User login
FDA wants annual COVID boosters, just like annual flu shots
The U.S. Food and Drug Administration is suggesting a single annual shot. The formulation would be selected in June targeting the most threatening COVID-19 strains, and then people could get a shot in the fall when people begin spending more time indoors and exposure increases.
Some people, such as those who are older or immunocompromised, may need more than one dose.
A national advisory committee is expected to vote on the proposal at a meeting Jan. 26.
People in the United States have been much less likely to get an updated COVID-19 booster shot, compared with widespread uptake of the primary vaccine series. In its proposal, the FDA indicated it hoped a single annual shot would overcome challenges created by the complexity of the process – both in messaging and administration – attributed to that low booster rate. Nine in 10 people age 12 or older got the primary vaccine series in the United States, but only 15% got the latest booster shot for COVID-19.
About half of children and adults in the U.S. get an annual flu shot, according to Centers for Disease Control and Prevention data.
The FDA also wants to move to a single COVID-19 vaccine formulation that would be used for primary vaccine series and for booster shots.
COVID-19 cases, hospitalizations, and deaths are trending downward, according to the data tracker from the New York Times. Cases are down 28%, with 47,290 tallied daily. Hospitalizations are down 22%, with 37,474 daily. Deaths are down 4%, with an average of 489 per day as of Jan. 22.
A version of this article originally appeared on WebMD.com.
The U.S. Food and Drug Administration is suggesting a single annual shot. The formulation would be selected in June targeting the most threatening COVID-19 strains, and then people could get a shot in the fall when people begin spending more time indoors and exposure increases.
Some people, such as those who are older or immunocompromised, may need more than one dose.
A national advisory committee is expected to vote on the proposal at a meeting Jan. 26.
People in the United States have been much less likely to get an updated COVID-19 booster shot, compared with widespread uptake of the primary vaccine series. In its proposal, the FDA indicated it hoped a single annual shot would overcome challenges created by the complexity of the process – both in messaging and administration – attributed to that low booster rate. Nine in 10 people age 12 or older got the primary vaccine series in the United States, but only 15% got the latest booster shot for COVID-19.
About half of children and adults in the U.S. get an annual flu shot, according to Centers for Disease Control and Prevention data.
The FDA also wants to move to a single COVID-19 vaccine formulation that would be used for primary vaccine series and for booster shots.
COVID-19 cases, hospitalizations, and deaths are trending downward, according to the data tracker from the New York Times. Cases are down 28%, with 47,290 tallied daily. Hospitalizations are down 22%, with 37,474 daily. Deaths are down 4%, with an average of 489 per day as of Jan. 22.
A version of this article originally appeared on WebMD.com.
The U.S. Food and Drug Administration is suggesting a single annual shot. The formulation would be selected in June targeting the most threatening COVID-19 strains, and then people could get a shot in the fall when people begin spending more time indoors and exposure increases.
Some people, such as those who are older or immunocompromised, may need more than one dose.
A national advisory committee is expected to vote on the proposal at a meeting Jan. 26.
People in the United States have been much less likely to get an updated COVID-19 booster shot, compared with widespread uptake of the primary vaccine series. In its proposal, the FDA indicated it hoped a single annual shot would overcome challenges created by the complexity of the process – both in messaging and administration – attributed to that low booster rate. Nine in 10 people age 12 or older got the primary vaccine series in the United States, but only 15% got the latest booster shot for COVID-19.
About half of children and adults in the U.S. get an annual flu shot, according to Centers for Disease Control and Prevention data.
The FDA also wants to move to a single COVID-19 vaccine formulation that would be used for primary vaccine series and for booster shots.
COVID-19 cases, hospitalizations, and deaths are trending downward, according to the data tracker from the New York Times. Cases are down 28%, with 47,290 tallied daily. Hospitalizations are down 22%, with 37,474 daily. Deaths are down 4%, with an average of 489 per day as of Jan. 22.
A version of this article originally appeared on WebMD.com.
Highly anticipated HIV vaccine fails in large trial
officials announced Wednesday.
The vaccine had been in development since 2019 and was given to 3,900 study participants through October 2022, but data shows it does not protect against HIV compared with a placebo, according to developer Janssen Pharmaceutical.
Experts estimate the failure means there won’t be another potential vaccine on the horizon for 3 to 5 years, the New York Times reported.
“It’s obviously disappointing,” Anthony Fauci, MD, former head of the National Institute of Allergy and Infectious Diseases, told MSNBC, noting that other areas of HIV treatment research are promising. “I don’t think that people should give up on the field of the HIV vaccine.”
No safety issues had been identified with the vaccine during the trial, which studied the experimental treatment in men who have sex with men or with transgender people.
There is no cure for HIV, but disease progression can be managed with existing treatments. HIV attacks the body’s immune system and destroys white blood cells, increasing the risk of other infections. More than 1.5 million people worldwide were infected with HIV in 2021 and 38.4 million people are living with the virus, according to UNAIDS.
A version of this article first appeared on WebMD.com.
officials announced Wednesday.
The vaccine had been in development since 2019 and was given to 3,900 study participants through October 2022, but data shows it does not protect against HIV compared with a placebo, according to developer Janssen Pharmaceutical.
Experts estimate the failure means there won’t be another potential vaccine on the horizon for 3 to 5 years, the New York Times reported.
“It’s obviously disappointing,” Anthony Fauci, MD, former head of the National Institute of Allergy and Infectious Diseases, told MSNBC, noting that other areas of HIV treatment research are promising. “I don’t think that people should give up on the field of the HIV vaccine.”
No safety issues had been identified with the vaccine during the trial, which studied the experimental treatment in men who have sex with men or with transgender people.
There is no cure for HIV, but disease progression can be managed with existing treatments. HIV attacks the body’s immune system and destroys white blood cells, increasing the risk of other infections. More than 1.5 million people worldwide were infected with HIV in 2021 and 38.4 million people are living with the virus, according to UNAIDS.
A version of this article first appeared on WebMD.com.
officials announced Wednesday.
The vaccine had been in development since 2019 and was given to 3,900 study participants through October 2022, but data shows it does not protect against HIV compared with a placebo, according to developer Janssen Pharmaceutical.
Experts estimate the failure means there won’t be another potential vaccine on the horizon for 3 to 5 years, the New York Times reported.
“It’s obviously disappointing,” Anthony Fauci, MD, former head of the National Institute of Allergy and Infectious Diseases, told MSNBC, noting that other areas of HIV treatment research are promising. “I don’t think that people should give up on the field of the HIV vaccine.”
No safety issues had been identified with the vaccine during the trial, which studied the experimental treatment in men who have sex with men or with transgender people.
There is no cure for HIV, but disease progression can be managed with existing treatments. HIV attacks the body’s immune system and destroys white blood cells, increasing the risk of other infections. More than 1.5 million people worldwide were infected with HIV in 2021 and 38.4 million people are living with the virus, according to UNAIDS.
A version of this article first appeared on WebMD.com.
Is it time for yet another COVID booster? It’s complicated
For some people who have received a two-dose primary series and all the recommended boosters, that could mean a sixth shot since COVID-19 vaccines became available. But is even that enough (or too much)?
At this point, no one knows for sure, but new guidance may be on the docket.
On Jan. 26, the FDA’s Vaccines and Related Biological Products Advisory Committee is meeting. On the agenda is discussion about plans for future vaccinations for COVID-19.The committee, made up of external advisers, evaluates data on vaccines and other products for the agency.
According to the FDA announcement, after the meeting, “the FDA will consider whether to recommend adjustments to the current authorizations and approvals, and the FDA will consider the most efficient and transparent process to use for selection of strains for inclusion in the primary and booster vaccines.”
From there, the CDC will take up the issue and decide on recommendations.
The issue is important, as more than 550 Americans a day are still dying from COVID-19, as of the week ending Jan. 13, the CDC reported. That’s up from 346 a day for the week ending Dec. 28.
Yet, uptake of the newest vaccine, the bivalent booster, has been slow. As of Jan. 11, just 15.9% of the population 5 years and up has gotten it; for those most vulnerable to COVID19 – those 65 and up – the number is just 39%.
COVID vaccines, 2023 and beyond
Meanwhile, infectious disease experts have widely differing views on what the vaccination landscape of 2023 and beyond should look like. Among the areas of disagreement are how effective the bivalent vaccine is, which people most need another shot, and what type of vaccine is best.
“I think we probably will need another booster,” says Peter Hotez, MD, PhD, dean of the National School of Tropical Medicine at Baylor College of Medicine, and codirector of the Center for Vaccine Development at Texas Children’s Hospital in Houston. “The question is, what is it going to be? Is it going to be the same bivalent that we just got, or will it be a new bivalent or even a trivalent?”
The trivalent booster, he suggested, might include something more protective against XBB.1.5.
The bivalent booster gives “broadened immunity” that is improved from the original booster shots, says Eric Topol, MD, founder and director of the Scripps Research Translational Institute in La Jolla, Calif., and editor-in-chief of Medscape, WebMD’s sister site for health professionals.
In his publication Ground Truths, Dr. Topol on Jan. 11 explained how new data caused him to reverse his previously skeptical view of how the FDA authorized the bivalent vaccine in September without data on how it affected humans at the time.
Paul Offit, MD, director of the Vaccine Education Center and a professor of pediatrics at the Children’s Hospital of Philadelphia, is a member of the FDA advisory committee for vaccines. He still takes a dimmer view of more bivalent booster vaccines, at least as a blanket recommendation.
While he acknowledges that boosters can help some groups – such as older adults, people with multiple health conditions, and those with compromised immune systems – he opposes a recommendation that’s population-wide.
“People who fall into those three groups do benefit,” he says, “but the recommendation is everyone over 6 months get the bivalent, and what I’m asking is, ‘Where is the data that a healthy 12-year-old boy needs a booster to stay out of the hospital?’ ”
Evolving research
“We are trying to understand how to stay one step ahead rather than several steps behind [the virus],“ says Michael Osterholm, PhD, director of the Center for Infectious Disease Research and Policy at the University of Minnesota.
Among the key questions: How well can a vaccine work against a single subvariant, when no one can say for sure what the next predominant subvariant will be?
Much more research has become available recently about the bivalent vaccine and its effectiveness, Dr. Osterholm says. “The bivalent vaccine is working as well as we could have expected,” he says, especially in high-risk people and in those over age 65. “The challenge we have is, what does that mean going forward?”
In his review, Dr. Topol concludes: “There is now more than ample, highly consistent evidence via lab studies and clinical outcomes to support the bivalent’s benefit over the original booster.”
Among other evidence, he looked at eight studies, including four that used a live virus as part of the research. Six of the eight studies showed the bivalent booster is more effective against the BA.5 variant, compared with the original booster shots. Two others showed no real difference.
“The four live virus studies offer consistent evidence of broadened immunity for the BA.5 vaccine that is improved over the original booster shots,” Dr. Topol wrote. The evidence also found the bivalent antibody response superior against XBB, he wrote.
Dr. Topol also cited CDC data that supports the benefits of the bivalent shot on hospitalization in older adults. During November, hospitalization of adults 65 and above was 2.5 times higher for those vaccinated who did not get the booster, compared to those who got the updated bivalent booster.
Boosters do matter, Dr. Offit says. “But not for all.” In a perspective published Jan. 11 in the New England Journal of Medicine – the same issue that published the two studies finding few differences between the original and bivalent – Dr. Offit wrote that boosting is best reserved for vulnerable groups.
Chasing the variants with a bivalent vaccine, he says, “has not panned out. There remains no evidence that a bivalent vaccine is any better than what we had. Please, show me the data that one is better than the other.”
Dr. Offit believes the goal should not be to prevent all symptomatic infections in healthy, young people by boosting them “with vaccines containing mRNA from strains that might disappear a few months later.”
The CDC needs to parse the data by subgroups, Dr. Offit says. “The critical question is, ‘Who gets hospitalized and who is dying? Who are they?’ ”
That data should take into account age, ethnicity, vaccine history, and other factors, Dr. Offit says, because right now, there is no great data to say, “OK, everyone gets a boost.”
Future vaccine costs
Another debate – for not only current boosters but future ones, too – centers on cost. Without congressional action to fund more vaccines, vaccine makers have suggested their prices may reach $130 a dose, compared with the average $20-per-dose cost the federal government pays now, according to a Kaiser Family Foundation report.
The government has spent more than $30 billion on COVID-19 vaccines, including the bivalent, to provide them free of charge.
The suggested price increase infuriated many. On Jan. 10, Sen. Bernie Sanders (I-Vt.), incoming chair of the Senate Committee on Health, Education, Labor and Pensions, sent a letter to Moderna CEO Stéphane Bancel, urging him to reconsider and refrain from any price increase.
“The huge increase in price that you have proposed will have a significantly negative impact on the budgets of Medicaid, Medicare and other government programs that will continue covering the vaccine without cost-sharing for patients.”
He pointed out, too, the $19 billion in profits Moderna has made over the past 2 years.
While most people with health insurance would likely still get the vaccines and booster for free, according to the Kaiser analysis, will a higher price discourage people from keeping up with recommended vaccinations, including a possible new booster?
“I think so, yes,” Dr. Hotez says, noting that vaccine reluctance is high as it is, even with free vaccinations and easy access.
“The government is balking at paying for the boosters,” he says. “I think it’s very tone deaf from the pharmaceutical companies [to increase the price]. Given all the help they’ve gotten from the American people, I think they should not be gouging at this point.”
He noted that the federal government provided not just money to the companies for the vaccines, but a “glide path” through the FDA for the vaccine approvals.
Are new, variant-specific boosters coming?
Are Moderna, Pfizer-BioNTech, and others developing more variant-specific vaccines, boosters, or other advances?
Novavax, approved in July 2022 as a primary series and in some cases as a booster, is “also developing an Omicron-containing bivalent vaccine at the direction of public health agencies,” says spokesperson Alison Chartan.
Pfizer responded: “When and if we have something to share we will let you know.”
Moderna did not respond.
A version of this article first appeared on WebMD.com.
For some people who have received a two-dose primary series and all the recommended boosters, that could mean a sixth shot since COVID-19 vaccines became available. But is even that enough (or too much)?
At this point, no one knows for sure, but new guidance may be on the docket.
On Jan. 26, the FDA’s Vaccines and Related Biological Products Advisory Committee is meeting. On the agenda is discussion about plans for future vaccinations for COVID-19.The committee, made up of external advisers, evaluates data on vaccines and other products for the agency.
According to the FDA announcement, after the meeting, “the FDA will consider whether to recommend adjustments to the current authorizations and approvals, and the FDA will consider the most efficient and transparent process to use for selection of strains for inclusion in the primary and booster vaccines.”
From there, the CDC will take up the issue and decide on recommendations.
The issue is important, as more than 550 Americans a day are still dying from COVID-19, as of the week ending Jan. 13, the CDC reported. That’s up from 346 a day for the week ending Dec. 28.
Yet, uptake of the newest vaccine, the bivalent booster, has been slow. As of Jan. 11, just 15.9% of the population 5 years and up has gotten it; for those most vulnerable to COVID19 – those 65 and up – the number is just 39%.
COVID vaccines, 2023 and beyond
Meanwhile, infectious disease experts have widely differing views on what the vaccination landscape of 2023 and beyond should look like. Among the areas of disagreement are how effective the bivalent vaccine is, which people most need another shot, and what type of vaccine is best.
“I think we probably will need another booster,” says Peter Hotez, MD, PhD, dean of the National School of Tropical Medicine at Baylor College of Medicine, and codirector of the Center for Vaccine Development at Texas Children’s Hospital in Houston. “The question is, what is it going to be? Is it going to be the same bivalent that we just got, or will it be a new bivalent or even a trivalent?”
The trivalent booster, he suggested, might include something more protective against XBB.1.5.
The bivalent booster gives “broadened immunity” that is improved from the original booster shots, says Eric Topol, MD, founder and director of the Scripps Research Translational Institute in La Jolla, Calif., and editor-in-chief of Medscape, WebMD’s sister site for health professionals.
In his publication Ground Truths, Dr. Topol on Jan. 11 explained how new data caused him to reverse his previously skeptical view of how the FDA authorized the bivalent vaccine in September without data on how it affected humans at the time.
Paul Offit, MD, director of the Vaccine Education Center and a professor of pediatrics at the Children’s Hospital of Philadelphia, is a member of the FDA advisory committee for vaccines. He still takes a dimmer view of more bivalent booster vaccines, at least as a blanket recommendation.
While he acknowledges that boosters can help some groups – such as older adults, people with multiple health conditions, and those with compromised immune systems – he opposes a recommendation that’s population-wide.
“People who fall into those three groups do benefit,” he says, “but the recommendation is everyone over 6 months get the bivalent, and what I’m asking is, ‘Where is the data that a healthy 12-year-old boy needs a booster to stay out of the hospital?’ ”
Evolving research
“We are trying to understand how to stay one step ahead rather than several steps behind [the virus],“ says Michael Osterholm, PhD, director of the Center for Infectious Disease Research and Policy at the University of Minnesota.
Among the key questions: How well can a vaccine work against a single subvariant, when no one can say for sure what the next predominant subvariant will be?
Much more research has become available recently about the bivalent vaccine and its effectiveness, Dr. Osterholm says. “The bivalent vaccine is working as well as we could have expected,” he says, especially in high-risk people and in those over age 65. “The challenge we have is, what does that mean going forward?”
In his review, Dr. Topol concludes: “There is now more than ample, highly consistent evidence via lab studies and clinical outcomes to support the bivalent’s benefit over the original booster.”
Among other evidence, he looked at eight studies, including four that used a live virus as part of the research. Six of the eight studies showed the bivalent booster is more effective against the BA.5 variant, compared with the original booster shots. Two others showed no real difference.
“The four live virus studies offer consistent evidence of broadened immunity for the BA.5 vaccine that is improved over the original booster shots,” Dr. Topol wrote. The evidence also found the bivalent antibody response superior against XBB, he wrote.
Dr. Topol also cited CDC data that supports the benefits of the bivalent shot on hospitalization in older adults. During November, hospitalization of adults 65 and above was 2.5 times higher for those vaccinated who did not get the booster, compared to those who got the updated bivalent booster.
Boosters do matter, Dr. Offit says. “But not for all.” In a perspective published Jan. 11 in the New England Journal of Medicine – the same issue that published the two studies finding few differences between the original and bivalent – Dr. Offit wrote that boosting is best reserved for vulnerable groups.
Chasing the variants with a bivalent vaccine, he says, “has not panned out. There remains no evidence that a bivalent vaccine is any better than what we had. Please, show me the data that one is better than the other.”
Dr. Offit believes the goal should not be to prevent all symptomatic infections in healthy, young people by boosting them “with vaccines containing mRNA from strains that might disappear a few months later.”
The CDC needs to parse the data by subgroups, Dr. Offit says. “The critical question is, ‘Who gets hospitalized and who is dying? Who are they?’ ”
That data should take into account age, ethnicity, vaccine history, and other factors, Dr. Offit says, because right now, there is no great data to say, “OK, everyone gets a boost.”
Future vaccine costs
Another debate – for not only current boosters but future ones, too – centers on cost. Without congressional action to fund more vaccines, vaccine makers have suggested their prices may reach $130 a dose, compared with the average $20-per-dose cost the federal government pays now, according to a Kaiser Family Foundation report.
The government has spent more than $30 billion on COVID-19 vaccines, including the bivalent, to provide them free of charge.
The suggested price increase infuriated many. On Jan. 10, Sen. Bernie Sanders (I-Vt.), incoming chair of the Senate Committee on Health, Education, Labor and Pensions, sent a letter to Moderna CEO Stéphane Bancel, urging him to reconsider and refrain from any price increase.
“The huge increase in price that you have proposed will have a significantly negative impact on the budgets of Medicaid, Medicare and other government programs that will continue covering the vaccine without cost-sharing for patients.”
He pointed out, too, the $19 billion in profits Moderna has made over the past 2 years.
While most people with health insurance would likely still get the vaccines and booster for free, according to the Kaiser analysis, will a higher price discourage people from keeping up with recommended vaccinations, including a possible new booster?
“I think so, yes,” Dr. Hotez says, noting that vaccine reluctance is high as it is, even with free vaccinations and easy access.
“The government is balking at paying for the boosters,” he says. “I think it’s very tone deaf from the pharmaceutical companies [to increase the price]. Given all the help they’ve gotten from the American people, I think they should not be gouging at this point.”
He noted that the federal government provided not just money to the companies for the vaccines, but a “glide path” through the FDA for the vaccine approvals.
Are new, variant-specific boosters coming?
Are Moderna, Pfizer-BioNTech, and others developing more variant-specific vaccines, boosters, or other advances?
Novavax, approved in July 2022 as a primary series and in some cases as a booster, is “also developing an Omicron-containing bivalent vaccine at the direction of public health agencies,” says spokesperson Alison Chartan.
Pfizer responded: “When and if we have something to share we will let you know.”
Moderna did not respond.
A version of this article first appeared on WebMD.com.
For some people who have received a two-dose primary series and all the recommended boosters, that could mean a sixth shot since COVID-19 vaccines became available. But is even that enough (or too much)?
At this point, no one knows for sure, but new guidance may be on the docket.
On Jan. 26, the FDA’s Vaccines and Related Biological Products Advisory Committee is meeting. On the agenda is discussion about plans for future vaccinations for COVID-19.The committee, made up of external advisers, evaluates data on vaccines and other products for the agency.
According to the FDA announcement, after the meeting, “the FDA will consider whether to recommend adjustments to the current authorizations and approvals, and the FDA will consider the most efficient and transparent process to use for selection of strains for inclusion in the primary and booster vaccines.”
From there, the CDC will take up the issue and decide on recommendations.
The issue is important, as more than 550 Americans a day are still dying from COVID-19, as of the week ending Jan. 13, the CDC reported. That’s up from 346 a day for the week ending Dec. 28.
Yet, uptake of the newest vaccine, the bivalent booster, has been slow. As of Jan. 11, just 15.9% of the population 5 years and up has gotten it; for those most vulnerable to COVID19 – those 65 and up – the number is just 39%.
COVID vaccines, 2023 and beyond
Meanwhile, infectious disease experts have widely differing views on what the vaccination landscape of 2023 and beyond should look like. Among the areas of disagreement are how effective the bivalent vaccine is, which people most need another shot, and what type of vaccine is best.
“I think we probably will need another booster,” says Peter Hotez, MD, PhD, dean of the National School of Tropical Medicine at Baylor College of Medicine, and codirector of the Center for Vaccine Development at Texas Children’s Hospital in Houston. “The question is, what is it going to be? Is it going to be the same bivalent that we just got, or will it be a new bivalent or even a trivalent?”
The trivalent booster, he suggested, might include something more protective against XBB.1.5.
The bivalent booster gives “broadened immunity” that is improved from the original booster shots, says Eric Topol, MD, founder and director of the Scripps Research Translational Institute in La Jolla, Calif., and editor-in-chief of Medscape, WebMD’s sister site for health professionals.
In his publication Ground Truths, Dr. Topol on Jan. 11 explained how new data caused him to reverse his previously skeptical view of how the FDA authorized the bivalent vaccine in September without data on how it affected humans at the time.
Paul Offit, MD, director of the Vaccine Education Center and a professor of pediatrics at the Children’s Hospital of Philadelphia, is a member of the FDA advisory committee for vaccines. He still takes a dimmer view of more bivalent booster vaccines, at least as a blanket recommendation.
While he acknowledges that boosters can help some groups – such as older adults, people with multiple health conditions, and those with compromised immune systems – he opposes a recommendation that’s population-wide.
“People who fall into those three groups do benefit,” he says, “but the recommendation is everyone over 6 months get the bivalent, and what I’m asking is, ‘Where is the data that a healthy 12-year-old boy needs a booster to stay out of the hospital?’ ”
Evolving research
“We are trying to understand how to stay one step ahead rather than several steps behind [the virus],“ says Michael Osterholm, PhD, director of the Center for Infectious Disease Research and Policy at the University of Minnesota.
Among the key questions: How well can a vaccine work against a single subvariant, when no one can say for sure what the next predominant subvariant will be?
Much more research has become available recently about the bivalent vaccine and its effectiveness, Dr. Osterholm says. “The bivalent vaccine is working as well as we could have expected,” he says, especially in high-risk people and in those over age 65. “The challenge we have is, what does that mean going forward?”
In his review, Dr. Topol concludes: “There is now more than ample, highly consistent evidence via lab studies and clinical outcomes to support the bivalent’s benefit over the original booster.”
Among other evidence, he looked at eight studies, including four that used a live virus as part of the research. Six of the eight studies showed the bivalent booster is more effective against the BA.5 variant, compared with the original booster shots. Two others showed no real difference.
“The four live virus studies offer consistent evidence of broadened immunity for the BA.5 vaccine that is improved over the original booster shots,” Dr. Topol wrote. The evidence also found the bivalent antibody response superior against XBB, he wrote.
Dr. Topol also cited CDC data that supports the benefits of the bivalent shot on hospitalization in older adults. During November, hospitalization of adults 65 and above was 2.5 times higher for those vaccinated who did not get the booster, compared to those who got the updated bivalent booster.
Boosters do matter, Dr. Offit says. “But not for all.” In a perspective published Jan. 11 in the New England Journal of Medicine – the same issue that published the two studies finding few differences between the original and bivalent – Dr. Offit wrote that boosting is best reserved for vulnerable groups.
Chasing the variants with a bivalent vaccine, he says, “has not panned out. There remains no evidence that a bivalent vaccine is any better than what we had. Please, show me the data that one is better than the other.”
Dr. Offit believes the goal should not be to prevent all symptomatic infections in healthy, young people by boosting them “with vaccines containing mRNA from strains that might disappear a few months later.”
The CDC needs to parse the data by subgroups, Dr. Offit says. “The critical question is, ‘Who gets hospitalized and who is dying? Who are they?’ ”
That data should take into account age, ethnicity, vaccine history, and other factors, Dr. Offit says, because right now, there is no great data to say, “OK, everyone gets a boost.”
Future vaccine costs
Another debate – for not only current boosters but future ones, too – centers on cost. Without congressional action to fund more vaccines, vaccine makers have suggested their prices may reach $130 a dose, compared with the average $20-per-dose cost the federal government pays now, according to a Kaiser Family Foundation report.
The government has spent more than $30 billion on COVID-19 vaccines, including the bivalent, to provide them free of charge.
The suggested price increase infuriated many. On Jan. 10, Sen. Bernie Sanders (I-Vt.), incoming chair of the Senate Committee on Health, Education, Labor and Pensions, sent a letter to Moderna CEO Stéphane Bancel, urging him to reconsider and refrain from any price increase.
“The huge increase in price that you have proposed will have a significantly negative impact on the budgets of Medicaid, Medicare and other government programs that will continue covering the vaccine without cost-sharing for patients.”
He pointed out, too, the $19 billion in profits Moderna has made over the past 2 years.
While most people with health insurance would likely still get the vaccines and booster for free, according to the Kaiser analysis, will a higher price discourage people from keeping up with recommended vaccinations, including a possible new booster?
“I think so, yes,” Dr. Hotez says, noting that vaccine reluctance is high as it is, even with free vaccinations and easy access.
“The government is balking at paying for the boosters,” he says. “I think it’s very tone deaf from the pharmaceutical companies [to increase the price]. Given all the help they’ve gotten from the American people, I think they should not be gouging at this point.”
He noted that the federal government provided not just money to the companies for the vaccines, but a “glide path” through the FDA for the vaccine approvals.
Are new, variant-specific boosters coming?
Are Moderna, Pfizer-BioNTech, and others developing more variant-specific vaccines, boosters, or other advances?
Novavax, approved in July 2022 as a primary series and in some cases as a booster, is “also developing an Omicron-containing bivalent vaccine at the direction of public health agencies,” says spokesperson Alison Chartan.
Pfizer responded: “When and if we have something to share we will let you know.”
Moderna did not respond.
A version of this article first appeared on WebMD.com.
Possible bivalent vaccine link to strokes in people over 65
who got the shot, the Centers for Disease Control and Prevention and the Food and Drug Administration said in a joint news release.
The release did not recommend people change their vaccine practices, saying the database finding probably did not represent a “true clinical risk.” The CDC said everybody, including people over 65, should stay up to date on their COVID vaccines, including the bivalent booster.
The news release said the Vaccine Safety Datalink (VSD), “a near real-time surveillance system,” raised a safety concern about the Pfizer/BioNTech booster.
“Rapid-response investigation of the signal in the VSD raised a question of whether people 65 and older who have received the Pfizer-BioNTech COVID-19 Vaccine, Bivalent were more likely to have an ischemic stroke in the 21 days following vaccination compared with days 22-44 following vaccination,” the news release said.
Ischemic strokes are blockages of blood to the brain, often caused by blood clots.
“Although the totality of the data currently suggests that it is very unlikely that the signal in VSD (Vaccine Safety Datalink) represents a true clinical risk, we believe it is important to share this information with the public, as we have in the past, when one of our safety monitoring systems detects a signal,” the release said.
No higher likelihood of strokes linked to the Pfizer bivalent vaccine had been found by Pfizer/BioNTech, the Department of Veterans Affairs, the Vaccine Adverse Event Reporting System maintained by the CDC and the FDA, or other agencies that monitor reactions of vaccines, the news release said. No safety issues about strokes have been identified with the Moderna bivalent vaccine.
CNN, citing a CDC official, reported that about 550,000 seniors who got Pfizer bivalent boosters were tracked by the VSD, and 130 of them had strokes within 3 weeks of getting the shot. None of those 130 people died, CNN said. The official spoke on the condition of anonymity because they weren’t authorized to share the data.
The issue will be discussed at the January meeting of the FDA’s Vaccines and Related Biological Products Advisory Committee.
In a joint statement, Pfizer and BioNTech said: “Neither Pfizer and BioNTech nor the CDC or FDA have observed similar findings across numerous other monitoring systems in the U.S. and globally and there is no evidence to conclude that ischemic stroke is associated with the use of the companies’ COVID-19 vaccines.”
Bivalent boosters contain two strains of vaccine – one to protect against the original COVID-19 virus and another targeting Omicron subvariants.
A version of this article first appeared on WebMD.com.
who got the shot, the Centers for Disease Control and Prevention and the Food and Drug Administration said in a joint news release.
The release did not recommend people change their vaccine practices, saying the database finding probably did not represent a “true clinical risk.” The CDC said everybody, including people over 65, should stay up to date on their COVID vaccines, including the bivalent booster.
The news release said the Vaccine Safety Datalink (VSD), “a near real-time surveillance system,” raised a safety concern about the Pfizer/BioNTech booster.
“Rapid-response investigation of the signal in the VSD raised a question of whether people 65 and older who have received the Pfizer-BioNTech COVID-19 Vaccine, Bivalent were more likely to have an ischemic stroke in the 21 days following vaccination compared with days 22-44 following vaccination,” the news release said.
Ischemic strokes are blockages of blood to the brain, often caused by blood clots.
“Although the totality of the data currently suggests that it is very unlikely that the signal in VSD (Vaccine Safety Datalink) represents a true clinical risk, we believe it is important to share this information with the public, as we have in the past, when one of our safety monitoring systems detects a signal,” the release said.
No higher likelihood of strokes linked to the Pfizer bivalent vaccine had been found by Pfizer/BioNTech, the Department of Veterans Affairs, the Vaccine Adverse Event Reporting System maintained by the CDC and the FDA, or other agencies that monitor reactions of vaccines, the news release said. No safety issues about strokes have been identified with the Moderna bivalent vaccine.
CNN, citing a CDC official, reported that about 550,000 seniors who got Pfizer bivalent boosters were tracked by the VSD, and 130 of them had strokes within 3 weeks of getting the shot. None of those 130 people died, CNN said. The official spoke on the condition of anonymity because they weren’t authorized to share the data.
The issue will be discussed at the January meeting of the FDA’s Vaccines and Related Biological Products Advisory Committee.
In a joint statement, Pfizer and BioNTech said: “Neither Pfizer and BioNTech nor the CDC or FDA have observed similar findings across numerous other monitoring systems in the U.S. and globally and there is no evidence to conclude that ischemic stroke is associated with the use of the companies’ COVID-19 vaccines.”
Bivalent boosters contain two strains of vaccine – one to protect against the original COVID-19 virus and another targeting Omicron subvariants.
A version of this article first appeared on WebMD.com.
who got the shot, the Centers for Disease Control and Prevention and the Food and Drug Administration said in a joint news release.
The release did not recommend people change their vaccine practices, saying the database finding probably did not represent a “true clinical risk.” The CDC said everybody, including people over 65, should stay up to date on their COVID vaccines, including the bivalent booster.
The news release said the Vaccine Safety Datalink (VSD), “a near real-time surveillance system,” raised a safety concern about the Pfizer/BioNTech booster.
“Rapid-response investigation of the signal in the VSD raised a question of whether people 65 and older who have received the Pfizer-BioNTech COVID-19 Vaccine, Bivalent were more likely to have an ischemic stroke in the 21 days following vaccination compared with days 22-44 following vaccination,” the news release said.
Ischemic strokes are blockages of blood to the brain, often caused by blood clots.
“Although the totality of the data currently suggests that it is very unlikely that the signal in VSD (Vaccine Safety Datalink) represents a true clinical risk, we believe it is important to share this information with the public, as we have in the past, when one of our safety monitoring systems detects a signal,” the release said.
No higher likelihood of strokes linked to the Pfizer bivalent vaccine had been found by Pfizer/BioNTech, the Department of Veterans Affairs, the Vaccine Adverse Event Reporting System maintained by the CDC and the FDA, or other agencies that monitor reactions of vaccines, the news release said. No safety issues about strokes have been identified with the Moderna bivalent vaccine.
CNN, citing a CDC official, reported that about 550,000 seniors who got Pfizer bivalent boosters were tracked by the VSD, and 130 of them had strokes within 3 weeks of getting the shot. None of those 130 people died, CNN said. The official spoke on the condition of anonymity because they weren’t authorized to share the data.
The issue will be discussed at the January meeting of the FDA’s Vaccines and Related Biological Products Advisory Committee.
In a joint statement, Pfizer and BioNTech said: “Neither Pfizer and BioNTech nor the CDC or FDA have observed similar findings across numerous other monitoring systems in the U.S. and globally and there is no evidence to conclude that ischemic stroke is associated with the use of the companies’ COVID-19 vaccines.”
Bivalent boosters contain two strains of vaccine – one to protect against the original COVID-19 virus and another targeting Omicron subvariants.
A version of this article first appeared on WebMD.com.
CDC frets over further dip in kindergarten vaccination rates
The percentage of kindergarteners in the United States who have received routine vaccines to protect against illnesses such as measles, whooping cough, and polio has declined for 2 straight years, a new study has found.
Drops in vaccine coverage leave communities more susceptible to outbreaks of vaccine-preventable diseases, such as those that occurred in 2022, public health officials said.
Coverage for four vaccines – against measles, mumps, and rubella (MMR); diphtheria, tetanus, and acellular pertussis (DTaP); poliovirus; and varicella – among kindergarten students was about 95% in 2019-2020.
The rate fell to 94% the following year.
For the 2021-2022 school year, coverage dropped another point, to 93%, according to the report, published online in Morbidity and Mortality Weekly Report.
The rate of vaccination overall remains high, but about 250,000 kindergarten students may not be protected against measles, the researchers estimate. Measles, which is highly infectious, can lead to serious illness and even death in children who have not been vaccinated against the virus.
“In 2022, two communities in the United States responded to outbreaks of measles where children have been hospitalized,” Georgina Peacock, MD, MPH, director of the immunization services division of the Centers for Disease Control and Prevention, said in a media briefing about the report. “One community reported a case of paralytic polio in an unvaccinated person. These outbreaks were preventable. The best way to prevent these diseases and their devastating impact on children is through vaccination.”
Exemptions steady
For the new study, Ranee Seither, MPH, with the CDC’s National Center for Immunization and Respiratory Diseases and her colleagues analyzed data reported by states to estimate nationwide coverage for the four routine vaccines.
The number of students with exemptions remained low, at 2.6%, but another 3.9% who were without exemptions were not up to date with the MMR vaccine, the investigators report.
In a separate study, researchers found that vaccination coverage for 2-year-olds has increased. Approximately 70% of children were up to date with a seven-vaccine series by age 24 months. The coverage rate was higher for children born during 2018-2019 than for those born during 2016-2017.
Although the COVID-19 pandemic was not associated with decreased vaccination rates in this younger age group overall, coverage fell by 4-5 percentage points for children living below the poverty level or in rural areas, according to the study.
In addition, uninsured children were eight times more likely than those with private insurance to not be vaccinated by their second birthday, the researchers found.
Strategies to increase vaccination coverage include enforcing school vaccination requirements and holding vaccination clinics at schools, the CDC said.
“Providers should review children’s histories and recommend needed vaccinations during every clinical encounter and address parental hesitancy to help reduce disparities and ensure that all children are protected from vaccine-preventable diseases,” the agency said.
To that end, the agency launched an initiative this week called Let’s RISE (Routine Immunizations on Schedule for Everyone) to provide clinicians with resources to help patients get on track with their immunizations.
Hundreds of thousands unprotected
MMR vaccination coverage for kindergartners is the lowest it has been in over a decade, Dr. Peacock noted. Decreased coverage for kindergarten students might be tied to pandemic-related disruptions in health care systems and schools, she said. School administrators and parents may have been less focused on routine vaccination paperwork amid the return to in-person learning, for instance.
Hesitancy about COVID vaccines could be affecting routine vaccinations. “That’s something that we are watching very closely,” Dr. Peacock said.
The 2-point decrease in vaccination coverage “translates to hundreds of thousands of children starting school without being fully protected” against preventable diseases that can spread easily in classrooms, Sean O’Leary, MD, chair of the American Academy of Pediatrics’ Committee on Infectious Diseases, said.
Despite the drop in coverage, Dr. O’Leary said he saw some encouraging signs in the data: Nonmedical exemptions for kindergarten students have not increased. And the vast majority of parents are still having their children vaccinated. At the same time, the reports highlight a need to address child poverty and improve vaccine access in rural areas, he said.
A version of this article first appeared on Medscape.com.
The percentage of kindergarteners in the United States who have received routine vaccines to protect against illnesses such as measles, whooping cough, and polio has declined for 2 straight years, a new study has found.
Drops in vaccine coverage leave communities more susceptible to outbreaks of vaccine-preventable diseases, such as those that occurred in 2022, public health officials said.
Coverage for four vaccines – against measles, mumps, and rubella (MMR); diphtheria, tetanus, and acellular pertussis (DTaP); poliovirus; and varicella – among kindergarten students was about 95% in 2019-2020.
The rate fell to 94% the following year.
For the 2021-2022 school year, coverage dropped another point, to 93%, according to the report, published online in Morbidity and Mortality Weekly Report.
The rate of vaccination overall remains high, but about 250,000 kindergarten students may not be protected against measles, the researchers estimate. Measles, which is highly infectious, can lead to serious illness and even death in children who have not been vaccinated against the virus.
“In 2022, two communities in the United States responded to outbreaks of measles where children have been hospitalized,” Georgina Peacock, MD, MPH, director of the immunization services division of the Centers for Disease Control and Prevention, said in a media briefing about the report. “One community reported a case of paralytic polio in an unvaccinated person. These outbreaks were preventable. The best way to prevent these diseases and their devastating impact on children is through vaccination.”
Exemptions steady
For the new study, Ranee Seither, MPH, with the CDC’s National Center for Immunization and Respiratory Diseases and her colleagues analyzed data reported by states to estimate nationwide coverage for the four routine vaccines.
The number of students with exemptions remained low, at 2.6%, but another 3.9% who were without exemptions were not up to date with the MMR vaccine, the investigators report.
In a separate study, researchers found that vaccination coverage for 2-year-olds has increased. Approximately 70% of children were up to date with a seven-vaccine series by age 24 months. The coverage rate was higher for children born during 2018-2019 than for those born during 2016-2017.
Although the COVID-19 pandemic was not associated with decreased vaccination rates in this younger age group overall, coverage fell by 4-5 percentage points for children living below the poverty level or in rural areas, according to the study.
In addition, uninsured children were eight times more likely than those with private insurance to not be vaccinated by their second birthday, the researchers found.
Strategies to increase vaccination coverage include enforcing school vaccination requirements and holding vaccination clinics at schools, the CDC said.
“Providers should review children’s histories and recommend needed vaccinations during every clinical encounter and address parental hesitancy to help reduce disparities and ensure that all children are protected from vaccine-preventable diseases,” the agency said.
To that end, the agency launched an initiative this week called Let’s RISE (Routine Immunizations on Schedule for Everyone) to provide clinicians with resources to help patients get on track with their immunizations.
Hundreds of thousands unprotected
MMR vaccination coverage for kindergartners is the lowest it has been in over a decade, Dr. Peacock noted. Decreased coverage for kindergarten students might be tied to pandemic-related disruptions in health care systems and schools, she said. School administrators and parents may have been less focused on routine vaccination paperwork amid the return to in-person learning, for instance.
Hesitancy about COVID vaccines could be affecting routine vaccinations. “That’s something that we are watching very closely,” Dr. Peacock said.
The 2-point decrease in vaccination coverage “translates to hundreds of thousands of children starting school without being fully protected” against preventable diseases that can spread easily in classrooms, Sean O’Leary, MD, chair of the American Academy of Pediatrics’ Committee on Infectious Diseases, said.
Despite the drop in coverage, Dr. O’Leary said he saw some encouraging signs in the data: Nonmedical exemptions for kindergarten students have not increased. And the vast majority of parents are still having their children vaccinated. At the same time, the reports highlight a need to address child poverty and improve vaccine access in rural areas, he said.
A version of this article first appeared on Medscape.com.
The percentage of kindergarteners in the United States who have received routine vaccines to protect against illnesses such as measles, whooping cough, and polio has declined for 2 straight years, a new study has found.
Drops in vaccine coverage leave communities more susceptible to outbreaks of vaccine-preventable diseases, such as those that occurred in 2022, public health officials said.
Coverage for four vaccines – against measles, mumps, and rubella (MMR); diphtheria, tetanus, and acellular pertussis (DTaP); poliovirus; and varicella – among kindergarten students was about 95% in 2019-2020.
The rate fell to 94% the following year.
For the 2021-2022 school year, coverage dropped another point, to 93%, according to the report, published online in Morbidity and Mortality Weekly Report.
The rate of vaccination overall remains high, but about 250,000 kindergarten students may not be protected against measles, the researchers estimate. Measles, which is highly infectious, can lead to serious illness and even death in children who have not been vaccinated against the virus.
“In 2022, two communities in the United States responded to outbreaks of measles where children have been hospitalized,” Georgina Peacock, MD, MPH, director of the immunization services division of the Centers for Disease Control and Prevention, said in a media briefing about the report. “One community reported a case of paralytic polio in an unvaccinated person. These outbreaks were preventable. The best way to prevent these diseases and their devastating impact on children is through vaccination.”
Exemptions steady
For the new study, Ranee Seither, MPH, with the CDC’s National Center for Immunization and Respiratory Diseases and her colleagues analyzed data reported by states to estimate nationwide coverage for the four routine vaccines.
The number of students with exemptions remained low, at 2.6%, but another 3.9% who were without exemptions were not up to date with the MMR vaccine, the investigators report.
In a separate study, researchers found that vaccination coverage for 2-year-olds has increased. Approximately 70% of children were up to date with a seven-vaccine series by age 24 months. The coverage rate was higher for children born during 2018-2019 than for those born during 2016-2017.
Although the COVID-19 pandemic was not associated with decreased vaccination rates in this younger age group overall, coverage fell by 4-5 percentage points for children living below the poverty level or in rural areas, according to the study.
In addition, uninsured children were eight times more likely than those with private insurance to not be vaccinated by their second birthday, the researchers found.
Strategies to increase vaccination coverage include enforcing school vaccination requirements and holding vaccination clinics at schools, the CDC said.
“Providers should review children’s histories and recommend needed vaccinations during every clinical encounter and address parental hesitancy to help reduce disparities and ensure that all children are protected from vaccine-preventable diseases,” the agency said.
To that end, the agency launched an initiative this week called Let’s RISE (Routine Immunizations on Schedule for Everyone) to provide clinicians with resources to help patients get on track with their immunizations.
Hundreds of thousands unprotected
MMR vaccination coverage for kindergartners is the lowest it has been in over a decade, Dr. Peacock noted. Decreased coverage for kindergarten students might be tied to pandemic-related disruptions in health care systems and schools, she said. School administrators and parents may have been less focused on routine vaccination paperwork amid the return to in-person learning, for instance.
Hesitancy about COVID vaccines could be affecting routine vaccinations. “That’s something that we are watching very closely,” Dr. Peacock said.
The 2-point decrease in vaccination coverage “translates to hundreds of thousands of children starting school without being fully protected” against preventable diseases that can spread easily in classrooms, Sean O’Leary, MD, chair of the American Academy of Pediatrics’ Committee on Infectious Diseases, said.
Despite the drop in coverage, Dr. O’Leary said he saw some encouraging signs in the data: Nonmedical exemptions for kindergarten students have not increased. And the vast majority of parents are still having their children vaccinated. At the same time, the reports highlight a need to address child poverty and improve vaccine access in rural areas, he said.
A version of this article first appeared on Medscape.com.
FROM THE MMWR
Cardiac Adverse Events Following COVID-19 Vaccination in Patients With Prior Vaccine-Associated Myocarditis
Vaccinations have substantially reduced morbidity and mortality from many infectious diseases. Despite the clear value of vaccinations in public health, efforts to better understand adverse events (AEs) following immunization are important to sustain public trust and vaccine confidence. Noninfectious inflammation of the heart may manifest as myocarditis or pericarditis, or occasionally, with shared signs and symptoms of each, as myopericarditis. This is a rare AE following some immunizations. Vaccine-associated myocarditis, pericarditis, or myopericarditis (VAMP) has been most clearly associated with smallpox vaccines and mRNA COVID-19 vaccines.1-6 Although extremely rare, VAMP also has been associated with other vaccines.7,8 Limited information exists to guide shared clinical decision making on COVID-19 vaccination in persons with a history of VAMP. It is unknown whether individuals with a history of VAMP are at higher risk for developing a recurrence or experiencing a more severe outcome following COVID-19 vaccination.
Methods
As part of the collaborative public health mission with the Centers for Disease Control and Prevention (CDC) for enhanced vaccine AE surveillance, the Defense Health Agency Immunization Healthcare Division (IHD) maintains a clinical database of service members and beneficiaries referred for suspected AEs following immunizations. A review of all AEs following immunization cases in this database from January 1, 2003, through February 28, 2022, identified individuals meeting the following criteria: (a) VAMP prior to receipt of COVID-19 vaccine; (b) receipt of COVID-19 vaccine in 2021; and (c) medical documentation in available electronic health records sufficient to describe health status at least 30 days following COVID-19 vaccination.9 If medical entries suggested cardiac symptoms following a COVID-19 vaccine, additional information was sought to verify VAMP based on current published criteria.10,11 Both the initial VAMP cases and the suspected COVID-19 VAMP cases were adjudicated by a team of vaccine experts and specialists in immunology, cardiology, and preventive medicine.
This retrospective review was approved and conducted in accordance with the Walter Reed National Military Medical Center Institutional Review Board protocol #20664. All individuals with recurrent VAMP consented to share their health records and clinical details.
Results
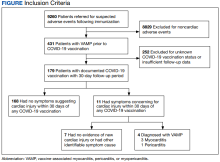
Among 9260 cases in the IHD database, 431 met the case definition for VAMP.
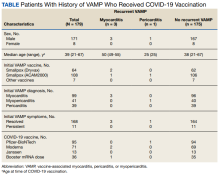
Among the 179 patients included in this analysis, 171 (96%) were male. Their median age was 39 years at the time of COVID-19 vaccination.
Within 1 month of receipt of any COVID-19 vaccine, 11 individuals had documented symptoms suggesting cardiac involvement, specifically, chest pain, palpitations, or dyspnea. After cardiac evaluation, 4 patients met the criteria for VAMP after COVID-19 vaccination.10,11 Seven patients either did not meet the criteria for VAMP or had alternative causes for their symptoms.
Two men aged 49 and 50 years with a history of vaccine-associated myocarditis following smallpox vaccination (Dryvax and ACAM2000) developed myocarditis 3 days after their second dose of the Moderna vaccine. One of these patients received a Pfizer-BioNTech booster 10 months later with no recurrence of symptoms. A 55-year-old man with a history of vaccine-associated myocarditis following Dryvax vaccination developed myocarditis 2 days after his Pfizer-BioNTech booster. None of the patients who developed post-COVID-19 VAMP reported residual symptoms from their initial VAMP episode, which occurred 12 to 18 years earlier. All were hospitalized briefly for observation and had complete symptom resolution within 6 weeks.
A 25-year-old man developed pericarditis 4 days after his second Pfizer-BioNTech vaccination. His previous ACAM2000 vaccine-associated myocarditis occurred 3 years earlier, with no residual symptoms. Of note, he had a mild COVID-19 infection 78 days before the onset of his pericarditis. After the onset of his COVID-19 vaccine-associated pericarditis, he continued to experience transient bouts of chest pressure and exertional dyspnea that resolved within 7 months of onset.
The median interval between COVID-19 vaccine doses in those who developed post-COVID-19 VAMP was within the recommended mRNA vaccine dosing intervals of 3 to 4 weeks and was consistent with the median mRNA vaccine dosing intervals among the entire cohort.
Due to the small cohort size and other limitations of this study, the suggested rate of cardiac injury in this review (4 cases in 179 persons, or 2.2%) is an imprecise estimate of risk in a small population (95% CI, 0.1%-4.4%). While this rate may seem higher than expected within the general population after COVID-19 vaccination, it is lower than the estimated lifetime risk of recurrent myocarditis from any cause.6,12
Discussion
To our knowledge, this is the first report describing cardiac outcomes after COVID-19 vaccination among a cohort of individuals with prior history of VAMP. Four cases of COVID-19 VAMP were identified among 179 patients with previous VAMP. All cases had experienced VAMP after the smallpox vaccine several years earlier, with complete resolution of symptoms. Three cases presented with recurrent VAMP after their second dose of an mRNA COVID-19 vaccine, and one after an mRNA booster dose. All fully recovered over the course of several months.
Myocarditis is a heterogeneous inflammatory injury with diverse, sometimes idiopathic, etiologies.13 In contrast to infection-related cardiac injury, prior reports of vaccine-associated myocarditis have suggested a hypersensitivity reaction characterized by patchy eosinophilic infiltrates, a benign clinical course, and good prognosis.2,3
There are several common features between VAMP after smallpox and COVID-19 vaccination. Cases occur predominantly in young men. The onset of symptoms after smallpox vaccine (mean, 10 days) and after mRNA COVID-19 vaccine (mean, 3 days) appears to correspond to the timing of peak postvaccination pro-inflammatory cytokine elevation.14 While all VAMP cases are serious events, the majority of patients appear to have a relatively benign clinical course with rapid and full recovery.13
Patients who have experienced an inflammatory cardiac injury may be at higher risk for recurrence, but quantifying risk of this rare phenomenon is challenging. Cases of VAMP after the COVID-19 vaccine have occasionally been reported in patients with previous cardiac injury unrelated to vaccination.15-17 The cases presented here represent the first report of recurrent VAMP following prior non-COVID-19 vaccinations.
Most patients with prior VAMP in this cohort did not experience cardiac-suggestive symptoms following COVID-19 vaccination. Among 11 patients who developed symptoms, 3 had confirmed myocarditis and 1 had confirmed pericarditis. The clinical course for these patients with recurrent VAMP was observed to be no different in severity or duration from those who experience new-onset VAMP.4 All other patients not meeting criteria for VAMP or having alternative explanations for their symptoms also had a benign clinical course. Nonetheless, of the study cohort of 179, recurrent VAMP was diagnosed in 4 of the 11 who developed cardiac-suggestive symptoms following COVID-19 vaccination. The importance of cardiac evaluation should be emphasized for any patient presenting with chest pain, dyspnea, or other cardiac-suggestive symptoms following vaccination.
Strengths and Limitations
The strength of this review of VAMP recurrence associated with COVID-19 vaccination derives from our large and unique longitudinal database of VAMP among current and prior service members. Additionally, the IHD’s ongoing enhanced vaccine AEs surveillance provides the opportunity to contact patients and review their electronic health records over an extended interval of time.
When interpreting this report’s implications, limitations inherent to any retrospective case review should be considered. The cohort of cases of prior VAMP included primarily healthy, fit, young service members; this population is not representative of the general population. The cohort included prior VAMP cases that generally occurred after smallpox vaccination. Experiences after smallpox vaccine may not apply to cardiac injury from other vaccines or etiologies. By the nature of this review, the population studied at the time of COVID-19 vaccination was somewhat older than those most likely to develop an initial bout of VAMP.2 This review was limited by information available in the electronic health records of a small number of patients. Subclinical cases of VAMP and cases without adequate clinical evaluation also could not be included.
Conclusions
Noninfectious inflammation of the heart (myocarditis, pericarditis, or myopericarditis) is a rare AE following certain vaccines, especially live replicating smallpox vaccine and mRNA COVID-19 vaccines. In this observational analysis, the majority of patients with previous VAMP successfully received a COVID-19 vaccine without recurrence. The 4 patients who were identified with recurrent VAMP following COVID-19 vaccination all recovered with supportive care. While the CDC endorses that individuals with a history of infectious myocarditis may receive COVID-19 vaccine after symptoms have resolved, there is currently insufficient safety data regarding COVID-19 vaccination of those with prior non-COVID-19 VAMP or following subsequent COVID-19 vaccination in those with prior VAMP related to COVID-19.10 For these individuals, COVID-19 vaccination is a precaution.10 Although insufficient to determine a precise level of risk, this report does provide data on which to base the CDC-recommended shared decision-making counseling of these patients. More research is needed to better define factors that increase risk for, or protection from, immune-mediated AEs following immunization, including VAMP. While benefits of vaccination have clearly outweighed risks during the COVID-19 pandemic, such research may optimize future vaccine recommendations.18
1. Decker MD, Garman PM, Hughes H, et al. Enhanced safety surveillance study of ACAM2000 smallpox vaccine among US military service members. Vaccine. 2021;39(39):5541-5547. doi:10.1016/j.vaccine.2021.08.041
2. Engler RJ, Nelson MR, Collins LC Jr, et al. A prospective study of the incidence of myocarditis/pericarditis and new onset cardiac symptoms following smallpox and influenza vaccination. PLoS One. 2015;10(3):e0118283. doi:10.1371/journal.pone.0118283
3. Faix DJ, Gordon DM, Perry LN, et al. Prospective safety surveillance study of ACAM2000 smallpox vaccine in deploying military personnel. Vaccine. 2020;38(46):7323-7330. doi:10.1016/j.vaccine.2020.09.037
4. Montgomery J, Ryan M, Engler R, et al. Myocarditis following immunization with mRNA COVID-19 vaccines in members of the US military. JAMA Cardiol. 2021;6(10):1202-1206. doi:10.1001/jamacardio.2021.2833
5. Witberg G, Barda N, Hoss S, et al. Myocarditis after Covid-19 vaccination in a large health care organization. N Engl J Med. 2021;385(23):2132-2139. doi:10.1056/NEJMoa2110737
6. Oster ME, Shay DK, Su JR, et al. Myocarditis cases reported after mRNA-based COVID-19 vaccination in the US from December 2020 to August 2021. JAMA. 2022;327(4):331-340. doi:10.1001/jama.2021.24110
7. Su JR, McNeil MM, Welsh KJ, et al. Myopericarditis after vaccination, Vaccine Adverse Event Reporting System (VAERS), 1990-2018. Vaccine. 2021;39(5):839-845. doi:10.1016/j.vaccine.2020.12.046
8. Mei R, Raschi E, Forcesi E, Diemberger I, De Ponti F, Poluzzi E. Myocarditis and pericarditis after immunization: gaining insights through the Vaccine Adverse Event Reporting System. Int J Cardiol. 2018;273:183-186. doi:10.1016/j.ijcard.2018.09.054
9. Centers for Disease Control and Prevention (CDC). Update: cardiac-related events during the civilian smallpox vaccination program—United States, 2003. MMWR Morb Mortal Wkly Rep. 2003;52(21):492-496.
10. Gargano JW, Wallace M, Hadler SC, et al. Use of mRNA COVID-19 vaccine after reports of myocarditis among vaccine recipients: update from the Advisory Committee on Immunization Practices—United States, June 2021. MMWR Morb Mortal Wkly Rep. 2021;70(27):977-982. doi:10.15585/mmwr.mm7027e2
11. Sexson Tejtel SK, Munoz FM, Al-Ammouri I, et al. Myocarditis and pericarditis: case definition and guidelines for data collection, analysis, and presentation of immunization safety data. Vaccine. 2022;40(10):1499-1511. doi:10.1016/j.vaccine.2021.11.074
12. Sagar S, Liu PP, Cooper LT Jr. Myocarditis. Lancet. 2012;379(9817):738-747. doi:10.1016/S0140-6736(11) 60648-X
13. Heymans S, Cooper LT. Myocarditis after COVID-19 mRNA vaccination: clinical observations and potential mechanisms. Nat Rev Cardiol. 2022;19(2):75-77. doi:10.1038/s41569-021-00662-w
14. Cohen JI, Hohman P, Fulton R, et al. Kinetics of serum cytokines after primary or repeat vaccination with the smallpox vaccine. J Infect Dis. 2010;201(8):1183-1191. doi:10.1086/651453
15. Minocha PK, Better D, Singh RK, Hoque T. Recurrence of acute myocarditis temporally associated with receipt of the mRNA COVID-19 vaccine in an adolescent male. J Pediatr. 2021;238:321-323. doi:10.1016/j.jpeds.2021.06.035
16. Umei TC, Kishino Y, Watanabe K, et al. Recurrence of myopericarditis following mRNA COVID-19 vaccination in a male adolescent. CJC Open. 2022;4(3):350-352. doi:10.1016/j.cjco.2021.12.002
17. Pasha MA, Isaac S, Khan Z. Recurrent myocarditis following COVID-19 infection and the mRNA vaccine. Cureus. 2022;14(7):e26650. doi:10.7759/cureus.26650
18. Block JP, Boehmer TK, Forrest CB, et al. Cardiac complications after SARS-CoV-2 infection and mRNA COVID-19 vaccination—PCORnet, United States, January 2021-January 2022. MMWR Morb Mortal Wkly Rep. 2022;71(14):517-523. Published 2022 Apr 8. doi:10.15585/mmwr.mm7114e1
Vaccinations have substantially reduced morbidity and mortality from many infectious diseases. Despite the clear value of vaccinations in public health, efforts to better understand adverse events (AEs) following immunization are important to sustain public trust and vaccine confidence. Noninfectious inflammation of the heart may manifest as myocarditis or pericarditis, or occasionally, with shared signs and symptoms of each, as myopericarditis. This is a rare AE following some immunizations. Vaccine-associated myocarditis, pericarditis, or myopericarditis (VAMP) has been most clearly associated with smallpox vaccines and mRNA COVID-19 vaccines.1-6 Although extremely rare, VAMP also has been associated with other vaccines.7,8 Limited information exists to guide shared clinical decision making on COVID-19 vaccination in persons with a history of VAMP. It is unknown whether individuals with a history of VAMP are at higher risk for developing a recurrence or experiencing a more severe outcome following COVID-19 vaccination.
Methods
As part of the collaborative public health mission with the Centers for Disease Control and Prevention (CDC) for enhanced vaccine AE surveillance, the Defense Health Agency Immunization Healthcare Division (IHD) maintains a clinical database of service members and beneficiaries referred for suspected AEs following immunizations. A review of all AEs following immunization cases in this database from January 1, 2003, through February 28, 2022, identified individuals meeting the following criteria: (a) VAMP prior to receipt of COVID-19 vaccine; (b) receipt of COVID-19 vaccine in 2021; and (c) medical documentation in available electronic health records sufficient to describe health status at least 30 days following COVID-19 vaccination.9 If medical entries suggested cardiac symptoms following a COVID-19 vaccine, additional information was sought to verify VAMP based on current published criteria.10,11 Both the initial VAMP cases and the suspected COVID-19 VAMP cases were adjudicated by a team of vaccine experts and specialists in immunology, cardiology, and preventive medicine.
This retrospective review was approved and conducted in accordance with the Walter Reed National Military Medical Center Institutional Review Board protocol #20664. All individuals with recurrent VAMP consented to share their health records and clinical details.
Results
Among 9260 cases in the IHD database, 431 met the case definition for VAMP.
Among the 179 patients included in this analysis, 171 (96%) were male. Their median age was 39 years at the time of COVID-19 vaccination.
Within 1 month of receipt of any COVID-19 vaccine, 11 individuals had documented symptoms suggesting cardiac involvement, specifically, chest pain, palpitations, or dyspnea. After cardiac evaluation, 4 patients met the criteria for VAMP after COVID-19 vaccination.10,11 Seven patients either did not meet the criteria for VAMP or had alternative causes for their symptoms.
Two men aged 49 and 50 years with a history of vaccine-associated myocarditis following smallpox vaccination (Dryvax and ACAM2000) developed myocarditis 3 days after their second dose of the Moderna vaccine. One of these patients received a Pfizer-BioNTech booster 10 months later with no recurrence of symptoms. A 55-year-old man with a history of vaccine-associated myocarditis following Dryvax vaccination developed myocarditis 2 days after his Pfizer-BioNTech booster. None of the patients who developed post-COVID-19 VAMP reported residual symptoms from their initial VAMP episode, which occurred 12 to 18 years earlier. All were hospitalized briefly for observation and had complete symptom resolution within 6 weeks.
A 25-year-old man developed pericarditis 4 days after his second Pfizer-BioNTech vaccination. His previous ACAM2000 vaccine-associated myocarditis occurred 3 years earlier, with no residual symptoms. Of note, he had a mild COVID-19 infection 78 days before the onset of his pericarditis. After the onset of his COVID-19 vaccine-associated pericarditis, he continued to experience transient bouts of chest pressure and exertional dyspnea that resolved within 7 months of onset.
The median interval between COVID-19 vaccine doses in those who developed post-COVID-19 VAMP was within the recommended mRNA vaccine dosing intervals of 3 to 4 weeks and was consistent with the median mRNA vaccine dosing intervals among the entire cohort.
Due to the small cohort size and other limitations of this study, the suggested rate of cardiac injury in this review (4 cases in 179 persons, or 2.2%) is an imprecise estimate of risk in a small population (95% CI, 0.1%-4.4%). While this rate may seem higher than expected within the general population after COVID-19 vaccination, it is lower than the estimated lifetime risk of recurrent myocarditis from any cause.6,12
Discussion
To our knowledge, this is the first report describing cardiac outcomes after COVID-19 vaccination among a cohort of individuals with prior history of VAMP. Four cases of COVID-19 VAMP were identified among 179 patients with previous VAMP. All cases had experienced VAMP after the smallpox vaccine several years earlier, with complete resolution of symptoms. Three cases presented with recurrent VAMP after their second dose of an mRNA COVID-19 vaccine, and one after an mRNA booster dose. All fully recovered over the course of several months.
Myocarditis is a heterogeneous inflammatory injury with diverse, sometimes idiopathic, etiologies.13 In contrast to infection-related cardiac injury, prior reports of vaccine-associated myocarditis have suggested a hypersensitivity reaction characterized by patchy eosinophilic infiltrates, a benign clinical course, and good prognosis.2,3
There are several common features between VAMP after smallpox and COVID-19 vaccination. Cases occur predominantly in young men. The onset of symptoms after smallpox vaccine (mean, 10 days) and after mRNA COVID-19 vaccine (mean, 3 days) appears to correspond to the timing of peak postvaccination pro-inflammatory cytokine elevation.14 While all VAMP cases are serious events, the majority of patients appear to have a relatively benign clinical course with rapid and full recovery.13
Patients who have experienced an inflammatory cardiac injury may be at higher risk for recurrence, but quantifying risk of this rare phenomenon is challenging. Cases of VAMP after the COVID-19 vaccine have occasionally been reported in patients with previous cardiac injury unrelated to vaccination.15-17 The cases presented here represent the first report of recurrent VAMP following prior non-COVID-19 vaccinations.
Most patients with prior VAMP in this cohort did not experience cardiac-suggestive symptoms following COVID-19 vaccination. Among 11 patients who developed symptoms, 3 had confirmed myocarditis and 1 had confirmed pericarditis. The clinical course for these patients with recurrent VAMP was observed to be no different in severity or duration from those who experience new-onset VAMP.4 All other patients not meeting criteria for VAMP or having alternative explanations for their symptoms also had a benign clinical course. Nonetheless, of the study cohort of 179, recurrent VAMP was diagnosed in 4 of the 11 who developed cardiac-suggestive symptoms following COVID-19 vaccination. The importance of cardiac evaluation should be emphasized for any patient presenting with chest pain, dyspnea, or other cardiac-suggestive symptoms following vaccination.
Strengths and Limitations
The strength of this review of VAMP recurrence associated with COVID-19 vaccination derives from our large and unique longitudinal database of VAMP among current and prior service members. Additionally, the IHD’s ongoing enhanced vaccine AEs surveillance provides the opportunity to contact patients and review their electronic health records over an extended interval of time.
When interpreting this report’s implications, limitations inherent to any retrospective case review should be considered. The cohort of cases of prior VAMP included primarily healthy, fit, young service members; this population is not representative of the general population. The cohort included prior VAMP cases that generally occurred after smallpox vaccination. Experiences after smallpox vaccine may not apply to cardiac injury from other vaccines or etiologies. By the nature of this review, the population studied at the time of COVID-19 vaccination was somewhat older than those most likely to develop an initial bout of VAMP.2 This review was limited by information available in the electronic health records of a small number of patients. Subclinical cases of VAMP and cases without adequate clinical evaluation also could not be included.
Conclusions
Noninfectious inflammation of the heart (myocarditis, pericarditis, or myopericarditis) is a rare AE following certain vaccines, especially live replicating smallpox vaccine and mRNA COVID-19 vaccines. In this observational analysis, the majority of patients with previous VAMP successfully received a COVID-19 vaccine without recurrence. The 4 patients who were identified with recurrent VAMP following COVID-19 vaccination all recovered with supportive care. While the CDC endorses that individuals with a history of infectious myocarditis may receive COVID-19 vaccine after symptoms have resolved, there is currently insufficient safety data regarding COVID-19 vaccination of those with prior non-COVID-19 VAMP or following subsequent COVID-19 vaccination in those with prior VAMP related to COVID-19.10 For these individuals, COVID-19 vaccination is a precaution.10 Although insufficient to determine a precise level of risk, this report does provide data on which to base the CDC-recommended shared decision-making counseling of these patients. More research is needed to better define factors that increase risk for, or protection from, immune-mediated AEs following immunization, including VAMP. While benefits of vaccination have clearly outweighed risks during the COVID-19 pandemic, such research may optimize future vaccine recommendations.18
Vaccinations have substantially reduced morbidity and mortality from many infectious diseases. Despite the clear value of vaccinations in public health, efforts to better understand adverse events (AEs) following immunization are important to sustain public trust and vaccine confidence. Noninfectious inflammation of the heart may manifest as myocarditis or pericarditis, or occasionally, with shared signs and symptoms of each, as myopericarditis. This is a rare AE following some immunizations. Vaccine-associated myocarditis, pericarditis, or myopericarditis (VAMP) has been most clearly associated with smallpox vaccines and mRNA COVID-19 vaccines.1-6 Although extremely rare, VAMP also has been associated with other vaccines.7,8 Limited information exists to guide shared clinical decision making on COVID-19 vaccination in persons with a history of VAMP. It is unknown whether individuals with a history of VAMP are at higher risk for developing a recurrence or experiencing a more severe outcome following COVID-19 vaccination.
Methods
As part of the collaborative public health mission with the Centers for Disease Control and Prevention (CDC) for enhanced vaccine AE surveillance, the Defense Health Agency Immunization Healthcare Division (IHD) maintains a clinical database of service members and beneficiaries referred for suspected AEs following immunizations. A review of all AEs following immunization cases in this database from January 1, 2003, through February 28, 2022, identified individuals meeting the following criteria: (a) VAMP prior to receipt of COVID-19 vaccine; (b) receipt of COVID-19 vaccine in 2021; and (c) medical documentation in available electronic health records sufficient to describe health status at least 30 days following COVID-19 vaccination.9 If medical entries suggested cardiac symptoms following a COVID-19 vaccine, additional information was sought to verify VAMP based on current published criteria.10,11 Both the initial VAMP cases and the suspected COVID-19 VAMP cases were adjudicated by a team of vaccine experts and specialists in immunology, cardiology, and preventive medicine.
This retrospective review was approved and conducted in accordance with the Walter Reed National Military Medical Center Institutional Review Board protocol #20664. All individuals with recurrent VAMP consented to share their health records and clinical details.
Results
Among 9260 cases in the IHD database, 431 met the case definition for VAMP.
Among the 179 patients included in this analysis, 171 (96%) were male. Their median age was 39 years at the time of COVID-19 vaccination.
Within 1 month of receipt of any COVID-19 vaccine, 11 individuals had documented symptoms suggesting cardiac involvement, specifically, chest pain, palpitations, or dyspnea. After cardiac evaluation, 4 patients met the criteria for VAMP after COVID-19 vaccination.10,11 Seven patients either did not meet the criteria for VAMP or had alternative causes for their symptoms.
Two men aged 49 and 50 years with a history of vaccine-associated myocarditis following smallpox vaccination (Dryvax and ACAM2000) developed myocarditis 3 days after their second dose of the Moderna vaccine. One of these patients received a Pfizer-BioNTech booster 10 months later with no recurrence of symptoms. A 55-year-old man with a history of vaccine-associated myocarditis following Dryvax vaccination developed myocarditis 2 days after his Pfizer-BioNTech booster. None of the patients who developed post-COVID-19 VAMP reported residual symptoms from their initial VAMP episode, which occurred 12 to 18 years earlier. All were hospitalized briefly for observation and had complete symptom resolution within 6 weeks.
A 25-year-old man developed pericarditis 4 days after his second Pfizer-BioNTech vaccination. His previous ACAM2000 vaccine-associated myocarditis occurred 3 years earlier, with no residual symptoms. Of note, he had a mild COVID-19 infection 78 days before the onset of his pericarditis. After the onset of his COVID-19 vaccine-associated pericarditis, he continued to experience transient bouts of chest pressure and exertional dyspnea that resolved within 7 months of onset.
The median interval between COVID-19 vaccine doses in those who developed post-COVID-19 VAMP was within the recommended mRNA vaccine dosing intervals of 3 to 4 weeks and was consistent with the median mRNA vaccine dosing intervals among the entire cohort.
Due to the small cohort size and other limitations of this study, the suggested rate of cardiac injury in this review (4 cases in 179 persons, or 2.2%) is an imprecise estimate of risk in a small population (95% CI, 0.1%-4.4%). While this rate may seem higher than expected within the general population after COVID-19 vaccination, it is lower than the estimated lifetime risk of recurrent myocarditis from any cause.6,12
Discussion
To our knowledge, this is the first report describing cardiac outcomes after COVID-19 vaccination among a cohort of individuals with prior history of VAMP. Four cases of COVID-19 VAMP were identified among 179 patients with previous VAMP. All cases had experienced VAMP after the smallpox vaccine several years earlier, with complete resolution of symptoms. Three cases presented with recurrent VAMP after their second dose of an mRNA COVID-19 vaccine, and one after an mRNA booster dose. All fully recovered over the course of several months.
Myocarditis is a heterogeneous inflammatory injury with diverse, sometimes idiopathic, etiologies.13 In contrast to infection-related cardiac injury, prior reports of vaccine-associated myocarditis have suggested a hypersensitivity reaction characterized by patchy eosinophilic infiltrates, a benign clinical course, and good prognosis.2,3
There are several common features between VAMP after smallpox and COVID-19 vaccination. Cases occur predominantly in young men. The onset of symptoms after smallpox vaccine (mean, 10 days) and after mRNA COVID-19 vaccine (mean, 3 days) appears to correspond to the timing of peak postvaccination pro-inflammatory cytokine elevation.14 While all VAMP cases are serious events, the majority of patients appear to have a relatively benign clinical course with rapid and full recovery.13
Patients who have experienced an inflammatory cardiac injury may be at higher risk for recurrence, but quantifying risk of this rare phenomenon is challenging. Cases of VAMP after the COVID-19 vaccine have occasionally been reported in patients with previous cardiac injury unrelated to vaccination.15-17 The cases presented here represent the first report of recurrent VAMP following prior non-COVID-19 vaccinations.
Most patients with prior VAMP in this cohort did not experience cardiac-suggestive symptoms following COVID-19 vaccination. Among 11 patients who developed symptoms, 3 had confirmed myocarditis and 1 had confirmed pericarditis. The clinical course for these patients with recurrent VAMP was observed to be no different in severity or duration from those who experience new-onset VAMP.4 All other patients not meeting criteria for VAMP or having alternative explanations for their symptoms also had a benign clinical course. Nonetheless, of the study cohort of 179, recurrent VAMP was diagnosed in 4 of the 11 who developed cardiac-suggestive symptoms following COVID-19 vaccination. The importance of cardiac evaluation should be emphasized for any patient presenting with chest pain, dyspnea, or other cardiac-suggestive symptoms following vaccination.
Strengths and Limitations
The strength of this review of VAMP recurrence associated with COVID-19 vaccination derives from our large and unique longitudinal database of VAMP among current and prior service members. Additionally, the IHD’s ongoing enhanced vaccine AEs surveillance provides the opportunity to contact patients and review their electronic health records over an extended interval of time.
When interpreting this report’s implications, limitations inherent to any retrospective case review should be considered. The cohort of cases of prior VAMP included primarily healthy, fit, young service members; this population is not representative of the general population. The cohort included prior VAMP cases that generally occurred after smallpox vaccination. Experiences after smallpox vaccine may not apply to cardiac injury from other vaccines or etiologies. By the nature of this review, the population studied at the time of COVID-19 vaccination was somewhat older than those most likely to develop an initial bout of VAMP.2 This review was limited by information available in the electronic health records of a small number of patients. Subclinical cases of VAMP and cases without adequate clinical evaluation also could not be included.
Conclusions
Noninfectious inflammation of the heart (myocarditis, pericarditis, or myopericarditis) is a rare AE following certain vaccines, especially live replicating smallpox vaccine and mRNA COVID-19 vaccines. In this observational analysis, the majority of patients with previous VAMP successfully received a COVID-19 vaccine without recurrence. The 4 patients who were identified with recurrent VAMP following COVID-19 vaccination all recovered with supportive care. While the CDC endorses that individuals with a history of infectious myocarditis may receive COVID-19 vaccine after symptoms have resolved, there is currently insufficient safety data regarding COVID-19 vaccination of those with prior non-COVID-19 VAMP or following subsequent COVID-19 vaccination in those with prior VAMP related to COVID-19.10 For these individuals, COVID-19 vaccination is a precaution.10 Although insufficient to determine a precise level of risk, this report does provide data on which to base the CDC-recommended shared decision-making counseling of these patients. More research is needed to better define factors that increase risk for, or protection from, immune-mediated AEs following immunization, including VAMP. While benefits of vaccination have clearly outweighed risks during the COVID-19 pandemic, such research may optimize future vaccine recommendations.18
1. Decker MD, Garman PM, Hughes H, et al. Enhanced safety surveillance study of ACAM2000 smallpox vaccine among US military service members. Vaccine. 2021;39(39):5541-5547. doi:10.1016/j.vaccine.2021.08.041
2. Engler RJ, Nelson MR, Collins LC Jr, et al. A prospective study of the incidence of myocarditis/pericarditis and new onset cardiac symptoms following smallpox and influenza vaccination. PLoS One. 2015;10(3):e0118283. doi:10.1371/journal.pone.0118283
3. Faix DJ, Gordon DM, Perry LN, et al. Prospective safety surveillance study of ACAM2000 smallpox vaccine in deploying military personnel. Vaccine. 2020;38(46):7323-7330. doi:10.1016/j.vaccine.2020.09.037
4. Montgomery J, Ryan M, Engler R, et al. Myocarditis following immunization with mRNA COVID-19 vaccines in members of the US military. JAMA Cardiol. 2021;6(10):1202-1206. doi:10.1001/jamacardio.2021.2833
5. Witberg G, Barda N, Hoss S, et al. Myocarditis after Covid-19 vaccination in a large health care organization. N Engl J Med. 2021;385(23):2132-2139. doi:10.1056/NEJMoa2110737
6. Oster ME, Shay DK, Su JR, et al. Myocarditis cases reported after mRNA-based COVID-19 vaccination in the US from December 2020 to August 2021. JAMA. 2022;327(4):331-340. doi:10.1001/jama.2021.24110
7. Su JR, McNeil MM, Welsh KJ, et al. Myopericarditis after vaccination, Vaccine Adverse Event Reporting System (VAERS), 1990-2018. Vaccine. 2021;39(5):839-845. doi:10.1016/j.vaccine.2020.12.046
8. Mei R, Raschi E, Forcesi E, Diemberger I, De Ponti F, Poluzzi E. Myocarditis and pericarditis after immunization: gaining insights through the Vaccine Adverse Event Reporting System. Int J Cardiol. 2018;273:183-186. doi:10.1016/j.ijcard.2018.09.054
9. Centers for Disease Control and Prevention (CDC). Update: cardiac-related events during the civilian smallpox vaccination program—United States, 2003. MMWR Morb Mortal Wkly Rep. 2003;52(21):492-496.
10. Gargano JW, Wallace M, Hadler SC, et al. Use of mRNA COVID-19 vaccine after reports of myocarditis among vaccine recipients: update from the Advisory Committee on Immunization Practices—United States, June 2021. MMWR Morb Mortal Wkly Rep. 2021;70(27):977-982. doi:10.15585/mmwr.mm7027e2
11. Sexson Tejtel SK, Munoz FM, Al-Ammouri I, et al. Myocarditis and pericarditis: case definition and guidelines for data collection, analysis, and presentation of immunization safety data. Vaccine. 2022;40(10):1499-1511. doi:10.1016/j.vaccine.2021.11.074
12. Sagar S, Liu PP, Cooper LT Jr. Myocarditis. Lancet. 2012;379(9817):738-747. doi:10.1016/S0140-6736(11) 60648-X
13. Heymans S, Cooper LT. Myocarditis after COVID-19 mRNA vaccination: clinical observations and potential mechanisms. Nat Rev Cardiol. 2022;19(2):75-77. doi:10.1038/s41569-021-00662-w
14. Cohen JI, Hohman P, Fulton R, et al. Kinetics of serum cytokines after primary or repeat vaccination with the smallpox vaccine. J Infect Dis. 2010;201(8):1183-1191. doi:10.1086/651453
15. Minocha PK, Better D, Singh RK, Hoque T. Recurrence of acute myocarditis temporally associated with receipt of the mRNA COVID-19 vaccine in an adolescent male. J Pediatr. 2021;238:321-323. doi:10.1016/j.jpeds.2021.06.035
16. Umei TC, Kishino Y, Watanabe K, et al. Recurrence of myopericarditis following mRNA COVID-19 vaccination in a male adolescent. CJC Open. 2022;4(3):350-352. doi:10.1016/j.cjco.2021.12.002
17. Pasha MA, Isaac S, Khan Z. Recurrent myocarditis following COVID-19 infection and the mRNA vaccine. Cureus. 2022;14(7):e26650. doi:10.7759/cureus.26650
18. Block JP, Boehmer TK, Forrest CB, et al. Cardiac complications after SARS-CoV-2 infection and mRNA COVID-19 vaccination—PCORnet, United States, January 2021-January 2022. MMWR Morb Mortal Wkly Rep. 2022;71(14):517-523. Published 2022 Apr 8. doi:10.15585/mmwr.mm7114e1
1. Decker MD, Garman PM, Hughes H, et al. Enhanced safety surveillance study of ACAM2000 smallpox vaccine among US military service members. Vaccine. 2021;39(39):5541-5547. doi:10.1016/j.vaccine.2021.08.041
2. Engler RJ, Nelson MR, Collins LC Jr, et al. A prospective study of the incidence of myocarditis/pericarditis and new onset cardiac symptoms following smallpox and influenza vaccination. PLoS One. 2015;10(3):e0118283. doi:10.1371/journal.pone.0118283
3. Faix DJ, Gordon DM, Perry LN, et al. Prospective safety surveillance study of ACAM2000 smallpox vaccine in deploying military personnel. Vaccine. 2020;38(46):7323-7330. doi:10.1016/j.vaccine.2020.09.037
4. Montgomery J, Ryan M, Engler R, et al. Myocarditis following immunization with mRNA COVID-19 vaccines in members of the US military. JAMA Cardiol. 2021;6(10):1202-1206. doi:10.1001/jamacardio.2021.2833
5. Witberg G, Barda N, Hoss S, et al. Myocarditis after Covid-19 vaccination in a large health care organization. N Engl J Med. 2021;385(23):2132-2139. doi:10.1056/NEJMoa2110737
6. Oster ME, Shay DK, Su JR, et al. Myocarditis cases reported after mRNA-based COVID-19 vaccination in the US from December 2020 to August 2021. JAMA. 2022;327(4):331-340. doi:10.1001/jama.2021.24110
7. Su JR, McNeil MM, Welsh KJ, et al. Myopericarditis after vaccination, Vaccine Adverse Event Reporting System (VAERS), 1990-2018. Vaccine. 2021;39(5):839-845. doi:10.1016/j.vaccine.2020.12.046
8. Mei R, Raschi E, Forcesi E, Diemberger I, De Ponti F, Poluzzi E. Myocarditis and pericarditis after immunization: gaining insights through the Vaccine Adverse Event Reporting System. Int J Cardiol. 2018;273:183-186. doi:10.1016/j.ijcard.2018.09.054
9. Centers for Disease Control and Prevention (CDC). Update: cardiac-related events during the civilian smallpox vaccination program—United States, 2003. MMWR Morb Mortal Wkly Rep. 2003;52(21):492-496.
10. Gargano JW, Wallace M, Hadler SC, et al. Use of mRNA COVID-19 vaccine after reports of myocarditis among vaccine recipients: update from the Advisory Committee on Immunization Practices—United States, June 2021. MMWR Morb Mortal Wkly Rep. 2021;70(27):977-982. doi:10.15585/mmwr.mm7027e2
11. Sexson Tejtel SK, Munoz FM, Al-Ammouri I, et al. Myocarditis and pericarditis: case definition and guidelines for data collection, analysis, and presentation of immunization safety data. Vaccine. 2022;40(10):1499-1511. doi:10.1016/j.vaccine.2021.11.074
12. Sagar S, Liu PP, Cooper LT Jr. Myocarditis. Lancet. 2012;379(9817):738-747. doi:10.1016/S0140-6736(11) 60648-X
13. Heymans S, Cooper LT. Myocarditis after COVID-19 mRNA vaccination: clinical observations and potential mechanisms. Nat Rev Cardiol. 2022;19(2):75-77. doi:10.1038/s41569-021-00662-w
14. Cohen JI, Hohman P, Fulton R, et al. Kinetics of serum cytokines after primary or repeat vaccination with the smallpox vaccine. J Infect Dis. 2010;201(8):1183-1191. doi:10.1086/651453
15. Minocha PK, Better D, Singh RK, Hoque T. Recurrence of acute myocarditis temporally associated with receipt of the mRNA COVID-19 vaccine in an adolescent male. J Pediatr. 2021;238:321-323. doi:10.1016/j.jpeds.2021.06.035
16. Umei TC, Kishino Y, Watanabe K, et al. Recurrence of myopericarditis following mRNA COVID-19 vaccination in a male adolescent. CJC Open. 2022;4(3):350-352. doi:10.1016/j.cjco.2021.12.002
17. Pasha MA, Isaac S, Khan Z. Recurrent myocarditis following COVID-19 infection and the mRNA vaccine. Cureus. 2022;14(7):e26650. doi:10.7759/cureus.26650
18. Block JP, Boehmer TK, Forrest CB, et al. Cardiac complications after SARS-CoV-2 infection and mRNA COVID-19 vaccination—PCORnet, United States, January 2021-January 2022. MMWR Morb Mortal Wkly Rep. 2022;71(14):517-523. Published 2022 Apr 8. doi:10.15585/mmwr.mm7114e1
How Well Does the Third Dose of COVID-19 Vaccine Work?
How effective are the COVID-19 vaccines, third time around? Researchers compared 2 large groups of veterans to find out how well a third dose protected against documented infection, symptomatic COVID-19, and COVID-19–related hospitalization, intensive care unit (ICU) admission, and death.
The research, published in Nature, used electronic health records of 65,196 veterans who received BNT162b2 (Pfizer-BioNTech) and 65,196 who received mRNA-1273 (Moderna). They chose to study the 16 weeks between October 20, 2021 and February 8, 2022, which included both Delta- and Omicron-variant waves.
During the follow-up (median, 77 days), 2994 COVID-19 infections were documented, of which 200 were detected as symptomatic, 194 required hospitalization, and 52 required ICU admission. Twenty-two patients died.
In a previous head-to-head trial comparing breakthrough COVID-19 outcomes after the first doses of the 2 vaccines (given when the Alpha and Delta variants were predominant), the researchers had found a low risk of documented infection and severe outcomes, but lower for the Moderna vaccine. They note that few head-to-head comparisons have been made of third-dose effectiveness.
As expected, in this trial, the researchers found a “nearly identical” pattern for the risk of the 2 vaccine groups. Although the risks for all of the measured outcomes over 16 weeks were low for both vaccines ≤ 4% for documented infection and < 0.03% for death in each group—those veterans who received the Pfizer-BioNTech vaccine had an excess of 45 documented infections and 11 hospitalizations per 10,000 persons, compared with the Moderna group. The Pfizer-BioNTech group also had a higher risk of documented infection over 9 weeks of follow-up, during which an Omicron-variant predominated.
Given the high effectiveness of a third dose of both vaccines, either vaccine is strongly recommended, the researchers conclude. They point to “evidence of clear and comparable benefits” for the most severe outcomes: The difference in estimated 16-week risk of death between the 2 groups was two-thousandths of 1 %.
They add that, while the differences in estimated risk for less severe outcomes between the 2 groups were small on the absolute scale, they may be meaningful when considering the population scale at which these vaccines are deployed.
How effective are the COVID-19 vaccines, third time around? Researchers compared 2 large groups of veterans to find out how well a third dose protected against documented infection, symptomatic COVID-19, and COVID-19–related hospitalization, intensive care unit (ICU) admission, and death.
The research, published in Nature, used electronic health records of 65,196 veterans who received BNT162b2 (Pfizer-BioNTech) and 65,196 who received mRNA-1273 (Moderna). They chose to study the 16 weeks between October 20, 2021 and February 8, 2022, which included both Delta- and Omicron-variant waves.
During the follow-up (median, 77 days), 2994 COVID-19 infections were documented, of which 200 were detected as symptomatic, 194 required hospitalization, and 52 required ICU admission. Twenty-two patients died.
In a previous head-to-head trial comparing breakthrough COVID-19 outcomes after the first doses of the 2 vaccines (given when the Alpha and Delta variants were predominant), the researchers had found a low risk of documented infection and severe outcomes, but lower for the Moderna vaccine. They note that few head-to-head comparisons have been made of third-dose effectiveness.
As expected, in this trial, the researchers found a “nearly identical” pattern for the risk of the 2 vaccine groups. Although the risks for all of the measured outcomes over 16 weeks were low for both vaccines ≤ 4% for documented infection and < 0.03% for death in each group—those veterans who received the Pfizer-BioNTech vaccine had an excess of 45 documented infections and 11 hospitalizations per 10,000 persons, compared with the Moderna group. The Pfizer-BioNTech group also had a higher risk of documented infection over 9 weeks of follow-up, during which an Omicron-variant predominated.
Given the high effectiveness of a third dose of both vaccines, either vaccine is strongly recommended, the researchers conclude. They point to “evidence of clear and comparable benefits” for the most severe outcomes: The difference in estimated 16-week risk of death between the 2 groups was two-thousandths of 1 %.
They add that, while the differences in estimated risk for less severe outcomes between the 2 groups were small on the absolute scale, they may be meaningful when considering the population scale at which these vaccines are deployed.
How effective are the COVID-19 vaccines, third time around? Researchers compared 2 large groups of veterans to find out how well a third dose protected against documented infection, symptomatic COVID-19, and COVID-19–related hospitalization, intensive care unit (ICU) admission, and death.
The research, published in Nature, used electronic health records of 65,196 veterans who received BNT162b2 (Pfizer-BioNTech) and 65,196 who received mRNA-1273 (Moderna). They chose to study the 16 weeks between October 20, 2021 and February 8, 2022, which included both Delta- and Omicron-variant waves.
During the follow-up (median, 77 days), 2994 COVID-19 infections were documented, of which 200 were detected as symptomatic, 194 required hospitalization, and 52 required ICU admission. Twenty-two patients died.
In a previous head-to-head trial comparing breakthrough COVID-19 outcomes after the first doses of the 2 vaccines (given when the Alpha and Delta variants were predominant), the researchers had found a low risk of documented infection and severe outcomes, but lower for the Moderna vaccine. They note that few head-to-head comparisons have been made of third-dose effectiveness.
As expected, in this trial, the researchers found a “nearly identical” pattern for the risk of the 2 vaccine groups. Although the risks for all of the measured outcomes over 16 weeks were low for both vaccines ≤ 4% for documented infection and < 0.03% for death in each group—those veterans who received the Pfizer-BioNTech vaccine had an excess of 45 documented infections and 11 hospitalizations per 10,000 persons, compared with the Moderna group. The Pfizer-BioNTech group also had a higher risk of documented infection over 9 weeks of follow-up, during which an Omicron-variant predominated.
Given the high effectiveness of a third dose of both vaccines, either vaccine is strongly recommended, the researchers conclude. They point to “evidence of clear and comparable benefits” for the most severe outcomes: The difference in estimated 16-week risk of death between the 2 groups was two-thousandths of 1 %.
They add that, while the differences in estimated risk for less severe outcomes between the 2 groups were small on the absolute scale, they may be meaningful when considering the population scale at which these vaccines are deployed.
COVID leading cause of death among law enforcement for third year
A new report says 70 officers died of COVID-related causes after getting the virus while on the job. The number is down dramatically from 2021, when 405 officer deaths were attributed to COVID.
The annual count was published Wednesday by the National Law Enforcement Officers Memorial Fund.
In total, 226 officers died in the line of duty in 2022, which is a decrease of 61% from 2021.
The decrease “is almost entirely related to the significant reduction in COVID-19 deaths,” the report stated. The authors said the decline was likely due to “reduced infection rates and the broad availability and use of vaccinations.”
Reported deaths included federal, state, tribal, and local law enforcement officers.
Firearms-related fatalities were the second-leading cause of death among officers, with 64 in 2022. That count sustains a 21% increase seen in 2021, up from the decade-long average of 53 firearms-related deaths annually from 2010 to 2020.
Traffic-related causes ranked third for cause of death in 2022, accounting for 56 deaths.
“While overall line-of-duty deaths are trending down, the continuing trend of greater-than-average firearms-related deaths continues to be a serious concern,” Marcia Ferranto, the organization’s chief executive officer, said in a news release. “Using and reporting on this data allows us to highlight the continuing cost of maintaining our democracy, regrettably measured in the lives of the many law enforcement professionals who sacrifice everything fulfilling their promise to serve and protect.”
A version of this article first appeared on WebMD.com.
A new report says 70 officers died of COVID-related causes after getting the virus while on the job. The number is down dramatically from 2021, when 405 officer deaths were attributed to COVID.
The annual count was published Wednesday by the National Law Enforcement Officers Memorial Fund.
In total, 226 officers died in the line of duty in 2022, which is a decrease of 61% from 2021.
The decrease “is almost entirely related to the significant reduction in COVID-19 deaths,” the report stated. The authors said the decline was likely due to “reduced infection rates and the broad availability and use of vaccinations.”
Reported deaths included federal, state, tribal, and local law enforcement officers.
Firearms-related fatalities were the second-leading cause of death among officers, with 64 in 2022. That count sustains a 21% increase seen in 2021, up from the decade-long average of 53 firearms-related deaths annually from 2010 to 2020.
Traffic-related causes ranked third for cause of death in 2022, accounting for 56 deaths.
“While overall line-of-duty deaths are trending down, the continuing trend of greater-than-average firearms-related deaths continues to be a serious concern,” Marcia Ferranto, the organization’s chief executive officer, said in a news release. “Using and reporting on this data allows us to highlight the continuing cost of maintaining our democracy, regrettably measured in the lives of the many law enforcement professionals who sacrifice everything fulfilling their promise to serve and protect.”
A version of this article first appeared on WebMD.com.
A new report says 70 officers died of COVID-related causes after getting the virus while on the job. The number is down dramatically from 2021, when 405 officer deaths were attributed to COVID.
The annual count was published Wednesday by the National Law Enforcement Officers Memorial Fund.
In total, 226 officers died in the line of duty in 2022, which is a decrease of 61% from 2021.
The decrease “is almost entirely related to the significant reduction in COVID-19 deaths,” the report stated. The authors said the decline was likely due to “reduced infection rates and the broad availability and use of vaccinations.”
Reported deaths included federal, state, tribal, and local law enforcement officers.
Firearms-related fatalities were the second-leading cause of death among officers, with 64 in 2022. That count sustains a 21% increase seen in 2021, up from the decade-long average of 53 firearms-related deaths annually from 2010 to 2020.
Traffic-related causes ranked third for cause of death in 2022, accounting for 56 deaths.
“While overall line-of-duty deaths are trending down, the continuing trend of greater-than-average firearms-related deaths continues to be a serious concern,” Marcia Ferranto, the organization’s chief executive officer, said in a news release. “Using and reporting on this data allows us to highlight the continuing cost of maintaining our democracy, regrettably measured in the lives of the many law enforcement professionals who sacrifice everything fulfilling their promise to serve and protect.”
A version of this article first appeared on WebMD.com.
FDA OKs Tdap shot in pregnancy to protect newborns from pertussis
The Food and Drug Administration has approved another Tdap vaccine option for use during pregnancy to protect newborns from whooping cough.
The agency on Jan. 9 licensed Adacel (Sanofi Pasteur) for immunization during the third trimester to prevent pertussis in infants younger than 2 months old.
The FDA in October approved a different Tdap vaccine, Boostrix (GlaxoSmithKline), for this indication. Boostrix was the first vaccine specifically approved to prevent a disease in newborns whose mothers receive the vaccine while pregnant.
The Centers for Disease Control and Prevention recommend that women receive a dose of Tdap vaccine during each pregnancy, preferably during gestational weeks 27-36 – and ideally toward the earlier end of that window – to help protect babies from whooping cough, the respiratory tract infection caused by Bordetella pertussis.
Providing a Tdap vaccine – tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis vaccine, adsorbed – in the third trimester confers passive immunity to the baby, according to the CDC. It also reduces the likelihood that the mother will get pertussis and pass it on to the infant.
One study found that providing Tdap vaccination during gestational weeks 27-36 was 85% more effective at preventing pertussis in infants younger than 2 months old, compared with providing Tdap vaccination to mothers in the hospital postpartum.
“On average, about 1,000 infants are hospitalized and typically between 5 and 15 infants die each year in the United States due to pertussis,” according to a CDC reference page. “Most of these deaths are among infants who are too young to be protected by the childhood pertussis vaccine series that starts when infants are 2 months old.”
The Food and Drug Administration has approved another Tdap vaccine option for use during pregnancy to protect newborns from whooping cough.
The agency on Jan. 9 licensed Adacel (Sanofi Pasteur) for immunization during the third trimester to prevent pertussis in infants younger than 2 months old.
The FDA in October approved a different Tdap vaccine, Boostrix (GlaxoSmithKline), for this indication. Boostrix was the first vaccine specifically approved to prevent a disease in newborns whose mothers receive the vaccine while pregnant.
The Centers for Disease Control and Prevention recommend that women receive a dose of Tdap vaccine during each pregnancy, preferably during gestational weeks 27-36 – and ideally toward the earlier end of that window – to help protect babies from whooping cough, the respiratory tract infection caused by Bordetella pertussis.
Providing a Tdap vaccine – tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis vaccine, adsorbed – in the third trimester confers passive immunity to the baby, according to the CDC. It also reduces the likelihood that the mother will get pertussis and pass it on to the infant.
One study found that providing Tdap vaccination during gestational weeks 27-36 was 85% more effective at preventing pertussis in infants younger than 2 months old, compared with providing Tdap vaccination to mothers in the hospital postpartum.
“On average, about 1,000 infants are hospitalized and typically between 5 and 15 infants die each year in the United States due to pertussis,” according to a CDC reference page. “Most of these deaths are among infants who are too young to be protected by the childhood pertussis vaccine series that starts when infants are 2 months old.”
The Food and Drug Administration has approved another Tdap vaccine option for use during pregnancy to protect newborns from whooping cough.
The agency on Jan. 9 licensed Adacel (Sanofi Pasteur) for immunization during the third trimester to prevent pertussis in infants younger than 2 months old.
The FDA in October approved a different Tdap vaccine, Boostrix (GlaxoSmithKline), for this indication. Boostrix was the first vaccine specifically approved to prevent a disease in newborns whose mothers receive the vaccine while pregnant.
The Centers for Disease Control and Prevention recommend that women receive a dose of Tdap vaccine during each pregnancy, preferably during gestational weeks 27-36 – and ideally toward the earlier end of that window – to help protect babies from whooping cough, the respiratory tract infection caused by Bordetella pertussis.
Providing a Tdap vaccine – tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis vaccine, adsorbed – in the third trimester confers passive immunity to the baby, according to the CDC. It also reduces the likelihood that the mother will get pertussis and pass it on to the infant.
One study found that providing Tdap vaccination during gestational weeks 27-36 was 85% more effective at preventing pertussis in infants younger than 2 months old, compared with providing Tdap vaccination to mothers in the hospital postpartum.
“On average, about 1,000 infants are hospitalized and typically between 5 and 15 infants die each year in the United States due to pertussis,” according to a CDC reference page. “Most of these deaths are among infants who are too young to be protected by the childhood pertussis vaccine series that starts when infants are 2 months old.”
Spikes out: A COVID mystery
Welcome to Impact Factor, your weekly dose of commentary on a new medical study. I’m Dr. F. Perry Wilson of the Yale School of Medicine.
To date, it has been a mystery, like “Glass Onion.” And in the spirit of all the great mysteries, to get to the bottom of this, we’ll need to round up the usual suspects.
Appearing in Circulation, a new study does a great job of systematically evaluating multiple hypotheses linking vaccination to myocarditis, and eliminating them, Poirot-style, one by one until only one remains. We’ll get there.
But first, let’s review the suspects. Why do the mRNA vaccines cause myocarditis in a small subset of people?
There are a few leading candidates.
Number one: antibody responses. There are two flavors here. The quantitative hypothesis suggests that some people simply generate too many antibodies to the vaccine, leading to increased inflammation and heart damage.
The qualitative hypothesis suggests that maybe it’s the nature of the antibodies generated rather than the amount; they might cross-react with some protein on the surface of heart cells for instance.
Or maybe it is driven by T-cell responses, which, of course, are independent of antibody levels.
There’s the idea that myocarditis is due to excessive cytokine release – sort of like what we see in the multisystem inflammatory syndrome in children.
Or it could be due to the viral antigens themselves – the spike protein the mRNA codes for that is generated after vaccination.
To tease all these possibilities apart, researchers led by Lael Yonker at Mass General performed a case-control study. Sixteen children with postvaccine myocarditis were matched by age to 45 control children who had been vaccinated without complications.
The matching was OK, but as you can see here, there were more boys in the myocarditis group, and the time from vaccination was a bit shorter in that group as well. We’ll keep that in mind as we go through the results.
OK, let’s start eliminating suspects.
First, quantitative antibodies. Seems unlikely. Absolute antibody titers were really no different in the myocarditis vs. the control group.
What about the quality of the antibodies? Would the kids with myocarditis have more self-recognizing antibodies present? It doesn’t appear so. Autoantibody levels were similar in the two groups.
Take antibodies off the list.
T-cell responses come next, and, again, no major differences here, save for one specific T-cell subtype that was moderately elevated in the myocarditis group. Not what I would call a smoking gun, frankly.
Cytokines give us a bit more to chew on. Levels of interleukin (IL)-8, IL-6, tumor necrosis factor (TNF)-alpha, and IL-10 were all substantially higher in the kids with myocarditis.
But the thing about cytokines is that they are not particularly specific. OK, kids with myocarditis have more systemic inflammation than kids without; that’s not really surprising. It still leaves us with the question of what is causing all this inflammation? Who is the arch-villain? The kingpin? The don?
It’s the analyses of antigens – the protein products of vaccination – that may hold the key here.
In 12 out of 16 kids with myocarditis, the researchers were able to measure free spike protein in the blood – that is to say spike protein, not bound by antispike antibodies.
These free spikes were present in – wait for it – zero of the 45 control patients. That makes spike protein itself our prime suspect. J’accuse free spike protein!
Of course, all good detectives need to wrap up the case with a good story: How was it all done?
And here’s where we could use Agatha Christie’s help. How could this all work? The vaccine gets injected; mRNA is taken up into cells, where spike protein is generated and released, generating antibody and T-cell responses all the while. Those responses rapidly clear that spike protein from the system – this has been demonstrated in multiple studies – in adults, at least. But in some small number of people, apparently, spike protein is not cleared. Why? It makes no damn sense. Compels me, though. Some have suggested that inadvertent intravenous injection of vaccine, compared with the appropriate intramuscular route, might distribute the vaccine to sites with less immune surveillance. But that is definitely not proven yet.
We are on the path for sure, but this is, as Benoit Blanc would say, a twisted web – and we are not finished untangling it. Not yet.
F. Perry Wilson, MD, MSCE, is an associate professor of medicine and director of Yale’s Clinical and Translational Research Accelerator. His science communication work can be found in the Huffington Post, on NPR, and here. He tweets @fperrywilson and his new book, “How Medicine Works and When It Doesn’t,” is available for preorder now. He reports no conflicts of interest.
A version of this article first appeared on Medscape.com.
Welcome to Impact Factor, your weekly dose of commentary on a new medical study. I’m Dr. F. Perry Wilson of the Yale School of Medicine.
To date, it has been a mystery, like “Glass Onion.” And in the spirit of all the great mysteries, to get to the bottom of this, we’ll need to round up the usual suspects.
Appearing in Circulation, a new study does a great job of systematically evaluating multiple hypotheses linking vaccination to myocarditis, and eliminating them, Poirot-style, one by one until only one remains. We’ll get there.
But first, let’s review the suspects. Why do the mRNA vaccines cause myocarditis in a small subset of people?
There are a few leading candidates.
Number one: antibody responses. There are two flavors here. The quantitative hypothesis suggests that some people simply generate too many antibodies to the vaccine, leading to increased inflammation and heart damage.
The qualitative hypothesis suggests that maybe it’s the nature of the antibodies generated rather than the amount; they might cross-react with some protein on the surface of heart cells for instance.
Or maybe it is driven by T-cell responses, which, of course, are independent of antibody levels.
There’s the idea that myocarditis is due to excessive cytokine release – sort of like what we see in the multisystem inflammatory syndrome in children.
Or it could be due to the viral antigens themselves – the spike protein the mRNA codes for that is generated after vaccination.
To tease all these possibilities apart, researchers led by Lael Yonker at Mass General performed a case-control study. Sixteen children with postvaccine myocarditis were matched by age to 45 control children who had been vaccinated without complications.
The matching was OK, but as you can see here, there were more boys in the myocarditis group, and the time from vaccination was a bit shorter in that group as well. We’ll keep that in mind as we go through the results.
OK, let’s start eliminating suspects.
First, quantitative antibodies. Seems unlikely. Absolute antibody titers were really no different in the myocarditis vs. the control group.
What about the quality of the antibodies? Would the kids with myocarditis have more self-recognizing antibodies present? It doesn’t appear so. Autoantibody levels were similar in the two groups.
Take antibodies off the list.
T-cell responses come next, and, again, no major differences here, save for one specific T-cell subtype that was moderately elevated in the myocarditis group. Not what I would call a smoking gun, frankly.
Cytokines give us a bit more to chew on. Levels of interleukin (IL)-8, IL-6, tumor necrosis factor (TNF)-alpha, and IL-10 were all substantially higher in the kids with myocarditis.
But the thing about cytokines is that they are not particularly specific. OK, kids with myocarditis have more systemic inflammation than kids without; that’s not really surprising. It still leaves us with the question of what is causing all this inflammation? Who is the arch-villain? The kingpin? The don?
It’s the analyses of antigens – the protein products of vaccination – that may hold the key here.
In 12 out of 16 kids with myocarditis, the researchers were able to measure free spike protein in the blood – that is to say spike protein, not bound by antispike antibodies.
These free spikes were present in – wait for it – zero of the 45 control patients. That makes spike protein itself our prime suspect. J’accuse free spike protein!
Of course, all good detectives need to wrap up the case with a good story: How was it all done?
And here’s where we could use Agatha Christie’s help. How could this all work? The vaccine gets injected; mRNA is taken up into cells, where spike protein is generated and released, generating antibody and T-cell responses all the while. Those responses rapidly clear that spike protein from the system – this has been demonstrated in multiple studies – in adults, at least. But in some small number of people, apparently, spike protein is not cleared. Why? It makes no damn sense. Compels me, though. Some have suggested that inadvertent intravenous injection of vaccine, compared with the appropriate intramuscular route, might distribute the vaccine to sites with less immune surveillance. But that is definitely not proven yet.
We are on the path for sure, but this is, as Benoit Blanc would say, a twisted web – and we are not finished untangling it. Not yet.
F. Perry Wilson, MD, MSCE, is an associate professor of medicine and director of Yale’s Clinical and Translational Research Accelerator. His science communication work can be found in the Huffington Post, on NPR, and here. He tweets @fperrywilson and his new book, “How Medicine Works and When It Doesn’t,” is available for preorder now. He reports no conflicts of interest.
A version of this article first appeared on Medscape.com.
Welcome to Impact Factor, your weekly dose of commentary on a new medical study. I’m Dr. F. Perry Wilson of the Yale School of Medicine.
To date, it has been a mystery, like “Glass Onion.” And in the spirit of all the great mysteries, to get to the bottom of this, we’ll need to round up the usual suspects.
Appearing in Circulation, a new study does a great job of systematically evaluating multiple hypotheses linking vaccination to myocarditis, and eliminating them, Poirot-style, one by one until only one remains. We’ll get there.
But first, let’s review the suspects. Why do the mRNA vaccines cause myocarditis in a small subset of people?
There are a few leading candidates.
Number one: antibody responses. There are two flavors here. The quantitative hypothesis suggests that some people simply generate too many antibodies to the vaccine, leading to increased inflammation and heart damage.
The qualitative hypothesis suggests that maybe it’s the nature of the antibodies generated rather than the amount; they might cross-react with some protein on the surface of heart cells for instance.
Or maybe it is driven by T-cell responses, which, of course, are independent of antibody levels.
There’s the idea that myocarditis is due to excessive cytokine release – sort of like what we see in the multisystem inflammatory syndrome in children.
Or it could be due to the viral antigens themselves – the spike protein the mRNA codes for that is generated after vaccination.
To tease all these possibilities apart, researchers led by Lael Yonker at Mass General performed a case-control study. Sixteen children with postvaccine myocarditis were matched by age to 45 control children who had been vaccinated without complications.
The matching was OK, but as you can see here, there were more boys in the myocarditis group, and the time from vaccination was a bit shorter in that group as well. We’ll keep that in mind as we go through the results.
OK, let’s start eliminating suspects.
First, quantitative antibodies. Seems unlikely. Absolute antibody titers were really no different in the myocarditis vs. the control group.
What about the quality of the antibodies? Would the kids with myocarditis have more self-recognizing antibodies present? It doesn’t appear so. Autoantibody levels were similar in the two groups.
Take antibodies off the list.
T-cell responses come next, and, again, no major differences here, save for one specific T-cell subtype that was moderately elevated in the myocarditis group. Not what I would call a smoking gun, frankly.
Cytokines give us a bit more to chew on. Levels of interleukin (IL)-8, IL-6, tumor necrosis factor (TNF)-alpha, and IL-10 were all substantially higher in the kids with myocarditis.
But the thing about cytokines is that they are not particularly specific. OK, kids with myocarditis have more systemic inflammation than kids without; that’s not really surprising. It still leaves us with the question of what is causing all this inflammation? Who is the arch-villain? The kingpin? The don?
It’s the analyses of antigens – the protein products of vaccination – that may hold the key here.
In 12 out of 16 kids with myocarditis, the researchers were able to measure free spike protein in the blood – that is to say spike protein, not bound by antispike antibodies.
These free spikes were present in – wait for it – zero of the 45 control patients. That makes spike protein itself our prime suspect. J’accuse free spike protein!
Of course, all good detectives need to wrap up the case with a good story: How was it all done?
And here’s where we could use Agatha Christie’s help. How could this all work? The vaccine gets injected; mRNA is taken up into cells, where spike protein is generated and released, generating antibody and T-cell responses all the while. Those responses rapidly clear that spike protein from the system – this has been demonstrated in multiple studies – in adults, at least. But in some small number of people, apparently, spike protein is not cleared. Why? It makes no damn sense. Compels me, though. Some have suggested that inadvertent intravenous injection of vaccine, compared with the appropriate intramuscular route, might distribute the vaccine to sites with less immune surveillance. But that is definitely not proven yet.
We are on the path for sure, but this is, as Benoit Blanc would say, a twisted web – and we are not finished untangling it. Not yet.
F. Perry Wilson, MD, MSCE, is an associate professor of medicine and director of Yale’s Clinical and Translational Research Accelerator. His science communication work can be found in the Huffington Post, on NPR, and here. He tweets @fperrywilson and his new book, “How Medicine Works and When It Doesn’t,” is available for preorder now. He reports no conflicts of interest.
A version of this article first appeared on Medscape.com.