User login
USPSTF recommends counseling for perinatal depression prevention
according to a B recommendation from the U.S. Preventive Services Task Force.
The Task Force determined that counseling interventions are effective in preventing perinatal depression, defined as a major or minor depressive episode during pregnancy or within the first year after delivery. The condition affects an estimated 12% of new mothers in the United States each year, according to lead author Susan J. Curry, PhD, of the University of Iowa, Iowa City, and her colleagues.
The recommendation, published in JAMA, applies to “pregnant persons and persons who are less than 1 year postpartum who do not have a current diagnosis of depression but are at increased risk of developing depression,” according to the authors (JAMA. 2019 Feb 12;321(6):580-7).
Risk factors for development of perinatal depression include:
- Past history of depression.
- Current depressive symptoms (that do not reach a diagnostic threshold).
- History of physical or sexual abuse.
- Unplanned or unwanted pregnancy.
- Stressful life events.
- Lack of social and financial support.
- Intimate partner violence.
- Pregestational or gestational diabetes.
- Complications during pregnancy.
- Adolescent parenthood.
- Low socioeconomic status.
- Lack of social support.
After reviewing the evidence, the USPSTF found a moderate net benefit for counseling interventions, particularly cognitive behavioral therapy and interpersonal therapy, for preventing perinatal depression in women at risk. Counseling sessions reviewed for this recommendation ranged from 4 to 20 meetings (median, 8 meetings).
The USPSTF found inadequate evidence to assess the harms and benefits of other noncounseling interventions, including pharmacologic therapy.
In the evidence review accompanying the recommendations, Elizabeth A. O’Connor, PhD, of Kaiser Permanente, Portland, Ore., and her colleagues analyzed data from 50 studies including 22,385 individuals; 20 of these studies were randomized, controlled trials of counseling interventions (JAMA. 2019 Feb 12;321(6):588-601).
Overall, the likelihood of perinatal depression was significantly lower among women who received counseling, compared with controls, among more than 3,000 women in those studies (pooled risk ratio 0.61). Absolute risk differences for perinatal depression ranged from a 1% increased reduction in controls to a 32% increased reduction among women who received counseling. The effects were strongest for cognitive behavioral therapy and interpersonal therapy as interventions. No adverse events were reported in the counseling intervention studies.
In three studies of health system interventions, the researchers found a benefit for interventions vs. controls, but the difference was not statistically significant.
Trials of most other alternative interventions including infant sleep advice, birth-experience postpartum debriefing, omega-3 fatty acid supplementation, expressive writing, antidepressants, and yoga did not show statistical significance in benefit for reducing perinatal depression.
Only one of three randomized controlled trials of physical activity found a statistically significant group difference.
A trial of nortriptyline to prevent perinatal depression showed no benefit, compared with placebo. A sertraline study of found “a smaller percentage of participants taking sertraline had a depression recurrence, compared with those taking placebo,” the investigators wrote. In these two studies, women who took nortriptyline showed no adverse effects, and those in a trial involving sertraline reported significantly more dizziness and drowsiness compared with placebo patients.
The evidence review was limited by the small number of quality studies, especially studies of alternative interventions. More research is needed; however, the findings support data from similar reviews and support the potential for counseling to prevent perinatal depression, particularly in women at increased risk for perinatal depression, Dr. O’Connor and her associates said.
The USPSTF is supported by the Agency for Healthcare Research and Quality. Coauthor Dr. Michelle L. Henninger reported receiving grants from Pfizer IGLC (Independent Grants for Learning & Change) outside the submitted work. Coauthor Dr. Bradley N. Gaynes reported receiving personal fees from LivaNova and Johnson & Johnson outside the submitted work. The remaining researchers had no financial conflicts to disclose.
SOURCE: Curry SJ et al. JAMA. 2019;321(6):580-7; O’Connor E et al. JAMA. 2019;321(6):588-601.
A proactive approach to prevention and management of perinatal depression as recommended by the USPSTF can potentially improve outcomes for new mothers and their babies, Marlene P. Freeman, MD, wrote in an accompanying editorial. She identified three key challenges to implementing the USPSTF recommendations: identifying women at risk, connecting them to evidence-based treatment, and assessing outcomes after treatment.
The development of screening tools would help clinicians identify women at risk for perinatal depression, Dr. Freeman said. No such tool currently exists, but in the meantime, “women at risk may be identified by targeting those with histories of depression, subthreshold depressive symptoms, and certain sociodemographic factors (i.e., economically disadvantaged, single/young, unplanned pregnancy).”
The counseling interventions shown to be effective in preventing perinatal depression – cognitive behavioral therapy and interpersonal psychotherapy – require education and training outside the time limitations and expertise of many clinicians providing obstetric care, she noted.
“The delivery of effective care on a large scale will require creative solutions,” such as the use of telehealth and smartphone platforms, and the involvement of multidisciplinary teams to care for women with severe illness, Dr. Freeman said. “In addition, a substantial number of reproductive-aged women have serious psychiatric disorders and will be identified as at risk for perinatal depression, although their needs may be more comprehensive,” she wrote. “Women who are identified as at risk for perinatal depression may have psychotic disorders, bipolar spectrum disorders, anxiety disorders, and substance use disorders, and there is comorbidity among psychiatric disorders. Therefore, systematic provisions for referral and treatment for other psychiatric disorders should be considered.” Further research is needed to explore treatment options including pharmacotherapy for women with severe psychiatric disorders.
However, she expressed optimism that the recommendations for screening and counseling for perinatal depression are valuable, and they “may return great dividends in the form of enhanced well-being of mothers and their offspring.”
Dr. Freeman is affiliated with the department of psychiatry at Massachusetts General Hospital, Boston. She commented in an editorial accompanying the article by Curry et al. (JAMA. 2019 Feb 12;321[6]:550-2). Dr. Freeman disclosed relationships with companies including Takeda, JayMac, Sage, Otsuka, Alkermes, Janssen, and Sunovion; she also disclosed serving on an independent data safety and monitoring committee for Janssen (Johnson & Johnson); and editing the GOED (Global Organization for EPA & DHA Omega-3) newsletter.
A proactive approach to prevention and management of perinatal depression as recommended by the USPSTF can potentially improve outcomes for new mothers and their babies, Marlene P. Freeman, MD, wrote in an accompanying editorial. She identified three key challenges to implementing the USPSTF recommendations: identifying women at risk, connecting them to evidence-based treatment, and assessing outcomes after treatment.
The development of screening tools would help clinicians identify women at risk for perinatal depression, Dr. Freeman said. No such tool currently exists, but in the meantime, “women at risk may be identified by targeting those with histories of depression, subthreshold depressive symptoms, and certain sociodemographic factors (i.e., economically disadvantaged, single/young, unplanned pregnancy).”
The counseling interventions shown to be effective in preventing perinatal depression – cognitive behavioral therapy and interpersonal psychotherapy – require education and training outside the time limitations and expertise of many clinicians providing obstetric care, she noted.
“The delivery of effective care on a large scale will require creative solutions,” such as the use of telehealth and smartphone platforms, and the involvement of multidisciplinary teams to care for women with severe illness, Dr. Freeman said. “In addition, a substantial number of reproductive-aged women have serious psychiatric disorders and will be identified as at risk for perinatal depression, although their needs may be more comprehensive,” she wrote. “Women who are identified as at risk for perinatal depression may have psychotic disorders, bipolar spectrum disorders, anxiety disorders, and substance use disorders, and there is comorbidity among psychiatric disorders. Therefore, systematic provisions for referral and treatment for other psychiatric disorders should be considered.” Further research is needed to explore treatment options including pharmacotherapy for women with severe psychiatric disorders.
However, she expressed optimism that the recommendations for screening and counseling for perinatal depression are valuable, and they “may return great dividends in the form of enhanced well-being of mothers and their offspring.”
Dr. Freeman is affiliated with the department of psychiatry at Massachusetts General Hospital, Boston. She commented in an editorial accompanying the article by Curry et al. (JAMA. 2019 Feb 12;321[6]:550-2). Dr. Freeman disclosed relationships with companies including Takeda, JayMac, Sage, Otsuka, Alkermes, Janssen, and Sunovion; she also disclosed serving on an independent data safety and monitoring committee for Janssen (Johnson & Johnson); and editing the GOED (Global Organization for EPA & DHA Omega-3) newsletter.
A proactive approach to prevention and management of perinatal depression as recommended by the USPSTF can potentially improve outcomes for new mothers and their babies, Marlene P. Freeman, MD, wrote in an accompanying editorial. She identified three key challenges to implementing the USPSTF recommendations: identifying women at risk, connecting them to evidence-based treatment, and assessing outcomes after treatment.
The development of screening tools would help clinicians identify women at risk for perinatal depression, Dr. Freeman said. No such tool currently exists, but in the meantime, “women at risk may be identified by targeting those with histories of depression, subthreshold depressive symptoms, and certain sociodemographic factors (i.e., economically disadvantaged, single/young, unplanned pregnancy).”
The counseling interventions shown to be effective in preventing perinatal depression – cognitive behavioral therapy and interpersonal psychotherapy – require education and training outside the time limitations and expertise of many clinicians providing obstetric care, she noted.
“The delivery of effective care on a large scale will require creative solutions,” such as the use of telehealth and smartphone platforms, and the involvement of multidisciplinary teams to care for women with severe illness, Dr. Freeman said. “In addition, a substantial number of reproductive-aged women have serious psychiatric disorders and will be identified as at risk for perinatal depression, although their needs may be more comprehensive,” she wrote. “Women who are identified as at risk for perinatal depression may have psychotic disorders, bipolar spectrum disorders, anxiety disorders, and substance use disorders, and there is comorbidity among psychiatric disorders. Therefore, systematic provisions for referral and treatment for other psychiatric disorders should be considered.” Further research is needed to explore treatment options including pharmacotherapy for women with severe psychiatric disorders.
However, she expressed optimism that the recommendations for screening and counseling for perinatal depression are valuable, and they “may return great dividends in the form of enhanced well-being of mothers and their offspring.”
Dr. Freeman is affiliated with the department of psychiatry at Massachusetts General Hospital, Boston. She commented in an editorial accompanying the article by Curry et al. (JAMA. 2019 Feb 12;321[6]:550-2). Dr. Freeman disclosed relationships with companies including Takeda, JayMac, Sage, Otsuka, Alkermes, Janssen, and Sunovion; she also disclosed serving on an independent data safety and monitoring committee for Janssen (Johnson & Johnson); and editing the GOED (Global Organization for EPA & DHA Omega-3) newsletter.
according to a B recommendation from the U.S. Preventive Services Task Force.
The Task Force determined that counseling interventions are effective in preventing perinatal depression, defined as a major or minor depressive episode during pregnancy or within the first year after delivery. The condition affects an estimated 12% of new mothers in the United States each year, according to lead author Susan J. Curry, PhD, of the University of Iowa, Iowa City, and her colleagues.
The recommendation, published in JAMA, applies to “pregnant persons and persons who are less than 1 year postpartum who do not have a current diagnosis of depression but are at increased risk of developing depression,” according to the authors (JAMA. 2019 Feb 12;321(6):580-7).
Risk factors for development of perinatal depression include:
- Past history of depression.
- Current depressive symptoms (that do not reach a diagnostic threshold).
- History of physical or sexual abuse.
- Unplanned or unwanted pregnancy.
- Stressful life events.
- Lack of social and financial support.
- Intimate partner violence.
- Pregestational or gestational diabetes.
- Complications during pregnancy.
- Adolescent parenthood.
- Low socioeconomic status.
- Lack of social support.
After reviewing the evidence, the USPSTF found a moderate net benefit for counseling interventions, particularly cognitive behavioral therapy and interpersonal therapy, for preventing perinatal depression in women at risk. Counseling sessions reviewed for this recommendation ranged from 4 to 20 meetings (median, 8 meetings).
The USPSTF found inadequate evidence to assess the harms and benefits of other noncounseling interventions, including pharmacologic therapy.
In the evidence review accompanying the recommendations, Elizabeth A. O’Connor, PhD, of Kaiser Permanente, Portland, Ore., and her colleagues analyzed data from 50 studies including 22,385 individuals; 20 of these studies were randomized, controlled trials of counseling interventions (JAMA. 2019 Feb 12;321(6):588-601).
Overall, the likelihood of perinatal depression was significantly lower among women who received counseling, compared with controls, among more than 3,000 women in those studies (pooled risk ratio 0.61). Absolute risk differences for perinatal depression ranged from a 1% increased reduction in controls to a 32% increased reduction among women who received counseling. The effects were strongest for cognitive behavioral therapy and interpersonal therapy as interventions. No adverse events were reported in the counseling intervention studies.
In three studies of health system interventions, the researchers found a benefit for interventions vs. controls, but the difference was not statistically significant.
Trials of most other alternative interventions including infant sleep advice, birth-experience postpartum debriefing, omega-3 fatty acid supplementation, expressive writing, antidepressants, and yoga did not show statistical significance in benefit for reducing perinatal depression.
Only one of three randomized controlled trials of physical activity found a statistically significant group difference.
A trial of nortriptyline to prevent perinatal depression showed no benefit, compared with placebo. A sertraline study of found “a smaller percentage of participants taking sertraline had a depression recurrence, compared with those taking placebo,” the investigators wrote. In these two studies, women who took nortriptyline showed no adverse effects, and those in a trial involving sertraline reported significantly more dizziness and drowsiness compared with placebo patients.
The evidence review was limited by the small number of quality studies, especially studies of alternative interventions. More research is needed; however, the findings support data from similar reviews and support the potential for counseling to prevent perinatal depression, particularly in women at increased risk for perinatal depression, Dr. O’Connor and her associates said.
The USPSTF is supported by the Agency for Healthcare Research and Quality. Coauthor Dr. Michelle L. Henninger reported receiving grants from Pfizer IGLC (Independent Grants for Learning & Change) outside the submitted work. Coauthor Dr. Bradley N. Gaynes reported receiving personal fees from LivaNova and Johnson & Johnson outside the submitted work. The remaining researchers had no financial conflicts to disclose.
SOURCE: Curry SJ et al. JAMA. 2019;321(6):580-7; O’Connor E et al. JAMA. 2019;321(6):588-601.
according to a B recommendation from the U.S. Preventive Services Task Force.
The Task Force determined that counseling interventions are effective in preventing perinatal depression, defined as a major or minor depressive episode during pregnancy or within the first year after delivery. The condition affects an estimated 12% of new mothers in the United States each year, according to lead author Susan J. Curry, PhD, of the University of Iowa, Iowa City, and her colleagues.
The recommendation, published in JAMA, applies to “pregnant persons and persons who are less than 1 year postpartum who do not have a current diagnosis of depression but are at increased risk of developing depression,” according to the authors (JAMA. 2019 Feb 12;321(6):580-7).
Risk factors for development of perinatal depression include:
- Past history of depression.
- Current depressive symptoms (that do not reach a diagnostic threshold).
- History of physical or sexual abuse.
- Unplanned or unwanted pregnancy.
- Stressful life events.
- Lack of social and financial support.
- Intimate partner violence.
- Pregestational or gestational diabetes.
- Complications during pregnancy.
- Adolescent parenthood.
- Low socioeconomic status.
- Lack of social support.
After reviewing the evidence, the USPSTF found a moderate net benefit for counseling interventions, particularly cognitive behavioral therapy and interpersonal therapy, for preventing perinatal depression in women at risk. Counseling sessions reviewed for this recommendation ranged from 4 to 20 meetings (median, 8 meetings).
The USPSTF found inadequate evidence to assess the harms and benefits of other noncounseling interventions, including pharmacologic therapy.
In the evidence review accompanying the recommendations, Elizabeth A. O’Connor, PhD, of Kaiser Permanente, Portland, Ore., and her colleagues analyzed data from 50 studies including 22,385 individuals; 20 of these studies were randomized, controlled trials of counseling interventions (JAMA. 2019 Feb 12;321(6):588-601).
Overall, the likelihood of perinatal depression was significantly lower among women who received counseling, compared with controls, among more than 3,000 women in those studies (pooled risk ratio 0.61). Absolute risk differences for perinatal depression ranged from a 1% increased reduction in controls to a 32% increased reduction among women who received counseling. The effects were strongest for cognitive behavioral therapy and interpersonal therapy as interventions. No adverse events were reported in the counseling intervention studies.
In three studies of health system interventions, the researchers found a benefit for interventions vs. controls, but the difference was not statistically significant.
Trials of most other alternative interventions including infant sleep advice, birth-experience postpartum debriefing, omega-3 fatty acid supplementation, expressive writing, antidepressants, and yoga did not show statistical significance in benefit for reducing perinatal depression.
Only one of three randomized controlled trials of physical activity found a statistically significant group difference.
A trial of nortriptyline to prevent perinatal depression showed no benefit, compared with placebo. A sertraline study of found “a smaller percentage of participants taking sertraline had a depression recurrence, compared with those taking placebo,” the investigators wrote. In these two studies, women who took nortriptyline showed no adverse effects, and those in a trial involving sertraline reported significantly more dizziness and drowsiness compared with placebo patients.
The evidence review was limited by the small number of quality studies, especially studies of alternative interventions. More research is needed; however, the findings support data from similar reviews and support the potential for counseling to prevent perinatal depression, particularly in women at increased risk for perinatal depression, Dr. O’Connor and her associates said.
The USPSTF is supported by the Agency for Healthcare Research and Quality. Coauthor Dr. Michelle L. Henninger reported receiving grants from Pfizer IGLC (Independent Grants for Learning & Change) outside the submitted work. Coauthor Dr. Bradley N. Gaynes reported receiving personal fees from LivaNova and Johnson & Johnson outside the submitted work. The remaining researchers had no financial conflicts to disclose.
SOURCE: Curry SJ et al. JAMA. 2019;321(6):580-7; O’Connor E et al. JAMA. 2019;321(6):588-601.
FROM JAMA
Interactive parenting, life skill intervention improves self-esteem in teen mothers
than did those who received standard care, according to Joanne E. Cox, MD, director of primary care at Boston Children’s Hospital and Harvard Medical School, and her associates.
A total of 140 mothers who were aged less than 19 years when they delivered and whose child was aged less than 12 months were included in the study published in Pediatrics. Of this group, 72 received the intervention, which included a series of five 1-hour, one-on-one, interactive modules adapted from the Nurturing and Ansell-Casey Life Skills curricula, delivered over the infant’s first 15 months, in addition to standard teen-tot clinic care. The remaining 68 mothers received teen-tot care alone.
While overall maternal self-esteem decreased in both the intervention and control groups when measured at 36 months, the intervention group experienced a significantly smaller decrease from baseline (P = .011). Similarly, the intervention group had higher scores regarding preparedness for motherhood (P = .011), acceptance of infant (P = .008), and expected relationship with infant (P = .029).
Of the 52 mothers in the intervention group and 48 mothers in the control group for whom pregnancy data was available at 36 months, 42% in the intervention group had a repeat pregnancy, compared with 67% in the control group (adjusted odds ratio, 0.20; 95% confidence interval, 0.06-0.75; P = .017).
The study findings “highlight the positive impact of pairing medical services with comprehensive social services and parenting education and can inform future policy and services for teen parents. These positive effects also have potential to improve long-term outcomes for teens and their children,” Dr. Cox and her associates concluded.
The study authors reported no conflicts of interest. The study was supported in part by a grant from the Office of Adolescent Pregnancy Programs, the Edgerley Family Endowment, a Leadership Education in Adolescent Health training grant, the Maternal and Child Health Bureau, and the Health Resources and Services Administration.
[email protected]
SOURCE: Cox JE et al. Pediatrics. 2019 Feb 12. doi: 10.1542/peds.2018-2303.
than did those who received standard care, according to Joanne E. Cox, MD, director of primary care at Boston Children’s Hospital and Harvard Medical School, and her associates.
A total of 140 mothers who were aged less than 19 years when they delivered and whose child was aged less than 12 months were included in the study published in Pediatrics. Of this group, 72 received the intervention, which included a series of five 1-hour, one-on-one, interactive modules adapted from the Nurturing and Ansell-Casey Life Skills curricula, delivered over the infant’s first 15 months, in addition to standard teen-tot clinic care. The remaining 68 mothers received teen-tot care alone.
While overall maternal self-esteem decreased in both the intervention and control groups when measured at 36 months, the intervention group experienced a significantly smaller decrease from baseline (P = .011). Similarly, the intervention group had higher scores regarding preparedness for motherhood (P = .011), acceptance of infant (P = .008), and expected relationship with infant (P = .029).
Of the 52 mothers in the intervention group and 48 mothers in the control group for whom pregnancy data was available at 36 months, 42% in the intervention group had a repeat pregnancy, compared with 67% in the control group (adjusted odds ratio, 0.20; 95% confidence interval, 0.06-0.75; P = .017).
The study findings “highlight the positive impact of pairing medical services with comprehensive social services and parenting education and can inform future policy and services for teen parents. These positive effects also have potential to improve long-term outcomes for teens and their children,” Dr. Cox and her associates concluded.
The study authors reported no conflicts of interest. The study was supported in part by a grant from the Office of Adolescent Pregnancy Programs, the Edgerley Family Endowment, a Leadership Education in Adolescent Health training grant, the Maternal and Child Health Bureau, and the Health Resources and Services Administration.
[email protected]
SOURCE: Cox JE et al. Pediatrics. 2019 Feb 12. doi: 10.1542/peds.2018-2303.
than did those who received standard care, according to Joanne E. Cox, MD, director of primary care at Boston Children’s Hospital and Harvard Medical School, and her associates.
A total of 140 mothers who were aged less than 19 years when they delivered and whose child was aged less than 12 months were included in the study published in Pediatrics. Of this group, 72 received the intervention, which included a series of five 1-hour, one-on-one, interactive modules adapted from the Nurturing and Ansell-Casey Life Skills curricula, delivered over the infant’s first 15 months, in addition to standard teen-tot clinic care. The remaining 68 mothers received teen-tot care alone.
While overall maternal self-esteem decreased in both the intervention and control groups when measured at 36 months, the intervention group experienced a significantly smaller decrease from baseline (P = .011). Similarly, the intervention group had higher scores regarding preparedness for motherhood (P = .011), acceptance of infant (P = .008), and expected relationship with infant (P = .029).
Of the 52 mothers in the intervention group and 48 mothers in the control group for whom pregnancy data was available at 36 months, 42% in the intervention group had a repeat pregnancy, compared with 67% in the control group (adjusted odds ratio, 0.20; 95% confidence interval, 0.06-0.75; P = .017).
The study findings “highlight the positive impact of pairing medical services with comprehensive social services and parenting education and can inform future policy and services for teen parents. These positive effects also have potential to improve long-term outcomes for teens and their children,” Dr. Cox and her associates concluded.
The study authors reported no conflicts of interest. The study was supported in part by a grant from the Office of Adolescent Pregnancy Programs, the Edgerley Family Endowment, a Leadership Education in Adolescent Health training grant, the Maternal and Child Health Bureau, and the Health Resources and Services Administration.
[email protected]
SOURCE: Cox JE et al. Pediatrics. 2019 Feb 12. doi: 10.1542/peds.2018-2303.
FROM PEDIATRICS
Supreme Court halts Louisiana abortion law from taking effect
The U.S. Supreme Court has temporarily barred a Louisiana law that would require stricter requirements for physicians who provide abortion care, the first abortion-related decision for the current conservative-leaning high court.
In a Feb. 7, 2019, order, Supreme Court justices stopped the law from moving forward until they can decide whether to accept the case for oral argument. The law in question would require Louisiana physicians who perform abortions to have admitting privileges at a hospital within 30 miles of the clinic where they offer abortion care.
A group of health professionals sued over the Louisiana statute after it was enacted in 2014, arguing that the requirement was unconstitutional because it placed an undue burden on women seeking abortions. A federal court agreed, concluding that the law would leave a significant number of Louisiana women unable to get an abortion. The state appealed to the 5th U.S. Circuit Court of Appeals, which reversed the decision in January 2019. The physician plaintiffs then urged the Supreme Court to stop the law, scheduled to take effect on Feb. 4 while the case continued through the courts. The health professionals argue that no physicians in Louisiana would be available to perform abortions after 17 weeks of pregnancy if the law proceeds and that only one physician in the state would be available to provide an abortion in the earlier stages of pregnancy. Attorneys for the state countered that health providers are overestimating the law’s effect and requested that the measure be allowed to go forward.
In a Feb. 1 order, the Supreme Court provided the plaintiffs a short stay while they reviewed briefs in the case. Then, in a 5-4 decision on Feb. 7, the majority court halted the legal challenge indefinitely until they can decide whether to take up the case.
Four justices – Clarence Thomas, Samuel Alito, Neil Gorsuch, and Brett Kavanaugh – dissented from the majority, writing that they would have allowed Louisiana to enforce the law. Chief Justice John Roberts joined the high court’s four more liberal justices in stopping the law’s enactment.
The plaintiffs’ petition to the Supreme Court is due in April. If the case is accepted, oral arguments would likely be scheduled for fall 2019 or winter 2020, according to court analysts.
The U.S. Supreme Court has temporarily barred a Louisiana law that would require stricter requirements for physicians who provide abortion care, the first abortion-related decision for the current conservative-leaning high court.
In a Feb. 7, 2019, order, Supreme Court justices stopped the law from moving forward until they can decide whether to accept the case for oral argument. The law in question would require Louisiana physicians who perform abortions to have admitting privileges at a hospital within 30 miles of the clinic where they offer abortion care.
A group of health professionals sued over the Louisiana statute after it was enacted in 2014, arguing that the requirement was unconstitutional because it placed an undue burden on women seeking abortions. A federal court agreed, concluding that the law would leave a significant number of Louisiana women unable to get an abortion. The state appealed to the 5th U.S. Circuit Court of Appeals, which reversed the decision in January 2019. The physician plaintiffs then urged the Supreme Court to stop the law, scheduled to take effect on Feb. 4 while the case continued through the courts. The health professionals argue that no physicians in Louisiana would be available to perform abortions after 17 weeks of pregnancy if the law proceeds and that only one physician in the state would be available to provide an abortion in the earlier stages of pregnancy. Attorneys for the state countered that health providers are overestimating the law’s effect and requested that the measure be allowed to go forward.
In a Feb. 1 order, the Supreme Court provided the plaintiffs a short stay while they reviewed briefs in the case. Then, in a 5-4 decision on Feb. 7, the majority court halted the legal challenge indefinitely until they can decide whether to take up the case.
Four justices – Clarence Thomas, Samuel Alito, Neil Gorsuch, and Brett Kavanaugh – dissented from the majority, writing that they would have allowed Louisiana to enforce the law. Chief Justice John Roberts joined the high court’s four more liberal justices in stopping the law’s enactment.
The plaintiffs’ petition to the Supreme Court is due in April. If the case is accepted, oral arguments would likely be scheduled for fall 2019 or winter 2020, according to court analysts.
The U.S. Supreme Court has temporarily barred a Louisiana law that would require stricter requirements for physicians who provide abortion care, the first abortion-related decision for the current conservative-leaning high court.
In a Feb. 7, 2019, order, Supreme Court justices stopped the law from moving forward until they can decide whether to accept the case for oral argument. The law in question would require Louisiana physicians who perform abortions to have admitting privileges at a hospital within 30 miles of the clinic where they offer abortion care.
A group of health professionals sued over the Louisiana statute after it was enacted in 2014, arguing that the requirement was unconstitutional because it placed an undue burden on women seeking abortions. A federal court agreed, concluding that the law would leave a significant number of Louisiana women unable to get an abortion. The state appealed to the 5th U.S. Circuit Court of Appeals, which reversed the decision in January 2019. The physician plaintiffs then urged the Supreme Court to stop the law, scheduled to take effect on Feb. 4 while the case continued through the courts. The health professionals argue that no physicians in Louisiana would be available to perform abortions after 17 weeks of pregnancy if the law proceeds and that only one physician in the state would be available to provide an abortion in the earlier stages of pregnancy. Attorneys for the state countered that health providers are overestimating the law’s effect and requested that the measure be allowed to go forward.
In a Feb. 1 order, the Supreme Court provided the plaintiffs a short stay while they reviewed briefs in the case. Then, in a 5-4 decision on Feb. 7, the majority court halted the legal challenge indefinitely until they can decide whether to take up the case.
Four justices – Clarence Thomas, Samuel Alito, Neil Gorsuch, and Brett Kavanaugh – dissented from the majority, writing that they would have allowed Louisiana to enforce the law. Chief Justice John Roberts joined the high court’s four more liberal justices in stopping the law’s enactment.
The plaintiffs’ petition to the Supreme Court is due in April. If the case is accepted, oral arguments would likely be scheduled for fall 2019 or winter 2020, according to court analysts.
Most pregnant women want guidance on prenatal whole-genome sequencing
according to results from a survey published in Obstetrics & Gynecology.
Nearly half said they would want clear guidance from clinicians before undergoing the noninvasive procedure.
“Prenatal whole-genome sequencing offers significantly more fetal information than women can currently receive, and it is not surprising that, when faced with a tremendous range of information, many women want recommendations from their clinicians,” Haley K. Sullivan from the National Institutes of Health Clinical Center and National Human Genome Research Institute and colleagues wrote. “Our data suggest that most women prefer a directive interaction with their clinician when deciding what types of genetic information to receive from prenatal whole-genome sequencing.”
Research coordinators from the Inova Translational Medicine Institute offered 805 pregnant women a survey on their preferences for prenatal whole-genome sequencing between June and August 2017; of these, 553 women answered (69% response rate). The women responded to questions about what type of information they would like to receive if they were to undergo prenatal whole-genome sequencing and what role a clinician would preferably play in the decision-making process. The researchers divided the survey into sections based on actionability, severity, prevalence, and age of onset.
According to the survey results, 90% of respondents wanted information on serious treatable childhood-onset diseases from prenatal whole-genome sequencing results, while 40% said they did not want to receive results based on nonmedical traits such as eye color, height, or athletic ability.
With regard to clinician role, 45% of women said they wanted all options presented with clear recommendations from a clinician on which tests to order, 26% wanted all options presented but with a joint decision-making process, 13% wanted all options presented but independent decision making, and 11% wanted the clear recommendation from clinicians alone.
The respondents said the most common reason for wanting to undergo prenatal whole-genome sequencing was to prepare “financially, medically, or psychologically” for a child with special needs, the researchers said.
“This represents a departure from the current state of genetic counseling, where nondirectiveness is a central tenet, and is contrary to the 45% of ob.gyns. who said in a previous survey that they should not be at all directive when counseling patients on prenatal whole-genome sequencing,” the authors wrote. “Given this clear patient desire for guidance, there is a vital opportunity for the American College of Obstetricians and Gynecologists to provide leadership and recommendations as prenatal whole-genome sequencing is adopted into clinical practice.”
Limitations in the study include asking the respondents to make hypothetical decisions, using examples to describe genetic conditions that might have skewed decision making; asking women to pick only one reason for wanting the sequencing information from a list of predetermined options, when many reasons may be important to them; social desirability bias in the responses, if women are reluctant to pick a choice they perceive as less socially acceptable; and a potential systematic difference between women who were and were not enrolled as survey participants. The respondents also were from the Northern Virginia area, which may not be generalizable to a national population of patients, the researchers said.
This study was supported by the Intramural Research Program of the National Human Genome Research Institute and the Clinical Center Department of Bioethics, National Institutes of Health. The authors reported no relevant conflicts of interest.
SOURCE: Sullivan HK et al. Obstet Gynecol. 2019 Mar. doi: 10.1097/AOG.0000000000003121.
according to results from a survey published in Obstetrics & Gynecology.
Nearly half said they would want clear guidance from clinicians before undergoing the noninvasive procedure.
“Prenatal whole-genome sequencing offers significantly more fetal information than women can currently receive, and it is not surprising that, when faced with a tremendous range of information, many women want recommendations from their clinicians,” Haley K. Sullivan from the National Institutes of Health Clinical Center and National Human Genome Research Institute and colleagues wrote. “Our data suggest that most women prefer a directive interaction with their clinician when deciding what types of genetic information to receive from prenatal whole-genome sequencing.”
Research coordinators from the Inova Translational Medicine Institute offered 805 pregnant women a survey on their preferences for prenatal whole-genome sequencing between June and August 2017; of these, 553 women answered (69% response rate). The women responded to questions about what type of information they would like to receive if they were to undergo prenatal whole-genome sequencing and what role a clinician would preferably play in the decision-making process. The researchers divided the survey into sections based on actionability, severity, prevalence, and age of onset.
According to the survey results, 90% of respondents wanted information on serious treatable childhood-onset diseases from prenatal whole-genome sequencing results, while 40% said they did not want to receive results based on nonmedical traits such as eye color, height, or athletic ability.
With regard to clinician role, 45% of women said they wanted all options presented with clear recommendations from a clinician on which tests to order, 26% wanted all options presented but with a joint decision-making process, 13% wanted all options presented but independent decision making, and 11% wanted the clear recommendation from clinicians alone.
The respondents said the most common reason for wanting to undergo prenatal whole-genome sequencing was to prepare “financially, medically, or psychologically” for a child with special needs, the researchers said.
“This represents a departure from the current state of genetic counseling, where nondirectiveness is a central tenet, and is contrary to the 45% of ob.gyns. who said in a previous survey that they should not be at all directive when counseling patients on prenatal whole-genome sequencing,” the authors wrote. “Given this clear patient desire for guidance, there is a vital opportunity for the American College of Obstetricians and Gynecologists to provide leadership and recommendations as prenatal whole-genome sequencing is adopted into clinical practice.”
Limitations in the study include asking the respondents to make hypothetical decisions, using examples to describe genetic conditions that might have skewed decision making; asking women to pick only one reason for wanting the sequencing information from a list of predetermined options, when many reasons may be important to them; social desirability bias in the responses, if women are reluctant to pick a choice they perceive as less socially acceptable; and a potential systematic difference between women who were and were not enrolled as survey participants. The respondents also were from the Northern Virginia area, which may not be generalizable to a national population of patients, the researchers said.
This study was supported by the Intramural Research Program of the National Human Genome Research Institute and the Clinical Center Department of Bioethics, National Institutes of Health. The authors reported no relevant conflicts of interest.
SOURCE: Sullivan HK et al. Obstet Gynecol. 2019 Mar. doi: 10.1097/AOG.0000000000003121.
according to results from a survey published in Obstetrics & Gynecology.
Nearly half said they would want clear guidance from clinicians before undergoing the noninvasive procedure.
“Prenatal whole-genome sequencing offers significantly more fetal information than women can currently receive, and it is not surprising that, when faced with a tremendous range of information, many women want recommendations from their clinicians,” Haley K. Sullivan from the National Institutes of Health Clinical Center and National Human Genome Research Institute and colleagues wrote. “Our data suggest that most women prefer a directive interaction with their clinician when deciding what types of genetic information to receive from prenatal whole-genome sequencing.”
Research coordinators from the Inova Translational Medicine Institute offered 805 pregnant women a survey on their preferences for prenatal whole-genome sequencing between June and August 2017; of these, 553 women answered (69% response rate). The women responded to questions about what type of information they would like to receive if they were to undergo prenatal whole-genome sequencing and what role a clinician would preferably play in the decision-making process. The researchers divided the survey into sections based on actionability, severity, prevalence, and age of onset.
According to the survey results, 90% of respondents wanted information on serious treatable childhood-onset diseases from prenatal whole-genome sequencing results, while 40% said they did not want to receive results based on nonmedical traits such as eye color, height, or athletic ability.
With regard to clinician role, 45% of women said they wanted all options presented with clear recommendations from a clinician on which tests to order, 26% wanted all options presented but with a joint decision-making process, 13% wanted all options presented but independent decision making, and 11% wanted the clear recommendation from clinicians alone.
The respondents said the most common reason for wanting to undergo prenatal whole-genome sequencing was to prepare “financially, medically, or psychologically” for a child with special needs, the researchers said.
“This represents a departure from the current state of genetic counseling, where nondirectiveness is a central tenet, and is contrary to the 45% of ob.gyns. who said in a previous survey that they should not be at all directive when counseling patients on prenatal whole-genome sequencing,” the authors wrote. “Given this clear patient desire for guidance, there is a vital opportunity for the American College of Obstetricians and Gynecologists to provide leadership and recommendations as prenatal whole-genome sequencing is adopted into clinical practice.”
Limitations in the study include asking the respondents to make hypothetical decisions, using examples to describe genetic conditions that might have skewed decision making; asking women to pick only one reason for wanting the sequencing information from a list of predetermined options, when many reasons may be important to them; social desirability bias in the responses, if women are reluctant to pick a choice they perceive as less socially acceptable; and a potential systematic difference between women who were and were not enrolled as survey participants. The respondents also were from the Northern Virginia area, which may not be generalizable to a national population of patients, the researchers said.
This study was supported by the Intramural Research Program of the National Human Genome Research Institute and the Clinical Center Department of Bioethics, National Institutes of Health. The authors reported no relevant conflicts of interest.
SOURCE: Sullivan HK et al. Obstet Gynecol. 2019 Mar. doi: 10.1097/AOG.0000000000003121.
FROM OBSTETRICS & GYNECOLOGY
Key clinical point: A majority of pregnant women surveyed said they wanted information on childhood-onset genetic diseases, with almost half wanting clear clinical recommendations before deciding to undergo noninvasive prenatal whole-genome sequencing.
Major finding: Of the respondents, 90% said they wanted information on serious treatable childhood-onset conditions.
Study details: A survey of 553 pregnant women coordinated by the Inova Translational Medicine Institute.
Disclosures: This study was supported by the Intramural Research Program of the National Human Genome Research Institute and the Clinical Center Department of Bioethics, National Institutes of Health. The authors reported no relevant conflicts of interest.
Source: Sullivan HK et al. Obstet Gynecol. 2019 Mar. doi: 10.1097/AOG.0000000000003121.
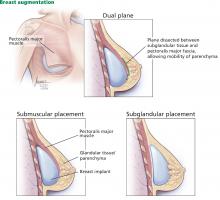
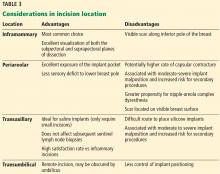
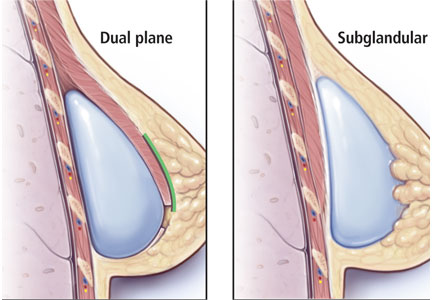
FDA: 246 new reports on breast implant-associated lymphoma
The Food and Drug Administration has identified 457 unique cases of breast implant–associated anaplastic large cell lymphoma (BIA-ALCL) and 9 related deaths since 2010, and received 246 new medical device reports (MDRs) regarding BIA-ALCL between September 2017 and September 2018, according to an update from the agency’s Center for Devices and Radiological Health.

That brings the total number of reports to 660; however, that number reflects duplicative cases, Binita Ashar, MD, a general surgeon and the director of the division of surgical devices at the center, said in a statement.
“These types of increases in the MDRs are to be expected and may include past cases that were not previously reported to the FDA,” Dr. Ashar said, addressing the high number of new reports. “The increased number of MDRs contributes to our evolving understanding of BIA-ALCL and represents a more thorough and comprehensive analysis.”
BIA-ALCL is a type of non-Hodgkin lymphoma and a known risk from breast implants that was first communicated by the FDA in 2011. Regular updates have been provided with respect to related medical device reports, cases, deaths, and known risks.
“We hope that this information prompts providers and patients to have important, informed conversations about breast implants and the risk of BIA-ALCL. At the same time, we remain committed to working in partnership with all stakeholders to continue to study, understand, and provide updates about this important public health issue,” Dr. Ashar said.
To that end, the center also issued a Letter to Health Care Providers to “encourage those who regularly treat patients, including primary care physicians and gynecologists, to learn about BIA-ALCL in patients with breast implants.”
Patients and providers are encouraged to file MDRs with the FDA via MedWatch, the FDA Safety Information and Adverse Event Reporting program, she said.
The Food and Drug Administration has identified 457 unique cases of breast implant–associated anaplastic large cell lymphoma (BIA-ALCL) and 9 related deaths since 2010, and received 246 new medical device reports (MDRs) regarding BIA-ALCL between September 2017 and September 2018, according to an update from the agency’s Center for Devices and Radiological Health.

That brings the total number of reports to 660; however, that number reflects duplicative cases, Binita Ashar, MD, a general surgeon and the director of the division of surgical devices at the center, said in a statement.
“These types of increases in the MDRs are to be expected and may include past cases that were not previously reported to the FDA,” Dr. Ashar said, addressing the high number of new reports. “The increased number of MDRs contributes to our evolving understanding of BIA-ALCL and represents a more thorough and comprehensive analysis.”
BIA-ALCL is a type of non-Hodgkin lymphoma and a known risk from breast implants that was first communicated by the FDA in 2011. Regular updates have been provided with respect to related medical device reports, cases, deaths, and known risks.
“We hope that this information prompts providers and patients to have important, informed conversations about breast implants and the risk of BIA-ALCL. At the same time, we remain committed to working in partnership with all stakeholders to continue to study, understand, and provide updates about this important public health issue,” Dr. Ashar said.
To that end, the center also issued a Letter to Health Care Providers to “encourage those who regularly treat patients, including primary care physicians and gynecologists, to learn about BIA-ALCL in patients with breast implants.”
Patients and providers are encouraged to file MDRs with the FDA via MedWatch, the FDA Safety Information and Adverse Event Reporting program, she said.
The Food and Drug Administration has identified 457 unique cases of breast implant–associated anaplastic large cell lymphoma (BIA-ALCL) and 9 related deaths since 2010, and received 246 new medical device reports (MDRs) regarding BIA-ALCL between September 2017 and September 2018, according to an update from the agency’s Center for Devices and Radiological Health.

That brings the total number of reports to 660; however, that number reflects duplicative cases, Binita Ashar, MD, a general surgeon and the director of the division of surgical devices at the center, said in a statement.
“These types of increases in the MDRs are to be expected and may include past cases that were not previously reported to the FDA,” Dr. Ashar said, addressing the high number of new reports. “The increased number of MDRs contributes to our evolving understanding of BIA-ALCL and represents a more thorough and comprehensive analysis.”
BIA-ALCL is a type of non-Hodgkin lymphoma and a known risk from breast implants that was first communicated by the FDA in 2011. Regular updates have been provided with respect to related medical device reports, cases, deaths, and known risks.
“We hope that this information prompts providers and patients to have important, informed conversations about breast implants and the risk of BIA-ALCL. At the same time, we remain committed to working in partnership with all stakeholders to continue to study, understand, and provide updates about this important public health issue,” Dr. Ashar said.
To that end, the center also issued a Letter to Health Care Providers to “encourage those who regularly treat patients, including primary care physicians and gynecologists, to learn about BIA-ALCL in patients with breast implants.”
Patients and providers are encouraged to file MDRs with the FDA via MedWatch, the FDA Safety Information and Adverse Event Reporting program, she said.
Obstetric hospitalists can screen for postpartum depression
Postpartum depression (PPD) is the most common complication of pregnancy, and onset can occur at any time from pregnancy until up to 1 year post partum.1,2 The immediate postpartum period is a time during which care is shared among multiple providers for both mother and child, and the transition from inpatient to outpatient postpartum care can impede communication between those caring for the patient in each setting. In 2018, the American College of Obstetricians and Gynecologists published a committee opinion emphasizing the importance of the “fourth trimester” and calling for health care providers to assist women in navigating the transition from pre- to postpartum care.3 An important consideration of perinatal care is mental health care for the mother, including screening and care for postpartum depression; however, the optimal role for the obstetric hospitalist in providing such services has been unclear.
Estimates of the prevalence of PPD in new mothers in the United States varied by state from 8% to 20% in 2012, with an overall average of 12%.2 Left untreated, PPD may result in significant negative outcomes for women, their children, and families. The depressive symptoms of PPD may persist for months or years afterward,4 with one study finding elevated depressive symptoms in women up to 11 years post partum.5 Suicide is also a leading cause of pregnancy-related mortality associated with depressive symptoms.6-9 In addition, maternal postpartum depression symptoms have been associated with impaired mother-infant bonding at 6 months of age10 and decreased cognitive and fine motor development of children at 18 months.11
Importance of screening
Evidence from the literature shows that, without proper screening, approximately 50% of cases of PPD go undiagnosed, and that increasing the number of women being screened by perinatal providers is an important first step to improving outcomes.12-18 Current recommendations for the timing and frequency of screening for PPD vary among the published guidelines. ACOG recommends screening at least once during the perinatal period for depression and anxiety using a standardized, validated tool; an update of the ACOG committee opinion in 2018 also states: “It is recommended that all obstetrician-gynecologists and other obstetric care providers complete a full assessment of mood and emotional well-being (including screening for PPD and anxiety with a validated instrument) during the comprehensive postpartum visit for each patient.”19 The American Medical Association adopted new policies in 2017 promoting the implementation of a routine protocol for depression screening of perinatal women.20 The American Academy of Pediatrics recommends more frequent screening, with assessments at the 1-, 2-, 4-, and 6-month visits.21 Finally, the U.S. Preventive Services Task Force recommends screening for depression in the general population including pregnant and postpartum women.22
Multiple standardized, validated screening instruments are available for detecting possible symptoms of PPD, including the most widely used tools: the Edinburgh Postnatal Depression Scale (EPDS)19,23 and the Patient Health Questionnaire (PHQ-9).24 Two recent studies have shown that screening women for symptoms of PPD with a validated tool may reduce the duration or severity of depressive symptoms,25,26 further reinforcing the need to ensure that women experiencing symptoms of PPD are identified and treated early.
The inpatient hospitalization for labor, delivery, and birth of a child has not traditionally been viewed as an opportunity for PPD screening. While private practitioners and obstetric medical group practices typically have inquired about and documented the individual patient’s mental health history and risk factors for PPD, the obstetric hospitalist is most commonly meeting a patient in labor or in a postpartum encounter for the first time. As obstetric practices grow ever more consolidated, and as obstetric hospitalist care is implemented for a variety of reasons including, but not limited to, preventing burnout among private practitioners, serving as a safety net for all inpatient obstetric services, and increasing standardization in obstetric triage and obstetric emergency departments,
Barriers remain
Despite the need for early detection of PPD, screening practices remain inconsistent. A literature review of health care provider practices showed only one in four physicians reported using screening tools; obstetrician-gynecologists were most likely (36%) to use screening tools, followed by family practitioners (31%), with pediatricians the least likely (7%).27 This low rate is at least partially the result of perceived barriers to screening among health care providers, which contributes to underdiagnosis. A survey of more than 200 physicians who were members of ACOG showed that the top three barriers restricting screening practices were time constraints, inadequate training, and a lack of knowledge of the diagnostic criteria.28
Since 2017, Dignity Health has instituted routine screening of all inpatient postpartum patients at its 29 birth centers in Arizona, California, and Nevada. In this program, of which I am a physician participant, more than 30,000 women have been screened with the EPDS. In addition to providing screening, Dignity Health staff (physicians, certified nurse midwives, nurse practitioners, registered nurses, social workers, mental health therapists, lactation consultants, health educators, and others) have received in-person Perinatal Mental Health training. In this way, the entire care team coordinates inpatient screening and referral to outpatient care providers – thus bridging the gap in postpartum mental health care. For those patients who screen positive while an inpatient, a psychiatric telemedicine appointment is provided and, if necessary, short-course medications can be prescribed until the patient has outpatient follow-up and continuity of care. While we as obstetric hospitalists and community obstetrician-gynecologists recognize that inpatient postpartum screening may be limited in its sensitivity for capturing all women who will go on to develop PPD, there is definitely a benefit to having a discussion about PPD and maternal mental health early and often throughout the postpartum period. For many women suffering in silence, a 6-week postpartum outpatient visit is too late, especially given that approximately one-third of women are lost to postpartum follow-up.29,30
Addressing barriers
A growing number of states have enacted policies to address the challenge of peripartum behavioral health needs, and several states – Illinois, Massachusetts, New Jersey, and West Virginia – now mandate routine PPD screening by health care providers.31 However, few of these laws or policies contain specific guidance, such as the optimal timing for screening, instead leaving the details to providers.32 The proper identification and management of PPD cannot be achieved by state-level policy mandates alone, but must include clinician buy-in and participation.
Obstetricians play an essential role in the identification and treatment of PPD. Among nonpsychiatric specialists, obstetrician-gynecologists are the most likely providers to see and screen during the perinatal period.33 In addition, women prefer to receive help for PPD from either their obstetric practitioners or a mental-health specialists located at the obstetric clinic, and are more likely to receive mental-health services if they are provided at the same location as that of the obstetric provider.34,35 According to ACOG’s new guidance on the fourth trimester, obstetricians are encouraged to take responsibility for women’s care immediately after birth, and this care would include contact with all mothers within the first 3 weeks post partum, at follow-up visits as needed, and for a comprehensive postpartum visit at 12 weeks.3
Our specialty has and will continue to evolve, and obstetric hospitalists will play an ever more essential role in the care of women during their inpatient obstetric admission. Whether we are a patient’s primary inpatient obstetric provider or a practice extender for single or multigroup practice, we are in a unique role to screen, begin treatment for, and offer anticipatory guidance for maternal mental health and postpartum depression disorders. Obstetric hospitalists can be a bridge between inpatient and outpatient follow-up and catalysts for implementing universal inpatient PPD screening. Our role presents an opportunity to start the discussion early and often in the fourth trimester and to make a significant difference in addressing this critical unmet need in postnatal care.
Dr. van Dis is the medical director of the Ob Hospitalist Group in Burbank, Calif. She disclosed she received editorial assistance from Erik MacLaren, PhD, of Boston Strategic Partners Inc., with funding support from Sage Therapeutics Inc. E-mail [email protected].
References
1. Centers for Disease Control and Prevention. Postpartum Depression. 2017.
2. Morb Mortal Wkly Rep. 2017;66(6):153-8.
3. Obstet Gynecol. 2018;131(5):e140-e150.
4. Harv Rev Psychiatry. 2014;22(1):1-22.
5. JAMA Psychiatry. 2018;75(3):247-53.
6. J Womens Health (Larchmt). 2016;25(12):1219-24.
7. J Psychiatr Res. 2017;84:284-91.
8. Br J Psychiatry. 2003;183:279-81.
9. Obstet Gynecol Surv. 2005;60(3):183-90.
10. Arch Womens Ment Health. 2016;19(1):87-94.
11. Soc Psychiatry Psychiatr Epidemiol. 2013;48(8):1335-45.
12. J Reprod Med. 1999;44(4):351-8.
13. J Behav Health Serv Res. 2004;31(2):117-33.
14. J Clin Psychiatry. 2016;77(9):1189-200.
15. Am J Obstet Gynecol. 2000;182(5):1080-2.
16. J Fam Pract. 2001;50(2):117-22.
17. Obstet Gynecol. 1999;93(5 Pt 1):653-7.
18. J Womens Health (Larchmt). 2010;19(3):477-90.
19. Obstet Gynecol. 2018;132:e208-12.
20. “Physicians back programs to address maternal mortality, depression,” AMA, Nov. 15, 2017
21. Pediatrics. 2019 Jan 1;143(1):e20183260.
22. JAMA. 2016;315(4):380-7.
23. Br J Psychiatry. 1987;150:782-6.
24. Ann Fam Med. 2009;7(1):63-70.
25. Obstet Gynecol. 2016;127(5):917-25.
26. Pediatrics. 2017 Oct;140(4). pii: e20170110.
27. Womens Health Issues. 2015;25(6):703-10.
28. J Psychosom Obstet Gynaecol. 2011;32(1):27-34.
29. Matern Child Health J. 2016;20(Suppl 1):22-7.
30. National Committee for Quality Assurance. Prenatal and Postpartum Care (PPC). 2018.
31. Psychiatr Serv. 2015;66(3):324-8.
32. Postpartum Support International. Legislation. 2018.
33. American Academy of Pediatrics, American College of Obstetricians and Gynecologists, eds. Guidelines for Perinatal Care. 7th ed. (Elk Grove Village, IL: Washington, DC: American Academy of Pediatrics; American College of Obstetricians and Gynecologists; Oct 2012.)
34. Birth. 2009;36(1):60-9.
35. Gen Hosp Psychiatry. 2009;31(2):155-62.
Postpartum depression (PPD) is the most common complication of pregnancy, and onset can occur at any time from pregnancy until up to 1 year post partum.1,2 The immediate postpartum period is a time during which care is shared among multiple providers for both mother and child, and the transition from inpatient to outpatient postpartum care can impede communication between those caring for the patient in each setting. In 2018, the American College of Obstetricians and Gynecologists published a committee opinion emphasizing the importance of the “fourth trimester” and calling for health care providers to assist women in navigating the transition from pre- to postpartum care.3 An important consideration of perinatal care is mental health care for the mother, including screening and care for postpartum depression; however, the optimal role for the obstetric hospitalist in providing such services has been unclear.
Estimates of the prevalence of PPD in new mothers in the United States varied by state from 8% to 20% in 2012, with an overall average of 12%.2 Left untreated, PPD may result in significant negative outcomes for women, their children, and families. The depressive symptoms of PPD may persist for months or years afterward,4 with one study finding elevated depressive symptoms in women up to 11 years post partum.5 Suicide is also a leading cause of pregnancy-related mortality associated with depressive symptoms.6-9 In addition, maternal postpartum depression symptoms have been associated with impaired mother-infant bonding at 6 months of age10 and decreased cognitive and fine motor development of children at 18 months.11
Importance of screening
Evidence from the literature shows that, without proper screening, approximately 50% of cases of PPD go undiagnosed, and that increasing the number of women being screened by perinatal providers is an important first step to improving outcomes.12-18 Current recommendations for the timing and frequency of screening for PPD vary among the published guidelines. ACOG recommends screening at least once during the perinatal period for depression and anxiety using a standardized, validated tool; an update of the ACOG committee opinion in 2018 also states: “It is recommended that all obstetrician-gynecologists and other obstetric care providers complete a full assessment of mood and emotional well-being (including screening for PPD and anxiety with a validated instrument) during the comprehensive postpartum visit for each patient.”19 The American Medical Association adopted new policies in 2017 promoting the implementation of a routine protocol for depression screening of perinatal women.20 The American Academy of Pediatrics recommends more frequent screening, with assessments at the 1-, 2-, 4-, and 6-month visits.21 Finally, the U.S. Preventive Services Task Force recommends screening for depression in the general population including pregnant and postpartum women.22
Multiple standardized, validated screening instruments are available for detecting possible symptoms of PPD, including the most widely used tools: the Edinburgh Postnatal Depression Scale (EPDS)19,23 and the Patient Health Questionnaire (PHQ-9).24 Two recent studies have shown that screening women for symptoms of PPD with a validated tool may reduce the duration or severity of depressive symptoms,25,26 further reinforcing the need to ensure that women experiencing symptoms of PPD are identified and treated early.
The inpatient hospitalization for labor, delivery, and birth of a child has not traditionally been viewed as an opportunity for PPD screening. While private practitioners and obstetric medical group practices typically have inquired about and documented the individual patient’s mental health history and risk factors for PPD, the obstetric hospitalist is most commonly meeting a patient in labor or in a postpartum encounter for the first time. As obstetric practices grow ever more consolidated, and as obstetric hospitalist care is implemented for a variety of reasons including, but not limited to, preventing burnout among private practitioners, serving as a safety net for all inpatient obstetric services, and increasing standardization in obstetric triage and obstetric emergency departments,
Barriers remain
Despite the need for early detection of PPD, screening practices remain inconsistent. A literature review of health care provider practices showed only one in four physicians reported using screening tools; obstetrician-gynecologists were most likely (36%) to use screening tools, followed by family practitioners (31%), with pediatricians the least likely (7%).27 This low rate is at least partially the result of perceived barriers to screening among health care providers, which contributes to underdiagnosis. A survey of more than 200 physicians who were members of ACOG showed that the top three barriers restricting screening practices were time constraints, inadequate training, and a lack of knowledge of the diagnostic criteria.28
Since 2017, Dignity Health has instituted routine screening of all inpatient postpartum patients at its 29 birth centers in Arizona, California, and Nevada. In this program, of which I am a physician participant, more than 30,000 women have been screened with the EPDS. In addition to providing screening, Dignity Health staff (physicians, certified nurse midwives, nurse practitioners, registered nurses, social workers, mental health therapists, lactation consultants, health educators, and others) have received in-person Perinatal Mental Health training. In this way, the entire care team coordinates inpatient screening and referral to outpatient care providers – thus bridging the gap in postpartum mental health care. For those patients who screen positive while an inpatient, a psychiatric telemedicine appointment is provided and, if necessary, short-course medications can be prescribed until the patient has outpatient follow-up and continuity of care. While we as obstetric hospitalists and community obstetrician-gynecologists recognize that inpatient postpartum screening may be limited in its sensitivity for capturing all women who will go on to develop PPD, there is definitely a benefit to having a discussion about PPD and maternal mental health early and often throughout the postpartum period. For many women suffering in silence, a 6-week postpartum outpatient visit is too late, especially given that approximately one-third of women are lost to postpartum follow-up.29,30
Addressing barriers
A growing number of states have enacted policies to address the challenge of peripartum behavioral health needs, and several states – Illinois, Massachusetts, New Jersey, and West Virginia – now mandate routine PPD screening by health care providers.31 However, few of these laws or policies contain specific guidance, such as the optimal timing for screening, instead leaving the details to providers.32 The proper identification and management of PPD cannot be achieved by state-level policy mandates alone, but must include clinician buy-in and participation.
Obstetricians play an essential role in the identification and treatment of PPD. Among nonpsychiatric specialists, obstetrician-gynecologists are the most likely providers to see and screen during the perinatal period.33 In addition, women prefer to receive help for PPD from either their obstetric practitioners or a mental-health specialists located at the obstetric clinic, and are more likely to receive mental-health services if they are provided at the same location as that of the obstetric provider.34,35 According to ACOG’s new guidance on the fourth trimester, obstetricians are encouraged to take responsibility for women’s care immediately after birth, and this care would include contact with all mothers within the first 3 weeks post partum, at follow-up visits as needed, and for a comprehensive postpartum visit at 12 weeks.3
Our specialty has and will continue to evolve, and obstetric hospitalists will play an ever more essential role in the care of women during their inpatient obstetric admission. Whether we are a patient’s primary inpatient obstetric provider or a practice extender for single or multigroup practice, we are in a unique role to screen, begin treatment for, and offer anticipatory guidance for maternal mental health and postpartum depression disorders. Obstetric hospitalists can be a bridge between inpatient and outpatient follow-up and catalysts for implementing universal inpatient PPD screening. Our role presents an opportunity to start the discussion early and often in the fourth trimester and to make a significant difference in addressing this critical unmet need in postnatal care.
Dr. van Dis is the medical director of the Ob Hospitalist Group in Burbank, Calif. She disclosed she received editorial assistance from Erik MacLaren, PhD, of Boston Strategic Partners Inc., with funding support from Sage Therapeutics Inc. E-mail [email protected].
References
1. Centers for Disease Control and Prevention. Postpartum Depression. 2017.
2. Morb Mortal Wkly Rep. 2017;66(6):153-8.
3. Obstet Gynecol. 2018;131(5):e140-e150.
4. Harv Rev Psychiatry. 2014;22(1):1-22.
5. JAMA Psychiatry. 2018;75(3):247-53.
6. J Womens Health (Larchmt). 2016;25(12):1219-24.
7. J Psychiatr Res. 2017;84:284-91.
8. Br J Psychiatry. 2003;183:279-81.
9. Obstet Gynecol Surv. 2005;60(3):183-90.
10. Arch Womens Ment Health. 2016;19(1):87-94.
11. Soc Psychiatry Psychiatr Epidemiol. 2013;48(8):1335-45.
12. J Reprod Med. 1999;44(4):351-8.
13. J Behav Health Serv Res. 2004;31(2):117-33.
14. J Clin Psychiatry. 2016;77(9):1189-200.
15. Am J Obstet Gynecol. 2000;182(5):1080-2.
16. J Fam Pract. 2001;50(2):117-22.
17. Obstet Gynecol. 1999;93(5 Pt 1):653-7.
18. J Womens Health (Larchmt). 2010;19(3):477-90.
19. Obstet Gynecol. 2018;132:e208-12.
20. “Physicians back programs to address maternal mortality, depression,” AMA, Nov. 15, 2017
21. Pediatrics. 2019 Jan 1;143(1):e20183260.
22. JAMA. 2016;315(4):380-7.
23. Br J Psychiatry. 1987;150:782-6.
24. Ann Fam Med. 2009;7(1):63-70.
25. Obstet Gynecol. 2016;127(5):917-25.
26. Pediatrics. 2017 Oct;140(4). pii: e20170110.
27. Womens Health Issues. 2015;25(6):703-10.
28. J Psychosom Obstet Gynaecol. 2011;32(1):27-34.
29. Matern Child Health J. 2016;20(Suppl 1):22-7.
30. National Committee for Quality Assurance. Prenatal and Postpartum Care (PPC). 2018.
31. Psychiatr Serv. 2015;66(3):324-8.
32. Postpartum Support International. Legislation. 2018.
33. American Academy of Pediatrics, American College of Obstetricians and Gynecologists, eds. Guidelines for Perinatal Care. 7th ed. (Elk Grove Village, IL: Washington, DC: American Academy of Pediatrics; American College of Obstetricians and Gynecologists; Oct 2012.)
34. Birth. 2009;36(1):60-9.
35. Gen Hosp Psychiatry. 2009;31(2):155-62.
Postpartum depression (PPD) is the most common complication of pregnancy, and onset can occur at any time from pregnancy until up to 1 year post partum.1,2 The immediate postpartum period is a time during which care is shared among multiple providers for both mother and child, and the transition from inpatient to outpatient postpartum care can impede communication between those caring for the patient in each setting. In 2018, the American College of Obstetricians and Gynecologists published a committee opinion emphasizing the importance of the “fourth trimester” and calling for health care providers to assist women in navigating the transition from pre- to postpartum care.3 An important consideration of perinatal care is mental health care for the mother, including screening and care for postpartum depression; however, the optimal role for the obstetric hospitalist in providing such services has been unclear.
Estimates of the prevalence of PPD in new mothers in the United States varied by state from 8% to 20% in 2012, with an overall average of 12%.2 Left untreated, PPD may result in significant negative outcomes for women, their children, and families. The depressive symptoms of PPD may persist for months or years afterward,4 with one study finding elevated depressive symptoms in women up to 11 years post partum.5 Suicide is also a leading cause of pregnancy-related mortality associated with depressive symptoms.6-9 In addition, maternal postpartum depression symptoms have been associated with impaired mother-infant bonding at 6 months of age10 and decreased cognitive and fine motor development of children at 18 months.11
Importance of screening
Evidence from the literature shows that, without proper screening, approximately 50% of cases of PPD go undiagnosed, and that increasing the number of women being screened by perinatal providers is an important first step to improving outcomes.12-18 Current recommendations for the timing and frequency of screening for PPD vary among the published guidelines. ACOG recommends screening at least once during the perinatal period for depression and anxiety using a standardized, validated tool; an update of the ACOG committee opinion in 2018 also states: “It is recommended that all obstetrician-gynecologists and other obstetric care providers complete a full assessment of mood and emotional well-being (including screening for PPD and anxiety with a validated instrument) during the comprehensive postpartum visit for each patient.”19 The American Medical Association adopted new policies in 2017 promoting the implementation of a routine protocol for depression screening of perinatal women.20 The American Academy of Pediatrics recommends more frequent screening, with assessments at the 1-, 2-, 4-, and 6-month visits.21 Finally, the U.S. Preventive Services Task Force recommends screening for depression in the general population including pregnant and postpartum women.22
Multiple standardized, validated screening instruments are available for detecting possible symptoms of PPD, including the most widely used tools: the Edinburgh Postnatal Depression Scale (EPDS)19,23 and the Patient Health Questionnaire (PHQ-9).24 Two recent studies have shown that screening women for symptoms of PPD with a validated tool may reduce the duration or severity of depressive symptoms,25,26 further reinforcing the need to ensure that women experiencing symptoms of PPD are identified and treated early.
The inpatient hospitalization for labor, delivery, and birth of a child has not traditionally been viewed as an opportunity for PPD screening. While private practitioners and obstetric medical group practices typically have inquired about and documented the individual patient’s mental health history and risk factors for PPD, the obstetric hospitalist is most commonly meeting a patient in labor or in a postpartum encounter for the first time. As obstetric practices grow ever more consolidated, and as obstetric hospitalist care is implemented for a variety of reasons including, but not limited to, preventing burnout among private practitioners, serving as a safety net for all inpatient obstetric services, and increasing standardization in obstetric triage and obstetric emergency departments,
Barriers remain
Despite the need for early detection of PPD, screening practices remain inconsistent. A literature review of health care provider practices showed only one in four physicians reported using screening tools; obstetrician-gynecologists were most likely (36%) to use screening tools, followed by family practitioners (31%), with pediatricians the least likely (7%).27 This low rate is at least partially the result of perceived barriers to screening among health care providers, which contributes to underdiagnosis. A survey of more than 200 physicians who were members of ACOG showed that the top three barriers restricting screening practices were time constraints, inadequate training, and a lack of knowledge of the diagnostic criteria.28
Since 2017, Dignity Health has instituted routine screening of all inpatient postpartum patients at its 29 birth centers in Arizona, California, and Nevada. In this program, of which I am a physician participant, more than 30,000 women have been screened with the EPDS. In addition to providing screening, Dignity Health staff (physicians, certified nurse midwives, nurse practitioners, registered nurses, social workers, mental health therapists, lactation consultants, health educators, and others) have received in-person Perinatal Mental Health training. In this way, the entire care team coordinates inpatient screening and referral to outpatient care providers – thus bridging the gap in postpartum mental health care. For those patients who screen positive while an inpatient, a psychiatric telemedicine appointment is provided and, if necessary, short-course medications can be prescribed until the patient has outpatient follow-up and continuity of care. While we as obstetric hospitalists and community obstetrician-gynecologists recognize that inpatient postpartum screening may be limited in its sensitivity for capturing all women who will go on to develop PPD, there is definitely a benefit to having a discussion about PPD and maternal mental health early and often throughout the postpartum period. For many women suffering in silence, a 6-week postpartum outpatient visit is too late, especially given that approximately one-third of women are lost to postpartum follow-up.29,30
Addressing barriers
A growing number of states have enacted policies to address the challenge of peripartum behavioral health needs, and several states – Illinois, Massachusetts, New Jersey, and West Virginia – now mandate routine PPD screening by health care providers.31 However, few of these laws or policies contain specific guidance, such as the optimal timing for screening, instead leaving the details to providers.32 The proper identification and management of PPD cannot be achieved by state-level policy mandates alone, but must include clinician buy-in and participation.
Obstetricians play an essential role in the identification and treatment of PPD. Among nonpsychiatric specialists, obstetrician-gynecologists are the most likely providers to see and screen during the perinatal period.33 In addition, women prefer to receive help for PPD from either their obstetric practitioners or a mental-health specialists located at the obstetric clinic, and are more likely to receive mental-health services if they are provided at the same location as that of the obstetric provider.34,35 According to ACOG’s new guidance on the fourth trimester, obstetricians are encouraged to take responsibility for women’s care immediately after birth, and this care would include contact with all mothers within the first 3 weeks post partum, at follow-up visits as needed, and for a comprehensive postpartum visit at 12 weeks.3
Our specialty has and will continue to evolve, and obstetric hospitalists will play an ever more essential role in the care of women during their inpatient obstetric admission. Whether we are a patient’s primary inpatient obstetric provider or a practice extender for single or multigroup practice, we are in a unique role to screen, begin treatment for, and offer anticipatory guidance for maternal mental health and postpartum depression disorders. Obstetric hospitalists can be a bridge between inpatient and outpatient follow-up and catalysts for implementing universal inpatient PPD screening. Our role presents an opportunity to start the discussion early and often in the fourth trimester and to make a significant difference in addressing this critical unmet need in postnatal care.
Dr. van Dis is the medical director of the Ob Hospitalist Group in Burbank, Calif. She disclosed she received editorial assistance from Erik MacLaren, PhD, of Boston Strategic Partners Inc., with funding support from Sage Therapeutics Inc. E-mail [email protected].
References
1. Centers for Disease Control and Prevention. Postpartum Depression. 2017.
2. Morb Mortal Wkly Rep. 2017;66(6):153-8.
3. Obstet Gynecol. 2018;131(5):e140-e150.
4. Harv Rev Psychiatry. 2014;22(1):1-22.
5. JAMA Psychiatry. 2018;75(3):247-53.
6. J Womens Health (Larchmt). 2016;25(12):1219-24.
7. J Psychiatr Res. 2017;84:284-91.
8. Br J Psychiatry. 2003;183:279-81.
9. Obstet Gynecol Surv. 2005;60(3):183-90.
10. Arch Womens Ment Health. 2016;19(1):87-94.
11. Soc Psychiatry Psychiatr Epidemiol. 2013;48(8):1335-45.
12. J Reprod Med. 1999;44(4):351-8.
13. J Behav Health Serv Res. 2004;31(2):117-33.
14. J Clin Psychiatry. 2016;77(9):1189-200.
15. Am J Obstet Gynecol. 2000;182(5):1080-2.
16. J Fam Pract. 2001;50(2):117-22.
17. Obstet Gynecol. 1999;93(5 Pt 1):653-7.
18. J Womens Health (Larchmt). 2010;19(3):477-90.
19. Obstet Gynecol. 2018;132:e208-12.
20. “Physicians back programs to address maternal mortality, depression,” AMA, Nov. 15, 2017
21. Pediatrics. 2019 Jan 1;143(1):e20183260.
22. JAMA. 2016;315(4):380-7.
23. Br J Psychiatry. 1987;150:782-6.
24. Ann Fam Med. 2009;7(1):63-70.
25. Obstet Gynecol. 2016;127(5):917-25.
26. Pediatrics. 2017 Oct;140(4). pii: e20170110.
27. Womens Health Issues. 2015;25(6):703-10.
28. J Psychosom Obstet Gynaecol. 2011;32(1):27-34.
29. Matern Child Health J. 2016;20(Suppl 1):22-7.
30. National Committee for Quality Assurance. Prenatal and Postpartum Care (PPC). 2018.
31. Psychiatr Serv. 2015;66(3):324-8.
32. Postpartum Support International. Legislation. 2018.
33. American Academy of Pediatrics, American College of Obstetricians and Gynecologists, eds. Guidelines for Perinatal Care. 7th ed. (Elk Grove Village, IL: Washington, DC: American Academy of Pediatrics; American College of Obstetricians and Gynecologists; Oct 2012.)
34. Birth. 2009;36(1):60-9.
35. Gen Hosp Psychiatry. 2009;31(2):155-62.
Biomarkers predict VTE risk with menopausal oral hormone therapy
CHICAGO – An elevated baseline D-dimer level is helpful to women and their physicians in clarifying decision making about oral hormone therapy for troublesome menopausal symptoms, Mary Cushman, MD, said at the American Heart Association scientific sessions.
She was lead investigator in a nested case-control study embedded in the landmark Women’s Health Initiative (WHI), which showed that participants who had a baseline D-dimer greater than 0.54 mg/L – putting them in the top 25% – and were randomized to oral menopausal hormone therapy had a 5-year incidence of venous thromboembolism (VTE) of 6%. That’s 500% higher than in women with a lower D-dimer randomized to placebo.
“The number needed to test for D-dimer in advance of prescribing in order to prevent one VTE over 5 years of hormone therapy was only 33. So this is potentially something in the toolbox you can use in counseling women about oral hormone therapy,” said Dr. Cushman, professor of medicine and pathology and medical director of the thrombosis and hemostasis program at the University of Vermont, Burlington.
The biomarker study included 1,082 WHI participants aged 50-79 years randomized to oral conjugated equine estrogen with or without medroxyprogesterone acetate or to placebo, 215 of whom experienced VTE during a mean 4.1 years of follow-up. Levels of a variety of biomarkers obtained at baseline were assessed in terms of their associated risk of future VTE. The biomarkers included C-reactive protein and procoagulant, anticoagulant, and fibrinolytic factors.
In a logistic regression analysis adjusted for age, race, body mass index, and hysterectomy, the strongest association with VTE was a high D-dimer. That 500% increased risk of VTE with hormone therapy in women with a D-dimer greater than 0.54 mg/L was comparable in magnitude with the risk Dr. Cushman and her coinvestigators previously reported for the combination of factor V Leiden and hormone therapy.
Dr. Cushman and her associates also took a first step towards developing a multibiomarker risk score. They found that WHI participants randomized to hormone therapy who had abnormal baseline values for any three or more of eight biomarkers had a 1,450% greater risk of future VTE than women with zero or one abnormal biomarker who were assigned to placebo. The eight-biomarker panel described in the recently published study comprised D-dimer, factor V Leiden, protein C, total protein S, free protein S, antithrombin, plasmin-antiplasmin complex, and fragment 1.2. However, the investigators indicated the risk score needs further study before it’s ready for adoption in clinical practice (Res Pract Thromb Haemost. 2018 Apr 17;2[2]:310-9).
Dr. Cushman noted that, although the main findings of the WHI have largely resulted in abandonment of menopausal hormone therapy for disease prevention, many women still want to take oral hormone therapy for relief of bothersome menopausal symptoms. She tries to steer them instead to safer nonoral formulations. Transdermal estrogen replacement has no associated risk of VTE and doesn’t activate anticoagulation. Neither does vaginal estradiol.
In offering what she called “the 30,000-foot view of the impact of venous thrombosis on women’s health,” Dr. Cushman noted that VTE is the third-most common vascular disease in the United States, with up to 900,000 cases per year. The lifetime risk in women after age 45 is 8.4%. Half of VTEs are provoked and therefore potentially preventable, with common triggers being surgery, cancer, pregnancy, trauma, and immobilization, especially during travel.
In addition, a retrospective study conducted in the Worcester, Mass., area showed that 1-month mortality after VTE remained static in the 5%-10% range during 1999-2009.
“This is a fatal disease, even though we treat it as an outpatient quite a lot,” Dr. Cushman observed.
Common nonfatal complications of VTE include major bleeding in 5%-10% of cases, a recurrence rate of 5%-10% annually, a 20%-40% of the burdensome and not infrequently disabling condition known as postthrombotic syndrome, and a 3%-4% incidence of chronic thromboembolic pulmonary hypertension. Yet despite the seriousness of VTE, awareness about VTE is poor among both patients and physicians, and appropriate prophylaxis is underutilized, she said.
The key to improved primary prevention of VTE, Dr. Cushman continued, is greater attention to modifiable behavioral risk factors, along with more use of prophylactic medication when needed.
The traditional cardiovascular risk factors, like hypertension, smoking, and hyperlipidemia, aren’t relevant to VTE risk. But obesity and sedentary lifestyle have come to be recognized as important modifiable risk factors. In one study of more than 30,000 Americans, the risk of VTE was shown to be reduced by 40% in individuals who exercised at least four times per week, compared with the physically inactive.
And in an analysis led by Dr. Cushman of nearly 21,000 participants over age 45 years with 12.6 years of follow-up in the Longitudinal Investigation of Thromboembolism Etiology (LITE), the investigators found that greater levels of all body size measures – not just body mass index, but calf circumference, waist-hip ratio, hip circumference, and others – were associated with increased VTE risk. These associations weren’t affected by levels of circulating biomarkers for inflammation or hypercoagulability, suggesting that it’s obesity per se, with its associated adverse impact on blood flow caused by physical factors, that explains the mechanism underlying obesity as a risk factor for VTE (Thromb Res. 2016 Aug;144:127-32).
At the meeting’s opening ceremonies, AHA President Ivor Benjamin, MD, of the Medical College of Wisconsin, Milwaukee, presented Dr. Cushman with the AHA Population Research Prize. She was honored for her “critically acclaimed research utilizing biomarker assessments in population studies to elucidate pathways of disease etiology for the three most common vascular diseases – coronary heart disease, stroke, and venous thromboembolism – as well as their risk factors,” said Dr. Benjamin.
Dr. Cushman reported having no financial conflicts regarding her D-dimer study, which was funded by the National Institutes of Health.
CHICAGO – An elevated baseline D-dimer level is helpful to women and their physicians in clarifying decision making about oral hormone therapy for troublesome menopausal symptoms, Mary Cushman, MD, said at the American Heart Association scientific sessions.
She was lead investigator in a nested case-control study embedded in the landmark Women’s Health Initiative (WHI), which showed that participants who had a baseline D-dimer greater than 0.54 mg/L – putting them in the top 25% – and were randomized to oral menopausal hormone therapy had a 5-year incidence of venous thromboembolism (VTE) of 6%. That’s 500% higher than in women with a lower D-dimer randomized to placebo.
“The number needed to test for D-dimer in advance of prescribing in order to prevent one VTE over 5 years of hormone therapy was only 33. So this is potentially something in the toolbox you can use in counseling women about oral hormone therapy,” said Dr. Cushman, professor of medicine and pathology and medical director of the thrombosis and hemostasis program at the University of Vermont, Burlington.
The biomarker study included 1,082 WHI participants aged 50-79 years randomized to oral conjugated equine estrogen with or without medroxyprogesterone acetate or to placebo, 215 of whom experienced VTE during a mean 4.1 years of follow-up. Levels of a variety of biomarkers obtained at baseline were assessed in terms of their associated risk of future VTE. The biomarkers included C-reactive protein and procoagulant, anticoagulant, and fibrinolytic factors.
In a logistic regression analysis adjusted for age, race, body mass index, and hysterectomy, the strongest association with VTE was a high D-dimer. That 500% increased risk of VTE with hormone therapy in women with a D-dimer greater than 0.54 mg/L was comparable in magnitude with the risk Dr. Cushman and her coinvestigators previously reported for the combination of factor V Leiden and hormone therapy.
Dr. Cushman and her associates also took a first step towards developing a multibiomarker risk score. They found that WHI participants randomized to hormone therapy who had abnormal baseline values for any three or more of eight biomarkers had a 1,450% greater risk of future VTE than women with zero or one abnormal biomarker who were assigned to placebo. The eight-biomarker panel described in the recently published study comprised D-dimer, factor V Leiden, protein C, total protein S, free protein S, antithrombin, plasmin-antiplasmin complex, and fragment 1.2. However, the investigators indicated the risk score needs further study before it’s ready for adoption in clinical practice (Res Pract Thromb Haemost. 2018 Apr 17;2[2]:310-9).
Dr. Cushman noted that, although the main findings of the WHI have largely resulted in abandonment of menopausal hormone therapy for disease prevention, many women still want to take oral hormone therapy for relief of bothersome menopausal symptoms. She tries to steer them instead to safer nonoral formulations. Transdermal estrogen replacement has no associated risk of VTE and doesn’t activate anticoagulation. Neither does vaginal estradiol.
In offering what she called “the 30,000-foot view of the impact of venous thrombosis on women’s health,” Dr. Cushman noted that VTE is the third-most common vascular disease in the United States, with up to 900,000 cases per year. The lifetime risk in women after age 45 is 8.4%. Half of VTEs are provoked and therefore potentially preventable, with common triggers being surgery, cancer, pregnancy, trauma, and immobilization, especially during travel.
In addition, a retrospective study conducted in the Worcester, Mass., area showed that 1-month mortality after VTE remained static in the 5%-10% range during 1999-2009.
“This is a fatal disease, even though we treat it as an outpatient quite a lot,” Dr. Cushman observed.
Common nonfatal complications of VTE include major bleeding in 5%-10% of cases, a recurrence rate of 5%-10% annually, a 20%-40% of the burdensome and not infrequently disabling condition known as postthrombotic syndrome, and a 3%-4% incidence of chronic thromboembolic pulmonary hypertension. Yet despite the seriousness of VTE, awareness about VTE is poor among both patients and physicians, and appropriate prophylaxis is underutilized, she said.
The key to improved primary prevention of VTE, Dr. Cushman continued, is greater attention to modifiable behavioral risk factors, along with more use of prophylactic medication when needed.
The traditional cardiovascular risk factors, like hypertension, smoking, and hyperlipidemia, aren’t relevant to VTE risk. But obesity and sedentary lifestyle have come to be recognized as important modifiable risk factors. In one study of more than 30,000 Americans, the risk of VTE was shown to be reduced by 40% in individuals who exercised at least four times per week, compared with the physically inactive.
And in an analysis led by Dr. Cushman of nearly 21,000 participants over age 45 years with 12.6 years of follow-up in the Longitudinal Investigation of Thromboembolism Etiology (LITE), the investigators found that greater levels of all body size measures – not just body mass index, but calf circumference, waist-hip ratio, hip circumference, and others – were associated with increased VTE risk. These associations weren’t affected by levels of circulating biomarkers for inflammation or hypercoagulability, suggesting that it’s obesity per se, with its associated adverse impact on blood flow caused by physical factors, that explains the mechanism underlying obesity as a risk factor for VTE (Thromb Res. 2016 Aug;144:127-32).
At the meeting’s opening ceremonies, AHA President Ivor Benjamin, MD, of the Medical College of Wisconsin, Milwaukee, presented Dr. Cushman with the AHA Population Research Prize. She was honored for her “critically acclaimed research utilizing biomarker assessments in population studies to elucidate pathways of disease etiology for the three most common vascular diseases – coronary heart disease, stroke, and venous thromboembolism – as well as their risk factors,” said Dr. Benjamin.
Dr. Cushman reported having no financial conflicts regarding her D-dimer study, which was funded by the National Institutes of Health.
CHICAGO – An elevated baseline D-dimer level is helpful to women and their physicians in clarifying decision making about oral hormone therapy for troublesome menopausal symptoms, Mary Cushman, MD, said at the American Heart Association scientific sessions.
She was lead investigator in a nested case-control study embedded in the landmark Women’s Health Initiative (WHI), which showed that participants who had a baseline D-dimer greater than 0.54 mg/L – putting them in the top 25% – and were randomized to oral menopausal hormone therapy had a 5-year incidence of venous thromboembolism (VTE) of 6%. That’s 500% higher than in women with a lower D-dimer randomized to placebo.
“The number needed to test for D-dimer in advance of prescribing in order to prevent one VTE over 5 years of hormone therapy was only 33. So this is potentially something in the toolbox you can use in counseling women about oral hormone therapy,” said Dr. Cushman, professor of medicine and pathology and medical director of the thrombosis and hemostasis program at the University of Vermont, Burlington.
The biomarker study included 1,082 WHI participants aged 50-79 years randomized to oral conjugated equine estrogen with or without medroxyprogesterone acetate or to placebo, 215 of whom experienced VTE during a mean 4.1 years of follow-up. Levels of a variety of biomarkers obtained at baseline were assessed in terms of their associated risk of future VTE. The biomarkers included C-reactive protein and procoagulant, anticoagulant, and fibrinolytic factors.
In a logistic regression analysis adjusted for age, race, body mass index, and hysterectomy, the strongest association with VTE was a high D-dimer. That 500% increased risk of VTE with hormone therapy in women with a D-dimer greater than 0.54 mg/L was comparable in magnitude with the risk Dr. Cushman and her coinvestigators previously reported for the combination of factor V Leiden and hormone therapy.
Dr. Cushman and her associates also took a first step towards developing a multibiomarker risk score. They found that WHI participants randomized to hormone therapy who had abnormal baseline values for any three or more of eight biomarkers had a 1,450% greater risk of future VTE than women with zero or one abnormal biomarker who were assigned to placebo. The eight-biomarker panel described in the recently published study comprised D-dimer, factor V Leiden, protein C, total protein S, free protein S, antithrombin, plasmin-antiplasmin complex, and fragment 1.2. However, the investigators indicated the risk score needs further study before it’s ready for adoption in clinical practice (Res Pract Thromb Haemost. 2018 Apr 17;2[2]:310-9).
Dr. Cushman noted that, although the main findings of the WHI have largely resulted in abandonment of menopausal hormone therapy for disease prevention, many women still want to take oral hormone therapy for relief of bothersome menopausal symptoms. She tries to steer them instead to safer nonoral formulations. Transdermal estrogen replacement has no associated risk of VTE and doesn’t activate anticoagulation. Neither does vaginal estradiol.
In offering what she called “the 30,000-foot view of the impact of venous thrombosis on women’s health,” Dr. Cushman noted that VTE is the third-most common vascular disease in the United States, with up to 900,000 cases per year. The lifetime risk in women after age 45 is 8.4%. Half of VTEs are provoked and therefore potentially preventable, with common triggers being surgery, cancer, pregnancy, trauma, and immobilization, especially during travel.
In addition, a retrospective study conducted in the Worcester, Mass., area showed that 1-month mortality after VTE remained static in the 5%-10% range during 1999-2009.
“This is a fatal disease, even though we treat it as an outpatient quite a lot,” Dr. Cushman observed.
Common nonfatal complications of VTE include major bleeding in 5%-10% of cases, a recurrence rate of 5%-10% annually, a 20%-40% of the burdensome and not infrequently disabling condition known as postthrombotic syndrome, and a 3%-4% incidence of chronic thromboembolic pulmonary hypertension. Yet despite the seriousness of VTE, awareness about VTE is poor among both patients and physicians, and appropriate prophylaxis is underutilized, she said.
The key to improved primary prevention of VTE, Dr. Cushman continued, is greater attention to modifiable behavioral risk factors, along with more use of prophylactic medication when needed.
The traditional cardiovascular risk factors, like hypertension, smoking, and hyperlipidemia, aren’t relevant to VTE risk. But obesity and sedentary lifestyle have come to be recognized as important modifiable risk factors. In one study of more than 30,000 Americans, the risk of VTE was shown to be reduced by 40% in individuals who exercised at least four times per week, compared with the physically inactive.
And in an analysis led by Dr. Cushman of nearly 21,000 participants over age 45 years with 12.6 years of follow-up in the Longitudinal Investigation of Thromboembolism Etiology (LITE), the investigators found that greater levels of all body size measures – not just body mass index, but calf circumference, waist-hip ratio, hip circumference, and others – were associated with increased VTE risk. These associations weren’t affected by levels of circulating biomarkers for inflammation or hypercoagulability, suggesting that it’s obesity per se, with its associated adverse impact on blood flow caused by physical factors, that explains the mechanism underlying obesity as a risk factor for VTE (Thromb Res. 2016 Aug;144:127-32).
At the meeting’s opening ceremonies, AHA President Ivor Benjamin, MD, of the Medical College of Wisconsin, Milwaukee, presented Dr. Cushman with the AHA Population Research Prize. She was honored for her “critically acclaimed research utilizing biomarker assessments in population studies to elucidate pathways of disease etiology for the three most common vascular diseases – coronary heart disease, stroke, and venous thromboembolism – as well as their risk factors,” said Dr. Benjamin.
Dr. Cushman reported having no financial conflicts regarding her D-dimer study, which was funded by the National Institutes of Health.
REPORTING FROM THE AHA SCIENTIFIC SESSIONS
Key clinical point:
Major finding: Women in the top 25% for D-dimer level before going on menopausal hormone therapy had a 6% incidence of venous thromboembolism over 5 years.
Study details: This was a nested case-control study focused on identifying biomarkers for venous thromboembolism risk which included 1,082 participants in the Women’s Health Initiative randomized to menopausal hormone therapy or placebo.
Disclosures: The presenter reported having no financial conflicts regarding the study, which was funded by the National Institutes of Health.
Click for Credit: Missed HIV screening opps; aspirin & preeclampsia; more
Here are 5 articles from the February issue of Clinician Reviews (individual articles are valid for one year from date of publication—expiration dates below):
1. Short-term lung function better predicts mortality risk in SSc
To take the posttest, go to: https://bit.ly/2RrRuIY
Expires November 26, 2019
2. Healthier lifestyle in midlife women reduces subclinical carotid atherosclerosis
To take the posttest, go to: https://bit.ly/2TvDH5G
Expires November 28, 2019
3. Three commonly used quick cognitive assessments often yield flawed results
To take the posttest, go to: https://bit.ly/2G1qkHn
Expires November 28, 2019
4. Missed HIV screening opportunities found among subsequently infected youth
To take the posttest, go to: https://bit.ly/2HGa8Nm
Expires November 29, 2019
5. Aspirin appears underused to prevent preeclampsia in SLE patients
To take the posttest, go to: https://bit.ly/2G0dU2v
Expires January 2, 2019
Here are 5 articles from the February issue of Clinician Reviews (individual articles are valid for one year from date of publication—expiration dates below):
1. Short-term lung function better predicts mortality risk in SSc
To take the posttest, go to: https://bit.ly/2RrRuIY
Expires November 26, 2019
2. Healthier lifestyle in midlife women reduces subclinical carotid atherosclerosis
To take the posttest, go to: https://bit.ly/2TvDH5G
Expires November 28, 2019
3. Three commonly used quick cognitive assessments often yield flawed results
To take the posttest, go to: https://bit.ly/2G1qkHn
Expires November 28, 2019
4. Missed HIV screening opportunities found among subsequently infected youth
To take the posttest, go to: https://bit.ly/2HGa8Nm
Expires November 29, 2019
5. Aspirin appears underused to prevent preeclampsia in SLE patients
To take the posttest, go to: https://bit.ly/2G0dU2v
Expires January 2, 2019
Here are 5 articles from the February issue of Clinician Reviews (individual articles are valid for one year from date of publication—expiration dates below):
1. Short-term lung function better predicts mortality risk in SSc
To take the posttest, go to: https://bit.ly/2RrRuIY
Expires November 26, 2019
2. Healthier lifestyle in midlife women reduces subclinical carotid atherosclerosis
To take the posttest, go to: https://bit.ly/2TvDH5G
Expires November 28, 2019
3. Three commonly used quick cognitive assessments often yield flawed results
To take the posttest, go to: https://bit.ly/2G1qkHn
Expires November 28, 2019
4. Missed HIV screening opportunities found among subsequently infected youth
To take the posttest, go to: https://bit.ly/2HGa8Nm
Expires November 29, 2019
5. Aspirin appears underused to prevent preeclampsia in SLE patients
To take the posttest, go to: https://bit.ly/2G0dU2v
Expires January 2, 2019
Pregnancy problems predict cardiovascular future
SNOWMASS, COLO. – Think of pregnancy as a cardiovascular stress test, Carole A. Warnes, MD, urged at the Annual Cardiovascular Conference at Snowmass sponsored by the American College of Cardiology.
Pregnancy complications may unmask a predisposition to premature cardiovascular disease. Yet a woman’s reproductive history is often overlooked in this regard, despite the fact that cardiovascular disease is the number-one cause of death in women, observed Dr. Warnes, the Snowmass conference director and professor of medicine at the Mayo Clinic in Rochester, Minn.
“I think reproductive history is often overlooked as a predictor of cardiovascular and even peripheral vascular events. I suspect many of us don’t routinely ask our patients about miscarriages and stillbirths. We might think about preeclampsia, but these are also hallmarks of trouble to come,” the cardiologist said.
Indeed, this point was underscored in a retrospective Danish national population-based cohort registry study of more than 1 million women followed for nearly 16 million person-years after one or more miscarriages, stillbirths, or live singleton births. Women with stillbirths were 2.69-fold more likely to have an MI, 2.42-fold more likely to develop renovascular hypertension, and 1.74-fold more likely to have a stroke during follow-up than those with no stillbirths.
Moreover, women with miscarriages were 1.13-, 1.2-, and 1.16-fold more likely to have an MI, renovascular hypertension, and stroke, respectively, than women with no miscarriages. And the risks were additive: For each additional miscarriage, the risks of MI, renovascular hypertension, and stroke increased by 9%, 19%, and 13%, respectively (Circulation. 2013;127[17]:1775-82).
The concept of maternal placental syndromes encompasses four events believed to originate from diseased placental blood vessels: preeclampsia, gestational hypertension, placental abruption, and placental infarction. In a population-based retrospective study known as CHAMPS (Cardiovascular Health After Maternal Placental Syndromes), conducted in more than 1 million Ontario women who were free from cardiovascular disease prior to their first delivery, 7% were diagnosed with a maternal placental syndrome. Their incidence of a composite endpoint comprised of hospitalization or revascularization for CAD, peripheral artery disease, or cerebrovascular disease at least 90 days after delivery discharge was double that of women without a maternal placental syndrome.
“These women manifested their first cardiovascular event at an average age of 38, not 50 or 60,” Dr. Warnes said.
The risk of premature cardiovascular disease was magnified 4.4-fold in women with a maternal placental syndrome plus an intrauterine fetal death, compared with those with neither, after adjustment for sociodemographic factors and other potential confounders, and by 3.1-fold in women with a maternal placental syndrome and poor fetal growth (Lancet. 2005;366[9499]:1797-803).
These findings were independently confirmed recently in a population-based retrospective study of nearly 303,000 Florida women free of prepregnancy hypertension, diabetes, heart disease, or renal disease who were followed for a median of 4.9 years after their first delivery. During that relative brief follow-up period, the adjusted risk of cardiovascular disease was increased by 19% in those with a maternal placental syndrome, compared with those without. And the risk was additive: women with more than one maternal placental syndrome had a 43% greater short-term risk of developing cardiovascular disease, compared with those with none. And when women with a maternal placental syndrome also had a preterm birth or a small-for-gestational age baby, their risk increased 45% (Am J Obstet Gynecol. 2016;215[4]:484.e1-484.e14).
It’s not just preeclampsia, which affects 3%-5% of all pregnancies, and gestational hypertension – defined as high blood pressure arising only after 20 weeks’ gestation and without proteinuria – that have been linked to future premature cardiovascular disease. In the Northern Finland Birth Cohort 1966, in which investigators have followed 10,314 women born in that year for 39 years, any form of high blood pressure during pregnancy was a harbinger of subsequent cardiovascular disease, diabetes, and chronic kidney disease. That included chronic isolated systolic and isolated diastolic hypertension (Circulation. 2013;127[6]:681-90).
The pathophysiologic processes involved in complicated pregnancies echo those of CAD and stroke: inflammation, altered angiogenesis, vasculopathy, thrombosis, and insulin resistance. Still unsettled, however, is the chicken-versus-egg question of whether preeclampsia and other pregnancy complications represent the initial expression of an adverse phenotype associated with early development of cardiovascular disease or the complications injure the vascular endothelium and thereby trigger accelerated atherosclerosis. In any case, markers of endothelial activation have been documented up to 15 years after an episode of preeclampsia, Dr. Warnes said.
All of these data underscore the importance of identifying at-risk women based upon reproductive history, scheduling additional medical checkups so they don’t drop off the radar for the next 20 years, encouraging lifestyle modification, and giving consideration to early initiation of antihypertensive and lipid-lowering therapies.
“Pregnancy complications give us a glimpse of this awful disease trajectory at a time when women are completely asymptomatic and we could intervene and perhaps change outcomes with targeted therapy when it might be expected to work better and patients might be more receptive to such interventions,” she said.
Dr. Warnes reported having no financial conflicts of interest.
SNOWMASS, COLO. – Think of pregnancy as a cardiovascular stress test, Carole A. Warnes, MD, urged at the Annual Cardiovascular Conference at Snowmass sponsored by the American College of Cardiology.
Pregnancy complications may unmask a predisposition to premature cardiovascular disease. Yet a woman’s reproductive history is often overlooked in this regard, despite the fact that cardiovascular disease is the number-one cause of death in women, observed Dr. Warnes, the Snowmass conference director and professor of medicine at the Mayo Clinic in Rochester, Minn.
“I think reproductive history is often overlooked as a predictor of cardiovascular and even peripheral vascular events. I suspect many of us don’t routinely ask our patients about miscarriages and stillbirths. We might think about preeclampsia, but these are also hallmarks of trouble to come,” the cardiologist said.
Indeed, this point was underscored in a retrospective Danish national population-based cohort registry study of more than 1 million women followed for nearly 16 million person-years after one or more miscarriages, stillbirths, or live singleton births. Women with stillbirths were 2.69-fold more likely to have an MI, 2.42-fold more likely to develop renovascular hypertension, and 1.74-fold more likely to have a stroke during follow-up than those with no stillbirths.
Moreover, women with miscarriages were 1.13-, 1.2-, and 1.16-fold more likely to have an MI, renovascular hypertension, and stroke, respectively, than women with no miscarriages. And the risks were additive: For each additional miscarriage, the risks of MI, renovascular hypertension, and stroke increased by 9%, 19%, and 13%, respectively (Circulation. 2013;127[17]:1775-82).
The concept of maternal placental syndromes encompasses four events believed to originate from diseased placental blood vessels: preeclampsia, gestational hypertension, placental abruption, and placental infarction. In a population-based retrospective study known as CHAMPS (Cardiovascular Health After Maternal Placental Syndromes), conducted in more than 1 million Ontario women who were free from cardiovascular disease prior to their first delivery, 7% were diagnosed with a maternal placental syndrome. Their incidence of a composite endpoint comprised of hospitalization or revascularization for CAD, peripheral artery disease, or cerebrovascular disease at least 90 days after delivery discharge was double that of women without a maternal placental syndrome.
“These women manifested their first cardiovascular event at an average age of 38, not 50 or 60,” Dr. Warnes said.
The risk of premature cardiovascular disease was magnified 4.4-fold in women with a maternal placental syndrome plus an intrauterine fetal death, compared with those with neither, after adjustment for sociodemographic factors and other potential confounders, and by 3.1-fold in women with a maternal placental syndrome and poor fetal growth (Lancet. 2005;366[9499]:1797-803).
These findings were independently confirmed recently in a population-based retrospective study of nearly 303,000 Florida women free of prepregnancy hypertension, diabetes, heart disease, or renal disease who were followed for a median of 4.9 years after their first delivery. During that relative brief follow-up period, the adjusted risk of cardiovascular disease was increased by 19% in those with a maternal placental syndrome, compared with those without. And the risk was additive: women with more than one maternal placental syndrome had a 43% greater short-term risk of developing cardiovascular disease, compared with those with none. And when women with a maternal placental syndrome also had a preterm birth or a small-for-gestational age baby, their risk increased 45% (Am J Obstet Gynecol. 2016;215[4]:484.e1-484.e14).
It’s not just preeclampsia, which affects 3%-5% of all pregnancies, and gestational hypertension – defined as high blood pressure arising only after 20 weeks’ gestation and without proteinuria – that have been linked to future premature cardiovascular disease. In the Northern Finland Birth Cohort 1966, in which investigators have followed 10,314 women born in that year for 39 years, any form of high blood pressure during pregnancy was a harbinger of subsequent cardiovascular disease, diabetes, and chronic kidney disease. That included chronic isolated systolic and isolated diastolic hypertension (Circulation. 2013;127[6]:681-90).
The pathophysiologic processes involved in complicated pregnancies echo those of CAD and stroke: inflammation, altered angiogenesis, vasculopathy, thrombosis, and insulin resistance. Still unsettled, however, is the chicken-versus-egg question of whether preeclampsia and other pregnancy complications represent the initial expression of an adverse phenotype associated with early development of cardiovascular disease or the complications injure the vascular endothelium and thereby trigger accelerated atherosclerosis. In any case, markers of endothelial activation have been documented up to 15 years after an episode of preeclampsia, Dr. Warnes said.
All of these data underscore the importance of identifying at-risk women based upon reproductive history, scheduling additional medical checkups so they don’t drop off the radar for the next 20 years, encouraging lifestyle modification, and giving consideration to early initiation of antihypertensive and lipid-lowering therapies.
“Pregnancy complications give us a glimpse of this awful disease trajectory at a time when women are completely asymptomatic and we could intervene and perhaps change outcomes with targeted therapy when it might be expected to work better and patients might be more receptive to such interventions,” she said.
Dr. Warnes reported having no financial conflicts of interest.
SNOWMASS, COLO. – Think of pregnancy as a cardiovascular stress test, Carole A. Warnes, MD, urged at the Annual Cardiovascular Conference at Snowmass sponsored by the American College of Cardiology.
Pregnancy complications may unmask a predisposition to premature cardiovascular disease. Yet a woman’s reproductive history is often overlooked in this regard, despite the fact that cardiovascular disease is the number-one cause of death in women, observed Dr. Warnes, the Snowmass conference director and professor of medicine at the Mayo Clinic in Rochester, Minn.
“I think reproductive history is often overlooked as a predictor of cardiovascular and even peripheral vascular events. I suspect many of us don’t routinely ask our patients about miscarriages and stillbirths. We might think about preeclampsia, but these are also hallmarks of trouble to come,” the cardiologist said.
Indeed, this point was underscored in a retrospective Danish national population-based cohort registry study of more than 1 million women followed for nearly 16 million person-years after one or more miscarriages, stillbirths, or live singleton births. Women with stillbirths were 2.69-fold more likely to have an MI, 2.42-fold more likely to develop renovascular hypertension, and 1.74-fold more likely to have a stroke during follow-up than those with no stillbirths.
Moreover, women with miscarriages were 1.13-, 1.2-, and 1.16-fold more likely to have an MI, renovascular hypertension, and stroke, respectively, than women with no miscarriages. And the risks were additive: For each additional miscarriage, the risks of MI, renovascular hypertension, and stroke increased by 9%, 19%, and 13%, respectively (Circulation. 2013;127[17]:1775-82).
The concept of maternal placental syndromes encompasses four events believed to originate from diseased placental blood vessels: preeclampsia, gestational hypertension, placental abruption, and placental infarction. In a population-based retrospective study known as CHAMPS (Cardiovascular Health After Maternal Placental Syndromes), conducted in more than 1 million Ontario women who were free from cardiovascular disease prior to their first delivery, 7% were diagnosed with a maternal placental syndrome. Their incidence of a composite endpoint comprised of hospitalization or revascularization for CAD, peripheral artery disease, or cerebrovascular disease at least 90 days after delivery discharge was double that of women without a maternal placental syndrome.
“These women manifested their first cardiovascular event at an average age of 38, not 50 or 60,” Dr. Warnes said.
The risk of premature cardiovascular disease was magnified 4.4-fold in women with a maternal placental syndrome plus an intrauterine fetal death, compared with those with neither, after adjustment for sociodemographic factors and other potential confounders, and by 3.1-fold in women with a maternal placental syndrome and poor fetal growth (Lancet. 2005;366[9499]:1797-803).
These findings were independently confirmed recently in a population-based retrospective study of nearly 303,000 Florida women free of prepregnancy hypertension, diabetes, heart disease, or renal disease who were followed for a median of 4.9 years after their first delivery. During that relative brief follow-up period, the adjusted risk of cardiovascular disease was increased by 19% in those with a maternal placental syndrome, compared with those without. And the risk was additive: women with more than one maternal placental syndrome had a 43% greater short-term risk of developing cardiovascular disease, compared with those with none. And when women with a maternal placental syndrome also had a preterm birth or a small-for-gestational age baby, their risk increased 45% (Am J Obstet Gynecol. 2016;215[4]:484.e1-484.e14).
It’s not just preeclampsia, which affects 3%-5% of all pregnancies, and gestational hypertension – defined as high blood pressure arising only after 20 weeks’ gestation and without proteinuria – that have been linked to future premature cardiovascular disease. In the Northern Finland Birth Cohort 1966, in which investigators have followed 10,314 women born in that year for 39 years, any form of high blood pressure during pregnancy was a harbinger of subsequent cardiovascular disease, diabetes, and chronic kidney disease. That included chronic isolated systolic and isolated diastolic hypertension (Circulation. 2013;127[6]:681-90).
The pathophysiologic processes involved in complicated pregnancies echo those of CAD and stroke: inflammation, altered angiogenesis, vasculopathy, thrombosis, and insulin resistance. Still unsettled, however, is the chicken-versus-egg question of whether preeclampsia and other pregnancy complications represent the initial expression of an adverse phenotype associated with early development of cardiovascular disease or the complications injure the vascular endothelium and thereby trigger accelerated atherosclerosis. In any case, markers of endothelial activation have been documented up to 15 years after an episode of preeclampsia, Dr. Warnes said.
All of these data underscore the importance of identifying at-risk women based upon reproductive history, scheduling additional medical checkups so they don’t drop off the radar for the next 20 years, encouraging lifestyle modification, and giving consideration to early initiation of antihypertensive and lipid-lowering therapies.
“Pregnancy complications give us a glimpse of this awful disease trajectory at a time when women are completely asymptomatic and we could intervene and perhaps change outcomes with targeted therapy when it might be expected to work better and patients might be more receptive to such interventions,” she said.
Dr. Warnes reported having no financial conflicts of interest.
EXPERT ANALYSIS FROM ACC SNOWMASS 2019
Breast augmentation surgery: Clinical considerations
At present, 300,000 US women undergo breast augmentation surgery each year,1 making this the second most common aesthetic procedure in women (after liposuction),2–4 and making it extremely likely that clinicians will encounter women who have breast implants. In addition, approximately 110,000 women undergo breast reconstructive surgery after mastectomy, of whom more than 88,000 (81%) receive implants (2016 data).5
This review discusses the evolution of breast implants, their complications, and key considerations with regard to aesthetic and reconstructive breast surgery, as the principles are similar.
EVOLUTION OF IMPLANTS
Reports of breast augmentation surgery, also known as augmentation mammoplasty, date back to 1895, when a fatty tumor (lipoma) was successfully transplanted from a patient’s back to a breast defect in a mastectomy patient.2,3,6,7 In the 1930s, implantation of a glass ball into a patient’s breast marked the first implant-based breast augmentation.6 By 1954, attempts at breast augmentation using local dermal-fat flaps, adipose tissue, and even omentum were described.
Alloplastic materials gained popularity throughout the 1950s and 1960s and included polyurethane, polytetrafluoroethylene (Teflon), and other synthetics. Adverse reactions associated with alloplastic materials were plentiful: local tissue reactions, distortion of the breast mound, increased firmness, and discomfort all contributed to the eventual discontinuation of their use. The history of alloplastic breast augmentation also included epoxy resin, shellac, beeswax, paraffin, rubber, petroleum jelly, and liquefied silicone. Outcomes were not good, and many patients ultimately needed mastectomy.7
The first modern breast prosthesis was developed in 1961, and since then, implant composition and design have evolved significantly.8
From silicone to saline, and back again
The first silicone gel implants, introduced in the early 1960s,8–19 had high complication rates—some centers reported an incidence of capsular contracture of up to 70%.8,11 This is a foreign body reaction in which pathologic scar tissue encases the implant, causing it to distort, appear misshapen, harden, and even become painful.11 Attempts to minimize this reaction led to later generations of silicone implants with polyurethane shells.12
Inflatable implants filled with sterile saline solution were originally developed in France in 1965. Unlike silicone implants, saline implants have undergone minimal changes since their inception, and grew in popularity during the 1970s in view of the high rates of capsular contracture with silicone implants.8 However, saline implants have their own problems, and as they became increasingly popular, deflation and the unnatural feel of saline sparked a renewed interest in silicone gel.
By the late 1980s, the thinner-shelled generation of silicone implants displayed its own frustrating complications including implant rupture, capsular contracture, infection, and possible systemic and disseminated granulomatous disease. From 1992 to 2006, the US Food and Drug Administration (FDA) placed a moratorium on silicone implants due to concerns about a possible link with autoimmune and connective tissue diseases and the possible carcinogenic nature of silicone.
While silicone implants were prohibited in the United States, development continued abroad, and eventually the moratorium was lifted after several meta-analyses failed to reveal any link regarding the aforementioned concerns.13
Today, silicone gel implants dominate the world market.14 In the United States, approximately 60% of implants contain silicone gel filler, and trends are similar in Europe.7
Table 1 summarizes the evolution of silicone breast implants over the last 50 years.2,6,11,12 Table 2 lists the advantages and disadvantages of silicone and saline breast implants.2,6,8,15
CURRENT IMPLANT OPTIONS
Currently, 3 companies (Allergan, Mentor, Sientra) manufacture and distribute breast implants and implant-associated products such as tissue expanders and sizers in the US market.6
Another company, Motiva, makes an implant that is available in Europe, Asia, and Australia, and the device is currently undergoing a 10-year clinical trial in the United States that began recruiting patients in 16 centers in April 2018.16 Pending final approval, the Cleveland Clinic Department of Plastic Surgery may be among the centers involved in the clinical trial of the Motiva implant. Innovations in the Motiva implant include a high-performance shell that maintains consistent strength and includes a proprietary barrier layer, improved silicone gel filler, 3-D imprinted surface texturing, and an implant shape that adapts with vertical and horizontal movement. It also contains radio-frequency identification transponders that can transmit data about the implant wirelessly.17–19
Surface (textured vs smooth)
Developed in the 1980s, texturing of the implant surface disrupts capsule formation around the prosthesis. Additionally, texturing stabilizes an anatomically shaped (teardrop) implant within the breast pocket, reducing malrotation.20,21
The first textured implants were covered with polyurethane foam, but they were ultimately withdrawn from the US market because of concern for in vivo degradation to carcinogenic compounds. The focus subsequently turned to texturing implant shells by mechanically creating pores of different sizes. Smooth implants, by contrast, are manufactured by repeatedly dipping the implant shell into liquid silicone.2
The capsular contraction rate has been shown to be lower with textured silicone than with smooth silicone (number needed to treat = 7–9), and evidence suggests a lower risk of needing a secondary procedure.21
Form-stable vs fluid-form
Silicone is a polymer. The physical properties of polymers vary greatly and depend on the length of the individual chains and the degree to which those chains are cross-linked. Liquid silicone contains short chains and sparse cross-linking, resulting in an oily compound well suited for lubrication. Silicone gel contains longer chains and more cross-linking and is therefore more viscous.
In “form-stable” implants, the silicone interior has sufficient chain length and cross-linking to retain the designed shape even at rest,2 but they require slightly larger incisions.7 “Fluid-form” refers to an implant with silicone filler with shorter chain length, less cross-linking, and more fluidity.6
Shell
As with silicone fillers, the properties of silicone implant shells also depend on chain length and cross-linking within the polymer. Silicone elastomer shells (Table 1) contain extensively cross-linked chains that impart a flexible yet rubbery character. Silicone elastomers can also be found in facial implants and tissue expanders.2
Implant shape (round vs anatomic)
The shape of an implant is determined by the gel distribution inside of it. To understand gel distribution and implant shape, one must understand the gel-shell ratio. This ratio increases as cohesivity of the filler increases, and it represents increased bonding of the gel filler to the shell and a preserved implant shape at rest.
The gel-shell ratio varies among manufacturers, and a less-viscous filler may be more prone to rippling or loss of upper pole fullness in some patients. For this reason, careful analysis, patient and implant selection, and discussion of complications remain paramount.2
No anatomically shaped implant is manufactured with a smooth shell, but rather with a textured shell that resists malrotation.6,15 However, in the United States, 95% of patients receive round implants.16
PATIENT ASSESSMENT
Before breast augmentation surgery, the surgeon assesses a number of factors—physical and psychosocial—and helps the patient choose a type and size of implant. The surgeon and patient also plan where the implants will be placed—ie, above or beneath the chest wall muscle—and where the incisions will be made. Every decision is made in close consultation with the patient, taking into account the patient’s desires and expectations, as well as what the patient’s anatomy allows. An integral component of this shared decision-making process is a discussion of the possible complications, and often photographs to better illustrate what to expect postoperatively.
Psychosocial factors
One must consider the patient’s psychology, motivations for surgery, and emotional stability. Here, we look for underlying body dysmorphic disorder; excessive or unusual encouragement to undergo the procedure by a spouse, friends, or others; a history of other aesthetic procedures; unrealistic expectations; and other factors influencing the desire to undergo this surgery.
Choosing an implant
Implant selection must take into account the patient’s height, weight,7 and overall body morphology: taller patients and those with wider hips or shoulders usually require larger implants. A reliable method for determining the appropriate implant must include the current breast shape, dimensions, volume, skin elasticity, soft-tissue thickness, and overall body habitus. Ultimately, the most important considerations include breast base diameter, implant volume,20 and soft-tissue envelope.
Preoperative sizing can involve placing sample implants within a brassiere so that the patient can preview possible outcomes. This method is particularly effective in minimizing dissatisfaction because it shares ownership of the decision-making process.15
A computerized implant selection program available in Europe suggests a “best-fit” implant based on a clinician’s measurements.7
Anatomic placement
Traditionally, plastic surgeons place breast implants either beneath the pectoralis major muscle (submuscular placement) or over the pectoralis8 but beneath the glandular breast parenchyma (subglandular placement) (Figure 2).7
Advantages of submuscular placement are a smoother transition of the upper breast pole from the chest wall and less rippling visible through the skin, due to the additional muscular coverage of the implant. Another advantage is that capsular contraction rates are lower with submuscular placement, likely due to possible contamination of implants by lactiferous ductal microbes when accessing the subglandular plane.14,20 Disadvantages are pronounced discomfort after surgery and animation deformities with muscle contraction, particularly in young, highly active patients.
A popular modification of submuscular placement involves creating a surgical dissection plane between the subglandular tissue and the pectoralis major fascia. This “dualplane” approach allows the parenchyma to retract superiorly and reduce breast ptosis.7
Incisions
Table 3 highlights important considerations with regard to incision location.15,20,21
ANTIBIOTICS
Many surgeons give a single prophylactic dose of antibiotic before surgery, a practice that some studies have shown to be effective in reducing the risk of infection.15 However, the benefit of routine postoperative use of antibiotics remains unsubstantiated15: postoperative antibiotic use does not appear to protect against infection, capsular contracture, or overall complications in primary or secondary breast augmentation surgery.20
PERIOPERATIVE PERIOD
At our institution, breast augmentation surgery is an ambulatory procedure—the patient goes home the same day unless circumstances such as pain control warrant admission. This is, however, according to surgeon preference, and differs on a case-by-case basis. General anesthesia is the standard of care.15
POSTOPERATIVE PERIOD
In the immediate postoperative period, patients are instructed to wear a surgical bra for up to 6 weeks to allow stable scarring. Early mobilization is encouraged.7,15 Depending on the patient’s situation, recovery, and healing, she may be out of work for about 1 week, sometimes more, sometimes less.
Additional instructions are surgeon-specific. However, the patient is instructed to avoid bathing, swimming, immersion in water, and wearing underwire brassieres that could impair healing of an inferior incision; instead, patients are often instructed to wear a surgical bra provided on the day of surgery until cleared in the clinic.
Showering is allowed the next day or the second day after surgery, and of course there is no driving while on narcotics. Additionally, patients are counseled extensively regarding hematoma formation and the signs and symptoms of infection.
Patients are typically seen in clinic 1 week after surgery.
The cost of surgery may be $5,000 to $6,000 but can vary significantly from center to center depending on who the patient sees and where, and whether the patient presents for breast reconstruction after cancer or repair of congenital anomalies, or in certain cases of transgender surgery. The patient is typically responsible for the fee, but again this depends on the patient, indications, and particular insurance concerns.
IMPLANT LONGEVITY AND RUPTURE
In the United States, implant rupture rates range from 1.1% to 17.7% at 6 to 10 years after primary augmentation, 2.9% to 14.7% after revision augmentation, 1.5% to 35.4% after primary breast reconstruction, and 0% to 19.6% after revision reconstruction.11
Unfortunately, the existence of multiple implant manufacturers, numerous implant generations, and poorly standardized screening protocols and reporting systems make the true rate of implant rupture difficult to assess without definitive imaging or implant retrieval.11
Damage from surgical instrumentation during implantation is the most common cause of silicone breast implant rupture (50% to 64% of cases).22 Other causes include underfilling and fold flaw from capsular contracture.
Leakage of silicone gel filler may be confined to the periprosthetic capsule (intracapsular rupture) or extend beyond and into the breast parenchyma (extracapsular rupture). One study reported that only 10% of intracapsular ruptures progressed extracapsularly, while 84% of patients with extracapsular involvement remained stable for up to 2 years,23 indicating that intracapsular rupture may not portend worsening disease.11
Implant rupture occurs silently in most cases, with no clinically detectable signs or symptoms. In other cases, patients may present with alterations in breast shape and size, sudden asymmetry, firmness, pronounced capsular contracture, contour irregularity, or pain.
Aside from physical examination, comprehensive diagnostic testing includes imaging—ultrasonography, mammography, computed tomography, and magnetic resonance imaging (MRI). Of these, MRI is the method of choice, with sensitivity and specificity exceeding 90% for detecting implant rupture.11 Classic findings on MRI include the “linguine” sign from a deflating implant shell, or the teardrop sign from implant sagging. Classic findings on ultrasonography include the “snowstorm” sign of extracapsular rupture and the “stepladder” sign of intracapsular rupture.
Mammography effectively detects free silicone in breast tissue with extracapsular rupture (25% of ruptures according to some studies)23; however, it cannot detect rupture within the implant capsule. As an aside, submuscular implant placement may interfere less with screening mammography than subglandular implants do.14,24
Current FDA recommendations to detect implant rupture encourage women with silicone breast implants to undergo screening 3 years after implantation and then every 2 years thereafter; no long-term monitoring is suggested for saline implants.15 Many plastic surgeons evaluate silicone breast implant patients every 1 to 2 years for contracture and rupture.8 Of note, capsular contracture impairs the effectiveness of ultrasonography and may require MRI confirmation.11
If implant rupture is confirmed, the current recommendation is to remove the implant and the capsule. Another implant may be placed depending on the patient’s preference. Rigorous washout remains a key feature of any surgical intervention for ruptured breast implants; however, in the event of extracapsular rupture, resection of silicone granulomas may also be required.11
Reoperation rates for primary breast augmentation surgery approach 20% and are even higher for secondary augmentation over a patient’s lifetime—the highest rate of all aesthetic procedures.7,14
CAPSULAR CONTRACTURE
Capsular contracture is the most common complication of breast augmentation,25 typically presenting within the first postoperative year,26,27 and the risk increases over time.28 It occurs with both silicone and saline breast implants.
In some studies, the incidence exceeded 4% in the first 2 years after surgery,29 and nearly 50% by 10 years.30 Other studies found rates of 0% to 20% over 13 years.20
The etiology is not well understood and is presumed to be multifactorial, with proposed mechanisms and factors that include bacterial contamination, surface texturing, the implant pocket selected, the incision type, drain placement, antibiotic use, and smoking.25
A meta-analysis from 17,000 implants found that the risk of capsular contracture was significantly higher when an implant was placed in a subglandular pocket than in a submuscular pocket,22,26 and that although texturing decreased capsular contracture compared with smooth implants, the effect was modest when a textured or smooth implant was placed in a submuscular location.28 With regard to incision location, studies have reported that the incidence of capsular contracture is highest with transaxillary and periareolar incisions, and lowest with inframammary incisions.20,21
The leading theory is that contamination of the implant (primarily from the mammary ducts) results in biofilm formation. Subclinical hematoma surrounding the implant may also provide key bacterial nutrients.20
Textured implants induce a greater inflammatory response in the capsular tissue, resulting in a thicker capsule; however, contracture rates remain lower with textured than with smooth implants.14,31 Interestingly, lower rates of capsular contracture have been observed with later-generation, cohesive-gel, form-stable implants than with those of earlier generations.12
Although more research is needed, silicone implants appear to confer a higher risk of capsular contracture than saline implants.14,20
Irrigating the breast pocket intraoperatively with triple antibiotic solution (bacitracin, cefazolin, and gentamicin) before placing the implant may decrease the capsular contracture rate.15,20
Treatments for capsular contracture include pocket modifications such as capsulotomy (making releasing, relaxing incisions in the scar capsule encasing the implant), capsulectomy (removing portions of or the entire capsule), and replacing the implant in the other pocket (ie, if the original implant was subglandular, the replacement is placed in the submuscular pocket). Patients who have contractures that fail to respond to these treatments may ultimately benefit from implant removal and autologous reconstruction (autoaugmentation) rather than implant replacement.32,33
ADDITIONAL COMPLICATIONS
Other complications include infection, malposition, rippling, seroma, hematoma, and sensory alterations.
Irrigation during the implantation procedure with a triple antibiotic solution consisting of bacitracin, gentamycin, and cephalexin in normal saline decreases infection and seroma rates.15,20,34
Some surgeons also choose to irrigate the pocket with a betadine solution, or to cleanse the skin with betadine and place sterile towels and redrape before inserting the implant. Additionally, many prefer using a sterile device much like a pastry funnel called a Keller funnel to insert the implant into the breast pocket.35
Infection is less common with cosmetic augmentations than with implant-based breast reconstruction, likely because of healthier, well-vascularized tissue in patients undergoing cosmetic surgery than in those undergoing mastectomy.14
Seroma is thought to be a consequence of texturing, and more so with macro- vs microtexturing. Though poorly understood, an association between texturing and double capsules has also been reported.12,20
After primary breast augmentation, 10-year follow-up rates of capsular contracture, seroma, rippling, and malposition vary across the 3 major silicone implant manufacturers.12 Hematoma and infection occur in less than 1% of primary augmentation patients.15
Malposition of the implant over time is less frequent with textured implants because of the higher coefficient of friction compared with smooth implants.6,8,15
Visible skin rippling may be a consequence of texturing and also of thin body habitus, eg, in patients with a body mass index less than 18.5 kg/m2. If the soft-tissue layer of the breast is thin, the natural rippling of smooth saline implant shells are more likely to show when placed in the subglandular pocket. Form-stable implants, by contrast, resist rippling.12,15
Large implants and extensive lateral dissection can cause alterations in nipple sensation and sensory loss within lower breast pole skin. Axillary incisions may traumatize or damage the intercostobrachial nerve, resulting in upper inner arm sensory aberrations.
Ultimately, the 10-year incidence of secondary surgery ranges from 0% to 36% and the 10-year incidence of capsular contracture ranges from 11% to 19%.15 Additional cosmetic complaints after augmentation with implants include enlargement of the areola and engorgement of breast veins.14
BREAST CANCER AND DETECTION
Patients with or without implants do not seem to differ with regard to breast cancer stage upon detection, tumor burden, recurrence, or survival. However, more patients with implants may present with palpable masses, invasive tumors, axillary metastasis, and falsely negative mammograms.
Breast implants may actually facilitate cancer detection on physical examination by providing a more dense or stable surface upon which to palpate the breast tissue. Although they do not necessarily impair mastectomy or breast reconstruction, they may result in an increased rate of revision surgery after breast conservation therapy.24,36 Mammography remains the standard of care for radiologic diagnosis but can be further supported by MRI and ultrasonography if necessary in patients with implants.
AUTOIMMUNE DISEASES
Although concerns persist, multiple studies have demonstrated the safety of fourth- and fifth-generation silicone breast implants with regard to autoimmune disease.7
In various clinical studies in mastectomy patients who underwent breast reconstruction with either silicone implants or autologous tissue, no difference was found with regard to the incidence of autoimmune diseases.2 Additionally, in meta-analyses of data from more than 87,000 women, no association was found between connective tissue disease and silicone breast implants.2,11 One study11,23 noted no increase in autoantibodies in patients with undamaged silicone implants vs patients who experienced rupture.
Studies have also demonstrated that in children born to mothers with breast implants, the risk of rheumatic disease, esophageal disorders, congenital malformations, and death during the perinatal period is comparable with that in controls.37 Another study, examining breastfeeding in women with silicone breast implants, showed no significant difference in silicon levels (used as a proxy for silicone) in breast milk compared with controls without implants; silicon levels were found to be significantly higher in cow’s milk and store-bought formulas.38
BREAST IMPLANT-ASSOCIATED ANAPLASTIC LARGE-CELL LYMPHOMA
Breast implant-associated anaplastic large-cell lymphoma (BIA-ALCL) is a subtype of T-cell lymphoma that develops in tissue adjacent to breast implants. It typically presents as breast swelling 2 to 38 years (mean of 8 years) after implant insertion.39,40 The swelling may be secondary to periprosthetic seroma formation or, more rarely, palpable disease in the axilla. Patients occasionally complain of pain and, rarely, constitutional symptoms.20 BIA-ALCL is not a disease of the surrounding breast tissue, but rather of the fibrous periprosthetic capsule.21
Of note, there is no documented case involving smooth implants,41–43 but it may be related to fifth-generation textured implants.6 At present, it is not possible to definitively state which implant is associated with this condition; hence, more data are needed, and this association is currently under study.
The absolute risk of BIA-ALCL was reported in a Dutch study39 as 1 in 35,000 by age 50, 1 in 12,000 by age 70, and 1 in 7,000 by age 75, with a number needed to harm of 6,920. Overall lifetime risk was estimated at 1 in 30,000 for women with textured implants in a 2015 US study.40 In comparison, breast cancer risk is about 1 in 8 women. There is no apparent predilection for patients who underwent cosmetic augmentation vs reconstruction, or who received silicone vs saline implants.
The diagnosis is confirmed by ultrasonographically guided fine-needle aspiration of seroma fluid and subsequent immunohistochemical testing for CD30-positive and ALK-negative T lymphocytes. Other than positron-emission tomography for staging after diagnosis confirmation, imaging is ineffective. Expert opinion does not recommend routine screening unless the aforementioned symptoms arise.
Treatment involves implant removal and total capsulectomy, with samples sent for pathology study with cytokeratin staining.12 Of note, in all cases of BIA-ALCL in which the disease was limited to the circumscribed scar tissue of the breast capsule, complete surgical excision has proved curative, whereas incomplete capsulectomy portends a greater risk of recurrence and decreased survival.44
In cases of advanced or recurrent ALCL, diagnosed late or inappropriately, the National Comprehensive Cancer Network recommends a multidisciplinary approach involving adjuvant chemotherapy and radiation.44 Anecdotally, at our institution, we have recently treated several cases of advanced ALCL presenting with invasive chest wall masses with extirpative surgery and subsequent reconstruction with the assistance of our thoracic surgery colleagues, as well as the aforementioned multidisciplinary approach using adjuvant therapy.
The mechanism of this malignancy is currently under investigation, but the current theory implicates an exaggerated lymphoproliferative response to bacterial contamination of the capsule superimposed upon genetic factors in susceptible patients.42,43
National societies advise plastic surgeons to discuss the risk of BIA-ALCL with all patients at the time of breast augmentation consultation and to report all confirmed cases to the PROFILE registry (Patient Registry and Outcomes for Breast Implants and Anaplastic Large Cell Lymphoma Etiology and Epidemiology).45
ARE PATIENTS HAPPIER AFTERWARD?
Studies have shown that after undergoing breast augmentation surgery, patients note improvement in body image, and satisfaction rates range from 85% to 95% with respect to self-confidence and body image.46 An evaluation of patient responses on the validated BREAST-Q Augmentation Questionnaire showed the following satisfaction rates: breasts 83%, psychosocial well-being 88%, and sexual functioning 81%.15
Although epidemiologic studies have reported higher suicide rates in women with cosmetic breast implants, this likely stems from preoperative psychological factors and underscores the role of psychiatric referral in patients with a mental health history or in those whom the surgeon deems it necessary.46
Several high-quality studies have demonstrated that quality of life and psychosocial functioning (including depression) markedly improve after breast augmentation surgery.47 Among a cohort of Norwegian patients, breast implant surgery resulted in improved motivation to perform daily activities, as well as improved quality of life from both a psychosocial and aesthetic perspective.48 Interestingly, a recent study reported that patients who underwent breast implant surgery alone reported greater satisfaction and psychosocial quality of life than patients who underwent combination breast augmentation and mastopexy (breast-lifting) surgery.49
Additional data are needed to refine our understanding of the complex interplay between psychosocial factors before and after surgery in patients seeking and undergoing breast augmentation procedures.
- Derby BM, Codner MA. Textured silicone breast implant use in primary augmentation: core data update and review. Plast Reconstr Surg 2015; 135(1):113–124. doi:10.1097/PRS.0000000000000832
- Maxwell GP, Gabriel A. Breast implant design. Gland Surg 2017; 6(2):148–153. doi:10.21037/gs.2016.11.09
- Gabriel A, Maxwell GP. The evolution of breast implants. Clin Plast Surg 2015; 42(4):399–404. doi:10.1016/j.cps.2015.06.015
- American Society of Plastic Surgeons. Procedural statistics trends 1992–2012. www.plasticsurgery.org/documents/News/Statistics/2012/plastic-surgery-statistics-full-report-2012.pdf. Accessed January 17, 2019.
- American Society of Plastic Surgeons. Plastic surgery statistics report 2016. www.plasticsurgery.org/documents/News/Statistics/2016/plastic-surgery-statistics-full-report-2016.pdf. Accessed January 17, 2019.
- Henderson PW, Nash D, Laskowski M, Grant RT. Objective comparison of commercially available breast implant devices. Aesthetic Plast Surg 2015; 39(5):724–732. doi:10.1007/s00266-015-0537-1
- Adams WP Jr, Mallucci P. Breast augmentation. Plast Reconstr Surg 2012; 130(4):597e–611e. doi:10.1097/PRS.0b013e318262f607
- Spear SL, Jespersen MR. Breast implants: saline or silicone? Aesthet Surg J 2010; 30(4):557–570. doi:10.1177/1090820X10380401
- Cronin TD, Gerow FJ. Augmentation mammaplasty: a new “natural feel” prosthesis. In: Transactions of the Third International Conference of Plastic Surgery: October 13–18, 1963, Washington, DC.
- Maxwell GP, Gabriel A. The evolution of breast implants. Plast Reconstr Surg 2014; 134(suppl 1):12S–17S. doi:10.1097/PRS.0000000000000348
- Hillard C, Fowler JD, Barta R, Cunningham B. Silicone breast implant rupture: a review. Gland Surg 2017; 6(2):163–168. doi:10.21037/gs.2016.09.12
- Derby BM, Codner MA. Textured silicone breast implant use in primary augmentation: core data update and review. Plast Reconstr Surg 2015; 135(1):113–124. doi:10.1097/PRS.0000000000000832
- Tugwell P, Wells G, Peterson J, et al. Do silicone breast implants cause rheumatologic disorders? A systematic review for a court-appointed national science panel. Arthritis Rheum 2001; 44(11):2477–2484. pmid:11710703
- Alpert BS, Lalonde DH. MOC-PS(SM) CME article: breast augmentation. Plast Reconstr Surg 2008; 121(suppl 4):1–7. doi:10.1097/01.prs.0000305933.31540.5d
- Hidalgo DA, Spector JA. Breast augmentation. Plast Reconstr Surg 2014; 133(4):567e–583e. doi:10.1097/PRS.0000000000000033
- ClinicalTrials.gov. Study of the safety and effectiveness of Motiva Implants®. https://clinicaltrials.gov/ct2/show/NCT03579901. Accessed January 17, 2019.
- Establishment Labs. Motiva Implants. https://motivaimplants.com/why-motiva/innovation-for-enhanced-safety/. Accessed January 17, 2019.
- Sforza M, Zaccheddu R, Alleruzzo A, et al. Preliminary 3-year evaluation of experience with silksurface and velvetsurface Motiva silicone breast implants: a single-center experience with 5813 consecutive breast augmentation cases. Aesthet Surg J 2018; 38(suppl 2):S62–S73. doi:10.1093/asj/sjx150
- Huemer GM, Wenny R, Aitzetmüller MM, Duscher D. Motiva ergonomix round silksurface silicone breast implants: outcome analysis of 100 primary breast augmentations over 3 years and technical considerations. Plast Reconstr Surg 2018; 141(6):831e–842e. doi:10.1097/PRS.0000000000004367
- Lista F, Ahmad J. Evidence-based medicine: augmentation mammaplasty. Plast Reconstr Surg 2013; 132(6):1684–1696. doi:10.1097/PRS.0b013e3182a80880
- Namnoum JD, Largent J, Kaplan HM, Oefelein MG, Brown MH. Primary breast augmentation clinical trial outcomes stratified by surgical incision, anatomical placement and implant device type. J Plast Reconstr Aesthet Surg 2013; 66(9):1165–1172. doi:10.1016/j.bjps.2013.04.046
- Handel N, Garcia ME, Wixtrom R. Breast implant rupture: causes, incidence, clinical impact, and management. Plast Reconstr Surg 2013; 132(5):1128–1137. doi:10.1097/PRS.0b013e3182a4c243
- Hölmich LR, Friis S, Fryzek JP, et al. Incidence of silicone breast implant rupture. Arch Surg 2003; 138(7):801–806. doi:10.1001/archsurg.138.7.801
- Mccarthy CM, Pusic AL, Disa JJ, Cordeiro PG, Cody HS 3rd, Mehrara B. Breast cancer in the previously augmented breast. Plast Reconstr Surg 2007; 119(1):49–58. doi:10.1097/01.prs.0000244748.38742.1f
- Egeberg A, Sørensen JA. The impact of breast implant location on the risk of capsular contraction. Ann Plast Surg 2016; 77(2):255–259. doi:10.1097/SAP.0000000000000227
- Wickman M. Rapid versus slow tissue expansion for breast reconstruction: a three-year follow-up. Plast Reconstr Surg 1995; 95(4):712–718. pmid:7892316
- Kjøller K, Hölmich LR, Jacobsen PH, et al. Epidemiological investigation of local complications after cosmetic breast implant surgery in Denmark. Ann Plast Surg 2002; 48(3):229–237. pmid:11862025
- Handel N, Jensen JA, Black Q, Waisman JR, Silverstein MJ. The fate of breast implants: a critical analysis of complications and outcomes. Plast Reconstr Surg 1995; 96(7):1521–1533. pmid:7480271
- Henriksen TF, Hölmich LR, Fryzek JP, et al. Incidence and severity of short-term complications after breast augmentation: results from a nationwide breast implant registry. Ann Plast Surg 2003; 51(6):531–539. doi:10.1097/01.sap.0000096446.44082.60
- Fernandes JR, Salinas HM, Broelsch GF, et al. Prevention of capsular contracture with photochemical tissue passivation. Plast Reconstr Surg 2014; 133(3):571–577. doi:10.1097/01.prs.0000438063.31043.79
- Wong CH, Samuel M, Tan BK, Song C. Capsular contracture in subglandular breast augmentation with textured versus smooth breast implants: a systematic review. Plast Reconstr Surg 2006; 118(5):1224–1236. doi:10.1097/01.prs.0000237013.50283.d2
- Gurunluoglu R, Sacak B, Arton J. Outcomes analysis of patients undergoing autoaugmentation after breast implant removal. Plast Reconstr Surg 2013; 132(2):304–315. doi:10.1097/PRS.0b013e31829e7d9e
- Gurunluoglu R, Shafighi M, Schwabegger A, Ninkovic M. Secondary breast reconstruction with deepithelialized free flaps from the lower abdomen for intractable capsular contracture and maintenance of breast volume. J Reconstr Microsurg 2005; 21(1):35–41. doi:10.1055/s-2005-862779
- Adams WP Jr, Rios JL, Smith SJ. Enhancing patient outcomes in aesthetic reconstructive breast surgery using triple antibiotic breast irrigation: six-year prospective clinical study. Plast Reconstru Surg 2006; 118(7 suppl):46S–52S. doi:10.1097/01.prs.0000185671.51993.7e
- Moyer HR, Ghazi B, Saunders N, Losken A. Contamination in smooth gel breast implant placement: testing a funnel versus digital insertion technique in a cadaver model. Aesthet Surg J 2012; 32(2):194–199. doi:10.1177/1090820X11434505
- Handel N. The effect of silicone implants on the diagnosis, prognosis, and treatment of breast cancer. Plast Reconstr Surg 2007; 120(7 suppl 1):81S–93S. doi:10.1097/01.prs.0000286578.94102.2b
- Kjøller K, Friis S, Lipworth L, Mclaughlin JK, Olsen JH. Adverse health outcomes in offspring of mothers with cosmetic breast implants: a review. Plast Reconstr Surg 2007; 120(7 suppl 1):129S–134S. doi:10.1097/01.prs.0000286571.93392.00
- Semple JL. Breast-feeding and silicone implants. Plast Reconstr Surg 2007; 120(7 suppl 1):123S–128S. doi:10.1097/01.prs.0000286579.27852.ed
- de Boer M, van leeuwen FE, Hauptmann M, et al. Breast implants and the risk of anaplastic large-cell lymphoma in the breast. JAMA Oncol 2018; 4(3):335–341. doi:10.1001/jamaoncol.2017.4510
- McCarthy CM, Horwitz SM. Association of breast implants with anaplastic large-cell lymphoma. JAMA Oncol 2018; 4(3):341–342. doi:10.1001/jamaoncol.2017.4467
- American Society of Plastic Surgeons. BIA-ALCL physician resources. www.plasticsurgery.org/for-medical-professionals/health-policy/bia-alcl-physician-resources. Accessed December 17, 2018.
- The American Society for Aesthetic Plastic Surgery, Inc. Member FAQs: latest information on ALCL. www.surgery.org/sites/default/files/Member-FAQs_1.pdf. Accessed January 17, 2019.
- The American Society of Plastic Surgeons. BIA-ALCL resources: summary and quick facts. www.plasticsurgery.org/for-medical-professionals/health-policy/bia-alcl-summary-and-quick-facts. Accessed January 17, 2019.
- National Comprehensive Cancer Network. T-cell lymphomas. www.nccn.org/professionals/physician_gls/pdf/t-cell.pdf.
- The Plastic Surgery Foundation PROFILE Registry. www.thepsf.org/research/registries/profile. Accessed January 17, 2019.
- Sarwer DB. The psychological aspects of cosmetic breast augmentation. Plast Reconstr Surg 2007; 120(7 suppl 1):110S–117S. doi:10.1097/01.prs.0000286591.05612.72
- Rohrich RJ, Adams WP, Potter JK. A review of psychological outcomes and suicide in aesthetic breast augmentation. Plast Reconstr Surg 2007; 119(1):401–408. doi:10.1097/01.prs.0000245342.06662.00
- Kalaaji A, Bjertness CB, Nordahl C, Olafsen K. Survey of breast implant patients: characteristics, depression rate, and quality of life. Aesthet Surg J 2013; 33(2):252–257. doi:10.1177/1090820X12473106
- Kalaaji A, Dreyer S, Brinkmann J, Maric I, Nordahl C, Olafsen K. Quality of life after breast enlargement with implants versus augmentation mastopexy: a comparative study. Aesthet Surg J 2018; 38(12):1304–1315. doi:10.1093/asj/sjy047
At present, 300,000 US women undergo breast augmentation surgery each year,1 making this the second most common aesthetic procedure in women (after liposuction),2–4 and making it extremely likely that clinicians will encounter women who have breast implants. In addition, approximately 110,000 women undergo breast reconstructive surgery after mastectomy, of whom more than 88,000 (81%) receive implants (2016 data).5
This review discusses the evolution of breast implants, their complications, and key considerations with regard to aesthetic and reconstructive breast surgery, as the principles are similar.
EVOLUTION OF IMPLANTS
Reports of breast augmentation surgery, also known as augmentation mammoplasty, date back to 1895, when a fatty tumor (lipoma) was successfully transplanted from a patient’s back to a breast defect in a mastectomy patient.2,3,6,7 In the 1930s, implantation of a glass ball into a patient’s breast marked the first implant-based breast augmentation.6 By 1954, attempts at breast augmentation using local dermal-fat flaps, adipose tissue, and even omentum were described.
Alloplastic materials gained popularity throughout the 1950s and 1960s and included polyurethane, polytetrafluoroethylene (Teflon), and other synthetics. Adverse reactions associated with alloplastic materials were plentiful: local tissue reactions, distortion of the breast mound, increased firmness, and discomfort all contributed to the eventual discontinuation of their use. The history of alloplastic breast augmentation also included epoxy resin, shellac, beeswax, paraffin, rubber, petroleum jelly, and liquefied silicone. Outcomes were not good, and many patients ultimately needed mastectomy.7
The first modern breast prosthesis was developed in 1961, and since then, implant composition and design have evolved significantly.8
From silicone to saline, and back again
The first silicone gel implants, introduced in the early 1960s,8–19 had high complication rates—some centers reported an incidence of capsular contracture of up to 70%.8,11 This is a foreign body reaction in which pathologic scar tissue encases the implant, causing it to distort, appear misshapen, harden, and even become painful.11 Attempts to minimize this reaction led to later generations of silicone implants with polyurethane shells.12
Inflatable implants filled with sterile saline solution were originally developed in France in 1965. Unlike silicone implants, saline implants have undergone minimal changes since their inception, and grew in popularity during the 1970s in view of the high rates of capsular contracture with silicone implants.8 However, saline implants have their own problems, and as they became increasingly popular, deflation and the unnatural feel of saline sparked a renewed interest in silicone gel.
By the late 1980s, the thinner-shelled generation of silicone implants displayed its own frustrating complications including implant rupture, capsular contracture, infection, and possible systemic and disseminated granulomatous disease. From 1992 to 2006, the US Food and Drug Administration (FDA) placed a moratorium on silicone implants due to concerns about a possible link with autoimmune and connective tissue diseases and the possible carcinogenic nature of silicone.
While silicone implants were prohibited in the United States, development continued abroad, and eventually the moratorium was lifted after several meta-analyses failed to reveal any link regarding the aforementioned concerns.13
Today, silicone gel implants dominate the world market.14 In the United States, approximately 60% of implants contain silicone gel filler, and trends are similar in Europe.7
Table 1 summarizes the evolution of silicone breast implants over the last 50 years.2,6,11,12 Table 2 lists the advantages and disadvantages of silicone and saline breast implants.2,6,8,15
CURRENT IMPLANT OPTIONS
Currently, 3 companies (Allergan, Mentor, Sientra) manufacture and distribute breast implants and implant-associated products such as tissue expanders and sizers in the US market.6
Another company, Motiva, makes an implant that is available in Europe, Asia, and Australia, and the device is currently undergoing a 10-year clinical trial in the United States that began recruiting patients in 16 centers in April 2018.16 Pending final approval, the Cleveland Clinic Department of Plastic Surgery may be among the centers involved in the clinical trial of the Motiva implant. Innovations in the Motiva implant include a high-performance shell that maintains consistent strength and includes a proprietary barrier layer, improved silicone gel filler, 3-D imprinted surface texturing, and an implant shape that adapts with vertical and horizontal movement. It also contains radio-frequency identification transponders that can transmit data about the implant wirelessly.17–19
Surface (textured vs smooth)
Developed in the 1980s, texturing of the implant surface disrupts capsule formation around the prosthesis. Additionally, texturing stabilizes an anatomically shaped (teardrop) implant within the breast pocket, reducing malrotation.20,21
The first textured implants were covered with polyurethane foam, but they were ultimately withdrawn from the US market because of concern for in vivo degradation to carcinogenic compounds. The focus subsequently turned to texturing implant shells by mechanically creating pores of different sizes. Smooth implants, by contrast, are manufactured by repeatedly dipping the implant shell into liquid silicone.2
The capsular contraction rate has been shown to be lower with textured silicone than with smooth silicone (number needed to treat = 7–9), and evidence suggests a lower risk of needing a secondary procedure.21
Form-stable vs fluid-form
Silicone is a polymer. The physical properties of polymers vary greatly and depend on the length of the individual chains and the degree to which those chains are cross-linked. Liquid silicone contains short chains and sparse cross-linking, resulting in an oily compound well suited for lubrication. Silicone gel contains longer chains and more cross-linking and is therefore more viscous.
In “form-stable” implants, the silicone interior has sufficient chain length and cross-linking to retain the designed shape even at rest,2 but they require slightly larger incisions.7 “Fluid-form” refers to an implant with silicone filler with shorter chain length, less cross-linking, and more fluidity.6
Shell
As with silicone fillers, the properties of silicone implant shells also depend on chain length and cross-linking within the polymer. Silicone elastomer shells (Table 1) contain extensively cross-linked chains that impart a flexible yet rubbery character. Silicone elastomers can also be found in facial implants and tissue expanders.2
Implant shape (round vs anatomic)
The shape of an implant is determined by the gel distribution inside of it. To understand gel distribution and implant shape, one must understand the gel-shell ratio. This ratio increases as cohesivity of the filler increases, and it represents increased bonding of the gel filler to the shell and a preserved implant shape at rest.
The gel-shell ratio varies among manufacturers, and a less-viscous filler may be more prone to rippling or loss of upper pole fullness in some patients. For this reason, careful analysis, patient and implant selection, and discussion of complications remain paramount.2
No anatomically shaped implant is manufactured with a smooth shell, but rather with a textured shell that resists malrotation.6,15 However, in the United States, 95% of patients receive round implants.16
PATIENT ASSESSMENT
Before breast augmentation surgery, the surgeon assesses a number of factors—physical and psychosocial—and helps the patient choose a type and size of implant. The surgeon and patient also plan where the implants will be placed—ie, above or beneath the chest wall muscle—and where the incisions will be made. Every decision is made in close consultation with the patient, taking into account the patient’s desires and expectations, as well as what the patient’s anatomy allows. An integral component of this shared decision-making process is a discussion of the possible complications, and often photographs to better illustrate what to expect postoperatively.
Psychosocial factors
One must consider the patient’s psychology, motivations for surgery, and emotional stability. Here, we look for underlying body dysmorphic disorder; excessive or unusual encouragement to undergo the procedure by a spouse, friends, or others; a history of other aesthetic procedures; unrealistic expectations; and other factors influencing the desire to undergo this surgery.
Choosing an implant
Implant selection must take into account the patient’s height, weight,7 and overall body morphology: taller patients and those with wider hips or shoulders usually require larger implants. A reliable method for determining the appropriate implant must include the current breast shape, dimensions, volume, skin elasticity, soft-tissue thickness, and overall body habitus. Ultimately, the most important considerations include breast base diameter, implant volume,20 and soft-tissue envelope.
Preoperative sizing can involve placing sample implants within a brassiere so that the patient can preview possible outcomes. This method is particularly effective in minimizing dissatisfaction because it shares ownership of the decision-making process.15
A computerized implant selection program available in Europe suggests a “best-fit” implant based on a clinician’s measurements.7
Anatomic placement
Traditionally, plastic surgeons place breast implants either beneath the pectoralis major muscle (submuscular placement) or over the pectoralis8 but beneath the glandular breast parenchyma (subglandular placement) (Figure 2).7
Advantages of submuscular placement are a smoother transition of the upper breast pole from the chest wall and less rippling visible through the skin, due to the additional muscular coverage of the implant. Another advantage is that capsular contraction rates are lower with submuscular placement, likely due to possible contamination of implants by lactiferous ductal microbes when accessing the subglandular plane.14,20 Disadvantages are pronounced discomfort after surgery and animation deformities with muscle contraction, particularly in young, highly active patients.
A popular modification of submuscular placement involves creating a surgical dissection plane between the subglandular tissue and the pectoralis major fascia. This “dualplane” approach allows the parenchyma to retract superiorly and reduce breast ptosis.7
Incisions
Table 3 highlights important considerations with regard to incision location.15,20,21
ANTIBIOTICS
Many surgeons give a single prophylactic dose of antibiotic before surgery, a practice that some studies have shown to be effective in reducing the risk of infection.15 However, the benefit of routine postoperative use of antibiotics remains unsubstantiated15: postoperative antibiotic use does not appear to protect against infection, capsular contracture, or overall complications in primary or secondary breast augmentation surgery.20
PERIOPERATIVE PERIOD
At our institution, breast augmentation surgery is an ambulatory procedure—the patient goes home the same day unless circumstances such as pain control warrant admission. This is, however, according to surgeon preference, and differs on a case-by-case basis. General anesthesia is the standard of care.15
POSTOPERATIVE PERIOD
In the immediate postoperative period, patients are instructed to wear a surgical bra for up to 6 weeks to allow stable scarring. Early mobilization is encouraged.7,15 Depending on the patient’s situation, recovery, and healing, she may be out of work for about 1 week, sometimes more, sometimes less.
Additional instructions are surgeon-specific. However, the patient is instructed to avoid bathing, swimming, immersion in water, and wearing underwire brassieres that could impair healing of an inferior incision; instead, patients are often instructed to wear a surgical bra provided on the day of surgery until cleared in the clinic.
Showering is allowed the next day or the second day after surgery, and of course there is no driving while on narcotics. Additionally, patients are counseled extensively regarding hematoma formation and the signs and symptoms of infection.
Patients are typically seen in clinic 1 week after surgery.
The cost of surgery may be $5,000 to $6,000 but can vary significantly from center to center depending on who the patient sees and where, and whether the patient presents for breast reconstruction after cancer or repair of congenital anomalies, or in certain cases of transgender surgery. The patient is typically responsible for the fee, but again this depends on the patient, indications, and particular insurance concerns.
IMPLANT LONGEVITY AND RUPTURE
In the United States, implant rupture rates range from 1.1% to 17.7% at 6 to 10 years after primary augmentation, 2.9% to 14.7% after revision augmentation, 1.5% to 35.4% after primary breast reconstruction, and 0% to 19.6% after revision reconstruction.11
Unfortunately, the existence of multiple implant manufacturers, numerous implant generations, and poorly standardized screening protocols and reporting systems make the true rate of implant rupture difficult to assess without definitive imaging or implant retrieval.11
Damage from surgical instrumentation during implantation is the most common cause of silicone breast implant rupture (50% to 64% of cases).22 Other causes include underfilling and fold flaw from capsular contracture.
Leakage of silicone gel filler may be confined to the periprosthetic capsule (intracapsular rupture) or extend beyond and into the breast parenchyma (extracapsular rupture). One study reported that only 10% of intracapsular ruptures progressed extracapsularly, while 84% of patients with extracapsular involvement remained stable for up to 2 years,23 indicating that intracapsular rupture may not portend worsening disease.11
Implant rupture occurs silently in most cases, with no clinically detectable signs or symptoms. In other cases, patients may present with alterations in breast shape and size, sudden asymmetry, firmness, pronounced capsular contracture, contour irregularity, or pain.
Aside from physical examination, comprehensive diagnostic testing includes imaging—ultrasonography, mammography, computed tomography, and magnetic resonance imaging (MRI). Of these, MRI is the method of choice, with sensitivity and specificity exceeding 90% for detecting implant rupture.11 Classic findings on MRI include the “linguine” sign from a deflating implant shell, or the teardrop sign from implant sagging. Classic findings on ultrasonography include the “snowstorm” sign of extracapsular rupture and the “stepladder” sign of intracapsular rupture.
Mammography effectively detects free silicone in breast tissue with extracapsular rupture (25% of ruptures according to some studies)23; however, it cannot detect rupture within the implant capsule. As an aside, submuscular implant placement may interfere less with screening mammography than subglandular implants do.14,24
Current FDA recommendations to detect implant rupture encourage women with silicone breast implants to undergo screening 3 years after implantation and then every 2 years thereafter; no long-term monitoring is suggested for saline implants.15 Many plastic surgeons evaluate silicone breast implant patients every 1 to 2 years for contracture and rupture.8 Of note, capsular contracture impairs the effectiveness of ultrasonography and may require MRI confirmation.11
If implant rupture is confirmed, the current recommendation is to remove the implant and the capsule. Another implant may be placed depending on the patient’s preference. Rigorous washout remains a key feature of any surgical intervention for ruptured breast implants; however, in the event of extracapsular rupture, resection of silicone granulomas may also be required.11
Reoperation rates for primary breast augmentation surgery approach 20% and are even higher for secondary augmentation over a patient’s lifetime—the highest rate of all aesthetic procedures.7,14
CAPSULAR CONTRACTURE
Capsular contracture is the most common complication of breast augmentation,25 typically presenting within the first postoperative year,26,27 and the risk increases over time.28 It occurs with both silicone and saline breast implants.
In some studies, the incidence exceeded 4% in the first 2 years after surgery,29 and nearly 50% by 10 years.30 Other studies found rates of 0% to 20% over 13 years.20
The etiology is not well understood and is presumed to be multifactorial, with proposed mechanisms and factors that include bacterial contamination, surface texturing, the implant pocket selected, the incision type, drain placement, antibiotic use, and smoking.25
A meta-analysis from 17,000 implants found that the risk of capsular contracture was significantly higher when an implant was placed in a subglandular pocket than in a submuscular pocket,22,26 and that although texturing decreased capsular contracture compared with smooth implants, the effect was modest when a textured or smooth implant was placed in a submuscular location.28 With regard to incision location, studies have reported that the incidence of capsular contracture is highest with transaxillary and periareolar incisions, and lowest with inframammary incisions.20,21
The leading theory is that contamination of the implant (primarily from the mammary ducts) results in biofilm formation. Subclinical hematoma surrounding the implant may also provide key bacterial nutrients.20
Textured implants induce a greater inflammatory response in the capsular tissue, resulting in a thicker capsule; however, contracture rates remain lower with textured than with smooth implants.14,31 Interestingly, lower rates of capsular contracture have been observed with later-generation, cohesive-gel, form-stable implants than with those of earlier generations.12
Although more research is needed, silicone implants appear to confer a higher risk of capsular contracture than saline implants.14,20
Irrigating the breast pocket intraoperatively with triple antibiotic solution (bacitracin, cefazolin, and gentamicin) before placing the implant may decrease the capsular contracture rate.15,20
Treatments for capsular contracture include pocket modifications such as capsulotomy (making releasing, relaxing incisions in the scar capsule encasing the implant), capsulectomy (removing portions of or the entire capsule), and replacing the implant in the other pocket (ie, if the original implant was subglandular, the replacement is placed in the submuscular pocket). Patients who have contractures that fail to respond to these treatments may ultimately benefit from implant removal and autologous reconstruction (autoaugmentation) rather than implant replacement.32,33
ADDITIONAL COMPLICATIONS
Other complications include infection, malposition, rippling, seroma, hematoma, and sensory alterations.
Irrigation during the implantation procedure with a triple antibiotic solution consisting of bacitracin, gentamycin, and cephalexin in normal saline decreases infection and seroma rates.15,20,34
Some surgeons also choose to irrigate the pocket with a betadine solution, or to cleanse the skin with betadine and place sterile towels and redrape before inserting the implant. Additionally, many prefer using a sterile device much like a pastry funnel called a Keller funnel to insert the implant into the breast pocket.35
Infection is less common with cosmetic augmentations than with implant-based breast reconstruction, likely because of healthier, well-vascularized tissue in patients undergoing cosmetic surgery than in those undergoing mastectomy.14
Seroma is thought to be a consequence of texturing, and more so with macro- vs microtexturing. Though poorly understood, an association between texturing and double capsules has also been reported.12,20
After primary breast augmentation, 10-year follow-up rates of capsular contracture, seroma, rippling, and malposition vary across the 3 major silicone implant manufacturers.12 Hematoma and infection occur in less than 1% of primary augmentation patients.15
Malposition of the implant over time is less frequent with textured implants because of the higher coefficient of friction compared with smooth implants.6,8,15
Visible skin rippling may be a consequence of texturing and also of thin body habitus, eg, in patients with a body mass index less than 18.5 kg/m2. If the soft-tissue layer of the breast is thin, the natural rippling of smooth saline implant shells are more likely to show when placed in the subglandular pocket. Form-stable implants, by contrast, resist rippling.12,15
Large implants and extensive lateral dissection can cause alterations in nipple sensation and sensory loss within lower breast pole skin. Axillary incisions may traumatize or damage the intercostobrachial nerve, resulting in upper inner arm sensory aberrations.
Ultimately, the 10-year incidence of secondary surgery ranges from 0% to 36% and the 10-year incidence of capsular contracture ranges from 11% to 19%.15 Additional cosmetic complaints after augmentation with implants include enlargement of the areola and engorgement of breast veins.14
BREAST CANCER AND DETECTION
Patients with or without implants do not seem to differ with regard to breast cancer stage upon detection, tumor burden, recurrence, or survival. However, more patients with implants may present with palpable masses, invasive tumors, axillary metastasis, and falsely negative mammograms.
Breast implants may actually facilitate cancer detection on physical examination by providing a more dense or stable surface upon which to palpate the breast tissue. Although they do not necessarily impair mastectomy or breast reconstruction, they may result in an increased rate of revision surgery after breast conservation therapy.24,36 Mammography remains the standard of care for radiologic diagnosis but can be further supported by MRI and ultrasonography if necessary in patients with implants.
AUTOIMMUNE DISEASES
Although concerns persist, multiple studies have demonstrated the safety of fourth- and fifth-generation silicone breast implants with regard to autoimmune disease.7
In various clinical studies in mastectomy patients who underwent breast reconstruction with either silicone implants or autologous tissue, no difference was found with regard to the incidence of autoimmune diseases.2 Additionally, in meta-analyses of data from more than 87,000 women, no association was found between connective tissue disease and silicone breast implants.2,11 One study11,23 noted no increase in autoantibodies in patients with undamaged silicone implants vs patients who experienced rupture.
Studies have also demonstrated that in children born to mothers with breast implants, the risk of rheumatic disease, esophageal disorders, congenital malformations, and death during the perinatal period is comparable with that in controls.37 Another study, examining breastfeeding in women with silicone breast implants, showed no significant difference in silicon levels (used as a proxy for silicone) in breast milk compared with controls without implants; silicon levels were found to be significantly higher in cow’s milk and store-bought formulas.38
BREAST IMPLANT-ASSOCIATED ANAPLASTIC LARGE-CELL LYMPHOMA
Breast implant-associated anaplastic large-cell lymphoma (BIA-ALCL) is a subtype of T-cell lymphoma that develops in tissue adjacent to breast implants. It typically presents as breast swelling 2 to 38 years (mean of 8 years) after implant insertion.39,40 The swelling may be secondary to periprosthetic seroma formation or, more rarely, palpable disease in the axilla. Patients occasionally complain of pain and, rarely, constitutional symptoms.20 BIA-ALCL is not a disease of the surrounding breast tissue, but rather of the fibrous periprosthetic capsule.21
Of note, there is no documented case involving smooth implants,41–43 but it may be related to fifth-generation textured implants.6 At present, it is not possible to definitively state which implant is associated with this condition; hence, more data are needed, and this association is currently under study.
The absolute risk of BIA-ALCL was reported in a Dutch study39 as 1 in 35,000 by age 50, 1 in 12,000 by age 70, and 1 in 7,000 by age 75, with a number needed to harm of 6,920. Overall lifetime risk was estimated at 1 in 30,000 for women with textured implants in a 2015 US study.40 In comparison, breast cancer risk is about 1 in 8 women. There is no apparent predilection for patients who underwent cosmetic augmentation vs reconstruction, or who received silicone vs saline implants.
The diagnosis is confirmed by ultrasonographically guided fine-needle aspiration of seroma fluid and subsequent immunohistochemical testing for CD30-positive and ALK-negative T lymphocytes. Other than positron-emission tomography for staging after diagnosis confirmation, imaging is ineffective. Expert opinion does not recommend routine screening unless the aforementioned symptoms arise.
Treatment involves implant removal and total capsulectomy, with samples sent for pathology study with cytokeratin staining.12 Of note, in all cases of BIA-ALCL in which the disease was limited to the circumscribed scar tissue of the breast capsule, complete surgical excision has proved curative, whereas incomplete capsulectomy portends a greater risk of recurrence and decreased survival.44
In cases of advanced or recurrent ALCL, diagnosed late or inappropriately, the National Comprehensive Cancer Network recommends a multidisciplinary approach involving adjuvant chemotherapy and radiation.44 Anecdotally, at our institution, we have recently treated several cases of advanced ALCL presenting with invasive chest wall masses with extirpative surgery and subsequent reconstruction with the assistance of our thoracic surgery colleagues, as well as the aforementioned multidisciplinary approach using adjuvant therapy.
The mechanism of this malignancy is currently under investigation, but the current theory implicates an exaggerated lymphoproliferative response to bacterial contamination of the capsule superimposed upon genetic factors in susceptible patients.42,43
National societies advise plastic surgeons to discuss the risk of BIA-ALCL with all patients at the time of breast augmentation consultation and to report all confirmed cases to the PROFILE registry (Patient Registry and Outcomes for Breast Implants and Anaplastic Large Cell Lymphoma Etiology and Epidemiology).45
ARE PATIENTS HAPPIER AFTERWARD?
Studies have shown that after undergoing breast augmentation surgery, patients note improvement in body image, and satisfaction rates range from 85% to 95% with respect to self-confidence and body image.46 An evaluation of patient responses on the validated BREAST-Q Augmentation Questionnaire showed the following satisfaction rates: breasts 83%, psychosocial well-being 88%, and sexual functioning 81%.15
Although epidemiologic studies have reported higher suicide rates in women with cosmetic breast implants, this likely stems from preoperative psychological factors and underscores the role of psychiatric referral in patients with a mental health history or in those whom the surgeon deems it necessary.46
Several high-quality studies have demonstrated that quality of life and psychosocial functioning (including depression) markedly improve after breast augmentation surgery.47 Among a cohort of Norwegian patients, breast implant surgery resulted in improved motivation to perform daily activities, as well as improved quality of life from both a psychosocial and aesthetic perspective.48 Interestingly, a recent study reported that patients who underwent breast implant surgery alone reported greater satisfaction and psychosocial quality of life than patients who underwent combination breast augmentation and mastopexy (breast-lifting) surgery.49
Additional data are needed to refine our understanding of the complex interplay between psychosocial factors before and after surgery in patients seeking and undergoing breast augmentation procedures.
At present, 300,000 US women undergo breast augmentation surgery each year,1 making this the second most common aesthetic procedure in women (after liposuction),2–4 and making it extremely likely that clinicians will encounter women who have breast implants. In addition, approximately 110,000 women undergo breast reconstructive surgery after mastectomy, of whom more than 88,000 (81%) receive implants (2016 data).5
This review discusses the evolution of breast implants, their complications, and key considerations with regard to aesthetic and reconstructive breast surgery, as the principles are similar.
EVOLUTION OF IMPLANTS
Reports of breast augmentation surgery, also known as augmentation mammoplasty, date back to 1895, when a fatty tumor (lipoma) was successfully transplanted from a patient’s back to a breast defect in a mastectomy patient.2,3,6,7 In the 1930s, implantation of a glass ball into a patient’s breast marked the first implant-based breast augmentation.6 By 1954, attempts at breast augmentation using local dermal-fat flaps, adipose tissue, and even omentum were described.
Alloplastic materials gained popularity throughout the 1950s and 1960s and included polyurethane, polytetrafluoroethylene (Teflon), and other synthetics. Adverse reactions associated with alloplastic materials were plentiful: local tissue reactions, distortion of the breast mound, increased firmness, and discomfort all contributed to the eventual discontinuation of their use. The history of alloplastic breast augmentation also included epoxy resin, shellac, beeswax, paraffin, rubber, petroleum jelly, and liquefied silicone. Outcomes were not good, and many patients ultimately needed mastectomy.7
The first modern breast prosthesis was developed in 1961, and since then, implant composition and design have evolved significantly.8
From silicone to saline, and back again
The first silicone gel implants, introduced in the early 1960s,8–19 had high complication rates—some centers reported an incidence of capsular contracture of up to 70%.8,11 This is a foreign body reaction in which pathologic scar tissue encases the implant, causing it to distort, appear misshapen, harden, and even become painful.11 Attempts to minimize this reaction led to later generations of silicone implants with polyurethane shells.12
Inflatable implants filled with sterile saline solution were originally developed in France in 1965. Unlike silicone implants, saline implants have undergone minimal changes since their inception, and grew in popularity during the 1970s in view of the high rates of capsular contracture with silicone implants.8 However, saline implants have their own problems, and as they became increasingly popular, deflation and the unnatural feel of saline sparked a renewed interest in silicone gel.
By the late 1980s, the thinner-shelled generation of silicone implants displayed its own frustrating complications including implant rupture, capsular contracture, infection, and possible systemic and disseminated granulomatous disease. From 1992 to 2006, the US Food and Drug Administration (FDA) placed a moratorium on silicone implants due to concerns about a possible link with autoimmune and connective tissue diseases and the possible carcinogenic nature of silicone.
While silicone implants were prohibited in the United States, development continued abroad, and eventually the moratorium was lifted after several meta-analyses failed to reveal any link regarding the aforementioned concerns.13
Today, silicone gel implants dominate the world market.14 In the United States, approximately 60% of implants contain silicone gel filler, and trends are similar in Europe.7
Table 1 summarizes the evolution of silicone breast implants over the last 50 years.2,6,11,12 Table 2 lists the advantages and disadvantages of silicone and saline breast implants.2,6,8,15
CURRENT IMPLANT OPTIONS
Currently, 3 companies (Allergan, Mentor, Sientra) manufacture and distribute breast implants and implant-associated products such as tissue expanders and sizers in the US market.6
Another company, Motiva, makes an implant that is available in Europe, Asia, and Australia, and the device is currently undergoing a 10-year clinical trial in the United States that began recruiting patients in 16 centers in April 2018.16 Pending final approval, the Cleveland Clinic Department of Plastic Surgery may be among the centers involved in the clinical trial of the Motiva implant. Innovations in the Motiva implant include a high-performance shell that maintains consistent strength and includes a proprietary barrier layer, improved silicone gel filler, 3-D imprinted surface texturing, and an implant shape that adapts with vertical and horizontal movement. It also contains radio-frequency identification transponders that can transmit data about the implant wirelessly.17–19
Surface (textured vs smooth)
Developed in the 1980s, texturing of the implant surface disrupts capsule formation around the prosthesis. Additionally, texturing stabilizes an anatomically shaped (teardrop) implant within the breast pocket, reducing malrotation.20,21
The first textured implants were covered with polyurethane foam, but they were ultimately withdrawn from the US market because of concern for in vivo degradation to carcinogenic compounds. The focus subsequently turned to texturing implant shells by mechanically creating pores of different sizes. Smooth implants, by contrast, are manufactured by repeatedly dipping the implant shell into liquid silicone.2
The capsular contraction rate has been shown to be lower with textured silicone than with smooth silicone (number needed to treat = 7–9), and evidence suggests a lower risk of needing a secondary procedure.21
Form-stable vs fluid-form
Silicone is a polymer. The physical properties of polymers vary greatly and depend on the length of the individual chains and the degree to which those chains are cross-linked. Liquid silicone contains short chains and sparse cross-linking, resulting in an oily compound well suited for lubrication. Silicone gel contains longer chains and more cross-linking and is therefore more viscous.
In “form-stable” implants, the silicone interior has sufficient chain length and cross-linking to retain the designed shape even at rest,2 but they require slightly larger incisions.7 “Fluid-form” refers to an implant with silicone filler with shorter chain length, less cross-linking, and more fluidity.6
Shell
As with silicone fillers, the properties of silicone implant shells also depend on chain length and cross-linking within the polymer. Silicone elastomer shells (Table 1) contain extensively cross-linked chains that impart a flexible yet rubbery character. Silicone elastomers can also be found in facial implants and tissue expanders.2
Implant shape (round vs anatomic)
The shape of an implant is determined by the gel distribution inside of it. To understand gel distribution and implant shape, one must understand the gel-shell ratio. This ratio increases as cohesivity of the filler increases, and it represents increased bonding of the gel filler to the shell and a preserved implant shape at rest.
The gel-shell ratio varies among manufacturers, and a less-viscous filler may be more prone to rippling or loss of upper pole fullness in some patients. For this reason, careful analysis, patient and implant selection, and discussion of complications remain paramount.2
No anatomically shaped implant is manufactured with a smooth shell, but rather with a textured shell that resists malrotation.6,15 However, in the United States, 95% of patients receive round implants.16
PATIENT ASSESSMENT
Before breast augmentation surgery, the surgeon assesses a number of factors—physical and psychosocial—and helps the patient choose a type and size of implant. The surgeon and patient also plan where the implants will be placed—ie, above or beneath the chest wall muscle—and where the incisions will be made. Every decision is made in close consultation with the patient, taking into account the patient’s desires and expectations, as well as what the patient’s anatomy allows. An integral component of this shared decision-making process is a discussion of the possible complications, and often photographs to better illustrate what to expect postoperatively.
Psychosocial factors
One must consider the patient’s psychology, motivations for surgery, and emotional stability. Here, we look for underlying body dysmorphic disorder; excessive or unusual encouragement to undergo the procedure by a spouse, friends, or others; a history of other aesthetic procedures; unrealistic expectations; and other factors influencing the desire to undergo this surgery.
Choosing an implant
Implant selection must take into account the patient’s height, weight,7 and overall body morphology: taller patients and those with wider hips or shoulders usually require larger implants. A reliable method for determining the appropriate implant must include the current breast shape, dimensions, volume, skin elasticity, soft-tissue thickness, and overall body habitus. Ultimately, the most important considerations include breast base diameter, implant volume,20 and soft-tissue envelope.
Preoperative sizing can involve placing sample implants within a brassiere so that the patient can preview possible outcomes. This method is particularly effective in minimizing dissatisfaction because it shares ownership of the decision-making process.15
A computerized implant selection program available in Europe suggests a “best-fit” implant based on a clinician’s measurements.7
Anatomic placement
Traditionally, plastic surgeons place breast implants either beneath the pectoralis major muscle (submuscular placement) or over the pectoralis8 but beneath the glandular breast parenchyma (subglandular placement) (Figure 2).7
Advantages of submuscular placement are a smoother transition of the upper breast pole from the chest wall and less rippling visible through the skin, due to the additional muscular coverage of the implant. Another advantage is that capsular contraction rates are lower with submuscular placement, likely due to possible contamination of implants by lactiferous ductal microbes when accessing the subglandular plane.14,20 Disadvantages are pronounced discomfort after surgery and animation deformities with muscle contraction, particularly in young, highly active patients.
A popular modification of submuscular placement involves creating a surgical dissection plane between the subglandular tissue and the pectoralis major fascia. This “dualplane” approach allows the parenchyma to retract superiorly and reduce breast ptosis.7
Incisions
Table 3 highlights important considerations with regard to incision location.15,20,21
ANTIBIOTICS
Many surgeons give a single prophylactic dose of antibiotic before surgery, a practice that some studies have shown to be effective in reducing the risk of infection.15 However, the benefit of routine postoperative use of antibiotics remains unsubstantiated15: postoperative antibiotic use does not appear to protect against infection, capsular contracture, or overall complications in primary or secondary breast augmentation surgery.20
PERIOPERATIVE PERIOD
At our institution, breast augmentation surgery is an ambulatory procedure—the patient goes home the same day unless circumstances such as pain control warrant admission. This is, however, according to surgeon preference, and differs on a case-by-case basis. General anesthesia is the standard of care.15
POSTOPERATIVE PERIOD
In the immediate postoperative period, patients are instructed to wear a surgical bra for up to 6 weeks to allow stable scarring. Early mobilization is encouraged.7,15 Depending on the patient’s situation, recovery, and healing, she may be out of work for about 1 week, sometimes more, sometimes less.
Additional instructions are surgeon-specific. However, the patient is instructed to avoid bathing, swimming, immersion in water, and wearing underwire brassieres that could impair healing of an inferior incision; instead, patients are often instructed to wear a surgical bra provided on the day of surgery until cleared in the clinic.
Showering is allowed the next day or the second day after surgery, and of course there is no driving while on narcotics. Additionally, patients are counseled extensively regarding hematoma formation and the signs and symptoms of infection.
Patients are typically seen in clinic 1 week after surgery.
The cost of surgery may be $5,000 to $6,000 but can vary significantly from center to center depending on who the patient sees and where, and whether the patient presents for breast reconstruction after cancer or repair of congenital anomalies, or in certain cases of transgender surgery. The patient is typically responsible for the fee, but again this depends on the patient, indications, and particular insurance concerns.
IMPLANT LONGEVITY AND RUPTURE
In the United States, implant rupture rates range from 1.1% to 17.7% at 6 to 10 years after primary augmentation, 2.9% to 14.7% after revision augmentation, 1.5% to 35.4% after primary breast reconstruction, and 0% to 19.6% after revision reconstruction.11
Unfortunately, the existence of multiple implant manufacturers, numerous implant generations, and poorly standardized screening protocols and reporting systems make the true rate of implant rupture difficult to assess without definitive imaging or implant retrieval.11
Damage from surgical instrumentation during implantation is the most common cause of silicone breast implant rupture (50% to 64% of cases).22 Other causes include underfilling and fold flaw from capsular contracture.
Leakage of silicone gel filler may be confined to the periprosthetic capsule (intracapsular rupture) or extend beyond and into the breast parenchyma (extracapsular rupture). One study reported that only 10% of intracapsular ruptures progressed extracapsularly, while 84% of patients with extracapsular involvement remained stable for up to 2 years,23 indicating that intracapsular rupture may not portend worsening disease.11
Implant rupture occurs silently in most cases, with no clinically detectable signs or symptoms. In other cases, patients may present with alterations in breast shape and size, sudden asymmetry, firmness, pronounced capsular contracture, contour irregularity, or pain.
Aside from physical examination, comprehensive diagnostic testing includes imaging—ultrasonography, mammography, computed tomography, and magnetic resonance imaging (MRI). Of these, MRI is the method of choice, with sensitivity and specificity exceeding 90% for detecting implant rupture.11 Classic findings on MRI include the “linguine” sign from a deflating implant shell, or the teardrop sign from implant sagging. Classic findings on ultrasonography include the “snowstorm” sign of extracapsular rupture and the “stepladder” sign of intracapsular rupture.
Mammography effectively detects free silicone in breast tissue with extracapsular rupture (25% of ruptures according to some studies)23; however, it cannot detect rupture within the implant capsule. As an aside, submuscular implant placement may interfere less with screening mammography than subglandular implants do.14,24
Current FDA recommendations to detect implant rupture encourage women with silicone breast implants to undergo screening 3 years after implantation and then every 2 years thereafter; no long-term monitoring is suggested for saline implants.15 Many plastic surgeons evaluate silicone breast implant patients every 1 to 2 years for contracture and rupture.8 Of note, capsular contracture impairs the effectiveness of ultrasonography and may require MRI confirmation.11
If implant rupture is confirmed, the current recommendation is to remove the implant and the capsule. Another implant may be placed depending on the patient’s preference. Rigorous washout remains a key feature of any surgical intervention for ruptured breast implants; however, in the event of extracapsular rupture, resection of silicone granulomas may also be required.11
Reoperation rates for primary breast augmentation surgery approach 20% and are even higher for secondary augmentation over a patient’s lifetime—the highest rate of all aesthetic procedures.7,14
CAPSULAR CONTRACTURE
Capsular contracture is the most common complication of breast augmentation,25 typically presenting within the first postoperative year,26,27 and the risk increases over time.28 It occurs with both silicone and saline breast implants.
In some studies, the incidence exceeded 4% in the first 2 years after surgery,29 and nearly 50% by 10 years.30 Other studies found rates of 0% to 20% over 13 years.20
The etiology is not well understood and is presumed to be multifactorial, with proposed mechanisms and factors that include bacterial contamination, surface texturing, the implant pocket selected, the incision type, drain placement, antibiotic use, and smoking.25
A meta-analysis from 17,000 implants found that the risk of capsular contracture was significantly higher when an implant was placed in a subglandular pocket than in a submuscular pocket,22,26 and that although texturing decreased capsular contracture compared with smooth implants, the effect was modest when a textured or smooth implant was placed in a submuscular location.28 With regard to incision location, studies have reported that the incidence of capsular contracture is highest with transaxillary and periareolar incisions, and lowest with inframammary incisions.20,21
The leading theory is that contamination of the implant (primarily from the mammary ducts) results in biofilm formation. Subclinical hematoma surrounding the implant may also provide key bacterial nutrients.20
Textured implants induce a greater inflammatory response in the capsular tissue, resulting in a thicker capsule; however, contracture rates remain lower with textured than with smooth implants.14,31 Interestingly, lower rates of capsular contracture have been observed with later-generation, cohesive-gel, form-stable implants than with those of earlier generations.12
Although more research is needed, silicone implants appear to confer a higher risk of capsular contracture than saline implants.14,20
Irrigating the breast pocket intraoperatively with triple antibiotic solution (bacitracin, cefazolin, and gentamicin) before placing the implant may decrease the capsular contracture rate.15,20
Treatments for capsular contracture include pocket modifications such as capsulotomy (making releasing, relaxing incisions in the scar capsule encasing the implant), capsulectomy (removing portions of or the entire capsule), and replacing the implant in the other pocket (ie, if the original implant was subglandular, the replacement is placed in the submuscular pocket). Patients who have contractures that fail to respond to these treatments may ultimately benefit from implant removal and autologous reconstruction (autoaugmentation) rather than implant replacement.32,33
ADDITIONAL COMPLICATIONS
Other complications include infection, malposition, rippling, seroma, hematoma, and sensory alterations.
Irrigation during the implantation procedure with a triple antibiotic solution consisting of bacitracin, gentamycin, and cephalexin in normal saline decreases infection and seroma rates.15,20,34
Some surgeons also choose to irrigate the pocket with a betadine solution, or to cleanse the skin with betadine and place sterile towels and redrape before inserting the implant. Additionally, many prefer using a sterile device much like a pastry funnel called a Keller funnel to insert the implant into the breast pocket.35
Infection is less common with cosmetic augmentations than with implant-based breast reconstruction, likely because of healthier, well-vascularized tissue in patients undergoing cosmetic surgery than in those undergoing mastectomy.14
Seroma is thought to be a consequence of texturing, and more so with macro- vs microtexturing. Though poorly understood, an association between texturing and double capsules has also been reported.12,20
After primary breast augmentation, 10-year follow-up rates of capsular contracture, seroma, rippling, and malposition vary across the 3 major silicone implant manufacturers.12 Hematoma and infection occur in less than 1% of primary augmentation patients.15
Malposition of the implant over time is less frequent with textured implants because of the higher coefficient of friction compared with smooth implants.6,8,15
Visible skin rippling may be a consequence of texturing and also of thin body habitus, eg, in patients with a body mass index less than 18.5 kg/m2. If the soft-tissue layer of the breast is thin, the natural rippling of smooth saline implant shells are more likely to show when placed in the subglandular pocket. Form-stable implants, by contrast, resist rippling.12,15
Large implants and extensive lateral dissection can cause alterations in nipple sensation and sensory loss within lower breast pole skin. Axillary incisions may traumatize or damage the intercostobrachial nerve, resulting in upper inner arm sensory aberrations.
Ultimately, the 10-year incidence of secondary surgery ranges from 0% to 36% and the 10-year incidence of capsular contracture ranges from 11% to 19%.15 Additional cosmetic complaints after augmentation with implants include enlargement of the areola and engorgement of breast veins.14
BREAST CANCER AND DETECTION
Patients with or without implants do not seem to differ with regard to breast cancer stage upon detection, tumor burden, recurrence, or survival. However, more patients with implants may present with palpable masses, invasive tumors, axillary metastasis, and falsely negative mammograms.
Breast implants may actually facilitate cancer detection on physical examination by providing a more dense or stable surface upon which to palpate the breast tissue. Although they do not necessarily impair mastectomy or breast reconstruction, they may result in an increased rate of revision surgery after breast conservation therapy.24,36 Mammography remains the standard of care for radiologic diagnosis but can be further supported by MRI and ultrasonography if necessary in patients with implants.
AUTOIMMUNE DISEASES
Although concerns persist, multiple studies have demonstrated the safety of fourth- and fifth-generation silicone breast implants with regard to autoimmune disease.7
In various clinical studies in mastectomy patients who underwent breast reconstruction with either silicone implants or autologous tissue, no difference was found with regard to the incidence of autoimmune diseases.2 Additionally, in meta-analyses of data from more than 87,000 women, no association was found between connective tissue disease and silicone breast implants.2,11 One study11,23 noted no increase in autoantibodies in patients with undamaged silicone implants vs patients who experienced rupture.
Studies have also demonstrated that in children born to mothers with breast implants, the risk of rheumatic disease, esophageal disorders, congenital malformations, and death during the perinatal period is comparable with that in controls.37 Another study, examining breastfeeding in women with silicone breast implants, showed no significant difference in silicon levels (used as a proxy for silicone) in breast milk compared with controls without implants; silicon levels were found to be significantly higher in cow’s milk and store-bought formulas.38
BREAST IMPLANT-ASSOCIATED ANAPLASTIC LARGE-CELL LYMPHOMA
Breast implant-associated anaplastic large-cell lymphoma (BIA-ALCL) is a subtype of T-cell lymphoma that develops in tissue adjacent to breast implants. It typically presents as breast swelling 2 to 38 years (mean of 8 years) after implant insertion.39,40 The swelling may be secondary to periprosthetic seroma formation or, more rarely, palpable disease in the axilla. Patients occasionally complain of pain and, rarely, constitutional symptoms.20 BIA-ALCL is not a disease of the surrounding breast tissue, but rather of the fibrous periprosthetic capsule.21
Of note, there is no documented case involving smooth implants,41–43 but it may be related to fifth-generation textured implants.6 At present, it is not possible to definitively state which implant is associated with this condition; hence, more data are needed, and this association is currently under study.
The absolute risk of BIA-ALCL was reported in a Dutch study39 as 1 in 35,000 by age 50, 1 in 12,000 by age 70, and 1 in 7,000 by age 75, with a number needed to harm of 6,920. Overall lifetime risk was estimated at 1 in 30,000 for women with textured implants in a 2015 US study.40 In comparison, breast cancer risk is about 1 in 8 women. There is no apparent predilection for patients who underwent cosmetic augmentation vs reconstruction, or who received silicone vs saline implants.
The diagnosis is confirmed by ultrasonographically guided fine-needle aspiration of seroma fluid and subsequent immunohistochemical testing for CD30-positive and ALK-negative T lymphocytes. Other than positron-emission tomography for staging after diagnosis confirmation, imaging is ineffective. Expert opinion does not recommend routine screening unless the aforementioned symptoms arise.
Treatment involves implant removal and total capsulectomy, with samples sent for pathology study with cytokeratin staining.12 Of note, in all cases of BIA-ALCL in which the disease was limited to the circumscribed scar tissue of the breast capsule, complete surgical excision has proved curative, whereas incomplete capsulectomy portends a greater risk of recurrence and decreased survival.44
In cases of advanced or recurrent ALCL, diagnosed late or inappropriately, the National Comprehensive Cancer Network recommends a multidisciplinary approach involving adjuvant chemotherapy and radiation.44 Anecdotally, at our institution, we have recently treated several cases of advanced ALCL presenting with invasive chest wall masses with extirpative surgery and subsequent reconstruction with the assistance of our thoracic surgery colleagues, as well as the aforementioned multidisciplinary approach using adjuvant therapy.
The mechanism of this malignancy is currently under investigation, but the current theory implicates an exaggerated lymphoproliferative response to bacterial contamination of the capsule superimposed upon genetic factors in susceptible patients.42,43
National societies advise plastic surgeons to discuss the risk of BIA-ALCL with all patients at the time of breast augmentation consultation and to report all confirmed cases to the PROFILE registry (Patient Registry and Outcomes for Breast Implants and Anaplastic Large Cell Lymphoma Etiology and Epidemiology).45
ARE PATIENTS HAPPIER AFTERWARD?
Studies have shown that after undergoing breast augmentation surgery, patients note improvement in body image, and satisfaction rates range from 85% to 95% with respect to self-confidence and body image.46 An evaluation of patient responses on the validated BREAST-Q Augmentation Questionnaire showed the following satisfaction rates: breasts 83%, psychosocial well-being 88%, and sexual functioning 81%.15
Although epidemiologic studies have reported higher suicide rates in women with cosmetic breast implants, this likely stems from preoperative psychological factors and underscores the role of psychiatric referral in patients with a mental health history or in those whom the surgeon deems it necessary.46
Several high-quality studies have demonstrated that quality of life and psychosocial functioning (including depression) markedly improve after breast augmentation surgery.47 Among a cohort of Norwegian patients, breast implant surgery resulted in improved motivation to perform daily activities, as well as improved quality of life from both a psychosocial and aesthetic perspective.48 Interestingly, a recent study reported that patients who underwent breast implant surgery alone reported greater satisfaction and psychosocial quality of life than patients who underwent combination breast augmentation and mastopexy (breast-lifting) surgery.49
Additional data are needed to refine our understanding of the complex interplay between psychosocial factors before and after surgery in patients seeking and undergoing breast augmentation procedures.
- Derby BM, Codner MA. Textured silicone breast implant use in primary augmentation: core data update and review. Plast Reconstr Surg 2015; 135(1):113–124. doi:10.1097/PRS.0000000000000832
- Maxwell GP, Gabriel A. Breast implant design. Gland Surg 2017; 6(2):148–153. doi:10.21037/gs.2016.11.09
- Gabriel A, Maxwell GP. The evolution of breast implants. Clin Plast Surg 2015; 42(4):399–404. doi:10.1016/j.cps.2015.06.015
- American Society of Plastic Surgeons. Procedural statistics trends 1992–2012. www.plasticsurgery.org/documents/News/Statistics/2012/plastic-surgery-statistics-full-report-2012.pdf. Accessed January 17, 2019.
- American Society of Plastic Surgeons. Plastic surgery statistics report 2016. www.plasticsurgery.org/documents/News/Statistics/2016/plastic-surgery-statistics-full-report-2016.pdf. Accessed January 17, 2019.
- Henderson PW, Nash D, Laskowski M, Grant RT. Objective comparison of commercially available breast implant devices. Aesthetic Plast Surg 2015; 39(5):724–732. doi:10.1007/s00266-015-0537-1
- Adams WP Jr, Mallucci P. Breast augmentation. Plast Reconstr Surg 2012; 130(4):597e–611e. doi:10.1097/PRS.0b013e318262f607
- Spear SL, Jespersen MR. Breast implants: saline or silicone? Aesthet Surg J 2010; 30(4):557–570. doi:10.1177/1090820X10380401
- Cronin TD, Gerow FJ. Augmentation mammaplasty: a new “natural feel” prosthesis. In: Transactions of the Third International Conference of Plastic Surgery: October 13–18, 1963, Washington, DC.
- Maxwell GP, Gabriel A. The evolution of breast implants. Plast Reconstr Surg 2014; 134(suppl 1):12S–17S. doi:10.1097/PRS.0000000000000348
- Hillard C, Fowler JD, Barta R, Cunningham B. Silicone breast implant rupture: a review. Gland Surg 2017; 6(2):163–168. doi:10.21037/gs.2016.09.12
- Derby BM, Codner MA. Textured silicone breast implant use in primary augmentation: core data update and review. Plast Reconstr Surg 2015; 135(1):113–124. doi:10.1097/PRS.0000000000000832
- Tugwell P, Wells G, Peterson J, et al. Do silicone breast implants cause rheumatologic disorders? A systematic review for a court-appointed national science panel. Arthritis Rheum 2001; 44(11):2477–2484. pmid:11710703
- Alpert BS, Lalonde DH. MOC-PS(SM) CME article: breast augmentation. Plast Reconstr Surg 2008; 121(suppl 4):1–7. doi:10.1097/01.prs.0000305933.31540.5d
- Hidalgo DA, Spector JA. Breast augmentation. Plast Reconstr Surg 2014; 133(4):567e–583e. doi:10.1097/PRS.0000000000000033
- ClinicalTrials.gov. Study of the safety and effectiveness of Motiva Implants®. https://clinicaltrials.gov/ct2/show/NCT03579901. Accessed January 17, 2019.
- Establishment Labs. Motiva Implants. https://motivaimplants.com/why-motiva/innovation-for-enhanced-safety/. Accessed January 17, 2019.
- Sforza M, Zaccheddu R, Alleruzzo A, et al. Preliminary 3-year evaluation of experience with silksurface and velvetsurface Motiva silicone breast implants: a single-center experience with 5813 consecutive breast augmentation cases. Aesthet Surg J 2018; 38(suppl 2):S62–S73. doi:10.1093/asj/sjx150
- Huemer GM, Wenny R, Aitzetmüller MM, Duscher D. Motiva ergonomix round silksurface silicone breast implants: outcome analysis of 100 primary breast augmentations over 3 years and technical considerations. Plast Reconstr Surg 2018; 141(6):831e–842e. doi:10.1097/PRS.0000000000004367
- Lista F, Ahmad J. Evidence-based medicine: augmentation mammaplasty. Plast Reconstr Surg 2013; 132(6):1684–1696. doi:10.1097/PRS.0b013e3182a80880
- Namnoum JD, Largent J, Kaplan HM, Oefelein MG, Brown MH. Primary breast augmentation clinical trial outcomes stratified by surgical incision, anatomical placement and implant device type. J Plast Reconstr Aesthet Surg 2013; 66(9):1165–1172. doi:10.1016/j.bjps.2013.04.046
- Handel N, Garcia ME, Wixtrom R. Breast implant rupture: causes, incidence, clinical impact, and management. Plast Reconstr Surg 2013; 132(5):1128–1137. doi:10.1097/PRS.0b013e3182a4c243
- Hölmich LR, Friis S, Fryzek JP, et al. Incidence of silicone breast implant rupture. Arch Surg 2003; 138(7):801–806. doi:10.1001/archsurg.138.7.801
- Mccarthy CM, Pusic AL, Disa JJ, Cordeiro PG, Cody HS 3rd, Mehrara B. Breast cancer in the previously augmented breast. Plast Reconstr Surg 2007; 119(1):49–58. doi:10.1097/01.prs.0000244748.38742.1f
- Egeberg A, Sørensen JA. The impact of breast implant location on the risk of capsular contraction. Ann Plast Surg 2016; 77(2):255–259. doi:10.1097/SAP.0000000000000227
- Wickman M. Rapid versus slow tissue expansion for breast reconstruction: a three-year follow-up. Plast Reconstr Surg 1995; 95(4):712–718. pmid:7892316
- Kjøller K, Hölmich LR, Jacobsen PH, et al. Epidemiological investigation of local complications after cosmetic breast implant surgery in Denmark. Ann Plast Surg 2002; 48(3):229–237. pmid:11862025
- Handel N, Jensen JA, Black Q, Waisman JR, Silverstein MJ. The fate of breast implants: a critical analysis of complications and outcomes. Plast Reconstr Surg 1995; 96(7):1521–1533. pmid:7480271
- Henriksen TF, Hölmich LR, Fryzek JP, et al. Incidence and severity of short-term complications after breast augmentation: results from a nationwide breast implant registry. Ann Plast Surg 2003; 51(6):531–539. doi:10.1097/01.sap.0000096446.44082.60
- Fernandes JR, Salinas HM, Broelsch GF, et al. Prevention of capsular contracture with photochemical tissue passivation. Plast Reconstr Surg 2014; 133(3):571–577. doi:10.1097/01.prs.0000438063.31043.79
- Wong CH, Samuel M, Tan BK, Song C. Capsular contracture in subglandular breast augmentation with textured versus smooth breast implants: a systematic review. Plast Reconstr Surg 2006; 118(5):1224–1236. doi:10.1097/01.prs.0000237013.50283.d2
- Gurunluoglu R, Sacak B, Arton J. Outcomes analysis of patients undergoing autoaugmentation after breast implant removal. Plast Reconstr Surg 2013; 132(2):304–315. doi:10.1097/PRS.0b013e31829e7d9e
- Gurunluoglu R, Shafighi M, Schwabegger A, Ninkovic M. Secondary breast reconstruction with deepithelialized free flaps from the lower abdomen for intractable capsular contracture and maintenance of breast volume. J Reconstr Microsurg 2005; 21(1):35–41. doi:10.1055/s-2005-862779
- Adams WP Jr, Rios JL, Smith SJ. Enhancing patient outcomes in aesthetic reconstructive breast surgery using triple antibiotic breast irrigation: six-year prospective clinical study. Plast Reconstru Surg 2006; 118(7 suppl):46S–52S. doi:10.1097/01.prs.0000185671.51993.7e
- Moyer HR, Ghazi B, Saunders N, Losken A. Contamination in smooth gel breast implant placement: testing a funnel versus digital insertion technique in a cadaver model. Aesthet Surg J 2012; 32(2):194–199. doi:10.1177/1090820X11434505
- Handel N. The effect of silicone implants on the diagnosis, prognosis, and treatment of breast cancer. Plast Reconstr Surg 2007; 120(7 suppl 1):81S–93S. doi:10.1097/01.prs.0000286578.94102.2b
- Kjøller K, Friis S, Lipworth L, Mclaughlin JK, Olsen JH. Adverse health outcomes in offspring of mothers with cosmetic breast implants: a review. Plast Reconstr Surg 2007; 120(7 suppl 1):129S–134S. doi:10.1097/01.prs.0000286571.93392.00
- Semple JL. Breast-feeding and silicone implants. Plast Reconstr Surg 2007; 120(7 suppl 1):123S–128S. doi:10.1097/01.prs.0000286579.27852.ed
- de Boer M, van leeuwen FE, Hauptmann M, et al. Breast implants and the risk of anaplastic large-cell lymphoma in the breast. JAMA Oncol 2018; 4(3):335–341. doi:10.1001/jamaoncol.2017.4510
- McCarthy CM, Horwitz SM. Association of breast implants with anaplastic large-cell lymphoma. JAMA Oncol 2018; 4(3):341–342. doi:10.1001/jamaoncol.2017.4467
- American Society of Plastic Surgeons. BIA-ALCL physician resources. www.plasticsurgery.org/for-medical-professionals/health-policy/bia-alcl-physician-resources. Accessed December 17, 2018.
- The American Society for Aesthetic Plastic Surgery, Inc. Member FAQs: latest information on ALCL. www.surgery.org/sites/default/files/Member-FAQs_1.pdf. Accessed January 17, 2019.
- The American Society of Plastic Surgeons. BIA-ALCL resources: summary and quick facts. www.plasticsurgery.org/for-medical-professionals/health-policy/bia-alcl-summary-and-quick-facts. Accessed January 17, 2019.
- National Comprehensive Cancer Network. T-cell lymphomas. www.nccn.org/professionals/physician_gls/pdf/t-cell.pdf.
- The Plastic Surgery Foundation PROFILE Registry. www.thepsf.org/research/registries/profile. Accessed January 17, 2019.
- Sarwer DB. The psychological aspects of cosmetic breast augmentation. Plast Reconstr Surg 2007; 120(7 suppl 1):110S–117S. doi:10.1097/01.prs.0000286591.05612.72
- Rohrich RJ, Adams WP, Potter JK. A review of psychological outcomes and suicide in aesthetic breast augmentation. Plast Reconstr Surg 2007; 119(1):401–408. doi:10.1097/01.prs.0000245342.06662.00
- Kalaaji A, Bjertness CB, Nordahl C, Olafsen K. Survey of breast implant patients: characteristics, depression rate, and quality of life. Aesthet Surg J 2013; 33(2):252–257. doi:10.1177/1090820X12473106
- Kalaaji A, Dreyer S, Brinkmann J, Maric I, Nordahl C, Olafsen K. Quality of life after breast enlargement with implants versus augmentation mastopexy: a comparative study. Aesthet Surg J 2018; 38(12):1304–1315. doi:10.1093/asj/sjy047
- Derby BM, Codner MA. Textured silicone breast implant use in primary augmentation: core data update and review. Plast Reconstr Surg 2015; 135(1):113–124. doi:10.1097/PRS.0000000000000832
- Maxwell GP, Gabriel A. Breast implant design. Gland Surg 2017; 6(2):148–153. doi:10.21037/gs.2016.11.09
- Gabriel A, Maxwell GP. The evolution of breast implants. Clin Plast Surg 2015; 42(4):399–404. doi:10.1016/j.cps.2015.06.015
- American Society of Plastic Surgeons. Procedural statistics trends 1992–2012. www.plasticsurgery.org/documents/News/Statistics/2012/plastic-surgery-statistics-full-report-2012.pdf. Accessed January 17, 2019.
- American Society of Plastic Surgeons. Plastic surgery statistics report 2016. www.plasticsurgery.org/documents/News/Statistics/2016/plastic-surgery-statistics-full-report-2016.pdf. Accessed January 17, 2019.
- Henderson PW, Nash D, Laskowski M, Grant RT. Objective comparison of commercially available breast implant devices. Aesthetic Plast Surg 2015; 39(5):724–732. doi:10.1007/s00266-015-0537-1
- Adams WP Jr, Mallucci P. Breast augmentation. Plast Reconstr Surg 2012; 130(4):597e–611e. doi:10.1097/PRS.0b013e318262f607
- Spear SL, Jespersen MR. Breast implants: saline or silicone? Aesthet Surg J 2010; 30(4):557–570. doi:10.1177/1090820X10380401
- Cronin TD, Gerow FJ. Augmentation mammaplasty: a new “natural feel” prosthesis. In: Transactions of the Third International Conference of Plastic Surgery: October 13–18, 1963, Washington, DC.
- Maxwell GP, Gabriel A. The evolution of breast implants. Plast Reconstr Surg 2014; 134(suppl 1):12S–17S. doi:10.1097/PRS.0000000000000348
- Hillard C, Fowler JD, Barta R, Cunningham B. Silicone breast implant rupture: a review. Gland Surg 2017; 6(2):163–168. doi:10.21037/gs.2016.09.12
- Derby BM, Codner MA. Textured silicone breast implant use in primary augmentation: core data update and review. Plast Reconstr Surg 2015; 135(1):113–124. doi:10.1097/PRS.0000000000000832
- Tugwell P, Wells G, Peterson J, et al. Do silicone breast implants cause rheumatologic disorders? A systematic review for a court-appointed national science panel. Arthritis Rheum 2001; 44(11):2477–2484. pmid:11710703
- Alpert BS, Lalonde DH. MOC-PS(SM) CME article: breast augmentation. Plast Reconstr Surg 2008; 121(suppl 4):1–7. doi:10.1097/01.prs.0000305933.31540.5d
- Hidalgo DA, Spector JA. Breast augmentation. Plast Reconstr Surg 2014; 133(4):567e–583e. doi:10.1097/PRS.0000000000000033
- ClinicalTrials.gov. Study of the safety and effectiveness of Motiva Implants®. https://clinicaltrials.gov/ct2/show/NCT03579901. Accessed January 17, 2019.
- Establishment Labs. Motiva Implants. https://motivaimplants.com/why-motiva/innovation-for-enhanced-safety/. Accessed January 17, 2019.
- Sforza M, Zaccheddu R, Alleruzzo A, et al. Preliminary 3-year evaluation of experience with silksurface and velvetsurface Motiva silicone breast implants: a single-center experience with 5813 consecutive breast augmentation cases. Aesthet Surg J 2018; 38(suppl 2):S62–S73. doi:10.1093/asj/sjx150
- Huemer GM, Wenny R, Aitzetmüller MM, Duscher D. Motiva ergonomix round silksurface silicone breast implants: outcome analysis of 100 primary breast augmentations over 3 years and technical considerations. Plast Reconstr Surg 2018; 141(6):831e–842e. doi:10.1097/PRS.0000000000004367
- Lista F, Ahmad J. Evidence-based medicine: augmentation mammaplasty. Plast Reconstr Surg 2013; 132(6):1684–1696. doi:10.1097/PRS.0b013e3182a80880
- Namnoum JD, Largent J, Kaplan HM, Oefelein MG, Brown MH. Primary breast augmentation clinical trial outcomes stratified by surgical incision, anatomical placement and implant device type. J Plast Reconstr Aesthet Surg 2013; 66(9):1165–1172. doi:10.1016/j.bjps.2013.04.046
- Handel N, Garcia ME, Wixtrom R. Breast implant rupture: causes, incidence, clinical impact, and management. Plast Reconstr Surg 2013; 132(5):1128–1137. doi:10.1097/PRS.0b013e3182a4c243
- Hölmich LR, Friis S, Fryzek JP, et al. Incidence of silicone breast implant rupture. Arch Surg 2003; 138(7):801–806. doi:10.1001/archsurg.138.7.801
- Mccarthy CM, Pusic AL, Disa JJ, Cordeiro PG, Cody HS 3rd, Mehrara B. Breast cancer in the previously augmented breast. Plast Reconstr Surg 2007; 119(1):49–58. doi:10.1097/01.prs.0000244748.38742.1f
- Egeberg A, Sørensen JA. The impact of breast implant location on the risk of capsular contraction. Ann Plast Surg 2016; 77(2):255–259. doi:10.1097/SAP.0000000000000227
- Wickman M. Rapid versus slow tissue expansion for breast reconstruction: a three-year follow-up. Plast Reconstr Surg 1995; 95(4):712–718. pmid:7892316
- Kjøller K, Hölmich LR, Jacobsen PH, et al. Epidemiological investigation of local complications after cosmetic breast implant surgery in Denmark. Ann Plast Surg 2002; 48(3):229–237. pmid:11862025
- Handel N, Jensen JA, Black Q, Waisman JR, Silverstein MJ. The fate of breast implants: a critical analysis of complications and outcomes. Plast Reconstr Surg 1995; 96(7):1521–1533. pmid:7480271
- Henriksen TF, Hölmich LR, Fryzek JP, et al. Incidence and severity of short-term complications after breast augmentation: results from a nationwide breast implant registry. Ann Plast Surg 2003; 51(6):531–539. doi:10.1097/01.sap.0000096446.44082.60
- Fernandes JR, Salinas HM, Broelsch GF, et al. Prevention of capsular contracture with photochemical tissue passivation. Plast Reconstr Surg 2014; 133(3):571–577. doi:10.1097/01.prs.0000438063.31043.79
- Wong CH, Samuel M, Tan BK, Song C. Capsular contracture in subglandular breast augmentation with textured versus smooth breast implants: a systematic review. Plast Reconstr Surg 2006; 118(5):1224–1236. doi:10.1097/01.prs.0000237013.50283.d2
- Gurunluoglu R, Sacak B, Arton J. Outcomes analysis of patients undergoing autoaugmentation after breast implant removal. Plast Reconstr Surg 2013; 132(2):304–315. doi:10.1097/PRS.0b013e31829e7d9e
- Gurunluoglu R, Shafighi M, Schwabegger A, Ninkovic M. Secondary breast reconstruction with deepithelialized free flaps from the lower abdomen for intractable capsular contracture and maintenance of breast volume. J Reconstr Microsurg 2005; 21(1):35–41. doi:10.1055/s-2005-862779
- Adams WP Jr, Rios JL, Smith SJ. Enhancing patient outcomes in aesthetic reconstructive breast surgery using triple antibiotic breast irrigation: six-year prospective clinical study. Plast Reconstru Surg 2006; 118(7 suppl):46S–52S. doi:10.1097/01.prs.0000185671.51993.7e
- Moyer HR, Ghazi B, Saunders N, Losken A. Contamination in smooth gel breast implant placement: testing a funnel versus digital insertion technique in a cadaver model. Aesthet Surg J 2012; 32(2):194–199. doi:10.1177/1090820X11434505
- Handel N. The effect of silicone implants on the diagnosis, prognosis, and treatment of breast cancer. Plast Reconstr Surg 2007; 120(7 suppl 1):81S–93S. doi:10.1097/01.prs.0000286578.94102.2b
- Kjøller K, Friis S, Lipworth L, Mclaughlin JK, Olsen JH. Adverse health outcomes in offspring of mothers with cosmetic breast implants: a review. Plast Reconstr Surg 2007; 120(7 suppl 1):129S–134S. doi:10.1097/01.prs.0000286571.93392.00
- Semple JL. Breast-feeding and silicone implants. Plast Reconstr Surg 2007; 120(7 suppl 1):123S–128S. doi:10.1097/01.prs.0000286579.27852.ed
- de Boer M, van leeuwen FE, Hauptmann M, et al. Breast implants and the risk of anaplastic large-cell lymphoma in the breast. JAMA Oncol 2018; 4(3):335–341. doi:10.1001/jamaoncol.2017.4510
- McCarthy CM, Horwitz SM. Association of breast implants with anaplastic large-cell lymphoma. JAMA Oncol 2018; 4(3):341–342. doi:10.1001/jamaoncol.2017.4467
- American Society of Plastic Surgeons. BIA-ALCL physician resources. www.plasticsurgery.org/for-medical-professionals/health-policy/bia-alcl-physician-resources. Accessed December 17, 2018.
- The American Society for Aesthetic Plastic Surgery, Inc. Member FAQs: latest information on ALCL. www.surgery.org/sites/default/files/Member-FAQs_1.pdf. Accessed January 17, 2019.
- The American Society of Plastic Surgeons. BIA-ALCL resources: summary and quick facts. www.plasticsurgery.org/for-medical-professionals/health-policy/bia-alcl-summary-and-quick-facts. Accessed January 17, 2019.
- National Comprehensive Cancer Network. T-cell lymphomas. www.nccn.org/professionals/physician_gls/pdf/t-cell.pdf.
- The Plastic Surgery Foundation PROFILE Registry. www.thepsf.org/research/registries/profile. Accessed January 17, 2019.
- Sarwer DB. The psychological aspects of cosmetic breast augmentation. Plast Reconstr Surg 2007; 120(7 suppl 1):110S–117S. doi:10.1097/01.prs.0000286591.05612.72
- Rohrich RJ, Adams WP, Potter JK. A review of psychological outcomes and suicide in aesthetic breast augmentation. Plast Reconstr Surg 2007; 119(1):401–408. doi:10.1097/01.prs.0000245342.06662.00
- Kalaaji A, Bjertness CB, Nordahl C, Olafsen K. Survey of breast implant patients: characteristics, depression rate, and quality of life. Aesthet Surg J 2013; 33(2):252–257. doi:10.1177/1090820X12473106
- Kalaaji A, Dreyer S, Brinkmann J, Maric I, Nordahl C, Olafsen K. Quality of life after breast enlargement with implants versus augmentation mastopexy: a comparative study. Aesthet Surg J 2018; 38(12):1304–1315. doi:10.1093/asj/sjy047
KEY POINTS
- Nearly 300,000 breast augmentation surgeries are performed annually, making this the second most common aesthetic procedure in US women (after liposuction).
- Today, silicone gel implants dominate the world market, and in the United States, approximately 60% of implants contain silicone gel filler.
- Capsular contracture is the most common complication of breast augmentation, typically presenting within the first postoperative year and with increasing risk over time. It occurs with both silicone and saline breast implants.
- Numerous studies have demonstrated the safety of silicone breast implants with regard to autoimmune disease incidence. However, the risk of associated anaplastic large-cell lymphoma must be discussed at every consultation, and confirmed cases should be reported to a national registry.