User login
Menstrual cup use with copper IUDs linked to higher expulsion rates
Citing menstrual cup use for menstrual hygiene as “increasingly popular,” researchers led by Jill Long, MD, MPH, studied women participating in a prospective contraceptive efficacy trial of two copper IUDs to evaluate the relationship between menstrual cup use and IUD expulsion over a period of 24 months. The findings were released ahead of the study’s scheduled presentation at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists. ACOG canceled the meeting and released abstracts for press coverage.
In the ongoing 3-year trial, which also was published in Obstetrics & Gynecology, 1,092 women were randomized to one of two copper IUDs. Dr. Long, project officer for the Contraceptive Clinical Trials Network, a project of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, Bethesda, Md. and colleagues conducted follow-up visits at 6 weeks after insertion in the first year, and then 3, 6, and 12 months after insertion. At the 9-month mark, the study counseling was amended to advise patients against concurrent use of the menstrual cup because of a higher risk of IUD expulsions noted in women using the cup.
Among the 1,092 women studied, 266 (24%) reported menstrual cup use. At 24 months after initiating enrollment, 43 cup users (17%) and 43 nonusers (5%) experienced expulsion (odds ratio, 3.81). Fourteen menstrual cup users with expulsion (30%) reported that the event occurred during menstrual cup removal. Dr. Long and colleagues found that, at year 1 of the study, expulsion rates among menstrual cup users and nonusers were 14% and 5%, respectively (P < .001). At the end of year 2, these rates rose to 23% and 7% (P < .001). The study won second place among abstracts in the category of current clinical and basic investigation.
“This outstanding abstract reflects an important study with results that should lead to changes in the way providers counsel patients about IUDs, namely that the risk of IUD expulsion is significantly higher in women who use menstrual cups than in those who use other menstrual hygiene products,” Eve Espey, MD, MPH, who was not affiliated with the study, said in an interview.
According to Dr. Espey, who chairs the department of obstetrics and gynecology at the University of New Mexico, Albuquerque, key strengths of the study include its prospective methodology and the relatively large number of patients with concurrent IUD and menstrual cup use.
“A limitation is the nonrandomized design for the current study’s aim, which would require randomizing women using the IUD to menstrual cup use versus nonuse,” said Dr. Espey, who is a member of the Ob.Gyn News editorial advisory board.* “Another limitation is that only copper IUDs were used, but it is plausible that this result would apply to other IUDs as well. The study is innovative and important in being the first prospective study to evaluate the association between menstrual cup use and IUD expulsion.”
Dr. Long and two coauthors reported having no financial disclosures, but the remaining three authors reported having numerous potential conflicts of interest. Dr. Espey reported having no financial disclosures.
SOURCE: Long J et al. Obstet Gynecol. 2020 May;135.1S. doi: 10.1097/01.AOG.0000662872.89062.83.
*The article was updated on 4/28/2020.
Citing menstrual cup use for menstrual hygiene as “increasingly popular,” researchers led by Jill Long, MD, MPH, studied women participating in a prospective contraceptive efficacy trial of two copper IUDs to evaluate the relationship between menstrual cup use and IUD expulsion over a period of 24 months. The findings were released ahead of the study’s scheduled presentation at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists. ACOG canceled the meeting and released abstracts for press coverage.
In the ongoing 3-year trial, which also was published in Obstetrics & Gynecology, 1,092 women were randomized to one of two copper IUDs. Dr. Long, project officer for the Contraceptive Clinical Trials Network, a project of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, Bethesda, Md. and colleagues conducted follow-up visits at 6 weeks after insertion in the first year, and then 3, 6, and 12 months after insertion. At the 9-month mark, the study counseling was amended to advise patients against concurrent use of the menstrual cup because of a higher risk of IUD expulsions noted in women using the cup.
Among the 1,092 women studied, 266 (24%) reported menstrual cup use. At 24 months after initiating enrollment, 43 cup users (17%) and 43 nonusers (5%) experienced expulsion (odds ratio, 3.81). Fourteen menstrual cup users with expulsion (30%) reported that the event occurred during menstrual cup removal. Dr. Long and colleagues found that, at year 1 of the study, expulsion rates among menstrual cup users and nonusers were 14% and 5%, respectively (P < .001). At the end of year 2, these rates rose to 23% and 7% (P < .001). The study won second place among abstracts in the category of current clinical and basic investigation.
“This outstanding abstract reflects an important study with results that should lead to changes in the way providers counsel patients about IUDs, namely that the risk of IUD expulsion is significantly higher in women who use menstrual cups than in those who use other menstrual hygiene products,” Eve Espey, MD, MPH, who was not affiliated with the study, said in an interview.
According to Dr. Espey, who chairs the department of obstetrics and gynecology at the University of New Mexico, Albuquerque, key strengths of the study include its prospective methodology and the relatively large number of patients with concurrent IUD and menstrual cup use.
“A limitation is the nonrandomized design for the current study’s aim, which would require randomizing women using the IUD to menstrual cup use versus nonuse,” said Dr. Espey, who is a member of the Ob.Gyn News editorial advisory board.* “Another limitation is that only copper IUDs were used, but it is plausible that this result would apply to other IUDs as well. The study is innovative and important in being the first prospective study to evaluate the association between menstrual cup use and IUD expulsion.”
Dr. Long and two coauthors reported having no financial disclosures, but the remaining three authors reported having numerous potential conflicts of interest. Dr. Espey reported having no financial disclosures.
SOURCE: Long J et al. Obstet Gynecol. 2020 May;135.1S. doi: 10.1097/01.AOG.0000662872.89062.83.
*The article was updated on 4/28/2020.
Citing menstrual cup use for menstrual hygiene as “increasingly popular,” researchers led by Jill Long, MD, MPH, studied women participating in a prospective contraceptive efficacy trial of two copper IUDs to evaluate the relationship between menstrual cup use and IUD expulsion over a period of 24 months. The findings were released ahead of the study’s scheduled presentation at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists. ACOG canceled the meeting and released abstracts for press coverage.
In the ongoing 3-year trial, which also was published in Obstetrics & Gynecology, 1,092 women were randomized to one of two copper IUDs. Dr. Long, project officer for the Contraceptive Clinical Trials Network, a project of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, Bethesda, Md. and colleagues conducted follow-up visits at 6 weeks after insertion in the first year, and then 3, 6, and 12 months after insertion. At the 9-month mark, the study counseling was amended to advise patients against concurrent use of the menstrual cup because of a higher risk of IUD expulsions noted in women using the cup.
Among the 1,092 women studied, 266 (24%) reported menstrual cup use. At 24 months after initiating enrollment, 43 cup users (17%) and 43 nonusers (5%) experienced expulsion (odds ratio, 3.81). Fourteen menstrual cup users with expulsion (30%) reported that the event occurred during menstrual cup removal. Dr. Long and colleagues found that, at year 1 of the study, expulsion rates among menstrual cup users and nonusers were 14% and 5%, respectively (P < .001). At the end of year 2, these rates rose to 23% and 7% (P < .001). The study won second place among abstracts in the category of current clinical and basic investigation.
“This outstanding abstract reflects an important study with results that should lead to changes in the way providers counsel patients about IUDs, namely that the risk of IUD expulsion is significantly higher in women who use menstrual cups than in those who use other menstrual hygiene products,” Eve Espey, MD, MPH, who was not affiliated with the study, said in an interview.
According to Dr. Espey, who chairs the department of obstetrics and gynecology at the University of New Mexico, Albuquerque, key strengths of the study include its prospective methodology and the relatively large number of patients with concurrent IUD and menstrual cup use.
“A limitation is the nonrandomized design for the current study’s aim, which would require randomizing women using the IUD to menstrual cup use versus nonuse,” said Dr. Espey, who is a member of the Ob.Gyn News editorial advisory board.* “Another limitation is that only copper IUDs were used, but it is plausible that this result would apply to other IUDs as well. The study is innovative and important in being the first prospective study to evaluate the association between menstrual cup use and IUD expulsion.”
Dr. Long and two coauthors reported having no financial disclosures, but the remaining three authors reported having numerous potential conflicts of interest. Dr. Espey reported having no financial disclosures.
SOURCE: Long J et al. Obstet Gynecol. 2020 May;135.1S. doi: 10.1097/01.AOG.0000662872.89062.83.
*The article was updated on 4/28/2020.
FROM ACOG 2020
FDA OKs new drug for triple-negative breast cancer
The US Food and Drug Administration (FDA) granted accelerated approval to sacituzumab govitecan (Trodelvy, Immunomedics) for the treatment of metastatic triple-negative breast cancer (TNBC).
Eligible patients must have received at least two prior therapies.
TNBC is so-called because it lacks the three cellular targets present in more common forms of breast cancer. It is usually treated with chemotherapy.
Sacituzumab govitecan offers a new approach – and it has a target.
Given intravenously, the new drug is an antibody-drug conjugate in which SN-38, an active metabolite of the chemotherapy drug irinotecan (multiple brands), is coupled to a monoclonal antibody that targets an antigen that has high expression in TNBC and induces cancer cell growth.
“Metastatic triple-negative breast cancer is an aggressive form of breast cancer with limited treatment options,” observed Richard Pazdur, MD, director of the FDA’s Oncology Center of Excellence and acting director of the Office of Oncologic Diseases in the FDA’s Center for Drug Evaluation and Research in a press statement. “There is intense interest in finding new medications” for this patient population, he added.
The new approval is based on safety and efficacy results from a phase 1/2 clinical trial of 108 patients (median age, 56 years) who had received at least two prior treatments for metastatic disease.
The overall response rate was 33% (n = 36), including three complete responses. Median duration of response was 7.7 months. Of responders, 55.6% maintained their response for at least 6 months and 16.7% for at least 12 months.
Median progression-free survival was 5.5 months, and median overall survival was 13.0 months.
The study data were published last year in the New England Journal of Medicine.
“It’s not every day that we see this sort of clinical activity in this aggressive subtype of breast cancer,” said senior study author Kevin Kalinsky, MD, in an interview at that time. He is a medical oncologist at New York–Presbyterian Hospital and Columbia University Medical Center in New York City.
The most common side effects of the new therapy were nausea, neutropenia, diarrhea, fatigue, anemia, vomiting, alopecia, constipation, decreased appetite, rash, and abdominal pain.
No peripheral neuropathy of grade 3 or higher was reported.
In the study, patients received sacituzumab govitecan intravenously (10 mg/kg body weight) on days 1 and 8 of each 21-day cycle until disease progression or unacceptable toxicity.
The 108 participants received a mean 18.7 doses of sacituzumab govitecan, or 9.6 cycles. The median duration of exposure was 5.1 months.
Three patients discontinued treatment because of adverse events, and two patients discontinued because of drug-related events.
The prescribing information includes a boxed warning regarding the risks of severe neutropenia and severe diarrhea. Blood cell counts should be monitored during treatment and granulocyte-colony stimulating factor (G-CSF) therapy should be considered. Anti-infective treatment should be initiated in the event of febrile neutropenia. Patients with reduced uridine diphosphate-glucuronosyltransferase 1A1 (UGT1A1) activity are at increased risk for neutropenia following initiation of treatment.
The new drug can also cause hypersensitivity reactions including severe anaphylactic reactions.
Women who are pregnant should not take sacituzumab govitecan.
This article first appeared on Medscape.com.
The US Food and Drug Administration (FDA) granted accelerated approval to sacituzumab govitecan (Trodelvy, Immunomedics) for the treatment of metastatic triple-negative breast cancer (TNBC).
Eligible patients must have received at least two prior therapies.
TNBC is so-called because it lacks the three cellular targets present in more common forms of breast cancer. It is usually treated with chemotherapy.
Sacituzumab govitecan offers a new approach – and it has a target.
Given intravenously, the new drug is an antibody-drug conjugate in which SN-38, an active metabolite of the chemotherapy drug irinotecan (multiple brands), is coupled to a monoclonal antibody that targets an antigen that has high expression in TNBC and induces cancer cell growth.
“Metastatic triple-negative breast cancer is an aggressive form of breast cancer with limited treatment options,” observed Richard Pazdur, MD, director of the FDA’s Oncology Center of Excellence and acting director of the Office of Oncologic Diseases in the FDA’s Center for Drug Evaluation and Research in a press statement. “There is intense interest in finding new medications” for this patient population, he added.
The new approval is based on safety and efficacy results from a phase 1/2 clinical trial of 108 patients (median age, 56 years) who had received at least two prior treatments for metastatic disease.
The overall response rate was 33% (n = 36), including three complete responses. Median duration of response was 7.7 months. Of responders, 55.6% maintained their response for at least 6 months and 16.7% for at least 12 months.
Median progression-free survival was 5.5 months, and median overall survival was 13.0 months.
The study data were published last year in the New England Journal of Medicine.
“It’s not every day that we see this sort of clinical activity in this aggressive subtype of breast cancer,” said senior study author Kevin Kalinsky, MD, in an interview at that time. He is a medical oncologist at New York–Presbyterian Hospital and Columbia University Medical Center in New York City.
The most common side effects of the new therapy were nausea, neutropenia, diarrhea, fatigue, anemia, vomiting, alopecia, constipation, decreased appetite, rash, and abdominal pain.
No peripheral neuropathy of grade 3 or higher was reported.
In the study, patients received sacituzumab govitecan intravenously (10 mg/kg body weight) on days 1 and 8 of each 21-day cycle until disease progression or unacceptable toxicity.
The 108 participants received a mean 18.7 doses of sacituzumab govitecan, or 9.6 cycles. The median duration of exposure was 5.1 months.
Three patients discontinued treatment because of adverse events, and two patients discontinued because of drug-related events.
The prescribing information includes a boxed warning regarding the risks of severe neutropenia and severe diarrhea. Blood cell counts should be monitored during treatment and granulocyte-colony stimulating factor (G-CSF) therapy should be considered. Anti-infective treatment should be initiated in the event of febrile neutropenia. Patients with reduced uridine diphosphate-glucuronosyltransferase 1A1 (UGT1A1) activity are at increased risk for neutropenia following initiation of treatment.
The new drug can also cause hypersensitivity reactions including severe anaphylactic reactions.
Women who are pregnant should not take sacituzumab govitecan.
This article first appeared on Medscape.com.
The US Food and Drug Administration (FDA) granted accelerated approval to sacituzumab govitecan (Trodelvy, Immunomedics) for the treatment of metastatic triple-negative breast cancer (TNBC).
Eligible patients must have received at least two prior therapies.
TNBC is so-called because it lacks the three cellular targets present in more common forms of breast cancer. It is usually treated with chemotherapy.
Sacituzumab govitecan offers a new approach – and it has a target.
Given intravenously, the new drug is an antibody-drug conjugate in which SN-38, an active metabolite of the chemotherapy drug irinotecan (multiple brands), is coupled to a monoclonal antibody that targets an antigen that has high expression in TNBC and induces cancer cell growth.
“Metastatic triple-negative breast cancer is an aggressive form of breast cancer with limited treatment options,” observed Richard Pazdur, MD, director of the FDA’s Oncology Center of Excellence and acting director of the Office of Oncologic Diseases in the FDA’s Center for Drug Evaluation and Research in a press statement. “There is intense interest in finding new medications” for this patient population, he added.
The new approval is based on safety and efficacy results from a phase 1/2 clinical trial of 108 patients (median age, 56 years) who had received at least two prior treatments for metastatic disease.
The overall response rate was 33% (n = 36), including three complete responses. Median duration of response was 7.7 months. Of responders, 55.6% maintained their response for at least 6 months and 16.7% for at least 12 months.
Median progression-free survival was 5.5 months, and median overall survival was 13.0 months.
The study data were published last year in the New England Journal of Medicine.
“It’s not every day that we see this sort of clinical activity in this aggressive subtype of breast cancer,” said senior study author Kevin Kalinsky, MD, in an interview at that time. He is a medical oncologist at New York–Presbyterian Hospital and Columbia University Medical Center in New York City.
The most common side effects of the new therapy were nausea, neutropenia, diarrhea, fatigue, anemia, vomiting, alopecia, constipation, decreased appetite, rash, and abdominal pain.
No peripheral neuropathy of grade 3 or higher was reported.
In the study, patients received sacituzumab govitecan intravenously (10 mg/kg body weight) on days 1 and 8 of each 21-day cycle until disease progression or unacceptable toxicity.
The 108 participants received a mean 18.7 doses of sacituzumab govitecan, or 9.6 cycles. The median duration of exposure was 5.1 months.
Three patients discontinued treatment because of adverse events, and two patients discontinued because of drug-related events.
The prescribing information includes a boxed warning regarding the risks of severe neutropenia and severe diarrhea. Blood cell counts should be monitored during treatment and granulocyte-colony stimulating factor (G-CSF) therapy should be considered. Anti-infective treatment should be initiated in the event of febrile neutropenia. Patients with reduced uridine diphosphate-glucuronosyltransferase 1A1 (UGT1A1) activity are at increased risk for neutropenia following initiation of treatment.
The new drug can also cause hypersensitivity reactions including severe anaphylactic reactions.
Women who are pregnant should not take sacituzumab govitecan.
This article first appeared on Medscape.com.
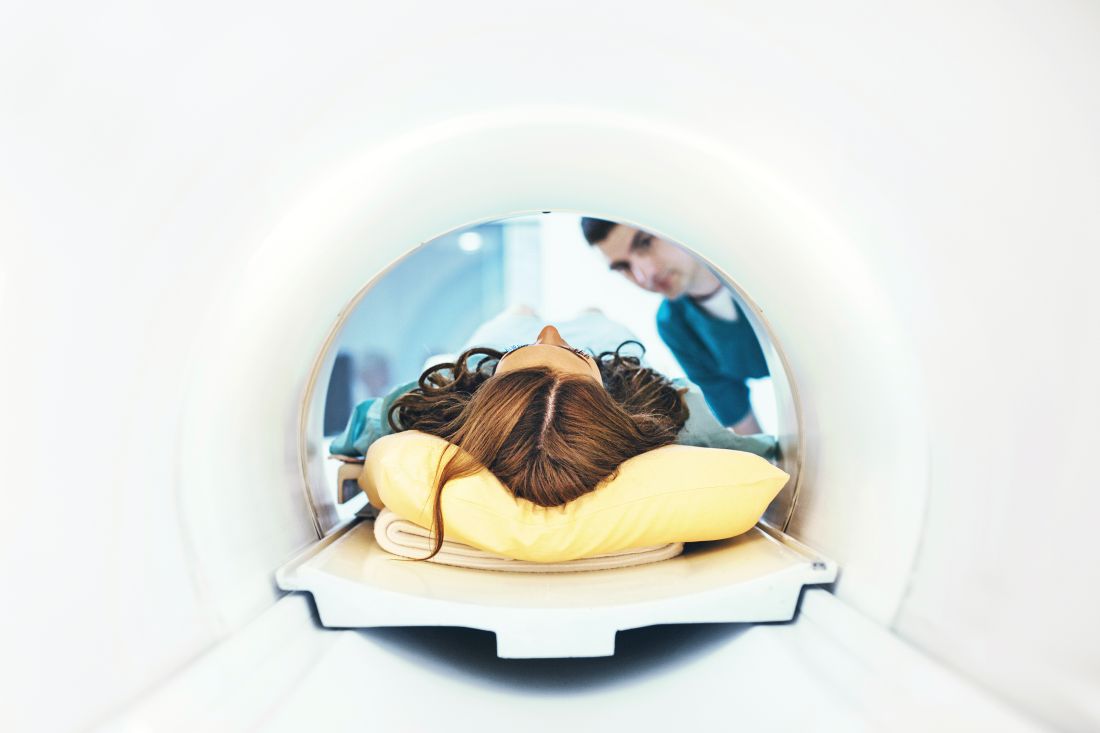
Sacroiliac bone marrow edema on MRI may be common postpartum
A “strikingly high number” of women have bone marrow edema on MRI of the sacroiliac joints postpartum, according to a prospective study of 35 patients published in Annals of the Rheumatic Diseases. Postpartum sacroiliac bone marrow edema decreases over time. Its occurrence may be associated with a shorter duration of labor and a lack of epidural anesthesia, the researchers wrote.
Sacroiliac bone marrow edema in postpartum women “persists mainly in subjects older than 30 years,” noted Thomas Renson, MD, of Ghent (Belgium) University Hospital and colleagues. “When suspecting AxSpA [axial spondyloarthritis], our data indicate the need to wait at least 6 months to perform an MRI [of the sacroiliac joints] in postpartum women and, if positive, repeat the MRI after 12 months.”
Sacroiliac bone marrow edema is a hallmark of axSpA. However, recent studies of young, active people without the disease have found a high prevalence of bone marrow edema meeting the Assessment of SpondyloArthritis International Society (ASAS) definition of a positive MRI for sacroiliitis. Researchers have reported sacroiliac bone marrow edema in women during pregnancy and after childbirth, but prior studies have not assessed the trajectory of sacroiliac bone marrow edema after delivery, Dr. Renson and his colleagues said.
To study this question, the researchers recruited 35 subjects from the department of obstetrics at Ghent University Hospital. All participants were aged 18-45 years and had an uncomplicated, vaginal childbirth. The investigators excluded patients with a diagnosis of spondyloarthritis, inflammatory bowel disease, severe scoliosis, treatment with anti–tumor necrosis factor-alpha agents, any contraindication for MRI, childbirth through cesarean section, or pregnancy with more than one fetus.
Researchers performed a baseline MRI within 10 days of when patients gave birth and another MRI after 6 months. If the second MRI fulfilled the ASAS definition of a positive MRI for sacroiliitis, another MRI was performed at 12 months. Bone marrow edema was scored using the Spondyloarthritis Research Consortium of Canada (SPARCC) method.
In all, 77% of the patients had sacroiliac bone marrow edema on MRI an average of 5 days postpartum, and 60% fulfilled the ASAS definition of a positive MRI. After 6 months, 46% had bone marrow edema on MRI, and 15% had a positive MRI according to the ASAS definition. After 12 months, MRI was positive in 12% of the subjects. There was a high prevalence of bone marrow edema “even in subjects without back pain,” the researchers said.
“Four subjects would have fulfilled the ASAS classification criteria if there was a suspicion of axSpA: Three fulfilled the ASAS definition of a positive MRI for sacroiliitis and had inflammatory back pain, [and] one had chronic back pain, a positive MRI, and skin psoriasis,” the researchers wrote.
Misdiagnosis of axSpA based on MRI findings entails risks, the authors noted. NSAIDs may be less effective in patients who do not have axSpA, and patients may be “subsequently more likely to receive ineffective biological therapy, which has significant potential side effects and encompasses high socioeconomic costs,” the investigators said.
The study was supported by an ASAS research grant. The authors declared having no competing interests.
SOURCE: Renson T et al. Ann Rheum Dis. 2020 Apr 16. doi: 10.1136/annrheumdis-2020-217095.
A “strikingly high number” of women have bone marrow edema on MRI of the sacroiliac joints postpartum, according to a prospective study of 35 patients published in Annals of the Rheumatic Diseases. Postpartum sacroiliac bone marrow edema decreases over time. Its occurrence may be associated with a shorter duration of labor and a lack of epidural anesthesia, the researchers wrote.
Sacroiliac bone marrow edema in postpartum women “persists mainly in subjects older than 30 years,” noted Thomas Renson, MD, of Ghent (Belgium) University Hospital and colleagues. “When suspecting AxSpA [axial spondyloarthritis], our data indicate the need to wait at least 6 months to perform an MRI [of the sacroiliac joints] in postpartum women and, if positive, repeat the MRI after 12 months.”
Sacroiliac bone marrow edema is a hallmark of axSpA. However, recent studies of young, active people without the disease have found a high prevalence of bone marrow edema meeting the Assessment of SpondyloArthritis International Society (ASAS) definition of a positive MRI for sacroiliitis. Researchers have reported sacroiliac bone marrow edema in women during pregnancy and after childbirth, but prior studies have not assessed the trajectory of sacroiliac bone marrow edema after delivery, Dr. Renson and his colleagues said.
To study this question, the researchers recruited 35 subjects from the department of obstetrics at Ghent University Hospital. All participants were aged 18-45 years and had an uncomplicated, vaginal childbirth. The investigators excluded patients with a diagnosis of spondyloarthritis, inflammatory bowel disease, severe scoliosis, treatment with anti–tumor necrosis factor-alpha agents, any contraindication for MRI, childbirth through cesarean section, or pregnancy with more than one fetus.
Researchers performed a baseline MRI within 10 days of when patients gave birth and another MRI after 6 months. If the second MRI fulfilled the ASAS definition of a positive MRI for sacroiliitis, another MRI was performed at 12 months. Bone marrow edema was scored using the Spondyloarthritis Research Consortium of Canada (SPARCC) method.
In all, 77% of the patients had sacroiliac bone marrow edema on MRI an average of 5 days postpartum, and 60% fulfilled the ASAS definition of a positive MRI. After 6 months, 46% had bone marrow edema on MRI, and 15% had a positive MRI according to the ASAS definition. After 12 months, MRI was positive in 12% of the subjects. There was a high prevalence of bone marrow edema “even in subjects without back pain,” the researchers said.
“Four subjects would have fulfilled the ASAS classification criteria if there was a suspicion of axSpA: Three fulfilled the ASAS definition of a positive MRI for sacroiliitis and had inflammatory back pain, [and] one had chronic back pain, a positive MRI, and skin psoriasis,” the researchers wrote.
Misdiagnosis of axSpA based on MRI findings entails risks, the authors noted. NSAIDs may be less effective in patients who do not have axSpA, and patients may be “subsequently more likely to receive ineffective biological therapy, which has significant potential side effects and encompasses high socioeconomic costs,” the investigators said.
The study was supported by an ASAS research grant. The authors declared having no competing interests.
SOURCE: Renson T et al. Ann Rheum Dis. 2020 Apr 16. doi: 10.1136/annrheumdis-2020-217095.
A “strikingly high number” of women have bone marrow edema on MRI of the sacroiliac joints postpartum, according to a prospective study of 35 patients published in Annals of the Rheumatic Diseases. Postpartum sacroiliac bone marrow edema decreases over time. Its occurrence may be associated with a shorter duration of labor and a lack of epidural anesthesia, the researchers wrote.
Sacroiliac bone marrow edema in postpartum women “persists mainly in subjects older than 30 years,” noted Thomas Renson, MD, of Ghent (Belgium) University Hospital and colleagues. “When suspecting AxSpA [axial spondyloarthritis], our data indicate the need to wait at least 6 months to perform an MRI [of the sacroiliac joints] in postpartum women and, if positive, repeat the MRI after 12 months.”
Sacroiliac bone marrow edema is a hallmark of axSpA. However, recent studies of young, active people without the disease have found a high prevalence of bone marrow edema meeting the Assessment of SpondyloArthritis International Society (ASAS) definition of a positive MRI for sacroiliitis. Researchers have reported sacroiliac bone marrow edema in women during pregnancy and after childbirth, but prior studies have not assessed the trajectory of sacroiliac bone marrow edema after delivery, Dr. Renson and his colleagues said.
To study this question, the researchers recruited 35 subjects from the department of obstetrics at Ghent University Hospital. All participants were aged 18-45 years and had an uncomplicated, vaginal childbirth. The investigators excluded patients with a diagnosis of spondyloarthritis, inflammatory bowel disease, severe scoliosis, treatment with anti–tumor necrosis factor-alpha agents, any contraindication for MRI, childbirth through cesarean section, or pregnancy with more than one fetus.
Researchers performed a baseline MRI within 10 days of when patients gave birth and another MRI after 6 months. If the second MRI fulfilled the ASAS definition of a positive MRI for sacroiliitis, another MRI was performed at 12 months. Bone marrow edema was scored using the Spondyloarthritis Research Consortium of Canada (SPARCC) method.
In all, 77% of the patients had sacroiliac bone marrow edema on MRI an average of 5 days postpartum, and 60% fulfilled the ASAS definition of a positive MRI. After 6 months, 46% had bone marrow edema on MRI, and 15% had a positive MRI according to the ASAS definition. After 12 months, MRI was positive in 12% of the subjects. There was a high prevalence of bone marrow edema “even in subjects without back pain,” the researchers said.
“Four subjects would have fulfilled the ASAS classification criteria if there was a suspicion of axSpA: Three fulfilled the ASAS definition of a positive MRI for sacroiliitis and had inflammatory back pain, [and] one had chronic back pain, a positive MRI, and skin psoriasis,” the researchers wrote.
Misdiagnosis of axSpA based on MRI findings entails risks, the authors noted. NSAIDs may be less effective in patients who do not have axSpA, and patients may be “subsequently more likely to receive ineffective biological therapy, which has significant potential side effects and encompasses high socioeconomic costs,” the investigators said.
The study was supported by an ASAS research grant. The authors declared having no competing interests.
SOURCE: Renson T et al. Ann Rheum Dis. 2020 Apr 16. doi: 10.1136/annrheumdis-2020-217095.
FROM ANNALS OF THE RHEUMATIC DISEASES
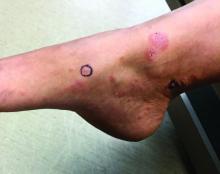
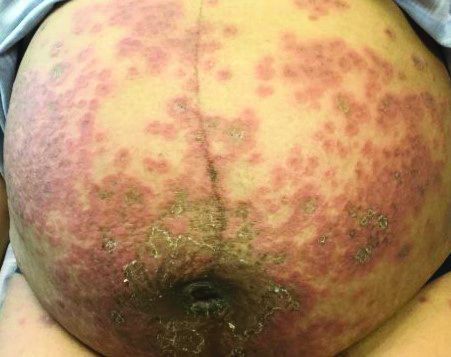
Itchy, vesicular rash
Pemphigoid gestationis
It typically presents with the abrupt onset of very pruritic urticarial plaques and papules, which start around the umbilicus and then spread to involve the trunk and extremities. The papules and plaques evolve to generalized tense blisters, which typically spare the face, palms, soles, and mucous membranes. Half of affected patients may present in an atypical distribution involving the extremities, palms, or soles. Patients may be at an increased risk for the development of Graves disease.
The cause of pemphigoid gestationis is a factor known as “herpes gestationis factor” that induces C3 deposition along the dermal-epidermal junction. As in bullous pemphigoid, patients with pemphigoid gestationis have antibodies to a transmembrane hemidesmosomal protein called BPAG2/BP180/collagen XVII.
Three-quarters of patients worsen at the time of delivery and up to 10% of newborns will have bullous lesions secondary to placental transfer of antibodies. In most cases, lesions will spontaneously resolve over a few weeks following delivery. Recurrence with future pregnancies is common, with severity increasing with each pregnancy. Recurrence with menstruation and with the use of oral contraceptives can also occur. Although there is no increase in maternal mortality, onset in the first or second trimester and presence of blisters is associated with decreased gestational age of baby at delivery and lower-birth-weight infants. There is no increase in fetal mortality.
Histopathology reveals a subepidermal vesicle and perivascular infiltrate consisting of lymphocytes and eosinophils. Diagnosis can be confirmed with direct immunofluorescence showing C3 in a linear band along the basement membrane zone. IgG may be present as well. Complement added indirect immunofluorescence reveals circulating anti–basement zone IgG, which allows differentiation from pruritic urticarial papules and plaques of pregnancy.
Treatment for localized disease includes class I topical steroids and oral antihistamines. More severe cases require systemic corticosteroid treatment. Systemic steroids may cause lower-birth-weight infants.
This case and the photos were submitted by Dr. Hanson of Associated Skin Care Specialists in Eden Prairie, Minn. The case was edited by Donna Bilu Martin, MD.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
Pemphigoid gestationis
It typically presents with the abrupt onset of very pruritic urticarial plaques and papules, which start around the umbilicus and then spread to involve the trunk and extremities. The papules and plaques evolve to generalized tense blisters, which typically spare the face, palms, soles, and mucous membranes. Half of affected patients may present in an atypical distribution involving the extremities, palms, or soles. Patients may be at an increased risk for the development of Graves disease.
The cause of pemphigoid gestationis is a factor known as “herpes gestationis factor” that induces C3 deposition along the dermal-epidermal junction. As in bullous pemphigoid, patients with pemphigoid gestationis have antibodies to a transmembrane hemidesmosomal protein called BPAG2/BP180/collagen XVII.
Three-quarters of patients worsen at the time of delivery and up to 10% of newborns will have bullous lesions secondary to placental transfer of antibodies. In most cases, lesions will spontaneously resolve over a few weeks following delivery. Recurrence with future pregnancies is common, with severity increasing with each pregnancy. Recurrence with menstruation and with the use of oral contraceptives can also occur. Although there is no increase in maternal mortality, onset in the first or second trimester and presence of blisters is associated with decreased gestational age of baby at delivery and lower-birth-weight infants. There is no increase in fetal mortality.
Histopathology reveals a subepidermal vesicle and perivascular infiltrate consisting of lymphocytes and eosinophils. Diagnosis can be confirmed with direct immunofluorescence showing C3 in a linear band along the basement membrane zone. IgG may be present as well. Complement added indirect immunofluorescence reveals circulating anti–basement zone IgG, which allows differentiation from pruritic urticarial papules and plaques of pregnancy.
Treatment for localized disease includes class I topical steroids and oral antihistamines. More severe cases require systemic corticosteroid treatment. Systemic steroids may cause lower-birth-weight infants.
This case and the photos were submitted by Dr. Hanson of Associated Skin Care Specialists in Eden Prairie, Minn. The case was edited by Donna Bilu Martin, MD.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
Pemphigoid gestationis
It typically presents with the abrupt onset of very pruritic urticarial plaques and papules, which start around the umbilicus and then spread to involve the trunk and extremities. The papules and plaques evolve to generalized tense blisters, which typically spare the face, palms, soles, and mucous membranes. Half of affected patients may present in an atypical distribution involving the extremities, palms, or soles. Patients may be at an increased risk for the development of Graves disease.
The cause of pemphigoid gestationis is a factor known as “herpes gestationis factor” that induces C3 deposition along the dermal-epidermal junction. As in bullous pemphigoid, patients with pemphigoid gestationis have antibodies to a transmembrane hemidesmosomal protein called BPAG2/BP180/collagen XVII.
Three-quarters of patients worsen at the time of delivery and up to 10% of newborns will have bullous lesions secondary to placental transfer of antibodies. In most cases, lesions will spontaneously resolve over a few weeks following delivery. Recurrence with future pregnancies is common, with severity increasing with each pregnancy. Recurrence with menstruation and with the use of oral contraceptives can also occur. Although there is no increase in maternal mortality, onset in the first or second trimester and presence of blisters is associated with decreased gestational age of baby at delivery and lower-birth-weight infants. There is no increase in fetal mortality.
Histopathology reveals a subepidermal vesicle and perivascular infiltrate consisting of lymphocytes and eosinophils. Diagnosis can be confirmed with direct immunofluorescence showing C3 in a linear band along the basement membrane zone. IgG may be present as well. Complement added indirect immunofluorescence reveals circulating anti–basement zone IgG, which allows differentiation from pruritic urticarial papules and plaques of pregnancy.
Treatment for localized disease includes class I topical steroids and oral antihistamines. More severe cases require systemic corticosteroid treatment. Systemic steroids may cause lower-birth-weight infants.
This case and the photos were submitted by Dr. Hanson of Associated Skin Care Specialists in Eden Prairie, Minn. The case was edited by Donna Bilu Martin, MD.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
FDA approves first new breast cancer drug with international group
The U.S. Food and Drug Administration has approved the oral therapy tucatinib (Tukysa, Seattle Genetics) for the treatment of advanced HER2-positive breast cancer. This is the first new drug approved under Project Orbis, an international collaboration.
Tucatinib, which is a small-molecule tyrosine kinase inhibitor, is approved in combination with trastuzumab and capecitabine to treat patients who have received one or more prior treatments for advanced disease.
The FDA collaborated with the regulatory authorities of Australia, Canada, Singapore, and Switzerland on this review. However, only the FDA has approved tucatinib; the application is still under review at the other agencies.
While working with Project Orbis in 2019, the FDA granted an accelerated, conditional approval to a drug combination that included previously approved agents.
“The FDA’s Project Orbis provides a framework for concurrent submission and review of oncology drug applications among the FDA’s international collaborators,” said Richard Pazdur, MD, acting director of the Office of Oncologic Diseases in the FDA’s Center for Drug Evaluation and Research, in a statement.
Collaboration among regulators may allow patients with cancer to receive earlier access to products in other countries where there may be significant delays in regulatory submissions, according to the FDA.
The new drug is a “valuable addition” to the roster of treatments for advanced HER2-positive breast cancer, said study investigator Eric Winer, MD, Dana-Farber Cancer Institute, Boston, Massachusetts, in a company press statement.
“With highly significant and clinically important results for overall and progression-free survival, the addition of [tucatinib] to trastuzumab and capecitabine has the potential to become a standard of care for people with HER2-positive metastatic breast cancer after having received one or more previous anti-HER2 therapies in the metastatic setting,” he said.
The new approval is based on safety and efficacy results from the phase 2 HER2CLIMB trial that enrolled 612 patients with HER2-positive unresectable locally advanced or metastatic breast cancer who had previously received, either separately or in combination, trastuzumab, pertuzumab, and ado-trastuzumab emtansine.
Nearly half (48%) of patients in the study had brain metastases at the start of the trial. The primary outcome measure was progression-free survival (PFS). All patients received trastuzumab and capecitabine and were randomly assigned to either tucatinib or placebo.
Median PFS in the tucatinib patient group was 7.8 months, compared with 5.6 months in the placebo group. The PFS results in the subgroup of patients with brain metastases were nearly the same.
Median overall survival in the tucatinib patient group was 21.9 months versus 17.4 months in the placebo group.
The new drug is a rare success in the treatment of breast cancer brain metastases, said Jawad Fares, MD, of Northwestern University, Chicago, Illinois, who spoke to Medscape Medical News when the phase 3 trial data were first presented at the 2019 San Antonio Breast Cancer Symposium.
“Outcomes in the field have been pretty dismal,” summarized Fares, who was not involved in the study.
The results of the HER2CLIMB study, which was funded by Seattle Genetics, were published in the New England Journal of Medicine last year.
According to the FDA, common side effects with tucatinib were diarrhea, palmar-plantar erythrodysesthesia syndrome, nausea, fatigue, hepatotoxicity, vomiting, stomatitis, decreased appetite, abdominal pain, headache, anemia, and rash.
Tucatinib can cause serious side effects, including diarrhea associated with dehydration, acute kidney injury, and death. Health care professionals should start antidiarrheals as clinically indicated if diarrhea occurs and should interrupt treatment or reduce the dosage. Tucatinib can also cause severe hepatotoxicity; patients should be monitored with liver tests.
This article first appeared on Medscape.com.
The U.S. Food and Drug Administration has approved the oral therapy tucatinib (Tukysa, Seattle Genetics) for the treatment of advanced HER2-positive breast cancer. This is the first new drug approved under Project Orbis, an international collaboration.
Tucatinib, which is a small-molecule tyrosine kinase inhibitor, is approved in combination with trastuzumab and capecitabine to treat patients who have received one or more prior treatments for advanced disease.
The FDA collaborated with the regulatory authorities of Australia, Canada, Singapore, and Switzerland on this review. However, only the FDA has approved tucatinib; the application is still under review at the other agencies.
While working with Project Orbis in 2019, the FDA granted an accelerated, conditional approval to a drug combination that included previously approved agents.
“The FDA’s Project Orbis provides a framework for concurrent submission and review of oncology drug applications among the FDA’s international collaborators,” said Richard Pazdur, MD, acting director of the Office of Oncologic Diseases in the FDA’s Center for Drug Evaluation and Research, in a statement.
Collaboration among regulators may allow patients with cancer to receive earlier access to products in other countries where there may be significant delays in regulatory submissions, according to the FDA.
The new drug is a “valuable addition” to the roster of treatments for advanced HER2-positive breast cancer, said study investigator Eric Winer, MD, Dana-Farber Cancer Institute, Boston, Massachusetts, in a company press statement.
“With highly significant and clinically important results for overall and progression-free survival, the addition of [tucatinib] to trastuzumab and capecitabine has the potential to become a standard of care for people with HER2-positive metastatic breast cancer after having received one or more previous anti-HER2 therapies in the metastatic setting,” he said.
The new approval is based on safety and efficacy results from the phase 2 HER2CLIMB trial that enrolled 612 patients with HER2-positive unresectable locally advanced or metastatic breast cancer who had previously received, either separately or in combination, trastuzumab, pertuzumab, and ado-trastuzumab emtansine.
Nearly half (48%) of patients in the study had brain metastases at the start of the trial. The primary outcome measure was progression-free survival (PFS). All patients received trastuzumab and capecitabine and were randomly assigned to either tucatinib or placebo.
Median PFS in the tucatinib patient group was 7.8 months, compared with 5.6 months in the placebo group. The PFS results in the subgroup of patients with brain metastases were nearly the same.
Median overall survival in the tucatinib patient group was 21.9 months versus 17.4 months in the placebo group.
The new drug is a rare success in the treatment of breast cancer brain metastases, said Jawad Fares, MD, of Northwestern University, Chicago, Illinois, who spoke to Medscape Medical News when the phase 3 trial data were first presented at the 2019 San Antonio Breast Cancer Symposium.
“Outcomes in the field have been pretty dismal,” summarized Fares, who was not involved in the study.
The results of the HER2CLIMB study, which was funded by Seattle Genetics, were published in the New England Journal of Medicine last year.
According to the FDA, common side effects with tucatinib were diarrhea, palmar-plantar erythrodysesthesia syndrome, nausea, fatigue, hepatotoxicity, vomiting, stomatitis, decreased appetite, abdominal pain, headache, anemia, and rash.
Tucatinib can cause serious side effects, including diarrhea associated with dehydration, acute kidney injury, and death. Health care professionals should start antidiarrheals as clinically indicated if diarrhea occurs and should interrupt treatment or reduce the dosage. Tucatinib can also cause severe hepatotoxicity; patients should be monitored with liver tests.
This article first appeared on Medscape.com.
The U.S. Food and Drug Administration has approved the oral therapy tucatinib (Tukysa, Seattle Genetics) for the treatment of advanced HER2-positive breast cancer. This is the first new drug approved under Project Orbis, an international collaboration.
Tucatinib, which is a small-molecule tyrosine kinase inhibitor, is approved in combination with trastuzumab and capecitabine to treat patients who have received one or more prior treatments for advanced disease.
The FDA collaborated with the regulatory authorities of Australia, Canada, Singapore, and Switzerland on this review. However, only the FDA has approved tucatinib; the application is still under review at the other agencies.
While working with Project Orbis in 2019, the FDA granted an accelerated, conditional approval to a drug combination that included previously approved agents.
“The FDA’s Project Orbis provides a framework for concurrent submission and review of oncology drug applications among the FDA’s international collaborators,” said Richard Pazdur, MD, acting director of the Office of Oncologic Diseases in the FDA’s Center for Drug Evaluation and Research, in a statement.
Collaboration among regulators may allow patients with cancer to receive earlier access to products in other countries where there may be significant delays in regulatory submissions, according to the FDA.
The new drug is a “valuable addition” to the roster of treatments for advanced HER2-positive breast cancer, said study investigator Eric Winer, MD, Dana-Farber Cancer Institute, Boston, Massachusetts, in a company press statement.
“With highly significant and clinically important results for overall and progression-free survival, the addition of [tucatinib] to trastuzumab and capecitabine has the potential to become a standard of care for people with HER2-positive metastatic breast cancer after having received one or more previous anti-HER2 therapies in the metastatic setting,” he said.
The new approval is based on safety and efficacy results from the phase 2 HER2CLIMB trial that enrolled 612 patients with HER2-positive unresectable locally advanced or metastatic breast cancer who had previously received, either separately or in combination, trastuzumab, pertuzumab, and ado-trastuzumab emtansine.
Nearly half (48%) of patients in the study had brain metastases at the start of the trial. The primary outcome measure was progression-free survival (PFS). All patients received trastuzumab and capecitabine and were randomly assigned to either tucatinib or placebo.
Median PFS in the tucatinib patient group was 7.8 months, compared with 5.6 months in the placebo group. The PFS results in the subgroup of patients with brain metastases were nearly the same.
Median overall survival in the tucatinib patient group was 21.9 months versus 17.4 months in the placebo group.
The new drug is a rare success in the treatment of breast cancer brain metastases, said Jawad Fares, MD, of Northwestern University, Chicago, Illinois, who spoke to Medscape Medical News when the phase 3 trial data were first presented at the 2019 San Antonio Breast Cancer Symposium.
“Outcomes in the field have been pretty dismal,” summarized Fares, who was not involved in the study.
The results of the HER2CLIMB study, which was funded by Seattle Genetics, were published in the New England Journal of Medicine last year.
According to the FDA, common side effects with tucatinib were diarrhea, palmar-plantar erythrodysesthesia syndrome, nausea, fatigue, hepatotoxicity, vomiting, stomatitis, decreased appetite, abdominal pain, headache, anemia, and rash.
Tucatinib can cause serious side effects, including diarrhea associated with dehydration, acute kidney injury, and death. Health care professionals should start antidiarrheals as clinically indicated if diarrhea occurs and should interrupt treatment or reduce the dosage. Tucatinib can also cause severe hepatotoxicity; patients should be monitored with liver tests.
This article first appeared on Medscape.com.
Addressing CVD’s role in U.S. maternal mortality: Multispecialty collaboration is needed
Nearly 700 women died from pregnancy-related complications in the United States in 2018, and almost a third of those deaths were associated with cardiovascular disease, according to the latest data from the Centers for Disease Control and Prevention.
Strikingly, studies suggest that up to half of cardiovascular disease–related maternal deaths are preventable, yet CVD remains the leading cause of maternal morbidity and mortality – and the incidence has been rising steadily for 2 decades.
The American College of Obstetricians and Gynecologists says that acquired heart disease is the likely culprit in the rise in incidence of maternal mortality as women enter pregnancy with an increasingly heavy burden of CVD risk factors, including older age, obesity, diabetes, and hypertension.
“They are entering pregnancy while already at risk, and that has led to an increase in morbidity and mortality during pregnancy,” Renee Patrice Bullock-Palmer, MD, a cardiologist and director of the Women’s Heart Center at Deborah Heart and Lung Center in Browns Mills, N.J., explained in an interview. “Unfortunately, among developed countries, the U.S. has the highest rates of maternal morbidity and mortality, and that’s shocking.”
It’s a problem that requires collaboration between obstetricians, cardiologists, and others involved in the care of pregnant women, she said.
The data and the depth of the crisis
The maternal mortality rate in 1987 – the year the CDC’s Pregnancy Mortality Surveillance System was implemented – was 7.2 per 100,000 live births. The rate in 2016 was more than double that at 16.9, and the rate in 2018, the most recent year for which data are available, was 17.4 – and significant racial and ethnic disparities in those rates have persisted over time.
In an August 2019 article published on the American Heart Association website, Dr. Bullock-Palmer addressed the cardiovascular state of health for pregnant women and the role of the cardiologists in their care, noting that there is a “role for increased collaboration between the cardiologist and the obstetrician with regards to a pregnancy heart team.”
“It is vital that mothers who are at increased risk for CVD or have established CVD be referred to a cardiologist for cardiovascular assessment and management,” she wrote, adding it is important to raise awareness among ob.gyns. and to improve cardiologists’ recognition of women at risk when they present for care for the first time.
These referrals should be made in the antepartum and early postpartum period, she said in an interview. More attention also must be paid to racial and ethnic disparities, and the role of cardiologists in addressing these disparities.
The CDC has emphasized racial and ethnic disparities in maternal mortality, noting in a 2019 Morbidity and Mortality Weekly report that, compared with white women, black and American Indian/Alaskan Native women aged over 30 years have a 300%-400% higher rate of pregnancy-related deaths (Morb Mortal Wkly Rep. 2019 Sep 6;68[35]:762-5).
With regard to disparities, Dr. Bullock-Palmer said the causes are multifold and may be related to a higher prevalence of CVD risk factors like obesity and hypertension in non-Hispanic black women.
“There may also be limited access to adequate postpartum care in this patient population,” she wrote, adding that some attention has been paid to addressing disparities, but that “there is a lot of work left to be done in resolving these inequities in maternal health care.”
Partnerships across specialties will help in addressing most of the factors associated with CVD and maternal death, she said.
The urgent need for these partnerships is underscored by the latest findings on CVD-related complications in pregnancy. A study published in March 2020 in the Journal of the American College of Cardiology, for example, looked specifically at the incidence of serious cardiac events (SCEs) in pregnant women with heart disease, and whether the events were preventable.
In a prospective cohort of 1,315 pregnancies among women with heart disease, Birgit Pfaller, MD, of the University of Toronto Pregnancy and Heart Disease Research Program, and colleagues found that SCEs occurred in 3.6% of cases (47 women) – most often during the antepartum period – that 49% were preventable, and that 74% were related to provider management factors.
The most common SCEs were cardiac death or arrest, heart failure, arrhythmias, and urgent intervention, and they were more likely to occur in women with acquired heart disease, severe aortic or mitral stenosis, mechanical valves, and systemic ventricular dysfunction. Adverse fetal and neonatal outcomes more than doubled in cases involving SCEs, compared with those without (62% vs. 29%), and adverse obstetric events occurred most often in women with severe preeclampsia.
“The majority of the preventable events occurred due to provider management factors, including: failure to identify the patient condition prior to pregnancy, failure to identify the patient as high risk, late recognition in cardiac deterioration, delay in treatment/intervention, inappropriate treatment, and lack of preconception counseling,” Melinda Davis, MD, of the University of Michigan, Ann Arbor, wrote in a summary and editorial published in the Journal of the American College of Cardiology.
Some preventable events were attributable to patient failure to seek care, noncompliance with care recommendations, and lack of access to care, Dr. Davis noted.
“These findings suggest that provider training, patient education, and health care advocacy are all important interventions to improve outcomes among pregnant women,” she wrote, adding that “the development of multidisciplinary cardio-obstetric clinics at tertiary care centers may also be helpful.”
Dr. Bullock-Palmer added the need for greater risk-prediction tools to the list, explaining that these are needed to assess CVD risk in the prenatal, antenatal, and postnatal period.
“The recently concluded Cardiac Disease in Pregnancy [CARPREG II] study indicated that there were 10 predictors that could be utilized to asses maternal CVD risk,” she noted.
The CARPREG II authors identified five general predictors (prior cardiac events or arrhythmias, poor functional class or cyanosis, high-risk valve disease/left ventricular outflow tract obstruction, systemic ventricular dysfunction, no prior cardiac interventions), four lesion-specific predictors (mechanical valves, high-risk aortopathies, pulmonary hypertension, coronary artery disease), and one delivery-of-care predictor (late pregnancy assessment), and incorporated them into a risk index.
“It is hopeful that these new initiatives will assist providers in improving their ability to appropriately risk stratify women,” Dr. Bullock-Palmer said.
Ongoing efforts
Efforts also are ongoing to develop the types of cardio-obstetric clinics mentioned by Dr. Davis and to establish collaborations and “pregnancy heart teams” as attention is increasingly focused on the U.S. maternal mortality crisis.
In fact, such teams are a cornerstone of ACOG’s guidance on pregnancy and heart disease. In May 2019 the college released a Practice Bulletin with 27 specific recommendations and conclusions relating to screening, diagnosis, and management of CVD for women during the prepregnancy period through the postpartum period.
Pregnant women and postpartum women with known or suspected CVD should undergo evaluation by a “pregnancy heart team that includes a cardiologist and maternal-fetal medicine subspecialist, or both, and other subspecialists as necessary,” according to the bulletin.
In a recent interview, Lisa Hollier, MD, immediate past president of ACOG and an instrumental figure in the push to better address maternal mortality – and in particular the cardiovascular contributors to the crisis – said she is “seeing a strengthening of that” with numerous organizations establishing pregnancy health teams.
Dr. Bullock-Palmer said she also is seeing progress, and added that collaboration should be prioritized even in the absence of dedicated pregnancy heart teams and clinics.
“Heart disease in pregnancy requires a multidisciplinary approach. You can’t just see the patient from the cardiac perspective – you also have to interact and team up with the obstetrician who is handling the pregnancy,” she said, adding that, without a dedicated team, coordination takes more effort, but is imperative for improving outcomes. “You have to collaborate at times when it is beyond the expertise of the institution or the physician; you have to know when to refer these higher-risk patients, particularly women with adult congenital heart disease.”
This referral should occur early – preferably in the antenatal period, she added.
The most important thing, however, is “recognizing these women ... even before the pregnancy,” Dr. Bullock-Palmer said, explaining that this can facilitate the necessary management – and in some cases, postponement – of pregnancy for women whose cardiac issues need to be addressed first.
Among other efforts to address maternal mortality are several programs developed by ACOG, and the Heart Outcomes in Pregnancy: Expectations for Mom and Baby Registry (HOPE) project of the Saint Luke’s Health System in Kansas.
“Hopefully the [HOPE] research collaborative ... which aims to address key clinical questions surrounding the preconception period, antenatal care, delivery planning and outcomes, and long-term postpartum care and outcomes of women will help to address the knowledge gaps and disparities in the care of women with heart disease in pregnancy,” Dr. Bullock-Palmer wrote in her article.
CVD-related risks in the post partum
Dr. Bullock-Palmer has particular concern for postpartum follow-up, given the increased risk for future heart disease among women with CVD-related pregnancy complications and the heightened risk of certain CVD-related events in the postpartum period.
That’s a component of the crisis that also was addressed during a press briefing at the 2019 ACOG annual meeting when the Pregnancy and Heart Disease Practice Bulletin was released.
James Martin, MD, chair of ACOG’s Pregnancy and Heart Disease Task Force and a past ACOG president, explained during the briefing that CVD-related risks may accelerate and persist in the days and weeks after delivery, underscoring the need for follow-up and postpartum care.
Cardiomyopathy is a particular concern during this time – it’s the major cause of maternal mortality after 42 days, he noted. An emphasis on postpartum care also is especially important given that some data suggest up to 40% of women don’t return for that care.
“That is a very sad statistic and perhaps it reflects on our need to change payment models so that physicians and patients realize the importance of coming back for continuing care, because this really may be the end of pregnancy, but it is the beginning of the rest of their life,” he said. “And if they have cardiovascular disease or the risk factors ... they are going to possibly become worse over the course of their lifetime.”
Nearly 700 women died from pregnancy-related complications in the United States in 2018, and almost a third of those deaths were associated with cardiovascular disease, according to the latest data from the Centers for Disease Control and Prevention.
Strikingly, studies suggest that up to half of cardiovascular disease–related maternal deaths are preventable, yet CVD remains the leading cause of maternal morbidity and mortality – and the incidence has been rising steadily for 2 decades.
The American College of Obstetricians and Gynecologists says that acquired heart disease is the likely culprit in the rise in incidence of maternal mortality as women enter pregnancy with an increasingly heavy burden of CVD risk factors, including older age, obesity, diabetes, and hypertension.
“They are entering pregnancy while already at risk, and that has led to an increase in morbidity and mortality during pregnancy,” Renee Patrice Bullock-Palmer, MD, a cardiologist and director of the Women’s Heart Center at Deborah Heart and Lung Center in Browns Mills, N.J., explained in an interview. “Unfortunately, among developed countries, the U.S. has the highest rates of maternal morbidity and mortality, and that’s shocking.”
It’s a problem that requires collaboration between obstetricians, cardiologists, and others involved in the care of pregnant women, she said.
The data and the depth of the crisis
The maternal mortality rate in 1987 – the year the CDC’s Pregnancy Mortality Surveillance System was implemented – was 7.2 per 100,000 live births. The rate in 2016 was more than double that at 16.9, and the rate in 2018, the most recent year for which data are available, was 17.4 – and significant racial and ethnic disparities in those rates have persisted over time.
In an August 2019 article published on the American Heart Association website, Dr. Bullock-Palmer addressed the cardiovascular state of health for pregnant women and the role of the cardiologists in their care, noting that there is a “role for increased collaboration between the cardiologist and the obstetrician with regards to a pregnancy heart team.”
“It is vital that mothers who are at increased risk for CVD or have established CVD be referred to a cardiologist for cardiovascular assessment and management,” she wrote, adding it is important to raise awareness among ob.gyns. and to improve cardiologists’ recognition of women at risk when they present for care for the first time.
These referrals should be made in the antepartum and early postpartum period, she said in an interview. More attention also must be paid to racial and ethnic disparities, and the role of cardiologists in addressing these disparities.
The CDC has emphasized racial and ethnic disparities in maternal mortality, noting in a 2019 Morbidity and Mortality Weekly report that, compared with white women, black and American Indian/Alaskan Native women aged over 30 years have a 300%-400% higher rate of pregnancy-related deaths (Morb Mortal Wkly Rep. 2019 Sep 6;68[35]:762-5).
With regard to disparities, Dr. Bullock-Palmer said the causes are multifold and may be related to a higher prevalence of CVD risk factors like obesity and hypertension in non-Hispanic black women.
“There may also be limited access to adequate postpartum care in this patient population,” she wrote, adding that some attention has been paid to addressing disparities, but that “there is a lot of work left to be done in resolving these inequities in maternal health care.”
Partnerships across specialties will help in addressing most of the factors associated with CVD and maternal death, she said.
The urgent need for these partnerships is underscored by the latest findings on CVD-related complications in pregnancy. A study published in March 2020 in the Journal of the American College of Cardiology, for example, looked specifically at the incidence of serious cardiac events (SCEs) in pregnant women with heart disease, and whether the events were preventable.
In a prospective cohort of 1,315 pregnancies among women with heart disease, Birgit Pfaller, MD, of the University of Toronto Pregnancy and Heart Disease Research Program, and colleagues found that SCEs occurred in 3.6% of cases (47 women) – most often during the antepartum period – that 49% were preventable, and that 74% were related to provider management factors.
The most common SCEs were cardiac death or arrest, heart failure, arrhythmias, and urgent intervention, and they were more likely to occur in women with acquired heart disease, severe aortic or mitral stenosis, mechanical valves, and systemic ventricular dysfunction. Adverse fetal and neonatal outcomes more than doubled in cases involving SCEs, compared with those without (62% vs. 29%), and adverse obstetric events occurred most often in women with severe preeclampsia.
“The majority of the preventable events occurred due to provider management factors, including: failure to identify the patient condition prior to pregnancy, failure to identify the patient as high risk, late recognition in cardiac deterioration, delay in treatment/intervention, inappropriate treatment, and lack of preconception counseling,” Melinda Davis, MD, of the University of Michigan, Ann Arbor, wrote in a summary and editorial published in the Journal of the American College of Cardiology.
Some preventable events were attributable to patient failure to seek care, noncompliance with care recommendations, and lack of access to care, Dr. Davis noted.
“These findings suggest that provider training, patient education, and health care advocacy are all important interventions to improve outcomes among pregnant women,” she wrote, adding that “the development of multidisciplinary cardio-obstetric clinics at tertiary care centers may also be helpful.”
Dr. Bullock-Palmer added the need for greater risk-prediction tools to the list, explaining that these are needed to assess CVD risk in the prenatal, antenatal, and postnatal period.
“The recently concluded Cardiac Disease in Pregnancy [CARPREG II] study indicated that there were 10 predictors that could be utilized to asses maternal CVD risk,” she noted.
The CARPREG II authors identified five general predictors (prior cardiac events or arrhythmias, poor functional class or cyanosis, high-risk valve disease/left ventricular outflow tract obstruction, systemic ventricular dysfunction, no prior cardiac interventions), four lesion-specific predictors (mechanical valves, high-risk aortopathies, pulmonary hypertension, coronary artery disease), and one delivery-of-care predictor (late pregnancy assessment), and incorporated them into a risk index.
“It is hopeful that these new initiatives will assist providers in improving their ability to appropriately risk stratify women,” Dr. Bullock-Palmer said.
Ongoing efforts
Efforts also are ongoing to develop the types of cardio-obstetric clinics mentioned by Dr. Davis and to establish collaborations and “pregnancy heart teams” as attention is increasingly focused on the U.S. maternal mortality crisis.
In fact, such teams are a cornerstone of ACOG’s guidance on pregnancy and heart disease. In May 2019 the college released a Practice Bulletin with 27 specific recommendations and conclusions relating to screening, diagnosis, and management of CVD for women during the prepregnancy period through the postpartum period.
Pregnant women and postpartum women with known or suspected CVD should undergo evaluation by a “pregnancy heart team that includes a cardiologist and maternal-fetal medicine subspecialist, or both, and other subspecialists as necessary,” according to the bulletin.
In a recent interview, Lisa Hollier, MD, immediate past president of ACOG and an instrumental figure in the push to better address maternal mortality – and in particular the cardiovascular contributors to the crisis – said she is “seeing a strengthening of that” with numerous organizations establishing pregnancy health teams.
Dr. Bullock-Palmer said she also is seeing progress, and added that collaboration should be prioritized even in the absence of dedicated pregnancy heart teams and clinics.
“Heart disease in pregnancy requires a multidisciplinary approach. You can’t just see the patient from the cardiac perspective – you also have to interact and team up with the obstetrician who is handling the pregnancy,” she said, adding that, without a dedicated team, coordination takes more effort, but is imperative for improving outcomes. “You have to collaborate at times when it is beyond the expertise of the institution or the physician; you have to know when to refer these higher-risk patients, particularly women with adult congenital heart disease.”
This referral should occur early – preferably in the antenatal period, she added.
The most important thing, however, is “recognizing these women ... even before the pregnancy,” Dr. Bullock-Palmer said, explaining that this can facilitate the necessary management – and in some cases, postponement – of pregnancy for women whose cardiac issues need to be addressed first.
Among other efforts to address maternal mortality are several programs developed by ACOG, and the Heart Outcomes in Pregnancy: Expectations for Mom and Baby Registry (HOPE) project of the Saint Luke’s Health System in Kansas.
“Hopefully the [HOPE] research collaborative ... which aims to address key clinical questions surrounding the preconception period, antenatal care, delivery planning and outcomes, and long-term postpartum care and outcomes of women will help to address the knowledge gaps and disparities in the care of women with heart disease in pregnancy,” Dr. Bullock-Palmer wrote in her article.
CVD-related risks in the post partum
Dr. Bullock-Palmer has particular concern for postpartum follow-up, given the increased risk for future heart disease among women with CVD-related pregnancy complications and the heightened risk of certain CVD-related events in the postpartum period.
That’s a component of the crisis that also was addressed during a press briefing at the 2019 ACOG annual meeting when the Pregnancy and Heart Disease Practice Bulletin was released.
James Martin, MD, chair of ACOG’s Pregnancy and Heart Disease Task Force and a past ACOG president, explained during the briefing that CVD-related risks may accelerate and persist in the days and weeks after delivery, underscoring the need for follow-up and postpartum care.
Cardiomyopathy is a particular concern during this time – it’s the major cause of maternal mortality after 42 days, he noted. An emphasis on postpartum care also is especially important given that some data suggest up to 40% of women don’t return for that care.
“That is a very sad statistic and perhaps it reflects on our need to change payment models so that physicians and patients realize the importance of coming back for continuing care, because this really may be the end of pregnancy, but it is the beginning of the rest of their life,” he said. “And if they have cardiovascular disease or the risk factors ... they are going to possibly become worse over the course of their lifetime.”
Nearly 700 women died from pregnancy-related complications in the United States in 2018, and almost a third of those deaths were associated with cardiovascular disease, according to the latest data from the Centers for Disease Control and Prevention.
Strikingly, studies suggest that up to half of cardiovascular disease–related maternal deaths are preventable, yet CVD remains the leading cause of maternal morbidity and mortality – and the incidence has been rising steadily for 2 decades.
The American College of Obstetricians and Gynecologists says that acquired heart disease is the likely culprit in the rise in incidence of maternal mortality as women enter pregnancy with an increasingly heavy burden of CVD risk factors, including older age, obesity, diabetes, and hypertension.
“They are entering pregnancy while already at risk, and that has led to an increase in morbidity and mortality during pregnancy,” Renee Patrice Bullock-Palmer, MD, a cardiologist and director of the Women’s Heart Center at Deborah Heart and Lung Center in Browns Mills, N.J., explained in an interview. “Unfortunately, among developed countries, the U.S. has the highest rates of maternal morbidity and mortality, and that’s shocking.”
It’s a problem that requires collaboration between obstetricians, cardiologists, and others involved in the care of pregnant women, she said.
The data and the depth of the crisis
The maternal mortality rate in 1987 – the year the CDC’s Pregnancy Mortality Surveillance System was implemented – was 7.2 per 100,000 live births. The rate in 2016 was more than double that at 16.9, and the rate in 2018, the most recent year for which data are available, was 17.4 – and significant racial and ethnic disparities in those rates have persisted over time.
In an August 2019 article published on the American Heart Association website, Dr. Bullock-Palmer addressed the cardiovascular state of health for pregnant women and the role of the cardiologists in their care, noting that there is a “role for increased collaboration between the cardiologist and the obstetrician with regards to a pregnancy heart team.”
“It is vital that mothers who are at increased risk for CVD or have established CVD be referred to a cardiologist for cardiovascular assessment and management,” she wrote, adding it is important to raise awareness among ob.gyns. and to improve cardiologists’ recognition of women at risk when they present for care for the first time.
These referrals should be made in the antepartum and early postpartum period, she said in an interview. More attention also must be paid to racial and ethnic disparities, and the role of cardiologists in addressing these disparities.
The CDC has emphasized racial and ethnic disparities in maternal mortality, noting in a 2019 Morbidity and Mortality Weekly report that, compared with white women, black and American Indian/Alaskan Native women aged over 30 years have a 300%-400% higher rate of pregnancy-related deaths (Morb Mortal Wkly Rep. 2019 Sep 6;68[35]:762-5).
With regard to disparities, Dr. Bullock-Palmer said the causes are multifold and may be related to a higher prevalence of CVD risk factors like obesity and hypertension in non-Hispanic black women.
“There may also be limited access to adequate postpartum care in this patient population,” she wrote, adding that some attention has been paid to addressing disparities, but that “there is a lot of work left to be done in resolving these inequities in maternal health care.”
Partnerships across specialties will help in addressing most of the factors associated with CVD and maternal death, she said.
The urgent need for these partnerships is underscored by the latest findings on CVD-related complications in pregnancy. A study published in March 2020 in the Journal of the American College of Cardiology, for example, looked specifically at the incidence of serious cardiac events (SCEs) in pregnant women with heart disease, and whether the events were preventable.
In a prospective cohort of 1,315 pregnancies among women with heart disease, Birgit Pfaller, MD, of the University of Toronto Pregnancy and Heart Disease Research Program, and colleagues found that SCEs occurred in 3.6% of cases (47 women) – most often during the antepartum period – that 49% were preventable, and that 74% were related to provider management factors.
The most common SCEs were cardiac death or arrest, heart failure, arrhythmias, and urgent intervention, and they were more likely to occur in women with acquired heart disease, severe aortic or mitral stenosis, mechanical valves, and systemic ventricular dysfunction. Adverse fetal and neonatal outcomes more than doubled in cases involving SCEs, compared with those without (62% vs. 29%), and adverse obstetric events occurred most often in women with severe preeclampsia.
“The majority of the preventable events occurred due to provider management factors, including: failure to identify the patient condition prior to pregnancy, failure to identify the patient as high risk, late recognition in cardiac deterioration, delay in treatment/intervention, inappropriate treatment, and lack of preconception counseling,” Melinda Davis, MD, of the University of Michigan, Ann Arbor, wrote in a summary and editorial published in the Journal of the American College of Cardiology.
Some preventable events were attributable to patient failure to seek care, noncompliance with care recommendations, and lack of access to care, Dr. Davis noted.
“These findings suggest that provider training, patient education, and health care advocacy are all important interventions to improve outcomes among pregnant women,” she wrote, adding that “the development of multidisciplinary cardio-obstetric clinics at tertiary care centers may also be helpful.”
Dr. Bullock-Palmer added the need for greater risk-prediction tools to the list, explaining that these are needed to assess CVD risk in the prenatal, antenatal, and postnatal period.
“The recently concluded Cardiac Disease in Pregnancy [CARPREG II] study indicated that there were 10 predictors that could be utilized to asses maternal CVD risk,” she noted.
The CARPREG II authors identified five general predictors (prior cardiac events or arrhythmias, poor functional class or cyanosis, high-risk valve disease/left ventricular outflow tract obstruction, systemic ventricular dysfunction, no prior cardiac interventions), four lesion-specific predictors (mechanical valves, high-risk aortopathies, pulmonary hypertension, coronary artery disease), and one delivery-of-care predictor (late pregnancy assessment), and incorporated them into a risk index.
“It is hopeful that these new initiatives will assist providers in improving their ability to appropriately risk stratify women,” Dr. Bullock-Palmer said.
Ongoing efforts
Efforts also are ongoing to develop the types of cardio-obstetric clinics mentioned by Dr. Davis and to establish collaborations and “pregnancy heart teams” as attention is increasingly focused on the U.S. maternal mortality crisis.
In fact, such teams are a cornerstone of ACOG’s guidance on pregnancy and heart disease. In May 2019 the college released a Practice Bulletin with 27 specific recommendations and conclusions relating to screening, diagnosis, and management of CVD for women during the prepregnancy period through the postpartum period.
Pregnant women and postpartum women with known or suspected CVD should undergo evaluation by a “pregnancy heart team that includes a cardiologist and maternal-fetal medicine subspecialist, or both, and other subspecialists as necessary,” according to the bulletin.
In a recent interview, Lisa Hollier, MD, immediate past president of ACOG and an instrumental figure in the push to better address maternal mortality – and in particular the cardiovascular contributors to the crisis – said she is “seeing a strengthening of that” with numerous organizations establishing pregnancy health teams.
Dr. Bullock-Palmer said she also is seeing progress, and added that collaboration should be prioritized even in the absence of dedicated pregnancy heart teams and clinics.
“Heart disease in pregnancy requires a multidisciplinary approach. You can’t just see the patient from the cardiac perspective – you also have to interact and team up with the obstetrician who is handling the pregnancy,” she said, adding that, without a dedicated team, coordination takes more effort, but is imperative for improving outcomes. “You have to collaborate at times when it is beyond the expertise of the institution or the physician; you have to know when to refer these higher-risk patients, particularly women with adult congenital heart disease.”
This referral should occur early – preferably in the antenatal period, she added.
The most important thing, however, is “recognizing these women ... even before the pregnancy,” Dr. Bullock-Palmer said, explaining that this can facilitate the necessary management – and in some cases, postponement – of pregnancy for women whose cardiac issues need to be addressed first.
Among other efforts to address maternal mortality are several programs developed by ACOG, and the Heart Outcomes in Pregnancy: Expectations for Mom and Baby Registry (HOPE) project of the Saint Luke’s Health System in Kansas.
“Hopefully the [HOPE] research collaborative ... which aims to address key clinical questions surrounding the preconception period, antenatal care, delivery planning and outcomes, and long-term postpartum care and outcomes of women will help to address the knowledge gaps and disparities in the care of women with heart disease in pregnancy,” Dr. Bullock-Palmer wrote in her article.
CVD-related risks in the post partum
Dr. Bullock-Palmer has particular concern for postpartum follow-up, given the increased risk for future heart disease among women with CVD-related pregnancy complications and the heightened risk of certain CVD-related events in the postpartum period.
That’s a component of the crisis that also was addressed during a press briefing at the 2019 ACOG annual meeting when the Pregnancy and Heart Disease Practice Bulletin was released.
James Martin, MD, chair of ACOG’s Pregnancy and Heart Disease Task Force and a past ACOG president, explained during the briefing that CVD-related risks may accelerate and persist in the days and weeks after delivery, underscoring the need for follow-up and postpartum care.
Cardiomyopathy is a particular concern during this time – it’s the major cause of maternal mortality after 42 days, he noted. An emphasis on postpartum care also is especially important given that some data suggest up to 40% of women don’t return for that care.
“That is a very sad statistic and perhaps it reflects on our need to change payment models so that physicians and patients realize the importance of coming back for continuing care, because this really may be the end of pregnancy, but it is the beginning of the rest of their life,” he said. “And if they have cardiovascular disease or the risk factors ... they are going to possibly become worse over the course of their lifetime.”
Bone density slow to rebound after lactation in women with HIV
Women with HIV had more bone mobilization during lactation, and attenuated skeletal recovery after lactation, compared with HIV-negative women, according to research presented during the Conference on Retroviruses & Opportunistic Infections, which was presented online this year. CROI organizers chose to hold a virtual meeting because of concerns about the spread of COVID-19.
The study “demonstrated that there were reductions as expected in BMD during breastfeeding, and there was recovery at the end of breastfeeding, which was higher among women who were not HIV-infected compared to HIV-infected women,” said Mary Glenn Fowler, MD, speaking in a video presentation during the virtual conference. The differences between women who had HIV and the HIV-negative reference group were statistically significant (P = .003 for lumbar spine and P less than .001 for whole-body aBMD).
“We also saw that for whole-body BMD, there was recovery at the end of breastfeeding for women who were not HIV infected, but a dampened response of recovery for BMD for HIV-infected women,” she went on, adding: “These findings held after adjustment for parity, age, body mass, breastfeeding practices, duration of breastfeeding, use of [injectable medroxyprogesterone acetate], and resumption of menses.”
Dr. Fowler presented the study’s results on behalf of lead author Florence Nabwire, PhD, an investigator scientist in the nutrition and bone health group of the United Kingdom’s Medical Research Council (Cambridge).
Although it’s known that antiretroviral therapy (ART) is associated with bone loss, Dr. Fowler explained that there are only limited data in HIV-positive women who are lactating. It’s important to see what happens during lactation for this group of women because of the potential sequelae later in life of insufficient recovery from the physiological bone mobilization that occurs during lactation. The study looked at changes in areal bone mineral density (aBMD) both during and after lactation for women with HIV living in Uganda who were taking Option B+ ART, a regimen that includes tenofovir, 3TC, and efavirenz. These women were compared with a reference group of HIV-negative women.
In all, 95 women with HIV and 96 HIV negative women were recruited into the study during pregnancy. Participants were followed postpartum at weeks 2, 14, and 26, and at a final visit that occurred 14 weeks after lactation stopped.
In addition to lumbar spine, total hip, and femoral neck aBMD measurements, the investigators also obtained whole body-less-head reading.
For total hip and femoral neck aBMD, the nadir of density was seen at 26 postpartum, when a drop of about 6% was seen from baseline readings. By the final post-lactation visit, women without HIV had recovered to their baseline; for women with HIV, some recovery also occurred, but the effect was dampened, with a persistent bone density deficit of about 3% from baseline. The differences between HIV-positive and HIV-negative women in these measurements were also statistically significant, at P less than .001 for total hip aBMD differences and P = .0008 for femoral neck differences. Again, correction for multiple confounders didn’t attenuate the results, said Dr. Fowler.
“In conclusion, these data showed accentuated mobilization of hip and whole body aBMD during lactation,” said Dr. Fowler, who also noted “slower skeletal recovery post lactation for HIV-infected women.” Clinical implications of these findings aren’t currently known, she said. Further ongoing studies are aiming to tease out both mechanisms and longer-term consequences for the bone health of HIV-infected women and their children, who may also see differences in bone mineral accretion and growth.
Session moderator Risa Hoffman, MD, in introductory remarks, set the findings in some context. “As we know, HIV-positive adults have low bone mineral density, and this appears to be a result of interactions of HIV, traditional risk factors for loss of bone density, and antiretroviral therapy,” said Dr. Hoffman, director of the global health program at the University of California, Los Angeles. She added that previous work had shown that “middle-aged HIV-positive women have higher 10-year fracture incidence compared to their HIV-negative counterparts.” The current study, she said, “has both short- and long-term implications for women as they go through multiple pregnancies and multiple periods of breastfeeding.”
The study was funded by the United Kingdom’s Medical Research Council and Department for International Development as well as the Alborada Trust and the Gates Cambridge Scholarship. The authors reported no conflicts of interest.
SOURCE: Nabwire F et al. CROI 2020, Abstract 768.
Women with HIV had more bone mobilization during lactation, and attenuated skeletal recovery after lactation, compared with HIV-negative women, according to research presented during the Conference on Retroviruses & Opportunistic Infections, which was presented online this year. CROI organizers chose to hold a virtual meeting because of concerns about the spread of COVID-19.
The study “demonstrated that there were reductions as expected in BMD during breastfeeding, and there was recovery at the end of breastfeeding, which was higher among women who were not HIV-infected compared to HIV-infected women,” said Mary Glenn Fowler, MD, speaking in a video presentation during the virtual conference. The differences between women who had HIV and the HIV-negative reference group were statistically significant (P = .003 for lumbar spine and P less than .001 for whole-body aBMD).
“We also saw that for whole-body BMD, there was recovery at the end of breastfeeding for women who were not HIV infected, but a dampened response of recovery for BMD for HIV-infected women,” she went on, adding: “These findings held after adjustment for parity, age, body mass, breastfeeding practices, duration of breastfeeding, use of [injectable medroxyprogesterone acetate], and resumption of menses.”
Dr. Fowler presented the study’s results on behalf of lead author Florence Nabwire, PhD, an investigator scientist in the nutrition and bone health group of the United Kingdom’s Medical Research Council (Cambridge).
Although it’s known that antiretroviral therapy (ART) is associated with bone loss, Dr. Fowler explained that there are only limited data in HIV-positive women who are lactating. It’s important to see what happens during lactation for this group of women because of the potential sequelae later in life of insufficient recovery from the physiological bone mobilization that occurs during lactation. The study looked at changes in areal bone mineral density (aBMD) both during and after lactation for women with HIV living in Uganda who were taking Option B+ ART, a regimen that includes tenofovir, 3TC, and efavirenz. These women were compared with a reference group of HIV-negative women.
In all, 95 women with HIV and 96 HIV negative women were recruited into the study during pregnancy. Participants were followed postpartum at weeks 2, 14, and 26, and at a final visit that occurred 14 weeks after lactation stopped.
In addition to lumbar spine, total hip, and femoral neck aBMD measurements, the investigators also obtained whole body-less-head reading.
For total hip and femoral neck aBMD, the nadir of density was seen at 26 postpartum, when a drop of about 6% was seen from baseline readings. By the final post-lactation visit, women without HIV had recovered to their baseline; for women with HIV, some recovery also occurred, but the effect was dampened, with a persistent bone density deficit of about 3% from baseline. The differences between HIV-positive and HIV-negative women in these measurements were also statistically significant, at P less than .001 for total hip aBMD differences and P = .0008 for femoral neck differences. Again, correction for multiple confounders didn’t attenuate the results, said Dr. Fowler.
“In conclusion, these data showed accentuated mobilization of hip and whole body aBMD during lactation,” said Dr. Fowler, who also noted “slower skeletal recovery post lactation for HIV-infected women.” Clinical implications of these findings aren’t currently known, she said. Further ongoing studies are aiming to tease out both mechanisms and longer-term consequences for the bone health of HIV-infected women and their children, who may also see differences in bone mineral accretion and growth.
Session moderator Risa Hoffman, MD, in introductory remarks, set the findings in some context. “As we know, HIV-positive adults have low bone mineral density, and this appears to be a result of interactions of HIV, traditional risk factors for loss of bone density, and antiretroviral therapy,” said Dr. Hoffman, director of the global health program at the University of California, Los Angeles. She added that previous work had shown that “middle-aged HIV-positive women have higher 10-year fracture incidence compared to their HIV-negative counterparts.” The current study, she said, “has both short- and long-term implications for women as they go through multiple pregnancies and multiple periods of breastfeeding.”
The study was funded by the United Kingdom’s Medical Research Council and Department for International Development as well as the Alborada Trust and the Gates Cambridge Scholarship. The authors reported no conflicts of interest.
SOURCE: Nabwire F et al. CROI 2020, Abstract 768.
Women with HIV had more bone mobilization during lactation, and attenuated skeletal recovery after lactation, compared with HIV-negative women, according to research presented during the Conference on Retroviruses & Opportunistic Infections, which was presented online this year. CROI organizers chose to hold a virtual meeting because of concerns about the spread of COVID-19.
The study “demonstrated that there were reductions as expected in BMD during breastfeeding, and there was recovery at the end of breastfeeding, which was higher among women who were not HIV-infected compared to HIV-infected women,” said Mary Glenn Fowler, MD, speaking in a video presentation during the virtual conference. The differences between women who had HIV and the HIV-negative reference group were statistically significant (P = .003 for lumbar spine and P less than .001 for whole-body aBMD).
“We also saw that for whole-body BMD, there was recovery at the end of breastfeeding for women who were not HIV infected, but a dampened response of recovery for BMD for HIV-infected women,” she went on, adding: “These findings held after adjustment for parity, age, body mass, breastfeeding practices, duration of breastfeeding, use of [injectable medroxyprogesterone acetate], and resumption of menses.”
Dr. Fowler presented the study’s results on behalf of lead author Florence Nabwire, PhD, an investigator scientist in the nutrition and bone health group of the United Kingdom’s Medical Research Council (Cambridge).
Although it’s known that antiretroviral therapy (ART) is associated with bone loss, Dr. Fowler explained that there are only limited data in HIV-positive women who are lactating. It’s important to see what happens during lactation for this group of women because of the potential sequelae later in life of insufficient recovery from the physiological bone mobilization that occurs during lactation. The study looked at changes in areal bone mineral density (aBMD) both during and after lactation for women with HIV living in Uganda who were taking Option B+ ART, a regimen that includes tenofovir, 3TC, and efavirenz. These women were compared with a reference group of HIV-negative women.
In all, 95 women with HIV and 96 HIV negative women were recruited into the study during pregnancy. Participants were followed postpartum at weeks 2, 14, and 26, and at a final visit that occurred 14 weeks after lactation stopped.
In addition to lumbar spine, total hip, and femoral neck aBMD measurements, the investigators also obtained whole body-less-head reading.
For total hip and femoral neck aBMD, the nadir of density was seen at 26 postpartum, when a drop of about 6% was seen from baseline readings. By the final post-lactation visit, women without HIV had recovered to their baseline; for women with HIV, some recovery also occurred, but the effect was dampened, with a persistent bone density deficit of about 3% from baseline. The differences between HIV-positive and HIV-negative women in these measurements were also statistically significant, at P less than .001 for total hip aBMD differences and P = .0008 for femoral neck differences. Again, correction for multiple confounders didn’t attenuate the results, said Dr. Fowler.
“In conclusion, these data showed accentuated mobilization of hip and whole body aBMD during lactation,” said Dr. Fowler, who also noted “slower skeletal recovery post lactation for HIV-infected women.” Clinical implications of these findings aren’t currently known, she said. Further ongoing studies are aiming to tease out both mechanisms and longer-term consequences for the bone health of HIV-infected women and their children, who may also see differences in bone mineral accretion and growth.
Session moderator Risa Hoffman, MD, in introductory remarks, set the findings in some context. “As we know, HIV-positive adults have low bone mineral density, and this appears to be a result of interactions of HIV, traditional risk factors for loss of bone density, and antiretroviral therapy,” said Dr. Hoffman, director of the global health program at the University of California, Los Angeles. She added that previous work had shown that “middle-aged HIV-positive women have higher 10-year fracture incidence compared to their HIV-negative counterparts.” The current study, she said, “has both short- and long-term implications for women as they go through multiple pregnancies and multiple periods of breastfeeding.”
The study was funded by the United Kingdom’s Medical Research Council and Department for International Development as well as the Alborada Trust and the Gates Cambridge Scholarship. The authors reported no conflicts of interest.
SOURCE: Nabwire F et al. CROI 2020, Abstract 768.
FROM CROI 2020
When to treat, delay, or omit breast cancer therapy in the face of COVID-19
Nothing is business as usual during the COVID-19 pandemic, and that includes breast cancer therapy. That’s why two groups have released guidance documents on treating breast cancer patients during the pandemic.
A guidance on surgery, drug therapy, and radiotherapy was created by the COVID-19 Pandemic Breast Cancer Consortium. This guidance is set to be published in Breast Cancer Research and Treatment and can be downloaded from the American College of Surgeons website.
A group from Memorial Sloan Kettering Cancer Center (MSKCC) created a guidance document on radiotherapy for breast cancer patients, and that guidance was recently published in Advances in Radiation Oncology.
Prioritizing certain patients and treatments
As hospital beds and clinics fill with coronavirus-infected patients, oncologists must balance the need for timely therapy for their patients with the imperative to protect vulnerable, immunosuppressed patients from exposure and keep clinical resources as free as possible.
“As we’re taking care of breast cancer patients during this unprecedented pandemic, what we’re all trying to do is balance the most effective treatments for our patients against the risk of additional exposures, either from other patients [or] from being outside, and considerations about the safety of our staff,” said Steven Isakoff, MD, PhD, of Massachusetts General Hospital Cancer Center in Boston, who is an author of the COVID-19 Pandemic Breast Cancer Consortium guidance.
The consortium’s guidance recommends prioritizing treatment according to patient needs and the disease type and stage. The three basic categories for considering when to treat are:
- Priority A: Patients who have immediately life-threatening conditions, are clinically unstable, or would experience a significant change in prognosis with even a short delay in treatment.
- Priority B: Deferring treatment for a short time (6-12 weeks) would not impact overall outcomes in these patients.
- Priority C: These patients are stable enough that treatment can be delayed for the duration of the COVID-19 pandemic.
“The consortium highly recommends multidisciplinary discussion regarding priority for elective surgery and adjuvant treatments for your breast cancer patients,” the guidance authors wrote. “The COVID-19 pandemic may vary in severity over time, and these recommendations are subject to change with changing COVID-19 pandemic severity.”
For example, depending on local circumstances, the guidance recommends limiting immediate outpatient visits to patients with potentially unstable conditions such as infection or hematoma. Established patients with new problems or patients with a new diagnosis of noninvasive cancer might be managed with telemedicine visits, and patients who are on follow-up with no new issues or who have benign lesions might have their visits safely postponed.
Surgery and drug recommendations
High-priority surgical procedures include operative drainage of a breast abscess in a septic patient and evacuation of expanding hematoma in a hemodynamically unstable patient, according to the consortium guidance.
Other surgical situations are more nuanced. For example, for patients with triple-negative breast cancer (TNBC) or HER2-positive disease, the guidance recommends neoadjuvant chemotherapy or HER2-targeted chemotherapy in some cases. In other cases, institutions may proceed with surgery before chemotherapy, but “these decisions will depend on institutional resources and patient factors,” according to the authors.
The guidance states that chemotherapy and other drug treatments should not be delayed in patients with oncologic emergencies, such as febrile neutropenia, hypercalcemia, intolerable pain, symptomatic pleural effusions, or brain metastases.
In addition, patients with inflammatory breast cancer, TNBC, or HER2-positive breast cancer should receive neoadjuvant/adjuvant chemotherapy. Patients with metastatic disease that is likely to benefit from therapy should start chemotherapy, endocrine therapy, or targeted therapy. And patients who have already started neoadjuvant/adjuvant chemotherapy or oral adjuvant endocrine therapy should continue on these treatments.
Radiation therapy recommendations
The consortium guidance recommends administering radiation to patients with bleeding or painful inoperable locoregional disease, those with symptomatic metastatic disease, and patients who progress on neoadjuvant chemotherapy.
In contrast, older patients (aged 65-70 years) with lower-risk, stage I, hormone receptor–positive, HER2-negative cancers who are on adjuvant endocrine therapy can safely defer or omit radiation without affecting their overall survival, according to the guidance. Patients with ductal carcinoma in situ, especially those with estrogen receptor–positive disease on endocrine therapy, can safely omit radiation.
“There are clearly conditions where radiation might reduce the risk of recurrence but not improve overall survival, where a delay in treatment really will have minimal or no impact,” Dr. Isakoff said.
The MSKCC guidance recommends omitting radiation for some patients with favorable-risk disease and truncating or accelerating regimens using hypofractionation for others who require whole-breast radiation or post-mastectomy treatment.
The MSKCC guidance also contains recommendations for prioritization of patients according to disease state and the urgency of care. It divides cases into high, intermediate, and low priority for breast radiotherapy, as follows:
- Tier 1 (high priority): Patients with inflammatory breast cancer, residual node-positive disease after neoadjuvant chemotherapy, four or more positive nodes (N2), recurrent disease, node-positive TNBC, or extensive lymphovascular invasion.
- Tier 2 (intermediate priority): Patients with estrogen receptor–positive disease with one to three positive nodes (N1a), pathologic stage N0 after neoadjuvant chemotherapy, lymphovascular invasion not otherwise specified, or node-negative TNBC.
- Tier 3 (low priority): Patients with early-stage estrogen receptor-positive breast cancer (especially patients of advanced age), patients with ductal carcinoma in situ, or those who otherwise do not meet the criteria for tiers 1 or 2.
The MSKCC guidance also contains recommended hypofractionated or accelerated radiotherapy regimens for partial and whole-breast irradiation, post-mastectomy treatment, and breast and regional node irradiation, including recommended techniques (for example, 3-D conformal or intensity modulated approaches).
The authors of the MSKCC guidance disclosed relationships with eContour, Volastra Therapeutics, Sanofi, the Prostate Cancer Foundation, and Cancer Research UK. The authors of the COVID-19 Pandemic Breast Cancer Consortium guidance did not disclose any conflicts and said there was no funding source for the guidance.
SOURCES: Braunstein LZ et al. Adv Radiat Oncol. 2020 Apr 1. doi:10.1016/j.adro.2020.03.013; Dietz JR et al. 2020 Apr. Recommendations for prioritization, treatment and triage of breast cancer patients during the COVID-19 pandemic. Accepted for publication in Breast Cancer Research and Treatment.
Nothing is business as usual during the COVID-19 pandemic, and that includes breast cancer therapy. That’s why two groups have released guidance documents on treating breast cancer patients during the pandemic.
A guidance on surgery, drug therapy, and radiotherapy was created by the COVID-19 Pandemic Breast Cancer Consortium. This guidance is set to be published in Breast Cancer Research and Treatment and can be downloaded from the American College of Surgeons website.
A group from Memorial Sloan Kettering Cancer Center (MSKCC) created a guidance document on radiotherapy for breast cancer patients, and that guidance was recently published in Advances in Radiation Oncology.
Prioritizing certain patients and treatments
As hospital beds and clinics fill with coronavirus-infected patients, oncologists must balance the need for timely therapy for their patients with the imperative to protect vulnerable, immunosuppressed patients from exposure and keep clinical resources as free as possible.
“As we’re taking care of breast cancer patients during this unprecedented pandemic, what we’re all trying to do is balance the most effective treatments for our patients against the risk of additional exposures, either from other patients [or] from being outside, and considerations about the safety of our staff,” said Steven Isakoff, MD, PhD, of Massachusetts General Hospital Cancer Center in Boston, who is an author of the COVID-19 Pandemic Breast Cancer Consortium guidance.
The consortium’s guidance recommends prioritizing treatment according to patient needs and the disease type and stage. The three basic categories for considering when to treat are:
- Priority A: Patients who have immediately life-threatening conditions, are clinically unstable, or would experience a significant change in prognosis with even a short delay in treatment.
- Priority B: Deferring treatment for a short time (6-12 weeks) would not impact overall outcomes in these patients.
- Priority C: These patients are stable enough that treatment can be delayed for the duration of the COVID-19 pandemic.
“The consortium highly recommends multidisciplinary discussion regarding priority for elective surgery and adjuvant treatments for your breast cancer patients,” the guidance authors wrote. “The COVID-19 pandemic may vary in severity over time, and these recommendations are subject to change with changing COVID-19 pandemic severity.”
For example, depending on local circumstances, the guidance recommends limiting immediate outpatient visits to patients with potentially unstable conditions such as infection or hematoma. Established patients with new problems or patients with a new diagnosis of noninvasive cancer might be managed with telemedicine visits, and patients who are on follow-up with no new issues or who have benign lesions might have their visits safely postponed.
Surgery and drug recommendations
High-priority surgical procedures include operative drainage of a breast abscess in a septic patient and evacuation of expanding hematoma in a hemodynamically unstable patient, according to the consortium guidance.
Other surgical situations are more nuanced. For example, for patients with triple-negative breast cancer (TNBC) or HER2-positive disease, the guidance recommends neoadjuvant chemotherapy or HER2-targeted chemotherapy in some cases. In other cases, institutions may proceed with surgery before chemotherapy, but “these decisions will depend on institutional resources and patient factors,” according to the authors.
The guidance states that chemotherapy and other drug treatments should not be delayed in patients with oncologic emergencies, such as febrile neutropenia, hypercalcemia, intolerable pain, symptomatic pleural effusions, or brain metastases.
In addition, patients with inflammatory breast cancer, TNBC, or HER2-positive breast cancer should receive neoadjuvant/adjuvant chemotherapy. Patients with metastatic disease that is likely to benefit from therapy should start chemotherapy, endocrine therapy, or targeted therapy. And patients who have already started neoadjuvant/adjuvant chemotherapy or oral adjuvant endocrine therapy should continue on these treatments.
Radiation therapy recommendations
The consortium guidance recommends administering radiation to patients with bleeding or painful inoperable locoregional disease, those with symptomatic metastatic disease, and patients who progress on neoadjuvant chemotherapy.
In contrast, older patients (aged 65-70 years) with lower-risk, stage I, hormone receptor–positive, HER2-negative cancers who are on adjuvant endocrine therapy can safely defer or omit radiation without affecting their overall survival, according to the guidance. Patients with ductal carcinoma in situ, especially those with estrogen receptor–positive disease on endocrine therapy, can safely omit radiation.
“There are clearly conditions where radiation might reduce the risk of recurrence but not improve overall survival, where a delay in treatment really will have minimal or no impact,” Dr. Isakoff said.
The MSKCC guidance recommends omitting radiation for some patients with favorable-risk disease and truncating or accelerating regimens using hypofractionation for others who require whole-breast radiation or post-mastectomy treatment.
The MSKCC guidance also contains recommendations for prioritization of patients according to disease state and the urgency of care. It divides cases into high, intermediate, and low priority for breast radiotherapy, as follows:
- Tier 1 (high priority): Patients with inflammatory breast cancer, residual node-positive disease after neoadjuvant chemotherapy, four or more positive nodes (N2), recurrent disease, node-positive TNBC, or extensive lymphovascular invasion.
- Tier 2 (intermediate priority): Patients with estrogen receptor–positive disease with one to three positive nodes (N1a), pathologic stage N0 after neoadjuvant chemotherapy, lymphovascular invasion not otherwise specified, or node-negative TNBC.
- Tier 3 (low priority): Patients with early-stage estrogen receptor-positive breast cancer (especially patients of advanced age), patients with ductal carcinoma in situ, or those who otherwise do not meet the criteria for tiers 1 or 2.
The MSKCC guidance also contains recommended hypofractionated or accelerated radiotherapy regimens for partial and whole-breast irradiation, post-mastectomy treatment, and breast and regional node irradiation, including recommended techniques (for example, 3-D conformal or intensity modulated approaches).
The authors of the MSKCC guidance disclosed relationships with eContour, Volastra Therapeutics, Sanofi, the Prostate Cancer Foundation, and Cancer Research UK. The authors of the COVID-19 Pandemic Breast Cancer Consortium guidance did not disclose any conflicts and said there was no funding source for the guidance.
SOURCES: Braunstein LZ et al. Adv Radiat Oncol. 2020 Apr 1. doi:10.1016/j.adro.2020.03.013; Dietz JR et al. 2020 Apr. Recommendations for prioritization, treatment and triage of breast cancer patients during the COVID-19 pandemic. Accepted for publication in Breast Cancer Research and Treatment.
Nothing is business as usual during the COVID-19 pandemic, and that includes breast cancer therapy. That’s why two groups have released guidance documents on treating breast cancer patients during the pandemic.
A guidance on surgery, drug therapy, and radiotherapy was created by the COVID-19 Pandemic Breast Cancer Consortium. This guidance is set to be published in Breast Cancer Research and Treatment and can be downloaded from the American College of Surgeons website.
A group from Memorial Sloan Kettering Cancer Center (MSKCC) created a guidance document on radiotherapy for breast cancer patients, and that guidance was recently published in Advances in Radiation Oncology.
Prioritizing certain patients and treatments
As hospital beds and clinics fill with coronavirus-infected patients, oncologists must balance the need for timely therapy for their patients with the imperative to protect vulnerable, immunosuppressed patients from exposure and keep clinical resources as free as possible.
“As we’re taking care of breast cancer patients during this unprecedented pandemic, what we’re all trying to do is balance the most effective treatments for our patients against the risk of additional exposures, either from other patients [or] from being outside, and considerations about the safety of our staff,” said Steven Isakoff, MD, PhD, of Massachusetts General Hospital Cancer Center in Boston, who is an author of the COVID-19 Pandemic Breast Cancer Consortium guidance.
The consortium’s guidance recommends prioritizing treatment according to patient needs and the disease type and stage. The three basic categories for considering when to treat are:
- Priority A: Patients who have immediately life-threatening conditions, are clinically unstable, or would experience a significant change in prognosis with even a short delay in treatment.
- Priority B: Deferring treatment for a short time (6-12 weeks) would not impact overall outcomes in these patients.
- Priority C: These patients are stable enough that treatment can be delayed for the duration of the COVID-19 pandemic.
“The consortium highly recommends multidisciplinary discussion regarding priority for elective surgery and adjuvant treatments for your breast cancer patients,” the guidance authors wrote. “The COVID-19 pandemic may vary in severity over time, and these recommendations are subject to change with changing COVID-19 pandemic severity.”
For example, depending on local circumstances, the guidance recommends limiting immediate outpatient visits to patients with potentially unstable conditions such as infection or hematoma. Established patients with new problems or patients with a new diagnosis of noninvasive cancer might be managed with telemedicine visits, and patients who are on follow-up with no new issues or who have benign lesions might have their visits safely postponed.
Surgery and drug recommendations
High-priority surgical procedures include operative drainage of a breast abscess in a septic patient and evacuation of expanding hematoma in a hemodynamically unstable patient, according to the consortium guidance.
Other surgical situations are more nuanced. For example, for patients with triple-negative breast cancer (TNBC) or HER2-positive disease, the guidance recommends neoadjuvant chemotherapy or HER2-targeted chemotherapy in some cases. In other cases, institutions may proceed with surgery before chemotherapy, but “these decisions will depend on institutional resources and patient factors,” according to the authors.
The guidance states that chemotherapy and other drug treatments should not be delayed in patients with oncologic emergencies, such as febrile neutropenia, hypercalcemia, intolerable pain, symptomatic pleural effusions, or brain metastases.
In addition, patients with inflammatory breast cancer, TNBC, or HER2-positive breast cancer should receive neoadjuvant/adjuvant chemotherapy. Patients with metastatic disease that is likely to benefit from therapy should start chemotherapy, endocrine therapy, or targeted therapy. And patients who have already started neoadjuvant/adjuvant chemotherapy or oral adjuvant endocrine therapy should continue on these treatments.
Radiation therapy recommendations
The consortium guidance recommends administering radiation to patients with bleeding or painful inoperable locoregional disease, those with symptomatic metastatic disease, and patients who progress on neoadjuvant chemotherapy.
In contrast, older patients (aged 65-70 years) with lower-risk, stage I, hormone receptor–positive, HER2-negative cancers who are on adjuvant endocrine therapy can safely defer or omit radiation without affecting their overall survival, according to the guidance. Patients with ductal carcinoma in situ, especially those with estrogen receptor–positive disease on endocrine therapy, can safely omit radiation.
“There are clearly conditions where radiation might reduce the risk of recurrence but not improve overall survival, where a delay in treatment really will have minimal or no impact,” Dr. Isakoff said.
The MSKCC guidance recommends omitting radiation for some patients with favorable-risk disease and truncating or accelerating regimens using hypofractionation for others who require whole-breast radiation or post-mastectomy treatment.
The MSKCC guidance also contains recommendations for prioritization of patients according to disease state and the urgency of care. It divides cases into high, intermediate, and low priority for breast radiotherapy, as follows:
- Tier 1 (high priority): Patients with inflammatory breast cancer, residual node-positive disease after neoadjuvant chemotherapy, four or more positive nodes (N2), recurrent disease, node-positive TNBC, or extensive lymphovascular invasion.
- Tier 2 (intermediate priority): Patients with estrogen receptor–positive disease with one to three positive nodes (N1a), pathologic stage N0 after neoadjuvant chemotherapy, lymphovascular invasion not otherwise specified, or node-negative TNBC.
- Tier 3 (low priority): Patients with early-stage estrogen receptor-positive breast cancer (especially patients of advanced age), patients with ductal carcinoma in situ, or those who otherwise do not meet the criteria for tiers 1 or 2.
The MSKCC guidance also contains recommended hypofractionated or accelerated radiotherapy regimens for partial and whole-breast irradiation, post-mastectomy treatment, and breast and regional node irradiation, including recommended techniques (for example, 3-D conformal or intensity modulated approaches).
The authors of the MSKCC guidance disclosed relationships with eContour, Volastra Therapeutics, Sanofi, the Prostate Cancer Foundation, and Cancer Research UK. The authors of the COVID-19 Pandemic Breast Cancer Consortium guidance did not disclose any conflicts and said there was no funding source for the guidance.
SOURCES: Braunstein LZ et al. Adv Radiat Oncol. 2020 Apr 1. doi:10.1016/j.adro.2020.03.013; Dietz JR et al. 2020 Apr. Recommendations for prioritization, treatment and triage of breast cancer patients during the COVID-19 pandemic. Accepted for publication in Breast Cancer Research and Treatment.
With mild or stable lupus, few patients flare during, after pregnancy
Approximately 26% of women with inactive or mild lupus at conception experienced flares at some point during pregnancy, based on data from 384 patients.
Active systemic lupus erythematosus (SLE) is a known predictor of poor pregnancy outcomes, including preterm birth, growth restriction, and fetal loss, but predictors of flares during and after pregnancy in women with SLE have not been well studied, wrote Julia Davis-Porada, MD, of the Hospital for Special Surgery, New York, and her colleagues.
In a study published in Arthritis Research & Therapy, the investigators reviewed data from the PROMISSE (Predictors of Pregnancy Outcome: Biomarkers in Antiphospholipid Antibody Syndrome and Systemic Lupus Erythematosus) study, a prospective study of pregnant women aged 18-45 years. The women were enrolled at less than 12 weeks’ gestation, and participants had a baseline hematocrit greater than 26%. Participants met criteria for inactive or mild/stable disease at the time of conception.
Overall, 20.8% of patients experienced at least one mild or moderate flare and 6.25% had one or more severe flares during pregnancy. Mild to moderate flares and severe flares occurred postpartum (2-6 months after the end of pregnancy) in 22.7% and 1.7% of patients, respectively.
Patients who were younger and those who had lower C4 at baseline and higher Physician Global Assessment scores at baseline were significantly more likely to have at least one flare during pregnancy (P = .003, P = .024, P = .0005, respectively).
In the analysis of postpartum flares, the incidence rates for mild to moderate and severe flares were 0.8 and 0.06 per person-year, respectively. “In contrast to the findings observed for flares that occurred during pregnancy, baseline patient characteristics were not correlated with postpartum flares,” the researchers wrote.
No medications were associated with flares during or after pregnancy.
The study findings were limited by several factors, including the exclusion of SLE patients with current nephritis and those who needed high-dose prednisone; the potential for missed flares; and the lack of postpartum data for approximately 10% of patients, the researchers noted. Also, “since many patients presented to this study only after conception, we have no data to review disease activity prior to pregnancy to determine whether pregnancy per se increased the risk for flare,” they said.
However, the results were strengthened by the large, multiethnic population and prospective study design, and support physicians in reassuring patients with SLE that pregnancy and postpartum flares are unlikely if they plan pregnancy during a time of mild or inactive disease, they concluded.
The study was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases. The researchers had no financial conflicts to disclose.
SOURCE: Davis-Porada J et al. Arthritis Res Ther. 2020 Mar 19. doi: 10.1186/s13075-020-2139-9.
Approximately 26% of women with inactive or mild lupus at conception experienced flares at some point during pregnancy, based on data from 384 patients.
Active systemic lupus erythematosus (SLE) is a known predictor of poor pregnancy outcomes, including preterm birth, growth restriction, and fetal loss, but predictors of flares during and after pregnancy in women with SLE have not been well studied, wrote Julia Davis-Porada, MD, of the Hospital for Special Surgery, New York, and her colleagues.
In a study published in Arthritis Research & Therapy, the investigators reviewed data from the PROMISSE (Predictors of Pregnancy Outcome: Biomarkers in Antiphospholipid Antibody Syndrome and Systemic Lupus Erythematosus) study, a prospective study of pregnant women aged 18-45 years. The women were enrolled at less than 12 weeks’ gestation, and participants had a baseline hematocrit greater than 26%. Participants met criteria for inactive or mild/stable disease at the time of conception.
Overall, 20.8% of patients experienced at least one mild or moderate flare and 6.25% had one or more severe flares during pregnancy. Mild to moderate flares and severe flares occurred postpartum (2-6 months after the end of pregnancy) in 22.7% and 1.7% of patients, respectively.
Patients who were younger and those who had lower C4 at baseline and higher Physician Global Assessment scores at baseline were significantly more likely to have at least one flare during pregnancy (P = .003, P = .024, P = .0005, respectively).
In the analysis of postpartum flares, the incidence rates for mild to moderate and severe flares were 0.8 and 0.06 per person-year, respectively. “In contrast to the findings observed for flares that occurred during pregnancy, baseline patient characteristics were not correlated with postpartum flares,” the researchers wrote.
No medications were associated with flares during or after pregnancy.
The study findings were limited by several factors, including the exclusion of SLE patients with current nephritis and those who needed high-dose prednisone; the potential for missed flares; and the lack of postpartum data for approximately 10% of patients, the researchers noted. Also, “since many patients presented to this study only after conception, we have no data to review disease activity prior to pregnancy to determine whether pregnancy per se increased the risk for flare,” they said.
However, the results were strengthened by the large, multiethnic population and prospective study design, and support physicians in reassuring patients with SLE that pregnancy and postpartum flares are unlikely if they plan pregnancy during a time of mild or inactive disease, they concluded.
The study was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases. The researchers had no financial conflicts to disclose.
SOURCE: Davis-Porada J et al. Arthritis Res Ther. 2020 Mar 19. doi: 10.1186/s13075-020-2139-9.
Approximately 26% of women with inactive or mild lupus at conception experienced flares at some point during pregnancy, based on data from 384 patients.
Active systemic lupus erythematosus (SLE) is a known predictor of poor pregnancy outcomes, including preterm birth, growth restriction, and fetal loss, but predictors of flares during and after pregnancy in women with SLE have not been well studied, wrote Julia Davis-Porada, MD, of the Hospital for Special Surgery, New York, and her colleagues.
In a study published in Arthritis Research & Therapy, the investigators reviewed data from the PROMISSE (Predictors of Pregnancy Outcome: Biomarkers in Antiphospholipid Antibody Syndrome and Systemic Lupus Erythematosus) study, a prospective study of pregnant women aged 18-45 years. The women were enrolled at less than 12 weeks’ gestation, and participants had a baseline hematocrit greater than 26%. Participants met criteria for inactive or mild/stable disease at the time of conception.
Overall, 20.8% of patients experienced at least one mild or moderate flare and 6.25% had one or more severe flares during pregnancy. Mild to moderate flares and severe flares occurred postpartum (2-6 months after the end of pregnancy) in 22.7% and 1.7% of patients, respectively.
Patients who were younger and those who had lower C4 at baseline and higher Physician Global Assessment scores at baseline were significantly more likely to have at least one flare during pregnancy (P = .003, P = .024, P = .0005, respectively).
In the analysis of postpartum flares, the incidence rates for mild to moderate and severe flares were 0.8 and 0.06 per person-year, respectively. “In contrast to the findings observed for flares that occurred during pregnancy, baseline patient characteristics were not correlated with postpartum flares,” the researchers wrote.
No medications were associated with flares during or after pregnancy.
The study findings were limited by several factors, including the exclusion of SLE patients with current nephritis and those who needed high-dose prednisone; the potential for missed flares; and the lack of postpartum data for approximately 10% of patients, the researchers noted. Also, “since many patients presented to this study only after conception, we have no data to review disease activity prior to pregnancy to determine whether pregnancy per se increased the risk for flare,” they said.
However, the results were strengthened by the large, multiethnic population and prospective study design, and support physicians in reassuring patients with SLE that pregnancy and postpartum flares are unlikely if they plan pregnancy during a time of mild or inactive disease, they concluded.
The study was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases. The researchers had no financial conflicts to disclose.
SOURCE: Davis-Porada J et al. Arthritis Res Ther. 2020 Mar 19. doi: 10.1186/s13075-020-2139-9.
FROM ARTHRITIS RESEARCH & THERAPY
Can drinking more water prevent urinary tract infections?
ILLUSTRATIVE CASE
A 23-year-old nonpregnant woman, whom you treated 3 times in the past year for cystitis, comes to you for follow-up. She wants to know what she can do to prevent another urinary tract infection other than taking prophylactic antibiotics. Should you recommend that this patient increase her daily water intake to prevent recurrent cystitis?
Urinary tract infection (UTI) is the most common bacterial infection encountered in the ambulatory setting. Half of all women report having had at least 1 UTI by the time they are 32 years old.2 Recurrence is also common, with 27% of women having 1 recurrence within 6 months of their first episode.2
Because of growing antimicrobial resistance, the World Health Organization has urged using novel antimicrobial-sparing approaches to infectious diseases.3 Physicians have long recommended behavioral, nonantimicrobial strategies for prevention of recurrent uncomplicated cystitis. Such behavioral recommendations include drinking fluids liberally, urinating after intercourse, not delaying urination, wiping front to back, and avoiding tight-fitting underwear. However, these behavior modification strategies have been studied largely in case-control trials that have yet to find an association between behavior modification and reduced risk of UTI.2 Although unproven as a prevention strategy, increasing daily fluid intake has long been a recommendation because of the belief that it helps to dilute and clear bactiuria.4 This study is the first non–case-control trial to examine the association between increased fluid intake and decreased UTIs.1
STUDY SUMMARY
RCT looks at whether more water leads to fewer UTIs
Hooton and colleagues1 conducted an open-label, randomized controlled trial (RCT) of premenopausal women with recurrent UTIs and low baseline fluid intake and compared increased fluid intake (an additional 1.5 L/d) with no additional fluids. Participants were provided three 500-mL bottles of water per day and were followed for 1 year. Screened women were included if they had 3 or more symptomatic UTIs in the previous year, 1 culture-confirmed UTI, self-reported fluid intake < 1.5 L /d, and were otherwise in good health. Fluid intake was verified by 24-hour urine collection, requiring a volume < 1.2 L and urine osmolality of ≥ 500 mOsm/kg. Exclusion criteria included a history of pyelonephritis within the past year, interstitial cystitis, pregnancy, or current symptoms of UTI.
The primary outcome was frequency of UTI during the study period, defined as 1 urinary symptom and at least 103 CFU/mL uropathogens in a urine culture. Secondary outcomes included the number of antimicrobial agents used, time to first UTI, mean time interval between cystitis episodes, and adverse events.1
A total of 140 participants were randomized with 70 in the water group and 70 in the control group. The mean age of the participants was 35.7 years, and the mean number of reported cystitis episodes was 3.3 in the 12 months prior to the study. By the end of the 12-month study period, mean daily fluid intake had increased by 1.7 L above baseline in the water group. During the 12-month study period, the mean (SD) number of cystitis episodes was 1.7 (95% confidence interval [CI], 1.5-1.8) in the water group compared with 3.2 (95% CI, 3-3.4) in the control group, with a difference in means of 1.5 (95% CI, 1.2-1.8; P < .001).
The mean number of antimicrobial agents used for UTI was 1.9 (95% CI, 1.7-2.2) in the water group and 3.6 (95% CI, 3.3-4) in the control group. The median time to first UTI episode was 148 days in the water group compared with 93.5 days in the control group (hazard ratio [HR] = 0.51; 95% CI, 0.36-0.74; P < .001) and the difference in means for the time interval between UTI episodes was 58.4 days (95% CI, 39.4-77.4; P < .001). No serious adverse events were reported.1
Continue to: WHAT'S NEW
WHAT’S NEW
Proof that increased fluid intake reduces the risk of recurrent UTI
Increasing daily fluid intake is a long-held but previously unproven recommendation. This is the first RCT to show increased daily water intake can reduce the risk of recurrent cystitis in premenopausal patients at high risk for UTI and with low fluid intake. No additional risk of adverse events was found.
CAVEATS
Is there a risk of overhydration?
The study did not address the effect of increasing water intake in women who do not have low-volume fluid intake. Case reports of overhydration emphasize the need to be cautious when making recommendations to hydrate.5 It is not known if physicians should screen for fluid intake at baseline to identify those (with low intake) who would be eligible for this intervention.
CHALLENGES TO IMPLEMENTATION
It’s unclear whether the strategy will work without monitoring
The intervention is both low-risk and low-cost to the patient. However, the intervention was supported by home delivery of water and monthly monitoring interventions that are not typical in normal care. Although the clinical intervention of drinking more fluids (primarily water) appears sound, it is not known whether a physician’s recommendation would result in the same adherence and risk reduction as water delivery and monitoring.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Hooton TM, Vecchio M, Iroz A, et al. Effect of increased daily water intake in premenopausal women with recurrent urinary tract infections: a randomized clinical trial. JAMA Intern Med. 2018;178:1509-1515.
2. Hooton TM. Clinical practice. Uncomplicated urinary tract infection. N Engl J Med. 2012;366:1028-1037.
3. WHO. Antimicrobial resistance: global report on surveillance. April 2014. www.who.int/drugresistance/documents/surveillancereport/en/. Accessed March 23, 2020.
4. Fasugba O, Mitchell BG, McInnes E, et al. Increased fluid intake for the prevention of urinary tract infection in adults and children in all settings: a systematic review. J Hosp Infect. 2020;104:68-77.
5. Lee LC, Noronha M. When plenty is too much: water intoxication in a patient with a simple urinary tract infection. BMJ Case Rep. 2016. doi:10.1136/bcr-2016-216882.
ILLUSTRATIVE CASE
A 23-year-old nonpregnant woman, whom you treated 3 times in the past year for cystitis, comes to you for follow-up. She wants to know what she can do to prevent another urinary tract infection other than taking prophylactic antibiotics. Should you recommend that this patient increase her daily water intake to prevent recurrent cystitis?
Urinary tract infection (UTI) is the most common bacterial infection encountered in the ambulatory setting. Half of all women report having had at least 1 UTI by the time they are 32 years old.2 Recurrence is also common, with 27% of women having 1 recurrence within 6 months of their first episode.2
Because of growing antimicrobial resistance, the World Health Organization has urged using novel antimicrobial-sparing approaches to infectious diseases.3 Physicians have long recommended behavioral, nonantimicrobial strategies for prevention of recurrent uncomplicated cystitis. Such behavioral recommendations include drinking fluids liberally, urinating after intercourse, not delaying urination, wiping front to back, and avoiding tight-fitting underwear. However, these behavior modification strategies have been studied largely in case-control trials that have yet to find an association between behavior modification and reduced risk of UTI.2 Although unproven as a prevention strategy, increasing daily fluid intake has long been a recommendation because of the belief that it helps to dilute and clear bactiuria.4 This study is the first non–case-control trial to examine the association between increased fluid intake and decreased UTIs.1
STUDY SUMMARY
RCT looks at whether more water leads to fewer UTIs
Hooton and colleagues1 conducted an open-label, randomized controlled trial (RCT) of premenopausal women with recurrent UTIs and low baseline fluid intake and compared increased fluid intake (an additional 1.5 L/d) with no additional fluids. Participants were provided three 500-mL bottles of water per day and were followed for 1 year. Screened women were included if they had 3 or more symptomatic UTIs in the previous year, 1 culture-confirmed UTI, self-reported fluid intake < 1.5 L /d, and were otherwise in good health. Fluid intake was verified by 24-hour urine collection, requiring a volume < 1.2 L and urine osmolality of ≥ 500 mOsm/kg. Exclusion criteria included a history of pyelonephritis within the past year, interstitial cystitis, pregnancy, or current symptoms of UTI.
The primary outcome was frequency of UTI during the study period, defined as 1 urinary symptom and at least 103 CFU/mL uropathogens in a urine culture. Secondary outcomes included the number of antimicrobial agents used, time to first UTI, mean time interval between cystitis episodes, and adverse events.1
A total of 140 participants were randomized with 70 in the water group and 70 in the control group. The mean age of the participants was 35.7 years, and the mean number of reported cystitis episodes was 3.3 in the 12 months prior to the study. By the end of the 12-month study period, mean daily fluid intake had increased by 1.7 L above baseline in the water group. During the 12-month study period, the mean (SD) number of cystitis episodes was 1.7 (95% confidence interval [CI], 1.5-1.8) in the water group compared with 3.2 (95% CI, 3-3.4) in the control group, with a difference in means of 1.5 (95% CI, 1.2-1.8; P < .001).
The mean number of antimicrobial agents used for UTI was 1.9 (95% CI, 1.7-2.2) in the water group and 3.6 (95% CI, 3.3-4) in the control group. The median time to first UTI episode was 148 days in the water group compared with 93.5 days in the control group (hazard ratio [HR] = 0.51; 95% CI, 0.36-0.74; P < .001) and the difference in means for the time interval between UTI episodes was 58.4 days (95% CI, 39.4-77.4; P < .001). No serious adverse events were reported.1
Continue to: WHAT'S NEW
WHAT’S NEW
Proof that increased fluid intake reduces the risk of recurrent UTI
Increasing daily fluid intake is a long-held but previously unproven recommendation. This is the first RCT to show increased daily water intake can reduce the risk of recurrent cystitis in premenopausal patients at high risk for UTI and with low fluid intake. No additional risk of adverse events was found.
CAVEATS
Is there a risk of overhydration?
The study did not address the effect of increasing water intake in women who do not have low-volume fluid intake. Case reports of overhydration emphasize the need to be cautious when making recommendations to hydrate.5 It is not known if physicians should screen for fluid intake at baseline to identify those (with low intake) who would be eligible for this intervention.
CHALLENGES TO IMPLEMENTATION
It’s unclear whether the strategy will work without monitoring
The intervention is both low-risk and low-cost to the patient. However, the intervention was supported by home delivery of water and monthly monitoring interventions that are not typical in normal care. Although the clinical intervention of drinking more fluids (primarily water) appears sound, it is not known whether a physician’s recommendation would result in the same adherence and risk reduction as water delivery and monitoring.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
ILLUSTRATIVE CASE
A 23-year-old nonpregnant woman, whom you treated 3 times in the past year for cystitis, comes to you for follow-up. She wants to know what she can do to prevent another urinary tract infection other than taking prophylactic antibiotics. Should you recommend that this patient increase her daily water intake to prevent recurrent cystitis?
Urinary tract infection (UTI) is the most common bacterial infection encountered in the ambulatory setting. Half of all women report having had at least 1 UTI by the time they are 32 years old.2 Recurrence is also common, with 27% of women having 1 recurrence within 6 months of their first episode.2
Because of growing antimicrobial resistance, the World Health Organization has urged using novel antimicrobial-sparing approaches to infectious diseases.3 Physicians have long recommended behavioral, nonantimicrobial strategies for prevention of recurrent uncomplicated cystitis. Such behavioral recommendations include drinking fluids liberally, urinating after intercourse, not delaying urination, wiping front to back, and avoiding tight-fitting underwear. However, these behavior modification strategies have been studied largely in case-control trials that have yet to find an association between behavior modification and reduced risk of UTI.2 Although unproven as a prevention strategy, increasing daily fluid intake has long been a recommendation because of the belief that it helps to dilute and clear bactiuria.4 This study is the first non–case-control trial to examine the association between increased fluid intake and decreased UTIs.1
STUDY SUMMARY
RCT looks at whether more water leads to fewer UTIs
Hooton and colleagues1 conducted an open-label, randomized controlled trial (RCT) of premenopausal women with recurrent UTIs and low baseline fluid intake and compared increased fluid intake (an additional 1.5 L/d) with no additional fluids. Participants were provided three 500-mL bottles of water per day and were followed for 1 year. Screened women were included if they had 3 or more symptomatic UTIs in the previous year, 1 culture-confirmed UTI, self-reported fluid intake < 1.5 L /d, and were otherwise in good health. Fluid intake was verified by 24-hour urine collection, requiring a volume < 1.2 L and urine osmolality of ≥ 500 mOsm/kg. Exclusion criteria included a history of pyelonephritis within the past year, interstitial cystitis, pregnancy, or current symptoms of UTI.
The primary outcome was frequency of UTI during the study period, defined as 1 urinary symptom and at least 103 CFU/mL uropathogens in a urine culture. Secondary outcomes included the number of antimicrobial agents used, time to first UTI, mean time interval between cystitis episodes, and adverse events.1
A total of 140 participants were randomized with 70 in the water group and 70 in the control group. The mean age of the participants was 35.7 years, and the mean number of reported cystitis episodes was 3.3 in the 12 months prior to the study. By the end of the 12-month study period, mean daily fluid intake had increased by 1.7 L above baseline in the water group. During the 12-month study period, the mean (SD) number of cystitis episodes was 1.7 (95% confidence interval [CI], 1.5-1.8) in the water group compared with 3.2 (95% CI, 3-3.4) in the control group, with a difference in means of 1.5 (95% CI, 1.2-1.8; P < .001).
The mean number of antimicrobial agents used for UTI was 1.9 (95% CI, 1.7-2.2) in the water group and 3.6 (95% CI, 3.3-4) in the control group. The median time to first UTI episode was 148 days in the water group compared with 93.5 days in the control group (hazard ratio [HR] = 0.51; 95% CI, 0.36-0.74; P < .001) and the difference in means for the time interval between UTI episodes was 58.4 days (95% CI, 39.4-77.4; P < .001). No serious adverse events were reported.1
Continue to: WHAT'S NEW
WHAT’S NEW
Proof that increased fluid intake reduces the risk of recurrent UTI
Increasing daily fluid intake is a long-held but previously unproven recommendation. This is the first RCT to show increased daily water intake can reduce the risk of recurrent cystitis in premenopausal patients at high risk for UTI and with low fluid intake. No additional risk of adverse events was found.
CAVEATS
Is there a risk of overhydration?
The study did not address the effect of increasing water intake in women who do not have low-volume fluid intake. Case reports of overhydration emphasize the need to be cautious when making recommendations to hydrate.5 It is not known if physicians should screen for fluid intake at baseline to identify those (with low intake) who would be eligible for this intervention.
CHALLENGES TO IMPLEMENTATION
It’s unclear whether the strategy will work without monitoring
The intervention is both low-risk and low-cost to the patient. However, the intervention was supported by home delivery of water and monthly monitoring interventions that are not typical in normal care. Although the clinical intervention of drinking more fluids (primarily water) appears sound, it is not known whether a physician’s recommendation would result in the same adherence and risk reduction as water delivery and monitoring.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Hooton TM, Vecchio M, Iroz A, et al. Effect of increased daily water intake in premenopausal women with recurrent urinary tract infections: a randomized clinical trial. JAMA Intern Med. 2018;178:1509-1515.
2. Hooton TM. Clinical practice. Uncomplicated urinary tract infection. N Engl J Med. 2012;366:1028-1037.
3. WHO. Antimicrobial resistance: global report on surveillance. April 2014. www.who.int/drugresistance/documents/surveillancereport/en/. Accessed March 23, 2020.
4. Fasugba O, Mitchell BG, McInnes E, et al. Increased fluid intake for the prevention of urinary tract infection in adults and children in all settings: a systematic review. J Hosp Infect. 2020;104:68-77.
5. Lee LC, Noronha M. When plenty is too much: water intoxication in a patient with a simple urinary tract infection. BMJ Case Rep. 2016. doi:10.1136/bcr-2016-216882.
1. Hooton TM, Vecchio M, Iroz A, et al. Effect of increased daily water intake in premenopausal women with recurrent urinary tract infections: a randomized clinical trial. JAMA Intern Med. 2018;178:1509-1515.
2. Hooton TM. Clinical practice. Uncomplicated urinary tract infection. N Engl J Med. 2012;366:1028-1037.
3. WHO. Antimicrobial resistance: global report on surveillance. April 2014. www.who.int/drugresistance/documents/surveillancereport/en/. Accessed March 23, 2020.
4. Fasugba O, Mitchell BG, McInnes E, et al. Increased fluid intake for the prevention of urinary tract infection in adults and children in all settings: a systematic review. J Hosp Infect. 2020;104:68-77.
5. Lee LC, Noronha M. When plenty is too much: water intoxication in a patient with a simple urinary tract infection. BMJ Case Rep. 2016. doi:10.1136/bcr-2016-216882.
PRACTICE CHANGER
Advise premenopausal women with recurrent urinary tract infections (UTIs) and low-volume fluid intake to increase their water intake by at least 1.5 liters daily to reduce the frequency of UTIs.1
STRENGTH OF RECOMMENDATION
A: Based on a single, high-quality randomized controlled trial.
Hooton TM, Vecchio M, Iroz A, et al. Effect of increased daily water intake in premenopausal women with recurrent urinary tract infections: a randomized clinical trial. JAMA Intern Med. 2018;178:1509-1515.