User login
FDA finalizes guidance for power morcellators in gynecologic surgery
The agency noted that physicians should conduct a thorough preoperative screening and that the devices should only be used for hysterectomies and myomectomies. Clinicians should not use the devices in cases involving uterine malignancy or suspected uterine malignancy.
In addition, clinicians should not use morcellators to remove uterine tissue containing suspected fibroids in women older than 50 years or who are postmenopausal. Nor should the devices be used for women who are “candidates for removal of tissue (en bloc) through the vagina or via a minilaparotomy incision,” the agency said.
The safety communication, which was issued on Dec. 29, 2020, updates previous guidance from February 2020. The updated recommendations are consistent with final labeling guidance for laparoscopic power morcellators, also issued by the FDA on Dec. 29.
Risk of disease spread
Prior evidence suggests that use of uncontained power morcellators in women with malignant uterine tissue can spread disease.
Even among women who do not have malignant uterine tissue, containment is important. The agency noted an association between uncontained power morcellation and the spread of benign uterine tissue, such as parasitic myomas and disseminated peritoneal leiomyomatosis, which could require additional surgeries.
In 2016, the FDA approved the PneumoLiner, a containment system for isolating uterine tissue that is not suspected of containing cancer.
“While unsuspected cancer can occur at any age, the prevalence of unsuspected cancer in women undergoing hysterectomy for fibroids increases with age such that the benefit-risk profile of using [laparoscopic power morcellators] is worse in older women when compared to younger women,” according to the new labeling guidance. “Also, the surgical technique of en bloc tissue removal eliminates the need to perform morcellation, thereby reducing the risk of iatrogenic dissemination and upstaging of an occult sarcoma. A thorough preoperative screening should be conducted; however, it is important to note that no screening procedure that can reliably detect sarcoma preoperatively has been identified.”
“The FDA will continue to review the latest data and scientific literature on laparoscopic power morcellation, including gathering real-world evidence from patients, providers and others, and encouraging innovation to better detect uterine cancer and develop containment systems for gynecologic surgery,” said Jeffrey Shuren, MD, JD, director of the FDA’s Center for Devices and Radiological Health, in a news release. “The FDA seeks to ensure women and their health care providers are fully informed when considering laparoscopic power morcellators for gynecologic surgeries.”
A version of this article first appeared on Medscape.com.
The agency noted that physicians should conduct a thorough preoperative screening and that the devices should only be used for hysterectomies and myomectomies. Clinicians should not use the devices in cases involving uterine malignancy or suspected uterine malignancy.
In addition, clinicians should not use morcellators to remove uterine tissue containing suspected fibroids in women older than 50 years or who are postmenopausal. Nor should the devices be used for women who are “candidates for removal of tissue (en bloc) through the vagina or via a minilaparotomy incision,” the agency said.
The safety communication, which was issued on Dec. 29, 2020, updates previous guidance from February 2020. The updated recommendations are consistent with final labeling guidance for laparoscopic power morcellators, also issued by the FDA on Dec. 29.
Risk of disease spread
Prior evidence suggests that use of uncontained power morcellators in women with malignant uterine tissue can spread disease.
Even among women who do not have malignant uterine tissue, containment is important. The agency noted an association between uncontained power morcellation and the spread of benign uterine tissue, such as parasitic myomas and disseminated peritoneal leiomyomatosis, which could require additional surgeries.
In 2016, the FDA approved the PneumoLiner, a containment system for isolating uterine tissue that is not suspected of containing cancer.
“While unsuspected cancer can occur at any age, the prevalence of unsuspected cancer in women undergoing hysterectomy for fibroids increases with age such that the benefit-risk profile of using [laparoscopic power morcellators] is worse in older women when compared to younger women,” according to the new labeling guidance. “Also, the surgical technique of en bloc tissue removal eliminates the need to perform morcellation, thereby reducing the risk of iatrogenic dissemination and upstaging of an occult sarcoma. A thorough preoperative screening should be conducted; however, it is important to note that no screening procedure that can reliably detect sarcoma preoperatively has been identified.”
“The FDA will continue to review the latest data and scientific literature on laparoscopic power morcellation, including gathering real-world evidence from patients, providers and others, and encouraging innovation to better detect uterine cancer and develop containment systems for gynecologic surgery,” said Jeffrey Shuren, MD, JD, director of the FDA’s Center for Devices and Radiological Health, in a news release. “The FDA seeks to ensure women and their health care providers are fully informed when considering laparoscopic power morcellators for gynecologic surgeries.”
A version of this article first appeared on Medscape.com.
The agency noted that physicians should conduct a thorough preoperative screening and that the devices should only be used for hysterectomies and myomectomies. Clinicians should not use the devices in cases involving uterine malignancy or suspected uterine malignancy.
In addition, clinicians should not use morcellators to remove uterine tissue containing suspected fibroids in women older than 50 years or who are postmenopausal. Nor should the devices be used for women who are “candidates for removal of tissue (en bloc) through the vagina or via a minilaparotomy incision,” the agency said.
The safety communication, which was issued on Dec. 29, 2020, updates previous guidance from February 2020. The updated recommendations are consistent with final labeling guidance for laparoscopic power morcellators, also issued by the FDA on Dec. 29.
Risk of disease spread
Prior evidence suggests that use of uncontained power morcellators in women with malignant uterine tissue can spread disease.
Even among women who do not have malignant uterine tissue, containment is important. The agency noted an association between uncontained power morcellation and the spread of benign uterine tissue, such as parasitic myomas and disseminated peritoneal leiomyomatosis, which could require additional surgeries.
In 2016, the FDA approved the PneumoLiner, a containment system for isolating uterine tissue that is not suspected of containing cancer.
“While unsuspected cancer can occur at any age, the prevalence of unsuspected cancer in women undergoing hysterectomy for fibroids increases with age such that the benefit-risk profile of using [laparoscopic power morcellators] is worse in older women when compared to younger women,” according to the new labeling guidance. “Also, the surgical technique of en bloc tissue removal eliminates the need to perform morcellation, thereby reducing the risk of iatrogenic dissemination and upstaging of an occult sarcoma. A thorough preoperative screening should be conducted; however, it is important to note that no screening procedure that can reliably detect sarcoma preoperatively has been identified.”
“The FDA will continue to review the latest data and scientific literature on laparoscopic power morcellation, including gathering real-world evidence from patients, providers and others, and encouraging innovation to better detect uterine cancer and develop containment systems for gynecologic surgery,” said Jeffrey Shuren, MD, JD, director of the FDA’s Center for Devices and Radiological Health, in a news release. “The FDA seeks to ensure women and their health care providers are fully informed when considering laparoscopic power morcellators for gynecologic surgeries.”
A version of this article first appeared on Medscape.com.
Complications and death within 30 days after noncardiac surgery
Background: There have been advances in perioperative care and technology for adults, but at the same time the patient population is increasingly medically complex. We do not know the current mortality risk of noncardiac surgery in adults.
Study design: Prospective cohort study.
Setting: Twenty-eight academic centers in 14 countries in North America, South America, Asia, Europe, Africa, and Australia. At least four academic centers represented each of these continents, except Africa, with one center reporting there.
Synopsis: The VISION study included 40,004 inpatients, aged 45 years and older, followed for 30-day mortality after noncardiac surgery. One-third of surgeries were considered low risk. A startling 99.1% of patients completed the study. Mortality rate was 1.8%, with 71% of patients dying during the index admission and 29% dying after discharge.
Nine events were independently associated with postoperative death, but the top three – major bleeding, myocardial injury after noncardiac surgery (MINS), and sepsis – accounted for 45% of the attributable fraction. These, on average, occurred within 1-6 days after surgery. The other events (infection, kidney injury with dialysis, stroke, venous thromboembolism, new atrial fibrillation, and congestive heart failure) constituted less than 3% of the attributable fraction. Findings suggest that closer monitoring in the hospital and post discharge might improve survival after noncardiac surgery.
Limitations for hospitalists include that patients were younger and less medically complex than our typically comanaged patients: More than half of patients were aged 45-64, less than 10% had chronic kidney disease stage 3b or greater, and only 20% had diabetes mellitus.
Bottom line: Postoperative and postdischarge bleeding, myocardial injury after noncardiac surgery, and sepsis are major risk factors for 30-day mortality in adults undergoing noncardiac surgery. Closer postoperative monitoring for these conditions should be explored.
Citation: The Vision Study Investigators (Spence J et al.) Association between complications and death within 30 days after noncardiac surgery. CMAJ. 2019 Jul 29;191(30):E830-7.
Dr. Brouillette is a med-peds hospitalist at Maine Medical Center in Portland.
Background: There have been advances in perioperative care and technology for adults, but at the same time the patient population is increasingly medically complex. We do not know the current mortality risk of noncardiac surgery in adults.
Study design: Prospective cohort study.
Setting: Twenty-eight academic centers in 14 countries in North America, South America, Asia, Europe, Africa, and Australia. At least four academic centers represented each of these continents, except Africa, with one center reporting there.
Synopsis: The VISION study included 40,004 inpatients, aged 45 years and older, followed for 30-day mortality after noncardiac surgery. One-third of surgeries were considered low risk. A startling 99.1% of patients completed the study. Mortality rate was 1.8%, with 71% of patients dying during the index admission and 29% dying after discharge.
Nine events were independently associated with postoperative death, but the top three – major bleeding, myocardial injury after noncardiac surgery (MINS), and sepsis – accounted for 45% of the attributable fraction. These, on average, occurred within 1-6 days after surgery. The other events (infection, kidney injury with dialysis, stroke, venous thromboembolism, new atrial fibrillation, and congestive heart failure) constituted less than 3% of the attributable fraction. Findings suggest that closer monitoring in the hospital and post discharge might improve survival after noncardiac surgery.
Limitations for hospitalists include that patients were younger and less medically complex than our typically comanaged patients: More than half of patients were aged 45-64, less than 10% had chronic kidney disease stage 3b or greater, and only 20% had diabetes mellitus.
Bottom line: Postoperative and postdischarge bleeding, myocardial injury after noncardiac surgery, and sepsis are major risk factors for 30-day mortality in adults undergoing noncardiac surgery. Closer postoperative monitoring for these conditions should be explored.
Citation: The Vision Study Investigators (Spence J et al.) Association between complications and death within 30 days after noncardiac surgery. CMAJ. 2019 Jul 29;191(30):E830-7.
Dr. Brouillette is a med-peds hospitalist at Maine Medical Center in Portland.
Background: There have been advances in perioperative care and technology for adults, but at the same time the patient population is increasingly medically complex. We do not know the current mortality risk of noncardiac surgery in adults.
Study design: Prospective cohort study.
Setting: Twenty-eight academic centers in 14 countries in North America, South America, Asia, Europe, Africa, and Australia. At least four academic centers represented each of these continents, except Africa, with one center reporting there.
Synopsis: The VISION study included 40,004 inpatients, aged 45 years and older, followed for 30-day mortality after noncardiac surgery. One-third of surgeries were considered low risk. A startling 99.1% of patients completed the study. Mortality rate was 1.8%, with 71% of patients dying during the index admission and 29% dying after discharge.
Nine events were independently associated with postoperative death, but the top three – major bleeding, myocardial injury after noncardiac surgery (MINS), and sepsis – accounted for 45% of the attributable fraction. These, on average, occurred within 1-6 days after surgery. The other events (infection, kidney injury with dialysis, stroke, venous thromboembolism, new atrial fibrillation, and congestive heart failure) constituted less than 3% of the attributable fraction. Findings suggest that closer monitoring in the hospital and post discharge might improve survival after noncardiac surgery.
Limitations for hospitalists include that patients were younger and less medically complex than our typically comanaged patients: More than half of patients were aged 45-64, less than 10% had chronic kidney disease stage 3b or greater, and only 20% had diabetes mellitus.
Bottom line: Postoperative and postdischarge bleeding, myocardial injury after noncardiac surgery, and sepsis are major risk factors for 30-day mortality in adults undergoing noncardiac surgery. Closer postoperative monitoring for these conditions should be explored.
Citation: The Vision Study Investigators (Spence J et al.) Association between complications and death within 30 days after noncardiac surgery. CMAJ. 2019 Jul 29;191(30):E830-7.
Dr. Brouillette is a med-peds hospitalist at Maine Medical Center in Portland.
Sentinel node biopsy cuts surgery time over lymphadenectomy
Sentinel node biopsy shortens operative time by 13% and may play a role in reducing recovery time and length of hospital stay, reported David L. Tait, MD, of the Levine Cancer Institute, Charlotte, N.C., and colleagues.
In an effort to compare the immediate perioperative outcomes for narcotic usage and use of hospital resources for patients having sentinel node dissection, Dr. Tait and his colleagues conducted a retrospective study of 241 consecutive cases of minimally invasive surgery performed between Jan. 1, 2018, and Aug. 31, 2019, on endometrial cancer patients.
A total of 156 (65%) patients received nodal dissection, including 93 (60%) who received sentinel node biopsy and 63 (40%) who underwent a full lymphadenectomy in accordance with pathological criteria established at the time of surgery. The authors noted no differences between the sentinel group and the lymphadenectomy group in terms of age, body mass index, estimated blood loss, use of a preoperative enhanced recovery after surgery (ERAS) program, tobacco use, or ethanol use. They also found no difference in primary outcome of intravenous narcotics dispensed in surgery, recovery, or total dose.
Sentinel node biopsy offers several advantages
Dr. Tate and colleagues noted that a significantly shorter surgery time, by 27 minutes, on average, was not unexpected with the sentinel node biopsy technique. With lymphadenectomy, surgical procedure and recovery times were longer (214.2 minutes vs. 185.2 minutes and 157.6 minutes vs. 125.2 minutes, respectively) than sentinel biopsy, a difference the researchers could not explain given the similar use of narcotics between the two procedures. Lymphadenectomy also resulted in longer hospital stay than sentinel biopsy (23.5 hours vs. 15.5 hours), with same-day discharge significantly less frequent (16% vs. 50%).
The differences in operative time, recovery time, and hospital stay “are important with respect to improving the efficiency of the operating room, which has become even more important in the era of the COVID-19 pandemic,” the authors noted. They also found noteworthy that recovery and hospital stay are longer after full lymphadenectomy even though there was no difference in overall narcotic administration. Although this suggests surgeon and staff bias, other factors that were not accounted for in the study include distance from hospital, social situation, and functional status.
Change in practice patterns over time and the introduction of a universal ERAS program during the study period were noted as possible limitations. It was also noted that the study did not collect data on functional status or long-term outcome of patients.
The authors did note that using the sentinel node technique was advantageous because it was performed on all patients regardless of risk factors for extra uterine spread since the injection must be performed before hysterectomy. What makes this so beneficial is the potential it offers for detecting nodal metastasis in low-risk patients who may not have otherwise qualified for dissection, said Dr. Tait and colleagues.
In a separate interview, Justin Chura, MD, director of gynecologic oncology and robotic surgery at the Cancer Treatment Centers of America in Philadelphia, observed that “sentinel lymph node [SLN] mapping has been around since the late 1970s. It is most validated in melanoma and breast cancers but has also seen application for gynecological cancers including vulva, cervix, and endometrium. More than 5 years ago, the Society of Gynecologic Oncology issued a clinical practice statement regarding the role of sentinel lymph node mapping for endometrial cancer. An SLN algorithm has been part of [National Comprehensive Cancer Network] guidelines for a similar time frame. The technique faced a lot of skepticism and criticism in the breast cancer literature until randomized studies demonstrated that full axillary adenopathy did not confer a survival benefit. For endometrial cancer, it is unlikely that we will have as robust data, so we often look to retrospective studies such as the one presented by Tait et al.
“The study utilized a data set that was originally collected to assess the impact of an ERAS protocol. So, it is important to note that the data set was not collected with the intent of evaluating SLN mapping versus full lymphadenectomy. This explains why pathological data regarding lymph node yield and final surgicopathologic staging are absent,” he said.
Adoption of sentinel node biopsy is gaining popularity
“Overall, SLN mapping is safe (from a surgical standpoint) and may decrease perioperative morbidity,” Dr. Chura said. “The adoption of SLN mapping also appears to be increasing. Some gyn oncologists (including myself) are even performing SLN mapping on patients with endometrial intraepithelial neoplasia given the risk of malignancy being identified on final pathology.
“The current study provides more of a glimpse into the practice patterns of the authors’ institution where ‘full lymphadenectomy was very dependent upon the surgeon (P < .001)’ than it demonstrates one technique is better than the other. The ultimate question is how we define ‘better?’ Survival? Less morbidity? Improved accuracy of nodal metastasis? Shorter length of stay?” Dr. Chura said.Dr. Tait and colleagues as well as Dr. Chura had no conflicts of interest and no relevant financial disclosures.
SOURCE: Tate DL et al. J Minim Invasive Gynecol. 2020 Dec 19. doi: 10.1016/jmig.2020.12.019.
Sentinel node biopsy shortens operative time by 13% and may play a role in reducing recovery time and length of hospital stay, reported David L. Tait, MD, of the Levine Cancer Institute, Charlotte, N.C., and colleagues.
In an effort to compare the immediate perioperative outcomes for narcotic usage and use of hospital resources for patients having sentinel node dissection, Dr. Tait and his colleagues conducted a retrospective study of 241 consecutive cases of minimally invasive surgery performed between Jan. 1, 2018, and Aug. 31, 2019, on endometrial cancer patients.
A total of 156 (65%) patients received nodal dissection, including 93 (60%) who received sentinel node biopsy and 63 (40%) who underwent a full lymphadenectomy in accordance with pathological criteria established at the time of surgery. The authors noted no differences between the sentinel group and the lymphadenectomy group in terms of age, body mass index, estimated blood loss, use of a preoperative enhanced recovery after surgery (ERAS) program, tobacco use, or ethanol use. They also found no difference in primary outcome of intravenous narcotics dispensed in surgery, recovery, or total dose.
Sentinel node biopsy offers several advantages
Dr. Tate and colleagues noted that a significantly shorter surgery time, by 27 minutes, on average, was not unexpected with the sentinel node biopsy technique. With lymphadenectomy, surgical procedure and recovery times were longer (214.2 minutes vs. 185.2 minutes and 157.6 minutes vs. 125.2 minutes, respectively) than sentinel biopsy, a difference the researchers could not explain given the similar use of narcotics between the two procedures. Lymphadenectomy also resulted in longer hospital stay than sentinel biopsy (23.5 hours vs. 15.5 hours), with same-day discharge significantly less frequent (16% vs. 50%).
The differences in operative time, recovery time, and hospital stay “are important with respect to improving the efficiency of the operating room, which has become even more important in the era of the COVID-19 pandemic,” the authors noted. They also found noteworthy that recovery and hospital stay are longer after full lymphadenectomy even though there was no difference in overall narcotic administration. Although this suggests surgeon and staff bias, other factors that were not accounted for in the study include distance from hospital, social situation, and functional status.
Change in practice patterns over time and the introduction of a universal ERAS program during the study period were noted as possible limitations. It was also noted that the study did not collect data on functional status or long-term outcome of patients.
The authors did note that using the sentinel node technique was advantageous because it was performed on all patients regardless of risk factors for extra uterine spread since the injection must be performed before hysterectomy. What makes this so beneficial is the potential it offers for detecting nodal metastasis in low-risk patients who may not have otherwise qualified for dissection, said Dr. Tait and colleagues.
In a separate interview, Justin Chura, MD, director of gynecologic oncology and robotic surgery at the Cancer Treatment Centers of America in Philadelphia, observed that “sentinel lymph node [SLN] mapping has been around since the late 1970s. It is most validated in melanoma and breast cancers but has also seen application for gynecological cancers including vulva, cervix, and endometrium. More than 5 years ago, the Society of Gynecologic Oncology issued a clinical practice statement regarding the role of sentinel lymph node mapping for endometrial cancer. An SLN algorithm has been part of [National Comprehensive Cancer Network] guidelines for a similar time frame. The technique faced a lot of skepticism and criticism in the breast cancer literature until randomized studies demonstrated that full axillary adenopathy did not confer a survival benefit. For endometrial cancer, it is unlikely that we will have as robust data, so we often look to retrospective studies such as the one presented by Tait et al.
“The study utilized a data set that was originally collected to assess the impact of an ERAS protocol. So, it is important to note that the data set was not collected with the intent of evaluating SLN mapping versus full lymphadenectomy. This explains why pathological data regarding lymph node yield and final surgicopathologic staging are absent,” he said.
Adoption of sentinel node biopsy is gaining popularity
“Overall, SLN mapping is safe (from a surgical standpoint) and may decrease perioperative morbidity,” Dr. Chura said. “The adoption of SLN mapping also appears to be increasing. Some gyn oncologists (including myself) are even performing SLN mapping on patients with endometrial intraepithelial neoplasia given the risk of malignancy being identified on final pathology.
“The current study provides more of a glimpse into the practice patterns of the authors’ institution where ‘full lymphadenectomy was very dependent upon the surgeon (P < .001)’ than it demonstrates one technique is better than the other. The ultimate question is how we define ‘better?’ Survival? Less morbidity? Improved accuracy of nodal metastasis? Shorter length of stay?” Dr. Chura said.Dr. Tait and colleagues as well as Dr. Chura had no conflicts of interest and no relevant financial disclosures.
SOURCE: Tate DL et al. J Minim Invasive Gynecol. 2020 Dec 19. doi: 10.1016/jmig.2020.12.019.
Sentinel node biopsy shortens operative time by 13% and may play a role in reducing recovery time and length of hospital stay, reported David L. Tait, MD, of the Levine Cancer Institute, Charlotte, N.C., and colleagues.
In an effort to compare the immediate perioperative outcomes for narcotic usage and use of hospital resources for patients having sentinel node dissection, Dr. Tait and his colleagues conducted a retrospective study of 241 consecutive cases of minimally invasive surgery performed between Jan. 1, 2018, and Aug. 31, 2019, on endometrial cancer patients.
A total of 156 (65%) patients received nodal dissection, including 93 (60%) who received sentinel node biopsy and 63 (40%) who underwent a full lymphadenectomy in accordance with pathological criteria established at the time of surgery. The authors noted no differences between the sentinel group and the lymphadenectomy group in terms of age, body mass index, estimated blood loss, use of a preoperative enhanced recovery after surgery (ERAS) program, tobacco use, or ethanol use. They also found no difference in primary outcome of intravenous narcotics dispensed in surgery, recovery, or total dose.
Sentinel node biopsy offers several advantages
Dr. Tate and colleagues noted that a significantly shorter surgery time, by 27 minutes, on average, was not unexpected with the sentinel node biopsy technique. With lymphadenectomy, surgical procedure and recovery times were longer (214.2 minutes vs. 185.2 minutes and 157.6 minutes vs. 125.2 minutes, respectively) than sentinel biopsy, a difference the researchers could not explain given the similar use of narcotics between the two procedures. Lymphadenectomy also resulted in longer hospital stay than sentinel biopsy (23.5 hours vs. 15.5 hours), with same-day discharge significantly less frequent (16% vs. 50%).
The differences in operative time, recovery time, and hospital stay “are important with respect to improving the efficiency of the operating room, which has become even more important in the era of the COVID-19 pandemic,” the authors noted. They also found noteworthy that recovery and hospital stay are longer after full lymphadenectomy even though there was no difference in overall narcotic administration. Although this suggests surgeon and staff bias, other factors that were not accounted for in the study include distance from hospital, social situation, and functional status.
Change in practice patterns over time and the introduction of a universal ERAS program during the study period were noted as possible limitations. It was also noted that the study did not collect data on functional status or long-term outcome of patients.
The authors did note that using the sentinel node technique was advantageous because it was performed on all patients regardless of risk factors for extra uterine spread since the injection must be performed before hysterectomy. What makes this so beneficial is the potential it offers for detecting nodal metastasis in low-risk patients who may not have otherwise qualified for dissection, said Dr. Tait and colleagues.
In a separate interview, Justin Chura, MD, director of gynecologic oncology and robotic surgery at the Cancer Treatment Centers of America in Philadelphia, observed that “sentinel lymph node [SLN] mapping has been around since the late 1970s. It is most validated in melanoma and breast cancers but has also seen application for gynecological cancers including vulva, cervix, and endometrium. More than 5 years ago, the Society of Gynecologic Oncology issued a clinical practice statement regarding the role of sentinel lymph node mapping for endometrial cancer. An SLN algorithm has been part of [National Comprehensive Cancer Network] guidelines for a similar time frame. The technique faced a lot of skepticism and criticism in the breast cancer literature until randomized studies demonstrated that full axillary adenopathy did not confer a survival benefit. For endometrial cancer, it is unlikely that we will have as robust data, so we often look to retrospective studies such as the one presented by Tait et al.
“The study utilized a data set that was originally collected to assess the impact of an ERAS protocol. So, it is important to note that the data set was not collected with the intent of evaluating SLN mapping versus full lymphadenectomy. This explains why pathological data regarding lymph node yield and final surgicopathologic staging are absent,” he said.
Adoption of sentinel node biopsy is gaining popularity
“Overall, SLN mapping is safe (from a surgical standpoint) and may decrease perioperative morbidity,” Dr. Chura said. “The adoption of SLN mapping also appears to be increasing. Some gyn oncologists (including myself) are even performing SLN mapping on patients with endometrial intraepithelial neoplasia given the risk of malignancy being identified on final pathology.
“The current study provides more of a glimpse into the practice patterns of the authors’ institution where ‘full lymphadenectomy was very dependent upon the surgeon (P < .001)’ than it demonstrates one technique is better than the other. The ultimate question is how we define ‘better?’ Survival? Less morbidity? Improved accuracy of nodal metastasis? Shorter length of stay?” Dr. Chura said.Dr. Tait and colleagues as well as Dr. Chura had no conflicts of interest and no relevant financial disclosures.
SOURCE: Tate DL et al. J Minim Invasive Gynecol. 2020 Dec 19. doi: 10.1016/jmig.2020.12.019.
FROM THE JOURNAL OF MINIMALLY INVASIVE GYNECOLOGY
Home pregnancy tests—Is ectopic always on your mind?
CASE Unidentified ectopic pregnancy leads to rupture*
A 33-year-old woman (G1 P0010) with 2 positive home pregnancy tests presents to the emergency department (ED) reporting intermittent vaginal bleeding for 3 days. Her last menstrual period was 10 weeks ago, but she reports that her menses are always irregular. She has a history of asymptomatic chlamydia, as well as spontaneous abortion 2 years prior. At present, she denies abdominal pain or vaginal discharge.
Upon examination her vital signs are: temperature, 98.3 °F; pulse, 112 bpm, with a resting rate of 16 bpm; blood pressure (BP), 142/91 mm Hg; pulse O2, 99%; height, 4’ 3”; weight, 115 lb. Her labs are: hemoglobin, 12.1 g/dL; hematocrit, 38%; serum human chorionic gonadotropin (hCG) 236 mIU/mL. Upon pelvic examination, no active bleeding is noted. She agrees to be followed up by her gynecologist and is given a prescription for serum hCG in 2 days. She is instructed to return to the ED should she have pain or increased vaginal bleeding.
Three days later, the patient follows up with her gynecologist reporting mild cramping. She notes having had an episode of heavy vaginal bleeding and a “weakly positive” home pregnancy test. Transvaginal ultrasonography notes endometrial thickness 0.59 mm and unremarkable adnexa. A urine pregnancy test performed in the office is positive; urinalysis is positive for nitrites. With the bleeding slowed, the gynecologist’s overall impression is that the patient has undergone complete spontaneous abortion. She prescribes Macrobid for the urinary tract infection. She does not obtain the ED-prescribed serum HCG levels, as she feels, since complete spontaneous abortion has occurred there is no need to obtain a follow-up serum HCG.
Five days later, the patient returns to the ED reporting abdominal pain after eating. Fever and productive cough of 2 days are noted. The patient states that she had a recent miscarriage. The overall impression of the patient’s condition is bronchitis, and it is noted on the patient’s record, “unlikely ectopic pregnancy and pregnancy test may be false positive,” hence a pregnancy test is not ordered. Examination reveals mild suprapubic tenderness with no rebound; no pelvic exam is performed. The patient is instructed to follow up with a health care clinic within a week, and to return to the ED with severe abdominal pain, higher fever, or any new concerning symptoms. A Zithromax Z-pak is prescribed.
Four days later, the patient is brought by ambulance to the ED of the local major medical center with severe abdominal pain involving the right lower quadrant. She states that she had a miscarriage 3 weeks prior and was recently treated for bronchitis. She has dizziness when standing. Her vital signs are: temperature, 97.8 °F; heart rate, 95 bpm; BP, 72/48 mm Hg; pulse O2, 100%. She reports her abdominal pain to be 6/10.
The patient is given a Lactated Ringer’s bolus of 1,000 mL for a hypotensive episode. Computed tomography is obtained and notes, “low attenuation in the left adnexa with a dilated fallopian tube.” A large heterogeneous collection of fluid in the pelvis is noted with active extravasation, consistent with an “acute bleed.”
The patient is brought to the operating room with a diagnosis of probable ruptured ectopic pregnancy. Intraoperatively she is noted to have a right ruptured ectopic and left tubo-ovarian abscess. The surgeon proceeds with right salpingectomy and left salpingo-oophorectomy. Three liters of hemoperitoneum is found.
She is followed postoperatively with serum hCG until levels are negative. Her postoperative course is uneventful. Her only future option for pregnancy is through assisted reproductive technology (ART) with in vitro fertilization (IVF). The patient sues the gynecologist and second ED physician for presumed inappropriate assessment for ectopic pregnancy.
*The “facts” of this case are a composite, drawn from several cases to illustrate medical and legal issues. The statement of facts should be considered hypothetical.
Continue to: WHAT’S THE VERDICT?...
WHAT’S THE VERDICT?
A defense verdict is returned.
Medical considerations
The incidence of ectopic pregnancy is 2% of all pregnancies, with a higher incidence (about 4%) among infertility patients.1 Up to 10% of ectopic pregnancies have no symptoms.2
Clinical presentations. Classic signs of ectopic pregnancy include:
- abdominal pain
- vaginal bleeding
- late menses (often noted).
A recent case of ectopic pregnancy presenting with chest pain was reported.3 Clinicians must never lose site of the fact that ectopic pregnancy is the most common cause of maternal mortality in the first trimester, with an incidence of 1% to 10% of all first-trimester deaths.4
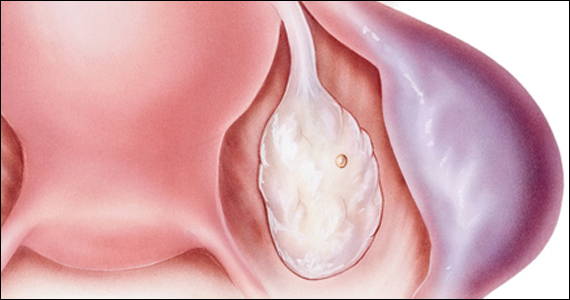
Risk factors include pelvic inflammatory disease, as demonstrated in the opening case. “The silent epidemic of chlamydia” comes to mind, and tobacco smoking can adversely affect tubal cilia, as can pelvic adhesions and/or prior tubal surgery. All of these factors can predispose a patient to ectopic pregnancy; in addition, intrauterine devices, endometriosis, tubal ligation (or ligation reversal), all can set the stage for an ectopic pregnancy.5 Appropriate serum hCG monitoring during early pregnancy can assist in sorting out pregnancies of unknown location (PUL; FIGURE). First trimester ultrasonography, at 5 weeks gestation, usually identifies early intrauterine gestation.

Imaging. With regard to pelvic sonography, the earliest sign of an intrauterine pregnancy (IUP) is a sac eccentrically located in the decidua.6 As the IUP progresses, it becomes equated with a “double decidual sign,” with double rings of tissue around the sac.6 If the pregnancy is located in an adnexal mass, it is frequently inhomogeneous or noncystic in appearance (ie, “the blob” sign); the positive predictive value (PPV) is 96%.2 The PPV of transvaginal ultrasound is 80%, as paratubal, paraovarian, ovarian cyst, and hydrosalpinx can affect the interpretation.7
Heterotopic pregnancy includes an intrauterine gestation and an ectopic pregnancy. This presentation includes the presence of a “pseudosac” in the endometrial cavity plus an extrauterine gestation. Heterotopic pregnancies have become somewhat more common as ART/IVF has unfolded, especially prior to the predominance of single embryo transfer.
Managing ectopic pregnancy
For cases of early pregnancy complicated by intermittent bleeding and/or pain, monitoring with serum hCG levels at 48-hour intervals to distinguish a viable IUP from an abnormal IUP or an ectopic is appropriate. The “discriminatory zone” collates serum hCG levels with findings on ultrasonography. Specific lower limits of serum hCG levels are not clear cut, with recommendations of 3,500 mIU/mL to provide sonographic evidence of an intrauterine gestation “to avoid misdiagnosis and possible interruption of intrauterine pregnancy,” as conveyed in the American College of Obstetricians and Gynecologists 2018 practice bulletin.8 Serum progesterone levels also have been suggested to complement hCG levels; a progesterone level of <20 nmol/L is consistent with an abnormal pregnancy, whereas levels >25 nmol/L are suggestive of a viable pregnancy.2 Inhibin A levels also have been suggested to be helpful, but they are not an ideal monitoring tool.
While most ectopic pregnancies are located in the fallopian tube, other locations also can be abdominal or ovarian. In addition, cesarean scar ectopic pregnancy can occur and often is associated with delay in diagnosis and greater morbidity due to such delay.9 With regard to ovarian ectopic, Spiegelberg criteria are established for diagnosis (TABLE 1).10
Appropriate management of an ectopic pregnancy is dependent upon the gestational age, serum hCG levels, and imaging findings, as well as the patient’s symptoms and exam findings. Treatment is established in large part on a case-by-case basis and includes, for early pregnancy, expectant management and use of methotrexate (TABLE 2).11 Dilation and curettage may be used to identify the pregnancy’s location when the serum hCG level is below 2,000 mIU/mL and there is no evidence of an IUP on ultrasound. Surgical treatment can include minimally invasive salpingostomy or salpingectomy and, depending on circumstance, laparotomy may be indicated.

Fertility following ectopic pregnancy varies and is affected by location, treatment, predisposing factors, total number of ectopic pregnancies, and other factors. Ectopic pregnancy, although rare, also can occur with use of IVF. Humans are not unique with regard to ectopic pregnancies, as they also occur in sheep.12
Continue to: Legal perspective...
Legal perspective
Lawsuits related to ectopic pregnancy are not a new phenomenon. In fact, in 1897, a physician in Ohio who misdiagnosed an “extrauterine pregnancy” as appendicitis was the center of a malpractice lawsuit.13 Unrecognized or mishandled ectopic pregnancy can result in serious injuries—in the range of 1% to 10% (see above) of maternal deaths are related to ectopic pregnancy.14 Ectopic pregnancy cases, therefore, have been the subject of substantial litigation over the years. An informal, noncomprehensive review of malpractice lawsuits brought from 2000 to 2019, found more than 300 ectopic pregnancy cases. Given the large number of malpractice claims against ObGyns,15 ectopic pregnancy cases are only a small portion of all ObGyn malpractice cases.16
A common claim: negligent diagnosis or treatment
The most common basis for lawsuits in cases of ectopic pregnancy is the clinician’s negligent failure to properly diagnose the ectopic nature of the pregnancy. There are also a number of cases claiming negligent treatment of an identified ectopic pregnancy. Not every missed diagnosis, or unsuccessful treatment, leads to liability, of course. It is only when a diagnosis or treatment fails to meet the standard of care within the profession that there should be liability. That standard of care is generally defined by what a reasonably prudent physician would do under the circumstances. Expert witnesses, who are familiar with the standard of practice within the specialty, are usually necessary to establish what that practice is. Both the plaintiff and the defense obtain experts, the former to prove what the standard of care is and that the standard was not met in the case at hand. The defense experts are usually arguing that the standard of care was met.17 Inadequate diagnosis of ectopic pregnancy or other condition may arise from a failure to take a sufficient history, conduct an appropriately thorough physical examination, recognize any of the symptoms that would suggest it is present, use and conduct ultrasound correctly, or follow-up appropriately with additional testing.18
A malpractice claim of negligent treatment can involve any the following circumstances19:
- failure to establish an appropriate treatment plan
- prescribing inappropriate medications for the patient (eg, methotrexate, when it is contraindicated)
- delivering the wrong medication or the wrong amount of the right medication
- performing a procedure badly
- undertaking a new treatment without adequate instruction and preparation.
Given the nature and risks of ectopic pregnancy, ongoing, frequent contact with the patient is essential from the point at which the condition is suspected. The greater the risk of harm (probability or consequence), the more careful any professional ought to be. Because ectopic pregnancy is not an uncommon occurrence, and because it can have devastating effects, including death, a reasonably prudent practitioner would be especially aware of the clinical presentations discussed above.20 In the opening case, the treatment plan was not well documented.
Negligence must lead to patient harm. In addition to negligence (proving that the physician did not act in accordance with the standard of care), to prevail in a malpractice case, the plaintiff-patient must prove that the negligence caused the injury, or worsened it. If the failure to make a diagnosis would not have made any difference in a harm the patient suffered, there are no damages and no liability. Suppose, for example, that a physician negligently failed to diagnose ectopic pregnancy, but performed surgery expecting to find the misdiagnosed condition. In the course of the surgery, however, the surgeon discovered and appropriately treated the ectopic pregnancy. (A version of this happened in the old 19th century case mentioned above.) The negligence of the physician did not cause harm, so there are no damages and no liability.
Continue to: Informed consent is vital...
Informed consent is vital
A part of malpractice is informed consent (or the absence of it)—issues that can arise in any medical care.21 It is wise to pay particular attention in cases where the nature of the illness is unknown, and where there are significant uncertainties and the nature of testing and treatment may change substantially over a period of a few days or few weeks. As always, informed consent should include a discussion of what process or procedure is proposed, its risks and benefits, alternative approaches that might be available, and the risk of doing nothing. Frequently, the uncertainty of ectopic pregnancy complicates the informed consent process.22
Because communication with the patient is an essential function of informed consent, the consent process should productively be used in PUL and similar cases to inform the patient about the uncertainty, and the testing and (nonsurgical) treatment that will occur. This is an opportunity to reinforce the message that the patient must maintain ongoing communication with the physician’s office about changes in her condition, and appear for each appointment scheduled. If more invasive procedures—notably surgery—become required, a separate consent process should be completed, because the risks and considerations are now meaningfully different than when treatment began. As a general matter, any possible treatment that may result in infertility or reduced reproductive capacity should specifically be included in the consent process.
In the hypothetical case, the gynecologist failed to obtain a follow-up serum hCG level. In addition, the record did not reflect ectopic pregnancy in the differential diagnosis. As noted above, the patient had predisposing factors for an ectopic pregnancy. The physician should have acknowledged the history of sexually transmitted disease predisposing her to an ectopic pregnancy. Monitoring of serum hCG levels until they are negative is appropriate with ectopic, or presumed ectopic, pregnancy management. Appropriate monitoring did not occur in this case. Each of these errors (following up on serum hCG levels and the inadequacy of notations about the possibility of ectopic pregnancy) seem inconsistent with the usual standard of care. Furthermore, as a result of the outcome, the only future option for the patient to pursue pregnancy was IVF.
Other legal issues
There are a number of other legal issues that are associated with the topic of ectopic pregnancy. There is evidence, for example, that Catholic and non-Catholic hospitals treat ectopic pregnancies differently,23 which may reflect different views on taking a life or the use of methotrexate and its association with abortion.24 In addition, the possibility of an increase in future ectopic pregnancies is one of the “risks” of abortion that pro-life organizations have pushed to see included in abortion informed consent.25 This has led some commentators to conclude that some Catholic hospitals violate federal law in managing ectopic pregnancy. There is also evidence of “overwhelming rates of medical misinformation on pregnancy center websites, including a link between abortion and ectopic pregnancy.”26
The fact that cesarean deliveries are related to an increased risk for ectopic pregnancy (because of the risk of cesarean scar ectopic pregnancy) also has been cited as information that should play a role in the consent process for cesarean delivery.27 In terms of liability, failed tubal ligation leads to a 33% risk of ectopic pregnancy.28 The risk of ectopic pregnancy is also commonly included in surrogacy contracts.29
Why the outcome was for the defense
The opening hypothetical case illustrates some of the uncertainties of medical malpractice cases. As noted, there appeared a deviation from the usual standard of care, particularly the failure to follow up on the serum hCG level. The weakness in the medical record, failing to note the possibility of ectopic pregnancy, also was probably an error but, apparently, the court felt that this did not result in any harm to the patient.
The question arises of how there would be a defense verdict in light of the failure to track consecutive serum hCG levels. A speculative explanation is that there are many uncertainties in most lawsuits. Procedural problems may result in a case being limited, expert witnesses are essential to both the plaintiff and defense, with the quality of their review and testimony possibly uneven. Judges and juries may rely on one expert witness rather than another, juries vary, and the quality of advocacy differs. Any of these situations can contribute to the unpredictability of the outcome of a case. In the case above, the liability was somewhat uncertain, and the various other factors tipped in favor of a defense verdict. ●
- Centers for Disease Control and Prevention. Ectopic pregnancy—United States, 1990‒1992. MMWR Morb Mortal Wkly Rep. 1995;44:46-48.
- Kirk E, Bottomley C, Bourne T. Diagnosing ectopic pregnancy and current concepts in the management of pregnancy of unknown location. Hum Reprod Update. 2012;20:250-261.
- Dichter E, Espinosa J, Baird J, Lucerna A. An unusual emergency department case: ruptured ectopic pregnancy presenting as chest pain. World J Emerg Med. 2017;8:71-73.
- Cecchino GN, Araujo E, Elito J. Methotrexate for ectopic pregnancy: when and how. Arch Gynecol Obstet. 2014;290:417- 423.
- Barnhart KT, Sammel MD, Cracia CR, et al. Risk factors for ectopic pregnancy in women with symptomatic firsttrimester pregnancies. Fertil Steril. 2006;86:36-43.
- Carusi D. Pregnancy of unknown location: evaluation and management. Semin Perinatol. 2019;43:95-100.
- Barnhart KT, Fay CA, Suescum M, et al. Clinical factors affecting the accuracy of ultrasonography in symptomatic first-trimester pregnancy. Obstet Gynecol. 2011;117:299-306.
- American College of Obstetricians and Gynecologists Practice Bulletin No. 193: tubal ectopic pregnancy. Obstet Gynecol. 2018;131:e91-e103.
- Bouyer J, Coste J, Fernandez H, et al. Sites of ectopic pregnancy: a 10-year population-based study of 1800 cases. Hum Reprod. 2002;17:3224-3230.
- Spiegelberg O. Zur casuistic der ovarial schwangerschaft. Arch Gynecol. 1978;13:73.
- OB Hospitalist Group. Methotrexate use for ectopic pregnancies guidelines. https://www.obhg.com/wp-content /uploads/2020/01/Methotrexate-Use-for-EctopicPregnancies_2016-updates.pdf. Accessed December 10, 2020.
- Brozos C, Kargiannis I, Kiossis E, et al. Ectopic pregnancy through a caesarean scar in a ewe. N Z Vet J. 2013;61:373-375.
- Tucker v. Gillette, 12 Ohio Cir. Dec. 401 (Cir. Ct. 1901).
- Creanga AA, Syverson C, Seed K, et al. Pregnancy-related mortality in the United States, 2011–2013. Obstet Gynecol. 2017;130:366-373.
- Matthews LR, Alvi FA, Milad MP. Reproductive surgery malpractice patterns. Fertil Steril. 2016;106:e42-e43.
- Kim B. The impact of malpractice risk on the use of obstetrics procedures. J Legal Studies. 2006;36:S79-S120.
- Abinader R, Warsof S. Complications involving obstetrical ultrasound. In: Warsof S, Shwayder JM, eds. Legal Concepts and Best Practices in Obstetrics: The Nuts and Bolts Guide to Mitigating Risk. 2019;45-48.
- Creanga AA, Shapiro-Mendoza CK, Bish CL, et al. Trends in ectopic pregnancy mortality in the United States: 1980-2007. Obstet Gynecol. 2011;117:837-843.
- Shwayder JM. IUP diagnosed and treated as ectopic: How bad can it get? Contemporary OB/GYN. 2019;64:49-46.
- Kaplan AI. Should this ectopic pregnancy have been diagnosed earlier? Contemporary OB/GYN. 2017;62:53.
- American College of Obstetricians and Gynecologists Committee on Ethics. Committee opinion 439: informed consent. Reaffirmed 2015. https://www.acog.org/clinical /clinical-guidance/committee-opinion/articles/2009/08 /informed-consent. Accessed December 9, 2020.
- Shwayder JM. Liability in ob/gyn ultrasound. Contemporary OB/GYN. 2017;62:32-49.
- Fisher LN. Institutional religious exemptions: a balancing approach. BYU Law Review. 2014;415-444.
- Makdisi J. Aquinas’s prohibition of killing reconsidered. J Catholic Legal Stud. 2019:57:67-128.
- Franzonello A. Remarks of Anna Franzonello. Alb Law J Sci Tech. 2012;23:519-530.
- Malcolm HE. Pregnancy centers and the limits of mandated disclosure. Columbia Law Rev. 2019;119:1133-1168.
- Kukura E. Contested care: the limitations of evidencebased maternity care reform. Berkeley J Gender Law Justice. 2016;31:241-298.
- Donley G. Contraceptive equity: curing the sex discrimination in the ACA’s mandate. Alabama Law Rev. 2019;71:499-560.
- Berk H. Savvy surrogates and rock star parents: compensation provisions, contracting practices, and the value of womb work. Law Social Inquiry. 2020;45:398-431.
CASE Unidentified ectopic pregnancy leads to rupture*
A 33-year-old woman (G1 P0010) with 2 positive home pregnancy tests presents to the emergency department (ED) reporting intermittent vaginal bleeding for 3 days. Her last menstrual period was 10 weeks ago, but she reports that her menses are always irregular. She has a history of asymptomatic chlamydia, as well as spontaneous abortion 2 years prior. At present, she denies abdominal pain or vaginal discharge.
Upon examination her vital signs are: temperature, 98.3 °F; pulse, 112 bpm, with a resting rate of 16 bpm; blood pressure (BP), 142/91 mm Hg; pulse O2, 99%; height, 4’ 3”; weight, 115 lb. Her labs are: hemoglobin, 12.1 g/dL; hematocrit, 38%; serum human chorionic gonadotropin (hCG) 236 mIU/mL. Upon pelvic examination, no active bleeding is noted. She agrees to be followed up by her gynecologist and is given a prescription for serum hCG in 2 days. She is instructed to return to the ED should she have pain or increased vaginal bleeding.
Three days later, the patient follows up with her gynecologist reporting mild cramping. She notes having had an episode of heavy vaginal bleeding and a “weakly positive” home pregnancy test. Transvaginal ultrasonography notes endometrial thickness 0.59 mm and unremarkable adnexa. A urine pregnancy test performed in the office is positive; urinalysis is positive for nitrites. With the bleeding slowed, the gynecologist’s overall impression is that the patient has undergone complete spontaneous abortion. She prescribes Macrobid for the urinary tract infection. She does not obtain the ED-prescribed serum HCG levels, as she feels, since complete spontaneous abortion has occurred there is no need to obtain a follow-up serum HCG.
Five days later, the patient returns to the ED reporting abdominal pain after eating. Fever and productive cough of 2 days are noted. The patient states that she had a recent miscarriage. The overall impression of the patient’s condition is bronchitis, and it is noted on the patient’s record, “unlikely ectopic pregnancy and pregnancy test may be false positive,” hence a pregnancy test is not ordered. Examination reveals mild suprapubic tenderness with no rebound; no pelvic exam is performed. The patient is instructed to follow up with a health care clinic within a week, and to return to the ED with severe abdominal pain, higher fever, or any new concerning symptoms. A Zithromax Z-pak is prescribed.
Four days later, the patient is brought by ambulance to the ED of the local major medical center with severe abdominal pain involving the right lower quadrant. She states that she had a miscarriage 3 weeks prior and was recently treated for bronchitis. She has dizziness when standing. Her vital signs are: temperature, 97.8 °F; heart rate, 95 bpm; BP, 72/48 mm Hg; pulse O2, 100%. She reports her abdominal pain to be 6/10.
The patient is given a Lactated Ringer’s bolus of 1,000 mL for a hypotensive episode. Computed tomography is obtained and notes, “low attenuation in the left adnexa with a dilated fallopian tube.” A large heterogeneous collection of fluid in the pelvis is noted with active extravasation, consistent with an “acute bleed.”
The patient is brought to the operating room with a diagnosis of probable ruptured ectopic pregnancy. Intraoperatively she is noted to have a right ruptured ectopic and left tubo-ovarian abscess. The surgeon proceeds with right salpingectomy and left salpingo-oophorectomy. Three liters of hemoperitoneum is found.
She is followed postoperatively with serum hCG until levels are negative. Her postoperative course is uneventful. Her only future option for pregnancy is through assisted reproductive technology (ART) with in vitro fertilization (IVF). The patient sues the gynecologist and second ED physician for presumed inappropriate assessment for ectopic pregnancy.
*The “facts” of this case are a composite, drawn from several cases to illustrate medical and legal issues. The statement of facts should be considered hypothetical.
Continue to: WHAT’S THE VERDICT?...
WHAT’S THE VERDICT?
A defense verdict is returned.
Medical considerations
The incidence of ectopic pregnancy is 2% of all pregnancies, with a higher incidence (about 4%) among infertility patients.1 Up to 10% of ectopic pregnancies have no symptoms.2
Clinical presentations. Classic signs of ectopic pregnancy include:
- abdominal pain
- vaginal bleeding
- late menses (often noted).
A recent case of ectopic pregnancy presenting with chest pain was reported.3 Clinicians must never lose site of the fact that ectopic pregnancy is the most common cause of maternal mortality in the first trimester, with an incidence of 1% to 10% of all first-trimester deaths.4
Risk factors include pelvic inflammatory disease, as demonstrated in the opening case. “The silent epidemic of chlamydia” comes to mind, and tobacco smoking can adversely affect tubal cilia, as can pelvic adhesions and/or prior tubal surgery. All of these factors can predispose a patient to ectopic pregnancy; in addition, intrauterine devices, endometriosis, tubal ligation (or ligation reversal), all can set the stage for an ectopic pregnancy.5 Appropriate serum hCG monitoring during early pregnancy can assist in sorting out pregnancies of unknown location (PUL; FIGURE). First trimester ultrasonography, at 5 weeks gestation, usually identifies early intrauterine gestation.

Imaging. With regard to pelvic sonography, the earliest sign of an intrauterine pregnancy (IUP) is a sac eccentrically located in the decidua.6 As the IUP progresses, it becomes equated with a “double decidual sign,” with double rings of tissue around the sac.6 If the pregnancy is located in an adnexal mass, it is frequently inhomogeneous or noncystic in appearance (ie, “the blob” sign); the positive predictive value (PPV) is 96%.2 The PPV of transvaginal ultrasound is 80%, as paratubal, paraovarian, ovarian cyst, and hydrosalpinx can affect the interpretation.7
Heterotopic pregnancy includes an intrauterine gestation and an ectopic pregnancy. This presentation includes the presence of a “pseudosac” in the endometrial cavity plus an extrauterine gestation. Heterotopic pregnancies have become somewhat more common as ART/IVF has unfolded, especially prior to the predominance of single embryo transfer.
Managing ectopic pregnancy
For cases of early pregnancy complicated by intermittent bleeding and/or pain, monitoring with serum hCG levels at 48-hour intervals to distinguish a viable IUP from an abnormal IUP or an ectopic is appropriate. The “discriminatory zone” collates serum hCG levels with findings on ultrasonography. Specific lower limits of serum hCG levels are not clear cut, with recommendations of 3,500 mIU/mL to provide sonographic evidence of an intrauterine gestation “to avoid misdiagnosis and possible interruption of intrauterine pregnancy,” as conveyed in the American College of Obstetricians and Gynecologists 2018 practice bulletin.8 Serum progesterone levels also have been suggested to complement hCG levels; a progesterone level of <20 nmol/L is consistent with an abnormal pregnancy, whereas levels >25 nmol/L are suggestive of a viable pregnancy.2 Inhibin A levels also have been suggested to be helpful, but they are not an ideal monitoring tool.
While most ectopic pregnancies are located in the fallopian tube, other locations also can be abdominal or ovarian. In addition, cesarean scar ectopic pregnancy can occur and often is associated with delay in diagnosis and greater morbidity due to such delay.9 With regard to ovarian ectopic, Spiegelberg criteria are established for diagnosis (TABLE 1).10
Appropriate management of an ectopic pregnancy is dependent upon the gestational age, serum hCG levels, and imaging findings, as well as the patient’s symptoms and exam findings. Treatment is established in large part on a case-by-case basis and includes, for early pregnancy, expectant management and use of methotrexate (TABLE 2).11 Dilation and curettage may be used to identify the pregnancy’s location when the serum hCG level is below 2,000 mIU/mL and there is no evidence of an IUP on ultrasound. Surgical treatment can include minimally invasive salpingostomy or salpingectomy and, depending on circumstance, laparotomy may be indicated.

Fertility following ectopic pregnancy varies and is affected by location, treatment, predisposing factors, total number of ectopic pregnancies, and other factors. Ectopic pregnancy, although rare, also can occur with use of IVF. Humans are not unique with regard to ectopic pregnancies, as they also occur in sheep.12
Continue to: Legal perspective...
Legal perspective
Lawsuits related to ectopic pregnancy are not a new phenomenon. In fact, in 1897, a physician in Ohio who misdiagnosed an “extrauterine pregnancy” as appendicitis was the center of a malpractice lawsuit.13 Unrecognized or mishandled ectopic pregnancy can result in serious injuries—in the range of 1% to 10% (see above) of maternal deaths are related to ectopic pregnancy.14 Ectopic pregnancy cases, therefore, have been the subject of substantial litigation over the years. An informal, noncomprehensive review of malpractice lawsuits brought from 2000 to 2019, found more than 300 ectopic pregnancy cases. Given the large number of malpractice claims against ObGyns,15 ectopic pregnancy cases are only a small portion of all ObGyn malpractice cases.16
A common claim: negligent diagnosis or treatment
The most common basis for lawsuits in cases of ectopic pregnancy is the clinician’s negligent failure to properly diagnose the ectopic nature of the pregnancy. There are also a number of cases claiming negligent treatment of an identified ectopic pregnancy. Not every missed diagnosis, or unsuccessful treatment, leads to liability, of course. It is only when a diagnosis or treatment fails to meet the standard of care within the profession that there should be liability. That standard of care is generally defined by what a reasonably prudent physician would do under the circumstances. Expert witnesses, who are familiar with the standard of practice within the specialty, are usually necessary to establish what that practice is. Both the plaintiff and the defense obtain experts, the former to prove what the standard of care is and that the standard was not met in the case at hand. The defense experts are usually arguing that the standard of care was met.17 Inadequate diagnosis of ectopic pregnancy or other condition may arise from a failure to take a sufficient history, conduct an appropriately thorough physical examination, recognize any of the symptoms that would suggest it is present, use and conduct ultrasound correctly, or follow-up appropriately with additional testing.18
A malpractice claim of negligent treatment can involve any the following circumstances19:
- failure to establish an appropriate treatment plan
- prescribing inappropriate medications for the patient (eg, methotrexate, when it is contraindicated)
- delivering the wrong medication or the wrong amount of the right medication
- performing a procedure badly
- undertaking a new treatment without adequate instruction and preparation.
Given the nature and risks of ectopic pregnancy, ongoing, frequent contact with the patient is essential from the point at which the condition is suspected. The greater the risk of harm (probability or consequence), the more careful any professional ought to be. Because ectopic pregnancy is not an uncommon occurrence, and because it can have devastating effects, including death, a reasonably prudent practitioner would be especially aware of the clinical presentations discussed above.20 In the opening case, the treatment plan was not well documented.
Negligence must lead to patient harm. In addition to negligence (proving that the physician did not act in accordance with the standard of care), to prevail in a malpractice case, the plaintiff-patient must prove that the negligence caused the injury, or worsened it. If the failure to make a diagnosis would not have made any difference in a harm the patient suffered, there are no damages and no liability. Suppose, for example, that a physician negligently failed to diagnose ectopic pregnancy, but performed surgery expecting to find the misdiagnosed condition. In the course of the surgery, however, the surgeon discovered and appropriately treated the ectopic pregnancy. (A version of this happened in the old 19th century case mentioned above.) The negligence of the physician did not cause harm, so there are no damages and no liability.
Continue to: Informed consent is vital...
Informed consent is vital
A part of malpractice is informed consent (or the absence of it)—issues that can arise in any medical care.21 It is wise to pay particular attention in cases where the nature of the illness is unknown, and where there are significant uncertainties and the nature of testing and treatment may change substantially over a period of a few days or few weeks. As always, informed consent should include a discussion of what process or procedure is proposed, its risks and benefits, alternative approaches that might be available, and the risk of doing nothing. Frequently, the uncertainty of ectopic pregnancy complicates the informed consent process.22
Because communication with the patient is an essential function of informed consent, the consent process should productively be used in PUL and similar cases to inform the patient about the uncertainty, and the testing and (nonsurgical) treatment that will occur. This is an opportunity to reinforce the message that the patient must maintain ongoing communication with the physician’s office about changes in her condition, and appear for each appointment scheduled. If more invasive procedures—notably surgery—become required, a separate consent process should be completed, because the risks and considerations are now meaningfully different than when treatment began. As a general matter, any possible treatment that may result in infertility or reduced reproductive capacity should specifically be included in the consent process.
In the hypothetical case, the gynecologist failed to obtain a follow-up serum hCG level. In addition, the record did not reflect ectopic pregnancy in the differential diagnosis. As noted above, the patient had predisposing factors for an ectopic pregnancy. The physician should have acknowledged the history of sexually transmitted disease predisposing her to an ectopic pregnancy. Monitoring of serum hCG levels until they are negative is appropriate with ectopic, or presumed ectopic, pregnancy management. Appropriate monitoring did not occur in this case. Each of these errors (following up on serum hCG levels and the inadequacy of notations about the possibility of ectopic pregnancy) seem inconsistent with the usual standard of care. Furthermore, as a result of the outcome, the only future option for the patient to pursue pregnancy was IVF.
Other legal issues
There are a number of other legal issues that are associated with the topic of ectopic pregnancy. There is evidence, for example, that Catholic and non-Catholic hospitals treat ectopic pregnancies differently,23 which may reflect different views on taking a life or the use of methotrexate and its association with abortion.24 In addition, the possibility of an increase in future ectopic pregnancies is one of the “risks” of abortion that pro-life organizations have pushed to see included in abortion informed consent.25 This has led some commentators to conclude that some Catholic hospitals violate federal law in managing ectopic pregnancy. There is also evidence of “overwhelming rates of medical misinformation on pregnancy center websites, including a link between abortion and ectopic pregnancy.”26
The fact that cesarean deliveries are related to an increased risk for ectopic pregnancy (because of the risk of cesarean scar ectopic pregnancy) also has been cited as information that should play a role in the consent process for cesarean delivery.27 In terms of liability, failed tubal ligation leads to a 33% risk of ectopic pregnancy.28 The risk of ectopic pregnancy is also commonly included in surrogacy contracts.29
Why the outcome was for the defense
The opening hypothetical case illustrates some of the uncertainties of medical malpractice cases. As noted, there appeared a deviation from the usual standard of care, particularly the failure to follow up on the serum hCG level. The weakness in the medical record, failing to note the possibility of ectopic pregnancy, also was probably an error but, apparently, the court felt that this did not result in any harm to the patient.
The question arises of how there would be a defense verdict in light of the failure to track consecutive serum hCG levels. A speculative explanation is that there are many uncertainties in most lawsuits. Procedural problems may result in a case being limited, expert witnesses are essential to both the plaintiff and defense, with the quality of their review and testimony possibly uneven. Judges and juries may rely on one expert witness rather than another, juries vary, and the quality of advocacy differs. Any of these situations can contribute to the unpredictability of the outcome of a case. In the case above, the liability was somewhat uncertain, and the various other factors tipped in favor of a defense verdict. ●
CASE Unidentified ectopic pregnancy leads to rupture*
A 33-year-old woman (G1 P0010) with 2 positive home pregnancy tests presents to the emergency department (ED) reporting intermittent vaginal bleeding for 3 days. Her last menstrual period was 10 weeks ago, but she reports that her menses are always irregular. She has a history of asymptomatic chlamydia, as well as spontaneous abortion 2 years prior. At present, she denies abdominal pain or vaginal discharge.
Upon examination her vital signs are: temperature, 98.3 °F; pulse, 112 bpm, with a resting rate of 16 bpm; blood pressure (BP), 142/91 mm Hg; pulse O2, 99%; height, 4’ 3”; weight, 115 lb. Her labs are: hemoglobin, 12.1 g/dL; hematocrit, 38%; serum human chorionic gonadotropin (hCG) 236 mIU/mL. Upon pelvic examination, no active bleeding is noted. She agrees to be followed up by her gynecologist and is given a prescription for serum hCG in 2 days. She is instructed to return to the ED should she have pain or increased vaginal bleeding.
Three days later, the patient follows up with her gynecologist reporting mild cramping. She notes having had an episode of heavy vaginal bleeding and a “weakly positive” home pregnancy test. Transvaginal ultrasonography notes endometrial thickness 0.59 mm and unremarkable adnexa. A urine pregnancy test performed in the office is positive; urinalysis is positive for nitrites. With the bleeding slowed, the gynecologist’s overall impression is that the patient has undergone complete spontaneous abortion. She prescribes Macrobid for the urinary tract infection. She does not obtain the ED-prescribed serum HCG levels, as she feels, since complete spontaneous abortion has occurred there is no need to obtain a follow-up serum HCG.
Five days later, the patient returns to the ED reporting abdominal pain after eating. Fever and productive cough of 2 days are noted. The patient states that she had a recent miscarriage. The overall impression of the patient’s condition is bronchitis, and it is noted on the patient’s record, “unlikely ectopic pregnancy and pregnancy test may be false positive,” hence a pregnancy test is not ordered. Examination reveals mild suprapubic tenderness with no rebound; no pelvic exam is performed. The patient is instructed to follow up with a health care clinic within a week, and to return to the ED with severe abdominal pain, higher fever, or any new concerning symptoms. A Zithromax Z-pak is prescribed.
Four days later, the patient is brought by ambulance to the ED of the local major medical center with severe abdominal pain involving the right lower quadrant. She states that she had a miscarriage 3 weeks prior and was recently treated for bronchitis. She has dizziness when standing. Her vital signs are: temperature, 97.8 °F; heart rate, 95 bpm; BP, 72/48 mm Hg; pulse O2, 100%. She reports her abdominal pain to be 6/10.
The patient is given a Lactated Ringer’s bolus of 1,000 mL for a hypotensive episode. Computed tomography is obtained and notes, “low attenuation in the left adnexa with a dilated fallopian tube.” A large heterogeneous collection of fluid in the pelvis is noted with active extravasation, consistent with an “acute bleed.”
The patient is brought to the operating room with a diagnosis of probable ruptured ectopic pregnancy. Intraoperatively she is noted to have a right ruptured ectopic and left tubo-ovarian abscess. The surgeon proceeds with right salpingectomy and left salpingo-oophorectomy. Three liters of hemoperitoneum is found.
She is followed postoperatively with serum hCG until levels are negative. Her postoperative course is uneventful. Her only future option for pregnancy is through assisted reproductive technology (ART) with in vitro fertilization (IVF). The patient sues the gynecologist and second ED physician for presumed inappropriate assessment for ectopic pregnancy.
*The “facts” of this case are a composite, drawn from several cases to illustrate medical and legal issues. The statement of facts should be considered hypothetical.
Continue to: WHAT’S THE VERDICT?...
WHAT’S THE VERDICT?
A defense verdict is returned.
Medical considerations
The incidence of ectopic pregnancy is 2% of all pregnancies, with a higher incidence (about 4%) among infertility patients.1 Up to 10% of ectopic pregnancies have no symptoms.2
Clinical presentations. Classic signs of ectopic pregnancy include:
- abdominal pain
- vaginal bleeding
- late menses (often noted).
A recent case of ectopic pregnancy presenting with chest pain was reported.3 Clinicians must never lose site of the fact that ectopic pregnancy is the most common cause of maternal mortality in the first trimester, with an incidence of 1% to 10% of all first-trimester deaths.4
Risk factors include pelvic inflammatory disease, as demonstrated in the opening case. “The silent epidemic of chlamydia” comes to mind, and tobacco smoking can adversely affect tubal cilia, as can pelvic adhesions and/or prior tubal surgery. All of these factors can predispose a patient to ectopic pregnancy; in addition, intrauterine devices, endometriosis, tubal ligation (or ligation reversal), all can set the stage for an ectopic pregnancy.5 Appropriate serum hCG monitoring during early pregnancy can assist in sorting out pregnancies of unknown location (PUL; FIGURE). First trimester ultrasonography, at 5 weeks gestation, usually identifies early intrauterine gestation.

Imaging. With regard to pelvic sonography, the earliest sign of an intrauterine pregnancy (IUP) is a sac eccentrically located in the decidua.6 As the IUP progresses, it becomes equated with a “double decidual sign,” with double rings of tissue around the sac.6 If the pregnancy is located in an adnexal mass, it is frequently inhomogeneous or noncystic in appearance (ie, “the blob” sign); the positive predictive value (PPV) is 96%.2 The PPV of transvaginal ultrasound is 80%, as paratubal, paraovarian, ovarian cyst, and hydrosalpinx can affect the interpretation.7
Heterotopic pregnancy includes an intrauterine gestation and an ectopic pregnancy. This presentation includes the presence of a “pseudosac” in the endometrial cavity plus an extrauterine gestation. Heterotopic pregnancies have become somewhat more common as ART/IVF has unfolded, especially prior to the predominance of single embryo transfer.
Managing ectopic pregnancy
For cases of early pregnancy complicated by intermittent bleeding and/or pain, monitoring with serum hCG levels at 48-hour intervals to distinguish a viable IUP from an abnormal IUP or an ectopic is appropriate. The “discriminatory zone” collates serum hCG levels with findings on ultrasonography. Specific lower limits of serum hCG levels are not clear cut, with recommendations of 3,500 mIU/mL to provide sonographic evidence of an intrauterine gestation “to avoid misdiagnosis and possible interruption of intrauterine pregnancy,” as conveyed in the American College of Obstetricians and Gynecologists 2018 practice bulletin.8 Serum progesterone levels also have been suggested to complement hCG levels; a progesterone level of <20 nmol/L is consistent with an abnormal pregnancy, whereas levels >25 nmol/L are suggestive of a viable pregnancy.2 Inhibin A levels also have been suggested to be helpful, but they are not an ideal monitoring tool.
While most ectopic pregnancies are located in the fallopian tube, other locations also can be abdominal or ovarian. In addition, cesarean scar ectopic pregnancy can occur and often is associated with delay in diagnosis and greater morbidity due to such delay.9 With regard to ovarian ectopic, Spiegelberg criteria are established for diagnosis (TABLE 1).10
Appropriate management of an ectopic pregnancy is dependent upon the gestational age, serum hCG levels, and imaging findings, as well as the patient’s symptoms and exam findings. Treatment is established in large part on a case-by-case basis and includes, for early pregnancy, expectant management and use of methotrexate (TABLE 2).11 Dilation and curettage may be used to identify the pregnancy’s location when the serum hCG level is below 2,000 mIU/mL and there is no evidence of an IUP on ultrasound. Surgical treatment can include minimally invasive salpingostomy or salpingectomy and, depending on circumstance, laparotomy may be indicated.

Fertility following ectopic pregnancy varies and is affected by location, treatment, predisposing factors, total number of ectopic pregnancies, and other factors. Ectopic pregnancy, although rare, also can occur with use of IVF. Humans are not unique with regard to ectopic pregnancies, as they also occur in sheep.12
Continue to: Legal perspective...
Legal perspective
Lawsuits related to ectopic pregnancy are not a new phenomenon. In fact, in 1897, a physician in Ohio who misdiagnosed an “extrauterine pregnancy” as appendicitis was the center of a malpractice lawsuit.13 Unrecognized or mishandled ectopic pregnancy can result in serious injuries—in the range of 1% to 10% (see above) of maternal deaths are related to ectopic pregnancy.14 Ectopic pregnancy cases, therefore, have been the subject of substantial litigation over the years. An informal, noncomprehensive review of malpractice lawsuits brought from 2000 to 2019, found more than 300 ectopic pregnancy cases. Given the large number of malpractice claims against ObGyns,15 ectopic pregnancy cases are only a small portion of all ObGyn malpractice cases.16
A common claim: negligent diagnosis or treatment
The most common basis for lawsuits in cases of ectopic pregnancy is the clinician’s negligent failure to properly diagnose the ectopic nature of the pregnancy. There are also a number of cases claiming negligent treatment of an identified ectopic pregnancy. Not every missed diagnosis, or unsuccessful treatment, leads to liability, of course. It is only when a diagnosis or treatment fails to meet the standard of care within the profession that there should be liability. That standard of care is generally defined by what a reasonably prudent physician would do under the circumstances. Expert witnesses, who are familiar with the standard of practice within the specialty, are usually necessary to establish what that practice is. Both the plaintiff and the defense obtain experts, the former to prove what the standard of care is and that the standard was not met in the case at hand. The defense experts are usually arguing that the standard of care was met.17 Inadequate diagnosis of ectopic pregnancy or other condition may arise from a failure to take a sufficient history, conduct an appropriately thorough physical examination, recognize any of the symptoms that would suggest it is present, use and conduct ultrasound correctly, or follow-up appropriately with additional testing.18
A malpractice claim of negligent treatment can involve any the following circumstances19:
- failure to establish an appropriate treatment plan
- prescribing inappropriate medications for the patient (eg, methotrexate, when it is contraindicated)
- delivering the wrong medication or the wrong amount of the right medication
- performing a procedure badly
- undertaking a new treatment without adequate instruction and preparation.
Given the nature and risks of ectopic pregnancy, ongoing, frequent contact with the patient is essential from the point at which the condition is suspected. The greater the risk of harm (probability or consequence), the more careful any professional ought to be. Because ectopic pregnancy is not an uncommon occurrence, and because it can have devastating effects, including death, a reasonably prudent practitioner would be especially aware of the clinical presentations discussed above.20 In the opening case, the treatment plan was not well documented.
Negligence must lead to patient harm. In addition to negligence (proving that the physician did not act in accordance with the standard of care), to prevail in a malpractice case, the plaintiff-patient must prove that the negligence caused the injury, or worsened it. If the failure to make a diagnosis would not have made any difference in a harm the patient suffered, there are no damages and no liability. Suppose, for example, that a physician negligently failed to diagnose ectopic pregnancy, but performed surgery expecting to find the misdiagnosed condition. In the course of the surgery, however, the surgeon discovered and appropriately treated the ectopic pregnancy. (A version of this happened in the old 19th century case mentioned above.) The negligence of the physician did not cause harm, so there are no damages and no liability.
Continue to: Informed consent is vital...
Informed consent is vital
A part of malpractice is informed consent (or the absence of it)—issues that can arise in any medical care.21 It is wise to pay particular attention in cases where the nature of the illness is unknown, and where there are significant uncertainties and the nature of testing and treatment may change substantially over a period of a few days or few weeks. As always, informed consent should include a discussion of what process or procedure is proposed, its risks and benefits, alternative approaches that might be available, and the risk of doing nothing. Frequently, the uncertainty of ectopic pregnancy complicates the informed consent process.22
Because communication with the patient is an essential function of informed consent, the consent process should productively be used in PUL and similar cases to inform the patient about the uncertainty, and the testing and (nonsurgical) treatment that will occur. This is an opportunity to reinforce the message that the patient must maintain ongoing communication with the physician’s office about changes in her condition, and appear for each appointment scheduled. If more invasive procedures—notably surgery—become required, a separate consent process should be completed, because the risks and considerations are now meaningfully different than when treatment began. As a general matter, any possible treatment that may result in infertility or reduced reproductive capacity should specifically be included in the consent process.
In the hypothetical case, the gynecologist failed to obtain a follow-up serum hCG level. In addition, the record did not reflect ectopic pregnancy in the differential diagnosis. As noted above, the patient had predisposing factors for an ectopic pregnancy. The physician should have acknowledged the history of sexually transmitted disease predisposing her to an ectopic pregnancy. Monitoring of serum hCG levels until they are negative is appropriate with ectopic, or presumed ectopic, pregnancy management. Appropriate monitoring did not occur in this case. Each of these errors (following up on serum hCG levels and the inadequacy of notations about the possibility of ectopic pregnancy) seem inconsistent with the usual standard of care. Furthermore, as a result of the outcome, the only future option for the patient to pursue pregnancy was IVF.
Other legal issues
There are a number of other legal issues that are associated with the topic of ectopic pregnancy. There is evidence, for example, that Catholic and non-Catholic hospitals treat ectopic pregnancies differently,23 which may reflect different views on taking a life or the use of methotrexate and its association with abortion.24 In addition, the possibility of an increase in future ectopic pregnancies is one of the “risks” of abortion that pro-life organizations have pushed to see included in abortion informed consent.25 This has led some commentators to conclude that some Catholic hospitals violate federal law in managing ectopic pregnancy. There is also evidence of “overwhelming rates of medical misinformation on pregnancy center websites, including a link between abortion and ectopic pregnancy.”26
The fact that cesarean deliveries are related to an increased risk for ectopic pregnancy (because of the risk of cesarean scar ectopic pregnancy) also has been cited as information that should play a role in the consent process for cesarean delivery.27 In terms of liability, failed tubal ligation leads to a 33% risk of ectopic pregnancy.28 The risk of ectopic pregnancy is also commonly included in surrogacy contracts.29
Why the outcome was for the defense
The opening hypothetical case illustrates some of the uncertainties of medical malpractice cases. As noted, there appeared a deviation from the usual standard of care, particularly the failure to follow up on the serum hCG level. The weakness in the medical record, failing to note the possibility of ectopic pregnancy, also was probably an error but, apparently, the court felt that this did not result in any harm to the patient.
The question arises of how there would be a defense verdict in light of the failure to track consecutive serum hCG levels. A speculative explanation is that there are many uncertainties in most lawsuits. Procedural problems may result in a case being limited, expert witnesses are essential to both the plaintiff and defense, with the quality of their review and testimony possibly uneven. Judges and juries may rely on one expert witness rather than another, juries vary, and the quality of advocacy differs. Any of these situations can contribute to the unpredictability of the outcome of a case. In the case above, the liability was somewhat uncertain, and the various other factors tipped in favor of a defense verdict. ●
- Centers for Disease Control and Prevention. Ectopic pregnancy—United States, 1990‒1992. MMWR Morb Mortal Wkly Rep. 1995;44:46-48.
- Kirk E, Bottomley C, Bourne T. Diagnosing ectopic pregnancy and current concepts in the management of pregnancy of unknown location. Hum Reprod Update. 2012;20:250-261.
- Dichter E, Espinosa J, Baird J, Lucerna A. An unusual emergency department case: ruptured ectopic pregnancy presenting as chest pain. World J Emerg Med. 2017;8:71-73.
- Cecchino GN, Araujo E, Elito J. Methotrexate for ectopic pregnancy: when and how. Arch Gynecol Obstet. 2014;290:417- 423.
- Barnhart KT, Sammel MD, Cracia CR, et al. Risk factors for ectopic pregnancy in women with symptomatic firsttrimester pregnancies. Fertil Steril. 2006;86:36-43.
- Carusi D. Pregnancy of unknown location: evaluation and management. Semin Perinatol. 2019;43:95-100.
- Barnhart KT, Fay CA, Suescum M, et al. Clinical factors affecting the accuracy of ultrasonography in symptomatic first-trimester pregnancy. Obstet Gynecol. 2011;117:299-306.
- American College of Obstetricians and Gynecologists Practice Bulletin No. 193: tubal ectopic pregnancy. Obstet Gynecol. 2018;131:e91-e103.
- Bouyer J, Coste J, Fernandez H, et al. Sites of ectopic pregnancy: a 10-year population-based study of 1800 cases. Hum Reprod. 2002;17:3224-3230.
- Spiegelberg O. Zur casuistic der ovarial schwangerschaft. Arch Gynecol. 1978;13:73.
- OB Hospitalist Group. Methotrexate use for ectopic pregnancies guidelines. https://www.obhg.com/wp-content /uploads/2020/01/Methotrexate-Use-for-EctopicPregnancies_2016-updates.pdf. Accessed December 10, 2020.
- Brozos C, Kargiannis I, Kiossis E, et al. Ectopic pregnancy through a caesarean scar in a ewe. N Z Vet J. 2013;61:373-375.
- Tucker v. Gillette, 12 Ohio Cir. Dec. 401 (Cir. Ct. 1901).
- Creanga AA, Syverson C, Seed K, et al. Pregnancy-related mortality in the United States, 2011–2013. Obstet Gynecol. 2017;130:366-373.
- Matthews LR, Alvi FA, Milad MP. Reproductive surgery malpractice patterns. Fertil Steril. 2016;106:e42-e43.
- Kim B. The impact of malpractice risk on the use of obstetrics procedures. J Legal Studies. 2006;36:S79-S120.
- Abinader R, Warsof S. Complications involving obstetrical ultrasound. In: Warsof S, Shwayder JM, eds. Legal Concepts and Best Practices in Obstetrics: The Nuts and Bolts Guide to Mitigating Risk. 2019;45-48.
- Creanga AA, Shapiro-Mendoza CK, Bish CL, et al. Trends in ectopic pregnancy mortality in the United States: 1980-2007. Obstet Gynecol. 2011;117:837-843.
- Shwayder JM. IUP diagnosed and treated as ectopic: How bad can it get? Contemporary OB/GYN. 2019;64:49-46.
- Kaplan AI. Should this ectopic pregnancy have been diagnosed earlier? Contemporary OB/GYN. 2017;62:53.
- American College of Obstetricians and Gynecologists Committee on Ethics. Committee opinion 439: informed consent. Reaffirmed 2015. https://www.acog.org/clinical /clinical-guidance/committee-opinion/articles/2009/08 /informed-consent. Accessed December 9, 2020.
- Shwayder JM. Liability in ob/gyn ultrasound. Contemporary OB/GYN. 2017;62:32-49.
- Fisher LN. Institutional religious exemptions: a balancing approach. BYU Law Review. 2014;415-444.
- Makdisi J. Aquinas’s prohibition of killing reconsidered. J Catholic Legal Stud. 2019:57:67-128.
- Franzonello A. Remarks of Anna Franzonello. Alb Law J Sci Tech. 2012;23:519-530.
- Malcolm HE. Pregnancy centers and the limits of mandated disclosure. Columbia Law Rev. 2019;119:1133-1168.
- Kukura E. Contested care: the limitations of evidencebased maternity care reform. Berkeley J Gender Law Justice. 2016;31:241-298.
- Donley G. Contraceptive equity: curing the sex discrimination in the ACA’s mandate. Alabama Law Rev. 2019;71:499-560.
- Berk H. Savvy surrogates and rock star parents: compensation provisions, contracting practices, and the value of womb work. Law Social Inquiry. 2020;45:398-431.
- Centers for Disease Control and Prevention. Ectopic pregnancy—United States, 1990‒1992. MMWR Morb Mortal Wkly Rep. 1995;44:46-48.
- Kirk E, Bottomley C, Bourne T. Diagnosing ectopic pregnancy and current concepts in the management of pregnancy of unknown location. Hum Reprod Update. 2012;20:250-261.
- Dichter E, Espinosa J, Baird J, Lucerna A. An unusual emergency department case: ruptured ectopic pregnancy presenting as chest pain. World J Emerg Med. 2017;8:71-73.
- Cecchino GN, Araujo E, Elito J. Methotrexate for ectopic pregnancy: when and how. Arch Gynecol Obstet. 2014;290:417- 423.
- Barnhart KT, Sammel MD, Cracia CR, et al. Risk factors for ectopic pregnancy in women with symptomatic firsttrimester pregnancies. Fertil Steril. 2006;86:36-43.
- Carusi D. Pregnancy of unknown location: evaluation and management. Semin Perinatol. 2019;43:95-100.
- Barnhart KT, Fay CA, Suescum M, et al. Clinical factors affecting the accuracy of ultrasonography in symptomatic first-trimester pregnancy. Obstet Gynecol. 2011;117:299-306.
- American College of Obstetricians and Gynecologists Practice Bulletin No. 193: tubal ectopic pregnancy. Obstet Gynecol. 2018;131:e91-e103.
- Bouyer J, Coste J, Fernandez H, et al. Sites of ectopic pregnancy: a 10-year population-based study of 1800 cases. Hum Reprod. 2002;17:3224-3230.
- Spiegelberg O. Zur casuistic der ovarial schwangerschaft. Arch Gynecol. 1978;13:73.
- OB Hospitalist Group. Methotrexate use for ectopic pregnancies guidelines. https://www.obhg.com/wp-content /uploads/2020/01/Methotrexate-Use-for-EctopicPregnancies_2016-updates.pdf. Accessed December 10, 2020.
- Brozos C, Kargiannis I, Kiossis E, et al. Ectopic pregnancy through a caesarean scar in a ewe. N Z Vet J. 2013;61:373-375.
- Tucker v. Gillette, 12 Ohio Cir. Dec. 401 (Cir. Ct. 1901).
- Creanga AA, Syverson C, Seed K, et al. Pregnancy-related mortality in the United States, 2011–2013. Obstet Gynecol. 2017;130:366-373.
- Matthews LR, Alvi FA, Milad MP. Reproductive surgery malpractice patterns. Fertil Steril. 2016;106:e42-e43.
- Kim B. The impact of malpractice risk on the use of obstetrics procedures. J Legal Studies. 2006;36:S79-S120.
- Abinader R, Warsof S. Complications involving obstetrical ultrasound. In: Warsof S, Shwayder JM, eds. Legal Concepts and Best Practices in Obstetrics: The Nuts and Bolts Guide to Mitigating Risk. 2019;45-48.
- Creanga AA, Shapiro-Mendoza CK, Bish CL, et al. Trends in ectopic pregnancy mortality in the United States: 1980-2007. Obstet Gynecol. 2011;117:837-843.
- Shwayder JM. IUP diagnosed and treated as ectopic: How bad can it get? Contemporary OB/GYN. 2019;64:49-46.
- Kaplan AI. Should this ectopic pregnancy have been diagnosed earlier? Contemporary OB/GYN. 2017;62:53.
- American College of Obstetricians and Gynecologists Committee on Ethics. Committee opinion 439: informed consent. Reaffirmed 2015. https://www.acog.org/clinical /clinical-guidance/committee-opinion/articles/2009/08 /informed-consent. Accessed December 9, 2020.
- Shwayder JM. Liability in ob/gyn ultrasound. Contemporary OB/GYN. 2017;62:32-49.
- Fisher LN. Institutional religious exemptions: a balancing approach. BYU Law Review. 2014;415-444.
- Makdisi J. Aquinas’s prohibition of killing reconsidered. J Catholic Legal Stud. 2019:57:67-128.
- Franzonello A. Remarks of Anna Franzonello. Alb Law J Sci Tech. 2012;23:519-530.
- Malcolm HE. Pregnancy centers and the limits of mandated disclosure. Columbia Law Rev. 2019;119:1133-1168.
- Kukura E. Contested care: the limitations of evidencebased maternity care reform. Berkeley J Gender Law Justice. 2016;31:241-298.
- Donley G. Contraceptive equity: curing the sex discrimination in the ACA’s mandate. Alabama Law Rev. 2019;71:499-560.
- Berk H. Savvy surrogates and rock star parents: compensation provisions, contracting practices, and the value of womb work. Law Social Inquiry. 2020;45:398-431.
Still happening: Pelvic exams on anesthetized patients. Why?
“When I was doing ob.gyn. as a med student, the attending would have me do a pelvic right after the patient was under and before we started surgery,” said one participant in an online forum. “We didn’t exactly get permission but it was for teaching purposes.”
Yet others don’t see what the commotion is about. “There are a hundred things that are done during a surgery that don’t require your specific consent (some of them much more ‘humiliating’ than a pelvic exam). ... There’s not really much left to be shy about during a gyn/rectal/prostate surgery, let me put it that way,” one doctor wrote.
However, many physicians are adamantly opposed to the practice, and laws intended to stop or limit it are being enacted throughout the nation.
Renewed concerns have prompted new state laws
A few states have required consent for pelvic exams for many years, beginning with California in 2003. But up until 2019, providing pelvic exams without informed consent was illegal in only six states.
Continuing reports of unauthorized pelvic exams indicate that the practice has not disappeared. University of Michigan professor Maya M. Hammoud, MD, past president of the Association of Professors of Gynecology and Obstetrics, and many others attribute renewed interest in the issue to a 2018 article in the journal Bioethics by Phoebe Friesen, a medical ethicist at McGill University, Montreal, that laid out the ethical arguments against the practice.
Starting in 2019, an outpouring of new state bills have been introduced, and nine more states have passed laws. In addition, 14 other states considered similar bills but did not pass them, in some cases because teaching institutions argued that they were already dealing with the issue. This happened in Connecticut and Massachusetts, after representatives of Yale University, New Haven, Conn., met with legislators.
Laws against the practice have been passed by 15 states, including California, Florida, Illinois, and New York. Some teaching institutions have recently been clamping down on the practice, while many teaching physicians insist that at this point, it has all but ended.
A practice that may still continue
For many years, ethicists, women’s rights groups, state legislators, and organized medicine have been trying to eliminate the practice of unauthorized pelvic exams by medical students. Several key medical groups have come out against it, including the American Medical Association, the Association of American Medical Colleges, and the American College of Obstetricians and Gynecologists.
“Fifteen years ago, studies found a substantial number of cases, but my sense is that most of that has stopped,” said Dr. Hammoud.
Yet despite these changes, there are some disturbing signs that the practice persists.
“I don’t have data, but anecdotally I see it still going on,” said Peter Ubel, MD, a professor at Duke University, Durham, N.C., who was involved in one of those early studies. “Every so often when I’m making a speech, a medical student tells me about performing a pelvic exam without getting permission.
“Perhaps in some cases the attending [physician] did get permission and didn’t tell the medical student, but that would also be a problem,” Dr. Ubel said. “The medical student should be informed that permission was given. This helps them be sensitive to the need to get consent.”
In a 2019 survey of medical students, 92% said they performed a pelvic exam on an anesthetized female patient, and of those, 61% did so without explicit patient consent.
The survey – involving 101 medical students at seven U.S. medical schools – also found that 11% of the medical students said they were extremely uncomfortable with the practice. But nearly one-third of the medical students said that opting out might jeopardize their grades and future careers.
“I tried to opt out once from doing a pelvic exam when I hadn’t met the patient beforehand,” one of them wrote. “The resident told me no.”
Some physicians defend the practice
Why do many medical students and doctors think that getting consent for pelvic exams is not necessary?
Some argue that patients implicitly give consent when they walk through the doors of a teaching hospital. “Sorry, but you inherently agree to that when you’re seen in an academic teaching hospital,” wrote one participant in a Student Doctor Network forum. “You agree to have residents and medical students participate in your care, not just an attending. If you just want an attending, then you are free to go to a nonteaching hospital. That’s the deal.”
Others argued that since the anesthetized patient couldn’t feel what was going on, it shouldn’t matter. “Things like pelvic exams, rectal exams, or even heroic trauma surgery occur for training purposes when there is no memory, no sensation and no harm to be done [and] society gains a better practitioner of the art of medicine,” a physician in Columbus, Ohio, wrote on Quora, an online forum.
Some doctors argue that they don’t ask for specific consent when they touch a variety of other body parts, and pelvic exams should be no different. Pelvic exams are needed before surgery of the pelvic area, but they have also been given to women undergoing surgery in a different part of the body.
In 2019 a woman told Deseret News in Utah that she had been recovering from stomach surgery when a resident physician mentioned something she had noticed “when we looked at your cervix.” When she asked why the physician had examined her cervix to prepare for stomach surgery, “no one could give her a good answer.”
A ‘positive goal’ doesn’t make it okay
What is missing in many defenses of the practice is any recognition that genitals are the most intimate part of the body, and that a patient’s desire for privacy ought to come first. In a survey of women undergoing gynecologic surgery, 72% expected to be asked for consent before medical students undertook pelvic examinations under anesthesia.
Overruling patients’ concerns about their own privacy is unethical, said Eli Y. Adashi, MD, professor of medical science and former dean of medicine and biological sciences at Brown University, Providence, R.I.
Dr. Adashi said the principle of patient autonomy in medical ethics directs that patients must be involved in decision-making about their care – even when caretakers are pursuing a positive goal, such as helping to educate future doctors.
“Conducting pelvic exams on unconscious women without their specific consent is simply untenable and never has been tenable, and it ought to be discontinued if it hasn’t been already,” says Dr. Adashi, who wrote an opinion piece on the issue for JAMA.
Furthermore, it has been shown that ignoring the need to get consent for pelvic exams makes physicians less concerned about getting patient consent in general. A study led by Dr. Ubel found that medical students who had completed an ob.gyn. clerkship thought getting patients’ consent was significantly less important than those who had not completed that clerkship.
Why give pelvic exams to anesthetized women?
Despite the controversy, a number of medical educators continue to direct medical students to perform pelvic exams on anesthetized women. Why is that?
“Pelvic exams are not easy to do,” Dr. Hammoud said. “Learners need to keep working on them; they have to do a lot of them in order to do them well.”
To teach pelvic exams, most medical schools provide standardized patients – paid volunteers who submit to exams and critique the medical student’s work afterwards – but these encounters are limited because of their cost, says Guy Benrubi, MD, professor and emeritus chair of the department of obstetrics and gynecology at the University of Florida, Jacksonville.
He said teaching programs therefore need to supplement exams on standardized patients with exams on unpaid volunteers who provide consent. Programs prefer anesthetized patients, Dr. Benrubi said, because they are easier for novices to work on. “With patients under anesthesia, the muscles are relaxed and it’s easier for learners to detect organs. All the same, you need to get consent.”
Teaching institutions stiffen consent requirements
Faced with growing opposition to pelvic exams without consent, teaching institutions as well as gynecologic educators have recently been tightening their policies.
Dr. Hammoud said she has always informed patients orally about the possibility of medical students performing pelvic exams on them, but now some institutions, including her own, want a more involved process. The university recently began consent in writing for pelvic exams.
In addition, the university also now requires that medical students meet patients before performing pelvic exams and that teaching physicians explain the students’ involvement.
Dr. Hammoud said some institutions now require a separate consent form for pelvic exams, but the University of Michigan simply directs that the possibility of the patient getting a pelvic exam be part of the consent form.
This requirement, called “explicit consent,” was endorsed by APGO. It differs from having a separate consent form for pelvic exams, which would highlight the possibility of a pelvic exam, as many women’s rights activists are calling for.
Why not have a separate form? Dr. Hammoud is concerned that it would unnecessarily alarm patients. “When you point out a certain issue, you’re in effect saying to the patient that this is not normal,” she said, noting that, when asked for consent to do the exams, most women agree to it.
New wave of state laws prompted by renewed concerns
Dr. Hammoud thinks the laws are unnecessary. “These laws are excessive for the vast majority of physicians who practice ethically. The profession should come up with its own standards rather than having a plethora of laws.”
Several of the more recent laws have a broader scope than the original laws. The original laws simply state that medical students or physicians must get informed consent, but they did not stipulate how informed consent should be obtained. (The laws also typically prohibit pelvic exams when surgery will be in a different area of the body.)
The new laws often follow this format, but some go well beyond it. Some also apply to rectal exams (Maine and Maryland), to men as well as women (Utah and Maryland) requires separate consent (Utah), and require consent for all pelvic exams (Florida).
The struggle over Florida’s law
The original Florida bill was drafted in 2019 by state Sen. Lauren Book, a Democrat who is a victims’ rights advocate working with women who have undergone sexual trauma. In written comments for this article, she says not getting consent for pelvic exams is still going on.
“This disturbing practice is commonplace at medical schools and teaching hospitals across the country – including several Florida universities, based on accounts from current and former medical students and faculty,” Sen. Book stated. “At best, these exams have been wrongful learning experiences for medical students or at worst, the equivalent of a sexual assault.”
Dr. Ubel took exception to linking the teaching activities to sexual assault. “I understand why many women would be horrified by this practice, but it’s not as bad as it seems,” he said. “There is nothing sexual or prurient about these exams, and they are motivated purely by a desire to teach people to be better doctors. That said, patients have the right to say, ‘I don’t want it done to me.’ ”
In early 2020, Dr. Benrubi was part of a coalition of medical groups that was trying to influence Sen. Book’s bill as it went through the legislature. Sen. Book’s original bill was relatively mild, “but then, late in the process, it was changed into a more sweeping bill with some unclear language,” he said.
The final version was passed and signed into law by Gov. Ron DeSantis, a conservative Republican, in June.
Dr. Benrubi said that a large number of state legislators, including Sen. Book, have been agreeable to fixing the bill. This was supposed to happen in a special session in the fall, but that never materialized, and so the fix will have to wait until the regular session in early 2021.
“The law should not apply to patients undergoing routine pelvic exams,” Dr. Benrubi said. “It should only apply to women patients under anesthesia.”
But while organized medicine wants to walk back the law, Dr. Book wants to expand it. “This upcoming session, I look forward to working with physicians to continue to hone this new law, and to work toward inclusion for males. Everyone has a right to consent.”
A version of this article first appeared on Medscape.com.
“When I was doing ob.gyn. as a med student, the attending would have me do a pelvic right after the patient was under and before we started surgery,” said one participant in an online forum. “We didn’t exactly get permission but it was for teaching purposes.”
Yet others don’t see what the commotion is about. “There are a hundred things that are done during a surgery that don’t require your specific consent (some of them much more ‘humiliating’ than a pelvic exam). ... There’s not really much left to be shy about during a gyn/rectal/prostate surgery, let me put it that way,” one doctor wrote.
However, many physicians are adamantly opposed to the practice, and laws intended to stop or limit it are being enacted throughout the nation.
Renewed concerns have prompted new state laws
A few states have required consent for pelvic exams for many years, beginning with California in 2003. But up until 2019, providing pelvic exams without informed consent was illegal in only six states.
Continuing reports of unauthorized pelvic exams indicate that the practice has not disappeared. University of Michigan professor Maya M. Hammoud, MD, past president of the Association of Professors of Gynecology and Obstetrics, and many others attribute renewed interest in the issue to a 2018 article in the journal Bioethics by Phoebe Friesen, a medical ethicist at McGill University, Montreal, that laid out the ethical arguments against the practice.
Starting in 2019, an outpouring of new state bills have been introduced, and nine more states have passed laws. In addition, 14 other states considered similar bills but did not pass them, in some cases because teaching institutions argued that they were already dealing with the issue. This happened in Connecticut and Massachusetts, after representatives of Yale University, New Haven, Conn., met with legislators.
Laws against the practice have been passed by 15 states, including California, Florida, Illinois, and New York. Some teaching institutions have recently been clamping down on the practice, while many teaching physicians insist that at this point, it has all but ended.
A practice that may still continue
For many years, ethicists, women’s rights groups, state legislators, and organized medicine have been trying to eliminate the practice of unauthorized pelvic exams by medical students. Several key medical groups have come out against it, including the American Medical Association, the Association of American Medical Colleges, and the American College of Obstetricians and Gynecologists.
“Fifteen years ago, studies found a substantial number of cases, but my sense is that most of that has stopped,” said Dr. Hammoud.
Yet despite these changes, there are some disturbing signs that the practice persists.
“I don’t have data, but anecdotally I see it still going on,” said Peter Ubel, MD, a professor at Duke University, Durham, N.C., who was involved in one of those early studies. “Every so often when I’m making a speech, a medical student tells me about performing a pelvic exam without getting permission.
“Perhaps in some cases the attending [physician] did get permission and didn’t tell the medical student, but that would also be a problem,” Dr. Ubel said. “The medical student should be informed that permission was given. This helps them be sensitive to the need to get consent.”
In a 2019 survey of medical students, 92% said they performed a pelvic exam on an anesthetized female patient, and of those, 61% did so without explicit patient consent.
The survey – involving 101 medical students at seven U.S. medical schools – also found that 11% of the medical students said they were extremely uncomfortable with the practice. But nearly one-third of the medical students said that opting out might jeopardize their grades and future careers.
“I tried to opt out once from doing a pelvic exam when I hadn’t met the patient beforehand,” one of them wrote. “The resident told me no.”
Some physicians defend the practice
Why do many medical students and doctors think that getting consent for pelvic exams is not necessary?
Some argue that patients implicitly give consent when they walk through the doors of a teaching hospital. “Sorry, but you inherently agree to that when you’re seen in an academic teaching hospital,” wrote one participant in a Student Doctor Network forum. “You agree to have residents and medical students participate in your care, not just an attending. If you just want an attending, then you are free to go to a nonteaching hospital. That’s the deal.”
Others argued that since the anesthetized patient couldn’t feel what was going on, it shouldn’t matter. “Things like pelvic exams, rectal exams, or even heroic trauma surgery occur for training purposes when there is no memory, no sensation and no harm to be done [and] society gains a better practitioner of the art of medicine,” a physician in Columbus, Ohio, wrote on Quora, an online forum.
Some doctors argue that they don’t ask for specific consent when they touch a variety of other body parts, and pelvic exams should be no different. Pelvic exams are needed before surgery of the pelvic area, but they have also been given to women undergoing surgery in a different part of the body.
In 2019 a woman told Deseret News in Utah that she had been recovering from stomach surgery when a resident physician mentioned something she had noticed “when we looked at your cervix.” When she asked why the physician had examined her cervix to prepare for stomach surgery, “no one could give her a good answer.”
A ‘positive goal’ doesn’t make it okay
What is missing in many defenses of the practice is any recognition that genitals are the most intimate part of the body, and that a patient’s desire for privacy ought to come first. In a survey of women undergoing gynecologic surgery, 72% expected to be asked for consent before medical students undertook pelvic examinations under anesthesia.
Overruling patients’ concerns about their own privacy is unethical, said Eli Y. Adashi, MD, professor of medical science and former dean of medicine and biological sciences at Brown University, Providence, R.I.
Dr. Adashi said the principle of patient autonomy in medical ethics directs that patients must be involved in decision-making about their care – even when caretakers are pursuing a positive goal, such as helping to educate future doctors.
“Conducting pelvic exams on unconscious women without their specific consent is simply untenable and never has been tenable, and it ought to be discontinued if it hasn’t been already,” says Dr. Adashi, who wrote an opinion piece on the issue for JAMA.
Furthermore, it has been shown that ignoring the need to get consent for pelvic exams makes physicians less concerned about getting patient consent in general. A study led by Dr. Ubel found that medical students who had completed an ob.gyn. clerkship thought getting patients’ consent was significantly less important than those who had not completed that clerkship.
Why give pelvic exams to anesthetized women?
Despite the controversy, a number of medical educators continue to direct medical students to perform pelvic exams on anesthetized women. Why is that?
“Pelvic exams are not easy to do,” Dr. Hammoud said. “Learners need to keep working on them; they have to do a lot of them in order to do them well.”
To teach pelvic exams, most medical schools provide standardized patients – paid volunteers who submit to exams and critique the medical student’s work afterwards – but these encounters are limited because of their cost, says Guy Benrubi, MD, professor and emeritus chair of the department of obstetrics and gynecology at the University of Florida, Jacksonville.
He said teaching programs therefore need to supplement exams on standardized patients with exams on unpaid volunteers who provide consent. Programs prefer anesthetized patients, Dr. Benrubi said, because they are easier for novices to work on. “With patients under anesthesia, the muscles are relaxed and it’s easier for learners to detect organs. All the same, you need to get consent.”
Teaching institutions stiffen consent requirements
Faced with growing opposition to pelvic exams without consent, teaching institutions as well as gynecologic educators have recently been tightening their policies.
Dr. Hammoud said she has always informed patients orally about the possibility of medical students performing pelvic exams on them, but now some institutions, including her own, want a more involved process. The university recently began consent in writing for pelvic exams.
In addition, the university also now requires that medical students meet patients before performing pelvic exams and that teaching physicians explain the students’ involvement.
Dr. Hammoud said some institutions now require a separate consent form for pelvic exams, but the University of Michigan simply directs that the possibility of the patient getting a pelvic exam be part of the consent form.
This requirement, called “explicit consent,” was endorsed by APGO. It differs from having a separate consent form for pelvic exams, which would highlight the possibility of a pelvic exam, as many women’s rights activists are calling for.
Why not have a separate form? Dr. Hammoud is concerned that it would unnecessarily alarm patients. “When you point out a certain issue, you’re in effect saying to the patient that this is not normal,” she said, noting that, when asked for consent to do the exams, most women agree to it.
New wave of state laws prompted by renewed concerns
Dr. Hammoud thinks the laws are unnecessary. “These laws are excessive for the vast majority of physicians who practice ethically. The profession should come up with its own standards rather than having a plethora of laws.”
Several of the more recent laws have a broader scope than the original laws. The original laws simply state that medical students or physicians must get informed consent, but they did not stipulate how informed consent should be obtained. (The laws also typically prohibit pelvic exams when surgery will be in a different area of the body.)
The new laws often follow this format, but some go well beyond it. Some also apply to rectal exams (Maine and Maryland), to men as well as women (Utah and Maryland) requires separate consent (Utah), and require consent for all pelvic exams (Florida).
The struggle over Florida’s law
The original Florida bill was drafted in 2019 by state Sen. Lauren Book, a Democrat who is a victims’ rights advocate working with women who have undergone sexual trauma. In written comments for this article, she says not getting consent for pelvic exams is still going on.
“This disturbing practice is commonplace at medical schools and teaching hospitals across the country – including several Florida universities, based on accounts from current and former medical students and faculty,” Sen. Book stated. “At best, these exams have been wrongful learning experiences for medical students or at worst, the equivalent of a sexual assault.”
Dr. Ubel took exception to linking the teaching activities to sexual assault. “I understand why many women would be horrified by this practice, but it’s not as bad as it seems,” he said. “There is nothing sexual or prurient about these exams, and they are motivated purely by a desire to teach people to be better doctors. That said, patients have the right to say, ‘I don’t want it done to me.’ ”
In early 2020, Dr. Benrubi was part of a coalition of medical groups that was trying to influence Sen. Book’s bill as it went through the legislature. Sen. Book’s original bill was relatively mild, “but then, late in the process, it was changed into a more sweeping bill with some unclear language,” he said.
The final version was passed and signed into law by Gov. Ron DeSantis, a conservative Republican, in June.
Dr. Benrubi said that a large number of state legislators, including Sen. Book, have been agreeable to fixing the bill. This was supposed to happen in a special session in the fall, but that never materialized, and so the fix will have to wait until the regular session in early 2021.
“The law should not apply to patients undergoing routine pelvic exams,” Dr. Benrubi said. “It should only apply to women patients under anesthesia.”
But while organized medicine wants to walk back the law, Dr. Book wants to expand it. “This upcoming session, I look forward to working with physicians to continue to hone this new law, and to work toward inclusion for males. Everyone has a right to consent.”
A version of this article first appeared on Medscape.com.
“When I was doing ob.gyn. as a med student, the attending would have me do a pelvic right after the patient was under and before we started surgery,” said one participant in an online forum. “We didn’t exactly get permission but it was for teaching purposes.”
Yet others don’t see what the commotion is about. “There are a hundred things that are done during a surgery that don’t require your specific consent (some of them much more ‘humiliating’ than a pelvic exam). ... There’s not really much left to be shy about during a gyn/rectal/prostate surgery, let me put it that way,” one doctor wrote.
However, many physicians are adamantly opposed to the practice, and laws intended to stop or limit it are being enacted throughout the nation.
Renewed concerns have prompted new state laws
A few states have required consent for pelvic exams for many years, beginning with California in 2003. But up until 2019, providing pelvic exams without informed consent was illegal in only six states.
Continuing reports of unauthorized pelvic exams indicate that the practice has not disappeared. University of Michigan professor Maya M. Hammoud, MD, past president of the Association of Professors of Gynecology and Obstetrics, and many others attribute renewed interest in the issue to a 2018 article in the journal Bioethics by Phoebe Friesen, a medical ethicist at McGill University, Montreal, that laid out the ethical arguments against the practice.
Starting in 2019, an outpouring of new state bills have been introduced, and nine more states have passed laws. In addition, 14 other states considered similar bills but did not pass them, in some cases because teaching institutions argued that they were already dealing with the issue. This happened in Connecticut and Massachusetts, after representatives of Yale University, New Haven, Conn., met with legislators.
Laws against the practice have been passed by 15 states, including California, Florida, Illinois, and New York. Some teaching institutions have recently been clamping down on the practice, while many teaching physicians insist that at this point, it has all but ended.
A practice that may still continue
For many years, ethicists, women’s rights groups, state legislators, and organized medicine have been trying to eliminate the practice of unauthorized pelvic exams by medical students. Several key medical groups have come out against it, including the American Medical Association, the Association of American Medical Colleges, and the American College of Obstetricians and Gynecologists.
“Fifteen years ago, studies found a substantial number of cases, but my sense is that most of that has stopped,” said Dr. Hammoud.
Yet despite these changes, there are some disturbing signs that the practice persists.
“I don’t have data, but anecdotally I see it still going on,” said Peter Ubel, MD, a professor at Duke University, Durham, N.C., who was involved in one of those early studies. “Every so often when I’m making a speech, a medical student tells me about performing a pelvic exam without getting permission.
“Perhaps in some cases the attending [physician] did get permission and didn’t tell the medical student, but that would also be a problem,” Dr. Ubel said. “The medical student should be informed that permission was given. This helps them be sensitive to the need to get consent.”
In a 2019 survey of medical students, 92% said they performed a pelvic exam on an anesthetized female patient, and of those, 61% did so without explicit patient consent.
The survey – involving 101 medical students at seven U.S. medical schools – also found that 11% of the medical students said they were extremely uncomfortable with the practice. But nearly one-third of the medical students said that opting out might jeopardize their grades and future careers.
“I tried to opt out once from doing a pelvic exam when I hadn’t met the patient beforehand,” one of them wrote. “The resident told me no.”
Some physicians defend the practice
Why do many medical students and doctors think that getting consent for pelvic exams is not necessary?
Some argue that patients implicitly give consent when they walk through the doors of a teaching hospital. “Sorry, but you inherently agree to that when you’re seen in an academic teaching hospital,” wrote one participant in a Student Doctor Network forum. “You agree to have residents and medical students participate in your care, not just an attending. If you just want an attending, then you are free to go to a nonteaching hospital. That’s the deal.”
Others argued that since the anesthetized patient couldn’t feel what was going on, it shouldn’t matter. “Things like pelvic exams, rectal exams, or even heroic trauma surgery occur for training purposes when there is no memory, no sensation and no harm to be done [and] society gains a better practitioner of the art of medicine,” a physician in Columbus, Ohio, wrote on Quora, an online forum.
Some doctors argue that they don’t ask for specific consent when they touch a variety of other body parts, and pelvic exams should be no different. Pelvic exams are needed before surgery of the pelvic area, but they have also been given to women undergoing surgery in a different part of the body.
In 2019 a woman told Deseret News in Utah that she had been recovering from stomach surgery when a resident physician mentioned something she had noticed “when we looked at your cervix.” When she asked why the physician had examined her cervix to prepare for stomach surgery, “no one could give her a good answer.”
A ‘positive goal’ doesn’t make it okay
What is missing in many defenses of the practice is any recognition that genitals are the most intimate part of the body, and that a patient’s desire for privacy ought to come first. In a survey of women undergoing gynecologic surgery, 72% expected to be asked for consent before medical students undertook pelvic examinations under anesthesia.
Overruling patients’ concerns about their own privacy is unethical, said Eli Y. Adashi, MD, professor of medical science and former dean of medicine and biological sciences at Brown University, Providence, R.I.
Dr. Adashi said the principle of patient autonomy in medical ethics directs that patients must be involved in decision-making about their care – even when caretakers are pursuing a positive goal, such as helping to educate future doctors.
“Conducting pelvic exams on unconscious women without their specific consent is simply untenable and never has been tenable, and it ought to be discontinued if it hasn’t been already,” says Dr. Adashi, who wrote an opinion piece on the issue for JAMA.
Furthermore, it has been shown that ignoring the need to get consent for pelvic exams makes physicians less concerned about getting patient consent in general. A study led by Dr. Ubel found that medical students who had completed an ob.gyn. clerkship thought getting patients’ consent was significantly less important than those who had not completed that clerkship.
Why give pelvic exams to anesthetized women?
Despite the controversy, a number of medical educators continue to direct medical students to perform pelvic exams on anesthetized women. Why is that?
“Pelvic exams are not easy to do,” Dr. Hammoud said. “Learners need to keep working on them; they have to do a lot of them in order to do them well.”
To teach pelvic exams, most medical schools provide standardized patients – paid volunteers who submit to exams and critique the medical student’s work afterwards – but these encounters are limited because of their cost, says Guy Benrubi, MD, professor and emeritus chair of the department of obstetrics and gynecology at the University of Florida, Jacksonville.
He said teaching programs therefore need to supplement exams on standardized patients with exams on unpaid volunteers who provide consent. Programs prefer anesthetized patients, Dr. Benrubi said, because they are easier for novices to work on. “With patients under anesthesia, the muscles are relaxed and it’s easier for learners to detect organs. All the same, you need to get consent.”
Teaching institutions stiffen consent requirements
Faced with growing opposition to pelvic exams without consent, teaching institutions as well as gynecologic educators have recently been tightening their policies.
Dr. Hammoud said she has always informed patients orally about the possibility of medical students performing pelvic exams on them, but now some institutions, including her own, want a more involved process. The university recently began consent in writing for pelvic exams.
In addition, the university also now requires that medical students meet patients before performing pelvic exams and that teaching physicians explain the students’ involvement.
Dr. Hammoud said some institutions now require a separate consent form for pelvic exams, but the University of Michigan simply directs that the possibility of the patient getting a pelvic exam be part of the consent form.
This requirement, called “explicit consent,” was endorsed by APGO. It differs from having a separate consent form for pelvic exams, which would highlight the possibility of a pelvic exam, as many women’s rights activists are calling for.
Why not have a separate form? Dr. Hammoud is concerned that it would unnecessarily alarm patients. “When you point out a certain issue, you’re in effect saying to the patient that this is not normal,” she said, noting that, when asked for consent to do the exams, most women agree to it.
New wave of state laws prompted by renewed concerns
Dr. Hammoud thinks the laws are unnecessary. “These laws are excessive for the vast majority of physicians who practice ethically. The profession should come up with its own standards rather than having a plethora of laws.”
Several of the more recent laws have a broader scope than the original laws. The original laws simply state that medical students or physicians must get informed consent, but they did not stipulate how informed consent should be obtained. (The laws also typically prohibit pelvic exams when surgery will be in a different area of the body.)
The new laws often follow this format, but some go well beyond it. Some also apply to rectal exams (Maine and Maryland), to men as well as women (Utah and Maryland) requires separate consent (Utah), and require consent for all pelvic exams (Florida).
The struggle over Florida’s law
The original Florida bill was drafted in 2019 by state Sen. Lauren Book, a Democrat who is a victims’ rights advocate working with women who have undergone sexual trauma. In written comments for this article, she says not getting consent for pelvic exams is still going on.
“This disturbing practice is commonplace at medical schools and teaching hospitals across the country – including several Florida universities, based on accounts from current and former medical students and faculty,” Sen. Book stated. “At best, these exams have been wrongful learning experiences for medical students or at worst, the equivalent of a sexual assault.”
Dr. Ubel took exception to linking the teaching activities to sexual assault. “I understand why many women would be horrified by this practice, but it’s not as bad as it seems,” he said. “There is nothing sexual or prurient about these exams, and they are motivated purely by a desire to teach people to be better doctors. That said, patients have the right to say, ‘I don’t want it done to me.’ ”
In early 2020, Dr. Benrubi was part of a coalition of medical groups that was trying to influence Sen. Book’s bill as it went through the legislature. Sen. Book’s original bill was relatively mild, “but then, late in the process, it was changed into a more sweeping bill with some unclear language,” he said.
The final version was passed and signed into law by Gov. Ron DeSantis, a conservative Republican, in June.
Dr. Benrubi said that a large number of state legislators, including Sen. Book, have been agreeable to fixing the bill. This was supposed to happen in a special session in the fall, but that never materialized, and so the fix will have to wait until the regular session in early 2021.
“The law should not apply to patients undergoing routine pelvic exams,” Dr. Benrubi said. “It should only apply to women patients under anesthesia.”
But while organized medicine wants to walk back the law, Dr. Book wants to expand it. “This upcoming session, I look forward to working with physicians to continue to hone this new law, and to work toward inclusion for males. Everyone has a right to consent.”
A version of this article first appeared on Medscape.com.
Is diagnostic hysteroscopy safe in patients with type 2 endometrial cancer?
Among women with type 2 endometrial cancer, diagnostic hysteroscopy may not be associated with increased odds of positive peritoneal cytology at the time of surgical staging or with decreased survival, according to a retrospective study of 127 patients.
a researcher said at the meeting sponsored by AAGL, held virtually this year.
Possible associations between cytology and procedures
Prior research has found that positive peritoneal cytology may correlate with greater likelihood of death among patients with endometrial cancer, and researchers have wondered whether pressure on the uterine cavity during hysteroscopy increases the presence of positive peritoneal cytology. “According to some systematic reviews ... it seems that it does,” said study author Luiz Brito, MD, PhD, associate professor of obstetrics and gynecology at the University of Campinas in Brazil.
Nevertheless, research suggests that “most of the time hysteroscopy does not have a powerful impact on the prognosis of these patients,” he said.
Studies have tended to focus on patients with type 1 endometrial cancer, however. Type 2 endometrial cancer, which is more aggressive, “is scarcely studied,” Dr. Brito said. One retrospective study that focused on type 2 endometrial cancer included 140 patients. Among patients who underwent hysteroscopy, 30% had positive cytology. In comparison, 12% of patients in the curettage group had positive cytology. But the difference in disease-specific survival between groups was not statistically significant, and about 33% of the patients in each group developed a recurrence.
To examine associations between diagnostic methods and outcomes in another group of patients with type 2 endometrial cancer, Dr. Brito and colleagues analyzed data from a hospital registry in Brazil.
The database included 1,183 patients with endometrial cancer between 2002 and 2017, including 235 patients with type 2 endometrial cancer. After excluding patients with synchronous tumor and those who did not undergo surgery or did not have peritoneal cytology performed, 127 patients remained for the analysis. The study included follow-up to December 2019.
The researchers compared the prevalence of positive peritoneal cytology among 43 patients who underwent hysteroscopy with that among 84 patients who underwent curettage. The groups had similar baseline characteristics.
Positive peritoneal cytology was more common in the curettage group than in the hysteroscopy group (10.7% vs. 4.6%), although the difference was not statistically significant. Lymphovascular invasion and advanced surgical staging were more common in the curettage group.
In a multivariate analysis, older age and advanced cancer staging were the only factors associated with decreased disease-free survival. Age, advanced cancer staging, and vascular invasion were associated with decreased disease-specific survival.
The researchers also had considered factors such as peritoneal cytology, diagnostic method, age of menarche, menopause time, parity, comorbidities, smoking status, body mass index, abnormal uterine bleeding, histological type, and adjuvant treatment.
A limitation of the study is that it relied on data from a public health system that often has long wait times for diagnosis and treatment, Dr. Brito noted.
Some doctors may forgo cytology
The available research raises questions about the role and relevance of peritoneal cytology in caring for patients with endometrial cancer, René Pareja, MD, a gynecologic oncologist at Instituto Nacional de Cancerología, Bogotá, Colombia, said in a discussion following the presentation.
Peritoneal cytology has not been part of endometrial cancer staging since 2009, Dr. Pareja said. Still, guidelines recommend that surgeons collect cytology during surgical staging, with the idea that the results could inform adjuvant treatment decisions.
“Peritoneal cytology is recommended in the guidelines, but there are no recommendations on how to proceed if it is positive,” Dr. Pareja said. “While some gynecologic oncologists continue to take cytology during endometrial cancer staging, some have stopped doing so. And in Colombia, most of us are not performing pelvic cytology.”
Although some studies indicate that hysteroscopy may increase the rate of positive cytology, positive cytology may not be associated with worse oncological outcomes independent of other risk factors for recurrence, said Dr. Pareja.
So far, studies have been retrospective. Furthermore, the sensitivity and specificity of pelvic cytology tests are not 100%. “Should we continue performing pelvic cytology given the results of this and other studies?” Dr. Pareja asked.
Despite limited knowledge about this variable, physicians may want to be aware if a patient has positive cytology, Dr. Brito suggested. “At least it will give us some red flags so we can be attentive to these patients.”
If researchers were to design a prospective study that incorporates hysteroscopic variables, it could provide more complete answers about the relationship between hysteroscopy and peritoneal cytology and clarify the importance of positive cytology, Dr. Brito said.
Dr. Brito had no relevant disclosures. Dr. Pareja disclosed consulting for Johnson & Johnson.
SOURCE: Oliveira Brito LG et al. J Minim Invasive Gynecol. 2020 Nov. doi: 10.1016/j.jmig.2020.08.356.
Among women with type 2 endometrial cancer, diagnostic hysteroscopy may not be associated with increased odds of positive peritoneal cytology at the time of surgical staging or with decreased survival, according to a retrospective study of 127 patients.
a researcher said at the meeting sponsored by AAGL, held virtually this year.
Possible associations between cytology and procedures
Prior research has found that positive peritoneal cytology may correlate with greater likelihood of death among patients with endometrial cancer, and researchers have wondered whether pressure on the uterine cavity during hysteroscopy increases the presence of positive peritoneal cytology. “According to some systematic reviews ... it seems that it does,” said study author Luiz Brito, MD, PhD, associate professor of obstetrics and gynecology at the University of Campinas in Brazil.
Nevertheless, research suggests that “most of the time hysteroscopy does not have a powerful impact on the prognosis of these patients,” he said.
Studies have tended to focus on patients with type 1 endometrial cancer, however. Type 2 endometrial cancer, which is more aggressive, “is scarcely studied,” Dr. Brito said. One retrospective study that focused on type 2 endometrial cancer included 140 patients. Among patients who underwent hysteroscopy, 30% had positive cytology. In comparison, 12% of patients in the curettage group had positive cytology. But the difference in disease-specific survival between groups was not statistically significant, and about 33% of the patients in each group developed a recurrence.
To examine associations between diagnostic methods and outcomes in another group of patients with type 2 endometrial cancer, Dr. Brito and colleagues analyzed data from a hospital registry in Brazil.
The database included 1,183 patients with endometrial cancer between 2002 and 2017, including 235 patients with type 2 endometrial cancer. After excluding patients with synchronous tumor and those who did not undergo surgery or did not have peritoneal cytology performed, 127 patients remained for the analysis. The study included follow-up to December 2019.
The researchers compared the prevalence of positive peritoneal cytology among 43 patients who underwent hysteroscopy with that among 84 patients who underwent curettage. The groups had similar baseline characteristics.
Positive peritoneal cytology was more common in the curettage group than in the hysteroscopy group (10.7% vs. 4.6%), although the difference was not statistically significant. Lymphovascular invasion and advanced surgical staging were more common in the curettage group.
In a multivariate analysis, older age and advanced cancer staging were the only factors associated with decreased disease-free survival. Age, advanced cancer staging, and vascular invasion were associated with decreased disease-specific survival.
The researchers also had considered factors such as peritoneal cytology, diagnostic method, age of menarche, menopause time, parity, comorbidities, smoking status, body mass index, abnormal uterine bleeding, histological type, and adjuvant treatment.
A limitation of the study is that it relied on data from a public health system that often has long wait times for diagnosis and treatment, Dr. Brito noted.
Some doctors may forgo cytology
The available research raises questions about the role and relevance of peritoneal cytology in caring for patients with endometrial cancer, René Pareja, MD, a gynecologic oncologist at Instituto Nacional de Cancerología, Bogotá, Colombia, said in a discussion following the presentation.
Peritoneal cytology has not been part of endometrial cancer staging since 2009, Dr. Pareja said. Still, guidelines recommend that surgeons collect cytology during surgical staging, with the idea that the results could inform adjuvant treatment decisions.
“Peritoneal cytology is recommended in the guidelines, but there are no recommendations on how to proceed if it is positive,” Dr. Pareja said. “While some gynecologic oncologists continue to take cytology during endometrial cancer staging, some have stopped doing so. And in Colombia, most of us are not performing pelvic cytology.”
Although some studies indicate that hysteroscopy may increase the rate of positive cytology, positive cytology may not be associated with worse oncological outcomes independent of other risk factors for recurrence, said Dr. Pareja.
So far, studies have been retrospective. Furthermore, the sensitivity and specificity of pelvic cytology tests are not 100%. “Should we continue performing pelvic cytology given the results of this and other studies?” Dr. Pareja asked.
Despite limited knowledge about this variable, physicians may want to be aware if a patient has positive cytology, Dr. Brito suggested. “At least it will give us some red flags so we can be attentive to these patients.”
If researchers were to design a prospective study that incorporates hysteroscopic variables, it could provide more complete answers about the relationship between hysteroscopy and peritoneal cytology and clarify the importance of positive cytology, Dr. Brito said.
Dr. Brito had no relevant disclosures. Dr. Pareja disclosed consulting for Johnson & Johnson.
SOURCE: Oliveira Brito LG et al. J Minim Invasive Gynecol. 2020 Nov. doi: 10.1016/j.jmig.2020.08.356.
Among women with type 2 endometrial cancer, diagnostic hysteroscopy may not be associated with increased odds of positive peritoneal cytology at the time of surgical staging or with decreased survival, according to a retrospective study of 127 patients.
a researcher said at the meeting sponsored by AAGL, held virtually this year.
Possible associations between cytology and procedures
Prior research has found that positive peritoneal cytology may correlate with greater likelihood of death among patients with endometrial cancer, and researchers have wondered whether pressure on the uterine cavity during hysteroscopy increases the presence of positive peritoneal cytology. “According to some systematic reviews ... it seems that it does,” said study author Luiz Brito, MD, PhD, associate professor of obstetrics and gynecology at the University of Campinas in Brazil.
Nevertheless, research suggests that “most of the time hysteroscopy does not have a powerful impact on the prognosis of these patients,” he said.
Studies have tended to focus on patients with type 1 endometrial cancer, however. Type 2 endometrial cancer, which is more aggressive, “is scarcely studied,” Dr. Brito said. One retrospective study that focused on type 2 endometrial cancer included 140 patients. Among patients who underwent hysteroscopy, 30% had positive cytology. In comparison, 12% of patients in the curettage group had positive cytology. But the difference in disease-specific survival between groups was not statistically significant, and about 33% of the patients in each group developed a recurrence.
To examine associations between diagnostic methods and outcomes in another group of patients with type 2 endometrial cancer, Dr. Brito and colleagues analyzed data from a hospital registry in Brazil.
The database included 1,183 patients with endometrial cancer between 2002 and 2017, including 235 patients with type 2 endometrial cancer. After excluding patients with synchronous tumor and those who did not undergo surgery or did not have peritoneal cytology performed, 127 patients remained for the analysis. The study included follow-up to December 2019.
The researchers compared the prevalence of positive peritoneal cytology among 43 patients who underwent hysteroscopy with that among 84 patients who underwent curettage. The groups had similar baseline characteristics.
Positive peritoneal cytology was more common in the curettage group than in the hysteroscopy group (10.7% vs. 4.6%), although the difference was not statistically significant. Lymphovascular invasion and advanced surgical staging were more common in the curettage group.
In a multivariate analysis, older age and advanced cancer staging were the only factors associated with decreased disease-free survival. Age, advanced cancer staging, and vascular invasion were associated with decreased disease-specific survival.
The researchers also had considered factors such as peritoneal cytology, diagnostic method, age of menarche, menopause time, parity, comorbidities, smoking status, body mass index, abnormal uterine bleeding, histological type, and adjuvant treatment.
A limitation of the study is that it relied on data from a public health system that often has long wait times for diagnosis and treatment, Dr. Brito noted.
Some doctors may forgo cytology
The available research raises questions about the role and relevance of peritoneal cytology in caring for patients with endometrial cancer, René Pareja, MD, a gynecologic oncologist at Instituto Nacional de Cancerología, Bogotá, Colombia, said in a discussion following the presentation.
Peritoneal cytology has not been part of endometrial cancer staging since 2009, Dr. Pareja said. Still, guidelines recommend that surgeons collect cytology during surgical staging, with the idea that the results could inform adjuvant treatment decisions.
“Peritoneal cytology is recommended in the guidelines, but there are no recommendations on how to proceed if it is positive,” Dr. Pareja said. “While some gynecologic oncologists continue to take cytology during endometrial cancer staging, some have stopped doing so. And in Colombia, most of us are not performing pelvic cytology.”
Although some studies indicate that hysteroscopy may increase the rate of positive cytology, positive cytology may not be associated with worse oncological outcomes independent of other risk factors for recurrence, said Dr. Pareja.
So far, studies have been retrospective. Furthermore, the sensitivity and specificity of pelvic cytology tests are not 100%. “Should we continue performing pelvic cytology given the results of this and other studies?” Dr. Pareja asked.
Despite limited knowledge about this variable, physicians may want to be aware if a patient has positive cytology, Dr. Brito suggested. “At least it will give us some red flags so we can be attentive to these patients.”
If researchers were to design a prospective study that incorporates hysteroscopic variables, it could provide more complete answers about the relationship between hysteroscopy and peritoneal cytology and clarify the importance of positive cytology, Dr. Brito said.
Dr. Brito had no relevant disclosures. Dr. Pareja disclosed consulting for Johnson & Johnson.
SOURCE: Oliveira Brito LG et al. J Minim Invasive Gynecol. 2020 Nov. doi: 10.1016/j.jmig.2020.08.356.
FROM AAGL GLOBAL CONGRESS
Etonogestrel implants may be bent, fractured by trauma or during sports
In 2017, Global Pediatric Health published a case report series associated with the use of long-acting reversible contraceptives, specifically the etonogestrel implant.
In November 2020, the makers of the etonogestrel implant (Merck) recommended a change in practice with the release of a notice to health care providers certified in the training of this product. This mass marketing blast included an updated warning and cautions for prescribers as well as patient information on the potential risks of migration, fracture, and bent devices attributable to trauma or sports. “Broken or Bent Implant (Section 5.16). The addition of the following underlined language: “There have been reports of broken or bent implants, which may be related to external forces (e.g., manipulation of the implant or contact sports) while in the patient’s arm. There have also been reports of migration of a broken implant fragment within the arm.”
Clearly the etonogestrel subdermal hormonal implant is an effective form of contraception and particularly beneficial in nonadherent sexually active teens who struggle to remember oral contraceptives. But it is important to be aware of this alert. Little is known about the type of trauma or rate of external force required to cause migration, fracture, or bend implants. This update requires adequate counseling of potential risks and complications of the etonogestrel implant, including the risk of migration, fracture, or bent devices specifically in the event of contact sports and trauma.
Ms. Thew is medical director of the department of adolescent medicine at Children’s Wisconsin in Milwaukee. She is a member of the Pediatric News editorial advisory board. She had no relevant financial disclosures. Email Ms. Thew at [email protected].
In 2017, Global Pediatric Health published a case report series associated with the use of long-acting reversible contraceptives, specifically the etonogestrel implant.
In November 2020, the makers of the etonogestrel implant (Merck) recommended a change in practice with the release of a notice to health care providers certified in the training of this product. This mass marketing blast included an updated warning and cautions for prescribers as well as patient information on the potential risks of migration, fracture, and bent devices attributable to trauma or sports. “Broken or Bent Implant (Section 5.16). The addition of the following underlined language: “There have been reports of broken or bent implants, which may be related to external forces (e.g., manipulation of the implant or contact sports) while in the patient’s arm. There have also been reports of migration of a broken implant fragment within the arm.”
Clearly the etonogestrel subdermal hormonal implant is an effective form of contraception and particularly beneficial in nonadherent sexually active teens who struggle to remember oral contraceptives. But it is important to be aware of this alert. Little is known about the type of trauma or rate of external force required to cause migration, fracture, or bend implants. This update requires adequate counseling of potential risks and complications of the etonogestrel implant, including the risk of migration, fracture, or bent devices specifically in the event of contact sports and trauma.
Ms. Thew is medical director of the department of adolescent medicine at Children’s Wisconsin in Milwaukee. She is a member of the Pediatric News editorial advisory board. She had no relevant financial disclosures. Email Ms. Thew at [email protected].
In 2017, Global Pediatric Health published a case report series associated with the use of long-acting reversible contraceptives, specifically the etonogestrel implant.
In November 2020, the makers of the etonogestrel implant (Merck) recommended a change in practice with the release of a notice to health care providers certified in the training of this product. This mass marketing blast included an updated warning and cautions for prescribers as well as patient information on the potential risks of migration, fracture, and bent devices attributable to trauma or sports. “Broken or Bent Implant (Section 5.16). The addition of the following underlined language: “There have been reports of broken or bent implants, which may be related to external forces (e.g., manipulation of the implant or contact sports) while in the patient’s arm. There have also been reports of migration of a broken implant fragment within the arm.”
Clearly the etonogestrel subdermal hormonal implant is an effective form of contraception and particularly beneficial in nonadherent sexually active teens who struggle to remember oral contraceptives. But it is important to be aware of this alert. Little is known about the type of trauma or rate of external force required to cause migration, fracture, or bend implants. This update requires adequate counseling of potential risks and complications of the etonogestrel implant, including the risk of migration, fracture, or bent devices specifically in the event of contact sports and trauma.
Ms. Thew is medical director of the department of adolescent medicine at Children’s Wisconsin in Milwaukee. She is a member of the Pediatric News editorial advisory board. She had no relevant financial disclosures. Email Ms. Thew at [email protected].
Replace routine preoperative testing with individualized risk assessment and indicated testing
CASE Patient questions need for preoperative tests
A healthy 42-year-old woman (G2P2) with abnormal uterine bleeding and a 2-cm endometrial polyp is scheduled for hysteroscopic polypectomy. After your preoperative clinic visit, the patient receives her paperwork containing information about preoperative lab work and diagnostic studies. You are asked to come into the room because she has further questions. When you arrive, the patient holds the papers out and asks, “Is all this blood work and a chest x-ray necessary? I thought I was healthy and this was a fairly simple surgery. Is there more I should be worried about?”
How would you respond?
The goal of preoperative testing is to determine which patients may be at an increased risk for experiencing an adverse perioperative event, taking into account both the inherent risks of the surgical procedure and the health of the individual patient. In the literature, the general consensus is that physicians rely too heavily on unnecessary laboratory and diagnostic testing during their preoperative assessment.1 More than 50% of patients who underwent preoperative evaluation had at least 1 unindicated test.2 These tests may result in a high frequency of abnormal findings, with less than 3% of abnormalities having clinical value or leading to a change in management.3
With health care costs accounting for almost 20% of the gross domestic product in the United States (totaling about $3.5 billion in 2017), performing unindicated preoperative testing contributes to the economic burden on health care systems, with an estimated cost of $3 to $18 million annually.4,5 In addition, unindicated tests can increase patient anxiety and necessitate follow-up testing, possibly exposing physicians to increased liability if abnormal results are not adequately investigated.6
It is time to rethink our use of routine preoperative testing.
Which tests to consider—or not: Evidence-based guidance
Professional societies, including the American Board of Internal Medicine’s Choosing Wisely campaign, promote a move away from routine testing to avoid unnecessary visits and studies. In addition, the American Society of Anesthesiologists (ASA) has published recommendations to guide preoperative testing.7 To stratify patients’ surgical risk according to their pre-existing health conditions, the ASA created a physical status classification system (TABLE 1).8
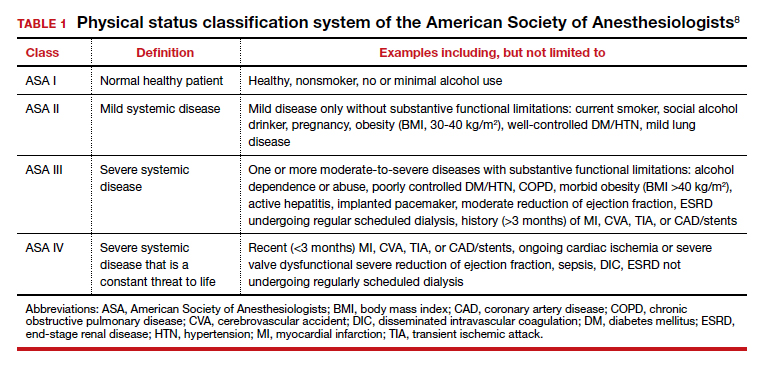
In addition to individual patient characteristics, some guidelines similarly stratify surgical procedures into minor, intermediate, and major risk. The modified Johns Hopkins surgical criteria allocates surgical risk based on expected blood loss, insensible loss, and the inherent risk of a procedure separate from anesthesia (TABLE 2).9 Despite these guidelines, physicians responsible for preoperative evaluations continue to order laboratory and diagnostic tests that are not indicated, often over concerns of case delays or cancellations.10,11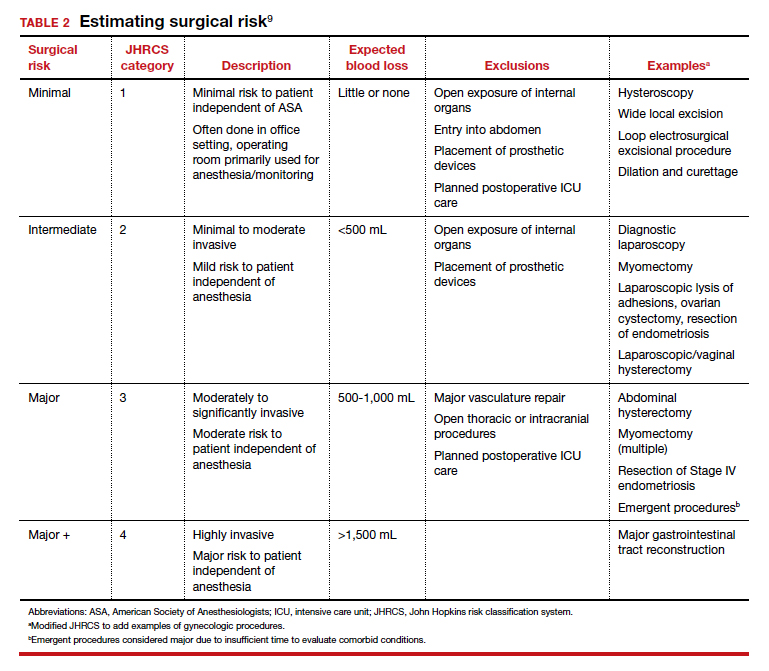
The following evidence-based recommendations provide guidance to gynecologists performing surgery for benign indications to determine which preoperative studies should be performed.
Serum chemistries
Basic metabolic panel (BMP). In both contemporary studies and earlier prospective studies, a preoperative BMP has a low likelihood of changing the surgical procedure or the patient’s management, especially in patients who are classified as ASA I and are undergoing minor- and intermediate-risk procedures.12,13 Therefore, we recommend a BMP for patients in class ASA II or higher who are undergoing intermediate-risk or major surgery.14
Thyroid function. A basic tenet of preoperative evaluation is that asymptomatic patients should not be diagnosed according to lab values prior to surgical intervention. Therefore, we do not recommend routine preoperative thyroid function testing in patients without a history of thyroid disease.10 For patients with known thyroid disease, a thyroid stimulating hormone (TSH) level should be evaluated prior to major surgery, or with any changes in medication dose or symptoms, within the past year.15
Liver function tests (LFTs). Routine screening of asymptomatic individuals without risk factors for liver disease is not recommended because there is a significantly lower incidence of abnormal lab values for LFTs than for other lab tests.16 We recommend LFTs only in symptomatic patients or patients diagnosed with severe liver disease undergoing intermediate-risk or major procedures.14
Hemoglobin A1c (HbA1c). Poorly controlled diabetes is a risk factor for poor wound healing, hospital readmission, prolonged hospitalization, and adverse events following surgery.17 We recommend that HbA1c levels be drawn only for patients with known diabetes undergoing intermediate-risk or major surgery who do not have an available lab value within the past 3 months.14
Continue to: Hematologic studies...
Hematologic studies
Complete blood count (CBC). Many patients undergoing gynecologic procedures may have unreported or undiagnosed anemia secondary to abnormal uterine bleeding, which also may encompass heavy menstrual bleeding. With an abnormal CBC likely to affect preoperative management, assessment of preoperative hemoglobin levels is critical so that hemoglobin levels can be appropriately corrected before surgery. We therefore recommend obtaining a CBC for patients in class ASA II or higher who are undergoing intermediate-risk or major surgery.10,14
Coagulation studies. Preoperative coagulation studies are unlikely to uncover previously undiagnosed inherited coagulopathies, which are generally uncommon in the general population, and they do not predict operative bleeding when ordered unnecessarily.18,19 Therefore, we recommend preoperative coagulation studies only in patients 1) currently on anticoagulation therapy undergoing intermediate-risk or major surgery or 2) in class ASA III or higher with bleeding disorders or cirrhosis undergoing intermediate-risk or major surgery.14
Type and screen (T&S). Complicated algorithms have been proposed to determine when a preoperative T&S is necessary, but these may be impractical for busy gynecologists.20 We recommend a T&S within 72 hours, or on the day, of surgery for all patients undergoing major surgery, including hysterectomy, or with an anticipated blood loss of more than 500 mL; routine crossmatching of blood is not recommended.10,14
Urologic studies
Urine pregnancy test. Although the probability of a positive pregnancy test is likely very low, its occurrence frequently leads to the cancellation of surgery. We therefore recommend a preoperative urine pregnancy test, particularly in reproductive-aged patients with unknown pregnancy status or unreliable contraceptive habits.14 Preoperative urine pregnancy testing, even in patients who report sexual inactivity, ideally should be individualized and based on risk of fetal harm during or subsequent to surgery. Surgeries involving the uterus, or those involving possible teratogens like radiation, also should be considered when making recommendations for testing.
Urinalysis and urine culture. In asymptomatic patients undergoing general gynecologic procedures, a routine preoperative urinalysis and urine culture are of little value.18 However, among patients undergoing a urogynecologic surgical procedure, the risk of a postoperative urinary tract infection is higher than among patients undergoing a nonurogynecologic procedure.21,22 Therefore, we typically do not recommend routine preoperative urinalysis or urine culture, but a preoperative urine culture may be beneficial in patients undergoing urogynecologic surgery.14
Continue to: Diagnostic studies...
Diagnostic studies
Electrocardiography (ECG). The absolute difference in cardiovascular death is less than 1% among patients with and without ECG abnormalities undergoing a noncardiac procedure with minimal to moderate risk; therefore, routine ECG for low-risk patients should not be performed.23 Instead, ECG should be performed in patients with known coronary artery disease or structural heart disease and in patients aged 65 years and older, since age older than 65 years is an independent predictor of significant ECG abnormalities.24,25 We therefore recommend that the following individuals have an ECG within the last 12 months: patients aged 65 years and older, patients in class ASA II or higher with cardiovascular disease, and patients in class ASA III or higher undergoing general anesthesia. If there is a change in cardiovascular health since the most recent ECG—even if it was performed within 12 months—a repeat ECG is warranted.10,14
Chest x-ray. Despite a high rate of abnormalities seen on routine and indicated chest x-rays, there is no significant difference in perioperative pulmonary complications among patients with a normal or abnormal chest x-ray.16 Rather than changing surgical management, these abnormal results are more likely to lead to the cancellation or postponement of a surgical procedure.7 We therefore recommend against routine preoperative chest x-ray.14
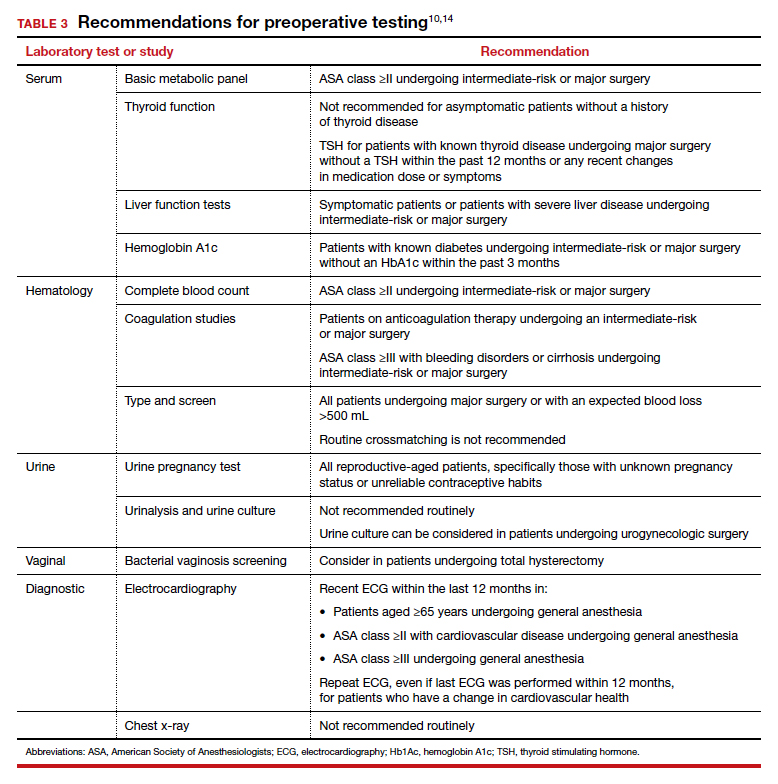
The bottom line
Preoperative testing serves as an additional component of surgical planning. The fact is, however, that abnormal test results are common and frequently do not correlate with surgical outcomes.26 Instead, they can lead to unnecessary surgical procedure cancellations or postponements, undue anxiety in patients, increased liability among physicians, and rising health care costs.5-7
Rather than overly relying on routine laboratory or diagnostic studies, the history and physical examination should continue to be the cornerstone for surgeons responsible for assessing surgical risk. With individualized risk assessment, specific, indicated testing rather than routine nonspecific testing can be obtained.10,14 In short, low-risk patients undergoing noncardiac surgery are unlikely to benefit from preoperative ECG, chest x-ray, or routine laboratory testing without clinical indication. ●
- Kachalia A, Berg A, Fagerlin A, et al. Overuse of testing in preoperative evaluation and syncope: a survey of hospitalists. Ann Intern Med. 2015;162:100-108.
- Onuoha OC, Hatch M, Miano TA, et al. The incidence of un-indicated preoperative testing in a tertiary academic ambulatory center: a retrospective cohort study. Perioper Med. 2015; 4:14.
- Kaplan EB, Sheiner LB, Boeckmann AJ, et al. The usefulness of preoperative laboratory screening. JAMA. 1985;253:3576-3581.
- Centers for Disease Control and Prevention National Center for Health Statistics. Table 42: Gross domestic product, national health expenditures, per capita amounts, percent distribution, and average annual percent change: United States, selected years 1960-2017. https://www.cdc.gov/nchs/ data/hus/2018/042.pdf. Accessed July 2020.
- Benarroch-Gampel J, Sheffield KM, Duncan CB, et al. Preoperative laboratory testing in patients undergoing elective, low-risk ambulatory surgery. Ann Surg. 2012;256:518-528.
- O’Neill F, Carter E, Pink N, et al. Routine preoperative tests for elective surgery: summary of updated NICE guidance. BMJ. 2016;354: i3292.
- Committee on Standards and Practice Parameters; Apfelbaum JL, Connis RT, Nickinovich DG, et al. Practice advisory for preanesthesia evaluation: an updated report by the American Society of Anesthesiologists Task Force on Preanesthesia Evaluation. Anesthesiology. 2012;116:522-538.
- American Society of Anesthesiologists. ASA physical status classification system. https://www.asahq.org/standardsand-guidelines/asa-physical-status-classification-system. Accessed July 2020.
- Pasternak LR, Johns A. Ambulatory gynaecological surgery: risk and assessment. Best Pract Res Clin Obstet Gynaecol. 2005;19:663-679.
- Shields J, Lupo A, Walsh T, et al. Preoperative evaluation for gynecologic surgery: a guide to judicious, evidence-based testing. Curr Opin Obstet Gynecol. 2018;30:252-259.
- Sigmund AE, Stevens ER, Blitz JD, et al. Use of preoperative testing and physicians’ response to professional society guidance. JAMA Intern Med. 2015;175:1352-1359.
- St Clair CM, Shah M, Diver EJ, et al. Adherence to evidence-based guidelines for preoperative testing in women undergoing gynecologic surgery. Obstet Gynecol. 2010;116:694-700.
- De Sousa Soares D, Brandao RR, Mourao MR, et al. Relevance of routine testing in low-risk patients undergoing minor and medium surgical procedures. Braz J Anesthesiol. 2013;63:197-201.
- Shields J, Kho KA. Preoperative evaluation for minimally invasive gynecologic surgery: what is the best evidence and recommendations for clinical practice. J Minim Invasive Gynecol. 2019;26:312-320.
- Palace MR. Perioperative management of thyroid dysfunction. Health Serv Insights. 2017;10:1178632916689677.
- Smetana GW, Macpherson DS. The case against routine preoperative laboratory testing. Med Clin North Am. 2003;87:7-40.
- Jehan F, Khan M, Sakran JV, et al. Perioperative glycemic control and postoperative complications in patients undergoing emergency general surgery: what is the role of plasma hemoglobin A1c? J Trauma Acute Care Surg. 2018;84:112-117.
- Feely MA, Collins CS, Daniels PR, et al. Preoperative testing before noncardiac surgery: guidelines and recommendations. Am Fam Physician. 2013;87:414-418.
- Rusk MH. Avoiding unnecessary preoperative testing. Med Clin North Am. 2016;100:1003-1008.
- Dexter F, Ledolter J, Davis E, et al. Systematic criteria for type and screen based on procedure’s probability of erythrocyte transfusion. Anesthesiology. 2012;116:768-778.
- Gehrich AP, Lustik MB, Mehr AA, et al. Risk of postoperative urinary tract infections following midurethral sling operations in women undergoing hysterectomy. Int Urogynecol J. 2016;27:483-490.
- American College of Obstetricians and Gynecologists. ACOG practice bulletin No. 195 summary: prevention of infection after gynecologic procedures. Obstet Gynecol. 2018;131:1177- 1179.
- Noordzij PG, Boersma E, Bax JJ, et al. Prognostic value of routine preoperative electrocardiography in patients undergoing noncardiac surgery. Am J Cardiol, 2006;97: 1103-1106.
- Fleisher LA, Fleischmann KE, Auerbach AD, et al. 2014 ACC/ AHA guideline on perioperative cardiovascular examination and management of patients undergoing noncardiac surgery: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;130:2215-2245.
- Correll DJ, Hepner DL, Chang C, et al. Preoperative electrocardiograms: patient factors predictive of abnormalities. Anesthesiology. 2009;110:1217-1122.
- Fritsch G, Flamm M, Hepner DL, et al. Abnormal preoperative tests, pathologic findings of medical history, and their predictive value for perioperative complications. Acta Anaesthesiol Scand. 2012;56:339-350.
CASE Patient questions need for preoperative tests
A healthy 42-year-old woman (G2P2) with abnormal uterine bleeding and a 2-cm endometrial polyp is scheduled for hysteroscopic polypectomy. After your preoperative clinic visit, the patient receives her paperwork containing information about preoperative lab work and diagnostic studies. You are asked to come into the room because she has further questions. When you arrive, the patient holds the papers out and asks, “Is all this blood work and a chest x-ray necessary? I thought I was healthy and this was a fairly simple surgery. Is there more I should be worried about?”
How would you respond?
The goal of preoperative testing is to determine which patients may be at an increased risk for experiencing an adverse perioperative event, taking into account both the inherent risks of the surgical procedure and the health of the individual patient. In the literature, the general consensus is that physicians rely too heavily on unnecessary laboratory and diagnostic testing during their preoperative assessment.1 More than 50% of patients who underwent preoperative evaluation had at least 1 unindicated test.2 These tests may result in a high frequency of abnormal findings, with less than 3% of abnormalities having clinical value or leading to a change in management.3
With health care costs accounting for almost 20% of the gross domestic product in the United States (totaling about $3.5 billion in 2017), performing unindicated preoperative testing contributes to the economic burden on health care systems, with an estimated cost of $3 to $18 million annually.4,5 In addition, unindicated tests can increase patient anxiety and necessitate follow-up testing, possibly exposing physicians to increased liability if abnormal results are not adequately investigated.6
It is time to rethink our use of routine preoperative testing.
Which tests to consider—or not: Evidence-based guidance
Professional societies, including the American Board of Internal Medicine’s Choosing Wisely campaign, promote a move away from routine testing to avoid unnecessary visits and studies. In addition, the American Society of Anesthesiologists (ASA) has published recommendations to guide preoperative testing.7 To stratify patients’ surgical risk according to their pre-existing health conditions, the ASA created a physical status classification system (TABLE 1).8

In addition to individual patient characteristics, some guidelines similarly stratify surgical procedures into minor, intermediate, and major risk. The modified Johns Hopkins surgical criteria allocates surgical risk based on expected blood loss, insensible loss, and the inherent risk of a procedure separate from anesthesia (TABLE 2).9 Despite these guidelines, physicians responsible for preoperative evaluations continue to order laboratory and diagnostic tests that are not indicated, often over concerns of case delays or cancellations.10,11
The following evidence-based recommendations provide guidance to gynecologists performing surgery for benign indications to determine which preoperative studies should be performed.
Serum chemistries
Basic metabolic panel (BMP). In both contemporary studies and earlier prospective studies, a preoperative BMP has a low likelihood of changing the surgical procedure or the patient’s management, especially in patients who are classified as ASA I and are undergoing minor- and intermediate-risk procedures.12,13 Therefore, we recommend a BMP for patients in class ASA II or higher who are undergoing intermediate-risk or major surgery.14
Thyroid function. A basic tenet of preoperative evaluation is that asymptomatic patients should not be diagnosed according to lab values prior to surgical intervention. Therefore, we do not recommend routine preoperative thyroid function testing in patients without a history of thyroid disease.10 For patients with known thyroid disease, a thyroid stimulating hormone (TSH) level should be evaluated prior to major surgery, or with any changes in medication dose or symptoms, within the past year.15
Liver function tests (LFTs). Routine screening of asymptomatic individuals without risk factors for liver disease is not recommended because there is a significantly lower incidence of abnormal lab values for LFTs than for other lab tests.16 We recommend LFTs only in symptomatic patients or patients diagnosed with severe liver disease undergoing intermediate-risk or major procedures.14
Hemoglobin A1c (HbA1c). Poorly controlled diabetes is a risk factor for poor wound healing, hospital readmission, prolonged hospitalization, and adverse events following surgery.17 We recommend that HbA1c levels be drawn only for patients with known diabetes undergoing intermediate-risk or major surgery who do not have an available lab value within the past 3 months.14
Continue to: Hematologic studies...
Hematologic studies
Complete blood count (CBC). Many patients undergoing gynecologic procedures may have unreported or undiagnosed anemia secondary to abnormal uterine bleeding, which also may encompass heavy menstrual bleeding. With an abnormal CBC likely to affect preoperative management, assessment of preoperative hemoglobin levels is critical so that hemoglobin levels can be appropriately corrected before surgery. We therefore recommend obtaining a CBC for patients in class ASA II or higher who are undergoing intermediate-risk or major surgery.10,14
Coagulation studies. Preoperative coagulation studies are unlikely to uncover previously undiagnosed inherited coagulopathies, which are generally uncommon in the general population, and they do not predict operative bleeding when ordered unnecessarily.18,19 Therefore, we recommend preoperative coagulation studies only in patients 1) currently on anticoagulation therapy undergoing intermediate-risk or major surgery or 2) in class ASA III or higher with bleeding disorders or cirrhosis undergoing intermediate-risk or major surgery.14
Type and screen (T&S). Complicated algorithms have been proposed to determine when a preoperative T&S is necessary, but these may be impractical for busy gynecologists.20 We recommend a T&S within 72 hours, or on the day, of surgery for all patients undergoing major surgery, including hysterectomy, or with an anticipated blood loss of more than 500 mL; routine crossmatching of blood is not recommended.10,14
Urologic studies
Urine pregnancy test. Although the probability of a positive pregnancy test is likely very low, its occurrence frequently leads to the cancellation of surgery. We therefore recommend a preoperative urine pregnancy test, particularly in reproductive-aged patients with unknown pregnancy status or unreliable contraceptive habits.14 Preoperative urine pregnancy testing, even in patients who report sexual inactivity, ideally should be individualized and based on risk of fetal harm during or subsequent to surgery. Surgeries involving the uterus, or those involving possible teratogens like radiation, also should be considered when making recommendations for testing.
Urinalysis and urine culture. In asymptomatic patients undergoing general gynecologic procedures, a routine preoperative urinalysis and urine culture are of little value.18 However, among patients undergoing a urogynecologic surgical procedure, the risk of a postoperative urinary tract infection is higher than among patients undergoing a nonurogynecologic procedure.21,22 Therefore, we typically do not recommend routine preoperative urinalysis or urine culture, but a preoperative urine culture may be beneficial in patients undergoing urogynecologic surgery.14
Continue to: Diagnostic studies...
Diagnostic studies
Electrocardiography (ECG). The absolute difference in cardiovascular death is less than 1% among patients with and without ECG abnormalities undergoing a noncardiac procedure with minimal to moderate risk; therefore, routine ECG for low-risk patients should not be performed.23 Instead, ECG should be performed in patients with known coronary artery disease or structural heart disease and in patients aged 65 years and older, since age older than 65 years is an independent predictor of significant ECG abnormalities.24,25 We therefore recommend that the following individuals have an ECG within the last 12 months: patients aged 65 years and older, patients in class ASA II or higher with cardiovascular disease, and patients in class ASA III or higher undergoing general anesthesia. If there is a change in cardiovascular health since the most recent ECG—even if it was performed within 12 months—a repeat ECG is warranted.10,14
Chest x-ray. Despite a high rate of abnormalities seen on routine and indicated chest x-rays, there is no significant difference in perioperative pulmonary complications among patients with a normal or abnormal chest x-ray.16 Rather than changing surgical management, these abnormal results are more likely to lead to the cancellation or postponement of a surgical procedure.7 We therefore recommend against routine preoperative chest x-ray.14

The bottom line
Preoperative testing serves as an additional component of surgical planning. The fact is, however, that abnormal test results are common and frequently do not correlate with surgical outcomes.26 Instead, they can lead to unnecessary surgical procedure cancellations or postponements, undue anxiety in patients, increased liability among physicians, and rising health care costs.5-7
Rather than overly relying on routine laboratory or diagnostic studies, the history and physical examination should continue to be the cornerstone for surgeons responsible for assessing surgical risk. With individualized risk assessment, specific, indicated testing rather than routine nonspecific testing can be obtained.10,14 In short, low-risk patients undergoing noncardiac surgery are unlikely to benefit from preoperative ECG, chest x-ray, or routine laboratory testing without clinical indication. ●
CASE Patient questions need for preoperative tests
A healthy 42-year-old woman (G2P2) with abnormal uterine bleeding and a 2-cm endometrial polyp is scheduled for hysteroscopic polypectomy. After your preoperative clinic visit, the patient receives her paperwork containing information about preoperative lab work and diagnostic studies. You are asked to come into the room because she has further questions. When you arrive, the patient holds the papers out and asks, “Is all this blood work and a chest x-ray necessary? I thought I was healthy and this was a fairly simple surgery. Is there more I should be worried about?”
How would you respond?
The goal of preoperative testing is to determine which patients may be at an increased risk for experiencing an adverse perioperative event, taking into account both the inherent risks of the surgical procedure and the health of the individual patient. In the literature, the general consensus is that physicians rely too heavily on unnecessary laboratory and diagnostic testing during their preoperative assessment.1 More than 50% of patients who underwent preoperative evaluation had at least 1 unindicated test.2 These tests may result in a high frequency of abnormal findings, with less than 3% of abnormalities having clinical value or leading to a change in management.3
With health care costs accounting for almost 20% of the gross domestic product in the United States (totaling about $3.5 billion in 2017), performing unindicated preoperative testing contributes to the economic burden on health care systems, with an estimated cost of $3 to $18 million annually.4,5 In addition, unindicated tests can increase patient anxiety and necessitate follow-up testing, possibly exposing physicians to increased liability if abnormal results are not adequately investigated.6
It is time to rethink our use of routine preoperative testing.
Which tests to consider—or not: Evidence-based guidance
Professional societies, including the American Board of Internal Medicine’s Choosing Wisely campaign, promote a move away from routine testing to avoid unnecessary visits and studies. In addition, the American Society of Anesthesiologists (ASA) has published recommendations to guide preoperative testing.7 To stratify patients’ surgical risk according to their pre-existing health conditions, the ASA created a physical status classification system (TABLE 1).8

In addition to individual patient characteristics, some guidelines similarly stratify surgical procedures into minor, intermediate, and major risk. The modified Johns Hopkins surgical criteria allocates surgical risk based on expected blood loss, insensible loss, and the inherent risk of a procedure separate from anesthesia (TABLE 2).9 Despite these guidelines, physicians responsible for preoperative evaluations continue to order laboratory and diagnostic tests that are not indicated, often over concerns of case delays or cancellations.10,11
The following evidence-based recommendations provide guidance to gynecologists performing surgery for benign indications to determine which preoperative studies should be performed.
Serum chemistries
Basic metabolic panel (BMP). In both contemporary studies and earlier prospective studies, a preoperative BMP has a low likelihood of changing the surgical procedure or the patient’s management, especially in patients who are classified as ASA I and are undergoing minor- and intermediate-risk procedures.12,13 Therefore, we recommend a BMP for patients in class ASA II or higher who are undergoing intermediate-risk or major surgery.14
Thyroid function. A basic tenet of preoperative evaluation is that asymptomatic patients should not be diagnosed according to lab values prior to surgical intervention. Therefore, we do not recommend routine preoperative thyroid function testing in patients without a history of thyroid disease.10 For patients with known thyroid disease, a thyroid stimulating hormone (TSH) level should be evaluated prior to major surgery, or with any changes in medication dose or symptoms, within the past year.15
Liver function tests (LFTs). Routine screening of asymptomatic individuals without risk factors for liver disease is not recommended because there is a significantly lower incidence of abnormal lab values for LFTs than for other lab tests.16 We recommend LFTs only in symptomatic patients or patients diagnosed with severe liver disease undergoing intermediate-risk or major procedures.14
Hemoglobin A1c (HbA1c). Poorly controlled diabetes is a risk factor for poor wound healing, hospital readmission, prolonged hospitalization, and adverse events following surgery.17 We recommend that HbA1c levels be drawn only for patients with known diabetes undergoing intermediate-risk or major surgery who do not have an available lab value within the past 3 months.14
Continue to: Hematologic studies...
Hematologic studies
Complete blood count (CBC). Many patients undergoing gynecologic procedures may have unreported or undiagnosed anemia secondary to abnormal uterine bleeding, which also may encompass heavy menstrual bleeding. With an abnormal CBC likely to affect preoperative management, assessment of preoperative hemoglobin levels is critical so that hemoglobin levels can be appropriately corrected before surgery. We therefore recommend obtaining a CBC for patients in class ASA II or higher who are undergoing intermediate-risk or major surgery.10,14
Coagulation studies. Preoperative coagulation studies are unlikely to uncover previously undiagnosed inherited coagulopathies, which are generally uncommon in the general population, and they do not predict operative bleeding when ordered unnecessarily.18,19 Therefore, we recommend preoperative coagulation studies only in patients 1) currently on anticoagulation therapy undergoing intermediate-risk or major surgery or 2) in class ASA III or higher with bleeding disorders or cirrhosis undergoing intermediate-risk or major surgery.14
Type and screen (T&S). Complicated algorithms have been proposed to determine when a preoperative T&S is necessary, but these may be impractical for busy gynecologists.20 We recommend a T&S within 72 hours, or on the day, of surgery for all patients undergoing major surgery, including hysterectomy, or with an anticipated blood loss of more than 500 mL; routine crossmatching of blood is not recommended.10,14
Urologic studies
Urine pregnancy test. Although the probability of a positive pregnancy test is likely very low, its occurrence frequently leads to the cancellation of surgery. We therefore recommend a preoperative urine pregnancy test, particularly in reproductive-aged patients with unknown pregnancy status or unreliable contraceptive habits.14 Preoperative urine pregnancy testing, even in patients who report sexual inactivity, ideally should be individualized and based on risk of fetal harm during or subsequent to surgery. Surgeries involving the uterus, or those involving possible teratogens like radiation, also should be considered when making recommendations for testing.
Urinalysis and urine culture. In asymptomatic patients undergoing general gynecologic procedures, a routine preoperative urinalysis and urine culture are of little value.18 However, among patients undergoing a urogynecologic surgical procedure, the risk of a postoperative urinary tract infection is higher than among patients undergoing a nonurogynecologic procedure.21,22 Therefore, we typically do not recommend routine preoperative urinalysis or urine culture, but a preoperative urine culture may be beneficial in patients undergoing urogynecologic surgery.14
Continue to: Diagnostic studies...
Diagnostic studies
Electrocardiography (ECG). The absolute difference in cardiovascular death is less than 1% among patients with and without ECG abnormalities undergoing a noncardiac procedure with minimal to moderate risk; therefore, routine ECG for low-risk patients should not be performed.23 Instead, ECG should be performed in patients with known coronary artery disease or structural heart disease and in patients aged 65 years and older, since age older than 65 years is an independent predictor of significant ECG abnormalities.24,25 We therefore recommend that the following individuals have an ECG within the last 12 months: patients aged 65 years and older, patients in class ASA II or higher with cardiovascular disease, and patients in class ASA III or higher undergoing general anesthesia. If there is a change in cardiovascular health since the most recent ECG—even if it was performed within 12 months—a repeat ECG is warranted.10,14
Chest x-ray. Despite a high rate of abnormalities seen on routine and indicated chest x-rays, there is no significant difference in perioperative pulmonary complications among patients with a normal or abnormal chest x-ray.16 Rather than changing surgical management, these abnormal results are more likely to lead to the cancellation or postponement of a surgical procedure.7 We therefore recommend against routine preoperative chest x-ray.14

The bottom line
Preoperative testing serves as an additional component of surgical planning. The fact is, however, that abnormal test results are common and frequently do not correlate with surgical outcomes.26 Instead, they can lead to unnecessary surgical procedure cancellations or postponements, undue anxiety in patients, increased liability among physicians, and rising health care costs.5-7
Rather than overly relying on routine laboratory or diagnostic studies, the history and physical examination should continue to be the cornerstone for surgeons responsible for assessing surgical risk. With individualized risk assessment, specific, indicated testing rather than routine nonspecific testing can be obtained.10,14 In short, low-risk patients undergoing noncardiac surgery are unlikely to benefit from preoperative ECG, chest x-ray, or routine laboratory testing without clinical indication. ●
- Kachalia A, Berg A, Fagerlin A, et al. Overuse of testing in preoperative evaluation and syncope: a survey of hospitalists. Ann Intern Med. 2015;162:100-108.
- Onuoha OC, Hatch M, Miano TA, et al. The incidence of un-indicated preoperative testing in a tertiary academic ambulatory center: a retrospective cohort study. Perioper Med. 2015; 4:14.
- Kaplan EB, Sheiner LB, Boeckmann AJ, et al. The usefulness of preoperative laboratory screening. JAMA. 1985;253:3576-3581.
- Centers for Disease Control and Prevention National Center for Health Statistics. Table 42: Gross domestic product, national health expenditures, per capita amounts, percent distribution, and average annual percent change: United States, selected years 1960-2017. https://www.cdc.gov/nchs/ data/hus/2018/042.pdf. Accessed July 2020.
- Benarroch-Gampel J, Sheffield KM, Duncan CB, et al. Preoperative laboratory testing in patients undergoing elective, low-risk ambulatory surgery. Ann Surg. 2012;256:518-528.
- O’Neill F, Carter E, Pink N, et al. Routine preoperative tests for elective surgery: summary of updated NICE guidance. BMJ. 2016;354: i3292.
- Committee on Standards and Practice Parameters; Apfelbaum JL, Connis RT, Nickinovich DG, et al. Practice advisory for preanesthesia evaluation: an updated report by the American Society of Anesthesiologists Task Force on Preanesthesia Evaluation. Anesthesiology. 2012;116:522-538.
- American Society of Anesthesiologists. ASA physical status classification system. https://www.asahq.org/standardsand-guidelines/asa-physical-status-classification-system. Accessed July 2020.
- Pasternak LR, Johns A. Ambulatory gynaecological surgery: risk and assessment. Best Pract Res Clin Obstet Gynaecol. 2005;19:663-679.
- Shields J, Lupo A, Walsh T, et al. Preoperative evaluation for gynecologic surgery: a guide to judicious, evidence-based testing. Curr Opin Obstet Gynecol. 2018;30:252-259.
- Sigmund AE, Stevens ER, Blitz JD, et al. Use of preoperative testing and physicians’ response to professional society guidance. JAMA Intern Med. 2015;175:1352-1359.
- St Clair CM, Shah M, Diver EJ, et al. Adherence to evidence-based guidelines for preoperative testing in women undergoing gynecologic surgery. Obstet Gynecol. 2010;116:694-700.
- De Sousa Soares D, Brandao RR, Mourao MR, et al. Relevance of routine testing in low-risk patients undergoing minor and medium surgical procedures. Braz J Anesthesiol. 2013;63:197-201.
- Shields J, Kho KA. Preoperative evaluation for minimally invasive gynecologic surgery: what is the best evidence and recommendations for clinical practice. J Minim Invasive Gynecol. 2019;26:312-320.
- Palace MR. Perioperative management of thyroid dysfunction. Health Serv Insights. 2017;10:1178632916689677.
- Smetana GW, Macpherson DS. The case against routine preoperative laboratory testing. Med Clin North Am. 2003;87:7-40.
- Jehan F, Khan M, Sakran JV, et al. Perioperative glycemic control and postoperative complications in patients undergoing emergency general surgery: what is the role of plasma hemoglobin A1c? J Trauma Acute Care Surg. 2018;84:112-117.
- Feely MA, Collins CS, Daniels PR, et al. Preoperative testing before noncardiac surgery: guidelines and recommendations. Am Fam Physician. 2013;87:414-418.
- Rusk MH. Avoiding unnecessary preoperative testing. Med Clin North Am. 2016;100:1003-1008.
- Dexter F, Ledolter J, Davis E, et al. Systematic criteria for type and screen based on procedure’s probability of erythrocyte transfusion. Anesthesiology. 2012;116:768-778.
- Gehrich AP, Lustik MB, Mehr AA, et al. Risk of postoperative urinary tract infections following midurethral sling operations in women undergoing hysterectomy. Int Urogynecol J. 2016;27:483-490.
- American College of Obstetricians and Gynecologists. ACOG practice bulletin No. 195 summary: prevention of infection after gynecologic procedures. Obstet Gynecol. 2018;131:1177- 1179.
- Noordzij PG, Boersma E, Bax JJ, et al. Prognostic value of routine preoperative electrocardiography in patients undergoing noncardiac surgery. Am J Cardiol, 2006;97: 1103-1106.
- Fleisher LA, Fleischmann KE, Auerbach AD, et al. 2014 ACC/ AHA guideline on perioperative cardiovascular examination and management of patients undergoing noncardiac surgery: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;130:2215-2245.
- Correll DJ, Hepner DL, Chang C, et al. Preoperative electrocardiograms: patient factors predictive of abnormalities. Anesthesiology. 2009;110:1217-1122.
- Fritsch G, Flamm M, Hepner DL, et al. Abnormal preoperative tests, pathologic findings of medical history, and their predictive value for perioperative complications. Acta Anaesthesiol Scand. 2012;56:339-350.
- Kachalia A, Berg A, Fagerlin A, et al. Overuse of testing in preoperative evaluation and syncope: a survey of hospitalists. Ann Intern Med. 2015;162:100-108.
- Onuoha OC, Hatch M, Miano TA, et al. The incidence of un-indicated preoperative testing in a tertiary academic ambulatory center: a retrospective cohort study. Perioper Med. 2015; 4:14.
- Kaplan EB, Sheiner LB, Boeckmann AJ, et al. The usefulness of preoperative laboratory screening. JAMA. 1985;253:3576-3581.
- Centers for Disease Control and Prevention National Center for Health Statistics. Table 42: Gross domestic product, national health expenditures, per capita amounts, percent distribution, and average annual percent change: United States, selected years 1960-2017. https://www.cdc.gov/nchs/ data/hus/2018/042.pdf. Accessed July 2020.
- Benarroch-Gampel J, Sheffield KM, Duncan CB, et al. Preoperative laboratory testing in patients undergoing elective, low-risk ambulatory surgery. Ann Surg. 2012;256:518-528.
- O’Neill F, Carter E, Pink N, et al. Routine preoperative tests for elective surgery: summary of updated NICE guidance. BMJ. 2016;354: i3292.
- Committee on Standards and Practice Parameters; Apfelbaum JL, Connis RT, Nickinovich DG, et al. Practice advisory for preanesthesia evaluation: an updated report by the American Society of Anesthesiologists Task Force on Preanesthesia Evaluation. Anesthesiology. 2012;116:522-538.
- American Society of Anesthesiologists. ASA physical status classification system. https://www.asahq.org/standardsand-guidelines/asa-physical-status-classification-system. Accessed July 2020.
- Pasternak LR, Johns A. Ambulatory gynaecological surgery: risk and assessment. Best Pract Res Clin Obstet Gynaecol. 2005;19:663-679.
- Shields J, Lupo A, Walsh T, et al. Preoperative evaluation for gynecologic surgery: a guide to judicious, evidence-based testing. Curr Opin Obstet Gynecol. 2018;30:252-259.
- Sigmund AE, Stevens ER, Blitz JD, et al. Use of preoperative testing and physicians’ response to professional society guidance. JAMA Intern Med. 2015;175:1352-1359.
- St Clair CM, Shah M, Diver EJ, et al. Adherence to evidence-based guidelines for preoperative testing in women undergoing gynecologic surgery. Obstet Gynecol. 2010;116:694-700.
- De Sousa Soares D, Brandao RR, Mourao MR, et al. Relevance of routine testing in low-risk patients undergoing minor and medium surgical procedures. Braz J Anesthesiol. 2013;63:197-201.
- Shields J, Kho KA. Preoperative evaluation for minimally invasive gynecologic surgery: what is the best evidence and recommendations for clinical practice. J Minim Invasive Gynecol. 2019;26:312-320.
- Palace MR. Perioperative management of thyroid dysfunction. Health Serv Insights. 2017;10:1178632916689677.
- Smetana GW, Macpherson DS. The case against routine preoperative laboratory testing. Med Clin North Am. 2003;87:7-40.
- Jehan F, Khan M, Sakran JV, et al. Perioperative glycemic control and postoperative complications in patients undergoing emergency general surgery: what is the role of plasma hemoglobin A1c? J Trauma Acute Care Surg. 2018;84:112-117.
- Feely MA, Collins CS, Daniels PR, et al. Preoperative testing before noncardiac surgery: guidelines and recommendations. Am Fam Physician. 2013;87:414-418.
- Rusk MH. Avoiding unnecessary preoperative testing. Med Clin North Am. 2016;100:1003-1008.
- Dexter F, Ledolter J, Davis E, et al. Systematic criteria for type and screen based on procedure’s probability of erythrocyte transfusion. Anesthesiology. 2012;116:768-778.
- Gehrich AP, Lustik MB, Mehr AA, et al. Risk of postoperative urinary tract infections following midurethral sling operations in women undergoing hysterectomy. Int Urogynecol J. 2016;27:483-490.
- American College of Obstetricians and Gynecologists. ACOG practice bulletin No. 195 summary: prevention of infection after gynecologic procedures. Obstet Gynecol. 2018;131:1177- 1179.
- Noordzij PG, Boersma E, Bax JJ, et al. Prognostic value of routine preoperative electrocardiography in patients undergoing noncardiac surgery. Am J Cardiol, 2006;97: 1103-1106.
- Fleisher LA, Fleischmann KE, Auerbach AD, et al. 2014 ACC/ AHA guideline on perioperative cardiovascular examination and management of patients undergoing noncardiac surgery: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;130:2215-2245.
- Correll DJ, Hepner DL, Chang C, et al. Preoperative electrocardiograms: patient factors predictive of abnormalities. Anesthesiology. 2009;110:1217-1122.
- Fritsch G, Flamm M, Hepner DL, et al. Abnormal preoperative tests, pathologic findings of medical history, and their predictive value for perioperative complications. Acta Anaesthesiol Scand. 2012;56:339-350.
Prophylactic antibiotics for myomectomy?
In the 1990s, researchers found that patients undergoing any type of surgical procedure were more than twice as likely to die if they developed postsurgical infection.1 Work to reduce surgical site infection (SSI) has and does continue, with perioperative antibiotics representing a good part of that effort. The American College of Obstetricians and Gynecologists currently recommends such antibiotic therapy for women undergoing laparotomy and laparoscopic hysterectomy.2 ACOG does not, however, recommend prophylactic antibiotics for myomectomy procedures.3 Rates of infection for hysterectomy have been reported to be 3.9% for abdominal and 1.4% for minimally invasive approaches.4
To determine the current use of antibiotics during myomectomy and associated rates of SSI at their institutions, Dipti Banerjee, MD, and colleagues conducted a retrospective analysis of women undergoing laparoscopic or abdominal myomectomy between February 2013 and December 2017 at the University of California, Los Angeles and Hoag Memorial Hospital in Orange County, California. They presented their study results at AAGL’s 49th Global Congress on MIGS, held virtually November 6-14, 2020.3
Rate of SSI after myomectomy
A total of 620 women underwent laparoscopic myomectomy and 563 underwent open myomectomy during the study period. Antibiotics were used in 76.9% of cases. SSI developed within 6 weeks of surgery in 34 women (2.9%) overall. The women undergoing abdominal myomectomy without antibiotics were more likely to experience SSI than the women who received antibiotics (odds ratio [OR], 4.89; confidence interval [CI], 1.80–13.27; P = .0006). For laparoscopic myomectomy, antibiotic use did not affect the odds of developing SSI (OR, 1.08; CI, 0.35–3.35).
Antibiotics were more likely to be used in certain cases
Antibiotics were more likely to be administered for patients who:
- were obese (body mass index ≥30 kg/m2) (P = .009)
- underwent previous abdominal surgery (P = .001)
- underwent laparotomy (P <.0001)
- had endometrial cavity entry (P <.0001)
- had >1 fibroid (P = .0004) or an aggregate fibroid weight >500 g (P <.0001).
More data on antibiotics for myomectomy
In a retrospective study conducted at 2 academic hospitals in Boston, Massachusetts, 1,211 women underwent myomectomy from 2009 to 2016. (Exclusions were use of vaginal or hysteroscopic myomectomy, chromopertubation, or conversion to hysterectomy.) More than 92% of the women received perioperative antibiotics at the time of surgery. Although demographics were similar between women receiving and not receiving antibiotics, women who received antibiotics were more likely to have longer operative times (median 140 vs 85 min), a greater myoma burden (7 vs 2 myomas removed and weight 255 vs 53 g), and lose blood during the procedure (137 vs 50 mL). These women also were 4 times less likely to have surgical site infection (adjusted OR, 3.77; 95% CI, 1.30–10.97; P = .015).5,6
Banerjee and colleagues say that their California study demonstrates “that the majority of surgeons elect to use antibiotics prophylactically” during myomectomy, despite current ACOG guidelines, and that their findings of benefit for abdominal myomectomy but not for laparoscopic myomectomy should inform future guidance on antibiotics for myomectomy surgery.3
- Kirkland KB, Briggs JP, Trivette SL, et al. The impact of surgical-site infections in the 1990s: attributable mortality, excess length of hospitalization, and extra costs. Infect Control Hosp Epidemiol. 1999;20:725-730.
- American College of Obstetricians and Gynecologists. Practice Bulletin No. 195: prevention of infection after gynecologic procedures. Obstet Gynecol. 2018;131:e172-e189.
- Banerjee D, Dejbakhsh S, Patel HH, et al. Perioperative antibiotic prophylaxis in myomectomy surgery. Paper presented at 49th Annual Meeting of the AAGL; November 2020.
- Uppal S, Harris J, Al-Niaimi A. Prophylactic antibiotic choice and risk of surgical site infection after hysterectomy. Obstet Gynecol. 2016;127:321-329.
- Kim AJ, Clark NV, Jansen LJ, et al. Perioperative antibiotic use and associated infectious outcomes at the time of myomectomy. Obstet Gynecol. 2019;133:626-635.
- Rebar RW. Should perioperative antibiotics at myomectomy be universal? NEJM J Watch. March 11, 2019.
In the 1990s, researchers found that patients undergoing any type of surgical procedure were more than twice as likely to die if they developed postsurgical infection.1 Work to reduce surgical site infection (SSI) has and does continue, with perioperative antibiotics representing a good part of that effort. The American College of Obstetricians and Gynecologists currently recommends such antibiotic therapy for women undergoing laparotomy and laparoscopic hysterectomy.2 ACOG does not, however, recommend prophylactic antibiotics for myomectomy procedures.3 Rates of infection for hysterectomy have been reported to be 3.9% for abdominal and 1.4% for minimally invasive approaches.4
To determine the current use of antibiotics during myomectomy and associated rates of SSI at their institutions, Dipti Banerjee, MD, and colleagues conducted a retrospective analysis of women undergoing laparoscopic or abdominal myomectomy between February 2013 and December 2017 at the University of California, Los Angeles and Hoag Memorial Hospital in Orange County, California. They presented their study results at AAGL’s 49th Global Congress on MIGS, held virtually November 6-14, 2020.3
Rate of SSI after myomectomy
A total of 620 women underwent laparoscopic myomectomy and 563 underwent open myomectomy during the study period. Antibiotics were used in 76.9% of cases. SSI developed within 6 weeks of surgery in 34 women (2.9%) overall. The women undergoing abdominal myomectomy without antibiotics were more likely to experience SSI than the women who received antibiotics (odds ratio [OR], 4.89; confidence interval [CI], 1.80–13.27; P = .0006). For laparoscopic myomectomy, antibiotic use did not affect the odds of developing SSI (OR, 1.08; CI, 0.35–3.35).
Antibiotics were more likely to be used in certain cases
Antibiotics were more likely to be administered for patients who:
- were obese (body mass index ≥30 kg/m2) (P = .009)
- underwent previous abdominal surgery (P = .001)
- underwent laparotomy (P <.0001)
- had endometrial cavity entry (P <.0001)
- had >1 fibroid (P = .0004) or an aggregate fibroid weight >500 g (P <.0001).
More data on antibiotics for myomectomy
In a retrospective study conducted at 2 academic hospitals in Boston, Massachusetts, 1,211 women underwent myomectomy from 2009 to 2016. (Exclusions were use of vaginal or hysteroscopic myomectomy, chromopertubation, or conversion to hysterectomy.) More than 92% of the women received perioperative antibiotics at the time of surgery. Although demographics were similar between women receiving and not receiving antibiotics, women who received antibiotics were more likely to have longer operative times (median 140 vs 85 min), a greater myoma burden (7 vs 2 myomas removed and weight 255 vs 53 g), and lose blood during the procedure (137 vs 50 mL). These women also were 4 times less likely to have surgical site infection (adjusted OR, 3.77; 95% CI, 1.30–10.97; P = .015).5,6
Banerjee and colleagues say that their California study demonstrates “that the majority of surgeons elect to use antibiotics prophylactically” during myomectomy, despite current ACOG guidelines, and that their findings of benefit for abdominal myomectomy but not for laparoscopic myomectomy should inform future guidance on antibiotics for myomectomy surgery.3
In the 1990s, researchers found that patients undergoing any type of surgical procedure were more than twice as likely to die if they developed postsurgical infection.1 Work to reduce surgical site infection (SSI) has and does continue, with perioperative antibiotics representing a good part of that effort. The American College of Obstetricians and Gynecologists currently recommends such antibiotic therapy for women undergoing laparotomy and laparoscopic hysterectomy.2 ACOG does not, however, recommend prophylactic antibiotics for myomectomy procedures.3 Rates of infection for hysterectomy have been reported to be 3.9% for abdominal and 1.4% for minimally invasive approaches.4
To determine the current use of antibiotics during myomectomy and associated rates of SSI at their institutions, Dipti Banerjee, MD, and colleagues conducted a retrospective analysis of women undergoing laparoscopic or abdominal myomectomy between February 2013 and December 2017 at the University of California, Los Angeles and Hoag Memorial Hospital in Orange County, California. They presented their study results at AAGL’s 49th Global Congress on MIGS, held virtually November 6-14, 2020.3
Rate of SSI after myomectomy
A total of 620 women underwent laparoscopic myomectomy and 563 underwent open myomectomy during the study period. Antibiotics were used in 76.9% of cases. SSI developed within 6 weeks of surgery in 34 women (2.9%) overall. The women undergoing abdominal myomectomy without antibiotics were more likely to experience SSI than the women who received antibiotics (odds ratio [OR], 4.89; confidence interval [CI], 1.80–13.27; P = .0006). For laparoscopic myomectomy, antibiotic use did not affect the odds of developing SSI (OR, 1.08; CI, 0.35–3.35).
Antibiotics were more likely to be used in certain cases
Antibiotics were more likely to be administered for patients who:
- were obese (body mass index ≥30 kg/m2) (P = .009)
- underwent previous abdominal surgery (P = .001)
- underwent laparotomy (P <.0001)
- had endometrial cavity entry (P <.0001)
- had >1 fibroid (P = .0004) or an aggregate fibroid weight >500 g (P <.0001).
More data on antibiotics for myomectomy
In a retrospective study conducted at 2 academic hospitals in Boston, Massachusetts, 1,211 women underwent myomectomy from 2009 to 2016. (Exclusions were use of vaginal or hysteroscopic myomectomy, chromopertubation, or conversion to hysterectomy.) More than 92% of the women received perioperative antibiotics at the time of surgery. Although demographics were similar between women receiving and not receiving antibiotics, women who received antibiotics were more likely to have longer operative times (median 140 vs 85 min), a greater myoma burden (7 vs 2 myomas removed and weight 255 vs 53 g), and lose blood during the procedure (137 vs 50 mL). These women also were 4 times less likely to have surgical site infection (adjusted OR, 3.77; 95% CI, 1.30–10.97; P = .015).5,6
Banerjee and colleagues say that their California study demonstrates “that the majority of surgeons elect to use antibiotics prophylactically” during myomectomy, despite current ACOG guidelines, and that their findings of benefit for abdominal myomectomy but not for laparoscopic myomectomy should inform future guidance on antibiotics for myomectomy surgery.3
- Kirkland KB, Briggs JP, Trivette SL, et al. The impact of surgical-site infections in the 1990s: attributable mortality, excess length of hospitalization, and extra costs. Infect Control Hosp Epidemiol. 1999;20:725-730.
- American College of Obstetricians and Gynecologists. Practice Bulletin No. 195: prevention of infection after gynecologic procedures. Obstet Gynecol. 2018;131:e172-e189.
- Banerjee D, Dejbakhsh S, Patel HH, et al. Perioperative antibiotic prophylaxis in myomectomy surgery. Paper presented at 49th Annual Meeting of the AAGL; November 2020.
- Uppal S, Harris J, Al-Niaimi A. Prophylactic antibiotic choice and risk of surgical site infection after hysterectomy. Obstet Gynecol. 2016;127:321-329.
- Kim AJ, Clark NV, Jansen LJ, et al. Perioperative antibiotic use and associated infectious outcomes at the time of myomectomy. Obstet Gynecol. 2019;133:626-635.
- Rebar RW. Should perioperative antibiotics at myomectomy be universal? NEJM J Watch. March 11, 2019.
- Kirkland KB, Briggs JP, Trivette SL, et al. The impact of surgical-site infections in the 1990s: attributable mortality, excess length of hospitalization, and extra costs. Infect Control Hosp Epidemiol. 1999;20:725-730.
- American College of Obstetricians and Gynecologists. Practice Bulletin No. 195: prevention of infection after gynecologic procedures. Obstet Gynecol. 2018;131:e172-e189.
- Banerjee D, Dejbakhsh S, Patel HH, et al. Perioperative antibiotic prophylaxis in myomectomy surgery. Paper presented at 49th Annual Meeting of the AAGL; November 2020.
- Uppal S, Harris J, Al-Niaimi A. Prophylactic antibiotic choice and risk of surgical site infection after hysterectomy. Obstet Gynecol. 2016;127:321-329.
- Kim AJ, Clark NV, Jansen LJ, et al. Perioperative antibiotic use and associated infectious outcomes at the time of myomectomy. Obstet Gynecol. 2019;133:626-635.
- Rebar RW. Should perioperative antibiotics at myomectomy be universal? NEJM J Watch. March 11, 2019.
Vaginal cleansing protocol curbs deep SSIs after cesarean
reported Johanna Quist-Nelson, MD, of the University of North Carolina, Chapel Hill.
“Surgical site infections after a cesarean delivery are more common if the patient is in labor or has ruptured membranes,” she said at the 2020 virtual meeting of the American College of Obstetricians and Gynecologists..
Two options to decrease the risk of SSIs after cesarean for those patients in labor or with ruptured membranes are vaginal cleansing and azithromycin, given in addition to preoperative antibiotics, Dr. Quist-Nelson said. She and her colleagues conducted a quality improvement study of the effects of a stepwise implementation of vaginal cleansing and azithromycin to reduce SSIs at cesarean delivery in this high-risk population. The data were collected from 2016 to 2019 at Thomas Jefferson University, Philadelphia.
“We aimed to decrease our SSI rate by 30% by adopting an intervention of cleansing followed by azithromycin,” she said.
The researchers added vaginal cleansing to the SSI prevention protocol in January 2017, with the addition of azithromycin in March 2018. Vaginal cleansing involved 30 seconds of anterior to posterior cleaning prior to urinary catheter placement. Azithromycin was given at a dose of 500 mg intravenously in addition to preoperative antibiotics and within an hour of cesarean delivery.
A total of 1,033 deliveries qualified for the study by being in labor or with ruptured membranes; of these 291 were performed prior to the interventions, 335 received vaginal cleansing only, and 407 received vaginal cleansing and azithromycin. The average age of the participants was 30 years; approximately 42% were Black, and 32% were White.
Cleansing protocol reduces SSIs
Overall, the rate of SSIs was 22% in the standard care group, 17% in the vaginal cleansing group, and 15% in the vaginal cleansing plus azithromycin group. When broken down by infection type, no deep SSI occurred in the vaginal cleansing or cleansing plus azithromycin group, compared with 2% of the standard care group (P = .009). In addition, endometritis, which is an organ-space SSI, was significantly lower in the cleansing group (10%) and the cleansing plus azithromycin group (11%), compared with the standard care group (16%).
The study findings were limited by factors including the use of EMRs for collection of data, and given that it is a quality improvement study, there is a potential lack of generalizability to other institutions. The study focused on patients at high risk for SSI and the use of the Plan-Do-Study-Act (PDSA) method of conducting the research, Dr. Quist-Nelson said. Compared with standard care, the implementation of vaginal cleansing reduced the SSI rate by 33%, with no significantly further change in SSI after the addition of azithromycin, she concluded.
Data sharing boosts compliance
In a question-and-answer session, Dr. Quist-Nelson noted that povidone iodine (Betadine) was chosen for vaginal cleansing because it was easily accessible at her institution, but that patients with allergies were given chlorhexidine. The cleansing itself was “primarily vaginal, not a full vulvar cleansing,” she clarified. The cleansing was performed immediately before catheter placement and included the urethra.
When asked about strategies to increase compliance, Dr. Quist-Nelson noted that sharing data was valuable, namely “reporting to our group the current compliance,” as well as sharing information by email and discussing it during multidisciplinary rounds.
The study was a quality improvement project and not a randomized trial, so the researchers were not able to tease out the impact of vaginal cleansing from the impact of azithromycin, Dr. Quist-Nelson said.
Based on her results, Dr. Quist-Nelson said she would recommend the protocol for use in patients who require cesarean delivery after being in labor or having ruptured membranes, and that “there are trials to support the use of both interventions.”
The results suggest opportunities for further randomized trials, including examination of the use of oral versus IV azithromycin, she added.
The study received no outside funding. Dr. Quist-Nelson had no financial conflicts to disclose.
reported Johanna Quist-Nelson, MD, of the University of North Carolina, Chapel Hill.
“Surgical site infections after a cesarean delivery are more common if the patient is in labor or has ruptured membranes,” she said at the 2020 virtual meeting of the American College of Obstetricians and Gynecologists..
Two options to decrease the risk of SSIs after cesarean for those patients in labor or with ruptured membranes are vaginal cleansing and azithromycin, given in addition to preoperative antibiotics, Dr. Quist-Nelson said. She and her colleagues conducted a quality improvement study of the effects of a stepwise implementation of vaginal cleansing and azithromycin to reduce SSIs at cesarean delivery in this high-risk population. The data were collected from 2016 to 2019 at Thomas Jefferson University, Philadelphia.
“We aimed to decrease our SSI rate by 30% by adopting an intervention of cleansing followed by azithromycin,” she said.
The researchers added vaginal cleansing to the SSI prevention protocol in January 2017, with the addition of azithromycin in March 2018. Vaginal cleansing involved 30 seconds of anterior to posterior cleaning prior to urinary catheter placement. Azithromycin was given at a dose of 500 mg intravenously in addition to preoperative antibiotics and within an hour of cesarean delivery.
A total of 1,033 deliveries qualified for the study by being in labor or with ruptured membranes; of these 291 were performed prior to the interventions, 335 received vaginal cleansing only, and 407 received vaginal cleansing and azithromycin. The average age of the participants was 30 years; approximately 42% were Black, and 32% were White.
Cleansing protocol reduces SSIs
Overall, the rate of SSIs was 22% in the standard care group, 17% in the vaginal cleansing group, and 15% in the vaginal cleansing plus azithromycin group. When broken down by infection type, no deep SSI occurred in the vaginal cleansing or cleansing plus azithromycin group, compared with 2% of the standard care group (P = .009). In addition, endometritis, which is an organ-space SSI, was significantly lower in the cleansing group (10%) and the cleansing plus azithromycin group (11%), compared with the standard care group (16%).
The study findings were limited by factors including the use of EMRs for collection of data, and given that it is a quality improvement study, there is a potential lack of generalizability to other institutions. The study focused on patients at high risk for SSI and the use of the Plan-Do-Study-Act (PDSA) method of conducting the research, Dr. Quist-Nelson said. Compared with standard care, the implementation of vaginal cleansing reduced the SSI rate by 33%, with no significantly further change in SSI after the addition of azithromycin, she concluded.
Data sharing boosts compliance
In a question-and-answer session, Dr. Quist-Nelson noted that povidone iodine (Betadine) was chosen for vaginal cleansing because it was easily accessible at her institution, but that patients with allergies were given chlorhexidine. The cleansing itself was “primarily vaginal, not a full vulvar cleansing,” she clarified. The cleansing was performed immediately before catheter placement and included the urethra.
When asked about strategies to increase compliance, Dr. Quist-Nelson noted that sharing data was valuable, namely “reporting to our group the current compliance,” as well as sharing information by email and discussing it during multidisciplinary rounds.
The study was a quality improvement project and not a randomized trial, so the researchers were not able to tease out the impact of vaginal cleansing from the impact of azithromycin, Dr. Quist-Nelson said.
Based on her results, Dr. Quist-Nelson said she would recommend the protocol for use in patients who require cesarean delivery after being in labor or having ruptured membranes, and that “there are trials to support the use of both interventions.”
The results suggest opportunities for further randomized trials, including examination of the use of oral versus IV azithromycin, she added.
The study received no outside funding. Dr. Quist-Nelson had no financial conflicts to disclose.
reported Johanna Quist-Nelson, MD, of the University of North Carolina, Chapel Hill.
“Surgical site infections after a cesarean delivery are more common if the patient is in labor or has ruptured membranes,” she said at the 2020 virtual meeting of the American College of Obstetricians and Gynecologists..
Two options to decrease the risk of SSIs after cesarean for those patients in labor or with ruptured membranes are vaginal cleansing and azithromycin, given in addition to preoperative antibiotics, Dr. Quist-Nelson said. She and her colleagues conducted a quality improvement study of the effects of a stepwise implementation of vaginal cleansing and azithromycin to reduce SSIs at cesarean delivery in this high-risk population. The data were collected from 2016 to 2019 at Thomas Jefferson University, Philadelphia.
“We aimed to decrease our SSI rate by 30% by adopting an intervention of cleansing followed by azithromycin,” she said.
The researchers added vaginal cleansing to the SSI prevention protocol in January 2017, with the addition of azithromycin in March 2018. Vaginal cleansing involved 30 seconds of anterior to posterior cleaning prior to urinary catheter placement. Azithromycin was given at a dose of 500 mg intravenously in addition to preoperative antibiotics and within an hour of cesarean delivery.
A total of 1,033 deliveries qualified for the study by being in labor or with ruptured membranes; of these 291 were performed prior to the interventions, 335 received vaginal cleansing only, and 407 received vaginal cleansing and azithromycin. The average age of the participants was 30 years; approximately 42% were Black, and 32% were White.
Cleansing protocol reduces SSIs
Overall, the rate of SSIs was 22% in the standard care group, 17% in the vaginal cleansing group, and 15% in the vaginal cleansing plus azithromycin group. When broken down by infection type, no deep SSI occurred in the vaginal cleansing or cleansing plus azithromycin group, compared with 2% of the standard care group (P = .009). In addition, endometritis, which is an organ-space SSI, was significantly lower in the cleansing group (10%) and the cleansing plus azithromycin group (11%), compared with the standard care group (16%).
The study findings were limited by factors including the use of EMRs for collection of data, and given that it is a quality improvement study, there is a potential lack of generalizability to other institutions. The study focused on patients at high risk for SSI and the use of the Plan-Do-Study-Act (PDSA) method of conducting the research, Dr. Quist-Nelson said. Compared with standard care, the implementation of vaginal cleansing reduced the SSI rate by 33%, with no significantly further change in SSI after the addition of azithromycin, she concluded.
Data sharing boosts compliance
In a question-and-answer session, Dr. Quist-Nelson noted that povidone iodine (Betadine) was chosen for vaginal cleansing because it was easily accessible at her institution, but that patients with allergies were given chlorhexidine. The cleansing itself was “primarily vaginal, not a full vulvar cleansing,” she clarified. The cleansing was performed immediately before catheter placement and included the urethra.
When asked about strategies to increase compliance, Dr. Quist-Nelson noted that sharing data was valuable, namely “reporting to our group the current compliance,” as well as sharing information by email and discussing it during multidisciplinary rounds.
The study was a quality improvement project and not a randomized trial, so the researchers were not able to tease out the impact of vaginal cleansing from the impact of azithromycin, Dr. Quist-Nelson said.
Based on her results, Dr. Quist-Nelson said she would recommend the protocol for use in patients who require cesarean delivery after being in labor or having ruptured membranes, and that “there are trials to support the use of both interventions.”
The results suggest opportunities for further randomized trials, including examination of the use of oral versus IV azithromycin, she added.
The study received no outside funding. Dr. Quist-Nelson had no financial conflicts to disclose.
FROM ACOG 2020