User login
Major increase in hidradenitis suppurativa cases anticipated
CHICAGO – Once thought to be a rare disease and largely neglected as a focus of research, and increase the number of patients seeking care, according to an expert interview conducted at the annual meeting of the Society for Investigational Dermatology.
“For decades ... we’ve really not understood how prevalent it is,” said Haley B. Naik, MD, of the department of dermatology at University of California, San Francisco, in a video interview. “Now, thanks to great population-based studies and new data, we know that HS is common and hugely impacts the lives of the people who suffer with this condition.”
Recapping some of the highlights of an update on this chronic inflammatory skin condition that she presented at the meeting, Dr. Naik said that HS has been mischaracterized as rare. Many patients, embarrassed by the symptoms or failing to receive adequate relief from previous health care encounters, are not currently seeking care.
This will change as treatments improve, according to Dr. Naik, who asserted that HS has become a hot topic. Progress in understanding the underlying pathophysiology has been driving new management strategies. She counted more than 15 clinical trials being conducted with new agents for this disease in a clinicaltrials.gov survey.
In the interview, Dr. Naik calls on dermatologists to increase their awareness of the signs and symptoms of HS so that they can diagnose and intervene earlier, a step that will be made easier as new therapies become available.
CHICAGO – Once thought to be a rare disease and largely neglected as a focus of research, and increase the number of patients seeking care, according to an expert interview conducted at the annual meeting of the Society for Investigational Dermatology.
“For decades ... we’ve really not understood how prevalent it is,” said Haley B. Naik, MD, of the department of dermatology at University of California, San Francisco, in a video interview. “Now, thanks to great population-based studies and new data, we know that HS is common and hugely impacts the lives of the people who suffer with this condition.”
Recapping some of the highlights of an update on this chronic inflammatory skin condition that she presented at the meeting, Dr. Naik said that HS has been mischaracterized as rare. Many patients, embarrassed by the symptoms or failing to receive adequate relief from previous health care encounters, are not currently seeking care.
This will change as treatments improve, according to Dr. Naik, who asserted that HS has become a hot topic. Progress in understanding the underlying pathophysiology has been driving new management strategies. She counted more than 15 clinical trials being conducted with new agents for this disease in a clinicaltrials.gov survey.
In the interview, Dr. Naik calls on dermatologists to increase their awareness of the signs and symptoms of HS so that they can diagnose and intervene earlier, a step that will be made easier as new therapies become available.
CHICAGO – Once thought to be a rare disease and largely neglected as a focus of research, and increase the number of patients seeking care, according to an expert interview conducted at the annual meeting of the Society for Investigational Dermatology.
“For decades ... we’ve really not understood how prevalent it is,” said Haley B. Naik, MD, of the department of dermatology at University of California, San Francisco, in a video interview. “Now, thanks to great population-based studies and new data, we know that HS is common and hugely impacts the lives of the people who suffer with this condition.”
Recapping some of the highlights of an update on this chronic inflammatory skin condition that she presented at the meeting, Dr. Naik said that HS has been mischaracterized as rare. Many patients, embarrassed by the symptoms or failing to receive adequate relief from previous health care encounters, are not currently seeking care.
This will change as treatments improve, according to Dr. Naik, who asserted that HS has become a hot topic. Progress in understanding the underlying pathophysiology has been driving new management strategies. She counted more than 15 clinical trials being conducted with new agents for this disease in a clinicaltrials.gov survey.
In the interview, Dr. Naik calls on dermatologists to increase their awareness of the signs and symptoms of HS so that they can diagnose and intervene earlier, a step that will be made easier as new therapies become available.
REPORTING FROM SID 2019
Molecular profiling shows promise for treating unusual skin rashes
CHICAGO – Although at a relatively early stage of research, according to research that was described at the annual meeting of the Society for Investigative Dermatology.
“We now have several cases that suggest single-cell molecular profiling can provide treatment guidance for atypical rashes, providing an opportunity for a rational treatment choice rather than just improvising in a difficult population,” reported Raymond Cho, MD, PhD, of the department of dermatology at the University of California, San Francisco.
Based on a growing cohort of patients with atypical rashes, one goal is to develop “a library of molecular fingerprints” for classifying rashes that are atypical when defined by morphology, histopathology, or therapeutic response, according to Dr. Cho.
“The big focus now is on expanding this patient cohort. We want to move from anecdotal cases to a larger patient population with which we can statistically prove that we can nominate the best first-line therapy through this approach,” he explained.
In describing work he is performing in collaboration with Jeffrey Cheng, MD, also with the department of dermatology at UCSF, Dr. Cho said the profiles are based on RNA sequencing from single immune cells and epitope measurements. Work already performed in rashes of known etiology supports the approach. For example, the profile for atopic dermatitis includes elevated expression of interleukin (IL)-4 and IL-13, whereas that of psoriasis includes elevated expression of IL-17, which fit with the expected molecular signatures of these diseases.
To be considered for inclusion in the cohort of atypical rashes, patients are required to have an idiopathic skin lesion of at least 6 months’ duration with at least two atypical features defined by such characteristics as morphology or location. Many of these patients have already consulted with multiple providers, have undergone multiple biopsies without a diagnosis, and have failed common treatments, such as steroids.
Examples selected from this cohort have already supported the premise that molecular profiles are relevant to treatment choice. Dr. Cho described one patient with unremitting generalized pruritus and another with nodular lesions on the legs. Both had symptoms of long duration that had failed multiple treatments.
In both cases, immune cell profiling identified lesions high in IL-13 expression. Both achieved complete or near complete resolution of their rash and symptoms when treated with dupilumab, a biologic that targets the IL-13 pathway. In one patient with a large symptom burden, Dr. Cho described the response as “remarkable.”
There are more than 40 patients in the expanding cohort, according to Dr. Cho, who emphasized that this work is timely because of “the armamentarium of immunomodulatory drugs that are coming on line.” He said this type of drug development in dermatology is the basis for a potential paradigm shift.
“Personalized therapy has been used in clinical oncology for almost 10 years now, but this is an approach that needs to find a home in our specialty as well,” Dr. Cho said. He cited data suggesting that nearly 15% of rashes are atypical and represent a major source of frustration to both patients and clinicians when conventional treatments fail.
Asked about cost, he acknowledged that the molecular profiling that he and Dr. Cheng are performing is expensive at the current time, but “we are hopeful that we can find cheaper markers and technologies” to bring this cost down. However, he noted that undiagnosed rashes consume a great deal of time of effort from clinicians while generating significant morbidity for patients, which is justifying novel strategies to find effective therapies.
“These are not happy patients,” Dr. Cho said. Although there are technical challenges for building a molecular library that has practical utility across the substantial heterogeneity of idiopathic rashes, he suggested that a larger patient sample is considered one of the important steps toward overcoming hurdles.
Dr. Cho reports no potential conflicts of interest.
CHICAGO – Although at a relatively early stage of research, according to research that was described at the annual meeting of the Society for Investigative Dermatology.
“We now have several cases that suggest single-cell molecular profiling can provide treatment guidance for atypical rashes, providing an opportunity for a rational treatment choice rather than just improvising in a difficult population,” reported Raymond Cho, MD, PhD, of the department of dermatology at the University of California, San Francisco.
Based on a growing cohort of patients with atypical rashes, one goal is to develop “a library of molecular fingerprints” for classifying rashes that are atypical when defined by morphology, histopathology, or therapeutic response, according to Dr. Cho.
“The big focus now is on expanding this patient cohort. We want to move from anecdotal cases to a larger patient population with which we can statistically prove that we can nominate the best first-line therapy through this approach,” he explained.
In describing work he is performing in collaboration with Jeffrey Cheng, MD, also with the department of dermatology at UCSF, Dr. Cho said the profiles are based on RNA sequencing from single immune cells and epitope measurements. Work already performed in rashes of known etiology supports the approach. For example, the profile for atopic dermatitis includes elevated expression of interleukin (IL)-4 and IL-13, whereas that of psoriasis includes elevated expression of IL-17, which fit with the expected molecular signatures of these diseases.
To be considered for inclusion in the cohort of atypical rashes, patients are required to have an idiopathic skin lesion of at least 6 months’ duration with at least two atypical features defined by such characteristics as morphology or location. Many of these patients have already consulted with multiple providers, have undergone multiple biopsies without a diagnosis, and have failed common treatments, such as steroids.
Examples selected from this cohort have already supported the premise that molecular profiles are relevant to treatment choice. Dr. Cho described one patient with unremitting generalized pruritus and another with nodular lesions on the legs. Both had symptoms of long duration that had failed multiple treatments.
In both cases, immune cell profiling identified lesions high in IL-13 expression. Both achieved complete or near complete resolution of their rash and symptoms when treated with dupilumab, a biologic that targets the IL-13 pathway. In one patient with a large symptom burden, Dr. Cho described the response as “remarkable.”
There are more than 40 patients in the expanding cohort, according to Dr. Cho, who emphasized that this work is timely because of “the armamentarium of immunomodulatory drugs that are coming on line.” He said this type of drug development in dermatology is the basis for a potential paradigm shift.
“Personalized therapy has been used in clinical oncology for almost 10 years now, but this is an approach that needs to find a home in our specialty as well,” Dr. Cho said. He cited data suggesting that nearly 15% of rashes are atypical and represent a major source of frustration to both patients and clinicians when conventional treatments fail.
Asked about cost, he acknowledged that the molecular profiling that he and Dr. Cheng are performing is expensive at the current time, but “we are hopeful that we can find cheaper markers and technologies” to bring this cost down. However, he noted that undiagnosed rashes consume a great deal of time of effort from clinicians while generating significant morbidity for patients, which is justifying novel strategies to find effective therapies.
“These are not happy patients,” Dr. Cho said. Although there are technical challenges for building a molecular library that has practical utility across the substantial heterogeneity of idiopathic rashes, he suggested that a larger patient sample is considered one of the important steps toward overcoming hurdles.
Dr. Cho reports no potential conflicts of interest.
CHICAGO – Although at a relatively early stage of research, according to research that was described at the annual meeting of the Society for Investigative Dermatology.
“We now have several cases that suggest single-cell molecular profiling can provide treatment guidance for atypical rashes, providing an opportunity for a rational treatment choice rather than just improvising in a difficult population,” reported Raymond Cho, MD, PhD, of the department of dermatology at the University of California, San Francisco.
Based on a growing cohort of patients with atypical rashes, one goal is to develop “a library of molecular fingerprints” for classifying rashes that are atypical when defined by morphology, histopathology, or therapeutic response, according to Dr. Cho.
“The big focus now is on expanding this patient cohort. We want to move from anecdotal cases to a larger patient population with which we can statistically prove that we can nominate the best first-line therapy through this approach,” he explained.
In describing work he is performing in collaboration with Jeffrey Cheng, MD, also with the department of dermatology at UCSF, Dr. Cho said the profiles are based on RNA sequencing from single immune cells and epitope measurements. Work already performed in rashes of known etiology supports the approach. For example, the profile for atopic dermatitis includes elevated expression of interleukin (IL)-4 and IL-13, whereas that of psoriasis includes elevated expression of IL-17, which fit with the expected molecular signatures of these diseases.
To be considered for inclusion in the cohort of atypical rashes, patients are required to have an idiopathic skin lesion of at least 6 months’ duration with at least two atypical features defined by such characteristics as morphology or location. Many of these patients have already consulted with multiple providers, have undergone multiple biopsies without a diagnosis, and have failed common treatments, such as steroids.
Examples selected from this cohort have already supported the premise that molecular profiles are relevant to treatment choice. Dr. Cho described one patient with unremitting generalized pruritus and another with nodular lesions on the legs. Both had symptoms of long duration that had failed multiple treatments.
In both cases, immune cell profiling identified lesions high in IL-13 expression. Both achieved complete or near complete resolution of their rash and symptoms when treated with dupilumab, a biologic that targets the IL-13 pathway. In one patient with a large symptom burden, Dr. Cho described the response as “remarkable.”
There are more than 40 patients in the expanding cohort, according to Dr. Cho, who emphasized that this work is timely because of “the armamentarium of immunomodulatory drugs that are coming on line.” He said this type of drug development in dermatology is the basis for a potential paradigm shift.
“Personalized therapy has been used in clinical oncology for almost 10 years now, but this is an approach that needs to find a home in our specialty as well,” Dr. Cho said. He cited data suggesting that nearly 15% of rashes are atypical and represent a major source of frustration to both patients and clinicians when conventional treatments fail.
Asked about cost, he acknowledged that the molecular profiling that he and Dr. Cheng are performing is expensive at the current time, but “we are hopeful that we can find cheaper markers and technologies” to bring this cost down. However, he noted that undiagnosed rashes consume a great deal of time of effort from clinicians while generating significant morbidity for patients, which is justifying novel strategies to find effective therapies.
“These are not happy patients,” Dr. Cho said. Although there are technical challenges for building a molecular library that has practical utility across the substantial heterogeneity of idiopathic rashes, he suggested that a larger patient sample is considered one of the important steps toward overcoming hurdles.
Dr. Cho reports no potential conflicts of interest.
EXPERT ANALYSIS FROM SID 2019
Belatacept may mitigate skin cancer risk in transplant patients
CHICAGO – Compared with that of calcineurin inhibitors, belatacept appears to be associated with a lower risk of keratinocyte carcinomas in solid organ transplant patients, based on results from a single-center analysis presented at the annual meeting of the Society for Investigative Dermatology.
“Belatacept may offer a better risk-benefit profile in regards to skin cancer,” reported Michael Wang, a medical student who conducted this research in collaboration with the senior author, Oscar Colegio, MD, PhD, an associate professor of dermatology, pathology, and surgery at Yale University, New Haven, Conn.
Belatacept, a CTLA-4 fusion protein, has been compared with calcineurin inhibitors in two previous studies. The results were equivocal in one, and the other found no difference in risk and could not rule out the possibility that skin cancer risk was even higher on belatacept.
This single-center chart review included 110 kidney transplant patients, median age 58 years, who were switched from a calcineurin inhibitor, such as cyclosporine or tacrolimus, to belatacept. Ultimately, the study was limited to the 66 patients with at least 2 years of dermatologic follow-up both before and after the switch from a calcineurin inhibitor.
The primary outcome was the number of keratinocyte carcinomas overall and, specifically, the number of squamous cell carcinomas (SCCs) before and after the switch. Over the course of this study there were 128 cutaneous malignancies, 83 of which were SCCs.
When patients were on a calcineurin inhibitor, the risk of keratinocyte carcinomas increased incrementally by 2.6 events per 100 patients per year of follow-up, and the risk of SCCs increased by 1.7 events per 100 patients per year of follow-up. In the first 6 months after the switch to belatacept, there was no change in the rising trajectory of skin cancers, but rates declined thereafter.
Relative to rates prior to and 6 months after the switch, “the incidence of SCCs decreased at a rate of 5.9 events per 100 patients per year (P = .0068), and the incidence of keratinocyte carcinomas decreased by 7.1 events per 100 patients per year (P = .003),” Mr. Wang reported. He noted, however, that the incidence of basal cell carcinomas and melanomas following the switch remained unchanged.
When patients switched to belatacept were compared with another group of patients who remained on a calcineurin inhibitor after developing a SCC, the hazard ratio for a new SCC was 0.42, indicating a greater than 50% reduction in risk.
In patients on calcineurin inhibitors, the risk of keratinocyte carcinomas appears to be related to a direct effect of these agents on keratinocyte dedifferentiation. Belatacept is not believed to have any direct effects on keratinocytes, according to Mr. Wang.
As the chart review was retrospective and limited to a single center, “we hope [the findings] will encourage a prospective trial,” Mr. Wang said.
SOURCE: Wang M. SID 2019, Abstract 532.
CHICAGO – Compared with that of calcineurin inhibitors, belatacept appears to be associated with a lower risk of keratinocyte carcinomas in solid organ transplant patients, based on results from a single-center analysis presented at the annual meeting of the Society for Investigative Dermatology.
“Belatacept may offer a better risk-benefit profile in regards to skin cancer,” reported Michael Wang, a medical student who conducted this research in collaboration with the senior author, Oscar Colegio, MD, PhD, an associate professor of dermatology, pathology, and surgery at Yale University, New Haven, Conn.
Belatacept, a CTLA-4 fusion protein, has been compared with calcineurin inhibitors in two previous studies. The results were equivocal in one, and the other found no difference in risk and could not rule out the possibility that skin cancer risk was even higher on belatacept.
This single-center chart review included 110 kidney transplant patients, median age 58 years, who were switched from a calcineurin inhibitor, such as cyclosporine or tacrolimus, to belatacept. Ultimately, the study was limited to the 66 patients with at least 2 years of dermatologic follow-up both before and after the switch from a calcineurin inhibitor.
The primary outcome was the number of keratinocyte carcinomas overall and, specifically, the number of squamous cell carcinomas (SCCs) before and after the switch. Over the course of this study there were 128 cutaneous malignancies, 83 of which were SCCs.
When patients were on a calcineurin inhibitor, the risk of keratinocyte carcinomas increased incrementally by 2.6 events per 100 patients per year of follow-up, and the risk of SCCs increased by 1.7 events per 100 patients per year of follow-up. In the first 6 months after the switch to belatacept, there was no change in the rising trajectory of skin cancers, but rates declined thereafter.
Relative to rates prior to and 6 months after the switch, “the incidence of SCCs decreased at a rate of 5.9 events per 100 patients per year (P = .0068), and the incidence of keratinocyte carcinomas decreased by 7.1 events per 100 patients per year (P = .003),” Mr. Wang reported. He noted, however, that the incidence of basal cell carcinomas and melanomas following the switch remained unchanged.
When patients switched to belatacept were compared with another group of patients who remained on a calcineurin inhibitor after developing a SCC, the hazard ratio for a new SCC was 0.42, indicating a greater than 50% reduction in risk.
In patients on calcineurin inhibitors, the risk of keratinocyte carcinomas appears to be related to a direct effect of these agents on keratinocyte dedifferentiation. Belatacept is not believed to have any direct effects on keratinocytes, according to Mr. Wang.
As the chart review was retrospective and limited to a single center, “we hope [the findings] will encourage a prospective trial,” Mr. Wang said.
SOURCE: Wang M. SID 2019, Abstract 532.
CHICAGO – Compared with that of calcineurin inhibitors, belatacept appears to be associated with a lower risk of keratinocyte carcinomas in solid organ transplant patients, based on results from a single-center analysis presented at the annual meeting of the Society for Investigative Dermatology.
“Belatacept may offer a better risk-benefit profile in regards to skin cancer,” reported Michael Wang, a medical student who conducted this research in collaboration with the senior author, Oscar Colegio, MD, PhD, an associate professor of dermatology, pathology, and surgery at Yale University, New Haven, Conn.
Belatacept, a CTLA-4 fusion protein, has been compared with calcineurin inhibitors in two previous studies. The results were equivocal in one, and the other found no difference in risk and could not rule out the possibility that skin cancer risk was even higher on belatacept.
This single-center chart review included 110 kidney transplant patients, median age 58 years, who were switched from a calcineurin inhibitor, such as cyclosporine or tacrolimus, to belatacept. Ultimately, the study was limited to the 66 patients with at least 2 years of dermatologic follow-up both before and after the switch from a calcineurin inhibitor.
The primary outcome was the number of keratinocyte carcinomas overall and, specifically, the number of squamous cell carcinomas (SCCs) before and after the switch. Over the course of this study there were 128 cutaneous malignancies, 83 of which were SCCs.
When patients were on a calcineurin inhibitor, the risk of keratinocyte carcinomas increased incrementally by 2.6 events per 100 patients per year of follow-up, and the risk of SCCs increased by 1.7 events per 100 patients per year of follow-up. In the first 6 months after the switch to belatacept, there was no change in the rising trajectory of skin cancers, but rates declined thereafter.
Relative to rates prior to and 6 months after the switch, “the incidence of SCCs decreased at a rate of 5.9 events per 100 patients per year (P = .0068), and the incidence of keratinocyte carcinomas decreased by 7.1 events per 100 patients per year (P = .003),” Mr. Wang reported. He noted, however, that the incidence of basal cell carcinomas and melanomas following the switch remained unchanged.
When patients switched to belatacept were compared with another group of patients who remained on a calcineurin inhibitor after developing a SCC, the hazard ratio for a new SCC was 0.42, indicating a greater than 50% reduction in risk.
In patients on calcineurin inhibitors, the risk of keratinocyte carcinomas appears to be related to a direct effect of these agents on keratinocyte dedifferentiation. Belatacept is not believed to have any direct effects on keratinocytes, according to Mr. Wang.
As the chart review was retrospective and limited to a single center, “we hope [the findings] will encourage a prospective trial,” Mr. Wang said.
SOURCE: Wang M. SID 2019, Abstract 532.
REPORTING FROM SID 2019
Prior authorizations for dermatology care nearly doubled in the last 2 years at one center
CHICAGO – Prior authorizations for the delivery of care in dermatology may be increasing steeply, judging from the experience of one large academic dermatology practice.
“About the same number of patients were seen in 2018 as in 2016, but the number of prior authorizations required to serve those patients went up substantially,” reported Ryan P. Carlisle, a medical student who performed this analysis under the guidance of Aaron M. Secrest, MD, PhD, of the University of Utah, Salt Lake City.
Tracking of prior authorizations for the delivery of dermatologic care was initiated in 2016 at the University of Utah. By a number of measures, the burden of prior authorizations has been increasing steadily since that time, Mr. Carlisle said at the annual meeting of the Society for Investigative Dermatology.
As an example, one prior authorization was required for every 15 patient visits (6.7%) over a 30-day period in September 2016. In comparison, one prior authorization was required for every 9 patient visits (11.1%) in a comparable 30-day period in September 2018. Further, the number of clinic visits during this more recent period was 2.4% higher than in the earlier one (9,743 vs. 9,512), so the number of prior authorizations increased by 73.8% (1,088 vs. 626), Mr. Carlisle reported.
Two full-time staff and eight part-time staff at the University of Utah handle prior authorizations for 40 dermatologists and 10 nonphysician clinicians. The substantial unreimbursed costs incurred by this labor can be huge, he said. In one specific case, 81% of the reimbursed cost for a patient visit was consumed by seeking a prior authorization.
Of prior authorizations tracked at the University of Utah, 39.1% were for nonbiologic therapies, 21.6% were for excisions, 16% were for Moh’s surgery, 11% were for biologics, and the remainder was for an array of other procedures or therapies.
Of these, prior authorizations for biologics “were the most burdensome both in time and cost” on a per-visit basis, Mr. Carlisle reported.
The proportions of prior authorizations that were denied were relatively low. The highest proportion of denials was for nonbiologic medications (25%). The rate of denials for biologics over the study period was just 11%. Moreover, of denials that were appealed, 56% were granted.
Importantly, some prior authorizations were rarely denied. This includes a 0% denial rate for Moh’s surgery and a 1% denial rate for incisional procedures. Mr. Carlisle questioned whether the requirement for prior authorizations makes sense in these situations.
“Dermatology should partner with insurers to reduce unnecessary prior authorizations and appeals,” Mr. Carlisle suggested. It “is likely that these are affecting patient care” as well as ultimately, and perhaps unnecessarily, reducing reimbursement.
“The process to get prior authorizations completed and get patients their medications has just gotten to be too burdensome,” said Mr. Carlisle, summarizing the Utah experience.
SOURCE: Carlisle RP. SID 2019, Abstract 247.
CHICAGO – Prior authorizations for the delivery of care in dermatology may be increasing steeply, judging from the experience of one large academic dermatology practice.
“About the same number of patients were seen in 2018 as in 2016, but the number of prior authorizations required to serve those patients went up substantially,” reported Ryan P. Carlisle, a medical student who performed this analysis under the guidance of Aaron M. Secrest, MD, PhD, of the University of Utah, Salt Lake City.
Tracking of prior authorizations for the delivery of dermatologic care was initiated in 2016 at the University of Utah. By a number of measures, the burden of prior authorizations has been increasing steadily since that time, Mr. Carlisle said at the annual meeting of the Society for Investigative Dermatology.
As an example, one prior authorization was required for every 15 patient visits (6.7%) over a 30-day period in September 2016. In comparison, one prior authorization was required for every 9 patient visits (11.1%) in a comparable 30-day period in September 2018. Further, the number of clinic visits during this more recent period was 2.4% higher than in the earlier one (9,743 vs. 9,512), so the number of prior authorizations increased by 73.8% (1,088 vs. 626), Mr. Carlisle reported.
Two full-time staff and eight part-time staff at the University of Utah handle prior authorizations for 40 dermatologists and 10 nonphysician clinicians. The substantial unreimbursed costs incurred by this labor can be huge, he said. In one specific case, 81% of the reimbursed cost for a patient visit was consumed by seeking a prior authorization.
Of prior authorizations tracked at the University of Utah, 39.1% were for nonbiologic therapies, 21.6% were for excisions, 16% were for Moh’s surgery, 11% were for biologics, and the remainder was for an array of other procedures or therapies.
Of these, prior authorizations for biologics “were the most burdensome both in time and cost” on a per-visit basis, Mr. Carlisle reported.
The proportions of prior authorizations that were denied were relatively low. The highest proportion of denials was for nonbiologic medications (25%). The rate of denials for biologics over the study period was just 11%. Moreover, of denials that were appealed, 56% were granted.
Importantly, some prior authorizations were rarely denied. This includes a 0% denial rate for Moh’s surgery and a 1% denial rate for incisional procedures. Mr. Carlisle questioned whether the requirement for prior authorizations makes sense in these situations.
“Dermatology should partner with insurers to reduce unnecessary prior authorizations and appeals,” Mr. Carlisle suggested. It “is likely that these are affecting patient care” as well as ultimately, and perhaps unnecessarily, reducing reimbursement.
“The process to get prior authorizations completed and get patients their medications has just gotten to be too burdensome,” said Mr. Carlisle, summarizing the Utah experience.
SOURCE: Carlisle RP. SID 2019, Abstract 247.
CHICAGO – Prior authorizations for the delivery of care in dermatology may be increasing steeply, judging from the experience of one large academic dermatology practice.
“About the same number of patients were seen in 2018 as in 2016, but the number of prior authorizations required to serve those patients went up substantially,” reported Ryan P. Carlisle, a medical student who performed this analysis under the guidance of Aaron M. Secrest, MD, PhD, of the University of Utah, Salt Lake City.
Tracking of prior authorizations for the delivery of dermatologic care was initiated in 2016 at the University of Utah. By a number of measures, the burden of prior authorizations has been increasing steadily since that time, Mr. Carlisle said at the annual meeting of the Society for Investigative Dermatology.
As an example, one prior authorization was required for every 15 patient visits (6.7%) over a 30-day period in September 2016. In comparison, one prior authorization was required for every 9 patient visits (11.1%) in a comparable 30-day period in September 2018. Further, the number of clinic visits during this more recent period was 2.4% higher than in the earlier one (9,743 vs. 9,512), so the number of prior authorizations increased by 73.8% (1,088 vs. 626), Mr. Carlisle reported.
Two full-time staff and eight part-time staff at the University of Utah handle prior authorizations for 40 dermatologists and 10 nonphysician clinicians. The substantial unreimbursed costs incurred by this labor can be huge, he said. In one specific case, 81% of the reimbursed cost for a patient visit was consumed by seeking a prior authorization.
Of prior authorizations tracked at the University of Utah, 39.1% were for nonbiologic therapies, 21.6% were for excisions, 16% were for Moh’s surgery, 11% were for biologics, and the remainder was for an array of other procedures or therapies.
Of these, prior authorizations for biologics “were the most burdensome both in time and cost” on a per-visit basis, Mr. Carlisle reported.
The proportions of prior authorizations that were denied were relatively low. The highest proportion of denials was for nonbiologic medications (25%). The rate of denials for biologics over the study period was just 11%. Moreover, of denials that were appealed, 56% were granted.
Importantly, some prior authorizations were rarely denied. This includes a 0% denial rate for Moh’s surgery and a 1% denial rate for incisional procedures. Mr. Carlisle questioned whether the requirement for prior authorizations makes sense in these situations.
“Dermatology should partner with insurers to reduce unnecessary prior authorizations and appeals,” Mr. Carlisle suggested. It “is likely that these are affecting patient care” as well as ultimately, and perhaps unnecessarily, reducing reimbursement.
“The process to get prior authorizations completed and get patients their medications has just gotten to be too burdensome,” said Mr. Carlisle, summarizing the Utah experience.
SOURCE: Carlisle RP. SID 2019, Abstract 247.
REPORTING FROM SID 2019
Atopic dermatitis in adults associated with increased risk of dementia
CHICAGO – Atopic dermatitis in adulthood was associated with a twofold increase in the risk of developing dementia late in life, based on results from a large longitudinal cohort study presented at the annual meeting of the Society for Investigative Dermatology.
“After adjusting for potential mediators such as smoking status, depression, cardiovascular disease, and asthma or rhinitis, the effect was decreased slightly but still remained strongly statistically significant,” reported Katrina Abuabara, MD, of the University of California, San Francisco.
Atopic dermatitis is the latest in growing list of chronic inflammatory conditions that have been associated with an increased risk of dementia, according to Dr. Abuabara, who cited a body of evidence suggesting that inflammation triggers or exacerbates the processes that drive risk of developing dementia late in life.
Interest in the potential association of atopic dermatitis and dementia has been triggered “by a paradigm shift in which we now think of atopic dermatitis as a systemic inflammatory condition.” Dr. Abuabara reported.
In a primary care database of more than 1 million patients, both atopic dermatitis and dementia were common in those aged 60 years or older. The two disorders were identified in 6.75% and 6.49% of patients, respectively.
Cox proportional hazard ratios were employed to determine the relationship between the presence of atopic dermatitis and subsequent development of dementia. The median follow-up was 8 years. Atopic dermatitis was classified as mild, moderate, or severe involvement based on treatment records.
Patients with dementia associated with infectious diseases such as HIV, alcoholism, and other exogenous toxins were excluded from the analysis.
For those with atopic dermatitis relative to those without, the unadjusted hazard ratios were 1.91 for dementia of any type, 2.14 for Alzheimer’s dementia, and 2.25 for vascular-related dementia. After adjustment for confounders such as age, sex, and socioeconomic status, these hazard ratios, respectively, were only somewhat lower and remained statistically significant.
There was a trend for greater dementia risk with greater atopic dermatitis severity, rising from 2.07 in those with mild atopic dermatitis to 2.72 to those with severe disease, according to Dr. Abuabara.
“The important next step is to determine if better control of atopic dermatitis results in a lower risk of dementia,” she said.
According to Dr. Abuabara, some experimental studies have supported the hypothesis that downregulation of systemic markers of inflammation may be protective.
“Even if you reduced risk by a small amount, it would translate into a large health impact because of the large and growing prevalence of dementia,” she said.
Dr. Abuabara is a consultant for the TARGET-DERM study, sponsored by Target PharmaSolutions.
CHICAGO – Atopic dermatitis in adulthood was associated with a twofold increase in the risk of developing dementia late in life, based on results from a large longitudinal cohort study presented at the annual meeting of the Society for Investigative Dermatology.
“After adjusting for potential mediators such as smoking status, depression, cardiovascular disease, and asthma or rhinitis, the effect was decreased slightly but still remained strongly statistically significant,” reported Katrina Abuabara, MD, of the University of California, San Francisco.
Atopic dermatitis is the latest in growing list of chronic inflammatory conditions that have been associated with an increased risk of dementia, according to Dr. Abuabara, who cited a body of evidence suggesting that inflammation triggers or exacerbates the processes that drive risk of developing dementia late in life.
Interest in the potential association of atopic dermatitis and dementia has been triggered “by a paradigm shift in which we now think of atopic dermatitis as a systemic inflammatory condition.” Dr. Abuabara reported.
In a primary care database of more than 1 million patients, both atopic dermatitis and dementia were common in those aged 60 years or older. The two disorders were identified in 6.75% and 6.49% of patients, respectively.
Cox proportional hazard ratios were employed to determine the relationship between the presence of atopic dermatitis and subsequent development of dementia. The median follow-up was 8 years. Atopic dermatitis was classified as mild, moderate, or severe involvement based on treatment records.
Patients with dementia associated with infectious diseases such as HIV, alcoholism, and other exogenous toxins were excluded from the analysis.
For those with atopic dermatitis relative to those without, the unadjusted hazard ratios were 1.91 for dementia of any type, 2.14 for Alzheimer’s dementia, and 2.25 for vascular-related dementia. After adjustment for confounders such as age, sex, and socioeconomic status, these hazard ratios, respectively, were only somewhat lower and remained statistically significant.
There was a trend for greater dementia risk with greater atopic dermatitis severity, rising from 2.07 in those with mild atopic dermatitis to 2.72 to those with severe disease, according to Dr. Abuabara.
“The important next step is to determine if better control of atopic dermatitis results in a lower risk of dementia,” she said.
According to Dr. Abuabara, some experimental studies have supported the hypothesis that downregulation of systemic markers of inflammation may be protective.
“Even if you reduced risk by a small amount, it would translate into a large health impact because of the large and growing prevalence of dementia,” she said.
Dr. Abuabara is a consultant for the TARGET-DERM study, sponsored by Target PharmaSolutions.
CHICAGO – Atopic dermatitis in adulthood was associated with a twofold increase in the risk of developing dementia late in life, based on results from a large longitudinal cohort study presented at the annual meeting of the Society for Investigative Dermatology.
“After adjusting for potential mediators such as smoking status, depression, cardiovascular disease, and asthma or rhinitis, the effect was decreased slightly but still remained strongly statistically significant,” reported Katrina Abuabara, MD, of the University of California, San Francisco.
Atopic dermatitis is the latest in growing list of chronic inflammatory conditions that have been associated with an increased risk of dementia, according to Dr. Abuabara, who cited a body of evidence suggesting that inflammation triggers or exacerbates the processes that drive risk of developing dementia late in life.
Interest in the potential association of atopic dermatitis and dementia has been triggered “by a paradigm shift in which we now think of atopic dermatitis as a systemic inflammatory condition.” Dr. Abuabara reported.
In a primary care database of more than 1 million patients, both atopic dermatitis and dementia were common in those aged 60 years or older. The two disorders were identified in 6.75% and 6.49% of patients, respectively.
Cox proportional hazard ratios were employed to determine the relationship between the presence of atopic dermatitis and subsequent development of dementia. The median follow-up was 8 years. Atopic dermatitis was classified as mild, moderate, or severe involvement based on treatment records.
Patients with dementia associated with infectious diseases such as HIV, alcoholism, and other exogenous toxins were excluded from the analysis.
For those with atopic dermatitis relative to those without, the unadjusted hazard ratios were 1.91 for dementia of any type, 2.14 for Alzheimer’s dementia, and 2.25 for vascular-related dementia. After adjustment for confounders such as age, sex, and socioeconomic status, these hazard ratios, respectively, were only somewhat lower and remained statistically significant.
There was a trend for greater dementia risk with greater atopic dermatitis severity, rising from 2.07 in those with mild atopic dermatitis to 2.72 to those with severe disease, according to Dr. Abuabara.
“The important next step is to determine if better control of atopic dermatitis results in a lower risk of dementia,” she said.
According to Dr. Abuabara, some experimental studies have supported the hypothesis that downregulation of systemic markers of inflammation may be protective.
“Even if you reduced risk by a small amount, it would translate into a large health impact because of the large and growing prevalence of dementia,” she said.
Dr. Abuabara is a consultant for the TARGET-DERM study, sponsored by Target PharmaSolutions.
REPORTING FROM SID 2019
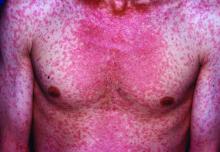
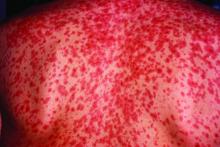
Measles complications in the U.S. unchanged in posteradication era
CHICAGO – An evaluation of the measles threat in the modern era gives no indication that the risk of complications or death is any different than it was before a vaccine became available, according to an analysis of inpatient complications between 2002 and 2013.
In 2000, measles was declared eliminated in the United States, but for those who have been infected since that time, the risk of serious complications and death has not diminished, noted Raj Chovatiya, MD, PhD, in a session at the annual meeting of the Society for Investigative Dermatology.
By eliminated, the Centers of Disease Control and Prevention – which reported 86 confirmed cases of measles in 2000 – was referring to a technical definition of no new endemic or continuous transmissions in the previous 12 months. It was expected that a modest number of cases of this reportable disease would continue to accrue for an infection that remains common elsewhere in the world.
“Worldwide there are about 20 million cases of measles annually with an estimated 100,000 deaths attributed to this cause,” said Dr. Chovatiya, who is a dermatology resident at Northwestern University, Chicago.
In the United States, posteradication infection rates remained at low levels for several years but were already rising from 2002 to 2013, when Dr. Chovatiya and his coinvestigators sought to describe the incidence, associations, comorbidities, and outcomes of hospitalizations for measles. Toward the end of the period the researchers were examining the incidence rates climbed more steeply.
“So far this year, 764 CDC cases of measles [were] reported. That is the most we have seen in the U.S. since 1994,” Dr. Chovatiya said.
Based on his analysis of hospitalizations from 2002 to 2013, the threat of these outbreaks is no different then that before the disease was declared eliminated or before a vaccine became available.
The cross-sectional study was conducted with data from the Nationwide Inpatient Sample, an all-payer database that is considered to be a representative of national trends.
Characteristic of measles, the majority of the 582 hospitalizations evaluated over this period occurred in children aged between 1 and 9 years. The proportion of patients with preexisting chronic comorbid conditions was low. Rather, “most were pretty healthy” prior to admission, according to Dr. Chovatiya, who said that the majority of admissions were from an emergency department.
Measles, which targets epithelial cells and depresses the immune system, is a potentially serious disease because of its ability to produce complications in essentially every organ of the body, including the lungs, kidneys, blood, and central nervous system. Consistent with past studies, the most common complication in this series was pneumonia, observed in 20% of patients. The list of other serious complications identified in this study period, including encephalitis and acute renal failure, was long.
“We observed death in 4.3% of our 582 cases, or about 25 cases,” reported Dr. Chovatiya. He indicated that this is a high percentage among a population composed largely of children who were well before hospitalization.
The mortality rate from measles was numerically but not statistically higher than that of overall hospital admissions during this period, but an admission for measles was associated with significantly longer average length of stay (3.7 vs. 3.5 days) and slightly but significantly higher direct costs ($18,907 vs. $18,474).
“I want to point out that these are just direct inpatient costs,” Dr. Chovatiya said. Extrapolating from published data about indirect expenses, he said that the total health cost burden “is absolutely staggering.”
Previous studies have suggested that about 25% of patients with measles require hospitalization and 1 in every 1,000 patients will die. The data collected by Dr. Chovatiya support these often-cited figures, indicating that they remain unchanged in the modern era.
particularly insufficient penetration of vaccination in many communities.
The vaccine “is inexpensive, extremely effective, and lifesaving,” said Dr. Chovatiya, making the point that all of the morbidity, mortality, and costs he described are largely avoidable.
Attempting to provide perspective of the measles threat and the impact of the vaccine, Dr. Chovatiya cited a hypothetical calculation that 732,000 deaths from measles would have been expected in the United States among the pool of children born between 1994 and 2013 had no vaccine been offered. Again, most of these deaths would have occurred in otherwise healthy children.
Dr. Chovatiya reported no potential conflicts of interest.
CHICAGO – An evaluation of the measles threat in the modern era gives no indication that the risk of complications or death is any different than it was before a vaccine became available, according to an analysis of inpatient complications between 2002 and 2013.
In 2000, measles was declared eliminated in the United States, but for those who have been infected since that time, the risk of serious complications and death has not diminished, noted Raj Chovatiya, MD, PhD, in a session at the annual meeting of the Society for Investigative Dermatology.
By eliminated, the Centers of Disease Control and Prevention – which reported 86 confirmed cases of measles in 2000 – was referring to a technical definition of no new endemic or continuous transmissions in the previous 12 months. It was expected that a modest number of cases of this reportable disease would continue to accrue for an infection that remains common elsewhere in the world.
“Worldwide there are about 20 million cases of measles annually with an estimated 100,000 deaths attributed to this cause,” said Dr. Chovatiya, who is a dermatology resident at Northwestern University, Chicago.
In the United States, posteradication infection rates remained at low levels for several years but were already rising from 2002 to 2013, when Dr. Chovatiya and his coinvestigators sought to describe the incidence, associations, comorbidities, and outcomes of hospitalizations for measles. Toward the end of the period the researchers were examining the incidence rates climbed more steeply.
“So far this year, 764 CDC cases of measles [were] reported. That is the most we have seen in the U.S. since 1994,” Dr. Chovatiya said.
Based on his analysis of hospitalizations from 2002 to 2013, the threat of these outbreaks is no different then that before the disease was declared eliminated or before a vaccine became available.
The cross-sectional study was conducted with data from the Nationwide Inpatient Sample, an all-payer database that is considered to be a representative of national trends.
Characteristic of measles, the majority of the 582 hospitalizations evaluated over this period occurred in children aged between 1 and 9 years. The proportion of patients with preexisting chronic comorbid conditions was low. Rather, “most were pretty healthy” prior to admission, according to Dr. Chovatiya, who said that the majority of admissions were from an emergency department.
Measles, which targets epithelial cells and depresses the immune system, is a potentially serious disease because of its ability to produce complications in essentially every organ of the body, including the lungs, kidneys, blood, and central nervous system. Consistent with past studies, the most common complication in this series was pneumonia, observed in 20% of patients. The list of other serious complications identified in this study period, including encephalitis and acute renal failure, was long.
“We observed death in 4.3% of our 582 cases, or about 25 cases,” reported Dr. Chovatiya. He indicated that this is a high percentage among a population composed largely of children who were well before hospitalization.
The mortality rate from measles was numerically but not statistically higher than that of overall hospital admissions during this period, but an admission for measles was associated with significantly longer average length of stay (3.7 vs. 3.5 days) and slightly but significantly higher direct costs ($18,907 vs. $18,474).
“I want to point out that these are just direct inpatient costs,” Dr. Chovatiya said. Extrapolating from published data about indirect expenses, he said that the total health cost burden “is absolutely staggering.”
Previous studies have suggested that about 25% of patients with measles require hospitalization and 1 in every 1,000 patients will die. The data collected by Dr. Chovatiya support these often-cited figures, indicating that they remain unchanged in the modern era.
particularly insufficient penetration of vaccination in many communities.
The vaccine “is inexpensive, extremely effective, and lifesaving,” said Dr. Chovatiya, making the point that all of the morbidity, mortality, and costs he described are largely avoidable.
Attempting to provide perspective of the measles threat and the impact of the vaccine, Dr. Chovatiya cited a hypothetical calculation that 732,000 deaths from measles would have been expected in the United States among the pool of children born between 1994 and 2013 had no vaccine been offered. Again, most of these deaths would have occurred in otherwise healthy children.
Dr. Chovatiya reported no potential conflicts of interest.
CHICAGO – An evaluation of the measles threat in the modern era gives no indication that the risk of complications or death is any different than it was before a vaccine became available, according to an analysis of inpatient complications between 2002 and 2013.
In 2000, measles was declared eliminated in the United States, but for those who have been infected since that time, the risk of serious complications and death has not diminished, noted Raj Chovatiya, MD, PhD, in a session at the annual meeting of the Society for Investigative Dermatology.
By eliminated, the Centers of Disease Control and Prevention – which reported 86 confirmed cases of measles in 2000 – was referring to a technical definition of no new endemic or continuous transmissions in the previous 12 months. It was expected that a modest number of cases of this reportable disease would continue to accrue for an infection that remains common elsewhere in the world.
“Worldwide there are about 20 million cases of measles annually with an estimated 100,000 deaths attributed to this cause,” said Dr. Chovatiya, who is a dermatology resident at Northwestern University, Chicago.
In the United States, posteradication infection rates remained at low levels for several years but were already rising from 2002 to 2013, when Dr. Chovatiya and his coinvestigators sought to describe the incidence, associations, comorbidities, and outcomes of hospitalizations for measles. Toward the end of the period the researchers were examining the incidence rates climbed more steeply.
“So far this year, 764 CDC cases of measles [were] reported. That is the most we have seen in the U.S. since 1994,” Dr. Chovatiya said.
Based on his analysis of hospitalizations from 2002 to 2013, the threat of these outbreaks is no different then that before the disease was declared eliminated or before a vaccine became available.
The cross-sectional study was conducted with data from the Nationwide Inpatient Sample, an all-payer database that is considered to be a representative of national trends.
Characteristic of measles, the majority of the 582 hospitalizations evaluated over this period occurred in children aged between 1 and 9 years. The proportion of patients with preexisting chronic comorbid conditions was low. Rather, “most were pretty healthy” prior to admission, according to Dr. Chovatiya, who said that the majority of admissions were from an emergency department.
Measles, which targets epithelial cells and depresses the immune system, is a potentially serious disease because of its ability to produce complications in essentially every organ of the body, including the lungs, kidneys, blood, and central nervous system. Consistent with past studies, the most common complication in this series was pneumonia, observed in 20% of patients. The list of other serious complications identified in this study period, including encephalitis and acute renal failure, was long.
“We observed death in 4.3% of our 582 cases, or about 25 cases,” reported Dr. Chovatiya. He indicated that this is a high percentage among a population composed largely of children who were well before hospitalization.
The mortality rate from measles was numerically but not statistically higher than that of overall hospital admissions during this period, but an admission for measles was associated with significantly longer average length of stay (3.7 vs. 3.5 days) and slightly but significantly higher direct costs ($18,907 vs. $18,474).
“I want to point out that these are just direct inpatient costs,” Dr. Chovatiya said. Extrapolating from published data about indirect expenses, he said that the total health cost burden “is absolutely staggering.”
Previous studies have suggested that about 25% of patients with measles require hospitalization and 1 in every 1,000 patients will die. The data collected by Dr. Chovatiya support these often-cited figures, indicating that they remain unchanged in the modern era.
particularly insufficient penetration of vaccination in many communities.
The vaccine “is inexpensive, extremely effective, and lifesaving,” said Dr. Chovatiya, making the point that all of the morbidity, mortality, and costs he described are largely avoidable.
Attempting to provide perspective of the measles threat and the impact of the vaccine, Dr. Chovatiya cited a hypothetical calculation that 732,000 deaths from measles would have been expected in the United States among the pool of children born between 1994 and 2013 had no vaccine been offered. Again, most of these deaths would have occurred in otherwise healthy children.
Dr. Chovatiya reported no potential conflicts of interest.
REPORTING FROM SID 2019
Relatively high starting infliximab doses recommended for hidradenitis suppurativa
CHICAGO – In patients with hidradenitis suppurativa (HS), infliximab started at 7.5 mg/kg was highly effective and well tolerated and may be a more appropriate starting dose than the 5 mg/kg that is recommended in the treatment of plaque psoriasis, according to a chart review presented as a late breaker at the annual meeting of the Society for Investigative Dermatology.
“Based on these data, we believe that a high starting dose of infliximab should be the new paradigm for this disease,” reported Justine Potemkin, a medical student at Albert Einstein College of Medicine, New York. The analysis was performed under the mentorship of Steven R. Cohen, MD, professor of dermatology and chief of the division of dermatology at Albert Einstein.
Infliximab, a tumor necrosis factor (TNF) blocker, is not approved for the treatment of HS, but it has been widely used following numerous reports of benefit in the literature, including a phase 2 randomized study published several years ago (J Am Acad Dermatol. 2010 Feb;62[2]:205-17), according to Ms. Potemkin.
“Based on dosing recommended for psoriasis, many hidradenitis suppurativa patients are started on 5 mg/kg, but doses of up to 10 mg/kg are within labeling for psoriasis, and our data support more aggressive initial treatment in patients with HS,” said Mondana Ghias, a research assistant in the department of dermatology at Albert Einstein, who was also involved in this study and joined Ms. Potemkin in presenting the data at the meeting.
In the chart review, 58 patients with moderate to severe HS initiated on 7.5 mg/kg of intravenous infliximab were identified. Due to a suboptimal response, 16 of these patients received 10 mg/kg infliximab in subsequent infusions, but the average response scores at 4 weeks compared favorably to previous reports with lower doses of infliximab, without increased toxicity.
“Starting with the 7.5 mg/kg dose with dose escalation if needed appears to provide a maximal response with minimal safety issues, based on our experience,” Ms. Potemkin reported.
At 4 weeks, a 10-point pain scale fell from a baseline mean of 5.65 to 1.34 (P less than .01). It reached 0.52 at week 12. The HS Physician Global Assessment (HS-PGA) fell from a baseline mean of 4.2 to 2.1 at week 4 (P less than .01) and then to 1.9 at week 12. The control of disease activity correlated with significant reductions in C-reactive protein and erythrocyte sedimentation rate.
The mean age in this patient population was 34.6 years. Slightly more than half (53.3%) were female. The average body mass index was 34.
“Obese patients also achieved a good response, which is consistent with weight-based dosing,” Ms. Potemkin reported.
The TNF blocker adalimumab is the only biologic and the only systemic therapy given regulatory approval for the treatment of HS, but Ms. Potemkin indicated that infliximab remains a widely used treatment option. She suggested that the responses observed in this patient series suggest infliximab is effective when used aggressively.
“No previous reports have examined the efficacy of high-dose infliximab,” Ms. Potemkin noted. “We think these findings are relevant to HS management.”
CHICAGO – In patients with hidradenitis suppurativa (HS), infliximab started at 7.5 mg/kg was highly effective and well tolerated and may be a more appropriate starting dose than the 5 mg/kg that is recommended in the treatment of plaque psoriasis, according to a chart review presented as a late breaker at the annual meeting of the Society for Investigative Dermatology.
“Based on these data, we believe that a high starting dose of infliximab should be the new paradigm for this disease,” reported Justine Potemkin, a medical student at Albert Einstein College of Medicine, New York. The analysis was performed under the mentorship of Steven R. Cohen, MD, professor of dermatology and chief of the division of dermatology at Albert Einstein.
Infliximab, a tumor necrosis factor (TNF) blocker, is not approved for the treatment of HS, but it has been widely used following numerous reports of benefit in the literature, including a phase 2 randomized study published several years ago (J Am Acad Dermatol. 2010 Feb;62[2]:205-17), according to Ms. Potemkin.
“Based on dosing recommended for psoriasis, many hidradenitis suppurativa patients are started on 5 mg/kg, but doses of up to 10 mg/kg are within labeling for psoriasis, and our data support more aggressive initial treatment in patients with HS,” said Mondana Ghias, a research assistant in the department of dermatology at Albert Einstein, who was also involved in this study and joined Ms. Potemkin in presenting the data at the meeting.
In the chart review, 58 patients with moderate to severe HS initiated on 7.5 mg/kg of intravenous infliximab were identified. Due to a suboptimal response, 16 of these patients received 10 mg/kg infliximab in subsequent infusions, but the average response scores at 4 weeks compared favorably to previous reports with lower doses of infliximab, without increased toxicity.
“Starting with the 7.5 mg/kg dose with dose escalation if needed appears to provide a maximal response with minimal safety issues, based on our experience,” Ms. Potemkin reported.
At 4 weeks, a 10-point pain scale fell from a baseline mean of 5.65 to 1.34 (P less than .01). It reached 0.52 at week 12. The HS Physician Global Assessment (HS-PGA) fell from a baseline mean of 4.2 to 2.1 at week 4 (P less than .01) and then to 1.9 at week 12. The control of disease activity correlated with significant reductions in C-reactive protein and erythrocyte sedimentation rate.
The mean age in this patient population was 34.6 years. Slightly more than half (53.3%) were female. The average body mass index was 34.
“Obese patients also achieved a good response, which is consistent with weight-based dosing,” Ms. Potemkin reported.
The TNF blocker adalimumab is the only biologic and the only systemic therapy given regulatory approval for the treatment of HS, but Ms. Potemkin indicated that infliximab remains a widely used treatment option. She suggested that the responses observed in this patient series suggest infliximab is effective when used aggressively.
“No previous reports have examined the efficacy of high-dose infliximab,” Ms. Potemkin noted. “We think these findings are relevant to HS management.”
CHICAGO – In patients with hidradenitis suppurativa (HS), infliximab started at 7.5 mg/kg was highly effective and well tolerated and may be a more appropriate starting dose than the 5 mg/kg that is recommended in the treatment of plaque psoriasis, according to a chart review presented as a late breaker at the annual meeting of the Society for Investigative Dermatology.
“Based on these data, we believe that a high starting dose of infliximab should be the new paradigm for this disease,” reported Justine Potemkin, a medical student at Albert Einstein College of Medicine, New York. The analysis was performed under the mentorship of Steven R. Cohen, MD, professor of dermatology and chief of the division of dermatology at Albert Einstein.
Infliximab, a tumor necrosis factor (TNF) blocker, is not approved for the treatment of HS, but it has been widely used following numerous reports of benefit in the literature, including a phase 2 randomized study published several years ago (J Am Acad Dermatol. 2010 Feb;62[2]:205-17), according to Ms. Potemkin.
“Based on dosing recommended for psoriasis, many hidradenitis suppurativa patients are started on 5 mg/kg, but doses of up to 10 mg/kg are within labeling for psoriasis, and our data support more aggressive initial treatment in patients with HS,” said Mondana Ghias, a research assistant in the department of dermatology at Albert Einstein, who was also involved in this study and joined Ms. Potemkin in presenting the data at the meeting.
In the chart review, 58 patients with moderate to severe HS initiated on 7.5 mg/kg of intravenous infliximab were identified. Due to a suboptimal response, 16 of these patients received 10 mg/kg infliximab in subsequent infusions, but the average response scores at 4 weeks compared favorably to previous reports with lower doses of infliximab, without increased toxicity.
“Starting with the 7.5 mg/kg dose with dose escalation if needed appears to provide a maximal response with minimal safety issues, based on our experience,” Ms. Potemkin reported.
At 4 weeks, a 10-point pain scale fell from a baseline mean of 5.65 to 1.34 (P less than .01). It reached 0.52 at week 12. The HS Physician Global Assessment (HS-PGA) fell from a baseline mean of 4.2 to 2.1 at week 4 (P less than .01) and then to 1.9 at week 12. The control of disease activity correlated with significant reductions in C-reactive protein and erythrocyte sedimentation rate.
The mean age in this patient population was 34.6 years. Slightly more than half (53.3%) were female. The average body mass index was 34.
“Obese patients also achieved a good response, which is consistent with weight-based dosing,” Ms. Potemkin reported.
The TNF blocker adalimumab is the only biologic and the only systemic therapy given regulatory approval for the treatment of HS, but Ms. Potemkin indicated that infliximab remains a widely used treatment option. She suggested that the responses observed in this patient series suggest infliximab is effective when used aggressively.
“No previous reports have examined the efficacy of high-dose infliximab,” Ms. Potemkin noted. “We think these findings are relevant to HS management.”
REPORTING FROM SID 2019
Baseline imaging recommended in all Merkel cell carcinoma patients
CHICAGO – , including those without palpable lymph nodes, according to results of an analysis of a large MCC registry presented at the annual meeting of the Society for Investigative Dermatology.
The results were a “surprise,” according to Neha Singh, a researcher in the division of dermatology at the University of Washington, Seattle. She contended that many treatment guidelines for MCC, including imaging at the time of diagnosis, are borrowed from those developed for melanoma but should not be.
“MCC is much more frequently metastatic to regional and distant sites than melanoma, so current melanoma guidelines may not be appropriate for use with MCC,” she said. According to the data she cited, 41% of MCC patients, versus 14% of melanoma patients, already have metastatic disease at the time of diagnosis.
The presence of more aggressive disease and the need for scanning was confirmed in the analysis of the MCC Registry in Seattle, which contains 1,439 patients. Of 586 patients who met inclusion criteria for this analysis, 493 MCC patients had no palpable lymph nodes at the time of diagnosis. Yet, 60 (12%) proved to already have regional or distant metastases on the basis of scans.
This contrasts starkly with melanoma data, according to Ms. Singh. Guidelines from the National Comprehensive Cancer Network (NCCN) do not recommend scans in melanoma patients without palpable lymph nodes based on evidence that only 1% of these will be upstaged by imaging. This figure was judged too small to justify routine scans, she said.
In melanoma patients with palpable lymph nodes, NCCN guidelines do recommend imaging at diagnosis because upstaging is common, and the same is true in MCC, Ms. Singh noted. In the Seattle registry, 10 (11%) of the 93 patients with palpable lymph nodes were upstaged for distant metastases found on imaging.
In those without palpable lymph nodes, “even a small tumor does not guarantee the absence of distant metastases,” Ms. Singh cautioned. Although the median tumor size in this group was 2.3 cm, tumors of less than 1 cm were still associated with distant disease.
The likelihood of distant disease in MCC patients without palpable lymph nodes might be even greater than that identified in this analysis. At least some of the patients in this series were evaluated with CT rather than PET imaging, which is more sensitive. Ms. Singh reported that no stratification to determine rates of distant disease by imaging type have yet been undertaken in this dataset.
Based on these findings, guidelines for MCC should include consideration of baseline imaging in all patients, Ms. Singh said. In making this point, she also emphasized the guidelines for melanoma should not be considered transferable to MCC.
“Why is this important?” Ms. Singh asked. Understaging MCC “may lead to inadequate surgery, overaggressive local therapy, and a potential delay to effective systemic therapy.”
CHICAGO – , including those without palpable lymph nodes, according to results of an analysis of a large MCC registry presented at the annual meeting of the Society for Investigative Dermatology.
The results were a “surprise,” according to Neha Singh, a researcher in the division of dermatology at the University of Washington, Seattle. She contended that many treatment guidelines for MCC, including imaging at the time of diagnosis, are borrowed from those developed for melanoma but should not be.
“MCC is much more frequently metastatic to regional and distant sites than melanoma, so current melanoma guidelines may not be appropriate for use with MCC,” she said. According to the data she cited, 41% of MCC patients, versus 14% of melanoma patients, already have metastatic disease at the time of diagnosis.
The presence of more aggressive disease and the need for scanning was confirmed in the analysis of the MCC Registry in Seattle, which contains 1,439 patients. Of 586 patients who met inclusion criteria for this analysis, 493 MCC patients had no palpable lymph nodes at the time of diagnosis. Yet, 60 (12%) proved to already have regional or distant metastases on the basis of scans.
This contrasts starkly with melanoma data, according to Ms. Singh. Guidelines from the National Comprehensive Cancer Network (NCCN) do not recommend scans in melanoma patients without palpable lymph nodes based on evidence that only 1% of these will be upstaged by imaging. This figure was judged too small to justify routine scans, she said.
In melanoma patients with palpable lymph nodes, NCCN guidelines do recommend imaging at diagnosis because upstaging is common, and the same is true in MCC, Ms. Singh noted. In the Seattle registry, 10 (11%) of the 93 patients with palpable lymph nodes were upstaged for distant metastases found on imaging.
In those without palpable lymph nodes, “even a small tumor does not guarantee the absence of distant metastases,” Ms. Singh cautioned. Although the median tumor size in this group was 2.3 cm, tumors of less than 1 cm were still associated with distant disease.
The likelihood of distant disease in MCC patients without palpable lymph nodes might be even greater than that identified in this analysis. At least some of the patients in this series were evaluated with CT rather than PET imaging, which is more sensitive. Ms. Singh reported that no stratification to determine rates of distant disease by imaging type have yet been undertaken in this dataset.
Based on these findings, guidelines for MCC should include consideration of baseline imaging in all patients, Ms. Singh said. In making this point, she also emphasized the guidelines for melanoma should not be considered transferable to MCC.
“Why is this important?” Ms. Singh asked. Understaging MCC “may lead to inadequate surgery, overaggressive local therapy, and a potential delay to effective systemic therapy.”
CHICAGO – , including those without palpable lymph nodes, according to results of an analysis of a large MCC registry presented at the annual meeting of the Society for Investigative Dermatology.
The results were a “surprise,” according to Neha Singh, a researcher in the division of dermatology at the University of Washington, Seattle. She contended that many treatment guidelines for MCC, including imaging at the time of diagnosis, are borrowed from those developed for melanoma but should not be.
“MCC is much more frequently metastatic to regional and distant sites than melanoma, so current melanoma guidelines may not be appropriate for use with MCC,” she said. According to the data she cited, 41% of MCC patients, versus 14% of melanoma patients, already have metastatic disease at the time of diagnosis.
The presence of more aggressive disease and the need for scanning was confirmed in the analysis of the MCC Registry in Seattle, which contains 1,439 patients. Of 586 patients who met inclusion criteria for this analysis, 493 MCC patients had no palpable lymph nodes at the time of diagnosis. Yet, 60 (12%) proved to already have regional or distant metastases on the basis of scans.
This contrasts starkly with melanoma data, according to Ms. Singh. Guidelines from the National Comprehensive Cancer Network (NCCN) do not recommend scans in melanoma patients without palpable lymph nodes based on evidence that only 1% of these will be upstaged by imaging. This figure was judged too small to justify routine scans, she said.
In melanoma patients with palpable lymph nodes, NCCN guidelines do recommend imaging at diagnosis because upstaging is common, and the same is true in MCC, Ms. Singh noted. In the Seattle registry, 10 (11%) of the 93 patients with palpable lymph nodes were upstaged for distant metastases found on imaging.
In those without palpable lymph nodes, “even a small tumor does not guarantee the absence of distant metastases,” Ms. Singh cautioned. Although the median tumor size in this group was 2.3 cm, tumors of less than 1 cm were still associated with distant disease.
The likelihood of distant disease in MCC patients without palpable lymph nodes might be even greater than that identified in this analysis. At least some of the patients in this series were evaluated with CT rather than PET imaging, which is more sensitive. Ms. Singh reported that no stratification to determine rates of distant disease by imaging type have yet been undertaken in this dataset.
Based on these findings, guidelines for MCC should include consideration of baseline imaging in all patients, Ms. Singh said. In making this point, she also emphasized the guidelines for melanoma should not be considered transferable to MCC.
“Why is this important?” Ms. Singh asked. Understaging MCC “may lead to inadequate surgery, overaggressive local therapy, and a potential delay to effective systemic therapy.”
REPORTING FROM SID 2019
Gentamicin restores wound healing in hereditary epidermolysis bullosa
Rare progress seen in challenging disease
CHICAGO – Topical gentamicin counters the nonsense mutations that inhibit production of laminin 332 in infants with Herlitz junctional epidermolysis bullosa (H-JEB) to allow lesion healing, according to results of a small clinical study presented at the annual meeting of the Society for Investigative Dermatology.
“All of the children treated so far have responded,” reported Andrew Kwong, who will soon graduate from the Keck School of Medicine at the University of Southern California, Los Angeles.
H-JEB is an inherited blistering skin disease associated with nonsense mutations in the LAMA3, LAMB3, or LAMC2 genes that result in impaired production of functional laminin 332, an essential protein for epidermal-dermal adherence. At this time there are no effective therapies, and the disease is fatal.
The small clinical study was initiated after in vitro studies demonstrated that gentamicin restored functional laminin 332 in cultured keratinocytes from infants with H-JEB. The dose-dependent effect was credited to the ability of gentamicin to induce readthrough of premature stop codons that block production of laminin 332.
Data were presented on the first three infants with H-JEB treated with oral gentamicin. In each child, lesions were treated with topical 0.5% gentamicin twice daily for two weeks. Biopsies were taken prior to the initiation of treatment and at one and three months after treatment. The primary outcome was change in laminin 332, but clinical improvement was also monitored.
Although none of the infants had measurable laminin 332 prior to treatment, all lesions treated with topical gentamicin developed localized laminin 332 at the dermal-epidermal junction of the skin, Mr. Kwong reported. This expression, which was about 40% to 60% of that seen in normal skin, still persisted when evaluated three months after treatment.
The expression was associated with resolution of existing lesions and a reduced risk of developing new lesions, according to Mr. Kwong. In lesions that went untreated, there was no change.
Other molecular changes in the skin, such as increased expression and polarization of beta-4 integrin, were consistent with the ability of gentamicin to address the underlying pathophysiology of H-JEB. There were no adverse events observed.
By restoring functional laminin 332 in the skin, topical gentamicin appears to address the underlying cause of the bullae associated with H-JEB, but Mr. Kwong said that the next step is to determine whether intravenous gentamicin can address the systemic effects. If so, this treatment has the potential to improve survival. He reported that an infant with H-JEB was recently started on intravenous treatment, and initial results were encouraging.
Asked whether he would recommend topical gentamicin on the basis of these findings, Mr. Kwong cautioned that the case series remains very small, but he noted that the uniformity of the positive response is encouraging. He expects that off-label use of this novel and low-cost approach might be warranted in a population that has very limited therapeutic options.
SOURCE: Kwong A. SID 2019;S102, Abstract 594. Annual meeting of the Society for Investigative Dermatology.
Rare progress seen in challenging disease
Rare progress seen in challenging disease
CHICAGO – Topical gentamicin counters the nonsense mutations that inhibit production of laminin 332 in infants with Herlitz junctional epidermolysis bullosa (H-JEB) to allow lesion healing, according to results of a small clinical study presented at the annual meeting of the Society for Investigative Dermatology.
“All of the children treated so far have responded,” reported Andrew Kwong, who will soon graduate from the Keck School of Medicine at the University of Southern California, Los Angeles.
H-JEB is an inherited blistering skin disease associated with nonsense mutations in the LAMA3, LAMB3, or LAMC2 genes that result in impaired production of functional laminin 332, an essential protein for epidermal-dermal adherence. At this time there are no effective therapies, and the disease is fatal.
The small clinical study was initiated after in vitro studies demonstrated that gentamicin restored functional laminin 332 in cultured keratinocytes from infants with H-JEB. The dose-dependent effect was credited to the ability of gentamicin to induce readthrough of premature stop codons that block production of laminin 332.
Data were presented on the first three infants with H-JEB treated with oral gentamicin. In each child, lesions were treated with topical 0.5% gentamicin twice daily for two weeks. Biopsies were taken prior to the initiation of treatment and at one and three months after treatment. The primary outcome was change in laminin 332, but clinical improvement was also monitored.
Although none of the infants had measurable laminin 332 prior to treatment, all lesions treated with topical gentamicin developed localized laminin 332 at the dermal-epidermal junction of the skin, Mr. Kwong reported. This expression, which was about 40% to 60% of that seen in normal skin, still persisted when evaluated three months after treatment.
The expression was associated with resolution of existing lesions and a reduced risk of developing new lesions, according to Mr. Kwong. In lesions that went untreated, there was no change.
Other molecular changes in the skin, such as increased expression and polarization of beta-4 integrin, were consistent with the ability of gentamicin to address the underlying pathophysiology of H-JEB. There were no adverse events observed.
By restoring functional laminin 332 in the skin, topical gentamicin appears to address the underlying cause of the bullae associated with H-JEB, but Mr. Kwong said that the next step is to determine whether intravenous gentamicin can address the systemic effects. If so, this treatment has the potential to improve survival. He reported that an infant with H-JEB was recently started on intravenous treatment, and initial results were encouraging.
Asked whether he would recommend topical gentamicin on the basis of these findings, Mr. Kwong cautioned that the case series remains very small, but he noted that the uniformity of the positive response is encouraging. He expects that off-label use of this novel and low-cost approach might be warranted in a population that has very limited therapeutic options.
SOURCE: Kwong A. SID 2019;S102, Abstract 594. Annual meeting of the Society for Investigative Dermatology.
CHICAGO – Topical gentamicin counters the nonsense mutations that inhibit production of laminin 332 in infants with Herlitz junctional epidermolysis bullosa (H-JEB) to allow lesion healing, according to results of a small clinical study presented at the annual meeting of the Society for Investigative Dermatology.
“All of the children treated so far have responded,” reported Andrew Kwong, who will soon graduate from the Keck School of Medicine at the University of Southern California, Los Angeles.
H-JEB is an inherited blistering skin disease associated with nonsense mutations in the LAMA3, LAMB3, or LAMC2 genes that result in impaired production of functional laminin 332, an essential protein for epidermal-dermal adherence. At this time there are no effective therapies, and the disease is fatal.
The small clinical study was initiated after in vitro studies demonstrated that gentamicin restored functional laminin 332 in cultured keratinocytes from infants with H-JEB. The dose-dependent effect was credited to the ability of gentamicin to induce readthrough of premature stop codons that block production of laminin 332.
Data were presented on the first three infants with H-JEB treated with oral gentamicin. In each child, lesions were treated with topical 0.5% gentamicin twice daily for two weeks. Biopsies were taken prior to the initiation of treatment and at one and three months after treatment. The primary outcome was change in laminin 332, but clinical improvement was also monitored.
Although none of the infants had measurable laminin 332 prior to treatment, all lesions treated with topical gentamicin developed localized laminin 332 at the dermal-epidermal junction of the skin, Mr. Kwong reported. This expression, which was about 40% to 60% of that seen in normal skin, still persisted when evaluated three months after treatment.
The expression was associated with resolution of existing lesions and a reduced risk of developing new lesions, according to Mr. Kwong. In lesions that went untreated, there was no change.
Other molecular changes in the skin, such as increased expression and polarization of beta-4 integrin, were consistent with the ability of gentamicin to address the underlying pathophysiology of H-JEB. There were no adverse events observed.
By restoring functional laminin 332 in the skin, topical gentamicin appears to address the underlying cause of the bullae associated with H-JEB, but Mr. Kwong said that the next step is to determine whether intravenous gentamicin can address the systemic effects. If so, this treatment has the potential to improve survival. He reported that an infant with H-JEB was recently started on intravenous treatment, and initial results were encouraging.
Asked whether he would recommend topical gentamicin on the basis of these findings, Mr. Kwong cautioned that the case series remains very small, but he noted that the uniformity of the positive response is encouraging. He expects that off-label use of this novel and low-cost approach might be warranted in a population that has very limited therapeutic options.
SOURCE: Kwong A. SID 2019;S102, Abstract 594. Annual meeting of the Society for Investigative Dermatology.
REPORTING FROM SID 2019