User login
Poor asthma control during pregnancy trims live birth rate
SAN FRANCISCO – and among the live births had a significantly increased rate of both preterm delivery and neonatal intensive care admissions, according to a review of insurance claims data for more than 1 million American women during 2011-2015.
On the other hand, asthma severity, which the researchers inferred based on the type and amount of treatment patients received, showed essentially no link with the live birth rate, Jennifer Yland said at the annual meeting of the American Academy of Allergy, Asthma, and Immunology.
“The findings add to the body of evidence that relate poor asthma control to an increased risk for pregnancy complications.” explained Michael X. Schatz, MD, an allergist at Kaiser Permanente of Southern California, in San Diego, and a coauthor of the study.
Results from several prior studies had shown links between asthma and an increased rate of preterm birth, “but the larger, more generalizable population is a strength of the current findings. Results from prior studies have less frequently shown a link between asthma during pregnancy and neonatal ICU admissions,” he added.“The findings strengthen the case for good asthma control during pregnancy.”
For their review, Ms. Yland and her coauthors used insurance claims data from privately-insured American women aged 12-55 years who were pregnant and had drug prescription records during the study period. The database included 996,861 women without an asthma diagnosis and 29,882 women diagnosed with asthma. The analysis excluded women diagnosed with chronic obstructive pulmonary disease at least twice during pregnancy.
To analyze the pregnancy outcomes by asthma severity Ms. Yland and her associates divided the asthma patients into five subgroups based on the drug regimens they were on during pregnancy as a surrogate marker of disease severity. This analysis showed no relationship between disease severity and live birth rate.
The researchers also ran an analysis that divided patients into the quality of their management during pregnancy – either good or poor – based on either of two markers of poor control: filling five or more prescriptions for a short-acting beta-antagonist, or at least one exacerbation episode defined as an asthma-related emergency department visit, hospitalization, or need for oral corticosteroid treatment. By these criteria 7,135 (24%) of the pregnant women with asthma were poorly controlled. The live birth rate was 74% among women without asthma, 71% among those with well-controlled asthma, and 68% among women with poorly-controlled asthma, reported Ms. Yland, a researcher at the Harvard T.H. Chan School of Public Health in Boston.
In a multivariate analysis that adjusted for demographic differences and comorbidities, women with poorly-controlled asthma had preterm delivery a statistically significant 30% more often than did women with well-controlled asthma, and the rate of neonatal ICU admissions was a significant 24% higher in women with poorly-controlled asthma, compared with women who had well-controlled asthma. However, the rates of small-for-gestational-age infants and infants with congenital malformations was not significantly different between the well-controlled and poorly-controlled subgroups.
The finding that almost a quarter of the pregnant women in the study were poorly controlled wasn’t surprising, Dr. Schatz said in an interview. In some studies as many as half the asthma patients have poor control.
The 24% rate of poor asthma control during pregnancy in the studied women is “most likely an underestimate of poor control in the general population” because the study used data from women with commercial health insurance, noted Sonia Hernandez-Diaz, MD, lead investigator for the study and professor of epidemiology at Harvard T.H. Chan School of Public Health. “More disadvantaged populations, such as pregnant women on Medicaid, tend to have worse control.”
Barriers to good asthma control during pregnancy include smoking, weight gain, undertreatment, poor adherence, and viral infection. The overall approach to managing asthma during pregnancy is the same as when women are not pregnant, although certain asthma medications have a better safety record during pregnancy. “The most reassuring data exist for albuterol and inhaled steroids, particularly budesonide and fluticasone. Reassuring data also exist for the long-acting beta agonists salmeterol and formoterol, which are combined with inhaled steroids, and for montelukast,” Dr. Schatz said.
This is the first study to assess the impact of asthma management on pregnancy outcome in such a large population. The large number of women included provided a lot of statistical power and allowed the analyses to control for several potential confounders, Ms. Yland noted in an interview. She plans to expand the analysis with Medicaid data to try to further increase the generalizability and precision of the findings.
The study was funded by GlaxoSmithKline, and a coauthor of the study is a company employee. Ms. Yland had no disclosures. Dr. Schatz has received research funding from ALK, AstraZeneca, Medimmune, GlaxoSmithKline, and Merck. Dr. Hernandez-Diaz has been a consultant to Boehringer Ingelheim, Roche, and UCB, and has received research funding from GlaxoSmithKline, Lilly, and Pfizer.
SOURCE: Yland J et al. J Allergy Clin Immunol. 2019 Feb;143[2]:AB422.
SAN FRANCISCO – and among the live births had a significantly increased rate of both preterm delivery and neonatal intensive care admissions, according to a review of insurance claims data for more than 1 million American women during 2011-2015.
On the other hand, asthma severity, which the researchers inferred based on the type and amount of treatment patients received, showed essentially no link with the live birth rate, Jennifer Yland said at the annual meeting of the American Academy of Allergy, Asthma, and Immunology.
“The findings add to the body of evidence that relate poor asthma control to an increased risk for pregnancy complications.” explained Michael X. Schatz, MD, an allergist at Kaiser Permanente of Southern California, in San Diego, and a coauthor of the study.
Results from several prior studies had shown links between asthma and an increased rate of preterm birth, “but the larger, more generalizable population is a strength of the current findings. Results from prior studies have less frequently shown a link between asthma during pregnancy and neonatal ICU admissions,” he added.“The findings strengthen the case for good asthma control during pregnancy.”
For their review, Ms. Yland and her coauthors used insurance claims data from privately-insured American women aged 12-55 years who were pregnant and had drug prescription records during the study period. The database included 996,861 women without an asthma diagnosis and 29,882 women diagnosed with asthma. The analysis excluded women diagnosed with chronic obstructive pulmonary disease at least twice during pregnancy.
To analyze the pregnancy outcomes by asthma severity Ms. Yland and her associates divided the asthma patients into five subgroups based on the drug regimens they were on during pregnancy as a surrogate marker of disease severity. This analysis showed no relationship between disease severity and live birth rate.
The researchers also ran an analysis that divided patients into the quality of their management during pregnancy – either good or poor – based on either of two markers of poor control: filling five or more prescriptions for a short-acting beta-antagonist, or at least one exacerbation episode defined as an asthma-related emergency department visit, hospitalization, or need for oral corticosteroid treatment. By these criteria 7,135 (24%) of the pregnant women with asthma were poorly controlled. The live birth rate was 74% among women without asthma, 71% among those with well-controlled asthma, and 68% among women with poorly-controlled asthma, reported Ms. Yland, a researcher at the Harvard T.H. Chan School of Public Health in Boston.
In a multivariate analysis that adjusted for demographic differences and comorbidities, women with poorly-controlled asthma had preterm delivery a statistically significant 30% more often than did women with well-controlled asthma, and the rate of neonatal ICU admissions was a significant 24% higher in women with poorly-controlled asthma, compared with women who had well-controlled asthma. However, the rates of small-for-gestational-age infants and infants with congenital malformations was not significantly different between the well-controlled and poorly-controlled subgroups.
The finding that almost a quarter of the pregnant women in the study were poorly controlled wasn’t surprising, Dr. Schatz said in an interview. In some studies as many as half the asthma patients have poor control.
The 24% rate of poor asthma control during pregnancy in the studied women is “most likely an underestimate of poor control in the general population” because the study used data from women with commercial health insurance, noted Sonia Hernandez-Diaz, MD, lead investigator for the study and professor of epidemiology at Harvard T.H. Chan School of Public Health. “More disadvantaged populations, such as pregnant women on Medicaid, tend to have worse control.”
Barriers to good asthma control during pregnancy include smoking, weight gain, undertreatment, poor adherence, and viral infection. The overall approach to managing asthma during pregnancy is the same as when women are not pregnant, although certain asthma medications have a better safety record during pregnancy. “The most reassuring data exist for albuterol and inhaled steroids, particularly budesonide and fluticasone. Reassuring data also exist for the long-acting beta agonists salmeterol and formoterol, which are combined with inhaled steroids, and for montelukast,” Dr. Schatz said.
This is the first study to assess the impact of asthma management on pregnancy outcome in such a large population. The large number of women included provided a lot of statistical power and allowed the analyses to control for several potential confounders, Ms. Yland noted in an interview. She plans to expand the analysis with Medicaid data to try to further increase the generalizability and precision of the findings.
The study was funded by GlaxoSmithKline, and a coauthor of the study is a company employee. Ms. Yland had no disclosures. Dr. Schatz has received research funding from ALK, AstraZeneca, Medimmune, GlaxoSmithKline, and Merck. Dr. Hernandez-Diaz has been a consultant to Boehringer Ingelheim, Roche, and UCB, and has received research funding from GlaxoSmithKline, Lilly, and Pfizer.
SOURCE: Yland J et al. J Allergy Clin Immunol. 2019 Feb;143[2]:AB422.
SAN FRANCISCO – and among the live births had a significantly increased rate of both preterm delivery and neonatal intensive care admissions, according to a review of insurance claims data for more than 1 million American women during 2011-2015.
On the other hand, asthma severity, which the researchers inferred based on the type and amount of treatment patients received, showed essentially no link with the live birth rate, Jennifer Yland said at the annual meeting of the American Academy of Allergy, Asthma, and Immunology.
“The findings add to the body of evidence that relate poor asthma control to an increased risk for pregnancy complications.” explained Michael X. Schatz, MD, an allergist at Kaiser Permanente of Southern California, in San Diego, and a coauthor of the study.
Results from several prior studies had shown links between asthma and an increased rate of preterm birth, “but the larger, more generalizable population is a strength of the current findings. Results from prior studies have less frequently shown a link between asthma during pregnancy and neonatal ICU admissions,” he added.“The findings strengthen the case for good asthma control during pregnancy.”
For their review, Ms. Yland and her coauthors used insurance claims data from privately-insured American women aged 12-55 years who were pregnant and had drug prescription records during the study period. The database included 996,861 women without an asthma diagnosis and 29,882 women diagnosed with asthma. The analysis excluded women diagnosed with chronic obstructive pulmonary disease at least twice during pregnancy.
To analyze the pregnancy outcomes by asthma severity Ms. Yland and her associates divided the asthma patients into five subgroups based on the drug regimens they were on during pregnancy as a surrogate marker of disease severity. This analysis showed no relationship between disease severity and live birth rate.
The researchers also ran an analysis that divided patients into the quality of their management during pregnancy – either good or poor – based on either of two markers of poor control: filling five or more prescriptions for a short-acting beta-antagonist, or at least one exacerbation episode defined as an asthma-related emergency department visit, hospitalization, or need for oral corticosteroid treatment. By these criteria 7,135 (24%) of the pregnant women with asthma were poorly controlled. The live birth rate was 74% among women without asthma, 71% among those with well-controlled asthma, and 68% among women with poorly-controlled asthma, reported Ms. Yland, a researcher at the Harvard T.H. Chan School of Public Health in Boston.
In a multivariate analysis that adjusted for demographic differences and comorbidities, women with poorly-controlled asthma had preterm delivery a statistically significant 30% more often than did women with well-controlled asthma, and the rate of neonatal ICU admissions was a significant 24% higher in women with poorly-controlled asthma, compared with women who had well-controlled asthma. However, the rates of small-for-gestational-age infants and infants with congenital malformations was not significantly different between the well-controlled and poorly-controlled subgroups.
The finding that almost a quarter of the pregnant women in the study were poorly controlled wasn’t surprising, Dr. Schatz said in an interview. In some studies as many as half the asthma patients have poor control.
The 24% rate of poor asthma control during pregnancy in the studied women is “most likely an underestimate of poor control in the general population” because the study used data from women with commercial health insurance, noted Sonia Hernandez-Diaz, MD, lead investigator for the study and professor of epidemiology at Harvard T.H. Chan School of Public Health. “More disadvantaged populations, such as pregnant women on Medicaid, tend to have worse control.”
Barriers to good asthma control during pregnancy include smoking, weight gain, undertreatment, poor adherence, and viral infection. The overall approach to managing asthma during pregnancy is the same as when women are not pregnant, although certain asthma medications have a better safety record during pregnancy. “The most reassuring data exist for albuterol and inhaled steroids, particularly budesonide and fluticasone. Reassuring data also exist for the long-acting beta agonists salmeterol and formoterol, which are combined with inhaled steroids, and for montelukast,” Dr. Schatz said.
This is the first study to assess the impact of asthma management on pregnancy outcome in such a large population. The large number of women included provided a lot of statistical power and allowed the analyses to control for several potential confounders, Ms. Yland noted in an interview. She plans to expand the analysis with Medicaid data to try to further increase the generalizability and precision of the findings.
The study was funded by GlaxoSmithKline, and a coauthor of the study is a company employee. Ms. Yland had no disclosures. Dr. Schatz has received research funding from ALK, AstraZeneca, Medimmune, GlaxoSmithKline, and Merck. Dr. Hernandez-Diaz has been a consultant to Boehringer Ingelheim, Roche, and UCB, and has received research funding from GlaxoSmithKline, Lilly, and Pfizer.
SOURCE: Yland J et al. J Allergy Clin Immunol. 2019 Feb;143[2]:AB422.
REPORTING FROM AAAAI 2019
A systematic approach to chronic abnormal uterine bleeding
Menstrual bleeding is considered normal when it occurs regularly (every 21-35 days), lasts 4 to 8 days, and is not associated with heavy bleeding.1 During the first few years after menarche, it is normal for girls to experience irregular menstrual cycles but, by the third year, 60% to 80% of girls have an adult pattern of menstrual bleeding.2
Menstrual flow without normal volume, duration, regularity, or frequency is considered abnormal uterine bleeding (AUB). The condition is considered acute if there is need for immediate intervention. In the absence of the need for immediate intervention, recurrent AUB is classified as chronic.3 Chronic AUB is the focus of this article.
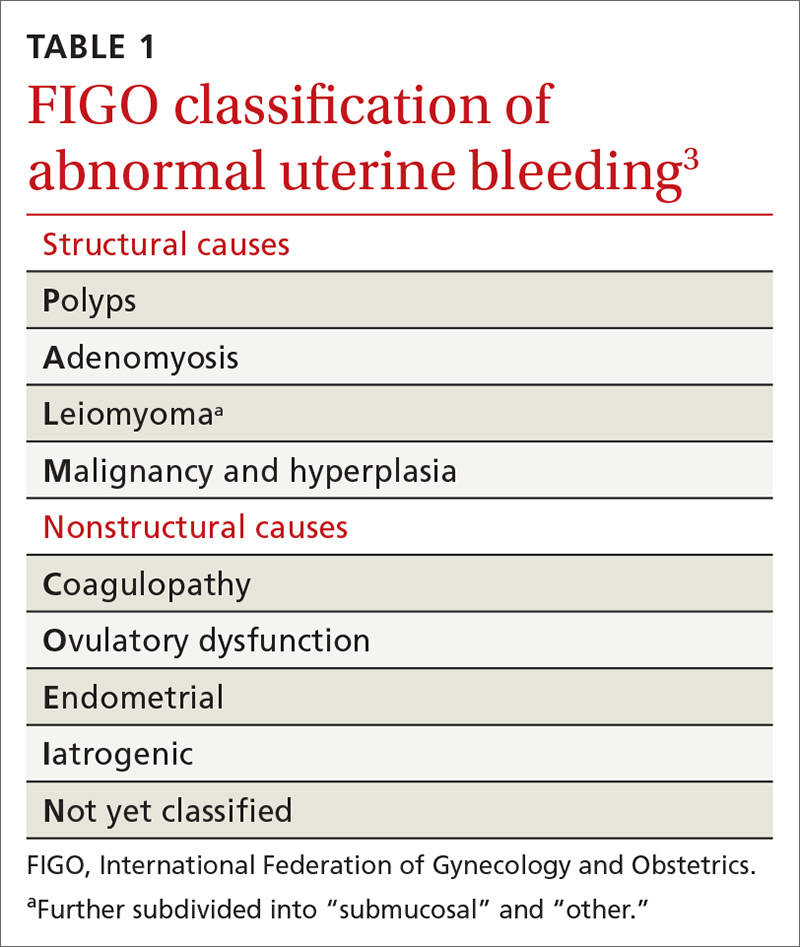
Invaluable tool: The FIGO classification
In 2011, the
- structural causes, recalled by “PALM” (Polyps, Adenomyosis, Leiomyoma, and Malignancy/hyperplasia)
- nonstructural causes, recalled by “COEIN” (Coagulopathy, Ovulatory dysfunction, Endometrial, Iatrogenic, and Not yet classified).
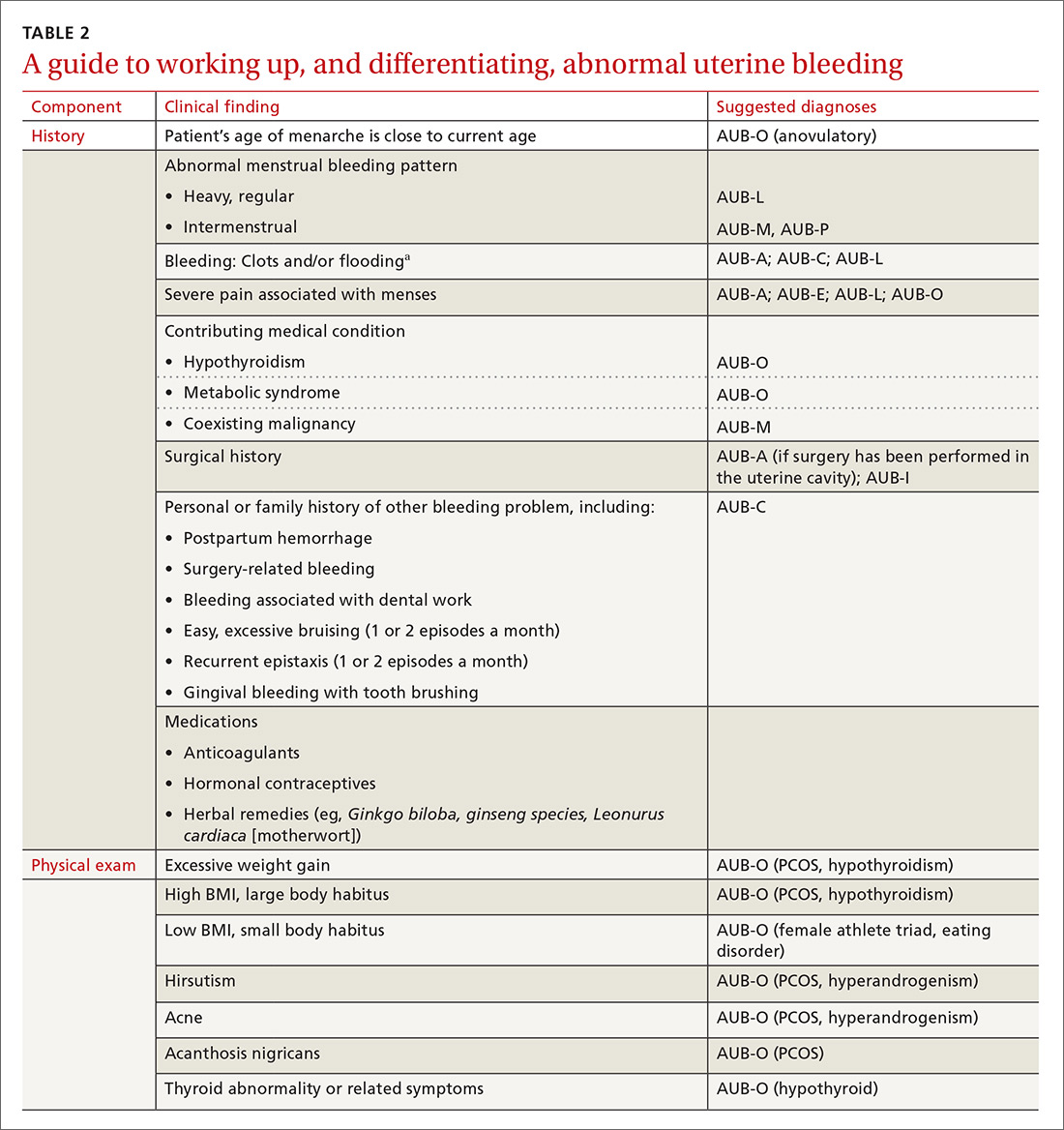
The PALM–COEIN system also uses descriptive terminology (heavy bleeding, intermenstrual bleeding) to characterize the bleeding pattern.3 The American College of Obstetricians and Gynecologists has adopted this classification system and recommends that such historically used terminology as “dysfunctional uterine bleeding,” “menorrhagia,” and “metrorrhagia” be abandoned.1
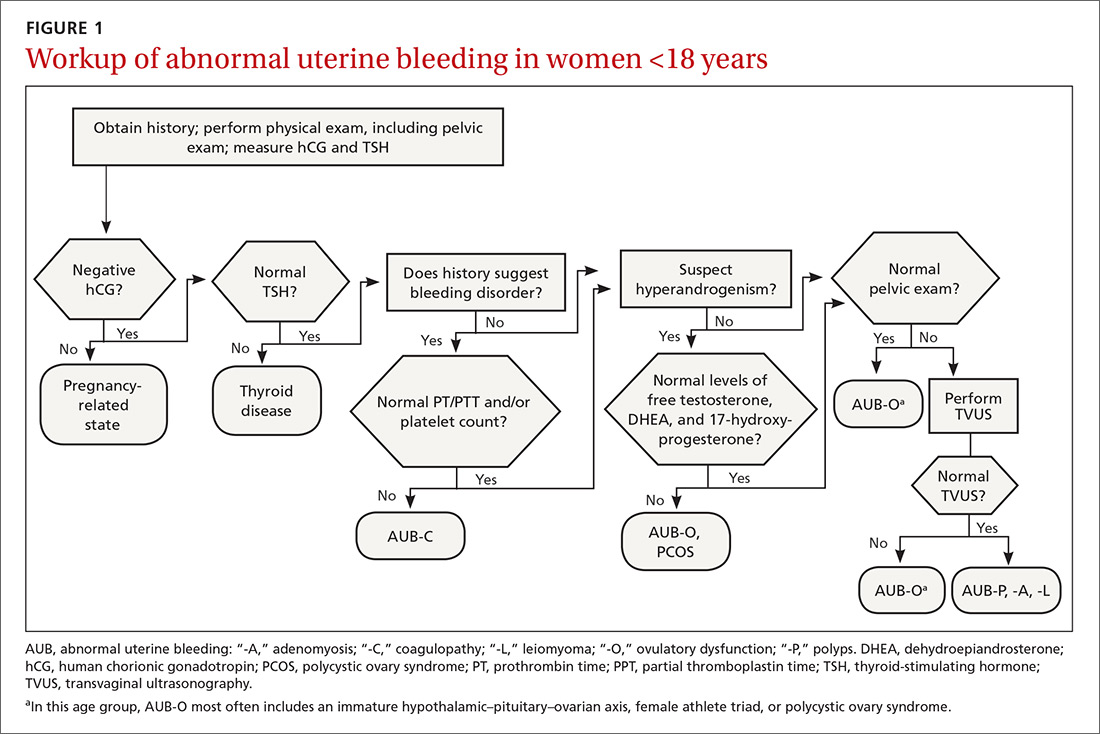
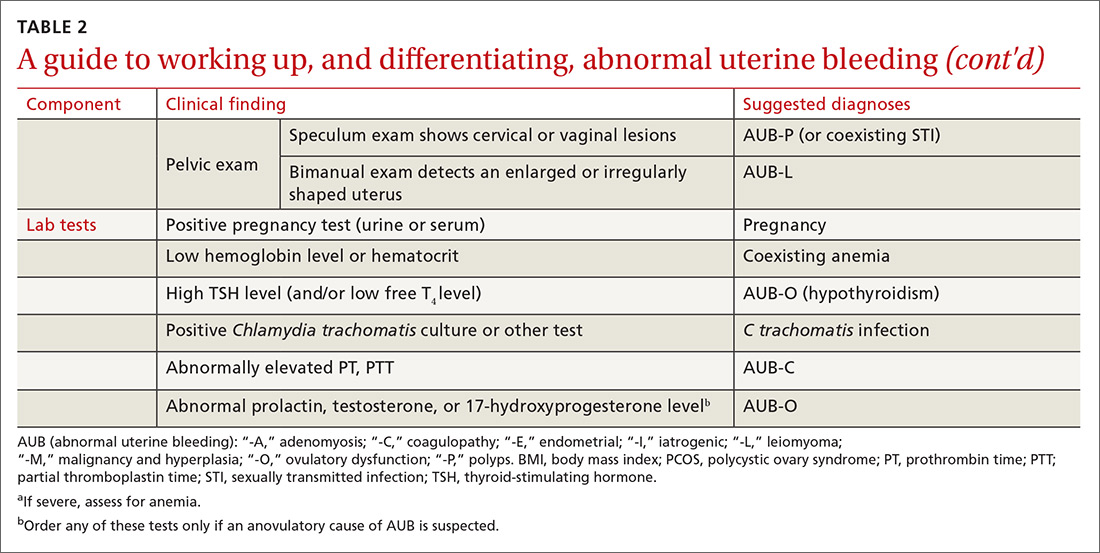
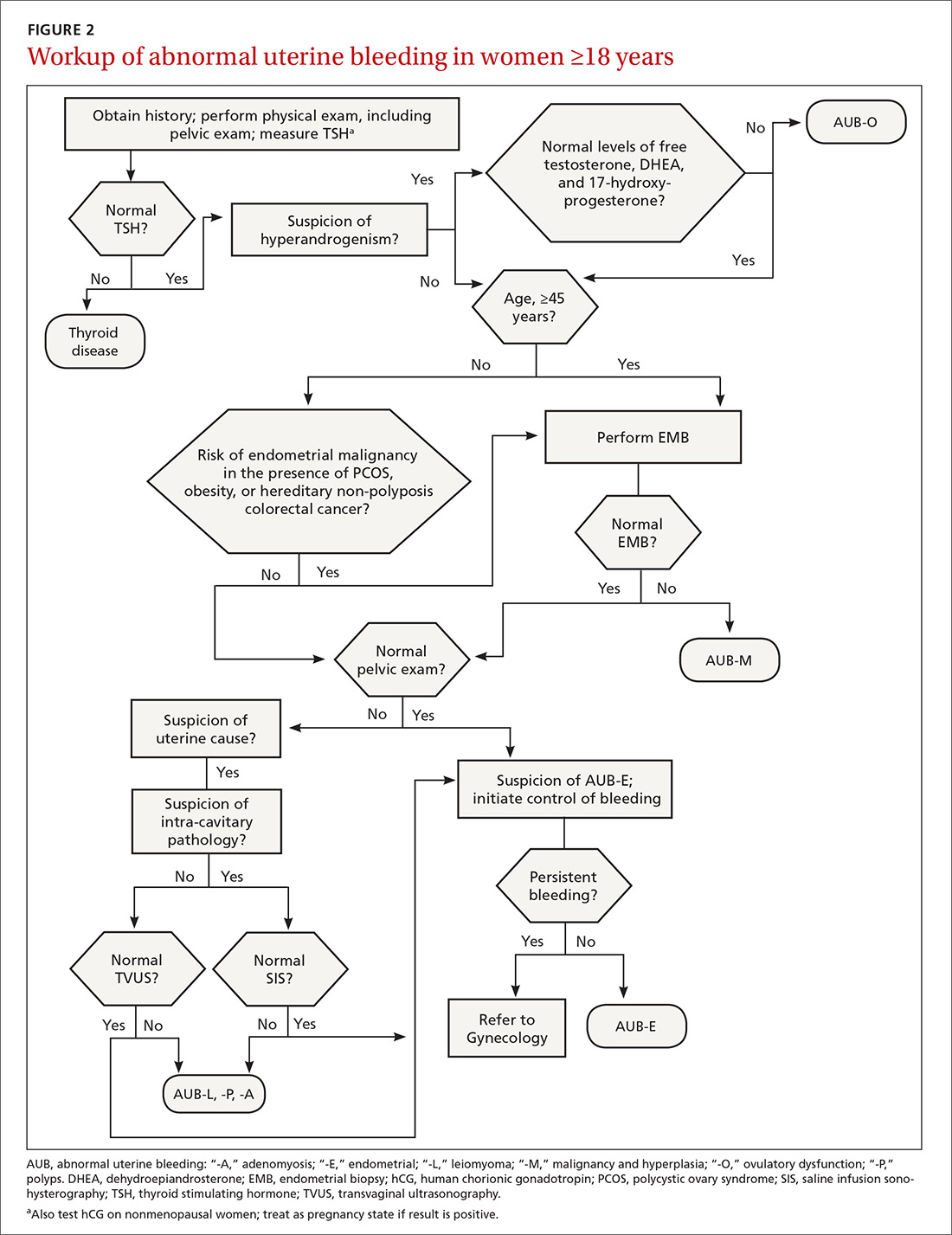
The initial workup of all causes of chronic uterine bleeding begins with a history; physical examination, including pelvic exam; and laboratory testing, including a urine pregnancy test, complete blood count, and a test of thyroid-stimulating hormone (TABLE 2). The need for additional laboratory testing, imaging, or endometrial biopsy depends on the suspected cause of AUB, detailed stepwise in FIGURE 1 (women <18 years) and FIGURE 2 (≥18 years).
We first briefly review the 9 categories of AUB in the PALM–COEIN system; discuss the most common causes in more detail; and review common treatment options (TABLE 3).

CASE 1
Marsha R, a 41-year-old-woman, complains of heavy menstrual bleeding for the past year that has become worse over the past 2 months. Her menstrual cycles have occurred every 28 days and last 10 days; she uses 10 to 12 pads a day.
Continue to: Recently, Ms. R reports...
Recently, Ms. R reports, she has been bleeding continuously for 14 days, with episodes of lighter bleeding followed by heavier bleeding. She also complains of fatigue.
Bimanual examination is notable for an enlarged uterus.
How would you proceed with the workup of this patient, to determine the cause of her bleeding and tailor management accordingly?
Structural AUB: The “PALM” mnemonic
A structural cause of AUB must be considered when you encounter an abnormality on physical exam (TABLE 1).3 In obese women or other patients in whom the physical exam is difficult, historical clues—including postcoital bleeding, intermenstrual bleeding, or pelvic pain or pressure—also suggest a structural abnormality.4
Transvaginal ultrasonography (TVUS) is the initial method of evaluation when a structural abnormality is suspected.1,4 However, although TVUS is excellent at visualizing the myometrium, lesions within the uterine cavity can be missed. If intracavitary pathology, such as submucosal fibroids or endometrial polyps, is suspected, additional imaging with saline infusion sonohysterography (SIS) should be performed. If a cavitary abnormality is confirmed, hysteroscopy is indicated.1 Magnetic resonance imaging (MRI) is reserved for cases in which a uterine cavity abnormality is found on TVUS but cannot be further characterized by SIS or hysteroscopy.1
Continue to: Endometrial biopsy...
Endometrial biopsy (EMB) is indicated as part of the initial evaluation of AUB in all women >45 years and in younger women who have risk factors for endometrial cancer, including polycystic ovary syndrome (PCOS), obesity, and hereditary nonpolyposis colorectal cancer. Such biopsy is necessary in these women whether or not another condition is the cause of the AUB and regardless of findings on TVUS.1-4 Endometrial biopsy should also be performed in women with AUB that persists despite medical management. If office EMB is nondiagnostic, hysteroscopy or SIS can be used to obtain tissue samples for further evaluation.5
Polyps. An endometrial polyp is a benign growth of endometrial tissue that is covered with epithelial cells. Polyps are often diagnosed by EMB or TVUS when these techniques are performed as part of the workup for AUB.6 Endometrial polyps are found more commonly in postmenopausal women, but should be considered as a cause of AUB in premenopausal women, too, especially those with intermenstrual bleeding or postcoital bleeding (or both) that is unresponsive to medical management.7 Risk factors for polyps include older age, obesity, and treatment with tamoxifen.7 The usual treatment for symptomatic endometrial polyps is removal by operative hysteroscopy.7
Adenomyosis. Ectopic endometrial tissue in the myometrium that leads to hypertrophy of the myometrium and uterine enlargement is known as adenomyosis. The disorder is most often diagnosed in women 40 to 50 years of age, who commonly complain of heavy uterine bleeding (40%-60% of cases) and dysmenorrhea (65%).8 Although definitive diagnosis is made histologically at hysterectomy, TVUS and MRI can be useful tools to help narrow the differential diagnosis in women with unexplained AUB.
According to a systematic review,9 the sensitivity and specificity of imaging in the diagnosis of adenomyosis is 72% and 81%, respectively, for TVUS and 77% and 89%, respectively, for MRI. Needle biopsy, performed hysteroscopically or laparoscopically, is less useful because the technique has low sensitivity (reported variously as 8%-56%) in diagnosing adenomyosis.8
Treatment options for adenomyosis are medical management with agents that reduce bleeding (eg, a combination oral contraceptive [OC], nonsteroidal anti-inflammatory drugs [NSAIDs], the antifibrinolytic tranexamic acid, and, when there is no distortion of the uterine cavity, a levonorgestrel intrauterine device [LNG-IUD]); uterine artery embolization; and hysterectomy.8
Continue to: Leiomyoma
Leiomyoma. Uterine fibroids, or leiomyomas, are benign, fibromuscular solid tumors, thought to be hormone-dependent because many regress after menopause. In women of reproductive age, uterine fibroids are the most common cause of structural AUB, with a cumulative incidence of 70% to 80% among women in this age group.3,10 Fibroids are more common in African-American women, women who experienced early menarche, and women who are obese, have PCOS, or had a late first pregnancy.3-10
Many fibroids are asymptomatic, and are found incidentally on sonographic examination performed for other reasons; in one-third of affected patients, the fibroids result in heavy menstrual bleeding.10 Intermenstrual bleeding and postcoital bleeding can occur, but are not common symptoms with fibroids. Consider other causes of AUB, such as endometrial polyps, when these symptoms are present.
Treatment of fibroids is medical or surgical. Medical management is a reasonable first-line option, especially in women who have not completed childbearing and who have small (<3 cm in diameter) fibroids. Options include a combination OC, NSAIDs, tranexamic acid, and, when the uterine cavity is not distorted, an LNG-IUD.4,10,11
For women with larger fibroids, those for whom the aforementioned medical treatments are unsuccessful, and those who are seeking more definitive treatment, uterine artery embolization, myomectomy, or hysterectomy can be considered.
› Uterine artery embolization is performed by an interventional radiologist under local anesthesia and, if necessary, moderate sedation.12 After the procedure, fibroids decrease in size due to avascular necrosis, but the remainder of the myometrium is relatively unaffected because collateral blood supply develops.13,14 Patients might experience abdominal cramping for 2 or 3 days following the procedure, which can be managed with an oral NSAID.12 Approximately 90% of women treated with embolization note improvement in AUB by 3 months after the procedure.15 Uterine artery embolization is not recommended in women who have not completed childbearing.12,16,17
Continue to: Myomectomy
› Myomectomy (removal of the leiomyoma) is the surgical treatment of choice for women who want to maintain fertility. Depending on the size and location of the fibroid(s), myomectomy can be performed as an open surgical procedure, laparoscopically, or hysteroscopically. At the discretion of the surgeon, leuprolide acetate, a gonadotropin-releasing hormone agonist, can be prescribed for 3 months before myomectomy to reduce intraoperative blood loss by decreasing the vascularity of the fibroids.4,18 Reduction in bleeding is reported in 70% to 90% of patients who undergo myomectomy.19
› Hysterectomy, the definitive treatment for uterine fibroids, should be reserved for women who have completed childbearing and who have failed (or have a contraindication to) other treatment options.
Malignancy/hyperplasia. EMB should be performed when endometrial malignancy/hyperplasia is suspected. As noted, endometrial cancer should be considered as a diagnostic possibility in women >45 years, in younger women with risk factors, and in women who have failed to respond to medical treatment for other suspected causes of AUB.5
When hyperplasia without atypia is diagnosed, the LNG-IUD or oral progesterone is an acceptable treatment option; note that fewer women who have an LNG-IUD eventually require hysterectomy, compared to women who take oral hormone therapy for AUB.20 When hyperplasia with atypia is diagnosed, hysterectomy is the treatment of choice. If a woman wishes to maintain fertility, however, oral progesterone therapy can be offered.21
When the diagnosis is cancer, the patient should be referred to a gynecologic oncologist for staging and treatment. Treatment varies depending on stage, but generally requires hysterectomy including bilateral salpingo-oophorectomy, with possible chemotherapy or radiation, or both.22
Continue to: CASE 1
CASE 1
Ms. R undergoes a sonogram that reveals a 4-cm fibroid in the uterine fundus that has not distorted the uterine cavity. Although she has completed childbearing, Ms. R is not interested in a surgical procedure at this time. You recommend insertion of an LNG-IUD; she accepts your advice.
CASE 2
Claire G, 27 years old, with a body mass index of 41,* complains of irregular menses for several months. Her menstrual cycle is irregular, as is the duration of menses and amount of bleeding. She has some mild fatigue without dizziness.
The physical exam is notable for mild hirsutism, without abnormalities on pelvic examination. Lab testing reveals iron-deficiency anemia; a pregnancy test is negative.
The questions that were raised by Ms. R’s case challenge you here, too: What is the appropriate workup of Ms. G’s bleeding? Once the cause is confirmed, how should you treat her?
Nonstructural AUB: The “COEIN” mnemonic
In the absence of abnormalities on a pelvic exam, and after excluding endometrial malignancy/hyperplasia in patients with the aforementioned risk factors, a nonstructural cause of AUB should be considered (TABLE 1).3 In women 20 to 40 years of age, the primary common cause of nonstructural uterine bleeding is ovulatory dysfunction, most often caused by PCOS or anovulatory bleeding.
Continue to: For nonstructual causes of AUB...
For nonstructural causes of AUB, the recommended laboratory workup varies with the suspected diagnosis. In addition, recently pregnant women should have a quantitative assay of β human chorionic gonadotropin to evaluate for trophoblastic disease.5,23
Imaging is not usually recommended when the cause of AUB is suspected to be nonstructural. However, when PCOS is suspected, TVUS can be used to confirm the presence of polycystic ovaries.23
As noted, EMB should be performed when AUB is present in women >45 years, in patients of any age group who fail to respond to medical therapy, and in those at increased risk for endometrial cancer.
Coagulopathy. When heavy bleeding has been present since the onset of menarche, inherited bleeding disorders must be considered, the most common of which is von Willebrand disease, a disorder of platelet adhesion.24 It is estimated that just under 50% of adolescents with abnormal uterine bleeding have a coagulopathy, most often a platelet function disorder.25 Additional clues to the presence of a coagulation disorder include a family history of bleeding disorder, a personal history of bleeding problems associated with surgery, and a history of iron-deficiency anemia.26 Abnormal uterine bleeding might resolve with treatment of the underlying coagulopathy; if it does not, consider consultation with a hematologist before prescribing an NSAID or an OC.
Heavy bleeding in patients taking an anticoagulant falls into the category of coagulopathy-related AUB. No further workup is generally needed for these women.3
Continue to: Ovulatory dysfunction
Ovulatory dysfunction. Abnormal uterine bleeding caused by ovulatory dysfunction is generally due to PCOS or anovulatory bleeding. Other causes, beyond the scope of this discussion, include hypothyroidism, hyperandrogenism, female athlete triad, stress, and hyperprolactinemia.
› Polycystic ovary syndrome. A diagnosis of PCOS is made using any of several recognized criteria. The commonly used Rotterdam 2003 criteria27 require that at least 2 of the following be present to make a diagnosis of PCOS:
- oligo-ovulation or anovulation
- hyperandrogenism
- polycystic ovaries seen on ultrasonography.
In addition, women with PCOS are frequently obese, show signs of insulin resistance (diabetes, prediabetes, acanthosis nigricans), or hyperandrogenism (hirsutism, acne). Even if these latter findings are not present at diagnosis, women with PCOS are at risk for a metabolic disorder. Once a diagnosis of PCOS has been established, therefore, screening tests for diabetes and cardiac risk factors (eg, dyslipidemia) should be performed.28.29
To evaluate for hyperandrogenism, free testosterone should be measured using a high-sensitivity immunoassay in all women in whom PCOS is suspected. Because of a higher prevalence of nonclassical (ie, late-onset) congenital adrenal hyperplasia (CAH) in women of Ashkenazi Jewish (estimated prevalence, 3.7%), Hispanic (1.9%), Slavic (1.6%), and Italian (0.3%) descent, screening for CAH as a possible cause of hyperandrogenism is also recommended, by a test of a morning 17-hydroxyprogesterone level.23,29,30 (Note: The general Caucasian population has an estimated prevalence of nonclassical CAH of 0.1%.30)
Treatment of PCOS should be individualized, based on a patient’s symptoms and comorbidities. For overweight and obese women, weight loss, exercise, and metformin (1500-2000 mg/d) are the mainstays of therapy, and might reduce AUB.29,31 If these measures do not reduce AUB, other options include an OC, an LNG-IUD, and NSAIDs.
Continue to: Information on treating other PCOS-related symptoms...
Information on treating other PCOS-related symptoms (acne, hirsutism) is available from many sources29; these treatments do not typically help the patient’s AUB, however, and are therefore not addressed in this article.
› Anovulatory bleeding. In adolescence, the most common cause of AUB is anovulation resulting from immaturity of the hypothalamic–pituitary–ovarian axis. During anovulatory cycles, the imbalance of estrogen and progesterone creates a fragile endometrium, leading to unpredictable bleeding and irregular cycles. Other less common causes of AUB, such as ovarian or adrenal tumor, should be considered in adolescents who have hirsutism but do not meet the criteria for PCOS.5
When seeing an adolescent for evaluation of AUB, be aware that emotional barriers might be present that make it difficult for her to talk about menses and sexual activity. Be patient and normalize the patient’s symptoms when appropriate. Pelvic exam can be deferred, especially in adolescents who have not yet had vaginal intercourse. When AUB occurs in an adolescent and the cause is thought to be immaturity of the hypothalamic–pituitary–ovarian axis, there is no need for laboratory testing or imaging studies, other than excluding hypothyroidism and pregnancy as the cause.
Oral contraceptives, NSAIDs, tranexamic acid, and the LNG-IUD are all options for treating patients who have anovulatory bleeding4,5 (TABLE 3). An OC has a major advantage for adolescents because it alleviates other complaints related to adolescent hormonal changes, such as acne, and provides contraception when taken on a regular basis.
Alternatively, the LNG-IUD has the benefit of ease of use once inserted, while still providing the added benefit of contraception. In women who have not yet had vaginal intercourse, an intrauterine device might not be the first choice of treatment, however, and should be prescribed only after discussion with the patient. For both OCs and the LNG-IUD, myths surrounding the use of these medications must be addressed with the patient and, if she is a minor, her parents or guardian.32
Continue to: NSAIDS can be effective because...
NSAIDs can be effective because they reduce bleeding by causing vasoconstriction, but they provide the greatest benefit when started before menses, which can be difficult for a patient who has irregular cycles.
Endometrial causes of AUB should be suspected when a patient has heavy menstrual bleeding with regular menstrual cycles and no other causes can be identified. Endometrial dysfunction as the cause of AUB stems from aberrations in the biochemical pathways of endometrial hemostasis and repair, and therefore is difficult to confirm by laboratory analysis or histologic evaluation.3 Medical management focuses on alleviating heavy menstrual bleeding (TABLE 3).
Iatrogenic. The most common type of iatrogenic AUB is unscheduled bleeding, also known as breakthrough bleeding, that occurs during hormonal treatment with an OC or during the first few months after insertion of an LNG-IUD or contraceptive implant.3 In most cases, no specific treatment is required; bleeding resolves upon continued use of the contraceptive.
Not yet classified. This category is difficult to define; it was created for causes of AUB that have not yet been identified and remain unclear. For example, a condition known as chronic endometritis is under study as a possible cause of AUB, but has not been assigned to a PALM–COEIN category.3 As more data become available and understanding of pathophysiologic mechanisms lead to better definitions of disease, this and other poorly understood conditions will be moved to an appropriate category in the FIGO classification system.
CASE 2
Ms. G is given a diagnosis of PCOS, based on her history. You recommend weight loss and exercise; screen her for diabetes and dyslipidemia; and prescribe metformin.
ACKNOWLEDGMENT
Barry D. Weiss, MD, University of Arizona College of Medicine, Department of Family and Community Medicine, Tucson, assisted with the editing of this manuscript.
CORRESPONDENCE
Melody A. Jordahl-Iafrato, MD, Community Hospital East Family Medicine Residency, 10122 East 10th Street, Suite 100, Indianapolis, IN 46229; [email protected].
1. Committee on Practice Bulletins—Gynecology. American College of Obstetricians and Gynecologists Practice Bulletin Number 128, July 2012: Diagnosis of abnormal uterine bleeding in reproductive-aged women. Obstet Gynecol. 2012;120:197-206.
2. American College of Obstetricians and Gynecologists Committee Opinion No. 651: Menstruation in girls and adolescents: using the menstrual cycle as a vital sign. Obstet Gynecol. 2015;126:e143-e146.
3. Munro MG, Critchley HO, Broder MS, et al; FIGO Working Group on Menstrual Disorders. FIGO classifcation system (PALM-COEIN) for causes of abnormal uterine bleeding in nongravid women of reproductive age. Int J Gynaecol Obstet. 2011;133:3-13.
4. National Institute for Health and Care Excellence (NICE). Heavy menstrual bleeding: assessment and management [NG88]. www.nice.org.uk/guidance/ng88. Accessed February 28, 2019.
5. Committee on Practice Bulletins—Gynecology. American College of Obstetricians and Gynecologists Practice Bulletin Number 136, July 2013: Management of abnormal uterine bleeding associated with ovulatory dysfunction. Obstet Gynecol. 2013;122:176-185.
6. Hassa H, Tekin B, Senses T, et al. Are the site, diameter, and number of endometrial polyps related with symptomatology? Am J Obstet Gynecol. 2006;194:718-721.
7. Salim S, Won H, Nesbitt-Hawes E, et al. Diagnosis and management of endometrial polyps: a critical review of the literature. J Minim Invasive Gynecol. 2011;18:569-581.
8. Struble J, Reid S, Bedaiwy MA. Adenomyosis: A clinical review of a challenging gynecologic condition. J Minim Invasive Gynecol. 2016;23:164-185.
9. Champaneria R, Abedin P, Daniels J, et al. Ultrasound scan and magnetic resonance imaging for the diagnosis of adenomyosis: systematic review comparing test accuracy. Acta Obstet Gynecol Scand. 2010;89:1374-1384.
10. Bartels CB, Cayton KC, Chuong FS, et al. An evidence-based approach to the medical management of fibroids: a systematic review. Clin Obstet Gynecol. 2016;59:30-52.
11. Lethaby A, Cooke I, Rees MC. Progesterone or progestogen-releasing intrauterine systems for heavy menstrual bleeding. Cochrane Database Syst Rev. 2005;(4):CD002126.
12. Spies JB. Current role of uterine artery embolization in the management of uterine fibroids. Clin Obstet Gynecol. 2016;59:93-102.
13. Gupta JK, Sinha A, Lumsden MA, et al. Uterine artery embolization for symptomatic uterine fibroids. Cochrane Database Syst Rev. 2014;(12):CD005073.
14. Edwards RD, Moss JG, Lumsden MA, et al; Committee of the Randomized Trial of Embolization versus Surgical Treatment for Fibroids. Uterine artery embolization versus surgery for symptomatic uterine fibroids. N Engl J Med. 2007;356:360-370.
15. Pron G, Bennett J, Common A, et al; Ontario Uterine Fibroid Embolization Collaboration Group. The Ontario Uterine Fibroid Embolization Trial. Part 2. Uterine fibroid reduction and symptom relief after uterine artery embolization for fibroids. Fertil Steril. 2003;79:120-127.
16. Torre A, Fauconnier A, Kahn V, et al. Fertility after uterine artery embolization for symptomatic multiple fibroids with no other infertility factors. Eur Radiol. 2017;27:2850-2859.
17. Mara M, Maskova J, Fucikova Z, et al. Midterm clinical and first reproductive results of a randomized controlled trial comparing uterine fibroid embolization and myomectomy. Cardiovasc Intervent Radiol. 2008;31:73-85.
18. Lethaby A, Vollenhoven B, Sowter M. Pre-operative GnRH analogue therapy before hysterectomy or myomectomy for uterine fibroids. Cochrane Database Syst Rev. 2001;(2): CD000547.
19. Capmas P, Levaillant JM, Fernandez H. Surgical techniques and outcome in the management of submucous fibroids. Curr Opin Obstet Gynecol. 2013;25:332-338.
20. Abu Hashim H, Ghayaty E, El Rakhawy M. Levonorgestrel-releasing intrauterine system vs oral progestins for non-atypical endometrial hyperplasia: a systematic review and metaanalysis of randomized trials. Am J Obstet Gynecol. 2015;213:469-478.
21. Reed SD, Voigt LF, Newton KM, et al. Weiss NS. Progestin therapy of complex endometrial hyperplasia with and without atypia. Obstet Gynecol. 2009;113;655-662.
22. Morice P, Leary A, Creutzberg C, et al. Endometrial cancer. Lancet. 2016;387:1094-1108.
23. Goodman NF, Cobin RH, Futterweit W, et al; American Association of Clinical Endocrinologists (AACE); American College of Endocrinology (ACE); Androgen Excess and PCOS Society (AES). American Association of Clinical Endocrinologists, American College of Endocrinology, and Androgen Excess and PCOS Society disease state clinical review: guide to the best practices in the evaluation and treatment of polycystic ovary syndrome—part 1. Endocr Pract. 2015;21:1291-1300.
24. Shankar M, Lee CA, Sabin CA, et al. von Willebrand disease in women with menorrhagia: a systematic review. BJOG. 2004;111:734-740.
25. Seravalli V, Linari S, Peruzzi E, et al. Prevalence of hemostatic disorders in adolescents with abnormal uterine bleeding. J Pediatr Adolesc Gynecol. 2013;26:285-289.
26. Philipp CS, Faiz A, Dowling NF, et al. Development of a screening tool for identifying women with menorrhagia for hemostatic evaluation. Am J Obstet Gynecol. 2008;198:163.e1-e8.
27. The Rotterdam ESHRE/ASRM-sponsored PCOS consensus workshop group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS). Hum Reprod. 2004;19:41-47.
28. Goodman NF, Cobin RH, Futterweit W, et al; American Association of Clinical Endocrinologists (AACE); American College of Endocrinology (ACE); Androgen Excess and PCOS Society (AES). American Association of Clinical Endocrinologists, American College of Endocrinology, and Androgen Excess and PCOS Society disease state clinical review: guide to the best practices in the evaluation and treatment of polycystic ovary syndrome—part 2. Endocr Pract. 2015;21:1415-26.
29. American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No 194: Polycystic ovary syndrome. Obstet Gynecol. 2018;131:e157-e171.
30. Speiser PW, Dupont B, Rubinstein P, et al. High frequency of nonclassical steroid 21-hydroxylase deficiency. Am J Hum Genet. 1985;37:650-667.
31. Naderpoor N, Shorakae S, de Courten B, et al. Metformin and lifestyle modification in polycystic ovary syndrome: systematic review and meta-analysis. Hum Reprod Update. 2015;21:560-574.
32. Kolman KB, Hadley SK, Jordahl-Iafrato MA. Long-acting reversible contraception: who, what, when, and how. J Fam Pract. 2015;64:479-484.
Menstrual bleeding is considered normal when it occurs regularly (every 21-35 days), lasts 4 to 8 days, and is not associated with heavy bleeding.1 During the first few years after menarche, it is normal for girls to experience irregular menstrual cycles but, by the third year, 60% to 80% of girls have an adult pattern of menstrual bleeding.2
Menstrual flow without normal volume, duration, regularity, or frequency is considered abnormal uterine bleeding (AUB). The condition is considered acute if there is need for immediate intervention. In the absence of the need for immediate intervention, recurrent AUB is classified as chronic.3 Chronic AUB is the focus of this article.

Invaluable tool: The FIGO classification
In 2011, the
- structural causes, recalled by “PALM” (Polyps, Adenomyosis, Leiomyoma, and Malignancy/hyperplasia)
- nonstructural causes, recalled by “COEIN” (Coagulopathy, Ovulatory dysfunction, Endometrial, Iatrogenic, and Not yet classified).


The PALM–COEIN system also uses descriptive terminology (heavy bleeding, intermenstrual bleeding) to characterize the bleeding pattern.3 The American College of Obstetricians and Gynecologists has adopted this classification system and recommends that such historically used terminology as “dysfunctional uterine bleeding,” “menorrhagia,” and “metrorrhagia” be abandoned.1

The initial workup of all causes of chronic uterine bleeding begins with a history; physical examination, including pelvic exam; and laboratory testing, including a urine pregnancy test, complete blood count, and a test of thyroid-stimulating hormone (TABLE 2). The need for additional laboratory testing, imaging, or endometrial biopsy depends on the suspected cause of AUB, detailed stepwise in FIGURE 1 (women <18 years) and FIGURE 2 (≥18 years).

We first briefly review the 9 categories of AUB in the PALM–COEIN system; discuss the most common causes in more detail; and review common treatment options (TABLE 3).

CASE 1
Marsha R, a 41-year-old-woman, complains of heavy menstrual bleeding for the past year that has become worse over the past 2 months. Her menstrual cycles have occurred every 28 days and last 10 days; she uses 10 to 12 pads a day.
Continue to: Recently, Ms. R reports...
Recently, Ms. R reports, she has been bleeding continuously for 14 days, with episodes of lighter bleeding followed by heavier bleeding. She also complains of fatigue.
Bimanual examination is notable for an enlarged uterus.
How would you proceed with the workup of this patient, to determine the cause of her bleeding and tailor management accordingly?
Structural AUB: The “PALM” mnemonic
A structural cause of AUB must be considered when you encounter an abnormality on physical exam (TABLE 1).3 In obese women or other patients in whom the physical exam is difficult, historical clues—including postcoital bleeding, intermenstrual bleeding, or pelvic pain or pressure—also suggest a structural abnormality.4
Transvaginal ultrasonography (TVUS) is the initial method of evaluation when a structural abnormality is suspected.1,4 However, although TVUS is excellent at visualizing the myometrium, lesions within the uterine cavity can be missed. If intracavitary pathology, such as submucosal fibroids or endometrial polyps, is suspected, additional imaging with saline infusion sonohysterography (SIS) should be performed. If a cavitary abnormality is confirmed, hysteroscopy is indicated.1 Magnetic resonance imaging (MRI) is reserved for cases in which a uterine cavity abnormality is found on TVUS but cannot be further characterized by SIS or hysteroscopy.1
Continue to: Endometrial biopsy...
Endometrial biopsy (EMB) is indicated as part of the initial evaluation of AUB in all women >45 years and in younger women who have risk factors for endometrial cancer, including polycystic ovary syndrome (PCOS), obesity, and hereditary nonpolyposis colorectal cancer. Such biopsy is necessary in these women whether or not another condition is the cause of the AUB and regardless of findings on TVUS.1-4 Endometrial biopsy should also be performed in women with AUB that persists despite medical management. If office EMB is nondiagnostic, hysteroscopy or SIS can be used to obtain tissue samples for further evaluation.5
Polyps. An endometrial polyp is a benign growth of endometrial tissue that is covered with epithelial cells. Polyps are often diagnosed by EMB or TVUS when these techniques are performed as part of the workup for AUB.6 Endometrial polyps are found more commonly in postmenopausal women, but should be considered as a cause of AUB in premenopausal women, too, especially those with intermenstrual bleeding or postcoital bleeding (or both) that is unresponsive to medical management.7 Risk factors for polyps include older age, obesity, and treatment with tamoxifen.7 The usual treatment for symptomatic endometrial polyps is removal by operative hysteroscopy.7
Adenomyosis. Ectopic endometrial tissue in the myometrium that leads to hypertrophy of the myometrium and uterine enlargement is known as adenomyosis. The disorder is most often diagnosed in women 40 to 50 years of age, who commonly complain of heavy uterine bleeding (40%-60% of cases) and dysmenorrhea (65%).8 Although definitive diagnosis is made histologically at hysterectomy, TVUS and MRI can be useful tools to help narrow the differential diagnosis in women with unexplained AUB.
According to a systematic review,9 the sensitivity and specificity of imaging in the diagnosis of adenomyosis is 72% and 81%, respectively, for TVUS and 77% and 89%, respectively, for MRI. Needle biopsy, performed hysteroscopically or laparoscopically, is less useful because the technique has low sensitivity (reported variously as 8%-56%) in diagnosing adenomyosis.8
Treatment options for adenomyosis are medical management with agents that reduce bleeding (eg, a combination oral contraceptive [OC], nonsteroidal anti-inflammatory drugs [NSAIDs], the antifibrinolytic tranexamic acid, and, when there is no distortion of the uterine cavity, a levonorgestrel intrauterine device [LNG-IUD]); uterine artery embolization; and hysterectomy.8
Continue to: Leiomyoma
Leiomyoma. Uterine fibroids, or leiomyomas, are benign, fibromuscular solid tumors, thought to be hormone-dependent because many regress after menopause. In women of reproductive age, uterine fibroids are the most common cause of structural AUB, with a cumulative incidence of 70% to 80% among women in this age group.3,10 Fibroids are more common in African-American women, women who experienced early menarche, and women who are obese, have PCOS, or had a late first pregnancy.3-10
Many fibroids are asymptomatic, and are found incidentally on sonographic examination performed for other reasons; in one-third of affected patients, the fibroids result in heavy menstrual bleeding.10 Intermenstrual bleeding and postcoital bleeding can occur, but are not common symptoms with fibroids. Consider other causes of AUB, such as endometrial polyps, when these symptoms are present.
Treatment of fibroids is medical or surgical. Medical management is a reasonable first-line option, especially in women who have not completed childbearing and who have small (<3 cm in diameter) fibroids. Options include a combination OC, NSAIDs, tranexamic acid, and, when the uterine cavity is not distorted, an LNG-IUD.4,10,11
For women with larger fibroids, those for whom the aforementioned medical treatments are unsuccessful, and those who are seeking more definitive treatment, uterine artery embolization, myomectomy, or hysterectomy can be considered.
› Uterine artery embolization is performed by an interventional radiologist under local anesthesia and, if necessary, moderate sedation.12 After the procedure, fibroids decrease in size due to avascular necrosis, but the remainder of the myometrium is relatively unaffected because collateral blood supply develops.13,14 Patients might experience abdominal cramping for 2 or 3 days following the procedure, which can be managed with an oral NSAID.12 Approximately 90% of women treated with embolization note improvement in AUB by 3 months after the procedure.15 Uterine artery embolization is not recommended in women who have not completed childbearing.12,16,17
Continue to: Myomectomy
› Myomectomy (removal of the leiomyoma) is the surgical treatment of choice for women who want to maintain fertility. Depending on the size and location of the fibroid(s), myomectomy can be performed as an open surgical procedure, laparoscopically, or hysteroscopically. At the discretion of the surgeon, leuprolide acetate, a gonadotropin-releasing hormone agonist, can be prescribed for 3 months before myomectomy to reduce intraoperative blood loss by decreasing the vascularity of the fibroids.4,18 Reduction in bleeding is reported in 70% to 90% of patients who undergo myomectomy.19
› Hysterectomy, the definitive treatment for uterine fibroids, should be reserved for women who have completed childbearing and who have failed (or have a contraindication to) other treatment options.
Malignancy/hyperplasia. EMB should be performed when endometrial malignancy/hyperplasia is suspected. As noted, endometrial cancer should be considered as a diagnostic possibility in women >45 years, in younger women with risk factors, and in women who have failed to respond to medical treatment for other suspected causes of AUB.5
When hyperplasia without atypia is diagnosed, the LNG-IUD or oral progesterone is an acceptable treatment option; note that fewer women who have an LNG-IUD eventually require hysterectomy, compared to women who take oral hormone therapy for AUB.20 When hyperplasia with atypia is diagnosed, hysterectomy is the treatment of choice. If a woman wishes to maintain fertility, however, oral progesterone therapy can be offered.21
When the diagnosis is cancer, the patient should be referred to a gynecologic oncologist for staging and treatment. Treatment varies depending on stage, but generally requires hysterectomy including bilateral salpingo-oophorectomy, with possible chemotherapy or radiation, or both.22
Continue to: CASE 1
CASE 1
Ms. R undergoes a sonogram that reveals a 4-cm fibroid in the uterine fundus that has not distorted the uterine cavity. Although she has completed childbearing, Ms. R is not interested in a surgical procedure at this time. You recommend insertion of an LNG-IUD; she accepts your advice.
CASE 2
Claire G, 27 years old, with a body mass index of 41,* complains of irregular menses for several months. Her menstrual cycle is irregular, as is the duration of menses and amount of bleeding. She has some mild fatigue without dizziness.
The physical exam is notable for mild hirsutism, without abnormalities on pelvic examination. Lab testing reveals iron-deficiency anemia; a pregnancy test is negative.
The questions that were raised by Ms. R’s case challenge you here, too: What is the appropriate workup of Ms. G’s bleeding? Once the cause is confirmed, how should you treat her?
Nonstructural AUB: The “COEIN” mnemonic
In the absence of abnormalities on a pelvic exam, and after excluding endometrial malignancy/hyperplasia in patients with the aforementioned risk factors, a nonstructural cause of AUB should be considered (TABLE 1).3 In women 20 to 40 years of age, the primary common cause of nonstructural uterine bleeding is ovulatory dysfunction, most often caused by PCOS or anovulatory bleeding.
Continue to: For nonstructual causes of AUB...
For nonstructural causes of AUB, the recommended laboratory workup varies with the suspected diagnosis. In addition, recently pregnant women should have a quantitative assay of β human chorionic gonadotropin to evaluate for trophoblastic disease.5,23
Imaging is not usually recommended when the cause of AUB is suspected to be nonstructural. However, when PCOS is suspected, TVUS can be used to confirm the presence of polycystic ovaries.23
As noted, EMB should be performed when AUB is present in women >45 years, in patients of any age group who fail to respond to medical therapy, and in those at increased risk for endometrial cancer.
Coagulopathy. When heavy bleeding has been present since the onset of menarche, inherited bleeding disorders must be considered, the most common of which is von Willebrand disease, a disorder of platelet adhesion.24 It is estimated that just under 50% of adolescents with abnormal uterine bleeding have a coagulopathy, most often a platelet function disorder.25 Additional clues to the presence of a coagulation disorder include a family history of bleeding disorder, a personal history of bleeding problems associated with surgery, and a history of iron-deficiency anemia.26 Abnormal uterine bleeding might resolve with treatment of the underlying coagulopathy; if it does not, consider consultation with a hematologist before prescribing an NSAID or an OC.
Heavy bleeding in patients taking an anticoagulant falls into the category of coagulopathy-related AUB. No further workup is generally needed for these women.3
Continue to: Ovulatory dysfunction
Ovulatory dysfunction. Abnormal uterine bleeding caused by ovulatory dysfunction is generally due to PCOS or anovulatory bleeding. Other causes, beyond the scope of this discussion, include hypothyroidism, hyperandrogenism, female athlete triad, stress, and hyperprolactinemia.
› Polycystic ovary syndrome. A diagnosis of PCOS is made using any of several recognized criteria. The commonly used Rotterdam 2003 criteria27 require that at least 2 of the following be present to make a diagnosis of PCOS:
- oligo-ovulation or anovulation
- hyperandrogenism
- polycystic ovaries seen on ultrasonography.
In addition, women with PCOS are frequently obese, show signs of insulin resistance (diabetes, prediabetes, acanthosis nigricans), or hyperandrogenism (hirsutism, acne). Even if these latter findings are not present at diagnosis, women with PCOS are at risk for a metabolic disorder. Once a diagnosis of PCOS has been established, therefore, screening tests for diabetes and cardiac risk factors (eg, dyslipidemia) should be performed.28.29
To evaluate for hyperandrogenism, free testosterone should be measured using a high-sensitivity immunoassay in all women in whom PCOS is suspected. Because of a higher prevalence of nonclassical (ie, late-onset) congenital adrenal hyperplasia (CAH) in women of Ashkenazi Jewish (estimated prevalence, 3.7%), Hispanic (1.9%), Slavic (1.6%), and Italian (0.3%) descent, screening for CAH as a possible cause of hyperandrogenism is also recommended, by a test of a morning 17-hydroxyprogesterone level.23,29,30 (Note: The general Caucasian population has an estimated prevalence of nonclassical CAH of 0.1%.30)
Treatment of PCOS should be individualized, based on a patient’s symptoms and comorbidities. For overweight and obese women, weight loss, exercise, and metformin (1500-2000 mg/d) are the mainstays of therapy, and might reduce AUB.29,31 If these measures do not reduce AUB, other options include an OC, an LNG-IUD, and NSAIDs.
Continue to: Information on treating other PCOS-related symptoms...
Information on treating other PCOS-related symptoms (acne, hirsutism) is available from many sources29; these treatments do not typically help the patient’s AUB, however, and are therefore not addressed in this article.
› Anovulatory bleeding. In adolescence, the most common cause of AUB is anovulation resulting from immaturity of the hypothalamic–pituitary–ovarian axis. During anovulatory cycles, the imbalance of estrogen and progesterone creates a fragile endometrium, leading to unpredictable bleeding and irregular cycles. Other less common causes of AUB, such as ovarian or adrenal tumor, should be considered in adolescents who have hirsutism but do not meet the criteria for PCOS.5
When seeing an adolescent for evaluation of AUB, be aware that emotional barriers might be present that make it difficult for her to talk about menses and sexual activity. Be patient and normalize the patient’s symptoms when appropriate. Pelvic exam can be deferred, especially in adolescents who have not yet had vaginal intercourse. When AUB occurs in an adolescent and the cause is thought to be immaturity of the hypothalamic–pituitary–ovarian axis, there is no need for laboratory testing or imaging studies, other than excluding hypothyroidism and pregnancy as the cause.
Oral contraceptives, NSAIDs, tranexamic acid, and the LNG-IUD are all options for treating patients who have anovulatory bleeding4,5 (TABLE 3). An OC has a major advantage for adolescents because it alleviates other complaints related to adolescent hormonal changes, such as acne, and provides contraception when taken on a regular basis.
Alternatively, the LNG-IUD has the benefit of ease of use once inserted, while still providing the added benefit of contraception. In women who have not yet had vaginal intercourse, an intrauterine device might not be the first choice of treatment, however, and should be prescribed only after discussion with the patient. For both OCs and the LNG-IUD, myths surrounding the use of these medications must be addressed with the patient and, if she is a minor, her parents or guardian.32
Continue to: NSAIDS can be effective because...
NSAIDs can be effective because they reduce bleeding by causing vasoconstriction, but they provide the greatest benefit when started before menses, which can be difficult for a patient who has irregular cycles.
Endometrial causes of AUB should be suspected when a patient has heavy menstrual bleeding with regular menstrual cycles and no other causes can be identified. Endometrial dysfunction as the cause of AUB stems from aberrations in the biochemical pathways of endometrial hemostasis and repair, and therefore is difficult to confirm by laboratory analysis or histologic evaluation.3 Medical management focuses on alleviating heavy menstrual bleeding (TABLE 3).
Iatrogenic. The most common type of iatrogenic AUB is unscheduled bleeding, also known as breakthrough bleeding, that occurs during hormonal treatment with an OC or during the first few months after insertion of an LNG-IUD or contraceptive implant.3 In most cases, no specific treatment is required; bleeding resolves upon continued use of the contraceptive.
Not yet classified. This category is difficult to define; it was created for causes of AUB that have not yet been identified and remain unclear. For example, a condition known as chronic endometritis is under study as a possible cause of AUB, but has not been assigned to a PALM–COEIN category.3 As more data become available and understanding of pathophysiologic mechanisms lead to better definitions of disease, this and other poorly understood conditions will be moved to an appropriate category in the FIGO classification system.
CASE 2
Ms. G is given a diagnosis of PCOS, based on her history. You recommend weight loss and exercise; screen her for diabetes and dyslipidemia; and prescribe metformin.
ACKNOWLEDGMENT
Barry D. Weiss, MD, University of Arizona College of Medicine, Department of Family and Community Medicine, Tucson, assisted with the editing of this manuscript.
CORRESPONDENCE
Melody A. Jordahl-Iafrato, MD, Community Hospital East Family Medicine Residency, 10122 East 10th Street, Suite 100, Indianapolis, IN 46229; [email protected].
Menstrual bleeding is considered normal when it occurs regularly (every 21-35 days), lasts 4 to 8 days, and is not associated with heavy bleeding.1 During the first few years after menarche, it is normal for girls to experience irregular menstrual cycles but, by the third year, 60% to 80% of girls have an adult pattern of menstrual bleeding.2
Menstrual flow without normal volume, duration, regularity, or frequency is considered abnormal uterine bleeding (AUB). The condition is considered acute if there is need for immediate intervention. In the absence of the need for immediate intervention, recurrent AUB is classified as chronic.3 Chronic AUB is the focus of this article.

Invaluable tool: The FIGO classification
In 2011, the
- structural causes, recalled by “PALM” (Polyps, Adenomyosis, Leiomyoma, and Malignancy/hyperplasia)
- nonstructural causes, recalled by “COEIN” (Coagulopathy, Ovulatory dysfunction, Endometrial, Iatrogenic, and Not yet classified).


The PALM–COEIN system also uses descriptive terminology (heavy bleeding, intermenstrual bleeding) to characterize the bleeding pattern.3 The American College of Obstetricians and Gynecologists has adopted this classification system and recommends that such historically used terminology as “dysfunctional uterine bleeding,” “menorrhagia,” and “metrorrhagia” be abandoned.1

The initial workup of all causes of chronic uterine bleeding begins with a history; physical examination, including pelvic exam; and laboratory testing, including a urine pregnancy test, complete blood count, and a test of thyroid-stimulating hormone (TABLE 2). The need for additional laboratory testing, imaging, or endometrial biopsy depends on the suspected cause of AUB, detailed stepwise in FIGURE 1 (women <18 years) and FIGURE 2 (≥18 years).

We first briefly review the 9 categories of AUB in the PALM–COEIN system; discuss the most common causes in more detail; and review common treatment options (TABLE 3).

CASE 1
Marsha R, a 41-year-old-woman, complains of heavy menstrual bleeding for the past year that has become worse over the past 2 months. Her menstrual cycles have occurred every 28 days and last 10 days; she uses 10 to 12 pads a day.
Continue to: Recently, Ms. R reports...
Recently, Ms. R reports, she has been bleeding continuously for 14 days, with episodes of lighter bleeding followed by heavier bleeding. She also complains of fatigue.
Bimanual examination is notable for an enlarged uterus.
How would you proceed with the workup of this patient, to determine the cause of her bleeding and tailor management accordingly?
Structural AUB: The “PALM” mnemonic
A structural cause of AUB must be considered when you encounter an abnormality on physical exam (TABLE 1).3 In obese women or other patients in whom the physical exam is difficult, historical clues—including postcoital bleeding, intermenstrual bleeding, or pelvic pain or pressure—also suggest a structural abnormality.4
Transvaginal ultrasonography (TVUS) is the initial method of evaluation when a structural abnormality is suspected.1,4 However, although TVUS is excellent at visualizing the myometrium, lesions within the uterine cavity can be missed. If intracavitary pathology, such as submucosal fibroids or endometrial polyps, is suspected, additional imaging with saline infusion sonohysterography (SIS) should be performed. If a cavitary abnormality is confirmed, hysteroscopy is indicated.1 Magnetic resonance imaging (MRI) is reserved for cases in which a uterine cavity abnormality is found on TVUS but cannot be further characterized by SIS or hysteroscopy.1
Continue to: Endometrial biopsy...
Endometrial biopsy (EMB) is indicated as part of the initial evaluation of AUB in all women >45 years and in younger women who have risk factors for endometrial cancer, including polycystic ovary syndrome (PCOS), obesity, and hereditary nonpolyposis colorectal cancer. Such biopsy is necessary in these women whether or not another condition is the cause of the AUB and regardless of findings on TVUS.1-4 Endometrial biopsy should also be performed in women with AUB that persists despite medical management. If office EMB is nondiagnostic, hysteroscopy or SIS can be used to obtain tissue samples for further evaluation.5
Polyps. An endometrial polyp is a benign growth of endometrial tissue that is covered with epithelial cells. Polyps are often diagnosed by EMB or TVUS when these techniques are performed as part of the workup for AUB.6 Endometrial polyps are found more commonly in postmenopausal women, but should be considered as a cause of AUB in premenopausal women, too, especially those with intermenstrual bleeding or postcoital bleeding (or both) that is unresponsive to medical management.7 Risk factors for polyps include older age, obesity, and treatment with tamoxifen.7 The usual treatment for symptomatic endometrial polyps is removal by operative hysteroscopy.7
Adenomyosis. Ectopic endometrial tissue in the myometrium that leads to hypertrophy of the myometrium and uterine enlargement is known as adenomyosis. The disorder is most often diagnosed in women 40 to 50 years of age, who commonly complain of heavy uterine bleeding (40%-60% of cases) and dysmenorrhea (65%).8 Although definitive diagnosis is made histologically at hysterectomy, TVUS and MRI can be useful tools to help narrow the differential diagnosis in women with unexplained AUB.
According to a systematic review,9 the sensitivity and specificity of imaging in the diagnosis of adenomyosis is 72% and 81%, respectively, for TVUS and 77% and 89%, respectively, for MRI. Needle biopsy, performed hysteroscopically or laparoscopically, is less useful because the technique has low sensitivity (reported variously as 8%-56%) in diagnosing adenomyosis.8
Treatment options for adenomyosis are medical management with agents that reduce bleeding (eg, a combination oral contraceptive [OC], nonsteroidal anti-inflammatory drugs [NSAIDs], the antifibrinolytic tranexamic acid, and, when there is no distortion of the uterine cavity, a levonorgestrel intrauterine device [LNG-IUD]); uterine artery embolization; and hysterectomy.8
Continue to: Leiomyoma
Leiomyoma. Uterine fibroids, or leiomyomas, are benign, fibromuscular solid tumors, thought to be hormone-dependent because many regress after menopause. In women of reproductive age, uterine fibroids are the most common cause of structural AUB, with a cumulative incidence of 70% to 80% among women in this age group.3,10 Fibroids are more common in African-American women, women who experienced early menarche, and women who are obese, have PCOS, or had a late first pregnancy.3-10
Many fibroids are asymptomatic, and are found incidentally on sonographic examination performed for other reasons; in one-third of affected patients, the fibroids result in heavy menstrual bleeding.10 Intermenstrual bleeding and postcoital bleeding can occur, but are not common symptoms with fibroids. Consider other causes of AUB, such as endometrial polyps, when these symptoms are present.
Treatment of fibroids is medical or surgical. Medical management is a reasonable first-line option, especially in women who have not completed childbearing and who have small (<3 cm in diameter) fibroids. Options include a combination OC, NSAIDs, tranexamic acid, and, when the uterine cavity is not distorted, an LNG-IUD.4,10,11
For women with larger fibroids, those for whom the aforementioned medical treatments are unsuccessful, and those who are seeking more definitive treatment, uterine artery embolization, myomectomy, or hysterectomy can be considered.
› Uterine artery embolization is performed by an interventional radiologist under local anesthesia and, if necessary, moderate sedation.12 After the procedure, fibroids decrease in size due to avascular necrosis, but the remainder of the myometrium is relatively unaffected because collateral blood supply develops.13,14 Patients might experience abdominal cramping for 2 or 3 days following the procedure, which can be managed with an oral NSAID.12 Approximately 90% of women treated with embolization note improvement in AUB by 3 months after the procedure.15 Uterine artery embolization is not recommended in women who have not completed childbearing.12,16,17
Continue to: Myomectomy
› Myomectomy (removal of the leiomyoma) is the surgical treatment of choice for women who want to maintain fertility. Depending on the size and location of the fibroid(s), myomectomy can be performed as an open surgical procedure, laparoscopically, or hysteroscopically. At the discretion of the surgeon, leuprolide acetate, a gonadotropin-releasing hormone agonist, can be prescribed for 3 months before myomectomy to reduce intraoperative blood loss by decreasing the vascularity of the fibroids.4,18 Reduction in bleeding is reported in 70% to 90% of patients who undergo myomectomy.19
› Hysterectomy, the definitive treatment for uterine fibroids, should be reserved for women who have completed childbearing and who have failed (or have a contraindication to) other treatment options.
Malignancy/hyperplasia. EMB should be performed when endometrial malignancy/hyperplasia is suspected. As noted, endometrial cancer should be considered as a diagnostic possibility in women >45 years, in younger women with risk factors, and in women who have failed to respond to medical treatment for other suspected causes of AUB.5
When hyperplasia without atypia is diagnosed, the LNG-IUD or oral progesterone is an acceptable treatment option; note that fewer women who have an LNG-IUD eventually require hysterectomy, compared to women who take oral hormone therapy for AUB.20 When hyperplasia with atypia is diagnosed, hysterectomy is the treatment of choice. If a woman wishes to maintain fertility, however, oral progesterone therapy can be offered.21
When the diagnosis is cancer, the patient should be referred to a gynecologic oncologist for staging and treatment. Treatment varies depending on stage, but generally requires hysterectomy including bilateral salpingo-oophorectomy, with possible chemotherapy or radiation, or both.22
Continue to: CASE 1
CASE 1
Ms. R undergoes a sonogram that reveals a 4-cm fibroid in the uterine fundus that has not distorted the uterine cavity. Although she has completed childbearing, Ms. R is not interested in a surgical procedure at this time. You recommend insertion of an LNG-IUD; she accepts your advice.
CASE 2
Claire G, 27 years old, with a body mass index of 41,* complains of irregular menses for several months. Her menstrual cycle is irregular, as is the duration of menses and amount of bleeding. She has some mild fatigue without dizziness.
The physical exam is notable for mild hirsutism, without abnormalities on pelvic examination. Lab testing reveals iron-deficiency anemia; a pregnancy test is negative.
The questions that were raised by Ms. R’s case challenge you here, too: What is the appropriate workup of Ms. G’s bleeding? Once the cause is confirmed, how should you treat her?
Nonstructural AUB: The “COEIN” mnemonic
In the absence of abnormalities on a pelvic exam, and after excluding endometrial malignancy/hyperplasia in patients with the aforementioned risk factors, a nonstructural cause of AUB should be considered (TABLE 1).3 In women 20 to 40 years of age, the primary common cause of nonstructural uterine bleeding is ovulatory dysfunction, most often caused by PCOS or anovulatory bleeding.
Continue to: For nonstructual causes of AUB...
For nonstructural causes of AUB, the recommended laboratory workup varies with the suspected diagnosis. In addition, recently pregnant women should have a quantitative assay of β human chorionic gonadotropin to evaluate for trophoblastic disease.5,23
Imaging is not usually recommended when the cause of AUB is suspected to be nonstructural. However, when PCOS is suspected, TVUS can be used to confirm the presence of polycystic ovaries.23
As noted, EMB should be performed when AUB is present in women >45 years, in patients of any age group who fail to respond to medical therapy, and in those at increased risk for endometrial cancer.
Coagulopathy. When heavy bleeding has been present since the onset of menarche, inherited bleeding disorders must be considered, the most common of which is von Willebrand disease, a disorder of platelet adhesion.24 It is estimated that just under 50% of adolescents with abnormal uterine bleeding have a coagulopathy, most often a platelet function disorder.25 Additional clues to the presence of a coagulation disorder include a family history of bleeding disorder, a personal history of bleeding problems associated with surgery, and a history of iron-deficiency anemia.26 Abnormal uterine bleeding might resolve with treatment of the underlying coagulopathy; if it does not, consider consultation with a hematologist before prescribing an NSAID or an OC.
Heavy bleeding in patients taking an anticoagulant falls into the category of coagulopathy-related AUB. No further workup is generally needed for these women.3
Continue to: Ovulatory dysfunction
Ovulatory dysfunction. Abnormal uterine bleeding caused by ovulatory dysfunction is generally due to PCOS or anovulatory bleeding. Other causes, beyond the scope of this discussion, include hypothyroidism, hyperandrogenism, female athlete triad, stress, and hyperprolactinemia.
› Polycystic ovary syndrome. A diagnosis of PCOS is made using any of several recognized criteria. The commonly used Rotterdam 2003 criteria27 require that at least 2 of the following be present to make a diagnosis of PCOS:
- oligo-ovulation or anovulation
- hyperandrogenism
- polycystic ovaries seen on ultrasonography.
In addition, women with PCOS are frequently obese, show signs of insulin resistance (diabetes, prediabetes, acanthosis nigricans), or hyperandrogenism (hirsutism, acne). Even if these latter findings are not present at diagnosis, women with PCOS are at risk for a metabolic disorder. Once a diagnosis of PCOS has been established, therefore, screening tests for diabetes and cardiac risk factors (eg, dyslipidemia) should be performed.28.29
To evaluate for hyperandrogenism, free testosterone should be measured using a high-sensitivity immunoassay in all women in whom PCOS is suspected. Because of a higher prevalence of nonclassical (ie, late-onset) congenital adrenal hyperplasia (CAH) in women of Ashkenazi Jewish (estimated prevalence, 3.7%), Hispanic (1.9%), Slavic (1.6%), and Italian (0.3%) descent, screening for CAH as a possible cause of hyperandrogenism is also recommended, by a test of a morning 17-hydroxyprogesterone level.23,29,30 (Note: The general Caucasian population has an estimated prevalence of nonclassical CAH of 0.1%.30)
Treatment of PCOS should be individualized, based on a patient’s symptoms and comorbidities. For overweight and obese women, weight loss, exercise, and metformin (1500-2000 mg/d) are the mainstays of therapy, and might reduce AUB.29,31 If these measures do not reduce AUB, other options include an OC, an LNG-IUD, and NSAIDs.
Continue to: Information on treating other PCOS-related symptoms...
Information on treating other PCOS-related symptoms (acne, hirsutism) is available from many sources29; these treatments do not typically help the patient’s AUB, however, and are therefore not addressed in this article.
› Anovulatory bleeding. In adolescence, the most common cause of AUB is anovulation resulting from immaturity of the hypothalamic–pituitary–ovarian axis. During anovulatory cycles, the imbalance of estrogen and progesterone creates a fragile endometrium, leading to unpredictable bleeding and irregular cycles. Other less common causes of AUB, such as ovarian or adrenal tumor, should be considered in adolescents who have hirsutism but do not meet the criteria for PCOS.5
When seeing an adolescent for evaluation of AUB, be aware that emotional barriers might be present that make it difficult for her to talk about menses and sexual activity. Be patient and normalize the patient’s symptoms when appropriate. Pelvic exam can be deferred, especially in adolescents who have not yet had vaginal intercourse. When AUB occurs in an adolescent and the cause is thought to be immaturity of the hypothalamic–pituitary–ovarian axis, there is no need for laboratory testing or imaging studies, other than excluding hypothyroidism and pregnancy as the cause.
Oral contraceptives, NSAIDs, tranexamic acid, and the LNG-IUD are all options for treating patients who have anovulatory bleeding4,5 (TABLE 3). An OC has a major advantage for adolescents because it alleviates other complaints related to adolescent hormonal changes, such as acne, and provides contraception when taken on a regular basis.
Alternatively, the LNG-IUD has the benefit of ease of use once inserted, while still providing the added benefit of contraception. In women who have not yet had vaginal intercourse, an intrauterine device might not be the first choice of treatment, however, and should be prescribed only after discussion with the patient. For both OCs and the LNG-IUD, myths surrounding the use of these medications must be addressed with the patient and, if she is a minor, her parents or guardian.32
Continue to: NSAIDS can be effective because...
NSAIDs can be effective because they reduce bleeding by causing vasoconstriction, but they provide the greatest benefit when started before menses, which can be difficult for a patient who has irregular cycles.
Endometrial causes of AUB should be suspected when a patient has heavy menstrual bleeding with regular menstrual cycles and no other causes can be identified. Endometrial dysfunction as the cause of AUB stems from aberrations in the biochemical pathways of endometrial hemostasis and repair, and therefore is difficult to confirm by laboratory analysis or histologic evaluation.3 Medical management focuses on alleviating heavy menstrual bleeding (TABLE 3).
Iatrogenic. The most common type of iatrogenic AUB is unscheduled bleeding, also known as breakthrough bleeding, that occurs during hormonal treatment with an OC or during the first few months after insertion of an LNG-IUD or contraceptive implant.3 In most cases, no specific treatment is required; bleeding resolves upon continued use of the contraceptive.
Not yet classified. This category is difficult to define; it was created for causes of AUB that have not yet been identified and remain unclear. For example, a condition known as chronic endometritis is under study as a possible cause of AUB, but has not been assigned to a PALM–COEIN category.3 As more data become available and understanding of pathophysiologic mechanisms lead to better definitions of disease, this and other poorly understood conditions will be moved to an appropriate category in the FIGO classification system.
CASE 2
Ms. G is given a diagnosis of PCOS, based on her history. You recommend weight loss and exercise; screen her for diabetes and dyslipidemia; and prescribe metformin.
ACKNOWLEDGMENT
Barry D. Weiss, MD, University of Arizona College of Medicine, Department of Family and Community Medicine, Tucson, assisted with the editing of this manuscript.
CORRESPONDENCE
Melody A. Jordahl-Iafrato, MD, Community Hospital East Family Medicine Residency, 10122 East 10th Street, Suite 100, Indianapolis, IN 46229; [email protected].
1. Committee on Practice Bulletins—Gynecology. American College of Obstetricians and Gynecologists Practice Bulletin Number 128, July 2012: Diagnosis of abnormal uterine bleeding in reproductive-aged women. Obstet Gynecol. 2012;120:197-206.
2. American College of Obstetricians and Gynecologists Committee Opinion No. 651: Menstruation in girls and adolescents: using the menstrual cycle as a vital sign. Obstet Gynecol. 2015;126:e143-e146.
3. Munro MG, Critchley HO, Broder MS, et al; FIGO Working Group on Menstrual Disorders. FIGO classifcation system (PALM-COEIN) for causes of abnormal uterine bleeding in nongravid women of reproductive age. Int J Gynaecol Obstet. 2011;133:3-13.
4. National Institute for Health and Care Excellence (NICE). Heavy menstrual bleeding: assessment and management [NG88]. www.nice.org.uk/guidance/ng88. Accessed February 28, 2019.
5. Committee on Practice Bulletins—Gynecology. American College of Obstetricians and Gynecologists Practice Bulletin Number 136, July 2013: Management of abnormal uterine bleeding associated with ovulatory dysfunction. Obstet Gynecol. 2013;122:176-185.
6. Hassa H, Tekin B, Senses T, et al. Are the site, diameter, and number of endometrial polyps related with symptomatology? Am J Obstet Gynecol. 2006;194:718-721.
7. Salim S, Won H, Nesbitt-Hawes E, et al. Diagnosis and management of endometrial polyps: a critical review of the literature. J Minim Invasive Gynecol. 2011;18:569-581.
8. Struble J, Reid S, Bedaiwy MA. Adenomyosis: A clinical review of a challenging gynecologic condition. J Minim Invasive Gynecol. 2016;23:164-185.
9. Champaneria R, Abedin P, Daniels J, et al. Ultrasound scan and magnetic resonance imaging for the diagnosis of adenomyosis: systematic review comparing test accuracy. Acta Obstet Gynecol Scand. 2010;89:1374-1384.
10. Bartels CB, Cayton KC, Chuong FS, et al. An evidence-based approach to the medical management of fibroids: a systematic review. Clin Obstet Gynecol. 2016;59:30-52.
11. Lethaby A, Cooke I, Rees MC. Progesterone or progestogen-releasing intrauterine systems for heavy menstrual bleeding. Cochrane Database Syst Rev. 2005;(4):CD002126.
12. Spies JB. Current role of uterine artery embolization in the management of uterine fibroids. Clin Obstet Gynecol. 2016;59:93-102.
13. Gupta JK, Sinha A, Lumsden MA, et al. Uterine artery embolization for symptomatic uterine fibroids. Cochrane Database Syst Rev. 2014;(12):CD005073.
14. Edwards RD, Moss JG, Lumsden MA, et al; Committee of the Randomized Trial of Embolization versus Surgical Treatment for Fibroids. Uterine artery embolization versus surgery for symptomatic uterine fibroids. N Engl J Med. 2007;356:360-370.
15. Pron G, Bennett J, Common A, et al; Ontario Uterine Fibroid Embolization Collaboration Group. The Ontario Uterine Fibroid Embolization Trial. Part 2. Uterine fibroid reduction and symptom relief after uterine artery embolization for fibroids. Fertil Steril. 2003;79:120-127.
16. Torre A, Fauconnier A, Kahn V, et al. Fertility after uterine artery embolization for symptomatic multiple fibroids with no other infertility factors. Eur Radiol. 2017;27:2850-2859.
17. Mara M, Maskova J, Fucikova Z, et al. Midterm clinical and first reproductive results of a randomized controlled trial comparing uterine fibroid embolization and myomectomy. Cardiovasc Intervent Radiol. 2008;31:73-85.
18. Lethaby A, Vollenhoven B, Sowter M. Pre-operative GnRH analogue therapy before hysterectomy or myomectomy for uterine fibroids. Cochrane Database Syst Rev. 2001;(2): CD000547.
19. Capmas P, Levaillant JM, Fernandez H. Surgical techniques and outcome in the management of submucous fibroids. Curr Opin Obstet Gynecol. 2013;25:332-338.
20. Abu Hashim H, Ghayaty E, El Rakhawy M. Levonorgestrel-releasing intrauterine system vs oral progestins for non-atypical endometrial hyperplasia: a systematic review and metaanalysis of randomized trials. Am J Obstet Gynecol. 2015;213:469-478.
21. Reed SD, Voigt LF, Newton KM, et al. Weiss NS. Progestin therapy of complex endometrial hyperplasia with and without atypia. Obstet Gynecol. 2009;113;655-662.
22. Morice P, Leary A, Creutzberg C, et al. Endometrial cancer. Lancet. 2016;387:1094-1108.
23. Goodman NF, Cobin RH, Futterweit W, et al; American Association of Clinical Endocrinologists (AACE); American College of Endocrinology (ACE); Androgen Excess and PCOS Society (AES). American Association of Clinical Endocrinologists, American College of Endocrinology, and Androgen Excess and PCOS Society disease state clinical review: guide to the best practices in the evaluation and treatment of polycystic ovary syndrome—part 1. Endocr Pract. 2015;21:1291-1300.
24. Shankar M, Lee CA, Sabin CA, et al. von Willebrand disease in women with menorrhagia: a systematic review. BJOG. 2004;111:734-740.
25. Seravalli V, Linari S, Peruzzi E, et al. Prevalence of hemostatic disorders in adolescents with abnormal uterine bleeding. J Pediatr Adolesc Gynecol. 2013;26:285-289.
26. Philipp CS, Faiz A, Dowling NF, et al. Development of a screening tool for identifying women with menorrhagia for hemostatic evaluation. Am J Obstet Gynecol. 2008;198:163.e1-e8.
27. The Rotterdam ESHRE/ASRM-sponsored PCOS consensus workshop group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS). Hum Reprod. 2004;19:41-47.
28. Goodman NF, Cobin RH, Futterweit W, et al; American Association of Clinical Endocrinologists (AACE); American College of Endocrinology (ACE); Androgen Excess and PCOS Society (AES). American Association of Clinical Endocrinologists, American College of Endocrinology, and Androgen Excess and PCOS Society disease state clinical review: guide to the best practices in the evaluation and treatment of polycystic ovary syndrome—part 2. Endocr Pract. 2015;21:1415-26.
29. American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No 194: Polycystic ovary syndrome. Obstet Gynecol. 2018;131:e157-e171.
30. Speiser PW, Dupont B, Rubinstein P, et al. High frequency of nonclassical steroid 21-hydroxylase deficiency. Am J Hum Genet. 1985;37:650-667.
31. Naderpoor N, Shorakae S, de Courten B, et al. Metformin and lifestyle modification in polycystic ovary syndrome: systematic review and meta-analysis. Hum Reprod Update. 2015;21:560-574.
32. Kolman KB, Hadley SK, Jordahl-Iafrato MA. Long-acting reversible contraception: who, what, when, and how. J Fam Pract. 2015;64:479-484.
1. Committee on Practice Bulletins—Gynecology. American College of Obstetricians and Gynecologists Practice Bulletin Number 128, July 2012: Diagnosis of abnormal uterine bleeding in reproductive-aged women. Obstet Gynecol. 2012;120:197-206.
2. American College of Obstetricians and Gynecologists Committee Opinion No. 651: Menstruation in girls and adolescents: using the menstrual cycle as a vital sign. Obstet Gynecol. 2015;126:e143-e146.
3. Munro MG, Critchley HO, Broder MS, et al; FIGO Working Group on Menstrual Disorders. FIGO classifcation system (PALM-COEIN) for causes of abnormal uterine bleeding in nongravid women of reproductive age. Int J Gynaecol Obstet. 2011;133:3-13.
4. National Institute for Health and Care Excellence (NICE). Heavy menstrual bleeding: assessment and management [NG88]. www.nice.org.uk/guidance/ng88. Accessed February 28, 2019.
5. Committee on Practice Bulletins—Gynecology. American College of Obstetricians and Gynecologists Practice Bulletin Number 136, July 2013: Management of abnormal uterine bleeding associated with ovulatory dysfunction. Obstet Gynecol. 2013;122:176-185.
6. Hassa H, Tekin B, Senses T, et al. Are the site, diameter, and number of endometrial polyps related with symptomatology? Am J Obstet Gynecol. 2006;194:718-721.
7. Salim S, Won H, Nesbitt-Hawes E, et al. Diagnosis and management of endometrial polyps: a critical review of the literature. J Minim Invasive Gynecol. 2011;18:569-581.
8. Struble J, Reid S, Bedaiwy MA. Adenomyosis: A clinical review of a challenging gynecologic condition. J Minim Invasive Gynecol. 2016;23:164-185.
9. Champaneria R, Abedin P, Daniels J, et al. Ultrasound scan and magnetic resonance imaging for the diagnosis of adenomyosis: systematic review comparing test accuracy. Acta Obstet Gynecol Scand. 2010;89:1374-1384.
10. Bartels CB, Cayton KC, Chuong FS, et al. An evidence-based approach to the medical management of fibroids: a systematic review. Clin Obstet Gynecol. 2016;59:30-52.
11. Lethaby A, Cooke I, Rees MC. Progesterone or progestogen-releasing intrauterine systems for heavy menstrual bleeding. Cochrane Database Syst Rev. 2005;(4):CD002126.
12. Spies JB. Current role of uterine artery embolization in the management of uterine fibroids. Clin Obstet Gynecol. 2016;59:93-102.
13. Gupta JK, Sinha A, Lumsden MA, et al. Uterine artery embolization for symptomatic uterine fibroids. Cochrane Database Syst Rev. 2014;(12):CD005073.
14. Edwards RD, Moss JG, Lumsden MA, et al; Committee of the Randomized Trial of Embolization versus Surgical Treatment for Fibroids. Uterine artery embolization versus surgery for symptomatic uterine fibroids. N Engl J Med. 2007;356:360-370.
15. Pron G, Bennett J, Common A, et al; Ontario Uterine Fibroid Embolization Collaboration Group. The Ontario Uterine Fibroid Embolization Trial. Part 2. Uterine fibroid reduction and symptom relief after uterine artery embolization for fibroids. Fertil Steril. 2003;79:120-127.
16. Torre A, Fauconnier A, Kahn V, et al. Fertility after uterine artery embolization for symptomatic multiple fibroids with no other infertility factors. Eur Radiol. 2017;27:2850-2859.
17. Mara M, Maskova J, Fucikova Z, et al. Midterm clinical and first reproductive results of a randomized controlled trial comparing uterine fibroid embolization and myomectomy. Cardiovasc Intervent Radiol. 2008;31:73-85.
18. Lethaby A, Vollenhoven B, Sowter M. Pre-operative GnRH analogue therapy before hysterectomy or myomectomy for uterine fibroids. Cochrane Database Syst Rev. 2001;(2): CD000547.
19. Capmas P, Levaillant JM, Fernandez H. Surgical techniques and outcome in the management of submucous fibroids. Curr Opin Obstet Gynecol. 2013;25:332-338.
20. Abu Hashim H, Ghayaty E, El Rakhawy M. Levonorgestrel-releasing intrauterine system vs oral progestins for non-atypical endometrial hyperplasia: a systematic review and metaanalysis of randomized trials. Am J Obstet Gynecol. 2015;213:469-478.
21. Reed SD, Voigt LF, Newton KM, et al. Weiss NS. Progestin therapy of complex endometrial hyperplasia with and without atypia. Obstet Gynecol. 2009;113;655-662.
22. Morice P, Leary A, Creutzberg C, et al. Endometrial cancer. Lancet. 2016;387:1094-1108.
23. Goodman NF, Cobin RH, Futterweit W, et al; American Association of Clinical Endocrinologists (AACE); American College of Endocrinology (ACE); Androgen Excess and PCOS Society (AES). American Association of Clinical Endocrinologists, American College of Endocrinology, and Androgen Excess and PCOS Society disease state clinical review: guide to the best practices in the evaluation and treatment of polycystic ovary syndrome—part 1. Endocr Pract. 2015;21:1291-1300.
24. Shankar M, Lee CA, Sabin CA, et al. von Willebrand disease in women with menorrhagia: a systematic review. BJOG. 2004;111:734-740.
25. Seravalli V, Linari S, Peruzzi E, et al. Prevalence of hemostatic disorders in adolescents with abnormal uterine bleeding. J Pediatr Adolesc Gynecol. 2013;26:285-289.
26. Philipp CS, Faiz A, Dowling NF, et al. Development of a screening tool for identifying women with menorrhagia for hemostatic evaluation. Am J Obstet Gynecol. 2008;198:163.e1-e8.
27. The Rotterdam ESHRE/ASRM-sponsored PCOS consensus workshop group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS). Hum Reprod. 2004;19:41-47.
28. Goodman NF, Cobin RH, Futterweit W, et al; American Association of Clinical Endocrinologists (AACE); American College of Endocrinology (ACE); Androgen Excess and PCOS Society (AES). American Association of Clinical Endocrinologists, American College of Endocrinology, and Androgen Excess and PCOS Society disease state clinical review: guide to the best practices in the evaluation and treatment of polycystic ovary syndrome—part 2. Endocr Pract. 2015;21:1415-26.
29. American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No 194: Polycystic ovary syndrome. Obstet Gynecol. 2018;131:e157-e171.
30. Speiser PW, Dupont B, Rubinstein P, et al. High frequency of nonclassical steroid 21-hydroxylase deficiency. Am J Hum Genet. 1985;37:650-667.
31. Naderpoor N, Shorakae S, de Courten B, et al. Metformin and lifestyle modification in polycystic ovary syndrome: systematic review and meta-analysis. Hum Reprod Update. 2015;21:560-574.
32. Kolman KB, Hadley SK, Jordahl-Iafrato MA. Long-acting reversible contraception: who, what, when, and how. J Fam Pract. 2015;64:479-484.
PRACTICE RECOMMENDATIONS
› Perform endometrial biopsy on all women who have abnormal uterine bleeding and risk factors for endometrial cancer and on all women ≥45 years, regardless of risk. C
› Initiate a workup for a coagulation disorder in women who are close to the onset of menarche and have a history of heavy menstrual bleeding. C
› Promote lifestyle changes and weight loss as primary treatments for polycystic ovary syndrome. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Severe maternal morbidity increasing in California
The according to a study covering almost 8.3 million births in California.
Changes in severe maternal morbidity (SMM) prevalence from 1997 to 2014 were fairly consistent by race/ethnicity, although increases for black (179%), Asian/Pacific Islander (175%), and Hispanic (173%) women were somewhat larger than for whites (163%), Stephanie A. Leonard, PhD, of Stanford (Calif.) University, and her associates reported in Annals of Epidemiology.
Differences between races/ethnicities over the entire study period were seen for SMM with and without transfusion-only cases. Individual-level factors such as cesarean birth, comorbidities, and anemia “contribute to, but do not fully explain, these disparities. Additionally, changes in the characteristics of pregnant women – including increases in comorbidities – have not affected racial/ethnic differences in severe maternal morbidity over time,” the investigators wrote.
The cohort study used data for 8,252,025 live births with birth certificates that were previously linked to delivery discharge records. SMM was measured using the Severe Maternity Morbidity Index. Because “blood transfusion is the only qualifying indicator for approximately half of SMM cases … we also studied a subset of SMM that excluded those cases for which the only indication was a blood transfusion,” they noted.
SOURCE: Leonard SA et al. Ann Epidemiol. 2019 Feb 28. doi: 10.1016/j.annepidem.2019.02.007.
The according to a study covering almost 8.3 million births in California.

Changes in severe maternal morbidity (SMM) prevalence from 1997 to 2014 were fairly consistent by race/ethnicity, although increases for black (179%), Asian/Pacific Islander (175%), and Hispanic (173%) women were somewhat larger than for whites (163%), Stephanie A. Leonard, PhD, of Stanford (Calif.) University, and her associates reported in Annals of Epidemiology.
Differences between races/ethnicities over the entire study period were seen for SMM with and without transfusion-only cases. Individual-level factors such as cesarean birth, comorbidities, and anemia “contribute to, but do not fully explain, these disparities. Additionally, changes in the characteristics of pregnant women – including increases in comorbidities – have not affected racial/ethnic differences in severe maternal morbidity over time,” the investigators wrote.
The cohort study used data for 8,252,025 live births with birth certificates that were previously linked to delivery discharge records. SMM was measured using the Severe Maternity Morbidity Index. Because “blood transfusion is the only qualifying indicator for approximately half of SMM cases … we also studied a subset of SMM that excluded those cases for which the only indication was a blood transfusion,” they noted.
SOURCE: Leonard SA et al. Ann Epidemiol. 2019 Feb 28. doi: 10.1016/j.annepidem.2019.02.007.
The according to a study covering almost 8.3 million births in California.

Changes in severe maternal morbidity (SMM) prevalence from 1997 to 2014 were fairly consistent by race/ethnicity, although increases for black (179%), Asian/Pacific Islander (175%), and Hispanic (173%) women were somewhat larger than for whites (163%), Stephanie A. Leonard, PhD, of Stanford (Calif.) University, and her associates reported in Annals of Epidemiology.
Differences between races/ethnicities over the entire study period were seen for SMM with and without transfusion-only cases. Individual-level factors such as cesarean birth, comorbidities, and anemia “contribute to, but do not fully explain, these disparities. Additionally, changes in the characteristics of pregnant women – including increases in comorbidities – have not affected racial/ethnic differences in severe maternal morbidity over time,” the investigators wrote.
The cohort study used data for 8,252,025 live births with birth certificates that were previously linked to delivery discharge records. SMM was measured using the Severe Maternity Morbidity Index. Because “blood transfusion is the only qualifying indicator for approximately half of SMM cases … we also studied a subset of SMM that excluded those cases for which the only indication was a blood transfusion,” they noted.
SOURCE: Leonard SA et al. Ann Epidemiol. 2019 Feb 28. doi: 10.1016/j.annepidem.2019.02.007.
FROM ANNALS OF EPIDEMIOLOGY
Attitudes of Women Toward the Gynecologic Examination
MS prevalence estimates reach highest point to date
“Our findings suggest that there has been a steady rise in the prevalence of MS over the past 5 decades, that the prevalence of MS remains higher for women than men, and that a north-south geographic gradient still persists,” wrote lead author Mitchell T. Wallin, MD, of Georgetown University, Washington, and his coauthors. The study was published in Neurology.
To determine adult cases of MS, Dr. Wallin and colleagues applied a validated algorithm to private, military, and public AHC datasets. Data from the 2010 U.S. Census were also used to standardize age and sex. In total, 125 million people over 18 years of age were captured in the study, nearly 45% of the U.S. population.
After adjustment, the 2010 prevalence for MS cumulated over 10 years was 309.2 per 100,000 adults (95% confidence interval, 308.1-310.1). This represented a total of 727,344 people with MS. The female to male ratio was 2.8, with a prevalence of 450.1 per 100,000 (95% CI, 448.1-451.6) for women versus a prevalence of 159.7 (95% CI, 158.7-160.6) for men. The age group with the highest estimated prevalence was 55-64 years old, and the prevalence in northern regions of the United States was statistically significantly higher than in southern regions.
The limitations of this study included not including children, the Indian Health Service, the U.S. prison system, or undocumented U.S. residents in the prevalence estimates. However, the authors did note that “these segments of the population are relatively small or, in the case of children, would contribute few cases.” In addition, they were unable to acquire more than 3 years of data for all insurance pools because of high costs.
The study was funded by a grant from the National Multiple Sclerosis Society. The authors reported numerous disclosures, including receiving consulting fees, researching funding, and grant support from various government agencies, foundations, and pharmaceutical companies.
SOURCE: Wallin MT et al. Neurology. 2019 Feb 15. doi: 10.1212/WNL.0000000000007035.

“Our findings suggest that there has been a steady rise in the prevalence of MS over the past 5 decades, that the prevalence of MS remains higher for women than men, and that a north-south geographic gradient still persists,” wrote lead author Mitchell T. Wallin, MD, of Georgetown University, Washington, and his coauthors. The study was published in Neurology.
To determine adult cases of MS, Dr. Wallin and colleagues applied a validated algorithm to private, military, and public AHC datasets. Data from the 2010 U.S. Census were also used to standardize age and sex. In total, 125 million people over 18 years of age were captured in the study, nearly 45% of the U.S. population.
After adjustment, the 2010 prevalence for MS cumulated over 10 years was 309.2 per 100,000 adults (95% confidence interval, 308.1-310.1). This represented a total of 727,344 people with MS. The female to male ratio was 2.8, with a prevalence of 450.1 per 100,000 (95% CI, 448.1-451.6) for women versus a prevalence of 159.7 (95% CI, 158.7-160.6) for men. The age group with the highest estimated prevalence was 55-64 years old, and the prevalence in northern regions of the United States was statistically significantly higher than in southern regions.
The limitations of this study included not including children, the Indian Health Service, the U.S. prison system, or undocumented U.S. residents in the prevalence estimates. However, the authors did note that “these segments of the population are relatively small or, in the case of children, would contribute few cases.” In addition, they were unable to acquire more than 3 years of data for all insurance pools because of high costs.
The study was funded by a grant from the National Multiple Sclerosis Society. The authors reported numerous disclosures, including receiving consulting fees, researching funding, and grant support from various government agencies, foundations, and pharmaceutical companies.
SOURCE: Wallin MT et al. Neurology. 2019 Feb 15. doi: 10.1212/WNL.0000000000007035.

“Our findings suggest that there has been a steady rise in the prevalence of MS over the past 5 decades, that the prevalence of MS remains higher for women than men, and that a north-south geographic gradient still persists,” wrote lead author Mitchell T. Wallin, MD, of Georgetown University, Washington, and his coauthors. The study was published in Neurology.
To determine adult cases of MS, Dr. Wallin and colleagues applied a validated algorithm to private, military, and public AHC datasets. Data from the 2010 U.S. Census were also used to standardize age and sex. In total, 125 million people over 18 years of age were captured in the study, nearly 45% of the U.S. population.
After adjustment, the 2010 prevalence for MS cumulated over 10 years was 309.2 per 100,000 adults (95% confidence interval, 308.1-310.1). This represented a total of 727,344 people with MS. The female to male ratio was 2.8, with a prevalence of 450.1 per 100,000 (95% CI, 448.1-451.6) for women versus a prevalence of 159.7 (95% CI, 158.7-160.6) for men. The age group with the highest estimated prevalence was 55-64 years old, and the prevalence in northern regions of the United States was statistically significantly higher than in southern regions.
The limitations of this study included not including children, the Indian Health Service, the U.S. prison system, or undocumented U.S. residents in the prevalence estimates. However, the authors did note that “these segments of the population are relatively small or, in the case of children, would contribute few cases.” In addition, they were unable to acquire more than 3 years of data for all insurance pools because of high costs.
The study was funded by a grant from the National Multiple Sclerosis Society. The authors reported numerous disclosures, including receiving consulting fees, researching funding, and grant support from various government agencies, foundations, and pharmaceutical companies.
SOURCE: Wallin MT et al. Neurology. 2019 Feb 15. doi: 10.1212/WNL.0000000000007035.
FROM NEUROLOGY
No increased pregnancy loss risk for women conceiving soon after stillbirth
according to authors of a large, international observational study.
There was no significantly increased risk of stillbirth, preterm birth, or small-for-gestational-age birth in the next pregnancy for women who conceived in that 12-month time period, according to results of the study, which was based on birth records for nearly 14,500 women in Finland, Norway, and Australia.
“We hope that our findings can provide reassurance to women who wish to become pregnant or unexpectedly become pregnant shortly after a stillbirth,” study author Annette K. Regan, PhD, of Curtin University, Perth, Australia, said in a statement on the study, which appears in The Lancet.
Judette Marie Louis, MD, MPH, said that while data are conflicting on optimal interpregnancy interval following stillbirth, large population-based studies such as this one may provide an indication of the relative safety of an interval of 12 months or less. (She was not involved in this study.)
“This paper is good news for a lot of women,” Dr. Louis, associate professor of obstetrics and gynecology at the University of South Florida, Tampa, said in an interview. “After a stillbirth, it’s such a traumatic experience that some do want to move on, and these findings suggest that, yes, you don’t have to wait that long to have a successful pregnancy.”
These results are for women living in relatively high-income countries, so the findings might not apply to every population, she added. Dr. Louis was the first author of a recent interpregnancy care consensus statement by the American College of Obstetricians and Gynecologists and the Society of Maternal-Fetal Medicine, and was asked to comment on this study.
The World Health Organization recommends interpregnancy intervals of 2 years or more after live births and 6 months or more after miscarriage, but currently has no specific recommendations on the optimal interpregnancy interval after a stillbirth, according to Dr. Regan and her colleagues.
“Because length of gestation might affect nutrient concentrations and health status in women, it is plausible that the optimal interval after stillbirth is somewhere between the optimal interval after miscarriage and live birth,” they said in their report.
Researchers for two previous studies also have reported on the link between the interpregnancy interval after stillbirth and birth outcomes in the next pregnancy, but neither was specifically designed to evaluate that outcome, they added.
Dr. Regan and her coauthors used birth record data spanning several decades from three high-income countries to identify 14,452 women who had stillbirths. Of those, 63% conceived within the next 12 months, and for 37%, it was as early as 6 months.
Overall, 2% of the subsequent pregnancies ended in stillbirth, while 9% were small-for-gestational-age and 18% were preterm, according to the report.
In analyses adjusted for variables such as age, smoking, and education level, there was no association between short interpregnancy intervals and subsequent stillbirths, compared with longer intervals (24-59 months), with odds ratios of 1.09 for an interval shorter than 6 months and 0.90 for 6-11 months.
Likewise, there was no link between shorter intervals and subsequent small-for-gestational-age birth, with odds ratios of 0.66 for less than 6 months and 0.64 for 6-11 months, and no link between interval and subsequent preterm births, with odds ratios of 0.91 for both short-interval groups.
That data could be useful to health care providers who do postpartum counseling after stillbirths, and could potentially inform future recommendations on pregnancy spacing, Dr. Regan and her coauthors said.
“These results apply to a large proportion of women conceiving after a stillbirth,” they noted.
This study included countries with access to universal health care, with populations that are mostly white, so the results may not apply to low- or middle-income countries without universal health care or with significant ethnic minority populations, they added.
Dr. Regan and her colleagues declared no competing interests related to the study, which was funded the National Health and Medical Research Council of Australia, among other sources.
SOURCE: Regan AK et al. Lancet. 2019 Feb 28. doi: 10.1016/S0140-6736(18)32266-9.
The interval between pregnancy loss and the next conception may be less important than previously assumed, based on the results of this and other recent studies, according to Mark A Klebanoff, MD, MPH.
“Rather than adhering to hard and fast rules, clinical recommendations should consider a woman’s current health status, her current age in conjunction with her desires regarding child spacing and ultimate family size, and particularly following a loss, her emotional readiness to become pregnant again,” he said in a commentary accompanying the article by Regan et al.
However, these results are specific to high-income countries, and might not extrapolate to women in “less favorable situations” where poor access to quality medical and obstetric care, malnutrition, and untreated chronic conditions are more common, Dr. Klebanoff added.
Another limitation of the study, acknowledged by Regan and coauthors, is the relatively small number of stillbirths in subsequent pregnancies (228) despite this being the largest study of its kind to date.
“The fairly small number of women included in this report dictates that replication, probably using other large, linked, population-level databases, is required,” Dr. Klebanoff said in his commentary.
Dr. Klebanoff is with the Center for Perinatal Research, The Research Institute at Nationwide Children’s Hospital; and the departments of pediatrics and obstetrics and gynecology at the College of Medicine, and division of epidemiology at the College of Public Health, at The Ohio State University, all in Columbus. This is a summarization of his commentary (Lancet. 2019 Feb 28. doi: 10.1016/S0140-6736(18)32430-9 ). Dr. Klebanoff said he had a pending grant application to the National Institutes of Health to study the association between interpregnancy interval and birth outcomes, and had no other competing interests.
The interval between pregnancy loss and the next conception may be less important than previously assumed, based on the results of this and other recent studies, according to Mark A Klebanoff, MD, MPH.
“Rather than adhering to hard and fast rules, clinical recommendations should consider a woman’s current health status, her current age in conjunction with her desires regarding child spacing and ultimate family size, and particularly following a loss, her emotional readiness to become pregnant again,” he said in a commentary accompanying the article by Regan et al.
However, these results are specific to high-income countries, and might not extrapolate to women in “less favorable situations” where poor access to quality medical and obstetric care, malnutrition, and untreated chronic conditions are more common, Dr. Klebanoff added.
Another limitation of the study, acknowledged by Regan and coauthors, is the relatively small number of stillbirths in subsequent pregnancies (228) despite this being the largest study of its kind to date.
“The fairly small number of women included in this report dictates that replication, probably using other large, linked, population-level databases, is required,” Dr. Klebanoff said in his commentary.
Dr. Klebanoff is with the Center for Perinatal Research, The Research Institute at Nationwide Children’s Hospital; and the departments of pediatrics and obstetrics and gynecology at the College of Medicine, and division of epidemiology at the College of Public Health, at The Ohio State University, all in Columbus. This is a summarization of his commentary (Lancet. 2019 Feb 28. doi: 10.1016/S0140-6736(18)32430-9 ). Dr. Klebanoff said he had a pending grant application to the National Institutes of Health to study the association between interpregnancy interval and birth outcomes, and had no other competing interests.
The interval between pregnancy loss and the next conception may be less important than previously assumed, based on the results of this and other recent studies, according to Mark A Klebanoff, MD, MPH.
“Rather than adhering to hard and fast rules, clinical recommendations should consider a woman’s current health status, her current age in conjunction with her desires regarding child spacing and ultimate family size, and particularly following a loss, her emotional readiness to become pregnant again,” he said in a commentary accompanying the article by Regan et al.
However, these results are specific to high-income countries, and might not extrapolate to women in “less favorable situations” where poor access to quality medical and obstetric care, malnutrition, and untreated chronic conditions are more common, Dr. Klebanoff added.
Another limitation of the study, acknowledged by Regan and coauthors, is the relatively small number of stillbirths in subsequent pregnancies (228) despite this being the largest study of its kind to date.
“The fairly small number of women included in this report dictates that replication, probably using other large, linked, population-level databases, is required,” Dr. Klebanoff said in his commentary.
Dr. Klebanoff is with the Center for Perinatal Research, The Research Institute at Nationwide Children’s Hospital; and the departments of pediatrics and obstetrics and gynecology at the College of Medicine, and division of epidemiology at the College of Public Health, at The Ohio State University, all in Columbus. This is a summarization of his commentary (Lancet. 2019 Feb 28. doi: 10.1016/S0140-6736(18)32430-9 ). Dr. Klebanoff said he had a pending grant application to the National Institutes of Health to study the association between interpregnancy interval and birth outcomes, and had no other competing interests.
according to authors of a large, international observational study.
There was no significantly increased risk of stillbirth, preterm birth, or small-for-gestational-age birth in the next pregnancy for women who conceived in that 12-month time period, according to results of the study, which was based on birth records for nearly 14,500 women in Finland, Norway, and Australia.
“We hope that our findings can provide reassurance to women who wish to become pregnant or unexpectedly become pregnant shortly after a stillbirth,” study author Annette K. Regan, PhD, of Curtin University, Perth, Australia, said in a statement on the study, which appears in The Lancet.
Judette Marie Louis, MD, MPH, said that while data are conflicting on optimal interpregnancy interval following stillbirth, large population-based studies such as this one may provide an indication of the relative safety of an interval of 12 months or less. (She was not involved in this study.)
“This paper is good news for a lot of women,” Dr. Louis, associate professor of obstetrics and gynecology at the University of South Florida, Tampa, said in an interview. “After a stillbirth, it’s such a traumatic experience that some do want to move on, and these findings suggest that, yes, you don’t have to wait that long to have a successful pregnancy.”
These results are for women living in relatively high-income countries, so the findings might not apply to every population, she added. Dr. Louis was the first author of a recent interpregnancy care consensus statement by the American College of Obstetricians and Gynecologists and the Society of Maternal-Fetal Medicine, and was asked to comment on this study.
The World Health Organization recommends interpregnancy intervals of 2 years or more after live births and 6 months or more after miscarriage, but currently has no specific recommendations on the optimal interpregnancy interval after a stillbirth, according to Dr. Regan and her colleagues.
“Because length of gestation might affect nutrient concentrations and health status in women, it is plausible that the optimal interval after stillbirth is somewhere between the optimal interval after miscarriage and live birth,” they said in their report.
Researchers for two previous studies also have reported on the link between the interpregnancy interval after stillbirth and birth outcomes in the next pregnancy, but neither was specifically designed to evaluate that outcome, they added.
Dr. Regan and her coauthors used birth record data spanning several decades from three high-income countries to identify 14,452 women who had stillbirths. Of those, 63% conceived within the next 12 months, and for 37%, it was as early as 6 months.
Overall, 2% of the subsequent pregnancies ended in stillbirth, while 9% were small-for-gestational-age and 18% were preterm, according to the report.
In analyses adjusted for variables such as age, smoking, and education level, there was no association between short interpregnancy intervals and subsequent stillbirths, compared with longer intervals (24-59 months), with odds ratios of 1.09 for an interval shorter than 6 months and 0.90 for 6-11 months.
Likewise, there was no link between shorter intervals and subsequent small-for-gestational-age birth, with odds ratios of 0.66 for less than 6 months and 0.64 for 6-11 months, and no link between interval and subsequent preterm births, with odds ratios of 0.91 for both short-interval groups.
That data could be useful to health care providers who do postpartum counseling after stillbirths, and could potentially inform future recommendations on pregnancy spacing, Dr. Regan and her coauthors said.
“These results apply to a large proportion of women conceiving after a stillbirth,” they noted.
This study included countries with access to universal health care, with populations that are mostly white, so the results may not apply to low- or middle-income countries without universal health care or with significant ethnic minority populations, they added.
Dr. Regan and her colleagues declared no competing interests related to the study, which was funded the National Health and Medical Research Council of Australia, among other sources.
SOURCE: Regan AK et al. Lancet. 2019 Feb 28. doi: 10.1016/S0140-6736(18)32266-9.
according to authors of a large, international observational study.
There was no significantly increased risk of stillbirth, preterm birth, or small-for-gestational-age birth in the next pregnancy for women who conceived in that 12-month time period, according to results of the study, which was based on birth records for nearly 14,500 women in Finland, Norway, and Australia.
“We hope that our findings can provide reassurance to women who wish to become pregnant or unexpectedly become pregnant shortly after a stillbirth,” study author Annette K. Regan, PhD, of Curtin University, Perth, Australia, said in a statement on the study, which appears in The Lancet.
Judette Marie Louis, MD, MPH, said that while data are conflicting on optimal interpregnancy interval following stillbirth, large population-based studies such as this one may provide an indication of the relative safety of an interval of 12 months or less. (She was not involved in this study.)
“This paper is good news for a lot of women,” Dr. Louis, associate professor of obstetrics and gynecology at the University of South Florida, Tampa, said in an interview. “After a stillbirth, it’s such a traumatic experience that some do want to move on, and these findings suggest that, yes, you don’t have to wait that long to have a successful pregnancy.”
These results are for women living in relatively high-income countries, so the findings might not apply to every population, she added. Dr. Louis was the first author of a recent interpregnancy care consensus statement by the American College of Obstetricians and Gynecologists and the Society of Maternal-Fetal Medicine, and was asked to comment on this study.
The World Health Organization recommends interpregnancy intervals of 2 years or more after live births and 6 months or more after miscarriage, but currently has no specific recommendations on the optimal interpregnancy interval after a stillbirth, according to Dr. Regan and her colleagues.
“Because length of gestation might affect nutrient concentrations and health status in women, it is plausible that the optimal interval after stillbirth is somewhere between the optimal interval after miscarriage and live birth,” they said in their report.
Researchers for two previous studies also have reported on the link between the interpregnancy interval after stillbirth and birth outcomes in the next pregnancy, but neither was specifically designed to evaluate that outcome, they added.
Dr. Regan and her coauthors used birth record data spanning several decades from three high-income countries to identify 14,452 women who had stillbirths. Of those, 63% conceived within the next 12 months, and for 37%, it was as early as 6 months.
Overall, 2% of the subsequent pregnancies ended in stillbirth, while 9% were small-for-gestational-age and 18% were preterm, according to the report.
In analyses adjusted for variables such as age, smoking, and education level, there was no association between short interpregnancy intervals and subsequent stillbirths, compared with longer intervals (24-59 months), with odds ratios of 1.09 for an interval shorter than 6 months and 0.90 for 6-11 months.
Likewise, there was no link between shorter intervals and subsequent small-for-gestational-age birth, with odds ratios of 0.66 for less than 6 months and 0.64 for 6-11 months, and no link between interval and subsequent preterm births, with odds ratios of 0.91 for both short-interval groups.
That data could be useful to health care providers who do postpartum counseling after stillbirths, and could potentially inform future recommendations on pregnancy spacing, Dr. Regan and her coauthors said.
“These results apply to a large proportion of women conceiving after a stillbirth,” they noted.
This study included countries with access to universal health care, with populations that are mostly white, so the results may not apply to low- or middle-income countries without universal health care or with significant ethnic minority populations, they added.
Dr. Regan and her colleagues declared no competing interests related to the study, which was funded the National Health and Medical Research Council of Australia, among other sources.
SOURCE: Regan AK et al. Lancet. 2019 Feb 28. doi: 10.1016/S0140-6736(18)32266-9.
FROM THE LANCET
Click for Credit: Endometriosis surgery benefits; diabetes & aging; more
Here are 5 articles from the March issue of Clinician Reviews (individual articles are valid for one year from date of publication—expiration dates below):
1. Endometriosis surgery: Women can expect years-long benefits
To take the posttest, go to: https://bit.ly/2Ez8mdu
Expires January 3, 2019
2. Cerebral small vessel disease progression linked to MCI in hypertensive patients
To take the posttest, go to: https://bit.ly/2ExDV7o
Expires January 4, 2019
3. Adult atopic dermatitis is fraught with dermatologic comorbidities
To take the posttest, go to: https://bit.ly/2Vl7E9a
Expires January 11, 2019
4. Antidepressants tied to greater hip fracture incidence in older adults
To take the posttest, go to: https://bit.ly/2GRfMeH
Expires January 4, 2019
5. Researchers exploring ways to mitigate aging’s impact on diabetes
To take the posttest, go to: https://bit.ly/2tFxF7v
Expires January 8, 2019
Here are 5 articles from the March issue of Clinician Reviews (individual articles are valid for one year from date of publication—expiration dates below):
1. Endometriosis surgery: Women can expect years-long benefits
To take the posttest, go to: https://bit.ly/2Ez8mdu
Expires January 3, 2019
2. Cerebral small vessel disease progression linked to MCI in hypertensive patients
To take the posttest, go to: https://bit.ly/2ExDV7o
Expires January 4, 2019
3. Adult atopic dermatitis is fraught with dermatologic comorbidities
To take the posttest, go to: https://bit.ly/2Vl7E9a
Expires January 11, 2019
4. Antidepressants tied to greater hip fracture incidence in older adults
To take the posttest, go to: https://bit.ly/2GRfMeH
Expires January 4, 2019
5. Researchers exploring ways to mitigate aging’s impact on diabetes
To take the posttest, go to: https://bit.ly/2tFxF7v
Expires January 8, 2019
Here are 5 articles from the March issue of Clinician Reviews (individual articles are valid for one year from date of publication—expiration dates below):
1. Endometriosis surgery: Women can expect years-long benefits
To take the posttest, go to: https://bit.ly/2Ez8mdu
Expires January 3, 2019
2. Cerebral small vessel disease progression linked to MCI in hypertensive patients
To take the posttest, go to: https://bit.ly/2ExDV7o
Expires January 4, 2019
3. Adult atopic dermatitis is fraught with dermatologic comorbidities
To take the posttest, go to: https://bit.ly/2Vl7E9a
Expires January 11, 2019
4. Antidepressants tied to greater hip fracture incidence in older adults
To take the posttest, go to: https://bit.ly/2GRfMeH
Expires January 4, 2019
5. Researchers exploring ways to mitigate aging’s impact on diabetes
To take the posttest, go to: https://bit.ly/2tFxF7v
Expires January 8, 2019
Many common dermatologic drugs can be safely used during pregnancy
WASHINGTON – With proper counseling and oversight, many drugs used for psoriasis, pemphigus, and atopic dermatitis are safe to use during pregnancy, Jenny Murase, MD, said at the annual meeting of the American Academy of Dermatology.
But it’s important to have an early talk about potential pregnancies, because most dermatologists don’t think about it unless a patient is taking a known teratogen, like isotretinoin, said Dr. Murase of the University of California, San Francisco. “It’s only about 10% of the time that the dermatologist brings it up. In patients with these chronic skin diseases, we need to address family planning proactively. Most women don’t discover they’re pregnant until they’re 2-5 weeks along, and by that time the development of major organs has already started.”
As part of her expertise in this topic, Dr. Murase published two comprehensive reports on the safety of dermatologic drugs in pregnancy and lactation. They were grouped according to the newest federal guidance, the Food and Drug Administration Pregnancy and Lactation Label Ruling. Issued in 2014, it requires the inclusion of any contact information for drug registries and covers reproductive risks or both males and females. Slowly being phased in as new drugs are approved, the ruling is replacing the old category A, B, and C.
The articles were published in the Journal of the American Academy of Dermatology and in the international Journal of Women’s Dermatology, an open-access journal Dr. Murase founded.
Corticosteroids
These dermatology workhorses are largely safe in pregnancy, Dr. Murase said. Some very early reports suggested that systemic cortisone might be associated with oral clefts, but that has never been borne out in prospective data. Prednisone may be the safest as it has the most limited placental transport; betamethasone and dexamethasone cross the placenta easily.
In a Cochrane review, only one study showed an increased risk of orofacial clefts. A 2013 study of about 10,000 women suggested an increased risk of low birth weight associated with a total dose of more than 300 grams during the pregnancy.
Antihistamines
First-generation antihistamines, including hydroxyzine, have been used as antiemetics in pregnant women. Hydroxyzine is generally considered safe, but should be discussed carefully, as it carries a slightly increased risk of congenital malformations (5.8% vs. 2.3% background risk).
Newborns exposed to large doses of hydroxyzine (150 mg or more daily) have also exhibited withdrawal symptoms at birth, including irritability, poor feeding, and tonic-clinic seizures.
“Antihistamines can exert oxytocin-like effects, especially in overdose or when given intravenously, so please avoid using them in the last month of pregnancy. There have also been a few reports of retrolental fibroplasia in preterm infants who were exposed to antihistamines within 2 weeks of delivery,” Dr. Murase said.
Immunosuppressants
Mycophenolate is not compatible with pregnancy. In 2007, the FDA changed the labeling of mycophenolate from category C to D, because of reports of congenital malformations arising from the U.S. National Transplantation Pregnancy Registry and other sources.
“You need to treat these patients like you do someone who is going on isotretinoin,” Dr. Murase said. “Any woman prescribed it should be on mandatory contraception at least 4 weeks before beginning the medication and for 6 weeks after completing treatment.”
Thus far there are no reported pregnancy-related safety issues with dupilumab, although data are scarce.
Pregnancy itself exerts a positive effect on psoriasis in many women, but not all. “About half the time a women will improve,” Dr. Murase said. “A quarter of the time, there’s no change and a quarter of the time, she’ll get worse. But the ones who do improve, often improve dramatically with about 80% body surface area clearance.”
She considers light therapy to be the safest treatment during pregnancy, with one caveat: Ultraviolet light can degrade some vitamins, including folic acid. “Every one of my patients of childbearing age I have on folic acid or a prenatal vitamin just in case. You have to be proactive here.”
Cyclosporine appears to be “quite safe,” she said. The possibility of intrauterine growth restriction seen in some studies is tough to tease out, because it was reported mainly in transplant populations among women with other medical comorbidities. Children from these pregnancies have been followed through toddlerhood and showed no neurodevelopmental or kidney issues.
Apremilast is a category C drug. Some animal data suggested increased spontaneous abortions and fetal demise with doses given at two to four times the human dose.
Biologics
Antibodies are an interesting lot, Dr. Murase noted. Maternal antibodies are transported to the fetus across the villi by Fc receptor; most of this transfer happens during the third trimester. The large, hydrophilic monoclonal antibodies infliximab, adalimumab, and ustekinumab travel this way as well. Cord blood can contain 50% higher serum levels than in maternal blood. Etanercept, however, is a fusion protein that diffuses across the placenta. Cord blood levels generally exceed maternal levels by less than 7%.
There is one published report of a fetal death associated with maternal infliximab for Crohn’s disease. The infant was healthy until it received a Bacillus Calmette–Guerin vaccine. It then developed widespread eczematous dermatitis, head lag, and poor weight gain and died at 4.5 months.
“This is another important counseling point,” Dr. Murase said. “Babies who have been exposed to infliximab in utero can’t have that vaccination in the first 9 months of life.”
Perhaps the safest bet for a pregnant women who needs a biologic is PEGylated certolizumab. “Certolizumab is the only PEGylated anti-TNF [tumor necrosis factor] without an Fc region; study of patients greater than 30 weeks pregnant certolizumab levels were below 0.032 mcg/mL in 13 of 14 infant samples at birth.”
Pemphigus
Pemphigus (impetigo herpetiformous) is a serious dermatologic disorder that can manifest in the third trimester, and affect the fetus as well as the mother. “You have to take even mild cases very seriously, because there’s no distinct correlation between the extent of neonatal involvement and the extent of maternal disease,” Dr. Murase said
Oral pemphigus in the mother is especially worrisome, she added. “Fetal skin shares the same desmoglein-3 profile as adult oral mucosa, and neonatal pemphigus is more likely if mother has oral disease. There’s an increased risk of fetal demise as well.”
Treatment would generally start with topical steroids, progressing to systemic low-dose corticosteroids. If more than 20 mg of prednisone a day is required, consider intravenous immunoglobulin (IVIG), azathioprine, dapsone, or rituximab.
“IVIG is very safe for pregnant women, and in fact reproductive endocrinologists use this to increase the chance of pregnancy for infertility cases,” Dr. Murase said.
She reported relationships with Regeneron, UCB, Dermira, and Genzyme/Sanofi.
WASHINGTON – With proper counseling and oversight, many drugs used for psoriasis, pemphigus, and atopic dermatitis are safe to use during pregnancy, Jenny Murase, MD, said at the annual meeting of the American Academy of Dermatology.
But it’s important to have an early talk about potential pregnancies, because most dermatologists don’t think about it unless a patient is taking a known teratogen, like isotretinoin, said Dr. Murase of the University of California, San Francisco. “It’s only about 10% of the time that the dermatologist brings it up. In patients with these chronic skin diseases, we need to address family planning proactively. Most women don’t discover they’re pregnant until they’re 2-5 weeks along, and by that time the development of major organs has already started.”
As part of her expertise in this topic, Dr. Murase published two comprehensive reports on the safety of dermatologic drugs in pregnancy and lactation. They were grouped according to the newest federal guidance, the Food and Drug Administration Pregnancy and Lactation Label Ruling. Issued in 2014, it requires the inclusion of any contact information for drug registries and covers reproductive risks or both males and females. Slowly being phased in as new drugs are approved, the ruling is replacing the old category A, B, and C.
The articles were published in the Journal of the American Academy of Dermatology and in the international Journal of Women’s Dermatology, an open-access journal Dr. Murase founded.
Corticosteroids
These dermatology workhorses are largely safe in pregnancy, Dr. Murase said. Some very early reports suggested that systemic cortisone might be associated with oral clefts, but that has never been borne out in prospective data. Prednisone may be the safest as it has the most limited placental transport; betamethasone and dexamethasone cross the placenta easily.
In a Cochrane review, only one study showed an increased risk of orofacial clefts. A 2013 study of about 10,000 women suggested an increased risk of low birth weight associated with a total dose of more than 300 grams during the pregnancy.
Antihistamines
First-generation antihistamines, including hydroxyzine, have been used as antiemetics in pregnant women. Hydroxyzine is generally considered safe, but should be discussed carefully, as it carries a slightly increased risk of congenital malformations (5.8% vs. 2.3% background risk).
Newborns exposed to large doses of hydroxyzine (150 mg or more daily) have also exhibited withdrawal symptoms at birth, including irritability, poor feeding, and tonic-clinic seizures.
“Antihistamines can exert oxytocin-like effects, especially in overdose or when given intravenously, so please avoid using them in the last month of pregnancy. There have also been a few reports of retrolental fibroplasia in preterm infants who were exposed to antihistamines within 2 weeks of delivery,” Dr. Murase said.
Immunosuppressants
Mycophenolate is not compatible with pregnancy. In 2007, the FDA changed the labeling of mycophenolate from category C to D, because of reports of congenital malformations arising from the U.S. National Transplantation Pregnancy Registry and other sources.
“You need to treat these patients like you do someone who is going on isotretinoin,” Dr. Murase said. “Any woman prescribed it should be on mandatory contraception at least 4 weeks before beginning the medication and for 6 weeks after completing treatment.”
Thus far there are no reported pregnancy-related safety issues with dupilumab, although data are scarce.
Pregnancy itself exerts a positive effect on psoriasis in many women, but not all. “About half the time a women will improve,” Dr. Murase said. “A quarter of the time, there’s no change and a quarter of the time, she’ll get worse. But the ones who do improve, often improve dramatically with about 80% body surface area clearance.”
She considers light therapy to be the safest treatment during pregnancy, with one caveat: Ultraviolet light can degrade some vitamins, including folic acid. “Every one of my patients of childbearing age I have on folic acid or a prenatal vitamin just in case. You have to be proactive here.”
Cyclosporine appears to be “quite safe,” she said. The possibility of intrauterine growth restriction seen in some studies is tough to tease out, because it was reported mainly in transplant populations among women with other medical comorbidities. Children from these pregnancies have been followed through toddlerhood and showed no neurodevelopmental or kidney issues.
Apremilast is a category C drug. Some animal data suggested increased spontaneous abortions and fetal demise with doses given at two to four times the human dose.
Biologics
Antibodies are an interesting lot, Dr. Murase noted. Maternal antibodies are transported to the fetus across the villi by Fc receptor; most of this transfer happens during the third trimester. The large, hydrophilic monoclonal antibodies infliximab, adalimumab, and ustekinumab travel this way as well. Cord blood can contain 50% higher serum levels than in maternal blood. Etanercept, however, is a fusion protein that diffuses across the placenta. Cord blood levels generally exceed maternal levels by less than 7%.
There is one published report of a fetal death associated with maternal infliximab for Crohn’s disease. The infant was healthy until it received a Bacillus Calmette–Guerin vaccine. It then developed widespread eczematous dermatitis, head lag, and poor weight gain and died at 4.5 months.
“This is another important counseling point,” Dr. Murase said. “Babies who have been exposed to infliximab in utero can’t have that vaccination in the first 9 months of life.”
Perhaps the safest bet for a pregnant women who needs a biologic is PEGylated certolizumab. “Certolizumab is the only PEGylated anti-TNF [tumor necrosis factor] without an Fc region; study of patients greater than 30 weeks pregnant certolizumab levels were below 0.032 mcg/mL in 13 of 14 infant samples at birth.”
Pemphigus
Pemphigus (impetigo herpetiformous) is a serious dermatologic disorder that can manifest in the third trimester, and affect the fetus as well as the mother. “You have to take even mild cases very seriously, because there’s no distinct correlation between the extent of neonatal involvement and the extent of maternal disease,” Dr. Murase said
Oral pemphigus in the mother is especially worrisome, she added. “Fetal skin shares the same desmoglein-3 profile as adult oral mucosa, and neonatal pemphigus is more likely if mother has oral disease. There’s an increased risk of fetal demise as well.”
Treatment would generally start with topical steroids, progressing to systemic low-dose corticosteroids. If more than 20 mg of prednisone a day is required, consider intravenous immunoglobulin (IVIG), azathioprine, dapsone, or rituximab.
“IVIG is very safe for pregnant women, and in fact reproductive endocrinologists use this to increase the chance of pregnancy for infertility cases,” Dr. Murase said.
She reported relationships with Regeneron, UCB, Dermira, and Genzyme/Sanofi.
WASHINGTON – With proper counseling and oversight, many drugs used for psoriasis, pemphigus, and atopic dermatitis are safe to use during pregnancy, Jenny Murase, MD, said at the annual meeting of the American Academy of Dermatology.
But it’s important to have an early talk about potential pregnancies, because most dermatologists don’t think about it unless a patient is taking a known teratogen, like isotretinoin, said Dr. Murase of the University of California, San Francisco. “It’s only about 10% of the time that the dermatologist brings it up. In patients with these chronic skin diseases, we need to address family planning proactively. Most women don’t discover they’re pregnant until they’re 2-5 weeks along, and by that time the development of major organs has already started.”
As part of her expertise in this topic, Dr. Murase published two comprehensive reports on the safety of dermatologic drugs in pregnancy and lactation. They were grouped according to the newest federal guidance, the Food and Drug Administration Pregnancy and Lactation Label Ruling. Issued in 2014, it requires the inclusion of any contact information for drug registries and covers reproductive risks or both males and females. Slowly being phased in as new drugs are approved, the ruling is replacing the old category A, B, and C.
The articles were published in the Journal of the American Academy of Dermatology and in the international Journal of Women’s Dermatology, an open-access journal Dr. Murase founded.
Corticosteroids
These dermatology workhorses are largely safe in pregnancy, Dr. Murase said. Some very early reports suggested that systemic cortisone might be associated with oral clefts, but that has never been borne out in prospective data. Prednisone may be the safest as it has the most limited placental transport; betamethasone and dexamethasone cross the placenta easily.
In a Cochrane review, only one study showed an increased risk of orofacial clefts. A 2013 study of about 10,000 women suggested an increased risk of low birth weight associated with a total dose of more than 300 grams during the pregnancy.
Antihistamines
First-generation antihistamines, including hydroxyzine, have been used as antiemetics in pregnant women. Hydroxyzine is generally considered safe, but should be discussed carefully, as it carries a slightly increased risk of congenital malformations (5.8% vs. 2.3% background risk).
Newborns exposed to large doses of hydroxyzine (150 mg or more daily) have also exhibited withdrawal symptoms at birth, including irritability, poor feeding, and tonic-clinic seizures.
“Antihistamines can exert oxytocin-like effects, especially in overdose or when given intravenously, so please avoid using them in the last month of pregnancy. There have also been a few reports of retrolental fibroplasia in preterm infants who were exposed to antihistamines within 2 weeks of delivery,” Dr. Murase said.
Immunosuppressants
Mycophenolate is not compatible with pregnancy. In 2007, the FDA changed the labeling of mycophenolate from category C to D, because of reports of congenital malformations arising from the U.S. National Transplantation Pregnancy Registry and other sources.
“You need to treat these patients like you do someone who is going on isotretinoin,” Dr. Murase said. “Any woman prescribed it should be on mandatory contraception at least 4 weeks before beginning the medication and for 6 weeks after completing treatment.”
Thus far there are no reported pregnancy-related safety issues with dupilumab, although data are scarce.
Pregnancy itself exerts a positive effect on psoriasis in many women, but not all. “About half the time a women will improve,” Dr. Murase said. “A quarter of the time, there’s no change and a quarter of the time, she’ll get worse. But the ones who do improve, often improve dramatically with about 80% body surface area clearance.”
She considers light therapy to be the safest treatment during pregnancy, with one caveat: Ultraviolet light can degrade some vitamins, including folic acid. “Every one of my patients of childbearing age I have on folic acid or a prenatal vitamin just in case. You have to be proactive here.”
Cyclosporine appears to be “quite safe,” she said. The possibility of intrauterine growth restriction seen in some studies is tough to tease out, because it was reported mainly in transplant populations among women with other medical comorbidities. Children from these pregnancies have been followed through toddlerhood and showed no neurodevelopmental or kidney issues.
Apremilast is a category C drug. Some animal data suggested increased spontaneous abortions and fetal demise with doses given at two to four times the human dose.
Biologics
Antibodies are an interesting lot, Dr. Murase noted. Maternal antibodies are transported to the fetus across the villi by Fc receptor; most of this transfer happens during the third trimester. The large, hydrophilic monoclonal antibodies infliximab, adalimumab, and ustekinumab travel this way as well. Cord blood can contain 50% higher serum levels than in maternal blood. Etanercept, however, is a fusion protein that diffuses across the placenta. Cord blood levels generally exceed maternal levels by less than 7%.
There is one published report of a fetal death associated with maternal infliximab for Crohn’s disease. The infant was healthy until it received a Bacillus Calmette–Guerin vaccine. It then developed widespread eczematous dermatitis, head lag, and poor weight gain and died at 4.5 months.
“This is another important counseling point,” Dr. Murase said. “Babies who have been exposed to infliximab in utero can’t have that vaccination in the first 9 months of life.”
Perhaps the safest bet for a pregnant women who needs a biologic is PEGylated certolizumab. “Certolizumab is the only PEGylated anti-TNF [tumor necrosis factor] without an Fc region; study of patients greater than 30 weeks pregnant certolizumab levels were below 0.032 mcg/mL in 13 of 14 infant samples at birth.”
Pemphigus
Pemphigus (impetigo herpetiformous) is a serious dermatologic disorder that can manifest in the third trimester, and affect the fetus as well as the mother. “You have to take even mild cases very seriously, because there’s no distinct correlation between the extent of neonatal involvement and the extent of maternal disease,” Dr. Murase said
Oral pemphigus in the mother is especially worrisome, she added. “Fetal skin shares the same desmoglein-3 profile as adult oral mucosa, and neonatal pemphigus is more likely if mother has oral disease. There’s an increased risk of fetal demise as well.”
Treatment would generally start with topical steroids, progressing to systemic low-dose corticosteroids. If more than 20 mg of prednisone a day is required, consider intravenous immunoglobulin (IVIG), azathioprine, dapsone, or rituximab.
“IVIG is very safe for pregnant women, and in fact reproductive endocrinologists use this to increase the chance of pregnancy for infertility cases,” Dr. Murase said.
She reported relationships with Regeneron, UCB, Dermira, and Genzyme/Sanofi.
EXPERT ANALYSIS FROM AAD 2019
ACOG: Avoid inductions before 39 weeks unless medically necessary
Babies should not be delivered before 39 0/7 weeks’ gestation by means besides spontaneous vaginal delivery, in the absence of medical indications for an earlier delivery.
wrote the authors of the new opinion, developed by the American College of Obstetricians and Gynecologists committee on obstetric practice and the Society for Maternal-Fetal Medicine.
The opinion, which replaces a 2013 statement, clarifies that their recommendations include avoiding cesarean delivery, labor induction, and cervical ripening before 39 0/7 weeks of gestation, unless a medical indication exists for earlier delivery.
The new opinion statement relied, in part, on a recent systematic review finding that late-preterm and early-term children do not fare as well as do term-delivered children in a variety of cognitive and educational domains. The opinion statement acknowledges that it’s not clear why children delivered earlier are showing performance difficulties and that it is possible that medical indications for an earlier delivery contributed to the differences.
Immediate outcomes for neonates delivered in the late preterm and early term period also are worse, compared with those delivered at term, according to several studies cited in the opinion. For example, composite morbidity was higher for infants delivered at both 37 and 38 weeks gestation, compared with those delivered at 39 weeks, with adjusted odds ratios for the composite outcome of 2.1 and 1.5, respectively (N Engl J Med. 2009 Jan 8;360[2]:111-20).
And lung maturity alone should not guide delivery, wrote the authors of the opinion. “Because nonrespiratory morbidities also are increased in early-term deliveries, documentation of fetal pulmonary maturity does not justify an early nonmedically indicated delivery,” they said, adding that physicians should not perform amniocentesis to determine lung maturity in an effort to guide delivery timing.
Because intrapartum demise remains a risk as long as a woman is pregnant, the potential for adverse neonatal outcomes with early delivery has to be balanced against the risk of stillbirth with continued gestation, the opinion authors acknowledged. But, they said, this question has been addressed by “multiple studies using national population level data,” which show that “even as the gestational age at term has increased in response to efforts to reduce early elective delivery, these efforts have not adversely affected stillbirth rates nationally or even in states with the greatest reductions in early elective delivery.”
Formal programs to reduce nonmedically indicated early-term deliveries have been successful. For example, the state of South Carolina achieved a reduction of almost 50% in nonmedically indicated early-term deliveries over the course of just 1 year. The South Carolina Birth Outcomes Initiative led a collaborative effort to institute a “hard-stop” policy against nonmedically indicated early-term deliveries that resulted in an absolute 4.7% decrease in late-preterm birth during 2011-2012. Similar efforts in Oregon and Ohio have reported significant reductions as well, with no increases in adverse neonatal outcomes.
Various policy approaches have been tried to achieve a reduction in nonmedically indicated late-preterm and early-term birth. These range from awareness raising and education, to “soft-stop” policies in which health care providers agree not to deliver before 39 weeks without medical indication, to “hard-stop” policies in which hospitals prohibit the nonindicated deliveries. In one comparative outcomes study, the hard-stop policy was the most effective, with a drop from 8.2% to 1.7% in nonindicated early deliveries, but the soft-stop policy also produced a decrease from 8.4% to 3.3% (P = .007 and .025, respectively). The educational approach didn’t produce a significant drop in nonmedically indicated early deliveries (Am J Obstet Gynecol. 2010 Nov;203[5]:449.e1-6).
In a separate, preexisting statement (Obstet Gynecol. 2019;133:e151-5), ACOG has outlined the management of medically indicated late-preterm and early-term deliveries and has developed an app (www.acog.org/acogapp) as a decision tool for indicated deliveries.
Examples cited by the current opinion statement authors of medical indications for early delivery include maternal factors such as preeclampsia, gestational hypertension, and poorly controlled diabetes. Placentation problems, fetal growth restriction, and prior cesarean deliveries also may warrant earlier delivery, as may a host of other complications. If an earlier delivery is planned, the committee authors recommend full discussion with the patient and clear documentation of the indications and discussion.
Babies should not be delivered before 39 0/7 weeks’ gestation by means besides spontaneous vaginal delivery, in the absence of medical indications for an earlier delivery.
wrote the authors of the new opinion, developed by the American College of Obstetricians and Gynecologists committee on obstetric practice and the Society for Maternal-Fetal Medicine.
The opinion, which replaces a 2013 statement, clarifies that their recommendations include avoiding cesarean delivery, labor induction, and cervical ripening before 39 0/7 weeks of gestation, unless a medical indication exists for earlier delivery.
The new opinion statement relied, in part, on a recent systematic review finding that late-preterm and early-term children do not fare as well as do term-delivered children in a variety of cognitive and educational domains. The opinion statement acknowledges that it’s not clear why children delivered earlier are showing performance difficulties and that it is possible that medical indications for an earlier delivery contributed to the differences.
Immediate outcomes for neonates delivered in the late preterm and early term period also are worse, compared with those delivered at term, according to several studies cited in the opinion. For example, composite morbidity was higher for infants delivered at both 37 and 38 weeks gestation, compared with those delivered at 39 weeks, with adjusted odds ratios for the composite outcome of 2.1 and 1.5, respectively (N Engl J Med. 2009 Jan 8;360[2]:111-20).
And lung maturity alone should not guide delivery, wrote the authors of the opinion. “Because nonrespiratory morbidities also are increased in early-term deliveries, documentation of fetal pulmonary maturity does not justify an early nonmedically indicated delivery,” they said, adding that physicians should not perform amniocentesis to determine lung maturity in an effort to guide delivery timing.
Because intrapartum demise remains a risk as long as a woman is pregnant, the potential for adverse neonatal outcomes with early delivery has to be balanced against the risk of stillbirth with continued gestation, the opinion authors acknowledged. But, they said, this question has been addressed by “multiple studies using national population level data,” which show that “even as the gestational age at term has increased in response to efforts to reduce early elective delivery, these efforts have not adversely affected stillbirth rates nationally or even in states with the greatest reductions in early elective delivery.”
Formal programs to reduce nonmedically indicated early-term deliveries have been successful. For example, the state of South Carolina achieved a reduction of almost 50% in nonmedically indicated early-term deliveries over the course of just 1 year. The South Carolina Birth Outcomes Initiative led a collaborative effort to institute a “hard-stop” policy against nonmedically indicated early-term deliveries that resulted in an absolute 4.7% decrease in late-preterm birth during 2011-2012. Similar efforts in Oregon and Ohio have reported significant reductions as well, with no increases in adverse neonatal outcomes.
Various policy approaches have been tried to achieve a reduction in nonmedically indicated late-preterm and early-term birth. These range from awareness raising and education, to “soft-stop” policies in which health care providers agree not to deliver before 39 weeks without medical indication, to “hard-stop” policies in which hospitals prohibit the nonindicated deliveries. In one comparative outcomes study, the hard-stop policy was the most effective, with a drop from 8.2% to 1.7% in nonindicated early deliveries, but the soft-stop policy also produced a decrease from 8.4% to 3.3% (P = .007 and .025, respectively). The educational approach didn’t produce a significant drop in nonmedically indicated early deliveries (Am J Obstet Gynecol. 2010 Nov;203[5]:449.e1-6).
In a separate, preexisting statement (Obstet Gynecol. 2019;133:e151-5), ACOG has outlined the management of medically indicated late-preterm and early-term deliveries and has developed an app (www.acog.org/acogapp) as a decision tool for indicated deliveries.
Examples cited by the current opinion statement authors of medical indications for early delivery include maternal factors such as preeclampsia, gestational hypertension, and poorly controlled diabetes. Placentation problems, fetal growth restriction, and prior cesarean deliveries also may warrant earlier delivery, as may a host of other complications. If an earlier delivery is planned, the committee authors recommend full discussion with the patient and clear documentation of the indications and discussion.
Babies should not be delivered before 39 0/7 weeks’ gestation by means besides spontaneous vaginal delivery, in the absence of medical indications for an earlier delivery.
wrote the authors of the new opinion, developed by the American College of Obstetricians and Gynecologists committee on obstetric practice and the Society for Maternal-Fetal Medicine.
The opinion, which replaces a 2013 statement, clarifies that their recommendations include avoiding cesarean delivery, labor induction, and cervical ripening before 39 0/7 weeks of gestation, unless a medical indication exists for earlier delivery.
The new opinion statement relied, in part, on a recent systematic review finding that late-preterm and early-term children do not fare as well as do term-delivered children in a variety of cognitive and educational domains. The opinion statement acknowledges that it’s not clear why children delivered earlier are showing performance difficulties and that it is possible that medical indications for an earlier delivery contributed to the differences.
Immediate outcomes for neonates delivered in the late preterm and early term period also are worse, compared with those delivered at term, according to several studies cited in the opinion. For example, composite morbidity was higher for infants delivered at both 37 and 38 weeks gestation, compared with those delivered at 39 weeks, with adjusted odds ratios for the composite outcome of 2.1 and 1.5, respectively (N Engl J Med. 2009 Jan 8;360[2]:111-20).
And lung maturity alone should not guide delivery, wrote the authors of the opinion. “Because nonrespiratory morbidities also are increased in early-term deliveries, documentation of fetal pulmonary maturity does not justify an early nonmedically indicated delivery,” they said, adding that physicians should not perform amniocentesis to determine lung maturity in an effort to guide delivery timing.
Because intrapartum demise remains a risk as long as a woman is pregnant, the potential for adverse neonatal outcomes with early delivery has to be balanced against the risk of stillbirth with continued gestation, the opinion authors acknowledged. But, they said, this question has been addressed by “multiple studies using national population level data,” which show that “even as the gestational age at term has increased in response to efforts to reduce early elective delivery, these efforts have not adversely affected stillbirth rates nationally or even in states with the greatest reductions in early elective delivery.”
Formal programs to reduce nonmedically indicated early-term deliveries have been successful. For example, the state of South Carolina achieved a reduction of almost 50% in nonmedically indicated early-term deliveries over the course of just 1 year. The South Carolina Birth Outcomes Initiative led a collaborative effort to institute a “hard-stop” policy against nonmedically indicated early-term deliveries that resulted in an absolute 4.7% decrease in late-preterm birth during 2011-2012. Similar efforts in Oregon and Ohio have reported significant reductions as well, with no increases in adverse neonatal outcomes.
Various policy approaches have been tried to achieve a reduction in nonmedically indicated late-preterm and early-term birth. These range from awareness raising and education, to “soft-stop” policies in which health care providers agree not to deliver before 39 weeks without medical indication, to “hard-stop” policies in which hospitals prohibit the nonindicated deliveries. In one comparative outcomes study, the hard-stop policy was the most effective, with a drop from 8.2% to 1.7% in nonindicated early deliveries, but the soft-stop policy also produced a decrease from 8.4% to 3.3% (P = .007 and .025, respectively). The educational approach didn’t produce a significant drop in nonmedically indicated early deliveries (Am J Obstet Gynecol. 2010 Nov;203[5]:449.e1-6).
In a separate, preexisting statement (Obstet Gynecol. 2019;133:e151-5), ACOG has outlined the management of medically indicated late-preterm and early-term deliveries and has developed an app (www.acog.org/acogapp) as a decision tool for indicated deliveries.
Examples cited by the current opinion statement authors of medical indications for early delivery include maternal factors such as preeclampsia, gestational hypertension, and poorly controlled diabetes. Placentation problems, fetal growth restriction, and prior cesarean deliveries also may warrant earlier delivery, as may a host of other complications. If an earlier delivery is planned, the committee authors recommend full discussion with the patient and clear documentation of the indications and discussion.
FROM OBSTETRICS & GYNECOLOGY
Malpractice suits are less frequent – but more costly
Lawsuits against physicians declined across virtually all specialties by more than a quarter over a 10-year span, but the cost to manage legal challenges went up, a recent analysis finds.
From 2007 to 2016, the rate of claims dropped by 27% per 100 doctors from 5.1 to 3.7, according to a review of 124,000 cases by CRICO Strategies, a division of CRICO, the medical liability insurance provider for the Harvard medical community. CRICO’s database of claims contains about 30% of legal cases filed against health providers across the U.S.
For internists, the rate of lawsuits decreased by 35% between 2007 and 2016, according to CRICO data provided to MDedge News. Ob.gyns. saw a 44% drop in claims over the 10-year period, and surgeons experienced a 23% rate decrease. The analysis did not break down the rate of claims by other single subspecialists. Claims decreased by a combined 29% for cardiologists, dermatologists, endocrinologists, family physicians, gastroenterologists, hematologists/oncologists, hospitalists, infectious disease specialists, internists, nephrologists, neurologists, pulmonologists, and rheumatologists/immunologists, according to the report published in February 2019 on CRICO’s website.
The findings are consistent with prior research on claim trends, said Seth Seabury, PhD, a medical liability researcher and director of the Keck-Schaeffer Initiative for Population Health Policy at the University of Southern California, Los Angeles.
“Malpractice claim frequency has been falling pretty steadily for a while now, reflecting a number of factors including the widespread adoption of tort reform and other measures to shield physicians from malpractice risk,” Dr. Seabury said in an interview. “Interestingly, the decline seems greatest in the claims with lower potential stakes, as you see average indemnity holding flat or rising. Some of this likely reflects the unwillingness of attorneys to take cases with lower potential payouts, because of the high cost of litigating a malpractice case.”
While frequency went down, the cost to manage a legal claim went up, according to CRICO data. The price of defending a malpractice lawsuit rose an average of 3.5% annually over the 10-year period from $36,000 to $46,000. For cases that ended with no payment (indemnity) to plaintiffs, the cost to manage a case rose an average of 5% annually.
The upward trends in case management expenses are striking, particularly since the time to resolve cases has decreased, said Michelle Mello, PhD, a health research and policy professor at Stanford (Calif.) University. From 2007 to 2016, the average time to resolve a case dropped from 29 to 27 months, the CRICO report found.
“CRICO nods to disclosure and apology approaches as perhaps underlying the more encouraging trend in time to resolution, but it was surprising to me that such approaches have not translated into lower defense costs,” Dr. Mello said in an interview. “In particular, a lot is still being spent to manage cases that never result in a payment to the patient. My hope was that, as hospitals got better at communicating with patients about adverse events, including the fact that about three-quarters of them are not due to substandard care, there would be fewer claims involving such events and also less money spent dealing with such claims when they do arise.”
For cases that do end in payment, high payouts are on the rise. Cases that ended in payments of $1 million or more increased 4% over the 10-year time frame, while payments of $3 million to $11 million increased 7% annually, according to the CRICO report. Cases that ended in payment lower than $1 million dropped over the 10-year span.
The reasons behind increasing plaintiff payouts is uncertain, Dr. Seabury said.
“It’s hard to say exactly why high payouts are on the rise, as payout levels reflect a number of factors – [such as] economic damages, clinical severity, pain and suffering – that can be difficult to disentangle,” he said. “But it is probably concerning for doctors in the sense that, while claims are becoming less likely, when they do happen, it could be more catastrophic in the sense of having large damages that exceed the policy limit.”
Lawsuits against physicians declined across virtually all specialties by more than a quarter over a 10-year span, but the cost to manage legal challenges went up, a recent analysis finds.
From 2007 to 2016, the rate of claims dropped by 27% per 100 doctors from 5.1 to 3.7, according to a review of 124,000 cases by CRICO Strategies, a division of CRICO, the medical liability insurance provider for the Harvard medical community. CRICO’s database of claims contains about 30% of legal cases filed against health providers across the U.S.
For internists, the rate of lawsuits decreased by 35% between 2007 and 2016, according to CRICO data provided to MDedge News. Ob.gyns. saw a 44% drop in claims over the 10-year period, and surgeons experienced a 23% rate decrease. The analysis did not break down the rate of claims by other single subspecialists. Claims decreased by a combined 29% for cardiologists, dermatologists, endocrinologists, family physicians, gastroenterologists, hematologists/oncologists, hospitalists, infectious disease specialists, internists, nephrologists, neurologists, pulmonologists, and rheumatologists/immunologists, according to the report published in February 2019 on CRICO’s website.
The findings are consistent with prior research on claim trends, said Seth Seabury, PhD, a medical liability researcher and director of the Keck-Schaeffer Initiative for Population Health Policy at the University of Southern California, Los Angeles.
“Malpractice claim frequency has been falling pretty steadily for a while now, reflecting a number of factors including the widespread adoption of tort reform and other measures to shield physicians from malpractice risk,” Dr. Seabury said in an interview. “Interestingly, the decline seems greatest in the claims with lower potential stakes, as you see average indemnity holding flat or rising. Some of this likely reflects the unwillingness of attorneys to take cases with lower potential payouts, because of the high cost of litigating a malpractice case.”
While frequency went down, the cost to manage a legal claim went up, according to CRICO data. The price of defending a malpractice lawsuit rose an average of 3.5% annually over the 10-year period from $36,000 to $46,000. For cases that ended with no payment (indemnity) to plaintiffs, the cost to manage a case rose an average of 5% annually.
The upward trends in case management expenses are striking, particularly since the time to resolve cases has decreased, said Michelle Mello, PhD, a health research and policy professor at Stanford (Calif.) University. From 2007 to 2016, the average time to resolve a case dropped from 29 to 27 months, the CRICO report found.
“CRICO nods to disclosure and apology approaches as perhaps underlying the more encouraging trend in time to resolution, but it was surprising to me that such approaches have not translated into lower defense costs,” Dr. Mello said in an interview. “In particular, a lot is still being spent to manage cases that never result in a payment to the patient. My hope was that, as hospitals got better at communicating with patients about adverse events, including the fact that about three-quarters of them are not due to substandard care, there would be fewer claims involving such events and also less money spent dealing with such claims when they do arise.”
For cases that do end in payment, high payouts are on the rise. Cases that ended in payments of $1 million or more increased 4% over the 10-year time frame, while payments of $3 million to $11 million increased 7% annually, according to the CRICO report. Cases that ended in payment lower than $1 million dropped over the 10-year span.
The reasons behind increasing plaintiff payouts is uncertain, Dr. Seabury said.
“It’s hard to say exactly why high payouts are on the rise, as payout levels reflect a number of factors – [such as] economic damages, clinical severity, pain and suffering – that can be difficult to disentangle,” he said. “But it is probably concerning for doctors in the sense that, while claims are becoming less likely, when they do happen, it could be more catastrophic in the sense of having large damages that exceed the policy limit.”
Lawsuits against physicians declined across virtually all specialties by more than a quarter over a 10-year span, but the cost to manage legal challenges went up, a recent analysis finds.
From 2007 to 2016, the rate of claims dropped by 27% per 100 doctors from 5.1 to 3.7, according to a review of 124,000 cases by CRICO Strategies, a division of CRICO, the medical liability insurance provider for the Harvard medical community. CRICO’s database of claims contains about 30% of legal cases filed against health providers across the U.S.
For internists, the rate of lawsuits decreased by 35% between 2007 and 2016, according to CRICO data provided to MDedge News. Ob.gyns. saw a 44% drop in claims over the 10-year period, and surgeons experienced a 23% rate decrease. The analysis did not break down the rate of claims by other single subspecialists. Claims decreased by a combined 29% for cardiologists, dermatologists, endocrinologists, family physicians, gastroenterologists, hematologists/oncologists, hospitalists, infectious disease specialists, internists, nephrologists, neurologists, pulmonologists, and rheumatologists/immunologists, according to the report published in February 2019 on CRICO’s website.
The findings are consistent with prior research on claim trends, said Seth Seabury, PhD, a medical liability researcher and director of the Keck-Schaeffer Initiative for Population Health Policy at the University of Southern California, Los Angeles.
“Malpractice claim frequency has been falling pretty steadily for a while now, reflecting a number of factors including the widespread adoption of tort reform and other measures to shield physicians from malpractice risk,” Dr. Seabury said in an interview. “Interestingly, the decline seems greatest in the claims with lower potential stakes, as you see average indemnity holding flat or rising. Some of this likely reflects the unwillingness of attorneys to take cases with lower potential payouts, because of the high cost of litigating a malpractice case.”
While frequency went down, the cost to manage a legal claim went up, according to CRICO data. The price of defending a malpractice lawsuit rose an average of 3.5% annually over the 10-year period from $36,000 to $46,000. For cases that ended with no payment (indemnity) to plaintiffs, the cost to manage a case rose an average of 5% annually.
The upward trends in case management expenses are striking, particularly since the time to resolve cases has decreased, said Michelle Mello, PhD, a health research and policy professor at Stanford (Calif.) University. From 2007 to 2016, the average time to resolve a case dropped from 29 to 27 months, the CRICO report found.
“CRICO nods to disclosure and apology approaches as perhaps underlying the more encouraging trend in time to resolution, but it was surprising to me that such approaches have not translated into lower defense costs,” Dr. Mello said in an interview. “In particular, a lot is still being spent to manage cases that never result in a payment to the patient. My hope was that, as hospitals got better at communicating with patients about adverse events, including the fact that about three-quarters of them are not due to substandard care, there would be fewer claims involving such events and also less money spent dealing with such claims when they do arise.”
For cases that do end in payment, high payouts are on the rise. Cases that ended in payments of $1 million or more increased 4% over the 10-year time frame, while payments of $3 million to $11 million increased 7% annually, according to the CRICO report. Cases that ended in payment lower than $1 million dropped over the 10-year span.
The reasons behind increasing plaintiff payouts is uncertain, Dr. Seabury said.
“It’s hard to say exactly why high payouts are on the rise, as payout levels reflect a number of factors – [such as] economic damages, clinical severity, pain and suffering – that can be difficult to disentangle,” he said. “But it is probably concerning for doctors in the sense that, while claims are becoming less likely, when they do happen, it could be more catastrophic in the sense of having large damages that exceed the policy limit.”