User login
Severe early-life respiratory infections heighten pediatric OSA risk
AIRWAYS DISORDERS NETWORK
Pediatric Chest Medicine Section
Children with severe lower respiratory tract infections (LRTIs) within the first 2 years of life had a 2.06-fold increased risk of developing pediatric OSA by age 5, according to a study comparing patients hospitalized with LRTI to controls without severe LRTI.1 Prior studies linked LRTI and OSA, but the impact of LRTI severity was unknown.2,3,4 They used Kaplan-Meier survival estimates and Cox proportional hazards models to evaluate the risk of OSA.
Compared with patients with severe LRTIs, controls were more likely to have been full-term births, delivered vaginally, and breastfed. The OSA rate was significantly higher among children with severe LRTIs compared with controls (14.7% vs 6.8%). In the adjusted model controlling for relevant maternal and infant covariables, severe LRTI was significantly associated with increased OSA risk (HR, 2.06; 95% CI, 1.41-3.02; P < .001). Other factors such as prematurity (HR, 1.34; 95% CI, 1.01-1.77; P = .039) and maternal obesity (HR, 1.82; 95% CI, 1.32-2.52; P < .001) were also associated with increased OSA risk.
Maria Gutierrez, MD, of the Division of Pediatric Allergy, Immunology, and Rheumatology at Johns Hopkins University School of Medicine in Baltimore led the research. The study was published in Pediatric Pulmonology (2023 Dec 2. doi: 10.1002/ppul.26810). Study limitations included the use of electronic medical record data and potential lack of generalizability. The BBC is supported by the NIH.
– Agnes S. Montgomery, MD
Fellow-in-Training
References
1. Gayoso-Liviac MG, Nino G, Montgomery AS, Hong X, Wang X, Gutierrez MJ. Infants hospitalized with lower respiratory tract infections during the first two years of life have increased risk of pediatric obstructive sleep apnea. Pediatr Pulmonol. 2024;59:679-687.
2. Snow A, Dayyat E, Montgomery‐Downs HE, Kheirandish‐Gozal L, Gozal D. Pediatric obstructive sleep apnea: a potential late consequence of respiratory syncytial virus bronchiolitis. Pediatr Pulmonol. 2009;44(12):1186‐1191.
3. Chen VC‐H, Yang Y‐H, Kuo T‐Y, et al. Increased incidence of obstructive sleep apnea in hospitalized children after enterovirus infection: a nationwide population‐based cohort study. Pediatr Infect Dis J. 2018;37(9):872‐879.
4. Gutierrez MJ, Nino G, Landeo‐Gutierrez JS, et al. Lower respiratory tract infections in early life are associated with obstructive sleep apnea diagnosis during childhood in a large birth cohort. Sleep. 2021;44:12.
AIRWAYS DISORDERS NETWORK
Pediatric Chest Medicine Section
Children with severe lower respiratory tract infections (LRTIs) within the first 2 years of life had a 2.06-fold increased risk of developing pediatric OSA by age 5, according to a study comparing patients hospitalized with LRTI to controls without severe LRTI.1 Prior studies linked LRTI and OSA, but the impact of LRTI severity was unknown.2,3,4 They used Kaplan-Meier survival estimates and Cox proportional hazards models to evaluate the risk of OSA.
Compared with patients with severe LRTIs, controls were more likely to have been full-term births, delivered vaginally, and breastfed. The OSA rate was significantly higher among children with severe LRTIs compared with controls (14.7% vs 6.8%). In the adjusted model controlling for relevant maternal and infant covariables, severe LRTI was significantly associated with increased OSA risk (HR, 2.06; 95% CI, 1.41-3.02; P < .001). Other factors such as prematurity (HR, 1.34; 95% CI, 1.01-1.77; P = .039) and maternal obesity (HR, 1.82; 95% CI, 1.32-2.52; P < .001) were also associated with increased OSA risk.
Maria Gutierrez, MD, of the Division of Pediatric Allergy, Immunology, and Rheumatology at Johns Hopkins University School of Medicine in Baltimore led the research. The study was published in Pediatric Pulmonology (2023 Dec 2. doi: 10.1002/ppul.26810). Study limitations included the use of electronic medical record data and potential lack of generalizability. The BBC is supported by the NIH.
– Agnes S. Montgomery, MD
Fellow-in-Training
References
1. Gayoso-Liviac MG, Nino G, Montgomery AS, Hong X, Wang X, Gutierrez MJ. Infants hospitalized with lower respiratory tract infections during the first two years of life have increased risk of pediatric obstructive sleep apnea. Pediatr Pulmonol. 2024;59:679-687.
2. Snow A, Dayyat E, Montgomery‐Downs HE, Kheirandish‐Gozal L, Gozal D. Pediatric obstructive sleep apnea: a potential late consequence of respiratory syncytial virus bronchiolitis. Pediatr Pulmonol. 2009;44(12):1186‐1191.
3. Chen VC‐H, Yang Y‐H, Kuo T‐Y, et al. Increased incidence of obstructive sleep apnea in hospitalized children after enterovirus infection: a nationwide population‐based cohort study. Pediatr Infect Dis J. 2018;37(9):872‐879.
4. Gutierrez MJ, Nino G, Landeo‐Gutierrez JS, et al. Lower respiratory tract infections in early life are associated with obstructive sleep apnea diagnosis during childhood in a large birth cohort. Sleep. 2021;44:12.
AIRWAYS DISORDERS NETWORK
Pediatric Chest Medicine Section
Children with severe lower respiratory tract infections (LRTIs) within the first 2 years of life had a 2.06-fold increased risk of developing pediatric OSA by age 5, according to a study comparing patients hospitalized with LRTI to controls without severe LRTI.1 Prior studies linked LRTI and OSA, but the impact of LRTI severity was unknown.2,3,4 They used Kaplan-Meier survival estimates and Cox proportional hazards models to evaluate the risk of OSA.
Compared with patients with severe LRTIs, controls were more likely to have been full-term births, delivered vaginally, and breastfed. The OSA rate was significantly higher among children with severe LRTIs compared with controls (14.7% vs 6.8%). In the adjusted model controlling for relevant maternal and infant covariables, severe LRTI was significantly associated with increased OSA risk (HR, 2.06; 95% CI, 1.41-3.02; P < .001). Other factors such as prematurity (HR, 1.34; 95% CI, 1.01-1.77; P = .039) and maternal obesity (HR, 1.82; 95% CI, 1.32-2.52; P < .001) were also associated with increased OSA risk.
Maria Gutierrez, MD, of the Division of Pediatric Allergy, Immunology, and Rheumatology at Johns Hopkins University School of Medicine in Baltimore led the research. The study was published in Pediatric Pulmonology (2023 Dec 2. doi: 10.1002/ppul.26810). Study limitations included the use of electronic medical record data and potential lack of generalizability. The BBC is supported by the NIH.
– Agnes S. Montgomery, MD
Fellow-in-Training
References
1. Gayoso-Liviac MG, Nino G, Montgomery AS, Hong X, Wang X, Gutierrez MJ. Infants hospitalized with lower respiratory tract infections during the first two years of life have increased risk of pediatric obstructive sleep apnea. Pediatr Pulmonol. 2024;59:679-687.
2. Snow A, Dayyat E, Montgomery‐Downs HE, Kheirandish‐Gozal L, Gozal D. Pediatric obstructive sleep apnea: a potential late consequence of respiratory syncytial virus bronchiolitis. Pediatr Pulmonol. 2009;44(12):1186‐1191.
3. Chen VC‐H, Yang Y‐H, Kuo T‐Y, et al. Increased incidence of obstructive sleep apnea in hospitalized children after enterovirus infection: a nationwide population‐based cohort study. Pediatr Infect Dis J. 2018;37(9):872‐879.
4. Gutierrez MJ, Nino G, Landeo‐Gutierrez JS, et al. Lower respiratory tract infections in early life are associated with obstructive sleep apnea diagnosis during childhood in a large birth cohort. Sleep. 2021;44:12.
NIH to Begin Long COVID Trial Focused on Sleep, Exercise
The National institutes of Health will soon start a clinical trial in an attempt to find potential treatments for symptoms of long COVID, focusing on sleep disturbances, problems with exercise, and post-exertional malaise.
“When people can’t get reliable sleep, can’t exert themselves and feel sick following tasks that used to be simple, the physical and mental anguish can lead to feelings of utter helplessness,” Walter J. Koroshetz, MD, director of the NIH’s National Institute of Neurological Disorders and Stroke, said in a statement. “We urgently need to come up with answers to help those struggling with long COVID feel whole again.”
The new trials will be part of the NIH’s Researching COVID to Enhance Recovery initiative, known as RECOVER. Since beginning enrollment in July 2023 for four trials, RECOVER now features eight trials across the country looking at all parts of long COVID. RECOVER is part of a $1.15 billion nationwide program that Congress approved in 2020 for the NIH to research and test treatments for long COVID.
While focused on sleep disturbances, the trial will test two Food and Drug Administration–approved drugs currently used to treat people with hypersomnia. There will also be a trial to test if melatonin helps people with long COVID-related sleep problems. Light therapy will also be tested.
The trials that deal with problems people have had with exercise will focus on personalized cardiopulmonary rehabilitation, where patients experiment with exercise training, strength and flexibility training, education, and social support.
Another trial will look at structured pacing, which is designed to help people with exercise problems identify, control, and ease long COVID symptoms.
A version of this article appeared on WebMD.com.
The National institutes of Health will soon start a clinical trial in an attempt to find potential treatments for symptoms of long COVID, focusing on sleep disturbances, problems with exercise, and post-exertional malaise.
“When people can’t get reliable sleep, can’t exert themselves and feel sick following tasks that used to be simple, the physical and mental anguish can lead to feelings of utter helplessness,” Walter J. Koroshetz, MD, director of the NIH’s National Institute of Neurological Disorders and Stroke, said in a statement. “We urgently need to come up with answers to help those struggling with long COVID feel whole again.”
The new trials will be part of the NIH’s Researching COVID to Enhance Recovery initiative, known as RECOVER. Since beginning enrollment in July 2023 for four trials, RECOVER now features eight trials across the country looking at all parts of long COVID. RECOVER is part of a $1.15 billion nationwide program that Congress approved in 2020 for the NIH to research and test treatments for long COVID.
While focused on sleep disturbances, the trial will test two Food and Drug Administration–approved drugs currently used to treat people with hypersomnia. There will also be a trial to test if melatonin helps people with long COVID-related sleep problems. Light therapy will also be tested.
The trials that deal with problems people have had with exercise will focus on personalized cardiopulmonary rehabilitation, where patients experiment with exercise training, strength and flexibility training, education, and social support.
Another trial will look at structured pacing, which is designed to help people with exercise problems identify, control, and ease long COVID symptoms.
A version of this article appeared on WebMD.com.
The National institutes of Health will soon start a clinical trial in an attempt to find potential treatments for symptoms of long COVID, focusing on sleep disturbances, problems with exercise, and post-exertional malaise.
“When people can’t get reliable sleep, can’t exert themselves and feel sick following tasks that used to be simple, the physical and mental anguish can lead to feelings of utter helplessness,” Walter J. Koroshetz, MD, director of the NIH’s National Institute of Neurological Disorders and Stroke, said in a statement. “We urgently need to come up with answers to help those struggling with long COVID feel whole again.”
The new trials will be part of the NIH’s Researching COVID to Enhance Recovery initiative, known as RECOVER. Since beginning enrollment in July 2023 for four trials, RECOVER now features eight trials across the country looking at all parts of long COVID. RECOVER is part of a $1.15 billion nationwide program that Congress approved in 2020 for the NIH to research and test treatments for long COVID.
While focused on sleep disturbances, the trial will test two Food and Drug Administration–approved drugs currently used to treat people with hypersomnia. There will also be a trial to test if melatonin helps people with long COVID-related sleep problems. Light therapy will also be tested.
The trials that deal with problems people have had with exercise will focus on personalized cardiopulmonary rehabilitation, where patients experiment with exercise training, strength and flexibility training, education, and social support.
Another trial will look at structured pacing, which is designed to help people with exercise problems identify, control, and ease long COVID symptoms.
A version of this article appeared on WebMD.com.
Healthy Sleep Linked to Lower Odds for Digestive Diseases
TOPLINE:
Healthier sleep is associated with lower odds of developing a wide range of gastrointestinal conditions, regardless of genetic susceptibility, new research revealed.
METHODOLOGY:
- Due to the widespread prevalence of sleep issues and a growing burden of digestive diseases globally, researchers investigated the association between sleep quality and digestive disorders in a prospective cohort study of 410,586 people in the UK Biobank.
- Five individual sleep behaviors were assessed: sleep duration, insomnia, snoring, daytime sleepiness, and chronotype.
- A healthy sleep was defined as a morning chronotype, 7-8 hours of sleep duration, no self-reported snoring, never or rare insomnia, and a low frequency of daytime sleepiness, for a score of 5/5.
- The study investigators tracked the development of 16 digestive diseases over a mean period of 13.2 years.
- As well as looking at healthy sleep scores, researchers considered genetic susceptibility to gastrointestinal conditions.
TAKEAWAY:
- Of the 16 digestive diseases looked at, the reduction of risk was highest for irritable bowel syndrome at 50% (HR, 0.50; 95% CI, 0.45-0.57).
- A healthy sleep score was also associated with 37% reduced odds for metabolic dysfunction–associated steatotic liver disease (formerly known as nonalcoholic fatty liver disease; HR, 0.63; 95% CI, 0.55-0.71), 35% lower chance for peptic ulcer (HR, 0.65; 95% CI, 0.058-0.74), 34% reduced chance for dyspepsia (HR, 0.66; 95% CI, 0.58-0.75), and a 25% lower risk for diverticulosis (HR, 0.75; 95% CI, 0.71-0.80).
- High genetic risk and poor sleep scores were also associated with increased odds (53% to > 200%) of developing digestive diseases.
- However, healthy sleep reduced the risk for digestive diseases regardless of genetic susceptibility.
IN PRACTICE:
“Our findings underscore the potential holistic impact of different sleep behaviors in mitigating the risk of digestive diseases in clinical practice,” wrote Shiyi Yu, MD, of Guangdong Provincial People’s Hospital, Guangzhou, Guangdong, China, and colleagues.
Poor sleep can also change our gut microbiome, Dr. Yu told this news organization. If you don’t sleep well, the repair of the gut lining cannot be finished during the night.
SOURCE:
The study was presented at the Digestive Disease Week® (DDW), 2024, annual meeting.
DISCLOSURES:
Dr. Yu had no relevant financial disclosures.
A version of this article appeared on Medscape.com.
TOPLINE:
Healthier sleep is associated with lower odds of developing a wide range of gastrointestinal conditions, regardless of genetic susceptibility, new research revealed.
METHODOLOGY:
- Due to the widespread prevalence of sleep issues and a growing burden of digestive diseases globally, researchers investigated the association between sleep quality and digestive disorders in a prospective cohort study of 410,586 people in the UK Biobank.
- Five individual sleep behaviors were assessed: sleep duration, insomnia, snoring, daytime sleepiness, and chronotype.
- A healthy sleep was defined as a morning chronotype, 7-8 hours of sleep duration, no self-reported snoring, never or rare insomnia, and a low frequency of daytime sleepiness, for a score of 5/5.
- The study investigators tracked the development of 16 digestive diseases over a mean period of 13.2 years.
- As well as looking at healthy sleep scores, researchers considered genetic susceptibility to gastrointestinal conditions.
TAKEAWAY:
- Of the 16 digestive diseases looked at, the reduction of risk was highest for irritable bowel syndrome at 50% (HR, 0.50; 95% CI, 0.45-0.57).
- A healthy sleep score was also associated with 37% reduced odds for metabolic dysfunction–associated steatotic liver disease (formerly known as nonalcoholic fatty liver disease; HR, 0.63; 95% CI, 0.55-0.71), 35% lower chance for peptic ulcer (HR, 0.65; 95% CI, 0.058-0.74), 34% reduced chance for dyspepsia (HR, 0.66; 95% CI, 0.58-0.75), and a 25% lower risk for diverticulosis (HR, 0.75; 95% CI, 0.71-0.80).
- High genetic risk and poor sleep scores were also associated with increased odds (53% to > 200%) of developing digestive diseases.
- However, healthy sleep reduced the risk for digestive diseases regardless of genetic susceptibility.
IN PRACTICE:
“Our findings underscore the potential holistic impact of different sleep behaviors in mitigating the risk of digestive diseases in clinical practice,” wrote Shiyi Yu, MD, of Guangdong Provincial People’s Hospital, Guangzhou, Guangdong, China, and colleagues.
Poor sleep can also change our gut microbiome, Dr. Yu told this news organization. If you don’t sleep well, the repair of the gut lining cannot be finished during the night.
SOURCE:
The study was presented at the Digestive Disease Week® (DDW), 2024, annual meeting.
DISCLOSURES:
Dr. Yu had no relevant financial disclosures.
A version of this article appeared on Medscape.com.
TOPLINE:
Healthier sleep is associated with lower odds of developing a wide range of gastrointestinal conditions, regardless of genetic susceptibility, new research revealed.
METHODOLOGY:
- Due to the widespread prevalence of sleep issues and a growing burden of digestive diseases globally, researchers investigated the association between sleep quality and digestive disorders in a prospective cohort study of 410,586 people in the UK Biobank.
- Five individual sleep behaviors were assessed: sleep duration, insomnia, snoring, daytime sleepiness, and chronotype.
- A healthy sleep was defined as a morning chronotype, 7-8 hours of sleep duration, no self-reported snoring, never or rare insomnia, and a low frequency of daytime sleepiness, for a score of 5/5.
- The study investigators tracked the development of 16 digestive diseases over a mean period of 13.2 years.
- As well as looking at healthy sleep scores, researchers considered genetic susceptibility to gastrointestinal conditions.
TAKEAWAY:
- Of the 16 digestive diseases looked at, the reduction of risk was highest for irritable bowel syndrome at 50% (HR, 0.50; 95% CI, 0.45-0.57).
- A healthy sleep score was also associated with 37% reduced odds for metabolic dysfunction–associated steatotic liver disease (formerly known as nonalcoholic fatty liver disease; HR, 0.63; 95% CI, 0.55-0.71), 35% lower chance for peptic ulcer (HR, 0.65; 95% CI, 0.058-0.74), 34% reduced chance for dyspepsia (HR, 0.66; 95% CI, 0.58-0.75), and a 25% lower risk for diverticulosis (HR, 0.75; 95% CI, 0.71-0.80).
- High genetic risk and poor sleep scores were also associated with increased odds (53% to > 200%) of developing digestive diseases.
- However, healthy sleep reduced the risk for digestive diseases regardless of genetic susceptibility.
IN PRACTICE:
“Our findings underscore the potential holistic impact of different sleep behaviors in mitigating the risk of digestive diseases in clinical practice,” wrote Shiyi Yu, MD, of Guangdong Provincial People’s Hospital, Guangzhou, Guangdong, China, and colleagues.
Poor sleep can also change our gut microbiome, Dr. Yu told this news organization. If you don’t sleep well, the repair of the gut lining cannot be finished during the night.
SOURCE:
The study was presented at the Digestive Disease Week® (DDW), 2024, annual meeting.
DISCLOSURES:
Dr. Yu had no relevant financial disclosures.
A version of this article appeared on Medscape.com.
CPAP Underperforms: The Sequel
A few months ago, I posted a column on continuous positive airway pressure (CPAP) with the title, “CPAP Oversells and Underperforms.” To date, it has 299 likes and 90 comments, which are almost all negative. I’m glad to see that it’s generated interest, and I’d like to address some of the themes expressed in the posts.
Most comments were personal testimonies to the miracles of CPAP. These are important, and the point deserves emphasis. CPAP can provide significant improvements in daytime sleepiness and quality of life. I closed the original piece by acknowledging this important fact. Readers can be forgiven for missing it given that the title and text were otherwise disparaging of CPAP.
But several comments warrant a more in-depth discussion. The original piece focuses on CPAP and cardiovascular (CV) outcomes but made no mention of atrial fibrillation (AF) or ejection fraction (EF). The effects of CPAP on each are touted by cardiologists and PAP-pushers alike and are drivers of frequent referrals. It›s my fault for omitting them from the discussion.
AF is easy. The data is identical to all other things CPAP and CV. Based on biologic plausibility alone, the likelihood of a relationship between AF and obstructive sleep apnea (OSA) is similar to the odds that the Celtics raise an 18th banner come June. There’s hypoxia, intrathoracic pressure swings, sympathetic surges, and sleep state disruptions. It’s easy to get from there to arrhythmogenesis. There’s lots of observational noise, too, but no randomized proof that CPAP alters this relationship.
I found four randomized controlled trials (RCTs) that tested CPAP’s effect on AF. I’ll save you the suspense; they were all negative. One even found a signal for more adverse events in the CPAP group. These studies have several positive qualities: They enrolled patients with moderate to severe sleep apnea and high oxygen desaturation indices, adherence averaged more than 4 hours across all groups in all trials, and the methods for assessing the AF outcomes differed slightly. There’s also a lot not to like: The sample sizes were small, only one trial enrolled “sleepy” patients (as assessed by the Epworth Sleepiness Score), and follow-up was short.
To paraphrase Carl Sagan, “absence of evidence does not equal evidence of absence.” As a statistician would say, type II error cannot be excluded by these RCTs. In medicine, however, the burden of proof falls on demonstrating efficacy. If we treat before concluding that a therapy works, we risk wasting time, money, medical resources, and the most precious of patient commodities: the energy required for behavior change. In their response to letters to the editor, the authors of the third RCT summarize the CPAP, AF, and CV disease data far better than I ever could. They sound the same words of caution and come out against screening patients with AF for OSA.
The story for CPAP’s effects on EF is similar though muddier. The American College of Cardiology (ACC)/American Heart Association (AHA) guidelines for heart failure cite a meta-analysis showing that CPAP improves left ventricular EF. In 2019, the American Academy of Sleep Medicine (AASM) CPAP guidelines included a systematic review and meta-analysis that found that CPAP has no effect on left ventricular EF in patients with or without heart failure.
There are a million reasons why two systematic reviews on the same topic might come to different conclusions. In this case, the included studies only partially overlap, and broadly speaking, it appears the authors made trade-offs. The review cited by the ACC/AHA had broader inclusion and significantly more patients and paid for it in heterogeneity (I2 in the 80%-90% range). The AASM analysis achieved 0% heterogeneity but limited inclusion to fewer than 100 patients. Across both, the improvement in EF was 2%- 5% at a minimally clinically important difference of 4%. Hardly convincing.
In summary, the road to negative trials and patient harm has always been paved with observational signal and biologic plausibility. Throw in some intellectual and academic bias, and you’ve created the perfect storm of therapeutic overconfidence.
Dr. Holley is a professor in the department of medicine, Uniformed Services University, Bethesda, Maryland, and a physician at Pulmonary/Sleep and Critical Care Medicine, MedStar Washington Hospital Center, Washington. He disclosed ties to Metapharm Inc., CHEST College, and WebMD.
A version of this article appeared on Medscape.com .
A few months ago, I posted a column on continuous positive airway pressure (CPAP) with the title, “CPAP Oversells and Underperforms.” To date, it has 299 likes and 90 comments, which are almost all negative. I’m glad to see that it’s generated interest, and I’d like to address some of the themes expressed in the posts.
Most comments were personal testimonies to the miracles of CPAP. These are important, and the point deserves emphasis. CPAP can provide significant improvements in daytime sleepiness and quality of life. I closed the original piece by acknowledging this important fact. Readers can be forgiven for missing it given that the title and text were otherwise disparaging of CPAP.
But several comments warrant a more in-depth discussion. The original piece focuses on CPAP and cardiovascular (CV) outcomes but made no mention of atrial fibrillation (AF) or ejection fraction (EF). The effects of CPAP on each are touted by cardiologists and PAP-pushers alike and are drivers of frequent referrals. It›s my fault for omitting them from the discussion.
AF is easy. The data is identical to all other things CPAP and CV. Based on biologic plausibility alone, the likelihood of a relationship between AF and obstructive sleep apnea (OSA) is similar to the odds that the Celtics raise an 18th banner come June. There’s hypoxia, intrathoracic pressure swings, sympathetic surges, and sleep state disruptions. It’s easy to get from there to arrhythmogenesis. There’s lots of observational noise, too, but no randomized proof that CPAP alters this relationship.
I found four randomized controlled trials (RCTs) that tested CPAP’s effect on AF. I’ll save you the suspense; they were all negative. One even found a signal for more adverse events in the CPAP group. These studies have several positive qualities: They enrolled patients with moderate to severe sleep apnea and high oxygen desaturation indices, adherence averaged more than 4 hours across all groups in all trials, and the methods for assessing the AF outcomes differed slightly. There’s also a lot not to like: The sample sizes were small, only one trial enrolled “sleepy” patients (as assessed by the Epworth Sleepiness Score), and follow-up was short.
To paraphrase Carl Sagan, “absence of evidence does not equal evidence of absence.” As a statistician would say, type II error cannot be excluded by these RCTs. In medicine, however, the burden of proof falls on demonstrating efficacy. If we treat before concluding that a therapy works, we risk wasting time, money, medical resources, and the most precious of patient commodities: the energy required for behavior change. In their response to letters to the editor, the authors of the third RCT summarize the CPAP, AF, and CV disease data far better than I ever could. They sound the same words of caution and come out against screening patients with AF for OSA.
The story for CPAP’s effects on EF is similar though muddier. The American College of Cardiology (ACC)/American Heart Association (AHA) guidelines for heart failure cite a meta-analysis showing that CPAP improves left ventricular EF. In 2019, the American Academy of Sleep Medicine (AASM) CPAP guidelines included a systematic review and meta-analysis that found that CPAP has no effect on left ventricular EF in patients with or without heart failure.
There are a million reasons why two systematic reviews on the same topic might come to different conclusions. In this case, the included studies only partially overlap, and broadly speaking, it appears the authors made trade-offs. The review cited by the ACC/AHA had broader inclusion and significantly more patients and paid for it in heterogeneity (I2 in the 80%-90% range). The AASM analysis achieved 0% heterogeneity but limited inclusion to fewer than 100 patients. Across both, the improvement in EF was 2%- 5% at a minimally clinically important difference of 4%. Hardly convincing.
In summary, the road to negative trials and patient harm has always been paved with observational signal and biologic plausibility. Throw in some intellectual and academic bias, and you’ve created the perfect storm of therapeutic overconfidence.
Dr. Holley is a professor in the department of medicine, Uniformed Services University, Bethesda, Maryland, and a physician at Pulmonary/Sleep and Critical Care Medicine, MedStar Washington Hospital Center, Washington. He disclosed ties to Metapharm Inc., CHEST College, and WebMD.
A version of this article appeared on Medscape.com .
A few months ago, I posted a column on continuous positive airway pressure (CPAP) with the title, “CPAP Oversells and Underperforms.” To date, it has 299 likes and 90 comments, which are almost all negative. I’m glad to see that it’s generated interest, and I’d like to address some of the themes expressed in the posts.
Most comments were personal testimonies to the miracles of CPAP. These are important, and the point deserves emphasis. CPAP can provide significant improvements in daytime sleepiness and quality of life. I closed the original piece by acknowledging this important fact. Readers can be forgiven for missing it given that the title and text were otherwise disparaging of CPAP.
But several comments warrant a more in-depth discussion. The original piece focuses on CPAP and cardiovascular (CV) outcomes but made no mention of atrial fibrillation (AF) or ejection fraction (EF). The effects of CPAP on each are touted by cardiologists and PAP-pushers alike and are drivers of frequent referrals. It›s my fault for omitting them from the discussion.
AF is easy. The data is identical to all other things CPAP and CV. Based on biologic plausibility alone, the likelihood of a relationship between AF and obstructive sleep apnea (OSA) is similar to the odds that the Celtics raise an 18th banner come June. There’s hypoxia, intrathoracic pressure swings, sympathetic surges, and sleep state disruptions. It’s easy to get from there to arrhythmogenesis. There’s lots of observational noise, too, but no randomized proof that CPAP alters this relationship.
I found four randomized controlled trials (RCTs) that tested CPAP’s effect on AF. I’ll save you the suspense; they were all negative. One even found a signal for more adverse events in the CPAP group. These studies have several positive qualities: They enrolled patients with moderate to severe sleep apnea and high oxygen desaturation indices, adherence averaged more than 4 hours across all groups in all trials, and the methods for assessing the AF outcomes differed slightly. There’s also a lot not to like: The sample sizes were small, only one trial enrolled “sleepy” patients (as assessed by the Epworth Sleepiness Score), and follow-up was short.
To paraphrase Carl Sagan, “absence of evidence does not equal evidence of absence.” As a statistician would say, type II error cannot be excluded by these RCTs. In medicine, however, the burden of proof falls on demonstrating efficacy. If we treat before concluding that a therapy works, we risk wasting time, money, medical resources, and the most precious of patient commodities: the energy required for behavior change. In their response to letters to the editor, the authors of the third RCT summarize the CPAP, AF, and CV disease data far better than I ever could. They sound the same words of caution and come out against screening patients with AF for OSA.
The story for CPAP’s effects on EF is similar though muddier. The American College of Cardiology (ACC)/American Heart Association (AHA) guidelines for heart failure cite a meta-analysis showing that CPAP improves left ventricular EF. In 2019, the American Academy of Sleep Medicine (AASM) CPAP guidelines included a systematic review and meta-analysis that found that CPAP has no effect on left ventricular EF in patients with or without heart failure.
There are a million reasons why two systematic reviews on the same topic might come to different conclusions. In this case, the included studies only partially overlap, and broadly speaking, it appears the authors made trade-offs. The review cited by the ACC/AHA had broader inclusion and significantly more patients and paid for it in heterogeneity (I2 in the 80%-90% range). The AASM analysis achieved 0% heterogeneity but limited inclusion to fewer than 100 patients. Across both, the improvement in EF was 2%- 5% at a minimally clinically important difference of 4%. Hardly convincing.
In summary, the road to negative trials and patient harm has always been paved with observational signal and biologic plausibility. Throw in some intellectual and academic bias, and you’ve created the perfect storm of therapeutic overconfidence.
Dr. Holley is a professor in the department of medicine, Uniformed Services University, Bethesda, Maryland, and a physician at Pulmonary/Sleep and Critical Care Medicine, MedStar Washington Hospital Center, Washington. He disclosed ties to Metapharm Inc., CHEST College, and WebMD.
A version of this article appeared on Medscape.com .
Nocturnal Hot Flashes and Alzheimer’s Risk
In a recent article in the American Journal of Obstetrics & Gynecology, Rebecca C. Thurston, PhD, and Pauline Maki, PhD, leading scientists in the area of menopause’s impact on brain function, presented data from their assessment of 248 late perimenopausal and postmenopausal women who reported hot flashes, also known as vasomotor symptoms (VMS).
Hot flashes are known to be associated with changes in brain white matter, carotid atherosclerosis, brain function, and memory. Dr. Thurston and colleagues objectively measured VMS over 24 hours, using skin conductance monitoring. Plasma concentrations of Alzheimer’s disease biomarkers, including the amyloid beta 42–to–amyloid beta 40 ratio, were assessed. The mean age of study participants was 59 years, and they experienced a mean of five objective VMS daily.
A key finding was that VMS, particularly those occurring during sleep, were associated with a significantly lower amyloid beta 42–to–beta 40 ratio. This finding suggests that nighttime VMS may be a marker of risk for Alzheimer’s disease.
Previous research has found that menopausal hormone therapy is associated with favorable changes in Alzheimer’s disease biomarkers. Likewise, large observational studies have shown a lower incidence of Alzheimer’s disease among women who initiate hormone therapy in their late perimenopausal or early postmenopausal years and continue such therapy long term.
The findings of this important study by Thurston and colleagues provide further evidence to support the tantalizing possibility that agents that reduce nighttime hot flashes (including hormone therapy) may lower the subsequent incidence of Alzheimer’s disease in high-risk women.
Dr. Kaunitz is a tenured professor and associate chair in the department of obstetrics and gynecology at the University of Florida College of Medicine–Jacksonville, and medical director and director of menopause and gynecologic ultrasound services at the University of Florida Southside Women’s Health, Jacksonville. He disclosed ties to Sumitomo Pharma America, Mithra, Viatris, Bayer, Merck, Mylan (Viatris), and UpToDate.
A version of this article appeared on Medscape.com.
In a recent article in the American Journal of Obstetrics & Gynecology, Rebecca C. Thurston, PhD, and Pauline Maki, PhD, leading scientists in the area of menopause’s impact on brain function, presented data from their assessment of 248 late perimenopausal and postmenopausal women who reported hot flashes, also known as vasomotor symptoms (VMS).
Hot flashes are known to be associated with changes in brain white matter, carotid atherosclerosis, brain function, and memory. Dr. Thurston and colleagues objectively measured VMS over 24 hours, using skin conductance monitoring. Plasma concentrations of Alzheimer’s disease biomarkers, including the amyloid beta 42–to–amyloid beta 40 ratio, were assessed. The mean age of study participants was 59 years, and they experienced a mean of five objective VMS daily.
A key finding was that VMS, particularly those occurring during sleep, were associated with a significantly lower amyloid beta 42–to–beta 40 ratio. This finding suggests that nighttime VMS may be a marker of risk for Alzheimer’s disease.
Previous research has found that menopausal hormone therapy is associated with favorable changes in Alzheimer’s disease biomarkers. Likewise, large observational studies have shown a lower incidence of Alzheimer’s disease among women who initiate hormone therapy in their late perimenopausal or early postmenopausal years and continue such therapy long term.
The findings of this important study by Thurston and colleagues provide further evidence to support the tantalizing possibility that agents that reduce nighttime hot flashes (including hormone therapy) may lower the subsequent incidence of Alzheimer’s disease in high-risk women.
Dr. Kaunitz is a tenured professor and associate chair in the department of obstetrics and gynecology at the University of Florida College of Medicine–Jacksonville, and medical director and director of menopause and gynecologic ultrasound services at the University of Florida Southside Women’s Health, Jacksonville. He disclosed ties to Sumitomo Pharma America, Mithra, Viatris, Bayer, Merck, Mylan (Viatris), and UpToDate.
A version of this article appeared on Medscape.com.
In a recent article in the American Journal of Obstetrics & Gynecology, Rebecca C. Thurston, PhD, and Pauline Maki, PhD, leading scientists in the area of menopause’s impact on brain function, presented data from their assessment of 248 late perimenopausal and postmenopausal women who reported hot flashes, also known as vasomotor symptoms (VMS).
Hot flashes are known to be associated with changes in brain white matter, carotid atherosclerosis, brain function, and memory. Dr. Thurston and colleagues objectively measured VMS over 24 hours, using skin conductance monitoring. Plasma concentrations of Alzheimer’s disease biomarkers, including the amyloid beta 42–to–amyloid beta 40 ratio, were assessed. The mean age of study participants was 59 years, and they experienced a mean of five objective VMS daily.
A key finding was that VMS, particularly those occurring during sleep, were associated with a significantly lower amyloid beta 42–to–beta 40 ratio. This finding suggests that nighttime VMS may be a marker of risk for Alzheimer’s disease.
Previous research has found that menopausal hormone therapy is associated with favorable changes in Alzheimer’s disease biomarkers. Likewise, large observational studies have shown a lower incidence of Alzheimer’s disease among women who initiate hormone therapy in their late perimenopausal or early postmenopausal years and continue such therapy long term.
The findings of this important study by Thurston and colleagues provide further evidence to support the tantalizing possibility that agents that reduce nighttime hot flashes (including hormone therapy) may lower the subsequent incidence of Alzheimer’s disease in high-risk women.
Dr. Kaunitz is a tenured professor and associate chair in the department of obstetrics and gynecology at the University of Florida College of Medicine–Jacksonville, and medical director and director of menopause and gynecologic ultrasound services at the University of Florida Southside Women’s Health, Jacksonville. He disclosed ties to Sumitomo Pharma America, Mithra, Viatris, Bayer, Merck, Mylan (Viatris), and UpToDate.
A version of this article appeared on Medscape.com.
Traffic Noise Negatively Impacts Health
New research by Thomas Münzel, MD, senior professor of cardiology at Johannes Gutenberg University Mainz in Mainz, Germany, and colleagues again emphasized the harmful effects of noise on the heart and blood vessels. An analysis of current epidemiologic data provided strong indications that transportation noise is closely related to cardiovascular and cerebrovascular diseases, according to a statement on the data analysis. The results were published in Circulation Research.
Morbidity and Mortality
Epidemiologic studies have shown that road, rail, or air traffic noise increases the risk for cardiovascular morbidity and mortality, with strong evidence for ischemic heart disease, heart failure, and stroke, according to the scientists. These factors could favor vascular (endothelial) dysfunction, inflammation, and hypertension, thereby increasing cardiovascular risk.
Consequences and Pathomechanisms
In the current publication, the authors provided an overview of epidemiologic research on the effects of transportation noise on cardiovascular risk factors and diseases, discussed mechanistic insights from the latest clinical and experimental studies, and proposed new risk markers to address noise-induced cardiovascular effects in the general population. An integrated analysis in the article demonstrated that for every 10 dB(A) increase, the risk for cardiovascular diseases such as heart attack, stroke, and heart failure significantly increases by 3.2%.
The authors also explained the possible effects of noise on changes in gene networks, epigenetic pathways, circadian rhythms, signal transmission along the neuronal-cardiovascular axis, oxidative stress, inflammation, and metabolism. Finally, current and future noise protection strategies are described, and the existing evidence on noise as a cardiovascular risk factor is discussed.
Confirmed Cardiovascular Risk Factor
“As an increasing proportion of the population is exposed to harmful traffic noise, efforts to reduce noise and laws for noise reduction are of great importance for future public health,” said Dr. Münzel. “It is also important for us that due to the strong evidence, traffic noise is finally recognized as a risk factor for cardiovascular diseases.”
Heart Attack Outcomes
Dr. Münzel and other researchers from Mainz have been studying the cardiovascular consequences of air pollution and traffic noise for several years. For example, they found that heart attacks in people and animals exposed to high noise levels earlier in life healed poorly. These results were published last year in Cardiovascular Research. According to the authors, the findings suggest that traffic noise may play a significant role in the development and course of coronary heart disease, such as after a heart attack.
The scientists initially found in animal experiments that exposure to aircraft noise for 4 days led to increased inflammation in the vessels. Compared with mice not exposed to aircraft noise, the noise-exposed animals showed an increase in free radicals; these animals exhibited a significant inflammatory response and had impaired vessel function.
The researchers explained that the experimental data showed aircraft noise alone triggers a proinflammatory transcription program that promotes the infiltration of immune cells into cardiovascular tissue in animals with acute myocardial infarction. They noted an increased infiltration of CD45+ cells into the vessels and heart, dominated by neutrophils in vessel tissue and Ly6Chigh monocytes in heart tissue. This infiltration creates a proinflammatory milieu that adversely affects the outcome after myocardial infarction by predisposing the heart tissue to greater ischemic damage and functional impairment. Exposure of animals to aircraft noise before induction of myocardial infarction by left anterior descending (LAD) coronary artery ligation impaired left ventricular function and increased infarct size after cardiac ischemia. In addition, noise exposure exacerbated infarct-induced endothelial dysfunction of peripheral vessels as early as 24 hours after LAD ligation.
Clinical Confirmation
These experimental results were confirmed by observations in the population-based Gutenberg Health Study. The researchers analyzed data from 100 patients with heart attack. The lead and senior authors of the study Michael Molitor, MD, and Philip Wenzel, MD, of the University of Mainz, explained, “From our studies, we have learned that exposure to aircraft noise before a heart attack significantly amplifies subsequent cardiovascular inflammation and exacerbates ischemic heart failure, which is favored by inflammation-promoting vascular conditioning. Our translational results show that people who have been exposed to noise in the past have a worse course if they experience a heart attack later in life.”
Study participants who had experienced a heart attack in their medical history had elevated levels of C-reactive protein if they had been exposed to aircraft noise in the past and subsequently developed noise annoyance reactions (0.305 vs 1.5; P = .0094). In addition, left ventricular ejection fraction in these patients after a heart attack was worse than that in patients with infarction without noise exposure in their medical history (62.5 vs 65.6; P = .0053).
The results suggest that measures to reduce environmental noise could help improve the clinical outcomes of heart attack patients, according to the authors.
Mental Health Effects
Traffic noise also may be associated with an increased risk for depression and anxiety disorders, as reported 2 years ago by the German Society for Psychosomatic Medicine and Medical Psychotherapy. Evolution has programmed the human organism to perceive noises as indicators of potential sources of danger — even during sleep. “Noise puts the body on alert,” explained Manfred E. Beutel, MD, director of the Clinic for Psychosomatic Medicine and Psychotherapy at the University of Mainz. As a result, the autonomic nervous system activates stress hormones such as adrenaline and cortisol, leading to an increase in heart rate and blood pressure. If noise becomes chronic, chronic diseases can develop. “Indeed, observational and experimental studies have shown that persistent noise annoyance promotes incident hypertension, cardiovascular diseases, and type 2 diabetes,” said Dr. Beutel.
Depression Risk Doubled
Among the negative effects of noise annoyance are also mental illnesses, as has become increasingly clear. “Noise annoyance disrupts daily activities and interferes with feelings and thoughts, sleep, and recovery,” said Dr. Beutel. The interruptions trigger negative emotional reactions such as anger, distress, exhaustion, flight impulses, and stress symptoms. “Such conditions promote the development of depression over time,” said Dr. Beutel. This observation was confirmed by the large-scale Gutenberg Health Study using the example of the Mainz population, which suffers to a large extent from noise annoyance because of the nearby Frankfurt Airport. “With increasing noise annoyance, the rates of depression and anxiety disorders steadily increased, until the risks eventually doubled with extreme annoyance,” said Dr. Beutel. Other studies point in the same direction. For example, a meta-analysis found a 12% increase in the risk for depression per 10-dB increase in noise. Another study found an association between nocturnal noise annoyance and the use of antidepressants.
Fine Particulate Matter
According to an evaluation of the Gutenberg Study, people perceive noise annoyance from aircraft noise as the most pronounced, followed by road, neighborhood, industrial, and railway noise. Noise occurs most frequently in urban areas that also produce air pollution such as fine particulate matter. “Fine particulate matter is also suspected of promoting anxiety and depression,” said Dr. Beutel, “because the small particles of fine particulate matter can enter the bloodstream and trigger inflammatory processes there, which in turn are closely related to depression.”
This story was translated from Univadis Germany, which is part of the Medscape professional network, using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
New research by Thomas Münzel, MD, senior professor of cardiology at Johannes Gutenberg University Mainz in Mainz, Germany, and colleagues again emphasized the harmful effects of noise on the heart and blood vessels. An analysis of current epidemiologic data provided strong indications that transportation noise is closely related to cardiovascular and cerebrovascular diseases, according to a statement on the data analysis. The results were published in Circulation Research.
Morbidity and Mortality
Epidemiologic studies have shown that road, rail, or air traffic noise increases the risk for cardiovascular morbidity and mortality, with strong evidence for ischemic heart disease, heart failure, and stroke, according to the scientists. These factors could favor vascular (endothelial) dysfunction, inflammation, and hypertension, thereby increasing cardiovascular risk.
Consequences and Pathomechanisms
In the current publication, the authors provided an overview of epidemiologic research on the effects of transportation noise on cardiovascular risk factors and diseases, discussed mechanistic insights from the latest clinical and experimental studies, and proposed new risk markers to address noise-induced cardiovascular effects in the general population. An integrated analysis in the article demonstrated that for every 10 dB(A) increase, the risk for cardiovascular diseases such as heart attack, stroke, and heart failure significantly increases by 3.2%.
The authors also explained the possible effects of noise on changes in gene networks, epigenetic pathways, circadian rhythms, signal transmission along the neuronal-cardiovascular axis, oxidative stress, inflammation, and metabolism. Finally, current and future noise protection strategies are described, and the existing evidence on noise as a cardiovascular risk factor is discussed.
Confirmed Cardiovascular Risk Factor
“As an increasing proportion of the population is exposed to harmful traffic noise, efforts to reduce noise and laws for noise reduction are of great importance for future public health,” said Dr. Münzel. “It is also important for us that due to the strong evidence, traffic noise is finally recognized as a risk factor for cardiovascular diseases.”
Heart Attack Outcomes
Dr. Münzel and other researchers from Mainz have been studying the cardiovascular consequences of air pollution and traffic noise for several years. For example, they found that heart attacks in people and animals exposed to high noise levels earlier in life healed poorly. These results were published last year in Cardiovascular Research. According to the authors, the findings suggest that traffic noise may play a significant role in the development and course of coronary heart disease, such as after a heart attack.
The scientists initially found in animal experiments that exposure to aircraft noise for 4 days led to increased inflammation in the vessels. Compared with mice not exposed to aircraft noise, the noise-exposed animals showed an increase in free radicals; these animals exhibited a significant inflammatory response and had impaired vessel function.
The researchers explained that the experimental data showed aircraft noise alone triggers a proinflammatory transcription program that promotes the infiltration of immune cells into cardiovascular tissue in animals with acute myocardial infarction. They noted an increased infiltration of CD45+ cells into the vessels and heart, dominated by neutrophils in vessel tissue and Ly6Chigh monocytes in heart tissue. This infiltration creates a proinflammatory milieu that adversely affects the outcome after myocardial infarction by predisposing the heart tissue to greater ischemic damage and functional impairment. Exposure of animals to aircraft noise before induction of myocardial infarction by left anterior descending (LAD) coronary artery ligation impaired left ventricular function and increased infarct size after cardiac ischemia. In addition, noise exposure exacerbated infarct-induced endothelial dysfunction of peripheral vessels as early as 24 hours after LAD ligation.
Clinical Confirmation
These experimental results were confirmed by observations in the population-based Gutenberg Health Study. The researchers analyzed data from 100 patients with heart attack. The lead and senior authors of the study Michael Molitor, MD, and Philip Wenzel, MD, of the University of Mainz, explained, “From our studies, we have learned that exposure to aircraft noise before a heart attack significantly amplifies subsequent cardiovascular inflammation and exacerbates ischemic heart failure, which is favored by inflammation-promoting vascular conditioning. Our translational results show that people who have been exposed to noise in the past have a worse course if they experience a heart attack later in life.”
Study participants who had experienced a heart attack in their medical history had elevated levels of C-reactive protein if they had been exposed to aircraft noise in the past and subsequently developed noise annoyance reactions (0.305 vs 1.5; P = .0094). In addition, left ventricular ejection fraction in these patients after a heart attack was worse than that in patients with infarction without noise exposure in their medical history (62.5 vs 65.6; P = .0053).
The results suggest that measures to reduce environmental noise could help improve the clinical outcomes of heart attack patients, according to the authors.
Mental Health Effects
Traffic noise also may be associated with an increased risk for depression and anxiety disorders, as reported 2 years ago by the German Society for Psychosomatic Medicine and Medical Psychotherapy. Evolution has programmed the human organism to perceive noises as indicators of potential sources of danger — even during sleep. “Noise puts the body on alert,” explained Manfred E. Beutel, MD, director of the Clinic for Psychosomatic Medicine and Psychotherapy at the University of Mainz. As a result, the autonomic nervous system activates stress hormones such as adrenaline and cortisol, leading to an increase in heart rate and blood pressure. If noise becomes chronic, chronic diseases can develop. “Indeed, observational and experimental studies have shown that persistent noise annoyance promotes incident hypertension, cardiovascular diseases, and type 2 diabetes,” said Dr. Beutel.
Depression Risk Doubled
Among the negative effects of noise annoyance are also mental illnesses, as has become increasingly clear. “Noise annoyance disrupts daily activities and interferes with feelings and thoughts, sleep, and recovery,” said Dr. Beutel. The interruptions trigger negative emotional reactions such as anger, distress, exhaustion, flight impulses, and stress symptoms. “Such conditions promote the development of depression over time,” said Dr. Beutel. This observation was confirmed by the large-scale Gutenberg Health Study using the example of the Mainz population, which suffers to a large extent from noise annoyance because of the nearby Frankfurt Airport. “With increasing noise annoyance, the rates of depression and anxiety disorders steadily increased, until the risks eventually doubled with extreme annoyance,” said Dr. Beutel. Other studies point in the same direction. For example, a meta-analysis found a 12% increase in the risk for depression per 10-dB increase in noise. Another study found an association between nocturnal noise annoyance and the use of antidepressants.
Fine Particulate Matter
According to an evaluation of the Gutenberg Study, people perceive noise annoyance from aircraft noise as the most pronounced, followed by road, neighborhood, industrial, and railway noise. Noise occurs most frequently in urban areas that also produce air pollution such as fine particulate matter. “Fine particulate matter is also suspected of promoting anxiety and depression,” said Dr. Beutel, “because the small particles of fine particulate matter can enter the bloodstream and trigger inflammatory processes there, which in turn are closely related to depression.”
This story was translated from Univadis Germany, which is part of the Medscape professional network, using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
New research by Thomas Münzel, MD, senior professor of cardiology at Johannes Gutenberg University Mainz in Mainz, Germany, and colleagues again emphasized the harmful effects of noise on the heart and blood vessels. An analysis of current epidemiologic data provided strong indications that transportation noise is closely related to cardiovascular and cerebrovascular diseases, according to a statement on the data analysis. The results were published in Circulation Research.
Morbidity and Mortality
Epidemiologic studies have shown that road, rail, or air traffic noise increases the risk for cardiovascular morbidity and mortality, with strong evidence for ischemic heart disease, heart failure, and stroke, according to the scientists. These factors could favor vascular (endothelial) dysfunction, inflammation, and hypertension, thereby increasing cardiovascular risk.
Consequences and Pathomechanisms
In the current publication, the authors provided an overview of epidemiologic research on the effects of transportation noise on cardiovascular risk factors and diseases, discussed mechanistic insights from the latest clinical and experimental studies, and proposed new risk markers to address noise-induced cardiovascular effects in the general population. An integrated analysis in the article demonstrated that for every 10 dB(A) increase, the risk for cardiovascular diseases such as heart attack, stroke, and heart failure significantly increases by 3.2%.
The authors also explained the possible effects of noise on changes in gene networks, epigenetic pathways, circadian rhythms, signal transmission along the neuronal-cardiovascular axis, oxidative stress, inflammation, and metabolism. Finally, current and future noise protection strategies are described, and the existing evidence on noise as a cardiovascular risk factor is discussed.
Confirmed Cardiovascular Risk Factor
“As an increasing proportion of the population is exposed to harmful traffic noise, efforts to reduce noise and laws for noise reduction are of great importance for future public health,” said Dr. Münzel. “It is also important for us that due to the strong evidence, traffic noise is finally recognized as a risk factor for cardiovascular diseases.”
Heart Attack Outcomes
Dr. Münzel and other researchers from Mainz have been studying the cardiovascular consequences of air pollution and traffic noise for several years. For example, they found that heart attacks in people and animals exposed to high noise levels earlier in life healed poorly. These results were published last year in Cardiovascular Research. According to the authors, the findings suggest that traffic noise may play a significant role in the development and course of coronary heart disease, such as after a heart attack.
The scientists initially found in animal experiments that exposure to aircraft noise for 4 days led to increased inflammation in the vessels. Compared with mice not exposed to aircraft noise, the noise-exposed animals showed an increase in free radicals; these animals exhibited a significant inflammatory response and had impaired vessel function.
The researchers explained that the experimental data showed aircraft noise alone triggers a proinflammatory transcription program that promotes the infiltration of immune cells into cardiovascular tissue in animals with acute myocardial infarction. They noted an increased infiltration of CD45+ cells into the vessels and heart, dominated by neutrophils in vessel tissue and Ly6Chigh monocytes in heart tissue. This infiltration creates a proinflammatory milieu that adversely affects the outcome after myocardial infarction by predisposing the heart tissue to greater ischemic damage and functional impairment. Exposure of animals to aircraft noise before induction of myocardial infarction by left anterior descending (LAD) coronary artery ligation impaired left ventricular function and increased infarct size after cardiac ischemia. In addition, noise exposure exacerbated infarct-induced endothelial dysfunction of peripheral vessels as early as 24 hours after LAD ligation.
Clinical Confirmation
These experimental results were confirmed by observations in the population-based Gutenberg Health Study. The researchers analyzed data from 100 patients with heart attack. The lead and senior authors of the study Michael Molitor, MD, and Philip Wenzel, MD, of the University of Mainz, explained, “From our studies, we have learned that exposure to aircraft noise before a heart attack significantly amplifies subsequent cardiovascular inflammation and exacerbates ischemic heart failure, which is favored by inflammation-promoting vascular conditioning. Our translational results show that people who have been exposed to noise in the past have a worse course if they experience a heart attack later in life.”
Study participants who had experienced a heart attack in their medical history had elevated levels of C-reactive protein if they had been exposed to aircraft noise in the past and subsequently developed noise annoyance reactions (0.305 vs 1.5; P = .0094). In addition, left ventricular ejection fraction in these patients after a heart attack was worse than that in patients with infarction without noise exposure in their medical history (62.5 vs 65.6; P = .0053).
The results suggest that measures to reduce environmental noise could help improve the clinical outcomes of heart attack patients, according to the authors.
Mental Health Effects
Traffic noise also may be associated with an increased risk for depression and anxiety disorders, as reported 2 years ago by the German Society for Psychosomatic Medicine and Medical Psychotherapy. Evolution has programmed the human organism to perceive noises as indicators of potential sources of danger — even during sleep. “Noise puts the body on alert,” explained Manfred E. Beutel, MD, director of the Clinic for Psychosomatic Medicine and Psychotherapy at the University of Mainz. As a result, the autonomic nervous system activates stress hormones such as adrenaline and cortisol, leading to an increase in heart rate and blood pressure. If noise becomes chronic, chronic diseases can develop. “Indeed, observational and experimental studies have shown that persistent noise annoyance promotes incident hypertension, cardiovascular diseases, and type 2 diabetes,” said Dr. Beutel.
Depression Risk Doubled
Among the negative effects of noise annoyance are also mental illnesses, as has become increasingly clear. “Noise annoyance disrupts daily activities and interferes with feelings and thoughts, sleep, and recovery,” said Dr. Beutel. The interruptions trigger negative emotional reactions such as anger, distress, exhaustion, flight impulses, and stress symptoms. “Such conditions promote the development of depression over time,” said Dr. Beutel. This observation was confirmed by the large-scale Gutenberg Health Study using the example of the Mainz population, which suffers to a large extent from noise annoyance because of the nearby Frankfurt Airport. “With increasing noise annoyance, the rates of depression and anxiety disorders steadily increased, until the risks eventually doubled with extreme annoyance,” said Dr. Beutel. Other studies point in the same direction. For example, a meta-analysis found a 12% increase in the risk for depression per 10-dB increase in noise. Another study found an association between nocturnal noise annoyance and the use of antidepressants.
Fine Particulate Matter
According to an evaluation of the Gutenberg Study, people perceive noise annoyance from aircraft noise as the most pronounced, followed by road, neighborhood, industrial, and railway noise. Noise occurs most frequently in urban areas that also produce air pollution such as fine particulate matter. “Fine particulate matter is also suspected of promoting anxiety and depression,” said Dr. Beutel, “because the small particles of fine particulate matter can enter the bloodstream and trigger inflammatory processes there, which in turn are closely related to depression.”
This story was translated from Univadis Germany, which is part of the Medscape professional network, using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
From Pharma’s Factories Direct to You
Pharmaceutical giant Eli Lilly recently announced that its newly approved weight loss medication Zepbound — a glucagon-like peptide 1 receptor agonist (GLP-1 RA) akin to Mounjaro, Ozempic, and Wegovy — will be prescribed by independent telehealth providers on a platform managed by the company itself. The drug can be subsequently shipped direct to consumer (DTC), allowing delivery straight to patients’ homes.
This arrangement raises serious concerns about an inherent conflict of interest, as we previously discussed. What happens when a pharmaceutical company influences access to remote providers who prescribe the very medications it manufactures?
Without new guardrails, the potential for misleading messaging to result in dangerous prescribing patterns looms large. The United States is one of only two countries to allow DTC advertising of prescription drugs, and the explosion in demand for GLP-1 RAs is partly attributable to this model (Oh, oh, Ozempic, anyone?). Americans spent over $78 billion on weight loss goods and services in 2019; time-intensive approaches such as diet and exercise are understandably difficult, and the public has always looked for a magic cure. Although GLP-1 RAs are promising, they may present a path to disaster without proper supervision.
LillyDirect, which in addition to Zepbound offers migraine medications and other products in the company’s catalogue, primarily aims to increase access to medication and reduce costs of the drugs for consumers. The stated mission is noble: By cutting out the middlemen of traditional pharmacies and benefit managers, administrative costs drop. LillyDirect goes a step further by reducing the need for patients to visit their regular family doctor to receive these medications.
On the surface, this design appears promising. Wait times for doctor’s appointments will fall. Patients can order drugs from the comfort of their home. Everyone benefits. Or do they?
Although easier access and reduced cost may be an apparent win for patients, DTC arrangements complicate the ethics of prescriptions and patient follow-up. This model reminds us of the roots of the opioid crisis, where powerful advertising and relationships between prescribers and drugmakers led to great harm. Providers often faced a conflict of interest when prescribing dangerous drugs to patients who requested them. We must learn from these mistakes to ensure there is critical oversight into the independence of prescribers used by LillyDirect and other DTC platforms.
Adding to these parallels, once a patient begins a GLP-1 medication such as Zepbound, stopping treatment will probably lead to regaining lost weight, serving as negative reinforcement. Hence, patients may decide never to discontinue these medications.
Obtaining what amounts to a lifelong prescription from a telehealth provider who may never follow a patient sets a dangerous precedent that will be difficult to unravel once begun. Recent challenges in access to medications such as Zepbound have been complicated by supply chain and manufacturing issues, leading to potential interruptions in patient access, ultimately affecting compliance. The rapid increase in online providers indicates competition for distribution channels has sharply increased and poses a threat to Lilly’s DTC site.
Furthermore, the lack of a regular physician to monitor patients introduces uncertainty in safety and continuity of care. These are important tenets in protecting patients, especially patients who are not diabetic and desire a quick fix. We have already seen a huge, arguably unrestrained, rise in prescriptions of GLP-1 RAs for weight loss — up to a 352% increase in 2023.
These drugs have shown great promise and are generally safe when used in the right patient, but important contraindications exist — namely, serious gastrointestinal side effects and low blood glucose in nondiabetic persons — that an astute physician must consider. Patients desiring these medications often must undergo comprehensive laboratory testing and cardiac evaluation, both before initiation and during regular follow-up, to check for comorbidities.
The American College of Physicians cautioned against such prescribing practices in a recent position statement, emphasizing that the lack of an established care provider could adversely affect patients. We note that the potential harms of DTC sales would concentrate in economically and racially underserved communities, where obesity, lack of insurance, and low health literacy are more common.
But the DTC genie is out of the pill bottle, and as such platforms become more common, patients will inherently take more ownership over their medical care. Remote providers will of course not be following these patients and evaluating for side effects. As a result, we in medical practice must be abreast of new downsides of these medications if and when they arise.
Every clinician must be aware of the medications a patient is taking, even those that they did not prescribe. They should educate their patients about drug-drug interactions and side effects and order lab tests to monitor for side effects.
Independent physicians abide by an underlying oath: First, do no harm. They serve as a trusted check on industry and a valuable long-term partner for patients. Where are the guardrails to protect patients and ensure that pharmaceutical companies are not essentially pushing prescriptions for their own products? Will traditional healthcare providers be effectively relegated to a bystander role in Lilly’s transactional approach to medication distribution? Unlike other commercial goods, pharmacologics have great nuance; not every approved medication is meant for every patient.
A version of this article appeared on Medscape.com.
Pharmaceutical giant Eli Lilly recently announced that its newly approved weight loss medication Zepbound — a glucagon-like peptide 1 receptor agonist (GLP-1 RA) akin to Mounjaro, Ozempic, and Wegovy — will be prescribed by independent telehealth providers on a platform managed by the company itself. The drug can be subsequently shipped direct to consumer (DTC), allowing delivery straight to patients’ homes.
This arrangement raises serious concerns about an inherent conflict of interest, as we previously discussed. What happens when a pharmaceutical company influences access to remote providers who prescribe the very medications it manufactures?
Without new guardrails, the potential for misleading messaging to result in dangerous prescribing patterns looms large. The United States is one of only two countries to allow DTC advertising of prescription drugs, and the explosion in demand for GLP-1 RAs is partly attributable to this model (Oh, oh, Ozempic, anyone?). Americans spent over $78 billion on weight loss goods and services in 2019; time-intensive approaches such as diet and exercise are understandably difficult, and the public has always looked for a magic cure. Although GLP-1 RAs are promising, they may present a path to disaster without proper supervision.
LillyDirect, which in addition to Zepbound offers migraine medications and other products in the company’s catalogue, primarily aims to increase access to medication and reduce costs of the drugs for consumers. The stated mission is noble: By cutting out the middlemen of traditional pharmacies and benefit managers, administrative costs drop. LillyDirect goes a step further by reducing the need for patients to visit their regular family doctor to receive these medications.
On the surface, this design appears promising. Wait times for doctor’s appointments will fall. Patients can order drugs from the comfort of their home. Everyone benefits. Or do they?
Although easier access and reduced cost may be an apparent win for patients, DTC arrangements complicate the ethics of prescriptions and patient follow-up. This model reminds us of the roots of the opioid crisis, where powerful advertising and relationships between prescribers and drugmakers led to great harm. Providers often faced a conflict of interest when prescribing dangerous drugs to patients who requested them. We must learn from these mistakes to ensure there is critical oversight into the independence of prescribers used by LillyDirect and other DTC platforms.
Adding to these parallels, once a patient begins a GLP-1 medication such as Zepbound, stopping treatment will probably lead to regaining lost weight, serving as negative reinforcement. Hence, patients may decide never to discontinue these medications.
Obtaining what amounts to a lifelong prescription from a telehealth provider who may never follow a patient sets a dangerous precedent that will be difficult to unravel once begun. Recent challenges in access to medications such as Zepbound have been complicated by supply chain and manufacturing issues, leading to potential interruptions in patient access, ultimately affecting compliance. The rapid increase in online providers indicates competition for distribution channels has sharply increased and poses a threat to Lilly’s DTC site.
Furthermore, the lack of a regular physician to monitor patients introduces uncertainty in safety and continuity of care. These are important tenets in protecting patients, especially patients who are not diabetic and desire a quick fix. We have already seen a huge, arguably unrestrained, rise in prescriptions of GLP-1 RAs for weight loss — up to a 352% increase in 2023.
These drugs have shown great promise and are generally safe when used in the right patient, but important contraindications exist — namely, serious gastrointestinal side effects and low blood glucose in nondiabetic persons — that an astute physician must consider. Patients desiring these medications often must undergo comprehensive laboratory testing and cardiac evaluation, both before initiation and during regular follow-up, to check for comorbidities.
The American College of Physicians cautioned against such prescribing practices in a recent position statement, emphasizing that the lack of an established care provider could adversely affect patients. We note that the potential harms of DTC sales would concentrate in economically and racially underserved communities, where obesity, lack of insurance, and low health literacy are more common.
But the DTC genie is out of the pill bottle, and as such platforms become more common, patients will inherently take more ownership over their medical care. Remote providers will of course not be following these patients and evaluating for side effects. As a result, we in medical practice must be abreast of new downsides of these medications if and when they arise.
Every clinician must be aware of the medications a patient is taking, even those that they did not prescribe. They should educate their patients about drug-drug interactions and side effects and order lab tests to monitor for side effects.
Independent physicians abide by an underlying oath: First, do no harm. They serve as a trusted check on industry and a valuable long-term partner for patients. Where are the guardrails to protect patients and ensure that pharmaceutical companies are not essentially pushing prescriptions for their own products? Will traditional healthcare providers be effectively relegated to a bystander role in Lilly’s transactional approach to medication distribution? Unlike other commercial goods, pharmacologics have great nuance; not every approved medication is meant for every patient.
A version of this article appeared on Medscape.com.
Pharmaceutical giant Eli Lilly recently announced that its newly approved weight loss medication Zepbound — a glucagon-like peptide 1 receptor agonist (GLP-1 RA) akin to Mounjaro, Ozempic, and Wegovy — will be prescribed by independent telehealth providers on a platform managed by the company itself. The drug can be subsequently shipped direct to consumer (DTC), allowing delivery straight to patients’ homes.
This arrangement raises serious concerns about an inherent conflict of interest, as we previously discussed. What happens when a pharmaceutical company influences access to remote providers who prescribe the very medications it manufactures?
Without new guardrails, the potential for misleading messaging to result in dangerous prescribing patterns looms large. The United States is one of only two countries to allow DTC advertising of prescription drugs, and the explosion in demand for GLP-1 RAs is partly attributable to this model (Oh, oh, Ozempic, anyone?). Americans spent over $78 billion on weight loss goods and services in 2019; time-intensive approaches such as diet and exercise are understandably difficult, and the public has always looked for a magic cure. Although GLP-1 RAs are promising, they may present a path to disaster without proper supervision.
LillyDirect, which in addition to Zepbound offers migraine medications and other products in the company’s catalogue, primarily aims to increase access to medication and reduce costs of the drugs for consumers. The stated mission is noble: By cutting out the middlemen of traditional pharmacies and benefit managers, administrative costs drop. LillyDirect goes a step further by reducing the need for patients to visit their regular family doctor to receive these medications.
On the surface, this design appears promising. Wait times for doctor’s appointments will fall. Patients can order drugs from the comfort of their home. Everyone benefits. Or do they?
Although easier access and reduced cost may be an apparent win for patients, DTC arrangements complicate the ethics of prescriptions and patient follow-up. This model reminds us of the roots of the opioid crisis, where powerful advertising and relationships between prescribers and drugmakers led to great harm. Providers often faced a conflict of interest when prescribing dangerous drugs to patients who requested them. We must learn from these mistakes to ensure there is critical oversight into the independence of prescribers used by LillyDirect and other DTC platforms.
Adding to these parallels, once a patient begins a GLP-1 medication such as Zepbound, stopping treatment will probably lead to regaining lost weight, serving as negative reinforcement. Hence, patients may decide never to discontinue these medications.
Obtaining what amounts to a lifelong prescription from a telehealth provider who may never follow a patient sets a dangerous precedent that will be difficult to unravel once begun. Recent challenges in access to medications such as Zepbound have been complicated by supply chain and manufacturing issues, leading to potential interruptions in patient access, ultimately affecting compliance. The rapid increase in online providers indicates competition for distribution channels has sharply increased and poses a threat to Lilly’s DTC site.
Furthermore, the lack of a regular physician to monitor patients introduces uncertainty in safety and continuity of care. These are important tenets in protecting patients, especially patients who are not diabetic and desire a quick fix. We have already seen a huge, arguably unrestrained, rise in prescriptions of GLP-1 RAs for weight loss — up to a 352% increase in 2023.
These drugs have shown great promise and are generally safe when used in the right patient, but important contraindications exist — namely, serious gastrointestinal side effects and low blood glucose in nondiabetic persons — that an astute physician must consider. Patients desiring these medications often must undergo comprehensive laboratory testing and cardiac evaluation, both before initiation and during regular follow-up, to check for comorbidities.
The American College of Physicians cautioned against such prescribing practices in a recent position statement, emphasizing that the lack of an established care provider could adversely affect patients. We note that the potential harms of DTC sales would concentrate in economically and racially underserved communities, where obesity, lack of insurance, and low health literacy are more common.
But the DTC genie is out of the pill bottle, and as such platforms become more common, patients will inherently take more ownership over their medical care. Remote providers will of course not be following these patients and evaluating for side effects. As a result, we in medical practice must be abreast of new downsides of these medications if and when they arise.
Every clinician must be aware of the medications a patient is taking, even those that they did not prescribe. They should educate their patients about drug-drug interactions and side effects and order lab tests to monitor for side effects.
Independent physicians abide by an underlying oath: First, do no harm. They serve as a trusted check on industry and a valuable long-term partner for patients. Where are the guardrails to protect patients and ensure that pharmaceutical companies are not essentially pushing prescriptions for their own products? Will traditional healthcare providers be effectively relegated to a bystander role in Lilly’s transactional approach to medication distribution? Unlike other commercial goods, pharmacologics have great nuance; not every approved medication is meant for every patient.
A version of this article appeared on Medscape.com.
Home ventilation consult
Specialist input on a sudden shift in device availability
Philips Respironics released a public statement on January 25, 2024, that would dramatically change the landscape of home mechanical ventilation and sleep-disordered breathing management in the United States. The company announced that, effective immediately in the US and US territories, Philips Respironics would stop production and sale of all hospital and home mechanical ventilation products, home and hospital ventilation devices, and oxygen concentrators.
There are many unknowns and uncertainties about how to proceed with care for patients requiring these devices. So we gathered an expert panel of clinicians from CHEST’s Home-Based Mechanical Ventilation and Neuromuscular Section within the Sleep Medicine Network to explain the current situation and offer suggestions on moving forward in caring for these patients.
Why is this happening?
John M. Coleman III, MD, FCCP: To understand the current Philips Respironics announcement, we must go back to June 2021. At that time, Philips recalled certain home mechanical ventilators, CPAP machines, and BiPAP machines due to potential health risks related to breakdown of the polyester-based polyurethane (PE-PUR) foam placed in these devices for noise reduction. Small and microscopic particles of this foam were at risk for being inhaled or ingested by patients using these devices. It was suspected that inhalation of these particles could potentially result in temporary or permanent injury. Machines in hot temperatures or using ozone cleaning were at increased risk. The US Food and Drug Administration (FDA) issued a class 1 recall, defined as “a situation in which there is reasonable probability that the use of or exposure to a violative product will cause serious adverse health consequences or death.”
In the months following the initial recall, there were additional recalls of both in-hospital and home ventilators related to the potential of these foam particles to move and block the air path, reducing airflow and causing the device to alarm.
Over the next few years, tens of thousands of medical device reports were filed about PE-PUR foam-related injuries, with some cases resulting in death. At this time, the Department of Justice began collaborating with the FDA on a consent decree. There were ongoing recalls of the CoughAssist T70 device, as well as the newest generation of Philips Respironics home ventilators, the Trilogy EVO.
Ultimately, after years of ongoing recalls and reports of numerous deaths and injuries, with multiple class action lawsuits, the consent decree was finalized. Philips Respironics agreed to stop production of all respiratory-related products in the US and US territories.
What devices does this apply to?
Jason Ackrivo, MD: This notice affects the devices shown in Table 1. All sales and device shipments have been discontinued as of January 25, 2024. Philips Respironics will continue to service the devices, subject to part availability, up to 5 years after sales discontinuation. However, Philips Respironics will continue to sell consumables and accessories, including masks.
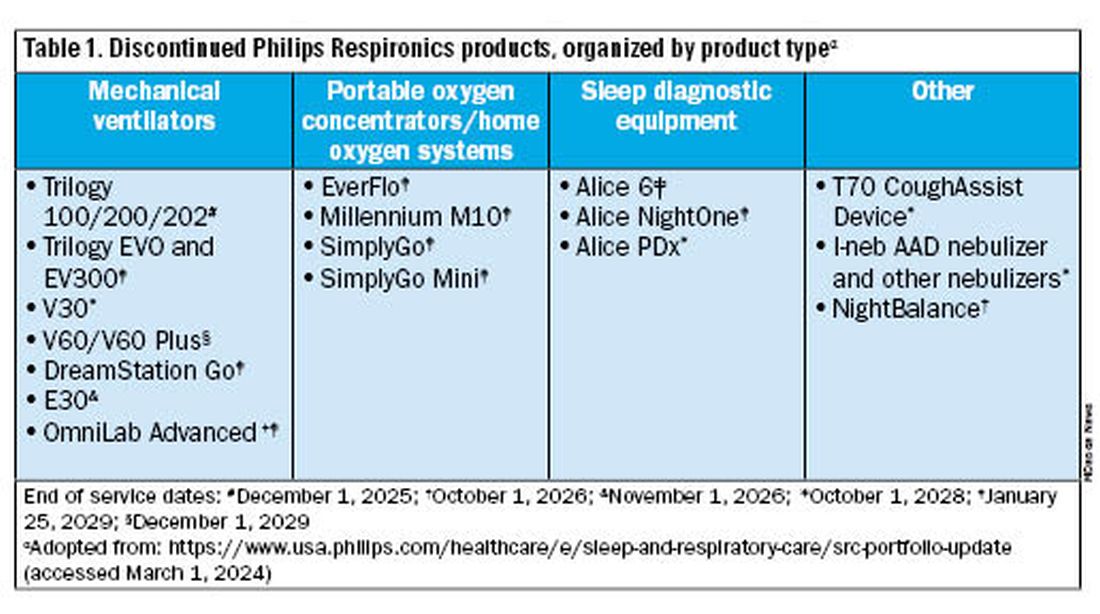
What are my options for home mechanical ventilators?
Bethany L. Lussier, MD, FCCP: In the US, alternative approved home mechanical ventilator (HMV) devices include Astral by ResMed, Vivo 45 and Vivo 65 by Breas, and VOCSN by Ventec. Additional options made available through emergency use authorization by the FDA between 2020 and 2022 included Luisa by Löwenstein Medical, the V+ by Ventec, and Life2000 by Baxter. Many of us expedite disposition from the hospital by prescribing HMVs rather than respiratory assist devices (RADs) because it is easier to meet qualifying criteria for insurance. In efforts to promote just allocation of resources, now might be the ideal time to reconsider higher utilization of RADs over HMVs. Reasonable RAD candidates are those who do not need autotitration of EPAP, dual mode therapy, or invasive ventilation. In these cases, the qualifying criteria and patient needs may be met with a RAD capable of VAPS or BPAP-ST mode.
How are these alternative devices similar to and different from the Trilogy EVO?
Dr. Ackrivo:All these devices are portable ventilators that can deliver noninvasive or invasive ventilation. They have internal batteries for enabling portability. They offer multiple programmable presets and mouthpiece ventilation, and some offer both oxygenation and CO2 monitoring (both TcCO2 and EtCO2).
All alternative portable ventilators include a proprietary ventilation mode analogous to the Trilogy AVAPS algorithm (Table 2). The ResMed Astral has a safety tidal volume feature that targets a minimum tidal volume in PS, S/T, or P(A)C modes. The ResMed iVAPS algorithm adjusts inspiratory pressure and respiratory rate to target an alveolar ventilation based on patient-entered height. The Breas Vivo can target a tidal volume (TgV) in either PSV or PCV mode.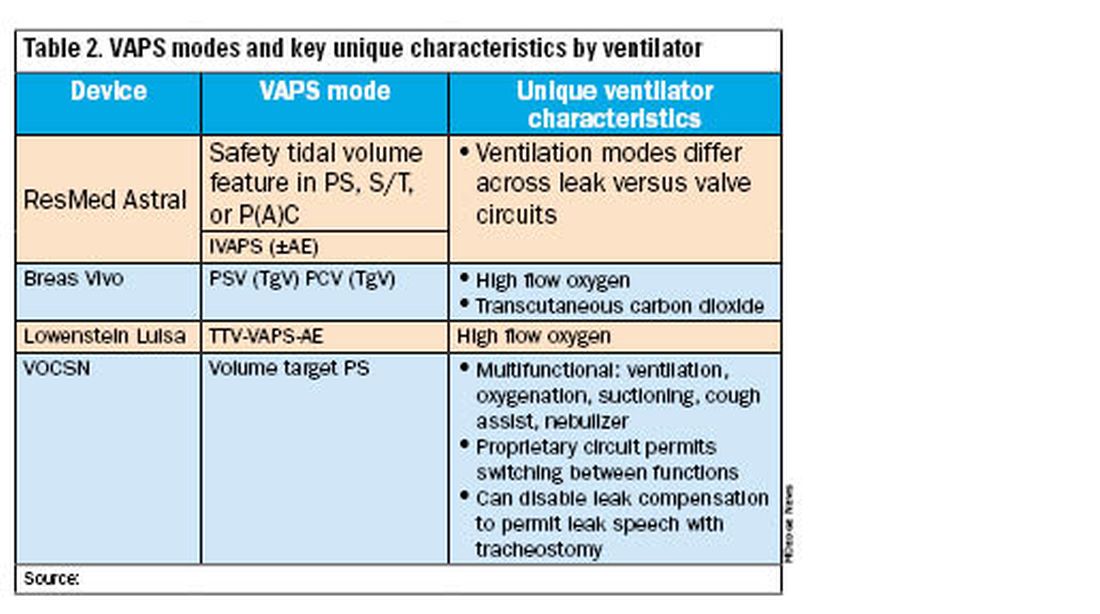
Unique ventilator characteristics are shown in Table 2. ResMed Astral mode options will differ between leak (passive) or valve (active) circuits. Both the Breas Vivo and Löwenstein Luisa enable high-flow oxygen delivery. Only the Breas Vivo enables connecting to a transcutaneous carbon dioxide monitor. The VOCSN name is an acronym for its multifunctional capabilities: ventilation, oxygenation, cough assist, suction, and nebulizer treatments. Lastly, the VOCSN can disable leak compensation, which may be advantageous for enabling leak speech with a tracheostomy.
I just provided my patient with a Trilogy EVO. Do I need to change this immediately?
Dr. Coleman: No, but you should start conversations with your patient/caregiving support and with your durable medical equipment (DME) provider about alternative options. The ripple effects of the Philips Respironics recall will be ongoing for years. The silver lining of this situation is that there are numerous HMV options on the market currently. It is important to review the differences between these new devices and consider what will work best for your patient and your practice. In addition, it is critical that your DME provider is familiar with these new devices, both for support and education, and is taking steps to make alternate devices available. We anticipate a push in coming months to switch patients off Trilogy EVO, so it important to get this process started.
For patients not interested in switching just yet, Philips Respironics will continue to service and offer supplies for these devices for up to 5 years, depending on part availability (Table 1). Refer to the Philips Respironics Sleep & Respiratory Product Portfolio Changes website for the most up-to-date information.
I have a patient on AVAPS, and I must change to iVAPS. What now?
Dr. Lussier: As mentioned previously by Dr. Ackrivo, the ResMed iVAPS algorithm adjusts inspiratory pressure and respiratory rate to target an alveolar ventilation based on patient-entered height. A download from a current VAPS setting can be helpful in defining target ventilation and pressure ranges for a tailored prescription. ResMed has an online iVAPS calculator (resmed.com) to assist in making this switch. Close clinical monitoring with data downloads is recommended to assure desired targets are still achieved.
What will happen to Philips Respironics’ cloud patient data?
Dr. Lussier: Representatives have reported that both providers and DME companies will have continued access to Care Orchestrator going forward. Currently, the logistics of data maintenance and ownership remain unclear, which poses additional questions about global access to patients’ data downloads.
----------
The recent discontinuation of Philips Respironics ventilation devices will induce a dramatic shift in home ventilation options in the US. Clinicians and DME companies should begin familiarizing themselves with alternative ventilators and their unique features. While significant uncertainty exists, we encourage a proactive approach to education and communication to ensure a smooth transition for patients on home ventilation.
John M. Coleman III, MD, FCCP, is Associate Professor, Division of Pulmonary & Critical Care Medicine, Department of Neurology, Northwestern University Feinberg School of Medicine. Bethany L. Lussier, MD, FCCP, is in the Department of Internal Medicine, Division of Pulmonary & Critical Care Medicine, Department of Neurology, Division of Neurocritical Care, UT Southwestern Medical Center. Jason Ackrivo, MD, is Assistant Professor of Medicine and Neurology, and Associate Director, Jay and Randy Fishman Program for Home Assisted Ventilation, Pulmonary, Allergy, and Critical Care Division, Perelman School of Medicine, University of Pennsylvania.
Specialist input on a sudden shift in device availability
Specialist input on a sudden shift in device availability
Philips Respironics released a public statement on January 25, 2024, that would dramatically change the landscape of home mechanical ventilation and sleep-disordered breathing management in the United States. The company announced that, effective immediately in the US and US territories, Philips Respironics would stop production and sale of all hospital and home mechanical ventilation products, home and hospital ventilation devices, and oxygen concentrators.
There are many unknowns and uncertainties about how to proceed with care for patients requiring these devices. So we gathered an expert panel of clinicians from CHEST’s Home-Based Mechanical Ventilation and Neuromuscular Section within the Sleep Medicine Network to explain the current situation and offer suggestions on moving forward in caring for these patients.
Why is this happening?
John M. Coleman III, MD, FCCP: To understand the current Philips Respironics announcement, we must go back to June 2021. At that time, Philips recalled certain home mechanical ventilators, CPAP machines, and BiPAP machines due to potential health risks related to breakdown of the polyester-based polyurethane (PE-PUR) foam placed in these devices for noise reduction. Small and microscopic particles of this foam were at risk for being inhaled or ingested by patients using these devices. It was suspected that inhalation of these particles could potentially result in temporary or permanent injury. Machines in hot temperatures or using ozone cleaning were at increased risk. The US Food and Drug Administration (FDA) issued a class 1 recall, defined as “a situation in which there is reasonable probability that the use of or exposure to a violative product will cause serious adverse health consequences or death.”
In the months following the initial recall, there were additional recalls of both in-hospital and home ventilators related to the potential of these foam particles to move and block the air path, reducing airflow and causing the device to alarm.
Over the next few years, tens of thousands of medical device reports were filed about PE-PUR foam-related injuries, with some cases resulting in death. At this time, the Department of Justice began collaborating with the FDA on a consent decree. There were ongoing recalls of the CoughAssist T70 device, as well as the newest generation of Philips Respironics home ventilators, the Trilogy EVO.
Ultimately, after years of ongoing recalls and reports of numerous deaths and injuries, with multiple class action lawsuits, the consent decree was finalized. Philips Respironics agreed to stop production of all respiratory-related products in the US and US territories.
What devices does this apply to?
Jason Ackrivo, MD: This notice affects the devices shown in Table 1. All sales and device shipments have been discontinued as of January 25, 2024. Philips Respironics will continue to service the devices, subject to part availability, up to 5 years after sales discontinuation. However, Philips Respironics will continue to sell consumables and accessories, including masks.

What are my options for home mechanical ventilators?
Bethany L. Lussier, MD, FCCP: In the US, alternative approved home mechanical ventilator (HMV) devices include Astral by ResMed, Vivo 45 and Vivo 65 by Breas, and VOCSN by Ventec. Additional options made available through emergency use authorization by the FDA between 2020 and 2022 included Luisa by Löwenstein Medical, the V+ by Ventec, and Life2000 by Baxter. Many of us expedite disposition from the hospital by prescribing HMVs rather than respiratory assist devices (RADs) because it is easier to meet qualifying criteria for insurance. In efforts to promote just allocation of resources, now might be the ideal time to reconsider higher utilization of RADs over HMVs. Reasonable RAD candidates are those who do not need autotitration of EPAP, dual mode therapy, or invasive ventilation. In these cases, the qualifying criteria and patient needs may be met with a RAD capable of VAPS or BPAP-ST mode.
How are these alternative devices similar to and different from the Trilogy EVO?
Dr. Ackrivo:All these devices are portable ventilators that can deliver noninvasive or invasive ventilation. They have internal batteries for enabling portability. They offer multiple programmable presets and mouthpiece ventilation, and some offer both oxygenation and CO2 monitoring (both TcCO2 and EtCO2).
All alternative portable ventilators include a proprietary ventilation mode analogous to the Trilogy AVAPS algorithm (Table 2). The ResMed Astral has a safety tidal volume feature that targets a minimum tidal volume in PS, S/T, or P(A)C modes. The ResMed iVAPS algorithm adjusts inspiratory pressure and respiratory rate to target an alveolar ventilation based on patient-entered height. The Breas Vivo can target a tidal volume (TgV) in either PSV or PCV mode.
Unique ventilator characteristics are shown in Table 2. ResMed Astral mode options will differ between leak (passive) or valve (active) circuits. Both the Breas Vivo and Löwenstein Luisa enable high-flow oxygen delivery. Only the Breas Vivo enables connecting to a transcutaneous carbon dioxide monitor. The VOCSN name is an acronym for its multifunctional capabilities: ventilation, oxygenation, cough assist, suction, and nebulizer treatments. Lastly, the VOCSN can disable leak compensation, which may be advantageous for enabling leak speech with a tracheostomy.
I just provided my patient with a Trilogy EVO. Do I need to change this immediately?
Dr. Coleman: No, but you should start conversations with your patient/caregiving support and with your durable medical equipment (DME) provider about alternative options. The ripple effects of the Philips Respironics recall will be ongoing for years. The silver lining of this situation is that there are numerous HMV options on the market currently. It is important to review the differences between these new devices and consider what will work best for your patient and your practice. In addition, it is critical that your DME provider is familiar with these new devices, both for support and education, and is taking steps to make alternate devices available. We anticipate a push in coming months to switch patients off Trilogy EVO, so it important to get this process started.
For patients not interested in switching just yet, Philips Respironics will continue to service and offer supplies for these devices for up to 5 years, depending on part availability (Table 1). Refer to the Philips Respironics Sleep & Respiratory Product Portfolio Changes website for the most up-to-date information.
I have a patient on AVAPS, and I must change to iVAPS. What now?
Dr. Lussier: As mentioned previously by Dr. Ackrivo, the ResMed iVAPS algorithm adjusts inspiratory pressure and respiratory rate to target an alveolar ventilation based on patient-entered height. A download from a current VAPS setting can be helpful in defining target ventilation and pressure ranges for a tailored prescription. ResMed has an online iVAPS calculator (resmed.com) to assist in making this switch. Close clinical monitoring with data downloads is recommended to assure desired targets are still achieved.
What will happen to Philips Respironics’ cloud patient data?
Dr. Lussier: Representatives have reported that both providers and DME companies will have continued access to Care Orchestrator going forward. Currently, the logistics of data maintenance and ownership remain unclear, which poses additional questions about global access to patients’ data downloads.
----------
The recent discontinuation of Philips Respironics ventilation devices will induce a dramatic shift in home ventilation options in the US. Clinicians and DME companies should begin familiarizing themselves with alternative ventilators and their unique features. While significant uncertainty exists, we encourage a proactive approach to education and communication to ensure a smooth transition for patients on home ventilation.
John M. Coleman III, MD, FCCP, is Associate Professor, Division of Pulmonary & Critical Care Medicine, Department of Neurology, Northwestern University Feinberg School of Medicine. Bethany L. Lussier, MD, FCCP, is in the Department of Internal Medicine, Division of Pulmonary & Critical Care Medicine, Department of Neurology, Division of Neurocritical Care, UT Southwestern Medical Center. Jason Ackrivo, MD, is Assistant Professor of Medicine and Neurology, and Associate Director, Jay and Randy Fishman Program for Home Assisted Ventilation, Pulmonary, Allergy, and Critical Care Division, Perelman School of Medicine, University of Pennsylvania.
Philips Respironics released a public statement on January 25, 2024, that would dramatically change the landscape of home mechanical ventilation and sleep-disordered breathing management in the United States. The company announced that, effective immediately in the US and US territories, Philips Respironics would stop production and sale of all hospital and home mechanical ventilation products, home and hospital ventilation devices, and oxygen concentrators.
There are many unknowns and uncertainties about how to proceed with care for patients requiring these devices. So we gathered an expert panel of clinicians from CHEST’s Home-Based Mechanical Ventilation and Neuromuscular Section within the Sleep Medicine Network to explain the current situation and offer suggestions on moving forward in caring for these patients.
Why is this happening?
John M. Coleman III, MD, FCCP: To understand the current Philips Respironics announcement, we must go back to June 2021. At that time, Philips recalled certain home mechanical ventilators, CPAP machines, and BiPAP machines due to potential health risks related to breakdown of the polyester-based polyurethane (PE-PUR) foam placed in these devices for noise reduction. Small and microscopic particles of this foam were at risk for being inhaled or ingested by patients using these devices. It was suspected that inhalation of these particles could potentially result in temporary or permanent injury. Machines in hot temperatures or using ozone cleaning were at increased risk. The US Food and Drug Administration (FDA) issued a class 1 recall, defined as “a situation in which there is reasonable probability that the use of or exposure to a violative product will cause serious adverse health consequences or death.”
In the months following the initial recall, there were additional recalls of both in-hospital and home ventilators related to the potential of these foam particles to move and block the air path, reducing airflow and causing the device to alarm.
Over the next few years, tens of thousands of medical device reports were filed about PE-PUR foam-related injuries, with some cases resulting in death. At this time, the Department of Justice began collaborating with the FDA on a consent decree. There were ongoing recalls of the CoughAssist T70 device, as well as the newest generation of Philips Respironics home ventilators, the Trilogy EVO.
Ultimately, after years of ongoing recalls and reports of numerous deaths and injuries, with multiple class action lawsuits, the consent decree was finalized. Philips Respironics agreed to stop production of all respiratory-related products in the US and US territories.
What devices does this apply to?
Jason Ackrivo, MD: This notice affects the devices shown in Table 1. All sales and device shipments have been discontinued as of January 25, 2024. Philips Respironics will continue to service the devices, subject to part availability, up to 5 years after sales discontinuation. However, Philips Respironics will continue to sell consumables and accessories, including masks.

What are my options for home mechanical ventilators?
Bethany L. Lussier, MD, FCCP: In the US, alternative approved home mechanical ventilator (HMV) devices include Astral by ResMed, Vivo 45 and Vivo 65 by Breas, and VOCSN by Ventec. Additional options made available through emergency use authorization by the FDA between 2020 and 2022 included Luisa by Löwenstein Medical, the V+ by Ventec, and Life2000 by Baxter. Many of us expedite disposition from the hospital by prescribing HMVs rather than respiratory assist devices (RADs) because it is easier to meet qualifying criteria for insurance. In efforts to promote just allocation of resources, now might be the ideal time to reconsider higher utilization of RADs over HMVs. Reasonable RAD candidates are those who do not need autotitration of EPAP, dual mode therapy, or invasive ventilation. In these cases, the qualifying criteria and patient needs may be met with a RAD capable of VAPS or BPAP-ST mode.
How are these alternative devices similar to and different from the Trilogy EVO?
Dr. Ackrivo:All these devices are portable ventilators that can deliver noninvasive or invasive ventilation. They have internal batteries for enabling portability. They offer multiple programmable presets and mouthpiece ventilation, and some offer both oxygenation and CO2 monitoring (both TcCO2 and EtCO2).
All alternative portable ventilators include a proprietary ventilation mode analogous to the Trilogy AVAPS algorithm (Table 2). The ResMed Astral has a safety tidal volume feature that targets a minimum tidal volume in PS, S/T, or P(A)C modes. The ResMed iVAPS algorithm adjusts inspiratory pressure and respiratory rate to target an alveolar ventilation based on patient-entered height. The Breas Vivo can target a tidal volume (TgV) in either PSV or PCV mode.
Unique ventilator characteristics are shown in Table 2. ResMed Astral mode options will differ between leak (passive) or valve (active) circuits. Both the Breas Vivo and Löwenstein Luisa enable high-flow oxygen delivery. Only the Breas Vivo enables connecting to a transcutaneous carbon dioxide monitor. The VOCSN name is an acronym for its multifunctional capabilities: ventilation, oxygenation, cough assist, suction, and nebulizer treatments. Lastly, the VOCSN can disable leak compensation, which may be advantageous for enabling leak speech with a tracheostomy.
I just provided my patient with a Trilogy EVO. Do I need to change this immediately?
Dr. Coleman: No, but you should start conversations with your patient/caregiving support and with your durable medical equipment (DME) provider about alternative options. The ripple effects of the Philips Respironics recall will be ongoing for years. The silver lining of this situation is that there are numerous HMV options on the market currently. It is important to review the differences between these new devices and consider what will work best for your patient and your practice. In addition, it is critical that your DME provider is familiar with these new devices, both for support and education, and is taking steps to make alternate devices available. We anticipate a push in coming months to switch patients off Trilogy EVO, so it important to get this process started.
For patients not interested in switching just yet, Philips Respironics will continue to service and offer supplies for these devices for up to 5 years, depending on part availability (Table 1). Refer to the Philips Respironics Sleep & Respiratory Product Portfolio Changes website for the most up-to-date information.
I have a patient on AVAPS, and I must change to iVAPS. What now?
Dr. Lussier: As mentioned previously by Dr. Ackrivo, the ResMed iVAPS algorithm adjusts inspiratory pressure and respiratory rate to target an alveolar ventilation based on patient-entered height. A download from a current VAPS setting can be helpful in defining target ventilation and pressure ranges for a tailored prescription. ResMed has an online iVAPS calculator (resmed.com) to assist in making this switch. Close clinical monitoring with data downloads is recommended to assure desired targets are still achieved.
What will happen to Philips Respironics’ cloud patient data?
Dr. Lussier: Representatives have reported that both providers and DME companies will have continued access to Care Orchestrator going forward. Currently, the logistics of data maintenance and ownership remain unclear, which poses additional questions about global access to patients’ data downloads.
----------
The recent discontinuation of Philips Respironics ventilation devices will induce a dramatic shift in home ventilation options in the US. Clinicians and DME companies should begin familiarizing themselves with alternative ventilators and their unique features. While significant uncertainty exists, we encourage a proactive approach to education and communication to ensure a smooth transition for patients on home ventilation.
John M. Coleman III, MD, FCCP, is Associate Professor, Division of Pulmonary & Critical Care Medicine, Department of Neurology, Northwestern University Feinberg School of Medicine. Bethany L. Lussier, MD, FCCP, is in the Department of Internal Medicine, Division of Pulmonary & Critical Care Medicine, Department of Neurology, Division of Neurocritical Care, UT Southwestern Medical Center. Jason Ackrivo, MD, is Assistant Professor of Medicine and Neurology, and Associate Director, Jay and Randy Fishman Program for Home Assisted Ventilation, Pulmonary, Allergy, and Critical Care Division, Perelman School of Medicine, University of Pennsylvania.
Robotic Pet Therapy in the Intensive Care Unit
Critical illness is commonly associated with interrelated conditions including pain, agitation, delirium, immobility, and sleep disruption (PADIS). Managing PADIS is often complex and includes pharmacologic and nonpharmacologic interventions.1 Incorporating multifaceted practices to enhance PADIS management has been shown to improve several intensive care unit (ICU)-related outcomes.2
Many pharmacologic PADIS treatments are ineffective or associated with adverse effects. For example, antipsychotics used for treating ICU-related delirium have not shown improved outcomes.3,4 Commonly used medications for agitation, such as benzodiazepines, increase delirium risk.5,6 Because of these limitations, several nonpharmacologic interventions for PADIS have been evaluated.
Pet therapy has been implemented in some ICU settings, but is not widely adopted.7 Also referred to as animal-assisted activities, animal-assisted therapy, or animal-assisted interventions, pet therapy typically involves interaction between a patient and a live animal (most commonly a dog) under the direction of an animal handler, with the intention of providing therapeutic benefit. Interactions frequently include meet and greet activities such as petting, but also could include walking or other activities. Pet therapy has been reported to reduce pain, agitation, and stress among ICU patients.8 Introducing a pet therapy program with live animals in the ICU could be challenging because of factors such as identifying trained, accredited animals and handlers, and managing infection control and other risks.9 As an alternative to live pets, robotic pet therapy has been shown to be beneficial—mostly outside the ICU—in settings such as long-term care.10,11 Although uncommon, robotic pets have been used in the ICU and hospital settings for therapeutic purposes.12 Robotic pets reduce many concerns associated with live animals while mimicking the behaviors of live animals and potentially offering many of the same benefits.
OBSERVATIONS
The North Florida/South Georgia Veterans Health System (NF/SGVHS) implemented a novel robotic pet therapy program for patients requiring ICU care to improve the treatment of PADIS. Funding was provided through a Veterans Health Administration Innovation Grant procured by a clinical pharmacy specialist as the program’s champion. Goals of the robotic pet therapy program include reductions in: distressing symptoms associated with PADIS, use of psychoactive drugs and physical restraints, and ICU length of stay. The ICU team developed standard operating procedures and an order menu, which were integrated into the ICU prescriber ordering menu. Patients were selected for pet therapy based on PADIS scores and potential for positive response to pet therapy as assessed by the ICU team.Patients in medical and surgical ICU settings were eligible for the program. The robotic pets used in the program were Joy for All Companion Pets (Ageless Innovation LLC). Robotic cats and dogs were available and pets were “adopted’ by each patient (Figure). As an infection control measure, pets were not reissued or shared amongpatients and pets could be cleaned with a disinfectant solution. Nurses were primarily responsible for monitoring and documenting responses to robotic pet therapy.
It was necessary to secure buy-in from several services to successfully implement the program. The critical care clinical pharmacy specialists were responsible for ordering, storing, and dispensing the robotic pets. The NF/SGVHS innovation specialist helped secure funding, procure the robotic pet, and promote the program. The standard operating procedures for the program were developed by a multidisciplinary team with input from critical care nurses, intensivists, pharmacists, patient safety, and infection control (Table 1). Success of the program also required buy-in from ICU team members.
Program Impact
A retrospective cohort study was conducted to assess for improvements in PADIS symptoms and medication use post-intervention. Patients were included if they received robotic pet therapy in the ICU from July 10, 2019, to February 1, 2021. Individuals aged < 18 years or > 89 years, were pregnant, or were not receiving ICU-level care were excluded. Outcomes assessed included improvement in pain scores, agitation scores, sleep quality, resolution of delirium, and use of pain or psychoactive medications during patients’ ICU stay.
Thirty patients were included in the study (Table 2). After receiving a robotic pet, 9 (30%) patients recorded decreased pain scores, 15 (50%) recorded decreased agitation scores, 8 (27%) had resolution of delirium, and 2 (7%) described improvement in sleep. Pain medication use decreased in 12 (40%) patients and psychoactive medication use was reduced in 7 (23%) patients.
Limitations
The robotic pet therapy program has shown promising results; however, some aspects merit discussion. Evaluation of this program is limited by factors such as the observational study design, single-center patient sample, and lack of comparator group. Although no known adverse effects of robotic pet therapy were seen, it is possible that some patients may not have a favorable response. Challenges of implementing a robotic pet therapy program include cost and additional operational activities (storage, ordering, dispensing) necessary to maintain the program. Additional research is needed to evaluate the impact of robotic pet therapy on other outcomes including cost, ICU length of stay, and patient satisfaction.
CONCLUSIONS
Robotic pet therapy can be successfully implemented in the ICU and appears to provide a simple, safe, beneficial, nonpharmacologic intervention for PADIS. This study showed that many patients had favorable response to robotic pet therapy, indicating that it may be a viable alternative to traditional pet therapy. Other health systems could benefit from implementing programs similar to the robotic pet therapy program at NF/SGVHS.
Acknowledgments
The author would like to acknowledge Simran Panesar, PharmD, and Theresa Faison, PharmD, for their contributions to this project.
1. Devlin JW, Skrobik Y, Gélinas C, et al. Clinical practice guidelines for the prevention and management of pain, agitation/sedation, delirium, immobility, and sleep disruption in adult patients in the ICU. Crit Care Med. 2018;46:e825-e873. doi:10.1097/CCM.0000000000003299
2. Pun BT, Balas MC, Barnes-Daly MA, et al. Caring for critically ill patients with the ABCDEF bundle: results of the ICU Liberation Collaborative in over 15,000 adults. Crit Care Med. 2019;47:3-14. doi:10.1097/CCM.0000000000003482
3. Andersen-Ranberg NC, Poulsen LM, Perner A, et al; AID-ICU Trial Group. Haloperidol for the treatment of delirium in ICU patients. N Engl J Med. 2022;387:2425-2435. doi:10.1056/NEJMoa2211868
4. Girard TD, Exline MC, Carson SS, et al; MIND-USA Investigators. Haloperidol and ziprasidone for treatment of delirium in critical illness. N Engl J Med. 2018;379:2506-2516. doi:10.1056/NEJMoa1808217
5. Riker RR, Shehabi Y, Bokesch PM, et al; SEDCOM (Safety and Efficacy of Dexmedetomidine Compared With Midazolam) Study Group. Dexmedetomidine vs midazolam for sedation of critically ill patients: a randomized trial. JAMA. 2009;301:489-499. doi:10.1001/jama.2009.56
6. Pandharipande P, Shintani A, Peterson J, et al. Lorazepam is an independent risk factor for transitioning to delirium in intensive care unit patients. Anesthesiology. 2006;104:21-26. doi:10.1097/00000542-200601000-00005
7. Society of Critical Care Medicine. ICU liberation bundle. Accessed February 27, 2024. https://www.sccm.org/ICULiberation/Home/ABCDEF-Bundles
8. Lovell T, Ranse K. Animal-assisted activities in the intensive care unit: a scoping review. Intensive Crit Care Nurs. 2022;73:103304. doi:10.1016/j.iccn.2022.103304
9. Hosey MM, Jaskulski J, Wegener ST, Chlan LL, Needham DM. Animal-assisted intervention in the ICU: a tool for humanization. Crit Care. 2018;22:22. doi:10.1186/s13054-018-1946-8
10. Jøranson N, Pedersen I, Rokstad AM, Ihlebæk C. Effects on symptoms of agitation and depression in persons with dementia participating in robot-assisted activity: a cluster-randomized controlled trial. J Am Med Dir Assoc. 2015;16:867-873. doi:10.1016/j.jamda.2015.05.002
11. Robinson H, Macdonald B, Kerse N, Broadbent E. The psychosocial effects of a companion robot: a randomized controlled trial. J Am Med Dir Assoc. 2013;14:661-667. doi:10.1016/j.jamda.2013.02.007
12. Schulman-Marcus J, Mookherjee S, Rice L, Lyubarova R. New approaches for the treatment of delirium: a case for robotic pets. Am J Med. 2019;132:781-782. doi:10.1016/j.amjmed.2018.12.039
Critical illness is commonly associated with interrelated conditions including pain, agitation, delirium, immobility, and sleep disruption (PADIS). Managing PADIS is often complex and includes pharmacologic and nonpharmacologic interventions.1 Incorporating multifaceted practices to enhance PADIS management has been shown to improve several intensive care unit (ICU)-related outcomes.2
Many pharmacologic PADIS treatments are ineffective or associated with adverse effects. For example, antipsychotics used for treating ICU-related delirium have not shown improved outcomes.3,4 Commonly used medications for agitation, such as benzodiazepines, increase delirium risk.5,6 Because of these limitations, several nonpharmacologic interventions for PADIS have been evaluated.
Pet therapy has been implemented in some ICU settings, but is not widely adopted.7 Also referred to as animal-assisted activities, animal-assisted therapy, or animal-assisted interventions, pet therapy typically involves interaction between a patient and a live animal (most commonly a dog) under the direction of an animal handler, with the intention of providing therapeutic benefit. Interactions frequently include meet and greet activities such as petting, but also could include walking or other activities. Pet therapy has been reported to reduce pain, agitation, and stress among ICU patients.8 Introducing a pet therapy program with live animals in the ICU could be challenging because of factors such as identifying trained, accredited animals and handlers, and managing infection control and other risks.9 As an alternative to live pets, robotic pet therapy has been shown to be beneficial—mostly outside the ICU—in settings such as long-term care.10,11 Although uncommon, robotic pets have been used in the ICU and hospital settings for therapeutic purposes.12 Robotic pets reduce many concerns associated with live animals while mimicking the behaviors of live animals and potentially offering many of the same benefits.
OBSERVATIONS
The North Florida/South Georgia Veterans Health System (NF/SGVHS) implemented a novel robotic pet therapy program for patients requiring ICU care to improve the treatment of PADIS. Funding was provided through a Veterans Health Administration Innovation Grant procured by a clinical pharmacy specialist as the program’s champion. Goals of the robotic pet therapy program include reductions in: distressing symptoms associated with PADIS, use of psychoactive drugs and physical restraints, and ICU length of stay. The ICU team developed standard operating procedures and an order menu, which were integrated into the ICU prescriber ordering menu. Patients were selected for pet therapy based on PADIS scores and potential for positive response to pet therapy as assessed by the ICU team.Patients in medical and surgical ICU settings were eligible for the program. The robotic pets used in the program were Joy for All Companion Pets (Ageless Innovation LLC). Robotic cats and dogs were available and pets were “adopted’ by each patient (Figure). As an infection control measure, pets were not reissued or shared amongpatients and pets could be cleaned with a disinfectant solution. Nurses were primarily responsible for monitoring and documenting responses to robotic pet therapy.
It was necessary to secure buy-in from several services to successfully implement the program. The critical care clinical pharmacy specialists were responsible for ordering, storing, and dispensing the robotic pets. The NF/SGVHS innovation specialist helped secure funding, procure the robotic pet, and promote the program. The standard operating procedures for the program were developed by a multidisciplinary team with input from critical care nurses, intensivists, pharmacists, patient safety, and infection control (Table 1). Success of the program also required buy-in from ICU team members.
Program Impact
A retrospective cohort study was conducted to assess for improvements in PADIS symptoms and medication use post-intervention. Patients were included if they received robotic pet therapy in the ICU from July 10, 2019, to February 1, 2021. Individuals aged < 18 years or > 89 years, were pregnant, or were not receiving ICU-level care were excluded. Outcomes assessed included improvement in pain scores, agitation scores, sleep quality, resolution of delirium, and use of pain or psychoactive medications during patients’ ICU stay.
Thirty patients were included in the study (Table 2). After receiving a robotic pet, 9 (30%) patients recorded decreased pain scores, 15 (50%) recorded decreased agitation scores, 8 (27%) had resolution of delirium, and 2 (7%) described improvement in sleep. Pain medication use decreased in 12 (40%) patients and psychoactive medication use was reduced in 7 (23%) patients.
Limitations
The robotic pet therapy program has shown promising results; however, some aspects merit discussion. Evaluation of this program is limited by factors such as the observational study design, single-center patient sample, and lack of comparator group. Although no known adverse effects of robotic pet therapy were seen, it is possible that some patients may not have a favorable response. Challenges of implementing a robotic pet therapy program include cost and additional operational activities (storage, ordering, dispensing) necessary to maintain the program. Additional research is needed to evaluate the impact of robotic pet therapy on other outcomes including cost, ICU length of stay, and patient satisfaction.
CONCLUSIONS
Robotic pet therapy can be successfully implemented in the ICU and appears to provide a simple, safe, beneficial, nonpharmacologic intervention for PADIS. This study showed that many patients had favorable response to robotic pet therapy, indicating that it may be a viable alternative to traditional pet therapy. Other health systems could benefit from implementing programs similar to the robotic pet therapy program at NF/SGVHS.
Acknowledgments
The author would like to acknowledge Simran Panesar, PharmD, and Theresa Faison, PharmD, for their contributions to this project.
Critical illness is commonly associated with interrelated conditions including pain, agitation, delirium, immobility, and sleep disruption (PADIS). Managing PADIS is often complex and includes pharmacologic and nonpharmacologic interventions.1 Incorporating multifaceted practices to enhance PADIS management has been shown to improve several intensive care unit (ICU)-related outcomes.2
Many pharmacologic PADIS treatments are ineffective or associated with adverse effects. For example, antipsychotics used for treating ICU-related delirium have not shown improved outcomes.3,4 Commonly used medications for agitation, such as benzodiazepines, increase delirium risk.5,6 Because of these limitations, several nonpharmacologic interventions for PADIS have been evaluated.
Pet therapy has been implemented in some ICU settings, but is not widely adopted.7 Also referred to as animal-assisted activities, animal-assisted therapy, or animal-assisted interventions, pet therapy typically involves interaction between a patient and a live animal (most commonly a dog) under the direction of an animal handler, with the intention of providing therapeutic benefit. Interactions frequently include meet and greet activities such as petting, but also could include walking or other activities. Pet therapy has been reported to reduce pain, agitation, and stress among ICU patients.8 Introducing a pet therapy program with live animals in the ICU could be challenging because of factors such as identifying trained, accredited animals and handlers, and managing infection control and other risks.9 As an alternative to live pets, robotic pet therapy has been shown to be beneficial—mostly outside the ICU—in settings such as long-term care.10,11 Although uncommon, robotic pets have been used in the ICU and hospital settings for therapeutic purposes.12 Robotic pets reduce many concerns associated with live animals while mimicking the behaviors of live animals and potentially offering many of the same benefits.
OBSERVATIONS
The North Florida/South Georgia Veterans Health System (NF/SGVHS) implemented a novel robotic pet therapy program for patients requiring ICU care to improve the treatment of PADIS. Funding was provided through a Veterans Health Administration Innovation Grant procured by a clinical pharmacy specialist as the program’s champion. Goals of the robotic pet therapy program include reductions in: distressing symptoms associated with PADIS, use of psychoactive drugs and physical restraints, and ICU length of stay. The ICU team developed standard operating procedures and an order menu, which were integrated into the ICU prescriber ordering menu. Patients were selected for pet therapy based on PADIS scores and potential for positive response to pet therapy as assessed by the ICU team.Patients in medical and surgical ICU settings were eligible for the program. The robotic pets used in the program were Joy for All Companion Pets (Ageless Innovation LLC). Robotic cats and dogs were available and pets were “adopted’ by each patient (Figure). As an infection control measure, pets were not reissued or shared amongpatients and pets could be cleaned with a disinfectant solution. Nurses were primarily responsible for monitoring and documenting responses to robotic pet therapy.
It was necessary to secure buy-in from several services to successfully implement the program. The critical care clinical pharmacy specialists were responsible for ordering, storing, and dispensing the robotic pets. The NF/SGVHS innovation specialist helped secure funding, procure the robotic pet, and promote the program. The standard operating procedures for the program were developed by a multidisciplinary team with input from critical care nurses, intensivists, pharmacists, patient safety, and infection control (Table 1). Success of the program also required buy-in from ICU team members.
Program Impact
A retrospective cohort study was conducted to assess for improvements in PADIS symptoms and medication use post-intervention. Patients were included if they received robotic pet therapy in the ICU from July 10, 2019, to February 1, 2021. Individuals aged < 18 years or > 89 years, were pregnant, or were not receiving ICU-level care were excluded. Outcomes assessed included improvement in pain scores, agitation scores, sleep quality, resolution of delirium, and use of pain or psychoactive medications during patients’ ICU stay.
Thirty patients were included in the study (Table 2). After receiving a robotic pet, 9 (30%) patients recorded decreased pain scores, 15 (50%) recorded decreased agitation scores, 8 (27%) had resolution of delirium, and 2 (7%) described improvement in sleep. Pain medication use decreased in 12 (40%) patients and psychoactive medication use was reduced in 7 (23%) patients.
Limitations
The robotic pet therapy program has shown promising results; however, some aspects merit discussion. Evaluation of this program is limited by factors such as the observational study design, single-center patient sample, and lack of comparator group. Although no known adverse effects of robotic pet therapy were seen, it is possible that some patients may not have a favorable response. Challenges of implementing a robotic pet therapy program include cost and additional operational activities (storage, ordering, dispensing) necessary to maintain the program. Additional research is needed to evaluate the impact of robotic pet therapy on other outcomes including cost, ICU length of stay, and patient satisfaction.
CONCLUSIONS
Robotic pet therapy can be successfully implemented in the ICU and appears to provide a simple, safe, beneficial, nonpharmacologic intervention for PADIS. This study showed that many patients had favorable response to robotic pet therapy, indicating that it may be a viable alternative to traditional pet therapy. Other health systems could benefit from implementing programs similar to the robotic pet therapy program at NF/SGVHS.
Acknowledgments
The author would like to acknowledge Simran Panesar, PharmD, and Theresa Faison, PharmD, for their contributions to this project.
1. Devlin JW, Skrobik Y, Gélinas C, et al. Clinical practice guidelines for the prevention and management of pain, agitation/sedation, delirium, immobility, and sleep disruption in adult patients in the ICU. Crit Care Med. 2018;46:e825-e873. doi:10.1097/CCM.0000000000003299
2. Pun BT, Balas MC, Barnes-Daly MA, et al. Caring for critically ill patients with the ABCDEF bundle: results of the ICU Liberation Collaborative in over 15,000 adults. Crit Care Med. 2019;47:3-14. doi:10.1097/CCM.0000000000003482
3. Andersen-Ranberg NC, Poulsen LM, Perner A, et al; AID-ICU Trial Group. Haloperidol for the treatment of delirium in ICU patients. N Engl J Med. 2022;387:2425-2435. doi:10.1056/NEJMoa2211868
4. Girard TD, Exline MC, Carson SS, et al; MIND-USA Investigators. Haloperidol and ziprasidone for treatment of delirium in critical illness. N Engl J Med. 2018;379:2506-2516. doi:10.1056/NEJMoa1808217
5. Riker RR, Shehabi Y, Bokesch PM, et al; SEDCOM (Safety and Efficacy of Dexmedetomidine Compared With Midazolam) Study Group. Dexmedetomidine vs midazolam for sedation of critically ill patients: a randomized trial. JAMA. 2009;301:489-499. doi:10.1001/jama.2009.56
6. Pandharipande P, Shintani A, Peterson J, et al. Lorazepam is an independent risk factor for transitioning to delirium in intensive care unit patients. Anesthesiology. 2006;104:21-26. doi:10.1097/00000542-200601000-00005
7. Society of Critical Care Medicine. ICU liberation bundle. Accessed February 27, 2024. https://www.sccm.org/ICULiberation/Home/ABCDEF-Bundles
8. Lovell T, Ranse K. Animal-assisted activities in the intensive care unit: a scoping review. Intensive Crit Care Nurs. 2022;73:103304. doi:10.1016/j.iccn.2022.103304
9. Hosey MM, Jaskulski J, Wegener ST, Chlan LL, Needham DM. Animal-assisted intervention in the ICU: a tool for humanization. Crit Care. 2018;22:22. doi:10.1186/s13054-018-1946-8
10. Jøranson N, Pedersen I, Rokstad AM, Ihlebæk C. Effects on symptoms of agitation and depression in persons with dementia participating in robot-assisted activity: a cluster-randomized controlled trial. J Am Med Dir Assoc. 2015;16:867-873. doi:10.1016/j.jamda.2015.05.002
11. Robinson H, Macdonald B, Kerse N, Broadbent E. The psychosocial effects of a companion robot: a randomized controlled trial. J Am Med Dir Assoc. 2013;14:661-667. doi:10.1016/j.jamda.2013.02.007
12. Schulman-Marcus J, Mookherjee S, Rice L, Lyubarova R. New approaches for the treatment of delirium: a case for robotic pets. Am J Med. 2019;132:781-782. doi:10.1016/j.amjmed.2018.12.039
1. Devlin JW, Skrobik Y, Gélinas C, et al. Clinical practice guidelines for the prevention and management of pain, agitation/sedation, delirium, immobility, and sleep disruption in adult patients in the ICU. Crit Care Med. 2018;46:e825-e873. doi:10.1097/CCM.0000000000003299
2. Pun BT, Balas MC, Barnes-Daly MA, et al. Caring for critically ill patients with the ABCDEF bundle: results of the ICU Liberation Collaborative in over 15,000 adults. Crit Care Med. 2019;47:3-14. doi:10.1097/CCM.0000000000003482
3. Andersen-Ranberg NC, Poulsen LM, Perner A, et al; AID-ICU Trial Group. Haloperidol for the treatment of delirium in ICU patients. N Engl J Med. 2022;387:2425-2435. doi:10.1056/NEJMoa2211868
4. Girard TD, Exline MC, Carson SS, et al; MIND-USA Investigators. Haloperidol and ziprasidone for treatment of delirium in critical illness. N Engl J Med. 2018;379:2506-2516. doi:10.1056/NEJMoa1808217
5. Riker RR, Shehabi Y, Bokesch PM, et al; SEDCOM (Safety and Efficacy of Dexmedetomidine Compared With Midazolam) Study Group. Dexmedetomidine vs midazolam for sedation of critically ill patients: a randomized trial. JAMA. 2009;301:489-499. doi:10.1001/jama.2009.56
6. Pandharipande P, Shintani A, Peterson J, et al. Lorazepam is an independent risk factor for transitioning to delirium in intensive care unit patients. Anesthesiology. 2006;104:21-26. doi:10.1097/00000542-200601000-00005
7. Society of Critical Care Medicine. ICU liberation bundle. Accessed February 27, 2024. https://www.sccm.org/ICULiberation/Home/ABCDEF-Bundles
8. Lovell T, Ranse K. Animal-assisted activities in the intensive care unit: a scoping review. Intensive Crit Care Nurs. 2022;73:103304. doi:10.1016/j.iccn.2022.103304
9. Hosey MM, Jaskulski J, Wegener ST, Chlan LL, Needham DM. Animal-assisted intervention in the ICU: a tool for humanization. Crit Care. 2018;22:22. doi:10.1186/s13054-018-1946-8
10. Jøranson N, Pedersen I, Rokstad AM, Ihlebæk C. Effects on symptoms of agitation and depression in persons with dementia participating in robot-assisted activity: a cluster-randomized controlled trial. J Am Med Dir Assoc. 2015;16:867-873. doi:10.1016/j.jamda.2015.05.002
11. Robinson H, Macdonald B, Kerse N, Broadbent E. The psychosocial effects of a companion robot: a randomized controlled trial. J Am Med Dir Assoc. 2013;14:661-667. doi:10.1016/j.jamda.2013.02.007
12. Schulman-Marcus J, Mookherjee S, Rice L, Lyubarova R. New approaches for the treatment of delirium: a case for robotic pets. Am J Med. 2019;132:781-782. doi:10.1016/j.amjmed.2018.12.039
Is it time to embrace a multinight sleep study?
Sleep Medicine Network
Respiratory-Related Sleep Disorders Section
Since the 1960s, sleep researchers have been intrigued by the first-night effect (FNE) in polysomnography (PSG) studies. A meta-analysis by Ding and colleagues revealed FNE’s impact on sleep metrics, like total sleep time and REM sleep, without affecting the apnea-hypopnea index, highlighting PSG’s limitations in simulating natural sleep patterns.1
Lechat and colleagues conducted a study using a home-based sleep analyzer on more than 67,000 individuals, averaging 170 nights each.2 This study found that single-night studies could lead to a 20% misdiagnosis rate in OSA, attributed to overlooking real sleep factors such as body posture, environmental effects, alcohol, and medication. Despite this, the wider use of multinight studies for accurate diagnosis is limited by insurance coverage issues.3
The last decade has seen substantial advances in health technology, particularly in consumer wearables capable of detecting various medical conditions. Devices employing techniques like actigraphy and accelerometry have reached a level of performance comparable with US Food and Drug Administration-approved clinical tools. However, these technologies are still in development for the diagnosis and classification of sleep-disordered breathing.
Tech companies are actively innovating sleep sensing technologies, smartwatches, bed sensors, wireless EEG, radiofrequency, and ultrasound sensors. With significant investments in this sector, these technologies could be ready for widespread use in the next 5 to 10 years. Health care professionals should consider data from sleep-tracking wearables when there are inconsistencies between a patient’s sleep study results and symptoms. The insights from these devices could provide crucial diagnostic information, enhancing the accuracy of sleep disorder diagnoses.
References
1. Ding L, Chen B, Dai Y, Li Y. A meta-analysis of the first-night effect in healthy individuals for the full age spectrum. Sleep Med. 2022;89:159-165. Preprint. Posted online December 17, 2021. PMID: 34998093. doi: 10.1016/j.sleep.2021.12.007
2. Lechat B, Naik G, Reynolds A, et al. Multinight prevalence, variability, and diagnostic misclassification of obstructive sleep apnea. Am J Respir Crit Care Med. 2022;205(5):563-569. PMID: 34904935; PMCID: PMC8906484. doi: 10.1164/rccm.202107-1761OC
3. Abreu A, Punjabi NM. How many nights are really needed to diagnose obstructive sleep apnea? Am J Respir Crit Care Med. 2022;206(1):125-126. PMID: 35476613; PMCID: PMC9954337. doi: 10.1164/rccm.202112-2837LE
Sleep Medicine Network
Respiratory-Related Sleep Disorders Section
Since the 1960s, sleep researchers have been intrigued by the first-night effect (FNE) in polysomnography (PSG) studies. A meta-analysis by Ding and colleagues revealed FNE’s impact on sleep metrics, like total sleep time and REM sleep, without affecting the apnea-hypopnea index, highlighting PSG’s limitations in simulating natural sleep patterns.1
Lechat and colleagues conducted a study using a home-based sleep analyzer on more than 67,000 individuals, averaging 170 nights each.2 This study found that single-night studies could lead to a 20% misdiagnosis rate in OSA, attributed to overlooking real sleep factors such as body posture, environmental effects, alcohol, and medication. Despite this, the wider use of multinight studies for accurate diagnosis is limited by insurance coverage issues.3
The last decade has seen substantial advances in health technology, particularly in consumer wearables capable of detecting various medical conditions. Devices employing techniques like actigraphy and accelerometry have reached a level of performance comparable with US Food and Drug Administration-approved clinical tools. However, these technologies are still in development for the diagnosis and classification of sleep-disordered breathing.
Tech companies are actively innovating sleep sensing technologies, smartwatches, bed sensors, wireless EEG, radiofrequency, and ultrasound sensors. With significant investments in this sector, these technologies could be ready for widespread use in the next 5 to 10 years. Health care professionals should consider data from sleep-tracking wearables when there are inconsistencies between a patient’s sleep study results and symptoms. The insights from these devices could provide crucial diagnostic information, enhancing the accuracy of sleep disorder diagnoses.
References
1. Ding L, Chen B, Dai Y, Li Y. A meta-analysis of the first-night effect in healthy individuals for the full age spectrum. Sleep Med. 2022;89:159-165. Preprint. Posted online December 17, 2021. PMID: 34998093. doi: 10.1016/j.sleep.2021.12.007
2. Lechat B, Naik G, Reynolds A, et al. Multinight prevalence, variability, and diagnostic misclassification of obstructive sleep apnea. Am J Respir Crit Care Med. 2022;205(5):563-569. PMID: 34904935; PMCID: PMC8906484. doi: 10.1164/rccm.202107-1761OC
3. Abreu A, Punjabi NM. How many nights are really needed to diagnose obstructive sleep apnea? Am J Respir Crit Care Med. 2022;206(1):125-126. PMID: 35476613; PMCID: PMC9954337. doi: 10.1164/rccm.202112-2837LE
Sleep Medicine Network
Respiratory-Related Sleep Disorders Section
Since the 1960s, sleep researchers have been intrigued by the first-night effect (FNE) in polysomnography (PSG) studies. A meta-analysis by Ding and colleagues revealed FNE’s impact on sleep metrics, like total sleep time and REM sleep, without affecting the apnea-hypopnea index, highlighting PSG’s limitations in simulating natural sleep patterns.1
Lechat and colleagues conducted a study using a home-based sleep analyzer on more than 67,000 individuals, averaging 170 nights each.2 This study found that single-night studies could lead to a 20% misdiagnosis rate in OSA, attributed to overlooking real sleep factors such as body posture, environmental effects, alcohol, and medication. Despite this, the wider use of multinight studies for accurate diagnosis is limited by insurance coverage issues.3
The last decade has seen substantial advances in health technology, particularly in consumer wearables capable of detecting various medical conditions. Devices employing techniques like actigraphy and accelerometry have reached a level of performance comparable with US Food and Drug Administration-approved clinical tools. However, these technologies are still in development for the diagnosis and classification of sleep-disordered breathing.
Tech companies are actively innovating sleep sensing technologies, smartwatches, bed sensors, wireless EEG, radiofrequency, and ultrasound sensors. With significant investments in this sector, these technologies could be ready for widespread use in the next 5 to 10 years. Health care professionals should consider data from sleep-tracking wearables when there are inconsistencies between a patient’s sleep study results and symptoms. The insights from these devices could provide crucial diagnostic information, enhancing the accuracy of sleep disorder diagnoses.
References
1. Ding L, Chen B, Dai Y, Li Y. A meta-analysis of the first-night effect in healthy individuals for the full age spectrum. Sleep Med. 2022;89:159-165. Preprint. Posted online December 17, 2021. PMID: 34998093. doi: 10.1016/j.sleep.2021.12.007
2. Lechat B, Naik G, Reynolds A, et al. Multinight prevalence, variability, and diagnostic misclassification of obstructive sleep apnea. Am J Respir Crit Care Med. 2022;205(5):563-569. PMID: 34904935; PMCID: PMC8906484. doi: 10.1164/rccm.202107-1761OC
3. Abreu A, Punjabi NM. How many nights are really needed to diagnose obstructive sleep apnea? Am J Respir Crit Care Med. 2022;206(1):125-126. PMID: 35476613; PMCID: PMC9954337. doi: 10.1164/rccm.202112-2837LE