User login
Human papillomavirus in 2019: An update on cervical cancer prevention and screening guidelines
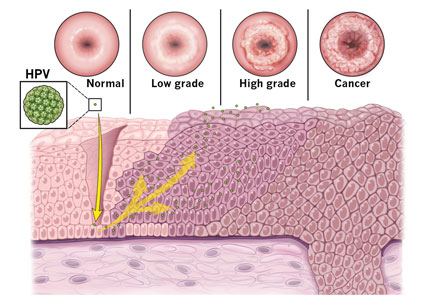
About 12% of women worldwide are infected with human papillomavirus (HPV).1 Persistent HPV infection with high-risk strains such as HPV 6, 11, 16, and 18 cause nearly all cases of cervical cancer and some anal, vaginal, penile, and oropharyngeal cancers.2 An estimated 13,000 cases of invasive cervical cancer will be diagnosed this year in the United States alone.3
Up to 70% of HPV-related cervical cancer cases can be prevented with vaccination. A number of changes have been made to the vaccination schedule within the past few years—patients younger than 15 need only 2 rather than 3 doses, and the vaccine itself can be used in adults up to age 45.
Vaccination and routine cervical cancer screening are both necessary to prevent this disease3 along with effective family and patient counseling. Here, we discuss the most up-to-date HPV vaccination recommendations, current cervical cancer screening guidelines, counseling techniques that increase vaccination acceptance rates, and follow-up protocols for abnormal cervical cancer screening results.
TYPES OF HPV VACCINES
HPV immunization can prevent up to 70% of cases of cervical cancer due to HPV as well as 90% of genital warts.4 The US Food and Drug Administration (FDA) has approved 3 HPV vaccines:
- Gardasil 9 targets HPV types 6, 11, 16, and 18 along with 31, 33, 45, 52, 58—these cause 90% of cervical cancer cases and most cases of genital warts5—making it the most effective vaccine available; Gardasil 9 is the only HPV vaccine currently available in the United States
- The bivalent vaccine (Cervarix) targeted HPV 16 and 18 only, and was discontinued in the United States in 2016
- The quadrivalent HPV vaccine (Gardasil) targeted HPV 16 and 18 as well as 6 and 11, which cause most cases of genital warts; the last available doses in the United States expired in May 2017; it has been replaced by Gardasil 9.
The incidence of cervical cancer in the United States dropped 29% among 15- to 24-year-olds from 2003–2006 when HPV vaccination first started to 2011–2014.6
VACCINE DOSING RECOMMENDATIONS FOR PRIMARY PREVENTION
The Advisory Committee on Immunization Practices (ACIP) revised its HPV vaccine schedule in 2016, when it decreased the necessary doses from 3 to 2 for patients under age 15 and addressed the needs of special patient populations.7 In late 2018, the FDA approved the use of the vaccine in men and women up to age 45. However, no change in guidelines have yet been made (Table 1).
In females, the ACIP recommends starting HPV vaccination at age 11 or 12, but it can be given as early as age 9. A 2-dose schedule is recommended for the 9-valent vaccine before the patient’s 15th birthday (the second dose 6 to 12 months after the first).7 For females who initiate HPV vaccination between ages 15 and 45, a 3-dose schedule is necessary (at 0, 1 to 2, and 6 months).7,8
The change to a 2-dose schedule was prompted by an evaluation of girls ages 9 to 13 randomized to receive either a 2- or 3-dose schedule. Antibody responses with a 2-dose schedule were not inferior to those of young women (ages 16 to 26) who received all 3 doses.9 The geometric mean titer ratios remained noninferior throughout the study period of 36 months.
However, a loss of noninferiority was noted for HPV-18 by 24 months and for HPV-6 by 36 months.9 Thus, further studies are needed to understand the duration of protection with a 2-dose schedule. Nevertheless, decreasing the number of doses makes it a more convenient and cost-effective option for many families.
The recommendations are the same for males except for one notable difference: in males ages 21 to 26, vaccination is not routinely recommended by the ACIP, but rather it is considered a “permissive use” recommendation: ie, the vaccine should be offered and final decisions on administration be made after individualized discussion with the patient.10 Permissive-use status also means the vaccine may not be covered by health insurance. Even though the vaccine is now available to men and women until age 45, many insurance plans do not cover it after age 26.
Children of either sex with a history of sexual abuse should receive their first vaccine dose beginning at age 9.7
Immunocompromised patients should follow the 3-dose schedule regardless of their sex or the age when vaccination was initiated.10
For transgender patients and for men not previously vaccinated who have sex with men, the 3-dose schedule vaccine should be given by the age of 26 (this is a routine recommendation, not a permissive one).8
CHALLENGES OF VACCINATION
Effective patient and family counseling is important. Even though the first HPV vaccine was approved in 2006, only 34.9% of US adolescents were fully vaccinated by 2015. This was in part because providers did not recommend it, were unfamiliar with it, or had concerns about its safety,11,12 and in part because some parents refused it.
The physician must address any myths regarding HPV vaccination and ensure that parents and patients understand that HPV vaccine is safe and effective. Studies have shown that with high-quality recommendations (ie, the care provider strongly endorses the HPV vaccine, encourages same-day vaccination, and discusses cancer prevention), patients are 9 times more likely to start the HPV vaccination schedule and 3 times more likely to follow through with subsequent doses.13
Providing good family and patient education does not necessarily require spending more counseling time. A recent study showed that spending less time discussing the HPV vaccine can lead to better vaccine coverage.14 The study compared parent HPV vaccine counseling techniques and found that simply informing patients and their families that the HPV vaccine was due was associated with a higher vaccine acceptance rate than inviting conversations about it.14 When providers announced that the vaccine was due, assuming the parents were ready to vaccinate, there was a 5.4% increase in HPV vaccination coverage.14
Conversely, physicians who engaged parents in open-ended discussions about the HPV vaccine did not improve HPV vaccination coverage.14 The authors suggested that providers approach HPV vaccination as if they were counseling patients and families about the need to avoid second-hand smoke or the need to use car seats. If parents or patients resist the presumptive announcement approach, expanded counseling and shared decision-making are appropriate. This includes addressing misconceptions that parents and patients may have about the HPV vaccine. The American Cancer Society lists 8 facts to reference (Table 2).15
SECONDARY PREVENTION: CERVICAL CANCER SCREENING
Since the introduction of the Papanicolaou (Pap) test, US cervical cancer incidence rates have decreased by more than 60%.16 Because almost all cervical cancer is preventable with proper screening, all women ages 21 to 65 should be screened.
Currently, there are 3 options available for cervical cancer screening: the Pap-only test, the Pap-HPV cotest, and the high-risk HPV-only test (Table 3). The latter 2 options detect high-risk HPV genotypes.
Several organizations have screening algorithms that recommend when to use these tests, but the 3 that shape today’s standard of care in cervical cancer screening come from the American College of Obstetricians and Gynecologists (ACOG), the American Society for Colposcopy and Cervical Pathology (ASCCP), and US Preventive Services Task Force (USPSTF).17–19
Pap-only testing is performed every 3 years to screen for cervical neoplasia that might indicate premalignancy.
Pap-HPV cotesting is performed every 5 years in women older than 30 with past normal screening. Until 2018, all 3 organizations recommended cotesting as the preferred screening algorithm for women ages 30 to 65.17–19 Patients with a history of abnormal test results require more frequent testing as recommended by the ASCCP.18
The high-risk HPV-only test utilizes real-time polymerase chain reaction to detect HPV 16, HPV 18, and 12 other HPV genotypes. Only 2 tests are approved by the FDA as stand-alone cervical cancer screening tests—the Roche Cobas HPV test approved in 2014 and the Becton Dickinson Onclarity HPV assay approved in 2018. Other HPV tests that are used in a cotesting strategy should not be used for high-risk HPV-only testing because their performance characteristics may differ.
In 2015, the Addressing the Need for Advanced HPV Diagnostics (ATHENA) study showed that 1 round of high-risk HPV-only screening for women older than 25 was more sensitive than Pap-only or cotesting for stage 3 cervical intraepithelial neoplasia or more severe disease (after 3 years of follow-up).20 Current guidelines from ASCCP18 and ACOG17 state that the high-risk HPV test can be repeated every 3 years (when used to screen by itself) if the woman is older than 25 and has had a normal test result.
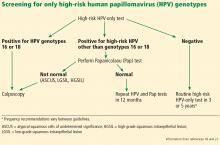
If the HPV test result is positive for high-risk HPV 16 or 18 genotypes, then immediate colposcopy is indicated; women who test positive for one of the other 12 high-risk subtypes will need to undergo a Pap test to determine the appropriate follow-up (Figure 1).18,21
In 2018, the USPSTF updated its recommendations, noting that for women age 30 to 65, Pap-only testing every 3 years, cotesting every 5 years, or high-risk HPV-only testing every 5 years are all appropriate screening strategies, with the Pap-only or high-risk HPV-only screenings being preferred.19 This is in contrast to ACOG and ASCCP recommendations for cotesting every 5 years, with alternative options of Pap-only or HPV-only testing being done every 3 years.17,18
Is there a best screening protocol?
The USPSTF reviewed large randomized and observational studies to summarize the effectiveness of the 3 screening strategies and commissioned a decision analysis model to compare the risks, benefits, and costs of the 3 screening algorithms. The guideline statement notes both cotesting and high-risk HPV testing offer similar cancer detection rates: each prevents 1 additional cancer per 1,000 women screened as opposed to Pap-only testing.19
Also, tests that incorporate high-risk HPV screening may offer better detection of cervical adenocarcinoma (which has a worse prognosis than the more common squamous cell carcinoma type). However, both HPV-based screening strategies are more likely to require additional colposcopies for follow-up than Pap-only screening (1,630 colposcopies required for each cancer prevented with high-risk HPV alone, 1,635 with cotesting). Colposcopy is a simple office procedure that causes minimal discomfort to the patient.
The USPSTF guideline also differs in the recommended frequency of high-risk HPV-only testing; a high-risk HPV result should be repeated every 5 years if normal (as opposed to every 3 years as recommended by ACOG and ASCCP).19 The 5-year recommendation is based on analysis modeling, which suggests that performing high-risk HPV-only testing more frequently is unlikely to improve detection rates but will increase the number of screening tests and colposcopies.19
No trial has directly compared cotesting with high-risk HPV testing for more than 2 rounds of screening. The updated USPSTF recommendations are based on modeling estimates and expert opinion, which assesses cost and benefit vs harm in the long term. Also, no high-risk HPV test is currently FDA-approved for every-5-year screening when used by itself.
All 3 cervical cancer screening methods provide highly effective cancer prevention, so it is important for providers to choose the strategy that best fits their practice. The most critical aspect of screening is getting all women screened, no matter which method is used.
It is critical to remember that the screening intervals are intended for patients without symptoms. Those who have new concerns such as bleeding should have a diagnostic Pap done to evaluate their symptoms.
Follow-up of abnormal results
Regardless of the pathway chosen, appropriate follow-up of any abnormal test result is critical to the early detection of cancer. Established follow-up guidelines exist,22,23 but accessing this information can be difficult for the busy clinician. The ASCCP has a mobile phone application that outlines the action steps corresponding to the patient’s age and results of any combination of Pap or HPV testing. The app also includes the best screening algorithms for a particular patient.24
All guidelines agree that cervical cancer screening should start at age 21, regardless of HPV vaccination status or age of sexual initiation.17,18,25 Screening can be discontinued at age 65 for women with normal screening results in the prior decade (3 consecutive negative Pap results or 2 consecutive negative cotest results).23
For women who have had a total hysterectomy and no history of cervical neoplasia, screening should be stopped immediately after the procedure. However, several high-risk groups of women will need continued screening past the age of 65, or after a hysterectomy.
For a woman with a history of stage 2 cervical intraepithelial neoplasia or higher grade lesions, routine screening is continued for an additional 20 years, even if she is over age 65. Pap-only testing every 3 years is acceptable, because the role of HPV testing is unclear after hysterectomy.23 Prior guidelines suggested annual screening in these patients, so the change to every 3 years is notable. Many gynecologic oncologists will recommend that women with a history of cervical cancer continue annual screening indefinitely.
Within the first 2 to 3 years after treatment for high-grade dysplastic changes, annual follow-up is done by the gynecologic oncology team. Providers who offer follow-up during this time frame should keep in communication with the oncology team to ensure appropriate, individualized care. These recommendations are based on expert opinion, so variations in clinical practice may be seen.
Women infected with the human immunodeficiency virus can have Pap-only testing every 3 years, after a series of 3 normal annual Pap results.26 But screening does not stop at age 65.23,26 For patients who are immunosuppressed or have a history of diethylstilbestrol exposure, screening should be done annually indefinitely.23
- Bruni L, Diaz M, Castellsagué X, Ferrer E, Bosch FX, de Sanjosé S. Cervical human papillomavirus prevalence in 5 continents: meta-analysis of 1 million women with normal cytological findings. J Infect Dis 2010; 202(12):1789–1799. doi:10.1086/657321
- de Martel C, Ferlay J, Franceschi S, et al. Global burden of cancer attributable to infections in 2008: a review and synthetic analysis. Lancet Oncol 2012; 13(6):607–615. doi:10.1016/S1470-2045(12)70137-7
- American Cancer Society. Key statistics for cervical cancer. www.cancer.org/cancer/cervical-cancer/about/key-statistics.html. Accessed February 14, 2019.
- Thaxton L, Waxman AG. Cervical cancer prevention: immunization and screening 2015. Med Clin North Am 2015; 99(3):469–477. doi:10.1016/j.mcna.2015.01.003
- McNamara M, Batur P, Walsh JME, Johnson KM. HPV update: vaccination, screening, and associated disease. J Gen Intern Med 2016; 31(11):1360–1366. doi:10.1007/s11606-016-3725-z
- Guo F, Cofie LE, Berenson AB. Cervical cancer incidence in young US females after human papillomavirus vaccine introduction. Am J Prev Med 2018; 55(2):197–204. doi:10.1016/j.amepre.2018.03.013
- Meites E, Kempe A, Markowitz LE. Use of a 2-dose schedule for human papillomavirus vaccination—updated recommendations of the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep 2016; 65(49):1405–1408. doi:10.15585/mmwr.mm6549a5
- Centers for Disease Control and Prevention (CDC). Supplemental information and guidance for vaccination providers regarding use of 9-valent HPV vaccine Information for persons who started an HPV vaccination series with quadrivalent or bivalent HPV vaccine. www.cdc.gov/hpv/downloads/9vhpv-guidance.pdf. Accessed February 14, 2019.
- Dobson SR, McNeil S, Dionne M, et al. Immunogenicity of 2 doses of HPV vaccine in younger adolescents vs 3 doses in young women: a randomized clinical trial. JAMA 2013; 309(17):1793–1802. doi:10.1001/jama.2013.1625
- Markowitz LE, Dunne EF, Saraiya M, et al; Centers for Disease Control and Prevention (CDC). Human papillomavirus vaccination: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2014; 63(RR-05):1–30. pmid:25167164
- Thompson EL, Rosen BL, Vamos CA, Kadono M, Daley EM. Human papillomavirus vaccination: what are the reasons for nonvaccination among US adolescents? J Adolesc Health 2017; 61(3):288–293. doi:10.1016/j.jadohealth.2017.05.015
- Reagan-Steiner S, Yankey D, Jeyarajah J, et al. National, regional, state, and selected local area vaccination coverage among adolescents aged 13-17 years—United States, 2015. MMWR Morb Mortal Wkly Rep 2016; 65(33):850–858. doi:10.15585/mmwr.mm6533a4
- Gilkey MB, Calo WA, Moss JL, Shah PD, Marciniak MW, Brewer NT. Provider communication and HPV vaccination: The impact of recommendation quality. Vaccine 2016; 34(9):1187–1192. doi:10.1016/j.vaccine.2016.01.023
- Brewer NT, Hall ME, Malo TL, Gilkey MB, Quinn B, Lathren C. Announcements versus conversations to improve HPV vaccination coverage: a randomized trial. Pediatrics 2017; 139(1):e20161764. doi:10.1542/peds.2016-1764
- American Cancer Society. HPV vaccine facts. www.cancer.org/cancer/cancer-causes/infectious-agents/hpv/hpv-vaccine-facts-and-fears.html. Accessed February 14, 2019.
- National Cancer Institute; Chasan R, Manrow R. Cervical cancer. https://report.nih.gov/nihfactsheets/viewfactsheet.aspx?csid=76. Accessed February 14, 2019.
- The American College of Obstetricians and Gynecologists (ACOG). Frequently asked questions. Cervical cancer screening. www.acog.org/Patients/FAQs/Cervical-Cancer-Screening. Accessed February 14, 2019.
- Saslow D, Solomon D, Lawson HW, et al; American Cancer Society; American Society for Colposcopy and Cervical Pathology; American Society for Clinical Pathology. American Cancer Society, American Society for Colposcopy and Cervical Pathology, and American Society for Clinical Pathology screening guidelines for the prevention and early detection of cervical cancer. Am J Clin Pathol 2012; 137(4):516–542. doi:10.1309/AJCPTGD94EVRSJCG
- US Preventive Services Task Force; Curry SJ, Krist AH, Owens DK, et al. Screening for cervical cancer: US Preventive Services Task Force Recommendation Statement. JAMA 2018; 320(7):674–686. doi:10.1001/jama.2018.10897
- Wright TC, Stoler MH, Behrens CM, Sharma A, Zhang G, Wright TL. Primary cervical cancer screening with human papillomavirus: end of study results from the ATHENA study using HPV as the first-line screening test. Gynecol Oncol 2015; 136(2):189–197. doi:10.1016/j.ygyno.2014.11.076
- Huh WK, Ault KA, Chelmow D, et al. Use of primary high-risk human papillomavirus testing for cervical cancer screening: interim clinical guidance. Obstet Gynecol 2015; 125(2):330–337. doi:10.1097/AOG.0000000000000669
- Massad LS, Einstein MH, Huh WK, et al; 2012 ASCCP Consensus Guidelines Conference. 2012 updated consensus guidelines for the management of abnormal cervical cancer screening tests and cancer precursors. Obstet Gynecol 2013; 121(4):829–846. doi:10.1097/AOG.0b013e3182883a34
- Committee on Practice Bulletins—Gynecology. Practice Bulletin No. 168: cervical cancer screening and prevention. Obstet Gynecol 2016; 128(4):e111–e130. doi:10.1097/AOG.0000000000001708
- ASCCP. Mobile app. http://www.asccp.org/store-detail2/asccp-mobile-app. Accessed February 14, 2019.
- USPSTF. Draft recommendation: cervical cancer: screening. www.uspreventiveservicestaskforce.org/Page/Document/draft-recommendation-statement/cervical-cancer-screening2. Accessed February 14, 2019.
- Masur H, Brooks JT, Benson CA, Holmes KK, Pau AK, Kaplan JE; National Institutes of Health; Centers for Disease Control and Prevention; HIV Medicine Association of the Infectious Diseases Society of America. Prevention and treatment of opportunistic infections in HIV-infected adults and adolescents: Updated guidelines from the Centers for Disease Control and Prevention, National Institutes of Health, and HIV Medicine Association of the Infectious Diseases Society of America. Clin Infect Dis 2014; 58(9):1308–1311. doi:10.1093/cid/ciu094
About 12% of women worldwide are infected with human papillomavirus (HPV).1 Persistent HPV infection with high-risk strains such as HPV 6, 11, 16, and 18 cause nearly all cases of cervical cancer and some anal, vaginal, penile, and oropharyngeal cancers.2 An estimated 13,000 cases of invasive cervical cancer will be diagnosed this year in the United States alone.3
Up to 70% of HPV-related cervical cancer cases can be prevented with vaccination. A number of changes have been made to the vaccination schedule within the past few years—patients younger than 15 need only 2 rather than 3 doses, and the vaccine itself can be used in adults up to age 45.
Vaccination and routine cervical cancer screening are both necessary to prevent this disease3 along with effective family and patient counseling. Here, we discuss the most up-to-date HPV vaccination recommendations, current cervical cancer screening guidelines, counseling techniques that increase vaccination acceptance rates, and follow-up protocols for abnormal cervical cancer screening results.
TYPES OF HPV VACCINES
HPV immunization can prevent up to 70% of cases of cervical cancer due to HPV as well as 90% of genital warts.4 The US Food and Drug Administration (FDA) has approved 3 HPV vaccines:
- Gardasil 9 targets HPV types 6, 11, 16, and 18 along with 31, 33, 45, 52, 58—these cause 90% of cervical cancer cases and most cases of genital warts5—making it the most effective vaccine available; Gardasil 9 is the only HPV vaccine currently available in the United States
- The bivalent vaccine (Cervarix) targeted HPV 16 and 18 only, and was discontinued in the United States in 2016
- The quadrivalent HPV vaccine (Gardasil) targeted HPV 16 and 18 as well as 6 and 11, which cause most cases of genital warts; the last available doses in the United States expired in May 2017; it has been replaced by Gardasil 9.
The incidence of cervical cancer in the United States dropped 29% among 15- to 24-year-olds from 2003–2006 when HPV vaccination first started to 2011–2014.6
VACCINE DOSING RECOMMENDATIONS FOR PRIMARY PREVENTION
The Advisory Committee on Immunization Practices (ACIP) revised its HPV vaccine schedule in 2016, when it decreased the necessary doses from 3 to 2 for patients under age 15 and addressed the needs of special patient populations.7 In late 2018, the FDA approved the use of the vaccine in men and women up to age 45. However, no change in guidelines have yet been made (Table 1).
In females, the ACIP recommends starting HPV vaccination at age 11 or 12, but it can be given as early as age 9. A 2-dose schedule is recommended for the 9-valent vaccine before the patient’s 15th birthday (the second dose 6 to 12 months after the first).7 For females who initiate HPV vaccination between ages 15 and 45, a 3-dose schedule is necessary (at 0, 1 to 2, and 6 months).7,8
The change to a 2-dose schedule was prompted by an evaluation of girls ages 9 to 13 randomized to receive either a 2- or 3-dose schedule. Antibody responses with a 2-dose schedule were not inferior to those of young women (ages 16 to 26) who received all 3 doses.9 The geometric mean titer ratios remained noninferior throughout the study period of 36 months.
However, a loss of noninferiority was noted for HPV-18 by 24 months and for HPV-6 by 36 months.9 Thus, further studies are needed to understand the duration of protection with a 2-dose schedule. Nevertheless, decreasing the number of doses makes it a more convenient and cost-effective option for many families.
The recommendations are the same for males except for one notable difference: in males ages 21 to 26, vaccination is not routinely recommended by the ACIP, but rather it is considered a “permissive use” recommendation: ie, the vaccine should be offered and final decisions on administration be made after individualized discussion with the patient.10 Permissive-use status also means the vaccine may not be covered by health insurance. Even though the vaccine is now available to men and women until age 45, many insurance plans do not cover it after age 26.
Children of either sex with a history of sexual abuse should receive their first vaccine dose beginning at age 9.7
Immunocompromised patients should follow the 3-dose schedule regardless of their sex or the age when vaccination was initiated.10
For transgender patients and for men not previously vaccinated who have sex with men, the 3-dose schedule vaccine should be given by the age of 26 (this is a routine recommendation, not a permissive one).8
CHALLENGES OF VACCINATION
Effective patient and family counseling is important. Even though the first HPV vaccine was approved in 2006, only 34.9% of US adolescents were fully vaccinated by 2015. This was in part because providers did not recommend it, were unfamiliar with it, or had concerns about its safety,11,12 and in part because some parents refused it.
The physician must address any myths regarding HPV vaccination and ensure that parents and patients understand that HPV vaccine is safe and effective. Studies have shown that with high-quality recommendations (ie, the care provider strongly endorses the HPV vaccine, encourages same-day vaccination, and discusses cancer prevention), patients are 9 times more likely to start the HPV vaccination schedule and 3 times more likely to follow through with subsequent doses.13
Providing good family and patient education does not necessarily require spending more counseling time. A recent study showed that spending less time discussing the HPV vaccine can lead to better vaccine coverage.14 The study compared parent HPV vaccine counseling techniques and found that simply informing patients and their families that the HPV vaccine was due was associated with a higher vaccine acceptance rate than inviting conversations about it.14 When providers announced that the vaccine was due, assuming the parents were ready to vaccinate, there was a 5.4% increase in HPV vaccination coverage.14
Conversely, physicians who engaged parents in open-ended discussions about the HPV vaccine did not improve HPV vaccination coverage.14 The authors suggested that providers approach HPV vaccination as if they were counseling patients and families about the need to avoid second-hand smoke or the need to use car seats. If parents or patients resist the presumptive announcement approach, expanded counseling and shared decision-making are appropriate. This includes addressing misconceptions that parents and patients may have about the HPV vaccine. The American Cancer Society lists 8 facts to reference (Table 2).15
SECONDARY PREVENTION: CERVICAL CANCER SCREENING
Since the introduction of the Papanicolaou (Pap) test, US cervical cancer incidence rates have decreased by more than 60%.16 Because almost all cervical cancer is preventable with proper screening, all women ages 21 to 65 should be screened.
Currently, there are 3 options available for cervical cancer screening: the Pap-only test, the Pap-HPV cotest, and the high-risk HPV-only test (Table 3). The latter 2 options detect high-risk HPV genotypes.
Several organizations have screening algorithms that recommend when to use these tests, but the 3 that shape today’s standard of care in cervical cancer screening come from the American College of Obstetricians and Gynecologists (ACOG), the American Society for Colposcopy and Cervical Pathology (ASCCP), and US Preventive Services Task Force (USPSTF).17–19
Pap-only testing is performed every 3 years to screen for cervical neoplasia that might indicate premalignancy.
Pap-HPV cotesting is performed every 5 years in women older than 30 with past normal screening. Until 2018, all 3 organizations recommended cotesting as the preferred screening algorithm for women ages 30 to 65.17–19 Patients with a history of abnormal test results require more frequent testing as recommended by the ASCCP.18
The high-risk HPV-only test utilizes real-time polymerase chain reaction to detect HPV 16, HPV 18, and 12 other HPV genotypes. Only 2 tests are approved by the FDA as stand-alone cervical cancer screening tests—the Roche Cobas HPV test approved in 2014 and the Becton Dickinson Onclarity HPV assay approved in 2018. Other HPV tests that are used in a cotesting strategy should not be used for high-risk HPV-only testing because their performance characteristics may differ.
In 2015, the Addressing the Need for Advanced HPV Diagnostics (ATHENA) study showed that 1 round of high-risk HPV-only screening for women older than 25 was more sensitive than Pap-only or cotesting for stage 3 cervical intraepithelial neoplasia or more severe disease (after 3 years of follow-up).20 Current guidelines from ASCCP18 and ACOG17 state that the high-risk HPV test can be repeated every 3 years (when used to screen by itself) if the woman is older than 25 and has had a normal test result.
If the HPV test result is positive for high-risk HPV 16 or 18 genotypes, then immediate colposcopy is indicated; women who test positive for one of the other 12 high-risk subtypes will need to undergo a Pap test to determine the appropriate follow-up (Figure 1).18,21
In 2018, the USPSTF updated its recommendations, noting that for women age 30 to 65, Pap-only testing every 3 years, cotesting every 5 years, or high-risk HPV-only testing every 5 years are all appropriate screening strategies, with the Pap-only or high-risk HPV-only screenings being preferred.19 This is in contrast to ACOG and ASCCP recommendations for cotesting every 5 years, with alternative options of Pap-only or HPV-only testing being done every 3 years.17,18
Is there a best screening protocol?
The USPSTF reviewed large randomized and observational studies to summarize the effectiveness of the 3 screening strategies and commissioned a decision analysis model to compare the risks, benefits, and costs of the 3 screening algorithms. The guideline statement notes both cotesting and high-risk HPV testing offer similar cancer detection rates: each prevents 1 additional cancer per 1,000 women screened as opposed to Pap-only testing.19
Also, tests that incorporate high-risk HPV screening may offer better detection of cervical adenocarcinoma (which has a worse prognosis than the more common squamous cell carcinoma type). However, both HPV-based screening strategies are more likely to require additional colposcopies for follow-up than Pap-only screening (1,630 colposcopies required for each cancer prevented with high-risk HPV alone, 1,635 with cotesting). Colposcopy is a simple office procedure that causes minimal discomfort to the patient.
The USPSTF guideline also differs in the recommended frequency of high-risk HPV-only testing; a high-risk HPV result should be repeated every 5 years if normal (as opposed to every 3 years as recommended by ACOG and ASCCP).19 The 5-year recommendation is based on analysis modeling, which suggests that performing high-risk HPV-only testing more frequently is unlikely to improve detection rates but will increase the number of screening tests and colposcopies.19
No trial has directly compared cotesting with high-risk HPV testing for more than 2 rounds of screening. The updated USPSTF recommendations are based on modeling estimates and expert opinion, which assesses cost and benefit vs harm in the long term. Also, no high-risk HPV test is currently FDA-approved for every-5-year screening when used by itself.
All 3 cervical cancer screening methods provide highly effective cancer prevention, so it is important for providers to choose the strategy that best fits their practice. The most critical aspect of screening is getting all women screened, no matter which method is used.
It is critical to remember that the screening intervals are intended for patients without symptoms. Those who have new concerns such as bleeding should have a diagnostic Pap done to evaluate their symptoms.
Follow-up of abnormal results
Regardless of the pathway chosen, appropriate follow-up of any abnormal test result is critical to the early detection of cancer. Established follow-up guidelines exist,22,23 but accessing this information can be difficult for the busy clinician. The ASCCP has a mobile phone application that outlines the action steps corresponding to the patient’s age and results of any combination of Pap or HPV testing. The app also includes the best screening algorithms for a particular patient.24
All guidelines agree that cervical cancer screening should start at age 21, regardless of HPV vaccination status or age of sexual initiation.17,18,25 Screening can be discontinued at age 65 for women with normal screening results in the prior decade (3 consecutive negative Pap results or 2 consecutive negative cotest results).23
For women who have had a total hysterectomy and no history of cervical neoplasia, screening should be stopped immediately after the procedure. However, several high-risk groups of women will need continued screening past the age of 65, or after a hysterectomy.
For a woman with a history of stage 2 cervical intraepithelial neoplasia or higher grade lesions, routine screening is continued for an additional 20 years, even if she is over age 65. Pap-only testing every 3 years is acceptable, because the role of HPV testing is unclear after hysterectomy.23 Prior guidelines suggested annual screening in these patients, so the change to every 3 years is notable. Many gynecologic oncologists will recommend that women with a history of cervical cancer continue annual screening indefinitely.
Within the first 2 to 3 years after treatment for high-grade dysplastic changes, annual follow-up is done by the gynecologic oncology team. Providers who offer follow-up during this time frame should keep in communication with the oncology team to ensure appropriate, individualized care. These recommendations are based on expert opinion, so variations in clinical practice may be seen.
Women infected with the human immunodeficiency virus can have Pap-only testing every 3 years, after a series of 3 normal annual Pap results.26 But screening does not stop at age 65.23,26 For patients who are immunosuppressed or have a history of diethylstilbestrol exposure, screening should be done annually indefinitely.23
About 12% of women worldwide are infected with human papillomavirus (HPV).1 Persistent HPV infection with high-risk strains such as HPV 6, 11, 16, and 18 cause nearly all cases of cervical cancer and some anal, vaginal, penile, and oropharyngeal cancers.2 An estimated 13,000 cases of invasive cervical cancer will be diagnosed this year in the United States alone.3
Up to 70% of HPV-related cervical cancer cases can be prevented with vaccination. A number of changes have been made to the vaccination schedule within the past few years—patients younger than 15 need only 2 rather than 3 doses, and the vaccine itself can be used in adults up to age 45.
Vaccination and routine cervical cancer screening are both necessary to prevent this disease3 along with effective family and patient counseling. Here, we discuss the most up-to-date HPV vaccination recommendations, current cervical cancer screening guidelines, counseling techniques that increase vaccination acceptance rates, and follow-up protocols for abnormal cervical cancer screening results.
TYPES OF HPV VACCINES
HPV immunization can prevent up to 70% of cases of cervical cancer due to HPV as well as 90% of genital warts.4 The US Food and Drug Administration (FDA) has approved 3 HPV vaccines:
- Gardasil 9 targets HPV types 6, 11, 16, and 18 along with 31, 33, 45, 52, 58—these cause 90% of cervical cancer cases and most cases of genital warts5—making it the most effective vaccine available; Gardasil 9 is the only HPV vaccine currently available in the United States
- The bivalent vaccine (Cervarix) targeted HPV 16 and 18 only, and was discontinued in the United States in 2016
- The quadrivalent HPV vaccine (Gardasil) targeted HPV 16 and 18 as well as 6 and 11, which cause most cases of genital warts; the last available doses in the United States expired in May 2017; it has been replaced by Gardasil 9.
The incidence of cervical cancer in the United States dropped 29% among 15- to 24-year-olds from 2003–2006 when HPV vaccination first started to 2011–2014.6
VACCINE DOSING RECOMMENDATIONS FOR PRIMARY PREVENTION
The Advisory Committee on Immunization Practices (ACIP) revised its HPV vaccine schedule in 2016, when it decreased the necessary doses from 3 to 2 for patients under age 15 and addressed the needs of special patient populations.7 In late 2018, the FDA approved the use of the vaccine in men and women up to age 45. However, no change in guidelines have yet been made (Table 1).
In females, the ACIP recommends starting HPV vaccination at age 11 or 12, but it can be given as early as age 9. A 2-dose schedule is recommended for the 9-valent vaccine before the patient’s 15th birthday (the second dose 6 to 12 months after the first).7 For females who initiate HPV vaccination between ages 15 and 45, a 3-dose schedule is necessary (at 0, 1 to 2, and 6 months).7,8
The change to a 2-dose schedule was prompted by an evaluation of girls ages 9 to 13 randomized to receive either a 2- or 3-dose schedule. Antibody responses with a 2-dose schedule were not inferior to those of young women (ages 16 to 26) who received all 3 doses.9 The geometric mean titer ratios remained noninferior throughout the study period of 36 months.
However, a loss of noninferiority was noted for HPV-18 by 24 months and for HPV-6 by 36 months.9 Thus, further studies are needed to understand the duration of protection with a 2-dose schedule. Nevertheless, decreasing the number of doses makes it a more convenient and cost-effective option for many families.
The recommendations are the same for males except for one notable difference: in males ages 21 to 26, vaccination is not routinely recommended by the ACIP, but rather it is considered a “permissive use” recommendation: ie, the vaccine should be offered and final decisions on administration be made after individualized discussion with the patient.10 Permissive-use status also means the vaccine may not be covered by health insurance. Even though the vaccine is now available to men and women until age 45, many insurance plans do not cover it after age 26.
Children of either sex with a history of sexual abuse should receive their first vaccine dose beginning at age 9.7
Immunocompromised patients should follow the 3-dose schedule regardless of their sex or the age when vaccination was initiated.10
For transgender patients and for men not previously vaccinated who have sex with men, the 3-dose schedule vaccine should be given by the age of 26 (this is a routine recommendation, not a permissive one).8
CHALLENGES OF VACCINATION
Effective patient and family counseling is important. Even though the first HPV vaccine was approved in 2006, only 34.9% of US adolescents were fully vaccinated by 2015. This was in part because providers did not recommend it, were unfamiliar with it, or had concerns about its safety,11,12 and in part because some parents refused it.
The physician must address any myths regarding HPV vaccination and ensure that parents and patients understand that HPV vaccine is safe and effective. Studies have shown that with high-quality recommendations (ie, the care provider strongly endorses the HPV vaccine, encourages same-day vaccination, and discusses cancer prevention), patients are 9 times more likely to start the HPV vaccination schedule and 3 times more likely to follow through with subsequent doses.13
Providing good family and patient education does not necessarily require spending more counseling time. A recent study showed that spending less time discussing the HPV vaccine can lead to better vaccine coverage.14 The study compared parent HPV vaccine counseling techniques and found that simply informing patients and their families that the HPV vaccine was due was associated with a higher vaccine acceptance rate than inviting conversations about it.14 When providers announced that the vaccine was due, assuming the parents were ready to vaccinate, there was a 5.4% increase in HPV vaccination coverage.14
Conversely, physicians who engaged parents in open-ended discussions about the HPV vaccine did not improve HPV vaccination coverage.14 The authors suggested that providers approach HPV vaccination as if they were counseling patients and families about the need to avoid second-hand smoke or the need to use car seats. If parents or patients resist the presumptive announcement approach, expanded counseling and shared decision-making are appropriate. This includes addressing misconceptions that parents and patients may have about the HPV vaccine. The American Cancer Society lists 8 facts to reference (Table 2).15
SECONDARY PREVENTION: CERVICAL CANCER SCREENING
Since the introduction of the Papanicolaou (Pap) test, US cervical cancer incidence rates have decreased by more than 60%.16 Because almost all cervical cancer is preventable with proper screening, all women ages 21 to 65 should be screened.
Currently, there are 3 options available for cervical cancer screening: the Pap-only test, the Pap-HPV cotest, and the high-risk HPV-only test (Table 3). The latter 2 options detect high-risk HPV genotypes.
Several organizations have screening algorithms that recommend when to use these tests, but the 3 that shape today’s standard of care in cervical cancer screening come from the American College of Obstetricians and Gynecologists (ACOG), the American Society for Colposcopy and Cervical Pathology (ASCCP), and US Preventive Services Task Force (USPSTF).17–19
Pap-only testing is performed every 3 years to screen for cervical neoplasia that might indicate premalignancy.
Pap-HPV cotesting is performed every 5 years in women older than 30 with past normal screening. Until 2018, all 3 organizations recommended cotesting as the preferred screening algorithm for women ages 30 to 65.17–19 Patients with a history of abnormal test results require more frequent testing as recommended by the ASCCP.18
The high-risk HPV-only test utilizes real-time polymerase chain reaction to detect HPV 16, HPV 18, and 12 other HPV genotypes. Only 2 tests are approved by the FDA as stand-alone cervical cancer screening tests—the Roche Cobas HPV test approved in 2014 and the Becton Dickinson Onclarity HPV assay approved in 2018. Other HPV tests that are used in a cotesting strategy should not be used for high-risk HPV-only testing because their performance characteristics may differ.
In 2015, the Addressing the Need for Advanced HPV Diagnostics (ATHENA) study showed that 1 round of high-risk HPV-only screening for women older than 25 was more sensitive than Pap-only or cotesting for stage 3 cervical intraepithelial neoplasia or more severe disease (after 3 years of follow-up).20 Current guidelines from ASCCP18 and ACOG17 state that the high-risk HPV test can be repeated every 3 years (when used to screen by itself) if the woman is older than 25 and has had a normal test result.
If the HPV test result is positive for high-risk HPV 16 or 18 genotypes, then immediate colposcopy is indicated; women who test positive for one of the other 12 high-risk subtypes will need to undergo a Pap test to determine the appropriate follow-up (Figure 1).18,21
In 2018, the USPSTF updated its recommendations, noting that for women age 30 to 65, Pap-only testing every 3 years, cotesting every 5 years, or high-risk HPV-only testing every 5 years are all appropriate screening strategies, with the Pap-only or high-risk HPV-only screenings being preferred.19 This is in contrast to ACOG and ASCCP recommendations for cotesting every 5 years, with alternative options of Pap-only or HPV-only testing being done every 3 years.17,18
Is there a best screening protocol?
The USPSTF reviewed large randomized and observational studies to summarize the effectiveness of the 3 screening strategies and commissioned a decision analysis model to compare the risks, benefits, and costs of the 3 screening algorithms. The guideline statement notes both cotesting and high-risk HPV testing offer similar cancer detection rates: each prevents 1 additional cancer per 1,000 women screened as opposed to Pap-only testing.19
Also, tests that incorporate high-risk HPV screening may offer better detection of cervical adenocarcinoma (which has a worse prognosis than the more common squamous cell carcinoma type). However, both HPV-based screening strategies are more likely to require additional colposcopies for follow-up than Pap-only screening (1,630 colposcopies required for each cancer prevented with high-risk HPV alone, 1,635 with cotesting). Colposcopy is a simple office procedure that causes minimal discomfort to the patient.
The USPSTF guideline also differs in the recommended frequency of high-risk HPV-only testing; a high-risk HPV result should be repeated every 5 years if normal (as opposed to every 3 years as recommended by ACOG and ASCCP).19 The 5-year recommendation is based on analysis modeling, which suggests that performing high-risk HPV-only testing more frequently is unlikely to improve detection rates but will increase the number of screening tests and colposcopies.19
No trial has directly compared cotesting with high-risk HPV testing for more than 2 rounds of screening. The updated USPSTF recommendations are based on modeling estimates and expert opinion, which assesses cost and benefit vs harm in the long term. Also, no high-risk HPV test is currently FDA-approved for every-5-year screening when used by itself.
All 3 cervical cancer screening methods provide highly effective cancer prevention, so it is important for providers to choose the strategy that best fits their practice. The most critical aspect of screening is getting all women screened, no matter which method is used.
It is critical to remember that the screening intervals are intended for patients without symptoms. Those who have new concerns such as bleeding should have a diagnostic Pap done to evaluate their symptoms.
Follow-up of abnormal results
Regardless of the pathway chosen, appropriate follow-up of any abnormal test result is critical to the early detection of cancer. Established follow-up guidelines exist,22,23 but accessing this information can be difficult for the busy clinician. The ASCCP has a mobile phone application that outlines the action steps corresponding to the patient’s age and results of any combination of Pap or HPV testing. The app also includes the best screening algorithms for a particular patient.24
All guidelines agree that cervical cancer screening should start at age 21, regardless of HPV vaccination status or age of sexual initiation.17,18,25 Screening can be discontinued at age 65 for women with normal screening results in the prior decade (3 consecutive negative Pap results or 2 consecutive negative cotest results).23
For women who have had a total hysterectomy and no history of cervical neoplasia, screening should be stopped immediately after the procedure. However, several high-risk groups of women will need continued screening past the age of 65, or after a hysterectomy.
For a woman with a history of stage 2 cervical intraepithelial neoplasia or higher grade lesions, routine screening is continued for an additional 20 years, even if she is over age 65. Pap-only testing every 3 years is acceptable, because the role of HPV testing is unclear after hysterectomy.23 Prior guidelines suggested annual screening in these patients, so the change to every 3 years is notable. Many gynecologic oncologists will recommend that women with a history of cervical cancer continue annual screening indefinitely.
Within the first 2 to 3 years after treatment for high-grade dysplastic changes, annual follow-up is done by the gynecologic oncology team. Providers who offer follow-up during this time frame should keep in communication with the oncology team to ensure appropriate, individualized care. These recommendations are based on expert opinion, so variations in clinical practice may be seen.
Women infected with the human immunodeficiency virus can have Pap-only testing every 3 years, after a series of 3 normal annual Pap results.26 But screening does not stop at age 65.23,26 For patients who are immunosuppressed or have a history of diethylstilbestrol exposure, screening should be done annually indefinitely.23
- Bruni L, Diaz M, Castellsagué X, Ferrer E, Bosch FX, de Sanjosé S. Cervical human papillomavirus prevalence in 5 continents: meta-analysis of 1 million women with normal cytological findings. J Infect Dis 2010; 202(12):1789–1799. doi:10.1086/657321
- de Martel C, Ferlay J, Franceschi S, et al. Global burden of cancer attributable to infections in 2008: a review and synthetic analysis. Lancet Oncol 2012; 13(6):607–615. doi:10.1016/S1470-2045(12)70137-7
- American Cancer Society. Key statistics for cervical cancer. www.cancer.org/cancer/cervical-cancer/about/key-statistics.html. Accessed February 14, 2019.
- Thaxton L, Waxman AG. Cervical cancer prevention: immunization and screening 2015. Med Clin North Am 2015; 99(3):469–477. doi:10.1016/j.mcna.2015.01.003
- McNamara M, Batur P, Walsh JME, Johnson KM. HPV update: vaccination, screening, and associated disease. J Gen Intern Med 2016; 31(11):1360–1366. doi:10.1007/s11606-016-3725-z
- Guo F, Cofie LE, Berenson AB. Cervical cancer incidence in young US females after human papillomavirus vaccine introduction. Am J Prev Med 2018; 55(2):197–204. doi:10.1016/j.amepre.2018.03.013
- Meites E, Kempe A, Markowitz LE. Use of a 2-dose schedule for human papillomavirus vaccination—updated recommendations of the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep 2016; 65(49):1405–1408. doi:10.15585/mmwr.mm6549a5
- Centers for Disease Control and Prevention (CDC). Supplemental information and guidance for vaccination providers regarding use of 9-valent HPV vaccine Information for persons who started an HPV vaccination series with quadrivalent or bivalent HPV vaccine. www.cdc.gov/hpv/downloads/9vhpv-guidance.pdf. Accessed February 14, 2019.
- Dobson SR, McNeil S, Dionne M, et al. Immunogenicity of 2 doses of HPV vaccine in younger adolescents vs 3 doses in young women: a randomized clinical trial. JAMA 2013; 309(17):1793–1802. doi:10.1001/jama.2013.1625
- Markowitz LE, Dunne EF, Saraiya M, et al; Centers for Disease Control and Prevention (CDC). Human papillomavirus vaccination: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2014; 63(RR-05):1–30. pmid:25167164
- Thompson EL, Rosen BL, Vamos CA, Kadono M, Daley EM. Human papillomavirus vaccination: what are the reasons for nonvaccination among US adolescents? J Adolesc Health 2017; 61(3):288–293. doi:10.1016/j.jadohealth.2017.05.015
- Reagan-Steiner S, Yankey D, Jeyarajah J, et al. National, regional, state, and selected local area vaccination coverage among adolescents aged 13-17 years—United States, 2015. MMWR Morb Mortal Wkly Rep 2016; 65(33):850–858. doi:10.15585/mmwr.mm6533a4
- Gilkey MB, Calo WA, Moss JL, Shah PD, Marciniak MW, Brewer NT. Provider communication and HPV vaccination: The impact of recommendation quality. Vaccine 2016; 34(9):1187–1192. doi:10.1016/j.vaccine.2016.01.023
- Brewer NT, Hall ME, Malo TL, Gilkey MB, Quinn B, Lathren C. Announcements versus conversations to improve HPV vaccination coverage: a randomized trial. Pediatrics 2017; 139(1):e20161764. doi:10.1542/peds.2016-1764
- American Cancer Society. HPV vaccine facts. www.cancer.org/cancer/cancer-causes/infectious-agents/hpv/hpv-vaccine-facts-and-fears.html. Accessed February 14, 2019.
- National Cancer Institute; Chasan R, Manrow R. Cervical cancer. https://report.nih.gov/nihfactsheets/viewfactsheet.aspx?csid=76. Accessed February 14, 2019.
- The American College of Obstetricians and Gynecologists (ACOG). Frequently asked questions. Cervical cancer screening. www.acog.org/Patients/FAQs/Cervical-Cancer-Screening. Accessed February 14, 2019.
- Saslow D, Solomon D, Lawson HW, et al; American Cancer Society; American Society for Colposcopy and Cervical Pathology; American Society for Clinical Pathology. American Cancer Society, American Society for Colposcopy and Cervical Pathology, and American Society for Clinical Pathology screening guidelines for the prevention and early detection of cervical cancer. Am J Clin Pathol 2012; 137(4):516–542. doi:10.1309/AJCPTGD94EVRSJCG
- US Preventive Services Task Force; Curry SJ, Krist AH, Owens DK, et al. Screening for cervical cancer: US Preventive Services Task Force Recommendation Statement. JAMA 2018; 320(7):674–686. doi:10.1001/jama.2018.10897
- Wright TC, Stoler MH, Behrens CM, Sharma A, Zhang G, Wright TL. Primary cervical cancer screening with human papillomavirus: end of study results from the ATHENA study using HPV as the first-line screening test. Gynecol Oncol 2015; 136(2):189–197. doi:10.1016/j.ygyno.2014.11.076
- Huh WK, Ault KA, Chelmow D, et al. Use of primary high-risk human papillomavirus testing for cervical cancer screening: interim clinical guidance. Obstet Gynecol 2015; 125(2):330–337. doi:10.1097/AOG.0000000000000669
- Massad LS, Einstein MH, Huh WK, et al; 2012 ASCCP Consensus Guidelines Conference. 2012 updated consensus guidelines for the management of abnormal cervical cancer screening tests and cancer precursors. Obstet Gynecol 2013; 121(4):829–846. doi:10.1097/AOG.0b013e3182883a34
- Committee on Practice Bulletins—Gynecology. Practice Bulletin No. 168: cervical cancer screening and prevention. Obstet Gynecol 2016; 128(4):e111–e130. doi:10.1097/AOG.0000000000001708
- ASCCP. Mobile app. http://www.asccp.org/store-detail2/asccp-mobile-app. Accessed February 14, 2019.
- USPSTF. Draft recommendation: cervical cancer: screening. www.uspreventiveservicestaskforce.org/Page/Document/draft-recommendation-statement/cervical-cancer-screening2. Accessed February 14, 2019.
- Masur H, Brooks JT, Benson CA, Holmes KK, Pau AK, Kaplan JE; National Institutes of Health; Centers for Disease Control and Prevention; HIV Medicine Association of the Infectious Diseases Society of America. Prevention and treatment of opportunistic infections in HIV-infected adults and adolescents: Updated guidelines from the Centers for Disease Control and Prevention, National Institutes of Health, and HIV Medicine Association of the Infectious Diseases Society of America. Clin Infect Dis 2014; 58(9):1308–1311. doi:10.1093/cid/ciu094
- Bruni L, Diaz M, Castellsagué X, Ferrer E, Bosch FX, de Sanjosé S. Cervical human papillomavirus prevalence in 5 continents: meta-analysis of 1 million women with normal cytological findings. J Infect Dis 2010; 202(12):1789–1799. doi:10.1086/657321
- de Martel C, Ferlay J, Franceschi S, et al. Global burden of cancer attributable to infections in 2008: a review and synthetic analysis. Lancet Oncol 2012; 13(6):607–615. doi:10.1016/S1470-2045(12)70137-7
- American Cancer Society. Key statistics for cervical cancer. www.cancer.org/cancer/cervical-cancer/about/key-statistics.html. Accessed February 14, 2019.
- Thaxton L, Waxman AG. Cervical cancer prevention: immunization and screening 2015. Med Clin North Am 2015; 99(3):469–477. doi:10.1016/j.mcna.2015.01.003
- McNamara M, Batur P, Walsh JME, Johnson KM. HPV update: vaccination, screening, and associated disease. J Gen Intern Med 2016; 31(11):1360–1366. doi:10.1007/s11606-016-3725-z
- Guo F, Cofie LE, Berenson AB. Cervical cancer incidence in young US females after human papillomavirus vaccine introduction. Am J Prev Med 2018; 55(2):197–204. doi:10.1016/j.amepre.2018.03.013
- Meites E, Kempe A, Markowitz LE. Use of a 2-dose schedule for human papillomavirus vaccination—updated recommendations of the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep 2016; 65(49):1405–1408. doi:10.15585/mmwr.mm6549a5
- Centers for Disease Control and Prevention (CDC). Supplemental information and guidance for vaccination providers regarding use of 9-valent HPV vaccine Information for persons who started an HPV vaccination series with quadrivalent or bivalent HPV vaccine. www.cdc.gov/hpv/downloads/9vhpv-guidance.pdf. Accessed February 14, 2019.
- Dobson SR, McNeil S, Dionne M, et al. Immunogenicity of 2 doses of HPV vaccine in younger adolescents vs 3 doses in young women: a randomized clinical trial. JAMA 2013; 309(17):1793–1802. doi:10.1001/jama.2013.1625
- Markowitz LE, Dunne EF, Saraiya M, et al; Centers for Disease Control and Prevention (CDC). Human papillomavirus vaccination: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2014; 63(RR-05):1–30. pmid:25167164
- Thompson EL, Rosen BL, Vamos CA, Kadono M, Daley EM. Human papillomavirus vaccination: what are the reasons for nonvaccination among US adolescents? J Adolesc Health 2017; 61(3):288–293. doi:10.1016/j.jadohealth.2017.05.015
- Reagan-Steiner S, Yankey D, Jeyarajah J, et al. National, regional, state, and selected local area vaccination coverage among adolescents aged 13-17 years—United States, 2015. MMWR Morb Mortal Wkly Rep 2016; 65(33):850–858. doi:10.15585/mmwr.mm6533a4
- Gilkey MB, Calo WA, Moss JL, Shah PD, Marciniak MW, Brewer NT. Provider communication and HPV vaccination: The impact of recommendation quality. Vaccine 2016; 34(9):1187–1192. doi:10.1016/j.vaccine.2016.01.023
- Brewer NT, Hall ME, Malo TL, Gilkey MB, Quinn B, Lathren C. Announcements versus conversations to improve HPV vaccination coverage: a randomized trial. Pediatrics 2017; 139(1):e20161764. doi:10.1542/peds.2016-1764
- American Cancer Society. HPV vaccine facts. www.cancer.org/cancer/cancer-causes/infectious-agents/hpv/hpv-vaccine-facts-and-fears.html. Accessed February 14, 2019.
- National Cancer Institute; Chasan R, Manrow R. Cervical cancer. https://report.nih.gov/nihfactsheets/viewfactsheet.aspx?csid=76. Accessed February 14, 2019.
- The American College of Obstetricians and Gynecologists (ACOG). Frequently asked questions. Cervical cancer screening. www.acog.org/Patients/FAQs/Cervical-Cancer-Screening. Accessed February 14, 2019.
- Saslow D, Solomon D, Lawson HW, et al; American Cancer Society; American Society for Colposcopy and Cervical Pathology; American Society for Clinical Pathology. American Cancer Society, American Society for Colposcopy and Cervical Pathology, and American Society for Clinical Pathology screening guidelines for the prevention and early detection of cervical cancer. Am J Clin Pathol 2012; 137(4):516–542. doi:10.1309/AJCPTGD94EVRSJCG
- US Preventive Services Task Force; Curry SJ, Krist AH, Owens DK, et al. Screening for cervical cancer: US Preventive Services Task Force Recommendation Statement. JAMA 2018; 320(7):674–686. doi:10.1001/jama.2018.10897
- Wright TC, Stoler MH, Behrens CM, Sharma A, Zhang G, Wright TL. Primary cervical cancer screening with human papillomavirus: end of study results from the ATHENA study using HPV as the first-line screening test. Gynecol Oncol 2015; 136(2):189–197. doi:10.1016/j.ygyno.2014.11.076
- Huh WK, Ault KA, Chelmow D, et al. Use of primary high-risk human papillomavirus testing for cervical cancer screening: interim clinical guidance. Obstet Gynecol 2015; 125(2):330–337. doi:10.1097/AOG.0000000000000669
- Massad LS, Einstein MH, Huh WK, et al; 2012 ASCCP Consensus Guidelines Conference. 2012 updated consensus guidelines for the management of abnormal cervical cancer screening tests and cancer precursors. Obstet Gynecol 2013; 121(4):829–846. doi:10.1097/AOG.0b013e3182883a34
- Committee on Practice Bulletins—Gynecology. Practice Bulletin No. 168: cervical cancer screening and prevention. Obstet Gynecol 2016; 128(4):e111–e130. doi:10.1097/AOG.0000000000001708
- ASCCP. Mobile app. http://www.asccp.org/store-detail2/asccp-mobile-app. Accessed February 14, 2019.
- USPSTF. Draft recommendation: cervical cancer: screening. www.uspreventiveservicestaskforce.org/Page/Document/draft-recommendation-statement/cervical-cancer-screening2. Accessed February 14, 2019.
- Masur H, Brooks JT, Benson CA, Holmes KK, Pau AK, Kaplan JE; National Institutes of Health; Centers for Disease Control and Prevention; HIV Medicine Association of the Infectious Diseases Society of America. Prevention and treatment of opportunistic infections in HIV-infected adults and adolescents: Updated guidelines from the Centers for Disease Control and Prevention, National Institutes of Health, and HIV Medicine Association of the Infectious Diseases Society of America. Clin Infect Dis 2014; 58(9):1308–1311. doi:10.1093/cid/ciu094
KEY POINTS
- Immunization against HPV can prevent up to 70% of HPV-related cervical cancer cases.
- Gardasil 9 is the only HPV vaccine currently available in the United States and is now approved for use in males and females between the ages of 9 and 45.
- In girls and boys younger than 15, a 2-dose schedule is recommended; patients ages 15 through 45 require 3 doses.
- Vaccine acceptance rates are highest when primary care providers announce that the vaccine is due rather than invite open-ended discussions.
- Regular cervical cancer screening is an important preventive tool and should be performed using the Papanicolaou (Pap) test, the high-risk HPV-only test, or the Pap-HPV cotest.
Cancer screening: A modest proposal for prevention
I have been assured by a very knowing American of my acquaintance in London, that a young healthy child well nursed is at a year old, a most delicious, nourishing, and wholesome food, whether stewed, roasted, baked, or boiled, and I make no doubt that it will equally serve in a fricassee, or ragout.
—Jonathan Swift, A Modest Proposal1
Large-scale cancer screening programs have the unintended consequences of false-positive results and overdiagnosis, leading to anxiety and overtreatment. The magnitude of these harms continues to be clarified after decades of screening.
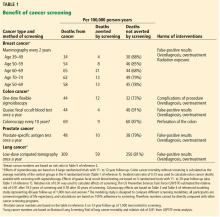
Recognizing the trade-off between benefits and harms, the US Preventive Services Task Force (USPSTF) has changed several of its recommendations in recent years. Breast cancer screening recommendations have gone from yearly mammograms starting at age 40 to biennial mammograms starting at age 50 for women at average risk.2 Prostate cancer screening is no longer recommended for men age 70 and older, and even for men between 55 and 69, screening is now an individual decision.3
Newer screening programs are targeting high-risk groups rather than the general population, with the aim of increasing the likelihood of benefits and limiting the harms. For example, lung cancer screening is recommended only for current smokers or smokers who have quit within the past 15 years, are between 55 and 80, and have at least a 30 pack-year smoking history.4
The movement toward less-frequent screening and screening in a narrower population has evoked strong reactions from advocates of cancer screening. One professor of radiology writes, “It borders on unethical to suggest that the benefit of having your life saved by screening and living another 40 years can be balanced against the ‘harm’ of being recalled for additional mammographic views for what proves to not be a cancer.”5 Another notes, “It does not make any sense to throw away the lives saved by screening to avoid over-treating a small number of cancers.”6 Both of these authors defend the position that the goal of screening is to minimize cause-specific mortality, irrespective of overdiagnosis, overtreatment, or false-positive results. In other words, harm should have little to no weight in screening recommendations.
Although the debate on cancer screening is moving toward a more balanced discussion of benefits and harms, many patients are still subjected to screening that is more aggressive than the USPSTF recommends, which may be due to an underlying belief that no harm is greater than the benefit of saving a life.
IS MORE-AGGRESSIVE SCREENING THE ANSWER?
One may wonder if more-aggressive screening could prevent deaths that occur despite standard screening. For example, more-frequent screening or use of additional screening methods such as ultrasonography or magnetic resonance imaging has been suggested for patients at high risk of breast cancer.
A MODEST PROPOSAL
If one holds the view that benefits alone should be considered when writing recommendations about screening, the logical conclusion extends beyond screening. We would therefore like to propose a different approach to reducing cancer deaths in the general population:
Why not just remove everybody’s breasts, prostate gland, and colon before cancer arises?
TO CUT IS TO PREVENT
Currently, we offer prophylactic surgery to patients at high risk of cancer. For example, women with BRCA1/BRCA2 mutations are offered prophylactic mastectomy as one of several options for reducing risk of breast cancer. In 2013, the first case of prophylactic prostatectomy was performed in a man who had a BRCA1/BRCA2 mutation. Total colectomy is considered in men and women who have hereditary nonpolyposis colon cancer, instead of segmental resection, to prevent future cancer.
If prophylactic surgery were extended to the general population, it would greatly reduce the number of cancer deaths. Assuming that removing an organ almost always precludes development of cancer, we may predict that prophylactic mastectomy, prostatectomy, or colectomy would save the lives of most of the patients who are still dying of cancer of these organs. The effectiveness rates would approach, but not reach 100%; such is the case with prophylactic mastectomy.
Consider prostate-specific antigen (PSA) screening. Even using the favorable estimate of the impact of PSA screening, arising from the European Randomised Study of Screening for Prostate Cancer trial, 27 men have to be diagnosed, most undergoing local therapy (the trial was conducted before active surveillance became routine), to avert 1 death from prostate cancer over 13 years.9
Contrast this “number needed to diagnose” with the number needed to treat for a strategy of routine prostate removal at age 45 or 50. Given that the lifetime risk of death from prostate cancer approaches 3%, and few cases arise before this age, a prophylactic surgical strategy would avert 1 death per 33 operations. If proponents of screening are willing to accept a number needed to diagnose of 27 over a 13-year interval, they may be willing to consider a number needed to treat of 33 over a lifetime.
There may be harms such as perioperative and postoperative complications. Mastectomy could lead to emotional stress from altered body image. Prostatectomy can have long-term complications such as urinary incontinence and sexual dysfunction. Nevertheless, prophylactic organ removal would save far more lives than current screening practices. It also could decrease mental burden, as patients could rest assured that they will never develop cancer, whereas screening often involves ambiguous test results, follow-up tests, and interventions, increasing patient anxiety.
FINDING THE BALANCE BETWEEN BENEFITS AND HARMS
In truth, we do not really advocate universal mastectomy, prostatectomy, and colectomy to prevent cancer, no more than Swift1 really wanted to eat the children of Ireland to alleviate poverty and famine in that country. Rather, we use it as an extreme proposal to highlight the scope and depth of harms that inevitably arise from screening.
If proponents of aggressive screening believe that the goal is to reduce cause-specific mortality as much as possible, giving little weight or consideration to overdiagnosis and overtreatment, then they ought to embrace universal prophylactic surgery as well. Recognition of this logical consequence reminds us that we must make screening recommendations that balance benefits and harms.
Considering an extreme perspective can help in recognizing our bias toward saving lives from cancer and discounting the harms. Aggravating this bias, it is impossible to know whether an individual patient has avoided fatal cancer or undergone unnecessary treatment. Moreover, changing practice is more difficult if it involves rolling back interventions that were once the standard.
Balancing benefits and harms is especially difficult when trying to compare the benefit of preventing a single cancer death against a harm that is less serious but more common. Medicine has always involved difficult trade-offs, as seen in cost-benefit analysis of new treatments or balancing quality of life with quantity of life in a single patient. In addition, each individual may place different values on benefits of screening and avoiding possible harms.
There is an undeniable trade-off with screening, and we must make a conscious decision on where to draw the line when harms outweigh the benefits. We must proceed with caution when subjecting large numbers of men and women to the possibility of psychological burden and decreased quality of life.
Given the growing appreciation of the harms of screening, it is likely that future guidance will continue to move toward less- frequent screening or focusing resources on high-risk populations, where the absolute magnitude of benefit is greater. Cancer screening is also likely to become an individual decision based on personal values and informed decisions.
- Swift J. A Modest Proposal for Preventing the Children of Poor People in Ireland, from Being a Burden on Their Parents or Country, and for Making Them Beneficial to the Publick. Dublin: S. Harding, 1729.
- Nelson HD, Cantor A, Humphrey L, et al. Screening for breast cancer: a systematic review to update the 2009 US Preventive Services Task Force Recommendation. www.ncbi.nlm.nih.gov/books/NBK343819. Accessed February 13, 2019.
- US Preventive Services Task Force; Grossman DC, Curry SJ, Owens DK, et al. Screening for prostate cancer: US Preventive Services Task Force Recommendation Statement. JAMA 2018; 319(18):1901–1913. doi:10.1001/jama.2018.3710
- Humphrey L, Deffebach M, Pappas M, et al. Screening for lung cancer: systematic review to update the US Preventive Services Task Force Recommendation. www.ncbi.nlm.nih.gov/books/NBK154610. Accessed February 13, 2019.
- Kopans DB. A review of: “Tipping the balance of benefits and harms to favor screening mammography starting at age 40 years.” www.sbi-online.org/Portals/0/downloads/documents/pdfs/A%20review%20of%20Tipping%20the%20Balance%20of%20Benefits%20and%20Harms%20to%20Favor%20Screening%20Mammography%20Starting%20at%20Age%2040%20Years%20-%20Kopans.pdf. Accessed February 13, 2019.
- Yaffe M, Gordon, P. Routine mammograms do save lives: U of T expert. U of T News. www.utoronto.ca/news/routine-mammograms-do-save-lives-u-t-expert. Accessed February 13, 2019.
- Lin JS, Piper MA, Perdue LA, et al. Screening for colorectal cancer: updated evidence report and systematic review for the US Preventive Services Task Force. JAMA 2016; 315(23):2576–2594. doi:10.1001/jama.2016.3332
- Knudsen AB, Zauber AG, Rutter CM, et al. Estimation of benefits, burden, and harms of colorectal cancer screening strategies: modeling study for the US Preventive Services Task Force. JAMA 2016; 315(23):2595–2609. doi:10.1001/jama.2016.6828
- Schröder FH, Hugosson J, Roobol MJ, et al; ERSPC Investigators. Screening and prostate cancer mortality: results of the European Randomised Study of Screening for Prostate Cancer (ERSPC) at 13 years of follow-up. Lancet 2014; 384(9959):2027–2035. doi:10.1016/S0140-6736(14)60525-0
I have been assured by a very knowing American of my acquaintance in London, that a young healthy child well nursed is at a year old, a most delicious, nourishing, and wholesome food, whether stewed, roasted, baked, or boiled, and I make no doubt that it will equally serve in a fricassee, or ragout.
—Jonathan Swift, A Modest Proposal1
Large-scale cancer screening programs have the unintended consequences of false-positive results and overdiagnosis, leading to anxiety and overtreatment. The magnitude of these harms continues to be clarified after decades of screening.
Recognizing the trade-off between benefits and harms, the US Preventive Services Task Force (USPSTF) has changed several of its recommendations in recent years. Breast cancer screening recommendations have gone from yearly mammograms starting at age 40 to biennial mammograms starting at age 50 for women at average risk.2 Prostate cancer screening is no longer recommended for men age 70 and older, and even for men between 55 and 69, screening is now an individual decision.3
Newer screening programs are targeting high-risk groups rather than the general population, with the aim of increasing the likelihood of benefits and limiting the harms. For example, lung cancer screening is recommended only for current smokers or smokers who have quit within the past 15 years, are between 55 and 80, and have at least a 30 pack-year smoking history.4
The movement toward less-frequent screening and screening in a narrower population has evoked strong reactions from advocates of cancer screening. One professor of radiology writes, “It borders on unethical to suggest that the benefit of having your life saved by screening and living another 40 years can be balanced against the ‘harm’ of being recalled for additional mammographic views for what proves to not be a cancer.”5 Another notes, “It does not make any sense to throw away the lives saved by screening to avoid over-treating a small number of cancers.”6 Both of these authors defend the position that the goal of screening is to minimize cause-specific mortality, irrespective of overdiagnosis, overtreatment, or false-positive results. In other words, harm should have little to no weight in screening recommendations.
Although the debate on cancer screening is moving toward a more balanced discussion of benefits and harms, many patients are still subjected to screening that is more aggressive than the USPSTF recommends, which may be due to an underlying belief that no harm is greater than the benefit of saving a life.
IS MORE-AGGRESSIVE SCREENING THE ANSWER?
One may wonder if more-aggressive screening could prevent deaths that occur despite standard screening. For example, more-frequent screening or use of additional screening methods such as ultrasonography or magnetic resonance imaging has been suggested for patients at high risk of breast cancer.
A MODEST PROPOSAL
If one holds the view that benefits alone should be considered when writing recommendations about screening, the logical conclusion extends beyond screening. We would therefore like to propose a different approach to reducing cancer deaths in the general population:
Why not just remove everybody’s breasts, prostate gland, and colon before cancer arises?
TO CUT IS TO PREVENT
Currently, we offer prophylactic surgery to patients at high risk of cancer. For example, women with BRCA1/BRCA2 mutations are offered prophylactic mastectomy as one of several options for reducing risk of breast cancer. In 2013, the first case of prophylactic prostatectomy was performed in a man who had a BRCA1/BRCA2 mutation. Total colectomy is considered in men and women who have hereditary nonpolyposis colon cancer, instead of segmental resection, to prevent future cancer.
If prophylactic surgery were extended to the general population, it would greatly reduce the number of cancer deaths. Assuming that removing an organ almost always precludes development of cancer, we may predict that prophylactic mastectomy, prostatectomy, or colectomy would save the lives of most of the patients who are still dying of cancer of these organs. The effectiveness rates would approach, but not reach 100%; such is the case with prophylactic mastectomy.
Consider prostate-specific antigen (PSA) screening. Even using the favorable estimate of the impact of PSA screening, arising from the European Randomised Study of Screening for Prostate Cancer trial, 27 men have to be diagnosed, most undergoing local therapy (the trial was conducted before active surveillance became routine), to avert 1 death from prostate cancer over 13 years.9
Contrast this “number needed to diagnose” with the number needed to treat for a strategy of routine prostate removal at age 45 or 50. Given that the lifetime risk of death from prostate cancer approaches 3%, and few cases arise before this age, a prophylactic surgical strategy would avert 1 death per 33 operations. If proponents of screening are willing to accept a number needed to diagnose of 27 over a 13-year interval, they may be willing to consider a number needed to treat of 33 over a lifetime.
There may be harms such as perioperative and postoperative complications. Mastectomy could lead to emotional stress from altered body image. Prostatectomy can have long-term complications such as urinary incontinence and sexual dysfunction. Nevertheless, prophylactic organ removal would save far more lives than current screening practices. It also could decrease mental burden, as patients could rest assured that they will never develop cancer, whereas screening often involves ambiguous test results, follow-up tests, and interventions, increasing patient anxiety.
FINDING THE BALANCE BETWEEN BENEFITS AND HARMS
In truth, we do not really advocate universal mastectomy, prostatectomy, and colectomy to prevent cancer, no more than Swift1 really wanted to eat the children of Ireland to alleviate poverty and famine in that country. Rather, we use it as an extreme proposal to highlight the scope and depth of harms that inevitably arise from screening.
If proponents of aggressive screening believe that the goal is to reduce cause-specific mortality as much as possible, giving little weight or consideration to overdiagnosis and overtreatment, then they ought to embrace universal prophylactic surgery as well. Recognition of this logical consequence reminds us that we must make screening recommendations that balance benefits and harms.
Considering an extreme perspective can help in recognizing our bias toward saving lives from cancer and discounting the harms. Aggravating this bias, it is impossible to know whether an individual patient has avoided fatal cancer or undergone unnecessary treatment. Moreover, changing practice is more difficult if it involves rolling back interventions that were once the standard.
Balancing benefits and harms is especially difficult when trying to compare the benefit of preventing a single cancer death against a harm that is less serious but more common. Medicine has always involved difficult trade-offs, as seen in cost-benefit analysis of new treatments or balancing quality of life with quantity of life in a single patient. In addition, each individual may place different values on benefits of screening and avoiding possible harms.
There is an undeniable trade-off with screening, and we must make a conscious decision on where to draw the line when harms outweigh the benefits. We must proceed with caution when subjecting large numbers of men and women to the possibility of psychological burden and decreased quality of life.
Given the growing appreciation of the harms of screening, it is likely that future guidance will continue to move toward less- frequent screening or focusing resources on high-risk populations, where the absolute magnitude of benefit is greater. Cancer screening is also likely to become an individual decision based on personal values and informed decisions.
I have been assured by a very knowing American of my acquaintance in London, that a young healthy child well nursed is at a year old, a most delicious, nourishing, and wholesome food, whether stewed, roasted, baked, or boiled, and I make no doubt that it will equally serve in a fricassee, or ragout.
—Jonathan Swift, A Modest Proposal1
Large-scale cancer screening programs have the unintended consequences of false-positive results and overdiagnosis, leading to anxiety and overtreatment. The magnitude of these harms continues to be clarified after decades of screening.
Recognizing the trade-off between benefits and harms, the US Preventive Services Task Force (USPSTF) has changed several of its recommendations in recent years. Breast cancer screening recommendations have gone from yearly mammograms starting at age 40 to biennial mammograms starting at age 50 for women at average risk.2 Prostate cancer screening is no longer recommended for men age 70 and older, and even for men between 55 and 69, screening is now an individual decision.3
Newer screening programs are targeting high-risk groups rather than the general population, with the aim of increasing the likelihood of benefits and limiting the harms. For example, lung cancer screening is recommended only for current smokers or smokers who have quit within the past 15 years, are between 55 and 80, and have at least a 30 pack-year smoking history.4
The movement toward less-frequent screening and screening in a narrower population has evoked strong reactions from advocates of cancer screening. One professor of radiology writes, “It borders on unethical to suggest that the benefit of having your life saved by screening and living another 40 years can be balanced against the ‘harm’ of being recalled for additional mammographic views for what proves to not be a cancer.”5 Another notes, “It does not make any sense to throw away the lives saved by screening to avoid over-treating a small number of cancers.”6 Both of these authors defend the position that the goal of screening is to minimize cause-specific mortality, irrespective of overdiagnosis, overtreatment, or false-positive results. In other words, harm should have little to no weight in screening recommendations.
Although the debate on cancer screening is moving toward a more balanced discussion of benefits and harms, many patients are still subjected to screening that is more aggressive than the USPSTF recommends, which may be due to an underlying belief that no harm is greater than the benefit of saving a life.
IS MORE-AGGRESSIVE SCREENING THE ANSWER?
One may wonder if more-aggressive screening could prevent deaths that occur despite standard screening. For example, more-frequent screening or use of additional screening methods such as ultrasonography or magnetic resonance imaging has been suggested for patients at high risk of breast cancer.
A MODEST PROPOSAL
If one holds the view that benefits alone should be considered when writing recommendations about screening, the logical conclusion extends beyond screening. We would therefore like to propose a different approach to reducing cancer deaths in the general population:
Why not just remove everybody’s breasts, prostate gland, and colon before cancer arises?
TO CUT IS TO PREVENT
Currently, we offer prophylactic surgery to patients at high risk of cancer. For example, women with BRCA1/BRCA2 mutations are offered prophylactic mastectomy as one of several options for reducing risk of breast cancer. In 2013, the first case of prophylactic prostatectomy was performed in a man who had a BRCA1/BRCA2 mutation. Total colectomy is considered in men and women who have hereditary nonpolyposis colon cancer, instead of segmental resection, to prevent future cancer.
If prophylactic surgery were extended to the general population, it would greatly reduce the number of cancer deaths. Assuming that removing an organ almost always precludes development of cancer, we may predict that prophylactic mastectomy, prostatectomy, or colectomy would save the lives of most of the patients who are still dying of cancer of these organs. The effectiveness rates would approach, but not reach 100%; such is the case with prophylactic mastectomy.
Consider prostate-specific antigen (PSA) screening. Even using the favorable estimate of the impact of PSA screening, arising from the European Randomised Study of Screening for Prostate Cancer trial, 27 men have to be diagnosed, most undergoing local therapy (the trial was conducted before active surveillance became routine), to avert 1 death from prostate cancer over 13 years.9
Contrast this “number needed to diagnose” with the number needed to treat for a strategy of routine prostate removal at age 45 or 50. Given that the lifetime risk of death from prostate cancer approaches 3%, and few cases arise before this age, a prophylactic surgical strategy would avert 1 death per 33 operations. If proponents of screening are willing to accept a number needed to diagnose of 27 over a 13-year interval, they may be willing to consider a number needed to treat of 33 over a lifetime.
There may be harms such as perioperative and postoperative complications. Mastectomy could lead to emotional stress from altered body image. Prostatectomy can have long-term complications such as urinary incontinence and sexual dysfunction. Nevertheless, prophylactic organ removal would save far more lives than current screening practices. It also could decrease mental burden, as patients could rest assured that they will never develop cancer, whereas screening often involves ambiguous test results, follow-up tests, and interventions, increasing patient anxiety.
FINDING THE BALANCE BETWEEN BENEFITS AND HARMS
In truth, we do not really advocate universal mastectomy, prostatectomy, and colectomy to prevent cancer, no more than Swift1 really wanted to eat the children of Ireland to alleviate poverty and famine in that country. Rather, we use it as an extreme proposal to highlight the scope and depth of harms that inevitably arise from screening.
If proponents of aggressive screening believe that the goal is to reduce cause-specific mortality as much as possible, giving little weight or consideration to overdiagnosis and overtreatment, then they ought to embrace universal prophylactic surgery as well. Recognition of this logical consequence reminds us that we must make screening recommendations that balance benefits and harms.
Considering an extreme perspective can help in recognizing our bias toward saving lives from cancer and discounting the harms. Aggravating this bias, it is impossible to know whether an individual patient has avoided fatal cancer or undergone unnecessary treatment. Moreover, changing practice is more difficult if it involves rolling back interventions that were once the standard.
Balancing benefits and harms is especially difficult when trying to compare the benefit of preventing a single cancer death against a harm that is less serious but more common. Medicine has always involved difficult trade-offs, as seen in cost-benefit analysis of new treatments or balancing quality of life with quantity of life in a single patient. In addition, each individual may place different values on benefits of screening and avoiding possible harms.
There is an undeniable trade-off with screening, and we must make a conscious decision on where to draw the line when harms outweigh the benefits. We must proceed with caution when subjecting large numbers of men and women to the possibility of psychological burden and decreased quality of life.
Given the growing appreciation of the harms of screening, it is likely that future guidance will continue to move toward less- frequent screening or focusing resources on high-risk populations, where the absolute magnitude of benefit is greater. Cancer screening is also likely to become an individual decision based on personal values and informed decisions.
- Swift J. A Modest Proposal for Preventing the Children of Poor People in Ireland, from Being a Burden on Their Parents or Country, and for Making Them Beneficial to the Publick. Dublin: S. Harding, 1729.
- Nelson HD, Cantor A, Humphrey L, et al. Screening for breast cancer: a systematic review to update the 2009 US Preventive Services Task Force Recommendation. www.ncbi.nlm.nih.gov/books/NBK343819. Accessed February 13, 2019.
- US Preventive Services Task Force; Grossman DC, Curry SJ, Owens DK, et al. Screening for prostate cancer: US Preventive Services Task Force Recommendation Statement. JAMA 2018; 319(18):1901–1913. doi:10.1001/jama.2018.3710
- Humphrey L, Deffebach M, Pappas M, et al. Screening for lung cancer: systematic review to update the US Preventive Services Task Force Recommendation. www.ncbi.nlm.nih.gov/books/NBK154610. Accessed February 13, 2019.
- Kopans DB. A review of: “Tipping the balance of benefits and harms to favor screening mammography starting at age 40 years.” www.sbi-online.org/Portals/0/downloads/documents/pdfs/A%20review%20of%20Tipping%20the%20Balance%20of%20Benefits%20and%20Harms%20to%20Favor%20Screening%20Mammography%20Starting%20at%20Age%2040%20Years%20-%20Kopans.pdf. Accessed February 13, 2019.
- Yaffe M, Gordon, P. Routine mammograms do save lives: U of T expert. U of T News. www.utoronto.ca/news/routine-mammograms-do-save-lives-u-t-expert. Accessed February 13, 2019.
- Lin JS, Piper MA, Perdue LA, et al. Screening for colorectal cancer: updated evidence report and systematic review for the US Preventive Services Task Force. JAMA 2016; 315(23):2576–2594. doi:10.1001/jama.2016.3332
- Knudsen AB, Zauber AG, Rutter CM, et al. Estimation of benefits, burden, and harms of colorectal cancer screening strategies: modeling study for the US Preventive Services Task Force. JAMA 2016; 315(23):2595–2609. doi:10.1001/jama.2016.6828
- Schröder FH, Hugosson J, Roobol MJ, et al; ERSPC Investigators. Screening and prostate cancer mortality: results of the European Randomised Study of Screening for Prostate Cancer (ERSPC) at 13 years of follow-up. Lancet 2014; 384(9959):2027–2035. doi:10.1016/S0140-6736(14)60525-0
- Swift J. A Modest Proposal for Preventing the Children of Poor People in Ireland, from Being a Burden on Their Parents or Country, and for Making Them Beneficial to the Publick. Dublin: S. Harding, 1729.
- Nelson HD, Cantor A, Humphrey L, et al. Screening for breast cancer: a systematic review to update the 2009 US Preventive Services Task Force Recommendation. www.ncbi.nlm.nih.gov/books/NBK343819. Accessed February 13, 2019.
- US Preventive Services Task Force; Grossman DC, Curry SJ, Owens DK, et al. Screening for prostate cancer: US Preventive Services Task Force Recommendation Statement. JAMA 2018; 319(18):1901–1913. doi:10.1001/jama.2018.3710
- Humphrey L, Deffebach M, Pappas M, et al. Screening for lung cancer: systematic review to update the US Preventive Services Task Force Recommendation. www.ncbi.nlm.nih.gov/books/NBK154610. Accessed February 13, 2019.
- Kopans DB. A review of: “Tipping the balance of benefits and harms to favor screening mammography starting at age 40 years.” www.sbi-online.org/Portals/0/downloads/documents/pdfs/A%20review%20of%20Tipping%20the%20Balance%20of%20Benefits%20and%20Harms%20to%20Favor%20Screening%20Mammography%20Starting%20at%20Age%2040%20Years%20-%20Kopans.pdf. Accessed February 13, 2019.
- Yaffe M, Gordon, P. Routine mammograms do save lives: U of T expert. U of T News. www.utoronto.ca/news/routine-mammograms-do-save-lives-u-t-expert. Accessed February 13, 2019.
- Lin JS, Piper MA, Perdue LA, et al. Screening for colorectal cancer: updated evidence report and systematic review for the US Preventive Services Task Force. JAMA 2016; 315(23):2576–2594. doi:10.1001/jama.2016.3332
- Knudsen AB, Zauber AG, Rutter CM, et al. Estimation of benefits, burden, and harms of colorectal cancer screening strategies: modeling study for the US Preventive Services Task Force. JAMA 2016; 315(23):2595–2609. doi:10.1001/jama.2016.6828
- Schröder FH, Hugosson J, Roobol MJ, et al; ERSPC Investigators. Screening and prostate cancer mortality: results of the European Randomised Study of Screening for Prostate Cancer (ERSPC) at 13 years of follow-up. Lancet 2014; 384(9959):2027–2035. doi:10.1016/S0140-6736(14)60525-0
Correction: Hypertension guidelines
In Aleyadeh W, Hutt-Centeno E, Ahmed HM, Shah NP. Hypertension guidelines: treat patients, not numbers. Cleve Clin J Med 2019; 86(1):47–56. doi:10.3949/ccjm.86a.18027, on page 50, the following statement was incorrect: “In 2017, the American College of Physicians (ACP) and the American Academy of Family Physicians (AAFP) recommended a relaxed systolic blood pressure target, ie, below 150 mm Hg, for adults over age 60, but a tighter goal of less than 130 mm Hg for the same age group if they have transient ischemic attack, stroke, or high cardiovascular risk.9” In fact, the ACP and AAFP recommended a tighter goal of less than 140 mm Hg for this higher-risk group. This has been corrected online.
In Aleyadeh W, Hutt-Centeno E, Ahmed HM, Shah NP. Hypertension guidelines: treat patients, not numbers. Cleve Clin J Med 2019; 86(1):47–56. doi:10.3949/ccjm.86a.18027, on page 50, the following statement was incorrect: “In 2017, the American College of Physicians (ACP) and the American Academy of Family Physicians (AAFP) recommended a relaxed systolic blood pressure target, ie, below 150 mm Hg, for adults over age 60, but a tighter goal of less than 130 mm Hg for the same age group if they have transient ischemic attack, stroke, or high cardiovascular risk.9” In fact, the ACP and AAFP recommended a tighter goal of less than 140 mm Hg for this higher-risk group. This has been corrected online.
In Aleyadeh W, Hutt-Centeno E, Ahmed HM, Shah NP. Hypertension guidelines: treat patients, not numbers. Cleve Clin J Med 2019; 86(1):47–56. doi:10.3949/ccjm.86a.18027, on page 50, the following statement was incorrect: “In 2017, the American College of Physicians (ACP) and the American Academy of Family Physicians (AAFP) recommended a relaxed systolic blood pressure target, ie, below 150 mm Hg, for adults over age 60, but a tighter goal of less than 130 mm Hg for the same age group if they have transient ischemic attack, stroke, or high cardiovascular risk.9” In fact, the ACP and AAFP recommended a tighter goal of less than 140 mm Hg for this higher-risk group. This has been corrected online.
Masterclass: Marlene Freeman on treating bipolar disorder in women
during pregnancy. Dr. Freeman, associate professor of psychiatry at Harvard Medical School, also shares preliminary data on the impact of exposure to atypical antipsychotics from the National Pregnancy Registry for Psychiatric Medications at Massachusetts General Hospital, where she also serves as associate director of Women’s Mental Health.
Amazon
Apple Podcasts
Google Podcasts
Spotify
during pregnancy. Dr. Freeman, associate professor of psychiatry at Harvard Medical School, also shares preliminary data on the impact of exposure to atypical antipsychotics from the National Pregnancy Registry for Psychiatric Medications at Massachusetts General Hospital, where she also serves as associate director of Women’s Mental Health.
Amazon
Apple Podcasts
Google Podcasts
Spotify
during pregnancy. Dr. Freeman, associate professor of psychiatry at Harvard Medical School, also shares preliminary data on the impact of exposure to atypical antipsychotics from the National Pregnancy Registry for Psychiatric Medications at Massachusetts General Hospital, where she also serves as associate director of Women’s Mental Health.
Amazon
Apple Podcasts
Google Podcasts
Spotify
Insulin-treated diabetes in pregnancy carries preterm risk
Women with insulin-treated diabetes are at significantly greater risk of preterm birth and of delivering babies who are large for gestational age (LGA), regardless of prepregnancy body weight, new findings suggest.
Researchers examined the role of maternal diabetes and weight on pregnancy outcomes in the population-based cohort study. The study comprised 649,043 live births in Finland between Jan. 1, 2004, and Dec. 31, 2014, including 4,000 in women with insulin-treated diabetes, 3,740 in women with type 2 diabetes, and 98,568 women with gestational diabetes.
Prepregnancy body mass index was normal for nearly 60% of mothers, while 4% were underweight, 21% were overweight, 8% were moderately obese, and 4% were severely obese.
Overall, the researchers found that women with insulin-treated diabetes had a 43-fold higher odds of having an LGA infant, compared with the reference group of women of normal BMI without diabetes (adjusted odds ratio [aOR], 43.80; 95% confidence interval, 40.88-46.93). And there was an 11-fold greater odds of having a preterm birth in this group (aOR, 11.17; 95% CI, 10.46-11.93).
The findings were published in JAMA Pediatrics.
“Smaller, but clearly statistically significant, increased LGA risks were found also for mothers with type 2 diabetes and gestational diabetes not treated with insulin, especially in combination with prepregnancy overweight or obesity that were stronger for type 2 diabetes than gestational diabetes,” wrote Linghua Kong, MSc, of the department of molecular medicine and surgery at Karolinska Institutet, and coauthors.
The aOR for LGA among women with type 2 diabetes was 9.57 (95% CI, 8.65-10.58), compared with the reference group. And for women with maternal gestational diabetes, the aOR for LGA was 3.80 (95% CI, 3.66-3.96).
Looking at the risk for preterm birth, the researchers found that the aOR among women with type 2 diabetes was 2.12 (95% CI, 1.90-2.36), while there was no association between gestational diabetes and preterm birth.
The researchers also reported that for women with gestational diabetes or no diabetes, the odds of preterm birth increased slightly as maternal prepregnancy BMI increased.
“Maternal glucose metabolism during pregnancy differs from that in the non-pregnant state; insulin resistance is increased, directing fat as the mother’s energy source to ensure adequate carbohydrate supply for the growing fetus,” the researchers wrote. “This increase in insulin resistance is mediated by a number of factors, such as increased levels of progesterone, estrogen, and human placental lactogen.”
The authors noted that their data did not include information on congenital anomalies, maternal complications such as preeclampsia, and grade of diabetes control during pregnancy. In addition, the data on maternal BMI was derived from a single time point.
“These findings may have implications for counseling and managing pregnancies to prevent adverse birth outcomes,” they wrote.
The study and some authors were supported by the THL National Institute for Health and Welfare, the Swedish Research Council, Stockholm County Council, the China Scholarship Council, and the Swedish Brain Foundation.
SOURCE: Kong L et al. JAMA Pediatr. 2019 Feb 25. doi: 10.1001/jamapediatrics.2018.5541.
Women with insulin-treated diabetes are at significantly greater risk of preterm birth and of delivering babies who are large for gestational age (LGA), regardless of prepregnancy body weight, new findings suggest.
Researchers examined the role of maternal diabetes and weight on pregnancy outcomes in the population-based cohort study. The study comprised 649,043 live births in Finland between Jan. 1, 2004, and Dec. 31, 2014, including 4,000 in women with insulin-treated diabetes, 3,740 in women with type 2 diabetes, and 98,568 women with gestational diabetes.
Prepregnancy body mass index was normal for nearly 60% of mothers, while 4% were underweight, 21% were overweight, 8% were moderately obese, and 4% were severely obese.
Overall, the researchers found that women with insulin-treated diabetes had a 43-fold higher odds of having an LGA infant, compared with the reference group of women of normal BMI without diabetes (adjusted odds ratio [aOR], 43.80; 95% confidence interval, 40.88-46.93). And there was an 11-fold greater odds of having a preterm birth in this group (aOR, 11.17; 95% CI, 10.46-11.93).
The findings were published in JAMA Pediatrics.
“Smaller, but clearly statistically significant, increased LGA risks were found also for mothers with type 2 diabetes and gestational diabetes not treated with insulin, especially in combination with prepregnancy overweight or obesity that were stronger for type 2 diabetes than gestational diabetes,” wrote Linghua Kong, MSc, of the department of molecular medicine and surgery at Karolinska Institutet, and coauthors.
The aOR for LGA among women with type 2 diabetes was 9.57 (95% CI, 8.65-10.58), compared with the reference group. And for women with maternal gestational diabetes, the aOR for LGA was 3.80 (95% CI, 3.66-3.96).
Looking at the risk for preterm birth, the researchers found that the aOR among women with type 2 diabetes was 2.12 (95% CI, 1.90-2.36), while there was no association between gestational diabetes and preterm birth.
The researchers also reported that for women with gestational diabetes or no diabetes, the odds of preterm birth increased slightly as maternal prepregnancy BMI increased.
“Maternal glucose metabolism during pregnancy differs from that in the non-pregnant state; insulin resistance is increased, directing fat as the mother’s energy source to ensure adequate carbohydrate supply for the growing fetus,” the researchers wrote. “This increase in insulin resistance is mediated by a number of factors, such as increased levels of progesterone, estrogen, and human placental lactogen.”
The authors noted that their data did not include information on congenital anomalies, maternal complications such as preeclampsia, and grade of diabetes control during pregnancy. In addition, the data on maternal BMI was derived from a single time point.
“These findings may have implications for counseling and managing pregnancies to prevent adverse birth outcomes,” they wrote.
The study and some authors were supported by the THL National Institute for Health and Welfare, the Swedish Research Council, Stockholm County Council, the China Scholarship Council, and the Swedish Brain Foundation.
SOURCE: Kong L et al. JAMA Pediatr. 2019 Feb 25. doi: 10.1001/jamapediatrics.2018.5541.
Women with insulin-treated diabetes are at significantly greater risk of preterm birth and of delivering babies who are large for gestational age (LGA), regardless of prepregnancy body weight, new findings suggest.
Researchers examined the role of maternal diabetes and weight on pregnancy outcomes in the population-based cohort study. The study comprised 649,043 live births in Finland between Jan. 1, 2004, and Dec. 31, 2014, including 4,000 in women with insulin-treated diabetes, 3,740 in women with type 2 diabetes, and 98,568 women with gestational diabetes.
Prepregnancy body mass index was normal for nearly 60% of mothers, while 4% were underweight, 21% were overweight, 8% were moderately obese, and 4% were severely obese.
Overall, the researchers found that women with insulin-treated diabetes had a 43-fold higher odds of having an LGA infant, compared with the reference group of women of normal BMI without diabetes (adjusted odds ratio [aOR], 43.80; 95% confidence interval, 40.88-46.93). And there was an 11-fold greater odds of having a preterm birth in this group (aOR, 11.17; 95% CI, 10.46-11.93).
The findings were published in JAMA Pediatrics.
“Smaller, but clearly statistically significant, increased LGA risks were found also for mothers with type 2 diabetes and gestational diabetes not treated with insulin, especially in combination with prepregnancy overweight or obesity that were stronger for type 2 diabetes than gestational diabetes,” wrote Linghua Kong, MSc, of the department of molecular medicine and surgery at Karolinska Institutet, and coauthors.
The aOR for LGA among women with type 2 diabetes was 9.57 (95% CI, 8.65-10.58), compared with the reference group. And for women with maternal gestational diabetes, the aOR for LGA was 3.80 (95% CI, 3.66-3.96).
Looking at the risk for preterm birth, the researchers found that the aOR among women with type 2 diabetes was 2.12 (95% CI, 1.90-2.36), while there was no association between gestational diabetes and preterm birth.
The researchers also reported that for women with gestational diabetes or no diabetes, the odds of preterm birth increased slightly as maternal prepregnancy BMI increased.
“Maternal glucose metabolism during pregnancy differs from that in the non-pregnant state; insulin resistance is increased, directing fat as the mother’s energy source to ensure adequate carbohydrate supply for the growing fetus,” the researchers wrote. “This increase in insulin resistance is mediated by a number of factors, such as increased levels of progesterone, estrogen, and human placental lactogen.”
The authors noted that their data did not include information on congenital anomalies, maternal complications such as preeclampsia, and grade of diabetes control during pregnancy. In addition, the data on maternal BMI was derived from a single time point.
“These findings may have implications for counseling and managing pregnancies to prevent adverse birth outcomes,” they wrote.
The study and some authors were supported by the THL National Institute for Health and Welfare, the Swedish Research Council, Stockholm County Council, the China Scholarship Council, and the Swedish Brain Foundation.
SOURCE: Kong L et al. JAMA Pediatr. 2019 Feb 25. doi: 10.1001/jamapediatrics.2018.5541.
FROM JAMA PEDIATRICS
Key clinical point:
Major finding: Pregnant women with insulin-treated diabetes have a 43-fold higher odds of having a child who is large for gestational age and 11-fold high risk for preterm birth.
Study details: A population-based cohort study of 649,043 live births in Finland between 2004 and 2014.
Disclosures: The study and some authors were supported by the THL National Institute for Health and Welfare, the Swedish Research Council, Stockholm County Council, the China Scholarship Council, and the Swedish Brain Foundation.
Source: Kong L et al. JAMA Pediatr. 2019 Feb 25. doi: 10.1001/jamapediatrics.2018.5541.
Female Sexual Dysfunction
Trump bars abortion referrals from family planning program
The U.S. Department of Health & Human Services has finalized sweeping changes to the federal Title X family planning program, pulling back funds from clinics that provide abortion counseling or that refer patients for abortion services, regardless of whether the money is used for other health care services.
Under the final rule, announced Feb. 22 by HHS, women’s health clinics are ineligible for Title X funding if they offer, promote, or support abortion as a method of family planning. Title X grants generally go to health centers that provide reproductive health care – such as STD-testing, cancer screenings, and contraception – to low-income families.
In a fact sheet, HHS stated the final rule will provide for clear financial and physical separation between Title X and non-Title X activities, reduce confusion on the part of Title X clinics and the public about permissible Title X activities, and improve program transparency by requiring more complete reporting by grantees about their partnerships with referral agencies.
“The final rule ensures compliance with statutory program integrity provisions governing the program and, in particular, the statutory prohibition on funding programs where abortion is a method of family planning,” department officials said in a statement. “The final rule amends the Title X regulation, which had not been substantially updated in nearly 2 decades, and makes notable improvements designed to increase the number of patients served and improve the quality of their care.”
Lisa Hollier, MD, president for the American College of Obstetricians and Gynecologists (ACOG) said the final rule threatens the ability of women’s health care providers to deliver medically accurate and comprehensive reproductive health care and poses significant harms to women’s health.
“As the only federal program exclusively dedicated to providing low-income patients with access to family planning and preventive health services and information, [Title X] plays a vital role in the landscape of women’s health care,” Dr. Hollier said during a Feb. 22 press conference. “By weakening the requirements for the scope of contraceptive care provided by grant recipients and restricting the types of care recipients can discuss with patients, the final rule fundamentally harms the scope and purpose of this historic program.”
The American Medical Association also expressed disappointment, referring to the final requirement as a “gag rule” between physicians and patients.
“This rule interferes with and imposes restrictions on the patient-physician relationship,” Barbara L. McAneny, MD, AMA President said in a statement. “For all intents and purposes, it imposes a gag rule on what information physicians can provide to their patients. The patient-physician relationship relies on trust, open conversation and informed decision making and the government should not be telling physicians what they can and cannot say to their patients.”
Under the rule, proposed last year, physicians are prohibited from discussing abortion options with pregnant women, from sharing abortion information, and from making abortion referrals if the clinic receives Title X funds. The regulation permits, but no longer requires, nondirective pregnancy counseling, including nondirective counseling on abortion. In its statement, HHS officials said the new rule ensures “conscience protections” for Title X health providers by eliminating the requirement for providers to counsel on and refer for abortion.
Susan B. Anthony List, an anti-abortion group, praised the final rule as a measure that disentangles taxpayers from the “big abortion industry led by Planned Parenthood.”
“The Protect Life Rule does not cut family planning funding by a single dime, and instead directs tax dollars to entities that provide health care to women but do not perform abortions,” said SBA List President Marjorie Dannenfelser in a statement. “The Title X program was not intended to be a slush fund for abortion businesses like Planned Parenthood, which violently ends the lives of more than 332,000 unborn babies a year and receives almost $60 million a year in Title X taxpayer dollars.”
Emily Stewart, vice president of public policy for the Planned Parenthood Federation of America indicated that the group plans to fight the rule in court.
“Since day one, the Trump-Pence administration has aggressively targeted the health, rights, and bodily autonomy of people of color, people with low incomes, and women,” she said in a statement. “We’re going to fight this rule through every possible avenue.”
The final rule has been submitted to the Federal Register for publication.
The U.S. Department of Health & Human Services has finalized sweeping changes to the federal Title X family planning program, pulling back funds from clinics that provide abortion counseling or that refer patients for abortion services, regardless of whether the money is used for other health care services.
Under the final rule, announced Feb. 22 by HHS, women’s health clinics are ineligible for Title X funding if they offer, promote, or support abortion as a method of family planning. Title X grants generally go to health centers that provide reproductive health care – such as STD-testing, cancer screenings, and contraception – to low-income families.
In a fact sheet, HHS stated the final rule will provide for clear financial and physical separation between Title X and non-Title X activities, reduce confusion on the part of Title X clinics and the public about permissible Title X activities, and improve program transparency by requiring more complete reporting by grantees about their partnerships with referral agencies.
“The final rule ensures compliance with statutory program integrity provisions governing the program and, in particular, the statutory prohibition on funding programs where abortion is a method of family planning,” department officials said in a statement. “The final rule amends the Title X regulation, which had not been substantially updated in nearly 2 decades, and makes notable improvements designed to increase the number of patients served and improve the quality of their care.”
Lisa Hollier, MD, president for the American College of Obstetricians and Gynecologists (ACOG) said the final rule threatens the ability of women’s health care providers to deliver medically accurate and comprehensive reproductive health care and poses significant harms to women’s health.
“As the only federal program exclusively dedicated to providing low-income patients with access to family planning and preventive health services and information, [Title X] plays a vital role in the landscape of women’s health care,” Dr. Hollier said during a Feb. 22 press conference. “By weakening the requirements for the scope of contraceptive care provided by grant recipients and restricting the types of care recipients can discuss with patients, the final rule fundamentally harms the scope and purpose of this historic program.”
The American Medical Association also expressed disappointment, referring to the final requirement as a “gag rule” between physicians and patients.
“This rule interferes with and imposes restrictions on the patient-physician relationship,” Barbara L. McAneny, MD, AMA President said in a statement. “For all intents and purposes, it imposes a gag rule on what information physicians can provide to their patients. The patient-physician relationship relies on trust, open conversation and informed decision making and the government should not be telling physicians what they can and cannot say to their patients.”
Under the rule, proposed last year, physicians are prohibited from discussing abortion options with pregnant women, from sharing abortion information, and from making abortion referrals if the clinic receives Title X funds. The regulation permits, but no longer requires, nondirective pregnancy counseling, including nondirective counseling on abortion. In its statement, HHS officials said the new rule ensures “conscience protections” for Title X health providers by eliminating the requirement for providers to counsel on and refer for abortion.
Susan B. Anthony List, an anti-abortion group, praised the final rule as a measure that disentangles taxpayers from the “big abortion industry led by Planned Parenthood.”
“The Protect Life Rule does not cut family planning funding by a single dime, and instead directs tax dollars to entities that provide health care to women but do not perform abortions,” said SBA List President Marjorie Dannenfelser in a statement. “The Title X program was not intended to be a slush fund for abortion businesses like Planned Parenthood, which violently ends the lives of more than 332,000 unborn babies a year and receives almost $60 million a year in Title X taxpayer dollars.”
Emily Stewart, vice president of public policy for the Planned Parenthood Federation of America indicated that the group plans to fight the rule in court.
“Since day one, the Trump-Pence administration has aggressively targeted the health, rights, and bodily autonomy of people of color, people with low incomes, and women,” she said in a statement. “We’re going to fight this rule through every possible avenue.”
The final rule has been submitted to the Federal Register for publication.
The U.S. Department of Health & Human Services has finalized sweeping changes to the federal Title X family planning program, pulling back funds from clinics that provide abortion counseling or that refer patients for abortion services, regardless of whether the money is used for other health care services.
Under the final rule, announced Feb. 22 by HHS, women’s health clinics are ineligible for Title X funding if they offer, promote, or support abortion as a method of family planning. Title X grants generally go to health centers that provide reproductive health care – such as STD-testing, cancer screenings, and contraception – to low-income families.
In a fact sheet, HHS stated the final rule will provide for clear financial and physical separation between Title X and non-Title X activities, reduce confusion on the part of Title X clinics and the public about permissible Title X activities, and improve program transparency by requiring more complete reporting by grantees about their partnerships with referral agencies.
“The final rule ensures compliance with statutory program integrity provisions governing the program and, in particular, the statutory prohibition on funding programs where abortion is a method of family planning,” department officials said in a statement. “The final rule amends the Title X regulation, which had not been substantially updated in nearly 2 decades, and makes notable improvements designed to increase the number of patients served and improve the quality of their care.”
Lisa Hollier, MD, president for the American College of Obstetricians and Gynecologists (ACOG) said the final rule threatens the ability of women’s health care providers to deliver medically accurate and comprehensive reproductive health care and poses significant harms to women’s health.
“As the only federal program exclusively dedicated to providing low-income patients with access to family planning and preventive health services and information, [Title X] plays a vital role in the landscape of women’s health care,” Dr. Hollier said during a Feb. 22 press conference. “By weakening the requirements for the scope of contraceptive care provided by grant recipients and restricting the types of care recipients can discuss with patients, the final rule fundamentally harms the scope and purpose of this historic program.”
The American Medical Association also expressed disappointment, referring to the final requirement as a “gag rule” between physicians and patients.
“This rule interferes with and imposes restrictions on the patient-physician relationship,” Barbara L. McAneny, MD, AMA President said in a statement. “For all intents and purposes, it imposes a gag rule on what information physicians can provide to their patients. The patient-physician relationship relies on trust, open conversation and informed decision making and the government should not be telling physicians what they can and cannot say to their patients.”
Under the rule, proposed last year, physicians are prohibited from discussing abortion options with pregnant women, from sharing abortion information, and from making abortion referrals if the clinic receives Title X funds. The regulation permits, but no longer requires, nondirective pregnancy counseling, including nondirective counseling on abortion. In its statement, HHS officials said the new rule ensures “conscience protections” for Title X health providers by eliminating the requirement for providers to counsel on and refer for abortion.
Susan B. Anthony List, an anti-abortion group, praised the final rule as a measure that disentangles taxpayers from the “big abortion industry led by Planned Parenthood.”
“The Protect Life Rule does not cut family planning funding by a single dime, and instead directs tax dollars to entities that provide health care to women but do not perform abortions,” said SBA List President Marjorie Dannenfelser in a statement. “The Title X program was not intended to be a slush fund for abortion businesses like Planned Parenthood, which violently ends the lives of more than 332,000 unborn babies a year and receives almost $60 million a year in Title X taxpayer dollars.”
Emily Stewart, vice president of public policy for the Planned Parenthood Federation of America indicated that the group plans to fight the rule in court.
“Since day one, the Trump-Pence administration has aggressively targeted the health, rights, and bodily autonomy of people of color, people with low incomes, and women,” she said in a statement. “We’re going to fight this rule through every possible avenue.”
The final rule has been submitted to the Federal Register for publication.
Combination model predicts imminent preeclampsia
A triple test was a significantly more effective predictor of preeclampsia than was either angiogenic placental growth factor (PlGF) alone or the antiangiogenic factor soluble fms-like tyrosine kinase-1(sFLT)/PlGF ratio, based on data from more than 15,000 pregnancies.
The use of either PlGF or sFLT/PlGF alone to predict preeclampsia fails to account for individual maternal risk factors or the measurement of blood pressure at presentation, wrote Anca Ciobanu, MD, of King’s College London, and her colleagues.
In a study published in the American Journal of Obstetrics & Gynecology, the researchers reviewed data from 15,247 singleton pregnancies with live births of healthy babies and compared the preeclampsia detection rates of PlGF, sFLT/PlGF and a competing risks model that included a combination of maternal factors and median values of PlGF, sFLT, and mean arterial pressure (triple test). Preeclampsia developed in 2.1% of pregnancies.
Overall, the triple-test detection rate for delivery with preeclampsia was 10% higher than the sFLT/PlGF ratio and 20% higher than PlGF alone based on assessment at 2 weeks or less or 4 weeks or less before delivery. The negative predictive value was similar for the three tests.
At 2 weeks or less before delivery, the area under the receiver operating characteristic curves (AUROC) for preeclampsia was significantly higher for the combination model (0.975), compared with PlGF (0.900) or the sFLT/PlGF ratio (0.932), with P less than .0001 in each case. Similarly, at 4 weeks or less before delivery, the AUROC for preeclampsia was 0.907 for the triple test, 0.827 for PlGF alone, and 0.857 for the sFLT/PlGF ratio, with P less than .0001 in each case.
The competing risks model allows clinicians more flexibility to identify patients at increased risk by considering factors including maternal characteristics and variations from normal blood pressure, Dr. Ciobanu and her associates noted. Also, the combination model, “can form the basis of future research that would quantify and incorporate into the model, symptoms such as headache and epigastric pain, as well as proteinuria, creatinine, liver enzymes and platelets.”
The study findings were limited by several factors including the potential predictive value of screening for women with hypertensive symptoms attending specialist clinics, and whether mean arterial pressure would be an effective measure in patients seen at these clinics, the researchers noted. However, the results support the value of the competing risks model, which “provides a personalized risk for delivery with preeclampsia that could lead to personalized stratification of the intensity of monitoring and timing of delivery.”
The study was supported by a grant from the Fetal Medicine Foundation; Thermo Fisher Scientific provided the reagents and equipment. The researchers had no financial conflicts of interest.
SOURCE: Ciobanu A et al. Am J Obstet Gynecol. 2019 Feb 7. doi. org/10.1016/j.ajog.2019.01.235.
A triple test was a significantly more effective predictor of preeclampsia than was either angiogenic placental growth factor (PlGF) alone or the antiangiogenic factor soluble fms-like tyrosine kinase-1(sFLT)/PlGF ratio, based on data from more than 15,000 pregnancies.
The use of either PlGF or sFLT/PlGF alone to predict preeclampsia fails to account for individual maternal risk factors or the measurement of blood pressure at presentation, wrote Anca Ciobanu, MD, of King’s College London, and her colleagues.
In a study published in the American Journal of Obstetrics & Gynecology, the researchers reviewed data from 15,247 singleton pregnancies with live births of healthy babies and compared the preeclampsia detection rates of PlGF, sFLT/PlGF and a competing risks model that included a combination of maternal factors and median values of PlGF, sFLT, and mean arterial pressure (triple test). Preeclampsia developed in 2.1% of pregnancies.
Overall, the triple-test detection rate for delivery with preeclampsia was 10% higher than the sFLT/PlGF ratio and 20% higher than PlGF alone based on assessment at 2 weeks or less or 4 weeks or less before delivery. The negative predictive value was similar for the three tests.
At 2 weeks or less before delivery, the area under the receiver operating characteristic curves (AUROC) for preeclampsia was significantly higher for the combination model (0.975), compared with PlGF (0.900) or the sFLT/PlGF ratio (0.932), with P less than .0001 in each case. Similarly, at 4 weeks or less before delivery, the AUROC for preeclampsia was 0.907 for the triple test, 0.827 for PlGF alone, and 0.857 for the sFLT/PlGF ratio, with P less than .0001 in each case.
The competing risks model allows clinicians more flexibility to identify patients at increased risk by considering factors including maternal characteristics and variations from normal blood pressure, Dr. Ciobanu and her associates noted. Also, the combination model, “can form the basis of future research that would quantify and incorporate into the model, symptoms such as headache and epigastric pain, as well as proteinuria, creatinine, liver enzymes and platelets.”
The study findings were limited by several factors including the potential predictive value of screening for women with hypertensive symptoms attending specialist clinics, and whether mean arterial pressure would be an effective measure in patients seen at these clinics, the researchers noted. However, the results support the value of the competing risks model, which “provides a personalized risk for delivery with preeclampsia that could lead to personalized stratification of the intensity of monitoring and timing of delivery.”
The study was supported by a grant from the Fetal Medicine Foundation; Thermo Fisher Scientific provided the reagents and equipment. The researchers had no financial conflicts of interest.
SOURCE: Ciobanu A et al. Am J Obstet Gynecol. 2019 Feb 7. doi. org/10.1016/j.ajog.2019.01.235.
A triple test was a significantly more effective predictor of preeclampsia than was either angiogenic placental growth factor (PlGF) alone or the antiangiogenic factor soluble fms-like tyrosine kinase-1(sFLT)/PlGF ratio, based on data from more than 15,000 pregnancies.
The use of either PlGF or sFLT/PlGF alone to predict preeclampsia fails to account for individual maternal risk factors or the measurement of blood pressure at presentation, wrote Anca Ciobanu, MD, of King’s College London, and her colleagues.
In a study published in the American Journal of Obstetrics & Gynecology, the researchers reviewed data from 15,247 singleton pregnancies with live births of healthy babies and compared the preeclampsia detection rates of PlGF, sFLT/PlGF and a competing risks model that included a combination of maternal factors and median values of PlGF, sFLT, and mean arterial pressure (triple test). Preeclampsia developed in 2.1% of pregnancies.
Overall, the triple-test detection rate for delivery with preeclampsia was 10% higher than the sFLT/PlGF ratio and 20% higher than PlGF alone based on assessment at 2 weeks or less or 4 weeks or less before delivery. The negative predictive value was similar for the three tests.
At 2 weeks or less before delivery, the area under the receiver operating characteristic curves (AUROC) for preeclampsia was significantly higher for the combination model (0.975), compared with PlGF (0.900) or the sFLT/PlGF ratio (0.932), with P less than .0001 in each case. Similarly, at 4 weeks or less before delivery, the AUROC for preeclampsia was 0.907 for the triple test, 0.827 for PlGF alone, and 0.857 for the sFLT/PlGF ratio, with P less than .0001 in each case.
The competing risks model allows clinicians more flexibility to identify patients at increased risk by considering factors including maternal characteristics and variations from normal blood pressure, Dr. Ciobanu and her associates noted. Also, the combination model, “can form the basis of future research that would quantify and incorporate into the model, symptoms such as headache and epigastric pain, as well as proteinuria, creatinine, liver enzymes and platelets.”
The study findings were limited by several factors including the potential predictive value of screening for women with hypertensive symptoms attending specialist clinics, and whether mean arterial pressure would be an effective measure in patients seen at these clinics, the researchers noted. However, the results support the value of the competing risks model, which “provides a personalized risk for delivery with preeclampsia that could lead to personalized stratification of the intensity of monitoring and timing of delivery.”
The study was supported by a grant from the Fetal Medicine Foundation; Thermo Fisher Scientific provided the reagents and equipment. The researchers had no financial conflicts of interest.
SOURCE: Ciobanu A et al. Am J Obstet Gynecol. 2019 Feb 7. doi. org/10.1016/j.ajog.2019.01.235.
FROM THE AMERICAN JOURNAL OF OBSTETRICS & GYNECOLOGY
Delayed cord clamping didn’t drop maternal hemoglobin in term cesarean deliveries
LAS VEGAS – according to a recent study.
The change in maternal hemoglobin from preoperative level to postoperative day 1, the study’s primary outcome measure, was not significantly different whether the umbilical cord was clamped within 15 seconds of delivery or clamping was delayed for 1 minute.
For the 56 women who received immediate cord clamping, hemoglobin dropped a mean 1.78 g/dL; for the 57 women who received delayed cord clamping, the drop was 1.85 g/dL (P = .69). Mean estimated blood loss for the delayed clamping group was numerically higher at 884 mL, compared with 830 mL for the immediate clamping group, but this was not a statistically significant difference (P = .13)
However, the practice did result in significantly greater neonatal hemoglobin measured at 24-72 hours post delivery. Hemoglobin data were available for 90 infants, or about 80% of participants. For the 44 infants in the immediate clamping group, mean hemoglobin was 16.4 g/dL; for the delayed clamping group, the figure was 18.1 g/dL (P less than .01).
Although delayed cord clamping has clear benefits to the neonate, whether the practice adversely affects women undergoing cesarean was not clear, said Stephanie Purisch, MD, who discussed the findings of the two-site, randomized, clinical trial during a fellows research session at the meeting sponsored by the Society for Maternal-Fetal Medicine.
“Maternal outcomes have not been a focus of research” in the cord clamping literature, said Dr. Purisch, from Columbia University, New York. A 2013 Cochrane review found that delayed cord clamping did not change postpartum hemoglobin levels or increase blood loss or the need for transfusion. However, she said, the review included only healthy women who expected a vaginal delivery, so cesarean deliveries were undersampled in the data.
Of the 3,911 deliveries included in all prior randomized, controlled trials of delayed cord clamping, just 87, or 2.2%, were cesarean deliveries, she said. In cesarean deliveries, mean blood loss is double that of vaginal deliveries. Delayed clamping could further increase bleeding because the hysterotomy closure is delayed, said Dr. Purisch, so the question of safety in cesarean deliveries is clinically important.
Faced with this knowledge gap, Dr. Purisch and her colleagues constructed a prospective, randomized, controlled trial of delayed cord clamping in cesarean delivery at term, with the hypothesis that maternal blood loss would be increased by the practice.
Patients were eligible if they had singleton gestations with cesarean deliveries scheduled at 37 weeks’ gestation or more. Patients with known placentation problems, significant maternal or known fetal anemia, maternal bleeding disorders, and preeclampsia were excluded. The study also did not include pregnancies with known fetal anomalies or intrauterine growth retardation, or those in which cord blood banking was planned or the mother would refuse blood products.
In an intention-to-treat analysis, Dr. Purisch and her colleagues randomly assigned participants 1:1 to immediate cord clamping, defined as clamping the cord by 15 seconds after delivery, or delayed cord clamping, in which the umbilical cord was clamped 1 minute after delivery.
Oxytocin was routinely administered to each group on delivery, and there was no umbilical cord milking in either group. For the delayed-clamping group, the infant was kept at the level of the placenta and tended by the pediatric team during the minute before clamping. Dr. Purisch explained that cord clamping was performed before 60 seconds in the intervention group if needed for neonatal resuscitation.
Participants were similar in the two study arms, with a median gestational age of 39.1 weeks at delivery. Most women (60%-64%) had one prior cesarean delivery, with about a quarter having two or more prior cesarean deliveries. Preoperative maternal hemoglobin was 11.6-12.0 g/dL. About 41% of participants were Hispanic, and the median prepregnancy body mass index for participants was about 27 kg/m2.
Looking at secondary outcome measures, there was no difference in rates of postpartum hemorrhage or uterotonic administration between the two groups (P = .99 for both). Hemoglobin levels at postoperative day 1 were numerically higher for the delayed cord clamping group, but the difference wasn’t significant (10.2 vs. 9.8 g/dL; P = .18). Just two women, both in the immediate cord clamping group, required blood transfusions.
Additional neonatal secondary outcome measures included birth weight, Apgar scores at 1 and 5 minutes, the need for phototherapy for jaundice, and umbilical cord artery pH. There were no between-group differences except that umbilical cord artery pH was slightly lower in the delayed group (7.2 vs. 7.3; P = .04).
“Delayed cord clamping is not associated with increased maternal blood loss … but it does achieve higher neonatal hemoglobin levels at 24-72 hours of life,” said Dr. Purisch. “These results provide support for the application of current [American College of Obstetricians and Gynecologists] recommendations to women planned for cesarean delivery.”
The study was funded by the Columbia Maternal-Fetal Medicine Fellow Research Fund. Dr. Purisch reported no conflicts of interest.
SOURCE: Purisch, S. et al. Am J Obstet Gynecol. 2019 Jan;220(1):S37-38, Abstract 47.
LAS VEGAS – according to a recent study.
The change in maternal hemoglobin from preoperative level to postoperative day 1, the study’s primary outcome measure, was not significantly different whether the umbilical cord was clamped within 15 seconds of delivery or clamping was delayed for 1 minute.
For the 56 women who received immediate cord clamping, hemoglobin dropped a mean 1.78 g/dL; for the 57 women who received delayed cord clamping, the drop was 1.85 g/dL (P = .69). Mean estimated blood loss for the delayed clamping group was numerically higher at 884 mL, compared with 830 mL for the immediate clamping group, but this was not a statistically significant difference (P = .13)
However, the practice did result in significantly greater neonatal hemoglobin measured at 24-72 hours post delivery. Hemoglobin data were available for 90 infants, or about 80% of participants. For the 44 infants in the immediate clamping group, mean hemoglobin was 16.4 g/dL; for the delayed clamping group, the figure was 18.1 g/dL (P less than .01).
Although delayed cord clamping has clear benefits to the neonate, whether the practice adversely affects women undergoing cesarean was not clear, said Stephanie Purisch, MD, who discussed the findings of the two-site, randomized, clinical trial during a fellows research session at the meeting sponsored by the Society for Maternal-Fetal Medicine.
“Maternal outcomes have not been a focus of research” in the cord clamping literature, said Dr. Purisch, from Columbia University, New York. A 2013 Cochrane review found that delayed cord clamping did not change postpartum hemoglobin levels or increase blood loss or the need for transfusion. However, she said, the review included only healthy women who expected a vaginal delivery, so cesarean deliveries were undersampled in the data.
Of the 3,911 deliveries included in all prior randomized, controlled trials of delayed cord clamping, just 87, or 2.2%, were cesarean deliveries, she said. In cesarean deliveries, mean blood loss is double that of vaginal deliveries. Delayed clamping could further increase bleeding because the hysterotomy closure is delayed, said Dr. Purisch, so the question of safety in cesarean deliveries is clinically important.
Faced with this knowledge gap, Dr. Purisch and her colleagues constructed a prospective, randomized, controlled trial of delayed cord clamping in cesarean delivery at term, with the hypothesis that maternal blood loss would be increased by the practice.
Patients were eligible if they had singleton gestations with cesarean deliveries scheduled at 37 weeks’ gestation or more. Patients with known placentation problems, significant maternal or known fetal anemia, maternal bleeding disorders, and preeclampsia were excluded. The study also did not include pregnancies with known fetal anomalies or intrauterine growth retardation, or those in which cord blood banking was planned or the mother would refuse blood products.
In an intention-to-treat analysis, Dr. Purisch and her colleagues randomly assigned participants 1:1 to immediate cord clamping, defined as clamping the cord by 15 seconds after delivery, or delayed cord clamping, in which the umbilical cord was clamped 1 minute after delivery.
Oxytocin was routinely administered to each group on delivery, and there was no umbilical cord milking in either group. For the delayed-clamping group, the infant was kept at the level of the placenta and tended by the pediatric team during the minute before clamping. Dr. Purisch explained that cord clamping was performed before 60 seconds in the intervention group if needed for neonatal resuscitation.
Participants were similar in the two study arms, with a median gestational age of 39.1 weeks at delivery. Most women (60%-64%) had one prior cesarean delivery, with about a quarter having two or more prior cesarean deliveries. Preoperative maternal hemoglobin was 11.6-12.0 g/dL. About 41% of participants were Hispanic, and the median prepregnancy body mass index for participants was about 27 kg/m2.
Looking at secondary outcome measures, there was no difference in rates of postpartum hemorrhage or uterotonic administration between the two groups (P = .99 for both). Hemoglobin levels at postoperative day 1 were numerically higher for the delayed cord clamping group, but the difference wasn’t significant (10.2 vs. 9.8 g/dL; P = .18). Just two women, both in the immediate cord clamping group, required blood transfusions.
Additional neonatal secondary outcome measures included birth weight, Apgar scores at 1 and 5 minutes, the need for phototherapy for jaundice, and umbilical cord artery pH. There were no between-group differences except that umbilical cord artery pH was slightly lower in the delayed group (7.2 vs. 7.3; P = .04).
“Delayed cord clamping is not associated with increased maternal blood loss … but it does achieve higher neonatal hemoglobin levels at 24-72 hours of life,” said Dr. Purisch. “These results provide support for the application of current [American College of Obstetricians and Gynecologists] recommendations to women planned for cesarean delivery.”
The study was funded by the Columbia Maternal-Fetal Medicine Fellow Research Fund. Dr. Purisch reported no conflicts of interest.
SOURCE: Purisch, S. et al. Am J Obstet Gynecol. 2019 Jan;220(1):S37-38, Abstract 47.
LAS VEGAS – according to a recent study.
The change in maternal hemoglobin from preoperative level to postoperative day 1, the study’s primary outcome measure, was not significantly different whether the umbilical cord was clamped within 15 seconds of delivery or clamping was delayed for 1 minute.
For the 56 women who received immediate cord clamping, hemoglobin dropped a mean 1.78 g/dL; for the 57 women who received delayed cord clamping, the drop was 1.85 g/dL (P = .69). Mean estimated blood loss for the delayed clamping group was numerically higher at 884 mL, compared with 830 mL for the immediate clamping group, but this was not a statistically significant difference (P = .13)
However, the practice did result in significantly greater neonatal hemoglobin measured at 24-72 hours post delivery. Hemoglobin data were available for 90 infants, or about 80% of participants. For the 44 infants in the immediate clamping group, mean hemoglobin was 16.4 g/dL; for the delayed clamping group, the figure was 18.1 g/dL (P less than .01).
Although delayed cord clamping has clear benefits to the neonate, whether the practice adversely affects women undergoing cesarean was not clear, said Stephanie Purisch, MD, who discussed the findings of the two-site, randomized, clinical trial during a fellows research session at the meeting sponsored by the Society for Maternal-Fetal Medicine.
“Maternal outcomes have not been a focus of research” in the cord clamping literature, said Dr. Purisch, from Columbia University, New York. A 2013 Cochrane review found that delayed cord clamping did not change postpartum hemoglobin levels or increase blood loss or the need for transfusion. However, she said, the review included only healthy women who expected a vaginal delivery, so cesarean deliveries were undersampled in the data.
Of the 3,911 deliveries included in all prior randomized, controlled trials of delayed cord clamping, just 87, or 2.2%, were cesarean deliveries, she said. In cesarean deliveries, mean blood loss is double that of vaginal deliveries. Delayed clamping could further increase bleeding because the hysterotomy closure is delayed, said Dr. Purisch, so the question of safety in cesarean deliveries is clinically important.
Faced with this knowledge gap, Dr. Purisch and her colleagues constructed a prospective, randomized, controlled trial of delayed cord clamping in cesarean delivery at term, with the hypothesis that maternal blood loss would be increased by the practice.
Patients were eligible if they had singleton gestations with cesarean deliveries scheduled at 37 weeks’ gestation or more. Patients with known placentation problems, significant maternal or known fetal anemia, maternal bleeding disorders, and preeclampsia were excluded. The study also did not include pregnancies with known fetal anomalies or intrauterine growth retardation, or those in which cord blood banking was planned or the mother would refuse blood products.
In an intention-to-treat analysis, Dr. Purisch and her colleagues randomly assigned participants 1:1 to immediate cord clamping, defined as clamping the cord by 15 seconds after delivery, or delayed cord clamping, in which the umbilical cord was clamped 1 minute after delivery.
Oxytocin was routinely administered to each group on delivery, and there was no umbilical cord milking in either group. For the delayed-clamping group, the infant was kept at the level of the placenta and tended by the pediatric team during the minute before clamping. Dr. Purisch explained that cord clamping was performed before 60 seconds in the intervention group if needed for neonatal resuscitation.
Participants were similar in the two study arms, with a median gestational age of 39.1 weeks at delivery. Most women (60%-64%) had one prior cesarean delivery, with about a quarter having two or more prior cesarean deliveries. Preoperative maternal hemoglobin was 11.6-12.0 g/dL. About 41% of participants were Hispanic, and the median prepregnancy body mass index for participants was about 27 kg/m2.
Looking at secondary outcome measures, there was no difference in rates of postpartum hemorrhage or uterotonic administration between the two groups (P = .99 for both). Hemoglobin levels at postoperative day 1 were numerically higher for the delayed cord clamping group, but the difference wasn’t significant (10.2 vs. 9.8 g/dL; P = .18). Just two women, both in the immediate cord clamping group, required blood transfusions.
Additional neonatal secondary outcome measures included birth weight, Apgar scores at 1 and 5 minutes, the need for phototherapy for jaundice, and umbilical cord artery pH. There were no between-group differences except that umbilical cord artery pH was slightly lower in the delayed group (7.2 vs. 7.3; P = .04).
“Delayed cord clamping is not associated with increased maternal blood loss … but it does achieve higher neonatal hemoglobin levels at 24-72 hours of life,” said Dr. Purisch. “These results provide support for the application of current [American College of Obstetricians and Gynecologists] recommendations to women planned for cesarean delivery.”
The study was funded by the Columbia Maternal-Fetal Medicine Fellow Research Fund. Dr. Purisch reported no conflicts of interest.
SOURCE: Purisch, S. et al. Am J Obstet Gynecol. 2019 Jan;220(1):S37-38, Abstract 47.
REPORTING FROM THE PREGNANCY MEETING
Dental device borrowed from sports world no help in pushing
LAS VEGAS –
In a randomized, controlled trial of 346 nulliparous women, the device, adapted from a design used in athletics, made no difference in the duration of the second stage of labor overall or in passive descent or active pushing time. The findings were presented on behalf of first author Eric Bergh, MD, by Patricia Rekawek, MD, at a fellows abstract session at the meeting sponsored by the Society for Maternal-Fetal Medicine. Dr. Rekawek and Dr. Bergh are maternal-fetal medicine fellows at the Icahn School of Medicine at Mount Sinai, New York.
The dental support device (DSD) was actually used by 128 (74%) of the patients who were randomized to DSD usage. Of these patients, two thirds (n = 85) used the device for all second stage contractions. About one quarter (27%; n = 35) used it less than half the time, and a small minority (6%; n = 8) used it more than half the time but not for all pushing, noted Dr. Rekawek.
Most women (61%) who used the device agreed or strongly agreed that it was helpful. Most also found it comfortable (67%), and would use it again in a future delivery (61%). Having a custom-fit DSD, or spending more time practicing before labor, might help with efficacy in shortening the second stage of labor and merits study, the investigators said.
Reducing time spent in the second stage of labor could benefit both mother and neonate because “a prolonged second stage of labor is associated with multiple maternal and neonatal complications,” including increased risk for chorioamnionitis, neonatal sepsis, low umbilical artery pH, shoulder dystocia, and third- and fourth-degree lacerations, said Dr. Rekawek.
In athletics, a DSD “raises the vertical dimension of the deep bite and increases the size of the oropharynx” by introducing a 3-mm bite plate between the upper and lower rear molars. The DSD mouthpiece integrates the bite plates with a retainer-like band that wraps around the front incisors; speaking and drinking are possible with the DSD in place, said Dr. Rekawek.
She added that athletic evidence suggests that the DSD can lead to increased oxygenation, less head and neck tension, less muscle fatigue, and improved exercise capacity in some sports.
If these benefits translated to the maternal Valsalva maneuver during the second stage of labor, the increased isometric endurance could increase uterine pressure and optimize the expulsive effect of pushing, explained Dr. Rekawek. However, mixed results were seen in previous work that had women using a DSD during the second stage of labor.
The current study was powered to detect a difference of 20% in the duration of the second stage of labor for nulliparous women who were randomized either to use or not use a DSD while pushing. Women and their caregivers learned their random allocation by opening a sealed envelope at the beginning of the second stage of labor.
The study included nulliparous women who were carrying a singleton pregnancy of at least 37 weeks’ gestation and without known fetal anomalies. Enrollment occurred before the second stage of labor, and 173 women participated in each arm of the study.
For those who were assigned to use the DSD, the second stage of labor lasted a median 84 minutes, whereas the median for the control group was 92 minutes (P = .11) in an intention-to-treat analysis. Passive descent and active pushing lasted a median 14 and 50 minutes, respectively, for the DSD group, compared with 13 and 55 minutes for the control group (P = .22 and P = .29, respectively).
Another analysis of the primary outcome measure used Kaplan-Meier curves to compare the curves of the women who remained pregnant in each group across the period of the second stage of labor. There was no significant difference between the groups when the data were examined in this manner (log rank P = .18).
Secondary maternal outcomes included the mode of delivery. Most women (83%-85%) in each group had a normal spontaneous vaginal delivery, with no differences in any mode of delivery (P = .93). Neonatal outcomes also were similar between groups, with no significant differences in sex, birth weight, arterial pH, and neonatal intensive care unit admissions.
The investigators tracked whether patients were admitted in spontaneous labor, for labor induction with active membranes, or with premature rupture of membranes. Also, they looked at the degree of cervical dilation at admission, whether oxytocin or epidural anesthesia were used in labor, and anesthetic administered during the second stage of labor. There were no differences between groups in these variables.
Dr. Rekawek noted that the study benefited from a large sample size and a prospective, randomized design. Blinding lasted only until pushing began. Also, although all patients were taught how to use the device, they didn’t have an opportunity to practice.
Dr. Rekawek presented on behalf of Dr. Bergh because he became a new father the day before the presentation. His wife, said Dr. Rekawek, didn’t use a dental support device.
The authors reported that they have no relevant financial disclosures.
SOURCE: Bergh E et al. Am J Obstet Gynecol. 2019 Jan;220(1):S39, Abstract 49.
LAS VEGAS –
In a randomized, controlled trial of 346 nulliparous women, the device, adapted from a design used in athletics, made no difference in the duration of the second stage of labor overall or in passive descent or active pushing time. The findings were presented on behalf of first author Eric Bergh, MD, by Patricia Rekawek, MD, at a fellows abstract session at the meeting sponsored by the Society for Maternal-Fetal Medicine. Dr. Rekawek and Dr. Bergh are maternal-fetal medicine fellows at the Icahn School of Medicine at Mount Sinai, New York.
The dental support device (DSD) was actually used by 128 (74%) of the patients who were randomized to DSD usage. Of these patients, two thirds (n = 85) used the device for all second stage contractions. About one quarter (27%; n = 35) used it less than half the time, and a small minority (6%; n = 8) used it more than half the time but not for all pushing, noted Dr. Rekawek.
Most women (61%) who used the device agreed or strongly agreed that it was helpful. Most also found it comfortable (67%), and would use it again in a future delivery (61%). Having a custom-fit DSD, or spending more time practicing before labor, might help with efficacy in shortening the second stage of labor and merits study, the investigators said.
Reducing time spent in the second stage of labor could benefit both mother and neonate because “a prolonged second stage of labor is associated with multiple maternal and neonatal complications,” including increased risk for chorioamnionitis, neonatal sepsis, low umbilical artery pH, shoulder dystocia, and third- and fourth-degree lacerations, said Dr. Rekawek.
In athletics, a DSD “raises the vertical dimension of the deep bite and increases the size of the oropharynx” by introducing a 3-mm bite plate between the upper and lower rear molars. The DSD mouthpiece integrates the bite plates with a retainer-like band that wraps around the front incisors; speaking and drinking are possible with the DSD in place, said Dr. Rekawek.
She added that athletic evidence suggests that the DSD can lead to increased oxygenation, less head and neck tension, less muscle fatigue, and improved exercise capacity in some sports.
If these benefits translated to the maternal Valsalva maneuver during the second stage of labor, the increased isometric endurance could increase uterine pressure and optimize the expulsive effect of pushing, explained Dr. Rekawek. However, mixed results were seen in previous work that had women using a DSD during the second stage of labor.
The current study was powered to detect a difference of 20% in the duration of the second stage of labor for nulliparous women who were randomized either to use or not use a DSD while pushing. Women and their caregivers learned their random allocation by opening a sealed envelope at the beginning of the second stage of labor.
The study included nulliparous women who were carrying a singleton pregnancy of at least 37 weeks’ gestation and without known fetal anomalies. Enrollment occurred before the second stage of labor, and 173 women participated in each arm of the study.
For those who were assigned to use the DSD, the second stage of labor lasted a median 84 minutes, whereas the median for the control group was 92 minutes (P = .11) in an intention-to-treat analysis. Passive descent and active pushing lasted a median 14 and 50 minutes, respectively, for the DSD group, compared with 13 and 55 minutes for the control group (P = .22 and P = .29, respectively).
Another analysis of the primary outcome measure used Kaplan-Meier curves to compare the curves of the women who remained pregnant in each group across the period of the second stage of labor. There was no significant difference between the groups when the data were examined in this manner (log rank P = .18).
Secondary maternal outcomes included the mode of delivery. Most women (83%-85%) in each group had a normal spontaneous vaginal delivery, with no differences in any mode of delivery (P = .93). Neonatal outcomes also were similar between groups, with no significant differences in sex, birth weight, arterial pH, and neonatal intensive care unit admissions.
The investigators tracked whether patients were admitted in spontaneous labor, for labor induction with active membranes, or with premature rupture of membranes. Also, they looked at the degree of cervical dilation at admission, whether oxytocin or epidural anesthesia were used in labor, and anesthetic administered during the second stage of labor. There were no differences between groups in these variables.
Dr. Rekawek noted that the study benefited from a large sample size and a prospective, randomized design. Blinding lasted only until pushing began. Also, although all patients were taught how to use the device, they didn’t have an opportunity to practice.
Dr. Rekawek presented on behalf of Dr. Bergh because he became a new father the day before the presentation. His wife, said Dr. Rekawek, didn’t use a dental support device.
The authors reported that they have no relevant financial disclosures.
SOURCE: Bergh E et al. Am J Obstet Gynecol. 2019 Jan;220(1):S39, Abstract 49.
LAS VEGAS –
In a randomized, controlled trial of 346 nulliparous women, the device, adapted from a design used in athletics, made no difference in the duration of the second stage of labor overall or in passive descent or active pushing time. The findings were presented on behalf of first author Eric Bergh, MD, by Patricia Rekawek, MD, at a fellows abstract session at the meeting sponsored by the Society for Maternal-Fetal Medicine. Dr. Rekawek and Dr. Bergh are maternal-fetal medicine fellows at the Icahn School of Medicine at Mount Sinai, New York.
The dental support device (DSD) was actually used by 128 (74%) of the patients who were randomized to DSD usage. Of these patients, two thirds (n = 85) used the device for all second stage contractions. About one quarter (27%; n = 35) used it less than half the time, and a small minority (6%; n = 8) used it more than half the time but not for all pushing, noted Dr. Rekawek.
Most women (61%) who used the device agreed or strongly agreed that it was helpful. Most also found it comfortable (67%), and would use it again in a future delivery (61%). Having a custom-fit DSD, or spending more time practicing before labor, might help with efficacy in shortening the second stage of labor and merits study, the investigators said.
Reducing time spent in the second stage of labor could benefit both mother and neonate because “a prolonged second stage of labor is associated with multiple maternal and neonatal complications,” including increased risk for chorioamnionitis, neonatal sepsis, low umbilical artery pH, shoulder dystocia, and third- and fourth-degree lacerations, said Dr. Rekawek.
In athletics, a DSD “raises the vertical dimension of the deep bite and increases the size of the oropharynx” by introducing a 3-mm bite plate between the upper and lower rear molars. The DSD mouthpiece integrates the bite plates with a retainer-like band that wraps around the front incisors; speaking and drinking are possible with the DSD in place, said Dr. Rekawek.
She added that athletic evidence suggests that the DSD can lead to increased oxygenation, less head and neck tension, less muscle fatigue, and improved exercise capacity in some sports.
If these benefits translated to the maternal Valsalva maneuver during the second stage of labor, the increased isometric endurance could increase uterine pressure and optimize the expulsive effect of pushing, explained Dr. Rekawek. However, mixed results were seen in previous work that had women using a DSD during the second stage of labor.
The current study was powered to detect a difference of 20% in the duration of the second stage of labor for nulliparous women who were randomized either to use or not use a DSD while pushing. Women and their caregivers learned their random allocation by opening a sealed envelope at the beginning of the second stage of labor.
The study included nulliparous women who were carrying a singleton pregnancy of at least 37 weeks’ gestation and without known fetal anomalies. Enrollment occurred before the second stage of labor, and 173 women participated in each arm of the study.
For those who were assigned to use the DSD, the second stage of labor lasted a median 84 minutes, whereas the median for the control group was 92 minutes (P = .11) in an intention-to-treat analysis. Passive descent and active pushing lasted a median 14 and 50 minutes, respectively, for the DSD group, compared with 13 and 55 minutes for the control group (P = .22 and P = .29, respectively).
Another analysis of the primary outcome measure used Kaplan-Meier curves to compare the curves of the women who remained pregnant in each group across the period of the second stage of labor. There was no significant difference between the groups when the data were examined in this manner (log rank P = .18).
Secondary maternal outcomes included the mode of delivery. Most women (83%-85%) in each group had a normal spontaneous vaginal delivery, with no differences in any mode of delivery (P = .93). Neonatal outcomes also were similar between groups, with no significant differences in sex, birth weight, arterial pH, and neonatal intensive care unit admissions.
The investigators tracked whether patients were admitted in spontaneous labor, for labor induction with active membranes, or with premature rupture of membranes. Also, they looked at the degree of cervical dilation at admission, whether oxytocin or epidural anesthesia were used in labor, and anesthetic administered during the second stage of labor. There were no differences between groups in these variables.
Dr. Rekawek noted that the study benefited from a large sample size and a prospective, randomized design. Blinding lasted only until pushing began. Also, although all patients were taught how to use the device, they didn’t have an opportunity to practice.
Dr. Rekawek presented on behalf of Dr. Bergh because he became a new father the day before the presentation. His wife, said Dr. Rekawek, didn’t use a dental support device.
The authors reported that they have no relevant financial disclosures.
SOURCE: Bergh E et al. Am J Obstet Gynecol. 2019 Jan;220(1):S39, Abstract 49.
REPORTING FROM THE PREGNANCY MEETING