User login
Autoimmune Progesterone Dermatitis
To the Editor:
Autoimmune progesterone dermatitis (APD) is a rare dermatologic condition that can be challenging to diagnose. The associated skin lesions are not only variable in physical presentation but also in the timing of the outbreak. The skin disorder stems from an internal reaction to elevated levels of progesterone during the luteal phase of the menstrual cycle. Autoimmune progesterone dermatitis can be difficult to detect; although the typical menstrual cycle is 28 days, many women have longer or shorter hormonal phases, leading to cyclical irregularity that can cause the lesions to appear sporadic in nature when in fact they are not.1
A 34-year-old woman with a history of endometriosis, psoriasis, and malignant melanoma presented to our dermatology clinic 2 days after a brief hospitalization during which she was diagnosed with a hypersensitivity reaction. Two days prior to her hospital admission, the patient developed a rash on the lower back with associated myalgia. The rash progressively worsened, spreading laterally to the flanks, which prompted her to seek medical attention. Blood work included a complete blood cell count with differential, complete metabolic panel, antinuclear antibody test, and erythrocyte sedimentation rate, which all were within reference range. A 4-mm punch biopsy from the left lateral flank was performed and was consistent with a neutrophilic dermatosis. The patient’s symptoms diminished and she was discharged the next day with instructions to follow up with a dermatologist.
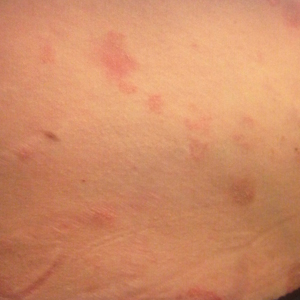
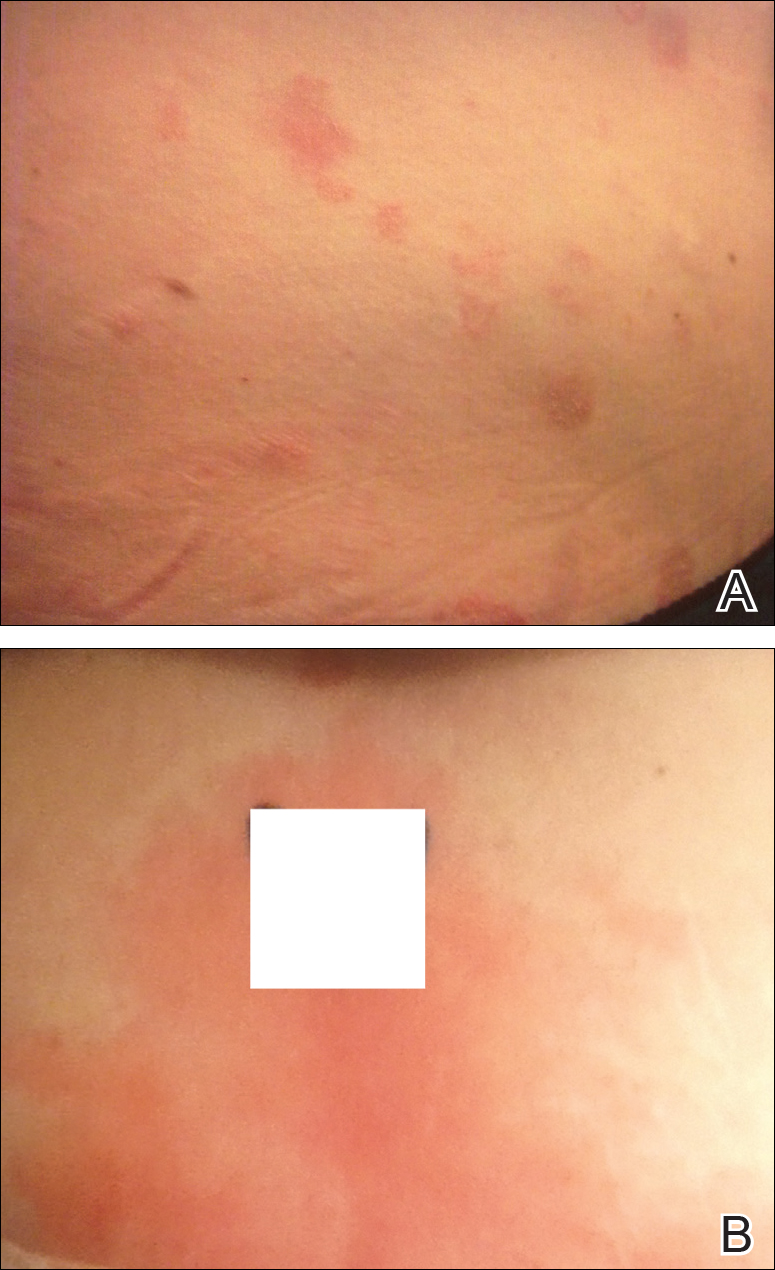
Physical examination at our clinic revealed multiple minimally indurated, erythematous plaques with superficial scaling along the left lower back and upper buttock (Figure 1). No other skin lesions were present, and palpation of the cervical, axillary, and inguinal lymph nodes was unremarkable. A repeat 6-mm punch biopsy was performed and she was sent for fasting blood work.
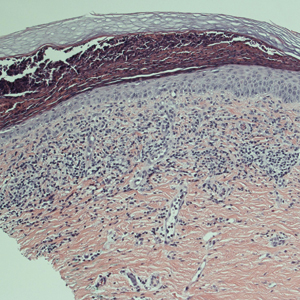
Histologic examination of the punch biopsy revealed a superficial and deep perivascular and interstitial dermatitis with scattered neutrophils and eosinophils. Findings were described as nonspecific, possibly representing a dermal hypersensitivity or urticarial reaction.
Glucose-6-phosphate dehydrogenase testing was within reference range, and therapy was initiated with oral dapsone 50 mg once daily as well as fexofenadine 180 mg once daily. The patient initially responded well to the oral therapy, but she experienced recurrence of the skin eruption at infrequent intervals over the next few months, requiring escalating doses of dapsone to control the symptoms. After further questioning at a subsequent visit a few months later, it was discovered that the eruption occurred near the onset of the patient’s irregular menstrual cycle.
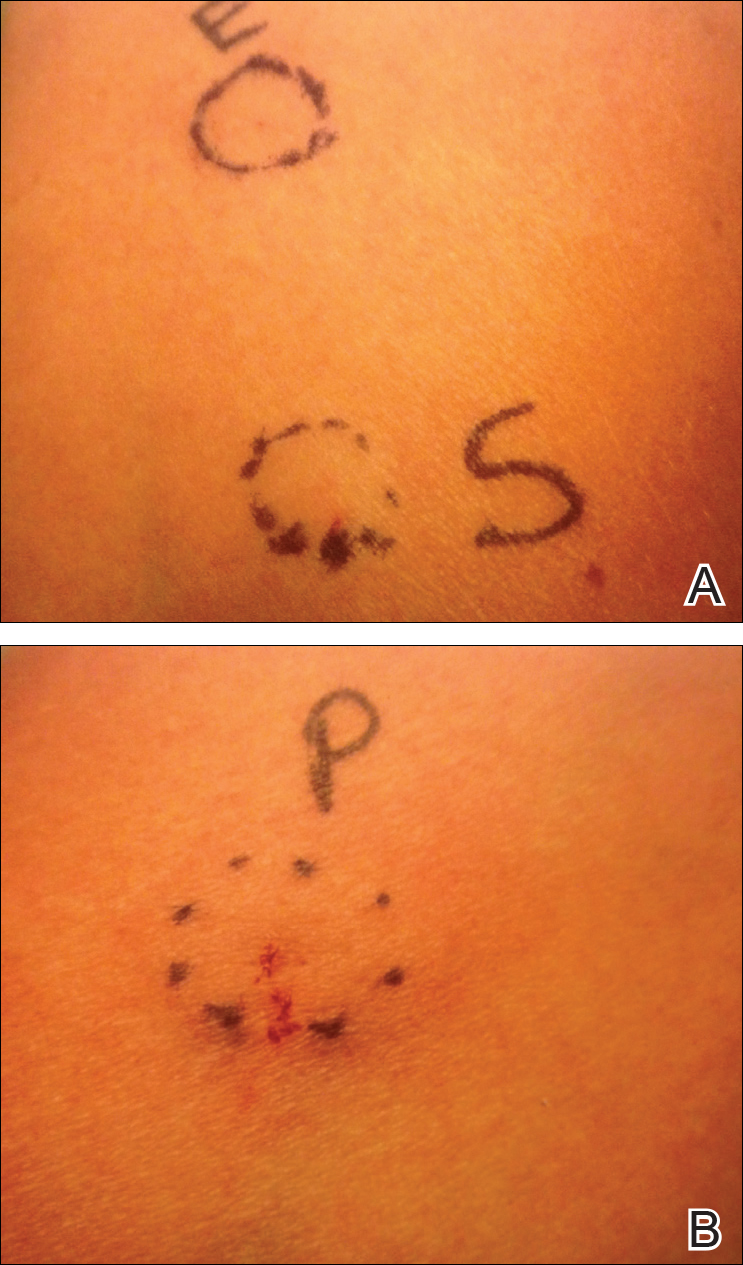
Approximately 1 year after her initial presentation, the patient returned for intradermal hormone injections to test for hormonally induced hypersensitivities. An injection of0.1 mL of a 50-mg/mL progesterone solution was administered in the right forearm as well as 0.1 mL of a 5-mg/mL estradiol solution and 0.1 mL of saline in the left forearm as a control. One hour after the injections, a strong positive reaction consisting of a 15-mm indurated plaque with surrounding wheal was noted at the site of the progesterone injection. The estradiol and saline control sites were clear of any dermal reaction (Figure 2). A diagnosis of APD was established, and the patient was referred to her gynecologist for treatment.
Due to the aggressive nature of her endometriosis, the gonadotropin-releasing hormone agonist leuprolide acetate was the first-line treatment prescribed by her gynecologist; however, after 8 months of therapy with leuprolide acetate, she was still experiencing breakthrough myalgia with her menstrual cycle and opted for a hysterectomy with a bilateral salpingo-oophorectomy. Within weeks of surgery, the myalgia ceased and the patient was completely asymptomatic.
Autoimmune progesterone dermatitis was first described in 1921.2 In affected women, the body reacts to the progesterone hormone surge during the luteal phase of the menstrual cycle. Symptoms begin approximately 3 to 4 days prior to menses and resolve 2 to 3 days after onset of flow. These progesterone hypersensitivity reactions can present within a spectrum of morphologies and severities. The lesions can appear eczematous, urticarial, as an angioedemalike reaction, as an erythema multiforme–like reaction with targetoid lesions, or in other nonspecific ways.1,3 Some patients experience a very mild, almost asymptomatic reaction, while others have a profound reaction progressing to anaphylaxis. Originally it was thought that exogenous exposure to progesterone led to a cross-reaction or hypersensitivity to the hormone; however, there have been cases reported in females as young as 12 years of age with no prior exposure.3,4 Reactions also can vary during pregnancy. There have been reports of spontaneous abortion in some affected females, but symptoms may dissipate in others, possibly due to a slow rise in progesterone causing a desensitization reaction.3,5
According to Bandino et al,6 there are 3 criteria for diagnosis of APD: (1) skin lesions related to the menstrual cycle, (2) positive response to intradermal testing with progesterone, and (3) symptomatic improvement after inhibiting progesterone secretions by suppressing ovulation.Areas checked with intradermal testing need to be evaluated 24 and 48 hours later for possible immediate or delayed-type hypersensitivity reactions. Biopsy typically is not helpful in this diagnosis because results usually are nonspecific.
Treatment of APD is targeted toward suppressing the internal hormonal surge. By suppressing the progesterone hormone, the symptoms are alleviated. The discomfort from the skin reaction typically is unresponsive to steroids or antihistamines. Oral contraceptives are first line in most cases because they suppress ovulation. Gonadotropin-releasing hormone analogues and tamoxifen also have been successful. For patients with severe disease that is recalcitrant to standard therapy or those who are postmenopausal, an oophorectemy is a curative option.2,4,5,7
Autoimmune progesterone dermatitis is a rare cyclical dermatologic condition in which the body responds to a surge of the patient’s own progesterone hormone. The disorder is difficult to diagnose because it can present with differing morphologies and biopsy is nonspecific. It also can be increasingly difficult to diagnose in women who do not have a typical 28-day menstrual cycle. In our patient, her irregular menstrual cycle may have caused a delay in diagnosis. Although the condition is rare, APD should be included in the differential diagnosis in females with a recurrent, cyclical, or recalcitrant cutaneous eruption.
- Wojnarowska F, Greaves MW, Peachey RD, et al. Progesterone-induced erythema multiforme. J R Soc Med. 1985;78:407-408.
- Lee MK, Lee WY, Yong SJ, et al. A case of autoimmune progesterone dermatitis misdiagnosed as allergic contact dermatitis [published online February 9, 2011]. Allergy Asthma Immunol Res. 2011;3:141-144.
- Baptist AP, Baldwin JL. Autoimmune progesterone dermatitis in a patient with endometriosis: a case report and review of the literature. Clin Mol Allergy. 2004;2:10.
- Baççıoğlu A, Kocak M, Bozdag O, et al. An unusual form of autoimmune progesterone dermatitis (ADP): the role of diagnostic challenge test. World Allergy Organ J. 2007;10:S52.
- George R, Badawy SZ. Autoimmune progesterone dermatitis: a case report [published online August 9, 2012]. Case Rep Obstet Gynecol. doi:10.1155/2012/757854.
- Bandino JP, Thoppil J, Kennedy JS, et al. Iatrogenic autoimmune progesterone dermatitis causes by 17α-hydroxyprogesterone caproate for preterm labor prevention. Cutis. 2011;88:241-243.
- Magen E, Feldman V. Autoimmune progesterone anaphylaxis in a 24-year-old woman. Isr Med Assoc J. 2012;14:518-519.
To the Editor:
Autoimmune progesterone dermatitis (APD) is a rare dermatologic condition that can be challenging to diagnose. The associated skin lesions are not only variable in physical presentation but also in the timing of the outbreak. The skin disorder stems from an internal reaction to elevated levels of progesterone during the luteal phase of the menstrual cycle. Autoimmune progesterone dermatitis can be difficult to detect; although the typical menstrual cycle is 28 days, many women have longer or shorter hormonal phases, leading to cyclical irregularity that can cause the lesions to appear sporadic in nature when in fact they are not.1
A 34-year-old woman with a history of endometriosis, psoriasis, and malignant melanoma presented to our dermatology clinic 2 days after a brief hospitalization during which she was diagnosed with a hypersensitivity reaction. Two days prior to her hospital admission, the patient developed a rash on the lower back with associated myalgia. The rash progressively worsened, spreading laterally to the flanks, which prompted her to seek medical attention. Blood work included a complete blood cell count with differential, complete metabolic panel, antinuclear antibody test, and erythrocyte sedimentation rate, which all were within reference range. A 4-mm punch biopsy from the left lateral flank was performed and was consistent with a neutrophilic dermatosis. The patient’s symptoms diminished and she was discharged the next day with instructions to follow up with a dermatologist.
Physical examination at our clinic revealed multiple minimally indurated, erythematous plaques with superficial scaling along the left lower back and upper buttock (Figure 1). No other skin lesions were present, and palpation of the cervical, axillary, and inguinal lymph nodes was unremarkable. A repeat 6-mm punch biopsy was performed and she was sent for fasting blood work.

Histologic examination of the punch biopsy revealed a superficial and deep perivascular and interstitial dermatitis with scattered neutrophils and eosinophils. Findings were described as nonspecific, possibly representing a dermal hypersensitivity or urticarial reaction.
Glucose-6-phosphate dehydrogenase testing was within reference range, and therapy was initiated with oral dapsone 50 mg once daily as well as fexofenadine 180 mg once daily. The patient initially responded well to the oral therapy, but she experienced recurrence of the skin eruption at infrequent intervals over the next few months, requiring escalating doses of dapsone to control the symptoms. After further questioning at a subsequent visit a few months later, it was discovered that the eruption occurred near the onset of the patient’s irregular menstrual cycle.
Approximately 1 year after her initial presentation, the patient returned for intradermal hormone injections to test for hormonally induced hypersensitivities. An injection of0.1 mL of a 50-mg/mL progesterone solution was administered in the right forearm as well as 0.1 mL of a 5-mg/mL estradiol solution and 0.1 mL of saline in the left forearm as a control. One hour after the injections, a strong positive reaction consisting of a 15-mm indurated plaque with surrounding wheal was noted at the site of the progesterone injection. The estradiol and saline control sites were clear of any dermal reaction (Figure 2). A diagnosis of APD was established, and the patient was referred to her gynecologist for treatment.

Due to the aggressive nature of her endometriosis, the gonadotropin-releasing hormone agonist leuprolide acetate was the first-line treatment prescribed by her gynecologist; however, after 8 months of therapy with leuprolide acetate, she was still experiencing breakthrough myalgia with her menstrual cycle and opted for a hysterectomy with a bilateral salpingo-oophorectomy. Within weeks of surgery, the myalgia ceased and the patient was completely asymptomatic.
Autoimmune progesterone dermatitis was first described in 1921.2 In affected women, the body reacts to the progesterone hormone surge during the luteal phase of the menstrual cycle. Symptoms begin approximately 3 to 4 days prior to menses and resolve 2 to 3 days after onset of flow. These progesterone hypersensitivity reactions can present within a spectrum of morphologies and severities. The lesions can appear eczematous, urticarial, as an angioedemalike reaction, as an erythema multiforme–like reaction with targetoid lesions, or in other nonspecific ways.1,3 Some patients experience a very mild, almost asymptomatic reaction, while others have a profound reaction progressing to anaphylaxis. Originally it was thought that exogenous exposure to progesterone led to a cross-reaction or hypersensitivity to the hormone; however, there have been cases reported in females as young as 12 years of age with no prior exposure.3,4 Reactions also can vary during pregnancy. There have been reports of spontaneous abortion in some affected females, but symptoms may dissipate in others, possibly due to a slow rise in progesterone causing a desensitization reaction.3,5
According to Bandino et al,6 there are 3 criteria for diagnosis of APD: (1) skin lesions related to the menstrual cycle, (2) positive response to intradermal testing with progesterone, and (3) symptomatic improvement after inhibiting progesterone secretions by suppressing ovulation.Areas checked with intradermal testing need to be evaluated 24 and 48 hours later for possible immediate or delayed-type hypersensitivity reactions. Biopsy typically is not helpful in this diagnosis because results usually are nonspecific.
Treatment of APD is targeted toward suppressing the internal hormonal surge. By suppressing the progesterone hormone, the symptoms are alleviated. The discomfort from the skin reaction typically is unresponsive to steroids or antihistamines. Oral contraceptives are first line in most cases because they suppress ovulation. Gonadotropin-releasing hormone analogues and tamoxifen also have been successful. For patients with severe disease that is recalcitrant to standard therapy or those who are postmenopausal, an oophorectemy is a curative option.2,4,5,7
Autoimmune progesterone dermatitis is a rare cyclical dermatologic condition in which the body responds to a surge of the patient’s own progesterone hormone. The disorder is difficult to diagnose because it can present with differing morphologies and biopsy is nonspecific. It also can be increasingly difficult to diagnose in women who do not have a typical 28-day menstrual cycle. In our patient, her irregular menstrual cycle may have caused a delay in diagnosis. Although the condition is rare, APD should be included in the differential diagnosis in females with a recurrent, cyclical, or recalcitrant cutaneous eruption.
To the Editor:
Autoimmune progesterone dermatitis (APD) is a rare dermatologic condition that can be challenging to diagnose. The associated skin lesions are not only variable in physical presentation but also in the timing of the outbreak. The skin disorder stems from an internal reaction to elevated levels of progesterone during the luteal phase of the menstrual cycle. Autoimmune progesterone dermatitis can be difficult to detect; although the typical menstrual cycle is 28 days, many women have longer or shorter hormonal phases, leading to cyclical irregularity that can cause the lesions to appear sporadic in nature when in fact they are not.1
A 34-year-old woman with a history of endometriosis, psoriasis, and malignant melanoma presented to our dermatology clinic 2 days after a brief hospitalization during which she was diagnosed with a hypersensitivity reaction. Two days prior to her hospital admission, the patient developed a rash on the lower back with associated myalgia. The rash progressively worsened, spreading laterally to the flanks, which prompted her to seek medical attention. Blood work included a complete blood cell count with differential, complete metabolic panel, antinuclear antibody test, and erythrocyte sedimentation rate, which all were within reference range. A 4-mm punch biopsy from the left lateral flank was performed and was consistent with a neutrophilic dermatosis. The patient’s symptoms diminished and she was discharged the next day with instructions to follow up with a dermatologist.
Physical examination at our clinic revealed multiple minimally indurated, erythematous plaques with superficial scaling along the left lower back and upper buttock (Figure 1). No other skin lesions were present, and palpation of the cervical, axillary, and inguinal lymph nodes was unremarkable. A repeat 6-mm punch biopsy was performed and she was sent for fasting blood work.

Histologic examination of the punch biopsy revealed a superficial and deep perivascular and interstitial dermatitis with scattered neutrophils and eosinophils. Findings were described as nonspecific, possibly representing a dermal hypersensitivity or urticarial reaction.
Glucose-6-phosphate dehydrogenase testing was within reference range, and therapy was initiated with oral dapsone 50 mg once daily as well as fexofenadine 180 mg once daily. The patient initially responded well to the oral therapy, but she experienced recurrence of the skin eruption at infrequent intervals over the next few months, requiring escalating doses of dapsone to control the symptoms. After further questioning at a subsequent visit a few months later, it was discovered that the eruption occurred near the onset of the patient’s irregular menstrual cycle.
Approximately 1 year after her initial presentation, the patient returned for intradermal hormone injections to test for hormonally induced hypersensitivities. An injection of0.1 mL of a 50-mg/mL progesterone solution was administered in the right forearm as well as 0.1 mL of a 5-mg/mL estradiol solution and 0.1 mL of saline in the left forearm as a control. One hour after the injections, a strong positive reaction consisting of a 15-mm indurated plaque with surrounding wheal was noted at the site of the progesterone injection. The estradiol and saline control sites were clear of any dermal reaction (Figure 2). A diagnosis of APD was established, and the patient was referred to her gynecologist for treatment.

Due to the aggressive nature of her endometriosis, the gonadotropin-releasing hormone agonist leuprolide acetate was the first-line treatment prescribed by her gynecologist; however, after 8 months of therapy with leuprolide acetate, she was still experiencing breakthrough myalgia with her menstrual cycle and opted for a hysterectomy with a bilateral salpingo-oophorectomy. Within weeks of surgery, the myalgia ceased and the patient was completely asymptomatic.
Autoimmune progesterone dermatitis was first described in 1921.2 In affected women, the body reacts to the progesterone hormone surge during the luteal phase of the menstrual cycle. Symptoms begin approximately 3 to 4 days prior to menses and resolve 2 to 3 days after onset of flow. These progesterone hypersensitivity reactions can present within a spectrum of morphologies and severities. The lesions can appear eczematous, urticarial, as an angioedemalike reaction, as an erythema multiforme–like reaction with targetoid lesions, or in other nonspecific ways.1,3 Some patients experience a very mild, almost asymptomatic reaction, while others have a profound reaction progressing to anaphylaxis. Originally it was thought that exogenous exposure to progesterone led to a cross-reaction or hypersensitivity to the hormone; however, there have been cases reported in females as young as 12 years of age with no prior exposure.3,4 Reactions also can vary during pregnancy. There have been reports of spontaneous abortion in some affected females, but symptoms may dissipate in others, possibly due to a slow rise in progesterone causing a desensitization reaction.3,5
According to Bandino et al,6 there are 3 criteria for diagnosis of APD: (1) skin lesions related to the menstrual cycle, (2) positive response to intradermal testing with progesterone, and (3) symptomatic improvement after inhibiting progesterone secretions by suppressing ovulation.Areas checked with intradermal testing need to be evaluated 24 and 48 hours later for possible immediate or delayed-type hypersensitivity reactions. Biopsy typically is not helpful in this diagnosis because results usually are nonspecific.
Treatment of APD is targeted toward suppressing the internal hormonal surge. By suppressing the progesterone hormone, the symptoms are alleviated. The discomfort from the skin reaction typically is unresponsive to steroids or antihistamines. Oral contraceptives are first line in most cases because they suppress ovulation. Gonadotropin-releasing hormone analogues and tamoxifen also have been successful. For patients with severe disease that is recalcitrant to standard therapy or those who are postmenopausal, an oophorectemy is a curative option.2,4,5,7
Autoimmune progesterone dermatitis is a rare cyclical dermatologic condition in which the body responds to a surge of the patient’s own progesterone hormone. The disorder is difficult to diagnose because it can present with differing morphologies and biopsy is nonspecific. It also can be increasingly difficult to diagnose in women who do not have a typical 28-day menstrual cycle. In our patient, her irregular menstrual cycle may have caused a delay in diagnosis. Although the condition is rare, APD should be included in the differential diagnosis in females with a recurrent, cyclical, or recalcitrant cutaneous eruption.
- Wojnarowska F, Greaves MW, Peachey RD, et al. Progesterone-induced erythema multiforme. J R Soc Med. 1985;78:407-408.
- Lee MK, Lee WY, Yong SJ, et al. A case of autoimmune progesterone dermatitis misdiagnosed as allergic contact dermatitis [published online February 9, 2011]. Allergy Asthma Immunol Res. 2011;3:141-144.
- Baptist AP, Baldwin JL. Autoimmune progesterone dermatitis in a patient with endometriosis: a case report and review of the literature. Clin Mol Allergy. 2004;2:10.
- Baççıoğlu A, Kocak M, Bozdag O, et al. An unusual form of autoimmune progesterone dermatitis (ADP): the role of diagnostic challenge test. World Allergy Organ J. 2007;10:S52.
- George R, Badawy SZ. Autoimmune progesterone dermatitis: a case report [published online August 9, 2012]. Case Rep Obstet Gynecol. doi:10.1155/2012/757854.
- Bandino JP, Thoppil J, Kennedy JS, et al. Iatrogenic autoimmune progesterone dermatitis causes by 17α-hydroxyprogesterone caproate for preterm labor prevention. Cutis. 2011;88:241-243.
- Magen E, Feldman V. Autoimmune progesterone anaphylaxis in a 24-year-old woman. Isr Med Assoc J. 2012;14:518-519.
- Wojnarowska F, Greaves MW, Peachey RD, et al. Progesterone-induced erythema multiforme. J R Soc Med. 1985;78:407-408.
- Lee MK, Lee WY, Yong SJ, et al. A case of autoimmune progesterone dermatitis misdiagnosed as allergic contact dermatitis [published online February 9, 2011]. Allergy Asthma Immunol Res. 2011;3:141-144.
- Baptist AP, Baldwin JL. Autoimmune progesterone dermatitis in a patient with endometriosis: a case report and review of the literature. Clin Mol Allergy. 2004;2:10.
- Baççıoğlu A, Kocak M, Bozdag O, et al. An unusual form of autoimmune progesterone dermatitis (ADP): the role of diagnostic challenge test. World Allergy Organ J. 2007;10:S52.
- George R, Badawy SZ. Autoimmune progesterone dermatitis: a case report [published online August 9, 2012]. Case Rep Obstet Gynecol. doi:10.1155/2012/757854.
- Bandino JP, Thoppil J, Kennedy JS, et al. Iatrogenic autoimmune progesterone dermatitis causes by 17α-hydroxyprogesterone caproate for preterm labor prevention. Cutis. 2011;88:241-243.
- Magen E, Feldman V. Autoimmune progesterone anaphylaxis in a 24-year-old woman. Isr Med Assoc J. 2012;14:518-519.
Practice Points
- Autoimmune progesterone dermatitis (APD) is a hypersensitivity reaction to the progesterone surge during a woman’s menstrual cycle.
- Patients with APD often are misdiagnosed for years due to the variability of each woman’s menstrual cycle, making the correlation difficult.
- It is important to keep APD in mind for any recalcitrant or recurrent rash in females. A thorough history is critical when formulating a diagnosis.
Metastatic Vulvovaginal Crohn Disease in the Setting of Well-Controlled Intestinal Disease
The cutaneous manifestations of Crohn disease (CD) are varied, including pyoderma gangrenosum, erythema nodosum, and metastatic CD (MCD). First described by Parks et al,1 MCD is defined as the occurrence of granulomatous lesions at a skin site distant from the gastrointestinal tract.1-20 Metastatic CD presents a diagnostic challenge because it is a rare component in the spectrum of inflammatory bowel disease complications, and many physicians are unaware of its existence. It may precede, coincide with, or develop after the diagnosis of intestinal disease.2-5 Vulvoperineal involvement is particularly problematic because a multitude of other, more likely disease processes are considered first. Typically it is initially diagnosed as a presumed infection prompting reflexive treatment with antivirals, antifungals, and antibiotics. Patients may experience symptoms for years prior to correct diagnosis and institution of proper therapy. A variety of clinical presentations have been described, including nonspecific pain and swelling, erythematous papules and plaques, and nonhealing ulcers. Skin biopsy characteristically confirms the diagnosis and reveals dermal noncaseating granulomas. Multiple oral and parenteral therapies are available, with surgical intervention reserved for resistant cases. We present a case of vulvovaginal MCD in the setting of well-controlled intestinal disease. We also provide a review of the literature regarding genital CD and emphasize the need to keep MCD in the differential of vulvoperineal pathology.
Case Report
A 29-year-old woman was referred to the dermatology clinic with vulvar pain, swelling, and pruritus of 14 months’ duration. Her medical history was remarkable for CD following a colectomy with colostomy. Prior therapies included methotrexate with infliximab for 5 years followed by a 2-year regimen with adalimumab, which induced remission of the intestinal disease.
The patient previously had taken a variety of topical and oral antimicrobials based on treatment from a primary care physician because fungal, bacterial, and viral infections initially were suspected; however, the vulvar disease persisted, and drug-induced immunosuppression was considered to be an underlying factor. Thus, adalimumab was discontinued. Despite elimination of the biologic, the vulvar disease progressed, which prompted referral to the dermatology clinic.
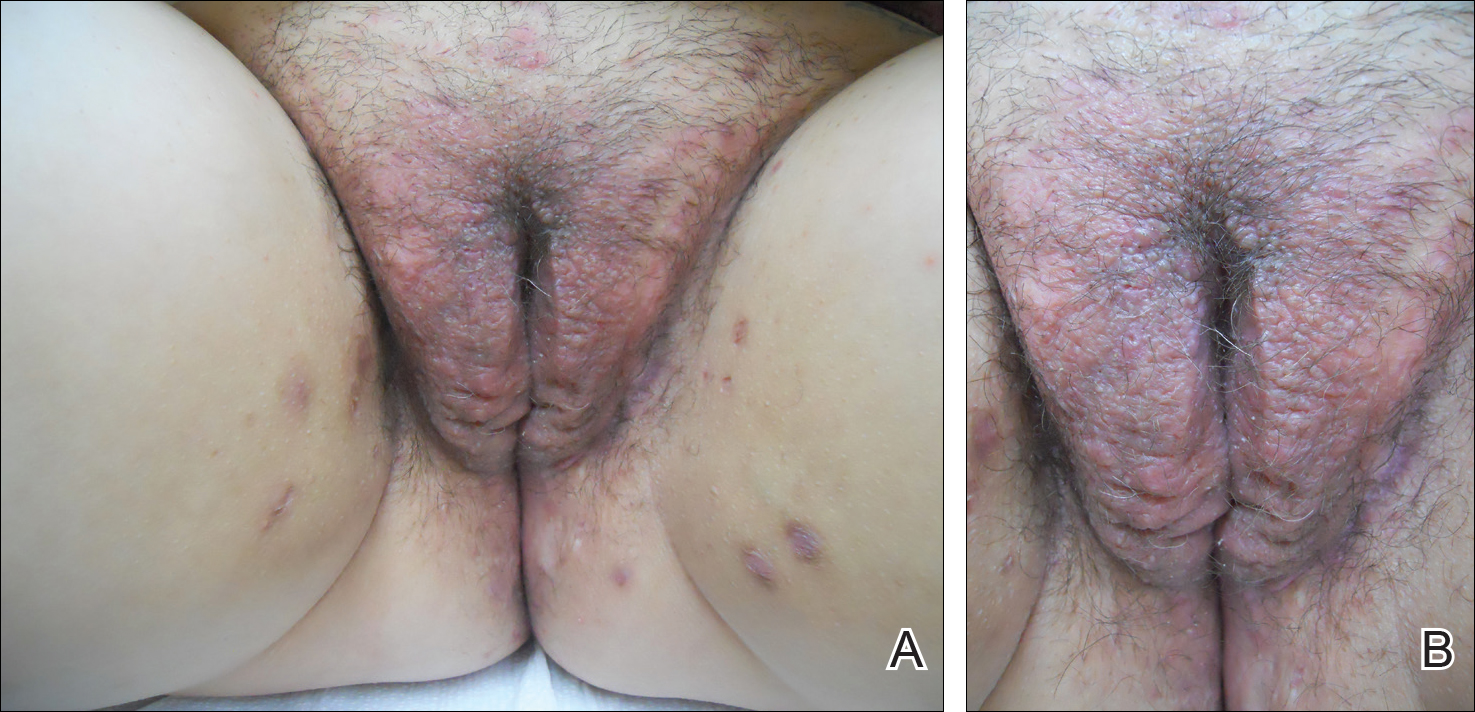
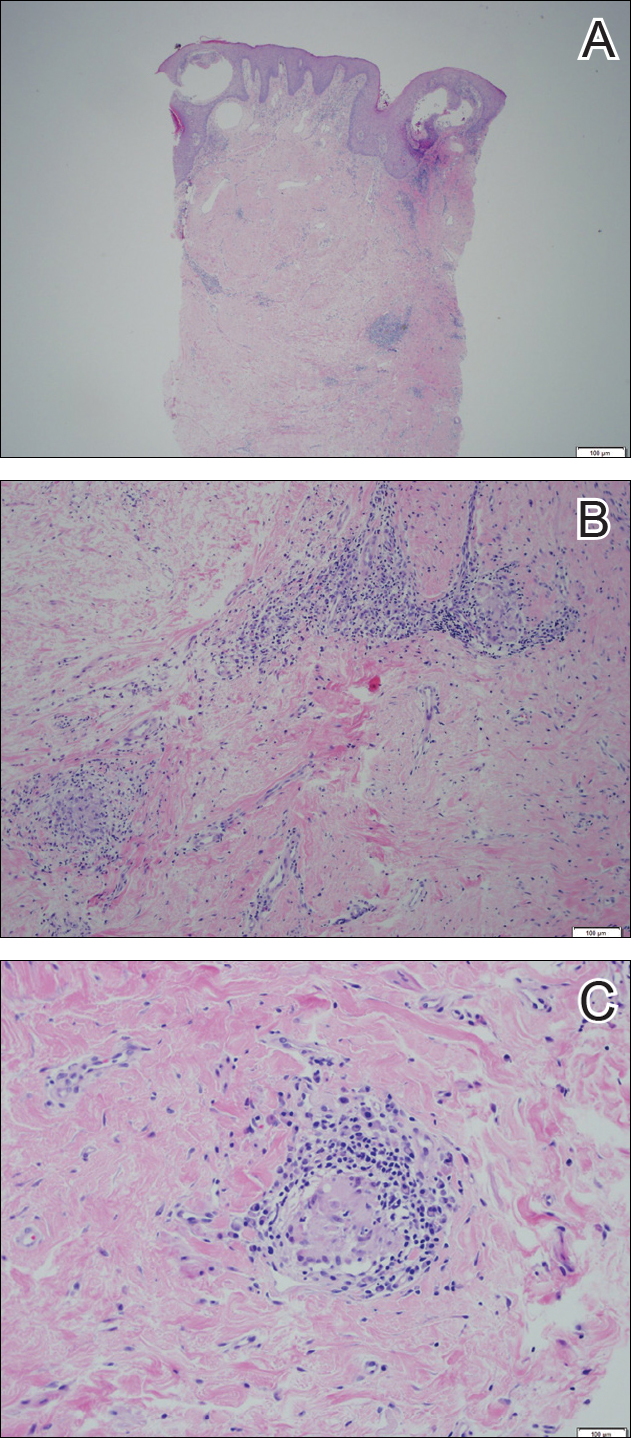
Physical examination revealed diffuse vulvar edema with overlying erythema and scale (Figure 1A). Upon closer inspection, scattered violaceous papules atop a backdrop of lichenification were evident, imparting a cobblestone appearance (Figure 1B). Additionally, a fissure was present on the gluteal cleft. Biopsy from the left labia majora demonstrated well-formed granulomas within a fibrotic reticular dermis (Figures 2A and 2B). The granulomas consisted of both mononucleated and multinucleated histiocytes, rimmed peripherally by lymphocytes and plasma cells (Figure 2C). Periodic acid–Schiff–diastase and acid-fast bacilli stains as well as polarizing microscopy were negative.
Given the patient’s history, a diagnosis of vulvoperineal MCD was rendered. The patient was started on oral metronidazole 250 mg 3 times daily with topical fluocinonide and tacrolimus. She responded well to this treatment regimen and was referred back to the gastroenterologist for management of the intestinal disease.
Comment
Crohn disease is an idiopathic chronic inflammatory condition that primarily affects the gastrointestinal tract, anywhere from the mouth to the anus. It is characterized by transmural inflammation and fissures that can extend beyond the muscularis propria.4,6 Extraintestinal manifestations are common.3
Cutaneous CD often presents as perianal, perifistular, or peristomal inflammation or ulceration.7 Other skin manifestations include pyoderma gangrenosum, erythema nodosum, erythema multiforme, epidermolysis bullosa acquisita, and palmar erythema.7 Metastatic CD involves skin noncontiguous with the gastrointestinal tract1-20 and may involve any portion of the cutis. Although rare, MCD is the typical etiology underlying vulvar CD.8
Approximately 20% of MCD patients have cutaneous lesions without a history of gastrointestinal disease. More than half of cases in adults and approximately two-thirds in children involve the genitalia. Although more common in adults, vulvar involvement has been reported in children as young as 6 years of age.2 Diagnosis is especially challenging when bowel symptoms are absent; those patients should be evaluated and followed for subsequent intestinal involvement.6
Clinically, symptoms may include general discomfort, pain, pruritus, and dyspareunia. Psychosocial and sexual dysfunction are prevalent and also should be addressed.9 Depending on the stage of the disease, physical examination may reveal erythema, edema, papules, pustules, nodules, condylomatous lesions, abscesses, fissures, fistulas, ulceration, acrochordons, and scarring.2-6,10,11
A host of infections (ie, mycobacterial, actinomycosis, deep fungal, sexually transmitted, schistosomiasis), inflammatory conditions (ie, sarcoid, hidradenitis suppurativa), foreign body reactions, Melkersson-Rosenthal syndrome, and sexual abuse should be included in the differential diagnosis.2,6,10-12 Once infection, sarcoid, and foreign body reaction have been ruled out, noncaseating granulomas in skin are highly suggestive of CD.7
Histopathologic findings of MCD reveal myriad morphological reaction patterns,5,13 including high-grade dysplasia and carcinoma of the vulva; therefore, it may be imprudent to withhold diagnosis based on the absence of the historically pathognomonic noncaseating granulomas.5
The etiopathogenesis of MCD remains an enigma. Dermatopathologic examinations consistently reveal a vascular injury syndrome,13 implicating a possible circulatory system contribution via deposition of immune complexes or antigens in skin.7 Bacterial infection has been implicated in the intestinal manifestations of CD; however, failure to detect microbial ribosomal RNA in MCD biopsies refutes theories of hematogenous spread of microbes.13 Another plausible explanation is that antibodies are formed to conserved microbial epitopes following loss of tolerance to gut flora, which results in an excessive immunologic response at distinct sites in susceptible individuals.13 A T-lymphocyte–mediated type IV hypersensitivity reaction also has been proposed via cross-reactivity of lymphocytes, with skin antigens precipitating extraintestinal granuloma formation and vascular injury.3 Clearly, further investigation is needed.
Magnetic resonanance imaging can identify the extent and anatomy of intestinal and pelvic disease and can assist in the diagnosis of vulvar CD.10,11,14 For these reasons, some experts propose that imaging should be instituted prior to therapy,12,15,16 especially when direct extension is suspected.17
Treatment is challenging and often involves collaboration among several specialties.12 Many treatment options exist because therapeutic responses vary and genital MCD is frequently recalcitrant to therapy.4 Medical therapy includes antibiotics such as metronidazole, corticosteroids (ie, topical, intralesional, systemic), and immune modulators (eg, azathioprine, 6-mercaptopurine, cyclosporine, methotrexate, mycophenolate mofetil, tumor necrosis factor α inhibitors).2,3,6,10,16,18 Thalidomide has been used for refractory cases.19 These treatments can be used alone or in combination. Patients should be monitored for side effects and informed that many treatment regimens may be required before a sustained response is achieved.4,16,18 Surgery is reserved for the most resistant cases. Extensive radical excision of the involved area is the best approach, as limited local excision often is followed by recurrence.20
Conclusion
Our case highlights that vulvar CD can develop in the setting of well-controlled intestinal disease. Vulvoperineal CD should be considered in the differential diagnosis of chronic vulvar pain, swelling, and pruritus, especially in cases resistant to standard therapies and regardless of whether or not gastrointestinal tract symptoms are present. Physicians must be cognizant that vulvar signs and symptoms may precede, coincide with, or follow the diagnosis of intestinal CD. Increased awareness of this entity may facilitate its early recognition and prompt more timely treatment among women with vulvar disease caused by MCD.
- Parks AG, Morson BC, Pegum JS. Crohn’s disease with cutaneous involvement. Proc R Soc Med. 1965;58:241-242.
- Ploysangam T, Heubi JE, Eisen D, et al. Cutaneous Crohn’s disease in children. J Am Acad Dermatol. 1997;36:697-704.
- Palamaras I, El-Jabbour J, Pietropaolo N, et al. Metastatic Crohn’s disease: a review. J Eur Acad Dermatol Venereol. 2008;22:1033-1043.
- Leu S, Sun PK, Collyer J, et al. Clinical spectrum of vulvar metastatic Crohn’s disease. Dig Dis Sci. 2009;54:1565-1571.
- Foo WC, Papalas JA, Robboy SJ, et al. Vulvar manifestations of Crohn’s disease. Am J Dermatopathol. 2001;33:588-593.
- Urbanek M, Neill SM, McKee PH. Vulval Crohn’s disease: difficulties in diagnosis. Clin Exp Dermatol. 1996;21:211-214.
- Burgdorf W. Cutaneous manifestations of Crohn’s disease. J Am Acad Dermatol. 1981;5:689-695.
- Andreani SM, Ratnasingham K, Dang HH, et al. Crohn’s disease of the vulva. Int J Surg. 2010;8:2-5.
- Feller E, Ribaudo S, Jackson N. Gynecologic aspects of Crohn’s disease. Am Fam Physician. 2001;64:1725-1728.
- Corbett SL, Walsh CM, Spitzer RF, et al. Vulvar inflammation as the only clinical manifestation of Crohn disease in an 8-year-old girl [published online May 10, 2010]. Pediatrics. 2010;125:E1518-E1522.
- Tonolini M, Villa C, Campari A, et al. Common and unusual urogenital Crohn’s disease complications: spectrum of cross-sectional imaging findings. Abdom Imaging. 2013;38:32-41.
- Bhaduri S, Jenkinson S, Lewis F. Vulval Crohn’s disease—a multi-specialty approach. Int J STD AIDS. 2005;16:512-514.
- Crowson AN, Nuovo GJ, Mihm MC Jr, et al. Cutaneous manifestations of Crohn’s disease, its spectrum, and its pathogenesis: intracellular consensus bacterial 16S rRNA is associated with the gastrointestinal but not the cutaneous manifestations of Crohn’s disease. Hum Pathol. 2003;34:1185-1192.
- Pai D, Dillman JR, Mahani MG, et al. MRI of vulvar Crohn disease. Pediatr Radiol. 2011;41:537-541.
- Madnani NA, Desai D, Gandhi N, et al. Isolated Crohn’s disease of the vulva. Indian J Dermatol Venereol Leprol. 2011;77:342-344.
- Makhija S, Trotter M, Wagner E, et al. Refractory Crohn’s disease of the vulva treated with infliximab: a case report. Can J Gastroenterol. 2007;21:835-837.
- Fahmy N, Kalidindi M, Khan R. Direct colo-labial Crohn’s abscess mimicking bartholinitis. Am J Obstret Gynecol. 2010;30:741-742.
- Preston PW, Hudson N, Lewis FM. Treatment of vulval Crohn’s disease with infliximab. Clin Exp Derm. 2006;31:378-380.
- Kolivras A, De Maubeuge J, André J, et al. Thalidomide in refractory vulvar ulcerations associated with Crohn’s disease. Dermatology. 2003;206:381-383.
- Kao MS, Paulson JD, Askin FB. Crohn’s disease of the vulva. Obstet Gynecol. 1975;46:329-333.
The cutaneous manifestations of Crohn disease (CD) are varied, including pyoderma gangrenosum, erythema nodosum, and metastatic CD (MCD). First described by Parks et al,1 MCD is defined as the occurrence of granulomatous lesions at a skin site distant from the gastrointestinal tract.1-20 Metastatic CD presents a diagnostic challenge because it is a rare component in the spectrum of inflammatory bowel disease complications, and many physicians are unaware of its existence. It may precede, coincide with, or develop after the diagnosis of intestinal disease.2-5 Vulvoperineal involvement is particularly problematic because a multitude of other, more likely disease processes are considered first. Typically it is initially diagnosed as a presumed infection prompting reflexive treatment with antivirals, antifungals, and antibiotics. Patients may experience symptoms for years prior to correct diagnosis and institution of proper therapy. A variety of clinical presentations have been described, including nonspecific pain and swelling, erythematous papules and plaques, and nonhealing ulcers. Skin biopsy characteristically confirms the diagnosis and reveals dermal noncaseating granulomas. Multiple oral and parenteral therapies are available, with surgical intervention reserved for resistant cases. We present a case of vulvovaginal MCD in the setting of well-controlled intestinal disease. We also provide a review of the literature regarding genital CD and emphasize the need to keep MCD in the differential of vulvoperineal pathology.
Case Report
A 29-year-old woman was referred to the dermatology clinic with vulvar pain, swelling, and pruritus of 14 months’ duration. Her medical history was remarkable for CD following a colectomy with colostomy. Prior therapies included methotrexate with infliximab for 5 years followed by a 2-year regimen with adalimumab, which induced remission of the intestinal disease.
The patient previously had taken a variety of topical and oral antimicrobials based on treatment from a primary care physician because fungal, bacterial, and viral infections initially were suspected; however, the vulvar disease persisted, and drug-induced immunosuppression was considered to be an underlying factor. Thus, adalimumab was discontinued. Despite elimination of the biologic, the vulvar disease progressed, which prompted referral to the dermatology clinic.
Physical examination revealed diffuse vulvar edema with overlying erythema and scale (Figure 1A). Upon closer inspection, scattered violaceous papules atop a backdrop of lichenification were evident, imparting a cobblestone appearance (Figure 1B). Additionally, a fissure was present on the gluteal cleft. Biopsy from the left labia majora demonstrated well-formed granulomas within a fibrotic reticular dermis (Figures 2A and 2B). The granulomas consisted of both mononucleated and multinucleated histiocytes, rimmed peripherally by lymphocytes and plasma cells (Figure 2C). Periodic acid–Schiff–diastase and acid-fast bacilli stains as well as polarizing microscopy were negative.


Given the patient’s history, a diagnosis of vulvoperineal MCD was rendered. The patient was started on oral metronidazole 250 mg 3 times daily with topical fluocinonide and tacrolimus. She responded well to this treatment regimen and was referred back to the gastroenterologist for management of the intestinal disease.
Comment
Crohn disease is an idiopathic chronic inflammatory condition that primarily affects the gastrointestinal tract, anywhere from the mouth to the anus. It is characterized by transmural inflammation and fissures that can extend beyond the muscularis propria.4,6 Extraintestinal manifestations are common.3
Cutaneous CD often presents as perianal, perifistular, or peristomal inflammation or ulceration.7 Other skin manifestations include pyoderma gangrenosum, erythema nodosum, erythema multiforme, epidermolysis bullosa acquisita, and palmar erythema.7 Metastatic CD involves skin noncontiguous with the gastrointestinal tract1-20 and may involve any portion of the cutis. Although rare, MCD is the typical etiology underlying vulvar CD.8
Approximately 20% of MCD patients have cutaneous lesions without a history of gastrointestinal disease. More than half of cases in adults and approximately two-thirds in children involve the genitalia. Although more common in adults, vulvar involvement has been reported in children as young as 6 years of age.2 Diagnosis is especially challenging when bowel symptoms are absent; those patients should be evaluated and followed for subsequent intestinal involvement.6
Clinically, symptoms may include general discomfort, pain, pruritus, and dyspareunia. Psychosocial and sexual dysfunction are prevalent and also should be addressed.9 Depending on the stage of the disease, physical examination may reveal erythema, edema, papules, pustules, nodules, condylomatous lesions, abscesses, fissures, fistulas, ulceration, acrochordons, and scarring.2-6,10,11
A host of infections (ie, mycobacterial, actinomycosis, deep fungal, sexually transmitted, schistosomiasis), inflammatory conditions (ie, sarcoid, hidradenitis suppurativa), foreign body reactions, Melkersson-Rosenthal syndrome, and sexual abuse should be included in the differential diagnosis.2,6,10-12 Once infection, sarcoid, and foreign body reaction have been ruled out, noncaseating granulomas in skin are highly suggestive of CD.7
Histopathologic findings of MCD reveal myriad morphological reaction patterns,5,13 including high-grade dysplasia and carcinoma of the vulva; therefore, it may be imprudent to withhold diagnosis based on the absence of the historically pathognomonic noncaseating granulomas.5
The etiopathogenesis of MCD remains an enigma. Dermatopathologic examinations consistently reveal a vascular injury syndrome,13 implicating a possible circulatory system contribution via deposition of immune complexes or antigens in skin.7 Bacterial infection has been implicated in the intestinal manifestations of CD; however, failure to detect microbial ribosomal RNA in MCD biopsies refutes theories of hematogenous spread of microbes.13 Another plausible explanation is that antibodies are formed to conserved microbial epitopes following loss of tolerance to gut flora, which results in an excessive immunologic response at distinct sites in susceptible individuals.13 A T-lymphocyte–mediated type IV hypersensitivity reaction also has been proposed via cross-reactivity of lymphocytes, with skin antigens precipitating extraintestinal granuloma formation and vascular injury.3 Clearly, further investigation is needed.
Magnetic resonanance imaging can identify the extent and anatomy of intestinal and pelvic disease and can assist in the diagnosis of vulvar CD.10,11,14 For these reasons, some experts propose that imaging should be instituted prior to therapy,12,15,16 especially when direct extension is suspected.17
Treatment is challenging and often involves collaboration among several specialties.12 Many treatment options exist because therapeutic responses vary and genital MCD is frequently recalcitrant to therapy.4 Medical therapy includes antibiotics such as metronidazole, corticosteroids (ie, topical, intralesional, systemic), and immune modulators (eg, azathioprine, 6-mercaptopurine, cyclosporine, methotrexate, mycophenolate mofetil, tumor necrosis factor α inhibitors).2,3,6,10,16,18 Thalidomide has been used for refractory cases.19 These treatments can be used alone or in combination. Patients should be monitored for side effects and informed that many treatment regimens may be required before a sustained response is achieved.4,16,18 Surgery is reserved for the most resistant cases. Extensive radical excision of the involved area is the best approach, as limited local excision often is followed by recurrence.20
Conclusion
Our case highlights that vulvar CD can develop in the setting of well-controlled intestinal disease. Vulvoperineal CD should be considered in the differential diagnosis of chronic vulvar pain, swelling, and pruritus, especially in cases resistant to standard therapies and regardless of whether or not gastrointestinal tract symptoms are present. Physicians must be cognizant that vulvar signs and symptoms may precede, coincide with, or follow the diagnosis of intestinal CD. Increased awareness of this entity may facilitate its early recognition and prompt more timely treatment among women with vulvar disease caused by MCD.
The cutaneous manifestations of Crohn disease (CD) are varied, including pyoderma gangrenosum, erythema nodosum, and metastatic CD (MCD). First described by Parks et al,1 MCD is defined as the occurrence of granulomatous lesions at a skin site distant from the gastrointestinal tract.1-20 Metastatic CD presents a diagnostic challenge because it is a rare component in the spectrum of inflammatory bowel disease complications, and many physicians are unaware of its existence. It may precede, coincide with, or develop after the diagnosis of intestinal disease.2-5 Vulvoperineal involvement is particularly problematic because a multitude of other, more likely disease processes are considered first. Typically it is initially diagnosed as a presumed infection prompting reflexive treatment with antivirals, antifungals, and antibiotics. Patients may experience symptoms for years prior to correct diagnosis and institution of proper therapy. A variety of clinical presentations have been described, including nonspecific pain and swelling, erythematous papules and plaques, and nonhealing ulcers. Skin biopsy characteristically confirms the diagnosis and reveals dermal noncaseating granulomas. Multiple oral and parenteral therapies are available, with surgical intervention reserved for resistant cases. We present a case of vulvovaginal MCD in the setting of well-controlled intestinal disease. We also provide a review of the literature regarding genital CD and emphasize the need to keep MCD in the differential of vulvoperineal pathology.
Case Report
A 29-year-old woman was referred to the dermatology clinic with vulvar pain, swelling, and pruritus of 14 months’ duration. Her medical history was remarkable for CD following a colectomy with colostomy. Prior therapies included methotrexate with infliximab for 5 years followed by a 2-year regimen with adalimumab, which induced remission of the intestinal disease.
The patient previously had taken a variety of topical and oral antimicrobials based on treatment from a primary care physician because fungal, bacterial, and viral infections initially were suspected; however, the vulvar disease persisted, and drug-induced immunosuppression was considered to be an underlying factor. Thus, adalimumab was discontinued. Despite elimination of the biologic, the vulvar disease progressed, which prompted referral to the dermatology clinic.
Physical examination revealed diffuse vulvar edema with overlying erythema and scale (Figure 1A). Upon closer inspection, scattered violaceous papules atop a backdrop of lichenification were evident, imparting a cobblestone appearance (Figure 1B). Additionally, a fissure was present on the gluteal cleft. Biopsy from the left labia majora demonstrated well-formed granulomas within a fibrotic reticular dermis (Figures 2A and 2B). The granulomas consisted of both mononucleated and multinucleated histiocytes, rimmed peripherally by lymphocytes and plasma cells (Figure 2C). Periodic acid–Schiff–diastase and acid-fast bacilli stains as well as polarizing microscopy were negative.


Given the patient’s history, a diagnosis of vulvoperineal MCD was rendered. The patient was started on oral metronidazole 250 mg 3 times daily with topical fluocinonide and tacrolimus. She responded well to this treatment regimen and was referred back to the gastroenterologist for management of the intestinal disease.
Comment
Crohn disease is an idiopathic chronic inflammatory condition that primarily affects the gastrointestinal tract, anywhere from the mouth to the anus. It is characterized by transmural inflammation and fissures that can extend beyond the muscularis propria.4,6 Extraintestinal manifestations are common.3
Cutaneous CD often presents as perianal, perifistular, or peristomal inflammation or ulceration.7 Other skin manifestations include pyoderma gangrenosum, erythema nodosum, erythema multiforme, epidermolysis bullosa acquisita, and palmar erythema.7 Metastatic CD involves skin noncontiguous with the gastrointestinal tract1-20 and may involve any portion of the cutis. Although rare, MCD is the typical etiology underlying vulvar CD.8
Approximately 20% of MCD patients have cutaneous lesions without a history of gastrointestinal disease. More than half of cases in adults and approximately two-thirds in children involve the genitalia. Although more common in adults, vulvar involvement has been reported in children as young as 6 years of age.2 Diagnosis is especially challenging when bowel symptoms are absent; those patients should be evaluated and followed for subsequent intestinal involvement.6
Clinically, symptoms may include general discomfort, pain, pruritus, and dyspareunia. Psychosocial and sexual dysfunction are prevalent and also should be addressed.9 Depending on the stage of the disease, physical examination may reveal erythema, edema, papules, pustules, nodules, condylomatous lesions, abscesses, fissures, fistulas, ulceration, acrochordons, and scarring.2-6,10,11
A host of infections (ie, mycobacterial, actinomycosis, deep fungal, sexually transmitted, schistosomiasis), inflammatory conditions (ie, sarcoid, hidradenitis suppurativa), foreign body reactions, Melkersson-Rosenthal syndrome, and sexual abuse should be included in the differential diagnosis.2,6,10-12 Once infection, sarcoid, and foreign body reaction have been ruled out, noncaseating granulomas in skin are highly suggestive of CD.7
Histopathologic findings of MCD reveal myriad morphological reaction patterns,5,13 including high-grade dysplasia and carcinoma of the vulva; therefore, it may be imprudent to withhold diagnosis based on the absence of the historically pathognomonic noncaseating granulomas.5
The etiopathogenesis of MCD remains an enigma. Dermatopathologic examinations consistently reveal a vascular injury syndrome,13 implicating a possible circulatory system contribution via deposition of immune complexes or antigens in skin.7 Bacterial infection has been implicated in the intestinal manifestations of CD; however, failure to detect microbial ribosomal RNA in MCD biopsies refutes theories of hematogenous spread of microbes.13 Another plausible explanation is that antibodies are formed to conserved microbial epitopes following loss of tolerance to gut flora, which results in an excessive immunologic response at distinct sites in susceptible individuals.13 A T-lymphocyte–mediated type IV hypersensitivity reaction also has been proposed via cross-reactivity of lymphocytes, with skin antigens precipitating extraintestinal granuloma formation and vascular injury.3 Clearly, further investigation is needed.
Magnetic resonanance imaging can identify the extent and anatomy of intestinal and pelvic disease and can assist in the diagnosis of vulvar CD.10,11,14 For these reasons, some experts propose that imaging should be instituted prior to therapy,12,15,16 especially when direct extension is suspected.17
Treatment is challenging and often involves collaboration among several specialties.12 Many treatment options exist because therapeutic responses vary and genital MCD is frequently recalcitrant to therapy.4 Medical therapy includes antibiotics such as metronidazole, corticosteroids (ie, topical, intralesional, systemic), and immune modulators (eg, azathioprine, 6-mercaptopurine, cyclosporine, methotrexate, mycophenolate mofetil, tumor necrosis factor α inhibitors).2,3,6,10,16,18 Thalidomide has been used for refractory cases.19 These treatments can be used alone or in combination. Patients should be monitored for side effects and informed that many treatment regimens may be required before a sustained response is achieved.4,16,18 Surgery is reserved for the most resistant cases. Extensive radical excision of the involved area is the best approach, as limited local excision often is followed by recurrence.20
Conclusion
Our case highlights that vulvar CD can develop in the setting of well-controlled intestinal disease. Vulvoperineal CD should be considered in the differential diagnosis of chronic vulvar pain, swelling, and pruritus, especially in cases resistant to standard therapies and regardless of whether or not gastrointestinal tract symptoms are present. Physicians must be cognizant that vulvar signs and symptoms may precede, coincide with, or follow the diagnosis of intestinal CD. Increased awareness of this entity may facilitate its early recognition and prompt more timely treatment among women with vulvar disease caused by MCD.
- Parks AG, Morson BC, Pegum JS. Crohn’s disease with cutaneous involvement. Proc R Soc Med. 1965;58:241-242.
- Ploysangam T, Heubi JE, Eisen D, et al. Cutaneous Crohn’s disease in children. J Am Acad Dermatol. 1997;36:697-704.
- Palamaras I, El-Jabbour J, Pietropaolo N, et al. Metastatic Crohn’s disease: a review. J Eur Acad Dermatol Venereol. 2008;22:1033-1043.
- Leu S, Sun PK, Collyer J, et al. Clinical spectrum of vulvar metastatic Crohn’s disease. Dig Dis Sci. 2009;54:1565-1571.
- Foo WC, Papalas JA, Robboy SJ, et al. Vulvar manifestations of Crohn’s disease. Am J Dermatopathol. 2001;33:588-593.
- Urbanek M, Neill SM, McKee PH. Vulval Crohn’s disease: difficulties in diagnosis. Clin Exp Dermatol. 1996;21:211-214.
- Burgdorf W. Cutaneous manifestations of Crohn’s disease. J Am Acad Dermatol. 1981;5:689-695.
- Andreani SM, Ratnasingham K, Dang HH, et al. Crohn’s disease of the vulva. Int J Surg. 2010;8:2-5.
- Feller E, Ribaudo S, Jackson N. Gynecologic aspects of Crohn’s disease. Am Fam Physician. 2001;64:1725-1728.
- Corbett SL, Walsh CM, Spitzer RF, et al. Vulvar inflammation as the only clinical manifestation of Crohn disease in an 8-year-old girl [published online May 10, 2010]. Pediatrics. 2010;125:E1518-E1522.
- Tonolini M, Villa C, Campari A, et al. Common and unusual urogenital Crohn’s disease complications: spectrum of cross-sectional imaging findings. Abdom Imaging. 2013;38:32-41.
- Bhaduri S, Jenkinson S, Lewis F. Vulval Crohn’s disease—a multi-specialty approach. Int J STD AIDS. 2005;16:512-514.
- Crowson AN, Nuovo GJ, Mihm MC Jr, et al. Cutaneous manifestations of Crohn’s disease, its spectrum, and its pathogenesis: intracellular consensus bacterial 16S rRNA is associated with the gastrointestinal but not the cutaneous manifestations of Crohn’s disease. Hum Pathol. 2003;34:1185-1192.
- Pai D, Dillman JR, Mahani MG, et al. MRI of vulvar Crohn disease. Pediatr Radiol. 2011;41:537-541.
- Madnani NA, Desai D, Gandhi N, et al. Isolated Crohn’s disease of the vulva. Indian J Dermatol Venereol Leprol. 2011;77:342-344.
- Makhija S, Trotter M, Wagner E, et al. Refractory Crohn’s disease of the vulva treated with infliximab: a case report. Can J Gastroenterol. 2007;21:835-837.
- Fahmy N, Kalidindi M, Khan R. Direct colo-labial Crohn’s abscess mimicking bartholinitis. Am J Obstret Gynecol. 2010;30:741-742.
- Preston PW, Hudson N, Lewis FM. Treatment of vulval Crohn’s disease with infliximab. Clin Exp Derm. 2006;31:378-380.
- Kolivras A, De Maubeuge J, André J, et al. Thalidomide in refractory vulvar ulcerations associated with Crohn’s disease. Dermatology. 2003;206:381-383.
- Kao MS, Paulson JD, Askin FB. Crohn’s disease of the vulva. Obstet Gynecol. 1975;46:329-333.
- Parks AG, Morson BC, Pegum JS. Crohn’s disease with cutaneous involvement. Proc R Soc Med. 1965;58:241-242.
- Ploysangam T, Heubi JE, Eisen D, et al. Cutaneous Crohn’s disease in children. J Am Acad Dermatol. 1997;36:697-704.
- Palamaras I, El-Jabbour J, Pietropaolo N, et al. Metastatic Crohn’s disease: a review. J Eur Acad Dermatol Venereol. 2008;22:1033-1043.
- Leu S, Sun PK, Collyer J, et al. Clinical spectrum of vulvar metastatic Crohn’s disease. Dig Dis Sci. 2009;54:1565-1571.
- Foo WC, Papalas JA, Robboy SJ, et al. Vulvar manifestations of Crohn’s disease. Am J Dermatopathol. 2001;33:588-593.
- Urbanek M, Neill SM, McKee PH. Vulval Crohn’s disease: difficulties in diagnosis. Clin Exp Dermatol. 1996;21:211-214.
- Burgdorf W. Cutaneous manifestations of Crohn’s disease. J Am Acad Dermatol. 1981;5:689-695.
- Andreani SM, Ratnasingham K, Dang HH, et al. Crohn’s disease of the vulva. Int J Surg. 2010;8:2-5.
- Feller E, Ribaudo S, Jackson N. Gynecologic aspects of Crohn’s disease. Am Fam Physician. 2001;64:1725-1728.
- Corbett SL, Walsh CM, Spitzer RF, et al. Vulvar inflammation as the only clinical manifestation of Crohn disease in an 8-year-old girl [published online May 10, 2010]. Pediatrics. 2010;125:E1518-E1522.
- Tonolini M, Villa C, Campari A, et al. Common and unusual urogenital Crohn’s disease complications: spectrum of cross-sectional imaging findings. Abdom Imaging. 2013;38:32-41.
- Bhaduri S, Jenkinson S, Lewis F. Vulval Crohn’s disease—a multi-specialty approach. Int J STD AIDS. 2005;16:512-514.
- Crowson AN, Nuovo GJ, Mihm MC Jr, et al. Cutaneous manifestations of Crohn’s disease, its spectrum, and its pathogenesis: intracellular consensus bacterial 16S rRNA is associated with the gastrointestinal but not the cutaneous manifestations of Crohn’s disease. Hum Pathol. 2003;34:1185-1192.
- Pai D, Dillman JR, Mahani MG, et al. MRI of vulvar Crohn disease. Pediatr Radiol. 2011;41:537-541.
- Madnani NA, Desai D, Gandhi N, et al. Isolated Crohn’s disease of the vulva. Indian J Dermatol Venereol Leprol. 2011;77:342-344.
- Makhija S, Trotter M, Wagner E, et al. Refractory Crohn’s disease of the vulva treated with infliximab: a case report. Can J Gastroenterol. 2007;21:835-837.
- Fahmy N, Kalidindi M, Khan R. Direct colo-labial Crohn’s abscess mimicking bartholinitis. Am J Obstret Gynecol. 2010;30:741-742.
- Preston PW, Hudson N, Lewis FM. Treatment of vulval Crohn’s disease with infliximab. Clin Exp Derm. 2006;31:378-380.
- Kolivras A, De Maubeuge J, André J, et al. Thalidomide in refractory vulvar ulcerations associated with Crohn’s disease. Dermatology. 2003;206:381-383.
- Kao MS, Paulson JD, Askin FB. Crohn’s disease of the vulva. Obstet Gynecol. 1975;46:329-333.
Eosinophilic Pustular Folliculitis With Underlying Mantle Cell Lymphoma
Eosinophilic pustular folliculitis (EPF) was originally described in 1965 and has since evolved into 3 distinct subtypes: classic, immunosuppressed (IS), and infantile types. Immunosuppressed EPF can be further subdivided into human immunodeficiency virus (HIV) associated (IS-HIV) and non-HIV associated. Human immunodeficiency virus–seronegative cases have been associated with underlying malignancies (IS-heme) or chronic immunosuppression, such as that seen in transplant patients.
Case Report
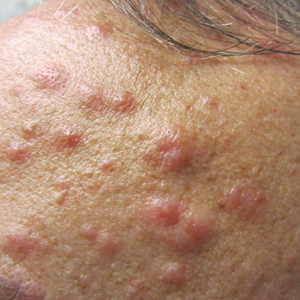
A 52-year-old man with a medical history limited to prostate adenocarcinoma treated with a robotic prostatectomy presented with a pruritic red rash on the face, neck, shoulders, and chest of 1 month’s duration. The patient previously completed a course of azithromycin 250 mg, intramuscular triamcinolone, and oral prednisone with only minor improvement. Physical examination demonstrated multiple pink folliculocentric papules and pustules scattered on the head (Figure 1A), neck, and chest (Figure 1B), as well as edematous pink papules and plaques on the forehead (Figures 1C and 1D). The palms, soles, and oral mucosa were clear.
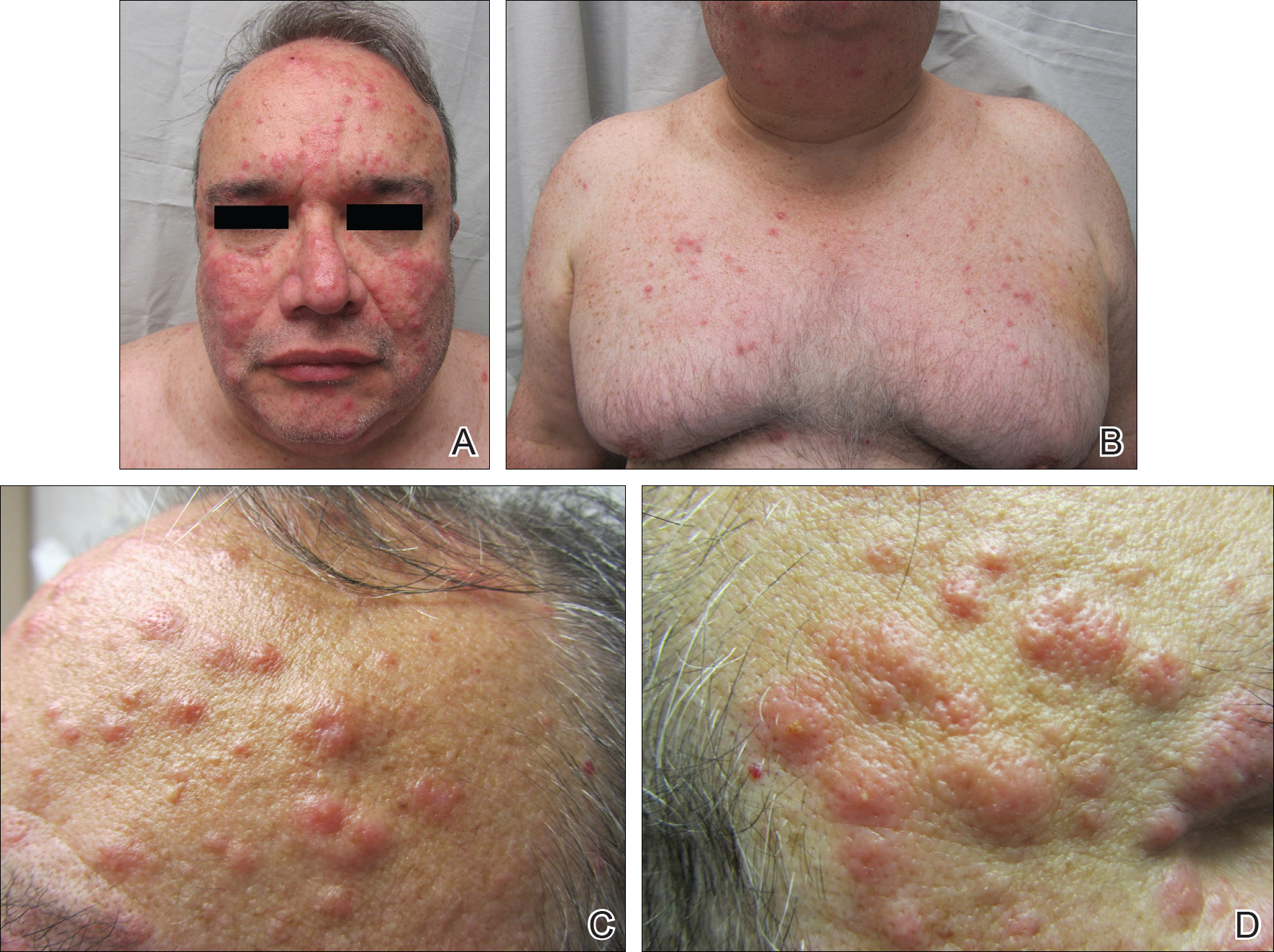
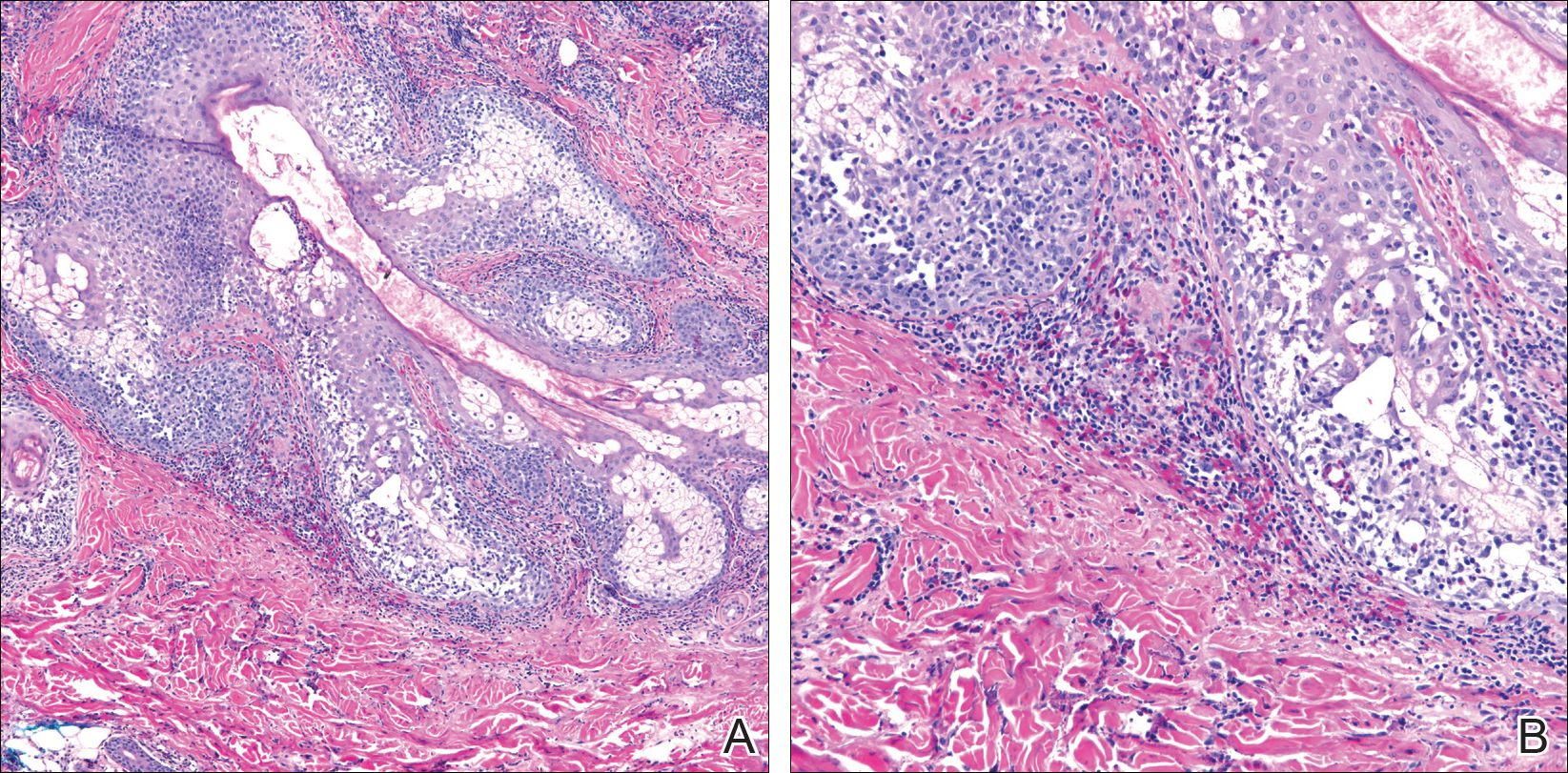
Initial biopsy of the right side of the chest was nonspecific and most consistent with a reaction to an arthropod bite. The patient was started on oral doxycycline 100 mg twice daily for 2 weeks. With no improvement seen, additional biopsies were obtained from the left side of the chest and forehead. The biopsy of the chest showed ruptured folliculitis with evidence of acute and chronic inflammation. The biopsy of the forehead demonstrated eosinophilic follicular spongiosis with intrafollicular Langerhans cell microgranulomas along with abundant eosinophils adjacent to follicles, consistent with EPF (Figure 2). Serum HIV testing was negative. Serum white blood cell count was normal at 6400/µL (reference range, 4500–11,000/µL) with mild elevation of eosinophils (8%). The remaining complete blood cell count and comprehensive metabolic panel were within reference range. The patient was subsequently started on oral indomethacin 25 mg twice daily and triamcinolone cream 0.1%. Within a few days he experienced initial improvement in his symptoms of pruritus and diminution in the number of inflammatory follicular papules.
Approximately 1 month after presentation, he began to experience symptoms of dysphagia and fatigue. In addition, tonsillar hypertrophy and palpable neck and axillary lymphadenopathy were present. Computed tomography of the neck, chest, and abdomen showed diffuse lymphadenopathy. Full-body positron emission tomography–computed tomography demonstrated extensive metabolically active lymphoma in multiple nodal groups above and below the diaphragm. There also was lymphomatous involvement of the spleen. An axillary lymph node biopsy was diagnostic for mantle cell lymphoma (CD4:CD8, 1:1; CD45 negative; CD20 positive; CD5 positive). He
Comment
Subtypes of EPF
Eosinophilic pustular folliculitis was first described in a Japanese female presenting with folliculocentric pustules distributed on the face, torso, and arms.1 This noninfectious eosinophilic infiltration of hair follicles predominantly seen in the Japanese population is now regarded as the classic form. Three distinct subtypes of EPF now exist, including the originally described classic variant (Ofuji disease), an IS variant, and a rare infantile form.1
All 3 subtypes of EPF are more commonly seen in men than women. The classic form has a peak incidence between the third and fourth decades of life. It presents as chronic annular papules and sterile pustules exhibiting peripheral extension, with individual lesions lasting for approximately 7 to 10 days with frequent relapses. The face is the most common area of involvement, followed by the trunk, extremities, and more rarely the palmoplantar surfaces. Concomitant leukocytosis with eosinophilia is seen in up to 35% of patients.1 The infantile type represents the rarest EPF form. The average age of onset is 5 months, with most cases resolving by 14 months of age.1
Clinically, EPF is characterized by recurrent papules and pustules predominantly on the scalp without annular or polycyclic ring formation, as seen in the classic type. The palms and soles may be involved, which can clinically mimic infantile acropustulosis and scabies infection. Most patients exhibit a concomitant peripheral eosinophilia.1,2
In the late 1980s, the IS variant of EPF was recognized in HIV-positive (IS-HIV) and HIV-negative malignancy-associated (IS-heme) populations.1,3 This newly characterized form differs morphologically and biologically from the classic and infantile subtypes. The IS subtype has a unique presentation including intensely pruritic, discrete, erythematous, follicular papules with palmoplantar sparing and infrequent annular or circinate plaque forms.1 Frequently, with the IS-HIV form, CD4+ T-cell counts are below 300 cells/mL, and 25% to 50% of patients have lymphopenia with eosinophilia.3 Highly active antiretroviral therapy has been associated with EPF resolution in HIV-positive individuals; however, it also has been shown to induce transient EPF during the first 3 to 6 months of initiation.1,3,4
Unlike the IS-HIV form, the IS-heme form has occurred solely in males and is predominantly associated with hematologic malignancies (eg, non-Hodgkin lymphoma, acute lymphoblastic leukemia, acute myeloid leukemia, myelodysplastic syndrome) 30 to 90 days following bone marrow transplant, peripheral blood stem cell transplant, or chemotherapy treatment.5,6 Unlike the chronic and persistent IS-HIV form, prior cases of IS-heme EPF have been predominantly self-limited. Interestingly, only 2 reported cases of EPF have occurred prior to the diagnosis of malignancy including B-cell leukemia and myelodysplastic syndrome.5
Histopathology
All 3 identified forms of EPF histopathologically show acute and chronic lymphoeosinophilic infiltrate concentrated at the follicular isthmus, which can lead to follicular destruction. Scattered mononuclear cells, eosinophils, and neutrophils are found within the pilar outer root sheath, sebaceous glands, and ducts. Approximately 40% of cases demonstrate follicular mucinosis.1 Histopathology of lesional palmar skin in classic-type EPF demonstrates intraepidermal pustule formation with abundant eosinophils and neutrophils adjacent to the acrosyringium.7,8
Pathogenesis
Although the pathophysiology of EPF is largely unknown, it is thought to represent a helper T cell (TH2) response involving IL-4, IL-5, and IL-13 cytokines.9 Chemoattractant receptor homologous molecule 2, which is expressed on eosinophils and lymphocytes, is believed to play a role in the pruritus, edema, and inflammatory response seen adjacent to pilosebaceous units in EPF.10 Moreover, immunohistochemical and flow cytometry analysis has revealed a prevalence of prostaglandin D2 within the perisebocyte infiltrate in EPF.9 Prostaglandin D2 induces eotaxin-3 production within sebocytes via peroxisome proliferator-activated receptor γ, which enhances chemoattraction of eosinophils. This pathogenesis represents a prostaglandin-based mechanism and potentially explains the efficacy of indomethacin treatment of EPF through its cyclooxygenase inhibition and reduction of chemoattractant receptor homologous molecule 2 expression.9-11
Treatment
Multiple therapeutic modalities have been reported for the treatment of EPF. For all 3 subtypes, moderate- to high-potency topical corticosteroids are considered first-line therapy. UVB phototherapy 2 to 3 times weekly remains the gold standard, given its consistent efficacy.1,12 Indomethacin (50–75 mg daily) remains first-line treatment of classic EPF.4,12 Previously reported cases of classic EPF and IS-EPF have responded well to oral prednisone (1 mg/kg daily).12,13 In a retrospective review of EPF treatment data, the following treatments also have been reported to be successful: psoralen plus UVA, oral cetirizine (20–40 mg daily, particularly for IS-EPF cases), metronidazole (250 mg 3 times daily), minocycline (150 mg daily), itraconazole (200–400 mg daily, dapsone (50–200 mg daily), systemic retinoids, tacrolimus ointment 0.1%, and permethrin cream.4,12
Malignancy
Although the entity of IS-heme EPF is rare, the morphology and treatment are unique and can potentially unmask an underlying hematologic malignancy. In patients with EPF and associated malignancy, such as our patient, a differential diagnosis to consider is eosinophilic dermatosis of hematologic malignancy (EDHM). Eosinophilic dermatosis of hematologic malignancy is most commonly associated with chronic lymphocytic leukemia and can be differentiated from EPF clinically, histopathologically, and by treatment response. Eosinophilic dermatosis of hematologic malignancy clinically presents with nonspecific papules, pustules, and/or vesicles on the head, trunk, and extremities. On histopathology, EDHM shows a superficial and deep perivascular and interstitial lymphoeosinophilic infiltration. Furthermore, EDHM patients typically exhibit a poor treatment response to oral indomethacin.14
Conclusion
Eosinophilic pustular folliculitis is a noninfectious folliculocentric process comprised of 3 distinct types. The histopathology shows follicular spongiosis with increased eosinophils. The pathogenesis is most likely related to a multifactorial immune system dysregulation involving TH2 T cells, prostaglandin D2, and eotaxin-3. The treatment of EPF may involve topical corticosteroids, UVB phototherapy, or most notably oral indomethacin. In patients with EPF and malignancy, EDHM is a differential diagnosis to consider. Our case serves as a reminder that rare eosinophilic dermatoses may represent manifestations of underlying hematopoietic malignancy and, when investigated early, can lead to appropriate life-saving treatment.
- Nervi J, Stephen. Eosinophilic pustular folliculitis: a 40 year retrospect. J Am Acad Dermatol. 2006;55:285-289.
- Hernández-Martín Á, Nuño-González A, Colmenero I, et al. Eosinophilic pustular folliculitis of infancy: a series of 15 cases and review of the literature [published online July 21, 2012]. J Am Acad Dermatol. 2013;68:150-155.
- Soepr
ono F, Schinella R. Eosinophilic pustular folliculitis in patients with acquired immunodeficiency syndrome. report of three cases. J Am Acad Dermatol. 1986;14:1020-1022. - Katoh
M, Nomura T, Miyachi Y, et al. Eosinophilic pustular folliculitis: a review of the Japanese published works. J Dermatol. 2013;40:15-20. - Keida
T, Hayashi N, Kawashima M. Eosinophilic pustular folliculitis following autologous peripheral blood stem-cell transplant. J Dermatol. 2004;31:21-26. - Goiriz R, Gul-Millán G, Peñas PF, et al. Eosinophilic folliculitis following allogeneic peripheral blood stem cell transplantation: case report and review. J Cutan Pathol. 2007;34(suppl 1):33-36.
- Satoh T, Ikeda H, Yokozeki H. Acrosyringeal involvement of palmoplantar lesions of eosinophilic pustular folliculitis. Acta Derm Venereol. 2013;93:99.
- Tsuboi H, Wakita K, Fujimura T, et al. Acral variant of eosinophilic pustular folliculitis (Ofuji’s disease). Clin Exp Dermatol. 2003;28:321-324.
- Nakahig
ashi K, Doi H, Otsuka A, et al. PGD2 induces eotaxin-3 via PPARgamma from sebocytes: a possible pathogenesis of eosinophilic pustular folliculitis. J Allergy Clin Immunol. 2012;129:536-543. - Satoh
T, Shimura C, Miyagishi C, et al. Indomethacin-induced reduction in CRTH2 in eosinophilic pustular folliculitis (Ofuji’s disease): a proposed mechanism of action. Acta Derm Venereol. 2010;90:18-22. - Hagiwara A, Fujimura T, Furudate S, et al. Induction of CD163(+)M2 macrophages in the lesional skin of eosinophilic pustular folliculitis. Acta Derm Venereol. 2014;94:104-106.
- Ellis
E, Scheinfeld N. Eosinophilic pustular folliculitis: a comprehensive review of treatment options. Am J Clin Dermatol. 2004;5:189-197. - Bull R
H, Harland CA, Fallowfield ME, et al. Eosinophilic folliculitis: a self-limiting illness in patients being treated for haematological malignancy. Br J Dermatol. 1993;129:178-182. - Farber M, Forgia S, Sahu J, et al. Eosinophilic dermatosis of hematologic malignancy. J Cutan Pathol. 2012;39:690-695.
Eosinophilic pustular folliculitis (EPF) was originally described in 1965 and has since evolved into 3 distinct subtypes: classic, immunosuppressed (IS), and infantile types. Immunosuppressed EPF can be further subdivided into human immunodeficiency virus (HIV) associated (IS-HIV) and non-HIV associated. Human immunodeficiency virus–seronegative cases have been associated with underlying malignancies (IS-heme) or chronic immunosuppression, such as that seen in transplant patients.
Case Report
A 52-year-old man with a medical history limited to prostate adenocarcinoma treated with a robotic prostatectomy presented with a pruritic red rash on the face, neck, shoulders, and chest of 1 month’s duration. The patient previously completed a course of azithromycin 250 mg, intramuscular triamcinolone, and oral prednisone with only minor improvement. Physical examination demonstrated multiple pink folliculocentric papules and pustules scattered on the head (Figure 1A), neck, and chest (Figure 1B), as well as edematous pink papules and plaques on the forehead (Figures 1C and 1D). The palms, soles, and oral mucosa were clear.

Initial biopsy of the right side of the chest was nonspecific and most consistent with a reaction to an arthropod bite. The patient was started on oral doxycycline 100 mg twice daily for 2 weeks. With no improvement seen, additional biopsies were obtained from the left side of the chest and forehead. The biopsy of the chest showed ruptured folliculitis with evidence of acute and chronic inflammation. The biopsy of the forehead demonstrated eosinophilic follicular spongiosis with intrafollicular Langerhans cell microgranulomas along with abundant eosinophils adjacent to follicles, consistent with EPF (Figure 2). Serum HIV testing was negative. Serum white blood cell count was normal at 6400/µL (reference range, 4500–11,000/µL) with mild elevation of eosinophils (8%). The remaining complete blood cell count and comprehensive metabolic panel were within reference range. The patient was subsequently started on oral indomethacin 25 mg twice daily and triamcinolone cream 0.1%. Within a few days he experienced initial improvement in his symptoms of pruritus and diminution in the number of inflammatory follicular papules.

Approximately 1 month after presentation, he began to experience symptoms of dysphagia and fatigue. In addition, tonsillar hypertrophy and palpable neck and axillary lymphadenopathy were present. Computed tomography of the neck, chest, and abdomen showed diffuse lymphadenopathy. Full-body positron emission tomography–computed tomography demonstrated extensive metabolically active lymphoma in multiple nodal groups above and below the diaphragm. There also was lymphomatous involvement of the spleen. An axillary lymph node biopsy was diagnostic for mantle cell lymphoma (CD4:CD8, 1:1; CD45 negative; CD20 positive; CD5 positive). He
Comment
Subtypes of EPF
Eosinophilic pustular folliculitis was first described in a Japanese female presenting with folliculocentric pustules distributed on the face, torso, and arms.1 This noninfectious eosinophilic infiltration of hair follicles predominantly seen in the Japanese population is now regarded as the classic form. Three distinct subtypes of EPF now exist, including the originally described classic variant (Ofuji disease), an IS variant, and a rare infantile form.1
All 3 subtypes of EPF are more commonly seen in men than women. The classic form has a peak incidence between the third and fourth decades of life. It presents as chronic annular papules and sterile pustules exhibiting peripheral extension, with individual lesions lasting for approximately 7 to 10 days with frequent relapses. The face is the most common area of involvement, followed by the trunk, extremities, and more rarely the palmoplantar surfaces. Concomitant leukocytosis with eosinophilia is seen in up to 35% of patients.1 The infantile type represents the rarest EPF form. The average age of onset is 5 months, with most cases resolving by 14 months of age.1
Clinically, EPF is characterized by recurrent papules and pustules predominantly on the scalp without annular or polycyclic ring formation, as seen in the classic type. The palms and soles may be involved, which can clinically mimic infantile acropustulosis and scabies infection. Most patients exhibit a concomitant peripheral eosinophilia.1,2
In the late 1980s, the IS variant of EPF was recognized in HIV-positive (IS-HIV) and HIV-negative malignancy-associated (IS-heme) populations.1,3 This newly characterized form differs morphologically and biologically from the classic and infantile subtypes. The IS subtype has a unique presentation including intensely pruritic, discrete, erythematous, follicular papules with palmoplantar sparing and infrequent annular or circinate plaque forms.1 Frequently, with the IS-HIV form, CD4+ T-cell counts are below 300 cells/mL, and 25% to 50% of patients have lymphopenia with eosinophilia.3 Highly active antiretroviral therapy has been associated with EPF resolution in HIV-positive individuals; however, it also has been shown to induce transient EPF during the first 3 to 6 months of initiation.1,3,4
Unlike the IS-HIV form, the IS-heme form has occurred solely in males and is predominantly associated with hematologic malignancies (eg, non-Hodgkin lymphoma, acute lymphoblastic leukemia, acute myeloid leukemia, myelodysplastic syndrome) 30 to 90 days following bone marrow transplant, peripheral blood stem cell transplant, or chemotherapy treatment.5,6 Unlike the chronic and persistent IS-HIV form, prior cases of IS-heme EPF have been predominantly self-limited. Interestingly, only 2 reported cases of EPF have occurred prior to the diagnosis of malignancy including B-cell leukemia and myelodysplastic syndrome.5
Histopathology
All 3 identified forms of EPF histopathologically show acute and chronic lymphoeosinophilic infiltrate concentrated at the follicular isthmus, which can lead to follicular destruction. Scattered mononuclear cells, eosinophils, and neutrophils are found within the pilar outer root sheath, sebaceous glands, and ducts. Approximately 40% of cases demonstrate follicular mucinosis.1 Histopathology of lesional palmar skin in classic-type EPF demonstrates intraepidermal pustule formation with abundant eosinophils and neutrophils adjacent to the acrosyringium.7,8
Pathogenesis
Although the pathophysiology of EPF is largely unknown, it is thought to represent a helper T cell (TH2) response involving IL-4, IL-5, and IL-13 cytokines.9 Chemoattractant receptor homologous molecule 2, which is expressed on eosinophils and lymphocytes, is believed to play a role in the pruritus, edema, and inflammatory response seen adjacent to pilosebaceous units in EPF.10 Moreover, immunohistochemical and flow cytometry analysis has revealed a prevalence of prostaglandin D2 within the perisebocyte infiltrate in EPF.9 Prostaglandin D2 induces eotaxin-3 production within sebocytes via peroxisome proliferator-activated receptor γ, which enhances chemoattraction of eosinophils. This pathogenesis represents a prostaglandin-based mechanism and potentially explains the efficacy of indomethacin treatment of EPF through its cyclooxygenase inhibition and reduction of chemoattractant receptor homologous molecule 2 expression.9-11
Treatment
Multiple therapeutic modalities have been reported for the treatment of EPF. For all 3 subtypes, moderate- to high-potency topical corticosteroids are considered first-line therapy. UVB phototherapy 2 to 3 times weekly remains the gold standard, given its consistent efficacy.1,12 Indomethacin (50–75 mg daily) remains first-line treatment of classic EPF.4,12 Previously reported cases of classic EPF and IS-EPF have responded well to oral prednisone (1 mg/kg daily).12,13 In a retrospective review of EPF treatment data, the following treatments also have been reported to be successful: psoralen plus UVA, oral cetirizine (20–40 mg daily, particularly for IS-EPF cases), metronidazole (250 mg 3 times daily), minocycline (150 mg daily), itraconazole (200–400 mg daily, dapsone (50–200 mg daily), systemic retinoids, tacrolimus ointment 0.1%, and permethrin cream.4,12
Malignancy
Although the entity of IS-heme EPF is rare, the morphology and treatment are unique and can potentially unmask an underlying hematologic malignancy. In patients with EPF and associated malignancy, such as our patient, a differential diagnosis to consider is eosinophilic dermatosis of hematologic malignancy (EDHM). Eosinophilic dermatosis of hematologic malignancy is most commonly associated with chronic lymphocytic leukemia and can be differentiated from EPF clinically, histopathologically, and by treatment response. Eosinophilic dermatosis of hematologic malignancy clinically presents with nonspecific papules, pustules, and/or vesicles on the head, trunk, and extremities. On histopathology, EDHM shows a superficial and deep perivascular and interstitial lymphoeosinophilic infiltration. Furthermore, EDHM patients typically exhibit a poor treatment response to oral indomethacin.14
Conclusion
Eosinophilic pustular folliculitis is a noninfectious folliculocentric process comprised of 3 distinct types. The histopathology shows follicular spongiosis with increased eosinophils. The pathogenesis is most likely related to a multifactorial immune system dysregulation involving TH2 T cells, prostaglandin D2, and eotaxin-3. The treatment of EPF may involve topical corticosteroids, UVB phototherapy, or most notably oral indomethacin. In patients with EPF and malignancy, EDHM is a differential diagnosis to consider. Our case serves as a reminder that rare eosinophilic dermatoses may represent manifestations of underlying hematopoietic malignancy and, when investigated early, can lead to appropriate life-saving treatment.
Eosinophilic pustular folliculitis (EPF) was originally described in 1965 and has since evolved into 3 distinct subtypes: classic, immunosuppressed (IS), and infantile types. Immunosuppressed EPF can be further subdivided into human immunodeficiency virus (HIV) associated (IS-HIV) and non-HIV associated. Human immunodeficiency virus–seronegative cases have been associated with underlying malignancies (IS-heme) or chronic immunosuppression, such as that seen in transplant patients.
Case Report
A 52-year-old man with a medical history limited to prostate adenocarcinoma treated with a robotic prostatectomy presented with a pruritic red rash on the face, neck, shoulders, and chest of 1 month’s duration. The patient previously completed a course of azithromycin 250 mg, intramuscular triamcinolone, and oral prednisone with only minor improvement. Physical examination demonstrated multiple pink folliculocentric papules and pustules scattered on the head (Figure 1A), neck, and chest (Figure 1B), as well as edematous pink papules and plaques on the forehead (Figures 1C and 1D). The palms, soles, and oral mucosa were clear.

Initial biopsy of the right side of the chest was nonspecific and most consistent with a reaction to an arthropod bite. The patient was started on oral doxycycline 100 mg twice daily for 2 weeks. With no improvement seen, additional biopsies were obtained from the left side of the chest and forehead. The biopsy of the chest showed ruptured folliculitis with evidence of acute and chronic inflammation. The biopsy of the forehead demonstrated eosinophilic follicular spongiosis with intrafollicular Langerhans cell microgranulomas along with abundant eosinophils adjacent to follicles, consistent with EPF (Figure 2). Serum HIV testing was negative. Serum white blood cell count was normal at 6400/µL (reference range, 4500–11,000/µL) with mild elevation of eosinophils (8%). The remaining complete blood cell count and comprehensive metabolic panel were within reference range. The patient was subsequently started on oral indomethacin 25 mg twice daily and triamcinolone cream 0.1%. Within a few days he experienced initial improvement in his symptoms of pruritus and diminution in the number of inflammatory follicular papules.

Approximately 1 month after presentation, he began to experience symptoms of dysphagia and fatigue. In addition, tonsillar hypertrophy and palpable neck and axillary lymphadenopathy were present. Computed tomography of the neck, chest, and abdomen showed diffuse lymphadenopathy. Full-body positron emission tomography–computed tomography demonstrated extensive metabolically active lymphoma in multiple nodal groups above and below the diaphragm. There also was lymphomatous involvement of the spleen. An axillary lymph node biopsy was diagnostic for mantle cell lymphoma (CD4:CD8, 1:1; CD45 negative; CD20 positive; CD5 positive). He
Comment
Subtypes of EPF
Eosinophilic pustular folliculitis was first described in a Japanese female presenting with folliculocentric pustules distributed on the face, torso, and arms.1 This noninfectious eosinophilic infiltration of hair follicles predominantly seen in the Japanese population is now regarded as the classic form. Three distinct subtypes of EPF now exist, including the originally described classic variant (Ofuji disease), an IS variant, and a rare infantile form.1
All 3 subtypes of EPF are more commonly seen in men than women. The classic form has a peak incidence between the third and fourth decades of life. It presents as chronic annular papules and sterile pustules exhibiting peripheral extension, with individual lesions lasting for approximately 7 to 10 days with frequent relapses. The face is the most common area of involvement, followed by the trunk, extremities, and more rarely the palmoplantar surfaces. Concomitant leukocytosis with eosinophilia is seen in up to 35% of patients.1 The infantile type represents the rarest EPF form. The average age of onset is 5 months, with most cases resolving by 14 months of age.1
Clinically, EPF is characterized by recurrent papules and pustules predominantly on the scalp without annular or polycyclic ring formation, as seen in the classic type. The palms and soles may be involved, which can clinically mimic infantile acropustulosis and scabies infection. Most patients exhibit a concomitant peripheral eosinophilia.1,2
In the late 1980s, the IS variant of EPF was recognized in HIV-positive (IS-HIV) and HIV-negative malignancy-associated (IS-heme) populations.1,3 This newly characterized form differs morphologically and biologically from the classic and infantile subtypes. The IS subtype has a unique presentation including intensely pruritic, discrete, erythematous, follicular papules with palmoplantar sparing and infrequent annular or circinate plaque forms.1 Frequently, with the IS-HIV form, CD4+ T-cell counts are below 300 cells/mL, and 25% to 50% of patients have lymphopenia with eosinophilia.3 Highly active antiretroviral therapy has been associated with EPF resolution in HIV-positive individuals; however, it also has been shown to induce transient EPF during the first 3 to 6 months of initiation.1,3,4
Unlike the IS-HIV form, the IS-heme form has occurred solely in males and is predominantly associated with hematologic malignancies (eg, non-Hodgkin lymphoma, acute lymphoblastic leukemia, acute myeloid leukemia, myelodysplastic syndrome) 30 to 90 days following bone marrow transplant, peripheral blood stem cell transplant, or chemotherapy treatment.5,6 Unlike the chronic and persistent IS-HIV form, prior cases of IS-heme EPF have been predominantly self-limited. Interestingly, only 2 reported cases of EPF have occurred prior to the diagnosis of malignancy including B-cell leukemia and myelodysplastic syndrome.5
Histopathology
All 3 identified forms of EPF histopathologically show acute and chronic lymphoeosinophilic infiltrate concentrated at the follicular isthmus, which can lead to follicular destruction. Scattered mononuclear cells, eosinophils, and neutrophils are found within the pilar outer root sheath, sebaceous glands, and ducts. Approximately 40% of cases demonstrate follicular mucinosis.1 Histopathology of lesional palmar skin in classic-type EPF demonstrates intraepidermal pustule formation with abundant eosinophils and neutrophils adjacent to the acrosyringium.7,8
Pathogenesis
Although the pathophysiology of EPF is largely unknown, it is thought to represent a helper T cell (TH2) response involving IL-4, IL-5, and IL-13 cytokines.9 Chemoattractant receptor homologous molecule 2, which is expressed on eosinophils and lymphocytes, is believed to play a role in the pruritus, edema, and inflammatory response seen adjacent to pilosebaceous units in EPF.10 Moreover, immunohistochemical and flow cytometry analysis has revealed a prevalence of prostaglandin D2 within the perisebocyte infiltrate in EPF.9 Prostaglandin D2 induces eotaxin-3 production within sebocytes via peroxisome proliferator-activated receptor γ, which enhances chemoattraction of eosinophils. This pathogenesis represents a prostaglandin-based mechanism and potentially explains the efficacy of indomethacin treatment of EPF through its cyclooxygenase inhibition and reduction of chemoattractant receptor homologous molecule 2 expression.9-11
Treatment
Multiple therapeutic modalities have been reported for the treatment of EPF. For all 3 subtypes, moderate- to high-potency topical corticosteroids are considered first-line therapy. UVB phototherapy 2 to 3 times weekly remains the gold standard, given its consistent efficacy.1,12 Indomethacin (50–75 mg daily) remains first-line treatment of classic EPF.4,12 Previously reported cases of classic EPF and IS-EPF have responded well to oral prednisone (1 mg/kg daily).12,13 In a retrospective review of EPF treatment data, the following treatments also have been reported to be successful: psoralen plus UVA, oral cetirizine (20–40 mg daily, particularly for IS-EPF cases), metronidazole (250 mg 3 times daily), minocycline (150 mg daily), itraconazole (200–400 mg daily, dapsone (50–200 mg daily), systemic retinoids, tacrolimus ointment 0.1%, and permethrin cream.4,12
Malignancy
Although the entity of IS-heme EPF is rare, the morphology and treatment are unique and can potentially unmask an underlying hematologic malignancy. In patients with EPF and associated malignancy, such as our patient, a differential diagnosis to consider is eosinophilic dermatosis of hematologic malignancy (EDHM). Eosinophilic dermatosis of hematologic malignancy is most commonly associated with chronic lymphocytic leukemia and can be differentiated from EPF clinically, histopathologically, and by treatment response. Eosinophilic dermatosis of hematologic malignancy clinically presents with nonspecific papules, pustules, and/or vesicles on the head, trunk, and extremities. On histopathology, EDHM shows a superficial and deep perivascular and interstitial lymphoeosinophilic infiltration. Furthermore, EDHM patients typically exhibit a poor treatment response to oral indomethacin.14
Conclusion
Eosinophilic pustular folliculitis is a noninfectious folliculocentric process comprised of 3 distinct types. The histopathology shows follicular spongiosis with increased eosinophils. The pathogenesis is most likely related to a multifactorial immune system dysregulation involving TH2 T cells, prostaglandin D2, and eotaxin-3. The treatment of EPF may involve topical corticosteroids, UVB phototherapy, or most notably oral indomethacin. In patients with EPF and malignancy, EDHM is a differential diagnosis to consider. Our case serves as a reminder that rare eosinophilic dermatoses may represent manifestations of underlying hematopoietic malignancy and, when investigated early, can lead to appropriate life-saving treatment.
- Nervi J, Stephen. Eosinophilic pustular folliculitis: a 40 year retrospect. J Am Acad Dermatol. 2006;55:285-289.
- Hernández-Martín Á, Nuño-González A, Colmenero I, et al. Eosinophilic pustular folliculitis of infancy: a series of 15 cases and review of the literature [published online July 21, 2012]. J Am Acad Dermatol. 2013;68:150-155.
- Soepr
ono F, Schinella R. Eosinophilic pustular folliculitis in patients with acquired immunodeficiency syndrome. report of three cases. J Am Acad Dermatol. 1986;14:1020-1022. - Katoh
M, Nomura T, Miyachi Y, et al. Eosinophilic pustular folliculitis: a review of the Japanese published works. J Dermatol. 2013;40:15-20. - Keida
T, Hayashi N, Kawashima M. Eosinophilic pustular folliculitis following autologous peripheral blood stem-cell transplant. J Dermatol. 2004;31:21-26. - Goiriz R, Gul-Millán G, Peñas PF, et al. Eosinophilic folliculitis following allogeneic peripheral blood stem cell transplantation: case report and review. J Cutan Pathol. 2007;34(suppl 1):33-36.
- Satoh T, Ikeda H, Yokozeki H. Acrosyringeal involvement of palmoplantar lesions of eosinophilic pustular folliculitis. Acta Derm Venereol. 2013;93:99.
- Tsuboi H, Wakita K, Fujimura T, et al. Acral variant of eosinophilic pustular folliculitis (Ofuji’s disease). Clin Exp Dermatol. 2003;28:321-324.
- Nakahig
ashi K, Doi H, Otsuka A, et al. PGD2 induces eotaxin-3 via PPARgamma from sebocytes: a possible pathogenesis of eosinophilic pustular folliculitis. J Allergy Clin Immunol. 2012;129:536-543. - Satoh
T, Shimura C, Miyagishi C, et al. Indomethacin-induced reduction in CRTH2 in eosinophilic pustular folliculitis (Ofuji’s disease): a proposed mechanism of action. Acta Derm Venereol. 2010;90:18-22. - Hagiwara A, Fujimura T, Furudate S, et al. Induction of CD163(+)M2 macrophages in the lesional skin of eosinophilic pustular folliculitis. Acta Derm Venereol. 2014;94:104-106.
- Ellis
E, Scheinfeld N. Eosinophilic pustular folliculitis: a comprehensive review of treatment options. Am J Clin Dermatol. 2004;5:189-197. - Bull R
H, Harland CA, Fallowfield ME, et al. Eosinophilic folliculitis: a self-limiting illness in patients being treated for haematological malignancy. Br J Dermatol. 1993;129:178-182. - Farber M, Forgia S, Sahu J, et al. Eosinophilic dermatosis of hematologic malignancy. J Cutan Pathol. 2012;39:690-695.
- Nervi J, Stephen. Eosinophilic pustular folliculitis: a 40 year retrospect. J Am Acad Dermatol. 2006;55:285-289.
- Hernández-Martín Á, Nuño-González A, Colmenero I, et al. Eosinophilic pustular folliculitis of infancy: a series of 15 cases and review of the literature [published online July 21, 2012]. J Am Acad Dermatol. 2013;68:150-155.
- Soepr
ono F, Schinella R. Eosinophilic pustular folliculitis in patients with acquired immunodeficiency syndrome. report of three cases. J Am Acad Dermatol. 1986;14:1020-1022. - Katoh
M, Nomura T, Miyachi Y, et al. Eosinophilic pustular folliculitis: a review of the Japanese published works. J Dermatol. 2013;40:15-20. - Keida
T, Hayashi N, Kawashima M. Eosinophilic pustular folliculitis following autologous peripheral blood stem-cell transplant. J Dermatol. 2004;31:21-26. - Goiriz R, Gul-Millán G, Peñas PF, et al. Eosinophilic folliculitis following allogeneic peripheral blood stem cell transplantation: case report and review. J Cutan Pathol. 2007;34(suppl 1):33-36.
- Satoh T, Ikeda H, Yokozeki H. Acrosyringeal involvement of palmoplantar lesions of eosinophilic pustular folliculitis. Acta Derm Venereol. 2013;93:99.
- Tsuboi H, Wakita K, Fujimura T, et al. Acral variant of eosinophilic pustular folliculitis (Ofuji’s disease). Clin Exp Dermatol. 2003;28:321-324.
- Nakahig
ashi K, Doi H, Otsuka A, et al. PGD2 induces eotaxin-3 via PPARgamma from sebocytes: a possible pathogenesis of eosinophilic pustular folliculitis. J Allergy Clin Immunol. 2012;129:536-543. - Satoh
T, Shimura C, Miyagishi C, et al. Indomethacin-induced reduction in CRTH2 in eosinophilic pustular folliculitis (Ofuji’s disease): a proposed mechanism of action. Acta Derm Venereol. 2010;90:18-22. - Hagiwara A, Fujimura T, Furudate S, et al. Induction of CD163(+)M2 macrophages in the lesional skin of eosinophilic pustular folliculitis. Acta Derm Venereol. 2014;94:104-106.
- Ellis
E, Scheinfeld N. Eosinophilic pustular folliculitis: a comprehensive review of treatment options. Am J Clin Dermatol. 2004;5:189-197. - Bull R
H, Harland CA, Fallowfield ME, et al. Eosinophilic folliculitis: a self-limiting illness in patients being treated for haematological malignancy. Br J Dermatol. 1993;129:178-182. - Farber M, Forgia S, Sahu J, et al. Eosinophilic dermatosis of hematologic malignancy. J Cutan Pathol. 2012;39:690-695.
Practice Points
- Recalcitrant folliculocentric papules and pustules involving the head, trunk, arms, and legs should raise suspicion of possible eosinophilic pustular folliculitis (EPF).
- Underlying hematopoietic malignancy may be associated with cases of EPF.
Acrodermatitis Enteropathica From Zinc-Deficient Total Parenteral Nutrition
Case Report
A 54-year-old woman presented with a pruritic and slightly painful skin eruption that began perinasally and progressed over 1 week to involve the labial commissures, finger webs, dorsal surfaces of the feet, heels, and bilateral gluteal folds. In addition, the eruption involved the left thigh at the donor site of a prior skin graft. She received no relief after an intramuscular steroid injection and hydrocortisone cream 1% prescribed by a primary care physician who diagnosed the rash as poison ivy contact dermatitis despite no exposure to plants. Review of systems was negative and she denied any new medication use. Her medical history was notable for extensive mesenteric injury secondary to a motor vehicle accident. She subsequently had multiple enterocutaneous fistulas that resulted in a complete small bowel enterectomy 10 months prior to presentation, which caused her to become dependent on total parenteral nutrition (TPN).
Physical examination revealed sharply demarcated, erythematous, scaly plaques perinasally, periorally, and on the bilateral gluteal folds (Figure 1). There were sharply demarcated, erythematous, scaly plaques on the right and left finger webs, dorsal surface of the right foot, and left upper thigh. Hemorrhagic bullae were appreciated on the left finger webs. Large flaccid bullae were present on the bilateral heels and dorsum of the right foot (Figure 2).
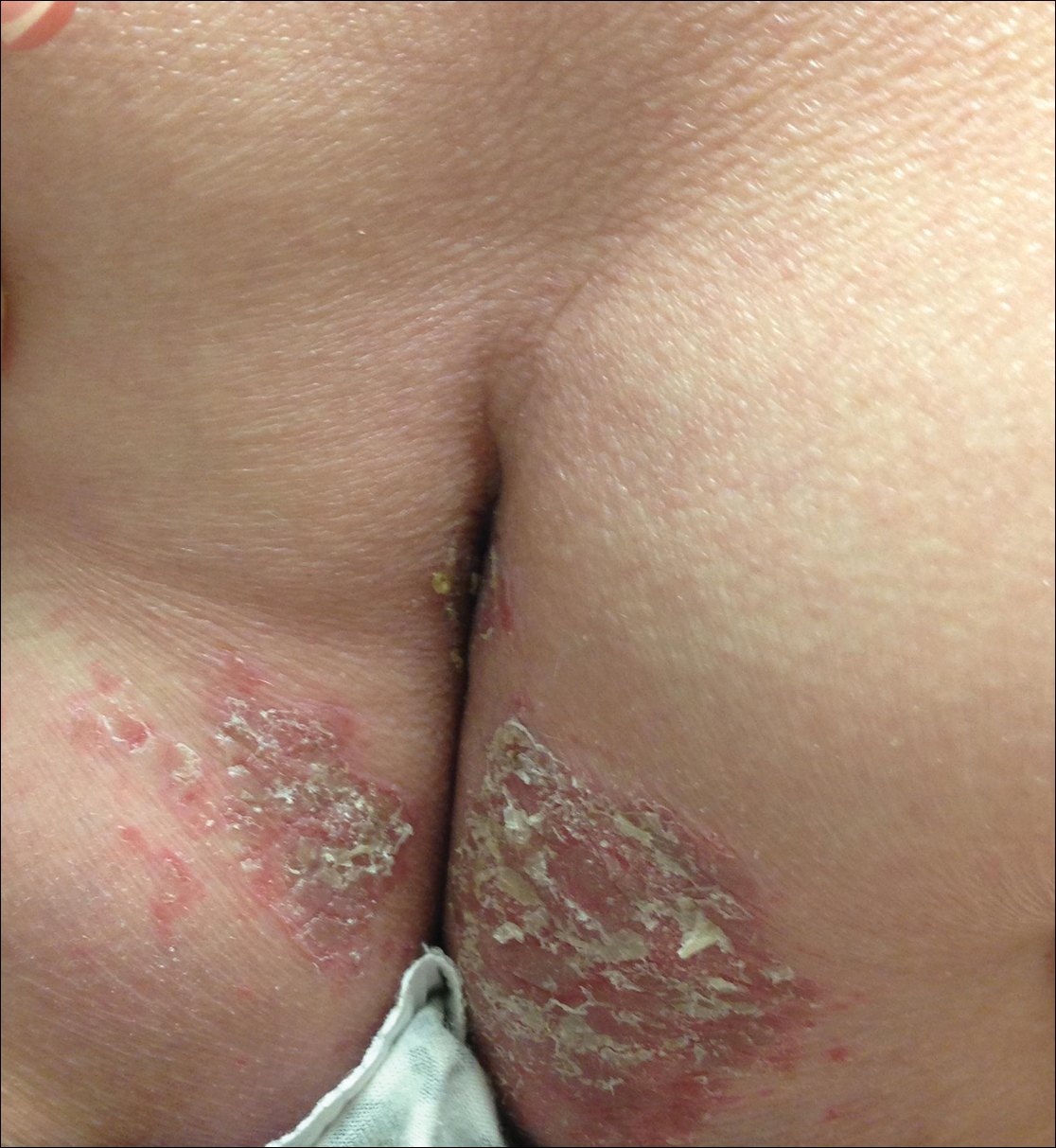
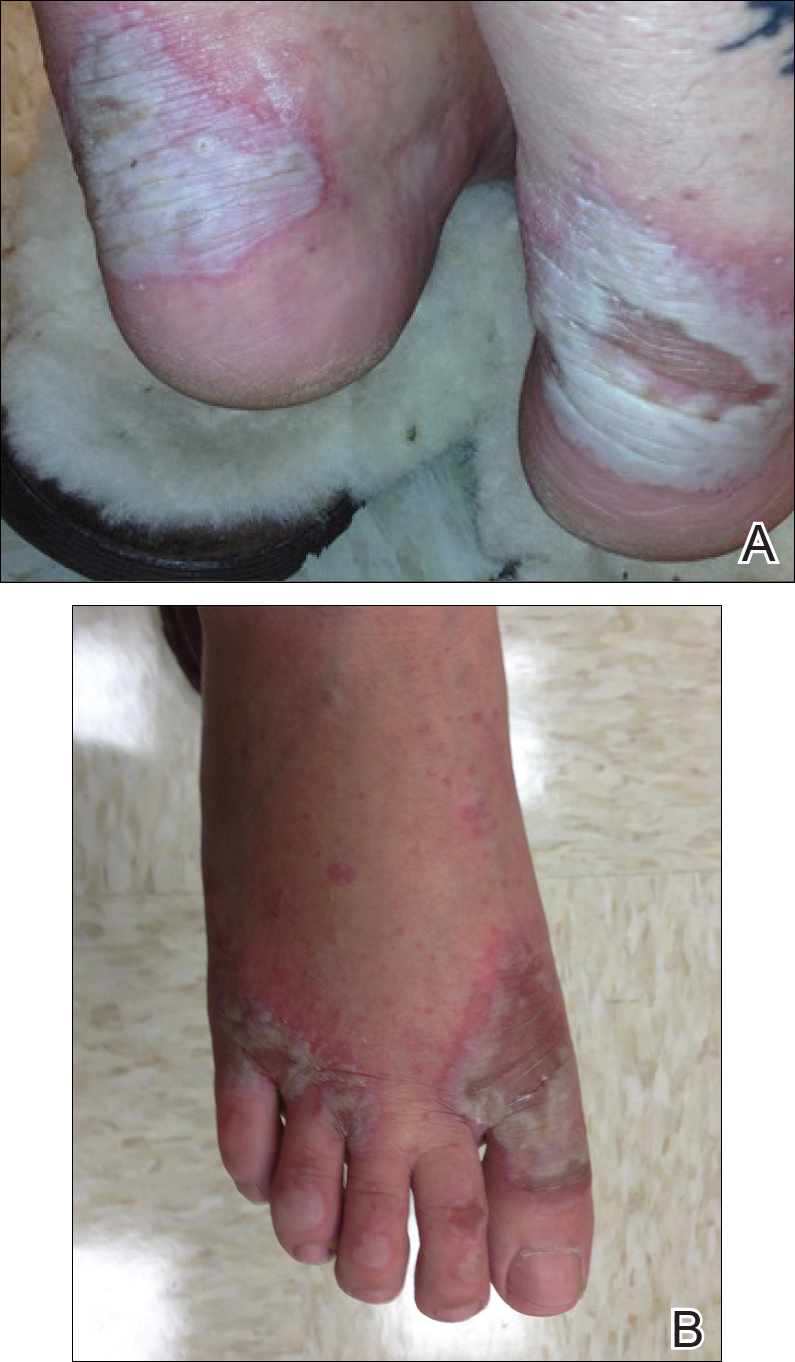
Suspecting a diagnosis of acrodermatitis enteropathica (AE), laboratory testing included a serum zinc level, which was 42 µg/dL (reference range, 70–130 µg/dL). The copper and selenium levels also were low with values of 71 µg/dL (reference range, 80–155 µg/dL) and 31 µg/dL (reference range, 79–326 µg/dL), respectively. No additional vitamin or mineral deficiencies were discovered. A complete blood cell count and comprehensive metabolic panel were performed and showed no abnormalities other than a mildly elevated sodium level of 147 mEq/L (reference range, 136–142 mEq/L).
A punch biopsy was performed. Histopathology revealed subcorneal neutrophils and neutrophilic crust, mild spongiosis, and a dense upper dermal mixed neutrophilic and lymphohistiocytic infiltrate. The specimen also exhibited mild intercellular edema and prominent capillaries (Figure 3).

After further investigation, the company providing the patient’s TPN confirmed that zinc had been removed several weeks prior to the onset of symptoms due to a critical national shortage of trace element additives. Zinc was supplemented at 15 mg daily to the TPN solution. Three days later a skin examination revealed dramatic changes with notable improvement of the finger web plaques and complete resolution of the facial lesions. The plaques and bullae on the lower extremities also had resolved (Figure 4).
Comment
Background
Acrodermatitis enteropathica is a rare autosomal-recessive disorder of zinc metabolism characterized by skin lesions predominantly distributed in acral and periorificial sites as well as alopecia and diarrhea. Acrodermatitis enteropathica was first described by Brandt1 in 1936 and later characterized by Danbolt and Closs2 in 1942 as a unique and often fatal disease of unknown etiology. More than 30 years later, the link between zinc deficiency and AE was illustrated by Moynahan3 who demonstrated clinical improvement with zinc supplementation. It was not until 2002 that the molecular pathogenesis of hypozincemia in patients with inherited AE was described. Küry et al4 identified a mutation in the SLC39A4 gene responsible for encoding the Zip4 protein, a zinc transporter found on enterocytes, particularly in the proximal small intestine.5,6 Classically, patients with inherited AE are children who present within days of birth or days to weeks after being weaned from breast milk to cow’s milk. The zinc in bovine milk is less bioavailable than breast milk, though both have similar total zinc concentrations, which results in the decreased plasma zinc levels seen in children with inherited AE.5-8 Occasionally, children present before weaning due to decreased maternal mammary zinc secretion (lactogenic AE).9,10
Clinical Presentation
Similar clinical findings are seen in patients with noninherited forms of zinc deficiency known as acquired AE. Acquired zinc deficiency may be broadly categorized as being from inadequate intake, deficient absorption, excess demand, or overexcretion.8 Such disturbances of zinc balance are most frequently seen in patients with restrictive diets, anorexia nervosa, intestinal bypass procedures, Crohn disease, pancreatic insufficiency, alcoholism, human immunodeficiency virus, and extensive cutaneous burns. Premature infants, mothers who are breastfeeding, and those dependent on TPN are at risk for developing acquired zinc deficiency.7-9,11
Differentiating Characteristics
Both acquired and inherited AE present as erythematous or pink eczematous scaly plaques with the variable presence of vesicular or bullous lesions involving periorificial, acral, and anogenital regions. Early manifestations of AE may include angular cheilitis and paronychia. Alopecia and diarrhea are characteristics of later disease. In fact, the complete triad of dermatitis, alopecia, and diarrhea is seen in only 20% of cases.7 Without treatment, patients may develop blepharitis, conjunctivitis, photophobia, irritability, anorexia, apathy, growth retardation, hypogonadism, hypogeusia, and mental slowing. Skin lesions frequently become secondarily infected with Candida albicans and/or bacteria.5,7,11
Histopathology
Histopathologic examination of skin biopsy specimens from AE lesions demonstrates nonspecific findings similar to other deficiency dermatoses, such as pellagra and glucagonoma-associated necrolytic migratory erythema. Histology typically reveals cytoplasmic pallor with vacuolization and ballooning degeneration of keratinocytes, followed by confluent keratinocyte necrosis within the stratum granulosum and stratum spinosum of the epidermis.5 Confluent parakeratosis with hypogranulosis variably associated with neutrophil crust also is seen. Scattered dyskeratotic keratinocytes may be found within all levels of the epidermis. In resolving or chronic AE lesions, psoriasiform hyperplasia is prevalent, though necrolysis may be minimal or absent.5,11
Diagnosis
Evaluation includes measurement of plasma zinc levels. Zinc levels less than 50 µg/dL are suggestive but not diagnostic of AE.5 Although plasma zinc measurement is the most useful indicator of zinc status, its utility in assessing the true total body store of zinc is limited. Plasma zinc is tightly regulated and only represents 0.1% of body stores.5,6 Additionally, zinc levels may decrease in proinflammatory states.12 Beyond zinc measurement, evaluation of alkaline phosphatase, a zinc-dependent enzyme, can provide useful diagnostic information.5,6
Zinc and TPN
Patients on TPN are at a unique risk for developing zinc and other nutritional deficiencies. Because the daily recommended dietary allowance for zinc is low (8 mg daily for adult women and 11 mg daily for adult men)5 and the element is found in a wide variety of foods, maintaining adequate zinc levels is easily achieved in healthy individuals with normal diets. Kay et al13 described 4 patients on parenteral nutrition who developed hypozincemia and an AE-like syndrome within weeks of TPN induction. The authors described rapid and drastic clinical improvement after initiating zinc supplementation, accentuating the importance of including zinc as a component of TPN.13,14 Brazin et al15 also reported a case of an AE-like syndrome from zinc-deficient hyperalimentation in a patient receiving TPN for short bowel syndrome. Chun et al16 described another case of acquired AE in a patient on TPN for acute pancreatitis. Both cases demonstrated prompt improvement of skin lesions after treatment with zinc supplementation. Other nutrient deficiencies may reveal themselves through similar dermatologic manifestations. For example, cases of scaly dermatitis secondary to the development of essential fatty acid deficiency from TPN formulations lacking adequate quantities of linoleic acid have been reported.Similar to our case, the resolution of skin lesions was seen after TPN was supplemented with the deficient nutrient.17 These cases exemplify the importance in considering deficiency dermatoses in the TPN-dependent patient population.
Conclusion
In our case, the development of skin lesions directly coincided with a recent removal of zinc from the patient’s TPN, which provided us with a unique opportunity to observe the causal relationship between decreased zinc intake and the development of clinical signs of acquired AE. This association was further elucidated by laboratory confirmation of low serum zinc levels and rapid improvement in all skin lesions after zinc supplementation was initiated.
- Brandt T. Dermatitis in children with disturbances of general condition and absorption of food. Acta Derm Venereol. 1936;17:513-537.
- Danbolt N, Closs K. Acrodermatitis enteropathica. Acta Derm Venereol. 1942;23:127-169.
- Moynahan E. Acrodermatitis enteropathica: a lethal inherited human zinc deficiency disorder. Lancet. 1974;2:299-400.
- Küry S, Dréno B, Bézieau S, et al. Identification of SLC39A4, a gene involved in acrodermatitis enteropathica. Nat Genet. 2002;31:238-240.
- Maverakis E, Fung MA, Lynch PJ, et al. Acrodermatitis enteropathica and an overview of zinc metabolism. J Am Acad Dermatol. 2007;56:116-124.
- Thrash B, Patel M, Shah KR, et al. Cutaneous manifestations of gastrointestinal disease: part II. J Am Acad Dermatol. 2013;68:211.e1-211.e33; quiz 244-246.
- Perafán-Riveros C, França LF, Alves AC, et al. Acrodermatitis enteropathica: case report and review of the literature. Pediatr Dermatol. 2002;19:426-431.
- Kumar P, Ranjan NR, Mondal AK. Zinc and skin: a brief summary. Dermatol Online J. 2012;18:1.
- Saritha M, Gupta D, Chandrashekar L, et al. Acquired zinc deficiency in an adult female. Indian J Dermatol. 2012;57:492-494.
- Neldner K, Hambidge K, Walravens P. Acrodermatitis enteropathica.Int J Dermatol. 1978;17:380-387.
- Gehrig K, Dinulos J. Acrodermatitis due to nutritional deficiency. Curr Opin Pediatr. 2010;22:107-112.
- Liuzzi JP, Lichten LA, Rivera S, et al. Interleukin-6 regulates the zinc transporter Zip14 in liver and contributes to hypozincemia of the acute-phase response. Proct Natl Acad Sci U S A. 2005;102:6843-6848.
- Kay RG, Tasman-Jones C, Pybus J, et al. A syndrome of acute zinc deficiency during total parenteral nutrition in man. Ann Surg. 1976;183:331-340.
- Jeejeebhoy K. Zinc: an essential trace element for parenteral nutrition. Gastroenterology. 2009;137(5 suppl):S7-S12.
- Brazin SA, Johnson WT, Abramson LJ. The acrodermatitis enteropathica-like syndrome. Arch Dermatol. 1979;115:597-599.
- Chun JH, Baek JH, Chung NG. Development of bullous acrodermatitis enteropathica during the course of chemotherapy for acute lymphocytic leukemia. Ann Dermatol. 2011;23(suppl 3):S326-S328.
- Roongpisuthipong W, Phanachet P, Roongpisuthipong C, et al. Essential fatty acid deficiency while a patient receiving fat regimen total parenteral nutrition [published June 14, 2012]. BMJ Case Rep. doi:10.1136/bcr.07.2011.4475.
Case Report
A 54-year-old woman presented with a pruritic and slightly painful skin eruption that began perinasally and progressed over 1 week to involve the labial commissures, finger webs, dorsal surfaces of the feet, heels, and bilateral gluteal folds. In addition, the eruption involved the left thigh at the donor site of a prior skin graft. She received no relief after an intramuscular steroid injection and hydrocortisone cream 1% prescribed by a primary care physician who diagnosed the rash as poison ivy contact dermatitis despite no exposure to plants. Review of systems was negative and she denied any new medication use. Her medical history was notable for extensive mesenteric injury secondary to a motor vehicle accident. She subsequently had multiple enterocutaneous fistulas that resulted in a complete small bowel enterectomy 10 months prior to presentation, which caused her to become dependent on total parenteral nutrition (TPN).
Physical examination revealed sharply demarcated, erythematous, scaly plaques perinasally, periorally, and on the bilateral gluteal folds (Figure 1). There were sharply demarcated, erythematous, scaly plaques on the right and left finger webs, dorsal surface of the right foot, and left upper thigh. Hemorrhagic bullae were appreciated on the left finger webs. Large flaccid bullae were present on the bilateral heels and dorsum of the right foot (Figure 2).


Suspecting a diagnosis of acrodermatitis enteropathica (AE), laboratory testing included a serum zinc level, which was 42 µg/dL (reference range, 70–130 µg/dL). The copper and selenium levels also were low with values of 71 µg/dL (reference range, 80–155 µg/dL) and 31 µg/dL (reference range, 79–326 µg/dL), respectively. No additional vitamin or mineral deficiencies were discovered. A complete blood cell count and comprehensive metabolic panel were performed and showed no abnormalities other than a mildly elevated sodium level of 147 mEq/L (reference range, 136–142 mEq/L).
A punch biopsy was performed. Histopathology revealed subcorneal neutrophils and neutrophilic crust, mild spongiosis, and a dense upper dermal mixed neutrophilic and lymphohistiocytic infiltrate. The specimen also exhibited mild intercellular edema and prominent capillaries (Figure 3).

After further investigation, the company providing the patient’s TPN confirmed that zinc had been removed several weeks prior to the onset of symptoms due to a critical national shortage of trace element additives. Zinc was supplemented at 15 mg daily to the TPN solution. Three days later a skin examination revealed dramatic changes with notable improvement of the finger web plaques and complete resolution of the facial lesions. The plaques and bullae on the lower extremities also had resolved (Figure 4).

Comment
Background
Acrodermatitis enteropathica is a rare autosomal-recessive disorder of zinc metabolism characterized by skin lesions predominantly distributed in acral and periorificial sites as well as alopecia and diarrhea. Acrodermatitis enteropathica was first described by Brandt1 in 1936 and later characterized by Danbolt and Closs2 in 1942 as a unique and often fatal disease of unknown etiology. More than 30 years later, the link between zinc deficiency and AE was illustrated by Moynahan3 who demonstrated clinical improvement with zinc supplementation. It was not until 2002 that the molecular pathogenesis of hypozincemia in patients with inherited AE was described. Küry et al4 identified a mutation in the SLC39A4 gene responsible for encoding the Zip4 protein, a zinc transporter found on enterocytes, particularly in the proximal small intestine.5,6 Classically, patients with inherited AE are children who present within days of birth or days to weeks after being weaned from breast milk to cow’s milk. The zinc in bovine milk is less bioavailable than breast milk, though both have similar total zinc concentrations, which results in the decreased plasma zinc levels seen in children with inherited AE.5-8 Occasionally, children present before weaning due to decreased maternal mammary zinc secretion (lactogenic AE).9,10
Clinical Presentation
Similar clinical findings are seen in patients with noninherited forms of zinc deficiency known as acquired AE. Acquired zinc deficiency may be broadly categorized as being from inadequate intake, deficient absorption, excess demand, or overexcretion.8 Such disturbances of zinc balance are most frequently seen in patients with restrictive diets, anorexia nervosa, intestinal bypass procedures, Crohn disease, pancreatic insufficiency, alcoholism, human immunodeficiency virus, and extensive cutaneous burns. Premature infants, mothers who are breastfeeding, and those dependent on TPN are at risk for developing acquired zinc deficiency.7-9,11
Differentiating Characteristics
Both acquired and inherited AE present as erythematous or pink eczematous scaly plaques with the variable presence of vesicular or bullous lesions involving periorificial, acral, and anogenital regions. Early manifestations of AE may include angular cheilitis and paronychia. Alopecia and diarrhea are characteristics of later disease. In fact, the complete triad of dermatitis, alopecia, and diarrhea is seen in only 20% of cases.7 Without treatment, patients may develop blepharitis, conjunctivitis, photophobia, irritability, anorexia, apathy, growth retardation, hypogonadism, hypogeusia, and mental slowing. Skin lesions frequently become secondarily infected with Candida albicans and/or bacteria.5,7,11
Histopathology
Histopathologic examination of skin biopsy specimens from AE lesions demonstrates nonspecific findings similar to other deficiency dermatoses, such as pellagra and glucagonoma-associated necrolytic migratory erythema. Histology typically reveals cytoplasmic pallor with vacuolization and ballooning degeneration of keratinocytes, followed by confluent keratinocyte necrosis within the stratum granulosum and stratum spinosum of the epidermis.5 Confluent parakeratosis with hypogranulosis variably associated with neutrophil crust also is seen. Scattered dyskeratotic keratinocytes may be found within all levels of the epidermis. In resolving or chronic AE lesions, psoriasiform hyperplasia is prevalent, though necrolysis may be minimal or absent.5,11
Diagnosis
Evaluation includes measurement of plasma zinc levels. Zinc levels less than 50 µg/dL are suggestive but not diagnostic of AE.5 Although plasma zinc measurement is the most useful indicator of zinc status, its utility in assessing the true total body store of zinc is limited. Plasma zinc is tightly regulated and only represents 0.1% of body stores.5,6 Additionally, zinc levels may decrease in proinflammatory states.12 Beyond zinc measurement, evaluation of alkaline phosphatase, a zinc-dependent enzyme, can provide useful diagnostic information.5,6
Zinc and TPN
Patients on TPN are at a unique risk for developing zinc and other nutritional deficiencies. Because the daily recommended dietary allowance for zinc is low (8 mg daily for adult women and 11 mg daily for adult men)5 and the element is found in a wide variety of foods, maintaining adequate zinc levels is easily achieved in healthy individuals with normal diets. Kay et al13 described 4 patients on parenteral nutrition who developed hypozincemia and an AE-like syndrome within weeks of TPN induction. The authors described rapid and drastic clinical improvement after initiating zinc supplementation, accentuating the importance of including zinc as a component of TPN.13,14 Brazin et al15 also reported a case of an AE-like syndrome from zinc-deficient hyperalimentation in a patient receiving TPN for short bowel syndrome. Chun et al16 described another case of acquired AE in a patient on TPN for acute pancreatitis. Both cases demonstrated prompt improvement of skin lesions after treatment with zinc supplementation. Other nutrient deficiencies may reveal themselves through similar dermatologic manifestations. For example, cases of scaly dermatitis secondary to the development of essential fatty acid deficiency from TPN formulations lacking adequate quantities of linoleic acid have been reported.Similar to our case, the resolution of skin lesions was seen after TPN was supplemented with the deficient nutrient.17 These cases exemplify the importance in considering deficiency dermatoses in the TPN-dependent patient population.
Conclusion
In our case, the development of skin lesions directly coincided with a recent removal of zinc from the patient’s TPN, which provided us with a unique opportunity to observe the causal relationship between decreased zinc intake and the development of clinical signs of acquired AE. This association was further elucidated by laboratory confirmation of low serum zinc levels and rapid improvement in all skin lesions after zinc supplementation was initiated.
Case Report
A 54-year-old woman presented with a pruritic and slightly painful skin eruption that began perinasally and progressed over 1 week to involve the labial commissures, finger webs, dorsal surfaces of the feet, heels, and bilateral gluteal folds. In addition, the eruption involved the left thigh at the donor site of a prior skin graft. She received no relief after an intramuscular steroid injection and hydrocortisone cream 1% prescribed by a primary care physician who diagnosed the rash as poison ivy contact dermatitis despite no exposure to plants. Review of systems was negative and she denied any new medication use. Her medical history was notable for extensive mesenteric injury secondary to a motor vehicle accident. She subsequently had multiple enterocutaneous fistulas that resulted in a complete small bowel enterectomy 10 months prior to presentation, which caused her to become dependent on total parenteral nutrition (TPN).
Physical examination revealed sharply demarcated, erythematous, scaly plaques perinasally, periorally, and on the bilateral gluteal folds (Figure 1). There were sharply demarcated, erythematous, scaly plaques on the right and left finger webs, dorsal surface of the right foot, and left upper thigh. Hemorrhagic bullae were appreciated on the left finger webs. Large flaccid bullae were present on the bilateral heels and dorsum of the right foot (Figure 2).


Suspecting a diagnosis of acrodermatitis enteropathica (AE), laboratory testing included a serum zinc level, which was 42 µg/dL (reference range, 70–130 µg/dL). The copper and selenium levels also were low with values of 71 µg/dL (reference range, 80–155 µg/dL) and 31 µg/dL (reference range, 79–326 µg/dL), respectively. No additional vitamin or mineral deficiencies were discovered. A complete blood cell count and comprehensive metabolic panel were performed and showed no abnormalities other than a mildly elevated sodium level of 147 mEq/L (reference range, 136–142 mEq/L).
A punch biopsy was performed. Histopathology revealed subcorneal neutrophils and neutrophilic crust, mild spongiosis, and a dense upper dermal mixed neutrophilic and lymphohistiocytic infiltrate. The specimen also exhibited mild intercellular edema and prominent capillaries (Figure 3).

After further investigation, the company providing the patient’s TPN confirmed that zinc had been removed several weeks prior to the onset of symptoms due to a critical national shortage of trace element additives. Zinc was supplemented at 15 mg daily to the TPN solution. Three days later a skin examination revealed dramatic changes with notable improvement of the finger web plaques and complete resolution of the facial lesions. The plaques and bullae on the lower extremities also had resolved (Figure 4).

Comment
Background
Acrodermatitis enteropathica is a rare autosomal-recessive disorder of zinc metabolism characterized by skin lesions predominantly distributed in acral and periorificial sites as well as alopecia and diarrhea. Acrodermatitis enteropathica was first described by Brandt1 in 1936 and later characterized by Danbolt and Closs2 in 1942 as a unique and often fatal disease of unknown etiology. More than 30 years later, the link between zinc deficiency and AE was illustrated by Moynahan3 who demonstrated clinical improvement with zinc supplementation. It was not until 2002 that the molecular pathogenesis of hypozincemia in patients with inherited AE was described. Küry et al4 identified a mutation in the SLC39A4 gene responsible for encoding the Zip4 protein, a zinc transporter found on enterocytes, particularly in the proximal small intestine.5,6 Classically, patients with inherited AE are children who present within days of birth or days to weeks after being weaned from breast milk to cow’s milk. The zinc in bovine milk is less bioavailable than breast milk, though both have similar total zinc concentrations, which results in the decreased plasma zinc levels seen in children with inherited AE.5-8 Occasionally, children present before weaning due to decreased maternal mammary zinc secretion (lactogenic AE).9,10
Clinical Presentation
Similar clinical findings are seen in patients with noninherited forms of zinc deficiency known as acquired AE. Acquired zinc deficiency may be broadly categorized as being from inadequate intake, deficient absorption, excess demand, or overexcretion.8 Such disturbances of zinc balance are most frequently seen in patients with restrictive diets, anorexia nervosa, intestinal bypass procedures, Crohn disease, pancreatic insufficiency, alcoholism, human immunodeficiency virus, and extensive cutaneous burns. Premature infants, mothers who are breastfeeding, and those dependent on TPN are at risk for developing acquired zinc deficiency.7-9,11
Differentiating Characteristics
Both acquired and inherited AE present as erythematous or pink eczematous scaly plaques with the variable presence of vesicular or bullous lesions involving periorificial, acral, and anogenital regions. Early manifestations of AE may include angular cheilitis and paronychia. Alopecia and diarrhea are characteristics of later disease. In fact, the complete triad of dermatitis, alopecia, and diarrhea is seen in only 20% of cases.7 Without treatment, patients may develop blepharitis, conjunctivitis, photophobia, irritability, anorexia, apathy, growth retardation, hypogonadism, hypogeusia, and mental slowing. Skin lesions frequently become secondarily infected with Candida albicans and/or bacteria.5,7,11
Histopathology
Histopathologic examination of skin biopsy specimens from AE lesions demonstrates nonspecific findings similar to other deficiency dermatoses, such as pellagra and glucagonoma-associated necrolytic migratory erythema. Histology typically reveals cytoplasmic pallor with vacuolization and ballooning degeneration of keratinocytes, followed by confluent keratinocyte necrosis within the stratum granulosum and stratum spinosum of the epidermis.5 Confluent parakeratosis with hypogranulosis variably associated with neutrophil crust also is seen. Scattered dyskeratotic keratinocytes may be found within all levels of the epidermis. In resolving or chronic AE lesions, psoriasiform hyperplasia is prevalent, though necrolysis may be minimal or absent.5,11
Diagnosis
Evaluation includes measurement of plasma zinc levels. Zinc levels less than 50 µg/dL are suggestive but not diagnostic of AE.5 Although plasma zinc measurement is the most useful indicator of zinc status, its utility in assessing the true total body store of zinc is limited. Plasma zinc is tightly regulated and only represents 0.1% of body stores.5,6 Additionally, zinc levels may decrease in proinflammatory states.12 Beyond zinc measurement, evaluation of alkaline phosphatase, a zinc-dependent enzyme, can provide useful diagnostic information.5,6
Zinc and TPN
Patients on TPN are at a unique risk for developing zinc and other nutritional deficiencies. Because the daily recommended dietary allowance for zinc is low (8 mg daily for adult women and 11 mg daily for adult men)5 and the element is found in a wide variety of foods, maintaining adequate zinc levels is easily achieved in healthy individuals with normal diets. Kay et al13 described 4 patients on parenteral nutrition who developed hypozincemia and an AE-like syndrome within weeks of TPN induction. The authors described rapid and drastic clinical improvement after initiating zinc supplementation, accentuating the importance of including zinc as a component of TPN.13,14 Brazin et al15 also reported a case of an AE-like syndrome from zinc-deficient hyperalimentation in a patient receiving TPN for short bowel syndrome. Chun et al16 described another case of acquired AE in a patient on TPN for acute pancreatitis. Both cases demonstrated prompt improvement of skin lesions after treatment with zinc supplementation. Other nutrient deficiencies may reveal themselves through similar dermatologic manifestations. For example, cases of scaly dermatitis secondary to the development of essential fatty acid deficiency from TPN formulations lacking adequate quantities of linoleic acid have been reported.Similar to our case, the resolution of skin lesions was seen after TPN was supplemented with the deficient nutrient.17 These cases exemplify the importance in considering deficiency dermatoses in the TPN-dependent patient population.
Conclusion
In our case, the development of skin lesions directly coincided with a recent removal of zinc from the patient’s TPN, which provided us with a unique opportunity to observe the causal relationship between decreased zinc intake and the development of clinical signs of acquired AE. This association was further elucidated by laboratory confirmation of low serum zinc levels and rapid improvement in all skin lesions after zinc supplementation was initiated.
- Brandt T. Dermatitis in children with disturbances of general condition and absorption of food. Acta Derm Venereol. 1936;17:513-537.
- Danbolt N, Closs K. Acrodermatitis enteropathica. Acta Derm Venereol. 1942;23:127-169.
- Moynahan E. Acrodermatitis enteropathica: a lethal inherited human zinc deficiency disorder. Lancet. 1974;2:299-400.
- Küry S, Dréno B, Bézieau S, et al. Identification of SLC39A4, a gene involved in acrodermatitis enteropathica. Nat Genet. 2002;31:238-240.
- Maverakis E, Fung MA, Lynch PJ, et al. Acrodermatitis enteropathica and an overview of zinc metabolism. J Am Acad Dermatol. 2007;56:116-124.
- Thrash B, Patel M, Shah KR, et al. Cutaneous manifestations of gastrointestinal disease: part II. J Am Acad Dermatol. 2013;68:211.e1-211.e33; quiz 244-246.
- Perafán-Riveros C, França LF, Alves AC, et al. Acrodermatitis enteropathica: case report and review of the literature. Pediatr Dermatol. 2002;19:426-431.
- Kumar P, Ranjan NR, Mondal AK. Zinc and skin: a brief summary. Dermatol Online J. 2012;18:1.
- Saritha M, Gupta D, Chandrashekar L, et al. Acquired zinc deficiency in an adult female. Indian J Dermatol. 2012;57:492-494.
- Neldner K, Hambidge K, Walravens P. Acrodermatitis enteropathica.Int J Dermatol. 1978;17:380-387.
- Gehrig K, Dinulos J. Acrodermatitis due to nutritional deficiency. Curr Opin Pediatr. 2010;22:107-112.
- Liuzzi JP, Lichten LA, Rivera S, et al. Interleukin-6 regulates the zinc transporter Zip14 in liver and contributes to hypozincemia of the acute-phase response. Proct Natl Acad Sci U S A. 2005;102:6843-6848.
- Kay RG, Tasman-Jones C, Pybus J, et al. A syndrome of acute zinc deficiency during total parenteral nutrition in man. Ann Surg. 1976;183:331-340.
- Jeejeebhoy K. Zinc: an essential trace element for parenteral nutrition. Gastroenterology. 2009;137(5 suppl):S7-S12.
- Brazin SA, Johnson WT, Abramson LJ. The acrodermatitis enteropathica-like syndrome. Arch Dermatol. 1979;115:597-599.
- Chun JH, Baek JH, Chung NG. Development of bullous acrodermatitis enteropathica during the course of chemotherapy for acute lymphocytic leukemia. Ann Dermatol. 2011;23(suppl 3):S326-S328.
- Roongpisuthipong W, Phanachet P, Roongpisuthipong C, et al. Essential fatty acid deficiency while a patient receiving fat regimen total parenteral nutrition [published June 14, 2012]. BMJ Case Rep. doi:10.1136/bcr.07.2011.4475.
- Brandt T. Dermatitis in children with disturbances of general condition and absorption of food. Acta Derm Venereol. 1936;17:513-537.
- Danbolt N, Closs K. Acrodermatitis enteropathica. Acta Derm Venereol. 1942;23:127-169.
- Moynahan E. Acrodermatitis enteropathica: a lethal inherited human zinc deficiency disorder. Lancet. 1974;2:299-400.
- Küry S, Dréno B, Bézieau S, et al. Identification of SLC39A4, a gene involved in acrodermatitis enteropathica. Nat Genet. 2002;31:238-240.
- Maverakis E, Fung MA, Lynch PJ, et al. Acrodermatitis enteropathica and an overview of zinc metabolism. J Am Acad Dermatol. 2007;56:116-124.
- Thrash B, Patel M, Shah KR, et al. Cutaneous manifestations of gastrointestinal disease: part II. J Am Acad Dermatol. 2013;68:211.e1-211.e33; quiz 244-246.
- Perafán-Riveros C, França LF, Alves AC, et al. Acrodermatitis enteropathica: case report and review of the literature. Pediatr Dermatol. 2002;19:426-431.
- Kumar P, Ranjan NR, Mondal AK. Zinc and skin: a brief summary. Dermatol Online J. 2012;18:1.
- Saritha M, Gupta D, Chandrashekar L, et al. Acquired zinc deficiency in an adult female. Indian J Dermatol. 2012;57:492-494.
- Neldner K, Hambidge K, Walravens P. Acrodermatitis enteropathica.Int J Dermatol. 1978;17:380-387.
- Gehrig K, Dinulos J. Acrodermatitis due to nutritional deficiency. Curr Opin Pediatr. 2010;22:107-112.
- Liuzzi JP, Lichten LA, Rivera S, et al. Interleukin-6 regulates the zinc transporter Zip14 in liver and contributes to hypozincemia of the acute-phase response. Proct Natl Acad Sci U S A. 2005;102:6843-6848.
- Kay RG, Tasman-Jones C, Pybus J, et al. A syndrome of acute zinc deficiency during total parenteral nutrition in man. Ann Surg. 1976;183:331-340.
- Jeejeebhoy K. Zinc: an essential trace element for parenteral nutrition. Gastroenterology. 2009;137(5 suppl):S7-S12.
- Brazin SA, Johnson WT, Abramson LJ. The acrodermatitis enteropathica-like syndrome. Arch Dermatol. 1979;115:597-599.
- Chun JH, Baek JH, Chung NG. Development of bullous acrodermatitis enteropathica during the course of chemotherapy for acute lymphocytic leukemia. Ann Dermatol. 2011;23(suppl 3):S326-S328.
- Roongpisuthipong W, Phanachet P, Roongpisuthipong C, et al. Essential fatty acid deficiency while a patient receiving fat regimen total parenteral nutrition [published June 14, 2012]. BMJ Case Rep. doi:10.1136/bcr.07.2011.4475.
Practice Points
- Acrodermatitis enteropathica (AE) may be acquired or due to a rare autosomal-recessive disorder of zinc absorption.
- Hereditary AE typically becomes symptomatic during infancy, while acquired AE may develop during hypozincemia in patients of any age.
- Both acquired and hereditary AE improve with zinc supplementation.
Teleconference is effective in assessing penicillin allergy
ORLANDO – and resulted in almost every patient having their allergy label removed, researchers reported.
In what the researchers said was the first study showing the utility of telemedicine in evaluating patient-reported penicillin allergies, allergy and immunology physicians did a secure telemedicine consultation with patients after they underwent penicillin skin testing with a physician assistant; an approach which, on average, took 123 minutes fewer each time than if the physician had done the consultation face-to-face. The teleconference can be done on a laptop or smartphone.
She said the approach is sensible and effective, and it is a good alternative to the traditional way of doing these tests. “What this takes out of that is the travel part. Someone else is doing the travel and the technique of testing,” added that people often are labeled in childhood after getting a rash that was thought to be related to penicillin, but actually was just a coincidence that was unrelated. Then the false allergy label is attached to them for life.
“This is so overlabeled,” Dr. Ramsey said. “Ten percent of the population thinks they’re allergic to penicillin, and 90% of them are not.”
A stark difference was found in the types of medicines administered before and after the evaluations, with aminopenicillin therapy jumping from 0 days of use to 188 days, and vancomycin – a more potent, but more costly alternative – dropping from 130 days of use to 16 days (P less than .05 for both).
Dr. Ramsey noted that, in part because of the time-consuming nature of the penicillin skin tests, they often are simply not done, so the false allergy labels are not caught, leading to pointless costs and exposure to more potent and potentially harmful forms of antibiotic therapy.
“Some hospitals don’t have allergists who will come in to do testing,” she said. “Sometimes patients are on medications that may interfere. And then a lot of times it’s just underrecognized – the implications of a penicillin allergy label. That is a very hot topic in our field and also in infectious disease.”
She hopes the telemedicine approach catches on more widely, which would help minimize the multitude of problems linked to penicillin allergy labels.
“Patients that avoid penicillin, they’re on more costly second-line antibiotics that are, in general, less effective, depending on which infection you’re talking about,” Dr. Ramsey said. “The [second-line antibiotics] have more side effects. And there’s data to show that patients with [a] penicillin allergy label have longer hospital stays, more costly hospital stays, are at risk for more resistant infections. And it breeds antimicrobial resistance in the long-term.”
Dr. Ramsey had no relevant financial disclosures.
SOURCE: Ramsey AC et al. AAAAI/WAO Joint Congress, Abstract 104.
ORLANDO – and resulted in almost every patient having their allergy label removed, researchers reported.
In what the researchers said was the first study showing the utility of telemedicine in evaluating patient-reported penicillin allergies, allergy and immunology physicians did a secure telemedicine consultation with patients after they underwent penicillin skin testing with a physician assistant; an approach which, on average, took 123 minutes fewer each time than if the physician had done the consultation face-to-face. The teleconference can be done on a laptop or smartphone.
She said the approach is sensible and effective, and it is a good alternative to the traditional way of doing these tests. “What this takes out of that is the travel part. Someone else is doing the travel and the technique of testing,” added that people often are labeled in childhood after getting a rash that was thought to be related to penicillin, but actually was just a coincidence that was unrelated. Then the false allergy label is attached to them for life.
“This is so overlabeled,” Dr. Ramsey said. “Ten percent of the population thinks they’re allergic to penicillin, and 90% of them are not.”
A stark difference was found in the types of medicines administered before and after the evaluations, with aminopenicillin therapy jumping from 0 days of use to 188 days, and vancomycin – a more potent, but more costly alternative – dropping from 130 days of use to 16 days (P less than .05 for both).
Dr. Ramsey noted that, in part because of the time-consuming nature of the penicillin skin tests, they often are simply not done, so the false allergy labels are not caught, leading to pointless costs and exposure to more potent and potentially harmful forms of antibiotic therapy.
“Some hospitals don’t have allergists who will come in to do testing,” she said. “Sometimes patients are on medications that may interfere. And then a lot of times it’s just underrecognized – the implications of a penicillin allergy label. That is a very hot topic in our field and also in infectious disease.”
She hopes the telemedicine approach catches on more widely, which would help minimize the multitude of problems linked to penicillin allergy labels.
“Patients that avoid penicillin, they’re on more costly second-line antibiotics that are, in general, less effective, depending on which infection you’re talking about,” Dr. Ramsey said. “The [second-line antibiotics] have more side effects. And there’s data to show that patients with [a] penicillin allergy label have longer hospital stays, more costly hospital stays, are at risk for more resistant infections. And it breeds antimicrobial resistance in the long-term.”
Dr. Ramsey had no relevant financial disclosures.
SOURCE: Ramsey AC et al. AAAAI/WAO Joint Congress, Abstract 104.
ORLANDO – and resulted in almost every patient having their allergy label removed, researchers reported.
In what the researchers said was the first study showing the utility of telemedicine in evaluating patient-reported penicillin allergies, allergy and immunology physicians did a secure telemedicine consultation with patients after they underwent penicillin skin testing with a physician assistant; an approach which, on average, took 123 minutes fewer each time than if the physician had done the consultation face-to-face. The teleconference can be done on a laptop or smartphone.
She said the approach is sensible and effective, and it is a good alternative to the traditional way of doing these tests. “What this takes out of that is the travel part. Someone else is doing the travel and the technique of testing,” added that people often are labeled in childhood after getting a rash that was thought to be related to penicillin, but actually was just a coincidence that was unrelated. Then the false allergy label is attached to them for life.
“This is so overlabeled,” Dr. Ramsey said. “Ten percent of the population thinks they’re allergic to penicillin, and 90% of them are not.”
A stark difference was found in the types of medicines administered before and after the evaluations, with aminopenicillin therapy jumping from 0 days of use to 188 days, and vancomycin – a more potent, but more costly alternative – dropping from 130 days of use to 16 days (P less than .05 for both).
Dr. Ramsey noted that, in part because of the time-consuming nature of the penicillin skin tests, they often are simply not done, so the false allergy labels are not caught, leading to pointless costs and exposure to more potent and potentially harmful forms of antibiotic therapy.
“Some hospitals don’t have allergists who will come in to do testing,” she said. “Sometimes patients are on medications that may interfere. And then a lot of times it’s just underrecognized – the implications of a penicillin allergy label. That is a very hot topic in our field and also in infectious disease.”
She hopes the telemedicine approach catches on more widely, which would help minimize the multitude of problems linked to penicillin allergy labels.
“Patients that avoid penicillin, they’re on more costly second-line antibiotics that are, in general, less effective, depending on which infection you’re talking about,” Dr. Ramsey said. “The [second-line antibiotics] have more side effects. And there’s data to show that patients with [a] penicillin allergy label have longer hospital stays, more costly hospital stays, are at risk for more resistant infections. And it breeds antimicrobial resistance in the long-term.”
Dr. Ramsey had no relevant financial disclosures.
SOURCE: Ramsey AC et al. AAAAI/WAO Joint Congress, Abstract 104.
REPORTING FROM AAAAI/WAO JOINT CONGRESS
Key clinical point: Teleconferencing to assess patient-reported penicillin allergies saves time and results in 9 out of 10 patients being delabeled.
Major finding: Of 50 patients prospectively assessed with this approach over a 4-month period last year, 46 were delabeled, with $23,000 in direct antibiotic cost savings, or $360 per patient.
Study details: A prospective study conducted over 4 months in 2017.
Disclosures: Dr. Ramsey had no relevant financial disclosures.
Source: Ramsey AC et al. AAAAI/WAO Joint Congress, Abstract 104.
Omalizumab for chronic urticaria quells suffocation fears
GENEVA – a widely underappreciated dimension of the impairment caused by this disease, Karsten Weller, MD, said at the annual congress of the European Academy of Dermatology and Venereology.
“Virtually all clinical studies of CSU in recent decades focus on the wheal and pruritus components and not on the angioedema component, even though angioedema is a frequent symptom in the disease. Roughly half of patients with CSU experience wheals and angioedema, and up to 13% experience angioedema only,” said Dr. Weller, a dermatologist at Charité University Hospital in Berlin.
“Angioedema is a major driver of quality-of-life impairment in CSU,” he continued. “We know that these are the patients who particularly suffer from the unpredictability of the disease, from disfigurement, from embarrassment. These are the patients who come to the emergency rooms, who lose working days, and these are the patients who often have the feeling of losing control over their lives.”
X-ACT was a multicenter German study which included 91 patients with moderate to severe CSU marked by at least four angioedema episodes during the 6 months prior to enrollment. Participants also had to be refractory to second-generation H1 antihistamines at two to four times the approved dose. The subjects were randomized to subcutaneous omalizumab at 300 mg every 4 weeks or placebo for 28 weeks; they were then further assessed for changes in quality of life during 8 weeks off omalizumab.
Because assessment of quality of life was such a major part of X-ACT, the investigators pulled out all the stops. Their multimodal evaluation included the Angioedema Quality of Life questionnaire – a patient-reported, 17-item instrument that is the first validated tool for evaluation of angioedema-specific quality of life – as well as the Dermatology Life Quality Index and the weekly Angioedema Activity Score.
Patients were also asked to rate on a 0-4 scale their degree of fearfulness of life-threatening swelling episodes and also their degree of fearfulness of angioedema-related suffocation. “To my knowledge, this is the first time this has been done in a randomized clinical trial,” Dr. Weller noted.
The patient reports were striking: At baseline, 49% indicated that they occasionally, often, or very often were afraid of suffocating caused by swelling episodes; only 4% of patients expressed that fear after 28 weeks on omalizumab, compared with 25% of placebo-treated controls. Similarly, at baseline two-thirds of patients reported occasionally, often, or very often being fearful of life-threatening swelling episodes, a rate that fell to 14% after 28 weeks on omalizumab, compared with 42% for controls.
Scores on the Angioedema Quality of Life Questionnaire improved continuously from a baseline of roughly 60 on a 0-100 scale – indicative of severe impairment – to less than 20 after 28 weeks on omalizumab; these scores steadily worsened again during the 8 weeks following treatment discontinuation. The Dermatology Life Quality Index scores dropped from a mean baseline of 15.6 down to 5 by week 4, remained in the 3-5 range for the remainder of the treatment period, then increased again when treatment was discontinued. The Angioedema Activity Score followed a similar pattern.
One audience member observed that the placebo response was quite strong in the study, with the percentage of patients reporting fear of suffocating caused by angioedema episodes falling from 49% at baseline to 25% after 28 weeks on placebo.
Dr. Weller replied that a potent placebo response is a consistent feature of all clinical trials of CSU therapies. The explanation, he added, is unknown.
He reported receiving research grants from and serving as a consultant to Novartis, which sponsored the X-ACT trial.
SOURCE: Weller K et al. EADV Congress 2017.
GENEVA – a widely underappreciated dimension of the impairment caused by this disease, Karsten Weller, MD, said at the annual congress of the European Academy of Dermatology and Venereology.
“Virtually all clinical studies of CSU in recent decades focus on the wheal and pruritus components and not on the angioedema component, even though angioedema is a frequent symptom in the disease. Roughly half of patients with CSU experience wheals and angioedema, and up to 13% experience angioedema only,” said Dr. Weller, a dermatologist at Charité University Hospital in Berlin.
“Angioedema is a major driver of quality-of-life impairment in CSU,” he continued. “We know that these are the patients who particularly suffer from the unpredictability of the disease, from disfigurement, from embarrassment. These are the patients who come to the emergency rooms, who lose working days, and these are the patients who often have the feeling of losing control over their lives.”
X-ACT was a multicenter German study which included 91 patients with moderate to severe CSU marked by at least four angioedema episodes during the 6 months prior to enrollment. Participants also had to be refractory to second-generation H1 antihistamines at two to four times the approved dose. The subjects were randomized to subcutaneous omalizumab at 300 mg every 4 weeks or placebo for 28 weeks; they were then further assessed for changes in quality of life during 8 weeks off omalizumab.
Because assessment of quality of life was such a major part of X-ACT, the investigators pulled out all the stops. Their multimodal evaluation included the Angioedema Quality of Life questionnaire – a patient-reported, 17-item instrument that is the first validated tool for evaluation of angioedema-specific quality of life – as well as the Dermatology Life Quality Index and the weekly Angioedema Activity Score.
Patients were also asked to rate on a 0-4 scale their degree of fearfulness of life-threatening swelling episodes and also their degree of fearfulness of angioedema-related suffocation. “To my knowledge, this is the first time this has been done in a randomized clinical trial,” Dr. Weller noted.
The patient reports were striking: At baseline, 49% indicated that they occasionally, often, or very often were afraid of suffocating caused by swelling episodes; only 4% of patients expressed that fear after 28 weeks on omalizumab, compared with 25% of placebo-treated controls. Similarly, at baseline two-thirds of patients reported occasionally, often, or very often being fearful of life-threatening swelling episodes, a rate that fell to 14% after 28 weeks on omalizumab, compared with 42% for controls.
Scores on the Angioedema Quality of Life Questionnaire improved continuously from a baseline of roughly 60 on a 0-100 scale – indicative of severe impairment – to less than 20 after 28 weeks on omalizumab; these scores steadily worsened again during the 8 weeks following treatment discontinuation. The Dermatology Life Quality Index scores dropped from a mean baseline of 15.6 down to 5 by week 4, remained in the 3-5 range for the remainder of the treatment period, then increased again when treatment was discontinued. The Angioedema Activity Score followed a similar pattern.
One audience member observed that the placebo response was quite strong in the study, with the percentage of patients reporting fear of suffocating caused by angioedema episodes falling from 49% at baseline to 25% after 28 weeks on placebo.
Dr. Weller replied that a potent placebo response is a consistent feature of all clinical trials of CSU therapies. The explanation, he added, is unknown.
He reported receiving research grants from and serving as a consultant to Novartis, which sponsored the X-ACT trial.
SOURCE: Weller K et al. EADV Congress 2017.
GENEVA – a widely underappreciated dimension of the impairment caused by this disease, Karsten Weller, MD, said at the annual congress of the European Academy of Dermatology and Venereology.
“Virtually all clinical studies of CSU in recent decades focus on the wheal and pruritus components and not on the angioedema component, even though angioedema is a frequent symptom in the disease. Roughly half of patients with CSU experience wheals and angioedema, and up to 13% experience angioedema only,” said Dr. Weller, a dermatologist at Charité University Hospital in Berlin.
“Angioedema is a major driver of quality-of-life impairment in CSU,” he continued. “We know that these are the patients who particularly suffer from the unpredictability of the disease, from disfigurement, from embarrassment. These are the patients who come to the emergency rooms, who lose working days, and these are the patients who often have the feeling of losing control over their lives.”
X-ACT was a multicenter German study which included 91 patients with moderate to severe CSU marked by at least four angioedema episodes during the 6 months prior to enrollment. Participants also had to be refractory to second-generation H1 antihistamines at two to four times the approved dose. The subjects were randomized to subcutaneous omalizumab at 300 mg every 4 weeks or placebo for 28 weeks; they were then further assessed for changes in quality of life during 8 weeks off omalizumab.
Because assessment of quality of life was such a major part of X-ACT, the investigators pulled out all the stops. Their multimodal evaluation included the Angioedema Quality of Life questionnaire – a patient-reported, 17-item instrument that is the first validated tool for evaluation of angioedema-specific quality of life – as well as the Dermatology Life Quality Index and the weekly Angioedema Activity Score.
Patients were also asked to rate on a 0-4 scale their degree of fearfulness of life-threatening swelling episodes and also their degree of fearfulness of angioedema-related suffocation. “To my knowledge, this is the first time this has been done in a randomized clinical trial,” Dr. Weller noted.
The patient reports were striking: At baseline, 49% indicated that they occasionally, often, or very often were afraid of suffocating caused by swelling episodes; only 4% of patients expressed that fear after 28 weeks on omalizumab, compared with 25% of placebo-treated controls. Similarly, at baseline two-thirds of patients reported occasionally, often, or very often being fearful of life-threatening swelling episodes, a rate that fell to 14% after 28 weeks on omalizumab, compared with 42% for controls.
Scores on the Angioedema Quality of Life Questionnaire improved continuously from a baseline of roughly 60 on a 0-100 scale – indicative of severe impairment – to less than 20 after 28 weeks on omalizumab; these scores steadily worsened again during the 8 weeks following treatment discontinuation. The Dermatology Life Quality Index scores dropped from a mean baseline of 15.6 down to 5 by week 4, remained in the 3-5 range for the remainder of the treatment period, then increased again when treatment was discontinued. The Angioedema Activity Score followed a similar pattern.
One audience member observed that the placebo response was quite strong in the study, with the percentage of patients reporting fear of suffocating caused by angioedema episodes falling from 49% at baseline to 25% after 28 weeks on placebo.
Dr. Weller replied that a potent placebo response is a consistent feature of all clinical trials of CSU therapies. The explanation, he added, is unknown.
He reported receiving research grants from and serving as a consultant to Novartis, which sponsored the X-ACT trial.
SOURCE: Weller K et al. EADV Congress 2017.
REPORTING FROM THE EADV CONGRESS
Key clinical point: Omalizumab relieves the heavy quality-of-life burden associated with CSU.
Major finding: At baseline, 49% of CSU patients indicated they occasionally, often, or very often were afraid of suffocating due to swelling episodes; after 28 weeks on omalizumab, only 4% expressed that fear.
Study details: The X-ACT trial was a phase 3 double-blind, multicenter, placebo-controlled randomized trial including 91 patients with CSU.
Disclosures: The presenter reported receiving research grants from and serving as a consultant to Novartis, which sponsored the X-ACT trial.
Source: Weller K et al. EADV Congress 2017.
Updosing omalizumab for chronic urticaria pays off
GENEVA – In real-world clinical practice, roughly – and for those who don’t, three-quarters will respond upon updosing to 450 or 600 mg every 4 weeks.
That’s the key message of an open-label study of 286 patients with chronic spontaneous urticaria (CSU) conducted at 15 hospitals by the Catalan and Balearic Chronic Urticaria Network (XUrCB), Jorge Spertino, MD, reported at the annual congress of the European Academy of Dermatology and Venereology.
In three published, pivotal, phase 3 randomized trials, the clinical response rate to omalizumab at 300 mg every 4 weeks, as defined by a weekly 7-day Urticaria Activity Score (UAS7) of 6 or less at 12 weeks, was 52% in ASTERIA I, 66% in ASTERIA II, and 52% in GLACIAL. But patients enrolled in formal randomized trials are often quite different from the broader group encountered in daily practice, and the Spanish dermatologists wanted to know if updosing in suboptimal responders was safe and effective. It turns out that it certainly is, according to Dr. Spertino of Teknon Medical Center in Barcelona.
The treatment algorithm followed by the XUrCB investigators was that, if after six doses at the approved dose of 300 mg every 4 weeks a patient didn’t have good control of disease activity, the dose was increased to 450 mg every 4 weeks. If after three doses at that level, there still wasn’t good control of the CSU, the dose was further increased to 600 mg every 4 weeks.
As in the pivotal phase 3 clinical trials, the XUrCB group defined good control of disease activity as a UAS7 score of 6 or less in accord with a study that demonstrated such a score on the 0- to 42-point UAS7 correlates well with minimal or no patient symptoms (Br J Dermatol. 2017 Oct;177[4]:1093-1101).
At baseline, the mean age of the 286 CSU patients was 44.6 years and the mean UAS7 score was 26.5; 74% were women. Forty-seven percent of patients experienced angioedema and 33% had inducible urticaria, most commonly brought forth by pressure or dermographism. One-third of patients had previously been on cyclosporine and half of the patients had a high d-dimer level.
Sixty-five percent of patients achieved good disease control on omalizumab at 300 mg every 4 weeks. Of the 99 patients (35%) who didn’t, 20 patients stopped treatment at their dermatologist’s request because their symptoms remained uncontrolled on the approved dose. But 59 of the 79 who updosed obtained good disease control: 43 on a dose of 450 mg and 16 on a dose of 600 mg.
In multivariate analysis, two predictors of treatment success with updosing were identified: previous treatment with cyclosporine and obesity. Among patients previously on cyclosporine – a marker for more severe disease – only 21% achieved a UAS7 score of 6 or less on the approved dose, while 41% did so upon updosing. And obesity was associated with a 3.7-fold increased likelihood of a favorable response to updosing after lack of treatment success at the approved dose.
Neither a high d-dimer or serum IgE level, baseline UAS7 score, gender, associated angioedema, nor inducible urticaria was significantly associated with an increased treatment success rate upon updosing.
Updosing proved to be safe. All adverse events were mild and infrequent, consisting of headache, local injection site reactions, and arthromyalgia s, each occurring in 1%-2% of patients. Frequencies were similar in updosed patients and those on the approved dosing schedule.
Session cochair Jorgen Serup, MD, DMsc, congratulated Dr. Spertino for supplying physicians with “very-much-needed data.”
“This is very convincing data and highly clinically relevant for those of us who have these patients in our practices,” said Dr. Serup, professor of dermatology at Copenhagen University.
Dr. Spertino reported having no financial conflicts of interest regarding his presentation.
SOURCE: Spertino J et al. EADV Congress
GENEVA – In real-world clinical practice, roughly – and for those who don’t, three-quarters will respond upon updosing to 450 or 600 mg every 4 weeks.
That’s the key message of an open-label study of 286 patients with chronic spontaneous urticaria (CSU) conducted at 15 hospitals by the Catalan and Balearic Chronic Urticaria Network (XUrCB), Jorge Spertino, MD, reported at the annual congress of the European Academy of Dermatology and Venereology.
In three published, pivotal, phase 3 randomized trials, the clinical response rate to omalizumab at 300 mg every 4 weeks, as defined by a weekly 7-day Urticaria Activity Score (UAS7) of 6 or less at 12 weeks, was 52% in ASTERIA I, 66% in ASTERIA II, and 52% in GLACIAL. But patients enrolled in formal randomized trials are often quite different from the broader group encountered in daily practice, and the Spanish dermatologists wanted to know if updosing in suboptimal responders was safe and effective. It turns out that it certainly is, according to Dr. Spertino of Teknon Medical Center in Barcelona.
The treatment algorithm followed by the XUrCB investigators was that, if after six doses at the approved dose of 300 mg every 4 weeks a patient didn’t have good control of disease activity, the dose was increased to 450 mg every 4 weeks. If after three doses at that level, there still wasn’t good control of the CSU, the dose was further increased to 600 mg every 4 weeks.
As in the pivotal phase 3 clinical trials, the XUrCB group defined good control of disease activity as a UAS7 score of 6 or less in accord with a study that demonstrated such a score on the 0- to 42-point UAS7 correlates well with minimal or no patient symptoms (Br J Dermatol. 2017 Oct;177[4]:1093-1101).
At baseline, the mean age of the 286 CSU patients was 44.6 years and the mean UAS7 score was 26.5; 74% were women. Forty-seven percent of patients experienced angioedema and 33% had inducible urticaria, most commonly brought forth by pressure or dermographism. One-third of patients had previously been on cyclosporine and half of the patients had a high d-dimer level.
Sixty-five percent of patients achieved good disease control on omalizumab at 300 mg every 4 weeks. Of the 99 patients (35%) who didn’t, 20 patients stopped treatment at their dermatologist’s request because their symptoms remained uncontrolled on the approved dose. But 59 of the 79 who updosed obtained good disease control: 43 on a dose of 450 mg and 16 on a dose of 600 mg.
In multivariate analysis, two predictors of treatment success with updosing were identified: previous treatment with cyclosporine and obesity. Among patients previously on cyclosporine – a marker for more severe disease – only 21% achieved a UAS7 score of 6 or less on the approved dose, while 41% did so upon updosing. And obesity was associated with a 3.7-fold increased likelihood of a favorable response to updosing after lack of treatment success at the approved dose.
Neither a high d-dimer or serum IgE level, baseline UAS7 score, gender, associated angioedema, nor inducible urticaria was significantly associated with an increased treatment success rate upon updosing.
Updosing proved to be safe. All adverse events were mild and infrequent, consisting of headache, local injection site reactions, and arthromyalgia s, each occurring in 1%-2% of patients. Frequencies were similar in updosed patients and those on the approved dosing schedule.
Session cochair Jorgen Serup, MD, DMsc, congratulated Dr. Spertino for supplying physicians with “very-much-needed data.”
“This is very convincing data and highly clinically relevant for those of us who have these patients in our practices,” said Dr. Serup, professor of dermatology at Copenhagen University.
Dr. Spertino reported having no financial conflicts of interest regarding his presentation.
SOURCE: Spertino J et al. EADV Congress
GENEVA – In real-world clinical practice, roughly – and for those who don’t, three-quarters will respond upon updosing to 450 or 600 mg every 4 weeks.
That’s the key message of an open-label study of 286 patients with chronic spontaneous urticaria (CSU) conducted at 15 hospitals by the Catalan and Balearic Chronic Urticaria Network (XUrCB), Jorge Spertino, MD, reported at the annual congress of the European Academy of Dermatology and Venereology.
In three published, pivotal, phase 3 randomized trials, the clinical response rate to omalizumab at 300 mg every 4 weeks, as defined by a weekly 7-day Urticaria Activity Score (UAS7) of 6 or less at 12 weeks, was 52% in ASTERIA I, 66% in ASTERIA II, and 52% in GLACIAL. But patients enrolled in formal randomized trials are often quite different from the broader group encountered in daily practice, and the Spanish dermatologists wanted to know if updosing in suboptimal responders was safe and effective. It turns out that it certainly is, according to Dr. Spertino of Teknon Medical Center in Barcelona.
The treatment algorithm followed by the XUrCB investigators was that, if after six doses at the approved dose of 300 mg every 4 weeks a patient didn’t have good control of disease activity, the dose was increased to 450 mg every 4 weeks. If after three doses at that level, there still wasn’t good control of the CSU, the dose was further increased to 600 mg every 4 weeks.
As in the pivotal phase 3 clinical trials, the XUrCB group defined good control of disease activity as a UAS7 score of 6 or less in accord with a study that demonstrated such a score on the 0- to 42-point UAS7 correlates well with minimal or no patient symptoms (Br J Dermatol. 2017 Oct;177[4]:1093-1101).
At baseline, the mean age of the 286 CSU patients was 44.6 years and the mean UAS7 score was 26.5; 74% were women. Forty-seven percent of patients experienced angioedema and 33% had inducible urticaria, most commonly brought forth by pressure or dermographism. One-third of patients had previously been on cyclosporine and half of the patients had a high d-dimer level.
Sixty-five percent of patients achieved good disease control on omalizumab at 300 mg every 4 weeks. Of the 99 patients (35%) who didn’t, 20 patients stopped treatment at their dermatologist’s request because their symptoms remained uncontrolled on the approved dose. But 59 of the 79 who updosed obtained good disease control: 43 on a dose of 450 mg and 16 on a dose of 600 mg.
In multivariate analysis, two predictors of treatment success with updosing were identified: previous treatment with cyclosporine and obesity. Among patients previously on cyclosporine – a marker for more severe disease – only 21% achieved a UAS7 score of 6 or less on the approved dose, while 41% did so upon updosing. And obesity was associated with a 3.7-fold increased likelihood of a favorable response to updosing after lack of treatment success at the approved dose.
Neither a high d-dimer or serum IgE level, baseline UAS7 score, gender, associated angioedema, nor inducible urticaria was significantly associated with an increased treatment success rate upon updosing.
Updosing proved to be safe. All adverse events were mild and infrequent, consisting of headache, local injection site reactions, and arthromyalgia s, each occurring in 1%-2% of patients. Frequencies were similar in updosed patients and those on the approved dosing schedule.
Session cochair Jorgen Serup, MD, DMsc, congratulated Dr. Spertino for supplying physicians with “very-much-needed data.”
“This is very convincing data and highly clinically relevant for those of us who have these patients in our practices,” said Dr. Serup, professor of dermatology at Copenhagen University.
Dr. Spertino reported having no financial conflicts of interest regarding his presentation.
SOURCE: Spertino J et al. EADV Congress
REPORTING FROM THE EADV CONGRESS
Key clinical point: Updosing of omalizumab to a maximum of twice the approved dose is safe and effective in chronic spontaneous urticaria patients unresponsive to the licensed dose.
Major finding: Upon updosing, 75% of nonresponders to the approved dose achieved good disease control with no increase in adverse events.
Study details: This multicenter study of an omalizumab updosing algorithm included 286 patients with chronic spontaneous urticaria.
Disclosures: The study presenter reported having no financial conflicts.
Evaluation of Patch Test Reactivities in Patients With Chronic Idiopathic Urticaria
Chronic urticaria (CU) is clinically defined as the daily or almost daily presence of wheals on the skin for at least 6 weeks.1 Chronic urticaria severely affects patients’ quality of life and can cause emotional disability and distress.2 In clinical practice, CU is one of the most common and challenging conditions for general practitioners, dermatologists, and allergists. It can be provoked by a wide variety of different causes or may be the clinical presentation of certain systemic diseases3,4; thus, CU often requires a detailed and time-consuming diagnostic procedure that includes screening for allergies, autoimmune diseases, parasites, malignancies, infections, and metabolic disorders.5,6 In many patients (up to 50% in some case series), the cause or pathogenic mechanism cannot be identified, and the disease is then classified as chronic idiopathic urticaria (CIU).7
It has previously been shown that contact sensitization could have some relation with CIU,8 which was further explored in this study. This study sought to evaluate if contact allergy may play a role in disease development in CIU patients in Saudi Arabia and if patch testing should be routinely performed for CIU patients to determine if any allergens can be avoided.
Methods
This prospective study was conducted at the King Khalid University Hospital Allergy Clinic (Riyadh, Saudi Arabia) in patients aged 18 to 60 years who had CU for more than 6 weeks. It was a clinic-based study conducted over a period of 2 years (March 2010 to February 2012). The study protocol was approved by the local ethics committee at King Khalid University Hospital. Valid written consent was obtained from each patient.
Patients were excluded if they had CU caused by physical factors (eg, hot or cold temperature, water, physical contact) or drug reactions that were possible causative factors or if they had taken oral prednisolone or other oral immunosuppressive drugs (eg, azathioprine, cyclosporine) in the last month. However, patients taking antihistamines were not excluded because it was impossible for the patients to discontinue their urticaria treatment. Other exclusion criteria included CU associated with any systemic disease, thyroid disease, diabetes mellitus, autoimmune disorder, or atopic dermatitis. Pregnant and lactating women were not included in this study.
All new adult CU patients (ie, disease duration >6 weeks) were worked up using the routine diagnostic tests that are typically performed for any new CU patient, including complete blood cell count with differential, erythrocyte sedimentation rate, liver function tests, urine analysis, and hepatitis B and C screenings. Further diagnostic tests also were carried out when appropriate according to the patient’s history and physical examination, including levels of urea, electrolytes, thyrotropin, thyroid antibodies (antithyroglobulin and antimicrosomal), and antinuclear antibodies, as well as a Helicobacter pylori test.
All of the patients enrolled in the study were evaluated by skin prick testing to establish the link between CU and its cause. Patch testing was performed in patients who were negative on skin prick testing.
Skin Prick Testing
All patients were advised to temporarily discontinue the use of antihistamines and corticosteroids 5 to 6 days prior to testing.
Patch Testing
Patch tests were carried out using a ready-to-use epicutaneous patch test system for the diagnosis of allergic contact dermatitis (ACD).10 A European standard series was used with the addition of 4 allergens of local relevance: black seed oil, local perfume mix, henna, and myrrh (a topical herbal medicine used to promote healing).
Assessment of Improvement
Assessment of urticaria severity using the Chronic Urticaria Severity Score (CUSS), a simple semiquantitative assessment of disease activity, was calculated as the sum of the number of wheals and the degree of itch severity graded from 0 (none) to 3 (severe), according to the guidelines established by the Dermatology Section of the European Academy of Allergology and Clinical Immunology, the Global Allergy and Asthma European Network, the European Dermatology Forum, and the World Allergy Organization.11 The avoidance group of patients was assessed at baseline and after 1 month to evaluate changes in their CUSS after allergen avoidance for 8 weeks.
Statistical Analysis
All of the statistical analyses were carried out using SPSS software version 16. Results were presented as the median with the range or the mean (SD).
Results
During the study period, a total of 120 CU patients were seen at the clinic. Ninety-three patients with CU met our selection criteria (77.5%) and were enrolled in the study. The mean age (SD) of the patients was 34.7 (12.4) years. Women comprised 68.8% (64/93) of the study population (Table 1).
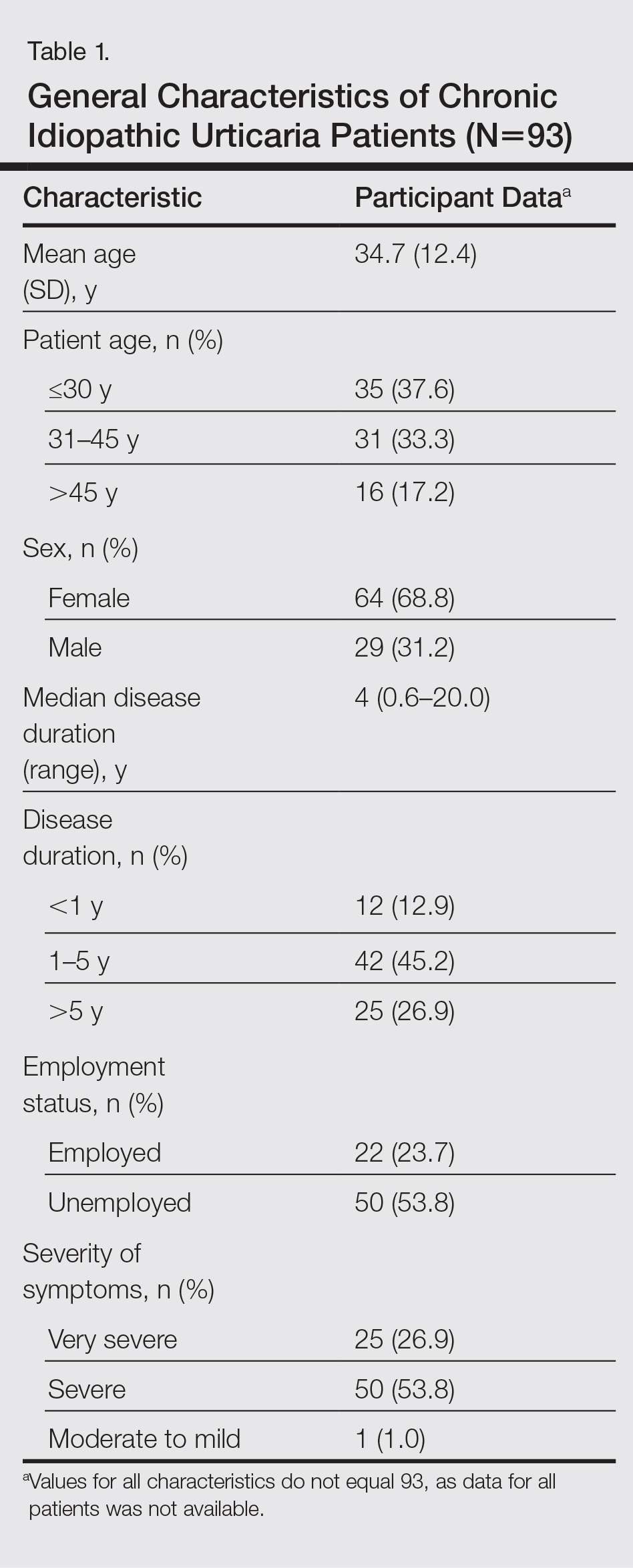
The duration of urticaria ranged from 0.6 to 20 years, with a median duration of 4 years. Approximately half of the patients (50/93) experienced severe symptoms of urticaria, but only 26.9% (25/93) had graded their urticaria as very severe.
Negative results from the skin prick test were reported in 62.4% (58/93) of patients and were subsequently patch tested. These patients also had no other etiologic factors (eg, infection; thyroid, autoimmune, or metabolic disease). Patients who had positive skin prick test results (35/93 [37.6%]) were not considered to be cases of CIU, according to diagnostic recommendations.12 Of the 58 CIU patients who were patch tested, 31 (53.4%) had positive results and 27 (46.5%) had negative results to both skin prick and patch tests (Figure).
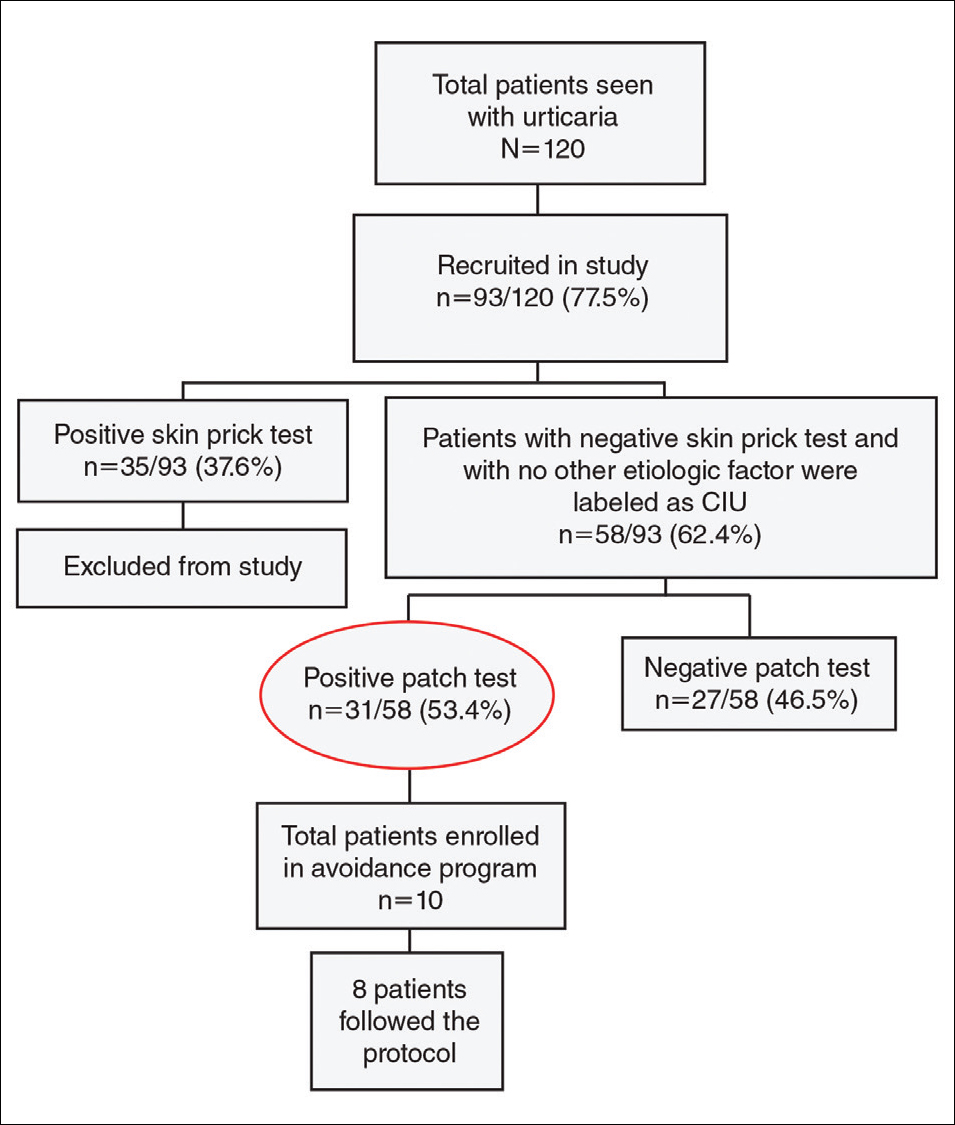
Univariate analysis revealed significant associations between age, gender, and duration of urticaria and patch test positivity (χ2 test, P<.05). T
Of the 31 patients with positive patch tests, there were 20 positive reactions to nickel, 6 to formaldehyde, 4 to phenylenediamine, 3 to cobalt, and 3 to a fragrance mix (Table 2). Some patients showed patch test reactivity to more than 1 allergen concomitantly. Overall, these 31 patients had positive reactions to 16 allergens. None of the patients showed actual signs of contact dermatitis (Table 2).
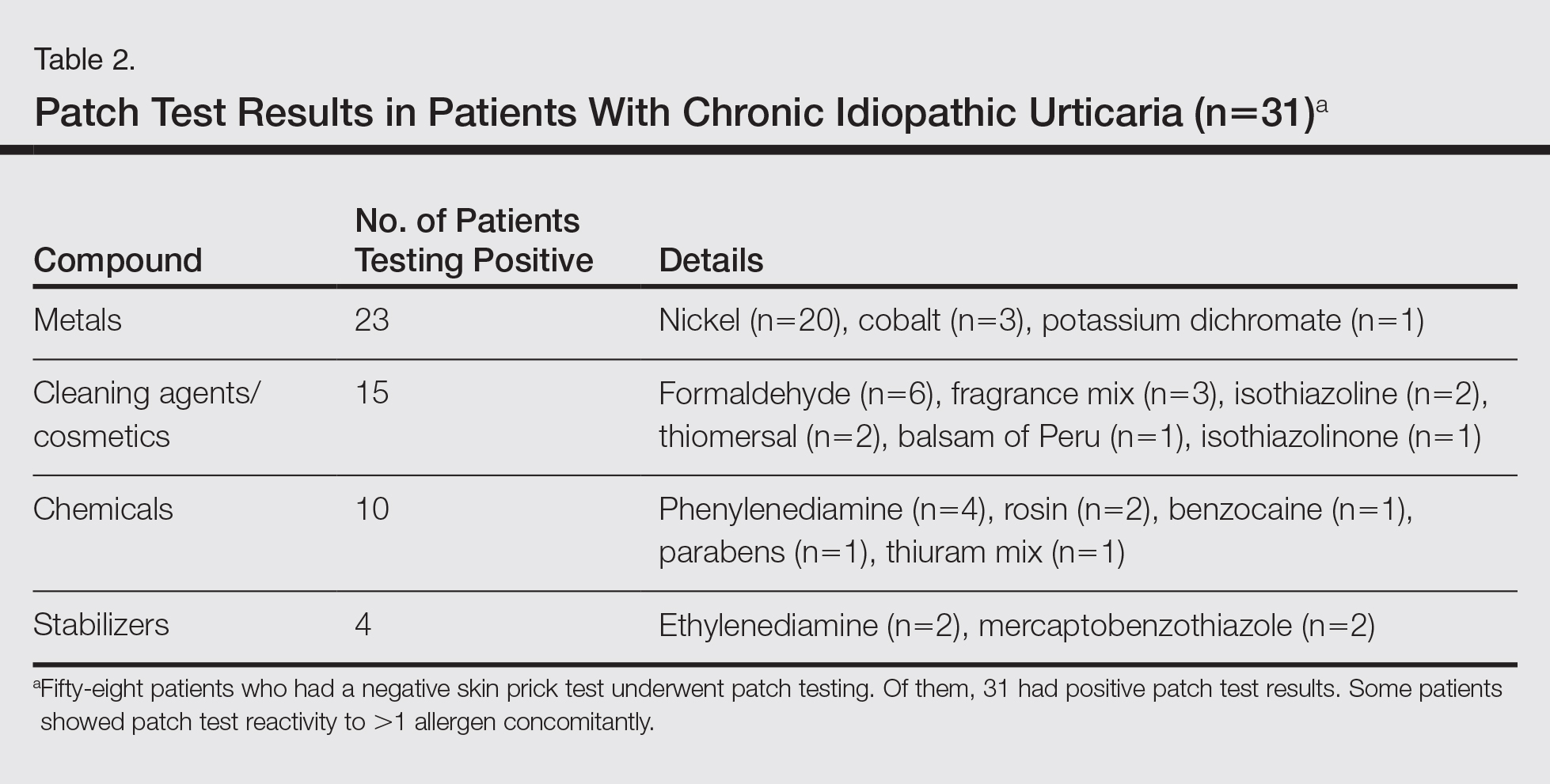
Of
Comment
Chronic idiopathic urticaria is the diagnosis given when urticarial vasculitis, physical urticaria, and all other possible etiologic factors have been excluded in patients with CU. Our study was designed to assess patch test reactivity in patients with CU without any identifiable systemic etiologic factor after detailed laboratory testing and negative skin prick tests.
Chronic idiopathic urticaria can be an extremely disabling and difficult-to-treat condition. Because the cause is unknown, the management of CIU often is frustrating. The
Patch testing is commonly performed to diagnose ACD, and if contact allergens are found via patch testing, patients can often be cured of their dermatitis by avoiding these agents. However, patch testing is not routinely performed in the evaluation of patients with CIU. It is a relatively inexpensive and safe procedure to determine a causal link between sensitization to a specific agent and ACD. In patch test clinics, agents often are tested in standard and screening series. Sensitization that is not suspected from the patient’s history and/or clinical examination can be detected in this manner. Requirements for the inclusion of a chemical in a standard series have been formulated by Bruze et al.13 In addition, ready-to-use materials relevant to the specific leisure activities and working conditions also can be selected for patch testing.
A study conducted in Saudi Arabia showed that the European standard series is suitable for patch testing patients in this community14; however, 3 allergens of local relevance were added in our study: black seed oil, local perfume mix, and henna. Moreover, in our study we added a local allergen known as myrrh, which is a topical herbal medicine used to promote healing that has been reported to cause ACD in some cases.15 We sought to determine if contact allergens can be identified with patch testing in patients with CU and if avoiding these contact allergens would resolve the CU.
Urticaria was once considered an IgE-mediated hypersensitivity reaction, but recent studies have demonstrated the existence of different subgroupsof urticaria, some with an autoimmune mechanism.1-4,11 In CU, skin prick tests are recommended for etiologic workup, while patch testing generally is not recommended.16
It has been observed in clinical practice that a substantial number of patients with CU are positive to patch tests, even without a clear clinical history or signs of contact dermatitis.17 In 2007, Guerra et al17 reported that of 121 patients with CU, 50 (41.3%) tested positive for contact allergens. In all of the patch test–positive patients, avoidance measures led to complete remission within 10 days to 1 month. Therefore, this result suggested that testing for contact sensitization could be helpful in the management of CU. Patients with nickel sensitivity were subsequently allowed to ingest small amounts of nickel-containing foods after 8 weeks of a completely nickel-free diet, and remission persisted.17
Contact dermatitis affects approximately 20% of the general population18; however, there has been little investigation (limited to nickel) into the relationship between contact allergens and CU,19,20 and the underlying mechanisms of the disease are unknown. It has been hypothesized that small amounts of the substances are absorbed through the skin or the digestive tract into the bloodstream over the long-term and are delivered to antigen-presenting cells in the skin, which provide the necessary signals for mast cell activation. Nonetheless, the reasons for a selectively cutaneous localization of the reaction remain largely unclear.
Management of CU is debated among physicians, and several diagnostic flowcharts have been proposed.1,2 In general, patch tests for contact dermatitis are not recommended as a fundamental part of the diagnostic procedure, but Guerra et al17 suggested that contact allergy often plays a role in CU.
There have been inadequate reports of CU found to be caused by common contact sensitizers.21-24 Interestingly, no signs of contact allergy were demonstrated in CU patients before urticarial attack.
Our findings supported our patient selection criteria and also confirmed that contact sensitization may be one of the many possible mechanisms involved in the etiology of CU. Urticaria may have a delayed-type hypersensitivity reaction element, and patients with CU without an obvious causal factor can have positive patch test results.
The
The main findings of our study were that 53.4% of patients with CIU had positive patch test results and that avoidance of the sensitizing substance was effective in 5 of 8 patients who completed an avoidance program. Almost all of the patients showed notable remission of symptoms after limiting their exposure to the offending allergens. This study clearly showed that a cause or pathogenesis for CIU could be identified, thus showing that CIU occurs less frequently than is usually assumed.
Our study had limitations. The first is our lack of a controlled challenge test, which is important to confirm an allergen as a cause of CIU.26 Nonetheless, avoidance of the revealed contact allergen was associated with comparable improvement of CIU severity after 1 month in 5 of 8 patients, though such measures were not tested in all 31 of 58 CIU patients who had positive patch test results.
Conclusion
We propose that patch tests should be performed while investigating CU because they give effective diagnostic and therapeutic results in a substantial number of patients. Urticaria, or at least a subgroup of the disease, may have a delayed-type reaction element, which may explain the disease etiology for many CIU patients. Patients with CU without a detectable underlying etiologic factor can have positive patch test results.
- Zuberbier T, Bindslev-Jensen C, Canonica W, et al. Guidelines, definition, classification and diagnosis of urticaria. Allergy. 2006;61:316-331.
- Kaplan AP. Chronic urticaria: pathogenesis and treatment. J Allergy Clin Immunol. 2004;114:465-474.
- Champion RH. Urticaria: then and now. Br J Dermatol. 1988;119:427-436.
- Green GA, Koelsche GA, Kierland R. Etiology and pathogenesis of chronic urticaria. Ann Allergy. 1965;23:30-36.
- Kaplan AP. Chronic urticaria and angioedema. N Engl J Med. 2002;346:175-179.
- Dreskin SC, Andrews KY. The thyroid and urticaria. Curr Opin Allergy Clin Immunol. 2005;5:408-412.
- Greaves M. Chronic urticaria. J Allergy Clin Immunol. 2000;105:664-672.
- Sharma AD. Use of patch testing for identifying allergen causing chronic urticaria. Indian J Dermatol Venereol Leprol. 2008;74:114-117.
- Li JT, Andrist D, Bamlet WR, et al. Accuracy of patient prediction of allergy skin test results. Ann Allergy Asthma Immunol. 2000;85:382-384.
- Nelson JL, Mowad CM. Allergic contact dermatitis: patch testing beyond the TRUE test. J Clin Aesthet Dermatol. 2010;3:36-41.
- Zuberbier T, Asero R, Bindslev-Jensen C, et al; Dermatology Section of the European Academy of Allergology and Clinical Immunology; Global Allergy and Asthma European Network; European Dermatology Forum; World Allergy Organization. EAACI/GA(2)LEN/EDF/WAO guideline: definition, classification and diagnosis of urticaria. Allergy. 2009;64:1417-1426.
- Bindslev-Jensen C, Finzi A, Greaves M, et al. Chronic urticaria: diagnostic recommendations. Eur Acad Dermatol Venereol. 2000;14:175-180.
- Bruze M, Conde-Slazar L, Goossens A, et al. Thoughts on sensitizers in a standard patch test series. Contact Dermatitis. 1999;41:241-250.
- Al-Sheikh OA, Gad El-Rab MO. Allergic contact dermatitis: clinical features and profile of sensitizing allergens in Riyadh, Saudi Arabia. Int J Dermatol. 1996;35:493-497.
- Al-Suwaidan SN, Gad El Rab MO, Al-Fakhiry S, et al. Allergic contact dermatitis from myrrh, a topical herbal medicine used to promote healing. Contact Dermatitis. 1998;39:137.
- Henz BM, Zuberbier T. Causes of urticaria. In: Henz B, Zuberbier T, Grabbe J, et al, eds. Urticaria: Clinical Diagnostic and Therapeutic Aspects. Berlin, Germany: Springer; 1998:19.
- Guerra L, Rogkakou A, Massacane P, et al. Role of contact sensitization in chronic urticaria. J Am Acad Dermatol. 2007;56:88-90.
- Thyssen JP, Linneberg A, Menné T, et al. The epidemiology of contact allergy in the general population—prevalence and main findings. Contact Dermatitis. 2007;57:287-299.
- Smart GA, Sherlock JC. Nickel in foods and the diet. Food Addit Contam. 1987;4:61-71.
- Abeck D, Traenckner I, Steinkraus V, et al. Chronic urticaria due to nickel intake. Acta Derm Venereol. 1993;73:438-439.
- Moneret-Vautrin DA. Allergic and pseudo-allergic reactions to foods in chronic urticaria [in French]. Ann Dermatol Venereol. 2003;130(Spec No 1):1S35-1S42.
- Wedi B, Raap U, Kapp A. Chronic urticaria and infections. Curr Opin Allergy Clin Immunol. 2004;4:387-396.
- Foti C, Nettis E, Cassano N, et al. Acute allergic reactions to Anisakis simplex after ingestion of anchovies. Acta Derm Venerol. 2002;82:121-123.
- Uter W, Hegewald J, Aberer W, et al. The European standard series in 9 European countries, 2002/2003: first results of the European Surveillance System on Contact Allergies. Contact Dermatitis. 2005;53:136-145.
- Magen E, Mishal J, Menachem S. Impact of contact sensitization in chronic spontaneous urticaria. Am J Med Sci. 2011;341:202-206.
- Antico A, Soana R. Chronic allergic-like dermatopathies in nickel sensitive patients: results of dietary restrictions and challenge with nickel salts. Allergy Asthma Proc. 1999;20:235-242.
Chronic urticaria (CU) is clinically defined as the daily or almost daily presence of wheals on the skin for at least 6 weeks.1 Chronic urticaria severely affects patients’ quality of life and can cause emotional disability and distress.2 In clinical practice, CU is one of the most common and challenging conditions for general practitioners, dermatologists, and allergists. It can be provoked by a wide variety of different causes or may be the clinical presentation of certain systemic diseases3,4; thus, CU often requires a detailed and time-consuming diagnostic procedure that includes screening for allergies, autoimmune diseases, parasites, malignancies, infections, and metabolic disorders.5,6 In many patients (up to 50% in some case series), the cause or pathogenic mechanism cannot be identified, and the disease is then classified as chronic idiopathic urticaria (CIU).7
It has previously been shown that contact sensitization could have some relation with CIU,8 which was further explored in this study. This study sought to evaluate if contact allergy may play a role in disease development in CIU patients in Saudi Arabia and if patch testing should be routinely performed for CIU patients to determine if any allergens can be avoided.
Methods
This prospective study was conducted at the King Khalid University Hospital Allergy Clinic (Riyadh, Saudi Arabia) in patients aged 18 to 60 years who had CU for more than 6 weeks. It was a clinic-based study conducted over a period of 2 years (March 2010 to February 2012). The study protocol was approved by the local ethics committee at King Khalid University Hospital. Valid written consent was obtained from each patient.
Patients were excluded if they had CU caused by physical factors (eg, hot or cold temperature, water, physical contact) or drug reactions that were possible causative factors or if they had taken oral prednisolone or other oral immunosuppressive drugs (eg, azathioprine, cyclosporine) in the last month. However, patients taking antihistamines were not excluded because it was impossible for the patients to discontinue their urticaria treatment. Other exclusion criteria included CU associated with any systemic disease, thyroid disease, diabetes mellitus, autoimmune disorder, or atopic dermatitis. Pregnant and lactating women were not included in this study.
All new adult CU patients (ie, disease duration >6 weeks) were worked up using the routine diagnostic tests that are typically performed for any new CU patient, including complete blood cell count with differential, erythrocyte sedimentation rate, liver function tests, urine analysis, and hepatitis B and C screenings. Further diagnostic tests also were carried out when appropriate according to the patient’s history and physical examination, including levels of urea, electrolytes, thyrotropin, thyroid antibodies (antithyroglobulin and antimicrosomal), and antinuclear antibodies, as well as a Helicobacter pylori test.
All of the patients enrolled in the study were evaluated by skin prick testing to establish the link between CU and its cause. Patch testing was performed in patients who were negative on skin prick testing.
Skin Prick Testing
All patients were advised to temporarily discontinue the use of antihistamines and corticosteroids 5 to 6 days prior to testing.
Patch Testing
Patch tests were carried out using a ready-to-use epicutaneous patch test system for the diagnosis of allergic contact dermatitis (ACD).10 A European standard series was used with the addition of 4 allergens of local relevance: black seed oil, local perfume mix, henna, and myrrh (a topical herbal medicine used to promote healing).
Assessment of Improvement
Assessment of urticaria severity using the Chronic Urticaria Severity Score (CUSS), a simple semiquantitative assessment of disease activity, was calculated as the sum of the number of wheals and the degree of itch severity graded from 0 (none) to 3 (severe), according to the guidelines established by the Dermatology Section of the European Academy of Allergology and Clinical Immunology, the Global Allergy and Asthma European Network, the European Dermatology Forum, and the World Allergy Organization.11 The avoidance group of patients was assessed at baseline and after 1 month to evaluate changes in their CUSS after allergen avoidance for 8 weeks.
Statistical Analysis
All of the statistical analyses were carried out using SPSS software version 16. Results were presented as the median with the range or the mean (SD).
Results
During the study period, a total of 120 CU patients were seen at the clinic. Ninety-three patients with CU met our selection criteria (77.5%) and were enrolled in the study. The mean age (SD) of the patients was 34.7 (12.4) years. Women comprised 68.8% (64/93) of the study population (Table 1).

The duration of urticaria ranged from 0.6 to 20 years, with a median duration of 4 years. Approximately half of the patients (50/93) experienced severe symptoms of urticaria, but only 26.9% (25/93) had graded their urticaria as very severe.
Negative results from the skin prick test were reported in 62.4% (58/93) of patients and were subsequently patch tested. These patients also had no other etiologic factors (eg, infection; thyroid, autoimmune, or metabolic disease). Patients who had positive skin prick test results (35/93 [37.6%]) were not considered to be cases of CIU, according to diagnostic recommendations.12 Of the 58 CIU patients who were patch tested, 31 (53.4%) had positive results and 27 (46.5%) had negative results to both skin prick and patch tests (Figure).

Univariate analysis revealed significant associations between age, gender, and duration of urticaria and patch test positivity (χ2 test, P<.05). T
Of the 31 patients with positive patch tests, there were 20 positive reactions to nickel, 6 to formaldehyde, 4 to phenylenediamine, 3 to cobalt, and 3 to a fragrance mix (Table 2). Some patients showed patch test reactivity to more than 1 allergen concomitantly. Overall, these 31 patients had positive reactions to 16 allergens. None of the patients showed actual signs of contact dermatitis (Table 2).

Of
Comment
Chronic idiopathic urticaria is the diagnosis given when urticarial vasculitis, physical urticaria, and all other possible etiologic factors have been excluded in patients with CU. Our study was designed to assess patch test reactivity in patients with CU without any identifiable systemic etiologic factor after detailed laboratory testing and negative skin prick tests.
Chronic idiopathic urticaria can be an extremely disabling and difficult-to-treat condition. Because the cause is unknown, the management of CIU often is frustrating. The
Patch testing is commonly performed to diagnose ACD, and if contact allergens are found via patch testing, patients can often be cured of their dermatitis by avoiding these agents. However, patch testing is not routinely performed in the evaluation of patients with CIU. It is a relatively inexpensive and safe procedure to determine a causal link between sensitization to a specific agent and ACD. In patch test clinics, agents often are tested in standard and screening series. Sensitization that is not suspected from the patient’s history and/or clinical examination can be detected in this manner. Requirements for the inclusion of a chemical in a standard series have been formulated by Bruze et al.13 In addition, ready-to-use materials relevant to the specific leisure activities and working conditions also can be selected for patch testing.
A study conducted in Saudi Arabia showed that the European standard series is suitable for patch testing patients in this community14; however, 3 allergens of local relevance were added in our study: black seed oil, local perfume mix, and henna. Moreover, in our study we added a local allergen known as myrrh, which is a topical herbal medicine used to promote healing that has been reported to cause ACD in some cases.15 We sought to determine if contact allergens can be identified with patch testing in patients with CU and if avoiding these contact allergens would resolve the CU.
Urticaria was once considered an IgE-mediated hypersensitivity reaction, but recent studies have demonstrated the existence of different subgroupsof urticaria, some with an autoimmune mechanism.1-4,11 In CU, skin prick tests are recommended for etiologic workup, while patch testing generally is not recommended.16
It has been observed in clinical practice that a substantial number of patients with CU are positive to patch tests, even without a clear clinical history or signs of contact dermatitis.17 In 2007, Guerra et al17 reported that of 121 patients with CU, 50 (41.3%) tested positive for contact allergens. In all of the patch test–positive patients, avoidance measures led to complete remission within 10 days to 1 month. Therefore, this result suggested that testing for contact sensitization could be helpful in the management of CU. Patients with nickel sensitivity were subsequently allowed to ingest small amounts of nickel-containing foods after 8 weeks of a completely nickel-free diet, and remission persisted.17
Contact dermatitis affects approximately 20% of the general population18; however, there has been little investigation (limited to nickel) into the relationship between contact allergens and CU,19,20 and the underlying mechanisms of the disease are unknown. It has been hypothesized that small amounts of the substances are absorbed through the skin or the digestive tract into the bloodstream over the long-term and are delivered to antigen-presenting cells in the skin, which provide the necessary signals for mast cell activation. Nonetheless, the reasons for a selectively cutaneous localization of the reaction remain largely unclear.
Management of CU is debated among physicians, and several diagnostic flowcharts have been proposed.1,2 In general, patch tests for contact dermatitis are not recommended as a fundamental part of the diagnostic procedure, but Guerra et al17 suggested that contact allergy often plays a role in CU.
There have been inadequate reports of CU found to be caused by common contact sensitizers.21-24 Interestingly, no signs of contact allergy were demonstrated in CU patients before urticarial attack.
Our findings supported our patient selection criteria and also confirmed that contact sensitization may be one of the many possible mechanisms involved in the etiology of CU. Urticaria may have a delayed-type hypersensitivity reaction element, and patients with CU without an obvious causal factor can have positive patch test results.
The
The main findings of our study were that 53.4% of patients with CIU had positive patch test results and that avoidance of the sensitizing substance was effective in 5 of 8 patients who completed an avoidance program. Almost all of the patients showed notable remission of symptoms after limiting their exposure to the offending allergens. This study clearly showed that a cause or pathogenesis for CIU could be identified, thus showing that CIU occurs less frequently than is usually assumed.
Our study had limitations. The first is our lack of a controlled challenge test, which is important to confirm an allergen as a cause of CIU.26 Nonetheless, avoidance of the revealed contact allergen was associated with comparable improvement of CIU severity after 1 month in 5 of 8 patients, though such measures were not tested in all 31 of 58 CIU patients who had positive patch test results.
Conclusion
We propose that patch tests should be performed while investigating CU because they give effective diagnostic and therapeutic results in a substantial number of patients. Urticaria, or at least a subgroup of the disease, may have a delayed-type reaction element, which may explain the disease etiology for many CIU patients. Patients with CU without a detectable underlying etiologic factor can have positive patch test results.
Chronic urticaria (CU) is clinically defined as the daily or almost daily presence of wheals on the skin for at least 6 weeks.1 Chronic urticaria severely affects patients’ quality of life and can cause emotional disability and distress.2 In clinical practice, CU is one of the most common and challenging conditions for general practitioners, dermatologists, and allergists. It can be provoked by a wide variety of different causes or may be the clinical presentation of certain systemic diseases3,4; thus, CU often requires a detailed and time-consuming diagnostic procedure that includes screening for allergies, autoimmune diseases, parasites, malignancies, infections, and metabolic disorders.5,6 In many patients (up to 50% in some case series), the cause or pathogenic mechanism cannot be identified, and the disease is then classified as chronic idiopathic urticaria (CIU).7
It has previously been shown that contact sensitization could have some relation with CIU,8 which was further explored in this study. This study sought to evaluate if contact allergy may play a role in disease development in CIU patients in Saudi Arabia and if patch testing should be routinely performed for CIU patients to determine if any allergens can be avoided.
Methods
This prospective study was conducted at the King Khalid University Hospital Allergy Clinic (Riyadh, Saudi Arabia) in patients aged 18 to 60 years who had CU for more than 6 weeks. It was a clinic-based study conducted over a period of 2 years (March 2010 to February 2012). The study protocol was approved by the local ethics committee at King Khalid University Hospital. Valid written consent was obtained from each patient.
Patients were excluded if they had CU caused by physical factors (eg, hot or cold temperature, water, physical contact) or drug reactions that were possible causative factors or if they had taken oral prednisolone or other oral immunosuppressive drugs (eg, azathioprine, cyclosporine) in the last month. However, patients taking antihistamines were not excluded because it was impossible for the patients to discontinue their urticaria treatment. Other exclusion criteria included CU associated with any systemic disease, thyroid disease, diabetes mellitus, autoimmune disorder, or atopic dermatitis. Pregnant and lactating women were not included in this study.
All new adult CU patients (ie, disease duration >6 weeks) were worked up using the routine diagnostic tests that are typically performed for any new CU patient, including complete blood cell count with differential, erythrocyte sedimentation rate, liver function tests, urine analysis, and hepatitis B and C screenings. Further diagnostic tests also were carried out when appropriate according to the patient’s history and physical examination, including levels of urea, electrolytes, thyrotropin, thyroid antibodies (antithyroglobulin and antimicrosomal), and antinuclear antibodies, as well as a Helicobacter pylori test.
All of the patients enrolled in the study were evaluated by skin prick testing to establish the link between CU and its cause. Patch testing was performed in patients who were negative on skin prick testing.
Skin Prick Testing
All patients were advised to temporarily discontinue the use of antihistamines and corticosteroids 5 to 6 days prior to testing.
Patch Testing
Patch tests were carried out using a ready-to-use epicutaneous patch test system for the diagnosis of allergic contact dermatitis (ACD).10 A European standard series was used with the addition of 4 allergens of local relevance: black seed oil, local perfume mix, henna, and myrrh (a topical herbal medicine used to promote healing).
Assessment of Improvement
Assessment of urticaria severity using the Chronic Urticaria Severity Score (CUSS), a simple semiquantitative assessment of disease activity, was calculated as the sum of the number of wheals and the degree of itch severity graded from 0 (none) to 3 (severe), according to the guidelines established by the Dermatology Section of the European Academy of Allergology and Clinical Immunology, the Global Allergy and Asthma European Network, the European Dermatology Forum, and the World Allergy Organization.11 The avoidance group of patients was assessed at baseline and after 1 month to evaluate changes in their CUSS after allergen avoidance for 8 weeks.
Statistical Analysis
All of the statistical analyses were carried out using SPSS software version 16. Results were presented as the median with the range or the mean (SD).
Results
During the study period, a total of 120 CU patients were seen at the clinic. Ninety-three patients with CU met our selection criteria (77.5%) and were enrolled in the study. The mean age (SD) of the patients was 34.7 (12.4) years. Women comprised 68.8% (64/93) of the study population (Table 1).

The duration of urticaria ranged from 0.6 to 20 years, with a median duration of 4 years. Approximately half of the patients (50/93) experienced severe symptoms of urticaria, but only 26.9% (25/93) had graded their urticaria as very severe.
Negative results from the skin prick test were reported in 62.4% (58/93) of patients and were subsequently patch tested. These patients also had no other etiologic factors (eg, infection; thyroid, autoimmune, or metabolic disease). Patients who had positive skin prick test results (35/93 [37.6%]) were not considered to be cases of CIU, according to diagnostic recommendations.12 Of the 58 CIU patients who were patch tested, 31 (53.4%) had positive results and 27 (46.5%) had negative results to both skin prick and patch tests (Figure).

Univariate analysis revealed significant associations between age, gender, and duration of urticaria and patch test positivity (χ2 test, P<.05). T
Of the 31 patients with positive patch tests, there were 20 positive reactions to nickel, 6 to formaldehyde, 4 to phenylenediamine, 3 to cobalt, and 3 to a fragrance mix (Table 2). Some patients showed patch test reactivity to more than 1 allergen concomitantly. Overall, these 31 patients had positive reactions to 16 allergens. None of the patients showed actual signs of contact dermatitis (Table 2).

Of
Comment
Chronic idiopathic urticaria is the diagnosis given when urticarial vasculitis, physical urticaria, and all other possible etiologic factors have been excluded in patients with CU. Our study was designed to assess patch test reactivity in patients with CU without any identifiable systemic etiologic factor after detailed laboratory testing and negative skin prick tests.
Chronic idiopathic urticaria can be an extremely disabling and difficult-to-treat condition. Because the cause is unknown, the management of CIU often is frustrating. The
Patch testing is commonly performed to diagnose ACD, and if contact allergens are found via patch testing, patients can often be cured of their dermatitis by avoiding these agents. However, patch testing is not routinely performed in the evaluation of patients with CIU. It is a relatively inexpensive and safe procedure to determine a causal link between sensitization to a specific agent and ACD. In patch test clinics, agents often are tested in standard and screening series. Sensitization that is not suspected from the patient’s history and/or clinical examination can be detected in this manner. Requirements for the inclusion of a chemical in a standard series have been formulated by Bruze et al.13 In addition, ready-to-use materials relevant to the specific leisure activities and working conditions also can be selected for patch testing.
A study conducted in Saudi Arabia showed that the European standard series is suitable for patch testing patients in this community14; however, 3 allergens of local relevance were added in our study: black seed oil, local perfume mix, and henna. Moreover, in our study we added a local allergen known as myrrh, which is a topical herbal medicine used to promote healing that has been reported to cause ACD in some cases.15 We sought to determine if contact allergens can be identified with patch testing in patients with CU and if avoiding these contact allergens would resolve the CU.
Urticaria was once considered an IgE-mediated hypersensitivity reaction, but recent studies have demonstrated the existence of different subgroupsof urticaria, some with an autoimmune mechanism.1-4,11 In CU, skin prick tests are recommended for etiologic workup, while patch testing generally is not recommended.16
It has been observed in clinical practice that a substantial number of patients with CU are positive to patch tests, even without a clear clinical history or signs of contact dermatitis.17 In 2007, Guerra et al17 reported that of 121 patients with CU, 50 (41.3%) tested positive for contact allergens. In all of the patch test–positive patients, avoidance measures led to complete remission within 10 days to 1 month. Therefore, this result suggested that testing for contact sensitization could be helpful in the management of CU. Patients with nickel sensitivity were subsequently allowed to ingest small amounts of nickel-containing foods after 8 weeks of a completely nickel-free diet, and remission persisted.17
Contact dermatitis affects approximately 20% of the general population18; however, there has been little investigation (limited to nickel) into the relationship between contact allergens and CU,19,20 and the underlying mechanisms of the disease are unknown. It has been hypothesized that small amounts of the substances are absorbed through the skin or the digestive tract into the bloodstream over the long-term and are delivered to antigen-presenting cells in the skin, which provide the necessary signals for mast cell activation. Nonetheless, the reasons for a selectively cutaneous localization of the reaction remain largely unclear.
Management of CU is debated among physicians, and several diagnostic flowcharts have been proposed.1,2 In general, patch tests for contact dermatitis are not recommended as a fundamental part of the diagnostic procedure, but Guerra et al17 suggested that contact allergy often plays a role in CU.
There have been inadequate reports of CU found to be caused by common contact sensitizers.21-24 Interestingly, no signs of contact allergy were demonstrated in CU patients before urticarial attack.
Our findings supported our patient selection criteria and also confirmed that contact sensitization may be one of the many possible mechanisms involved in the etiology of CU. Urticaria may have a delayed-type hypersensitivity reaction element, and patients with CU without an obvious causal factor can have positive patch test results.
The
The main findings of our study were that 53.4% of patients with CIU had positive patch test results and that avoidance of the sensitizing substance was effective in 5 of 8 patients who completed an avoidance program. Almost all of the patients showed notable remission of symptoms after limiting their exposure to the offending allergens. This study clearly showed that a cause or pathogenesis for CIU could be identified, thus showing that CIU occurs less frequently than is usually assumed.
Our study had limitations. The first is our lack of a controlled challenge test, which is important to confirm an allergen as a cause of CIU.26 Nonetheless, avoidance of the revealed contact allergen was associated with comparable improvement of CIU severity after 1 month in 5 of 8 patients, though such measures were not tested in all 31 of 58 CIU patients who had positive patch test results.
Conclusion
We propose that patch tests should be performed while investigating CU because they give effective diagnostic and therapeutic results in a substantial number of patients. Urticaria, or at least a subgroup of the disease, may have a delayed-type reaction element, which may explain the disease etiology for many CIU patients. Patients with CU without a detectable underlying etiologic factor can have positive patch test results.
- Zuberbier T, Bindslev-Jensen C, Canonica W, et al. Guidelines, definition, classification and diagnosis of urticaria. Allergy. 2006;61:316-331.
- Kaplan AP. Chronic urticaria: pathogenesis and treatment. J Allergy Clin Immunol. 2004;114:465-474.
- Champion RH. Urticaria: then and now. Br J Dermatol. 1988;119:427-436.
- Green GA, Koelsche GA, Kierland R. Etiology and pathogenesis of chronic urticaria. Ann Allergy. 1965;23:30-36.
- Kaplan AP. Chronic urticaria and angioedema. N Engl J Med. 2002;346:175-179.
- Dreskin SC, Andrews KY. The thyroid and urticaria. Curr Opin Allergy Clin Immunol. 2005;5:408-412.
- Greaves M. Chronic urticaria. J Allergy Clin Immunol. 2000;105:664-672.
- Sharma AD. Use of patch testing for identifying allergen causing chronic urticaria. Indian J Dermatol Venereol Leprol. 2008;74:114-117.
- Li JT, Andrist D, Bamlet WR, et al. Accuracy of patient prediction of allergy skin test results. Ann Allergy Asthma Immunol. 2000;85:382-384.
- Nelson JL, Mowad CM. Allergic contact dermatitis: patch testing beyond the TRUE test. J Clin Aesthet Dermatol. 2010;3:36-41.
- Zuberbier T, Asero R, Bindslev-Jensen C, et al; Dermatology Section of the European Academy of Allergology and Clinical Immunology; Global Allergy and Asthma European Network; European Dermatology Forum; World Allergy Organization. EAACI/GA(2)LEN/EDF/WAO guideline: definition, classification and diagnosis of urticaria. Allergy. 2009;64:1417-1426.
- Bindslev-Jensen C, Finzi A, Greaves M, et al. Chronic urticaria: diagnostic recommendations. Eur Acad Dermatol Venereol. 2000;14:175-180.
- Bruze M, Conde-Slazar L, Goossens A, et al. Thoughts on sensitizers in a standard patch test series. Contact Dermatitis. 1999;41:241-250.
- Al-Sheikh OA, Gad El-Rab MO. Allergic contact dermatitis: clinical features and profile of sensitizing allergens in Riyadh, Saudi Arabia. Int J Dermatol. 1996;35:493-497.
- Al-Suwaidan SN, Gad El Rab MO, Al-Fakhiry S, et al. Allergic contact dermatitis from myrrh, a topical herbal medicine used to promote healing. Contact Dermatitis. 1998;39:137.
- Henz BM, Zuberbier T. Causes of urticaria. In: Henz B, Zuberbier T, Grabbe J, et al, eds. Urticaria: Clinical Diagnostic and Therapeutic Aspects. Berlin, Germany: Springer; 1998:19.
- Guerra L, Rogkakou A, Massacane P, et al. Role of contact sensitization in chronic urticaria. J Am Acad Dermatol. 2007;56:88-90.
- Thyssen JP, Linneberg A, Menné T, et al. The epidemiology of contact allergy in the general population—prevalence and main findings. Contact Dermatitis. 2007;57:287-299.
- Smart GA, Sherlock JC. Nickel in foods and the diet. Food Addit Contam. 1987;4:61-71.
- Abeck D, Traenckner I, Steinkraus V, et al. Chronic urticaria due to nickel intake. Acta Derm Venereol. 1993;73:438-439.
- Moneret-Vautrin DA. Allergic and pseudo-allergic reactions to foods in chronic urticaria [in French]. Ann Dermatol Venereol. 2003;130(Spec No 1):1S35-1S42.
- Wedi B, Raap U, Kapp A. Chronic urticaria and infections. Curr Opin Allergy Clin Immunol. 2004;4:387-396.
- Foti C, Nettis E, Cassano N, et al. Acute allergic reactions to Anisakis simplex after ingestion of anchovies. Acta Derm Venerol. 2002;82:121-123.
- Uter W, Hegewald J, Aberer W, et al. The European standard series in 9 European countries, 2002/2003: first results of the European Surveillance System on Contact Allergies. Contact Dermatitis. 2005;53:136-145.
- Magen E, Mishal J, Menachem S. Impact of contact sensitization in chronic spontaneous urticaria. Am J Med Sci. 2011;341:202-206.
- Antico A, Soana R. Chronic allergic-like dermatopathies in nickel sensitive patients: results of dietary restrictions and challenge with nickel salts. Allergy Asthma Proc. 1999;20:235-242.
- Zuberbier T, Bindslev-Jensen C, Canonica W, et al. Guidelines, definition, classification and diagnosis of urticaria. Allergy. 2006;61:316-331.
- Kaplan AP. Chronic urticaria: pathogenesis and treatment. J Allergy Clin Immunol. 2004;114:465-474.
- Champion RH. Urticaria: then and now. Br J Dermatol. 1988;119:427-436.
- Green GA, Koelsche GA, Kierland R. Etiology and pathogenesis of chronic urticaria. Ann Allergy. 1965;23:30-36.
- Kaplan AP. Chronic urticaria and angioedema. N Engl J Med. 2002;346:175-179.
- Dreskin SC, Andrews KY. The thyroid and urticaria. Curr Opin Allergy Clin Immunol. 2005;5:408-412.
- Greaves M. Chronic urticaria. J Allergy Clin Immunol. 2000;105:664-672.
- Sharma AD. Use of patch testing for identifying allergen causing chronic urticaria. Indian J Dermatol Venereol Leprol. 2008;74:114-117.
- Li JT, Andrist D, Bamlet WR, et al. Accuracy of patient prediction of allergy skin test results. Ann Allergy Asthma Immunol. 2000;85:382-384.
- Nelson JL, Mowad CM. Allergic contact dermatitis: patch testing beyond the TRUE test. J Clin Aesthet Dermatol. 2010;3:36-41.
- Zuberbier T, Asero R, Bindslev-Jensen C, et al; Dermatology Section of the European Academy of Allergology and Clinical Immunology; Global Allergy and Asthma European Network; European Dermatology Forum; World Allergy Organization. EAACI/GA(2)LEN/EDF/WAO guideline: definition, classification and diagnosis of urticaria. Allergy. 2009;64:1417-1426.
- Bindslev-Jensen C, Finzi A, Greaves M, et al. Chronic urticaria: diagnostic recommendations. Eur Acad Dermatol Venereol. 2000;14:175-180.
- Bruze M, Conde-Slazar L, Goossens A, et al. Thoughts on sensitizers in a standard patch test series. Contact Dermatitis. 1999;41:241-250.
- Al-Sheikh OA, Gad El-Rab MO. Allergic contact dermatitis: clinical features and profile of sensitizing allergens in Riyadh, Saudi Arabia. Int J Dermatol. 1996;35:493-497.
- Al-Suwaidan SN, Gad El Rab MO, Al-Fakhiry S, et al. Allergic contact dermatitis from myrrh, a topical herbal medicine used to promote healing. Contact Dermatitis. 1998;39:137.
- Henz BM, Zuberbier T. Causes of urticaria. In: Henz B, Zuberbier T, Grabbe J, et al, eds. Urticaria: Clinical Diagnostic and Therapeutic Aspects. Berlin, Germany: Springer; 1998:19.
- Guerra L, Rogkakou A, Massacane P, et al. Role of contact sensitization in chronic urticaria. J Am Acad Dermatol. 2007;56:88-90.
- Thyssen JP, Linneberg A, Menné T, et al. The epidemiology of contact allergy in the general population—prevalence and main findings. Contact Dermatitis. 2007;57:287-299.
- Smart GA, Sherlock JC. Nickel in foods and the diet. Food Addit Contam. 1987;4:61-71.
- Abeck D, Traenckner I, Steinkraus V, et al. Chronic urticaria due to nickel intake. Acta Derm Venereol. 1993;73:438-439.
- Moneret-Vautrin DA. Allergic and pseudo-allergic reactions to foods in chronic urticaria [in French]. Ann Dermatol Venereol. 2003;130(Spec No 1):1S35-1S42.
- Wedi B, Raap U, Kapp A. Chronic urticaria and infections. Curr Opin Allergy Clin Immunol. 2004;4:387-396.
- Foti C, Nettis E, Cassano N, et al. Acute allergic reactions to Anisakis simplex after ingestion of anchovies. Acta Derm Venerol. 2002;82:121-123.
- Uter W, Hegewald J, Aberer W, et al. The European standard series in 9 European countries, 2002/2003: first results of the European Surveillance System on Contact Allergies. Contact Dermatitis. 2005;53:136-145.
- Magen E, Mishal J, Menachem S. Impact of contact sensitization in chronic spontaneous urticaria. Am J Med Sci. 2011;341:202-206.
- Antico A, Soana R. Chronic allergic-like dermatopathies in nickel sensitive patients: results of dietary restrictions and challenge with nickel salts. Allergy Asthma Proc. 1999;20:235-242.
Practice Points
- Patients with chronic urticaria (CU) without a detectable underlying etiologic factor can have positive patch test results.
- Avoidance of the sensitizing substance can be effective in CU patients and remission of symptoms can be possible after limiting their exposure to the offending allergens.
Leukocytoclastic Vasculitis Resolution With Topical Dapsone
Leukocytoclastic vasculitis (LCV) is a disease characterized by inflammation of small vessels with characteristic clinical findings of petechiae and palpable purpura.1 Numerous etiologies have been described, but the disease commonly remains idiopathic.2,3 Leukocytoclastic vasculitis often spontaneously resolves within weeks and requires only symptomatic treatment. Chronic or severe disease can require systemic medical treatment with agents such as colchicine, dapsone, and corticosteroids. These agents are effective but carry risks of serious side effects.4,5 These side effects and/or medical contraindications prevent some patients from taking systemic medications for LCV. We present a case of LCV that resolved after treatment with topical dapsone, highlighting a potential new treatment ofLCV with a markedly better side-effect profile.
Case Report
A 60-year-old woman with recent upper respiratory tract and sinus infections presented to our dermatology clinic with painful palpable purpura on the bilateral shins, thighs, and dorsal aspects of the feet of several months’ duration (Figure, A). Her primary care provider initiated treatment with amoxicillin and doxycycline for the infections. When the rash developed approximately 1.5 weeks following initiation of her symptoms, the patient was referred to the dermatology and rheumatology departments at our institution. The treating dermatologist (M.B.T.) obtained a 4-mm punch biopsy from the right lower leg and LCV was shown on histology. The patient completed a 14-day course of doxycycline and amoxicillin without resolution of the eruption. After an extensive investigation, the treating rheumatologist concluded that the LCV was idiopathic or secondary to an infection or drug exposure. The rheumatologist started the patient on oral prednisone for the chronic symptomatic LCV, but she was intolerant of this medication and discontinued it after 1 week. Our dermatology clinic started her on triamcinolone cream 0.1% twice daily, but she continued to experience new and worsening lesions. At her follow-up appointment 1 month later, triamcinolone cream was discontinued and dapsone gel 5% twice daily was started. She experienced resolution of her previously recalcitrant LCV within 3 weeks (Figure, B).
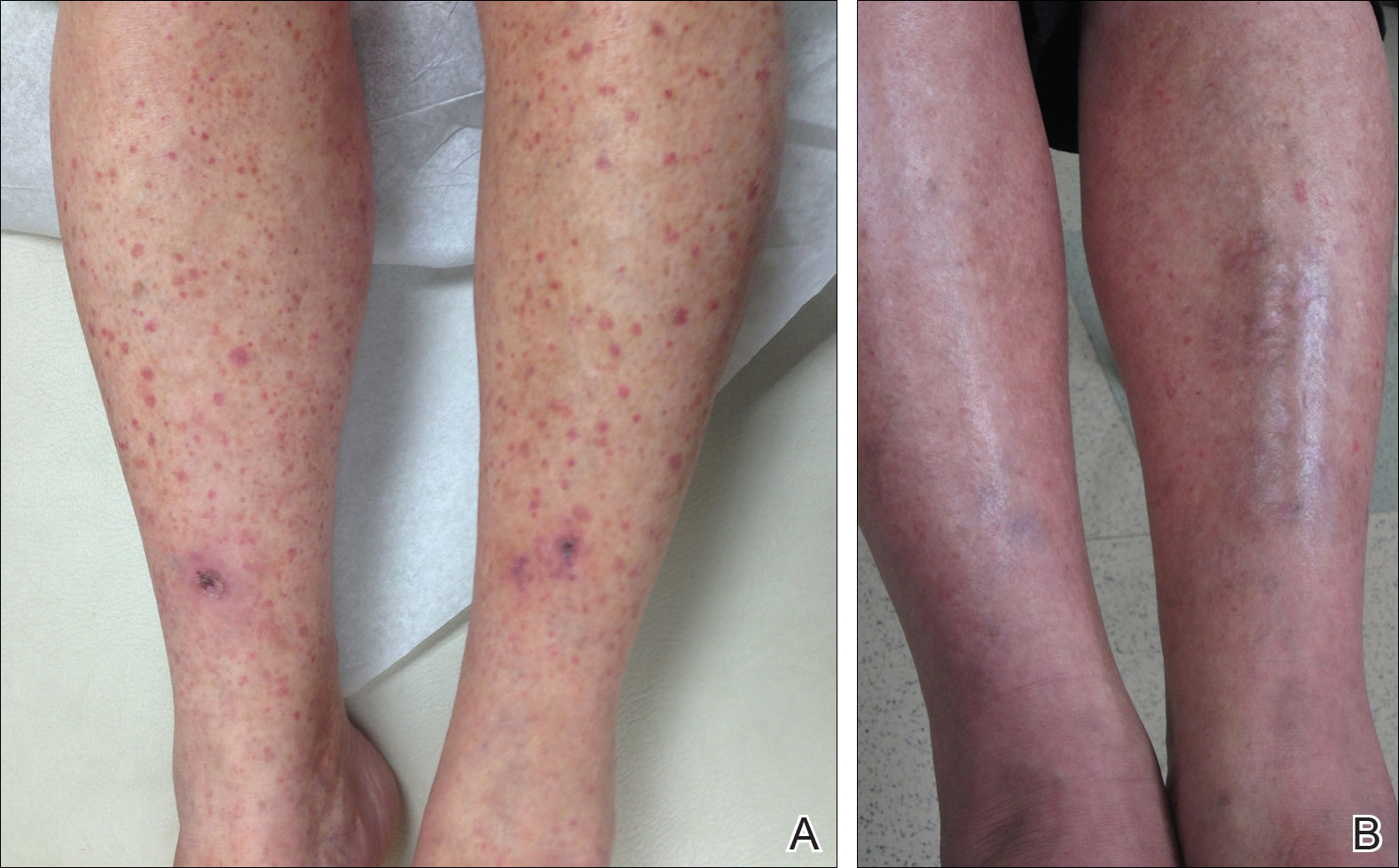
Comment
Established therapies for LCV carry serious side-effect profiles, which can preclude their use.5 Therefore, a topical therapeutic alternative for LCV would be ideal. Systemic prednisone is the first-line therapy for chronic and/or symptomatic LCV, but its side effects include suppression of the hypothalamic-pituitary-adrenal axis, immunosuppression, osteonecrosis, and glucose intolerance.5 Colchicine therapy carries risks for blood dyscrasia, immunosuppression, and gastrointestinal tract upset. Systemic dapsone also is an effective therapy for chronic and/or symptomatic LCV.5,6 However, systemic dapsone requires glucose-6-phosphate dehydrogenase deficiency screening and routine monitoring of blood counts, and it also carries the risk for serious adverse effects including neuropathy, blood dyscrasia, and hypersensitivity syndrome.5,6 Topical dapsone may provide similar efficacy with far fewer adverse effects and has proven to be a safe treatment of acne, even when used in patients with glucose-6-phosphate dehydrogenase deficiency. It displays low systemic absorption and does not accumulate over time once a steady state is reached.7 It also has been shown to be beneficial in other vasculopathies such as erythema elevatum diutinum and in other neutrophilic inflammatory disorders such as pyoderma gangrenosum.8,9 A case of methemoglobinemia due to topical dapsone has been reported.10 Although this effect is rare, clinicians should be aware of such adverse effects when using medications for off-label purposes.
Leukocytoclastic vasculitis can spontaneously resolve; however, our patient’s disease was chronic for several months, and she continued to develop new lesions without signs of resolution. After initiating topical dapsone, she experienced resolution within 3 weeks.
Conclusion
Topical dapsone is a novel approach for treating LCV. Given this drug’s favorable side-effect profile compared to the currently available therapeutic alternatives, we believe it is a reasonable option in select patients. Further investigation is needed to prove its efficacy, but it could be an ideal alternative for patients with contraindications to traditional therapies and/or for those unable to tolerate systemic therapy.
- Koutkia P, Mylonakis E, Rounds S, et al. Leucocytoclastic vasculitis: an update for the clinician. Scand J Rheumatol. 2001;30:315-322.
- Af Ekenstam E, Callen JP. Cutaneous leukocytoclastic vasculitis. clinical and laboratory features of 82 patients seen in private practice. Arch Dermatol. 1984;120:484-489.
- Gyselbrecht L, de Keyser F, Ongenae K, et al. Etiological factors and underlying conditions in patientswith leucocytoclastic vasculitis. Clin Exp Rheumatol. 1996;14:665-668.
- Sais G, Vidaller A, Jucglà A, et al. Colchicine in the treatment of cutaneous leukocytoclastic vasculitis. results of a prospective, randomized controlled trial. Arch Dermatol. 1995;131:1399-1402.
- Sunderkotter C, Bonsmann G, Sindrilaru A, et al. Management of leukocytoclastic vasculitis: clinical review. J Dermatol Treat. 2005;16:193-206.
- Zhu YI, Stiller MJ. Dapsone and sulfones in dermatology: overview and update. J Am Acad Dermatol. 2001;45:420-434.
- Stotland M, Shalita AR, Kissling RF. Dapsone 5% gel: a review of its efficacy and safety in the treatment of acne vulgaris. Am J Clin Dermatol. 2009;10:221-227.
- Frieling GW, Williams NL, Lim SJ, et al. Novel use of topical dapsone 5% gel for erythema elevatum diutinum: safer and effective. J Drugs Dermatol. 2013;12:481-484.
- Handler MZ, Hamilton H, Aires D. Treatment of peristomal pyoderma gangrenosum with topical crushed dapsone. J Drugs Dermatol. 2011;10:1059-1061.
- Swartzentruber GS, Yanta JH, Pizon AF. Methemoglobi-nemia as a complication of topical dapsone. N Engl J Med. 2015;372:491-492.
Leukocytoclastic vasculitis (LCV) is a disease characterized by inflammation of small vessels with characteristic clinical findings of petechiae and palpable purpura.1 Numerous etiologies have been described, but the disease commonly remains idiopathic.2,3 Leukocytoclastic vasculitis often spontaneously resolves within weeks and requires only symptomatic treatment. Chronic or severe disease can require systemic medical treatment with agents such as colchicine, dapsone, and corticosteroids. These agents are effective but carry risks of serious side effects.4,5 These side effects and/or medical contraindications prevent some patients from taking systemic medications for LCV. We present a case of LCV that resolved after treatment with topical dapsone, highlighting a potential new treatment ofLCV with a markedly better side-effect profile.
Case Report
A 60-year-old woman with recent upper respiratory tract and sinus infections presented to our dermatology clinic with painful palpable purpura on the bilateral shins, thighs, and dorsal aspects of the feet of several months’ duration (Figure, A). Her primary care provider initiated treatment with amoxicillin and doxycycline for the infections. When the rash developed approximately 1.5 weeks following initiation of her symptoms, the patient was referred to the dermatology and rheumatology departments at our institution. The treating dermatologist (M.B.T.) obtained a 4-mm punch biopsy from the right lower leg and LCV was shown on histology. The patient completed a 14-day course of doxycycline and amoxicillin without resolution of the eruption. After an extensive investigation, the treating rheumatologist concluded that the LCV was idiopathic or secondary to an infection or drug exposure. The rheumatologist started the patient on oral prednisone for the chronic symptomatic LCV, but she was intolerant of this medication and discontinued it after 1 week. Our dermatology clinic started her on triamcinolone cream 0.1% twice daily, but she continued to experience new and worsening lesions. At her follow-up appointment 1 month later, triamcinolone cream was discontinued and dapsone gel 5% twice daily was started. She experienced resolution of her previously recalcitrant LCV within 3 weeks (Figure, B).

Comment
Established therapies for LCV carry serious side-effect profiles, which can preclude their use.5 Therefore, a topical therapeutic alternative for LCV would be ideal. Systemic prednisone is the first-line therapy for chronic and/or symptomatic LCV, but its side effects include suppression of the hypothalamic-pituitary-adrenal axis, immunosuppression, osteonecrosis, and glucose intolerance.5 Colchicine therapy carries risks for blood dyscrasia, immunosuppression, and gastrointestinal tract upset. Systemic dapsone also is an effective therapy for chronic and/or symptomatic LCV.5,6 However, systemic dapsone requires glucose-6-phosphate dehydrogenase deficiency screening and routine monitoring of blood counts, and it also carries the risk for serious adverse effects including neuropathy, blood dyscrasia, and hypersensitivity syndrome.5,6 Topical dapsone may provide similar efficacy with far fewer adverse effects and has proven to be a safe treatment of acne, even when used in patients with glucose-6-phosphate dehydrogenase deficiency. It displays low systemic absorption and does not accumulate over time once a steady state is reached.7 It also has been shown to be beneficial in other vasculopathies such as erythema elevatum diutinum and in other neutrophilic inflammatory disorders such as pyoderma gangrenosum.8,9 A case of methemoglobinemia due to topical dapsone has been reported.10 Although this effect is rare, clinicians should be aware of such adverse effects when using medications for off-label purposes.
Leukocytoclastic vasculitis can spontaneously resolve; however, our patient’s disease was chronic for several months, and she continued to develop new lesions without signs of resolution. After initiating topical dapsone, she experienced resolution within 3 weeks.
Conclusion
Topical dapsone is a novel approach for treating LCV. Given this drug’s favorable side-effect profile compared to the currently available therapeutic alternatives, we believe it is a reasonable option in select patients. Further investigation is needed to prove its efficacy, but it could be an ideal alternative for patients with contraindications to traditional therapies and/or for those unable to tolerate systemic therapy.
Leukocytoclastic vasculitis (LCV) is a disease characterized by inflammation of small vessels with characteristic clinical findings of petechiae and palpable purpura.1 Numerous etiologies have been described, but the disease commonly remains idiopathic.2,3 Leukocytoclastic vasculitis often spontaneously resolves within weeks and requires only symptomatic treatment. Chronic or severe disease can require systemic medical treatment with agents such as colchicine, dapsone, and corticosteroids. These agents are effective but carry risks of serious side effects.4,5 These side effects and/or medical contraindications prevent some patients from taking systemic medications for LCV. We present a case of LCV that resolved after treatment with topical dapsone, highlighting a potential new treatment ofLCV with a markedly better side-effect profile.
Case Report
A 60-year-old woman with recent upper respiratory tract and sinus infections presented to our dermatology clinic with painful palpable purpura on the bilateral shins, thighs, and dorsal aspects of the feet of several months’ duration (Figure, A). Her primary care provider initiated treatment with amoxicillin and doxycycline for the infections. When the rash developed approximately 1.5 weeks following initiation of her symptoms, the patient was referred to the dermatology and rheumatology departments at our institution. The treating dermatologist (M.B.T.) obtained a 4-mm punch biopsy from the right lower leg and LCV was shown on histology. The patient completed a 14-day course of doxycycline and amoxicillin without resolution of the eruption. After an extensive investigation, the treating rheumatologist concluded that the LCV was idiopathic or secondary to an infection or drug exposure. The rheumatologist started the patient on oral prednisone for the chronic symptomatic LCV, but she was intolerant of this medication and discontinued it after 1 week. Our dermatology clinic started her on triamcinolone cream 0.1% twice daily, but she continued to experience new and worsening lesions. At her follow-up appointment 1 month later, triamcinolone cream was discontinued and dapsone gel 5% twice daily was started. She experienced resolution of her previously recalcitrant LCV within 3 weeks (Figure, B).

Comment
Established therapies for LCV carry serious side-effect profiles, which can preclude their use.5 Therefore, a topical therapeutic alternative for LCV would be ideal. Systemic prednisone is the first-line therapy for chronic and/or symptomatic LCV, but its side effects include suppression of the hypothalamic-pituitary-adrenal axis, immunosuppression, osteonecrosis, and glucose intolerance.5 Colchicine therapy carries risks for blood dyscrasia, immunosuppression, and gastrointestinal tract upset. Systemic dapsone also is an effective therapy for chronic and/or symptomatic LCV.5,6 However, systemic dapsone requires glucose-6-phosphate dehydrogenase deficiency screening and routine monitoring of blood counts, and it also carries the risk for serious adverse effects including neuropathy, blood dyscrasia, and hypersensitivity syndrome.5,6 Topical dapsone may provide similar efficacy with far fewer adverse effects and has proven to be a safe treatment of acne, even when used in patients with glucose-6-phosphate dehydrogenase deficiency. It displays low systemic absorption and does not accumulate over time once a steady state is reached.7 It also has been shown to be beneficial in other vasculopathies such as erythema elevatum diutinum and in other neutrophilic inflammatory disorders such as pyoderma gangrenosum.8,9 A case of methemoglobinemia due to topical dapsone has been reported.10 Although this effect is rare, clinicians should be aware of such adverse effects when using medications for off-label purposes.
Leukocytoclastic vasculitis can spontaneously resolve; however, our patient’s disease was chronic for several months, and she continued to develop new lesions without signs of resolution. After initiating topical dapsone, she experienced resolution within 3 weeks.
Conclusion
Topical dapsone is a novel approach for treating LCV. Given this drug’s favorable side-effect profile compared to the currently available therapeutic alternatives, we believe it is a reasonable option in select patients. Further investigation is needed to prove its efficacy, but it could be an ideal alternative for patients with contraindications to traditional therapies and/or for those unable to tolerate systemic therapy.
- Koutkia P, Mylonakis E, Rounds S, et al. Leucocytoclastic vasculitis: an update for the clinician. Scand J Rheumatol. 2001;30:315-322.
- Af Ekenstam E, Callen JP. Cutaneous leukocytoclastic vasculitis. clinical and laboratory features of 82 patients seen in private practice. Arch Dermatol. 1984;120:484-489.
- Gyselbrecht L, de Keyser F, Ongenae K, et al. Etiological factors and underlying conditions in patientswith leucocytoclastic vasculitis. Clin Exp Rheumatol. 1996;14:665-668.
- Sais G, Vidaller A, Jucglà A, et al. Colchicine in the treatment of cutaneous leukocytoclastic vasculitis. results of a prospective, randomized controlled trial. Arch Dermatol. 1995;131:1399-1402.
- Sunderkotter C, Bonsmann G, Sindrilaru A, et al. Management of leukocytoclastic vasculitis: clinical review. J Dermatol Treat. 2005;16:193-206.
- Zhu YI, Stiller MJ. Dapsone and sulfones in dermatology: overview and update. J Am Acad Dermatol. 2001;45:420-434.
- Stotland M, Shalita AR, Kissling RF. Dapsone 5% gel: a review of its efficacy and safety in the treatment of acne vulgaris. Am J Clin Dermatol. 2009;10:221-227.
- Frieling GW, Williams NL, Lim SJ, et al. Novel use of topical dapsone 5% gel for erythema elevatum diutinum: safer and effective. J Drugs Dermatol. 2013;12:481-484.
- Handler MZ, Hamilton H, Aires D. Treatment of peristomal pyoderma gangrenosum with topical crushed dapsone. J Drugs Dermatol. 2011;10:1059-1061.
- Swartzentruber GS, Yanta JH, Pizon AF. Methemoglobi-nemia as a complication of topical dapsone. N Engl J Med. 2015;372:491-492.
- Koutkia P, Mylonakis E, Rounds S, et al. Leucocytoclastic vasculitis: an update for the clinician. Scand J Rheumatol. 2001;30:315-322.
- Af Ekenstam E, Callen JP. Cutaneous leukocytoclastic vasculitis. clinical and laboratory features of 82 patients seen in private practice. Arch Dermatol. 1984;120:484-489.
- Gyselbrecht L, de Keyser F, Ongenae K, et al. Etiological factors and underlying conditions in patientswith leucocytoclastic vasculitis. Clin Exp Rheumatol. 1996;14:665-668.
- Sais G, Vidaller A, Jucglà A, et al. Colchicine in the treatment of cutaneous leukocytoclastic vasculitis. results of a prospective, randomized controlled trial. Arch Dermatol. 1995;131:1399-1402.
- Sunderkotter C, Bonsmann G, Sindrilaru A, et al. Management of leukocytoclastic vasculitis: clinical review. J Dermatol Treat. 2005;16:193-206.
- Zhu YI, Stiller MJ. Dapsone and sulfones in dermatology: overview and update. J Am Acad Dermatol. 2001;45:420-434.
- Stotland M, Shalita AR, Kissling RF. Dapsone 5% gel: a review of its efficacy and safety in the treatment of acne vulgaris. Am J Clin Dermatol. 2009;10:221-227.
- Frieling GW, Williams NL, Lim SJ, et al. Novel use of topical dapsone 5% gel for erythema elevatum diutinum: safer and effective. J Drugs Dermatol. 2013;12:481-484.
- Handler MZ, Hamilton H, Aires D. Treatment of peristomal pyoderma gangrenosum with topical crushed dapsone. J Drugs Dermatol. 2011;10:1059-1061.
- Swartzentruber GS, Yanta JH, Pizon AF. Methemoglobi-nemia as a complication of topical dapsone. N Engl J Med. 2015;372:491-492.
Practice Points
- Leukocytoclastic vasculitis is characterized by inflammation of small vessels with characteristic clinical findings of petechiae and palpable purpura.
- Leukocytoclastic vasculitis often spontaneously resolves within weeks and requires only symptomatic treatment, but chronic or severe disease can require systemic medical treatment with agents such as colchicine, dapsone, and corticosteroids.
Low-histamine diet reduces disease activity in chronic urticaria
A low-histamine diet could decrease symptoms and improve the quality of life for people with chronic spontaneous urticaria (CsU), according to Nicola Wagner, MD, of the department of dermatology at the Clinical Center Darmstadt (Germany) GmbH, Darmstadt, and her coauthors.
In their prospective study of 56 patients with a 3-month history of CsU (average 25 months) who followed a low-histamine diet for 3 weeks, 42 (75%) showed improvements in the urticaria activity score (UAS), compared to baseline. In nine patients (16%), disease activity remained the same, and five patients (9%) experienced worsening symptoms.
The primary endpoint, which was at least a three-point improvement in urticaria activity score, was reached by 34 patients (61%). Among these 34 patients, the average reduction in the UAS score during the last 4 days of the diet compared with the 4 days before starting the diet was 8.59 points (P less than .001), and dropped from 9.05 to 4.23 (P = .004) across the entire group (J Eur Acad Dermatol Venereol. 2017 Apr;31[4]:650-5).
The low-histamine diet omitted food such as cheese, preserved meats, strawberries, raspberries, citrus fruit, bananas, kiwis, plums, papaya, and alcohol, and included foods such as dairy, vegetables, fresh meat, eggs, bread, pasta, rice, and certain varieties of fish. “Many patients with CsU complain of worsening of symptoms by consuming histamine-rich food, like red wine or matured cheese, but to the best of our knowledge, until now no studies were available supporting these observations,” the authors wrote.
The low-histamine diet was also associated with an average improvement in Dermatological Life Quality Instrument Questionnaire from baseline of 2.08 points across all participants, while Chronic Urticaria Quality of Life Questionnaire scores improved by an average of 5.46 score points in the 52 patients for whom these evaluations were available.
“Quality of life showed an improvement by a low-histamine diet, which was surprising, because dieting may decrease quality of life,” the authors noted.
There was also a reduction in antihistamine use among participants taking antihistamines at baseline, with 39% (22 of 56) reducing their intake and just over half of these patients (12 of the 22) stopping antihistamines altogether.
The study measured levels of diamine oxidase – the enzyme responsible for disintegrating histamine – both before and after, and saw no significant change across the patient group. However, patients with higher urticaria activity scores at baseline did show a decline in diamine oxidase activity with the diet.
Addressing concerns that these improvements could simply reflect spontaneous remission of the disease, the authors pointed out that a previous study had found a remission rate of just 3.4% over 6 months.
Based on the results, they concluded that a low-histamine diet “might be recommended for a period of 3-4 weeks in CsU patients in order to reduce symptoms and antihistamine intake as well as to improve quality of life.”
The authors had no funding source or conflicts of interest to declare.
A low-histamine diet could decrease symptoms and improve the quality of life for people with chronic spontaneous urticaria (CsU), according to Nicola Wagner, MD, of the department of dermatology at the Clinical Center Darmstadt (Germany) GmbH, Darmstadt, and her coauthors.
In their prospective study of 56 patients with a 3-month history of CsU (average 25 months) who followed a low-histamine diet for 3 weeks, 42 (75%) showed improvements in the urticaria activity score (UAS), compared to baseline. In nine patients (16%), disease activity remained the same, and five patients (9%) experienced worsening symptoms.
The primary endpoint, which was at least a three-point improvement in urticaria activity score, was reached by 34 patients (61%). Among these 34 patients, the average reduction in the UAS score during the last 4 days of the diet compared with the 4 days before starting the diet was 8.59 points (P less than .001), and dropped from 9.05 to 4.23 (P = .004) across the entire group (J Eur Acad Dermatol Venereol. 2017 Apr;31[4]:650-5).
The low-histamine diet omitted food such as cheese, preserved meats, strawberries, raspberries, citrus fruit, bananas, kiwis, plums, papaya, and alcohol, and included foods such as dairy, vegetables, fresh meat, eggs, bread, pasta, rice, and certain varieties of fish. “Many patients with CsU complain of worsening of symptoms by consuming histamine-rich food, like red wine or matured cheese, but to the best of our knowledge, until now no studies were available supporting these observations,” the authors wrote.
The low-histamine diet was also associated with an average improvement in Dermatological Life Quality Instrument Questionnaire from baseline of 2.08 points across all participants, while Chronic Urticaria Quality of Life Questionnaire scores improved by an average of 5.46 score points in the 52 patients for whom these evaluations were available.
“Quality of life showed an improvement by a low-histamine diet, which was surprising, because dieting may decrease quality of life,” the authors noted.
There was also a reduction in antihistamine use among participants taking antihistamines at baseline, with 39% (22 of 56) reducing their intake and just over half of these patients (12 of the 22) stopping antihistamines altogether.
The study measured levels of diamine oxidase – the enzyme responsible for disintegrating histamine – both before and after, and saw no significant change across the patient group. However, patients with higher urticaria activity scores at baseline did show a decline in diamine oxidase activity with the diet.
Addressing concerns that these improvements could simply reflect spontaneous remission of the disease, the authors pointed out that a previous study had found a remission rate of just 3.4% over 6 months.
Based on the results, they concluded that a low-histamine diet “might be recommended for a period of 3-4 weeks in CsU patients in order to reduce symptoms and antihistamine intake as well as to improve quality of life.”
The authors had no funding source or conflicts of interest to declare.
A low-histamine diet could decrease symptoms and improve the quality of life for people with chronic spontaneous urticaria (CsU), according to Nicola Wagner, MD, of the department of dermatology at the Clinical Center Darmstadt (Germany) GmbH, Darmstadt, and her coauthors.
In their prospective study of 56 patients with a 3-month history of CsU (average 25 months) who followed a low-histamine diet for 3 weeks, 42 (75%) showed improvements in the urticaria activity score (UAS), compared to baseline. In nine patients (16%), disease activity remained the same, and five patients (9%) experienced worsening symptoms.
The primary endpoint, which was at least a three-point improvement in urticaria activity score, was reached by 34 patients (61%). Among these 34 patients, the average reduction in the UAS score during the last 4 days of the diet compared with the 4 days before starting the diet was 8.59 points (P less than .001), and dropped from 9.05 to 4.23 (P = .004) across the entire group (J Eur Acad Dermatol Venereol. 2017 Apr;31[4]:650-5).
The low-histamine diet omitted food such as cheese, preserved meats, strawberries, raspberries, citrus fruit, bananas, kiwis, plums, papaya, and alcohol, and included foods such as dairy, vegetables, fresh meat, eggs, bread, pasta, rice, and certain varieties of fish. “Many patients with CsU complain of worsening of symptoms by consuming histamine-rich food, like red wine or matured cheese, but to the best of our knowledge, until now no studies were available supporting these observations,” the authors wrote.
The low-histamine diet was also associated with an average improvement in Dermatological Life Quality Instrument Questionnaire from baseline of 2.08 points across all participants, while Chronic Urticaria Quality of Life Questionnaire scores improved by an average of 5.46 score points in the 52 patients for whom these evaluations were available.
“Quality of life showed an improvement by a low-histamine diet, which was surprising, because dieting may decrease quality of life,” the authors noted.
There was also a reduction in antihistamine use among participants taking antihistamines at baseline, with 39% (22 of 56) reducing their intake and just over half of these patients (12 of the 22) stopping antihistamines altogether.
The study measured levels of diamine oxidase – the enzyme responsible for disintegrating histamine – both before and after, and saw no significant change across the patient group. However, patients with higher urticaria activity scores at baseline did show a decline in diamine oxidase activity with the diet.
Addressing concerns that these improvements could simply reflect spontaneous remission of the disease, the authors pointed out that a previous study had found a remission rate of just 3.4% over 6 months.
Based on the results, they concluded that a low-histamine diet “might be recommended for a period of 3-4 weeks in CsU patients in order to reduce symptoms and antihistamine intake as well as to improve quality of life.”
The authors had no funding source or conflicts of interest to declare.
FROM THE JOURNAL OF THE EUROPEAN ACADEMY OF DERMATOLOGY AND VENEREOLOGY
Key clinical point: A low-histamine diet for 3-4 weeks may be a simple therapeutic option for patients suffering with chronic spontaneous urticaria (CsU).
Major finding: Three-quarters of patients with CsU showed an improvement in disease activity score after 3 weeks on a low-histamine diet.
Data source: A prospective 3-week study evaluating the impact of a low-histamine diet on 56 patients with CsU.
Disclosures: No conflicts of interest or study funding source were declared.