User login
CDC: 20% of people in the U.S. are infected with an STD
Among the more than 320 million people in the United States, there was a prevalence estimate of 67.6 million sexually transmitted infections at the time of assessment in 2018, according to the results of an epidemiologic study using multiple data sources, including the National Health and Nutrition Examination Survey (NHANES).
In addition, almost half of the incident STIs occurred in the 15- to 24-year age bracket, according to a report published online in Sexually Transmitted Diseases. Researchers estimated the combined number of prevalent and incident infections of eight STIs in the United States in 2018: chlamydia, gonorrhea, trichomoniasis, syphilis, genital herpes (caused by herpes simplex virus type 2 [HSV-2]), human papillomavirus (HPV), sexually transmitted hepatitis B virus (HBV), and sexually transmitted HIV.
The estimated incidences of these STIs in this update, the first since 2008, were made using more recent data and improved estimation methods to provide updated STI prevalence and incidence estimates for 2018, both overall and by disease. “Having a combined estimate is crucial for policy purposes to illustrate the importance of STIs in the United States,” according to Kristen M. Kreisel, PhD, an epidemiologist at the Centers for Disease Control and Prevention, division of STD prevention, and colleagues.
The number of prevalent and incident infections were obtained by multiplying each STI’s updated per capita estimates by the 2018 full resident population estimates from the American Community Survey.
Detailed results
Chlamydia. The prevalence of chlamydia was estimated using 2015-2018 NHANES data, which was then used to create a modeled prevalence in 2018, according to the authors. There were an estimated 2.4 million prevalent urogenital chlamydial infections among persons aged 15-39 years in 2018; 1.1 and 1.3 million infections among men and women, respectively. Individuals aged 15-24 years comprised 56.7% and 75.8% of all infections in men and women respectively.
Gonorrhea. The prevalence of gonorrhea was estimated using ordinary differential equation based modeling. The number of prevalent urogenital gonococcal infections in 2018 among 15- to 39-year-olds was 209,000 overall; 50,000 in men and 155,000 in women. Of these, 113,000 (54.1%) occurred in 15- to 24-year-olds.
Trichomoniasis. The prevalence of trichomoniasis was estimated using 2015-2018 NHANES data, which was then used to create a modeled prevalence in 2018, according to the authors. The number of prevalent Trichomonas infections among 15- to 59-year-olds was 2.6 million, with 470,000 in men and 2.1 million in women. Persons aged 15-24 years comprised 15.6% of all prevalent infections, according to the authors.
Syphilis. The number of estimated prevalent syphilitic infections (all stages) among 14- to 49-year-old persons in 2018 was 156,000, with infections in men comprising 71.8% of all infections. Infections in both men and women aged 14-24 years accounted for about 25% of all infections, with 36,000 total prevalent syphilitic infections among 14- to 24-year-olds in 2018.
Genital herpes. The prevalence of genital herpes (caused by HSV-2) was estimated using 2015-2018 NHANES data, according to the authors. In persons aged 15-49 years in 2018, there were 18.6 million prevalent HSV-2 infections; 6.4 million among men and 12.2 million among women. Infections in 15- to 24-year-olds comprised 7.1% of all prevalent HSV-2 infections.
HPV. The prevalence of HPV was estimated using 2013-2016 NHANES data, which was assumed to reflect stable prevalence in 2018, according to the authors. Among 15- to 59-year-olds, the estimated number of persons, men, and women infected with one or more disease-associated HPV types in 2018 was 42.5, 23.4, and 19.2 million, respectively, with an estimated 9.0 million (21%) 15- to 24-year-olds infected,
HBV. NHANES 2013-2018 data were used to estimate the prevalence of sexually transmitted chronic HBV infections in 2018, according to the authors. The estimated number of infections among persons aged 15 years and older in 2018 was 103,000 (51,000 men and 52,000 women). There small sample size of individuals aged 15-24 years in the NHANES database made it impossible to obtain an accurate estimate for this group, according to the authors.
HIV. Data from the National HIV Surveillance System were used to estimate the prevalence and incidence of sexually transmitted HIV infections for persons aged 13 years and older in 2018. A total of 984,000 individuals aged 13 years and older were estimated to be living with sexually transmitted HIV at the end of 2018, according to the authors. Nearly 80% were men. In the 13- to 24-year-old age bracket, there were an estimated 45,400 living with sexually transmitted HIV.
Billions in costs
Commenting on the study by the CDC researchers, Raul Romaguera, acting director for CDC’s division of STD prevention, stated in a press release: “There are significant human and financial costs associated with these infections, and we know from other studies that cuts in STI prevention efforts result in higher costs down the road. Preventing STIs could save billions in medical costs, but more importantly, prevention would improve the health and lives of millions of people.”
“About 20% of the total U.S. population had an STI at a given point in 2018, while nearly half of all incident infections occurred in people aged 15-24 years. Focusing STI prevention efforts on the 15- to 24-year-old population may be key to lowering the STI burden in the U.S.,” the researchers concluded.
The authors reported that they had no disclosures.
Among the more than 320 million people in the United States, there was a prevalence estimate of 67.6 million sexually transmitted infections at the time of assessment in 2018, according to the results of an epidemiologic study using multiple data sources, including the National Health and Nutrition Examination Survey (NHANES).
In addition, almost half of the incident STIs occurred in the 15- to 24-year age bracket, according to a report published online in Sexually Transmitted Diseases. Researchers estimated the combined number of prevalent and incident infections of eight STIs in the United States in 2018: chlamydia, gonorrhea, trichomoniasis, syphilis, genital herpes (caused by herpes simplex virus type 2 [HSV-2]), human papillomavirus (HPV), sexually transmitted hepatitis B virus (HBV), and sexually transmitted HIV.
The estimated incidences of these STIs in this update, the first since 2008, were made using more recent data and improved estimation methods to provide updated STI prevalence and incidence estimates for 2018, both overall and by disease. “Having a combined estimate is crucial for policy purposes to illustrate the importance of STIs in the United States,” according to Kristen M. Kreisel, PhD, an epidemiologist at the Centers for Disease Control and Prevention, division of STD prevention, and colleagues.
The number of prevalent and incident infections were obtained by multiplying each STI’s updated per capita estimates by the 2018 full resident population estimates from the American Community Survey.
Detailed results
Chlamydia. The prevalence of chlamydia was estimated using 2015-2018 NHANES data, which was then used to create a modeled prevalence in 2018, according to the authors. There were an estimated 2.4 million prevalent urogenital chlamydial infections among persons aged 15-39 years in 2018; 1.1 and 1.3 million infections among men and women, respectively. Individuals aged 15-24 years comprised 56.7% and 75.8% of all infections in men and women respectively.
Gonorrhea. The prevalence of gonorrhea was estimated using ordinary differential equation based modeling. The number of prevalent urogenital gonococcal infections in 2018 among 15- to 39-year-olds was 209,000 overall; 50,000 in men and 155,000 in women. Of these, 113,000 (54.1%) occurred in 15- to 24-year-olds.
Trichomoniasis. The prevalence of trichomoniasis was estimated using 2015-2018 NHANES data, which was then used to create a modeled prevalence in 2018, according to the authors. The number of prevalent Trichomonas infections among 15- to 59-year-olds was 2.6 million, with 470,000 in men and 2.1 million in women. Persons aged 15-24 years comprised 15.6% of all prevalent infections, according to the authors.
Syphilis. The number of estimated prevalent syphilitic infections (all stages) among 14- to 49-year-old persons in 2018 was 156,000, with infections in men comprising 71.8% of all infections. Infections in both men and women aged 14-24 years accounted for about 25% of all infections, with 36,000 total prevalent syphilitic infections among 14- to 24-year-olds in 2018.
Genital herpes. The prevalence of genital herpes (caused by HSV-2) was estimated using 2015-2018 NHANES data, according to the authors. In persons aged 15-49 years in 2018, there were 18.6 million prevalent HSV-2 infections; 6.4 million among men and 12.2 million among women. Infections in 15- to 24-year-olds comprised 7.1% of all prevalent HSV-2 infections.
HPV. The prevalence of HPV was estimated using 2013-2016 NHANES data, which was assumed to reflect stable prevalence in 2018, according to the authors. Among 15- to 59-year-olds, the estimated number of persons, men, and women infected with one or more disease-associated HPV types in 2018 was 42.5, 23.4, and 19.2 million, respectively, with an estimated 9.0 million (21%) 15- to 24-year-olds infected,
HBV. NHANES 2013-2018 data were used to estimate the prevalence of sexually transmitted chronic HBV infections in 2018, according to the authors. The estimated number of infections among persons aged 15 years and older in 2018 was 103,000 (51,000 men and 52,000 women). There small sample size of individuals aged 15-24 years in the NHANES database made it impossible to obtain an accurate estimate for this group, according to the authors.
HIV. Data from the National HIV Surveillance System were used to estimate the prevalence and incidence of sexually transmitted HIV infections for persons aged 13 years and older in 2018. A total of 984,000 individuals aged 13 years and older were estimated to be living with sexually transmitted HIV at the end of 2018, according to the authors. Nearly 80% were men. In the 13- to 24-year-old age bracket, there were an estimated 45,400 living with sexually transmitted HIV.
Billions in costs
Commenting on the study by the CDC researchers, Raul Romaguera, acting director for CDC’s division of STD prevention, stated in a press release: “There are significant human and financial costs associated with these infections, and we know from other studies that cuts in STI prevention efforts result in higher costs down the road. Preventing STIs could save billions in medical costs, but more importantly, prevention would improve the health and lives of millions of people.”
“About 20% of the total U.S. population had an STI at a given point in 2018, while nearly half of all incident infections occurred in people aged 15-24 years. Focusing STI prevention efforts on the 15- to 24-year-old population may be key to lowering the STI burden in the U.S.,” the researchers concluded.
The authors reported that they had no disclosures.
Among the more than 320 million people in the United States, there was a prevalence estimate of 67.6 million sexually transmitted infections at the time of assessment in 2018, according to the results of an epidemiologic study using multiple data sources, including the National Health and Nutrition Examination Survey (NHANES).
In addition, almost half of the incident STIs occurred in the 15- to 24-year age bracket, according to a report published online in Sexually Transmitted Diseases. Researchers estimated the combined number of prevalent and incident infections of eight STIs in the United States in 2018: chlamydia, gonorrhea, trichomoniasis, syphilis, genital herpes (caused by herpes simplex virus type 2 [HSV-2]), human papillomavirus (HPV), sexually transmitted hepatitis B virus (HBV), and sexually transmitted HIV.
The estimated incidences of these STIs in this update, the first since 2008, were made using more recent data and improved estimation methods to provide updated STI prevalence and incidence estimates for 2018, both overall and by disease. “Having a combined estimate is crucial for policy purposes to illustrate the importance of STIs in the United States,” according to Kristen M. Kreisel, PhD, an epidemiologist at the Centers for Disease Control and Prevention, division of STD prevention, and colleagues.
The number of prevalent and incident infections were obtained by multiplying each STI’s updated per capita estimates by the 2018 full resident population estimates from the American Community Survey.
Detailed results
Chlamydia. The prevalence of chlamydia was estimated using 2015-2018 NHANES data, which was then used to create a modeled prevalence in 2018, according to the authors. There were an estimated 2.4 million prevalent urogenital chlamydial infections among persons aged 15-39 years in 2018; 1.1 and 1.3 million infections among men and women, respectively. Individuals aged 15-24 years comprised 56.7% and 75.8% of all infections in men and women respectively.
Gonorrhea. The prevalence of gonorrhea was estimated using ordinary differential equation based modeling. The number of prevalent urogenital gonococcal infections in 2018 among 15- to 39-year-olds was 209,000 overall; 50,000 in men and 155,000 in women. Of these, 113,000 (54.1%) occurred in 15- to 24-year-olds.
Trichomoniasis. The prevalence of trichomoniasis was estimated using 2015-2018 NHANES data, which was then used to create a modeled prevalence in 2018, according to the authors. The number of prevalent Trichomonas infections among 15- to 59-year-olds was 2.6 million, with 470,000 in men and 2.1 million in women. Persons aged 15-24 years comprised 15.6% of all prevalent infections, according to the authors.
Syphilis. The number of estimated prevalent syphilitic infections (all stages) among 14- to 49-year-old persons in 2018 was 156,000, with infections in men comprising 71.8% of all infections. Infections in both men and women aged 14-24 years accounted for about 25% of all infections, with 36,000 total prevalent syphilitic infections among 14- to 24-year-olds in 2018.
Genital herpes. The prevalence of genital herpes (caused by HSV-2) was estimated using 2015-2018 NHANES data, according to the authors. In persons aged 15-49 years in 2018, there were 18.6 million prevalent HSV-2 infections; 6.4 million among men and 12.2 million among women. Infections in 15- to 24-year-olds comprised 7.1% of all prevalent HSV-2 infections.
HPV. The prevalence of HPV was estimated using 2013-2016 NHANES data, which was assumed to reflect stable prevalence in 2018, according to the authors. Among 15- to 59-year-olds, the estimated number of persons, men, and women infected with one or more disease-associated HPV types in 2018 was 42.5, 23.4, and 19.2 million, respectively, with an estimated 9.0 million (21%) 15- to 24-year-olds infected,
HBV. NHANES 2013-2018 data were used to estimate the prevalence of sexually transmitted chronic HBV infections in 2018, according to the authors. The estimated number of infections among persons aged 15 years and older in 2018 was 103,000 (51,000 men and 52,000 women). There small sample size of individuals aged 15-24 years in the NHANES database made it impossible to obtain an accurate estimate for this group, according to the authors.
HIV. Data from the National HIV Surveillance System were used to estimate the prevalence and incidence of sexually transmitted HIV infections for persons aged 13 years and older in 2018. A total of 984,000 individuals aged 13 years and older were estimated to be living with sexually transmitted HIV at the end of 2018, according to the authors. Nearly 80% were men. In the 13- to 24-year-old age bracket, there were an estimated 45,400 living with sexually transmitted HIV.
Billions in costs
Commenting on the study by the CDC researchers, Raul Romaguera, acting director for CDC’s division of STD prevention, stated in a press release: “There are significant human and financial costs associated with these infections, and we know from other studies that cuts in STI prevention efforts result in higher costs down the road. Preventing STIs could save billions in medical costs, but more importantly, prevention would improve the health and lives of millions of people.”
“About 20% of the total U.S. population had an STI at a given point in 2018, while nearly half of all incident infections occurred in people aged 15-24 years. Focusing STI prevention efforts on the 15- to 24-year-old population may be key to lowering the STI burden in the U.S.,” the researchers concluded.
The authors reported that they had no disclosures.
FROM SEXUALLY TRANSMITTED DISEASES
Family physicians can help achieve national goals on STIs
Among these are the U.S. Department of Health and Human Services’ first “Sexually Transmitted Infections (STIs) National Strategic Plan for the United States,” which has a strong encompassing vision.
“The United States will be a place where sexually transmitted infections are prevented and where every person has high-quality STI prevention care, and treatment while living free from stigma and discrimination. The vision includes all people, regardless of age, sex, gender identity, sexual orientation, race, ethnicity, religion, disability, geographic location, or socioeconomic circumstance,” the new HHS plan states.1
Family physicians can and should play important roles in helping our country meet this plan’s goals particularly by following two important updated clinical guidelines, one from the U.S. Preventive Services Task Force (USPSTF) and another from the Centers for Disease Control and Prevention (CDC).
This strategic plan includes the following five overarching goals with associated objectives:
- Prevent New STIs.
- Improve the health of people by reducing adverse outcomes of STIs.
- Accelerate progress in STI research, technology, and innovation.
- Reduce STI-related health disparities and health inequities.
- Achieve integrated, coordinated efforts that address the STI epidemic.1
In my opinion, family physicians have important roles to play in order for each of these goals to be achieved.Unfortunately, there are approximately 20 million new cases of STIs each year, and the U.S. has seen increases in the rates of STIs in the past decade.
“Sexually transmitted infections are frequently asymptomatic, which may delay diagnosis and treatment and lead persons to unknowingly transmit STIs to others,” according to a new recommendation statement from the USPSTF.2 STIs may lead to serious health consequences for patients, cause harms to a mother and infant during pregnancy, and lead to cases of cancer among other concerning outcomes. As such, following the HHS new national strategic plan is critical for us to address the needs of our communities.
Preventing new STIs
Family physicians can be vital in achieving the first goal of the plan by helping to prevent new STIs. In August 2020, the USPSTF updated its guideline on behavioral counseling interventions to prevent STIs. In my opinion, the USPSTF offers some practical improvements from the earlier version of this guideline.
The task force provides a grade B recommendation that all sexually active adolescents and adults at increased risk for STIs be provided with behavioral counseling to prevent STIs. The guideline indicates that behavioral counseling interventions reduce the likelihood of those at increased risk for acquiring STIs.2
The 2014 guideline had recommended intensive interventions with a minimum of 30 minutes of counseling. Many family physicians may have found this previous recommendation impractical to implement. These updated recommendations now include a variety of interventions, such as those that take less than 30 minutes.
Although interventions with more than 120 minutes of contact time had the most effect, those with less than 30 minutes still demonstrated statistically significant fewer acquisitions of STIs during follow-up. These options include in-person counseling, and providing written materials, websites, videos, and telephone and text support to patients. These interventions can be delivered directly by the family physician, or patients may be referred to other settings or the media interventions.
The task force’s updated recommendation statement refers to a variety of resources that can be used to identify these interventions. Many of the studies reviewed for this guideline were conducted in STI clinics, and the guideline authors recommended further studies in primary care as opportunities for more generalizability.
In addition to behavioral counseling for STI prevention, family physicians can help prevent STIs in their patients through HPV vaccination and HIV pre-exposure prophylaxis (PrEP provision) within their practices. As the first contact for health care for many patients, we have an opportunity to significantly impact this first goal of prevention.
Treating STIs
Within the second goal of the national strategic plan is treatment of STIs, which family physicians should include in their practices as well as the diagnosis of STIs.
In December 2020, an update to the CDC’s treatment guideline for gonococcal infection was released. Prior to the publishing of this updated recommendation, the CDC recommended combination therapy of 250 mg intramuscular (IM) dose of ceftriaxone and either doxycycline or azithromycin. This recommendation has been changed to a single 500-mg IM dose of ceftriaxone for uncomplicated urogenital, anorectal, and pharyngeal gonorrhea. If chlamydia cannot be excluded, then the addition of oral doxycycline 100 mg twice daily for 7 days is recommended for nonpregnant persons, and 1 g oral azithromycin for pregnant persons. The previous treatment was recommended based on a concern for gonococcal resistance.
This updated guideline reflects increasing concerns for antimicrobial stewardship and emerging azithromycin resistance. It does not recommend a test-of-cure for urogenital or rectal gonorrhea, though did recommend a test-of-cure 7-14 days after treatment of pharyngeal gonorrhea. The guideline also recommends testing for reinfection 3-12 months after treatment as the rate of reinfection ranges from 7% to 12% among those previously treated.3
For some offices, the provision of the IM injection may be challenging, though having this medication in stock with the possibility of provision can greatly improve access and ease of treatment for patients. Family physicians can incorporate these updated recommendations along with those for other STIs such as chlamydia and syphilis with standing orders for treatment and testing within their offices.
Accelerating progress in STI research
Family physicians can also support the national strategic plan by participating in studies looking at the impact of behavioral counseling in the primary care office as opposed to in STI clinics. In addition, by following the STI treatment and screening guidelines, family physicians will contribute to the body of knowledge of prevalence, treatment failure, and reinfection rates of STIs. We can also help advance the research by providing feedback on interventions that have success within our practices.
Reducing STI-related health disparities and inequities
Family physicians are also in important places to support the strategic plan’s fourth goal of reducing health disparities and health inequities.
If we continue to ask the questions to identify those at high risk and ensure that we are offering appropriate STI prevention, care, and treatment services within our clinics, we can expand access to all who need services and improve equity. By offering these services within the primary care office, we may be able to decrease the stigma some may feel going to an STI clinic for services.
By incorporating additional screening and counseling in our practices we may identify some patients who were not aware that they were at risk for an STI and offer them preventive services.
Achieving integrated and coordinated efforts
Finally, as many family physicians have integrated practices, we are uniquely poised to support the fifth goal of the strategic plan of achieving integrated and coordinated efforts addressing the STI epidemic. In our practices we can participate in, lead, and refer to programs for substance use disorders, viral hepatitis, STIs, and HIV as part of full scope primary care.
Family physicians and other primary care providers should work to support the entire strategic plan to ensure that we are fully caring for our patients and communities and stopping the past decade’s increase in STIs. We have an opportunity to use this strategy and make a large impact in our communities.
Dr. Wheat is a family physician at Erie Family Health Center in Chicago. She is program director of Northwestern’s McGaw Family Medicine residency program at Humboldt Park, Chicago. Dr. Wheat serves on the editorial advisory board of Family Practice News. You can contact her at [email protected].
References
1. U.S. Department of Health and Human Services. 2020. Sexually Transmitted Infections National Strategic Plan for the United States: 2021-2025. Washington.
2. U.S. Preventive Services Task Force. Behavioral counseling interventions to prevent sexually transmitted infections: U.S. Preventive Services Task Force Recommendation Statement. JAMA. 2020;324(7):674-81. doi: 10.1001/jama.2020.13095.
3. St. Cyr S et al. Update to CDC’s Treatment Guideline for Gonococcal Infection, 2020. MMWR Morb Mortal Wkly Rep 2020;69:1911-6. doi: 10.15585/mmwr.mm6950a6external_icon.
Among these are the U.S. Department of Health and Human Services’ first “Sexually Transmitted Infections (STIs) National Strategic Plan for the United States,” which has a strong encompassing vision.
“The United States will be a place where sexually transmitted infections are prevented and where every person has high-quality STI prevention care, and treatment while living free from stigma and discrimination. The vision includes all people, regardless of age, sex, gender identity, sexual orientation, race, ethnicity, religion, disability, geographic location, or socioeconomic circumstance,” the new HHS plan states.1
Family physicians can and should play important roles in helping our country meet this plan’s goals particularly by following two important updated clinical guidelines, one from the U.S. Preventive Services Task Force (USPSTF) and another from the Centers for Disease Control and Prevention (CDC).
This strategic plan includes the following five overarching goals with associated objectives:
- Prevent New STIs.
- Improve the health of people by reducing adverse outcomes of STIs.
- Accelerate progress in STI research, technology, and innovation.
- Reduce STI-related health disparities and health inequities.
- Achieve integrated, coordinated efforts that address the STI epidemic.1
In my opinion, family physicians have important roles to play in order for each of these goals to be achieved.Unfortunately, there are approximately 20 million new cases of STIs each year, and the U.S. has seen increases in the rates of STIs in the past decade.
“Sexually transmitted infections are frequently asymptomatic, which may delay diagnosis and treatment and lead persons to unknowingly transmit STIs to others,” according to a new recommendation statement from the USPSTF.2 STIs may lead to serious health consequences for patients, cause harms to a mother and infant during pregnancy, and lead to cases of cancer among other concerning outcomes. As such, following the HHS new national strategic plan is critical for us to address the needs of our communities.
Preventing new STIs
Family physicians can be vital in achieving the first goal of the plan by helping to prevent new STIs. In August 2020, the USPSTF updated its guideline on behavioral counseling interventions to prevent STIs. In my opinion, the USPSTF offers some practical improvements from the earlier version of this guideline.
The task force provides a grade B recommendation that all sexually active adolescents and adults at increased risk for STIs be provided with behavioral counseling to prevent STIs. The guideline indicates that behavioral counseling interventions reduce the likelihood of those at increased risk for acquiring STIs.2
The 2014 guideline had recommended intensive interventions with a minimum of 30 minutes of counseling. Many family physicians may have found this previous recommendation impractical to implement. These updated recommendations now include a variety of interventions, such as those that take less than 30 minutes.
Although interventions with more than 120 minutes of contact time had the most effect, those with less than 30 minutes still demonstrated statistically significant fewer acquisitions of STIs during follow-up. These options include in-person counseling, and providing written materials, websites, videos, and telephone and text support to patients. These interventions can be delivered directly by the family physician, or patients may be referred to other settings or the media interventions.
The task force’s updated recommendation statement refers to a variety of resources that can be used to identify these interventions. Many of the studies reviewed for this guideline were conducted in STI clinics, and the guideline authors recommended further studies in primary care as opportunities for more generalizability.
In addition to behavioral counseling for STI prevention, family physicians can help prevent STIs in their patients through HPV vaccination and HIV pre-exposure prophylaxis (PrEP provision) within their practices. As the first contact for health care for many patients, we have an opportunity to significantly impact this first goal of prevention.
Treating STIs
Within the second goal of the national strategic plan is treatment of STIs, which family physicians should include in their practices as well as the diagnosis of STIs.
In December 2020, an update to the CDC’s treatment guideline for gonococcal infection was released. Prior to the publishing of this updated recommendation, the CDC recommended combination therapy of 250 mg intramuscular (IM) dose of ceftriaxone and either doxycycline or azithromycin. This recommendation has been changed to a single 500-mg IM dose of ceftriaxone for uncomplicated urogenital, anorectal, and pharyngeal gonorrhea. If chlamydia cannot be excluded, then the addition of oral doxycycline 100 mg twice daily for 7 days is recommended for nonpregnant persons, and 1 g oral azithromycin for pregnant persons. The previous treatment was recommended based on a concern for gonococcal resistance.
This updated guideline reflects increasing concerns for antimicrobial stewardship and emerging azithromycin resistance. It does not recommend a test-of-cure for urogenital or rectal gonorrhea, though did recommend a test-of-cure 7-14 days after treatment of pharyngeal gonorrhea. The guideline also recommends testing for reinfection 3-12 months after treatment as the rate of reinfection ranges from 7% to 12% among those previously treated.3
For some offices, the provision of the IM injection may be challenging, though having this medication in stock with the possibility of provision can greatly improve access and ease of treatment for patients. Family physicians can incorporate these updated recommendations along with those for other STIs such as chlamydia and syphilis with standing orders for treatment and testing within their offices.
Accelerating progress in STI research
Family physicians can also support the national strategic plan by participating in studies looking at the impact of behavioral counseling in the primary care office as opposed to in STI clinics. In addition, by following the STI treatment and screening guidelines, family physicians will contribute to the body of knowledge of prevalence, treatment failure, and reinfection rates of STIs. We can also help advance the research by providing feedback on interventions that have success within our practices.
Reducing STI-related health disparities and inequities
Family physicians are also in important places to support the strategic plan’s fourth goal of reducing health disparities and health inequities.
If we continue to ask the questions to identify those at high risk and ensure that we are offering appropriate STI prevention, care, and treatment services within our clinics, we can expand access to all who need services and improve equity. By offering these services within the primary care office, we may be able to decrease the stigma some may feel going to an STI clinic for services.
By incorporating additional screening and counseling in our practices we may identify some patients who were not aware that they were at risk for an STI and offer them preventive services.
Achieving integrated and coordinated efforts
Finally, as many family physicians have integrated practices, we are uniquely poised to support the fifth goal of the strategic plan of achieving integrated and coordinated efforts addressing the STI epidemic. In our practices we can participate in, lead, and refer to programs for substance use disorders, viral hepatitis, STIs, and HIV as part of full scope primary care.
Family physicians and other primary care providers should work to support the entire strategic plan to ensure that we are fully caring for our patients and communities and stopping the past decade’s increase in STIs. We have an opportunity to use this strategy and make a large impact in our communities.
Dr. Wheat is a family physician at Erie Family Health Center in Chicago. She is program director of Northwestern’s McGaw Family Medicine residency program at Humboldt Park, Chicago. Dr. Wheat serves on the editorial advisory board of Family Practice News. You can contact her at [email protected].
References
1. U.S. Department of Health and Human Services. 2020. Sexually Transmitted Infections National Strategic Plan for the United States: 2021-2025. Washington.
2. U.S. Preventive Services Task Force. Behavioral counseling interventions to prevent sexually transmitted infections: U.S. Preventive Services Task Force Recommendation Statement. JAMA. 2020;324(7):674-81. doi: 10.1001/jama.2020.13095.
3. St. Cyr S et al. Update to CDC’s Treatment Guideline for Gonococcal Infection, 2020. MMWR Morb Mortal Wkly Rep 2020;69:1911-6. doi: 10.15585/mmwr.mm6950a6external_icon.
Among these are the U.S. Department of Health and Human Services’ first “Sexually Transmitted Infections (STIs) National Strategic Plan for the United States,” which has a strong encompassing vision.
“The United States will be a place where sexually transmitted infections are prevented and where every person has high-quality STI prevention care, and treatment while living free from stigma and discrimination. The vision includes all people, regardless of age, sex, gender identity, sexual orientation, race, ethnicity, religion, disability, geographic location, or socioeconomic circumstance,” the new HHS plan states.1
Family physicians can and should play important roles in helping our country meet this plan’s goals particularly by following two important updated clinical guidelines, one from the U.S. Preventive Services Task Force (USPSTF) and another from the Centers for Disease Control and Prevention (CDC).
This strategic plan includes the following five overarching goals with associated objectives:
- Prevent New STIs.
- Improve the health of people by reducing adverse outcomes of STIs.
- Accelerate progress in STI research, technology, and innovation.
- Reduce STI-related health disparities and health inequities.
- Achieve integrated, coordinated efforts that address the STI epidemic.1
In my opinion, family physicians have important roles to play in order for each of these goals to be achieved.Unfortunately, there are approximately 20 million new cases of STIs each year, and the U.S. has seen increases in the rates of STIs in the past decade.
“Sexually transmitted infections are frequently asymptomatic, which may delay diagnosis and treatment and lead persons to unknowingly transmit STIs to others,” according to a new recommendation statement from the USPSTF.2 STIs may lead to serious health consequences for patients, cause harms to a mother and infant during pregnancy, and lead to cases of cancer among other concerning outcomes. As such, following the HHS new national strategic plan is critical for us to address the needs of our communities.
Preventing new STIs
Family physicians can be vital in achieving the first goal of the plan by helping to prevent new STIs. In August 2020, the USPSTF updated its guideline on behavioral counseling interventions to prevent STIs. In my opinion, the USPSTF offers some practical improvements from the earlier version of this guideline.
The task force provides a grade B recommendation that all sexually active adolescents and adults at increased risk for STIs be provided with behavioral counseling to prevent STIs. The guideline indicates that behavioral counseling interventions reduce the likelihood of those at increased risk for acquiring STIs.2
The 2014 guideline had recommended intensive interventions with a minimum of 30 minutes of counseling. Many family physicians may have found this previous recommendation impractical to implement. These updated recommendations now include a variety of interventions, such as those that take less than 30 minutes.
Although interventions with more than 120 minutes of contact time had the most effect, those with less than 30 minutes still demonstrated statistically significant fewer acquisitions of STIs during follow-up. These options include in-person counseling, and providing written materials, websites, videos, and telephone and text support to patients. These interventions can be delivered directly by the family physician, or patients may be referred to other settings or the media interventions.
The task force’s updated recommendation statement refers to a variety of resources that can be used to identify these interventions. Many of the studies reviewed for this guideline were conducted in STI clinics, and the guideline authors recommended further studies in primary care as opportunities for more generalizability.
In addition to behavioral counseling for STI prevention, family physicians can help prevent STIs in their patients through HPV vaccination and HIV pre-exposure prophylaxis (PrEP provision) within their practices. As the first contact for health care for many patients, we have an opportunity to significantly impact this first goal of prevention.
Treating STIs
Within the second goal of the national strategic plan is treatment of STIs, which family physicians should include in their practices as well as the diagnosis of STIs.
In December 2020, an update to the CDC’s treatment guideline for gonococcal infection was released. Prior to the publishing of this updated recommendation, the CDC recommended combination therapy of 250 mg intramuscular (IM) dose of ceftriaxone and either doxycycline or azithromycin. This recommendation has been changed to a single 500-mg IM dose of ceftriaxone for uncomplicated urogenital, anorectal, and pharyngeal gonorrhea. If chlamydia cannot be excluded, then the addition of oral doxycycline 100 mg twice daily for 7 days is recommended for nonpregnant persons, and 1 g oral azithromycin for pregnant persons. The previous treatment was recommended based on a concern for gonococcal resistance.
This updated guideline reflects increasing concerns for antimicrobial stewardship and emerging azithromycin resistance. It does not recommend a test-of-cure for urogenital or rectal gonorrhea, though did recommend a test-of-cure 7-14 days after treatment of pharyngeal gonorrhea. The guideline also recommends testing for reinfection 3-12 months after treatment as the rate of reinfection ranges from 7% to 12% among those previously treated.3
For some offices, the provision of the IM injection may be challenging, though having this medication in stock with the possibility of provision can greatly improve access and ease of treatment for patients. Family physicians can incorporate these updated recommendations along with those for other STIs such as chlamydia and syphilis with standing orders for treatment and testing within their offices.
Accelerating progress in STI research
Family physicians can also support the national strategic plan by participating in studies looking at the impact of behavioral counseling in the primary care office as opposed to in STI clinics. In addition, by following the STI treatment and screening guidelines, family physicians will contribute to the body of knowledge of prevalence, treatment failure, and reinfection rates of STIs. We can also help advance the research by providing feedback on interventions that have success within our practices.
Reducing STI-related health disparities and inequities
Family physicians are also in important places to support the strategic plan’s fourth goal of reducing health disparities and health inequities.
If we continue to ask the questions to identify those at high risk and ensure that we are offering appropriate STI prevention, care, and treatment services within our clinics, we can expand access to all who need services and improve equity. By offering these services within the primary care office, we may be able to decrease the stigma some may feel going to an STI clinic for services.
By incorporating additional screening and counseling in our practices we may identify some patients who were not aware that they were at risk for an STI and offer them preventive services.
Achieving integrated and coordinated efforts
Finally, as many family physicians have integrated practices, we are uniquely poised to support the fifth goal of the strategic plan of achieving integrated and coordinated efforts addressing the STI epidemic. In our practices we can participate in, lead, and refer to programs for substance use disorders, viral hepatitis, STIs, and HIV as part of full scope primary care.
Family physicians and other primary care providers should work to support the entire strategic plan to ensure that we are fully caring for our patients and communities and stopping the past decade’s increase in STIs. We have an opportunity to use this strategy and make a large impact in our communities.
Dr. Wheat is a family physician at Erie Family Health Center in Chicago. She is program director of Northwestern’s McGaw Family Medicine residency program at Humboldt Park, Chicago. Dr. Wheat serves on the editorial advisory board of Family Practice News. You can contact her at [email protected].
References
1. U.S. Department of Health and Human Services. 2020. Sexually Transmitted Infections National Strategic Plan for the United States: 2021-2025. Washington.
2. U.S. Preventive Services Task Force. Behavioral counseling interventions to prevent sexually transmitted infections: U.S. Preventive Services Task Force Recommendation Statement. JAMA. 2020;324(7):674-81. doi: 10.1001/jama.2020.13095.
3. St. Cyr S et al. Update to CDC’s Treatment Guideline for Gonococcal Infection, 2020. MMWR Morb Mortal Wkly Rep 2020;69:1911-6. doi: 10.15585/mmwr.mm6950a6external_icon.
Prophylactic HIV treatment in female STI patients is rare
reported Kirk D. Henny, PhD, and colleagues of the Centers for Disease Control and Prevention.
In an effort to quantify HIV testing rates as well as the rate of pre-exposure prophylaxis (PrEP) among women with gonorrhea or syphilis, Dr. Henny and his colleagues performed a multivariate logistic regression analysis of 13,074 female patients aged 15-64 diagnosed with a STI in the absence of HIV. Data was pulled in 2017 from the IBM MarketScan commercial and Medicaid insurance databases, and the research was published in Obstetrics & Gynecology.
Medicaid patients were more likely to be tested for HIV
A total of 3,709 patients with commercial insurance were diagnosed with gonorrhea and 1,696 with syphilis. Among those with Medicaid, 6,172 were diagnosed with gonorrhea and 1,497 with syphilis. Medicaid patients diagnosed with either STI were more likely to be tested for HIV than the commercially insured patients. With an adjusted prevalence ratio, patients commercially insured with had either STI were more likely to be tested for HIV than patients who had no STI. Prophylactic treatment rates were similar in both insurance groups: 0.15% in the commercial insurance group and 0.26% in the Medicaid group. No patient from either group who was diagnosed with gonorrhea or syphilis and subsequently tested for HIV received pre-exposure prophylactic (PrEP) treatment.
STI diagnosis is a significant indicator of future HIV
Female patients diagnosed with either STI are more likely to contract HIV, the researchers noted. They cautioned that their findings of low HIV testing rates and the absence of prophylactic treatment means that “these missed opportunities for health care professionals to intervene with female patients diagnosed with gonorrhea or syphilis might have contributed to HIV infections that could have been averted.”
The researchers also pointed out that, in a recent analysis of pharmacy data, prophylactic prescribing for female patients with clinical indications for PrEP was 6.6%, less than one-third the coverage provided to male patients.
Future research should target understanding “individual and contextual factors associated with low HIV testing” and PrEP treatment in female patients, especially those with STIs, Dr. Henny and his colleagues advised.
In a separate interview, Constance Bohon, MD FACOG, observed: “The authors present data to document the low incidence of pre-exposure prophylaxis in women who are at substantial risk of acquiring HIV and possible causes for the low utilization of this treatment.” It is important to identify barriers to diagnosis, counseling, and treatment, she advised.
“Multicenter studies to determine the best methodologies to improve the identification, management, and treatment of these at-risk women need to be done, and the conclusions disseminated to health care providers caring for women,” Dr. Bohon said.
PrEP is an important, simple strategy for reducing HIV transmission
“Pre-exposure prophylaxis has been demonstrated to decrease HIV acquisition in those at risk by up to 90% when taken appropriately,” and yet prescribing rates are extremely low (2%-6%) in at-risk women and especially women of color. These disparities have only grown over time, with prophylactic prescriptions for women at 5% between 2012 and 2017, compared with 68% for men, Catherine S. Eppes, MD, MPH, and Jennifer McKinney, MD, MPH, said in a related editorial commenting on the Research Letter by Dr. Henny and colleagues in Obstetrics & Gynecology (2020 Dec;136[6]:1080-2).
Given the abundant research demonstrating the importance and ease of prescribing PrEP, the question remains: “why does preexposure prophylaxis uptake remain so low, especially for women and women of color? There are three important issues about preexposure prophylaxis raised by this study: the research gap, the implementation gap, and the effect of systemic racism and bias,” noted Dr. Eppes and Dr. McKinney.
Women constitute a significant portion of the population that would benefit from HIV-prevention strategies, yet they continue to be excluded from research, they noted. “Much focus on research into barriers and implementation interventions for preexposure prophylaxis have focused on men who have sex with men and transgender women,” the authors of the editorial wrote.
Most women eligible for treatment would be willing to consider it if they were aware of the option, but numerous studies have cited a lack of awareness, especially among high-risk women of color in the United States, Dr. Eppes and Dr. McKinney noted.
Clinicians also need to add it to their growing checklist of mandatory appointment discussion topics, the editorialists said. “We propose standardized inclusion of preexposure prophylaxis counseling during reproductive healthcare visits. This could be aided through an electronic medical record-based best practice advisory alert. … Standardized order sets with the medication and laboratory studies necessary for safe monitoring could facilitate ease of incorporating into routine visits,” they suggested.
“Preexposure prophylaxis is extremely effective in preventing HIV, is safe, and is the only prevention method that leaves control entirely in the hands of the female partner. As a specialty, we have a responsibility to make sure our patients know about this option,” the editorialists concluded.
The authors had no financial disclosures to report. Dr. Bohon had no conflicts of interest to report.
SOURCE: Henny KD et al. Obstet Gynecol. 2020 Dec;136(6):1083-5.
reported Kirk D. Henny, PhD, and colleagues of the Centers for Disease Control and Prevention.
In an effort to quantify HIV testing rates as well as the rate of pre-exposure prophylaxis (PrEP) among women with gonorrhea or syphilis, Dr. Henny and his colleagues performed a multivariate logistic regression analysis of 13,074 female patients aged 15-64 diagnosed with a STI in the absence of HIV. Data was pulled in 2017 from the IBM MarketScan commercial and Medicaid insurance databases, and the research was published in Obstetrics & Gynecology.
Medicaid patients were more likely to be tested for HIV
A total of 3,709 patients with commercial insurance were diagnosed with gonorrhea and 1,696 with syphilis. Among those with Medicaid, 6,172 were diagnosed with gonorrhea and 1,497 with syphilis. Medicaid patients diagnosed with either STI were more likely to be tested for HIV than the commercially insured patients. With an adjusted prevalence ratio, patients commercially insured with had either STI were more likely to be tested for HIV than patients who had no STI. Prophylactic treatment rates were similar in both insurance groups: 0.15% in the commercial insurance group and 0.26% in the Medicaid group. No patient from either group who was diagnosed with gonorrhea or syphilis and subsequently tested for HIV received pre-exposure prophylactic (PrEP) treatment.
STI diagnosis is a significant indicator of future HIV
Female patients diagnosed with either STI are more likely to contract HIV, the researchers noted. They cautioned that their findings of low HIV testing rates and the absence of prophylactic treatment means that “these missed opportunities for health care professionals to intervene with female patients diagnosed with gonorrhea or syphilis might have contributed to HIV infections that could have been averted.”
The researchers also pointed out that, in a recent analysis of pharmacy data, prophylactic prescribing for female patients with clinical indications for PrEP was 6.6%, less than one-third the coverage provided to male patients.
Future research should target understanding “individual and contextual factors associated with low HIV testing” and PrEP treatment in female patients, especially those with STIs, Dr. Henny and his colleagues advised.
In a separate interview, Constance Bohon, MD FACOG, observed: “The authors present data to document the low incidence of pre-exposure prophylaxis in women who are at substantial risk of acquiring HIV and possible causes for the low utilization of this treatment.” It is important to identify barriers to diagnosis, counseling, and treatment, she advised.
“Multicenter studies to determine the best methodologies to improve the identification, management, and treatment of these at-risk women need to be done, and the conclusions disseminated to health care providers caring for women,” Dr. Bohon said.
PrEP is an important, simple strategy for reducing HIV transmission
“Pre-exposure prophylaxis has been demonstrated to decrease HIV acquisition in those at risk by up to 90% when taken appropriately,” and yet prescribing rates are extremely low (2%-6%) in at-risk women and especially women of color. These disparities have only grown over time, with prophylactic prescriptions for women at 5% between 2012 and 2017, compared with 68% for men, Catherine S. Eppes, MD, MPH, and Jennifer McKinney, MD, MPH, said in a related editorial commenting on the Research Letter by Dr. Henny and colleagues in Obstetrics & Gynecology (2020 Dec;136[6]:1080-2).
Given the abundant research demonstrating the importance and ease of prescribing PrEP, the question remains: “why does preexposure prophylaxis uptake remain so low, especially for women and women of color? There are three important issues about preexposure prophylaxis raised by this study: the research gap, the implementation gap, and the effect of systemic racism and bias,” noted Dr. Eppes and Dr. McKinney.
Women constitute a significant portion of the population that would benefit from HIV-prevention strategies, yet they continue to be excluded from research, they noted. “Much focus on research into barriers and implementation interventions for preexposure prophylaxis have focused on men who have sex with men and transgender women,” the authors of the editorial wrote.
Most women eligible for treatment would be willing to consider it if they were aware of the option, but numerous studies have cited a lack of awareness, especially among high-risk women of color in the United States, Dr. Eppes and Dr. McKinney noted.
Clinicians also need to add it to their growing checklist of mandatory appointment discussion topics, the editorialists said. “We propose standardized inclusion of preexposure prophylaxis counseling during reproductive healthcare visits. This could be aided through an electronic medical record-based best practice advisory alert. … Standardized order sets with the medication and laboratory studies necessary for safe monitoring could facilitate ease of incorporating into routine visits,” they suggested.
“Preexposure prophylaxis is extremely effective in preventing HIV, is safe, and is the only prevention method that leaves control entirely in the hands of the female partner. As a specialty, we have a responsibility to make sure our patients know about this option,” the editorialists concluded.
The authors had no financial disclosures to report. Dr. Bohon had no conflicts of interest to report.
SOURCE: Henny KD et al. Obstet Gynecol. 2020 Dec;136(6):1083-5.
reported Kirk D. Henny, PhD, and colleagues of the Centers for Disease Control and Prevention.
In an effort to quantify HIV testing rates as well as the rate of pre-exposure prophylaxis (PrEP) among women with gonorrhea or syphilis, Dr. Henny and his colleagues performed a multivariate logistic regression analysis of 13,074 female patients aged 15-64 diagnosed with a STI in the absence of HIV. Data was pulled in 2017 from the IBM MarketScan commercial and Medicaid insurance databases, and the research was published in Obstetrics & Gynecology.
Medicaid patients were more likely to be tested for HIV
A total of 3,709 patients with commercial insurance were diagnosed with gonorrhea and 1,696 with syphilis. Among those with Medicaid, 6,172 were diagnosed with gonorrhea and 1,497 with syphilis. Medicaid patients diagnosed with either STI were more likely to be tested for HIV than the commercially insured patients. With an adjusted prevalence ratio, patients commercially insured with had either STI were more likely to be tested for HIV than patients who had no STI. Prophylactic treatment rates were similar in both insurance groups: 0.15% in the commercial insurance group and 0.26% in the Medicaid group. No patient from either group who was diagnosed with gonorrhea or syphilis and subsequently tested for HIV received pre-exposure prophylactic (PrEP) treatment.
STI diagnosis is a significant indicator of future HIV
Female patients diagnosed with either STI are more likely to contract HIV, the researchers noted. They cautioned that their findings of low HIV testing rates and the absence of prophylactic treatment means that “these missed opportunities for health care professionals to intervene with female patients diagnosed with gonorrhea or syphilis might have contributed to HIV infections that could have been averted.”
The researchers also pointed out that, in a recent analysis of pharmacy data, prophylactic prescribing for female patients with clinical indications for PrEP was 6.6%, less than one-third the coverage provided to male patients.
Future research should target understanding “individual and contextual factors associated with low HIV testing” and PrEP treatment in female patients, especially those with STIs, Dr. Henny and his colleagues advised.
In a separate interview, Constance Bohon, MD FACOG, observed: “The authors present data to document the low incidence of pre-exposure prophylaxis in women who are at substantial risk of acquiring HIV and possible causes for the low utilization of this treatment.” It is important to identify barriers to diagnosis, counseling, and treatment, she advised.
“Multicenter studies to determine the best methodologies to improve the identification, management, and treatment of these at-risk women need to be done, and the conclusions disseminated to health care providers caring for women,” Dr. Bohon said.
PrEP is an important, simple strategy for reducing HIV transmission
“Pre-exposure prophylaxis has been demonstrated to decrease HIV acquisition in those at risk by up to 90% when taken appropriately,” and yet prescribing rates are extremely low (2%-6%) in at-risk women and especially women of color. These disparities have only grown over time, with prophylactic prescriptions for women at 5% between 2012 and 2017, compared with 68% for men, Catherine S. Eppes, MD, MPH, and Jennifer McKinney, MD, MPH, said in a related editorial commenting on the Research Letter by Dr. Henny and colleagues in Obstetrics & Gynecology (2020 Dec;136[6]:1080-2).
Given the abundant research demonstrating the importance and ease of prescribing PrEP, the question remains: “why does preexposure prophylaxis uptake remain so low, especially for women and women of color? There are three important issues about preexposure prophylaxis raised by this study: the research gap, the implementation gap, and the effect of systemic racism and bias,” noted Dr. Eppes and Dr. McKinney.
Women constitute a significant portion of the population that would benefit from HIV-prevention strategies, yet they continue to be excluded from research, they noted. “Much focus on research into barriers and implementation interventions for preexposure prophylaxis have focused on men who have sex with men and transgender women,” the authors of the editorial wrote.
Most women eligible for treatment would be willing to consider it if they were aware of the option, but numerous studies have cited a lack of awareness, especially among high-risk women of color in the United States, Dr. Eppes and Dr. McKinney noted.
Clinicians also need to add it to their growing checklist of mandatory appointment discussion topics, the editorialists said. “We propose standardized inclusion of preexposure prophylaxis counseling during reproductive healthcare visits. This could be aided through an electronic medical record-based best practice advisory alert. … Standardized order sets with the medication and laboratory studies necessary for safe monitoring could facilitate ease of incorporating into routine visits,” they suggested.
“Preexposure prophylaxis is extremely effective in preventing HIV, is safe, and is the only prevention method that leaves control entirely in the hands of the female partner. As a specialty, we have a responsibility to make sure our patients know about this option,” the editorialists concluded.
The authors had no financial disclosures to report. Dr. Bohon had no conflicts of interest to report.
SOURCE: Henny KD et al. Obstet Gynecol. 2020 Dec;136(6):1083-5.
FROM OBSTETRICS & GYNECOLOGY
Harnessing the HIV care continuum model to improve HCV treatment success
Better linkage to care with providers who are familiar with both the HCV and HIV treatment cascade may not only improve access to HCV treatment, but it may also support patient retention, treatment adherence, and achievement of sustained virologic response (SVR) and viral suppression, said Stephanie LaMoy, CAN Community Health, North Point, Florida. She presented the results of a pilot study at the virtual Association of Nurses in AIDS Care 2020 Annual Meeting.
In an effort to identify strategies most important for improving care access among their patients with HCV, LaMoy and her colleagues assessed 12-month patient data collected from three of their clinics. These data were evaluated for HCV treatment access, engagement, and outcomes.
The pilot study included 126 patients who were reactive and another 24 HCV-positive patients who were referred from other sources. Active HCV infections requiring treatment were reported in 144 patients.
A total of 59 patients were linked to care but did not initiate treatment for their active infection. LaMoy said there were multiple causes, including homelessness, substance abuse, and inability to maintain contact.
In contrast, 85 patients with HCV infection started treatment, but 35 of these patients did not complete their regimen. Out of the 50 patients who reported completing treatment, 30 did not return to the clinic to confirm sustained viral suppression.
According to LaMoy, this raised a red flag, causing the investigators to consider a different approach to care.
HIV care continuum model and its role in HCV
To improve the rate at which patients with HCV infection complete treatment within their clinics, the researchers formed a panel to determine necessary interventions that could reduce barriers to care.
The HIV care continuum came into play. They chose this model based on knowledge that HCV and HIV share the same care continuum with similar goals in diagnosis, linkage to care, retention, and suppression.
Based on the consensus of the panel and consideration of the HIV care continuum model, they identified a number of interventions needed to mitigate HCV treatment barriers. These included the incorporation of peer navigators or linkage-to-care (LCC) coordinators, use of the mobile medical unit, greater implementation of onsite lab visits, and medication-assisted treatment.
The LCC coordinators proved to be particularly important, as these team members helped assist patients with social and financial support to address challenges with access to treatment. These coordinators can also help patients gain access to specialized providers, ultimately improving the chance of successful HCV management.
Additionally, LCC coordinators may help identify and reduce barriers associated with housing, transportation, and nutrition. Frequent patient contact by the LCC coordinators can encourage adherence and promote risk reduction education, such as providing referrals to needle exchange services.
“Linking individuals to care with providers who are familiar with the treatment cascade could help improve retention and should be a top priority for those involved in HCV screening and treatment,” said LaMoy. “An environment with knowledge, lack of judgment, and a tenacious need to heal the community that welcomes those with barriers to care is exactly what is needed for the patients in our program.”
National, community challenges fuel barriers to HCV treatment access
Substance use, trauma histories, and mental health problems can negatively affect care engagement and must be addressed before the benefits of HCV therapy can be realized.
Addressing these issues isn’t always easy, said Kathleen Bernock, FNP-BC, AACRN, AAHIVS, of the Bedford-Stuyvesant Family Health Center in New York City, in an email to Medscape Medical News. She pointed out that several states have harsh restrictions on who is able to access HCV treatment, and some states will not approve certain medications for people who actively use drugs.
“Even for states without these restrictions, many health systems are difficult to navigate and may not be welcoming to persons actively using,” said Bernock. Trauma-informed care can also be difficult to translate into clinics, she added.
“Decentralizing care to the communities most affected would greatly help mitigate these barriers,” suggested Bernock. Decentralization, she explained, might include co-locating services such as syringe exchanges, utilizing community health workers and patient navigators, and expanding capacity-to-treat to community-based providers.
“[And] with the expansion of telehealth services in the US,” said Bernock, “we now have even more avenues to reach people that we never had before.”
LaMoy and Bernock have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Better linkage to care with providers who are familiar with both the HCV and HIV treatment cascade may not only improve access to HCV treatment, but it may also support patient retention, treatment adherence, and achievement of sustained virologic response (SVR) and viral suppression, said Stephanie LaMoy, CAN Community Health, North Point, Florida. She presented the results of a pilot study at the virtual Association of Nurses in AIDS Care 2020 Annual Meeting.
In an effort to identify strategies most important for improving care access among their patients with HCV, LaMoy and her colleagues assessed 12-month patient data collected from three of their clinics. These data were evaluated for HCV treatment access, engagement, and outcomes.
The pilot study included 126 patients who were reactive and another 24 HCV-positive patients who were referred from other sources. Active HCV infections requiring treatment were reported in 144 patients.
A total of 59 patients were linked to care but did not initiate treatment for their active infection. LaMoy said there were multiple causes, including homelessness, substance abuse, and inability to maintain contact.
In contrast, 85 patients with HCV infection started treatment, but 35 of these patients did not complete their regimen. Out of the 50 patients who reported completing treatment, 30 did not return to the clinic to confirm sustained viral suppression.
According to LaMoy, this raised a red flag, causing the investigators to consider a different approach to care.
HIV care continuum model and its role in HCV
To improve the rate at which patients with HCV infection complete treatment within their clinics, the researchers formed a panel to determine necessary interventions that could reduce barriers to care.
The HIV care continuum came into play. They chose this model based on knowledge that HCV and HIV share the same care continuum with similar goals in diagnosis, linkage to care, retention, and suppression.
Based on the consensus of the panel and consideration of the HIV care continuum model, they identified a number of interventions needed to mitigate HCV treatment barriers. These included the incorporation of peer navigators or linkage-to-care (LCC) coordinators, use of the mobile medical unit, greater implementation of onsite lab visits, and medication-assisted treatment.
The LCC coordinators proved to be particularly important, as these team members helped assist patients with social and financial support to address challenges with access to treatment. These coordinators can also help patients gain access to specialized providers, ultimately improving the chance of successful HCV management.
Additionally, LCC coordinators may help identify and reduce barriers associated with housing, transportation, and nutrition. Frequent patient contact by the LCC coordinators can encourage adherence and promote risk reduction education, such as providing referrals to needle exchange services.
“Linking individuals to care with providers who are familiar with the treatment cascade could help improve retention and should be a top priority for those involved in HCV screening and treatment,” said LaMoy. “An environment with knowledge, lack of judgment, and a tenacious need to heal the community that welcomes those with barriers to care is exactly what is needed for the patients in our program.”
National, community challenges fuel barriers to HCV treatment access
Substance use, trauma histories, and mental health problems can negatively affect care engagement and must be addressed before the benefits of HCV therapy can be realized.
Addressing these issues isn’t always easy, said Kathleen Bernock, FNP-BC, AACRN, AAHIVS, of the Bedford-Stuyvesant Family Health Center in New York City, in an email to Medscape Medical News. She pointed out that several states have harsh restrictions on who is able to access HCV treatment, and some states will not approve certain medications for people who actively use drugs.
“Even for states without these restrictions, many health systems are difficult to navigate and may not be welcoming to persons actively using,” said Bernock. Trauma-informed care can also be difficult to translate into clinics, she added.
“Decentralizing care to the communities most affected would greatly help mitigate these barriers,” suggested Bernock. Decentralization, she explained, might include co-locating services such as syringe exchanges, utilizing community health workers and patient navigators, and expanding capacity-to-treat to community-based providers.
“[And] with the expansion of telehealth services in the US,” said Bernock, “we now have even more avenues to reach people that we never had before.”
LaMoy and Bernock have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Better linkage to care with providers who are familiar with both the HCV and HIV treatment cascade may not only improve access to HCV treatment, but it may also support patient retention, treatment adherence, and achievement of sustained virologic response (SVR) and viral suppression, said Stephanie LaMoy, CAN Community Health, North Point, Florida. She presented the results of a pilot study at the virtual Association of Nurses in AIDS Care 2020 Annual Meeting.
In an effort to identify strategies most important for improving care access among their patients with HCV, LaMoy and her colleagues assessed 12-month patient data collected from three of their clinics. These data were evaluated for HCV treatment access, engagement, and outcomes.
The pilot study included 126 patients who were reactive and another 24 HCV-positive patients who were referred from other sources. Active HCV infections requiring treatment were reported in 144 patients.
A total of 59 patients were linked to care but did not initiate treatment for their active infection. LaMoy said there were multiple causes, including homelessness, substance abuse, and inability to maintain contact.
In contrast, 85 patients with HCV infection started treatment, but 35 of these patients did not complete their regimen. Out of the 50 patients who reported completing treatment, 30 did not return to the clinic to confirm sustained viral suppression.
According to LaMoy, this raised a red flag, causing the investigators to consider a different approach to care.
HIV care continuum model and its role in HCV
To improve the rate at which patients with HCV infection complete treatment within their clinics, the researchers formed a panel to determine necessary interventions that could reduce barriers to care.
The HIV care continuum came into play. They chose this model based on knowledge that HCV and HIV share the same care continuum with similar goals in diagnosis, linkage to care, retention, and suppression.
Based on the consensus of the panel and consideration of the HIV care continuum model, they identified a number of interventions needed to mitigate HCV treatment barriers. These included the incorporation of peer navigators or linkage-to-care (LCC) coordinators, use of the mobile medical unit, greater implementation of onsite lab visits, and medication-assisted treatment.
The LCC coordinators proved to be particularly important, as these team members helped assist patients with social and financial support to address challenges with access to treatment. These coordinators can also help patients gain access to specialized providers, ultimately improving the chance of successful HCV management.
Additionally, LCC coordinators may help identify and reduce barriers associated with housing, transportation, and nutrition. Frequent patient contact by the LCC coordinators can encourage adherence and promote risk reduction education, such as providing referrals to needle exchange services.
“Linking individuals to care with providers who are familiar with the treatment cascade could help improve retention and should be a top priority for those involved in HCV screening and treatment,” said LaMoy. “An environment with knowledge, lack of judgment, and a tenacious need to heal the community that welcomes those with barriers to care is exactly what is needed for the patients in our program.”
National, community challenges fuel barriers to HCV treatment access
Substance use, trauma histories, and mental health problems can negatively affect care engagement and must be addressed before the benefits of HCV therapy can be realized.
Addressing these issues isn’t always easy, said Kathleen Bernock, FNP-BC, AACRN, AAHIVS, of the Bedford-Stuyvesant Family Health Center in New York City, in an email to Medscape Medical News. She pointed out that several states have harsh restrictions on who is able to access HCV treatment, and some states will not approve certain medications for people who actively use drugs.
“Even for states without these restrictions, many health systems are difficult to navigate and may not be welcoming to persons actively using,” said Bernock. Trauma-informed care can also be difficult to translate into clinics, she added.
“Decentralizing care to the communities most affected would greatly help mitigate these barriers,” suggested Bernock. Decentralization, she explained, might include co-locating services such as syringe exchanges, utilizing community health workers and patient navigators, and expanding capacity-to-treat to community-based providers.
“[And] with the expansion of telehealth services in the US,” said Bernock, “we now have even more avenues to reach people that we never had before.”
LaMoy and Bernock have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Abnormal anal paps in people with HIV can go more than a year without follow-up
That delay “revealed missed opportunities for a better experience on the patient, clinic, and provider level,” Jessica Wells, PhD, research assistant professor at the Nell Hodgson Woodruff School of Nursing at Emory University, Atlanta, said in an interview. After all, “a lot can happen in that 1 year,” including early development of human papillomavirus (HPV)–associated anal cancer.
Although it’s too soon to say how significant that delay is with respect to the natural history of anal cancer, Dr. Wells said the data are a potential signal of disparities.
“The findings from my study may foreshadow potential disparities if we don’t have the necessary resources in place to promote follow-up care after an abnormal Pap test, similar to the disparities that we see in cervical cancer,” she said during the virtual Association of Nurses in AIDS Care 2020 Annual Meeting.
Single-center study
In the United States, people living with HIV are 19 times more likely to develop anal cancer than the general population, according to a 2018 article in the Journal of Clinical Oncology. Another single-center study from Yale University found that, in minority communities, anal cancer rates were 75% higher than in White communities. Anal cancer rates were 72% higher in communities with greater poverty. As a result, many clinics are beginning to administer Pap tests to determine early signs of HPV infection and associated changes.
In Dr. Wells’ study, which was conducted from 2012 to 2015, 150 adults with HIV who were aged 21 and older were recruited from Grady Ponce De Leon Center in Atlanta. According to a 2018 study from that center, a large minority of participants had late-stage HIV and suppressed immune systems.
All participants had been referred for HRA after a recent abnormal anal Pap test. Participants filled out questionnaires on sociodemographics, internalized HIV-related stigma, depression, risk behaviors, social support, and knowledge about HPV and anal cancer.
Participants were disproportionately older (mean age, 50.9 years); cisgender (86.7%), Black (78%); and gay, lesbian, or bisexual (84.3%). Slightly more than 1 in 10 participants (11.3%) were transgender women.
Although for 6% of participants, Pap test results indicated high-grade squamous intraepithelial lesions (HSIL), an additional 8% had atypical Pap findings that couldn’t exclude HSIL – the kinds of results that are one step away from a cancer diagnosis. More than 80% of participants had low-grade or inconclusive results. Nearly half (44%) of participants’ Pap tests revealed low-grade squamous cell intraepithelial cell lesions (LSIL); 42% indicated atypical squamous cells of undetermined significance.
When Dr. Wells looked at how long participants had waited to undergo HRA, she found something that surprised her: although some participants underwent follow-up assessment in 17 days, for many, it took much longer. The longest wait was 2,350 days – more than 6 years.
“There were quite a few patients who had follow-up beyond 1,000-plus days,” Dr. Wells said in an interview. “I didn›t think the delays were that long — at most, I would say that patients will get scheduled and come back within a few weeks or months.”
What’s more, she discovered through the HPV knowledge questionnaire that many participants did not understand why they were having a follow-up appointment. Anecdotally, some confused HPV with HIV.
“There’s education to be done to inform this target population that those living with HIV are more prone or at increased risk of this virus causing cancer later,” she said. “There are a lot of campaigns around women living with HIV, that they need to do cervical cancer screening. I think we need to really expand this campaign to include that HPV can also cause anal cancer.”
Dr. Wells had planned to primarily investigate the impact of psychosocial factors on wait time to follow-up, but none of those factors were associated with longer wait times.
Systems-level factors
That led Ann Gakumo, PhD, chair of nursing at the College of Nursing and Health Sciences of the University of Massachusetts, Boston, to ask what other factors could account for the delay.
There were several, Dr. Wells said. Precarious housing, for example, could have influenced this lag in follow-up. About one in four participants were in transient housing, and one participant reported having been incarcerated. She gathered street addresses and plans to analyze that data to see whether the cases occurred in clusters in specific neighborhoods, as the Yale data indicated.
In addition, the anoscopy clinic was only available to receive patients one day a week and was staffed with only one clinician who was trained to perform HRA. Wait times could stretch for hours. Sometimes, participants had to leave the clinic to attend to other business, and their appointments needed to be rescheduled, Wells said.
In addition to the sometimes poor understanding of the importance of the follow-up test, Dr. Wells said, “we start to see a layering of these barriers. That’s where we start seeing breakdowns. So I’m hoping in a larger study I can address some of these barriers on a multilevel approach.”
This resonated with Dr. Gakumo.
“Oftentimes, we put so much of the responsibility for this on the part of the client and not enough on the part of the provider or on the systems level,” she said.
Guiding guidelines
Guidelines on follow-up for abnormal anal Pap test results are scarce, mostly because, unlike cervical cancer, the natural history of HPV-related anal cancers hasn’t been established. The HIV Medical Association does recommend anal Pap tests, but only in cases in which “access to appropriate referral for follow-up, including high-resolution anoscopy, is available.”
In an interview, Cecile Lahiri, MD, assistant professor of infectious disease at Emory University, said that, at Ponce De Leon Center, they recommend an anal Pap for women with HIV who have a history of cervical dysplasia.
There is a reliable association between high-grade abnormal Pap tests and cervical cancer, although low-grade changes can resolve on their own. In the case of anal cancer, especially in patients with HIV, low-grade cell changes are predictive; moreover, for such patients, anal cancer is more likely to recur and is harder to treat, Dr. Lahiri said.
“The cervical environment and the anal environment are very different,” said Dr. Lahiri, who works at the Grady Ponce De Leon Center but was not involved in Dr. Wells’ study. Dr. Lahiri is also a coinvestigator of the multisite, randomized, controlled Anal Cancer HSIL Outcomes Research (ANCHOR) study, which seeks to establish whether early treatment of high-grade anal Pap changes is better than a watch-and-wait approach.
Dr. Lahiri said that when the results of that trial become available, they are more likely to know how important early anoscopy and treatment are. The findings should inform guidelines and insurance coverage of anal Pap tests and anoscopy.
In the meantime, she said, she suspected that, with the ANCHOR trial in 2015, many sites’ capacity for anoscopy may have increased, and the wait times may have gone down.
“One of the most important pieces of the study is actually the time period in which it was conducted,” said Dr. Lahiri, who in 2015 became the clinic’s second physician trained in anoscopy. Currently, more than 200 people at the Ponce De Leon Center are enrolled in the ANCHOR trial. In addition, the general capacity for performing anoscopies has gone up nationwide as a result of the trial, which required that more providers learn how to properly perform an HRA. Many clinicians are not routinely trained in performing HRA, including gastroenterologists and surgeons, Dr. Lahiri said.
“It would be interesting to look at the differences, with the start of ANCHOR being the time point for before and after,” she said.
This article first appeared on Medscape.com.
That delay “revealed missed opportunities for a better experience on the patient, clinic, and provider level,” Jessica Wells, PhD, research assistant professor at the Nell Hodgson Woodruff School of Nursing at Emory University, Atlanta, said in an interview. After all, “a lot can happen in that 1 year,” including early development of human papillomavirus (HPV)–associated anal cancer.
Although it’s too soon to say how significant that delay is with respect to the natural history of anal cancer, Dr. Wells said the data are a potential signal of disparities.
“The findings from my study may foreshadow potential disparities if we don’t have the necessary resources in place to promote follow-up care after an abnormal Pap test, similar to the disparities that we see in cervical cancer,” she said during the virtual Association of Nurses in AIDS Care 2020 Annual Meeting.
Single-center study
In the United States, people living with HIV are 19 times more likely to develop anal cancer than the general population, according to a 2018 article in the Journal of Clinical Oncology. Another single-center study from Yale University found that, in minority communities, anal cancer rates were 75% higher than in White communities. Anal cancer rates were 72% higher in communities with greater poverty. As a result, many clinics are beginning to administer Pap tests to determine early signs of HPV infection and associated changes.
In Dr. Wells’ study, which was conducted from 2012 to 2015, 150 adults with HIV who were aged 21 and older were recruited from Grady Ponce De Leon Center in Atlanta. According to a 2018 study from that center, a large minority of participants had late-stage HIV and suppressed immune systems.
All participants had been referred for HRA after a recent abnormal anal Pap test. Participants filled out questionnaires on sociodemographics, internalized HIV-related stigma, depression, risk behaviors, social support, and knowledge about HPV and anal cancer.
Participants were disproportionately older (mean age, 50.9 years); cisgender (86.7%), Black (78%); and gay, lesbian, or bisexual (84.3%). Slightly more than 1 in 10 participants (11.3%) were transgender women.
Although for 6% of participants, Pap test results indicated high-grade squamous intraepithelial lesions (HSIL), an additional 8% had atypical Pap findings that couldn’t exclude HSIL – the kinds of results that are one step away from a cancer diagnosis. More than 80% of participants had low-grade or inconclusive results. Nearly half (44%) of participants’ Pap tests revealed low-grade squamous cell intraepithelial cell lesions (LSIL); 42% indicated atypical squamous cells of undetermined significance.
When Dr. Wells looked at how long participants had waited to undergo HRA, she found something that surprised her: although some participants underwent follow-up assessment in 17 days, for many, it took much longer. The longest wait was 2,350 days – more than 6 years.
“There were quite a few patients who had follow-up beyond 1,000-plus days,” Dr. Wells said in an interview. “I didn›t think the delays were that long — at most, I would say that patients will get scheduled and come back within a few weeks or months.”
What’s more, she discovered through the HPV knowledge questionnaire that many participants did not understand why they were having a follow-up appointment. Anecdotally, some confused HPV with HIV.
“There’s education to be done to inform this target population that those living with HIV are more prone or at increased risk of this virus causing cancer later,” she said. “There are a lot of campaigns around women living with HIV, that they need to do cervical cancer screening. I think we need to really expand this campaign to include that HPV can also cause anal cancer.”
Dr. Wells had planned to primarily investigate the impact of psychosocial factors on wait time to follow-up, but none of those factors were associated with longer wait times.
Systems-level factors
That led Ann Gakumo, PhD, chair of nursing at the College of Nursing and Health Sciences of the University of Massachusetts, Boston, to ask what other factors could account for the delay.
There were several, Dr. Wells said. Precarious housing, for example, could have influenced this lag in follow-up. About one in four participants were in transient housing, and one participant reported having been incarcerated. She gathered street addresses and plans to analyze that data to see whether the cases occurred in clusters in specific neighborhoods, as the Yale data indicated.
In addition, the anoscopy clinic was only available to receive patients one day a week and was staffed with only one clinician who was trained to perform HRA. Wait times could stretch for hours. Sometimes, participants had to leave the clinic to attend to other business, and their appointments needed to be rescheduled, Wells said.
In addition to the sometimes poor understanding of the importance of the follow-up test, Dr. Wells said, “we start to see a layering of these barriers. That’s where we start seeing breakdowns. So I’m hoping in a larger study I can address some of these barriers on a multilevel approach.”
This resonated with Dr. Gakumo.
“Oftentimes, we put so much of the responsibility for this on the part of the client and not enough on the part of the provider or on the systems level,” she said.
Guiding guidelines
Guidelines on follow-up for abnormal anal Pap test results are scarce, mostly because, unlike cervical cancer, the natural history of HPV-related anal cancers hasn’t been established. The HIV Medical Association does recommend anal Pap tests, but only in cases in which “access to appropriate referral for follow-up, including high-resolution anoscopy, is available.”
In an interview, Cecile Lahiri, MD, assistant professor of infectious disease at Emory University, said that, at Ponce De Leon Center, they recommend an anal Pap for women with HIV who have a history of cervical dysplasia.
There is a reliable association between high-grade abnormal Pap tests and cervical cancer, although low-grade changes can resolve on their own. In the case of anal cancer, especially in patients with HIV, low-grade cell changes are predictive; moreover, for such patients, anal cancer is more likely to recur and is harder to treat, Dr. Lahiri said.
“The cervical environment and the anal environment are very different,” said Dr. Lahiri, who works at the Grady Ponce De Leon Center but was not involved in Dr. Wells’ study. Dr. Lahiri is also a coinvestigator of the multisite, randomized, controlled Anal Cancer HSIL Outcomes Research (ANCHOR) study, which seeks to establish whether early treatment of high-grade anal Pap changes is better than a watch-and-wait approach.
Dr. Lahiri said that when the results of that trial become available, they are more likely to know how important early anoscopy and treatment are. The findings should inform guidelines and insurance coverage of anal Pap tests and anoscopy.
In the meantime, she said, she suspected that, with the ANCHOR trial in 2015, many sites’ capacity for anoscopy may have increased, and the wait times may have gone down.
“One of the most important pieces of the study is actually the time period in which it was conducted,” said Dr. Lahiri, who in 2015 became the clinic’s second physician trained in anoscopy. Currently, more than 200 people at the Ponce De Leon Center are enrolled in the ANCHOR trial. In addition, the general capacity for performing anoscopies has gone up nationwide as a result of the trial, which required that more providers learn how to properly perform an HRA. Many clinicians are not routinely trained in performing HRA, including gastroenterologists and surgeons, Dr. Lahiri said.
“It would be interesting to look at the differences, with the start of ANCHOR being the time point for before and after,” she said.
This article first appeared on Medscape.com.
That delay “revealed missed opportunities for a better experience on the patient, clinic, and provider level,” Jessica Wells, PhD, research assistant professor at the Nell Hodgson Woodruff School of Nursing at Emory University, Atlanta, said in an interview. After all, “a lot can happen in that 1 year,” including early development of human papillomavirus (HPV)–associated anal cancer.
Although it’s too soon to say how significant that delay is with respect to the natural history of anal cancer, Dr. Wells said the data are a potential signal of disparities.
“The findings from my study may foreshadow potential disparities if we don’t have the necessary resources in place to promote follow-up care after an abnormal Pap test, similar to the disparities that we see in cervical cancer,” she said during the virtual Association of Nurses in AIDS Care 2020 Annual Meeting.
Single-center study
In the United States, people living with HIV are 19 times more likely to develop anal cancer than the general population, according to a 2018 article in the Journal of Clinical Oncology. Another single-center study from Yale University found that, in minority communities, anal cancer rates were 75% higher than in White communities. Anal cancer rates were 72% higher in communities with greater poverty. As a result, many clinics are beginning to administer Pap tests to determine early signs of HPV infection and associated changes.
In Dr. Wells’ study, which was conducted from 2012 to 2015, 150 adults with HIV who were aged 21 and older were recruited from Grady Ponce De Leon Center in Atlanta. According to a 2018 study from that center, a large minority of participants had late-stage HIV and suppressed immune systems.
All participants had been referred for HRA after a recent abnormal anal Pap test. Participants filled out questionnaires on sociodemographics, internalized HIV-related stigma, depression, risk behaviors, social support, and knowledge about HPV and anal cancer.
Participants were disproportionately older (mean age, 50.9 years); cisgender (86.7%), Black (78%); and gay, lesbian, or bisexual (84.3%). Slightly more than 1 in 10 participants (11.3%) were transgender women.
Although for 6% of participants, Pap test results indicated high-grade squamous intraepithelial lesions (HSIL), an additional 8% had atypical Pap findings that couldn’t exclude HSIL – the kinds of results that are one step away from a cancer diagnosis. More than 80% of participants had low-grade or inconclusive results. Nearly half (44%) of participants’ Pap tests revealed low-grade squamous cell intraepithelial cell lesions (LSIL); 42% indicated atypical squamous cells of undetermined significance.
When Dr. Wells looked at how long participants had waited to undergo HRA, she found something that surprised her: although some participants underwent follow-up assessment in 17 days, for many, it took much longer. The longest wait was 2,350 days – more than 6 years.
“There were quite a few patients who had follow-up beyond 1,000-plus days,” Dr. Wells said in an interview. “I didn›t think the delays were that long — at most, I would say that patients will get scheduled and come back within a few weeks or months.”
What’s more, she discovered through the HPV knowledge questionnaire that many participants did not understand why they were having a follow-up appointment. Anecdotally, some confused HPV with HIV.
“There’s education to be done to inform this target population that those living with HIV are more prone or at increased risk of this virus causing cancer later,” she said. “There are a lot of campaigns around women living with HIV, that they need to do cervical cancer screening. I think we need to really expand this campaign to include that HPV can also cause anal cancer.”
Dr. Wells had planned to primarily investigate the impact of psychosocial factors on wait time to follow-up, but none of those factors were associated with longer wait times.
Systems-level factors
That led Ann Gakumo, PhD, chair of nursing at the College of Nursing and Health Sciences of the University of Massachusetts, Boston, to ask what other factors could account for the delay.
There were several, Dr. Wells said. Precarious housing, for example, could have influenced this lag in follow-up. About one in four participants were in transient housing, and one participant reported having been incarcerated. She gathered street addresses and plans to analyze that data to see whether the cases occurred in clusters in specific neighborhoods, as the Yale data indicated.
In addition, the anoscopy clinic was only available to receive patients one day a week and was staffed with only one clinician who was trained to perform HRA. Wait times could stretch for hours. Sometimes, participants had to leave the clinic to attend to other business, and their appointments needed to be rescheduled, Wells said.
In addition to the sometimes poor understanding of the importance of the follow-up test, Dr. Wells said, “we start to see a layering of these barriers. That’s where we start seeing breakdowns. So I’m hoping in a larger study I can address some of these barriers on a multilevel approach.”
This resonated with Dr. Gakumo.
“Oftentimes, we put so much of the responsibility for this on the part of the client and not enough on the part of the provider or on the systems level,” she said.
Guiding guidelines
Guidelines on follow-up for abnormal anal Pap test results are scarce, mostly because, unlike cervical cancer, the natural history of HPV-related anal cancers hasn’t been established. The HIV Medical Association does recommend anal Pap tests, but only in cases in which “access to appropriate referral for follow-up, including high-resolution anoscopy, is available.”
In an interview, Cecile Lahiri, MD, assistant professor of infectious disease at Emory University, said that, at Ponce De Leon Center, they recommend an anal Pap for women with HIV who have a history of cervical dysplasia.
There is a reliable association between high-grade abnormal Pap tests and cervical cancer, although low-grade changes can resolve on their own. In the case of anal cancer, especially in patients with HIV, low-grade cell changes are predictive; moreover, for such patients, anal cancer is more likely to recur and is harder to treat, Dr. Lahiri said.
“The cervical environment and the anal environment are very different,” said Dr. Lahiri, who works at the Grady Ponce De Leon Center but was not involved in Dr. Wells’ study. Dr. Lahiri is also a coinvestigator of the multisite, randomized, controlled Anal Cancer HSIL Outcomes Research (ANCHOR) study, which seeks to establish whether early treatment of high-grade anal Pap changes is better than a watch-and-wait approach.
Dr. Lahiri said that when the results of that trial become available, they are more likely to know how important early anoscopy and treatment are. The findings should inform guidelines and insurance coverage of anal Pap tests and anoscopy.
In the meantime, she said, she suspected that, with the ANCHOR trial in 2015, many sites’ capacity for anoscopy may have increased, and the wait times may have gone down.
“One of the most important pieces of the study is actually the time period in which it was conducted,” said Dr. Lahiri, who in 2015 became the clinic’s second physician trained in anoscopy. Currently, more than 200 people at the Ponce De Leon Center are enrolled in the ANCHOR trial. In addition, the general capacity for performing anoscopies has gone up nationwide as a result of the trial, which required that more providers learn how to properly perform an HRA. Many clinicians are not routinely trained in performing HRA, including gastroenterologists and surgeons, Dr. Lahiri said.
“It would be interesting to look at the differences, with the start of ANCHOR being the time point for before and after,” she said.
This article first appeared on Medscape.com.
‘Uptake is only the first step’ for effective HIV PrEP protection
To Amanda Allmacher, DNP, RN, nurse practitioner at the Detroit Public Health STD Clinic, that means that same-day PrEP prescribing works and is acceptable. But there’s more work to do on the clinic and pharmacy side to make HIV protection a reality for most of her patients. Allmacher presented her data at the Association of Nurses in AIDS Care 2020 virtual annual meeting.
Dawn K. Smith, MD, epidemiologist and medical officer in the Division of HIV/AIDS Prevention at the Centers for Disease Control and Prevention’s National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention, said this adds to other data to show that we’re now entering the next phase of PrEP implementation.
“Our original focus was on uptake — informing folks what PrEP is, why they might benefit from its use, and then prescribing it if accepted,” Smith told Medscape Medical News via email. “Whether standard or same-day [PrEP prescribing], it is clear that uptake is only the first step.”
Nurses help navigate
Patients who attended the Detroit Public Health STD Clinic are more likely to be younger, have no insurance, and otherwise “have little to no contact with the healthcare system,” Allmacher said in her presentation. They also tend to come from communities that bear the greatest burden of HIV in the US — in other words, they are often the people most missed in PrEP rollouts thus far.
In response, the clinic implemented a same-day PrEP protocol, in which registered nurses trained in HIV risk assessment identify clients who might most benefit from PrEP. Criteria often include the presence of other STIs. Once the nurse explains what PrEP is and how it works, if the patient is interested, clients meet with a nurse practitioner right then to get the prescription for PrEP. The clinic also does labs to rule out current HIV infection, hepatitis B, metabolic issues, and other STI screening.
But it doesn’t stop there. The clinic used grant funding to offer PrEP navigation and financial counseling services, which help clients navigate the sometimes-thorny process of paying for PrEP. Payment comes either through Medicaid, which in Michigan charges $3 a month for a PrEP prescription, through patient assistance programs, or through private insurance. With clients under age 18 who are interested in PrEP, the clinic works to find a way to access PrEP without having to inform their parents. These same navigators schedule follow-up appointments, offer appointment reminders, and contact clients when they miss an appointment.
“Our navigators and financial counselors are a huge support for our same-day PrEP starts, helping with financial assistance, prior authorization, navigating different plans, and helping patients apply for Medicaid when appropriate,” she said.
The clinic also offers community outreach and incentives, which can include gift cards, bus passes, and pill containers, among other things.
This was a key lesson in setting up the program, Allmacher told Medscape Medical News.
“Starting PrEP at that initial visit allows for clinicians to meet patients where they are and administer care in a more equitable manner,” Allmacher said via email. “Use all available resources and funding sources. We have a versatile team working together to increase access for patients and promote HIV prevention and risk reduction.”
Script vs. follow-up
This approach is common, used in places like New York City and San Francisco. So once it was set up Allmacher sat back and waited to see how the program helped clients protect themselves from HIV.
Of the 451 clients eligible for PrEP in 2019, 336 were gay and bisexual men, 6 were transgender women, 61 were heterosexual, cisgender men, and 48 were cisgender women. One transgender man also screened as eligible. Allmacher did not break down data by race.
Uptake was high: 70% of all eligible clients did receive a prescription for PrEP, either generic tenofovir disoproxil fumarate/emtricitabine (Truvada) or tenofovir alafenamide/emtricitabine (Descovy). And uptake was high among people most at risk: 80% of gay and bisexual men who were eligible got a prescription, 60% of eligible cisgender women, 50% of the small number of transgender women, and 32.7% of heterosexual cisgender men did as well. The 1 transgender man also received a prescription.
This is a higher rate than found in a recent PrEP demonstration project, which found that despite gay and bisexual men, transgender adults, and Black people having the highest risk for HIV in the US, state health departments were more likely to refer heterosexual adults for PrEP.
That high uptake rate is encouraging, but follow-up? Not so much. After initial intake, clients are meant to return in a month to double-check their labs, ask about side effects, and start their 90-day supply of the medication. But just 40% showed up for their 30-day appointment, Allmacher said. And only one third of those showed up for the follow-up in 90 days.
By the end of 2019, just 73 of the original 451 clients screened were still taking PrEP.
“It was surprising to see just how significant the follow-up dropped off after that first visit, when the patient initially accepted the prescription,” Allmacher said.
And while it’s possible that some clients get their follow-up care from their primary care providers, “our clinic serves individuals regardless of insurance status and many do not identify having access to primary care for any type of service, PrEP or otherwise,” she said.
5 HIV acquisitions
In addition, the program review identified five clients who had been offered PrEP or had taken PrEP briefly who later acquired HIV. Those clients were offered same-day antiretroviral treatment, Allmacher said.
“So we’re finding people who are at high risk for HIV and we can prevent them, but we’re still not quite doing enough,” Allmacher said of those acquisitions. “Clearly we have a lot of work to do to focus on HIV prevention, and we are looking to create a more formal follow-up process” from the clinic’s side.
For instance, clinic staff call clients 1 week after their initial visit to share lab results. “This was identified as a missed opportunity for us to ask about their status, whether they filled their prescription, or if they need further assistance,” she said. “This is an area where our registered nurses are going to be taking on a greater role moving forward.”
Allmacher and team also discovered that, despite PrEP navigators arranging insurance coverage for clients on the day they receive their prescription, sometimes there were still barriers when the client showed up at the pharmacy to pick up their meds. The clinic does not have an in-house pharmacy and does not currently have the funding that would allow them to hand patients a bottle of the appropriate medication when they leave the clinic.
“Navigating the copays and the insurance coverage and using financial assistance through the drug manufacturer — even though we have the support in the clinic, it seems like there’s a disconnect between our clinic and getting to the pharmacy. Not every pharmacy is super familiar with navigating those,” she said. “So we have started to identify some area pharmacies near our clinic that are great at navigating these, and we really try to get our patients to go to places we know can give them assistance.”
A version of this story originally appeared on Medscape.com.
To Amanda Allmacher, DNP, RN, nurse practitioner at the Detroit Public Health STD Clinic, that means that same-day PrEP prescribing works and is acceptable. But there’s more work to do on the clinic and pharmacy side to make HIV protection a reality for most of her patients. Allmacher presented her data at the Association of Nurses in AIDS Care 2020 virtual annual meeting.
Dawn K. Smith, MD, epidemiologist and medical officer in the Division of HIV/AIDS Prevention at the Centers for Disease Control and Prevention’s National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention, said this adds to other data to show that we’re now entering the next phase of PrEP implementation.
“Our original focus was on uptake — informing folks what PrEP is, why they might benefit from its use, and then prescribing it if accepted,” Smith told Medscape Medical News via email. “Whether standard or same-day [PrEP prescribing], it is clear that uptake is only the first step.”
Nurses help navigate
Patients who attended the Detroit Public Health STD Clinic are more likely to be younger, have no insurance, and otherwise “have little to no contact with the healthcare system,” Allmacher said in her presentation. They also tend to come from communities that bear the greatest burden of HIV in the US — in other words, they are often the people most missed in PrEP rollouts thus far.
In response, the clinic implemented a same-day PrEP protocol, in which registered nurses trained in HIV risk assessment identify clients who might most benefit from PrEP. Criteria often include the presence of other STIs. Once the nurse explains what PrEP is and how it works, if the patient is interested, clients meet with a nurse practitioner right then to get the prescription for PrEP. The clinic also does labs to rule out current HIV infection, hepatitis B, metabolic issues, and other STI screening.
But it doesn’t stop there. The clinic used grant funding to offer PrEP navigation and financial counseling services, which help clients navigate the sometimes-thorny process of paying for PrEP. Payment comes either through Medicaid, which in Michigan charges $3 a month for a PrEP prescription, through patient assistance programs, or through private insurance. With clients under age 18 who are interested in PrEP, the clinic works to find a way to access PrEP without having to inform their parents. These same navigators schedule follow-up appointments, offer appointment reminders, and contact clients when they miss an appointment.
“Our navigators and financial counselors are a huge support for our same-day PrEP starts, helping with financial assistance, prior authorization, navigating different plans, and helping patients apply for Medicaid when appropriate,” she said.
The clinic also offers community outreach and incentives, which can include gift cards, bus passes, and pill containers, among other things.
This was a key lesson in setting up the program, Allmacher told Medscape Medical News.
“Starting PrEP at that initial visit allows for clinicians to meet patients where they are and administer care in a more equitable manner,” Allmacher said via email. “Use all available resources and funding sources. We have a versatile team working together to increase access for patients and promote HIV prevention and risk reduction.”
Script vs. follow-up
This approach is common, used in places like New York City and San Francisco. So once it was set up Allmacher sat back and waited to see how the program helped clients protect themselves from HIV.
Of the 451 clients eligible for PrEP in 2019, 336 were gay and bisexual men, 6 were transgender women, 61 were heterosexual, cisgender men, and 48 were cisgender women. One transgender man also screened as eligible. Allmacher did not break down data by race.
Uptake was high: 70% of all eligible clients did receive a prescription for PrEP, either generic tenofovir disoproxil fumarate/emtricitabine (Truvada) or tenofovir alafenamide/emtricitabine (Descovy). And uptake was high among people most at risk: 80% of gay and bisexual men who were eligible got a prescription, 60% of eligible cisgender women, 50% of the small number of transgender women, and 32.7% of heterosexual cisgender men did as well. The 1 transgender man also received a prescription.
This is a higher rate than found in a recent PrEP demonstration project, which found that despite gay and bisexual men, transgender adults, and Black people having the highest risk for HIV in the US, state health departments were more likely to refer heterosexual adults for PrEP.
That high uptake rate is encouraging, but follow-up? Not so much. After initial intake, clients are meant to return in a month to double-check their labs, ask about side effects, and start their 90-day supply of the medication. But just 40% showed up for their 30-day appointment, Allmacher said. And only one third of those showed up for the follow-up in 90 days.
By the end of 2019, just 73 of the original 451 clients screened were still taking PrEP.
“It was surprising to see just how significant the follow-up dropped off after that first visit, when the patient initially accepted the prescription,” Allmacher said.
And while it’s possible that some clients get their follow-up care from their primary care providers, “our clinic serves individuals regardless of insurance status and many do not identify having access to primary care for any type of service, PrEP or otherwise,” she said.
5 HIV acquisitions
In addition, the program review identified five clients who had been offered PrEP or had taken PrEP briefly who later acquired HIV. Those clients were offered same-day antiretroviral treatment, Allmacher said.
“So we’re finding people who are at high risk for HIV and we can prevent them, but we’re still not quite doing enough,” Allmacher said of those acquisitions. “Clearly we have a lot of work to do to focus on HIV prevention, and we are looking to create a more formal follow-up process” from the clinic’s side.
For instance, clinic staff call clients 1 week after their initial visit to share lab results. “This was identified as a missed opportunity for us to ask about their status, whether they filled their prescription, or if they need further assistance,” she said. “This is an area where our registered nurses are going to be taking on a greater role moving forward.”
Allmacher and team also discovered that, despite PrEP navigators arranging insurance coverage for clients on the day they receive their prescription, sometimes there were still barriers when the client showed up at the pharmacy to pick up their meds. The clinic does not have an in-house pharmacy and does not currently have the funding that would allow them to hand patients a bottle of the appropriate medication when they leave the clinic.
“Navigating the copays and the insurance coverage and using financial assistance through the drug manufacturer — even though we have the support in the clinic, it seems like there’s a disconnect between our clinic and getting to the pharmacy. Not every pharmacy is super familiar with navigating those,” she said. “So we have started to identify some area pharmacies near our clinic that are great at navigating these, and we really try to get our patients to go to places we know can give them assistance.”
A version of this story originally appeared on Medscape.com.
To Amanda Allmacher, DNP, RN, nurse practitioner at the Detroit Public Health STD Clinic, that means that same-day PrEP prescribing works and is acceptable. But there’s more work to do on the clinic and pharmacy side to make HIV protection a reality for most of her patients. Allmacher presented her data at the Association of Nurses in AIDS Care 2020 virtual annual meeting.
Dawn K. Smith, MD, epidemiologist and medical officer in the Division of HIV/AIDS Prevention at the Centers for Disease Control and Prevention’s National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention, said this adds to other data to show that we’re now entering the next phase of PrEP implementation.
“Our original focus was on uptake — informing folks what PrEP is, why they might benefit from its use, and then prescribing it if accepted,” Smith told Medscape Medical News via email. “Whether standard or same-day [PrEP prescribing], it is clear that uptake is only the first step.”
Nurses help navigate
Patients who attended the Detroit Public Health STD Clinic are more likely to be younger, have no insurance, and otherwise “have little to no contact with the healthcare system,” Allmacher said in her presentation. They also tend to come from communities that bear the greatest burden of HIV in the US — in other words, they are often the people most missed in PrEP rollouts thus far.
In response, the clinic implemented a same-day PrEP protocol, in which registered nurses trained in HIV risk assessment identify clients who might most benefit from PrEP. Criteria often include the presence of other STIs. Once the nurse explains what PrEP is and how it works, if the patient is interested, clients meet with a nurse practitioner right then to get the prescription for PrEP. The clinic also does labs to rule out current HIV infection, hepatitis B, metabolic issues, and other STI screening.
But it doesn’t stop there. The clinic used grant funding to offer PrEP navigation and financial counseling services, which help clients navigate the sometimes-thorny process of paying for PrEP. Payment comes either through Medicaid, which in Michigan charges $3 a month for a PrEP prescription, through patient assistance programs, or through private insurance. With clients under age 18 who are interested in PrEP, the clinic works to find a way to access PrEP without having to inform their parents. These same navigators schedule follow-up appointments, offer appointment reminders, and contact clients when they miss an appointment.
“Our navigators and financial counselors are a huge support for our same-day PrEP starts, helping with financial assistance, prior authorization, navigating different plans, and helping patients apply for Medicaid when appropriate,” she said.
The clinic also offers community outreach and incentives, which can include gift cards, bus passes, and pill containers, among other things.
This was a key lesson in setting up the program, Allmacher told Medscape Medical News.
“Starting PrEP at that initial visit allows for clinicians to meet patients where they are and administer care in a more equitable manner,” Allmacher said via email. “Use all available resources and funding sources. We have a versatile team working together to increase access for patients and promote HIV prevention and risk reduction.”
Script vs. follow-up
This approach is common, used in places like New York City and San Francisco. So once it was set up Allmacher sat back and waited to see how the program helped clients protect themselves from HIV.
Of the 451 clients eligible for PrEP in 2019, 336 were gay and bisexual men, 6 were transgender women, 61 were heterosexual, cisgender men, and 48 were cisgender women. One transgender man also screened as eligible. Allmacher did not break down data by race.
Uptake was high: 70% of all eligible clients did receive a prescription for PrEP, either generic tenofovir disoproxil fumarate/emtricitabine (Truvada) or tenofovir alafenamide/emtricitabine (Descovy). And uptake was high among people most at risk: 80% of gay and bisexual men who were eligible got a prescription, 60% of eligible cisgender women, 50% of the small number of transgender women, and 32.7% of heterosexual cisgender men did as well. The 1 transgender man also received a prescription.
This is a higher rate than found in a recent PrEP demonstration project, which found that despite gay and bisexual men, transgender adults, and Black people having the highest risk for HIV in the US, state health departments were more likely to refer heterosexual adults for PrEP.
That high uptake rate is encouraging, but follow-up? Not so much. After initial intake, clients are meant to return in a month to double-check their labs, ask about side effects, and start their 90-day supply of the medication. But just 40% showed up for their 30-day appointment, Allmacher said. And only one third of those showed up for the follow-up in 90 days.
By the end of 2019, just 73 of the original 451 clients screened were still taking PrEP.
“It was surprising to see just how significant the follow-up dropped off after that first visit, when the patient initially accepted the prescription,” Allmacher said.
And while it’s possible that some clients get their follow-up care from their primary care providers, “our clinic serves individuals regardless of insurance status and many do not identify having access to primary care for any type of service, PrEP or otherwise,” she said.
5 HIV acquisitions
In addition, the program review identified five clients who had been offered PrEP or had taken PrEP briefly who later acquired HIV. Those clients were offered same-day antiretroviral treatment, Allmacher said.
“So we’re finding people who are at high risk for HIV and we can prevent them, but we’re still not quite doing enough,” Allmacher said of those acquisitions. “Clearly we have a lot of work to do to focus on HIV prevention, and we are looking to create a more formal follow-up process” from the clinic’s side.
For instance, clinic staff call clients 1 week after their initial visit to share lab results. “This was identified as a missed opportunity for us to ask about their status, whether they filled their prescription, or if they need further assistance,” she said. “This is an area where our registered nurses are going to be taking on a greater role moving forward.”
Allmacher and team also discovered that, despite PrEP navigators arranging insurance coverage for clients on the day they receive their prescription, sometimes there were still barriers when the client showed up at the pharmacy to pick up their meds. The clinic does not have an in-house pharmacy and does not currently have the funding that would allow them to hand patients a bottle of the appropriate medication when they leave the clinic.
“Navigating the copays and the insurance coverage and using financial assistance through the drug manufacturer — even though we have the support in the clinic, it seems like there’s a disconnect between our clinic and getting to the pharmacy. Not every pharmacy is super familiar with navigating those,” she said. “So we have started to identify some area pharmacies near our clinic that are great at navigating these, and we really try to get our patients to go to places we know can give them assistance.”
A version of this story originally appeared on Medscape.com.
HPV vaccination remains below Healthy People goals despite increases
and vary widely across states based on data from a nested cohort study including more than 7 million children.
“Understanding regional and temporal variations in HPV vaccination coverage may help improve HPV vaccination uptake by informing public health policy,” Szu-Ta Chen, MD, of Harvard University, Boston, and colleagues wrote in Pediatrics.
To identify trends in one-dose and two-dose human papillomavirus (HPV) vaccination coverage, the researchers reviewed data from the MarketScan health care database between January 2003 and December 2017 that included 7,837,480 children and 19,843,737 person-years. The children were followed starting at age 9, when HPV vaccination could begin, and ending at one of the following: the first or second vaccination, insurance disenrollment, December 2017, or the end of the year in which they turned 17.
Overall, the proportion of 15-year-old girls and boys with at least a one-dose HPV vaccination increased from 38% and 5%, respectively, in 2011 to 57% and 51%, respectively, in 2017. The comparable proportions of girls and boys with at least a two-dose vaccination increased from 30% and 2%, respectively, in 2011 to 46% and 39%, respectively, in 2017.
Coverage lacks consistency across states
However, the vaccination coverage varied widely across states; two-dose HPV vaccination coverage ranged from 80% of girls in the District of Columbia to 15% of boys in Mississippi. In general, states with more HPV vaccine interventions had higher levels of vaccination, the researchers noted.
Legislation to improve vaccination education showed the strongest association with coverage; an 8.8% increase in coverage for girls and an 8.7% increase for boys. Pediatrician availability also was a factor associated with a 1.1% increase in coverage estimated for every pediatrician per 10,000 children.
Cumulative HPV vaccinations seen among children continuously enrolled in the study were similar to the primary analysis, the Dr. Chen and associates said. “After the initial HPV vaccination, 87% of girls and 82% of boys received a second dose by age 17 in the most recent cohorts.”
However, the HPV vaccination coverage remains below the Healthy People 2020 goal of 80% of children vaccinated by age 15 years, the researchers said. Barriers to vaccination may include a lack of routine clinical encounters in adolescents aged 11-17 years. HPV vaccination coverage was higher in urban populations, compared with rural, which may be related to a lack of providers in rural areas.
“Thus, measures beyond recommending routine vaccination at annual check-ups might be necessary to attain sufficient HPV vaccine coverage, and the optimal strategy may differ by state characteristics,” they wrote.
The study findings were limited by several factors including the use of data from only commercially-insured children and lack of data on vaccines received outside of insurance, the researchers noted.
However, the results were strengthened by the large, population-based sample, and support the need for increased efforts in HPV vaccination. “Most states will not achieve the Healthy People 2020 goal of 80% coverage with at least two HPV vaccine doses by 2020,” Dr. Chen and associates concluded.
Vaccination goals are possible with effort in the right places
The fact of below-target vaccination for HPV in the United States may be old news, but the current study offers new insights on HPV uptake, Amanda F. Dempsey, MD, PhD, of the University of Colorado at Denver, in Aurora, wrote in an accompanying editorial.
“A unique feature of this study is the ability of its researchers to study individuals over time, particularly at a national scope,” which yielded two key messages, she said.
The longitudinal examination of vaccination levels among birth cohorts showed that similar vaccination levels were achieved more quickly each year.
“For example, among the birth cohort from the year 2000, representing 17-year-olds at the time data were abstracted for the study, 40% vaccination coverage was achieved when this group was 14 years old. In contrast, among the birth cohort from the year 2005, representing 12-year-olds at the time of data abstraction, 40% vaccination coverage was reached at the age of 12,” Dr. Dempsey explained.
In addition, the study design allowed the researchers to model future vaccine coverage based on current trends, said Dr. Dempsey. “The authors estimate that, by the year 2022, the 2012 birth cohort will have reached 80% coverage for the first dose in the HPV vaccine series.”
Dr. Dempsey said she was surprised that the models did not support the hypothesis that school mandates for vaccination would increase coverage; however, there were few states in this category.
Although the findings were limited by the lack of data on uninsured children and those insured by Medicaid, the state-by-state results show that the achievement of national vaccination goals is possible, Dr. Dempsey said. In addition, the findings “warrant close consideration by policy makers and the medical community at large regarding vaccination policies and workforce,” she emphasized.The study received no outside funding. Dr. Chen had no financial conflicts to disclose. Several coauthors reported research grants to their institutions from pharmaceutical companies or being consultants to such companies. Dr. Dempsey disclosed serving on the advisory boards for Merck, Pfizer, and Sanofi Pasteur.
SOURCE: Chen S-T et al. Pediatrics. 2020 Sep 14. doi: 10.1542/peds.2019-3557.
and vary widely across states based on data from a nested cohort study including more than 7 million children.
“Understanding regional and temporal variations in HPV vaccination coverage may help improve HPV vaccination uptake by informing public health policy,” Szu-Ta Chen, MD, of Harvard University, Boston, and colleagues wrote in Pediatrics.
To identify trends in one-dose and two-dose human papillomavirus (HPV) vaccination coverage, the researchers reviewed data from the MarketScan health care database between January 2003 and December 2017 that included 7,837,480 children and 19,843,737 person-years. The children were followed starting at age 9, when HPV vaccination could begin, and ending at one of the following: the first or second vaccination, insurance disenrollment, December 2017, or the end of the year in which they turned 17.
Overall, the proportion of 15-year-old girls and boys with at least a one-dose HPV vaccination increased from 38% and 5%, respectively, in 2011 to 57% and 51%, respectively, in 2017. The comparable proportions of girls and boys with at least a two-dose vaccination increased from 30% and 2%, respectively, in 2011 to 46% and 39%, respectively, in 2017.
Coverage lacks consistency across states
However, the vaccination coverage varied widely across states; two-dose HPV vaccination coverage ranged from 80% of girls in the District of Columbia to 15% of boys in Mississippi. In general, states with more HPV vaccine interventions had higher levels of vaccination, the researchers noted.
Legislation to improve vaccination education showed the strongest association with coverage; an 8.8% increase in coverage for girls and an 8.7% increase for boys. Pediatrician availability also was a factor associated with a 1.1% increase in coverage estimated for every pediatrician per 10,000 children.
Cumulative HPV vaccinations seen among children continuously enrolled in the study were similar to the primary analysis, the Dr. Chen and associates said. “After the initial HPV vaccination, 87% of girls and 82% of boys received a second dose by age 17 in the most recent cohorts.”
However, the HPV vaccination coverage remains below the Healthy People 2020 goal of 80% of children vaccinated by age 15 years, the researchers said. Barriers to vaccination may include a lack of routine clinical encounters in adolescents aged 11-17 years. HPV vaccination coverage was higher in urban populations, compared with rural, which may be related to a lack of providers in rural areas.
“Thus, measures beyond recommending routine vaccination at annual check-ups might be necessary to attain sufficient HPV vaccine coverage, and the optimal strategy may differ by state characteristics,” they wrote.
The study findings were limited by several factors including the use of data from only commercially-insured children and lack of data on vaccines received outside of insurance, the researchers noted.
However, the results were strengthened by the large, population-based sample, and support the need for increased efforts in HPV vaccination. “Most states will not achieve the Healthy People 2020 goal of 80% coverage with at least two HPV vaccine doses by 2020,” Dr. Chen and associates concluded.
Vaccination goals are possible with effort in the right places
The fact of below-target vaccination for HPV in the United States may be old news, but the current study offers new insights on HPV uptake, Amanda F. Dempsey, MD, PhD, of the University of Colorado at Denver, in Aurora, wrote in an accompanying editorial.
“A unique feature of this study is the ability of its researchers to study individuals over time, particularly at a national scope,” which yielded two key messages, she said.
The longitudinal examination of vaccination levels among birth cohorts showed that similar vaccination levels were achieved more quickly each year.
“For example, among the birth cohort from the year 2000, representing 17-year-olds at the time data were abstracted for the study, 40% vaccination coverage was achieved when this group was 14 years old. In contrast, among the birth cohort from the year 2005, representing 12-year-olds at the time of data abstraction, 40% vaccination coverage was reached at the age of 12,” Dr. Dempsey explained.
In addition, the study design allowed the researchers to model future vaccine coverage based on current trends, said Dr. Dempsey. “The authors estimate that, by the year 2022, the 2012 birth cohort will have reached 80% coverage for the first dose in the HPV vaccine series.”
Dr. Dempsey said she was surprised that the models did not support the hypothesis that school mandates for vaccination would increase coverage; however, there were few states in this category.
Although the findings were limited by the lack of data on uninsured children and those insured by Medicaid, the state-by-state results show that the achievement of national vaccination goals is possible, Dr. Dempsey said. In addition, the findings “warrant close consideration by policy makers and the medical community at large regarding vaccination policies and workforce,” she emphasized.The study received no outside funding. Dr. Chen had no financial conflicts to disclose. Several coauthors reported research grants to their institutions from pharmaceutical companies or being consultants to such companies. Dr. Dempsey disclosed serving on the advisory boards for Merck, Pfizer, and Sanofi Pasteur.
SOURCE: Chen S-T et al. Pediatrics. 2020 Sep 14. doi: 10.1542/peds.2019-3557.
and vary widely across states based on data from a nested cohort study including more than 7 million children.
“Understanding regional and temporal variations in HPV vaccination coverage may help improve HPV vaccination uptake by informing public health policy,” Szu-Ta Chen, MD, of Harvard University, Boston, and colleagues wrote in Pediatrics.
To identify trends in one-dose and two-dose human papillomavirus (HPV) vaccination coverage, the researchers reviewed data from the MarketScan health care database between January 2003 and December 2017 that included 7,837,480 children and 19,843,737 person-years. The children were followed starting at age 9, when HPV vaccination could begin, and ending at one of the following: the first or second vaccination, insurance disenrollment, December 2017, or the end of the year in which they turned 17.
Overall, the proportion of 15-year-old girls and boys with at least a one-dose HPV vaccination increased from 38% and 5%, respectively, in 2011 to 57% and 51%, respectively, in 2017. The comparable proportions of girls and boys with at least a two-dose vaccination increased from 30% and 2%, respectively, in 2011 to 46% and 39%, respectively, in 2017.
Coverage lacks consistency across states
However, the vaccination coverage varied widely across states; two-dose HPV vaccination coverage ranged from 80% of girls in the District of Columbia to 15% of boys in Mississippi. In general, states with more HPV vaccine interventions had higher levels of vaccination, the researchers noted.
Legislation to improve vaccination education showed the strongest association with coverage; an 8.8% increase in coverage for girls and an 8.7% increase for boys. Pediatrician availability also was a factor associated with a 1.1% increase in coverage estimated for every pediatrician per 10,000 children.
Cumulative HPV vaccinations seen among children continuously enrolled in the study were similar to the primary analysis, the Dr. Chen and associates said. “After the initial HPV vaccination, 87% of girls and 82% of boys received a second dose by age 17 in the most recent cohorts.”
However, the HPV vaccination coverage remains below the Healthy People 2020 goal of 80% of children vaccinated by age 15 years, the researchers said. Barriers to vaccination may include a lack of routine clinical encounters in adolescents aged 11-17 years. HPV vaccination coverage was higher in urban populations, compared with rural, which may be related to a lack of providers in rural areas.
“Thus, measures beyond recommending routine vaccination at annual check-ups might be necessary to attain sufficient HPV vaccine coverage, and the optimal strategy may differ by state characteristics,” they wrote.
The study findings were limited by several factors including the use of data from only commercially-insured children and lack of data on vaccines received outside of insurance, the researchers noted.
However, the results were strengthened by the large, population-based sample, and support the need for increased efforts in HPV vaccination. “Most states will not achieve the Healthy People 2020 goal of 80% coverage with at least two HPV vaccine doses by 2020,” Dr. Chen and associates concluded.
Vaccination goals are possible with effort in the right places
The fact of below-target vaccination for HPV in the United States may be old news, but the current study offers new insights on HPV uptake, Amanda F. Dempsey, MD, PhD, of the University of Colorado at Denver, in Aurora, wrote in an accompanying editorial.
“A unique feature of this study is the ability of its researchers to study individuals over time, particularly at a national scope,” which yielded two key messages, she said.
The longitudinal examination of vaccination levels among birth cohorts showed that similar vaccination levels were achieved more quickly each year.
“For example, among the birth cohort from the year 2000, representing 17-year-olds at the time data were abstracted for the study, 40% vaccination coverage was achieved when this group was 14 years old. In contrast, among the birth cohort from the year 2005, representing 12-year-olds at the time of data abstraction, 40% vaccination coverage was reached at the age of 12,” Dr. Dempsey explained.
In addition, the study design allowed the researchers to model future vaccine coverage based on current trends, said Dr. Dempsey. “The authors estimate that, by the year 2022, the 2012 birth cohort will have reached 80% coverage for the first dose in the HPV vaccine series.”
Dr. Dempsey said she was surprised that the models did not support the hypothesis that school mandates for vaccination would increase coverage; however, there were few states in this category.
Although the findings were limited by the lack of data on uninsured children and those insured by Medicaid, the state-by-state results show that the achievement of national vaccination goals is possible, Dr. Dempsey said. In addition, the findings “warrant close consideration by policy makers and the medical community at large regarding vaccination policies and workforce,” she emphasized.The study received no outside funding. Dr. Chen had no financial conflicts to disclose. Several coauthors reported research grants to their institutions from pharmaceutical companies or being consultants to such companies. Dr. Dempsey disclosed serving on the advisory boards for Merck, Pfizer, and Sanofi Pasteur.
SOURCE: Chen S-T et al. Pediatrics. 2020 Sep 14. doi: 10.1542/peds.2019-3557.
FROM PEDIATRICS
ID dermatology: Advancements, but new challenges, over 50 years
When Stephen Tyring, MD, PhD, an infectious disease dermatologist, started his career in the early 1980s, he said “we were diagnosing Kaposi’s sarcoma right and left. We would see a new case every day or two.”
It was the early days of the HIV/AIDS epidemic, and dermatologists were at the forefront because HIV/AIDS often presented with skin manifestations. Dr. Tyring, clinical professor in the departments of dermatology, microbiology & molecular genetics and internal medicine at the University of Texas Health Science Center, Houston, and his colleagues referred Kaposi’s patients for chemotherapy and radiation, but the outlook was often grim, especially if lesions developed in the lungs.
Dermatologist don’t see much Kaposi’s anymore because of highly effective treatments for HIV.
Members of the original editorial advisory board saw it coming. In a feature in which board members provided their prediction for the 1970s that appeared in the first issue, New York dermatologist Norman Orentreich, MD, counted the “probable introduction of virucidal agents” as one of the “significant advances or changes that I foresee in the next 10 years.” J. Lamar Callaway, MD, professor of dermatology at Duke University, Durham, N.C., predicted that “the next 10 years should develop effective anti-viral agents for warts, herpes simplex, and herpes zoster.”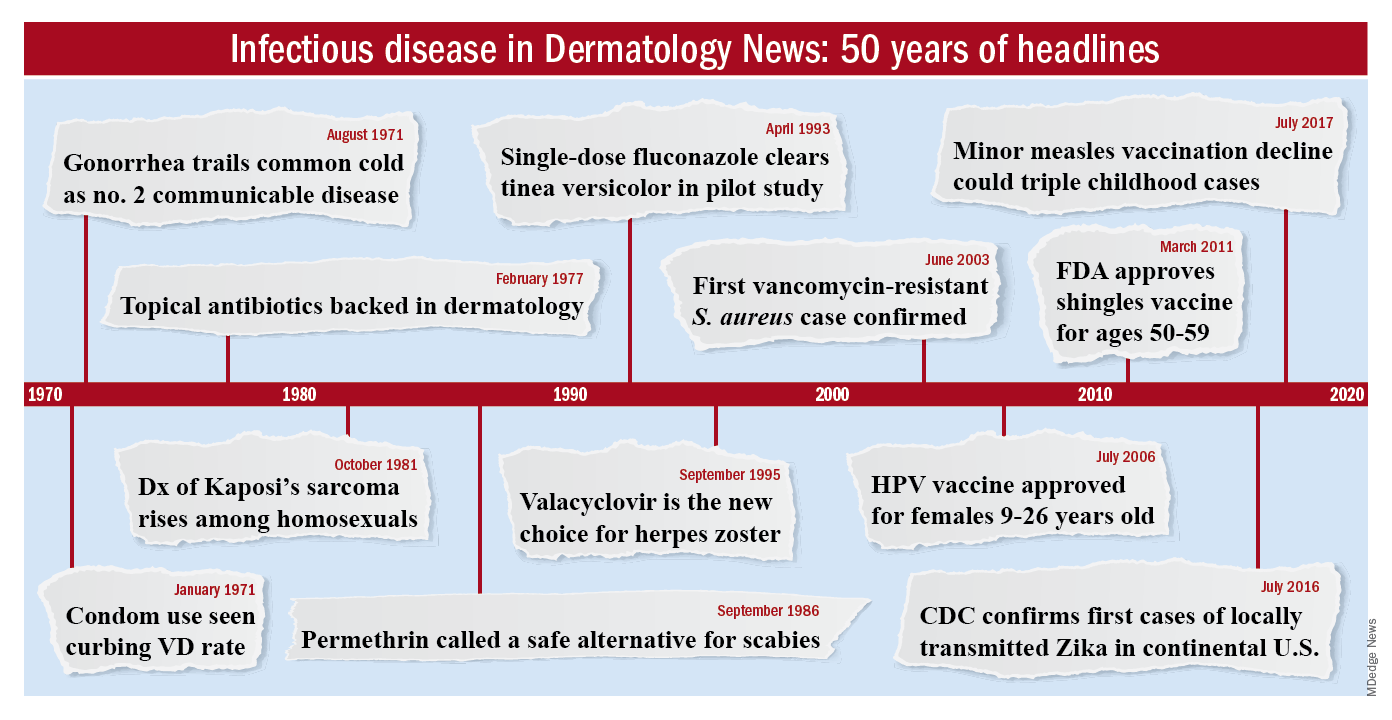
To celebrate the 50th anniversary of Dermatology News, we are looking back at how the field has changed since that first issue. The focus this month is infectious disease. There’s a lot to be grateful for but there are also challenges like antibiotic resistance that weren’t on the radar screens of Dr. Orentreich, Dr. Callaway, and their peers in 1970.
All in all, “the only thing I wish we did the old way is sit at the bedside and talk to patients more. We rely so much on technology now that we sometimes lose the art of medicine, which is comforting to the patient,” said Theodore Rosen, MD, an ID dermatologist and professor of dermatology at Baylor College of Medicine, Houston, who’s been in practice for 42 years.
“A lot of advancements against herpes viruses”
One of the biggest wins for ID dermatology over the last 5 decades has been the management of herpes, both herpes simplex virus 1 and 2, as well as herpes zoster virus. It started with the approval of acyclovir in 1981. Before then, “we had no direct therapy for genital herpes, herpes zoster, or disseminated herpes in immunosuppressed or cancer patients,” Dr. Rosen said.
“I can remember doing an interview with Good Morning America when I gave the first IV dose of acyclovir in the city of Houston for really bad disseminated herpes” in an HIV patient, he said, and it worked.
Two derivatives, valacyclovir and famciclovir, became available in the mid-1990s, so today “we have three drugs and some others at the periphery that are all highly effective not only” against herpes, but also for preventing outbreaks; valacyclovir can even prevent asymptomatic shedding, therefore possibly preventing new infections. “That’s a concept we didn’t even have 40 years ago,” Dr. Rosen said.
Cidofovir has also made a difference. The IV formulation was approved for AIDS-associated cytomegalovirus retinitis in 1996 but discontinued a few years later amid concerns of severe renal toxicity. It’s found a new home in dermatology since then, explained ID dermatologist Carrie Kovarik, MD, associate professor of dermatology at the University of Pennsylvania, Philadelphia.
Dermatologists see acyclovir-resistant herpes “heaped up on the genitals in HIV patients,” and there weren’t many options in the past. A few years ago, “we [tried] injecting cidofovir directly into the skin lesions, and it’s been remarkably successful. It is a good way to treat these lesions” if dermatologists can get it compounded, she said.
Shingles vaccines, first the live attenuated zoster vaccine (Zostavax) approved by the Food and Drug Administration in 2006 and the more effective recombinant zoster vaccine (Shingrix) approved in 2017, have also had a significant impact.
Dr. Rosen remembers what it was like when he first started practicing over 40 years ago. Not uncommonly, “we saw horrible cases of shingles,” including one in his uncle, who was left with permanent hand pain long after the rash subsided.
Today, “I see much less shingles, and when I do see it, it’s in a much-attenuated form. [Shingrix], even if it doesn’t prevent the disease, often prevents postherpetic neuralgia,” he said.
Also, with pediatric vaccinations against chicken pox, “we’re probably going to see a whole new generation without shingles, which is huge. We’ve made a lot of advancements against herpes viruses,” Dr. Kovarik said.
“We finally found something that helps”
“We’ve [also] come a really long way with genital wart treatment,” Dr. Kovarik said.
It started with approval of topical imiquimod in 1997. “Before that, we were just killing one wart here and one wart there” but they would often come back and pop up in other areas. Injectable interferon was an option at the time, but people didn’t like all the needles.
With imiquimod, “we finally [had] a way to target HPV [human papillomavirus] and not just scrape” or freeze one wart at a time, and “we were able to generate an inflammatory response in the whole area to clear the virus.” Working with HIV patients, “I see sheets and sheets of confluent warts throughout the whole genital area; to try to freeze that is impossible. Now I have a way to get rid of [genital] warts and keep them away even if you have a big cluster,” she said.
“Sometimes, we’ll do both liquid nitrogen and imiquimod. That’s a good way to tackle people who have a high burden of warts,” Dr. Kovarik noted. Other effective treatments have come out as well, including an ointment formulation of sinecatechins, extracted from green tea, “but you have to put it on several times a day, and insurance companies don’t cover it often,” she said.
Intralesional cidofovir is also proving to be boon for potentially malignant refractory warts in HIV and transplant patients. “It’s an incredible treatment. We can inject that antiviral into warts and get rid of them. We finally found something that helps” these people, Dr. Kovarik said.
The HPV vaccine Gardasil is making a difference, as well. In addition to cervical dysplasia and anogenital cancers, it protects against two condyloma strains. Dr. Rosen said he’s seeing fewer cases of genital warts now than when he started practicing, likely because of the vaccine.
“Organisms that weren’t pathogens are now pathogens”
Antibiotic resistance probably tops the list for what’s changed in a bad way in ID dermatology since 1970. Dr. Rosen remembers at the start of his career that “we never worried about antibiotic resistance. We’d put people on antibiotics for acne, rosacea, and we’d keep them on them for 3 years, 6 years”; resistance wasn’t on the radar screen and was not mentioned once in the first issue of Dermatology News, which was packed with articles and ran 24 pages.
The situation is different now. Driven by decades of overuse in agriculture and the medical system, antibiotic resistance is a concern throughout medicine, and unfortunately, “we have not come nearly as far as fast with antibiotics,” at least the ones dermatologists use, “as we have with antivirals,” Dr. Tyring said.
For instance, methicillin-resistant Staphylococcus aureus (MRSA), first described in the United States in 1968, is “no longer the exception to the rule, but the rule” itself, he said, with carbuncles, furuncles, and abscesses not infrequently growing out MRSA. There are also new drug-resistant forms of old problems like gonorrhea and tuberculosis, among other developments, and impetigo has shifted since 1970 from mostly a Streptococcus infection easily treated with penicillin to often a Staphylococcus disease that’s resistant to it. There’s also been a steady march of new pathogens, including the latest one, SARS-CoV-2, the virus that causes COVID-19, which has been recognized as having a variety of skin manifestations.
“No matter how smart we think we are, nature has a way of putting us back in our place,” Dr. Rosen said.
The bright spot is that “we’ve become very adept at identifying and characterizing” microbes “based on techniques we didn’t even have when I started practicing,” such as polymerase chain reaction. “It has taken a lot of guess work out of treating infectious diseases,” he said.
The widespread use of immunosuppressives such as cyclophosphamide, mycophenolate, azathioprine, rituximab, and other agents used in conjunction with solid organ transplantation, has also been a challenge. “We are seeing infections with really odd organisms. Just recently, I had a patient with fusarium in the skin; it’s a fungus that lives in the dirt. I saw a patient with a species of algae” that normally lives in stagnant water, he commented. “We used to get [things like that] back on reports, and we’d throw them away. You can’t do that anymore. Organisms that weren’t pathogens in the past are now pathogens,” particularly in immunosuppressed people, Dr. Rosen said.
Venereologists no more
There’s been another big change in the field. “Back in the not too distant past, dermatologists in the U.S. were referred to as ‘dermatologist-venereologists.’ ” It goes back to the time when syphilis wasn’t diagnosed and treated early, so patients often presented with secondary skin complications and went to dermatologists for help. As a result, “dermatologists became the most experienced at treating it,” Dr. Tyring said.
That’s faded from practice. Part of the reason is that as late as 2000, syphilis seemed to be on the way out; the Centers for Disease and Control and Prevention even raised the possibility of elimination. Dermatologists turned their attention to other areas.
It might have been short-sighted, Dr. Rosen said. Syphilis has made a strong comeback, and drug-resistant gonorrhea has also emerged globally and in at least a few states. No other medical field has stepped in to take up the slack. “Ob.gyns. are busy delivering babies, ID [physicians are] concerned about HIV, and urologists are worried about kidney stones and cancer.” Other than herpes and genital warts, “we have not done well” with management of sexually transmitted diseases, he said.
“I could sense” his frustration
The first issue of Dermatology News carried an article and photospread about scabies that could run today, except that topical permethrin and oral ivermectin have largely replaced benzyl benzoate and sulfur ointments for treatment in the United States. In the article, Scottish dermatologist J. O’D. Alexander, MD, called scabies “the scourge of mankind” and blamed it’s prevalence on “an offhand attitude to the disease which makes control very difficult.”
“I could sense this man’s frustration that people were not recognizing scabies,” Dr. Kovarik said, and it’s no closer to being eradicated than it was in 1970. “It’s still around, and we see it in our clinics. It’s a horrible disease in kids we see in dermatology not infrequently,” and treatment has only advanced a bit.
The article highlights what hasn’t changed much in ID dermatology over the years. Common warts are another one. “With all the evolution in medicine, we don’t have any better treatments approved for common warts than we ever had.” Injecting cidofovir “works great,” but access is a problem, Dr. Tyring said.
Onychomycosis has also proven a tough nut to crack. Readers back in 1970 counted the introduction of the antifungal, griseofulvin, as a major advancement in the 1960s; it’s still a go-to for tinea capitis, but it didn’t work very well for toenail fungus. Terbinafine (Lamisil), approved in 1993, and subsequent developments have helped, but the field still awaits more effective options; a few potential new agents are in the pipeline.
Although there have been major advancements for serious systemic fungal infections, “we’ve mainly seen small steps forward” in ID dermatology, Dr. Tyring said.
Dr. Tyring, Dr. Kovarik, and Dr. Rosen said they had no relevant disclosures.
When Stephen Tyring, MD, PhD, an infectious disease dermatologist, started his career in the early 1980s, he said “we were diagnosing Kaposi’s sarcoma right and left. We would see a new case every day or two.”
It was the early days of the HIV/AIDS epidemic, and dermatologists were at the forefront because HIV/AIDS often presented with skin manifestations. Dr. Tyring, clinical professor in the departments of dermatology, microbiology & molecular genetics and internal medicine at the University of Texas Health Science Center, Houston, and his colleagues referred Kaposi’s patients for chemotherapy and radiation, but the outlook was often grim, especially if lesions developed in the lungs.
Dermatologist don’t see much Kaposi’s anymore because of highly effective treatments for HIV.
Members of the original editorial advisory board saw it coming. In a feature in which board members provided their prediction for the 1970s that appeared in the first issue, New York dermatologist Norman Orentreich, MD, counted the “probable introduction of virucidal agents” as one of the “significant advances or changes that I foresee in the next 10 years.” J. Lamar Callaway, MD, professor of dermatology at Duke University, Durham, N.C., predicted that “the next 10 years should develop effective anti-viral agents for warts, herpes simplex, and herpes zoster.”
To celebrate the 50th anniversary of Dermatology News, we are looking back at how the field has changed since that first issue. The focus this month is infectious disease. There’s a lot to be grateful for but there are also challenges like antibiotic resistance that weren’t on the radar screens of Dr. Orentreich, Dr. Callaway, and their peers in 1970.
All in all, “the only thing I wish we did the old way is sit at the bedside and talk to patients more. We rely so much on technology now that we sometimes lose the art of medicine, which is comforting to the patient,” said Theodore Rosen, MD, an ID dermatologist and professor of dermatology at Baylor College of Medicine, Houston, who’s been in practice for 42 years.
“A lot of advancements against herpes viruses”
One of the biggest wins for ID dermatology over the last 5 decades has been the management of herpes, both herpes simplex virus 1 and 2, as well as herpes zoster virus. It started with the approval of acyclovir in 1981. Before then, “we had no direct therapy for genital herpes, herpes zoster, or disseminated herpes in immunosuppressed or cancer patients,” Dr. Rosen said.
“I can remember doing an interview with Good Morning America when I gave the first IV dose of acyclovir in the city of Houston for really bad disseminated herpes” in an HIV patient, he said, and it worked.
Two derivatives, valacyclovir and famciclovir, became available in the mid-1990s, so today “we have three drugs and some others at the periphery that are all highly effective not only” against herpes, but also for preventing outbreaks; valacyclovir can even prevent asymptomatic shedding, therefore possibly preventing new infections. “That’s a concept we didn’t even have 40 years ago,” Dr. Rosen said.
Cidofovir has also made a difference. The IV formulation was approved for AIDS-associated cytomegalovirus retinitis in 1996 but discontinued a few years later amid concerns of severe renal toxicity. It’s found a new home in dermatology since then, explained ID dermatologist Carrie Kovarik, MD, associate professor of dermatology at the University of Pennsylvania, Philadelphia.
Dermatologists see acyclovir-resistant herpes “heaped up on the genitals in HIV patients,” and there weren’t many options in the past. A few years ago, “we [tried] injecting cidofovir directly into the skin lesions, and it’s been remarkably successful. It is a good way to treat these lesions” if dermatologists can get it compounded, she said.
Shingles vaccines, first the live attenuated zoster vaccine (Zostavax) approved by the Food and Drug Administration in 2006 and the more effective recombinant zoster vaccine (Shingrix) approved in 2017, have also had a significant impact.
Dr. Rosen remembers what it was like when he first started practicing over 40 years ago. Not uncommonly, “we saw horrible cases of shingles,” including one in his uncle, who was left with permanent hand pain long after the rash subsided.
Today, “I see much less shingles, and when I do see it, it’s in a much-attenuated form. [Shingrix], even if it doesn’t prevent the disease, often prevents postherpetic neuralgia,” he said.
Also, with pediatric vaccinations against chicken pox, “we’re probably going to see a whole new generation without shingles, which is huge. We’ve made a lot of advancements against herpes viruses,” Dr. Kovarik said.
“We finally found something that helps”
“We’ve [also] come a really long way with genital wart treatment,” Dr. Kovarik said.
It started with approval of topical imiquimod in 1997. “Before that, we were just killing one wart here and one wart there” but they would often come back and pop up in other areas. Injectable interferon was an option at the time, but people didn’t like all the needles.
With imiquimod, “we finally [had] a way to target HPV [human papillomavirus] and not just scrape” or freeze one wart at a time, and “we were able to generate an inflammatory response in the whole area to clear the virus.” Working with HIV patients, “I see sheets and sheets of confluent warts throughout the whole genital area; to try to freeze that is impossible. Now I have a way to get rid of [genital] warts and keep them away even if you have a big cluster,” she said.
“Sometimes, we’ll do both liquid nitrogen and imiquimod. That’s a good way to tackle people who have a high burden of warts,” Dr. Kovarik noted. Other effective treatments have come out as well, including an ointment formulation of sinecatechins, extracted from green tea, “but you have to put it on several times a day, and insurance companies don’t cover it often,” she said.
Intralesional cidofovir is also proving to be boon for potentially malignant refractory warts in HIV and transplant patients. “It’s an incredible treatment. We can inject that antiviral into warts and get rid of them. We finally found something that helps” these people, Dr. Kovarik said.
The HPV vaccine Gardasil is making a difference, as well. In addition to cervical dysplasia and anogenital cancers, it protects against two condyloma strains. Dr. Rosen said he’s seeing fewer cases of genital warts now than when he started practicing, likely because of the vaccine.
“Organisms that weren’t pathogens are now pathogens”
Antibiotic resistance probably tops the list for what’s changed in a bad way in ID dermatology since 1970. Dr. Rosen remembers at the start of his career that “we never worried about antibiotic resistance. We’d put people on antibiotics for acne, rosacea, and we’d keep them on them for 3 years, 6 years”; resistance wasn’t on the radar screen and was not mentioned once in the first issue of Dermatology News, which was packed with articles and ran 24 pages.
The situation is different now. Driven by decades of overuse in agriculture and the medical system, antibiotic resistance is a concern throughout medicine, and unfortunately, “we have not come nearly as far as fast with antibiotics,” at least the ones dermatologists use, “as we have with antivirals,” Dr. Tyring said.
For instance, methicillin-resistant Staphylococcus aureus (MRSA), first described in the United States in 1968, is “no longer the exception to the rule, but the rule” itself, he said, with carbuncles, furuncles, and abscesses not infrequently growing out MRSA. There are also new drug-resistant forms of old problems like gonorrhea and tuberculosis, among other developments, and impetigo has shifted since 1970 from mostly a Streptococcus infection easily treated with penicillin to often a Staphylococcus disease that’s resistant to it. There’s also been a steady march of new pathogens, including the latest one, SARS-CoV-2, the virus that causes COVID-19, which has been recognized as having a variety of skin manifestations.
“No matter how smart we think we are, nature has a way of putting us back in our place,” Dr. Rosen said.
The bright spot is that “we’ve become very adept at identifying and characterizing” microbes “based on techniques we didn’t even have when I started practicing,” such as polymerase chain reaction. “It has taken a lot of guess work out of treating infectious diseases,” he said.
The widespread use of immunosuppressives such as cyclophosphamide, mycophenolate, azathioprine, rituximab, and other agents used in conjunction with solid organ transplantation, has also been a challenge. “We are seeing infections with really odd organisms. Just recently, I had a patient with fusarium in the skin; it’s a fungus that lives in the dirt. I saw a patient with a species of algae” that normally lives in stagnant water, he commented. “We used to get [things like that] back on reports, and we’d throw them away. You can’t do that anymore. Organisms that weren’t pathogens in the past are now pathogens,” particularly in immunosuppressed people, Dr. Rosen said.
Venereologists no more
There’s been another big change in the field. “Back in the not too distant past, dermatologists in the U.S. were referred to as ‘dermatologist-venereologists.’ ” It goes back to the time when syphilis wasn’t diagnosed and treated early, so patients often presented with secondary skin complications and went to dermatologists for help. As a result, “dermatologists became the most experienced at treating it,” Dr. Tyring said.
That’s faded from practice. Part of the reason is that as late as 2000, syphilis seemed to be on the way out; the Centers for Disease and Control and Prevention even raised the possibility of elimination. Dermatologists turned their attention to other areas.
It might have been short-sighted, Dr. Rosen said. Syphilis has made a strong comeback, and drug-resistant gonorrhea has also emerged globally and in at least a few states. No other medical field has stepped in to take up the slack. “Ob.gyns. are busy delivering babies, ID [physicians are] concerned about HIV, and urologists are worried about kidney stones and cancer.” Other than herpes and genital warts, “we have not done well” with management of sexually transmitted diseases, he said.
“I could sense” his frustration
The first issue of Dermatology News carried an article and photospread about scabies that could run today, except that topical permethrin and oral ivermectin have largely replaced benzyl benzoate and sulfur ointments for treatment in the United States. In the article, Scottish dermatologist J. O’D. Alexander, MD, called scabies “the scourge of mankind” and blamed it’s prevalence on “an offhand attitude to the disease which makes control very difficult.”
“I could sense this man’s frustration that people were not recognizing scabies,” Dr. Kovarik said, and it’s no closer to being eradicated than it was in 1970. “It’s still around, and we see it in our clinics. It’s a horrible disease in kids we see in dermatology not infrequently,” and treatment has only advanced a bit.
The article highlights what hasn’t changed much in ID dermatology over the years. Common warts are another one. “With all the evolution in medicine, we don’t have any better treatments approved for common warts than we ever had.” Injecting cidofovir “works great,” but access is a problem, Dr. Tyring said.
Onychomycosis has also proven a tough nut to crack. Readers back in 1970 counted the introduction of the antifungal, griseofulvin, as a major advancement in the 1960s; it’s still a go-to for tinea capitis, but it didn’t work very well for toenail fungus. Terbinafine (Lamisil), approved in 1993, and subsequent developments have helped, but the field still awaits more effective options; a few potential new agents are in the pipeline.
Although there have been major advancements for serious systemic fungal infections, “we’ve mainly seen small steps forward” in ID dermatology, Dr. Tyring said.
Dr. Tyring, Dr. Kovarik, and Dr. Rosen said they had no relevant disclosures.
When Stephen Tyring, MD, PhD, an infectious disease dermatologist, started his career in the early 1980s, he said “we were diagnosing Kaposi’s sarcoma right and left. We would see a new case every day or two.”
It was the early days of the HIV/AIDS epidemic, and dermatologists were at the forefront because HIV/AIDS often presented with skin manifestations. Dr. Tyring, clinical professor in the departments of dermatology, microbiology & molecular genetics and internal medicine at the University of Texas Health Science Center, Houston, and his colleagues referred Kaposi’s patients for chemotherapy and radiation, but the outlook was often grim, especially if lesions developed in the lungs.
Dermatologist don’t see much Kaposi’s anymore because of highly effective treatments for HIV.
Members of the original editorial advisory board saw it coming. In a feature in which board members provided their prediction for the 1970s that appeared in the first issue, New York dermatologist Norman Orentreich, MD, counted the “probable introduction of virucidal agents” as one of the “significant advances or changes that I foresee in the next 10 years.” J. Lamar Callaway, MD, professor of dermatology at Duke University, Durham, N.C., predicted that “the next 10 years should develop effective anti-viral agents for warts, herpes simplex, and herpes zoster.”
To celebrate the 50th anniversary of Dermatology News, we are looking back at how the field has changed since that first issue. The focus this month is infectious disease. There’s a lot to be grateful for but there are also challenges like antibiotic resistance that weren’t on the radar screens of Dr. Orentreich, Dr. Callaway, and their peers in 1970.
All in all, “the only thing I wish we did the old way is sit at the bedside and talk to patients more. We rely so much on technology now that we sometimes lose the art of medicine, which is comforting to the patient,” said Theodore Rosen, MD, an ID dermatologist and professor of dermatology at Baylor College of Medicine, Houston, who’s been in practice for 42 years.
“A lot of advancements against herpes viruses”
One of the biggest wins for ID dermatology over the last 5 decades has been the management of herpes, both herpes simplex virus 1 and 2, as well as herpes zoster virus. It started with the approval of acyclovir in 1981. Before then, “we had no direct therapy for genital herpes, herpes zoster, or disseminated herpes in immunosuppressed or cancer patients,” Dr. Rosen said.
“I can remember doing an interview with Good Morning America when I gave the first IV dose of acyclovir in the city of Houston for really bad disseminated herpes” in an HIV patient, he said, and it worked.
Two derivatives, valacyclovir and famciclovir, became available in the mid-1990s, so today “we have three drugs and some others at the periphery that are all highly effective not only” against herpes, but also for preventing outbreaks; valacyclovir can even prevent asymptomatic shedding, therefore possibly preventing new infections. “That’s a concept we didn’t even have 40 years ago,” Dr. Rosen said.
Cidofovir has also made a difference. The IV formulation was approved for AIDS-associated cytomegalovirus retinitis in 1996 but discontinued a few years later amid concerns of severe renal toxicity. It’s found a new home in dermatology since then, explained ID dermatologist Carrie Kovarik, MD, associate professor of dermatology at the University of Pennsylvania, Philadelphia.
Dermatologists see acyclovir-resistant herpes “heaped up on the genitals in HIV patients,” and there weren’t many options in the past. A few years ago, “we [tried] injecting cidofovir directly into the skin lesions, and it’s been remarkably successful. It is a good way to treat these lesions” if dermatologists can get it compounded, she said.
Shingles vaccines, first the live attenuated zoster vaccine (Zostavax) approved by the Food and Drug Administration in 2006 and the more effective recombinant zoster vaccine (Shingrix) approved in 2017, have also had a significant impact.
Dr. Rosen remembers what it was like when he first started practicing over 40 years ago. Not uncommonly, “we saw horrible cases of shingles,” including one in his uncle, who was left with permanent hand pain long after the rash subsided.
Today, “I see much less shingles, and when I do see it, it’s in a much-attenuated form. [Shingrix], even if it doesn’t prevent the disease, often prevents postherpetic neuralgia,” he said.
Also, with pediatric vaccinations against chicken pox, “we’re probably going to see a whole new generation without shingles, which is huge. We’ve made a lot of advancements against herpes viruses,” Dr. Kovarik said.
“We finally found something that helps”
“We’ve [also] come a really long way with genital wart treatment,” Dr. Kovarik said.
It started with approval of topical imiquimod in 1997. “Before that, we were just killing one wart here and one wart there” but they would often come back and pop up in other areas. Injectable interferon was an option at the time, but people didn’t like all the needles.
With imiquimod, “we finally [had] a way to target HPV [human papillomavirus] and not just scrape” or freeze one wart at a time, and “we were able to generate an inflammatory response in the whole area to clear the virus.” Working with HIV patients, “I see sheets and sheets of confluent warts throughout the whole genital area; to try to freeze that is impossible. Now I have a way to get rid of [genital] warts and keep them away even if you have a big cluster,” she said.
“Sometimes, we’ll do both liquid nitrogen and imiquimod. That’s a good way to tackle people who have a high burden of warts,” Dr. Kovarik noted. Other effective treatments have come out as well, including an ointment formulation of sinecatechins, extracted from green tea, “but you have to put it on several times a day, and insurance companies don’t cover it often,” she said.
Intralesional cidofovir is also proving to be boon for potentially malignant refractory warts in HIV and transplant patients. “It’s an incredible treatment. We can inject that antiviral into warts and get rid of them. We finally found something that helps” these people, Dr. Kovarik said.
The HPV vaccine Gardasil is making a difference, as well. In addition to cervical dysplasia and anogenital cancers, it protects against two condyloma strains. Dr. Rosen said he’s seeing fewer cases of genital warts now than when he started practicing, likely because of the vaccine.
“Organisms that weren’t pathogens are now pathogens”
Antibiotic resistance probably tops the list for what’s changed in a bad way in ID dermatology since 1970. Dr. Rosen remembers at the start of his career that “we never worried about antibiotic resistance. We’d put people on antibiotics for acne, rosacea, and we’d keep them on them for 3 years, 6 years”; resistance wasn’t on the radar screen and was not mentioned once in the first issue of Dermatology News, which was packed with articles and ran 24 pages.
The situation is different now. Driven by decades of overuse in agriculture and the medical system, antibiotic resistance is a concern throughout medicine, and unfortunately, “we have not come nearly as far as fast with antibiotics,” at least the ones dermatologists use, “as we have with antivirals,” Dr. Tyring said.
For instance, methicillin-resistant Staphylococcus aureus (MRSA), first described in the United States in 1968, is “no longer the exception to the rule, but the rule” itself, he said, with carbuncles, furuncles, and abscesses not infrequently growing out MRSA. There are also new drug-resistant forms of old problems like gonorrhea and tuberculosis, among other developments, and impetigo has shifted since 1970 from mostly a Streptococcus infection easily treated with penicillin to often a Staphylococcus disease that’s resistant to it. There’s also been a steady march of new pathogens, including the latest one, SARS-CoV-2, the virus that causes COVID-19, which has been recognized as having a variety of skin manifestations.
“No matter how smart we think we are, nature has a way of putting us back in our place,” Dr. Rosen said.
The bright spot is that “we’ve become very adept at identifying and characterizing” microbes “based on techniques we didn’t even have when I started practicing,” such as polymerase chain reaction. “It has taken a lot of guess work out of treating infectious diseases,” he said.
The widespread use of immunosuppressives such as cyclophosphamide, mycophenolate, azathioprine, rituximab, and other agents used in conjunction with solid organ transplantation, has also been a challenge. “We are seeing infections with really odd organisms. Just recently, I had a patient with fusarium in the skin; it’s a fungus that lives in the dirt. I saw a patient with a species of algae” that normally lives in stagnant water, he commented. “We used to get [things like that] back on reports, and we’d throw them away. You can’t do that anymore. Organisms that weren’t pathogens in the past are now pathogens,” particularly in immunosuppressed people, Dr. Rosen said.
Venereologists no more
There’s been another big change in the field. “Back in the not too distant past, dermatologists in the U.S. were referred to as ‘dermatologist-venereologists.’ ” It goes back to the time when syphilis wasn’t diagnosed and treated early, so patients often presented with secondary skin complications and went to dermatologists for help. As a result, “dermatologists became the most experienced at treating it,” Dr. Tyring said.
That’s faded from practice. Part of the reason is that as late as 2000, syphilis seemed to be on the way out; the Centers for Disease and Control and Prevention even raised the possibility of elimination. Dermatologists turned their attention to other areas.
It might have been short-sighted, Dr. Rosen said. Syphilis has made a strong comeback, and drug-resistant gonorrhea has also emerged globally and in at least a few states. No other medical field has stepped in to take up the slack. “Ob.gyns. are busy delivering babies, ID [physicians are] concerned about HIV, and urologists are worried about kidney stones and cancer.” Other than herpes and genital warts, “we have not done well” with management of sexually transmitted diseases, he said.
“I could sense” his frustration
The first issue of Dermatology News carried an article and photospread about scabies that could run today, except that topical permethrin and oral ivermectin have largely replaced benzyl benzoate and sulfur ointments for treatment in the United States. In the article, Scottish dermatologist J. O’D. Alexander, MD, called scabies “the scourge of mankind” and blamed it’s prevalence on “an offhand attitude to the disease which makes control very difficult.”
“I could sense this man’s frustration that people were not recognizing scabies,” Dr. Kovarik said, and it’s no closer to being eradicated than it was in 1970. “It’s still around, and we see it in our clinics. It’s a horrible disease in kids we see in dermatology not infrequently,” and treatment has only advanced a bit.
The article highlights what hasn’t changed much in ID dermatology over the years. Common warts are another one. “With all the evolution in medicine, we don’t have any better treatments approved for common warts than we ever had.” Injecting cidofovir “works great,” but access is a problem, Dr. Tyring said.
Onychomycosis has also proven a tough nut to crack. Readers back in 1970 counted the introduction of the antifungal, griseofulvin, as a major advancement in the 1960s; it’s still a go-to for tinea capitis, but it didn’t work very well for toenail fungus. Terbinafine (Lamisil), approved in 1993, and subsequent developments have helped, but the field still awaits more effective options; a few potential new agents are in the pipeline.
Although there have been major advancements for serious systemic fungal infections, “we’ve mainly seen small steps forward” in ID dermatology, Dr. Tyring said.
Dr. Tyring, Dr. Kovarik, and Dr. Rosen said they had no relevant disclosures.
World first: Saliva test detects occult HPV-driven oropharyngeal cancer
A saliva test for detecting oropharyngeal cancer caused by human papillomavirus–16 (HPV-16) has scored a world first: It detected such a cancer in an asymptomatic adult.
If the finding can be replicated in a larger cohort of healthy asymptomatic individuals, widespread screening for HPV-16 – the main driver behind skyrocketing rates of oropharyngeal cancer – could be one step closer, the researchers suggested.
“Oropharyngeal squamous cell carcinomas often presents at a late stage with patients suffering huge morbidity as a result of treatment, [so] we must find strategies to detect these cancers earlier,” senior author Chamindie Punyadeera, PhD, Queensland University of Technology in Brisbane, Australia, told Medscape Medical News in an email.
“This study, for the first time, provides a solid scientific foundation to initiate a screening trial in high-risk individuals to detect HPV-driven oropharyngeal cancer, she added. “Saliva testing could be broadly implemented and [used] in a screening trial in the future,” she said.
The case report was published online March 31 in Frontiers in Oncology.
The saliva test was developed by Dr. Punyadeera and first author Kai Dun Tang, PhD, also from Queensland University of Technology. It is administered as an oral rinse: the individual swishes a saline solution around in his or her mouth for a minute or 2, and then spits the sample into a tube.
Prevalence Study
The saliva test was being scrutinized in an ongoing HPV-16 DNA prevalence study, which involved 650 healthy participants being tested for oral HPV-16 DNA.
“Of these, 3 have been identified to have persistent oral HPV-16 DNA infection,” the investigators reported.
After having approached these three participants, one middle-aged male who had been consistently HPV-16 DNA positive for a period of 36 months – and whose HPV-16 viral load had been steadily rising over time – was invited to attend an ear, nose, and throat clinic for assessment.
“Initial clinical examination of the oropharynx including palpation and white light revealed no significant abnormalities,” the researchers emphasized.
As Dr. Punyadeera explained, standard clinical assessment for oropharyngeal malignancy includes white light examination for masses, detection of irregularities or asymmetry of the underlying structures and palpation of the tonsil and tongue base.
Cross-sectional imaging with CT or MRI can be helpful as well, she said, but these imaging studies are unable to detect lesions smaller than a few millimeters in size.
In the case of this individual, salivary oral rinse samples had been collected at baseline, and again at 6, 12, and 36 months after study enrollment as well as 2 weeks after the patient decided to undergo a bilateral tonsillectomy.
DNA was extracted from the salivary oral rinse samples, as well as from the tonsillar tissue obtained after resection. HPV-16 DNA genotyping and viral loads were analyzed with a PCR assay.
Results from the salivary samples indicated that the patient’s HPV-16 DNA viral load had increased exponentially across the 36 months of follow-up, from 3.43 copies/50 ng at baseline to 1281.69 copies/50 ng at 36 months.
On surgery, the patient was found to have a 2 mm squamous cell carcinoma in the left tonsil, but all other oropharyngeal tissues were normal and HPV-16 DNA negative.
Two weeks after undergoing the tonsillectomy, the patient’s HPV-16 DNA viral load in the saliva samples became undetectable.
This case report demonstrates that salivary HPV can both detect smaller lesions than either clinical examination or even radiological investigation, and that the same salivary test can likely also be used to monitor treatment response, Dr. Punyadeera commented.
Long-term persistence
As researchers explained in their paper, long-term persistence of HPV-16 infection is most likely a prerequisite for the development of subsequent malignancy.
Unlike cervical cancer caused by HPV-16 infection, the natural history of HPV infection in the oropharyngeal cavity is not known.
However, clinical assessment of patients with either persistent HPV infection or microscopic carcinoma has failed to detect any identifiable abnormalities.
Thus, this is the first report of a histologically confirmed diagnosis of an asymptomatic occult oropharyngeal cancer detected by a screening test through serial measurements of HPV-16 DNA, the investigators emphasized.
The report also demonstrated that very early lesions can be eradicated with minimal morbidity. Unfortunately, most oropharyngeal cancer is currently diagnosed at much later stages, and surgical removal of these is often associated with significant disabilities including difficulties with swallowing and even communicating.
“It’s amazing to think that this man was cured of his disease with a 15-minute procedure which left him with no lasting issues at all,” Dr. Punyadeera commented. “We need to try and make this the norm, not the exception.
“So we must have a well-designed screening study using all the insights we have gained from this case. We owe it to patients to explore these findings to their fullest,” Dr. Punyadeera emphasized.
The authors have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
A saliva test for detecting oropharyngeal cancer caused by human papillomavirus–16 (HPV-16) has scored a world first: It detected such a cancer in an asymptomatic adult.
If the finding can be replicated in a larger cohort of healthy asymptomatic individuals, widespread screening for HPV-16 – the main driver behind skyrocketing rates of oropharyngeal cancer – could be one step closer, the researchers suggested.
“Oropharyngeal squamous cell carcinomas often presents at a late stage with patients suffering huge morbidity as a result of treatment, [so] we must find strategies to detect these cancers earlier,” senior author Chamindie Punyadeera, PhD, Queensland University of Technology in Brisbane, Australia, told Medscape Medical News in an email.
“This study, for the first time, provides a solid scientific foundation to initiate a screening trial in high-risk individuals to detect HPV-driven oropharyngeal cancer, she added. “Saliva testing could be broadly implemented and [used] in a screening trial in the future,” she said.
The case report was published online March 31 in Frontiers in Oncology.
The saliva test was developed by Dr. Punyadeera and first author Kai Dun Tang, PhD, also from Queensland University of Technology. It is administered as an oral rinse: the individual swishes a saline solution around in his or her mouth for a minute or 2, and then spits the sample into a tube.
Prevalence Study
The saliva test was being scrutinized in an ongoing HPV-16 DNA prevalence study, which involved 650 healthy participants being tested for oral HPV-16 DNA.
“Of these, 3 have been identified to have persistent oral HPV-16 DNA infection,” the investigators reported.
After having approached these three participants, one middle-aged male who had been consistently HPV-16 DNA positive for a period of 36 months – and whose HPV-16 viral load had been steadily rising over time – was invited to attend an ear, nose, and throat clinic for assessment.
“Initial clinical examination of the oropharynx including palpation and white light revealed no significant abnormalities,” the researchers emphasized.
As Dr. Punyadeera explained, standard clinical assessment for oropharyngeal malignancy includes white light examination for masses, detection of irregularities or asymmetry of the underlying structures and palpation of the tonsil and tongue base.
Cross-sectional imaging with CT or MRI can be helpful as well, she said, but these imaging studies are unable to detect lesions smaller than a few millimeters in size.
In the case of this individual, salivary oral rinse samples had been collected at baseline, and again at 6, 12, and 36 months after study enrollment as well as 2 weeks after the patient decided to undergo a bilateral tonsillectomy.
DNA was extracted from the salivary oral rinse samples, as well as from the tonsillar tissue obtained after resection. HPV-16 DNA genotyping and viral loads were analyzed with a PCR assay.
Results from the salivary samples indicated that the patient’s HPV-16 DNA viral load had increased exponentially across the 36 months of follow-up, from 3.43 copies/50 ng at baseline to 1281.69 copies/50 ng at 36 months.
On surgery, the patient was found to have a 2 mm squamous cell carcinoma in the left tonsil, but all other oropharyngeal tissues were normal and HPV-16 DNA negative.
Two weeks after undergoing the tonsillectomy, the patient’s HPV-16 DNA viral load in the saliva samples became undetectable.
This case report demonstrates that salivary HPV can both detect smaller lesions than either clinical examination or even radiological investigation, and that the same salivary test can likely also be used to monitor treatment response, Dr. Punyadeera commented.
Long-term persistence
As researchers explained in their paper, long-term persistence of HPV-16 infection is most likely a prerequisite for the development of subsequent malignancy.
Unlike cervical cancer caused by HPV-16 infection, the natural history of HPV infection in the oropharyngeal cavity is not known.
However, clinical assessment of patients with either persistent HPV infection or microscopic carcinoma has failed to detect any identifiable abnormalities.
Thus, this is the first report of a histologically confirmed diagnosis of an asymptomatic occult oropharyngeal cancer detected by a screening test through serial measurements of HPV-16 DNA, the investigators emphasized.
The report also demonstrated that very early lesions can be eradicated with minimal morbidity. Unfortunately, most oropharyngeal cancer is currently diagnosed at much later stages, and surgical removal of these is often associated with significant disabilities including difficulties with swallowing and even communicating.
“It’s amazing to think that this man was cured of his disease with a 15-minute procedure which left him with no lasting issues at all,” Dr. Punyadeera commented. “We need to try and make this the norm, not the exception.
“So we must have a well-designed screening study using all the insights we have gained from this case. We owe it to patients to explore these findings to their fullest,” Dr. Punyadeera emphasized.
The authors have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
A saliva test for detecting oropharyngeal cancer caused by human papillomavirus–16 (HPV-16) has scored a world first: It detected such a cancer in an asymptomatic adult.
If the finding can be replicated in a larger cohort of healthy asymptomatic individuals, widespread screening for HPV-16 – the main driver behind skyrocketing rates of oropharyngeal cancer – could be one step closer, the researchers suggested.
“Oropharyngeal squamous cell carcinomas often presents at a late stage with patients suffering huge morbidity as a result of treatment, [so] we must find strategies to detect these cancers earlier,” senior author Chamindie Punyadeera, PhD, Queensland University of Technology in Brisbane, Australia, told Medscape Medical News in an email.
“This study, for the first time, provides a solid scientific foundation to initiate a screening trial in high-risk individuals to detect HPV-driven oropharyngeal cancer, she added. “Saliva testing could be broadly implemented and [used] in a screening trial in the future,” she said.
The case report was published online March 31 in Frontiers in Oncology.
The saliva test was developed by Dr. Punyadeera and first author Kai Dun Tang, PhD, also from Queensland University of Technology. It is administered as an oral rinse: the individual swishes a saline solution around in his or her mouth for a minute or 2, and then spits the sample into a tube.
Prevalence Study
The saliva test was being scrutinized in an ongoing HPV-16 DNA prevalence study, which involved 650 healthy participants being tested for oral HPV-16 DNA.
“Of these, 3 have been identified to have persistent oral HPV-16 DNA infection,” the investigators reported.
After having approached these three participants, one middle-aged male who had been consistently HPV-16 DNA positive for a period of 36 months – and whose HPV-16 viral load had been steadily rising over time – was invited to attend an ear, nose, and throat clinic for assessment.
“Initial clinical examination of the oropharynx including palpation and white light revealed no significant abnormalities,” the researchers emphasized.
As Dr. Punyadeera explained, standard clinical assessment for oropharyngeal malignancy includes white light examination for masses, detection of irregularities or asymmetry of the underlying structures and palpation of the tonsil and tongue base.
Cross-sectional imaging with CT or MRI can be helpful as well, she said, but these imaging studies are unable to detect lesions smaller than a few millimeters in size.
In the case of this individual, salivary oral rinse samples had been collected at baseline, and again at 6, 12, and 36 months after study enrollment as well as 2 weeks after the patient decided to undergo a bilateral tonsillectomy.
DNA was extracted from the salivary oral rinse samples, as well as from the tonsillar tissue obtained after resection. HPV-16 DNA genotyping and viral loads were analyzed with a PCR assay.
Results from the salivary samples indicated that the patient’s HPV-16 DNA viral load had increased exponentially across the 36 months of follow-up, from 3.43 copies/50 ng at baseline to 1281.69 copies/50 ng at 36 months.
On surgery, the patient was found to have a 2 mm squamous cell carcinoma in the left tonsil, but all other oropharyngeal tissues were normal and HPV-16 DNA negative.
Two weeks after undergoing the tonsillectomy, the patient’s HPV-16 DNA viral load in the saliva samples became undetectable.
This case report demonstrates that salivary HPV can both detect smaller lesions than either clinical examination or even radiological investigation, and that the same salivary test can likely also be used to monitor treatment response, Dr. Punyadeera commented.
Long-term persistence
As researchers explained in their paper, long-term persistence of HPV-16 infection is most likely a prerequisite for the development of subsequent malignancy.
Unlike cervical cancer caused by HPV-16 infection, the natural history of HPV infection in the oropharyngeal cavity is not known.
However, clinical assessment of patients with either persistent HPV infection or microscopic carcinoma has failed to detect any identifiable abnormalities.
Thus, this is the first report of a histologically confirmed diagnosis of an asymptomatic occult oropharyngeal cancer detected by a screening test through serial measurements of HPV-16 DNA, the investigators emphasized.
The report also demonstrated that very early lesions can be eradicated with minimal morbidity. Unfortunately, most oropharyngeal cancer is currently diagnosed at much later stages, and surgical removal of these is often associated with significant disabilities including difficulties with swallowing and even communicating.
“It’s amazing to think that this man was cured of his disease with a 15-minute procedure which left him with no lasting issues at all,” Dr. Punyadeera commented. “We need to try and make this the norm, not the exception.
“So we must have a well-designed screening study using all the insights we have gained from this case. We owe it to patients to explore these findings to their fullest,” Dr. Punyadeera emphasized.
The authors have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Less pain with a cancer drug to treat anal HPV, but it’s expensive
At the end of 6 months of low-dose pomalidomide (Pomalyst), more than half of men who have sex with men had partial or complete clearance of long-standing, grade 3 anal lesions from human papillomavirus, irrespective of HIV status; the number increased to almost two-thirds when they were checked at 12 months, according to a 26-subject study said in video presentation of his research during the Conference on Retroviruses & Opportunistic Infections, which was presented online this year. CROI organizers chose to hold a virtual meeting because of concerns about the spread of COVID-19.
“Therapy induced durable and continuous clearance of anal HSIL [high-grade squamous intraepithelial lesions]. Further study in HPV-associated premalignancy is warranted to follow up this small, single arm study,” said study lead Mark Polizzotto, MD, PhD, head of the therapeutic and vaccine research program at the Kirby Institute in Sydney.
HPV anal lesions, and subsequent HSIL and progression to anal cancer, are prevalent among men who have sex with men. The risk increases with chronic lesions and concomitant HIV infection.
Pomalidomide is potentially a less painful alternative to options such as freezing and laser ablation, and it may have a lower rate of recurrence, but it’s expensive. Copays range upward from $5,000 for a month supply, according to GoodRx. Celgene, the maker of the drug, offers financial assistance.
Pomalidomide is a derivative of thalidomide that’s approved for multiple myeloma and also has shown effect against a viral lesion associated with HIV, Kaposi sarcoma. The drug is a T-cell activator, and since T-cell activation also is key to spontaneous anal HSIL clearance, Dr. Polizzotto and team wanted to take a look to see if it could help, he said.
The men in the study were at high risk for progression to anal cancer. With a median lesion duration of more than 3 years, and at least one case out past 7 years, spontaneous clearance wasn’t in the cards. The lesions were all grade 3 HSIL, which means severe dysplasia, and more than half of the subjects had HPV genotype 16, and the rest had other risky genotypes. Ten subjects also had HIV, which also increases the risk of anal cancer.
Pomalidomide was given in back-to-back cycles for 6 months, each consisting of 2 mg orally for 3 weeks, then 1 week off, along with a thrombolytic, usually aspirin, given the black box warning of blood clots. The dose was half the 5-mg cycle for Kaposi’s.
The overall response rate – complete clearance or a partial clearance of at least a 50% on high-resolution anoscopy – was 50% at 6 months (12/24), including four complete responses (4/15, 27%) in subjects without HIV, as well as four in the HIV group (4/9, 44%).
On follow-up at month 12, “we saw something we did not expect. Strikingly, with no additional therapy in the interim, we saw a deepening of response in a number of subjects.” The overall response rate climbed to 63% (15/24), including 33% complete response in the HIV-free group (5/15) and HIV-positive group (3/9).
Some did lose their response in the interim, however, and the study team is working to figure out if it was do to a recurrence or a new infection.
A general pattern of immune activation on treatment, including increased systemic CD4+ T-cell responses to HPV during therapy, supported the investigator’s hunch of an immunologic mechanism of action, Dr. Polizzotto said.
There were four instances of grade 3 neutropenia over eight treatment cycles, and one possibly related angina attack, but other than that, adverse reactions were generally mild and self-limited, mostly to grade 1 or 2 neutropenia, constipation, fatigue, and rash, with no idiosyncratic reactions in the HIV group or loss of viral suppression, and no discontinuations because of side effects.
The men in the study were aged 40-50 years, with a median age of 54 years; all but one were white. The median lesion involved a quarter of the anal ring, but sometimes more than half.
The work was funded by the Cancer Institute of New South Wales, the Australian National Health and Medical Research Council, and Celgene. Dr. Polizzotto disclosed patents with Celgene and research funding from the company.
SOURCE: Polizzotto M et al. CROI 2020. Abstract 70
At the end of 6 months of low-dose pomalidomide (Pomalyst), more than half of men who have sex with men had partial or complete clearance of long-standing, grade 3 anal lesions from human papillomavirus, irrespective of HIV status; the number increased to almost two-thirds when they were checked at 12 months, according to a 26-subject study said in video presentation of his research during the Conference on Retroviruses & Opportunistic Infections, which was presented online this year. CROI organizers chose to hold a virtual meeting because of concerns about the spread of COVID-19.
“Therapy induced durable and continuous clearance of anal HSIL [high-grade squamous intraepithelial lesions]. Further study in HPV-associated premalignancy is warranted to follow up this small, single arm study,” said study lead Mark Polizzotto, MD, PhD, head of the therapeutic and vaccine research program at the Kirby Institute in Sydney.
HPV anal lesions, and subsequent HSIL and progression to anal cancer, are prevalent among men who have sex with men. The risk increases with chronic lesions and concomitant HIV infection.
Pomalidomide is potentially a less painful alternative to options such as freezing and laser ablation, and it may have a lower rate of recurrence, but it’s expensive. Copays range upward from $5,000 for a month supply, according to GoodRx. Celgene, the maker of the drug, offers financial assistance.
Pomalidomide is a derivative of thalidomide that’s approved for multiple myeloma and also has shown effect against a viral lesion associated with HIV, Kaposi sarcoma. The drug is a T-cell activator, and since T-cell activation also is key to spontaneous anal HSIL clearance, Dr. Polizzotto and team wanted to take a look to see if it could help, he said.
The men in the study were at high risk for progression to anal cancer. With a median lesion duration of more than 3 years, and at least one case out past 7 years, spontaneous clearance wasn’t in the cards. The lesions were all grade 3 HSIL, which means severe dysplasia, and more than half of the subjects had HPV genotype 16, and the rest had other risky genotypes. Ten subjects also had HIV, which also increases the risk of anal cancer.
Pomalidomide was given in back-to-back cycles for 6 months, each consisting of 2 mg orally for 3 weeks, then 1 week off, along with a thrombolytic, usually aspirin, given the black box warning of blood clots. The dose was half the 5-mg cycle for Kaposi’s.
The overall response rate – complete clearance or a partial clearance of at least a 50% on high-resolution anoscopy – was 50% at 6 months (12/24), including four complete responses (4/15, 27%) in subjects without HIV, as well as four in the HIV group (4/9, 44%).
On follow-up at month 12, “we saw something we did not expect. Strikingly, with no additional therapy in the interim, we saw a deepening of response in a number of subjects.” The overall response rate climbed to 63% (15/24), including 33% complete response in the HIV-free group (5/15) and HIV-positive group (3/9).
Some did lose their response in the interim, however, and the study team is working to figure out if it was do to a recurrence or a new infection.
A general pattern of immune activation on treatment, including increased systemic CD4+ T-cell responses to HPV during therapy, supported the investigator’s hunch of an immunologic mechanism of action, Dr. Polizzotto said.
There were four instances of grade 3 neutropenia over eight treatment cycles, and one possibly related angina attack, but other than that, adverse reactions were generally mild and self-limited, mostly to grade 1 or 2 neutropenia, constipation, fatigue, and rash, with no idiosyncratic reactions in the HIV group or loss of viral suppression, and no discontinuations because of side effects.
The men in the study were aged 40-50 years, with a median age of 54 years; all but one were white. The median lesion involved a quarter of the anal ring, but sometimes more than half.
The work was funded by the Cancer Institute of New South Wales, the Australian National Health and Medical Research Council, and Celgene. Dr. Polizzotto disclosed patents with Celgene and research funding from the company.
SOURCE: Polizzotto M et al. CROI 2020. Abstract 70
At the end of 6 months of low-dose pomalidomide (Pomalyst), more than half of men who have sex with men had partial or complete clearance of long-standing, grade 3 anal lesions from human papillomavirus, irrespective of HIV status; the number increased to almost two-thirds when they were checked at 12 months, according to a 26-subject study said in video presentation of his research during the Conference on Retroviruses & Opportunistic Infections, which was presented online this year. CROI organizers chose to hold a virtual meeting because of concerns about the spread of COVID-19.
“Therapy induced durable and continuous clearance of anal HSIL [high-grade squamous intraepithelial lesions]. Further study in HPV-associated premalignancy is warranted to follow up this small, single arm study,” said study lead Mark Polizzotto, MD, PhD, head of the therapeutic and vaccine research program at the Kirby Institute in Sydney.
HPV anal lesions, and subsequent HSIL and progression to anal cancer, are prevalent among men who have sex with men. The risk increases with chronic lesions and concomitant HIV infection.
Pomalidomide is potentially a less painful alternative to options such as freezing and laser ablation, and it may have a lower rate of recurrence, but it’s expensive. Copays range upward from $5,000 for a month supply, according to GoodRx. Celgene, the maker of the drug, offers financial assistance.
Pomalidomide is a derivative of thalidomide that’s approved for multiple myeloma and also has shown effect against a viral lesion associated with HIV, Kaposi sarcoma. The drug is a T-cell activator, and since T-cell activation also is key to spontaneous anal HSIL clearance, Dr. Polizzotto and team wanted to take a look to see if it could help, he said.
The men in the study were at high risk for progression to anal cancer. With a median lesion duration of more than 3 years, and at least one case out past 7 years, spontaneous clearance wasn’t in the cards. The lesions were all grade 3 HSIL, which means severe dysplasia, and more than half of the subjects had HPV genotype 16, and the rest had other risky genotypes. Ten subjects also had HIV, which also increases the risk of anal cancer.
Pomalidomide was given in back-to-back cycles for 6 months, each consisting of 2 mg orally for 3 weeks, then 1 week off, along with a thrombolytic, usually aspirin, given the black box warning of blood clots. The dose was half the 5-mg cycle for Kaposi’s.
The overall response rate – complete clearance or a partial clearance of at least a 50% on high-resolution anoscopy – was 50% at 6 months (12/24), including four complete responses (4/15, 27%) in subjects without HIV, as well as four in the HIV group (4/9, 44%).
On follow-up at month 12, “we saw something we did not expect. Strikingly, with no additional therapy in the interim, we saw a deepening of response in a number of subjects.” The overall response rate climbed to 63% (15/24), including 33% complete response in the HIV-free group (5/15) and HIV-positive group (3/9).
Some did lose their response in the interim, however, and the study team is working to figure out if it was do to a recurrence or a new infection.
A general pattern of immune activation on treatment, including increased systemic CD4+ T-cell responses to HPV during therapy, supported the investigator’s hunch of an immunologic mechanism of action, Dr. Polizzotto said.
There were four instances of grade 3 neutropenia over eight treatment cycles, and one possibly related angina attack, but other than that, adverse reactions were generally mild and self-limited, mostly to grade 1 or 2 neutropenia, constipation, fatigue, and rash, with no idiosyncratic reactions in the HIV group or loss of viral suppression, and no discontinuations because of side effects.
The men in the study were aged 40-50 years, with a median age of 54 years; all but one were white. The median lesion involved a quarter of the anal ring, but sometimes more than half.
The work was funded by the Cancer Institute of New South Wales, the Australian National Health and Medical Research Council, and Celgene. Dr. Polizzotto disclosed patents with Celgene and research funding from the company.
SOURCE: Polizzotto M et al. CROI 2020. Abstract 70
FROM CROI 2020