User login
A breath of objectivity
How many minutes do you spend each day trying to coax new parents off the guilt train? They have delayed their childbearing until they felt comfortable economically and emotionally ready to raise a child. Convinced that up to this point they have done enough correctly to be considered successful, they see no reason that they won’t be able to tackle parenthood just as easily. Their black lab is a model of obedience. Housebreaking him was a breeze. They are skilled at using the Internet and social media to gather the information they will need for raising a child.
However, at some point in the first 72 hours after the birth of their child, most parents are going to hit the wall of reality. It may be that breastfeeding doesn’t work as well their cousin told them it would or simply that babies cry, often for no discernible reason. Desperately wanting to do what’s right for their child, guilt creeps in as the little failures and fatigue begin to accumulate.
In their search for answers, new parents naturally come to us as pediatricians and family practitioners for the facts, but they also will search the Internet, talk to lactation consultants, and be bombarded by unsolicited advice from family members and neighbors. Every source they turn to, including physicians, will be filtered through its own bias.
I recently came across the most sensible advice for new parents I have read in a long time, and it came not from a pediatrician but from an economics professor at Brown University. Emily Oster, PhD, writing in the New York Times, examines the available data on the topics of breastfeeding, sleep training, and parents working out of the home with the objectivity of an economist and the sensitivity of a mother who has been there and done that (“The Data All Guilt-Ridden Parents Need,” New York Times, April 19, 2019).
For example, she observes that many of the benefits of breastfeeding are supported by some evidence, “just not always especially good evidence. And even when the evidence is good, the benefits are smaller than many people realize.” She points out that “most studies of breastfeeding are biased by the fact that women who breastfeed are typically different from those who do not.” I will leave it to you to read her full discussion that includes a comparison of random trials versus observational studies. But she concludes that, if one relies on only good evidence, the only demonstrable benefit of breastfeeding is for mothers who nurse longer than 12 months who may have a 20%-30% decrease in breast cancer risk.
Using the same kind of careful analysis, Dr. Oster finds that sleep training may have a short-term benefit for parents who will have improved sleep and less maternal depression, but in the long run children who were sleep trained were no different than those that weren’t.
She also finds that, when it comes to the “optimal configuration of adult work hours” for a household, there is “no compelling evidence that proves that having a stay-at-home parent affects child outcomes, positively or negatively.” It is up to each household what works best for all it members, not just the child.
I found it particularly helpful as a practitioner who has often felt shackled, or at least disadvantaged, by the American Academy of Pediatrics’ overly simplistic and sometimes biased recommendations on issues that send my patients’ parents on unfortunate and avoidable guilt trips.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Email him at [email protected].
How many minutes do you spend each day trying to coax new parents off the guilt train? They have delayed their childbearing until they felt comfortable economically and emotionally ready to raise a child. Convinced that up to this point they have done enough correctly to be considered successful, they see no reason that they won’t be able to tackle parenthood just as easily. Their black lab is a model of obedience. Housebreaking him was a breeze. They are skilled at using the Internet and social media to gather the information they will need for raising a child.
However, at some point in the first 72 hours after the birth of their child, most parents are going to hit the wall of reality. It may be that breastfeeding doesn’t work as well their cousin told them it would or simply that babies cry, often for no discernible reason. Desperately wanting to do what’s right for their child, guilt creeps in as the little failures and fatigue begin to accumulate.
In their search for answers, new parents naturally come to us as pediatricians and family practitioners for the facts, but they also will search the Internet, talk to lactation consultants, and be bombarded by unsolicited advice from family members and neighbors. Every source they turn to, including physicians, will be filtered through its own bias.
I recently came across the most sensible advice for new parents I have read in a long time, and it came not from a pediatrician but from an economics professor at Brown University. Emily Oster, PhD, writing in the New York Times, examines the available data on the topics of breastfeeding, sleep training, and parents working out of the home with the objectivity of an economist and the sensitivity of a mother who has been there and done that (“The Data All Guilt-Ridden Parents Need,” New York Times, April 19, 2019).
For example, she observes that many of the benefits of breastfeeding are supported by some evidence, “just not always especially good evidence. And even when the evidence is good, the benefits are smaller than many people realize.” She points out that “most studies of breastfeeding are biased by the fact that women who breastfeed are typically different from those who do not.” I will leave it to you to read her full discussion that includes a comparison of random trials versus observational studies. But she concludes that, if one relies on only good evidence, the only demonstrable benefit of breastfeeding is for mothers who nurse longer than 12 months who may have a 20%-30% decrease in breast cancer risk.
Using the same kind of careful analysis, Dr. Oster finds that sleep training may have a short-term benefit for parents who will have improved sleep and less maternal depression, but in the long run children who were sleep trained were no different than those that weren’t.
She also finds that, when it comes to the “optimal configuration of adult work hours” for a household, there is “no compelling evidence that proves that having a stay-at-home parent affects child outcomes, positively or negatively.” It is up to each household what works best for all it members, not just the child.
I found it particularly helpful as a practitioner who has often felt shackled, or at least disadvantaged, by the American Academy of Pediatrics’ overly simplistic and sometimes biased recommendations on issues that send my patients’ parents on unfortunate and avoidable guilt trips.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Email him at [email protected].
How many minutes do you spend each day trying to coax new parents off the guilt train? They have delayed their childbearing until they felt comfortable economically and emotionally ready to raise a child. Convinced that up to this point they have done enough correctly to be considered successful, they see no reason that they won’t be able to tackle parenthood just as easily. Their black lab is a model of obedience. Housebreaking him was a breeze. They are skilled at using the Internet and social media to gather the information they will need for raising a child.
However, at some point in the first 72 hours after the birth of their child, most parents are going to hit the wall of reality. It may be that breastfeeding doesn’t work as well their cousin told them it would or simply that babies cry, often for no discernible reason. Desperately wanting to do what’s right for their child, guilt creeps in as the little failures and fatigue begin to accumulate.
In their search for answers, new parents naturally come to us as pediatricians and family practitioners for the facts, but they also will search the Internet, talk to lactation consultants, and be bombarded by unsolicited advice from family members and neighbors. Every source they turn to, including physicians, will be filtered through its own bias.
I recently came across the most sensible advice for new parents I have read in a long time, and it came not from a pediatrician but from an economics professor at Brown University. Emily Oster, PhD, writing in the New York Times, examines the available data on the topics of breastfeeding, sleep training, and parents working out of the home with the objectivity of an economist and the sensitivity of a mother who has been there and done that (“The Data All Guilt-Ridden Parents Need,” New York Times, April 19, 2019).
For example, she observes that many of the benefits of breastfeeding are supported by some evidence, “just not always especially good evidence. And even when the evidence is good, the benefits are smaller than many people realize.” She points out that “most studies of breastfeeding are biased by the fact that women who breastfeed are typically different from those who do not.” I will leave it to you to read her full discussion that includes a comparison of random trials versus observational studies. But she concludes that, if one relies on only good evidence, the only demonstrable benefit of breastfeeding is for mothers who nurse longer than 12 months who may have a 20%-30% decrease in breast cancer risk.
Using the same kind of careful analysis, Dr. Oster finds that sleep training may have a short-term benefit for parents who will have improved sleep and less maternal depression, but in the long run children who were sleep trained were no different than those that weren’t.
She also finds that, when it comes to the “optimal configuration of adult work hours” for a household, there is “no compelling evidence that proves that having a stay-at-home parent affects child outcomes, positively or negatively.” It is up to each household what works best for all it members, not just the child.
I found it particularly helpful as a practitioner who has often felt shackled, or at least disadvantaged, by the American Academy of Pediatrics’ overly simplistic and sometimes biased recommendations on issues that send my patients’ parents on unfortunate and avoidable guilt trips.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Email him at [email protected].
Pediatrician knowledge of tampon safety is low
BALTIMORE – and a remarkably high proportion of them lack adequate knowledge themselves about the topic, a new survey-based study found.
“Significant knowledge gaps [were] noted, for instance, [such as] the maximum time a tampon can safely remain in the body,” Miriam Singer of Cohen Children’s Medical Center of New York told attendees of the Pediatric Academic Societies annual meeting.
More than 80% of females aged 17-21 years have used tampons by themselves or with pads, Ms. Singer noted in her background information, yet many teens have low knowledge about their use and safety.
Past research has found that only 35% of high school junior and senior girls heard about tampon use from their mothers, yet many of these mothers showed low knowledge about proper tampon use as well. That same research found that less than 15% of girls aged 10-19 years reported getting information from a health professional about products for menstruation despite recommendations from the American Academy of Pediatrics to instruct girls on feminine hygiene product usage.
Other research has found minimal to no education about menstruation in schools “due to time constraints and stigma associated with menstruation,” Ms. Singer said.
She and her colleagues emailed 2,500 AAP members in November-December 2018 a 53-question online questionnaire about their self-rated and measured knowledge of proper tampon usage and safety and how frequently they discussed tampons with their female adolescent patients. The survey included questions asking pediatricians to self-rate their knowledge about tampon use and safety on a Likert scale of 1 (not at all knowledgeable) to 5 (extremely knowledgeable).
Two incentives provided for completing the survey were a Feminine Hygiene Fact Sheet offered in the first email and an ADHD Medication Guide offered in the third and final email.
Among the 518 pediatricians who responded (21% response rate), 462 met the inclusion criteria of being a primary care pediatrician currently practicing in the United States. Most were women (79%) and white (79%). Just over half of the pediatricians worked only in private practice (54%) and in a suburban area (52%). About a quarter (26%) were in an urban area and 20% in a rural area. Distribution of years in practice (from 1-5 years to over 25 years in 5-year increments) was fairly even across respondents.
Only 9% of respondents reported they very often or almost always talk to their female adolescent patients about how to insert a tampon. The most common tampon-related conversation pediatricians reported was how often to change tampons, which only 35% of respondents said they very often or almost always do.
Yet a similar proportion, 36%, rarely or almost never discuss how often to change tampons, and 62% said they rarely or almost never discuss how to insert a tampon or talk about using tampons while sleeping. Half of respondents (51%) almost never discuss using tampons while swimming (only 21% very often or almost always do), and 77% have not discussed how tampons might affect the hymen with their patients.
More pediatricians (36%) reported almost never discussing the risks of tampon use with female teens than those who sometimes (32%) or very often/almost always (31%) discussed risks.
Respondents also were generally much more willing to discuss tampons with older adolescents than younger ones. Only 18% of respondents said they were highly likely to discuss them with 12- and 13-year-olds, compared with almost twice as many (33%) who would discuss tampons with 16- and 17-year-olds (P less than .001).
Male pediatricians were significantly less likely to discuss any of these topics with their female adolescent patients than female pediatricians (P less than .001 for all questions except risks [P = .01] and hymen [P = .04]). They also rated their knowledge about tampons as significantly lower than self ratings by female pediatricians (P less than .001). Less than half of pediatricians (43%) rated their knowledge about tampons as high or very high, and one in five (20%) rated it as low.
Actual measured knowledge reflected the self-ratings, but still revealed substantial gaps in knowledge among male and female providers. Just over half of male pediatricians (52%) answered all questions about tampon use and safety correctly; however, female pediatricians were only slightly better, with 71% answering all questions correctly (P less than .001). Less than half of male and female pediatricians knew the maximum time a tampon could stay in before it should be removed to reduce risk of toxic shock syndrome (8 hours).
The only two questions that more than half of male pediatricians answered correctly were that girls can swim in the ocean while wearing a tampon and that it can, rarely but not typically, tear the hymen. Less than half knew girls could sleep while wearing a tampon and that a girl could start using a tampon with her first menstruation.
More than half of female pediatricians answered all these questions correctly, although only about two-thirds gave correct answers on how tampons can affect the hymen (the only question that more male pediatricians than female answered correctly), whether a girl can sleep in a tampon, and that patients should use the lowest effective absorbency tampon to minimize toxic shock syndrome risk.
Although the study is limited by a nonvalidated knowledge assessment instrument, self-reporting and potential selection bias means the study may not accurately represent U.S. primary care pediatricians nationwide; however, the findings still demonstrate notably low self-rated and measured knowledge about tampons.
“Given the AAP’s recommendation that pediatricians instruct girls on the use of feminine products, pediatricians must take steps to ensure they are educating patients about tampons,” Ms. Singer said. She also recommended the development of web-based resources targeting the improvement of pediatrician knowledge about tampon use and safety, and the need for the AAP to raise awareness about the importance of discussing tampons with female adolescent patients.
The study did not use external funding, and the authors reported no relevant financial disclosures.
BALTIMORE – and a remarkably high proportion of them lack adequate knowledge themselves about the topic, a new survey-based study found.
“Significant knowledge gaps [were] noted, for instance, [such as] the maximum time a tampon can safely remain in the body,” Miriam Singer of Cohen Children’s Medical Center of New York told attendees of the Pediatric Academic Societies annual meeting.
More than 80% of females aged 17-21 years have used tampons by themselves or with pads, Ms. Singer noted in her background information, yet many teens have low knowledge about their use and safety.
Past research has found that only 35% of high school junior and senior girls heard about tampon use from their mothers, yet many of these mothers showed low knowledge about proper tampon use as well. That same research found that less than 15% of girls aged 10-19 years reported getting information from a health professional about products for menstruation despite recommendations from the American Academy of Pediatrics to instruct girls on feminine hygiene product usage.
Other research has found minimal to no education about menstruation in schools “due to time constraints and stigma associated with menstruation,” Ms. Singer said.
She and her colleagues emailed 2,500 AAP members in November-December 2018 a 53-question online questionnaire about their self-rated and measured knowledge of proper tampon usage and safety and how frequently they discussed tampons with their female adolescent patients. The survey included questions asking pediatricians to self-rate their knowledge about tampon use and safety on a Likert scale of 1 (not at all knowledgeable) to 5 (extremely knowledgeable).
Two incentives provided for completing the survey were a Feminine Hygiene Fact Sheet offered in the first email and an ADHD Medication Guide offered in the third and final email.
Among the 518 pediatricians who responded (21% response rate), 462 met the inclusion criteria of being a primary care pediatrician currently practicing in the United States. Most were women (79%) and white (79%). Just over half of the pediatricians worked only in private practice (54%) and in a suburban area (52%). About a quarter (26%) were in an urban area and 20% in a rural area. Distribution of years in practice (from 1-5 years to over 25 years in 5-year increments) was fairly even across respondents.
Only 9% of respondents reported they very often or almost always talk to their female adolescent patients about how to insert a tampon. The most common tampon-related conversation pediatricians reported was how often to change tampons, which only 35% of respondents said they very often or almost always do.
Yet a similar proportion, 36%, rarely or almost never discuss how often to change tampons, and 62% said they rarely or almost never discuss how to insert a tampon or talk about using tampons while sleeping. Half of respondents (51%) almost never discuss using tampons while swimming (only 21% very often or almost always do), and 77% have not discussed how tampons might affect the hymen with their patients.
More pediatricians (36%) reported almost never discussing the risks of tampon use with female teens than those who sometimes (32%) or very often/almost always (31%) discussed risks.
Respondents also were generally much more willing to discuss tampons with older adolescents than younger ones. Only 18% of respondents said they were highly likely to discuss them with 12- and 13-year-olds, compared with almost twice as many (33%) who would discuss tampons with 16- and 17-year-olds (P less than .001).
Male pediatricians were significantly less likely to discuss any of these topics with their female adolescent patients than female pediatricians (P less than .001 for all questions except risks [P = .01] and hymen [P = .04]). They also rated their knowledge about tampons as significantly lower than self ratings by female pediatricians (P less than .001). Less than half of pediatricians (43%) rated their knowledge about tampons as high or very high, and one in five (20%) rated it as low.
Actual measured knowledge reflected the self-ratings, but still revealed substantial gaps in knowledge among male and female providers. Just over half of male pediatricians (52%) answered all questions about tampon use and safety correctly; however, female pediatricians were only slightly better, with 71% answering all questions correctly (P less than .001). Less than half of male and female pediatricians knew the maximum time a tampon could stay in before it should be removed to reduce risk of toxic shock syndrome (8 hours).
The only two questions that more than half of male pediatricians answered correctly were that girls can swim in the ocean while wearing a tampon and that it can, rarely but not typically, tear the hymen. Less than half knew girls could sleep while wearing a tampon and that a girl could start using a tampon with her first menstruation.
More than half of female pediatricians answered all these questions correctly, although only about two-thirds gave correct answers on how tampons can affect the hymen (the only question that more male pediatricians than female answered correctly), whether a girl can sleep in a tampon, and that patients should use the lowest effective absorbency tampon to minimize toxic shock syndrome risk.
Although the study is limited by a nonvalidated knowledge assessment instrument, self-reporting and potential selection bias means the study may not accurately represent U.S. primary care pediatricians nationwide; however, the findings still demonstrate notably low self-rated and measured knowledge about tampons.
“Given the AAP’s recommendation that pediatricians instruct girls on the use of feminine products, pediatricians must take steps to ensure they are educating patients about tampons,” Ms. Singer said. She also recommended the development of web-based resources targeting the improvement of pediatrician knowledge about tampon use and safety, and the need for the AAP to raise awareness about the importance of discussing tampons with female adolescent patients.
The study did not use external funding, and the authors reported no relevant financial disclosures.
BALTIMORE – and a remarkably high proportion of them lack adequate knowledge themselves about the topic, a new survey-based study found.
“Significant knowledge gaps [were] noted, for instance, [such as] the maximum time a tampon can safely remain in the body,” Miriam Singer of Cohen Children’s Medical Center of New York told attendees of the Pediatric Academic Societies annual meeting.
More than 80% of females aged 17-21 years have used tampons by themselves or with pads, Ms. Singer noted in her background information, yet many teens have low knowledge about their use and safety.
Past research has found that only 35% of high school junior and senior girls heard about tampon use from their mothers, yet many of these mothers showed low knowledge about proper tampon use as well. That same research found that less than 15% of girls aged 10-19 years reported getting information from a health professional about products for menstruation despite recommendations from the American Academy of Pediatrics to instruct girls on feminine hygiene product usage.
Other research has found minimal to no education about menstruation in schools “due to time constraints and stigma associated with menstruation,” Ms. Singer said.
She and her colleagues emailed 2,500 AAP members in November-December 2018 a 53-question online questionnaire about their self-rated and measured knowledge of proper tampon usage and safety and how frequently they discussed tampons with their female adolescent patients. The survey included questions asking pediatricians to self-rate their knowledge about tampon use and safety on a Likert scale of 1 (not at all knowledgeable) to 5 (extremely knowledgeable).
Two incentives provided for completing the survey were a Feminine Hygiene Fact Sheet offered in the first email and an ADHD Medication Guide offered in the third and final email.
Among the 518 pediatricians who responded (21% response rate), 462 met the inclusion criteria of being a primary care pediatrician currently practicing in the United States. Most were women (79%) and white (79%). Just over half of the pediatricians worked only in private practice (54%) and in a suburban area (52%). About a quarter (26%) were in an urban area and 20% in a rural area. Distribution of years in practice (from 1-5 years to over 25 years in 5-year increments) was fairly even across respondents.
Only 9% of respondents reported they very often or almost always talk to their female adolescent patients about how to insert a tampon. The most common tampon-related conversation pediatricians reported was how often to change tampons, which only 35% of respondents said they very often or almost always do.
Yet a similar proportion, 36%, rarely or almost never discuss how often to change tampons, and 62% said they rarely or almost never discuss how to insert a tampon or talk about using tampons while sleeping. Half of respondents (51%) almost never discuss using tampons while swimming (only 21% very often or almost always do), and 77% have not discussed how tampons might affect the hymen with their patients.
More pediatricians (36%) reported almost never discussing the risks of tampon use with female teens than those who sometimes (32%) or very often/almost always (31%) discussed risks.
Respondents also were generally much more willing to discuss tampons with older adolescents than younger ones. Only 18% of respondents said they were highly likely to discuss them with 12- and 13-year-olds, compared with almost twice as many (33%) who would discuss tampons with 16- and 17-year-olds (P less than .001).
Male pediatricians were significantly less likely to discuss any of these topics with their female adolescent patients than female pediatricians (P less than .001 for all questions except risks [P = .01] and hymen [P = .04]). They also rated their knowledge about tampons as significantly lower than self ratings by female pediatricians (P less than .001). Less than half of pediatricians (43%) rated their knowledge about tampons as high or very high, and one in five (20%) rated it as low.
Actual measured knowledge reflected the self-ratings, but still revealed substantial gaps in knowledge among male and female providers. Just over half of male pediatricians (52%) answered all questions about tampon use and safety correctly; however, female pediatricians were only slightly better, with 71% answering all questions correctly (P less than .001). Less than half of male and female pediatricians knew the maximum time a tampon could stay in before it should be removed to reduce risk of toxic shock syndrome (8 hours).
The only two questions that more than half of male pediatricians answered correctly were that girls can swim in the ocean while wearing a tampon and that it can, rarely but not typically, tear the hymen. Less than half knew girls could sleep while wearing a tampon and that a girl could start using a tampon with her first menstruation.
More than half of female pediatricians answered all these questions correctly, although only about two-thirds gave correct answers on how tampons can affect the hymen (the only question that more male pediatricians than female answered correctly), whether a girl can sleep in a tampon, and that patients should use the lowest effective absorbency tampon to minimize toxic shock syndrome risk.
Although the study is limited by a nonvalidated knowledge assessment instrument, self-reporting and potential selection bias means the study may not accurately represent U.S. primary care pediatricians nationwide; however, the findings still demonstrate notably low self-rated and measured knowledge about tampons.
“Given the AAP’s recommendation that pediatricians instruct girls on the use of feminine products, pediatricians must take steps to ensure they are educating patients about tampons,” Ms. Singer said. She also recommended the development of web-based resources targeting the improvement of pediatrician knowledge about tampon use and safety, and the need for the AAP to raise awareness about the importance of discussing tampons with female adolescent patients.
The study did not use external funding, and the authors reported no relevant financial disclosures.
REPORTING FROM PAS 2019
Key clinical point: U.S. pediatricians have low knowledge of and willingness to discuss proper tampon use and safety with adolescent patients.
Major finding: 35% of U.S. pediatricians reported they very often/almost always discuss how long to wear a tampon before removing it.
Study details: The findings are based on a survey of 462 U.S. pediatricians who responded to a 53-question online survey.
Disclosures: The study did not use external funding, and the authors reported no relevant financial disclosures.
Magnetic beads functionalized with VEGF could treat preeclampsia
A method of apheresis using vascular endothelial growth factor functionalized magnetic beads reduced levels of the soluble form of the vascular endothelial growth factor 1 in blood from women with preeclampsia, according to recent research published in the journal Hypertension.
The approach both reduces levels of the soluble form of the vascular endothelial growth factor 1 (sFlt-1) and releases placental growth factor (PlGF), which could help restore endothelial function in women with preeclampsia. The researchers said they chose sFlt-1 as a target because of “mounting evidence of its involvement in the pathogenesis of preeclampsia.” sFlt-1 has been suspected of inhibiting angiogenic signaling through “direct sequestration of angiogenic ligands” vascular endothelial growth factor (VEGF) and PlGF as well as “dominant-negative heterodimerization with surface VEGFRs.”
“During normal pregnancy, massive amounts of PlGF are produced by the placenta, reaching concentrations of free PlGF around 400 pg/mL, whereas during preeclampsia, free PlGF is extremely low due to the release of sFlt-1 into the maternal circulation,” the researchers said.
Using VEGF-functionalized magnetic beads, the researchers performed static and dynamic experiments using phosphate buffered saline (PBS), conditioned media, and plasma from women with preeclampsia. Under static conditions, there was a decrease of 33% for sFlt-1 and an increase of 27% for PlGF, while in dynamic conditions, there was a 40% decrease in sFlt-1 and a twofold increase in freed PlGF. When tested with plasma from women with preeclampsia, the ratio of sFlt-1/PlGF decreased by 63%, and VEGF release was associated with apheresis.
“This was a proof of concept study and our approach aims to restore physiologic levels of angiogenic factors,” Vassilis Tsatsaris, MD, PhD, of Cochin Hospital, Paris, said in a press release. “The reduction of sFlt-1 and the release of angiogenic factors is very significant and promising.”
Dr. Tsatsaris and his colleagues noted their next steps are to optimize the process of reducing sFlt-1 and examining how the approach works in an animal model.
“During normal pregnancy, circulating free VEGF levels are very low, almost undetectable with noncompetitive [enzyme-linked immunosorbent assay] ELISA. Whether these extremely low levels of VEGF have a physiological role during pregnancy is not known,” they wrote.
This study was funded by Agence Nationale pour la recherche, Institut Pierre Gilles de Gennes and the PremUP Foundation. One author reported receiving a grant from the Ecole Normale Supérieure and a second author reported receiving a grant from the Fondation pour la Recherche Médicale. The other authors report no relevant conflicts of interest.
SOURCE: Trapiella-Alfonso L et al. Hypertension. 2019. doi: 10.1161/HYPERTENSIONAHA.118.12380.
A method of apheresis using vascular endothelial growth factor functionalized magnetic beads reduced levels of the soluble form of the vascular endothelial growth factor 1 in blood from women with preeclampsia, according to recent research published in the journal Hypertension.
The approach both reduces levels of the soluble form of the vascular endothelial growth factor 1 (sFlt-1) and releases placental growth factor (PlGF), which could help restore endothelial function in women with preeclampsia. The researchers said they chose sFlt-1 as a target because of “mounting evidence of its involvement in the pathogenesis of preeclampsia.” sFlt-1 has been suspected of inhibiting angiogenic signaling through “direct sequestration of angiogenic ligands” vascular endothelial growth factor (VEGF) and PlGF as well as “dominant-negative heterodimerization with surface VEGFRs.”
“During normal pregnancy, massive amounts of PlGF are produced by the placenta, reaching concentrations of free PlGF around 400 pg/mL, whereas during preeclampsia, free PlGF is extremely low due to the release of sFlt-1 into the maternal circulation,” the researchers said.
Using VEGF-functionalized magnetic beads, the researchers performed static and dynamic experiments using phosphate buffered saline (PBS), conditioned media, and plasma from women with preeclampsia. Under static conditions, there was a decrease of 33% for sFlt-1 and an increase of 27% for PlGF, while in dynamic conditions, there was a 40% decrease in sFlt-1 and a twofold increase in freed PlGF. When tested with plasma from women with preeclampsia, the ratio of sFlt-1/PlGF decreased by 63%, and VEGF release was associated with apheresis.
“This was a proof of concept study and our approach aims to restore physiologic levels of angiogenic factors,” Vassilis Tsatsaris, MD, PhD, of Cochin Hospital, Paris, said in a press release. “The reduction of sFlt-1 and the release of angiogenic factors is very significant and promising.”
Dr. Tsatsaris and his colleagues noted their next steps are to optimize the process of reducing sFlt-1 and examining how the approach works in an animal model.
“During normal pregnancy, circulating free VEGF levels are very low, almost undetectable with noncompetitive [enzyme-linked immunosorbent assay] ELISA. Whether these extremely low levels of VEGF have a physiological role during pregnancy is not known,” they wrote.
This study was funded by Agence Nationale pour la recherche, Institut Pierre Gilles de Gennes and the PremUP Foundation. One author reported receiving a grant from the Ecole Normale Supérieure and a second author reported receiving a grant from the Fondation pour la Recherche Médicale. The other authors report no relevant conflicts of interest.
SOURCE: Trapiella-Alfonso L et al. Hypertension. 2019. doi: 10.1161/HYPERTENSIONAHA.118.12380.
A method of apheresis using vascular endothelial growth factor functionalized magnetic beads reduced levels of the soluble form of the vascular endothelial growth factor 1 in blood from women with preeclampsia, according to recent research published in the journal Hypertension.
The approach both reduces levels of the soluble form of the vascular endothelial growth factor 1 (sFlt-1) and releases placental growth factor (PlGF), which could help restore endothelial function in women with preeclampsia. The researchers said they chose sFlt-1 as a target because of “mounting evidence of its involvement in the pathogenesis of preeclampsia.” sFlt-1 has been suspected of inhibiting angiogenic signaling through “direct sequestration of angiogenic ligands” vascular endothelial growth factor (VEGF) and PlGF as well as “dominant-negative heterodimerization with surface VEGFRs.”
“During normal pregnancy, massive amounts of PlGF are produced by the placenta, reaching concentrations of free PlGF around 400 pg/mL, whereas during preeclampsia, free PlGF is extremely low due to the release of sFlt-1 into the maternal circulation,” the researchers said.
Using VEGF-functionalized magnetic beads, the researchers performed static and dynamic experiments using phosphate buffered saline (PBS), conditioned media, and plasma from women with preeclampsia. Under static conditions, there was a decrease of 33% for sFlt-1 and an increase of 27% for PlGF, while in dynamic conditions, there was a 40% decrease in sFlt-1 and a twofold increase in freed PlGF. When tested with plasma from women with preeclampsia, the ratio of sFlt-1/PlGF decreased by 63%, and VEGF release was associated with apheresis.
“This was a proof of concept study and our approach aims to restore physiologic levels of angiogenic factors,” Vassilis Tsatsaris, MD, PhD, of Cochin Hospital, Paris, said in a press release. “The reduction of sFlt-1 and the release of angiogenic factors is very significant and promising.”
Dr. Tsatsaris and his colleagues noted their next steps are to optimize the process of reducing sFlt-1 and examining how the approach works in an animal model.
“During normal pregnancy, circulating free VEGF levels are very low, almost undetectable with noncompetitive [enzyme-linked immunosorbent assay] ELISA. Whether these extremely low levels of VEGF have a physiological role during pregnancy is not known,” they wrote.
This study was funded by Agence Nationale pour la recherche, Institut Pierre Gilles de Gennes and the PremUP Foundation. One author reported receiving a grant from the Ecole Normale Supérieure and a second author reported receiving a grant from the Fondation pour la Recherche Médicale. The other authors report no relevant conflicts of interest.
SOURCE: Trapiella-Alfonso L et al. Hypertension. 2019. doi: 10.1161/HYPERTENSIONAHA.118.12380.
FROM HYPERTENSION
Key clinical point: Use of magnetic beads functionalized with vascular endothelial growth factor (VEGF) reduced the soluble form of endothelial growth factor 1 (sFlt-1) in the blood of women with preeclampsia.
Major finding: sFlt-1 was reduced by 40% under dynamic conditions, and there was a twofold increase in the amount of freed placental growth factor.
Study details: A proof-of-concept study using VEGF-functionalized magnetic beads and phosphate buffered saline (PBS), conditioned media, and plasma from women with preeclampsia.
Disclosures: This study was funded by Agence Nationale pour la recherche, Institut Pierre Gilles de Gennes, and the PremUP Foundation. One author reported receiving a grant from the Ecole Normale Supérieure and a second author reported receiving a grant from the Fondation pour la Recherche Médicale. The other authors reported no relevant conflicts of interest.
Source: Trapiella-Alfonso L et al. Hypertension. 2019. doi: 10.1161/HYPERTENSIONAHA.118.12380.
2019 Update: Contraceptives and unintended pregnancy rates
NASHVILLE, TENN. – The unintended pregnancy rate is declining after years of hovering at close to 50%.
While the rates among women of color remain high – currently at 58 and 79 per 1,000 women aged 15-44 years for Hispanic and black women, respectively – they have declined from 79 and 92 per 1,000 Hispanic and black women in that age group in 2008, and the overall rate is now at about 45%, Eve Espey, MD, said at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists.
“Considering the scope and number of women affected by unplanned pregnancy, this is actually a huge public health achievement,” said Dr. Espey, professor and chair of the department of obstetrics & gynecology at the University of New Mexico, Albuquerque.
she noted, adding that “another enormous determinant of this decrease in unintended pregnancy is the use of long-acting reversible contraception [LARC].” About 2% of women used contraceptives in 2002, and now, based on the latest cycle of data from 2015-2017, 16% of women use contraceptives.
In this video interview, Dr. Espey discusses the main points of her talk entitled “Contraceptives: What you need to know in 2019,” including:
- The importance of “following reproductive justice–based principles and counseling” when it comes to prescribing contraceptives.
- The latest data showing that certain LARC methods remain safe and effective beyond their approved duration of use.
- Trends with respect to tubal ligation and salpingectomy.
- The value of the Centers for Disease Control and Prevention’s U.S. Medical Eligibility Criteria (MEC) for evidence-based guidance on selecting contraceptives based on patients’ individual needs.
“[MEC] is something every ob.gyn. should consider using,” she said, noting that access is available through a free app. “As our patients are more complex and have more comorbidities, it’s particularly helpful for matching up patients and their conditions with recommendations for specific contraceptive methods.”
Dr. Espey reported having no financial disclosures.
NASHVILLE, TENN. – The unintended pregnancy rate is declining after years of hovering at close to 50%.
While the rates among women of color remain high – currently at 58 and 79 per 1,000 women aged 15-44 years for Hispanic and black women, respectively – they have declined from 79 and 92 per 1,000 Hispanic and black women in that age group in 2008, and the overall rate is now at about 45%, Eve Espey, MD, said at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists.
“Considering the scope and number of women affected by unplanned pregnancy, this is actually a huge public health achievement,” said Dr. Espey, professor and chair of the department of obstetrics & gynecology at the University of New Mexico, Albuquerque.
she noted, adding that “another enormous determinant of this decrease in unintended pregnancy is the use of long-acting reversible contraception [LARC].” About 2% of women used contraceptives in 2002, and now, based on the latest cycle of data from 2015-2017, 16% of women use contraceptives.
In this video interview, Dr. Espey discusses the main points of her talk entitled “Contraceptives: What you need to know in 2019,” including:
- The importance of “following reproductive justice–based principles and counseling” when it comes to prescribing contraceptives.
- The latest data showing that certain LARC methods remain safe and effective beyond their approved duration of use.
- Trends with respect to tubal ligation and salpingectomy.
- The value of the Centers for Disease Control and Prevention’s U.S. Medical Eligibility Criteria (MEC) for evidence-based guidance on selecting contraceptives based on patients’ individual needs.
“[MEC] is something every ob.gyn. should consider using,” she said, noting that access is available through a free app. “As our patients are more complex and have more comorbidities, it’s particularly helpful for matching up patients and their conditions with recommendations for specific contraceptive methods.”
Dr. Espey reported having no financial disclosures.
NASHVILLE, TENN. – The unintended pregnancy rate is declining after years of hovering at close to 50%.
While the rates among women of color remain high – currently at 58 and 79 per 1,000 women aged 15-44 years for Hispanic and black women, respectively – they have declined from 79 and 92 per 1,000 Hispanic and black women in that age group in 2008, and the overall rate is now at about 45%, Eve Espey, MD, said at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists.
“Considering the scope and number of women affected by unplanned pregnancy, this is actually a huge public health achievement,” said Dr. Espey, professor and chair of the department of obstetrics & gynecology at the University of New Mexico, Albuquerque.
she noted, adding that “another enormous determinant of this decrease in unintended pregnancy is the use of long-acting reversible contraception [LARC].” About 2% of women used contraceptives in 2002, and now, based on the latest cycle of data from 2015-2017, 16% of women use contraceptives.
In this video interview, Dr. Espey discusses the main points of her talk entitled “Contraceptives: What you need to know in 2019,” including:
- The importance of “following reproductive justice–based principles and counseling” when it comes to prescribing contraceptives.
- The latest data showing that certain LARC methods remain safe and effective beyond their approved duration of use.
- Trends with respect to tubal ligation and salpingectomy.
- The value of the Centers for Disease Control and Prevention’s U.S. Medical Eligibility Criteria (MEC) for evidence-based guidance on selecting contraceptives based on patients’ individual needs.
“[MEC] is something every ob.gyn. should consider using,” she said, noting that access is available through a free app. “As our patients are more complex and have more comorbidities, it’s particularly helpful for matching up patients and their conditions with recommendations for specific contraceptive methods.”
Dr. Espey reported having no financial disclosures.
EXPERT ANALYSIS FROM ACOG 2019
Managing 2nd trimester loss: Shared decision making, honor patient preference
NASHVILLE, TENN. – according to Sara W. Prager, MD.
Information transfer between the physician and patient, as opposed to a provider-driven or patient-driven decision-making process, better ensures that “the best possible decision” will be reached, Dr. Prager, director of the family planning division and family planning fellowship at the University of Washington in Seattle, said at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists.
Engaging the patient in the process – actively involving and supporting her in health care and treatment decision-making activities – is critically important, especially when dealing with pregnancy loss, which involves an acute sense of powerlessness, she said. Patient engagement is essential for respecting her autonomy, enhancing her agency, improving health status, reducing decisional conflict, and improving overall satisfaction.
Shared decision making requires a discussion about how the two approaches compare, particularly with respect to specific complications associated with each, Dr. Prager said, noting that discussion of values also should be encouraged.
Although surgical management is used more often, both approaches are safe and effective, and in the absence of clear contraindications in settings where both medication and a practitioner skilled in dilatation and evacuation are available, patient preference should honored, she said.
In this video interview, Dr. Prager further explains her position. “Using evidence-based medicine to have a shared decision-making process ... is extremely helpful for patients to feel like they have some control in this out-of-control situation where they’re experiencing a pregnancy loss.”
She also discussed how the use of mifepristone plus misoprostol for medical management of second-trimester loss has the potential to improve access.
“This is medication that, because of stigma surrounding abortion, is not always available ... so actually using it for non–abortion-related activities can be a way to help reduce that stigma around the medication itself, and get it into clinical sites, because it really does meaningfully improve management in the second trimester, as well as in the first trimester.”
In fact, the combination can cut nearly in half the amount of time it takes from the start of an induction until the end of the induction process, she said.
Dr. Prager also discussed surgical training resources and how to advocate for patient access to family planning experts who have the appropriate training.
Dr. Prager said she had no relevant financial disclosures.
NASHVILLE, TENN. – according to Sara W. Prager, MD.
Information transfer between the physician and patient, as opposed to a provider-driven or patient-driven decision-making process, better ensures that “the best possible decision” will be reached, Dr. Prager, director of the family planning division and family planning fellowship at the University of Washington in Seattle, said at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists.
Engaging the patient in the process – actively involving and supporting her in health care and treatment decision-making activities – is critically important, especially when dealing with pregnancy loss, which involves an acute sense of powerlessness, she said. Patient engagement is essential for respecting her autonomy, enhancing her agency, improving health status, reducing decisional conflict, and improving overall satisfaction.
Shared decision making requires a discussion about how the two approaches compare, particularly with respect to specific complications associated with each, Dr. Prager said, noting that discussion of values also should be encouraged.
Although surgical management is used more often, both approaches are safe and effective, and in the absence of clear contraindications in settings where both medication and a practitioner skilled in dilatation and evacuation are available, patient preference should honored, she said.
In this video interview, Dr. Prager further explains her position. “Using evidence-based medicine to have a shared decision-making process ... is extremely helpful for patients to feel like they have some control in this out-of-control situation where they’re experiencing a pregnancy loss.”
She also discussed how the use of mifepristone plus misoprostol for medical management of second-trimester loss has the potential to improve access.
“This is medication that, because of stigma surrounding abortion, is not always available ... so actually using it for non–abortion-related activities can be a way to help reduce that stigma around the medication itself, and get it into clinical sites, because it really does meaningfully improve management in the second trimester, as well as in the first trimester.”
In fact, the combination can cut nearly in half the amount of time it takes from the start of an induction until the end of the induction process, she said.
Dr. Prager also discussed surgical training resources and how to advocate for patient access to family planning experts who have the appropriate training.
Dr. Prager said she had no relevant financial disclosures.
NASHVILLE, TENN. – according to Sara W. Prager, MD.
Information transfer between the physician and patient, as opposed to a provider-driven or patient-driven decision-making process, better ensures that “the best possible decision” will be reached, Dr. Prager, director of the family planning division and family planning fellowship at the University of Washington in Seattle, said at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists.
Engaging the patient in the process – actively involving and supporting her in health care and treatment decision-making activities – is critically important, especially when dealing with pregnancy loss, which involves an acute sense of powerlessness, she said. Patient engagement is essential for respecting her autonomy, enhancing her agency, improving health status, reducing decisional conflict, and improving overall satisfaction.
Shared decision making requires a discussion about how the two approaches compare, particularly with respect to specific complications associated with each, Dr. Prager said, noting that discussion of values also should be encouraged.
Although surgical management is used more often, both approaches are safe and effective, and in the absence of clear contraindications in settings where both medication and a practitioner skilled in dilatation and evacuation are available, patient preference should honored, she said.
In this video interview, Dr. Prager further explains her position. “Using evidence-based medicine to have a shared decision-making process ... is extremely helpful for patients to feel like they have some control in this out-of-control situation where they’re experiencing a pregnancy loss.”
She also discussed how the use of mifepristone plus misoprostol for medical management of second-trimester loss has the potential to improve access.
“This is medication that, because of stigma surrounding abortion, is not always available ... so actually using it for non–abortion-related activities can be a way to help reduce that stigma around the medication itself, and get it into clinical sites, because it really does meaningfully improve management in the second trimester, as well as in the first trimester.”
In fact, the combination can cut nearly in half the amount of time it takes from the start of an induction until the end of the induction process, she said.
Dr. Prager also discussed surgical training resources and how to advocate for patient access to family planning experts who have the appropriate training.
Dr. Prager said she had no relevant financial disclosures.
EXPERT ANALYSIS FROM ACOG 2019
Is routine induction of labor in a healthy pregnancy at 39 weeks reasonable?
NASHVILLE, TENN. – Aaron B. Caughey, MD, PhD, discussed this in a video at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists.
In a healthy pregnancy, with no medical indications for induction of labor, 39-40 weeks’ gestation is a time when there is a relatively low risk of stillbirth, although the risk is not zero, Dr. Caughey explained. The same is true for neonatal death. This gestational age is a time when there is a low risk for respiratory complications and a low risk for meconium.
“This might be a nice time to have a baby,” said Dr. Caughey, professor and chair of the department of obstetrics and gynecology at Oregon Health & Science University, Portland. “The trade-off is intervention. Don’t you increase the risk of C-sections?”
Actually, numerous retrospective studies have shown that there is either no difference or a decreased rate of C-sections with induction of labor at 39-40 weeks’ gestation, compared with expectant management.
These findings led to a prospective, randomized study by William A. Grobman, MD, and associates for the Eunice Kennedy Shriver National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network called the ARRIVE trial (N Engl J Med. 2018;379:513-23). In that trial, the investigators randomized 3,062 women to induction of labor and 3,044 to expectant management. A significantly lower percentage of women in the induction of labor group underwent C-section than did women randomized to expectant management: 19% vs. 22% (relative risk, 0.84; P less than .001) – that is, 16% fewer C-sections. Also, 36% fewer women in the induction of labor group experienced preeclampsia. No significant differences were found between the two groups in terms of neonatal outcomes.
However, this is just one study, Dr. Caughey noted. What does is mean for a local community hospital? What does it mean for a busy private obstetrics practice?
Watch this video for his answer.
NASHVILLE, TENN. – Aaron B. Caughey, MD, PhD, discussed this in a video at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists.
In a healthy pregnancy, with no medical indications for induction of labor, 39-40 weeks’ gestation is a time when there is a relatively low risk of stillbirth, although the risk is not zero, Dr. Caughey explained. The same is true for neonatal death. This gestational age is a time when there is a low risk for respiratory complications and a low risk for meconium.
“This might be a nice time to have a baby,” said Dr. Caughey, professor and chair of the department of obstetrics and gynecology at Oregon Health & Science University, Portland. “The trade-off is intervention. Don’t you increase the risk of C-sections?”
Actually, numerous retrospective studies have shown that there is either no difference or a decreased rate of C-sections with induction of labor at 39-40 weeks’ gestation, compared with expectant management.
These findings led to a prospective, randomized study by William A. Grobman, MD, and associates for the Eunice Kennedy Shriver National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network called the ARRIVE trial (N Engl J Med. 2018;379:513-23). In that trial, the investigators randomized 3,062 women to induction of labor and 3,044 to expectant management. A significantly lower percentage of women in the induction of labor group underwent C-section than did women randomized to expectant management: 19% vs. 22% (relative risk, 0.84; P less than .001) – that is, 16% fewer C-sections. Also, 36% fewer women in the induction of labor group experienced preeclampsia. No significant differences were found between the two groups in terms of neonatal outcomes.
However, this is just one study, Dr. Caughey noted. What does is mean for a local community hospital? What does it mean for a busy private obstetrics practice?
Watch this video for his answer.
NASHVILLE, TENN. – Aaron B. Caughey, MD, PhD, discussed this in a video at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists.
In a healthy pregnancy, with no medical indications for induction of labor, 39-40 weeks’ gestation is a time when there is a relatively low risk of stillbirth, although the risk is not zero, Dr. Caughey explained. The same is true for neonatal death. This gestational age is a time when there is a low risk for respiratory complications and a low risk for meconium.
“This might be a nice time to have a baby,” said Dr. Caughey, professor and chair of the department of obstetrics and gynecology at Oregon Health & Science University, Portland. “The trade-off is intervention. Don’t you increase the risk of C-sections?”
Actually, numerous retrospective studies have shown that there is either no difference or a decreased rate of C-sections with induction of labor at 39-40 weeks’ gestation, compared with expectant management.
These findings led to a prospective, randomized study by William A. Grobman, MD, and associates for the Eunice Kennedy Shriver National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network called the ARRIVE trial (N Engl J Med. 2018;379:513-23). In that trial, the investigators randomized 3,062 women to induction of labor and 3,044 to expectant management. A significantly lower percentage of women in the induction of labor group underwent C-section than did women randomized to expectant management: 19% vs. 22% (relative risk, 0.84; P less than .001) – that is, 16% fewer C-sections. Also, 36% fewer women in the induction of labor group experienced preeclampsia. No significant differences were found between the two groups in terms of neonatal outcomes.
However, this is just one study, Dr. Caughey noted. What does is mean for a local community hospital? What does it mean for a busy private obstetrics practice?
Watch this video for his answer.
REPORTING FROM ACOG 2019
2019 USPSTF update
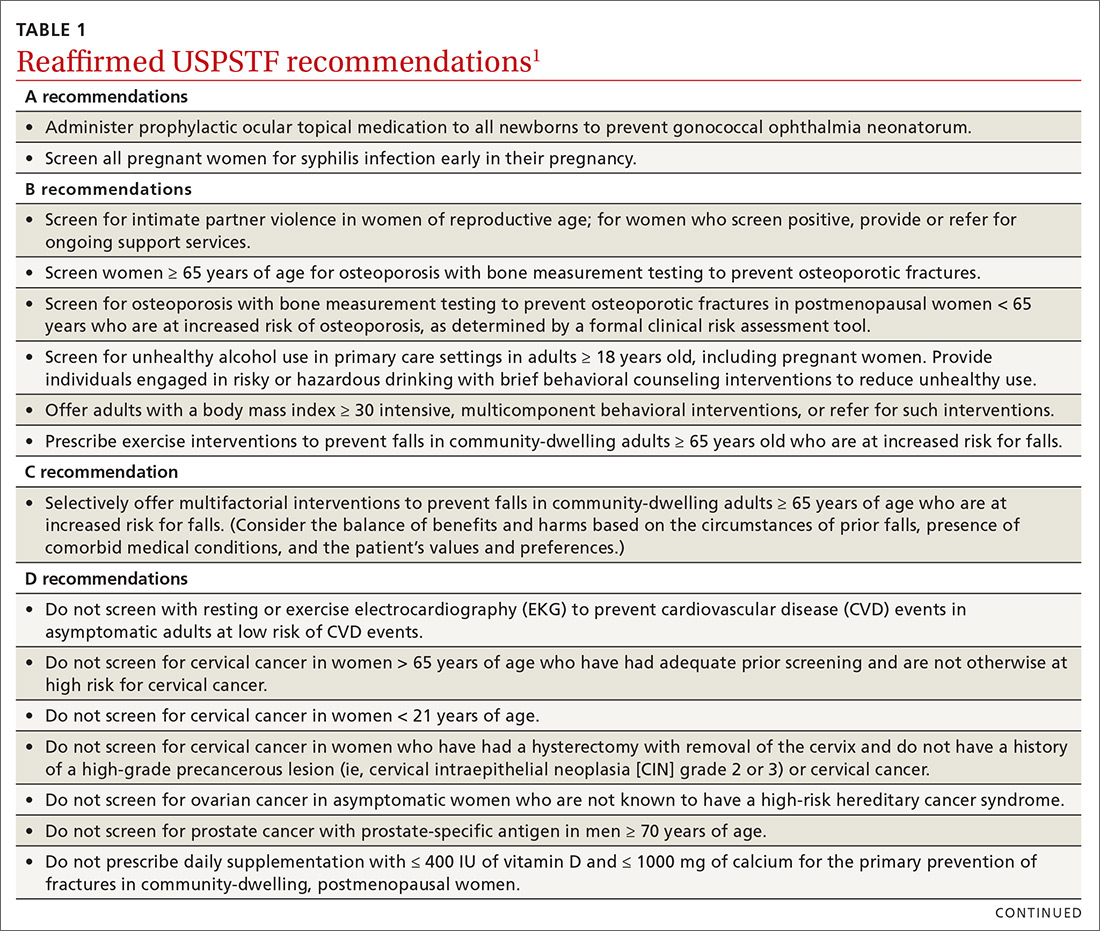
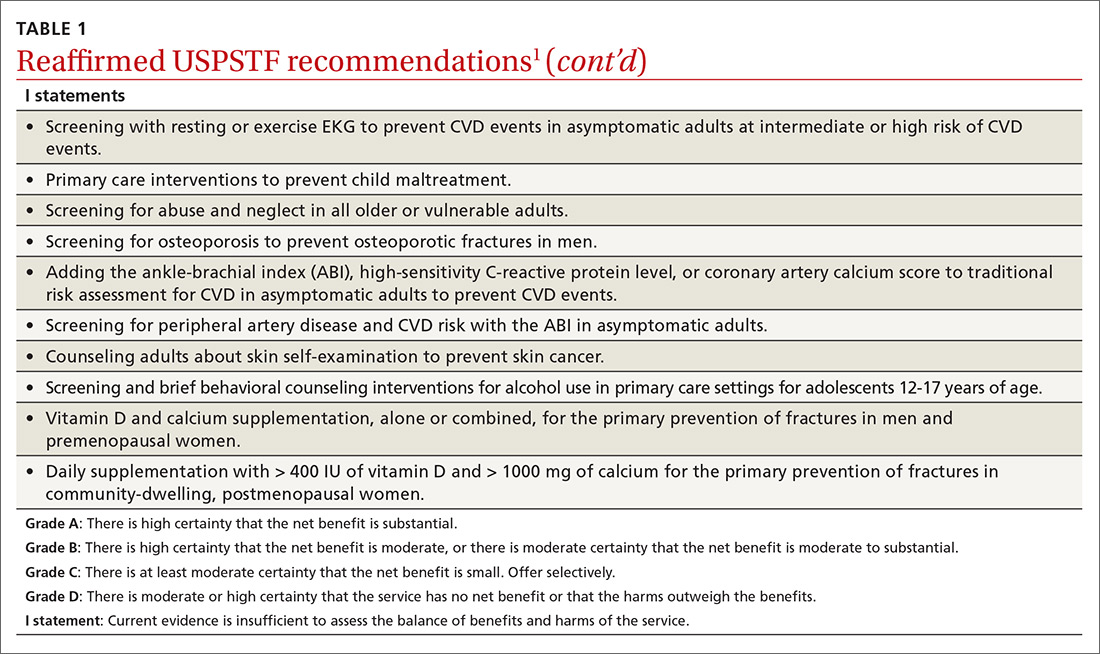
Over the past year through early 2019, the US Preventive Services Task Force made 34 recommendations on 19 different topics. Twenty-six were reaffirmations of recommendations made in previous years (TABLE 11); the Task Force attempts to reassess topics every 7 years. Two new topics were addressed with 2 new recommendations, and 6 previous recommendations were revised or reversed (TABLE 22-9).
This Practice Alert discusses the new and the changed recommendations. (In 2018, the Practice Alert podcast series covered screening for ovarian cancer [April], prostate cancer [June], and cervical cancer [October], and EKG screening for cardiovascular disease [November].) All current Task Force recommendations are available on the USPSTF Web site.1
New topics
Perinatal depression prevention
The Task Force recommends that clinicians counsel pregnant women and women in the first year postpartum who are at increased risk for perinatal depression, or refer for such services. The recommendation applies to those who are not diagnosed with depression but are at increased risk.
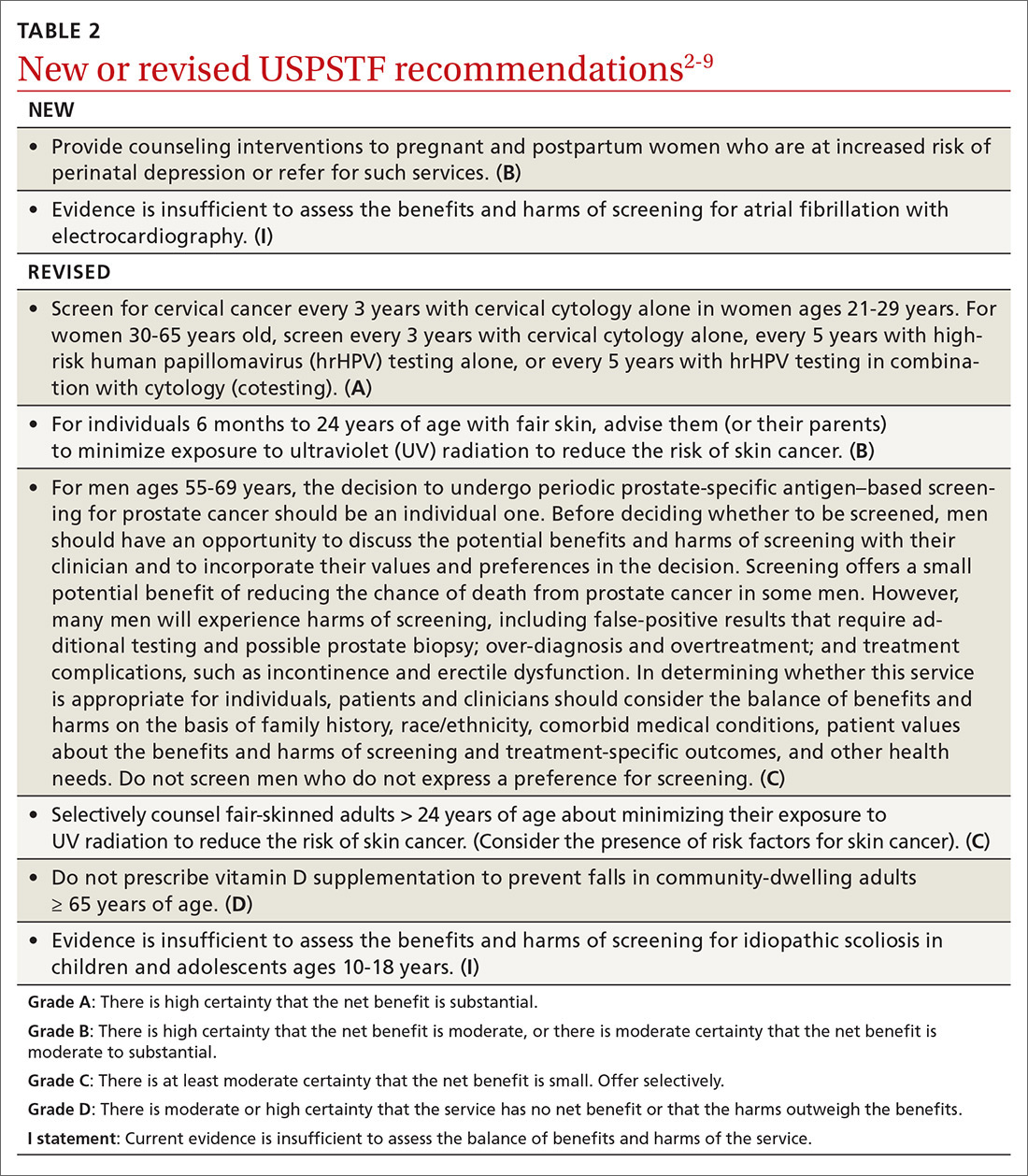
Perinatal depression can negatively affect both mother and child in several ways and occurs at a rate close to 9% during pregnancy and 37% during the first year postpartum.2 The interventions studied by the Task Force included cognitive behavioral therapy and interpersonal therapy; most sessions were initiated in the second trimester of pregnancy and varied in number of sessions and intensity. The Task Force includes the following in the list of risks that should prompt a referral: a history of depression, current depressive symptoms that fall short of that needed for a depression diagnosis, low income, adolescent or single parenthood, recent intimate partner violence, elevated anxiety symptoms, physical or sexual abuse, or a history of significant negative life events. (See “Postpartum anxiety: More common than you think,” in the April issue.)
Atrial fibrillation
The Task Force found insufficient evidence to recommend for or against the use of electrocardiography (EKG) to screen for atrial fibrillation (AF).3
Revisions of previous recommendations
Cervical cancer screening
Skin cancer prevention
The Task Force made 2 revisions to the 2012 recommendation on preventing skin cancer through behavioral counseling to avoid ultraviolet (UV) radiation.6 These recommendations continue to focus on those with fair skin. The first revision: The earliest age at which children (through their guardians) can benefit from counseling on UV avoidance has been lowered from age 10 years to 6 months. The second revision: Some adults older than age 24 can also benefit from such counseling if they have fair skin and other skin cancer risks such as using tanning beds, having a history of sunburns or previous skin cancer, having an increased number of nevi (moles) and atypical nevi, having human immunodeficiency virus (HIV) infection, having received an organ transplant, or having a family history of skin cancer.
Continue to: Those at risk...
Those at risk can reduce their chances of skin cancer by using broad-spectrum sunscreens and sun-protective clothing, and by avoiding sun exposure and indoor tanning beds.
Fall prevention
In a reversal of its 2012 recommendation, the Task Force now recommends against the use of vitamin D supplementation to prevent falls in community-dwelling adults 65 years or older.7 In a reanalysis of previous studies on this topic, along with new evidence, the Task Force concluded that vitamin D supplementation offers no benefit for preventing falls in adults who are not vitamin D deficient.
Screening for scoliosis in adolescents
In 2004 the USPSTF recommended against screening for idiopathic scoliosis in children and adolescents 10 to 18 years of age. In its most recent review, the Task Force continued to find no direct evidence of the benefit of screening and inadequate evidence on the long-term benefits of reduction in spinal curvature through exercise, surgery, and bracing. However, following a reanalysis of the potential harms of these treatments and the use of a new analytic framework, the Task Force concluded it is not possible at this time to assess the balance of benefits and harms of screening.8
Prostate cancer screening
In its most controversial action, the Task Force reversed its 2012 recommendation against routine prostate-specific antigen–based screening for prostate cancer in men ages 55 to 69 years and now lists this as a “C” recommendation.9 The potential benefits of screening include preventing 1.3 deaths from prostate cancer per 1000 men screened over 13 years and approximately 3 cases of metastatic prostate cancer. However, no trials have found a reduction in all-cause mortality from screening. Contrast that with the known harms of screening: 15% false positive results over 10 years; 1% hospitalization rate among those undergoing a prostate biopsy; over-diagnosis and resultant treatment of 20% to 50% of men diagnosed with prostate cancer through screening; and incontinence and erectile dysfunction in 20% and 67%, respectively, of men following prostatectomy.9
Based on these outcomes, the Task Force “does not recommend screening for prostate cancer unless men express a preference for screening after being informed of and understanding the benefits and risks.”9 The Task Force continues to recommend against screening men ages 70 years and older.
Continue to: The change in this recommendation...
The change in this recommendation and its wording present dilemmas for family physicians: whether to discuss potential screening with all men ages 55 to 69; to selectively discuss it with those at high risk (principally African Americans and those with a strong family history of prostate cancer); or to address the issue only if a patient asks about it. In addition, if a man requests screening, how often should it be performed? Most clinical trials have found equal benefit from testing less frequently than every year, with fewer harms. The Task Force provided little or no guidance on these issues.
Final advice: D recommendations
The Task Force reaffirmed that 7 services have either no benefit or cause more harm than benefit (TABLE 11). Family physicians should be familiar with these services, as well as all Task Force D recommendations, and avoid recommending them or providing them. High quality preventive care involves both providing services of proven benefit and avoiding those that do not.
1. USPSTF. Published recommendations. https://www.uspreventiveservicestaskforce.org/BrowseRec/Index/browse-recommendations. Accessed March 25, 2019.
2. USPSTF. Final recommendation statement. Perinatal depression: preventive interventions. https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/perinatal-depression-preventive-interventions. Accessed March 25, 2019.
3. USPSTF. Atrial fibrillation: screening with electrocardiography. https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/atrial-fibrillation-screening-with-electrocardiography. Accessed March 25, 2019.
4. USPSTF. Screening for atrial fibrillation with electrocardiography. JAMA. 2018;320:478-484.
5. USPSTF. Cervical cancer: screening. https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/cervical-cancer-screening2. Accessed March 25, 2019.
6. USPSTF. Skin cancer prevention: behavioral counseling. https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/skin-cancer-counseling2. Accessed March 25, 2019.
7. USPSTF. Falls prevention in community-dwelling older adults: interventions. https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/falls-prevention-in-older-adults-interventions1. Accessed March 25, 2019.
8. USPSTF. Adolescent idiopathic scoliosis: screening. https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/adolescent-idiopathic-scoliosis-screening1. Accessed March 25, 2019.
9. USPSTF. Prostate cancer: screening. https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/prostate-cancer-screening1#consider. Accessed March 25, 2019.
Over the past year through early 2019, the US Preventive Services Task Force made 34 recommendations on 19 different topics. Twenty-six were reaffirmations of recommendations made in previous years (TABLE 11); the Task Force attempts to reassess topics every 7 years. Two new topics were addressed with 2 new recommendations, and 6 previous recommendations were revised or reversed (TABLE 22-9).

This Practice Alert discusses the new and the changed recommendations. (In 2018, the Practice Alert podcast series covered screening for ovarian cancer [April], prostate cancer [June], and cervical cancer [October], and EKG screening for cardiovascular disease [November].) All current Task Force recommendations are available on the USPSTF Web site.1

New topics
Perinatal depression prevention
The Task Force recommends that clinicians counsel pregnant women and women in the first year postpartum who are at increased risk for perinatal depression, or refer for such services. The recommendation applies to those who are not diagnosed with depression but are at increased risk.

Perinatal depression can negatively affect both mother and child in several ways and occurs at a rate close to 9% during pregnancy and 37% during the first year postpartum.2 The interventions studied by the Task Force included cognitive behavioral therapy and interpersonal therapy; most sessions were initiated in the second trimester of pregnancy and varied in number of sessions and intensity. The Task Force includes the following in the list of risks that should prompt a referral: a history of depression, current depressive symptoms that fall short of that needed for a depression diagnosis, low income, adolescent or single parenthood, recent intimate partner violence, elevated anxiety symptoms, physical or sexual abuse, or a history of significant negative life events. (See “Postpartum anxiety: More common than you think,” in the April issue.)
Atrial fibrillation
The Task Force found insufficient evidence to recommend for or against the use of electrocardiography (EKG) to screen for atrial fibrillation (AF).3
Revisions of previous recommendations
Cervical cancer screening
Skin cancer prevention
The Task Force made 2 revisions to the 2012 recommendation on preventing skin cancer through behavioral counseling to avoid ultraviolet (UV) radiation.6 These recommendations continue to focus on those with fair skin. The first revision: The earliest age at which children (through their guardians) can benefit from counseling on UV avoidance has been lowered from age 10 years to 6 months. The second revision: Some adults older than age 24 can also benefit from such counseling if they have fair skin and other skin cancer risks such as using tanning beds, having a history of sunburns or previous skin cancer, having an increased number of nevi (moles) and atypical nevi, having human immunodeficiency virus (HIV) infection, having received an organ transplant, or having a family history of skin cancer.
Continue to: Those at risk...
Those at risk can reduce their chances of skin cancer by using broad-spectrum sunscreens and sun-protective clothing, and by avoiding sun exposure and indoor tanning beds.
Fall prevention
In a reversal of its 2012 recommendation, the Task Force now recommends against the use of vitamin D supplementation to prevent falls in community-dwelling adults 65 years or older.7 In a reanalysis of previous studies on this topic, along with new evidence, the Task Force concluded that vitamin D supplementation offers no benefit for preventing falls in adults who are not vitamin D deficient.
Screening for scoliosis in adolescents
In 2004 the USPSTF recommended against screening for idiopathic scoliosis in children and adolescents 10 to 18 years of age. In its most recent review, the Task Force continued to find no direct evidence of the benefit of screening and inadequate evidence on the long-term benefits of reduction in spinal curvature through exercise, surgery, and bracing. However, following a reanalysis of the potential harms of these treatments and the use of a new analytic framework, the Task Force concluded it is not possible at this time to assess the balance of benefits and harms of screening.8
Prostate cancer screening
In its most controversial action, the Task Force reversed its 2012 recommendation against routine prostate-specific antigen–based screening for prostate cancer in men ages 55 to 69 years and now lists this as a “C” recommendation.9 The potential benefits of screening include preventing 1.3 deaths from prostate cancer per 1000 men screened over 13 years and approximately 3 cases of metastatic prostate cancer. However, no trials have found a reduction in all-cause mortality from screening. Contrast that with the known harms of screening: 15% false positive results over 10 years; 1% hospitalization rate among those undergoing a prostate biopsy; over-diagnosis and resultant treatment of 20% to 50% of men diagnosed with prostate cancer through screening; and incontinence and erectile dysfunction in 20% and 67%, respectively, of men following prostatectomy.9
Based on these outcomes, the Task Force “does not recommend screening for prostate cancer unless men express a preference for screening after being informed of and understanding the benefits and risks.”9 The Task Force continues to recommend against screening men ages 70 years and older.
Continue to: The change in this recommendation...
The change in this recommendation and its wording present dilemmas for family physicians: whether to discuss potential screening with all men ages 55 to 69; to selectively discuss it with those at high risk (principally African Americans and those with a strong family history of prostate cancer); or to address the issue only if a patient asks about it. In addition, if a man requests screening, how often should it be performed? Most clinical trials have found equal benefit from testing less frequently than every year, with fewer harms. The Task Force provided little or no guidance on these issues.
Final advice: D recommendations
The Task Force reaffirmed that 7 services have either no benefit or cause more harm than benefit (TABLE 11). Family physicians should be familiar with these services, as well as all Task Force D recommendations, and avoid recommending them or providing them. High quality preventive care involves both providing services of proven benefit and avoiding those that do not.
Over the past year through early 2019, the US Preventive Services Task Force made 34 recommendations on 19 different topics. Twenty-six were reaffirmations of recommendations made in previous years (TABLE 11); the Task Force attempts to reassess topics every 7 years. Two new topics were addressed with 2 new recommendations, and 6 previous recommendations were revised or reversed (TABLE 22-9).

This Practice Alert discusses the new and the changed recommendations. (In 2018, the Practice Alert podcast series covered screening for ovarian cancer [April], prostate cancer [June], and cervical cancer [October], and EKG screening for cardiovascular disease [November].) All current Task Force recommendations are available on the USPSTF Web site.1

New topics
Perinatal depression prevention
The Task Force recommends that clinicians counsel pregnant women and women in the first year postpartum who are at increased risk for perinatal depression, or refer for such services. The recommendation applies to those who are not diagnosed with depression but are at increased risk.

Perinatal depression can negatively affect both mother and child in several ways and occurs at a rate close to 9% during pregnancy and 37% during the first year postpartum.2 The interventions studied by the Task Force included cognitive behavioral therapy and interpersonal therapy; most sessions were initiated in the second trimester of pregnancy and varied in number of sessions and intensity. The Task Force includes the following in the list of risks that should prompt a referral: a history of depression, current depressive symptoms that fall short of that needed for a depression diagnosis, low income, adolescent or single parenthood, recent intimate partner violence, elevated anxiety symptoms, physical or sexual abuse, or a history of significant negative life events. (See “Postpartum anxiety: More common than you think,” in the April issue.)
Atrial fibrillation
The Task Force found insufficient evidence to recommend for or against the use of electrocardiography (EKG) to screen for atrial fibrillation (AF).3
Revisions of previous recommendations
Cervical cancer screening
Skin cancer prevention
The Task Force made 2 revisions to the 2012 recommendation on preventing skin cancer through behavioral counseling to avoid ultraviolet (UV) radiation.6 These recommendations continue to focus on those with fair skin. The first revision: The earliest age at which children (through their guardians) can benefit from counseling on UV avoidance has been lowered from age 10 years to 6 months. The second revision: Some adults older than age 24 can also benefit from such counseling if they have fair skin and other skin cancer risks such as using tanning beds, having a history of sunburns or previous skin cancer, having an increased number of nevi (moles) and atypical nevi, having human immunodeficiency virus (HIV) infection, having received an organ transplant, or having a family history of skin cancer.
Continue to: Those at risk...
Those at risk can reduce their chances of skin cancer by using broad-spectrum sunscreens and sun-protective clothing, and by avoiding sun exposure and indoor tanning beds.
Fall prevention
In a reversal of its 2012 recommendation, the Task Force now recommends against the use of vitamin D supplementation to prevent falls in community-dwelling adults 65 years or older.7 In a reanalysis of previous studies on this topic, along with new evidence, the Task Force concluded that vitamin D supplementation offers no benefit for preventing falls in adults who are not vitamin D deficient.
Screening for scoliosis in adolescents
In 2004 the USPSTF recommended against screening for idiopathic scoliosis in children and adolescents 10 to 18 years of age. In its most recent review, the Task Force continued to find no direct evidence of the benefit of screening and inadequate evidence on the long-term benefits of reduction in spinal curvature through exercise, surgery, and bracing. However, following a reanalysis of the potential harms of these treatments and the use of a new analytic framework, the Task Force concluded it is not possible at this time to assess the balance of benefits and harms of screening.8
Prostate cancer screening
In its most controversial action, the Task Force reversed its 2012 recommendation against routine prostate-specific antigen–based screening for prostate cancer in men ages 55 to 69 years and now lists this as a “C” recommendation.9 The potential benefits of screening include preventing 1.3 deaths from prostate cancer per 1000 men screened over 13 years and approximately 3 cases of metastatic prostate cancer. However, no trials have found a reduction in all-cause mortality from screening. Contrast that with the known harms of screening: 15% false positive results over 10 years; 1% hospitalization rate among those undergoing a prostate biopsy; over-diagnosis and resultant treatment of 20% to 50% of men diagnosed with prostate cancer through screening; and incontinence and erectile dysfunction in 20% and 67%, respectively, of men following prostatectomy.9
Based on these outcomes, the Task Force “does not recommend screening for prostate cancer unless men express a preference for screening after being informed of and understanding the benefits and risks.”9 The Task Force continues to recommend against screening men ages 70 years and older.
Continue to: The change in this recommendation...
The change in this recommendation and its wording present dilemmas for family physicians: whether to discuss potential screening with all men ages 55 to 69; to selectively discuss it with those at high risk (principally African Americans and those with a strong family history of prostate cancer); or to address the issue only if a patient asks about it. In addition, if a man requests screening, how often should it be performed? Most clinical trials have found equal benefit from testing less frequently than every year, with fewer harms. The Task Force provided little or no guidance on these issues.
Final advice: D recommendations
The Task Force reaffirmed that 7 services have either no benefit or cause more harm than benefit (TABLE 11). Family physicians should be familiar with these services, as well as all Task Force D recommendations, and avoid recommending them or providing them. High quality preventive care involves both providing services of proven benefit and avoiding those that do not.
1. USPSTF. Published recommendations. https://www.uspreventiveservicestaskforce.org/BrowseRec/Index/browse-recommendations. Accessed March 25, 2019.
2. USPSTF. Final recommendation statement. Perinatal depression: preventive interventions. https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/perinatal-depression-preventive-interventions. Accessed March 25, 2019.
3. USPSTF. Atrial fibrillation: screening with electrocardiography. https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/atrial-fibrillation-screening-with-electrocardiography. Accessed March 25, 2019.
4. USPSTF. Screening for atrial fibrillation with electrocardiography. JAMA. 2018;320:478-484.
5. USPSTF. Cervical cancer: screening. https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/cervical-cancer-screening2. Accessed March 25, 2019.
6. USPSTF. Skin cancer prevention: behavioral counseling. https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/skin-cancer-counseling2. Accessed March 25, 2019.
7. USPSTF. Falls prevention in community-dwelling older adults: interventions. https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/falls-prevention-in-older-adults-interventions1. Accessed March 25, 2019.
8. USPSTF. Adolescent idiopathic scoliosis: screening. https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/adolescent-idiopathic-scoliosis-screening1. Accessed March 25, 2019.
9. USPSTF. Prostate cancer: screening. https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/prostate-cancer-screening1#consider. Accessed March 25, 2019.
1. USPSTF. Published recommendations. https://www.uspreventiveservicestaskforce.org/BrowseRec/Index/browse-recommendations. Accessed March 25, 2019.
2. USPSTF. Final recommendation statement. Perinatal depression: preventive interventions. https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/perinatal-depression-preventive-interventions. Accessed March 25, 2019.
3. USPSTF. Atrial fibrillation: screening with electrocardiography. https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/atrial-fibrillation-screening-with-electrocardiography. Accessed March 25, 2019.
4. USPSTF. Screening for atrial fibrillation with electrocardiography. JAMA. 2018;320:478-484.
5. USPSTF. Cervical cancer: screening. https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/cervical-cancer-screening2. Accessed March 25, 2019.
6. USPSTF. Skin cancer prevention: behavioral counseling. https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/skin-cancer-counseling2. Accessed March 25, 2019.
7. USPSTF. Falls prevention in community-dwelling older adults: interventions. https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/falls-prevention-in-older-adults-interventions1. Accessed March 25, 2019.
8. USPSTF. Adolescent idiopathic scoliosis: screening. https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/adolescent-idiopathic-scoliosis-screening1. Accessed March 25, 2019.
9. USPSTF. Prostate cancer: screening. https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/prostate-cancer-screening1#consider. Accessed March 25, 2019.
Most pregnancy-related deaths are preventable
according to the Centers for Disease Control and Prevention.
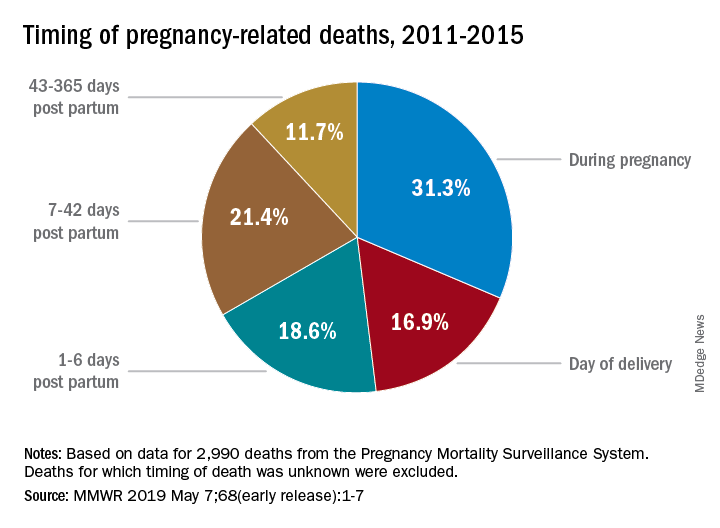
Deaths from pregnancy-related complications can occur “up to a year after delivery,” the CDC emphasized in a report released May 7. Indeed, 31% of pregnancy-related deaths happen during pregnancy, 36% happen at delivery or in the week after, and 33% happen 1 week to 1 year post partum. Yet detailed data from 13 state maternal mortality review committees (MMRCs) showed that 60% of such deaths are preventable.
There were 17 pregnancy-related deaths per 100,000 live births during 2011-2015, based on another data source: 3,410 pregnancy-related deaths (an average of 682 deaths per year) in the CDC’s Pregnancy Mortality Surveillance System. That pregnancy-related mortality ratio varied by race/ethnicity, Emily E. Peterson, MD, and the other CDC investigators reported in Morbidity and Mortality Weekly Report: Hispanic (11 deaths per 100,000), white (13), Asian/Pacific Islander (14), American Indian/Alaska Native (33), and black (43).
One aspect of the disparity was addressed by Wanda Barfield, MD, MPH, director of the CDC’s division of reproductive health and assistant surgeon general in the U.S. Public Health Service. “Recent studies have shown that racial and ethnic minority women deliver at different and lower-quality hospitals than white women and that these hospitals disproportionately care for black women at delivery,” she said at a CDC telebriefing.
Analysis of the timing of 2,990 deaths from the Pregnancy Mortality Surveillance System showed that almost a third (31.3%) occurred during pregnancy and 16.9% occurred on the day of delivery. Dr. Peterson and her CDC associates noted that more than half of pregnancy-related deaths, however, took place later: 1-6 days post partum (18.6%), 7-42 days (21.4%), and 43-365 days (11.7%).
The data on preventability were collected by the state MMRCs and included 232 deaths that occurred during 2013-2017. The MMRCs considered deaths preventable if they could be “averted by one or more reasonable changes to patient, community, provider, health facility, and/or system factors.”
“Our new analysis underscores the need for access to quality services, risk awareness, and early diagnosis, but it also highlights opportunities for preventing future pregnancy-related deaths,” Dr. Barfield said. “By identifying and promptly responding to warning signs not just during pregnancy, but even up to a year after delivery, we can save lives.”
according to the Centers for Disease Control and Prevention.

Deaths from pregnancy-related complications can occur “up to a year after delivery,” the CDC emphasized in a report released May 7. Indeed, 31% of pregnancy-related deaths happen during pregnancy, 36% happen at delivery or in the week after, and 33% happen 1 week to 1 year post partum. Yet detailed data from 13 state maternal mortality review committees (MMRCs) showed that 60% of such deaths are preventable.
There were 17 pregnancy-related deaths per 100,000 live births during 2011-2015, based on another data source: 3,410 pregnancy-related deaths (an average of 682 deaths per year) in the CDC’s Pregnancy Mortality Surveillance System. That pregnancy-related mortality ratio varied by race/ethnicity, Emily E. Peterson, MD, and the other CDC investigators reported in Morbidity and Mortality Weekly Report: Hispanic (11 deaths per 100,000), white (13), Asian/Pacific Islander (14), American Indian/Alaska Native (33), and black (43).
One aspect of the disparity was addressed by Wanda Barfield, MD, MPH, director of the CDC’s division of reproductive health and assistant surgeon general in the U.S. Public Health Service. “Recent studies have shown that racial and ethnic minority women deliver at different and lower-quality hospitals than white women and that these hospitals disproportionately care for black women at delivery,” she said at a CDC telebriefing.
Analysis of the timing of 2,990 deaths from the Pregnancy Mortality Surveillance System showed that almost a third (31.3%) occurred during pregnancy and 16.9% occurred on the day of delivery. Dr. Peterson and her CDC associates noted that more than half of pregnancy-related deaths, however, took place later: 1-6 days post partum (18.6%), 7-42 days (21.4%), and 43-365 days (11.7%).
The data on preventability were collected by the state MMRCs and included 232 deaths that occurred during 2013-2017. The MMRCs considered deaths preventable if they could be “averted by one or more reasonable changes to patient, community, provider, health facility, and/or system factors.”
“Our new analysis underscores the need for access to quality services, risk awareness, and early diagnosis, but it also highlights opportunities for preventing future pregnancy-related deaths,” Dr. Barfield said. “By identifying and promptly responding to warning signs not just during pregnancy, but even up to a year after delivery, we can save lives.”
according to the Centers for Disease Control and Prevention.

Deaths from pregnancy-related complications can occur “up to a year after delivery,” the CDC emphasized in a report released May 7. Indeed, 31% of pregnancy-related deaths happen during pregnancy, 36% happen at delivery or in the week after, and 33% happen 1 week to 1 year post partum. Yet detailed data from 13 state maternal mortality review committees (MMRCs) showed that 60% of such deaths are preventable.
There were 17 pregnancy-related deaths per 100,000 live births during 2011-2015, based on another data source: 3,410 pregnancy-related deaths (an average of 682 deaths per year) in the CDC’s Pregnancy Mortality Surveillance System. That pregnancy-related mortality ratio varied by race/ethnicity, Emily E. Peterson, MD, and the other CDC investigators reported in Morbidity and Mortality Weekly Report: Hispanic (11 deaths per 100,000), white (13), Asian/Pacific Islander (14), American Indian/Alaska Native (33), and black (43).
One aspect of the disparity was addressed by Wanda Barfield, MD, MPH, director of the CDC’s division of reproductive health and assistant surgeon general in the U.S. Public Health Service. “Recent studies have shown that racial and ethnic minority women deliver at different and lower-quality hospitals than white women and that these hospitals disproportionately care for black women at delivery,” she said at a CDC telebriefing.
Analysis of the timing of 2,990 deaths from the Pregnancy Mortality Surveillance System showed that almost a third (31.3%) occurred during pregnancy and 16.9% occurred on the day of delivery. Dr. Peterson and her CDC associates noted that more than half of pregnancy-related deaths, however, took place later: 1-6 days post partum (18.6%), 7-42 days (21.4%), and 43-365 days (11.7%).
The data on preventability were collected by the state MMRCs and included 232 deaths that occurred during 2013-2017. The MMRCs considered deaths preventable if they could be “averted by one or more reasonable changes to patient, community, provider, health facility, and/or system factors.”
“Our new analysis underscores the need for access to quality services, risk awareness, and early diagnosis, but it also highlights opportunities for preventing future pregnancy-related deaths,” Dr. Barfield said. “By identifying and promptly responding to warning signs not just during pregnancy, but even up to a year after delivery, we can save lives.”
Online counseling clarifies treatment options for menopausal women
NASHVILLE, TENN. – a post-intervention survey showed.
Of 36 women who completed the counseling, 72% were able to express a clear preference for a particular treatment. Additionally, 90% to 100% of those who completed the final survey said the various components of the counseling–such as an educational brochure or a telephone call from a research nurse–made them feel more prepared to speak with their provider about treatment options, Sandra Dayaratna, MD, reported during a poster session at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists.
Among 18 women with vasomotor symptoms, 6 (33%) preferred non-hormone treatment, 7 (39%) preferred hormone treatment, and 5 (28%) were unsure; among 18 with genitourinary symptoms of menopause, 6 (33%) preferred non-prescription treatment, 7 (39%) preferred topical hormone therapy, and 5 (28%) were unsure, Dr. Dayaratna, division director and clinical associate professor at Thomas Jefferson University Hospital and Sidney Kimmel Medical College, Philadelphia, found.
Of women who were not being treated for vasomotor symptoms, 5 (56%) expressed a clear treatment preference after counseling, whereas 8 (80%) of those not receiving treatment for genitourinary symptoms expressed a clear preference. Among 7 women receiving systemic hormone therapy for vasomotor symptoms, 86% preferred this treatment after counseling, and 3 women (50%) receiving topical hormone therapy preferred topical treatment after counseling.
The study included women aged 36-50 years from diverse educational and racial backgrounds who were referred to the counseling program after reporting menopausal symptoms, completing a baseline survey, and providing consent. The counseling, which was adapted from a tool developed at Thomas Jefferson University Hospital for the assessment of patients with colon and cancer or lung cancer, involved an educational brochure that was mailed to participants. It also offered access to an online tool that provided information about systemic hormone therapy, non-hormone prescription therapy, topical hormone treatment, and non-prescription treatment for vasomotor and genitourinary symptoms of menopause.
A research nurse contacted participants by phone and used the online program to review the brochure, clarify treatment preference, and produce a summary of the results.
In an interview, Dr. Dayaratna noted that the concept of “shared decision making” is often misunderstood to mean that patients are provided with information about options and then they make a choice.
Actually, this study demonstrates that shared decision making really involves a “values clarification” component, she explained. In this study, counseling that incorporates this type of shared decision making helped women feel more prepared to speak with their providers about treatment and thus may add value to care of menopausal women, she concluded.
“This is relevant because we know that over 50% of patients within 90 minutes of leaving their doctor’s office have forgotten what they were told, and over 50% do not comply with their treatment prescriptions,” she said. “So if patients can do this process ahead of time, when they come to speak to their physician about their symptoms and selection of medication, it’s a more effective and efficient conversation.”
Further research should evaluate impact on the subsequent office visit, she added.
This study was funded by an educational grant from Pfizer, Inc. and by the National Cancer Institute. Dr. Dayaratna reported having no disclosures.
SOURCE: Dayaratna S et al., ACOG 2019: Abstract 21M.
NASHVILLE, TENN. – a post-intervention survey showed.
Of 36 women who completed the counseling, 72% were able to express a clear preference for a particular treatment. Additionally, 90% to 100% of those who completed the final survey said the various components of the counseling–such as an educational brochure or a telephone call from a research nurse–made them feel more prepared to speak with their provider about treatment options, Sandra Dayaratna, MD, reported during a poster session at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists.
Among 18 women with vasomotor symptoms, 6 (33%) preferred non-hormone treatment, 7 (39%) preferred hormone treatment, and 5 (28%) were unsure; among 18 with genitourinary symptoms of menopause, 6 (33%) preferred non-prescription treatment, 7 (39%) preferred topical hormone therapy, and 5 (28%) were unsure, Dr. Dayaratna, division director and clinical associate professor at Thomas Jefferson University Hospital and Sidney Kimmel Medical College, Philadelphia, found.
Of women who were not being treated for vasomotor symptoms, 5 (56%) expressed a clear treatment preference after counseling, whereas 8 (80%) of those not receiving treatment for genitourinary symptoms expressed a clear preference. Among 7 women receiving systemic hormone therapy for vasomotor symptoms, 86% preferred this treatment after counseling, and 3 women (50%) receiving topical hormone therapy preferred topical treatment after counseling.
The study included women aged 36-50 years from diverse educational and racial backgrounds who were referred to the counseling program after reporting menopausal symptoms, completing a baseline survey, and providing consent. The counseling, which was adapted from a tool developed at Thomas Jefferson University Hospital for the assessment of patients with colon and cancer or lung cancer, involved an educational brochure that was mailed to participants. It also offered access to an online tool that provided information about systemic hormone therapy, non-hormone prescription therapy, topical hormone treatment, and non-prescription treatment for vasomotor and genitourinary symptoms of menopause.
A research nurse contacted participants by phone and used the online program to review the brochure, clarify treatment preference, and produce a summary of the results.
In an interview, Dr. Dayaratna noted that the concept of “shared decision making” is often misunderstood to mean that patients are provided with information about options and then they make a choice.
Actually, this study demonstrates that shared decision making really involves a “values clarification” component, she explained. In this study, counseling that incorporates this type of shared decision making helped women feel more prepared to speak with their providers about treatment and thus may add value to care of menopausal women, she concluded.
“This is relevant because we know that over 50% of patients within 90 minutes of leaving their doctor’s office have forgotten what they were told, and over 50% do not comply with their treatment prescriptions,” she said. “So if patients can do this process ahead of time, when they come to speak to their physician about their symptoms and selection of medication, it’s a more effective and efficient conversation.”
Further research should evaluate impact on the subsequent office visit, she added.
This study was funded by an educational grant from Pfizer, Inc. and by the National Cancer Institute. Dr. Dayaratna reported having no disclosures.
SOURCE: Dayaratna S et al., ACOG 2019: Abstract 21M.
NASHVILLE, TENN. – a post-intervention survey showed.
Of 36 women who completed the counseling, 72% were able to express a clear preference for a particular treatment. Additionally, 90% to 100% of those who completed the final survey said the various components of the counseling–such as an educational brochure or a telephone call from a research nurse–made them feel more prepared to speak with their provider about treatment options, Sandra Dayaratna, MD, reported during a poster session at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists.
Among 18 women with vasomotor symptoms, 6 (33%) preferred non-hormone treatment, 7 (39%) preferred hormone treatment, and 5 (28%) were unsure; among 18 with genitourinary symptoms of menopause, 6 (33%) preferred non-prescription treatment, 7 (39%) preferred topical hormone therapy, and 5 (28%) were unsure, Dr. Dayaratna, division director and clinical associate professor at Thomas Jefferson University Hospital and Sidney Kimmel Medical College, Philadelphia, found.
Of women who were not being treated for vasomotor symptoms, 5 (56%) expressed a clear treatment preference after counseling, whereas 8 (80%) of those not receiving treatment for genitourinary symptoms expressed a clear preference. Among 7 women receiving systemic hormone therapy for vasomotor symptoms, 86% preferred this treatment after counseling, and 3 women (50%) receiving topical hormone therapy preferred topical treatment after counseling.
The study included women aged 36-50 years from diverse educational and racial backgrounds who were referred to the counseling program after reporting menopausal symptoms, completing a baseline survey, and providing consent. The counseling, which was adapted from a tool developed at Thomas Jefferson University Hospital for the assessment of patients with colon and cancer or lung cancer, involved an educational brochure that was mailed to participants. It also offered access to an online tool that provided information about systemic hormone therapy, non-hormone prescription therapy, topical hormone treatment, and non-prescription treatment for vasomotor and genitourinary symptoms of menopause.
A research nurse contacted participants by phone and used the online program to review the brochure, clarify treatment preference, and produce a summary of the results.
In an interview, Dr. Dayaratna noted that the concept of “shared decision making” is often misunderstood to mean that patients are provided with information about options and then they make a choice.
Actually, this study demonstrates that shared decision making really involves a “values clarification” component, she explained. In this study, counseling that incorporates this type of shared decision making helped women feel more prepared to speak with their providers about treatment and thus may add value to care of menopausal women, she concluded.
“This is relevant because we know that over 50% of patients within 90 minutes of leaving their doctor’s office have forgotten what they were told, and over 50% do not comply with their treatment prescriptions,” she said. “So if patients can do this process ahead of time, when they come to speak to their physician about their symptoms and selection of medication, it’s a more effective and efficient conversation.”
Further research should evaluate impact on the subsequent office visit, she added.
This study was funded by an educational grant from Pfizer, Inc. and by the National Cancer Institute. Dr. Dayaratna reported having no disclosures.
SOURCE: Dayaratna S et al., ACOG 2019: Abstract 21M.
REPORTING FROM ACOG 2019
Liletta IUD efficacy extends to 6 years
NASHVILLE, TENN. – The 52 mg levonorgestrel intrauterine system Liletta, which is currently approved as a contraceptive for up to 5 years of use, remains highly effective and safe for an additional year of use, according to findings from an ongoing 10-year trial.
Of 1,714 women enrolled in the multicenter phase 3 trial and for whom demographic information is available, 379 have completed 6 years of use. Nine pregnancies have occurred to date, including 2 in year 1, 4 in year 2, and 1 each in years 3-5, for a cumulative life-table pregnancy rate through year 6 of 0.87, Carolyn L. Westhoff, MD, MSc, reported during a poster session at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists.
Six (67%) of the pregnancies were ectopic, noted Dr. Westhoff of Columbia University, N.Y.
Study subjects include 1,568 women aged 16-35 years at enrollment (986 were nulliparous and 433 were obese) and 146 aged 36 to 45 years. All were followed after device placement, with 138 having completed 8 years of use.
Two perforations occurred following device placement–both within the first year, and none have occurred since, Dr. Westhoff noted.
Other adverse events included expulsion in 68 women (4.0%), with 50 of those (73.5%) occurring in the first year of use, and pelvic infection in 15 women (0.9%), with 11 (73.3%) of those occurring after 6 or more months of use.
Only 40 women (2.3%) discontinued the study due to bleeding, and 30 of those (75%) did so in the first 2 years.
in both nulliparous and parous women, and in both non-obese and obese women, Dr. Westhoff concluded. She added that “there were no additional pregnancies in 6 years, and this study will be continuing to look all the way to 10-year effectiveness.”
This study is funded by Medicines360, a non-profit pharmaceutical company. Dr. Westhoff is a consultant or advisory board member for Agile and Cooper Surgical, and a Data and Safety Monitoring Board member for phase 4 studies for Bayer and Merck.
SOURCE: Westhoff C et al., ACOG 2019: Abstract 13M.
NASHVILLE, TENN. – The 52 mg levonorgestrel intrauterine system Liletta, which is currently approved as a contraceptive for up to 5 years of use, remains highly effective and safe for an additional year of use, according to findings from an ongoing 10-year trial.
Of 1,714 women enrolled in the multicenter phase 3 trial and for whom demographic information is available, 379 have completed 6 years of use. Nine pregnancies have occurred to date, including 2 in year 1, 4 in year 2, and 1 each in years 3-5, for a cumulative life-table pregnancy rate through year 6 of 0.87, Carolyn L. Westhoff, MD, MSc, reported during a poster session at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists.
Six (67%) of the pregnancies were ectopic, noted Dr. Westhoff of Columbia University, N.Y.
Study subjects include 1,568 women aged 16-35 years at enrollment (986 were nulliparous and 433 were obese) and 146 aged 36 to 45 years. All were followed after device placement, with 138 having completed 8 years of use.
Two perforations occurred following device placement–both within the first year, and none have occurred since, Dr. Westhoff noted.
Other adverse events included expulsion in 68 women (4.0%), with 50 of those (73.5%) occurring in the first year of use, and pelvic infection in 15 women (0.9%), with 11 (73.3%) of those occurring after 6 or more months of use.
Only 40 women (2.3%) discontinued the study due to bleeding, and 30 of those (75%) did so in the first 2 years.
in both nulliparous and parous women, and in both non-obese and obese women, Dr. Westhoff concluded. She added that “there were no additional pregnancies in 6 years, and this study will be continuing to look all the way to 10-year effectiveness.”
This study is funded by Medicines360, a non-profit pharmaceutical company. Dr. Westhoff is a consultant or advisory board member for Agile and Cooper Surgical, and a Data and Safety Monitoring Board member for phase 4 studies for Bayer and Merck.
SOURCE: Westhoff C et al., ACOG 2019: Abstract 13M.
NASHVILLE, TENN. – The 52 mg levonorgestrel intrauterine system Liletta, which is currently approved as a contraceptive for up to 5 years of use, remains highly effective and safe for an additional year of use, according to findings from an ongoing 10-year trial.
Of 1,714 women enrolled in the multicenter phase 3 trial and for whom demographic information is available, 379 have completed 6 years of use. Nine pregnancies have occurred to date, including 2 in year 1, 4 in year 2, and 1 each in years 3-5, for a cumulative life-table pregnancy rate through year 6 of 0.87, Carolyn L. Westhoff, MD, MSc, reported during a poster session at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists.
Six (67%) of the pregnancies were ectopic, noted Dr. Westhoff of Columbia University, N.Y.
Study subjects include 1,568 women aged 16-35 years at enrollment (986 were nulliparous and 433 were obese) and 146 aged 36 to 45 years. All were followed after device placement, with 138 having completed 8 years of use.
Two perforations occurred following device placement–both within the first year, and none have occurred since, Dr. Westhoff noted.
Other adverse events included expulsion in 68 women (4.0%), with 50 of those (73.5%) occurring in the first year of use, and pelvic infection in 15 women (0.9%), with 11 (73.3%) of those occurring after 6 or more months of use.
Only 40 women (2.3%) discontinued the study due to bleeding, and 30 of those (75%) did so in the first 2 years.
in both nulliparous and parous women, and in both non-obese and obese women, Dr. Westhoff concluded. She added that “there were no additional pregnancies in 6 years, and this study will be continuing to look all the way to 10-year effectiveness.”
This study is funded by Medicines360, a non-profit pharmaceutical company. Dr. Westhoff is a consultant or advisory board member for Agile and Cooper Surgical, and a Data and Safety Monitoring Board member for phase 4 studies for Bayer and Merck.
SOURCE: Westhoff C et al., ACOG 2019: Abstract 13M.
REPORTING FROM ACOG 2019