User login
Interval Throwing and Hitting Programs in Baseball: Biomechanics and Rehabilitation
Throwing and batting each require repetitive motions that can result in injuries unique to baseball. Fortuantely, advances in operative and nonoperative treatments have allowed players to return to competition after sustaining what previously would have been considered a career-ending injury. Once a player has been deemed ready to return to throwing or hitting, a comprehensive, multiphased approach to rehabilitation is necessary to reintroduce the athlete back to baseball activities and avoid re-injury. This article reviews the biomechanics of both throwing and hitting, and outlines the phases of rehabilitation necessary to allow the athlete to return to competition.
Throwing
Biomechanical Overview
The overhead throwing motion is complex and involves full body coordination from the initial force generation through the follow-through phase of throwing. The “kinetic chain”—the concept that movements in the body are connected through segments culminating with the highest energy in the final segment—is paramount to achieving the force and energy needed for throwing.1-8 The kinetic chain begins in the lower body and trunk and transmits the energy distally to the shoulder, elbow, and hand, ending with kinetic energy transfer to the ball.3-5,7 The progression of motion through the kinetic chain during throwing includes stride, pelvis rotation, upper torso rotation, elbow extension, shoulder internal rotation, and wrist flexion. Disruptions in this chain due to muscle imbalance or weakness can lead to injury downstream, particularly in the upper extremity.3,7,9
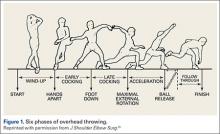
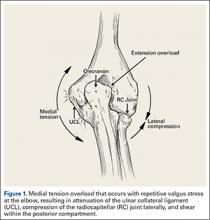
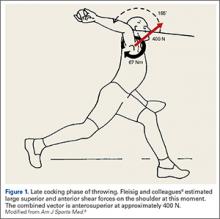
The importance of the kinetic chain can be highlighted in the 6 phases of throwing motion. These include wind-up, early arm cocking, late arm cocking, arm acceleration, arm deceleration, and follow-through (Figure 1).1,2,9,10
The wind-up phase starts with initiation of motion and ends with maximal knee lift of the lead leg; its objective is to place the body in an optimal stance to throw.3-5,7 There are minimal forces, torques, and muscle activity in the upper extremity during this phase, but up to 50% of throw speed is created through stride and trunk rotation.6 During the early cocking phase, the thrower keeps his stance foot planted and drives his lead leg towards the target, while bringing both arms into abduction. This is coupled with internal rotation of the stance hip, external rotation of the lead hip, and external rotation of the throwing shoulder. This creates linear velocity by maximizing the length of the elastic components of the body. Elbow, wrist, and finger extensors are also contracting during this phase to control elbow flexion and wrist hyperextension.3
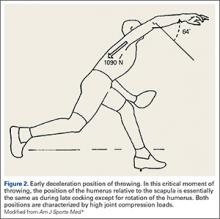
The late cocking phase begins when the lead foot contacts the ground and ends with maximum shoulder external rotation.3-5 Lead foot contact is followed by quadriceps contraction to decelerate and stabilize the lead leg. This is followed by rotation of the pelvis and upper torso. The result is energy transfer to the throwing arm with a shear force across the anterior shoulder of 400 N.4 The shoulder stays in 90° of abduction, 15° of horizontal adduction, and externally rotates to between 150° and 180°. This produces a maximum horizontal adduction moment of 100 N.m and internal rotation torque of 70 N.m.4 Simultaneously, the elbow generates maximum flexion and a 65 N.m varus torque.7 Forces about the elbow are generated to resist the large angular velocity experienced (up to 3000°/second). This places an extreme amount of valgus stress along the medial elbow, particularly on the ulnar collateral ligament. The shoulder girdle and rotator cuff muscles simultaneously act to stabilize the scapula and glenohumeral joint.
The arm acceleration phase is from maximal shoulder external rotation until ball release.3-5 In this phase, the thrower flexes his trunk from an extended position, returning to neutral by the time of ball release while the lead leg straightens. The shoulder stays abducted at 90° throughout while the rotator cuff internal rotators and scapular stabilizers contract to explosively internally rotate the shoulder, creating a maximal internal rotation velocity greater than 7000°/second by ball release.1,4,7 The elbow also begins to extend, reaching maximum velocity during mid-acceleration phase from a combination of triceps contraction and torque generated from rotation at the shoulder and upper trunk.3 Finally, the wrist flexors contract to move the wrist to a neutral position from hyperextension as the ball is released.
During arm deceleration, the shoulder achieves maximum internal rotation until reaching a neutral position and horizontally adducts across the body. This is controlled by contraction of the shoulder girdle musculature; the teres minor has the highest activity.3,4 The greatest forces produced during the throwing motion act at the shoulder and elbow during deceleration and can contribute to injury.2 These include compressive forces of greater than 1000 N, posterior shear forces of 400 N, and inferior shear forces of 300 N.4,7
The final phase, the follow-through phase, starts at shoulder maximum internal rotation and ends when the arm assumes a balanced position across the trunk. Lower extremity extension and trunk flexion help distribute forces throughout the body, taking stress away from the throwing arm. The posterior shoulder musculature and scapular protractors contribute to continued deceleration and muscle firing returns to resting levels. This complex motion of throwing fueled by the kinetic chain lasts less than 2 seconds and can result in ball release speeds as high as 100 miles per hour.3,4
Return to Throwing: Principles
Nonoperative and postoperative rehabilitation programs allow restoration of motion, strength, static and dynamic stability, and neuromuscular control. The initiation of an interval throwing program (ITP) is based on the assumption that tissue healing is complete and a complete physical examination has been conducted to the treating physician’s approval.11 An ITP progressively applies forces along the kinetic chain in a controlled manner through graduated throwing distances, while minimizing the risk of re-injury.
Reinold and colleagues12 described guidelines that were used in the development of the ITP.12 These factors include: (1) The act of throwing a baseball involves the transfer of energy from the feet up to the hand and therefore careful attention must be paid along the entire kinetic chain; (2) gradual progression of interval throwing decreases the chance for re-injury; (3) proper warm-up; and (4) proper throwing mechanics minimizes the chance of re-injury.
Variability. Unlike traditional rehabilitation programs that advance an athlete based on a specific timetable, the ITP requires that each level or phase to be completed pain-free or without complications prior to starting the next level. Therefore, an ITP can be used for overhead athletes of varying skill levels because progression will be different from one athlete to another. It is also important to have the athlete adhere strictly to the program, as over-eagerness to complete the ITP as quickly as possible can increase the chance of re-injury and thus slow the rehabilitation process.12
Warm-up. An adequate warm-up is recommended prior to initiating ITP. An athlete should jog or cycle to develop a light sweat and then progress to stretching and flexibility exercises. As emphasized before, throwing involves nearly all the muscles in the body. Therefore, all muscle groups should be stretched beginning with the legs and working distally along the kinetic chain.
Mechanics. Analysis, correction, and maintenance of proper throwing mechanics is essential throughout the early phases of rehabilitation and ITP. Improper pitching mechanics places increased stress on the throwing arm, potentially leading to re-injury. Therefore, it would be valuable to have a pitching coach available to emphasize proper mechanics throughout the rehabilitation process.
The Interval Throwing Program
For a PDF patient handout that summarizes the phases of this program, see Appendix 1.
Phase 1. We have adopted the ITP as described by Reinold and colleagues.12 Phase begins with the overhead athlete throwing on flat ground. He or she begins tossing from 45 feet and gradually progresses to 60, 90, 120, 150, and 180 feet.
As discussed earlier, it is critical to use proper mechanics throughout the ITP. The “crow hop” method simulates a throwing act and helps maintain proper pitching mechanics. Crow hop has 3 components: hop, skip, and throw. Using this technique, the pitcher begins warm-up throws at a comfortable distance (generally 30 feet) and then progresses to the distance as indicated on the ITP. The athlete will then need to perform each step 2 times, with 1 day of rest between steps, before advancing to the next step. The ball should be thrown with an arc and have only enough momentum to reach the desired distance.
For example, Step 1 calls for the athlete to perform 2 sets of 25 throws at 45 feet, with adequate rest (5 minutes) between sets. This step will be repeated following 1 day of rest. If the athlete demonstrates the ability to throw at the prescribed distance without pain, he or she can progress to Step 2, which calls for 3 sets of 25 throws at 45 feet. If pain is present at any step, the thrower returns to the previous asymptomatic step and can progress once he is pain-free.
Positional players are instructed to complete Phase 1 prior to starting position-specific drills. Pitchers, on the other hand, are instructed to stop once they reach and complete 120 feet. They will then progress to tossing at progressive distances of 60, 90, and 120 feet, followed by throwing at 60 feet 6 inches with normal pitching mechanics, initiating straight line throws with little to no arc.
Phase II (Throwing off the Mound). Once a pitcher completes Phase 1 without pain or complications, he is ready to begin throwing off the mound. The same principle remains in Phase 2: pitchers must complete each step pain-free before advancing to the next stage. Pitchers should first throw fastballs at 50% effort and progress to 75% and 100% effort. Because athletes often find it difficult to gauge their own effort, it is important to emphasize the importance of strictly adhering to the program. Fleisig and colleagues13 studied healthy pitchers’ ability to estimate their throwing effort. When targeting 50% effort, athletes generated ball speeds of 85% with forces and torque approaching 75% of maximum. A radar gun may be valuable in guiding effort control.
As the player advances through Phase 2, he will increase the volume of pitches as well as the effort in a gradual manner. The player may introduce breaking ball pitches once he demonstrates the ability to throw light batting practice. Phase 2 concludes with the pitcher throwing simulated games, progressing by 15 throws per workout.
Hitting
Biomechanics Overview
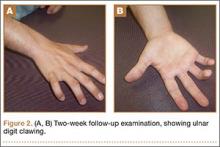
The mechanics of hitting a baseball can be broken down into 6 phases: the preparatory phase, stance phase, stride phase, drive phase, bat acceleration phase, and follow-through phase.14 While progressing through a return-to-play protocol, it is important to understand and teach the player proper swing mechanics during each phase in order to minimize the risk of re-injury (Figure 2).
The preparatory phase occurs as the player positions himself into the batter’s box. This phase is highly individualized, depending on each player’s personal preference. Though significant variability in approach exists, there are 3 basic stances a player can take in preparation to bat. In the closed stance, the batter’s front foot is positioned closer to the plate than the back foot. A more popular stance is the open stance, where the player’s back foot is placed closer to the plate than the front foot. The square batting stance is the most common stance. This stance is where both feet are in line with the pitcher and parallel with the edge of the batter’s box. Most authors agree that the square stance is the optimal position because it provides batters the best opportunity to hit pitches anywhere in the strike zone and limits compensatory or extra motion to their swing.15
Once the player begins the swing, he has entered the loading period, which is divided into the stance, stride, and drive phases. The loading period, also known as coiling or triggering, begins as the athlete eccentrically stretches agonist muscles and rotates the body away from the incoming ball. The elastic energy stored during this stretching is released during the concentric contraction of the same muscles and transferred through the entire kinetic chain as different segments of the body are rotated; it culminates in effort directed at hitting the baseball.16
In each phase of the loading period, certain critical motions should be monitored and corrected in order to return the player to his previous level of competition. Stride length has been shown to be critical in the timing of a batter’s swing. A short stride length can cause early initiation of the swing, while a longer stride can produce delayed activation of hip rotation. As the player enters the drive phase, he should have increased elbow flexion in the back elbow compared to the front elbow. The bat should be placed at a position approximately 45° in the frontal plane, and the bat should bisect the batter’s helmet. The back elbow should be down, both upper extremities should be positioned close to the hitter’s body, and the proximal interphalangeal joints of the hands should align on the handle of the bat. Athletic trainers and coaches should be aware that subtle compensations due to deficits during these movements could cause injury during the swing by disrupting the body’s natural motion.
The bat acceleration phase occurs from maximal bat loading through striking the ball. In this time, the linear force that has been exerted by the player must be transferred into rotational force through the trunk and upper extremities. When the lead leg contacts the ground, the player has created a closed kinetic chain, where the elastic energy gathered during the loading period is used to produce segmental rotation beginning in the hips and rising through the trunk and out to the arms and hands, finally producing contact with the baseball.16 To produce effective bat velocity, each segment must rotate in a sequential manner. If the upper extremities reach peak velocity before any lower segment, then the player has lost the ability to efficiently transfer kinetic energy up the kinetic chain.
Finally, the follow-through phase occurs after contact with the baseball and ends with complete deceleration, completing the swing. In order to achieve optimal effort, full hip rotation is needed, which is aided by rotation of the trail foot. Both hips and back laces should face the pitcher upon completion of the swing producing maximum power output.15
Return to Hitting: Principles
As with the initiation of the ITP, an interval hitting protocol (IHP) is designed to begin only after the player has been assessed on impairment measures, physical performance measures, and self-assessment.17 The player should have minimum to no pain, have no tenderness to palpation, and show adequate range of motion and strength to meet the demands of performing a full hitting cycle.12 It is recommended that before beginning a return-to-play protocol, the involved extremity should be at least 80% as strong as the uninvolved extremity.18 Physical measures challenging an athlete’s ability to perform tasks specific to hitting a baseball must also be considered through standardized examinations of the involved area.19 Finally, the athlete’s self-perception of functional abilities must be taken into account. This gives a subjective account of what the hitter perceives they are able to perform, providing useful insight into whether they are mentally prepared to participate in the protocol.
Like the ITP, progression through the IHP is also based on the player’s level of pain and soreness rather than following a specific timetable (Table). The program features a 1 day on, 1 day off schedule during which the player completes 1 step per day. The athlete must remain pain-free to progress to the next step and monitor his level of soreness during their workout. If pain or soreness persists, the player should rest for 2 days and be reevaluated upon return.17
The same principles of proper warm-up and mechanics apply in the IHP. An athlete should jog or cycle for a minimum of 10 minutes and perform stretching exercises focused on both upper and lower extremity muscles, as batting involves whole body movement. As the athlete progresses through the IHP, having a hitting coach to analyze, correct and maintain proper swing mechanics is valuable in enhancing performance as well as decreasing risk of re-injury.
The Interval Hitting Program
For a PDF patient handout that summarizes the phases of this program, see Appendix 2.
Phase 1 (Dry Swings). Only the most basic fundamentals are stressed during this phase. The player should focus on properly moving from one phase of the swing to the next, without the goal of hitting the baseball. Trainers should measure critical points in the swing and correct deficits early.
Phase 2 (Batting Off a Tee). In this phase, the player is reintroduced to batting at low intensity with a fixed position target. The initial steps have the batter swing in a position of greatest comfort and natural movement, while the final steps in this phase test the athlete’s range of motion and confidence in the previous, healed injury.
Phase 3 (Soft Toss). As the player progresses to this phase, a baseball with trajectory is used to simulate differences in placement of pitches used during a game. As the hitter is able to pick up differences in target position, his performance and confidence should both increase.20 The coach should sit about 30 feet away, facing the hitter at an angle of 45°, and toss the ball in an underhand motion.
Phase 4 (Simulated Hitting). In this phase, the player and coach should focus on the timing of sequential body movements in order to elicit proper loading and force production. With the randomized pitch delivery and increased velocity, the hitter will practice against pitches similar to those delivered in competition.
Conclusion
Interval throwing and hitting programs are designed to allow the athlete to return to competition through a gradual, stepwise program. This permits the player to prepare his body for the unique stresses associated with throwing and hitting. The medical personnel should familiarize themselves with the philosophy of the interval throwing and hitting programs and individualize them to each athlete. Emphasis on proper warm-up, mechanics, and effort control is paramount in expediting return to play while preventing re-injury.
1. Dillman CJ, Fleisig GS, Andrews JR. Biomechanics of pitching with emphasis upon shoulder kinematics. J Orthop Sports Phys Ther. 1993;18(2):402-408.
2. Fleisig GS, Andrews JR, Dillman CJ, Escamilla RF. Kinetics of baseball pitching with implications about injury mechanisms. Am J Sports Med. 1995;23(2):233-239.
3. Fleisig GS, Barrentine SW, Escamilla RF, Andrews JR. Biomechanics of overhand throwing with implications for injuries. Sports Med Auckl NZ. 1996;21(6):421-437.
4. Meister K. Injuries to the shoulder in the throwing athlete. Part one: Biomechanics/pathophysiology/classification of injury. Am J Sports Med. 2000;28(2):265-275.
5. Kaczmarek PK, Lubiatowski P, Cisowski P, et al. Shoulder problems in overhead sports. Part I - biomechanics of throwing. Pol Orthop Traumatol. 2014;79:50-58.
6. Toyoshima S, Hoshikawa T, Miyashita M, Oguri T. Contribution of the body parts to throwing performance. Biomech IV. 1974;5:169-174.
7. Weber AE, Kontaxis A, O’Brien SJ, Bedi A. The biomechanics of throwing: simplified and cogent. Sports Med Arthrosc Rev. 2014;22(2):72-79.
8. Werner SL, Fleisig GS, Dillman CJ, Andrews JR. Biomechanics of the elbow during baseball pitching. J Orthop Sports Phys Ther. 1993;17(6):274-278.
9. Chang ES, Greco NJ, McClincy MP, Bradley JP. Posterior shoulder instability in overhead athletes. Orthop Clin North Am. 2016;47(1):179-187.
10. Digiovine NM, Jobe FW, Pink M, Perry J. An electromyographic analysis of the upper extremity in pitching. J Shoulder Elbow Surg. 1992;1(1):15-25.
11. Axe M, Hurd W, Snyder-Mackler L. Data-based interval throwing programs for baseball players. Sports Health. 2009;1(2):145-153.
12. Reinold MM, Wilk KE, Reed J, Crenshaw K, Andrews JR. Interval sport programs: guidelines for baseball, tennis, and golf. J Orthop Sports Phys Ther. 2002;32(6):293-298.
13. Fleisig GS, Zheng N, Barrentine SW, Escamilla RF, Andrews JR, Lemak LF. Kinematic and kinetic comparison of full and partial effort baseball pitching. Conference proceedings of the 20th Annual Meeting. Atlanta, GA: American Society of Biomechanics; 1996:151-152.
14. Fleisig GS, Hsu WK, Fortenbaugh D, Cordover A, Press JM. Trunk axial rotation in baseball pitching and batting. Sports Biomech. 2013;12(4):324-333.
15. Monti R. Return to hitting: an interval hitting progression and overview of hitting mechanics following injury. Int J Sports Phys Ther. 2015;10(7):1059-1073.
16. Welch CM, Banks SA, Cook FF, Draovitch P. Hitting a baseball: a biomechanical description. J Orthop Sports Phys Ther. 1995;22(5):193-201.
17. Axe MJ, Snyder-Mackler L, Konin JG, Strube MJ. Development of a distance-based interval throwing program for Little League-aged athletes. Am J Sports Med. 1996;24(5):594-602.
18. Fitzgerald GK, Axe MJ, Snyder-Mackler L. Proposed practice guidelines for nonoperative anterior cruciate ligament rehabilitation of physically active individuals. J Orthop Sports Phys Ther. 2000;30(4):194-203.
19. Hegedus EJ, McDonough S, Bleakley C, Cook CE, Baxter GD. Clinician-friendly lower extremity physical performance measures in athletes: a systematic review of measurement properties and correlation with injury, part 1. The tests for knee function including the hop tests. Br J Sports Med. 2015;49(10):642-648.
20. Higuchi T, Nagami T, Morohoshi J, Nakata H, Kanosue K. Disturbance in hitting accuracy by professional and collegiate baseball players due to intentional change of target position. Percept Mot Skills. 2013;116(2):627-639.
Throwing and batting each require repetitive motions that can result in injuries unique to baseball. Fortuantely, advances in operative and nonoperative treatments have allowed players to return to competition after sustaining what previously would have been considered a career-ending injury. Once a player has been deemed ready to return to throwing or hitting, a comprehensive, multiphased approach to rehabilitation is necessary to reintroduce the athlete back to baseball activities and avoid re-injury. This article reviews the biomechanics of both throwing and hitting, and outlines the phases of rehabilitation necessary to allow the athlete to return to competition.
Throwing
Biomechanical Overview
The overhead throwing motion is complex and involves full body coordination from the initial force generation through the follow-through phase of throwing. The “kinetic chain”—the concept that movements in the body are connected through segments culminating with the highest energy in the final segment—is paramount to achieving the force and energy needed for throwing.1-8 The kinetic chain begins in the lower body and trunk and transmits the energy distally to the shoulder, elbow, and hand, ending with kinetic energy transfer to the ball.3-5,7 The progression of motion through the kinetic chain during throwing includes stride, pelvis rotation, upper torso rotation, elbow extension, shoulder internal rotation, and wrist flexion. Disruptions in this chain due to muscle imbalance or weakness can lead to injury downstream, particularly in the upper extremity.3,7,9
The importance of the kinetic chain can be highlighted in the 6 phases of throwing motion. These include wind-up, early arm cocking, late arm cocking, arm acceleration, arm deceleration, and follow-through (Figure 1).1,2,9,10
The wind-up phase starts with initiation of motion and ends with maximal knee lift of the lead leg; its objective is to place the body in an optimal stance to throw.3-5,7 There are minimal forces, torques, and muscle activity in the upper extremity during this phase, but up to 50% of throw speed is created through stride and trunk rotation.6 During the early cocking phase, the thrower keeps his stance foot planted and drives his lead leg towards the target, while bringing both arms into abduction. This is coupled with internal rotation of the stance hip, external rotation of the lead hip, and external rotation of the throwing shoulder. This creates linear velocity by maximizing the length of the elastic components of the body. Elbow, wrist, and finger extensors are also contracting during this phase to control elbow flexion and wrist hyperextension.3
The late cocking phase begins when the lead foot contacts the ground and ends with maximum shoulder external rotation.3-5 Lead foot contact is followed by quadriceps contraction to decelerate and stabilize the lead leg. This is followed by rotation of the pelvis and upper torso. The result is energy transfer to the throwing arm with a shear force across the anterior shoulder of 400 N.4 The shoulder stays in 90° of abduction, 15° of horizontal adduction, and externally rotates to between 150° and 180°. This produces a maximum horizontal adduction moment of 100 N.m and internal rotation torque of 70 N.m.4 Simultaneously, the elbow generates maximum flexion and a 65 N.m varus torque.7 Forces about the elbow are generated to resist the large angular velocity experienced (up to 3000°/second). This places an extreme amount of valgus stress along the medial elbow, particularly on the ulnar collateral ligament. The shoulder girdle and rotator cuff muscles simultaneously act to stabilize the scapula and glenohumeral joint.
The arm acceleration phase is from maximal shoulder external rotation until ball release.3-5 In this phase, the thrower flexes his trunk from an extended position, returning to neutral by the time of ball release while the lead leg straightens. The shoulder stays abducted at 90° throughout while the rotator cuff internal rotators and scapular stabilizers contract to explosively internally rotate the shoulder, creating a maximal internal rotation velocity greater than 7000°/second by ball release.1,4,7 The elbow also begins to extend, reaching maximum velocity during mid-acceleration phase from a combination of triceps contraction and torque generated from rotation at the shoulder and upper trunk.3 Finally, the wrist flexors contract to move the wrist to a neutral position from hyperextension as the ball is released.
During arm deceleration, the shoulder achieves maximum internal rotation until reaching a neutral position and horizontally adducts across the body. This is controlled by contraction of the shoulder girdle musculature; the teres minor has the highest activity.3,4 The greatest forces produced during the throwing motion act at the shoulder and elbow during deceleration and can contribute to injury.2 These include compressive forces of greater than 1000 N, posterior shear forces of 400 N, and inferior shear forces of 300 N.4,7
The final phase, the follow-through phase, starts at shoulder maximum internal rotation and ends when the arm assumes a balanced position across the trunk. Lower extremity extension and trunk flexion help distribute forces throughout the body, taking stress away from the throwing arm. The posterior shoulder musculature and scapular protractors contribute to continued deceleration and muscle firing returns to resting levels. This complex motion of throwing fueled by the kinetic chain lasts less than 2 seconds and can result in ball release speeds as high as 100 miles per hour.3,4
Return to Throwing: Principles
Nonoperative and postoperative rehabilitation programs allow restoration of motion, strength, static and dynamic stability, and neuromuscular control. The initiation of an interval throwing program (ITP) is based on the assumption that tissue healing is complete and a complete physical examination has been conducted to the treating physician’s approval.11 An ITP progressively applies forces along the kinetic chain in a controlled manner through graduated throwing distances, while minimizing the risk of re-injury.
Reinold and colleagues12 described guidelines that were used in the development of the ITP.12 These factors include: (1) The act of throwing a baseball involves the transfer of energy from the feet up to the hand and therefore careful attention must be paid along the entire kinetic chain; (2) gradual progression of interval throwing decreases the chance for re-injury; (3) proper warm-up; and (4) proper throwing mechanics minimizes the chance of re-injury.
Variability. Unlike traditional rehabilitation programs that advance an athlete based on a specific timetable, the ITP requires that each level or phase to be completed pain-free or without complications prior to starting the next level. Therefore, an ITP can be used for overhead athletes of varying skill levels because progression will be different from one athlete to another. It is also important to have the athlete adhere strictly to the program, as over-eagerness to complete the ITP as quickly as possible can increase the chance of re-injury and thus slow the rehabilitation process.12
Warm-up. An adequate warm-up is recommended prior to initiating ITP. An athlete should jog or cycle to develop a light sweat and then progress to stretching and flexibility exercises. As emphasized before, throwing involves nearly all the muscles in the body. Therefore, all muscle groups should be stretched beginning with the legs and working distally along the kinetic chain.
Mechanics. Analysis, correction, and maintenance of proper throwing mechanics is essential throughout the early phases of rehabilitation and ITP. Improper pitching mechanics places increased stress on the throwing arm, potentially leading to re-injury. Therefore, it would be valuable to have a pitching coach available to emphasize proper mechanics throughout the rehabilitation process.
The Interval Throwing Program
For a PDF patient handout that summarizes the phases of this program, see Appendix 1.
Phase 1. We have adopted the ITP as described by Reinold and colleagues.12 Phase begins with the overhead athlete throwing on flat ground. He or she begins tossing from 45 feet and gradually progresses to 60, 90, 120, 150, and 180 feet.
As discussed earlier, it is critical to use proper mechanics throughout the ITP. The “crow hop” method simulates a throwing act and helps maintain proper pitching mechanics. Crow hop has 3 components: hop, skip, and throw. Using this technique, the pitcher begins warm-up throws at a comfortable distance (generally 30 feet) and then progresses to the distance as indicated on the ITP. The athlete will then need to perform each step 2 times, with 1 day of rest between steps, before advancing to the next step. The ball should be thrown with an arc and have only enough momentum to reach the desired distance.
For example, Step 1 calls for the athlete to perform 2 sets of 25 throws at 45 feet, with adequate rest (5 minutes) between sets. This step will be repeated following 1 day of rest. If the athlete demonstrates the ability to throw at the prescribed distance without pain, he or she can progress to Step 2, which calls for 3 sets of 25 throws at 45 feet. If pain is present at any step, the thrower returns to the previous asymptomatic step and can progress once he is pain-free.
Positional players are instructed to complete Phase 1 prior to starting position-specific drills. Pitchers, on the other hand, are instructed to stop once they reach and complete 120 feet. They will then progress to tossing at progressive distances of 60, 90, and 120 feet, followed by throwing at 60 feet 6 inches with normal pitching mechanics, initiating straight line throws with little to no arc.
Phase II (Throwing off the Mound). Once a pitcher completes Phase 1 without pain or complications, he is ready to begin throwing off the mound. The same principle remains in Phase 2: pitchers must complete each step pain-free before advancing to the next stage. Pitchers should first throw fastballs at 50% effort and progress to 75% and 100% effort. Because athletes often find it difficult to gauge their own effort, it is important to emphasize the importance of strictly adhering to the program. Fleisig and colleagues13 studied healthy pitchers’ ability to estimate their throwing effort. When targeting 50% effort, athletes generated ball speeds of 85% with forces and torque approaching 75% of maximum. A radar gun may be valuable in guiding effort control.
As the player advances through Phase 2, he will increase the volume of pitches as well as the effort in a gradual manner. The player may introduce breaking ball pitches once he demonstrates the ability to throw light batting practice. Phase 2 concludes with the pitcher throwing simulated games, progressing by 15 throws per workout.
Hitting
Biomechanics Overview
The mechanics of hitting a baseball can be broken down into 6 phases: the preparatory phase, stance phase, stride phase, drive phase, bat acceleration phase, and follow-through phase.14 While progressing through a return-to-play protocol, it is important to understand and teach the player proper swing mechanics during each phase in order to minimize the risk of re-injury (Figure 2).
The preparatory phase occurs as the player positions himself into the batter’s box. This phase is highly individualized, depending on each player’s personal preference. Though significant variability in approach exists, there are 3 basic stances a player can take in preparation to bat. In the closed stance, the batter’s front foot is positioned closer to the plate than the back foot. A more popular stance is the open stance, where the player’s back foot is placed closer to the plate than the front foot. The square batting stance is the most common stance. This stance is where both feet are in line with the pitcher and parallel with the edge of the batter’s box. Most authors agree that the square stance is the optimal position because it provides batters the best opportunity to hit pitches anywhere in the strike zone and limits compensatory or extra motion to their swing.15
Once the player begins the swing, he has entered the loading period, which is divided into the stance, stride, and drive phases. The loading period, also known as coiling or triggering, begins as the athlete eccentrically stretches agonist muscles and rotates the body away from the incoming ball. The elastic energy stored during this stretching is released during the concentric contraction of the same muscles and transferred through the entire kinetic chain as different segments of the body are rotated; it culminates in effort directed at hitting the baseball.16
In each phase of the loading period, certain critical motions should be monitored and corrected in order to return the player to his previous level of competition. Stride length has been shown to be critical in the timing of a batter’s swing. A short stride length can cause early initiation of the swing, while a longer stride can produce delayed activation of hip rotation. As the player enters the drive phase, he should have increased elbow flexion in the back elbow compared to the front elbow. The bat should be placed at a position approximately 45° in the frontal plane, and the bat should bisect the batter’s helmet. The back elbow should be down, both upper extremities should be positioned close to the hitter’s body, and the proximal interphalangeal joints of the hands should align on the handle of the bat. Athletic trainers and coaches should be aware that subtle compensations due to deficits during these movements could cause injury during the swing by disrupting the body’s natural motion.
The bat acceleration phase occurs from maximal bat loading through striking the ball. In this time, the linear force that has been exerted by the player must be transferred into rotational force through the trunk and upper extremities. When the lead leg contacts the ground, the player has created a closed kinetic chain, where the elastic energy gathered during the loading period is used to produce segmental rotation beginning in the hips and rising through the trunk and out to the arms and hands, finally producing contact with the baseball.16 To produce effective bat velocity, each segment must rotate in a sequential manner. If the upper extremities reach peak velocity before any lower segment, then the player has lost the ability to efficiently transfer kinetic energy up the kinetic chain.
Finally, the follow-through phase occurs after contact with the baseball and ends with complete deceleration, completing the swing. In order to achieve optimal effort, full hip rotation is needed, which is aided by rotation of the trail foot. Both hips and back laces should face the pitcher upon completion of the swing producing maximum power output.15
Return to Hitting: Principles
As with the initiation of the ITP, an interval hitting protocol (IHP) is designed to begin only after the player has been assessed on impairment measures, physical performance measures, and self-assessment.17 The player should have minimum to no pain, have no tenderness to palpation, and show adequate range of motion and strength to meet the demands of performing a full hitting cycle.12 It is recommended that before beginning a return-to-play protocol, the involved extremity should be at least 80% as strong as the uninvolved extremity.18 Physical measures challenging an athlete’s ability to perform tasks specific to hitting a baseball must also be considered through standardized examinations of the involved area.19 Finally, the athlete’s self-perception of functional abilities must be taken into account. This gives a subjective account of what the hitter perceives they are able to perform, providing useful insight into whether they are mentally prepared to participate in the protocol.
Like the ITP, progression through the IHP is also based on the player’s level of pain and soreness rather than following a specific timetable (Table). The program features a 1 day on, 1 day off schedule during which the player completes 1 step per day. The athlete must remain pain-free to progress to the next step and monitor his level of soreness during their workout. If pain or soreness persists, the player should rest for 2 days and be reevaluated upon return.17
The same principles of proper warm-up and mechanics apply in the IHP. An athlete should jog or cycle for a minimum of 10 minutes and perform stretching exercises focused on both upper and lower extremity muscles, as batting involves whole body movement. As the athlete progresses through the IHP, having a hitting coach to analyze, correct and maintain proper swing mechanics is valuable in enhancing performance as well as decreasing risk of re-injury.
The Interval Hitting Program
For a PDF patient handout that summarizes the phases of this program, see Appendix 2.
Phase 1 (Dry Swings). Only the most basic fundamentals are stressed during this phase. The player should focus on properly moving from one phase of the swing to the next, without the goal of hitting the baseball. Trainers should measure critical points in the swing and correct deficits early.
Phase 2 (Batting Off a Tee). In this phase, the player is reintroduced to batting at low intensity with a fixed position target. The initial steps have the batter swing in a position of greatest comfort and natural movement, while the final steps in this phase test the athlete’s range of motion and confidence in the previous, healed injury.
Phase 3 (Soft Toss). As the player progresses to this phase, a baseball with trajectory is used to simulate differences in placement of pitches used during a game. As the hitter is able to pick up differences in target position, his performance and confidence should both increase.20 The coach should sit about 30 feet away, facing the hitter at an angle of 45°, and toss the ball in an underhand motion.
Phase 4 (Simulated Hitting). In this phase, the player and coach should focus on the timing of sequential body movements in order to elicit proper loading and force production. With the randomized pitch delivery and increased velocity, the hitter will practice against pitches similar to those delivered in competition.
Conclusion
Interval throwing and hitting programs are designed to allow the athlete to return to competition through a gradual, stepwise program. This permits the player to prepare his body for the unique stresses associated with throwing and hitting. The medical personnel should familiarize themselves with the philosophy of the interval throwing and hitting programs and individualize them to each athlete. Emphasis on proper warm-up, mechanics, and effort control is paramount in expediting return to play while preventing re-injury.
Throwing and batting each require repetitive motions that can result in injuries unique to baseball. Fortuantely, advances in operative and nonoperative treatments have allowed players to return to competition after sustaining what previously would have been considered a career-ending injury. Once a player has been deemed ready to return to throwing or hitting, a comprehensive, multiphased approach to rehabilitation is necessary to reintroduce the athlete back to baseball activities and avoid re-injury. This article reviews the biomechanics of both throwing and hitting, and outlines the phases of rehabilitation necessary to allow the athlete to return to competition.
Throwing
Biomechanical Overview
The overhead throwing motion is complex and involves full body coordination from the initial force generation through the follow-through phase of throwing. The “kinetic chain”—the concept that movements in the body are connected through segments culminating with the highest energy in the final segment—is paramount to achieving the force and energy needed for throwing.1-8 The kinetic chain begins in the lower body and trunk and transmits the energy distally to the shoulder, elbow, and hand, ending with kinetic energy transfer to the ball.3-5,7 The progression of motion through the kinetic chain during throwing includes stride, pelvis rotation, upper torso rotation, elbow extension, shoulder internal rotation, and wrist flexion. Disruptions in this chain due to muscle imbalance or weakness can lead to injury downstream, particularly in the upper extremity.3,7,9
The importance of the kinetic chain can be highlighted in the 6 phases of throwing motion. These include wind-up, early arm cocking, late arm cocking, arm acceleration, arm deceleration, and follow-through (Figure 1).1,2,9,10
The wind-up phase starts with initiation of motion and ends with maximal knee lift of the lead leg; its objective is to place the body in an optimal stance to throw.3-5,7 There are minimal forces, torques, and muscle activity in the upper extremity during this phase, but up to 50% of throw speed is created through stride and trunk rotation.6 During the early cocking phase, the thrower keeps his stance foot planted and drives his lead leg towards the target, while bringing both arms into abduction. This is coupled with internal rotation of the stance hip, external rotation of the lead hip, and external rotation of the throwing shoulder. This creates linear velocity by maximizing the length of the elastic components of the body. Elbow, wrist, and finger extensors are also contracting during this phase to control elbow flexion and wrist hyperextension.3
The late cocking phase begins when the lead foot contacts the ground and ends with maximum shoulder external rotation.3-5 Lead foot contact is followed by quadriceps contraction to decelerate and stabilize the lead leg. This is followed by rotation of the pelvis and upper torso. The result is energy transfer to the throwing arm with a shear force across the anterior shoulder of 400 N.4 The shoulder stays in 90° of abduction, 15° of horizontal adduction, and externally rotates to between 150° and 180°. This produces a maximum horizontal adduction moment of 100 N.m and internal rotation torque of 70 N.m.4 Simultaneously, the elbow generates maximum flexion and a 65 N.m varus torque.7 Forces about the elbow are generated to resist the large angular velocity experienced (up to 3000°/second). This places an extreme amount of valgus stress along the medial elbow, particularly on the ulnar collateral ligament. The shoulder girdle and rotator cuff muscles simultaneously act to stabilize the scapula and glenohumeral joint.
The arm acceleration phase is from maximal shoulder external rotation until ball release.3-5 In this phase, the thrower flexes his trunk from an extended position, returning to neutral by the time of ball release while the lead leg straightens. The shoulder stays abducted at 90° throughout while the rotator cuff internal rotators and scapular stabilizers contract to explosively internally rotate the shoulder, creating a maximal internal rotation velocity greater than 7000°/second by ball release.1,4,7 The elbow also begins to extend, reaching maximum velocity during mid-acceleration phase from a combination of triceps contraction and torque generated from rotation at the shoulder and upper trunk.3 Finally, the wrist flexors contract to move the wrist to a neutral position from hyperextension as the ball is released.
During arm deceleration, the shoulder achieves maximum internal rotation until reaching a neutral position and horizontally adducts across the body. This is controlled by contraction of the shoulder girdle musculature; the teres minor has the highest activity.3,4 The greatest forces produced during the throwing motion act at the shoulder and elbow during deceleration and can contribute to injury.2 These include compressive forces of greater than 1000 N, posterior shear forces of 400 N, and inferior shear forces of 300 N.4,7
The final phase, the follow-through phase, starts at shoulder maximum internal rotation and ends when the arm assumes a balanced position across the trunk. Lower extremity extension and trunk flexion help distribute forces throughout the body, taking stress away from the throwing arm. The posterior shoulder musculature and scapular protractors contribute to continued deceleration and muscle firing returns to resting levels. This complex motion of throwing fueled by the kinetic chain lasts less than 2 seconds and can result in ball release speeds as high as 100 miles per hour.3,4
Return to Throwing: Principles
Nonoperative and postoperative rehabilitation programs allow restoration of motion, strength, static and dynamic stability, and neuromuscular control. The initiation of an interval throwing program (ITP) is based on the assumption that tissue healing is complete and a complete physical examination has been conducted to the treating physician’s approval.11 An ITP progressively applies forces along the kinetic chain in a controlled manner through graduated throwing distances, while minimizing the risk of re-injury.
Reinold and colleagues12 described guidelines that were used in the development of the ITP.12 These factors include: (1) The act of throwing a baseball involves the transfer of energy from the feet up to the hand and therefore careful attention must be paid along the entire kinetic chain; (2) gradual progression of interval throwing decreases the chance for re-injury; (3) proper warm-up; and (4) proper throwing mechanics minimizes the chance of re-injury.
Variability. Unlike traditional rehabilitation programs that advance an athlete based on a specific timetable, the ITP requires that each level or phase to be completed pain-free or without complications prior to starting the next level. Therefore, an ITP can be used for overhead athletes of varying skill levels because progression will be different from one athlete to another. It is also important to have the athlete adhere strictly to the program, as over-eagerness to complete the ITP as quickly as possible can increase the chance of re-injury and thus slow the rehabilitation process.12
Warm-up. An adequate warm-up is recommended prior to initiating ITP. An athlete should jog or cycle to develop a light sweat and then progress to stretching and flexibility exercises. As emphasized before, throwing involves nearly all the muscles in the body. Therefore, all muscle groups should be stretched beginning with the legs and working distally along the kinetic chain.
Mechanics. Analysis, correction, and maintenance of proper throwing mechanics is essential throughout the early phases of rehabilitation and ITP. Improper pitching mechanics places increased stress on the throwing arm, potentially leading to re-injury. Therefore, it would be valuable to have a pitching coach available to emphasize proper mechanics throughout the rehabilitation process.
The Interval Throwing Program
For a PDF patient handout that summarizes the phases of this program, see Appendix 1.
Phase 1. We have adopted the ITP as described by Reinold and colleagues.12 Phase begins with the overhead athlete throwing on flat ground. He or she begins tossing from 45 feet and gradually progresses to 60, 90, 120, 150, and 180 feet.
As discussed earlier, it is critical to use proper mechanics throughout the ITP. The “crow hop” method simulates a throwing act and helps maintain proper pitching mechanics. Crow hop has 3 components: hop, skip, and throw. Using this technique, the pitcher begins warm-up throws at a comfortable distance (generally 30 feet) and then progresses to the distance as indicated on the ITP. The athlete will then need to perform each step 2 times, with 1 day of rest between steps, before advancing to the next step. The ball should be thrown with an arc and have only enough momentum to reach the desired distance.
For example, Step 1 calls for the athlete to perform 2 sets of 25 throws at 45 feet, with adequate rest (5 minutes) between sets. This step will be repeated following 1 day of rest. If the athlete demonstrates the ability to throw at the prescribed distance without pain, he or she can progress to Step 2, which calls for 3 sets of 25 throws at 45 feet. If pain is present at any step, the thrower returns to the previous asymptomatic step and can progress once he is pain-free.
Positional players are instructed to complete Phase 1 prior to starting position-specific drills. Pitchers, on the other hand, are instructed to stop once they reach and complete 120 feet. They will then progress to tossing at progressive distances of 60, 90, and 120 feet, followed by throwing at 60 feet 6 inches with normal pitching mechanics, initiating straight line throws with little to no arc.
Phase II (Throwing off the Mound). Once a pitcher completes Phase 1 without pain or complications, he is ready to begin throwing off the mound. The same principle remains in Phase 2: pitchers must complete each step pain-free before advancing to the next stage. Pitchers should first throw fastballs at 50% effort and progress to 75% and 100% effort. Because athletes often find it difficult to gauge their own effort, it is important to emphasize the importance of strictly adhering to the program. Fleisig and colleagues13 studied healthy pitchers’ ability to estimate their throwing effort. When targeting 50% effort, athletes generated ball speeds of 85% with forces and torque approaching 75% of maximum. A radar gun may be valuable in guiding effort control.
As the player advances through Phase 2, he will increase the volume of pitches as well as the effort in a gradual manner. The player may introduce breaking ball pitches once he demonstrates the ability to throw light batting practice. Phase 2 concludes with the pitcher throwing simulated games, progressing by 15 throws per workout.
Hitting
Biomechanics Overview
The mechanics of hitting a baseball can be broken down into 6 phases: the preparatory phase, stance phase, stride phase, drive phase, bat acceleration phase, and follow-through phase.14 While progressing through a return-to-play protocol, it is important to understand and teach the player proper swing mechanics during each phase in order to minimize the risk of re-injury (Figure 2).
The preparatory phase occurs as the player positions himself into the batter’s box. This phase is highly individualized, depending on each player’s personal preference. Though significant variability in approach exists, there are 3 basic stances a player can take in preparation to bat. In the closed stance, the batter’s front foot is positioned closer to the plate than the back foot. A more popular stance is the open stance, where the player’s back foot is placed closer to the plate than the front foot. The square batting stance is the most common stance. This stance is where both feet are in line with the pitcher and parallel with the edge of the batter’s box. Most authors agree that the square stance is the optimal position because it provides batters the best opportunity to hit pitches anywhere in the strike zone and limits compensatory or extra motion to their swing.15
Once the player begins the swing, he has entered the loading period, which is divided into the stance, stride, and drive phases. The loading period, also known as coiling or triggering, begins as the athlete eccentrically stretches agonist muscles and rotates the body away from the incoming ball. The elastic energy stored during this stretching is released during the concentric contraction of the same muscles and transferred through the entire kinetic chain as different segments of the body are rotated; it culminates in effort directed at hitting the baseball.16
In each phase of the loading period, certain critical motions should be monitored and corrected in order to return the player to his previous level of competition. Stride length has been shown to be critical in the timing of a batter’s swing. A short stride length can cause early initiation of the swing, while a longer stride can produce delayed activation of hip rotation. As the player enters the drive phase, he should have increased elbow flexion in the back elbow compared to the front elbow. The bat should be placed at a position approximately 45° in the frontal plane, and the bat should bisect the batter’s helmet. The back elbow should be down, both upper extremities should be positioned close to the hitter’s body, and the proximal interphalangeal joints of the hands should align on the handle of the bat. Athletic trainers and coaches should be aware that subtle compensations due to deficits during these movements could cause injury during the swing by disrupting the body’s natural motion.
The bat acceleration phase occurs from maximal bat loading through striking the ball. In this time, the linear force that has been exerted by the player must be transferred into rotational force through the trunk and upper extremities. When the lead leg contacts the ground, the player has created a closed kinetic chain, where the elastic energy gathered during the loading period is used to produce segmental rotation beginning in the hips and rising through the trunk and out to the arms and hands, finally producing contact with the baseball.16 To produce effective bat velocity, each segment must rotate in a sequential manner. If the upper extremities reach peak velocity before any lower segment, then the player has lost the ability to efficiently transfer kinetic energy up the kinetic chain.
Finally, the follow-through phase occurs after contact with the baseball and ends with complete deceleration, completing the swing. In order to achieve optimal effort, full hip rotation is needed, which is aided by rotation of the trail foot. Both hips and back laces should face the pitcher upon completion of the swing producing maximum power output.15
Return to Hitting: Principles
As with the initiation of the ITP, an interval hitting protocol (IHP) is designed to begin only after the player has been assessed on impairment measures, physical performance measures, and self-assessment.17 The player should have minimum to no pain, have no tenderness to palpation, and show adequate range of motion and strength to meet the demands of performing a full hitting cycle.12 It is recommended that before beginning a return-to-play protocol, the involved extremity should be at least 80% as strong as the uninvolved extremity.18 Physical measures challenging an athlete’s ability to perform tasks specific to hitting a baseball must also be considered through standardized examinations of the involved area.19 Finally, the athlete’s self-perception of functional abilities must be taken into account. This gives a subjective account of what the hitter perceives they are able to perform, providing useful insight into whether they are mentally prepared to participate in the protocol.
Like the ITP, progression through the IHP is also based on the player’s level of pain and soreness rather than following a specific timetable (Table). The program features a 1 day on, 1 day off schedule during which the player completes 1 step per day. The athlete must remain pain-free to progress to the next step and monitor his level of soreness during their workout. If pain or soreness persists, the player should rest for 2 days and be reevaluated upon return.17
The same principles of proper warm-up and mechanics apply in the IHP. An athlete should jog or cycle for a minimum of 10 minutes and perform stretching exercises focused on both upper and lower extremity muscles, as batting involves whole body movement. As the athlete progresses through the IHP, having a hitting coach to analyze, correct and maintain proper swing mechanics is valuable in enhancing performance as well as decreasing risk of re-injury.
The Interval Hitting Program
For a PDF patient handout that summarizes the phases of this program, see Appendix 2.
Phase 1 (Dry Swings). Only the most basic fundamentals are stressed during this phase. The player should focus on properly moving from one phase of the swing to the next, without the goal of hitting the baseball. Trainers should measure critical points in the swing and correct deficits early.
Phase 2 (Batting Off a Tee). In this phase, the player is reintroduced to batting at low intensity with a fixed position target. The initial steps have the batter swing in a position of greatest comfort and natural movement, while the final steps in this phase test the athlete’s range of motion and confidence in the previous, healed injury.
Phase 3 (Soft Toss). As the player progresses to this phase, a baseball with trajectory is used to simulate differences in placement of pitches used during a game. As the hitter is able to pick up differences in target position, his performance and confidence should both increase.20 The coach should sit about 30 feet away, facing the hitter at an angle of 45°, and toss the ball in an underhand motion.
Phase 4 (Simulated Hitting). In this phase, the player and coach should focus on the timing of sequential body movements in order to elicit proper loading and force production. With the randomized pitch delivery and increased velocity, the hitter will practice against pitches similar to those delivered in competition.
Conclusion
Interval throwing and hitting programs are designed to allow the athlete to return to competition through a gradual, stepwise program. This permits the player to prepare his body for the unique stresses associated with throwing and hitting. The medical personnel should familiarize themselves with the philosophy of the interval throwing and hitting programs and individualize them to each athlete. Emphasis on proper warm-up, mechanics, and effort control is paramount in expediting return to play while preventing re-injury.
1. Dillman CJ, Fleisig GS, Andrews JR. Biomechanics of pitching with emphasis upon shoulder kinematics. J Orthop Sports Phys Ther. 1993;18(2):402-408.
2. Fleisig GS, Andrews JR, Dillman CJ, Escamilla RF. Kinetics of baseball pitching with implications about injury mechanisms. Am J Sports Med. 1995;23(2):233-239.
3. Fleisig GS, Barrentine SW, Escamilla RF, Andrews JR. Biomechanics of overhand throwing with implications for injuries. Sports Med Auckl NZ. 1996;21(6):421-437.
4. Meister K. Injuries to the shoulder in the throwing athlete. Part one: Biomechanics/pathophysiology/classification of injury. Am J Sports Med. 2000;28(2):265-275.
5. Kaczmarek PK, Lubiatowski P, Cisowski P, et al. Shoulder problems in overhead sports. Part I - biomechanics of throwing. Pol Orthop Traumatol. 2014;79:50-58.
6. Toyoshima S, Hoshikawa T, Miyashita M, Oguri T. Contribution of the body parts to throwing performance. Biomech IV. 1974;5:169-174.
7. Weber AE, Kontaxis A, O’Brien SJ, Bedi A. The biomechanics of throwing: simplified and cogent. Sports Med Arthrosc Rev. 2014;22(2):72-79.
8. Werner SL, Fleisig GS, Dillman CJ, Andrews JR. Biomechanics of the elbow during baseball pitching. J Orthop Sports Phys Ther. 1993;17(6):274-278.
9. Chang ES, Greco NJ, McClincy MP, Bradley JP. Posterior shoulder instability in overhead athletes. Orthop Clin North Am. 2016;47(1):179-187.
10. Digiovine NM, Jobe FW, Pink M, Perry J. An electromyographic analysis of the upper extremity in pitching. J Shoulder Elbow Surg. 1992;1(1):15-25.
11. Axe M, Hurd W, Snyder-Mackler L. Data-based interval throwing programs for baseball players. Sports Health. 2009;1(2):145-153.
12. Reinold MM, Wilk KE, Reed J, Crenshaw K, Andrews JR. Interval sport programs: guidelines for baseball, tennis, and golf. J Orthop Sports Phys Ther. 2002;32(6):293-298.
13. Fleisig GS, Zheng N, Barrentine SW, Escamilla RF, Andrews JR, Lemak LF. Kinematic and kinetic comparison of full and partial effort baseball pitching. Conference proceedings of the 20th Annual Meeting. Atlanta, GA: American Society of Biomechanics; 1996:151-152.
14. Fleisig GS, Hsu WK, Fortenbaugh D, Cordover A, Press JM. Trunk axial rotation in baseball pitching and batting. Sports Biomech. 2013;12(4):324-333.
15. Monti R. Return to hitting: an interval hitting progression and overview of hitting mechanics following injury. Int J Sports Phys Ther. 2015;10(7):1059-1073.
16. Welch CM, Banks SA, Cook FF, Draovitch P. Hitting a baseball: a biomechanical description. J Orthop Sports Phys Ther. 1995;22(5):193-201.
17. Axe MJ, Snyder-Mackler L, Konin JG, Strube MJ. Development of a distance-based interval throwing program for Little League-aged athletes. Am J Sports Med. 1996;24(5):594-602.
18. Fitzgerald GK, Axe MJ, Snyder-Mackler L. Proposed practice guidelines for nonoperative anterior cruciate ligament rehabilitation of physically active individuals. J Orthop Sports Phys Ther. 2000;30(4):194-203.
19. Hegedus EJ, McDonough S, Bleakley C, Cook CE, Baxter GD. Clinician-friendly lower extremity physical performance measures in athletes: a systematic review of measurement properties and correlation with injury, part 1. The tests for knee function including the hop tests. Br J Sports Med. 2015;49(10):642-648.
20. Higuchi T, Nagami T, Morohoshi J, Nakata H, Kanosue K. Disturbance in hitting accuracy by professional and collegiate baseball players due to intentional change of target position. Percept Mot Skills. 2013;116(2):627-639.
1. Dillman CJ, Fleisig GS, Andrews JR. Biomechanics of pitching with emphasis upon shoulder kinematics. J Orthop Sports Phys Ther. 1993;18(2):402-408.
2. Fleisig GS, Andrews JR, Dillman CJ, Escamilla RF. Kinetics of baseball pitching with implications about injury mechanisms. Am J Sports Med. 1995;23(2):233-239.
3. Fleisig GS, Barrentine SW, Escamilla RF, Andrews JR. Biomechanics of overhand throwing with implications for injuries. Sports Med Auckl NZ. 1996;21(6):421-437.
4. Meister K. Injuries to the shoulder in the throwing athlete. Part one: Biomechanics/pathophysiology/classification of injury. Am J Sports Med. 2000;28(2):265-275.
5. Kaczmarek PK, Lubiatowski P, Cisowski P, et al. Shoulder problems in overhead sports. Part I - biomechanics of throwing. Pol Orthop Traumatol. 2014;79:50-58.
6. Toyoshima S, Hoshikawa T, Miyashita M, Oguri T. Contribution of the body parts to throwing performance. Biomech IV. 1974;5:169-174.
7. Weber AE, Kontaxis A, O’Brien SJ, Bedi A. The biomechanics of throwing: simplified and cogent. Sports Med Arthrosc Rev. 2014;22(2):72-79.
8. Werner SL, Fleisig GS, Dillman CJ, Andrews JR. Biomechanics of the elbow during baseball pitching. J Orthop Sports Phys Ther. 1993;17(6):274-278.
9. Chang ES, Greco NJ, McClincy MP, Bradley JP. Posterior shoulder instability in overhead athletes. Orthop Clin North Am. 2016;47(1):179-187.
10. Digiovine NM, Jobe FW, Pink M, Perry J. An electromyographic analysis of the upper extremity in pitching. J Shoulder Elbow Surg. 1992;1(1):15-25.
11. Axe M, Hurd W, Snyder-Mackler L. Data-based interval throwing programs for baseball players. Sports Health. 2009;1(2):145-153.
12. Reinold MM, Wilk KE, Reed J, Crenshaw K, Andrews JR. Interval sport programs: guidelines for baseball, tennis, and golf. J Orthop Sports Phys Ther. 2002;32(6):293-298.
13. Fleisig GS, Zheng N, Barrentine SW, Escamilla RF, Andrews JR, Lemak LF. Kinematic and kinetic comparison of full and partial effort baseball pitching. Conference proceedings of the 20th Annual Meeting. Atlanta, GA: American Society of Biomechanics; 1996:151-152.
14. Fleisig GS, Hsu WK, Fortenbaugh D, Cordover A, Press JM. Trunk axial rotation in baseball pitching and batting. Sports Biomech. 2013;12(4):324-333.
15. Monti R. Return to hitting: an interval hitting progression and overview of hitting mechanics following injury. Int J Sports Phys Ther. 2015;10(7):1059-1073.
16. Welch CM, Banks SA, Cook FF, Draovitch P. Hitting a baseball: a biomechanical description. J Orthop Sports Phys Ther. 1995;22(5):193-201.
17. Axe MJ, Snyder-Mackler L, Konin JG, Strube MJ. Development of a distance-based interval throwing program for Little League-aged athletes. Am J Sports Med. 1996;24(5):594-602.
18. Fitzgerald GK, Axe MJ, Snyder-Mackler L. Proposed practice guidelines for nonoperative anterior cruciate ligament rehabilitation of physically active individuals. J Orthop Sports Phys Ther. 2000;30(4):194-203.
19. Hegedus EJ, McDonough S, Bleakley C, Cook CE, Baxter GD. Clinician-friendly lower extremity physical performance measures in athletes: a systematic review of measurement properties and correlation with injury, part 1. The tests for knee function including the hop tests. Br J Sports Med. 2015;49(10):642-648.
20. Higuchi T, Nagami T, Morohoshi J, Nakata H, Kanosue K. Disturbance in hitting accuracy by professional and collegiate baseball players due to intentional change of target position. Percept Mot Skills. 2013;116(2):627-639.
Predicting and Preventing Injury in Major League Baseball
Major league baseball (MLB) is one of the most popular sports in the United States, with an average annual viewership of 11 million for the All-Star game and almost 14 million for the World Series.1 MLB has an average annual revenue of almost $10 billion, while the net worth of all 30 MLB teams combined is estimated at $36 billion; an increase of 48% from 1 year ago.2 As the sport continues to grow in popularity and receives more social media coverage, several issues, specifically injuries to its players, have come to the forefront of the news. Injuries to MLB players, specifically pitchers, have become a significant concern in recent years. The active and extended rosters in MLB include 750 and 1200 athletes, respectively, with approximately 360 active spots taken up by pitchers.3 Hence, MLB employs a large number of elite athletes within its organization. It is important to understand not only what injuries are occurring in these athletes, but also how these injuries may be prevented.
Epidemiology
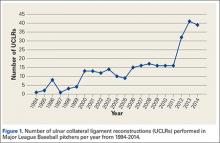
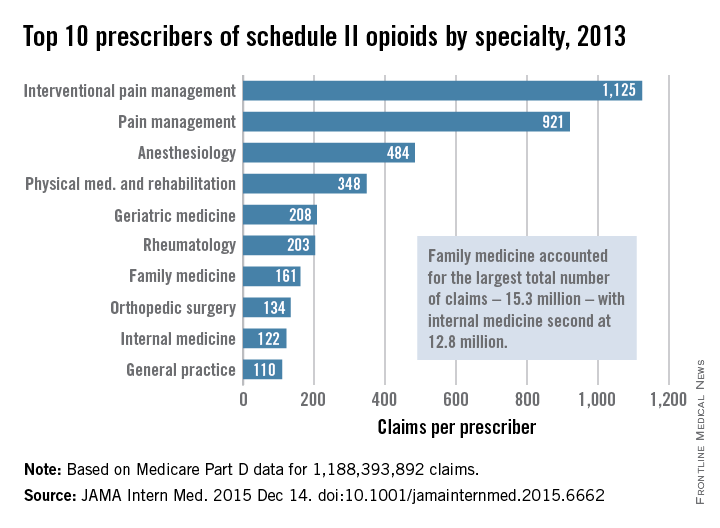
Injuries to MLB players, specifically pitchers, have increased over the past several years.4 Between 2005 and 2008, there was an overall increase of 37% in total number of injuries, with more injuries occurring in pitchers than any other position.5 While position players are more likely to sustain an injury to the lower extremity, pitchers are more likely to sustain an injury to the upper extremity.5 The month with the most injuries to MLB players was April, while the fewest number of injuries occurred in September.5 One injury that has been in the spotlight due to its dramatically increasing incidence is tear of the ulnar collateral ligament (UCL). Several studies have shown that the number of pitchers undergoing ulnar collateral ligament reconstruction (UCLR), commonly known as Tommy John surgery, has significantly increased over the past 20 years (Figure 1).4,6 Between 25% to 33% of all MLB pitchers have undergone UCLR.
While the number of primary UCLR in MLB pitchers has become a significant concern, an even more pressing concern is the number of pitchers undergoing revision UCLR, as this number has increased over the past several years.7 Currently, there is some debate as to how to best address the UCL during primary UCLR (graft type, exposure, treatment of the ulnar nerve, and graft fixation methods) because no study has shown one fixation method or graft type to be superior to others. Similarly, no study has definitively proven how to best manage the ulnar nerve (transpose in all patients, only transpose if preoperative symptoms of numbness/tingling, subluxation, etc. exist). Unfortunately, the results following revision UCLR are inferior to those following primary UCLR.4,7,8 Hence, given this information, it is imperative to both determine and implement strategies aimed at minimizing the need for revision.
Risk Factors for Injury
Although MLB has received more media attention than lower levels of baseball competition, there is relatively sparse evidence surrounding injury risk factors among MLB players. The majority of studies performed have evaluated risk factors for injury in younger baseball athletes (adolescent, high school, and college). The number of athletes at these lower levels sustaining injuries has increased over the past several years as well.9 Several large prospective studies have evaluated risk factors for shoulder and elbow injuries in adolescent baseball players. The risk factors include pitching year-round, pitching more than 100 innings per year, high pitch counts, pitching for multiple teams, geography, pitching on consecutive days, pitching while fatigued, breaking pitches, higher elbow valgus torque, pitching with higher velocity, pitching with supraspinatus weakness, and pitching with a glenohumeral internal rotation deficit (GIRD).10-17 The large majority of these risk factors are essentially part of a pitcher’s cumulative work, which consists of number of games pitched, total pitches thrown, total innings pitched, innings pitched per game, and pitches thrown per game. One prior study has evaluated cumulative work as a predictor for injury in MLB pitchers.18 While there were several issues with the study methodology, the authors found no correlation between a MLB pitcher’s cumulative work and risk for injury.
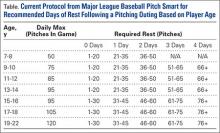
Given our current understanding of repetitive microtrauma as the pathophysiology behind these injuries, it remains unclear why cumulative work would be predictive of injury in youth pitchers but not in MLB pitchers.16 Several potential reasons exist as to why cumulative work may relate to risk of injury in youth pitchers and not MLB pitchers. Achieving MLB status may infer the element of natural selection based on technique and talent that supersedes the effect of “cumulative trauma” in many players. In MLB pitchers, cumulative work is closely monitored. In addition, these players are only playing for a single team and are not pitching competitively year-round, while many youth players play for multiple teams and may pitch year-round. To combat youth injuries, MLB Pitch Smart has developed recommendations on pitch counts and days of rest for pitchers of all age groups (Table).19 While data do not yet exist to clearly demonstrate the effectiveness of these guidelines, given the risk factors previously mentioned, it seems that these recommendations will show some reduction in youth injuries in years to come.
Some studies have evaluated anatomic variation among pitchers as a risk factor for injury. Polster and colleagues20 performed computed tomography (CT) scans with 3-dimensional reconstructions on the humeri of both the throwing and non-throwing arms of 25 MLB pitchers to determine if humeral torsion was related to the incidence and severity of upper extremity injuries in these athletes. The authors defined a severe injury as those which kept the player out for >30 days. Overall, 11 pitchers were injured during the 2-year study period. There was a strong inverse relationship between torsion and injury severity such that lower degrees of dominant humeral torsion correlated with higher injury severity (P = .005). However, neither throwing arm humeral torsion nor the difference in torsion between throwing and non-throwing humeri were predictive of overall injury incidence. While this is a nonmodifiable risk factor, it is important to understand how the pitcher’s anatomy plays a role in risk of injury.20 Understanding nonmodifiable risk factors may be helpful in the future to risk stratify, prognosticate, and modulate modifiable risk factors such as cumulative work.
Elbow
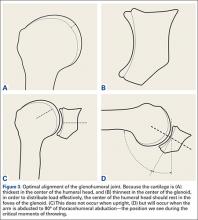
Injuries to the elbow have become more common in recent years amongst MLB players, although the literature regarding risk factors for elbow injuries is sparse.4,6 Wilk and colleagues21 performed a prospective study to determine if deficits in glenohumeral passive range of motion (ROM) increased the risk of elbow injury in MLB pitchers. Between 2005-2012, the authors measured passive shoulder ROM of both the throwing and non-throwing shoulder of 296 major and minor league pitchers and followed them for a median of 53.4 months. In total, 38 players suffered 49 elbow injuries and required 8 surgeries, accounting for a total of 2551 days spent on the disabled list (DL). GIRD and external rotation insufficiency were not correlated with elbow injuries. However, pitchers with deficits of >5° in total rotation between the throwing and non-throwing shoulders had a 2.6 times greater risk for injury (P = .007) and pitchers with deficits of ≥5° in flexion of the throwing shoulder compared to the non-throwing shoulder had a 2.8 times greater risk for injury (P = .008).21 Prior studies have demonstrated trends towards increased elbow injury in professional baseball pitchers with an increase in both elbow valgus torque as well as shoulder external rotation torque; maximum pitch velocity was also shown to be an independent risk factor for elbow injury in professional baseball pitchers.10,11 These injuries typically occur during the late cocking/early acceleration phase of the pitching cycle, when the shoulder and elbow experience the most significant force of any point in time during a pitch (Figure 2).17 At our institution, there are several ongoing studies to determine the relative contributions of pitch velocity, number, and type to elbow injury rates. Prospective studies are also ongoing at other institutions.
Shoulder
Shoulder injuries are one of the most common injuries seen in MLB players, specifically pitchers. Similar to the prior study, Wilk and colleagues22 recently performed a prospective study to determine if passive ROM of the glenohumeral joint in MLB pitchers was predictive of shoulder injury or shoulder surgery. As in the previous study, the authors’ measured passive shoulder ROM of the throwing and non-throwing shoulder of 296 major and minor league pitchers during spring training between 2005-2012 and obtained an average follow-up of 48.4 months. The authors found a total of 75 shoulder injuries and 20 surgeries among 51 pitchers (17%) that resulted in 5570 days on the DL. While total rotation deficit, GIRD, and flexion deficit had no relation to shoulder injury or surgery, pitchers with <5° greater external rotation in the throwing shoulder compared to the non-throwing shoulder were more than 2 times more likely to be placed on the DL for a shoulder injury (P = .014) and were 4 times more likely to require shoulder surgery (P = .009).22 The authors concluded that an insufficient side-to-side difference in external rotation of the throwing shoulder increased a pitcher’s likelihood of shoulder injury as well as surgery.
Other
One area that has not received as much attention as repetitive use injuries of the shoulder and elbow is acute collision injuries. Collision injuries include concussions, hyperextension injuries to the knees, shoulder dislocations, fractures of the foot and ankle, and others.23 Catchers and base runners during scoring plays are at a high risk for collision injury. Recent evidence has shown that catchers average approximately 2.75 collision injuries per 1000 athletic exposures (AE), accounting for an average of 39.1 days on the DL per collision injury.23 However, despite these collision injuries, catchers spend more time on the DL from non-collision injuries (specifically shoulder injuries requiring surgical intervention), as studies have shown 19 different non-collision injuries that accounted for >100 days on the DL for catchers compared to no collision injuries that caused a catcher to be on the DL for >100 days.23 The position of catcher is not an independent risk factor for sustaining an injury in MLB players.5
Preventative Measures
Given that recent evidence has identified certain modifiable risk factors, largely regarding shoulder ROM, for injuries to MLB pitchers, it stands to reason that by modifying these risk factors, the number of injuries to MLB pitchers can be decreased.21,22 However, to the authors’ knowledge, there have been no studies in the current literature that have clearly demonstrated the ability to prevent injuries in MLB players. Based on the prior studies, it seems logical that lowering peak pitch velocity and ensuring proper shoulder ROM would help prevent injuries in MLB players, but this remains speculative. Stretching techniques that have been shown to increase posterior shoulder soft tissue flexibility, including sleeper stretches and modified cross-body stretches, as well as closely monitoring ROM may be helpful in modifying these risk factors.24-26
Although the number of collision injuries is significantly lower than non-collision repetitive use injuries, MLB has implemented rule changes in recent years to prevent injuries to catchers and base runners alike.23,27 The rule change, which went into effect in 2014, prohibits catchers from blocking home plate unless they are actively fielding the ball or are in possession of the ball. Similarly, base runners are not allowed to deviate from their path to collide with the catcher while attempting to score.27 However, no study has analyzed whether this rule change has decreased the number of collision injuries sustained by MLB catchers, so it is unclear if this rule change has accomplished its goal.
Outcomes Following Injuries
One of the driving forces behind injury prevention in MLB players is to allow players to reach and maintain their full potential while minimizing time missed because of injury. Furthermore, as with any sport, the clinical outcomes and return to sport (RTS) rates for MLB players following injuries, especially injuries requiring surgical intervention, can be improved.4,28,29 Several studies have evaluated MLB pitchers following UCLR and have shown that over 80% of pitchers are able to RTS following surgery.4,30 When critically evaluated in multiple statistical parameters upon RTS, these players perform better in some areas and worse in others.4,30 However, the results following revision UCLR are not as encouraging as those following primary UCLR in MLB pitchers.7 Following revision UCLR, only 65% of pitchers were able to RTS, and those who were able to RTS pitched, on average, almost 1 year less than matched controls.7 Unfortunately, results following surgeries about the shoulder in MLB players have been worse than those about the elbow. Cohen and colleagues28 reported on 22 MLB players who underwent labral repair of the shoulder and found that only 32% were able to return to the same or higher level following surgery, while over 45% retired from baseball following surgery. Hence, it is imperative these injuries are prevented, as the RTS rate following treatment is less than ideal.
Future Directions
Although a concerted effort has been made over the past several years to mitigate the number of injuries sustained by MLB players, there is still significant room for improvement. New products are in development/early stages of use that attempt to determine when a pitcher begins to show signs of fatigue to allow the coach to remove him from the game. The mTHROW sleeve (Motus Global), currently used by several MLB teams, is an elastic sleeve that is worn by pitchers on their dominant arm. The sleeve approximates torque, velocity, and workload based upon an accelerometer positioned at the medial elbow and sends this information to a smart phone in real time. This technology theoretically allows players to be intensively monitored and thus may prevent injuries to the UCL by preventing pitchers from throwing while fatigued. However, elbow kinematic parameters may not change significantly as pitchers fatigue, which suggests that this strategy may be suboptimal. Trunk mechanics do change as pitchers become fatigued, opening up the possibility for shoulder and elbow injury.17,31,32 Further products that track hip-to-shoulder separation and trunk fatigue may be necessary to truly lower injury rates. However, no study has proven modifying either parameter leads to a decrease in injury rates.
Conclusion
Injuries to MLB pitchers and position players have become a significant concern over the past several years. Several risk factors for injury have been identified, including loss of shoulder ROM and pitch velocity. Further studies are necessary to determine the effectiveness of modifying these parameters on injury prevention.
1. Statista. Major League Baseball average TV viewership - selected games 2014 season (in million viewers) 2015 [cited 2015 December 12]. Available at: http://www.statista.com/statistics/251536/average-tv-viewership-of-selected-major-league-baseball-games/. Accessed December 12, 2015.
2. Ozanian M. MLB worth $36 billion as team values hit record $1.2 billion average. Forbes website. Available at: http://www.forbes.com/sites/mikeozanian/2015/03/25/mlb-worth-36-billion-as-team-values-hit-record-1-2-billion-average/. Accessed December 12, 2015.
3. Castrovince A. Equitable roster rules needed for September. Major League Baseball website. Available at: http://m.mlb.com/news/article/39009416. Accessed December 12, 2015.
4. Erickson BJ, Gupta AK, Harris JD, et al. Rate of return to pitching and performance after Tommy John Surgery in Major League Baseball pitchers. Am J Sports Med. 2014;42(3):536-543.
5. Posner M, Cameron KL, Wolf JM, Belmont PJ Jr, Owens BD. Epidemiology of Major League Baseball injuries. Am J Sports Med. 2011;39(8):1676-1680.
6. Conte SA, Fleisig GS, Dines JS, et al. Prevalence of ulnar collateral ligament surgery in professional baseball players. Am J Sports Med. 2015;43(7):1764-1769.
7. Marshall NE, Keller RA, Lynch JR, Bey MJ, Moutzouros V. Pitching performance and longevity after revision ulnar collateral ligament reconstruction in Major League Baseball pitchers. Am J Sports Med. 2015;43(5):1051-1056.
8. Wilson AT, Pidgeon TS, Morrell NT, DaSilva MF. Trends in revision elbow ulnar collateral ligament reconstruction in professional baseball pitchers. J Hand Surg Am. 2015;40(11):2249-2254.
9. Cain EL Jr, Andrews JR, Dugas JR, et al. Outcome of ulnar collateral ligament reconstruction of the elbow in 1281 athletes: Results in 743 athletes with minimum 2-year follow-up. Am J Sports Med. 2010;38(12):2426-2434.
10. Anz AW, Bushnell BD, Griffin LP, Noonan TJ, Torry MR, Hawkins RJ. Correlation of torque and elbow injury in professional baseball pitchers. Am J Sports Med. 2010;38(7):1368-1374.
11. Bushnell BD, Anz AW, Noonan TJ, Torry MR, Hawkins RJ. Association of maximum pitch velocity and elbow injury in professional baseball pitchers. Am J Sports Med 2010;38(4):728-732.
12. Byram IR, Bushnell BD, Dugger K, Charron K, Harrell FE Jr, Noonan TJ. Preseason shoulder strength measurements in professional baseball pitchers: identifying players at risk for injury. Am J Sports Med. 2010;38(7):1375-1382.
13. Dines JS, Frank JB, Akerman M, Yocum LA. Glenohumeral internal rotation deficits in baseball players with ulnar collateral ligament insufficiency. Am J Sports Med. 2009;37(3):566-570.
14. Petty DH, Andrews JR, Fleisig GS, Cain EL. Ulnar collateral ligament reconstruction in high school baseball players: clinical results and injury risk factors. Am J Sports Med. 2004;32(5):1158-1164.
15. Lyman S, Fleisig GS, Andrews JR, Osinski ED. Effect of pitch type, pitch count, and pitching mechanics on risk of elbow and shoulder pain in youth baseball pitchers. Am J Sports Med. 2002;30(4):463-468.
16. Fleisig GS, Andrews JR, Cutter GR, et al. Risk of serious injury for young baseball pitchers: a 10-year prospective study. Am J Sports Med. 2011;39(2):253-257.
17. Fleisig GS, Andrews JR, Dillman CJ, Escamilla RF. Kinetics of baseball pitching with implications about injury mechanisms. Am J Sports Med. 1995;23(2):233-239.
18. Karakolis T, Bhan S, Crotin RL. An inferential and descriptive statistical examination of the relationship between cumulative work metrics and injury in Major League Baseball pitchers. J Strength Cond Res. 2013;27(8):2113-2118.
19. Smart MP. Guidelines for youth and adolescent pitchers. Major League Baseball website. Available at: http://m.mlb.com/pitchsmart/pitching-guidelines/. Accessed January 3, 2016.
20. Polster JM, Bullen J, Obuchowski NA, Bryan JA, Soloff L, Schickendantz MS. Relationship between humeral torsion and injury in professional baseball pitchers. Am J Sports Med. 2013;41(9):2015-2021.
21. Wilk KE, Macrina LC, Fleisig GS, et al. Deficits in glenohumeral passive range of motion increase risk of elbow injury in professional baseball pitchers: a prospective study. Am J Sports Med. 2014;42(9):2075-2081.
22. Wilk KE, Macrina LC, Fleisig GS, et al. Deficits in glenohumeral passive range of motion increase risk of shoulder injury in professional baseball pitchers: a prospective study. Am J Sports Med. 2015;43(10):2379-2385.
23. Kilcoyne KG, Ebel BG, Bancells RL, Wilckens JH, McFarland EG. Epidemiology of injuries in Major League Baseball catchers. Am J Sports Med. 2015;43(10):2496-2500.
24. Wilk KE, Hooks TR, Macrina LC. The modified sleeper stretch and modified cross-body stretch to increase shoulder internal rotation range of motion in the overhead throwing athlete. J Orthop Sports Phys Ther. 2013;43(12):891-894.
25. Laudner KG, Sipes RC, Wilson JT. The acute effects of sleeper stretches on shoulder range of motion. J Athl Train. 2008;43(4):359-363.
26. McClure P, Balaicuis J, Heiland D, Broersma ME, Thorndike CK, Wood A. A randomized controlled comparison of stretching procedures for posterior shoulder tightness. J Orthop Sports Phys Ther. 2007;37(3):108-114.
27. Major League Baseball. MLB, MLBPA adopt experimental rule 7.13 on home plate collisions. Major League Baseball website. Available from: http://m.mlb.com/news/article/68268622/mlb-mlbpa-adopt-experimental-rule-713-on-home-plate-collisions. Accessed December 2, 2015.
28. Cohen SB, Sheridan S, Ciccotti MG. Return to sports for professional baseball players after surgery of the shoulder or elbow. Sports Health. 2011;3(1):105-111.
29. Wasserman EB, Abar B, Shah MN, Wasserman D, Bazarian JJ. Concussions are associated with decreased batting performance among Major League Baseball Players. Am J Sports Med. 2015;43(5):1127-1133.
30. Jiang JJ, Leland JM. Analysis of pitching velocity in major league baseball players before and after ulnar collateral ligament reconstruction. Am J Sports Med. 2014;42(4):880-885.
31. Crotin RL, Kozlowski K, Horvath P, Ramsey DK. Altered stride length in response to increasing exertion among baseball pitchers. Med Sci Sports Exerc. 2014;46(3):565-571.
32. Escamilla RF, Barrentine SW, Fleisig GS, et al. Pitching biomechanics as a pitcher approaches muscular fatigue during a simulated baseball game. Am J Sports Med. 2007;35(1):23-33.
Major league baseball (MLB) is one of the most popular sports in the United States, with an average annual viewership of 11 million for the All-Star game and almost 14 million for the World Series.1 MLB has an average annual revenue of almost $10 billion, while the net worth of all 30 MLB teams combined is estimated at $36 billion; an increase of 48% from 1 year ago.2 As the sport continues to grow in popularity and receives more social media coverage, several issues, specifically injuries to its players, have come to the forefront of the news. Injuries to MLB players, specifically pitchers, have become a significant concern in recent years. The active and extended rosters in MLB include 750 and 1200 athletes, respectively, with approximately 360 active spots taken up by pitchers.3 Hence, MLB employs a large number of elite athletes within its organization. It is important to understand not only what injuries are occurring in these athletes, but also how these injuries may be prevented.
Epidemiology
Injuries to MLB players, specifically pitchers, have increased over the past several years.4 Between 2005 and 2008, there was an overall increase of 37% in total number of injuries, with more injuries occurring in pitchers than any other position.5 While position players are more likely to sustain an injury to the lower extremity, pitchers are more likely to sustain an injury to the upper extremity.5 The month with the most injuries to MLB players was April, while the fewest number of injuries occurred in September.5 One injury that has been in the spotlight due to its dramatically increasing incidence is tear of the ulnar collateral ligament (UCL). Several studies have shown that the number of pitchers undergoing ulnar collateral ligament reconstruction (UCLR), commonly known as Tommy John surgery, has significantly increased over the past 20 years (Figure 1).4,6 Between 25% to 33% of all MLB pitchers have undergone UCLR.
While the number of primary UCLR in MLB pitchers has become a significant concern, an even more pressing concern is the number of pitchers undergoing revision UCLR, as this number has increased over the past several years.7 Currently, there is some debate as to how to best address the UCL during primary UCLR (graft type, exposure, treatment of the ulnar nerve, and graft fixation methods) because no study has shown one fixation method or graft type to be superior to others. Similarly, no study has definitively proven how to best manage the ulnar nerve (transpose in all patients, only transpose if preoperative symptoms of numbness/tingling, subluxation, etc. exist). Unfortunately, the results following revision UCLR are inferior to those following primary UCLR.4,7,8 Hence, given this information, it is imperative to both determine and implement strategies aimed at minimizing the need for revision.
Risk Factors for Injury
Although MLB has received more media attention than lower levels of baseball competition, there is relatively sparse evidence surrounding injury risk factors among MLB players. The majority of studies performed have evaluated risk factors for injury in younger baseball athletes (adolescent, high school, and college). The number of athletes at these lower levels sustaining injuries has increased over the past several years as well.9 Several large prospective studies have evaluated risk factors for shoulder and elbow injuries in adolescent baseball players. The risk factors include pitching year-round, pitching more than 100 innings per year, high pitch counts, pitching for multiple teams, geography, pitching on consecutive days, pitching while fatigued, breaking pitches, higher elbow valgus torque, pitching with higher velocity, pitching with supraspinatus weakness, and pitching with a glenohumeral internal rotation deficit (GIRD).10-17 The large majority of these risk factors are essentially part of a pitcher’s cumulative work, which consists of number of games pitched, total pitches thrown, total innings pitched, innings pitched per game, and pitches thrown per game. One prior study has evaluated cumulative work as a predictor for injury in MLB pitchers.18 While there were several issues with the study methodology, the authors found no correlation between a MLB pitcher’s cumulative work and risk for injury.
Given our current understanding of repetitive microtrauma as the pathophysiology behind these injuries, it remains unclear why cumulative work would be predictive of injury in youth pitchers but not in MLB pitchers.16 Several potential reasons exist as to why cumulative work may relate to risk of injury in youth pitchers and not MLB pitchers. Achieving MLB status may infer the element of natural selection based on technique and talent that supersedes the effect of “cumulative trauma” in many players. In MLB pitchers, cumulative work is closely monitored. In addition, these players are only playing for a single team and are not pitching competitively year-round, while many youth players play for multiple teams and may pitch year-round. To combat youth injuries, MLB Pitch Smart has developed recommendations on pitch counts and days of rest for pitchers of all age groups (Table).19 While data do not yet exist to clearly demonstrate the effectiveness of these guidelines, given the risk factors previously mentioned, it seems that these recommendations will show some reduction in youth injuries in years to come.
Some studies have evaluated anatomic variation among pitchers as a risk factor for injury. Polster and colleagues20 performed computed tomography (CT) scans with 3-dimensional reconstructions on the humeri of both the throwing and non-throwing arms of 25 MLB pitchers to determine if humeral torsion was related to the incidence and severity of upper extremity injuries in these athletes. The authors defined a severe injury as those which kept the player out for >30 days. Overall, 11 pitchers were injured during the 2-year study period. There was a strong inverse relationship between torsion and injury severity such that lower degrees of dominant humeral torsion correlated with higher injury severity (P = .005). However, neither throwing arm humeral torsion nor the difference in torsion between throwing and non-throwing humeri were predictive of overall injury incidence. While this is a nonmodifiable risk factor, it is important to understand how the pitcher’s anatomy plays a role in risk of injury.20 Understanding nonmodifiable risk factors may be helpful in the future to risk stratify, prognosticate, and modulate modifiable risk factors such as cumulative work.
Elbow
Injuries to the elbow have become more common in recent years amongst MLB players, although the literature regarding risk factors for elbow injuries is sparse.4,6 Wilk and colleagues21 performed a prospective study to determine if deficits in glenohumeral passive range of motion (ROM) increased the risk of elbow injury in MLB pitchers. Between 2005-2012, the authors measured passive shoulder ROM of both the throwing and non-throwing shoulder of 296 major and minor league pitchers and followed them for a median of 53.4 months. In total, 38 players suffered 49 elbow injuries and required 8 surgeries, accounting for a total of 2551 days spent on the disabled list (DL). GIRD and external rotation insufficiency were not correlated with elbow injuries. However, pitchers with deficits of >5° in total rotation between the throwing and non-throwing shoulders had a 2.6 times greater risk for injury (P = .007) and pitchers with deficits of ≥5° in flexion of the throwing shoulder compared to the non-throwing shoulder had a 2.8 times greater risk for injury (P = .008).21 Prior studies have demonstrated trends towards increased elbow injury in professional baseball pitchers with an increase in both elbow valgus torque as well as shoulder external rotation torque; maximum pitch velocity was also shown to be an independent risk factor for elbow injury in professional baseball pitchers.10,11 These injuries typically occur during the late cocking/early acceleration phase of the pitching cycle, when the shoulder and elbow experience the most significant force of any point in time during a pitch (Figure 2).17 At our institution, there are several ongoing studies to determine the relative contributions of pitch velocity, number, and type to elbow injury rates. Prospective studies are also ongoing at other institutions.
Shoulder
Shoulder injuries are one of the most common injuries seen in MLB players, specifically pitchers. Similar to the prior study, Wilk and colleagues22 recently performed a prospective study to determine if passive ROM of the glenohumeral joint in MLB pitchers was predictive of shoulder injury or shoulder surgery. As in the previous study, the authors’ measured passive shoulder ROM of the throwing and non-throwing shoulder of 296 major and minor league pitchers during spring training between 2005-2012 and obtained an average follow-up of 48.4 months. The authors found a total of 75 shoulder injuries and 20 surgeries among 51 pitchers (17%) that resulted in 5570 days on the DL. While total rotation deficit, GIRD, and flexion deficit had no relation to shoulder injury or surgery, pitchers with <5° greater external rotation in the throwing shoulder compared to the non-throwing shoulder were more than 2 times more likely to be placed on the DL for a shoulder injury (P = .014) and were 4 times more likely to require shoulder surgery (P = .009).22 The authors concluded that an insufficient side-to-side difference in external rotation of the throwing shoulder increased a pitcher’s likelihood of shoulder injury as well as surgery.
Other
One area that has not received as much attention as repetitive use injuries of the shoulder and elbow is acute collision injuries. Collision injuries include concussions, hyperextension injuries to the knees, shoulder dislocations, fractures of the foot and ankle, and others.23 Catchers and base runners during scoring plays are at a high risk for collision injury. Recent evidence has shown that catchers average approximately 2.75 collision injuries per 1000 athletic exposures (AE), accounting for an average of 39.1 days on the DL per collision injury.23 However, despite these collision injuries, catchers spend more time on the DL from non-collision injuries (specifically shoulder injuries requiring surgical intervention), as studies have shown 19 different non-collision injuries that accounted for >100 days on the DL for catchers compared to no collision injuries that caused a catcher to be on the DL for >100 days.23 The position of catcher is not an independent risk factor for sustaining an injury in MLB players.5
Preventative Measures
Given that recent evidence has identified certain modifiable risk factors, largely regarding shoulder ROM, for injuries to MLB pitchers, it stands to reason that by modifying these risk factors, the number of injuries to MLB pitchers can be decreased.21,22 However, to the authors’ knowledge, there have been no studies in the current literature that have clearly demonstrated the ability to prevent injuries in MLB players. Based on the prior studies, it seems logical that lowering peak pitch velocity and ensuring proper shoulder ROM would help prevent injuries in MLB players, but this remains speculative. Stretching techniques that have been shown to increase posterior shoulder soft tissue flexibility, including sleeper stretches and modified cross-body stretches, as well as closely monitoring ROM may be helpful in modifying these risk factors.24-26
Although the number of collision injuries is significantly lower than non-collision repetitive use injuries, MLB has implemented rule changes in recent years to prevent injuries to catchers and base runners alike.23,27 The rule change, which went into effect in 2014, prohibits catchers from blocking home plate unless they are actively fielding the ball or are in possession of the ball. Similarly, base runners are not allowed to deviate from their path to collide with the catcher while attempting to score.27 However, no study has analyzed whether this rule change has decreased the number of collision injuries sustained by MLB catchers, so it is unclear if this rule change has accomplished its goal.
Outcomes Following Injuries
One of the driving forces behind injury prevention in MLB players is to allow players to reach and maintain their full potential while minimizing time missed because of injury. Furthermore, as with any sport, the clinical outcomes and return to sport (RTS) rates for MLB players following injuries, especially injuries requiring surgical intervention, can be improved.4,28,29 Several studies have evaluated MLB pitchers following UCLR and have shown that over 80% of pitchers are able to RTS following surgery.4,30 When critically evaluated in multiple statistical parameters upon RTS, these players perform better in some areas and worse in others.4,30 However, the results following revision UCLR are not as encouraging as those following primary UCLR in MLB pitchers.7 Following revision UCLR, only 65% of pitchers were able to RTS, and those who were able to RTS pitched, on average, almost 1 year less than matched controls.7 Unfortunately, results following surgeries about the shoulder in MLB players have been worse than those about the elbow. Cohen and colleagues28 reported on 22 MLB players who underwent labral repair of the shoulder and found that only 32% were able to return to the same or higher level following surgery, while over 45% retired from baseball following surgery. Hence, it is imperative these injuries are prevented, as the RTS rate following treatment is less than ideal.
Future Directions
Although a concerted effort has been made over the past several years to mitigate the number of injuries sustained by MLB players, there is still significant room for improvement. New products are in development/early stages of use that attempt to determine when a pitcher begins to show signs of fatigue to allow the coach to remove him from the game. The mTHROW sleeve (Motus Global), currently used by several MLB teams, is an elastic sleeve that is worn by pitchers on their dominant arm. The sleeve approximates torque, velocity, and workload based upon an accelerometer positioned at the medial elbow and sends this information to a smart phone in real time. This technology theoretically allows players to be intensively monitored and thus may prevent injuries to the UCL by preventing pitchers from throwing while fatigued. However, elbow kinematic parameters may not change significantly as pitchers fatigue, which suggests that this strategy may be suboptimal. Trunk mechanics do change as pitchers become fatigued, opening up the possibility for shoulder and elbow injury.17,31,32 Further products that track hip-to-shoulder separation and trunk fatigue may be necessary to truly lower injury rates. However, no study has proven modifying either parameter leads to a decrease in injury rates.
Conclusion
Injuries to MLB pitchers and position players have become a significant concern over the past several years. Several risk factors for injury have been identified, including loss of shoulder ROM and pitch velocity. Further studies are necessary to determine the effectiveness of modifying these parameters on injury prevention.
Major league baseball (MLB) is one of the most popular sports in the United States, with an average annual viewership of 11 million for the All-Star game and almost 14 million for the World Series.1 MLB has an average annual revenue of almost $10 billion, while the net worth of all 30 MLB teams combined is estimated at $36 billion; an increase of 48% from 1 year ago.2 As the sport continues to grow in popularity and receives more social media coverage, several issues, specifically injuries to its players, have come to the forefront of the news. Injuries to MLB players, specifically pitchers, have become a significant concern in recent years. The active and extended rosters in MLB include 750 and 1200 athletes, respectively, with approximately 360 active spots taken up by pitchers.3 Hence, MLB employs a large number of elite athletes within its organization. It is important to understand not only what injuries are occurring in these athletes, but also how these injuries may be prevented.
Epidemiology
Injuries to MLB players, specifically pitchers, have increased over the past several years.4 Between 2005 and 2008, there was an overall increase of 37% in total number of injuries, with more injuries occurring in pitchers than any other position.5 While position players are more likely to sustain an injury to the lower extremity, pitchers are more likely to sustain an injury to the upper extremity.5 The month with the most injuries to MLB players was April, while the fewest number of injuries occurred in September.5 One injury that has been in the spotlight due to its dramatically increasing incidence is tear of the ulnar collateral ligament (UCL). Several studies have shown that the number of pitchers undergoing ulnar collateral ligament reconstruction (UCLR), commonly known as Tommy John surgery, has significantly increased over the past 20 years (Figure 1).4,6 Between 25% to 33% of all MLB pitchers have undergone UCLR.
While the number of primary UCLR in MLB pitchers has become a significant concern, an even more pressing concern is the number of pitchers undergoing revision UCLR, as this number has increased over the past several years.7 Currently, there is some debate as to how to best address the UCL during primary UCLR (graft type, exposure, treatment of the ulnar nerve, and graft fixation methods) because no study has shown one fixation method or graft type to be superior to others. Similarly, no study has definitively proven how to best manage the ulnar nerve (transpose in all patients, only transpose if preoperative symptoms of numbness/tingling, subluxation, etc. exist). Unfortunately, the results following revision UCLR are inferior to those following primary UCLR.4,7,8 Hence, given this information, it is imperative to both determine and implement strategies aimed at minimizing the need for revision.
Risk Factors for Injury
Although MLB has received more media attention than lower levels of baseball competition, there is relatively sparse evidence surrounding injury risk factors among MLB players. The majority of studies performed have evaluated risk factors for injury in younger baseball athletes (adolescent, high school, and college). The number of athletes at these lower levels sustaining injuries has increased over the past several years as well.9 Several large prospective studies have evaluated risk factors for shoulder and elbow injuries in adolescent baseball players. The risk factors include pitching year-round, pitching more than 100 innings per year, high pitch counts, pitching for multiple teams, geography, pitching on consecutive days, pitching while fatigued, breaking pitches, higher elbow valgus torque, pitching with higher velocity, pitching with supraspinatus weakness, and pitching with a glenohumeral internal rotation deficit (GIRD).10-17 The large majority of these risk factors are essentially part of a pitcher’s cumulative work, which consists of number of games pitched, total pitches thrown, total innings pitched, innings pitched per game, and pitches thrown per game. One prior study has evaluated cumulative work as a predictor for injury in MLB pitchers.18 While there were several issues with the study methodology, the authors found no correlation between a MLB pitcher’s cumulative work and risk for injury.
Given our current understanding of repetitive microtrauma as the pathophysiology behind these injuries, it remains unclear why cumulative work would be predictive of injury in youth pitchers but not in MLB pitchers.16 Several potential reasons exist as to why cumulative work may relate to risk of injury in youth pitchers and not MLB pitchers. Achieving MLB status may infer the element of natural selection based on technique and talent that supersedes the effect of “cumulative trauma” in many players. In MLB pitchers, cumulative work is closely monitored. In addition, these players are only playing for a single team and are not pitching competitively year-round, while many youth players play for multiple teams and may pitch year-round. To combat youth injuries, MLB Pitch Smart has developed recommendations on pitch counts and days of rest for pitchers of all age groups (Table).19 While data do not yet exist to clearly demonstrate the effectiveness of these guidelines, given the risk factors previously mentioned, it seems that these recommendations will show some reduction in youth injuries in years to come.
Some studies have evaluated anatomic variation among pitchers as a risk factor for injury. Polster and colleagues20 performed computed tomography (CT) scans with 3-dimensional reconstructions on the humeri of both the throwing and non-throwing arms of 25 MLB pitchers to determine if humeral torsion was related to the incidence and severity of upper extremity injuries in these athletes. The authors defined a severe injury as those which kept the player out for >30 days. Overall, 11 pitchers were injured during the 2-year study period. There was a strong inverse relationship between torsion and injury severity such that lower degrees of dominant humeral torsion correlated with higher injury severity (P = .005). However, neither throwing arm humeral torsion nor the difference in torsion between throwing and non-throwing humeri were predictive of overall injury incidence. While this is a nonmodifiable risk factor, it is important to understand how the pitcher’s anatomy plays a role in risk of injury.20 Understanding nonmodifiable risk factors may be helpful in the future to risk stratify, prognosticate, and modulate modifiable risk factors such as cumulative work.
Elbow
Injuries to the elbow have become more common in recent years amongst MLB players, although the literature regarding risk factors for elbow injuries is sparse.4,6 Wilk and colleagues21 performed a prospective study to determine if deficits in glenohumeral passive range of motion (ROM) increased the risk of elbow injury in MLB pitchers. Between 2005-2012, the authors measured passive shoulder ROM of both the throwing and non-throwing shoulder of 296 major and minor league pitchers and followed them for a median of 53.4 months. In total, 38 players suffered 49 elbow injuries and required 8 surgeries, accounting for a total of 2551 days spent on the disabled list (DL). GIRD and external rotation insufficiency were not correlated with elbow injuries. However, pitchers with deficits of >5° in total rotation between the throwing and non-throwing shoulders had a 2.6 times greater risk for injury (P = .007) and pitchers with deficits of ≥5° in flexion of the throwing shoulder compared to the non-throwing shoulder had a 2.8 times greater risk for injury (P = .008).21 Prior studies have demonstrated trends towards increased elbow injury in professional baseball pitchers with an increase in both elbow valgus torque as well as shoulder external rotation torque; maximum pitch velocity was also shown to be an independent risk factor for elbow injury in professional baseball pitchers.10,11 These injuries typically occur during the late cocking/early acceleration phase of the pitching cycle, when the shoulder and elbow experience the most significant force of any point in time during a pitch (Figure 2).17 At our institution, there are several ongoing studies to determine the relative contributions of pitch velocity, number, and type to elbow injury rates. Prospective studies are also ongoing at other institutions.
Shoulder
Shoulder injuries are one of the most common injuries seen in MLB players, specifically pitchers. Similar to the prior study, Wilk and colleagues22 recently performed a prospective study to determine if passive ROM of the glenohumeral joint in MLB pitchers was predictive of shoulder injury or shoulder surgery. As in the previous study, the authors’ measured passive shoulder ROM of the throwing and non-throwing shoulder of 296 major and minor league pitchers during spring training between 2005-2012 and obtained an average follow-up of 48.4 months. The authors found a total of 75 shoulder injuries and 20 surgeries among 51 pitchers (17%) that resulted in 5570 days on the DL. While total rotation deficit, GIRD, and flexion deficit had no relation to shoulder injury or surgery, pitchers with <5° greater external rotation in the throwing shoulder compared to the non-throwing shoulder were more than 2 times more likely to be placed on the DL for a shoulder injury (P = .014) and were 4 times more likely to require shoulder surgery (P = .009).22 The authors concluded that an insufficient side-to-side difference in external rotation of the throwing shoulder increased a pitcher’s likelihood of shoulder injury as well as surgery.
Other
One area that has not received as much attention as repetitive use injuries of the shoulder and elbow is acute collision injuries. Collision injuries include concussions, hyperextension injuries to the knees, shoulder dislocations, fractures of the foot and ankle, and others.23 Catchers and base runners during scoring plays are at a high risk for collision injury. Recent evidence has shown that catchers average approximately 2.75 collision injuries per 1000 athletic exposures (AE), accounting for an average of 39.1 days on the DL per collision injury.23 However, despite these collision injuries, catchers spend more time on the DL from non-collision injuries (specifically shoulder injuries requiring surgical intervention), as studies have shown 19 different non-collision injuries that accounted for >100 days on the DL for catchers compared to no collision injuries that caused a catcher to be on the DL for >100 days.23 The position of catcher is not an independent risk factor for sustaining an injury in MLB players.5
Preventative Measures
Given that recent evidence has identified certain modifiable risk factors, largely regarding shoulder ROM, for injuries to MLB pitchers, it stands to reason that by modifying these risk factors, the number of injuries to MLB pitchers can be decreased.21,22 However, to the authors’ knowledge, there have been no studies in the current literature that have clearly demonstrated the ability to prevent injuries in MLB players. Based on the prior studies, it seems logical that lowering peak pitch velocity and ensuring proper shoulder ROM would help prevent injuries in MLB players, but this remains speculative. Stretching techniques that have been shown to increase posterior shoulder soft tissue flexibility, including sleeper stretches and modified cross-body stretches, as well as closely monitoring ROM may be helpful in modifying these risk factors.24-26
Although the number of collision injuries is significantly lower than non-collision repetitive use injuries, MLB has implemented rule changes in recent years to prevent injuries to catchers and base runners alike.23,27 The rule change, which went into effect in 2014, prohibits catchers from blocking home plate unless they are actively fielding the ball or are in possession of the ball. Similarly, base runners are not allowed to deviate from their path to collide with the catcher while attempting to score.27 However, no study has analyzed whether this rule change has decreased the number of collision injuries sustained by MLB catchers, so it is unclear if this rule change has accomplished its goal.
Outcomes Following Injuries
One of the driving forces behind injury prevention in MLB players is to allow players to reach and maintain their full potential while minimizing time missed because of injury. Furthermore, as with any sport, the clinical outcomes and return to sport (RTS) rates for MLB players following injuries, especially injuries requiring surgical intervention, can be improved.4,28,29 Several studies have evaluated MLB pitchers following UCLR and have shown that over 80% of pitchers are able to RTS following surgery.4,30 When critically evaluated in multiple statistical parameters upon RTS, these players perform better in some areas and worse in others.4,30 However, the results following revision UCLR are not as encouraging as those following primary UCLR in MLB pitchers.7 Following revision UCLR, only 65% of pitchers were able to RTS, and those who were able to RTS pitched, on average, almost 1 year less than matched controls.7 Unfortunately, results following surgeries about the shoulder in MLB players have been worse than those about the elbow. Cohen and colleagues28 reported on 22 MLB players who underwent labral repair of the shoulder and found that only 32% were able to return to the same or higher level following surgery, while over 45% retired from baseball following surgery. Hence, it is imperative these injuries are prevented, as the RTS rate following treatment is less than ideal.
Future Directions
Although a concerted effort has been made over the past several years to mitigate the number of injuries sustained by MLB players, there is still significant room for improvement. New products are in development/early stages of use that attempt to determine when a pitcher begins to show signs of fatigue to allow the coach to remove him from the game. The mTHROW sleeve (Motus Global), currently used by several MLB teams, is an elastic sleeve that is worn by pitchers on their dominant arm. The sleeve approximates torque, velocity, and workload based upon an accelerometer positioned at the medial elbow and sends this information to a smart phone in real time. This technology theoretically allows players to be intensively monitored and thus may prevent injuries to the UCL by preventing pitchers from throwing while fatigued. However, elbow kinematic parameters may not change significantly as pitchers fatigue, which suggests that this strategy may be suboptimal. Trunk mechanics do change as pitchers become fatigued, opening up the possibility for shoulder and elbow injury.17,31,32 Further products that track hip-to-shoulder separation and trunk fatigue may be necessary to truly lower injury rates. However, no study has proven modifying either parameter leads to a decrease in injury rates.
Conclusion
Injuries to MLB pitchers and position players have become a significant concern over the past several years. Several risk factors for injury have been identified, including loss of shoulder ROM and pitch velocity. Further studies are necessary to determine the effectiveness of modifying these parameters on injury prevention.
1. Statista. Major League Baseball average TV viewership - selected games 2014 season (in million viewers) 2015 [cited 2015 December 12]. Available at: http://www.statista.com/statistics/251536/average-tv-viewership-of-selected-major-league-baseball-games/. Accessed December 12, 2015.
2. Ozanian M. MLB worth $36 billion as team values hit record $1.2 billion average. Forbes website. Available at: http://www.forbes.com/sites/mikeozanian/2015/03/25/mlb-worth-36-billion-as-team-values-hit-record-1-2-billion-average/. Accessed December 12, 2015.
3. Castrovince A. Equitable roster rules needed for September. Major League Baseball website. Available at: http://m.mlb.com/news/article/39009416. Accessed December 12, 2015.
4. Erickson BJ, Gupta AK, Harris JD, et al. Rate of return to pitching and performance after Tommy John Surgery in Major League Baseball pitchers. Am J Sports Med. 2014;42(3):536-543.
5. Posner M, Cameron KL, Wolf JM, Belmont PJ Jr, Owens BD. Epidemiology of Major League Baseball injuries. Am J Sports Med. 2011;39(8):1676-1680.
6. Conte SA, Fleisig GS, Dines JS, et al. Prevalence of ulnar collateral ligament surgery in professional baseball players. Am J Sports Med. 2015;43(7):1764-1769.
7. Marshall NE, Keller RA, Lynch JR, Bey MJ, Moutzouros V. Pitching performance and longevity after revision ulnar collateral ligament reconstruction in Major League Baseball pitchers. Am J Sports Med. 2015;43(5):1051-1056.
8. Wilson AT, Pidgeon TS, Morrell NT, DaSilva MF. Trends in revision elbow ulnar collateral ligament reconstruction in professional baseball pitchers. J Hand Surg Am. 2015;40(11):2249-2254.
9. Cain EL Jr, Andrews JR, Dugas JR, et al. Outcome of ulnar collateral ligament reconstruction of the elbow in 1281 athletes: Results in 743 athletes with minimum 2-year follow-up. Am J Sports Med. 2010;38(12):2426-2434.
10. Anz AW, Bushnell BD, Griffin LP, Noonan TJ, Torry MR, Hawkins RJ. Correlation of torque and elbow injury in professional baseball pitchers. Am J Sports Med. 2010;38(7):1368-1374.
11. Bushnell BD, Anz AW, Noonan TJ, Torry MR, Hawkins RJ. Association of maximum pitch velocity and elbow injury in professional baseball pitchers. Am J Sports Med 2010;38(4):728-732.
12. Byram IR, Bushnell BD, Dugger K, Charron K, Harrell FE Jr, Noonan TJ. Preseason shoulder strength measurements in professional baseball pitchers: identifying players at risk for injury. Am J Sports Med. 2010;38(7):1375-1382.
13. Dines JS, Frank JB, Akerman M, Yocum LA. Glenohumeral internal rotation deficits in baseball players with ulnar collateral ligament insufficiency. Am J Sports Med. 2009;37(3):566-570.
14. Petty DH, Andrews JR, Fleisig GS, Cain EL. Ulnar collateral ligament reconstruction in high school baseball players: clinical results and injury risk factors. Am J Sports Med. 2004;32(5):1158-1164.
15. Lyman S, Fleisig GS, Andrews JR, Osinski ED. Effect of pitch type, pitch count, and pitching mechanics on risk of elbow and shoulder pain in youth baseball pitchers. Am J Sports Med. 2002;30(4):463-468.
16. Fleisig GS, Andrews JR, Cutter GR, et al. Risk of serious injury for young baseball pitchers: a 10-year prospective study. Am J Sports Med. 2011;39(2):253-257.
17. Fleisig GS, Andrews JR, Dillman CJ, Escamilla RF. Kinetics of baseball pitching with implications about injury mechanisms. Am J Sports Med. 1995;23(2):233-239.
18. Karakolis T, Bhan S, Crotin RL. An inferential and descriptive statistical examination of the relationship between cumulative work metrics and injury in Major League Baseball pitchers. J Strength Cond Res. 2013;27(8):2113-2118.
19. Smart MP. Guidelines for youth and adolescent pitchers. Major League Baseball website. Available at: http://m.mlb.com/pitchsmart/pitching-guidelines/. Accessed January 3, 2016.
20. Polster JM, Bullen J, Obuchowski NA, Bryan JA, Soloff L, Schickendantz MS. Relationship between humeral torsion and injury in professional baseball pitchers. Am J Sports Med. 2013;41(9):2015-2021.
21. Wilk KE, Macrina LC, Fleisig GS, et al. Deficits in glenohumeral passive range of motion increase risk of elbow injury in professional baseball pitchers: a prospective study. Am J Sports Med. 2014;42(9):2075-2081.
22. Wilk KE, Macrina LC, Fleisig GS, et al. Deficits in glenohumeral passive range of motion increase risk of shoulder injury in professional baseball pitchers: a prospective study. Am J Sports Med. 2015;43(10):2379-2385.
23. Kilcoyne KG, Ebel BG, Bancells RL, Wilckens JH, McFarland EG. Epidemiology of injuries in Major League Baseball catchers. Am J Sports Med. 2015;43(10):2496-2500.
24. Wilk KE, Hooks TR, Macrina LC. The modified sleeper stretch and modified cross-body stretch to increase shoulder internal rotation range of motion in the overhead throwing athlete. J Orthop Sports Phys Ther. 2013;43(12):891-894.
25. Laudner KG, Sipes RC, Wilson JT. The acute effects of sleeper stretches on shoulder range of motion. J Athl Train. 2008;43(4):359-363.
26. McClure P, Balaicuis J, Heiland D, Broersma ME, Thorndike CK, Wood A. A randomized controlled comparison of stretching procedures for posterior shoulder tightness. J Orthop Sports Phys Ther. 2007;37(3):108-114.
27. Major League Baseball. MLB, MLBPA adopt experimental rule 7.13 on home plate collisions. Major League Baseball website. Available from: http://m.mlb.com/news/article/68268622/mlb-mlbpa-adopt-experimental-rule-713-on-home-plate-collisions. Accessed December 2, 2015.
28. Cohen SB, Sheridan S, Ciccotti MG. Return to sports for professional baseball players after surgery of the shoulder or elbow. Sports Health. 2011;3(1):105-111.
29. Wasserman EB, Abar B, Shah MN, Wasserman D, Bazarian JJ. Concussions are associated with decreased batting performance among Major League Baseball Players. Am J Sports Med. 2015;43(5):1127-1133.
30. Jiang JJ, Leland JM. Analysis of pitching velocity in major league baseball players before and after ulnar collateral ligament reconstruction. Am J Sports Med. 2014;42(4):880-885.
31. Crotin RL, Kozlowski K, Horvath P, Ramsey DK. Altered stride length in response to increasing exertion among baseball pitchers. Med Sci Sports Exerc. 2014;46(3):565-571.
32. Escamilla RF, Barrentine SW, Fleisig GS, et al. Pitching biomechanics as a pitcher approaches muscular fatigue during a simulated baseball game. Am J Sports Med. 2007;35(1):23-33.
1. Statista. Major League Baseball average TV viewership - selected games 2014 season (in million viewers) 2015 [cited 2015 December 12]. Available at: http://www.statista.com/statistics/251536/average-tv-viewership-of-selected-major-league-baseball-games/. Accessed December 12, 2015.
2. Ozanian M. MLB worth $36 billion as team values hit record $1.2 billion average. Forbes website. Available at: http://www.forbes.com/sites/mikeozanian/2015/03/25/mlb-worth-36-billion-as-team-values-hit-record-1-2-billion-average/. Accessed December 12, 2015.
3. Castrovince A. Equitable roster rules needed for September. Major League Baseball website. Available at: http://m.mlb.com/news/article/39009416. Accessed December 12, 2015.
4. Erickson BJ, Gupta AK, Harris JD, et al. Rate of return to pitching and performance after Tommy John Surgery in Major League Baseball pitchers. Am J Sports Med. 2014;42(3):536-543.
5. Posner M, Cameron KL, Wolf JM, Belmont PJ Jr, Owens BD. Epidemiology of Major League Baseball injuries. Am J Sports Med. 2011;39(8):1676-1680.
6. Conte SA, Fleisig GS, Dines JS, et al. Prevalence of ulnar collateral ligament surgery in professional baseball players. Am J Sports Med. 2015;43(7):1764-1769.
7. Marshall NE, Keller RA, Lynch JR, Bey MJ, Moutzouros V. Pitching performance and longevity after revision ulnar collateral ligament reconstruction in Major League Baseball pitchers. Am J Sports Med. 2015;43(5):1051-1056.
8. Wilson AT, Pidgeon TS, Morrell NT, DaSilva MF. Trends in revision elbow ulnar collateral ligament reconstruction in professional baseball pitchers. J Hand Surg Am. 2015;40(11):2249-2254.
9. Cain EL Jr, Andrews JR, Dugas JR, et al. Outcome of ulnar collateral ligament reconstruction of the elbow in 1281 athletes: Results in 743 athletes with minimum 2-year follow-up. Am J Sports Med. 2010;38(12):2426-2434.
10. Anz AW, Bushnell BD, Griffin LP, Noonan TJ, Torry MR, Hawkins RJ. Correlation of torque and elbow injury in professional baseball pitchers. Am J Sports Med. 2010;38(7):1368-1374.
11. Bushnell BD, Anz AW, Noonan TJ, Torry MR, Hawkins RJ. Association of maximum pitch velocity and elbow injury in professional baseball pitchers. Am J Sports Med 2010;38(4):728-732.
12. Byram IR, Bushnell BD, Dugger K, Charron K, Harrell FE Jr, Noonan TJ. Preseason shoulder strength measurements in professional baseball pitchers: identifying players at risk for injury. Am J Sports Med. 2010;38(7):1375-1382.
13. Dines JS, Frank JB, Akerman M, Yocum LA. Glenohumeral internal rotation deficits in baseball players with ulnar collateral ligament insufficiency. Am J Sports Med. 2009;37(3):566-570.
14. Petty DH, Andrews JR, Fleisig GS, Cain EL. Ulnar collateral ligament reconstruction in high school baseball players: clinical results and injury risk factors. Am J Sports Med. 2004;32(5):1158-1164.
15. Lyman S, Fleisig GS, Andrews JR, Osinski ED. Effect of pitch type, pitch count, and pitching mechanics on risk of elbow and shoulder pain in youth baseball pitchers. Am J Sports Med. 2002;30(4):463-468.
16. Fleisig GS, Andrews JR, Cutter GR, et al. Risk of serious injury for young baseball pitchers: a 10-year prospective study. Am J Sports Med. 2011;39(2):253-257.
17. Fleisig GS, Andrews JR, Dillman CJ, Escamilla RF. Kinetics of baseball pitching with implications about injury mechanisms. Am J Sports Med. 1995;23(2):233-239.
18. Karakolis T, Bhan S, Crotin RL. An inferential and descriptive statistical examination of the relationship between cumulative work metrics and injury in Major League Baseball pitchers. J Strength Cond Res. 2013;27(8):2113-2118.
19. Smart MP. Guidelines for youth and adolescent pitchers. Major League Baseball website. Available at: http://m.mlb.com/pitchsmart/pitching-guidelines/. Accessed January 3, 2016.
20. Polster JM, Bullen J, Obuchowski NA, Bryan JA, Soloff L, Schickendantz MS. Relationship between humeral torsion and injury in professional baseball pitchers. Am J Sports Med. 2013;41(9):2015-2021.
21. Wilk KE, Macrina LC, Fleisig GS, et al. Deficits in glenohumeral passive range of motion increase risk of elbow injury in professional baseball pitchers: a prospective study. Am J Sports Med. 2014;42(9):2075-2081.
22. Wilk KE, Macrina LC, Fleisig GS, et al. Deficits in glenohumeral passive range of motion increase risk of shoulder injury in professional baseball pitchers: a prospective study. Am J Sports Med. 2015;43(10):2379-2385.
23. Kilcoyne KG, Ebel BG, Bancells RL, Wilckens JH, McFarland EG. Epidemiology of injuries in Major League Baseball catchers. Am J Sports Med. 2015;43(10):2496-2500.
24. Wilk KE, Hooks TR, Macrina LC. The modified sleeper stretch and modified cross-body stretch to increase shoulder internal rotation range of motion in the overhead throwing athlete. J Orthop Sports Phys Ther. 2013;43(12):891-894.
25. Laudner KG, Sipes RC, Wilson JT. The acute effects of sleeper stretches on shoulder range of motion. J Athl Train. 2008;43(4):359-363.
26. McClure P, Balaicuis J, Heiland D, Broersma ME, Thorndike CK, Wood A. A randomized controlled comparison of stretching procedures for posterior shoulder tightness. J Orthop Sports Phys Ther. 2007;37(3):108-114.
27. Major League Baseball. MLB, MLBPA adopt experimental rule 7.13 on home plate collisions. Major League Baseball website. Available from: http://m.mlb.com/news/article/68268622/mlb-mlbpa-adopt-experimental-rule-713-on-home-plate-collisions. Accessed December 2, 2015.
28. Cohen SB, Sheridan S, Ciccotti MG. Return to sports for professional baseball players after surgery of the shoulder or elbow. Sports Health. 2011;3(1):105-111.
29. Wasserman EB, Abar B, Shah MN, Wasserman D, Bazarian JJ. Concussions are associated with decreased batting performance among Major League Baseball Players. Am J Sports Med. 2015;43(5):1127-1133.
30. Jiang JJ, Leland JM. Analysis of pitching velocity in major league baseball players before and after ulnar collateral ligament reconstruction. Am J Sports Med. 2014;42(4):880-885.
31. Crotin RL, Kozlowski K, Horvath P, Ramsey DK. Altered stride length in response to increasing exertion among baseball pitchers. Med Sci Sports Exerc. 2014;46(3):565-571.
32. Escamilla RF, Barrentine SW, Fleisig GS, et al. Pitching biomechanics as a pitcher approaches muscular fatigue during a simulated baseball game. Am J Sports Med. 2007;35(1):23-33.
Valgus Extension Overload in Baseball Players
The supraphysiological demands imposed on the elbow of a throwing athlete result in predictable patterns of injury. This is especially true of baseball pitchers. Knowledge of elbow anatomy, as well as the biomechanics of throwing, assist in making diagnostic and therapeutic decisions and also influence surgical technique when surgery is required. During the late cocking and early acceleration phases of throwing, valgus torque can reach 65 Nm with angular velocities of the forearm reaching 5000°/sec, which is considered the fasted recorded human movment.1 The valgus torque and rapid extension synergistically create 3 major forces placed on the elbow. The first is a tensile stress along the medial aspect of elbow affecting the ulnar collateral ligament (UCL), flexor pronator mass, and medial epicondyle. Secondly, compression forces affect the lateral aspect of the elbow at the radiocapitellar joint. Finally, a shearing stress occurs in the posterior compartment at the posterior medial tip of the olecranon and the olecranon fossa.
These forces generated on the elbow result in predictable pathology. The recurring tensile forces applied on the medial aspect on the elbow can compromise the integrity of the UCL. It is well known that injury to the UCL leads to valgus instability. Individuals with valgus instability who continue to throw may trigger and/or aggravate injury in the posterior and lateral components of the elbow. Lateral compression forces can often reach 500 N, resulting in radiocapitellar overload syndrome, which occurs in combination with medial ligament instability and valgus extension overload.2 Radiocapitellar compression may cause chondral or osteochondral fracture with resulting intra-articular loose bodes. This compression also contributes to the etiology of osteochondritis dissecans (OCD) in skeletally immature athletes. In the posterior elbow, throwing forcefully and repeatedly pushes the olecranon into the olecranon fossa. Shear stress on the medial olecranon tip and fossa, due to combined valgus and extension forces, lead to the development of osteophytes. This collection of injuries in the medial, lateral, and posterior aspects of the elbow is known as “valgus extension overload syndrome” or VEO. Symptoms in VEO can be the result of chondral lesions, loose bodies, and marginal exostosis.3
The aim of this review is to provide understanding regarding both the relevant anatomy and pathomechanics of VEO, key aspects to clinical evaluation, and effective treatment options.
Functional Anatomy
A functional comprehension of elbow anatomy and biomechanics is essential to understanding the constellation of injuries in VEO. The osseous anatomy of the elbow permits a variety of movements. These include flexion-extension and pronation-supination, which are mediated by the ulnohumeral and radiocapitellar articulations. While in full extension, the elbow has a normal valgus carrying angle of 11° to 16°. It is important to know that 50% of the elbow’s stability is attributed to the configuration of the bones.4-6 This is especially true in varus stress while the elbow is in full extension. The soft tissues, including muscle and ligaments such as the UCL, lateral UCL, and radial UCL complexes, provide the remaining elbow stability.4-6
The UCL complex is composed of 3 main segments known as the anterior, posterior, and oblique bundles (transverse ligament). Collectively, these bundles are responsible for providing medial elbow stability. However, each of these bundles contributes to medial elbow stability in its own way. The first and arguably the most important bundle is the anterior bundle; its most important function is providing stability against valgus stress.4,5,7 It is composed of parallel fibers inserting on the medial coronoid process.4,5,7 Furthermore, its eccentric location with respect to the axis of elbow allows it to provide stability throughout the full range of elbow motion.6 The anterior bundle can be further divided into individual anterior and posterior bands that have reciprocal functionality.5,8,9 The anterior band acts as the chief restraint to valgus stress up to 90° of flexion.9 Any flexion beyond 90° renders the anterior band’s role secondary in resisting valgus stress.9 The posterior band’s function in resisting valgus stress is most important between 60° and full flexion, while having a secondary role in lesser degrees of flexion.8,9 Notably, the posterior band is isometric and is more important in the overhead-throwing athlete due to the fact its primary role in resisting valgus stress occurs at higher degrees of flexion.10
The remaining posterior and oblique bundles of the UCL complex have lesser roles in maintaining elbow stability. The posterior bundle of the UCL complex is fan-shaped, originates from the medial epicondyle, and inserts onto the medial margin the semi-lunar notch. It is more slender and frailer than the anterior bundle. This is reflected in its functionality, as it plays a secondary role in elbow stability during elbow flexion beyond 90°.4,5,8 In contrast to the anterior and posterior bundles, the oblique bundle, also known as the transverse ligament, does not cross the elbow joint. It is a thickening of the caudal most aspect of the joint capsule, which extends from the medial olecranon to the inferior medial coronoid process and as a result functions in expanding the greater sigmoid notch.6
The musculotendinous components of the elbow are essential to providing dynamic functional resistance to valgus stress.11 These components are flexor-pronator musculature that originate from the medial epicondyle. Listed proximally to distally, the flexor-pronator muscles include pronator teres, flexor carpi radialis (FCR), palmaris longus, flexor digitorum superficialis, and the flexor carpi ulnaris (FCU).
Pathomechanics
Once familiarized with the relevant function anatomy, it is crucial to understand the mechanics of throwing in order to understand the pathomechanics of VEO. The action of overhead throwing has been divided into 6 phases.6,12-16 Phase 4, acceleration, is the most relevant when discussing forces on elbow, since the majority of forces are generated during this state. Phase 4 represents a rapid acceleration of the upper extremity with a large forward-directed force on the arm generated by the shoulder muscles. Additionally, there is internal rotation and adduction of the humerus with rapid elbow extension terminating with ball release. The elbow accelerates up to 600,000°/sec2 in a miniscule time frame of 40 to 50 milliseconds.1,5 Immense valgus forces are exerted on the medial aspect of the elbow. The anterior bundle of the UCL bears the majority of the force, with the flexor pronator mass enabling the transmission.11 The majority of injuries occur during stage 4 as a result of the stress load on the medial elbow structures like the UCL. The proceeding phases 5 (deceleration), and 6 (follow-through) involve eventual dissipation of excess kinetic energy as the elbow completely extends. The deceleration during phase 5 is rapid and powerful, occurring at about 500,000°/sec2 in the short span of 50 milliseconds.1,6,12-16 High-velocity throwing, such as baseball pitching, generates forces in the elbow that are opposed by the articular, ligamentous, and muscular portions of the arm. The ulnohumeral articulation stabilizes motion of the arm from 0° to 20° of flexion and beyond 120° of flexion. Static and dynamic soft tissues maintain stability during the remaining of 100° arc of motion.
During deceleration, the elbow undergoes terminal extension resulting in the posteromedial olecranon contacting the trochlea and the olecranon fossa with subsequent dissipation of the combined valgus force and angular moment (Figure 1). This dissipation of force creates pathologic shear and compressive forces in the posterior elbow. Poor muscular control and the traumatic abutment that occurs in the posterior compartment may further add to the pathologic forces. Reactive bone formation is induced by the repetitive compression and shear, resulting in osteophytes on the posteromedial tip of the olecranon (Figure 2). Consequent “kissing lesions” of chondromalacia may occur in the olecranon fossa and posteromedial trochlea. The subsequent development of loose bodies may also occur. The presence of osteophytes and/or loose bodies may result in posteromedial impingement (PMI).
The association between PMI of the olecranon and valgus instability has been elucidated in both clinical and biomechanical investigations.17,18,19,20 Conway18 identified tip exostosis in 24% of lateral radiographs of 135 asymptomatic professional pitchers. Approximately one-fifth (21%) of these pitchers had >1.0 mm increased relative valgus laxity on stress radiographs. Roughly one-third (34%) of players with exostosis had >1.0 mm of increased relative valgus laxity, compared to 16% of players without exostosis formation. These results provide evidence for a probable association between PMI and valgus laxity. In biomechanical research, Ahmad and colleagues17 studied the effect of partial and full thickness UCL injuries on contact forces of the posterior elbow. Posteromedial compartments of cadaver specimens were subjected to physiologic valgus stresses while placed on pressure-senstive film. Contact area and pressure between posteromedial trochlea and olecranon were altered in the setting of UCL insufficiency, helping explain how posteromedial osteophyte formation occurs.
Additional biomechanical studies have also investigated the posteromedial olecranon’s role in functioning as a stabilizing buttress to medial tensile forces. Treating PMI with aggressive bone removal may increase valgus instability as well as strain on the UCL, leading to UCL injury following olecranon resection.19,20 Kamineni and colleagues19 investigated strain on anterior bundle of UCL as a function of increasing applied torque and posteromedial resections of the olecranon. This investigation was done utilizing an electromagnetic tracking placed in cadaver elbows. A nonuniform change in strain was found at 3 mm of resection during flexion and valgus testing. This nonuniform change implied that removal of posteromedial olecranon beyond 3 mm made the UCL more vulnerable to injury. Follow-up investigations looked at kinematic effects of increasing valgus and varus torques and sequential posteromedial olecranon resections.20 Valgus angulation of the elbow increased with all resection levels but no critical amount of olecranon resection was identified. The consensus in the literature indicates that posteromedial articulation of the elbow is a significant stabilizer to valgus stress.17-22 Thus, normal bone should be preserved and only osteophytes should be removed during treatment.
In addition, VEO may lead to injury in the lateral compartment as well. After attenuation and insufficiency of the UCL due to repetitive stress, excessive force transmission to the lateral aspect of the elbow occurs. Compressive and rotatory forces escalate within the radiocapitellar joint, causing synovitis and osteochondral lesions.3,23 These osteochondral lesions include osteochondritis dissecans and osteochondral fractures that may fragment and become loose bodies.
Evaluation of VEO
History
Patients will typically have a history of repetitive throwing or other repetitive overhead activity. VEO is most common in baseball pitchers but may also occur in other sports, such as tennis, football, lacrosse, gymnastics, and javelin throwing. In baseball pitchers, clinical presentation is often preceded by a decrease in pitch velocity, control, and early fatigability. It presents with elbow pain localized to the posteromedial aspect of olecranon after release of the ball, when the elbow reaches terminal extension. Patients also report limited extension, due to impinging posterior osteophytes. Also, locking and catching caused by loose bodies and chondromalacia may be present. VEO may also occur in combination with concomitant valgus instability, as well as in a patient with a prior history of valgus instability. Flexor pronator injury, ulnar neuritis, and subluxation may also be present in a patient with VEO.
Physical Examination
VEO may occur in an isolated fashion or with concomitant pathology. Therefore, a comprehensive physical examination includes evaluating the entire kinetic chain of throwing and a focused examination covering VEO and associated valgus instability. Patients may exhibit crepitus and tenderness over the posteromedial olecranon and a loss of extension with a firm end point. The extension impingement test should be performed where the elbow is snapped into terminal extension. This typically elicits pain in the posterior compartment in a patient with VEO. The arm bar test involves positioning the patient’s shoulder at 90° of forward flexion, full internal rotation, with the patient’s hand placed on the examiner’s shoulder.24 The examiner pulls down on the olecranon, simulating forced extension; pain is indicative of a positive test. It is important to note if there are signs of ulnar neuritis or subluxing ulnar nerve, especially if planning to utilize medial portals during arthroscopic treatment.
Examination maneuvers for valgus instability should also be conducted during evaluation of VEO. The physical examination for valgus instability in the elbow is ideally performed with the patient seated. Secure the patient’s wrist between the examiner’s forearm and trunk, and flex the patient’s elbow between 20° and 30° to unlock the olecranon from its fossa. Proceed to apply valgus stress. This stresses the anterior band of the anterior bundle of the UCL.6,25,26 Palpate the UCL from the medial epicondyle to the proximal ulna as valgus stress is applied. Occasionally, valgus laxity can be appreciated when compared to contralateral side. The milking maneuver is a helpful test to determine UCL injury. Pull on the patient’s thumb while the forearm is supinated, shoulder extended, and the elbow flexed beyond 90°.6 The milking maneuver exerts valgus stress on a flexed elbow. A patient with an injured UCL will experience the subjective feeling of apprehension and instability, with medial elbow pain.
The most sensitive test is the moving valgus stress test. This is performed with the patient in the upright position and the shoulder abducted 90°. Starting with the arm in full flexion, the examiner applies a constant valgus torque to the elbow and then rapidly extends the elbow. Reproduction of pain during range of motion from 120° to 70° represents UCL injury, while pain with extension beyond 70° represents chondral injury to the ulnohumeral joint. Be aware that the absence of increased pain with wrist flexion, along with pain localized slightly posterior to the common flexor origin, differentiates a UCL injury from flexor-pronator muscle injury.6,26,27Examine range of motion in affected and unaffected elbows. Loss of terminal extension may be present, along with secondary to flexion contracture due to repeated attempts at healing and stabilization.25
Imaging
Imaging is essential to the accurate diagnosis of VEO and related conditions. Anterior posterior (AP), lateral, and oblique radiographs of elbow (Figures 3A-3C) may show posteromedial olecranon osteophytes and/or loose bodies. Calcification of ligaments or other soft tissues may also be seen. An AP radiograph with 140° of external rotation may best visualize osteophytes on posteromedial olecranon.18 A computed tomography scan with 2-dimensional sagittal and coronal reconstruction and 3-dimensional surface rendering (Figures 4A, 4B) may best demonstrate morphological abnormalities, loose bodies, and osteophytes. Magnetic resonance imaging (MRI) is essential for assessment of soft tissues and chondral injuries. MRI may detect UCL compromise, synovial plicae, bone edema, olecranon, or stress fractures.
Treatment
Nonoperative Treatment
Treatment consists of both nonoperative and operative modalities. Nonoperative treatment methods are first line in treating VEO. Patients should modify their physical activity and rest from throwing activities. Nonsteroid anti-inflammatory drugs are appropriate to treat pain along with intra-articular corticosteroid injections of the elbow. A wide assessment of pitching mechanics should be performed in an attempt to correct errors in throwing technique and address muscular imbalances. After cessation of the resting period, the patient may initiate a progressive throwing program supervised by an experienced therapist and trainer. A plan for returning to competition should be made upon completion of the throwing program.
Operative Treatment
Surgical treatment is reserved for patients who fail nonoperative treatment. These patients have persistent symptoms of posteromedial impingement and desire to return to pre-injury level of performance. Posteromedial decompression is not recommended when provocative physical examination maneuvers are negative, regardless of presence of olecranon osteophytes on imaging. Osteophytes are an asymptomatic finding typically seen in professional baseball players and do not warrant surgical treatment.18,28 UCL compromise is a relative contraindication to olecranon debridement as UCL injury could become symptomatic following surgery. Surgical options in the appropriate patient to decompress posterior compartment include arthroscopic olecranon debridement or limited incision arthrotomy. Excessive resection of posteromedial osteophytes must be avoided. Arthroscopy has limited morbidity and allows for complete diagnostic assessment. UCL reconstruction should also be considered in combination with posteromedial debridement when the UCL is torn. More challenging indications for UCL reconstruction occur when the UCL is partially torn or torn and asymptomatic. Isolated posteromedial decompression in this setting risks future development of UCL symptoms that would then need to be addressed.
Surgical Technique
As previously mentioned, elbow arthroscopy or limited excision arthrotomy are the preferred operative methods for decompression of the posterior compartment and thus treatment of VEO. Anesthesia and patient positioning should be selected based on the surgeon’s preference. The patient should be positioned supine, prone, or in lateral decubitis. When a UCL reconstruction is expected, supine position is advantageous to avoid repositioning after completing the arthroscopic portion of the procedure. However, arthroscopy can be performed in the lateral position with subsequent repositioning, repeat prepping, and draping for UCL reconstruction (Figures 5A, 5B).
Prepare for elbow arthroscopy by distending the elbow joint with normal saline to aid in protection of neurovascular structures and simplify the insertion of the scope trocar. Perform diagnostic anterior arthroscopy via the proximal anteromedial portal. Assess for presence of loose bodies and osteochondral lesions of the radiocapitellar joint, as well as osteophytes of the coronoid tip and fossa. Utilizing a spinal needle under direct visualization establish a proximal lateral portal with adequate view of the anterior compartment. Proceed to visualize the medial compartment and assess for UCL injury. Apply valgus stress while in 70° of flexion. Visualize the coronoid process and look for medial trochlea gapping of 3 mm or greater, which indicates UCL insufficiency.30
Establish the posterolateral port for visualization of the posterior compartment. A posterior portal is established through the triceps tendon. Proceed to shave and ablate synovitis in order to create an adequate working space. Inspect the posteromedial olecranon, looking for any osteophytes or chondromalacia in the area (Figure 6). Examine the posterior radiocapitellar joint, looking specifically for loose bodies. The presence of loose bodies may require creating an extra mid lateral portal for removal. The ulnar nerve is located superficial to the elbow capsule and can be damaged by instruments utilized in the posteromedial gutter. As a precaution, be sure to remove suction attached to shaver. Place a curved articulating retractor in an accessory posterolateral portal to assist in protecting the ulnar nerve by retracting the capsule away from the surgical field (Figures 7A, 7B).
The osteophyte may be encased in soft tissue. Using a combination of ablation devices and shavers, the osteophyte can be exposed. The olecranon osteophyte can be removed with a small osteotome located at the border of the osteophyte and the normal olecranon. A motorized shaver or burr may also be introduced through the direct posterior portal or the posterolateral portal to complete the contouring of the olecranon (Figures 8A, 8B). Intraoperative lateral radiographs may be obtained for guidance in adequate bone removal and to ensure no bone debris is left in the soft tissues. It is critical that only pathologic osteophyte is removed and that normal olecranon is not compromised. This prevents an increase in UCL strain during valgus loading.19 However, in some non-throwing athletes, more aggressive debridement can be performed due to a smaller risk of UCL injury after posterior decompression.
Often, with the presence of osteophytes on the olecranon, there may be associated chondromalacia of the trochlea. These kissing lesions must be addressed after debridement of osteophytes. Loose flaps or frayed edges are carefully debrided and for any significant lesion the edges are contoured to a stable rim using shavers and curettes. Once altered to a well-shouldered lesion, microfracture is performed. Anterograde drilling of the lesion with perforations separated by 2 to 3 mm allow for the release of marrow elements and induction of a fibrocartilage healing response.
For an isolated posteromedial decompression, early rehabilitation begins with simple elbow flexion and extension exercises. It is important to restore flexor-pronator strength. Six weeks postoperatively, a progressive throwing program that includes plyometric exercises, neuromuscular training, and endurance exercises can be initiated. Patients can typically return to competition 3 to 4 months after surgery, if they have successfully regained preoperative range of motion, preoperative strength in the elbow, and there is no pain or tenderness on stress testing or palpation.
Outcomes
Safety and Advances in Arthroscopy
A clearer understanding of portal placement and proximity to neurovasculature in conjunction with advances in equipment have allowed for continual improvements in elbow arthroscopy techniques. There is plenty of literature indicating that arthroscopic posteromedial decompression is a safe, reliable, effective procedure, with a high rate of patient satisfaction.22,30-34] Andrews and Carson30 published one of the earliest investigations indicating the effectiveness of elbow arthroscopy utilizing objective and subjective outcome scores. They found that preoperative scores indicating patient satisfaction increased from 50% to 83%. Patients who underwent only loose body removal had the best outcomes. Andrews and Timmerman31 later evaluated the results of 72 professional baseball players who underwent either arthroscopic or open elbow surgery. They found that posteromedial olecranon osteophytes and intraarticular loose bodies were the most common diagnoses, present in 65% and 54% of players, respectively. In addition, a 41% reoperation rate was reported after posteromedial olecranon resection, along with 25% a rate of valgus instability necessitating UCL reconstruction. Andrews and Timmerman31 propose that the incidence of UCL injuries is underestimated and that UCL pathology must be treated prior to treating its secondary effects. Recently, Reddy and colleagues32 reviewed the results of 187 arthroscopic procedures. Posterior impingement, loose bodies, and osteoarthritis were the most common problems, occurring in 51%, 31%, and 22% of patients, respectively. Reported results were encouraging, with 87% good to excellent results and 85% of baseball players returning to preinjury levels.
Conclusion
An understanding of the relevant functional anatomy and the biomechanics of throwing is essential to understanding VEO. Potential concomitant valgus instability and UCL injury must be carefully assessed. Only symptomatic patients who have failed conservative treatment should undergo surgery. It is critical to avoid exacerbating and/or causing valgus instability by surgical excessively removing normal bone from the olecranon. Arthroscopy has been shown to be a safe and effective method to treat refractory cases of VEO.
1. Pappas AM, Zawacki RM, Sullivan TJ. Biomechanics of baseball pitching. A preliminary report. Am J Sports Med. 1985;13(4):216-222.
2. Fleisig GS, Barrentine SW, Escamilla RF, Andrews JR. Biomechanics of overhand throwing with implications for injuries. Sports Med. 1996;21(6):421-437.
3. Wilson FD, Andrews JR, Blackburn TA, McCluskey G. Valgus extension overload in the pitching elbow. Am J Sports Med. 1983;11(2):83-88.
4. Morrey BF. Applied anatomy and biomechanics of the elbow joint. Instr Course Lect. 1986,35:59-68.
5. Schwab GH, Bennett JB, Woods GW, Tullos HS. Biomechanics of elbow instability: the role of medial collateral ligament. Clin Orthop Relat Res. 1980;146:42-52.
6. Jobe FW, Kvitne RS. Elbow instability in the athlete. Instr Course Lect. 1991;40:17-23.
7. Søjbjerg JO, Ovesen J, Nielsen S. Experimental elbow instability after transection of the medial collateral ligament. Clin Orthop Relat Res. 1987;218:186-190.
8. Regan WD, Korinek SL, Morrey BF, An KN. Biomechanical study of ligaments around the elbow joint. Clin Orthop Relat Res. 1991;271:170-179.
9. Callaway GH, Field LD, Deng XH, et al. Biomechanical evaluation of the medial collateral ligament of the elbow. J Bone Joint Surg Am. 1997;79(8):1223-1231.
10. Chen FS, Rokito AS, Jobe FW. Medial elbow problems in the overhead-throwing athlete. J Am Acad Orthop Surg. 2001;9(2):99-113.
11. Davidson PA, Pink M, Perry J, Jobe FW. Functional anatomy of the flexor pronator muscle group in relation to the medial collateral ligament of the elbow. Am J Sports Med. 1995;23(2):245-250.
12. Jobe FW, Moynes DR, Tibone JE, Perry J. An EMG analysis of the shoulder in pitching. A second report. Am J Sports Med. 1984;12(3):218-220.
13. Hamilton CD, Glousman RE, Jobe FW, Brault J, Pink M, Perry J. Dynamic stability of the elbow: electromyographic analysis of the flexor pronator group and the extensor group in pitchers with valgus instability. J Shoulder Elbow Surg. 1996;5(5):347-354.
14. Glousman RE, Barron J, Jobe FW, Perry J, Pink M. An electromyographic analysis of the elbow in normal and injured pitchers with medial collateral ligament insufficiency. Am J Sports Med. 1992;20(3):311-317.
15. DiGiovine NM, Jobe FW, Pink M, Perry J. An electromyographic analysis of the upper extremity in pitching. J Shoulder Elbow Surg. 1992;1(1):15-25.
16. Sisto DJ, Jobe FW, Moynes DR, Antonelli DJ. An electromyographic analysis of the elbow in pitching. Am J Sports Med. 1987;15(3):260-263.
17. Ahmad CS, Park MC, Elattrache NS. Elbow medial ulnar collateral ligament insufficiency alters posteromedial olecranon contact. Am J Sports Med. 2004;32(7):1607–1612.
18. Ahmad CS, Conway J. Elbow arthroscopy: beginners to advanced: valgus extension overload. In: Egol, ed. Instructional Course Lectures; vol 60. Rosemont, IL: American Academy of Orthopaedic Surgeons; submitted 2009.
19. Kamineni S, ElAttrache NS, O’Driscoll S W, et al. Medial collateral ligament strain with partial posteromedial olecranon resection. A biomechanical study. J Bone Joint Surg Am. 2004;86-A(11):2424–2430.
20. Kamineni S, Hirahara H, Pomianowski S, et al. Partial posteromedial olecranon resection: a kinematic study. J Bone Joint Surg Am. 2003;85-A(6):1005–1011.
21. Morrey BF, An KN. Articular and ligamentous contributions to the stability of the elbow joint. Am J Sports Med. 1983;11(5):315–319.
22. O’Driscoll SW, Morrey BF. Arthroscopy of the elbow. Diagnostic and therapeutic benefits and hazards. J Bone Joint Surg Am. 1992;74(1):84–94.
23. Miller CD, Savoie FH 3rd. Valgus extension injuries of the elbow in the throwing athlete. J Am Acad Orthop Surg. 1994;2(5):261-269.
24. O’Driscoll SW. Valgus extension overload and plica. In: Levine WN, ed. The Athlete’s Elbow. Rosemont, IL: American Academy of Orthopaedic Surgeons; 2008:71-83.
25. Boatright JR, D’Alessandro DF. Nerve entrapment syndromes at the elbow. In Jobe FW, Pink MM, Glousman RE, Kvitne RE, Zemel NP, eds. Operative Techniques in Upper Extremity Sports Injuries. St. Louis, MO: Mosby-Year Book; 1996:518-537.
26. Conway JE, Jobe FW, Glousman RE, Pink M. Medial instability of the elbow in throwing athletes. Treatment by repair or reconstruction of the ulnar collateral ligament. J Bone Joint Surg Am. 1992;74(1):67-83.
27. Jobe FW, Stark H, Lombardo SJ. Reconstruction of the ulnar collateral ligament in athletes. J Bone Joint Surg Am. 1986;68(8):1158-1163.
28. Kooima CL, Anderson K, Craig JV, Teeter DM, van Holsbeeck M. Evidence of subclinical medial collateral ligament injury and posteromedial impingement in professional baseball players. Am J Sports Med. 2004;32(7):1602-1606.
29. Field LD, Altchek DW. Evaluation of the arthroscopic valgus instability test of the elbow. Am J Sports Med. 1996;24(2):177–181.
30. Andrews JR, Carson WG. Arthroscopy of the elbow. Arthroscopy. 1985;1(2):97-107.
31. Andrews JR, Timmerman LA. Outcome of elbow surgery in professional baseball players. Am J Sports Med. 1995;23(4):407-413.
32. Reddy AS, Kvitne RE, Yocum LA, Elattrache NS, Glousman RE, Jobe FW. Arthroscopy of the elbow: a long-term clinical review. Arthroscopy. 2000;16(6):588-594.
33. Rosenwasser MP, Steinmann S. Elbow arthroscopy in the treatment of posterior olecranon impingement. Paper present at: AANA Annual Meeting; 1991; San Diego, CA.
34. Wilson FD, Andrews JR, Blackburn TA, McCluskey G. Valgus extension overload in the pitching elbow. Am J Sports Med. 1983;11(2):83-88.
The supraphysiological demands imposed on the elbow of a throwing athlete result in predictable patterns of injury. This is especially true of baseball pitchers. Knowledge of elbow anatomy, as well as the biomechanics of throwing, assist in making diagnostic and therapeutic decisions and also influence surgical technique when surgery is required. During the late cocking and early acceleration phases of throwing, valgus torque can reach 65 Nm with angular velocities of the forearm reaching 5000°/sec, which is considered the fasted recorded human movment.1 The valgus torque and rapid extension synergistically create 3 major forces placed on the elbow. The first is a tensile stress along the medial aspect of elbow affecting the ulnar collateral ligament (UCL), flexor pronator mass, and medial epicondyle. Secondly, compression forces affect the lateral aspect of the elbow at the radiocapitellar joint. Finally, a shearing stress occurs in the posterior compartment at the posterior medial tip of the olecranon and the olecranon fossa.
These forces generated on the elbow result in predictable pathology. The recurring tensile forces applied on the medial aspect on the elbow can compromise the integrity of the UCL. It is well known that injury to the UCL leads to valgus instability. Individuals with valgus instability who continue to throw may trigger and/or aggravate injury in the posterior and lateral components of the elbow. Lateral compression forces can often reach 500 N, resulting in radiocapitellar overload syndrome, which occurs in combination with medial ligament instability and valgus extension overload.2 Radiocapitellar compression may cause chondral or osteochondral fracture with resulting intra-articular loose bodes. This compression also contributes to the etiology of osteochondritis dissecans (OCD) in skeletally immature athletes. In the posterior elbow, throwing forcefully and repeatedly pushes the olecranon into the olecranon fossa. Shear stress on the medial olecranon tip and fossa, due to combined valgus and extension forces, lead to the development of osteophytes. This collection of injuries in the medial, lateral, and posterior aspects of the elbow is known as “valgus extension overload syndrome” or VEO. Symptoms in VEO can be the result of chondral lesions, loose bodies, and marginal exostosis.3
The aim of this review is to provide understanding regarding both the relevant anatomy and pathomechanics of VEO, key aspects to clinical evaluation, and effective treatment options.
Functional Anatomy
A functional comprehension of elbow anatomy and biomechanics is essential to understanding the constellation of injuries in VEO. The osseous anatomy of the elbow permits a variety of movements. These include flexion-extension and pronation-supination, which are mediated by the ulnohumeral and radiocapitellar articulations. While in full extension, the elbow has a normal valgus carrying angle of 11° to 16°. It is important to know that 50% of the elbow’s stability is attributed to the configuration of the bones.4-6 This is especially true in varus stress while the elbow is in full extension. The soft tissues, including muscle and ligaments such as the UCL, lateral UCL, and radial UCL complexes, provide the remaining elbow stability.4-6
The UCL complex is composed of 3 main segments known as the anterior, posterior, and oblique bundles (transverse ligament). Collectively, these bundles are responsible for providing medial elbow stability. However, each of these bundles contributes to medial elbow stability in its own way. The first and arguably the most important bundle is the anterior bundle; its most important function is providing stability against valgus stress.4,5,7 It is composed of parallel fibers inserting on the medial coronoid process.4,5,7 Furthermore, its eccentric location with respect to the axis of elbow allows it to provide stability throughout the full range of elbow motion.6 The anterior bundle can be further divided into individual anterior and posterior bands that have reciprocal functionality.5,8,9 The anterior band acts as the chief restraint to valgus stress up to 90° of flexion.9 Any flexion beyond 90° renders the anterior band’s role secondary in resisting valgus stress.9 The posterior band’s function in resisting valgus stress is most important between 60° and full flexion, while having a secondary role in lesser degrees of flexion.8,9 Notably, the posterior band is isometric and is more important in the overhead-throwing athlete due to the fact its primary role in resisting valgus stress occurs at higher degrees of flexion.10
The remaining posterior and oblique bundles of the UCL complex have lesser roles in maintaining elbow stability. The posterior bundle of the UCL complex is fan-shaped, originates from the medial epicondyle, and inserts onto the medial margin the semi-lunar notch. It is more slender and frailer than the anterior bundle. This is reflected in its functionality, as it plays a secondary role in elbow stability during elbow flexion beyond 90°.4,5,8 In contrast to the anterior and posterior bundles, the oblique bundle, also known as the transverse ligament, does not cross the elbow joint. It is a thickening of the caudal most aspect of the joint capsule, which extends from the medial olecranon to the inferior medial coronoid process and as a result functions in expanding the greater sigmoid notch.6
The musculotendinous components of the elbow are essential to providing dynamic functional resistance to valgus stress.11 These components are flexor-pronator musculature that originate from the medial epicondyle. Listed proximally to distally, the flexor-pronator muscles include pronator teres, flexor carpi radialis (FCR), palmaris longus, flexor digitorum superficialis, and the flexor carpi ulnaris (FCU).
Pathomechanics
Once familiarized with the relevant function anatomy, it is crucial to understand the mechanics of throwing in order to understand the pathomechanics of VEO. The action of overhead throwing has been divided into 6 phases.6,12-16 Phase 4, acceleration, is the most relevant when discussing forces on elbow, since the majority of forces are generated during this state. Phase 4 represents a rapid acceleration of the upper extremity with a large forward-directed force on the arm generated by the shoulder muscles. Additionally, there is internal rotation and adduction of the humerus with rapid elbow extension terminating with ball release. The elbow accelerates up to 600,000°/sec2 in a miniscule time frame of 40 to 50 milliseconds.1,5 Immense valgus forces are exerted on the medial aspect of the elbow. The anterior bundle of the UCL bears the majority of the force, with the flexor pronator mass enabling the transmission.11 The majority of injuries occur during stage 4 as a result of the stress load on the medial elbow structures like the UCL. The proceeding phases 5 (deceleration), and 6 (follow-through) involve eventual dissipation of excess kinetic energy as the elbow completely extends. The deceleration during phase 5 is rapid and powerful, occurring at about 500,000°/sec2 in the short span of 50 milliseconds.1,6,12-16 High-velocity throwing, such as baseball pitching, generates forces in the elbow that are opposed by the articular, ligamentous, and muscular portions of the arm. The ulnohumeral articulation stabilizes motion of the arm from 0° to 20° of flexion and beyond 120° of flexion. Static and dynamic soft tissues maintain stability during the remaining of 100° arc of motion.
During deceleration, the elbow undergoes terminal extension resulting in the posteromedial olecranon contacting the trochlea and the olecranon fossa with subsequent dissipation of the combined valgus force and angular moment (Figure 1). This dissipation of force creates pathologic shear and compressive forces in the posterior elbow. Poor muscular control and the traumatic abutment that occurs in the posterior compartment may further add to the pathologic forces. Reactive bone formation is induced by the repetitive compression and shear, resulting in osteophytes on the posteromedial tip of the olecranon (Figure 2). Consequent “kissing lesions” of chondromalacia may occur in the olecranon fossa and posteromedial trochlea. The subsequent development of loose bodies may also occur. The presence of osteophytes and/or loose bodies may result in posteromedial impingement (PMI).
The association between PMI of the olecranon and valgus instability has been elucidated in both clinical and biomechanical investigations.17,18,19,20 Conway18 identified tip exostosis in 24% of lateral radiographs of 135 asymptomatic professional pitchers. Approximately one-fifth (21%) of these pitchers had >1.0 mm increased relative valgus laxity on stress radiographs. Roughly one-third (34%) of players with exostosis had >1.0 mm of increased relative valgus laxity, compared to 16% of players without exostosis formation. These results provide evidence for a probable association between PMI and valgus laxity. In biomechanical research, Ahmad and colleagues17 studied the effect of partial and full thickness UCL injuries on contact forces of the posterior elbow. Posteromedial compartments of cadaver specimens were subjected to physiologic valgus stresses while placed on pressure-senstive film. Contact area and pressure between posteromedial trochlea and olecranon were altered in the setting of UCL insufficiency, helping explain how posteromedial osteophyte formation occurs.
Additional biomechanical studies have also investigated the posteromedial olecranon’s role in functioning as a stabilizing buttress to medial tensile forces. Treating PMI with aggressive bone removal may increase valgus instability as well as strain on the UCL, leading to UCL injury following olecranon resection.19,20 Kamineni and colleagues19 investigated strain on anterior bundle of UCL as a function of increasing applied torque and posteromedial resections of the olecranon. This investigation was done utilizing an electromagnetic tracking placed in cadaver elbows. A nonuniform change in strain was found at 3 mm of resection during flexion and valgus testing. This nonuniform change implied that removal of posteromedial olecranon beyond 3 mm made the UCL more vulnerable to injury. Follow-up investigations looked at kinematic effects of increasing valgus and varus torques and sequential posteromedial olecranon resections.20 Valgus angulation of the elbow increased with all resection levels but no critical amount of olecranon resection was identified. The consensus in the literature indicates that posteromedial articulation of the elbow is a significant stabilizer to valgus stress.17-22 Thus, normal bone should be preserved and only osteophytes should be removed during treatment.
In addition, VEO may lead to injury in the lateral compartment as well. After attenuation and insufficiency of the UCL due to repetitive stress, excessive force transmission to the lateral aspect of the elbow occurs. Compressive and rotatory forces escalate within the radiocapitellar joint, causing synovitis and osteochondral lesions.3,23 These osteochondral lesions include osteochondritis dissecans and osteochondral fractures that may fragment and become loose bodies.
Evaluation of VEO
History
Patients will typically have a history of repetitive throwing or other repetitive overhead activity. VEO is most common in baseball pitchers but may also occur in other sports, such as tennis, football, lacrosse, gymnastics, and javelin throwing. In baseball pitchers, clinical presentation is often preceded by a decrease in pitch velocity, control, and early fatigability. It presents with elbow pain localized to the posteromedial aspect of olecranon after release of the ball, when the elbow reaches terminal extension. Patients also report limited extension, due to impinging posterior osteophytes. Also, locking and catching caused by loose bodies and chondromalacia may be present. VEO may also occur in combination with concomitant valgus instability, as well as in a patient with a prior history of valgus instability. Flexor pronator injury, ulnar neuritis, and subluxation may also be present in a patient with VEO.
Physical Examination
VEO may occur in an isolated fashion or with concomitant pathology. Therefore, a comprehensive physical examination includes evaluating the entire kinetic chain of throwing and a focused examination covering VEO and associated valgus instability. Patients may exhibit crepitus and tenderness over the posteromedial olecranon and a loss of extension with a firm end point. The extension impingement test should be performed where the elbow is snapped into terminal extension. This typically elicits pain in the posterior compartment in a patient with VEO. The arm bar test involves positioning the patient’s shoulder at 90° of forward flexion, full internal rotation, with the patient’s hand placed on the examiner’s shoulder.24 The examiner pulls down on the olecranon, simulating forced extension; pain is indicative of a positive test. It is important to note if there are signs of ulnar neuritis or subluxing ulnar nerve, especially if planning to utilize medial portals during arthroscopic treatment.
Examination maneuvers for valgus instability should also be conducted during evaluation of VEO. The physical examination for valgus instability in the elbow is ideally performed with the patient seated. Secure the patient’s wrist between the examiner’s forearm and trunk, and flex the patient’s elbow between 20° and 30° to unlock the olecranon from its fossa. Proceed to apply valgus stress. This stresses the anterior band of the anterior bundle of the UCL.6,25,26 Palpate the UCL from the medial epicondyle to the proximal ulna as valgus stress is applied. Occasionally, valgus laxity can be appreciated when compared to contralateral side. The milking maneuver is a helpful test to determine UCL injury. Pull on the patient’s thumb while the forearm is supinated, shoulder extended, and the elbow flexed beyond 90°.6 The milking maneuver exerts valgus stress on a flexed elbow. A patient with an injured UCL will experience the subjective feeling of apprehension and instability, with medial elbow pain.
The most sensitive test is the moving valgus stress test. This is performed with the patient in the upright position and the shoulder abducted 90°. Starting with the arm in full flexion, the examiner applies a constant valgus torque to the elbow and then rapidly extends the elbow. Reproduction of pain during range of motion from 120° to 70° represents UCL injury, while pain with extension beyond 70° represents chondral injury to the ulnohumeral joint. Be aware that the absence of increased pain with wrist flexion, along with pain localized slightly posterior to the common flexor origin, differentiates a UCL injury from flexor-pronator muscle injury.6,26,27Examine range of motion in affected and unaffected elbows. Loss of terminal extension may be present, along with secondary to flexion contracture due to repeated attempts at healing and stabilization.25
Imaging
Imaging is essential to the accurate diagnosis of VEO and related conditions. Anterior posterior (AP), lateral, and oblique radiographs of elbow (Figures 3A-3C) may show posteromedial olecranon osteophytes and/or loose bodies. Calcification of ligaments or other soft tissues may also be seen. An AP radiograph with 140° of external rotation may best visualize osteophytes on posteromedial olecranon.18 A computed tomography scan with 2-dimensional sagittal and coronal reconstruction and 3-dimensional surface rendering (Figures 4A, 4B) may best demonstrate morphological abnormalities, loose bodies, and osteophytes. Magnetic resonance imaging (MRI) is essential for assessment of soft tissues and chondral injuries. MRI may detect UCL compromise, synovial plicae, bone edema, olecranon, or stress fractures.
Treatment
Nonoperative Treatment
Treatment consists of both nonoperative and operative modalities. Nonoperative treatment methods are first line in treating VEO. Patients should modify their physical activity and rest from throwing activities. Nonsteroid anti-inflammatory drugs are appropriate to treat pain along with intra-articular corticosteroid injections of the elbow. A wide assessment of pitching mechanics should be performed in an attempt to correct errors in throwing technique and address muscular imbalances. After cessation of the resting period, the patient may initiate a progressive throwing program supervised by an experienced therapist and trainer. A plan for returning to competition should be made upon completion of the throwing program.
Operative Treatment
Surgical treatment is reserved for patients who fail nonoperative treatment. These patients have persistent symptoms of posteromedial impingement and desire to return to pre-injury level of performance. Posteromedial decompression is not recommended when provocative physical examination maneuvers are negative, regardless of presence of olecranon osteophytes on imaging. Osteophytes are an asymptomatic finding typically seen in professional baseball players and do not warrant surgical treatment.18,28 UCL compromise is a relative contraindication to olecranon debridement as UCL injury could become symptomatic following surgery. Surgical options in the appropriate patient to decompress posterior compartment include arthroscopic olecranon debridement or limited incision arthrotomy. Excessive resection of posteromedial osteophytes must be avoided. Arthroscopy has limited morbidity and allows for complete diagnostic assessment. UCL reconstruction should also be considered in combination with posteromedial debridement when the UCL is torn. More challenging indications for UCL reconstruction occur when the UCL is partially torn or torn and asymptomatic. Isolated posteromedial decompression in this setting risks future development of UCL symptoms that would then need to be addressed.
Surgical Technique
As previously mentioned, elbow arthroscopy or limited excision arthrotomy are the preferred operative methods for decompression of the posterior compartment and thus treatment of VEO. Anesthesia and patient positioning should be selected based on the surgeon’s preference. The patient should be positioned supine, prone, or in lateral decubitis. When a UCL reconstruction is expected, supine position is advantageous to avoid repositioning after completing the arthroscopic portion of the procedure. However, arthroscopy can be performed in the lateral position with subsequent repositioning, repeat prepping, and draping for UCL reconstruction (Figures 5A, 5B).
Prepare for elbow arthroscopy by distending the elbow joint with normal saline to aid in protection of neurovascular structures and simplify the insertion of the scope trocar. Perform diagnostic anterior arthroscopy via the proximal anteromedial portal. Assess for presence of loose bodies and osteochondral lesions of the radiocapitellar joint, as well as osteophytes of the coronoid tip and fossa. Utilizing a spinal needle under direct visualization establish a proximal lateral portal with adequate view of the anterior compartment. Proceed to visualize the medial compartment and assess for UCL injury. Apply valgus stress while in 70° of flexion. Visualize the coronoid process and look for medial trochlea gapping of 3 mm or greater, which indicates UCL insufficiency.30
Establish the posterolateral port for visualization of the posterior compartment. A posterior portal is established through the triceps tendon. Proceed to shave and ablate synovitis in order to create an adequate working space. Inspect the posteromedial olecranon, looking for any osteophytes or chondromalacia in the area (Figure 6). Examine the posterior radiocapitellar joint, looking specifically for loose bodies. The presence of loose bodies may require creating an extra mid lateral portal for removal. The ulnar nerve is located superficial to the elbow capsule and can be damaged by instruments utilized in the posteromedial gutter. As a precaution, be sure to remove suction attached to shaver. Place a curved articulating retractor in an accessory posterolateral portal to assist in protecting the ulnar nerve by retracting the capsule away from the surgical field (Figures 7A, 7B).
The osteophyte may be encased in soft tissue. Using a combination of ablation devices and shavers, the osteophyte can be exposed. The olecranon osteophyte can be removed with a small osteotome located at the border of the osteophyte and the normal olecranon. A motorized shaver or burr may also be introduced through the direct posterior portal or the posterolateral portal to complete the contouring of the olecranon (Figures 8A, 8B). Intraoperative lateral radiographs may be obtained for guidance in adequate bone removal and to ensure no bone debris is left in the soft tissues. It is critical that only pathologic osteophyte is removed and that normal olecranon is not compromised. This prevents an increase in UCL strain during valgus loading.19 However, in some non-throwing athletes, more aggressive debridement can be performed due to a smaller risk of UCL injury after posterior decompression.
Often, with the presence of osteophytes on the olecranon, there may be associated chondromalacia of the trochlea. These kissing lesions must be addressed after debridement of osteophytes. Loose flaps or frayed edges are carefully debrided and for any significant lesion the edges are contoured to a stable rim using shavers and curettes. Once altered to a well-shouldered lesion, microfracture is performed. Anterograde drilling of the lesion with perforations separated by 2 to 3 mm allow for the release of marrow elements and induction of a fibrocartilage healing response.
For an isolated posteromedial decompression, early rehabilitation begins with simple elbow flexion and extension exercises. It is important to restore flexor-pronator strength. Six weeks postoperatively, a progressive throwing program that includes plyometric exercises, neuromuscular training, and endurance exercises can be initiated. Patients can typically return to competition 3 to 4 months after surgery, if they have successfully regained preoperative range of motion, preoperative strength in the elbow, and there is no pain or tenderness on stress testing or palpation.
Outcomes
Safety and Advances in Arthroscopy
A clearer understanding of portal placement and proximity to neurovasculature in conjunction with advances in equipment have allowed for continual improvements in elbow arthroscopy techniques. There is plenty of literature indicating that arthroscopic posteromedial decompression is a safe, reliable, effective procedure, with a high rate of patient satisfaction.22,30-34] Andrews and Carson30 published one of the earliest investigations indicating the effectiveness of elbow arthroscopy utilizing objective and subjective outcome scores. They found that preoperative scores indicating patient satisfaction increased from 50% to 83%. Patients who underwent only loose body removal had the best outcomes. Andrews and Timmerman31 later evaluated the results of 72 professional baseball players who underwent either arthroscopic or open elbow surgery. They found that posteromedial olecranon osteophytes and intraarticular loose bodies were the most common diagnoses, present in 65% and 54% of players, respectively. In addition, a 41% reoperation rate was reported after posteromedial olecranon resection, along with 25% a rate of valgus instability necessitating UCL reconstruction. Andrews and Timmerman31 propose that the incidence of UCL injuries is underestimated and that UCL pathology must be treated prior to treating its secondary effects. Recently, Reddy and colleagues32 reviewed the results of 187 arthroscopic procedures. Posterior impingement, loose bodies, and osteoarthritis were the most common problems, occurring in 51%, 31%, and 22% of patients, respectively. Reported results were encouraging, with 87% good to excellent results and 85% of baseball players returning to preinjury levels.
Conclusion
An understanding of the relevant functional anatomy and the biomechanics of throwing is essential to understanding VEO. Potential concomitant valgus instability and UCL injury must be carefully assessed. Only symptomatic patients who have failed conservative treatment should undergo surgery. It is critical to avoid exacerbating and/or causing valgus instability by surgical excessively removing normal bone from the olecranon. Arthroscopy has been shown to be a safe and effective method to treat refractory cases of VEO.
The supraphysiological demands imposed on the elbow of a throwing athlete result in predictable patterns of injury. This is especially true of baseball pitchers. Knowledge of elbow anatomy, as well as the biomechanics of throwing, assist in making diagnostic and therapeutic decisions and also influence surgical technique when surgery is required. During the late cocking and early acceleration phases of throwing, valgus torque can reach 65 Nm with angular velocities of the forearm reaching 5000°/sec, which is considered the fasted recorded human movment.1 The valgus torque and rapid extension synergistically create 3 major forces placed on the elbow. The first is a tensile stress along the medial aspect of elbow affecting the ulnar collateral ligament (UCL), flexor pronator mass, and medial epicondyle. Secondly, compression forces affect the lateral aspect of the elbow at the radiocapitellar joint. Finally, a shearing stress occurs in the posterior compartment at the posterior medial tip of the olecranon and the olecranon fossa.
These forces generated on the elbow result in predictable pathology. The recurring tensile forces applied on the medial aspect on the elbow can compromise the integrity of the UCL. It is well known that injury to the UCL leads to valgus instability. Individuals with valgus instability who continue to throw may trigger and/or aggravate injury in the posterior and lateral components of the elbow. Lateral compression forces can often reach 500 N, resulting in radiocapitellar overload syndrome, which occurs in combination with medial ligament instability and valgus extension overload.2 Radiocapitellar compression may cause chondral or osteochondral fracture with resulting intra-articular loose bodes. This compression also contributes to the etiology of osteochondritis dissecans (OCD) in skeletally immature athletes. In the posterior elbow, throwing forcefully and repeatedly pushes the olecranon into the olecranon fossa. Shear stress on the medial olecranon tip and fossa, due to combined valgus and extension forces, lead to the development of osteophytes. This collection of injuries in the medial, lateral, and posterior aspects of the elbow is known as “valgus extension overload syndrome” or VEO. Symptoms in VEO can be the result of chondral lesions, loose bodies, and marginal exostosis.3
The aim of this review is to provide understanding regarding both the relevant anatomy and pathomechanics of VEO, key aspects to clinical evaluation, and effective treatment options.
Functional Anatomy
A functional comprehension of elbow anatomy and biomechanics is essential to understanding the constellation of injuries in VEO. The osseous anatomy of the elbow permits a variety of movements. These include flexion-extension and pronation-supination, which are mediated by the ulnohumeral and radiocapitellar articulations. While in full extension, the elbow has a normal valgus carrying angle of 11° to 16°. It is important to know that 50% of the elbow’s stability is attributed to the configuration of the bones.4-6 This is especially true in varus stress while the elbow is in full extension. The soft tissues, including muscle and ligaments such as the UCL, lateral UCL, and radial UCL complexes, provide the remaining elbow stability.4-6
The UCL complex is composed of 3 main segments known as the anterior, posterior, and oblique bundles (transverse ligament). Collectively, these bundles are responsible for providing medial elbow stability. However, each of these bundles contributes to medial elbow stability in its own way. The first and arguably the most important bundle is the anterior bundle; its most important function is providing stability against valgus stress.4,5,7 It is composed of parallel fibers inserting on the medial coronoid process.4,5,7 Furthermore, its eccentric location with respect to the axis of elbow allows it to provide stability throughout the full range of elbow motion.6 The anterior bundle can be further divided into individual anterior and posterior bands that have reciprocal functionality.5,8,9 The anterior band acts as the chief restraint to valgus stress up to 90° of flexion.9 Any flexion beyond 90° renders the anterior band’s role secondary in resisting valgus stress.9 The posterior band’s function in resisting valgus stress is most important between 60° and full flexion, while having a secondary role in lesser degrees of flexion.8,9 Notably, the posterior band is isometric and is more important in the overhead-throwing athlete due to the fact its primary role in resisting valgus stress occurs at higher degrees of flexion.10
The remaining posterior and oblique bundles of the UCL complex have lesser roles in maintaining elbow stability. The posterior bundle of the UCL complex is fan-shaped, originates from the medial epicondyle, and inserts onto the medial margin the semi-lunar notch. It is more slender and frailer than the anterior bundle. This is reflected in its functionality, as it plays a secondary role in elbow stability during elbow flexion beyond 90°.4,5,8 In contrast to the anterior and posterior bundles, the oblique bundle, also known as the transverse ligament, does not cross the elbow joint. It is a thickening of the caudal most aspect of the joint capsule, which extends from the medial olecranon to the inferior medial coronoid process and as a result functions in expanding the greater sigmoid notch.6
The musculotendinous components of the elbow are essential to providing dynamic functional resistance to valgus stress.11 These components are flexor-pronator musculature that originate from the medial epicondyle. Listed proximally to distally, the flexor-pronator muscles include pronator teres, flexor carpi radialis (FCR), palmaris longus, flexor digitorum superficialis, and the flexor carpi ulnaris (FCU).
Pathomechanics
Once familiarized with the relevant function anatomy, it is crucial to understand the mechanics of throwing in order to understand the pathomechanics of VEO. The action of overhead throwing has been divided into 6 phases.6,12-16 Phase 4, acceleration, is the most relevant when discussing forces on elbow, since the majority of forces are generated during this state. Phase 4 represents a rapid acceleration of the upper extremity with a large forward-directed force on the arm generated by the shoulder muscles. Additionally, there is internal rotation and adduction of the humerus with rapid elbow extension terminating with ball release. The elbow accelerates up to 600,000°/sec2 in a miniscule time frame of 40 to 50 milliseconds.1,5 Immense valgus forces are exerted on the medial aspect of the elbow. The anterior bundle of the UCL bears the majority of the force, with the flexor pronator mass enabling the transmission.11 The majority of injuries occur during stage 4 as a result of the stress load on the medial elbow structures like the UCL. The proceeding phases 5 (deceleration), and 6 (follow-through) involve eventual dissipation of excess kinetic energy as the elbow completely extends. The deceleration during phase 5 is rapid and powerful, occurring at about 500,000°/sec2 in the short span of 50 milliseconds.1,6,12-16 High-velocity throwing, such as baseball pitching, generates forces in the elbow that are opposed by the articular, ligamentous, and muscular portions of the arm. The ulnohumeral articulation stabilizes motion of the arm from 0° to 20° of flexion and beyond 120° of flexion. Static and dynamic soft tissues maintain stability during the remaining of 100° arc of motion.
During deceleration, the elbow undergoes terminal extension resulting in the posteromedial olecranon contacting the trochlea and the olecranon fossa with subsequent dissipation of the combined valgus force and angular moment (Figure 1). This dissipation of force creates pathologic shear and compressive forces in the posterior elbow. Poor muscular control and the traumatic abutment that occurs in the posterior compartment may further add to the pathologic forces. Reactive bone formation is induced by the repetitive compression and shear, resulting in osteophytes on the posteromedial tip of the olecranon (Figure 2). Consequent “kissing lesions” of chondromalacia may occur in the olecranon fossa and posteromedial trochlea. The subsequent development of loose bodies may also occur. The presence of osteophytes and/or loose bodies may result in posteromedial impingement (PMI).
The association between PMI of the olecranon and valgus instability has been elucidated in both clinical and biomechanical investigations.17,18,19,20 Conway18 identified tip exostosis in 24% of lateral radiographs of 135 asymptomatic professional pitchers. Approximately one-fifth (21%) of these pitchers had >1.0 mm increased relative valgus laxity on stress radiographs. Roughly one-third (34%) of players with exostosis had >1.0 mm of increased relative valgus laxity, compared to 16% of players without exostosis formation. These results provide evidence for a probable association between PMI and valgus laxity. In biomechanical research, Ahmad and colleagues17 studied the effect of partial and full thickness UCL injuries on contact forces of the posterior elbow. Posteromedial compartments of cadaver specimens were subjected to physiologic valgus stresses while placed on pressure-senstive film. Contact area and pressure between posteromedial trochlea and olecranon were altered in the setting of UCL insufficiency, helping explain how posteromedial osteophyte formation occurs.
Additional biomechanical studies have also investigated the posteromedial olecranon’s role in functioning as a stabilizing buttress to medial tensile forces. Treating PMI with aggressive bone removal may increase valgus instability as well as strain on the UCL, leading to UCL injury following olecranon resection.19,20 Kamineni and colleagues19 investigated strain on anterior bundle of UCL as a function of increasing applied torque and posteromedial resections of the olecranon. This investigation was done utilizing an electromagnetic tracking placed in cadaver elbows. A nonuniform change in strain was found at 3 mm of resection during flexion and valgus testing. This nonuniform change implied that removal of posteromedial olecranon beyond 3 mm made the UCL more vulnerable to injury. Follow-up investigations looked at kinematic effects of increasing valgus and varus torques and sequential posteromedial olecranon resections.20 Valgus angulation of the elbow increased with all resection levels but no critical amount of olecranon resection was identified. The consensus in the literature indicates that posteromedial articulation of the elbow is a significant stabilizer to valgus stress.17-22 Thus, normal bone should be preserved and only osteophytes should be removed during treatment.
In addition, VEO may lead to injury in the lateral compartment as well. After attenuation and insufficiency of the UCL due to repetitive stress, excessive force transmission to the lateral aspect of the elbow occurs. Compressive and rotatory forces escalate within the radiocapitellar joint, causing synovitis and osteochondral lesions.3,23 These osteochondral lesions include osteochondritis dissecans and osteochondral fractures that may fragment and become loose bodies.
Evaluation of VEO
History
Patients will typically have a history of repetitive throwing or other repetitive overhead activity. VEO is most common in baseball pitchers but may also occur in other sports, such as tennis, football, lacrosse, gymnastics, and javelin throwing. In baseball pitchers, clinical presentation is often preceded by a decrease in pitch velocity, control, and early fatigability. It presents with elbow pain localized to the posteromedial aspect of olecranon after release of the ball, when the elbow reaches terminal extension. Patients also report limited extension, due to impinging posterior osteophytes. Also, locking and catching caused by loose bodies and chondromalacia may be present. VEO may also occur in combination with concomitant valgus instability, as well as in a patient with a prior history of valgus instability. Flexor pronator injury, ulnar neuritis, and subluxation may also be present in a patient with VEO.
Physical Examination
VEO may occur in an isolated fashion or with concomitant pathology. Therefore, a comprehensive physical examination includes evaluating the entire kinetic chain of throwing and a focused examination covering VEO and associated valgus instability. Patients may exhibit crepitus and tenderness over the posteromedial olecranon and a loss of extension with a firm end point. The extension impingement test should be performed where the elbow is snapped into terminal extension. This typically elicits pain in the posterior compartment in a patient with VEO. The arm bar test involves positioning the patient’s shoulder at 90° of forward flexion, full internal rotation, with the patient’s hand placed on the examiner’s shoulder.24 The examiner pulls down on the olecranon, simulating forced extension; pain is indicative of a positive test. It is important to note if there are signs of ulnar neuritis or subluxing ulnar nerve, especially if planning to utilize medial portals during arthroscopic treatment.
Examination maneuvers for valgus instability should also be conducted during evaluation of VEO. The physical examination for valgus instability in the elbow is ideally performed with the patient seated. Secure the patient’s wrist between the examiner’s forearm and trunk, and flex the patient’s elbow between 20° and 30° to unlock the olecranon from its fossa. Proceed to apply valgus stress. This stresses the anterior band of the anterior bundle of the UCL.6,25,26 Palpate the UCL from the medial epicondyle to the proximal ulna as valgus stress is applied. Occasionally, valgus laxity can be appreciated when compared to contralateral side. The milking maneuver is a helpful test to determine UCL injury. Pull on the patient’s thumb while the forearm is supinated, shoulder extended, and the elbow flexed beyond 90°.6 The milking maneuver exerts valgus stress on a flexed elbow. A patient with an injured UCL will experience the subjective feeling of apprehension and instability, with medial elbow pain.
The most sensitive test is the moving valgus stress test. This is performed with the patient in the upright position and the shoulder abducted 90°. Starting with the arm in full flexion, the examiner applies a constant valgus torque to the elbow and then rapidly extends the elbow. Reproduction of pain during range of motion from 120° to 70° represents UCL injury, while pain with extension beyond 70° represents chondral injury to the ulnohumeral joint. Be aware that the absence of increased pain with wrist flexion, along with pain localized slightly posterior to the common flexor origin, differentiates a UCL injury from flexor-pronator muscle injury.6,26,27Examine range of motion in affected and unaffected elbows. Loss of terminal extension may be present, along with secondary to flexion contracture due to repeated attempts at healing and stabilization.25
Imaging
Imaging is essential to the accurate diagnosis of VEO and related conditions. Anterior posterior (AP), lateral, and oblique radiographs of elbow (Figures 3A-3C) may show posteromedial olecranon osteophytes and/or loose bodies. Calcification of ligaments or other soft tissues may also be seen. An AP radiograph with 140° of external rotation may best visualize osteophytes on posteromedial olecranon.18 A computed tomography scan with 2-dimensional sagittal and coronal reconstruction and 3-dimensional surface rendering (Figures 4A, 4B) may best demonstrate morphological abnormalities, loose bodies, and osteophytes. Magnetic resonance imaging (MRI) is essential for assessment of soft tissues and chondral injuries. MRI may detect UCL compromise, synovial plicae, bone edema, olecranon, or stress fractures.
Treatment
Nonoperative Treatment
Treatment consists of both nonoperative and operative modalities. Nonoperative treatment methods are first line in treating VEO. Patients should modify their physical activity and rest from throwing activities. Nonsteroid anti-inflammatory drugs are appropriate to treat pain along with intra-articular corticosteroid injections of the elbow. A wide assessment of pitching mechanics should be performed in an attempt to correct errors in throwing technique and address muscular imbalances. After cessation of the resting period, the patient may initiate a progressive throwing program supervised by an experienced therapist and trainer. A plan for returning to competition should be made upon completion of the throwing program.
Operative Treatment
Surgical treatment is reserved for patients who fail nonoperative treatment. These patients have persistent symptoms of posteromedial impingement and desire to return to pre-injury level of performance. Posteromedial decompression is not recommended when provocative physical examination maneuvers are negative, regardless of presence of olecranon osteophytes on imaging. Osteophytes are an asymptomatic finding typically seen in professional baseball players and do not warrant surgical treatment.18,28 UCL compromise is a relative contraindication to olecranon debridement as UCL injury could become symptomatic following surgery. Surgical options in the appropriate patient to decompress posterior compartment include arthroscopic olecranon debridement or limited incision arthrotomy. Excessive resection of posteromedial osteophytes must be avoided. Arthroscopy has limited morbidity and allows for complete diagnostic assessment. UCL reconstruction should also be considered in combination with posteromedial debridement when the UCL is torn. More challenging indications for UCL reconstruction occur when the UCL is partially torn or torn and asymptomatic. Isolated posteromedial decompression in this setting risks future development of UCL symptoms that would then need to be addressed.
Surgical Technique
As previously mentioned, elbow arthroscopy or limited excision arthrotomy are the preferred operative methods for decompression of the posterior compartment and thus treatment of VEO. Anesthesia and patient positioning should be selected based on the surgeon’s preference. The patient should be positioned supine, prone, or in lateral decubitis. When a UCL reconstruction is expected, supine position is advantageous to avoid repositioning after completing the arthroscopic portion of the procedure. However, arthroscopy can be performed in the lateral position with subsequent repositioning, repeat prepping, and draping for UCL reconstruction (Figures 5A, 5B).
Prepare for elbow arthroscopy by distending the elbow joint with normal saline to aid in protection of neurovascular structures and simplify the insertion of the scope trocar. Perform diagnostic anterior arthroscopy via the proximal anteromedial portal. Assess for presence of loose bodies and osteochondral lesions of the radiocapitellar joint, as well as osteophytes of the coronoid tip and fossa. Utilizing a spinal needle under direct visualization establish a proximal lateral portal with adequate view of the anterior compartment. Proceed to visualize the medial compartment and assess for UCL injury. Apply valgus stress while in 70° of flexion. Visualize the coronoid process and look for medial trochlea gapping of 3 mm or greater, which indicates UCL insufficiency.30
Establish the posterolateral port for visualization of the posterior compartment. A posterior portal is established through the triceps tendon. Proceed to shave and ablate synovitis in order to create an adequate working space. Inspect the posteromedial olecranon, looking for any osteophytes or chondromalacia in the area (Figure 6). Examine the posterior radiocapitellar joint, looking specifically for loose bodies. The presence of loose bodies may require creating an extra mid lateral portal for removal. The ulnar nerve is located superficial to the elbow capsule and can be damaged by instruments utilized in the posteromedial gutter. As a precaution, be sure to remove suction attached to shaver. Place a curved articulating retractor in an accessory posterolateral portal to assist in protecting the ulnar nerve by retracting the capsule away from the surgical field (Figures 7A, 7B).
The osteophyte may be encased in soft tissue. Using a combination of ablation devices and shavers, the osteophyte can be exposed. The olecranon osteophyte can be removed with a small osteotome located at the border of the osteophyte and the normal olecranon. A motorized shaver or burr may also be introduced through the direct posterior portal or the posterolateral portal to complete the contouring of the olecranon (Figures 8A, 8B). Intraoperative lateral radiographs may be obtained for guidance in adequate bone removal and to ensure no bone debris is left in the soft tissues. It is critical that only pathologic osteophyte is removed and that normal olecranon is not compromised. This prevents an increase in UCL strain during valgus loading.19 However, in some non-throwing athletes, more aggressive debridement can be performed due to a smaller risk of UCL injury after posterior decompression.
Often, with the presence of osteophytes on the olecranon, there may be associated chondromalacia of the trochlea. These kissing lesions must be addressed after debridement of osteophytes. Loose flaps or frayed edges are carefully debrided and for any significant lesion the edges are contoured to a stable rim using shavers and curettes. Once altered to a well-shouldered lesion, microfracture is performed. Anterograde drilling of the lesion with perforations separated by 2 to 3 mm allow for the release of marrow elements and induction of a fibrocartilage healing response.
For an isolated posteromedial decompression, early rehabilitation begins with simple elbow flexion and extension exercises. It is important to restore flexor-pronator strength. Six weeks postoperatively, a progressive throwing program that includes plyometric exercises, neuromuscular training, and endurance exercises can be initiated. Patients can typically return to competition 3 to 4 months after surgery, if they have successfully regained preoperative range of motion, preoperative strength in the elbow, and there is no pain or tenderness on stress testing or palpation.
Outcomes
Safety and Advances in Arthroscopy
A clearer understanding of portal placement and proximity to neurovasculature in conjunction with advances in equipment have allowed for continual improvements in elbow arthroscopy techniques. There is plenty of literature indicating that arthroscopic posteromedial decompression is a safe, reliable, effective procedure, with a high rate of patient satisfaction.22,30-34] Andrews and Carson30 published one of the earliest investigations indicating the effectiveness of elbow arthroscopy utilizing objective and subjective outcome scores. They found that preoperative scores indicating patient satisfaction increased from 50% to 83%. Patients who underwent only loose body removal had the best outcomes. Andrews and Timmerman31 later evaluated the results of 72 professional baseball players who underwent either arthroscopic or open elbow surgery. They found that posteromedial olecranon osteophytes and intraarticular loose bodies were the most common diagnoses, present in 65% and 54% of players, respectively. In addition, a 41% reoperation rate was reported after posteromedial olecranon resection, along with 25% a rate of valgus instability necessitating UCL reconstruction. Andrews and Timmerman31 propose that the incidence of UCL injuries is underestimated and that UCL pathology must be treated prior to treating its secondary effects. Recently, Reddy and colleagues32 reviewed the results of 187 arthroscopic procedures. Posterior impingement, loose bodies, and osteoarthritis were the most common problems, occurring in 51%, 31%, and 22% of patients, respectively. Reported results were encouraging, with 87% good to excellent results and 85% of baseball players returning to preinjury levels.
Conclusion
An understanding of the relevant functional anatomy and the biomechanics of throwing is essential to understanding VEO. Potential concomitant valgus instability and UCL injury must be carefully assessed. Only symptomatic patients who have failed conservative treatment should undergo surgery. It is critical to avoid exacerbating and/or causing valgus instability by surgical excessively removing normal bone from the olecranon. Arthroscopy has been shown to be a safe and effective method to treat refractory cases of VEO.
1. Pappas AM, Zawacki RM, Sullivan TJ. Biomechanics of baseball pitching. A preliminary report. Am J Sports Med. 1985;13(4):216-222.
2. Fleisig GS, Barrentine SW, Escamilla RF, Andrews JR. Biomechanics of overhand throwing with implications for injuries. Sports Med. 1996;21(6):421-437.
3. Wilson FD, Andrews JR, Blackburn TA, McCluskey G. Valgus extension overload in the pitching elbow. Am J Sports Med. 1983;11(2):83-88.
4. Morrey BF. Applied anatomy and biomechanics of the elbow joint. Instr Course Lect. 1986,35:59-68.
5. Schwab GH, Bennett JB, Woods GW, Tullos HS. Biomechanics of elbow instability: the role of medial collateral ligament. Clin Orthop Relat Res. 1980;146:42-52.
6. Jobe FW, Kvitne RS. Elbow instability in the athlete. Instr Course Lect. 1991;40:17-23.
7. Søjbjerg JO, Ovesen J, Nielsen S. Experimental elbow instability after transection of the medial collateral ligament. Clin Orthop Relat Res. 1987;218:186-190.
8. Regan WD, Korinek SL, Morrey BF, An KN. Biomechanical study of ligaments around the elbow joint. Clin Orthop Relat Res. 1991;271:170-179.
9. Callaway GH, Field LD, Deng XH, et al. Biomechanical evaluation of the medial collateral ligament of the elbow. J Bone Joint Surg Am. 1997;79(8):1223-1231.
10. Chen FS, Rokito AS, Jobe FW. Medial elbow problems in the overhead-throwing athlete. J Am Acad Orthop Surg. 2001;9(2):99-113.
11. Davidson PA, Pink M, Perry J, Jobe FW. Functional anatomy of the flexor pronator muscle group in relation to the medial collateral ligament of the elbow. Am J Sports Med. 1995;23(2):245-250.
12. Jobe FW, Moynes DR, Tibone JE, Perry J. An EMG analysis of the shoulder in pitching. A second report. Am J Sports Med. 1984;12(3):218-220.
13. Hamilton CD, Glousman RE, Jobe FW, Brault J, Pink M, Perry J. Dynamic stability of the elbow: electromyographic analysis of the flexor pronator group and the extensor group in pitchers with valgus instability. J Shoulder Elbow Surg. 1996;5(5):347-354.
14. Glousman RE, Barron J, Jobe FW, Perry J, Pink M. An electromyographic analysis of the elbow in normal and injured pitchers with medial collateral ligament insufficiency. Am J Sports Med. 1992;20(3):311-317.
15. DiGiovine NM, Jobe FW, Pink M, Perry J. An electromyographic analysis of the upper extremity in pitching. J Shoulder Elbow Surg. 1992;1(1):15-25.
16. Sisto DJ, Jobe FW, Moynes DR, Antonelli DJ. An electromyographic analysis of the elbow in pitching. Am J Sports Med. 1987;15(3):260-263.
17. Ahmad CS, Park MC, Elattrache NS. Elbow medial ulnar collateral ligament insufficiency alters posteromedial olecranon contact. Am J Sports Med. 2004;32(7):1607–1612.
18. Ahmad CS, Conway J. Elbow arthroscopy: beginners to advanced: valgus extension overload. In: Egol, ed. Instructional Course Lectures; vol 60. Rosemont, IL: American Academy of Orthopaedic Surgeons; submitted 2009.
19. Kamineni S, ElAttrache NS, O’Driscoll S W, et al. Medial collateral ligament strain with partial posteromedial olecranon resection. A biomechanical study. J Bone Joint Surg Am. 2004;86-A(11):2424–2430.
20. Kamineni S, Hirahara H, Pomianowski S, et al. Partial posteromedial olecranon resection: a kinematic study. J Bone Joint Surg Am. 2003;85-A(6):1005–1011.
21. Morrey BF, An KN. Articular and ligamentous contributions to the stability of the elbow joint. Am J Sports Med. 1983;11(5):315–319.
22. O’Driscoll SW, Morrey BF. Arthroscopy of the elbow. Diagnostic and therapeutic benefits and hazards. J Bone Joint Surg Am. 1992;74(1):84–94.
23. Miller CD, Savoie FH 3rd. Valgus extension injuries of the elbow in the throwing athlete. J Am Acad Orthop Surg. 1994;2(5):261-269.
24. O’Driscoll SW. Valgus extension overload and plica. In: Levine WN, ed. The Athlete’s Elbow. Rosemont, IL: American Academy of Orthopaedic Surgeons; 2008:71-83.
25. Boatright JR, D’Alessandro DF. Nerve entrapment syndromes at the elbow. In Jobe FW, Pink MM, Glousman RE, Kvitne RE, Zemel NP, eds. Operative Techniques in Upper Extremity Sports Injuries. St. Louis, MO: Mosby-Year Book; 1996:518-537.
26. Conway JE, Jobe FW, Glousman RE, Pink M. Medial instability of the elbow in throwing athletes. Treatment by repair or reconstruction of the ulnar collateral ligament. J Bone Joint Surg Am. 1992;74(1):67-83.
27. Jobe FW, Stark H, Lombardo SJ. Reconstruction of the ulnar collateral ligament in athletes. J Bone Joint Surg Am. 1986;68(8):1158-1163.
28. Kooima CL, Anderson K, Craig JV, Teeter DM, van Holsbeeck M. Evidence of subclinical medial collateral ligament injury and posteromedial impingement in professional baseball players. Am J Sports Med. 2004;32(7):1602-1606.
29. Field LD, Altchek DW. Evaluation of the arthroscopic valgus instability test of the elbow. Am J Sports Med. 1996;24(2):177–181.
30. Andrews JR, Carson WG. Arthroscopy of the elbow. Arthroscopy. 1985;1(2):97-107.
31. Andrews JR, Timmerman LA. Outcome of elbow surgery in professional baseball players. Am J Sports Med. 1995;23(4):407-413.
32. Reddy AS, Kvitne RE, Yocum LA, Elattrache NS, Glousman RE, Jobe FW. Arthroscopy of the elbow: a long-term clinical review. Arthroscopy. 2000;16(6):588-594.
33. Rosenwasser MP, Steinmann S. Elbow arthroscopy in the treatment of posterior olecranon impingement. Paper present at: AANA Annual Meeting; 1991; San Diego, CA.
34. Wilson FD, Andrews JR, Blackburn TA, McCluskey G. Valgus extension overload in the pitching elbow. Am J Sports Med. 1983;11(2):83-88.
1. Pappas AM, Zawacki RM, Sullivan TJ. Biomechanics of baseball pitching. A preliminary report. Am J Sports Med. 1985;13(4):216-222.
2. Fleisig GS, Barrentine SW, Escamilla RF, Andrews JR. Biomechanics of overhand throwing with implications for injuries. Sports Med. 1996;21(6):421-437.
3. Wilson FD, Andrews JR, Blackburn TA, McCluskey G. Valgus extension overload in the pitching elbow. Am J Sports Med. 1983;11(2):83-88.
4. Morrey BF. Applied anatomy and biomechanics of the elbow joint. Instr Course Lect. 1986,35:59-68.
5. Schwab GH, Bennett JB, Woods GW, Tullos HS. Biomechanics of elbow instability: the role of medial collateral ligament. Clin Orthop Relat Res. 1980;146:42-52.
6. Jobe FW, Kvitne RS. Elbow instability in the athlete. Instr Course Lect. 1991;40:17-23.
7. Søjbjerg JO, Ovesen J, Nielsen S. Experimental elbow instability after transection of the medial collateral ligament. Clin Orthop Relat Res. 1987;218:186-190.
8. Regan WD, Korinek SL, Morrey BF, An KN. Biomechanical study of ligaments around the elbow joint. Clin Orthop Relat Res. 1991;271:170-179.
9. Callaway GH, Field LD, Deng XH, et al. Biomechanical evaluation of the medial collateral ligament of the elbow. J Bone Joint Surg Am. 1997;79(8):1223-1231.
10. Chen FS, Rokito AS, Jobe FW. Medial elbow problems in the overhead-throwing athlete. J Am Acad Orthop Surg. 2001;9(2):99-113.
11. Davidson PA, Pink M, Perry J, Jobe FW. Functional anatomy of the flexor pronator muscle group in relation to the medial collateral ligament of the elbow. Am J Sports Med. 1995;23(2):245-250.
12. Jobe FW, Moynes DR, Tibone JE, Perry J. An EMG analysis of the shoulder in pitching. A second report. Am J Sports Med. 1984;12(3):218-220.
13. Hamilton CD, Glousman RE, Jobe FW, Brault J, Pink M, Perry J. Dynamic stability of the elbow: electromyographic analysis of the flexor pronator group and the extensor group in pitchers with valgus instability. J Shoulder Elbow Surg. 1996;5(5):347-354.
14. Glousman RE, Barron J, Jobe FW, Perry J, Pink M. An electromyographic analysis of the elbow in normal and injured pitchers with medial collateral ligament insufficiency. Am J Sports Med. 1992;20(3):311-317.
15. DiGiovine NM, Jobe FW, Pink M, Perry J. An electromyographic analysis of the upper extremity in pitching. J Shoulder Elbow Surg. 1992;1(1):15-25.
16. Sisto DJ, Jobe FW, Moynes DR, Antonelli DJ. An electromyographic analysis of the elbow in pitching. Am J Sports Med. 1987;15(3):260-263.
17. Ahmad CS, Park MC, Elattrache NS. Elbow medial ulnar collateral ligament insufficiency alters posteromedial olecranon contact. Am J Sports Med. 2004;32(7):1607–1612.
18. Ahmad CS, Conway J. Elbow arthroscopy: beginners to advanced: valgus extension overload. In: Egol, ed. Instructional Course Lectures; vol 60. Rosemont, IL: American Academy of Orthopaedic Surgeons; submitted 2009.
19. Kamineni S, ElAttrache NS, O’Driscoll S W, et al. Medial collateral ligament strain with partial posteromedial olecranon resection. A biomechanical study. J Bone Joint Surg Am. 2004;86-A(11):2424–2430.
20. Kamineni S, Hirahara H, Pomianowski S, et al. Partial posteromedial olecranon resection: a kinematic study. J Bone Joint Surg Am. 2003;85-A(6):1005–1011.
21. Morrey BF, An KN. Articular and ligamentous contributions to the stability of the elbow joint. Am J Sports Med. 1983;11(5):315–319.
22. O’Driscoll SW, Morrey BF. Arthroscopy of the elbow. Diagnostic and therapeutic benefits and hazards. J Bone Joint Surg Am. 1992;74(1):84–94.
23. Miller CD, Savoie FH 3rd. Valgus extension injuries of the elbow in the throwing athlete. J Am Acad Orthop Surg. 1994;2(5):261-269.
24. O’Driscoll SW. Valgus extension overload and plica. In: Levine WN, ed. The Athlete’s Elbow. Rosemont, IL: American Academy of Orthopaedic Surgeons; 2008:71-83.
25. Boatright JR, D’Alessandro DF. Nerve entrapment syndromes at the elbow. In Jobe FW, Pink MM, Glousman RE, Kvitne RE, Zemel NP, eds. Operative Techniques in Upper Extremity Sports Injuries. St. Louis, MO: Mosby-Year Book; 1996:518-537.
26. Conway JE, Jobe FW, Glousman RE, Pink M. Medial instability of the elbow in throwing athletes. Treatment by repair or reconstruction of the ulnar collateral ligament. J Bone Joint Surg Am. 1992;74(1):67-83.
27. Jobe FW, Stark H, Lombardo SJ. Reconstruction of the ulnar collateral ligament in athletes. J Bone Joint Surg Am. 1986;68(8):1158-1163.
28. Kooima CL, Anderson K, Craig JV, Teeter DM, van Holsbeeck M. Evidence of subclinical medial collateral ligament injury and posteromedial impingement in professional baseball players. Am J Sports Med. 2004;32(7):1602-1606.
29. Field LD, Altchek DW. Evaluation of the arthroscopic valgus instability test of the elbow. Am J Sports Med. 1996;24(2):177–181.
30. Andrews JR, Carson WG. Arthroscopy of the elbow. Arthroscopy. 1985;1(2):97-107.
31. Andrews JR, Timmerman LA. Outcome of elbow surgery in professional baseball players. Am J Sports Med. 1995;23(4):407-413.
32. Reddy AS, Kvitne RE, Yocum LA, Elattrache NS, Glousman RE, Jobe FW. Arthroscopy of the elbow: a long-term clinical review. Arthroscopy. 2000;16(6):588-594.
33. Rosenwasser MP, Steinmann S. Elbow arthroscopy in the treatment of posterior olecranon impingement. Paper present at: AANA Annual Meeting; 1991; San Diego, CA.
34. Wilson FD, Andrews JR, Blackburn TA, McCluskey G. Valgus extension overload in the pitching elbow. Am J Sports Med. 1983;11(2):83-88.
Epidemiology, Treatment, and Prevention of Lumbar Spine Injuries in Major League Baseball Players
For the last 20 years, injuries resulting in time out of play have been on the rise in Major League Baseball (MLB), and those affecting the back are no exception.1,2 In the first comprehensive report on injuries in MLB players, back injuries resulted in a mean of 1016 disabled list days per season from 1995 to 1999.1 Similarly, core and back injuries were responsible for 359 disabled list designations from 2002 to 2008. This represented 11.7% of all injuries resulting in time out of play during that time span.2 During that time, back injury prevalence ranked 6th highest of all possible body regions (out of 17), and both position players and pitchers were similarly affected (7.8% and 7.4% of all injuries, respectively).2 These injuries often result in a significant time out of play and can have a tremendous impact on player health. A healthy, stable, and well-functioning lumbar spine is a prerequisite for nearly all baseball-related activities, including pitching, throwing, batting, and running. Accordingly, even minor lumbar spine injuries may profoundly influence baseball performance. Despite this, less is currently known about the true epidemiology and impact of back injuries in professional baseball compared to other professional sporting organizations.3
The most common causes of low back pain and injury in elite baseball players include muscle strains, stress fractures (spondylolysis), annular tears, disc herniation, stenosis, transverse process fractures, facetogenic pain, and sacroiliac (SI) joint arthropathy.4-8 These injuries present in a variety of ways with varying symptomatology. Accordingly, a thorough understanding and comprehensive approach to the diagnosis and treatment of these injuries is necessary. The purpose of this article is to discuss the current state of lumbar spine injuries in professional baseball players. Specifically, we will discuss the critical role of the spine in baseball activities, common causes of injury, tips for making the diagnosis, treatment options, outcomes, and injury prevention and rehabilitation strategies.
Role of the Spine in Baseball
The spine and core musculature are responsible for positioning the head, shoulders, and upper extremities in space over the hips and lower extremities. Proper maintenance of this relationship is required during all phases of throwing, pitching, running, and hitting. During these activities, the spine may dynamically flex, extend, rotate, and laterally bend as needed to keep the body balanced with the head centered over the trunk.
Pitching and Throwing
Whether pitching from the wind-up or the stretch, the head begins centered over the hips and pelvis. As the pitching motion progresses, the hips undergo rotation, flexion, extension, abduction, and circumduction. While this is occurring, the shoulders and upper truck must bend, rotate, and translate toward home plate with the body. Just prior to front foot contact, trunk rotation averages 55 ± 6° with a maximal mean angular acceleration of 11,600 ± 3100°/s2. 9 In order for the body to remain balanced, controlled, and synchronized throughout this delivery, the lumbar spine and core musculature must work diligently to stabilize the entire kinetic chain. Of all the trunk muscles (paraspinal, rectus abdominis, obliques, and glutei), the lumbar paraspinal muscles often work the hardest during the pitching motion, demonstrating activity increases ranging from 100% to 400%.10 Accordingly, it is not uncommon for pitchers to develop SI joint or lumbar facet joint pain due to this high degree of torsional strain exerted on the low back.4 Poor lumbopelvic control has been shown to be a predictor of subsequent injury, and the degree of lumbopelvic dysfunction is proportional to injury severity in MLB pitchers.5
Hitting
Similar to pitching, hitting involves a complex combination of movements from the upper and lower extremities that must be balanced by the core and spine. Although numerous movements occur simultaneously, rotational motion is primarily responsible for generating power. The trunk rotates an average of 46 ± 9° during the swing and reaches a maximal angular acceleration of 7200 ± 2800°/s2 just after contact.9 During this period of rapid torsion, the spine must rotate in conjunction with the hips and shoulders to create a stable cylinder and axis of rotation. The spine and core are responsible for synchronizing rotation to ensure that hip and shoulder parallelism is maintained from swing initiation to ball contact. If the body does not rotate as a unit, the position of the head is affected and the batter’s ability to see the ball may be compromised. Additionally, if delivery of the shoulders lags too far behind that of the hips, the position of the hands (and bat) in space is adversely affected. The entire kinetic chain must remain balanced, coordinated, precisely timed, and standardized throughout the entire swing from initial trigger to final follow-through. The lumbar spine plays a critical role in each of these steps. If lumbar spine mechanics are not sound, this can have significant adverse effects on batting performance and may predispose hitters to injury.4
Common Etiologies for Spinal Injury
The vast majority of baseball players who experience lumbar pain will have injuries that can be classified as mechanical back pain (ie, spondylolysis, annular tears, facetogenic pain, SI joint arthropathy, or muscle injuries) (Table). Although less likely to occur, nerve entrapment or impingement syndromes (ie, disc herniation, stenosis, and peripheral nerve entrapment) have been observed in professional baseball players. Finally, more concerning pathologies such as infection and tumor are extremely rare, but they must not be overlooked in this high-demand patient population.
Stress Fracture or Spondylolysis
In young athletic patients, up to one-third of those with low back pain may have evidence of a lumbar stress fracture on bone scan.11,12 This is particularly true for athletes who undergo repetitive lumbar extension and rotation, such as linemen, gymnasts, wrestlers, weight lifters, and baseball players.4,13 Although the majority of lumbar stress fractures occur at the pars interarticularis, they can occur in the pedicle or articular process (Figure 1). Most spondylolytic lesions do not progress to spondylolisthesis, especially once patients reach skeletal maturity. Because the fifth lumbar vertebra represents the transition from the lumbar to the sacral spine, most stress fractures occur at L5. These typically present as localized low back pain that worsens with flexion, extension, and rotation.
Muscle Injury
One of the most common causes of low back pain in athletes is muscle strains and spasms. Because the lumbar paraspinal muscles are extremely active during throwing and hitting,10 they are particularly susceptible to injury. This is particularly true in deconditioned athletes or those who report to spring training having not adequately maintained strength and flexibility through the off-season.4,5 These injuries typically present in an acute fashion with an obvious inciting incident. Players may have a history of similar muscle injuries in the past. On examination, they tend to have difficulty maintaining normal posture or ranging the spine through a full arc of motion. Localized, superficial tenderness to palpation in the injured muscle is a key component of the diagnosis.
Annular Tears and Disc Herniation
These injuries typically occur as the result of a combination of compressive and rotary forces on the lumbar spine that overcome the ability of the annulus fibrosus to resist hoop stresses. Patients with annular tears typically present with severe lower back pain that may be accompanied by spasm and pain radiation into the buttock or lower extremities. Pain is usually worsened by valsalva, coughing, sneezing, or bearing down.4 Although annular tears can occur in isolation, they can also lead to herniation of the nucleus pulposus into the spinal canal (Figure 2). Depending on the location and severity of the herniation, nerve entrapment or impingement can occur. This may initially present as pain that radiates into the lower extremities in a dermatomal fashion. As the herniation progresses, decreased sensation and weakness may develop.
Facet Joint Pain
Facetogenic pain can occur as the result of degenerative changes, trauma, or joint inflammation. Facet injury typically occurs during rotation while the back is extended.4 This results in localized pain and tenderness that can be reproduced by loading the facet joint (lumbar extension) during the examination, and patients will often demonstrate discomfort and altered motion when extending the flexed back.
Sacroiliac Joint Pain
Although pain in the region of the SI joint is very common, much of this may actually be referred from more centrally located neuromotion segments.4 SI joint pathology can occur as a result of trauma, degeneration, or inflammatory processes as is seen in ankylosing spondylitis (AS). Patients with AS typically present with a gradual onset of progressive stiffness and pain in the low back and hips that is worse in the morning or following periods of inactivity. It is most common in Caucasian males in their second to fourth decades.14 Although 80% to 95% of patients with AS will test positive for human leukocyte antigen B27 (HLA-B27), it is important to note that the vast majority of people with HLA-B27 do not go on to develop AS.14 Regardless of the cause, SI joint pain can be very debilitating and negatively impact all baseball-related activities.
Stenosis
Lumbar stenosis may develop from arthritic changes, disc protrusion, facet hypertrophy, or ligament ossification. In this young, athletic population, congenital stenosis should also be a consideration. Patients with congenital stenosis at baseline are at increased risk for developing neurologic symptoms from disc protrusion or other acquired spinal pathology. Lumbar stenosis generally manifests as a gradual onset of progressive low back pain with radicular symptoms or neurogenic claudication.4
Making the Diagnosis
History
When identifying the cause of any musculoskeletal complaint, the diagnosis begins with a thorough history. In addition to the standard components of the history, such as timing, severity, relation to activity, exacerbating factors, associated symptoms, and prior treatments, Watkins and colleagues4 have outlined a number of key factors that should be determined when specifically evaluating the athlete with low back pain.These include quantification of the morbidity, identification of contributing psychosocial factors, ruling out of urgent diagnoses (ie, neoplasm, infection, rapidly progressive neurologic deficits, cauda equina, and paralysis), determination of injury type and duration, identification of the clinical syndrome/etiology, pinpointing the location of the pathology (what nerve at what level?), and quantification of back versus leg symptoms. Answers to these questions will set the framework for an appropriately directed physical examination, imaging, and diagnostic tests.
Physical Examination
The physical examination begins by observing the patient or player walk across the playing field, training room, or examination room, paying attention to posture, gait, and overall body movement. Many patients with lumbar injuries will demonstrate adaptive patterns of motion in an attempt to accommodate their pain. This may be seen during baseball-related activities such as throwing, batting, or running. The spine should be visualized and palpated for malalignment while standing erect and during forward bending. If possible, motion should be assessed in rotation, lateral bending, and the flexion and extension planes. Special attention should be paid to any positions or maneuvers that reproduce pain or neurologic symptoms. Areas of tenderness and radiating pain should be fully palpated. A full neurologic examination consisting of manual muscle testing, sensory examination, and reflex evaluation of both the upper and lower extremities should be performed. Numerous special tests and neurologic stretch maneuvers that assess specific lumbar nerve roots have been described.15
Imaging and Diagnostic Tests
Depending on the history and physical examination, imaging of the lumbar spine is not always warranted in the acute setting. This is especially the case if muscle injury, herniation, or annular tears are suspected. In cases of persistent pain, trauma, or suspected neoplasia, imaging is generally warranted. When x-rays are negative and spondylolysis is suspected, bone scan with lumbar single photon emission computed tomography (SPECT) is the most sensitive test.16 SPECT scans are positive in active spondylolysis because the radio-nucleotide is taken up by active, bone-forming osteoblasts. Quiescent stress fractures that are not apparent on SPECT scans are generally chronic and painless.4 If the SPECT scan is positive, the injury can be further characterized by computed tomography (CT) (Figure 1), which can distinguish between spondylolysis, osteoid osteoma, osteoblastoma, acute fracture, or arthritic degeneration. When the SPECT is negative, or if neural impingement is suspected, magnetic resonance imaging (MRI) (Figure 2) is likely the best diagnostic imaging tool. MRI allows identification of bone edema, disc herniation, annular disruption, disc desiccation, stenosis, and nerve entrapment. Finally, when attempting to distinguish between central and peripheral nerve entrapment syndromes, an electromyogram (EMG) or nerve conduction study (NCS) is a reliable way to identify the location of injury.
Treatment and Outcomes
The approach to a patient with low back pain begins with identification of the etiology and discontinuation of the activities that reproduce pain.4 Trunk stabilization exercises and anti-inflammatory medications are the mainstays of treatment regardless of the cause of the lumbar spinal injury in the baseball player.4
Stress Fracture or Spondylolysis
Management of symptomatic spondylolysis or spondylolisthesis in the athlete initially consists of conservative treatment, which achieves good to excellent long-term outcomes and return to play in 70% to 90% of athletes, especially for acute injuries.17-19 After stopping the activity that causes the pain, trunk stabilization exercises should be started as soon as tolerated with the use of non-steroidal anti-inflammatory medications (NSAIDs), oral steroids, and spinal injections to control symptoms and permit initiation of the rehabilitation program.4 Although bracing is a commonly used adjunctive treatment, a recent meta-analysis did not demonstrate any difference in clinical outcomes between patients treated with a brace compared to non-braced controls.20
Surgical indications for the treatment of spondylolysis or spondylolisthesis are limited; however, failure of nonoperative treatment after 6 months is a reasonable time to consider surgery.17 The spondylolytic defect can often be repaired directly using hook screws, translaminar screws, wiring, pedicle screws, or image-guided lag screws across the lesion with grafting.4 Lumbar spinal fusion is less successful in professional athletes due to the high demands placed on adjacent levels as well as the time required for the fusion to heal.4 Bony union can be determined by a CT scan at 6 months postoperatively if the patient has met appropriate return to play criteria.4
Muscle Injury
Management of lumbar sprains and strains typically includes restricting painful postures and a rehabilitation program that focuses on core strengthening within a pain-free arc of motion.21 Because acute injuries typically resolve quickly and spontaneously, a short interval of decreased activity, icing, NSAIDs, and stretching followed by focused strength training is appropriate before return to sports activity.22
Annular Tears and Disc Herniation
Initial management of baseball players with acute lumbar disc herniation and/or annular tears consists of rest for up to 5 days followed by physical therapy and NSAIDs, Medrol Dose pak, or epidural injections.4 Professional baseball players return to play at high rates following a herniated lumbar disc.6 Earhart and colleagues6 found that 97.1% of players returned to play at an average time of 6.6 months from the time of injury. When stratified by position, all pitchers (29 of 29) returned to competitive play after operative or nonoperative management, while 38 of 40 hitters returned.6 The average career length after lumbar disc herniation in the professional baseball player is between 4.1 and 5.3 years or between 256 and 471 games.6,23 Other work has suggested that players undergoing operative treatment for lumbar herniation had shorter career lengths; however, patients in the operative group tended to be older at the time of injury.23
Emphasis should be placed on nonoperative management of baseball players with disc pathology except in cases of cauda equina syndrome.4 Hitters and pitchers who require surgery have demonstrated decreased 1-year and 3-year postoperative statistical performance compared to preinjury levels.6 No significant changes in any performance statistic were seen in baseball following nonoperative management.6 Consequently, indications for surgery in the baseball player with lumbar disc pathology includes cauda equina syndrome, progressive neurologic deficit, sufficient morbidity, failure of conservative care, a lesion that can be corrected safely with surgery, and the ability for the patient to comply with a comprehensive postoperative rehabilitation program.4 Operative treatment typically consists of a lumbar microdiscectomy and/or laminotomy. 4,6
Facet Joint Pain
The mainstay of therapy in patients with facet joint pain consists of analgesia and a trunk stabilization program.24 Lumbar zygapophysial joint injections and radiofrequency denervation can be considered if the patient fails 4 weeks of directed conservative treatment.24,25 Injections may be useful in select patients; however, the literature supporting the use of lumbar facet joint injections or radiofrequency denervation for facetogenic pain is limited.24,25
Sacroiliac Joint Pain
Acute injury of the SI joint can be treated with NSAIDs, icing, and relative rest.26 Mobilization of the SI joint in addition to correcting any asymmetries in muscle length or stiffness should be started and progressed as soon as tolerated within a pain-free range of motion.26 Rehabilitation should correct biomechanical deficits and maladaptation with a special focus on agonist and antagonist muscle groups across the sacrum and ilium.26 Treatment of AS in the athlete should emphasize symptom control, as there is no definite treatment. For patients with AS, other long-term therapeutic options include sulfasalazine, methotrexate, thalidomide, and anti-tumor necrosis factor therapies.14
Stenosis
Lumbar spinal stenosis, whether congenital or acquired, should initially be managed conservatively.27 Although they do not alter the progression of the disease, epidural steroids and local injections may temporarily decrease symptoms in approximately 40% of cases.27 Those who fail conservative therapy after 3 months may be candidates for surgical decompression and/or fusion.27,28 However, surgical treatment for lumbar spinal stenosis in elite baseball players has not been thoroughly studied, so the long-term prognosis is not well documented.27
Rehabilitation and Prevention of Injuries
After an appropriate diagnosis has been made, a structured rehabilitation process should commence. During rehabilitation, it is of primary importance that deep core stabilization is established. As an initial step in this process, athletes are trained to initiate deep core stabilization with breathing techniques in a static, supine position.29 Proper diaphragm activation with co-contractions of the transverse abdominis (TA) and pelvic floor has been shown to increase lumbar spine stability.30 This will allow for an increase in intra-abdominal pressure (IAP) and improved stabilization of the lumbar spine, creating a muscular cylinder between the bottom of the rib cage and top of the pelvis. These activities are initiated in the supine position but are soon advanced as upper and lower extremity movement against resistance is added. It is important to make sure IAP and contraction of the TA is maintained throughout this sequence of progression.
Once deep core stabilization has been established, athletes are progressed to global muscle training and kinetic linking in all 3 planes of movement. This is an important phase, as lumbar stability is a result of coordinated muscle activation involving many muscles.31 This program progresses from supine breathing exercises to a modified side bridge position to enhance core activation along with frontal plane stability. Next, athletes are progressed to a half kneeling position and then on to standing. Rotational activities are introduced starting with isometric holds progressing to chops/lifts and rotational medicine ball toss. During these tasks, focus should be on quality of movement and maintenance of core activation. Endurance of these muscles should be trained during this process. Appropriate pain-free and safe cardiovascular exercise, such as walking, biking, swimming, and jogging, should be performed throughout each stage in the rehabilitation process. Activities should be halted with any increase in pain. At the completion of the rehabilitation process, it is important to observe the athlete while performing sport-specific tasks. Spinal stabilization must be translational and monitored by observing maintenance of the “cylinder” from the training room to sports specific movements.
Since poor lumbar control has been associated with increased amount of time on the disabled list,5 it would be ideal to identify those at risk of injury before problems arise. Conte and colleagues32 have shown that core muscle strains could be a result of muscle imbalance or improper pitching or hitting technique. Other work has demonstrated that pitchers with poor lumbopelvic control did not perform as well as those with superior control.33 By assessing spinal stability and biomechanics at baseline, we may be able to identify those at risk. Pitchers with suboptimal spinal stabilization can present with an unstable balance phase, increased amounts of hyperextension of the lumbar spine from the moment of max cocking through ball release, as well as increased lateral trunk tilt at ball release. Correcting these flaws and increasing deep core stabilization can prevent injuries and improve performance.
Summary
A stable, well-functioning lumbar spine is vital to nearly every baseball-related activity, including pitching, throwing, batting, fielding, and running. The spine serves as a critical link in the kinetic chain between the upper and lower extremities. Due to the high demand on the lumbar spine, injuries to this area represent a significant amount of time out of play in MLB. Initial treatment typically consists of a comprehensive nonoperative rehabilitation process involving analgesics, rest, and therapy focusing on core stabilization. Because poor lumbopelvic control and mechanics have been demonstrated to increase injury risk, preemptive spinal and core stabilization is likely an appropriate step towards injury prevention.
1. Conte S, Requa RK, Garrick JG. Disability days in major league baseball. Am J Sports Med. 2001;29(4):431-436.
2. Posner M, Cameron KL, Wolf JM, Belmont PJ, Owens BD. Epidemiology of Major League Baseball injuries. Am J Sports Med. 2011;39(8):1676-1680.
3. Makhni EC, Buza JA, Byram I, Ahmad CS. Sports reporting: a comprehensive review of the medical literature regarding North American professional sports. Phys Sportsmed. 2014;42(2):154-162.
4. Watkins RG III, Watkins RG IV. Chapter 36: Lumbar injuries. In: Sports Medicine of Baseball. Dines JS, Altchek DW, Andrews JR, ElAttrache NS, Wilk KE, Yocum LA, eds. Philadelphia, PA: Lippincott Williams & Wilkins; 2012; 383-398.
5. Chaudhari AMW, McKenzie CS, Pan X, Oñate JA. Lumbopelvic control and days missed because of injury in professional baseball pitchers. Am J Sports Med. 2014;42(11):2734-2740.
6. Earhart JS, Roberts D, Roc G, Gryzlo S, Hsu W. Effects of lumbar disk herniation on the careers of professional baseball players. Orthopedics. 2012;35(1):43-49.
7. Hamid KS, Nwachukwu BU, Hsu E, Edgerton CA, Hobson DR, Lang JE. Orthopedic resident work-shift analysis: Are we making the best use of resident work hours? J Surg Educ. 2014;71(2):205-210.
8. Nair R, Kahlenberg CA, Hsu WK. Outcomes of lumbar discectomy in elite athletes: the need for high-level evidence. Clin Orthop Relat Res. 2015;473(6):1971-1977.
9. Fleisig GS, Hsu WK, Fortenbaugh D, Cordover A, Press JM. Trunk axial rotation in baseball pitching and batting. Sports Biomech. 2013;12(4):324-333.
10. Watkins RG, Dennis S, Dillin WH, et al. Dynamic EMG analysis of torque transfer in professional baseball pitchers. Spine (Phila Pa 1976). 1989;14(4):404-408.
11. Micheli LJ. Back injuries in gymnastics. Clin Sports Med. 1985;4(1):85-93.
12. Papanicolaou N, Wilkinson RH, Emans JB, Treves S, Micheli LJ. Bone scintigraphy and radiography in young athletes with low back pain. AJR Am J Roentgenol. 1985;145(5):1039-1044.
13. Elliott S, Hutson MA, Wastie ML. Bone scintigraphy in the assessment of spondylolysis in patients attending a sports injury clinic. Clin Radiol. 1988;39(3):269-272.
14. Kubiak EN, Moskovich R, Errico TJ, Di Cesare PE. Orthopaedic management of ankylosing spondylitis. J Am Acad Orthop Surg. 2005;13(4):267-278.
15. Miller KJ. Physical assessment of lower extremity radiculopathy and sciatica. J Chiropr Med. 2007;6(2):75-82.
16. Bellah RD, Summerville DA, Treves ST, Micheli LJ. Low-back pain in adolescent athletes: detection of stress injury to the pars interarticularis with SPECT. Radiology. 1991;180(2):509-512.
17. Radcliff KE, Kalantar SB, Reitman CA. Surgical management of spondylolysis and spondylolisthesis in athletes: indications and return to play. Curr Sports Med Rep. 8(1):35-40.
18. Morita T, Ikata T, Katoh S, Miyake R. Lumbar spondylolysis in children and adolescents. J Bone Joint Surg Br. 1995;77(4):620-625.
19. Hu SS, Tribus CB, Diab M, Ghanayem AJ. Spondylolisthesis and spondylolysis. J Bone Joint Surg Am. 2008;90(3):656-671.
20. Klein G, Mehlman CT, McCarty M. Nonoperative treatment of spondylolysis and grade I spondylolisthesis in children and young adults: a meta-analysis of observational studies. J Pediatr Orthop. 2009;29(2):146-156.
21. Bono CM. Low-back pain in athletes. J Bone Joint Surg Am. 2004;86-A(2):382-396.
22. Dreisinger TE, Nelson B. Management of back pain in athletes. Sports Med. 1996;21(4):313-320.
23. Hsu WK, McCarthy KJ, Savage JW, et al. The Professional Athlete Spine Initiative: outcomes after lumbar disc herniation in 342 elite professional athletes. Spine J. 2011;11(3):180-186.
24. Dreyfuss PH, Dreyer SJ; NASS. Lumbar zygapophysial (facet) joint injections. Spine J. 2003;3(3 Suppl):50S-59S.
25. Slipman CW, Bhat AL, Gilchrist R V, Issac Z, Chou L, Lenrow DA. A critical review of the evidence for the use of zygapophysial injections and radiofrequency denervation in the treatment of low back pain. Spine J. 2003;3(4):310-316.
26. Prather H. Sacroiliac joint pain: practical management. Clin J Sport Med. 2003;13(4):252-255.
27. Graw BP, Wiesel SW. Low back pain in the aging athlete. Sports Med Arthrosc. 2008;16(1):39-46.
28. Melancia JL, Francisco AF, Antunes JL. Spinal stenosis. Handb Clin Neurol. 2014;119:541-549.
29. Frank C, Kobesova A, Kolar P. Dynamic neuromuscular stabilization & sports rehabilitation. Int J Sports Phys Ther. 2013;8(1):62-73.
30. Cholewicki J, Juluru K, McGill SM. Intra-abdominal pressure mechanism for stabilizing the lumbar spine. J Biomech. 1999;32(1):13-17.
31. McGill SM, Grenier S, Kavcic N, Cholewicki J. Coordination of muscle activity to assure stability of the lumbar spine. J Electromyogr Kinesiol. 2003;13(4):353-359.
32. Conte SA, Thompson MM, Marks MA, Dines JS. Abdominal muscle strains in professional baseball: 1991-2010. Am J Sports Med. 2012;40(3):650-656.
33. Chaudhari AMW, McKenzie CS, Borchers JR, Best TM. Lumbopelvic control and pitching performance of professional baseball pitchers. J Strength Cond Res. 2011;25(8):2127-2132.
For the last 20 years, injuries resulting in time out of play have been on the rise in Major League Baseball (MLB), and those affecting the back are no exception.1,2 In the first comprehensive report on injuries in MLB players, back injuries resulted in a mean of 1016 disabled list days per season from 1995 to 1999.1 Similarly, core and back injuries were responsible for 359 disabled list designations from 2002 to 2008. This represented 11.7% of all injuries resulting in time out of play during that time span.2 During that time, back injury prevalence ranked 6th highest of all possible body regions (out of 17), and both position players and pitchers were similarly affected (7.8% and 7.4% of all injuries, respectively).2 These injuries often result in a significant time out of play and can have a tremendous impact on player health. A healthy, stable, and well-functioning lumbar spine is a prerequisite for nearly all baseball-related activities, including pitching, throwing, batting, and running. Accordingly, even minor lumbar spine injuries may profoundly influence baseball performance. Despite this, less is currently known about the true epidemiology and impact of back injuries in professional baseball compared to other professional sporting organizations.3
The most common causes of low back pain and injury in elite baseball players include muscle strains, stress fractures (spondylolysis), annular tears, disc herniation, stenosis, transverse process fractures, facetogenic pain, and sacroiliac (SI) joint arthropathy.4-8 These injuries present in a variety of ways with varying symptomatology. Accordingly, a thorough understanding and comprehensive approach to the diagnosis and treatment of these injuries is necessary. The purpose of this article is to discuss the current state of lumbar spine injuries in professional baseball players. Specifically, we will discuss the critical role of the spine in baseball activities, common causes of injury, tips for making the diagnosis, treatment options, outcomes, and injury prevention and rehabilitation strategies.
Role of the Spine in Baseball
The spine and core musculature are responsible for positioning the head, shoulders, and upper extremities in space over the hips and lower extremities. Proper maintenance of this relationship is required during all phases of throwing, pitching, running, and hitting. During these activities, the spine may dynamically flex, extend, rotate, and laterally bend as needed to keep the body balanced with the head centered over the trunk.
Pitching and Throwing
Whether pitching from the wind-up or the stretch, the head begins centered over the hips and pelvis. As the pitching motion progresses, the hips undergo rotation, flexion, extension, abduction, and circumduction. While this is occurring, the shoulders and upper truck must bend, rotate, and translate toward home plate with the body. Just prior to front foot contact, trunk rotation averages 55 ± 6° with a maximal mean angular acceleration of 11,600 ± 3100°/s2. 9 In order for the body to remain balanced, controlled, and synchronized throughout this delivery, the lumbar spine and core musculature must work diligently to stabilize the entire kinetic chain. Of all the trunk muscles (paraspinal, rectus abdominis, obliques, and glutei), the lumbar paraspinal muscles often work the hardest during the pitching motion, demonstrating activity increases ranging from 100% to 400%.10 Accordingly, it is not uncommon for pitchers to develop SI joint or lumbar facet joint pain due to this high degree of torsional strain exerted on the low back.4 Poor lumbopelvic control has been shown to be a predictor of subsequent injury, and the degree of lumbopelvic dysfunction is proportional to injury severity in MLB pitchers.5
Hitting
Similar to pitching, hitting involves a complex combination of movements from the upper and lower extremities that must be balanced by the core and spine. Although numerous movements occur simultaneously, rotational motion is primarily responsible for generating power. The trunk rotates an average of 46 ± 9° during the swing and reaches a maximal angular acceleration of 7200 ± 2800°/s2 just after contact.9 During this period of rapid torsion, the spine must rotate in conjunction with the hips and shoulders to create a stable cylinder and axis of rotation. The spine and core are responsible for synchronizing rotation to ensure that hip and shoulder parallelism is maintained from swing initiation to ball contact. If the body does not rotate as a unit, the position of the head is affected and the batter’s ability to see the ball may be compromised. Additionally, if delivery of the shoulders lags too far behind that of the hips, the position of the hands (and bat) in space is adversely affected. The entire kinetic chain must remain balanced, coordinated, precisely timed, and standardized throughout the entire swing from initial trigger to final follow-through. The lumbar spine plays a critical role in each of these steps. If lumbar spine mechanics are not sound, this can have significant adverse effects on batting performance and may predispose hitters to injury.4
Common Etiologies for Spinal Injury
The vast majority of baseball players who experience lumbar pain will have injuries that can be classified as mechanical back pain (ie, spondylolysis, annular tears, facetogenic pain, SI joint arthropathy, or muscle injuries) (Table). Although less likely to occur, nerve entrapment or impingement syndromes (ie, disc herniation, stenosis, and peripheral nerve entrapment) have been observed in professional baseball players. Finally, more concerning pathologies such as infection and tumor are extremely rare, but they must not be overlooked in this high-demand patient population.
Stress Fracture or Spondylolysis
In young athletic patients, up to one-third of those with low back pain may have evidence of a lumbar stress fracture on bone scan.11,12 This is particularly true for athletes who undergo repetitive lumbar extension and rotation, such as linemen, gymnasts, wrestlers, weight lifters, and baseball players.4,13 Although the majority of lumbar stress fractures occur at the pars interarticularis, they can occur in the pedicle or articular process (Figure 1). Most spondylolytic lesions do not progress to spondylolisthesis, especially once patients reach skeletal maturity. Because the fifth lumbar vertebra represents the transition from the lumbar to the sacral spine, most stress fractures occur at L5. These typically present as localized low back pain that worsens with flexion, extension, and rotation.
Muscle Injury
One of the most common causes of low back pain in athletes is muscle strains and spasms. Because the lumbar paraspinal muscles are extremely active during throwing and hitting,10 they are particularly susceptible to injury. This is particularly true in deconditioned athletes or those who report to spring training having not adequately maintained strength and flexibility through the off-season.4,5 These injuries typically present in an acute fashion with an obvious inciting incident. Players may have a history of similar muscle injuries in the past. On examination, they tend to have difficulty maintaining normal posture or ranging the spine through a full arc of motion. Localized, superficial tenderness to palpation in the injured muscle is a key component of the diagnosis.
Annular Tears and Disc Herniation
These injuries typically occur as the result of a combination of compressive and rotary forces on the lumbar spine that overcome the ability of the annulus fibrosus to resist hoop stresses. Patients with annular tears typically present with severe lower back pain that may be accompanied by spasm and pain radiation into the buttock or lower extremities. Pain is usually worsened by valsalva, coughing, sneezing, or bearing down.4 Although annular tears can occur in isolation, they can also lead to herniation of the nucleus pulposus into the spinal canal (Figure 2). Depending on the location and severity of the herniation, nerve entrapment or impingement can occur. This may initially present as pain that radiates into the lower extremities in a dermatomal fashion. As the herniation progresses, decreased sensation and weakness may develop.
Facet Joint Pain
Facetogenic pain can occur as the result of degenerative changes, trauma, or joint inflammation. Facet injury typically occurs during rotation while the back is extended.4 This results in localized pain and tenderness that can be reproduced by loading the facet joint (lumbar extension) during the examination, and patients will often demonstrate discomfort and altered motion when extending the flexed back.
Sacroiliac Joint Pain
Although pain in the region of the SI joint is very common, much of this may actually be referred from more centrally located neuromotion segments.4 SI joint pathology can occur as a result of trauma, degeneration, or inflammatory processes as is seen in ankylosing spondylitis (AS). Patients with AS typically present with a gradual onset of progressive stiffness and pain in the low back and hips that is worse in the morning or following periods of inactivity. It is most common in Caucasian males in their second to fourth decades.14 Although 80% to 95% of patients with AS will test positive for human leukocyte antigen B27 (HLA-B27), it is important to note that the vast majority of people with HLA-B27 do not go on to develop AS.14 Regardless of the cause, SI joint pain can be very debilitating and negatively impact all baseball-related activities.
Stenosis
Lumbar stenosis may develop from arthritic changes, disc protrusion, facet hypertrophy, or ligament ossification. In this young, athletic population, congenital stenosis should also be a consideration. Patients with congenital stenosis at baseline are at increased risk for developing neurologic symptoms from disc protrusion or other acquired spinal pathology. Lumbar stenosis generally manifests as a gradual onset of progressive low back pain with radicular symptoms or neurogenic claudication.4
Making the Diagnosis
History
When identifying the cause of any musculoskeletal complaint, the diagnosis begins with a thorough history. In addition to the standard components of the history, such as timing, severity, relation to activity, exacerbating factors, associated symptoms, and prior treatments, Watkins and colleagues4 have outlined a number of key factors that should be determined when specifically evaluating the athlete with low back pain.These include quantification of the morbidity, identification of contributing psychosocial factors, ruling out of urgent diagnoses (ie, neoplasm, infection, rapidly progressive neurologic deficits, cauda equina, and paralysis), determination of injury type and duration, identification of the clinical syndrome/etiology, pinpointing the location of the pathology (what nerve at what level?), and quantification of back versus leg symptoms. Answers to these questions will set the framework for an appropriately directed physical examination, imaging, and diagnostic tests.
Physical Examination
The physical examination begins by observing the patient or player walk across the playing field, training room, or examination room, paying attention to posture, gait, and overall body movement. Many patients with lumbar injuries will demonstrate adaptive patterns of motion in an attempt to accommodate their pain. This may be seen during baseball-related activities such as throwing, batting, or running. The spine should be visualized and palpated for malalignment while standing erect and during forward bending. If possible, motion should be assessed in rotation, lateral bending, and the flexion and extension planes. Special attention should be paid to any positions or maneuvers that reproduce pain or neurologic symptoms. Areas of tenderness and radiating pain should be fully palpated. A full neurologic examination consisting of manual muscle testing, sensory examination, and reflex evaluation of both the upper and lower extremities should be performed. Numerous special tests and neurologic stretch maneuvers that assess specific lumbar nerve roots have been described.15
Imaging and Diagnostic Tests
Depending on the history and physical examination, imaging of the lumbar spine is not always warranted in the acute setting. This is especially the case if muscle injury, herniation, or annular tears are suspected. In cases of persistent pain, trauma, or suspected neoplasia, imaging is generally warranted. When x-rays are negative and spondylolysis is suspected, bone scan with lumbar single photon emission computed tomography (SPECT) is the most sensitive test.16 SPECT scans are positive in active spondylolysis because the radio-nucleotide is taken up by active, bone-forming osteoblasts. Quiescent stress fractures that are not apparent on SPECT scans are generally chronic and painless.4 If the SPECT scan is positive, the injury can be further characterized by computed tomography (CT) (Figure 1), which can distinguish between spondylolysis, osteoid osteoma, osteoblastoma, acute fracture, or arthritic degeneration. When the SPECT is negative, or if neural impingement is suspected, magnetic resonance imaging (MRI) (Figure 2) is likely the best diagnostic imaging tool. MRI allows identification of bone edema, disc herniation, annular disruption, disc desiccation, stenosis, and nerve entrapment. Finally, when attempting to distinguish between central and peripheral nerve entrapment syndromes, an electromyogram (EMG) or nerve conduction study (NCS) is a reliable way to identify the location of injury.
Treatment and Outcomes
The approach to a patient with low back pain begins with identification of the etiology and discontinuation of the activities that reproduce pain.4 Trunk stabilization exercises and anti-inflammatory medications are the mainstays of treatment regardless of the cause of the lumbar spinal injury in the baseball player.4
Stress Fracture or Spondylolysis
Management of symptomatic spondylolysis or spondylolisthesis in the athlete initially consists of conservative treatment, which achieves good to excellent long-term outcomes and return to play in 70% to 90% of athletes, especially for acute injuries.17-19 After stopping the activity that causes the pain, trunk stabilization exercises should be started as soon as tolerated with the use of non-steroidal anti-inflammatory medications (NSAIDs), oral steroids, and spinal injections to control symptoms and permit initiation of the rehabilitation program.4 Although bracing is a commonly used adjunctive treatment, a recent meta-analysis did not demonstrate any difference in clinical outcomes between patients treated with a brace compared to non-braced controls.20
Surgical indications for the treatment of spondylolysis or spondylolisthesis are limited; however, failure of nonoperative treatment after 6 months is a reasonable time to consider surgery.17 The spondylolytic defect can often be repaired directly using hook screws, translaminar screws, wiring, pedicle screws, or image-guided lag screws across the lesion with grafting.4 Lumbar spinal fusion is less successful in professional athletes due to the high demands placed on adjacent levels as well as the time required for the fusion to heal.4 Bony union can be determined by a CT scan at 6 months postoperatively if the patient has met appropriate return to play criteria.4
Muscle Injury
Management of lumbar sprains and strains typically includes restricting painful postures and a rehabilitation program that focuses on core strengthening within a pain-free arc of motion.21 Because acute injuries typically resolve quickly and spontaneously, a short interval of decreased activity, icing, NSAIDs, and stretching followed by focused strength training is appropriate before return to sports activity.22
Annular Tears and Disc Herniation
Initial management of baseball players with acute lumbar disc herniation and/or annular tears consists of rest for up to 5 days followed by physical therapy and NSAIDs, Medrol Dose pak, or epidural injections.4 Professional baseball players return to play at high rates following a herniated lumbar disc.6 Earhart and colleagues6 found that 97.1% of players returned to play at an average time of 6.6 months from the time of injury. When stratified by position, all pitchers (29 of 29) returned to competitive play after operative or nonoperative management, while 38 of 40 hitters returned.6 The average career length after lumbar disc herniation in the professional baseball player is between 4.1 and 5.3 years or between 256 and 471 games.6,23 Other work has suggested that players undergoing operative treatment for lumbar herniation had shorter career lengths; however, patients in the operative group tended to be older at the time of injury.23
Emphasis should be placed on nonoperative management of baseball players with disc pathology except in cases of cauda equina syndrome.4 Hitters and pitchers who require surgery have demonstrated decreased 1-year and 3-year postoperative statistical performance compared to preinjury levels.6 No significant changes in any performance statistic were seen in baseball following nonoperative management.6 Consequently, indications for surgery in the baseball player with lumbar disc pathology includes cauda equina syndrome, progressive neurologic deficit, sufficient morbidity, failure of conservative care, a lesion that can be corrected safely with surgery, and the ability for the patient to comply with a comprehensive postoperative rehabilitation program.4 Operative treatment typically consists of a lumbar microdiscectomy and/or laminotomy. 4,6
Facet Joint Pain
The mainstay of therapy in patients with facet joint pain consists of analgesia and a trunk stabilization program.24 Lumbar zygapophysial joint injections and radiofrequency denervation can be considered if the patient fails 4 weeks of directed conservative treatment.24,25 Injections may be useful in select patients; however, the literature supporting the use of lumbar facet joint injections or radiofrequency denervation for facetogenic pain is limited.24,25
Sacroiliac Joint Pain
Acute injury of the SI joint can be treated with NSAIDs, icing, and relative rest.26 Mobilization of the SI joint in addition to correcting any asymmetries in muscle length or stiffness should be started and progressed as soon as tolerated within a pain-free range of motion.26 Rehabilitation should correct biomechanical deficits and maladaptation with a special focus on agonist and antagonist muscle groups across the sacrum and ilium.26 Treatment of AS in the athlete should emphasize symptom control, as there is no definite treatment. For patients with AS, other long-term therapeutic options include sulfasalazine, methotrexate, thalidomide, and anti-tumor necrosis factor therapies.14
Stenosis
Lumbar spinal stenosis, whether congenital or acquired, should initially be managed conservatively.27 Although they do not alter the progression of the disease, epidural steroids and local injections may temporarily decrease symptoms in approximately 40% of cases.27 Those who fail conservative therapy after 3 months may be candidates for surgical decompression and/or fusion.27,28 However, surgical treatment for lumbar spinal stenosis in elite baseball players has not been thoroughly studied, so the long-term prognosis is not well documented.27
Rehabilitation and Prevention of Injuries
After an appropriate diagnosis has been made, a structured rehabilitation process should commence. During rehabilitation, it is of primary importance that deep core stabilization is established. As an initial step in this process, athletes are trained to initiate deep core stabilization with breathing techniques in a static, supine position.29 Proper diaphragm activation with co-contractions of the transverse abdominis (TA) and pelvic floor has been shown to increase lumbar spine stability.30 This will allow for an increase in intra-abdominal pressure (IAP) and improved stabilization of the lumbar spine, creating a muscular cylinder between the bottom of the rib cage and top of the pelvis. These activities are initiated in the supine position but are soon advanced as upper and lower extremity movement against resistance is added. It is important to make sure IAP and contraction of the TA is maintained throughout this sequence of progression.
Once deep core stabilization has been established, athletes are progressed to global muscle training and kinetic linking in all 3 planes of movement. This is an important phase, as lumbar stability is a result of coordinated muscle activation involving many muscles.31 This program progresses from supine breathing exercises to a modified side bridge position to enhance core activation along with frontal plane stability. Next, athletes are progressed to a half kneeling position and then on to standing. Rotational activities are introduced starting with isometric holds progressing to chops/lifts and rotational medicine ball toss. During these tasks, focus should be on quality of movement and maintenance of core activation. Endurance of these muscles should be trained during this process. Appropriate pain-free and safe cardiovascular exercise, such as walking, biking, swimming, and jogging, should be performed throughout each stage in the rehabilitation process. Activities should be halted with any increase in pain. At the completion of the rehabilitation process, it is important to observe the athlete while performing sport-specific tasks. Spinal stabilization must be translational and monitored by observing maintenance of the “cylinder” from the training room to sports specific movements.
Since poor lumbar control has been associated with increased amount of time on the disabled list,5 it would be ideal to identify those at risk of injury before problems arise. Conte and colleagues32 have shown that core muscle strains could be a result of muscle imbalance or improper pitching or hitting technique. Other work has demonstrated that pitchers with poor lumbopelvic control did not perform as well as those with superior control.33 By assessing spinal stability and biomechanics at baseline, we may be able to identify those at risk. Pitchers with suboptimal spinal stabilization can present with an unstable balance phase, increased amounts of hyperextension of the lumbar spine from the moment of max cocking through ball release, as well as increased lateral trunk tilt at ball release. Correcting these flaws and increasing deep core stabilization can prevent injuries and improve performance.
Summary
A stable, well-functioning lumbar spine is vital to nearly every baseball-related activity, including pitching, throwing, batting, fielding, and running. The spine serves as a critical link in the kinetic chain between the upper and lower extremities. Due to the high demand on the lumbar spine, injuries to this area represent a significant amount of time out of play in MLB. Initial treatment typically consists of a comprehensive nonoperative rehabilitation process involving analgesics, rest, and therapy focusing on core stabilization. Because poor lumbopelvic control and mechanics have been demonstrated to increase injury risk, preemptive spinal and core stabilization is likely an appropriate step towards injury prevention.
For the last 20 years, injuries resulting in time out of play have been on the rise in Major League Baseball (MLB), and those affecting the back are no exception.1,2 In the first comprehensive report on injuries in MLB players, back injuries resulted in a mean of 1016 disabled list days per season from 1995 to 1999.1 Similarly, core and back injuries were responsible for 359 disabled list designations from 2002 to 2008. This represented 11.7% of all injuries resulting in time out of play during that time span.2 During that time, back injury prevalence ranked 6th highest of all possible body regions (out of 17), and both position players and pitchers were similarly affected (7.8% and 7.4% of all injuries, respectively).2 These injuries often result in a significant time out of play and can have a tremendous impact on player health. A healthy, stable, and well-functioning lumbar spine is a prerequisite for nearly all baseball-related activities, including pitching, throwing, batting, and running. Accordingly, even minor lumbar spine injuries may profoundly influence baseball performance. Despite this, less is currently known about the true epidemiology and impact of back injuries in professional baseball compared to other professional sporting organizations.3
The most common causes of low back pain and injury in elite baseball players include muscle strains, stress fractures (spondylolysis), annular tears, disc herniation, stenosis, transverse process fractures, facetogenic pain, and sacroiliac (SI) joint arthropathy.4-8 These injuries present in a variety of ways with varying symptomatology. Accordingly, a thorough understanding and comprehensive approach to the diagnosis and treatment of these injuries is necessary. The purpose of this article is to discuss the current state of lumbar spine injuries in professional baseball players. Specifically, we will discuss the critical role of the spine in baseball activities, common causes of injury, tips for making the diagnosis, treatment options, outcomes, and injury prevention and rehabilitation strategies.
Role of the Spine in Baseball
The spine and core musculature are responsible for positioning the head, shoulders, and upper extremities in space over the hips and lower extremities. Proper maintenance of this relationship is required during all phases of throwing, pitching, running, and hitting. During these activities, the spine may dynamically flex, extend, rotate, and laterally bend as needed to keep the body balanced with the head centered over the trunk.
Pitching and Throwing
Whether pitching from the wind-up or the stretch, the head begins centered over the hips and pelvis. As the pitching motion progresses, the hips undergo rotation, flexion, extension, abduction, and circumduction. While this is occurring, the shoulders and upper truck must bend, rotate, and translate toward home plate with the body. Just prior to front foot contact, trunk rotation averages 55 ± 6° with a maximal mean angular acceleration of 11,600 ± 3100°/s2. 9 In order for the body to remain balanced, controlled, and synchronized throughout this delivery, the lumbar spine and core musculature must work diligently to stabilize the entire kinetic chain. Of all the trunk muscles (paraspinal, rectus abdominis, obliques, and glutei), the lumbar paraspinal muscles often work the hardest during the pitching motion, demonstrating activity increases ranging from 100% to 400%.10 Accordingly, it is not uncommon for pitchers to develop SI joint or lumbar facet joint pain due to this high degree of torsional strain exerted on the low back.4 Poor lumbopelvic control has been shown to be a predictor of subsequent injury, and the degree of lumbopelvic dysfunction is proportional to injury severity in MLB pitchers.5
Hitting
Similar to pitching, hitting involves a complex combination of movements from the upper and lower extremities that must be balanced by the core and spine. Although numerous movements occur simultaneously, rotational motion is primarily responsible for generating power. The trunk rotates an average of 46 ± 9° during the swing and reaches a maximal angular acceleration of 7200 ± 2800°/s2 just after contact.9 During this period of rapid torsion, the spine must rotate in conjunction with the hips and shoulders to create a stable cylinder and axis of rotation. The spine and core are responsible for synchronizing rotation to ensure that hip and shoulder parallelism is maintained from swing initiation to ball contact. If the body does not rotate as a unit, the position of the head is affected and the batter’s ability to see the ball may be compromised. Additionally, if delivery of the shoulders lags too far behind that of the hips, the position of the hands (and bat) in space is adversely affected. The entire kinetic chain must remain balanced, coordinated, precisely timed, and standardized throughout the entire swing from initial trigger to final follow-through. The lumbar spine plays a critical role in each of these steps. If lumbar spine mechanics are not sound, this can have significant adverse effects on batting performance and may predispose hitters to injury.4
Common Etiologies for Spinal Injury
The vast majority of baseball players who experience lumbar pain will have injuries that can be classified as mechanical back pain (ie, spondylolysis, annular tears, facetogenic pain, SI joint arthropathy, or muscle injuries) (Table). Although less likely to occur, nerve entrapment or impingement syndromes (ie, disc herniation, stenosis, and peripheral nerve entrapment) have been observed in professional baseball players. Finally, more concerning pathologies such as infection and tumor are extremely rare, but they must not be overlooked in this high-demand patient population.
Stress Fracture or Spondylolysis
In young athletic patients, up to one-third of those with low back pain may have evidence of a lumbar stress fracture on bone scan.11,12 This is particularly true for athletes who undergo repetitive lumbar extension and rotation, such as linemen, gymnasts, wrestlers, weight lifters, and baseball players.4,13 Although the majority of lumbar stress fractures occur at the pars interarticularis, they can occur in the pedicle or articular process (Figure 1). Most spondylolytic lesions do not progress to spondylolisthesis, especially once patients reach skeletal maturity. Because the fifth lumbar vertebra represents the transition from the lumbar to the sacral spine, most stress fractures occur at L5. These typically present as localized low back pain that worsens with flexion, extension, and rotation.
Muscle Injury
One of the most common causes of low back pain in athletes is muscle strains and spasms. Because the lumbar paraspinal muscles are extremely active during throwing and hitting,10 they are particularly susceptible to injury. This is particularly true in deconditioned athletes or those who report to spring training having not adequately maintained strength and flexibility through the off-season.4,5 These injuries typically present in an acute fashion with an obvious inciting incident. Players may have a history of similar muscle injuries in the past. On examination, they tend to have difficulty maintaining normal posture or ranging the spine through a full arc of motion. Localized, superficial tenderness to palpation in the injured muscle is a key component of the diagnosis.
Annular Tears and Disc Herniation
These injuries typically occur as the result of a combination of compressive and rotary forces on the lumbar spine that overcome the ability of the annulus fibrosus to resist hoop stresses. Patients with annular tears typically present with severe lower back pain that may be accompanied by spasm and pain radiation into the buttock or lower extremities. Pain is usually worsened by valsalva, coughing, sneezing, or bearing down.4 Although annular tears can occur in isolation, they can also lead to herniation of the nucleus pulposus into the spinal canal (Figure 2). Depending on the location and severity of the herniation, nerve entrapment or impingement can occur. This may initially present as pain that radiates into the lower extremities in a dermatomal fashion. As the herniation progresses, decreased sensation and weakness may develop.
Facet Joint Pain
Facetogenic pain can occur as the result of degenerative changes, trauma, or joint inflammation. Facet injury typically occurs during rotation while the back is extended.4 This results in localized pain and tenderness that can be reproduced by loading the facet joint (lumbar extension) during the examination, and patients will often demonstrate discomfort and altered motion when extending the flexed back.
Sacroiliac Joint Pain
Although pain in the region of the SI joint is very common, much of this may actually be referred from more centrally located neuromotion segments.4 SI joint pathology can occur as a result of trauma, degeneration, or inflammatory processes as is seen in ankylosing spondylitis (AS). Patients with AS typically present with a gradual onset of progressive stiffness and pain in the low back and hips that is worse in the morning or following periods of inactivity. It is most common in Caucasian males in their second to fourth decades.14 Although 80% to 95% of patients with AS will test positive for human leukocyte antigen B27 (HLA-B27), it is important to note that the vast majority of people with HLA-B27 do not go on to develop AS.14 Regardless of the cause, SI joint pain can be very debilitating and negatively impact all baseball-related activities.
Stenosis
Lumbar stenosis may develop from arthritic changes, disc protrusion, facet hypertrophy, or ligament ossification. In this young, athletic population, congenital stenosis should also be a consideration. Patients with congenital stenosis at baseline are at increased risk for developing neurologic symptoms from disc protrusion or other acquired spinal pathology. Lumbar stenosis generally manifests as a gradual onset of progressive low back pain with radicular symptoms or neurogenic claudication.4
Making the Diagnosis
History
When identifying the cause of any musculoskeletal complaint, the diagnosis begins with a thorough history. In addition to the standard components of the history, such as timing, severity, relation to activity, exacerbating factors, associated symptoms, and prior treatments, Watkins and colleagues4 have outlined a number of key factors that should be determined when specifically evaluating the athlete with low back pain.These include quantification of the morbidity, identification of contributing psychosocial factors, ruling out of urgent diagnoses (ie, neoplasm, infection, rapidly progressive neurologic deficits, cauda equina, and paralysis), determination of injury type and duration, identification of the clinical syndrome/etiology, pinpointing the location of the pathology (what nerve at what level?), and quantification of back versus leg symptoms. Answers to these questions will set the framework for an appropriately directed physical examination, imaging, and diagnostic tests.
Physical Examination
The physical examination begins by observing the patient or player walk across the playing field, training room, or examination room, paying attention to posture, gait, and overall body movement. Many patients with lumbar injuries will demonstrate adaptive patterns of motion in an attempt to accommodate their pain. This may be seen during baseball-related activities such as throwing, batting, or running. The spine should be visualized and palpated for malalignment while standing erect and during forward bending. If possible, motion should be assessed in rotation, lateral bending, and the flexion and extension planes. Special attention should be paid to any positions or maneuvers that reproduce pain or neurologic symptoms. Areas of tenderness and radiating pain should be fully palpated. A full neurologic examination consisting of manual muscle testing, sensory examination, and reflex evaluation of both the upper and lower extremities should be performed. Numerous special tests and neurologic stretch maneuvers that assess specific lumbar nerve roots have been described.15
Imaging and Diagnostic Tests
Depending on the history and physical examination, imaging of the lumbar spine is not always warranted in the acute setting. This is especially the case if muscle injury, herniation, or annular tears are suspected. In cases of persistent pain, trauma, or suspected neoplasia, imaging is generally warranted. When x-rays are negative and spondylolysis is suspected, bone scan with lumbar single photon emission computed tomography (SPECT) is the most sensitive test.16 SPECT scans are positive in active spondylolysis because the radio-nucleotide is taken up by active, bone-forming osteoblasts. Quiescent stress fractures that are not apparent on SPECT scans are generally chronic and painless.4 If the SPECT scan is positive, the injury can be further characterized by computed tomography (CT) (Figure 1), which can distinguish between spondylolysis, osteoid osteoma, osteoblastoma, acute fracture, or arthritic degeneration. When the SPECT is negative, or if neural impingement is suspected, magnetic resonance imaging (MRI) (Figure 2) is likely the best diagnostic imaging tool. MRI allows identification of bone edema, disc herniation, annular disruption, disc desiccation, stenosis, and nerve entrapment. Finally, when attempting to distinguish between central and peripheral nerve entrapment syndromes, an electromyogram (EMG) or nerve conduction study (NCS) is a reliable way to identify the location of injury.
Treatment and Outcomes
The approach to a patient with low back pain begins with identification of the etiology and discontinuation of the activities that reproduce pain.4 Trunk stabilization exercises and anti-inflammatory medications are the mainstays of treatment regardless of the cause of the lumbar spinal injury in the baseball player.4
Stress Fracture or Spondylolysis
Management of symptomatic spondylolysis or spondylolisthesis in the athlete initially consists of conservative treatment, which achieves good to excellent long-term outcomes and return to play in 70% to 90% of athletes, especially for acute injuries.17-19 After stopping the activity that causes the pain, trunk stabilization exercises should be started as soon as tolerated with the use of non-steroidal anti-inflammatory medications (NSAIDs), oral steroids, and spinal injections to control symptoms and permit initiation of the rehabilitation program.4 Although bracing is a commonly used adjunctive treatment, a recent meta-analysis did not demonstrate any difference in clinical outcomes between patients treated with a brace compared to non-braced controls.20
Surgical indications for the treatment of spondylolysis or spondylolisthesis are limited; however, failure of nonoperative treatment after 6 months is a reasonable time to consider surgery.17 The spondylolytic defect can often be repaired directly using hook screws, translaminar screws, wiring, pedicle screws, or image-guided lag screws across the lesion with grafting.4 Lumbar spinal fusion is less successful in professional athletes due to the high demands placed on adjacent levels as well as the time required for the fusion to heal.4 Bony union can be determined by a CT scan at 6 months postoperatively if the patient has met appropriate return to play criteria.4
Muscle Injury
Management of lumbar sprains and strains typically includes restricting painful postures and a rehabilitation program that focuses on core strengthening within a pain-free arc of motion.21 Because acute injuries typically resolve quickly and spontaneously, a short interval of decreased activity, icing, NSAIDs, and stretching followed by focused strength training is appropriate before return to sports activity.22
Annular Tears and Disc Herniation
Initial management of baseball players with acute lumbar disc herniation and/or annular tears consists of rest for up to 5 days followed by physical therapy and NSAIDs, Medrol Dose pak, or epidural injections.4 Professional baseball players return to play at high rates following a herniated lumbar disc.6 Earhart and colleagues6 found that 97.1% of players returned to play at an average time of 6.6 months from the time of injury. When stratified by position, all pitchers (29 of 29) returned to competitive play after operative or nonoperative management, while 38 of 40 hitters returned.6 The average career length after lumbar disc herniation in the professional baseball player is between 4.1 and 5.3 years or between 256 and 471 games.6,23 Other work has suggested that players undergoing operative treatment for lumbar herniation had shorter career lengths; however, patients in the operative group tended to be older at the time of injury.23
Emphasis should be placed on nonoperative management of baseball players with disc pathology except in cases of cauda equina syndrome.4 Hitters and pitchers who require surgery have demonstrated decreased 1-year and 3-year postoperative statistical performance compared to preinjury levels.6 No significant changes in any performance statistic were seen in baseball following nonoperative management.6 Consequently, indications for surgery in the baseball player with lumbar disc pathology includes cauda equina syndrome, progressive neurologic deficit, sufficient morbidity, failure of conservative care, a lesion that can be corrected safely with surgery, and the ability for the patient to comply with a comprehensive postoperative rehabilitation program.4 Operative treatment typically consists of a lumbar microdiscectomy and/or laminotomy. 4,6
Facet Joint Pain
The mainstay of therapy in patients with facet joint pain consists of analgesia and a trunk stabilization program.24 Lumbar zygapophysial joint injections and radiofrequency denervation can be considered if the patient fails 4 weeks of directed conservative treatment.24,25 Injections may be useful in select patients; however, the literature supporting the use of lumbar facet joint injections or radiofrequency denervation for facetogenic pain is limited.24,25
Sacroiliac Joint Pain
Acute injury of the SI joint can be treated with NSAIDs, icing, and relative rest.26 Mobilization of the SI joint in addition to correcting any asymmetries in muscle length or stiffness should be started and progressed as soon as tolerated within a pain-free range of motion.26 Rehabilitation should correct biomechanical deficits and maladaptation with a special focus on agonist and antagonist muscle groups across the sacrum and ilium.26 Treatment of AS in the athlete should emphasize symptom control, as there is no definite treatment. For patients with AS, other long-term therapeutic options include sulfasalazine, methotrexate, thalidomide, and anti-tumor necrosis factor therapies.14
Stenosis
Lumbar spinal stenosis, whether congenital or acquired, should initially be managed conservatively.27 Although they do not alter the progression of the disease, epidural steroids and local injections may temporarily decrease symptoms in approximately 40% of cases.27 Those who fail conservative therapy after 3 months may be candidates for surgical decompression and/or fusion.27,28 However, surgical treatment for lumbar spinal stenosis in elite baseball players has not been thoroughly studied, so the long-term prognosis is not well documented.27
Rehabilitation and Prevention of Injuries
After an appropriate diagnosis has been made, a structured rehabilitation process should commence. During rehabilitation, it is of primary importance that deep core stabilization is established. As an initial step in this process, athletes are trained to initiate deep core stabilization with breathing techniques in a static, supine position.29 Proper diaphragm activation with co-contractions of the transverse abdominis (TA) and pelvic floor has been shown to increase lumbar spine stability.30 This will allow for an increase in intra-abdominal pressure (IAP) and improved stabilization of the lumbar spine, creating a muscular cylinder between the bottom of the rib cage and top of the pelvis. These activities are initiated in the supine position but are soon advanced as upper and lower extremity movement against resistance is added. It is important to make sure IAP and contraction of the TA is maintained throughout this sequence of progression.
Once deep core stabilization has been established, athletes are progressed to global muscle training and kinetic linking in all 3 planes of movement. This is an important phase, as lumbar stability is a result of coordinated muscle activation involving many muscles.31 This program progresses from supine breathing exercises to a modified side bridge position to enhance core activation along with frontal plane stability. Next, athletes are progressed to a half kneeling position and then on to standing. Rotational activities are introduced starting with isometric holds progressing to chops/lifts and rotational medicine ball toss. During these tasks, focus should be on quality of movement and maintenance of core activation. Endurance of these muscles should be trained during this process. Appropriate pain-free and safe cardiovascular exercise, such as walking, biking, swimming, and jogging, should be performed throughout each stage in the rehabilitation process. Activities should be halted with any increase in pain. At the completion of the rehabilitation process, it is important to observe the athlete while performing sport-specific tasks. Spinal stabilization must be translational and monitored by observing maintenance of the “cylinder” from the training room to sports specific movements.
Since poor lumbar control has been associated with increased amount of time on the disabled list,5 it would be ideal to identify those at risk of injury before problems arise. Conte and colleagues32 have shown that core muscle strains could be a result of muscle imbalance or improper pitching or hitting technique. Other work has demonstrated that pitchers with poor lumbopelvic control did not perform as well as those with superior control.33 By assessing spinal stability and biomechanics at baseline, we may be able to identify those at risk. Pitchers with suboptimal spinal stabilization can present with an unstable balance phase, increased amounts of hyperextension of the lumbar spine from the moment of max cocking through ball release, as well as increased lateral trunk tilt at ball release. Correcting these flaws and increasing deep core stabilization can prevent injuries and improve performance.
Summary
A stable, well-functioning lumbar spine is vital to nearly every baseball-related activity, including pitching, throwing, batting, fielding, and running. The spine serves as a critical link in the kinetic chain between the upper and lower extremities. Due to the high demand on the lumbar spine, injuries to this area represent a significant amount of time out of play in MLB. Initial treatment typically consists of a comprehensive nonoperative rehabilitation process involving analgesics, rest, and therapy focusing on core stabilization. Because poor lumbopelvic control and mechanics have been demonstrated to increase injury risk, preemptive spinal and core stabilization is likely an appropriate step towards injury prevention.
1. Conte S, Requa RK, Garrick JG. Disability days in major league baseball. Am J Sports Med. 2001;29(4):431-436.
2. Posner M, Cameron KL, Wolf JM, Belmont PJ, Owens BD. Epidemiology of Major League Baseball injuries. Am J Sports Med. 2011;39(8):1676-1680.
3. Makhni EC, Buza JA, Byram I, Ahmad CS. Sports reporting: a comprehensive review of the medical literature regarding North American professional sports. Phys Sportsmed. 2014;42(2):154-162.
4. Watkins RG III, Watkins RG IV. Chapter 36: Lumbar injuries. In: Sports Medicine of Baseball. Dines JS, Altchek DW, Andrews JR, ElAttrache NS, Wilk KE, Yocum LA, eds. Philadelphia, PA: Lippincott Williams & Wilkins; 2012; 383-398.
5. Chaudhari AMW, McKenzie CS, Pan X, Oñate JA. Lumbopelvic control and days missed because of injury in professional baseball pitchers. Am J Sports Med. 2014;42(11):2734-2740.
6. Earhart JS, Roberts D, Roc G, Gryzlo S, Hsu W. Effects of lumbar disk herniation on the careers of professional baseball players. Orthopedics. 2012;35(1):43-49.
7. Hamid KS, Nwachukwu BU, Hsu E, Edgerton CA, Hobson DR, Lang JE. Orthopedic resident work-shift analysis: Are we making the best use of resident work hours? J Surg Educ. 2014;71(2):205-210.
8. Nair R, Kahlenberg CA, Hsu WK. Outcomes of lumbar discectomy in elite athletes: the need for high-level evidence. Clin Orthop Relat Res. 2015;473(6):1971-1977.
9. Fleisig GS, Hsu WK, Fortenbaugh D, Cordover A, Press JM. Trunk axial rotation in baseball pitching and batting. Sports Biomech. 2013;12(4):324-333.
10. Watkins RG, Dennis S, Dillin WH, et al. Dynamic EMG analysis of torque transfer in professional baseball pitchers. Spine (Phila Pa 1976). 1989;14(4):404-408.
11. Micheli LJ. Back injuries in gymnastics. Clin Sports Med. 1985;4(1):85-93.
12. Papanicolaou N, Wilkinson RH, Emans JB, Treves S, Micheli LJ. Bone scintigraphy and radiography in young athletes with low back pain. AJR Am J Roentgenol. 1985;145(5):1039-1044.
13. Elliott S, Hutson MA, Wastie ML. Bone scintigraphy in the assessment of spondylolysis in patients attending a sports injury clinic. Clin Radiol. 1988;39(3):269-272.
14. Kubiak EN, Moskovich R, Errico TJ, Di Cesare PE. Orthopaedic management of ankylosing spondylitis. J Am Acad Orthop Surg. 2005;13(4):267-278.
15. Miller KJ. Physical assessment of lower extremity radiculopathy and sciatica. J Chiropr Med. 2007;6(2):75-82.
16. Bellah RD, Summerville DA, Treves ST, Micheli LJ. Low-back pain in adolescent athletes: detection of stress injury to the pars interarticularis with SPECT. Radiology. 1991;180(2):509-512.
17. Radcliff KE, Kalantar SB, Reitman CA. Surgical management of spondylolysis and spondylolisthesis in athletes: indications and return to play. Curr Sports Med Rep. 8(1):35-40.
18. Morita T, Ikata T, Katoh S, Miyake R. Lumbar spondylolysis in children and adolescents. J Bone Joint Surg Br. 1995;77(4):620-625.
19. Hu SS, Tribus CB, Diab M, Ghanayem AJ. Spondylolisthesis and spondylolysis. J Bone Joint Surg Am. 2008;90(3):656-671.
20. Klein G, Mehlman CT, McCarty M. Nonoperative treatment of spondylolysis and grade I spondylolisthesis in children and young adults: a meta-analysis of observational studies. J Pediatr Orthop. 2009;29(2):146-156.
21. Bono CM. Low-back pain in athletes. J Bone Joint Surg Am. 2004;86-A(2):382-396.
22. Dreisinger TE, Nelson B. Management of back pain in athletes. Sports Med. 1996;21(4):313-320.
23. Hsu WK, McCarthy KJ, Savage JW, et al. The Professional Athlete Spine Initiative: outcomes after lumbar disc herniation in 342 elite professional athletes. Spine J. 2011;11(3):180-186.
24. Dreyfuss PH, Dreyer SJ; NASS. Lumbar zygapophysial (facet) joint injections. Spine J. 2003;3(3 Suppl):50S-59S.
25. Slipman CW, Bhat AL, Gilchrist R V, Issac Z, Chou L, Lenrow DA. A critical review of the evidence for the use of zygapophysial injections and radiofrequency denervation in the treatment of low back pain. Spine J. 2003;3(4):310-316.
26. Prather H. Sacroiliac joint pain: practical management. Clin J Sport Med. 2003;13(4):252-255.
27. Graw BP, Wiesel SW. Low back pain in the aging athlete. Sports Med Arthrosc. 2008;16(1):39-46.
28. Melancia JL, Francisco AF, Antunes JL. Spinal stenosis. Handb Clin Neurol. 2014;119:541-549.
29. Frank C, Kobesova A, Kolar P. Dynamic neuromuscular stabilization & sports rehabilitation. Int J Sports Phys Ther. 2013;8(1):62-73.
30. Cholewicki J, Juluru K, McGill SM. Intra-abdominal pressure mechanism for stabilizing the lumbar spine. J Biomech. 1999;32(1):13-17.
31. McGill SM, Grenier S, Kavcic N, Cholewicki J. Coordination of muscle activity to assure stability of the lumbar spine. J Electromyogr Kinesiol. 2003;13(4):353-359.
32. Conte SA, Thompson MM, Marks MA, Dines JS. Abdominal muscle strains in professional baseball: 1991-2010. Am J Sports Med. 2012;40(3):650-656.
33. Chaudhari AMW, McKenzie CS, Borchers JR, Best TM. Lumbopelvic control and pitching performance of professional baseball pitchers. J Strength Cond Res. 2011;25(8):2127-2132.
1. Conte S, Requa RK, Garrick JG. Disability days in major league baseball. Am J Sports Med. 2001;29(4):431-436.
2. Posner M, Cameron KL, Wolf JM, Belmont PJ, Owens BD. Epidemiology of Major League Baseball injuries. Am J Sports Med. 2011;39(8):1676-1680.
3. Makhni EC, Buza JA, Byram I, Ahmad CS. Sports reporting: a comprehensive review of the medical literature regarding North American professional sports. Phys Sportsmed. 2014;42(2):154-162.
4. Watkins RG III, Watkins RG IV. Chapter 36: Lumbar injuries. In: Sports Medicine of Baseball. Dines JS, Altchek DW, Andrews JR, ElAttrache NS, Wilk KE, Yocum LA, eds. Philadelphia, PA: Lippincott Williams & Wilkins; 2012; 383-398.
5. Chaudhari AMW, McKenzie CS, Pan X, Oñate JA. Lumbopelvic control and days missed because of injury in professional baseball pitchers. Am J Sports Med. 2014;42(11):2734-2740.
6. Earhart JS, Roberts D, Roc G, Gryzlo S, Hsu W. Effects of lumbar disk herniation on the careers of professional baseball players. Orthopedics. 2012;35(1):43-49.
7. Hamid KS, Nwachukwu BU, Hsu E, Edgerton CA, Hobson DR, Lang JE. Orthopedic resident work-shift analysis: Are we making the best use of resident work hours? J Surg Educ. 2014;71(2):205-210.
8. Nair R, Kahlenberg CA, Hsu WK. Outcomes of lumbar discectomy in elite athletes: the need for high-level evidence. Clin Orthop Relat Res. 2015;473(6):1971-1977.
9. Fleisig GS, Hsu WK, Fortenbaugh D, Cordover A, Press JM. Trunk axial rotation in baseball pitching and batting. Sports Biomech. 2013;12(4):324-333.
10. Watkins RG, Dennis S, Dillin WH, et al. Dynamic EMG analysis of torque transfer in professional baseball pitchers. Spine (Phila Pa 1976). 1989;14(4):404-408.
11. Micheli LJ. Back injuries in gymnastics. Clin Sports Med. 1985;4(1):85-93.
12. Papanicolaou N, Wilkinson RH, Emans JB, Treves S, Micheli LJ. Bone scintigraphy and radiography in young athletes with low back pain. AJR Am J Roentgenol. 1985;145(5):1039-1044.
13. Elliott S, Hutson MA, Wastie ML. Bone scintigraphy in the assessment of spondylolysis in patients attending a sports injury clinic. Clin Radiol. 1988;39(3):269-272.
14. Kubiak EN, Moskovich R, Errico TJ, Di Cesare PE. Orthopaedic management of ankylosing spondylitis. J Am Acad Orthop Surg. 2005;13(4):267-278.
15. Miller KJ. Physical assessment of lower extremity radiculopathy and sciatica. J Chiropr Med. 2007;6(2):75-82.
16. Bellah RD, Summerville DA, Treves ST, Micheli LJ. Low-back pain in adolescent athletes: detection of stress injury to the pars interarticularis with SPECT. Radiology. 1991;180(2):509-512.
17. Radcliff KE, Kalantar SB, Reitman CA. Surgical management of spondylolysis and spondylolisthesis in athletes: indications and return to play. Curr Sports Med Rep. 8(1):35-40.
18. Morita T, Ikata T, Katoh S, Miyake R. Lumbar spondylolysis in children and adolescents. J Bone Joint Surg Br. 1995;77(4):620-625.
19. Hu SS, Tribus CB, Diab M, Ghanayem AJ. Spondylolisthesis and spondylolysis. J Bone Joint Surg Am. 2008;90(3):656-671.
20. Klein G, Mehlman CT, McCarty M. Nonoperative treatment of spondylolysis and grade I spondylolisthesis in children and young adults: a meta-analysis of observational studies. J Pediatr Orthop. 2009;29(2):146-156.
21. Bono CM. Low-back pain in athletes. J Bone Joint Surg Am. 2004;86-A(2):382-396.
22. Dreisinger TE, Nelson B. Management of back pain in athletes. Sports Med. 1996;21(4):313-320.
23. Hsu WK, McCarthy KJ, Savage JW, et al. The Professional Athlete Spine Initiative: outcomes after lumbar disc herniation in 342 elite professional athletes. Spine J. 2011;11(3):180-186.
24. Dreyfuss PH, Dreyer SJ; NASS. Lumbar zygapophysial (facet) joint injections. Spine J. 2003;3(3 Suppl):50S-59S.
25. Slipman CW, Bhat AL, Gilchrist R V, Issac Z, Chou L, Lenrow DA. A critical review of the evidence for the use of zygapophysial injections and radiofrequency denervation in the treatment of low back pain. Spine J. 2003;3(4):310-316.
26. Prather H. Sacroiliac joint pain: practical management. Clin J Sport Med. 2003;13(4):252-255.
27. Graw BP, Wiesel SW. Low back pain in the aging athlete. Sports Med Arthrosc. 2008;16(1):39-46.
28. Melancia JL, Francisco AF, Antunes JL. Spinal stenosis. Handb Clin Neurol. 2014;119:541-549.
29. Frank C, Kobesova A, Kolar P. Dynamic neuromuscular stabilization & sports rehabilitation. Int J Sports Phys Ther. 2013;8(1):62-73.
30. Cholewicki J, Juluru K, McGill SM. Intra-abdominal pressure mechanism for stabilizing the lumbar spine. J Biomech. 1999;32(1):13-17.
31. McGill SM, Grenier S, Kavcic N, Cholewicki J. Coordination of muscle activity to assure stability of the lumbar spine. J Electromyogr Kinesiol. 2003;13(4):353-359.
32. Conte SA, Thompson MM, Marks MA, Dines JS. Abdominal muscle strains in professional baseball: 1991-2010. Am J Sports Med. 2012;40(3):650-656.
33. Chaudhari AMW, McKenzie CS, Borchers JR, Best TM. Lumbopelvic control and pitching performance of professional baseball pitchers. J Strength Cond Res. 2011;25(8):2127-2132.
Hand Blisters in Major League Baseball Pitchers: Current Concepts and Management
Friction blisters result from repetitive friction and strain forces that develop between the skin and various objects. Blisters form in areas where the stratum corneum and stratum granulosum are sufficiently robust (Figure), such as the palmar and plantar surfaces of the hand and feet. Thus, these layers are capable of transmitting the surface forces to the underlying layer, the stratum spinosum. In areas without strong stratum corneum and stratum granulosum layers, an abrasion forms instead.1
It has been shown that the transmitted frictional forces disrupt the stratum spinosum, with the blister roof being composed of the 2 upper epidermal layers as well as prickle cells of the traumatically disrupted stratum spinosum. The basal cell layer typically shows little damage and the dermal-epidermal junction remains intact.1
Early experimental studies in humans using repeatedly cycled probes demonstrated the pathologic sequence of events in blister formation. First, there is slight exfoliation of the stratum corneum layer, accompanied by a reddened area in the zone of rubbing (erythroderma). This is followed by a pale, narrow demarcation, which forms around the reddened region. Subsequently, this pale area fills in toward the center to occupy the entire affected area, which becomes the blister lesion.1,2
Hydrostatic pressure then causes blister fluid to accumulate within 1 to 2 hours following the trauma. Compared to plasma, the blister fluid has a lower protein level and similar electrolyte content.3,4 Cells in the blister cavity continue to degrade for about 4 hours following the injury, with resumption of cellular activity beginning at 6 hours. Mitotic activity is increased after 24 to 30 hours, and at 48 hours, a new granular layer is present. By 120 hours post-injury, a new stratum corneum is formed.5,6
A number of factors have been found to affect blister formation. Frictional force magnitude and number of cycles play the most obvious role. There is an inverse relationship between the two: as the frictional force increases, fewer cycles are required for blister formation.7 This is likely the reason blisters occur most commonly in areas where the fingertips are in contact with a seam, as opposed to the smoother surface of a baseball.
Many authors have examined moisture’s effect on frictional forces, and found that very dry and very wet skin produce low frictional forces, whereas moist skin produces the highest frictional force.1,2,8-10 In the case of dry skin, this is thought to be due to exfoliation and sloughing of cells from the stratum corneum, which produces a dry lubrication similar to graphite. Very wet skin has a fluid layer that lubricates the 2 surfaces. In the case of moist skin, however, it is hypothesized that surface tension impedes the movement of squamous cells, increasing the frictional forces.2,9 This moist environment is most commonly produced by sweating.
Other factors include skin temperature, which, when elevated, mildly predisposes the skin to blister formation. Some studies have shown temperatures as high as 50°C in rubbing experiments2,7,8,11; however, it should be noted that friction blisters do not resemble second-degree burns, either histologically or clinically.12,13
Blisters in Baseball Pitchers
Blisters in baseball pitchers are a well-known and frequently publicized problem; however, there is a paucity of literature describing the incidence or treatment of such blisters.14,15 The digital pulp experiences frictional forces from the baseball stiches as well as from the distal margin of the nail plate during release of the ball. Forces are transmitted to the ball predominately through the thumb, index, and long fingers. While the thumb acts mainly as a post, the index and long fingers impart the “action” on the ball. Not surprisingly, blisters form most commonly on these 2 fingers. While relatively small in size and significance, the impact of such a blister on a pitcher’s ability to maintain the fine control of his pitches cannot be overlooked. Biomechanical studies have shown that maximum gripping strength is attained when the fingers grasp a dynamometer handle at the level of the distal interphalangeal joint.16 Contact pressure mapping during gripping of a cylindrical object has shown that phalanges 2, 3, and 4 experience the highest forces in gripping and pulling activities.17 No study has specifically addressed phalangeal pressure generation in pitchers, however.
Blister Prevention
Blister prevention and treatment methods in baseball pitchers are steeped in folklore and tradition. Methods for drying out blisters and hardening calluses have included the use of pickle juice, urine, bags of rice, and superglue.14,15 Superglue, surgical glue, or any other foreign substance is not allowed during a game on the finger or hands of pitchers by Major League Baseball rules. Other anecdotal options include the use of compounded medicines that are marketed as creams and sprays designed to toughen skin.
As with other injuries, it is important to recognize any predisposing factors and ways to avoid them. Dampness and temperature (>104°F) have been identified as chief factors that substantially increase the friction coefficient and increase blister incidence.18 While temperature and perspiration are impossible to avoid during competition, steps can be taken to keep the pitcher’s hand dry on the mound as well as between innings, such as a rosin bag, a dry towel, and a rice bucket.
Maintaining fingernail length plays an important role in preventing blister formation. The nail can both protect the adjacent skin by decreasing the frictional force on the skin as well as lead to the development of blisters on the other fingers by repetitive abrasion. Nail length and contour need to be tailored to each pitcher specifically. The length of the nail can protect the finger pulp by minimally “elevating” the ball off of the finger itself. However, too long of a nail may come at the cost of abrading the abutting finger as the spin is imparted onto the ball. The shape of the nail is generally kept well contoured to avoid any sharp edges, which can act as local irritants. In the instance of soft, cracked, or torn nails, some pitchers have used acrylic nails. Maintaining proper fingernail shape and length is an essential preventive measure that requires regular use of clippers and emery boards.
Callus care is also paramount in preventing blister formation. It is believed that development of a callus is inevitable with repetitive throwing and likely protective of the underlying skin. The size and shape of the callus, like that of the nail, needs to be carefully monitored. A callus that becomes overly prominent can lead to increased friction with a baseball seam. This can lead to blister development. A small, smooth callus without edges or loose borders is the goal. The free edges of a callus can be trimmed with clean clippers. Contouring is best performed with careful use of an emery board.
Treatment of Finger Blisters
Blister management is determined by the size of the blister as well as the integrity of the overlying callus. Small blisters with intact skin coverage can be sterilely drained with a needle or a No. 11 blade.6,19-21 This allows apposition of the skin layers and quicker healing. The free edge of the blister can then be repaired with surgical glue. In these instances, a starting pitcher may be required to miss a start to allow further healing. In most cases, there is no need to place the player on the disabled list (DL).
Larger blisters, or those that traumatically open, represent a more concerning issue. The loose layers of skin can be removed, and the raw bed can then be treated with antibiotic ointment for the first 2 to 3 days. Subsequently, benzoin tincture, a commonly used paste of benzoin and alum, can be utilized to toughen the raw skin. Bulky dressings can be applied early in treatment but should then be discouraged, as the underlying skin softens due to the presence of moisture. These instances generally lead to lost time on the field. It is not uncommon that the pitcher requires placement on the 15-day DL.
Summary
Blisters on the fingertips of professional baseball players can lead to significant pain and decreased performance. Prevention of blister formation represents the goal of the player and the medical staff. Skin and nail care requires daily evaluation. When blisters do form, appropriate management can minimize lost time.
1. Sulzberger MB, Cortese TA, Fishman L, Wiley HS. Studies on blisters produced by friction. I. Results of linear rubbing and twisting technics. J Invest Dermatol. 1966;47(5):456-465.
2. Naylor PFD. Experimental friction blisters. Brit J Dermatol. 1955;67(10):327-342.
3. Cortese TA, Mitchell W, Sulzberger MB. Studies on blisters produced by friction. II. The blister fluid. J Invest Dermatol. 1968;50(1):47-53.
4. Schmidt P. Quantification of specific proteins in blister fluid. J Invest Dermatol. 1970;55(4):244-248.
5. Epstein WL, Fukuyama K, Cortese TA. Autographic study of friction blisters. RNA, DNA, and protein synthesis. Arch Dermatol. 1969;99(1):94-106.
6. Cortese TA Jr, Fukuyama K, Epstein W, Sulzberger MB. Treatment of friction blisters. An experimental study. Arch Dermatol. 1968;97(6):717-721.
7. Comaish JS. Epidermal fatigue as a cause of friction blisters. Lancet. 1973;1(7794):81-83.
8. Akers WA, Sulzberger MB. The friction blister. Mil Med. 1972;137(1):l-7.
9. Highley DR, Coomey M, DenBeste M, Wolfman LJ. Frictional properties of skin. J Invest Dermatol. 1977;69(3):303-305.
10. Nacht S, Close J, Yeung D, et al. Skin friction coefficient: changes induced by skin hydration and emollient application and correlation with perceived skin feel. J Soc Cosmet Chern. 1981;32:55-65.
11. Griffin TB, Corqese TA, Layton LL, et al. Inverse time and temperature relationship in experimental friction blisters. J Invest Dermatol. 1969;52:391.
12. Shupp JW, Nazabzadeh TJ, Rosenthal DS, Jordan MH, Fidler P, Jeng JC. A review of the local pathophysiologic bases of burn wound progression. J Burn Care Res. 2010;31(6):849-873.
13. Knapik JJ, Reynolds KL, Duplantis KL, Jones BH. Friction blisters: pathophysiology, prevention and treatment. Sports Med. 1995;20(3):136-147.
14. Sielski M. C.J. Wilson on pitching-hand care. The Wall Street Journal. October 30, 2010. Available at: http://blogs.wsj.com/dailyfix/2010/10/30/cj-wilson-on-caring-for-his-pitching-hand Accessed January 10, 2016.
15. Trezza J. Blisters are normal part of pitching for Lynn. St. Louis Post-Dispatch. July 4, 2014. Available at: http://www.stltoday.com/sports/baseball/professional/blisters-are-normal-part-of-pitching-for-lynn/article_9743c6f9-14b2-5c50-83b4-fa6a3c34cb83.html Accessed January 10, 2016.
16. Kaufmann RA, Kozin SH, Mirarchi A, Holland B, Porter S. Biomechanical analysis of flexor digitorum profundus and superficialis in grip-strenth generation. Am J Orthop. 2007;36(9):E128-E132.
17. Nicholas JW, Corvese RJ, Woolley C, Armstrong TJ. Quantification of hand grasp force using a pressure mapping system. Work. 2012;41(Suppl 1):605-612.
18. Knapik JJ, Reynolds KL. Risk factors for foot blisters during road marching: tobacco use, ethnicity, foot type, previous illness and others. Mil Med. 1999;164(2):92-97.
19. Emer J, Sivek R, Marciniak B. Sports Dermatology: Part 1 of 2. Traumatic or mechanical injuries, inflammatory conditions, and exacerbations of pre-existing conditions. J Clin Aesthet Dermatol. 2015;8(4):31-43.
20. De Luca JF, Adams BB, Yosipovitch G. Skin manifestations of athletes competing in the summer olympics: what a sports medicine physician should know. Sports Med. 2012;42(5):399-413.
21. Helm TN, Bergfeld WF. Sports dermatology. Clin Dermatol. 199;16(1):159-165.
Friction blisters result from repetitive friction and strain forces that develop between the skin and various objects. Blisters form in areas where the stratum corneum and stratum granulosum are sufficiently robust (Figure), such as the palmar and plantar surfaces of the hand and feet. Thus, these layers are capable of transmitting the surface forces to the underlying layer, the stratum spinosum. In areas without strong stratum corneum and stratum granulosum layers, an abrasion forms instead.1
It has been shown that the transmitted frictional forces disrupt the stratum spinosum, with the blister roof being composed of the 2 upper epidermal layers as well as prickle cells of the traumatically disrupted stratum spinosum. The basal cell layer typically shows little damage and the dermal-epidermal junction remains intact.1
Early experimental studies in humans using repeatedly cycled probes demonstrated the pathologic sequence of events in blister formation. First, there is slight exfoliation of the stratum corneum layer, accompanied by a reddened area in the zone of rubbing (erythroderma). This is followed by a pale, narrow demarcation, which forms around the reddened region. Subsequently, this pale area fills in toward the center to occupy the entire affected area, which becomes the blister lesion.1,2
Hydrostatic pressure then causes blister fluid to accumulate within 1 to 2 hours following the trauma. Compared to plasma, the blister fluid has a lower protein level and similar electrolyte content.3,4 Cells in the blister cavity continue to degrade for about 4 hours following the injury, with resumption of cellular activity beginning at 6 hours. Mitotic activity is increased after 24 to 30 hours, and at 48 hours, a new granular layer is present. By 120 hours post-injury, a new stratum corneum is formed.5,6
A number of factors have been found to affect blister formation. Frictional force magnitude and number of cycles play the most obvious role. There is an inverse relationship between the two: as the frictional force increases, fewer cycles are required for blister formation.7 This is likely the reason blisters occur most commonly in areas where the fingertips are in contact with a seam, as opposed to the smoother surface of a baseball.
Many authors have examined moisture’s effect on frictional forces, and found that very dry and very wet skin produce low frictional forces, whereas moist skin produces the highest frictional force.1,2,8-10 In the case of dry skin, this is thought to be due to exfoliation and sloughing of cells from the stratum corneum, which produces a dry lubrication similar to graphite. Very wet skin has a fluid layer that lubricates the 2 surfaces. In the case of moist skin, however, it is hypothesized that surface tension impedes the movement of squamous cells, increasing the frictional forces.2,9 This moist environment is most commonly produced by sweating.
Other factors include skin temperature, which, when elevated, mildly predisposes the skin to blister formation. Some studies have shown temperatures as high as 50°C in rubbing experiments2,7,8,11; however, it should be noted that friction blisters do not resemble second-degree burns, either histologically or clinically.12,13
Blisters in Baseball Pitchers
Blisters in baseball pitchers are a well-known and frequently publicized problem; however, there is a paucity of literature describing the incidence or treatment of such blisters.14,15 The digital pulp experiences frictional forces from the baseball stiches as well as from the distal margin of the nail plate during release of the ball. Forces are transmitted to the ball predominately through the thumb, index, and long fingers. While the thumb acts mainly as a post, the index and long fingers impart the “action” on the ball. Not surprisingly, blisters form most commonly on these 2 fingers. While relatively small in size and significance, the impact of such a blister on a pitcher’s ability to maintain the fine control of his pitches cannot be overlooked. Biomechanical studies have shown that maximum gripping strength is attained when the fingers grasp a dynamometer handle at the level of the distal interphalangeal joint.16 Contact pressure mapping during gripping of a cylindrical object has shown that phalanges 2, 3, and 4 experience the highest forces in gripping and pulling activities.17 No study has specifically addressed phalangeal pressure generation in pitchers, however.
Blister Prevention
Blister prevention and treatment methods in baseball pitchers are steeped in folklore and tradition. Methods for drying out blisters and hardening calluses have included the use of pickle juice, urine, bags of rice, and superglue.14,15 Superglue, surgical glue, or any other foreign substance is not allowed during a game on the finger or hands of pitchers by Major League Baseball rules. Other anecdotal options include the use of compounded medicines that are marketed as creams and sprays designed to toughen skin.
As with other injuries, it is important to recognize any predisposing factors and ways to avoid them. Dampness and temperature (>104°F) have been identified as chief factors that substantially increase the friction coefficient and increase blister incidence.18 While temperature and perspiration are impossible to avoid during competition, steps can be taken to keep the pitcher’s hand dry on the mound as well as between innings, such as a rosin bag, a dry towel, and a rice bucket.
Maintaining fingernail length plays an important role in preventing blister formation. The nail can both protect the adjacent skin by decreasing the frictional force on the skin as well as lead to the development of blisters on the other fingers by repetitive abrasion. Nail length and contour need to be tailored to each pitcher specifically. The length of the nail can protect the finger pulp by minimally “elevating” the ball off of the finger itself. However, too long of a nail may come at the cost of abrading the abutting finger as the spin is imparted onto the ball. The shape of the nail is generally kept well contoured to avoid any sharp edges, which can act as local irritants. In the instance of soft, cracked, or torn nails, some pitchers have used acrylic nails. Maintaining proper fingernail shape and length is an essential preventive measure that requires regular use of clippers and emery boards.
Callus care is also paramount in preventing blister formation. It is believed that development of a callus is inevitable with repetitive throwing and likely protective of the underlying skin. The size and shape of the callus, like that of the nail, needs to be carefully monitored. A callus that becomes overly prominent can lead to increased friction with a baseball seam. This can lead to blister development. A small, smooth callus without edges or loose borders is the goal. The free edges of a callus can be trimmed with clean clippers. Contouring is best performed with careful use of an emery board.
Treatment of Finger Blisters
Blister management is determined by the size of the blister as well as the integrity of the overlying callus. Small blisters with intact skin coverage can be sterilely drained with a needle or a No. 11 blade.6,19-21 This allows apposition of the skin layers and quicker healing. The free edge of the blister can then be repaired with surgical glue. In these instances, a starting pitcher may be required to miss a start to allow further healing. In most cases, there is no need to place the player on the disabled list (DL).
Larger blisters, or those that traumatically open, represent a more concerning issue. The loose layers of skin can be removed, and the raw bed can then be treated with antibiotic ointment for the first 2 to 3 days. Subsequently, benzoin tincture, a commonly used paste of benzoin and alum, can be utilized to toughen the raw skin. Bulky dressings can be applied early in treatment but should then be discouraged, as the underlying skin softens due to the presence of moisture. These instances generally lead to lost time on the field. It is not uncommon that the pitcher requires placement on the 15-day DL.
Summary
Blisters on the fingertips of professional baseball players can lead to significant pain and decreased performance. Prevention of blister formation represents the goal of the player and the medical staff. Skin and nail care requires daily evaluation. When blisters do form, appropriate management can minimize lost time.
Friction blisters result from repetitive friction and strain forces that develop between the skin and various objects. Blisters form in areas where the stratum corneum and stratum granulosum are sufficiently robust (Figure), such as the palmar and plantar surfaces of the hand and feet. Thus, these layers are capable of transmitting the surface forces to the underlying layer, the stratum spinosum. In areas without strong stratum corneum and stratum granulosum layers, an abrasion forms instead.1
It has been shown that the transmitted frictional forces disrupt the stratum spinosum, with the blister roof being composed of the 2 upper epidermal layers as well as prickle cells of the traumatically disrupted stratum spinosum. The basal cell layer typically shows little damage and the dermal-epidermal junction remains intact.1
Early experimental studies in humans using repeatedly cycled probes demonstrated the pathologic sequence of events in blister formation. First, there is slight exfoliation of the stratum corneum layer, accompanied by a reddened area in the zone of rubbing (erythroderma). This is followed by a pale, narrow demarcation, which forms around the reddened region. Subsequently, this pale area fills in toward the center to occupy the entire affected area, which becomes the blister lesion.1,2
Hydrostatic pressure then causes blister fluid to accumulate within 1 to 2 hours following the trauma. Compared to plasma, the blister fluid has a lower protein level and similar electrolyte content.3,4 Cells in the blister cavity continue to degrade for about 4 hours following the injury, with resumption of cellular activity beginning at 6 hours. Mitotic activity is increased after 24 to 30 hours, and at 48 hours, a new granular layer is present. By 120 hours post-injury, a new stratum corneum is formed.5,6
A number of factors have been found to affect blister formation. Frictional force magnitude and number of cycles play the most obvious role. There is an inverse relationship between the two: as the frictional force increases, fewer cycles are required for blister formation.7 This is likely the reason blisters occur most commonly in areas where the fingertips are in contact with a seam, as opposed to the smoother surface of a baseball.
Many authors have examined moisture’s effect on frictional forces, and found that very dry and very wet skin produce low frictional forces, whereas moist skin produces the highest frictional force.1,2,8-10 In the case of dry skin, this is thought to be due to exfoliation and sloughing of cells from the stratum corneum, which produces a dry lubrication similar to graphite. Very wet skin has a fluid layer that lubricates the 2 surfaces. In the case of moist skin, however, it is hypothesized that surface tension impedes the movement of squamous cells, increasing the frictional forces.2,9 This moist environment is most commonly produced by sweating.
Other factors include skin temperature, which, when elevated, mildly predisposes the skin to blister formation. Some studies have shown temperatures as high as 50°C in rubbing experiments2,7,8,11; however, it should be noted that friction blisters do not resemble second-degree burns, either histologically or clinically.12,13
Blisters in Baseball Pitchers
Blisters in baseball pitchers are a well-known and frequently publicized problem; however, there is a paucity of literature describing the incidence or treatment of such blisters.14,15 The digital pulp experiences frictional forces from the baseball stiches as well as from the distal margin of the nail plate during release of the ball. Forces are transmitted to the ball predominately through the thumb, index, and long fingers. While the thumb acts mainly as a post, the index and long fingers impart the “action” on the ball. Not surprisingly, blisters form most commonly on these 2 fingers. While relatively small in size and significance, the impact of such a blister on a pitcher’s ability to maintain the fine control of his pitches cannot be overlooked. Biomechanical studies have shown that maximum gripping strength is attained when the fingers grasp a dynamometer handle at the level of the distal interphalangeal joint.16 Contact pressure mapping during gripping of a cylindrical object has shown that phalanges 2, 3, and 4 experience the highest forces in gripping and pulling activities.17 No study has specifically addressed phalangeal pressure generation in pitchers, however.
Blister Prevention
Blister prevention and treatment methods in baseball pitchers are steeped in folklore and tradition. Methods for drying out blisters and hardening calluses have included the use of pickle juice, urine, bags of rice, and superglue.14,15 Superglue, surgical glue, or any other foreign substance is not allowed during a game on the finger or hands of pitchers by Major League Baseball rules. Other anecdotal options include the use of compounded medicines that are marketed as creams and sprays designed to toughen skin.
As with other injuries, it is important to recognize any predisposing factors and ways to avoid them. Dampness and temperature (>104°F) have been identified as chief factors that substantially increase the friction coefficient and increase blister incidence.18 While temperature and perspiration are impossible to avoid during competition, steps can be taken to keep the pitcher’s hand dry on the mound as well as between innings, such as a rosin bag, a dry towel, and a rice bucket.
Maintaining fingernail length plays an important role in preventing blister formation. The nail can both protect the adjacent skin by decreasing the frictional force on the skin as well as lead to the development of blisters on the other fingers by repetitive abrasion. Nail length and contour need to be tailored to each pitcher specifically. The length of the nail can protect the finger pulp by minimally “elevating” the ball off of the finger itself. However, too long of a nail may come at the cost of abrading the abutting finger as the spin is imparted onto the ball. The shape of the nail is generally kept well contoured to avoid any sharp edges, which can act as local irritants. In the instance of soft, cracked, or torn nails, some pitchers have used acrylic nails. Maintaining proper fingernail shape and length is an essential preventive measure that requires regular use of clippers and emery boards.
Callus care is also paramount in preventing blister formation. It is believed that development of a callus is inevitable with repetitive throwing and likely protective of the underlying skin. The size and shape of the callus, like that of the nail, needs to be carefully monitored. A callus that becomes overly prominent can lead to increased friction with a baseball seam. This can lead to blister development. A small, smooth callus without edges or loose borders is the goal. The free edges of a callus can be trimmed with clean clippers. Contouring is best performed with careful use of an emery board.
Treatment of Finger Blisters
Blister management is determined by the size of the blister as well as the integrity of the overlying callus. Small blisters with intact skin coverage can be sterilely drained with a needle or a No. 11 blade.6,19-21 This allows apposition of the skin layers and quicker healing. The free edge of the blister can then be repaired with surgical glue. In these instances, a starting pitcher may be required to miss a start to allow further healing. In most cases, there is no need to place the player on the disabled list (DL).
Larger blisters, or those that traumatically open, represent a more concerning issue. The loose layers of skin can be removed, and the raw bed can then be treated with antibiotic ointment for the first 2 to 3 days. Subsequently, benzoin tincture, a commonly used paste of benzoin and alum, can be utilized to toughen the raw skin. Bulky dressings can be applied early in treatment but should then be discouraged, as the underlying skin softens due to the presence of moisture. These instances generally lead to lost time on the field. It is not uncommon that the pitcher requires placement on the 15-day DL.
Summary
Blisters on the fingertips of professional baseball players can lead to significant pain and decreased performance. Prevention of blister formation represents the goal of the player and the medical staff. Skin and nail care requires daily evaluation. When blisters do form, appropriate management can minimize lost time.
1. Sulzberger MB, Cortese TA, Fishman L, Wiley HS. Studies on blisters produced by friction. I. Results of linear rubbing and twisting technics. J Invest Dermatol. 1966;47(5):456-465.
2. Naylor PFD. Experimental friction blisters. Brit J Dermatol. 1955;67(10):327-342.
3. Cortese TA, Mitchell W, Sulzberger MB. Studies on blisters produced by friction. II. The blister fluid. J Invest Dermatol. 1968;50(1):47-53.
4. Schmidt P. Quantification of specific proteins in blister fluid. J Invest Dermatol. 1970;55(4):244-248.
5. Epstein WL, Fukuyama K, Cortese TA. Autographic study of friction blisters. RNA, DNA, and protein synthesis. Arch Dermatol. 1969;99(1):94-106.
6. Cortese TA Jr, Fukuyama K, Epstein W, Sulzberger MB. Treatment of friction blisters. An experimental study. Arch Dermatol. 1968;97(6):717-721.
7. Comaish JS. Epidermal fatigue as a cause of friction blisters. Lancet. 1973;1(7794):81-83.
8. Akers WA, Sulzberger MB. The friction blister. Mil Med. 1972;137(1):l-7.
9. Highley DR, Coomey M, DenBeste M, Wolfman LJ. Frictional properties of skin. J Invest Dermatol. 1977;69(3):303-305.
10. Nacht S, Close J, Yeung D, et al. Skin friction coefficient: changes induced by skin hydration and emollient application and correlation with perceived skin feel. J Soc Cosmet Chern. 1981;32:55-65.
11. Griffin TB, Corqese TA, Layton LL, et al. Inverse time and temperature relationship in experimental friction blisters. J Invest Dermatol. 1969;52:391.
12. Shupp JW, Nazabzadeh TJ, Rosenthal DS, Jordan MH, Fidler P, Jeng JC. A review of the local pathophysiologic bases of burn wound progression. J Burn Care Res. 2010;31(6):849-873.
13. Knapik JJ, Reynolds KL, Duplantis KL, Jones BH. Friction blisters: pathophysiology, prevention and treatment. Sports Med. 1995;20(3):136-147.
14. Sielski M. C.J. Wilson on pitching-hand care. The Wall Street Journal. October 30, 2010. Available at: http://blogs.wsj.com/dailyfix/2010/10/30/cj-wilson-on-caring-for-his-pitching-hand Accessed January 10, 2016.
15. Trezza J. Blisters are normal part of pitching for Lynn. St. Louis Post-Dispatch. July 4, 2014. Available at: http://www.stltoday.com/sports/baseball/professional/blisters-are-normal-part-of-pitching-for-lynn/article_9743c6f9-14b2-5c50-83b4-fa6a3c34cb83.html Accessed January 10, 2016.
16. Kaufmann RA, Kozin SH, Mirarchi A, Holland B, Porter S. Biomechanical analysis of flexor digitorum profundus and superficialis in grip-strenth generation. Am J Orthop. 2007;36(9):E128-E132.
17. Nicholas JW, Corvese RJ, Woolley C, Armstrong TJ. Quantification of hand grasp force using a pressure mapping system. Work. 2012;41(Suppl 1):605-612.
18. Knapik JJ, Reynolds KL. Risk factors for foot blisters during road marching: tobacco use, ethnicity, foot type, previous illness and others. Mil Med. 1999;164(2):92-97.
19. Emer J, Sivek R, Marciniak B. Sports Dermatology: Part 1 of 2. Traumatic or mechanical injuries, inflammatory conditions, and exacerbations of pre-existing conditions. J Clin Aesthet Dermatol. 2015;8(4):31-43.
20. De Luca JF, Adams BB, Yosipovitch G. Skin manifestations of athletes competing in the summer olympics: what a sports medicine physician should know. Sports Med. 2012;42(5):399-413.
21. Helm TN, Bergfeld WF. Sports dermatology. Clin Dermatol. 199;16(1):159-165.
1. Sulzberger MB, Cortese TA, Fishman L, Wiley HS. Studies on blisters produced by friction. I. Results of linear rubbing and twisting technics. J Invest Dermatol. 1966;47(5):456-465.
2. Naylor PFD. Experimental friction blisters. Brit J Dermatol. 1955;67(10):327-342.
3. Cortese TA, Mitchell W, Sulzberger MB. Studies on blisters produced by friction. II. The blister fluid. J Invest Dermatol. 1968;50(1):47-53.
4. Schmidt P. Quantification of specific proteins in blister fluid. J Invest Dermatol. 1970;55(4):244-248.
5. Epstein WL, Fukuyama K, Cortese TA. Autographic study of friction blisters. RNA, DNA, and protein synthesis. Arch Dermatol. 1969;99(1):94-106.
6. Cortese TA Jr, Fukuyama K, Epstein W, Sulzberger MB. Treatment of friction blisters. An experimental study. Arch Dermatol. 1968;97(6):717-721.
7. Comaish JS. Epidermal fatigue as a cause of friction blisters. Lancet. 1973;1(7794):81-83.
8. Akers WA, Sulzberger MB. The friction blister. Mil Med. 1972;137(1):l-7.
9. Highley DR, Coomey M, DenBeste M, Wolfman LJ. Frictional properties of skin. J Invest Dermatol. 1977;69(3):303-305.
10. Nacht S, Close J, Yeung D, et al. Skin friction coefficient: changes induced by skin hydration and emollient application and correlation with perceived skin feel. J Soc Cosmet Chern. 1981;32:55-65.
11. Griffin TB, Corqese TA, Layton LL, et al. Inverse time and temperature relationship in experimental friction blisters. J Invest Dermatol. 1969;52:391.
12. Shupp JW, Nazabzadeh TJ, Rosenthal DS, Jordan MH, Fidler P, Jeng JC. A review of the local pathophysiologic bases of burn wound progression. J Burn Care Res. 2010;31(6):849-873.
13. Knapik JJ, Reynolds KL, Duplantis KL, Jones BH. Friction blisters: pathophysiology, prevention and treatment. Sports Med. 1995;20(3):136-147.
14. Sielski M. C.J. Wilson on pitching-hand care. The Wall Street Journal. October 30, 2010. Available at: http://blogs.wsj.com/dailyfix/2010/10/30/cj-wilson-on-caring-for-his-pitching-hand Accessed January 10, 2016.
15. Trezza J. Blisters are normal part of pitching for Lynn. St. Louis Post-Dispatch. July 4, 2014. Available at: http://www.stltoday.com/sports/baseball/professional/blisters-are-normal-part-of-pitching-for-lynn/article_9743c6f9-14b2-5c50-83b4-fa6a3c34cb83.html Accessed January 10, 2016.
16. Kaufmann RA, Kozin SH, Mirarchi A, Holland B, Porter S. Biomechanical analysis of flexor digitorum profundus and superficialis in grip-strenth generation. Am J Orthop. 2007;36(9):E128-E132.
17. Nicholas JW, Corvese RJ, Woolley C, Armstrong TJ. Quantification of hand grasp force using a pressure mapping system. Work. 2012;41(Suppl 1):605-612.
18. Knapik JJ, Reynolds KL. Risk factors for foot blisters during road marching: tobacco use, ethnicity, foot type, previous illness and others. Mil Med. 1999;164(2):92-97.
19. Emer J, Sivek R, Marciniak B. Sports Dermatology: Part 1 of 2. Traumatic or mechanical injuries, inflammatory conditions, and exacerbations of pre-existing conditions. J Clin Aesthet Dermatol. 2015;8(4):31-43.
20. De Luca JF, Adams BB, Yosipovitch G. Skin manifestations of athletes competing in the summer olympics: what a sports medicine physician should know. Sports Med. 2012;42(5):399-413.
21. Helm TN, Bergfeld WF. Sports dermatology. Clin Dermatol. 199;16(1):159-165.
Throwing, the Shoulder, and Human Evolution
Charles Darwin once said that apes “...are quite unable, as I have myself seen, to throw a stone with precision”.1 Yet humans can throw with precision and speed, a skill that likely had significant advantages: throwing can affect change at a distance—something few species can do. Throwing can provide protection against predators and can allow for predation for food resources. Throwing would be important in contesting other hominids for scarce resources. As such, throwing has been critically important in human evolution and likely is a skill that has been promoted through natural selection.2-5
In the orthopedic literature, most published work on throwing will ask proximate questions: “how, what, who, when, and where?” Evolutionary biologists are concerned with ultimate questions6,7: “why?” Asking ultimate questions provides insight into how a behavior might offer advantages under natural selection, which can then improve our understanding of the proximate questions for that behavior.
With regard to the shoulder, a number of mysteries exist that, to date, proximate studies have not been able to solve. This article argues that the human shoulder has evolved for throwing and by using this frame of reference, many of the mysteries surrounding the anatomy of the shoulder can be understood.
Pitching Kinematics
The mechanics of pitching have been analyzed extensively. Fleisig and colleagues8 performed kinematic and electromyographic analyses of pitchers to identify the critical moments of pitching (defined as where the forces are highest and injury is most likely going to occur). They found 2 moments where the forces about the shoulder are highest during pitching: the late cocking phase (defined by the point where the humerus reaches maximal external rotation); and the early deceleration phase (defined by the point when the ball is released). If throwing is important in natural selection of humans, then the shoulder anatomy should be optimized to withstand the forces generated in these positions.
Late Cocking Phase of Throwing
The early phases of throwing are attempting to maximize external rotation of the abducted arm as the velocity of the pitched ball correlates to the amount of external rotation achieved.9-11 In this position, kinetic energy in external rotation is stored and converted into kinetic energy in internal rotation.12 The position of the shoulder during late cocking is 94 ± 21° of thoracohumeral abduction, 11 ± 11° of horizontal adduction, and a remarkable 165 ± 11° of thoracohumeral external rotation (Figure 1).8
Fleisig and colleagues8 estimated the torque and forces about the shoulder, which are quite high for joint compression (480 ± 130 N). They also analyzed the shear forces and while trying to describe the origin of superior labrum anterior to posterior (SLAP) lesions and anterior labral tears, broke down the major shear vector into an anterior force vector (310 ± 100 N) and a superior force (250 ± 80 N).8 Note that the resulting shear vector is in an anterosuperior direction and is approximately 400 N.
Early Deceleration Phase of Throwing
Interestingly, the position of the humerus during this critical moment of throwing is not much different than the position during the late cocking phase of throwing, with 93 ± 10° of thoracohumeral abduction, 6 ± 8° of horizontal adduction.8 The major difference in the position of the arm is found in the amount of thoracohumeral rotation, which is now 64 ± 35° of external rotation (Figure 2).8
The forces in early deceleration are tremendous, with an estimated 1090 ± 110 N joint compression force, and an anteroinferior shear force of approximately 130 N.8
Clearly, if throwing is an important skill in human evolution, adaptations must exist in the shoulder to withstand the high forces in these 2 critical moments of throwing.
Solving Mysteries of Shoulder Anatomy in the Context of Throwing
There are many anatomic features of the shoulder that remain poorly understood. These include the alignment of the glenohumeral joint, the function of the glenohumeral ligaments, the function of the coracoacromial ligament, the depression of the human greater tuberosity, and the nature and function of the very tendinous subscapularis and long head of the biceps. These mysteries of the human shoulder can be solved if one considers the hypothesis that the shoulder has evolved to throw.
Glenohumeral Joint Alignment
The cartilage of the humeral head is thickest at its center, and thinnest at the periphery (Figure 3A).13,14 Conversely, the cartilage of the glenoid is thinnest at the fovea and thickest in the periphery (Figure 3B).14 It seems obvious that in order to maximally distribute high loads across this joint, the center of the humeral head should rest in the center of the glenoid. Interestingly, this does not occur during most positions of the shoulder. When upright, the center of the humeral head is directed above the glenoid in the coronal plane (Figure 3C). In order to align the glenohumeral joint optimally for the distribution of loads across the joint, the humerus must be abducted approximately 60° relative to the scapula. Assuming a 2:1 glenohumeral to scapulothoracic abduction for arm abduction relative to the thorax,15 this equates to approximately 90° of thoracohumeral abduction—the exact kinematic position of the shoulder during both critical moments of throwing (Figure 3D).
Function of the Glenohumeral Ligaments
The glenohumeral joint capsule has thickenings that help to stabilize the joint. The function of these glenohumeral ligaments has been evaluated biomechanically for their role in preventing translation and instability by a number of authors. The inferior glenohumeral ligament has classically been described as resisting anterior translation of the abducted arm.16 The coracohumeral ligament has been described as important to prevent inferior translation of the adducted arm.17
Interestingly, these ligaments are also the most important ligaments in resisting external rotation of the adducted arm.18 The dominant arm of throwing athletes has been shown to have increased inferior translation19 and increase external rotation.19-22 While the external rotation is partly related to bony adaptation,23,24 the ligamentous restraints to external rotation are likely under tremendous load, which may explain why Dr. Frank Jobe revolutionized the surgical treatment of the throwing athlete by performing an “instability” operation,25,26 as he believed these athletes had “subtle instability” that produced pain, but not symptoms of looseness.27
While these ligaments may exist in part to prevent translation and instability, current thinking suggests that “over-rotation” may lead to internal impingement and may be responsible for symptoms in the thrower’s shoulder,28 as SLAP lesions seem to occur easier with external rotation.29 Again, the importance of maximizing external rotation in throwing and the finding that this position is a critical moment with very high forces suggests that these ligaments may represent an adaptation to restrain external rotation while throwing.
Coracoacromial Ligament
The coracoacromial ligament is unique in that it connects 2 pieces of the same bone, and is only seen in hominids—not other primates.30 Its function has been debated for decades. This ligament is generally thought to limit superior translation of the humeral head,31,32 an effect that is critically important in patients with rotator cuff tears 33,34 Its importance is demonstrated by the fact that it seems to regenerate after it has been resected.35,36 Yet release or resection of this ligament has been a standard treatment for shoulder pain for decades.
Its function becomes clear if one examines the coracoacromial ligament with respect to the kinematics of throwing. As mentioned above, in the late cocking phase of throwing, tremendous shear forces exist in the shoulder. Fleisig and colleagues8 estimated a superior force of 250 ± 80 N, and an anterior shear force of 310 ± 100 N. While Fleisig and colleagues8 analyzed these shear forces with respect to the development of superior and anterior labral tears, it is important to note that these shear forces are vectors that should be combined. When one does this, it becomes apparent that in the late cocking phase of throwing there is shear force in an anterosuperior direction of approximately 400 N (Figure 1). The coracoacromial ligament is positioned to restrain this tremendous force. If throwing is an important adaptation in the evolution of humans, then the function of this ligament and its importance becomes clear.
Depressed Greater Tuberosity and the Pear-Shaped Glenoid
Compared to other primates, the greater tuberosity in humans sits significantly lower (Figure 4). This depression effectively decreases the moment arm of the muscle tendon unit, making the supraspinatus less powerful for raising the arm.37 In addition, by tenting the supraspinatus tendon over the humeral head, a watershed zone is created with decreased vascularity, which is thought to contribute to rotator cuff disease.38 What would be the advantage of the depressed tuberosity?
In primates, a lower tuberosity allows for more motion, particularly for arboreal travel.37 In order to throw with velocity, the humerus must achieve extremes of external rotation. A large tuberosity would limit external rotation of the abducted arm. Similarly, the pear-shaped glenoid cavity allows for the depressed tuberosity to achieve maximal external rotation. It is conceivable that a depressed greater tuberosity that allows for throwing would be an adaptation that could be favorable despite its proclivity toward rotator cuff disease in senescence.
Nature of the Subscapularis and the Role of the Long Head of the Biceps
The subscapularis is unique among rotator cuff muscles in that the upper two-thirds of the muscle is surprisingly tendinous.39 Why should this rotator cuff muscle have so much tendon material? Why is the tendon missing from the inferior one-third of the muscle? This situation is not optimal to prevent anterior glenohumeral instability, where inferior tendon material would be preferred.40
The function of the tendon of the long head of the biceps has long been debated and remains unclear.41-43 Cadaver experiments suggest the long head of the biceps provides glenohumeral joint stability in a variety of directions and positions, yet in vivo studies may not show this effect. Electromyography studies show little activity of the long head of the biceps with shoulder motion when the elbow is immobilized, leading some to suggest it is important as a passive restraint.43 This lack of understanding has led some to believe the biceps is not important and can be sacrificed without much concern.42,43
Again, these questions can be answered if one considers them in the context of throwing. At the point of maximal external rotation, the shoulder quickly moves from external rotation to internal rotation. This occurs by converting kinetic energy of external rotation into stored potential energy in the tissues. This energy is then converted into internal rotation. This elastic energy storage is critical for developing the necessary velocities to launch a projectile. While many structures are responsible for storing this energy,12 the subscapularis and long head of the biceps are particularly important. In fact, these 2 structures are important restraints to external rotation of the abducted arm–and become increasingly important with increased external rotation.45,46
One can think of the long head of the biceps as a spring (muscle), a cable (the long tendon), and a pulley (the bicipital groove). Similarly, one can consider the subscapularis as a similar structure, with the coracoid process serving as the pulley. In the late cocking phase of throwing, an interesting alignment occurs such that the pulleys (coracoid process and bicipital groove) are on opposite sides of the joint, providing glenohumeral joint stability. This system, with the inferior glenohumeral ligament (which is the primary restraint to external rotation of the abducted arm18), produces an incredibly stable envelope, preventing the humeral head from over-rotating and translating during the late cocking phase of throwing when the forces about the shoulder are extremely high. Because the muscles serve as springs, this system is also capable of storing kinetic energy during the late cocking phase of throwing and converting it into kinetic energy for internal rotation.
Summary
While throwing is not as critical to survival in today’s culture, the ability to throw was clearly an important adaptation in human evolution. With this in mind, we can approach human anatomy with this perspective, and in fact, many other lines of thinking suggest that throwing was important in the evolution of the hand,47 the brain,48 bipedalism,49 and even human society.50 The shoulder was highly influenced through natural selection to promote the throwing skill. With this perspective, many of the mysteries about the shoulder can be answered.
1. Darwin C. The Descent of Man, and Selection in Relation to Sex. 2nd ed. London, UK: John Murray; 1874:35.
2. Issac B. Throwing and human evolution. African Archeol Record. 1987;5:3-17.
3. Kirschann E. The human throw and a new model of hominid evolution (German). Homo. 1999;50(1):80-85.
4. Knusel CJ. The throwing hypothesis and hominid origins. Human Evolution. 1992;7(1):1-7.
5. Dunsworth H, Challis J, Walker A. The evolution of throwing: a new look at an old idea. Courier Forschungsinstitut Senckenberg. 2003;243:105-110.
6. Mayr E. Animal Species and Evolution. Cambridge, MA: Harvard University Press; 1963.
7. Tinbergen N. On the aims and methods of ethology. Zeitschrift für Tierpsychologie. 1963;20:410-433.
8. Fleisig GS, Andrews JR, Dillman CJ, Escamilla RF. Kinetics of baseball pitching with implications about injury mechanisms. Am J Sports Med. 1995;23(2):233-239.
9. Atwater AE. Biomechanics of overarm throwing movements and of throwing injuries. Exerc Sport Sci Rev. 1975;7:43-85.
10. Matsuo T, Escamila RF, Fleisig GS, Barrentine SW, Andrews JR. Comparison of kinematic and temporal parameters between different pitch velocity groups. J Appl Biomech. 2001;17:1-13.
11. Wang YT, Ford HT III, Ford HT Jr, Shin DM. Three-dimensional kinematic analysis of baseball pitching in acceleration phase. Percept Mot Skills. 1995;80:43-48.
12. Roach NT, Venkadesan M, Rainbow MJ, Lieberman DE. Elastic energy storage in the shoulder and the evolution of high-speed throwing in Homo. Nature. 2013;498(7455):483-486.
13. Fox JA, Cole BJ, Romeo AA, et al. Articular cartilage thickness of the humeral head: an anatomic study. Orthopedics. 2008;31(3):216.
14. Zumstein V, Kraljevic M, Conzen A, Hoechel S, Müller-Gerbl M. Thickness distribution of the glenohumeral joint cartilage: a quantitative study using computed tomography. Surg Radiol Anat. 2014;36(4):327-331.
15. Inman VT, Saunders M, Abbott LC. Observations on the function of the shoulder joint. J Bone Joint Surg Am. 1944;26:1-30.
16. O’Brien SJ, Schwartz RS, Warren RF, Torzilli PA. Capsular restraints to anterior posterior motion of the abducted shoulder: A biomechanical study. J Shoulder Elbow Surg. 1995;4(4):298-308.
17. Warner JJ, Deng XH, Warren RF, Torzilli PA. Static capsuloligamentous restraints to superior-inferior translation of the glenohumeral joint. Am J Sports Med. 1992;20(6):675-685.
18. Kuhn JE, Bey MJ, Huston LJ, Blasier RB, Soslowsky LJ. Ligamentous restraints to external rotation of the humerus in the late-cocking phase of throwing. A cadaveric biomechanical investigation. Am J Sports Med. 2000;28(2):200-205.
19. Bigliani LU, Codd TP, Connor PM, Levine WN, Littlefield MA, Hershon SJ. Shoulder motion and laxity in the professional baseball player. Am J Sports Med. 1997;25(5):609-613.
20. Borsa PA, Dover GC, Wilk KE, Reinold MM. Glenohumeral range of motion and stiffness in professional baseball pitchers. Med Sci Sports Exerc. 2006;38(1):21-26.
21. Hurd WJ, Kaplan KM, Eiattrache NS, Jobe FW, Morrey BF, Kaufman KR. A profile of glenohumeral internal and external rotation motion in the uninjured high school baseball pitcher, part I: motion. J Athl Train. 2011;46(3):282-288.
22. Wilk KE, Macrina LC, Arrigo C. Passive range of motion characteristics in the overhead baseball pitcher and their implications for rehabilitation. Clin Orthop Relat Res. 2012;470(6):1586-1594.
23. Osbahr DC, Cannon DL, Speer KP. Retroversion of the humerus in the throwing shoulder of college baseball pitchers. Am J Sports Med. 2002;30(3):347-353.
24. Greenberg EM, Fernandez-Fernandez A, Lawrence JT, McClure P. The development of humeral retrotorsion and its relationship to throwing sports. Sports Health. 2015;7(6):489-496.
25. Jobe FW, Pink M. The athlete’s shoulder. J Hand Ther. 1994;7(2):107-110.
26. Montgomery WH 3rd, Jobe FW. Functional outcomes in athletes after modified anterior capsulolabral reconstruction. Am J Sports Med. 1994;22(3):352-358.
27. Jobe FW, Kvitne RS, Giangarra CE. Shoulder pain in the overhand or throwing athlete. The relationship of anterior instability and rotator cuff impingement. Orthop Rev. 1989;18(9):963-975.
28. Reinhold MM, Wilk KE, Dugas JR, Andrews JR. Chapter 11. Internal Impingement. In: Wilk K, Reinold MM, Andrews JR, eds. The Athlete’s Shoulder. 2nd ed. Philadelphia, PA: Churchill Livingstone Elsevier; 2009:126.
29. Kuhn JE, Lindholm SR, Huston LJ, Soslowsky LJ, Blasier RB. Failure of the biceps superior labral complex: a cadaveric biomechanical investigation comparing the late cocking and early deceleration positions of throwing. Arthroscopy. 2003;19(4):373-379.
30. Ciochon RL, Corruccini RS. The coraco-acromial ligament and projection index in man and other anthropoid primates. J Anat. 1977;124(Pt 3):627-632.
31. Moorman CT, Warren RF, Deng XH, Wickiewicz TL, Torzilli PA. Role of coracoacromial ligament and related structures in glenohumeral stability: a cadaveric study. J Surg Orthop Adv. 2012;21(4):210-217.
32. Su WR, Budoff JE, Luo ZP. The effect of coracoacromial ligament excision and acromioplasty on superior and anterosuperior glenohumeral stability. Arthroscopy. 2009;25(1):13-18.
33. Wellmann M, Petersen W, Zantop T, Schanz S, Raschke MJ, Hurschler C. Effect of coracoacromial ligament resection on glenohumeral stability under active muscle loading in an in vitro model. Arthroscopy. 2008;24(11):1258-1264.
34. Fagelman M, Sartori M, Freedman KB, Patwardhan AG, Carandang G, Marra G. Biomechanics of coracoacromial arch modification. J Shoulder Elbow Surg. 2007;16(1):101-116.
35. Bak K, Spring IB, Henderson IP. Re-formation of the coracoacromial ligament after open resection or arthroscopic release. J Shoulder Elbow Surg. 2000;9:289-293.
36. Levy O, Copeland SA. Regeneration of the coracoacromial ligament after acromioplasty and arthroscopic subacromial decompression. J Shoulder Elbow Surg. 2001;10(4):317-320.
37. Larson SG, Stern JT Jr. Role of supraspinatus in the quadrupedal locomotion of vervets (Cercopithecus aethiops): Implications for interpretation of humeral morphology. Am J Phys Anthropol. 1989;79(3):369-377.
38. Chansky HA, Iannotti JP. The vascularity of the rotator cuff. Clin Sports Med. 1991;10(4):807-822.
39. Klapper RC, Jobe FW, Matsuura P. Subscapularis muscle and its glenohumeral ligament-like bands. A histomorphologic study. Am J Sports Med. 1992;20(3):307-310.
40. Halder A, Zobitz ME, Schultz E, An KN. Structural properties of the subscapularis tendon. J Orthop Res. 2000;18(5):
829-834.
41. Elser F, Braun S, Dewing CB, Giphart JE, Millett PJ. Anatomy, function, injuries, and treatment of the long head of the biceps brachii tendon. Arthroscopy. 2011;27(4):581-592.
42. Pill SG, Walch G, Hawkins RJ, Kissenberth MJ. The role of the biceps tendon in massive rotator cuff tears. Instr Course Lect. 2012;61:113-120.
43. Krupp RJ, Kevern MA, Gaines MD, Kotara S, Singleton SB. Long head of the biceps tendon pain: differential diagnosis and treatment. J Orthop Sports Phys Ther. 2009;39(2):55-70.
44. Levy AS, Kelly BT, Lintner SA, Osbahr DC, Speer KP. Function of the long head of the biceps at the shoulder: electromyographic analysis. J Shoulder Elbow Surg. 2001;10(3):250-255.
45. Kuhn JE, Huston LJ, Soslowsky LJ, Shyr Y, Blasier RB. External rotation of the glenohumeral joint: ligament restraints and muscle effects in the neutral and abducted positions.
J Shoulder Elbow Surg. 2005;14(1 Suppl S):39S-48S.
46. McGarry MH, Nguyen ML, Quigley RJ, Hanypsiak B, Gupta R, Lee TQ. The effect of long and short head biceps loading on glenohumeral joint rotational range of motion and humeral head postion. Knee Surge Sports Traumatol Arthrosc. 2014 Sep 26. [Epub ahead of print].
47. Young RW. Evolution of the human hand: The role of throwing and clubbing. J Anat. 2003;202:165-174.
48. Calvin WH. Did throwing stones shape hominid brain evolution? Ethology and Sociobiology. 1982;3:115-124.
49. Fifer FC. The adoption of bipedalism by the hominids: A new hypothesis. Human Evolution. 1987;2(2):135-147.
50. Darlington PJ. Group selection, altruism, reinforcement, and throwing in human evolution. Proc Nat Acad Sci. 1973;72(9):3748-3752.
Charles Darwin once said that apes “...are quite unable, as I have myself seen, to throw a stone with precision”.1 Yet humans can throw with precision and speed, a skill that likely had significant advantages: throwing can affect change at a distance—something few species can do. Throwing can provide protection against predators and can allow for predation for food resources. Throwing would be important in contesting other hominids for scarce resources. As such, throwing has been critically important in human evolution and likely is a skill that has been promoted through natural selection.2-5
In the orthopedic literature, most published work on throwing will ask proximate questions: “how, what, who, when, and where?” Evolutionary biologists are concerned with ultimate questions6,7: “why?” Asking ultimate questions provides insight into how a behavior might offer advantages under natural selection, which can then improve our understanding of the proximate questions for that behavior.
With regard to the shoulder, a number of mysteries exist that, to date, proximate studies have not been able to solve. This article argues that the human shoulder has evolved for throwing and by using this frame of reference, many of the mysteries surrounding the anatomy of the shoulder can be understood.
Pitching Kinematics
The mechanics of pitching have been analyzed extensively. Fleisig and colleagues8 performed kinematic and electromyographic analyses of pitchers to identify the critical moments of pitching (defined as where the forces are highest and injury is most likely going to occur). They found 2 moments where the forces about the shoulder are highest during pitching: the late cocking phase (defined by the point where the humerus reaches maximal external rotation); and the early deceleration phase (defined by the point when the ball is released). If throwing is important in natural selection of humans, then the shoulder anatomy should be optimized to withstand the forces generated in these positions.
Late Cocking Phase of Throwing
The early phases of throwing are attempting to maximize external rotation of the abducted arm as the velocity of the pitched ball correlates to the amount of external rotation achieved.9-11 In this position, kinetic energy in external rotation is stored and converted into kinetic energy in internal rotation.12 The position of the shoulder during late cocking is 94 ± 21° of thoracohumeral abduction, 11 ± 11° of horizontal adduction, and a remarkable 165 ± 11° of thoracohumeral external rotation (Figure 1).8
Fleisig and colleagues8 estimated the torque and forces about the shoulder, which are quite high for joint compression (480 ± 130 N). They also analyzed the shear forces and while trying to describe the origin of superior labrum anterior to posterior (SLAP) lesions and anterior labral tears, broke down the major shear vector into an anterior force vector (310 ± 100 N) and a superior force (250 ± 80 N).8 Note that the resulting shear vector is in an anterosuperior direction and is approximately 400 N.
Early Deceleration Phase of Throwing
Interestingly, the position of the humerus during this critical moment of throwing is not much different than the position during the late cocking phase of throwing, with 93 ± 10° of thoracohumeral abduction, 6 ± 8° of horizontal adduction.8 The major difference in the position of the arm is found in the amount of thoracohumeral rotation, which is now 64 ± 35° of external rotation (Figure 2).8
The forces in early deceleration are tremendous, with an estimated 1090 ± 110 N joint compression force, and an anteroinferior shear force of approximately 130 N.8
Clearly, if throwing is an important skill in human evolution, adaptations must exist in the shoulder to withstand the high forces in these 2 critical moments of throwing.
Solving Mysteries of Shoulder Anatomy in the Context of Throwing
There are many anatomic features of the shoulder that remain poorly understood. These include the alignment of the glenohumeral joint, the function of the glenohumeral ligaments, the function of the coracoacromial ligament, the depression of the human greater tuberosity, and the nature and function of the very tendinous subscapularis and long head of the biceps. These mysteries of the human shoulder can be solved if one considers the hypothesis that the shoulder has evolved to throw.
Glenohumeral Joint Alignment
The cartilage of the humeral head is thickest at its center, and thinnest at the periphery (Figure 3A).13,14 Conversely, the cartilage of the glenoid is thinnest at the fovea and thickest in the periphery (Figure 3B).14 It seems obvious that in order to maximally distribute high loads across this joint, the center of the humeral head should rest in the center of the glenoid. Interestingly, this does not occur during most positions of the shoulder. When upright, the center of the humeral head is directed above the glenoid in the coronal plane (Figure 3C). In order to align the glenohumeral joint optimally for the distribution of loads across the joint, the humerus must be abducted approximately 60° relative to the scapula. Assuming a 2:1 glenohumeral to scapulothoracic abduction for arm abduction relative to the thorax,15 this equates to approximately 90° of thoracohumeral abduction—the exact kinematic position of the shoulder during both critical moments of throwing (Figure 3D).
Function of the Glenohumeral Ligaments
The glenohumeral joint capsule has thickenings that help to stabilize the joint. The function of these glenohumeral ligaments has been evaluated biomechanically for their role in preventing translation and instability by a number of authors. The inferior glenohumeral ligament has classically been described as resisting anterior translation of the abducted arm.16 The coracohumeral ligament has been described as important to prevent inferior translation of the adducted arm.17
Interestingly, these ligaments are also the most important ligaments in resisting external rotation of the adducted arm.18 The dominant arm of throwing athletes has been shown to have increased inferior translation19 and increase external rotation.19-22 While the external rotation is partly related to bony adaptation,23,24 the ligamentous restraints to external rotation are likely under tremendous load, which may explain why Dr. Frank Jobe revolutionized the surgical treatment of the throwing athlete by performing an “instability” operation,25,26 as he believed these athletes had “subtle instability” that produced pain, but not symptoms of looseness.27
While these ligaments may exist in part to prevent translation and instability, current thinking suggests that “over-rotation” may lead to internal impingement and may be responsible for symptoms in the thrower’s shoulder,28 as SLAP lesions seem to occur easier with external rotation.29 Again, the importance of maximizing external rotation in throwing and the finding that this position is a critical moment with very high forces suggests that these ligaments may represent an adaptation to restrain external rotation while throwing.
Coracoacromial Ligament
The coracoacromial ligament is unique in that it connects 2 pieces of the same bone, and is only seen in hominids—not other primates.30 Its function has been debated for decades. This ligament is generally thought to limit superior translation of the humeral head,31,32 an effect that is critically important in patients with rotator cuff tears 33,34 Its importance is demonstrated by the fact that it seems to regenerate after it has been resected.35,36 Yet release or resection of this ligament has been a standard treatment for shoulder pain for decades.
Its function becomes clear if one examines the coracoacromial ligament with respect to the kinematics of throwing. As mentioned above, in the late cocking phase of throwing, tremendous shear forces exist in the shoulder. Fleisig and colleagues8 estimated a superior force of 250 ± 80 N, and an anterior shear force of 310 ± 100 N. While Fleisig and colleagues8 analyzed these shear forces with respect to the development of superior and anterior labral tears, it is important to note that these shear forces are vectors that should be combined. When one does this, it becomes apparent that in the late cocking phase of throwing there is shear force in an anterosuperior direction of approximately 400 N (Figure 1). The coracoacromial ligament is positioned to restrain this tremendous force. If throwing is an important adaptation in the evolution of humans, then the function of this ligament and its importance becomes clear.
Depressed Greater Tuberosity and the Pear-Shaped Glenoid
Compared to other primates, the greater tuberosity in humans sits significantly lower (Figure 4). This depression effectively decreases the moment arm of the muscle tendon unit, making the supraspinatus less powerful for raising the arm.37 In addition, by tenting the supraspinatus tendon over the humeral head, a watershed zone is created with decreased vascularity, which is thought to contribute to rotator cuff disease.38 What would be the advantage of the depressed tuberosity?
In primates, a lower tuberosity allows for more motion, particularly for arboreal travel.37 In order to throw with velocity, the humerus must achieve extremes of external rotation. A large tuberosity would limit external rotation of the abducted arm. Similarly, the pear-shaped glenoid cavity allows for the depressed tuberosity to achieve maximal external rotation. It is conceivable that a depressed greater tuberosity that allows for throwing would be an adaptation that could be favorable despite its proclivity toward rotator cuff disease in senescence.
Nature of the Subscapularis and the Role of the Long Head of the Biceps
The subscapularis is unique among rotator cuff muscles in that the upper two-thirds of the muscle is surprisingly tendinous.39 Why should this rotator cuff muscle have so much tendon material? Why is the tendon missing from the inferior one-third of the muscle? This situation is not optimal to prevent anterior glenohumeral instability, where inferior tendon material would be preferred.40
The function of the tendon of the long head of the biceps has long been debated and remains unclear.41-43 Cadaver experiments suggest the long head of the biceps provides glenohumeral joint stability in a variety of directions and positions, yet in vivo studies may not show this effect. Electromyography studies show little activity of the long head of the biceps with shoulder motion when the elbow is immobilized, leading some to suggest it is important as a passive restraint.43 This lack of understanding has led some to believe the biceps is not important and can be sacrificed without much concern.42,43
Again, these questions can be answered if one considers them in the context of throwing. At the point of maximal external rotation, the shoulder quickly moves from external rotation to internal rotation. This occurs by converting kinetic energy of external rotation into stored potential energy in the tissues. This energy is then converted into internal rotation. This elastic energy storage is critical for developing the necessary velocities to launch a projectile. While many structures are responsible for storing this energy,12 the subscapularis and long head of the biceps are particularly important. In fact, these 2 structures are important restraints to external rotation of the abducted arm–and become increasingly important with increased external rotation.45,46
One can think of the long head of the biceps as a spring (muscle), a cable (the long tendon), and a pulley (the bicipital groove). Similarly, one can consider the subscapularis as a similar structure, with the coracoid process serving as the pulley. In the late cocking phase of throwing, an interesting alignment occurs such that the pulleys (coracoid process and bicipital groove) are on opposite sides of the joint, providing glenohumeral joint stability. This system, with the inferior glenohumeral ligament (which is the primary restraint to external rotation of the abducted arm18), produces an incredibly stable envelope, preventing the humeral head from over-rotating and translating during the late cocking phase of throwing when the forces about the shoulder are extremely high. Because the muscles serve as springs, this system is also capable of storing kinetic energy during the late cocking phase of throwing and converting it into kinetic energy for internal rotation.
Summary
While throwing is not as critical to survival in today’s culture, the ability to throw was clearly an important adaptation in human evolution. With this in mind, we can approach human anatomy with this perspective, and in fact, many other lines of thinking suggest that throwing was important in the evolution of the hand,47 the brain,48 bipedalism,49 and even human society.50 The shoulder was highly influenced through natural selection to promote the throwing skill. With this perspective, many of the mysteries about the shoulder can be answered.
Charles Darwin once said that apes “...are quite unable, as I have myself seen, to throw a stone with precision”.1 Yet humans can throw with precision and speed, a skill that likely had significant advantages: throwing can affect change at a distance—something few species can do. Throwing can provide protection against predators and can allow for predation for food resources. Throwing would be important in contesting other hominids for scarce resources. As such, throwing has been critically important in human evolution and likely is a skill that has been promoted through natural selection.2-5
In the orthopedic literature, most published work on throwing will ask proximate questions: “how, what, who, when, and where?” Evolutionary biologists are concerned with ultimate questions6,7: “why?” Asking ultimate questions provides insight into how a behavior might offer advantages under natural selection, which can then improve our understanding of the proximate questions for that behavior.
With regard to the shoulder, a number of mysteries exist that, to date, proximate studies have not been able to solve. This article argues that the human shoulder has evolved for throwing and by using this frame of reference, many of the mysteries surrounding the anatomy of the shoulder can be understood.
Pitching Kinematics
The mechanics of pitching have been analyzed extensively. Fleisig and colleagues8 performed kinematic and electromyographic analyses of pitchers to identify the critical moments of pitching (defined as where the forces are highest and injury is most likely going to occur). They found 2 moments where the forces about the shoulder are highest during pitching: the late cocking phase (defined by the point where the humerus reaches maximal external rotation); and the early deceleration phase (defined by the point when the ball is released). If throwing is important in natural selection of humans, then the shoulder anatomy should be optimized to withstand the forces generated in these positions.
Late Cocking Phase of Throwing
The early phases of throwing are attempting to maximize external rotation of the abducted arm as the velocity of the pitched ball correlates to the amount of external rotation achieved.9-11 In this position, kinetic energy in external rotation is stored and converted into kinetic energy in internal rotation.12 The position of the shoulder during late cocking is 94 ± 21° of thoracohumeral abduction, 11 ± 11° of horizontal adduction, and a remarkable 165 ± 11° of thoracohumeral external rotation (Figure 1).8
Fleisig and colleagues8 estimated the torque and forces about the shoulder, which are quite high for joint compression (480 ± 130 N). They also analyzed the shear forces and while trying to describe the origin of superior labrum anterior to posterior (SLAP) lesions and anterior labral tears, broke down the major shear vector into an anterior force vector (310 ± 100 N) and a superior force (250 ± 80 N).8 Note that the resulting shear vector is in an anterosuperior direction and is approximately 400 N.
Early Deceleration Phase of Throwing
Interestingly, the position of the humerus during this critical moment of throwing is not much different than the position during the late cocking phase of throwing, with 93 ± 10° of thoracohumeral abduction, 6 ± 8° of horizontal adduction.8 The major difference in the position of the arm is found in the amount of thoracohumeral rotation, which is now 64 ± 35° of external rotation (Figure 2).8
The forces in early deceleration are tremendous, with an estimated 1090 ± 110 N joint compression force, and an anteroinferior shear force of approximately 130 N.8
Clearly, if throwing is an important skill in human evolution, adaptations must exist in the shoulder to withstand the high forces in these 2 critical moments of throwing.
Solving Mysteries of Shoulder Anatomy in the Context of Throwing
There are many anatomic features of the shoulder that remain poorly understood. These include the alignment of the glenohumeral joint, the function of the glenohumeral ligaments, the function of the coracoacromial ligament, the depression of the human greater tuberosity, and the nature and function of the very tendinous subscapularis and long head of the biceps. These mysteries of the human shoulder can be solved if one considers the hypothesis that the shoulder has evolved to throw.
Glenohumeral Joint Alignment
The cartilage of the humeral head is thickest at its center, and thinnest at the periphery (Figure 3A).13,14 Conversely, the cartilage of the glenoid is thinnest at the fovea and thickest in the periphery (Figure 3B).14 It seems obvious that in order to maximally distribute high loads across this joint, the center of the humeral head should rest in the center of the glenoid. Interestingly, this does not occur during most positions of the shoulder. When upright, the center of the humeral head is directed above the glenoid in the coronal plane (Figure 3C). In order to align the glenohumeral joint optimally for the distribution of loads across the joint, the humerus must be abducted approximately 60° relative to the scapula. Assuming a 2:1 glenohumeral to scapulothoracic abduction for arm abduction relative to the thorax,15 this equates to approximately 90° of thoracohumeral abduction—the exact kinematic position of the shoulder during both critical moments of throwing (Figure 3D).
Function of the Glenohumeral Ligaments
The glenohumeral joint capsule has thickenings that help to stabilize the joint. The function of these glenohumeral ligaments has been evaluated biomechanically for their role in preventing translation and instability by a number of authors. The inferior glenohumeral ligament has classically been described as resisting anterior translation of the abducted arm.16 The coracohumeral ligament has been described as important to prevent inferior translation of the adducted arm.17
Interestingly, these ligaments are also the most important ligaments in resisting external rotation of the adducted arm.18 The dominant arm of throwing athletes has been shown to have increased inferior translation19 and increase external rotation.19-22 While the external rotation is partly related to bony adaptation,23,24 the ligamentous restraints to external rotation are likely under tremendous load, which may explain why Dr. Frank Jobe revolutionized the surgical treatment of the throwing athlete by performing an “instability” operation,25,26 as he believed these athletes had “subtle instability” that produced pain, but not symptoms of looseness.27
While these ligaments may exist in part to prevent translation and instability, current thinking suggests that “over-rotation” may lead to internal impingement and may be responsible for symptoms in the thrower’s shoulder,28 as SLAP lesions seem to occur easier with external rotation.29 Again, the importance of maximizing external rotation in throwing and the finding that this position is a critical moment with very high forces suggests that these ligaments may represent an adaptation to restrain external rotation while throwing.
Coracoacromial Ligament
The coracoacromial ligament is unique in that it connects 2 pieces of the same bone, and is only seen in hominids—not other primates.30 Its function has been debated for decades. This ligament is generally thought to limit superior translation of the humeral head,31,32 an effect that is critically important in patients with rotator cuff tears 33,34 Its importance is demonstrated by the fact that it seems to regenerate after it has been resected.35,36 Yet release or resection of this ligament has been a standard treatment for shoulder pain for decades.
Its function becomes clear if one examines the coracoacromial ligament with respect to the kinematics of throwing. As mentioned above, in the late cocking phase of throwing, tremendous shear forces exist in the shoulder. Fleisig and colleagues8 estimated a superior force of 250 ± 80 N, and an anterior shear force of 310 ± 100 N. While Fleisig and colleagues8 analyzed these shear forces with respect to the development of superior and anterior labral tears, it is important to note that these shear forces are vectors that should be combined. When one does this, it becomes apparent that in the late cocking phase of throwing there is shear force in an anterosuperior direction of approximately 400 N (Figure 1). The coracoacromial ligament is positioned to restrain this tremendous force. If throwing is an important adaptation in the evolution of humans, then the function of this ligament and its importance becomes clear.
Depressed Greater Tuberosity and the Pear-Shaped Glenoid
Compared to other primates, the greater tuberosity in humans sits significantly lower (Figure 4). This depression effectively decreases the moment arm of the muscle tendon unit, making the supraspinatus less powerful for raising the arm.37 In addition, by tenting the supraspinatus tendon over the humeral head, a watershed zone is created with decreased vascularity, which is thought to contribute to rotator cuff disease.38 What would be the advantage of the depressed tuberosity?
In primates, a lower tuberosity allows for more motion, particularly for arboreal travel.37 In order to throw with velocity, the humerus must achieve extremes of external rotation. A large tuberosity would limit external rotation of the abducted arm. Similarly, the pear-shaped glenoid cavity allows for the depressed tuberosity to achieve maximal external rotation. It is conceivable that a depressed greater tuberosity that allows for throwing would be an adaptation that could be favorable despite its proclivity toward rotator cuff disease in senescence.
Nature of the Subscapularis and the Role of the Long Head of the Biceps
The subscapularis is unique among rotator cuff muscles in that the upper two-thirds of the muscle is surprisingly tendinous.39 Why should this rotator cuff muscle have so much tendon material? Why is the tendon missing from the inferior one-third of the muscle? This situation is not optimal to prevent anterior glenohumeral instability, where inferior tendon material would be preferred.40
The function of the tendon of the long head of the biceps has long been debated and remains unclear.41-43 Cadaver experiments suggest the long head of the biceps provides glenohumeral joint stability in a variety of directions and positions, yet in vivo studies may not show this effect. Electromyography studies show little activity of the long head of the biceps with shoulder motion when the elbow is immobilized, leading some to suggest it is important as a passive restraint.43 This lack of understanding has led some to believe the biceps is not important and can be sacrificed without much concern.42,43
Again, these questions can be answered if one considers them in the context of throwing. At the point of maximal external rotation, the shoulder quickly moves from external rotation to internal rotation. This occurs by converting kinetic energy of external rotation into stored potential energy in the tissues. This energy is then converted into internal rotation. This elastic energy storage is critical for developing the necessary velocities to launch a projectile. While many structures are responsible for storing this energy,12 the subscapularis and long head of the biceps are particularly important. In fact, these 2 structures are important restraints to external rotation of the abducted arm–and become increasingly important with increased external rotation.45,46
One can think of the long head of the biceps as a spring (muscle), a cable (the long tendon), and a pulley (the bicipital groove). Similarly, one can consider the subscapularis as a similar structure, with the coracoid process serving as the pulley. In the late cocking phase of throwing, an interesting alignment occurs such that the pulleys (coracoid process and bicipital groove) are on opposite sides of the joint, providing glenohumeral joint stability. This system, with the inferior glenohumeral ligament (which is the primary restraint to external rotation of the abducted arm18), produces an incredibly stable envelope, preventing the humeral head from over-rotating and translating during the late cocking phase of throwing when the forces about the shoulder are extremely high. Because the muscles serve as springs, this system is also capable of storing kinetic energy during the late cocking phase of throwing and converting it into kinetic energy for internal rotation.
Summary
While throwing is not as critical to survival in today’s culture, the ability to throw was clearly an important adaptation in human evolution. With this in mind, we can approach human anatomy with this perspective, and in fact, many other lines of thinking suggest that throwing was important in the evolution of the hand,47 the brain,48 bipedalism,49 and even human society.50 The shoulder was highly influenced through natural selection to promote the throwing skill. With this perspective, many of the mysteries about the shoulder can be answered.
1. Darwin C. The Descent of Man, and Selection in Relation to Sex. 2nd ed. London, UK: John Murray; 1874:35.
2. Issac B. Throwing and human evolution. African Archeol Record. 1987;5:3-17.
3. Kirschann E. The human throw and a new model of hominid evolution (German). Homo. 1999;50(1):80-85.
4. Knusel CJ. The throwing hypothesis and hominid origins. Human Evolution. 1992;7(1):1-7.
5. Dunsworth H, Challis J, Walker A. The evolution of throwing: a new look at an old idea. Courier Forschungsinstitut Senckenberg. 2003;243:105-110.
6. Mayr E. Animal Species and Evolution. Cambridge, MA: Harvard University Press; 1963.
7. Tinbergen N. On the aims and methods of ethology. Zeitschrift für Tierpsychologie. 1963;20:410-433.
8. Fleisig GS, Andrews JR, Dillman CJ, Escamilla RF. Kinetics of baseball pitching with implications about injury mechanisms. Am J Sports Med. 1995;23(2):233-239.
9. Atwater AE. Biomechanics of overarm throwing movements and of throwing injuries. Exerc Sport Sci Rev. 1975;7:43-85.
10. Matsuo T, Escamila RF, Fleisig GS, Barrentine SW, Andrews JR. Comparison of kinematic and temporal parameters between different pitch velocity groups. J Appl Biomech. 2001;17:1-13.
11. Wang YT, Ford HT III, Ford HT Jr, Shin DM. Three-dimensional kinematic analysis of baseball pitching in acceleration phase. Percept Mot Skills. 1995;80:43-48.
12. Roach NT, Venkadesan M, Rainbow MJ, Lieberman DE. Elastic energy storage in the shoulder and the evolution of high-speed throwing in Homo. Nature. 2013;498(7455):483-486.
13. Fox JA, Cole BJ, Romeo AA, et al. Articular cartilage thickness of the humeral head: an anatomic study. Orthopedics. 2008;31(3):216.
14. Zumstein V, Kraljevic M, Conzen A, Hoechel S, Müller-Gerbl M. Thickness distribution of the glenohumeral joint cartilage: a quantitative study using computed tomography. Surg Radiol Anat. 2014;36(4):327-331.
15. Inman VT, Saunders M, Abbott LC. Observations on the function of the shoulder joint. J Bone Joint Surg Am. 1944;26:1-30.
16. O’Brien SJ, Schwartz RS, Warren RF, Torzilli PA. Capsular restraints to anterior posterior motion of the abducted shoulder: A biomechanical study. J Shoulder Elbow Surg. 1995;4(4):298-308.
17. Warner JJ, Deng XH, Warren RF, Torzilli PA. Static capsuloligamentous restraints to superior-inferior translation of the glenohumeral joint. Am J Sports Med. 1992;20(6):675-685.
18. Kuhn JE, Bey MJ, Huston LJ, Blasier RB, Soslowsky LJ. Ligamentous restraints to external rotation of the humerus in the late-cocking phase of throwing. A cadaveric biomechanical investigation. Am J Sports Med. 2000;28(2):200-205.
19. Bigliani LU, Codd TP, Connor PM, Levine WN, Littlefield MA, Hershon SJ. Shoulder motion and laxity in the professional baseball player. Am J Sports Med. 1997;25(5):609-613.
20. Borsa PA, Dover GC, Wilk KE, Reinold MM. Glenohumeral range of motion and stiffness in professional baseball pitchers. Med Sci Sports Exerc. 2006;38(1):21-26.
21. Hurd WJ, Kaplan KM, Eiattrache NS, Jobe FW, Morrey BF, Kaufman KR. A profile of glenohumeral internal and external rotation motion in the uninjured high school baseball pitcher, part I: motion. J Athl Train. 2011;46(3):282-288.
22. Wilk KE, Macrina LC, Arrigo C. Passive range of motion characteristics in the overhead baseball pitcher and their implications for rehabilitation. Clin Orthop Relat Res. 2012;470(6):1586-1594.
23. Osbahr DC, Cannon DL, Speer KP. Retroversion of the humerus in the throwing shoulder of college baseball pitchers. Am J Sports Med. 2002;30(3):347-353.
24. Greenberg EM, Fernandez-Fernandez A, Lawrence JT, McClure P. The development of humeral retrotorsion and its relationship to throwing sports. Sports Health. 2015;7(6):489-496.
25. Jobe FW, Pink M. The athlete’s shoulder. J Hand Ther. 1994;7(2):107-110.
26. Montgomery WH 3rd, Jobe FW. Functional outcomes in athletes after modified anterior capsulolabral reconstruction. Am J Sports Med. 1994;22(3):352-358.
27. Jobe FW, Kvitne RS, Giangarra CE. Shoulder pain in the overhand or throwing athlete. The relationship of anterior instability and rotator cuff impingement. Orthop Rev. 1989;18(9):963-975.
28. Reinhold MM, Wilk KE, Dugas JR, Andrews JR. Chapter 11. Internal Impingement. In: Wilk K, Reinold MM, Andrews JR, eds. The Athlete’s Shoulder. 2nd ed. Philadelphia, PA: Churchill Livingstone Elsevier; 2009:126.
29. Kuhn JE, Lindholm SR, Huston LJ, Soslowsky LJ, Blasier RB. Failure of the biceps superior labral complex: a cadaveric biomechanical investigation comparing the late cocking and early deceleration positions of throwing. Arthroscopy. 2003;19(4):373-379.
30. Ciochon RL, Corruccini RS. The coraco-acromial ligament and projection index in man and other anthropoid primates. J Anat. 1977;124(Pt 3):627-632.
31. Moorman CT, Warren RF, Deng XH, Wickiewicz TL, Torzilli PA. Role of coracoacromial ligament and related structures in glenohumeral stability: a cadaveric study. J Surg Orthop Adv. 2012;21(4):210-217.
32. Su WR, Budoff JE, Luo ZP. The effect of coracoacromial ligament excision and acromioplasty on superior and anterosuperior glenohumeral stability. Arthroscopy. 2009;25(1):13-18.
33. Wellmann M, Petersen W, Zantop T, Schanz S, Raschke MJ, Hurschler C. Effect of coracoacromial ligament resection on glenohumeral stability under active muscle loading in an in vitro model. Arthroscopy. 2008;24(11):1258-1264.
34. Fagelman M, Sartori M, Freedman KB, Patwardhan AG, Carandang G, Marra G. Biomechanics of coracoacromial arch modification. J Shoulder Elbow Surg. 2007;16(1):101-116.
35. Bak K, Spring IB, Henderson IP. Re-formation of the coracoacromial ligament after open resection or arthroscopic release. J Shoulder Elbow Surg. 2000;9:289-293.
36. Levy O, Copeland SA. Regeneration of the coracoacromial ligament after acromioplasty and arthroscopic subacromial decompression. J Shoulder Elbow Surg. 2001;10(4):317-320.
37. Larson SG, Stern JT Jr. Role of supraspinatus in the quadrupedal locomotion of vervets (Cercopithecus aethiops): Implications for interpretation of humeral morphology. Am J Phys Anthropol. 1989;79(3):369-377.
38. Chansky HA, Iannotti JP. The vascularity of the rotator cuff. Clin Sports Med. 1991;10(4):807-822.
39. Klapper RC, Jobe FW, Matsuura P. Subscapularis muscle and its glenohumeral ligament-like bands. A histomorphologic study. Am J Sports Med. 1992;20(3):307-310.
40. Halder A, Zobitz ME, Schultz E, An KN. Structural properties of the subscapularis tendon. J Orthop Res. 2000;18(5):
829-834.
41. Elser F, Braun S, Dewing CB, Giphart JE, Millett PJ. Anatomy, function, injuries, and treatment of the long head of the biceps brachii tendon. Arthroscopy. 2011;27(4):581-592.
42. Pill SG, Walch G, Hawkins RJ, Kissenberth MJ. The role of the biceps tendon in massive rotator cuff tears. Instr Course Lect. 2012;61:113-120.
43. Krupp RJ, Kevern MA, Gaines MD, Kotara S, Singleton SB. Long head of the biceps tendon pain: differential diagnosis and treatment. J Orthop Sports Phys Ther. 2009;39(2):55-70.
44. Levy AS, Kelly BT, Lintner SA, Osbahr DC, Speer KP. Function of the long head of the biceps at the shoulder: electromyographic analysis. J Shoulder Elbow Surg. 2001;10(3):250-255.
45. Kuhn JE, Huston LJ, Soslowsky LJ, Shyr Y, Blasier RB. External rotation of the glenohumeral joint: ligament restraints and muscle effects in the neutral and abducted positions.
J Shoulder Elbow Surg. 2005;14(1 Suppl S):39S-48S.
46. McGarry MH, Nguyen ML, Quigley RJ, Hanypsiak B, Gupta R, Lee TQ. The effect of long and short head biceps loading on glenohumeral joint rotational range of motion and humeral head postion. Knee Surge Sports Traumatol Arthrosc. 2014 Sep 26. [Epub ahead of print].
47. Young RW. Evolution of the human hand: The role of throwing and clubbing. J Anat. 2003;202:165-174.
48. Calvin WH. Did throwing stones shape hominid brain evolution? Ethology and Sociobiology. 1982;3:115-124.
49. Fifer FC. The adoption of bipedalism by the hominids: A new hypothesis. Human Evolution. 1987;2(2):135-147.
50. Darlington PJ. Group selection, altruism, reinforcement, and throwing in human evolution. Proc Nat Acad Sci. 1973;72(9):3748-3752.
1. Darwin C. The Descent of Man, and Selection in Relation to Sex. 2nd ed. London, UK: John Murray; 1874:35.
2. Issac B. Throwing and human evolution. African Archeol Record. 1987;5:3-17.
3. Kirschann E. The human throw and a new model of hominid evolution (German). Homo. 1999;50(1):80-85.
4. Knusel CJ. The throwing hypothesis and hominid origins. Human Evolution. 1992;7(1):1-7.
5. Dunsworth H, Challis J, Walker A. The evolution of throwing: a new look at an old idea. Courier Forschungsinstitut Senckenberg. 2003;243:105-110.
6. Mayr E. Animal Species and Evolution. Cambridge, MA: Harvard University Press; 1963.
7. Tinbergen N. On the aims and methods of ethology. Zeitschrift für Tierpsychologie. 1963;20:410-433.
8. Fleisig GS, Andrews JR, Dillman CJ, Escamilla RF. Kinetics of baseball pitching with implications about injury mechanisms. Am J Sports Med. 1995;23(2):233-239.
9. Atwater AE. Biomechanics of overarm throwing movements and of throwing injuries. Exerc Sport Sci Rev. 1975;7:43-85.
10. Matsuo T, Escamila RF, Fleisig GS, Barrentine SW, Andrews JR. Comparison of kinematic and temporal parameters between different pitch velocity groups. J Appl Biomech. 2001;17:1-13.
11. Wang YT, Ford HT III, Ford HT Jr, Shin DM. Three-dimensional kinematic analysis of baseball pitching in acceleration phase. Percept Mot Skills. 1995;80:43-48.
12. Roach NT, Venkadesan M, Rainbow MJ, Lieberman DE. Elastic energy storage in the shoulder and the evolution of high-speed throwing in Homo. Nature. 2013;498(7455):483-486.
13. Fox JA, Cole BJ, Romeo AA, et al. Articular cartilage thickness of the humeral head: an anatomic study. Orthopedics. 2008;31(3):216.
14. Zumstein V, Kraljevic M, Conzen A, Hoechel S, Müller-Gerbl M. Thickness distribution of the glenohumeral joint cartilage: a quantitative study using computed tomography. Surg Radiol Anat. 2014;36(4):327-331.
15. Inman VT, Saunders M, Abbott LC. Observations on the function of the shoulder joint. J Bone Joint Surg Am. 1944;26:1-30.
16. O’Brien SJ, Schwartz RS, Warren RF, Torzilli PA. Capsular restraints to anterior posterior motion of the abducted shoulder: A biomechanical study. J Shoulder Elbow Surg. 1995;4(4):298-308.
17. Warner JJ, Deng XH, Warren RF, Torzilli PA. Static capsuloligamentous restraints to superior-inferior translation of the glenohumeral joint. Am J Sports Med. 1992;20(6):675-685.
18. Kuhn JE, Bey MJ, Huston LJ, Blasier RB, Soslowsky LJ. Ligamentous restraints to external rotation of the humerus in the late-cocking phase of throwing. A cadaveric biomechanical investigation. Am J Sports Med. 2000;28(2):200-205.
19. Bigliani LU, Codd TP, Connor PM, Levine WN, Littlefield MA, Hershon SJ. Shoulder motion and laxity in the professional baseball player. Am J Sports Med. 1997;25(5):609-613.
20. Borsa PA, Dover GC, Wilk KE, Reinold MM. Glenohumeral range of motion and stiffness in professional baseball pitchers. Med Sci Sports Exerc. 2006;38(1):21-26.
21. Hurd WJ, Kaplan KM, Eiattrache NS, Jobe FW, Morrey BF, Kaufman KR. A profile of glenohumeral internal and external rotation motion in the uninjured high school baseball pitcher, part I: motion. J Athl Train. 2011;46(3):282-288.
22. Wilk KE, Macrina LC, Arrigo C. Passive range of motion characteristics in the overhead baseball pitcher and their implications for rehabilitation. Clin Orthop Relat Res. 2012;470(6):1586-1594.
23. Osbahr DC, Cannon DL, Speer KP. Retroversion of the humerus in the throwing shoulder of college baseball pitchers. Am J Sports Med. 2002;30(3):347-353.
24. Greenberg EM, Fernandez-Fernandez A, Lawrence JT, McClure P. The development of humeral retrotorsion and its relationship to throwing sports. Sports Health. 2015;7(6):489-496.
25. Jobe FW, Pink M. The athlete’s shoulder. J Hand Ther. 1994;7(2):107-110.
26. Montgomery WH 3rd, Jobe FW. Functional outcomes in athletes after modified anterior capsulolabral reconstruction. Am J Sports Med. 1994;22(3):352-358.
27. Jobe FW, Kvitne RS, Giangarra CE. Shoulder pain in the overhand or throwing athlete. The relationship of anterior instability and rotator cuff impingement. Orthop Rev. 1989;18(9):963-975.
28. Reinhold MM, Wilk KE, Dugas JR, Andrews JR. Chapter 11. Internal Impingement. In: Wilk K, Reinold MM, Andrews JR, eds. The Athlete’s Shoulder. 2nd ed. Philadelphia, PA: Churchill Livingstone Elsevier; 2009:126.
29. Kuhn JE, Lindholm SR, Huston LJ, Soslowsky LJ, Blasier RB. Failure of the biceps superior labral complex: a cadaveric biomechanical investigation comparing the late cocking and early deceleration positions of throwing. Arthroscopy. 2003;19(4):373-379.
30. Ciochon RL, Corruccini RS. The coraco-acromial ligament and projection index in man and other anthropoid primates. J Anat. 1977;124(Pt 3):627-632.
31. Moorman CT, Warren RF, Deng XH, Wickiewicz TL, Torzilli PA. Role of coracoacromial ligament and related structures in glenohumeral stability: a cadaveric study. J Surg Orthop Adv. 2012;21(4):210-217.
32. Su WR, Budoff JE, Luo ZP. The effect of coracoacromial ligament excision and acromioplasty on superior and anterosuperior glenohumeral stability. Arthroscopy. 2009;25(1):13-18.
33. Wellmann M, Petersen W, Zantop T, Schanz S, Raschke MJ, Hurschler C. Effect of coracoacromial ligament resection on glenohumeral stability under active muscle loading in an in vitro model. Arthroscopy. 2008;24(11):1258-1264.
34. Fagelman M, Sartori M, Freedman KB, Patwardhan AG, Carandang G, Marra G. Biomechanics of coracoacromial arch modification. J Shoulder Elbow Surg. 2007;16(1):101-116.
35. Bak K, Spring IB, Henderson IP. Re-formation of the coracoacromial ligament after open resection or arthroscopic release. J Shoulder Elbow Surg. 2000;9:289-293.
36. Levy O, Copeland SA. Regeneration of the coracoacromial ligament after acromioplasty and arthroscopic subacromial decompression. J Shoulder Elbow Surg. 2001;10(4):317-320.
37. Larson SG, Stern JT Jr. Role of supraspinatus in the quadrupedal locomotion of vervets (Cercopithecus aethiops): Implications for interpretation of humeral morphology. Am J Phys Anthropol. 1989;79(3):369-377.
38. Chansky HA, Iannotti JP. The vascularity of the rotator cuff. Clin Sports Med. 1991;10(4):807-822.
39. Klapper RC, Jobe FW, Matsuura P. Subscapularis muscle and its glenohumeral ligament-like bands. A histomorphologic study. Am J Sports Med. 1992;20(3):307-310.
40. Halder A, Zobitz ME, Schultz E, An KN. Structural properties of the subscapularis tendon. J Orthop Res. 2000;18(5):
829-834.
41. Elser F, Braun S, Dewing CB, Giphart JE, Millett PJ. Anatomy, function, injuries, and treatment of the long head of the biceps brachii tendon. Arthroscopy. 2011;27(4):581-592.
42. Pill SG, Walch G, Hawkins RJ, Kissenberth MJ. The role of the biceps tendon in massive rotator cuff tears. Instr Course Lect. 2012;61:113-120.
43. Krupp RJ, Kevern MA, Gaines MD, Kotara S, Singleton SB. Long head of the biceps tendon pain: differential diagnosis and treatment. J Orthop Sports Phys Ther. 2009;39(2):55-70.
44. Levy AS, Kelly BT, Lintner SA, Osbahr DC, Speer KP. Function of the long head of the biceps at the shoulder: electromyographic analysis. J Shoulder Elbow Surg. 2001;10(3):250-255.
45. Kuhn JE, Huston LJ, Soslowsky LJ, Shyr Y, Blasier RB. External rotation of the glenohumeral joint: ligament restraints and muscle effects in the neutral and abducted positions.
J Shoulder Elbow Surg. 2005;14(1 Suppl S):39S-48S.
46. McGarry MH, Nguyen ML, Quigley RJ, Hanypsiak B, Gupta R, Lee TQ. The effect of long and short head biceps loading on glenohumeral joint rotational range of motion and humeral head postion. Knee Surge Sports Traumatol Arthrosc. 2014 Sep 26. [Epub ahead of print].
47. Young RW. Evolution of the human hand: The role of throwing and clubbing. J Anat. 2003;202:165-174.
48. Calvin WH. Did throwing stones shape hominid brain evolution? Ethology and Sociobiology. 1982;3:115-124.
49. Fifer FC. The adoption of bipedalism by the hominids: A new hypothesis. Human Evolution. 1987;2(2):135-147.
50. Darlington PJ. Group selection, altruism, reinforcement, and throwing in human evolution. Proc Nat Acad Sci. 1973;72(9):3748-3752.
Make Room on Your Shelves
As orthopedic surgeons, we’ve made a commitment to lifelong learning. I can’t think of a single surgery that I perform the same way I did when I was in training. With rapidly evolving technology, continuously advancing procedures, and ever-increasing documentation requirements, it’s hard to stay on top of it all. We know your time is precious and that you have less of it than ever before. What little time you have that is not dedicated to work is reserved for your family or your hobbies. There’s no time to read every orthopedic journal, many filled with articles that have no practical value to your practice. That’s why we’ve created the new AJO. Our goal, as an editorial staff, is to provide a journal where every article, column, and feature contains information that directly benefits your practice, your patients, or your bottom line, and keeps you informed of the latest techniques, procedures, and products. We will help surgeons “work smarter, not harder,” implement new technologies into their practices, and find creative revenue streams that are both legal and compliant.
We’ve assembled a team of talented editors to accomplish this task, and will introduce them throughout the coming year. In this issue, you will meet our Deputy Editors-in-Chief and some of our new Associate Editors who’ve collaborated to bring you the “new AJO”.
At this year’s Academy, the AJO launched an extensive rebranding. We have a new look, a new logo, and a new creative directive. The journal will now feature new columns, invited articles, and innovative surgical techniques. We will publish 5 issues for the remainder of 2016. Our March/April issue is a special edition dedicated to baseball. In time for Spring Training/Opening Day, this issue includes articles from Major League Baseball’s physicians and trainers, a “Codes to Know” segment, “Tips of the Trade,” and a “Tools of the Trade” feature. “The Baseball Issue” will set the tone for what readers can expect from the “new AJO”.
Our first feature article, written by Jed Kuhn, takes a philosophical look at the evolution of the throwing shoulder, and invites the reader to help unlock some of the great shoulder anatomy mysteries by viewing them from a time when throwing was an activity of daily living. In ancient times, if you couldn’t throw, you couldn’t eat. We know that children who play baseball remodel their shoulder to allow for increased external rotation. Read Dr. Kuhn’s article and imagine when a shoulder optimized for throwing was a competitive advantage for survival.
Our second feature article is written by Stan Conte, a legend of the game and longtime trainer for the Los Angeles Dodgers. Dr. Conte studied injury trends in baseball over the past 18 seasons and provides an analysis of the staggering cost of placing players on the disabled list.
A baseball issue could not be complete without an article on Tommy John surgery. In this issue, AJO shares a revolutionary new technique for treating players with MUCL tears by author Jeffrey Dugas. Named the “Internal Brace”, Dr. Dugas shares his technique for augmenting the injured MUCL and we are proud to bring it to you first.
A recurring feature in the new AJO will be a section we refer to as “Codes to Know.” In partnership with Karen Zupko, AJO will present little-known coding secrets and proper coding techniques to help you get reimbursed appropriately for your work. This month, in the first article of a 3-part series, Alan Hirahara teaches us how to properly code for a diagnostic ultrasound examination of the shoulder. The article includes templates available for download to assist you with proper documentation. Parts 2 and 3 will provide a tutorial on the proper technique for examinations and injections.
While shoulder and elbow injuries get more attention, Major League Baseball’s Injury Panel has produced a look at the staggering amount of knee injuries over the 2011-2014 seasons, inspiring us to feature the knee in our 2 “Trade” Columns.
The “Tips of the Trade” column will continue, featuring this month a guide to identifying and treating meniscal root tears. A new segment, referred to as “Tools of the Trade,” reviews the latest products for all-inside meniscal repair. Our “Tools” section will feature announcements and reviews of the hottest new products, with a buying guide and surgical pearls from the surgeons who know them best.
While we are discussing the lower extremity, we should point out that we plan to do the “leg work” for you. Each AJO issue will have handouts that can be downloaded from our website and utilized in your practice. Read Robin West’s article entitled “Interval Throwing and Hitting Programs in Baseball: Biomechanics and Rehabilitation,” and download Return to Throwing and Hitting programs your patients and therapists can use.
Finally, I’d like to thank our previous Editor-in-Chief Dr. Peter McCann for his stewardship the last 10 years and recognize him for his dedication to the journal.
Thank you for reading AJO and for continuing to do so in the future. I know that collectively, we can turn AJO into a product worthy of its title. We know our past reputation. We are no longer that journal. Spend some time to get to know the “new AJO”, and make some room on your shelves, because the information between the covers will provide a template to implement new technologies and revenue streams into your practice and help fulfill your commitment to learning.
As orthopedic surgeons, we’ve made a commitment to lifelong learning. I can’t think of a single surgery that I perform the same way I did when I was in training. With rapidly evolving technology, continuously advancing procedures, and ever-increasing documentation requirements, it’s hard to stay on top of it all. We know your time is precious and that you have less of it than ever before. What little time you have that is not dedicated to work is reserved for your family or your hobbies. There’s no time to read every orthopedic journal, many filled with articles that have no practical value to your practice. That’s why we’ve created the new AJO. Our goal, as an editorial staff, is to provide a journal where every article, column, and feature contains information that directly benefits your practice, your patients, or your bottom line, and keeps you informed of the latest techniques, procedures, and products. We will help surgeons “work smarter, not harder,” implement new technologies into their practices, and find creative revenue streams that are both legal and compliant.
We’ve assembled a team of talented editors to accomplish this task, and will introduce them throughout the coming year. In this issue, you will meet our Deputy Editors-in-Chief and some of our new Associate Editors who’ve collaborated to bring you the “new AJO”.
At this year’s Academy, the AJO launched an extensive rebranding. We have a new look, a new logo, and a new creative directive. The journal will now feature new columns, invited articles, and innovative surgical techniques. We will publish 5 issues for the remainder of 2016. Our March/April issue is a special edition dedicated to baseball. In time for Spring Training/Opening Day, this issue includes articles from Major League Baseball’s physicians and trainers, a “Codes to Know” segment, “Tips of the Trade,” and a “Tools of the Trade” feature. “The Baseball Issue” will set the tone for what readers can expect from the “new AJO”.
Our first feature article, written by Jed Kuhn, takes a philosophical look at the evolution of the throwing shoulder, and invites the reader to help unlock some of the great shoulder anatomy mysteries by viewing them from a time when throwing was an activity of daily living. In ancient times, if you couldn’t throw, you couldn’t eat. We know that children who play baseball remodel their shoulder to allow for increased external rotation. Read Dr. Kuhn’s article and imagine when a shoulder optimized for throwing was a competitive advantage for survival.
Our second feature article is written by Stan Conte, a legend of the game and longtime trainer for the Los Angeles Dodgers. Dr. Conte studied injury trends in baseball over the past 18 seasons and provides an analysis of the staggering cost of placing players on the disabled list.
A baseball issue could not be complete without an article on Tommy John surgery. In this issue, AJO shares a revolutionary new technique for treating players with MUCL tears by author Jeffrey Dugas. Named the “Internal Brace”, Dr. Dugas shares his technique for augmenting the injured MUCL and we are proud to bring it to you first.
A recurring feature in the new AJO will be a section we refer to as “Codes to Know.” In partnership with Karen Zupko, AJO will present little-known coding secrets and proper coding techniques to help you get reimbursed appropriately for your work. This month, in the first article of a 3-part series, Alan Hirahara teaches us how to properly code for a diagnostic ultrasound examination of the shoulder. The article includes templates available for download to assist you with proper documentation. Parts 2 and 3 will provide a tutorial on the proper technique for examinations and injections.
While shoulder and elbow injuries get more attention, Major League Baseball’s Injury Panel has produced a look at the staggering amount of knee injuries over the 2011-2014 seasons, inspiring us to feature the knee in our 2 “Trade” Columns.
The “Tips of the Trade” column will continue, featuring this month a guide to identifying and treating meniscal root tears. A new segment, referred to as “Tools of the Trade,” reviews the latest products for all-inside meniscal repair. Our “Tools” section will feature announcements and reviews of the hottest new products, with a buying guide and surgical pearls from the surgeons who know them best.
While we are discussing the lower extremity, we should point out that we plan to do the “leg work” for you. Each AJO issue will have handouts that can be downloaded from our website and utilized in your practice. Read Robin West’s article entitled “Interval Throwing and Hitting Programs in Baseball: Biomechanics and Rehabilitation,” and download Return to Throwing and Hitting programs your patients and therapists can use.
Finally, I’d like to thank our previous Editor-in-Chief Dr. Peter McCann for his stewardship the last 10 years and recognize him for his dedication to the journal.
Thank you for reading AJO and for continuing to do so in the future. I know that collectively, we can turn AJO into a product worthy of its title. We know our past reputation. We are no longer that journal. Spend some time to get to know the “new AJO”, and make some room on your shelves, because the information between the covers will provide a template to implement new technologies and revenue streams into your practice and help fulfill your commitment to learning.
As orthopedic surgeons, we’ve made a commitment to lifelong learning. I can’t think of a single surgery that I perform the same way I did when I was in training. With rapidly evolving technology, continuously advancing procedures, and ever-increasing documentation requirements, it’s hard to stay on top of it all. We know your time is precious and that you have less of it than ever before. What little time you have that is not dedicated to work is reserved for your family or your hobbies. There’s no time to read every orthopedic journal, many filled with articles that have no practical value to your practice. That’s why we’ve created the new AJO. Our goal, as an editorial staff, is to provide a journal where every article, column, and feature contains information that directly benefits your practice, your patients, or your bottom line, and keeps you informed of the latest techniques, procedures, and products. We will help surgeons “work smarter, not harder,” implement new technologies into their practices, and find creative revenue streams that are both legal and compliant.
We’ve assembled a team of talented editors to accomplish this task, and will introduce them throughout the coming year. In this issue, you will meet our Deputy Editors-in-Chief and some of our new Associate Editors who’ve collaborated to bring you the “new AJO”.
At this year’s Academy, the AJO launched an extensive rebranding. We have a new look, a new logo, and a new creative directive. The journal will now feature new columns, invited articles, and innovative surgical techniques. We will publish 5 issues for the remainder of 2016. Our March/April issue is a special edition dedicated to baseball. In time for Spring Training/Opening Day, this issue includes articles from Major League Baseball’s physicians and trainers, a “Codes to Know” segment, “Tips of the Trade,” and a “Tools of the Trade” feature. “The Baseball Issue” will set the tone for what readers can expect from the “new AJO”.
Our first feature article, written by Jed Kuhn, takes a philosophical look at the evolution of the throwing shoulder, and invites the reader to help unlock some of the great shoulder anatomy mysteries by viewing them from a time when throwing was an activity of daily living. In ancient times, if you couldn’t throw, you couldn’t eat. We know that children who play baseball remodel their shoulder to allow for increased external rotation. Read Dr. Kuhn’s article and imagine when a shoulder optimized for throwing was a competitive advantage for survival.
Our second feature article is written by Stan Conte, a legend of the game and longtime trainer for the Los Angeles Dodgers. Dr. Conte studied injury trends in baseball over the past 18 seasons and provides an analysis of the staggering cost of placing players on the disabled list.
A baseball issue could not be complete without an article on Tommy John surgery. In this issue, AJO shares a revolutionary new technique for treating players with MUCL tears by author Jeffrey Dugas. Named the “Internal Brace”, Dr. Dugas shares his technique for augmenting the injured MUCL and we are proud to bring it to you first.
A recurring feature in the new AJO will be a section we refer to as “Codes to Know.” In partnership with Karen Zupko, AJO will present little-known coding secrets and proper coding techniques to help you get reimbursed appropriately for your work. This month, in the first article of a 3-part series, Alan Hirahara teaches us how to properly code for a diagnostic ultrasound examination of the shoulder. The article includes templates available for download to assist you with proper documentation. Parts 2 and 3 will provide a tutorial on the proper technique for examinations and injections.
While shoulder and elbow injuries get more attention, Major League Baseball’s Injury Panel has produced a look at the staggering amount of knee injuries over the 2011-2014 seasons, inspiring us to feature the knee in our 2 “Trade” Columns.
The “Tips of the Trade” column will continue, featuring this month a guide to identifying and treating meniscal root tears. A new segment, referred to as “Tools of the Trade,” reviews the latest products for all-inside meniscal repair. Our “Tools” section will feature announcements and reviews of the hottest new products, with a buying guide and surgical pearls from the surgeons who know them best.
While we are discussing the lower extremity, we should point out that we plan to do the “leg work” for you. Each AJO issue will have handouts that can be downloaded from our website and utilized in your practice. Read Robin West’s article entitled “Interval Throwing and Hitting Programs in Baseball: Biomechanics and Rehabilitation,” and download Return to Throwing and Hitting programs your patients and therapists can use.
Finally, I’d like to thank our previous Editor-in-Chief Dr. Peter McCann for his stewardship the last 10 years and recognize him for his dedication to the journal.
Thank you for reading AJO and for continuing to do so in the future. I know that collectively, we can turn AJO into a product worthy of its title. We know our past reputation. We are no longer that journal. Spend some time to get to know the “new AJO”, and make some room on your shelves, because the information between the covers will provide a template to implement new technologies and revenue streams into your practice and help fulfill your commitment to learning.
Distal Ulna Fracture With Delayed Ulnar Nerve Palsy in a Baseball Player
Ulnar nerve injury leads to clawing of the ulnar digits and loss of digital abduction and adduction because of paralysis of the ulnar innervated extrinsic and intrinsic muscles. Isolated motor paralysis without sensory deficit can occur from compression within the Guyon canal.1 Cubital tunnel at the elbow is the most common site for ulnar nerve compression.2 Compression at both levels can be encountered in sports-related activities. Nerve compression in the Guyon canal can occur with bicycling and is known as cyclist’s palsy,3-6 but it can also develop from canoeing.7 Cubital tunnel syndrome is the most common neuropathy of the elbow among throwing athletes, especially in baseball pitchers and can result from nerve traction and compression within the fibro-osseous tunnel or subluxation out of the tunnel.2 Both compression syndromes can develop from repetitive stress and/or pressure to the nerve in the retrocondylar groove.
Ulnar nerve palsy may be associated with forearm fractures, which is usually caused by simultaneous ulna and radius fractures, especially in children.8-12 To our knowledge, there are no reports in the literature of an ulnar nerve palsy associated with an isolated ulnar shaft fracture in an adult. We report a case of delayed ulnar nerve palsy after an ulnar shaft fracture in a baseball player. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 19-year-old, right hand–dominant college baseball player was batting right-handed in an intrasquad scrimmage when a high and inside pitched ball from a right-handed pitcher struck the volar-ulnar aspect of his right forearm. Examination in the training room and emergency department revealed moderate swelling and ecchymosis over the distal third of the ulna. He had a normal neurovascular examination, including normal sensation to light touch and normal finger abduction/adduction and wrist flexion/extension. He was otherwise healthy. Radiographs of the right forearm showed a minimally displaced transverse fracture of the distal third of the ulna (Figures 1A, 1B).
The patient was initially treated with a well-padded, removable, long-arm posterior splint for 2 weeks with serial examinations each day in the training room. At 2-week follow-up, he reported less pain and swelling but stated that his hand had “felt funny” the past several days. Examination revealed clawing of the ulnar digits with paresthesias in the ulnar nerve distribution (Figures 2A, 2B). His extrinsic muscle function was normal. Radiographs showed stable fracture alignment. Ulnar neuropathy was diagnosed, and treatment was observation with a plan for electromyography (EMG) at 6 weeks after injury if there were no signs of nerve recovery. Physical therapy was instituted and focused on improving intrinsic muscle and proprioceptive functions with the goal of an expeditious, but safe, return to playing baseball. Three weeks after his injury, the patient had decreased tenderness at his fracture site and was given a forearm pad and sleeve for light, noncontact baseball activity (Figure 3). A long velcro wrist splint was used during conditioning and when not playing baseball. Forearm supination and pronation were limited initially because of patient discomfort and to prevent torsional fracture displacement or delayed healing. Six weeks after his injury, the patient returned to hitting and was showing early signs of improved sensation and intrinsic hand strength. He had progressed to a light throwing program and reported difficult hand coordination, poor ball control, and overall difficulty in accurately throwing over the next 3 to 4 months. Because of his difficulty with ball control, the patient began a progressive return to full-game activity over 6 weeks, which initially included a return to batting only, then playing in the outfield, and, eventually, a return to his normal position in the infield. Serial radiographs continued to show good fracture alignment with appropriate new bone formation (Figures 4A, 4B). Normal motor strength was noted at 3 months after injury and normal sensation at 4 months after injury.
By the end of his summer league, 6 months after his injury, the patient was named Most Valuable Player and had a batting average over .400. He reported near-normal hand function. One year after injury, his examination revealed normal hand function (Figure 5), including normal sensation to light touch, 5/5 intrinsic hand function, and symmetric grip strength. Radiographs showed a healed fracture (Figures 6A, 6B). The patient has gone on to play more than 9 years of professional baseball.
Discussion
The ulnar nerve has a course that runs down the volar compartment of the distal forearm. The flexor carpi ulnaris provides coverage to the nerve in this area. Proximal to the wrist, the nerve emerges from under the flexor carpi ulnaris tendon and passes deep to the flexor retinaculum, which is the distal extension of the antebrachial fascia and blends distally into the palmar carpal ligament.13 In our patient, the most likely cause of this presentation of ulnar neuropathy was the direct blow to the nerve from the high-intensity impact of a thrown baseball to this superficial and exposed area of the forearm. Since the patient presented with delayed paresthesias and ulnar clawing 2 weeks after injury, possible contributing causes could be evolving pressure or nerve damage from a perineural hematoma and/or intraneural hematoma or increased local pressure from intramuscular hemorrhage.14 There are both acute and chronic cases of ulnar nerve entrapment by bone or scar tissue that resolved by surgical decompression.8-12 Surgical exploration was not deemed necessary in our case because the fracture was minimally displaced, and the patient regained sensation and motor function over the course of 3 to 4 months.
Nerve injuries can be classified as neurapraxia, axonotmesis, or neurotmesis. Neurapraxia is the mildest form of nerve injury and neurotmesis the most severe. Neurapraxia may be associated with a temporary block to conduction or nerve demyelination without axonal disruption. Spontaneous recovery takes 2 weeks to 2 months. Axonotmesis involves an actual loss of axonal continuity; however, connective tissue supporting structures remain intact and allow axonal regeneration. Finally, neurotmesis is transection of the peripheral nerve, and spontaneous regeneration is not possible. The mechanism of injury in our patient suggests that the pathology was neurapraxia.1,15
Management of these injuries should proceed according to basic extremity injury–care practices. Initial care should include thorough neurovascular and radiographic evaluations. If nerve deficits are present with a closed injury and minimal fracture displacement, treatment can include observation and serial examinations with a baseline EMG, or waiting until 4 to 6 weeks after injury to obtain an EMG if there are no signs of nerve recovery. Early EMG testing and surgical exploration may be warranted if there is a concern for nerve disruption or entrapment, such as marked fracture displacement or an open injury. Additional early-care measures should include swelling control modalities and immobilization based on the type of fracture. Ultrasound was not readily available at the time of our patient’s injury, but it may be a helpful adjunct in guiding decision-making regarding whether to perform early surgical exploration for hematoma evacuation or nerve injury.16-18 Our case report was intended to provide an awareness of the unusual association between an isolated ulnar shaft fracture and a delayed ulnar nerve palsy in an athlete. Nerve injuries may be unrecognized in some patients in a trauma situation, since the focus is usually on the fracture and the typical patient does not have to return to high-demand, coordinated athletic activity, such as throwing a ball. Because of the possible delayed presentation of these nerve injuries, close observation of nerve function after ulna fractures from blunt trauma is warranted.
1. Dhillon MS, Chu ML, Posner MA. Demyelinating focal motor neuropathy of the ulnar nerve masquerading as compression in Guyon’s canal: a case report. J Hand Surg Am. 2003;28(1):48-51.
2. Hariri S, McAdams TR. Nerve injuries about the elbow. Clin Sports Med. 2010;29(4):655-675.
3. Akuthota V, Plastaras C, Lindberg K, Tobey J, Press J, Garvan C. The effect of long-distance bicycling on ulnar and median nerves: an electrophysiologic evaluation of cyclist palsy. Am J Sports Med. 2005;33(8):1224-1230.
4. Capitani D, Beer S. Handlebar palsy--a compression syndrome of the deep terminal (motor) branch of the ulnar nerve in biking. J Neurol. 2002;249(10):1441-1445.
5. Patterson JM, Jaggars MM, Boyer MI. Ulnar and median nerve palsy in long-distance cyclists. A prospective study. Am J Sports Med. 2003;31(4):585-589.
6. Slane J, Timmerman M, Ploeg HL, Thelen DG. The influence of glove and hand position on pressure over the ulnar nerve during cycling. Clin Biomech (Bristol, Avon). 2011;26(6):642-648.
7. Paul F, Diesta FJ, Ratzlaff T, Vogel HP, Zipp F. Combined ulnar nerve palsy in Guyon’s canal and distal median nerve irritation following excessive canoeing. Clinical Neurophysiology. 2007;118(4):e81-e82.
8. Hirasawa H, Sakai A, Toba N, Kamiuttanai M, Nakamura T, Tanaka K. Bony entrapment of ulnar nerve after closed forearm fracture: a case report. J Orthop Surg (Hong Kong). 2004;12(1):122-125.
9. Dahlin LB, Düppe H. Injuries to the nerves associated with fractured forearms in children. Scand J Plast Reconstr Surg Hand Surg. 2007;41(4):207-210.
10. Neiman R, Maiocco B, Deeney VF. Ulnar nerve injury after closed forearm fractures in children. J Pediatr Orthop. 1998;18(5):683-685.
11. Pai VS. Injury of the ulnar nerve associated with fracture of the ulna: A case report. J Orthop Surgery. 1999;7(2):73.
12. Suganuma S, Tada K, Hayashi H, Segawa T, Tsuchiya H. Ulnar nerve palsy associated with closed midshaft forearm fractures. Orthopedics. 2012;35(11):e1680-e1683.
13. Ombaba J, Kuo M, Rayan G. Anatomy of the ulnar tunnel and the influence of wrist motion on its morphology. J Hand Surg Am. 2010;35A:760-768.
14. Vijayakumar R, Nesathurai S, Abbott KM, Eustace S. Ulnar neuropathy resulting from diffuse intramuscular hemorrhage: a case report. Arch Phys Med Rehabil. 2000;81(8):1127-1130.
15. Browner, Bruce. Skeletal Trauma: Basic Science, Management, and Reconstruction [eBook]. 4th ed. Philadelphia, PA: WB Saunders Company; 2009:1487.
16. Koenig RW, Pedro MT, Heinen CP, et al. High-resolution ultrasonography in evaluating peripheral nerve entrapment and trauma. Neurosurg Focus. 2009;26(2):E13.
17. Zhu J, Liu F, Li D, Shao J, Hu B. Preliminary study of the types of traumatic peripheral nerve injuries by ultrasound. Eur Radiol. 2011;21(5):1097-1101.
18. Lee FC, Singh H, Nazarian LN, Ratliff JK. High-resolution ultrasonography in the diagnosis and intra-operative management of peripheral nerve lesions. J Neurosurg. 2011;114(1):206-221.
Ulnar nerve injury leads to clawing of the ulnar digits and loss of digital abduction and adduction because of paralysis of the ulnar innervated extrinsic and intrinsic muscles. Isolated motor paralysis without sensory deficit can occur from compression within the Guyon canal.1 Cubital tunnel at the elbow is the most common site for ulnar nerve compression.2 Compression at both levels can be encountered in sports-related activities. Nerve compression in the Guyon canal can occur with bicycling and is known as cyclist’s palsy,3-6 but it can also develop from canoeing.7 Cubital tunnel syndrome is the most common neuropathy of the elbow among throwing athletes, especially in baseball pitchers and can result from nerve traction and compression within the fibro-osseous tunnel or subluxation out of the tunnel.2 Both compression syndromes can develop from repetitive stress and/or pressure to the nerve in the retrocondylar groove.
Ulnar nerve palsy may be associated with forearm fractures, which is usually caused by simultaneous ulna and radius fractures, especially in children.8-12 To our knowledge, there are no reports in the literature of an ulnar nerve palsy associated with an isolated ulnar shaft fracture in an adult. We report a case of delayed ulnar nerve palsy after an ulnar shaft fracture in a baseball player. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 19-year-old, right hand–dominant college baseball player was batting right-handed in an intrasquad scrimmage when a high and inside pitched ball from a right-handed pitcher struck the volar-ulnar aspect of his right forearm. Examination in the training room and emergency department revealed moderate swelling and ecchymosis over the distal third of the ulna. He had a normal neurovascular examination, including normal sensation to light touch and normal finger abduction/adduction and wrist flexion/extension. He was otherwise healthy. Radiographs of the right forearm showed a minimally displaced transverse fracture of the distal third of the ulna (Figures 1A, 1B).
The patient was initially treated with a well-padded, removable, long-arm posterior splint for 2 weeks with serial examinations each day in the training room. At 2-week follow-up, he reported less pain and swelling but stated that his hand had “felt funny” the past several days. Examination revealed clawing of the ulnar digits with paresthesias in the ulnar nerve distribution (Figures 2A, 2B). His extrinsic muscle function was normal. Radiographs showed stable fracture alignment. Ulnar neuropathy was diagnosed, and treatment was observation with a plan for electromyography (EMG) at 6 weeks after injury if there were no signs of nerve recovery. Physical therapy was instituted and focused on improving intrinsic muscle and proprioceptive functions with the goal of an expeditious, but safe, return to playing baseball. Three weeks after his injury, the patient had decreased tenderness at his fracture site and was given a forearm pad and sleeve for light, noncontact baseball activity (Figure 3). A long velcro wrist splint was used during conditioning and when not playing baseball. Forearm supination and pronation were limited initially because of patient discomfort and to prevent torsional fracture displacement or delayed healing. Six weeks after his injury, the patient returned to hitting and was showing early signs of improved sensation and intrinsic hand strength. He had progressed to a light throwing program and reported difficult hand coordination, poor ball control, and overall difficulty in accurately throwing over the next 3 to 4 months. Because of his difficulty with ball control, the patient began a progressive return to full-game activity over 6 weeks, which initially included a return to batting only, then playing in the outfield, and, eventually, a return to his normal position in the infield. Serial radiographs continued to show good fracture alignment with appropriate new bone formation (Figures 4A, 4B). Normal motor strength was noted at 3 months after injury and normal sensation at 4 months after injury.
By the end of his summer league, 6 months after his injury, the patient was named Most Valuable Player and had a batting average over .400. He reported near-normal hand function. One year after injury, his examination revealed normal hand function (Figure 5), including normal sensation to light touch, 5/5 intrinsic hand function, and symmetric grip strength. Radiographs showed a healed fracture (Figures 6A, 6B). The patient has gone on to play more than 9 years of professional baseball.
Discussion
The ulnar nerve has a course that runs down the volar compartment of the distal forearm. The flexor carpi ulnaris provides coverage to the nerve in this area. Proximal to the wrist, the nerve emerges from under the flexor carpi ulnaris tendon and passes deep to the flexor retinaculum, which is the distal extension of the antebrachial fascia and blends distally into the palmar carpal ligament.13 In our patient, the most likely cause of this presentation of ulnar neuropathy was the direct blow to the nerve from the high-intensity impact of a thrown baseball to this superficial and exposed area of the forearm. Since the patient presented with delayed paresthesias and ulnar clawing 2 weeks after injury, possible contributing causes could be evolving pressure or nerve damage from a perineural hematoma and/or intraneural hematoma or increased local pressure from intramuscular hemorrhage.14 There are both acute and chronic cases of ulnar nerve entrapment by bone or scar tissue that resolved by surgical decompression.8-12 Surgical exploration was not deemed necessary in our case because the fracture was minimally displaced, and the patient regained sensation and motor function over the course of 3 to 4 months.
Nerve injuries can be classified as neurapraxia, axonotmesis, or neurotmesis. Neurapraxia is the mildest form of nerve injury and neurotmesis the most severe. Neurapraxia may be associated with a temporary block to conduction or nerve demyelination without axonal disruption. Spontaneous recovery takes 2 weeks to 2 months. Axonotmesis involves an actual loss of axonal continuity; however, connective tissue supporting structures remain intact and allow axonal regeneration. Finally, neurotmesis is transection of the peripheral nerve, and spontaneous regeneration is not possible. The mechanism of injury in our patient suggests that the pathology was neurapraxia.1,15
Management of these injuries should proceed according to basic extremity injury–care practices. Initial care should include thorough neurovascular and radiographic evaluations. If nerve deficits are present with a closed injury and minimal fracture displacement, treatment can include observation and serial examinations with a baseline EMG, or waiting until 4 to 6 weeks after injury to obtain an EMG if there are no signs of nerve recovery. Early EMG testing and surgical exploration may be warranted if there is a concern for nerve disruption or entrapment, such as marked fracture displacement or an open injury. Additional early-care measures should include swelling control modalities and immobilization based on the type of fracture. Ultrasound was not readily available at the time of our patient’s injury, but it may be a helpful adjunct in guiding decision-making regarding whether to perform early surgical exploration for hematoma evacuation or nerve injury.16-18 Our case report was intended to provide an awareness of the unusual association between an isolated ulnar shaft fracture and a delayed ulnar nerve palsy in an athlete. Nerve injuries may be unrecognized in some patients in a trauma situation, since the focus is usually on the fracture and the typical patient does not have to return to high-demand, coordinated athletic activity, such as throwing a ball. Because of the possible delayed presentation of these nerve injuries, close observation of nerve function after ulna fractures from blunt trauma is warranted.
Ulnar nerve injury leads to clawing of the ulnar digits and loss of digital abduction and adduction because of paralysis of the ulnar innervated extrinsic and intrinsic muscles. Isolated motor paralysis without sensory deficit can occur from compression within the Guyon canal.1 Cubital tunnel at the elbow is the most common site for ulnar nerve compression.2 Compression at both levels can be encountered in sports-related activities. Nerve compression in the Guyon canal can occur with bicycling and is known as cyclist’s palsy,3-6 but it can also develop from canoeing.7 Cubital tunnel syndrome is the most common neuropathy of the elbow among throwing athletes, especially in baseball pitchers and can result from nerve traction and compression within the fibro-osseous tunnel or subluxation out of the tunnel.2 Both compression syndromes can develop from repetitive stress and/or pressure to the nerve in the retrocondylar groove.
Ulnar nerve palsy may be associated with forearm fractures, which is usually caused by simultaneous ulna and radius fractures, especially in children.8-12 To our knowledge, there are no reports in the literature of an ulnar nerve palsy associated with an isolated ulnar shaft fracture in an adult. We report a case of delayed ulnar nerve palsy after an ulnar shaft fracture in a baseball player. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 19-year-old, right hand–dominant college baseball player was batting right-handed in an intrasquad scrimmage when a high and inside pitched ball from a right-handed pitcher struck the volar-ulnar aspect of his right forearm. Examination in the training room and emergency department revealed moderate swelling and ecchymosis over the distal third of the ulna. He had a normal neurovascular examination, including normal sensation to light touch and normal finger abduction/adduction and wrist flexion/extension. He was otherwise healthy. Radiographs of the right forearm showed a minimally displaced transverse fracture of the distal third of the ulna (Figures 1A, 1B).
The patient was initially treated with a well-padded, removable, long-arm posterior splint for 2 weeks with serial examinations each day in the training room. At 2-week follow-up, he reported less pain and swelling but stated that his hand had “felt funny” the past several days. Examination revealed clawing of the ulnar digits with paresthesias in the ulnar nerve distribution (Figures 2A, 2B). His extrinsic muscle function was normal. Radiographs showed stable fracture alignment. Ulnar neuropathy was diagnosed, and treatment was observation with a plan for electromyography (EMG) at 6 weeks after injury if there were no signs of nerve recovery. Physical therapy was instituted and focused on improving intrinsic muscle and proprioceptive functions with the goal of an expeditious, but safe, return to playing baseball. Three weeks after his injury, the patient had decreased tenderness at his fracture site and was given a forearm pad and sleeve for light, noncontact baseball activity (Figure 3). A long velcro wrist splint was used during conditioning and when not playing baseball. Forearm supination and pronation were limited initially because of patient discomfort and to prevent torsional fracture displacement or delayed healing. Six weeks after his injury, the patient returned to hitting and was showing early signs of improved sensation and intrinsic hand strength. He had progressed to a light throwing program and reported difficult hand coordination, poor ball control, and overall difficulty in accurately throwing over the next 3 to 4 months. Because of his difficulty with ball control, the patient began a progressive return to full-game activity over 6 weeks, which initially included a return to batting only, then playing in the outfield, and, eventually, a return to his normal position in the infield. Serial radiographs continued to show good fracture alignment with appropriate new bone formation (Figures 4A, 4B). Normal motor strength was noted at 3 months after injury and normal sensation at 4 months after injury.
By the end of his summer league, 6 months after his injury, the patient was named Most Valuable Player and had a batting average over .400. He reported near-normal hand function. One year after injury, his examination revealed normal hand function (Figure 5), including normal sensation to light touch, 5/5 intrinsic hand function, and symmetric grip strength. Radiographs showed a healed fracture (Figures 6A, 6B). The patient has gone on to play more than 9 years of professional baseball.
Discussion
The ulnar nerve has a course that runs down the volar compartment of the distal forearm. The flexor carpi ulnaris provides coverage to the nerve in this area. Proximal to the wrist, the nerve emerges from under the flexor carpi ulnaris tendon and passes deep to the flexor retinaculum, which is the distal extension of the antebrachial fascia and blends distally into the palmar carpal ligament.13 In our patient, the most likely cause of this presentation of ulnar neuropathy was the direct blow to the nerve from the high-intensity impact of a thrown baseball to this superficial and exposed area of the forearm. Since the patient presented with delayed paresthesias and ulnar clawing 2 weeks after injury, possible contributing causes could be evolving pressure or nerve damage from a perineural hematoma and/or intraneural hematoma or increased local pressure from intramuscular hemorrhage.14 There are both acute and chronic cases of ulnar nerve entrapment by bone or scar tissue that resolved by surgical decompression.8-12 Surgical exploration was not deemed necessary in our case because the fracture was minimally displaced, and the patient regained sensation and motor function over the course of 3 to 4 months.
Nerve injuries can be classified as neurapraxia, axonotmesis, or neurotmesis. Neurapraxia is the mildest form of nerve injury and neurotmesis the most severe. Neurapraxia may be associated with a temporary block to conduction or nerve demyelination without axonal disruption. Spontaneous recovery takes 2 weeks to 2 months. Axonotmesis involves an actual loss of axonal continuity; however, connective tissue supporting structures remain intact and allow axonal regeneration. Finally, neurotmesis is transection of the peripheral nerve, and spontaneous regeneration is not possible. The mechanism of injury in our patient suggests that the pathology was neurapraxia.1,15
Management of these injuries should proceed according to basic extremity injury–care practices. Initial care should include thorough neurovascular and radiographic evaluations. If nerve deficits are present with a closed injury and minimal fracture displacement, treatment can include observation and serial examinations with a baseline EMG, or waiting until 4 to 6 weeks after injury to obtain an EMG if there are no signs of nerve recovery. Early EMG testing and surgical exploration may be warranted if there is a concern for nerve disruption or entrapment, such as marked fracture displacement or an open injury. Additional early-care measures should include swelling control modalities and immobilization based on the type of fracture. Ultrasound was not readily available at the time of our patient’s injury, but it may be a helpful adjunct in guiding decision-making regarding whether to perform early surgical exploration for hematoma evacuation or nerve injury.16-18 Our case report was intended to provide an awareness of the unusual association between an isolated ulnar shaft fracture and a delayed ulnar nerve palsy in an athlete. Nerve injuries may be unrecognized in some patients in a trauma situation, since the focus is usually on the fracture and the typical patient does not have to return to high-demand, coordinated athletic activity, such as throwing a ball. Because of the possible delayed presentation of these nerve injuries, close observation of nerve function after ulna fractures from blunt trauma is warranted.
1. Dhillon MS, Chu ML, Posner MA. Demyelinating focal motor neuropathy of the ulnar nerve masquerading as compression in Guyon’s canal: a case report. J Hand Surg Am. 2003;28(1):48-51.
2. Hariri S, McAdams TR. Nerve injuries about the elbow. Clin Sports Med. 2010;29(4):655-675.
3. Akuthota V, Plastaras C, Lindberg K, Tobey J, Press J, Garvan C. The effect of long-distance bicycling on ulnar and median nerves: an electrophysiologic evaluation of cyclist palsy. Am J Sports Med. 2005;33(8):1224-1230.
4. Capitani D, Beer S. Handlebar palsy--a compression syndrome of the deep terminal (motor) branch of the ulnar nerve in biking. J Neurol. 2002;249(10):1441-1445.
5. Patterson JM, Jaggars MM, Boyer MI. Ulnar and median nerve palsy in long-distance cyclists. A prospective study. Am J Sports Med. 2003;31(4):585-589.
6. Slane J, Timmerman M, Ploeg HL, Thelen DG. The influence of glove and hand position on pressure over the ulnar nerve during cycling. Clin Biomech (Bristol, Avon). 2011;26(6):642-648.
7. Paul F, Diesta FJ, Ratzlaff T, Vogel HP, Zipp F. Combined ulnar nerve palsy in Guyon’s canal and distal median nerve irritation following excessive canoeing. Clinical Neurophysiology. 2007;118(4):e81-e82.
8. Hirasawa H, Sakai A, Toba N, Kamiuttanai M, Nakamura T, Tanaka K. Bony entrapment of ulnar nerve after closed forearm fracture: a case report. J Orthop Surg (Hong Kong). 2004;12(1):122-125.
9. Dahlin LB, Düppe H. Injuries to the nerves associated with fractured forearms in children. Scand J Plast Reconstr Surg Hand Surg. 2007;41(4):207-210.
10. Neiman R, Maiocco B, Deeney VF. Ulnar nerve injury after closed forearm fractures in children. J Pediatr Orthop. 1998;18(5):683-685.
11. Pai VS. Injury of the ulnar nerve associated with fracture of the ulna: A case report. J Orthop Surgery. 1999;7(2):73.
12. Suganuma S, Tada K, Hayashi H, Segawa T, Tsuchiya H. Ulnar nerve palsy associated with closed midshaft forearm fractures. Orthopedics. 2012;35(11):e1680-e1683.
13. Ombaba J, Kuo M, Rayan G. Anatomy of the ulnar tunnel and the influence of wrist motion on its morphology. J Hand Surg Am. 2010;35A:760-768.
14. Vijayakumar R, Nesathurai S, Abbott KM, Eustace S. Ulnar neuropathy resulting from diffuse intramuscular hemorrhage: a case report. Arch Phys Med Rehabil. 2000;81(8):1127-1130.
15. Browner, Bruce. Skeletal Trauma: Basic Science, Management, and Reconstruction [eBook]. 4th ed. Philadelphia, PA: WB Saunders Company; 2009:1487.
16. Koenig RW, Pedro MT, Heinen CP, et al. High-resolution ultrasonography in evaluating peripheral nerve entrapment and trauma. Neurosurg Focus. 2009;26(2):E13.
17. Zhu J, Liu F, Li D, Shao J, Hu B. Preliminary study of the types of traumatic peripheral nerve injuries by ultrasound. Eur Radiol. 2011;21(5):1097-1101.
18. Lee FC, Singh H, Nazarian LN, Ratliff JK. High-resolution ultrasonography in the diagnosis and intra-operative management of peripheral nerve lesions. J Neurosurg. 2011;114(1):206-221.
1. Dhillon MS, Chu ML, Posner MA. Demyelinating focal motor neuropathy of the ulnar nerve masquerading as compression in Guyon’s canal: a case report. J Hand Surg Am. 2003;28(1):48-51.
2. Hariri S, McAdams TR. Nerve injuries about the elbow. Clin Sports Med. 2010;29(4):655-675.
3. Akuthota V, Plastaras C, Lindberg K, Tobey J, Press J, Garvan C. The effect of long-distance bicycling on ulnar and median nerves: an electrophysiologic evaluation of cyclist palsy. Am J Sports Med. 2005;33(8):1224-1230.
4. Capitani D, Beer S. Handlebar palsy--a compression syndrome of the deep terminal (motor) branch of the ulnar nerve in biking. J Neurol. 2002;249(10):1441-1445.
5. Patterson JM, Jaggars MM, Boyer MI. Ulnar and median nerve palsy in long-distance cyclists. A prospective study. Am J Sports Med. 2003;31(4):585-589.
6. Slane J, Timmerman M, Ploeg HL, Thelen DG. The influence of glove and hand position on pressure over the ulnar nerve during cycling. Clin Biomech (Bristol, Avon). 2011;26(6):642-648.
7. Paul F, Diesta FJ, Ratzlaff T, Vogel HP, Zipp F. Combined ulnar nerve palsy in Guyon’s canal and distal median nerve irritation following excessive canoeing. Clinical Neurophysiology. 2007;118(4):e81-e82.
8. Hirasawa H, Sakai A, Toba N, Kamiuttanai M, Nakamura T, Tanaka K. Bony entrapment of ulnar nerve after closed forearm fracture: a case report. J Orthop Surg (Hong Kong). 2004;12(1):122-125.
9. Dahlin LB, Düppe H. Injuries to the nerves associated with fractured forearms in children. Scand J Plast Reconstr Surg Hand Surg. 2007;41(4):207-210.
10. Neiman R, Maiocco B, Deeney VF. Ulnar nerve injury after closed forearm fractures in children. J Pediatr Orthop. 1998;18(5):683-685.
11. Pai VS. Injury of the ulnar nerve associated with fracture of the ulna: A case report. J Orthop Surgery. 1999;7(2):73.
12. Suganuma S, Tada K, Hayashi H, Segawa T, Tsuchiya H. Ulnar nerve palsy associated with closed midshaft forearm fractures. Orthopedics. 2012;35(11):e1680-e1683.
13. Ombaba J, Kuo M, Rayan G. Anatomy of the ulnar tunnel and the influence of wrist motion on its morphology. J Hand Surg Am. 2010;35A:760-768.
14. Vijayakumar R, Nesathurai S, Abbott KM, Eustace S. Ulnar neuropathy resulting from diffuse intramuscular hemorrhage: a case report. Arch Phys Med Rehabil. 2000;81(8):1127-1130.
15. Browner, Bruce. Skeletal Trauma: Basic Science, Management, and Reconstruction [eBook]. 4th ed. Philadelphia, PA: WB Saunders Company; 2009:1487.
16. Koenig RW, Pedro MT, Heinen CP, et al. High-resolution ultrasonography in evaluating peripheral nerve entrapment and trauma. Neurosurg Focus. 2009;26(2):E13.
17. Zhu J, Liu F, Li D, Shao J, Hu B. Preliminary study of the types of traumatic peripheral nerve injuries by ultrasound. Eur Radiol. 2011;21(5):1097-1101.
18. Lee FC, Singh H, Nazarian LN, Ratliff JK. High-resolution ultrasonography in the diagnosis and intra-operative management of peripheral nerve lesions. J Neurosurg. 2011;114(1):206-221.
Female Athletes: Unique Challenges Facing Women Warriors
Since Title IX passed in 1972, women have become exponentially more involved in competitive sports, from high school to professional levels. With more women engaging in serious athletics, the specific challenges they face have come to the forefront of sports medicine. These problems include the female athlete triad, concussions, exercise safety in pregnancy, anterior cruciate ligament (ACL) injuries, and continued sex discrimination and social injustice. Orthopedists treating female athletes should be aware of these problems, each of which is discussed in this review.
1. Female athlete triad
In 1992, the term female athlete triad was coined to describe 3 problems that often coexist in high-intensity female athletes.1 Since then, the definition has evolved, but the problem has remained essentially the same. The modern definition incorporates menstrual abnormalities, low energy availability with or without disordered eating, and decreased bone mineral density (BMD).2
With intense exercise and weight loss comes a variety of menstrual disturbances.3 In affected athletes, the hypothalamus is underactivated, and changes in gonadotropin-releasing hormone and luteinizing hormone lead to decreased estrogen production. Research suggests abnormal menses result from having inadequate energy and insufficient caloric intake to support extensive exercise.1 This phenomenon can occur in athletes in any sport but is most prevalent in lean-body sports, such as swimming, gymnastics, and ballet. The incidence of abnormal menses is as high as 79% in ballet dancers but only 5% in the general population.3 Menstrual abnormalities indicate hormonal abnormalities that can interfere with growth and maturation in young athletes.
Although full-blown eating disorders are uncommon among female athletes, disordered eating patterns are often found among women in competitive sports. Disordered eating can involve a spectrum of inadequate caloric intake and purging behavior, such as vomiting or laxative abuse, and has been reported in up to 25% of collegiate female athletes.4 Physicians must recognize these conditions and initiate counseling and treatment when appropriate. Women with disordered eating are at risk for developing electrolyte imbalances, malnutrition syndromes, and osteopenia.
Although careful evaluation and counseling are important, physicians must note that, in most cases, athletics participation may also protect against disordered eating and body image difficulties. A study of 146 college-age women found better body satisfaction among athletes than among nonathletes.5 Lean-sport athletes (eg, swimmers, gymnasts) were at higher risk for disordered eating and body image problems than other athletes were. Similarly, other studies have found that a majority of athletes have healthy eating habits.4
For poorly nourished and hormonally imbalanced female athletes, decreased BMD poses substantial risk. One study found a significant difference in BMD between athletes with amenorrhea and athletes with normal menses.6 In a cohort of female Navy recruits, those with amenorrhea were at 91% higher risk for stress fractures; calcium and vitamin D supplementation reduced risk by 20%.7 Osteopenia may be a special problem for prepubescent athletes. Girls who engage in intense exercise and have delayed menarche may have a low estrogen state, predisposing them to low BMD.3 Osteopenia and osteoporosis are difficult to reverse and can put these athletes at risk for stress fractures the rest of their lives. If unrecognized, stress fractures can end an athlete’s career.
Recommendations for dual-energy X-ray absorptiometry (DXA) include testing female athletes who have a diagnosed eating disorder, body mass index under 17.5, history of delayed menarche, oligomenorrhea, 2 prior stress fractures, or prior abnormal DXA scan. Complete testing recommendations appear in the 2014 consensus statement on the female athlete triad and return to sport.2,8
Orthopedists performing physical examinations for sports participation can screen for the female athlete triad through thoughtful questioning about menstrual history, nutrition habits, and stress fracture symptoms. Best treatment for a diagnosed case of the triad is multidisciplinary care with strong social support. When abnormal menses are an issue, referral to a gynecologist or endocrinologist and consideration of estrogen replacement should be discussed. Some cases require a psychiatrist’s assistance in treating disordered eating. Athletic trainers, coaches, and parents should be involved over the treatment course.1 Orthopedists must counsel women with osteopenia and osteoporosis about decreasing exercise to a safe level, improving nutritional intake, and supplementing with calcium (1200-1500 mg/d) and vitamin D (600-800 IU/d).3,7
2. Concussions
Increasing awareness of males’ sport-related concussions, particularly of concussions that occur during National Football League practice and games, has made physicians and researchers more aware of the rate of concussion in female athletes. That rate has increased, and, according to some reports, the risk for sport-related injury is higher for female athletes.9 A study of high school athletes found that the rate of concussion in girl’s soccer was second only to that in football.10
Concussions are categorized as mild traumatic brain injuries, and manifestations of the diagnosis are divided into physical, emotional, cognitive, and observed symptoms. The spectrum of symptoms is wide, ranging from difficulty concentrating and thinking clearly to headaches and dizziness.11 Compared with male athletes who sustain a concussion, female athletes report more of these concussive symptoms and have worse visual memory scores.12
Efforts to change sports at the player level have been resisted. Helmets have been proposed for field hockey and lacrosse but have not passed stringent concussion testing. In soccer, which has a high rate of concussion, a reform to eliminate heading the ball has been considered. Resistance to these suggestions stems from the thought that changes could alter the traditions of the games. Some individuals have indicated that helmets may give players a false sense of security and thereby cause them to play more aggressively.
Orthopedic surgeons must be aware of concussion symptoms. Multiple concussions may have a cumulative effect on functional ability and emotional well-being and may lead to chronic traumatic encephalopathy.13 Concern about the long-term effects of concussion has led to the implementation of universal “return to play” laws. These laws vary by state but have 3 steps in common: Educate coaches, players, and athletes; remove athletes from play; and obtain health care professionals’ permission to return to play.14 These guidelines set up an action plan for treating an athlete who has sustained a concussion.
Encouraging results of educating coaches have been noted. Coaches who were given Centers for Disease Control and Prevention–sponsored material on preventing, recognizing, and responding to concussions were able to effectively address concussions; 6 months later, 63% were better able to appreciate the severity of concussions.15 Continued education of athletic communities should help bring this injury to the attention of those treating female athletes.
3. Exercise safety in pregnancy
Women in sports can continue their athletic regimens during pregnancy. It is important to address challenges to the pregnant woman and to the fetus when assessing the risks of exercise.
The physiologic changes that occur during pregnancy may affect how a pregnant athlete responds to stress. Plasma volume, red blood cell volume, and cardiac function and output all increase during normal pregnancy.3,16 Abnormal heart rate during pregnancy can adversely affect the fetus. During and after exercise, fetal bradycardia can occur. Therefore, recommendations should include not exceeding pre-pregnancy activity levels.3 Careful monitoring of exercise intensity is recommended by the American College of Obstetrics and Gynecology; the guideline is to maintain less than 70% of maximal heart rate.17,18
The negative effects of exercise on the pregnant athlete are limited, but it is important to educate patients and to consider preventive strategies. One physiologic change that occurs during pregnancy is ligamentous laxity, which is caused by the hormone relaxin.16 Ligamentous laxity has the potential to put pregnant athletes at risk for soft-tissue and bony injury during impact sports. However, the positive effects of exercise during pregnancy include improved appetite, sleep, and emotional health.19 Aerobic exercise during pregnancy may reverse insulin resistance as demonstrated in animal studies; though this outcome has not been demonstrated in human studies,20 women should be reassured that moderate exercise has overall beneficial effects.
Some research suggests that exercise may expose the fetus to hyperthermia, blood sugar changes, physical injury, and premature labor.16 Typically, fetal heat is dissipated from the mother. After intense exercise, maternal body temperature rises and leads to some degree of fetal hyperthermia.16 Animal model studies have suggested that hyperthermia may result in a slightly higher rate of congenital abnormalities. Pregnant women should keep their exercise routines to less than 60 minutes, should exercise in a thermally regulated environment, and should keep themselves hydrated to avoid fetal hyperthermia.18
Reduced blood flow, accompanied by a deficit of oxygen to the uterus and the developing fetus, is another concern for pregnant athletes. During exercise, when more blood is flowing to the muscles, less is flowing to the uterus.16 Furthermore, during the third trimester, women should avoid supine exercise, as venous outflow is poor with the body in that position.21
Elite athletes who continue training during pregnancy should be carefully counseled about adjusting their training regimens. Because of increased cardiac output and blood volume, the heart rate will be lower than usual, demanding an adjustment in interpretation. Blood cell counts do not increase as much as plasma volume does—often leading to relative anemia. For elite athletes, this means iron supplementation is crucial.22 Thermal regulation may be more difficult, as training regimens may demand prolonged exercise. Physicians should recommend adequate hydration for these athletes.18
Although continued exercise is generally safe for a pregnant athlete and her fetus, caution is required when there is increased risk for premature delivery, or other special conditions exist. Multiple gestation, placenta previa, history of early labor or premature births, and incompetent cervix all contraindicate aerobic exercise during pregnancy.18 With these exceptions in mind, physicians can safely counsel pregnant women to do moderate exercise 30 minutes every day.17,18 Other recommendations are listed at the American College of Obstetricians and Gynecologists website.23
4. Anterior cruciate ligament injuries
ACL injuries affect a staggering number of athletes. In the United States, approximately 100,000 people sustain these injuries annually.24 As they occur up to 8 times more often in women than in men, ACL injuries are a top concern for physicians treating female athletes.
This disproportionate injury rate is influenced by differences between male and female anatomy. The width and shape of the femoral intercondylar notch have been studied as potential variables influencing the risk for ACL injury. Analysis of notch-view radiographs revealed a significant inverse relationship between notch width and ACL injury.25 A-shaped notches, notches with a significantly larger base and a narrowed roof, were more prevalent in women but did not correlate with increased risk for ACL injury. Studies have shown that female athletes with a noncontact ACL injury have a higher lateral tibial plateau posterior slope; this slope is associated with increased peak anteromedial ACL strain, which may contribute to injury.26 An analysis of magnetic resonance imaging scans in patients with and without ACL injury revealed that, for female patients, decreased femoral intercondylar notch width at the anterior outlet combined with increased lateral compartment posterior slope correlated best with risk for ACL injury.27
Although static anatomical factors contribute to ACL injuries in female athletes, dynamic neuromuscular influences are potential opportunities for intervention. Female athletes with high relative quadriceps strength and weak hamstring strength may be at increased risk for ACL injury.28 This “quadriceps dominance” becomes important in sports involving high-risk activities, such as running, cutting, pivoting, and jumping. In addition, compared with male athletes, female athletes demonstrate increased lateral trunk motion and knee valgus torque while landing during noncontact ACL tears, making core stability a factor in ACL injury.29
The collaborative efforts of physicians, physical therapists, athletic trainers, and coaches have yielded multifactorial neuromuscular training programs for the prevention of noncontact ACL injuries. Ideal ACL prevention protocols involve sessions that last for at least 10 minutes and take place 3 times a week. At these sessions, exercises are focused on strengthening, balance, and proprioceptive training.30 The programs last about 8 weeks, but sustained benefits require maintenance after the program has been completed and during the off-season. Program adherence must be encouraged and can be facilitated by varying workouts and raising risk awareness. The most effective programs have reduced the relative risk of noncontact ACL injuries by 75% to 100%.31 These promising results have led to increased focus on program implementation in an effort to prevent ACL injury.
5. Continued sex discrimination and social injustice
In 1972, Title IX was passed as part of the Education Amendments Act. Title IX states, “No person in the United States shall, on the basis of sex, be excluded from participation in, be denied the benefits of, or be subjected to discrimination under any educational program or activity receiving Federal financial assistance.” Passage of this law, which has implications outside of athletic participation, marked an important turning point in women’s ability to participate equally in college sports.32,33 The Civil Rights Restoration Act, passed in 1988, strengthened Title IX and made it applicable to all institutions receiving federal funding.34 Before the 1970s, women typically were restricted to club sports, and funding and participation opportunities were weighted heavily toward men. Over the past 40 years, women’s participation in high school, college, and professional sports has taken a huge leap forward.32 For example, the number of women participating in high school sports increased from 294,000 (7.4% of all athletes) in 1972 to 3.4 million (>41% of all athletes) in 2014.
Despite advances in women’s civil rights, examples of inequality in US schools remain, particularly in the distribution of funding, which still strongly favors men’s football.32 Men’s sports receive 90% of media coverage.33 In 2002, women represented 55% of college students but only 42% of varsity athletes.34 The schools that have complied the least with Title IX are schools in the Midwest and the South and those with football teams.34 Women are underrepresented as coaches, and funding continues to be disproportionately spent on men’s sports.
For women, the benefits of participating in sports are far-reaching and significant. These benefits include improvements in academic success, mental health, and responsible behavior.33 Women’s gaining acceptance and respect throughout the athletic world seems to have carried over elsewhere. Although many institutions remain noncompliant with Title IX, efforts continue to have a strongly positive effect on gender equality in the United States.
1. Nattiv A, Loucks AB, Manore MM, Sanborn CF, Sundgot-Borgen J, Warren MP; American College of Sports Medicine. American College of Sports Medicine position stand. The female athlete triad. Med Sci Sports Exerc. 2007;39(10):1867-1882.
2. De Souza MJ, Nattiv A, Joy E, et al; Expert Panel. 2014 Female Athlete Triad Coalition consensus statement on treatment and return to play of the female athlete triad: 1st international conference held in San Francisco, California, May 2012 and 2nd international conference held in Indianapolis, Indiana, May 2013. Br J Sports Med. 2014;48(4):289.
3. Warren MP, Shantha S. The female athlete. Baillieres Best Pract Res Clin Endocrinol Metab. 2000;14(1):37-53.
4. Greenleaf C, Petrie TA, Carter J, Reel JJ. Female collegiate athletes: prevalence of eating disorders and disordered eating behaviors. J Am Coll Health. 2009;57(5):489-495.
5. Reinking MF, Alexander LE. Prevalence of disordered-eating behaviors in undergraduate female collegiate athletes and nonathletes. J Athl Train. 2005;40(1):47-51.
6. Rencken ML, Chesnut CH 3rd, Drinkwater BL. Bone density at multiple skeletal sites in amenorrheic athletes. JAMA. 1996;276(3):238-240.
7. Lappe J, Cullen D, Haynatzki G, Recker R, Ahlf R, Thompson K. Calcium and vitamin D supplementation decreases incidence of stress fractures in female Navy recruits. J Bone Miner Res. 2008;23(5):741-749.
8. De Souza MJ. 2014 Female athlete triad consensus statement on guidelines for treatment and return to play. National Collegiate Athletic Association (NCAA) website. http://www.ncaa.org/health-and-safety/nutrition-and-performance/2014-female-athlete-triad-consensus-statement-guidelines. Accessed November 24, 2015.
9. Preiss-Farzanegan SJ, Chapman B, Wong TM, Wu J, Bazarian JJ. The relationship between gender and postconcussion symptoms after sport-related mild traumatic brain injury. PM R. 2009;1(3):245-253.
10. Marar M, McIlvain NM, Fields SK, Comstock RD. Epidemiology of concussions among United States high school athletes in 20 sports. Am J Sports Med. 2012;40(4):747-755.
11. Uhl RL, Rosenbaum AJ, Czajka C, Mulligan M, King C. Minor traumatic brain injury: a primer for the orthopaedic surgeon. J Am Acad Orthop Surg. 2013;21(10):624-631.
12. Covassin T, Elbin RJ, Harris W, Parker T, Kontos A. The role of age and sex in symptoms, neurocognitive performance, and postural stability in athletes after concussion. Am J Sports Med. 2012;40(6):1303-1312.
13. Covassin T, Moran R, Wilhelm K. Concussion symptoms and neurocognitive performance of high school and college athletes who incur multiple concussions. Am J Sports Med. 2013;41(12):2885-2889.
14. Sports concussion policies and laws: information for parents, coaches, and school & sports professionals. Centers for Disease Control and Prevention website. http://www.cdc.gov/headsup/policy/index.html. Updated February 16, 2015. Accessed November 24, 2015.
15. Covassin T, Elbin RJ, Sarmiento K. Educating coaches about concussion in sports: evaluation of the CDC’s “Heads Up: concussion in youth sports” initiative. J Sch Health. 2012;82(5):233-238.
16. Lumbers ER. Exercise in pregnancy: physiological basis of exercise prescription for the pregnant woman. J Sci Med Sport. 2002;5(1):20-31.
17. ACOG Committee Obstetric Practice. ACOG Committee opinion. Number 267, January 2002: exercise during pregnancy and the postpartum period. Obstet Gynecol. 2002;99(1):171-173.
18. Artal R, O’Toole M. Guidelines of the American College of Obstetricians and Gynecologists for exercise during pregnancy and the postpartum period. Br J Sports Med. 2003;37(1):6-12.
19. Kramer MS. Regular aerobic exercise during pregnancy. Cochrane Database Syst Rev. 2000;(2):CD000180. Update in: Cochrane Database Syst Rev. 2002;(2):CD000180.
20. Stafne SN, Salvesen KA, Romundstad PR, Stuge B, Morkved S. Does regular exercise during pregnancy influence lumbopelvic pain? A randomized controlled trial. Acta Obstet Gynecol Scand. 2012;91(5):552-559.
21. Nascimento SL, Surita FG, Cecatti JG. Physical exercise during pregnancy: a systematic review. Curr Opin Obstet Gynecol. 2012;24(6):387-394.
22. Hale RW, Milne L. The elite athlete and exercise in pregnancy. Semin Perinatol. 1996;20(4):277-284.
23. Exercise during pregnancy. American College of Obstetricians and Gynecologists website. http://www.acog.org/Patients/FAQs/Exercise-During-Pregnancy. Published August 2011. Accessed November 24, 2015.
24. Giugliano DN, Solomon JL. ACL tears in female athletes. Phys Med Rehabil Clin North Am. 2007;18(3):417-438, viii.
25. Ireland ML, Ballantyne BT, Little K, McClay IS. A radiographic analysis of the relationship between the size and shape of the intercondylar notch and anterior cruciate ligament injury. Knee Surg Sports Traumatol Arthrosc. 2001;9(4):200-205.
26. Lipps DB, Oh YK, Ashton-Miller JA, Wojtys EM. Morphologic characteristics help explain the gender difference in peak anterior cruciate ligament strain during a simulated pivot landing. Am J Sports Med. 2012;40(1):32-40.
27. Sturnick DR, Vacek PM, DeSarno MJ, et al. Combined anatomic factors predicting risk of anterior cruciate ligament injury for males and females. Am J Sports Med. 2015;43(4):839-847.
28. Myer GD, Ford KR, Barber Foss KD, Liu C, Nick TG, Hewett TE. The relationship of hamstrings and quadriceps strength to anterior cruciate ligament injury in female athletes. Clin J Sport Med. 2009;19(1):3-8.
29. Hewett TE, Torg JS, Boden BP. Video analysis of trunk and knee motion during non-contact anterior cruciate ligament injury in female athletes: lateral trunk and knee abduction motion are combined components of the injury mechanism. Br J Sports Med. 2009;43(6):417-422.
30. Sutton KM, Bullock JM. Anterior cruciate ligament rupture: differences between males and females. J Am Acad Orthop Surg. 2013;21(1):41-50.
31. Noyes FR, Barber-Westin SD. Neuromuscular retraining intervention programs: do they reduce noncontact anterior cruciate ligament injury rates in adolescent female athletes? Arthroscopy. 2014;30(2):245-255.
32. Ladd AL. The sports bra, the ACL, and Title IX—the game in play. Clin Orthop Relat Res. 2014;472(6):1681-1684.
33. Lopiano DA. Modern history of women in sports. Twenty-five years of Title IX. Clin Sports Med. 2000;19(2):163-173, vii.
34. Anderson DJ, Cheslock JJ, Ehrenberg RG. Gender equity in intercollegiate athletics: determinants of Title IX compliance. J High Educ. 2006;77(2):225-250.
Since Title IX passed in 1972, women have become exponentially more involved in competitive sports, from high school to professional levels. With more women engaging in serious athletics, the specific challenges they face have come to the forefront of sports medicine. These problems include the female athlete triad, concussions, exercise safety in pregnancy, anterior cruciate ligament (ACL) injuries, and continued sex discrimination and social injustice. Orthopedists treating female athletes should be aware of these problems, each of which is discussed in this review.
1. Female athlete triad
In 1992, the term female athlete triad was coined to describe 3 problems that often coexist in high-intensity female athletes.1 Since then, the definition has evolved, but the problem has remained essentially the same. The modern definition incorporates menstrual abnormalities, low energy availability with or without disordered eating, and decreased bone mineral density (BMD).2
With intense exercise and weight loss comes a variety of menstrual disturbances.3 In affected athletes, the hypothalamus is underactivated, and changes in gonadotropin-releasing hormone and luteinizing hormone lead to decreased estrogen production. Research suggests abnormal menses result from having inadequate energy and insufficient caloric intake to support extensive exercise.1 This phenomenon can occur in athletes in any sport but is most prevalent in lean-body sports, such as swimming, gymnastics, and ballet. The incidence of abnormal menses is as high as 79% in ballet dancers but only 5% in the general population.3 Menstrual abnormalities indicate hormonal abnormalities that can interfere with growth and maturation in young athletes.
Although full-blown eating disorders are uncommon among female athletes, disordered eating patterns are often found among women in competitive sports. Disordered eating can involve a spectrum of inadequate caloric intake and purging behavior, such as vomiting or laxative abuse, and has been reported in up to 25% of collegiate female athletes.4 Physicians must recognize these conditions and initiate counseling and treatment when appropriate. Women with disordered eating are at risk for developing electrolyte imbalances, malnutrition syndromes, and osteopenia.
Although careful evaluation and counseling are important, physicians must note that, in most cases, athletics participation may also protect against disordered eating and body image difficulties. A study of 146 college-age women found better body satisfaction among athletes than among nonathletes.5 Lean-sport athletes (eg, swimmers, gymnasts) were at higher risk for disordered eating and body image problems than other athletes were. Similarly, other studies have found that a majority of athletes have healthy eating habits.4
For poorly nourished and hormonally imbalanced female athletes, decreased BMD poses substantial risk. One study found a significant difference in BMD between athletes with amenorrhea and athletes with normal menses.6 In a cohort of female Navy recruits, those with amenorrhea were at 91% higher risk for stress fractures; calcium and vitamin D supplementation reduced risk by 20%.7 Osteopenia may be a special problem for prepubescent athletes. Girls who engage in intense exercise and have delayed menarche may have a low estrogen state, predisposing them to low BMD.3 Osteopenia and osteoporosis are difficult to reverse and can put these athletes at risk for stress fractures the rest of their lives. If unrecognized, stress fractures can end an athlete’s career.
Recommendations for dual-energy X-ray absorptiometry (DXA) include testing female athletes who have a diagnosed eating disorder, body mass index under 17.5, history of delayed menarche, oligomenorrhea, 2 prior stress fractures, or prior abnormal DXA scan. Complete testing recommendations appear in the 2014 consensus statement on the female athlete triad and return to sport.2,8
Orthopedists performing physical examinations for sports participation can screen for the female athlete triad through thoughtful questioning about menstrual history, nutrition habits, and stress fracture symptoms. Best treatment for a diagnosed case of the triad is multidisciplinary care with strong social support. When abnormal menses are an issue, referral to a gynecologist or endocrinologist and consideration of estrogen replacement should be discussed. Some cases require a psychiatrist’s assistance in treating disordered eating. Athletic trainers, coaches, and parents should be involved over the treatment course.1 Orthopedists must counsel women with osteopenia and osteoporosis about decreasing exercise to a safe level, improving nutritional intake, and supplementing with calcium (1200-1500 mg/d) and vitamin D (600-800 IU/d).3,7
2. Concussions
Increasing awareness of males’ sport-related concussions, particularly of concussions that occur during National Football League practice and games, has made physicians and researchers more aware of the rate of concussion in female athletes. That rate has increased, and, according to some reports, the risk for sport-related injury is higher for female athletes.9 A study of high school athletes found that the rate of concussion in girl’s soccer was second only to that in football.10
Concussions are categorized as mild traumatic brain injuries, and manifestations of the diagnosis are divided into physical, emotional, cognitive, and observed symptoms. The spectrum of symptoms is wide, ranging from difficulty concentrating and thinking clearly to headaches and dizziness.11 Compared with male athletes who sustain a concussion, female athletes report more of these concussive symptoms and have worse visual memory scores.12
Efforts to change sports at the player level have been resisted. Helmets have been proposed for field hockey and lacrosse but have not passed stringent concussion testing. In soccer, which has a high rate of concussion, a reform to eliminate heading the ball has been considered. Resistance to these suggestions stems from the thought that changes could alter the traditions of the games. Some individuals have indicated that helmets may give players a false sense of security and thereby cause them to play more aggressively.
Orthopedic surgeons must be aware of concussion symptoms. Multiple concussions may have a cumulative effect on functional ability and emotional well-being and may lead to chronic traumatic encephalopathy.13 Concern about the long-term effects of concussion has led to the implementation of universal “return to play” laws. These laws vary by state but have 3 steps in common: Educate coaches, players, and athletes; remove athletes from play; and obtain health care professionals’ permission to return to play.14 These guidelines set up an action plan for treating an athlete who has sustained a concussion.
Encouraging results of educating coaches have been noted. Coaches who were given Centers for Disease Control and Prevention–sponsored material on preventing, recognizing, and responding to concussions were able to effectively address concussions; 6 months later, 63% were better able to appreciate the severity of concussions.15 Continued education of athletic communities should help bring this injury to the attention of those treating female athletes.
3. Exercise safety in pregnancy
Women in sports can continue their athletic regimens during pregnancy. It is important to address challenges to the pregnant woman and to the fetus when assessing the risks of exercise.
The physiologic changes that occur during pregnancy may affect how a pregnant athlete responds to stress. Plasma volume, red blood cell volume, and cardiac function and output all increase during normal pregnancy.3,16 Abnormal heart rate during pregnancy can adversely affect the fetus. During and after exercise, fetal bradycardia can occur. Therefore, recommendations should include not exceeding pre-pregnancy activity levels.3 Careful monitoring of exercise intensity is recommended by the American College of Obstetrics and Gynecology; the guideline is to maintain less than 70% of maximal heart rate.17,18
The negative effects of exercise on the pregnant athlete are limited, but it is important to educate patients and to consider preventive strategies. One physiologic change that occurs during pregnancy is ligamentous laxity, which is caused by the hormone relaxin.16 Ligamentous laxity has the potential to put pregnant athletes at risk for soft-tissue and bony injury during impact sports. However, the positive effects of exercise during pregnancy include improved appetite, sleep, and emotional health.19 Aerobic exercise during pregnancy may reverse insulin resistance as demonstrated in animal studies; though this outcome has not been demonstrated in human studies,20 women should be reassured that moderate exercise has overall beneficial effects.
Some research suggests that exercise may expose the fetus to hyperthermia, blood sugar changes, physical injury, and premature labor.16 Typically, fetal heat is dissipated from the mother. After intense exercise, maternal body temperature rises and leads to some degree of fetal hyperthermia.16 Animal model studies have suggested that hyperthermia may result in a slightly higher rate of congenital abnormalities. Pregnant women should keep their exercise routines to less than 60 minutes, should exercise in a thermally regulated environment, and should keep themselves hydrated to avoid fetal hyperthermia.18
Reduced blood flow, accompanied by a deficit of oxygen to the uterus and the developing fetus, is another concern for pregnant athletes. During exercise, when more blood is flowing to the muscles, less is flowing to the uterus.16 Furthermore, during the third trimester, women should avoid supine exercise, as venous outflow is poor with the body in that position.21
Elite athletes who continue training during pregnancy should be carefully counseled about adjusting their training regimens. Because of increased cardiac output and blood volume, the heart rate will be lower than usual, demanding an adjustment in interpretation. Blood cell counts do not increase as much as plasma volume does—often leading to relative anemia. For elite athletes, this means iron supplementation is crucial.22 Thermal regulation may be more difficult, as training regimens may demand prolonged exercise. Physicians should recommend adequate hydration for these athletes.18
Although continued exercise is generally safe for a pregnant athlete and her fetus, caution is required when there is increased risk for premature delivery, or other special conditions exist. Multiple gestation, placenta previa, history of early labor or premature births, and incompetent cervix all contraindicate aerobic exercise during pregnancy.18 With these exceptions in mind, physicians can safely counsel pregnant women to do moderate exercise 30 minutes every day.17,18 Other recommendations are listed at the American College of Obstetricians and Gynecologists website.23
4. Anterior cruciate ligament injuries
ACL injuries affect a staggering number of athletes. In the United States, approximately 100,000 people sustain these injuries annually.24 As they occur up to 8 times more often in women than in men, ACL injuries are a top concern for physicians treating female athletes.
This disproportionate injury rate is influenced by differences between male and female anatomy. The width and shape of the femoral intercondylar notch have been studied as potential variables influencing the risk for ACL injury. Analysis of notch-view radiographs revealed a significant inverse relationship between notch width and ACL injury.25 A-shaped notches, notches with a significantly larger base and a narrowed roof, were more prevalent in women but did not correlate with increased risk for ACL injury. Studies have shown that female athletes with a noncontact ACL injury have a higher lateral tibial plateau posterior slope; this slope is associated with increased peak anteromedial ACL strain, which may contribute to injury.26 An analysis of magnetic resonance imaging scans in patients with and without ACL injury revealed that, for female patients, decreased femoral intercondylar notch width at the anterior outlet combined with increased lateral compartment posterior slope correlated best with risk for ACL injury.27
Although static anatomical factors contribute to ACL injuries in female athletes, dynamic neuromuscular influences are potential opportunities for intervention. Female athletes with high relative quadriceps strength and weak hamstring strength may be at increased risk for ACL injury.28 This “quadriceps dominance” becomes important in sports involving high-risk activities, such as running, cutting, pivoting, and jumping. In addition, compared with male athletes, female athletes demonstrate increased lateral trunk motion and knee valgus torque while landing during noncontact ACL tears, making core stability a factor in ACL injury.29
The collaborative efforts of physicians, physical therapists, athletic trainers, and coaches have yielded multifactorial neuromuscular training programs for the prevention of noncontact ACL injuries. Ideal ACL prevention protocols involve sessions that last for at least 10 minutes and take place 3 times a week. At these sessions, exercises are focused on strengthening, balance, and proprioceptive training.30 The programs last about 8 weeks, but sustained benefits require maintenance after the program has been completed and during the off-season. Program adherence must be encouraged and can be facilitated by varying workouts and raising risk awareness. The most effective programs have reduced the relative risk of noncontact ACL injuries by 75% to 100%.31 These promising results have led to increased focus on program implementation in an effort to prevent ACL injury.
5. Continued sex discrimination and social injustice
In 1972, Title IX was passed as part of the Education Amendments Act. Title IX states, “No person in the United States shall, on the basis of sex, be excluded from participation in, be denied the benefits of, or be subjected to discrimination under any educational program or activity receiving Federal financial assistance.” Passage of this law, which has implications outside of athletic participation, marked an important turning point in women’s ability to participate equally in college sports.32,33 The Civil Rights Restoration Act, passed in 1988, strengthened Title IX and made it applicable to all institutions receiving federal funding.34 Before the 1970s, women typically were restricted to club sports, and funding and participation opportunities were weighted heavily toward men. Over the past 40 years, women’s participation in high school, college, and professional sports has taken a huge leap forward.32 For example, the number of women participating in high school sports increased from 294,000 (7.4% of all athletes) in 1972 to 3.4 million (>41% of all athletes) in 2014.
Despite advances in women’s civil rights, examples of inequality in US schools remain, particularly in the distribution of funding, which still strongly favors men’s football.32 Men’s sports receive 90% of media coverage.33 In 2002, women represented 55% of college students but only 42% of varsity athletes.34 The schools that have complied the least with Title IX are schools in the Midwest and the South and those with football teams.34 Women are underrepresented as coaches, and funding continues to be disproportionately spent on men’s sports.
For women, the benefits of participating in sports are far-reaching and significant. These benefits include improvements in academic success, mental health, and responsible behavior.33 Women’s gaining acceptance and respect throughout the athletic world seems to have carried over elsewhere. Although many institutions remain noncompliant with Title IX, efforts continue to have a strongly positive effect on gender equality in the United States.
Since Title IX passed in 1972, women have become exponentially more involved in competitive sports, from high school to professional levels. With more women engaging in serious athletics, the specific challenges they face have come to the forefront of sports medicine. These problems include the female athlete triad, concussions, exercise safety in pregnancy, anterior cruciate ligament (ACL) injuries, and continued sex discrimination and social injustice. Orthopedists treating female athletes should be aware of these problems, each of which is discussed in this review.
1. Female athlete triad
In 1992, the term female athlete triad was coined to describe 3 problems that often coexist in high-intensity female athletes.1 Since then, the definition has evolved, but the problem has remained essentially the same. The modern definition incorporates menstrual abnormalities, low energy availability with or without disordered eating, and decreased bone mineral density (BMD).2
With intense exercise and weight loss comes a variety of menstrual disturbances.3 In affected athletes, the hypothalamus is underactivated, and changes in gonadotropin-releasing hormone and luteinizing hormone lead to decreased estrogen production. Research suggests abnormal menses result from having inadequate energy and insufficient caloric intake to support extensive exercise.1 This phenomenon can occur in athletes in any sport but is most prevalent in lean-body sports, such as swimming, gymnastics, and ballet. The incidence of abnormal menses is as high as 79% in ballet dancers but only 5% in the general population.3 Menstrual abnormalities indicate hormonal abnormalities that can interfere with growth and maturation in young athletes.
Although full-blown eating disorders are uncommon among female athletes, disordered eating patterns are often found among women in competitive sports. Disordered eating can involve a spectrum of inadequate caloric intake and purging behavior, such as vomiting or laxative abuse, and has been reported in up to 25% of collegiate female athletes.4 Physicians must recognize these conditions and initiate counseling and treatment when appropriate. Women with disordered eating are at risk for developing electrolyte imbalances, malnutrition syndromes, and osteopenia.
Although careful evaluation and counseling are important, physicians must note that, in most cases, athletics participation may also protect against disordered eating and body image difficulties. A study of 146 college-age women found better body satisfaction among athletes than among nonathletes.5 Lean-sport athletes (eg, swimmers, gymnasts) were at higher risk for disordered eating and body image problems than other athletes were. Similarly, other studies have found that a majority of athletes have healthy eating habits.4
For poorly nourished and hormonally imbalanced female athletes, decreased BMD poses substantial risk. One study found a significant difference in BMD between athletes with amenorrhea and athletes with normal menses.6 In a cohort of female Navy recruits, those with amenorrhea were at 91% higher risk for stress fractures; calcium and vitamin D supplementation reduced risk by 20%.7 Osteopenia may be a special problem for prepubescent athletes. Girls who engage in intense exercise and have delayed menarche may have a low estrogen state, predisposing them to low BMD.3 Osteopenia and osteoporosis are difficult to reverse and can put these athletes at risk for stress fractures the rest of their lives. If unrecognized, stress fractures can end an athlete’s career.
Recommendations for dual-energy X-ray absorptiometry (DXA) include testing female athletes who have a diagnosed eating disorder, body mass index under 17.5, history of delayed menarche, oligomenorrhea, 2 prior stress fractures, or prior abnormal DXA scan. Complete testing recommendations appear in the 2014 consensus statement on the female athlete triad and return to sport.2,8
Orthopedists performing physical examinations for sports participation can screen for the female athlete triad through thoughtful questioning about menstrual history, nutrition habits, and stress fracture symptoms. Best treatment for a diagnosed case of the triad is multidisciplinary care with strong social support. When abnormal menses are an issue, referral to a gynecologist or endocrinologist and consideration of estrogen replacement should be discussed. Some cases require a psychiatrist’s assistance in treating disordered eating. Athletic trainers, coaches, and parents should be involved over the treatment course.1 Orthopedists must counsel women with osteopenia and osteoporosis about decreasing exercise to a safe level, improving nutritional intake, and supplementing with calcium (1200-1500 mg/d) and vitamin D (600-800 IU/d).3,7
2. Concussions
Increasing awareness of males’ sport-related concussions, particularly of concussions that occur during National Football League practice and games, has made physicians and researchers more aware of the rate of concussion in female athletes. That rate has increased, and, according to some reports, the risk for sport-related injury is higher for female athletes.9 A study of high school athletes found that the rate of concussion in girl’s soccer was second only to that in football.10
Concussions are categorized as mild traumatic brain injuries, and manifestations of the diagnosis are divided into physical, emotional, cognitive, and observed symptoms. The spectrum of symptoms is wide, ranging from difficulty concentrating and thinking clearly to headaches and dizziness.11 Compared with male athletes who sustain a concussion, female athletes report more of these concussive symptoms and have worse visual memory scores.12
Efforts to change sports at the player level have been resisted. Helmets have been proposed for field hockey and lacrosse but have not passed stringent concussion testing. In soccer, which has a high rate of concussion, a reform to eliminate heading the ball has been considered. Resistance to these suggestions stems from the thought that changes could alter the traditions of the games. Some individuals have indicated that helmets may give players a false sense of security and thereby cause them to play more aggressively.
Orthopedic surgeons must be aware of concussion symptoms. Multiple concussions may have a cumulative effect on functional ability and emotional well-being and may lead to chronic traumatic encephalopathy.13 Concern about the long-term effects of concussion has led to the implementation of universal “return to play” laws. These laws vary by state but have 3 steps in common: Educate coaches, players, and athletes; remove athletes from play; and obtain health care professionals’ permission to return to play.14 These guidelines set up an action plan for treating an athlete who has sustained a concussion.
Encouraging results of educating coaches have been noted. Coaches who were given Centers for Disease Control and Prevention–sponsored material on preventing, recognizing, and responding to concussions were able to effectively address concussions; 6 months later, 63% were better able to appreciate the severity of concussions.15 Continued education of athletic communities should help bring this injury to the attention of those treating female athletes.
3. Exercise safety in pregnancy
Women in sports can continue their athletic regimens during pregnancy. It is important to address challenges to the pregnant woman and to the fetus when assessing the risks of exercise.
The physiologic changes that occur during pregnancy may affect how a pregnant athlete responds to stress. Plasma volume, red blood cell volume, and cardiac function and output all increase during normal pregnancy.3,16 Abnormal heart rate during pregnancy can adversely affect the fetus. During and after exercise, fetal bradycardia can occur. Therefore, recommendations should include not exceeding pre-pregnancy activity levels.3 Careful monitoring of exercise intensity is recommended by the American College of Obstetrics and Gynecology; the guideline is to maintain less than 70% of maximal heart rate.17,18
The negative effects of exercise on the pregnant athlete are limited, but it is important to educate patients and to consider preventive strategies. One physiologic change that occurs during pregnancy is ligamentous laxity, which is caused by the hormone relaxin.16 Ligamentous laxity has the potential to put pregnant athletes at risk for soft-tissue and bony injury during impact sports. However, the positive effects of exercise during pregnancy include improved appetite, sleep, and emotional health.19 Aerobic exercise during pregnancy may reverse insulin resistance as demonstrated in animal studies; though this outcome has not been demonstrated in human studies,20 women should be reassured that moderate exercise has overall beneficial effects.
Some research suggests that exercise may expose the fetus to hyperthermia, blood sugar changes, physical injury, and premature labor.16 Typically, fetal heat is dissipated from the mother. After intense exercise, maternal body temperature rises and leads to some degree of fetal hyperthermia.16 Animal model studies have suggested that hyperthermia may result in a slightly higher rate of congenital abnormalities. Pregnant women should keep their exercise routines to less than 60 minutes, should exercise in a thermally regulated environment, and should keep themselves hydrated to avoid fetal hyperthermia.18
Reduced blood flow, accompanied by a deficit of oxygen to the uterus and the developing fetus, is another concern for pregnant athletes. During exercise, when more blood is flowing to the muscles, less is flowing to the uterus.16 Furthermore, during the third trimester, women should avoid supine exercise, as venous outflow is poor with the body in that position.21
Elite athletes who continue training during pregnancy should be carefully counseled about adjusting their training regimens. Because of increased cardiac output and blood volume, the heart rate will be lower than usual, demanding an adjustment in interpretation. Blood cell counts do not increase as much as plasma volume does—often leading to relative anemia. For elite athletes, this means iron supplementation is crucial.22 Thermal regulation may be more difficult, as training regimens may demand prolonged exercise. Physicians should recommend adequate hydration for these athletes.18
Although continued exercise is generally safe for a pregnant athlete and her fetus, caution is required when there is increased risk for premature delivery, or other special conditions exist. Multiple gestation, placenta previa, history of early labor or premature births, and incompetent cervix all contraindicate aerobic exercise during pregnancy.18 With these exceptions in mind, physicians can safely counsel pregnant women to do moderate exercise 30 minutes every day.17,18 Other recommendations are listed at the American College of Obstetricians and Gynecologists website.23
4. Anterior cruciate ligament injuries
ACL injuries affect a staggering number of athletes. In the United States, approximately 100,000 people sustain these injuries annually.24 As they occur up to 8 times more often in women than in men, ACL injuries are a top concern for physicians treating female athletes.
This disproportionate injury rate is influenced by differences between male and female anatomy. The width and shape of the femoral intercondylar notch have been studied as potential variables influencing the risk for ACL injury. Analysis of notch-view radiographs revealed a significant inverse relationship between notch width and ACL injury.25 A-shaped notches, notches with a significantly larger base and a narrowed roof, were more prevalent in women but did not correlate with increased risk for ACL injury. Studies have shown that female athletes with a noncontact ACL injury have a higher lateral tibial plateau posterior slope; this slope is associated with increased peak anteromedial ACL strain, which may contribute to injury.26 An analysis of magnetic resonance imaging scans in patients with and without ACL injury revealed that, for female patients, decreased femoral intercondylar notch width at the anterior outlet combined with increased lateral compartment posterior slope correlated best with risk for ACL injury.27
Although static anatomical factors contribute to ACL injuries in female athletes, dynamic neuromuscular influences are potential opportunities for intervention. Female athletes with high relative quadriceps strength and weak hamstring strength may be at increased risk for ACL injury.28 This “quadriceps dominance” becomes important in sports involving high-risk activities, such as running, cutting, pivoting, and jumping. In addition, compared with male athletes, female athletes demonstrate increased lateral trunk motion and knee valgus torque while landing during noncontact ACL tears, making core stability a factor in ACL injury.29
The collaborative efforts of physicians, physical therapists, athletic trainers, and coaches have yielded multifactorial neuromuscular training programs for the prevention of noncontact ACL injuries. Ideal ACL prevention protocols involve sessions that last for at least 10 minutes and take place 3 times a week. At these sessions, exercises are focused on strengthening, balance, and proprioceptive training.30 The programs last about 8 weeks, but sustained benefits require maintenance after the program has been completed and during the off-season. Program adherence must be encouraged and can be facilitated by varying workouts and raising risk awareness. The most effective programs have reduced the relative risk of noncontact ACL injuries by 75% to 100%.31 These promising results have led to increased focus on program implementation in an effort to prevent ACL injury.
5. Continued sex discrimination and social injustice
In 1972, Title IX was passed as part of the Education Amendments Act. Title IX states, “No person in the United States shall, on the basis of sex, be excluded from participation in, be denied the benefits of, or be subjected to discrimination under any educational program or activity receiving Federal financial assistance.” Passage of this law, which has implications outside of athletic participation, marked an important turning point in women’s ability to participate equally in college sports.32,33 The Civil Rights Restoration Act, passed in 1988, strengthened Title IX and made it applicable to all institutions receiving federal funding.34 Before the 1970s, women typically were restricted to club sports, and funding and participation opportunities were weighted heavily toward men. Over the past 40 years, women’s participation in high school, college, and professional sports has taken a huge leap forward.32 For example, the number of women participating in high school sports increased from 294,000 (7.4% of all athletes) in 1972 to 3.4 million (>41% of all athletes) in 2014.
Despite advances in women’s civil rights, examples of inequality in US schools remain, particularly in the distribution of funding, which still strongly favors men’s football.32 Men’s sports receive 90% of media coverage.33 In 2002, women represented 55% of college students but only 42% of varsity athletes.34 The schools that have complied the least with Title IX are schools in the Midwest and the South and those with football teams.34 Women are underrepresented as coaches, and funding continues to be disproportionately spent on men’s sports.
For women, the benefits of participating in sports are far-reaching and significant. These benefits include improvements in academic success, mental health, and responsible behavior.33 Women’s gaining acceptance and respect throughout the athletic world seems to have carried over elsewhere. Although many institutions remain noncompliant with Title IX, efforts continue to have a strongly positive effect on gender equality in the United States.
1. Nattiv A, Loucks AB, Manore MM, Sanborn CF, Sundgot-Borgen J, Warren MP; American College of Sports Medicine. American College of Sports Medicine position stand. The female athlete triad. Med Sci Sports Exerc. 2007;39(10):1867-1882.
2. De Souza MJ, Nattiv A, Joy E, et al; Expert Panel. 2014 Female Athlete Triad Coalition consensus statement on treatment and return to play of the female athlete triad: 1st international conference held in San Francisco, California, May 2012 and 2nd international conference held in Indianapolis, Indiana, May 2013. Br J Sports Med. 2014;48(4):289.
3. Warren MP, Shantha S. The female athlete. Baillieres Best Pract Res Clin Endocrinol Metab. 2000;14(1):37-53.
4. Greenleaf C, Petrie TA, Carter J, Reel JJ. Female collegiate athletes: prevalence of eating disorders and disordered eating behaviors. J Am Coll Health. 2009;57(5):489-495.
5. Reinking MF, Alexander LE. Prevalence of disordered-eating behaviors in undergraduate female collegiate athletes and nonathletes. J Athl Train. 2005;40(1):47-51.
6. Rencken ML, Chesnut CH 3rd, Drinkwater BL. Bone density at multiple skeletal sites in amenorrheic athletes. JAMA. 1996;276(3):238-240.
7. Lappe J, Cullen D, Haynatzki G, Recker R, Ahlf R, Thompson K. Calcium and vitamin D supplementation decreases incidence of stress fractures in female Navy recruits. J Bone Miner Res. 2008;23(5):741-749.
8. De Souza MJ. 2014 Female athlete triad consensus statement on guidelines for treatment and return to play. National Collegiate Athletic Association (NCAA) website. http://www.ncaa.org/health-and-safety/nutrition-and-performance/2014-female-athlete-triad-consensus-statement-guidelines. Accessed November 24, 2015.
9. Preiss-Farzanegan SJ, Chapman B, Wong TM, Wu J, Bazarian JJ. The relationship between gender and postconcussion symptoms after sport-related mild traumatic brain injury. PM R. 2009;1(3):245-253.
10. Marar M, McIlvain NM, Fields SK, Comstock RD. Epidemiology of concussions among United States high school athletes in 20 sports. Am J Sports Med. 2012;40(4):747-755.
11. Uhl RL, Rosenbaum AJ, Czajka C, Mulligan M, King C. Minor traumatic brain injury: a primer for the orthopaedic surgeon. J Am Acad Orthop Surg. 2013;21(10):624-631.
12. Covassin T, Elbin RJ, Harris W, Parker T, Kontos A. The role of age and sex in symptoms, neurocognitive performance, and postural stability in athletes after concussion. Am J Sports Med. 2012;40(6):1303-1312.
13. Covassin T, Moran R, Wilhelm K. Concussion symptoms and neurocognitive performance of high school and college athletes who incur multiple concussions. Am J Sports Med. 2013;41(12):2885-2889.
14. Sports concussion policies and laws: information for parents, coaches, and school & sports professionals. Centers for Disease Control and Prevention website. http://www.cdc.gov/headsup/policy/index.html. Updated February 16, 2015. Accessed November 24, 2015.
15. Covassin T, Elbin RJ, Sarmiento K. Educating coaches about concussion in sports: evaluation of the CDC’s “Heads Up: concussion in youth sports” initiative. J Sch Health. 2012;82(5):233-238.
16. Lumbers ER. Exercise in pregnancy: physiological basis of exercise prescription for the pregnant woman. J Sci Med Sport. 2002;5(1):20-31.
17. ACOG Committee Obstetric Practice. ACOG Committee opinion. Number 267, January 2002: exercise during pregnancy and the postpartum period. Obstet Gynecol. 2002;99(1):171-173.
18. Artal R, O’Toole M. Guidelines of the American College of Obstetricians and Gynecologists for exercise during pregnancy and the postpartum period. Br J Sports Med. 2003;37(1):6-12.
19. Kramer MS. Regular aerobic exercise during pregnancy. Cochrane Database Syst Rev. 2000;(2):CD000180. Update in: Cochrane Database Syst Rev. 2002;(2):CD000180.
20. Stafne SN, Salvesen KA, Romundstad PR, Stuge B, Morkved S. Does regular exercise during pregnancy influence lumbopelvic pain? A randomized controlled trial. Acta Obstet Gynecol Scand. 2012;91(5):552-559.
21. Nascimento SL, Surita FG, Cecatti JG. Physical exercise during pregnancy: a systematic review. Curr Opin Obstet Gynecol. 2012;24(6):387-394.
22. Hale RW, Milne L. The elite athlete and exercise in pregnancy. Semin Perinatol. 1996;20(4):277-284.
23. Exercise during pregnancy. American College of Obstetricians and Gynecologists website. http://www.acog.org/Patients/FAQs/Exercise-During-Pregnancy. Published August 2011. Accessed November 24, 2015.
24. Giugliano DN, Solomon JL. ACL tears in female athletes. Phys Med Rehabil Clin North Am. 2007;18(3):417-438, viii.
25. Ireland ML, Ballantyne BT, Little K, McClay IS. A radiographic analysis of the relationship between the size and shape of the intercondylar notch and anterior cruciate ligament injury. Knee Surg Sports Traumatol Arthrosc. 2001;9(4):200-205.
26. Lipps DB, Oh YK, Ashton-Miller JA, Wojtys EM. Morphologic characteristics help explain the gender difference in peak anterior cruciate ligament strain during a simulated pivot landing. Am J Sports Med. 2012;40(1):32-40.
27. Sturnick DR, Vacek PM, DeSarno MJ, et al. Combined anatomic factors predicting risk of anterior cruciate ligament injury for males and females. Am J Sports Med. 2015;43(4):839-847.
28. Myer GD, Ford KR, Barber Foss KD, Liu C, Nick TG, Hewett TE. The relationship of hamstrings and quadriceps strength to anterior cruciate ligament injury in female athletes. Clin J Sport Med. 2009;19(1):3-8.
29. Hewett TE, Torg JS, Boden BP. Video analysis of trunk and knee motion during non-contact anterior cruciate ligament injury in female athletes: lateral trunk and knee abduction motion are combined components of the injury mechanism. Br J Sports Med. 2009;43(6):417-422.
30. Sutton KM, Bullock JM. Anterior cruciate ligament rupture: differences between males and females. J Am Acad Orthop Surg. 2013;21(1):41-50.
31. Noyes FR, Barber-Westin SD. Neuromuscular retraining intervention programs: do they reduce noncontact anterior cruciate ligament injury rates in adolescent female athletes? Arthroscopy. 2014;30(2):245-255.
32. Ladd AL. The sports bra, the ACL, and Title IX—the game in play. Clin Orthop Relat Res. 2014;472(6):1681-1684.
33. Lopiano DA. Modern history of women in sports. Twenty-five years of Title IX. Clin Sports Med. 2000;19(2):163-173, vii.
34. Anderson DJ, Cheslock JJ, Ehrenberg RG. Gender equity in intercollegiate athletics: determinants of Title IX compliance. J High Educ. 2006;77(2):225-250.
1. Nattiv A, Loucks AB, Manore MM, Sanborn CF, Sundgot-Borgen J, Warren MP; American College of Sports Medicine. American College of Sports Medicine position stand. The female athlete triad. Med Sci Sports Exerc. 2007;39(10):1867-1882.
2. De Souza MJ, Nattiv A, Joy E, et al; Expert Panel. 2014 Female Athlete Triad Coalition consensus statement on treatment and return to play of the female athlete triad: 1st international conference held in San Francisco, California, May 2012 and 2nd international conference held in Indianapolis, Indiana, May 2013. Br J Sports Med. 2014;48(4):289.
3. Warren MP, Shantha S. The female athlete. Baillieres Best Pract Res Clin Endocrinol Metab. 2000;14(1):37-53.
4. Greenleaf C, Petrie TA, Carter J, Reel JJ. Female collegiate athletes: prevalence of eating disorders and disordered eating behaviors. J Am Coll Health. 2009;57(5):489-495.
5. Reinking MF, Alexander LE. Prevalence of disordered-eating behaviors in undergraduate female collegiate athletes and nonathletes. J Athl Train. 2005;40(1):47-51.
6. Rencken ML, Chesnut CH 3rd, Drinkwater BL. Bone density at multiple skeletal sites in amenorrheic athletes. JAMA. 1996;276(3):238-240.
7. Lappe J, Cullen D, Haynatzki G, Recker R, Ahlf R, Thompson K. Calcium and vitamin D supplementation decreases incidence of stress fractures in female Navy recruits. J Bone Miner Res. 2008;23(5):741-749.
8. De Souza MJ. 2014 Female athlete triad consensus statement on guidelines for treatment and return to play. National Collegiate Athletic Association (NCAA) website. http://www.ncaa.org/health-and-safety/nutrition-and-performance/2014-female-athlete-triad-consensus-statement-guidelines. Accessed November 24, 2015.
9. Preiss-Farzanegan SJ, Chapman B, Wong TM, Wu J, Bazarian JJ. The relationship between gender and postconcussion symptoms after sport-related mild traumatic brain injury. PM R. 2009;1(3):245-253.
10. Marar M, McIlvain NM, Fields SK, Comstock RD. Epidemiology of concussions among United States high school athletes in 20 sports. Am J Sports Med. 2012;40(4):747-755.
11. Uhl RL, Rosenbaum AJ, Czajka C, Mulligan M, King C. Minor traumatic brain injury: a primer for the orthopaedic surgeon. J Am Acad Orthop Surg. 2013;21(10):624-631.
12. Covassin T, Elbin RJ, Harris W, Parker T, Kontos A. The role of age and sex in symptoms, neurocognitive performance, and postural stability in athletes after concussion. Am J Sports Med. 2012;40(6):1303-1312.
13. Covassin T, Moran R, Wilhelm K. Concussion symptoms and neurocognitive performance of high school and college athletes who incur multiple concussions. Am J Sports Med. 2013;41(12):2885-2889.
14. Sports concussion policies and laws: information for parents, coaches, and school & sports professionals. Centers for Disease Control and Prevention website. http://www.cdc.gov/headsup/policy/index.html. Updated February 16, 2015. Accessed November 24, 2015.
15. Covassin T, Elbin RJ, Sarmiento K. Educating coaches about concussion in sports: evaluation of the CDC’s “Heads Up: concussion in youth sports” initiative. J Sch Health. 2012;82(5):233-238.
16. Lumbers ER. Exercise in pregnancy: physiological basis of exercise prescription for the pregnant woman. J Sci Med Sport. 2002;5(1):20-31.
17. ACOG Committee Obstetric Practice. ACOG Committee opinion. Number 267, January 2002: exercise during pregnancy and the postpartum period. Obstet Gynecol. 2002;99(1):171-173.
18. Artal R, O’Toole M. Guidelines of the American College of Obstetricians and Gynecologists for exercise during pregnancy and the postpartum period. Br J Sports Med. 2003;37(1):6-12.
19. Kramer MS. Regular aerobic exercise during pregnancy. Cochrane Database Syst Rev. 2000;(2):CD000180. Update in: Cochrane Database Syst Rev. 2002;(2):CD000180.
20. Stafne SN, Salvesen KA, Romundstad PR, Stuge B, Morkved S. Does regular exercise during pregnancy influence lumbopelvic pain? A randomized controlled trial. Acta Obstet Gynecol Scand. 2012;91(5):552-559.
21. Nascimento SL, Surita FG, Cecatti JG. Physical exercise during pregnancy: a systematic review. Curr Opin Obstet Gynecol. 2012;24(6):387-394.
22. Hale RW, Milne L. The elite athlete and exercise in pregnancy. Semin Perinatol. 1996;20(4):277-284.
23. Exercise during pregnancy. American College of Obstetricians and Gynecologists website. http://www.acog.org/Patients/FAQs/Exercise-During-Pregnancy. Published August 2011. Accessed November 24, 2015.
24. Giugliano DN, Solomon JL. ACL tears in female athletes. Phys Med Rehabil Clin North Am. 2007;18(3):417-438, viii.
25. Ireland ML, Ballantyne BT, Little K, McClay IS. A radiographic analysis of the relationship between the size and shape of the intercondylar notch and anterior cruciate ligament injury. Knee Surg Sports Traumatol Arthrosc. 2001;9(4):200-205.
26. Lipps DB, Oh YK, Ashton-Miller JA, Wojtys EM. Morphologic characteristics help explain the gender difference in peak anterior cruciate ligament strain during a simulated pivot landing. Am J Sports Med. 2012;40(1):32-40.
27. Sturnick DR, Vacek PM, DeSarno MJ, et al. Combined anatomic factors predicting risk of anterior cruciate ligament injury for males and females. Am J Sports Med. 2015;43(4):839-847.
28. Myer GD, Ford KR, Barber Foss KD, Liu C, Nick TG, Hewett TE. The relationship of hamstrings and quadriceps strength to anterior cruciate ligament injury in female athletes. Clin J Sport Med. 2009;19(1):3-8.
29. Hewett TE, Torg JS, Boden BP. Video analysis of trunk and knee motion during non-contact anterior cruciate ligament injury in female athletes: lateral trunk and knee abduction motion are combined components of the injury mechanism. Br J Sports Med. 2009;43(6):417-422.
30. Sutton KM, Bullock JM. Anterior cruciate ligament rupture: differences between males and females. J Am Acad Orthop Surg. 2013;21(1):41-50.
31. Noyes FR, Barber-Westin SD. Neuromuscular retraining intervention programs: do they reduce noncontact anterior cruciate ligament injury rates in adolescent female athletes? Arthroscopy. 2014;30(2):245-255.
32. Ladd AL. The sports bra, the ACL, and Title IX—the game in play. Clin Orthop Relat Res. 2014;472(6):1681-1684.
33. Lopiano DA. Modern history of women in sports. Twenty-five years of Title IX. Clin Sports Med. 2000;19(2):163-173, vii.
34. Anderson DJ, Cheslock JJ, Ehrenberg RG. Gender equity in intercollegiate athletics: determinants of Title IX compliance. J High Educ. 2006;77(2):225-250.
Review: Opioid prescriptions are the work of many physicians
A “broad swath” of Medicare providers wrote scripts for opioids in 2013, contradicting the idea that the overdose epidemic is mainly the work of “small groups of prolific prescribers and corrupt pill mills,” investigators wrote online in JAMA Internal Medicine.
“Contrary to the California workers’ compensation data showing a small subset of prescribers accounting for a disproportionately large percentage of opioid prescribing, Medicare opioid prescribing is distributed across many prescribers and is, if anything, less skewed than all drug prescribing,” said Dr. Jonathan H. Chen of the Veterans Affairs Palo Alto (Calif.) Health Care System, and his associates.
Their study included 808,020 prescribers and almost 1.2 billion Medicare Part D claims worth nearly $81 billion dollars. They focused on schedule II opioid prescriptions containing oxycodone, fentanyl, hydrocodone, morphine, methadone, hydromorphone, oxymorphone, meperidine, codeine, opium, or levorphanol (JAMA Intern Med. 2015 Dec 14. doi: 10.1001/jamainternmed.2015.6662).
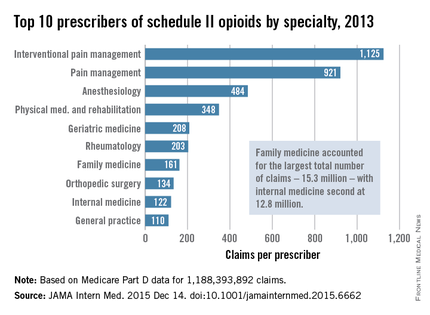
Not surprisingly, specialists in pain management, anesthesia, and physical medicine wrote the most prescriptions per provider. But family practitioners, internists, nurse practitioners, and physician assistants wrote 35,268,234 prescriptions – more than all other specialties combined. “The trends hold up across state lines, with negligible geographic variability,” the researchers said.
The findings contradict an analysis of California workers’ compensation data, in which 1% of prescribers accounted for a third of schedule II opioid prescriptions, and 10% of prescribers accounted for 80% of prescriptions, the investigators noted. Nonetheless, 10% of Medicare prescribers in Dr. Chen’s study accounted for 78% of the total cost of opioids, possibly because they were prescribing pricier formulations or higher doses.
Overall, the findings suggest that opioid prescribing is “widespread” and “relatively indifferent to individual physicians, specialty, or region” – and that efforts to stem the tide must be equally broad, the researchers concluded.
Their study was supported by the VA Office of Academic Affiliations, the VA Health Services Research and Development Service, the National Institute of General Medical Sciences, and the Peter F. McManus Charitable Trust. The researchers had no disclosures.
A “broad swath” of Medicare providers wrote scripts for opioids in 2013, contradicting the idea that the overdose epidemic is mainly the work of “small groups of prolific prescribers and corrupt pill mills,” investigators wrote online in JAMA Internal Medicine.
“Contrary to the California workers’ compensation data showing a small subset of prescribers accounting for a disproportionately large percentage of opioid prescribing, Medicare opioid prescribing is distributed across many prescribers and is, if anything, less skewed than all drug prescribing,” said Dr. Jonathan H. Chen of the Veterans Affairs Palo Alto (Calif.) Health Care System, and his associates.
Their study included 808,020 prescribers and almost 1.2 billion Medicare Part D claims worth nearly $81 billion dollars. They focused on schedule II opioid prescriptions containing oxycodone, fentanyl, hydrocodone, morphine, methadone, hydromorphone, oxymorphone, meperidine, codeine, opium, or levorphanol (JAMA Intern Med. 2015 Dec 14. doi: 10.1001/jamainternmed.2015.6662).

Not surprisingly, specialists in pain management, anesthesia, and physical medicine wrote the most prescriptions per provider. But family practitioners, internists, nurse practitioners, and physician assistants wrote 35,268,234 prescriptions – more than all other specialties combined. “The trends hold up across state lines, with negligible geographic variability,” the researchers said.
The findings contradict an analysis of California workers’ compensation data, in which 1% of prescribers accounted for a third of schedule II opioid prescriptions, and 10% of prescribers accounted for 80% of prescriptions, the investigators noted. Nonetheless, 10% of Medicare prescribers in Dr. Chen’s study accounted for 78% of the total cost of opioids, possibly because they were prescribing pricier formulations or higher doses.
Overall, the findings suggest that opioid prescribing is “widespread” and “relatively indifferent to individual physicians, specialty, or region” – and that efforts to stem the tide must be equally broad, the researchers concluded.
Their study was supported by the VA Office of Academic Affiliations, the VA Health Services Research and Development Service, the National Institute of General Medical Sciences, and the Peter F. McManus Charitable Trust. The researchers had no disclosures.
A “broad swath” of Medicare providers wrote scripts for opioids in 2013, contradicting the idea that the overdose epidemic is mainly the work of “small groups of prolific prescribers and corrupt pill mills,” investigators wrote online in JAMA Internal Medicine.
“Contrary to the California workers’ compensation data showing a small subset of prescribers accounting for a disproportionately large percentage of opioid prescribing, Medicare opioid prescribing is distributed across many prescribers and is, if anything, less skewed than all drug prescribing,” said Dr. Jonathan H. Chen of the Veterans Affairs Palo Alto (Calif.) Health Care System, and his associates.
Their study included 808,020 prescribers and almost 1.2 billion Medicare Part D claims worth nearly $81 billion dollars. They focused on schedule II opioid prescriptions containing oxycodone, fentanyl, hydrocodone, morphine, methadone, hydromorphone, oxymorphone, meperidine, codeine, opium, or levorphanol (JAMA Intern Med. 2015 Dec 14. doi: 10.1001/jamainternmed.2015.6662).

Not surprisingly, specialists in pain management, anesthesia, and physical medicine wrote the most prescriptions per provider. But family practitioners, internists, nurse practitioners, and physician assistants wrote 35,268,234 prescriptions – more than all other specialties combined. “The trends hold up across state lines, with negligible geographic variability,” the researchers said.
The findings contradict an analysis of California workers’ compensation data, in which 1% of prescribers accounted for a third of schedule II opioid prescriptions, and 10% of prescribers accounted for 80% of prescriptions, the investigators noted. Nonetheless, 10% of Medicare prescribers in Dr. Chen’s study accounted for 78% of the total cost of opioids, possibly because they were prescribing pricier formulations or higher doses.
Overall, the findings suggest that opioid prescribing is “widespread” and “relatively indifferent to individual physicians, specialty, or region” – and that efforts to stem the tide must be equally broad, the researchers concluded.
Their study was supported by the VA Office of Academic Affiliations, the VA Health Services Research and Development Service, the National Institute of General Medical Sciences, and the Peter F. McManus Charitable Trust. The researchers had no disclosures.
FROM JAMA INTERNAL MEDICINE
Key clinical point: Many different types of general practitioners and specialists often prescribe opioids to Medicare beneficiaries.
Major finding: Family practitioners, internists, nurse practitioners, and physician assistants wrote 35,268,234 prescriptions – more than all other specialties combined.
Data source: An analysis of nearly 1.2 billion Medicare part D claims from 2013.
Disclosures: The study was supported by the VA Office of Academic Affiliations, the VA Health Services Research and Development Service, the National Institute of General Medical Sciences, and the Peter F. McManus Charitable Trust. The researchers had no disclosures.