User login
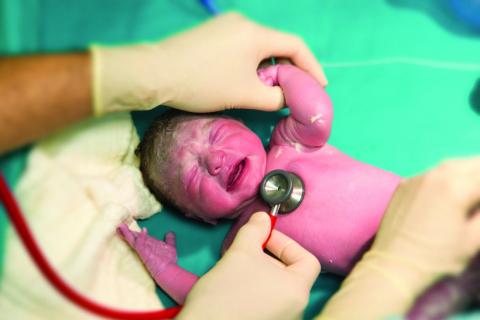
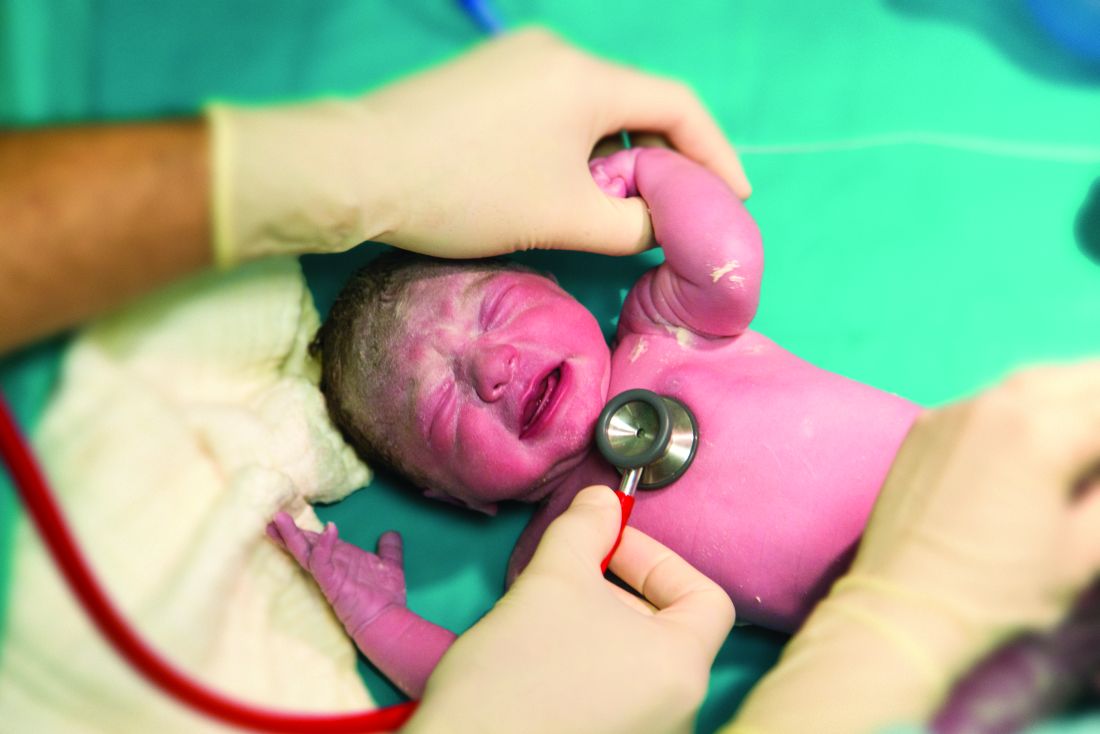
Sildenafil associated with persistent pulmonary hypertension in neonates with early IUGR
LAS VEGAS – Increased rates of persistent neonatal pulmonary hypertension in neonates put the brakes on STRIDER, an international placebo-controlled study looking at sildenafil as a treatment for early-onset intrauterine growth restriction (IUGR).
The study’s independent data safety monitoring board halted STRIDER (Sildenafil Therapy in Dismal Prognosis Early-Onset Fetal Growth Restriction) last July, after an interim safety analysis identified possible fetal harm and no signal of benefit over placebo, Dr. Anouk Pels reported at the annual meeting of the Society for Maternal-Fetal Medicine. The late-breaking presentation at the meeting revealed the first outcome data details.
The board had “serious concerns that sildenafil may cause harm to newborn children. … Given the results , it is extremely unlikely that any benefit could be shown on the primary endpoint if the trial is continued to its completion,” said Dr. Pels of the University of Amsterdam. “Our recommendation is not to use sildenafil for this indication in pregnant women.”
Although the link remains as-yet unproven, pulmonary hypertension among sildenafil-exposed neonates is biologically plausible, she said. It could have been a symptomatic rebound response to the discontinuation of constant intrauterine sildenafil exposure – or it could have been a hint of something more profound. Like the genital vasculature, pulmonary vasculature is a target of the drug. Intrauterine exposure to sildenafil could theoretically alter its development.
“It’s possible that sildenafil may be causing structural changes in the pulmonary vasculature of fetuses. This needs to be explored further, and we will do so by performing additional analyses on autopsy data and placental histology.”
STRIDER involved 261 pregnant women diagnosed with severe early-onset fetal growth restriction. They were randomized to sildenafil 25 mg or placebo three times daily until delivery or 32 weeks’ gestation. A safety analysis was conducted after every 50 patients were enrolled. The preplanned interim analysis was conducted after half of the cohort had been enrolled and received at least one dose of the study medication.
The primary outcome was a composite measure of neonatal mortality or major neonatal morbidity at hospital discharge.
Gestational age at baseline was about 24.6 weeks, and the estimated fetal weight by ultrasound, 465 g. About 45% of pregnancies had evidence of a notching in the uterine artery. In about 42%, the pulsatility index of the umbilical artery was above the 95th percentile; the pulsatility index of the middle cerebral artery was below the 5% percentile in about 70% of cases.
About a quarter of the women had a diagnosis of pregnancy-induced hypertension, and another quarter, preeclampsia. Women used the study medication for a mean of 22 days.
There were no significant between-group differences in maternal outcomes. Median gestational age at delivery was 28 weeks in both groups. About 10% in each group experienced new-onset pregnancy-induced hypertension; About a quarter of each group developed new-onset preeclampsia or HELLP (hemolysis, elevated liver enzymes, low platelet count). There were no between-group differences in the number of maternal antihypertensives prescribed.
The primary combined outcome of neonatal mortality or major neonatal morbidity occurred in 66 of the sildenafil-exposed neonates and 58 of the placebo-exposed infants (61% vs. 54%) – not a significant difference. Fetal death occurred in 23 and 29 pregnancies, respectively (21% and 27%); neonatal death occurred in 21 and 11, respectively (19% and 10%). Overall, fetal/neonatal mortality was similar between the sildenafil and placebo groups (41% and 37%, respectively).
Of the 64 sildenafil-exposed neonates who survived to hospital discharge, 22 exhibited clinically relevant morbidity. Of the 67 in the placebo-treated group who survived to hospital discharge, 18 had clinically relevant morbidity. Overall, 42 in the sildenafil group and 49 of the placebo group survived to hospital discharge without relevant morbidity.
There were a number of secondary outcomes, none exhibiting any significant between-group differences. These included the median weights of those who experienced intrauterine death (425 g and 350 g), median live birth weight (725 g and 783 g), intraventricular hemorrhage of grade II or IV (3% and 2%), periventricular hemorrhage grade II or higher (0%, both groups), necrotizing enterocolitis grade II or higher (7% and 8%), and at least one culture-proven or clinical infection (41% and 33%).
The significantly higher rates of pulmonary hypertension in the sildenafil-exposed neonates was the showstopper. Almost half of the 21 who died had proven pulmonary hypertension (10), as did 6 of the 64 who survived – an excess of 16 cases. Of proven cases, 11 were persistent pulmonary hypertension. There were also two cases of sepsis-associated pulmonary hypertension and four cases of bronchopulmonary dysplasia associated with the disorder. In the placebo-exposed group, pulmonary hypertension occurred in 3 of the 11 deaths and 1 of the 67 who survived. Of proven cases, two were persistent pulmonary hypertension. There was no sepsis-associated pulmonary hypertension, but there were three cases of bronchopulmonary dysplasia associated with the disorder. Some children had both persistent pulmonary hypertension and bronchopulmonary dysplasia.
“While there was no difference in the primary outcomes or in overall mortality, there were more cases of pulmonary hypertension in the sildenafil group,” Dr. Pels said. “We can speculate on the cause, whether it was related to sildenafil and why, or whether it was simply chance. This is the reason we need to conduct more in-depth analyses of these data.”
Funding came from federal health agencies and universities in the countries where STRIDER was conducted, including New Zealand, Australia, the United Kingdom, Ireland, and the Netherlands. Dr. Pels had no relevant financial disclosures.
SOURCE: Pels A et al. The Pregnancy Meeting, Late-Breaker 2.
LAS VEGAS – Increased rates of persistent neonatal pulmonary hypertension in neonates put the brakes on STRIDER, an international placebo-controlled study looking at sildenafil as a treatment for early-onset intrauterine growth restriction (IUGR).
The study’s independent data safety monitoring board halted STRIDER (Sildenafil Therapy in Dismal Prognosis Early-Onset Fetal Growth Restriction) last July, after an interim safety analysis identified possible fetal harm and no signal of benefit over placebo, Dr. Anouk Pels reported at the annual meeting of the Society for Maternal-Fetal Medicine. The late-breaking presentation at the meeting revealed the first outcome data details.
The board had “serious concerns that sildenafil may cause harm to newborn children. … Given the results , it is extremely unlikely that any benefit could be shown on the primary endpoint if the trial is continued to its completion,” said Dr. Pels of the University of Amsterdam. “Our recommendation is not to use sildenafil for this indication in pregnant women.”
Although the link remains as-yet unproven, pulmonary hypertension among sildenafil-exposed neonates is biologically plausible, she said. It could have been a symptomatic rebound response to the discontinuation of constant intrauterine sildenafil exposure – or it could have been a hint of something more profound. Like the genital vasculature, pulmonary vasculature is a target of the drug. Intrauterine exposure to sildenafil could theoretically alter its development.
“It’s possible that sildenafil may be causing structural changes in the pulmonary vasculature of fetuses. This needs to be explored further, and we will do so by performing additional analyses on autopsy data and placental histology.”
STRIDER involved 261 pregnant women diagnosed with severe early-onset fetal growth restriction. They were randomized to sildenafil 25 mg or placebo three times daily until delivery or 32 weeks’ gestation. A safety analysis was conducted after every 50 patients were enrolled. The preplanned interim analysis was conducted after half of the cohort had been enrolled and received at least one dose of the study medication.
The primary outcome was a composite measure of neonatal mortality or major neonatal morbidity at hospital discharge.
Gestational age at baseline was about 24.6 weeks, and the estimated fetal weight by ultrasound, 465 g. About 45% of pregnancies had evidence of a notching in the uterine artery. In about 42%, the pulsatility index of the umbilical artery was above the 95th percentile; the pulsatility index of the middle cerebral artery was below the 5% percentile in about 70% of cases.
About a quarter of the women had a diagnosis of pregnancy-induced hypertension, and another quarter, preeclampsia. Women used the study medication for a mean of 22 days.
There were no significant between-group differences in maternal outcomes. Median gestational age at delivery was 28 weeks in both groups. About 10% in each group experienced new-onset pregnancy-induced hypertension; About a quarter of each group developed new-onset preeclampsia or HELLP (hemolysis, elevated liver enzymes, low platelet count). There were no between-group differences in the number of maternal antihypertensives prescribed.
The primary combined outcome of neonatal mortality or major neonatal morbidity occurred in 66 of the sildenafil-exposed neonates and 58 of the placebo-exposed infants (61% vs. 54%) – not a significant difference. Fetal death occurred in 23 and 29 pregnancies, respectively (21% and 27%); neonatal death occurred in 21 and 11, respectively (19% and 10%). Overall, fetal/neonatal mortality was similar between the sildenafil and placebo groups (41% and 37%, respectively).
Of the 64 sildenafil-exposed neonates who survived to hospital discharge, 22 exhibited clinically relevant morbidity. Of the 67 in the placebo-treated group who survived to hospital discharge, 18 had clinically relevant morbidity. Overall, 42 in the sildenafil group and 49 of the placebo group survived to hospital discharge without relevant morbidity.
There were a number of secondary outcomes, none exhibiting any significant between-group differences. These included the median weights of those who experienced intrauterine death (425 g and 350 g), median live birth weight (725 g and 783 g), intraventricular hemorrhage of grade II or IV (3% and 2%), periventricular hemorrhage grade II or higher (0%, both groups), necrotizing enterocolitis grade II or higher (7% and 8%), and at least one culture-proven or clinical infection (41% and 33%).
The significantly higher rates of pulmonary hypertension in the sildenafil-exposed neonates was the showstopper. Almost half of the 21 who died had proven pulmonary hypertension (10), as did 6 of the 64 who survived – an excess of 16 cases. Of proven cases, 11 were persistent pulmonary hypertension. There were also two cases of sepsis-associated pulmonary hypertension and four cases of bronchopulmonary dysplasia associated with the disorder. In the placebo-exposed group, pulmonary hypertension occurred in 3 of the 11 deaths and 1 of the 67 who survived. Of proven cases, two were persistent pulmonary hypertension. There was no sepsis-associated pulmonary hypertension, but there were three cases of bronchopulmonary dysplasia associated with the disorder. Some children had both persistent pulmonary hypertension and bronchopulmonary dysplasia.
“While there was no difference in the primary outcomes or in overall mortality, there were more cases of pulmonary hypertension in the sildenafil group,” Dr. Pels said. “We can speculate on the cause, whether it was related to sildenafil and why, or whether it was simply chance. This is the reason we need to conduct more in-depth analyses of these data.”
Funding came from federal health agencies and universities in the countries where STRIDER was conducted, including New Zealand, Australia, the United Kingdom, Ireland, and the Netherlands. Dr. Pels had no relevant financial disclosures.
SOURCE: Pels A et al. The Pregnancy Meeting, Late-Breaker 2.
LAS VEGAS – Increased rates of persistent neonatal pulmonary hypertension in neonates put the brakes on STRIDER, an international placebo-controlled study looking at sildenafil as a treatment for early-onset intrauterine growth restriction (IUGR).
The study’s independent data safety monitoring board halted STRIDER (Sildenafil Therapy in Dismal Prognosis Early-Onset Fetal Growth Restriction) last July, after an interim safety analysis identified possible fetal harm and no signal of benefit over placebo, Dr. Anouk Pels reported at the annual meeting of the Society for Maternal-Fetal Medicine. The late-breaking presentation at the meeting revealed the first outcome data details.
The board had “serious concerns that sildenafil may cause harm to newborn children. … Given the results , it is extremely unlikely that any benefit could be shown on the primary endpoint if the trial is continued to its completion,” said Dr. Pels of the University of Amsterdam. “Our recommendation is not to use sildenafil for this indication in pregnant women.”
Although the link remains as-yet unproven, pulmonary hypertension among sildenafil-exposed neonates is biologically plausible, she said. It could have been a symptomatic rebound response to the discontinuation of constant intrauterine sildenafil exposure – or it could have been a hint of something more profound. Like the genital vasculature, pulmonary vasculature is a target of the drug. Intrauterine exposure to sildenafil could theoretically alter its development.
“It’s possible that sildenafil may be causing structural changes in the pulmonary vasculature of fetuses. This needs to be explored further, and we will do so by performing additional analyses on autopsy data and placental histology.”
STRIDER involved 261 pregnant women diagnosed with severe early-onset fetal growth restriction. They were randomized to sildenafil 25 mg or placebo three times daily until delivery or 32 weeks’ gestation. A safety analysis was conducted after every 50 patients were enrolled. The preplanned interim analysis was conducted after half of the cohort had been enrolled and received at least one dose of the study medication.
The primary outcome was a composite measure of neonatal mortality or major neonatal morbidity at hospital discharge.
Gestational age at baseline was about 24.6 weeks, and the estimated fetal weight by ultrasound, 465 g. About 45% of pregnancies had evidence of a notching in the uterine artery. In about 42%, the pulsatility index of the umbilical artery was above the 95th percentile; the pulsatility index of the middle cerebral artery was below the 5% percentile in about 70% of cases.
About a quarter of the women had a diagnosis of pregnancy-induced hypertension, and another quarter, preeclampsia. Women used the study medication for a mean of 22 days.
There were no significant between-group differences in maternal outcomes. Median gestational age at delivery was 28 weeks in both groups. About 10% in each group experienced new-onset pregnancy-induced hypertension; About a quarter of each group developed new-onset preeclampsia or HELLP (hemolysis, elevated liver enzymes, low platelet count). There were no between-group differences in the number of maternal antihypertensives prescribed.
The primary combined outcome of neonatal mortality or major neonatal morbidity occurred in 66 of the sildenafil-exposed neonates and 58 of the placebo-exposed infants (61% vs. 54%) – not a significant difference. Fetal death occurred in 23 and 29 pregnancies, respectively (21% and 27%); neonatal death occurred in 21 and 11, respectively (19% and 10%). Overall, fetal/neonatal mortality was similar between the sildenafil and placebo groups (41% and 37%, respectively).
Of the 64 sildenafil-exposed neonates who survived to hospital discharge, 22 exhibited clinically relevant morbidity. Of the 67 in the placebo-treated group who survived to hospital discharge, 18 had clinically relevant morbidity. Overall, 42 in the sildenafil group and 49 of the placebo group survived to hospital discharge without relevant morbidity.
There were a number of secondary outcomes, none exhibiting any significant between-group differences. These included the median weights of those who experienced intrauterine death (425 g and 350 g), median live birth weight (725 g and 783 g), intraventricular hemorrhage of grade II or IV (3% and 2%), periventricular hemorrhage grade II or higher (0%, both groups), necrotizing enterocolitis grade II or higher (7% and 8%), and at least one culture-proven or clinical infection (41% and 33%).
The significantly higher rates of pulmonary hypertension in the sildenafil-exposed neonates was the showstopper. Almost half of the 21 who died had proven pulmonary hypertension (10), as did 6 of the 64 who survived – an excess of 16 cases. Of proven cases, 11 were persistent pulmonary hypertension. There were also two cases of sepsis-associated pulmonary hypertension and four cases of bronchopulmonary dysplasia associated with the disorder. In the placebo-exposed group, pulmonary hypertension occurred in 3 of the 11 deaths and 1 of the 67 who survived. Of proven cases, two were persistent pulmonary hypertension. There was no sepsis-associated pulmonary hypertension, but there were three cases of bronchopulmonary dysplasia associated with the disorder. Some children had both persistent pulmonary hypertension and bronchopulmonary dysplasia.
“While there was no difference in the primary outcomes or in overall mortality, there were more cases of pulmonary hypertension in the sildenafil group,” Dr. Pels said. “We can speculate on the cause, whether it was related to sildenafil and why, or whether it was simply chance. This is the reason we need to conduct more in-depth analyses of these data.”
Funding came from federal health agencies and universities in the countries where STRIDER was conducted, including New Zealand, Australia, the United Kingdom, Ireland, and the Netherlands. Dr. Pels had no relevant financial disclosures.
SOURCE: Pels A et al. The Pregnancy Meeting, Late-Breaker 2.
REPORTING FROM THE PREGNANCY MEETING
Key clinical point:
Major finding: There were 16 cases of persistent pulmonary hypertension in the treated group and four in the placebo group.
Study details: The randomized study involved 261 pregnant women treated with sildenafil for early-onset intrauterine growth restriction.
Disclosures: Funding agencies and universities in the countries involved in the trial contributed to funding. Dr. Pels had no relevant financial disclosures.
Source: Pels A et al. The Pregnancy Meeting, Late-Breaker 2.
One postdelivery antibiotic dose nearly halves infection in operative delivery
LAS VEGAS – A randomized controlled trial comparing a single postdelivery intravenous dose of antibiotic after operative delivery found that antibiotics nearly halved the risk for maternal infection.
For women who received a single dose of amoxicillin-clavulanic acid, the risk ratio was 0.58 for suspected or confirmed infection, compared with those who received an intravenous dose of saline solution (95% confidence interval, 0.49-0.69, P less than .001). Culture-confirmed systemic infections were similarly reduced by a risk ratio (RR) of 0.44 (95% CI, 0.22-0.89; P =.018).
Superficial and deep incisional infections were also significantly less likely in the women who had received antibiotics (RRs 0.53 and 0.46, respectively; P less than .001 for both). Although sepsis occurred in numerically fewer women who received antibiotics, the numbers were, overall, small and not statistically significant.
By 6 weeks after delivery, patients receiving antibiotics were less likely to have outpatient or home visits for perineal problems or concerns as well (P less than .001).
“This trial shows clear benefit of a single dose of prophylactic antibiotic after operative vaginal birth, and this should be introduced into routine practice,” said Marian Knight, MBChB, DPhil.
Dr. Knight presented findings of the randomized trial, dubbed ANODE, at a late-breaking abstract session of the meeting, which was sponsored by the Society for Maternal-Fetal Medicine. Dr. Knight, professor of maternal and child population health at the University of Oxford (England), explained that ANODE aimed to determine whether a single dose of prophylactic amoxicillin-clavulanic acid was clinically effective in preventing confirmed or suspected maternal infection after operative vaginal birth.
The study tips the scales in favor of the anti-infective properties of the single antibiotic dose after operative delivery, and comes at a time when unacceptable levels of maternal morbidity and mortality coexist with pressing worries about antibiotic resistance, Dr. Knight said.
The multicenter randomized, blinded, placebo-controlled trial was conducted at 27 sites in the United Kingdom between March 2016 and June 2018.
Women at the study sites who underwent operative delivery, whether by forceps or vacuum extraction, received either a single dose of intravenous amoxicillin-clavulanic acid (1 gm/200 mg), or a placebo dose of saline solution. Antibiotics were given in the window of 0 to 6 hours post-delivery.
The primary outcome measure was confirmed or suspected maternal infection within 6 weeks of delivery. Women were positive for infection if they were prescribed antibiotics for perineal wound infections, if they experienced endometrial or uterine infections, if they had urinary tract infections with “systemic features, or if they had other systemic infections. Other criteria for infection were culture-confirmed systemic infection, or endometritis by criteria established by the Centers for Disease Control and Prevention.
Dr. Knight and her colleagues used an intention-to-treat analysis that looked at the primary outcome as a risk ratio, with a 95% CI. Secondary outcomes, also presented as risk ratios, were considered with a 99% CI.
A total of 3,427 women were randomized. In all, 1,715 women in the active arm and 1,705 in the placebo arm were included in the outcomes analyses. Women were interviewed by telephone, they completed questionnaires, and they received a questionnaire by mail or completed one online. Slightly more than 1,500 women in each arm completed the phone interview, and nearly 1,300 in each arm completed the initial questionnaire.
The mean age was 30 years, and most of the participants (84%-87%) were white. Most were of normal weight, with a median body mass index at the initial prenatal visit of 25 kg/m2.
Though women with multiple pregnancies were included in the study, just 11 in the active arm and 9 in the placebo arm delivered twins. There were no triplets. Most women (76%-78%) were primiparous, and just 7%-8% of women had prior cesarean delivery.
The mode of operative delivery for most of the participants (63%-67%) was forceps, with all but 10 of the remaining women receiving vacuum extraction (the remaining 10 had spontaneous vaginal deliveries).
The reasons for instrumental delivery were approximately evenly divided between failure of labor to progress and fetal compromise.
Nearly 90% of the women – more than 1,500 in each study arm – received episiotomies, a figure that Dr. Knight said she found surprising. She noted that mediolateral incisions are the standard of care in the United Kingdom. Still, 29%-33% of the women experienced a tear, with most being second-degree tears. Third- and fourth-degree tears occurred in two women overall. Almost all of the women (99%) had their wounds sutured.
Three serious adverse events were reported. One woman in the placebo arm required intensive care unit admission for severe sepsis, and another placebo participant required a transfusion after postpartum hemorrhage. One patient who received antibiotic had immediate diffuse itching and a swollen throat. However, antibiotic side effects were reported in only 2 of the 1,715 active arm participants, Dr. Knight said.
The competing concerns of maternal safety and antibiotic stewardship are weighed against a global backdrop of high maternal infection rates, Dr. Knight said. Sepsis causes 11% of global maternal deaths, a rate that drops to about 5% in higher-income nations. However, she pointed out, that figure rises to about 13% in the United States.
“For every woman that dies from pregnancy-related infection, a further 70 have severe infection and survive,” she said.
Known risk factors for infection include operative vaginal delivery and cesarean deliveries. For cesareans performed after the onset of labor, the adjusted odds ratio reaches 6.7 for severe infection, Dr. Knight said (PLoS Med. 2014;11:e1001672). A systematic review estimated that the rate for any infection following cesarean delivery approaches one in four women, she said (Cochrane Database Syst Rev. 2014 Oct 28;[10]:CD007482).
The same systematic review found that prophylactic antibiotics reduced incidence of wound infection, endometritis, and serious maternal wound infection after cesarean delivery (RR 0.40, 0.38, and 0.31, respectively).
For operative vaginal deliveries, however, a Cochrane review found one study of 393 women. Although no women given antibiotics developed endometritis compared with seven cases of endometritis in the no-antibiotics group for a RR of .07, the 95% confidence interval in the Cochrane analysis included zero, so the findings weren’t statistically significant. Hospital length of stay didn’t differ between the two groups (Cochrane Database Syst Rev. 2014 Oct 13;[10]:CD004455).
Citing this review, the United Kingdom’s Royal College of Gynecologists had concluded that evidence was insufficient to support routine antibiotic prophylaxis in operative deliveries. The American College of Obstetricians and Gynecologists make no mention of antibiotic prophylaxis or postdelivery infection in its guidelines for operative delivery, Dr. Knight said.
Since confirmed or suspected infection was still seen in 11% of women who received antibiotics, further analysis “is needed to investigate whether early administration, prenatal administration, or repeated administration is more likely to be effective,” she said. Women in the ANODE trial received their dose at a median of 3 hours after delivery.
“Until these analyses are completed, there is no indication for administration of more than a single dose of prophylactic antibiotic, or for predelivery administration,” she said.
Dr. Knight reported that ANODE was funded by the U.K.’s National Institute for Health Research. She reported that she had no conflicts of interest.
SOURCE: Knight M et al. Am J Obstet Gynecol. 2019 Jan;220;1:S685. Abstract LB 3.
LAS VEGAS – A randomized controlled trial comparing a single postdelivery intravenous dose of antibiotic after operative delivery found that antibiotics nearly halved the risk for maternal infection.
For women who received a single dose of amoxicillin-clavulanic acid, the risk ratio was 0.58 for suspected or confirmed infection, compared with those who received an intravenous dose of saline solution (95% confidence interval, 0.49-0.69, P less than .001). Culture-confirmed systemic infections were similarly reduced by a risk ratio (RR) of 0.44 (95% CI, 0.22-0.89; P =.018).
Superficial and deep incisional infections were also significantly less likely in the women who had received antibiotics (RRs 0.53 and 0.46, respectively; P less than .001 for both). Although sepsis occurred in numerically fewer women who received antibiotics, the numbers were, overall, small and not statistically significant.
By 6 weeks after delivery, patients receiving antibiotics were less likely to have outpatient or home visits for perineal problems or concerns as well (P less than .001).
“This trial shows clear benefit of a single dose of prophylactic antibiotic after operative vaginal birth, and this should be introduced into routine practice,” said Marian Knight, MBChB, DPhil.
Dr. Knight presented findings of the randomized trial, dubbed ANODE, at a late-breaking abstract session of the meeting, which was sponsored by the Society for Maternal-Fetal Medicine. Dr. Knight, professor of maternal and child population health at the University of Oxford (England), explained that ANODE aimed to determine whether a single dose of prophylactic amoxicillin-clavulanic acid was clinically effective in preventing confirmed or suspected maternal infection after operative vaginal birth.
The study tips the scales in favor of the anti-infective properties of the single antibiotic dose after operative delivery, and comes at a time when unacceptable levels of maternal morbidity and mortality coexist with pressing worries about antibiotic resistance, Dr. Knight said.
The multicenter randomized, blinded, placebo-controlled trial was conducted at 27 sites in the United Kingdom between March 2016 and June 2018.
Women at the study sites who underwent operative delivery, whether by forceps or vacuum extraction, received either a single dose of intravenous amoxicillin-clavulanic acid (1 gm/200 mg), or a placebo dose of saline solution. Antibiotics were given in the window of 0 to 6 hours post-delivery.
The primary outcome measure was confirmed or suspected maternal infection within 6 weeks of delivery. Women were positive for infection if they were prescribed antibiotics for perineal wound infections, if they experienced endometrial or uterine infections, if they had urinary tract infections with “systemic features, or if they had other systemic infections. Other criteria for infection were culture-confirmed systemic infection, or endometritis by criteria established by the Centers for Disease Control and Prevention.
Dr. Knight and her colleagues used an intention-to-treat analysis that looked at the primary outcome as a risk ratio, with a 95% CI. Secondary outcomes, also presented as risk ratios, were considered with a 99% CI.
A total of 3,427 women were randomized. In all, 1,715 women in the active arm and 1,705 in the placebo arm were included in the outcomes analyses. Women were interviewed by telephone, they completed questionnaires, and they received a questionnaire by mail or completed one online. Slightly more than 1,500 women in each arm completed the phone interview, and nearly 1,300 in each arm completed the initial questionnaire.
The mean age was 30 years, and most of the participants (84%-87%) were white. Most were of normal weight, with a median body mass index at the initial prenatal visit of 25 kg/m2.
Though women with multiple pregnancies were included in the study, just 11 in the active arm and 9 in the placebo arm delivered twins. There were no triplets. Most women (76%-78%) were primiparous, and just 7%-8% of women had prior cesarean delivery.
The mode of operative delivery for most of the participants (63%-67%) was forceps, with all but 10 of the remaining women receiving vacuum extraction (the remaining 10 had spontaneous vaginal deliveries).
The reasons for instrumental delivery were approximately evenly divided between failure of labor to progress and fetal compromise.
Nearly 90% of the women – more than 1,500 in each study arm – received episiotomies, a figure that Dr. Knight said she found surprising. She noted that mediolateral incisions are the standard of care in the United Kingdom. Still, 29%-33% of the women experienced a tear, with most being second-degree tears. Third- and fourth-degree tears occurred in two women overall. Almost all of the women (99%) had their wounds sutured.
Three serious adverse events were reported. One woman in the placebo arm required intensive care unit admission for severe sepsis, and another placebo participant required a transfusion after postpartum hemorrhage. One patient who received antibiotic had immediate diffuse itching and a swollen throat. However, antibiotic side effects were reported in only 2 of the 1,715 active arm participants, Dr. Knight said.
The competing concerns of maternal safety and antibiotic stewardship are weighed against a global backdrop of high maternal infection rates, Dr. Knight said. Sepsis causes 11% of global maternal deaths, a rate that drops to about 5% in higher-income nations. However, she pointed out, that figure rises to about 13% in the United States.
“For every woman that dies from pregnancy-related infection, a further 70 have severe infection and survive,” she said.
Known risk factors for infection include operative vaginal delivery and cesarean deliveries. For cesareans performed after the onset of labor, the adjusted odds ratio reaches 6.7 for severe infection, Dr. Knight said (PLoS Med. 2014;11:e1001672). A systematic review estimated that the rate for any infection following cesarean delivery approaches one in four women, she said (Cochrane Database Syst Rev. 2014 Oct 28;[10]:CD007482).
The same systematic review found that prophylactic antibiotics reduced incidence of wound infection, endometritis, and serious maternal wound infection after cesarean delivery (RR 0.40, 0.38, and 0.31, respectively).
For operative vaginal deliveries, however, a Cochrane review found one study of 393 women. Although no women given antibiotics developed endometritis compared with seven cases of endometritis in the no-antibiotics group for a RR of .07, the 95% confidence interval in the Cochrane analysis included zero, so the findings weren’t statistically significant. Hospital length of stay didn’t differ between the two groups (Cochrane Database Syst Rev. 2014 Oct 13;[10]:CD004455).
Citing this review, the United Kingdom’s Royal College of Gynecologists had concluded that evidence was insufficient to support routine antibiotic prophylaxis in operative deliveries. The American College of Obstetricians and Gynecologists make no mention of antibiotic prophylaxis or postdelivery infection in its guidelines for operative delivery, Dr. Knight said.
Since confirmed or suspected infection was still seen in 11% of women who received antibiotics, further analysis “is needed to investigate whether early administration, prenatal administration, or repeated administration is more likely to be effective,” she said. Women in the ANODE trial received their dose at a median of 3 hours after delivery.
“Until these analyses are completed, there is no indication for administration of more than a single dose of prophylactic antibiotic, or for predelivery administration,” she said.
Dr. Knight reported that ANODE was funded by the U.K.’s National Institute for Health Research. She reported that she had no conflicts of interest.
SOURCE: Knight M et al. Am J Obstet Gynecol. 2019 Jan;220;1:S685. Abstract LB 3.
LAS VEGAS – A randomized controlled trial comparing a single postdelivery intravenous dose of antibiotic after operative delivery found that antibiotics nearly halved the risk for maternal infection.
For women who received a single dose of amoxicillin-clavulanic acid, the risk ratio was 0.58 for suspected or confirmed infection, compared with those who received an intravenous dose of saline solution (95% confidence interval, 0.49-0.69, P less than .001). Culture-confirmed systemic infections were similarly reduced by a risk ratio (RR) of 0.44 (95% CI, 0.22-0.89; P =.018).
Superficial and deep incisional infections were also significantly less likely in the women who had received antibiotics (RRs 0.53 and 0.46, respectively; P less than .001 for both). Although sepsis occurred in numerically fewer women who received antibiotics, the numbers were, overall, small and not statistically significant.
By 6 weeks after delivery, patients receiving antibiotics were less likely to have outpatient or home visits for perineal problems or concerns as well (P less than .001).
“This trial shows clear benefit of a single dose of prophylactic antibiotic after operative vaginal birth, and this should be introduced into routine practice,” said Marian Knight, MBChB, DPhil.
Dr. Knight presented findings of the randomized trial, dubbed ANODE, at a late-breaking abstract session of the meeting, which was sponsored by the Society for Maternal-Fetal Medicine. Dr. Knight, professor of maternal and child population health at the University of Oxford (England), explained that ANODE aimed to determine whether a single dose of prophylactic amoxicillin-clavulanic acid was clinically effective in preventing confirmed or suspected maternal infection after operative vaginal birth.
The study tips the scales in favor of the anti-infective properties of the single antibiotic dose after operative delivery, and comes at a time when unacceptable levels of maternal morbidity and mortality coexist with pressing worries about antibiotic resistance, Dr. Knight said.
The multicenter randomized, blinded, placebo-controlled trial was conducted at 27 sites in the United Kingdom between March 2016 and June 2018.
Women at the study sites who underwent operative delivery, whether by forceps or vacuum extraction, received either a single dose of intravenous amoxicillin-clavulanic acid (1 gm/200 mg), or a placebo dose of saline solution. Antibiotics were given in the window of 0 to 6 hours post-delivery.
The primary outcome measure was confirmed or suspected maternal infection within 6 weeks of delivery. Women were positive for infection if they were prescribed antibiotics for perineal wound infections, if they experienced endometrial or uterine infections, if they had urinary tract infections with “systemic features, or if they had other systemic infections. Other criteria for infection were culture-confirmed systemic infection, or endometritis by criteria established by the Centers for Disease Control and Prevention.
Dr. Knight and her colleagues used an intention-to-treat analysis that looked at the primary outcome as a risk ratio, with a 95% CI. Secondary outcomes, also presented as risk ratios, were considered with a 99% CI.
A total of 3,427 women were randomized. In all, 1,715 women in the active arm and 1,705 in the placebo arm were included in the outcomes analyses. Women were interviewed by telephone, they completed questionnaires, and they received a questionnaire by mail or completed one online. Slightly more than 1,500 women in each arm completed the phone interview, and nearly 1,300 in each arm completed the initial questionnaire.
The mean age was 30 years, and most of the participants (84%-87%) were white. Most were of normal weight, with a median body mass index at the initial prenatal visit of 25 kg/m2.
Though women with multiple pregnancies were included in the study, just 11 in the active arm and 9 in the placebo arm delivered twins. There were no triplets. Most women (76%-78%) were primiparous, and just 7%-8% of women had prior cesarean delivery.
The mode of operative delivery for most of the participants (63%-67%) was forceps, with all but 10 of the remaining women receiving vacuum extraction (the remaining 10 had spontaneous vaginal deliveries).
The reasons for instrumental delivery were approximately evenly divided between failure of labor to progress and fetal compromise.
Nearly 90% of the women – more than 1,500 in each study arm – received episiotomies, a figure that Dr. Knight said she found surprising. She noted that mediolateral incisions are the standard of care in the United Kingdom. Still, 29%-33% of the women experienced a tear, with most being second-degree tears. Third- and fourth-degree tears occurred in two women overall. Almost all of the women (99%) had their wounds sutured.
Three serious adverse events were reported. One woman in the placebo arm required intensive care unit admission for severe sepsis, and another placebo participant required a transfusion after postpartum hemorrhage. One patient who received antibiotic had immediate diffuse itching and a swollen throat. However, antibiotic side effects were reported in only 2 of the 1,715 active arm participants, Dr. Knight said.
The competing concerns of maternal safety and antibiotic stewardship are weighed against a global backdrop of high maternal infection rates, Dr. Knight said. Sepsis causes 11% of global maternal deaths, a rate that drops to about 5% in higher-income nations. However, she pointed out, that figure rises to about 13% in the United States.
“For every woman that dies from pregnancy-related infection, a further 70 have severe infection and survive,” she said.
Known risk factors for infection include operative vaginal delivery and cesarean deliveries. For cesareans performed after the onset of labor, the adjusted odds ratio reaches 6.7 for severe infection, Dr. Knight said (PLoS Med. 2014;11:e1001672). A systematic review estimated that the rate for any infection following cesarean delivery approaches one in four women, she said (Cochrane Database Syst Rev. 2014 Oct 28;[10]:CD007482).
The same systematic review found that prophylactic antibiotics reduced incidence of wound infection, endometritis, and serious maternal wound infection after cesarean delivery (RR 0.40, 0.38, and 0.31, respectively).
For operative vaginal deliveries, however, a Cochrane review found one study of 393 women. Although no women given antibiotics developed endometritis compared with seven cases of endometritis in the no-antibiotics group for a RR of .07, the 95% confidence interval in the Cochrane analysis included zero, so the findings weren’t statistically significant. Hospital length of stay didn’t differ between the two groups (Cochrane Database Syst Rev. 2014 Oct 13;[10]:CD004455).
Citing this review, the United Kingdom’s Royal College of Gynecologists had concluded that evidence was insufficient to support routine antibiotic prophylaxis in operative deliveries. The American College of Obstetricians and Gynecologists make no mention of antibiotic prophylaxis or postdelivery infection in its guidelines for operative delivery, Dr. Knight said.
Since confirmed or suspected infection was still seen in 11% of women who received antibiotics, further analysis “is needed to investigate whether early administration, prenatal administration, or repeated administration is more likely to be effective,” she said. Women in the ANODE trial received their dose at a median of 3 hours after delivery.
“Until these analyses are completed, there is no indication for administration of more than a single dose of prophylactic antibiotic, or for predelivery administration,” she said.
Dr. Knight reported that ANODE was funded by the U.K.’s National Institute for Health Research. She reported that she had no conflicts of interest.
SOURCE: Knight M et al. Am J Obstet Gynecol. 2019 Jan;220;1:S685. Abstract LB 3.
REPORTING FROM THE PREGNANCY MEETING
Mediterranean diet cut Parkinson’s risk
‘Telereferrals’ improved mental health referral follow-through for children. How to take action to cut cardiovascular disease risk in rheumatoid patients. And the U.S. Preventive Services Task Force recommends counseling for perinatal depression prevention.
Amazon Alexa
Apple Podcasts
Google Podcasts
Spotify
‘Telereferrals’ improved mental health referral follow-through for children. How to take action to cut cardiovascular disease risk in rheumatoid patients. And the U.S. Preventive Services Task Force recommends counseling for perinatal depression prevention.
Amazon Alexa
Apple Podcasts
Google Podcasts
Spotify
‘Telereferrals’ improved mental health referral follow-through for children. How to take action to cut cardiovascular disease risk in rheumatoid patients. And the U.S. Preventive Services Task Force recommends counseling for perinatal depression prevention.
Amazon Alexa
Apple Podcasts
Google Podcasts
Spotify
Umbilical cord milking tied to severe IVH in very premature neonates
Delayed cord clamping and cutting is safer
LAS VEGAS – Umbilical cord milking can cause severe intraventricular hemorrhage (IVH) in very premature neonates and should not be performed on these cerebrovascularly fragile premature babies.
Just six of these procedures would be needed to cause a case of severe IVH in neonates born at 23-27 weeks’ gestation, Michael W. Varner, MD, said at the meeting sponsored by the Society for Maternal-Fetal Medicine.
“Centers practicing umbilical cord milking should consider discontinuing this practice in infants 23-27 weeks’ gestation,” said Dr. Varner of the University of Utah, Salt Lake City.
The damage to the brains of very young preemies appears to be a direct result of the fluid overload caused by milking, he said. “From a mechanistic perspective, we can intuit that these findings are consistent with cord milking. This causes increasing venous return to the right atrium where it enters the foramen ovale and aorta. These very premature babies have more pulmonary vasoconstriction, which shunts more blood toward the brain. This results in fluctuations in flow in an immature brain with fragile germinal matrices and perhaps further compromised by chorioamnionitis inflammation, resulting in IVH.”
Premature Infants Receiving Milking or Delayed Cord Clamping (PREMOD2) was a noninferiority trial of umbilical cord milking compared to delayed cord clamping and cutting in preterm infants. Conducted at 11 sites in the United States and Europe, the study was halted prematurely when the data safety monitoring board determined that cord milking increased the risk of IVH in younger preemies and was no better than delayed cutting in the older preemies. The analysis presented at the meeting is the first public discussion of the data details.
The trial involved 474 premature neonates. They were randomized to placental transfusion via a 60-second delay in cord clamping and cutting or to umbilical cord milking, which involved grasping the cord and manually pushing the cord blood toward the infant four times before clamping. All participating sites received a video demonstrating the proper procedure. The cohort also was divided by gestational age: 23-27 weeks and 28-31 weeks.
The primary endpoint was a combination of severe IVH (grade 3 or higher) and neonatal death. Overall, the primary endpoint occurred in 29 of those randomized to cord milking (12%) and 20 randomized to delayed clamping (8%) – a significant difference.
This finding was largely driven by the treatment differences in the 23-27 week group, Dr. Varner said. Severe IVH occurred in 20 (22%) of those randomized to cord milking and five (6%) of those randomized to delayed clamping – a highly statistically significant difference with a P value of 0.0019.
In the 28-31 week group, there were no cases of severe IVH in the cord milking group, and three cases in the delayed clamping group; the difference was not statistically significant.
Overall, deaths were similar between the cord milking and cord clamping groups (17 and 15, respectively). Most of these deaths occurred in the younger group (14 in the cord milking group and 13 in the clamping group). There were five deaths in the older group: three in the cord milking group and two in the clamping group. None of these differences were statistically significant.
After seeing these data in a preplanned interim safety analysis, the Data Safety Monitoring Board stopped the study, saying that the intervention appeared dangerous for the younger babies, and no better than the delayed cutting and clamping for the older group, Dr. Varner said.
Since the trial was halted, investigators have been dissecting the data to identify any other intracranial hemorrhage risks particular to the infants. They found no significant differences in maternal characteristics at baseline, and – other than age and randomization– nothing significantly different between the infant groups. Severe persistent IVH occurred in almost 70% of the infants born at 23 weeks’ gestation but in only 7% in the delayed cord clamping group. The risks declined rapidly with increasing gestational age, although they were at all times greater than the risk of IVH in the cord clamping group.
“Looking at the data by gestational age, it’s clear that the majority of the severe IVH occurrences were in the 23 weekers, and also occurred in the first 7 days of life,” Dr. Varner said.
The cohort will be followed for at least another year, he added, as investigators track neurodevelopmental outcomes.
Investigators are particularly interested in differences in motor and language skills, as well as general cognitive development.
The study was sponsored by theEunice Kennedy Shriver National Institute of Child Health and Development. Neither Dr. Varner nor any of the coauthors had any financial declarations.
SOURCE: Katheria AC et al. The Pregnancy Meeting, late breaking abstract 1.
Delayed cord clamping and cutting is safer
Delayed cord clamping and cutting is safer
LAS VEGAS – Umbilical cord milking can cause severe intraventricular hemorrhage (IVH) in very premature neonates and should not be performed on these cerebrovascularly fragile premature babies.
Just six of these procedures would be needed to cause a case of severe IVH in neonates born at 23-27 weeks’ gestation, Michael W. Varner, MD, said at the meeting sponsored by the Society for Maternal-Fetal Medicine.
“Centers practicing umbilical cord milking should consider discontinuing this practice in infants 23-27 weeks’ gestation,” said Dr. Varner of the University of Utah, Salt Lake City.
The damage to the brains of very young preemies appears to be a direct result of the fluid overload caused by milking, he said. “From a mechanistic perspective, we can intuit that these findings are consistent with cord milking. This causes increasing venous return to the right atrium where it enters the foramen ovale and aorta. These very premature babies have more pulmonary vasoconstriction, which shunts more blood toward the brain. This results in fluctuations in flow in an immature brain with fragile germinal matrices and perhaps further compromised by chorioamnionitis inflammation, resulting in IVH.”
Premature Infants Receiving Milking or Delayed Cord Clamping (PREMOD2) was a noninferiority trial of umbilical cord milking compared to delayed cord clamping and cutting in preterm infants. Conducted at 11 sites in the United States and Europe, the study was halted prematurely when the data safety monitoring board determined that cord milking increased the risk of IVH in younger preemies and was no better than delayed cutting in the older preemies. The analysis presented at the meeting is the first public discussion of the data details.
The trial involved 474 premature neonates. They were randomized to placental transfusion via a 60-second delay in cord clamping and cutting or to umbilical cord milking, which involved grasping the cord and manually pushing the cord blood toward the infant four times before clamping. All participating sites received a video demonstrating the proper procedure. The cohort also was divided by gestational age: 23-27 weeks and 28-31 weeks.
The primary endpoint was a combination of severe IVH (grade 3 or higher) and neonatal death. Overall, the primary endpoint occurred in 29 of those randomized to cord milking (12%) and 20 randomized to delayed clamping (8%) – a significant difference.
This finding was largely driven by the treatment differences in the 23-27 week group, Dr. Varner said. Severe IVH occurred in 20 (22%) of those randomized to cord milking and five (6%) of those randomized to delayed clamping – a highly statistically significant difference with a P value of 0.0019.
In the 28-31 week group, there were no cases of severe IVH in the cord milking group, and three cases in the delayed clamping group; the difference was not statistically significant.
Overall, deaths were similar between the cord milking and cord clamping groups (17 and 15, respectively). Most of these deaths occurred in the younger group (14 in the cord milking group and 13 in the clamping group). There were five deaths in the older group: three in the cord milking group and two in the clamping group. None of these differences were statistically significant.
After seeing these data in a preplanned interim safety analysis, the Data Safety Monitoring Board stopped the study, saying that the intervention appeared dangerous for the younger babies, and no better than the delayed cutting and clamping for the older group, Dr. Varner said.
Since the trial was halted, investigators have been dissecting the data to identify any other intracranial hemorrhage risks particular to the infants. They found no significant differences in maternal characteristics at baseline, and – other than age and randomization– nothing significantly different between the infant groups. Severe persistent IVH occurred in almost 70% of the infants born at 23 weeks’ gestation but in only 7% in the delayed cord clamping group. The risks declined rapidly with increasing gestational age, although they were at all times greater than the risk of IVH in the cord clamping group.
“Looking at the data by gestational age, it’s clear that the majority of the severe IVH occurrences were in the 23 weekers, and also occurred in the first 7 days of life,” Dr. Varner said.
The cohort will be followed for at least another year, he added, as investigators track neurodevelopmental outcomes.
Investigators are particularly interested in differences in motor and language skills, as well as general cognitive development.
The study was sponsored by theEunice Kennedy Shriver National Institute of Child Health and Development. Neither Dr. Varner nor any of the coauthors had any financial declarations.
SOURCE: Katheria AC et al. The Pregnancy Meeting, late breaking abstract 1.
LAS VEGAS – Umbilical cord milking can cause severe intraventricular hemorrhage (IVH) in very premature neonates and should not be performed on these cerebrovascularly fragile premature babies.
Just six of these procedures would be needed to cause a case of severe IVH in neonates born at 23-27 weeks’ gestation, Michael W. Varner, MD, said at the meeting sponsored by the Society for Maternal-Fetal Medicine.
“Centers practicing umbilical cord milking should consider discontinuing this practice in infants 23-27 weeks’ gestation,” said Dr. Varner of the University of Utah, Salt Lake City.
The damage to the brains of very young preemies appears to be a direct result of the fluid overload caused by milking, he said. “From a mechanistic perspective, we can intuit that these findings are consistent with cord milking. This causes increasing venous return to the right atrium where it enters the foramen ovale and aorta. These very premature babies have more pulmonary vasoconstriction, which shunts more blood toward the brain. This results in fluctuations in flow in an immature brain with fragile germinal matrices and perhaps further compromised by chorioamnionitis inflammation, resulting in IVH.”
Premature Infants Receiving Milking or Delayed Cord Clamping (PREMOD2) was a noninferiority trial of umbilical cord milking compared to delayed cord clamping and cutting in preterm infants. Conducted at 11 sites in the United States and Europe, the study was halted prematurely when the data safety monitoring board determined that cord milking increased the risk of IVH in younger preemies and was no better than delayed cutting in the older preemies. The analysis presented at the meeting is the first public discussion of the data details.
The trial involved 474 premature neonates. They were randomized to placental transfusion via a 60-second delay in cord clamping and cutting or to umbilical cord milking, which involved grasping the cord and manually pushing the cord blood toward the infant four times before clamping. All participating sites received a video demonstrating the proper procedure. The cohort also was divided by gestational age: 23-27 weeks and 28-31 weeks.
The primary endpoint was a combination of severe IVH (grade 3 or higher) and neonatal death. Overall, the primary endpoint occurred in 29 of those randomized to cord milking (12%) and 20 randomized to delayed clamping (8%) – a significant difference.
This finding was largely driven by the treatment differences in the 23-27 week group, Dr. Varner said. Severe IVH occurred in 20 (22%) of those randomized to cord milking and five (6%) of those randomized to delayed clamping – a highly statistically significant difference with a P value of 0.0019.
In the 28-31 week group, there were no cases of severe IVH in the cord milking group, and three cases in the delayed clamping group; the difference was not statistically significant.
Overall, deaths were similar between the cord milking and cord clamping groups (17 and 15, respectively). Most of these deaths occurred in the younger group (14 in the cord milking group and 13 in the clamping group). There were five deaths in the older group: three in the cord milking group and two in the clamping group. None of these differences were statistically significant.
After seeing these data in a preplanned interim safety analysis, the Data Safety Monitoring Board stopped the study, saying that the intervention appeared dangerous for the younger babies, and no better than the delayed cutting and clamping for the older group, Dr. Varner said.
Since the trial was halted, investigators have been dissecting the data to identify any other intracranial hemorrhage risks particular to the infants. They found no significant differences in maternal characteristics at baseline, and – other than age and randomization– nothing significantly different between the infant groups. Severe persistent IVH occurred in almost 70% of the infants born at 23 weeks’ gestation but in only 7% in the delayed cord clamping group. The risks declined rapidly with increasing gestational age, although they were at all times greater than the risk of IVH in the cord clamping group.
“Looking at the data by gestational age, it’s clear that the majority of the severe IVH occurrences were in the 23 weekers, and also occurred in the first 7 days of life,” Dr. Varner said.
The cohort will be followed for at least another year, he added, as investigators track neurodevelopmental outcomes.
Investigators are particularly interested in differences in motor and language skills, as well as general cognitive development.
The study was sponsored by theEunice Kennedy Shriver National Institute of Child Health and Development. Neither Dr. Varner nor any of the coauthors had any financial declarations.
SOURCE: Katheria AC et al. The Pregnancy Meeting, late breaking abstract 1.
REPORTING FROM THE PREGNANCY MEETING
Ehlers-Danlos syndrome: Increased IUGR risk reported
LAS VEGAS – Women with Ehlers-Danlos syndrome who became pregnant were more likely to experience antepartum hemorrhage, placenta previa, cervical incompetence, and preterm birth, according to a retrospective cohort study of national birth data. Long hospital stays also were more likely among these women.
Infants born to women with Ehlers-Danlos syndrome (EDS) were significantly more likely to have intrauterine growth retardation (IUGR) as well, an unexpected and as-yet unexplained finding, said the study’s first author, Laura Nicholls-Dempsey, MD, speaking at a poster session at the meeting sponsored by the Society for Maternal-Fetal Medicine.
Complications were infrequent overall, with a very low rate of intrauterine demise and no maternal mortality seen in the 910 women with EDS who were studied, said Dr. Nicholls-Dempsey, an ob.gyn. resident at McGill University, Montreal.
In counseling women with EDS, Dr. Nicholls-Dempsey said that she would advise them that “these are the types of things we’re going to watch out for, and we’ll see how the pregnancy goes. But we have to be careful about these: preterm birth, antepartum bleeding, placenta previa. We’ll watch the growth of the baby; we just have to be more careful about these specific things.”
Compared with women without EDS, those with the inherited connective tissue disorder had adjusted odds ratios (AORs) of 3.2 for cervical incompetence (95% confidence interval, 2.0-5.1) and 2.2 for placenta previa (95% CI, 1.3-3.9; P less than .01 for both). Absolute rates for these complications were 0.8% and 0.7% for women without EDS and 2.1% and 1.4% for women with EDS, respectively.
Women with EDS also had AORs of 1.8 for antepartum hemorrhage (2.8% versus 1.6%; 95% CI, 1.2-2.7; P less than .01). Cesarean delivery was more likely in women with EDS, with an AOR of 1.6 (37.4% versus 26.9%; 95% CI, 2.0-5.1); conversely, instrumental vaginal delivery was less likely in women with EDS (AOR = 0.5; 95% CI, 0.4-0.7; P less than .01 for both), meaning that spontaneous vaginal delivery was less likely in the EDS cohort.
The higher frequency of Cesarean deliveries may be attributable to anticipatory management by physicians seeking to avoid such complications as antepartum hemorrhage, as well as to the increased rate of placenta previa seen among the EDS cohort, Dr. Nicholls-Dempsey said.
After statistical adjustment, women in the EDS cohort were more than three times as likely to have hospital stays of both more than 7 days and 14 days (5.7% versus 2.1%, AOR = 3.1 for 7 days; 2.3% versus 0.7%, AOR = 3.8 for 14 days; P less than .01 for both).
Rates of some other maternal complications, such as pre-eclampsia, eclampsia, and gestational hypertension, were not elevated in the EDS cohort. Rates of premature rupture of membranes, chorioamnionitis, uterine rupture, postpartum hemorrhage, perineal laceration, and venous thromboembolism were also similar between groups.
However, not only was the AOR for preterm birth 1.5 for infants of women with EDS, but IUGR was more common in these neonates as well (AOR = 1.7, P less than .01 for both). The latter finding was unexpected, and Dr. Nicholls-Dempsey and her colleagues currently don’t have a mechanistic explanation for the higher IUGR rate.
Dr. Nicholls-Dempsey explained that she and her colleagues used data from the United States’ Health Care Cost and Utilization Project’s Nationwide Inpatient Sample (HCUP-NIS) to compare outcomes of women with EDS with the national sample as a whole.
Between 1999 and 2013, 13,881,592 births occurred in the HCUP-NIS cohort, with 910 deliveries to women who had EDS. These women were identified by ICD-9 codes, she said.
Comparing women with EDS to the non-EDS cohort, women with EDS were more likely to be Caucasian, have a higher income, and to be smokers; the cohorts were otherwise similar.
Ehlers-Danlos syndrome is a heterogeneous disorder involving abnormalities of collagen synthesis, with 13 known subtypes not captured in the HCUP-NIS data, Dr. Nicholls-Dempsey acknowledged. She characterized this as both a limitation but also a potential strength of the study.
“I really like this study because ... we know there’s 13 types of EDS that are genetically different ... They have their overlapping symptoms, but each one is different,” she said. “In an ideal world, we would have each subtype, and we would run this type of analysis on each subtype, to really be able to say to a patient, ‘You have this mutation, and this complication is going to be a big problem for you.’” The numbers of each subtype are so small that this is infeasible, she noted.
Still, the national sample acquired over many years offers real-world outcomes that clinicians can use in shared decision-making with EDS patients who are contemplating pregnancy or are already pregnant. Also, knowing which complications are more likely in patients with EDS can help plan optimal management of labor and delivery, Dr. Nicholls-Dempsey said.
Over the study’s 14-year span, the overall arc of EDS pregnancy outcomes is well captured regardless of mutation type. “It’s very applicable to the general population” of individuals with EDS, she noted. “Because it’s not type-specific, it’s really a good overview of what you can expect in EDS patients, regardless of the type.”
Dr. Nicholls-Dempsey reported no conflicts of interest and no outside sources of funding.
SOURCE: Nicholls-Dempsey L et al. Am J Obstet Gynecol. 2019 Jan;220(1):S381-382. Abstract 574
LAS VEGAS – Women with Ehlers-Danlos syndrome who became pregnant were more likely to experience antepartum hemorrhage, placenta previa, cervical incompetence, and preterm birth, according to a retrospective cohort study of national birth data. Long hospital stays also were more likely among these women.
Infants born to women with Ehlers-Danlos syndrome (EDS) were significantly more likely to have intrauterine growth retardation (IUGR) as well, an unexpected and as-yet unexplained finding, said the study’s first author, Laura Nicholls-Dempsey, MD, speaking at a poster session at the meeting sponsored by the Society for Maternal-Fetal Medicine.
Complications were infrequent overall, with a very low rate of intrauterine demise and no maternal mortality seen in the 910 women with EDS who were studied, said Dr. Nicholls-Dempsey, an ob.gyn. resident at McGill University, Montreal.
In counseling women with EDS, Dr. Nicholls-Dempsey said that she would advise them that “these are the types of things we’re going to watch out for, and we’ll see how the pregnancy goes. But we have to be careful about these: preterm birth, antepartum bleeding, placenta previa. We’ll watch the growth of the baby; we just have to be more careful about these specific things.”
Compared with women without EDS, those with the inherited connective tissue disorder had adjusted odds ratios (AORs) of 3.2 for cervical incompetence (95% confidence interval, 2.0-5.1) and 2.2 for placenta previa (95% CI, 1.3-3.9; P less than .01 for both). Absolute rates for these complications were 0.8% and 0.7% for women without EDS and 2.1% and 1.4% for women with EDS, respectively.
Women with EDS also had AORs of 1.8 for antepartum hemorrhage (2.8% versus 1.6%; 95% CI, 1.2-2.7; P less than .01). Cesarean delivery was more likely in women with EDS, with an AOR of 1.6 (37.4% versus 26.9%; 95% CI, 2.0-5.1); conversely, instrumental vaginal delivery was less likely in women with EDS (AOR = 0.5; 95% CI, 0.4-0.7; P less than .01 for both), meaning that spontaneous vaginal delivery was less likely in the EDS cohort.
The higher frequency of Cesarean deliveries may be attributable to anticipatory management by physicians seeking to avoid such complications as antepartum hemorrhage, as well as to the increased rate of placenta previa seen among the EDS cohort, Dr. Nicholls-Dempsey said.
After statistical adjustment, women in the EDS cohort were more than three times as likely to have hospital stays of both more than 7 days and 14 days (5.7% versus 2.1%, AOR = 3.1 for 7 days; 2.3% versus 0.7%, AOR = 3.8 for 14 days; P less than .01 for both).
Rates of some other maternal complications, such as pre-eclampsia, eclampsia, and gestational hypertension, were not elevated in the EDS cohort. Rates of premature rupture of membranes, chorioamnionitis, uterine rupture, postpartum hemorrhage, perineal laceration, and venous thromboembolism were also similar between groups.
However, not only was the AOR for preterm birth 1.5 for infants of women with EDS, but IUGR was more common in these neonates as well (AOR = 1.7, P less than .01 for both). The latter finding was unexpected, and Dr. Nicholls-Dempsey and her colleagues currently don’t have a mechanistic explanation for the higher IUGR rate.
Dr. Nicholls-Dempsey explained that she and her colleagues used data from the United States’ Health Care Cost and Utilization Project’s Nationwide Inpatient Sample (HCUP-NIS) to compare outcomes of women with EDS with the national sample as a whole.
Between 1999 and 2013, 13,881,592 births occurred in the HCUP-NIS cohort, with 910 deliveries to women who had EDS. These women were identified by ICD-9 codes, she said.
Comparing women with EDS to the non-EDS cohort, women with EDS were more likely to be Caucasian, have a higher income, and to be smokers; the cohorts were otherwise similar.
Ehlers-Danlos syndrome is a heterogeneous disorder involving abnormalities of collagen synthesis, with 13 known subtypes not captured in the HCUP-NIS data, Dr. Nicholls-Dempsey acknowledged. She characterized this as both a limitation but also a potential strength of the study.
“I really like this study because ... we know there’s 13 types of EDS that are genetically different ... They have their overlapping symptoms, but each one is different,” she said. “In an ideal world, we would have each subtype, and we would run this type of analysis on each subtype, to really be able to say to a patient, ‘You have this mutation, and this complication is going to be a big problem for you.’” The numbers of each subtype are so small that this is infeasible, she noted.
Still, the national sample acquired over many years offers real-world outcomes that clinicians can use in shared decision-making with EDS patients who are contemplating pregnancy or are already pregnant. Also, knowing which complications are more likely in patients with EDS can help plan optimal management of labor and delivery, Dr. Nicholls-Dempsey said.
Over the study’s 14-year span, the overall arc of EDS pregnancy outcomes is well captured regardless of mutation type. “It’s very applicable to the general population” of individuals with EDS, she noted. “Because it’s not type-specific, it’s really a good overview of what you can expect in EDS patients, regardless of the type.”
Dr. Nicholls-Dempsey reported no conflicts of interest and no outside sources of funding.
SOURCE: Nicholls-Dempsey L et al. Am J Obstet Gynecol. 2019 Jan;220(1):S381-382. Abstract 574
LAS VEGAS – Women with Ehlers-Danlos syndrome who became pregnant were more likely to experience antepartum hemorrhage, placenta previa, cervical incompetence, and preterm birth, according to a retrospective cohort study of national birth data. Long hospital stays also were more likely among these women.
Infants born to women with Ehlers-Danlos syndrome (EDS) were significantly more likely to have intrauterine growth retardation (IUGR) as well, an unexpected and as-yet unexplained finding, said the study’s first author, Laura Nicholls-Dempsey, MD, speaking at a poster session at the meeting sponsored by the Society for Maternal-Fetal Medicine.
Complications were infrequent overall, with a very low rate of intrauterine demise and no maternal mortality seen in the 910 women with EDS who were studied, said Dr. Nicholls-Dempsey, an ob.gyn. resident at McGill University, Montreal.
In counseling women with EDS, Dr. Nicholls-Dempsey said that she would advise them that “these are the types of things we’re going to watch out for, and we’ll see how the pregnancy goes. But we have to be careful about these: preterm birth, antepartum bleeding, placenta previa. We’ll watch the growth of the baby; we just have to be more careful about these specific things.”
Compared with women without EDS, those with the inherited connective tissue disorder had adjusted odds ratios (AORs) of 3.2 for cervical incompetence (95% confidence interval, 2.0-5.1) and 2.2 for placenta previa (95% CI, 1.3-3.9; P less than .01 for both). Absolute rates for these complications were 0.8% and 0.7% for women without EDS and 2.1% and 1.4% for women with EDS, respectively.
Women with EDS also had AORs of 1.8 for antepartum hemorrhage (2.8% versus 1.6%; 95% CI, 1.2-2.7; P less than .01). Cesarean delivery was more likely in women with EDS, with an AOR of 1.6 (37.4% versus 26.9%; 95% CI, 2.0-5.1); conversely, instrumental vaginal delivery was less likely in women with EDS (AOR = 0.5; 95% CI, 0.4-0.7; P less than .01 for both), meaning that spontaneous vaginal delivery was less likely in the EDS cohort.
The higher frequency of Cesarean deliveries may be attributable to anticipatory management by physicians seeking to avoid such complications as antepartum hemorrhage, as well as to the increased rate of placenta previa seen among the EDS cohort, Dr. Nicholls-Dempsey said.
After statistical adjustment, women in the EDS cohort were more than three times as likely to have hospital stays of both more than 7 days and 14 days (5.7% versus 2.1%, AOR = 3.1 for 7 days; 2.3% versus 0.7%, AOR = 3.8 for 14 days; P less than .01 for both).
Rates of some other maternal complications, such as pre-eclampsia, eclampsia, and gestational hypertension, were not elevated in the EDS cohort. Rates of premature rupture of membranes, chorioamnionitis, uterine rupture, postpartum hemorrhage, perineal laceration, and venous thromboembolism were also similar between groups.
However, not only was the AOR for preterm birth 1.5 for infants of women with EDS, but IUGR was more common in these neonates as well (AOR = 1.7, P less than .01 for both). The latter finding was unexpected, and Dr. Nicholls-Dempsey and her colleagues currently don’t have a mechanistic explanation for the higher IUGR rate.
Dr. Nicholls-Dempsey explained that she and her colleagues used data from the United States’ Health Care Cost and Utilization Project’s Nationwide Inpatient Sample (HCUP-NIS) to compare outcomes of women with EDS with the national sample as a whole.
Between 1999 and 2013, 13,881,592 births occurred in the HCUP-NIS cohort, with 910 deliveries to women who had EDS. These women were identified by ICD-9 codes, she said.
Comparing women with EDS to the non-EDS cohort, women with EDS were more likely to be Caucasian, have a higher income, and to be smokers; the cohorts were otherwise similar.
Ehlers-Danlos syndrome is a heterogeneous disorder involving abnormalities of collagen synthesis, with 13 known subtypes not captured in the HCUP-NIS data, Dr. Nicholls-Dempsey acknowledged. She characterized this as both a limitation but also a potential strength of the study.
“I really like this study because ... we know there’s 13 types of EDS that are genetically different ... They have their overlapping symptoms, but each one is different,” she said. “In an ideal world, we would have each subtype, and we would run this type of analysis on each subtype, to really be able to say to a patient, ‘You have this mutation, and this complication is going to be a big problem for you.’” The numbers of each subtype are so small that this is infeasible, she noted.
Still, the national sample acquired over many years offers real-world outcomes that clinicians can use in shared decision-making with EDS patients who are contemplating pregnancy or are already pregnant. Also, knowing which complications are more likely in patients with EDS can help plan optimal management of labor and delivery, Dr. Nicholls-Dempsey said.
Over the study’s 14-year span, the overall arc of EDS pregnancy outcomes is well captured regardless of mutation type. “It’s very applicable to the general population” of individuals with EDS, she noted. “Because it’s not type-specific, it’s really a good overview of what you can expect in EDS patients, regardless of the type.”
Dr. Nicholls-Dempsey reported no conflicts of interest and no outside sources of funding.
SOURCE: Nicholls-Dempsey L et al. Am J Obstet Gynecol. 2019 Jan;220(1):S381-382. Abstract 574
REPORTING FROM THE PREGNANCY MEETING
Obstetric patients with opioid use disorder fare well with medication-assisted treatment
LAS VEGAS – A prospective study of pregnant women with opioid use disorder showed good success with tapering or discontinuation of medication-assisted treatment (MAT) for women who wished to reduce or eliminate opioids while pregnant.
In related work, naltrexone showed promise for MAT in pregnancy, with rapid fetal clearance and no neonatal abstinence syndrome (NAS) seen in women who were adherent to naltrexone.
Presenting early experiences from an obstetric clinic dedicated to care of women with opioid use disorder (OUD), Craig Towers, MD, said that the clinic began seeing patients in November, 2016. “Eastern Tennessee has a high rate of opioid use disorder,” so the clinic at the University of Tennessee, Knoxville, fills an unmet need, he said, speaking during a poster session at the meeting sponsored by the Society for Maternal-Fetal Medicine.
Women who enroll at the clinic are offered MAT; those who are stable on MAT and adherent to prenatal care also are offered the choice to taper from MAT and detoxify, said Dr. Towers, professor in the division of maternal-fetal medicine in the department of obstetrics and gynecology at the University of Tennessee – Knoxville.
The prospective observational cohort study found that, of a total of 367 compliant patients, 286 (78%) chose opioid tapering/detoxification, and of these, 152 (53%) did detoxify fully. Of these patients, 126 (83%) were taking no opioids at delivery, and their infants experienced no NAS.
At the time of delivery, 26 patients (17%) were taking opioids at delivery; 18 were back on MAT, and 8 were using illicit opioids. A total of 116 patients chose to continue MAT, whether they stayed on the regimen or converted back to stable MAT doses after beginning to taper.
Another option offered women at the OUD-dedicated obstetric clinic is the use of the Bridge device, a percutaneous nerve field stimulator, for OUD. Dr. Tower said that, in early use in 14 patients, the Bridge was successful in helping women transition off opioids completely in 10 (71%) patients.
About the larger study, Dr. Towers said, “This is the first study to prospectively report outcome data on a designated OUD clinic that offers detoxification during pregnancy.
“With a structured program that includes behavioral health and offers the option of detoxification, fetal risks are negligible, relapse rates by delivery are low, and NAS rates are greatly decreased,” wrote Dr. Towers and colleagues in the abstract accompanying the study.
In a related poster presentation, Dr. Towers reported outcomes for a subset of pregnant women with OUD who received naltrexone as MAT. Previously, said Dr. Towers, retrospective work showed no significant harm using naltrexone, which is one of the three approved options for MAT to manage OUD, along with buprenorphine and methadone. Naltrexone has the advantage of helping reduce cravings.
Of the 108 patients, 82 (76%) remained on naltrexone until delivery; in these pregnancies, there were no cases of NAS. However, among the 26 pregnancies in which women stopped taking naltrexone before delivery, there were 6 (23%) NAS cases.
Fifty-one patients were started on naltrexone before 24 weeks’ gestation, and of those patients, no changes were seen with fetal monitoring. There were no instances of spontaneous abortion or intrauterine demise in any participants.
The investigators tracked gestational age at the point of full detoxification and gestational age at the point naltrexone was started. Additionally, fetal response to naltrexone and maternal and neonatal outcomes were recorded.
“This is the first prospective study and largest to date on the use of naltrexone in pregnancy,” Dr. Towers and his colleagues wrote in the poster accompanying the presentation. “These data demonstrate that naltrexone MAT is a viable option for managing OUD in pregnancy.”
Dr. Towers reported no conflicts of interest and no outside sources of funding.
LAS VEGAS – A prospective study of pregnant women with opioid use disorder showed good success with tapering or discontinuation of medication-assisted treatment (MAT) for women who wished to reduce or eliminate opioids while pregnant.
In related work, naltrexone showed promise for MAT in pregnancy, with rapid fetal clearance and no neonatal abstinence syndrome (NAS) seen in women who were adherent to naltrexone.
Presenting early experiences from an obstetric clinic dedicated to care of women with opioid use disorder (OUD), Craig Towers, MD, said that the clinic began seeing patients in November, 2016. “Eastern Tennessee has a high rate of opioid use disorder,” so the clinic at the University of Tennessee, Knoxville, fills an unmet need, he said, speaking during a poster session at the meeting sponsored by the Society for Maternal-Fetal Medicine.
Women who enroll at the clinic are offered MAT; those who are stable on MAT and adherent to prenatal care also are offered the choice to taper from MAT and detoxify, said Dr. Towers, professor in the division of maternal-fetal medicine in the department of obstetrics and gynecology at the University of Tennessee – Knoxville.
The prospective observational cohort study found that, of a total of 367 compliant patients, 286 (78%) chose opioid tapering/detoxification, and of these, 152 (53%) did detoxify fully. Of these patients, 126 (83%) were taking no opioids at delivery, and their infants experienced no NAS.
At the time of delivery, 26 patients (17%) were taking opioids at delivery; 18 were back on MAT, and 8 were using illicit opioids. A total of 116 patients chose to continue MAT, whether they stayed on the regimen or converted back to stable MAT doses after beginning to taper.
Another option offered women at the OUD-dedicated obstetric clinic is the use of the Bridge device, a percutaneous nerve field stimulator, for OUD. Dr. Tower said that, in early use in 14 patients, the Bridge was successful in helping women transition off opioids completely in 10 (71%) patients.
About the larger study, Dr. Towers said, “This is the first study to prospectively report outcome data on a designated OUD clinic that offers detoxification during pregnancy.
“With a structured program that includes behavioral health and offers the option of detoxification, fetal risks are negligible, relapse rates by delivery are low, and NAS rates are greatly decreased,” wrote Dr. Towers and colleagues in the abstract accompanying the study.
In a related poster presentation, Dr. Towers reported outcomes for a subset of pregnant women with OUD who received naltrexone as MAT. Previously, said Dr. Towers, retrospective work showed no significant harm using naltrexone, which is one of the three approved options for MAT to manage OUD, along with buprenorphine and methadone. Naltrexone has the advantage of helping reduce cravings.
Of the 108 patients, 82 (76%) remained on naltrexone until delivery; in these pregnancies, there were no cases of NAS. However, among the 26 pregnancies in which women stopped taking naltrexone before delivery, there were 6 (23%) NAS cases.
Fifty-one patients were started on naltrexone before 24 weeks’ gestation, and of those patients, no changes were seen with fetal monitoring. There were no instances of spontaneous abortion or intrauterine demise in any participants.
The investigators tracked gestational age at the point of full detoxification and gestational age at the point naltrexone was started. Additionally, fetal response to naltrexone and maternal and neonatal outcomes were recorded.
“This is the first prospective study and largest to date on the use of naltrexone in pregnancy,” Dr. Towers and his colleagues wrote in the poster accompanying the presentation. “These data demonstrate that naltrexone MAT is a viable option for managing OUD in pregnancy.”
Dr. Towers reported no conflicts of interest and no outside sources of funding.
LAS VEGAS – A prospective study of pregnant women with opioid use disorder showed good success with tapering or discontinuation of medication-assisted treatment (MAT) for women who wished to reduce or eliminate opioids while pregnant.
In related work, naltrexone showed promise for MAT in pregnancy, with rapid fetal clearance and no neonatal abstinence syndrome (NAS) seen in women who were adherent to naltrexone.
Presenting early experiences from an obstetric clinic dedicated to care of women with opioid use disorder (OUD), Craig Towers, MD, said that the clinic began seeing patients in November, 2016. “Eastern Tennessee has a high rate of opioid use disorder,” so the clinic at the University of Tennessee, Knoxville, fills an unmet need, he said, speaking during a poster session at the meeting sponsored by the Society for Maternal-Fetal Medicine.
Women who enroll at the clinic are offered MAT; those who are stable on MAT and adherent to prenatal care also are offered the choice to taper from MAT and detoxify, said Dr. Towers, professor in the division of maternal-fetal medicine in the department of obstetrics and gynecology at the University of Tennessee – Knoxville.
The prospective observational cohort study found that, of a total of 367 compliant patients, 286 (78%) chose opioid tapering/detoxification, and of these, 152 (53%) did detoxify fully. Of these patients, 126 (83%) were taking no opioids at delivery, and their infants experienced no NAS.
At the time of delivery, 26 patients (17%) were taking opioids at delivery; 18 were back on MAT, and 8 were using illicit opioids. A total of 116 patients chose to continue MAT, whether they stayed on the regimen or converted back to stable MAT doses after beginning to taper.
Another option offered women at the OUD-dedicated obstetric clinic is the use of the Bridge device, a percutaneous nerve field stimulator, for OUD. Dr. Tower said that, in early use in 14 patients, the Bridge was successful in helping women transition off opioids completely in 10 (71%) patients.
About the larger study, Dr. Towers said, “This is the first study to prospectively report outcome data on a designated OUD clinic that offers detoxification during pregnancy.
“With a structured program that includes behavioral health and offers the option of detoxification, fetal risks are negligible, relapse rates by delivery are low, and NAS rates are greatly decreased,” wrote Dr. Towers and colleagues in the abstract accompanying the study.
In a related poster presentation, Dr. Towers reported outcomes for a subset of pregnant women with OUD who received naltrexone as MAT. Previously, said Dr. Towers, retrospective work showed no significant harm using naltrexone, which is one of the three approved options for MAT to manage OUD, along with buprenorphine and methadone. Naltrexone has the advantage of helping reduce cravings.
Of the 108 patients, 82 (76%) remained on naltrexone until delivery; in these pregnancies, there were no cases of NAS. However, among the 26 pregnancies in which women stopped taking naltrexone before delivery, there were 6 (23%) NAS cases.
Fifty-one patients were started on naltrexone before 24 weeks’ gestation, and of those patients, no changes were seen with fetal monitoring. There were no instances of spontaneous abortion or intrauterine demise in any participants.
The investigators tracked gestational age at the point of full detoxification and gestational age at the point naltrexone was started. Additionally, fetal response to naltrexone and maternal and neonatal outcomes were recorded.
“This is the first prospective study and largest to date on the use of naltrexone in pregnancy,” Dr. Towers and his colleagues wrote in the poster accompanying the presentation. “These data demonstrate that naltrexone MAT is a viable option for managing OUD in pregnancy.”
Dr. Towers reported no conflicts of interest and no outside sources of funding.
REPORTING FROM THE PREGNANCY MEETING
Key clinical point: Over two-thirds of MAT-adherent patients chose detoxification.
Major finding:
Study details: Prospective single-center cohort study of 367 pregnant women with opioid use disorder.
Disclosures: Dr. Towers reported no outside sources of funding and no relevant conflicts of interest.
Source: Towers C. et al. SMFM 2019, Posters 141 & 142.
Postpartum depression often tricky to diagnose
LAS VEGAS – Diagnosing postpartum depression can be tricky because of the wide range of body changes that occur during the postpartum period, but vigilance is warranted with mothers who express a lack of sleep and a lack of social support.
at an annual psychopharmacology update held by the Nevada Psychiatric Association. “This gives you information about depression and insomnia. Make sure to ask about anxiety symptoms. Also ask about any thoughts of suicide or harming the infant, and support from family and friends when she’s under stress and taking care of the baby.”
According to Dr. Friedman, a perinatal and forensic psychiatrist at Case Western Reserve University, Cleveland, social risk factors for postpartum depression (PPD) include being a victim of intimate partner violence and/or abuse, negative life events, decreased social support, relationship issues, and socioeconomic status. Psychological risk factors include anxiety/depression in pregnancy, personal or family history of PPD, and substance misuse. Biological risk factors include medical illness, multiple births, and having an infant with low birth weight/prematurity.
PPD affects 10%-20% of new mothers and peaks at 12 weeks. Postpartum psychosis, meanwhile, occurs in about 1-2 of every 1,000 deliveries. Anxiety comorbidity is common.
In the neonatal intensive care unit (NICU), PPD rates might increase from 28% to 70% depending on the study. Risk factors include personal or family history, disturbed relationships, unfavorable socioeconomic factors, and stressful life events. Obstetrical risk factors might include conception by assisted reproductive technologies and having a stillbirth in the year before conception. NICU-specific risk factors include less-effective coping strategies, greater perception of maternal role disruption, and decreased perception of nursing support. “A lot of mothers [in the NICU] talk to me about being on a roller roaster every day about what’s going to happen with their baby,” Dr. Friedman said.
The most widely used measure to screen for PPD is the 10-item self-rating Edinburgh Postnatal Depression Scale . A total score of 10 or more is considered a flag for the need to follow up for possible depressive symptoms. She advises clinicians to pay particular attention to how patients respond to item No. 10 on the scale, which reads, “The thought of harming myself has occurred to me.” (Optional answers range from “Yes, quite often” to “Never.”) She also recommends administering the screen at both pediatric and obstetrical office visits, “because mothers are more likely to attend a pediatrics appointment than her own [postpartum] follow-up.”
The differential diagnosis of PPD includes the baby blues, postpartum psychosis, postpartum anxiety/PTSD, medical causes, substance use disorder, and PPD in bipolar disorder. Baby blues is not synonymous with PPD. It affects the majority (50%-80%) of new mothers and is characterized by emotional sensitivity, mood lability, and irritability. It usually occurs within 5 days and resolves by the second week post partum.
Postpartum psychosis (PPP) occurs in about 1-2 of every 1,000 deliveries, typically in the first 2 weeks after delivery. The onset occurs rapidly, and PPP is most frequently correlated with bipolar disorder over time. PPP itself is characterized by grandiose bizarre delusions, mood lability, hallucinations, confusion, and disorganized behavior. “This can occur as a new onset of mental illness as well, so getting collateral information about her behaviors is important,” she said.
Dr. Friedman explained that those events occur post partum largely because of sleep deprivation and increasing stress as the woman adjusts to a mothering role. Hormonal shifts also occur, with a drop in estrogen levels. Obstetrical complications also might factor in.
Postpartum obsessive-compulsive disorder (OCD) is commonly comorbid with PPD and is distinguished by ego-dystonic intrusive thoughts. The mother might have intense distress that she is going to harm the infant and might start to avoid holding the baby out of concern. “Common things I’ve heard from women with postpartum OCD are: ‘I’m afraid I’m going to put the baby in the microwave or in the oven instead of dinner’ or ‘I’m afraid I’m going to leave the baby in the car overnight and she’ll freeze to death,’ ” she said.
Postpartum PTSD can be triggered by a traumatic event experience in the birthing process, such as an emergency C-section. Affected mothers avoid the infant and hospital, “reexperience” the trauma, are easily startled, irritable, and disconnected. Dr. Friedman also noted that early parental PTSD symptoms predict sleep and eating problems in childhood and less sensitive/more controlling maternal behaviors.
Medical conditions that mimic PPD include anemia, thyroid disease, hypoactive delirium, infections, and alcohol/substance use disorder.
The best available data show that mothers with PPD are more withdrawn, disengaged, display more hostility, and are more likely to have disrupted attachment with their babies, Dr. Friedman said. They also are less likely to employ healthy child development practices and to breastfeed. Untreated depression might lead to psychotic symptoms, suicide, or homicide. Paternal PPD also occurs in an estimated 10% of fathers and is moderately correlated with maternal PPD.
Potential risks of PPD include impaired bonding, attachment disturbance, language development, cognitive skills, and behavior problems.
Potential risks of untreated PPD include child neglect or abuse because of active symptoms, suicide, and psychotic or maltreatment-related infanticide. “If the mother is taking about harming herself, I often ask: ‘Have you thought of what would happen to your baby if you were to take your own life?’ ” Dr. Friedman offered. Peripartum suicide risk is lower than in the general female population, but it represents about 20% of peripartum deaths. Overdose is the most common method. “However, uncommon and dramatic methods are more common in this population,” she said. “Teens and stigmatized single mothers are at greater risk.”
Dr. Friedman noted that clinicians face risk of a malpractice lawsuit if they fail to treat, abandon the patient, fail to provide informed consent, and if there are bad outcomes. The best approach is to proactively communicate with the patient, partner, pediatrics, and obstetrics. “Conduct an individual risk-benefit assessment with the individual patient’s history,” she advised. “Don’t do anything knee jerk. Consult when needed, document, and consider lactation and future pregnancy possibility in women of reproductive age.”
Nonpharmacologic therapy might be the first line of treatment for mild to moderate symptoms. Options include cognitive-behavioral therapy, interpersonal psychotherapy, family therapy, psychodynamic psychotherapy, and supportive psychotherapy. She recommends close follow-up and conducting a careful medication history. Electroconvulsive therapy remains a possibility.
If medication use is warranted, “weigh the benefits of breastfeeding with the usually low drug exposure of the infant,” Dr. Friedman advised. “We want to use the least number of medications at an effective dose to optimize treatment. Newer medications have less perinatal data. Sertraline and paroxetine are usually the preferred selective serotonin reuptake inhibitors in lactation. However, fluoxetine or citalopram might be used depending on the patient’s response history/use in pregnancy.”
Dr. Friedman reported no disclosures.
LAS VEGAS – Diagnosing postpartum depression can be tricky because of the wide range of body changes that occur during the postpartum period, but vigilance is warranted with mothers who express a lack of sleep and a lack of social support.
at an annual psychopharmacology update held by the Nevada Psychiatric Association. “This gives you information about depression and insomnia. Make sure to ask about anxiety symptoms. Also ask about any thoughts of suicide or harming the infant, and support from family and friends when she’s under stress and taking care of the baby.”
According to Dr. Friedman, a perinatal and forensic psychiatrist at Case Western Reserve University, Cleveland, social risk factors for postpartum depression (PPD) include being a victim of intimate partner violence and/or abuse, negative life events, decreased social support, relationship issues, and socioeconomic status. Psychological risk factors include anxiety/depression in pregnancy, personal or family history of PPD, and substance misuse. Biological risk factors include medical illness, multiple births, and having an infant with low birth weight/prematurity.
PPD affects 10%-20% of new mothers and peaks at 12 weeks. Postpartum psychosis, meanwhile, occurs in about 1-2 of every 1,000 deliveries. Anxiety comorbidity is common.
In the neonatal intensive care unit (NICU), PPD rates might increase from 28% to 70% depending on the study. Risk factors include personal or family history, disturbed relationships, unfavorable socioeconomic factors, and stressful life events. Obstetrical risk factors might include conception by assisted reproductive technologies and having a stillbirth in the year before conception. NICU-specific risk factors include less-effective coping strategies, greater perception of maternal role disruption, and decreased perception of nursing support. “A lot of mothers [in the NICU] talk to me about being on a roller roaster every day about what’s going to happen with their baby,” Dr. Friedman said.
The most widely used measure to screen for PPD is the 10-item self-rating Edinburgh Postnatal Depression Scale . A total score of 10 or more is considered a flag for the need to follow up for possible depressive symptoms. She advises clinicians to pay particular attention to how patients respond to item No. 10 on the scale, which reads, “The thought of harming myself has occurred to me.” (Optional answers range from “Yes, quite often” to “Never.”) She also recommends administering the screen at both pediatric and obstetrical office visits, “because mothers are more likely to attend a pediatrics appointment than her own [postpartum] follow-up.”
The differential diagnosis of PPD includes the baby blues, postpartum psychosis, postpartum anxiety/PTSD, medical causes, substance use disorder, and PPD in bipolar disorder. Baby blues is not synonymous with PPD. It affects the majority (50%-80%) of new mothers and is characterized by emotional sensitivity, mood lability, and irritability. It usually occurs within 5 days and resolves by the second week post partum.
Postpartum psychosis (PPP) occurs in about 1-2 of every 1,000 deliveries, typically in the first 2 weeks after delivery. The onset occurs rapidly, and PPP is most frequently correlated with bipolar disorder over time. PPP itself is characterized by grandiose bizarre delusions, mood lability, hallucinations, confusion, and disorganized behavior. “This can occur as a new onset of mental illness as well, so getting collateral information about her behaviors is important,” she said.
Dr. Friedman explained that those events occur post partum largely because of sleep deprivation and increasing stress as the woman adjusts to a mothering role. Hormonal shifts also occur, with a drop in estrogen levels. Obstetrical complications also might factor in.
Postpartum obsessive-compulsive disorder (OCD) is commonly comorbid with PPD and is distinguished by ego-dystonic intrusive thoughts. The mother might have intense distress that she is going to harm the infant and might start to avoid holding the baby out of concern. “Common things I’ve heard from women with postpartum OCD are: ‘I’m afraid I’m going to put the baby in the microwave or in the oven instead of dinner’ or ‘I’m afraid I’m going to leave the baby in the car overnight and she’ll freeze to death,’ ” she said.
Postpartum PTSD can be triggered by a traumatic event experience in the birthing process, such as an emergency C-section. Affected mothers avoid the infant and hospital, “reexperience” the trauma, are easily startled, irritable, and disconnected. Dr. Friedman also noted that early parental PTSD symptoms predict sleep and eating problems in childhood and less sensitive/more controlling maternal behaviors.
Medical conditions that mimic PPD include anemia, thyroid disease, hypoactive delirium, infections, and alcohol/substance use disorder.
The best available data show that mothers with PPD are more withdrawn, disengaged, display more hostility, and are more likely to have disrupted attachment with their babies, Dr. Friedman said. They also are less likely to employ healthy child development practices and to breastfeed. Untreated depression might lead to psychotic symptoms, suicide, or homicide. Paternal PPD also occurs in an estimated 10% of fathers and is moderately correlated with maternal PPD.
Potential risks of PPD include impaired bonding, attachment disturbance, language development, cognitive skills, and behavior problems.
Potential risks of untreated PPD include child neglect or abuse because of active symptoms, suicide, and psychotic or maltreatment-related infanticide. “If the mother is taking about harming herself, I often ask: ‘Have you thought of what would happen to your baby if you were to take your own life?’ ” Dr. Friedman offered. Peripartum suicide risk is lower than in the general female population, but it represents about 20% of peripartum deaths. Overdose is the most common method. “However, uncommon and dramatic methods are more common in this population,” she said. “Teens and stigmatized single mothers are at greater risk.”
Dr. Friedman noted that clinicians face risk of a malpractice lawsuit if they fail to treat, abandon the patient, fail to provide informed consent, and if there are bad outcomes. The best approach is to proactively communicate with the patient, partner, pediatrics, and obstetrics. “Conduct an individual risk-benefit assessment with the individual patient’s history,” she advised. “Don’t do anything knee jerk. Consult when needed, document, and consider lactation and future pregnancy possibility in women of reproductive age.”
Nonpharmacologic therapy might be the first line of treatment for mild to moderate symptoms. Options include cognitive-behavioral therapy, interpersonal psychotherapy, family therapy, psychodynamic psychotherapy, and supportive psychotherapy. She recommends close follow-up and conducting a careful medication history. Electroconvulsive therapy remains a possibility.
If medication use is warranted, “weigh the benefits of breastfeeding with the usually low drug exposure of the infant,” Dr. Friedman advised. “We want to use the least number of medications at an effective dose to optimize treatment. Newer medications have less perinatal data. Sertraline and paroxetine are usually the preferred selective serotonin reuptake inhibitors in lactation. However, fluoxetine or citalopram might be used depending on the patient’s response history/use in pregnancy.”
Dr. Friedman reported no disclosures.
LAS VEGAS – Diagnosing postpartum depression can be tricky because of the wide range of body changes that occur during the postpartum period, but vigilance is warranted with mothers who express a lack of sleep and a lack of social support.
at an annual psychopharmacology update held by the Nevada Psychiatric Association. “This gives you information about depression and insomnia. Make sure to ask about anxiety symptoms. Also ask about any thoughts of suicide or harming the infant, and support from family and friends when she’s under stress and taking care of the baby.”
According to Dr. Friedman, a perinatal and forensic psychiatrist at Case Western Reserve University, Cleveland, social risk factors for postpartum depression (PPD) include being a victim of intimate partner violence and/or abuse, negative life events, decreased social support, relationship issues, and socioeconomic status. Psychological risk factors include anxiety/depression in pregnancy, personal or family history of PPD, and substance misuse. Biological risk factors include medical illness, multiple births, and having an infant with low birth weight/prematurity.
PPD affects 10%-20% of new mothers and peaks at 12 weeks. Postpartum psychosis, meanwhile, occurs in about 1-2 of every 1,000 deliveries. Anxiety comorbidity is common.
In the neonatal intensive care unit (NICU), PPD rates might increase from 28% to 70% depending on the study. Risk factors include personal or family history, disturbed relationships, unfavorable socioeconomic factors, and stressful life events. Obstetrical risk factors might include conception by assisted reproductive technologies and having a stillbirth in the year before conception. NICU-specific risk factors include less-effective coping strategies, greater perception of maternal role disruption, and decreased perception of nursing support. “A lot of mothers [in the NICU] talk to me about being on a roller roaster every day about what’s going to happen with their baby,” Dr. Friedman said.
The most widely used measure to screen for PPD is the 10-item self-rating Edinburgh Postnatal Depression Scale . A total score of 10 or more is considered a flag for the need to follow up for possible depressive symptoms. She advises clinicians to pay particular attention to how patients respond to item No. 10 on the scale, which reads, “The thought of harming myself has occurred to me.” (Optional answers range from “Yes, quite often” to “Never.”) She also recommends administering the screen at both pediatric and obstetrical office visits, “because mothers are more likely to attend a pediatrics appointment than her own [postpartum] follow-up.”
The differential diagnosis of PPD includes the baby blues, postpartum psychosis, postpartum anxiety/PTSD, medical causes, substance use disorder, and PPD in bipolar disorder. Baby blues is not synonymous with PPD. It affects the majority (50%-80%) of new mothers and is characterized by emotional sensitivity, mood lability, and irritability. It usually occurs within 5 days and resolves by the second week post partum.
Postpartum psychosis (PPP) occurs in about 1-2 of every 1,000 deliveries, typically in the first 2 weeks after delivery. The onset occurs rapidly, and PPP is most frequently correlated with bipolar disorder over time. PPP itself is characterized by grandiose bizarre delusions, mood lability, hallucinations, confusion, and disorganized behavior. “This can occur as a new onset of mental illness as well, so getting collateral information about her behaviors is important,” she said.
Dr. Friedman explained that those events occur post partum largely because of sleep deprivation and increasing stress as the woman adjusts to a mothering role. Hormonal shifts also occur, with a drop in estrogen levels. Obstetrical complications also might factor in.
Postpartum obsessive-compulsive disorder (OCD) is commonly comorbid with PPD and is distinguished by ego-dystonic intrusive thoughts. The mother might have intense distress that she is going to harm the infant and might start to avoid holding the baby out of concern. “Common things I’ve heard from women with postpartum OCD are: ‘I’m afraid I’m going to put the baby in the microwave or in the oven instead of dinner’ or ‘I’m afraid I’m going to leave the baby in the car overnight and she’ll freeze to death,’ ” she said.
Postpartum PTSD can be triggered by a traumatic event experience in the birthing process, such as an emergency C-section. Affected mothers avoid the infant and hospital, “reexperience” the trauma, are easily startled, irritable, and disconnected. Dr. Friedman also noted that early parental PTSD symptoms predict sleep and eating problems in childhood and less sensitive/more controlling maternal behaviors.
Medical conditions that mimic PPD include anemia, thyroid disease, hypoactive delirium, infections, and alcohol/substance use disorder.
The best available data show that mothers with PPD are more withdrawn, disengaged, display more hostility, and are more likely to have disrupted attachment with their babies, Dr. Friedman said. They also are less likely to employ healthy child development practices and to breastfeed. Untreated depression might lead to psychotic symptoms, suicide, or homicide. Paternal PPD also occurs in an estimated 10% of fathers and is moderately correlated with maternal PPD.
Potential risks of PPD include impaired bonding, attachment disturbance, language development, cognitive skills, and behavior problems.
Potential risks of untreated PPD include child neglect or abuse because of active symptoms, suicide, and psychotic or maltreatment-related infanticide. “If the mother is taking about harming herself, I often ask: ‘Have you thought of what would happen to your baby if you were to take your own life?’ ” Dr. Friedman offered. Peripartum suicide risk is lower than in the general female population, but it represents about 20% of peripartum deaths. Overdose is the most common method. “However, uncommon and dramatic methods are more common in this population,” she said. “Teens and stigmatized single mothers are at greater risk.”
Dr. Friedman noted that clinicians face risk of a malpractice lawsuit if they fail to treat, abandon the patient, fail to provide informed consent, and if there are bad outcomes. The best approach is to proactively communicate with the patient, partner, pediatrics, and obstetrics. “Conduct an individual risk-benefit assessment with the individual patient’s history,” she advised. “Don’t do anything knee jerk. Consult when needed, document, and consider lactation and future pregnancy possibility in women of reproductive age.”
Nonpharmacologic therapy might be the first line of treatment for mild to moderate symptoms. Options include cognitive-behavioral therapy, interpersonal psychotherapy, family therapy, psychodynamic psychotherapy, and supportive psychotherapy. She recommends close follow-up and conducting a careful medication history. Electroconvulsive therapy remains a possibility.
If medication use is warranted, “weigh the benefits of breastfeeding with the usually low drug exposure of the infant,” Dr. Friedman advised. “We want to use the least number of medications at an effective dose to optimize treatment. Newer medications have less perinatal data. Sertraline and paroxetine are usually the preferred selective serotonin reuptake inhibitors in lactation. However, fluoxetine or citalopram might be used depending on the patient’s response history/use in pregnancy.”
Dr. Friedman reported no disclosures.
EXPERT ANALYSIS FROM NPA 2019
Statin adherence lower in women, minorities
Women, younger patients, and individuals from minority groups are significantly less likely to be adherent with their statins, and are at greater risk of hospitalization and death, a study has found.
In JAMA Cardiology, researchers report the outcomes of a retrospective cohort analysis involving 347,104 adults with atherosclerotic cardiovascular disease and stable statin prescriptions, who were treated within the Veterans Affairs Health System.
Statin adherence – defined as a medication possession ratio of 80% or above – was 87.7%. Patients on a moderate intensity of statin therapy were slightly more adherent than were patients on low- or high-intensity therapy.
The lowest levels of adherence were seen in the youngest patients. Those aged under 35 years had a 60% lower likelihood of adherence compared with the reference group aged 65-74 years, and those aged 35-44 years had a 47% lower likelihood of adherence. From age 55 on, adherence improved.
Women were 11% less likely to be adherent to statin therapy than were men. Adherence was significantly different among persons of different racial backgrounds and ethnicities: black patients were 42% less likely to be adherent compared with non-Hispanic whites, Asian patients were 18% less likely to be adherent, and Hispanic patients were 27% less likely to be adherent.
. Among patients with a medication possession ratio less than 50%, 13.4% were hospitalized for ischemic heart disease or ischemic stroke, compared with 11.5% of patients who had a medication possession ratio of 90% or above, even after adjustment for baseline characteristics.
Researchers saw a dose-response association between lower adherence and higher mortality. The incidence of death in the first year was 8.8% in patients with a medication possession ration below 50%, 7.5% for those with a ratio of 50%-69%, 6.3% for those with a ratio between 70% and 89%, and 5.7% among those with a medication possession ratio at or above 90%.
This effect was slightly attenuated by adjustment for adherence to other cardiac medications, but the association remained significant.
“Although statins are among the most effective drugs for the secondary prevention of [atherosclerotic cardiovascular disease], low adherence is a common problem,” wrote Dr. Fatima Rodriguez of Stanford (Calif.) University’s Division of Cardiovascular Medicine, and her coauthors. “Minorities have not been well represented in statin-related trials and more work is needed to implement strategies to improve guideline adherence in these populations.”
Stanford’s Division of Cardiovascular Medicine supported the study. The investigators have received funding from the Doris Duke Charitable Trust, the Department of Veterans Affairs Health Services Research and Development Service, the National Lipid Association, and Duke Clinical Research Institute.
SOURCE: Rodriguez F et al. JAMA Cardiol. 2019 Feb 13. doi: 10.1001/jamacardio.2018.4936.
Women, younger patients, and individuals from minority groups are significantly less likely to be adherent with their statins, and are at greater risk of hospitalization and death, a study has found.
In JAMA Cardiology, researchers report the outcomes of a retrospective cohort analysis involving 347,104 adults with atherosclerotic cardiovascular disease and stable statin prescriptions, who were treated within the Veterans Affairs Health System.
Statin adherence – defined as a medication possession ratio of 80% or above – was 87.7%. Patients on a moderate intensity of statin therapy were slightly more adherent than were patients on low- or high-intensity therapy.
The lowest levels of adherence were seen in the youngest patients. Those aged under 35 years had a 60% lower likelihood of adherence compared with the reference group aged 65-74 years, and those aged 35-44 years had a 47% lower likelihood of adherence. From age 55 on, adherence improved.
Women were 11% less likely to be adherent to statin therapy than were men. Adherence was significantly different among persons of different racial backgrounds and ethnicities: black patients were 42% less likely to be adherent compared with non-Hispanic whites, Asian patients were 18% less likely to be adherent, and Hispanic patients were 27% less likely to be adherent.
. Among patients with a medication possession ratio less than 50%, 13.4% were hospitalized for ischemic heart disease or ischemic stroke, compared with 11.5% of patients who had a medication possession ratio of 90% or above, even after adjustment for baseline characteristics.
Researchers saw a dose-response association between lower adherence and higher mortality. The incidence of death in the first year was 8.8% in patients with a medication possession ration below 50%, 7.5% for those with a ratio of 50%-69%, 6.3% for those with a ratio between 70% and 89%, and 5.7% among those with a medication possession ratio at or above 90%.
This effect was slightly attenuated by adjustment for adherence to other cardiac medications, but the association remained significant.
“Although statins are among the most effective drugs for the secondary prevention of [atherosclerotic cardiovascular disease], low adherence is a common problem,” wrote Dr. Fatima Rodriguez of Stanford (Calif.) University’s Division of Cardiovascular Medicine, and her coauthors. “Minorities have not been well represented in statin-related trials and more work is needed to implement strategies to improve guideline adherence in these populations.”
Stanford’s Division of Cardiovascular Medicine supported the study. The investigators have received funding from the Doris Duke Charitable Trust, the Department of Veterans Affairs Health Services Research and Development Service, the National Lipid Association, and Duke Clinical Research Institute.
SOURCE: Rodriguez F et al. JAMA Cardiol. 2019 Feb 13. doi: 10.1001/jamacardio.2018.4936.
Women, younger patients, and individuals from minority groups are significantly less likely to be adherent with their statins, and are at greater risk of hospitalization and death, a study has found.
In JAMA Cardiology, researchers report the outcomes of a retrospective cohort analysis involving 347,104 adults with atherosclerotic cardiovascular disease and stable statin prescriptions, who were treated within the Veterans Affairs Health System.
Statin adherence – defined as a medication possession ratio of 80% or above – was 87.7%. Patients on a moderate intensity of statin therapy were slightly more adherent than were patients on low- or high-intensity therapy.
The lowest levels of adherence were seen in the youngest patients. Those aged under 35 years had a 60% lower likelihood of adherence compared with the reference group aged 65-74 years, and those aged 35-44 years had a 47% lower likelihood of adherence. From age 55 on, adherence improved.
Women were 11% less likely to be adherent to statin therapy than were men. Adherence was significantly different among persons of different racial backgrounds and ethnicities: black patients were 42% less likely to be adherent compared with non-Hispanic whites, Asian patients were 18% less likely to be adherent, and Hispanic patients were 27% less likely to be adherent.
. Among patients with a medication possession ratio less than 50%, 13.4% were hospitalized for ischemic heart disease or ischemic stroke, compared with 11.5% of patients who had a medication possession ratio of 90% or above, even after adjustment for baseline characteristics.
Researchers saw a dose-response association between lower adherence and higher mortality. The incidence of death in the first year was 8.8% in patients with a medication possession ration below 50%, 7.5% for those with a ratio of 50%-69%, 6.3% for those with a ratio between 70% and 89%, and 5.7% among those with a medication possession ratio at or above 90%.
This effect was slightly attenuated by adjustment for adherence to other cardiac medications, but the association remained significant.
“Although statins are among the most effective drugs for the secondary prevention of [atherosclerotic cardiovascular disease], low adherence is a common problem,” wrote Dr. Fatima Rodriguez of Stanford (Calif.) University’s Division of Cardiovascular Medicine, and her coauthors. “Minorities have not been well represented in statin-related trials and more work is needed to implement strategies to improve guideline adherence in these populations.”
Stanford’s Division of Cardiovascular Medicine supported the study. The investigators have received funding from the Doris Duke Charitable Trust, the Department of Veterans Affairs Health Services Research and Development Service, the National Lipid Association, and Duke Clinical Research Institute.
SOURCE: Rodriguez F et al. JAMA Cardiol. 2019 Feb 13. doi: 10.1001/jamacardio.2018.4936.
FROM JAMA CARDIOLOGY
Key clinical point: Women, nonwhite, and younger patients are significantly less likely to be adherent to statin therapy.
Major finding: Women are 11% less likely than are men to adhere to statin therapy.
Study details: Retrospective cohort analysis involving 347,104 adults with atherosclerotic cardiovascular disease.
Disclosures: The Division of Cardiovascular Medicine at Stanford (Calif.) University supported the study. The investigators have received funding from the Doris Duke Charitable Trust, the Department of Veterans Affairs Health Services Research and Development Service, the National Lipid Association, and Duke Clinical Research Institute.
Source: Rodriguez F et al. JAMA Cardiol. 2019, Feb 13. doi: 10.1001/jamacardio.2018.4936.
Tranexamic acid shows improvements in heavy menstrual bleeding
new research suggests.
Writing in the Journal of Pediatric & Adolescent Gynecology, Sarah H. O’Brien, MD, from Nationwide Children’s Hospital and the Ohio State University, both in Columbus, and her coauthors presented the results of an open-label efficacy study of the competitive plasminogen inhibitor in 25 adolescent girls aged 10-19 years who attended pediatric hematology clinics for evaluation or management of heavy menstrual bleeding. The study participants were instructed to take 1,300 mg of tranexamic acid (two tablets) three times a day for up to 5 days during their monthly menstruation for three cycles.
The study found a significant improvement in mean menstrual impact questionnaire (MIQ) scores, which improved from a mean of 3 at baseline to 1.91 (P less than .001). Two-thirds of patients reported at least a one-point improvement from baseline, and all reported that this was clinically meaningful. At baseline, 84% of patients reported heavy to very heavy blood loss, but this decreased to 23% after treatment with tranexamic acid (P less than .001).
The study population included ten individuals (40%) with bleeding disorders. However, the researchers did not see a significant difference in response between those with bleeding disorders and those without.
While the treatment did not significantly affect school attendance (only 24% reported that their heavy bleeding limited school attendance), researchers did see a significant improvement in limitations on physical activities and on social and leisure activities. Patients who reported at baseline that their menstrual bleeding significantly affected their social and leisure activities had an average score improvement of 1.74, a greater than or equal to one point improvement. Participants also reported significant improvements in their Pictorial Blood Assessment Chart scores, which dropped from an average of 255 to 155 (P less than .001).
The treatment did not show any significant effects on hemoglobin or ferritin. The most common adverse events were sinonasal symptoms, such as nasal congestion, headache, and sinus pain, but no thrombotic or ocular adverse events were seen.
Dr. O’Brien and her coauthors wrote that one limitation of their study was using the MIQ score as their primary endpoint as opposed to a more objective measure, such as change in measured blood loss.
“However, a major factor that motivates patients with heavy menstrual bleeding to seek medical care is the negative impact of heavy menstrual bleeding on daily life,” they wrote.
The study drug was supplied by Ferring pharmaceuticals, and the study was supported by the Hemostasis and Thrombosis Research Society. One author disclosed receiving the Joan Fellowship in Pediatric Hemostasis and Thrombosis at Nationwide Children’s Hospital; no other authors said they had relevant financial disclosures.
SOURCE: O’Brien SH et al. J Pediatr Adol Gynec. 2019 Feb 4. doi: 10.1016/j.jpag.2019.01.009.
new research suggests.
Writing in the Journal of Pediatric & Adolescent Gynecology, Sarah H. O’Brien, MD, from Nationwide Children’s Hospital and the Ohio State University, both in Columbus, and her coauthors presented the results of an open-label efficacy study of the competitive plasminogen inhibitor in 25 adolescent girls aged 10-19 years who attended pediatric hematology clinics for evaluation or management of heavy menstrual bleeding. The study participants were instructed to take 1,300 mg of tranexamic acid (two tablets) three times a day for up to 5 days during their monthly menstruation for three cycles.
The study found a significant improvement in mean menstrual impact questionnaire (MIQ) scores, which improved from a mean of 3 at baseline to 1.91 (P less than .001). Two-thirds of patients reported at least a one-point improvement from baseline, and all reported that this was clinically meaningful. At baseline, 84% of patients reported heavy to very heavy blood loss, but this decreased to 23% after treatment with tranexamic acid (P less than .001).
The study population included ten individuals (40%) with bleeding disorders. However, the researchers did not see a significant difference in response between those with bleeding disorders and those without.
While the treatment did not significantly affect school attendance (only 24% reported that their heavy bleeding limited school attendance), researchers did see a significant improvement in limitations on physical activities and on social and leisure activities. Patients who reported at baseline that their menstrual bleeding significantly affected their social and leisure activities had an average score improvement of 1.74, a greater than or equal to one point improvement. Participants also reported significant improvements in their Pictorial Blood Assessment Chart scores, which dropped from an average of 255 to 155 (P less than .001).
The treatment did not show any significant effects on hemoglobin or ferritin. The most common adverse events were sinonasal symptoms, such as nasal congestion, headache, and sinus pain, but no thrombotic or ocular adverse events were seen.
Dr. O’Brien and her coauthors wrote that one limitation of their study was using the MIQ score as their primary endpoint as opposed to a more objective measure, such as change in measured blood loss.
“However, a major factor that motivates patients with heavy menstrual bleeding to seek medical care is the negative impact of heavy menstrual bleeding on daily life,” they wrote.
The study drug was supplied by Ferring pharmaceuticals, and the study was supported by the Hemostasis and Thrombosis Research Society. One author disclosed receiving the Joan Fellowship in Pediatric Hemostasis and Thrombosis at Nationwide Children’s Hospital; no other authors said they had relevant financial disclosures.
SOURCE: O’Brien SH et al. J Pediatr Adol Gynec. 2019 Feb 4. doi: 10.1016/j.jpag.2019.01.009.
new research suggests.
Writing in the Journal of Pediatric & Adolescent Gynecology, Sarah H. O’Brien, MD, from Nationwide Children’s Hospital and the Ohio State University, both in Columbus, and her coauthors presented the results of an open-label efficacy study of the competitive plasminogen inhibitor in 25 adolescent girls aged 10-19 years who attended pediatric hematology clinics for evaluation or management of heavy menstrual bleeding. The study participants were instructed to take 1,300 mg of tranexamic acid (two tablets) three times a day for up to 5 days during their monthly menstruation for three cycles.
The study found a significant improvement in mean menstrual impact questionnaire (MIQ) scores, which improved from a mean of 3 at baseline to 1.91 (P less than .001). Two-thirds of patients reported at least a one-point improvement from baseline, and all reported that this was clinically meaningful. At baseline, 84% of patients reported heavy to very heavy blood loss, but this decreased to 23% after treatment with tranexamic acid (P less than .001).
The study population included ten individuals (40%) with bleeding disorders. However, the researchers did not see a significant difference in response between those with bleeding disorders and those without.
While the treatment did not significantly affect school attendance (only 24% reported that their heavy bleeding limited school attendance), researchers did see a significant improvement in limitations on physical activities and on social and leisure activities. Patients who reported at baseline that their menstrual bleeding significantly affected their social and leisure activities had an average score improvement of 1.74, a greater than or equal to one point improvement. Participants also reported significant improvements in their Pictorial Blood Assessment Chart scores, which dropped from an average of 255 to 155 (P less than .001).
The treatment did not show any significant effects on hemoglobin or ferritin. The most common adverse events were sinonasal symptoms, such as nasal congestion, headache, and sinus pain, but no thrombotic or ocular adverse events were seen.
Dr. O’Brien and her coauthors wrote that one limitation of their study was using the MIQ score as their primary endpoint as opposed to a more objective measure, such as change in measured blood loss.
“However, a major factor that motivates patients with heavy menstrual bleeding to seek medical care is the negative impact of heavy menstrual bleeding on daily life,” they wrote.
The study drug was supplied by Ferring pharmaceuticals, and the study was supported by the Hemostasis and Thrombosis Research Society. One author disclosed receiving the Joan Fellowship in Pediatric Hemostasis and Thrombosis at Nationwide Children’s Hospital; no other authors said they had relevant financial disclosures.
SOURCE: O’Brien SH et al. J Pediatr Adol Gynec. 2019 Feb 4. doi: 10.1016/j.jpag.2019.01.009.
FROM THE JOURNAL OF PEDIATRIC & ADOLESCENT GYNECOLOGY
Key clinical point: Tranexamic acid appears to improve quality of life for adolescents with heavy menstrual bleeding.
Major finding: Patients treated with tranexamic acid reported significant improvements in mean menstrual impact questionnaire scores.
Study details: Open-label efficacy study in 25 adolescent girls with heavy menstrual bleeding.
Disclosures: The study drug was supplied by Ferring pharmaceuticals, and the study was supported by the Hemostasis and Thrombosis Research Society. One author disclosed receiving the Joan Fellowship in Pediatric Hemostasis and Thrombosis at Nationwide Children’s Hospital; no other authors said they had relevant financial disclosures.
Source: O’Brien SH et al. J Pediatr Adol Gynec. 2019 Feb 4. doi: 10.1016/j.jpag.2019.01.009.
Survey: Reproductive counseling is often MIA in IBD
LAS VEGAS – Inflammatory bowel disease (IBD) can disrupt both fertility and pregnancy, especially if it’s not fully controlled, and there’s a risk that the condition can be passed onto an unborn child. Still a new study suggests many patients with IBD don’t receive appropriate reproductive counseling.
Nearly two-thirds of 100 patients surveyed at a single center reported that no physician had talked to them about reproductive topics, and some said they’d considered not having children because of the condition. “Really fundamental subjects have not made their way into the interactions between patients and their care teams,” coauthor and gastroenterologist Sarah Streett, MD, AGAF, of Stanford (Calif.) University, said in an interview before the study was presented at the annual congress of the Crohn’s & Colitis Foundation and the American Gastroenterological Association.
IBD can lower fertility in both sexes and boost complications in pregnancy. “The good news is that almost all the medications used for IBD appear safe,” Dr. Streett said. “In fact, the safety risks for the baby and the pregnancy revolve around not having IBD under good control.”
Unfortunately, she said, misinformation is common. “Patients who become pregnant or are trying to become pregnant, and are worried about potential harm to the baby, will stop the medications due to incorrect information. Or they’ll be told by their health care team to stop their medications.”
Dr. Streett and study lead author Aarti Rao, MD, a gastroenterology fellow at Stanford, launched their study of IBD clinic patients to gain more understanding about patient knowledge. “We know from research already published that those with IBD have a lot of concerns about starting families and don’t have a lot of information to base their decision making on,” Dr. Streett said. “We wanted to evaluate that in our population and see how much people knew and what the need was.”
In 2018 and 2019, Dr. Streett and Dr. Rao gave an anonymous, validated 17-question survey to patients aged 18-45 with IBD. One hundred patients responded (median age = 30, 54% female, 59% white, 66% with incomes over $100,000, 52% with ulcerative colitis, 21% with prior IBD surgery, 71% with prior IBD hospitalization).
Just over a third – 35% – of the patients said they’d been counseled about reproductive health by a physician. This finding reflects findings in previous research, said Dr. Rao, who spoke in an interview.
Just 15% of those who’d undergone IBD surgery reported getting guidance about the effects of surgery on fertility.
More than a third (35%) of women and 15% of men said they’d considered not having children because of their IBD. In fact, “most potential dads and moms have the chance to do very well,” Dr. Streett said.
Without reproductive counseling, she added, parents won’t know about the risks of passing on IBD. According to Dr. Rao, there’s an estimated less than 5% chance that IBD will be passed on to children if one parent has the condition; the risk is much higher if both parents have it.
Going forward, “there’s a really urgent need for proactive counseling on the part of gastroenterologists and health care teams to give people of childbearing age the right information so they can be informed to make the best decisions,” Dr. Streett said.
The study was funded by a philanthropic grant. The study authors report no relevant disclosures.
SOURCE: Rao A et al. Crohn’s & Colitis Congress, Abstract P009.
LAS VEGAS – Inflammatory bowel disease (IBD) can disrupt both fertility and pregnancy, especially if it’s not fully controlled, and there’s a risk that the condition can be passed onto an unborn child. Still a new study suggests many patients with IBD don’t receive appropriate reproductive counseling.
Nearly two-thirds of 100 patients surveyed at a single center reported that no physician had talked to them about reproductive topics, and some said they’d considered not having children because of the condition. “Really fundamental subjects have not made their way into the interactions between patients and their care teams,” coauthor and gastroenterologist Sarah Streett, MD, AGAF, of Stanford (Calif.) University, said in an interview before the study was presented at the annual congress of the Crohn’s & Colitis Foundation and the American Gastroenterological Association.
IBD can lower fertility in both sexes and boost complications in pregnancy. “The good news is that almost all the medications used for IBD appear safe,” Dr. Streett said. “In fact, the safety risks for the baby and the pregnancy revolve around not having IBD under good control.”
Unfortunately, she said, misinformation is common. “Patients who become pregnant or are trying to become pregnant, and are worried about potential harm to the baby, will stop the medications due to incorrect information. Or they’ll be told by their health care team to stop their medications.”
Dr. Streett and study lead author Aarti Rao, MD, a gastroenterology fellow at Stanford, launched their study of IBD clinic patients to gain more understanding about patient knowledge. “We know from research already published that those with IBD have a lot of concerns about starting families and don’t have a lot of information to base their decision making on,” Dr. Streett said. “We wanted to evaluate that in our population and see how much people knew and what the need was.”
In 2018 and 2019, Dr. Streett and Dr. Rao gave an anonymous, validated 17-question survey to patients aged 18-45 with IBD. One hundred patients responded (median age = 30, 54% female, 59% white, 66% with incomes over $100,000, 52% with ulcerative colitis, 21% with prior IBD surgery, 71% with prior IBD hospitalization).
Just over a third – 35% – of the patients said they’d been counseled about reproductive health by a physician. This finding reflects findings in previous research, said Dr. Rao, who spoke in an interview.
Just 15% of those who’d undergone IBD surgery reported getting guidance about the effects of surgery on fertility.
More than a third (35%) of women and 15% of men said they’d considered not having children because of their IBD. In fact, “most potential dads and moms have the chance to do very well,” Dr. Streett said.
Without reproductive counseling, she added, parents won’t know about the risks of passing on IBD. According to Dr. Rao, there’s an estimated less than 5% chance that IBD will be passed on to children if one parent has the condition; the risk is much higher if both parents have it.
Going forward, “there’s a really urgent need for proactive counseling on the part of gastroenterologists and health care teams to give people of childbearing age the right information so they can be informed to make the best decisions,” Dr. Streett said.
The study was funded by a philanthropic grant. The study authors report no relevant disclosures.
SOURCE: Rao A et al. Crohn’s & Colitis Congress, Abstract P009.
LAS VEGAS – Inflammatory bowel disease (IBD) can disrupt both fertility and pregnancy, especially if it’s not fully controlled, and there’s a risk that the condition can be passed onto an unborn child. Still a new study suggests many patients with IBD don’t receive appropriate reproductive counseling.
Nearly two-thirds of 100 patients surveyed at a single center reported that no physician had talked to them about reproductive topics, and some said they’d considered not having children because of the condition. “Really fundamental subjects have not made their way into the interactions between patients and their care teams,” coauthor and gastroenterologist Sarah Streett, MD, AGAF, of Stanford (Calif.) University, said in an interview before the study was presented at the annual congress of the Crohn’s & Colitis Foundation and the American Gastroenterological Association.
IBD can lower fertility in both sexes and boost complications in pregnancy. “The good news is that almost all the medications used for IBD appear safe,” Dr. Streett said. “In fact, the safety risks for the baby and the pregnancy revolve around not having IBD under good control.”
Unfortunately, she said, misinformation is common. “Patients who become pregnant or are trying to become pregnant, and are worried about potential harm to the baby, will stop the medications due to incorrect information. Or they’ll be told by their health care team to stop their medications.”
Dr. Streett and study lead author Aarti Rao, MD, a gastroenterology fellow at Stanford, launched their study of IBD clinic patients to gain more understanding about patient knowledge. “We know from research already published that those with IBD have a lot of concerns about starting families and don’t have a lot of information to base their decision making on,” Dr. Streett said. “We wanted to evaluate that in our population and see how much people knew and what the need was.”
In 2018 and 2019, Dr. Streett and Dr. Rao gave an anonymous, validated 17-question survey to patients aged 18-45 with IBD. One hundred patients responded (median age = 30, 54% female, 59% white, 66% with incomes over $100,000, 52% with ulcerative colitis, 21% with prior IBD surgery, 71% with prior IBD hospitalization).
Just over a third – 35% – of the patients said they’d been counseled about reproductive health by a physician. This finding reflects findings in previous research, said Dr. Rao, who spoke in an interview.
Just 15% of those who’d undergone IBD surgery reported getting guidance about the effects of surgery on fertility.
More than a third (35%) of women and 15% of men said they’d considered not having children because of their IBD. In fact, “most potential dads and moms have the chance to do very well,” Dr. Streett said.
Without reproductive counseling, she added, parents won’t know about the risks of passing on IBD. According to Dr. Rao, there’s an estimated less than 5% chance that IBD will be passed on to children if one parent has the condition; the risk is much higher if both parents have it.
Going forward, “there’s a really urgent need for proactive counseling on the part of gastroenterologists and health care teams to give people of childbearing age the right information so they can be informed to make the best decisions,” Dr. Streett said.
The study was funded by a philanthropic grant. The study authors report no relevant disclosures.
SOURCE: Rao A et al. Crohn’s & Colitis Congress, Abstract P009.
REPORTING FROM THE CROHN’S & COLITIS CONGRESS
Key clinical point: Patients with inflammatory bowel disease aren’t getting proper guidance regarding fertility, pregnancy, and genetic risks.
Major finding: Among surveyed patients, 65% said they’d never received reproductive counseling from a physician.
Study details: Single-center survey of 100 patients (median age = 30, 54% female).
Disclosures: The study was funded by a philanthropic grant. The study authors report no relevant disclosures.
Source: Rao A et al. Crohn’s & Colitis Congress 2019, Abstract P009.