User login
Managing patients who are somatizing
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Nautical metaphors build physician resilience, beat burnout
SCOTTSDALE, ARIZ. – Linda L.M. Worley, MD, was stunned when a meeting she’d requested with her supervisor to address a shortage of beds turned into a rebuke.
“You’re on the tenure track, Linda. If you want to keep your job 6 years from now, you’d best pick up the pace. You need to see 20 private patients a week, and get moving on your research and publications,” Dr. Worley remembers the supervisor saying. At the time, she was a 32-year-old mother of two, wife, academic faculty physician, and sole attending running a general hospital consultation liaison psychiatry department and the college of medicine student mental health service. She also worked as the 24/7 on-call psychiatrist for a week at a time, said Dr. Worley, now a staff psychiatrist in the Fayetteville, Ark., Veterans Health Care System of the Ozarks and chief mental health officer for South Central VA Health Care Network.
Over the decades of an academic medical career complete with tenure, and dozens of published articles and book chapters, Dr. Worley has developed a system for achieving success while avoiding burnout, based on nautical references. In a session cofacilitated by Cynthia M. Stonnington, MD, chair of psychiatry and psychology at the Mayo Clinic’s campus in Scottsdale, Ariz., Dr. Worley presented her tips for self-care at the annual meeting of the American College of Psychiatrists.
“I use the nautical framework as a bio-psycho-social-spiritual model,” Dr. Worley said in an interview. “I teach it to medical students; I teach it to residents; I teach it to distressed physicians. I even teach it to patients when I am explaining a framework for a necessary treatment approach. With sailing, you have to stay in balance. That’s the same with taking good care of ourselves so we are less likely to get sick physically and mentally,” said Dr. Worley, who commutes to Nashville, Tenn., several times a year as part of her appointment as an adjunct professor of medicine at Vanderbilt University.
Her “Smooth Sailing Life” seminars have evolved over the past 20 years and are rooted in her training in psychosomatic medicine, which she said emphasizes the complexity of the entire person. “It’s about the biology and about the emotions, and the bridge between them,” according to Dr. Worley, who has a website, SmoothSailingLife.com, and is working on a book aimed at helping to meet what she said has been a steadily growing thirst for her approach to developing resilience.
“I am not studying anyone, but I am helping people to self-diagnose. I teach people how to avoid having to see a psychiatrist or a mental health provider but also to feel good about reaching out for help when necessary,” she said. “Life is far too short to suffer needlessly.”
In the interview, Dr. Worley said she adapts her presentations to the venue and the time allowed. Key aspects of her system include:
• Care for your yacht, which is the body, including the brain. “You only get one, and if you’re going to have a chance of winning the regatta, you have to take care of it. This means getting good sleep, nutrition, exercise, preventive care, rest, and rejuvenation, including vacation,” Dr. Worley said.
• Chart your course; have a navigational plan that includes your life goals and aspirations. Identify and rely upon “landmarks,” such as being a good spouse, mother, physician, or friend for the most authentic definition of personal success. “These are like buoys that keep us sailing in the right direction,” she said.
• Reef in your sails, meaning mind the “winds that come at us from every side,” she said. This includes triaging tasks and not letting perfectionism get in the way. “Perfectionists take too long to tack; they don’t know when it’s time to turn in the other direction,” she said. “If you want to finish the race, you have to do the best you can in the time you have.” This was the lesson Dr. Worley said she learned that day when she was a young physician feeling overwhelmed.
• Empty your bilge, the nautical term for removing waste water from within the hull. Dr. Worley uses this as a metaphor for identifying and expressing negative emotions of fear, anxiety, sadness, and frustration. “These vital emotions are giving us important messages. It is important to recognize that they are present. Name and accept them, and understand what they are trying to tell us. Is it a symptom of an underlying illness that needs treatment? A conflict in a relationship? A need not being met? Are you living your deepest values? Express the emotions and sort through the best response,” she said. “It’s all part of emotional intelligence.”
• Keep an even keel, which is Dr. Worley’s way of stating the importance of being connected to love and to living your deepest values. “The keel is your character, your connection to meaning, a spiritual connection. In medicine, we shy away from that. I have only lately ventured into talking about this,” she said, noting that this connection can come in numerous ways, such as meditation, and being in nature or with animals. “It’s very personal. It’s hard to quantify, but I have witnessed it and its healing power within the therapeutic alliance.”
In break-out sessions during her well-attended talk at the meeting, Dr. Worley listened as psychiatrists of all levels of experience and responsibility, ranging from medical directors to those in private community practice, shared the kinds of concerns she said she often encounters in her role as a core faculty member of the Program for Distressed Physicians at the Vanderbilt Center for Professional Health.
“Changes in medicine have been so frustrating; physicians are at their wits’ end. We don’t recruit people into medicine because they have a skill set for expressing their emotions, or taking care of themselves, or dealing with conflict,” she said. “That’s okay. They can learn it.”
[email protected]
On Twitter @whitneymcknight
SCOTTSDALE, ARIZ. – Linda L.M. Worley, MD, was stunned when a meeting she’d requested with her supervisor to address a shortage of beds turned into a rebuke.
“You’re on the tenure track, Linda. If you want to keep your job 6 years from now, you’d best pick up the pace. You need to see 20 private patients a week, and get moving on your research and publications,” Dr. Worley remembers the supervisor saying. At the time, she was a 32-year-old mother of two, wife, academic faculty physician, and sole attending running a general hospital consultation liaison psychiatry department and the college of medicine student mental health service. She also worked as the 24/7 on-call psychiatrist for a week at a time, said Dr. Worley, now a staff psychiatrist in the Fayetteville, Ark., Veterans Health Care System of the Ozarks and chief mental health officer for South Central VA Health Care Network.
Over the decades of an academic medical career complete with tenure, and dozens of published articles and book chapters, Dr. Worley has developed a system for achieving success while avoiding burnout, based on nautical references. In a session cofacilitated by Cynthia M. Stonnington, MD, chair of psychiatry and psychology at the Mayo Clinic’s campus in Scottsdale, Ariz., Dr. Worley presented her tips for self-care at the annual meeting of the American College of Psychiatrists.
“I use the nautical framework as a bio-psycho-social-spiritual model,” Dr. Worley said in an interview. “I teach it to medical students; I teach it to residents; I teach it to distressed physicians. I even teach it to patients when I am explaining a framework for a necessary treatment approach. With sailing, you have to stay in balance. That’s the same with taking good care of ourselves so we are less likely to get sick physically and mentally,” said Dr. Worley, who commutes to Nashville, Tenn., several times a year as part of her appointment as an adjunct professor of medicine at Vanderbilt University.
Her “Smooth Sailing Life” seminars have evolved over the past 20 years and are rooted in her training in psychosomatic medicine, which she said emphasizes the complexity of the entire person. “It’s about the biology and about the emotions, and the bridge between them,” according to Dr. Worley, who has a website, SmoothSailingLife.com, and is working on a book aimed at helping to meet what she said has been a steadily growing thirst for her approach to developing resilience.
“I am not studying anyone, but I am helping people to self-diagnose. I teach people how to avoid having to see a psychiatrist or a mental health provider but also to feel good about reaching out for help when necessary,” she said. “Life is far too short to suffer needlessly.”
In the interview, Dr. Worley said she adapts her presentations to the venue and the time allowed. Key aspects of her system include:
• Care for your yacht, which is the body, including the brain. “You only get one, and if you’re going to have a chance of winning the regatta, you have to take care of it. This means getting good sleep, nutrition, exercise, preventive care, rest, and rejuvenation, including vacation,” Dr. Worley said.
• Chart your course; have a navigational plan that includes your life goals and aspirations. Identify and rely upon “landmarks,” such as being a good spouse, mother, physician, or friend for the most authentic definition of personal success. “These are like buoys that keep us sailing in the right direction,” she said.
• Reef in your sails, meaning mind the “winds that come at us from every side,” she said. This includes triaging tasks and not letting perfectionism get in the way. “Perfectionists take too long to tack; they don’t know when it’s time to turn in the other direction,” she said. “If you want to finish the race, you have to do the best you can in the time you have.” This was the lesson Dr. Worley said she learned that day when she was a young physician feeling overwhelmed.
• Empty your bilge, the nautical term for removing waste water from within the hull. Dr. Worley uses this as a metaphor for identifying and expressing negative emotions of fear, anxiety, sadness, and frustration. “These vital emotions are giving us important messages. It is important to recognize that they are present. Name and accept them, and understand what they are trying to tell us. Is it a symptom of an underlying illness that needs treatment? A conflict in a relationship? A need not being met? Are you living your deepest values? Express the emotions and sort through the best response,” she said. “It’s all part of emotional intelligence.”
• Keep an even keel, which is Dr. Worley’s way of stating the importance of being connected to love and to living your deepest values. “The keel is your character, your connection to meaning, a spiritual connection. In medicine, we shy away from that. I have only lately ventured into talking about this,” she said, noting that this connection can come in numerous ways, such as meditation, and being in nature or with animals. “It’s very personal. It’s hard to quantify, but I have witnessed it and its healing power within the therapeutic alliance.”
In break-out sessions during her well-attended talk at the meeting, Dr. Worley listened as psychiatrists of all levels of experience and responsibility, ranging from medical directors to those in private community practice, shared the kinds of concerns she said she often encounters in her role as a core faculty member of the Program for Distressed Physicians at the Vanderbilt Center for Professional Health.
“Changes in medicine have been so frustrating; physicians are at their wits’ end. We don’t recruit people into medicine because they have a skill set for expressing their emotions, or taking care of themselves, or dealing with conflict,” she said. “That’s okay. They can learn it.”
[email protected]
On Twitter @whitneymcknight
SCOTTSDALE, ARIZ. – Linda L.M. Worley, MD, was stunned when a meeting she’d requested with her supervisor to address a shortage of beds turned into a rebuke.
“You’re on the tenure track, Linda. If you want to keep your job 6 years from now, you’d best pick up the pace. You need to see 20 private patients a week, and get moving on your research and publications,” Dr. Worley remembers the supervisor saying. At the time, she was a 32-year-old mother of two, wife, academic faculty physician, and sole attending running a general hospital consultation liaison psychiatry department and the college of medicine student mental health service. She also worked as the 24/7 on-call psychiatrist for a week at a time, said Dr. Worley, now a staff psychiatrist in the Fayetteville, Ark., Veterans Health Care System of the Ozarks and chief mental health officer for South Central VA Health Care Network.
Over the decades of an academic medical career complete with tenure, and dozens of published articles and book chapters, Dr. Worley has developed a system for achieving success while avoiding burnout, based on nautical references. In a session cofacilitated by Cynthia M. Stonnington, MD, chair of psychiatry and psychology at the Mayo Clinic’s campus in Scottsdale, Ariz., Dr. Worley presented her tips for self-care at the annual meeting of the American College of Psychiatrists.
“I use the nautical framework as a bio-psycho-social-spiritual model,” Dr. Worley said in an interview. “I teach it to medical students; I teach it to residents; I teach it to distressed physicians. I even teach it to patients when I am explaining a framework for a necessary treatment approach. With sailing, you have to stay in balance. That’s the same with taking good care of ourselves so we are less likely to get sick physically and mentally,” said Dr. Worley, who commutes to Nashville, Tenn., several times a year as part of her appointment as an adjunct professor of medicine at Vanderbilt University.
Her “Smooth Sailing Life” seminars have evolved over the past 20 years and are rooted in her training in psychosomatic medicine, which she said emphasizes the complexity of the entire person. “It’s about the biology and about the emotions, and the bridge between them,” according to Dr. Worley, who has a website, SmoothSailingLife.com, and is working on a book aimed at helping to meet what she said has been a steadily growing thirst for her approach to developing resilience.
“I am not studying anyone, but I am helping people to self-diagnose. I teach people how to avoid having to see a psychiatrist or a mental health provider but also to feel good about reaching out for help when necessary,” she said. “Life is far too short to suffer needlessly.”
In the interview, Dr. Worley said she adapts her presentations to the venue and the time allowed. Key aspects of her system include:
• Care for your yacht, which is the body, including the brain. “You only get one, and if you’re going to have a chance of winning the regatta, you have to take care of it. This means getting good sleep, nutrition, exercise, preventive care, rest, and rejuvenation, including vacation,” Dr. Worley said.
• Chart your course; have a navigational plan that includes your life goals and aspirations. Identify and rely upon “landmarks,” such as being a good spouse, mother, physician, or friend for the most authentic definition of personal success. “These are like buoys that keep us sailing in the right direction,” she said.
• Reef in your sails, meaning mind the “winds that come at us from every side,” she said. This includes triaging tasks and not letting perfectionism get in the way. “Perfectionists take too long to tack; they don’t know when it’s time to turn in the other direction,” she said. “If you want to finish the race, you have to do the best you can in the time you have.” This was the lesson Dr. Worley said she learned that day when she was a young physician feeling overwhelmed.
• Empty your bilge, the nautical term for removing waste water from within the hull. Dr. Worley uses this as a metaphor for identifying and expressing negative emotions of fear, anxiety, sadness, and frustration. “These vital emotions are giving us important messages. It is important to recognize that they are present. Name and accept them, and understand what they are trying to tell us. Is it a symptom of an underlying illness that needs treatment? A conflict in a relationship? A need not being met? Are you living your deepest values? Express the emotions and sort through the best response,” she said. “It’s all part of emotional intelligence.”
• Keep an even keel, which is Dr. Worley’s way of stating the importance of being connected to love and to living your deepest values. “The keel is your character, your connection to meaning, a spiritual connection. In medicine, we shy away from that. I have only lately ventured into talking about this,” she said, noting that this connection can come in numerous ways, such as meditation, and being in nature or with animals. “It’s very personal. It’s hard to quantify, but I have witnessed it and its healing power within the therapeutic alliance.”
In break-out sessions during her well-attended talk at the meeting, Dr. Worley listened as psychiatrists of all levels of experience and responsibility, ranging from medical directors to those in private community practice, shared the kinds of concerns she said she often encounters in her role as a core faculty member of the Program for Distressed Physicians at the Vanderbilt Center for Professional Health.
“Changes in medicine have been so frustrating; physicians are at their wits’ end. We don’t recruit people into medicine because they have a skill set for expressing their emotions, or taking care of themselves, or dealing with conflict,” she said. “That’s okay. They can learn it.”
[email protected]
On Twitter @whitneymcknight
EXPERT ANALYSIS FROM THE AMERICAN COLLEGE OF PSYCHIATRISTS MEETING
Valbenazine for tardive dyskinesia
Despite improvements in the tolerability of antipsychotic medications, the development of tardive dyskinesia (TD) still is a significant area of concern; however, clinicians have had few treatment options. Valbenazine, a vesicular monoamine transport type 2 (VMAT2) inhibitor, is the only FDA-approved medication for TD (Table 1).1 By modulating dopamine transport into presynaptic vesicles, synaptic dopamine release is decreased, thereby reducing the postsynaptic stimulation of D2 receptors and the severity of dyskinetic movements.

In the pivotal 6-week clinical trial, valbenazine significantly reduced TD severity as measured by Abnormal Involuntary Movement Scale (AIMS) ratings.2 Study completion rates were high (87.6%), with only 2 dropouts because of adverse events in each of the placebo (n = 78) and 40-mg (n = 76) arms, and 3 in the 80-mg group (n = 80).
Before the development of valbenazine, tetrabenazine was the only effective option for treating TD. Despite tetrabenazine’s known efficacy for TD, it was not available in the United States until 2008 with the sole indication for movements related to Huntington’s disease. U.S. patients often were subjected to a litany of ineffective medications for TD, often at great expense. Moreover, tetrabenazine involved multiple daily dosing, required cytochrome P450 (CYP) 2D6 genotyping for doses >50 mg/d, had significant tolerability issues, and a monthly cost of $8,000 to $10,000. The availability of an agent that is effective for TD and does not have tetrabenazine’s kinetic limitations, adverse effect profile, or CYP2D6 monitoring requirements represents an enormous advance in the treatment of TD.
Clinical implications
Tardive dyskinesia remains a significant public health concern because of the increasing use of antipsychotics for disorders beyond the core indication for schizophrenia. Although exposure to dopamine D2 antagonism could result in postsynaptic receptor upregulation and supersensitivity, this process best explains what underlies withdrawal dyskinesia.3 The persistence of TD symptoms in 66% to 80% of patients after discontinuing offending agents has led to hypotheses that the underlying pathophysiology of TD might best be conceptualized as a problem with neuroplasticity. As with many disorders, environmental contributions (eg, oxidative stress) and genetic predisposition might play a role beyond that related to exposure to D2 antagonism.3
There have been trials of numerous agents, but no medication has been FDA-approved for treating TD, and limited data support the efficacy of a few existing medications (clonazepam, amantadine, and ginkgo biloba extract [EGb-761]),4 albeit with small effect sizes. A medical food, consisting of branched-chain amino acids, received FDA approval for the dietary management of TD in males, but is no longer commercially available except from compounding pharmacies.5
Tetrabenazine, a molecule developed in the mid-1950s to improve on the tolerability of reserpine, was associated with significant adverse effects such as orthostasis.6 Like reserpine, tetrabenazine subsequently was found to be effective for TD7 but without the peripheral adverse effects of reserpine. However, the kinetics of tetrabenazine necessitated multiple daily doses, and required CYP2D6 genotyping for doses >50 mg/d.8
Receptor blocking. The mechanism that differentiated reserpine’s and tetrabenazine’s clinical properties became clearer in the 1980s when researchers discovered that transporters were necessary to package neurotransmitters into the synaptic vesicles of presynaptic neurons.9 The vesicular monoamine transporter (VMAT) exists in 2 isoforms (VMAT1 and VMAT2) that vary in distribution, with VMAT1 expressed mainly in the peripheral nervous system and VMAT2 expressed mainly in monoaminergic cells of the central nervous system.10
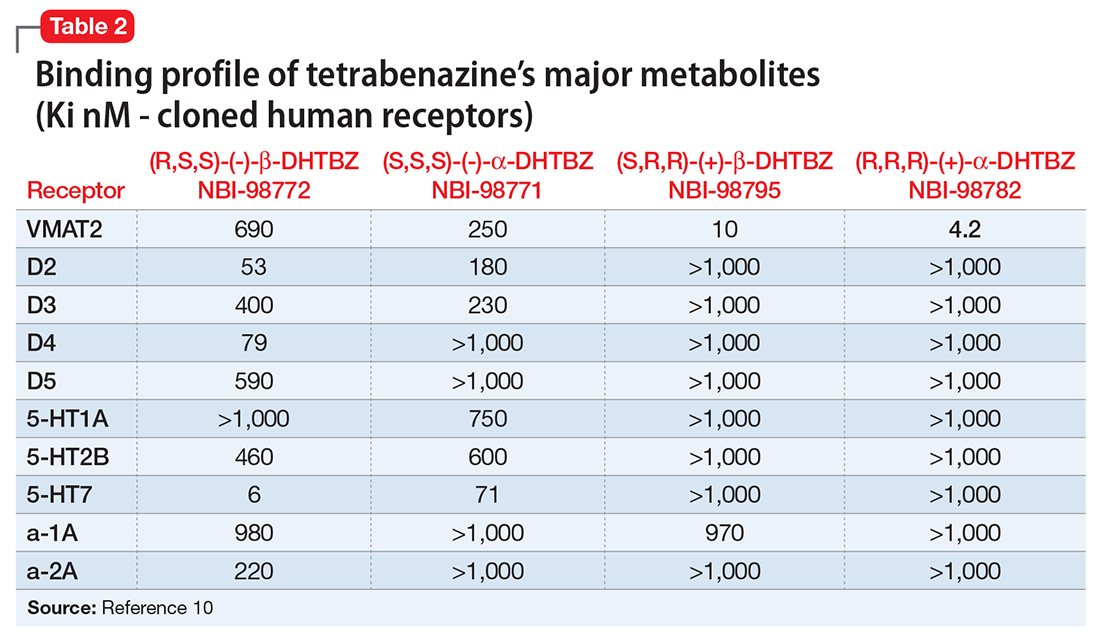
Tetrabenazine’s improved tolerability profile was related to the fact that it is a specific and reversible VMAT2 inhibitor, while reserpine is an irreversible and nonselective antagonist of both VMAT isoforms. Investigation of tetrabenazine’s metabolism revealed that it is rapidly and extensively converted into 2 isomers, α-dihydrotetrabenazine (DH-TBZ) and β-DH-TBZ. The isomeric forms of DH-TBZ have multiple chiral centers, and therefore numerous forms of which only 2 are significantly active at VMAT2.3 The α–DH-TBZ isomer is metabolized via CYP2D6 and 3A4 into inactive metabolites, while β-DH-TBZ is metabolized solely via 2D6.3 Because of the short half-life of DH-TBZ when generated from oral tetrabenazine, the existence of 2D6 polymorphisms, and the predominant activity deriving from only 2 isomers, a molecule was synthesized (valbenazine), that when metabolized would slowly be converted into the most active isomer of α–DH-TBZ designated as NBI-98782 (Table 2). This slower conversion to NBI-98782 from valbenazine (compared with its formation from oral tetrabenazine) yielded improved kinetics and permitted once-daily dosing; moreover, because the metabolism of NBI-98782 is not solely dependent on CYP2D6, the need for genotyping was removed. Neither of the 2 metabolites from valbenazine NBI-98782 and NB-136110 have significant affinity for targets other than VMAT2.11
Use in tardive dyskinesia. Recommended starting dosage is 40 mg once daily with or without food, increased to 80 mg after 1 week, based on the design and results from the phase-III clinical trial.12 The FDA granted breakthrough therapy designation for this compound, and only 1 phase-III trial was performed. Valbenazine produced significant improvement on the AIMS, with a mean 30% reduction in AIMS scores at the Week 6 endpoint from baseline of 10.4 ± 3.6.2 The effect size was large (Cohen’s d = 0.90) for the 80-mg dosage. Continuation of 40 mg/d may be considered for some patients based on tolerability, including those who are known CYP2D6 poor metabolizers, and those taking strong CYP2D6 inhibitors. Patients taking strong 3A4 inhibitors should not exceed 40 mg/d. The maximum daily dose is 40 mg for those who have moderate or severe hepatic impairment (Child-Pugh score, 7 to 15). Dosage adjustment is not required for mild to moderate renal impairment (creatinine clearance, 30 to 90 mL/min).
Pharmacologic profile, adverse reactions
Valbenazine and its 2 metabolites lack affinity for receptors other than VMAT2, leading to an absence of orthostasis in clinical trials.1,2 In the phase-II trial, 76% of participants receiving valbenazine (n = 51) were titrated to the maximum dosage of 75 mg/d. Common adverse reactions (incidence ≥5% and at least twice the rate of placebo) were headache (9.8% vs 4.1% placebo), fatigue (9.8% vs 4.1% placebo), and somnolence (5.9% vs 2% placebo).1 In the phase-III trial, participants were randomized 1:1:1 to valbenazine, 40 mg (n = 72), valbenazine, 80 mg (n = 79), or placebo (n = 76). In the clinical studies the most common diagnosis was schizophrenia or schizoaffective disorder, and 40% and 85% of participants in the phase-II and phase-III studies, respectively, remained on antipsychotics.1,2 There were no adverse effects with an incidence ≥5% and at least twice the rate of placebo in the phase-III trial.2
When data from all placebo-controlled studies were pooled, only 1 adverse effect occurred with an incidence ≥5% and twice that of placebo, somnolence with a rate of 10.9% for valbenazine vs 4.2% for placebo. The incidence of akathisia in the pooled analysis was 2.7% for valbenazine vs 0.5% for placebo. Importantly, in neither study was there a safety signal related to depression, suicidal ideation and behavior, or parkinsonism. There also were no clinically significant changes in measures of schizophrenia symptoms.
The mean QT prolongation for valbenazine in healthy participants was 6.7 milliseconds, with the upper bound of the double-sided 90% confidence interval reaching 8.4 milliseconds. For those taking strong 2D6 or 3A4 inhibitors, or known 2D6 poor metabolizers, the mean QT prolongation was 11.7 milliseconds (14.7 milliseconds upper bound of double-sided 90% CI). In the controlled trials, there was a dose-related increase in prolactin, alkaline phosphatase, and bilirubin. Overall, 3% of valbenazine-treated patients and 2% of placebo-treated patients discontinued because of adverse reactions.
As noted above, there were no adverse effects with an incidence ≥5% and at least twice the rate of placebo in the phase-III valbenazine trial. Aggregate data across all placebo-controlled studies found that somnolence was the only adverse effect that occurred with an incidence ≥5% and twice that of placebo (10.9% for valbenazine vs 4.2% for placebo).2 As a comparsion, rates of sedation and akathisia for tetrabenazine were higher in the pivotal Huntington’s disease trial: sedation/somnolence 31% vs 3% for placebo, and akathisia 19% vs 0% for placebo.8
How it works
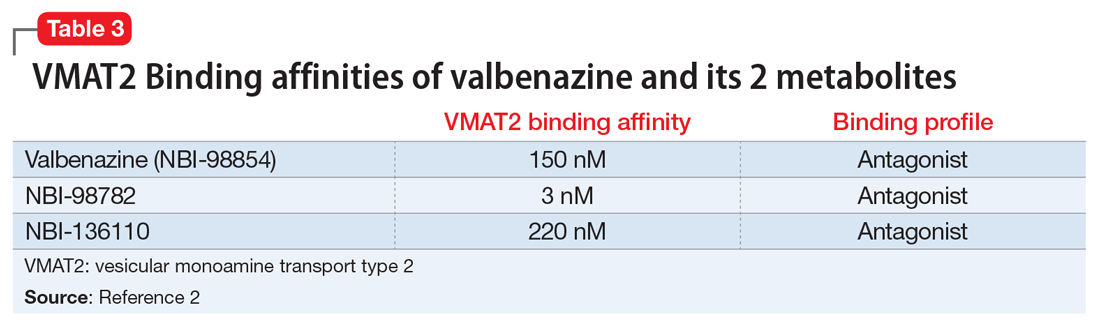
Tetrabenazine, a selective VMAT2 inhibitor, is the only agent that has demonstrated significant efficacy and tolerability for TD management; however, its complex metabolism generates numerous isomers of the metabolites α-DH-TBZ and β-DH-TBZ, of which only 2 are significantly active (Table 3). By choosing an active isomer (NBI-98782) as the metabolite of interest because of its selective and potent activity at VMAT2 and having a metabolism not solely dependent on CYP2D6, a compound was generated (valbenazine) that when metabolized slowly converts into NBI-98782.
Pharmacokinetics
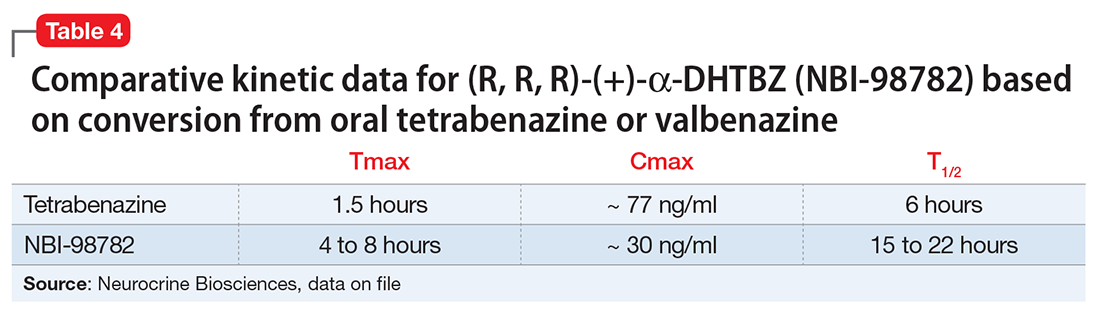
Valbenazine demonstrates dose-proportional pharmacokinetics after single oral dosages from 40 to 300 mg with no impact of food or fasting status on levels of the active metabolite. Valbenazine has a Tmax of 0.5 to 1.0 hours, with 49% oral bioavailability. The plasma half-life for valbenazine and for NBI-98782 ranges from 15 to 22 hours. The Tmax for NBI-98782 when formed from valbenazine occurs between 4 and 8 hours, with a Cmax of approximately 30 ng/mL. It should be noted that when NBI-98782 is generated from oral tetrabenazine, the mean half-life and Tmax are considerably shorter (6 hours and 1.5 hours, respectively), while the Cmax is much higher (approximately 77 ng/mL) (Table 4).
Valbenazine is metabolized through endogenous esterases to NBI-98782 and NBI-136110. NBI-98782, the active metabolite, is further metabolized through multiple CYP pathways, predominantly 3A4 and 2D6. Neither valbenazine nor its metabolites are inhibitors or inducers of major CYP enzymes. Aside from VMAT2, the results of in vitro studies suggest that valbenazine and its active metabolite are unlikely to inhibit most major drug transporters at clinically relevant concentrations. However, valbenazine increased digoxin levels because of inhibition of intestinal P-glycoprotein; therefore plasma digoxin level monitoring is recommended when these 2 are co-administered.
Efficacy
Efficacy was established in a 6-week, fixed-dosage, double-blind, placebo-controlled trial of adult patients with TD. Eligible participants had:
- DSM-IV diagnosis of antipsychotic-induced TD for ≥3 months before screening and moderate or severe TD, as indicated by AIMS item 8 (severity of abnormal movement), which was rated by a blinded, external reviewer using a video of the participant’s AIMS assessment at screening
- a DSM-IV diagnosis of schizophrenia or schizoaffective disorder or mood disorder (and stable per investigator)
- Brief Psychiatric Rating Scale score <50 at screening.
Exclusion criteria included clinically significant and unstable medical conditions within 1 month before screening; comorbid movement disorder (eg, parkinsonism, akathisia, truncal dystonia) that was more prominent than TD; and significant risk for active suicidal ideation, suicidal behavior, or violent behavior.2 Participants had a mean age of 56, 52% were male, and 65.7% of participants in the valbenazine 40-mg group had a schizophrenia spectrum disorder diagnosis, as did 65.8% in both the placebo and valbenazine 80-mg arms.
Antipsychotic treatments were permitted during the trial and >85% of participants continued taking these medications during the study. Participants (N = 234) were randomly allocated in a 1:1:1 manner to valbenazine 40 mg, 80 mg, or matched placebo. The primary outcome was change in AIMS total score (items 1 to 7) assessed by central, independent raters. Baseline AIMS scores were 9.9 ± 4.3 in the placebo group, and 9.8 ± 4.1 and 10.4 ± 3.6 in the valbenazine 40-mg and 80-mg arms, respectively.2
Outcome. A fixed-sequence testing procedure to control for family-wise error rate and multiplicity was employed, and the primary endpoint was change from baseline to Week 6 in AIMS total score (items 1 to 7) for valbenazine 80 mg vs placebo. Valbenazine, 40 mg, was associated with a 1.9 point decrease in AIMS score, while valbenazine, 80 mg, was associated with a 3.2 point decrease in AIMS score, compared with 0.1 point decrease for placebo (P < .05 for valbenazine, 40 mg, P < .001 for valbenazine, 80 mg). This difference for the 40-mg dosage did not meet the prespecified analysis endpoints; however, for the 80-mg valbenazine dosage, the effect size for this difference (Cohen’s d) was large 0.90. There also were statistically significant differences between 40 mg and 80 mg at weeks 2, 4, and 6 in the intent-to-treat population. Of the 79 participants, 43 taking the 80-mg dosage completed a 48-week extension. Efficacy was sustained in this group; however, when valbenazine was discontinued at Week 48, AIMS scores returned to baseline after 4 weeks.
Tolerability
Of the 234 randomized patients, 205 (87.6%) completed the 6-week trial. Discontinuations due to adverse events were low across all treatment groups: 2.6% and 2.8% in the placebo and valbenazine 40-mg arms, respectively, and 3.8% in valbenazine 80-mg cohort. There was no safety signal based on changes in depression, suicidality, parkinsonism rating, or changes in schizophrenia symptoms. Because valbenazine can cause somnolence, patients should not perform activities requiring mental alertness (eg, operating a vehicle or hazardous machinery) until they know how they will be affected by valbenazine.
Valbenazine should be avoided in patients with congenital long QT syndrome or with arrhythmias associated with a prolonged QT interval. For patients at increased risk of a prolonged QT interval, assess the QT interval before increasing the dosage.
Clinical considerations
Unique properties. Valbenazine is metabolized slowly to a potent, selective VMAT2 antagonist (NBI-98782) in a manner that permits once daily dosing, removes the need for CYP2D6 genotyping, and provides significant efficacy.
Why Rx? The reasons to prescribe valbenazine for TD patients include:
- currently the only agent with FDA approval for TD
- fewer tolerability issues seen with the only other effective agent, tetrabenazine
- no signal for effects on mood parameters or rates of parkinsonism
- lack of multiple daily dosing and possible need for 2D6 genotyping involved with TBZ prescribing.
Dosing
The recommended dosage of valbenazine is 80 mg/d administered as a single dose with or without food, starting at 40 mg once daily for 1 week. There is no dosage adjustment required in those with mild to moderate renal impairment; however, valbenazine is not recommended in those with severe renal impairment. The maximum dose is 40 mg/d for those who with moderate or severe hepatic impairment (Child-Pugh score, 7 to 15) however, valbenazine is not recommended for patients with severe renal impairment (creatinine clearance <30 mL/min) because the exposure to the active metabolite is reduced by approximately 75%. The combined efficacy and tolerability of dosages >80 mg/d has not been evaluated. Adverse effects seen with tetrabenazine at higher dosages include akathisia, anxiety, insomnia, parkinsonism, fatigue, and depression.
A daily dose of 40 mg may be considered for some patients based on tolerability, including those who are known CYP 2D6 poor metabolizers, and those taking strong CYP2D6 inhibitors.2 For those taking strong 3A4 inhibitors, the maximum daily dose is 40 mg. Concomitant use of valbenazine with strong 3A4 inducers is not recommended as the exposure to the active metabolite is reduced by approximately 75%.2 Lastly, because VMAT2 inhibition may alter synaptic levels of other monoamines, it is recommended that valbenazine not be administered with monoamine oxidase inhibitors, such as isocarboxazid, phenelzine, or selegiline.
Contraindications
There are no reported contraindications for valbenazine. As with most medications, there is limited available data on valbenazine use in pregnant women; however, administration of valbenazine to pregnant rats during organogenesis through lactation produced an increase in the number of stillborn pups and postnatal pup mortalities at doses under the maximum recommended human dose (MRHD) using body surface area based dosing (mg/m2). Pregnant women should be advised of the potential risk to a fetus. Valbenazine and its metabolites have been detected in rat milk at concentrations higher than in plasma after oral administration of valbenazine at doses 0.1 to 1.2 times the MRHD (based on mg/m2). Based on animal findings of increased perinatal mortality in exposed fetuses and pups, woman are advised not to breastfeed during valbenazine treatment and for 5 days after the final dose. No dosage adjustment is required for geriatric patients.
1. O’Brien CF, Jimenez R, Hauser RA, et al. NBI-98854, a selective monoamine transport inhibitor for the treatment of tardive dyskinesia: a randomized, double-blind, placebo-controlled study. Mov Disord. 2015;30(12):1681-1687.
2. Ingrezza [package insert]. San Diego, CA: Neurocrine Biosciences Inc.; 2017.
3. Marder S, Knesevich MA, Hauser RA, et al. KINECT 3: A randomized, double-blind, placebo-controlled phase 3 trial of valbenazine (NBI-98854) for tardive dyskinesia. Poster presented at the American Psychiatric Association Annual Meeting; May 14-18, 2016; Atlanta, GA.
4. Kazamatsuri H, Chien C, Cole JO. Treatment of tardive dyskinesia. I. Clinical efficacy of a dopamine-depleting agent, tetrabenazine. Arch Gen Psychiatry. 1972;27(1):95-99.
5. Richardson MA, Bevans ML, Read LL, et al. Efficacy of the branched-chain amino acids in the treatment of tardive dyskinesia in men. Am J Psychiatry. 2003;160(6):1117-1124.
6. Jankovic J, Clarence-Smith K. Tetrabenazine for the treatment of chorea and other hyperkinetic movement disorders. Expert Rev Neurother. 2011;11(11):1509-1523.
7. Meyer JM. Forgotten but not gone: new developments in the understanding and treatment of tardive dyskinesia. CNS Spectr. 2016;21(S1):13-24.
8. Bhidayasiri R, Fahn S, Weiner WJ, et al; American Academy of Neurology. Evidence-based guideline: treatment of tardive syndromes: report of the Guideline Development Subcommittee of the American Academy of Neurology. Neurology. 2013;81(5):463-469.
9. Quinn GP, Shore PA, Brodie BB. Biochemical and pharmacological studies of RO 1-9569 (tetrabenazine), a nonindole tranquilizing agent with reserpine-like effects. J Pharmacol Exp Ther. 1959;127:103-109.
10. Scherman D, Weber MJ. Characterization of the vesicular monoamine transporter in cultured rat sympathetic neurons: persistence upon induction of cholinergic phenotypic traits. Dev Biol. 1987;119(1):68-74.
11. Erickson JD, Schafer MK, Bonner TI, et al. Distinct pharmacological properties and distribution in neurons and endocrine cells of two isoforms of the human vesicular monoamine transporter. Proc Natl Acad Sci U S A. 1996;93(10):5166-5171.
12. Grigoriadis DE, Smith E, Madan A, et al. Pharmacologic characteristics of valbenazine (NBI-98854) and its metabolites. Poster presented at the U.S. Psychiatric & Mental Health Congress, October 21-24, 2016; San Antonio, TX.
Despite improvements in the tolerability of antipsychotic medications, the development of tardive dyskinesia (TD) still is a significant area of concern; however, clinicians have had few treatment options. Valbenazine, a vesicular monoamine transport type 2 (VMAT2) inhibitor, is the only FDA-approved medication for TD (Table 1).1 By modulating dopamine transport into presynaptic vesicles, synaptic dopamine release is decreased, thereby reducing the postsynaptic stimulation of D2 receptors and the severity of dyskinetic movements.

In the pivotal 6-week clinical trial, valbenazine significantly reduced TD severity as measured by Abnormal Involuntary Movement Scale (AIMS) ratings.2 Study completion rates were high (87.6%), with only 2 dropouts because of adverse events in each of the placebo (n = 78) and 40-mg (n = 76) arms, and 3 in the 80-mg group (n = 80).
Before the development of valbenazine, tetrabenazine was the only effective option for treating TD. Despite tetrabenazine’s known efficacy for TD, it was not available in the United States until 2008 with the sole indication for movements related to Huntington’s disease. U.S. patients often were subjected to a litany of ineffective medications for TD, often at great expense. Moreover, tetrabenazine involved multiple daily dosing, required cytochrome P450 (CYP) 2D6 genotyping for doses >50 mg/d, had significant tolerability issues, and a monthly cost of $8,000 to $10,000. The availability of an agent that is effective for TD and does not have tetrabenazine’s kinetic limitations, adverse effect profile, or CYP2D6 monitoring requirements represents an enormous advance in the treatment of TD.
Clinical implications
Tardive dyskinesia remains a significant public health concern because of the increasing use of antipsychotics for disorders beyond the core indication for schizophrenia. Although exposure to dopamine D2 antagonism could result in postsynaptic receptor upregulation and supersensitivity, this process best explains what underlies withdrawal dyskinesia.3 The persistence of TD symptoms in 66% to 80% of patients after discontinuing offending agents has led to hypotheses that the underlying pathophysiology of TD might best be conceptualized as a problem with neuroplasticity. As with many disorders, environmental contributions (eg, oxidative stress) and genetic predisposition might play a role beyond that related to exposure to D2 antagonism.3
There have been trials of numerous agents, but no medication has been FDA-approved for treating TD, and limited data support the efficacy of a few existing medications (clonazepam, amantadine, and ginkgo biloba extract [EGb-761]),4 albeit with small effect sizes. A medical food, consisting of branched-chain amino acids, received FDA approval for the dietary management of TD in males, but is no longer commercially available except from compounding pharmacies.5
Tetrabenazine, a molecule developed in the mid-1950s to improve on the tolerability of reserpine, was associated with significant adverse effects such as orthostasis.6 Like reserpine, tetrabenazine subsequently was found to be effective for TD7 but without the peripheral adverse effects of reserpine. However, the kinetics of tetrabenazine necessitated multiple daily doses, and required CYP2D6 genotyping for doses >50 mg/d.8
Receptor blocking. The mechanism that differentiated reserpine’s and tetrabenazine’s clinical properties became clearer in the 1980s when researchers discovered that transporters were necessary to package neurotransmitters into the synaptic vesicles of presynaptic neurons.9 The vesicular monoamine transporter (VMAT) exists in 2 isoforms (VMAT1 and VMAT2) that vary in distribution, with VMAT1 expressed mainly in the peripheral nervous system and VMAT2 expressed mainly in monoaminergic cells of the central nervous system.10
Tetrabenazine’s improved tolerability profile was related to the fact that it is a specific and reversible VMAT2 inhibitor, while reserpine is an irreversible and nonselective antagonist of both VMAT isoforms. Investigation of tetrabenazine’s metabolism revealed that it is rapidly and extensively converted into 2 isomers, α-dihydrotetrabenazine (DH-TBZ) and β-DH-TBZ. The isomeric forms of DH-TBZ have multiple chiral centers, and therefore numerous forms of which only 2 are significantly active at VMAT2.3 The α–DH-TBZ isomer is metabolized via CYP2D6 and 3A4 into inactive metabolites, while β-DH-TBZ is metabolized solely via 2D6.3 Because of the short half-life of DH-TBZ when generated from oral tetrabenazine, the existence of 2D6 polymorphisms, and the predominant activity deriving from only 2 isomers, a molecule was synthesized (valbenazine), that when metabolized would slowly be converted into the most active isomer of α–DH-TBZ designated as NBI-98782 (Table 2). This slower conversion to NBI-98782 from valbenazine (compared with its formation from oral tetrabenazine) yielded improved kinetics and permitted once-daily dosing; moreover, because the metabolism of NBI-98782 is not solely dependent on CYP2D6, the need for genotyping was removed. Neither of the 2 metabolites from valbenazine NBI-98782 and NB-136110 have significant affinity for targets other than VMAT2.11

Use in tardive dyskinesia. Recommended starting dosage is 40 mg once daily with or without food, increased to 80 mg after 1 week, based on the design and results from the phase-III clinical trial.12 The FDA granted breakthrough therapy designation for this compound, and only 1 phase-III trial was performed. Valbenazine produced significant improvement on the AIMS, with a mean 30% reduction in AIMS scores at the Week 6 endpoint from baseline of 10.4 ± 3.6.2 The effect size was large (Cohen’s d = 0.90) for the 80-mg dosage. Continuation of 40 mg/d may be considered for some patients based on tolerability, including those who are known CYP2D6 poor metabolizers, and those taking strong CYP2D6 inhibitors. Patients taking strong 3A4 inhibitors should not exceed 40 mg/d. The maximum daily dose is 40 mg for those who have moderate or severe hepatic impairment (Child-Pugh score, 7 to 15). Dosage adjustment is not required for mild to moderate renal impairment (creatinine clearance, 30 to 90 mL/min).
Pharmacologic profile, adverse reactions
Valbenazine and its 2 metabolites lack affinity for receptors other than VMAT2, leading to an absence of orthostasis in clinical trials.1,2 In the phase-II trial, 76% of participants receiving valbenazine (n = 51) were titrated to the maximum dosage of 75 mg/d. Common adverse reactions (incidence ≥5% and at least twice the rate of placebo) were headache (9.8% vs 4.1% placebo), fatigue (9.8% vs 4.1% placebo), and somnolence (5.9% vs 2% placebo).1 In the phase-III trial, participants were randomized 1:1:1 to valbenazine, 40 mg (n = 72), valbenazine, 80 mg (n = 79), or placebo (n = 76). In the clinical studies the most common diagnosis was schizophrenia or schizoaffective disorder, and 40% and 85% of participants in the phase-II and phase-III studies, respectively, remained on antipsychotics.1,2 There were no adverse effects with an incidence ≥5% and at least twice the rate of placebo in the phase-III trial.2
When data from all placebo-controlled studies were pooled, only 1 adverse effect occurred with an incidence ≥5% and twice that of placebo, somnolence with a rate of 10.9% for valbenazine vs 4.2% for placebo. The incidence of akathisia in the pooled analysis was 2.7% for valbenazine vs 0.5% for placebo. Importantly, in neither study was there a safety signal related to depression, suicidal ideation and behavior, or parkinsonism. There also were no clinically significant changes in measures of schizophrenia symptoms.
The mean QT prolongation for valbenazine in healthy participants was 6.7 milliseconds, with the upper bound of the double-sided 90% confidence interval reaching 8.4 milliseconds. For those taking strong 2D6 or 3A4 inhibitors, or known 2D6 poor metabolizers, the mean QT prolongation was 11.7 milliseconds (14.7 milliseconds upper bound of double-sided 90% CI). In the controlled trials, there was a dose-related increase in prolactin, alkaline phosphatase, and bilirubin. Overall, 3% of valbenazine-treated patients and 2% of placebo-treated patients discontinued because of adverse reactions.
As noted above, there were no adverse effects with an incidence ≥5% and at least twice the rate of placebo in the phase-III valbenazine trial. Aggregate data across all placebo-controlled studies found that somnolence was the only adverse effect that occurred with an incidence ≥5% and twice that of placebo (10.9% for valbenazine vs 4.2% for placebo).2 As a comparsion, rates of sedation and akathisia for tetrabenazine were higher in the pivotal Huntington’s disease trial: sedation/somnolence 31% vs 3% for placebo, and akathisia 19% vs 0% for placebo.8
How it works
Tetrabenazine, a selective VMAT2 inhibitor, is the only agent that has demonstrated significant efficacy and tolerability for TD management; however, its complex metabolism generates numerous isomers of the metabolites α-DH-TBZ and β-DH-TBZ, of which only 2 are significantly active (Table 3). By choosing an active isomer (NBI-98782) as the metabolite of interest because of its selective and potent activity at VMAT2 and having a metabolism not solely dependent on CYP2D6, a compound was generated (valbenazine) that when metabolized slowly converts into NBI-98782.

Pharmacokinetics
Valbenazine demonstrates dose-proportional pharmacokinetics after single oral dosages from 40 to 300 mg with no impact of food or fasting status on levels of the active metabolite. Valbenazine has a Tmax of 0.5 to 1.0 hours, with 49% oral bioavailability. The plasma half-life for valbenazine and for NBI-98782 ranges from 15 to 22 hours. The Tmax for NBI-98782 when formed from valbenazine occurs between 4 and 8 hours, with a Cmax of approximately 30 ng/mL. It should be noted that when NBI-98782 is generated from oral tetrabenazine, the mean half-life and Tmax are considerably shorter (6 hours and 1.5 hours, respectively), while the Cmax is much higher (approximately 77 ng/mL) (Table 4).

Valbenazine is metabolized through endogenous esterases to NBI-98782 and NBI-136110. NBI-98782, the active metabolite, is further metabolized through multiple CYP pathways, predominantly 3A4 and 2D6. Neither valbenazine nor its metabolites are inhibitors or inducers of major CYP enzymes. Aside from VMAT2, the results of in vitro studies suggest that valbenazine and its active metabolite are unlikely to inhibit most major drug transporters at clinically relevant concentrations. However, valbenazine increased digoxin levels because of inhibition of intestinal P-glycoprotein; therefore plasma digoxin level monitoring is recommended when these 2 are co-administered.
Efficacy
Efficacy was established in a 6-week, fixed-dosage, double-blind, placebo-controlled trial of adult patients with TD. Eligible participants had:
- DSM-IV diagnosis of antipsychotic-induced TD for ≥3 months before screening and moderate or severe TD, as indicated by AIMS item 8 (severity of abnormal movement), which was rated by a blinded, external reviewer using a video of the participant’s AIMS assessment at screening
- a DSM-IV diagnosis of schizophrenia or schizoaffective disorder or mood disorder (and stable per investigator)
- Brief Psychiatric Rating Scale score <50 at screening.
Exclusion criteria included clinically significant and unstable medical conditions within 1 month before screening; comorbid movement disorder (eg, parkinsonism, akathisia, truncal dystonia) that was more prominent than TD; and significant risk for active suicidal ideation, suicidal behavior, or violent behavior.2 Participants had a mean age of 56, 52% were male, and 65.7% of participants in the valbenazine 40-mg group had a schizophrenia spectrum disorder diagnosis, as did 65.8% in both the placebo and valbenazine 80-mg arms.
Antipsychotic treatments were permitted during the trial and >85% of participants continued taking these medications during the study. Participants (N = 234) were randomly allocated in a 1:1:1 manner to valbenazine 40 mg, 80 mg, or matched placebo. The primary outcome was change in AIMS total score (items 1 to 7) assessed by central, independent raters. Baseline AIMS scores were 9.9 ± 4.3 in the placebo group, and 9.8 ± 4.1 and 10.4 ± 3.6 in the valbenazine 40-mg and 80-mg arms, respectively.2
Outcome. A fixed-sequence testing procedure to control for family-wise error rate and multiplicity was employed, and the primary endpoint was change from baseline to Week 6 in AIMS total score (items 1 to 7) for valbenazine 80 mg vs placebo. Valbenazine, 40 mg, was associated with a 1.9 point decrease in AIMS score, while valbenazine, 80 mg, was associated with a 3.2 point decrease in AIMS score, compared with 0.1 point decrease for placebo (P < .05 for valbenazine, 40 mg, P < .001 for valbenazine, 80 mg). This difference for the 40-mg dosage did not meet the prespecified analysis endpoints; however, for the 80-mg valbenazine dosage, the effect size for this difference (Cohen’s d) was large 0.90. There also were statistically significant differences between 40 mg and 80 mg at weeks 2, 4, and 6 in the intent-to-treat population. Of the 79 participants, 43 taking the 80-mg dosage completed a 48-week extension. Efficacy was sustained in this group; however, when valbenazine was discontinued at Week 48, AIMS scores returned to baseline after 4 weeks.
Tolerability
Of the 234 randomized patients, 205 (87.6%) completed the 6-week trial. Discontinuations due to adverse events were low across all treatment groups: 2.6% and 2.8% in the placebo and valbenazine 40-mg arms, respectively, and 3.8% in valbenazine 80-mg cohort. There was no safety signal based on changes in depression, suicidality, parkinsonism rating, or changes in schizophrenia symptoms. Because valbenazine can cause somnolence, patients should not perform activities requiring mental alertness (eg, operating a vehicle or hazardous machinery) until they know how they will be affected by valbenazine.
Valbenazine should be avoided in patients with congenital long QT syndrome or with arrhythmias associated with a prolonged QT interval. For patients at increased risk of a prolonged QT interval, assess the QT interval before increasing the dosage.
Clinical considerations
Unique properties. Valbenazine is metabolized slowly to a potent, selective VMAT2 antagonist (NBI-98782) in a manner that permits once daily dosing, removes the need for CYP2D6 genotyping, and provides significant efficacy.
Why Rx? The reasons to prescribe valbenazine for TD patients include:
- currently the only agent with FDA approval for TD
- fewer tolerability issues seen with the only other effective agent, tetrabenazine
- no signal for effects on mood parameters or rates of parkinsonism
- lack of multiple daily dosing and possible need for 2D6 genotyping involved with TBZ prescribing.
Dosing
The recommended dosage of valbenazine is 80 mg/d administered as a single dose with or without food, starting at 40 mg once daily for 1 week. There is no dosage adjustment required in those with mild to moderate renal impairment; however, valbenazine is not recommended in those with severe renal impairment. The maximum dose is 40 mg/d for those who with moderate or severe hepatic impairment (Child-Pugh score, 7 to 15) however, valbenazine is not recommended for patients with severe renal impairment (creatinine clearance <30 mL/min) because the exposure to the active metabolite is reduced by approximately 75%. The combined efficacy and tolerability of dosages >80 mg/d has not been evaluated. Adverse effects seen with tetrabenazine at higher dosages include akathisia, anxiety, insomnia, parkinsonism, fatigue, and depression.
A daily dose of 40 mg may be considered for some patients based on tolerability, including those who are known CYP 2D6 poor metabolizers, and those taking strong CYP2D6 inhibitors.2 For those taking strong 3A4 inhibitors, the maximum daily dose is 40 mg. Concomitant use of valbenazine with strong 3A4 inducers is not recommended as the exposure to the active metabolite is reduced by approximately 75%.2 Lastly, because VMAT2 inhibition may alter synaptic levels of other monoamines, it is recommended that valbenazine not be administered with monoamine oxidase inhibitors, such as isocarboxazid, phenelzine, or selegiline.
Contraindications
There are no reported contraindications for valbenazine. As with most medications, there is limited available data on valbenazine use in pregnant women; however, administration of valbenazine to pregnant rats during organogenesis through lactation produced an increase in the number of stillborn pups and postnatal pup mortalities at doses under the maximum recommended human dose (MRHD) using body surface area based dosing (mg/m2). Pregnant women should be advised of the potential risk to a fetus. Valbenazine and its metabolites have been detected in rat milk at concentrations higher than in plasma after oral administration of valbenazine at doses 0.1 to 1.2 times the MRHD (based on mg/m2). Based on animal findings of increased perinatal mortality in exposed fetuses and pups, woman are advised not to breastfeed during valbenazine treatment and for 5 days after the final dose. No dosage adjustment is required for geriatric patients.
Despite improvements in the tolerability of antipsychotic medications, the development of tardive dyskinesia (TD) still is a significant area of concern; however, clinicians have had few treatment options. Valbenazine, a vesicular monoamine transport type 2 (VMAT2) inhibitor, is the only FDA-approved medication for TD (Table 1).1 By modulating dopamine transport into presynaptic vesicles, synaptic dopamine release is decreased, thereby reducing the postsynaptic stimulation of D2 receptors and the severity of dyskinetic movements.

In the pivotal 6-week clinical trial, valbenazine significantly reduced TD severity as measured by Abnormal Involuntary Movement Scale (AIMS) ratings.2 Study completion rates were high (87.6%), with only 2 dropouts because of adverse events in each of the placebo (n = 78) and 40-mg (n = 76) arms, and 3 in the 80-mg group (n = 80).
Before the development of valbenazine, tetrabenazine was the only effective option for treating TD. Despite tetrabenazine’s known efficacy for TD, it was not available in the United States until 2008 with the sole indication for movements related to Huntington’s disease. U.S. patients often were subjected to a litany of ineffective medications for TD, often at great expense. Moreover, tetrabenazine involved multiple daily dosing, required cytochrome P450 (CYP) 2D6 genotyping for doses >50 mg/d, had significant tolerability issues, and a monthly cost of $8,000 to $10,000. The availability of an agent that is effective for TD and does not have tetrabenazine’s kinetic limitations, adverse effect profile, or CYP2D6 monitoring requirements represents an enormous advance in the treatment of TD.
Clinical implications
Tardive dyskinesia remains a significant public health concern because of the increasing use of antipsychotics for disorders beyond the core indication for schizophrenia. Although exposure to dopamine D2 antagonism could result in postsynaptic receptor upregulation and supersensitivity, this process best explains what underlies withdrawal dyskinesia.3 The persistence of TD symptoms in 66% to 80% of patients after discontinuing offending agents has led to hypotheses that the underlying pathophysiology of TD might best be conceptualized as a problem with neuroplasticity. As with many disorders, environmental contributions (eg, oxidative stress) and genetic predisposition might play a role beyond that related to exposure to D2 antagonism.3
There have been trials of numerous agents, but no medication has been FDA-approved for treating TD, and limited data support the efficacy of a few existing medications (clonazepam, amantadine, and ginkgo biloba extract [EGb-761]),4 albeit with small effect sizes. A medical food, consisting of branched-chain amino acids, received FDA approval for the dietary management of TD in males, but is no longer commercially available except from compounding pharmacies.5
Tetrabenazine, a molecule developed in the mid-1950s to improve on the tolerability of reserpine, was associated with significant adverse effects such as orthostasis.6 Like reserpine, tetrabenazine subsequently was found to be effective for TD7 but without the peripheral adverse effects of reserpine. However, the kinetics of tetrabenazine necessitated multiple daily doses, and required CYP2D6 genotyping for doses >50 mg/d.8
Receptor blocking. The mechanism that differentiated reserpine’s and tetrabenazine’s clinical properties became clearer in the 1980s when researchers discovered that transporters were necessary to package neurotransmitters into the synaptic vesicles of presynaptic neurons.9 The vesicular monoamine transporter (VMAT) exists in 2 isoforms (VMAT1 and VMAT2) that vary in distribution, with VMAT1 expressed mainly in the peripheral nervous system and VMAT2 expressed mainly in monoaminergic cells of the central nervous system.10
Tetrabenazine’s improved tolerability profile was related to the fact that it is a specific and reversible VMAT2 inhibitor, while reserpine is an irreversible and nonselective antagonist of both VMAT isoforms. Investigation of tetrabenazine’s metabolism revealed that it is rapidly and extensively converted into 2 isomers, α-dihydrotetrabenazine (DH-TBZ) and β-DH-TBZ. The isomeric forms of DH-TBZ have multiple chiral centers, and therefore numerous forms of which only 2 are significantly active at VMAT2.3 The α–DH-TBZ isomer is metabolized via CYP2D6 and 3A4 into inactive metabolites, while β-DH-TBZ is metabolized solely via 2D6.3 Because of the short half-life of DH-TBZ when generated from oral tetrabenazine, the existence of 2D6 polymorphisms, and the predominant activity deriving from only 2 isomers, a molecule was synthesized (valbenazine), that when metabolized would slowly be converted into the most active isomer of α–DH-TBZ designated as NBI-98782 (Table 2). This slower conversion to NBI-98782 from valbenazine (compared with its formation from oral tetrabenazine) yielded improved kinetics and permitted once-daily dosing; moreover, because the metabolism of NBI-98782 is not solely dependent on CYP2D6, the need for genotyping was removed. Neither of the 2 metabolites from valbenazine NBI-98782 and NB-136110 have significant affinity for targets other than VMAT2.11

Use in tardive dyskinesia. Recommended starting dosage is 40 mg once daily with or without food, increased to 80 mg after 1 week, based on the design and results from the phase-III clinical trial.12 The FDA granted breakthrough therapy designation for this compound, and only 1 phase-III trial was performed. Valbenazine produced significant improvement on the AIMS, with a mean 30% reduction in AIMS scores at the Week 6 endpoint from baseline of 10.4 ± 3.6.2 The effect size was large (Cohen’s d = 0.90) for the 80-mg dosage. Continuation of 40 mg/d may be considered for some patients based on tolerability, including those who are known CYP2D6 poor metabolizers, and those taking strong CYP2D6 inhibitors. Patients taking strong 3A4 inhibitors should not exceed 40 mg/d. The maximum daily dose is 40 mg for those who have moderate or severe hepatic impairment (Child-Pugh score, 7 to 15). Dosage adjustment is not required for mild to moderate renal impairment (creatinine clearance, 30 to 90 mL/min).
Pharmacologic profile, adverse reactions
Valbenazine and its 2 metabolites lack affinity for receptors other than VMAT2, leading to an absence of orthostasis in clinical trials.1,2 In the phase-II trial, 76% of participants receiving valbenazine (n = 51) were titrated to the maximum dosage of 75 mg/d. Common adverse reactions (incidence ≥5% and at least twice the rate of placebo) were headache (9.8% vs 4.1% placebo), fatigue (9.8% vs 4.1% placebo), and somnolence (5.9% vs 2% placebo).1 In the phase-III trial, participants were randomized 1:1:1 to valbenazine, 40 mg (n = 72), valbenazine, 80 mg (n = 79), or placebo (n = 76). In the clinical studies the most common diagnosis was schizophrenia or schizoaffective disorder, and 40% and 85% of participants in the phase-II and phase-III studies, respectively, remained on antipsychotics.1,2 There were no adverse effects with an incidence ≥5% and at least twice the rate of placebo in the phase-III trial.2
When data from all placebo-controlled studies were pooled, only 1 adverse effect occurred with an incidence ≥5% and twice that of placebo, somnolence with a rate of 10.9% for valbenazine vs 4.2% for placebo. The incidence of akathisia in the pooled analysis was 2.7% for valbenazine vs 0.5% for placebo. Importantly, in neither study was there a safety signal related to depression, suicidal ideation and behavior, or parkinsonism. There also were no clinically significant changes in measures of schizophrenia symptoms.
The mean QT prolongation for valbenazine in healthy participants was 6.7 milliseconds, with the upper bound of the double-sided 90% confidence interval reaching 8.4 milliseconds. For those taking strong 2D6 or 3A4 inhibitors, or known 2D6 poor metabolizers, the mean QT prolongation was 11.7 milliseconds (14.7 milliseconds upper bound of double-sided 90% CI). In the controlled trials, there was a dose-related increase in prolactin, alkaline phosphatase, and bilirubin. Overall, 3% of valbenazine-treated patients and 2% of placebo-treated patients discontinued because of adverse reactions.
As noted above, there were no adverse effects with an incidence ≥5% and at least twice the rate of placebo in the phase-III valbenazine trial. Aggregate data across all placebo-controlled studies found that somnolence was the only adverse effect that occurred with an incidence ≥5% and twice that of placebo (10.9% for valbenazine vs 4.2% for placebo).2 As a comparsion, rates of sedation and akathisia for tetrabenazine were higher in the pivotal Huntington’s disease trial: sedation/somnolence 31% vs 3% for placebo, and akathisia 19% vs 0% for placebo.8
How it works
Tetrabenazine, a selective VMAT2 inhibitor, is the only agent that has demonstrated significant efficacy and tolerability for TD management; however, its complex metabolism generates numerous isomers of the metabolites α-DH-TBZ and β-DH-TBZ, of which only 2 are significantly active (Table 3). By choosing an active isomer (NBI-98782) as the metabolite of interest because of its selective and potent activity at VMAT2 and having a metabolism not solely dependent on CYP2D6, a compound was generated (valbenazine) that when metabolized slowly converts into NBI-98782.

Pharmacokinetics
Valbenazine demonstrates dose-proportional pharmacokinetics after single oral dosages from 40 to 300 mg with no impact of food or fasting status on levels of the active metabolite. Valbenazine has a Tmax of 0.5 to 1.0 hours, with 49% oral bioavailability. The plasma half-life for valbenazine and for NBI-98782 ranges from 15 to 22 hours. The Tmax for NBI-98782 when formed from valbenazine occurs between 4 and 8 hours, with a Cmax of approximately 30 ng/mL. It should be noted that when NBI-98782 is generated from oral tetrabenazine, the mean half-life and Tmax are considerably shorter (6 hours and 1.5 hours, respectively), while the Cmax is much higher (approximately 77 ng/mL) (Table 4).

Valbenazine is metabolized through endogenous esterases to NBI-98782 and NBI-136110. NBI-98782, the active metabolite, is further metabolized through multiple CYP pathways, predominantly 3A4 and 2D6. Neither valbenazine nor its metabolites are inhibitors or inducers of major CYP enzymes. Aside from VMAT2, the results of in vitro studies suggest that valbenazine and its active metabolite are unlikely to inhibit most major drug transporters at clinically relevant concentrations. However, valbenazine increased digoxin levels because of inhibition of intestinal P-glycoprotein; therefore plasma digoxin level monitoring is recommended when these 2 are co-administered.
Efficacy
Efficacy was established in a 6-week, fixed-dosage, double-blind, placebo-controlled trial of adult patients with TD. Eligible participants had:
- DSM-IV diagnosis of antipsychotic-induced TD for ≥3 months before screening and moderate or severe TD, as indicated by AIMS item 8 (severity of abnormal movement), which was rated by a blinded, external reviewer using a video of the participant’s AIMS assessment at screening
- a DSM-IV diagnosis of schizophrenia or schizoaffective disorder or mood disorder (and stable per investigator)
- Brief Psychiatric Rating Scale score <50 at screening.
Exclusion criteria included clinically significant and unstable medical conditions within 1 month before screening; comorbid movement disorder (eg, parkinsonism, akathisia, truncal dystonia) that was more prominent than TD; and significant risk for active suicidal ideation, suicidal behavior, or violent behavior.2 Participants had a mean age of 56, 52% were male, and 65.7% of participants in the valbenazine 40-mg group had a schizophrenia spectrum disorder diagnosis, as did 65.8% in both the placebo and valbenazine 80-mg arms.
Antipsychotic treatments were permitted during the trial and >85% of participants continued taking these medications during the study. Participants (N = 234) were randomly allocated in a 1:1:1 manner to valbenazine 40 mg, 80 mg, or matched placebo. The primary outcome was change in AIMS total score (items 1 to 7) assessed by central, independent raters. Baseline AIMS scores were 9.9 ± 4.3 in the placebo group, and 9.8 ± 4.1 and 10.4 ± 3.6 in the valbenazine 40-mg and 80-mg arms, respectively.2
Outcome. A fixed-sequence testing procedure to control for family-wise error rate and multiplicity was employed, and the primary endpoint was change from baseline to Week 6 in AIMS total score (items 1 to 7) for valbenazine 80 mg vs placebo. Valbenazine, 40 mg, was associated with a 1.9 point decrease in AIMS score, while valbenazine, 80 mg, was associated with a 3.2 point decrease in AIMS score, compared with 0.1 point decrease for placebo (P < .05 for valbenazine, 40 mg, P < .001 for valbenazine, 80 mg). This difference for the 40-mg dosage did not meet the prespecified analysis endpoints; however, for the 80-mg valbenazine dosage, the effect size for this difference (Cohen’s d) was large 0.90. There also were statistically significant differences between 40 mg and 80 mg at weeks 2, 4, and 6 in the intent-to-treat population. Of the 79 participants, 43 taking the 80-mg dosage completed a 48-week extension. Efficacy was sustained in this group; however, when valbenazine was discontinued at Week 48, AIMS scores returned to baseline after 4 weeks.
Tolerability
Of the 234 randomized patients, 205 (87.6%) completed the 6-week trial. Discontinuations due to adverse events were low across all treatment groups: 2.6% and 2.8% in the placebo and valbenazine 40-mg arms, respectively, and 3.8% in valbenazine 80-mg cohort. There was no safety signal based on changes in depression, suicidality, parkinsonism rating, or changes in schizophrenia symptoms. Because valbenazine can cause somnolence, patients should not perform activities requiring mental alertness (eg, operating a vehicle or hazardous machinery) until they know how they will be affected by valbenazine.
Valbenazine should be avoided in patients with congenital long QT syndrome or with arrhythmias associated with a prolonged QT interval. For patients at increased risk of a prolonged QT interval, assess the QT interval before increasing the dosage.
Clinical considerations
Unique properties. Valbenazine is metabolized slowly to a potent, selective VMAT2 antagonist (NBI-98782) in a manner that permits once daily dosing, removes the need for CYP2D6 genotyping, and provides significant efficacy.
Why Rx? The reasons to prescribe valbenazine for TD patients include:
- currently the only agent with FDA approval for TD
- fewer tolerability issues seen with the only other effective agent, tetrabenazine
- no signal for effects on mood parameters or rates of parkinsonism
- lack of multiple daily dosing and possible need for 2D6 genotyping involved with TBZ prescribing.
Dosing
The recommended dosage of valbenazine is 80 mg/d administered as a single dose with or without food, starting at 40 mg once daily for 1 week. There is no dosage adjustment required in those with mild to moderate renal impairment; however, valbenazine is not recommended in those with severe renal impairment. The maximum dose is 40 mg/d for those who with moderate or severe hepatic impairment (Child-Pugh score, 7 to 15) however, valbenazine is not recommended for patients with severe renal impairment (creatinine clearance <30 mL/min) because the exposure to the active metabolite is reduced by approximately 75%. The combined efficacy and tolerability of dosages >80 mg/d has not been evaluated. Adverse effects seen with tetrabenazine at higher dosages include akathisia, anxiety, insomnia, parkinsonism, fatigue, and depression.
A daily dose of 40 mg may be considered for some patients based on tolerability, including those who are known CYP 2D6 poor metabolizers, and those taking strong CYP2D6 inhibitors.2 For those taking strong 3A4 inhibitors, the maximum daily dose is 40 mg. Concomitant use of valbenazine with strong 3A4 inducers is not recommended as the exposure to the active metabolite is reduced by approximately 75%.2 Lastly, because VMAT2 inhibition may alter synaptic levels of other monoamines, it is recommended that valbenazine not be administered with monoamine oxidase inhibitors, such as isocarboxazid, phenelzine, or selegiline.
Contraindications
There are no reported contraindications for valbenazine. As with most medications, there is limited available data on valbenazine use in pregnant women; however, administration of valbenazine to pregnant rats during organogenesis through lactation produced an increase in the number of stillborn pups and postnatal pup mortalities at doses under the maximum recommended human dose (MRHD) using body surface area based dosing (mg/m2). Pregnant women should be advised of the potential risk to a fetus. Valbenazine and its metabolites have been detected in rat milk at concentrations higher than in plasma after oral administration of valbenazine at doses 0.1 to 1.2 times the MRHD (based on mg/m2). Based on animal findings of increased perinatal mortality in exposed fetuses and pups, woman are advised not to breastfeed during valbenazine treatment and for 5 days after the final dose. No dosage adjustment is required for geriatric patients.
1. O’Brien CF, Jimenez R, Hauser RA, et al. NBI-98854, a selective monoamine transport inhibitor for the treatment of tardive dyskinesia: a randomized, double-blind, placebo-controlled study. Mov Disord. 2015;30(12):1681-1687.
2. Ingrezza [package insert]. San Diego, CA: Neurocrine Biosciences Inc.; 2017.
3. Marder S, Knesevich MA, Hauser RA, et al. KINECT 3: A randomized, double-blind, placebo-controlled phase 3 trial of valbenazine (NBI-98854) for tardive dyskinesia. Poster presented at the American Psychiatric Association Annual Meeting; May 14-18, 2016; Atlanta, GA.
4. Kazamatsuri H, Chien C, Cole JO. Treatment of tardive dyskinesia. I. Clinical efficacy of a dopamine-depleting agent, tetrabenazine. Arch Gen Psychiatry. 1972;27(1):95-99.
5. Richardson MA, Bevans ML, Read LL, et al. Efficacy of the branched-chain amino acids in the treatment of tardive dyskinesia in men. Am J Psychiatry. 2003;160(6):1117-1124.
6. Jankovic J, Clarence-Smith K. Tetrabenazine for the treatment of chorea and other hyperkinetic movement disorders. Expert Rev Neurother. 2011;11(11):1509-1523.
7. Meyer JM. Forgotten but not gone: new developments in the understanding and treatment of tardive dyskinesia. CNS Spectr. 2016;21(S1):13-24.
8. Bhidayasiri R, Fahn S, Weiner WJ, et al; American Academy of Neurology. Evidence-based guideline: treatment of tardive syndromes: report of the Guideline Development Subcommittee of the American Academy of Neurology. Neurology. 2013;81(5):463-469.
9. Quinn GP, Shore PA, Brodie BB. Biochemical and pharmacological studies of RO 1-9569 (tetrabenazine), a nonindole tranquilizing agent with reserpine-like effects. J Pharmacol Exp Ther. 1959;127:103-109.
10. Scherman D, Weber MJ. Characterization of the vesicular monoamine transporter in cultured rat sympathetic neurons: persistence upon induction of cholinergic phenotypic traits. Dev Biol. 1987;119(1):68-74.
11. Erickson JD, Schafer MK, Bonner TI, et al. Distinct pharmacological properties and distribution in neurons and endocrine cells of two isoforms of the human vesicular monoamine transporter. Proc Natl Acad Sci U S A. 1996;93(10):5166-5171.
12. Grigoriadis DE, Smith E, Madan A, et al. Pharmacologic characteristics of valbenazine (NBI-98854) and its metabolites. Poster presented at the U.S. Psychiatric & Mental Health Congress, October 21-24, 2016; San Antonio, TX.
1. O’Brien CF, Jimenez R, Hauser RA, et al. NBI-98854, a selective monoamine transport inhibitor for the treatment of tardive dyskinesia: a randomized, double-blind, placebo-controlled study. Mov Disord. 2015;30(12):1681-1687.
2. Ingrezza [package insert]. San Diego, CA: Neurocrine Biosciences Inc.; 2017.
3. Marder S, Knesevich MA, Hauser RA, et al. KINECT 3: A randomized, double-blind, placebo-controlled phase 3 trial of valbenazine (NBI-98854) for tardive dyskinesia. Poster presented at the American Psychiatric Association Annual Meeting; May 14-18, 2016; Atlanta, GA.
4. Kazamatsuri H, Chien C, Cole JO. Treatment of tardive dyskinesia. I. Clinical efficacy of a dopamine-depleting agent, tetrabenazine. Arch Gen Psychiatry. 1972;27(1):95-99.
5. Richardson MA, Bevans ML, Read LL, et al. Efficacy of the branched-chain amino acids in the treatment of tardive dyskinesia in men. Am J Psychiatry. 2003;160(6):1117-1124.
6. Jankovic J, Clarence-Smith K. Tetrabenazine for the treatment of chorea and other hyperkinetic movement disorders. Expert Rev Neurother. 2011;11(11):1509-1523.
7. Meyer JM. Forgotten but not gone: new developments in the understanding and treatment of tardive dyskinesia. CNS Spectr. 2016;21(S1):13-24.
8. Bhidayasiri R, Fahn S, Weiner WJ, et al; American Academy of Neurology. Evidence-based guideline: treatment of tardive syndromes: report of the Guideline Development Subcommittee of the American Academy of Neurology. Neurology. 2013;81(5):463-469.
9. Quinn GP, Shore PA, Brodie BB. Biochemical and pharmacological studies of RO 1-9569 (tetrabenazine), a nonindole tranquilizing agent with reserpine-like effects. J Pharmacol Exp Ther. 1959;127:103-109.
10. Scherman D, Weber MJ. Characterization of the vesicular monoamine transporter in cultured rat sympathetic neurons: persistence upon induction of cholinergic phenotypic traits. Dev Biol. 1987;119(1):68-74.
11. Erickson JD, Schafer MK, Bonner TI, et al. Distinct pharmacological properties and distribution in neurons and endocrine cells of two isoforms of the human vesicular monoamine transporter. Proc Natl Acad Sci U S A. 1996;93(10):5166-5171.
12. Grigoriadis DE, Smith E, Madan A, et al. Pharmacologic characteristics of valbenazine (NBI-98854) and its metabolites. Poster presented at the U.S. Psychiatric & Mental Health Congress, October 21-24, 2016; San Antonio, TX.
People often think functional neurological symptoms are feigned
Clinicians, other caregivers, and even patients often think that functional neurological symptoms and movement disorders are feigned or somewhat amenable to the patient’s control, according to two separate survey studies published online in the Journal of Neurology, Neurosurgery & Psychiatry.
In the first study, Sandra M. A. van der Salm, MD, and her associates reported their assessments of clinicians’ and patients’ views about “free will” pertaining to three movement disorders: functional (“psychogenic”) movement disorders, myoclonus, and tics. They administered survey questionnaires to 39 expert clinicians, 28 patients with functional movement disorders, 15 patients with myoclonus, 17 patients with tics, and 22 healthy control subjects.
Clinicians differed markedly from patients in their perceptions of patient control over movements and symptoms. The majority rated “raising one’s hand to vote” as completely voluntary, functional movement disorders as largely involuntary, tics as slightly more involuntary than voluntary, and myoclonus as completely involuntary. In contrast, most patients (other than some who said they were sometimes able to suppress tics) felt their movements and symptoms were well beyond their control, reported Dr. van der Salm of the department of neurology and clinical neurophysiology, Academic Medical Center, Amsterdam, and her associates (J Neurol Neurosurg Psychiatry. 2017 March 11. doi: 10.1136/jnnp-2016-315152).
“For clinicians, it may be helpful to realize that doctors and patients may have different views of voluntariness in movement disorders, in particular regarding tics and functional movement disorders. Keeping this gap in mind during consultation could prevent misunderstandings that might jeopardize the doctor-patient relationship,” the investigators wrote.
“Our results suggest that patients with functional movement disorders probably require different cognitive behavioral techniques than those used for tics, because patients with functional movement disorders experience less behavioral control” of their symptoms, they wrote.
In the second study, Richard A. A. Kanaan, MD, and Juen Mei Ding, MD, both in the department of psychiatry at the University of Melbourne, reported the results of their survey of 172 “people waiting in a general hospital outpatient department,” including patients, family members, and caregivers.
The questionnaire presented a hypothetical scenario to the respondent in which he or she had “leg weakness” for which all tests had come back negative. A doctor presented seven possible terms for the symptoms and then asked respondents whether such patients would be imagining their symptoms, faking their symptoms, mentally ill, or having a medical condition. The seven diagnoses were functional weakness, psychogenic weakness, medically unexplained weakness, somatic symptom disorder, dissociative disorder, conversion disorder, and stroke.
A total of 26% of the respondents said that at least one of the diagnoses connoted feigning, “confirming that suspicions of feigning in functional neurological symptoms remain a problem among patients and the public,” wrote Dr. Kanaan and Dr. Ding (J Neurol Neurosurg Psychiatry. 2017 March 9. doi: 10.1136/jnnp-2016-315224).
There was a strong correlation between the respondents’ opinions of “faking” and their level of education. Study participants with only a primary-school education were the most likely to think symptoms were feigned (32 of 94), while those with a tertiary education were very unlikely to think so (9 of 73), the investigators said.
It was encouraging that this correlation disappeared after a “simple remedy” was applied: briefly explaining the meaning of each diagnosis. Thus, “the problem appears to be largely one of public ignorance, rather than some inherent stigmatization of mental health,” Dr. Kanaan and Dr. Ding wrote.
Dr. van der Salm’s study was funded by the Academic Medical Center graduate school. No funding source was cited for the study by Dr. Kanaan and Dr. Ding. Both research groups reported having no relevant financial disclosures.
These two studies make interesting and worthwhile observations. They do make it clear that both the public and many physicians consider that many functional movement disorder patients have some voluntariness and hence are faking it. This is a stigma. A number of us have been trying to educate at least the physicians that almost all these patients do have involuntary movements. It is a problem for doctor-patient relationships and for patients to accept their diagnosis. We still have work to do!
Mark Hallett, MD, is chief of the National Institute of Neurological Disorders and Stroke’s Medical Neurology Branch and chief of its Human Motor Control Section. He is now president of the International Federation of Clinical Neurophysiology. He has been president of the Movement Disorder Society and vice president of the American Academy of Neurology.
These two studies make interesting and worthwhile observations. They do make it clear that both the public and many physicians consider that many functional movement disorder patients have some voluntariness and hence are faking it. This is a stigma. A number of us have been trying to educate at least the physicians that almost all these patients do have involuntary movements. It is a problem for doctor-patient relationships and for patients to accept their diagnosis. We still have work to do!
Mark Hallett, MD, is chief of the National Institute of Neurological Disorders and Stroke’s Medical Neurology Branch and chief of its Human Motor Control Section. He is now president of the International Federation of Clinical Neurophysiology. He has been president of the Movement Disorder Society and vice president of the American Academy of Neurology.
These two studies make interesting and worthwhile observations. They do make it clear that both the public and many physicians consider that many functional movement disorder patients have some voluntariness and hence are faking it. This is a stigma. A number of us have been trying to educate at least the physicians that almost all these patients do have involuntary movements. It is a problem for doctor-patient relationships and for patients to accept their diagnosis. We still have work to do!
Mark Hallett, MD, is chief of the National Institute of Neurological Disorders and Stroke’s Medical Neurology Branch and chief of its Human Motor Control Section. He is now president of the International Federation of Clinical Neurophysiology. He has been president of the Movement Disorder Society and vice president of the American Academy of Neurology.
Clinicians, other caregivers, and even patients often think that functional neurological symptoms and movement disorders are feigned or somewhat amenable to the patient’s control, according to two separate survey studies published online in the Journal of Neurology, Neurosurgery & Psychiatry.
In the first study, Sandra M. A. van der Salm, MD, and her associates reported their assessments of clinicians’ and patients’ views about “free will” pertaining to three movement disorders: functional (“psychogenic”) movement disorders, myoclonus, and tics. They administered survey questionnaires to 39 expert clinicians, 28 patients with functional movement disorders, 15 patients with myoclonus, 17 patients with tics, and 22 healthy control subjects.
Clinicians differed markedly from patients in their perceptions of patient control over movements and symptoms. The majority rated “raising one’s hand to vote” as completely voluntary, functional movement disorders as largely involuntary, tics as slightly more involuntary than voluntary, and myoclonus as completely involuntary. In contrast, most patients (other than some who said they were sometimes able to suppress tics) felt their movements and symptoms were well beyond their control, reported Dr. van der Salm of the department of neurology and clinical neurophysiology, Academic Medical Center, Amsterdam, and her associates (J Neurol Neurosurg Psychiatry. 2017 March 11. doi: 10.1136/jnnp-2016-315152).
“For clinicians, it may be helpful to realize that doctors and patients may have different views of voluntariness in movement disorders, in particular regarding tics and functional movement disorders. Keeping this gap in mind during consultation could prevent misunderstandings that might jeopardize the doctor-patient relationship,” the investigators wrote.
“Our results suggest that patients with functional movement disorders probably require different cognitive behavioral techniques than those used for tics, because patients with functional movement disorders experience less behavioral control” of their symptoms, they wrote.
In the second study, Richard A. A. Kanaan, MD, and Juen Mei Ding, MD, both in the department of psychiatry at the University of Melbourne, reported the results of their survey of 172 “people waiting in a general hospital outpatient department,” including patients, family members, and caregivers.
The questionnaire presented a hypothetical scenario to the respondent in which he or she had “leg weakness” for which all tests had come back negative. A doctor presented seven possible terms for the symptoms and then asked respondents whether such patients would be imagining their symptoms, faking their symptoms, mentally ill, or having a medical condition. The seven diagnoses were functional weakness, psychogenic weakness, medically unexplained weakness, somatic symptom disorder, dissociative disorder, conversion disorder, and stroke.
A total of 26% of the respondents said that at least one of the diagnoses connoted feigning, “confirming that suspicions of feigning in functional neurological symptoms remain a problem among patients and the public,” wrote Dr. Kanaan and Dr. Ding (J Neurol Neurosurg Psychiatry. 2017 March 9. doi: 10.1136/jnnp-2016-315224).
There was a strong correlation between the respondents’ opinions of “faking” and their level of education. Study participants with only a primary-school education were the most likely to think symptoms were feigned (32 of 94), while those with a tertiary education were very unlikely to think so (9 of 73), the investigators said.
It was encouraging that this correlation disappeared after a “simple remedy” was applied: briefly explaining the meaning of each diagnosis. Thus, “the problem appears to be largely one of public ignorance, rather than some inherent stigmatization of mental health,” Dr. Kanaan and Dr. Ding wrote.
Dr. van der Salm’s study was funded by the Academic Medical Center graduate school. No funding source was cited for the study by Dr. Kanaan and Dr. Ding. Both research groups reported having no relevant financial disclosures.
Clinicians, other caregivers, and even patients often think that functional neurological symptoms and movement disorders are feigned or somewhat amenable to the patient’s control, according to two separate survey studies published online in the Journal of Neurology, Neurosurgery & Psychiatry.
In the first study, Sandra M. A. van der Salm, MD, and her associates reported their assessments of clinicians’ and patients’ views about “free will” pertaining to three movement disorders: functional (“psychogenic”) movement disorders, myoclonus, and tics. They administered survey questionnaires to 39 expert clinicians, 28 patients with functional movement disorders, 15 patients with myoclonus, 17 patients with tics, and 22 healthy control subjects.
Clinicians differed markedly from patients in their perceptions of patient control over movements and symptoms. The majority rated “raising one’s hand to vote” as completely voluntary, functional movement disorders as largely involuntary, tics as slightly more involuntary than voluntary, and myoclonus as completely involuntary. In contrast, most patients (other than some who said they were sometimes able to suppress tics) felt their movements and symptoms were well beyond their control, reported Dr. van der Salm of the department of neurology and clinical neurophysiology, Academic Medical Center, Amsterdam, and her associates (J Neurol Neurosurg Psychiatry. 2017 March 11. doi: 10.1136/jnnp-2016-315152).
“For clinicians, it may be helpful to realize that doctors and patients may have different views of voluntariness in movement disorders, in particular regarding tics and functional movement disorders. Keeping this gap in mind during consultation could prevent misunderstandings that might jeopardize the doctor-patient relationship,” the investigators wrote.
“Our results suggest that patients with functional movement disorders probably require different cognitive behavioral techniques than those used for tics, because patients with functional movement disorders experience less behavioral control” of their symptoms, they wrote.
In the second study, Richard A. A. Kanaan, MD, and Juen Mei Ding, MD, both in the department of psychiatry at the University of Melbourne, reported the results of their survey of 172 “people waiting in a general hospital outpatient department,” including patients, family members, and caregivers.
The questionnaire presented a hypothetical scenario to the respondent in which he or she had “leg weakness” for which all tests had come back negative. A doctor presented seven possible terms for the symptoms and then asked respondents whether such patients would be imagining their symptoms, faking their symptoms, mentally ill, or having a medical condition. The seven diagnoses were functional weakness, psychogenic weakness, medically unexplained weakness, somatic symptom disorder, dissociative disorder, conversion disorder, and stroke.
A total of 26% of the respondents said that at least one of the diagnoses connoted feigning, “confirming that suspicions of feigning in functional neurological symptoms remain a problem among patients and the public,” wrote Dr. Kanaan and Dr. Ding (J Neurol Neurosurg Psychiatry. 2017 March 9. doi: 10.1136/jnnp-2016-315224).
There was a strong correlation between the respondents’ opinions of “faking” and their level of education. Study participants with only a primary-school education were the most likely to think symptoms were feigned (32 of 94), while those with a tertiary education were very unlikely to think so (9 of 73), the investigators said.
It was encouraging that this correlation disappeared after a “simple remedy” was applied: briefly explaining the meaning of each diagnosis. Thus, “the problem appears to be largely one of public ignorance, rather than some inherent stigmatization of mental health,” Dr. Kanaan and Dr. Ding wrote.
Dr. van der Salm’s study was funded by the Academic Medical Center graduate school. No funding source was cited for the study by Dr. Kanaan and Dr. Ding. Both research groups reported having no relevant financial disclosures.
FROM JOURNAL OF NEUROLOGY, NEUROSURGERY & PSYCHIATRY
Key clinical point:
Major finding: Most clinicians rated “raising one’s hand to vote” as completely voluntary, functional movement disorders as largely voluntary, tics as slightly more involuntary than voluntary, and myoclonus as completely involuntary.
Data source: Two separate survey questionnaires involving 172 respondents and 121 respondents, respectively.
Disclosures: Dr. van der Salm’s study was funded by the Academic Medical Center graduate school. No funding source was cited for the study by Dr. Kanaan and Dr. Ding. Both research groups reported having no relevant financial disclosures.
Treating tardive dyskinesia
Hepatitis C among the mentally ill: Review and treatment update
At approximately 3 to 4 million patients, hepatitis C virus (HCV) is the most common viral hepatitis in the United States. Patients with mental illness are disproportionately affected by HCV and the management of their disease poses particular challenges.
HCV is commonly transmitted via IV drug use and blood transfusions; transmission through sexual contact is rare. Most patients with HCV are asymptomatic, although some do develop symptoms of acute hepatitis. Most HCV infections become chronic, with a high incidence of liver failure requiring liver transplantation.
Hepatitis refers to inflammation of the liver, which could have various etiologies, including viral infections, alcohol abuse, or autoimmune disease. Viral hepatitis refers to infection from 5 distinct groups of virus, coined A through E.1 This article will focus on chronic HCV (Table 1).
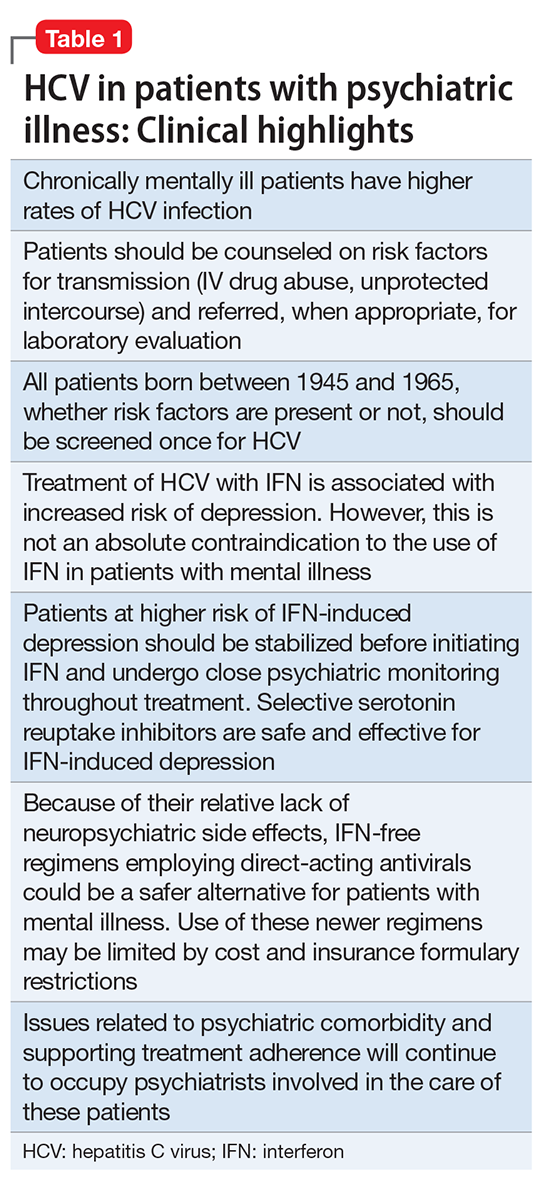
CASE Bipolar disorder, stress, history of IV drug use
Ms. S, age 48, has bipolar I disorder and has been hospitalized 4 times in the past, including once for a suicide attempt. She has 3 children and works as a cashier. Her psychiatric symptoms have been stable on lurasidone, 80 mg/d, and escitalopram, 10 mg/d. Recently, Ms. S has been under more stress at her job. Sometimes she misses doses of her medication, and then becomes more irritable and impulsive. Her husband, noting that she has used IV heroin in the past, comes with her today and is concerned that she is “not acting right.” What is Ms. S’s risk for HCV?
HCV in mental illness
Compared with the general population, HCV is more prevalent among chronically mentally ill persons. In one study, HCV occurred twice as often in men vs women with chronic mental illness.2 Up to 50% of patients with HCV have a history of mental illness and nearly 90% have a history of substance use disorders.3 Among 668 chronically mentally ill patients at 4 public sector clinics, risk factors for HCV were common and included use of injection drugs (>20%), sharing needles (14%), and crack cocaine use (>20%).4 Higher rates of HCV were reported in hospitalized patients with schizophrenia and comorbid psychoactive substance abuse in Japan.5 Because of the high prevalence in this population, it is essential to assess for substance use disorders. Employing a non-judgmental approach with motivational interviewing techniques can be effective.6
Individuals with mental illness should be screened for HCV risk factors, such as unprotected intercourse with high-risk partners and sharing needles used for illicit drug use. Patients frequently underreport these activities. At-risk individuals should undergo laboratory testing for the HIV-1 antibody, hepatitis C antibodies, and hepatitis B antibodies. Mental health providers should counsel patients about risk reduction (eg, avoiding unprotected sexual intercourse and sharing of drug paraphernalia). Educating patients about complications of viral hepatitis, such as liver failure, could be motivation to change risky behaviors.
CASE continued
During your interview with Ms. S, she becomes irritable and tells you that you are asking too many questions. It is clear that she is not taking her medications consistently, but she agrees to do so because she does not want to lose custody of her children. She denies current use of heroin but her husband says, “I don’t know what she is doing.” In addition to advising her on reducing risk factors, you order appropriate screening tests, including hepatitis and HIV antibody tests.
Screening guidelines
The U.S. Preventive Services Task Force and the CDC both recommend a 1-time screening for HCV in asymptomatic or low-risk patients born between 1945 and 1965.1,7 Furthermore, both organizations recommend screening for HCV in persons at high risk, including:
- those with a history of injection drug use
- persons with recognizable exposure, such as needlesticks
- persons who received blood transfusions before 1992
- medical conditions, such as long-term dialysis.
There is no vaccine for HCV; however, patients with HCV should receive vaccination against hepatitis B.
Diagnosis
Acute symptoms include fever, fatigue, headache, cough, nausea, and vomiting. Jaundice could develop, often accompanied by pain in the right upper quadrant. If there is suspicion of viral hepatitis, psychiatrists can initiate the laboratory evaluation. Chronic hepatitis, on the other hand, often is asymptomatic, although stigmata of chronic liver disease (eg, jaundice, ascites, peripheral edema) might be detected on physical exam.8 Elevated serum transaminases are seen with acute viral hepatitis, although levels could vary in chronic cases. Serologic detection of anti-HCV antibodies establishes a HCV diagnosis.
Treatment recommendations
All patients who test positive for HCV should be evaluated and treated by a hepatologist. Goals of therapy are to reduce complications from chronic viral hepatitis, including cirrhosis and hepatic failure. Duration and optimal regimen depends on the HCV genotype.8 Treatment outcomes are measured by virological parameters, including serum aminotransferases, HCV RNA levels, and histology. The most important parameter in treating chronic HCV is the sustained virological response (SVR), which is the absence of HCV RNA 12 weeks after completing therapy.9
Treatment is recommended for all persons with chronic HCV infection, according to current treatment guidelines, which are updated regularly by the American Association for the Study of Liver Diseases and the Infectious Diseases Society of America.10 Until recently, treatment consisted of IV pegylated interferon (PEG-IFN) in combination with oral ribavirin. Success rates with this regimen are approximately 40% to 50%. The advent of direct-acting antivirals (DAAs) has revolutionized treatment of chronic HCV. These agents include simeprevir, sofosbuvir, ledipasvir, and the combination of ombitasvir-paritaprevir-ritonavir plus dasabuvir (brand name, Viekira Pak). Advantages of these agents are oral administration, high treatment success rates (>90%), shorter treatment duration (12 weeks vs up to 48 weeks with older regimens), and few serious adverse effects9-11; drawbacks include the pricing of these regimens, which could cost upward of ≥$100,000 for a 12-week course, and a lack of coverage under some health insurance plans.12 The manufacturers of 2 agents, telaprevir and boceprevir, removed them from the market because of decreased demand related to their unfavorable side-effect profile and the availability of better tolerated agents.
Treatment considerations for interferon in psychiatric patients
Various neuropsychiatric symptoms have been reported with the use of PEG-IFN. The range of reported symptoms include:
- depressed mood
- anxiety
- hostility
- slowness
- fatigue
- sleep disturbance
- lethargy
- irritability
- emotional lability
- social withdrawal
- poor concentration.13,14
Depressive symptoms can present as early as 1 month after starting treatment, but typically occur at 8 to 12 weeks. A systematic review and meta-analysis of 26 observational studies found a cumulative 25% risk of interferon (IFN)-induced depression in the general HCV population.15 Risk factors for IFN-induced depression include:
- female sex
- history of major depression or other psychiatric disorder
- low educational level
- the presence of baseline subthreshold depressive symptoms.
Because of the risk of inducing depression, there was initial hesitation with providing IFN treatment to patients with psychiatric disorders. However, there is evidence that individuals with chronic psychiatric illness can be treated safely with IFN-based regimens and achieve results similar to non-psychiatric populations.16,17 For example, patients with schizophrenia in a small Veterans Affairs database who received IFN for HCV did not experience higher rates of symptoms of schizophrenia, depression, or mania over 8 years of follow-up.18 Furthermore, those with schizophrenia were just as likely to reach SVR as patients without psychiatric illness.19 Other encouraging results have been reported in depressed patients. One study found similar rates of treatment completion and SVR in patients with a history of major depressive disorder compared with those without depression.20 No difference in frequency of neuropsychiatric side effects was found between the groups.
Presence of a psychiatric disorder is no longer an absolute contraindication to IFN treatment for HCV. Optimal control of psychiatric symptoms should be attained in all patients before starting HCV treatment, and close clinical monitoring is warranted. A review of 9 studies showed benefit of antidepressants for HCV patients with elevated baseline depression or a history of IFN-induced depression.21 The largest body of evidence supports the safety and efficacy of selective serotonin reuptake inhibitors for treating IFN-induced depression. Although no antidepressants are FDA-approved for this indication, the best-studied agents include citalopram, escitalopram, sertraline, and paroxetine.
A review of 6 studies on using antidepressants to prevent IFN-induced depression concluded there was inadequate evidence to support this approach in all patients.22 Pretreatment primarily is indicated for those with elevated depressive symptoms at baseline or those with a history of IFN-induced depression. The prevailing approach to IFN-induced depression assessment, prevention, and treatment is summarized in Table 2.
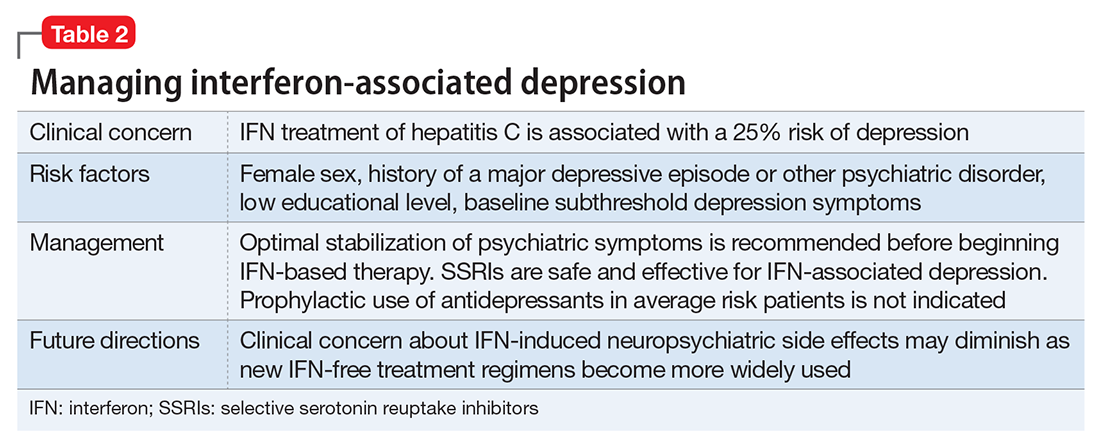
CASE continued
Ms. S tests positive for the HCV antibody but negative for HIV and hepatitis B. She immediately receives the hepatitis B vaccine series. Her sister discourages her from receiving treatment for HCV, warning her, “it will make you crazy depressed.” As a result, Ms. S avoids following up with the hepatologist. Her psychiatrist, aware that she now was taking her psychotropic medication and seeing that her mood is stable, educates her about new treatment options for HCV that do not cause depression. Ms. S finally agrees to see a hepatologist to discuss her treatment options.
IFN-free regimens
With the arrival of the DAAs, the potential now exists to use IFN-free treatment regimens,10 which could eliminate concerns about IFN-induced depression.
Clinical trials of the DAAs and real-world use so far do not indicate an elevated risk for neuropsychiatric symptoms, including depression.11 As a result, more patients with severe psychiatric illness likely will be eligible to receive treatment for HCV. However, as clinical experience builds with these new agents, it is important to monitor the experience of patients with psychiatric comorbidity. Current treatment guidelines for HCV genotype 1, which is most common in the United States, do not include IFN-based regimens.10 Treatment of genotype 3, which affects 6% of the U.S. population, still includes IFN. Therefore, the risk of IFN-induced depression still exists for some patients with HCV. Table 310 describes current treatment regimens in use for HCV without cirrhosis (see Related Resources for treating HCV with cirrhosis).
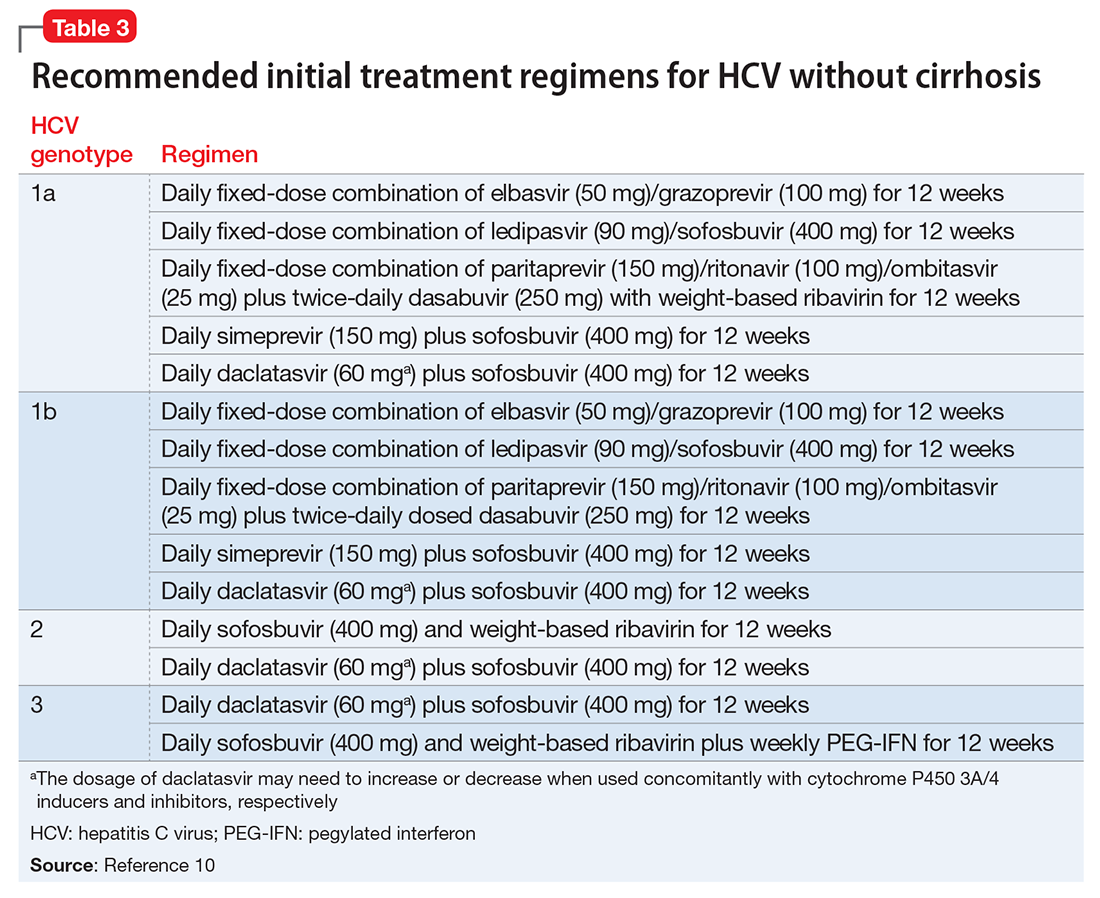
Evolving role of the psychiatrist
The availability of shorter, better-tolerated regimens means that the psychiatric contraindications to HCV treatment will be eased. With the emergence of non-IFN treatment regimens, the role of mental health providers could shift toward assisting with treatment adherence, monitoring drug–drug interactions, and managing comorbid substance use disorders.10
The psychiatrist’s role might shift away from the psychosocial assessment of factors affecting treatment eligibility, such as IFN-associated depressive symptoms. Clinical focus will likely shift to supporting adherence to HCV treatment regimens.23 Because depression and substance use disorders are risk factors for non-adherence, mental health providers may be called upon to optimize treatment of these conditions before beginning DAA regimens. A multi-dose regimen might be complicated for those with severe mental illness, and increased psychiatric and community support could be needed in these patients.23 Furthermore, models of care that integrate an HCV specialist with psychiatric care have demonstrated benefits.6,23 Long-term follow-up with a mental health provider will be key to provide ongoing psychiatric support, especially for those who do not achieve SVR.
Psychotropic drug–drug interactions with DAAs
Both sofosbuvir and ledipasvir are substrates of P-glycoprotein and not metabolized by cytochrome P450 (CYP) enzymes.24 Therefore, there are no known contraindications with psychotropic medications. However, co-administration of P-glycoprotein inducers, such as St. John’s wort, could reduce sofosbuvir and ledipasvir levels leading to reduced therapeutic efficacy.
Because it has been used for many years as an HIV treatment, drug interactions with ritonavir have been well-described. This agent is a “pan-inhibitor” and inhibits the CYP3A4, 2D6, 2C9, and 2C19 enzymes and could increase levels of any psychotropic metabolized by these enzymes.25 After several weeks of treatment, it also could induce CYP3A4, which could lead to reduced efficacy of oral contraceptives because ethinylestradiol is metabolized by CYP3A4. Ritonavir is primarily metabolized by CYP3A4 (and CYP2D6 to a smaller degree). Carbamazepine induces CYP3A4, which may lead to decreased levels of ritonavir.23 This, in turn, could reduce the likelihood of attaining SVR and successful treatment of HCV.
Boceprevir, telaprevir, and simeprevir inhibit CYP3A4 to varying degrees and therefore could affect psychotropic medications metabolized by this enzyme.23,26,27 These DAAs are metabolized by CYP3A4; therefore CYP3A4 inducers, such as carbamazepine, could lower DAA blood levels, increasing risk of HCV treatment failure and viral resistance.
Daclatasvir is a substrate of CYP3A4 and an inhibitor of P-glycoprotein.28 Concomitant buprenorphine or buprenorphine/naloxone levels may be increased, although the manufacturer does not recommend dosage adjustment. Elbasvir and grazoprevir are metabolized by CYP3A4.29 Drug–drug interactions therefore may result when administered with either CYP3A4 inducers or inhibitors.
CASE Conclusion
Ms. S sees her new hepatologist, Dr. Smith. She decides to try a 12-week course of ledipasvir/sofosbuvir. Dr. Smith collaborates frequently with Ms. S’s psychiatrist to discuss her case and to help monitor her psychiatric symptoms. She follows up closely with her psychiatrist for symptom monitoring and to help ensure treatment compliance. Ms. S does well with the IFN-free treatment regimen and experiences no worsening of her psychiatric symptoms during treatment.
1. Centers for Disease Control and Prevention. Viral hepatitis. http://www.cdc.gov/hepatitis. Updated December 9, 2016. Accessed February 9, 2017.
2. Butterfield MI, Bosworth HB, Meador KG, et al. Five-Site Health and Risk Study Research Committee. Gender differences in hepatitis C infection and risks among persons with severe mental illness. Psychiatr Serv. 2003;54(6):848-853.
3. Rifai MA, Gleason OC, Sabouni D. Psychiatric care of the patient with hepatitis C: a review of the literature. Prim Care Companion J Clin Psychiatry. 2010;12(6):PCC.09r00877. doi: 10.4088/PCC.09r00877whi.
4. Dinwiddie SH, Shicker L, Newman T. Prevalence of hepatitis C among psychiatric patients in the public sector. Am J Psychiatry. 2003;160(1):172-174.
5. Nakamura Y, Koh M, Miyoshi E, et al. High prevalence of the hepatitis C virus infection among the inpatients of schizophrenia and psychoactive substance abuse in Japan. Prog Neuropsychopharmacol Biol Psychiatry. 2004;28(3):591-597.
6. Sockalingam S, Blank D, Banga CA, et al. A novel program for treating patients with trimorbidity: hepatitis C, serious mental illness, and substance abuse. Eur J Gastroenterol Hepatol. 2013;25(12):1377-1384.
7. U.S. Preventive Services Task Force. Screening for hepatitis C virus infection: recommendation summary. https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/hepatitis-c-screening. Published June 2013. Accessed February 9, 2017.
8. Longo DL, Fauci AS, Kasper DL. Harrison’s principles of internal medicine. 18th ed. New York, NY: McGraw-Hill; 2012.
9. Belousova V, Abd-Rabou AA, Mousa SA. Recent advances and future directions in the management of hepatitis C infections. Pharmacol Ther. 2015;145:92-102.
10. American Association for the Study of Liver Diseases (AASLD); The Infectious Diseases Society of America (IDSA). HCV guidance: recommendations for testing, managing, and treating hepatitis C. http://www.hcvguidelines.org. Accessed February 9, 2017.
11. Rowan PJ, Bhulani N. Psychosocial assessment and monitoring in the new era of non-interferon-alpha hepatitis C treatments. World J Hepatol. 2015;7(19):2209-2213.
12. Good Rx, Inc. http://www.goodrx.com. Accessed October 9, 2015.
13. Raison CL, Borisov AS, Broadwell SD, et al. Depression during pegylated interferon-alpha plus ribavirin therapy: prevalence and prediction. J Clin Psychiatry. 2005;66(1):41-48.
14. Lotrich FE, Rabinovitz M, Gironda P, et al. Depression following pegylated interferon-alpha: characteristics and vulnerability. J Psychosom Res. 2007;63(2):131-135.
15. Udina M, Castellví P, Moreno-España J, et al. Interferon-induced depression in chronic hepatitis C: a systematic review and meta-analysis. J Clin Psychiatry. 2012;73(8):1128-1138.
16. Mustafa MZ, Schofield J, Mills PR, et al. The efficacy and safety of treating hepatitis C in patients with a diagnosis of schizophrenia. J Viral Hepat. 2014;21(7):e48-e51.
17. Huckans M, Mitchell A, Pavawalla S, et al. The influence of antiviral therapy on psychiatric symptoms among patients with hepatitis C and schizophrenia. Antivir Ther. 2010;15(1):111-119.
18. Huckans MS, Blackwell AD, Harms TA, et al. Management of hepatitis C disease among VA patients with schizophrenia and substance use disorders. Psychiatr Serv. 2006;57(3):403-406.
19. Huckans M, Mitchell A, Ruimy S, et al. Antiviral therapy completion and response rates among hepatitis C patients with and without schizophrenia. Schizophr Bull. 2010;36(1):165-172.
20. Hauser P, Morasco BJ, Linke A, et al. Antiviral completion rates and sustained viral response in hepatitis C patient with and without preexisting major depressive disorder. Psychosomatics. 2009;50(5):500-505.
21. Sockalingam S, Abbey SE. Managing depression during hepatitis C treatment. Can J Psychiatry. 2009;54(9):614-625.
22. Galvão-de Almeida A, Guindalini C, Batista-Neves S, et al. Can antidepressants prevent interferon-alpha-induced depression? A review of the literature. Gen Hosp Psychiatry. 2010;32(4):401-405.
23. Sockalingam S, Sheehan K, Feld JJ, et al. Psychiatric care during hepatitis c treatment: the changing role of psychiatrists in the era of direct-acting antivirals. Am J Psychiatry. 2015;172(6):512-516.
24. Harvoni [package insert]. Foster City, CA: Gilead Sciences, Inc.; 2016.
25. Wynn GH, Oesterheld, JR, Cozza KL, et al. Clinical manual of drug interactions principles for medical practice. Arlington, VA: American Psychiatric Publishing; 2009.
26. Olysio [package insert]. Titusville, NJ: Janssen Therapeutics; 2016.
27. Victrelis [package insert]. Whitehouse Station, NJ: Merck & Co.; 2017.
28. Daklinza [package insert]. Princeton, NJ: Bristol-Myers Squibb; 2016.
29. Zepatier [package insert]. Whitehouse Station, NJ: Merck & Co.; 2017.
At approximately 3 to 4 million patients, hepatitis C virus (HCV) is the most common viral hepatitis in the United States. Patients with mental illness are disproportionately affected by HCV and the management of their disease poses particular challenges.
HCV is commonly transmitted via IV drug use and blood transfusions; transmission through sexual contact is rare. Most patients with HCV are asymptomatic, although some do develop symptoms of acute hepatitis. Most HCV infections become chronic, with a high incidence of liver failure requiring liver transplantation.
Hepatitis refers to inflammation of the liver, which could have various etiologies, including viral infections, alcohol abuse, or autoimmune disease. Viral hepatitis refers to infection from 5 distinct groups of virus, coined A through E.1 This article will focus on chronic HCV (Table 1).

CASE Bipolar disorder, stress, history of IV drug use
Ms. S, age 48, has bipolar I disorder and has been hospitalized 4 times in the past, including once for a suicide attempt. She has 3 children and works as a cashier. Her psychiatric symptoms have been stable on lurasidone, 80 mg/d, and escitalopram, 10 mg/d. Recently, Ms. S has been under more stress at her job. Sometimes she misses doses of her medication, and then becomes more irritable and impulsive. Her husband, noting that she has used IV heroin in the past, comes with her today and is concerned that she is “not acting right.” What is Ms. S’s risk for HCV?
HCV in mental illness
Compared with the general population, HCV is more prevalent among chronically mentally ill persons. In one study, HCV occurred twice as often in men vs women with chronic mental illness.2 Up to 50% of patients with HCV have a history of mental illness and nearly 90% have a history of substance use disorders.3 Among 668 chronically mentally ill patients at 4 public sector clinics, risk factors for HCV were common and included use of injection drugs (>20%), sharing needles (14%), and crack cocaine use (>20%).4 Higher rates of HCV were reported in hospitalized patients with schizophrenia and comorbid psychoactive substance abuse in Japan.5 Because of the high prevalence in this population, it is essential to assess for substance use disorders. Employing a non-judgmental approach with motivational interviewing techniques can be effective.6
Individuals with mental illness should be screened for HCV risk factors, such as unprotected intercourse with high-risk partners and sharing needles used for illicit drug use. Patients frequently underreport these activities. At-risk individuals should undergo laboratory testing for the HIV-1 antibody, hepatitis C antibodies, and hepatitis B antibodies. Mental health providers should counsel patients about risk reduction (eg, avoiding unprotected sexual intercourse and sharing of drug paraphernalia). Educating patients about complications of viral hepatitis, such as liver failure, could be motivation to change risky behaviors.
CASE continued
During your interview with Ms. S, she becomes irritable and tells you that you are asking too many questions. It is clear that she is not taking her medications consistently, but she agrees to do so because she does not want to lose custody of her children. She denies current use of heroin but her husband says, “I don’t know what she is doing.” In addition to advising her on reducing risk factors, you order appropriate screening tests, including hepatitis and HIV antibody tests.
Screening guidelines
The U.S. Preventive Services Task Force and the CDC both recommend a 1-time screening for HCV in asymptomatic or low-risk patients born between 1945 and 1965.1,7 Furthermore, both organizations recommend screening for HCV in persons at high risk, including:
- those with a history of injection drug use
- persons with recognizable exposure, such as needlesticks
- persons who received blood transfusions before 1992
- medical conditions, such as long-term dialysis.
There is no vaccine for HCV; however, patients with HCV should receive vaccination against hepatitis B.
Diagnosis
Acute symptoms include fever, fatigue, headache, cough, nausea, and vomiting. Jaundice could develop, often accompanied by pain in the right upper quadrant. If there is suspicion of viral hepatitis, psychiatrists can initiate the laboratory evaluation. Chronic hepatitis, on the other hand, often is asymptomatic, although stigmata of chronic liver disease (eg, jaundice, ascites, peripheral edema) might be detected on physical exam.8 Elevated serum transaminases are seen with acute viral hepatitis, although levels could vary in chronic cases. Serologic detection of anti-HCV antibodies establishes a HCV diagnosis.
Treatment recommendations
All patients who test positive for HCV should be evaluated and treated by a hepatologist. Goals of therapy are to reduce complications from chronic viral hepatitis, including cirrhosis and hepatic failure. Duration and optimal regimen depends on the HCV genotype.8 Treatment outcomes are measured by virological parameters, including serum aminotransferases, HCV RNA levels, and histology. The most important parameter in treating chronic HCV is the sustained virological response (SVR), which is the absence of HCV RNA 12 weeks after completing therapy.9
Treatment is recommended for all persons with chronic HCV infection, according to current treatment guidelines, which are updated regularly by the American Association for the Study of Liver Diseases and the Infectious Diseases Society of America.10 Until recently, treatment consisted of IV pegylated interferon (PEG-IFN) in combination with oral ribavirin. Success rates with this regimen are approximately 40% to 50%. The advent of direct-acting antivirals (DAAs) has revolutionized treatment of chronic HCV. These agents include simeprevir, sofosbuvir, ledipasvir, and the combination of ombitasvir-paritaprevir-ritonavir plus dasabuvir (brand name, Viekira Pak). Advantages of these agents are oral administration, high treatment success rates (>90%), shorter treatment duration (12 weeks vs up to 48 weeks with older regimens), and few serious adverse effects9-11; drawbacks include the pricing of these regimens, which could cost upward of ≥$100,000 for a 12-week course, and a lack of coverage under some health insurance plans.12 The manufacturers of 2 agents, telaprevir and boceprevir, removed them from the market because of decreased demand related to their unfavorable side-effect profile and the availability of better tolerated agents.
Treatment considerations for interferon in psychiatric patients
Various neuropsychiatric symptoms have been reported with the use of PEG-IFN. The range of reported symptoms include:
- depressed mood
- anxiety
- hostility
- slowness
- fatigue
- sleep disturbance
- lethargy
- irritability
- emotional lability
- social withdrawal
- poor concentration.13,14
Depressive symptoms can present as early as 1 month after starting treatment, but typically occur at 8 to 12 weeks. A systematic review and meta-analysis of 26 observational studies found a cumulative 25% risk of interferon (IFN)-induced depression in the general HCV population.15 Risk factors for IFN-induced depression include:
- female sex
- history of major depression or other psychiatric disorder
- low educational level
- the presence of baseline subthreshold depressive symptoms.
Because of the risk of inducing depression, there was initial hesitation with providing IFN treatment to patients with psychiatric disorders. However, there is evidence that individuals with chronic psychiatric illness can be treated safely with IFN-based regimens and achieve results similar to non-psychiatric populations.16,17 For example, patients with schizophrenia in a small Veterans Affairs database who received IFN for HCV did not experience higher rates of symptoms of schizophrenia, depression, or mania over 8 years of follow-up.18 Furthermore, those with schizophrenia were just as likely to reach SVR as patients without psychiatric illness.19 Other encouraging results have been reported in depressed patients. One study found similar rates of treatment completion and SVR in patients with a history of major depressive disorder compared with those without depression.20 No difference in frequency of neuropsychiatric side effects was found between the groups.
Presence of a psychiatric disorder is no longer an absolute contraindication to IFN treatment for HCV. Optimal control of psychiatric symptoms should be attained in all patients before starting HCV treatment, and close clinical monitoring is warranted. A review of 9 studies showed benefit of antidepressants for HCV patients with elevated baseline depression or a history of IFN-induced depression.21 The largest body of evidence supports the safety and efficacy of selective serotonin reuptake inhibitors for treating IFN-induced depression. Although no antidepressants are FDA-approved for this indication, the best-studied agents include citalopram, escitalopram, sertraline, and paroxetine.
A review of 6 studies on using antidepressants to prevent IFN-induced depression concluded there was inadequate evidence to support this approach in all patients.22 Pretreatment primarily is indicated for those with elevated depressive symptoms at baseline or those with a history of IFN-induced depression. The prevailing approach to IFN-induced depression assessment, prevention, and treatment is summarized in Table 2.

CASE continued
Ms. S tests positive for the HCV antibody but negative for HIV and hepatitis B. She immediately receives the hepatitis B vaccine series. Her sister discourages her from receiving treatment for HCV, warning her, “it will make you crazy depressed.” As a result, Ms. S avoids following up with the hepatologist. Her psychiatrist, aware that she now was taking her psychotropic medication and seeing that her mood is stable, educates her about new treatment options for HCV that do not cause depression. Ms. S finally agrees to see a hepatologist to discuss her treatment options.
IFN-free regimens
With the arrival of the DAAs, the potential now exists to use IFN-free treatment regimens,10 which could eliminate concerns about IFN-induced depression.
Clinical trials of the DAAs and real-world use so far do not indicate an elevated risk for neuropsychiatric symptoms, including depression.11 As a result, more patients with severe psychiatric illness likely will be eligible to receive treatment for HCV. However, as clinical experience builds with these new agents, it is important to monitor the experience of patients with psychiatric comorbidity. Current treatment guidelines for HCV genotype 1, which is most common in the United States, do not include IFN-based regimens.10 Treatment of genotype 3, which affects 6% of the U.S. population, still includes IFN. Therefore, the risk of IFN-induced depression still exists for some patients with HCV. Table 310 describes current treatment regimens in use for HCV without cirrhosis (see Related Resources for treating HCV with cirrhosis).

Evolving role of the psychiatrist
The availability of shorter, better-tolerated regimens means that the psychiatric contraindications to HCV treatment will be eased. With the emergence of non-IFN treatment regimens, the role of mental health providers could shift toward assisting with treatment adherence, monitoring drug–drug interactions, and managing comorbid substance use disorders.10
The psychiatrist’s role might shift away from the psychosocial assessment of factors affecting treatment eligibility, such as IFN-associated depressive symptoms. Clinical focus will likely shift to supporting adherence to HCV treatment regimens.23 Because depression and substance use disorders are risk factors for non-adherence, mental health providers may be called upon to optimize treatment of these conditions before beginning DAA regimens. A multi-dose regimen might be complicated for those with severe mental illness, and increased psychiatric and community support could be needed in these patients.23 Furthermore, models of care that integrate an HCV specialist with psychiatric care have demonstrated benefits.6,23 Long-term follow-up with a mental health provider will be key to provide ongoing psychiatric support, especially for those who do not achieve SVR.
Psychotropic drug–drug interactions with DAAs
Both sofosbuvir and ledipasvir are substrates of P-glycoprotein and not metabolized by cytochrome P450 (CYP) enzymes.24 Therefore, there are no known contraindications with psychotropic medications. However, co-administration of P-glycoprotein inducers, such as St. John’s wort, could reduce sofosbuvir and ledipasvir levels leading to reduced therapeutic efficacy.
Because it has been used for many years as an HIV treatment, drug interactions with ritonavir have been well-described. This agent is a “pan-inhibitor” and inhibits the CYP3A4, 2D6, 2C9, and 2C19 enzymes and could increase levels of any psychotropic metabolized by these enzymes.25 After several weeks of treatment, it also could induce CYP3A4, which could lead to reduced efficacy of oral contraceptives because ethinylestradiol is metabolized by CYP3A4. Ritonavir is primarily metabolized by CYP3A4 (and CYP2D6 to a smaller degree). Carbamazepine induces CYP3A4, which may lead to decreased levels of ritonavir.23 This, in turn, could reduce the likelihood of attaining SVR and successful treatment of HCV.
Boceprevir, telaprevir, and simeprevir inhibit CYP3A4 to varying degrees and therefore could affect psychotropic medications metabolized by this enzyme.23,26,27 These DAAs are metabolized by CYP3A4; therefore CYP3A4 inducers, such as carbamazepine, could lower DAA blood levels, increasing risk of HCV treatment failure and viral resistance.
Daclatasvir is a substrate of CYP3A4 and an inhibitor of P-glycoprotein.28 Concomitant buprenorphine or buprenorphine/naloxone levels may be increased, although the manufacturer does not recommend dosage adjustment. Elbasvir and grazoprevir are metabolized by CYP3A4.29 Drug–drug interactions therefore may result when administered with either CYP3A4 inducers or inhibitors.
CASE Conclusion
Ms. S sees her new hepatologist, Dr. Smith. She decides to try a 12-week course of ledipasvir/sofosbuvir. Dr. Smith collaborates frequently with Ms. S’s psychiatrist to discuss her case and to help monitor her psychiatric symptoms. She follows up closely with her psychiatrist for symptom monitoring and to help ensure treatment compliance. Ms. S does well with the IFN-free treatment regimen and experiences no worsening of her psychiatric symptoms during treatment.
At approximately 3 to 4 million patients, hepatitis C virus (HCV) is the most common viral hepatitis in the United States. Patients with mental illness are disproportionately affected by HCV and the management of their disease poses particular challenges.
HCV is commonly transmitted via IV drug use and blood transfusions; transmission through sexual contact is rare. Most patients with HCV are asymptomatic, although some do develop symptoms of acute hepatitis. Most HCV infections become chronic, with a high incidence of liver failure requiring liver transplantation.
Hepatitis refers to inflammation of the liver, which could have various etiologies, including viral infections, alcohol abuse, or autoimmune disease. Viral hepatitis refers to infection from 5 distinct groups of virus, coined A through E.1 This article will focus on chronic HCV (Table 1).

CASE Bipolar disorder, stress, history of IV drug use
Ms. S, age 48, has bipolar I disorder and has been hospitalized 4 times in the past, including once for a suicide attempt. She has 3 children and works as a cashier. Her psychiatric symptoms have been stable on lurasidone, 80 mg/d, and escitalopram, 10 mg/d. Recently, Ms. S has been under more stress at her job. Sometimes she misses doses of her medication, and then becomes more irritable and impulsive. Her husband, noting that she has used IV heroin in the past, comes with her today and is concerned that she is “not acting right.” What is Ms. S’s risk for HCV?
HCV in mental illness
Compared with the general population, HCV is more prevalent among chronically mentally ill persons. In one study, HCV occurred twice as often in men vs women with chronic mental illness.2 Up to 50% of patients with HCV have a history of mental illness and nearly 90% have a history of substance use disorders.3 Among 668 chronically mentally ill patients at 4 public sector clinics, risk factors for HCV were common and included use of injection drugs (>20%), sharing needles (14%), and crack cocaine use (>20%).4 Higher rates of HCV were reported in hospitalized patients with schizophrenia and comorbid psychoactive substance abuse in Japan.5 Because of the high prevalence in this population, it is essential to assess for substance use disorders. Employing a non-judgmental approach with motivational interviewing techniques can be effective.6
Individuals with mental illness should be screened for HCV risk factors, such as unprotected intercourse with high-risk partners and sharing needles used for illicit drug use. Patients frequently underreport these activities. At-risk individuals should undergo laboratory testing for the HIV-1 antibody, hepatitis C antibodies, and hepatitis B antibodies. Mental health providers should counsel patients about risk reduction (eg, avoiding unprotected sexual intercourse and sharing of drug paraphernalia). Educating patients about complications of viral hepatitis, such as liver failure, could be motivation to change risky behaviors.
CASE continued
During your interview with Ms. S, she becomes irritable and tells you that you are asking too many questions. It is clear that she is not taking her medications consistently, but she agrees to do so because she does not want to lose custody of her children. She denies current use of heroin but her husband says, “I don’t know what she is doing.” In addition to advising her on reducing risk factors, you order appropriate screening tests, including hepatitis and HIV antibody tests.
Screening guidelines
The U.S. Preventive Services Task Force and the CDC both recommend a 1-time screening for HCV in asymptomatic or low-risk patients born between 1945 and 1965.1,7 Furthermore, both organizations recommend screening for HCV in persons at high risk, including:
- those with a history of injection drug use
- persons with recognizable exposure, such as needlesticks
- persons who received blood transfusions before 1992
- medical conditions, such as long-term dialysis.
There is no vaccine for HCV; however, patients with HCV should receive vaccination against hepatitis B.
Diagnosis
Acute symptoms include fever, fatigue, headache, cough, nausea, and vomiting. Jaundice could develop, often accompanied by pain in the right upper quadrant. If there is suspicion of viral hepatitis, psychiatrists can initiate the laboratory evaluation. Chronic hepatitis, on the other hand, often is asymptomatic, although stigmata of chronic liver disease (eg, jaundice, ascites, peripheral edema) might be detected on physical exam.8 Elevated serum transaminases are seen with acute viral hepatitis, although levels could vary in chronic cases. Serologic detection of anti-HCV antibodies establishes a HCV diagnosis.
Treatment recommendations
All patients who test positive for HCV should be evaluated and treated by a hepatologist. Goals of therapy are to reduce complications from chronic viral hepatitis, including cirrhosis and hepatic failure. Duration and optimal regimen depends on the HCV genotype.8 Treatment outcomes are measured by virological parameters, including serum aminotransferases, HCV RNA levels, and histology. The most important parameter in treating chronic HCV is the sustained virological response (SVR), which is the absence of HCV RNA 12 weeks after completing therapy.9
Treatment is recommended for all persons with chronic HCV infection, according to current treatment guidelines, which are updated regularly by the American Association for the Study of Liver Diseases and the Infectious Diseases Society of America.10 Until recently, treatment consisted of IV pegylated interferon (PEG-IFN) in combination with oral ribavirin. Success rates with this regimen are approximately 40% to 50%. The advent of direct-acting antivirals (DAAs) has revolutionized treatment of chronic HCV. These agents include simeprevir, sofosbuvir, ledipasvir, and the combination of ombitasvir-paritaprevir-ritonavir plus dasabuvir (brand name, Viekira Pak). Advantages of these agents are oral administration, high treatment success rates (>90%), shorter treatment duration (12 weeks vs up to 48 weeks with older regimens), and few serious adverse effects9-11; drawbacks include the pricing of these regimens, which could cost upward of ≥$100,000 for a 12-week course, and a lack of coverage under some health insurance plans.12 The manufacturers of 2 agents, telaprevir and boceprevir, removed them from the market because of decreased demand related to their unfavorable side-effect profile and the availability of better tolerated agents.
Treatment considerations for interferon in psychiatric patients
Various neuropsychiatric symptoms have been reported with the use of PEG-IFN. The range of reported symptoms include:
- depressed mood
- anxiety
- hostility
- slowness
- fatigue
- sleep disturbance
- lethargy
- irritability
- emotional lability
- social withdrawal
- poor concentration.13,14
Depressive symptoms can present as early as 1 month after starting treatment, but typically occur at 8 to 12 weeks. A systematic review and meta-analysis of 26 observational studies found a cumulative 25% risk of interferon (IFN)-induced depression in the general HCV population.15 Risk factors for IFN-induced depression include:
- female sex
- history of major depression or other psychiatric disorder
- low educational level
- the presence of baseline subthreshold depressive symptoms.
Because of the risk of inducing depression, there was initial hesitation with providing IFN treatment to patients with psychiatric disorders. However, there is evidence that individuals with chronic psychiatric illness can be treated safely with IFN-based regimens and achieve results similar to non-psychiatric populations.16,17 For example, patients with schizophrenia in a small Veterans Affairs database who received IFN for HCV did not experience higher rates of symptoms of schizophrenia, depression, or mania over 8 years of follow-up.18 Furthermore, those with schizophrenia were just as likely to reach SVR as patients without psychiatric illness.19 Other encouraging results have been reported in depressed patients. One study found similar rates of treatment completion and SVR in patients with a history of major depressive disorder compared with those without depression.20 No difference in frequency of neuropsychiatric side effects was found between the groups.
Presence of a psychiatric disorder is no longer an absolute contraindication to IFN treatment for HCV. Optimal control of psychiatric symptoms should be attained in all patients before starting HCV treatment, and close clinical monitoring is warranted. A review of 9 studies showed benefit of antidepressants for HCV patients with elevated baseline depression or a history of IFN-induced depression.21 The largest body of evidence supports the safety and efficacy of selective serotonin reuptake inhibitors for treating IFN-induced depression. Although no antidepressants are FDA-approved for this indication, the best-studied agents include citalopram, escitalopram, sertraline, and paroxetine.
A review of 6 studies on using antidepressants to prevent IFN-induced depression concluded there was inadequate evidence to support this approach in all patients.22 Pretreatment primarily is indicated for those with elevated depressive symptoms at baseline or those with a history of IFN-induced depression. The prevailing approach to IFN-induced depression assessment, prevention, and treatment is summarized in Table 2.

CASE continued
Ms. S tests positive for the HCV antibody but negative for HIV and hepatitis B. She immediately receives the hepatitis B vaccine series. Her sister discourages her from receiving treatment for HCV, warning her, “it will make you crazy depressed.” As a result, Ms. S avoids following up with the hepatologist. Her psychiatrist, aware that she now was taking her psychotropic medication and seeing that her mood is stable, educates her about new treatment options for HCV that do not cause depression. Ms. S finally agrees to see a hepatologist to discuss her treatment options.
IFN-free regimens
With the arrival of the DAAs, the potential now exists to use IFN-free treatment regimens,10 which could eliminate concerns about IFN-induced depression.
Clinical trials of the DAAs and real-world use so far do not indicate an elevated risk for neuropsychiatric symptoms, including depression.11 As a result, more patients with severe psychiatric illness likely will be eligible to receive treatment for HCV. However, as clinical experience builds with these new agents, it is important to monitor the experience of patients with psychiatric comorbidity. Current treatment guidelines for HCV genotype 1, which is most common in the United States, do not include IFN-based regimens.10 Treatment of genotype 3, which affects 6% of the U.S. population, still includes IFN. Therefore, the risk of IFN-induced depression still exists for some patients with HCV. Table 310 describes current treatment regimens in use for HCV without cirrhosis (see Related Resources for treating HCV with cirrhosis).

Evolving role of the psychiatrist
The availability of shorter, better-tolerated regimens means that the psychiatric contraindications to HCV treatment will be eased. With the emergence of non-IFN treatment regimens, the role of mental health providers could shift toward assisting with treatment adherence, monitoring drug–drug interactions, and managing comorbid substance use disorders.10
The psychiatrist’s role might shift away from the psychosocial assessment of factors affecting treatment eligibility, such as IFN-associated depressive symptoms. Clinical focus will likely shift to supporting adherence to HCV treatment regimens.23 Because depression and substance use disorders are risk factors for non-adherence, mental health providers may be called upon to optimize treatment of these conditions before beginning DAA regimens. A multi-dose regimen might be complicated for those with severe mental illness, and increased psychiatric and community support could be needed in these patients.23 Furthermore, models of care that integrate an HCV specialist with psychiatric care have demonstrated benefits.6,23 Long-term follow-up with a mental health provider will be key to provide ongoing psychiatric support, especially for those who do not achieve SVR.
Psychotropic drug–drug interactions with DAAs
Both sofosbuvir and ledipasvir are substrates of P-glycoprotein and not metabolized by cytochrome P450 (CYP) enzymes.24 Therefore, there are no known contraindications with psychotropic medications. However, co-administration of P-glycoprotein inducers, such as St. John’s wort, could reduce sofosbuvir and ledipasvir levels leading to reduced therapeutic efficacy.
Because it has been used for many years as an HIV treatment, drug interactions with ritonavir have been well-described. This agent is a “pan-inhibitor” and inhibits the CYP3A4, 2D6, 2C9, and 2C19 enzymes and could increase levels of any psychotropic metabolized by these enzymes.25 After several weeks of treatment, it also could induce CYP3A4, which could lead to reduced efficacy of oral contraceptives because ethinylestradiol is metabolized by CYP3A4. Ritonavir is primarily metabolized by CYP3A4 (and CYP2D6 to a smaller degree). Carbamazepine induces CYP3A4, which may lead to decreased levels of ritonavir.23 This, in turn, could reduce the likelihood of attaining SVR and successful treatment of HCV.
Boceprevir, telaprevir, and simeprevir inhibit CYP3A4 to varying degrees and therefore could affect psychotropic medications metabolized by this enzyme.23,26,27 These DAAs are metabolized by CYP3A4; therefore CYP3A4 inducers, such as carbamazepine, could lower DAA blood levels, increasing risk of HCV treatment failure and viral resistance.
Daclatasvir is a substrate of CYP3A4 and an inhibitor of P-glycoprotein.28 Concomitant buprenorphine or buprenorphine/naloxone levels may be increased, although the manufacturer does not recommend dosage adjustment. Elbasvir and grazoprevir are metabolized by CYP3A4.29 Drug–drug interactions therefore may result when administered with either CYP3A4 inducers or inhibitors.
CASE Conclusion
Ms. S sees her new hepatologist, Dr. Smith. She decides to try a 12-week course of ledipasvir/sofosbuvir. Dr. Smith collaborates frequently with Ms. S’s psychiatrist to discuss her case and to help monitor her psychiatric symptoms. She follows up closely with her psychiatrist for symptom monitoring and to help ensure treatment compliance. Ms. S does well with the IFN-free treatment regimen and experiences no worsening of her psychiatric symptoms during treatment.
1. Centers for Disease Control and Prevention. Viral hepatitis. http://www.cdc.gov/hepatitis. Updated December 9, 2016. Accessed February 9, 2017.
2. Butterfield MI, Bosworth HB, Meador KG, et al. Five-Site Health and Risk Study Research Committee. Gender differences in hepatitis C infection and risks among persons with severe mental illness. Psychiatr Serv. 2003;54(6):848-853.
3. Rifai MA, Gleason OC, Sabouni D. Psychiatric care of the patient with hepatitis C: a review of the literature. Prim Care Companion J Clin Psychiatry. 2010;12(6):PCC.09r00877. doi: 10.4088/PCC.09r00877whi.
4. Dinwiddie SH, Shicker L, Newman T. Prevalence of hepatitis C among psychiatric patients in the public sector. Am J Psychiatry. 2003;160(1):172-174.
5. Nakamura Y, Koh M, Miyoshi E, et al. High prevalence of the hepatitis C virus infection among the inpatients of schizophrenia and psychoactive substance abuse in Japan. Prog Neuropsychopharmacol Biol Psychiatry. 2004;28(3):591-597.
6. Sockalingam S, Blank D, Banga CA, et al. A novel program for treating patients with trimorbidity: hepatitis C, serious mental illness, and substance abuse. Eur J Gastroenterol Hepatol. 2013;25(12):1377-1384.
7. U.S. Preventive Services Task Force. Screening for hepatitis C virus infection: recommendation summary. https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/hepatitis-c-screening. Published June 2013. Accessed February 9, 2017.
8. Longo DL, Fauci AS, Kasper DL. Harrison’s principles of internal medicine. 18th ed. New York, NY: McGraw-Hill; 2012.
9. Belousova V, Abd-Rabou AA, Mousa SA. Recent advances and future directions in the management of hepatitis C infections. Pharmacol Ther. 2015;145:92-102.
10. American Association for the Study of Liver Diseases (AASLD); The Infectious Diseases Society of America (IDSA). HCV guidance: recommendations for testing, managing, and treating hepatitis C. http://www.hcvguidelines.org. Accessed February 9, 2017.
11. Rowan PJ, Bhulani N. Psychosocial assessment and monitoring in the new era of non-interferon-alpha hepatitis C treatments. World J Hepatol. 2015;7(19):2209-2213.
12. Good Rx, Inc. http://www.goodrx.com. Accessed October 9, 2015.
13. Raison CL, Borisov AS, Broadwell SD, et al. Depression during pegylated interferon-alpha plus ribavirin therapy: prevalence and prediction. J Clin Psychiatry. 2005;66(1):41-48.
14. Lotrich FE, Rabinovitz M, Gironda P, et al. Depression following pegylated interferon-alpha: characteristics and vulnerability. J Psychosom Res. 2007;63(2):131-135.
15. Udina M, Castellví P, Moreno-España J, et al. Interferon-induced depression in chronic hepatitis C: a systematic review and meta-analysis. J Clin Psychiatry. 2012;73(8):1128-1138.
16. Mustafa MZ, Schofield J, Mills PR, et al. The efficacy and safety of treating hepatitis C in patients with a diagnosis of schizophrenia. J Viral Hepat. 2014;21(7):e48-e51.
17. Huckans M, Mitchell A, Pavawalla S, et al. The influence of antiviral therapy on psychiatric symptoms among patients with hepatitis C and schizophrenia. Antivir Ther. 2010;15(1):111-119.
18. Huckans MS, Blackwell AD, Harms TA, et al. Management of hepatitis C disease among VA patients with schizophrenia and substance use disorders. Psychiatr Serv. 2006;57(3):403-406.
19. Huckans M, Mitchell A, Ruimy S, et al. Antiviral therapy completion and response rates among hepatitis C patients with and without schizophrenia. Schizophr Bull. 2010;36(1):165-172.
20. Hauser P, Morasco BJ, Linke A, et al. Antiviral completion rates and sustained viral response in hepatitis C patient with and without preexisting major depressive disorder. Psychosomatics. 2009;50(5):500-505.
21. Sockalingam S, Abbey SE. Managing depression during hepatitis C treatment. Can J Psychiatry. 2009;54(9):614-625.
22. Galvão-de Almeida A, Guindalini C, Batista-Neves S, et al. Can antidepressants prevent interferon-alpha-induced depression? A review of the literature. Gen Hosp Psychiatry. 2010;32(4):401-405.
23. Sockalingam S, Sheehan K, Feld JJ, et al. Psychiatric care during hepatitis c treatment: the changing role of psychiatrists in the era of direct-acting antivirals. Am J Psychiatry. 2015;172(6):512-516.
24. Harvoni [package insert]. Foster City, CA: Gilead Sciences, Inc.; 2016.
25. Wynn GH, Oesterheld, JR, Cozza KL, et al. Clinical manual of drug interactions principles for medical practice. Arlington, VA: American Psychiatric Publishing; 2009.
26. Olysio [package insert]. Titusville, NJ: Janssen Therapeutics; 2016.
27. Victrelis [package insert]. Whitehouse Station, NJ: Merck & Co.; 2017.
28. Daklinza [package insert]. Princeton, NJ: Bristol-Myers Squibb; 2016.
29. Zepatier [package insert]. Whitehouse Station, NJ: Merck & Co.; 2017.
1. Centers for Disease Control and Prevention. Viral hepatitis. http://www.cdc.gov/hepatitis. Updated December 9, 2016. Accessed February 9, 2017.
2. Butterfield MI, Bosworth HB, Meador KG, et al. Five-Site Health and Risk Study Research Committee. Gender differences in hepatitis C infection and risks among persons with severe mental illness. Psychiatr Serv. 2003;54(6):848-853.
3. Rifai MA, Gleason OC, Sabouni D. Psychiatric care of the patient with hepatitis C: a review of the literature. Prim Care Companion J Clin Psychiatry. 2010;12(6):PCC.09r00877. doi: 10.4088/PCC.09r00877whi.
4. Dinwiddie SH, Shicker L, Newman T. Prevalence of hepatitis C among psychiatric patients in the public sector. Am J Psychiatry. 2003;160(1):172-174.
5. Nakamura Y, Koh M, Miyoshi E, et al. High prevalence of the hepatitis C virus infection among the inpatients of schizophrenia and psychoactive substance abuse in Japan. Prog Neuropsychopharmacol Biol Psychiatry. 2004;28(3):591-597.
6. Sockalingam S, Blank D, Banga CA, et al. A novel program for treating patients with trimorbidity: hepatitis C, serious mental illness, and substance abuse. Eur J Gastroenterol Hepatol. 2013;25(12):1377-1384.
7. U.S. Preventive Services Task Force. Screening for hepatitis C virus infection: recommendation summary. https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/hepatitis-c-screening. Published June 2013. Accessed February 9, 2017.
8. Longo DL, Fauci AS, Kasper DL. Harrison’s principles of internal medicine. 18th ed. New York, NY: McGraw-Hill; 2012.
9. Belousova V, Abd-Rabou AA, Mousa SA. Recent advances and future directions in the management of hepatitis C infections. Pharmacol Ther. 2015;145:92-102.
10. American Association for the Study of Liver Diseases (AASLD); The Infectious Diseases Society of America (IDSA). HCV guidance: recommendations for testing, managing, and treating hepatitis C. http://www.hcvguidelines.org. Accessed February 9, 2017.
11. Rowan PJ, Bhulani N. Psychosocial assessment and monitoring in the new era of non-interferon-alpha hepatitis C treatments. World J Hepatol. 2015;7(19):2209-2213.
12. Good Rx, Inc. http://www.goodrx.com. Accessed October 9, 2015.
13. Raison CL, Borisov AS, Broadwell SD, et al. Depression during pegylated interferon-alpha plus ribavirin therapy: prevalence and prediction. J Clin Psychiatry. 2005;66(1):41-48.
14. Lotrich FE, Rabinovitz M, Gironda P, et al. Depression following pegylated interferon-alpha: characteristics and vulnerability. J Psychosom Res. 2007;63(2):131-135.
15. Udina M, Castellví P, Moreno-España J, et al. Interferon-induced depression in chronic hepatitis C: a systematic review and meta-analysis. J Clin Psychiatry. 2012;73(8):1128-1138.
16. Mustafa MZ, Schofield J, Mills PR, et al. The efficacy and safety of treating hepatitis C in patients with a diagnosis of schizophrenia. J Viral Hepat. 2014;21(7):e48-e51.
17. Huckans M, Mitchell A, Pavawalla S, et al. The influence of antiviral therapy on psychiatric symptoms among patients with hepatitis C and schizophrenia. Antivir Ther. 2010;15(1):111-119.
18. Huckans MS, Blackwell AD, Harms TA, et al. Management of hepatitis C disease among VA patients with schizophrenia and substance use disorders. Psychiatr Serv. 2006;57(3):403-406.
19. Huckans M, Mitchell A, Ruimy S, et al. Antiviral therapy completion and response rates among hepatitis C patients with and without schizophrenia. Schizophr Bull. 2010;36(1):165-172.
20. Hauser P, Morasco BJ, Linke A, et al. Antiviral completion rates and sustained viral response in hepatitis C patient with and without preexisting major depressive disorder. Psychosomatics. 2009;50(5):500-505.
21. Sockalingam S, Abbey SE. Managing depression during hepatitis C treatment. Can J Psychiatry. 2009;54(9):614-625.
22. Galvão-de Almeida A, Guindalini C, Batista-Neves S, et al. Can antidepressants prevent interferon-alpha-induced depression? A review of the literature. Gen Hosp Psychiatry. 2010;32(4):401-405.
23. Sockalingam S, Sheehan K, Feld JJ, et al. Psychiatric care during hepatitis c treatment: the changing role of psychiatrists in the era of direct-acting antivirals. Am J Psychiatry. 2015;172(6):512-516.
24. Harvoni [package insert]. Foster City, CA: Gilead Sciences, Inc.; 2016.
25. Wynn GH, Oesterheld, JR, Cozza KL, et al. Clinical manual of drug interactions principles for medical practice. Arlington, VA: American Psychiatric Publishing; 2009.
26. Olysio [package insert]. Titusville, NJ: Janssen Therapeutics; 2016.
27. Victrelis [package insert]. Whitehouse Station, NJ: Merck & Co.; 2017.
28. Daklinza [package insert]. Princeton, NJ: Bristol-Myers Squibb; 2016.
29. Zepatier [package insert]. Whitehouse Station, NJ: Merck & Co.; 2017.
Confused with ataxia and urinary and fecal incontinence
CASE Paranoia, ataxia
Ms. S, age 46, is admitted to the hospital for cellulitis and gait disturbance. She has been living in her car for the past week and presents to the local fire department to get help for housing. She is referred to this hospital where she was found to have cellulitis in her buttock secondary to urinary and fecal incontinence. She also was noted to have difficulty ambulating and a wide-based gait. Two weeks earlier, a hotel clerk found her on the floor, unable to get up. Ms. S was seen in a local emergency room (ER) and discharged after her glucose level was found to be normal.
At admission, she has an intact sensorium and is described as disheveled, illogical, rambling, and paranoid. Her mental status exam shows she is alert and oriented to person and time, with guarded and childlike behavior. Her affect/mood is irritable and oddly related, and her thought processes are concrete and simple with some thought-blocking and paranoid content. She denies thoughts of harming herself or others, and her insight is limited and judgment is poor.
Neurology is consulted to evaluate her gait disturbance. Ms. S has decreased muscle bulk in both calves, with brisk knee reflexes bilaterally. CT imaging shows nonspecific scattered periventricular white matter hypodensities consistent with microvascular ischemic diagnosis, but a demyelinating process could not be ruled out. Ms. S reports that the gait disturbance began in childhood, and that her grandmother had the same gait disturbance. Neurology recommends an electromyogram and MRI.
During her stay in the hospital, she is unwilling to cooperate with exams, declines to answer questions regarding her past, and appears suspicious of her acute care treatment team. The psychiatric team is consulted for evaluation of her paranoia and “seeming disorganization,” and she is transferred to the psychiatric unit. She appears to be repulsed by the fact that she was in a psychiatric ward stating, “I don’t belong here” and “I’m scared of the other people here.” She denies any psychiatric history, previous hospitalizations, or substance use, and no documentation of inpatient or outpatient care was found in the county’s computerized record system. Although she is willing to take a small dose of tranquilizer (eg, lorazepam) she refuses to take antipsychotic medications saying, “My mother told me not to take [antipsychotics]. I’m not psychotic.”
What is your diagnosis at this point?
a) normal pressure hydrocephalus
b) Charcot-Marie-Tooth disease
c) schizophrenia spectrum disorder
d) multiple sclerosis (MS)
e) vascular dementia
f) cord lesion compression
The authors’ observations
The neurology team initially suspected Charcot-Marie-Tooth disease because her clinical presentation included pes cavus, distal lower extremity weakness, and lower extremity muscle atrophy with a self-reported family history of similar gait disturbance, all of which are consistent with Charcot-Marie-Tooth disease.
Subcortical syndrome—a feature of vascular dementia—is characterized by focal motor deficits, gait disturbance, history of unsteadiness with frequent falls, urinary symptoms, personality and mood changes, and cognitive dysfunction.1-3 Subcortical syndrome is caused by chronic ischemia and lacunar infarctions that affect cerebral nuclei and white matter pathways.1 On imaging, subcortical vascular dementia is characterized by leukoaraiosis, which are hypointense spherical-like lesions on CT and hyperintense lesions in periventricular areas on T2 MRI.4
Although normal pressure hydrocephalus could be suspected given her clinical presentation of the Hakim-Adams triad (ie,“wacky, wobbly, and wet”), her head CT did not show any changes consistent with this condition.
Her clinical presentation does not align with schizophrenia spectrum disorder because of her history of higher functioning, acute later onset, and the absence of hallucinations, fixed delusions, or markedly disorganized speech. Although she is paranoid of her surroundings, her delusions were ill-formed. A cord lesion compression cannot be ruled out, and MRI is required urgently.
HISTORY High functioning
When asked, Ms. S states that she was admitted to the hospital because “someone who looked like a fake police officer [a member of the fire department] told me it was nice here.” She indicates that she initially thought it would be a nice place to live temporarily but later regretted coming after realizing that she was in a psychiatry unit. Available documentation from her recent hospitalization indicated that she was living in a motel on her own. Ms. S says that she works as an actress and has had minor roles in famous movies. She says that she studied at a well-known performance arts school and that her parents are famous musicians; however, she refuses to identify her parents or give permission to contact them—or any other collateral informant—because she is embarrassed about her current situation stating, “They would never believe it.”
During this interview, Ms. S appears confused as well as disorganized—which was a challenge to clearly delineate—disheveled, and guarded with hypoverbal and hypophonic speech. Her thought process is circumstantial, and she seems to be confabulating. She denies visual or auditory hallucinations but appears paranoid and states that she thinks we are experimenting on her. Except for the neurological exam, the rest of her physical exam is within normal limits. Urine toxicology screen and labs are negative except for a positive antinuclear antibody homogenous pattern with a titer of 1:640; B12 vitamin levels are not tested.
MRI is ordered, however, she does not consent to the scan saying, “It’s creepy, I don’t want people looking at my brain.” The team makes several attempts to encourage her for consent but she refuses. Because of the clinical urgency (ie, possible cord compression) and her refusal to provide a surrogate decision maker, the team felt the situation is urgent, confirmed by 2 physicians, which led them to perform the MRI on an emergent basis. The MRI reveals multiple periventricular, juxtacortical, infratentorial, and likely cervical spinal cord T2 hyperintense lesions (Figure).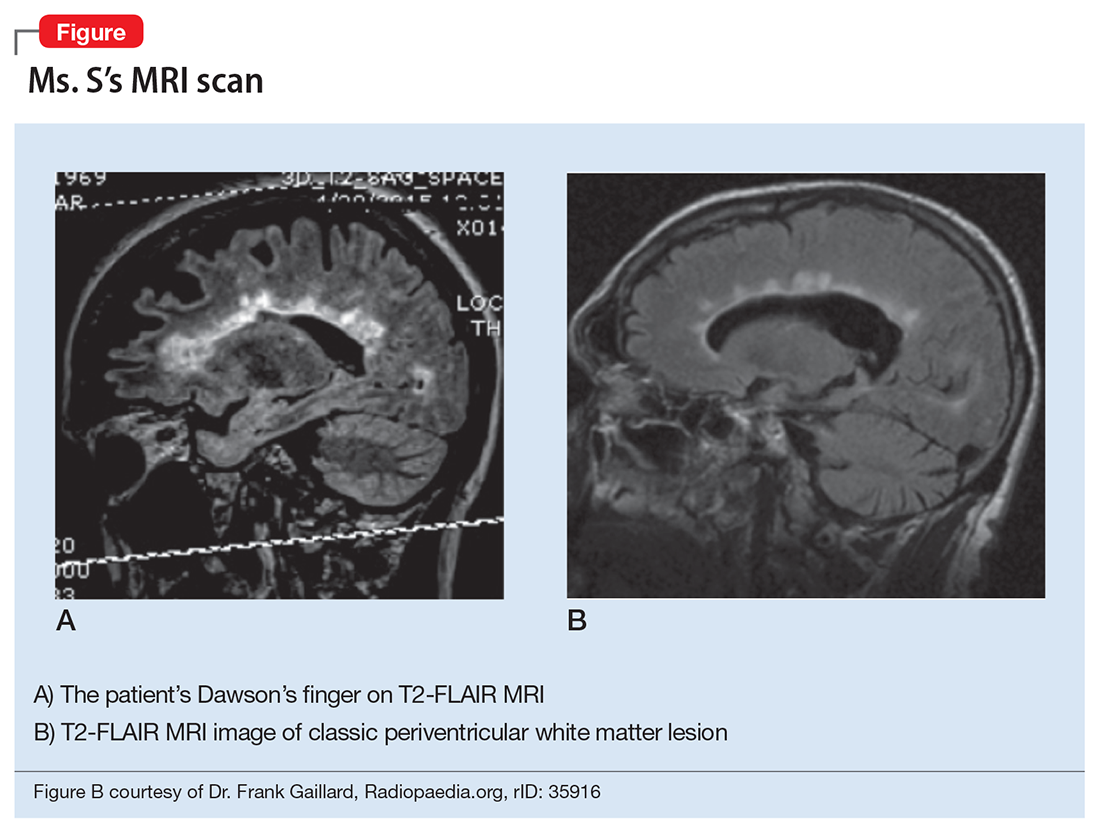
What would be your differential diagnosis at this time?
a) acute disseminated encephalomyelitis (ADEM)
b) systemic lupus erythematous
c) multiple sclerosis
d) vascular dementia
e) vitamin B deficiency
The authors’ observations
Psychosis in the presence of white matter demyelination could be associated with autoimmune, vascular, or nutritional disturbances. Deficiencies in vitamins B6, 9, and 12 (pyridoxine, folate, cobalamin) have been shown to cause neuropsychiatric symptoms and white matter lesions.5 Low levels of vitamins B6, 9, and 12 are associated with elevated homocysteine, which can cause small vessel ischemia leading to white matter lesions similar to changes seen in vascular dementia.5 The exact pathophysiology of ADEM is unclear, however, it is thought that after an infection, antiviral antibodies cross react with autoantigens on myelin causing an autoimmune demyelinating disease. Another hypothesized mechanism is that circulating immune complexes and humoral factors increase vascular permeability and inflammation thereby opening the blood–brain barrier. Once it is open, cells such as lymphocytes, phagocytes, and microglia cause gliosis and demyelination. Case reports have described ADEM associated with psychotic features.6
Likewise, systemic lupus erythematous has been associated with psychosis and neuropsychiatric symptoms in 14% to 75% of patients. Of these patients, 40% will experience neuropsychiatric symptoms before onset of lupus symptoms.7 One study found the most common MRI finding in neuropsychiatric systemic lupus erythematous was leukoaraiosis, which appeared in 57.1% of patients.8 Ms. S’s MRI results strongly suggest a diagnosis of MS.
EVALUATION Questionable story
Ms. S appears delusional and grandiose when she meets with the psychiatry team. She states that before her hospitalization, she was an actress and could ambulate, rent a motel room, and drive a car without assistance. However, during the examination, she cannot walk without 2 staff members for support, and overall her self-reported history sounds questionable. There were several pieces of evidence that corroborate portions of her story: (1) a screen actors guild card was found among personal belongings; (2) she was transported to the ER from a local motel; (3) she had recently visited another hospital and, at that time, was deemed stable enough to be discharged.
On the Montreal Cognitive Assessment (MoCA) Ms. S scored 19/30, with deficits mainly in executive/visuospatial and delayed recall memory. An alternate form of the MoCA is administered 1 day later, and she scores 20/30 with similar deficits. After obtaining medication consent, she is given risperidone, up to 2 mg/d, and becomes more cooperative with the treatment team.
The authors’ observations
Approximately 40% to 65% of MS patients experience cognitive impairment.9 Cognitive dysfunction in a depressed patient with MS might appear as pseudo-dementia, but other possible diagnoses include:
- true dementia
- encephalitis or infection
- medication- or substance-induced.
White matter demyelination is associated with subcortical dementia, which is characterized by slowness of information processing, forgetfulness, apathy, depression, and impaired cognition. According to meta-analyses, the most prominent neuropsychological deficits in MS are found in the areas of verbal fluency, information processing speed, working memory, and long-term memory.10 Relapsing-remitting type MS patients generally have less cognitive impairment than those with the chronic progressive type of the disease.
EVALUATION Cognitive deficits
Because of her acute condition and resistance to the evaluation, a modified screening neuropsychological battery is used. During the evaluation Ms. S is guarded and demonstrates paucity of speech; her responses are odd at times or contain word-substitution errors. Hand stiffness, tremor, and imprecision are noted during writing and drawing. Results of testing indicate average-range premorbid intellectual ability, with impairments in memory and information processing speed and a mild weakness in phonemic verbal fluency. Ms. S endorses statements reflecting paranoia and hostility on a self-report measure of emotional and personality functioning, consistent with her behavioral presentation. However, her responses on other subscales, including depression and psychotic symptoms, are within normal limits. Her cognitive deficits would be unusual if she had a psychiatric illness alone and are likely associated with her positive neuroimaging findings that suggest a demyelinating process. Overall, the results of the evaluation support a MS diagnosis.
The authors’ observations
Psychosis is found at a higher rate among MS patients (2% to 3%) than the general population (0.5% to 1%).9 Although rare, psychosis often can cloud the diagnosis of MS. Psychiatric symptoms that can occur in MS include:
- hallucinations and delusions (>50%)
- irritability and agitation (20%)
- grandiosity (15%)
- confusion, blunted affect, flight of ideas, depression, reduced self-care, and pressured speech (10%).11
A review of 10 studies found that depression was the most prevalent symptom in MS, and that schizophrenia occurred in up to 7% of MS patients.12 There are currently 3 theories about the relationship between psychosis and MS:
- MS and psychosis are thought to share the same pathophysiological process.
- Psychotic symptoms arise from regional demyelination simultaneously with MS.
- Psychosis is caused by medical treatment of MS.9
Other causes of psychiatric symptoms in MS include:
- depression associated with brain atrophy and lesions
- depression and anxiety as a result of chronic illness
- depression resulting from inflammatory changes
- corticosteroid treatment causing depression, mania, or psychosis.12
The link between psychosis and MS is still poorly understood and further investigation is needed.
How would you treat Ms. S?
a) haloperidol
b) risperidone
c) corticosteroids
d) selective serotonin reuptake inhibitors
Treating psychiatric symptoms in the context of MS
The literature, mainly case reports, suggests several treatment modalities for psychosis with MS. Clozapine has been shown to be beneficial in several case reports, and risperidone9 and ziprasidone13 also have been effective. Other studies recommended low-dose chlorpromazine.9
For MS patients with cognitive impairment, one study showed that interferon beta-1b (IFN-1b) treatment resulted in significant improvement in concentration, attention, visual learning, and recall after 1 year compared with control patients.9 However, there are also case reports of IFN-1b and glucocorticoid-induced psychosis in patients, which resolved after discontinuing treatment.9
Psychotic symptoms have been shown to resolve after corticosteroid treatment of MS.14 In another case report, mania and delusions subsided 3 days after IV methylprednisolone, whereas risperidone had no effect on psychotic features. However, it was unclear whether risperidone was discontinued when methylprednisolone was administered, therefore the specific effect of methylprednisolone is difficult to discern.15 Finally, in a case of a patient who has chronic MS for 16 years and presented with acute onset paranoid psychosis, symptoms resolved with aripiprazole, 10 to 20 mg/d.16 Because of the limited utility of case reports, there is a need for further research in medical management of psychiatric symptoms in MS.
1. de Groot JC, de Leeuw FE, Oudkerk M, et al. Cerebral white matter lesions and cognitive function: the Rotterdam Scan Study. Ann Neurol. 2000;47(2):145-151.
2. Tatemichi TK, Desmond DW, Prohovnik I, et al. Confusion and memory loss from capsular genu infarction: a thalamocortical disconnection syndrome? Neurology. 1992;42(10):1966-1979.
3. Staekenborg SS, van der Flier WM, van Straaten EC, et al. Neurological signs in relation to type of cerebrovascular disease in vascular dementia. Stroke. 2008;39(2):317-322.
4. Mortimer A, Likeman M, Lewis T. Neuroimaging in dementia: a practical guide. Pract Neurol. 2013;13(2):92-103.
5. Xiong YY, Mok V. Age-related white matter changes. J Aging Res. 2011;2011:617927. doi:10.4061/2011/617927.
6. Habek M, Brinar M, Brinar VV, et al. Psychiatric manifestations of multiple sclerosis and acute disseminated encephalomyelitis. Clin Neurol Neurosug. 2006;108(3);290-294.
7. Benros ME, Eaton WW, Mortensen PB. The epidemiologic evidence linking autoimmune disease and psychosis. Biol Psychiatry. 2014;75(4);300-306.
8. Jeong HW, Her M, Bae JS, et al. Brain MRI in neuropsychiatric lupus: associations with the 1999 ACR case definitions. Rheumatol Int. 2014;35(5):861-869.
9. Haussleiter IS, Brüne M, Juckel G. Psychopathology in multiple sclerosis: diagnosis, prevalence and treatment. Ther Adv Neurol Disord. 2009;2(1):13-29.
10. Thornton AE, DeFreitas VG. The neuropsychology of multiple sclerosis. In: Grant I, Adams KM, eds. Neuropsychological assessment of neuropsychiatric and neuromedical disorders. New York, NY: Oxford University Press; 2009:280-305.
11. Kosmidis MH, Giannakou M, Messinis L, et al. Psychotic features associated with multiple sclerosis. Int Rev Psychiatry. 2010;22(1):55-66.
12. Marrie RA, Reingold S, Cohen J, et al. The incidence and prevalence of psychiatric disorders in multiple sclerosis: a systematic review. Mult Scler. 2015;21(3):305-317.
13. Davids E, Hartwig U, Gastpar M. Antipsychotic treatment of psychosis associated with multiple sclerosis. Prog Neuropsychopharmacol Biol Psychiatry. 2004;28(4):734-744.
14. Thöne J, Kessler E. Improvement of neuropsychiatric symptoms in multiple sclerosis subsequent to high-dose corticosteroid treatment. Prim Care Companion J Clin Psychiatry. 2008;10(2):163-164.
15. Hoiter S, Maltete D, Bourre B, et al. A manic episode with psychotic features improved by methylprednisolone in a patient with multiple sclerosis. Gen Hosp Psychiatry. 2015;37(6):621.e1-621.e2.
16. Muzyk AJ, Christopher EJ, Gagliardi JP, et al. Use of aripiprazole in a patient with multiple sclerosis presenting with paranoid psychosis. J Psychiatr Pract. 2010;16(6):420-424.
CASE Paranoia, ataxia
Ms. S, age 46, is admitted to the hospital for cellulitis and gait disturbance. She has been living in her car for the past week and presents to the local fire department to get help for housing. She is referred to this hospital where she was found to have cellulitis in her buttock secondary to urinary and fecal incontinence. She also was noted to have difficulty ambulating and a wide-based gait. Two weeks earlier, a hotel clerk found her on the floor, unable to get up. Ms. S was seen in a local emergency room (ER) and discharged after her glucose level was found to be normal.
At admission, she has an intact sensorium and is described as disheveled, illogical, rambling, and paranoid. Her mental status exam shows she is alert and oriented to person and time, with guarded and childlike behavior. Her affect/mood is irritable and oddly related, and her thought processes are concrete and simple with some thought-blocking and paranoid content. She denies thoughts of harming herself or others, and her insight is limited and judgment is poor.
Neurology is consulted to evaluate her gait disturbance. Ms. S has decreased muscle bulk in both calves, with brisk knee reflexes bilaterally. CT imaging shows nonspecific scattered periventricular white matter hypodensities consistent with microvascular ischemic diagnosis, but a demyelinating process could not be ruled out. Ms. S reports that the gait disturbance began in childhood, and that her grandmother had the same gait disturbance. Neurology recommends an electromyogram and MRI.
During her stay in the hospital, she is unwilling to cooperate with exams, declines to answer questions regarding her past, and appears suspicious of her acute care treatment team. The psychiatric team is consulted for evaluation of her paranoia and “seeming disorganization,” and she is transferred to the psychiatric unit. She appears to be repulsed by the fact that she was in a psychiatric ward stating, “I don’t belong here” and “I’m scared of the other people here.” She denies any psychiatric history, previous hospitalizations, or substance use, and no documentation of inpatient or outpatient care was found in the county’s computerized record system. Although she is willing to take a small dose of tranquilizer (eg, lorazepam) she refuses to take antipsychotic medications saying, “My mother told me not to take [antipsychotics]. I’m not psychotic.”
What is your diagnosis at this point?
a) normal pressure hydrocephalus
b) Charcot-Marie-Tooth disease
c) schizophrenia spectrum disorder
d) multiple sclerosis (MS)
e) vascular dementia
f) cord lesion compression
The authors’ observations
The neurology team initially suspected Charcot-Marie-Tooth disease because her clinical presentation included pes cavus, distal lower extremity weakness, and lower extremity muscle atrophy with a self-reported family history of similar gait disturbance, all of which are consistent with Charcot-Marie-Tooth disease.
Subcortical syndrome—a feature of vascular dementia—is characterized by focal motor deficits, gait disturbance, history of unsteadiness with frequent falls, urinary symptoms, personality and mood changes, and cognitive dysfunction.1-3 Subcortical syndrome is caused by chronic ischemia and lacunar infarctions that affect cerebral nuclei and white matter pathways.1 On imaging, subcortical vascular dementia is characterized by leukoaraiosis, which are hypointense spherical-like lesions on CT and hyperintense lesions in periventricular areas on T2 MRI.4
Although normal pressure hydrocephalus could be suspected given her clinical presentation of the Hakim-Adams triad (ie,“wacky, wobbly, and wet”), her head CT did not show any changes consistent with this condition.
Her clinical presentation does not align with schizophrenia spectrum disorder because of her history of higher functioning, acute later onset, and the absence of hallucinations, fixed delusions, or markedly disorganized speech. Although she is paranoid of her surroundings, her delusions were ill-formed. A cord lesion compression cannot be ruled out, and MRI is required urgently.
HISTORY High functioning
When asked, Ms. S states that she was admitted to the hospital because “someone who looked like a fake police officer [a member of the fire department] told me it was nice here.” She indicates that she initially thought it would be a nice place to live temporarily but later regretted coming after realizing that she was in a psychiatry unit. Available documentation from her recent hospitalization indicated that she was living in a motel on her own. Ms. S says that she works as an actress and has had minor roles in famous movies. She says that she studied at a well-known performance arts school and that her parents are famous musicians; however, she refuses to identify her parents or give permission to contact them—or any other collateral informant—because she is embarrassed about her current situation stating, “They would never believe it.”
During this interview, Ms. S appears confused as well as disorganized—which was a challenge to clearly delineate—disheveled, and guarded with hypoverbal and hypophonic speech. Her thought process is circumstantial, and she seems to be confabulating. She denies visual or auditory hallucinations but appears paranoid and states that she thinks we are experimenting on her. Except for the neurological exam, the rest of her physical exam is within normal limits. Urine toxicology screen and labs are negative except for a positive antinuclear antibody homogenous pattern with a titer of 1:640; B12 vitamin levels are not tested.
MRI is ordered, however, she does not consent to the scan saying, “It’s creepy, I don’t want people looking at my brain.” The team makes several attempts to encourage her for consent but she refuses. Because of the clinical urgency (ie, possible cord compression) and her refusal to provide a surrogate decision maker, the team felt the situation is urgent, confirmed by 2 physicians, which led them to perform the MRI on an emergent basis. The MRI reveals multiple periventricular, juxtacortical, infratentorial, and likely cervical spinal cord T2 hyperintense lesions (Figure).
What would be your differential diagnosis at this time?
a) acute disseminated encephalomyelitis (ADEM)
b) systemic lupus erythematous
c) multiple sclerosis
d) vascular dementia
e) vitamin B deficiency
The authors’ observations
Psychosis in the presence of white matter demyelination could be associated with autoimmune, vascular, or nutritional disturbances. Deficiencies in vitamins B6, 9, and 12 (pyridoxine, folate, cobalamin) have been shown to cause neuropsychiatric symptoms and white matter lesions.5 Low levels of vitamins B6, 9, and 12 are associated with elevated homocysteine, which can cause small vessel ischemia leading to white matter lesions similar to changes seen in vascular dementia.5 The exact pathophysiology of ADEM is unclear, however, it is thought that after an infection, antiviral antibodies cross react with autoantigens on myelin causing an autoimmune demyelinating disease. Another hypothesized mechanism is that circulating immune complexes and humoral factors increase vascular permeability and inflammation thereby opening the blood–brain barrier. Once it is open, cells such as lymphocytes, phagocytes, and microglia cause gliosis and demyelination. Case reports have described ADEM associated with psychotic features.6
Likewise, systemic lupus erythematous has been associated with psychosis and neuropsychiatric symptoms in 14% to 75% of patients. Of these patients, 40% will experience neuropsychiatric symptoms before onset of lupus symptoms.7 One study found the most common MRI finding in neuropsychiatric systemic lupus erythematous was leukoaraiosis, which appeared in 57.1% of patients.8 Ms. S’s MRI results strongly suggest a diagnosis of MS.
EVALUATION Questionable story
Ms. S appears delusional and grandiose when she meets with the psychiatry team. She states that before her hospitalization, she was an actress and could ambulate, rent a motel room, and drive a car without assistance. However, during the examination, she cannot walk without 2 staff members for support, and overall her self-reported history sounds questionable. There were several pieces of evidence that corroborate portions of her story: (1) a screen actors guild card was found among personal belongings; (2) she was transported to the ER from a local motel; (3) she had recently visited another hospital and, at that time, was deemed stable enough to be discharged.
On the Montreal Cognitive Assessment (MoCA) Ms. S scored 19/30, with deficits mainly in executive/visuospatial and delayed recall memory. An alternate form of the MoCA is administered 1 day later, and she scores 20/30 with similar deficits. After obtaining medication consent, she is given risperidone, up to 2 mg/d, and becomes more cooperative with the treatment team.
The authors’ observations
Approximately 40% to 65% of MS patients experience cognitive impairment.9 Cognitive dysfunction in a depressed patient with MS might appear as pseudo-dementia, but other possible diagnoses include:
- true dementia
- encephalitis or infection
- medication- or substance-induced.
White matter demyelination is associated with subcortical dementia, which is characterized by slowness of information processing, forgetfulness, apathy, depression, and impaired cognition. According to meta-analyses, the most prominent neuropsychological deficits in MS are found in the areas of verbal fluency, information processing speed, working memory, and long-term memory.10 Relapsing-remitting type MS patients generally have less cognitive impairment than those with the chronic progressive type of the disease.
EVALUATION Cognitive deficits
Because of her acute condition and resistance to the evaluation, a modified screening neuropsychological battery is used. During the evaluation Ms. S is guarded and demonstrates paucity of speech; her responses are odd at times or contain word-substitution errors. Hand stiffness, tremor, and imprecision are noted during writing and drawing. Results of testing indicate average-range premorbid intellectual ability, with impairments in memory and information processing speed and a mild weakness in phonemic verbal fluency. Ms. S endorses statements reflecting paranoia and hostility on a self-report measure of emotional and personality functioning, consistent with her behavioral presentation. However, her responses on other subscales, including depression and psychotic symptoms, are within normal limits. Her cognitive deficits would be unusual if she had a psychiatric illness alone and are likely associated with her positive neuroimaging findings that suggest a demyelinating process. Overall, the results of the evaluation support a MS diagnosis.
The authors’ observations
Psychosis is found at a higher rate among MS patients (2% to 3%) than the general population (0.5% to 1%).9 Although rare, psychosis often can cloud the diagnosis of MS. Psychiatric symptoms that can occur in MS include:
- hallucinations and delusions (>50%)
- irritability and agitation (20%)
- grandiosity (15%)
- confusion, blunted affect, flight of ideas, depression, reduced self-care, and pressured speech (10%).11
A review of 10 studies found that depression was the most prevalent symptom in MS, and that schizophrenia occurred in up to 7% of MS patients.12 There are currently 3 theories about the relationship between psychosis and MS:
- MS and psychosis are thought to share the same pathophysiological process.
- Psychotic symptoms arise from regional demyelination simultaneously with MS.
- Psychosis is caused by medical treatment of MS.9
Other causes of psychiatric symptoms in MS include:
- depression associated with brain atrophy and lesions
- depression and anxiety as a result of chronic illness
- depression resulting from inflammatory changes
- corticosteroid treatment causing depression, mania, or psychosis.12
The link between psychosis and MS is still poorly understood and further investigation is needed.
How would you treat Ms. S?
a) haloperidol
b) risperidone
c) corticosteroids
d) selective serotonin reuptake inhibitors
Treating psychiatric symptoms in the context of MS
The literature, mainly case reports, suggests several treatment modalities for psychosis with MS. Clozapine has been shown to be beneficial in several case reports, and risperidone9 and ziprasidone13 also have been effective. Other studies recommended low-dose chlorpromazine.9
For MS patients with cognitive impairment, one study showed that interferon beta-1b (IFN-1b) treatment resulted in significant improvement in concentration, attention, visual learning, and recall after 1 year compared with control patients.9 However, there are also case reports of IFN-1b and glucocorticoid-induced psychosis in patients, which resolved after discontinuing treatment.9
Psychotic symptoms have been shown to resolve after corticosteroid treatment of MS.14 In another case report, mania and delusions subsided 3 days after IV methylprednisolone, whereas risperidone had no effect on psychotic features. However, it was unclear whether risperidone was discontinued when methylprednisolone was administered, therefore the specific effect of methylprednisolone is difficult to discern.15 Finally, in a case of a patient who has chronic MS for 16 years and presented with acute onset paranoid psychosis, symptoms resolved with aripiprazole, 10 to 20 mg/d.16 Because of the limited utility of case reports, there is a need for further research in medical management of psychiatric symptoms in MS.
CASE Paranoia, ataxia
Ms. S, age 46, is admitted to the hospital for cellulitis and gait disturbance. She has been living in her car for the past week and presents to the local fire department to get help for housing. She is referred to this hospital where she was found to have cellulitis in her buttock secondary to urinary and fecal incontinence. She also was noted to have difficulty ambulating and a wide-based gait. Two weeks earlier, a hotel clerk found her on the floor, unable to get up. Ms. S was seen in a local emergency room (ER) and discharged after her glucose level was found to be normal.
At admission, she has an intact sensorium and is described as disheveled, illogical, rambling, and paranoid. Her mental status exam shows she is alert and oriented to person and time, with guarded and childlike behavior. Her affect/mood is irritable and oddly related, and her thought processes are concrete and simple with some thought-blocking and paranoid content. She denies thoughts of harming herself or others, and her insight is limited and judgment is poor.
Neurology is consulted to evaluate her gait disturbance. Ms. S has decreased muscle bulk in both calves, with brisk knee reflexes bilaterally. CT imaging shows nonspecific scattered periventricular white matter hypodensities consistent with microvascular ischemic diagnosis, but a demyelinating process could not be ruled out. Ms. S reports that the gait disturbance began in childhood, and that her grandmother had the same gait disturbance. Neurology recommends an electromyogram and MRI.
During her stay in the hospital, she is unwilling to cooperate with exams, declines to answer questions regarding her past, and appears suspicious of her acute care treatment team. The psychiatric team is consulted for evaluation of her paranoia and “seeming disorganization,” and she is transferred to the psychiatric unit. She appears to be repulsed by the fact that she was in a psychiatric ward stating, “I don’t belong here” and “I’m scared of the other people here.” She denies any psychiatric history, previous hospitalizations, or substance use, and no documentation of inpatient or outpatient care was found in the county’s computerized record system. Although she is willing to take a small dose of tranquilizer (eg, lorazepam) she refuses to take antipsychotic medications saying, “My mother told me not to take [antipsychotics]. I’m not psychotic.”
What is your diagnosis at this point?
a) normal pressure hydrocephalus
b) Charcot-Marie-Tooth disease
c) schizophrenia spectrum disorder
d) multiple sclerosis (MS)
e) vascular dementia
f) cord lesion compression
The authors’ observations
The neurology team initially suspected Charcot-Marie-Tooth disease because her clinical presentation included pes cavus, distal lower extremity weakness, and lower extremity muscle atrophy with a self-reported family history of similar gait disturbance, all of which are consistent with Charcot-Marie-Tooth disease.
Subcortical syndrome—a feature of vascular dementia—is characterized by focal motor deficits, gait disturbance, history of unsteadiness with frequent falls, urinary symptoms, personality and mood changes, and cognitive dysfunction.1-3 Subcortical syndrome is caused by chronic ischemia and lacunar infarctions that affect cerebral nuclei and white matter pathways.1 On imaging, subcortical vascular dementia is characterized by leukoaraiosis, which are hypointense spherical-like lesions on CT and hyperintense lesions in periventricular areas on T2 MRI.4
Although normal pressure hydrocephalus could be suspected given her clinical presentation of the Hakim-Adams triad (ie,“wacky, wobbly, and wet”), her head CT did not show any changes consistent with this condition.
Her clinical presentation does not align with schizophrenia spectrum disorder because of her history of higher functioning, acute later onset, and the absence of hallucinations, fixed delusions, or markedly disorganized speech. Although she is paranoid of her surroundings, her delusions were ill-formed. A cord lesion compression cannot be ruled out, and MRI is required urgently.
HISTORY High functioning
When asked, Ms. S states that she was admitted to the hospital because “someone who looked like a fake police officer [a member of the fire department] told me it was nice here.” She indicates that she initially thought it would be a nice place to live temporarily but later regretted coming after realizing that she was in a psychiatry unit. Available documentation from her recent hospitalization indicated that she was living in a motel on her own. Ms. S says that she works as an actress and has had minor roles in famous movies. She says that she studied at a well-known performance arts school and that her parents are famous musicians; however, she refuses to identify her parents or give permission to contact them—or any other collateral informant—because she is embarrassed about her current situation stating, “They would never believe it.”
During this interview, Ms. S appears confused as well as disorganized—which was a challenge to clearly delineate—disheveled, and guarded with hypoverbal and hypophonic speech. Her thought process is circumstantial, and she seems to be confabulating. She denies visual or auditory hallucinations but appears paranoid and states that she thinks we are experimenting on her. Except for the neurological exam, the rest of her physical exam is within normal limits. Urine toxicology screen and labs are negative except for a positive antinuclear antibody homogenous pattern with a titer of 1:640; B12 vitamin levels are not tested.
MRI is ordered, however, she does not consent to the scan saying, “It’s creepy, I don’t want people looking at my brain.” The team makes several attempts to encourage her for consent but she refuses. Because of the clinical urgency (ie, possible cord compression) and her refusal to provide a surrogate decision maker, the team felt the situation is urgent, confirmed by 2 physicians, which led them to perform the MRI on an emergent basis. The MRI reveals multiple periventricular, juxtacortical, infratentorial, and likely cervical spinal cord T2 hyperintense lesions (Figure).
What would be your differential diagnosis at this time?
a) acute disseminated encephalomyelitis (ADEM)
b) systemic lupus erythematous
c) multiple sclerosis
d) vascular dementia
e) vitamin B deficiency
The authors’ observations
Psychosis in the presence of white matter demyelination could be associated with autoimmune, vascular, or nutritional disturbances. Deficiencies in vitamins B6, 9, and 12 (pyridoxine, folate, cobalamin) have been shown to cause neuropsychiatric symptoms and white matter lesions.5 Low levels of vitamins B6, 9, and 12 are associated with elevated homocysteine, which can cause small vessel ischemia leading to white matter lesions similar to changes seen in vascular dementia.5 The exact pathophysiology of ADEM is unclear, however, it is thought that after an infection, antiviral antibodies cross react with autoantigens on myelin causing an autoimmune demyelinating disease. Another hypothesized mechanism is that circulating immune complexes and humoral factors increase vascular permeability and inflammation thereby opening the blood–brain barrier. Once it is open, cells such as lymphocytes, phagocytes, and microglia cause gliosis and demyelination. Case reports have described ADEM associated with psychotic features.6
Likewise, systemic lupus erythematous has been associated with psychosis and neuropsychiatric symptoms in 14% to 75% of patients. Of these patients, 40% will experience neuropsychiatric symptoms before onset of lupus symptoms.7 One study found the most common MRI finding in neuropsychiatric systemic lupus erythematous was leukoaraiosis, which appeared in 57.1% of patients.8 Ms. S’s MRI results strongly suggest a diagnosis of MS.
EVALUATION Questionable story
Ms. S appears delusional and grandiose when she meets with the psychiatry team. She states that before her hospitalization, she was an actress and could ambulate, rent a motel room, and drive a car without assistance. However, during the examination, she cannot walk without 2 staff members for support, and overall her self-reported history sounds questionable. There were several pieces of evidence that corroborate portions of her story: (1) a screen actors guild card was found among personal belongings; (2) she was transported to the ER from a local motel; (3) she had recently visited another hospital and, at that time, was deemed stable enough to be discharged.
On the Montreal Cognitive Assessment (MoCA) Ms. S scored 19/30, with deficits mainly in executive/visuospatial and delayed recall memory. An alternate form of the MoCA is administered 1 day later, and she scores 20/30 with similar deficits. After obtaining medication consent, she is given risperidone, up to 2 mg/d, and becomes more cooperative with the treatment team.
The authors’ observations
Approximately 40% to 65% of MS patients experience cognitive impairment.9 Cognitive dysfunction in a depressed patient with MS might appear as pseudo-dementia, but other possible diagnoses include:
- true dementia
- encephalitis or infection
- medication- or substance-induced.
White matter demyelination is associated with subcortical dementia, which is characterized by slowness of information processing, forgetfulness, apathy, depression, and impaired cognition. According to meta-analyses, the most prominent neuropsychological deficits in MS are found in the areas of verbal fluency, information processing speed, working memory, and long-term memory.10 Relapsing-remitting type MS patients generally have less cognitive impairment than those with the chronic progressive type of the disease.
EVALUATION Cognitive deficits
Because of her acute condition and resistance to the evaluation, a modified screening neuropsychological battery is used. During the evaluation Ms. S is guarded and demonstrates paucity of speech; her responses are odd at times or contain word-substitution errors. Hand stiffness, tremor, and imprecision are noted during writing and drawing. Results of testing indicate average-range premorbid intellectual ability, with impairments in memory and information processing speed and a mild weakness in phonemic verbal fluency. Ms. S endorses statements reflecting paranoia and hostility on a self-report measure of emotional and personality functioning, consistent with her behavioral presentation. However, her responses on other subscales, including depression and psychotic symptoms, are within normal limits. Her cognitive deficits would be unusual if she had a psychiatric illness alone and are likely associated with her positive neuroimaging findings that suggest a demyelinating process. Overall, the results of the evaluation support a MS diagnosis.
The authors’ observations
Psychosis is found at a higher rate among MS patients (2% to 3%) than the general population (0.5% to 1%).9 Although rare, psychosis often can cloud the diagnosis of MS. Psychiatric symptoms that can occur in MS include:
- hallucinations and delusions (>50%)
- irritability and agitation (20%)
- grandiosity (15%)
- confusion, blunted affect, flight of ideas, depression, reduced self-care, and pressured speech (10%).11
A review of 10 studies found that depression was the most prevalent symptom in MS, and that schizophrenia occurred in up to 7% of MS patients.12 There are currently 3 theories about the relationship between psychosis and MS:
- MS and psychosis are thought to share the same pathophysiological process.
- Psychotic symptoms arise from regional demyelination simultaneously with MS.
- Psychosis is caused by medical treatment of MS.9
Other causes of psychiatric symptoms in MS include:
- depression associated with brain atrophy and lesions
- depression and anxiety as a result of chronic illness
- depression resulting from inflammatory changes
- corticosteroid treatment causing depression, mania, or psychosis.12
The link between psychosis and MS is still poorly understood and further investigation is needed.
How would you treat Ms. S?
a) haloperidol
b) risperidone
c) corticosteroids
d) selective serotonin reuptake inhibitors
Treating psychiatric symptoms in the context of MS
The literature, mainly case reports, suggests several treatment modalities for psychosis with MS. Clozapine has been shown to be beneficial in several case reports, and risperidone9 and ziprasidone13 also have been effective. Other studies recommended low-dose chlorpromazine.9
For MS patients with cognitive impairment, one study showed that interferon beta-1b (IFN-1b) treatment resulted in significant improvement in concentration, attention, visual learning, and recall after 1 year compared with control patients.9 However, there are also case reports of IFN-1b and glucocorticoid-induced psychosis in patients, which resolved after discontinuing treatment.9
Psychotic symptoms have been shown to resolve after corticosteroid treatment of MS.14 In another case report, mania and delusions subsided 3 days after IV methylprednisolone, whereas risperidone had no effect on psychotic features. However, it was unclear whether risperidone was discontinued when methylprednisolone was administered, therefore the specific effect of methylprednisolone is difficult to discern.15 Finally, in a case of a patient who has chronic MS for 16 years and presented with acute onset paranoid psychosis, symptoms resolved with aripiprazole, 10 to 20 mg/d.16 Because of the limited utility of case reports, there is a need for further research in medical management of psychiatric symptoms in MS.
1. de Groot JC, de Leeuw FE, Oudkerk M, et al. Cerebral white matter lesions and cognitive function: the Rotterdam Scan Study. Ann Neurol. 2000;47(2):145-151.
2. Tatemichi TK, Desmond DW, Prohovnik I, et al. Confusion and memory loss from capsular genu infarction: a thalamocortical disconnection syndrome? Neurology. 1992;42(10):1966-1979.
3. Staekenborg SS, van der Flier WM, van Straaten EC, et al. Neurological signs in relation to type of cerebrovascular disease in vascular dementia. Stroke. 2008;39(2):317-322.
4. Mortimer A, Likeman M, Lewis T. Neuroimaging in dementia: a practical guide. Pract Neurol. 2013;13(2):92-103.
5. Xiong YY, Mok V. Age-related white matter changes. J Aging Res. 2011;2011:617927. doi:10.4061/2011/617927.
6. Habek M, Brinar M, Brinar VV, et al. Psychiatric manifestations of multiple sclerosis and acute disseminated encephalomyelitis. Clin Neurol Neurosug. 2006;108(3);290-294.
7. Benros ME, Eaton WW, Mortensen PB. The epidemiologic evidence linking autoimmune disease and psychosis. Biol Psychiatry. 2014;75(4);300-306.
8. Jeong HW, Her M, Bae JS, et al. Brain MRI in neuropsychiatric lupus: associations with the 1999 ACR case definitions. Rheumatol Int. 2014;35(5):861-869.
9. Haussleiter IS, Brüne M, Juckel G. Psychopathology in multiple sclerosis: diagnosis, prevalence and treatment. Ther Adv Neurol Disord. 2009;2(1):13-29.
10. Thornton AE, DeFreitas VG. The neuropsychology of multiple sclerosis. In: Grant I, Adams KM, eds. Neuropsychological assessment of neuropsychiatric and neuromedical disorders. New York, NY: Oxford University Press; 2009:280-305.
11. Kosmidis MH, Giannakou M, Messinis L, et al. Psychotic features associated with multiple sclerosis. Int Rev Psychiatry. 2010;22(1):55-66.
12. Marrie RA, Reingold S, Cohen J, et al. The incidence and prevalence of psychiatric disorders in multiple sclerosis: a systematic review. Mult Scler. 2015;21(3):305-317.
13. Davids E, Hartwig U, Gastpar M. Antipsychotic treatment of psychosis associated with multiple sclerosis. Prog Neuropsychopharmacol Biol Psychiatry. 2004;28(4):734-744.
14. Thöne J, Kessler E. Improvement of neuropsychiatric symptoms in multiple sclerosis subsequent to high-dose corticosteroid treatment. Prim Care Companion J Clin Psychiatry. 2008;10(2):163-164.
15. Hoiter S, Maltete D, Bourre B, et al. A manic episode with psychotic features improved by methylprednisolone in a patient with multiple sclerosis. Gen Hosp Psychiatry. 2015;37(6):621.e1-621.e2.
16. Muzyk AJ, Christopher EJ, Gagliardi JP, et al. Use of aripiprazole in a patient with multiple sclerosis presenting with paranoid psychosis. J Psychiatr Pract. 2010;16(6):420-424.
1. de Groot JC, de Leeuw FE, Oudkerk M, et al. Cerebral white matter lesions and cognitive function: the Rotterdam Scan Study. Ann Neurol. 2000;47(2):145-151.
2. Tatemichi TK, Desmond DW, Prohovnik I, et al. Confusion and memory loss from capsular genu infarction: a thalamocortical disconnection syndrome? Neurology. 1992;42(10):1966-1979.
3. Staekenborg SS, van der Flier WM, van Straaten EC, et al. Neurological signs in relation to type of cerebrovascular disease in vascular dementia. Stroke. 2008;39(2):317-322.
4. Mortimer A, Likeman M, Lewis T. Neuroimaging in dementia: a practical guide. Pract Neurol. 2013;13(2):92-103.
5. Xiong YY, Mok V. Age-related white matter changes. J Aging Res. 2011;2011:617927. doi:10.4061/2011/617927.
6. Habek M, Brinar M, Brinar VV, et al. Psychiatric manifestations of multiple sclerosis and acute disseminated encephalomyelitis. Clin Neurol Neurosug. 2006;108(3);290-294.
7. Benros ME, Eaton WW, Mortensen PB. The epidemiologic evidence linking autoimmune disease and psychosis. Biol Psychiatry. 2014;75(4);300-306.
8. Jeong HW, Her M, Bae JS, et al. Brain MRI in neuropsychiatric lupus: associations with the 1999 ACR case definitions. Rheumatol Int. 2014;35(5):861-869.
9. Haussleiter IS, Brüne M, Juckel G. Psychopathology in multiple sclerosis: diagnosis, prevalence and treatment. Ther Adv Neurol Disord. 2009;2(1):13-29.
10. Thornton AE, DeFreitas VG. The neuropsychology of multiple sclerosis. In: Grant I, Adams KM, eds. Neuropsychological assessment of neuropsychiatric and neuromedical disorders. New York, NY: Oxford University Press; 2009:280-305.
11. Kosmidis MH, Giannakou M, Messinis L, et al. Psychotic features associated with multiple sclerosis. Int Rev Psychiatry. 2010;22(1):55-66.
12. Marrie RA, Reingold S, Cohen J, et al. The incidence and prevalence of psychiatric disorders in multiple sclerosis: a systematic review. Mult Scler. 2015;21(3):305-317.
13. Davids E, Hartwig U, Gastpar M. Antipsychotic treatment of psychosis associated with multiple sclerosis. Prog Neuropsychopharmacol Biol Psychiatry. 2004;28(4):734-744.
14. Thöne J, Kessler E. Improvement of neuropsychiatric symptoms in multiple sclerosis subsequent to high-dose corticosteroid treatment. Prim Care Companion J Clin Psychiatry. 2008;10(2):163-164.
15. Hoiter S, Maltete D, Bourre B, et al. A manic episode with psychotic features improved by methylprednisolone in a patient with multiple sclerosis. Gen Hosp Psychiatry. 2015;37(6):621.e1-621.e2.
16. Muzyk AJ, Christopher EJ, Gagliardi JP, et al. Use of aripiprazole in a patient with multiple sclerosis presenting with paranoid psychosis. J Psychiatr Pract. 2010;16(6):420-424.
Treatment adherence makes big impact in psychogenic nonepileptic seizures
HOUSTON – Patients with psychogenic nonepileptic seizures who stick with evidence-based treatment have significantly fewer seizures and have less associated disability than do those who don’t make it to therapy and psychiatry visits, a study showed.
Reporting preliminary data from 59 patients in a 123-patient study, Benjamin Tolchin, MD, and his colleagues said that patients who adhered to their treatment plans were significantly more likely to experience a reduction in seizure frequency of more than 50%, compared with nonadherent patients (P = .018). Treatment dropout was positively associated with having a prior psychogenic nonepileptic seizure (PNES) diagnosis and with having less concern about the illness.
These figures, he said, are consistent with what’s been reported in the PNES literature. Others have found that after diagnosis, 20%-30% of patients don’t attend their first appointment, although psychiatric treatment and therapy constitute evidence-based care that is effective in treating PNES.
Dr. Tolchin said previous studies have found that “over 71% of patients were found to have seizures and associated disability at the 4-year follow-up mark.”
In addition to tracking adherence, Dr. Tolchin and his coinvestigators attempted to identify risk factors for nonadherence among their patient cohort, all of whom had documented PNES. Study participants provided general demographic data, and investigators also gathered information about PNES event frequency; any prior diagnosis of PNES or other psychiatric comorbidities; history of physical, emotional, or sexual abuse; and health care resource utilization. Patients also were asked about their quality of life and time from symptom onset to receiving the PNES diagnosis.
Finally, patients filled out the Brief Illness Perception Questionnaire (BIPQ). This instrument measures various aspects of patients’ cognitive and emotional representations of illness, using a nine-item questionnaire. Higher scores indicate that the patient sees the illness as more concerning.
All patients were referred for both psychotherapy and four follow-up visits with a psychiatrist. The first psychiatric visit was to occur within 1-2 months after receiving the PNES diagnosis, with the next two visits occurring at 1.5- to 3-month intervals following the first visit. The final scheduled follow-up visit was to occur 6-9 months after the third visit.
Most patients (85%) were female and non-Hispanic white (77%), with a mean age of 38 years (range, 18-80). About one-third of patients were single, and another third were married. The remainder were evenly split between having a live-in partner and being separated or divorced, with just 2% being widowed.
By self-report, more than one-third of patients (37%) were on disability, and nearly one-quarter (24%) were unemployed. Just 18% were working full time; another 11% worked part time, and 8% were students.
The median weekly number of PNES episodes per patient was two, although reported events per week ranged from 0 to 350.
Psychiatric comorbidities were very frequent: 94% of patients reported some variety of psychiatric disorder. Depressive disorders were reported by 78% of patients, anxiety disorders by 61%, and posttraumatic stress disorder by 54%. Other commonly reported psychiatric diagnoses included panic disorder (40%), phobias (38%), and personality disorders (31%).
Almost a quarter of patients (23%) had attempted suicide in the past, and the same percentage reported a history of substance abuse. Patient reports of emotional (57%), physical (45%), and sexual (42%) abuse were also common.
Having a prior diagnosis of PNES was identified as a significant risk factor for dropping out of treatment (hazard ratio, 1.57; 95% confidence interval, 1.01-2.46; P = .046]. Patients with a higher concern for their illness, as evidenced by a higher BIPQ score, were less likely to drop out of treatment (HR, 0.77 for 10-point increment; 95% CI, 0.64-0.93; P = .008).
“Neurologists and behavioral health specialists need new interventions to improve adherence with treatment and prevent long-term disability,” Dr. Tolchin said.
The study, which won the Kaufman Honor for the highest-ranking abstract in the comorbidities topic category at the meeting, was supported by a practice research training fellowship from the American Academy of Neurology and the American Brain Foundation. Dr. Tolchin reported no other disclosures.
[email protected]
On Twitter @karioakes
HOUSTON – Patients with psychogenic nonepileptic seizures who stick with evidence-based treatment have significantly fewer seizures and have less associated disability than do those who don’t make it to therapy and psychiatry visits, a study showed.
Reporting preliminary data from 59 patients in a 123-patient study, Benjamin Tolchin, MD, and his colleagues said that patients who adhered to their treatment plans were significantly more likely to experience a reduction in seizure frequency of more than 50%, compared with nonadherent patients (P = .018). Treatment dropout was positively associated with having a prior psychogenic nonepileptic seizure (PNES) diagnosis and with having less concern about the illness.
These figures, he said, are consistent with what’s been reported in the PNES literature. Others have found that after diagnosis, 20%-30% of patients don’t attend their first appointment, although psychiatric treatment and therapy constitute evidence-based care that is effective in treating PNES.
Dr. Tolchin said previous studies have found that “over 71% of patients were found to have seizures and associated disability at the 4-year follow-up mark.”
In addition to tracking adherence, Dr. Tolchin and his coinvestigators attempted to identify risk factors for nonadherence among their patient cohort, all of whom had documented PNES. Study participants provided general demographic data, and investigators also gathered information about PNES event frequency; any prior diagnosis of PNES or other psychiatric comorbidities; history of physical, emotional, or sexual abuse; and health care resource utilization. Patients also were asked about their quality of life and time from symptom onset to receiving the PNES diagnosis.
Finally, patients filled out the Brief Illness Perception Questionnaire (BIPQ). This instrument measures various aspects of patients’ cognitive and emotional representations of illness, using a nine-item questionnaire. Higher scores indicate that the patient sees the illness as more concerning.
All patients were referred for both psychotherapy and four follow-up visits with a psychiatrist. The first psychiatric visit was to occur within 1-2 months after receiving the PNES diagnosis, with the next two visits occurring at 1.5- to 3-month intervals following the first visit. The final scheduled follow-up visit was to occur 6-9 months after the third visit.
Most patients (85%) were female and non-Hispanic white (77%), with a mean age of 38 years (range, 18-80). About one-third of patients were single, and another third were married. The remainder were evenly split between having a live-in partner and being separated or divorced, with just 2% being widowed.
By self-report, more than one-third of patients (37%) were on disability, and nearly one-quarter (24%) were unemployed. Just 18% were working full time; another 11% worked part time, and 8% were students.
The median weekly number of PNES episodes per patient was two, although reported events per week ranged from 0 to 350.
Psychiatric comorbidities were very frequent: 94% of patients reported some variety of psychiatric disorder. Depressive disorders were reported by 78% of patients, anxiety disorders by 61%, and posttraumatic stress disorder by 54%. Other commonly reported psychiatric diagnoses included panic disorder (40%), phobias (38%), and personality disorders (31%).
Almost a quarter of patients (23%) had attempted suicide in the past, and the same percentage reported a history of substance abuse. Patient reports of emotional (57%), physical (45%), and sexual (42%) abuse were also common.
Having a prior diagnosis of PNES was identified as a significant risk factor for dropping out of treatment (hazard ratio, 1.57; 95% confidence interval, 1.01-2.46; P = .046]. Patients with a higher concern for their illness, as evidenced by a higher BIPQ score, were less likely to drop out of treatment (HR, 0.77 for 10-point increment; 95% CI, 0.64-0.93; P = .008).
“Neurologists and behavioral health specialists need new interventions to improve adherence with treatment and prevent long-term disability,” Dr. Tolchin said.
The study, which won the Kaufman Honor for the highest-ranking abstract in the comorbidities topic category at the meeting, was supported by a practice research training fellowship from the American Academy of Neurology and the American Brain Foundation. Dr. Tolchin reported no other disclosures.
[email protected]
On Twitter @karioakes
HOUSTON – Patients with psychogenic nonepileptic seizures who stick with evidence-based treatment have significantly fewer seizures and have less associated disability than do those who don’t make it to therapy and psychiatry visits, a study showed.
Reporting preliminary data from 59 patients in a 123-patient study, Benjamin Tolchin, MD, and his colleagues said that patients who adhered to their treatment plans were significantly more likely to experience a reduction in seizure frequency of more than 50%, compared with nonadherent patients (P = .018). Treatment dropout was positively associated with having a prior psychogenic nonepileptic seizure (PNES) diagnosis and with having less concern about the illness.
These figures, he said, are consistent with what’s been reported in the PNES literature. Others have found that after diagnosis, 20%-30% of patients don’t attend their first appointment, although psychiatric treatment and therapy constitute evidence-based care that is effective in treating PNES.
Dr. Tolchin said previous studies have found that “over 71% of patients were found to have seizures and associated disability at the 4-year follow-up mark.”
In addition to tracking adherence, Dr. Tolchin and his coinvestigators attempted to identify risk factors for nonadherence among their patient cohort, all of whom had documented PNES. Study participants provided general demographic data, and investigators also gathered information about PNES event frequency; any prior diagnosis of PNES or other psychiatric comorbidities; history of physical, emotional, or sexual abuse; and health care resource utilization. Patients also were asked about their quality of life and time from symptom onset to receiving the PNES diagnosis.
Finally, patients filled out the Brief Illness Perception Questionnaire (BIPQ). This instrument measures various aspects of patients’ cognitive and emotional representations of illness, using a nine-item questionnaire. Higher scores indicate that the patient sees the illness as more concerning.
All patients were referred for both psychotherapy and four follow-up visits with a psychiatrist. The first psychiatric visit was to occur within 1-2 months after receiving the PNES diagnosis, with the next two visits occurring at 1.5- to 3-month intervals following the first visit. The final scheduled follow-up visit was to occur 6-9 months after the third visit.
Most patients (85%) were female and non-Hispanic white (77%), with a mean age of 38 years (range, 18-80). About one-third of patients were single, and another third were married. The remainder were evenly split between having a live-in partner and being separated or divorced, with just 2% being widowed.
By self-report, more than one-third of patients (37%) were on disability, and nearly one-quarter (24%) were unemployed. Just 18% were working full time; another 11% worked part time, and 8% were students.
The median weekly number of PNES episodes per patient was two, although reported events per week ranged from 0 to 350.
Psychiatric comorbidities were very frequent: 94% of patients reported some variety of psychiatric disorder. Depressive disorders were reported by 78% of patients, anxiety disorders by 61%, and posttraumatic stress disorder by 54%. Other commonly reported psychiatric diagnoses included panic disorder (40%), phobias (38%), and personality disorders (31%).
Almost a quarter of patients (23%) had attempted suicide in the past, and the same percentage reported a history of substance abuse. Patient reports of emotional (57%), physical (45%), and sexual (42%) abuse were also common.
Having a prior diagnosis of PNES was identified as a significant risk factor for dropping out of treatment (hazard ratio, 1.57; 95% confidence interval, 1.01-2.46; P = .046]. Patients with a higher concern for their illness, as evidenced by a higher BIPQ score, were less likely to drop out of treatment (HR, 0.77 for 10-point increment; 95% CI, 0.64-0.93; P = .008).
“Neurologists and behavioral health specialists need new interventions to improve adherence with treatment and prevent long-term disability,” Dr. Tolchin said.
The study, which won the Kaufman Honor for the highest-ranking abstract in the comorbidities topic category at the meeting, was supported by a practice research training fellowship from the American Academy of Neurology and the American Brain Foundation. Dr. Tolchin reported no other disclosures.
[email protected]
On Twitter @karioakes
AT AES 2016
Key clinical point:
Major finding: Adherent patients were more likely to reduce their seizures by half or more (P = .018).
Data source: A study of 123 patients with documented PNES.
Disclosures: The study was funded by a practice research training fellowship from the American Academy of Neurology and the American Brain Foundation. Dr. Tolchin reported no other disclosures.
Treating depression after TBI
Evaluating the risk of sexually transmitted infections in mentally ill patients
Sexually transmitted infections (STIs) continue to be a significant public health problem with potentially serious complications.1 The incidence of new STIs, including viral STIs, in the United States is estimated at 19 million cases per year.2Chlamydia trachomatis remains the most common bacterial STI with an estimated annual incidence of 2.8 million cases in the United States and 50 million worldwide. Second in prevalence is gonococcal infection. Herpes simplex virus is one of the most common viral STIs, but the incidence of human papillomavirus virus (HPV), which is associated with cervical cancer, has steadily increased worldwide.3 Young persons age 15 to 24 are at the highest risk of acquiring new STIs with almost 50% of new cases reported among this age group.4
STIs can have serious complications and sequelae. For example, 20% to 40% of women who have chlamydia infections and 10% to 20% of women who have gonococcal infections develop pelvic inflammatory disease (PID),2 which increases the risk for ectopic pregnancy, infertility, and chronic pelvic pain.
Patients with mental illness are at high risk of acquiring STIs. In the United States, the prevalence of HIV among patients with psychiatric illness is 10 to 20 times higher than in the general population.4,5 Factors contributing to increased vulnerability to STIs among psychiatric patients include:
- impaired autonomy
- increased impulsivity
- increased susceptibility to coerced sex.6
Furthermore, a higher incidence of poverty, placement in risky environments, and overall poor health and medical care also contribute to the high prevalence of STIs and their complications in this population (Table 1). Because of risk factors specific to psychiatric illness, standard STI prevention interventions are not always successful and novel and innovative behavioral approaches are necessary.7
Case Abdominal pain and fever
Ms. K, age 25, has a history of bipolar disorder treated with lithium and presents to the community psychiatrist with lower abdominal pain. She recently recovered from a manic episode and has started to reintegrate with the community mental health team. She refuses to see her primary care physician and is adamant that she wishes to see her psychiatrist, who is the only doctor she has rapport with.
Ms. K reports lower abdominal pain for 3 or 4 days and fever for 1 day. The pain is dull in character. She denies diarrhea, vomiting, or urinary symptoms, but on further questioning describes new-onset, foul-smelling vaginal discharge without vaginal bleeding. Her menstrual cycle usually is regular, but her last menstrual period occurred 2 months ago. Her medical history includes an appendectomy at age 10 and she is a current cigarette smoker. Chart notes taken during her manic episode describe high-risk behavior, including having unprotected sexual intercourse with several partners. On examination, she is febrile and tachycardic with a tender lower abdomen.
Diagnosing STIs
To diagnose an STI, first a clinician must consider its likelihood. Taking a thorough sexual history allows assessment of the need for further investigation and provides an opportunity to discuss risk reduction. In accordance with recent guidelines,8 all health care providers are encouraged to consider the sexual history a routine aspect of the clinical encounter. The Centers for Disease Control and Prevention’s (CDC’s) “Five Ps” approach (Table 2) is an excellent tool for guiding investigation and counseling.9
The Figure provides health care providers with an algorithm to guide testing for STIs among psychiatric patients. Note that chlamydia, gonorrhea, syphilis, chancroid, viral hepatitis, and HIV must be reported to state public health agencies and the CDC.
Modern laboratory techniques make diagnosing STIs easier. Analysis of urine or serum reduces the need for invasive sampling. If swabs are required for diagnosis, patient self-collection of urethral, vulvovaginal, rectal, or pharyngeal specimens is as accurate as clinician collected samples and is better tolerated.8 Because of variation in diagnostic assays, we recommend contacting the laboratory before sending non-standard samples to ensure accurate collection and analysis.

Guidelines for preventing and screening for STIs
There are no prevention guidelines for STIs specific to the psychiatric population, although there is a clear need for focused intervention in this vulnerable patient group.10 Rates of STI screening generally are low in the psychiatric setting,11 which results in a considerable burden of disease. All psychiatric patients should be encouraged to engage with STI screening programs that are in line with national guidelines. In the inpatient psychiatric or medical environment, clinicians have a responsibility to ensure that STI screening is considered for each patient.
Patients with mental illness should be assumed to be sexually active, even if they do not volunteer this information to clinicians. Employ a low threshold for recommending safer sex practices including condom use. Encourage women to develop a relationship with a family practitioner, internist, or gynecologist. Advise men who have sex with men (MSM) to visit a doctor regularly for screening of HIV and rectal, anal, and oral STIs as behavior and symptoms dictate.
There is general agreement about STI screening among the United States Preventive Services Task Force (USPSTF), CDC, American Academy of Family Physicians, American Academy of Pediatrics, and American College of Obstetricians and Gynecologists. USPSTF guidelines are summarized in Table 3.12
In addition to these guidelines, the CDC suggests that all adults and adolescents be tested at least once for HIV.13 The CDC also recommends annual testing of MSM for HIV, syphilis, chlamydia, and gonorrhea. In MSM who have multiple partners or who have sex while using illicit drugs, testing should occur more frequently, such as every 3 to 6 months.14
HPV. Routine HPV screening is not recommended; however, 2 vaccines are available to prevent oncogenic HPV (types 16 and 18). All females age 13 to 26 should receive 3 doses of HPV vaccine over a 6-month period. The quadrivalent vaccine (Gardasil) also protects against HPV types 6 and 11, which cause 90% of genital warts and is preferred when available. Males age 9 to 26 also can receive the vaccine, although ideally it should be administered before sexual activity begins.15 Women still should attend routine cervical cancer screening even if they have the vaccine because 30% of cervical cancers are not caused by HPV 16/18. However, this means that 70% of cervical cancers are associated with HPV 16/18, making screening and the vaccine an important public health initiative. There also is a link between HPV and oral cancers.
Treating STIs among mentally ill individuals
Treatment of STIs among mentally ill individuals is important to prevent medical complications and to reduce transmission. Here are a few additional questions to keep in mind when treating a patient with psychiatric illness:
Does the patient have a primary psychiatric disorder, or is the patient’s current psychiatric presentation a result of the infection?
Some STIs can manifest with psychiatric symptoms—for example, neurosyphilis and HIV-associated neurocognitive disorders—and pose a diagnostic challenge. Obtaining a longitudinal history of the patient’s mental health, age of onset, and family history can help clarify the cause.
Are there any psychiatric adverse effects of STI treatment?
Most drugs used for treating common STIs are not known to cause psychiatric adverse effects (See the American Psychiatric Association16 and Sockalingham et al17 for a thorough discussion of HIV and hepatitis C treatment). The exception is fluoroquinolones, which could be prescribed for PID if cephalosporin therapy is not feasible. CNS effects of fluoroquinolones include insomnia, restlessness, confusion, and, in rare cases, mania and psychosis.
What are possible medication interactions to keep in mind when treating a psychiatric patient?
Nonsteroidal anti-inflammatory drugs (NSAIDs), other than sulindac, could increase serum lithium levels. Although NSAIDs are not contraindicated in patients taking lithium, other pain relievers, such as acetaminophen, may be preferred as a first-line choice.
Carbamazepine could lower serum levels of doxycycline.18
Azithromycin and other macrolides, as well as fluoroquinolones, could have QTc prolonging effects and has been associated with torsades de pointes.19 Several psychiatric medications, in particular, atypical antipsychotics, also could prolong the QTc interval. This could be a consideration in patients with underlying long QT intervals at baseline or a family history of sudden cardiac death.
Psychiatric patients might refuse or not adhere to their medication. Refusals could be the result of grandiose delusions (“I don’t need treatment”) or paranoia (“The doctor is trying to poison me”). Consider 1-time doses of antibiotics that can be given in the clinic for uncomplicated infections when adherence is an issue. Because psychiatric patients are at higher risk for acquiring STIs, education and counseling—especially substance abuse counseling—are vital as both primary and secondary prevention strategies. Treatment of STIs should be accompanied by referrals to the social work team or a therapist when appropriate.
Finally, as with any proposed treatment, it is important to consider whether the patient has capacity to consent to or refuse treatment. To assess for capacity, a patient must be able to:
- communicate a choice
- understand the relevant information
- appreciate the medical consequences of the decision
- demonstrate the ability to reason about treatment choices.20
Case continued
In the emergency department, Ms. K’s vital signs are: temperature 39.5°C; pulse 110 beats per minute; blood pressure 96/67 mm Hg; and breathing 20 respirations per minute. She complains of nausea and has 2 episodes of emesis. She allows clinicians to perform a complete physical examination, including pelvic exam. Her cervix is inflamed, and she is noted to have adnexal and cervical motion tenderness.
Labs and imaging confirm a diagnosis of PID due to gonorrhea and she is admitted to the hospital for IV antibiotics. She continues to experience nausea and vomiting, but also complains of dizziness and diarrhea. Her speech is slurred and a coarse tremor is noticed in her hands. Renal function tests show slight impairment, probably due to dehydration. A pregnancy test is negative.
Lithium is held. Her nausea, vomiting, and diarrhea resolve quickly, and Ms. K asks to leave. When she is told that she is not ready for discharge, Ms. K becomes upset and rips out her IV yelling, “I don’t need treatment from you guys!” A psychiatry consult is called to assess for her capacity to refuse treatment. The team determines that she has capacity, but she becomes agreeable to remaining in the hospital after a phone conversation with her community mental health team.
Ms. K improves with antibiotic treatment. HIV and syphilis serology tests are negative. Before discharge, both the community psychiatrist and her primary care physicians are informed her lithium was held during hospitalization and restarted before discharge. Ms. K also is educated about the signs and symptoms of lithium toxicity, as well as common STIs.
Clinical considerations
- Physicians should have a low threshold of suspicion for PID in a sexually active young woman who presents with abdominal pain and shuffling gait, which is a natural attempt to reduce cervical irritation and is associated with PID.
- Ask about sexual history and symptoms of STIs.
- Rule out STIs in men presenting with urinary tract infections.
- If chlamydia is diagnosed, treatment for gonorrhea also is essential, and vice versa.
- Always think about HIV and hepatatis B and C in a patient with a STI.
- Treatment with single-dose medications can be effective.
- Risk of STIs is higher during episodes of mania or psychosis.
- Consider hospitalization if medically indicated or if you suspect non-adherence to therapy. It is important to remember that all kinds of systemic infections—including PID—can result in dehydration and alter renal metabolism leading to lithium accumulation.
- Mentally ill patients might require placement under involuntary commitment if they are found to be a danger to themselves or others. It is important to liaise with both the community psychiatry team and primary care physician both during hospitalization and before discharge to ensure a smooth transition.
1. Fenton KA, Lowndes CM. Recent trends in the epidemiology of sexually transmitted infections in the European Union. Sex Transm Infect. 2004;80(4):255-263.
2. Trigg BG, Kerndt PR, Aynalem G. Sexually transmitted infections and pelvic inflammatory disease in women. Med Clin North Am. 2008;92(5):1083-1113, x.
3. Frenkl TL, Potts J. Sexually transmitted infections. Urol Clin North Am. 2008;35(1):33-46; vi.
4. Weinstock H, Berman S, Cates W Jr. Sexually transmitted diseases among American youth: incidence and prevalence estimates, 2000. Perspect Sex Reprod Health. 2004;36(1):6-10.
5. Rosenberg SD, Goodman LA, Osher FC, et al. Prevalence of HIV, hepatitis B, and hepatitis C in people with severe mental illness. Am J Public Health. 2001;91(1):31-37.
6. King C, Feldman J, Waithaka Y, et al. Sexual risk behaviors and sexually transmitted infection prevalence in an outpatient psychiatry clinic. Sex Transm Dis. 2008;35(10):877-882.
7. Erbelding EJ, Hutton HE, Zenilman JM, et al. The prevalence of psychiatric disorders in sexually transmitted disease clinic patients and their association with sexually transmitted disease risk. Sex Transm Dis. 2004;31(1):8-12.
8. Freeman AH, Bernstein KT, Kohn RP, et al. Evaluation of self-collected versus clinician-collected swabs for the detection of Chlamydia trachomatis and Neisseria gonorrhoeae pharyngeal infection among men who have sex with men. Sex Transm Dis. 2011;38(11):1036-1039.
9. Workowski KA, Berman S; Centers for Disease Control and Prevention (CDC). Sexually transmitted diseases treatment guidelines, 2010. MMWR Recomm Rep. 2010;59(RR-12):1-110.
10. Rein DB, Anderson LA, Irwin KL. Mental health disorders and sexually transmitted diseases in a privately insured population. Am J Manag Care. 2004;10(12):917-924.
11. Rothbard AB, Blank MB, Staab JP, et al. Previously undetected metabolic syndromes and infectious diseases among psychiatric inpatients. Psychiatr Serv. 2009;60(4):534-537.
12. Meyers D, Wolff T, Gregory K, et al. USPSTF recommendations for STI screening. Am Fam Physician. 2008;77(6):819-824.
13. Branson BM, Handsfield HH, Lampe MA, et al; Centers for Disease Control and Prevention (CDC). Revised recommendations for HIV testing of adults, adolescents, and pregnant women in health-care settings. MMWR Recomm Rep. 2006;55(RR-14):1-17; quiz CE1-CE 4.
14. Centers for Disease Control and Prevention. Incidence, prevalence, and cost of sexually transmitted infections in the United States. https://npin.cdc.gov/publication/incidence-prevalence-and-cost-sexually-transmitted-infections-united-states. Published February 2013. Accessed December 12, 2016.
15. Centers for Disease Control and Prevention (CDC). Recommendations on the use of quadrivalent human papillomavirus vaccine in males—Advisory Committee on Immunization Practices (ACIP), 2011. MMWR Morb Mortal Wkly Rep. 2011;60(50):1705-1708.
16. American Psychiatric Association. HIV psychiatry. https://www.psychiatry.org/psychiatrists/practice/professional-interests/hiv-psychiatry. Accessed December 13, 2016.
17. Sockalingam S, Sheehan K, Feld JJ, et al. Psychiatric care during hepatitis C treatment: the changing role of psychiatrists in the era of direct-acting antivirals. Am J Psychiatry. 2015;172(6):512-516.
18. Neuvonen PJ, Pentikäinen PJ, Gothoni G. Inhibition of iron absorption by tetracycline. Br J Clin Pharmacol. 1975;2(1):94-96.
19. Sears SP, Getz TW, Austin CO, et al. Incidence of sustained ventricular tachycardia in patients with prolonged QTc after the administration of azithromycin: a retrospective study. Drugs Real World Outcomes. 2016;3:99-105.
20. Appelbaum PS. Clinical practice. Assessment of patients’ competence to consent to treatment. N Engl J Med. 2007;357(18):1834-1840.
Sexually transmitted infections (STIs) continue to be a significant public health problem with potentially serious complications.1 The incidence of new STIs, including viral STIs, in the United States is estimated at 19 million cases per year.2Chlamydia trachomatis remains the most common bacterial STI with an estimated annual incidence of 2.8 million cases in the United States and 50 million worldwide. Second in prevalence is gonococcal infection. Herpes simplex virus is one of the most common viral STIs, but the incidence of human papillomavirus virus (HPV), which is associated with cervical cancer, has steadily increased worldwide.3 Young persons age 15 to 24 are at the highest risk of acquiring new STIs with almost 50% of new cases reported among this age group.4
STIs can have serious complications and sequelae. For example, 20% to 40% of women who have chlamydia infections and 10% to 20% of women who have gonococcal infections develop pelvic inflammatory disease (PID),2 which increases the risk for ectopic pregnancy, infertility, and chronic pelvic pain.
Patients with mental illness are at high risk of acquiring STIs. In the United States, the prevalence of HIV among patients with psychiatric illness is 10 to 20 times higher than in the general population.4,5 Factors contributing to increased vulnerability to STIs among psychiatric patients include:
- impaired autonomy
- increased impulsivity
- increased susceptibility to coerced sex.6
Furthermore, a higher incidence of poverty, placement in risky environments, and overall poor health and medical care also contribute to the high prevalence of STIs and their complications in this population (Table 1). Because of risk factors specific to psychiatric illness, standard STI prevention interventions are not always successful and novel and innovative behavioral approaches are necessary.7
Case Abdominal pain and fever
Ms. K, age 25, has a history of bipolar disorder treated with lithium and presents to the community psychiatrist with lower abdominal pain. She recently recovered from a manic episode and has started to reintegrate with the community mental health team. She refuses to see her primary care physician and is adamant that she wishes to see her psychiatrist, who is the only doctor she has rapport with.
Ms. K reports lower abdominal pain for 3 or 4 days and fever for 1 day. The pain is dull in character. She denies diarrhea, vomiting, or urinary symptoms, but on further questioning describes new-onset, foul-smelling vaginal discharge without vaginal bleeding. Her menstrual cycle usually is regular, but her last menstrual period occurred 2 months ago. Her medical history includes an appendectomy at age 10 and she is a current cigarette smoker. Chart notes taken during her manic episode describe high-risk behavior, including having unprotected sexual intercourse with several partners. On examination, she is febrile and tachycardic with a tender lower abdomen.
Diagnosing STIs
To diagnose an STI, first a clinician must consider its likelihood. Taking a thorough sexual history allows assessment of the need for further investigation and provides an opportunity to discuss risk reduction. In accordance with recent guidelines,8 all health care providers are encouraged to consider the sexual history a routine aspect of the clinical encounter. The Centers for Disease Control and Prevention’s (CDC’s) “Five Ps” approach (Table 2) is an excellent tool for guiding investigation and counseling.9
The Figure provides health care providers with an algorithm to guide testing for STIs among psychiatric patients. Note that chlamydia, gonorrhea, syphilis, chancroid, viral hepatitis, and HIV must be reported to state public health agencies and the CDC.
Modern laboratory techniques make diagnosing STIs easier. Analysis of urine or serum reduces the need for invasive sampling. If swabs are required for diagnosis, patient self-collection of urethral, vulvovaginal, rectal, or pharyngeal specimens is as accurate as clinician collected samples and is better tolerated.8 Because of variation in diagnostic assays, we recommend contacting the laboratory before sending non-standard samples to ensure accurate collection and analysis.

Guidelines for preventing and screening for STIs
There are no prevention guidelines for STIs specific to the psychiatric population, although there is a clear need for focused intervention in this vulnerable patient group.10 Rates of STI screening generally are low in the psychiatric setting,11 which results in a considerable burden of disease. All psychiatric patients should be encouraged to engage with STI screening programs that are in line with national guidelines. In the inpatient psychiatric or medical environment, clinicians have a responsibility to ensure that STI screening is considered for each patient.
Patients with mental illness should be assumed to be sexually active, even if they do not volunteer this information to clinicians. Employ a low threshold for recommending safer sex practices including condom use. Encourage women to develop a relationship with a family practitioner, internist, or gynecologist. Advise men who have sex with men (MSM) to visit a doctor regularly for screening of HIV and rectal, anal, and oral STIs as behavior and symptoms dictate.
There is general agreement about STI screening among the United States Preventive Services Task Force (USPSTF), CDC, American Academy of Family Physicians, American Academy of Pediatrics, and American College of Obstetricians and Gynecologists. USPSTF guidelines are summarized in Table 3.12
In addition to these guidelines, the CDC suggests that all adults and adolescents be tested at least once for HIV.13 The CDC also recommends annual testing of MSM for HIV, syphilis, chlamydia, and gonorrhea. In MSM who have multiple partners or who have sex while using illicit drugs, testing should occur more frequently, such as every 3 to 6 months.14
HPV. Routine HPV screening is not recommended; however, 2 vaccines are available to prevent oncogenic HPV (types 16 and 18). All females age 13 to 26 should receive 3 doses of HPV vaccine over a 6-month period. The quadrivalent vaccine (Gardasil) also protects against HPV types 6 and 11, which cause 90% of genital warts and is preferred when available. Males age 9 to 26 also can receive the vaccine, although ideally it should be administered before sexual activity begins.15 Women still should attend routine cervical cancer screening even if they have the vaccine because 30% of cervical cancers are not caused by HPV 16/18. However, this means that 70% of cervical cancers are associated with HPV 16/18, making screening and the vaccine an important public health initiative. There also is a link between HPV and oral cancers.
Treating STIs among mentally ill individuals
Treatment of STIs among mentally ill individuals is important to prevent medical complications and to reduce transmission. Here are a few additional questions to keep in mind when treating a patient with psychiatric illness:
Does the patient have a primary psychiatric disorder, or is the patient’s current psychiatric presentation a result of the infection?
Some STIs can manifest with psychiatric symptoms—for example, neurosyphilis and HIV-associated neurocognitive disorders—and pose a diagnostic challenge. Obtaining a longitudinal history of the patient’s mental health, age of onset, and family history can help clarify the cause.
Are there any psychiatric adverse effects of STI treatment?
Most drugs used for treating common STIs are not known to cause psychiatric adverse effects (See the American Psychiatric Association16 and Sockalingham et al17 for a thorough discussion of HIV and hepatitis C treatment). The exception is fluoroquinolones, which could be prescribed for PID if cephalosporin therapy is not feasible. CNS effects of fluoroquinolones include insomnia, restlessness, confusion, and, in rare cases, mania and psychosis.
What are possible medication interactions to keep in mind when treating a psychiatric patient?
Nonsteroidal anti-inflammatory drugs (NSAIDs), other than sulindac, could increase serum lithium levels. Although NSAIDs are not contraindicated in patients taking lithium, other pain relievers, such as acetaminophen, may be preferred as a first-line choice.
Carbamazepine could lower serum levels of doxycycline.18
Azithromycin and other macrolides, as well as fluoroquinolones, could have QTc prolonging effects and has been associated with torsades de pointes.19 Several psychiatric medications, in particular, atypical antipsychotics, also could prolong the QTc interval. This could be a consideration in patients with underlying long QT intervals at baseline or a family history of sudden cardiac death.
Psychiatric patients might refuse or not adhere to their medication. Refusals could be the result of grandiose delusions (“I don’t need treatment”) or paranoia (“The doctor is trying to poison me”). Consider 1-time doses of antibiotics that can be given in the clinic for uncomplicated infections when adherence is an issue. Because psychiatric patients are at higher risk for acquiring STIs, education and counseling—especially substance abuse counseling—are vital as both primary and secondary prevention strategies. Treatment of STIs should be accompanied by referrals to the social work team or a therapist when appropriate.
Finally, as with any proposed treatment, it is important to consider whether the patient has capacity to consent to or refuse treatment. To assess for capacity, a patient must be able to:
- communicate a choice
- understand the relevant information
- appreciate the medical consequences of the decision
- demonstrate the ability to reason about treatment choices.20
Case continued
In the emergency department, Ms. K’s vital signs are: temperature 39.5°C; pulse 110 beats per minute; blood pressure 96/67 mm Hg; and breathing 20 respirations per minute. She complains of nausea and has 2 episodes of emesis. She allows clinicians to perform a complete physical examination, including pelvic exam. Her cervix is inflamed, and she is noted to have adnexal and cervical motion tenderness.
Labs and imaging confirm a diagnosis of PID due to gonorrhea and she is admitted to the hospital for IV antibiotics. She continues to experience nausea and vomiting, but also complains of dizziness and diarrhea. Her speech is slurred and a coarse tremor is noticed in her hands. Renal function tests show slight impairment, probably due to dehydration. A pregnancy test is negative.
Lithium is held. Her nausea, vomiting, and diarrhea resolve quickly, and Ms. K asks to leave. When she is told that she is not ready for discharge, Ms. K becomes upset and rips out her IV yelling, “I don’t need treatment from you guys!” A psychiatry consult is called to assess for her capacity to refuse treatment. The team determines that she has capacity, but she becomes agreeable to remaining in the hospital after a phone conversation with her community mental health team.
Ms. K improves with antibiotic treatment. HIV and syphilis serology tests are negative. Before discharge, both the community psychiatrist and her primary care physicians are informed her lithium was held during hospitalization and restarted before discharge. Ms. K also is educated about the signs and symptoms of lithium toxicity, as well as common STIs.
Clinical considerations
- Physicians should have a low threshold of suspicion for PID in a sexually active young woman who presents with abdominal pain and shuffling gait, which is a natural attempt to reduce cervical irritation and is associated with PID.
- Ask about sexual history and symptoms of STIs.
- Rule out STIs in men presenting with urinary tract infections.
- If chlamydia is diagnosed, treatment for gonorrhea also is essential, and vice versa.
- Always think about HIV and hepatatis B and C in a patient with a STI.
- Treatment with single-dose medications can be effective.
- Risk of STIs is higher during episodes of mania or psychosis.
- Consider hospitalization if medically indicated or if you suspect non-adherence to therapy. It is important to remember that all kinds of systemic infections—including PID—can result in dehydration and alter renal metabolism leading to lithium accumulation.
- Mentally ill patients might require placement under involuntary commitment if they are found to be a danger to themselves or others. It is important to liaise with both the community psychiatry team and primary care physician both during hospitalization and before discharge to ensure a smooth transition.
Sexually transmitted infections (STIs) continue to be a significant public health problem with potentially serious complications.1 The incidence of new STIs, including viral STIs, in the United States is estimated at 19 million cases per year.2Chlamydia trachomatis remains the most common bacterial STI with an estimated annual incidence of 2.8 million cases in the United States and 50 million worldwide. Second in prevalence is gonococcal infection. Herpes simplex virus is one of the most common viral STIs, but the incidence of human papillomavirus virus (HPV), which is associated with cervical cancer, has steadily increased worldwide.3 Young persons age 15 to 24 are at the highest risk of acquiring new STIs with almost 50% of new cases reported among this age group.4
STIs can have serious complications and sequelae. For example, 20% to 40% of women who have chlamydia infections and 10% to 20% of women who have gonococcal infections develop pelvic inflammatory disease (PID),2 which increases the risk for ectopic pregnancy, infertility, and chronic pelvic pain.
Patients with mental illness are at high risk of acquiring STIs. In the United States, the prevalence of HIV among patients with psychiatric illness is 10 to 20 times higher than in the general population.4,5 Factors contributing to increased vulnerability to STIs among psychiatric patients include:
- impaired autonomy
- increased impulsivity
- increased susceptibility to coerced sex.6
Furthermore, a higher incidence of poverty, placement in risky environments, and overall poor health and medical care also contribute to the high prevalence of STIs and their complications in this population (Table 1). Because of risk factors specific to psychiatric illness, standard STI prevention interventions are not always successful and novel and innovative behavioral approaches are necessary.7
Case Abdominal pain and fever
Ms. K, age 25, has a history of bipolar disorder treated with lithium and presents to the community psychiatrist with lower abdominal pain. She recently recovered from a manic episode and has started to reintegrate with the community mental health team. She refuses to see her primary care physician and is adamant that she wishes to see her psychiatrist, who is the only doctor she has rapport with.
Ms. K reports lower abdominal pain for 3 or 4 days and fever for 1 day. The pain is dull in character. She denies diarrhea, vomiting, or urinary symptoms, but on further questioning describes new-onset, foul-smelling vaginal discharge without vaginal bleeding. Her menstrual cycle usually is regular, but her last menstrual period occurred 2 months ago. Her medical history includes an appendectomy at age 10 and she is a current cigarette smoker. Chart notes taken during her manic episode describe high-risk behavior, including having unprotected sexual intercourse with several partners. On examination, she is febrile and tachycardic with a tender lower abdomen.
Diagnosing STIs
To diagnose an STI, first a clinician must consider its likelihood. Taking a thorough sexual history allows assessment of the need for further investigation and provides an opportunity to discuss risk reduction. In accordance with recent guidelines,8 all health care providers are encouraged to consider the sexual history a routine aspect of the clinical encounter. The Centers for Disease Control and Prevention’s (CDC’s) “Five Ps” approach (Table 2) is an excellent tool for guiding investigation and counseling.9
The Figure provides health care providers with an algorithm to guide testing for STIs among psychiatric patients. Note that chlamydia, gonorrhea, syphilis, chancroid, viral hepatitis, and HIV must be reported to state public health agencies and the CDC.
Modern laboratory techniques make diagnosing STIs easier. Analysis of urine or serum reduces the need for invasive sampling. If swabs are required for diagnosis, patient self-collection of urethral, vulvovaginal, rectal, or pharyngeal specimens is as accurate as clinician collected samples and is better tolerated.8 Because of variation in diagnostic assays, we recommend contacting the laboratory before sending non-standard samples to ensure accurate collection and analysis.

Guidelines for preventing and screening for STIs
There are no prevention guidelines for STIs specific to the psychiatric population, although there is a clear need for focused intervention in this vulnerable patient group.10 Rates of STI screening generally are low in the psychiatric setting,11 which results in a considerable burden of disease. All psychiatric patients should be encouraged to engage with STI screening programs that are in line with national guidelines. In the inpatient psychiatric or medical environment, clinicians have a responsibility to ensure that STI screening is considered for each patient.
Patients with mental illness should be assumed to be sexually active, even if they do not volunteer this information to clinicians. Employ a low threshold for recommending safer sex practices including condom use. Encourage women to develop a relationship with a family practitioner, internist, or gynecologist. Advise men who have sex with men (MSM) to visit a doctor regularly for screening of HIV and rectal, anal, and oral STIs as behavior and symptoms dictate.
There is general agreement about STI screening among the United States Preventive Services Task Force (USPSTF), CDC, American Academy of Family Physicians, American Academy of Pediatrics, and American College of Obstetricians and Gynecologists. USPSTF guidelines are summarized in Table 3.12
In addition to these guidelines, the CDC suggests that all adults and adolescents be tested at least once for HIV.13 The CDC also recommends annual testing of MSM for HIV, syphilis, chlamydia, and gonorrhea. In MSM who have multiple partners or who have sex while using illicit drugs, testing should occur more frequently, such as every 3 to 6 months.14
HPV. Routine HPV screening is not recommended; however, 2 vaccines are available to prevent oncogenic HPV (types 16 and 18). All females age 13 to 26 should receive 3 doses of HPV vaccine over a 6-month period. The quadrivalent vaccine (Gardasil) also protects against HPV types 6 and 11, which cause 90% of genital warts and is preferred when available. Males age 9 to 26 also can receive the vaccine, although ideally it should be administered before sexual activity begins.15 Women still should attend routine cervical cancer screening even if they have the vaccine because 30% of cervical cancers are not caused by HPV 16/18. However, this means that 70% of cervical cancers are associated with HPV 16/18, making screening and the vaccine an important public health initiative. There also is a link between HPV and oral cancers.
Treating STIs among mentally ill individuals
Treatment of STIs among mentally ill individuals is important to prevent medical complications and to reduce transmission. Here are a few additional questions to keep in mind when treating a patient with psychiatric illness:
Does the patient have a primary psychiatric disorder, or is the patient’s current psychiatric presentation a result of the infection?
Some STIs can manifest with psychiatric symptoms—for example, neurosyphilis and HIV-associated neurocognitive disorders—and pose a diagnostic challenge. Obtaining a longitudinal history of the patient’s mental health, age of onset, and family history can help clarify the cause.
Are there any psychiatric adverse effects of STI treatment?
Most drugs used for treating common STIs are not known to cause psychiatric adverse effects (See the American Psychiatric Association16 and Sockalingham et al17 for a thorough discussion of HIV and hepatitis C treatment). The exception is fluoroquinolones, which could be prescribed for PID if cephalosporin therapy is not feasible. CNS effects of fluoroquinolones include insomnia, restlessness, confusion, and, in rare cases, mania and psychosis.
What are possible medication interactions to keep in mind when treating a psychiatric patient?
Nonsteroidal anti-inflammatory drugs (NSAIDs), other than sulindac, could increase serum lithium levels. Although NSAIDs are not contraindicated in patients taking lithium, other pain relievers, such as acetaminophen, may be preferred as a first-line choice.
Carbamazepine could lower serum levels of doxycycline.18
Azithromycin and other macrolides, as well as fluoroquinolones, could have QTc prolonging effects and has been associated with torsades de pointes.19 Several psychiatric medications, in particular, atypical antipsychotics, also could prolong the QTc interval. This could be a consideration in patients with underlying long QT intervals at baseline or a family history of sudden cardiac death.
Psychiatric patients might refuse or not adhere to their medication. Refusals could be the result of grandiose delusions (“I don’t need treatment”) or paranoia (“The doctor is trying to poison me”). Consider 1-time doses of antibiotics that can be given in the clinic for uncomplicated infections when adherence is an issue. Because psychiatric patients are at higher risk for acquiring STIs, education and counseling—especially substance abuse counseling—are vital as both primary and secondary prevention strategies. Treatment of STIs should be accompanied by referrals to the social work team or a therapist when appropriate.
Finally, as with any proposed treatment, it is important to consider whether the patient has capacity to consent to or refuse treatment. To assess for capacity, a patient must be able to:
- communicate a choice
- understand the relevant information
- appreciate the medical consequences of the decision
- demonstrate the ability to reason about treatment choices.20
Case continued
In the emergency department, Ms. K’s vital signs are: temperature 39.5°C; pulse 110 beats per minute; blood pressure 96/67 mm Hg; and breathing 20 respirations per minute. She complains of nausea and has 2 episodes of emesis. She allows clinicians to perform a complete physical examination, including pelvic exam. Her cervix is inflamed, and she is noted to have adnexal and cervical motion tenderness.
Labs and imaging confirm a diagnosis of PID due to gonorrhea and she is admitted to the hospital for IV antibiotics. She continues to experience nausea and vomiting, but also complains of dizziness and diarrhea. Her speech is slurred and a coarse tremor is noticed in her hands. Renal function tests show slight impairment, probably due to dehydration. A pregnancy test is negative.
Lithium is held. Her nausea, vomiting, and diarrhea resolve quickly, and Ms. K asks to leave. When she is told that she is not ready for discharge, Ms. K becomes upset and rips out her IV yelling, “I don’t need treatment from you guys!” A psychiatry consult is called to assess for her capacity to refuse treatment. The team determines that she has capacity, but she becomes agreeable to remaining in the hospital after a phone conversation with her community mental health team.
Ms. K improves with antibiotic treatment. HIV and syphilis serology tests are negative. Before discharge, both the community psychiatrist and her primary care physicians are informed her lithium was held during hospitalization and restarted before discharge. Ms. K also is educated about the signs and symptoms of lithium toxicity, as well as common STIs.
Clinical considerations
- Physicians should have a low threshold of suspicion for PID in a sexually active young woman who presents with abdominal pain and shuffling gait, which is a natural attempt to reduce cervical irritation and is associated with PID.
- Ask about sexual history and symptoms of STIs.
- Rule out STIs in men presenting with urinary tract infections.
- If chlamydia is diagnosed, treatment for gonorrhea also is essential, and vice versa.
- Always think about HIV and hepatatis B and C in a patient with a STI.
- Treatment with single-dose medications can be effective.
- Risk of STIs is higher during episodes of mania or psychosis.
- Consider hospitalization if medically indicated or if you suspect non-adherence to therapy. It is important to remember that all kinds of systemic infections—including PID—can result in dehydration and alter renal metabolism leading to lithium accumulation.
- Mentally ill patients might require placement under involuntary commitment if they are found to be a danger to themselves or others. It is important to liaise with both the community psychiatry team and primary care physician both during hospitalization and before discharge to ensure a smooth transition.
1. Fenton KA, Lowndes CM. Recent trends in the epidemiology of sexually transmitted infections in the European Union. Sex Transm Infect. 2004;80(4):255-263.
2. Trigg BG, Kerndt PR, Aynalem G. Sexually transmitted infections and pelvic inflammatory disease in women. Med Clin North Am. 2008;92(5):1083-1113, x.
3. Frenkl TL, Potts J. Sexually transmitted infections. Urol Clin North Am. 2008;35(1):33-46; vi.
4. Weinstock H, Berman S, Cates W Jr. Sexually transmitted diseases among American youth: incidence and prevalence estimates, 2000. Perspect Sex Reprod Health. 2004;36(1):6-10.
5. Rosenberg SD, Goodman LA, Osher FC, et al. Prevalence of HIV, hepatitis B, and hepatitis C in people with severe mental illness. Am J Public Health. 2001;91(1):31-37.
6. King C, Feldman J, Waithaka Y, et al. Sexual risk behaviors and sexually transmitted infection prevalence in an outpatient psychiatry clinic. Sex Transm Dis. 2008;35(10):877-882.
7. Erbelding EJ, Hutton HE, Zenilman JM, et al. The prevalence of psychiatric disorders in sexually transmitted disease clinic patients and their association with sexually transmitted disease risk. Sex Transm Dis. 2004;31(1):8-12.
8. Freeman AH, Bernstein KT, Kohn RP, et al. Evaluation of self-collected versus clinician-collected swabs for the detection of Chlamydia trachomatis and Neisseria gonorrhoeae pharyngeal infection among men who have sex with men. Sex Transm Dis. 2011;38(11):1036-1039.
9. Workowski KA, Berman S; Centers for Disease Control and Prevention (CDC). Sexually transmitted diseases treatment guidelines, 2010. MMWR Recomm Rep. 2010;59(RR-12):1-110.
10. Rein DB, Anderson LA, Irwin KL. Mental health disorders and sexually transmitted diseases in a privately insured population. Am J Manag Care. 2004;10(12):917-924.
11. Rothbard AB, Blank MB, Staab JP, et al. Previously undetected metabolic syndromes and infectious diseases among psychiatric inpatients. Psychiatr Serv. 2009;60(4):534-537.
12. Meyers D, Wolff T, Gregory K, et al. USPSTF recommendations for STI screening. Am Fam Physician. 2008;77(6):819-824.
13. Branson BM, Handsfield HH, Lampe MA, et al; Centers for Disease Control and Prevention (CDC). Revised recommendations for HIV testing of adults, adolescents, and pregnant women in health-care settings. MMWR Recomm Rep. 2006;55(RR-14):1-17; quiz CE1-CE 4.
14. Centers for Disease Control and Prevention. Incidence, prevalence, and cost of sexually transmitted infections in the United States. https://npin.cdc.gov/publication/incidence-prevalence-and-cost-sexually-transmitted-infections-united-states. Published February 2013. Accessed December 12, 2016.
15. Centers for Disease Control and Prevention (CDC). Recommendations on the use of quadrivalent human papillomavirus vaccine in males—Advisory Committee on Immunization Practices (ACIP), 2011. MMWR Morb Mortal Wkly Rep. 2011;60(50):1705-1708.
16. American Psychiatric Association. HIV psychiatry. https://www.psychiatry.org/psychiatrists/practice/professional-interests/hiv-psychiatry. Accessed December 13, 2016.
17. Sockalingam S, Sheehan K, Feld JJ, et al. Psychiatric care during hepatitis C treatment: the changing role of psychiatrists in the era of direct-acting antivirals. Am J Psychiatry. 2015;172(6):512-516.
18. Neuvonen PJ, Pentikäinen PJ, Gothoni G. Inhibition of iron absorption by tetracycline. Br J Clin Pharmacol. 1975;2(1):94-96.
19. Sears SP, Getz TW, Austin CO, et al. Incidence of sustained ventricular tachycardia in patients with prolonged QTc after the administration of azithromycin: a retrospective study. Drugs Real World Outcomes. 2016;3:99-105.
20. Appelbaum PS. Clinical practice. Assessment of patients’ competence to consent to treatment. N Engl J Med. 2007;357(18):1834-1840.
1. Fenton KA, Lowndes CM. Recent trends in the epidemiology of sexually transmitted infections in the European Union. Sex Transm Infect. 2004;80(4):255-263.
2. Trigg BG, Kerndt PR, Aynalem G. Sexually transmitted infections and pelvic inflammatory disease in women. Med Clin North Am. 2008;92(5):1083-1113, x.
3. Frenkl TL, Potts J. Sexually transmitted infections. Urol Clin North Am. 2008;35(1):33-46; vi.
4. Weinstock H, Berman S, Cates W Jr. Sexually transmitted diseases among American youth: incidence and prevalence estimates, 2000. Perspect Sex Reprod Health. 2004;36(1):6-10.
5. Rosenberg SD, Goodman LA, Osher FC, et al. Prevalence of HIV, hepatitis B, and hepatitis C in people with severe mental illness. Am J Public Health. 2001;91(1):31-37.
6. King C, Feldman J, Waithaka Y, et al. Sexual risk behaviors and sexually transmitted infection prevalence in an outpatient psychiatry clinic. Sex Transm Dis. 2008;35(10):877-882.
7. Erbelding EJ, Hutton HE, Zenilman JM, et al. The prevalence of psychiatric disorders in sexually transmitted disease clinic patients and their association with sexually transmitted disease risk. Sex Transm Dis. 2004;31(1):8-12.
8. Freeman AH, Bernstein KT, Kohn RP, et al. Evaluation of self-collected versus clinician-collected swabs for the detection of Chlamydia trachomatis and Neisseria gonorrhoeae pharyngeal infection among men who have sex with men. Sex Transm Dis. 2011;38(11):1036-1039.
9. Workowski KA, Berman S; Centers for Disease Control and Prevention (CDC). Sexually transmitted diseases treatment guidelines, 2010. MMWR Recomm Rep. 2010;59(RR-12):1-110.
10. Rein DB, Anderson LA, Irwin KL. Mental health disorders and sexually transmitted diseases in a privately insured population. Am J Manag Care. 2004;10(12):917-924.
11. Rothbard AB, Blank MB, Staab JP, et al. Previously undetected metabolic syndromes and infectious diseases among psychiatric inpatients. Psychiatr Serv. 2009;60(4):534-537.
12. Meyers D, Wolff T, Gregory K, et al. USPSTF recommendations for STI screening. Am Fam Physician. 2008;77(6):819-824.
13. Branson BM, Handsfield HH, Lampe MA, et al; Centers for Disease Control and Prevention (CDC). Revised recommendations for HIV testing of adults, adolescents, and pregnant women in health-care settings. MMWR Recomm Rep. 2006;55(RR-14):1-17; quiz CE1-CE 4.
14. Centers for Disease Control and Prevention. Incidence, prevalence, and cost of sexually transmitted infections in the United States. https://npin.cdc.gov/publication/incidence-prevalence-and-cost-sexually-transmitted-infections-united-states. Published February 2013. Accessed December 12, 2016.
15. Centers for Disease Control and Prevention (CDC). Recommendations on the use of quadrivalent human papillomavirus vaccine in males—Advisory Committee on Immunization Practices (ACIP), 2011. MMWR Morb Mortal Wkly Rep. 2011;60(50):1705-1708.
16. American Psychiatric Association. HIV psychiatry. https://www.psychiatry.org/psychiatrists/practice/professional-interests/hiv-psychiatry. Accessed December 13, 2016.
17. Sockalingam S, Sheehan K, Feld JJ, et al. Psychiatric care during hepatitis C treatment: the changing role of psychiatrists in the era of direct-acting antivirals. Am J Psychiatry. 2015;172(6):512-516.
18. Neuvonen PJ, Pentikäinen PJ, Gothoni G. Inhibition of iron absorption by tetracycline. Br J Clin Pharmacol. 1975;2(1):94-96.
19. Sears SP, Getz TW, Austin CO, et al. Incidence of sustained ventricular tachycardia in patients with prolonged QTc after the administration of azithromycin: a retrospective study. Drugs Real World Outcomes. 2016;3:99-105.
20. Appelbaum PS. Clinical practice. Assessment of patients’ competence to consent to treatment. N Engl J Med. 2007;357(18):1834-1840.