk/guidance/ta139. Revised February 2012. Accessed October 28, 2019.
12. Qaseem A, Holty JE, Owens DK, et al. Management of obstructive sleep apnea in adults: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2013;159:471-483.
13. Netzer NC, Ancoli-Israel S, Bliwise DL, et al. Principles of practice parameters for the treatment of sleep disordered breathing in the elderly and frail elderly: the consensus of the International Geriatric Sleep Medicine Task Force. Eur Respir J. 2016;48:992-1018.
14. Phillips CL, Grunstein RR, Darendeliler MA, et al. Health outcomes of continuous positive airway pressure versus oral appliance treatment for obstructive sleep apnea: a randomized controlled trial. Am J Respir Crit Care Med. 2013;187:879-887.
15. Bratton DJ, Gaisl T, Schlatzer C, et al. Comparison of the effects of continuous positive airway pressure and mandibular advancement devices on sleepiness in patients with obstructive sleep apnoea: a network meta-analysis. Lancet Respir Med. 2015;3:869-878.
16. Ng SSS, Chan RSM, Woo J, et al. A randomized controlled study to examine the effect of a lifestyle modification program in OSA. Chest. 2015;148:1193-1203.
17. Sundaram S, Bridgman SA, Lim J, et al. Surgery for obstructive sleep apnoea. Cochrane Database Syst Rev. 2005;4:CD001004.
18. Certal VF, Zaghi S, Riaz M, et al. Hypoglossal nerve stimulation in the treatment of obstructive sleep apnea: a systematic review and meta-analysis. Laryngoscope. 2015; 125:1254-1264.
19. Lewis EF, Rui W, Punjabi N, et al. Impact of continuous positive airway pressure and oxygen on health status in patients with coronary heart disease, cardiovascular risk factors, and obstructive sleep apnea: A Heart Biomarker Evaluation in Apnea Treatment (HEARTBEAT) analysis. Am Heart J. 2017;189:59-67.
20. Martinez-Garcia MA, Chiner E, Hernandez L, et al. Obstructive sleep apnoea in the elderly: role of continuous positive airway pressure treatment. Eur Respir J. 2015;46:142-151.
21. McMillan A, Bratton DJ, Faria R, et al. Continuous positive airway pressure in older people with obstructive sleep apnoea syndrome (PREDICT): a 12-month, multicentre, randomised trial. Lancet Respir Med. 2014;2:804-812.
22. Crawford-Achour E, Dauphinot V, Martin MS, et al. Protective effect of long-term CPAP therapy on cognitive performance in elderly patients with severe OSA: the PROOF study. J Clin Sleep Med. 2015;11:519-524.
23. Ancoli-Israel S, Palmer BW, Cooke JR, et al. Cognitive effects of treating obstructive sleep apnea in Alzheimer’s disease: a randomized controlled study. J Am Geriatr Soc. 2008;56:2076-2081.
24. Troussière AC, Charley CM, Salleron J, et al. Treatment of sleep apnoea syndrome decreases cognitive decline in patients with Alzheimer’s disease. J Neurol Neurosurg Psychiatry. 2014;85:1405-1408.
25. Haentjens P, Van Meerhaeghe A, Moscariello A, et al. The impact of continuous positive airway pressure on blood pressure in patients with obstructive sleep apnea syndrome: evidence from a meta-analysis of placebo-controlled randomized trials. Arch Intern Med. 2007;167:757-764.
26. Montesi SB, Edwards BA, Malhotra A, et al. The effect of continuous positive airway pressure treatment on blood pressure: a systematic review and meta-analysis of randomized controlled trials. J Clin Sleep Med. 2012;8:587-596.
27. Barbé F, Durán-Cantolla J, Capote F, et al. Long-term effect of continuous positive airway pressure in hypertensive patients with sleep apnea. Am J Respir Crit Care Med. 2010;181:718-726.
28. Bratton DJ, Gaisl T, Wons AM, et al. CPAP vs mandibular advancement devices and blood pressure in patients with obstructive sleep apnea: a systematic review and meta-analysis. JAMA. 2015;314:2280-2293.
29. McEvoy RD, Antic NA, Heeley E, et al. CPAP for prevention of cardiovascular events in obstructive sleep apnea. N Engl J Med. 2016;375:919-931.
30. Barbé F, Durán-Cantolla J, Sánchez-de-la-Torre M, et al. Effect of continuous positive airway pressure on the incidence of hypertension and cardiovascular events in nonsleepy patients with obstructive sleep apnea: a randomized controlled trial. JAMA. 2012;307:2161-2168.
31. Peker Y, Glantz H, Eulenburg C, et al. Effect of positive airway pressure on cardiovascular outcomes in coronary artery disease patients with nonsleepy obstructive sleep apnea. The RICCADSA Randomized Controlled Trial. Am J Respir Crit Care Med. 2016;194:613-620.
32. Parra O, Sánchez-Armengol Á, Capote F, et al. Efficacy of continuous positive airway pressure treatment on 5-year survival in patients with ischaemic stroke and obstructive sleep apnea: a randomized controlled trial. J Sleep Res. 2015;24:47-53.
33. Abuzaid AS, Al Ashray HS, Elbadaway A, et al. Meta-analysis of cardiovascular outcomes with continuous positive airway pressure in patients with obstructive sleep apnea. Am J Card. 2017;120:693-699.
34. Yu J, Zhou Z, McEvoy D, et al. Association of positive airway pressure with cardiovascular events and death in adults with sleep apnea: a systematic review and meta-analysis. JAMA. 2017;318:156-166.
35. Gami AS, Hodge DO, Herges RM, et al. Obstructive sleep apnea, obesity, and the incident risk of atrial fibrillation. J Amer Coll of Card. 2007;49:565-571.
36. Holmqvist F, Guan N, Zhu Z, et al. Impact of obstructive sleep apnea and continuous positive airway pressure therapy on outcomes in patients with atrial fibrillation—results from the Outcomes Registry for Better Informed Treatment of Atrial Fibrillation (ORBIT-AF). Am Heart J. 2015;169:647-654.e2.
37. Nalliah CJ, Sanders P, Kalman JM. Obstructive sleep apnea treatment and atrial fibrillation: a need for definitive evidence. J Cardiovasc Electrophysiol. 2016;27:1001-1010.
38. Kaneko Y, Floras JS, Usui K, et al. Cardiovascular effects of continuous positive airway pressure in patients with heart failure and obstructive sleep apnea. N Engl J Med. 2003;348:1233-1241
39. Pamidi S, Wroblewski K, Stepien M, et al. Eight hours of nightly continuous positive airway pressure treatment of obstructive sleep apnea improves glucose metabolism in patients with prediabetes: a randomized controlled trial. Am J Respir Crit Care Med. 2015;192:96-105.
40. Feng Y, Zhang Z, Dong ZZ. Effects of continuous positive airway pressure therapy on glycaemic control, insulin sensitivity and body mass index in patients with obstructive sleep apnoea and type 2 diabetes: a systematic review and meta-analysis. NPJ Prim Care Respir Med. 2015;25:15005.
41. Jennum P, Tonnesen P, Ibsen R, et al. Obstructive sleep apnea: effect of comorbidities and positive airway pressure on all-cause mortality. Sleep Med. 2017;36:62-66.
42. Lisan Q, Van Sloten T, Marques Vidal P, et al. Association of positive airway pressure prescription with mortality in patients with obesity and severe obstructive sleep apnea: the sleep heart health study. JAMA Otolaryngol Head Neck Surg. 2019;145:509-515.
43. Mazza S, Pépin JL, Naëgelé B, et al. Driving ability in sleep apnoea patients before and after CPAP treatment: evaluation on a road safety platform. Eur Respir J. 2006;28:1020-1028.
44. Antonopoulos CN, Sergentanis TN, Daskalopoulou SS, et al. Nasal continuous positive airway pressure (nCPAP) treatment for obstructive sleep apnea, road traffic accidents and driving simulator performance: a meta-analysis. Sleep Med Rev. 2011;15:301-310.
45. Minai OA, Ricaurte B, Kaw R, et al. Frequency and impact of pulmonary hypertension in patients with obstructive sleep apnea syndrome. Am J Cardiol. 2009;104:1300-1306.
46. Imran TF, Ghazipura M, Liu S, et al. Effect of continuous positive airway pressure treatment on pulmonary artery pressure in patients with isolated obstructive sleep apnea: a meta-analysis. Heart Fail Rev. 2016;21:591-598.
PRACTICE RECOMMENDATIONS
› Treat patients with symptomatic obstructive sleep apnea (OSA) with positive airway pressure (PAP) or oral appliances to reduce daytime sleepiness, improve quality-of-life scores, and modestly reduce blood pressure in patients with hypertension. A
› Consider recommending at least 4 hours of PAP every night for asymptomatic patients (those without daytime sleepiness) with severe OSA and other conditions, including resistant hypertension, atrial fibrillation, congestive heart failure, cognitive impairment, obesity, and stroke. B
› Do not screen asymptomatic patients for OSA. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Beyond depression: Other uses for tricyclic antidepressants
Most tricyclic antidepressants (TCAs) have US Food and Drug Administration approval for treatment of depression and anxiety disorders, but they are also a viable off-label option that should be considered by clinicians in specialties beyond psychiatry, especially for treating pain syndromes. Given the ongoing epidemic of opioid use disorder, increasing attention has been drawn to alternative strategies for chronic pain management, renewing an interest in the use of TCAs.
This review summarizes the pharmacologic properties of TCAs, their potential indications in conditions other than depression, and safety considerations.
BRIEF HISTORY OF TRICYCLICS
TCAs were originally designed in the 1950s and marketed later for treating depression. Due to their adverse effects and lethality in overdose quantities, over time they have been largely replaced by selective serotonin reuptake inhibitors (SSRIs) and serotonin-norepinephrine reuptake inhibitors (SNRIs) in depression management. However, TCAs have been applied to conditions other than depression with varying degrees of efficacy and safety.
TCA PHARMACOLOGY
TCAs are absorbed in the small intestine and undergo first-pass metabolism in the liver. They bind extensively to proteins, leading to interactions with other protein-bound drugs. They are widely distributed throughout the systemic circulation because they are highly lipophilic, resulting in systemic effects including central nervous system manifestations.
Peak plasma concentration is at about 2 to 6 hours, and elimination half-life is around 24 hours for most agents, providing a long duration of action. Clearance depends on cytochrome P450 oxidative enzymes.1
MECHANISMS OF ACTION
TCAs inhibit reuptake of norepinephrine and serotonin, resulting in accumulation of these neurotransmitters in the presynaptic cleft. They also block postsynaptic histamine, alpha-adrenergic, and muscarinic-acetylcholine receptors, causing a variety of adverse effects, including dry mouth, confusion, cognitive impairment, hypotension, orthostasis, blurred vision, urinary retention, drowsiness, and sedation.1
Research suggests that TCAs relieve pain centrally through a descending pathway that inhibits transmission of pain signals in the spinal cord, as well as peripherally through complex anti-neuroimmune actions.2 Norepinephrine appears to play a more important role in this process than serotonin, although both are deemed necessary for the “dual action” often cited in pain management,1 which is also the rationale for widespread use of SNRIs to control pain.
Table 1 compares neurotransmitter reuptake mechanisms, adverse effect profiles, and typical dosages for depression for commonly prescribed TCAs.
POTENTIAL USES
Headache and migraine
TCAs have been shown to be effective for managing and preventing chronic headache syndromes.3,4 Amitriptyline has been the most studied of the TCAs for both chronic daily and episodic migraine headache, showing the most efficacy among diverse drug classes (angiotensin II receptor blockers, anticonvulsants, beta-blockers, SSRIs) compared with placebo. However, in head-to-head trials, amitriptyline was no more effective than SSRIs, venlafaxine, topiramate, or propranolol.4 Jackson et al4 suggested that prophylactic medication choices should be tailored to patient characteristics and expected adverse effects, and specifically recommended that TCAs—particularly amitriptyline—be reserved for patients who have both migraine and depression.
Neuropathic pain
Neuropathic pain is defined as pain secondary to a lesion or disease of the somatosensory nervous system5 and is the pathomechanistic component of a number of conditions, including postherpetic neuralgia,6 diabetic and nondiabetic painful polyneuropathy,7 posttraumatic or postsurgical neuropathic pain8 (including plexus avulsion and complex regional pain syndrome9), central poststroke pain,10 spinal cord injury pain,11 and multiple sclerosis-associated pain.12
As a group, TCAs appear to have a role as first-line agents for managing these varied neuropathic pain syndromes. In a recent meta-analysis,13 16 (89%) of 18 placebo-controlled trials of TCAs (mainly amitriptyline at 25–150 mg/day) for these pain conditions were positive, with a combined number needed to treat of 3.6, suggesting a role for TCAs in these conditions. Of note, the TCAs desipramine14 and nortriptyline15 have demonstrated little evidence of efficacy in neuropathic pain syndromes.
Chronic low back pain
Chronic low back pain is a leading cause of loss of work, excessive healthcare expenditure, and disability in the United States. It can be due to numerous spinal conditions, including degenerative disk disease, spinal stenosis, lumbar spondylosis, and spinal arthropathy.
TCAs have been used to treat chronic low back pain for decades and have been repeatedly shown to be more effective than placebo in reducing pain severity.16,17 A double-blind controlled trial18 from 1999 compared the effects of the TCA maprotiline (up to 150 mg daily), the SSRI paroxetine (up to 30 mg daily), and placebo and found a statistically significant reduction in back pain with maprotiline compared with paroxetine and placebo. However, a 2008 meta-analysis suggested little evidence that TCAs were superior to placebo.19
Evidence of TCA efficacy for back pain was reported in 2018 with a well-designed 6-month double-blind randomized controlled trial20 comparing low-dose amitriptyline (25 mg) with an active comparator (benztropine 1 mg). The authors reported that amitriptyline was effective in reducing pain and pain-related disability without incurring serious adverse effects. They suggested continued use of TCAs for chronic low back pain if complicated with pain-related disability, insomnia, depression, or other comorbidity, although they called for further large-scale studies. They also cautioned that patients started the trial with symptoms similar to the adverse effects of TCAs themselves; this has implications for monitoring of symptoms as well as TCA adverse effects while using these drugs.
Fibromyalgia and chronic widespread pain
Fibromyalgia is a common, frustrating, noninflammatory pain syndrome characterized by diffuse hyperalgesia and multiple comorbidities.21 Although sleep hygiene, exercise, cognitive-behavioral therapy, some gabapentinoids (pregabalin), and a combination of these therapies have demonstrated efficacy, TCAs also offer robust benefits.
A meta-analysis of 9 placebo-controlled TCA trials showed large effect sizes for pain reduction, fatigue reduction, improved sleep quality, and reduced stiffness and tenderness, with the most significant of these improvements being for sleep.22 A separate meta-analysis calculated that the number needed to treat with amitriptyline for a positive outcome is 4.9.23 Recent systematic reviews have supported these findings, listing TCAs as second-line agents after pregabalin, duloxetine, and milnacipran.24
Of note, TCA monotherapy rarely produces a complete response in patients with moderate to severe fibromyalgia, chronic widespread pain, or significant comorbidities (depression, anxiety). Supplementation with cognitive-behavioral therapy, physical therapy, functional restoration, and other modalities is strongly recommended.
Abdominal and gastrointestinal pain
TCAs have been applied to a number of gastrointestinal syndromes with or without pain. Patients with irritable bowel syndrome have long been known to benefit from TCAs; the number needed to treat for symptomatic benefit over placebo is 3.5.25,26
Although there is no substantial evidence that TCAs are useful in reducing active inflammation in inflammatory bowel disease, a study involving 81 patients found that residual noninflammatory gastrointestinal symptoms (such as diarrhea and pain) responded to TCAs, including nortriptyline and amitriptyline, with greater benefit for ulcerative colitis than for Crohn disease.27
TCAs have also shown prophylactic benefit in cyclic vomiting syndrome, with a clinical response in over 75% of patients in controlled cohort studies.28
The efficacy of TCAs in other abdominal or gastrointestinal syndromes is unclear or modest at best.29 However, few alternative treatments exist for these conditions. Amitriptyline may help symptoms of functional dyspepsia,30 but nortriptyline has proven ineffective in gastroparesis.31 Nonetheless, some authors29 suggest considering TCAs on an individualized basis, with proper monitoring, in many if not most functional gastrointestinal disorders, especially when paired with behavioral therapies.
Pelvic and urogynecologic symptoms
Chronic pelvic pain affects up to 24% of women32 and 5% to 10% of men.33 TCAs have shown efficacy in treating chronic pelvic pain with or without comorbid depression.34 Amitriptyline and to a lesser extent nortriptyline are the TCAs most often prescribed. Pain relief appears to be independent of antidepressant effects and may be achieved at low doses; initial dosing ranges from 10 to 25 mg at bedtime, which may be increased to 100 mg as tolerated.34
Based on a randomized, double-blind trial,35 amitriptyline was recommended as a treatment option for interstitial cystitis or bladder pain, with the greatest symptom improvement in patients tolerating a daily dose of 50 mg.
Another study36 randomized 56 women with chronic pelvic pain to amitriptyline or gabapentin, or a combination of the drugs for 24 months. Although each regimen resulted in significant reduction in pain, fewer adverse effects occurred with gabapentin than amitriptyline. Poor compliance and early discontinuation of amitriptyline were common due to anticholinergic effects.
In small uncontrolled studies,37 about half of women with chronic pelvic pain became pain-free after 8 weeks of treatment with nortriptyline and imipramine.
Randomized controlled studies are needed to confirm potential benefits of TCAs in chronic urologic and pelvic pain.
Insomnia
Insomnia affects 23% to 56% of people in the United States, Europe, and Asia38 and is the reason for more than 5.5 million primary care visits annually.39 TCAs (especially doxepin, maprotiline, and amitriptyline40) have been shown to be an effective treatment, with an 82% increase in somnolence compared with placebo, as well as measurably improved total sleep time, enhanced sleep efficiency, reduced latency to persistent sleep, and decreased wake times after sleep onset.38
Dosing should be kept at a minimum to minimize harsh anticholinergic effects and avoid daytime sedation. Patients should be advised to take new doses or dose escalations earlier in the night to ensure less hangover sedation the next morning.
For patients with insomnia and comorbid depression, the American Academy of Sleep Medicine suggests the addition of a low dose (eg, 10–25 mg) of a TCA at nighttime to complement preexisting, full-dose, non-TCA antidepressants, while monitoring for serotonin syndrome and other potential but exceedingly rare drug-drug interactions.41
Psychiatric indications other than depression
Beyond the known benefits in major depressive disorder, TCAs have been shown to be effective for obsessive-compulsive disorder, panic disorder, posttraumatic stress disorder, bulimia nervosa, and childhood enuresis.42 Given the shortage of mental health clinicians and the high prevalence of these conditions, nonpsychiatrist physicians should be familiar with the therapeutic potential of TCAs for these indications.
ADVERSE EFFECTS
Adverse effects vary among TCAs. Common ones include blurred vision, dry mouth, constipation, urinary retention, hypotension, tachycardia, tremor, weight gain, and sexual dysfunction.43 Tertiary amines are generally more sedating than secondary amines and cause more anticholinergic effects (Table 1).
Despite widespread perceptions that TCAs are less tolerable than newer antidepressants, studies repeatedly suggest that they have an adverse-effect burden similar to that of SSRIs and SNRIs, although SSRIs have a greater tendency to produce nausea, whereas TCAs are more likely to cause constipation.44
Discontinuation syndrome
Abrupt discontinuation or unintentionally missed doses of TCAs have been associated with a discontinuation syndrome in about 40% of users.45 Patients should be warned about this possibility and the syndrome’s potential effects: dizziness, insomnia, headaches, nausea, vomiting, flulike achiness, and restlessness. Rebound depression, anxiety, panic, or other psychiatric symptoms may also occur. Symptoms generally present within 2 to 5 days after dose discontinuation and last 7 to 14 days.45
However, all TCAs have a long half-life, allowing for sufficient coverage with once-daily dosing and thus carry a lower risk of discontinuation syndrome than many other antidepressants (78% with venlafaxine; 55% with paroxetine).45
To discontinue therapy safely, the dosage should be reduced gradually. As is pharmacologically expected, the greatest likelihood of discontinuation syndrome is associated with longer duration of continuous treatment.
CONTRAINDICATIONS
Cardiac conduction abnormalities
TCAs should not be prescribed to patients who have right bundle branch block, a severe electrolyte disturbance, or other cardiac conduction deficit or arrhythmia that can prolong the QTc interval and elevate the risk of lethal arrhythmia.46,47 Cardiac effects from TCAs are largely dose-dependent. Nevertheless, a baseline electrocardiogram can be obtained to assess cardiac risk, and dose escalation can proceed if results are normal (eg, appropriate conduction intervals, QTc ≤ 450 ms).
Advanced age
For elderly patients, TCAs should be prescribed with caution and sometimes not at all,48 because anticholinergic effects may worsen preexisting urinary retention (including benign prostatic hyperplasia), narrow-angle glaucoma, imbalance and gait issues, and cognitive impairment and dementia. Dehydration and orthostatic hypotension are contraindications for TCAs, as they may precipitate falls or hypotensive shock.
Epilepsy
TCAs should also be used with caution in patients with epilepsy, as they lower the seizure threshold.
Concomitant monoamine oxidase inhibitor treatment
Giving TCAs together with monoamine oxidase inhibitor antidepressants should be avoided, given the risk of hypertensive crisis.
Suicide risk
TCAs are dangerous and potentially lethal in overdose and so should not be prescribed to suicidal or otherwise impulsive patients.
Pregnancy
TCAs are in pregnancy risk category C (animal studies show adverse effects on fetus; no adequate or well-controlled studies in humans; potential benefits may warrant use despite risks). Using TCAs during pregnancy has very rarely led to neonatal withdrawal such as irritability, jitteriness, and convulsions, as well as fetal QTc interval prolongation.49
The American College of Obstetricians and Gynecologists recommends that therapy for depression during pregnancy be individualized, incorporating the expertise of the patient’s mental health clinician, obstetrician, primary healthcare provider, and pediatrician. In general, they recommend that TCAs should be avoided if possible and that alternatives such as SSRIs or SNRIs should be considered.50
TCAs are excreted in breast milk, but they have not been detected in the serum of nursing infants, and no adverse events have been reported.
OVERDOSE IS HIGHLY DANGEROUS
Severe morbidity and death are associated with TCA overdose, characterized by convulsions, cardiac arrest, and coma (the “3 Cs”). These dangers occur at much higher rates with TCAs than with other antidepressants.43 Signs and symptoms of toxicity develop rapidly, usually within the first hour of overdose. Manifestations of overdose include prolonged QTc, cardiac arrhythmias, tachycardia, hypertension, severe hypotension, agitation, seizures, central nervous system depression, hallucinations, seizures, and coma.
Overdose management includes activated charcoal, seizure control, cardioversion, hydration, electrolyte stabilization, and other intensive care.
OFF-LABEL TCA MANAGEMENT
Dosing recommendations for off-label use of TCAs vary based on the condition, the medication, and the suggestions of individual authors and researchers. In general, dosing ranges for pain and other nondepression indications may be lower than for severe depression (Table 2).1
As with any pharmacologic titration, monitoring for rate-limiting adverse effects is recommended. We suggest caution, tailoring the approach to the patient, and routinely assessing for adverse effects and other safety considerations.
In addition, we strongly recommend supplementing TCA therapy with nonpharmacologic strategies such as lifestyle changes, dietary modifications, exercise, physical therapy, and mental health optimization.
- Obata H. Analgesic mechanisms of antidepressants for neuropathic pain. Int J Mol Sci 2017; 18(11). doi:10.3390/ijms18112483
- Kremer M, Yalcin I, Goumon Y, et al. A dual noradrenergic mechanism for the relief of neuropathic allodynia by the antidepressant drugs duloxetine and amitriptyline. J Neurosci 2018; 38(46):9934–9954. doi:10.1523/JNEUROSCI.1004-18.2018
- Tomkins GE, Jackson JL, O’Malley PG, Balden E, Santoro JE. Treatment of chronic headache with antidepressants: a meta-analysis. Am J Med 2001; 111(1):54–63. doi:10.1016/s0002-9343(01)00762-8
- Jackson JL, Cogbill E, Santana-Davila R, et al. A comparative effectiveness meta-analysis of drugs for the prophylaxis of migraine headache. PLoS One 2015; 10(7):e0130733. doi:10.1371/journal.pone.0130733
- International Association for the Study of Pain (IASP). IASP Terminology. www.iasp-pain.org/Education/Content.aspx?ItemNumber=1698&navItemNumber=576. Accessed November 20, 2019.
- Feller L, Khammissa RAG, Fourie J, Bouckaert M, Lemmer J. Postherpectic neuralgia and trigeminal neuralgia. Pain Res Treat 2017; 2017:1681765. doi:10.1155/2017/1681765
- Shillo P, Sloan G, Greig M, et al. Painful and painless diabetic neuropathies: what is the difference? Curr Diab Rep 2019; 19(6):32. doi:10.1007/s11892-019-1150-5
- Schwartzman RJ, Maleki J. Postinjury neuropathic pain syndromes. Med Clin North Am 1999; 83(3):597–626. doi:10.1016/s0025-7125(05)70126-7
- Oaklander AL, Horowitz SH. The complex regional pain syndrome. Handb Clin Neurol 2015; 131:481–503. doi:10.1016/B978-0-444-62627-1.00026-3
- Akyuz G, Kuru P. Systematic review of central post stroke pain: what is happening in the central nervous system? Am J Phys Med Rehabil 2016; 95(8):618–627. doi:10.1097/PHM.0000000000000542
- Shiao R, Lee-Kubli CA. Neuropathic pain after spinal cord injury: challenges and research perspectives. Neurotherapeutics 2018; 15(3):635–653. doi:10.1007/s13311-018-0633-4
- Ceruti S. What role does multiple sclerosis play in the development of untreatable painful conditions? Pain Manag 2018; 8(1):37–44. doi:10.2217/pmt-2017-0038
- Finnerup NB, Attal N, Haroutounian S, et al. Pharmacotherapy for neuropathic pain in adults: a systematic review and meta-analysis. Lancet Neurol 2015; 14(2):162–173. doi:10.1016/S1474-4422(14)70251-0
- Hearn L, Moore RA, Derry S, Wiffen PJ, Phillips T. Desipramine for neuropathic pain in adults. Cochrane Database Syst Rev 2014; (9):CD011003. doi:10.1002/14651858.CD011003.pub2
- Derry S, Whiffen PJ, Aldington D, Moore RA. Nortriptyline for neuropathic pain in adults. Cochrane Database Syst Rev 2015; 1:CD011209. doi:10.1002/14651858.CD011209.pub2
- Salerno SM, Browning R, Jackson JL. The effect of antidepressant treatment on chronic back pain: a meta-analysis. Arch Intern Med 2002; 162(1):19–24. doi:10.1001/archinte.162.1.19
- Staiger TO, Gaster B, Sullivan MD, Deyo RA. Systematic review of antidepressants in the treatment of chronic low back pain. Spine (Phila Pa 1976) 2003; 28(22):2540–2545. doi:10.1097/01.BRS.0000092372.73527.BA
- Atkinson JH, Slater MA, Wahlgren DR, et al. Effects of noradrenergic and serotonergic antidepressants on chronic low back pain intensity. Pain 1999; 83(2):137–145. doi:10.1016/s0304-3959(99)00082-2
- Urquhart DM, Hoving JL, Assendelft WW, Roland M, van Tulder MW. Antidepressants for non-specific back pain. Cochrane Database Syst Rev 2008; (1):CD001703. doi:10.1002/14651858.CD001703.pub3
- Urquhart DM, Wluka AE, van Tulder M, et al. Efficacy of low-dose amitriptyline for chronic low back pain: a randomized clinical trial. JAMA Intern Med 2018; 178(11):1474–1481. doi:10.1001/jamainternmed.2018.4222
- Clauw DJ. Fibromyalgia: a clinical review. JAMA 2014; 311(15):1547–1555. doi:10.1001/jama.2014.3266
- Arnold LM, Keck PE Jr, Welge JA. Antidepressant treatment of fibromyalgia. A meta-analysis and review. Psychosomatics 2000; 41(2):104–113. pmid:10749947
- Hauser W, Wolfe F, Tolle T, Uceyler N, Sommer C. The role of antidepressants in the management of fibromyalgia syndrome: a systematic review and meta-analysis. CNS Drugs 2012; 26(4):297–307. doi:10.2165/11598970-000000000-00000
- Calandre EP, Rico-Villademoros F, Slim M. An update on pharmacotherapy for the treatment of fibromyalgia. Expert Opin Pharmacother 2015; 16(9):1347–1368. doi:10.1517/14656566.2015.1047343
- Jackson JL, O’Malley PG, Tomkins G, Balden E, Santoro J, Kroenke K. Treatment of functional gastrointestinal disorders with antidepressant medications: a meta-analysis. Am J Med 2000; 108(1):65–72. doi:10.1016/s0002-9343(99)00299-5
- Rahimi R, Nikfar S, Rezaie A, Abdollahi M. Efficacy of tricyclic antidepressants in irritable bowel syndrome: a meta-analysis. World J Gastroenterol 2009; 15(13):1548–1553. doi:10.3748/wjg.15.1548
- Iskandar HN, Cassell B, Kanuri N, et al. Tricyclic antidepressants for management of residual symptoms in inflammatory bowel disease. J Clin Gastroenterol 2014; 48(5):423–429. doi:10.1097/MCG.0000000000000049
- Lee LY, Abbott L, Mahlangu B, Moodie SJ, Anderson S. The management of cyclic vomiting syndrome: a systematic review. Eur J Gastroenterol Hepatol 2012; 24(9):1001–1006. doi:10.1097/MEG.0b013e328355638f
- Thorkelson G, Bielefeldt K, Szigethy E. Empirically supported use of psychiatric medications in adolescents and adults with IBD. Inflamm Bowel Dis 2016; 22(6):1509–1522. doi:10.1097/MIB.0000000000000734
- Braak B, Klooker TK, Wouters MM, et al. Randomised clinical trial: the effects of amitriptyline on drinking capacity and symptoms in patients with functional dyspepsia, a double-blind placebo-controlled study. Aliment Pharmacol Ther 2011; 34(6):638–648. doi:10.1111/j.1365-2036.2011.04775.x
- Parkman HP, Van Natta ML, Abell TL, et al. Effect of nortriptyline on symptoms of idiopathic gastroparesis: the NORIG randomized clinical trial. JAMA 2013; 310(24):2640–2649. doi:10.1001/jama.2013.282833
- Latthe P, Latthe M, Say L, Gulmezoglu M, Khan KS. WHO systematic review of prevalence of chronic pelvic pain: a neglected reproductive health morbidity. BMC Public Health 2006; 6:177. doi:10.1186/1471-2458-6-177
- Moise G, Capodice J, Winfree CJ. Treatment of chronic pelvic pain in men and women. Expert Rev Neurother 2007; 7(5):507–520. doi:10.1586/14737175.7.5.507
- Lai HH. Management of interstitial cystitis/bladder pain syndrome with tricyclic antidepressants. In: Moldwin RM, ed. Urological and Gynaecological Chronic Pelvic Pain. Cham, Switzerland: Springer; 2017:107–118.
- American Urological Association. Diagnosis and treatment interstitial cystitis/bladder pain syndrome (2014). www.auanet.org/guidelines/interstitial-cystitis/bladder-pain-syndrome-(2011-amended-2014). Accessed November 19, 2019.
- Carey ET, As-Sanie S. New developments in the pharmacotherapy of neuropathic chronic pelvic pain. Future Sci OA 2016; 2(4):FSO148. doi:10.4155/fsoa-2016-0048
- Papandreou C, Skapinakis P, Giannakis D, Sofikitis N, Mavreas V. Antidepressant drugs for chronic urological pelvic pain: an evidence-based review. Adv Urol 2009; 2009:797031. doi:10.1155/2009/797031
- Liu Y, Xu X, Dong M, Jia S, Wei Y. Treatment of insomnia with tricyclic antidepressants: a meta-analysis of polysomnographic randomized controlled trials. Sleep Med 2017; 34:126–133. doi:10.1016/j.sleep.2017.03.007
- Matheson E, Hainer BL. Insomnia: pharmacologic therapy. Am Fam Physician 2017; 96(1):29–35. pmid:28671376
- McCall C, McCall WV. What is the role of sedating antidepressants, antipsychotics, and anticonvulsants in the management of insomnia? Curr Psychiatry Rep 2012; 14(5):494–502. doi:10.1007/s11920-012-0302-y
- Clark MS, Smith PO, Jamieson B. FPIN’s clinical inquiries: antidepressants for the treatment of insomnia in patients with depression. Am Fam Physician 2011; 84(9):1–2. pmid:22164891
- Sadock BJ, Sadock VA, Ruiz P. Kaplan and Sadock’s Synopsis of Psychiatry. New York, NY: Lippincott Williams & Wilkins; 2014.
- Wang SM, Han C, Bahk WM, et al. Addressing the side effects of contemporary antidepressant drugs: a comprehensive review. Chonnam Med J 2018; 54(2):101–112. doi:10.4068/cmj.2018.54.2.101.
- Trindade E, Menon D, Topfer LA, Coloma C. Adverse effects associated with selective serotonin reuptake inhibitors and tricyclic antidepressants: a meta-analysis. CMAJ 1998; 159(10):1245–1252. pmid:9861221
- Fava M. Prospective studies of adverse events related to antidepressant discontinuation. J Clin Psychiatry 2006; 67(suppl 4):14–21. pmid:16683858
- Gintant G. An evaluation of hERG current assay performance: translating preclinical safety studies to clinical QT prolongation. Pharmacol Ther 2011; 129(2):109–119. doi:10.1016/j.pharmthera.2010.08.008.
- Beach SR, Celano CM, Noseworthy PA, Januzzi JL, Huffman JC. QTc prolongation, torsades de pointes, and psychotropic medications. Psychosomatics 2013; 54(1):1–13. doi:10.1016/j.psym.2012.11.001
- American Geriatrics Society 2015 Beers Criteria Update Expert Panel. American Geriatrics Society 2015 updated Beers Criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc 2015; 63(11):2227–2246. doi:10.1111/jgs.13702
- Fukushima N, Nanao K, Fukushima H, Namera A, Miura M. A neonatal prolonged QT syndrome due to maternal use of oral tricyclic antidepressants. Eur J Pediatr 2016; 175(8):1129–1132. doi:10.1007/s00431-016-2722-x
- ACOG Committee on Practice Bulletins—Obstetrics. ACOG Practice Bulletin: Clinical management guidelines for obstetrician-gynecologists number 92, April 2008 (replaces practice bulletin number 87, November 2007). Use of psychiatric medications during pregnancy and lactation. Obstet Gynecol 2008; 111(4):1001–1020. doi:10.1097/AOG.0b013e31816fd910
Most tricyclic antidepressants (TCAs) have US Food and Drug Administration approval for treatment of depression and anxiety disorders, but they are also a viable off-label option that should be considered by clinicians in specialties beyond psychiatry, especially for treating pain syndromes. Given the ongoing epidemic of opioid use disorder, increasing attention has been drawn to alternative strategies for chronic pain management, renewing an interest in the use of TCAs.
This review summarizes the pharmacologic properties of TCAs, their potential indications in conditions other than depression, and safety considerations.
BRIEF HISTORY OF TRICYCLICS
TCAs were originally designed in the 1950s and marketed later for treating depression. Due to their adverse effects and lethality in overdose quantities, over time they have been largely replaced by selective serotonin reuptake inhibitors (SSRIs) and serotonin-norepinephrine reuptake inhibitors (SNRIs) in depression management. However, TCAs have been applied to conditions other than depression with varying degrees of efficacy and safety.
TCA PHARMACOLOGY
TCAs are absorbed in the small intestine and undergo first-pass metabolism in the liver. They bind extensively to proteins, leading to interactions with other protein-bound drugs. They are widely distributed throughout the systemic circulation because they are highly lipophilic, resulting in systemic effects including central nervous system manifestations.
Peak plasma concentration is at about 2 to 6 hours, and elimination half-life is around 24 hours for most agents, providing a long duration of action. Clearance depends on cytochrome P450 oxidative enzymes.1
MECHANISMS OF ACTION
TCAs inhibit reuptake of norepinephrine and serotonin, resulting in accumulation of these neurotransmitters in the presynaptic cleft. They also block postsynaptic histamine, alpha-adrenergic, and muscarinic-acetylcholine receptors, causing a variety of adverse effects, including dry mouth, confusion, cognitive impairment, hypotension, orthostasis, blurred vision, urinary retention, drowsiness, and sedation.1
Research suggests that TCAs relieve pain centrally through a descending pathway that inhibits transmission of pain signals in the spinal cord, as well as peripherally through complex anti-neuroimmune actions.2 Norepinephrine appears to play a more important role in this process than serotonin, although both are deemed necessary for the “dual action” often cited in pain management,1 which is also the rationale for widespread use of SNRIs to control pain.
Table 1 compares neurotransmitter reuptake mechanisms, adverse effect profiles, and typical dosages for depression for commonly prescribed TCAs.
POTENTIAL USES
Headache and migraine
TCAs have been shown to be effective for managing and preventing chronic headache syndromes.3,4 Amitriptyline has been the most studied of the TCAs for both chronic daily and episodic migraine headache, showing the most efficacy among diverse drug classes (angiotensin II receptor blockers, anticonvulsants, beta-blockers, SSRIs) compared with placebo. However, in head-to-head trials, amitriptyline was no more effective than SSRIs, venlafaxine, topiramate, or propranolol.4 Jackson et al4 suggested that prophylactic medication choices should be tailored to patient characteristics and expected adverse effects, and specifically recommended that TCAs—particularly amitriptyline—be reserved for patients who have both migraine and depression.
Neuropathic pain
Neuropathic pain is defined as pain secondary to a lesion or disease of the somatosensory nervous system5 and is the pathomechanistic component of a number of conditions, including postherpetic neuralgia,6 diabetic and nondiabetic painful polyneuropathy,7 posttraumatic or postsurgical neuropathic pain8 (including plexus avulsion and complex regional pain syndrome9), central poststroke pain,10 spinal cord injury pain,11 and multiple sclerosis-associated pain.12
As a group, TCAs appear to have a role as first-line agents for managing these varied neuropathic pain syndromes. In a recent meta-analysis,13 16 (89%) of 18 placebo-controlled trials of TCAs (mainly amitriptyline at 25–150 mg/day) for these pain conditions were positive, with a combined number needed to treat of 3.6, suggesting a role for TCAs in these conditions. Of note, the TCAs desipramine14 and nortriptyline15 have demonstrated little evidence of efficacy in neuropathic pain syndromes.
Chronic low back pain
Chronic low back pain is a leading cause of loss of work, excessive healthcare expenditure, and disability in the United States. It can be due to numerous spinal conditions, including degenerative disk disease, spinal stenosis, lumbar spondylosis, and spinal arthropathy.
TCAs have been used to treat chronic low back pain for decades and have been repeatedly shown to be more effective than placebo in reducing pain severity.16,17 A double-blind controlled trial18 from 1999 compared the effects of the TCA maprotiline (up to 150 mg daily), the SSRI paroxetine (up to 30 mg daily), and placebo and found a statistically significant reduction in back pain with maprotiline compared with paroxetine and placebo. However, a 2008 meta-analysis suggested little evidence that TCAs were superior to placebo.19
Evidence of TCA efficacy for back pain was reported in 2018 with a well-designed 6-month double-blind randomized controlled trial20 comparing low-dose amitriptyline (25 mg) with an active comparator (benztropine 1 mg). The authors reported that amitriptyline was effective in reducing pain and pain-related disability without incurring serious adverse effects. They suggested continued use of TCAs for chronic low back pain if complicated with pain-related disability, insomnia, depression, or other comorbidity, although they called for further large-scale studies. They also cautioned that patients started the trial with symptoms similar to the adverse effects of TCAs themselves; this has implications for monitoring of symptoms as well as TCA adverse effects while using these drugs.
Fibromyalgia and chronic widespread pain
Fibromyalgia is a common, frustrating, noninflammatory pain syndrome characterized by diffuse hyperalgesia and multiple comorbidities.21 Although sleep hygiene, exercise, cognitive-behavioral therapy, some gabapentinoids (pregabalin), and a combination of these therapies have demonstrated efficacy, TCAs also offer robust benefits.
A meta-analysis of 9 placebo-controlled TCA trials showed large effect sizes for pain reduction, fatigue reduction, improved sleep quality, and reduced stiffness and tenderness, with the most significant of these improvements being for sleep.22 A separate meta-analysis calculated that the number needed to treat with amitriptyline for a positive outcome is 4.9.23 Recent systematic reviews have supported these findings, listing TCAs as second-line agents after pregabalin, duloxetine, and milnacipran.24
Of note, TCA monotherapy rarely produces a complete response in patients with moderate to severe fibromyalgia, chronic widespread pain, or significant comorbidities (depression, anxiety). Supplementation with cognitive-behavioral therapy, physical therapy, functional restoration, and other modalities is strongly recommended.
Abdominal and gastrointestinal pain
TCAs have been applied to a number of gastrointestinal syndromes with or without pain. Patients with irritable bowel syndrome have long been known to benefit from TCAs; the number needed to treat for symptomatic benefit over placebo is 3.5.25,26
Although there is no substantial evidence that TCAs are useful in reducing active inflammation in inflammatory bowel disease, a study involving 81 patients found that residual noninflammatory gastrointestinal symptoms (such as diarrhea and pain) responded to TCAs, including nortriptyline and amitriptyline, with greater benefit for ulcerative colitis than for Crohn disease.27
TCAs have also shown prophylactic benefit in cyclic vomiting syndrome, with a clinical response in over 75% of patients in controlled cohort studies.28
The efficacy of TCAs in other abdominal or gastrointestinal syndromes is unclear or modest at best.29 However, few alternative treatments exist for these conditions. Amitriptyline may help symptoms of functional dyspepsia,30 but nortriptyline has proven ineffective in gastroparesis.31 Nonetheless, some authors29 suggest considering TCAs on an individualized basis, with proper monitoring, in many if not most functional gastrointestinal disorders, especially when paired with behavioral therapies.
Pelvic and urogynecologic symptoms
Chronic pelvic pain affects up to 24% of women32 and 5% to 10% of men.33 TCAs have shown efficacy in treating chronic pelvic pain with or without comorbid depression.34 Amitriptyline and to a lesser extent nortriptyline are the TCAs most often prescribed. Pain relief appears to be independent of antidepressant effects and may be achieved at low doses; initial dosing ranges from 10 to 25 mg at bedtime, which may be increased to 100 mg as tolerated.34
Based on a randomized, double-blind trial,35 amitriptyline was recommended as a treatment option for interstitial cystitis or bladder pain, with the greatest symptom improvement in patients tolerating a daily dose of 50 mg.
Another study36 randomized 56 women with chronic pelvic pain to amitriptyline or gabapentin, or a combination of the drugs for 24 months. Although each regimen resulted in significant reduction in pain, fewer adverse effects occurred with gabapentin than amitriptyline. Poor compliance and early discontinuation of amitriptyline were common due to anticholinergic effects.
In small uncontrolled studies,37 about half of women with chronic pelvic pain became pain-free after 8 weeks of treatment with nortriptyline and imipramine.
Randomized controlled studies are needed to confirm potential benefits of TCAs in chronic urologic and pelvic pain.
Insomnia
Insomnia affects 23% to 56% of people in the United States, Europe, and Asia38 and is the reason for more than 5.5 million primary care visits annually.39 TCAs (especially doxepin, maprotiline, and amitriptyline40) have been shown to be an effective treatment, with an 82% increase in somnolence compared with placebo, as well as measurably improved total sleep time, enhanced sleep efficiency, reduced latency to persistent sleep, and decreased wake times after sleep onset.38
Dosing should be kept at a minimum to minimize harsh anticholinergic effects and avoid daytime sedation. Patients should be advised to take new doses or dose escalations earlier in the night to ensure less hangover sedation the next morning.
For patients with insomnia and comorbid depression, the American Academy of Sleep Medicine suggests the addition of a low dose (eg, 10–25 mg) of a TCA at nighttime to complement preexisting, full-dose, non-TCA antidepressants, while monitoring for serotonin syndrome and other potential but exceedingly rare drug-drug interactions.41
Psychiatric indications other than depression
Beyond the known benefits in major depressive disorder, TCAs have been shown to be effective for obsessive-compulsive disorder, panic disorder, posttraumatic stress disorder, bulimia nervosa, and childhood enuresis.42 Given the shortage of mental health clinicians and the high prevalence of these conditions, nonpsychiatrist physicians should be familiar with the therapeutic potential of TCAs for these indications.
ADVERSE EFFECTS
Adverse effects vary among TCAs. Common ones include blurred vision, dry mouth, constipation, urinary retention, hypotension, tachycardia, tremor, weight gain, and sexual dysfunction.43 Tertiary amines are generally more sedating than secondary amines and cause more anticholinergic effects (Table 1).
Despite widespread perceptions that TCAs are less tolerable than newer antidepressants, studies repeatedly suggest that they have an adverse-effect burden similar to that of SSRIs and SNRIs, although SSRIs have a greater tendency to produce nausea, whereas TCAs are more likely to cause constipation.44
Discontinuation syndrome
Abrupt discontinuation or unintentionally missed doses of TCAs have been associated with a discontinuation syndrome in about 40% of users.45 Patients should be warned about this possibility and the syndrome’s potential effects: dizziness, insomnia, headaches, nausea, vomiting, flulike achiness, and restlessness. Rebound depression, anxiety, panic, or other psychiatric symptoms may also occur. Symptoms generally present within 2 to 5 days after dose discontinuation and last 7 to 14 days.45
However, all TCAs have a long half-life, allowing for sufficient coverage with once-daily dosing and thus carry a lower risk of discontinuation syndrome than many other antidepressants (78% with venlafaxine; 55% with paroxetine).45
To discontinue therapy safely, the dosage should be reduced gradually. As is pharmacologically expected, the greatest likelihood of discontinuation syndrome is associated with longer duration of continuous treatment.
CONTRAINDICATIONS
Cardiac conduction abnormalities
TCAs should not be prescribed to patients who have right bundle branch block, a severe electrolyte disturbance, or other cardiac conduction deficit or arrhythmia that can prolong the QTc interval and elevate the risk of lethal arrhythmia.46,47 Cardiac effects from TCAs are largely dose-dependent. Nevertheless, a baseline electrocardiogram can be obtained to assess cardiac risk, and dose escalation can proceed if results are normal (eg, appropriate conduction intervals, QTc ≤ 450 ms).
Advanced age
For elderly patients, TCAs should be prescribed with caution and sometimes not at all,48 because anticholinergic effects may worsen preexisting urinary retention (including benign prostatic hyperplasia), narrow-angle glaucoma, imbalance and gait issues, and cognitive impairment and dementia. Dehydration and orthostatic hypotension are contraindications for TCAs, as they may precipitate falls or hypotensive shock.
Epilepsy
TCAs should also be used with caution in patients with epilepsy, as they lower the seizure threshold.
Concomitant monoamine oxidase inhibitor treatment
Giving TCAs together with monoamine oxidase inhibitor antidepressants should be avoided, given the risk of hypertensive crisis.
Suicide risk
TCAs are dangerous and potentially lethal in overdose and so should not be prescribed to suicidal or otherwise impulsive patients.
Pregnancy
TCAs are in pregnancy risk category C (animal studies show adverse effects on fetus; no adequate or well-controlled studies in humans; potential benefits may warrant use despite risks). Using TCAs during pregnancy has very rarely led to neonatal withdrawal such as irritability, jitteriness, and convulsions, as well as fetal QTc interval prolongation.49
The American College of Obstetricians and Gynecologists recommends that therapy for depression during pregnancy be individualized, incorporating the expertise of the patient’s mental health clinician, obstetrician, primary healthcare provider, and pediatrician. In general, they recommend that TCAs should be avoided if possible and that alternatives such as SSRIs or SNRIs should be considered.50
TCAs are excreted in breast milk, but they have not been detected in the serum of nursing infants, and no adverse events have been reported.
OVERDOSE IS HIGHLY DANGEROUS
Severe morbidity and death are associated with TCA overdose, characterized by convulsions, cardiac arrest, and coma (the “3 Cs”). These dangers occur at much higher rates with TCAs than with other antidepressants.43 Signs and symptoms of toxicity develop rapidly, usually within the first hour of overdose. Manifestations of overdose include prolonged QTc, cardiac arrhythmias, tachycardia, hypertension, severe hypotension, agitation, seizures, central nervous system depression, hallucinations, seizures, and coma.
Overdose management includes activated charcoal, seizure control, cardioversion, hydration, electrolyte stabilization, and other intensive care.
OFF-LABEL TCA MANAGEMENT
Dosing recommendations for off-label use of TCAs vary based on the condition, the medication, and the suggestions of individual authors and researchers. In general, dosing ranges for pain and other nondepression indications may be lower than for severe depression (Table 2).1
As with any pharmacologic titration, monitoring for rate-limiting adverse effects is recommended. We suggest caution, tailoring the approach to the patient, and routinely assessing for adverse effects and other safety considerations.
In addition, we strongly recommend supplementing TCA therapy with nonpharmacologic strategies such as lifestyle changes, dietary modifications, exercise, physical therapy, and mental health optimization.
Most tricyclic antidepressants (TCAs) have US Food and Drug Administration approval for treatment of depression and anxiety disorders, but they are also a viable off-label option that should be considered by clinicians in specialties beyond psychiatry, especially for treating pain syndromes. Given the ongoing epidemic of opioid use disorder, increasing attention has been drawn to alternative strategies for chronic pain management, renewing an interest in the use of TCAs.
This review summarizes the pharmacologic properties of TCAs, their potential indications in conditions other than depression, and safety considerations.
BRIEF HISTORY OF TRICYCLICS
TCAs were originally designed in the 1950s and marketed later for treating depression. Due to their adverse effects and lethality in overdose quantities, over time they have been largely replaced by selective serotonin reuptake inhibitors (SSRIs) and serotonin-norepinephrine reuptake inhibitors (SNRIs) in depression management. However, TCAs have been applied to conditions other than depression with varying degrees of efficacy and safety.
TCA PHARMACOLOGY
TCAs are absorbed in the small intestine and undergo first-pass metabolism in the liver. They bind extensively to proteins, leading to interactions with other protein-bound drugs. They are widely distributed throughout the systemic circulation because they are highly lipophilic, resulting in systemic effects including central nervous system manifestations.
Peak plasma concentration is at about 2 to 6 hours, and elimination half-life is around 24 hours for most agents, providing a long duration of action. Clearance depends on cytochrome P450 oxidative enzymes.1
MECHANISMS OF ACTION
TCAs inhibit reuptake of norepinephrine and serotonin, resulting in accumulation of these neurotransmitters in the presynaptic cleft. They also block postsynaptic histamine, alpha-adrenergic, and muscarinic-acetylcholine receptors, causing a variety of adverse effects, including dry mouth, confusion, cognitive impairment, hypotension, orthostasis, blurred vision, urinary retention, drowsiness, and sedation.1
Research suggests that TCAs relieve pain centrally through a descending pathway that inhibits transmission of pain signals in the spinal cord, as well as peripherally through complex anti-neuroimmune actions.2 Norepinephrine appears to play a more important role in this process than serotonin, although both are deemed necessary for the “dual action” often cited in pain management,1 which is also the rationale for widespread use of SNRIs to control pain.
Table 1 compares neurotransmitter reuptake mechanisms, adverse effect profiles, and typical dosages for depression for commonly prescribed TCAs.
POTENTIAL USES
Headache and migraine
TCAs have been shown to be effective for managing and preventing chronic headache syndromes.3,4 Amitriptyline has been the most studied of the TCAs for both chronic daily and episodic migraine headache, showing the most efficacy among diverse drug classes (angiotensin II receptor blockers, anticonvulsants, beta-blockers, SSRIs) compared with placebo. However, in head-to-head trials, amitriptyline was no more effective than SSRIs, venlafaxine, topiramate, or propranolol.4 Jackson et al4 suggested that prophylactic medication choices should be tailored to patient characteristics and expected adverse effects, and specifically recommended that TCAs—particularly amitriptyline—be reserved for patients who have both migraine and depression.
Neuropathic pain
Neuropathic pain is defined as pain secondary to a lesion or disease of the somatosensory nervous system5 and is the pathomechanistic component of a number of conditions, including postherpetic neuralgia,6 diabetic and nondiabetic painful polyneuropathy,7 posttraumatic or postsurgical neuropathic pain8 (including plexus avulsion and complex regional pain syndrome9), central poststroke pain,10 spinal cord injury pain,11 and multiple sclerosis-associated pain.12
As a group, TCAs appear to have a role as first-line agents for managing these varied neuropathic pain syndromes. In a recent meta-analysis,13 16 (89%) of 18 placebo-controlled trials of TCAs (mainly amitriptyline at 25–150 mg/day) for these pain conditions were positive, with a combined number needed to treat of 3.6, suggesting a role for TCAs in these conditions. Of note, the TCAs desipramine14 and nortriptyline15 have demonstrated little evidence of efficacy in neuropathic pain syndromes.
Chronic low back pain
Chronic low back pain is a leading cause of loss of work, excessive healthcare expenditure, and disability in the United States. It can be due to numerous spinal conditions, including degenerative disk disease, spinal stenosis, lumbar spondylosis, and spinal arthropathy.
TCAs have been used to treat chronic low back pain for decades and have been repeatedly shown to be more effective than placebo in reducing pain severity.16,17 A double-blind controlled trial18 from 1999 compared the effects of the TCA maprotiline (up to 150 mg daily), the SSRI paroxetine (up to 30 mg daily), and placebo and found a statistically significant reduction in back pain with maprotiline compared with paroxetine and placebo. However, a 2008 meta-analysis suggested little evidence that TCAs were superior to placebo.19
Evidence of TCA efficacy for back pain was reported in 2018 with a well-designed 6-month double-blind randomized controlled trial20 comparing low-dose amitriptyline (25 mg) with an active comparator (benztropine 1 mg). The authors reported that amitriptyline was effective in reducing pain and pain-related disability without incurring serious adverse effects. They suggested continued use of TCAs for chronic low back pain if complicated with pain-related disability, insomnia, depression, or other comorbidity, although they called for further large-scale studies. They also cautioned that patients started the trial with symptoms similar to the adverse effects of TCAs themselves; this has implications for monitoring of symptoms as well as TCA adverse effects while using these drugs.
Fibromyalgia and chronic widespread pain
Fibromyalgia is a common, frustrating, noninflammatory pain syndrome characterized by diffuse hyperalgesia and multiple comorbidities.21 Although sleep hygiene, exercise, cognitive-behavioral therapy, some gabapentinoids (pregabalin), and a combination of these therapies have demonstrated efficacy, TCAs also offer robust benefits.
A meta-analysis of 9 placebo-controlled TCA trials showed large effect sizes for pain reduction, fatigue reduction, improved sleep quality, and reduced stiffness and tenderness, with the most significant of these improvements being for sleep.22 A separate meta-analysis calculated that the number needed to treat with amitriptyline for a positive outcome is 4.9.23 Recent systematic reviews have supported these findings, listing TCAs as second-line agents after pregabalin, duloxetine, and milnacipran.24
Of note, TCA monotherapy rarely produces a complete response in patients with moderate to severe fibromyalgia, chronic widespread pain, or significant comorbidities (depression, anxiety). Supplementation with cognitive-behavioral therapy, physical therapy, functional restoration, and other modalities is strongly recommended.
Abdominal and gastrointestinal pain
TCAs have been applied to a number of gastrointestinal syndromes with or without pain. Patients with irritable bowel syndrome have long been known to benefit from TCAs; the number needed to treat for symptomatic benefit over placebo is 3.5.25,26
Although there is no substantial evidence that TCAs are useful in reducing active inflammation in inflammatory bowel disease, a study involving 81 patients found that residual noninflammatory gastrointestinal symptoms (such as diarrhea and pain) responded to TCAs, including nortriptyline and amitriptyline, with greater benefit for ulcerative colitis than for Crohn disease.27
TCAs have also shown prophylactic benefit in cyclic vomiting syndrome, with a clinical response in over 75% of patients in controlled cohort studies.28
The efficacy of TCAs in other abdominal or gastrointestinal syndromes is unclear or modest at best.29 However, few alternative treatments exist for these conditions. Amitriptyline may help symptoms of functional dyspepsia,30 but nortriptyline has proven ineffective in gastroparesis.31 Nonetheless, some authors29 suggest considering TCAs on an individualized basis, with proper monitoring, in many if not most functional gastrointestinal disorders, especially when paired with behavioral therapies.
Pelvic and urogynecologic symptoms
Chronic pelvic pain affects up to 24% of women32 and 5% to 10% of men.33 TCAs have shown efficacy in treating chronic pelvic pain with or without comorbid depression.34 Amitriptyline and to a lesser extent nortriptyline are the TCAs most often prescribed. Pain relief appears to be independent of antidepressant effects and may be achieved at low doses; initial dosing ranges from 10 to 25 mg at bedtime, which may be increased to 100 mg as tolerated.34
Based on a randomized, double-blind trial,35 amitriptyline was recommended as a treatment option for interstitial cystitis or bladder pain, with the greatest symptom improvement in patients tolerating a daily dose of 50 mg.
Another study36 randomized 56 women with chronic pelvic pain to amitriptyline or gabapentin, or a combination of the drugs for 24 months. Although each regimen resulted in significant reduction in pain, fewer adverse effects occurred with gabapentin than amitriptyline. Poor compliance and early discontinuation of amitriptyline were common due to anticholinergic effects.
In small uncontrolled studies,37 about half of women with chronic pelvic pain became pain-free after 8 weeks of treatment with nortriptyline and imipramine.
Randomized controlled studies are needed to confirm potential benefits of TCAs in chronic urologic and pelvic pain.
Insomnia
Insomnia affects 23% to 56% of people in the United States, Europe, and Asia38 and is the reason for more than 5.5 million primary care visits annually.39 TCAs (especially doxepin, maprotiline, and amitriptyline40) have been shown to be an effective treatment, with an 82% increase in somnolence compared with placebo, as well as measurably improved total sleep time, enhanced sleep efficiency, reduced latency to persistent sleep, and decreased wake times after sleep onset.38
Dosing should be kept at a minimum to minimize harsh anticholinergic effects and avoid daytime sedation. Patients should be advised to take new doses or dose escalations earlier in the night to ensure less hangover sedation the next morning.
For patients with insomnia and comorbid depression, the American Academy of Sleep Medicine suggests the addition of a low dose (eg, 10–25 mg) of a TCA at nighttime to complement preexisting, full-dose, non-TCA antidepressants, while monitoring for serotonin syndrome and other potential but exceedingly rare drug-drug interactions.41
Psychiatric indications other than depression
Beyond the known benefits in major depressive disorder, TCAs have been shown to be effective for obsessive-compulsive disorder, panic disorder, posttraumatic stress disorder, bulimia nervosa, and childhood enuresis.42 Given the shortage of mental health clinicians and the high prevalence of these conditions, nonpsychiatrist physicians should be familiar with the therapeutic potential of TCAs for these indications.
ADVERSE EFFECTS
Adverse effects vary among TCAs. Common ones include blurred vision, dry mouth, constipation, urinary retention, hypotension, tachycardia, tremor, weight gain, and sexual dysfunction.43 Tertiary amines are generally more sedating than secondary amines and cause more anticholinergic effects (Table 1).
Despite widespread perceptions that TCAs are less tolerable than newer antidepressants, studies repeatedly suggest that they have an adverse-effect burden similar to that of SSRIs and SNRIs, although SSRIs have a greater tendency to produce nausea, whereas TCAs are more likely to cause constipation.44
Discontinuation syndrome
Abrupt discontinuation or unintentionally missed doses of TCAs have been associated with a discontinuation syndrome in about 40% of users.45 Patients should be warned about this possibility and the syndrome’s potential effects: dizziness, insomnia, headaches, nausea, vomiting, flulike achiness, and restlessness. Rebound depression, anxiety, panic, or other psychiatric symptoms may also occur. Symptoms generally present within 2 to 5 days after dose discontinuation and last 7 to 14 days.45
However, all TCAs have a long half-life, allowing for sufficient coverage with once-daily dosing and thus carry a lower risk of discontinuation syndrome than many other antidepressants (78% with venlafaxine; 55% with paroxetine).45
To discontinue therapy safely, the dosage should be reduced gradually. As is pharmacologically expected, the greatest likelihood of discontinuation syndrome is associated with longer duration of continuous treatment.
CONTRAINDICATIONS
Cardiac conduction abnormalities
TCAs should not be prescribed to patients who have right bundle branch block, a severe electrolyte disturbance, or other cardiac conduction deficit or arrhythmia that can prolong the QTc interval and elevate the risk of lethal arrhythmia.46,47 Cardiac effects from TCAs are largely dose-dependent. Nevertheless, a baseline electrocardiogram can be obtained to assess cardiac risk, and dose escalation can proceed if results are normal (eg, appropriate conduction intervals, QTc ≤ 450 ms).
Advanced age
For elderly patients, TCAs should be prescribed with caution and sometimes not at all,48 because anticholinergic effects may worsen preexisting urinary retention (including benign prostatic hyperplasia), narrow-angle glaucoma, imbalance and gait issues, and cognitive impairment and dementia. Dehydration and orthostatic hypotension are contraindications for TCAs, as they may precipitate falls or hypotensive shock.
Epilepsy
TCAs should also be used with caution in patients with epilepsy, as they lower the seizure threshold.
Concomitant monoamine oxidase inhibitor treatment
Giving TCAs together with monoamine oxidase inhibitor antidepressants should be avoided, given the risk of hypertensive crisis.
Suicide risk
TCAs are dangerous and potentially lethal in overdose and so should not be prescribed to suicidal or otherwise impulsive patients.
Pregnancy
TCAs are in pregnancy risk category C (animal studies show adverse effects on fetus; no adequate or well-controlled studies in humans; potential benefits may warrant use despite risks). Using TCAs during pregnancy has very rarely led to neonatal withdrawal such as irritability, jitteriness, and convulsions, as well as fetal QTc interval prolongation.49
The American College of Obstetricians and Gynecologists recommends that therapy for depression during pregnancy be individualized, incorporating the expertise of the patient’s mental health clinician, obstetrician, primary healthcare provider, and pediatrician. In general, they recommend that TCAs should be avoided if possible and that alternatives such as SSRIs or SNRIs should be considered.50
TCAs are excreted in breast milk, but they have not been detected in the serum of nursing infants, and no adverse events have been reported.
OVERDOSE IS HIGHLY DANGEROUS
Severe morbidity and death are associated with TCA overdose, characterized by convulsions, cardiac arrest, and coma (the “3 Cs”). These dangers occur at much higher rates with TCAs than with other antidepressants.43 Signs and symptoms of toxicity develop rapidly, usually within the first hour of overdose. Manifestations of overdose include prolonged QTc, cardiac arrhythmias, tachycardia, hypertension, severe hypotension, agitation, seizures, central nervous system depression, hallucinations, seizures, and coma.
Overdose management includes activated charcoal, seizure control, cardioversion, hydration, electrolyte stabilization, and other intensive care.
OFF-LABEL TCA MANAGEMENT
Dosing recommendations for off-label use of TCAs vary based on the condition, the medication, and the suggestions of individual authors and researchers. In general, dosing ranges for pain and other nondepression indications may be lower than for severe depression (Table 2).1
As with any pharmacologic titration, monitoring for rate-limiting adverse effects is recommended. We suggest caution, tailoring the approach to the patient, and routinely assessing for adverse effects and other safety considerations.
In addition, we strongly recommend supplementing TCA therapy with nonpharmacologic strategies such as lifestyle changes, dietary modifications, exercise, physical therapy, and mental health optimization.
- Obata H. Analgesic mechanisms of antidepressants for neuropathic pain. Int J Mol Sci 2017; 18(11). doi:10.3390/ijms18112483
- Kremer M, Yalcin I, Goumon Y, et al. A dual noradrenergic mechanism for the relief of neuropathic allodynia by the antidepressant drugs duloxetine and amitriptyline. J Neurosci 2018; 38(46):9934–9954. doi:10.1523/JNEUROSCI.1004-18.2018
- Tomkins GE, Jackson JL, O’Malley PG, Balden E, Santoro JE. Treatment of chronic headache with antidepressants: a meta-analysis. Am J Med 2001; 111(1):54–63. doi:10.1016/s0002-9343(01)00762-8
- Jackson JL, Cogbill E, Santana-Davila R, et al. A comparative effectiveness meta-analysis of drugs for the prophylaxis of migraine headache. PLoS One 2015; 10(7):e0130733. doi:10.1371/journal.pone.0130733
- International Association for the Study of Pain (IASP). IASP Terminology. www.iasp-pain.org/Education/Content.aspx?ItemNumber=1698&navItemNumber=576. Accessed November 20, 2019.
- Feller L, Khammissa RAG, Fourie J, Bouckaert M, Lemmer J. Postherpectic neuralgia and trigeminal neuralgia. Pain Res Treat 2017; 2017:1681765. doi:10.1155/2017/1681765
- Shillo P, Sloan G, Greig M, et al. Painful and painless diabetic neuropathies: what is the difference? Curr Diab Rep 2019; 19(6):32. doi:10.1007/s11892-019-1150-5
- Schwartzman RJ, Maleki J. Postinjury neuropathic pain syndromes. Med Clin North Am 1999; 83(3):597–626. doi:10.1016/s0025-7125(05)70126-7
- Oaklander AL, Horowitz SH. The complex regional pain syndrome. Handb Clin Neurol 2015; 131:481–503. doi:10.1016/B978-0-444-62627-1.00026-3
- Akyuz G, Kuru P. Systematic review of central post stroke pain: what is happening in the central nervous system? Am J Phys Med Rehabil 2016; 95(8):618–627. doi:10.1097/PHM.0000000000000542
- Shiao R, Lee-Kubli CA. Neuropathic pain after spinal cord injury: challenges and research perspectives. Neurotherapeutics 2018; 15(3):635–653. doi:10.1007/s13311-018-0633-4
- Ceruti S. What role does multiple sclerosis play in the development of untreatable painful conditions? Pain Manag 2018; 8(1):37–44. doi:10.2217/pmt-2017-0038
- Finnerup NB, Attal N, Haroutounian S, et al. Pharmacotherapy for neuropathic pain in adults: a systematic review and meta-analysis. Lancet Neurol 2015; 14(2):162–173. doi:10.1016/S1474-4422(14)70251-0
- Hearn L, Moore RA, Derry S, Wiffen PJ, Phillips T. Desipramine for neuropathic pain in adults. Cochrane Database Syst Rev 2014; (9):CD011003. doi:10.1002/14651858.CD011003.pub2
- Derry S, Whiffen PJ, Aldington D, Moore RA. Nortriptyline for neuropathic pain in adults. Cochrane Database Syst Rev 2015; 1:CD011209. doi:10.1002/14651858.CD011209.pub2
- Salerno SM, Browning R, Jackson JL. The effect of antidepressant treatment on chronic back pain: a meta-analysis. Arch Intern Med 2002; 162(1):19–24. doi:10.1001/archinte.162.1.19
- Staiger TO, Gaster B, Sullivan MD, Deyo RA. Systematic review of antidepressants in the treatment of chronic low back pain. Spine (Phila Pa 1976) 2003; 28(22):2540–2545. doi:10.1097/01.BRS.0000092372.73527.BA
- Atkinson JH, Slater MA, Wahlgren DR, et al. Effects of noradrenergic and serotonergic antidepressants on chronic low back pain intensity. Pain 1999; 83(2):137–145. doi:10.1016/s0304-3959(99)00082-2
- Urquhart DM, Hoving JL, Assendelft WW, Roland M, van Tulder MW. Antidepressants for non-specific back pain. Cochrane Database Syst Rev 2008; (1):CD001703. doi:10.1002/14651858.CD001703.pub3
- Urquhart DM, Wluka AE, van Tulder M, et al. Efficacy of low-dose amitriptyline for chronic low back pain: a randomized clinical trial. JAMA Intern Med 2018; 178(11):1474–1481. doi:10.1001/jamainternmed.2018.4222
- Clauw DJ. Fibromyalgia: a clinical review. JAMA 2014; 311(15):1547–1555. doi:10.1001/jama.2014.3266
- Arnold LM, Keck PE Jr, Welge JA. Antidepressant treatment of fibromyalgia. A meta-analysis and review. Psychosomatics 2000; 41(2):104–113. pmid:10749947
- Hauser W, Wolfe F, Tolle T, Uceyler N, Sommer C. The role of antidepressants in the management of fibromyalgia syndrome: a systematic review and meta-analysis. CNS Drugs 2012; 26(4):297–307. doi:10.2165/11598970-000000000-00000
- Calandre EP, Rico-Villademoros F, Slim M. An update on pharmacotherapy for the treatment of fibromyalgia. Expert Opin Pharmacother 2015; 16(9):1347–1368. doi:10.1517/14656566.2015.1047343
- Jackson JL, O’Malley PG, Tomkins G, Balden E, Santoro J, Kroenke K. Treatment of functional gastrointestinal disorders with antidepressant medications: a meta-analysis. Am J Med 2000; 108(1):65–72. doi:10.1016/s0002-9343(99)00299-5
- Rahimi R, Nikfar S, Rezaie A, Abdollahi M. Efficacy of tricyclic antidepressants in irritable bowel syndrome: a meta-analysis. World J Gastroenterol 2009; 15(13):1548–1553. doi:10.3748/wjg.15.1548
- Iskandar HN, Cassell B, Kanuri N, et al. Tricyclic antidepressants for management of residual symptoms in inflammatory bowel disease. J Clin Gastroenterol 2014; 48(5):423–429. doi:10.1097/MCG.0000000000000049
- Lee LY, Abbott L, Mahlangu B, Moodie SJ, Anderson S. The management of cyclic vomiting syndrome: a systematic review. Eur J Gastroenterol Hepatol 2012; 24(9):1001–1006. doi:10.1097/MEG.0b013e328355638f
- Thorkelson G, Bielefeldt K, Szigethy E. Empirically supported use of psychiatric medications in adolescents and adults with IBD. Inflamm Bowel Dis 2016; 22(6):1509–1522. doi:10.1097/MIB.0000000000000734
- Braak B, Klooker TK, Wouters MM, et al. Randomised clinical trial: the effects of amitriptyline on drinking capacity and symptoms in patients with functional dyspepsia, a double-blind placebo-controlled study. Aliment Pharmacol Ther 2011; 34(6):638–648. doi:10.1111/j.1365-2036.2011.04775.x
- Parkman HP, Van Natta ML, Abell TL, et al. Effect of nortriptyline on symptoms of idiopathic gastroparesis: the NORIG randomized clinical trial. JAMA 2013; 310(24):2640–2649. doi:10.1001/jama.2013.282833
- Latthe P, Latthe M, Say L, Gulmezoglu M, Khan KS. WHO systematic review of prevalence of chronic pelvic pain: a neglected reproductive health morbidity. BMC Public Health 2006; 6:177. doi:10.1186/1471-2458-6-177
- Moise G, Capodice J, Winfree CJ. Treatment of chronic pelvic pain in men and women. Expert Rev Neurother 2007; 7(5):507–520. doi:10.1586/14737175.7.5.507
- Lai HH. Management of interstitial cystitis/bladder pain syndrome with tricyclic antidepressants. In: Moldwin RM, ed. Urological and Gynaecological Chronic Pelvic Pain. Cham, Switzerland: Springer; 2017:107–118.
- American Urological Association. Diagnosis and treatment interstitial cystitis/bladder pain syndrome (2014). www.auanet.org/guidelines/interstitial-cystitis/bladder-pain-syndrome-(2011-amended-2014). Accessed November 19, 2019.
- Carey ET, As-Sanie S. New developments in the pharmacotherapy of neuropathic chronic pelvic pain. Future Sci OA 2016; 2(4):FSO148. doi:10.4155/fsoa-2016-0048
- Papandreou C, Skapinakis P, Giannakis D, Sofikitis N, Mavreas V. Antidepressant drugs for chronic urological pelvic pain: an evidence-based review. Adv Urol 2009; 2009:797031. doi:10.1155/2009/797031
- Liu Y, Xu X, Dong M, Jia S, Wei Y. Treatment of insomnia with tricyclic antidepressants: a meta-analysis of polysomnographic randomized controlled trials. Sleep Med 2017; 34:126–133. doi:10.1016/j.sleep.2017.03.007
- Matheson E, Hainer BL. Insomnia: pharmacologic therapy. Am Fam Physician 2017; 96(1):29–35. pmid:28671376
- McCall C, McCall WV. What is the role of sedating antidepressants, antipsychotics, and anticonvulsants in the management of insomnia? Curr Psychiatry Rep 2012; 14(5):494–502. doi:10.1007/s11920-012-0302-y
- Clark MS, Smith PO, Jamieson B. FPIN’s clinical inquiries: antidepressants for the treatment of insomnia in patients with depression. Am Fam Physician 2011; 84(9):1–2. pmid:22164891
- Sadock BJ, Sadock VA, Ruiz P. Kaplan and Sadock’s Synopsis of Psychiatry. New York, NY: Lippincott Williams & Wilkins; 2014.
- Wang SM, Han C, Bahk WM, et al. Addressing the side effects of contemporary antidepressant drugs: a comprehensive review. Chonnam Med J 2018; 54(2):101–112. doi:10.4068/cmj.2018.54.2.101.
- Trindade E, Menon D, Topfer LA, Coloma C. Adverse effects associated with selective serotonin reuptake inhibitors and tricyclic antidepressants: a meta-analysis. CMAJ 1998; 159(10):1245–1252. pmid:9861221
- Fava M. Prospective studies of adverse events related to antidepressant discontinuation. J Clin Psychiatry 2006; 67(suppl 4):14–21. pmid:16683858
- Gintant G. An evaluation of hERG current assay performance: translating preclinical safety studies to clinical QT prolongation. Pharmacol Ther 2011; 129(2):109–119. doi:10.1016/j.pharmthera.2010.08.008.
- Beach SR, Celano CM, Noseworthy PA, Januzzi JL, Huffman JC. QTc prolongation, torsades de pointes, and psychotropic medications. Psychosomatics 2013; 54(1):1–13. doi:10.1016/j.psym.2012.11.001
- American Geriatrics Society 2015 Beers Criteria Update Expert Panel. American Geriatrics Society 2015 updated Beers Criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc 2015; 63(11):2227–2246. doi:10.1111/jgs.13702
- Fukushima N, Nanao K, Fukushima H, Namera A, Miura M. A neonatal prolonged QT syndrome due to maternal use of oral tricyclic antidepressants. Eur J Pediatr 2016; 175(8):1129–1132. doi:10.1007/s00431-016-2722-x
- ACOG Committee on Practice Bulletins—Obstetrics. ACOG Practice Bulletin: Clinical management guidelines for obstetrician-gynecologists number 92, April 2008 (replaces practice bulletin number 87, November 2007). Use of psychiatric medications during pregnancy and lactation. Obstet Gynecol 2008; 111(4):1001–1020. doi:10.1097/AOG.0b013e31816fd910
- Obata H. Analgesic mechanisms of antidepressants for neuropathic pain. Int J Mol Sci 2017; 18(11). doi:10.3390/ijms18112483
- Kremer M, Yalcin I, Goumon Y, et al. A dual noradrenergic mechanism for the relief of neuropathic allodynia by the antidepressant drugs duloxetine and amitriptyline. J Neurosci 2018; 38(46):9934–9954. doi:10.1523/JNEUROSCI.1004-18.2018
- Tomkins GE, Jackson JL, O’Malley PG, Balden E, Santoro JE. Treatment of chronic headache with antidepressants: a meta-analysis. Am J Med 2001; 111(1):54–63. doi:10.1016/s0002-9343(01)00762-8
- Jackson JL, Cogbill E, Santana-Davila R, et al. A comparative effectiveness meta-analysis of drugs for the prophylaxis of migraine headache. PLoS One 2015; 10(7):e0130733. doi:10.1371/journal.pone.0130733
- International Association for the Study of Pain (IASP). IASP Terminology. www.iasp-pain.org/Education/Content.aspx?ItemNumber=1698&navItemNumber=576. Accessed November 20, 2019.
- Feller L, Khammissa RAG, Fourie J, Bouckaert M, Lemmer J. Postherpectic neuralgia and trigeminal neuralgia. Pain Res Treat 2017; 2017:1681765. doi:10.1155/2017/1681765
- Shillo P, Sloan G, Greig M, et al. Painful and painless diabetic neuropathies: what is the difference? Curr Diab Rep 2019; 19(6):32. doi:10.1007/s11892-019-1150-5
- Schwartzman RJ, Maleki J. Postinjury neuropathic pain syndromes. Med Clin North Am 1999; 83(3):597–626. doi:10.1016/s0025-7125(05)70126-7
- Oaklander AL, Horowitz SH. The complex regional pain syndrome. Handb Clin Neurol 2015; 131:481–503. doi:10.1016/B978-0-444-62627-1.00026-3
- Akyuz G, Kuru P. Systematic review of central post stroke pain: what is happening in the central nervous system? Am J Phys Med Rehabil 2016; 95(8):618–627. doi:10.1097/PHM.0000000000000542
- Shiao R, Lee-Kubli CA. Neuropathic pain after spinal cord injury: challenges and research perspectives. Neurotherapeutics 2018; 15(3):635–653. doi:10.1007/s13311-018-0633-4
- Ceruti S. What role does multiple sclerosis play in the development of untreatable painful conditions? Pain Manag 2018; 8(1):37–44. doi:10.2217/pmt-2017-0038
- Finnerup NB, Attal N, Haroutounian S, et al. Pharmacotherapy for neuropathic pain in adults: a systematic review and meta-analysis. Lancet Neurol 2015; 14(2):162–173. doi:10.1016/S1474-4422(14)70251-0
- Hearn L, Moore RA, Derry S, Wiffen PJ, Phillips T. Desipramine for neuropathic pain in adults. Cochrane Database Syst Rev 2014; (9):CD011003. doi:10.1002/14651858.CD011003.pub2
- Derry S, Whiffen PJ, Aldington D, Moore RA. Nortriptyline for neuropathic pain in adults. Cochrane Database Syst Rev 2015; 1:CD011209. doi:10.1002/14651858.CD011209.pub2
- Salerno SM, Browning R, Jackson JL. The effect of antidepressant treatment on chronic back pain: a meta-analysis. Arch Intern Med 2002; 162(1):19–24. doi:10.1001/archinte.162.1.19
- Staiger TO, Gaster B, Sullivan MD, Deyo RA. Systematic review of antidepressants in the treatment of chronic low back pain. Spine (Phila Pa 1976) 2003; 28(22):2540–2545. doi:10.1097/01.BRS.0000092372.73527.BA
- Atkinson JH, Slater MA, Wahlgren DR, et al. Effects of noradrenergic and serotonergic antidepressants on chronic low back pain intensity. Pain 1999; 83(2):137–145. doi:10.1016/s0304-3959(99)00082-2
- Urquhart DM, Hoving JL, Assendelft WW, Roland M, van Tulder MW. Antidepressants for non-specific back pain. Cochrane Database Syst Rev 2008; (1):CD001703. doi:10.1002/14651858.CD001703.pub3
- Urquhart DM, Wluka AE, van Tulder M, et al. Efficacy of low-dose amitriptyline for chronic low back pain: a randomized clinical trial. JAMA Intern Med 2018; 178(11):1474–1481. doi:10.1001/jamainternmed.2018.4222
- Clauw DJ. Fibromyalgia: a clinical review. JAMA 2014; 311(15):1547–1555. doi:10.1001/jama.2014.3266
- Arnold LM, Keck PE Jr, Welge JA. Antidepressant treatment of fibromyalgia. A meta-analysis and review. Psychosomatics 2000; 41(2):104–113. pmid:10749947
- Hauser W, Wolfe F, Tolle T, Uceyler N, Sommer C. The role of antidepressants in the management of fibromyalgia syndrome: a systematic review and meta-analysis. CNS Drugs 2012; 26(4):297–307. doi:10.2165/11598970-000000000-00000
- Calandre EP, Rico-Villademoros F, Slim M. An update on pharmacotherapy for the treatment of fibromyalgia. Expert Opin Pharmacother 2015; 16(9):1347–1368. doi:10.1517/14656566.2015.1047343
- Jackson JL, O’Malley PG, Tomkins G, Balden E, Santoro J, Kroenke K. Treatment of functional gastrointestinal disorders with antidepressant medications: a meta-analysis. Am J Med 2000; 108(1):65–72. doi:10.1016/s0002-9343(99)00299-5
- Rahimi R, Nikfar S, Rezaie A, Abdollahi M. Efficacy of tricyclic antidepressants in irritable bowel syndrome: a meta-analysis. World J Gastroenterol 2009; 15(13):1548–1553. doi:10.3748/wjg.15.1548
- Iskandar HN, Cassell B, Kanuri N, et al. Tricyclic antidepressants for management of residual symptoms in inflammatory bowel disease. J Clin Gastroenterol 2014; 48(5):423–429. doi:10.1097/MCG.0000000000000049
- Lee LY, Abbott L, Mahlangu B, Moodie SJ, Anderson S. The management of cyclic vomiting syndrome: a systematic review. Eur J Gastroenterol Hepatol 2012; 24(9):1001–1006. doi:10.1097/MEG.0b013e328355638f
- Thorkelson G, Bielefeldt K, Szigethy E. Empirically supported use of psychiatric medications in adolescents and adults with IBD. Inflamm Bowel Dis 2016; 22(6):1509–1522. doi:10.1097/MIB.0000000000000734
- Braak B, Klooker TK, Wouters MM, et al. Randomised clinical trial: the effects of amitriptyline on drinking capacity and symptoms in patients with functional dyspepsia, a double-blind placebo-controlled study. Aliment Pharmacol Ther 2011; 34(6):638–648. doi:10.1111/j.1365-2036.2011.04775.x
- Parkman HP, Van Natta ML, Abell TL, et al. Effect of nortriptyline on symptoms of idiopathic gastroparesis: the NORIG randomized clinical trial. JAMA 2013; 310(24):2640–2649. doi:10.1001/jama.2013.282833
- Latthe P, Latthe M, Say L, Gulmezoglu M, Khan KS. WHO systematic review of prevalence of chronic pelvic pain: a neglected reproductive health morbidity. BMC Public Health 2006; 6:177. doi:10.1186/1471-2458-6-177
- Moise G, Capodice J, Winfree CJ. Treatment of chronic pelvic pain in men and women. Expert Rev Neurother 2007; 7(5):507–520. doi:10.1586/14737175.7.5.507
- Lai HH. Management of interstitial cystitis/bladder pain syndrome with tricyclic antidepressants. In: Moldwin RM, ed. Urological and Gynaecological Chronic Pelvic Pain. Cham, Switzerland: Springer; 2017:107–118.
- American Urological Association. Diagnosis and treatment interstitial cystitis/bladder pain syndrome (2014). www.auanet.org/guidelines/interstitial-cystitis/bladder-pain-syndrome-(2011-amended-2014). Accessed November 19, 2019.
- Carey ET, As-Sanie S. New developments in the pharmacotherapy of neuropathic chronic pelvic pain. Future Sci OA 2016; 2(4):FSO148. doi:10.4155/fsoa-2016-0048
- Papandreou C, Skapinakis P, Giannakis D, Sofikitis N, Mavreas V. Antidepressant drugs for chronic urological pelvic pain: an evidence-based review. Adv Urol 2009; 2009:797031. doi:10.1155/2009/797031
- Liu Y, Xu X, Dong M, Jia S, Wei Y. Treatment of insomnia with tricyclic antidepressants: a meta-analysis of polysomnographic randomized controlled trials. Sleep Med 2017; 34:126–133. doi:10.1016/j.sleep.2017.03.007
- Matheson E, Hainer BL. Insomnia: pharmacologic therapy. Am Fam Physician 2017; 96(1):29–35. pmid:28671376
- McCall C, McCall WV. What is the role of sedating antidepressants, antipsychotics, and anticonvulsants in the management of insomnia? Curr Psychiatry Rep 2012; 14(5):494–502. doi:10.1007/s11920-012-0302-y
- Clark MS, Smith PO, Jamieson B. FPIN’s clinical inquiries: antidepressants for the treatment of insomnia in patients with depression. Am Fam Physician 2011; 84(9):1–2. pmid:22164891
- Sadock BJ, Sadock VA, Ruiz P. Kaplan and Sadock’s Synopsis of Psychiatry. New York, NY: Lippincott Williams & Wilkins; 2014.
- Wang SM, Han C, Bahk WM, et al. Addressing the side effects of contemporary antidepressant drugs: a comprehensive review. Chonnam Med J 2018; 54(2):101–112. doi:10.4068/cmj.2018.54.2.101.
- Trindade E, Menon D, Topfer LA, Coloma C. Adverse effects associated with selective serotonin reuptake inhibitors and tricyclic antidepressants: a meta-analysis. CMAJ 1998; 159(10):1245–1252. pmid:9861221
- Fava M. Prospective studies of adverse events related to antidepressant discontinuation. J Clin Psychiatry 2006; 67(suppl 4):14–21. pmid:16683858
- Gintant G. An evaluation of hERG current assay performance: translating preclinical safety studies to clinical QT prolongation. Pharmacol Ther 2011; 129(2):109–119. doi:10.1016/j.pharmthera.2010.08.008.
- Beach SR, Celano CM, Noseworthy PA, Januzzi JL, Huffman JC. QTc prolongation, torsades de pointes, and psychotropic medications. Psychosomatics 2013; 54(1):1–13. doi:10.1016/j.psym.2012.11.001
- American Geriatrics Society 2015 Beers Criteria Update Expert Panel. American Geriatrics Society 2015 updated Beers Criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc 2015; 63(11):2227–2246. doi:10.1111/jgs.13702
- Fukushima N, Nanao K, Fukushima H, Namera A, Miura M. A neonatal prolonged QT syndrome due to maternal use of oral tricyclic antidepressants. Eur J Pediatr 2016; 175(8):1129–1132. doi:10.1007/s00431-016-2722-x
- ACOG Committee on Practice Bulletins—Obstetrics. ACOG Practice Bulletin: Clinical management guidelines for obstetrician-gynecologists number 92, April 2008 (replaces practice bulletin number 87, November 2007). Use of psychiatric medications during pregnancy and lactation. Obstet Gynecol 2008; 111(4):1001–1020. doi:10.1097/AOG.0b013e31816fd910
KEY POINTS
- Amitriptyline is the most useful TCA for many painful conditions.
- TCAs can be especially helpful for patients with a pain syndrome or insomnia with comorbid depression, although their benefits appear to be independent of antidepressant effects.
- TCAs have long half-lives and so can be taken once a day.
- Effective dosages for symptom control in many conditions are lower than for severe depression; dosage should start low and be gradually increased while monitoring efficacy and adverse effects.
- TCAs should not be used concurrently with a monoamine oxidase inhibitor and by certain patient groups: the elderly, pregnant women, and patients with certain cardiac conduction abnormalities, epilepsy, or risk of suicide.
57 Varieties: Sleep-Disordered Breathing Linked to Changes in 1 Important Gene
Blood oxygen levels drop during sleep, but how much and when that happens is mainly hereditary? Now researchers from Brigham and Women’s Hospital and Case Western Reserve University are getting closer to finding the genetic reasons for the fluctuations, which could help people with sleep apnea and other lung illnesses.
In their study, funded by the National Heart, Lung, and Blood Institute (NHLBI), the researchers analyzed whole genome sequence data from the NHLBI’s Trans-Omics for Precision Medicine (TOPMed) program. They also incorporated results for family-based linkage analysis, which maps genes with hereditary traits to their location in the genome.
The researchers identified 57 genetic variations of DLC1, a gene consistently associated with average arterial oxyhemoglobin saturation during sleep. The variants explain almost 1% of the variability in the oxygen levels in European Americans. That is high for complex genetic phenotypes, the researchers say. Of the 57 variants, 51 influence and regulate human lung fibroblast cells.
“This study highlights the advantage of using family data in searching for rare variants,” says James Kiley, PhD, director of the Division of Lung Diseases at NHLBI. “It showed that, when guided by family linkage data, whole genome sequence analysis can identify rare variants that signal disease risks, even with a small sample. In this case, the initial discovery was done with fewer than 500 samples.”
Blood oxygen levels drop during sleep, but how much and when that happens is mainly hereditary? Now researchers from Brigham and Women’s Hospital and Case Western Reserve University are getting closer to finding the genetic reasons for the fluctuations, which could help people with sleep apnea and other lung illnesses.
In their study, funded by the National Heart, Lung, and Blood Institute (NHLBI), the researchers analyzed whole genome sequence data from the NHLBI’s Trans-Omics for Precision Medicine (TOPMed) program. They also incorporated results for family-based linkage analysis, which maps genes with hereditary traits to their location in the genome.
The researchers identified 57 genetic variations of DLC1, a gene consistently associated with average arterial oxyhemoglobin saturation during sleep. The variants explain almost 1% of the variability in the oxygen levels in European Americans. That is high for complex genetic phenotypes, the researchers say. Of the 57 variants, 51 influence and regulate human lung fibroblast cells.
“This study highlights the advantage of using family data in searching for rare variants,” says James Kiley, PhD, director of the Division of Lung Diseases at NHLBI. “It showed that, when guided by family linkage data, whole genome sequence analysis can identify rare variants that signal disease risks, even with a small sample. In this case, the initial discovery was done with fewer than 500 samples.”
Blood oxygen levels drop during sleep, but how much and when that happens is mainly hereditary? Now researchers from Brigham and Women’s Hospital and Case Western Reserve University are getting closer to finding the genetic reasons for the fluctuations, which could help people with sleep apnea and other lung illnesses.
In their study, funded by the National Heart, Lung, and Blood Institute (NHLBI), the researchers analyzed whole genome sequence data from the NHLBI’s Trans-Omics for Precision Medicine (TOPMed) program. They also incorporated results for family-based linkage analysis, which maps genes with hereditary traits to their location in the genome.
The researchers identified 57 genetic variations of DLC1, a gene consistently associated with average arterial oxyhemoglobin saturation during sleep. The variants explain almost 1% of the variability in the oxygen levels in European Americans. That is high for complex genetic phenotypes, the researchers say. Of the 57 variants, 51 influence and regulate human lung fibroblast cells.
“This study highlights the advantage of using family data in searching for rare variants,” says James Kiley, PhD, director of the Division of Lung Diseases at NHLBI. “It showed that, when guided by family linkage data, whole genome sequence analysis can identify rare variants that signal disease risks, even with a small sample. In this case, the initial discovery was done with fewer than 500 samples.”
Helping adolescents get enough quality sleep
NEW ORLEANS – Social media and electronics aren’t the only barriers to a good night’s sleep for teens, according to Adiaha I. A. Spinks-Franklin, MD, MPH, a pediatrician at Texas Children’s Hospital in Houston.
Another half-dozen “sleep enemies” interfere with adolescents’ sleep and can contribute to insomnia or other sleep disorders, she told attendees at the annual meeting of the American Academy of Pediatrics.
Knowing what normal sleep physiology looks like in youth and understanding the most common sleep enemies and sleep-behavior problems can help you use effective interventions to help your patients get the sleep they need, she said.
Infants need the most sleep, about 12-16 hours each 24-hour period, including naps, for those aged 4-12 months. As they grow into toddlerhood and preschool age, children gradually need less: Children aged 1-2 years need 11-14 hours and children aged 3-5 years need 10-13 hours, including naps. By the time children are in school, ages 6-12, they should have dropped their naps and need 9-12 hours a night.
In fact, 75% of high school seniors get less than 8 hours of sleep a day and live with a chronic sleep debt, Dr. Spinks-Franklin said.
Although social media use and electronics in the bedroom – TVs, computers, cell phones, and video games – can certainly contribute to inadequate sleep, a heavy academic load and extracurricular activities can be just as problematic, Dr. Spinks-Franklin said. Teens who work after school also may have difficulty getting enough sleep, especially if they also have to balance a heavier academic load or even one or two extracurricular activities.
Socializing with friends also can interfere with sleep, especially when get-togethers run late; drinking caffeinated drinks in the afternoon onward can make it difficult for adolescents to get the sleep they need as well. Less-modifiable contributors to too little sleep are stress and early school start times, Dr. Spinks-Franklin said.
The two most common sleep problems seen in teens are insomnia and delayed sleep phase syndrome. Addressing these is important because the effects of chronic insufficient sleep can have far-reaching consequences. Obesity and related chronic health conditions are associated with inadequate sleep, as are poor academic performance, poor judgment, poor executive functioning, and mental health disorders like depression.
Short-term effects of insufficient sleep also can be problematic and can exacerbate existing sleep problems, such as sleeping in on the weekends to “catch up” on sleep or drinking more caffeine to try to stay awake during the day. Increased caffeine intake can interfere with non-REM deep sleep, Dr. Spinks-Franklin said, and therefore reduce the quality of sleep even if the person gets the total hours they need.
Insomnia in adolescents
Insomnia can refer to difficulty falling asleep, staying asleep, sleeping for long enough, or getting enough sleep in one period of time even when the opportunity is there. Some people may have no trouble falling asleep, but they wake up too early – before they have had gotten the sleep they need – and cannot return to sleep.
To be insomnia, the problem must occur “despite having enough time available for sleep,” Dr. Spinks-Franklin said. “Patient who restrict the amount of time for sleep due to work or social commitments may have trouble sleeping and daytime sleepiness but do not have insomnia.”
Daytime impairment also is part of the American Academy of Sleep Medicine’s definition of insomnia. The rare teen who doesn’t need as much sleep as average and functions without difficulty during the day does not necessarily have insomnia.
But the impairment may not necessarily just be fatigue or sleepiness. In fact, many of the symptoms are the same as those seen with ADHD.
Daytime consequences of insomnia can include the following:
- Depression, feeling sad or “blue,” or emotional hypersensitivity.
- Mood swings, crankiness, or irritability.
- Difficulty concentrating or paying attention, poor memory, mind wandering, or even inability to sit still.
- Job or school problems, such as not being able to finish homework, not finishing tasks they start, or forgetfulness.
- Difficulty in social situations, such as discomfort with others or problems with friends.
- Daytime sleepiness, even when unable to actually take a nap.
- Behavioral problems, such as hyperactivity, impulsivity, or aggression.
- Frequent mistakes, especially at work, at school, or while driving (often “errors of omission,” such as not seeing a street sign or not hearing an instruction).
- Lower levels of motivation or initiative, feeling less energetic.
- Excessive worry about sleep.
Evaluation of insomnia can be framed with “the three-factor model,” which includes predisposing factors, precipitating factors, and perpetuating factors.
Predisposing factors – those that indicate a person already may be at risk for insomnia – include potential genetic influences as well as their typical response to stress. “Do they sleep more or less?” Dr. Spinks-Franklin said. Even teens predisposed to insomnia may not develop it, however, without a precipitating trigger.
These triggers could include stress, anxiety, poor initial sleep hygiene that becomes a pattern, dietary intake or behaviors (such as drinking caffeine or eating too much or too late in the evening), changes to their schedule, or side effects of medications.
Once insomnia begins, various factors can then perpetuate the cycle, including some of those that triggered it, such as anxiety or a school or work schedule. Sometimes it can be difficult to pinpoint the factor prolonging insomnia, such as the unconscious reward of going to work or school late with few or no consequences.
Delayed sleep phase syndrome
Delayed sleep phase syndrome occurs when someone has a delayed onset of melatonin secretion that pushes back the time when they can fall asleep. Melatonin is the neurotransmitter produced by the pineal gland that signals the start of nighttime. Although it has a hereditary component, delayed sleep phase syndrome also can result from a pattern of poor sleep onset and sleeping in on the weekends.
Dr. Spinks-Franklin described the typical cycle: A teen doesn’t go to sleep until after midnight and then wants to sleep in later in the morning. Because they have to wake up early for school, they sleep in on the weekends to try to regain the sleep they lost. Sleeping in pushes their circadian rhythm even later, perpetuating the problem.
Interventions for sleep disorders
The recommended treatment for insomnia is cognitive-behavior therapy for insomnia, for which strong evidence exists. Before seeking cognitive-behavior therapy, however, families can work to improve sleep hygiene and reduce stimuli that contribute to insomnia.
Teens should avoid screens for at least 1 hour before bedtime and avoid caffeine and exercise for at least 4 hours before going to bed. They also need to develop a schedule with a consistent bedtime and wake-up time, including on the weekends. They should avoid sleeping in on the weekends or taking naps during the day, Dr. Spinks-Franklin said.
Delayed sleep phase syndrome is more resistant to treatment and has a high recurrence rate, she said, and it requires commitment from the parent and their child to address it successfully. Teens with this condition also can start with sleep hygiene practices: a consistent wake-up time that they maintain on the weekends and no daytime naps. Phototherapy in the morning can be added to hopefully induce an earlier onset of melatonin release in the evening.
The next step is making changes to the youth’s schedule, particularly evening and/or weekend activities. They can try to gradually advance their biological clock by changing their sleep schedule.
Dr. Spinks-Franklin also briefly addressed the use of over-the-counter melatonin supplements for treating sleep problems. Melatonin can be effective for treating insomnia by improving sleep onset and sleep quality, particularly in children and teens with autism spectrum disorder or ADHD.
Dr. Spinks-Franklin had no disclosures, and her presentation used no outside funding.
NEW ORLEANS – Social media and electronics aren’t the only barriers to a good night’s sleep for teens, according to Adiaha I. A. Spinks-Franklin, MD, MPH, a pediatrician at Texas Children’s Hospital in Houston.
Another half-dozen “sleep enemies” interfere with adolescents’ sleep and can contribute to insomnia or other sleep disorders, she told attendees at the annual meeting of the American Academy of Pediatrics.
Knowing what normal sleep physiology looks like in youth and understanding the most common sleep enemies and sleep-behavior problems can help you use effective interventions to help your patients get the sleep they need, she said.
Infants need the most sleep, about 12-16 hours each 24-hour period, including naps, for those aged 4-12 months. As they grow into toddlerhood and preschool age, children gradually need less: Children aged 1-2 years need 11-14 hours and children aged 3-5 years need 10-13 hours, including naps. By the time children are in school, ages 6-12, they should have dropped their naps and need 9-12 hours a night.
In fact, 75% of high school seniors get less than 8 hours of sleep a day and live with a chronic sleep debt, Dr. Spinks-Franklin said.
Although social media use and electronics in the bedroom – TVs, computers, cell phones, and video games – can certainly contribute to inadequate sleep, a heavy academic load and extracurricular activities can be just as problematic, Dr. Spinks-Franklin said. Teens who work after school also may have difficulty getting enough sleep, especially if they also have to balance a heavier academic load or even one or two extracurricular activities.
Socializing with friends also can interfere with sleep, especially when get-togethers run late; drinking caffeinated drinks in the afternoon onward can make it difficult for adolescents to get the sleep they need as well. Less-modifiable contributors to too little sleep are stress and early school start times, Dr. Spinks-Franklin said.
The two most common sleep problems seen in teens are insomnia and delayed sleep phase syndrome. Addressing these is important because the effects of chronic insufficient sleep can have far-reaching consequences. Obesity and related chronic health conditions are associated with inadequate sleep, as are poor academic performance, poor judgment, poor executive functioning, and mental health disorders like depression.
Short-term effects of insufficient sleep also can be problematic and can exacerbate existing sleep problems, such as sleeping in on the weekends to “catch up” on sleep or drinking more caffeine to try to stay awake during the day. Increased caffeine intake can interfere with non-REM deep sleep, Dr. Spinks-Franklin said, and therefore reduce the quality of sleep even if the person gets the total hours they need.
Insomnia in adolescents
Insomnia can refer to difficulty falling asleep, staying asleep, sleeping for long enough, or getting enough sleep in one period of time even when the opportunity is there. Some people may have no trouble falling asleep, but they wake up too early – before they have had gotten the sleep they need – and cannot return to sleep.
To be insomnia, the problem must occur “despite having enough time available for sleep,” Dr. Spinks-Franklin said. “Patient who restrict the amount of time for sleep due to work or social commitments may have trouble sleeping and daytime sleepiness but do not have insomnia.”
Daytime impairment also is part of the American Academy of Sleep Medicine’s definition of insomnia. The rare teen who doesn’t need as much sleep as average and functions without difficulty during the day does not necessarily have insomnia.
But the impairment may not necessarily just be fatigue or sleepiness. In fact, many of the symptoms are the same as those seen with ADHD.
Daytime consequences of insomnia can include the following:
- Depression, feeling sad or “blue,” or emotional hypersensitivity.
- Mood swings, crankiness, or irritability.
- Difficulty concentrating or paying attention, poor memory, mind wandering, or even inability to sit still.
- Job or school problems, such as not being able to finish homework, not finishing tasks they start, or forgetfulness.
- Difficulty in social situations, such as discomfort with others or problems with friends.
- Daytime sleepiness, even when unable to actually take a nap.
- Behavioral problems, such as hyperactivity, impulsivity, or aggression.
- Frequent mistakes, especially at work, at school, or while driving (often “errors of omission,” such as not seeing a street sign or not hearing an instruction).
- Lower levels of motivation or initiative, feeling less energetic.
- Excessive worry about sleep.
Evaluation of insomnia can be framed with “the three-factor model,” which includes predisposing factors, precipitating factors, and perpetuating factors.
Predisposing factors – those that indicate a person already may be at risk for insomnia – include potential genetic influences as well as their typical response to stress. “Do they sleep more or less?” Dr. Spinks-Franklin said. Even teens predisposed to insomnia may not develop it, however, without a precipitating trigger.
These triggers could include stress, anxiety, poor initial sleep hygiene that becomes a pattern, dietary intake or behaviors (such as drinking caffeine or eating too much or too late in the evening), changes to their schedule, or side effects of medications.
Once insomnia begins, various factors can then perpetuate the cycle, including some of those that triggered it, such as anxiety or a school or work schedule. Sometimes it can be difficult to pinpoint the factor prolonging insomnia, such as the unconscious reward of going to work or school late with few or no consequences.
Delayed sleep phase syndrome
Delayed sleep phase syndrome occurs when someone has a delayed onset of melatonin secretion that pushes back the time when they can fall asleep. Melatonin is the neurotransmitter produced by the pineal gland that signals the start of nighttime. Although it has a hereditary component, delayed sleep phase syndrome also can result from a pattern of poor sleep onset and sleeping in on the weekends.
Dr. Spinks-Franklin described the typical cycle: A teen doesn’t go to sleep until after midnight and then wants to sleep in later in the morning. Because they have to wake up early for school, they sleep in on the weekends to try to regain the sleep they lost. Sleeping in pushes their circadian rhythm even later, perpetuating the problem.
Interventions for sleep disorders
The recommended treatment for insomnia is cognitive-behavior therapy for insomnia, for which strong evidence exists. Before seeking cognitive-behavior therapy, however, families can work to improve sleep hygiene and reduce stimuli that contribute to insomnia.
Teens should avoid screens for at least 1 hour before bedtime and avoid caffeine and exercise for at least 4 hours before going to bed. They also need to develop a schedule with a consistent bedtime and wake-up time, including on the weekends. They should avoid sleeping in on the weekends or taking naps during the day, Dr. Spinks-Franklin said.
Delayed sleep phase syndrome is more resistant to treatment and has a high recurrence rate, she said, and it requires commitment from the parent and their child to address it successfully. Teens with this condition also can start with sleep hygiene practices: a consistent wake-up time that they maintain on the weekends and no daytime naps. Phototherapy in the morning can be added to hopefully induce an earlier onset of melatonin release in the evening.
The next step is making changes to the youth’s schedule, particularly evening and/or weekend activities. They can try to gradually advance their biological clock by changing their sleep schedule.
Dr. Spinks-Franklin also briefly addressed the use of over-the-counter melatonin supplements for treating sleep problems. Melatonin can be effective for treating insomnia by improving sleep onset and sleep quality, particularly in children and teens with autism spectrum disorder or ADHD.
Dr. Spinks-Franklin had no disclosures, and her presentation used no outside funding.
NEW ORLEANS – Social media and electronics aren’t the only barriers to a good night’s sleep for teens, according to Adiaha I. A. Spinks-Franklin, MD, MPH, a pediatrician at Texas Children’s Hospital in Houston.
Another half-dozen “sleep enemies” interfere with adolescents’ sleep and can contribute to insomnia or other sleep disorders, she told attendees at the annual meeting of the American Academy of Pediatrics.
Knowing what normal sleep physiology looks like in youth and understanding the most common sleep enemies and sleep-behavior problems can help you use effective interventions to help your patients get the sleep they need, she said.
Infants need the most sleep, about 12-16 hours each 24-hour period, including naps, for those aged 4-12 months. As they grow into toddlerhood and preschool age, children gradually need less: Children aged 1-2 years need 11-14 hours and children aged 3-5 years need 10-13 hours, including naps. By the time children are in school, ages 6-12, they should have dropped their naps and need 9-12 hours a night.
In fact, 75% of high school seniors get less than 8 hours of sleep a day and live with a chronic sleep debt, Dr. Spinks-Franklin said.
Although social media use and electronics in the bedroom – TVs, computers, cell phones, and video games – can certainly contribute to inadequate sleep, a heavy academic load and extracurricular activities can be just as problematic, Dr. Spinks-Franklin said. Teens who work after school also may have difficulty getting enough sleep, especially if they also have to balance a heavier academic load or even one or two extracurricular activities.
Socializing with friends also can interfere with sleep, especially when get-togethers run late; drinking caffeinated drinks in the afternoon onward can make it difficult for adolescents to get the sleep they need as well. Less-modifiable contributors to too little sleep are stress and early school start times, Dr. Spinks-Franklin said.
The two most common sleep problems seen in teens are insomnia and delayed sleep phase syndrome. Addressing these is important because the effects of chronic insufficient sleep can have far-reaching consequences. Obesity and related chronic health conditions are associated with inadequate sleep, as are poor academic performance, poor judgment, poor executive functioning, and mental health disorders like depression.
Short-term effects of insufficient sleep also can be problematic and can exacerbate existing sleep problems, such as sleeping in on the weekends to “catch up” on sleep or drinking more caffeine to try to stay awake during the day. Increased caffeine intake can interfere with non-REM deep sleep, Dr. Spinks-Franklin said, and therefore reduce the quality of sleep even if the person gets the total hours they need.
Insomnia in adolescents
Insomnia can refer to difficulty falling asleep, staying asleep, sleeping for long enough, or getting enough sleep in one period of time even when the opportunity is there. Some people may have no trouble falling asleep, but they wake up too early – before they have had gotten the sleep they need – and cannot return to sleep.
To be insomnia, the problem must occur “despite having enough time available for sleep,” Dr. Spinks-Franklin said. “Patient who restrict the amount of time for sleep due to work or social commitments may have trouble sleeping and daytime sleepiness but do not have insomnia.”
Daytime impairment also is part of the American Academy of Sleep Medicine’s definition of insomnia. The rare teen who doesn’t need as much sleep as average and functions without difficulty during the day does not necessarily have insomnia.
But the impairment may not necessarily just be fatigue or sleepiness. In fact, many of the symptoms are the same as those seen with ADHD.
Daytime consequences of insomnia can include the following:
- Depression, feeling sad or “blue,” or emotional hypersensitivity.
- Mood swings, crankiness, or irritability.
- Difficulty concentrating or paying attention, poor memory, mind wandering, or even inability to sit still.
- Job or school problems, such as not being able to finish homework, not finishing tasks they start, or forgetfulness.
- Difficulty in social situations, such as discomfort with others or problems with friends.
- Daytime sleepiness, even when unable to actually take a nap.
- Behavioral problems, such as hyperactivity, impulsivity, or aggression.
- Frequent mistakes, especially at work, at school, or while driving (often “errors of omission,” such as not seeing a street sign or not hearing an instruction).
- Lower levels of motivation or initiative, feeling less energetic.
- Excessive worry about sleep.
Evaluation of insomnia can be framed with “the three-factor model,” which includes predisposing factors, precipitating factors, and perpetuating factors.
Predisposing factors – those that indicate a person already may be at risk for insomnia – include potential genetic influences as well as their typical response to stress. “Do they sleep more or less?” Dr. Spinks-Franklin said. Even teens predisposed to insomnia may not develop it, however, without a precipitating trigger.
These triggers could include stress, anxiety, poor initial sleep hygiene that becomes a pattern, dietary intake or behaviors (such as drinking caffeine or eating too much or too late in the evening), changes to their schedule, or side effects of medications.
Once insomnia begins, various factors can then perpetuate the cycle, including some of those that triggered it, such as anxiety or a school or work schedule. Sometimes it can be difficult to pinpoint the factor prolonging insomnia, such as the unconscious reward of going to work or school late with few or no consequences.
Delayed sleep phase syndrome
Delayed sleep phase syndrome occurs when someone has a delayed onset of melatonin secretion that pushes back the time when they can fall asleep. Melatonin is the neurotransmitter produced by the pineal gland that signals the start of nighttime. Although it has a hereditary component, delayed sleep phase syndrome also can result from a pattern of poor sleep onset and sleeping in on the weekends.
Dr. Spinks-Franklin described the typical cycle: A teen doesn’t go to sleep until after midnight and then wants to sleep in later in the morning. Because they have to wake up early for school, they sleep in on the weekends to try to regain the sleep they lost. Sleeping in pushes their circadian rhythm even later, perpetuating the problem.
Interventions for sleep disorders
The recommended treatment for insomnia is cognitive-behavior therapy for insomnia, for which strong evidence exists. Before seeking cognitive-behavior therapy, however, families can work to improve sleep hygiene and reduce stimuli that contribute to insomnia.
Teens should avoid screens for at least 1 hour before bedtime and avoid caffeine and exercise for at least 4 hours before going to bed. They also need to develop a schedule with a consistent bedtime and wake-up time, including on the weekends. They should avoid sleeping in on the weekends or taking naps during the day, Dr. Spinks-Franklin said.
Delayed sleep phase syndrome is more resistant to treatment and has a high recurrence rate, she said, and it requires commitment from the parent and their child to address it successfully. Teens with this condition also can start with sleep hygiene practices: a consistent wake-up time that they maintain on the weekends and no daytime naps. Phototherapy in the morning can be added to hopefully induce an earlier onset of melatonin release in the evening.
The next step is making changes to the youth’s schedule, particularly evening and/or weekend activities. They can try to gradually advance their biological clock by changing their sleep schedule.
Dr. Spinks-Franklin also briefly addressed the use of over-the-counter melatonin supplements for treating sleep problems. Melatonin can be effective for treating insomnia by improving sleep onset and sleep quality, particularly in children and teens with autism spectrum disorder or ADHD.
Dr. Spinks-Franklin had no disclosures, and her presentation used no outside funding.
EXPERT ANALYSIS FROM AAP 19
CPAP vs noninvasive ventilation for obesity hypoventilation syndrome
The conventional approach to treat hypoventilation has been to use noninvasive ventilation (NIV), while continuous positive airway pressure (CPAP) that does not augment alveolar ventilation improves gas exchange by maintaining upper airway patency and increasing functional residual capacity. To understand this rationale, it is important to first review the pathophysiology of OHS.
The hallmark of OHS is resting daytime awake arterial PaCO2of 45 mm Hg or greater in an obese patient (BMI > 30 kg/m2) in absence of any other identifiable cause. To recognize why only some but not all obese subjects develop OHS, it is important to understand the different components of pathophysiology that contribute to hypoventilation: (1) obesity-related reduction in functional residual capacity and lung compliance with resultant increase in work of breathing; (2) central hypoventilation related to leptin resistance and reduction in respiratory drive with REM hypoventilation; and (3) upper airway obstruction caused by upper airway fat deposition along with low FRC contributing to pharyngeal airway narrowing and increased airway collapsibility (Masa JF, et al. Eur Respir Rev. 2019; 28:180097).
CPAP vs NIV for OHS
Let us examine some of the studies that have compared the short-term efficacy of CPAP vs NIV in patients with OHS. In a small randomized controlled trial (RCT), the effectiveness of CPAP and NIV was compared in 36 patients with OHS (Piper AJ, et al. Thorax. 2008;63:395). Reduction in PaCO2 at 3 months was similar between the two groups. However, patients with persistent nocturnal desaturation despite optimal CPAP were excluded from the study. In another RCT of 60 patients with OHS who were either in stable condition or after an episode of acute on chronic hypercapnic respiratory failure, the use of CPAP or NIV showed similar improvements at 3 months in daytime PaCO2, quality of life, and sleep parameters (Howard ME, et al. Thorax. 2017;72:437).
In one of the largest randomized control trials, the Spanish Pickwick study randomized 221 patients with OHS and AHI >30/h to NIV, CPAP, and lifestyle modification (Masa JF, et al. Am J Respir Crit Care Med. 2015:192:86). PAP therapy included NIV that consisted of in-lab titration with bilevel PAP therapy targeted to tidal volume 5-6 mL/kg of actual body weight or CPAP. Life style modification served as the control group. Primary outcome was the change in PaCO2 at 2 months. Secondary outcomes were symptoms, HRQOL, polysomnographic parameters, spirometry, and 6-min walk distance (6 MWD). Mean AHI was 69/h, and mean PAP settings for NIV and CPAP were 20/7.7 cm and 11 cm H2O, respectively. NIV provided the greatest improvement in PaCO2 and serum HCO3 as compared with control group but not relative to CPAP group. CPAP improved PaCO2 as compared with control group only after adjustment of PAP use. Spirometry and 6 MWD and some HRQOL measures improved slightly more with NIV as compared to CPAP. Improvement in symptoms and polysomnographic parameters was similar between the two groups.
In another related study by the same group (Masa JF, et al. Thorax. 2016;71:899), 86 patients with OHS and mild OSA (AHI <30/h), were randomized to NIV and lifestyle modification. Mean AHI was 14/h and mean baseline PaCO2 was 49 +/-4 mm Hg. The NIV group with mean PAP adherence at 6 hours showed greater improvement in PaCO2 as compared with lifestyle modification (6 mm vs 2.8 mm Hg). They concluded that NIV was better than lifestyle modification in patients with OHS and mild OSA.
To determine the long-term clinical effectiveness of CPAP vs NIV, patients in the Pickwick study, who were initially assigned to either CPAP or NIV treatment group, were continued on their respective treatments, while subjects in the control group were again randomized at 2 months to either CPAP or NIV (Masa JF, et al. Lancet. 2019;393:1721). All subjects (CPAP n=107; NIV n=97) were followed for a minimum of 3 years. CPAP and NIV settings (pressure-targeted to desired tidal volume) were determined by in-lab titration without transcutaneous CO2 monitor, and daytime adjustment of PAP to improve oxygen saturation. Primary outcome was the number of hospitalization days per year. Mean CPAP was 10.7 cm H2O pressure and NIV 19.7/8.18 cm H2O pressure with an average respiratory rate of 14/min. Median PAP use and adherence > 4 h, respectively, were similar between the two groups (CPAP 6.0 h, adherence > 4 h 67% vs NIV 6.0/h, adherence >4 h 61%). Median duration of follow-up was 5.44 years (IOR 4.45-6.37 years) for both groups. Mean hospitalization days per patient-year were similar between the two groups (CPAP 1.63 vs NIV 1.44 days; adj RR 0.78, 95% CI 0.34-1.77; p=0.561). Overall mortality, adverse cardiovascular events, and arterial blood gas parameters were similar between the two groups, suggesting equal efficacy of CPAP and NIV in this group of stable patients with OHS with an AHI >30/h. Given the low complexity and cost of CPAP vs NIV, the authors concluded that CPAP may be the preferred PAP treatment modality until more studies are available.
An accompanying editorial (Murphy PB, et al. Lancet. 2019; 393:1674), discussed that since this study was powered for superiority as opposed to noninferiority of NIV (20% reduction in hospitalization with NIV when compared with CPAP), superiority could not be shown, due to the low event rate for hospitalization (NIV 1.44 days vs CPAP 1.63 days). It is also possible optimum NIV titration may not have been determined since TCO2 was not used. Furthermore, since this study was done only in patients with OHS and AHI >30/h, these results may not be applicable to patients with OHS and low AHI < 30/h that are more likely to have central hypoventilation and comorbidities, and this group may benefit from NIV as compared with CPAP.
Novel modes of bi-level PAP therapy
There are limited data on the use of the new bi-level PAP modalities, such as volume-targeted pressure support ventilation (PS) with fixed or auto-EPAP. The use of intelligent volume-assured pressure support ventilation (iVAPS) vs standard fixed pressure support ventilation in select OHS patients (n=18) showed equivalent control of chronic respiratory failure with no worsening of sleep quality and better PAP adherence (Kelly JL, et al. Respirology. 2014;19:596). In another small randomized, double-blind, crossover study, done on two consecutive nights in 11 patients with OHS, the use of auto-adjusting EPAP was noninferior to fixed EPAP (10.8 cm vs 11.8 cm H2O pressure), with no differences in sleep quality and patient preference (McArdle N. Sleep. 2017;40:1). Although the data are limited, these small studies suggest the use of new PAP modalities, such as variable PS to deliver target volumes and auto EPAP could offer the potential to initiate bi-level PAP therapy in outpatients without the in-lab titration. More studies are needed before bi-level PAP therapy can be safely initiated in outpatients with OHS.
Summary
In summary, how can we utilize the most effective PAP therapy for patients with OHS? Can we use a phenotype-dependent approach to PAP treatment options? The answer is probably yes. Recently published ATS Clinical Practice Guideline (Am J Respir Crit Care Med. 2019;200:e6-e24) suggests the use of PAP therapy for stable ambulatory patients with OHS as compared with no PAP therapy, and patients with OHS with AHI >30/h (approximately 70% of the OHS patients) can be initially started on CPAP instead of NIV. Patients who have persistent nocturnal desaturation despite optimum CPAP can be switched to NIV. On the other hand, data are limited on the use of CPAP in patients with OHS with AHI <30/h, and these patients can be started on NIV. PAP adherence >5-6 h, and weight loss using a multidisciplinary approach should be encouraged for all patients with OHS.
Dr. Dewan is Professor and Program Director, Sleep Medicine; Division of Pulmonary, Critical Care and Sleep Medicine; Chief, Pulmonary Section VA Medical Center; Creighton University, Omaha, Nebraska.
The conventional approach to treat hypoventilation has been to use noninvasive ventilation (NIV), while continuous positive airway pressure (CPAP) that does not augment alveolar ventilation improves gas exchange by maintaining upper airway patency and increasing functional residual capacity. To understand this rationale, it is important to first review the pathophysiology of OHS.
The hallmark of OHS is resting daytime awake arterial PaCO2of 45 mm Hg or greater in an obese patient (BMI > 30 kg/m2) in absence of any other identifiable cause. To recognize why only some but not all obese subjects develop OHS, it is important to understand the different components of pathophysiology that contribute to hypoventilation: (1) obesity-related reduction in functional residual capacity and lung compliance with resultant increase in work of breathing; (2) central hypoventilation related to leptin resistance and reduction in respiratory drive with REM hypoventilation; and (3) upper airway obstruction caused by upper airway fat deposition along with low FRC contributing to pharyngeal airway narrowing and increased airway collapsibility (Masa JF, et al. Eur Respir Rev. 2019; 28:180097).
CPAP vs NIV for OHS
Let us examine some of the studies that have compared the short-term efficacy of CPAP vs NIV in patients with OHS. In a small randomized controlled trial (RCT), the effectiveness of CPAP and NIV was compared in 36 patients with OHS (Piper AJ, et al. Thorax. 2008;63:395). Reduction in PaCO2 at 3 months was similar between the two groups. However, patients with persistent nocturnal desaturation despite optimal CPAP were excluded from the study. In another RCT of 60 patients with OHS who were either in stable condition or after an episode of acute on chronic hypercapnic respiratory failure, the use of CPAP or NIV showed similar improvements at 3 months in daytime PaCO2, quality of life, and sleep parameters (Howard ME, et al. Thorax. 2017;72:437).
In one of the largest randomized control trials, the Spanish Pickwick study randomized 221 patients with OHS and AHI >30/h to NIV, CPAP, and lifestyle modification (Masa JF, et al. Am J Respir Crit Care Med. 2015:192:86). PAP therapy included NIV that consisted of in-lab titration with bilevel PAP therapy targeted to tidal volume 5-6 mL/kg of actual body weight or CPAP. Life style modification served as the control group. Primary outcome was the change in PaCO2 at 2 months. Secondary outcomes were symptoms, HRQOL, polysomnographic parameters, spirometry, and 6-min walk distance (6 MWD). Mean AHI was 69/h, and mean PAP settings for NIV and CPAP were 20/7.7 cm and 11 cm H2O, respectively. NIV provided the greatest improvement in PaCO2 and serum HCO3 as compared with control group but not relative to CPAP group. CPAP improved PaCO2 as compared with control group only after adjustment of PAP use. Spirometry and 6 MWD and some HRQOL measures improved slightly more with NIV as compared to CPAP. Improvement in symptoms and polysomnographic parameters was similar between the two groups.
In another related study by the same group (Masa JF, et al. Thorax. 2016;71:899), 86 patients with OHS and mild OSA (AHI <30/h), were randomized to NIV and lifestyle modification. Mean AHI was 14/h and mean baseline PaCO2 was 49 +/-4 mm Hg. The NIV group with mean PAP adherence at 6 hours showed greater improvement in PaCO2 as compared with lifestyle modification (6 mm vs 2.8 mm Hg). They concluded that NIV was better than lifestyle modification in patients with OHS and mild OSA.
To determine the long-term clinical effectiveness of CPAP vs NIV, patients in the Pickwick study, who were initially assigned to either CPAP or NIV treatment group, were continued on their respective treatments, while subjects in the control group were again randomized at 2 months to either CPAP or NIV (Masa JF, et al. Lancet. 2019;393:1721). All subjects (CPAP n=107; NIV n=97) were followed for a minimum of 3 years. CPAP and NIV settings (pressure-targeted to desired tidal volume) were determined by in-lab titration without transcutaneous CO2 monitor, and daytime adjustment of PAP to improve oxygen saturation. Primary outcome was the number of hospitalization days per year. Mean CPAP was 10.7 cm H2O pressure and NIV 19.7/8.18 cm H2O pressure with an average respiratory rate of 14/min. Median PAP use and adherence > 4 h, respectively, were similar between the two groups (CPAP 6.0 h, adherence > 4 h 67% vs NIV 6.0/h, adherence >4 h 61%). Median duration of follow-up was 5.44 years (IOR 4.45-6.37 years) for both groups. Mean hospitalization days per patient-year were similar between the two groups (CPAP 1.63 vs NIV 1.44 days; adj RR 0.78, 95% CI 0.34-1.77; p=0.561). Overall mortality, adverse cardiovascular events, and arterial blood gas parameters were similar between the two groups, suggesting equal efficacy of CPAP and NIV in this group of stable patients with OHS with an AHI >30/h. Given the low complexity and cost of CPAP vs NIV, the authors concluded that CPAP may be the preferred PAP treatment modality until more studies are available.
An accompanying editorial (Murphy PB, et al. Lancet. 2019; 393:1674), discussed that since this study was powered for superiority as opposed to noninferiority of NIV (20% reduction in hospitalization with NIV when compared with CPAP), superiority could not be shown, due to the low event rate for hospitalization (NIV 1.44 days vs CPAP 1.63 days). It is also possible optimum NIV titration may not have been determined since TCO2 was not used. Furthermore, since this study was done only in patients with OHS and AHI >30/h, these results may not be applicable to patients with OHS and low AHI < 30/h that are more likely to have central hypoventilation and comorbidities, and this group may benefit from NIV as compared with CPAP.
Novel modes of bi-level PAP therapy
There are limited data on the use of the new bi-level PAP modalities, such as volume-targeted pressure support ventilation (PS) with fixed or auto-EPAP. The use of intelligent volume-assured pressure support ventilation (iVAPS) vs standard fixed pressure support ventilation in select OHS patients (n=18) showed equivalent control of chronic respiratory failure with no worsening of sleep quality and better PAP adherence (Kelly JL, et al. Respirology. 2014;19:596). In another small randomized, double-blind, crossover study, done on two consecutive nights in 11 patients with OHS, the use of auto-adjusting EPAP was noninferior to fixed EPAP (10.8 cm vs 11.8 cm H2O pressure), with no differences in sleep quality and patient preference (McArdle N. Sleep. 2017;40:1). Although the data are limited, these small studies suggest the use of new PAP modalities, such as variable PS to deliver target volumes and auto EPAP could offer the potential to initiate bi-level PAP therapy in outpatients without the in-lab titration. More studies are needed before bi-level PAP therapy can be safely initiated in outpatients with OHS.
Summary
In summary, how can we utilize the most effective PAP therapy for patients with OHS? Can we use a phenotype-dependent approach to PAP treatment options? The answer is probably yes. Recently published ATS Clinical Practice Guideline (Am J Respir Crit Care Med. 2019;200:e6-e24) suggests the use of PAP therapy for stable ambulatory patients with OHS as compared with no PAP therapy, and patients with OHS with AHI >30/h (approximately 70% of the OHS patients) can be initially started on CPAP instead of NIV. Patients who have persistent nocturnal desaturation despite optimum CPAP can be switched to NIV. On the other hand, data are limited on the use of CPAP in patients with OHS with AHI <30/h, and these patients can be started on NIV. PAP adherence >5-6 h, and weight loss using a multidisciplinary approach should be encouraged for all patients with OHS.
Dr. Dewan is Professor and Program Director, Sleep Medicine; Division of Pulmonary, Critical Care and Sleep Medicine; Chief, Pulmonary Section VA Medical Center; Creighton University, Omaha, Nebraska.
The conventional approach to treat hypoventilation has been to use noninvasive ventilation (NIV), while continuous positive airway pressure (CPAP) that does not augment alveolar ventilation improves gas exchange by maintaining upper airway patency and increasing functional residual capacity. To understand this rationale, it is important to first review the pathophysiology of OHS.
The hallmark of OHS is resting daytime awake arterial PaCO2of 45 mm Hg or greater in an obese patient (BMI > 30 kg/m2) in absence of any other identifiable cause. To recognize why only some but not all obese subjects develop OHS, it is important to understand the different components of pathophysiology that contribute to hypoventilation: (1) obesity-related reduction in functional residual capacity and lung compliance with resultant increase in work of breathing; (2) central hypoventilation related to leptin resistance and reduction in respiratory drive with REM hypoventilation; and (3) upper airway obstruction caused by upper airway fat deposition along with low FRC contributing to pharyngeal airway narrowing and increased airway collapsibility (Masa JF, et al. Eur Respir Rev. 2019; 28:180097).
CPAP vs NIV for OHS
Let us examine some of the studies that have compared the short-term efficacy of CPAP vs NIV in patients with OHS. In a small randomized controlled trial (RCT), the effectiveness of CPAP and NIV was compared in 36 patients with OHS (Piper AJ, et al. Thorax. 2008;63:395). Reduction in PaCO2 at 3 months was similar between the two groups. However, patients with persistent nocturnal desaturation despite optimal CPAP were excluded from the study. In another RCT of 60 patients with OHS who were either in stable condition or after an episode of acute on chronic hypercapnic respiratory failure, the use of CPAP or NIV showed similar improvements at 3 months in daytime PaCO2, quality of life, and sleep parameters (Howard ME, et al. Thorax. 2017;72:437).
In one of the largest randomized control trials, the Spanish Pickwick study randomized 221 patients with OHS and AHI >30/h to NIV, CPAP, and lifestyle modification (Masa JF, et al. Am J Respir Crit Care Med. 2015:192:86). PAP therapy included NIV that consisted of in-lab titration with bilevel PAP therapy targeted to tidal volume 5-6 mL/kg of actual body weight or CPAP. Life style modification served as the control group. Primary outcome was the change in PaCO2 at 2 months. Secondary outcomes were symptoms, HRQOL, polysomnographic parameters, spirometry, and 6-min walk distance (6 MWD). Mean AHI was 69/h, and mean PAP settings for NIV and CPAP were 20/7.7 cm and 11 cm H2O, respectively. NIV provided the greatest improvement in PaCO2 and serum HCO3 as compared with control group but not relative to CPAP group. CPAP improved PaCO2 as compared with control group only after adjustment of PAP use. Spirometry and 6 MWD and some HRQOL measures improved slightly more with NIV as compared to CPAP. Improvement in symptoms and polysomnographic parameters was similar between the two groups.
In another related study by the same group (Masa JF, et al. Thorax. 2016;71:899), 86 patients with OHS and mild OSA (AHI <30/h), were randomized to NIV and lifestyle modification. Mean AHI was 14/h and mean baseline PaCO2 was 49 +/-4 mm Hg. The NIV group with mean PAP adherence at 6 hours showed greater improvement in PaCO2 as compared with lifestyle modification (6 mm vs 2.8 mm Hg). They concluded that NIV was better than lifestyle modification in patients with OHS and mild OSA.
To determine the long-term clinical effectiveness of CPAP vs NIV, patients in the Pickwick study, who were initially assigned to either CPAP or NIV treatment group, were continued on their respective treatments, while subjects in the control group were again randomized at 2 months to either CPAP or NIV (Masa JF, et al. Lancet. 2019;393:1721). All subjects (CPAP n=107; NIV n=97) were followed for a minimum of 3 years. CPAP and NIV settings (pressure-targeted to desired tidal volume) were determined by in-lab titration without transcutaneous CO2 monitor, and daytime adjustment of PAP to improve oxygen saturation. Primary outcome was the number of hospitalization days per year. Mean CPAP was 10.7 cm H2O pressure and NIV 19.7/8.18 cm H2O pressure with an average respiratory rate of 14/min. Median PAP use and adherence > 4 h, respectively, were similar between the two groups (CPAP 6.0 h, adherence > 4 h 67% vs NIV 6.0/h, adherence >4 h 61%). Median duration of follow-up was 5.44 years (IOR 4.45-6.37 years) for both groups. Mean hospitalization days per patient-year were similar between the two groups (CPAP 1.63 vs NIV 1.44 days; adj RR 0.78, 95% CI 0.34-1.77; p=0.561). Overall mortality, adverse cardiovascular events, and arterial blood gas parameters were similar between the two groups, suggesting equal efficacy of CPAP and NIV in this group of stable patients with OHS with an AHI >30/h. Given the low complexity and cost of CPAP vs NIV, the authors concluded that CPAP may be the preferred PAP treatment modality until more studies are available.
An accompanying editorial (Murphy PB, et al. Lancet. 2019; 393:1674), discussed that since this study was powered for superiority as opposed to noninferiority of NIV (20% reduction in hospitalization with NIV when compared with CPAP), superiority could not be shown, due to the low event rate for hospitalization (NIV 1.44 days vs CPAP 1.63 days). It is also possible optimum NIV titration may not have been determined since TCO2 was not used. Furthermore, since this study was done only in patients with OHS and AHI >30/h, these results may not be applicable to patients with OHS and low AHI < 30/h that are more likely to have central hypoventilation and comorbidities, and this group may benefit from NIV as compared with CPAP.
Novel modes of bi-level PAP therapy
There are limited data on the use of the new bi-level PAP modalities, such as volume-targeted pressure support ventilation (PS) with fixed or auto-EPAP. The use of intelligent volume-assured pressure support ventilation (iVAPS) vs standard fixed pressure support ventilation in select OHS patients (n=18) showed equivalent control of chronic respiratory failure with no worsening of sleep quality and better PAP adherence (Kelly JL, et al. Respirology. 2014;19:596). In another small randomized, double-blind, crossover study, done on two consecutive nights in 11 patients with OHS, the use of auto-adjusting EPAP was noninferior to fixed EPAP (10.8 cm vs 11.8 cm H2O pressure), with no differences in sleep quality and patient preference (McArdle N. Sleep. 2017;40:1). Although the data are limited, these small studies suggest the use of new PAP modalities, such as variable PS to deliver target volumes and auto EPAP could offer the potential to initiate bi-level PAP therapy in outpatients without the in-lab titration. More studies are needed before bi-level PAP therapy can be safely initiated in outpatients with OHS.
Summary
In summary, how can we utilize the most effective PAP therapy for patients with OHS? Can we use a phenotype-dependent approach to PAP treatment options? The answer is probably yes. Recently published ATS Clinical Practice Guideline (Am J Respir Crit Care Med. 2019;200:e6-e24) suggests the use of PAP therapy for stable ambulatory patients with OHS as compared with no PAP therapy, and patients with OHS with AHI >30/h (approximately 70% of the OHS patients) can be initially started on CPAP instead of NIV. Patients who have persistent nocturnal desaturation despite optimum CPAP can be switched to NIV. On the other hand, data are limited on the use of CPAP in patients with OHS with AHI <30/h, and these patients can be started on NIV. PAP adherence >5-6 h, and weight loss using a multidisciplinary approach should be encouraged for all patients with OHS.
Dr. Dewan is Professor and Program Director, Sleep Medicine; Division of Pulmonary, Critical Care and Sleep Medicine; Chief, Pulmonary Section VA Medical Center; Creighton University, Omaha, Nebraska.