User login
Better postpartum BP control with self-monitoring: POP-HT
, new research suggests.
In a randomized trial of 220 women with preeclampsia or gestational hypertension, those who took daily postpartum BP readings and received clinician-guided advice for titrating antihypertensives had a 5 mm Hg–lower average diastolic BP at 9 months, compared with those receiving usual care.
Jamie Kitt, DPhil, from the University of Oxford (England) presented these findings from the Physicians Optimized Postpartum Hypertension Treatment (POP-HT, NCT04273854) clinical trial at the American Heart Association scientific sessions. The study was simultaneously published online in JAMA, and a cardiac imaging substudy was published online in Circulation.
“This trial identifies a potential need for a paradigm shift in the way women affected by hypertensive pregnancy are managed postnatally,” Dr. Kitt said. “If a 5–mm Hg improvement in BP is maintained longer term, it can result in about a 20% reduction in lifetime cardiovascular risk.”
The imaging substudy suggests that short-term postnatal optimization of BP control following hypertensive pregnancy through self-monitoring and physician-guided antihypertensive titration is linked with better cardiac remodeling changes seen by cardiovascular magnetic resonance and echocardiography.
POP-HT “proves for the first time that the first few weeks after delivery are a critical time that can determine the long-term cardiovascular health of the mother,” senior author Paul Leeson, PhD, also from the University of Oxford, who presented the findings in a press briefing, said in an interview.
“Interventions during this period can have long-term beneficial impacts on cardiovascular health,” he said. “These findings rewrite the textbook on our understanding of how and why hypertensive pregnancies associate with later cardiovascular disease in the mother.”
Next, Dr. Leeson said, “We need to work out the best ways to implement these interventions “at scale. Then we can ensure all women who have hypertensive pregnancies can get access to the long-term cardiovascular benefits we have demonstrated are possible through improving postpartum cardiac care,” he said, adding that “this is entirely achievable using current available technologies.”
Hypertension in pregnancy
About 1 in 10 pregnant women develop hypertension in pregnancy (preeclampsia or gestational hypertension), and 1 in 3 such women go on to develop chronic hypertension within 10 years, “when they are usually still in their 30s or 40s,” Dr. Leeson said.
During pregnancy, the heart remodels to cope with pregnancy, and it undergoes more severe changes if BP is high. Then during the 6 weeks after giving birth, this remodeling rapidly reverses.
Higher blood pressure in young adulthood is associated with a twofold higher risk of subsequent myocardial infarction and stroke. And abnormal cardiac remodeling postpartum is also linked with higher cardiovascular risk.
Self-monitoring blood pressure during the postpartum period may be a “critical window” for intervention.
Previously, the research group performed a pilot study, the Self-Management of Postnatal Antihypertensive Treatment (SNAP-HT) trial and the SNAP-extension trial, which compared a BP self-monitoring intervention with usual care in 91 women with gestational hypertension or preeclampsia requiring postnatal antihypertensive treatment.
Diastolic BP, which drives cardiovascular risk in younger populations, was 4.5–mm Hg lower at 6 months postpartum and 7–mm Hg lower at 4 years post partum in patients randomly assigned to BP self-management vs. usual care – even after they were no longer taking antihypertensives.
Building on these findings, the POP-HT trial enrolled 220 pregnant women seen at Oxford University Hospitals in the United Kingdom who were age 18 years or older, had either gestational hypertension or preeclampsia, and still required antihypertensives when they were being discharged from hospital after giving birth.
Following a baseline visit at day 1-6 after delivery, while in the postnatal ward, the patients were randomly assigned 1:1 to the intervention group (112 women) or usual-care group (108 women).
They had an average age of 32.6 years; 40% had gestational hypertension, and 60% had preeclampsia.
Women in the usual-care group typically received a BP review at 7-10 days after hospital discharge with a community midwife, and another at 6-8 weeks with their general practitioner.
The women in the intervention group were given and taught to use a Bluetooth-enabled OMRON Evolv BP monitor (Omron Healthcare Europe) while on the postnatal ward, and they installed a smartphone app on their mobile phones that transmitted self-monitored BP readings to a National Health Service-hosted, web-based platform.
They were instructed to take daily BP measurements (twice daily if out of target range). Dose titration of antihypertensives after hospital discharge was guided remotely by research clinicians, according to a guideline-based algorithm.
Patients in both groups had four study visits when their BP was measured: visit 1 (baseline) between days 1 and 6 post partum; visit 2 at week 1; visit 3 at week 6; and visit 4 between months 6 and 9 post partum.
Similar antihypertensive classes were prescribed in each group (enalapril 57%, nifedipine 27%, and labetalol 30% for intervention vs. enalapril 43%, nifedipine 30%, and labetalol 27% for control).
At 6 weeks, approximately 30% of participants in each group were still taking medication; this dropped to approximately 12% by visit 4.
The primary outcome – the mean 24-hour diastolic BP at visit 4 (roughly 9 months post partum), adjusted for baseline postnatal diastolic blood pressure – was 5.8–mm Hg lower in the intervention group than in the control group (71.2 mm Hg vs. 76.6 mm Hg; P < .001).
Secondary outcomes – between-group differences in systolic BP at 9 months, BP-related postnatal admission, and cardiac remodeling assessed by cardiac magnetic resonance – were all better in the intervention group.
The mean 24-hour average systolic BP at 9 months post partum, adjusted for baseline postnatal systolic BP was 6.5–mm Hg lower in the intervention group than in the control group (114.0 mm Hg vs. 120.3 mm Hg; P < .001).
There was an absolute risk reduction of 20% and a relative risk reduction of 73.5% in postnatal readmission. The number needed to treat to avoid one postnatal readmission was five, which “has potential for big cost savings,” said Dr. Leeson.
Blood pressure post partum can be improved with self-monitoring and physician-guided medication adjustment, Dr. Leeson summarized. The blood pressure remains low for at least 9 months, even when medication is stopped, and the intervention leads to beneficial cardiac remodeling.
U.S. pilot study
Non-Hispanic Black adults have a high hypertension and cardiovascular disease burden, and a related small U.S. study showed benefits of BP self-monitoring in a population comprising mainly Black women, Keith Ferdinand, MD, discussant of the POP-HT trial in the press briefing, said in an interview.
Dr. Ferdinand, from Tulane University, New Orleans, Louisiana, was lead author of the Text My Hypertension BP Meds NOLA pilot study that was published in February in the American Heart Journal Plus: Cardiology Research and Practice.
The study showed that text-messaging and social support increased hypertension medication adherence.
They enrolled 36 individuals, of whom 32 (89%) were non-Hispanic Black, and 23 (64%) were women. The participants received validated Bluetooth-enabled BP-monitoring devices that were synced to smartphones via a secured cloud-based application. The participants could send and receive messages to health care practitioners.
This intervention significantly improved medication adherence and systolic BP without modifying pharmacotherapy.
‘Need to be passionate about monitoring BP’
“The take-home messages from these exciting findings is that physicians and women who have had high BP during pregnancy need to be passionate about monitoring and controlling their blood pressure and not ignore it,” Anastasia Mihailidou, PhD, Royal North Shore Hospital, Sydney, the assigned discussant in the late-breaking trial session, said in an interview.
“It also resulted in fewer postpartum hospital readmissions for high blood pressure and benefit at 9 months in the structure and function of the heart and blood vessels of the women,” she said.
“While we need to see further studies in ethnically diverse women to see that they are reproducible, there are simple measures that clinicians can implement, and women can ask to have their BP monitored more frequently than the current practice. In the U.K. it is 5-10 days after delivery and then at 6-8 weeks after giving birth when changes in heart structure have already started,” Dr. Mihailidou noted.
“The procedure will need to be modified if there are no telemedicine facilities, but that should not stop having close monitoring of BP and treating it adequately. Monitoring requires an accurate BP monitor. There also has to be monitoring BP for the children.”
The trial was funded by a BHF Clinical Research Training Fellowship to Dr. Kitt, with additional support from the NIHR Oxford Biomedical Research Centre and Oxford BHF Centre for Research Excellence.
A version of this article first appeared on Medscape.com.
, new research suggests.
In a randomized trial of 220 women with preeclampsia or gestational hypertension, those who took daily postpartum BP readings and received clinician-guided advice for titrating antihypertensives had a 5 mm Hg–lower average diastolic BP at 9 months, compared with those receiving usual care.
Jamie Kitt, DPhil, from the University of Oxford (England) presented these findings from the Physicians Optimized Postpartum Hypertension Treatment (POP-HT, NCT04273854) clinical trial at the American Heart Association scientific sessions. The study was simultaneously published online in JAMA, and a cardiac imaging substudy was published online in Circulation.
“This trial identifies a potential need for a paradigm shift in the way women affected by hypertensive pregnancy are managed postnatally,” Dr. Kitt said. “If a 5–mm Hg improvement in BP is maintained longer term, it can result in about a 20% reduction in lifetime cardiovascular risk.”
The imaging substudy suggests that short-term postnatal optimization of BP control following hypertensive pregnancy through self-monitoring and physician-guided antihypertensive titration is linked with better cardiac remodeling changes seen by cardiovascular magnetic resonance and echocardiography.
POP-HT “proves for the first time that the first few weeks after delivery are a critical time that can determine the long-term cardiovascular health of the mother,” senior author Paul Leeson, PhD, also from the University of Oxford, who presented the findings in a press briefing, said in an interview.
“Interventions during this period can have long-term beneficial impacts on cardiovascular health,” he said. “These findings rewrite the textbook on our understanding of how and why hypertensive pregnancies associate with later cardiovascular disease in the mother.”
Next, Dr. Leeson said, “We need to work out the best ways to implement these interventions “at scale. Then we can ensure all women who have hypertensive pregnancies can get access to the long-term cardiovascular benefits we have demonstrated are possible through improving postpartum cardiac care,” he said, adding that “this is entirely achievable using current available technologies.”
Hypertension in pregnancy
About 1 in 10 pregnant women develop hypertension in pregnancy (preeclampsia or gestational hypertension), and 1 in 3 such women go on to develop chronic hypertension within 10 years, “when they are usually still in their 30s or 40s,” Dr. Leeson said.
During pregnancy, the heart remodels to cope with pregnancy, and it undergoes more severe changes if BP is high. Then during the 6 weeks after giving birth, this remodeling rapidly reverses.
Higher blood pressure in young adulthood is associated with a twofold higher risk of subsequent myocardial infarction and stroke. And abnormal cardiac remodeling postpartum is also linked with higher cardiovascular risk.
Self-monitoring blood pressure during the postpartum period may be a “critical window” for intervention.
Previously, the research group performed a pilot study, the Self-Management of Postnatal Antihypertensive Treatment (SNAP-HT) trial and the SNAP-extension trial, which compared a BP self-monitoring intervention with usual care in 91 women with gestational hypertension or preeclampsia requiring postnatal antihypertensive treatment.
Diastolic BP, which drives cardiovascular risk in younger populations, was 4.5–mm Hg lower at 6 months postpartum and 7–mm Hg lower at 4 years post partum in patients randomly assigned to BP self-management vs. usual care – even after they were no longer taking antihypertensives.
Building on these findings, the POP-HT trial enrolled 220 pregnant women seen at Oxford University Hospitals in the United Kingdom who were age 18 years or older, had either gestational hypertension or preeclampsia, and still required antihypertensives when they were being discharged from hospital after giving birth.
Following a baseline visit at day 1-6 after delivery, while in the postnatal ward, the patients were randomly assigned 1:1 to the intervention group (112 women) or usual-care group (108 women).
They had an average age of 32.6 years; 40% had gestational hypertension, and 60% had preeclampsia.
Women in the usual-care group typically received a BP review at 7-10 days after hospital discharge with a community midwife, and another at 6-8 weeks with their general practitioner.
The women in the intervention group were given and taught to use a Bluetooth-enabled OMRON Evolv BP monitor (Omron Healthcare Europe) while on the postnatal ward, and they installed a smartphone app on their mobile phones that transmitted self-monitored BP readings to a National Health Service-hosted, web-based platform.
They were instructed to take daily BP measurements (twice daily if out of target range). Dose titration of antihypertensives after hospital discharge was guided remotely by research clinicians, according to a guideline-based algorithm.
Patients in both groups had four study visits when their BP was measured: visit 1 (baseline) between days 1 and 6 post partum; visit 2 at week 1; visit 3 at week 6; and visit 4 between months 6 and 9 post partum.
Similar antihypertensive classes were prescribed in each group (enalapril 57%, nifedipine 27%, and labetalol 30% for intervention vs. enalapril 43%, nifedipine 30%, and labetalol 27% for control).
At 6 weeks, approximately 30% of participants in each group were still taking medication; this dropped to approximately 12% by visit 4.
The primary outcome – the mean 24-hour diastolic BP at visit 4 (roughly 9 months post partum), adjusted for baseline postnatal diastolic blood pressure – was 5.8–mm Hg lower in the intervention group than in the control group (71.2 mm Hg vs. 76.6 mm Hg; P < .001).
Secondary outcomes – between-group differences in systolic BP at 9 months, BP-related postnatal admission, and cardiac remodeling assessed by cardiac magnetic resonance – were all better in the intervention group.
The mean 24-hour average systolic BP at 9 months post partum, adjusted for baseline postnatal systolic BP was 6.5–mm Hg lower in the intervention group than in the control group (114.0 mm Hg vs. 120.3 mm Hg; P < .001).
There was an absolute risk reduction of 20% and a relative risk reduction of 73.5% in postnatal readmission. The number needed to treat to avoid one postnatal readmission was five, which “has potential for big cost savings,” said Dr. Leeson.
Blood pressure post partum can be improved with self-monitoring and physician-guided medication adjustment, Dr. Leeson summarized. The blood pressure remains low for at least 9 months, even when medication is stopped, and the intervention leads to beneficial cardiac remodeling.
U.S. pilot study
Non-Hispanic Black adults have a high hypertension and cardiovascular disease burden, and a related small U.S. study showed benefits of BP self-monitoring in a population comprising mainly Black women, Keith Ferdinand, MD, discussant of the POP-HT trial in the press briefing, said in an interview.
Dr. Ferdinand, from Tulane University, New Orleans, Louisiana, was lead author of the Text My Hypertension BP Meds NOLA pilot study that was published in February in the American Heart Journal Plus: Cardiology Research and Practice.
The study showed that text-messaging and social support increased hypertension medication adherence.
They enrolled 36 individuals, of whom 32 (89%) were non-Hispanic Black, and 23 (64%) were women. The participants received validated Bluetooth-enabled BP-monitoring devices that were synced to smartphones via a secured cloud-based application. The participants could send and receive messages to health care practitioners.
This intervention significantly improved medication adherence and systolic BP without modifying pharmacotherapy.
‘Need to be passionate about monitoring BP’
“The take-home messages from these exciting findings is that physicians and women who have had high BP during pregnancy need to be passionate about monitoring and controlling their blood pressure and not ignore it,” Anastasia Mihailidou, PhD, Royal North Shore Hospital, Sydney, the assigned discussant in the late-breaking trial session, said in an interview.
“It also resulted in fewer postpartum hospital readmissions for high blood pressure and benefit at 9 months in the structure and function of the heart and blood vessels of the women,” she said.
“While we need to see further studies in ethnically diverse women to see that they are reproducible, there are simple measures that clinicians can implement, and women can ask to have their BP monitored more frequently than the current practice. In the U.K. it is 5-10 days after delivery and then at 6-8 weeks after giving birth when changes in heart structure have already started,” Dr. Mihailidou noted.
“The procedure will need to be modified if there are no telemedicine facilities, but that should not stop having close monitoring of BP and treating it adequately. Monitoring requires an accurate BP monitor. There also has to be monitoring BP for the children.”
The trial was funded by a BHF Clinical Research Training Fellowship to Dr. Kitt, with additional support from the NIHR Oxford Biomedical Research Centre and Oxford BHF Centre for Research Excellence.
A version of this article first appeared on Medscape.com.
, new research suggests.
In a randomized trial of 220 women with preeclampsia or gestational hypertension, those who took daily postpartum BP readings and received clinician-guided advice for titrating antihypertensives had a 5 mm Hg–lower average diastolic BP at 9 months, compared with those receiving usual care.
Jamie Kitt, DPhil, from the University of Oxford (England) presented these findings from the Physicians Optimized Postpartum Hypertension Treatment (POP-HT, NCT04273854) clinical trial at the American Heart Association scientific sessions. The study was simultaneously published online in JAMA, and a cardiac imaging substudy was published online in Circulation.
“This trial identifies a potential need for a paradigm shift in the way women affected by hypertensive pregnancy are managed postnatally,” Dr. Kitt said. “If a 5–mm Hg improvement in BP is maintained longer term, it can result in about a 20% reduction in lifetime cardiovascular risk.”
The imaging substudy suggests that short-term postnatal optimization of BP control following hypertensive pregnancy through self-monitoring and physician-guided antihypertensive titration is linked with better cardiac remodeling changes seen by cardiovascular magnetic resonance and echocardiography.
POP-HT “proves for the first time that the first few weeks after delivery are a critical time that can determine the long-term cardiovascular health of the mother,” senior author Paul Leeson, PhD, also from the University of Oxford, who presented the findings in a press briefing, said in an interview.
“Interventions during this period can have long-term beneficial impacts on cardiovascular health,” he said. “These findings rewrite the textbook on our understanding of how and why hypertensive pregnancies associate with later cardiovascular disease in the mother.”
Next, Dr. Leeson said, “We need to work out the best ways to implement these interventions “at scale. Then we can ensure all women who have hypertensive pregnancies can get access to the long-term cardiovascular benefits we have demonstrated are possible through improving postpartum cardiac care,” he said, adding that “this is entirely achievable using current available technologies.”
Hypertension in pregnancy
About 1 in 10 pregnant women develop hypertension in pregnancy (preeclampsia or gestational hypertension), and 1 in 3 such women go on to develop chronic hypertension within 10 years, “when they are usually still in their 30s or 40s,” Dr. Leeson said.
During pregnancy, the heart remodels to cope with pregnancy, and it undergoes more severe changes if BP is high. Then during the 6 weeks after giving birth, this remodeling rapidly reverses.
Higher blood pressure in young adulthood is associated with a twofold higher risk of subsequent myocardial infarction and stroke. And abnormal cardiac remodeling postpartum is also linked with higher cardiovascular risk.
Self-monitoring blood pressure during the postpartum period may be a “critical window” for intervention.
Previously, the research group performed a pilot study, the Self-Management of Postnatal Antihypertensive Treatment (SNAP-HT) trial and the SNAP-extension trial, which compared a BP self-monitoring intervention with usual care in 91 women with gestational hypertension or preeclampsia requiring postnatal antihypertensive treatment.
Diastolic BP, which drives cardiovascular risk in younger populations, was 4.5–mm Hg lower at 6 months postpartum and 7–mm Hg lower at 4 years post partum in patients randomly assigned to BP self-management vs. usual care – even after they were no longer taking antihypertensives.
Building on these findings, the POP-HT trial enrolled 220 pregnant women seen at Oxford University Hospitals in the United Kingdom who were age 18 years or older, had either gestational hypertension or preeclampsia, and still required antihypertensives when they were being discharged from hospital after giving birth.
Following a baseline visit at day 1-6 after delivery, while in the postnatal ward, the patients were randomly assigned 1:1 to the intervention group (112 women) or usual-care group (108 women).
They had an average age of 32.6 years; 40% had gestational hypertension, and 60% had preeclampsia.
Women in the usual-care group typically received a BP review at 7-10 days after hospital discharge with a community midwife, and another at 6-8 weeks with their general practitioner.
The women in the intervention group were given and taught to use a Bluetooth-enabled OMRON Evolv BP monitor (Omron Healthcare Europe) while on the postnatal ward, and they installed a smartphone app on their mobile phones that transmitted self-monitored BP readings to a National Health Service-hosted, web-based platform.
They were instructed to take daily BP measurements (twice daily if out of target range). Dose titration of antihypertensives after hospital discharge was guided remotely by research clinicians, according to a guideline-based algorithm.
Patients in both groups had four study visits when their BP was measured: visit 1 (baseline) between days 1 and 6 post partum; visit 2 at week 1; visit 3 at week 6; and visit 4 between months 6 and 9 post partum.
Similar antihypertensive classes were prescribed in each group (enalapril 57%, nifedipine 27%, and labetalol 30% for intervention vs. enalapril 43%, nifedipine 30%, and labetalol 27% for control).
At 6 weeks, approximately 30% of participants in each group were still taking medication; this dropped to approximately 12% by visit 4.
The primary outcome – the mean 24-hour diastolic BP at visit 4 (roughly 9 months post partum), adjusted for baseline postnatal diastolic blood pressure – was 5.8–mm Hg lower in the intervention group than in the control group (71.2 mm Hg vs. 76.6 mm Hg; P < .001).
Secondary outcomes – between-group differences in systolic BP at 9 months, BP-related postnatal admission, and cardiac remodeling assessed by cardiac magnetic resonance – were all better in the intervention group.
The mean 24-hour average systolic BP at 9 months post partum, adjusted for baseline postnatal systolic BP was 6.5–mm Hg lower in the intervention group than in the control group (114.0 mm Hg vs. 120.3 mm Hg; P < .001).
There was an absolute risk reduction of 20% and a relative risk reduction of 73.5% in postnatal readmission. The number needed to treat to avoid one postnatal readmission was five, which “has potential for big cost savings,” said Dr. Leeson.
Blood pressure post partum can be improved with self-monitoring and physician-guided medication adjustment, Dr. Leeson summarized. The blood pressure remains low for at least 9 months, even when medication is stopped, and the intervention leads to beneficial cardiac remodeling.
U.S. pilot study
Non-Hispanic Black adults have a high hypertension and cardiovascular disease burden, and a related small U.S. study showed benefits of BP self-monitoring in a population comprising mainly Black women, Keith Ferdinand, MD, discussant of the POP-HT trial in the press briefing, said in an interview.
Dr. Ferdinand, from Tulane University, New Orleans, Louisiana, was lead author of the Text My Hypertension BP Meds NOLA pilot study that was published in February in the American Heart Journal Plus: Cardiology Research and Practice.
The study showed that text-messaging and social support increased hypertension medication adherence.
They enrolled 36 individuals, of whom 32 (89%) were non-Hispanic Black, and 23 (64%) were women. The participants received validated Bluetooth-enabled BP-monitoring devices that were synced to smartphones via a secured cloud-based application. The participants could send and receive messages to health care practitioners.
This intervention significantly improved medication adherence and systolic BP without modifying pharmacotherapy.
‘Need to be passionate about monitoring BP’
“The take-home messages from these exciting findings is that physicians and women who have had high BP during pregnancy need to be passionate about monitoring and controlling their blood pressure and not ignore it,” Anastasia Mihailidou, PhD, Royal North Shore Hospital, Sydney, the assigned discussant in the late-breaking trial session, said in an interview.
“It also resulted in fewer postpartum hospital readmissions for high blood pressure and benefit at 9 months in the structure and function of the heart and blood vessels of the women,” she said.
“While we need to see further studies in ethnically diverse women to see that they are reproducible, there are simple measures that clinicians can implement, and women can ask to have their BP monitored more frequently than the current practice. In the U.K. it is 5-10 days after delivery and then at 6-8 weeks after giving birth when changes in heart structure have already started,” Dr. Mihailidou noted.
“The procedure will need to be modified if there are no telemedicine facilities, but that should not stop having close monitoring of BP and treating it adequately. Monitoring requires an accurate BP monitor. There also has to be monitoring BP for the children.”
The trial was funded by a BHF Clinical Research Training Fellowship to Dr. Kitt, with additional support from the NIHR Oxford Biomedical Research Centre and Oxford BHF Centre for Research Excellence.
A version of this article first appeared on Medscape.com.
FROM AHA 2023
TNF blockers not associated with poorer pregnancy outcomes
SAN DIEGO – Continuing a tumor necrosis factor inhibitor (TNFi) during pregnancy does not increase risk of worse fetal or obstetric outcomes, according to new research presented at the annual meeting of the American College of Rheumatology.
Patients who continued a TNFi also had fewer severe infections requiring hospitalization, compared with those who stopped taking the medication during their pregnancy.
“The main message is that patients continuing were not doing worse than the patients stopping. It’s an important clinical message for rheumatologists who are not really confident in dealing with these drugs during pregnancy,” said Anna Moltó, MD, PhD, a rheumatologist at Cochin Hospital, Paris, who led the research. “It adds to the data that it seems to be safe,” she added in an interview.
Previous research, largely from pregnant patients with inflammatory bowel disease, suggests that taking a TNFi during pregnancy is safe, and 2020 ACR guidelines conditionally recommend continuing therapy prior to and during pregnancy; however, many people still stop taking the drugs during pregnancy for fear of potentially harming the fetus.
To better understand how TNFi use affected pregnancy outcomes, Dr. Moltó and colleagues analyzed data from a French nationwide health insurance database to identify adult women with chronic rheumatic inflammatory disease. All women included in the cohort had a singleton pregnancy between 2008 and 2017 and were taking a TNFi upon pregnancy diagnosis.
Patients who restarted TNFi after initially pausing because of pregnancy were included in the continuation group.
Researchers identified more than 2,000 pregnancies, including 1,503 in individuals with spondyloarthritis and 579 individuals with rheumatoid arthritis. Patients were, on average, 31 years old and were diagnosed with a rheumatic disease 4 years prior to their pregnancy.
About 72% (n = 1,497) discontinued TNFi after learning they were pregnant, and 584 individuals continued treatment. Dr. Moltó noted that data from more recent years might have captured lower discontinuation rates among pregnant individuals, but those data were not available for the study.
There was no difference in unfavorable obstetrical or infant outcomes, including spontaneous abortion, preeclampsia, gestational diabetes, major congenital malformation, and severe infection of the infant requiring hospitalization. Somewhat surprisingly, the data showed that women who discontinued a TNFi were more likely to be hospitalized for infection either during their pregnancy or up to 6 weeks after delivery, compared with those who continued therapy (1.3% vs. 0.2%, respectively).
Dr. Moltó is currently looking into what could be behind this counterintuitive result, but she hypothesizes that patients who had stopped TNFi may have been taking more glucocorticoids.
“At our institution, there is generally a comfort level with continuing TNF inhibitors during pregnancy, at least until about 36 weeks,” said Sara K. Tedeschi, MD, MPH, a rheumatologist at Brigham and Women’s Hospital and assistant professor of medicine at Harvard Medical School, both in Boston. Sometimes, there is concern for risk of infection to the infant, depending on the type of TNFi being used, she added during a press conference.
“I think that these are really informative and supportive data to let women know that they probably have a really good chance of doing very well during the pregnancy if they continue” their TNFi, said Dr. Tedeschi, who was not involved with the study.
TNF discontinuation on the decline
In a related study, researchers at McGill University, Montreal, found that TNFi discontinuation prior to pregnancy had decreased over time in individuals with chronic inflammatory diseases.
Using a database of U.S. insurance claims, they identified 3,372 women with RA, ankylosing spondylitis (AS), psoriasis/psoriatic arthritis (PsA), and/or inflammatory bowel disease (IBD) who previously used a TNFi and gave birth between 2011 and 2019. A patient was considered to have used a TNFi if she had filled a prescription or had an infusion procedure insurance claim within 12 weeks before the gestational period or anytime during pregnancy. Researchers did not have time-specific data to account for women who stopped treatment at pregnancy diagnosis.
Nearly half (47%) of all identified pregnancies were in individuals with IBD, and the rest included patients with RA (24%), psoriasis or PsA (16%), AS (3%), or more than one diagnosis (10%).
In total, 14% of women discontinued TNFi use in the 12 weeks before becoming pregnant and did not restart. From 2011 to 2013, 19% of patients stopped their TNFi, but this proportion decreased overtime, with 10% of patients stopping therapy from 2017 to 2019 (P < .0001).
This decline “possibly reflects the increase in real-world evidence about the safety of TNFi in pregnancy. That research, in turn, led to new guidelines recommending the continuation of TNFi during pregnancy,” first author Leah Flatman, a PhD candidate in epidemiology at McGill, said in an interview. “I think we can see this potentially as good news.”
More patients with RA, psoriasis/PsA, and AS discontinued TNFi therapy prior to conception (23%-25%), compared with those with IBD (5%).
Ms. Flatman noted that her study and Moltó’s study complement each other by providing data on individuals stopping TNFi prior to conception versus those stopping treatment after pregnancy diagnosis.
“These findings demonstrate that continuing TNFi during pregnancy appears not to be associated with an increase in adverse obstetrical or infant outcomes,” Ms. Flatman said of Dr. Moltó’s study. “As guidelines currently recommend continuing TNFi, studies like this help demonstrate that the guideline changes do not appear to be associated with an increase in adverse events.”
Dr. Moltó and Ms. Flatman disclosed no relevant financial relationships. Dr. Tedeschi has worked as a consultant for Novartis.
A version of this article appeared on Medscape.com.
SAN DIEGO – Continuing a tumor necrosis factor inhibitor (TNFi) during pregnancy does not increase risk of worse fetal or obstetric outcomes, according to new research presented at the annual meeting of the American College of Rheumatology.
Patients who continued a TNFi also had fewer severe infections requiring hospitalization, compared with those who stopped taking the medication during their pregnancy.
“The main message is that patients continuing were not doing worse than the patients stopping. It’s an important clinical message for rheumatologists who are not really confident in dealing with these drugs during pregnancy,” said Anna Moltó, MD, PhD, a rheumatologist at Cochin Hospital, Paris, who led the research. “It adds to the data that it seems to be safe,” she added in an interview.
Previous research, largely from pregnant patients with inflammatory bowel disease, suggests that taking a TNFi during pregnancy is safe, and 2020 ACR guidelines conditionally recommend continuing therapy prior to and during pregnancy; however, many people still stop taking the drugs during pregnancy for fear of potentially harming the fetus.
To better understand how TNFi use affected pregnancy outcomes, Dr. Moltó and colleagues analyzed data from a French nationwide health insurance database to identify adult women with chronic rheumatic inflammatory disease. All women included in the cohort had a singleton pregnancy between 2008 and 2017 and were taking a TNFi upon pregnancy diagnosis.
Patients who restarted TNFi after initially pausing because of pregnancy were included in the continuation group.
Researchers identified more than 2,000 pregnancies, including 1,503 in individuals with spondyloarthritis and 579 individuals with rheumatoid arthritis. Patients were, on average, 31 years old and were diagnosed with a rheumatic disease 4 years prior to their pregnancy.
About 72% (n = 1,497) discontinued TNFi after learning they were pregnant, and 584 individuals continued treatment. Dr. Moltó noted that data from more recent years might have captured lower discontinuation rates among pregnant individuals, but those data were not available for the study.
There was no difference in unfavorable obstetrical or infant outcomes, including spontaneous abortion, preeclampsia, gestational diabetes, major congenital malformation, and severe infection of the infant requiring hospitalization. Somewhat surprisingly, the data showed that women who discontinued a TNFi were more likely to be hospitalized for infection either during their pregnancy or up to 6 weeks after delivery, compared with those who continued therapy (1.3% vs. 0.2%, respectively).
Dr. Moltó is currently looking into what could be behind this counterintuitive result, but she hypothesizes that patients who had stopped TNFi may have been taking more glucocorticoids.
“At our institution, there is generally a comfort level with continuing TNF inhibitors during pregnancy, at least until about 36 weeks,” said Sara K. Tedeschi, MD, MPH, a rheumatologist at Brigham and Women’s Hospital and assistant professor of medicine at Harvard Medical School, both in Boston. Sometimes, there is concern for risk of infection to the infant, depending on the type of TNFi being used, she added during a press conference.
“I think that these are really informative and supportive data to let women know that they probably have a really good chance of doing very well during the pregnancy if they continue” their TNFi, said Dr. Tedeschi, who was not involved with the study.
TNF discontinuation on the decline
In a related study, researchers at McGill University, Montreal, found that TNFi discontinuation prior to pregnancy had decreased over time in individuals with chronic inflammatory diseases.
Using a database of U.S. insurance claims, they identified 3,372 women with RA, ankylosing spondylitis (AS), psoriasis/psoriatic arthritis (PsA), and/or inflammatory bowel disease (IBD) who previously used a TNFi and gave birth between 2011 and 2019. A patient was considered to have used a TNFi if she had filled a prescription or had an infusion procedure insurance claim within 12 weeks before the gestational period or anytime during pregnancy. Researchers did not have time-specific data to account for women who stopped treatment at pregnancy diagnosis.
Nearly half (47%) of all identified pregnancies were in individuals with IBD, and the rest included patients with RA (24%), psoriasis or PsA (16%), AS (3%), or more than one diagnosis (10%).
In total, 14% of women discontinued TNFi use in the 12 weeks before becoming pregnant and did not restart. From 2011 to 2013, 19% of patients stopped their TNFi, but this proportion decreased overtime, with 10% of patients stopping therapy from 2017 to 2019 (P < .0001).
This decline “possibly reflects the increase in real-world evidence about the safety of TNFi in pregnancy. That research, in turn, led to new guidelines recommending the continuation of TNFi during pregnancy,” first author Leah Flatman, a PhD candidate in epidemiology at McGill, said in an interview. “I think we can see this potentially as good news.”
More patients with RA, psoriasis/PsA, and AS discontinued TNFi therapy prior to conception (23%-25%), compared with those with IBD (5%).
Ms. Flatman noted that her study and Moltó’s study complement each other by providing data on individuals stopping TNFi prior to conception versus those stopping treatment after pregnancy diagnosis.
“These findings demonstrate that continuing TNFi during pregnancy appears not to be associated with an increase in adverse obstetrical or infant outcomes,” Ms. Flatman said of Dr. Moltó’s study. “As guidelines currently recommend continuing TNFi, studies like this help demonstrate that the guideline changes do not appear to be associated with an increase in adverse events.”
Dr. Moltó and Ms. Flatman disclosed no relevant financial relationships. Dr. Tedeschi has worked as a consultant for Novartis.
A version of this article appeared on Medscape.com.
SAN DIEGO – Continuing a tumor necrosis factor inhibitor (TNFi) during pregnancy does not increase risk of worse fetal or obstetric outcomes, according to new research presented at the annual meeting of the American College of Rheumatology.
Patients who continued a TNFi also had fewer severe infections requiring hospitalization, compared with those who stopped taking the medication during their pregnancy.
“The main message is that patients continuing were not doing worse than the patients stopping. It’s an important clinical message for rheumatologists who are not really confident in dealing with these drugs during pregnancy,” said Anna Moltó, MD, PhD, a rheumatologist at Cochin Hospital, Paris, who led the research. “It adds to the data that it seems to be safe,” she added in an interview.
Previous research, largely from pregnant patients with inflammatory bowel disease, suggests that taking a TNFi during pregnancy is safe, and 2020 ACR guidelines conditionally recommend continuing therapy prior to and during pregnancy; however, many people still stop taking the drugs during pregnancy for fear of potentially harming the fetus.
To better understand how TNFi use affected pregnancy outcomes, Dr. Moltó and colleagues analyzed data from a French nationwide health insurance database to identify adult women with chronic rheumatic inflammatory disease. All women included in the cohort had a singleton pregnancy between 2008 and 2017 and were taking a TNFi upon pregnancy diagnosis.
Patients who restarted TNFi after initially pausing because of pregnancy were included in the continuation group.
Researchers identified more than 2,000 pregnancies, including 1,503 in individuals with spondyloarthritis and 579 individuals with rheumatoid arthritis. Patients were, on average, 31 years old and were diagnosed with a rheumatic disease 4 years prior to their pregnancy.
About 72% (n = 1,497) discontinued TNFi after learning they were pregnant, and 584 individuals continued treatment. Dr. Moltó noted that data from more recent years might have captured lower discontinuation rates among pregnant individuals, but those data were not available for the study.
There was no difference in unfavorable obstetrical or infant outcomes, including spontaneous abortion, preeclampsia, gestational diabetes, major congenital malformation, and severe infection of the infant requiring hospitalization. Somewhat surprisingly, the data showed that women who discontinued a TNFi were more likely to be hospitalized for infection either during their pregnancy or up to 6 weeks after delivery, compared with those who continued therapy (1.3% vs. 0.2%, respectively).
Dr. Moltó is currently looking into what could be behind this counterintuitive result, but she hypothesizes that patients who had stopped TNFi may have been taking more glucocorticoids.
“At our institution, there is generally a comfort level with continuing TNF inhibitors during pregnancy, at least until about 36 weeks,” said Sara K. Tedeschi, MD, MPH, a rheumatologist at Brigham and Women’s Hospital and assistant professor of medicine at Harvard Medical School, both in Boston. Sometimes, there is concern for risk of infection to the infant, depending on the type of TNFi being used, she added during a press conference.
“I think that these are really informative and supportive data to let women know that they probably have a really good chance of doing very well during the pregnancy if they continue” their TNFi, said Dr. Tedeschi, who was not involved with the study.
TNF discontinuation on the decline
In a related study, researchers at McGill University, Montreal, found that TNFi discontinuation prior to pregnancy had decreased over time in individuals with chronic inflammatory diseases.
Using a database of U.S. insurance claims, they identified 3,372 women with RA, ankylosing spondylitis (AS), psoriasis/psoriatic arthritis (PsA), and/or inflammatory bowel disease (IBD) who previously used a TNFi and gave birth between 2011 and 2019. A patient was considered to have used a TNFi if she had filled a prescription or had an infusion procedure insurance claim within 12 weeks before the gestational period or anytime during pregnancy. Researchers did not have time-specific data to account for women who stopped treatment at pregnancy diagnosis.
Nearly half (47%) of all identified pregnancies were in individuals with IBD, and the rest included patients with RA (24%), psoriasis or PsA (16%), AS (3%), or more than one diagnosis (10%).
In total, 14% of women discontinued TNFi use in the 12 weeks before becoming pregnant and did not restart. From 2011 to 2013, 19% of patients stopped their TNFi, but this proportion decreased overtime, with 10% of patients stopping therapy from 2017 to 2019 (P < .0001).
This decline “possibly reflects the increase in real-world evidence about the safety of TNFi in pregnancy. That research, in turn, led to new guidelines recommending the continuation of TNFi during pregnancy,” first author Leah Flatman, a PhD candidate in epidemiology at McGill, said in an interview. “I think we can see this potentially as good news.”
More patients with RA, psoriasis/PsA, and AS discontinued TNFi therapy prior to conception (23%-25%), compared with those with IBD (5%).
Ms. Flatman noted that her study and Moltó’s study complement each other by providing data on individuals stopping TNFi prior to conception versus those stopping treatment after pregnancy diagnosis.
“These findings demonstrate that continuing TNFi during pregnancy appears not to be associated with an increase in adverse obstetrical or infant outcomes,” Ms. Flatman said of Dr. Moltó’s study. “As guidelines currently recommend continuing TNFi, studies like this help demonstrate that the guideline changes do not appear to be associated with an increase in adverse events.”
Dr. Moltó and Ms. Flatman disclosed no relevant financial relationships. Dr. Tedeschi has worked as a consultant for Novartis.
A version of this article appeared on Medscape.com.
AT ACR 2023
A nurse’s view: Women desperately need information about pelvic floor disorders
Pelvic floor disorders are embarrassing, annoying, painful, and extremely disruptive to a woman’s life, often resulting in depression, anxiety, and a poor self-image. According to a 2021 study, approximately 75% of peripartum women and 68% of postmenopausal women feel insufficiently informed about pelvic floor disorders.1
Consequently, a large majority of women are not seeking care for these disorders. This drives health care costs higher as women wait until their symptoms are unbearable until finally seeking help. Many of these women don’t know they have options.
Who is at risk?
To understand the scope of this growing problem, it is vital to see who is most at risk. Parity, age, body mass index, and race are significant factors, although any woman can have a pelvic floor disorder (PFD).
Urinary incontinence (UI), pelvic floor prolapses (POP), and fecal incontinence (FI) are three of the most common pelvic floor disorders. Pregnancy and childbirth, specifically a vaginal birth, greatly contribute to this population’s risk. In pregnancy, the increase in plasma volume and glomerular filtration rate, along with hormone changes impacting urethral pressure and the growing gravid uterus, cause urinary frequency and nocturia. This can result in urinary incontinence during and after pregnancy.
Indeed, 76% of women with urinary incontinence at 3 months postpartum report it 12 years later.1 Third- and fourth-degree lacerations during delivery are uncommon (3.3%), but can cause fecal incontinence, often requiring surgery.1 Independently, all of these symptoms have been correlated with sexual dysfunction and postpartum depression.
One-third of all women and 50% of women over the age of 55 are currently affected by a PFD. Contributing factors include hormone changes with menopause that affect the pelvic floor muscles and connective tissue, prior childbirth and pregnancy, constipation, heavy lifting, prior pelvic surgery, and obesity. These women are vulnerable to pelvic organ prolapse from the weakened pelvic floor muscles. They will often present with a vague complaint of “something is protruding out of my vagina.” These women also present with urinary incontinence or leakage, proclaiming they have to wear a diaper or a pad. Without proper knowledge, aging women think these issues are normal and nothing can be done.
The woman with a BMI above 30 may have damaged tissues supporting the uterus and bladder, weakening those organs, and causing a prolapse. Incontinence is a result of poor muscle and connective tissue of the vagina that support the urethra. Obese women can suffer from both urinary and bowel incontinence. By the year 2030, it is projected that one in two adults will be obese.2 This will greatly impact health care costs.
To date, there is little conclusive evidence on the impact of race on pelvic floor disorders. A study in Scientific Reports did find that Asian women have a significantly lower risk for any PFD.2 Some research has found that Black and Hispanic women have less risk for UI but are at higher risk for FI and other PFDs.3 Understandably, women of certain cultures and demographics may be less likely to report incontinence to their clinicians and may be less informed as well.
What can we do?
The American College of Obstetricians and Gynecologists (ACOG) has acknowledged the deficiencies and lack of standard care of pelvic health in pregnancy and postpartum.1 There are differences in definitions across clinical practice and in the medical literature. Inconsistent patient reporting of PFD symptoms occurs due to nonstandard methods (questionnaire, interview, physical exam). With the often-short time allotted for visits with health care providers, women may neglect to discuss their symptoms, especially if they have other more pressing matters to address.
At the first OB appointment, a pregnant woman should be given information on what are normal and abnormal symptoms, from the beginning through postpartum. At each visit, she should be given ample opportunity to discuss symptoms of pelvic health. Clinicians should continue assessing, questioning, and discussing treatment options as applicable. Women need to know that early recognition and treatment can have a positive affect on their pelvic health for years to come.
ACOG recommends all postpartum patients see an obstetric provider within 3 weeks of delivery.1 Most are seen at 6 weeks. Pelvic health should be discussed at this final postpartum appointment, including normal and abnormal symptoms within the next few months and beyond.
Regardless of pregnancy status, women need a safe and supportive place to describe their pelvic floor issues. There is a validated questionnaire tool available for postpartum, but one is desperately needed for all women, especially women at risk. A pelvic health assessment must be included in every annual exam.
Women need to know there are multiple treatment modalities including simple exercises, physical therapy, a variety of pessaries, medications, and surgery. Sometimes, all that is needed are a few lifestyle changes: avoiding pushing or straining while urinating or having a bowel movement, maintaining a healthy diet rich in high fiber foods, and drinking plenty of fluids.
The National Public Health Service in the United Kingdom recently announced a government-funded program for pelvic health services to begin in April 2024.4 This program will address the pelvic floor needs, assessment, education and treatment for women after childbirth.
There are multiple clinics in the United States focusing on women’s health that feature urogynecologists – specialists in pelvic floor disorders. These specialists do a thorough health and physical assessment, explain types of pelvic floor disorders, and suggest appropriate treatment options. Most importantly, urogynecologists listen and address a woman’s concerns and fears.
There is no reason for women to feel compromised at any age. We, as health care providers, just need to assess, educate, treat, and follow up.
Ms. Barnett is a registered nurse in the department of obstetrics, Mills-Peninsula Medical Center, Burlingame, Calif. She has disclosed no relevant financial relationships.
References
1. Madsen AM et al. Recognition and management of pelvic floor disorders in pregnancy and the postpartum period. Obstet Gynecol Clin North Am. 2021 Sep;48(3):571-84. doi: 10.1016/j.ogc.2021.05.009.
2. Kenne KA et al. Prevalence of pelvic floor disorders in adult women being seen in a primary care setting and associated risk factors. Sci Rep. 2022 June; (12):9878. doi: 10.1038/s41598-022-13501-w.
3. Nygaard I et al. Prevalence of symptomatic pelvic floor disorders in US women. JAMA. 2008;300(11):1311-6. doi: 10.1001/jama.300.11.1311.
4. United Kingdom Department of Health and Social Care. “National pelvic health service to support women.” 2023 Oct 19.
Pelvic floor disorders are embarrassing, annoying, painful, and extremely disruptive to a woman’s life, often resulting in depression, anxiety, and a poor self-image. According to a 2021 study, approximately 75% of peripartum women and 68% of postmenopausal women feel insufficiently informed about pelvic floor disorders.1
Consequently, a large majority of women are not seeking care for these disorders. This drives health care costs higher as women wait until their symptoms are unbearable until finally seeking help. Many of these women don’t know they have options.
Who is at risk?
To understand the scope of this growing problem, it is vital to see who is most at risk. Parity, age, body mass index, and race are significant factors, although any woman can have a pelvic floor disorder (PFD).
Urinary incontinence (UI), pelvic floor prolapses (POP), and fecal incontinence (FI) are three of the most common pelvic floor disorders. Pregnancy and childbirth, specifically a vaginal birth, greatly contribute to this population’s risk. In pregnancy, the increase in plasma volume and glomerular filtration rate, along with hormone changes impacting urethral pressure and the growing gravid uterus, cause urinary frequency and nocturia. This can result in urinary incontinence during and after pregnancy.
Indeed, 76% of women with urinary incontinence at 3 months postpartum report it 12 years later.1 Third- and fourth-degree lacerations during delivery are uncommon (3.3%), but can cause fecal incontinence, often requiring surgery.1 Independently, all of these symptoms have been correlated with sexual dysfunction and postpartum depression.
One-third of all women and 50% of women over the age of 55 are currently affected by a PFD. Contributing factors include hormone changes with menopause that affect the pelvic floor muscles and connective tissue, prior childbirth and pregnancy, constipation, heavy lifting, prior pelvic surgery, and obesity. These women are vulnerable to pelvic organ prolapse from the weakened pelvic floor muscles. They will often present with a vague complaint of “something is protruding out of my vagina.” These women also present with urinary incontinence or leakage, proclaiming they have to wear a diaper or a pad. Without proper knowledge, aging women think these issues are normal and nothing can be done.
The woman with a BMI above 30 may have damaged tissues supporting the uterus and bladder, weakening those organs, and causing a prolapse. Incontinence is a result of poor muscle and connective tissue of the vagina that support the urethra. Obese women can suffer from both urinary and bowel incontinence. By the year 2030, it is projected that one in two adults will be obese.2 This will greatly impact health care costs.
To date, there is little conclusive evidence on the impact of race on pelvic floor disorders. A study in Scientific Reports did find that Asian women have a significantly lower risk for any PFD.2 Some research has found that Black and Hispanic women have less risk for UI but are at higher risk for FI and other PFDs.3 Understandably, women of certain cultures and demographics may be less likely to report incontinence to their clinicians and may be less informed as well.
What can we do?
The American College of Obstetricians and Gynecologists (ACOG) has acknowledged the deficiencies and lack of standard care of pelvic health in pregnancy and postpartum.1 There are differences in definitions across clinical practice and in the medical literature. Inconsistent patient reporting of PFD symptoms occurs due to nonstandard methods (questionnaire, interview, physical exam). With the often-short time allotted for visits with health care providers, women may neglect to discuss their symptoms, especially if they have other more pressing matters to address.
At the first OB appointment, a pregnant woman should be given information on what are normal and abnormal symptoms, from the beginning through postpartum. At each visit, she should be given ample opportunity to discuss symptoms of pelvic health. Clinicians should continue assessing, questioning, and discussing treatment options as applicable. Women need to know that early recognition and treatment can have a positive affect on their pelvic health for years to come.
ACOG recommends all postpartum patients see an obstetric provider within 3 weeks of delivery.1 Most are seen at 6 weeks. Pelvic health should be discussed at this final postpartum appointment, including normal and abnormal symptoms within the next few months and beyond.
Regardless of pregnancy status, women need a safe and supportive place to describe their pelvic floor issues. There is a validated questionnaire tool available for postpartum, but one is desperately needed for all women, especially women at risk. A pelvic health assessment must be included in every annual exam.
Women need to know there are multiple treatment modalities including simple exercises, physical therapy, a variety of pessaries, medications, and surgery. Sometimes, all that is needed are a few lifestyle changes: avoiding pushing or straining while urinating or having a bowel movement, maintaining a healthy diet rich in high fiber foods, and drinking plenty of fluids.
The National Public Health Service in the United Kingdom recently announced a government-funded program for pelvic health services to begin in April 2024.4 This program will address the pelvic floor needs, assessment, education and treatment for women after childbirth.
There are multiple clinics in the United States focusing on women’s health that feature urogynecologists – specialists in pelvic floor disorders. These specialists do a thorough health and physical assessment, explain types of pelvic floor disorders, and suggest appropriate treatment options. Most importantly, urogynecologists listen and address a woman’s concerns and fears.
There is no reason for women to feel compromised at any age. We, as health care providers, just need to assess, educate, treat, and follow up.
Ms. Barnett is a registered nurse in the department of obstetrics, Mills-Peninsula Medical Center, Burlingame, Calif. She has disclosed no relevant financial relationships.
References
1. Madsen AM et al. Recognition and management of pelvic floor disorders in pregnancy and the postpartum period. Obstet Gynecol Clin North Am. 2021 Sep;48(3):571-84. doi: 10.1016/j.ogc.2021.05.009.
2. Kenne KA et al. Prevalence of pelvic floor disorders in adult women being seen in a primary care setting and associated risk factors. Sci Rep. 2022 June; (12):9878. doi: 10.1038/s41598-022-13501-w.
3. Nygaard I et al. Prevalence of symptomatic pelvic floor disorders in US women. JAMA. 2008;300(11):1311-6. doi: 10.1001/jama.300.11.1311.
4. United Kingdom Department of Health and Social Care. “National pelvic health service to support women.” 2023 Oct 19.
Pelvic floor disorders are embarrassing, annoying, painful, and extremely disruptive to a woman’s life, often resulting in depression, anxiety, and a poor self-image. According to a 2021 study, approximately 75% of peripartum women and 68% of postmenopausal women feel insufficiently informed about pelvic floor disorders.1
Consequently, a large majority of women are not seeking care for these disorders. This drives health care costs higher as women wait until their symptoms are unbearable until finally seeking help. Many of these women don’t know they have options.
Who is at risk?
To understand the scope of this growing problem, it is vital to see who is most at risk. Parity, age, body mass index, and race are significant factors, although any woman can have a pelvic floor disorder (PFD).
Urinary incontinence (UI), pelvic floor prolapses (POP), and fecal incontinence (FI) are three of the most common pelvic floor disorders. Pregnancy and childbirth, specifically a vaginal birth, greatly contribute to this population’s risk. In pregnancy, the increase in plasma volume and glomerular filtration rate, along with hormone changes impacting urethral pressure and the growing gravid uterus, cause urinary frequency and nocturia. This can result in urinary incontinence during and after pregnancy.
Indeed, 76% of women with urinary incontinence at 3 months postpartum report it 12 years later.1 Third- and fourth-degree lacerations during delivery are uncommon (3.3%), but can cause fecal incontinence, often requiring surgery.1 Independently, all of these symptoms have been correlated with sexual dysfunction and postpartum depression.
One-third of all women and 50% of women over the age of 55 are currently affected by a PFD. Contributing factors include hormone changes with menopause that affect the pelvic floor muscles and connective tissue, prior childbirth and pregnancy, constipation, heavy lifting, prior pelvic surgery, and obesity. These women are vulnerable to pelvic organ prolapse from the weakened pelvic floor muscles. They will often present with a vague complaint of “something is protruding out of my vagina.” These women also present with urinary incontinence or leakage, proclaiming they have to wear a diaper or a pad. Without proper knowledge, aging women think these issues are normal and nothing can be done.
The woman with a BMI above 30 may have damaged tissues supporting the uterus and bladder, weakening those organs, and causing a prolapse. Incontinence is a result of poor muscle and connective tissue of the vagina that support the urethra. Obese women can suffer from both urinary and bowel incontinence. By the year 2030, it is projected that one in two adults will be obese.2 This will greatly impact health care costs.
To date, there is little conclusive evidence on the impact of race on pelvic floor disorders. A study in Scientific Reports did find that Asian women have a significantly lower risk for any PFD.2 Some research has found that Black and Hispanic women have less risk for UI but are at higher risk for FI and other PFDs.3 Understandably, women of certain cultures and demographics may be less likely to report incontinence to their clinicians and may be less informed as well.
What can we do?
The American College of Obstetricians and Gynecologists (ACOG) has acknowledged the deficiencies and lack of standard care of pelvic health in pregnancy and postpartum.1 There are differences in definitions across clinical practice and in the medical literature. Inconsistent patient reporting of PFD symptoms occurs due to nonstandard methods (questionnaire, interview, physical exam). With the often-short time allotted for visits with health care providers, women may neglect to discuss their symptoms, especially if they have other more pressing matters to address.
At the first OB appointment, a pregnant woman should be given information on what are normal and abnormal symptoms, from the beginning through postpartum. At each visit, she should be given ample opportunity to discuss symptoms of pelvic health. Clinicians should continue assessing, questioning, and discussing treatment options as applicable. Women need to know that early recognition and treatment can have a positive affect on their pelvic health for years to come.
ACOG recommends all postpartum patients see an obstetric provider within 3 weeks of delivery.1 Most are seen at 6 weeks. Pelvic health should be discussed at this final postpartum appointment, including normal and abnormal symptoms within the next few months and beyond.
Regardless of pregnancy status, women need a safe and supportive place to describe their pelvic floor issues. There is a validated questionnaire tool available for postpartum, but one is desperately needed for all women, especially women at risk. A pelvic health assessment must be included in every annual exam.
Women need to know there are multiple treatment modalities including simple exercises, physical therapy, a variety of pessaries, medications, and surgery. Sometimes, all that is needed are a few lifestyle changes: avoiding pushing or straining while urinating or having a bowel movement, maintaining a healthy diet rich in high fiber foods, and drinking plenty of fluids.
The National Public Health Service in the United Kingdom recently announced a government-funded program for pelvic health services to begin in April 2024.4 This program will address the pelvic floor needs, assessment, education and treatment for women after childbirth.
There are multiple clinics in the United States focusing on women’s health that feature urogynecologists – specialists in pelvic floor disorders. These specialists do a thorough health and physical assessment, explain types of pelvic floor disorders, and suggest appropriate treatment options. Most importantly, urogynecologists listen and address a woman’s concerns and fears.
There is no reason for women to feel compromised at any age. We, as health care providers, just need to assess, educate, treat, and follow up.
Ms. Barnett is a registered nurse in the department of obstetrics, Mills-Peninsula Medical Center, Burlingame, Calif. She has disclosed no relevant financial relationships.
References
1. Madsen AM et al. Recognition and management of pelvic floor disorders in pregnancy and the postpartum period. Obstet Gynecol Clin North Am. 2021 Sep;48(3):571-84. doi: 10.1016/j.ogc.2021.05.009.
2. Kenne KA et al. Prevalence of pelvic floor disorders in adult women being seen in a primary care setting and associated risk factors. Sci Rep. 2022 June; (12):9878. doi: 10.1038/s41598-022-13501-w.
3. Nygaard I et al. Prevalence of symptomatic pelvic floor disorders in US women. JAMA. 2008;300(11):1311-6. doi: 10.1001/jama.300.11.1311.
4. United Kingdom Department of Health and Social Care. “National pelvic health service to support women.” 2023 Oct 19.
Pregnancy in rheumatic disease quadruples risk of cardiovascular events
SAN DIEGO – Pregnant individuals with autoimmune rheumatic diseases (ARDs) are at least four times more likely to experience an acute cardiovascular event (CVE) than are pregnant individuals without these conditions, according to new research presented at the annual meeting of the American College of Rheumatology. Pregnant individuals with primary antiphospholipid syndrome (APS) had a 15-fold increase in CVE risk.
Patients who experienced CVEs were also more likely to experience preterm birth and other adverse pregnancy outcomes (APOs).
Rashmi Dhital, MD, a rheumatology fellow at the University of California, San Diego, and colleagues examined the medical records of pregnant individuals in California who had delivered singleton live-born infants from 2005 to 2020. Using data from the Study of Outcomes in Mothers and Infants (SOMI) database, an administrative population-based birth cohort in California, they identified more than 7 million individuals, 19,340 with ARDs and 7,758 with APS.
They then analyzed how many patients experienced an acute CVE during pregnancy and up to 6 weeks after giving birth.
CVEs occurred in 2.0% of patients with ARDs, 6.9% of individuals with APS, and 0.4% of women without these conditions. CVE risk was four times higher in the ARDs group (adjusted relative risk, 4.1; 95% confidence interval, 3.7-4.5) and nearly 15 times higher in the APS group (aRR, 14.7; 95% CI, 13.5-16.0) than in the comparison group. Patients with systemic lupus erythematosus (SLE) had a sixfold higher risk of CVE, which was further exacerbated by concomitant APS (18-fold higher risk) or lupus nephritis (15-fold higher risk).
Dr. Dhital also classified CVEs as either venous thromboembolism and non-VTE events. Pregnant patients with APS had a high risk for VTE-only CVE (40-fold greater) and a 3.7-fold higher risk of non-VTE events, compared with pregnant patients without these conditions. Patients with SLE along with lupus nephritis had a 20-fold increased risk of VTE-only CVE and an 11-fold higher risk of non-VTE CVE.
Although the study grouped rheumatic diseases together, “lupus is generally driving these results,” Sharon Kolasinski, MD, of the University of Pennsylvania, Philadelphia, noted in an interview. She moderated the plenary session where the research was presented. “If you take out lupus, then what is the risk? That would be an interesting question.”
Between 25% and 30% of all CVEs occurred in the postpartum period, highlighting the importance of close monitoring of cardiovascular risks and events in women with ARDs or APS both during pregnancy and postpartum, Dr. Dhital noted.
Recognizing these risks “can sometimes be challenging due to a lower suspicion of CVE in younger patients, and also symptoms overlap with normal pregnancy,” Dr. Dhital said during her plenary presentation. Working with other clinical teams could help physicians detect these risks in patients.
“It’s important for us to remember that there’s increased risk of cardiovascular events in pregnancy in our patients. It’s uncommon, but it’s not zero,” added Dr. Kolasinski, and this study highlighted when physicians should be more focused about that risk.
Dr. Dhital noted there were some limitations to the study that are inherent in using administrative databases for research that relies on ICD codes, including “the availability of information on disease activity, medications, and labs, which may restrict clinical interpretation.”
SOMI data reinforced by National Inpatient Sample study
The findings were complemented by a study using the National Inpatient Sample database to explore CVE risk in pregnant individuals with various rheumatic diseases. Lead author Karun Shrestha, MD, a resident physician at St. Barnabas Hospital in New York, and colleagues identified delivery hospitalizations from 2016 to 2019 for individuals with SLE, RA, and systemic vasculitis and looked for CVEs including preeclampsia, peripartum cardiomyopathy (PPCM), heart failure, stroke, cardiac arrhythmias, and VTE.
Out of over 3.4 million delivery hospitalizations, researchers identified 5,900 individuals with SLE, 4,895 with RA, and 325 with vasculitis. After adjusting for confounding factors such as race, age, insurance, and other comorbidities, SLE was identified as an independent risk factor for preeclampsia (odds ratio, 1.5; 95% CI, 1.1-2.1), arrhythmia (OR, 3.17; 95% CI, 1.73-5.79), and venous thrombosis (OR, 8.4; 95% CI, 2.9-22.1). Vasculitis was tied to increased risk for preeclampsia (OR, 4.7; 95% CI, 2-11.3), stroke (OR, 513.3; 95% CI, 114-2,284), heart failure (OR, 24.17; 95% CI, 4.68-124.6), and PPCM (OR, 66.7; 95% CI, 8.7-509.4). RA was tied to an increased risk for preeclampsia (OR, 1.5; 95% CI, 1.05-2.1).
Patients with SLE or vasculitis had longer, more costly hospital stays, compared with those without these conditions, and they experienced higher rates of in-hospital mortality. While previous research has demonstrated that patients with SLE have higher risk of cardiac events, there is less literature on CVE risk in pregnancies for vasculitis, Dr. Shrestha said in an interview.
“It’s something to work on,” he said.
Adverse pregnancy outcomes higher with ARDs, APS
In a second abstract also led by Dr. Dhital using SOMI data, researchers found that pregnant individuals with ARDs or APS had a higher risk of experiencing an APO – preterm birth or small-for-gestational age – than individuals without these conditions. CVEs exacerbated that risk, regardless of underlying chronic health conditions.
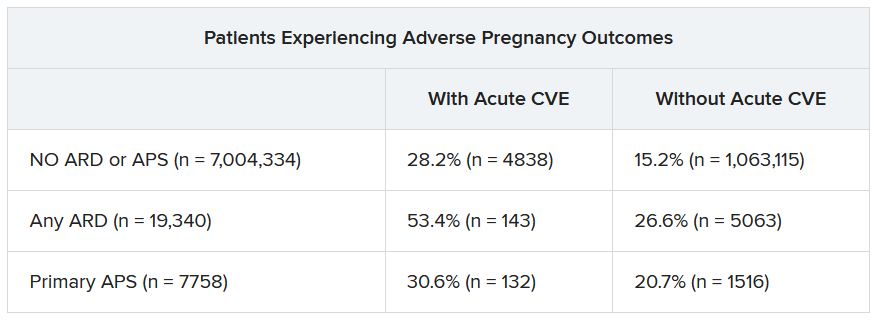
Over half of patients with an ARD and a CVE during pregnancy experienced an APO – most commonly preterm birth. More than one in four pregnant individuals without ARD or APS who experienced a CVE also had an APO.
After differentiating CVEs as either VTE and non-VTE events, patients with ARD and a non-VTE CVE had a fivefold greater risk of early preterm birth (< 32 weeks) and a threefold higher risk of moderate preterm birth (32 to < 34 weeks).
“These findings highlight the need for close monitoring and management of pregnant women, not only for adverse outcomes, but also for cardiovascular risks and events, in order to identify those at the highest risk for adverse outcomes,” the authors wrote. “This need is particularly significant for individuals with ARDs, as 53.4% of our population with an ARD and CVE in pregnancy experienced an APO.”
Dr. Dhital, Dr. Kolasinski, and Dr. Shrestha disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
SAN DIEGO – Pregnant individuals with autoimmune rheumatic diseases (ARDs) are at least four times more likely to experience an acute cardiovascular event (CVE) than are pregnant individuals without these conditions, according to new research presented at the annual meeting of the American College of Rheumatology. Pregnant individuals with primary antiphospholipid syndrome (APS) had a 15-fold increase in CVE risk.
Patients who experienced CVEs were also more likely to experience preterm birth and other adverse pregnancy outcomes (APOs).
Rashmi Dhital, MD, a rheumatology fellow at the University of California, San Diego, and colleagues examined the medical records of pregnant individuals in California who had delivered singleton live-born infants from 2005 to 2020. Using data from the Study of Outcomes in Mothers and Infants (SOMI) database, an administrative population-based birth cohort in California, they identified more than 7 million individuals, 19,340 with ARDs and 7,758 with APS.
They then analyzed how many patients experienced an acute CVE during pregnancy and up to 6 weeks after giving birth.
CVEs occurred in 2.0% of patients with ARDs, 6.9% of individuals with APS, and 0.4% of women without these conditions. CVE risk was four times higher in the ARDs group (adjusted relative risk, 4.1; 95% confidence interval, 3.7-4.5) and nearly 15 times higher in the APS group (aRR, 14.7; 95% CI, 13.5-16.0) than in the comparison group. Patients with systemic lupus erythematosus (SLE) had a sixfold higher risk of CVE, which was further exacerbated by concomitant APS (18-fold higher risk) or lupus nephritis (15-fold higher risk).
Dr. Dhital also classified CVEs as either venous thromboembolism and non-VTE events. Pregnant patients with APS had a high risk for VTE-only CVE (40-fold greater) and a 3.7-fold higher risk of non-VTE events, compared with pregnant patients without these conditions. Patients with SLE along with lupus nephritis had a 20-fold increased risk of VTE-only CVE and an 11-fold higher risk of non-VTE CVE.
Although the study grouped rheumatic diseases together, “lupus is generally driving these results,” Sharon Kolasinski, MD, of the University of Pennsylvania, Philadelphia, noted in an interview. She moderated the plenary session where the research was presented. “If you take out lupus, then what is the risk? That would be an interesting question.”
Between 25% and 30% of all CVEs occurred in the postpartum period, highlighting the importance of close monitoring of cardiovascular risks and events in women with ARDs or APS both during pregnancy and postpartum, Dr. Dhital noted.
Recognizing these risks “can sometimes be challenging due to a lower suspicion of CVE in younger patients, and also symptoms overlap with normal pregnancy,” Dr. Dhital said during her plenary presentation. Working with other clinical teams could help physicians detect these risks in patients.
“It’s important for us to remember that there’s increased risk of cardiovascular events in pregnancy in our patients. It’s uncommon, but it’s not zero,” added Dr. Kolasinski, and this study highlighted when physicians should be more focused about that risk.
Dr. Dhital noted there were some limitations to the study that are inherent in using administrative databases for research that relies on ICD codes, including “the availability of information on disease activity, medications, and labs, which may restrict clinical interpretation.”
SOMI data reinforced by National Inpatient Sample study
The findings were complemented by a study using the National Inpatient Sample database to explore CVE risk in pregnant individuals with various rheumatic diseases. Lead author Karun Shrestha, MD, a resident physician at St. Barnabas Hospital in New York, and colleagues identified delivery hospitalizations from 2016 to 2019 for individuals with SLE, RA, and systemic vasculitis and looked for CVEs including preeclampsia, peripartum cardiomyopathy (PPCM), heart failure, stroke, cardiac arrhythmias, and VTE.
Out of over 3.4 million delivery hospitalizations, researchers identified 5,900 individuals with SLE, 4,895 with RA, and 325 with vasculitis. After adjusting for confounding factors such as race, age, insurance, and other comorbidities, SLE was identified as an independent risk factor for preeclampsia (odds ratio, 1.5; 95% CI, 1.1-2.1), arrhythmia (OR, 3.17; 95% CI, 1.73-5.79), and venous thrombosis (OR, 8.4; 95% CI, 2.9-22.1). Vasculitis was tied to increased risk for preeclampsia (OR, 4.7; 95% CI, 2-11.3), stroke (OR, 513.3; 95% CI, 114-2,284), heart failure (OR, 24.17; 95% CI, 4.68-124.6), and PPCM (OR, 66.7; 95% CI, 8.7-509.4). RA was tied to an increased risk for preeclampsia (OR, 1.5; 95% CI, 1.05-2.1).
Patients with SLE or vasculitis had longer, more costly hospital stays, compared with those without these conditions, and they experienced higher rates of in-hospital mortality. While previous research has demonstrated that patients with SLE have higher risk of cardiac events, there is less literature on CVE risk in pregnancies for vasculitis, Dr. Shrestha said in an interview.
“It’s something to work on,” he said.
Adverse pregnancy outcomes higher with ARDs, APS
In a second abstract also led by Dr. Dhital using SOMI data, researchers found that pregnant individuals with ARDs or APS had a higher risk of experiencing an APO – preterm birth or small-for-gestational age – than individuals without these conditions. CVEs exacerbated that risk, regardless of underlying chronic health conditions.

Over half of patients with an ARD and a CVE during pregnancy experienced an APO – most commonly preterm birth. More than one in four pregnant individuals without ARD or APS who experienced a CVE also had an APO.
After differentiating CVEs as either VTE and non-VTE events, patients with ARD and a non-VTE CVE had a fivefold greater risk of early preterm birth (< 32 weeks) and a threefold higher risk of moderate preterm birth (32 to < 34 weeks).
“These findings highlight the need for close monitoring and management of pregnant women, not only for adverse outcomes, but also for cardiovascular risks and events, in order to identify those at the highest risk for adverse outcomes,” the authors wrote. “This need is particularly significant for individuals with ARDs, as 53.4% of our population with an ARD and CVE in pregnancy experienced an APO.”
Dr. Dhital, Dr. Kolasinski, and Dr. Shrestha disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
SAN DIEGO – Pregnant individuals with autoimmune rheumatic diseases (ARDs) are at least four times more likely to experience an acute cardiovascular event (CVE) than are pregnant individuals without these conditions, according to new research presented at the annual meeting of the American College of Rheumatology. Pregnant individuals with primary antiphospholipid syndrome (APS) had a 15-fold increase in CVE risk.
Patients who experienced CVEs were also more likely to experience preterm birth and other adverse pregnancy outcomes (APOs).
Rashmi Dhital, MD, a rheumatology fellow at the University of California, San Diego, and colleagues examined the medical records of pregnant individuals in California who had delivered singleton live-born infants from 2005 to 2020. Using data from the Study of Outcomes in Mothers and Infants (SOMI) database, an administrative population-based birth cohort in California, they identified more than 7 million individuals, 19,340 with ARDs and 7,758 with APS.
They then analyzed how many patients experienced an acute CVE during pregnancy and up to 6 weeks after giving birth.
CVEs occurred in 2.0% of patients with ARDs, 6.9% of individuals with APS, and 0.4% of women without these conditions. CVE risk was four times higher in the ARDs group (adjusted relative risk, 4.1; 95% confidence interval, 3.7-4.5) and nearly 15 times higher in the APS group (aRR, 14.7; 95% CI, 13.5-16.0) than in the comparison group. Patients with systemic lupus erythematosus (SLE) had a sixfold higher risk of CVE, which was further exacerbated by concomitant APS (18-fold higher risk) or lupus nephritis (15-fold higher risk).
Dr. Dhital also classified CVEs as either venous thromboembolism and non-VTE events. Pregnant patients with APS had a high risk for VTE-only CVE (40-fold greater) and a 3.7-fold higher risk of non-VTE events, compared with pregnant patients without these conditions. Patients with SLE along with lupus nephritis had a 20-fold increased risk of VTE-only CVE and an 11-fold higher risk of non-VTE CVE.
Although the study grouped rheumatic diseases together, “lupus is generally driving these results,” Sharon Kolasinski, MD, of the University of Pennsylvania, Philadelphia, noted in an interview. She moderated the plenary session where the research was presented. “If you take out lupus, then what is the risk? That would be an interesting question.”
Between 25% and 30% of all CVEs occurred in the postpartum period, highlighting the importance of close monitoring of cardiovascular risks and events in women with ARDs or APS both during pregnancy and postpartum, Dr. Dhital noted.
Recognizing these risks “can sometimes be challenging due to a lower suspicion of CVE in younger patients, and also symptoms overlap with normal pregnancy,” Dr. Dhital said during her plenary presentation. Working with other clinical teams could help physicians detect these risks in patients.
“It’s important for us to remember that there’s increased risk of cardiovascular events in pregnancy in our patients. It’s uncommon, but it’s not zero,” added Dr. Kolasinski, and this study highlighted when physicians should be more focused about that risk.
Dr. Dhital noted there were some limitations to the study that are inherent in using administrative databases for research that relies on ICD codes, including “the availability of information on disease activity, medications, and labs, which may restrict clinical interpretation.”
SOMI data reinforced by National Inpatient Sample study
The findings were complemented by a study using the National Inpatient Sample database to explore CVE risk in pregnant individuals with various rheumatic diseases. Lead author Karun Shrestha, MD, a resident physician at St. Barnabas Hospital in New York, and colleagues identified delivery hospitalizations from 2016 to 2019 for individuals with SLE, RA, and systemic vasculitis and looked for CVEs including preeclampsia, peripartum cardiomyopathy (PPCM), heart failure, stroke, cardiac arrhythmias, and VTE.
Out of over 3.4 million delivery hospitalizations, researchers identified 5,900 individuals with SLE, 4,895 with RA, and 325 with vasculitis. After adjusting for confounding factors such as race, age, insurance, and other comorbidities, SLE was identified as an independent risk factor for preeclampsia (odds ratio, 1.5; 95% CI, 1.1-2.1), arrhythmia (OR, 3.17; 95% CI, 1.73-5.79), and venous thrombosis (OR, 8.4; 95% CI, 2.9-22.1). Vasculitis was tied to increased risk for preeclampsia (OR, 4.7; 95% CI, 2-11.3), stroke (OR, 513.3; 95% CI, 114-2,284), heart failure (OR, 24.17; 95% CI, 4.68-124.6), and PPCM (OR, 66.7; 95% CI, 8.7-509.4). RA was tied to an increased risk for preeclampsia (OR, 1.5; 95% CI, 1.05-2.1).
Patients with SLE or vasculitis had longer, more costly hospital stays, compared with those without these conditions, and they experienced higher rates of in-hospital mortality. While previous research has demonstrated that patients with SLE have higher risk of cardiac events, there is less literature on CVE risk in pregnancies for vasculitis, Dr. Shrestha said in an interview.
“It’s something to work on,” he said.
Adverse pregnancy outcomes higher with ARDs, APS
In a second abstract also led by Dr. Dhital using SOMI data, researchers found that pregnant individuals with ARDs or APS had a higher risk of experiencing an APO – preterm birth or small-for-gestational age – than individuals without these conditions. CVEs exacerbated that risk, regardless of underlying chronic health conditions.

Over half of patients with an ARD and a CVE during pregnancy experienced an APO – most commonly preterm birth. More than one in four pregnant individuals without ARD or APS who experienced a CVE also had an APO.
After differentiating CVEs as either VTE and non-VTE events, patients with ARD and a non-VTE CVE had a fivefold greater risk of early preterm birth (< 32 weeks) and a threefold higher risk of moderate preterm birth (32 to < 34 weeks).
“These findings highlight the need for close monitoring and management of pregnant women, not only for adverse outcomes, but also for cardiovascular risks and events, in order to identify those at the highest risk for adverse outcomes,” the authors wrote. “This need is particularly significant for individuals with ARDs, as 53.4% of our population with an ARD and CVE in pregnancy experienced an APO.”
Dr. Dhital, Dr. Kolasinski, and Dr. Shrestha disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
AT ACR 2023
Pregnancies with low anti-SSA/Ro autoantibody levels: Forgo fetal heart rhythm monitoring?
SAN DIEGO – Pregnant women with anti-SSA/Ro autoantibodies at titer levels of less than 1,000 ELISA units per mL are at minimal to no risk for fetal atrioventricular (AV) block and may be able to forgo traditional echocardiographic heart rhythm monitoring, results from an ongoing, prospective, multicenter trial demonstrated.
However, pregnant patients with higher titer antibodies seem to be at greatest risk for fetal AV block and may benefit from ambulatory fetal heart rhythm monitoring (FHRM), which can detect emergent AV block, according to the study findings. The findings were published online in Arthritis & Rheumatology and will be presented Nov. 13 at the American College of Rheumatology (ACR) 2023 Annual Meeting by Jill P. Buyon, MD, a rheumatologist who directs the division of rheumatology and the Lupus Center at NYU Langone Health in New York.
“While anti-Ro antibodies have been known to be associated with AV block for decades, it has become increasingly clear that antibody titers matter,” Dr. Buyon said in an interview.
For the investigation, which is the largest of its kind, researchers at 22 sites drew from the large multiracial national study of pregnant women, Surveillance To Prevent AV Block Likely to Occur Quickly (STOP BLOQ), to address the impact of anti-Ro titers and use of frequent ambulatory FHRM on outcomes in women with no previously affected children and those at risk for recurrence. Monitoring occurred during the second trimester of pregnancy (from 17 weeks through 26 weeks) and consisted of daily fetal home testing by mothers using handheld, commercially available Doppler devices.
These were followed up by weekly or biweekly echocardiograms, and ultrasound tests to evaluate fetal heart rhythm and function, as well as to show any structural problems. Three times per day, the pregnant women texted the Doppler sound recordings in real time to a pediatric cardiologist, who immediately ordered an additional echocardiogram in cases of irregular or slowing fetal heart rates. If second-degree heart block was detected, drug therapy was initiated.
No AV block seen with low anti-Ro titers
Dr. Buyon, who led the study with Bettina Cuneo, MD, clinical scholar and professor of surgery and pediatrics at the University of Arizona in Tucson, presented findings from 413 pregnant subjects with a mean age of 33 years who finished monitoring surveillance: 152 women had low titers of both anti-Ro60 and –Ro52 (defined as < 1,000 ELISA units per mL), and 261 women with titers above the threshold for either antibody (defined as ≥ 1,000 ELISA units per mL). Of the 152 women with low titers of both anti-Ro60 and –Ro52, none of the pregnancies past 26 weeks resulted in AV block. Of the 261 women with titers above the threshold for either antibody, 10 of the pregnancies resulted in AV block (3.8%). The incidence of AV block increased with higher antibody titer levels, reaching 7.7% for those in the top quartile for anti–60-kD SSA/Ro; this increased to 27.3% in study participants with a previous child who had AV block, although numbers in this category were small.
Analysis of cumulative FHRM recordings between surveillance echocardiograms revealed that no case of second-degree or third-degree AV block was missed. In addition, 70% of AV blocks detected by FHRM were second-degree and all occurred less than 12 hours from normal FHRM and within another 45 minutes to 4.5 hours to echocardiogram. The one case of second/third-degree and two cases of third-degree AV block were diagnosed by urgent echocardiogram more than 17 to 72 hours from a previously normal FHRM episode.
Other factors besides high anti-Ro titer likely play a role
“STOP BLOQ nicely demonstrates that low titer is associated with a very low risk AV block, and intense monitoring may not be needed,” Dr. Buyon told this news organization. “However, high titer is not the whole answer since even women with the very highest titers can have healthy babies. This report also shows that titers stay constant through pregnancies in the same mother, whether there is the complication of AV block or not. This suggests other factors contribute to AV block.”
She added that FHRM can be easily performed by the mother, but at this time is still best interpreted by a cardiologist. “FHRM detected all cases of AV block, which can happen in hours,” she said. “FHRM should decrease the need for frequent echocardiograms. Some mothers do have more difficulty in deciding whether the baby’s heart is beating irregularly. We need [to improve our teaching] and for how best to have a cardiologist or trained listener interpret. FHRM can be done by the mother but needs interpretation by a cardiologist until we develop a device which can identify abnormalities.”
She acknowledged certain limitations of the study, including the fact that a commercial test for anti-SSA/Ro antibody levels is not available to all clinicians. “Try to find a lab that measures high titer anti-Ro antibodies, but if not, then use one of the common commercial tests such as the BioPlex 2000 autoimmune panels and consider decreased surveillance if titer is < 8,” Dr. Buyon advised.
Vaneet K. Sandhu, MD, a rheumatologist with Loma Linda (Calif.) Medical Center, who was asked to comment on the work, said that the study not only justifies the limited use of FHRM in those with high titer antibodies (followed by urgent fetal echocardiography where indicated), but also risk stratification for fetal AV block.
“For years, we have recommended frequent fetal echocardiography testing in pregnant women with positive anti-SSA/Ro,” Dr. Sandhu said. “This study tells us we need to look deeper. On one hand, recognizing that low titer anti-Ro antibodies do not confer a risk of AV block is cost effective. On the other hand, while the titer of the antibody appears to contribute to fetal AV block, we need to delve deeper into additional factors contributing to fetal AV block risk in order to better navigate our surveillance methods.”
The study was supported by NIH grants from the National Institute of Child Health and Human Development and the National Institute of Arthritis and Musculoskeletal and Skin Diseases. Dr. Sandhu has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
SAN DIEGO – Pregnant women with anti-SSA/Ro autoantibodies at titer levels of less than 1,000 ELISA units per mL are at minimal to no risk for fetal atrioventricular (AV) block and may be able to forgo traditional echocardiographic heart rhythm monitoring, results from an ongoing, prospective, multicenter trial demonstrated.
However, pregnant patients with higher titer antibodies seem to be at greatest risk for fetal AV block and may benefit from ambulatory fetal heart rhythm monitoring (FHRM), which can detect emergent AV block, according to the study findings. The findings were published online in Arthritis & Rheumatology and will be presented Nov. 13 at the American College of Rheumatology (ACR) 2023 Annual Meeting by Jill P. Buyon, MD, a rheumatologist who directs the division of rheumatology and the Lupus Center at NYU Langone Health in New York.
“While anti-Ro antibodies have been known to be associated with AV block for decades, it has become increasingly clear that antibody titers matter,” Dr. Buyon said in an interview.
For the investigation, which is the largest of its kind, researchers at 22 sites drew from the large multiracial national study of pregnant women, Surveillance To Prevent AV Block Likely to Occur Quickly (STOP BLOQ), to address the impact of anti-Ro titers and use of frequent ambulatory FHRM on outcomes in women with no previously affected children and those at risk for recurrence. Monitoring occurred during the second trimester of pregnancy (from 17 weeks through 26 weeks) and consisted of daily fetal home testing by mothers using handheld, commercially available Doppler devices.
These were followed up by weekly or biweekly echocardiograms, and ultrasound tests to evaluate fetal heart rhythm and function, as well as to show any structural problems. Three times per day, the pregnant women texted the Doppler sound recordings in real time to a pediatric cardiologist, who immediately ordered an additional echocardiogram in cases of irregular or slowing fetal heart rates. If second-degree heart block was detected, drug therapy was initiated.
No AV block seen with low anti-Ro titers
Dr. Buyon, who led the study with Bettina Cuneo, MD, clinical scholar and professor of surgery and pediatrics at the University of Arizona in Tucson, presented findings from 413 pregnant subjects with a mean age of 33 years who finished monitoring surveillance: 152 women had low titers of both anti-Ro60 and –Ro52 (defined as < 1,000 ELISA units per mL), and 261 women with titers above the threshold for either antibody (defined as ≥ 1,000 ELISA units per mL). Of the 152 women with low titers of both anti-Ro60 and –Ro52, none of the pregnancies past 26 weeks resulted in AV block. Of the 261 women with titers above the threshold for either antibody, 10 of the pregnancies resulted in AV block (3.8%). The incidence of AV block increased with higher antibody titer levels, reaching 7.7% for those in the top quartile for anti–60-kD SSA/Ro; this increased to 27.3% in study participants with a previous child who had AV block, although numbers in this category were small.
Analysis of cumulative FHRM recordings between surveillance echocardiograms revealed that no case of second-degree or third-degree AV block was missed. In addition, 70% of AV blocks detected by FHRM were second-degree and all occurred less than 12 hours from normal FHRM and within another 45 minutes to 4.5 hours to echocardiogram. The one case of second/third-degree and two cases of third-degree AV block were diagnosed by urgent echocardiogram more than 17 to 72 hours from a previously normal FHRM episode.
Other factors besides high anti-Ro titer likely play a role
“STOP BLOQ nicely demonstrates that low titer is associated with a very low risk AV block, and intense monitoring may not be needed,” Dr. Buyon told this news organization. “However, high titer is not the whole answer since even women with the very highest titers can have healthy babies. This report also shows that titers stay constant through pregnancies in the same mother, whether there is the complication of AV block or not. This suggests other factors contribute to AV block.”
She added that FHRM can be easily performed by the mother, but at this time is still best interpreted by a cardiologist. “FHRM detected all cases of AV block, which can happen in hours,” she said. “FHRM should decrease the need for frequent echocardiograms. Some mothers do have more difficulty in deciding whether the baby’s heart is beating irregularly. We need [to improve our teaching] and for how best to have a cardiologist or trained listener interpret. FHRM can be done by the mother but needs interpretation by a cardiologist until we develop a device which can identify abnormalities.”
She acknowledged certain limitations of the study, including the fact that a commercial test for anti-SSA/Ro antibody levels is not available to all clinicians. “Try to find a lab that measures high titer anti-Ro antibodies, but if not, then use one of the common commercial tests such as the BioPlex 2000 autoimmune panels and consider decreased surveillance if titer is < 8,” Dr. Buyon advised.
Vaneet K. Sandhu, MD, a rheumatologist with Loma Linda (Calif.) Medical Center, who was asked to comment on the work, said that the study not only justifies the limited use of FHRM in those with high titer antibodies (followed by urgent fetal echocardiography where indicated), but also risk stratification for fetal AV block.
“For years, we have recommended frequent fetal echocardiography testing in pregnant women with positive anti-SSA/Ro,” Dr. Sandhu said. “This study tells us we need to look deeper. On one hand, recognizing that low titer anti-Ro antibodies do not confer a risk of AV block is cost effective. On the other hand, while the titer of the antibody appears to contribute to fetal AV block, we need to delve deeper into additional factors contributing to fetal AV block risk in order to better navigate our surveillance methods.”
The study was supported by NIH grants from the National Institute of Child Health and Human Development and the National Institute of Arthritis and Musculoskeletal and Skin Diseases. Dr. Sandhu has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
SAN DIEGO – Pregnant women with anti-SSA/Ro autoantibodies at titer levels of less than 1,000 ELISA units per mL are at minimal to no risk for fetal atrioventricular (AV) block and may be able to forgo traditional echocardiographic heart rhythm monitoring, results from an ongoing, prospective, multicenter trial demonstrated.
However, pregnant patients with higher titer antibodies seem to be at greatest risk for fetal AV block and may benefit from ambulatory fetal heart rhythm monitoring (FHRM), which can detect emergent AV block, according to the study findings. The findings were published online in Arthritis & Rheumatology and will be presented Nov. 13 at the American College of Rheumatology (ACR) 2023 Annual Meeting by Jill P. Buyon, MD, a rheumatologist who directs the division of rheumatology and the Lupus Center at NYU Langone Health in New York.
“While anti-Ro antibodies have been known to be associated with AV block for decades, it has become increasingly clear that antibody titers matter,” Dr. Buyon said in an interview.
For the investigation, which is the largest of its kind, researchers at 22 sites drew from the large multiracial national study of pregnant women, Surveillance To Prevent AV Block Likely to Occur Quickly (STOP BLOQ), to address the impact of anti-Ro titers and use of frequent ambulatory FHRM on outcomes in women with no previously affected children and those at risk for recurrence. Monitoring occurred during the second trimester of pregnancy (from 17 weeks through 26 weeks) and consisted of daily fetal home testing by mothers using handheld, commercially available Doppler devices.
These were followed up by weekly or biweekly echocardiograms, and ultrasound tests to evaluate fetal heart rhythm and function, as well as to show any structural problems. Three times per day, the pregnant women texted the Doppler sound recordings in real time to a pediatric cardiologist, who immediately ordered an additional echocardiogram in cases of irregular or slowing fetal heart rates. If second-degree heart block was detected, drug therapy was initiated.
No AV block seen with low anti-Ro titers
Dr. Buyon, who led the study with Bettina Cuneo, MD, clinical scholar and professor of surgery and pediatrics at the University of Arizona in Tucson, presented findings from 413 pregnant subjects with a mean age of 33 years who finished monitoring surveillance: 152 women had low titers of both anti-Ro60 and –Ro52 (defined as < 1,000 ELISA units per mL), and 261 women with titers above the threshold for either antibody (defined as ≥ 1,000 ELISA units per mL). Of the 152 women with low titers of both anti-Ro60 and –Ro52, none of the pregnancies past 26 weeks resulted in AV block. Of the 261 women with titers above the threshold for either antibody, 10 of the pregnancies resulted in AV block (3.8%). The incidence of AV block increased with higher antibody titer levels, reaching 7.7% for those in the top quartile for anti–60-kD SSA/Ro; this increased to 27.3% in study participants with a previous child who had AV block, although numbers in this category were small.
Analysis of cumulative FHRM recordings between surveillance echocardiograms revealed that no case of second-degree or third-degree AV block was missed. In addition, 70% of AV blocks detected by FHRM were second-degree and all occurred less than 12 hours from normal FHRM and within another 45 minutes to 4.5 hours to echocardiogram. The one case of second/third-degree and two cases of third-degree AV block were diagnosed by urgent echocardiogram more than 17 to 72 hours from a previously normal FHRM episode.
Other factors besides high anti-Ro titer likely play a role
“STOP BLOQ nicely demonstrates that low titer is associated with a very low risk AV block, and intense monitoring may not be needed,” Dr. Buyon told this news organization. “However, high titer is not the whole answer since even women with the very highest titers can have healthy babies. This report also shows that titers stay constant through pregnancies in the same mother, whether there is the complication of AV block or not. This suggests other factors contribute to AV block.”
She added that FHRM can be easily performed by the mother, but at this time is still best interpreted by a cardiologist. “FHRM detected all cases of AV block, which can happen in hours,” she said. “FHRM should decrease the need for frequent echocardiograms. Some mothers do have more difficulty in deciding whether the baby’s heart is beating irregularly. We need [to improve our teaching] and for how best to have a cardiologist or trained listener interpret. FHRM can be done by the mother but needs interpretation by a cardiologist until we develop a device which can identify abnormalities.”
She acknowledged certain limitations of the study, including the fact that a commercial test for anti-SSA/Ro antibody levels is not available to all clinicians. “Try to find a lab that measures high titer anti-Ro antibodies, but if not, then use one of the common commercial tests such as the BioPlex 2000 autoimmune panels and consider decreased surveillance if titer is < 8,” Dr. Buyon advised.
Vaneet K. Sandhu, MD, a rheumatologist with Loma Linda (Calif.) Medical Center, who was asked to comment on the work, said that the study not only justifies the limited use of FHRM in those with high titer antibodies (followed by urgent fetal echocardiography where indicated), but also risk stratification for fetal AV block.
“For years, we have recommended frequent fetal echocardiography testing in pregnant women with positive anti-SSA/Ro,” Dr. Sandhu said. “This study tells us we need to look deeper. On one hand, recognizing that low titer anti-Ro antibodies do not confer a risk of AV block is cost effective. On the other hand, while the titer of the antibody appears to contribute to fetal AV block, we need to delve deeper into additional factors contributing to fetal AV block risk in order to better navigate our surveillance methods.”
The study was supported by NIH grants from the National Institute of Child Health and Human Development and the National Institute of Arthritis and Musculoskeletal and Skin Diseases. Dr. Sandhu has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
AT ACR 2023
Does vaginal estrogen use increase the risk for adverse cardiovascular outcomes?
Evidence summary
Cohort studies demonstrate no adverse CV outcomes
A 2020 systematic review and meta-analysis evaluated randomized controlled trials (RCTs) and observational studies to examine the association between menopausal hormone therapy and CV disease.1 The 26 RCTs primarily evaluated oral hormone administration. The observational studies comprised 30 cohort studies, 13 case-control studies, and 5 nested case-control studies, primarily in Europe and North America; 21 reported the route of administration. The trials evaluated women ages 49 to 77 years (mean, 61 years), and follow-up ranged from 1 to 21.5 years (mean, 7 years). In subgroup analyses of the observational studies, nonoral hormone therapy was associated with a lower risk for stroke and MI compared to oral administration (see TABLE1). Study limitations included enrollment of patients with few comorbidities, from limited geographic regions. Results in the meta-analysis were not stratified by the type of nonoral hormone therapy; only 4 studies evaluated vaginal estrogen use.
Two large cohort studies included in the systematic review provided more specific data on vaginal estrogens. The first used data from the Women’s Health Initiative in a subset of women ages 50 to 79 years (n = 46,566) who were not already on systemic hormone therapy and who did not have prior history of breast, endometrial, or ovarian cancer.2 Data were collected from self-assessment questionnaires and medical record reviews. The median duration of vaginal estrogen use was 2 years, and median follow-up duration was 7.2 years. Vaginal estrogen users had a 48% lower risk for CHD (adjusted hazard ratio [aHR] = 0.52; 95% CI, 0.31-0.85) than nonusers. Rates for all-cause mortality (aHR = 0.78; 95% CI, 0.58-1.04), stroke (aHR = 0.78; 95% CI, 0.49-1.24), and DVT/PE (aHR = 0.68; 95% CI, 0.36-1.28) were similar. In this and the other cohort studies to be discussed, outcome data for all vaginal estrogen preparations (eg, cream, ring, tablet) were combined.
The other large cohort study in the systematic review evaluated data on postmenopausal women from the Nurses’ Health Study.3 The authors evaluated health reports on 53,797 women as they transitioned through menopause. Patients with systemic hormone therapy use, history of cancer, and self-reported CV disease were excluded. After adjusting for covariates, the authors found no statistically significant difference between users and nonusers of vaginal estrogen and risk for total MI (aHR = 0.73; 95% CI, 0.47-1.13), stroke (aHR = 0.85; 95% CI, 0.56-1.29), or DVT/PE (aHR = 1.06; 95% CI, 0.58-1.93). Study limitations included low prevalence of vaginal estrogen use (< 3%), short duration of use (mean, 37.5 months), and lack of data on the type or dose of vaginal estrogen used. The study only included health professionals, which limits generalizability.
A Finnish cohort study (excluded from the systematic review because it used historical controls) compared rates of CHD and stroke in postmenopausal women who used vaginal estrogen against an age-matched background population. Researchers collected data from a nationwide prescription registry for women at least 50 years old who had purchased vaginal estrogens between 1994 and 2009 (n = 195,756).4 Women who purchased systemic hormone therapy at any point were excluded. After 3 to 5 years of exposure, use of vaginal estrogen was associated with a decreased risk for mortality from CHD (relative risk [RR] = 0.64; 95% CI, 0.57-0.70) and stroke (RR = 0.79; 95% CI, 0.69-0.91). However, after 10 years, these benefits were not seen (CHD: RR = 0.95; 95% CI, 0.90-1.00; stroke: RR = 0.93; 95% CI, 0.85-1.01). All confidence interval data were presented graphically. Key weaknesses of this study included use of both vaginal and systemic estrogen in the comparator background population, and the failure to collect data for other CV risk variables such as weight, tobacco exposure, and blood pressure.
Recommendations from others
In 2022, the North American Menopause Society issued a Hormone Therapy Position Statement that acknowledged the lack of clinical trials directly comparing risk for adverse CV endpoints with different estrogen administration routes.5 They stated nonoral routes of administration might offer advantages by bypassing first-pass hepatic metabolism.
Similarly, the 2015 Endocrine Society Clinical Practice Guideline on the Treatment of Symptoms of the Menopause also stated that the effects of low-dose vaginal estrogen therapy on CV disease or DVT/PE risk had not been adequately studied.6
A 2013 opinion by the American College of Obstetricians and Gynecologists stated that topical estrogen vaginal creams, tablets, and rings had low levels of systemic absorption and were not associated with an increased risk for DVT/PE.7
Editor’s takeaway
The available evidence on vaginal estrogen replacement reassures us of its safety. After decades spent studying hormone replacement therapy with vacillating conclusions and opinions, these cohorts—the best evidence we may ever get—along with a consensus of expert opinions, consistently demonstrate no adverse CV outcomes.
1. Kim JE, Chang JH, Jeong MJ, et al. A systematic review and meta-analysis of effects of menopausal hormone therapy on cardiovascular diseases. Sci Rep. 2020;10:20631. doi: 10.1038/s41598-020-77534-9
2. Crandall CJ, Hovey KM, Andrews CA, et al. Breast cancer, endometrial cancer, and cardiovascular events in participants who used vaginal estrogen in the WHI Observational Study. Menopause. 2018;25:11-20. doi: 10.1097/GME.0000000000000956
3. Bhupathiraju SN, Grodstein F, Stampfer MJ, et al. Vaginal estrogen use and chronic disease risk in the Nurses’ Health Study. Menopause. 2018;26:603-610. doi: 10.1097/GME.0000000000001284
4. Mikkola TS, Tuomikoski P, Lyytinen H, et al. Vaginal estrogen use and the risk for cardiovascular mortality. Human Reproduction. 2016;31:804-809. doi: 10.1093/humrep/dew014
5. North American Menopause Society. The 2022 hormone therapy position statement of The North American Menopause Society. Menopause. 2022;29:767-794. doi: 10.1097/GME.0000000000002028
6. Stuenkel CA, Davis SR, Gompel A, et al. Treatment of symptoms of the menopause: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2015;100:3975-4011. doi: 10.1210/jc.2015-2236
7. American College of Obstetricians and Gynecologists. Committee Opinion No 565: hormone therapy and heart disease. Obstet Gynecol. 2013;121:1407-1410. doi: 10.1097/01.AOG.0000431053.33593.2d
Evidence summary
Cohort studies demonstrate no adverse CV outcomes
A 2020 systematic review and meta-analysis evaluated randomized controlled trials (RCTs) and observational studies to examine the association between menopausal hormone therapy and CV disease.1 The 26 RCTs primarily evaluated oral hormone administration. The observational studies comprised 30 cohort studies, 13 case-control studies, and 5 nested case-control studies, primarily in Europe and North America; 21 reported the route of administration. The trials evaluated women ages 49 to 77 years (mean, 61 years), and follow-up ranged from 1 to 21.5 years (mean, 7 years). In subgroup analyses of the observational studies, nonoral hormone therapy was associated with a lower risk for stroke and MI compared to oral administration (see TABLE1). Study limitations included enrollment of patients with few comorbidities, from limited geographic regions. Results in the meta-analysis were not stratified by the type of nonoral hormone therapy; only 4 studies evaluated vaginal estrogen use.

Two large cohort studies included in the systematic review provided more specific data on vaginal estrogens. The first used data from the Women’s Health Initiative in a subset of women ages 50 to 79 years (n = 46,566) who were not already on systemic hormone therapy and who did not have prior history of breast, endometrial, or ovarian cancer.2 Data were collected from self-assessment questionnaires and medical record reviews. The median duration of vaginal estrogen use was 2 years, and median follow-up duration was 7.2 years. Vaginal estrogen users had a 48% lower risk for CHD (adjusted hazard ratio [aHR] = 0.52; 95% CI, 0.31-0.85) than nonusers. Rates for all-cause mortality (aHR = 0.78; 95% CI, 0.58-1.04), stroke (aHR = 0.78; 95% CI, 0.49-1.24), and DVT/PE (aHR = 0.68; 95% CI, 0.36-1.28) were similar. In this and the other cohort studies to be discussed, outcome data for all vaginal estrogen preparations (eg, cream, ring, tablet) were combined.
The other large cohort study in the systematic review evaluated data on postmenopausal women from the Nurses’ Health Study.3 The authors evaluated health reports on 53,797 women as they transitioned through menopause. Patients with systemic hormone therapy use, history of cancer, and self-reported CV disease were excluded. After adjusting for covariates, the authors found no statistically significant difference between users and nonusers of vaginal estrogen and risk for total MI (aHR = 0.73; 95% CI, 0.47-1.13), stroke (aHR = 0.85; 95% CI, 0.56-1.29), or DVT/PE (aHR = 1.06; 95% CI, 0.58-1.93). Study limitations included low prevalence of vaginal estrogen use (< 3%), short duration of use (mean, 37.5 months), and lack of data on the type or dose of vaginal estrogen used. The study only included health professionals, which limits generalizability.
A Finnish cohort study (excluded from the systematic review because it used historical controls) compared rates of CHD and stroke in postmenopausal women who used vaginal estrogen against an age-matched background population. Researchers collected data from a nationwide prescription registry for women at least 50 years old who had purchased vaginal estrogens between 1994 and 2009 (n = 195,756).4 Women who purchased systemic hormone therapy at any point were excluded. After 3 to 5 years of exposure, use of vaginal estrogen was associated with a decreased risk for mortality from CHD (relative risk [RR] = 0.64; 95% CI, 0.57-0.70) and stroke (RR = 0.79; 95% CI, 0.69-0.91). However, after 10 years, these benefits were not seen (CHD: RR = 0.95; 95% CI, 0.90-1.00; stroke: RR = 0.93; 95% CI, 0.85-1.01). All confidence interval data were presented graphically. Key weaknesses of this study included use of both vaginal and systemic estrogen in the comparator background population, and the failure to collect data for other CV risk variables such as weight, tobacco exposure, and blood pressure.
Recommendations from others
In 2022, the North American Menopause Society issued a Hormone Therapy Position Statement that acknowledged the lack of clinical trials directly comparing risk for adverse CV endpoints with different estrogen administration routes.5 They stated nonoral routes of administration might offer advantages by bypassing first-pass hepatic metabolism.
Similarly, the 2015 Endocrine Society Clinical Practice Guideline on the Treatment of Symptoms of the Menopause also stated that the effects of low-dose vaginal estrogen therapy on CV disease or DVT/PE risk had not been adequately studied.6
A 2013 opinion by the American College of Obstetricians and Gynecologists stated that topical estrogen vaginal creams, tablets, and rings had low levels of systemic absorption and were not associated with an increased risk for DVT/PE.7
Editor’s takeaway
The available evidence on vaginal estrogen replacement reassures us of its safety. After decades spent studying hormone replacement therapy with vacillating conclusions and opinions, these cohorts—the best evidence we may ever get—along with a consensus of expert opinions, consistently demonstrate no adverse CV outcomes.
Evidence summary
Cohort studies demonstrate no adverse CV outcomes
A 2020 systematic review and meta-analysis evaluated randomized controlled trials (RCTs) and observational studies to examine the association between menopausal hormone therapy and CV disease.1 The 26 RCTs primarily evaluated oral hormone administration. The observational studies comprised 30 cohort studies, 13 case-control studies, and 5 nested case-control studies, primarily in Europe and North America; 21 reported the route of administration. The trials evaluated women ages 49 to 77 years (mean, 61 years), and follow-up ranged from 1 to 21.5 years (mean, 7 years). In subgroup analyses of the observational studies, nonoral hormone therapy was associated with a lower risk for stroke and MI compared to oral administration (see TABLE1). Study limitations included enrollment of patients with few comorbidities, from limited geographic regions. Results in the meta-analysis were not stratified by the type of nonoral hormone therapy; only 4 studies evaluated vaginal estrogen use.

Two large cohort studies included in the systematic review provided more specific data on vaginal estrogens. The first used data from the Women’s Health Initiative in a subset of women ages 50 to 79 years (n = 46,566) who were not already on systemic hormone therapy and who did not have prior history of breast, endometrial, or ovarian cancer.2 Data were collected from self-assessment questionnaires and medical record reviews. The median duration of vaginal estrogen use was 2 years, and median follow-up duration was 7.2 years. Vaginal estrogen users had a 48% lower risk for CHD (adjusted hazard ratio [aHR] = 0.52; 95% CI, 0.31-0.85) than nonusers. Rates for all-cause mortality (aHR = 0.78; 95% CI, 0.58-1.04), stroke (aHR = 0.78; 95% CI, 0.49-1.24), and DVT/PE (aHR = 0.68; 95% CI, 0.36-1.28) were similar. In this and the other cohort studies to be discussed, outcome data for all vaginal estrogen preparations (eg, cream, ring, tablet) were combined.
The other large cohort study in the systematic review evaluated data on postmenopausal women from the Nurses’ Health Study.3 The authors evaluated health reports on 53,797 women as they transitioned through menopause. Patients with systemic hormone therapy use, history of cancer, and self-reported CV disease were excluded. After adjusting for covariates, the authors found no statistically significant difference between users and nonusers of vaginal estrogen and risk for total MI (aHR = 0.73; 95% CI, 0.47-1.13), stroke (aHR = 0.85; 95% CI, 0.56-1.29), or DVT/PE (aHR = 1.06; 95% CI, 0.58-1.93). Study limitations included low prevalence of vaginal estrogen use (< 3%), short duration of use (mean, 37.5 months), and lack of data on the type or dose of vaginal estrogen used. The study only included health professionals, which limits generalizability.
A Finnish cohort study (excluded from the systematic review because it used historical controls) compared rates of CHD and stroke in postmenopausal women who used vaginal estrogen against an age-matched background population. Researchers collected data from a nationwide prescription registry for women at least 50 years old who had purchased vaginal estrogens between 1994 and 2009 (n = 195,756).4 Women who purchased systemic hormone therapy at any point were excluded. After 3 to 5 years of exposure, use of vaginal estrogen was associated with a decreased risk for mortality from CHD (relative risk [RR] = 0.64; 95% CI, 0.57-0.70) and stroke (RR = 0.79; 95% CI, 0.69-0.91). However, after 10 years, these benefits were not seen (CHD: RR = 0.95; 95% CI, 0.90-1.00; stroke: RR = 0.93; 95% CI, 0.85-1.01). All confidence interval data were presented graphically. Key weaknesses of this study included use of both vaginal and systemic estrogen in the comparator background population, and the failure to collect data for other CV risk variables such as weight, tobacco exposure, and blood pressure.
Recommendations from others
In 2022, the North American Menopause Society issued a Hormone Therapy Position Statement that acknowledged the lack of clinical trials directly comparing risk for adverse CV endpoints with different estrogen administration routes.5 They stated nonoral routes of administration might offer advantages by bypassing first-pass hepatic metabolism.
Similarly, the 2015 Endocrine Society Clinical Practice Guideline on the Treatment of Symptoms of the Menopause also stated that the effects of low-dose vaginal estrogen therapy on CV disease or DVT/PE risk had not been adequately studied.6
A 2013 opinion by the American College of Obstetricians and Gynecologists stated that topical estrogen vaginal creams, tablets, and rings had low levels of systemic absorption and were not associated with an increased risk for DVT/PE.7
Editor’s takeaway
The available evidence on vaginal estrogen replacement reassures us of its safety. After decades spent studying hormone replacement therapy with vacillating conclusions and opinions, these cohorts—the best evidence we may ever get—along with a consensus of expert opinions, consistently demonstrate no adverse CV outcomes.
1. Kim JE, Chang JH, Jeong MJ, et al. A systematic review and meta-analysis of effects of menopausal hormone therapy on cardiovascular diseases. Sci Rep. 2020;10:20631. doi: 10.1038/s41598-020-77534-9
2. Crandall CJ, Hovey KM, Andrews CA, et al. Breast cancer, endometrial cancer, and cardiovascular events in participants who used vaginal estrogen in the WHI Observational Study. Menopause. 2018;25:11-20. doi: 10.1097/GME.0000000000000956
3. Bhupathiraju SN, Grodstein F, Stampfer MJ, et al. Vaginal estrogen use and chronic disease risk in the Nurses’ Health Study. Menopause. 2018;26:603-610. doi: 10.1097/GME.0000000000001284
4. Mikkola TS, Tuomikoski P, Lyytinen H, et al. Vaginal estrogen use and the risk for cardiovascular mortality. Human Reproduction. 2016;31:804-809. doi: 10.1093/humrep/dew014
5. North American Menopause Society. The 2022 hormone therapy position statement of The North American Menopause Society. Menopause. 2022;29:767-794. doi: 10.1097/GME.0000000000002028
6. Stuenkel CA, Davis SR, Gompel A, et al. Treatment of symptoms of the menopause: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2015;100:3975-4011. doi: 10.1210/jc.2015-2236
7. American College of Obstetricians and Gynecologists. Committee Opinion No 565: hormone therapy and heart disease. Obstet Gynecol. 2013;121:1407-1410. doi: 10.1097/01.AOG.0000431053.33593.2d
1. Kim JE, Chang JH, Jeong MJ, et al. A systematic review and meta-analysis of effects of menopausal hormone therapy on cardiovascular diseases. Sci Rep. 2020;10:20631. doi: 10.1038/s41598-020-77534-9
2. Crandall CJ, Hovey KM, Andrews CA, et al. Breast cancer, endometrial cancer, and cardiovascular events in participants who used vaginal estrogen in the WHI Observational Study. Menopause. 2018;25:11-20. doi: 10.1097/GME.0000000000000956
3. Bhupathiraju SN, Grodstein F, Stampfer MJ, et al. Vaginal estrogen use and chronic disease risk in the Nurses’ Health Study. Menopause. 2018;26:603-610. doi: 10.1097/GME.0000000000001284
4. Mikkola TS, Tuomikoski P, Lyytinen H, et al. Vaginal estrogen use and the risk for cardiovascular mortality. Human Reproduction. 2016;31:804-809. doi: 10.1093/humrep/dew014
5. North American Menopause Society. The 2022 hormone therapy position statement of The North American Menopause Society. Menopause. 2022;29:767-794. doi: 10.1097/GME.0000000000002028
6. Stuenkel CA, Davis SR, Gompel A, et al. Treatment of symptoms of the menopause: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2015;100:3975-4011. doi: 10.1210/jc.2015-2236
7. American College of Obstetricians and Gynecologists. Committee Opinion No 565: hormone therapy and heart disease. Obstet Gynecol. 2013;121:1407-1410. doi: 10.1097/01.AOG.0000431053.33593.2d
EVIDENCE-BASED ANSWER:
NO. In general, nonoral estrogen use for menopausal symptoms is associated with a lower cardiovascular (CV) risk profile than oral estrogen use (strength of recommendation [SOR], B; meta-analysis of cohort studies). Vaginal estrogen use is associated with lower risk for coronary heart disease (CHD) and similar risk for myocardial infarction (MI), stroke, and deep vein thrombosis/pulmonary embolism (DVT/PE) compared with nonuse (SOR, B; cohort studies). Vaginal estrogen therapy also is associated with lower CV-related mortality for 3 to 5 years compared with nonuse (SOR, B; cohort study). No high-quality randomized trials address this topic.
Two biomarkers promising for preeclampsia prediction
Two biomarkers – pregnancy-associated plasma protein A2 (PAPP-A2) and activin A – when added to relevant clinical information have a better positive predictive value than and a comparable negative predictive value to the currently used ratio of soluble fms-like tyrosine kinase 1 (sFlt-1) to placental growth factor (PlGF), new research suggests.
The third-trimester sFlt-1:PlGF ratio can predict short-term absence of preeclampsia. By contrast, , according to the authors.
Preeclampsia has “potentially devastating maternal and fetal complications, [including] significantly increased cardiovascular risk for affected women later in life,” study author Stella S. Daskalopoulou, MD, PhD, associate professor of medicine at McGill University Health Centre in Montreal, said in an interview.
“A more accurate prediction of preeclampsia is expected to improve risk stratification and clinical care and shape clinical practice guidelines,” she said.
The study was published online in the Canadian Journal of Cardiology.
Better predictive value
For a prospective cohort study, the investigators recruited 192 women with first-trimester high-risk singleton pregnancies from tertiary obstetric clinics in Montreal.
At baseline, they collected clinical information, including height, prepregnancy weight, personal and family medical history, and medication use.
At each trimester, blood pressure was measured, and blood samples were collected to quantify sFlt-1, PlGF, PAPP-A2, PAPP-A, activin A, inhibin A, follistatin, and glycosylated fibronectin. For the sFlt-1:PlGF ratio, the researchers used a cutoff point of 38, based on prior evidence. Because there are no agreed-upon cutoff points for the other biomarkers, they chose cutoff points that maximized sensitivity and specificity.
Pregnancies were considered high risk if the mother had any of the following conditions: prepregnancy BMI ≥ 25, maternal age ≥ 35 years, chronic hypertension, diabetes, renal disease, conception via in vitro fertilization, or maternal or first-degree family history of preeclampsia.
The primary outcome was preeclampsia, which was defined according to the Society of Obstetrics and Gynecology guidelines as systolic blood pressure ≥ 140 mm Hg or diastolic blood pressure of ≥ mm Hg together with either proteinuria or maternal end-organ dysfunction.
A total of 18 women (9.38%) developed preeclampsia. Those women had higher blood pressure at baseline (although it was within normal limits) and were more likely to have preexisting diabetes or a previous pregnancy with preeclampsia. They were also more likely to report Black race. Serum levels of PAPP-A, PAPP-A2, activin A, and inhibin A were significantly different between patients who developed preeclampsia and those who did not. These levels were increased throughout pregnancy.
Alongside the sFlt-1:PlGF ratio, two biomarkers, PAPP-A2 (odds ratio, 1.78) and activin A (OR, 1.84), were significantly associated with the primary outcome after adjustment for age, prepregnancy BMI, race, and mean arterial pressure.
When added to a model that included those clinical factors, a positive third-trimester result for both PAPP-A2 and activin A had a better positive predictive value than the sFlt-1:PlGF ratio added to the clinical model (91.67% vs. 66.67%). The two biomarkers also had a negative predictive value that was comparable to that of the sFlt-1:PlGF ratio (97.69% vs. 96%).
Study limitations include the small sample size and missing covariates for some participants. Furthermore, the findings cannot be generalized to low-risk populations.
“Whereas the third-trimester sFlt-1:PlGF ratio can predict short-term absence of preeclampsia, PAPP-A2 and activin A had both high positive and negative predictive values and thus could serve as biomarkers to predict the occurrence (and absence) of preeclampsia; these findings will be validated in future studies,” the authors concluded.
Dr. Daskalopoulou said that her group is currently performing a large multinational study, PULSE, “which will be the ideal platform to validate and extend our findings. The aim of the study is to predict preeclampsia using a multimodal approach that includes arterial stiffness measurements and blood biomarkers.”
She expanded on the potential benefits of this research. “Finding an accurate predictive tool would not only help design appropriate early care plans for truly high-risk pregnant women, including monitoring and delivery planning, but also facilitate the development of novel strategies for the prevention and treatment of preeclampsia, improving the life of millions of young mothers and their offspring around the world.”
Promising biomarkers
Commenting on the study, Nieca Goldberg, MD, clinical associate professor of medicine at NYU Langone Health and medical director of Atria, both in New York, said, “These biomarkers are promising, as the current biomarker, sFlt-1:PlGF, is good at ruling out preeclampsia in the short term, while the new biomarkers show that they are better at ruling in preeclampsia” as well as ruling it out. Dr. Goldberg was not involved in the research.
“The current study is small, some participant data points are missing, and the researchers only studied high-risk pregnancies,” she added. “We need larger studies of all the risk markers, in both high- and low-risk pregnancies that are followed throughout pregnancy.”
This work was supported by the Fonds de recherche du Québec Santé (FRQS), Heart and Stroke Foundation of Canada, McGill University Department of Obstetrics and Gynecology Academic Enrichment Fund, and Canadian Foundation for Women›s Health. Dr. Daskalopoulou is a senior clinician-scientist supported by a FRQS Clinician Scientist-Senior salary award. Dr. Daskalopoulou and Dr. Goldberg disclosed no conflicts of interest.
A version of this article appeared on Medscape.com.
Two biomarkers – pregnancy-associated plasma protein A2 (PAPP-A2) and activin A – when added to relevant clinical information have a better positive predictive value than and a comparable negative predictive value to the currently used ratio of soluble fms-like tyrosine kinase 1 (sFlt-1) to placental growth factor (PlGF), new research suggests.
The third-trimester sFlt-1:PlGF ratio can predict short-term absence of preeclampsia. By contrast, , according to the authors.
Preeclampsia has “potentially devastating maternal and fetal complications, [including] significantly increased cardiovascular risk for affected women later in life,” study author Stella S. Daskalopoulou, MD, PhD, associate professor of medicine at McGill University Health Centre in Montreal, said in an interview.
“A more accurate prediction of preeclampsia is expected to improve risk stratification and clinical care and shape clinical practice guidelines,” she said.
The study was published online in the Canadian Journal of Cardiology.
Better predictive value
For a prospective cohort study, the investigators recruited 192 women with first-trimester high-risk singleton pregnancies from tertiary obstetric clinics in Montreal.
At baseline, they collected clinical information, including height, prepregnancy weight, personal and family medical history, and medication use.
At each trimester, blood pressure was measured, and blood samples were collected to quantify sFlt-1, PlGF, PAPP-A2, PAPP-A, activin A, inhibin A, follistatin, and glycosylated fibronectin. For the sFlt-1:PlGF ratio, the researchers used a cutoff point of 38, based on prior evidence. Because there are no agreed-upon cutoff points for the other biomarkers, they chose cutoff points that maximized sensitivity and specificity.
Pregnancies were considered high risk if the mother had any of the following conditions: prepregnancy BMI ≥ 25, maternal age ≥ 35 years, chronic hypertension, diabetes, renal disease, conception via in vitro fertilization, or maternal or first-degree family history of preeclampsia.
The primary outcome was preeclampsia, which was defined according to the Society of Obstetrics and Gynecology guidelines as systolic blood pressure ≥ 140 mm Hg or diastolic blood pressure of ≥ mm Hg together with either proteinuria or maternal end-organ dysfunction.
A total of 18 women (9.38%) developed preeclampsia. Those women had higher blood pressure at baseline (although it was within normal limits) and were more likely to have preexisting diabetes or a previous pregnancy with preeclampsia. They were also more likely to report Black race. Serum levels of PAPP-A, PAPP-A2, activin A, and inhibin A were significantly different between patients who developed preeclampsia and those who did not. These levels were increased throughout pregnancy.
Alongside the sFlt-1:PlGF ratio, two biomarkers, PAPP-A2 (odds ratio, 1.78) and activin A (OR, 1.84), were significantly associated with the primary outcome after adjustment for age, prepregnancy BMI, race, and mean arterial pressure.
When added to a model that included those clinical factors, a positive third-trimester result for both PAPP-A2 and activin A had a better positive predictive value than the sFlt-1:PlGF ratio added to the clinical model (91.67% vs. 66.67%). The two biomarkers also had a negative predictive value that was comparable to that of the sFlt-1:PlGF ratio (97.69% vs. 96%).
Study limitations include the small sample size and missing covariates for some participants. Furthermore, the findings cannot be generalized to low-risk populations.
“Whereas the third-trimester sFlt-1:PlGF ratio can predict short-term absence of preeclampsia, PAPP-A2 and activin A had both high positive and negative predictive values and thus could serve as biomarkers to predict the occurrence (and absence) of preeclampsia; these findings will be validated in future studies,” the authors concluded.
Dr. Daskalopoulou said that her group is currently performing a large multinational study, PULSE, “which will be the ideal platform to validate and extend our findings. The aim of the study is to predict preeclampsia using a multimodal approach that includes arterial stiffness measurements and blood biomarkers.”
She expanded on the potential benefits of this research. “Finding an accurate predictive tool would not only help design appropriate early care plans for truly high-risk pregnant women, including monitoring and delivery planning, but also facilitate the development of novel strategies for the prevention and treatment of preeclampsia, improving the life of millions of young mothers and their offspring around the world.”
Promising biomarkers
Commenting on the study, Nieca Goldberg, MD, clinical associate professor of medicine at NYU Langone Health and medical director of Atria, both in New York, said, “These biomarkers are promising, as the current biomarker, sFlt-1:PlGF, is good at ruling out preeclampsia in the short term, while the new biomarkers show that they are better at ruling in preeclampsia” as well as ruling it out. Dr. Goldberg was not involved in the research.
“The current study is small, some participant data points are missing, and the researchers only studied high-risk pregnancies,” she added. “We need larger studies of all the risk markers, in both high- and low-risk pregnancies that are followed throughout pregnancy.”
This work was supported by the Fonds de recherche du Québec Santé (FRQS), Heart and Stroke Foundation of Canada, McGill University Department of Obstetrics and Gynecology Academic Enrichment Fund, and Canadian Foundation for Women›s Health. Dr. Daskalopoulou is a senior clinician-scientist supported by a FRQS Clinician Scientist-Senior salary award. Dr. Daskalopoulou and Dr. Goldberg disclosed no conflicts of interest.
A version of this article appeared on Medscape.com.
Two biomarkers – pregnancy-associated plasma protein A2 (PAPP-A2) and activin A – when added to relevant clinical information have a better positive predictive value than and a comparable negative predictive value to the currently used ratio of soluble fms-like tyrosine kinase 1 (sFlt-1) to placental growth factor (PlGF), new research suggests.
The third-trimester sFlt-1:PlGF ratio can predict short-term absence of preeclampsia. By contrast, , according to the authors.
Preeclampsia has “potentially devastating maternal and fetal complications, [including] significantly increased cardiovascular risk for affected women later in life,” study author Stella S. Daskalopoulou, MD, PhD, associate professor of medicine at McGill University Health Centre in Montreal, said in an interview.
“A more accurate prediction of preeclampsia is expected to improve risk stratification and clinical care and shape clinical practice guidelines,” she said.
The study was published online in the Canadian Journal of Cardiology.
Better predictive value
For a prospective cohort study, the investigators recruited 192 women with first-trimester high-risk singleton pregnancies from tertiary obstetric clinics in Montreal.
At baseline, they collected clinical information, including height, prepregnancy weight, personal and family medical history, and medication use.
At each trimester, blood pressure was measured, and blood samples were collected to quantify sFlt-1, PlGF, PAPP-A2, PAPP-A, activin A, inhibin A, follistatin, and glycosylated fibronectin. For the sFlt-1:PlGF ratio, the researchers used a cutoff point of 38, based on prior evidence. Because there are no agreed-upon cutoff points for the other biomarkers, they chose cutoff points that maximized sensitivity and specificity.
Pregnancies were considered high risk if the mother had any of the following conditions: prepregnancy BMI ≥ 25, maternal age ≥ 35 years, chronic hypertension, diabetes, renal disease, conception via in vitro fertilization, or maternal or first-degree family history of preeclampsia.
The primary outcome was preeclampsia, which was defined according to the Society of Obstetrics and Gynecology guidelines as systolic blood pressure ≥ 140 mm Hg or diastolic blood pressure of ≥ mm Hg together with either proteinuria or maternal end-organ dysfunction.
A total of 18 women (9.38%) developed preeclampsia. Those women had higher blood pressure at baseline (although it was within normal limits) and were more likely to have preexisting diabetes or a previous pregnancy with preeclampsia. They were also more likely to report Black race. Serum levels of PAPP-A, PAPP-A2, activin A, and inhibin A were significantly different between patients who developed preeclampsia and those who did not. These levels were increased throughout pregnancy.
Alongside the sFlt-1:PlGF ratio, two biomarkers, PAPP-A2 (odds ratio, 1.78) and activin A (OR, 1.84), were significantly associated with the primary outcome after adjustment for age, prepregnancy BMI, race, and mean arterial pressure.
When added to a model that included those clinical factors, a positive third-trimester result for both PAPP-A2 and activin A had a better positive predictive value than the sFlt-1:PlGF ratio added to the clinical model (91.67% vs. 66.67%). The two biomarkers also had a negative predictive value that was comparable to that of the sFlt-1:PlGF ratio (97.69% vs. 96%).
Study limitations include the small sample size and missing covariates for some participants. Furthermore, the findings cannot be generalized to low-risk populations.
“Whereas the third-trimester sFlt-1:PlGF ratio can predict short-term absence of preeclampsia, PAPP-A2 and activin A had both high positive and negative predictive values and thus could serve as biomarkers to predict the occurrence (and absence) of preeclampsia; these findings will be validated in future studies,” the authors concluded.
Dr. Daskalopoulou said that her group is currently performing a large multinational study, PULSE, “which will be the ideal platform to validate and extend our findings. The aim of the study is to predict preeclampsia using a multimodal approach that includes arterial stiffness measurements and blood biomarkers.”
She expanded on the potential benefits of this research. “Finding an accurate predictive tool would not only help design appropriate early care plans for truly high-risk pregnant women, including monitoring and delivery planning, but also facilitate the development of novel strategies for the prevention and treatment of preeclampsia, improving the life of millions of young mothers and their offspring around the world.”
Promising biomarkers
Commenting on the study, Nieca Goldberg, MD, clinical associate professor of medicine at NYU Langone Health and medical director of Atria, both in New York, said, “These biomarkers are promising, as the current biomarker, sFlt-1:PlGF, is good at ruling out preeclampsia in the short term, while the new biomarkers show that they are better at ruling in preeclampsia” as well as ruling it out. Dr. Goldberg was not involved in the research.
“The current study is small, some participant data points are missing, and the researchers only studied high-risk pregnancies,” she added. “We need larger studies of all the risk markers, in both high- and low-risk pregnancies that are followed throughout pregnancy.”
This work was supported by the Fonds de recherche du Québec Santé (FRQS), Heart and Stroke Foundation of Canada, McGill University Department of Obstetrics and Gynecology Academic Enrichment Fund, and Canadian Foundation for Women›s Health. Dr. Daskalopoulou is a senior clinician-scientist supported by a FRQS Clinician Scientist-Senior salary award. Dr. Daskalopoulou and Dr. Goldberg disclosed no conflicts of interest.
A version of this article appeared on Medscape.com.
FROM THE CANADIAN JOURNAL OF CARDIOLOGY
AI algorithm aids egg retrieval date during fertility treatment cycles
According to the researchers, such an algorithm is needed due to the increased demand for fertility treatments, as well as the high day-to-day variability in lab workload.
According to the study investigators, predicting retrieval dates in advance for ongoing cycles is of major importance for both patients and clinicians.
“The population requiring fertility treatments, including genetic testing and fertility preservation, has massively increased, and this causes many more cycles and a high day-to-day variability in IVF activity, especially in the lab workload,” said Rohi Hourvitz, MBA, from FertilAI, an Israeli health care company focused on developing technologies that improve fertility treatments.
“We also need to accommodate and reschedule for non-working days, which causes a big issue with managing the workload in many clinics around the world,” added Mr. Hourvitz, who presented the research highlighting AI’s growing role in reproductive medicine.
In addition, AI has recently emerged as an effective tool for assisting in clinical decision-making in assisted reproductive technology, prompting further research in this space, he said.
The new study used a dataset of 9,550 predictable antagonist cycles (defined as having all necessary data) gathered from one lab with over 50 physicians between August 2018 and October 2022. The data were split into two subsets: one for training the AI model and the other for prospective testing.
To train and test the AI model, data from nearly 6,000 predictable antagonist cycles were used. Key factors used for each cycle included estrogen levels, mean follicle size, primary follicle size, and various patient demographics. Other features were considered, but Mr. Hourvitz noted that primary follicle size influenced the algorithm most, “because that is what most of us use when we want to trigger.”
Mr. Hourvitz explained that these patient data were run through an algorithm that produced a graph predicting the most probable date for a cycle retrieval.
“We could accurately predict when those ‘peak days’ were going to be happening in the clinic, and we could also give a pretty good estimate on how many cycles you’re going to have every day,” Mr. Hourvitz said, explaining that this information could help clinics more efficiently allocate resources and manage patients.
According to Mr. Hourvitz, the predictions derived from this study could improve various aspects of fertility treatments and related procedures, including better staff planning and caseload management in IVF labs, as well as higher-quality eggs at retrieval. Patients would have a clearer timeline for their treatment cycles.
Nikica Zaninovic, PhD, MS, director of the embryology lab at Weill Cornell Medical College, New York City, cautioned that the new findings are not yet ready for clinical application but emphasized the importance of more AI research focusing on the quality of oocytes, not only embryos.
“We’re so focused on the end of the process: the embryo,” Dr. Zaninovic, who was not involved in the research, said in an interview. “I think the focus should be on the beginning – the quality of eggs and sperm, not just the quantity – because that’s what the embryos will depend on.”
He noted the increasing numbers of young women in the United States undergoing egg freezing.
“Cornell is the largest academic IVF center in the United States; 20%-30% of all of the patients that we treat are actually freezing their eggs,” he said. “It’s a huge population.”
“When they come to us, they ask how many eggs they’ll need to guarantee one or two children in the future,” Dr. Zaninovic continued. “We don’t have that answer, so we always tell them [we’ll retrieve] as many as we can. That’s not the answer; we need to be more precise. We’re still lacking these tools, and I think that’s where the research will go.”
The study was funded by FertilAI. Mr. Hourvitz is a shareholder and CEO of FertilAI. Dr. Zaninovic is president of the AI Fertility Society.
A version of this article appeared on Medscape.com.
According to the researchers, such an algorithm is needed due to the increased demand for fertility treatments, as well as the high day-to-day variability in lab workload.
According to the study investigators, predicting retrieval dates in advance for ongoing cycles is of major importance for both patients and clinicians.
“The population requiring fertility treatments, including genetic testing and fertility preservation, has massively increased, and this causes many more cycles and a high day-to-day variability in IVF activity, especially in the lab workload,” said Rohi Hourvitz, MBA, from FertilAI, an Israeli health care company focused on developing technologies that improve fertility treatments.
“We also need to accommodate and reschedule for non-working days, which causes a big issue with managing the workload in many clinics around the world,” added Mr. Hourvitz, who presented the research highlighting AI’s growing role in reproductive medicine.
In addition, AI has recently emerged as an effective tool for assisting in clinical decision-making in assisted reproductive technology, prompting further research in this space, he said.
The new study used a dataset of 9,550 predictable antagonist cycles (defined as having all necessary data) gathered from one lab with over 50 physicians between August 2018 and October 2022. The data were split into two subsets: one for training the AI model and the other for prospective testing.
To train and test the AI model, data from nearly 6,000 predictable antagonist cycles were used. Key factors used for each cycle included estrogen levels, mean follicle size, primary follicle size, and various patient demographics. Other features were considered, but Mr. Hourvitz noted that primary follicle size influenced the algorithm most, “because that is what most of us use when we want to trigger.”
Mr. Hourvitz explained that these patient data were run through an algorithm that produced a graph predicting the most probable date for a cycle retrieval.
“We could accurately predict when those ‘peak days’ were going to be happening in the clinic, and we could also give a pretty good estimate on how many cycles you’re going to have every day,” Mr. Hourvitz said, explaining that this information could help clinics more efficiently allocate resources and manage patients.
According to Mr. Hourvitz, the predictions derived from this study could improve various aspects of fertility treatments and related procedures, including better staff planning and caseload management in IVF labs, as well as higher-quality eggs at retrieval. Patients would have a clearer timeline for their treatment cycles.
Nikica Zaninovic, PhD, MS, director of the embryology lab at Weill Cornell Medical College, New York City, cautioned that the new findings are not yet ready for clinical application but emphasized the importance of more AI research focusing on the quality of oocytes, not only embryos.
“We’re so focused on the end of the process: the embryo,” Dr. Zaninovic, who was not involved in the research, said in an interview. “I think the focus should be on the beginning – the quality of eggs and sperm, not just the quantity – because that’s what the embryos will depend on.”
He noted the increasing numbers of young women in the United States undergoing egg freezing.
“Cornell is the largest academic IVF center in the United States; 20%-30% of all of the patients that we treat are actually freezing their eggs,” he said. “It’s a huge population.”
“When they come to us, they ask how many eggs they’ll need to guarantee one or two children in the future,” Dr. Zaninovic continued. “We don’t have that answer, so we always tell them [we’ll retrieve] as many as we can. That’s not the answer; we need to be more precise. We’re still lacking these tools, and I think that’s where the research will go.”
The study was funded by FertilAI. Mr. Hourvitz is a shareholder and CEO of FertilAI. Dr. Zaninovic is president of the AI Fertility Society.
A version of this article appeared on Medscape.com.
According to the researchers, such an algorithm is needed due to the increased demand for fertility treatments, as well as the high day-to-day variability in lab workload.
According to the study investigators, predicting retrieval dates in advance for ongoing cycles is of major importance for both patients and clinicians.
“The population requiring fertility treatments, including genetic testing and fertility preservation, has massively increased, and this causes many more cycles and a high day-to-day variability in IVF activity, especially in the lab workload,” said Rohi Hourvitz, MBA, from FertilAI, an Israeli health care company focused on developing technologies that improve fertility treatments.
“We also need to accommodate and reschedule for non-working days, which causes a big issue with managing the workload in many clinics around the world,” added Mr. Hourvitz, who presented the research highlighting AI’s growing role in reproductive medicine.
In addition, AI has recently emerged as an effective tool for assisting in clinical decision-making in assisted reproductive technology, prompting further research in this space, he said.
The new study used a dataset of 9,550 predictable antagonist cycles (defined as having all necessary data) gathered from one lab with over 50 physicians between August 2018 and October 2022. The data were split into two subsets: one for training the AI model and the other for prospective testing.
To train and test the AI model, data from nearly 6,000 predictable antagonist cycles were used. Key factors used for each cycle included estrogen levels, mean follicle size, primary follicle size, and various patient demographics. Other features were considered, but Mr. Hourvitz noted that primary follicle size influenced the algorithm most, “because that is what most of us use when we want to trigger.”
Mr. Hourvitz explained that these patient data were run through an algorithm that produced a graph predicting the most probable date for a cycle retrieval.
“We could accurately predict when those ‘peak days’ were going to be happening in the clinic, and we could also give a pretty good estimate on how many cycles you’re going to have every day,” Mr. Hourvitz said, explaining that this information could help clinics more efficiently allocate resources and manage patients.
According to Mr. Hourvitz, the predictions derived from this study could improve various aspects of fertility treatments and related procedures, including better staff planning and caseload management in IVF labs, as well as higher-quality eggs at retrieval. Patients would have a clearer timeline for their treatment cycles.
Nikica Zaninovic, PhD, MS, director of the embryology lab at Weill Cornell Medical College, New York City, cautioned that the new findings are not yet ready for clinical application but emphasized the importance of more AI research focusing on the quality of oocytes, not only embryos.
“We’re so focused on the end of the process: the embryo,” Dr. Zaninovic, who was not involved in the research, said in an interview. “I think the focus should be on the beginning – the quality of eggs and sperm, not just the quantity – because that’s what the embryos will depend on.”
He noted the increasing numbers of young women in the United States undergoing egg freezing.
“Cornell is the largest academic IVF center in the United States; 20%-30% of all of the patients that we treat are actually freezing their eggs,” he said. “It’s a huge population.”
“When they come to us, they ask how many eggs they’ll need to guarantee one or two children in the future,” Dr. Zaninovic continued. “We don’t have that answer, so we always tell them [we’ll retrieve] as many as we can. That’s not the answer; we need to be more precise. We’re still lacking these tools, and I think that’s where the research will go.”
The study was funded by FertilAI. Mr. Hourvitz is a shareholder and CEO of FertilAI. Dr. Zaninovic is president of the AI Fertility Society.
A version of this article appeared on Medscape.com.
FROM ASRM 2023
Hypertensive disorders of pregnancy and high stroke risk in Black women
I’d like to talk with you about a recent report from the large-scale Black Women’s Health Study, published in the new journal NEJM Evidence.
This study looked at the association between hypertensive disorders of pregnancy, including preeclampsia and gestational hypertension, and the risk for stroke over the next 20 (median, 22) years. Previous studies have linked hypertensive disorders of pregnancy with an increased risk for stroke. However, most of these studies have been done in White women of European ancestry, and evidence in Black women has been very limited, despite a disproportionately high risk of having a hypertensive disorder of pregnancy and also of stroke.
– overall, a 66% increased risk, an 80% increased risk with gestational hypertension, and about a 50% increased risk with preeclampsia.
We know that pregnancy itself can lead to some remodeling of the vascular system, but we don’t know whether a direct causal relationship exists between preeclampsia or gestational hypertension and subsequent stroke. Another potential explanation is that these complications of pregnancy serve as a window into a woman’s future cardiometabolic health and a marker of her cardiovascular risk.
Regardless, the clinical implications are the same. First, we would want to prevent these complications of pregnancy whenever possible. Some women will be candidates for the use of aspirin if they are at high risk for preeclampsia, and certainly for monitoring blood pressure very closely during pregnancy. It will also be important to maintain blood pressure control in the postpartum period and during the subsequent years of adulthood to minimize risk for stroke, because hypertension is such a powerful risk factor for stroke.
It will also be tremendously important to intensify lifestyle modifications such as increasing physical activity and having a heart-healthy diet. These complications of pregnancy have also been linked in other studies to an increased risk for subsequent coronary heart disease events and heart failure.
This transcript has been edited for clarity.
Dr. Manson is professor of medicine and the Michael and Lee Bell Professor of Women’s Health, Harvard Medical School, and chief of the division of preventive medicine, Brigham and Women’s Hospital, both in Boston, and past president, North American Menopause Society, 2011-2012. She disclosed receiving study pill donation and infrastructure support from Mars Symbioscience (for the COSMOS trial).
A version of this article appeared on Medscape.com.
I’d like to talk with you about a recent report from the large-scale Black Women’s Health Study, published in the new journal NEJM Evidence.
This study looked at the association between hypertensive disorders of pregnancy, including preeclampsia and gestational hypertension, and the risk for stroke over the next 20 (median, 22) years. Previous studies have linked hypertensive disorders of pregnancy with an increased risk for stroke. However, most of these studies have been done in White women of European ancestry, and evidence in Black women has been very limited, despite a disproportionately high risk of having a hypertensive disorder of pregnancy and also of stroke.
– overall, a 66% increased risk, an 80% increased risk with gestational hypertension, and about a 50% increased risk with preeclampsia.
We know that pregnancy itself can lead to some remodeling of the vascular system, but we don’t know whether a direct causal relationship exists between preeclampsia or gestational hypertension and subsequent stroke. Another potential explanation is that these complications of pregnancy serve as a window into a woman’s future cardiometabolic health and a marker of her cardiovascular risk.
Regardless, the clinical implications are the same. First, we would want to prevent these complications of pregnancy whenever possible. Some women will be candidates for the use of aspirin if they are at high risk for preeclampsia, and certainly for monitoring blood pressure very closely during pregnancy. It will also be important to maintain blood pressure control in the postpartum period and during the subsequent years of adulthood to minimize risk for stroke, because hypertension is such a powerful risk factor for stroke.
It will also be tremendously important to intensify lifestyle modifications such as increasing physical activity and having a heart-healthy diet. These complications of pregnancy have also been linked in other studies to an increased risk for subsequent coronary heart disease events and heart failure.
This transcript has been edited for clarity.
Dr. Manson is professor of medicine and the Michael and Lee Bell Professor of Women’s Health, Harvard Medical School, and chief of the division of preventive medicine, Brigham and Women’s Hospital, both in Boston, and past president, North American Menopause Society, 2011-2012. She disclosed receiving study pill donation and infrastructure support from Mars Symbioscience (for the COSMOS trial).
A version of this article appeared on Medscape.com.
I’d like to talk with you about a recent report from the large-scale Black Women’s Health Study, published in the new journal NEJM Evidence.
This study looked at the association between hypertensive disorders of pregnancy, including preeclampsia and gestational hypertension, and the risk for stroke over the next 20 (median, 22) years. Previous studies have linked hypertensive disorders of pregnancy with an increased risk for stroke. However, most of these studies have been done in White women of European ancestry, and evidence in Black women has been very limited, despite a disproportionately high risk of having a hypertensive disorder of pregnancy and also of stroke.
– overall, a 66% increased risk, an 80% increased risk with gestational hypertension, and about a 50% increased risk with preeclampsia.
We know that pregnancy itself can lead to some remodeling of the vascular system, but we don’t know whether a direct causal relationship exists between preeclampsia or gestational hypertension and subsequent stroke. Another potential explanation is that these complications of pregnancy serve as a window into a woman’s future cardiometabolic health and a marker of her cardiovascular risk.
Regardless, the clinical implications are the same. First, we would want to prevent these complications of pregnancy whenever possible. Some women will be candidates for the use of aspirin if they are at high risk for preeclampsia, and certainly for monitoring blood pressure very closely during pregnancy. It will also be important to maintain blood pressure control in the postpartum period and during the subsequent years of adulthood to minimize risk for stroke, because hypertension is such a powerful risk factor for stroke.
It will also be tremendously important to intensify lifestyle modifications such as increasing physical activity and having a heart-healthy diet. These complications of pregnancy have also been linked in other studies to an increased risk for subsequent coronary heart disease events and heart failure.
This transcript has been edited for clarity.
Dr. Manson is professor of medicine and the Michael and Lee Bell Professor of Women’s Health, Harvard Medical School, and chief of the division of preventive medicine, Brigham and Women’s Hospital, both in Boston, and past president, North American Menopause Society, 2011-2012. She disclosed receiving study pill donation and infrastructure support from Mars Symbioscience (for the COSMOS trial).
A version of this article appeared on Medscape.com.
Digital tool clarifies menopause symptoms
GLASGOW – An interactive digital decision tool that individualizes menopause care received praise from primary care clinicians in the United Kingdom, who said it could improve patient care and streamline office visits.
“Access to hormone replacement therapy [HRT], as well as decision-making around treatment for menopausal symptoms, is often complicated by concerns around its safety, and there is still a knowledge and a confidence gap among health care professionals causing reluctance to prescribe HRT,” said Aini Kamal, MSc, from University College London. Ms. Kamal presented results of a survey about the tool at the annual meeting of the Royal College of General Practitioners.
For the study, Ms. Kamal, Daniel Reisel, MBBS, PhD, a gynecologist at UCL, and colleagues evaluated Wellspring with doctors, nurses, and pharmacists.
“Ensuring that women receive education around symptoms, so that they are empowered, is a key part of optimizing their care and sharing decision-making,” Dr. Reisel said in an interview. He added that U.K. primary care had seen an increase in cases of women presenting with symptoms associated with the perimenopause and menopause at a time when U.K. Members of Parliament are debating whether to make it mandatory for all women to have menopause check-up in their early 40s.
The online survey was completed by 280 participants, and respondents were primarily GPs with several years of relevant prescribing practice. Of those, 93% found information from national guidelines to be accurately presented in the tool, and 97% said they would recommend this decision aid to other health care professionals, Ms. Kamal reported.
Nearly all participants said they could see themselves using the tool with patients in the clinic or as an adjunct to virtual sessions. “This [finding] was particularly important because it demonstrates the clinical potential this tool has,” she said.
One consult, too many problems
Louise Newson, MBChB, who runs the U.K.’s largest menopause clinic, said primary care appointments are often time-pressured and follow a “’one problem-one consultation’” policy. As such, women are often thinking ‘Do I go with my joint pains, or my palpitations, tinnitus, or what?’ If a patient presents with tinnitus, a doctor might focus on the potential of an inner ear problem rather than a hormone deficiency, but I do know that if the woman is perimenopausal or menopausal, we often look to replace the missing hormones, and then if the tinnitus doesn’t improve we can revisit the ear problem.”
Dr. Newson noted that 17% of women in her clinics have had more than six GP visits in the year before she sees them, but in the year following, this figure drops to 1%. Acknowledging that a menopause consultation for a GP is time-consuming, Dr. Newson pointed out that taking time initially with the patient “means it will reduce the number of future consultations quickly, but more importantly, we also know that taking HRT reduces long-term risk of serious diseases, including heart disease and osteoporosis.”
The digital tool can be used by both doctors and patients to help women work through their symptoms and equip them with knowledge so their GP visits are more productive.
“When we see women who are empowered with knowledge [about menopause symptoms], then the consultations are quicker and essentially place the patient central to the discussion,” Dr. Newson said.
Ed Russell-Smith, MBChB, a GP in Scotland who moderated the session, said the tool “lays out a nicely structured approach and provides modern treatment options and resources for patients.”
However, he added “we also need to remember there are potential harms to be done from HRT too. It’s vitally important that while patients might see HRT as a panacea, doctors need to balance this with the risks involved for each individual. As a tool, I think Wellspring can help us in this respect to apply general principles to that patient and individualize treatment.”
Dr. Reisel, Dr. Newson, Ms. Kamal, and Dr. Russell-Smith disclosed no relevant financial relationships. The Wellspring Decision Aid was supported by UCL’s Institute for Women’s Health. The Newson Health clinic is fully private, but research is done via the nonprofit arm, which is supported by the clinic. There is no pharma involvement.
A version of this article first appeared on Medscape.com.
GLASGOW – An interactive digital decision tool that individualizes menopause care received praise from primary care clinicians in the United Kingdom, who said it could improve patient care and streamline office visits.
“Access to hormone replacement therapy [HRT], as well as decision-making around treatment for menopausal symptoms, is often complicated by concerns around its safety, and there is still a knowledge and a confidence gap among health care professionals causing reluctance to prescribe HRT,” said Aini Kamal, MSc, from University College London. Ms. Kamal presented results of a survey about the tool at the annual meeting of the Royal College of General Practitioners.
For the study, Ms. Kamal, Daniel Reisel, MBBS, PhD, a gynecologist at UCL, and colleagues evaluated Wellspring with doctors, nurses, and pharmacists.
“Ensuring that women receive education around symptoms, so that they are empowered, is a key part of optimizing their care and sharing decision-making,” Dr. Reisel said in an interview. He added that U.K. primary care had seen an increase in cases of women presenting with symptoms associated with the perimenopause and menopause at a time when U.K. Members of Parliament are debating whether to make it mandatory for all women to have menopause check-up in their early 40s.
The online survey was completed by 280 participants, and respondents were primarily GPs with several years of relevant prescribing practice. Of those, 93% found information from national guidelines to be accurately presented in the tool, and 97% said they would recommend this decision aid to other health care professionals, Ms. Kamal reported.
Nearly all participants said they could see themselves using the tool with patients in the clinic or as an adjunct to virtual sessions. “This [finding] was particularly important because it demonstrates the clinical potential this tool has,” she said.
One consult, too many problems
Louise Newson, MBChB, who runs the U.K.’s largest menopause clinic, said primary care appointments are often time-pressured and follow a “’one problem-one consultation’” policy. As such, women are often thinking ‘Do I go with my joint pains, or my palpitations, tinnitus, or what?’ If a patient presents with tinnitus, a doctor might focus on the potential of an inner ear problem rather than a hormone deficiency, but I do know that if the woman is perimenopausal or menopausal, we often look to replace the missing hormones, and then if the tinnitus doesn’t improve we can revisit the ear problem.”
Dr. Newson noted that 17% of women in her clinics have had more than six GP visits in the year before she sees them, but in the year following, this figure drops to 1%. Acknowledging that a menopause consultation for a GP is time-consuming, Dr. Newson pointed out that taking time initially with the patient “means it will reduce the number of future consultations quickly, but more importantly, we also know that taking HRT reduces long-term risk of serious diseases, including heart disease and osteoporosis.”
The digital tool can be used by both doctors and patients to help women work through their symptoms and equip them with knowledge so their GP visits are more productive.
“When we see women who are empowered with knowledge [about menopause symptoms], then the consultations are quicker and essentially place the patient central to the discussion,” Dr. Newson said.
Ed Russell-Smith, MBChB, a GP in Scotland who moderated the session, said the tool “lays out a nicely structured approach and provides modern treatment options and resources for patients.”
However, he added “we also need to remember there are potential harms to be done from HRT too. It’s vitally important that while patients might see HRT as a panacea, doctors need to balance this with the risks involved for each individual. As a tool, I think Wellspring can help us in this respect to apply general principles to that patient and individualize treatment.”
Dr. Reisel, Dr. Newson, Ms. Kamal, and Dr. Russell-Smith disclosed no relevant financial relationships. The Wellspring Decision Aid was supported by UCL’s Institute for Women’s Health. The Newson Health clinic is fully private, but research is done via the nonprofit arm, which is supported by the clinic. There is no pharma involvement.
A version of this article first appeared on Medscape.com.
GLASGOW – An interactive digital decision tool that individualizes menopause care received praise from primary care clinicians in the United Kingdom, who said it could improve patient care and streamline office visits.
“Access to hormone replacement therapy [HRT], as well as decision-making around treatment for menopausal symptoms, is often complicated by concerns around its safety, and there is still a knowledge and a confidence gap among health care professionals causing reluctance to prescribe HRT,” said Aini Kamal, MSc, from University College London. Ms. Kamal presented results of a survey about the tool at the annual meeting of the Royal College of General Practitioners.
For the study, Ms. Kamal, Daniel Reisel, MBBS, PhD, a gynecologist at UCL, and colleagues evaluated Wellspring with doctors, nurses, and pharmacists.
“Ensuring that women receive education around symptoms, so that they are empowered, is a key part of optimizing their care and sharing decision-making,” Dr. Reisel said in an interview. He added that U.K. primary care had seen an increase in cases of women presenting with symptoms associated with the perimenopause and menopause at a time when U.K. Members of Parliament are debating whether to make it mandatory for all women to have menopause check-up in their early 40s.
The online survey was completed by 280 participants, and respondents were primarily GPs with several years of relevant prescribing practice. Of those, 93% found information from national guidelines to be accurately presented in the tool, and 97% said they would recommend this decision aid to other health care professionals, Ms. Kamal reported.
Nearly all participants said they could see themselves using the tool with patients in the clinic or as an adjunct to virtual sessions. “This [finding] was particularly important because it demonstrates the clinical potential this tool has,” she said.
One consult, too many problems
Louise Newson, MBChB, who runs the U.K.’s largest menopause clinic, said primary care appointments are often time-pressured and follow a “’one problem-one consultation’” policy. As such, women are often thinking ‘Do I go with my joint pains, or my palpitations, tinnitus, or what?’ If a patient presents with tinnitus, a doctor might focus on the potential of an inner ear problem rather than a hormone deficiency, but I do know that if the woman is perimenopausal or menopausal, we often look to replace the missing hormones, and then if the tinnitus doesn’t improve we can revisit the ear problem.”
Dr. Newson noted that 17% of women in her clinics have had more than six GP visits in the year before she sees them, but in the year following, this figure drops to 1%. Acknowledging that a menopause consultation for a GP is time-consuming, Dr. Newson pointed out that taking time initially with the patient “means it will reduce the number of future consultations quickly, but more importantly, we also know that taking HRT reduces long-term risk of serious diseases, including heart disease and osteoporosis.”
The digital tool can be used by both doctors and patients to help women work through their symptoms and equip them with knowledge so their GP visits are more productive.
“When we see women who are empowered with knowledge [about menopause symptoms], then the consultations are quicker and essentially place the patient central to the discussion,” Dr. Newson said.
Ed Russell-Smith, MBChB, a GP in Scotland who moderated the session, said the tool “lays out a nicely structured approach and provides modern treatment options and resources for patients.”
However, he added “we also need to remember there are potential harms to be done from HRT too. It’s vitally important that while patients might see HRT as a panacea, doctors need to balance this with the risks involved for each individual. As a tool, I think Wellspring can help us in this respect to apply general principles to that patient and individualize treatment.”
Dr. Reisel, Dr. Newson, Ms. Kamal, and Dr. Russell-Smith disclosed no relevant financial relationships. The Wellspring Decision Aid was supported by UCL’s Institute for Women’s Health. The Newson Health clinic is fully private, but research is done via the nonprofit arm, which is supported by the clinic. There is no pharma involvement.
A version of this article first appeared on Medscape.com.
AT RCGP 2023