User login
Adding a Cream May Enhance Flu Vaccine Effectiveness
Can a cream help a flu vaccine work better? In a phase 1 clinical trial, researchers from Baylor College of Medicine in Houston, Texas, are testing whether imiquimod cream—commonly used to treat genital warts and some skin cancers—can boost the immune response to an H5N1 flu vaccine. Studies have shown imiquimod generates significantly more robust immune responses.
Participants in the Vaccine and Treatment Evaluation Units trial will be given 2 intradermal doses of an H5N1 vaccine, 21 days apart. In one group, participants will have Aldara (imiquimod cream) applied to their upper arm before each vaccination; in the control group, a placebo cream will be applied.
Participants will return at regular intervals over 7 months to have blood drawn; they also will keep diaries to record symptoms.
The first participant was vaccinated in June. Early results are expected by the end of the year.
Can a cream help a flu vaccine work better? In a phase 1 clinical trial, researchers from Baylor College of Medicine in Houston, Texas, are testing whether imiquimod cream—commonly used to treat genital warts and some skin cancers—can boost the immune response to an H5N1 flu vaccine. Studies have shown imiquimod generates significantly more robust immune responses.
Participants in the Vaccine and Treatment Evaluation Units trial will be given 2 intradermal doses of an H5N1 vaccine, 21 days apart. In one group, participants will have Aldara (imiquimod cream) applied to their upper arm before each vaccination; in the control group, a placebo cream will be applied.
Participants will return at regular intervals over 7 months to have blood drawn; they also will keep diaries to record symptoms.
The first participant was vaccinated in June. Early results are expected by the end of the year.
Can a cream help a flu vaccine work better? In a phase 1 clinical trial, researchers from Baylor College of Medicine in Houston, Texas, are testing whether imiquimod cream—commonly used to treat genital warts and some skin cancers—can boost the immune response to an H5N1 flu vaccine. Studies have shown imiquimod generates significantly more robust immune responses.
Participants in the Vaccine and Treatment Evaluation Units trial will be given 2 intradermal doses of an H5N1 vaccine, 21 days apart. In one group, participants will have Aldara (imiquimod cream) applied to their upper arm before each vaccination; in the control group, a placebo cream will be applied.
Participants will return at regular intervals over 7 months to have blood drawn; they also will keep diaries to record symptoms.
The first participant was vaccinated in June. Early results are expected by the end of the year.
CDC: Trivalent adjuvanted influenza vaccine aIIV3 safe in elderly adults
ATLANTA – according to an analysis of reports to the Vaccine Adverse Event Reporting System (VAERS) during July 2016 through March 2018.
VAERS received 630 reports related to the vaccine (aIIV3; FLUAD) during the study period, of which 521 involved adults aged 65 years and older.
“Eighteen (3%) were serious reports, including two death reports (0.4%), all in adults aged [at least] 65 years,” Penina Haber and her colleagues at the Immunization Safety Office at the Centers for Disease Control and Prevention reported in a poster at the International Conference on Emerging Infectious Diseases.
The deaths included a 75-year-old man who died from Sjögren’s syndrome and a 65-year-old man who died from a myocardial infarction. The other serious events included five neurologic disorders (two cases of Guillain-Barré syndrome and one each of Bell’s palsy, Bickerstaff encephalitis, and lower-extremity weakness), five musculoskeletal and connective tissue disorders (three with shoulder pain and two with arm pain), three general disorders and administration site conditions (two cases of fever/chills and one case of cellulitis/bursitis), and one case each of a gastrointestinal disorder (acute diarrhea/gastroenteritis), an injury (a fall), and a skin/subcutaneous tissue disorder (keratosis pilaris rubra), according to the investigators.
There were no reports of anaphylaxis.
For the sake of comparison, the investigators also looked at reports associated with IIV3-HD and IIV3/IIV4 vaccines in adults aged 65 years and older during the same time period; they found that patient characteristics and reported events were similar for all the vaccines. For example, the percentages of reports involving patients aged 65 years and older were 65% or 66% for each, and those involving patients aged 75-84 years were 27%-29%. Further, 0.2%-0.6% of reports for each vaccine involved death.
The most frequently reported events for aIIV3, IIV3-HD, and IIV3/IIV4, respectively, were extremity pain (21%, 17%, and 15%, respectively), injection site erythema (18%, 19%, and 15%), and injection site pain (15%, 16%, and 16%), they said.
The aIIV3 vaccine – the first seasonal inactivated trivalent influenza vaccine produced from three influenza virus strains (two subtype A strains and one type B strain) – was approved by the Food and Drug Administration in 2015 for adults aged 65 years and older. It was the first influenza vaccine containing the adjuvant MF59 – a purified oil-in-water emulsion of squalene oil added to boost immune response in that population. Its safety was assessed in 15 randomized, controlled clinical studies, and several trials in older adults supported its efficacy and safety over nonadjuvanted influenza vaccines, the investigators reported. They noted that the Advisory Committee on Immunization Practices (ACIP) recommended the vaccine as an option for routine use in adults aged 65 years and older during the 2016-2017 flu seasons.
For the 2018-2019 flu season, ACIP determined that “For persons aged ≥65 years, any age-appropriate IIV formulation (standard-dose or high-dose, trivalent or quadrivalent, unadjuvanted or adjuvanted) or RIV4 are acceptable options.”
The findings of the analysis of the 2017-2018 flu season data are consistent with prelicensure studies, Ms. Haber and her colleagues concluded, noting that data mining did not detect disproportional reporting of any unexpected adverse event.
“[There were] no safety concerns following aIIV3 when compared to the nonadjuvanted influenza vaccines (IIV3-HD or IIV3/IIV4),” they wrote, adding that the “CDC and FDA will continue to monitor and ensure the safety of aIIV3.”
Ms. Haber reported having no disclosures
SOURCE: Haber P et al. ICEID 2018, Board 320.
ATLANTA – according to an analysis of reports to the Vaccine Adverse Event Reporting System (VAERS) during July 2016 through March 2018.
VAERS received 630 reports related to the vaccine (aIIV3; FLUAD) during the study period, of which 521 involved adults aged 65 years and older.
“Eighteen (3%) were serious reports, including two death reports (0.4%), all in adults aged [at least] 65 years,” Penina Haber and her colleagues at the Immunization Safety Office at the Centers for Disease Control and Prevention reported in a poster at the International Conference on Emerging Infectious Diseases.
The deaths included a 75-year-old man who died from Sjögren’s syndrome and a 65-year-old man who died from a myocardial infarction. The other serious events included five neurologic disorders (two cases of Guillain-Barré syndrome and one each of Bell’s palsy, Bickerstaff encephalitis, and lower-extremity weakness), five musculoskeletal and connective tissue disorders (three with shoulder pain and two with arm pain), three general disorders and administration site conditions (two cases of fever/chills and one case of cellulitis/bursitis), and one case each of a gastrointestinal disorder (acute diarrhea/gastroenteritis), an injury (a fall), and a skin/subcutaneous tissue disorder (keratosis pilaris rubra), according to the investigators.
There were no reports of anaphylaxis.
For the sake of comparison, the investigators also looked at reports associated with IIV3-HD and IIV3/IIV4 vaccines in adults aged 65 years and older during the same time period; they found that patient characteristics and reported events were similar for all the vaccines. For example, the percentages of reports involving patients aged 65 years and older were 65% or 66% for each, and those involving patients aged 75-84 years were 27%-29%. Further, 0.2%-0.6% of reports for each vaccine involved death.
The most frequently reported events for aIIV3, IIV3-HD, and IIV3/IIV4, respectively, were extremity pain (21%, 17%, and 15%, respectively), injection site erythema (18%, 19%, and 15%), and injection site pain (15%, 16%, and 16%), they said.
The aIIV3 vaccine – the first seasonal inactivated trivalent influenza vaccine produced from three influenza virus strains (two subtype A strains and one type B strain) – was approved by the Food and Drug Administration in 2015 for adults aged 65 years and older. It was the first influenza vaccine containing the adjuvant MF59 – a purified oil-in-water emulsion of squalene oil added to boost immune response in that population. Its safety was assessed in 15 randomized, controlled clinical studies, and several trials in older adults supported its efficacy and safety over nonadjuvanted influenza vaccines, the investigators reported. They noted that the Advisory Committee on Immunization Practices (ACIP) recommended the vaccine as an option for routine use in adults aged 65 years and older during the 2016-2017 flu seasons.
For the 2018-2019 flu season, ACIP determined that “For persons aged ≥65 years, any age-appropriate IIV formulation (standard-dose or high-dose, trivalent or quadrivalent, unadjuvanted or adjuvanted) or RIV4 are acceptable options.”
The findings of the analysis of the 2017-2018 flu season data are consistent with prelicensure studies, Ms. Haber and her colleagues concluded, noting that data mining did not detect disproportional reporting of any unexpected adverse event.
“[There were] no safety concerns following aIIV3 when compared to the nonadjuvanted influenza vaccines (IIV3-HD or IIV3/IIV4),” they wrote, adding that the “CDC and FDA will continue to monitor and ensure the safety of aIIV3.”
Ms. Haber reported having no disclosures
SOURCE: Haber P et al. ICEID 2018, Board 320.
ATLANTA – according to an analysis of reports to the Vaccine Adverse Event Reporting System (VAERS) during July 2016 through March 2018.
VAERS received 630 reports related to the vaccine (aIIV3; FLUAD) during the study period, of which 521 involved adults aged 65 years and older.
“Eighteen (3%) were serious reports, including two death reports (0.4%), all in adults aged [at least] 65 years,” Penina Haber and her colleagues at the Immunization Safety Office at the Centers for Disease Control and Prevention reported in a poster at the International Conference on Emerging Infectious Diseases.
The deaths included a 75-year-old man who died from Sjögren’s syndrome and a 65-year-old man who died from a myocardial infarction. The other serious events included five neurologic disorders (two cases of Guillain-Barré syndrome and one each of Bell’s palsy, Bickerstaff encephalitis, and lower-extremity weakness), five musculoskeletal and connective tissue disorders (three with shoulder pain and two with arm pain), three general disorders and administration site conditions (two cases of fever/chills and one case of cellulitis/bursitis), and one case each of a gastrointestinal disorder (acute diarrhea/gastroenteritis), an injury (a fall), and a skin/subcutaneous tissue disorder (keratosis pilaris rubra), according to the investigators.
There were no reports of anaphylaxis.
For the sake of comparison, the investigators also looked at reports associated with IIV3-HD and IIV3/IIV4 vaccines in adults aged 65 years and older during the same time period; they found that patient characteristics and reported events were similar for all the vaccines. For example, the percentages of reports involving patients aged 65 years and older were 65% or 66% for each, and those involving patients aged 75-84 years were 27%-29%. Further, 0.2%-0.6% of reports for each vaccine involved death.
The most frequently reported events for aIIV3, IIV3-HD, and IIV3/IIV4, respectively, were extremity pain (21%, 17%, and 15%, respectively), injection site erythema (18%, 19%, and 15%), and injection site pain (15%, 16%, and 16%), they said.
The aIIV3 vaccine – the first seasonal inactivated trivalent influenza vaccine produced from three influenza virus strains (two subtype A strains and one type B strain) – was approved by the Food and Drug Administration in 2015 for adults aged 65 years and older. It was the first influenza vaccine containing the adjuvant MF59 – a purified oil-in-water emulsion of squalene oil added to boost immune response in that population. Its safety was assessed in 15 randomized, controlled clinical studies, and several trials in older adults supported its efficacy and safety over nonadjuvanted influenza vaccines, the investigators reported. They noted that the Advisory Committee on Immunization Practices (ACIP) recommended the vaccine as an option for routine use in adults aged 65 years and older during the 2016-2017 flu seasons.
For the 2018-2019 flu season, ACIP determined that “For persons aged ≥65 years, any age-appropriate IIV formulation (standard-dose or high-dose, trivalent or quadrivalent, unadjuvanted or adjuvanted) or RIV4 are acceptable options.”
The findings of the analysis of the 2017-2018 flu season data are consistent with prelicensure studies, Ms. Haber and her colleagues concluded, noting that data mining did not detect disproportional reporting of any unexpected adverse event.
“[There were] no safety concerns following aIIV3 when compared to the nonadjuvanted influenza vaccines (IIV3-HD or IIV3/IIV4),” they wrote, adding that the “CDC and FDA will continue to monitor and ensure the safety of aIIV3.”
Ms. Haber reported having no disclosures
SOURCE: Haber P et al. ICEID 2018, Board 320.
REPORTING FROM ICEID 2018
Key clinical point: No new or unexpected adverse events were reported among the 630 reports related to the vaccine during the study period, of which 521 involved adults aged 65 years and older.
Major finding: Of 521 reports, 18 were serious, and there were two deaths.
Study details: A review of 521 reports to the Vaccine Adverse Event Reporting System in 2017-2018.
Disclosures: Ms. Haber reported having no disclosures.
Source: Haber P et al. ICEID 2018, Board 320.
PCV13 moderately effective in older adults
ATLANTA – (IPD) caused by PCV13 vaccine serotypes in adults aged 65 years and older, according to a case-control study involving Medicare beneficiaries.
Conversely, the 23-valent pneumococcal polysaccharide vaccine (PPSV23) showed limited effectiveness against serotypes unique to that vaccine in the study, which included 699 cases and more than 10,000 controls, Olivia Almendares, an epidemiologist at the Centers for Disease Control and Prevention, Atlanta, and her colleagues reported in a poster at the International Conference on Emerging Infectious Diseases.
“Vaccine efficacy against PCV13 [plus 6C type, which has cross-reactivity with serotype 6A] was 47% in those who received PCV13 vaccine only,” Ms. Almendares said in an interview, noting that efficacy was 26% against serotype 3 and 67% against other PCV13 serotypes (plus 6C). “Vaccine efficacy against PPSV23-unique types was 36% for those who received only PPSV23.”
Neither vaccine showed effectiveness against serotypes not included in the respective vaccines, she said.
The findings are timely given that the Advisory Committee on Immunization Practices (ACIP) is reevaluating its PCV13 recommendation for adults aged 65 years and older, she added.
“Specifically, ACIP is addressing whether PCV13 should be recommended routinely for all immunocompetent adults aged 65 and older given sustained indirect effects,” she said, explaining that, in 2014 when ACIP recommended routine use of the vaccine in series with PPSV23 for adults aged 65 years and older, the committee recognized that herd immunity effects from PCV13 use in children might eventually limit the utility of this recommendation, and therefore it proposed reevaluation and revision as needed after 4 years.
For the current study, she and her colleagues linked IPD cases in persons aged 65 years and older, which were identified through Active Bacterial Core surveillance during 2015-2016, to records for Centers for Medicare & Medicaid Services (CMS) beneficiaries. Vaccination and medical histories were obtained through medical records, and vaccine effectiveness was estimated as one minus the odds ratio for vaccination with PCV13 only or PPSV23 only versus neither vaccine using conditional logistic regression, with adjustment for sex and underlying medical conditions.
Of 2,246 IPD cases, 1,017 (45%) were matched to Medicare beneficiaries, and 699 were included in the analysis after those with noncontinuous enrollment in Medicare, long-term care residence, and missing census tract data were excluded. The cases were matched based on age, census tract of residence, and length of Medicare enrollment to 10,152 matched controls identified through CMS.
IPD associated with PCV13 (plus type 6C) accounted for 164 (23% of cases), of which 88 (12% of cases) involved serotype 3, and invasive pneumococcal disease associated with PPSV23 accounted for 350 cases (50%), she said.
PCV13 vaccine was given alone in 14% and 18% of cases and controls, respectively; PPSV23 alone was given in 22% and 21% of case patients and controls, respectively; and both vaccines were given in 8% of cases and controls.
Compared with controls, case patients were more likely to be of nonwhite race (16% vs. 11%), to have more than one chronic medical condition (88% vs. 58%), and to have one or more immunocompromising conditions (54% vs. 32%), she and her colleagues reported.
“PCV13 showed moderate overall effectiveness in preventing IPD caused by PCV13 (including 6C), but effectiveness may be lower for serotype 3 than for other PCV13 types,” she said.
“These results are in agreement with those from CAPiTA – a large clinical trial conducted in the Netherlands, which showed PCV13 to be effective against IPD caused by vaccine serotypes among community-dwelling adults aged 65 and older,” she noted. “Additionally, data from CDC surveillance suggest that PCV13-serotype [invasive pneumococcal disease] among children and adults aged 65 and older has declined dramatically following PCV13 introduction for children in 2010, as predicted.”
In fact, among adults aged 65 years and older, PCV13-serotype invasive pneumococcal disease declined by 40% after the vaccine was introduced in children. This corresponds to a change in the annual PCV13-serotype incidence from 14 cases per 100,000 population in 2010 to five cases per 100,000 population in 2014, she said; she added that IPD incidence plateaued in 2014-2016 with vaccine serotypes contributing to a small proportion of overall IPD burden among adults aged 65 years and older.
ACIP’s reevaluation of the PCV13 recommendation is ongoing and will be addressed at upcoming meetings.
“As part of the review process, we look at changes in disease incidence focusing primarily on invasive pneumococcal disease and noninvasive pneumonia, vaccine efficacy and effectiveness, and vaccine safety,” she said. She noted that ACIP currently has no plans to consider revising PCV13 recommendations for adults who have immunocompromising conditions, for whom PCV13 has been recommended since 2012.
Ms. Almendares reported having no disclosures.
SOURCE: Almendares O et al. ICEID 2018, Board 376.
ATLANTA – (IPD) caused by PCV13 vaccine serotypes in adults aged 65 years and older, according to a case-control study involving Medicare beneficiaries.
Conversely, the 23-valent pneumococcal polysaccharide vaccine (PPSV23) showed limited effectiveness against serotypes unique to that vaccine in the study, which included 699 cases and more than 10,000 controls, Olivia Almendares, an epidemiologist at the Centers for Disease Control and Prevention, Atlanta, and her colleagues reported in a poster at the International Conference on Emerging Infectious Diseases.
“Vaccine efficacy against PCV13 [plus 6C type, which has cross-reactivity with serotype 6A] was 47% in those who received PCV13 vaccine only,” Ms. Almendares said in an interview, noting that efficacy was 26% against serotype 3 and 67% against other PCV13 serotypes (plus 6C). “Vaccine efficacy against PPSV23-unique types was 36% for those who received only PPSV23.”
Neither vaccine showed effectiveness against serotypes not included in the respective vaccines, she said.
The findings are timely given that the Advisory Committee on Immunization Practices (ACIP) is reevaluating its PCV13 recommendation for adults aged 65 years and older, she added.
“Specifically, ACIP is addressing whether PCV13 should be recommended routinely for all immunocompetent adults aged 65 and older given sustained indirect effects,” she said, explaining that, in 2014 when ACIP recommended routine use of the vaccine in series with PPSV23 for adults aged 65 years and older, the committee recognized that herd immunity effects from PCV13 use in children might eventually limit the utility of this recommendation, and therefore it proposed reevaluation and revision as needed after 4 years.
For the current study, she and her colleagues linked IPD cases in persons aged 65 years and older, which were identified through Active Bacterial Core surveillance during 2015-2016, to records for Centers for Medicare & Medicaid Services (CMS) beneficiaries. Vaccination and medical histories were obtained through medical records, and vaccine effectiveness was estimated as one minus the odds ratio for vaccination with PCV13 only or PPSV23 only versus neither vaccine using conditional logistic regression, with adjustment for sex and underlying medical conditions.
Of 2,246 IPD cases, 1,017 (45%) were matched to Medicare beneficiaries, and 699 were included in the analysis after those with noncontinuous enrollment in Medicare, long-term care residence, and missing census tract data were excluded. The cases were matched based on age, census tract of residence, and length of Medicare enrollment to 10,152 matched controls identified through CMS.
IPD associated with PCV13 (plus type 6C) accounted for 164 (23% of cases), of which 88 (12% of cases) involved serotype 3, and invasive pneumococcal disease associated with PPSV23 accounted for 350 cases (50%), she said.
PCV13 vaccine was given alone in 14% and 18% of cases and controls, respectively; PPSV23 alone was given in 22% and 21% of case patients and controls, respectively; and both vaccines were given in 8% of cases and controls.
Compared with controls, case patients were more likely to be of nonwhite race (16% vs. 11%), to have more than one chronic medical condition (88% vs. 58%), and to have one or more immunocompromising conditions (54% vs. 32%), she and her colleagues reported.
“PCV13 showed moderate overall effectiveness in preventing IPD caused by PCV13 (including 6C), but effectiveness may be lower for serotype 3 than for other PCV13 types,” she said.
“These results are in agreement with those from CAPiTA – a large clinical trial conducted in the Netherlands, which showed PCV13 to be effective against IPD caused by vaccine serotypes among community-dwelling adults aged 65 and older,” she noted. “Additionally, data from CDC surveillance suggest that PCV13-serotype [invasive pneumococcal disease] among children and adults aged 65 and older has declined dramatically following PCV13 introduction for children in 2010, as predicted.”
In fact, among adults aged 65 years and older, PCV13-serotype invasive pneumococcal disease declined by 40% after the vaccine was introduced in children. This corresponds to a change in the annual PCV13-serotype incidence from 14 cases per 100,000 population in 2010 to five cases per 100,000 population in 2014, she said; she added that IPD incidence plateaued in 2014-2016 with vaccine serotypes contributing to a small proportion of overall IPD burden among adults aged 65 years and older.
ACIP’s reevaluation of the PCV13 recommendation is ongoing and will be addressed at upcoming meetings.
“As part of the review process, we look at changes in disease incidence focusing primarily on invasive pneumococcal disease and noninvasive pneumonia, vaccine efficacy and effectiveness, and vaccine safety,” she said. She noted that ACIP currently has no plans to consider revising PCV13 recommendations for adults who have immunocompromising conditions, for whom PCV13 has been recommended since 2012.
Ms. Almendares reported having no disclosures.
SOURCE: Almendares O et al. ICEID 2018, Board 376.
ATLANTA – (IPD) caused by PCV13 vaccine serotypes in adults aged 65 years and older, according to a case-control study involving Medicare beneficiaries.
Conversely, the 23-valent pneumococcal polysaccharide vaccine (PPSV23) showed limited effectiveness against serotypes unique to that vaccine in the study, which included 699 cases and more than 10,000 controls, Olivia Almendares, an epidemiologist at the Centers for Disease Control and Prevention, Atlanta, and her colleagues reported in a poster at the International Conference on Emerging Infectious Diseases.
“Vaccine efficacy against PCV13 [plus 6C type, which has cross-reactivity with serotype 6A] was 47% in those who received PCV13 vaccine only,” Ms. Almendares said in an interview, noting that efficacy was 26% against serotype 3 and 67% against other PCV13 serotypes (plus 6C). “Vaccine efficacy against PPSV23-unique types was 36% for those who received only PPSV23.”
Neither vaccine showed effectiveness against serotypes not included in the respective vaccines, she said.
The findings are timely given that the Advisory Committee on Immunization Practices (ACIP) is reevaluating its PCV13 recommendation for adults aged 65 years and older, she added.
“Specifically, ACIP is addressing whether PCV13 should be recommended routinely for all immunocompetent adults aged 65 and older given sustained indirect effects,” she said, explaining that, in 2014 when ACIP recommended routine use of the vaccine in series with PPSV23 for adults aged 65 years and older, the committee recognized that herd immunity effects from PCV13 use in children might eventually limit the utility of this recommendation, and therefore it proposed reevaluation and revision as needed after 4 years.
For the current study, she and her colleagues linked IPD cases in persons aged 65 years and older, which were identified through Active Bacterial Core surveillance during 2015-2016, to records for Centers for Medicare & Medicaid Services (CMS) beneficiaries. Vaccination and medical histories were obtained through medical records, and vaccine effectiveness was estimated as one minus the odds ratio for vaccination with PCV13 only or PPSV23 only versus neither vaccine using conditional logistic regression, with adjustment for sex and underlying medical conditions.
Of 2,246 IPD cases, 1,017 (45%) were matched to Medicare beneficiaries, and 699 were included in the analysis after those with noncontinuous enrollment in Medicare, long-term care residence, and missing census tract data were excluded. The cases were matched based on age, census tract of residence, and length of Medicare enrollment to 10,152 matched controls identified through CMS.
IPD associated with PCV13 (plus type 6C) accounted for 164 (23% of cases), of which 88 (12% of cases) involved serotype 3, and invasive pneumococcal disease associated with PPSV23 accounted for 350 cases (50%), she said.
PCV13 vaccine was given alone in 14% and 18% of cases and controls, respectively; PPSV23 alone was given in 22% and 21% of case patients and controls, respectively; and both vaccines were given in 8% of cases and controls.
Compared with controls, case patients were more likely to be of nonwhite race (16% vs. 11%), to have more than one chronic medical condition (88% vs. 58%), and to have one or more immunocompromising conditions (54% vs. 32%), she and her colleagues reported.
“PCV13 showed moderate overall effectiveness in preventing IPD caused by PCV13 (including 6C), but effectiveness may be lower for serotype 3 than for other PCV13 types,” she said.
“These results are in agreement with those from CAPiTA – a large clinical trial conducted in the Netherlands, which showed PCV13 to be effective against IPD caused by vaccine serotypes among community-dwelling adults aged 65 and older,” she noted. “Additionally, data from CDC surveillance suggest that PCV13-serotype [invasive pneumococcal disease] among children and adults aged 65 and older has declined dramatically following PCV13 introduction for children in 2010, as predicted.”
In fact, among adults aged 65 years and older, PCV13-serotype invasive pneumococcal disease declined by 40% after the vaccine was introduced in children. This corresponds to a change in the annual PCV13-serotype incidence from 14 cases per 100,000 population in 2010 to five cases per 100,000 population in 2014, she said; she added that IPD incidence plateaued in 2014-2016 with vaccine serotypes contributing to a small proportion of overall IPD burden among adults aged 65 years and older.
ACIP’s reevaluation of the PCV13 recommendation is ongoing and will be addressed at upcoming meetings.
“As part of the review process, we look at changes in disease incidence focusing primarily on invasive pneumococcal disease and noninvasive pneumonia, vaccine efficacy and effectiveness, and vaccine safety,” she said. She noted that ACIP currently has no plans to consider revising PCV13 recommendations for adults who have immunocompromising conditions, for whom PCV13 has been recommended since 2012.
Ms. Almendares reported having no disclosures.
SOURCE: Almendares O et al. ICEID 2018, Board 376.
REPORTING FROM ICEID 2018
Take these steps to improve your flu season preparedness
Last year’s influenza season was severe enough that hospitals around the United States set up special evaluation areas beyond their emergency departments, at times spilling over to tents or other temporary structures in what otherwise would be parking lots. The scale and potential severity of the annual epidemic can be difficult to convey to our patients, who sometimes say “just the flu” to refer to an illness responsible for more than 170 pediatric deaths in the United States this past year.1 The Centers for Disease Control and Prevention (CDC) recently updated its 5-year estimates of influenza-related deaths in the United States; influenza mortality ranges from about 12,000 deaths in a mild season (such as 2011-2012) to 56,000 in a more severe season (eg, 2012-2013).2
Although influenza cannot be completely prevented, the following strategies can help reduce the risk for the illness and limit its severity if contracted.
Prevention
Strategy 1: Vaccinate against influenza
While the efficacy of vaccines varies from year to year, vaccination remains the core of influenza prevention efforts. In this decade, vaccine effectiveness has ranged from 19% to 60%.3 However, models suggest that even when the vaccine is only 20% effective, vaccinating 140 million people (the average number of doses delivered annually in the United States over the past 5 seasons) prevents 21 million infections, 130,000 hospitalizations, and more than 61,000 deaths.4 In a case-control study, Flannery et al found that vaccination was 65% effective in preventing laboratory-confirmed influenza-associated death in children over 4 seasons (July 2010 through June 2014).5
Deciding who should be vaccinated is simpler than in prior years: Rather than targeting people who are at higher risk (those ages 65 and older, or those with comorbidities), the current CDC recommendation is to vaccinate nearly everyone ages 6 months or older, with limited exceptions.6,7 (See Table 18).
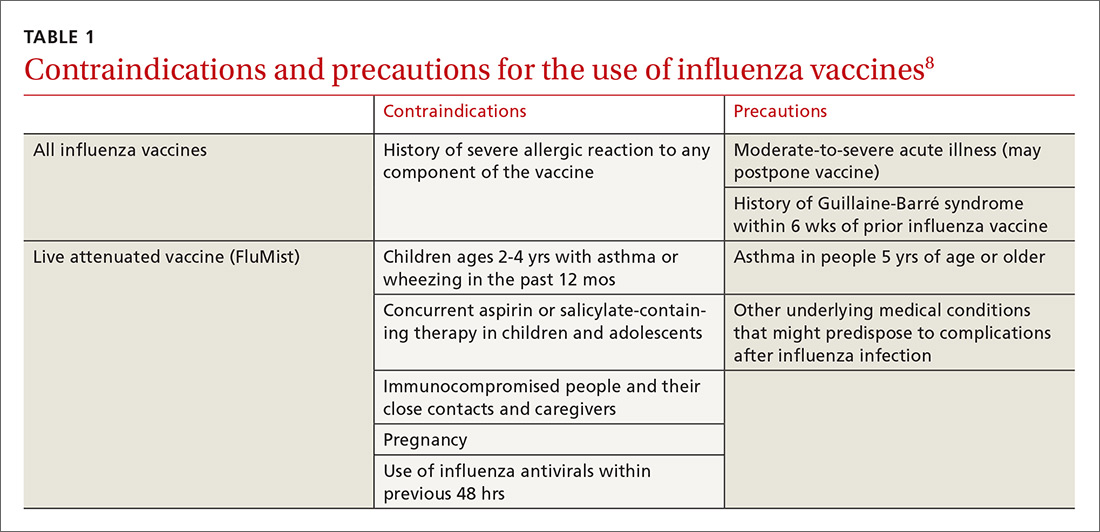
Formulations. Many types of influenza vaccine are approved for use in the United States; these differ in the number of strains included (3 or 4), the amount of antigen present for each strain, the presence of an adjuvant, the growth medium used for the virus, and the route of administration (see Table 29). The relative merits of each type are a matter of some debate. There is ongoing research into the comparative efficacy of vaccines comprised of egg- vs cell-based cultures, as well as studies comparing high-dose or adjuvant vaccines to standard-dose inactivated vaccines.
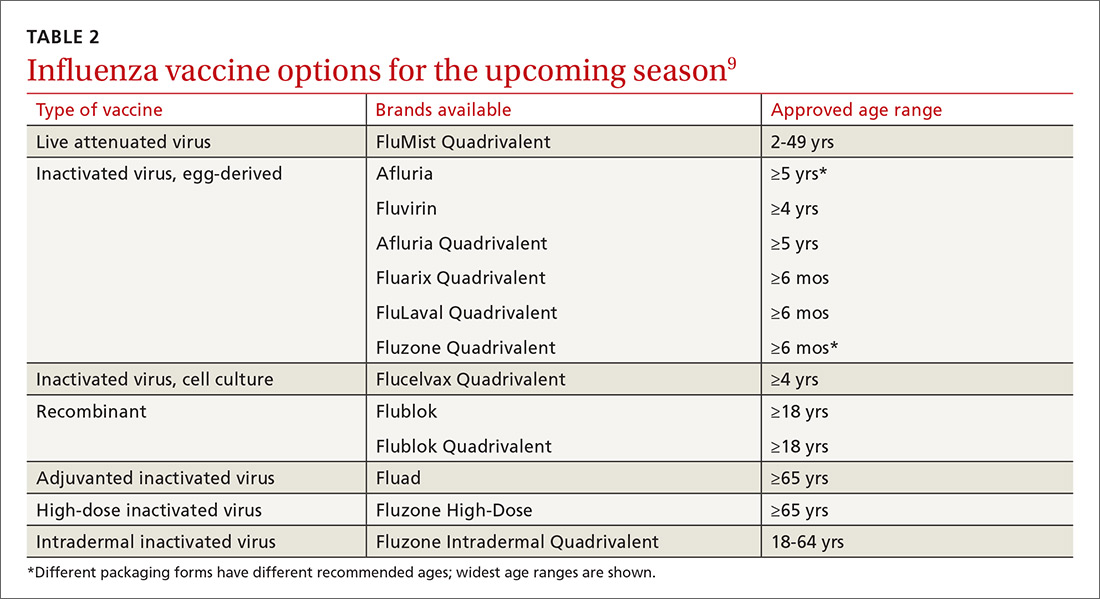
Previously, the CDC has recommended preferential use (or avoidance) of some vaccine types, based on their efficacy. For the 2018-2019 flu season, however, the CDC has rescinded its recommendation against vaccine containing live attenuated virus (LAIV; FluMist brand) and expresses no preference for any vaccine formulation for patients of appropriate age and health status.10 The American Academy of Pediatrics (AAP), however, is recommends that LAIV be used only if patients and their families decline injectable vaccines.11
Timing. Influenza vaccines are now distributed as early as July to some locations, raising concerns about waning immunity from early vaccination (July/August) if the influenza season does not peak until February or March.8,12,13 Currently, the CDC recommends balancing the possible benefit of delayed vaccination against the risks of missed opportunities to vaccinate, a possible early season, and logistical problems related to vaccinating the same number of people in a smaller time interval. Offering vaccination by the end of October, if possible, is recommended in order for immunity to develop by mid-November.8 Note: Children ages 6 months to 8 years will need to receive their initial vaccination in 2 half-doses administered at least 28 days apart; completing their vaccination by the end of October would require starting the process weeks earlier.
[polldaddy:10124269]
Continue to: Strategy 2
Strategy 2: Make use of chemoprophylaxis
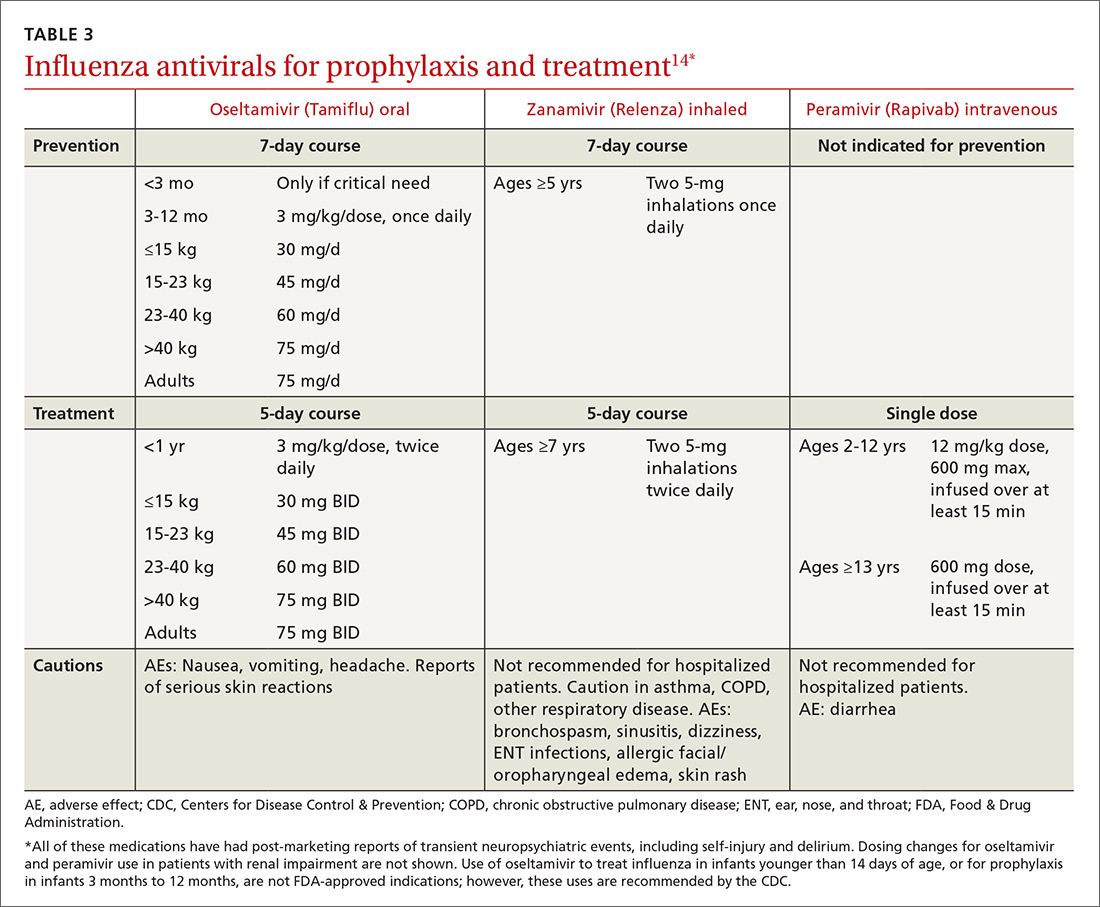
Preventive use of antiviral medication (chemoprophylaxis) may be a useful adjunct or alternative to vaccination in certain circumstances: if the patient is at high risk for complications, has been exposed to someone with influenza, has contraindications to vaccination, or received the vaccine within the past 2 weeks. The CDC also suggests that chemoprophylaxis be considered for those with immune deficiencies or who are otherwise immunosuppressed after exposure.14 Antivirals can also be used to control outbreaks in long-term care facilities; in these cases, the recommendedregimen is daily administration for at least 2 weeks, continuing until at least 7 days after the identification of the last case.14 Oseltamivir (Tamiflu) and zanamivir (Relenza) are the recommended prophylactic agents; a related intravenous medication, peramivir (Rapivab), is recommend for treatment only (see Table 314).
Strategy 3: Prevent comorbidities and opportunistic infections
Morbidity associated with influenza often comes from secondary infection. Pneumonia is among the most common complications, so influenza season is a good time to ensure that patients are appropriately vaccinated against pneumococcus, as well. Pneumococcal conjugate vaccine (Prevnar or PCV13) is recommended for children younger than 2 years of age, to be administered in a series of 4 doses: at 2, 4, 6, and 12-15 months. Vaccination with PCV13 is also recommended for those ages 65 or older, to be followed at least one year later with pneumococcal polysaccharide vaccine (Pneumovax or PPSV23).15 Additional doses of PCV13, PPSV23, or both may be indicated, depending on health status.
Strategy 4: Encourage good hygiene
The availability of immunizations and antivirals does not replace good hygiene. Frequent handwashing reduces the transmission of respiratory viruses, including influenza.16 Few studies have evaluated the use of alcohol-based hand sanitizers, but available evidence suggests they are effective in lowering viral transmission.16
Barriers, such as masks, gloves, and gowns, are helpful for health care workers.16 Surgical masks are often considered more comfortable to wear than N95 respirators. It may therefore be welcome news that when a 2009 randomized study assessed their use by hospital-based nurses, masks were non-inferior in protecting these health care workers against influenza.17
Presenteeism, the practice of going to work while sick, should be discouraged. People at risk for influenza may wish to avoid crowds during flu season; those with symptoms should be encouraged to stay home and limit contact with others.
Continue to: Treatment
Treatment
Strategy 1: Make prompt use of antivirals
Despite available preventive measures, tens of millions of people in the United States develop influenza every year. Use of antiviral medication, begun early in the course of illness, can reduce the duration of symptoms and may reduce the risk for complications.
The neuraminidase inhibitor (NI) group of antivirals—oseltamivir, zanamivir, and peramivir—is effective against influenza types A and B and current resistance rates are low.
The adamantine family of antivirals, amantadine and rimantadine, treat type A only. Since the circulating influenza strains in the past several seasons have demonstrated resistance >99%, these medications are not currently recommended.14
NIs reduce the duration of influenza symptoms by 10% to 20%, shortening the illness by 6 to 24 hours.18,19 In otherwise healthy patients, this benefit must be balanced against the increased risk for nausea and vomiting (oseltamivir), bronchospasm and sinusitis (zanamivir), and diarrhea (peramivir). In adults, NIs reduce the risk for lower respiratory tract complications and hospitalization. A 2015 meta-analysis by Dobson et al found a relative risk for hospitalization among those prescribed oseltamivir vs placebo of 37%.18
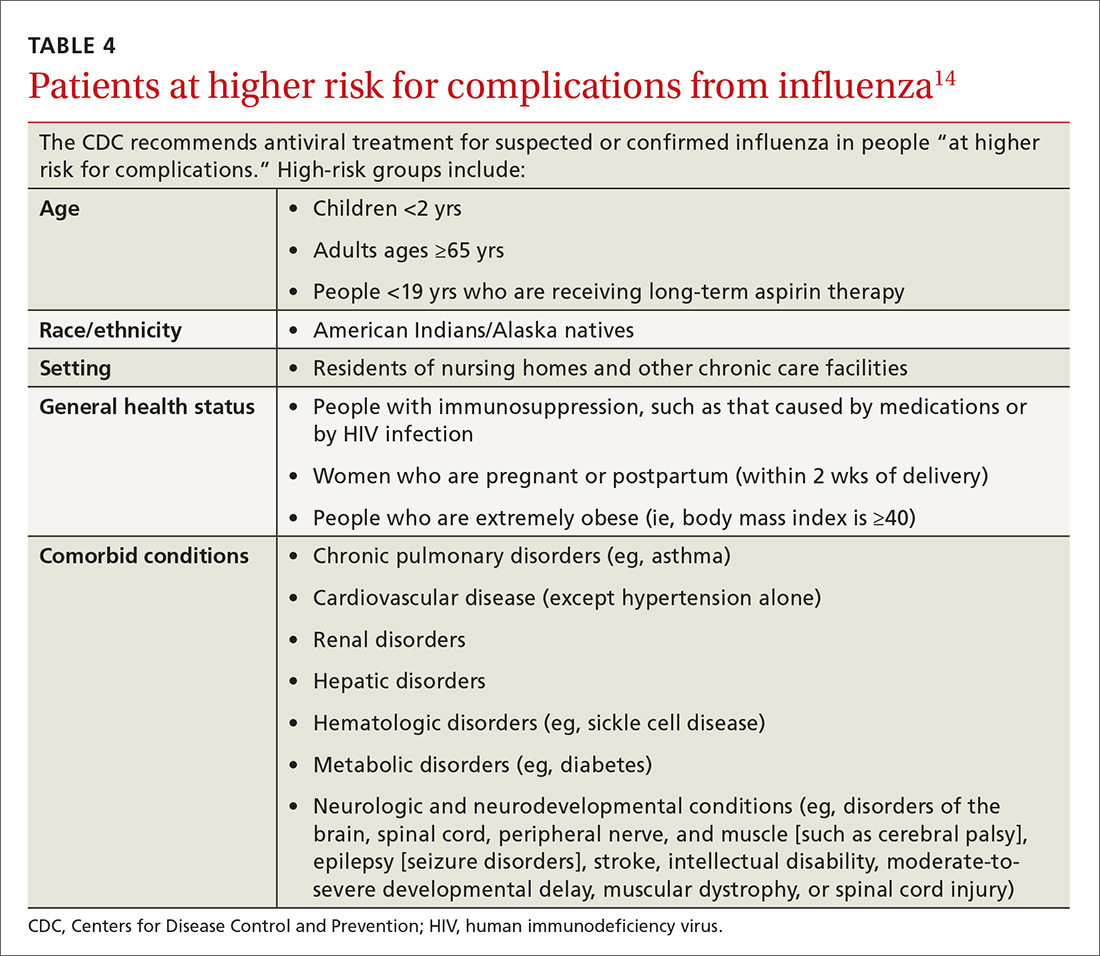
In the past, antivirals were used only in high-risk patients, such as children younger than 2 years, adults older than 65 years, and those with chronic health conditions.14 Now, antivirals are recommended for those who are at higher risk for complications (see Table 4), those with “severe, complicated, or progressive illness,” and hospitalized patients.14
Continue to: Antiviral treatment may have some value...
Antiviral treatment may have some value for hospitalized patients when started even 5 days after symptom onset. Treatment may be extended beyond the usual recommendations (5 days for oseltamivir or zanamivir) in immunosuppressed patients or the critically ill. Additionally, recent guidelines include consideration of antiviral treatment in outpatients who are at normal risk if treatment can be started within 48 hours of symptom onset.14
The CDC currently recommends use of oseltamivir rather than other antivirals for most hospitalized patients, based on the availability of data on its use in this setting.14 Intravenous peramivir is recommended for patients who cannot tolerate or absorb oral medication; inhaled zanamivir or IV peramivir are preferred for patients with end-stage renal disease who are not undergoing dialysis (see Table 3).14
Strategy 2: Exercise caution when it comes to supportive care
There are other medications that may offer symptom relief or prevent complications, especially when antivirals are contraindicated or unavailable.
Corticosteroids are recommended as part of the treatment of community-acquired pneumonia,20 but their role in influenza is controversial. A 2016 Cochrane review21 found no randomized controlled trials on the topic. Although the balance of available data from observational studies indicated that use of corticosteroids was associated with increased mortality, the authors also noted that all the studies included in their meta-analysis were of “very low quality.” They concluded that “the use of steroids in influenza remains a clinical judgement call.”
Statins may be associated with improved outcomes in influenza and pneumonia. Studies thus far have given contradictory results,22,23 and a planned Cochrane review of the question has been withdrawn.24
Continue to: Over-the-counter medications...
Over-the-counter medications, such as aspirin, acetaminophen, and ibuprofen are often used to manage the fever and myalgia associated with influenza. Patients should be cautioned against using the same ingredient in multiple different branded medications. Acetaminophen, for example, is not limited to Tylenol-branded products. To avoid Reye’s syndrome, children and teens with febrile illness, such as influenza, should not use aspirin.
CORRESPONDENCE
Jennifer L. Hamilton, MD, PhD, Drexel Family Medicine, 10 Shurs Lane, Suite 301, Philadelphia, PA 19127; [email protected].
1. CDC. Weekly US influenza surveillance report. https://www.cdc.gov/flu/weekly/index.htm. Published June 8, 2018. Accessed August 22, 2018.
2. CDC. Estimated influenza illnesses, medical visits, hospitalizations, and deaths averted by vaccination in the United States. Published April 19, 2017. https://www.cdc.gov/flu/about/disease/2015-16.htm. Accessed Setptember 18, 2018.
3. CDC. Seasonal influenza vaccine effectiveness, 2005-2018. https://www.cdc.gov/flu/professionals/vaccination/effectiveness-studies.htm. Published February 15, 2018. Accessed August 22, 2018.
4. Sah P, Medlock J, Fitzpatrick MC, et al. Optimizing the impact of low-efficacy influenza vaccines. Proc Natl Acad Sci. 2018:201802479.
5. Flannery B, Reynolds SB, Blanton L, et al. Influenza vaccine effectiveness against pediatric deaths: 2010-2014. Pediatrics. 2017;139: e20164244.
6. Kim DK, Riley LE, Hunter P. Advisory Committee on Immunization Practices recommended immunization schedule for adults aged 19 years or older—United States, 2018. MMWR Morb Mortal Wkly Rep. 2018;67:158–160.
7. Robinson CL, Romero JR, Kempe A, et al. Advisory Committee on Immunization Practices recommended immunization schedule for children and adolescents aged 18 years or younger—United States, 2018. MMWR Morb Mortal Wkly Rep. 2018;67:156–157.
8. Grohskopf LA, Sokolow LZ, Broder KR, et al. Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices—United States, 2017-18 influenza season. MMWR Recomm Rep. 2017;66:1-20.
9. CDC. Influenza vaccines—United States, 2017–18 influenza season. https://www.cdc.gov/flu/protect/vaccine/vaccines.htm. Published May 16, 2018. Accessed August 22, 2018.
10. Grohskopf LA, Sokolow LZ, Fry AM, et al. Update: ACIP recommendations for the use of quadrivalent live attenuated influenza vaccine (LAIV4)—United States, 2018-19 influenza season. MMWR Morb Mortal Wkly Rep. 2018;67:643–645.
11. Jenco M. AAP: Give children IIV flu shot; use LAIV as last resort. AAP News. May 21, 2018. http://www.aappublications.org/news/2018/05/21/fluvaccine051818. Accessed August 22, 2018.
12. Glinka ER, Smith DM, Johns ST. Timing matters—influenza vaccination to HIV-infected patients. HIV Med. 2016;17:601-604.
13. Castilla J, Martínez-Baz I, Martínez-Artola V, et al. Decline in influenza vaccine effectiveness with time after vaccination, Navarre, Spain, season 2011/12. Euro Surveill. 2013;18:20388.
14. CDC. Influenza antiviral medications: summary for clinicians. https://www.cdc.gov/flu/professionals/antivirals/summary-clinicians.htm. Published May 11, 2018. Accessed August 22, 2018.
15. CDC. Pneumococcal vaccination summary: who and when to vaccinate. https://www.cdc.gov/vaccines/vpd/pneumo/hcp/who-when-to-vaccinate.html. Published February 28, 2018. Accessed August 22, 2018.
16. Jefferson T, Del Mar CB, Dooley L, et al. Physical interventions to interrupt or reduce the spread of respiratory viruses. Cochrane Database Syst Rev. 2011;(7):CD006207.
17. Loeb M, Dafoe N, Mahony J, et al. Surgical mask vs N95 respirator for preventing influenza among health care workers: a randomized trial. JAMA. 2009;302:1865-1871.
18. Dobson J, Whitley RJ, Pocock S, Monto AS. Oseltamivir treatment for influenza in adults: a meta-analysis of randomised controlled trials. Lancet. 2015;385:1729-1737.
19. Ghebrehewet S, MacPherson P, Ho A. Influenza. BMJ. 2016;355:i6258.
20. Kaysin A, Viera AJ. Community-acquired pneumonia in adults: diagnosis and management. Am Fam Physician. 2016;94:698-706.
21. Rodrigo C, Leonardi‐Bee J, Nguyen‐Van‐Tam J, et al. Corticosteroids as adjunctive therapy in the treatment of influenza. Cochrane Database Syst Rev. 2016;3:CD010406.
22. Brassard P, Wu JW, Ernst P, et al. The effect of statins on influenza-like illness morbidity and mortality. Pharmacoepidemiol Drug Saf. 2017;26:63-70.
23. Fedson DS. Treating influenza with statins and other immunomodulatory agents. Antiviral Res. 2013;99:417-435.
24. Khandaker G, Rashid H, Chow MY, et al. Statins for influenza and pneumonia. Cochrane Database Syst Rev. January 9, 2017 [withdrawn].
Last year’s influenza season was severe enough that hospitals around the United States set up special evaluation areas beyond their emergency departments, at times spilling over to tents or other temporary structures in what otherwise would be parking lots. The scale and potential severity of the annual epidemic can be difficult to convey to our patients, who sometimes say “just the flu” to refer to an illness responsible for more than 170 pediatric deaths in the United States this past year.1 The Centers for Disease Control and Prevention (CDC) recently updated its 5-year estimates of influenza-related deaths in the United States; influenza mortality ranges from about 12,000 deaths in a mild season (such as 2011-2012) to 56,000 in a more severe season (eg, 2012-2013).2
Although influenza cannot be completely prevented, the following strategies can help reduce the risk for the illness and limit its severity if contracted.
Prevention
Strategy 1: Vaccinate against influenza
While the efficacy of vaccines varies from year to year, vaccination remains the core of influenza prevention efforts. In this decade, vaccine effectiveness has ranged from 19% to 60%.3 However, models suggest that even when the vaccine is only 20% effective, vaccinating 140 million people (the average number of doses delivered annually in the United States over the past 5 seasons) prevents 21 million infections, 130,000 hospitalizations, and more than 61,000 deaths.4 In a case-control study, Flannery et al found that vaccination was 65% effective in preventing laboratory-confirmed influenza-associated death in children over 4 seasons (July 2010 through June 2014).5
Deciding who should be vaccinated is simpler than in prior years: Rather than targeting people who are at higher risk (those ages 65 and older, or those with comorbidities), the current CDC recommendation is to vaccinate nearly everyone ages 6 months or older, with limited exceptions.6,7 (See Table 18).

Formulations. Many types of influenza vaccine are approved for use in the United States; these differ in the number of strains included (3 or 4), the amount of antigen present for each strain, the presence of an adjuvant, the growth medium used for the virus, and the route of administration (see Table 29). The relative merits of each type are a matter of some debate. There is ongoing research into the comparative efficacy of vaccines comprised of egg- vs cell-based cultures, as well as studies comparing high-dose or adjuvant vaccines to standard-dose inactivated vaccines.

Previously, the CDC has recommended preferential use (or avoidance) of some vaccine types, based on their efficacy. For the 2018-2019 flu season, however, the CDC has rescinded its recommendation against vaccine containing live attenuated virus (LAIV; FluMist brand) and expresses no preference for any vaccine formulation for patients of appropriate age and health status.10 The American Academy of Pediatrics (AAP), however, is recommends that LAIV be used only if patients and their families decline injectable vaccines.11
Timing. Influenza vaccines are now distributed as early as July to some locations, raising concerns about waning immunity from early vaccination (July/August) if the influenza season does not peak until February or March.8,12,13 Currently, the CDC recommends balancing the possible benefit of delayed vaccination against the risks of missed opportunities to vaccinate, a possible early season, and logistical problems related to vaccinating the same number of people in a smaller time interval. Offering vaccination by the end of October, if possible, is recommended in order for immunity to develop by mid-November.8 Note: Children ages 6 months to 8 years will need to receive their initial vaccination in 2 half-doses administered at least 28 days apart; completing their vaccination by the end of October would require starting the process weeks earlier.
[polldaddy:10124269]
Continue to: Strategy 2
Strategy 2: Make use of chemoprophylaxis
Preventive use of antiviral medication (chemoprophylaxis) may be a useful adjunct or alternative to vaccination in certain circumstances: if the patient is at high risk for complications, has been exposed to someone with influenza, has contraindications to vaccination, or received the vaccine within the past 2 weeks. The CDC also suggests that chemoprophylaxis be considered for those with immune deficiencies or who are otherwise immunosuppressed after exposure.14 Antivirals can also be used to control outbreaks in long-term care facilities; in these cases, the recommendedregimen is daily administration for at least 2 weeks, continuing until at least 7 days after the identification of the last case.14 Oseltamivir (Tamiflu) and zanamivir (Relenza) are the recommended prophylactic agents; a related intravenous medication, peramivir (Rapivab), is recommend for treatment only (see Table 314).

Strategy 3: Prevent comorbidities and opportunistic infections
Morbidity associated with influenza often comes from secondary infection. Pneumonia is among the most common complications, so influenza season is a good time to ensure that patients are appropriately vaccinated against pneumococcus, as well. Pneumococcal conjugate vaccine (Prevnar or PCV13) is recommended for children younger than 2 years of age, to be administered in a series of 4 doses: at 2, 4, 6, and 12-15 months. Vaccination with PCV13 is also recommended for those ages 65 or older, to be followed at least one year later with pneumococcal polysaccharide vaccine (Pneumovax or PPSV23).15 Additional doses of PCV13, PPSV23, or both may be indicated, depending on health status.
Strategy 4: Encourage good hygiene
The availability of immunizations and antivirals does not replace good hygiene. Frequent handwashing reduces the transmission of respiratory viruses, including influenza.16 Few studies have evaluated the use of alcohol-based hand sanitizers, but available evidence suggests they are effective in lowering viral transmission.16
Barriers, such as masks, gloves, and gowns, are helpful for health care workers.16 Surgical masks are often considered more comfortable to wear than N95 respirators. It may therefore be welcome news that when a 2009 randomized study assessed their use by hospital-based nurses, masks were non-inferior in protecting these health care workers against influenza.17
Presenteeism, the practice of going to work while sick, should be discouraged. People at risk for influenza may wish to avoid crowds during flu season; those with symptoms should be encouraged to stay home and limit contact with others.
Continue to: Treatment
Treatment
Strategy 1: Make prompt use of antivirals
Despite available preventive measures, tens of millions of people in the United States develop influenza every year. Use of antiviral medication, begun early in the course of illness, can reduce the duration of symptoms and may reduce the risk for complications.
The neuraminidase inhibitor (NI) group of antivirals—oseltamivir, zanamivir, and peramivir—is effective against influenza types A and B and current resistance rates are low.
The adamantine family of antivirals, amantadine and rimantadine, treat type A only. Since the circulating influenza strains in the past several seasons have demonstrated resistance >99%, these medications are not currently recommended.14
NIs reduce the duration of influenza symptoms by 10% to 20%, shortening the illness by 6 to 24 hours.18,19 In otherwise healthy patients, this benefit must be balanced against the increased risk for nausea and vomiting (oseltamivir), bronchospasm and sinusitis (zanamivir), and diarrhea (peramivir). In adults, NIs reduce the risk for lower respiratory tract complications and hospitalization. A 2015 meta-analysis by Dobson et al found a relative risk for hospitalization among those prescribed oseltamivir vs placebo of 37%.18
In the past, antivirals were used only in high-risk patients, such as children younger than 2 years, adults older than 65 years, and those with chronic health conditions.14 Now, antivirals are recommended for those who are at higher risk for complications (see Table 4), those with “severe, complicated, or progressive illness,” and hospitalized patients.14

Continue to: Antiviral treatment may have some value...
Antiviral treatment may have some value for hospitalized patients when started even 5 days after symptom onset. Treatment may be extended beyond the usual recommendations (5 days for oseltamivir or zanamivir) in immunosuppressed patients or the critically ill. Additionally, recent guidelines include consideration of antiviral treatment in outpatients who are at normal risk if treatment can be started within 48 hours of symptom onset.14
The CDC currently recommends use of oseltamivir rather than other antivirals for most hospitalized patients, based on the availability of data on its use in this setting.14 Intravenous peramivir is recommended for patients who cannot tolerate or absorb oral medication; inhaled zanamivir or IV peramivir are preferred for patients with end-stage renal disease who are not undergoing dialysis (see Table 3).14
Strategy 2: Exercise caution when it comes to supportive care
There are other medications that may offer symptom relief or prevent complications, especially when antivirals are contraindicated or unavailable.
Corticosteroids are recommended as part of the treatment of community-acquired pneumonia,20 but their role in influenza is controversial. A 2016 Cochrane review21 found no randomized controlled trials on the topic. Although the balance of available data from observational studies indicated that use of corticosteroids was associated with increased mortality, the authors also noted that all the studies included in their meta-analysis were of “very low quality.” They concluded that “the use of steroids in influenza remains a clinical judgement call.”
Statins may be associated with improved outcomes in influenza and pneumonia. Studies thus far have given contradictory results,22,23 and a planned Cochrane review of the question has been withdrawn.24
Continue to: Over-the-counter medications...
Over-the-counter medications, such as aspirin, acetaminophen, and ibuprofen are often used to manage the fever and myalgia associated with influenza. Patients should be cautioned against using the same ingredient in multiple different branded medications. Acetaminophen, for example, is not limited to Tylenol-branded products. To avoid Reye’s syndrome, children and teens with febrile illness, such as influenza, should not use aspirin.
CORRESPONDENCE
Jennifer L. Hamilton, MD, PhD, Drexel Family Medicine, 10 Shurs Lane, Suite 301, Philadelphia, PA 19127; [email protected].
Last year’s influenza season was severe enough that hospitals around the United States set up special evaluation areas beyond their emergency departments, at times spilling over to tents or other temporary structures in what otherwise would be parking lots. The scale and potential severity of the annual epidemic can be difficult to convey to our patients, who sometimes say “just the flu” to refer to an illness responsible for more than 170 pediatric deaths in the United States this past year.1 The Centers for Disease Control and Prevention (CDC) recently updated its 5-year estimates of influenza-related deaths in the United States; influenza mortality ranges from about 12,000 deaths in a mild season (such as 2011-2012) to 56,000 in a more severe season (eg, 2012-2013).2
Although influenza cannot be completely prevented, the following strategies can help reduce the risk for the illness and limit its severity if contracted.
Prevention
Strategy 1: Vaccinate against influenza
While the efficacy of vaccines varies from year to year, vaccination remains the core of influenza prevention efforts. In this decade, vaccine effectiveness has ranged from 19% to 60%.3 However, models suggest that even when the vaccine is only 20% effective, vaccinating 140 million people (the average number of doses delivered annually in the United States over the past 5 seasons) prevents 21 million infections, 130,000 hospitalizations, and more than 61,000 deaths.4 In a case-control study, Flannery et al found that vaccination was 65% effective in preventing laboratory-confirmed influenza-associated death in children over 4 seasons (July 2010 through June 2014).5
Deciding who should be vaccinated is simpler than in prior years: Rather than targeting people who are at higher risk (those ages 65 and older, or those with comorbidities), the current CDC recommendation is to vaccinate nearly everyone ages 6 months or older, with limited exceptions.6,7 (See Table 18).

Formulations. Many types of influenza vaccine are approved for use in the United States; these differ in the number of strains included (3 or 4), the amount of antigen present for each strain, the presence of an adjuvant, the growth medium used for the virus, and the route of administration (see Table 29). The relative merits of each type are a matter of some debate. There is ongoing research into the comparative efficacy of vaccines comprised of egg- vs cell-based cultures, as well as studies comparing high-dose or adjuvant vaccines to standard-dose inactivated vaccines.

Previously, the CDC has recommended preferential use (or avoidance) of some vaccine types, based on their efficacy. For the 2018-2019 flu season, however, the CDC has rescinded its recommendation against vaccine containing live attenuated virus (LAIV; FluMist brand) and expresses no preference for any vaccine formulation for patients of appropriate age and health status.10 The American Academy of Pediatrics (AAP), however, is recommends that LAIV be used only if patients and their families decline injectable vaccines.11
Timing. Influenza vaccines are now distributed as early as July to some locations, raising concerns about waning immunity from early vaccination (July/August) if the influenza season does not peak until February or March.8,12,13 Currently, the CDC recommends balancing the possible benefit of delayed vaccination against the risks of missed opportunities to vaccinate, a possible early season, and logistical problems related to vaccinating the same number of people in a smaller time interval. Offering vaccination by the end of October, if possible, is recommended in order for immunity to develop by mid-November.8 Note: Children ages 6 months to 8 years will need to receive their initial vaccination in 2 half-doses administered at least 28 days apart; completing their vaccination by the end of October would require starting the process weeks earlier.
[polldaddy:10124269]
Continue to: Strategy 2
Strategy 2: Make use of chemoprophylaxis
Preventive use of antiviral medication (chemoprophylaxis) may be a useful adjunct or alternative to vaccination in certain circumstances: if the patient is at high risk for complications, has been exposed to someone with influenza, has contraindications to vaccination, or received the vaccine within the past 2 weeks. The CDC also suggests that chemoprophylaxis be considered for those with immune deficiencies or who are otherwise immunosuppressed after exposure.14 Antivirals can also be used to control outbreaks in long-term care facilities; in these cases, the recommendedregimen is daily administration for at least 2 weeks, continuing until at least 7 days after the identification of the last case.14 Oseltamivir (Tamiflu) and zanamivir (Relenza) are the recommended prophylactic agents; a related intravenous medication, peramivir (Rapivab), is recommend for treatment only (see Table 314).

Strategy 3: Prevent comorbidities and opportunistic infections
Morbidity associated with influenza often comes from secondary infection. Pneumonia is among the most common complications, so influenza season is a good time to ensure that patients are appropriately vaccinated against pneumococcus, as well. Pneumococcal conjugate vaccine (Prevnar or PCV13) is recommended for children younger than 2 years of age, to be administered in a series of 4 doses: at 2, 4, 6, and 12-15 months. Vaccination with PCV13 is also recommended for those ages 65 or older, to be followed at least one year later with pneumococcal polysaccharide vaccine (Pneumovax or PPSV23).15 Additional doses of PCV13, PPSV23, or both may be indicated, depending on health status.
Strategy 4: Encourage good hygiene
The availability of immunizations and antivirals does not replace good hygiene. Frequent handwashing reduces the transmission of respiratory viruses, including influenza.16 Few studies have evaluated the use of alcohol-based hand sanitizers, but available evidence suggests they are effective in lowering viral transmission.16
Barriers, such as masks, gloves, and gowns, are helpful for health care workers.16 Surgical masks are often considered more comfortable to wear than N95 respirators. It may therefore be welcome news that when a 2009 randomized study assessed their use by hospital-based nurses, masks were non-inferior in protecting these health care workers against influenza.17
Presenteeism, the practice of going to work while sick, should be discouraged. People at risk for influenza may wish to avoid crowds during flu season; those with symptoms should be encouraged to stay home and limit contact with others.
Continue to: Treatment
Treatment
Strategy 1: Make prompt use of antivirals
Despite available preventive measures, tens of millions of people in the United States develop influenza every year. Use of antiviral medication, begun early in the course of illness, can reduce the duration of symptoms and may reduce the risk for complications.
The neuraminidase inhibitor (NI) group of antivirals—oseltamivir, zanamivir, and peramivir—is effective against influenza types A and B and current resistance rates are low.
The adamantine family of antivirals, amantadine and rimantadine, treat type A only. Since the circulating influenza strains in the past several seasons have demonstrated resistance >99%, these medications are not currently recommended.14
NIs reduce the duration of influenza symptoms by 10% to 20%, shortening the illness by 6 to 24 hours.18,19 In otherwise healthy patients, this benefit must be balanced against the increased risk for nausea and vomiting (oseltamivir), bronchospasm and sinusitis (zanamivir), and diarrhea (peramivir). In adults, NIs reduce the risk for lower respiratory tract complications and hospitalization. A 2015 meta-analysis by Dobson et al found a relative risk for hospitalization among those prescribed oseltamivir vs placebo of 37%.18
In the past, antivirals were used only in high-risk patients, such as children younger than 2 years, adults older than 65 years, and those with chronic health conditions.14 Now, antivirals are recommended for those who are at higher risk for complications (see Table 4), those with “severe, complicated, or progressive illness,” and hospitalized patients.14

Continue to: Antiviral treatment may have some value...
Antiviral treatment may have some value for hospitalized patients when started even 5 days after symptom onset. Treatment may be extended beyond the usual recommendations (5 days for oseltamivir or zanamivir) in immunosuppressed patients or the critically ill. Additionally, recent guidelines include consideration of antiviral treatment in outpatients who are at normal risk if treatment can be started within 48 hours of symptom onset.14
The CDC currently recommends use of oseltamivir rather than other antivirals for most hospitalized patients, based on the availability of data on its use in this setting.14 Intravenous peramivir is recommended for patients who cannot tolerate or absorb oral medication; inhaled zanamivir or IV peramivir are preferred for patients with end-stage renal disease who are not undergoing dialysis (see Table 3).14
Strategy 2: Exercise caution when it comes to supportive care
There are other medications that may offer symptom relief or prevent complications, especially when antivirals are contraindicated or unavailable.
Corticosteroids are recommended as part of the treatment of community-acquired pneumonia,20 but their role in influenza is controversial. A 2016 Cochrane review21 found no randomized controlled trials on the topic. Although the balance of available data from observational studies indicated that use of corticosteroids was associated with increased mortality, the authors also noted that all the studies included in their meta-analysis were of “very low quality.” They concluded that “the use of steroids in influenza remains a clinical judgement call.”
Statins may be associated with improved outcomes in influenza and pneumonia. Studies thus far have given contradictory results,22,23 and a planned Cochrane review of the question has been withdrawn.24
Continue to: Over-the-counter medications...
Over-the-counter medications, such as aspirin, acetaminophen, and ibuprofen are often used to manage the fever and myalgia associated with influenza. Patients should be cautioned against using the same ingredient in multiple different branded medications. Acetaminophen, for example, is not limited to Tylenol-branded products. To avoid Reye’s syndrome, children and teens with febrile illness, such as influenza, should not use aspirin.
CORRESPONDENCE
Jennifer L. Hamilton, MD, PhD, Drexel Family Medicine, 10 Shurs Lane, Suite 301, Philadelphia, PA 19127; [email protected].
1. CDC. Weekly US influenza surveillance report. https://www.cdc.gov/flu/weekly/index.htm. Published June 8, 2018. Accessed August 22, 2018.
2. CDC. Estimated influenza illnesses, medical visits, hospitalizations, and deaths averted by vaccination in the United States. Published April 19, 2017. https://www.cdc.gov/flu/about/disease/2015-16.htm. Accessed Setptember 18, 2018.
3. CDC. Seasonal influenza vaccine effectiveness, 2005-2018. https://www.cdc.gov/flu/professionals/vaccination/effectiveness-studies.htm. Published February 15, 2018. Accessed August 22, 2018.
4. Sah P, Medlock J, Fitzpatrick MC, et al. Optimizing the impact of low-efficacy influenza vaccines. Proc Natl Acad Sci. 2018:201802479.
5. Flannery B, Reynolds SB, Blanton L, et al. Influenza vaccine effectiveness against pediatric deaths: 2010-2014. Pediatrics. 2017;139: e20164244.
6. Kim DK, Riley LE, Hunter P. Advisory Committee on Immunization Practices recommended immunization schedule for adults aged 19 years or older—United States, 2018. MMWR Morb Mortal Wkly Rep. 2018;67:158–160.
7. Robinson CL, Romero JR, Kempe A, et al. Advisory Committee on Immunization Practices recommended immunization schedule for children and adolescents aged 18 years or younger—United States, 2018. MMWR Morb Mortal Wkly Rep. 2018;67:156–157.
8. Grohskopf LA, Sokolow LZ, Broder KR, et al. Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices—United States, 2017-18 influenza season. MMWR Recomm Rep. 2017;66:1-20.
9. CDC. Influenza vaccines—United States, 2017–18 influenza season. https://www.cdc.gov/flu/protect/vaccine/vaccines.htm. Published May 16, 2018. Accessed August 22, 2018.
10. Grohskopf LA, Sokolow LZ, Fry AM, et al. Update: ACIP recommendations for the use of quadrivalent live attenuated influenza vaccine (LAIV4)—United States, 2018-19 influenza season. MMWR Morb Mortal Wkly Rep. 2018;67:643–645.
11. Jenco M. AAP: Give children IIV flu shot; use LAIV as last resort. AAP News. May 21, 2018. http://www.aappublications.org/news/2018/05/21/fluvaccine051818. Accessed August 22, 2018.
12. Glinka ER, Smith DM, Johns ST. Timing matters—influenza vaccination to HIV-infected patients. HIV Med. 2016;17:601-604.
13. Castilla J, Martínez-Baz I, Martínez-Artola V, et al. Decline in influenza vaccine effectiveness with time after vaccination, Navarre, Spain, season 2011/12. Euro Surveill. 2013;18:20388.
14. CDC. Influenza antiviral medications: summary for clinicians. https://www.cdc.gov/flu/professionals/antivirals/summary-clinicians.htm. Published May 11, 2018. Accessed August 22, 2018.
15. CDC. Pneumococcal vaccination summary: who and when to vaccinate. https://www.cdc.gov/vaccines/vpd/pneumo/hcp/who-when-to-vaccinate.html. Published February 28, 2018. Accessed August 22, 2018.
16. Jefferson T, Del Mar CB, Dooley L, et al. Physical interventions to interrupt or reduce the spread of respiratory viruses. Cochrane Database Syst Rev. 2011;(7):CD006207.
17. Loeb M, Dafoe N, Mahony J, et al. Surgical mask vs N95 respirator for preventing influenza among health care workers: a randomized trial. JAMA. 2009;302:1865-1871.
18. Dobson J, Whitley RJ, Pocock S, Monto AS. Oseltamivir treatment for influenza in adults: a meta-analysis of randomised controlled trials. Lancet. 2015;385:1729-1737.
19. Ghebrehewet S, MacPherson P, Ho A. Influenza. BMJ. 2016;355:i6258.
20. Kaysin A, Viera AJ. Community-acquired pneumonia in adults: diagnosis and management. Am Fam Physician. 2016;94:698-706.
21. Rodrigo C, Leonardi‐Bee J, Nguyen‐Van‐Tam J, et al. Corticosteroids as adjunctive therapy in the treatment of influenza. Cochrane Database Syst Rev. 2016;3:CD010406.
22. Brassard P, Wu JW, Ernst P, et al. The effect of statins on influenza-like illness morbidity and mortality. Pharmacoepidemiol Drug Saf. 2017;26:63-70.
23. Fedson DS. Treating influenza with statins and other immunomodulatory agents. Antiviral Res. 2013;99:417-435.
24. Khandaker G, Rashid H, Chow MY, et al. Statins for influenza and pneumonia. Cochrane Database Syst Rev. January 9, 2017 [withdrawn].
1. CDC. Weekly US influenza surveillance report. https://www.cdc.gov/flu/weekly/index.htm. Published June 8, 2018. Accessed August 22, 2018.
2. CDC. Estimated influenza illnesses, medical visits, hospitalizations, and deaths averted by vaccination in the United States. Published April 19, 2017. https://www.cdc.gov/flu/about/disease/2015-16.htm. Accessed Setptember 18, 2018.
3. CDC. Seasonal influenza vaccine effectiveness, 2005-2018. https://www.cdc.gov/flu/professionals/vaccination/effectiveness-studies.htm. Published February 15, 2018. Accessed August 22, 2018.
4. Sah P, Medlock J, Fitzpatrick MC, et al. Optimizing the impact of low-efficacy influenza vaccines. Proc Natl Acad Sci. 2018:201802479.
5. Flannery B, Reynolds SB, Blanton L, et al. Influenza vaccine effectiveness against pediatric deaths: 2010-2014. Pediatrics. 2017;139: e20164244.
6. Kim DK, Riley LE, Hunter P. Advisory Committee on Immunization Practices recommended immunization schedule for adults aged 19 years or older—United States, 2018. MMWR Morb Mortal Wkly Rep. 2018;67:158–160.
7. Robinson CL, Romero JR, Kempe A, et al. Advisory Committee on Immunization Practices recommended immunization schedule for children and adolescents aged 18 years or younger—United States, 2018. MMWR Morb Mortal Wkly Rep. 2018;67:156–157.
8. Grohskopf LA, Sokolow LZ, Broder KR, et al. Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices—United States, 2017-18 influenza season. MMWR Recomm Rep. 2017;66:1-20.
9. CDC. Influenza vaccines—United States, 2017–18 influenza season. https://www.cdc.gov/flu/protect/vaccine/vaccines.htm. Published May 16, 2018. Accessed August 22, 2018.
10. Grohskopf LA, Sokolow LZ, Fry AM, et al. Update: ACIP recommendations for the use of quadrivalent live attenuated influenza vaccine (LAIV4)—United States, 2018-19 influenza season. MMWR Morb Mortal Wkly Rep. 2018;67:643–645.
11. Jenco M. AAP: Give children IIV flu shot; use LAIV as last resort. AAP News. May 21, 2018. http://www.aappublications.org/news/2018/05/21/fluvaccine051818. Accessed August 22, 2018.
12. Glinka ER, Smith DM, Johns ST. Timing matters—influenza vaccination to HIV-infected patients. HIV Med. 2016;17:601-604.
13. Castilla J, Martínez-Baz I, Martínez-Artola V, et al. Decline in influenza vaccine effectiveness with time after vaccination, Navarre, Spain, season 2011/12. Euro Surveill. 2013;18:20388.
14. CDC. Influenza antiviral medications: summary for clinicians. https://www.cdc.gov/flu/professionals/antivirals/summary-clinicians.htm. Published May 11, 2018. Accessed August 22, 2018.
15. CDC. Pneumococcal vaccination summary: who and when to vaccinate. https://www.cdc.gov/vaccines/vpd/pneumo/hcp/who-when-to-vaccinate.html. Published February 28, 2018. Accessed August 22, 2018.
16. Jefferson T, Del Mar CB, Dooley L, et al. Physical interventions to interrupt or reduce the spread of respiratory viruses. Cochrane Database Syst Rev. 2011;(7):CD006207.
17. Loeb M, Dafoe N, Mahony J, et al. Surgical mask vs N95 respirator for preventing influenza among health care workers: a randomized trial. JAMA. 2009;302:1865-1871.
18. Dobson J, Whitley RJ, Pocock S, Monto AS. Oseltamivir treatment for influenza in adults: a meta-analysis of randomised controlled trials. Lancet. 2015;385:1729-1737.
19. Ghebrehewet S, MacPherson P, Ho A. Influenza. BMJ. 2016;355:i6258.
20. Kaysin A, Viera AJ. Community-acquired pneumonia in adults: diagnosis and management. Am Fam Physician. 2016;94:698-706.
21. Rodrigo C, Leonardi‐Bee J, Nguyen‐Van‐Tam J, et al. Corticosteroids as adjunctive therapy in the treatment of influenza. Cochrane Database Syst Rev. 2016;3:CD010406.
22. Brassard P, Wu JW, Ernst P, et al. The effect of statins on influenza-like illness morbidity and mortality. Pharmacoepidemiol Drug Saf. 2017;26:63-70.
23. Fedson DS. Treating influenza with statins and other immunomodulatory agents. Antiviral Res. 2013;99:417-435.
24. Khandaker G, Rashid H, Chow MY, et al. Statins for influenza and pneumonia. Cochrane Database Syst Rev. January 9, 2017 [withdrawn].
PRACTICE RECOMMENDATIONS
› Recommend influenza vaccination for all patients at least 6 months old unless a specific contraindication exists. A
› Recommend pneumococcal vaccination to appropriate patients to reduce the risk for a common complication of influenza. A
› Encourage hygiene-based measures to limit infection, including frequent handwashing or use of a hand sanitizer. B
› Prescribe oseltamivir to hospitalized influenza patients to limit the duration and severity of infection. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Trials need standardized reporting of pediatric fever after flu vaccine
Researchers found a lower rate of pediatric fever after applying a standard definition of fever across three different clinical trials of pediatric patients receiving influenza vaccinations, according to research published in the Pediatric Infectious Disease Journal.
Investigators in future studies must adopt a standardized definition of pediatric fever after an influenza vaccination. “Our study demonstrates the variability in results which occur due to minor differences in the definition of fever, methods of analysis and reporting of results,” Jean Li-Kim-Moy, MBBS, of the University of Sydney, and colleagues wrote.
Dr. Li-Kim-Moy and colleagues analyzed pediatric datasets from three different clinical trials using trivalent influenza vaccine (TIV); the primary trial included 3,317 children aged 6-35 months who were randomized to receive Fluarix at 0.25 mL or 0.5 mL, or receive 0.25 mL of Fluzone. The other two trials studied children receiving TIV between 6 months–17 years and 3-17 years. The researchers also performed a multivariable regression analysis to determine the relationship between immunogenicity, antipyretic use, and postvaccination fever.
The primary study initially reported the fever rate 0 days–3 days after vaccination was between 6% and 7%. After reporting the rate of fever separately for each dose and changing the criteria to “defining fever as greater than or equal to 38.0°C by any route of measurement” for the primary study, the researchers found a rate of any-cause fever was 3%-4% for the first dose and 4%-5% for the second dose. The rate of vaccine-related fever in the primary study was 3% for the first dose and 3%-4% for the second dose, with researchers noting vaccine-related fever occurred significantly earlier compared with any-cause fever (mean 1 days vs. 2 days after vaccination; P equals .04).
Impact of fever, antipyretics
The researchers also performed a pooled immunogenicity analysis of 5,902 children from all three trials and found a strong association between fever after vaccination and increased geometric mean titer (GMT) ratios (1.21-1.39; P less than or equal to .01) and an association between antipyretic use and reduced GMT ratios (0.80-0.87; P less than .0006).
“Our pooled analysis of the three trials demonstrated highly significant associations, for all strains, between postvaccination fever and up to 39% higher GMT; Similarly, strong evidence of associations in the opposite direction was found between postvaccination antipyretic use (days 0-3), adjusting for all other factors including fever, and decreased immunogenicity against all vaccine strains in children,” Dr. Li-Kim-Moy and colleagues said.
Antipyretic use was common in the primary study, occurring in one in six of the children, they said. These findings of “significant associations between fever and increased vaccine immunogenicity, and between antipyretic use and reduced immunogenicity in children after influenza vaccination” suggest the need for further study, especially because parents often give antipyretics if their children are febrile after vaccinations.
“There is uncertainty whether our findings, and those of others, on immunogenicity translate into clinically significant effects,” they wrote. “However, the fact that influenza vaccine, unlike many routine childhood vaccines, is only moderately protective may mean that modest reductions in antibody response are more likely to correlate to less protection.”
Dr. Wood reported receiving a fellowship from the National Health and Medical Research Council (NHMRC) and being an investigator for GlaxoSmithKline trials. Dr. Booy reported being an advisor for influenza vaccine manufacturing, an advisory board member, on the speaker’s bureau, and a researcher of vaccines for several manufacturers. The other authors reported no relevant conflicts of interest.
SOURCE: Li-Kim-Moy J et al. Pediatr Infect Dis J. 2018 Oct;37(10):971-5.
Researchers found a lower rate of pediatric fever after applying a standard definition of fever across three different clinical trials of pediatric patients receiving influenza vaccinations, according to research published in the Pediatric Infectious Disease Journal.
Investigators in future studies must adopt a standardized definition of pediatric fever after an influenza vaccination. “Our study demonstrates the variability in results which occur due to minor differences in the definition of fever, methods of analysis and reporting of results,” Jean Li-Kim-Moy, MBBS, of the University of Sydney, and colleagues wrote.
Dr. Li-Kim-Moy and colleagues analyzed pediatric datasets from three different clinical trials using trivalent influenza vaccine (TIV); the primary trial included 3,317 children aged 6-35 months who were randomized to receive Fluarix at 0.25 mL or 0.5 mL, or receive 0.25 mL of Fluzone. The other two trials studied children receiving TIV between 6 months–17 years and 3-17 years. The researchers also performed a multivariable regression analysis to determine the relationship between immunogenicity, antipyretic use, and postvaccination fever.
The primary study initially reported the fever rate 0 days–3 days after vaccination was between 6% and 7%. After reporting the rate of fever separately for each dose and changing the criteria to “defining fever as greater than or equal to 38.0°C by any route of measurement” for the primary study, the researchers found a rate of any-cause fever was 3%-4% for the first dose and 4%-5% for the second dose. The rate of vaccine-related fever in the primary study was 3% for the first dose and 3%-4% for the second dose, with researchers noting vaccine-related fever occurred significantly earlier compared with any-cause fever (mean 1 days vs. 2 days after vaccination; P equals .04).
Impact of fever, antipyretics
The researchers also performed a pooled immunogenicity analysis of 5,902 children from all three trials and found a strong association between fever after vaccination and increased geometric mean titer (GMT) ratios (1.21-1.39; P less than or equal to .01) and an association between antipyretic use and reduced GMT ratios (0.80-0.87; P less than .0006).
“Our pooled analysis of the three trials demonstrated highly significant associations, for all strains, between postvaccination fever and up to 39% higher GMT; Similarly, strong evidence of associations in the opposite direction was found between postvaccination antipyretic use (days 0-3), adjusting for all other factors including fever, and decreased immunogenicity against all vaccine strains in children,” Dr. Li-Kim-Moy and colleagues said.
Antipyretic use was common in the primary study, occurring in one in six of the children, they said. These findings of “significant associations between fever and increased vaccine immunogenicity, and between antipyretic use and reduced immunogenicity in children after influenza vaccination” suggest the need for further study, especially because parents often give antipyretics if their children are febrile after vaccinations.
“There is uncertainty whether our findings, and those of others, on immunogenicity translate into clinically significant effects,” they wrote. “However, the fact that influenza vaccine, unlike many routine childhood vaccines, is only moderately protective may mean that modest reductions in antibody response are more likely to correlate to less protection.”
Dr. Wood reported receiving a fellowship from the National Health and Medical Research Council (NHMRC) and being an investigator for GlaxoSmithKline trials. Dr. Booy reported being an advisor for influenza vaccine manufacturing, an advisory board member, on the speaker’s bureau, and a researcher of vaccines for several manufacturers. The other authors reported no relevant conflicts of interest.
SOURCE: Li-Kim-Moy J et al. Pediatr Infect Dis J. 2018 Oct;37(10):971-5.
Researchers found a lower rate of pediatric fever after applying a standard definition of fever across three different clinical trials of pediatric patients receiving influenza vaccinations, according to research published in the Pediatric Infectious Disease Journal.
Investigators in future studies must adopt a standardized definition of pediatric fever after an influenza vaccination. “Our study demonstrates the variability in results which occur due to minor differences in the definition of fever, methods of analysis and reporting of results,” Jean Li-Kim-Moy, MBBS, of the University of Sydney, and colleagues wrote.
Dr. Li-Kim-Moy and colleagues analyzed pediatric datasets from three different clinical trials using trivalent influenza vaccine (TIV); the primary trial included 3,317 children aged 6-35 months who were randomized to receive Fluarix at 0.25 mL or 0.5 mL, or receive 0.25 mL of Fluzone. The other two trials studied children receiving TIV between 6 months–17 years and 3-17 years. The researchers also performed a multivariable regression analysis to determine the relationship between immunogenicity, antipyretic use, and postvaccination fever.
The primary study initially reported the fever rate 0 days–3 days after vaccination was between 6% and 7%. After reporting the rate of fever separately for each dose and changing the criteria to “defining fever as greater than or equal to 38.0°C by any route of measurement” for the primary study, the researchers found a rate of any-cause fever was 3%-4% for the first dose and 4%-5% for the second dose. The rate of vaccine-related fever in the primary study was 3% for the first dose and 3%-4% for the second dose, with researchers noting vaccine-related fever occurred significantly earlier compared with any-cause fever (mean 1 days vs. 2 days after vaccination; P equals .04).
Impact of fever, antipyretics
The researchers also performed a pooled immunogenicity analysis of 5,902 children from all three trials and found a strong association between fever after vaccination and increased geometric mean titer (GMT) ratios (1.21-1.39; P less than or equal to .01) and an association between antipyretic use and reduced GMT ratios (0.80-0.87; P less than .0006).
“Our pooled analysis of the three trials demonstrated highly significant associations, for all strains, between postvaccination fever and up to 39% higher GMT; Similarly, strong evidence of associations in the opposite direction was found between postvaccination antipyretic use (days 0-3), adjusting for all other factors including fever, and decreased immunogenicity against all vaccine strains in children,” Dr. Li-Kim-Moy and colleagues said.
Antipyretic use was common in the primary study, occurring in one in six of the children, they said. These findings of “significant associations between fever and increased vaccine immunogenicity, and between antipyretic use and reduced immunogenicity in children after influenza vaccination” suggest the need for further study, especially because parents often give antipyretics if their children are febrile after vaccinations.
“There is uncertainty whether our findings, and those of others, on immunogenicity translate into clinically significant effects,” they wrote. “However, the fact that influenza vaccine, unlike many routine childhood vaccines, is only moderately protective may mean that modest reductions in antibody response are more likely to correlate to less protection.”
Dr. Wood reported receiving a fellowship from the National Health and Medical Research Council (NHMRC) and being an investigator for GlaxoSmithKline trials. Dr. Booy reported being an advisor for influenza vaccine manufacturing, an advisory board member, on the speaker’s bureau, and a researcher of vaccines for several manufacturers. The other authors reported no relevant conflicts of interest.
SOURCE: Li-Kim-Moy J et al. Pediatr Infect Dis J. 2018 Oct;37(10):971-5.
FROM THE PEDIATRIC INFECTIOUS DISEASE JOURNAL
Key clinical point: There is variability in reporting and analysis of pediatric fever rates after administration of the influenza vaccine.
Major finding: Applying the Brighton Collaboration standardized definition for vaccine-related fever to three clinical trials yielded significantly lower rates of fever (3%-4%), compared with the rates reported in the trials (6%-7%).
Study details: An analysis of pediatric fever data from three different clinical trials using Brighton Collaboration criteria.
Disclosures: Dr. Wood reported receiving a fellowship from the National Health and Medical Research Council and being an investigator for GlaxoSmithKline trials. Dr. Booy reported being an advisor for influenza vaccine manufacturing, an advisory board member, on the speaker’s bureau, and a researcher of vaccines for several manufacturers. The other authors reported no relevant conflicts of interest.
Source: Li-Kim-Moy J et al. Pediatr Infect Dis J. 2018 Oct;37(10):971-5.
ID experts urge widespread flu vaccination for 2018-2019 season
WASHINGTON – The flu vaccine may not be perfect, but it can reduce the severity of illness and curb the risk of spreading the disease to others, William Schaffner, MD, emphasized at a press conference held by the National Foundation for Infectious Diseases.
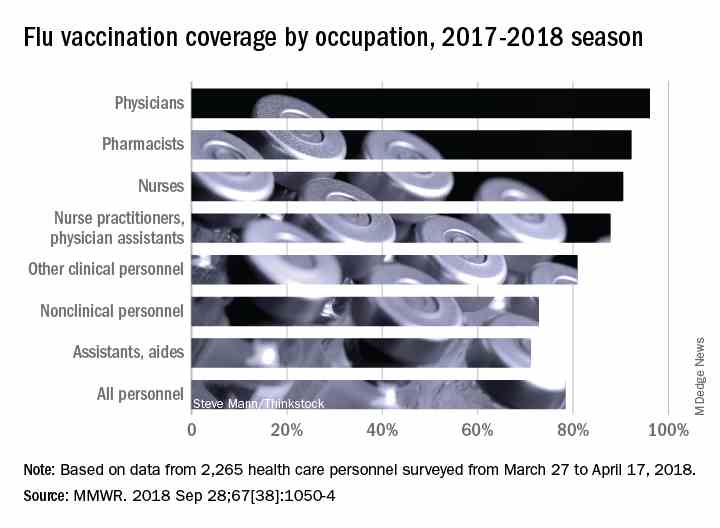
“Give the vaccine credit for softening the blow,” said Dr. Schaffner, medical director of NFID and a professor at Vanderbilt University in Nashville.
Dr. Schaffner and a panel of experts including U.S. Surgeon General Jerome M. Adams, MD, encouraged the public and the health care community to follow recommendation from the Centers for Disease Control & Prevention that everyone aged 6 months and older receive an influenza vaccine.
Dr. Schaffner shared recent data showing that complications from the flu don’t stop when the acute illness resolves. Acute influenza causes a whole-body inflammatory reaction, and consequently “there is an increased risk of heart attack and stroke during the 2-4 weeks of recovery from acute influenza,” he said. In addition, older adults who experience acute flu and are already frail may never regain their pre-flu level of function, as the flu can start a “domino effect of decline and disability.”
Despite last year’s severe flu season that included 180 deaths in children, vaccination remains the most effective protection against the flu, Dr. Adams said.
This year, between 163 million and 168 million doses of vaccine will be available in the United States. The vaccine is available in a range of settings including doctors’ offices, pharmacies, grocery stores, and workplaces, said Dr. Adams.
Flu vaccine choices this year include a return of the live-attenuated influenza vaccine (LAIV) given via nasal spray, along with the standard influenza vaccine that includes either three influenza viruses (trivalent, with two influenza A and one influenza B) or four influenza viruses (quadrivalent, with two influenza A and two influenza B). Other options are adjuvanted vaccine and high-dose vaccine for adults aged 65 years and older, and a cell-based and recombinant vaccine as alternatives to egg-based vaccines.
Dr. Adams emphasized the importance of healthy people getting vaccinated to protect the community. “All the people who died from the flu caught it from someone else,” he said.
The message to health care providers remains the same: Recommend the flu vaccine to patients at every opportunity, and lead by example and get vaccinated yourself, Dr. Adams said. He noted this year’s strategies to promote flu vaccination on social media, and encouraged clinicians to recommend the flu shot to their patients and to showcase their own shots via the #FightFlu hashtag.
Vaccination among health care personnel last year was approximately 78%, which is a plateau over the past several years (MMWR 2018; 67:1050-54).
Be prepared to offer antivirals to patients as appropriate, and to promote the pneumococcal vaccine to eligible older adults as well, to protect not only themselves, but their contacts and the community, Dr. Adams emphasized. Currently approved antiviral drugs recommended for the 2018-2019 flu season: oseltamivir, zanamivir, and peramivir.
Wendy Sue Swanson, MD, of Seattle Children’s Hospital, stressed the importance of flu vaccination for all children, given their ability to spread viral infections. She noted a concerning 2% drop in vaccinations for children aged 6 months to 4 years, although vaccination coverage in this group was highest among children overall, at approximately 68% last season.
Last year, approximately 80% of the child deaths from flu occurred in unvaccinated children, but the vaccine has been shown to reduce the likelihood of hospitalization or death even if a child does become ill, Dr. Swanson said.
Laura E. Riley, MD, of Weill Cornell Medical Center, noted that vaccination of pregnant women has plateaued in recent years, and was 49% last year. “Our goal is 80% plus,” she said. Data show that pregnant women who received flu vaccination were 40% less likely to be hospitalized for the flu, she noted. The American College of Obstetricians and Gynecologists recommends flu vaccination as safe during any trimester, and valuable to both mothers and newborns because it provides protective antibodies during the first 6 months of life before babies can receive their own vaccinations, Dr. Riley said.
More information about this year’s flu season is available from the CDC and NFID.
WASHINGTON – The flu vaccine may not be perfect, but it can reduce the severity of illness and curb the risk of spreading the disease to others, William Schaffner, MD, emphasized at a press conference held by the National Foundation for Infectious Diseases.

“Give the vaccine credit for softening the blow,” said Dr. Schaffner, medical director of NFID and a professor at Vanderbilt University in Nashville.
Dr. Schaffner and a panel of experts including U.S. Surgeon General Jerome M. Adams, MD, encouraged the public and the health care community to follow recommendation from the Centers for Disease Control & Prevention that everyone aged 6 months and older receive an influenza vaccine.
Dr. Schaffner shared recent data showing that complications from the flu don’t stop when the acute illness resolves. Acute influenza causes a whole-body inflammatory reaction, and consequently “there is an increased risk of heart attack and stroke during the 2-4 weeks of recovery from acute influenza,” he said. In addition, older adults who experience acute flu and are already frail may never regain their pre-flu level of function, as the flu can start a “domino effect of decline and disability.”
Despite last year’s severe flu season that included 180 deaths in children, vaccination remains the most effective protection against the flu, Dr. Adams said.
This year, between 163 million and 168 million doses of vaccine will be available in the United States. The vaccine is available in a range of settings including doctors’ offices, pharmacies, grocery stores, and workplaces, said Dr. Adams.
Flu vaccine choices this year include a return of the live-attenuated influenza vaccine (LAIV) given via nasal spray, along with the standard influenza vaccine that includes either three influenza viruses (trivalent, with two influenza A and one influenza B) or four influenza viruses (quadrivalent, with two influenza A and two influenza B). Other options are adjuvanted vaccine and high-dose vaccine for adults aged 65 years and older, and a cell-based and recombinant vaccine as alternatives to egg-based vaccines.
Dr. Adams emphasized the importance of healthy people getting vaccinated to protect the community. “All the people who died from the flu caught it from someone else,” he said.
The message to health care providers remains the same: Recommend the flu vaccine to patients at every opportunity, and lead by example and get vaccinated yourself, Dr. Adams said. He noted this year’s strategies to promote flu vaccination on social media, and encouraged clinicians to recommend the flu shot to their patients and to showcase their own shots via the #FightFlu hashtag.
Vaccination among health care personnel last year was approximately 78%, which is a plateau over the past several years (MMWR 2018; 67:1050-54).
Be prepared to offer antivirals to patients as appropriate, and to promote the pneumococcal vaccine to eligible older adults as well, to protect not only themselves, but their contacts and the community, Dr. Adams emphasized. Currently approved antiviral drugs recommended for the 2018-2019 flu season: oseltamivir, zanamivir, and peramivir.
Wendy Sue Swanson, MD, of Seattle Children’s Hospital, stressed the importance of flu vaccination for all children, given their ability to spread viral infections. She noted a concerning 2% drop in vaccinations for children aged 6 months to 4 years, although vaccination coverage in this group was highest among children overall, at approximately 68% last season.
Last year, approximately 80% of the child deaths from flu occurred in unvaccinated children, but the vaccine has been shown to reduce the likelihood of hospitalization or death even if a child does become ill, Dr. Swanson said.
Laura E. Riley, MD, of Weill Cornell Medical Center, noted that vaccination of pregnant women has plateaued in recent years, and was 49% last year. “Our goal is 80% plus,” she said. Data show that pregnant women who received flu vaccination were 40% less likely to be hospitalized for the flu, she noted. The American College of Obstetricians and Gynecologists recommends flu vaccination as safe during any trimester, and valuable to both mothers and newborns because it provides protective antibodies during the first 6 months of life before babies can receive their own vaccinations, Dr. Riley said.
More information about this year’s flu season is available from the CDC and NFID.
WASHINGTON – The flu vaccine may not be perfect, but it can reduce the severity of illness and curb the risk of spreading the disease to others, William Schaffner, MD, emphasized at a press conference held by the National Foundation for Infectious Diseases.

“Give the vaccine credit for softening the blow,” said Dr. Schaffner, medical director of NFID and a professor at Vanderbilt University in Nashville.
Dr. Schaffner and a panel of experts including U.S. Surgeon General Jerome M. Adams, MD, encouraged the public and the health care community to follow recommendation from the Centers for Disease Control & Prevention that everyone aged 6 months and older receive an influenza vaccine.
Dr. Schaffner shared recent data showing that complications from the flu don’t stop when the acute illness resolves. Acute influenza causes a whole-body inflammatory reaction, and consequently “there is an increased risk of heart attack and stroke during the 2-4 weeks of recovery from acute influenza,” he said. In addition, older adults who experience acute flu and are already frail may never regain their pre-flu level of function, as the flu can start a “domino effect of decline and disability.”
Despite last year’s severe flu season that included 180 deaths in children, vaccination remains the most effective protection against the flu, Dr. Adams said.
This year, between 163 million and 168 million doses of vaccine will be available in the United States. The vaccine is available in a range of settings including doctors’ offices, pharmacies, grocery stores, and workplaces, said Dr. Adams.
Flu vaccine choices this year include a return of the live-attenuated influenza vaccine (LAIV) given via nasal spray, along with the standard influenza vaccine that includes either three influenza viruses (trivalent, with two influenza A and one influenza B) or four influenza viruses (quadrivalent, with two influenza A and two influenza B). Other options are adjuvanted vaccine and high-dose vaccine for adults aged 65 years and older, and a cell-based and recombinant vaccine as alternatives to egg-based vaccines.
Dr. Adams emphasized the importance of healthy people getting vaccinated to protect the community. “All the people who died from the flu caught it from someone else,” he said.
The message to health care providers remains the same: Recommend the flu vaccine to patients at every opportunity, and lead by example and get vaccinated yourself, Dr. Adams said. He noted this year’s strategies to promote flu vaccination on social media, and encouraged clinicians to recommend the flu shot to their patients and to showcase their own shots via the #FightFlu hashtag.
Vaccination among health care personnel last year was approximately 78%, which is a plateau over the past several years (MMWR 2018; 67:1050-54).
Be prepared to offer antivirals to patients as appropriate, and to promote the pneumococcal vaccine to eligible older adults as well, to protect not only themselves, but their contacts and the community, Dr. Adams emphasized. Currently approved antiviral drugs recommended for the 2018-2019 flu season: oseltamivir, zanamivir, and peramivir.
Wendy Sue Swanson, MD, of Seattle Children’s Hospital, stressed the importance of flu vaccination for all children, given their ability to spread viral infections. She noted a concerning 2% drop in vaccinations for children aged 6 months to 4 years, although vaccination coverage in this group was highest among children overall, at approximately 68% last season.
Last year, approximately 80% of the child deaths from flu occurred in unvaccinated children, but the vaccine has been shown to reduce the likelihood of hospitalization or death even if a child does become ill, Dr. Swanson said.
Laura E. Riley, MD, of Weill Cornell Medical Center, noted that vaccination of pregnant women has plateaued in recent years, and was 49% last year. “Our goal is 80% plus,” she said. Data show that pregnant women who received flu vaccination were 40% less likely to be hospitalized for the flu, she noted. The American College of Obstetricians and Gynecologists recommends flu vaccination as safe during any trimester, and valuable to both mothers and newborns because it provides protective antibodies during the first 6 months of life before babies can receive their own vaccinations, Dr. Riley said.
More information about this year’s flu season is available from the CDC and NFID.
FROM AN NFID PRESS CONFERENCE
Recommending HPV vaccination: How would you grade yourself?
A few weeks ago, a patient asked whether he could get my opinion on something unrelated to his yellow fever vaccine visit: He asked what I thought about the human papillomavirus (HPV) vaccine. His daughter’s primary care physician (PCP) had recommended it, but he “heard that it wasn’t safe.” We had a brief discussion.
My pediatric training days have long since ended, but I was taught never to miss an opportunity to immunize. In this case, it was to help a parent decide to immunize. This type of encounter is not unusual because, as part of preparing persons for international travel, I review their routine immunizations. When documentation of a vaccine is absent, it is pointed out and often remedied after a brief discussion.
Unfortunately, with HPV, too often parents state “my primary care physician said” it was optional, it was not required, or it was never recommended. Some were told to wait until their child was older, and several have safety concerns as did the parent above. I sometimes hear, “it’s not necessary for my child”; this is usually a clue indicating that the issue is more likely about how HPV is transmitted than what HPV vaccine can prevent. Most have welcomed the opportunity to discuss the vaccine, hear about its benefits, and have their questions answered. All leave with HPV information and are directed to websites that provide accurate information. They are referred to their PCP – hopefully to be immunized.
Three vaccines – meningococcal conjugate vaccine (MCV), Tdap, and HPV vaccine – all are recommended for administration at 11-12 years of age. A booster of MCV is recommended at 16 years. However, let’s focus on HPV. In 2007, HPV administration was recommended by the Advisory Committee on Immunization Practices (ACIP) for girls; by 2011, the recommendation was extended to boys. It was a three-dose schedule expected to be completed by age 13 years. In December 2016, a two-dose schedule administered at least 6 months apart was recommended for teens who initiated immunization at less than 15 years. Three doses were still recommended for those initiating HPV after 15 years. This was the only time the number of doses to complete a vaccine series had been decreased based on postlicensure data. So
Vaccine coverage
The National Immunization Survey–Teen (NIS-Teen) monitors vaccine coverage annually amongst adolescents aged 13-17 years. Data are obtained from individuals from every state, as well as the District of Columbia, the U.S. Virgin Islands, and six major urban areas.
According to the Centers for Disease Control and Prevention’s Morbidity and Mortality Weekly Report (2018 Aug 24;67[33]:909-17), HPV vaccination continues to lag behind Tdap and MCV in 2018. Among all adolescents, coverage with one or more doses of HPV was 66%, with up-to-date HPV status in 49%. In contrast, 82% received a dose of MCV, and 89% received a dose of Tdap.
Coverage for receiving one or more doses of HPV among females was 69%, and up-to-date HPV status was 53%; among males, coverage with one or more doses was 63%, and up-to-date HPV status was 44%.
Up-to-date HPV coverage status differed geographically, ranging from 29% in Mississippi to 78% in DC. Overall, eight states and the District of Columbia reported increases in up-to-date status (District of Columbia, Louisiana, Massachusetts, Nebraska, North Carolina, South Carolina, Texas, Vermont, and Virginia). Kudos to Virginia for having the largest increase (20 percentage points).
Coverage also differed between urban and rural areas: one or more doses at 70% vs. 59% and up-to-date status at 52% vs. 42%.
HPV coverage differed by poverty level as well. It was higher for persons living below the poverty level, with one or more doses in 73% and up-to-date status in 54%, compared with persons living at or above poverty level at 63% and 47%, respectively.
HPV-related cancers

The most recent CDC data regarding types of HPV-associated cancers during 2011-2015 suggest that HPV types 16 and 18 account for the majority of cervical (78%) and oropharyngeal (86%) cancers.

Currently, there are more cases of oropharyngeal cancer than cervical, and we have no screening tool for the former.
Safety
Safety has been well documented. Since licensure, no serious safety concerns have been identified, contrary to what has been reported on various social and news media outlets. Yet it remains a concern for many parents who have delayed initiation of vaccine. Efficacy also has been documented in the United States and abroad.
Suggestions for improving HPV immunization coverage
Here are eight suggestions to help you recommend the vaccine and convince hesitant parents of its necessity:
1. Focus on your delivery of the HPV immunization recommendation. Clinician recommendation is the No. 1 reason parents vaccinate. The tone you use and how you make the recommendation can affect how the parent perceives the importance of this vaccine. The following are components of a high-quality recommendation (Academic Pediatrics. 2018;18:S23-S27):
- Routinely recommend vaccine at 11-12 years.
- Recommend vaccine for all preteens, not just those you feel are at risk for infection.
- Recommend the vaccine be given the same day it is discussed.
- Use language that expresses the importance of the HPV vaccine.
2. Use the “announcement or presumptive approach.” You expect the parent to agree with your recommendation. You don’t want to convey that it is an option.
3. Remind parents that immunizing on time means only two doses of HPV.
4. Revisit the topic again during another visit if a parent declines. Data suggest secondary acceptance can be as high as 66%.
5. Consider using a motivational interviewing approach for parents who are very hesitant to vaccinate. Most people want to comply with recommended health interventions.
6. Educate your staff about the importance of HPV vaccine and how it prevents cancer.
7. Determine how well your practice immunizes adolescents. This would be a perfect quality improvement project.
8. Explore “Answering Parents’ Questions” and other resources at www.cdc.gov/hpv to find quick answers to HPV vaccine–related questions .
Why is HPV coverage, a vaccine to prevent cancer, still lagging behind Tdap and MCV? I am as puzzled as others. What I do know is this: Our children will mature and one day become sexually active. They can be exposed to and get infected with HPV, and we can’t predict which ones will not clear the virus and end up developing an HPV-related cancer in the future. At the end of the day, HPV vaccination is cancer prevention.
Dr. Word is a pediatric infectious disease specialist and director of the Houston Travel Medicine Clinic. She said she had no relevant financial disclosures. Email her at [email protected].
A few weeks ago, a patient asked whether he could get my opinion on something unrelated to his yellow fever vaccine visit: He asked what I thought about the human papillomavirus (HPV) vaccine. His daughter’s primary care physician (PCP) had recommended it, but he “heard that it wasn’t safe.” We had a brief discussion.
My pediatric training days have long since ended, but I was taught never to miss an opportunity to immunize. In this case, it was to help a parent decide to immunize. This type of encounter is not unusual because, as part of preparing persons for international travel, I review their routine immunizations. When documentation of a vaccine is absent, it is pointed out and often remedied after a brief discussion.
Unfortunately, with HPV, too often parents state “my primary care physician said” it was optional, it was not required, or it was never recommended. Some were told to wait until their child was older, and several have safety concerns as did the parent above. I sometimes hear, “it’s not necessary for my child”; this is usually a clue indicating that the issue is more likely about how HPV is transmitted than what HPV vaccine can prevent. Most have welcomed the opportunity to discuss the vaccine, hear about its benefits, and have their questions answered. All leave with HPV information and are directed to websites that provide accurate information. They are referred to their PCP – hopefully to be immunized.
Three vaccines – meningococcal conjugate vaccine (MCV), Tdap, and HPV vaccine – all are recommended for administration at 11-12 years of age. A booster of MCV is recommended at 16 years. However, let’s focus on HPV. In 2007, HPV administration was recommended by the Advisory Committee on Immunization Practices (ACIP) for girls; by 2011, the recommendation was extended to boys. It was a three-dose schedule expected to be completed by age 13 years. In December 2016, a two-dose schedule administered at least 6 months apart was recommended for teens who initiated immunization at less than 15 years. Three doses were still recommended for those initiating HPV after 15 years. This was the only time the number of doses to complete a vaccine series had been decreased based on postlicensure data. So
Vaccine coverage
The National Immunization Survey–Teen (NIS-Teen) monitors vaccine coverage annually amongst adolescents aged 13-17 years. Data are obtained from individuals from every state, as well as the District of Columbia, the U.S. Virgin Islands, and six major urban areas.
According to the Centers for Disease Control and Prevention’s Morbidity and Mortality Weekly Report (2018 Aug 24;67[33]:909-17), HPV vaccination continues to lag behind Tdap and MCV in 2018. Among all adolescents, coverage with one or more doses of HPV was 66%, with up-to-date HPV status in 49%. In contrast, 82% received a dose of MCV, and 89% received a dose of Tdap.
Coverage for receiving one or more doses of HPV among females was 69%, and up-to-date HPV status was 53%; among males, coverage with one or more doses was 63%, and up-to-date HPV status was 44%.
Up-to-date HPV coverage status differed geographically, ranging from 29% in Mississippi to 78% in DC. Overall, eight states and the District of Columbia reported increases in up-to-date status (District of Columbia, Louisiana, Massachusetts, Nebraska, North Carolina, South Carolina, Texas, Vermont, and Virginia). Kudos to Virginia for having the largest increase (20 percentage points).
Coverage also differed between urban and rural areas: one or more doses at 70% vs. 59% and up-to-date status at 52% vs. 42%.
HPV coverage differed by poverty level as well. It was higher for persons living below the poverty level, with one or more doses in 73% and up-to-date status in 54%, compared with persons living at or above poverty level at 63% and 47%, respectively.
HPV-related cancers

The most recent CDC data regarding types of HPV-associated cancers during 2011-2015 suggest that HPV types 16 and 18 account for the majority of cervical (78%) and oropharyngeal (86%) cancers.

Currently, there are more cases of oropharyngeal cancer than cervical, and we have no screening tool for the former.
Safety
Safety has been well documented. Since licensure, no serious safety concerns have been identified, contrary to what has been reported on various social and news media outlets. Yet it remains a concern for many parents who have delayed initiation of vaccine. Efficacy also has been documented in the United States and abroad.
Suggestions for improving HPV immunization coverage
Here are eight suggestions to help you recommend the vaccine and convince hesitant parents of its necessity:
1. Focus on your delivery of the HPV immunization recommendation. Clinician recommendation is the No. 1 reason parents vaccinate. The tone you use and how you make the recommendation can affect how the parent perceives the importance of this vaccine. The following are components of a high-quality recommendation (Academic Pediatrics. 2018;18:S23-S27):
- Routinely recommend vaccine at 11-12 years.
- Recommend vaccine for all preteens, not just those you feel are at risk for infection.
- Recommend the vaccine be given the same day it is discussed.
- Use language that expresses the importance of the HPV vaccine.
2. Use the “announcement or presumptive approach.” You expect the parent to agree with your recommendation. You don’t want to convey that it is an option.
3. Remind parents that immunizing on time means only two doses of HPV.
4. Revisit the topic again during another visit if a parent declines. Data suggest secondary acceptance can be as high as 66%.
5. Consider using a motivational interviewing approach for parents who are very hesitant to vaccinate. Most people want to comply with recommended health interventions.
6. Educate your staff about the importance of HPV vaccine and how it prevents cancer.
7. Determine how well your practice immunizes adolescents. This would be a perfect quality improvement project.
8. Explore “Answering Parents’ Questions” and other resources at www.cdc.gov/hpv to find quick answers to HPV vaccine–related questions .
Why is HPV coverage, a vaccine to prevent cancer, still lagging behind Tdap and MCV? I am as puzzled as others. What I do know is this: Our children will mature and one day become sexually active. They can be exposed to and get infected with HPV, and we can’t predict which ones will not clear the virus and end up developing an HPV-related cancer in the future. At the end of the day, HPV vaccination is cancer prevention.
Dr. Word is a pediatric infectious disease specialist and director of the Houston Travel Medicine Clinic. She said she had no relevant financial disclosures. Email her at [email protected].
A few weeks ago, a patient asked whether he could get my opinion on something unrelated to his yellow fever vaccine visit: He asked what I thought about the human papillomavirus (HPV) vaccine. His daughter’s primary care physician (PCP) had recommended it, but he “heard that it wasn’t safe.” We had a brief discussion.
My pediatric training days have long since ended, but I was taught never to miss an opportunity to immunize. In this case, it was to help a parent decide to immunize. This type of encounter is not unusual because, as part of preparing persons for international travel, I review their routine immunizations. When documentation of a vaccine is absent, it is pointed out and often remedied after a brief discussion.
Unfortunately, with HPV, too often parents state “my primary care physician said” it was optional, it was not required, or it was never recommended. Some were told to wait until their child was older, and several have safety concerns as did the parent above. I sometimes hear, “it’s not necessary for my child”; this is usually a clue indicating that the issue is more likely about how HPV is transmitted than what HPV vaccine can prevent. Most have welcomed the opportunity to discuss the vaccine, hear about its benefits, and have their questions answered. All leave with HPV information and are directed to websites that provide accurate information. They are referred to their PCP – hopefully to be immunized.
Three vaccines – meningococcal conjugate vaccine (MCV), Tdap, and HPV vaccine – all are recommended for administration at 11-12 years of age. A booster of MCV is recommended at 16 years. However, let’s focus on HPV. In 2007, HPV administration was recommended by the Advisory Committee on Immunization Practices (ACIP) for girls; by 2011, the recommendation was extended to boys. It was a three-dose schedule expected to be completed by age 13 years. In December 2016, a two-dose schedule administered at least 6 months apart was recommended for teens who initiated immunization at less than 15 years. Three doses were still recommended for those initiating HPV after 15 years. This was the only time the number of doses to complete a vaccine series had been decreased based on postlicensure data. So
Vaccine coverage
The National Immunization Survey–Teen (NIS-Teen) monitors vaccine coverage annually amongst adolescents aged 13-17 years. Data are obtained from individuals from every state, as well as the District of Columbia, the U.S. Virgin Islands, and six major urban areas.
According to the Centers for Disease Control and Prevention’s Morbidity and Mortality Weekly Report (2018 Aug 24;67[33]:909-17), HPV vaccination continues to lag behind Tdap and MCV in 2018. Among all adolescents, coverage with one or more doses of HPV was 66%, with up-to-date HPV status in 49%. In contrast, 82% received a dose of MCV, and 89% received a dose of Tdap.
Coverage for receiving one or more doses of HPV among females was 69%, and up-to-date HPV status was 53%; among males, coverage with one or more doses was 63%, and up-to-date HPV status was 44%.
Up-to-date HPV coverage status differed geographically, ranging from 29% in Mississippi to 78% in DC. Overall, eight states and the District of Columbia reported increases in up-to-date status (District of Columbia, Louisiana, Massachusetts, Nebraska, North Carolina, South Carolina, Texas, Vermont, and Virginia). Kudos to Virginia for having the largest increase (20 percentage points).
Coverage also differed between urban and rural areas: one or more doses at 70% vs. 59% and up-to-date status at 52% vs. 42%.
HPV coverage differed by poverty level as well. It was higher for persons living below the poverty level, with one or more doses in 73% and up-to-date status in 54%, compared with persons living at or above poverty level at 63% and 47%, respectively.
HPV-related cancers

The most recent CDC data regarding types of HPV-associated cancers during 2011-2015 suggest that HPV types 16 and 18 account for the majority of cervical (78%) and oropharyngeal (86%) cancers.

Currently, there are more cases of oropharyngeal cancer than cervical, and we have no screening tool for the former.
Safety
Safety has been well documented. Since licensure, no serious safety concerns have been identified, contrary to what has been reported on various social and news media outlets. Yet it remains a concern for many parents who have delayed initiation of vaccine. Efficacy also has been documented in the United States and abroad.
Suggestions for improving HPV immunization coverage
Here are eight suggestions to help you recommend the vaccine and convince hesitant parents of its necessity:
1. Focus on your delivery of the HPV immunization recommendation. Clinician recommendation is the No. 1 reason parents vaccinate. The tone you use and how you make the recommendation can affect how the parent perceives the importance of this vaccine. The following are components of a high-quality recommendation (Academic Pediatrics. 2018;18:S23-S27):
- Routinely recommend vaccine at 11-12 years.
- Recommend vaccine for all preteens, not just those you feel are at risk for infection.
- Recommend the vaccine be given the same day it is discussed.
- Use language that expresses the importance of the HPV vaccine.
2. Use the “announcement or presumptive approach.” You expect the parent to agree with your recommendation. You don’t want to convey that it is an option.
3. Remind parents that immunizing on time means only two doses of HPV.
4. Revisit the topic again during another visit if a parent declines. Data suggest secondary acceptance can be as high as 66%.
5. Consider using a motivational interviewing approach for parents who are very hesitant to vaccinate. Most people want to comply with recommended health interventions.
6. Educate your staff about the importance of HPV vaccine and how it prevents cancer.
7. Determine how well your practice immunizes adolescents. This would be a perfect quality improvement project.
8. Explore “Answering Parents’ Questions” and other resources at www.cdc.gov/hpv to find quick answers to HPV vaccine–related questions .
Why is HPV coverage, a vaccine to prevent cancer, still lagging behind Tdap and MCV? I am as puzzled as others. What I do know is this: Our children will mature and one day become sexually active. They can be exposed to and get infected with HPV, and we can’t predict which ones will not clear the virus and end up developing an HPV-related cancer in the future. At the end of the day, HPV vaccination is cancer prevention.
Dr. Word is a pediatric infectious disease specialist and director of the Houston Travel Medicine Clinic. She said she had no relevant financial disclosures. Email her at [email protected].
Pertussis vaccine at birth shows immune response, tolerability
compared with a group receiving only the hepatitis B vaccine, a randomized clinical trial from Australia has found.
“These results indicate that a birth dose of aP vaccine is immunogenic in newborns and significantly narrows the immunity gap between birth and 14 days after receipt of DTaP at 6 or 8 weeks of age, marking the critical period when infants are most vulnerable to severe pertussis infection,” reported Nicholas Wood, PhD, of the National Centre for Immunisation Research and Surveillance of Vaccine Preventable Diseases in New South Wales, Australia, and his colleagues.
“Administration of the acellular pertussis vaccine at birth has the potential to reduce severe morbidity from Bordetella pertussis infection in the first 3 months of life, especially for infants of mothers who have not received a pertussis vaccine during pregnancy,” the researchers concluded in JAMA Pediatrics.
The researchers enrolled 417 infants from Sydney, Melbourne, Adelaide, and Perth between June 2010 and March 2013 and randomized them to receive either the hepatitis B vaccine alone (n = 205) or the hepatitis B vaccine with a monovalent acellular pertussis vaccine (n = 212) within the first 5 days after birth. The randomization was stratified for mothers’ receipt of the Tdap before pregnancy.
The Centers for Disease Control and Prevention currently recommends all newborns receive the hepatitis B vaccine shortly after birth and that pregnant women receive the Tdap vaccine during each pregnancy. There is not currently a monovalent acellular pertussis vaccine licensed in the United States.
The study infants then received the hexavalent DTaP-Hib-hep B-polio vaccine and the 10-valent pneumococcal conjugate vaccine at 6 weeks, 4 months, and 6 months.
The primary outcome was detectable levels of IgG antibody to pertussis toxin and pertactin at 10 weeks old.
Of the 206 infants receiving the pertussis vaccine at birth, 93% had detectable antibodies to pertussis toxin and pertactin at 10 weeks, compared with 51% of the 193 infants who received only the hepatitis B shot (P less than .001). Geometric mean concentration for pertussis toxin IgG also was four times higher in infants who received the pertussis vaccine at birth.
Adverse events were similar in the two groups both at birth and at 32 weeks, demonstrating that the pertussis birth dose is safe and tolerable.
“More important, in this study, the prevalence of fever after receipt of the birth dose, which can mistakenly be associated with potential sepsis and result in additional investigations in the neonatal period, was similar in both the group that received the aP vaccine at birth and the control group,” the authors reported.
A remaining question is the potential impact of maternal antibodies on protection from pertussis.
“The presence of maternal pertussis antibodies at birth can negatively affect postprimary responses to pertussis, diphtheria, and diphtheria-related CRM197 conjugate vaccines with a variety of infant immunization schedules and vaccines,” the authors noted. “The clinical significance of reductions in pertussis antibody related to maternal interference will require ongoing clinical evaluation, because there are no accepted serologic correlates of protection.”
The research was funded by a Australian National Health and Medical Research Council (NHMRC) grant, and several authors received NHMRC grants. One author also was supported by a Murdoch Children’s Research Institute Career Development Award. GlaxoSmithKline provided the vaccine and conducted the serologic assays. The authors reported having no conflicts of interest.
SOURCE: Wood N et al, JAMA Pediatr. 2018 Sep 10. doi: 10.1001/jamapediatrics.2018.2349.
Pertussis is most likely to cause morbidity or kill neonates between birth and when they are given their first pertussis vaccine at 6-8 weeks of age. This is well known.
In the current study giving the acellular pertussis (aP) vaccine at birth led to “significantly higher antibody titers to pertussis antigens at 10 weeks of age,” compared with those who did not receive it. Those infants who received the birth dose of aP vaccine also had higher pertussis antibodies at 6 weeks, whether or not their mothers had received Tdap within 5 years prior to delivery.
When this study began in 2009, maternal immunization was not a well accepted concept, but this attitude has changed, in part due to the safe vaccination of pregnant women with the pandemic flu vaccine. Despite this, Centers for Disease Control and Prevention 2016 data showed that only 49% of pregnant women in the United Stated received Tdap. These rates need to increase.
Administering the aP vaccine with the existing hepatitis B vaccine at birth to infants whose mothers who did not receive Tdap during pregnancy would be a practical solution, if the aP vaccine were universally available.
But the aP vaccine currently is not available in the United States and many other countries as a standalone vaccine, and the administration of DTaP as a birth dose has been linked with “significant immune interference.” The aP vaccine could have a place in countries where it is available, and there is no maternal immunization program. Otherwise, boosting maternal immunization appears to be the primary approach for now.
Kathryn M. Edwards, MD, is the Sarah H. Sell and Cornelius Vanderbilt Chair in Pediatrics at Vanderbilt University, Nashville. She specializes in pediatric infectious diseases. These comments are a summary of her editorial accompanying the article by Wood et al. (Pediatrics. 2018 Sep 10. doi: 10.1001/jamapediatrics.2018.2363). Dr. Edwards said she had no conflicts of interest.
Pertussis is most likely to cause morbidity or kill neonates between birth and when they are given their first pertussis vaccine at 6-8 weeks of age. This is well known.
In the current study giving the acellular pertussis (aP) vaccine at birth led to “significantly higher antibody titers to pertussis antigens at 10 weeks of age,” compared with those who did not receive it. Those infants who received the birth dose of aP vaccine also had higher pertussis antibodies at 6 weeks, whether or not their mothers had received Tdap within 5 years prior to delivery.
When this study began in 2009, maternal immunization was not a well accepted concept, but this attitude has changed, in part due to the safe vaccination of pregnant women with the pandemic flu vaccine. Despite this, Centers for Disease Control and Prevention 2016 data showed that only 49% of pregnant women in the United Stated received Tdap. These rates need to increase.
Administering the aP vaccine with the existing hepatitis B vaccine at birth to infants whose mothers who did not receive Tdap during pregnancy would be a practical solution, if the aP vaccine were universally available.
But the aP vaccine currently is not available in the United States and many other countries as a standalone vaccine, and the administration of DTaP as a birth dose has been linked with “significant immune interference.” The aP vaccine could have a place in countries where it is available, and there is no maternal immunization program. Otherwise, boosting maternal immunization appears to be the primary approach for now.
Kathryn M. Edwards, MD, is the Sarah H. Sell and Cornelius Vanderbilt Chair in Pediatrics at Vanderbilt University, Nashville. She specializes in pediatric infectious diseases. These comments are a summary of her editorial accompanying the article by Wood et al. (Pediatrics. 2018 Sep 10. doi: 10.1001/jamapediatrics.2018.2363). Dr. Edwards said she had no conflicts of interest.
Pertussis is most likely to cause morbidity or kill neonates between birth and when they are given their first pertussis vaccine at 6-8 weeks of age. This is well known.
In the current study giving the acellular pertussis (aP) vaccine at birth led to “significantly higher antibody titers to pertussis antigens at 10 weeks of age,” compared with those who did not receive it. Those infants who received the birth dose of aP vaccine also had higher pertussis antibodies at 6 weeks, whether or not their mothers had received Tdap within 5 years prior to delivery.
When this study began in 2009, maternal immunization was not a well accepted concept, but this attitude has changed, in part due to the safe vaccination of pregnant women with the pandemic flu vaccine. Despite this, Centers for Disease Control and Prevention 2016 data showed that only 49% of pregnant women in the United Stated received Tdap. These rates need to increase.
Administering the aP vaccine with the existing hepatitis B vaccine at birth to infants whose mothers who did not receive Tdap during pregnancy would be a practical solution, if the aP vaccine were universally available.
But the aP vaccine currently is not available in the United States and many other countries as a standalone vaccine, and the administration of DTaP as a birth dose has been linked with “significant immune interference.” The aP vaccine could have a place in countries where it is available, and there is no maternal immunization program. Otherwise, boosting maternal immunization appears to be the primary approach for now.
Kathryn M. Edwards, MD, is the Sarah H. Sell and Cornelius Vanderbilt Chair in Pediatrics at Vanderbilt University, Nashville. She specializes in pediatric infectious diseases. These comments are a summary of her editorial accompanying the article by Wood et al. (Pediatrics. 2018 Sep 10. doi: 10.1001/jamapediatrics.2018.2363). Dr. Edwards said she had no conflicts of interest.
compared with a group receiving only the hepatitis B vaccine, a randomized clinical trial from Australia has found.
“These results indicate that a birth dose of aP vaccine is immunogenic in newborns and significantly narrows the immunity gap between birth and 14 days after receipt of DTaP at 6 or 8 weeks of age, marking the critical period when infants are most vulnerable to severe pertussis infection,” reported Nicholas Wood, PhD, of the National Centre for Immunisation Research and Surveillance of Vaccine Preventable Diseases in New South Wales, Australia, and his colleagues.
“Administration of the acellular pertussis vaccine at birth has the potential to reduce severe morbidity from Bordetella pertussis infection in the first 3 months of life, especially for infants of mothers who have not received a pertussis vaccine during pregnancy,” the researchers concluded in JAMA Pediatrics.
The researchers enrolled 417 infants from Sydney, Melbourne, Adelaide, and Perth between June 2010 and March 2013 and randomized them to receive either the hepatitis B vaccine alone (n = 205) or the hepatitis B vaccine with a monovalent acellular pertussis vaccine (n = 212) within the first 5 days after birth. The randomization was stratified for mothers’ receipt of the Tdap before pregnancy.
The Centers for Disease Control and Prevention currently recommends all newborns receive the hepatitis B vaccine shortly after birth and that pregnant women receive the Tdap vaccine during each pregnancy. There is not currently a monovalent acellular pertussis vaccine licensed in the United States.
The study infants then received the hexavalent DTaP-Hib-hep B-polio vaccine and the 10-valent pneumococcal conjugate vaccine at 6 weeks, 4 months, and 6 months.
The primary outcome was detectable levels of IgG antibody to pertussis toxin and pertactin at 10 weeks old.
Of the 206 infants receiving the pertussis vaccine at birth, 93% had detectable antibodies to pertussis toxin and pertactin at 10 weeks, compared with 51% of the 193 infants who received only the hepatitis B shot (P less than .001). Geometric mean concentration for pertussis toxin IgG also was four times higher in infants who received the pertussis vaccine at birth.
Adverse events were similar in the two groups both at birth and at 32 weeks, demonstrating that the pertussis birth dose is safe and tolerable.
“More important, in this study, the prevalence of fever after receipt of the birth dose, which can mistakenly be associated with potential sepsis and result in additional investigations in the neonatal period, was similar in both the group that received the aP vaccine at birth and the control group,” the authors reported.
A remaining question is the potential impact of maternal antibodies on protection from pertussis.
“The presence of maternal pertussis antibodies at birth can negatively affect postprimary responses to pertussis, diphtheria, and diphtheria-related CRM197 conjugate vaccines with a variety of infant immunization schedules and vaccines,” the authors noted. “The clinical significance of reductions in pertussis antibody related to maternal interference will require ongoing clinical evaluation, because there are no accepted serologic correlates of protection.”
The research was funded by a Australian National Health and Medical Research Council (NHMRC) grant, and several authors received NHMRC grants. One author also was supported by a Murdoch Children’s Research Institute Career Development Award. GlaxoSmithKline provided the vaccine and conducted the serologic assays. The authors reported having no conflicts of interest.
SOURCE: Wood N et al, JAMA Pediatr. 2018 Sep 10. doi: 10.1001/jamapediatrics.2018.2349.
compared with a group receiving only the hepatitis B vaccine, a randomized clinical trial from Australia has found.
“These results indicate that a birth dose of aP vaccine is immunogenic in newborns and significantly narrows the immunity gap between birth and 14 days after receipt of DTaP at 6 or 8 weeks of age, marking the critical period when infants are most vulnerable to severe pertussis infection,” reported Nicholas Wood, PhD, of the National Centre for Immunisation Research and Surveillance of Vaccine Preventable Diseases in New South Wales, Australia, and his colleagues.
“Administration of the acellular pertussis vaccine at birth has the potential to reduce severe morbidity from Bordetella pertussis infection in the first 3 months of life, especially for infants of mothers who have not received a pertussis vaccine during pregnancy,” the researchers concluded in JAMA Pediatrics.
The researchers enrolled 417 infants from Sydney, Melbourne, Adelaide, and Perth between June 2010 and March 2013 and randomized them to receive either the hepatitis B vaccine alone (n = 205) or the hepatitis B vaccine with a monovalent acellular pertussis vaccine (n = 212) within the first 5 days after birth. The randomization was stratified for mothers’ receipt of the Tdap before pregnancy.
The Centers for Disease Control and Prevention currently recommends all newborns receive the hepatitis B vaccine shortly after birth and that pregnant women receive the Tdap vaccine during each pregnancy. There is not currently a monovalent acellular pertussis vaccine licensed in the United States.
The study infants then received the hexavalent DTaP-Hib-hep B-polio vaccine and the 10-valent pneumococcal conjugate vaccine at 6 weeks, 4 months, and 6 months.
The primary outcome was detectable levels of IgG antibody to pertussis toxin and pertactin at 10 weeks old.
Of the 206 infants receiving the pertussis vaccine at birth, 93% had detectable antibodies to pertussis toxin and pertactin at 10 weeks, compared with 51% of the 193 infants who received only the hepatitis B shot (P less than .001). Geometric mean concentration for pertussis toxin IgG also was four times higher in infants who received the pertussis vaccine at birth.
Adverse events were similar in the two groups both at birth and at 32 weeks, demonstrating that the pertussis birth dose is safe and tolerable.
“More important, in this study, the prevalence of fever after receipt of the birth dose, which can mistakenly be associated with potential sepsis and result in additional investigations in the neonatal period, was similar in both the group that received the aP vaccine at birth and the control group,” the authors reported.
A remaining question is the potential impact of maternal antibodies on protection from pertussis.
“The presence of maternal pertussis antibodies at birth can negatively affect postprimary responses to pertussis, diphtheria, and diphtheria-related CRM197 conjugate vaccines with a variety of infant immunization schedules and vaccines,” the authors noted. “The clinical significance of reductions in pertussis antibody related to maternal interference will require ongoing clinical evaluation, because there are no accepted serologic correlates of protection.”
The research was funded by a Australian National Health and Medical Research Council (NHMRC) grant, and several authors received NHMRC grants. One author also was supported by a Murdoch Children’s Research Institute Career Development Award. GlaxoSmithKline provided the vaccine and conducted the serologic assays. The authors reported having no conflicts of interest.
SOURCE: Wood N et al, JAMA Pediatr. 2018 Sep 10. doi: 10.1001/jamapediatrics.2018.2349.
FROM JAMA PEDIATRICS
Key clinical point: A monovalent acellular pertussis vaccine dose at birth appears safe, tolerable, and effective.
Major finding: 93% of 212 newborns receiving an acellular pertussis vaccine at birth showed antibodies against pertussis toxin and pertactin at 10 weeks, compared with 51% of 205 newborns without the birth dose.
Study details: The findings are based on a randomized controlled trial involving 417 healthy term newborns in four Australian cities from June 2010 to March 2013.
Disclosures: The research was funded by an Australian National Health and Medical Research Council (NHMRC) grant, and several authors received NHMRC grants. One author also was supported by a Murdoch Children’s Research Institute Career Development Award. GlaxoSmithKline provided the vaccine and conducted the serologic assays. The authors reporting having no conflicts of interest.
Source: Wood N et al. JAMA Pediatr. 2018 Sep. 10. doi: 10.1001/jamapediatrics.2018.2349.
CDC recommendations for the 2018-2019 influenza season
The 2017-2018 influenza season was one of the most severe in this century, according to every indicator measured by the Centers for Disease Control and Prevention (CDC). The proportion of outpatient visits due to influenza-like illness (ILI) was elevated nationally above a baseline of 2.2% for 19 straight weeks, and for 3 weeks it was over 7%.1 High ILI activity was widespread and included all 50 states in January.
From October 2017 through April 2018, the CDC estimates that the influenza-related hospitalization rate was 106.6 per 100,000 population, with the highest rates among children 0 to 4 years (74.3/100,000), adults 50 to 64 years (115.7/100,000), and adults 65 years and older (460.9/100,000). More than 90% of adults hospitalized had a chronic condition, such as heart or lung disease, diabetes, or obesity, placing them at high risk for influenza complications.1
Influenza severity is also measured as the proportion of deaths due to pneumonia and influenza, which was above the epidemic threshold for 16 weeks in 2017-2018 and was above 10% for 4 weeks in January.1 Based on all of these indicators, the 2017-2018 influenza season was classified as high severity overall and for all age groups, the first time this has happened since the 2003-2004 season. There were 171 pediatric deaths attributed to influenza, and more than three-quarters of vaccine-eligible children who died from influenza this season had not received influenza vaccine.1
The type of influenza predominating last season was influenza A from early- through mid-season, and was influenza B later in the season (see https://stacks.cdc.gov/view/cdc/54974).1 For the entire season, 71.2% of specimens that tested positive for influenza in public health labs were Influenza A and 84.9% of these were H3N2.1
Effectiveness of influenza vaccine last season. As measured by preventing respiratory illness needing medical attention, vaccine effectiveness was 36% overall: 25% against influenza A (H3N2), 67% against influenza A (H1N1), and 42% against influenza B.1 Effectiveness varied by age, being the highest in those 8 years and younger.2 Effectiveness was questionable in those older than 65, with an estimated effectiveness of 23% but confidence intervals including 0.2
While the effectiveness of influenza vaccines remains suboptimal, the morbidity and mortality they prevent is still considerable. The CDC estimates that in 2016-2017, more than 5 million influenza illnesses, 2.6 million medical visits, and 84,700 hospitalizations were prevented.3 And effectiveness last season was similar to, or better than, what has been seen in each of the past 10 years (FIGURE).4

Three drugs were recommended for use to treat influenza in 2017-1018 (oseltamivir, peramivir, and zanamivir), and no resistance was found except in 1% of influenza A (H1N1) tested.1 No resistance was found in other A or any B viruses tested.1
Continue to: Safety
Safety
The safety of influenza vaccines is studied each year by both the CDC and US Food and Drug Administration (FDA). This past year, studies were conducted using the CDC-supported Safety Datalink System, looking for increased rates of acute disseminated encephalomyelitis, anaphylaxis, Bell’s palsy, encephalitis, Guillain-Barré syndrome (GBS), seizures, and transverse myelitis.5 No safety signals were detected. However, for some of the newer vaccines, the numbers of vaccinated individuals studied were small. The FDA studied the incidence of GBS using Medicare data and found no increased rates in those vaccinated.5
2018-2019 Recommendations
There are only a few changes to the recommendations for the upcoming influenza season. The Advisory Committee on Immunization Practices (ACIP) still recommends universal vaccination for anyone age 6 months and older who does not have a contraindication (TABLE 16). Two of the antigens in the vaccines for this coming season are slightly different from last season (TABLE 27).
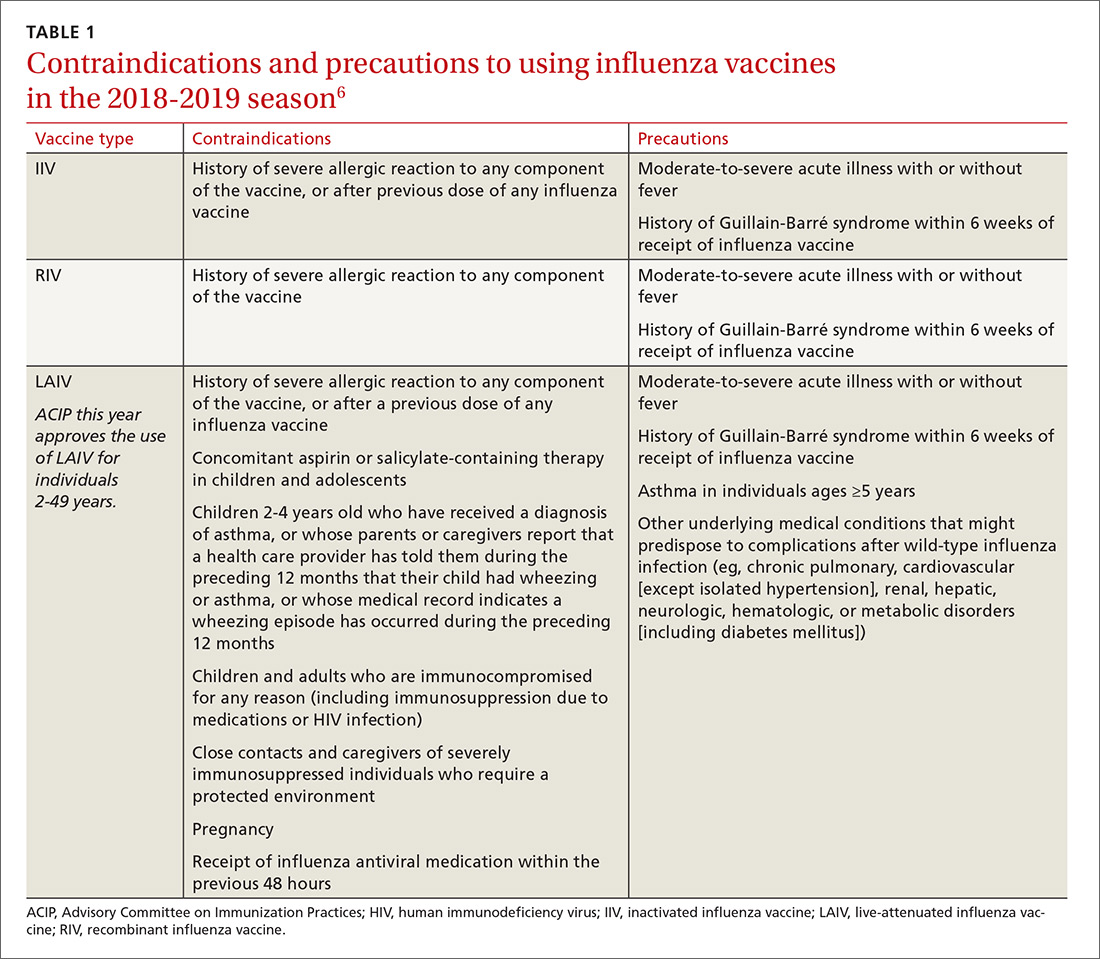
After 2 years of recommending against the use of live attenuated influenza vaccine (LAIV) because of its low effectiveness in children against influenza A (H1N1), ACIP now includes it as an option for the upcoming season in individuals ages 2 through 49 years.8 The basis of this revised recommendation was 2-fold: 1) evidence of LAIV effectiveness comparable to that of inactivated products against A (H3N2) and B viruses; and 2) evidence that a new strain of A (H1N1) now used to produce the vaccine (A/Slovenia) produces a significantly higher antibody response than the strain (A/Bolivia) used in the years when the vaccine was not effective against A (H1N1).

However, the new formulation’s clinical effectiveness against A (H1N1) has not been demonstrated, leading the American Academy of Pediatrics to recommend that LAIV should be used in children only if other options are not available or if injectable vaccine is refused.9 Contraindications to the use of LAIV remain the same as the previous version of the vaccine (TABLE 16).
Individuals with non-severe egg allergies can receive any licensed, recommended age-appropriate influenza vaccine and no longer have to be monitored for 30 minutes after receiving the vaccine. People who have severe egg allergies should be vaccinated with an egg-free product or in a medical setting and be supervised by a health care provider who is able to recognize and manage severe allergic conditions.
Continue to: Children 6 months through 8 years...
Children 6 months through 8 years who have previously received an influenza vaccine, either trivalent or quadrivalent, need only 1 dose; those who have not received vaccination need 2 doses separated by at least 4 weeks.
Available vaccine products
A table found on the CDC influenza Web site lists the vaccine products available in the United States and the ages for which they are approved.6 The options now include 2 standard-dose trivalent inactivated influenza vaccines (IIV3), 4 standard-dose quadrivalent inactivated influenza vaccines (IIV4), one cell culture-based IIV4 (ccIIV4), one standard dose IIV4 intradermal option, a trivalent and a quadrivalent recombinant influenza vaccine (RIV3, RIV4), one LAIV, and 2 products for those 65 years and older—an adjuvanted IIV3 (aIIV3) and a high dose IIV3. Three of these products do not depend on egg-based technology: RIV3, RIV4, and ccIIV4.
Comparative effectiveness studies of these vaccine options, including those available for the elderly, are being conducted. Studies presented at the June 2018 ACIP meeting show comparable effectiveness of egg-based and non–egg-based products.6 At this time, ACIP does not make a preferential recommendation for any influenza vaccine product for any age group.
1. Garten R, Blanton L, Elal AIA, eta al. Update: Influenza activity in the United States during the 2017-18 season and composition of the 2018-2019 influenza vaccine. MMWR Morb Mortal Wkly Rep. 2018;67;634-642.
2. Flannery B, Chung JR, Belongia EA, et al. Interim estimates of 2017-18 seasonal influenza vaccine effectiveness – United States, February 2018. MMWR Morb Mortal Wkly Rep. 2018;67:180-185.
3. Flannery B, Chung J, Ferdinands J. Preliminary estimates of 2017-2018 seasonal influenza vaccine effectiveness against laboratory-confirmed influenza from the US Flu VE and HAIVEN network. Meeting of the Advisory Committee on Immunization Practices; June 20, 2018; Atlanta, Ga. Available at: https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2018-06/flu-02-Flannery-508.pdf. Accessed August 11, 2018.
4. CDC. Seasonal influenza vaccine effectiveness, 2005-2018. Available at: https://www.cdc.gov/flu/professionals/vaccination/effectiveness-studies.htm. Accessed July 27, 2018.
5. Shimabukuro T. End-of-season update: 2017-2018 influenza vaccine safety monitoring. Presented at: meeting of the Advisory Committee on Immunization Practices; June 20, 2018; Atlanta, Ga. Available at: https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2018-06/flu-04-Shimabukuro-508.pdf. Accessed August 11, 2018.
6. CDC. Prevention and Control of Seasonal Influenza with Vaccines: Recommendations of the Advisory Committee on Immunization Practices—United States, 2018–19 Influenza Season. Available at: https://www.cdc.gov/mmwr/volumes/67/rr/rr6703a1.htm?s_cid=rr6703a1_w. Accessed August 23, 2018.
7. CDC. Update: Influenza activity in the United States during the 2017-18 season and composition of the 2018-19 influenza vaccine. Available at: https://www.cdc.gov/mmwr/volumes/67/wr/mm6722a4.htm. Accessed July 27, 2018.
8. Grohskopf LA, Sokolow LZ, Fry AM, et al. Update: ACIP recommendations for the use of quadrivalent live attenuated influenza vaccine (LAIV4) — United States, 2018–19 influenza season. MMWR Morb Mortal Wkly Rep. 2018;67:643-645.
9. Jenco M. AAP: Give children IIV flu shot; use LAIV as last resort. Available at: http://www.aappublications.org/news/2018/05/21/fluvaccine051818. Accessed August 1, 2018.
The 2017-2018 influenza season was one of the most severe in this century, according to every indicator measured by the Centers for Disease Control and Prevention (CDC). The proportion of outpatient visits due to influenza-like illness (ILI) was elevated nationally above a baseline of 2.2% for 19 straight weeks, and for 3 weeks it was over 7%.1 High ILI activity was widespread and included all 50 states in January.
From October 2017 through April 2018, the CDC estimates that the influenza-related hospitalization rate was 106.6 per 100,000 population, with the highest rates among children 0 to 4 years (74.3/100,000), adults 50 to 64 years (115.7/100,000), and adults 65 years and older (460.9/100,000). More than 90% of adults hospitalized had a chronic condition, such as heart or lung disease, diabetes, or obesity, placing them at high risk for influenza complications.1
Influenza severity is also measured as the proportion of deaths due to pneumonia and influenza, which was above the epidemic threshold for 16 weeks in 2017-2018 and was above 10% for 4 weeks in January.1 Based on all of these indicators, the 2017-2018 influenza season was classified as high severity overall and for all age groups, the first time this has happened since the 2003-2004 season. There were 171 pediatric deaths attributed to influenza, and more than three-quarters of vaccine-eligible children who died from influenza this season had not received influenza vaccine.1
The type of influenza predominating last season was influenza A from early- through mid-season, and was influenza B later in the season (see https://stacks.cdc.gov/view/cdc/54974).1 For the entire season, 71.2% of specimens that tested positive for influenza in public health labs were Influenza A and 84.9% of these were H3N2.1
Effectiveness of influenza vaccine last season. As measured by preventing respiratory illness needing medical attention, vaccine effectiveness was 36% overall: 25% against influenza A (H3N2), 67% against influenza A (H1N1), and 42% against influenza B.1 Effectiveness varied by age, being the highest in those 8 years and younger.2 Effectiveness was questionable in those older than 65, with an estimated effectiveness of 23% but confidence intervals including 0.2
While the effectiveness of influenza vaccines remains suboptimal, the morbidity and mortality they prevent is still considerable. The CDC estimates that in 2016-2017, more than 5 million influenza illnesses, 2.6 million medical visits, and 84,700 hospitalizations were prevented.3 And effectiveness last season was similar to, or better than, what has been seen in each of the past 10 years (FIGURE).4

Three drugs were recommended for use to treat influenza in 2017-1018 (oseltamivir, peramivir, and zanamivir), and no resistance was found except in 1% of influenza A (H1N1) tested.1 No resistance was found in other A or any B viruses tested.1
Continue to: Safety
Safety
The safety of influenza vaccines is studied each year by both the CDC and US Food and Drug Administration (FDA). This past year, studies were conducted using the CDC-supported Safety Datalink System, looking for increased rates of acute disseminated encephalomyelitis, anaphylaxis, Bell’s palsy, encephalitis, Guillain-Barré syndrome (GBS), seizures, and transverse myelitis.5 No safety signals were detected. However, for some of the newer vaccines, the numbers of vaccinated individuals studied were small. The FDA studied the incidence of GBS using Medicare data and found no increased rates in those vaccinated.5
2018-2019 Recommendations
There are only a few changes to the recommendations for the upcoming influenza season. The Advisory Committee on Immunization Practices (ACIP) still recommends universal vaccination for anyone age 6 months and older who does not have a contraindication (TABLE 16). Two of the antigens in the vaccines for this coming season are slightly different from last season (TABLE 27).

After 2 years of recommending against the use of live attenuated influenza vaccine (LAIV) because of its low effectiveness in children against influenza A (H1N1), ACIP now includes it as an option for the upcoming season in individuals ages 2 through 49 years.8 The basis of this revised recommendation was 2-fold: 1) evidence of LAIV effectiveness comparable to that of inactivated products against A (H3N2) and B viruses; and 2) evidence that a new strain of A (H1N1) now used to produce the vaccine (A/Slovenia) produces a significantly higher antibody response than the strain (A/Bolivia) used in the years when the vaccine was not effective against A (H1N1).

However, the new formulation’s clinical effectiveness against A (H1N1) has not been demonstrated, leading the American Academy of Pediatrics to recommend that LAIV should be used in children only if other options are not available or if injectable vaccine is refused.9 Contraindications to the use of LAIV remain the same as the previous version of the vaccine (TABLE 16).
Individuals with non-severe egg allergies can receive any licensed, recommended age-appropriate influenza vaccine and no longer have to be monitored for 30 minutes after receiving the vaccine. People who have severe egg allergies should be vaccinated with an egg-free product or in a medical setting and be supervised by a health care provider who is able to recognize and manage severe allergic conditions.
Continue to: Children 6 months through 8 years...
Children 6 months through 8 years who have previously received an influenza vaccine, either trivalent or quadrivalent, need only 1 dose; those who have not received vaccination need 2 doses separated by at least 4 weeks.
Available vaccine products
A table found on the CDC influenza Web site lists the vaccine products available in the United States and the ages for which they are approved.6 The options now include 2 standard-dose trivalent inactivated influenza vaccines (IIV3), 4 standard-dose quadrivalent inactivated influenza vaccines (IIV4), one cell culture-based IIV4 (ccIIV4), one standard dose IIV4 intradermal option, a trivalent and a quadrivalent recombinant influenza vaccine (RIV3, RIV4), one LAIV, and 2 products for those 65 years and older—an adjuvanted IIV3 (aIIV3) and a high dose IIV3. Three of these products do not depend on egg-based technology: RIV3, RIV4, and ccIIV4.
Comparative effectiveness studies of these vaccine options, including those available for the elderly, are being conducted. Studies presented at the June 2018 ACIP meeting show comparable effectiveness of egg-based and non–egg-based products.6 At this time, ACIP does not make a preferential recommendation for any influenza vaccine product for any age group.
The 2017-2018 influenza season was one of the most severe in this century, according to every indicator measured by the Centers for Disease Control and Prevention (CDC). The proportion of outpatient visits due to influenza-like illness (ILI) was elevated nationally above a baseline of 2.2% for 19 straight weeks, and for 3 weeks it was over 7%.1 High ILI activity was widespread and included all 50 states in January.
From October 2017 through April 2018, the CDC estimates that the influenza-related hospitalization rate was 106.6 per 100,000 population, with the highest rates among children 0 to 4 years (74.3/100,000), adults 50 to 64 years (115.7/100,000), and adults 65 years and older (460.9/100,000). More than 90% of adults hospitalized had a chronic condition, such as heart or lung disease, diabetes, or obesity, placing them at high risk for influenza complications.1
Influenza severity is also measured as the proportion of deaths due to pneumonia and influenza, which was above the epidemic threshold for 16 weeks in 2017-2018 and was above 10% for 4 weeks in January.1 Based on all of these indicators, the 2017-2018 influenza season was classified as high severity overall and for all age groups, the first time this has happened since the 2003-2004 season. There were 171 pediatric deaths attributed to influenza, and more than three-quarters of vaccine-eligible children who died from influenza this season had not received influenza vaccine.1
The type of influenza predominating last season was influenza A from early- through mid-season, and was influenza B later in the season (see https://stacks.cdc.gov/view/cdc/54974).1 For the entire season, 71.2% of specimens that tested positive for influenza in public health labs were Influenza A and 84.9% of these were H3N2.1
Effectiveness of influenza vaccine last season. As measured by preventing respiratory illness needing medical attention, vaccine effectiveness was 36% overall: 25% against influenza A (H3N2), 67% against influenza A (H1N1), and 42% against influenza B.1 Effectiveness varied by age, being the highest in those 8 years and younger.2 Effectiveness was questionable in those older than 65, with an estimated effectiveness of 23% but confidence intervals including 0.2
While the effectiveness of influenza vaccines remains suboptimal, the morbidity and mortality they prevent is still considerable. The CDC estimates that in 2016-2017, more than 5 million influenza illnesses, 2.6 million medical visits, and 84,700 hospitalizations were prevented.3 And effectiveness last season was similar to, or better than, what has been seen in each of the past 10 years (FIGURE).4

Three drugs were recommended for use to treat influenza in 2017-1018 (oseltamivir, peramivir, and zanamivir), and no resistance was found except in 1% of influenza A (H1N1) tested.1 No resistance was found in other A or any B viruses tested.1
Continue to: Safety
Safety
The safety of influenza vaccines is studied each year by both the CDC and US Food and Drug Administration (FDA). This past year, studies were conducted using the CDC-supported Safety Datalink System, looking for increased rates of acute disseminated encephalomyelitis, anaphylaxis, Bell’s palsy, encephalitis, Guillain-Barré syndrome (GBS), seizures, and transverse myelitis.5 No safety signals were detected. However, for some of the newer vaccines, the numbers of vaccinated individuals studied were small. The FDA studied the incidence of GBS using Medicare data and found no increased rates in those vaccinated.5
2018-2019 Recommendations
There are only a few changes to the recommendations for the upcoming influenza season. The Advisory Committee on Immunization Practices (ACIP) still recommends universal vaccination for anyone age 6 months and older who does not have a contraindication (TABLE 16). Two of the antigens in the vaccines for this coming season are slightly different from last season (TABLE 27).

After 2 years of recommending against the use of live attenuated influenza vaccine (LAIV) because of its low effectiveness in children against influenza A (H1N1), ACIP now includes it as an option for the upcoming season in individuals ages 2 through 49 years.8 The basis of this revised recommendation was 2-fold: 1) evidence of LAIV effectiveness comparable to that of inactivated products against A (H3N2) and B viruses; and 2) evidence that a new strain of A (H1N1) now used to produce the vaccine (A/Slovenia) produces a significantly higher antibody response than the strain (A/Bolivia) used in the years when the vaccine was not effective against A (H1N1).

However, the new formulation’s clinical effectiveness against A (H1N1) has not been demonstrated, leading the American Academy of Pediatrics to recommend that LAIV should be used in children only if other options are not available or if injectable vaccine is refused.9 Contraindications to the use of LAIV remain the same as the previous version of the vaccine (TABLE 16).
Individuals with non-severe egg allergies can receive any licensed, recommended age-appropriate influenza vaccine and no longer have to be monitored for 30 minutes after receiving the vaccine. People who have severe egg allergies should be vaccinated with an egg-free product or in a medical setting and be supervised by a health care provider who is able to recognize and manage severe allergic conditions.
Continue to: Children 6 months through 8 years...
Children 6 months through 8 years who have previously received an influenza vaccine, either trivalent or quadrivalent, need only 1 dose; those who have not received vaccination need 2 doses separated by at least 4 weeks.
Available vaccine products
A table found on the CDC influenza Web site lists the vaccine products available in the United States and the ages for which they are approved.6 The options now include 2 standard-dose trivalent inactivated influenza vaccines (IIV3), 4 standard-dose quadrivalent inactivated influenza vaccines (IIV4), one cell culture-based IIV4 (ccIIV4), one standard dose IIV4 intradermal option, a trivalent and a quadrivalent recombinant influenza vaccine (RIV3, RIV4), one LAIV, and 2 products for those 65 years and older—an adjuvanted IIV3 (aIIV3) and a high dose IIV3. Three of these products do not depend on egg-based technology: RIV3, RIV4, and ccIIV4.
Comparative effectiveness studies of these vaccine options, including those available for the elderly, are being conducted. Studies presented at the June 2018 ACIP meeting show comparable effectiveness of egg-based and non–egg-based products.6 At this time, ACIP does not make a preferential recommendation for any influenza vaccine product for any age group.
1. Garten R, Blanton L, Elal AIA, eta al. Update: Influenza activity in the United States during the 2017-18 season and composition of the 2018-2019 influenza vaccine. MMWR Morb Mortal Wkly Rep. 2018;67;634-642.
2. Flannery B, Chung JR, Belongia EA, et al. Interim estimates of 2017-18 seasonal influenza vaccine effectiveness – United States, February 2018. MMWR Morb Mortal Wkly Rep. 2018;67:180-185.
3. Flannery B, Chung J, Ferdinands J. Preliminary estimates of 2017-2018 seasonal influenza vaccine effectiveness against laboratory-confirmed influenza from the US Flu VE and HAIVEN network. Meeting of the Advisory Committee on Immunization Practices; June 20, 2018; Atlanta, Ga. Available at: https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2018-06/flu-02-Flannery-508.pdf. Accessed August 11, 2018.
4. CDC. Seasonal influenza vaccine effectiveness, 2005-2018. Available at: https://www.cdc.gov/flu/professionals/vaccination/effectiveness-studies.htm. Accessed July 27, 2018.
5. Shimabukuro T. End-of-season update: 2017-2018 influenza vaccine safety monitoring. Presented at: meeting of the Advisory Committee on Immunization Practices; June 20, 2018; Atlanta, Ga. Available at: https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2018-06/flu-04-Shimabukuro-508.pdf. Accessed August 11, 2018.
6. CDC. Prevention and Control of Seasonal Influenza with Vaccines: Recommendations of the Advisory Committee on Immunization Practices—United States, 2018–19 Influenza Season. Available at: https://www.cdc.gov/mmwr/volumes/67/rr/rr6703a1.htm?s_cid=rr6703a1_w. Accessed August 23, 2018.
7. CDC. Update: Influenza activity in the United States during the 2017-18 season and composition of the 2018-19 influenza vaccine. Available at: https://www.cdc.gov/mmwr/volumes/67/wr/mm6722a4.htm. Accessed July 27, 2018.
8. Grohskopf LA, Sokolow LZ, Fry AM, et al. Update: ACIP recommendations for the use of quadrivalent live attenuated influenza vaccine (LAIV4) — United States, 2018–19 influenza season. MMWR Morb Mortal Wkly Rep. 2018;67:643-645.
9. Jenco M. AAP: Give children IIV flu shot; use LAIV as last resort. Available at: http://www.aappublications.org/news/2018/05/21/fluvaccine051818. Accessed August 1, 2018.
1. Garten R, Blanton L, Elal AIA, eta al. Update: Influenza activity in the United States during the 2017-18 season and composition of the 2018-2019 influenza vaccine. MMWR Morb Mortal Wkly Rep. 2018;67;634-642.
2. Flannery B, Chung JR, Belongia EA, et al. Interim estimates of 2017-18 seasonal influenza vaccine effectiveness – United States, February 2018. MMWR Morb Mortal Wkly Rep. 2018;67:180-185.
3. Flannery B, Chung J, Ferdinands J. Preliminary estimates of 2017-2018 seasonal influenza vaccine effectiveness against laboratory-confirmed influenza from the US Flu VE and HAIVEN network. Meeting of the Advisory Committee on Immunization Practices; June 20, 2018; Atlanta, Ga. Available at: https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2018-06/flu-02-Flannery-508.pdf. Accessed August 11, 2018.
4. CDC. Seasonal influenza vaccine effectiveness, 2005-2018. Available at: https://www.cdc.gov/flu/professionals/vaccination/effectiveness-studies.htm. Accessed July 27, 2018.
5. Shimabukuro T. End-of-season update: 2017-2018 influenza vaccine safety monitoring. Presented at: meeting of the Advisory Committee on Immunization Practices; June 20, 2018; Atlanta, Ga. Available at: https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2018-06/flu-04-Shimabukuro-508.pdf. Accessed August 11, 2018.
6. CDC. Prevention and Control of Seasonal Influenza with Vaccines: Recommendations of the Advisory Committee on Immunization Practices—United States, 2018–19 Influenza Season. Available at: https://www.cdc.gov/mmwr/volumes/67/rr/rr6703a1.htm?s_cid=rr6703a1_w. Accessed August 23, 2018.
7. CDC. Update: Influenza activity in the United States during the 2017-18 season and composition of the 2018-19 influenza vaccine. Available at: https://www.cdc.gov/mmwr/volumes/67/wr/mm6722a4.htm. Accessed July 27, 2018.
8. Grohskopf LA, Sokolow LZ, Fry AM, et al. Update: ACIP recommendations for the use of quadrivalent live attenuated influenza vaccine (LAIV4) — United States, 2018–19 influenza season. MMWR Morb Mortal Wkly Rep. 2018;67:643-645.
9. Jenco M. AAP: Give children IIV flu shot; use LAIV as last resort. Available at: http://www.aappublications.org/news/2018/05/21/fluvaccine051818. Accessed August 1, 2018.
Live attenuated flu vaccine gets ACIP nod for 2018-2019 season
The latest seasonal influenza vaccine recommendations from the Advisory Committee on Immunization Practices provide several key updates that will impact clinical practice in the 2018-2019 influenza season.
Of note, , following two seasons in which the committee recommended it not be used.
ACIP also updated its recommendations for individuals with a history of egg allergy, described the vaccine strains chosen for 2018-2019 season, and detailed the changes in age indications for Afluria Quadrivalent and Fluarix Quadrivalent that have been made since publication of its previous guidelines.
Published in MMWR Recommendations and Reports, the updated ACIP recommendations reflect discussions and decisions from the three public meetings of ACIP that have taken place since the last annual update.
All individuals 6 months of age and older who have no contraindications to influenza vaccine should receive routine annual influenza vaccine, ACIP also said in its report, reinforcing a key recommendation that has been in place since 2010.
“To avoid missed opportunities for vaccination, providers should offer vaccination during routine health care visits and hospitalizations,” wrote authors of the report, including lead author Lisa A. Grohskopf, MD, of the National Center for Immunization and Respiratory Diseases, Centers for Disease Control and Prevention, Atlanta.
Dr. Grohskopf and coauthors made no specific recommendations on which vaccine to use. They said providers should choose licensed, age-appropriate recommended vaccines expected to be available for the 2018-2019 season, including inactivated influenza vaccines (IIV), a recombinant influenza vaccine (RIV4), and the LAIV option.
FluMist Quadrivalent, the one LAIV product expected to be available for the 2018-2019 season, is licensed for individuals aged 2-49 years.
In its deliberations over the updated LAIV recommendation, ACIP reviewed observational data from previous seasons suggesting that the vaccine was poorly effective, and significantly less effective than IIV, against influenza A(H1N1) pdm09 viruses.
The current formulation of FluMist includes a new H1N1pdm09-like vaccine virus. While no effectiveness estimates were available at the time of review, ACIP said it did consider manufacturer data on shedding and immunogenicity for the current vaccine in children between the ages of 24 months through less than 4 years.
“These data suggest that this new H1N1pdm09-like virus has improved replicative fitness over previous H1N1pdm09-like viruses included in LAIV,” Dr. Grohskopf and colleagues wrote.
Individuals with an egg allergy history also can receive any licensed, recommended, age-appropriate IIV, RIV, or LAIV vaccine, said ACIP. This updated recommendation was based in part on the committee’s review and discussion of three studies that showed no cases of anaphylaxis in egg-allergic children receiving LAIV.
The ACIP recommendation update also outlines the strains selected earlier this year for the 2018-2019 season. Trivalent influenza vaccines in the United States will include an A/Michigan/45/2015 (H1N1) pdm09–like virus, an A/Singapore/INFIMH-16-0019/2016 (H3N2)-like virus, and a B/Colorado/06/2017–like virus (Victoria lineage). Quadrivalent vaccines will include those strains plus a B/Phuket/3073/2013–like virus (Yamagata lineage).
The report also acknowledges the recent expansion of age indication for two vaccines that have occurred since the last ACIP recommendations.
Afluria Quadrivalent was previously licensed for individuals 18 years of age and older. In August 2017, the Food and Drug Administration approved expansion of the indication to individuals 5 years of age or older. In January 2018, FDA approved expansion of the Fluarix Quadrivalent indication, previously licensed for age 3 and older, to individuals 6 months and older.
Report coauthor Emmanuel B. Walter disclosed grants from Novavax and Merck. The remaining report authors reported no relevant financial disclosures.
SOURCE: Grohskopf LA et al. MMWR Recomm Rep. 2018 Aug 24;67(3):1-20.
The latest seasonal influenza vaccine recommendations from the Advisory Committee on Immunization Practices provide several key updates that will impact clinical practice in the 2018-2019 influenza season.
Of note, , following two seasons in which the committee recommended it not be used.
ACIP also updated its recommendations for individuals with a history of egg allergy, described the vaccine strains chosen for 2018-2019 season, and detailed the changes in age indications for Afluria Quadrivalent and Fluarix Quadrivalent that have been made since publication of its previous guidelines.
Published in MMWR Recommendations and Reports, the updated ACIP recommendations reflect discussions and decisions from the three public meetings of ACIP that have taken place since the last annual update.
All individuals 6 months of age and older who have no contraindications to influenza vaccine should receive routine annual influenza vaccine, ACIP also said in its report, reinforcing a key recommendation that has been in place since 2010.
“To avoid missed opportunities for vaccination, providers should offer vaccination during routine health care visits and hospitalizations,” wrote authors of the report, including lead author Lisa A. Grohskopf, MD, of the National Center for Immunization and Respiratory Diseases, Centers for Disease Control and Prevention, Atlanta.
Dr. Grohskopf and coauthors made no specific recommendations on which vaccine to use. They said providers should choose licensed, age-appropriate recommended vaccines expected to be available for the 2018-2019 season, including inactivated influenza vaccines (IIV), a recombinant influenza vaccine (RIV4), and the LAIV option.
FluMist Quadrivalent, the one LAIV product expected to be available for the 2018-2019 season, is licensed for individuals aged 2-49 years.
In its deliberations over the updated LAIV recommendation, ACIP reviewed observational data from previous seasons suggesting that the vaccine was poorly effective, and significantly less effective than IIV, against influenza A(H1N1) pdm09 viruses.
The current formulation of FluMist includes a new H1N1pdm09-like vaccine virus. While no effectiveness estimates were available at the time of review, ACIP said it did consider manufacturer data on shedding and immunogenicity for the current vaccine in children between the ages of 24 months through less than 4 years.
“These data suggest that this new H1N1pdm09-like virus has improved replicative fitness over previous H1N1pdm09-like viruses included in LAIV,” Dr. Grohskopf and colleagues wrote.
Individuals with an egg allergy history also can receive any licensed, recommended, age-appropriate IIV, RIV, or LAIV vaccine, said ACIP. This updated recommendation was based in part on the committee’s review and discussion of three studies that showed no cases of anaphylaxis in egg-allergic children receiving LAIV.
The ACIP recommendation update also outlines the strains selected earlier this year for the 2018-2019 season. Trivalent influenza vaccines in the United States will include an A/Michigan/45/2015 (H1N1) pdm09–like virus, an A/Singapore/INFIMH-16-0019/2016 (H3N2)-like virus, and a B/Colorado/06/2017–like virus (Victoria lineage). Quadrivalent vaccines will include those strains plus a B/Phuket/3073/2013–like virus (Yamagata lineage).
The report also acknowledges the recent expansion of age indication for two vaccines that have occurred since the last ACIP recommendations.
Afluria Quadrivalent was previously licensed for individuals 18 years of age and older. In August 2017, the Food and Drug Administration approved expansion of the indication to individuals 5 years of age or older. In January 2018, FDA approved expansion of the Fluarix Quadrivalent indication, previously licensed for age 3 and older, to individuals 6 months and older.
Report coauthor Emmanuel B. Walter disclosed grants from Novavax and Merck. The remaining report authors reported no relevant financial disclosures.
SOURCE: Grohskopf LA et al. MMWR Recomm Rep. 2018 Aug 24;67(3):1-20.
The latest seasonal influenza vaccine recommendations from the Advisory Committee on Immunization Practices provide several key updates that will impact clinical practice in the 2018-2019 influenza season.
Of note, , following two seasons in which the committee recommended it not be used.
ACIP also updated its recommendations for individuals with a history of egg allergy, described the vaccine strains chosen for 2018-2019 season, and detailed the changes in age indications for Afluria Quadrivalent and Fluarix Quadrivalent that have been made since publication of its previous guidelines.
Published in MMWR Recommendations and Reports, the updated ACIP recommendations reflect discussions and decisions from the three public meetings of ACIP that have taken place since the last annual update.
All individuals 6 months of age and older who have no contraindications to influenza vaccine should receive routine annual influenza vaccine, ACIP also said in its report, reinforcing a key recommendation that has been in place since 2010.
“To avoid missed opportunities for vaccination, providers should offer vaccination during routine health care visits and hospitalizations,” wrote authors of the report, including lead author Lisa A. Grohskopf, MD, of the National Center for Immunization and Respiratory Diseases, Centers for Disease Control and Prevention, Atlanta.
Dr. Grohskopf and coauthors made no specific recommendations on which vaccine to use. They said providers should choose licensed, age-appropriate recommended vaccines expected to be available for the 2018-2019 season, including inactivated influenza vaccines (IIV), a recombinant influenza vaccine (RIV4), and the LAIV option.
FluMist Quadrivalent, the one LAIV product expected to be available for the 2018-2019 season, is licensed for individuals aged 2-49 years.
In its deliberations over the updated LAIV recommendation, ACIP reviewed observational data from previous seasons suggesting that the vaccine was poorly effective, and significantly less effective than IIV, against influenza A(H1N1) pdm09 viruses.
The current formulation of FluMist includes a new H1N1pdm09-like vaccine virus. While no effectiveness estimates were available at the time of review, ACIP said it did consider manufacturer data on shedding and immunogenicity for the current vaccine in children between the ages of 24 months through less than 4 years.
“These data suggest that this new H1N1pdm09-like virus has improved replicative fitness over previous H1N1pdm09-like viruses included in LAIV,” Dr. Grohskopf and colleagues wrote.
Individuals with an egg allergy history also can receive any licensed, recommended, age-appropriate IIV, RIV, or LAIV vaccine, said ACIP. This updated recommendation was based in part on the committee’s review and discussion of three studies that showed no cases of anaphylaxis in egg-allergic children receiving LAIV.
The ACIP recommendation update also outlines the strains selected earlier this year for the 2018-2019 season. Trivalent influenza vaccines in the United States will include an A/Michigan/45/2015 (H1N1) pdm09–like virus, an A/Singapore/INFIMH-16-0019/2016 (H3N2)-like virus, and a B/Colorado/06/2017–like virus (Victoria lineage). Quadrivalent vaccines will include those strains plus a B/Phuket/3073/2013–like virus (Yamagata lineage).
The report also acknowledges the recent expansion of age indication for two vaccines that have occurred since the last ACIP recommendations.
Afluria Quadrivalent was previously licensed for individuals 18 years of age and older. In August 2017, the Food and Drug Administration approved expansion of the indication to individuals 5 years of age or older. In January 2018, FDA approved expansion of the Fluarix Quadrivalent indication, previously licensed for age 3 and older, to individuals 6 months and older.
Report coauthor Emmanuel B. Walter disclosed grants from Novavax and Merck. The remaining report authors reported no relevant financial disclosures.
SOURCE: Grohskopf LA et al. MMWR Recomm Rep. 2018 Aug 24;67(3):1-20.
FROM MMWR