User login
Managing hyperhidrosis, HS: Ask questions first
A wide variety of medications exists for treating hyperhidrosis, a dermatologist told colleagues, but before prescribing anything to a pediatric patient, he recommended, ask the patient a simple question: “What bothers you the most?”
The answer will provide guidance for developing a step-by-step treatment strategy and help provide the patient “a set of realistic expectations in terms of what the response will look like,” George Hightower, MD, PhD, a pediatric dermatologist at Rady Children’s Hospital and the University of California, San Diego, said at MedscapeLive’s Women’s & Pediatric Dermatology Seminar.
A similar question-based approach will help guide therapy for patients with hidradenitis suppurativa (HS), he said.
With regards to hyperhidrosis, Dr. Hightower said that patients most commonly complain that their underarms are too smelly, too sweaty, and red, itchy, or painful. Causes, he said, can include irritation/contact dermatitis, folliculitis, and seborrheic dermatitis, as well as hyperhidrosis or HS.
Primary focal axillary hyperhidrosis is defined as focal, visible, excessive sweating for at least 6 months without an apparent cause plus at least two of the following characteristics: Sweating is bilateral and relatively symmetric, it impairs daily activities, it starts before the age of 25 with at least one episode per week (many patients have it daily), a family history of idiopathic hyperhidrosis is present, and focal sweating does not occur during sleep.
Secondary hyperhidrosis can be linked to other conditions, such as a spinal column injury, Dr. Hightower noted.
The first step on the treatment ladder is topical 20% aluminum chloride, which is available over the counter. This should be applied nightly for 1 week then every 1-2 weeks, Dr. Hightower recommended. All of his patients with hyperhidrosis have had at least one trial of this treatment.
The next option is daily topical treatment with 2.4% glycopyrronium tosylate (Qbrexza) cloths, approved by the Food and Drug Administration in 2018 for primary axillary hyperhidrosis in patients aged 9 and older. According to the prescribing information, dry mouth was by far the most common treatment-associated adverse effect in clinical trials (24% versus almost 6% among those on vehicle). As for skin reactions, erythema occurred in about 17% of both the intervention and vehicle groups, and burning/stinging occurred in 14% of those on treatment and almost 17% of those on vehicle.
“If they’re not able to get access to the cloths due to [insurance] coverage issues, or they don’t allow them to reach the clinical endpoint desired, then I use an oral daily glycopyrrolate pill,” Dr. Hightower said.
He recommends 1 mg to 6 mg daily of the anticholinergic drug, which has been used off-label for hyperhidrosis for several years. A 2012 study of 31 children with hyperhidrosis, he noted, supported the use of the drug. The retrospective study found that 90% of the patients, at a mean daily dose of 2 mg, experienced improvements, reported as major in 71%. In addition, patients experienced improvement within hours of taking the medication, and benefits disappeared within a day of stopping the medication. In the study, patients were on the treatment for an average of 2.1 years, and 29% experienced side effects, which were dose related; the most common were dry mouth in 26% and dry eyes in 10%.
According to goodrx.com, a month’s supply of 2 mg of the drug costs as little as $13 with a discount or coupon.
The next steps in treatment are procedural interventions such as microwave-based therapies.
Dr. Hightower said that patients should be advised that treatment may take years, and to encourage them to return for follow-up. He suggested this helpful message: “We’re still trying to find the best treatment for you, and we’ll need to see you back in the office.”
Hidradenitis suppurativa
Dr. Hightower said that too often, HS goes undiagnosed for a significant period of time, preventing patients from seeing a dermatologist for treatment. Hallmarks of HS include inflammatory nodules, abscesses, and scarring, he said. “It can be disfiguring, painful, embarrassing, and associated with significantly decreased quality of life. Early recognition in terms of making and solidifying the diagnosis is important so we can prevent further worsening of the disease.”
The goal of treatment include preventing scars and unnecessary emergency department visits, and stopping flares from worsening, Dr. Hightower said. For specifics, he pointed to clinical management guidelines released by the United States and Canadian hidradenitis suppurativa foundations in 2019.
Make sure to set individualized treatment goals and understand the impact of treatment on the patient’s interactions with family, school, and peers, he said. And keep in mind that “parent-defined goals may be different from patient-defined goals.”
Dr. Hightower reported no relevant disclosures. MedscapeLive and this news organization are owned by the same parent company
A wide variety of medications exists for treating hyperhidrosis, a dermatologist told colleagues, but before prescribing anything to a pediatric patient, he recommended, ask the patient a simple question: “What bothers you the most?”
The answer will provide guidance for developing a step-by-step treatment strategy and help provide the patient “a set of realistic expectations in terms of what the response will look like,” George Hightower, MD, PhD, a pediatric dermatologist at Rady Children’s Hospital and the University of California, San Diego, said at MedscapeLive’s Women’s & Pediatric Dermatology Seminar.
A similar question-based approach will help guide therapy for patients with hidradenitis suppurativa (HS), he said.
With regards to hyperhidrosis, Dr. Hightower said that patients most commonly complain that their underarms are too smelly, too sweaty, and red, itchy, or painful. Causes, he said, can include irritation/contact dermatitis, folliculitis, and seborrheic dermatitis, as well as hyperhidrosis or HS.
Primary focal axillary hyperhidrosis is defined as focal, visible, excessive sweating for at least 6 months without an apparent cause plus at least two of the following characteristics: Sweating is bilateral and relatively symmetric, it impairs daily activities, it starts before the age of 25 with at least one episode per week (many patients have it daily), a family history of idiopathic hyperhidrosis is present, and focal sweating does not occur during sleep.
Secondary hyperhidrosis can be linked to other conditions, such as a spinal column injury, Dr. Hightower noted.
The first step on the treatment ladder is topical 20% aluminum chloride, which is available over the counter. This should be applied nightly for 1 week then every 1-2 weeks, Dr. Hightower recommended. All of his patients with hyperhidrosis have had at least one trial of this treatment.
The next option is daily topical treatment with 2.4% glycopyrronium tosylate (Qbrexza) cloths, approved by the Food and Drug Administration in 2018 for primary axillary hyperhidrosis in patients aged 9 and older. According to the prescribing information, dry mouth was by far the most common treatment-associated adverse effect in clinical trials (24% versus almost 6% among those on vehicle). As for skin reactions, erythema occurred in about 17% of both the intervention and vehicle groups, and burning/stinging occurred in 14% of those on treatment and almost 17% of those on vehicle.
“If they’re not able to get access to the cloths due to [insurance] coverage issues, or they don’t allow them to reach the clinical endpoint desired, then I use an oral daily glycopyrrolate pill,” Dr. Hightower said.
He recommends 1 mg to 6 mg daily of the anticholinergic drug, which has been used off-label for hyperhidrosis for several years. A 2012 study of 31 children with hyperhidrosis, he noted, supported the use of the drug. The retrospective study found that 90% of the patients, at a mean daily dose of 2 mg, experienced improvements, reported as major in 71%. In addition, patients experienced improvement within hours of taking the medication, and benefits disappeared within a day of stopping the medication. In the study, patients were on the treatment for an average of 2.1 years, and 29% experienced side effects, which were dose related; the most common were dry mouth in 26% and dry eyes in 10%.
According to goodrx.com, a month’s supply of 2 mg of the drug costs as little as $13 with a discount or coupon.
The next steps in treatment are procedural interventions such as microwave-based therapies.
Dr. Hightower said that patients should be advised that treatment may take years, and to encourage them to return for follow-up. He suggested this helpful message: “We’re still trying to find the best treatment for you, and we’ll need to see you back in the office.”
Hidradenitis suppurativa
Dr. Hightower said that too often, HS goes undiagnosed for a significant period of time, preventing patients from seeing a dermatologist for treatment. Hallmarks of HS include inflammatory nodules, abscesses, and scarring, he said. “It can be disfiguring, painful, embarrassing, and associated with significantly decreased quality of life. Early recognition in terms of making and solidifying the diagnosis is important so we can prevent further worsening of the disease.”
The goal of treatment include preventing scars and unnecessary emergency department visits, and stopping flares from worsening, Dr. Hightower said. For specifics, he pointed to clinical management guidelines released by the United States and Canadian hidradenitis suppurativa foundations in 2019.
Make sure to set individualized treatment goals and understand the impact of treatment on the patient’s interactions with family, school, and peers, he said. And keep in mind that “parent-defined goals may be different from patient-defined goals.”
Dr. Hightower reported no relevant disclosures. MedscapeLive and this news organization are owned by the same parent company
A wide variety of medications exists for treating hyperhidrosis, a dermatologist told colleagues, but before prescribing anything to a pediatric patient, he recommended, ask the patient a simple question: “What bothers you the most?”
The answer will provide guidance for developing a step-by-step treatment strategy and help provide the patient “a set of realistic expectations in terms of what the response will look like,” George Hightower, MD, PhD, a pediatric dermatologist at Rady Children’s Hospital and the University of California, San Diego, said at MedscapeLive’s Women’s & Pediatric Dermatology Seminar.
A similar question-based approach will help guide therapy for patients with hidradenitis suppurativa (HS), he said.
With regards to hyperhidrosis, Dr. Hightower said that patients most commonly complain that their underarms are too smelly, too sweaty, and red, itchy, or painful. Causes, he said, can include irritation/contact dermatitis, folliculitis, and seborrheic dermatitis, as well as hyperhidrosis or HS.
Primary focal axillary hyperhidrosis is defined as focal, visible, excessive sweating for at least 6 months without an apparent cause plus at least two of the following characteristics: Sweating is bilateral and relatively symmetric, it impairs daily activities, it starts before the age of 25 with at least one episode per week (many patients have it daily), a family history of idiopathic hyperhidrosis is present, and focal sweating does not occur during sleep.
Secondary hyperhidrosis can be linked to other conditions, such as a spinal column injury, Dr. Hightower noted.
The first step on the treatment ladder is topical 20% aluminum chloride, which is available over the counter. This should be applied nightly for 1 week then every 1-2 weeks, Dr. Hightower recommended. All of his patients with hyperhidrosis have had at least one trial of this treatment.
The next option is daily topical treatment with 2.4% glycopyrronium tosylate (Qbrexza) cloths, approved by the Food and Drug Administration in 2018 for primary axillary hyperhidrosis in patients aged 9 and older. According to the prescribing information, dry mouth was by far the most common treatment-associated adverse effect in clinical trials (24% versus almost 6% among those on vehicle). As for skin reactions, erythema occurred in about 17% of both the intervention and vehicle groups, and burning/stinging occurred in 14% of those on treatment and almost 17% of those on vehicle.
“If they’re not able to get access to the cloths due to [insurance] coverage issues, or they don’t allow them to reach the clinical endpoint desired, then I use an oral daily glycopyrrolate pill,” Dr. Hightower said.
He recommends 1 mg to 6 mg daily of the anticholinergic drug, which has been used off-label for hyperhidrosis for several years. A 2012 study of 31 children with hyperhidrosis, he noted, supported the use of the drug. The retrospective study found that 90% of the patients, at a mean daily dose of 2 mg, experienced improvements, reported as major in 71%. In addition, patients experienced improvement within hours of taking the medication, and benefits disappeared within a day of stopping the medication. In the study, patients were on the treatment for an average of 2.1 years, and 29% experienced side effects, which were dose related; the most common were dry mouth in 26% and dry eyes in 10%.
According to goodrx.com, a month’s supply of 2 mg of the drug costs as little as $13 with a discount or coupon.
The next steps in treatment are procedural interventions such as microwave-based therapies.
Dr. Hightower said that patients should be advised that treatment may take years, and to encourage them to return for follow-up. He suggested this helpful message: “We’re still trying to find the best treatment for you, and we’ll need to see you back in the office.”
Hidradenitis suppurativa
Dr. Hightower said that too often, HS goes undiagnosed for a significant period of time, preventing patients from seeing a dermatologist for treatment. Hallmarks of HS include inflammatory nodules, abscesses, and scarring, he said. “It can be disfiguring, painful, embarrassing, and associated with significantly decreased quality of life. Early recognition in terms of making and solidifying the diagnosis is important so we can prevent further worsening of the disease.”
The goal of treatment include preventing scars and unnecessary emergency department visits, and stopping flares from worsening, Dr. Hightower said. For specifics, he pointed to clinical management guidelines released by the United States and Canadian hidradenitis suppurativa foundations in 2019.
Make sure to set individualized treatment goals and understand the impact of treatment on the patient’s interactions with family, school, and peers, he said. And keep in mind that “parent-defined goals may be different from patient-defined goals.”
Dr. Hightower reported no relevant disclosures. MedscapeLive and this news organization are owned by the same parent company
FROM MEDSCAPELIVE WOMEN’S & PEDIATRIC DERMATOLOGY SEMINAR
Is there liability if you don’t test for BRCA?
CASE Young woman with family history of breast cancer detects lump
Two weeks after noting a lump on her breast when her cat happened to jump on her in that spot, a 28-year-old woman (G0) went to her primary care provider. She was referred to her gynecologist; breast imaging, ultrasonography, and mammography were obtained, with microcalcifications noted. A fine needle aspiration diagnosed intraductal malignancy. The surgical breast tissue specimen was estrogen receptor (ER)- and progestogen receptor (PR)-positive and HER2-negative. Other tumor markers were obtained, including carcinoembryonic antigen, and tissue polypeptide specific antigen, p53, cathepsin D, cyclin E, and nestin, but results were not available.
With regard to family history, the woman’s mother and maternal grandmother had a history of breast cancer. The patient and her family underwent gene testing. The patient was found to be BRCA1- and BRCA2-positive; her mother was BRCA1-positive, an older sister was BRCA2-positive, and her grandmother was not tested.
The question arose in light of her family history as to why she was not tested for BRCA and appropriately counseled by her gynecologist prior to the cancer diagnosis. Litigation was initiated. While the case did not go forward regarding litigation, it is indeed a case in point. (Please note that this is a hypothetical case. It is based on a composite of several cases.)
Medical considerations
Breast cancer is the most common type of cancer affecting women in the Western world.1 Advances in clinical testing for gene mutations have escalated and allowed for identification of patients at increased risk for breast and ovarian cancer. Along with these advances come professional liability risk. After looking at the medical considerations for BRCA1 and 2 testing, we will consider a number of important legal issues. In the view of some commentators, the failure to diagnose genetic mutations in patients predisposed to cancer is “poised to become the next wave of medical professional liability lawsuits.”2
BRCA1 and BRCA2 genes provide tumor suppressor proteins, and assessment for mutations is recommended for individuals at high risk for breast and/or ovarian cancer; mutations in BRCA genes cause DNA damage, which increases the chance of developing cancer. The other way to look at it is, BRCA1 and 2 are tumor suppressor genes that are integrally involved with DNA damage control. Once there is a mutation, it adversely affects the beneficial effects of the gene. Mutations in these genes account for 5% to 10% of all hereditary breast cancers.3 Of note, men with BRCA2 are at increased risk for prostate cancer.
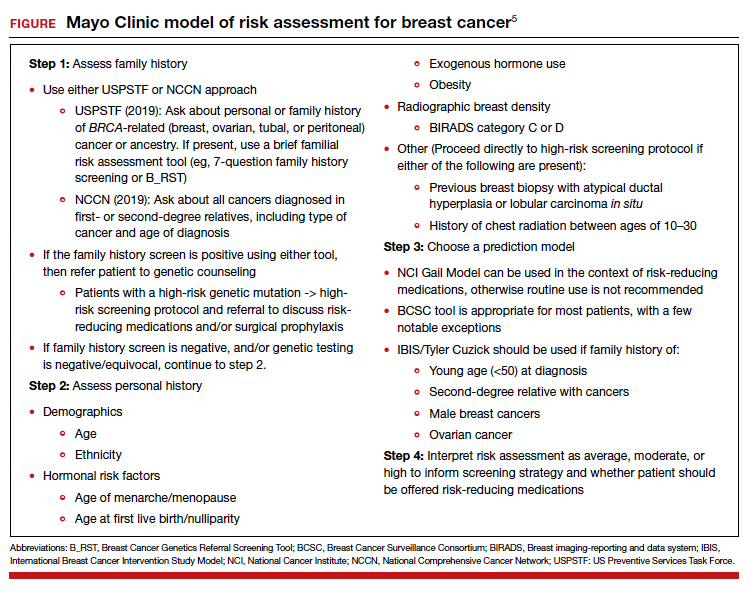
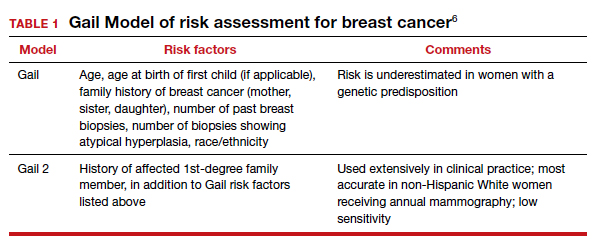
A patient who presents to her gynecologist stating that there is a family history of breast cancer, without knowledge of genetic components, presents a challenge (and a medicolegal risk) for the provider to assess. Prediction models have been used to determine specific patient risk for carrying a genetic mutation with resultant breast cancer development.4 Risk prediction models do not appear to be a good answer to predicting who is more likely to develop breast or ovarian cancer, however. A Mayo model may assist (FIGURE).5 Clinicians should also be aware of other models of risk assessment, including the Gail Model (TABLE 1).6
Continue to: Guidelines for genetic testing...
Guidelines for genetic testing
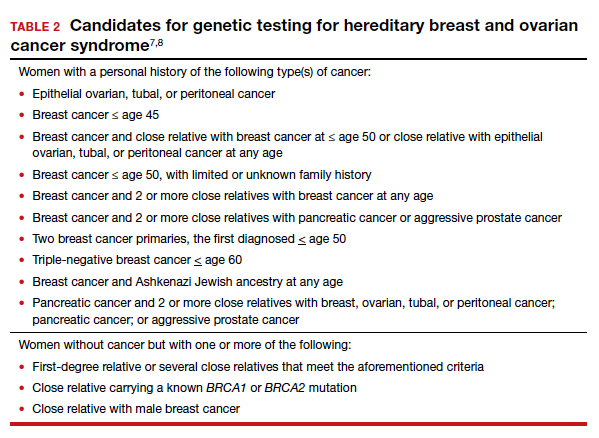
The American College of Obstetricians and Gynecologists states that patient medical history and family history are paramount in obtaining information regarding risk for breast and ovarian cancer. First- and second-degree relatives are allocated to this category. Information regarding age of diagnosis, maternal and paternal lineage, and ethnic background can imply a need for genetic testing (TABLE 2).7,8 A number of genetics national organizations have participated in recommendations and include the American College of Medical Genetics and Genomics, the National Society for Genetic Counselors, and the Society of Gynecologic Oncology.7
The question always surfaces, could the clinical outcome of the cancer when diagnosed have been changed if screening were undertaken, with earlier diagnosis, or prevented with prophylactic mastectomy, and changed the end result. In addition, it is well known that breast augmentation mammoplasty alters the ability to accurately evaluate mammograms. Patients considering this type of plastic surgery, ideally, should be counselled accordingly.9
Bottom line, we as clinicians must be cognizant of both ACOG and United States Preventive Services Task Force (USPSTF) recommendations regarding screening and gene testing for women considered high risk for breast cancer based on family history.7
Legal considerations
The case presented demonstrates that the discovery of the BRCA1 and BRCA2 genes, and reliable tests for determining the existence of the genes, brought with them legal issues as well as medical advantages. We look at professional liability (malpractice) questions this technology raises, and then consider the outcome of the hypothetical case. (BRCA is used here to apply broadly—not only to BRCA1 and 2 but also to PALB2, CHEK2, and similar genetic abnormalities.)
To date, the most visible BRCA legal issues covered in cases and law reviews have focused more on patent law than malpractice. The most important of these was a decision of the US Supreme Court in Association for Molecular Pathology v Myriad Genetics.10 The US Patent Office was granting patents to companies finding useful, naturally occurring segments of human DNA, and had granted Myriad several patents on BRCA1 and BRCA2 genes. This patent policy had the potential to seriously interfere with broad scientific use of these genes.11 Fortunately, the Supreme Court stepped in and unanimously invalidated such patents. It held that a “naturally occurring DNA segment is a product of nature and not patent eligible merely because it has been isolated.” The Court noted, “Finding the location of the BRCA1 and BRCA2 genes does not render the genes patent eligible ‘new . . . composition[s] of matter.’”8 The Court did allow the patenting of tests for specific gene structures, and artificial changes in naturally occurring genes.
Malpractice and BRCA
While the BRCA patent wars have lingered, the potential for a significant increase in BRCA-related malpractice cases is of increasing concern. Like most malpractice liability, these new claims are based on very old principles of negligence.12 To prevail, the plaintiff (ordinarily, an injured patient) must demonstrate 4 things:
- A duty. That is, the physician owed a duty to the injured party. Usually (but not always) that requires a professional relationship between the physician and the person injured.
- A breach of that duty. Malpractice liability is based on the fact that the physician did something that a reasonably careful physician (generally, of the same specialty) would not have done, or that the physician failed to do something that a reasonable physician would have done. This usually means that the profession itself sees what the physician did (or did not do) as medically inappropriate. In medical malpractice cases, that is ordinarily measured by what the usual or common practice is among prudent physicians. In rare circumstances, courts have found the standard practice of a profession to be negligent. Where, for example, it was custom for a professional not to give an eye pressure test to anyone under age 40, a court found that common standard to be inappropriate.13 In the words of Judge Learned Hand (speaking about a different case), “a whole calling may have unduly lagged in the adoption of new and available devices. It never may set its own tests.”14 Underlying negligence is a cost-benefit analysis (discussed below).
- Damages. There must have been some damage that courts recognize, usually loss of money or opportunity to work, the cost of care, pain and suffering, or loss of enjoyment/quality of life. In malpractice, many states now recognize the “loss of chance” or the “loss of a chance.” That means, if a “physician negligently fails to diagnose a curable disease, and the patient is harmed by the disease, the physician should be liable for causing the ‘loss of a chance of a cure.’”15 (Delay in diagnosis is the most common reason for claims in breast cancer care.)16
- Causation. The breach of duty (negligence) must have caused the damages. The causation must have been reasonably close. If a driver drives through a stop sign, or a physician misreads a test, and someone is injured but there is no connection between the negligence and the injury, there is not tort liability.
The 4 elements of malpractice just described are raised in some way in the possible liability associated with BRCA testing. We next look at the ways in which liability may arise from that testing (or lack of it).
Underlying much of the following discussion is the “cost-benefit” consideration noted above. This concept is that the total cost (financial and health) of testing should be compared with the value of the benefits of testing, taking into account the probabilities that the testing will result in better health outcomes. BRCA testing, for example, is essentially cost-free in terms of physical risk. Its financial cost, while not trivial, is not great, and it is commonly covered by health insurance.17 In terms of benefits, the testing has the potential for providing critical information in making treatment decisions for a meaningful percentage of patients and their families. There are many ways of analyzing the liability risks of genetic malpractice,7,18 and the following is intended to discuss some of the greatest risks related to BRCA testing.
Continue to: Areas of liability...
Areas of liability
The failure to recommend a test. The circumstances in which BRCA testing should be undertaken are set out by professional organizations (noted above). These recommendations are not static, however. They change from time to time. Given the potential harm caused by the failure to test in relevant circumstances, malpractice liability is certainly a possibility when the failure to recommend a test to a patient results in a cancer that might have been prevented had the genetic problem been identified in a timely manner. The circumstances in which testing should be considered continue to change, placing an obligation on clinicians to stay well informed of changing genetic understandings. Another risk is that one specialist may assume that it is the job of another specialist to order the test. Whatever the cause of the failure to test, or unnecessary delay in testing, it appears to be the primary basis for BRCA liability.
The failure to properly interpret a test. Any test that is misinterpreted may lead to harm for the patient. A false negative, of course, may mean that preventive treatment that could have been undertaken will be foregone, as a “loss of a chance.” On the other hand, a false positive can lead to radical, unnecessary surgery or treatment. If a misinterpretation occurred because of carelessness by the testing organization, or confusion by a practitioner, there is a likelihood of negligence.19
A different form of “misinterpretation” could be reasonable—and not negligent. Advances in scientific-medical understanding may result in the outcome of tests being reconsidered and changed. That has been the case with genetic testing and breast cancer. The availability of multiple breast cancer SNPs (single nucleotide polymorphisms), and combining this information with other risk factors for example, results in a polygenic risk score that may be at odds with the level of risk from earlier testing.20,21 This naturally leads to the question of when later, updated testing should be recommended to look for a better current interpretation.22,23
The failure to act on BRCA test results. Testing is of no value, of course, if the results are not used properly. Test results or analyses that are not sent to the proper physicians, or are somehow ignored when properly directed, is a “never” event—it should never happen. It almost always would be considered negligence, and if the patient were injured, could lead to liability. Amazingly, one study found that, in genetic testing liability cases, nearly 20% of the claims arose from failure to return test results to patients.24 In addition, when a patient is found to be BRCA-positive, there is an obligation to discuss the options for dealing with the increased risk associated with the gene mutation(s), as well as to recommend the prudent course of action or to refer the patient to someone who will have that discussion.
Informed consent to the patient. BRCA testing requires informed consent. The physical risks of the testing process are minimal, of course, but it carries a number of other emotional and family risks. The informed consent process is an invitation to an honest discussion between clinicians and patients. It should be an opportunity to discuss what the testing is, and is not, and what the test may mean for treatment. It may also be an opportunity to discuss the implications for other members of the patient’s family (noted below).
One element of informed consent is a discussion of the consequences of failure to consent, or to undertake one of the alternatives. In the case of BRCA testing, this is especially important in cases in which a patient expresses a hesitancy to be tested with an “I’d rather not know philosophy.” Although clinicians should not practice law, some patient concerns about discrimination may be addressed by the protection that the federal Genetic Information Nondiscrimination Act (GINA) and other laws provide (which prohibit insurance and employment discrimination based on genetic information). A good source of information about GINA and related nondiscrimination laws is provided by the National Human Genome Research Institute.25 In addition, the National Institutes of Health has a website that may be helpful to many patients26 (and a much more complex site for health professionals).27 At the same time, courts have resisted plaintiffs/patients who have tried to use informed consent as a way of suing for failure to offer genetic testing.28,29
The failure to refer. In some cases, a patient should be formally referred for genetics consultation. The considerations here are similar to other circumstances in modern, fast developing medical practice that require special sensitivity to those occasions in which a patient will benefit from additional expertise. It is a principle that the AMA Council on Ethical and Judicial Affairs has expressed this way: “In the absence of adequate expertise in pretest and posttest counseling, a physician should refer the patient to an appropriate specialist.”30 The failure to refer, when that deviates from acceptable practice, may result in liability.
Informing others. BRCA testing is an area of medicine in which results may be of great significance not only to the patient but also to the patient’s family.31 Physicians should counsel patients on the importance of informing relatives about relevant results and “should make themselves available to assist patients in communicating with relatives to discuss opportunities for counseling and testing, as appropriate.”30 The question may arise, however, of whether in some circumstances physicians should go a step further in ensuring relatives receive important information regarding their loved one’s health.32 The law has been reluctant to impose liability to “third parties” (someone not a patient). Duties usually arise through the physician-patient relationship. There are exceptions. Perhaps the best known has been the obligation of mental health professionals to take action to protect third parties from patients who have made believable threats against identifiable victims.33 There are indications that some courts could find, in extreme circumstances, a “duty to warn” nonpatients in some instances where it is essential to inform third parties that they should receive a specific form of genetic testing.34,35 Such a duty would, of course, have to protect the privacy rights of the patient to the maximum extent possible. A general duty of this type has not been established widely, but may be part of the future.
Continue to: Was there liability in our example case?...
Was there liability in our example case?
The hypothetical case provided above suggests that there could be liability. Routine medical history by the primary care physician would have produced the fact that the patient’s mother, sister, and maternal grandmother had breast cancer. That would clearly have put her in a category of those who should have received genetic testing. Yet, she was not tested until after her cancer was found. From the limited facts we have, it appears that this timeline of events would have been outside accepted practice—and negligent. The case was not pursued by the patient, however, and this may represent the current state of liability for BRCA issues.
The extent of liability seems to be significant
Our discussion of liability suggests that there is significant potential for BRCA testing negligence within practice, and that the damages in these cases could be substantial. Yet the predicted “tsunami” of malpractice lawsuits related to genetic testing has not appeared.36,37 One study of cases in the United States (through 2016) found a “slowly rising tide” of liability cases instead of a tsunami,24 as the number of claims made was low. On the other hand, the payments where damages were awarded were an order of magnitude larger than other malpractice cases—a mean of $5.3 million and median of $2 million. This is compared with mean values in the range of $275,000 to $600,000 in other areas of malpractice.
The majority of the genetic malpractice cases involve prenatal and newborn testing, and diagnosis/susceptibility/pharmacogenomic accounting for about 25% of cases. In terms of type of errors claimed, approximately 50% were diagnostic-interpretation errors, 30% failure to offer testing, nearly 20% failure to return test results to the patients, and a few remaining cases of failure to properly treat in light of genetic testing.24
Despite a few very large payments, however, the fact remains that there is a surprisingly low number of genetics malpractice cases. Gary Marchant and colleagues suggest that several reasons may account for this:
- the clinical implementation of genetic science has been slower than expected
- the lack of expertise of many physicians in genetic science
- expert witnesses have sometimes been hard to find
- the lack of understanding by plaintiffs’ attorneys of genetic malpractice
- potential plaintiffs’ lack of understanding of the nature of genetic testing and the harms resulting from genetic negligence.17,24,37
The tide is slowly coming in
By all appearances, there is every reason to think that genetic malpractice will be increasing, and that the recent past of much higher damages per claim paid in the genetics area will be part of that tide. The National Human Genome Research LawSeq project has suggested a number of useful ways of dealing with the liability issues.18 In addition to the BRCA issues that we have considered in this article for ObGyns, there are other critical issues of prenatal and newborn genetic testing.38 But those are topics for another day. ●
- Sevilla C, Moatti JP, Reynier CJ, et al. Testing for BRCA1 mutations: a cost-effective analysis. Europ J Human Genetics. 2002;10:599-606.
- Cotton V, Kirkpatrick D. Failure to recommend genetic counseling in breast cancer: is the next wave of medical professional liability lawsuits? Contemp OB/GYN. June 1, 2017.
- Suryavanshi M, Kumar D, Panigrahi M, et al. Detection of false positive mutations in BRCA gene by next generation sequencing. Fam Cancer. 2017;16:311-317.
- Black L, Knoppers B, Avard D, et al. Legal liability and the uncertain nature of risk prediction: the case of breast cancer risk prediction models. Public Health Genomics. 2012;15:335-340.
- McClintock A, Gollab A, Laya M. Breast cancer risk assessment, a step-wise approach for primary care physicians on the front lines of shared decision making. Mayo Clin Proc. 2020;95:1268-1275.
- National Cancer Institute. The Breast Cancer Risk Assessment Tool. https://bcrisktool.cancer.gov/. Accessed February 25, 2021.
- Neff J, Richardson G, Phelps J. Legal liabilities associated with hereditary breast and ovarian cancers. J Reprod Med. 2020;65:227-230.
- American College of Obstetricians and Gynecologists. Practice Bulletin No 182: hereditary breast and ovarian cancer syndrome. Obstet Gynecol. 2017;130:e110-e126.
- Sá dos Reis C, Gremion I, and Meystre NR. Study of breast implants mammography examinations for identification of suitable image quality criteria. Insights Imaging. 2020;11:3.
- Association for Molecular Pathology v Myriad Genetics, 569 U.S. 576 (2013).
- Smith SR. The Supreme Court 2012-2013: dogs, DNA, and DOMA. Register Rep. 2013;39(Fall):26-33.
- Bal BS. An introduction to medical malpractice in the United States. Clin Orthop Relat Res. 2009;467:339-347.
- Helling v Carey, 83 Wn.2d 514, 519 P.2d 981 (1974).
- The T.J. Hooper, 60 F.2d 737, 740 (2d Cir.1932), cert. denied 287 U.S. 662 (1932).
- Fischer DA. Tort recovery for loss of a chance. Wake Forest L Rev. 2001;36:605-655.
- Murphy BL, Ray-Zack MD, Reddy PN, et al. Breast cancer litigation in the 21st century. Ann Surg Oncol. 2018;25:2939- 2947.
- Prince AE. Prevention for those who can pay: insurance reimbursement of genetic-based preventive interventions in the liminal state between health and disease. J Law Biosci. 2015;2:365-395.
- Marchant G, Barnes M, Evans JP, et al; LawSeq Liability Task Force. From genetics to genomics: facing the liability implications in clinical care. J Law Med Ethics. 2020;48:11-43.
- Complaint, Held v Ambry Genetics Corp., No. 15-CV-8683, 2015 WL 6750024 (S.D.N.Y. Nov. 4, 2015); Order of Dismissal, Held v Ambry Genetics Corp., No. 15-CV-8683, (S.D.N.Y. Dec. 6, 2016).
- Pederson HJ. Breast cancer risk assessment and treatment: current concepts in genetics and genomics. Contemp OB/ GYN. 2017; 62:A1-A4.
- Pederson HJ. Who needs breast cancer genetics testing? OBG Manag. 2018;30:34-39.
- Roberts JL, Foulkes A. Genetic duties. William Mary L Rev. 2020;62:143-212.
- Thorogood A, Cook-Deegan R, Knoppers B. Public variant databases: liability? Genet Med. 2017;19:838–841.
- Marchant G, Lindor R. Genomic malpractice: an emerging tide or gentle ripple? Food Drug Law J. 2018;73:1-37.
- National Human Genome Research Institute. Genetic discrimination. https://www.genome.gov/about-genomics /policy-issues/Genetic-Discrimination. Updated September 16, 2020. Accessed February 25, 2021.
- National Cancer Institute. BRCA mutations: cancer risk and genetic testing. https://www.cancer.gov/about-cancer /causes-prevention/genetics/brca-fact-sheet. Reviewed November 19, 2020. Accessed February 25, 2021.
- National Cancer Institute. Genetics of breast and gynecologic cancers (PDQ®)–Health Professional Version. https://www .cancer.gov/types/breast/hp/breast-ovarian-genetics-pdq. Updated February 12, 2021. Accessed February 25, 2021.
- Reed v Campagnolo, 630 A.2d 1145, 1152–54 (Md. 1993).
- Munro v Regents of Univ. of Cal.,263 Cal. Rptr. 878, 885, 988 (1989).
- AMA Council on Ethical and Judicial Affairs. AMA Code of Medical Ethics’ opinions on genetic testing. Opinion 2.131. 2009;11:683-685. https://journalofethics.ama-assn .org/article/ama-code-medical-ethics-opinions-genetictesting/2009-09.
- Gilbar R, Barnoy S. Disclosing genetic test results to the patient’ relatives: how does the law influence clinical practice? J Law Technol Policy. 2019;125-168.
- Song K. Warning third parties of genetic risks in the era of personalized medicine. U.C. Davis L Rev. 2016;49:1987-2018.
- Tarasoff v Regents of the University of California, 551 P.2d 334, 131 Cal. Rptr. 14 (Cal. 1976).
- Safer v Estate of Pack, 677 A.2d 1188 (N.J. App. 1996), cert. denied, 683 A.2d 1163 (N.J. 1996).
- Pate v Threlkel, 661 So.2d 278 (Fla. 1995).
- Rothstein MA. Liability issues in pharmacogenomics. Louisiana L Rev. 2005;66:117-124.
- Marchant G, Lindor R. Personalized medicine and genetic malpractice. Genet Med. 2013;15:921-922.
- Westbrook M. Transforming the physician’s standard of care in the context of whole genome sequencing technologies: finding guidance in best practice standards. Saint Louis U J Health Law Policy. 2015;9:111-148.
CASE Young woman with family history of breast cancer detects lump
Two weeks after noting a lump on her breast when her cat happened to jump on her in that spot, a 28-year-old woman (G0) went to her primary care provider. She was referred to her gynecologist; breast imaging, ultrasonography, and mammography were obtained, with microcalcifications noted. A fine needle aspiration diagnosed intraductal malignancy. The surgical breast tissue specimen was estrogen receptor (ER)- and progestogen receptor (PR)-positive and HER2-negative. Other tumor markers were obtained, including carcinoembryonic antigen, and tissue polypeptide specific antigen, p53, cathepsin D, cyclin E, and nestin, but results were not available.
With regard to family history, the woman’s mother and maternal grandmother had a history of breast cancer. The patient and her family underwent gene testing. The patient was found to be BRCA1- and BRCA2-positive; her mother was BRCA1-positive, an older sister was BRCA2-positive, and her grandmother was not tested.
The question arose in light of her family history as to why she was not tested for BRCA and appropriately counseled by her gynecologist prior to the cancer diagnosis. Litigation was initiated. While the case did not go forward regarding litigation, it is indeed a case in point. (Please note that this is a hypothetical case. It is based on a composite of several cases.)
Medical considerations
Breast cancer is the most common type of cancer affecting women in the Western world.1 Advances in clinical testing for gene mutations have escalated and allowed for identification of patients at increased risk for breast and ovarian cancer. Along with these advances come professional liability risk. After looking at the medical considerations for BRCA1 and 2 testing, we will consider a number of important legal issues. In the view of some commentators, the failure to diagnose genetic mutations in patients predisposed to cancer is “poised to become the next wave of medical professional liability lawsuits.”2
BRCA1 and BRCA2 genes provide tumor suppressor proteins, and assessment for mutations is recommended for individuals at high risk for breast and/or ovarian cancer; mutations in BRCA genes cause DNA damage, which increases the chance of developing cancer. The other way to look at it is, BRCA1 and 2 are tumor suppressor genes that are integrally involved with DNA damage control. Once there is a mutation, it adversely affects the beneficial effects of the gene. Mutations in these genes account for 5% to 10% of all hereditary breast cancers.3 Of note, men with BRCA2 are at increased risk for prostate cancer.
A patient who presents to her gynecologist stating that there is a family history of breast cancer, without knowledge of genetic components, presents a challenge (and a medicolegal risk) for the provider to assess. Prediction models have been used to determine specific patient risk for carrying a genetic mutation with resultant breast cancer development.4 Risk prediction models do not appear to be a good answer to predicting who is more likely to develop breast or ovarian cancer, however. A Mayo model may assist (FIGURE).5 Clinicians should also be aware of other models of risk assessment, including the Gail Model (TABLE 1).6


Continue to: Guidelines for genetic testing...
Guidelines for genetic testing
The American College of Obstetricians and Gynecologists states that patient medical history and family history are paramount in obtaining information regarding risk for breast and ovarian cancer. First- and second-degree relatives are allocated to this category. Information regarding age of diagnosis, maternal and paternal lineage, and ethnic background can imply a need for genetic testing (TABLE 2).7,8 A number of genetics national organizations have participated in recommendations and include the American College of Medical Genetics and Genomics, the National Society for Genetic Counselors, and the Society of Gynecologic Oncology.7

The question always surfaces, could the clinical outcome of the cancer when diagnosed have been changed if screening were undertaken, with earlier diagnosis, or prevented with prophylactic mastectomy, and changed the end result. In addition, it is well known that breast augmentation mammoplasty alters the ability to accurately evaluate mammograms. Patients considering this type of plastic surgery, ideally, should be counselled accordingly.9
Bottom line, we as clinicians must be cognizant of both ACOG and United States Preventive Services Task Force (USPSTF) recommendations regarding screening and gene testing for women considered high risk for breast cancer based on family history.7
Legal considerations
The case presented demonstrates that the discovery of the BRCA1 and BRCA2 genes, and reliable tests for determining the existence of the genes, brought with them legal issues as well as medical advantages. We look at professional liability (malpractice) questions this technology raises, and then consider the outcome of the hypothetical case. (BRCA is used here to apply broadly—not only to BRCA1 and 2 but also to PALB2, CHEK2, and similar genetic abnormalities.)
To date, the most visible BRCA legal issues covered in cases and law reviews have focused more on patent law than malpractice. The most important of these was a decision of the US Supreme Court in Association for Molecular Pathology v Myriad Genetics.10 The US Patent Office was granting patents to companies finding useful, naturally occurring segments of human DNA, and had granted Myriad several patents on BRCA1 and BRCA2 genes. This patent policy had the potential to seriously interfere with broad scientific use of these genes.11 Fortunately, the Supreme Court stepped in and unanimously invalidated such patents. It held that a “naturally occurring DNA segment is a product of nature and not patent eligible merely because it has been isolated.” The Court noted, “Finding the location of the BRCA1 and BRCA2 genes does not render the genes patent eligible ‘new . . . composition[s] of matter.’”8 The Court did allow the patenting of tests for specific gene structures, and artificial changes in naturally occurring genes.
Malpractice and BRCA
While the BRCA patent wars have lingered, the potential for a significant increase in BRCA-related malpractice cases is of increasing concern. Like most malpractice liability, these new claims are based on very old principles of negligence.12 To prevail, the plaintiff (ordinarily, an injured patient) must demonstrate 4 things:
- A duty. That is, the physician owed a duty to the injured party. Usually (but not always) that requires a professional relationship between the physician and the person injured.
- A breach of that duty. Malpractice liability is based on the fact that the physician did something that a reasonably careful physician (generally, of the same specialty) would not have done, or that the physician failed to do something that a reasonable physician would have done. This usually means that the profession itself sees what the physician did (or did not do) as medically inappropriate. In medical malpractice cases, that is ordinarily measured by what the usual or common practice is among prudent physicians. In rare circumstances, courts have found the standard practice of a profession to be negligent. Where, for example, it was custom for a professional not to give an eye pressure test to anyone under age 40, a court found that common standard to be inappropriate.13 In the words of Judge Learned Hand (speaking about a different case), “a whole calling may have unduly lagged in the adoption of new and available devices. It never may set its own tests.”14 Underlying negligence is a cost-benefit analysis (discussed below).
- Damages. There must have been some damage that courts recognize, usually loss of money or opportunity to work, the cost of care, pain and suffering, or loss of enjoyment/quality of life. In malpractice, many states now recognize the “loss of chance” or the “loss of a chance.” That means, if a “physician negligently fails to diagnose a curable disease, and the patient is harmed by the disease, the physician should be liable for causing the ‘loss of a chance of a cure.’”15 (Delay in diagnosis is the most common reason for claims in breast cancer care.)16
- Causation. The breach of duty (negligence) must have caused the damages. The causation must have been reasonably close. If a driver drives through a stop sign, or a physician misreads a test, and someone is injured but there is no connection between the negligence and the injury, there is not tort liability.
The 4 elements of malpractice just described are raised in some way in the possible liability associated with BRCA testing. We next look at the ways in which liability may arise from that testing (or lack of it).
Underlying much of the following discussion is the “cost-benefit” consideration noted above. This concept is that the total cost (financial and health) of testing should be compared with the value of the benefits of testing, taking into account the probabilities that the testing will result in better health outcomes. BRCA testing, for example, is essentially cost-free in terms of physical risk. Its financial cost, while not trivial, is not great, and it is commonly covered by health insurance.17 In terms of benefits, the testing has the potential for providing critical information in making treatment decisions for a meaningful percentage of patients and their families. There are many ways of analyzing the liability risks of genetic malpractice,7,18 and the following is intended to discuss some of the greatest risks related to BRCA testing.
Continue to: Areas of liability...
Areas of liability
The failure to recommend a test. The circumstances in which BRCA testing should be undertaken are set out by professional organizations (noted above). These recommendations are not static, however. They change from time to time. Given the potential harm caused by the failure to test in relevant circumstances, malpractice liability is certainly a possibility when the failure to recommend a test to a patient results in a cancer that might have been prevented had the genetic problem been identified in a timely manner. The circumstances in which testing should be considered continue to change, placing an obligation on clinicians to stay well informed of changing genetic understandings. Another risk is that one specialist may assume that it is the job of another specialist to order the test. Whatever the cause of the failure to test, or unnecessary delay in testing, it appears to be the primary basis for BRCA liability.
The failure to properly interpret a test. Any test that is misinterpreted may lead to harm for the patient. A false negative, of course, may mean that preventive treatment that could have been undertaken will be foregone, as a “loss of a chance.” On the other hand, a false positive can lead to radical, unnecessary surgery or treatment. If a misinterpretation occurred because of carelessness by the testing organization, or confusion by a practitioner, there is a likelihood of negligence.19
A different form of “misinterpretation” could be reasonable—and not negligent. Advances in scientific-medical understanding may result in the outcome of tests being reconsidered and changed. That has been the case with genetic testing and breast cancer. The availability of multiple breast cancer SNPs (single nucleotide polymorphisms), and combining this information with other risk factors for example, results in a polygenic risk score that may be at odds with the level of risk from earlier testing.20,21 This naturally leads to the question of when later, updated testing should be recommended to look for a better current interpretation.22,23
The failure to act on BRCA test results. Testing is of no value, of course, if the results are not used properly. Test results or analyses that are not sent to the proper physicians, or are somehow ignored when properly directed, is a “never” event—it should never happen. It almost always would be considered negligence, and if the patient were injured, could lead to liability. Amazingly, one study found that, in genetic testing liability cases, nearly 20% of the claims arose from failure to return test results to patients.24 In addition, when a patient is found to be BRCA-positive, there is an obligation to discuss the options for dealing with the increased risk associated with the gene mutation(s), as well as to recommend the prudent course of action or to refer the patient to someone who will have that discussion.
Informed consent to the patient. BRCA testing requires informed consent. The physical risks of the testing process are minimal, of course, but it carries a number of other emotional and family risks. The informed consent process is an invitation to an honest discussion between clinicians and patients. It should be an opportunity to discuss what the testing is, and is not, and what the test may mean for treatment. It may also be an opportunity to discuss the implications for other members of the patient’s family (noted below).
One element of informed consent is a discussion of the consequences of failure to consent, or to undertake one of the alternatives. In the case of BRCA testing, this is especially important in cases in which a patient expresses a hesitancy to be tested with an “I’d rather not know philosophy.” Although clinicians should not practice law, some patient concerns about discrimination may be addressed by the protection that the federal Genetic Information Nondiscrimination Act (GINA) and other laws provide (which prohibit insurance and employment discrimination based on genetic information). A good source of information about GINA and related nondiscrimination laws is provided by the National Human Genome Research Institute.25 In addition, the National Institutes of Health has a website that may be helpful to many patients26 (and a much more complex site for health professionals).27 At the same time, courts have resisted plaintiffs/patients who have tried to use informed consent as a way of suing for failure to offer genetic testing.28,29
The failure to refer. In some cases, a patient should be formally referred for genetics consultation. The considerations here are similar to other circumstances in modern, fast developing medical practice that require special sensitivity to those occasions in which a patient will benefit from additional expertise. It is a principle that the AMA Council on Ethical and Judicial Affairs has expressed this way: “In the absence of adequate expertise in pretest and posttest counseling, a physician should refer the patient to an appropriate specialist.”30 The failure to refer, when that deviates from acceptable practice, may result in liability.
Informing others. BRCA testing is an area of medicine in which results may be of great significance not only to the patient but also to the patient’s family.31 Physicians should counsel patients on the importance of informing relatives about relevant results and “should make themselves available to assist patients in communicating with relatives to discuss opportunities for counseling and testing, as appropriate.”30 The question may arise, however, of whether in some circumstances physicians should go a step further in ensuring relatives receive important information regarding their loved one’s health.32 The law has been reluctant to impose liability to “third parties” (someone not a patient). Duties usually arise through the physician-patient relationship. There are exceptions. Perhaps the best known has been the obligation of mental health professionals to take action to protect third parties from patients who have made believable threats against identifiable victims.33 There are indications that some courts could find, in extreme circumstances, a “duty to warn” nonpatients in some instances where it is essential to inform third parties that they should receive a specific form of genetic testing.34,35 Such a duty would, of course, have to protect the privacy rights of the patient to the maximum extent possible. A general duty of this type has not been established widely, but may be part of the future.
Continue to: Was there liability in our example case?...
Was there liability in our example case?
The hypothetical case provided above suggests that there could be liability. Routine medical history by the primary care physician would have produced the fact that the patient’s mother, sister, and maternal grandmother had breast cancer. That would clearly have put her in a category of those who should have received genetic testing. Yet, she was not tested until after her cancer was found. From the limited facts we have, it appears that this timeline of events would have been outside accepted practice—and negligent. The case was not pursued by the patient, however, and this may represent the current state of liability for BRCA issues.
The extent of liability seems to be significant
Our discussion of liability suggests that there is significant potential for BRCA testing negligence within practice, and that the damages in these cases could be substantial. Yet the predicted “tsunami” of malpractice lawsuits related to genetic testing has not appeared.36,37 One study of cases in the United States (through 2016) found a “slowly rising tide” of liability cases instead of a tsunami,24 as the number of claims made was low. On the other hand, the payments where damages were awarded were an order of magnitude larger than other malpractice cases—a mean of $5.3 million and median of $2 million. This is compared with mean values in the range of $275,000 to $600,000 in other areas of malpractice.
The majority of the genetic malpractice cases involve prenatal and newborn testing, and diagnosis/susceptibility/pharmacogenomic accounting for about 25% of cases. In terms of type of errors claimed, approximately 50% were diagnostic-interpretation errors, 30% failure to offer testing, nearly 20% failure to return test results to the patients, and a few remaining cases of failure to properly treat in light of genetic testing.24
Despite a few very large payments, however, the fact remains that there is a surprisingly low number of genetics malpractice cases. Gary Marchant and colleagues suggest that several reasons may account for this:
- the clinical implementation of genetic science has been slower than expected
- the lack of expertise of many physicians in genetic science
- expert witnesses have sometimes been hard to find
- the lack of understanding by plaintiffs’ attorneys of genetic malpractice
- potential plaintiffs’ lack of understanding of the nature of genetic testing and the harms resulting from genetic negligence.17,24,37
The tide is slowly coming in
By all appearances, there is every reason to think that genetic malpractice will be increasing, and that the recent past of much higher damages per claim paid in the genetics area will be part of that tide. The National Human Genome Research LawSeq project has suggested a number of useful ways of dealing with the liability issues.18 In addition to the BRCA issues that we have considered in this article for ObGyns, there are other critical issues of prenatal and newborn genetic testing.38 But those are topics for another day. ●
CASE Young woman with family history of breast cancer detects lump
Two weeks after noting a lump on her breast when her cat happened to jump on her in that spot, a 28-year-old woman (G0) went to her primary care provider. She was referred to her gynecologist; breast imaging, ultrasonography, and mammography were obtained, with microcalcifications noted. A fine needle aspiration diagnosed intraductal malignancy. The surgical breast tissue specimen was estrogen receptor (ER)- and progestogen receptor (PR)-positive and HER2-negative. Other tumor markers were obtained, including carcinoembryonic antigen, and tissue polypeptide specific antigen, p53, cathepsin D, cyclin E, and nestin, but results were not available.
With regard to family history, the woman’s mother and maternal grandmother had a history of breast cancer. The patient and her family underwent gene testing. The patient was found to be BRCA1- and BRCA2-positive; her mother was BRCA1-positive, an older sister was BRCA2-positive, and her grandmother was not tested.
The question arose in light of her family history as to why she was not tested for BRCA and appropriately counseled by her gynecologist prior to the cancer diagnosis. Litigation was initiated. While the case did not go forward regarding litigation, it is indeed a case in point. (Please note that this is a hypothetical case. It is based on a composite of several cases.)
Medical considerations
Breast cancer is the most common type of cancer affecting women in the Western world.1 Advances in clinical testing for gene mutations have escalated and allowed for identification of patients at increased risk for breast and ovarian cancer. Along with these advances come professional liability risk. After looking at the medical considerations for BRCA1 and 2 testing, we will consider a number of important legal issues. In the view of some commentators, the failure to diagnose genetic mutations in patients predisposed to cancer is “poised to become the next wave of medical professional liability lawsuits.”2
BRCA1 and BRCA2 genes provide tumor suppressor proteins, and assessment for mutations is recommended for individuals at high risk for breast and/or ovarian cancer; mutations in BRCA genes cause DNA damage, which increases the chance of developing cancer. The other way to look at it is, BRCA1 and 2 are tumor suppressor genes that are integrally involved with DNA damage control. Once there is a mutation, it adversely affects the beneficial effects of the gene. Mutations in these genes account for 5% to 10% of all hereditary breast cancers.3 Of note, men with BRCA2 are at increased risk for prostate cancer.
A patient who presents to her gynecologist stating that there is a family history of breast cancer, without knowledge of genetic components, presents a challenge (and a medicolegal risk) for the provider to assess. Prediction models have been used to determine specific patient risk for carrying a genetic mutation with resultant breast cancer development.4 Risk prediction models do not appear to be a good answer to predicting who is more likely to develop breast or ovarian cancer, however. A Mayo model may assist (FIGURE).5 Clinicians should also be aware of other models of risk assessment, including the Gail Model (TABLE 1).6


Continue to: Guidelines for genetic testing...
Guidelines for genetic testing
The American College of Obstetricians and Gynecologists states that patient medical history and family history are paramount in obtaining information regarding risk for breast and ovarian cancer. First- and second-degree relatives are allocated to this category. Information regarding age of diagnosis, maternal and paternal lineage, and ethnic background can imply a need for genetic testing (TABLE 2).7,8 A number of genetics national organizations have participated in recommendations and include the American College of Medical Genetics and Genomics, the National Society for Genetic Counselors, and the Society of Gynecologic Oncology.7

The question always surfaces, could the clinical outcome of the cancer when diagnosed have been changed if screening were undertaken, with earlier diagnosis, or prevented with prophylactic mastectomy, and changed the end result. In addition, it is well known that breast augmentation mammoplasty alters the ability to accurately evaluate mammograms. Patients considering this type of plastic surgery, ideally, should be counselled accordingly.9
Bottom line, we as clinicians must be cognizant of both ACOG and United States Preventive Services Task Force (USPSTF) recommendations regarding screening and gene testing for women considered high risk for breast cancer based on family history.7
Legal considerations
The case presented demonstrates that the discovery of the BRCA1 and BRCA2 genes, and reliable tests for determining the existence of the genes, brought with them legal issues as well as medical advantages. We look at professional liability (malpractice) questions this technology raises, and then consider the outcome of the hypothetical case. (BRCA is used here to apply broadly—not only to BRCA1 and 2 but also to PALB2, CHEK2, and similar genetic abnormalities.)
To date, the most visible BRCA legal issues covered in cases and law reviews have focused more on patent law than malpractice. The most important of these was a decision of the US Supreme Court in Association for Molecular Pathology v Myriad Genetics.10 The US Patent Office was granting patents to companies finding useful, naturally occurring segments of human DNA, and had granted Myriad several patents on BRCA1 and BRCA2 genes. This patent policy had the potential to seriously interfere with broad scientific use of these genes.11 Fortunately, the Supreme Court stepped in and unanimously invalidated such patents. It held that a “naturally occurring DNA segment is a product of nature and not patent eligible merely because it has been isolated.” The Court noted, “Finding the location of the BRCA1 and BRCA2 genes does not render the genes patent eligible ‘new . . . composition[s] of matter.’”8 The Court did allow the patenting of tests for specific gene structures, and artificial changes in naturally occurring genes.
Malpractice and BRCA
While the BRCA patent wars have lingered, the potential for a significant increase in BRCA-related malpractice cases is of increasing concern. Like most malpractice liability, these new claims are based on very old principles of negligence.12 To prevail, the plaintiff (ordinarily, an injured patient) must demonstrate 4 things:
- A duty. That is, the physician owed a duty to the injured party. Usually (but not always) that requires a professional relationship between the physician and the person injured.
- A breach of that duty. Malpractice liability is based on the fact that the physician did something that a reasonably careful physician (generally, of the same specialty) would not have done, or that the physician failed to do something that a reasonable physician would have done. This usually means that the profession itself sees what the physician did (or did not do) as medically inappropriate. In medical malpractice cases, that is ordinarily measured by what the usual or common practice is among prudent physicians. In rare circumstances, courts have found the standard practice of a profession to be negligent. Where, for example, it was custom for a professional not to give an eye pressure test to anyone under age 40, a court found that common standard to be inappropriate.13 In the words of Judge Learned Hand (speaking about a different case), “a whole calling may have unduly lagged in the adoption of new and available devices. It never may set its own tests.”14 Underlying negligence is a cost-benefit analysis (discussed below).
- Damages. There must have been some damage that courts recognize, usually loss of money or opportunity to work, the cost of care, pain and suffering, or loss of enjoyment/quality of life. In malpractice, many states now recognize the “loss of chance” or the “loss of a chance.” That means, if a “physician negligently fails to diagnose a curable disease, and the patient is harmed by the disease, the physician should be liable for causing the ‘loss of a chance of a cure.’”15 (Delay in diagnosis is the most common reason for claims in breast cancer care.)16
- Causation. The breach of duty (negligence) must have caused the damages. The causation must have been reasonably close. If a driver drives through a stop sign, or a physician misreads a test, and someone is injured but there is no connection between the negligence and the injury, there is not tort liability.
The 4 elements of malpractice just described are raised in some way in the possible liability associated with BRCA testing. We next look at the ways in which liability may arise from that testing (or lack of it).
Underlying much of the following discussion is the “cost-benefit” consideration noted above. This concept is that the total cost (financial and health) of testing should be compared with the value of the benefits of testing, taking into account the probabilities that the testing will result in better health outcomes. BRCA testing, for example, is essentially cost-free in terms of physical risk. Its financial cost, while not trivial, is not great, and it is commonly covered by health insurance.17 In terms of benefits, the testing has the potential for providing critical information in making treatment decisions for a meaningful percentage of patients and their families. There are many ways of analyzing the liability risks of genetic malpractice,7,18 and the following is intended to discuss some of the greatest risks related to BRCA testing.
Continue to: Areas of liability...
Areas of liability
The failure to recommend a test. The circumstances in which BRCA testing should be undertaken are set out by professional organizations (noted above). These recommendations are not static, however. They change from time to time. Given the potential harm caused by the failure to test in relevant circumstances, malpractice liability is certainly a possibility when the failure to recommend a test to a patient results in a cancer that might have been prevented had the genetic problem been identified in a timely manner. The circumstances in which testing should be considered continue to change, placing an obligation on clinicians to stay well informed of changing genetic understandings. Another risk is that one specialist may assume that it is the job of another specialist to order the test. Whatever the cause of the failure to test, or unnecessary delay in testing, it appears to be the primary basis for BRCA liability.
The failure to properly interpret a test. Any test that is misinterpreted may lead to harm for the patient. A false negative, of course, may mean that preventive treatment that could have been undertaken will be foregone, as a “loss of a chance.” On the other hand, a false positive can lead to radical, unnecessary surgery or treatment. If a misinterpretation occurred because of carelessness by the testing organization, or confusion by a practitioner, there is a likelihood of negligence.19
A different form of “misinterpretation” could be reasonable—and not negligent. Advances in scientific-medical understanding may result in the outcome of tests being reconsidered and changed. That has been the case with genetic testing and breast cancer. The availability of multiple breast cancer SNPs (single nucleotide polymorphisms), and combining this information with other risk factors for example, results in a polygenic risk score that may be at odds with the level of risk from earlier testing.20,21 This naturally leads to the question of when later, updated testing should be recommended to look for a better current interpretation.22,23
The failure to act on BRCA test results. Testing is of no value, of course, if the results are not used properly. Test results or analyses that are not sent to the proper physicians, or are somehow ignored when properly directed, is a “never” event—it should never happen. It almost always would be considered negligence, and if the patient were injured, could lead to liability. Amazingly, one study found that, in genetic testing liability cases, nearly 20% of the claims arose from failure to return test results to patients.24 In addition, when a patient is found to be BRCA-positive, there is an obligation to discuss the options for dealing with the increased risk associated with the gene mutation(s), as well as to recommend the prudent course of action or to refer the patient to someone who will have that discussion.
Informed consent to the patient. BRCA testing requires informed consent. The physical risks of the testing process are minimal, of course, but it carries a number of other emotional and family risks. The informed consent process is an invitation to an honest discussion between clinicians and patients. It should be an opportunity to discuss what the testing is, and is not, and what the test may mean for treatment. It may also be an opportunity to discuss the implications for other members of the patient’s family (noted below).
One element of informed consent is a discussion of the consequences of failure to consent, or to undertake one of the alternatives. In the case of BRCA testing, this is especially important in cases in which a patient expresses a hesitancy to be tested with an “I’d rather not know philosophy.” Although clinicians should not practice law, some patient concerns about discrimination may be addressed by the protection that the federal Genetic Information Nondiscrimination Act (GINA) and other laws provide (which prohibit insurance and employment discrimination based on genetic information). A good source of information about GINA and related nondiscrimination laws is provided by the National Human Genome Research Institute.25 In addition, the National Institutes of Health has a website that may be helpful to many patients26 (and a much more complex site for health professionals).27 At the same time, courts have resisted plaintiffs/patients who have tried to use informed consent as a way of suing for failure to offer genetic testing.28,29
The failure to refer. In some cases, a patient should be formally referred for genetics consultation. The considerations here are similar to other circumstances in modern, fast developing medical practice that require special sensitivity to those occasions in which a patient will benefit from additional expertise. It is a principle that the AMA Council on Ethical and Judicial Affairs has expressed this way: “In the absence of adequate expertise in pretest and posttest counseling, a physician should refer the patient to an appropriate specialist.”30 The failure to refer, when that deviates from acceptable practice, may result in liability.
Informing others. BRCA testing is an area of medicine in which results may be of great significance not only to the patient but also to the patient’s family.31 Physicians should counsel patients on the importance of informing relatives about relevant results and “should make themselves available to assist patients in communicating with relatives to discuss opportunities for counseling and testing, as appropriate.”30 The question may arise, however, of whether in some circumstances physicians should go a step further in ensuring relatives receive important information regarding their loved one’s health.32 The law has been reluctant to impose liability to “third parties” (someone not a patient). Duties usually arise through the physician-patient relationship. There are exceptions. Perhaps the best known has been the obligation of mental health professionals to take action to protect third parties from patients who have made believable threats against identifiable victims.33 There are indications that some courts could find, in extreme circumstances, a “duty to warn” nonpatients in some instances where it is essential to inform third parties that they should receive a specific form of genetic testing.34,35 Such a duty would, of course, have to protect the privacy rights of the patient to the maximum extent possible. A general duty of this type has not been established widely, but may be part of the future.
Continue to: Was there liability in our example case?...
Was there liability in our example case?
The hypothetical case provided above suggests that there could be liability. Routine medical history by the primary care physician would have produced the fact that the patient’s mother, sister, and maternal grandmother had breast cancer. That would clearly have put her in a category of those who should have received genetic testing. Yet, she was not tested until after her cancer was found. From the limited facts we have, it appears that this timeline of events would have been outside accepted practice—and negligent. The case was not pursued by the patient, however, and this may represent the current state of liability for BRCA issues.
The extent of liability seems to be significant
Our discussion of liability suggests that there is significant potential for BRCA testing negligence within practice, and that the damages in these cases could be substantial. Yet the predicted “tsunami” of malpractice lawsuits related to genetic testing has not appeared.36,37 One study of cases in the United States (through 2016) found a “slowly rising tide” of liability cases instead of a tsunami,24 as the number of claims made was low. On the other hand, the payments where damages were awarded were an order of magnitude larger than other malpractice cases—a mean of $5.3 million and median of $2 million. This is compared with mean values in the range of $275,000 to $600,000 in other areas of malpractice.
The majority of the genetic malpractice cases involve prenatal and newborn testing, and diagnosis/susceptibility/pharmacogenomic accounting for about 25% of cases. In terms of type of errors claimed, approximately 50% were diagnostic-interpretation errors, 30% failure to offer testing, nearly 20% failure to return test results to the patients, and a few remaining cases of failure to properly treat in light of genetic testing.24
Despite a few very large payments, however, the fact remains that there is a surprisingly low number of genetics malpractice cases. Gary Marchant and colleagues suggest that several reasons may account for this:
- the clinical implementation of genetic science has been slower than expected
- the lack of expertise of many physicians in genetic science
- expert witnesses have sometimes been hard to find
- the lack of understanding by plaintiffs’ attorneys of genetic malpractice
- potential plaintiffs’ lack of understanding of the nature of genetic testing and the harms resulting from genetic negligence.17,24,37
The tide is slowly coming in
By all appearances, there is every reason to think that genetic malpractice will be increasing, and that the recent past of much higher damages per claim paid in the genetics area will be part of that tide. The National Human Genome Research LawSeq project has suggested a number of useful ways of dealing with the liability issues.18 In addition to the BRCA issues that we have considered in this article for ObGyns, there are other critical issues of prenatal and newborn genetic testing.38 But those are topics for another day. ●
- Sevilla C, Moatti JP, Reynier CJ, et al. Testing for BRCA1 mutations: a cost-effective analysis. Europ J Human Genetics. 2002;10:599-606.
- Cotton V, Kirkpatrick D. Failure to recommend genetic counseling in breast cancer: is the next wave of medical professional liability lawsuits? Contemp OB/GYN. June 1, 2017.
- Suryavanshi M, Kumar D, Panigrahi M, et al. Detection of false positive mutations in BRCA gene by next generation sequencing. Fam Cancer. 2017;16:311-317.
- Black L, Knoppers B, Avard D, et al. Legal liability and the uncertain nature of risk prediction: the case of breast cancer risk prediction models. Public Health Genomics. 2012;15:335-340.
- McClintock A, Gollab A, Laya M. Breast cancer risk assessment, a step-wise approach for primary care physicians on the front lines of shared decision making. Mayo Clin Proc. 2020;95:1268-1275.
- National Cancer Institute. The Breast Cancer Risk Assessment Tool. https://bcrisktool.cancer.gov/. Accessed February 25, 2021.
- Neff J, Richardson G, Phelps J. Legal liabilities associated with hereditary breast and ovarian cancers. J Reprod Med. 2020;65:227-230.
- American College of Obstetricians and Gynecologists. Practice Bulletin No 182: hereditary breast and ovarian cancer syndrome. Obstet Gynecol. 2017;130:e110-e126.
- Sá dos Reis C, Gremion I, and Meystre NR. Study of breast implants mammography examinations for identification of suitable image quality criteria. Insights Imaging. 2020;11:3.
- Association for Molecular Pathology v Myriad Genetics, 569 U.S. 576 (2013).
- Smith SR. The Supreme Court 2012-2013: dogs, DNA, and DOMA. Register Rep. 2013;39(Fall):26-33.
- Bal BS. An introduction to medical malpractice in the United States. Clin Orthop Relat Res. 2009;467:339-347.
- Helling v Carey, 83 Wn.2d 514, 519 P.2d 981 (1974).
- The T.J. Hooper, 60 F.2d 737, 740 (2d Cir.1932), cert. denied 287 U.S. 662 (1932).
- Fischer DA. Tort recovery for loss of a chance. Wake Forest L Rev. 2001;36:605-655.
- Murphy BL, Ray-Zack MD, Reddy PN, et al. Breast cancer litigation in the 21st century. Ann Surg Oncol. 2018;25:2939- 2947.
- Prince AE. Prevention for those who can pay: insurance reimbursement of genetic-based preventive interventions in the liminal state between health and disease. J Law Biosci. 2015;2:365-395.
- Marchant G, Barnes M, Evans JP, et al; LawSeq Liability Task Force. From genetics to genomics: facing the liability implications in clinical care. J Law Med Ethics. 2020;48:11-43.
- Complaint, Held v Ambry Genetics Corp., No. 15-CV-8683, 2015 WL 6750024 (S.D.N.Y. Nov. 4, 2015); Order of Dismissal, Held v Ambry Genetics Corp., No. 15-CV-8683, (S.D.N.Y. Dec. 6, 2016).
- Pederson HJ. Breast cancer risk assessment and treatment: current concepts in genetics and genomics. Contemp OB/ GYN. 2017; 62:A1-A4.
- Pederson HJ. Who needs breast cancer genetics testing? OBG Manag. 2018;30:34-39.
- Roberts JL, Foulkes A. Genetic duties. William Mary L Rev. 2020;62:143-212.
- Thorogood A, Cook-Deegan R, Knoppers B. Public variant databases: liability? Genet Med. 2017;19:838–841.
- Marchant G, Lindor R. Genomic malpractice: an emerging tide or gentle ripple? Food Drug Law J. 2018;73:1-37.
- National Human Genome Research Institute. Genetic discrimination. https://www.genome.gov/about-genomics /policy-issues/Genetic-Discrimination. Updated September 16, 2020. Accessed February 25, 2021.
- National Cancer Institute. BRCA mutations: cancer risk and genetic testing. https://www.cancer.gov/about-cancer /causes-prevention/genetics/brca-fact-sheet. Reviewed November 19, 2020. Accessed February 25, 2021.
- National Cancer Institute. Genetics of breast and gynecologic cancers (PDQ®)–Health Professional Version. https://www .cancer.gov/types/breast/hp/breast-ovarian-genetics-pdq. Updated February 12, 2021. Accessed February 25, 2021.
- Reed v Campagnolo, 630 A.2d 1145, 1152–54 (Md. 1993).
- Munro v Regents of Univ. of Cal.,263 Cal. Rptr. 878, 885, 988 (1989).
- AMA Council on Ethical and Judicial Affairs. AMA Code of Medical Ethics’ opinions on genetic testing. Opinion 2.131. 2009;11:683-685. https://journalofethics.ama-assn .org/article/ama-code-medical-ethics-opinions-genetictesting/2009-09.
- Gilbar R, Barnoy S. Disclosing genetic test results to the patient’ relatives: how does the law influence clinical practice? J Law Technol Policy. 2019;125-168.
- Song K. Warning third parties of genetic risks in the era of personalized medicine. U.C. Davis L Rev. 2016;49:1987-2018.
- Tarasoff v Regents of the University of California, 551 P.2d 334, 131 Cal. Rptr. 14 (Cal. 1976).
- Safer v Estate of Pack, 677 A.2d 1188 (N.J. App. 1996), cert. denied, 683 A.2d 1163 (N.J. 1996).
- Pate v Threlkel, 661 So.2d 278 (Fla. 1995).
- Rothstein MA. Liability issues in pharmacogenomics. Louisiana L Rev. 2005;66:117-124.
- Marchant G, Lindor R. Personalized medicine and genetic malpractice. Genet Med. 2013;15:921-922.
- Westbrook M. Transforming the physician’s standard of care in the context of whole genome sequencing technologies: finding guidance in best practice standards. Saint Louis U J Health Law Policy. 2015;9:111-148.
- Sevilla C, Moatti JP, Reynier CJ, et al. Testing for BRCA1 mutations: a cost-effective analysis. Europ J Human Genetics. 2002;10:599-606.
- Cotton V, Kirkpatrick D. Failure to recommend genetic counseling in breast cancer: is the next wave of medical professional liability lawsuits? Contemp OB/GYN. June 1, 2017.
- Suryavanshi M, Kumar D, Panigrahi M, et al. Detection of false positive mutations in BRCA gene by next generation sequencing. Fam Cancer. 2017;16:311-317.
- Black L, Knoppers B, Avard D, et al. Legal liability and the uncertain nature of risk prediction: the case of breast cancer risk prediction models. Public Health Genomics. 2012;15:335-340.
- McClintock A, Gollab A, Laya M. Breast cancer risk assessment, a step-wise approach for primary care physicians on the front lines of shared decision making. Mayo Clin Proc. 2020;95:1268-1275.
- National Cancer Institute. The Breast Cancer Risk Assessment Tool. https://bcrisktool.cancer.gov/. Accessed February 25, 2021.
- Neff J, Richardson G, Phelps J. Legal liabilities associated with hereditary breast and ovarian cancers. J Reprod Med. 2020;65:227-230.
- American College of Obstetricians and Gynecologists. Practice Bulletin No 182: hereditary breast and ovarian cancer syndrome. Obstet Gynecol. 2017;130:e110-e126.
- Sá dos Reis C, Gremion I, and Meystre NR. Study of breast implants mammography examinations for identification of suitable image quality criteria. Insights Imaging. 2020;11:3.
- Association for Molecular Pathology v Myriad Genetics, 569 U.S. 576 (2013).
- Smith SR. The Supreme Court 2012-2013: dogs, DNA, and DOMA. Register Rep. 2013;39(Fall):26-33.
- Bal BS. An introduction to medical malpractice in the United States. Clin Orthop Relat Res. 2009;467:339-347.
- Helling v Carey, 83 Wn.2d 514, 519 P.2d 981 (1974).
- The T.J. Hooper, 60 F.2d 737, 740 (2d Cir.1932), cert. denied 287 U.S. 662 (1932).
- Fischer DA. Tort recovery for loss of a chance. Wake Forest L Rev. 2001;36:605-655.
- Murphy BL, Ray-Zack MD, Reddy PN, et al. Breast cancer litigation in the 21st century. Ann Surg Oncol. 2018;25:2939- 2947.
- Prince AE. Prevention for those who can pay: insurance reimbursement of genetic-based preventive interventions in the liminal state between health and disease. J Law Biosci. 2015;2:365-395.
- Marchant G, Barnes M, Evans JP, et al; LawSeq Liability Task Force. From genetics to genomics: facing the liability implications in clinical care. J Law Med Ethics. 2020;48:11-43.
- Complaint, Held v Ambry Genetics Corp., No. 15-CV-8683, 2015 WL 6750024 (S.D.N.Y. Nov. 4, 2015); Order of Dismissal, Held v Ambry Genetics Corp., No. 15-CV-8683, (S.D.N.Y. Dec. 6, 2016).
- Pederson HJ. Breast cancer risk assessment and treatment: current concepts in genetics and genomics. Contemp OB/ GYN. 2017; 62:A1-A4.
- Pederson HJ. Who needs breast cancer genetics testing? OBG Manag. 2018;30:34-39.
- Roberts JL, Foulkes A. Genetic duties. William Mary L Rev. 2020;62:143-212.
- Thorogood A, Cook-Deegan R, Knoppers B. Public variant databases: liability? Genet Med. 2017;19:838–841.
- Marchant G, Lindor R. Genomic malpractice: an emerging tide or gentle ripple? Food Drug Law J. 2018;73:1-37.
- National Human Genome Research Institute. Genetic discrimination. https://www.genome.gov/about-genomics /policy-issues/Genetic-Discrimination. Updated September 16, 2020. Accessed February 25, 2021.
- National Cancer Institute. BRCA mutations: cancer risk and genetic testing. https://www.cancer.gov/about-cancer /causes-prevention/genetics/brca-fact-sheet. Reviewed November 19, 2020. Accessed February 25, 2021.
- National Cancer Institute. Genetics of breast and gynecologic cancers (PDQ®)–Health Professional Version. https://www .cancer.gov/types/breast/hp/breast-ovarian-genetics-pdq. Updated February 12, 2021. Accessed February 25, 2021.
- Reed v Campagnolo, 630 A.2d 1145, 1152–54 (Md. 1993).
- Munro v Regents of Univ. of Cal.,263 Cal. Rptr. 878, 885, 988 (1989).
- AMA Council on Ethical and Judicial Affairs. AMA Code of Medical Ethics’ opinions on genetic testing. Opinion 2.131. 2009;11:683-685. https://journalofethics.ama-assn .org/article/ama-code-medical-ethics-opinions-genetictesting/2009-09.
- Gilbar R, Barnoy S. Disclosing genetic test results to the patient’ relatives: how does the law influence clinical practice? J Law Technol Policy. 2019;125-168.
- Song K. Warning third parties of genetic risks in the era of personalized medicine. U.C. Davis L Rev. 2016;49:1987-2018.
- Tarasoff v Regents of the University of California, 551 P.2d 334, 131 Cal. Rptr. 14 (Cal. 1976).
- Safer v Estate of Pack, 677 A.2d 1188 (N.J. App. 1996), cert. denied, 683 A.2d 1163 (N.J. 1996).
- Pate v Threlkel, 661 So.2d 278 (Fla. 1995).
- Rothstein MA. Liability issues in pharmacogenomics. Louisiana L Rev. 2005;66:117-124.
- Marchant G, Lindor R. Personalized medicine and genetic malpractice. Genet Med. 2013;15:921-922.
- Westbrook M. Transforming the physician’s standard of care in the context of whole genome sequencing technologies: finding guidance in best practice standards. Saint Louis U J Health Law Policy. 2015;9:111-148.
FDA supports robotic device as hysterectomy helper
Surgeons have a new tool for use in benign hysterectomies with the Food & Drug Administration’s authorization for marketing of the Hominis Surgical System, a robotic-assisted surgical device. The marketing authorization was granted to Memic Innovative Surgery.
The FDA reviewed the device through the De Novo classification review process, a regulatory pathway for low- to moderate-risk devices of a new type.
The robotically assisted surgical device (RASD) is designed to facilitate transvaginal hysterectomy procedures and salpingo-oophorectomy procedures in patients without cancer.
RASDs are not robots and require human control, but they allow a surgeon to use computer technology to control and move surgical instruments inserted through incisions or orifices. “RASD technology facilitates performing minimally invasive surgery and complex tasks in confined areas inside the body,” according to an FDA press release announcing the authorization.
“The FDA continues to support advancements in safe and effective medical devices that can improve patient experiences when undergoing surgical procedures,” Binita Ashar, MD, of the Office of Surgical and Infection Control Devices in the FDA’s Center for Devices and Radiological Health, said in the press release. The device represents another minimally invasive option for noncancerous conditions requiring gynecologic surgery.
The FDA also is establishing controls to ensure safety and effectiveness for RASDs, including labeling and performance testing requirements. “When met, the special controls, along with general controls, provide reasonable assurance of safety and effectiveness for devices of this type,” according to the press release.
The Hominis Surgical System involves the use of minimally invasive surgical instruments inserted through the vagina. A video camera is inserted laparoscopically through an abdominal incision; the camera allows the surgeon to visualize the instruments inside the patient.
“The FDA will require the manufacturer to develop and provide a comprehensive training program for surgeons and operating room staff to complete before operation of the device,” according to the press release.
The FDA reviewed data from a clinical study of 30 patients aged 37-79 years who underwent transvaginal total hysterectomy with salpingo-oophorectomy or salpingectomy for benign conditions.
Observed adverse events included minor blood loss, urinary tract infection and delayed healing of the closure made at the top of the vagina (vaginal cuff) that is done as part of a hysterectomy, according to the FDA. However, all 30 procedures were completed with no need for conversion to an open or other procedure.
Surgeons have a new tool for use in benign hysterectomies with the Food & Drug Administration’s authorization for marketing of the Hominis Surgical System, a robotic-assisted surgical device. The marketing authorization was granted to Memic Innovative Surgery.
The FDA reviewed the device through the De Novo classification review process, a regulatory pathway for low- to moderate-risk devices of a new type.
The robotically assisted surgical device (RASD) is designed to facilitate transvaginal hysterectomy procedures and salpingo-oophorectomy procedures in patients without cancer.
RASDs are not robots and require human control, but they allow a surgeon to use computer technology to control and move surgical instruments inserted through incisions or orifices. “RASD technology facilitates performing minimally invasive surgery and complex tasks in confined areas inside the body,” according to an FDA press release announcing the authorization.
“The FDA continues to support advancements in safe and effective medical devices that can improve patient experiences when undergoing surgical procedures,” Binita Ashar, MD, of the Office of Surgical and Infection Control Devices in the FDA’s Center for Devices and Radiological Health, said in the press release. The device represents another minimally invasive option for noncancerous conditions requiring gynecologic surgery.
The FDA also is establishing controls to ensure safety and effectiveness for RASDs, including labeling and performance testing requirements. “When met, the special controls, along with general controls, provide reasonable assurance of safety and effectiveness for devices of this type,” according to the press release.
The Hominis Surgical System involves the use of minimally invasive surgical instruments inserted through the vagina. A video camera is inserted laparoscopically through an abdominal incision; the camera allows the surgeon to visualize the instruments inside the patient.
“The FDA will require the manufacturer to develop and provide a comprehensive training program for surgeons and operating room staff to complete before operation of the device,” according to the press release.
The FDA reviewed data from a clinical study of 30 patients aged 37-79 years who underwent transvaginal total hysterectomy with salpingo-oophorectomy or salpingectomy for benign conditions.
Observed adverse events included minor blood loss, urinary tract infection and delayed healing of the closure made at the top of the vagina (vaginal cuff) that is done as part of a hysterectomy, according to the FDA. However, all 30 procedures were completed with no need for conversion to an open or other procedure.
Surgeons have a new tool for use in benign hysterectomies with the Food & Drug Administration’s authorization for marketing of the Hominis Surgical System, a robotic-assisted surgical device. The marketing authorization was granted to Memic Innovative Surgery.
The FDA reviewed the device through the De Novo classification review process, a regulatory pathway for low- to moderate-risk devices of a new type.
The robotically assisted surgical device (RASD) is designed to facilitate transvaginal hysterectomy procedures and salpingo-oophorectomy procedures in patients without cancer.
RASDs are not robots and require human control, but they allow a surgeon to use computer technology to control and move surgical instruments inserted through incisions or orifices. “RASD technology facilitates performing minimally invasive surgery and complex tasks in confined areas inside the body,” according to an FDA press release announcing the authorization.
“The FDA continues to support advancements in safe and effective medical devices that can improve patient experiences when undergoing surgical procedures,” Binita Ashar, MD, of the Office of Surgical and Infection Control Devices in the FDA’s Center for Devices and Radiological Health, said in the press release. The device represents another minimally invasive option for noncancerous conditions requiring gynecologic surgery.
The FDA also is establishing controls to ensure safety and effectiveness for RASDs, including labeling and performance testing requirements. “When met, the special controls, along with general controls, provide reasonable assurance of safety and effectiveness for devices of this type,” according to the press release.
The Hominis Surgical System involves the use of minimally invasive surgical instruments inserted through the vagina. A video camera is inserted laparoscopically through an abdominal incision; the camera allows the surgeon to visualize the instruments inside the patient.
“The FDA will require the manufacturer to develop and provide a comprehensive training program for surgeons and operating room staff to complete before operation of the device,” according to the press release.
The FDA reviewed data from a clinical study of 30 patients aged 37-79 years who underwent transvaginal total hysterectomy with salpingo-oophorectomy or salpingectomy for benign conditions.
Observed adverse events included minor blood loss, urinary tract infection and delayed healing of the closure made at the top of the vagina (vaginal cuff) that is done as part of a hysterectomy, according to the FDA. However, all 30 procedures were completed with no need for conversion to an open or other procedure.
Prophylactic NPWT may not improve complication rate after gynecologic surgery
Use of prophylactic negative pressure wound therapy may not be appropriate in surgical cases where women undergo a laparotomy for presumed gynecologic malignancy, according to recent research in Obstetrics & Gynecology.
“The results of our randomized trial do not support the routine use of prophylactic negative pressure wound therapy at the time of laparotomy incision closure in women who are undergoing surgery for gynecologic malignancies or in morbidly obese women who are undergoing laparotomy for benign indications,” Mario M. Leitao Jr., MD, of Memorial Sloan Kettering Cancer Center, New York, and colleagues wrote.
Dr. Leitao and colleagues randomized 663 patients, stratified by body mass index (BMI) after skin closure, to receive negative pressure wound therapy (NPWT) or standard gauze after undergoing a laparotomy for gynecological surgery between March 2016 and August 2019. Patients in the study were aged a median 61 years with a median BMI of 26 kg/m2, but 32 patients with a BMI of 40 or higher who underwent a laparotomy for gynecologic surgery regardless of indication were also included in the study. Most women (80%-82%) were undergoing surgery to treat ovary, fallopian tube, or peritoneal cancer. The most common medical comorbidities in both groups were hypertension (34%-35%) and diabetes (8%-14%). Information on race of patients was not included in the baseline characteristics for the study.
In total, 505 patients were available for evaluation after surgery, which consisted of 254 patients in the NPWT group and 251 patients in the standard gauze group, with 495 patients (98%) having a malignant indication. The researchers examined the incidence of wound complication up to 30 days after surgery.
The results showed a similar rate of wound complications in the NPWT group (44 patients; 17.3%), compared with the group receiving standard gauze (41 patients; 16.3%), with an absolute risk difference between groups of 1% (90% confidence interval, –4.5 to 6.5%; P = .77). Nearly all patients who developed wound complications in both NPWT (92%) and standard gauze (95%) groups had the wound complication diagnosis occur after discharge from the hospital. Dr. Leitao and colleagues noted similarities between groups with regard to wound complications, with most patients having grade 1 complications, and said there were no instances of patients requiring surgery for complications. Among patients in the NPWT group, 33 patients developed skin blistering, compared with 3 patients in the standard gauze group (13% vs. 1.2%; P < .001). After an interim analysis consisting of 444 patients, the study was halted because of “low probability of showing a difference between the two groups at the end of the study.”
The analysis of patients with a BMI of 40 or higher showed 7 of 15 patients (47%) developed wound complications in the NPWT group and 6 of 17 patients (35%) in the standard gauze group (P = .51). In post hoc analyses, the researchers found a median BMI of 26 (range, 17-60) was significantly associated with not developing a wound complication, compared with a BMI of 32 (range, 17-56) (P < .001), and that 41% of patients with a BMI of at least 40 experienced wound complications, compared with 15% of patients with a BMI of less than 40 (P < .001). There was an independent association between developing a wound complication and increasing BMI, according to a multivariate analysis (adjusted odds ratio, 1.10; 95% CI, 1.06-1.14).
Applicability of results unclear for patients with higher BMI
Sarah M. Temkin, MD, a gynecologic oncologist who was not involved with the study, said in an interview that the results by Dr. Leitao and colleagues answer the question of whether patients undergoing surgery for gynecologic malignancy require NPWT, but raised questions about patient selection in the study.
“I think it’s hard to take data from this type of high-end surgical practice and apply it to the general population,” she said, who noted the median BMI of 26 for patients included in the study. A study that included only patients with a BMI of 40 or higher “would have made these results more applicable.”
The low rate of wound complications in the study could potentially be explained by patient selection, Dr. Temkin explained. She cited her own retrospective study from 2016 that showed a wound complication rate of 27.3% for patients receiving prophylactic NPWT where the BMI for the group was 41.29, compared with a complication rate of 19.7% for patients receiving standard care who had a BMI of 30.67.
“It’s hard to cross-trial compare, but that’s significantly higher than what they saw in this prospective study, and I would say that’s a difference with the patient population,” she said. “I think the question of how to reduce surgical-site infections and wound complications in the heavy patient with comorbidities is still unanswered.”
The question is important because patients with a higher BMI and medical comorbidities “still need cancer surgery and methods to reduce the morbidity of that surgery,” Dr. Temkin said. “I think this is an unmet need.”
This study was funded in part by a support grant from the National Institutes of Health/National Cancer Institute Cancer Center, and KCI/Acelity provided part of the study protocol. Nine authors reported personal and institutional relationships in the form of personal fees, grants, stock ownership, consultancies, and speaker’s bureau positions with AstraZeneca, Biom’Up, Bovie Medical, C Surgeries, CMR, ConMed, Covidien, Ethicon, GlaxoSmithKline, GRAIL, Intuitive Surgical, JNJ, Medtronic, Merck, Mylan, Olympus, Stryker/Novadaq, TransEnterix, UpToDate, and Verthermia. Dr. Temkin reported no relevant financial disclosures.
Use of prophylactic negative pressure wound therapy may not be appropriate in surgical cases where women undergo a laparotomy for presumed gynecologic malignancy, according to recent research in Obstetrics & Gynecology.
“The results of our randomized trial do not support the routine use of prophylactic negative pressure wound therapy at the time of laparotomy incision closure in women who are undergoing surgery for gynecologic malignancies or in morbidly obese women who are undergoing laparotomy for benign indications,” Mario M. Leitao Jr., MD, of Memorial Sloan Kettering Cancer Center, New York, and colleagues wrote.
Dr. Leitao and colleagues randomized 663 patients, stratified by body mass index (BMI) after skin closure, to receive negative pressure wound therapy (NPWT) or standard gauze after undergoing a laparotomy for gynecological surgery between March 2016 and August 2019. Patients in the study were aged a median 61 years with a median BMI of 26 kg/m2, but 32 patients with a BMI of 40 or higher who underwent a laparotomy for gynecologic surgery regardless of indication were also included in the study. Most women (80%-82%) were undergoing surgery to treat ovary, fallopian tube, or peritoneal cancer. The most common medical comorbidities in both groups were hypertension (34%-35%) and diabetes (8%-14%). Information on race of patients was not included in the baseline characteristics for the study.
In total, 505 patients were available for evaluation after surgery, which consisted of 254 patients in the NPWT group and 251 patients in the standard gauze group, with 495 patients (98%) having a malignant indication. The researchers examined the incidence of wound complication up to 30 days after surgery.
The results showed a similar rate of wound complications in the NPWT group (44 patients; 17.3%), compared with the group receiving standard gauze (41 patients; 16.3%), with an absolute risk difference between groups of 1% (90% confidence interval, –4.5 to 6.5%; P = .77). Nearly all patients who developed wound complications in both NPWT (92%) and standard gauze (95%) groups had the wound complication diagnosis occur after discharge from the hospital. Dr. Leitao and colleagues noted similarities between groups with regard to wound complications, with most patients having grade 1 complications, and said there were no instances of patients requiring surgery for complications. Among patients in the NPWT group, 33 patients developed skin blistering, compared with 3 patients in the standard gauze group (13% vs. 1.2%; P < .001). After an interim analysis consisting of 444 patients, the study was halted because of “low probability of showing a difference between the two groups at the end of the study.”
The analysis of patients with a BMI of 40 or higher showed 7 of 15 patients (47%) developed wound complications in the NPWT group and 6 of 17 patients (35%) in the standard gauze group (P = .51). In post hoc analyses, the researchers found a median BMI of 26 (range, 17-60) was significantly associated with not developing a wound complication, compared with a BMI of 32 (range, 17-56) (P < .001), and that 41% of patients with a BMI of at least 40 experienced wound complications, compared with 15% of patients with a BMI of less than 40 (P < .001). There was an independent association between developing a wound complication and increasing BMI, according to a multivariate analysis (adjusted odds ratio, 1.10; 95% CI, 1.06-1.14).
Applicability of results unclear for patients with higher BMI
Sarah M. Temkin, MD, a gynecologic oncologist who was not involved with the study, said in an interview that the results by Dr. Leitao and colleagues answer the question of whether patients undergoing surgery for gynecologic malignancy require NPWT, but raised questions about patient selection in the study.
“I think it’s hard to take data from this type of high-end surgical practice and apply it to the general population,” she said, who noted the median BMI of 26 for patients included in the study. A study that included only patients with a BMI of 40 or higher “would have made these results more applicable.”
The low rate of wound complications in the study could potentially be explained by patient selection, Dr. Temkin explained. She cited her own retrospective study from 2016 that showed a wound complication rate of 27.3% for patients receiving prophylactic NPWT where the BMI for the group was 41.29, compared with a complication rate of 19.7% for patients receiving standard care who had a BMI of 30.67.
“It’s hard to cross-trial compare, but that’s significantly higher than what they saw in this prospective study, and I would say that’s a difference with the patient population,” she said. “I think the question of how to reduce surgical-site infections and wound complications in the heavy patient with comorbidities is still unanswered.”
The question is important because patients with a higher BMI and medical comorbidities “still need cancer surgery and methods to reduce the morbidity of that surgery,” Dr. Temkin said. “I think this is an unmet need.”
This study was funded in part by a support grant from the National Institutes of Health/National Cancer Institute Cancer Center, and KCI/Acelity provided part of the study protocol. Nine authors reported personal and institutional relationships in the form of personal fees, grants, stock ownership, consultancies, and speaker’s bureau positions with AstraZeneca, Biom’Up, Bovie Medical, C Surgeries, CMR, ConMed, Covidien, Ethicon, GlaxoSmithKline, GRAIL, Intuitive Surgical, JNJ, Medtronic, Merck, Mylan, Olympus, Stryker/Novadaq, TransEnterix, UpToDate, and Verthermia. Dr. Temkin reported no relevant financial disclosures.
Use of prophylactic negative pressure wound therapy may not be appropriate in surgical cases where women undergo a laparotomy for presumed gynecologic malignancy, according to recent research in Obstetrics & Gynecology.
“The results of our randomized trial do not support the routine use of prophylactic negative pressure wound therapy at the time of laparotomy incision closure in women who are undergoing surgery for gynecologic malignancies or in morbidly obese women who are undergoing laparotomy for benign indications,” Mario M. Leitao Jr., MD, of Memorial Sloan Kettering Cancer Center, New York, and colleagues wrote.
Dr. Leitao and colleagues randomized 663 patients, stratified by body mass index (BMI) after skin closure, to receive negative pressure wound therapy (NPWT) or standard gauze after undergoing a laparotomy for gynecological surgery between March 2016 and August 2019. Patients in the study were aged a median 61 years with a median BMI of 26 kg/m2, but 32 patients with a BMI of 40 or higher who underwent a laparotomy for gynecologic surgery regardless of indication were also included in the study. Most women (80%-82%) were undergoing surgery to treat ovary, fallopian tube, or peritoneal cancer. The most common medical comorbidities in both groups were hypertension (34%-35%) and diabetes (8%-14%). Information on race of patients was not included in the baseline characteristics for the study.
In total, 505 patients were available for evaluation after surgery, which consisted of 254 patients in the NPWT group and 251 patients in the standard gauze group, with 495 patients (98%) having a malignant indication. The researchers examined the incidence of wound complication up to 30 days after surgery.
The results showed a similar rate of wound complications in the NPWT group (44 patients; 17.3%), compared with the group receiving standard gauze (41 patients; 16.3%), with an absolute risk difference between groups of 1% (90% confidence interval, –4.5 to 6.5%; P = .77). Nearly all patients who developed wound complications in both NPWT (92%) and standard gauze (95%) groups had the wound complication diagnosis occur after discharge from the hospital. Dr. Leitao and colleagues noted similarities between groups with regard to wound complications, with most patients having grade 1 complications, and said there were no instances of patients requiring surgery for complications. Among patients in the NPWT group, 33 patients developed skin blistering, compared with 3 patients in the standard gauze group (13% vs. 1.2%; P < .001). After an interim analysis consisting of 444 patients, the study was halted because of “low probability of showing a difference between the two groups at the end of the study.”
The analysis of patients with a BMI of 40 or higher showed 7 of 15 patients (47%) developed wound complications in the NPWT group and 6 of 17 patients (35%) in the standard gauze group (P = .51). In post hoc analyses, the researchers found a median BMI of 26 (range, 17-60) was significantly associated with not developing a wound complication, compared with a BMI of 32 (range, 17-56) (P < .001), and that 41% of patients with a BMI of at least 40 experienced wound complications, compared with 15% of patients with a BMI of less than 40 (P < .001). There was an independent association between developing a wound complication and increasing BMI, according to a multivariate analysis (adjusted odds ratio, 1.10; 95% CI, 1.06-1.14).
Applicability of results unclear for patients with higher BMI
Sarah M. Temkin, MD, a gynecologic oncologist who was not involved with the study, said in an interview that the results by Dr. Leitao and colleagues answer the question of whether patients undergoing surgery for gynecologic malignancy require NPWT, but raised questions about patient selection in the study.
“I think it’s hard to take data from this type of high-end surgical practice and apply it to the general population,” she said, who noted the median BMI of 26 for patients included in the study. A study that included only patients with a BMI of 40 or higher “would have made these results more applicable.”
The low rate of wound complications in the study could potentially be explained by patient selection, Dr. Temkin explained. She cited her own retrospective study from 2016 that showed a wound complication rate of 27.3% for patients receiving prophylactic NPWT where the BMI for the group was 41.29, compared with a complication rate of 19.7% for patients receiving standard care who had a BMI of 30.67.
“It’s hard to cross-trial compare, but that’s significantly higher than what they saw in this prospective study, and I would say that’s a difference with the patient population,” she said. “I think the question of how to reduce surgical-site infections and wound complications in the heavy patient with comorbidities is still unanswered.”
The question is important because patients with a higher BMI and medical comorbidities “still need cancer surgery and methods to reduce the morbidity of that surgery,” Dr. Temkin said. “I think this is an unmet need.”
This study was funded in part by a support grant from the National Institutes of Health/National Cancer Institute Cancer Center, and KCI/Acelity provided part of the study protocol. Nine authors reported personal and institutional relationships in the form of personal fees, grants, stock ownership, consultancies, and speaker’s bureau positions with AstraZeneca, Biom’Up, Bovie Medical, C Surgeries, CMR, ConMed, Covidien, Ethicon, GlaxoSmithKline, GRAIL, Intuitive Surgical, JNJ, Medtronic, Merck, Mylan, Olympus, Stryker/Novadaq, TransEnterix, UpToDate, and Verthermia. Dr. Temkin reported no relevant financial disclosures.
FROM OBSTETRICS & GYNECOLOGY
Prophylactic NPWT may not improve complication rate after gynecologic surgery
Use of prophylactic negative pressure wound therapy may not be appropriate in surgical cases where women undergo a laparotomy for presumed gynecologic malignancy, according to recent research published in Obstetrics & Gynecology.
“The results of our randomized trial do not support the routine use of prophylactic negative pressure wound therapy at the time of laparotomy incision closure in women who are undergoing surgery for gynecologic malignancies or in morbidly obese women who are undergoing laparotomy for benign indications,” wrote Mario M. Leitao Jr., MD, of Memorial Sloan Kettering Cancer Center in New York, and colleagues.
Dr. Leitao and colleagues randomized 663 patients, stratified by body mass index after skin closure, to receive negative pressure wound therapy (NPWT) or standard gauze after undergoing a laparotomy for gynecologic surgery between March 2016 and August 2019.
The median age of the patients was 61 years and median BMI was 26 kg/m2. Thirty-two patients with a BMI of 40 kg/m2 or higher who underwent a laparotomy for gynecologic surgery regardless of indication were also included in the study. Most women (80%-82%) were undergoing surgery to treat ovary, fallopian tube, or peritoneal cancer. The most common medical comorbidities in both groups were hypertension (34%-35%) and diabetes (8%-14%). Information on race of patients was not included in the baseline characteristics for the study.
In total, 505 patients were available for evaluation after surgery, which consisted of 254 patients in the NPWT group and 251 patients in the standard gauze group, with 495 patients (98%) having a malignant indication. The researchers examined the incidence of wound complication up to 30 days after surgery.
The results showed a similar rate of wound complications in the NPWT group (44 patients; 17.3%) compared with the group receiving standard gauze (41 patients; 16.3%), with an absolute risk difference between groups of 1% (90% confidence interval, –4.5-6.5%; P = .77). Nearly all patients who developed wound complications in both NPWT (92%) and standard gauze (95%) groups had the wound complication diagnosis occur after discharge from the hospital. Dr. Leitao and colleagues noted similarities between groups with regard to wound complications, with most patients having grade 1 complications, and said there were no instances of patients requiring surgery for complications. Among patients in the NPWT group, 33 patients developed skin blistering compared with 3 patients in the standard gauze group (13% vs. 1.2%; P < .001). After an interim analysis consisting of 444 patients, the study was halted because of “low probability of showing a difference between the two groups at the end of the study.”
The analysis of patients with a BMI of 40 kg/m2 or higher showed 7 of 15 patients (47%) developed wound complications in the NPWT group and 6 of 17 patients (35%) did so in the standard gauze group (P = .51). In post hoc analyses, the researchers found a median BMI of 26 kg/m2 (range, 17-60 kg/m2) was significantly associated with not developing a wound complication compared with a BMI of 32 kg/m2 (range, 17-56 kg/m2) (P < .001), and that 41% of patients with a BMI of at least 40 kg/m2 experienced wound complications compared with 15% of patients with a BMI of less than 40 kg/m2 (P < .001). There was an independent association between developing a wound complication and increasing BMI, according to a multivariate analysis (adjusted odds ratio, 1.10; 95% confidence interval, 1.06–1.14).
Applicability of results unclear for patients with higher BMI
Sarah M. Temkin, MD, a gynecologic oncologist who was not involved with the study, said in an interview that the results by Leitao and colleagues answer the question of whether patients undergoing surgery for gynecologic malignancy require NPWT, but raised questions about patient selection in the study.
“I think it’s hard to take data from this type of high-end surgical practice and apply it to the general population,” she said, noting the median BMI of 26 kg/m2 for patients included in the study. A study that included only patients with a BMI of 40 kg/m2 or higher “would have made these results more applicable,” she said.
The low rate of wound complications in the study could be explained by patient selection, Dr. Temkin explained. She cited her own retrospective study from 2016 that showed a wound complication rate of 27.3% for patients receiving prophylactic NPWT where the BMI for the group was 41.29 kg/m2 compared with a complication rate of 19.7% for patients receiving standard care who had a BMI of 30.67 kg/m2.
“It’s hard to cross trial compare, but that’s significantly higher than what they saw in this prospective study, and I would say that’s a difference with the patient population,” she said. “I think the question of how to reduce surgical site infections and wound complications in the heavy patient with comorbidities is still unanswered.”
The question is important because patients with a higher BMI and medical comorbidities “still need cancer surgery and methods to reduce the morbidity of that surgery,” Dr. Temkin said. “I think this is an unmet need.”
This study was funded in part by a support grant from NIH/NCI Cancer Center, and KCI/Acelity provided part of the study protocol. Nine authors reported personal and institutional relationships in the form of personal fees, grants, stock ownership, consultancies, and speakers bureau positions with AstraZeneca, Biom’Up, Bovie Medical Co., C Surgeries, CMR, ConMed, Covidien, Ethicon, GlaxoSmithKline, GRAIL, Intuitive Surgical Inc., JNJ, Medtronic, Merck, Mylan, Olympus, Stryker/Novadaq, TransEnterix Inc., UpToDate, and Verthermia Inc. Dr. Temkin reported no relevant financial disclosures.
Use of prophylactic negative pressure wound therapy may not be appropriate in surgical cases where women undergo a laparotomy for presumed gynecologic malignancy, according to recent research published in Obstetrics & Gynecology.
“The results of our randomized trial do not support the routine use of prophylactic negative pressure wound therapy at the time of laparotomy incision closure in women who are undergoing surgery for gynecologic malignancies or in morbidly obese women who are undergoing laparotomy for benign indications,” wrote Mario M. Leitao Jr., MD, of Memorial Sloan Kettering Cancer Center in New York, and colleagues.
Dr. Leitao and colleagues randomized 663 patients, stratified by body mass index after skin closure, to receive negative pressure wound therapy (NPWT) or standard gauze after undergoing a laparotomy for gynecologic surgery between March 2016 and August 2019.
The median age of the patients was 61 years and median BMI was 26 kg/m2. Thirty-two patients with a BMI of 40 kg/m2 or higher who underwent a laparotomy for gynecologic surgery regardless of indication were also included in the study. Most women (80%-82%) were undergoing surgery to treat ovary, fallopian tube, or peritoneal cancer. The most common medical comorbidities in both groups were hypertension (34%-35%) and diabetes (8%-14%). Information on race of patients was not included in the baseline characteristics for the study.
In total, 505 patients were available for evaluation after surgery, which consisted of 254 patients in the NPWT group and 251 patients in the standard gauze group, with 495 patients (98%) having a malignant indication. The researchers examined the incidence of wound complication up to 30 days after surgery.
The results showed a similar rate of wound complications in the NPWT group (44 patients; 17.3%) compared with the group receiving standard gauze (41 patients; 16.3%), with an absolute risk difference between groups of 1% (90% confidence interval, –4.5-6.5%; P = .77). Nearly all patients who developed wound complications in both NPWT (92%) and standard gauze (95%) groups had the wound complication diagnosis occur after discharge from the hospital. Dr. Leitao and colleagues noted similarities between groups with regard to wound complications, with most patients having grade 1 complications, and said there were no instances of patients requiring surgery for complications. Among patients in the NPWT group, 33 patients developed skin blistering compared with 3 patients in the standard gauze group (13% vs. 1.2%; P < .001). After an interim analysis consisting of 444 patients, the study was halted because of “low probability of showing a difference between the two groups at the end of the study.”
The analysis of patients with a BMI of 40 kg/m2 or higher showed 7 of 15 patients (47%) developed wound complications in the NPWT group and 6 of 17 patients (35%) did so in the standard gauze group (P = .51). In post hoc analyses, the researchers found a median BMI of 26 kg/m2 (range, 17-60 kg/m2) was significantly associated with not developing a wound complication compared with a BMI of 32 kg/m2 (range, 17-56 kg/m2) (P < .001), and that 41% of patients with a BMI of at least 40 kg/m2 experienced wound complications compared with 15% of patients with a BMI of less than 40 kg/m2 (P < .001). There was an independent association between developing a wound complication and increasing BMI, according to a multivariate analysis (adjusted odds ratio, 1.10; 95% confidence interval, 1.06–1.14).
Applicability of results unclear for patients with higher BMI
Sarah M. Temkin, MD, a gynecologic oncologist who was not involved with the study, said in an interview that the results by Leitao and colleagues answer the question of whether patients undergoing surgery for gynecologic malignancy require NPWT, but raised questions about patient selection in the study.
“I think it’s hard to take data from this type of high-end surgical practice and apply it to the general population,” she said, noting the median BMI of 26 kg/m2 for patients included in the study. A study that included only patients with a BMI of 40 kg/m2 or higher “would have made these results more applicable,” she said.
The low rate of wound complications in the study could be explained by patient selection, Dr. Temkin explained. She cited her own retrospective study from 2016 that showed a wound complication rate of 27.3% for patients receiving prophylactic NPWT where the BMI for the group was 41.29 kg/m2 compared with a complication rate of 19.7% for patients receiving standard care who had a BMI of 30.67 kg/m2.
“It’s hard to cross trial compare, but that’s significantly higher than what they saw in this prospective study, and I would say that’s a difference with the patient population,” she said. “I think the question of how to reduce surgical site infections and wound complications in the heavy patient with comorbidities is still unanswered.”
The question is important because patients with a higher BMI and medical comorbidities “still need cancer surgery and methods to reduce the morbidity of that surgery,” Dr. Temkin said. “I think this is an unmet need.”
This study was funded in part by a support grant from NIH/NCI Cancer Center, and KCI/Acelity provided part of the study protocol. Nine authors reported personal and institutional relationships in the form of personal fees, grants, stock ownership, consultancies, and speakers bureau positions with AstraZeneca, Biom’Up, Bovie Medical Co., C Surgeries, CMR, ConMed, Covidien, Ethicon, GlaxoSmithKline, GRAIL, Intuitive Surgical Inc., JNJ, Medtronic, Merck, Mylan, Olympus, Stryker/Novadaq, TransEnterix Inc., UpToDate, and Verthermia Inc. Dr. Temkin reported no relevant financial disclosures.
Use of prophylactic negative pressure wound therapy may not be appropriate in surgical cases where women undergo a laparotomy for presumed gynecologic malignancy, according to recent research published in Obstetrics & Gynecology.
“The results of our randomized trial do not support the routine use of prophylactic negative pressure wound therapy at the time of laparotomy incision closure in women who are undergoing surgery for gynecologic malignancies or in morbidly obese women who are undergoing laparotomy for benign indications,” wrote Mario M. Leitao Jr., MD, of Memorial Sloan Kettering Cancer Center in New York, and colleagues.
Dr. Leitao and colleagues randomized 663 patients, stratified by body mass index after skin closure, to receive negative pressure wound therapy (NPWT) or standard gauze after undergoing a laparotomy for gynecologic surgery between March 2016 and August 2019.
The median age of the patients was 61 years and median BMI was 26 kg/m2. Thirty-two patients with a BMI of 40 kg/m2 or higher who underwent a laparotomy for gynecologic surgery regardless of indication were also included in the study. Most women (80%-82%) were undergoing surgery to treat ovary, fallopian tube, or peritoneal cancer. The most common medical comorbidities in both groups were hypertension (34%-35%) and diabetes (8%-14%). Information on race of patients was not included in the baseline characteristics for the study.
In total, 505 patients were available for evaluation after surgery, which consisted of 254 patients in the NPWT group and 251 patients in the standard gauze group, with 495 patients (98%) having a malignant indication. The researchers examined the incidence of wound complication up to 30 days after surgery.
The results showed a similar rate of wound complications in the NPWT group (44 patients; 17.3%) compared with the group receiving standard gauze (41 patients; 16.3%), with an absolute risk difference between groups of 1% (90% confidence interval, –4.5-6.5%; P = .77). Nearly all patients who developed wound complications in both NPWT (92%) and standard gauze (95%) groups had the wound complication diagnosis occur after discharge from the hospital. Dr. Leitao and colleagues noted similarities between groups with regard to wound complications, with most patients having grade 1 complications, and said there were no instances of patients requiring surgery for complications. Among patients in the NPWT group, 33 patients developed skin blistering compared with 3 patients in the standard gauze group (13% vs. 1.2%; P < .001). After an interim analysis consisting of 444 patients, the study was halted because of “low probability of showing a difference between the two groups at the end of the study.”
The analysis of patients with a BMI of 40 kg/m2 or higher showed 7 of 15 patients (47%) developed wound complications in the NPWT group and 6 of 17 patients (35%) did so in the standard gauze group (P = .51). In post hoc analyses, the researchers found a median BMI of 26 kg/m2 (range, 17-60 kg/m2) was significantly associated with not developing a wound complication compared with a BMI of 32 kg/m2 (range, 17-56 kg/m2) (P < .001), and that 41% of patients with a BMI of at least 40 kg/m2 experienced wound complications compared with 15% of patients with a BMI of less than 40 kg/m2 (P < .001). There was an independent association between developing a wound complication and increasing BMI, according to a multivariate analysis (adjusted odds ratio, 1.10; 95% confidence interval, 1.06–1.14).
Applicability of results unclear for patients with higher BMI
Sarah M. Temkin, MD, a gynecologic oncologist who was not involved with the study, said in an interview that the results by Leitao and colleagues answer the question of whether patients undergoing surgery for gynecologic malignancy require NPWT, but raised questions about patient selection in the study.
“I think it’s hard to take data from this type of high-end surgical practice and apply it to the general population,” she said, noting the median BMI of 26 kg/m2 for patients included in the study. A study that included only patients with a BMI of 40 kg/m2 or higher “would have made these results more applicable,” she said.
The low rate of wound complications in the study could be explained by patient selection, Dr. Temkin explained. She cited her own retrospective study from 2016 that showed a wound complication rate of 27.3% for patients receiving prophylactic NPWT where the BMI for the group was 41.29 kg/m2 compared with a complication rate of 19.7% for patients receiving standard care who had a BMI of 30.67 kg/m2.
“It’s hard to cross trial compare, but that’s significantly higher than what they saw in this prospective study, and I would say that’s a difference with the patient population,” she said. “I think the question of how to reduce surgical site infections and wound complications in the heavy patient with comorbidities is still unanswered.”
The question is important because patients with a higher BMI and medical comorbidities “still need cancer surgery and methods to reduce the morbidity of that surgery,” Dr. Temkin said. “I think this is an unmet need.”
This study was funded in part by a support grant from NIH/NCI Cancer Center, and KCI/Acelity provided part of the study protocol. Nine authors reported personal and institutional relationships in the form of personal fees, grants, stock ownership, consultancies, and speakers bureau positions with AstraZeneca, Biom’Up, Bovie Medical Co., C Surgeries, CMR, ConMed, Covidien, Ethicon, GlaxoSmithKline, GRAIL, Intuitive Surgical Inc., JNJ, Medtronic, Merck, Mylan, Olympus, Stryker/Novadaq, TransEnterix Inc., UpToDate, and Verthermia Inc. Dr. Temkin reported no relevant financial disclosures.
FROM OBSTETRICS & GYNECOLOGY
Cesarean myomectomy: Safe operation or surgical folly?

Uterine leiomyomata (fibroids) are the most common pelvic tumor of women. When women are planning to conceive, and their fibroid(s) are clinically significant, causing abnormal uterine bleeding or bulk symptoms, it is often optimal to remove the uterine tumor(s) before conception. Advances in minimally invasive surgery offer women the option of laparoscopic or robot-assisted myomectomy with a low rate of operative complications, including excessive blood loss and hysterectomy, and a low rate of postoperative complications, including major pelvic adhesions and uterine rupture during subsequent pregnancy.1-3 However, many women become pregnant when they have clinically significant fibroids, and at least one-third of these women will have a cesarean birth.
Important clinical issues are the relative benefits and risks of performing a myomectomy at the time of the cesarean birth, so called cesarean myomectomy. Cesarean myomectomy offers carefully selected women the opportunity to have a cesarean birth and myomectomy in one operation, thereby avoiding a second major operation. Over the past 6 decades, most experts in the United States and the United Kingdom have strongly recommended against myomectomy at the time of cesarean delivery because of the risk of excessive blood loss and hysterectomy. Recently, expert opinion has shifted, especially in continental Europe and Asia, and cesarean myomectomy is now viewed as an acceptable surgical option in a limited number of clinical situations, including removal of pedunculated fibroids, excision of large solitary subserosal fibroids, and to achieve optimal management of the hysterotomy incision.
Decades of expert guidance: Avoid cesarean myomectomy at all costs
Dr. K.S.J. Olah succinctly captured the standard teaching that cesarean myomectomy should be avoided in this personal vignette:
Many years ago as a trainee I removed a subserosal fibroid during a cesarean section that was hanging by a thin stalk on the back of the uterus. The berating I received was severe and disproportionate to the crime. The rule was that myomectomy performed at cesarean section was not just frowned upon but expressly forbidden. It has always been considered foolish to consider removing fibroids at cesarean section, mostly because of the associated morbidity and the risk of haemorrhage requiring hysterectomy.4
Dr. Olah quoted guidance from Shaw’s Textbook of Operative Gynaecology,5 “It should be stressed that myomectomy in pregnancy should be avoided at all costs, including at caesarean section.” However, large case series published over the past 10 years report that, in limited clinical situations, cesarean myomectomy is a viable surgical option, where benefit may outweigh risk.6-14 The current literature has many weaknesses, including failure to specifically identify the indication for the cesarean myomectomy and lack of controlled prospective clinical trials. In almost all cases, cesarean myomectomy is performed after delivery of the fetus and placenta.
Continue to: The pedunculated, FIGO type 7 fibroid...
The pedunculated, FIGO type 7 fibroid
The International Federation of Gynecology and Obstetrics (FIGO) leiomyoma classification system identifies subserosal pedunculated fibroids as type 7 (FIGURE).15 Pedunculated fibroids are attached to the uterus by a stalk that is ≤10% of the mean of the 3 diameters of the fibroid. When a clinically significant pedunculated fibroid, causing bulk symptoms, is encountered at cesarean birth, I recommend that it be removed. This will save many patients a second major operation to perform a myomectomy. The surgical risk of removing a pedunculated is low.

The solitary FIGO type 6 fibroid
Type 6 fibroids are subserosal fibroids with less than 50% of their mass being subserosal. The type 6 fibroid is relatively easy to enucleate from the uterus. Following removal of a type 6 fibroid, closure of the serosal defect is relatively straightforward. In carefully selected cases, if the type 6 fibroid is causing bulk symptoms, cesarean myomectomy may be indicated with a low risk of operative complications.
The FIGO type 2-5 fibroid
The type 2-5 fibroid is a transmural fibroid with significant mass abutting both the endometrial cavity and serosal surface. Excision of a type 2-5 fibroid is likely to result in a large transmyometrial defect that will be more difficult to close and could be associated with greater blood loss. Although data are limited, I would recommend against cesarean myomectomy for type 2-5 fibroids in most clinical situations.
Myomectomy to achieve optimal management of the cesarean hysterotomy incision
Many surgeons performing a cesarean birth for a woman with clinically significant fibroids will plan the hysterotomy incision to avoid the fibroids. However, following delivery and contraction of the uterus, proper closure of the hysterotomy incision may be very difficult without removing a fibroid that is abutting the hysterotomy incision. Surgeons have reported performing myomectomy on lower uterine segment fibroids before making the hysterotomy incision in order to facilitate the hysterotomy incision and closure.16 Myomectomy prior to delivery of the newborn must be associated with additional risks to the fetus. I would prefer to identify an optimal site to perform a hysterotomy, deliver the newborn and placenta, and then consider myomectomy.
Complications associated with cesarean myomectomy
The evidence concerning the complications of cesarean birth plus myomectomy compared with cesarean birth alone in women with fibroids is limited to case series. There are no reported controlled clinical trials to guide practice. The largest single case series reported on 1,242 women with fibroids who had a cesarean birth plus myomectomy compared with 3 control groups, including 200 women without fibroids who had a cesarean birth, 145 women with fibroids who had a cesarean birth and no myomectomy, and 51 women with fibroids who had a cesarean hysterectomy. The investigators reported no significant differences in preoperative to postoperative hemoglobin change, incidence of postoperative fever, or length of hospital stay among the 4 groups.8 The authors concluded that myomectomy during cesarean birth was a safe and effective procedure.
Continue to: A systematic review and meta-analysis reported...
A systematic review and meta-analysis reported on the results of 17 studies which included 4,702 women who had a cesarean myomectomy and 1,843 women with cesarean birth without myomectomy.17 The authors of the meta-analysis noted that most reported case series had excluded women with a high risk of bleeding, including women with placenta previa, placenta accreta, coagulation disorders, and a history of multiple myomectomy operations. The investigators reported that, compared with the control women, the women undergoing cesarean myomectomy had a statistically significant but clinically insignificant decrease in mean hemoglobin concentration (-0.27 g/dL), a significant increase in mean operative time (+15 minutes) and a significant increase in the length of hospital stay (+0.36 days). There was an increase in the need for blood transfusion (risk ratio, 1.45; 95% confidence interval, 1.05–1.99), but only 3% of women undergoing cesarean myomectomy received a blood transfusion. There was no significant difference between the two groups in the incidence of postoperative fever. The authors concluded that cesarean myomectomy is a safe procedure when performed by experienced surgeons with appropriate hemostatic techniques.
Techniques to reduce blood loss at the time of cesarean myomectomy
A detailed review of all the available techniques to reduce blood loss at the time of cesarean myomectomy is beyond the scope of this editorial. All gynecologists know that control of uterine blood flow through the uterine artery, infundibulopelvic vessels and internal iliac artery can help to reduce bleeding at the time of myomectomy. Tourniquets, vascular clamps, and artery ligation all have been reported to be useful at the time of cesarean myomectomy. In addition, intravenous infusion of oxytocin and tranexamic acid is often used at the time of cesarean myomectomy. Direct injection of uterotonics, including carbetocin, oxytocin, and vasopressin, into the uterus also has been reported. Cell saver blood salvage technology has been utilized in a limited number of cases of cesarean myomectomy.8,18,19
Medicine is not a static field
Discoveries and new data help guide advances in medical practice. After 6 decades of strict adherence to the advice that myomectomy in pregnancy should be avoided at all costs, including at caesarean delivery, new data indicate that in carefully selected cases cesarean myomectomy is an acceptable operation. ●
- Pitter MC, Gargiulo AR, Bonaventura LM, et al. Pregnancy outcomes following robot-assisted myomectomy. Hum Reprod. 2013;28:99-108.
- Pitter MC, Srouji SS, Gargiulo AR, et al. Fertility and symptom relief following robot-assisted laparoscopic myomectomy. Obstet Gynecol Int. 2015;2015:967568.
- Huberlant S, Lenot J, Neron M, et al. Fertility and obstetric outcomes after robot-assisted laparoscopic myomectomy. Int J Med Robot. 2020;16:e2059.
- Olah KSJ. Caesarean myomectomy: TE or not TE? BJOG. 2018;125:501.
- Shaw, et al. Textbook of Operative Gynaecology. Edinburgh: Churchill Livingston; 1977.
- Burton CA, Grimes DA, March CM. Surgical management of leiomyomata during pregnancy. Obstet Gynecol. 1989;74:707-709.
- Ortac F, Gungor M, Sonmezer M. Myomectomy during cesarean section. Int J Gynaecol Obstet. 1999;67:189-193.
- Li H, Du J, Jin L, et al. Myomectomy during cesarean section. Acta Obstetricia et Gynecologica. 2009;88:183-186.
- Kwon DH, Song JE, Yoon KR, et al. Obstet Gynecol Sci. 2014;57:367-372.
- Senturk MB, Polat M, Dogan O, et al. Outcome of cesarean myomectomy: is it a safe procedure? Geburtshilfe Frauenheilkd. 2017;77:1200-1206.
- Chauhan AR. Cesarean myomectomy: necessity or opportunity? J Obstet Gynecol India. 2018;68:432-436.
- Sparic R, Kadija S, Stefanovic A, et al. Cesarean myomectomy in modern obstetrics: more light and fewer shadows. J Obstet Gynaecol Res. 2017;43:798-804.
- Ramya T, Sabnis SS, Chitra TV, et al. Cesarean myomectomy: an experience from a tertiary care teaching hospital. J Obstet Gynaecol India. 2019;69:426-430.
- Zhao R, Wang X, Zou L, et al. Outcomes of myomectomy at the time of cesarean section among pregnant women with uterine fibroids: a retrospective cohort study. Biomed Res Int. 2019;7576934.
- Munro MG, Critchley HOD, Fraser IS; FIGO Menstrual Disorders Committee. The two FIGO systems for normal and abnormal uterine bleeding symptoms and classification of causes of abnormal uterine bleeding in the reproductive years: 2018 revisions. In J Gynaecol Obstet. 2018;143:393.
- Omar SZ, Sivanesaratnam V, Damodaran P. Large lower segment myoma—myomectomy at lower segment caesarean section—a report of two cases. Singapore Med J. 1999;40:109-110.
- Goyal M, Dawood AS, Elbohoty SB, et al. Cesarean myomectomy in the last ten years; A true shift from contraindication to indication: a systematic review and meta-analysis. Eur J Obstet Gynecol Reprod Biol. 2021;256:145-157.
- Lin JY, Lee WL, Wang PH, et al. Uterine artery occlusion and myomectomy for treatment of pregnant women with uterine leiomyomas who are undergoing caesarean section. J Obstet Gynecol Res. 2010;36:284-290.
- Alfred E, Joy G, Uduak O, et al. Cesarean myomectomy outcome in a Nigerian hospital district hospital. J Basic Clin Reprod Sci. 2013;2:115-118.

Uterine leiomyomata (fibroids) are the most common pelvic tumor of women. When women are planning to conceive, and their fibroid(s) are clinically significant, causing abnormal uterine bleeding or bulk symptoms, it is often optimal to remove the uterine tumor(s) before conception. Advances in minimally invasive surgery offer women the option of laparoscopic or robot-assisted myomectomy with a low rate of operative complications, including excessive blood loss and hysterectomy, and a low rate of postoperative complications, including major pelvic adhesions and uterine rupture during subsequent pregnancy.1-3 However, many women become pregnant when they have clinically significant fibroids, and at least one-third of these women will have a cesarean birth.
Important clinical issues are the relative benefits and risks of performing a myomectomy at the time of the cesarean birth, so called cesarean myomectomy. Cesarean myomectomy offers carefully selected women the opportunity to have a cesarean birth and myomectomy in one operation, thereby avoiding a second major operation. Over the past 6 decades, most experts in the United States and the United Kingdom have strongly recommended against myomectomy at the time of cesarean delivery because of the risk of excessive blood loss and hysterectomy. Recently, expert opinion has shifted, especially in continental Europe and Asia, and cesarean myomectomy is now viewed as an acceptable surgical option in a limited number of clinical situations, including removal of pedunculated fibroids, excision of large solitary subserosal fibroids, and to achieve optimal management of the hysterotomy incision.
Decades of expert guidance: Avoid cesarean myomectomy at all costs
Dr. K.S.J. Olah succinctly captured the standard teaching that cesarean myomectomy should be avoided in this personal vignette:
Many years ago as a trainee I removed a subserosal fibroid during a cesarean section that was hanging by a thin stalk on the back of the uterus. The berating I received was severe and disproportionate to the crime. The rule was that myomectomy performed at cesarean section was not just frowned upon but expressly forbidden. It has always been considered foolish to consider removing fibroids at cesarean section, mostly because of the associated morbidity and the risk of haemorrhage requiring hysterectomy.4
Dr. Olah quoted guidance from Shaw’s Textbook of Operative Gynaecology,5 “It should be stressed that myomectomy in pregnancy should be avoided at all costs, including at caesarean section.” However, large case series published over the past 10 years report that, in limited clinical situations, cesarean myomectomy is a viable surgical option, where benefit may outweigh risk.6-14 The current literature has many weaknesses, including failure to specifically identify the indication for the cesarean myomectomy and lack of controlled prospective clinical trials. In almost all cases, cesarean myomectomy is performed after delivery of the fetus and placenta.
Continue to: The pedunculated, FIGO type 7 fibroid...
The pedunculated, FIGO type 7 fibroid
The International Federation of Gynecology and Obstetrics (FIGO) leiomyoma classification system identifies subserosal pedunculated fibroids as type 7 (FIGURE).15 Pedunculated fibroids are attached to the uterus by a stalk that is ≤10% of the mean of the 3 diameters of the fibroid. When a clinically significant pedunculated fibroid, causing bulk symptoms, is encountered at cesarean birth, I recommend that it be removed. This will save many patients a second major operation to perform a myomectomy. The surgical risk of removing a pedunculated is low.

The solitary FIGO type 6 fibroid
Type 6 fibroids are subserosal fibroids with less than 50% of their mass being subserosal. The type 6 fibroid is relatively easy to enucleate from the uterus. Following removal of a type 6 fibroid, closure of the serosal defect is relatively straightforward. In carefully selected cases, if the type 6 fibroid is causing bulk symptoms, cesarean myomectomy may be indicated with a low risk of operative complications.
The FIGO type 2-5 fibroid
The type 2-5 fibroid is a transmural fibroid with significant mass abutting both the endometrial cavity and serosal surface. Excision of a type 2-5 fibroid is likely to result in a large transmyometrial defect that will be more difficult to close and could be associated with greater blood loss. Although data are limited, I would recommend against cesarean myomectomy for type 2-5 fibroids in most clinical situations.
Myomectomy to achieve optimal management of the cesarean hysterotomy incision
Many surgeons performing a cesarean birth for a woman with clinically significant fibroids will plan the hysterotomy incision to avoid the fibroids. However, following delivery and contraction of the uterus, proper closure of the hysterotomy incision may be very difficult without removing a fibroid that is abutting the hysterotomy incision. Surgeons have reported performing myomectomy on lower uterine segment fibroids before making the hysterotomy incision in order to facilitate the hysterotomy incision and closure.16 Myomectomy prior to delivery of the newborn must be associated with additional risks to the fetus. I would prefer to identify an optimal site to perform a hysterotomy, deliver the newborn and placenta, and then consider myomectomy.
Complications associated with cesarean myomectomy
The evidence concerning the complications of cesarean birth plus myomectomy compared with cesarean birth alone in women with fibroids is limited to case series. There are no reported controlled clinical trials to guide practice. The largest single case series reported on 1,242 women with fibroids who had a cesarean birth plus myomectomy compared with 3 control groups, including 200 women without fibroids who had a cesarean birth, 145 women with fibroids who had a cesarean birth and no myomectomy, and 51 women with fibroids who had a cesarean hysterectomy. The investigators reported no significant differences in preoperative to postoperative hemoglobin change, incidence of postoperative fever, or length of hospital stay among the 4 groups.8 The authors concluded that myomectomy during cesarean birth was a safe and effective procedure.
Continue to: A systematic review and meta-analysis reported...
A systematic review and meta-analysis reported on the results of 17 studies which included 4,702 women who had a cesarean myomectomy and 1,843 women with cesarean birth without myomectomy.17 The authors of the meta-analysis noted that most reported case series had excluded women with a high risk of bleeding, including women with placenta previa, placenta accreta, coagulation disorders, and a history of multiple myomectomy operations. The investigators reported that, compared with the control women, the women undergoing cesarean myomectomy had a statistically significant but clinically insignificant decrease in mean hemoglobin concentration (-0.27 g/dL), a significant increase in mean operative time (+15 minutes) and a significant increase in the length of hospital stay (+0.36 days). There was an increase in the need for blood transfusion (risk ratio, 1.45; 95% confidence interval, 1.05–1.99), but only 3% of women undergoing cesarean myomectomy received a blood transfusion. There was no significant difference between the two groups in the incidence of postoperative fever. The authors concluded that cesarean myomectomy is a safe procedure when performed by experienced surgeons with appropriate hemostatic techniques.
Techniques to reduce blood loss at the time of cesarean myomectomy
A detailed review of all the available techniques to reduce blood loss at the time of cesarean myomectomy is beyond the scope of this editorial. All gynecologists know that control of uterine blood flow through the uterine artery, infundibulopelvic vessels and internal iliac artery can help to reduce bleeding at the time of myomectomy. Tourniquets, vascular clamps, and artery ligation all have been reported to be useful at the time of cesarean myomectomy. In addition, intravenous infusion of oxytocin and tranexamic acid is often used at the time of cesarean myomectomy. Direct injection of uterotonics, including carbetocin, oxytocin, and vasopressin, into the uterus also has been reported. Cell saver blood salvage technology has been utilized in a limited number of cases of cesarean myomectomy.8,18,19
Medicine is not a static field
Discoveries and new data help guide advances in medical practice. After 6 decades of strict adherence to the advice that myomectomy in pregnancy should be avoided at all costs, including at caesarean delivery, new data indicate that in carefully selected cases cesarean myomectomy is an acceptable operation. ●

Uterine leiomyomata (fibroids) are the most common pelvic tumor of women. When women are planning to conceive, and their fibroid(s) are clinically significant, causing abnormal uterine bleeding or bulk symptoms, it is often optimal to remove the uterine tumor(s) before conception. Advances in minimally invasive surgery offer women the option of laparoscopic or robot-assisted myomectomy with a low rate of operative complications, including excessive blood loss and hysterectomy, and a low rate of postoperative complications, including major pelvic adhesions and uterine rupture during subsequent pregnancy.1-3 However, many women become pregnant when they have clinically significant fibroids, and at least one-third of these women will have a cesarean birth.
Important clinical issues are the relative benefits and risks of performing a myomectomy at the time of the cesarean birth, so called cesarean myomectomy. Cesarean myomectomy offers carefully selected women the opportunity to have a cesarean birth and myomectomy in one operation, thereby avoiding a second major operation. Over the past 6 decades, most experts in the United States and the United Kingdom have strongly recommended against myomectomy at the time of cesarean delivery because of the risk of excessive blood loss and hysterectomy. Recently, expert opinion has shifted, especially in continental Europe and Asia, and cesarean myomectomy is now viewed as an acceptable surgical option in a limited number of clinical situations, including removal of pedunculated fibroids, excision of large solitary subserosal fibroids, and to achieve optimal management of the hysterotomy incision.
Decades of expert guidance: Avoid cesarean myomectomy at all costs
Dr. K.S.J. Olah succinctly captured the standard teaching that cesarean myomectomy should be avoided in this personal vignette:
Many years ago as a trainee I removed a subserosal fibroid during a cesarean section that was hanging by a thin stalk on the back of the uterus. The berating I received was severe and disproportionate to the crime. The rule was that myomectomy performed at cesarean section was not just frowned upon but expressly forbidden. It has always been considered foolish to consider removing fibroids at cesarean section, mostly because of the associated morbidity and the risk of haemorrhage requiring hysterectomy.4
Dr. Olah quoted guidance from Shaw’s Textbook of Operative Gynaecology,5 “It should be stressed that myomectomy in pregnancy should be avoided at all costs, including at caesarean section.” However, large case series published over the past 10 years report that, in limited clinical situations, cesarean myomectomy is a viable surgical option, where benefit may outweigh risk.6-14 The current literature has many weaknesses, including failure to specifically identify the indication for the cesarean myomectomy and lack of controlled prospective clinical trials. In almost all cases, cesarean myomectomy is performed after delivery of the fetus and placenta.
Continue to: The pedunculated, FIGO type 7 fibroid...
The pedunculated, FIGO type 7 fibroid
The International Federation of Gynecology and Obstetrics (FIGO) leiomyoma classification system identifies subserosal pedunculated fibroids as type 7 (FIGURE).15 Pedunculated fibroids are attached to the uterus by a stalk that is ≤10% of the mean of the 3 diameters of the fibroid. When a clinically significant pedunculated fibroid, causing bulk symptoms, is encountered at cesarean birth, I recommend that it be removed. This will save many patients a second major operation to perform a myomectomy. The surgical risk of removing a pedunculated is low.

The solitary FIGO type 6 fibroid
Type 6 fibroids are subserosal fibroids with less than 50% of their mass being subserosal. The type 6 fibroid is relatively easy to enucleate from the uterus. Following removal of a type 6 fibroid, closure of the serosal defect is relatively straightforward. In carefully selected cases, if the type 6 fibroid is causing bulk symptoms, cesarean myomectomy may be indicated with a low risk of operative complications.
The FIGO type 2-5 fibroid
The type 2-5 fibroid is a transmural fibroid with significant mass abutting both the endometrial cavity and serosal surface. Excision of a type 2-5 fibroid is likely to result in a large transmyometrial defect that will be more difficult to close and could be associated with greater blood loss. Although data are limited, I would recommend against cesarean myomectomy for type 2-5 fibroids in most clinical situations.
Myomectomy to achieve optimal management of the cesarean hysterotomy incision
Many surgeons performing a cesarean birth for a woman with clinically significant fibroids will plan the hysterotomy incision to avoid the fibroids. However, following delivery and contraction of the uterus, proper closure of the hysterotomy incision may be very difficult without removing a fibroid that is abutting the hysterotomy incision. Surgeons have reported performing myomectomy on lower uterine segment fibroids before making the hysterotomy incision in order to facilitate the hysterotomy incision and closure.16 Myomectomy prior to delivery of the newborn must be associated with additional risks to the fetus. I would prefer to identify an optimal site to perform a hysterotomy, deliver the newborn and placenta, and then consider myomectomy.
Complications associated with cesarean myomectomy
The evidence concerning the complications of cesarean birth plus myomectomy compared with cesarean birth alone in women with fibroids is limited to case series. There are no reported controlled clinical trials to guide practice. The largest single case series reported on 1,242 women with fibroids who had a cesarean birth plus myomectomy compared with 3 control groups, including 200 women without fibroids who had a cesarean birth, 145 women with fibroids who had a cesarean birth and no myomectomy, and 51 women with fibroids who had a cesarean hysterectomy. The investigators reported no significant differences in preoperative to postoperative hemoglobin change, incidence of postoperative fever, or length of hospital stay among the 4 groups.8 The authors concluded that myomectomy during cesarean birth was a safe and effective procedure.
Continue to: A systematic review and meta-analysis reported...
A systematic review and meta-analysis reported on the results of 17 studies which included 4,702 women who had a cesarean myomectomy and 1,843 women with cesarean birth without myomectomy.17 The authors of the meta-analysis noted that most reported case series had excluded women with a high risk of bleeding, including women with placenta previa, placenta accreta, coagulation disorders, and a history of multiple myomectomy operations. The investigators reported that, compared with the control women, the women undergoing cesarean myomectomy had a statistically significant but clinically insignificant decrease in mean hemoglobin concentration (-0.27 g/dL), a significant increase in mean operative time (+15 minutes) and a significant increase in the length of hospital stay (+0.36 days). There was an increase in the need for blood transfusion (risk ratio, 1.45; 95% confidence interval, 1.05–1.99), but only 3% of women undergoing cesarean myomectomy received a blood transfusion. There was no significant difference between the two groups in the incidence of postoperative fever. The authors concluded that cesarean myomectomy is a safe procedure when performed by experienced surgeons with appropriate hemostatic techniques.
Techniques to reduce blood loss at the time of cesarean myomectomy
A detailed review of all the available techniques to reduce blood loss at the time of cesarean myomectomy is beyond the scope of this editorial. All gynecologists know that control of uterine blood flow through the uterine artery, infundibulopelvic vessels and internal iliac artery can help to reduce bleeding at the time of myomectomy. Tourniquets, vascular clamps, and artery ligation all have been reported to be useful at the time of cesarean myomectomy. In addition, intravenous infusion of oxytocin and tranexamic acid is often used at the time of cesarean myomectomy. Direct injection of uterotonics, including carbetocin, oxytocin, and vasopressin, into the uterus also has been reported. Cell saver blood salvage technology has been utilized in a limited number of cases of cesarean myomectomy.8,18,19
Medicine is not a static field
Discoveries and new data help guide advances in medical practice. After 6 decades of strict adherence to the advice that myomectomy in pregnancy should be avoided at all costs, including at caesarean delivery, new data indicate that in carefully selected cases cesarean myomectomy is an acceptable operation. ●
- Pitter MC, Gargiulo AR, Bonaventura LM, et al. Pregnancy outcomes following robot-assisted myomectomy. Hum Reprod. 2013;28:99-108.
- Pitter MC, Srouji SS, Gargiulo AR, et al. Fertility and symptom relief following robot-assisted laparoscopic myomectomy. Obstet Gynecol Int. 2015;2015:967568.
- Huberlant S, Lenot J, Neron M, et al. Fertility and obstetric outcomes after robot-assisted laparoscopic myomectomy. Int J Med Robot. 2020;16:e2059.
- Olah KSJ. Caesarean myomectomy: TE or not TE? BJOG. 2018;125:501.
- Shaw, et al. Textbook of Operative Gynaecology. Edinburgh: Churchill Livingston; 1977.
- Burton CA, Grimes DA, March CM. Surgical management of leiomyomata during pregnancy. Obstet Gynecol. 1989;74:707-709.
- Ortac F, Gungor M, Sonmezer M. Myomectomy during cesarean section. Int J Gynaecol Obstet. 1999;67:189-193.
- Li H, Du J, Jin L, et al. Myomectomy during cesarean section. Acta Obstetricia et Gynecologica. 2009;88:183-186.
- Kwon DH, Song JE, Yoon KR, et al. Obstet Gynecol Sci. 2014;57:367-372.
- Senturk MB, Polat M, Dogan O, et al. Outcome of cesarean myomectomy: is it a safe procedure? Geburtshilfe Frauenheilkd. 2017;77:1200-1206.
- Chauhan AR. Cesarean myomectomy: necessity or opportunity? J Obstet Gynecol India. 2018;68:432-436.
- Sparic R, Kadija S, Stefanovic A, et al. Cesarean myomectomy in modern obstetrics: more light and fewer shadows. J Obstet Gynaecol Res. 2017;43:798-804.
- Ramya T, Sabnis SS, Chitra TV, et al. Cesarean myomectomy: an experience from a tertiary care teaching hospital. J Obstet Gynaecol India. 2019;69:426-430.
- Zhao R, Wang X, Zou L, et al. Outcomes of myomectomy at the time of cesarean section among pregnant women with uterine fibroids: a retrospective cohort study. Biomed Res Int. 2019;7576934.
- Munro MG, Critchley HOD, Fraser IS; FIGO Menstrual Disorders Committee. The two FIGO systems for normal and abnormal uterine bleeding symptoms and classification of causes of abnormal uterine bleeding in the reproductive years: 2018 revisions. In J Gynaecol Obstet. 2018;143:393.
- Omar SZ, Sivanesaratnam V, Damodaran P. Large lower segment myoma—myomectomy at lower segment caesarean section—a report of two cases. Singapore Med J. 1999;40:109-110.
- Goyal M, Dawood AS, Elbohoty SB, et al. Cesarean myomectomy in the last ten years; A true shift from contraindication to indication: a systematic review and meta-analysis. Eur J Obstet Gynecol Reprod Biol. 2021;256:145-157.
- Lin JY, Lee WL, Wang PH, et al. Uterine artery occlusion and myomectomy for treatment of pregnant women with uterine leiomyomas who are undergoing caesarean section. J Obstet Gynecol Res. 2010;36:284-290.
- Alfred E, Joy G, Uduak O, et al. Cesarean myomectomy outcome in a Nigerian hospital district hospital. J Basic Clin Reprod Sci. 2013;2:115-118.
- Pitter MC, Gargiulo AR, Bonaventura LM, et al. Pregnancy outcomes following robot-assisted myomectomy. Hum Reprod. 2013;28:99-108.
- Pitter MC, Srouji SS, Gargiulo AR, et al. Fertility and symptom relief following robot-assisted laparoscopic myomectomy. Obstet Gynecol Int. 2015;2015:967568.
- Huberlant S, Lenot J, Neron M, et al. Fertility and obstetric outcomes after robot-assisted laparoscopic myomectomy. Int J Med Robot. 2020;16:e2059.
- Olah KSJ. Caesarean myomectomy: TE or not TE? BJOG. 2018;125:501.
- Shaw, et al. Textbook of Operative Gynaecology. Edinburgh: Churchill Livingston; 1977.
- Burton CA, Grimes DA, March CM. Surgical management of leiomyomata during pregnancy. Obstet Gynecol. 1989;74:707-709.
- Ortac F, Gungor M, Sonmezer M. Myomectomy during cesarean section. Int J Gynaecol Obstet. 1999;67:189-193.
- Li H, Du J, Jin L, et al. Myomectomy during cesarean section. Acta Obstetricia et Gynecologica. 2009;88:183-186.
- Kwon DH, Song JE, Yoon KR, et al. Obstet Gynecol Sci. 2014;57:367-372.
- Senturk MB, Polat M, Dogan O, et al. Outcome of cesarean myomectomy: is it a safe procedure? Geburtshilfe Frauenheilkd. 2017;77:1200-1206.
- Chauhan AR. Cesarean myomectomy: necessity or opportunity? J Obstet Gynecol India. 2018;68:432-436.
- Sparic R, Kadija S, Stefanovic A, et al. Cesarean myomectomy in modern obstetrics: more light and fewer shadows. J Obstet Gynaecol Res. 2017;43:798-804.
- Ramya T, Sabnis SS, Chitra TV, et al. Cesarean myomectomy: an experience from a tertiary care teaching hospital. J Obstet Gynaecol India. 2019;69:426-430.
- Zhao R, Wang X, Zou L, et al. Outcomes of myomectomy at the time of cesarean section among pregnant women with uterine fibroids: a retrospective cohort study. Biomed Res Int. 2019;7576934.
- Munro MG, Critchley HOD, Fraser IS; FIGO Menstrual Disorders Committee. The two FIGO systems for normal and abnormal uterine bleeding symptoms and classification of causes of abnormal uterine bleeding in the reproductive years: 2018 revisions. In J Gynaecol Obstet. 2018;143:393.
- Omar SZ, Sivanesaratnam V, Damodaran P. Large lower segment myoma—myomectomy at lower segment caesarean section—a report of two cases. Singapore Med J. 1999;40:109-110.
- Goyal M, Dawood AS, Elbohoty SB, et al. Cesarean myomectomy in the last ten years; A true shift from contraindication to indication: a systematic review and meta-analysis. Eur J Obstet Gynecol Reprod Biol. 2021;256:145-157.
- Lin JY, Lee WL, Wang PH, et al. Uterine artery occlusion and myomectomy for treatment of pregnant women with uterine leiomyomas who are undergoing caesarean section. J Obstet Gynecol Res. 2010;36:284-290.
- Alfred E, Joy G, Uduak O, et al. Cesarean myomectomy outcome in a Nigerian hospital district hospital. J Basic Clin Reprod Sci. 2013;2:115-118.
A Preoperative Transthoracic Echocardiography Protocol to Reduce Time to Hip Fracture Surgery
From Dignity Health Methodist Hospital of Sacramento Family Medicine Residency Program, Sacramento, CA (Dr. Oldach); Nationwide Children’s Hospital, Columbus, OH (Dr. Irwin); OhioHealth Research Institute, Columbus, OH (Dr. Pershing); Department of Clinical Transformation, OhioHealth, Columbus, OH (Dr. Zigmont and Dr. Gascon); and Department of Geriatrics, OhioHealth, Columbus, OH (Dr. Skully).
Abstract
Objective: An interdisciplinary committee was formed to identify factors contributing to surgical delays in urgent hip fracture repair at an urban, level 1 trauma center, with the goal of reducing preoperative time to less than 24 hours. Surgical optimization was identified as a primary, modifiable factor, as surgeons were reluctant to clear patients for surgery without cardiac consultation. Preoperative transthoracic echocardiogram (TTE) was recommended as a safe alternative to cardiac consultation in most patients.
Methods: A retrospective review was conducted for patients who underwent urgent hip fracture repair between January 2010 and April 2014 (n = 316). Time to medical optimization, time to surgery, hospital length of stay, and anesthesia induction were compared for 3 patient groups of interest: those who received (1) neither TTE nor cardiology consultation (ie, direct to surgery); (2) a preoperative TTE; or (3) preoperative cardiac consultation.
Results: There were significant between-group differences in medical optimization time (P = 0.001) and mean time to surgery (P < 0.001) when comparing the 3 groups of interest. Patients in the preoperative cardiac consult group had the longest times, followed by the TTE and direct-to-surgery groups. There were no differences in the type of induction agent used across treatment groups when stratifying by ejection fraction.
Conclusion: Preoperative TTE allows for decreased preoperative time compared to a cardiology consultation. It provides an easily implemented inter-departmental, intra-institutional intervention to decrease preoperative time in patients presenting with hip fractures.
Keywords: surgical delay; preoperative risk stratification; process improvement.
Hip fractures are common, expensive, and associated with poor outcomes.1,2 Ample literature suggests that morbidity, mortality, and cost of care may be reduced by minimizing surgical delays.3-5 While individual reports indicate mixed evidence, in a 2010 meta-analysis, surgery within 72 hours was associated with significant reductions in pneumonia and pressure sores, as well as a 19% reduction in all-cause mortality through 1 year.6 Additional reviews suggest evidence of improved patient outcomes (pain, length of stay, non-union, and/or mortality) when surgery occurs early, within 12 to 72 hours after injury.4,6,7 Regardless of the definition of “early surgery” used, surgical delay remains a challenge, often due to organizational factors, including admission day of the week and hospital staffing, and patient characteristics, such as comorbidities, echocardiographic findings, age, and insurance status.7-9
Among factors that contribute to surgical delays, the need for preoperative cardiovascular risk stratification is significantly modifiable.10 The American College of Cardiology (ACC)/American Heart Association (AHA) Task Force risk stratification framework for preoperative cardiac testing assists clinicians in determining surgical urgency, active cardiac conditions, cardiovascular risk factors, and functional capacity of each patient, and is well established for low- or intermediate-risk patients.11 Specifically, metabolic equivalents (METs) measurements are used to identify medically stable patients with good or excellent functional capacity versus poor or unknown functional status. Patients with ≥ 4 METs may proceed to surgery without further testing; patients with < 4 METs may either proceed with planned surgery or undergo additional testing. Patients with a perceived increased risk profile who require urgent or semi-urgent hip fracture repair may be confounded by disagreement about required preoperative cardiac testing.
At OhioHealth Grant Medical Center (GMC), an urban, level 1 trauma center, the consideration of further preoperative noninvasive testing frequently contributed to surgical delays. In 2009, hip fracture patients arriving to the emergency department (ED) waited an average of 51 hours before being transferred to the operating room (OR) for surgery. Presuming prompt surgery is both desirable and feasible, the Grant Hip Fracture Management Committee (GHFMC) was developed in order to expedite surgeries in hip fracture patients. The GHFMC recommended a preoperative hip fracture protocol, and the outcomes from protocol implementation are described in this article.
Methods
This study was approved by the OhioHealth Institutional Review Board, with a waiver of the informed consent requirement. Medical records from patients treated at GMC during the time period between January 2010 and April 2014 (ie, following implementation of GHFMC recommendations) were retrospectively reviewed to identify the extent to which the use of preoperative transthoracic echocardiography (TTE) reduced average time to surgery and total length of stay, compared to cardiac consultation. This chart review included 316 participants and was used to identify primary induction agent utilized, time to medical optimization, time to surgery, and total length of hospital stay.
Intervention
The GHFMC conducted a 9-month quality improvement project to decrease ED-to-OR time to less than 24 hours for hip fracture patients. The multidisciplinary committee consisted of physicians from orthopedic surgery, anesthesia, hospital medicine, and geriatrics, along with key administrators and nurse outcomes managers. While there is lack of complete clarity surrounding optimal surgical timing, the committee decided that surgery within 24 hours would be beneficial for the majority of patients and therefore was considered a prudent goal.
Based on identified barriers that contributed to surgical delays, several process improvement strategies were implemented, including admitting patients to the hospitalist service, engaging the orthopedic trauma team, and implementing pre- and postoperative protocols and order sets (eg, ED and pain management order sets). Specific emphasis was placed on establishing guidelines for determining medical optimization. In the absence of established guidelines, medical optimization was determined at the discretion of the attending physician. The necessity of preoperative cardiac assessment was based, in part, on physician concerns about determining safe anesthesia protocols and hemodynamically managing patients who may have occult heart disease, specifically those patients with low functional capacity (< 4 METs) and/or inability to accurately communicate their medical history.
Many hip fractures result from a fall, and it may be unclear whether the fall causing a fracture was purely mechanical or indicative of a distinct acute or chronic illness. As a result, many patients received cardiac consultations, with or without pharmacologic stress testing, adding another 24 to 36 hours to preoperative time. As invasive preoperative cardiac procedures generally result in surgical delays without improving outcomes,11 the committee recommended that clinicians reserve preoperative cardiac consultation for patients with active cardiac conditions.
In lieu of cardiac consultation, the committee suggested preoperative TTE. While use of TTE has not been shown to improve preoperative risk stratification in routine noncardiac surgeries, it has been shown to provide clinically useful information in patients at high risk for cardiac complications.11 There was consensus for incorporating preoperative TTE for several reasons: (1) the patients with hip fractures were not “routine,” and often did not have a reliable medical history; (2) a large percentage of patients had cardiac risk factors; (3) patients with undiagnosed aortic stenosis, severe left ventricular dysfunction, or severe pulmonary hypertension would likely have altered intraoperative fluid management; and (4) in supplanting cardiac consultations, TTE would likely expedite patients’ ED-to-OR times. Therefore, the GHFMC created a recommendation of ordering urgent TTE for patients who were unable to exercise at ≥ 4 METs but needed urgent hip fracture surgery.
In order to evaluate the success of the new protocol, the ED-to-OR times were calculated for a cohort of patients who underwent surgery for hip fracture following algorithm implementation.
Participants
A chart review was conducted for patients admitted to GMC between January 2010 and April 2014 for operative treatment of a hip fracture. Exclusion criteria included lack of radiologist-diagnosed hip fracture, periprosthetic hip fracture, or multiple traumas. Electronic patient charts were reviewed by investigators (KI and BO) using a standardized, electronic abstraction form for 3 groups of patients who (1) proceeded directly to planned surgery without TTE or cardiac consultation (direct-to-surgery group); (2) received preoperative TTE but not a cardiac consultation (TTE-only group); or (3) received preoperative cardiac consultation (cardiac consult group).
Measures
Demographics, comorbid conditions, MET score, anesthesia protocol, and in-hospital morbidity and mortality were extracted from medical charts. Medical optimization time was determined by the latest time stamp of 1 of the following: time that the final consulting specialist stated that the patient was stable for surgery; time that the hospitalist described the patient as being ready for surgery; time that the TTE report was certified by the reading cardiologist; or time that the hospitalist described the outcome of completed preoperative risk stratification. Time elapsed prior to medical optimization, surgery, and discharge were calculated using differences between the patient’s arrival date and time at the ED, first recorded time of medical optimization, surgical start time (from the surgical report), and discharge time, respectively.
To assess whether the TTE protocol may have affected anesthesia selection, the induction agent (etomidate or propofol) was abstracted from anesthesia reports and stratified by the ejection fraction of each patient: very low (≤ 35%), low (36%–50%), or normal (> 50%). Patients without an echocardiogram report were assumed to have a normal ejection fraction for this analysis.
Analysis
Descriptive statistics were produced using mean and standard deviation (SD) for continuous variables and frequency and percentage for categorical variables. To determine whether statistically significant differences existed between the 3 groups, the Kruskal-Wallis test was used to compare skewed continuous variables, and Pearson’s chi-square test was used to compare categorical variables. Due to differences in baseline patient characteristics across the 3 treatment groups, inverse probability weights were used to adjust for group differences (using a multinomial logit treatment model) while comparing differences in outcome variables. This modeling strategy does not rely on any assumptions for the distribution of the outcome variable. Covariates were considered for inclusion in the treatment or outcome model if they were significantly associated (P < 0.05) with the group variable. Additionally, anesthetic agent (etomidate or propofol) was compared across the treatment groups after stratifying by ejection fraction to identify whether any differences existed in anesthesia regimen. Patients who were prescribed more than 1 anesthetic agent (n = 2) or an agent that was not of interest were removed from the analysis (n = 13). Stata (version 14) was used for analysis. All other missing data with respect to the tested variables were omitted in the analysis for that variable. Any disagreements about abstraction were resolved through consensus between the investigators.
Results
A total of 316 cases met inclusion criteria, including 108 direct-to-surgery patients, 143 preoperative TTE patients, and 65 cardiac consult patients. Patient demographics and preoperative characteristics are shown in Table 1. The average age for all patients was 76.5 years of age (SD, 12.89; IQR, 34-97); however, direct-to-surgery patients were significantly (P < 0.001) younger (71.2 years; SD, 14.2; interquartile range [IQR], 34-95 years) than TTE-only patients (79.0 years; SD, 11.5; IQR, 35-97 years) and cardiac consult patients (79.57 years; SD, 10.63; IQR, 49-97 years). The majority of patients were female (69.9%) and experienced a fall prior to admission (94%). Almost three-fourths of patients had 1 or more cardiac risk factors (73.7%), including history of congestive heart failure (CHF; 19%), coronary artery disease (CAD; 26.3%), chronic obstructive pulmonary disease (COPD; 19.3%), or aortic stenosis (AS; 3.5%). Due to between-group differences in these comorbid conditions, confounding factors were adjusted for in subsequent analyses.

As shown in Table 2, before adjustment for confounding factors, there were significant between-group differences in medical optimization time for patients in all 3 groups. After adjustment for treatment differences using age and number of comorbid diseases, and medical optimization time differences using age and COPD, fewer between-group differences were statistically significant. Patients who received a cardiac consult had an 18.44-hour longer medical optimization time compared to patients who went directly to surgery (29.136 vs 10.696 hours; P = 0.001). Optimization remained approximately 5 hours longer for the TTE-only group than for the direct-to-surgery group; however, this difference was not significant (P = 0.075).
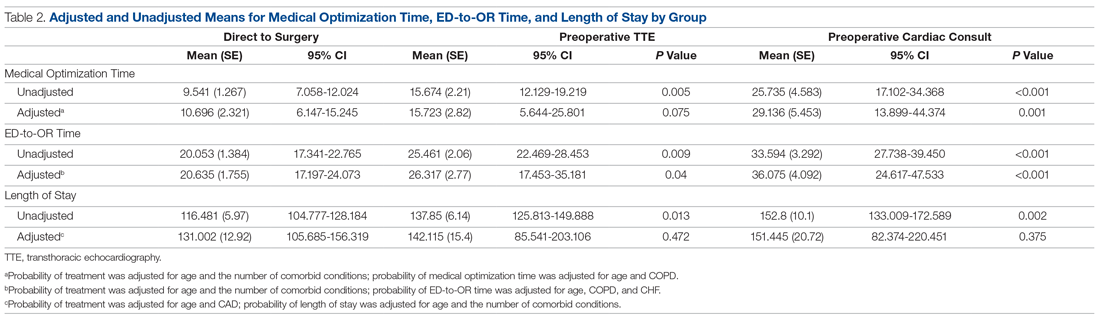
When comparing differences in ED-to-OR time for the 3 groups after adjusting the probability of treatment for age and the number of comorbid conditions, and adjusting the probability of ED-to-OR time for age, COPD, and CHF, significant differences remained in ED-to-OR times across all groups. Specifically, patients in the direct-to-surgery group experienced the shortest time (mean, 20.64 hours), compared to patients in the TTE-only group (mean, 26.32; P = 0.04) or patients in the cardiac consult group (mean, 36.08; P < 0.001). TTE-only patients had a longer time of 5.68 hours, compared to the direct-to-surgery group, and patients in the preoperative cardiac consult group were on average 15.44 hours longer than the direct-to-surgery group.
When comparing differences in the length of stay for the 3 groups before statistical adjustments, differences were observed; however, after removing the confounding factors related to treatment (age and CAD) and the outcome (age and the number of comorbid conditions), there were no statistically significant differences in the length of stay for the 3 groups. Average length of stay was 131 hours for direct-to-surgery patients, 142 hours for TTE-only patients, and 141 hours for cardiac consult patients.
The use of different anesthetic agents was compared for patients in the 3 groups. The majority of patients in the study (87.7%) were given propofol, and there were no differences after stratifying by ejection fraction (Table 3).
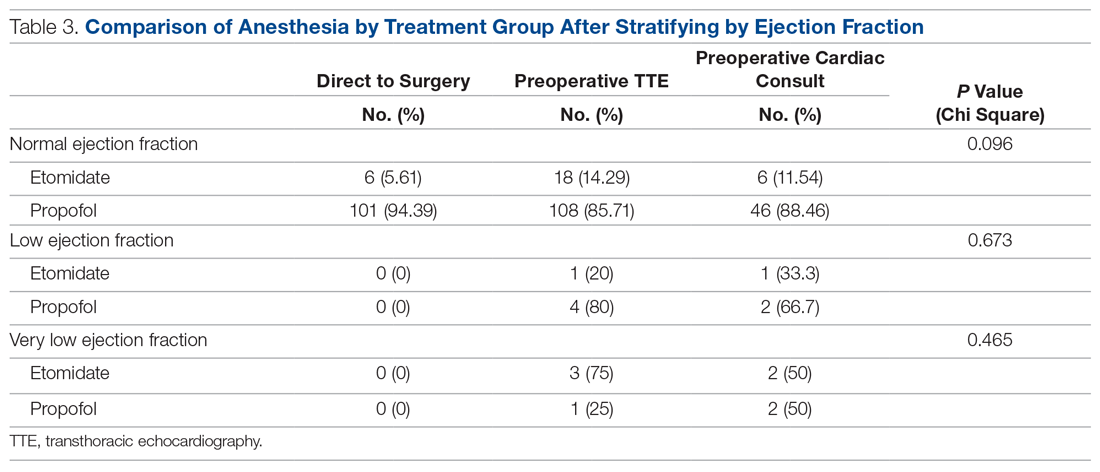
Discussion
The GHFMC was created to reduce surgical delays for hip fracture. Medical optimization was considered a primary, modifiable factor given that surgeons were reluctant to proceed without a cardiac consult. To address this gap, the committee recommended a preoperative TTE for patients with low or unknown functional status. This threshold provides a quick and easy method for stratifying patients who previously required risk stratification by a cardiologist, which often resulted in surgery delays.
In their recommendations for implementation of hip fracture quality improvement projects, the Geriatric Fracture Center emphasizes the importance of multidisciplinary physician leadership along with standardization of approach across patients.12 This recommendation is supported by increasing evidence that orthogeriatric collaborations are associated with decreased mortality and length of stay.13 The GHFMC and subsequent interventions reflect this approach, allowing for collaboration to identify cross-disciplinary procedural barriers to care. In our institution, addressing identified procedural barriers to care was associated with a reduction in the average time to surgery from 51 hours to 25.3 hours.
Multiple approaches have been attempted to decrease presurgical time in hip fracture patients in various settings. Prehospital interventions, such as providing ambulances with checklists and ability to bypass the ED, have not been shown to decrease time to surgery for hip fracture patients, though similar strategies have been successful in other conditions, such as stroke.14,15 In-hospital procedures, such as implementation of a hip fracture protocol and reduction of preoperative interventions, have more consistently been found to decrease time to surgery and in-hospital mortality.16,17 However, reduced delays have not been found universally. Luttrell and Nana found that preoperative TTE resulted in approximately 30.8-hour delays from the ED to OR, compared to patients who did not receive a preoperative TTE.18 However, in that study hospitalists used TTE at their own discretion, and there may have been confounding factors contributing to delays. When used as part of a protocol targeting patients with poor or unknown functional capacity, we believe that preoperative TTE results in modest surgical delays yet provides clinically useful information about each patient.
ACC/AHA preoperative guidelines were updated after we implemented our intervention and now recommend that patients with poor or unknown functional capacity in whom stress testing will not influence care proceed to surgery “according to guideline-directed medical care.”11 While routine use of preoperative evaluation of left ventricular function is not recommended, assessing left ventricular function may be reasonable for patients with heart failure with a change in clinical status. Guidelines also recommend that patients with clinically suspected valvular stenosis undergo preoperative echocardiography.11
Limitations
This study has several limitations. First, due to resource limitations, a substantial period of time elapsed between implementation of the new protocol and the analysis of the data set. That is, the hip fracture protocol assessed in this paper occurred from January 2010 through April 2014, and final analysis of the data set occurred in April 2020. This limitation precludes our ability to formally assess any pre- or post-protocol changes in patient outcomes. Second, randomization was not used to create groups that were balanced in differing health characteristics (ie, patients with noncardiac-related surgeries, patients in different age groups); however, the use of inverse probability treatment regression analysis was a way to statistically address these between-group differences. Moreover, this study is limited by the factors that were measured; unmeasured factors cannot be accounted for. Third, health care providers working at the hospital during this time were aware of the goal to decrease presurgical time, possibly creating exaggerated effects compared to a blinded trial. Finally, although this intervention is likely translatable to other centers, these results represent the experiences of a single level 1 trauma center and may not be replicable elsewhere.
Conclusion
Preoperative TTE in lieu of cardiac consultation has several advantages. First, it requires interdepartmental collaboration for implementation, but can be implemented through a single hospital or hospital system. Unlike prehospital interventions, preoperative urgent TTE for patients with low functional capacity does not require the support of emergency medical technicians, ambulance services, or other hospitals in the region. Second, while costs are associated with TTE, they are offset by a reduction in expensive consultations with specialists, surgical delays, and longer lengths of stay. Third, despite likely increased ED-to-OR times compared to no intervention, urgent TTE decreases time to surgery compared with cardiology consultation. Prior to the GHFMC, the ED-to-OR time at our institution was 51 hours. In contrast, the mean time following the GHFMC-led protocol was less than half that, at 25.3 hours (SD, 19.1 hours). In fact, nearly two-thirds (65.2%) of the patients evaluated in this study underwent surgery within 24 hours of admission. This improvement in presurgical time was attributed, in part, to the implementation of preoperative TTE over cardiology consultations.
Acknowledgments: The authors thank Jenny Williams, RN, who was instrumental in obtaining the data set for analysis, and Shauna Ayres, MPH, from the OhioHealth Research Institute, who provided writing and technical assistance.
Corresponding author: Robert Skully, MD, OhioHealth Family Medicine Grant, 290 East Town St., Columbus, OH 43215; [email protected].
Funding: This work was supported by the OhioHealth Summer Research Externship Program.
Financial disclosures: None.
1. Brauer CA, Coca-Perraillon M, Cutler DM, Rosen AB. Incidence and mortality of hip fractures in the United States. JAMA. 2009;302:1573-1579.
2. Lewiecki EM, Wright NC, Curtis JR, et al. Hip fracture trends in the United States 2002 to 2015. Osteoporos Int. 2018;29:717-722.
3. Colais P, Di Martino M, Fusco D, et al. The effect of early surgery after hip fracture on 1-year mortality. BMC Geriatr. 2015;15:141.
4. Nyholm AM, Gromov K, Palm H, et al. Time to surgery is associated with thirty-day and ninety-day mortality after proximal femoral fracture: a retrospective observational study on prospectively collected data from the Danish Fracture Database Collaborators. J Bone Joint Surg Am. 2015;97:1333-1339.
5. Judd KT, Christianson E. Expedited operative care of hip fractures results in significantly lower cost of treatment. Iowa Orthop J. 2015;35:62-64.
6. Simunovic N, Devereaux PJ, Sprague S, et al. Effect of early surgery after hip fracture on mortality and complications: systematic review and meta-analysis. CMAJ. 2010;182:1609-1616.
7. Ryan DJ, Yoshihara H, Yoneoka D, et al. Delay in hip fracture surgery: an analysis of patient-specific and hospital-specific risk factors. J Orthop Trauma. 2015;29:343-348.
8. Ricci WM, Brandt A, McAndrew C, Gardner MJ. Factors affecting delay to surgery and length of stay for patients with hip fracture. J Orthop Trauma. 2015;29:e109-e114.
9. Hagino T, Ochiai S, Senga S, et al. Efficacy of early surgery and causes of surgical delay in patients with hip fracture. J Orthop. 2015;12:142-146.
10. Rafiq A, Sklyar E, Bella JN. Cardiac evaluation and monitoring of patients undergoing noncardiac surgery. Health Serv Insights. 2017;9:1178632916686074.
11. Fleisher LA, Fleischmann KE, Auerbach AD, et al. 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;64:e77-e137.
12. Basu N, Natour M, Mounasamy V, Kates SL. Geriatric hip fracture management: keys to providing a successful program. Eur J Trauma Emerg Surg. 2016;42:565-569.
13. Grigoryan KV, Javedan H, Rudolph JL. Orthogeriatric care models and outcomes in hip fracture patients: a systematic review and meta-analysis. J Orthop Trauma. 2014;28:e49-e55.
14. Tai YJ, Yan B. Minimising time to treatment: targeted strategies to minimise time to thrombolysis for acute ischaemic stroke. Intern Med J. 2013;43:1176-1182.
15. Larsson G, Stromberg RU, Rogmark C, Nilsdotter A. Prehospital fast track care for patients with hip fracture: Impact on time to surgery, hospital stay, post-operative complications and mortality a randomised, controlled trial. Injury. 2016;47:881-886.
16. Bohm E, Loucks L, Wittmeier K, et al. Reduced time to surgery improves mortality and length of stay following hip fracture: results from an intervention study in a Canadian health authority. Can J Surg. 2015;58:257-263.
17. Ventura C, Trombetti S, Pioli G, et al. Impact of multidisciplinary hip fracture program on timing of surgery in elderly patients. Osteoporos Int J. 2014;25:2591-2597.
18. Luttrell K, Nana A. Effect of preoperative transthoracic echocardiogram on mortality and surgical timing in elderly adults with hip fracture. J Am Geriatr Soc. 2015;63:2505-2509.
From Dignity Health Methodist Hospital of Sacramento Family Medicine Residency Program, Sacramento, CA (Dr. Oldach); Nationwide Children’s Hospital, Columbus, OH (Dr. Irwin); OhioHealth Research Institute, Columbus, OH (Dr. Pershing); Department of Clinical Transformation, OhioHealth, Columbus, OH (Dr. Zigmont and Dr. Gascon); and Department of Geriatrics, OhioHealth, Columbus, OH (Dr. Skully).
Abstract
Objective: An interdisciplinary committee was formed to identify factors contributing to surgical delays in urgent hip fracture repair at an urban, level 1 trauma center, with the goal of reducing preoperative time to less than 24 hours. Surgical optimization was identified as a primary, modifiable factor, as surgeons were reluctant to clear patients for surgery without cardiac consultation. Preoperative transthoracic echocardiogram (TTE) was recommended as a safe alternative to cardiac consultation in most patients.
Methods: A retrospective review was conducted for patients who underwent urgent hip fracture repair between January 2010 and April 2014 (n = 316). Time to medical optimization, time to surgery, hospital length of stay, and anesthesia induction were compared for 3 patient groups of interest: those who received (1) neither TTE nor cardiology consultation (ie, direct to surgery); (2) a preoperative TTE; or (3) preoperative cardiac consultation.
Results: There were significant between-group differences in medical optimization time (P = 0.001) and mean time to surgery (P < 0.001) when comparing the 3 groups of interest. Patients in the preoperative cardiac consult group had the longest times, followed by the TTE and direct-to-surgery groups. There were no differences in the type of induction agent used across treatment groups when stratifying by ejection fraction.
Conclusion: Preoperative TTE allows for decreased preoperative time compared to a cardiology consultation. It provides an easily implemented inter-departmental, intra-institutional intervention to decrease preoperative time in patients presenting with hip fractures.
Keywords: surgical delay; preoperative risk stratification; process improvement.
Hip fractures are common, expensive, and associated with poor outcomes.1,2 Ample literature suggests that morbidity, mortality, and cost of care may be reduced by minimizing surgical delays.3-5 While individual reports indicate mixed evidence, in a 2010 meta-analysis, surgery within 72 hours was associated with significant reductions in pneumonia and pressure sores, as well as a 19% reduction in all-cause mortality through 1 year.6 Additional reviews suggest evidence of improved patient outcomes (pain, length of stay, non-union, and/or mortality) when surgery occurs early, within 12 to 72 hours after injury.4,6,7 Regardless of the definition of “early surgery” used, surgical delay remains a challenge, often due to organizational factors, including admission day of the week and hospital staffing, and patient characteristics, such as comorbidities, echocardiographic findings, age, and insurance status.7-9
Among factors that contribute to surgical delays, the need for preoperative cardiovascular risk stratification is significantly modifiable.10 The American College of Cardiology (ACC)/American Heart Association (AHA) Task Force risk stratification framework for preoperative cardiac testing assists clinicians in determining surgical urgency, active cardiac conditions, cardiovascular risk factors, and functional capacity of each patient, and is well established for low- or intermediate-risk patients.11 Specifically, metabolic equivalents (METs) measurements are used to identify medically stable patients with good or excellent functional capacity versus poor or unknown functional status. Patients with ≥ 4 METs may proceed to surgery without further testing; patients with < 4 METs may either proceed with planned surgery or undergo additional testing. Patients with a perceived increased risk profile who require urgent or semi-urgent hip fracture repair may be confounded by disagreement about required preoperative cardiac testing.
At OhioHealth Grant Medical Center (GMC), an urban, level 1 trauma center, the consideration of further preoperative noninvasive testing frequently contributed to surgical delays. In 2009, hip fracture patients arriving to the emergency department (ED) waited an average of 51 hours before being transferred to the operating room (OR) for surgery. Presuming prompt surgery is both desirable and feasible, the Grant Hip Fracture Management Committee (GHFMC) was developed in order to expedite surgeries in hip fracture patients. The GHFMC recommended a preoperative hip fracture protocol, and the outcomes from protocol implementation are described in this article.
Methods
This study was approved by the OhioHealth Institutional Review Board, with a waiver of the informed consent requirement. Medical records from patients treated at GMC during the time period between January 2010 and April 2014 (ie, following implementation of GHFMC recommendations) were retrospectively reviewed to identify the extent to which the use of preoperative transthoracic echocardiography (TTE) reduced average time to surgery and total length of stay, compared to cardiac consultation. This chart review included 316 participants and was used to identify primary induction agent utilized, time to medical optimization, time to surgery, and total length of hospital stay.
Intervention
The GHFMC conducted a 9-month quality improvement project to decrease ED-to-OR time to less than 24 hours for hip fracture patients. The multidisciplinary committee consisted of physicians from orthopedic surgery, anesthesia, hospital medicine, and geriatrics, along with key administrators and nurse outcomes managers. While there is lack of complete clarity surrounding optimal surgical timing, the committee decided that surgery within 24 hours would be beneficial for the majority of patients and therefore was considered a prudent goal.
Based on identified barriers that contributed to surgical delays, several process improvement strategies were implemented, including admitting patients to the hospitalist service, engaging the orthopedic trauma team, and implementing pre- and postoperative protocols and order sets (eg, ED and pain management order sets). Specific emphasis was placed on establishing guidelines for determining medical optimization. In the absence of established guidelines, medical optimization was determined at the discretion of the attending physician. The necessity of preoperative cardiac assessment was based, in part, on physician concerns about determining safe anesthesia protocols and hemodynamically managing patients who may have occult heart disease, specifically those patients with low functional capacity (< 4 METs) and/or inability to accurately communicate their medical history.
Many hip fractures result from a fall, and it may be unclear whether the fall causing a fracture was purely mechanical or indicative of a distinct acute or chronic illness. As a result, many patients received cardiac consultations, with or without pharmacologic stress testing, adding another 24 to 36 hours to preoperative time. As invasive preoperative cardiac procedures generally result in surgical delays without improving outcomes,11 the committee recommended that clinicians reserve preoperative cardiac consultation for patients with active cardiac conditions.
In lieu of cardiac consultation, the committee suggested preoperative TTE. While use of TTE has not been shown to improve preoperative risk stratification in routine noncardiac surgeries, it has been shown to provide clinically useful information in patients at high risk for cardiac complications.11 There was consensus for incorporating preoperative TTE for several reasons: (1) the patients with hip fractures were not “routine,” and often did not have a reliable medical history; (2) a large percentage of patients had cardiac risk factors; (3) patients with undiagnosed aortic stenosis, severe left ventricular dysfunction, or severe pulmonary hypertension would likely have altered intraoperative fluid management; and (4) in supplanting cardiac consultations, TTE would likely expedite patients’ ED-to-OR times. Therefore, the GHFMC created a recommendation of ordering urgent TTE for patients who were unable to exercise at ≥ 4 METs but needed urgent hip fracture surgery.
In order to evaluate the success of the new protocol, the ED-to-OR times were calculated for a cohort of patients who underwent surgery for hip fracture following algorithm implementation.
Participants
A chart review was conducted for patients admitted to GMC between January 2010 and April 2014 for operative treatment of a hip fracture. Exclusion criteria included lack of radiologist-diagnosed hip fracture, periprosthetic hip fracture, or multiple traumas. Electronic patient charts were reviewed by investigators (KI and BO) using a standardized, electronic abstraction form for 3 groups of patients who (1) proceeded directly to planned surgery without TTE or cardiac consultation (direct-to-surgery group); (2) received preoperative TTE but not a cardiac consultation (TTE-only group); or (3) received preoperative cardiac consultation (cardiac consult group).
Measures
Demographics, comorbid conditions, MET score, anesthesia protocol, and in-hospital morbidity and mortality were extracted from medical charts. Medical optimization time was determined by the latest time stamp of 1 of the following: time that the final consulting specialist stated that the patient was stable for surgery; time that the hospitalist described the patient as being ready for surgery; time that the TTE report was certified by the reading cardiologist; or time that the hospitalist described the outcome of completed preoperative risk stratification. Time elapsed prior to medical optimization, surgery, and discharge were calculated using differences between the patient’s arrival date and time at the ED, first recorded time of medical optimization, surgical start time (from the surgical report), and discharge time, respectively.
To assess whether the TTE protocol may have affected anesthesia selection, the induction agent (etomidate or propofol) was abstracted from anesthesia reports and stratified by the ejection fraction of each patient: very low (≤ 35%), low (36%–50%), or normal (> 50%). Patients without an echocardiogram report were assumed to have a normal ejection fraction for this analysis.
Analysis
Descriptive statistics were produced using mean and standard deviation (SD) for continuous variables and frequency and percentage for categorical variables. To determine whether statistically significant differences existed between the 3 groups, the Kruskal-Wallis test was used to compare skewed continuous variables, and Pearson’s chi-square test was used to compare categorical variables. Due to differences in baseline patient characteristics across the 3 treatment groups, inverse probability weights were used to adjust for group differences (using a multinomial logit treatment model) while comparing differences in outcome variables. This modeling strategy does not rely on any assumptions for the distribution of the outcome variable. Covariates were considered for inclusion in the treatment or outcome model if they were significantly associated (P < 0.05) with the group variable. Additionally, anesthetic agent (etomidate or propofol) was compared across the treatment groups after stratifying by ejection fraction to identify whether any differences existed in anesthesia regimen. Patients who were prescribed more than 1 anesthetic agent (n = 2) or an agent that was not of interest were removed from the analysis (n = 13). Stata (version 14) was used for analysis. All other missing data with respect to the tested variables were omitted in the analysis for that variable. Any disagreements about abstraction were resolved through consensus between the investigators.
Results
A total of 316 cases met inclusion criteria, including 108 direct-to-surgery patients, 143 preoperative TTE patients, and 65 cardiac consult patients. Patient demographics and preoperative characteristics are shown in Table 1. The average age for all patients was 76.5 years of age (SD, 12.89; IQR, 34-97); however, direct-to-surgery patients were significantly (P < 0.001) younger (71.2 years; SD, 14.2; interquartile range [IQR], 34-95 years) than TTE-only patients (79.0 years; SD, 11.5; IQR, 35-97 years) and cardiac consult patients (79.57 years; SD, 10.63; IQR, 49-97 years). The majority of patients were female (69.9%) and experienced a fall prior to admission (94%). Almost three-fourths of patients had 1 or more cardiac risk factors (73.7%), including history of congestive heart failure (CHF; 19%), coronary artery disease (CAD; 26.3%), chronic obstructive pulmonary disease (COPD; 19.3%), or aortic stenosis (AS; 3.5%). Due to between-group differences in these comorbid conditions, confounding factors were adjusted for in subsequent analyses.

As shown in Table 2, before adjustment for confounding factors, there were significant between-group differences in medical optimization time for patients in all 3 groups. After adjustment for treatment differences using age and number of comorbid diseases, and medical optimization time differences using age and COPD, fewer between-group differences were statistically significant. Patients who received a cardiac consult had an 18.44-hour longer medical optimization time compared to patients who went directly to surgery (29.136 vs 10.696 hours; P = 0.001). Optimization remained approximately 5 hours longer for the TTE-only group than for the direct-to-surgery group; however, this difference was not significant (P = 0.075).

When comparing differences in ED-to-OR time for the 3 groups after adjusting the probability of treatment for age and the number of comorbid conditions, and adjusting the probability of ED-to-OR time for age, COPD, and CHF, significant differences remained in ED-to-OR times across all groups. Specifically, patients in the direct-to-surgery group experienced the shortest time (mean, 20.64 hours), compared to patients in the TTE-only group (mean, 26.32; P = 0.04) or patients in the cardiac consult group (mean, 36.08; P < 0.001). TTE-only patients had a longer time of 5.68 hours, compared to the direct-to-surgery group, and patients in the preoperative cardiac consult group were on average 15.44 hours longer than the direct-to-surgery group.
When comparing differences in the length of stay for the 3 groups before statistical adjustments, differences were observed; however, after removing the confounding factors related to treatment (age and CAD) and the outcome (age and the number of comorbid conditions), there were no statistically significant differences in the length of stay for the 3 groups. Average length of stay was 131 hours for direct-to-surgery patients, 142 hours for TTE-only patients, and 141 hours for cardiac consult patients.
The use of different anesthetic agents was compared for patients in the 3 groups. The majority of patients in the study (87.7%) were given propofol, and there were no differences after stratifying by ejection fraction (Table 3).

Discussion
The GHFMC was created to reduce surgical delays for hip fracture. Medical optimization was considered a primary, modifiable factor given that surgeons were reluctant to proceed without a cardiac consult. To address this gap, the committee recommended a preoperative TTE for patients with low or unknown functional status. This threshold provides a quick and easy method for stratifying patients who previously required risk stratification by a cardiologist, which often resulted in surgery delays.
In their recommendations for implementation of hip fracture quality improvement projects, the Geriatric Fracture Center emphasizes the importance of multidisciplinary physician leadership along with standardization of approach across patients.12 This recommendation is supported by increasing evidence that orthogeriatric collaborations are associated with decreased mortality and length of stay.13 The GHFMC and subsequent interventions reflect this approach, allowing for collaboration to identify cross-disciplinary procedural barriers to care. In our institution, addressing identified procedural barriers to care was associated with a reduction in the average time to surgery from 51 hours to 25.3 hours.
Multiple approaches have been attempted to decrease presurgical time in hip fracture patients in various settings. Prehospital interventions, such as providing ambulances with checklists and ability to bypass the ED, have not been shown to decrease time to surgery for hip fracture patients, though similar strategies have been successful in other conditions, such as stroke.14,15 In-hospital procedures, such as implementation of a hip fracture protocol and reduction of preoperative interventions, have more consistently been found to decrease time to surgery and in-hospital mortality.16,17 However, reduced delays have not been found universally. Luttrell and Nana found that preoperative TTE resulted in approximately 30.8-hour delays from the ED to OR, compared to patients who did not receive a preoperative TTE.18 However, in that study hospitalists used TTE at their own discretion, and there may have been confounding factors contributing to delays. When used as part of a protocol targeting patients with poor or unknown functional capacity, we believe that preoperative TTE results in modest surgical delays yet provides clinically useful information about each patient.
ACC/AHA preoperative guidelines were updated after we implemented our intervention and now recommend that patients with poor or unknown functional capacity in whom stress testing will not influence care proceed to surgery “according to guideline-directed medical care.”11 While routine use of preoperative evaluation of left ventricular function is not recommended, assessing left ventricular function may be reasonable for patients with heart failure with a change in clinical status. Guidelines also recommend that patients with clinically suspected valvular stenosis undergo preoperative echocardiography.11
Limitations
This study has several limitations. First, due to resource limitations, a substantial period of time elapsed between implementation of the new protocol and the analysis of the data set. That is, the hip fracture protocol assessed in this paper occurred from January 2010 through April 2014, and final analysis of the data set occurred in April 2020. This limitation precludes our ability to formally assess any pre- or post-protocol changes in patient outcomes. Second, randomization was not used to create groups that were balanced in differing health characteristics (ie, patients with noncardiac-related surgeries, patients in different age groups); however, the use of inverse probability treatment regression analysis was a way to statistically address these between-group differences. Moreover, this study is limited by the factors that were measured; unmeasured factors cannot be accounted for. Third, health care providers working at the hospital during this time were aware of the goal to decrease presurgical time, possibly creating exaggerated effects compared to a blinded trial. Finally, although this intervention is likely translatable to other centers, these results represent the experiences of a single level 1 trauma center and may not be replicable elsewhere.
Conclusion
Preoperative TTE in lieu of cardiac consultation has several advantages. First, it requires interdepartmental collaboration for implementation, but can be implemented through a single hospital or hospital system. Unlike prehospital interventions, preoperative urgent TTE for patients with low functional capacity does not require the support of emergency medical technicians, ambulance services, or other hospitals in the region. Second, while costs are associated with TTE, they are offset by a reduction in expensive consultations with specialists, surgical delays, and longer lengths of stay. Third, despite likely increased ED-to-OR times compared to no intervention, urgent TTE decreases time to surgery compared with cardiology consultation. Prior to the GHFMC, the ED-to-OR time at our institution was 51 hours. In contrast, the mean time following the GHFMC-led protocol was less than half that, at 25.3 hours (SD, 19.1 hours). In fact, nearly two-thirds (65.2%) of the patients evaluated in this study underwent surgery within 24 hours of admission. This improvement in presurgical time was attributed, in part, to the implementation of preoperative TTE over cardiology consultations.
Acknowledgments: The authors thank Jenny Williams, RN, who was instrumental in obtaining the data set for analysis, and Shauna Ayres, MPH, from the OhioHealth Research Institute, who provided writing and technical assistance.
Corresponding author: Robert Skully, MD, OhioHealth Family Medicine Grant, 290 East Town St., Columbus, OH 43215; [email protected].
Funding: This work was supported by the OhioHealth Summer Research Externship Program.
Financial disclosures: None.
From Dignity Health Methodist Hospital of Sacramento Family Medicine Residency Program, Sacramento, CA (Dr. Oldach); Nationwide Children’s Hospital, Columbus, OH (Dr. Irwin); OhioHealth Research Institute, Columbus, OH (Dr. Pershing); Department of Clinical Transformation, OhioHealth, Columbus, OH (Dr. Zigmont and Dr. Gascon); and Department of Geriatrics, OhioHealth, Columbus, OH (Dr. Skully).
Abstract
Objective: An interdisciplinary committee was formed to identify factors contributing to surgical delays in urgent hip fracture repair at an urban, level 1 trauma center, with the goal of reducing preoperative time to less than 24 hours. Surgical optimization was identified as a primary, modifiable factor, as surgeons were reluctant to clear patients for surgery without cardiac consultation. Preoperative transthoracic echocardiogram (TTE) was recommended as a safe alternative to cardiac consultation in most patients.
Methods: A retrospective review was conducted for patients who underwent urgent hip fracture repair between January 2010 and April 2014 (n = 316). Time to medical optimization, time to surgery, hospital length of stay, and anesthesia induction were compared for 3 patient groups of interest: those who received (1) neither TTE nor cardiology consultation (ie, direct to surgery); (2) a preoperative TTE; or (3) preoperative cardiac consultation.
Results: There were significant between-group differences in medical optimization time (P = 0.001) and mean time to surgery (P < 0.001) when comparing the 3 groups of interest. Patients in the preoperative cardiac consult group had the longest times, followed by the TTE and direct-to-surgery groups. There were no differences in the type of induction agent used across treatment groups when stratifying by ejection fraction.
Conclusion: Preoperative TTE allows for decreased preoperative time compared to a cardiology consultation. It provides an easily implemented inter-departmental, intra-institutional intervention to decrease preoperative time in patients presenting with hip fractures.
Keywords: surgical delay; preoperative risk stratification; process improvement.
Hip fractures are common, expensive, and associated with poor outcomes.1,2 Ample literature suggests that morbidity, mortality, and cost of care may be reduced by minimizing surgical delays.3-5 While individual reports indicate mixed evidence, in a 2010 meta-analysis, surgery within 72 hours was associated with significant reductions in pneumonia and pressure sores, as well as a 19% reduction in all-cause mortality through 1 year.6 Additional reviews suggest evidence of improved patient outcomes (pain, length of stay, non-union, and/or mortality) when surgery occurs early, within 12 to 72 hours after injury.4,6,7 Regardless of the definition of “early surgery” used, surgical delay remains a challenge, often due to organizational factors, including admission day of the week and hospital staffing, and patient characteristics, such as comorbidities, echocardiographic findings, age, and insurance status.7-9
Among factors that contribute to surgical delays, the need for preoperative cardiovascular risk stratification is significantly modifiable.10 The American College of Cardiology (ACC)/American Heart Association (AHA) Task Force risk stratification framework for preoperative cardiac testing assists clinicians in determining surgical urgency, active cardiac conditions, cardiovascular risk factors, and functional capacity of each patient, and is well established for low- or intermediate-risk patients.11 Specifically, metabolic equivalents (METs) measurements are used to identify medically stable patients with good or excellent functional capacity versus poor or unknown functional status. Patients with ≥ 4 METs may proceed to surgery without further testing; patients with < 4 METs may either proceed with planned surgery or undergo additional testing. Patients with a perceived increased risk profile who require urgent or semi-urgent hip fracture repair may be confounded by disagreement about required preoperative cardiac testing.
At OhioHealth Grant Medical Center (GMC), an urban, level 1 trauma center, the consideration of further preoperative noninvasive testing frequently contributed to surgical delays. In 2009, hip fracture patients arriving to the emergency department (ED) waited an average of 51 hours before being transferred to the operating room (OR) for surgery. Presuming prompt surgery is both desirable and feasible, the Grant Hip Fracture Management Committee (GHFMC) was developed in order to expedite surgeries in hip fracture patients. The GHFMC recommended a preoperative hip fracture protocol, and the outcomes from protocol implementation are described in this article.
Methods
This study was approved by the OhioHealth Institutional Review Board, with a waiver of the informed consent requirement. Medical records from patients treated at GMC during the time period between January 2010 and April 2014 (ie, following implementation of GHFMC recommendations) were retrospectively reviewed to identify the extent to which the use of preoperative transthoracic echocardiography (TTE) reduced average time to surgery and total length of stay, compared to cardiac consultation. This chart review included 316 participants and was used to identify primary induction agent utilized, time to medical optimization, time to surgery, and total length of hospital stay.
Intervention
The GHFMC conducted a 9-month quality improvement project to decrease ED-to-OR time to less than 24 hours for hip fracture patients. The multidisciplinary committee consisted of physicians from orthopedic surgery, anesthesia, hospital medicine, and geriatrics, along with key administrators and nurse outcomes managers. While there is lack of complete clarity surrounding optimal surgical timing, the committee decided that surgery within 24 hours would be beneficial for the majority of patients and therefore was considered a prudent goal.
Based on identified barriers that contributed to surgical delays, several process improvement strategies were implemented, including admitting patients to the hospitalist service, engaging the orthopedic trauma team, and implementing pre- and postoperative protocols and order sets (eg, ED and pain management order sets). Specific emphasis was placed on establishing guidelines for determining medical optimization. In the absence of established guidelines, medical optimization was determined at the discretion of the attending physician. The necessity of preoperative cardiac assessment was based, in part, on physician concerns about determining safe anesthesia protocols and hemodynamically managing patients who may have occult heart disease, specifically those patients with low functional capacity (< 4 METs) and/or inability to accurately communicate their medical history.
Many hip fractures result from a fall, and it may be unclear whether the fall causing a fracture was purely mechanical or indicative of a distinct acute or chronic illness. As a result, many patients received cardiac consultations, with or without pharmacologic stress testing, adding another 24 to 36 hours to preoperative time. As invasive preoperative cardiac procedures generally result in surgical delays without improving outcomes,11 the committee recommended that clinicians reserve preoperative cardiac consultation for patients with active cardiac conditions.
In lieu of cardiac consultation, the committee suggested preoperative TTE. While use of TTE has not been shown to improve preoperative risk stratification in routine noncardiac surgeries, it has been shown to provide clinically useful information in patients at high risk for cardiac complications.11 There was consensus for incorporating preoperative TTE for several reasons: (1) the patients with hip fractures were not “routine,” and often did not have a reliable medical history; (2) a large percentage of patients had cardiac risk factors; (3) patients with undiagnosed aortic stenosis, severe left ventricular dysfunction, or severe pulmonary hypertension would likely have altered intraoperative fluid management; and (4) in supplanting cardiac consultations, TTE would likely expedite patients’ ED-to-OR times. Therefore, the GHFMC created a recommendation of ordering urgent TTE for patients who were unable to exercise at ≥ 4 METs but needed urgent hip fracture surgery.
In order to evaluate the success of the new protocol, the ED-to-OR times were calculated for a cohort of patients who underwent surgery for hip fracture following algorithm implementation.
Participants
A chart review was conducted for patients admitted to GMC between January 2010 and April 2014 for operative treatment of a hip fracture. Exclusion criteria included lack of radiologist-diagnosed hip fracture, periprosthetic hip fracture, or multiple traumas. Electronic patient charts were reviewed by investigators (KI and BO) using a standardized, electronic abstraction form for 3 groups of patients who (1) proceeded directly to planned surgery without TTE or cardiac consultation (direct-to-surgery group); (2) received preoperative TTE but not a cardiac consultation (TTE-only group); or (3) received preoperative cardiac consultation (cardiac consult group).
Measures
Demographics, comorbid conditions, MET score, anesthesia protocol, and in-hospital morbidity and mortality were extracted from medical charts. Medical optimization time was determined by the latest time stamp of 1 of the following: time that the final consulting specialist stated that the patient was stable for surgery; time that the hospitalist described the patient as being ready for surgery; time that the TTE report was certified by the reading cardiologist; or time that the hospitalist described the outcome of completed preoperative risk stratification. Time elapsed prior to medical optimization, surgery, and discharge were calculated using differences between the patient’s arrival date and time at the ED, first recorded time of medical optimization, surgical start time (from the surgical report), and discharge time, respectively.
To assess whether the TTE protocol may have affected anesthesia selection, the induction agent (etomidate or propofol) was abstracted from anesthesia reports and stratified by the ejection fraction of each patient: very low (≤ 35%), low (36%–50%), or normal (> 50%). Patients without an echocardiogram report were assumed to have a normal ejection fraction for this analysis.
Analysis
Descriptive statistics were produced using mean and standard deviation (SD) for continuous variables and frequency and percentage for categorical variables. To determine whether statistically significant differences existed between the 3 groups, the Kruskal-Wallis test was used to compare skewed continuous variables, and Pearson’s chi-square test was used to compare categorical variables. Due to differences in baseline patient characteristics across the 3 treatment groups, inverse probability weights were used to adjust for group differences (using a multinomial logit treatment model) while comparing differences in outcome variables. This modeling strategy does not rely on any assumptions for the distribution of the outcome variable. Covariates were considered for inclusion in the treatment or outcome model if they were significantly associated (P < 0.05) with the group variable. Additionally, anesthetic agent (etomidate or propofol) was compared across the treatment groups after stratifying by ejection fraction to identify whether any differences existed in anesthesia regimen. Patients who were prescribed more than 1 anesthetic agent (n = 2) or an agent that was not of interest were removed from the analysis (n = 13). Stata (version 14) was used for analysis. All other missing data with respect to the tested variables were omitted in the analysis for that variable. Any disagreements about abstraction were resolved through consensus between the investigators.
Results
A total of 316 cases met inclusion criteria, including 108 direct-to-surgery patients, 143 preoperative TTE patients, and 65 cardiac consult patients. Patient demographics and preoperative characteristics are shown in Table 1. The average age for all patients was 76.5 years of age (SD, 12.89; IQR, 34-97); however, direct-to-surgery patients were significantly (P < 0.001) younger (71.2 years; SD, 14.2; interquartile range [IQR], 34-95 years) than TTE-only patients (79.0 years; SD, 11.5; IQR, 35-97 years) and cardiac consult patients (79.57 years; SD, 10.63; IQR, 49-97 years). The majority of patients were female (69.9%) and experienced a fall prior to admission (94%). Almost three-fourths of patients had 1 or more cardiac risk factors (73.7%), including history of congestive heart failure (CHF; 19%), coronary artery disease (CAD; 26.3%), chronic obstructive pulmonary disease (COPD; 19.3%), or aortic stenosis (AS; 3.5%). Due to between-group differences in these comorbid conditions, confounding factors were adjusted for in subsequent analyses.

As shown in Table 2, before adjustment for confounding factors, there were significant between-group differences in medical optimization time for patients in all 3 groups. After adjustment for treatment differences using age and number of comorbid diseases, and medical optimization time differences using age and COPD, fewer between-group differences were statistically significant. Patients who received a cardiac consult had an 18.44-hour longer medical optimization time compared to patients who went directly to surgery (29.136 vs 10.696 hours; P = 0.001). Optimization remained approximately 5 hours longer for the TTE-only group than for the direct-to-surgery group; however, this difference was not significant (P = 0.075).

When comparing differences in ED-to-OR time for the 3 groups after adjusting the probability of treatment for age and the number of comorbid conditions, and adjusting the probability of ED-to-OR time for age, COPD, and CHF, significant differences remained in ED-to-OR times across all groups. Specifically, patients in the direct-to-surgery group experienced the shortest time (mean, 20.64 hours), compared to patients in the TTE-only group (mean, 26.32; P = 0.04) or patients in the cardiac consult group (mean, 36.08; P < 0.001). TTE-only patients had a longer time of 5.68 hours, compared to the direct-to-surgery group, and patients in the preoperative cardiac consult group were on average 15.44 hours longer than the direct-to-surgery group.
When comparing differences in the length of stay for the 3 groups before statistical adjustments, differences were observed; however, after removing the confounding factors related to treatment (age and CAD) and the outcome (age and the number of comorbid conditions), there were no statistically significant differences in the length of stay for the 3 groups. Average length of stay was 131 hours for direct-to-surgery patients, 142 hours for TTE-only patients, and 141 hours for cardiac consult patients.
The use of different anesthetic agents was compared for patients in the 3 groups. The majority of patients in the study (87.7%) were given propofol, and there were no differences after stratifying by ejection fraction (Table 3).

Discussion
The GHFMC was created to reduce surgical delays for hip fracture. Medical optimization was considered a primary, modifiable factor given that surgeons were reluctant to proceed without a cardiac consult. To address this gap, the committee recommended a preoperative TTE for patients with low or unknown functional status. This threshold provides a quick and easy method for stratifying patients who previously required risk stratification by a cardiologist, which often resulted in surgery delays.
In their recommendations for implementation of hip fracture quality improvement projects, the Geriatric Fracture Center emphasizes the importance of multidisciplinary physician leadership along with standardization of approach across patients.12 This recommendation is supported by increasing evidence that orthogeriatric collaborations are associated with decreased mortality and length of stay.13 The GHFMC and subsequent interventions reflect this approach, allowing for collaboration to identify cross-disciplinary procedural barriers to care. In our institution, addressing identified procedural barriers to care was associated with a reduction in the average time to surgery from 51 hours to 25.3 hours.
Multiple approaches have been attempted to decrease presurgical time in hip fracture patients in various settings. Prehospital interventions, such as providing ambulances with checklists and ability to bypass the ED, have not been shown to decrease time to surgery for hip fracture patients, though similar strategies have been successful in other conditions, such as stroke.14,15 In-hospital procedures, such as implementation of a hip fracture protocol and reduction of preoperative interventions, have more consistently been found to decrease time to surgery and in-hospital mortality.16,17 However, reduced delays have not been found universally. Luttrell and Nana found that preoperative TTE resulted in approximately 30.8-hour delays from the ED to OR, compared to patients who did not receive a preoperative TTE.18 However, in that study hospitalists used TTE at their own discretion, and there may have been confounding factors contributing to delays. When used as part of a protocol targeting patients with poor or unknown functional capacity, we believe that preoperative TTE results in modest surgical delays yet provides clinically useful information about each patient.
ACC/AHA preoperative guidelines were updated after we implemented our intervention and now recommend that patients with poor or unknown functional capacity in whom stress testing will not influence care proceed to surgery “according to guideline-directed medical care.”11 While routine use of preoperative evaluation of left ventricular function is not recommended, assessing left ventricular function may be reasonable for patients with heart failure with a change in clinical status. Guidelines also recommend that patients with clinically suspected valvular stenosis undergo preoperative echocardiography.11
Limitations
This study has several limitations. First, due to resource limitations, a substantial period of time elapsed between implementation of the new protocol and the analysis of the data set. That is, the hip fracture protocol assessed in this paper occurred from January 2010 through April 2014, and final analysis of the data set occurred in April 2020. This limitation precludes our ability to formally assess any pre- or post-protocol changes in patient outcomes. Second, randomization was not used to create groups that were balanced in differing health characteristics (ie, patients with noncardiac-related surgeries, patients in different age groups); however, the use of inverse probability treatment regression analysis was a way to statistically address these between-group differences. Moreover, this study is limited by the factors that were measured; unmeasured factors cannot be accounted for. Third, health care providers working at the hospital during this time were aware of the goal to decrease presurgical time, possibly creating exaggerated effects compared to a blinded trial. Finally, although this intervention is likely translatable to other centers, these results represent the experiences of a single level 1 trauma center and may not be replicable elsewhere.
Conclusion
Preoperative TTE in lieu of cardiac consultation has several advantages. First, it requires interdepartmental collaboration for implementation, but can be implemented through a single hospital or hospital system. Unlike prehospital interventions, preoperative urgent TTE for patients with low functional capacity does not require the support of emergency medical technicians, ambulance services, or other hospitals in the region. Second, while costs are associated with TTE, they are offset by a reduction in expensive consultations with specialists, surgical delays, and longer lengths of stay. Third, despite likely increased ED-to-OR times compared to no intervention, urgent TTE decreases time to surgery compared with cardiology consultation. Prior to the GHFMC, the ED-to-OR time at our institution was 51 hours. In contrast, the mean time following the GHFMC-led protocol was less than half that, at 25.3 hours (SD, 19.1 hours). In fact, nearly two-thirds (65.2%) of the patients evaluated in this study underwent surgery within 24 hours of admission. This improvement in presurgical time was attributed, in part, to the implementation of preoperative TTE over cardiology consultations.
Acknowledgments: The authors thank Jenny Williams, RN, who was instrumental in obtaining the data set for analysis, and Shauna Ayres, MPH, from the OhioHealth Research Institute, who provided writing and technical assistance.
Corresponding author: Robert Skully, MD, OhioHealth Family Medicine Grant, 290 East Town St., Columbus, OH 43215; [email protected].
Funding: This work was supported by the OhioHealth Summer Research Externship Program.
Financial disclosures: None.
1. Brauer CA, Coca-Perraillon M, Cutler DM, Rosen AB. Incidence and mortality of hip fractures in the United States. JAMA. 2009;302:1573-1579.
2. Lewiecki EM, Wright NC, Curtis JR, et al. Hip fracture trends in the United States 2002 to 2015. Osteoporos Int. 2018;29:717-722.
3. Colais P, Di Martino M, Fusco D, et al. The effect of early surgery after hip fracture on 1-year mortality. BMC Geriatr. 2015;15:141.
4. Nyholm AM, Gromov K, Palm H, et al. Time to surgery is associated with thirty-day and ninety-day mortality after proximal femoral fracture: a retrospective observational study on prospectively collected data from the Danish Fracture Database Collaborators. J Bone Joint Surg Am. 2015;97:1333-1339.
5. Judd KT, Christianson E. Expedited operative care of hip fractures results in significantly lower cost of treatment. Iowa Orthop J. 2015;35:62-64.
6. Simunovic N, Devereaux PJ, Sprague S, et al. Effect of early surgery after hip fracture on mortality and complications: systematic review and meta-analysis. CMAJ. 2010;182:1609-1616.
7. Ryan DJ, Yoshihara H, Yoneoka D, et al. Delay in hip fracture surgery: an analysis of patient-specific and hospital-specific risk factors. J Orthop Trauma. 2015;29:343-348.
8. Ricci WM, Brandt A, McAndrew C, Gardner MJ. Factors affecting delay to surgery and length of stay for patients with hip fracture. J Orthop Trauma. 2015;29:e109-e114.
9. Hagino T, Ochiai S, Senga S, et al. Efficacy of early surgery and causes of surgical delay in patients with hip fracture. J Orthop. 2015;12:142-146.
10. Rafiq A, Sklyar E, Bella JN. Cardiac evaluation and monitoring of patients undergoing noncardiac surgery. Health Serv Insights. 2017;9:1178632916686074.
11. Fleisher LA, Fleischmann KE, Auerbach AD, et al. 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;64:e77-e137.
12. Basu N, Natour M, Mounasamy V, Kates SL. Geriatric hip fracture management: keys to providing a successful program. Eur J Trauma Emerg Surg. 2016;42:565-569.
13. Grigoryan KV, Javedan H, Rudolph JL. Orthogeriatric care models and outcomes in hip fracture patients: a systematic review and meta-analysis. J Orthop Trauma. 2014;28:e49-e55.
14. Tai YJ, Yan B. Minimising time to treatment: targeted strategies to minimise time to thrombolysis for acute ischaemic stroke. Intern Med J. 2013;43:1176-1182.
15. Larsson G, Stromberg RU, Rogmark C, Nilsdotter A. Prehospital fast track care for patients with hip fracture: Impact on time to surgery, hospital stay, post-operative complications and mortality a randomised, controlled trial. Injury. 2016;47:881-886.
16. Bohm E, Loucks L, Wittmeier K, et al. Reduced time to surgery improves mortality and length of stay following hip fracture: results from an intervention study in a Canadian health authority. Can J Surg. 2015;58:257-263.
17. Ventura C, Trombetti S, Pioli G, et al. Impact of multidisciplinary hip fracture program on timing of surgery in elderly patients. Osteoporos Int J. 2014;25:2591-2597.
18. Luttrell K, Nana A. Effect of preoperative transthoracic echocardiogram on mortality and surgical timing in elderly adults with hip fracture. J Am Geriatr Soc. 2015;63:2505-2509.
1. Brauer CA, Coca-Perraillon M, Cutler DM, Rosen AB. Incidence and mortality of hip fractures in the United States. JAMA. 2009;302:1573-1579.
2. Lewiecki EM, Wright NC, Curtis JR, et al. Hip fracture trends in the United States 2002 to 2015. Osteoporos Int. 2018;29:717-722.
3. Colais P, Di Martino M, Fusco D, et al. The effect of early surgery after hip fracture on 1-year mortality. BMC Geriatr. 2015;15:141.
4. Nyholm AM, Gromov K, Palm H, et al. Time to surgery is associated with thirty-day and ninety-day mortality after proximal femoral fracture: a retrospective observational study on prospectively collected data from the Danish Fracture Database Collaborators. J Bone Joint Surg Am. 2015;97:1333-1339.
5. Judd KT, Christianson E. Expedited operative care of hip fractures results in significantly lower cost of treatment. Iowa Orthop J. 2015;35:62-64.
6. Simunovic N, Devereaux PJ, Sprague S, et al. Effect of early surgery after hip fracture on mortality and complications: systematic review and meta-analysis. CMAJ. 2010;182:1609-1616.
7. Ryan DJ, Yoshihara H, Yoneoka D, et al. Delay in hip fracture surgery: an analysis of patient-specific and hospital-specific risk factors. J Orthop Trauma. 2015;29:343-348.
8. Ricci WM, Brandt A, McAndrew C, Gardner MJ. Factors affecting delay to surgery and length of stay for patients with hip fracture. J Orthop Trauma. 2015;29:e109-e114.
9. Hagino T, Ochiai S, Senga S, et al. Efficacy of early surgery and causes of surgical delay in patients with hip fracture. J Orthop. 2015;12:142-146.
10. Rafiq A, Sklyar E, Bella JN. Cardiac evaluation and monitoring of patients undergoing noncardiac surgery. Health Serv Insights. 2017;9:1178632916686074.
11. Fleisher LA, Fleischmann KE, Auerbach AD, et al. 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;64:e77-e137.
12. Basu N, Natour M, Mounasamy V, Kates SL. Geriatric hip fracture management: keys to providing a successful program. Eur J Trauma Emerg Surg. 2016;42:565-569.
13. Grigoryan KV, Javedan H, Rudolph JL. Orthogeriatric care models and outcomes in hip fracture patients: a systematic review and meta-analysis. J Orthop Trauma. 2014;28:e49-e55.
14. Tai YJ, Yan B. Minimising time to treatment: targeted strategies to minimise time to thrombolysis for acute ischaemic stroke. Intern Med J. 2013;43:1176-1182.
15. Larsson G, Stromberg RU, Rogmark C, Nilsdotter A. Prehospital fast track care for patients with hip fracture: Impact on time to surgery, hospital stay, post-operative complications and mortality a randomised, controlled trial. Injury. 2016;47:881-886.
16. Bohm E, Loucks L, Wittmeier K, et al. Reduced time to surgery improves mortality and length of stay following hip fracture: results from an intervention study in a Canadian health authority. Can J Surg. 2015;58:257-263.
17. Ventura C, Trombetti S, Pioli G, et al. Impact of multidisciplinary hip fracture program on timing of surgery in elderly patients. Osteoporos Int J. 2014;25:2591-2597.
18. Luttrell K, Nana A. Effect of preoperative transthoracic echocardiogram on mortality and surgical timing in elderly adults with hip fracture. J Am Geriatr Soc. 2015;63:2505-2509.
Limiting antibiotic therapy after surgical drainage for native joint bacterial arthritis
Background: Currently the recommended duration of antibiotic therapy for native joint bacterial arthritis is 3-6 weeks based on expert opinion.
Study design: Prospective, unblinded, randomized, noninferiority.
Setting: Single center in Geneva.
Synopsis: In total, 154 patients were randomized to either 2 weeks or 4 weeks of antibiotic regimen selected in consultation with infectious disease specialists after surgical drainage of native joint bacterial arthritis.
The study population was 38% women with a median age of 51 years. Sites of infection were majority hand and wrist arthritis (64%). The most frequent pathogen was Staphylococcus aureus (31%) with no methicillin-resistant strains. There was a low incidence of patients with bacteremia (4%) and chronic immune compromise (10%). Antibiotic regimen varied with 13 different initial intravenous regimens and 11 different oral regimens.
The primary study outcome was rate of recurrent infection within 2 years, which was low with only one recurrence in the 2-week arm and two recurrences in the 4-week arm. This difference was well within the 10% noninferiority margin selected by the authors.
The study was underpowered for nonhand and nonwrist cases, limiting generalizability.
Bottom line: Consider a shorter duration of antibiotic therapy after surgical drainage for native joint bacterial arthritis of the hand and wrist in an otherwise healthy patient.
Citation: Gjika E et al. Two weeks versus four weeks of antibiotic therapy after surgical drainage for native joint bacterial arthritis: a prospective, randomized, non-inferiority trial. Ann Rheum Dis. 2019 Aug;78(8):1114-21.
Dr. Zarookian is a hospitalist at Maine Medical Center in Portland and Stephens Memorial Hospital in Norway, Maine.
Background: Currently the recommended duration of antibiotic therapy for native joint bacterial arthritis is 3-6 weeks based on expert opinion.
Study design: Prospective, unblinded, randomized, noninferiority.
Setting: Single center in Geneva.
Synopsis: In total, 154 patients were randomized to either 2 weeks or 4 weeks of antibiotic regimen selected in consultation with infectious disease specialists after surgical drainage of native joint bacterial arthritis.
The study population was 38% women with a median age of 51 years. Sites of infection were majority hand and wrist arthritis (64%). The most frequent pathogen was Staphylococcus aureus (31%) with no methicillin-resistant strains. There was a low incidence of patients with bacteremia (4%) and chronic immune compromise (10%). Antibiotic regimen varied with 13 different initial intravenous regimens and 11 different oral regimens.
The primary study outcome was rate of recurrent infection within 2 years, which was low with only one recurrence in the 2-week arm and two recurrences in the 4-week arm. This difference was well within the 10% noninferiority margin selected by the authors.
The study was underpowered for nonhand and nonwrist cases, limiting generalizability.
Bottom line: Consider a shorter duration of antibiotic therapy after surgical drainage for native joint bacterial arthritis of the hand and wrist in an otherwise healthy patient.
Citation: Gjika E et al. Two weeks versus four weeks of antibiotic therapy after surgical drainage for native joint bacterial arthritis: a prospective, randomized, non-inferiority trial. Ann Rheum Dis. 2019 Aug;78(8):1114-21.
Dr. Zarookian is a hospitalist at Maine Medical Center in Portland and Stephens Memorial Hospital in Norway, Maine.
Background: Currently the recommended duration of antibiotic therapy for native joint bacterial arthritis is 3-6 weeks based on expert opinion.
Study design: Prospective, unblinded, randomized, noninferiority.
Setting: Single center in Geneva.
Synopsis: In total, 154 patients were randomized to either 2 weeks or 4 weeks of antibiotic regimen selected in consultation with infectious disease specialists after surgical drainage of native joint bacterial arthritis.
The study population was 38% women with a median age of 51 years. Sites of infection were majority hand and wrist arthritis (64%). The most frequent pathogen was Staphylococcus aureus (31%) with no methicillin-resistant strains. There was a low incidence of patients with bacteremia (4%) and chronic immune compromise (10%). Antibiotic regimen varied with 13 different initial intravenous regimens and 11 different oral regimens.
The primary study outcome was rate of recurrent infection within 2 years, which was low with only one recurrence in the 2-week arm and two recurrences in the 4-week arm. This difference was well within the 10% noninferiority margin selected by the authors.
The study was underpowered for nonhand and nonwrist cases, limiting generalizability.
Bottom line: Consider a shorter duration of antibiotic therapy after surgical drainage for native joint bacterial arthritis of the hand and wrist in an otherwise healthy patient.
Citation: Gjika E et al. Two weeks versus four weeks of antibiotic therapy after surgical drainage for native joint bacterial arthritis: a prospective, randomized, non-inferiority trial. Ann Rheum Dis. 2019 Aug;78(8):1114-21.
Dr. Zarookian is a hospitalist at Maine Medical Center in Portland and Stephens Memorial Hospital in Norway, Maine.
Von Willebrand disease guidelines address women’s bleeding concerns
New guidelines issued jointly by four major international hematology groups focus on the management of patients with von Willebrand disease (VWD), the most common bleeding disorder in the world.
The evidence-based guidelines, published in Blood Advances, were developed in collaboration by the American Society of Hematology (ASH), the International Society on Thrombosis and Haemostasis, the National Hemophilia Foundation, and the World Federation of Hemophilia. They outline key recommendations spanning the care of patients with a broad range of therapeutic needs.
“We addressed some of the questions that were most important to the community, but certainly there are a lot of areas that we couldn’t cover” said coauthor Veronica H. Flood, MD, of the Medical College of Wisconsin in Milwaukee.
The guidelines process began with a survey sent to the von Willebrand disease community, including patients, caregivers, nurses, physicians, and scientists. The respondents were asked to prioritize issues that they felt should be addressed in the guidelines.
“Interestingly, some of the issues were the same between patients and caregivers and physicians, and some were different, but there were obviously some areas that we just couldn’t cover,” she said in an interview.
One of the areas of greatest concern for respondents was bleeding in women, and many of the recommendations include specific considerations for management of gynecologic and obstetric patients, Dr. Flood said.
“We also tried to make the questions applicable to as many patients with von Willebrand disease as possible,” she added.
Some of the questions, such as recommendation 1, regarding prophylaxis, are geared toward management of patients with severe disease, while others, such as recommendations for treatment of menstrual bleeding, are more suited for patients with milder VWD.
All of the recommendations in the guidelines are “conditional” (suggested), due to very low certainty in the evidence of effects, the authors noted.
Prophylaxis
The guidelines suggest long-term prophylaxis for patients with a history of severe and frequent bleeds, with periodic assessment of the need for prophylaxis.
Desmopressin
For those patients who may benefit from the use of desmopressin, primarily those with type 1 VWD, and who have a baseline von Willebrand factor (VWF) level below 0.30 IU/mL, the panel issued a conditional recommendation for a desmopressin trial with treatment based on the patient’s results compared with not performing a trial and treating with tranexamic acid or factor concentrate. The guidelines also advise against treating with desmopressin in the absence of a trial. In a section of “good practice statements,” the guidelines indicate that using desmopressin in patients with type 2B VWD is generally contraindicated, because of the risk of thrombocytopenia as a result of increased platelet binding. In addition, desmopressin is generally contraindicated in patients with active cardiovascular disease, patients with seizure disorders, patients less than 2 years old, and patients with type 1C VWD in the setting of surgery.
Antithrombotic therapy
The guideline panelists conditionally recommend antithrombotic therapy with either antiplatelet agents or anticoagulants, with an emphasis on reassessing bleeding risk throughout the course of treatment.
An accompanying good practice statement calls for individualized assessments of risks and benefits of specific antithrombotic therapies by a multidisciplinary team including hematologists, cardiovascular specialists, and the patient.
Major surgery
This section includes a recommendation for targeting both factor VIII and VWF activity levels to a minimum of 50 IU/mL for at least 3 days after surgery, and a suggestion against using factor VIII target levels alone.
Minor surgery/invasive procedures
The panelists suggest increasing VWF activity levels to a minimum of 0.50 IU/mL with desmopressin or factor concentrate with the addition of tranexamic acid over raising VWF levels to at least 0.50 IU/mL with desmopressin or factor concentrate alone.
In addition, the panelists suggest “giving tranexamic acid alone over increasing VWF activity levels to a minimum threshold of 0.50 IU/mL with any intervention in patients with type 1 VWD with baseline VWF activity levels of 0.30 IU/mL and a mild bleeding phenotype undergoing minor mucosal procedures.”
Heavy menstrual bleeding
In women with heavy menstrual bleeding who do not plan to conceive, the panel suggests either combined hormonal therapy or levonorgestrel-releasing intrauterine system, or tranexamic acid over desmopressin.
In women who wish to conceive, the panel suggests using tranexamic acid over desmopressin.
Neuraxial anesthesia during labor
For women in labor for whom neuraxial anesthesia is considered, the guidelines suggest targeting a VWF activity level from 0.50 to 1.50 IU/mL over targeting a level above 1.50 IU/mL.
Postpartum management
“The guideline panel suggests the use of tranexamic acid over not using it in women with type 1 VWD or low VWF levels (and this may also apply to types 2 and 3 VWD) during the postpartum period,” the guidelines say.
An accompanying good practice statement says that tranexamic acid can be provided orally or intravenously. The oral dose is 25 mg/kg three times daily for 10-14 days, or longer if blood loss remains heavy.
Dr. Flood said that the guidelines were developed under the assumption that they would apply to care of patients in regions with a high or moderately high degree of clinical resources.
“We recognize that this eliminates a great deal of the globe, and our hope is that ASH and the other sponsoring organizations are going to let us revise this and do a version for lower-resourced settings,” she said.
New guidelines issued jointly by four major international hematology groups focus on the management of patients with von Willebrand disease (VWD), the most common bleeding disorder in the world.
The evidence-based guidelines, published in Blood Advances, were developed in collaboration by the American Society of Hematology (ASH), the International Society on Thrombosis and Haemostasis, the National Hemophilia Foundation, and the World Federation of Hemophilia. They outline key recommendations spanning the care of patients with a broad range of therapeutic needs.
“We addressed some of the questions that were most important to the community, but certainly there are a lot of areas that we couldn’t cover” said coauthor Veronica H. Flood, MD, of the Medical College of Wisconsin in Milwaukee.
The guidelines process began with a survey sent to the von Willebrand disease community, including patients, caregivers, nurses, physicians, and scientists. The respondents were asked to prioritize issues that they felt should be addressed in the guidelines.
“Interestingly, some of the issues were the same between patients and caregivers and physicians, and some were different, but there were obviously some areas that we just couldn’t cover,” she said in an interview.
One of the areas of greatest concern for respondents was bleeding in women, and many of the recommendations include specific considerations for management of gynecologic and obstetric patients, Dr. Flood said.
“We also tried to make the questions applicable to as many patients with von Willebrand disease as possible,” she added.
Some of the questions, such as recommendation 1, regarding prophylaxis, are geared toward management of patients with severe disease, while others, such as recommendations for treatment of menstrual bleeding, are more suited for patients with milder VWD.
All of the recommendations in the guidelines are “conditional” (suggested), due to very low certainty in the evidence of effects, the authors noted.
Prophylaxis
The guidelines suggest long-term prophylaxis for patients with a history of severe and frequent bleeds, with periodic assessment of the need for prophylaxis.
Desmopressin
For those patients who may benefit from the use of desmopressin, primarily those with type 1 VWD, and who have a baseline von Willebrand factor (VWF) level below 0.30 IU/mL, the panel issued a conditional recommendation for a desmopressin trial with treatment based on the patient’s results compared with not performing a trial and treating with tranexamic acid or factor concentrate. The guidelines also advise against treating with desmopressin in the absence of a trial. In a section of “good practice statements,” the guidelines indicate that using desmopressin in patients with type 2B VWD is generally contraindicated, because of the risk of thrombocytopenia as a result of increased platelet binding. In addition, desmopressin is generally contraindicated in patients with active cardiovascular disease, patients with seizure disorders, patients less than 2 years old, and patients with type 1C VWD in the setting of surgery.
Antithrombotic therapy
The guideline panelists conditionally recommend antithrombotic therapy with either antiplatelet agents or anticoagulants, with an emphasis on reassessing bleeding risk throughout the course of treatment.
An accompanying good practice statement calls for individualized assessments of risks and benefits of specific antithrombotic therapies by a multidisciplinary team including hematologists, cardiovascular specialists, and the patient.
Major surgery
This section includes a recommendation for targeting both factor VIII and VWF activity levels to a minimum of 50 IU/mL for at least 3 days after surgery, and a suggestion against using factor VIII target levels alone.
Minor surgery/invasive procedures
The panelists suggest increasing VWF activity levels to a minimum of 0.50 IU/mL with desmopressin or factor concentrate with the addition of tranexamic acid over raising VWF levels to at least 0.50 IU/mL with desmopressin or factor concentrate alone.
In addition, the panelists suggest “giving tranexamic acid alone over increasing VWF activity levels to a minimum threshold of 0.50 IU/mL with any intervention in patients with type 1 VWD with baseline VWF activity levels of 0.30 IU/mL and a mild bleeding phenotype undergoing minor mucosal procedures.”
Heavy menstrual bleeding
In women with heavy menstrual bleeding who do not plan to conceive, the panel suggests either combined hormonal therapy or levonorgestrel-releasing intrauterine system, or tranexamic acid over desmopressin.
In women who wish to conceive, the panel suggests using tranexamic acid over desmopressin.
Neuraxial anesthesia during labor
For women in labor for whom neuraxial anesthesia is considered, the guidelines suggest targeting a VWF activity level from 0.50 to 1.50 IU/mL over targeting a level above 1.50 IU/mL.
Postpartum management
“The guideline panel suggests the use of tranexamic acid over not using it in women with type 1 VWD or low VWF levels (and this may also apply to types 2 and 3 VWD) during the postpartum period,” the guidelines say.
An accompanying good practice statement says that tranexamic acid can be provided orally or intravenously. The oral dose is 25 mg/kg three times daily for 10-14 days, or longer if blood loss remains heavy.
Dr. Flood said that the guidelines were developed under the assumption that they would apply to care of patients in regions with a high or moderately high degree of clinical resources.
“We recognize that this eliminates a great deal of the globe, and our hope is that ASH and the other sponsoring organizations are going to let us revise this and do a version for lower-resourced settings,” she said.
New guidelines issued jointly by four major international hematology groups focus on the management of patients with von Willebrand disease (VWD), the most common bleeding disorder in the world.
The evidence-based guidelines, published in Blood Advances, were developed in collaboration by the American Society of Hematology (ASH), the International Society on Thrombosis and Haemostasis, the National Hemophilia Foundation, and the World Federation of Hemophilia. They outline key recommendations spanning the care of patients with a broad range of therapeutic needs.
“We addressed some of the questions that were most important to the community, but certainly there are a lot of areas that we couldn’t cover” said coauthor Veronica H. Flood, MD, of the Medical College of Wisconsin in Milwaukee.
The guidelines process began with a survey sent to the von Willebrand disease community, including patients, caregivers, nurses, physicians, and scientists. The respondents were asked to prioritize issues that they felt should be addressed in the guidelines.
“Interestingly, some of the issues were the same between patients and caregivers and physicians, and some were different, but there were obviously some areas that we just couldn’t cover,” she said in an interview.
One of the areas of greatest concern for respondents was bleeding in women, and many of the recommendations include specific considerations for management of gynecologic and obstetric patients, Dr. Flood said.
“We also tried to make the questions applicable to as many patients with von Willebrand disease as possible,” she added.
Some of the questions, such as recommendation 1, regarding prophylaxis, are geared toward management of patients with severe disease, while others, such as recommendations for treatment of menstrual bleeding, are more suited for patients with milder VWD.
All of the recommendations in the guidelines are “conditional” (suggested), due to very low certainty in the evidence of effects, the authors noted.
Prophylaxis
The guidelines suggest long-term prophylaxis for patients with a history of severe and frequent bleeds, with periodic assessment of the need for prophylaxis.
Desmopressin
For those patients who may benefit from the use of desmopressin, primarily those with type 1 VWD, and who have a baseline von Willebrand factor (VWF) level below 0.30 IU/mL, the panel issued a conditional recommendation for a desmopressin trial with treatment based on the patient’s results compared with not performing a trial and treating with tranexamic acid or factor concentrate. The guidelines also advise against treating with desmopressin in the absence of a trial. In a section of “good practice statements,” the guidelines indicate that using desmopressin in patients with type 2B VWD is generally contraindicated, because of the risk of thrombocytopenia as a result of increased platelet binding. In addition, desmopressin is generally contraindicated in patients with active cardiovascular disease, patients with seizure disorders, patients less than 2 years old, and patients with type 1C VWD in the setting of surgery.
Antithrombotic therapy
The guideline panelists conditionally recommend antithrombotic therapy with either antiplatelet agents or anticoagulants, with an emphasis on reassessing bleeding risk throughout the course of treatment.
An accompanying good practice statement calls for individualized assessments of risks and benefits of specific antithrombotic therapies by a multidisciplinary team including hematologists, cardiovascular specialists, and the patient.
Major surgery
This section includes a recommendation for targeting both factor VIII and VWF activity levels to a minimum of 50 IU/mL for at least 3 days after surgery, and a suggestion against using factor VIII target levels alone.
Minor surgery/invasive procedures
The panelists suggest increasing VWF activity levels to a minimum of 0.50 IU/mL with desmopressin or factor concentrate with the addition of tranexamic acid over raising VWF levels to at least 0.50 IU/mL with desmopressin or factor concentrate alone.
In addition, the panelists suggest “giving tranexamic acid alone over increasing VWF activity levels to a minimum threshold of 0.50 IU/mL with any intervention in patients with type 1 VWD with baseline VWF activity levels of 0.30 IU/mL and a mild bleeding phenotype undergoing minor mucosal procedures.”
Heavy menstrual bleeding
In women with heavy menstrual bleeding who do not plan to conceive, the panel suggests either combined hormonal therapy or levonorgestrel-releasing intrauterine system, or tranexamic acid over desmopressin.
In women who wish to conceive, the panel suggests using tranexamic acid over desmopressin.
Neuraxial anesthesia during labor
For women in labor for whom neuraxial anesthesia is considered, the guidelines suggest targeting a VWF activity level from 0.50 to 1.50 IU/mL over targeting a level above 1.50 IU/mL.
Postpartum management
“The guideline panel suggests the use of tranexamic acid over not using it in women with type 1 VWD or low VWF levels (and this may also apply to types 2 and 3 VWD) during the postpartum period,” the guidelines say.
An accompanying good practice statement says that tranexamic acid can be provided orally or intravenously. The oral dose is 25 mg/kg three times daily for 10-14 days, or longer if blood loss remains heavy.
Dr. Flood said that the guidelines were developed under the assumption that they would apply to care of patients in regions with a high or moderately high degree of clinical resources.
“We recognize that this eliminates a great deal of the globe, and our hope is that ASH and the other sponsoring organizations are going to let us revise this and do a version for lower-resourced settings,” she said.
FROM BLOOD ADVANCES
Covert stroke after noncardiac surgery linked with cognitive decline
Background: Prior studies have established an increased risk of overt stroke after noncardiac surgery, with significant associated morbidity and mortality. Similarly, covert stroke in the nonsurgical population is well described and has been shown to be associated with cognitive decline.
Study design: Prospective cohort study.
Setting: Academic centers in nine countries.
Synopsis: This study evaluated 1,114 patients older than 65 years who were hospitalized for noncardiac surgery, excluding patients with carotid and neurosurgical procedures. All enrolled participants completed diffusion-weight MRI of the brain within 9 days of surgery. Follow-up rates for clinical outcomes (1,112; greater than 99%) were excellent, and the primary outcome measure, follow-up Montreal Cognitive Assessment (MOCA) at 1 year, was defined in 1,001 (90%) of the study subjects.
Covert stroke was detected in 78 (7%) of the study participants. Those with covert stroke had a higher incidence of cognitive decline at 1 year (adjusted odds ratio, 1.98; 95% confidence interval, 1.22-3.2) with an absolute risk increase of 13%. Patients with covert stroke also had a higher rate of delirium within 3 days of surgery (hazard ratio, 2.24; 95% CI, 1.06-4.73) and a higher rate of overt stroke and transient ischemic attack at 1 year (HR, 4.13; 95% CI, 1.14-14.99).
This study helps to establish the incidence of covert stroke after noncardiac surgery and provides support for covert stroke as a risk factor for cognitive impairment.
Bottom line: Covert stroke following noncardiac surgery is common, affecting 1 in 14 patients in this study, and it is associated with an increased risk of cognitive decline, perioperative delirium, and subsequent overt stroke.
Citation: The NeuroVISION Investigators (Mrkobrada M et al.). Perioperative covert stroke in patients undergoing noncardiac surgery (NeuroVISION): a prospective cohort study. Lancet. 2019;394(10203):1022-9.
Dr. Herrle is a hospitalist at Maine Medical Center in Portland and at Stephens Memorial Hospital in Norway, Maine.
Background: Prior studies have established an increased risk of overt stroke after noncardiac surgery, with significant associated morbidity and mortality. Similarly, covert stroke in the nonsurgical population is well described and has been shown to be associated with cognitive decline.
Study design: Prospective cohort study.
Setting: Academic centers in nine countries.
Synopsis: This study evaluated 1,114 patients older than 65 years who were hospitalized for noncardiac surgery, excluding patients with carotid and neurosurgical procedures. All enrolled participants completed diffusion-weight MRI of the brain within 9 days of surgery. Follow-up rates for clinical outcomes (1,112; greater than 99%) were excellent, and the primary outcome measure, follow-up Montreal Cognitive Assessment (MOCA) at 1 year, was defined in 1,001 (90%) of the study subjects.
Covert stroke was detected in 78 (7%) of the study participants. Those with covert stroke had a higher incidence of cognitive decline at 1 year (adjusted odds ratio, 1.98; 95% confidence interval, 1.22-3.2) with an absolute risk increase of 13%. Patients with covert stroke also had a higher rate of delirium within 3 days of surgery (hazard ratio, 2.24; 95% CI, 1.06-4.73) and a higher rate of overt stroke and transient ischemic attack at 1 year (HR, 4.13; 95% CI, 1.14-14.99).
This study helps to establish the incidence of covert stroke after noncardiac surgery and provides support for covert stroke as a risk factor for cognitive impairment.
Bottom line: Covert stroke following noncardiac surgery is common, affecting 1 in 14 patients in this study, and it is associated with an increased risk of cognitive decline, perioperative delirium, and subsequent overt stroke.
Citation: The NeuroVISION Investigators (Mrkobrada M et al.). Perioperative covert stroke in patients undergoing noncardiac surgery (NeuroVISION): a prospective cohort study. Lancet. 2019;394(10203):1022-9.
Dr. Herrle is a hospitalist at Maine Medical Center in Portland and at Stephens Memorial Hospital in Norway, Maine.
Background: Prior studies have established an increased risk of overt stroke after noncardiac surgery, with significant associated morbidity and mortality. Similarly, covert stroke in the nonsurgical population is well described and has been shown to be associated with cognitive decline.
Study design: Prospective cohort study.
Setting: Academic centers in nine countries.
Synopsis: This study evaluated 1,114 patients older than 65 years who were hospitalized for noncardiac surgery, excluding patients with carotid and neurosurgical procedures. All enrolled participants completed diffusion-weight MRI of the brain within 9 days of surgery. Follow-up rates for clinical outcomes (1,112; greater than 99%) were excellent, and the primary outcome measure, follow-up Montreal Cognitive Assessment (MOCA) at 1 year, was defined in 1,001 (90%) of the study subjects.
Covert stroke was detected in 78 (7%) of the study participants. Those with covert stroke had a higher incidence of cognitive decline at 1 year (adjusted odds ratio, 1.98; 95% confidence interval, 1.22-3.2) with an absolute risk increase of 13%. Patients with covert stroke also had a higher rate of delirium within 3 days of surgery (hazard ratio, 2.24; 95% CI, 1.06-4.73) and a higher rate of overt stroke and transient ischemic attack at 1 year (HR, 4.13; 95% CI, 1.14-14.99).
This study helps to establish the incidence of covert stroke after noncardiac surgery and provides support for covert stroke as a risk factor for cognitive impairment.
Bottom line: Covert stroke following noncardiac surgery is common, affecting 1 in 14 patients in this study, and it is associated with an increased risk of cognitive decline, perioperative delirium, and subsequent overt stroke.
Citation: The NeuroVISION Investigators (Mrkobrada M et al.). Perioperative covert stroke in patients undergoing noncardiac surgery (NeuroVISION): a prospective cohort study. Lancet. 2019;394(10203):1022-9.
Dr. Herrle is a hospitalist at Maine Medical Center in Portland and at Stephens Memorial Hospital in Norway, Maine.