User login
Cutaneous Id Reaction After Using Cyanoacrylate for Wound Closure
To the Editor:
In 1998, 2-octyl-cyanoacrylate (2-CA) tissue adhesive gained US Food and Drug Administration approval for topical application to easily hold closed approximated skin edges from surgical excisions and simple trauma-induced lacerations.1 It has since been employed for a number of off-label indications, including sutureless circumcision,2 skin graft fixation,3 pericatheter leakage,4 and intracorporeal use to control air leaks during lung resection.5 Animal investigations additionally have attempted to elucidate potential future uses of 2-CA for procedures such as inguinal hernia repair,6 bowel anastomosis,7 incisional hernia repair with mesh,8 and microvascular anastomosis.9 Compared to sutures, 2-CA offers ease and rapidity of application, a water-resistant barrier, and equivalent cosmetic results, as well as eliminates the need for suture removal.10 As 2-CA is used with increasing frequency across a variety of settings, there arises a greater need to be mindful of the potential complications of its use, such as irritant contact dermatitis (ICD), allergic contact dermatitis (ACD), and cutaneous id reaction.
A 14-year-old adolescent boy with no notable medical history and no known allergies underwent a minimally invasive Nuss procedure11 (performed by P.L.G.) for the repair of severe pectus excavatum. Two 4-cm incisions were made—one in each lateral chest wall at the approximately eighth intercostal space—to facilitate the introduction of the Nuss bar. The surgical wounds were closed with 2 layers of running polyglactin 910 suture before 2-CA was applied topically to the incision sites. The surgery was well tolerated, and the patient’s wounds healed without incident. When the patient was evaluated for Nuss bar removal 3 years later, incision sites were noted to be well healed, and he exhibited no other skin lesions. The original incision sites (bilateral chest walls) were utilized to facilitate surgical Nuss bar removal. The wounds were closed in 4 layers and 2-CA was again applied topically to the incision sites. There were no intraoperative complications; no devices, drains, or tissue implants were left in the patient at the conclusion of the procedure.
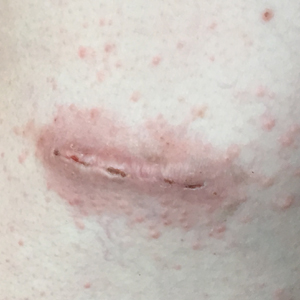
One week later, via text message and digital photographs, the patient reported intense pruritus at the bilateral chest wall incision sites, which were now surrounded by symmetric 1-cm erythematous plaques and associated sparse erythematous satellite papules (Figure 1). The patient denied any fevers, pain, swelling, or purulent discharge from the wounds. He was started on hydrocortisone cream 1% twice daily as well as oral diphenhydramine 25 mg at bedtime with initial good effect.
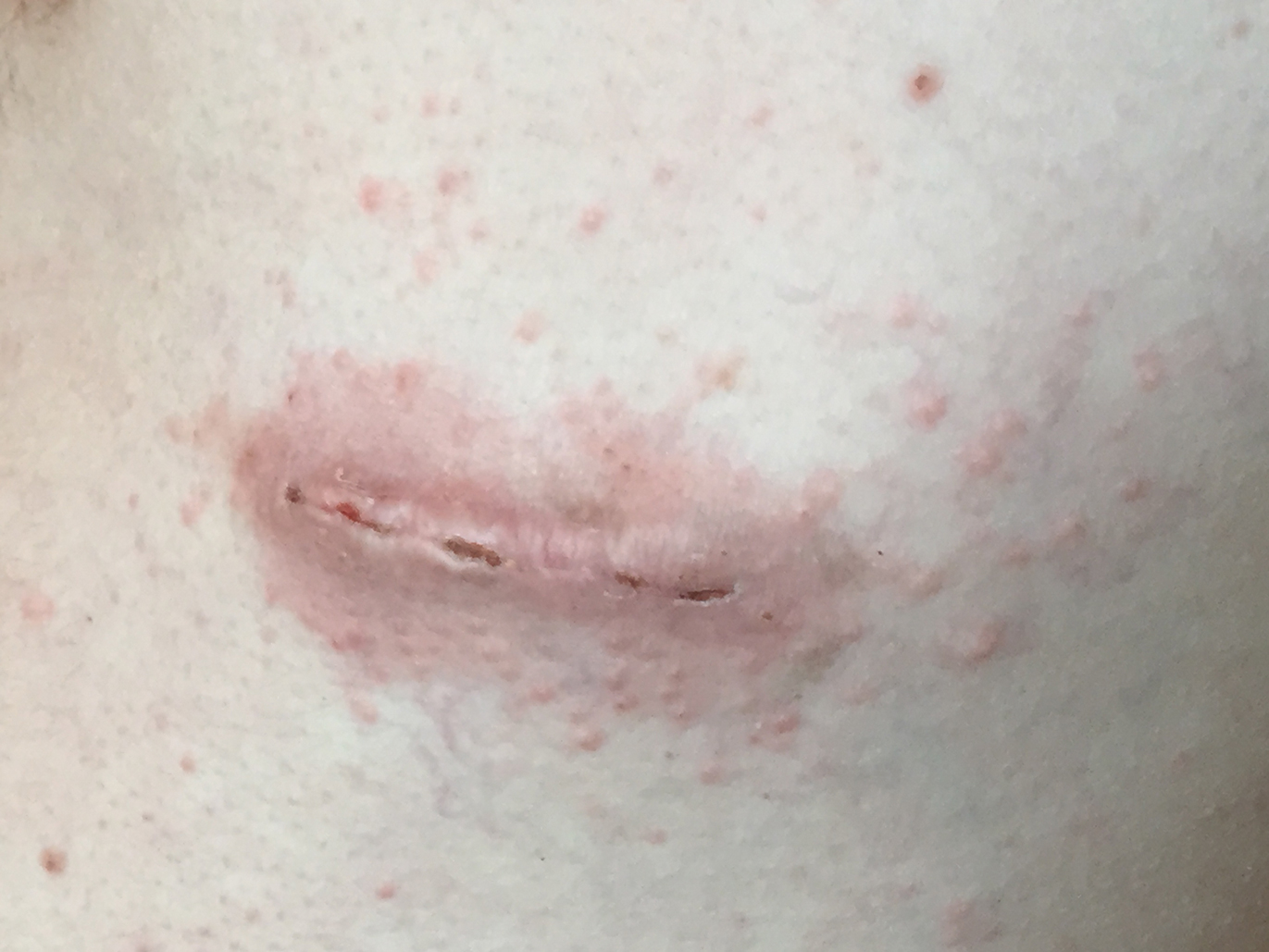
Three days later, the patient sent digital photographs of a morphologically similar–appearing rash that had progressed beyond the lateral chest walls to include the central chest and bilateral upper and lower extremities (Figure 2). He continued to deny any local or systemic signs of infection. Dermatology was consulted, and a diagnosis of ACD with cutaneous id reaction was made. The patient’s medication regimen was modified to include triamcinolone acetonide cream 0.1% applied twice daily to the rash away from the wounds, clobetasol propionate ointment 0.05% applied twice daily to the rash at the wound sites, oral levocetirizine 5 mg once daily, and oral hydroxyzine 25 to 50 mg every 6 hours as needed for pruritus. Additional recommendations included the use of a fragrance-free soap and application of an over-the-counter anti-itch lotion containing menthol and camphor applied as needed. Within 24 hours of starting this modified treatment regimen, the patient began to notice an improvement in symptoms, with full resolution over the course of the ensuing 2 weeks. The patient was counseled to inform his physicians—present and future—of his allergy to 2-CA.

Contact dermatitis associated with the use of 2-CA has been described in the literature.12-15 We report progression to an id reaction, which is characterized by the diffuse symmetric spread of a cutaneous eruption at a site distant from the primary localized dermatitis that develops within a few days of the primary lesion and exhibits the same morphologic and histopathologic findings.16,17 In our patient, pruritic erythematous papules and plaques symmetrically distributed on the arms, legs, and chest appeared 3 days after he first reported a similar eruption at the 2-CA application sites. It is theorized that id reactions develop when the sensitization phase of a type IV hypersensitivity reaction generates a population of T cells that not only recognizes a hapten but also recognizes keratinocyte-derived epitopes.16 A hapten is a small molecule (<500 Da) that is capable of penetrating the stratum corneum and binding skin components. A contact allergen is a hapten that has bound epidermal proteins to create a new antigenic determinant.18 The secondary dermatitis that characterizes id reactions results from an abnormal autoimmune response. Id reactions associated with exposure to adhesive material are rare.19
Allergic contact dermatitis is a type IV hypersensitivity reaction that appears after initial sensitization to an allergen followed by re-exposure. Our patient presented with symmetric erythematous plaques at the surgical incision sites 1 week after 2-CA had been applied. During this interval, sensitization to the inciting allergen occurred. The allergen is taken up by antigen-presenting cells, which then migrate to lymph nodes where they encounter naïve T lymphocytes that subsequently undergo clonal expansion to produce a cohort of T cells that are capable of recognizing the allergen. If subsequent exposure to the specific allergen takes place, an elicitation phase occurs in which primed T cells are incited to release mediators of inflammation that engender the manifestations of ACD within 24 to 72 hours.18,20 Sensitization may be promoted by skin barrier impairments such as dermatitis or a frank wound.12,20 In most cases, the patient is unaware that sensitization has occurred, though a primary ACD within 5 to 15 days after initial exposure to the inciting allergen rarely may be observed.18 Although our patient had 2-CA applied to his surgical wounds at 14 years of age, it was unlikely that sensitization took place at that time, as it was 1 week rather than 1 to 3 days before he experienced the cutaneous eruption associated with his second 2-CA exposure at 17 years of age.
Cyanoacrylate tissue adhesive also may cause ICD resulting from histotoxic degradation products such as formaldehyde and cyanoacetate that are capable of compromising cutaneous barrier function. Keratinocytes that have had their membranes disturbed release proinflammatory cytokines, which recruit cells of the innate immune system as well as T lymphocytes to the site of insult to facilitate the inflammatory response. The manifestations of ICD include erythema, edema, and local necrosis that can compromise wound healing.20 The speed at which a given cyanoacrylate adhesive degrades is proportional to the length of its carbon side chain. Those with shorter side chains—ethyl and methyl cyanoacrylate—degrade more rapidly into formaldehyde and cyanoacetate; 2-CA possesses a longer side chain and therefore degrades more slowly, which should, in theory, lessen its potential to cause ICD.20 Because it may take 7 to 14 days before 2-CA will spontaneously peel from the application site, however, its potential to evoke ICD nevertheless exists.
Treatment of ICD entails removing the irritant while concurrently working to restore the skin’s barrier with emollients. Although topical corticosteroids often are reflexively prescribed to treat rashes, some believe that their use should be avoided in cases of ICD, as their inhibitory effects on epidermal lipid synthesis may further impair the skin’s barrier.21 For cases of ACD, with or without an accompanying id reaction, topical corticosteroids are the mainstay of therapy. It is customary to start with a higher-potency topical steroid such as clobetasol and taper to lower-potency steroids as the patient’s condition improves. Steroid ointments are petroleum based and are capable of causing 2-CA to separate from the skin.10 As a result, they should be used with care when being applied to an area where 2-CA is maintaining dermal closure. Systemic corticosteroids may be warranted in cases with involvement of more than 20% of the body surface area and should start to provide relief within 12 to 24 hours.22 Oral antihistamines and cold water compresses can be added to help address pruritus and discomfort in both ACD and ICD.
Instances of contact dermatitis caused by 2-CA are rare, and progression to an id reaction is rarer still. Physicians should be aware of the possibility of encountering a patient that manifests one or both of these complications whenever 2-CA is employed for skin closure. Physicians who employ 2-CA for skin closure should first ask patients about prior cutaneous reactions to cyanoacrylates including 2-CA and other commonly encountered acrylate-containing products including adhesive wound dressings, dental cements and prostheses, superglue, artificial nails, and adhesives for wigs and false eyelashes. Still, many patients who exhibit acrylate-induced contact dermatitis, with or without an associated id reaction, will not attest to a history of adverse reactions; they simply may not recognize acrylate as the inciting agent. Practitioners across a range of specialties outside of dermatology—surgeons, emergency physicians, and primary care providers—should be prepared to both recognize contact dermatitis and id reaction arising from the use of 2-CA and implement a basic treatment plan that will bring the patient relief without compromising wound closure.
- US Food and Drug Administration. Premarket approval (PMA). https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpma/pma.cfm?id=p960052. Accessed March 4, 2020.
- Elmore JM, Smith EA, Kirsch AJ. Sutureless circumcision using 2-octyl cyanoacrylate (Dermabond): appraisal after 18-month experience. Urology. 2007;70:803-806.
- Kilic A, Ozdengil E. Skin graft fixation by applying cyanoacrylate without any complication. Plast Reconstr Surg. 2002;110:370-371.
- Gurnaney H, Kraemer FW, Ganesh A. Dermabond decreases pericatheter local anesthetic leakage after continuous perineural infusions. Anesth Analg. 2011;113:206.
- Carr JA. The intracorporeal use of 2-octyl cyanoacrylate resin to control air leaks after lung resection. Eur J Cardiothorac Surg. 2011;39:579-583.
- Miyano G, Yamataka A, Kato Y, et al. Laparoscopic injection of Dermabond tissue adhesive for the repair of inguinal hernia: short- and long-term follow-up. J Pediatr Surg. 2004;39:1867-1870.
- Paral J, Subrt Z, Lochman P, et al. Suture-free anastomosis of the colon. experimental comparison of two cyanoacrylate adhesives. J Gastrointest Surg. 2011;15:451-459.
- Birch DW, Park A. Octylcyanoacrylate tissue adhesive as an alternative to mechanical fixation of expanded polytetrafluoroethylene prosthesis. Am Surg. 2001;67:974-978.
- Ang ES, Tan KC, Tan LH, et al. 2-octylcyanoacrylate-assisted microvascular anastomosis: comparison with a conventional suture technique in rat femoral arteries. J Reconstr Microsurg. 2001;17:193-201.
- Bruns TB, Worthington JM. Using tissue adhesive for wound repair: a practical guide to Dermabond. Am Fam Physician. 2000;61:1383-1388.
- Nuss D, Kelly RE Jr, Croitoru DP, et al. A 10-year review of a minimally invasive technique for the correction of pectus excavatum. J Pediatr Surg. 1998;33:545-552.
- Hivnor CM, Hudkins ML. Allergic contact dermatitis after postsurgical repair with 2-octylcyanoacrylate. Arch Dermatol. 2008;144:814-815.
- Howard BK, Downey SE. Contact dermatitis from Dermabond. Plast Reconstr Surg. 2010;125:E252-E253.
- Perry AW, Sosin M. Severe allergic reaction to Dermabond. Aesthet Surg J. 2009;29:314-316.
- Sachse MM, Junghans T, Rose C, et al. Allergic contact dermatitis caused by topical 2-octyl-cyanoacrylate. Contact Dermatitis. 2013;68:317-319.
- Fehr BS, Takashima A, Bergstresser PR, et al. T cells reactive to keratinocyte antigens are generated during induction of contact hypersensitivity in mice. a model for autoeczematization in humans? Am J Contact Dermat. 2000;11:145-154.
- Gonzalez-Amaro R, Baranda L, Abud-Mendoza C, et al. Autoeczematization is associated with abnormal immune recognition of autologous skin antigens. J Am Acad Dermatol. 1993;28:56-60.
- Vocanson M, Hennino A, Rozières A, et al. Effector and regulatory mechanisms in allergic contact dermatitis. Allergy. 2009;64:1699-1714.
- Sommer LL, Hejazi EZ, Heymann WR. An acute linear pruritic eruption following allergic contact dermatitis. J Clin Aesthet Dermatol. 2014;7:42-44.
- Rietschel RL, Fowler JF. Plastics, adhesives, and synthetic resins. In: Rietschek RL, Fowler JF, eds. Fisher’s Contact Dermatitis. Hamilton, BC: Decker Inc; 2008:542-560.
- Kao JS, Fluhr JW, Man M, et al. Short-term glucocorticoid treatment compromises both permeability barrier homeostasis and stratum corneum integrity: inhibition of epidermal lipid synthesis accounts for functional abnormalities. J Invest Dermatol. 2003;120:456-464.
- American Academy of Allergy, Asthma and Immunology; American College of Allergy, Asthma and Immunology. Contact dermatitis: a practice parameter. Ann Allergy Asthma Immunol. 2006;97(3 suppl 2):S1-S38.
To the Editor:
In 1998, 2-octyl-cyanoacrylate (2-CA) tissue adhesive gained US Food and Drug Administration approval for topical application to easily hold closed approximated skin edges from surgical excisions and simple trauma-induced lacerations.1 It has since been employed for a number of off-label indications, including sutureless circumcision,2 skin graft fixation,3 pericatheter leakage,4 and intracorporeal use to control air leaks during lung resection.5 Animal investigations additionally have attempted to elucidate potential future uses of 2-CA for procedures such as inguinal hernia repair,6 bowel anastomosis,7 incisional hernia repair with mesh,8 and microvascular anastomosis.9 Compared to sutures, 2-CA offers ease and rapidity of application, a water-resistant barrier, and equivalent cosmetic results, as well as eliminates the need for suture removal.10 As 2-CA is used with increasing frequency across a variety of settings, there arises a greater need to be mindful of the potential complications of its use, such as irritant contact dermatitis (ICD), allergic contact dermatitis (ACD), and cutaneous id reaction.
A 14-year-old adolescent boy with no notable medical history and no known allergies underwent a minimally invasive Nuss procedure11 (performed by P.L.G.) for the repair of severe pectus excavatum. Two 4-cm incisions were made—one in each lateral chest wall at the approximately eighth intercostal space—to facilitate the introduction of the Nuss bar. The surgical wounds were closed with 2 layers of running polyglactin 910 suture before 2-CA was applied topically to the incision sites. The surgery was well tolerated, and the patient’s wounds healed without incident. When the patient was evaluated for Nuss bar removal 3 years later, incision sites were noted to be well healed, and he exhibited no other skin lesions. The original incision sites (bilateral chest walls) were utilized to facilitate surgical Nuss bar removal. The wounds were closed in 4 layers and 2-CA was again applied topically to the incision sites. There were no intraoperative complications; no devices, drains, or tissue implants were left in the patient at the conclusion of the procedure.
One week later, via text message and digital photographs, the patient reported intense pruritus at the bilateral chest wall incision sites, which were now surrounded by symmetric 1-cm erythematous plaques and associated sparse erythematous satellite papules (Figure 1). The patient denied any fevers, pain, swelling, or purulent discharge from the wounds. He was started on hydrocortisone cream 1% twice daily as well as oral diphenhydramine 25 mg at bedtime with initial good effect.

Three days later, the patient sent digital photographs of a morphologically similar–appearing rash that had progressed beyond the lateral chest walls to include the central chest and bilateral upper and lower extremities (Figure 2). He continued to deny any local or systemic signs of infection. Dermatology was consulted, and a diagnosis of ACD with cutaneous id reaction was made. The patient’s medication regimen was modified to include triamcinolone acetonide cream 0.1% applied twice daily to the rash away from the wounds, clobetasol propionate ointment 0.05% applied twice daily to the rash at the wound sites, oral levocetirizine 5 mg once daily, and oral hydroxyzine 25 to 50 mg every 6 hours as needed for pruritus. Additional recommendations included the use of a fragrance-free soap and application of an over-the-counter anti-itch lotion containing menthol and camphor applied as needed. Within 24 hours of starting this modified treatment regimen, the patient began to notice an improvement in symptoms, with full resolution over the course of the ensuing 2 weeks. The patient was counseled to inform his physicians—present and future—of his allergy to 2-CA.

Contact dermatitis associated with the use of 2-CA has been described in the literature.12-15 We report progression to an id reaction, which is characterized by the diffuse symmetric spread of a cutaneous eruption at a site distant from the primary localized dermatitis that develops within a few days of the primary lesion and exhibits the same morphologic and histopathologic findings.16,17 In our patient, pruritic erythematous papules and plaques symmetrically distributed on the arms, legs, and chest appeared 3 days after he first reported a similar eruption at the 2-CA application sites. It is theorized that id reactions develop when the sensitization phase of a type IV hypersensitivity reaction generates a population of T cells that not only recognizes a hapten but also recognizes keratinocyte-derived epitopes.16 A hapten is a small molecule (<500 Da) that is capable of penetrating the stratum corneum and binding skin components. A contact allergen is a hapten that has bound epidermal proteins to create a new antigenic determinant.18 The secondary dermatitis that characterizes id reactions results from an abnormal autoimmune response. Id reactions associated with exposure to adhesive material are rare.19
Allergic contact dermatitis is a type IV hypersensitivity reaction that appears after initial sensitization to an allergen followed by re-exposure. Our patient presented with symmetric erythematous plaques at the surgical incision sites 1 week after 2-CA had been applied. During this interval, sensitization to the inciting allergen occurred. The allergen is taken up by antigen-presenting cells, which then migrate to lymph nodes where they encounter naïve T lymphocytes that subsequently undergo clonal expansion to produce a cohort of T cells that are capable of recognizing the allergen. If subsequent exposure to the specific allergen takes place, an elicitation phase occurs in which primed T cells are incited to release mediators of inflammation that engender the manifestations of ACD within 24 to 72 hours.18,20 Sensitization may be promoted by skin barrier impairments such as dermatitis or a frank wound.12,20 In most cases, the patient is unaware that sensitization has occurred, though a primary ACD within 5 to 15 days after initial exposure to the inciting allergen rarely may be observed.18 Although our patient had 2-CA applied to his surgical wounds at 14 years of age, it was unlikely that sensitization took place at that time, as it was 1 week rather than 1 to 3 days before he experienced the cutaneous eruption associated with his second 2-CA exposure at 17 years of age.
Cyanoacrylate tissue adhesive also may cause ICD resulting from histotoxic degradation products such as formaldehyde and cyanoacetate that are capable of compromising cutaneous barrier function. Keratinocytes that have had their membranes disturbed release proinflammatory cytokines, which recruit cells of the innate immune system as well as T lymphocytes to the site of insult to facilitate the inflammatory response. The manifestations of ICD include erythema, edema, and local necrosis that can compromise wound healing.20 The speed at which a given cyanoacrylate adhesive degrades is proportional to the length of its carbon side chain. Those with shorter side chains—ethyl and methyl cyanoacrylate—degrade more rapidly into formaldehyde and cyanoacetate; 2-CA possesses a longer side chain and therefore degrades more slowly, which should, in theory, lessen its potential to cause ICD.20 Because it may take 7 to 14 days before 2-CA will spontaneously peel from the application site, however, its potential to evoke ICD nevertheless exists.
Treatment of ICD entails removing the irritant while concurrently working to restore the skin’s barrier with emollients. Although topical corticosteroids often are reflexively prescribed to treat rashes, some believe that their use should be avoided in cases of ICD, as their inhibitory effects on epidermal lipid synthesis may further impair the skin’s barrier.21 For cases of ACD, with or without an accompanying id reaction, topical corticosteroids are the mainstay of therapy. It is customary to start with a higher-potency topical steroid such as clobetasol and taper to lower-potency steroids as the patient’s condition improves. Steroid ointments are petroleum based and are capable of causing 2-CA to separate from the skin.10 As a result, they should be used with care when being applied to an area where 2-CA is maintaining dermal closure. Systemic corticosteroids may be warranted in cases with involvement of more than 20% of the body surface area and should start to provide relief within 12 to 24 hours.22 Oral antihistamines and cold water compresses can be added to help address pruritus and discomfort in both ACD and ICD.
Instances of contact dermatitis caused by 2-CA are rare, and progression to an id reaction is rarer still. Physicians should be aware of the possibility of encountering a patient that manifests one or both of these complications whenever 2-CA is employed for skin closure. Physicians who employ 2-CA for skin closure should first ask patients about prior cutaneous reactions to cyanoacrylates including 2-CA and other commonly encountered acrylate-containing products including adhesive wound dressings, dental cements and prostheses, superglue, artificial nails, and adhesives for wigs and false eyelashes. Still, many patients who exhibit acrylate-induced contact dermatitis, with or without an associated id reaction, will not attest to a history of adverse reactions; they simply may not recognize acrylate as the inciting agent. Practitioners across a range of specialties outside of dermatology—surgeons, emergency physicians, and primary care providers—should be prepared to both recognize contact dermatitis and id reaction arising from the use of 2-CA and implement a basic treatment plan that will bring the patient relief without compromising wound closure.
To the Editor:
In 1998, 2-octyl-cyanoacrylate (2-CA) tissue adhesive gained US Food and Drug Administration approval for topical application to easily hold closed approximated skin edges from surgical excisions and simple trauma-induced lacerations.1 It has since been employed for a number of off-label indications, including sutureless circumcision,2 skin graft fixation,3 pericatheter leakage,4 and intracorporeal use to control air leaks during lung resection.5 Animal investigations additionally have attempted to elucidate potential future uses of 2-CA for procedures such as inguinal hernia repair,6 bowel anastomosis,7 incisional hernia repair with mesh,8 and microvascular anastomosis.9 Compared to sutures, 2-CA offers ease and rapidity of application, a water-resistant barrier, and equivalent cosmetic results, as well as eliminates the need for suture removal.10 As 2-CA is used with increasing frequency across a variety of settings, there arises a greater need to be mindful of the potential complications of its use, such as irritant contact dermatitis (ICD), allergic contact dermatitis (ACD), and cutaneous id reaction.
A 14-year-old adolescent boy with no notable medical history and no known allergies underwent a minimally invasive Nuss procedure11 (performed by P.L.G.) for the repair of severe pectus excavatum. Two 4-cm incisions were made—one in each lateral chest wall at the approximately eighth intercostal space—to facilitate the introduction of the Nuss bar. The surgical wounds were closed with 2 layers of running polyglactin 910 suture before 2-CA was applied topically to the incision sites. The surgery was well tolerated, and the patient’s wounds healed without incident. When the patient was evaluated for Nuss bar removal 3 years later, incision sites were noted to be well healed, and he exhibited no other skin lesions. The original incision sites (bilateral chest walls) were utilized to facilitate surgical Nuss bar removal. The wounds were closed in 4 layers and 2-CA was again applied topically to the incision sites. There were no intraoperative complications; no devices, drains, or tissue implants were left in the patient at the conclusion of the procedure.
One week later, via text message and digital photographs, the patient reported intense pruritus at the bilateral chest wall incision sites, which were now surrounded by symmetric 1-cm erythematous plaques and associated sparse erythematous satellite papules (Figure 1). The patient denied any fevers, pain, swelling, or purulent discharge from the wounds. He was started on hydrocortisone cream 1% twice daily as well as oral diphenhydramine 25 mg at bedtime with initial good effect.

Three days later, the patient sent digital photographs of a morphologically similar–appearing rash that had progressed beyond the lateral chest walls to include the central chest and bilateral upper and lower extremities (Figure 2). He continued to deny any local or systemic signs of infection. Dermatology was consulted, and a diagnosis of ACD with cutaneous id reaction was made. The patient’s medication regimen was modified to include triamcinolone acetonide cream 0.1% applied twice daily to the rash away from the wounds, clobetasol propionate ointment 0.05% applied twice daily to the rash at the wound sites, oral levocetirizine 5 mg once daily, and oral hydroxyzine 25 to 50 mg every 6 hours as needed for pruritus. Additional recommendations included the use of a fragrance-free soap and application of an over-the-counter anti-itch lotion containing menthol and camphor applied as needed. Within 24 hours of starting this modified treatment regimen, the patient began to notice an improvement in symptoms, with full resolution over the course of the ensuing 2 weeks. The patient was counseled to inform his physicians—present and future—of his allergy to 2-CA.

Contact dermatitis associated with the use of 2-CA has been described in the literature.12-15 We report progression to an id reaction, which is characterized by the diffuse symmetric spread of a cutaneous eruption at a site distant from the primary localized dermatitis that develops within a few days of the primary lesion and exhibits the same morphologic and histopathologic findings.16,17 In our patient, pruritic erythematous papules and plaques symmetrically distributed on the arms, legs, and chest appeared 3 days after he first reported a similar eruption at the 2-CA application sites. It is theorized that id reactions develop when the sensitization phase of a type IV hypersensitivity reaction generates a population of T cells that not only recognizes a hapten but also recognizes keratinocyte-derived epitopes.16 A hapten is a small molecule (<500 Da) that is capable of penetrating the stratum corneum and binding skin components. A contact allergen is a hapten that has bound epidermal proteins to create a new antigenic determinant.18 The secondary dermatitis that characterizes id reactions results from an abnormal autoimmune response. Id reactions associated with exposure to adhesive material are rare.19
Allergic contact dermatitis is a type IV hypersensitivity reaction that appears after initial sensitization to an allergen followed by re-exposure. Our patient presented with symmetric erythematous plaques at the surgical incision sites 1 week after 2-CA had been applied. During this interval, sensitization to the inciting allergen occurred. The allergen is taken up by antigen-presenting cells, which then migrate to lymph nodes where they encounter naïve T lymphocytes that subsequently undergo clonal expansion to produce a cohort of T cells that are capable of recognizing the allergen. If subsequent exposure to the specific allergen takes place, an elicitation phase occurs in which primed T cells are incited to release mediators of inflammation that engender the manifestations of ACD within 24 to 72 hours.18,20 Sensitization may be promoted by skin barrier impairments such as dermatitis or a frank wound.12,20 In most cases, the patient is unaware that sensitization has occurred, though a primary ACD within 5 to 15 days after initial exposure to the inciting allergen rarely may be observed.18 Although our patient had 2-CA applied to his surgical wounds at 14 years of age, it was unlikely that sensitization took place at that time, as it was 1 week rather than 1 to 3 days before he experienced the cutaneous eruption associated with his second 2-CA exposure at 17 years of age.
Cyanoacrylate tissue adhesive also may cause ICD resulting from histotoxic degradation products such as formaldehyde and cyanoacetate that are capable of compromising cutaneous barrier function. Keratinocytes that have had their membranes disturbed release proinflammatory cytokines, which recruit cells of the innate immune system as well as T lymphocytes to the site of insult to facilitate the inflammatory response. The manifestations of ICD include erythema, edema, and local necrosis that can compromise wound healing.20 The speed at which a given cyanoacrylate adhesive degrades is proportional to the length of its carbon side chain. Those with shorter side chains—ethyl and methyl cyanoacrylate—degrade more rapidly into formaldehyde and cyanoacetate; 2-CA possesses a longer side chain and therefore degrades more slowly, which should, in theory, lessen its potential to cause ICD.20 Because it may take 7 to 14 days before 2-CA will spontaneously peel from the application site, however, its potential to evoke ICD nevertheless exists.
Treatment of ICD entails removing the irritant while concurrently working to restore the skin’s barrier with emollients. Although topical corticosteroids often are reflexively prescribed to treat rashes, some believe that their use should be avoided in cases of ICD, as their inhibitory effects on epidermal lipid synthesis may further impair the skin’s barrier.21 For cases of ACD, with or without an accompanying id reaction, topical corticosteroids are the mainstay of therapy. It is customary to start with a higher-potency topical steroid such as clobetasol and taper to lower-potency steroids as the patient’s condition improves. Steroid ointments are petroleum based and are capable of causing 2-CA to separate from the skin.10 As a result, they should be used with care when being applied to an area where 2-CA is maintaining dermal closure. Systemic corticosteroids may be warranted in cases with involvement of more than 20% of the body surface area and should start to provide relief within 12 to 24 hours.22 Oral antihistamines and cold water compresses can be added to help address pruritus and discomfort in both ACD and ICD.
Instances of contact dermatitis caused by 2-CA are rare, and progression to an id reaction is rarer still. Physicians should be aware of the possibility of encountering a patient that manifests one or both of these complications whenever 2-CA is employed for skin closure. Physicians who employ 2-CA for skin closure should first ask patients about prior cutaneous reactions to cyanoacrylates including 2-CA and other commonly encountered acrylate-containing products including adhesive wound dressings, dental cements and prostheses, superglue, artificial nails, and adhesives for wigs and false eyelashes. Still, many patients who exhibit acrylate-induced contact dermatitis, with or without an associated id reaction, will not attest to a history of adverse reactions; they simply may not recognize acrylate as the inciting agent. Practitioners across a range of specialties outside of dermatology—surgeons, emergency physicians, and primary care providers—should be prepared to both recognize contact dermatitis and id reaction arising from the use of 2-CA and implement a basic treatment plan that will bring the patient relief without compromising wound closure.
- US Food and Drug Administration. Premarket approval (PMA). https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpma/pma.cfm?id=p960052. Accessed March 4, 2020.
- Elmore JM, Smith EA, Kirsch AJ. Sutureless circumcision using 2-octyl cyanoacrylate (Dermabond): appraisal after 18-month experience. Urology. 2007;70:803-806.
- Kilic A, Ozdengil E. Skin graft fixation by applying cyanoacrylate without any complication. Plast Reconstr Surg. 2002;110:370-371.
- Gurnaney H, Kraemer FW, Ganesh A. Dermabond decreases pericatheter local anesthetic leakage after continuous perineural infusions. Anesth Analg. 2011;113:206.
- Carr JA. The intracorporeal use of 2-octyl cyanoacrylate resin to control air leaks after lung resection. Eur J Cardiothorac Surg. 2011;39:579-583.
- Miyano G, Yamataka A, Kato Y, et al. Laparoscopic injection of Dermabond tissue adhesive for the repair of inguinal hernia: short- and long-term follow-up. J Pediatr Surg. 2004;39:1867-1870.
- Paral J, Subrt Z, Lochman P, et al. Suture-free anastomosis of the colon. experimental comparison of two cyanoacrylate adhesives. J Gastrointest Surg. 2011;15:451-459.
- Birch DW, Park A. Octylcyanoacrylate tissue adhesive as an alternative to mechanical fixation of expanded polytetrafluoroethylene prosthesis. Am Surg. 2001;67:974-978.
- Ang ES, Tan KC, Tan LH, et al. 2-octylcyanoacrylate-assisted microvascular anastomosis: comparison with a conventional suture technique in rat femoral arteries. J Reconstr Microsurg. 2001;17:193-201.
- Bruns TB, Worthington JM. Using tissue adhesive for wound repair: a practical guide to Dermabond. Am Fam Physician. 2000;61:1383-1388.
- Nuss D, Kelly RE Jr, Croitoru DP, et al. A 10-year review of a minimally invasive technique for the correction of pectus excavatum. J Pediatr Surg. 1998;33:545-552.
- Hivnor CM, Hudkins ML. Allergic contact dermatitis after postsurgical repair with 2-octylcyanoacrylate. Arch Dermatol. 2008;144:814-815.
- Howard BK, Downey SE. Contact dermatitis from Dermabond. Plast Reconstr Surg. 2010;125:E252-E253.
- Perry AW, Sosin M. Severe allergic reaction to Dermabond. Aesthet Surg J. 2009;29:314-316.
- Sachse MM, Junghans T, Rose C, et al. Allergic contact dermatitis caused by topical 2-octyl-cyanoacrylate. Contact Dermatitis. 2013;68:317-319.
- Fehr BS, Takashima A, Bergstresser PR, et al. T cells reactive to keratinocyte antigens are generated during induction of contact hypersensitivity in mice. a model for autoeczematization in humans? Am J Contact Dermat. 2000;11:145-154.
- Gonzalez-Amaro R, Baranda L, Abud-Mendoza C, et al. Autoeczematization is associated with abnormal immune recognition of autologous skin antigens. J Am Acad Dermatol. 1993;28:56-60.
- Vocanson M, Hennino A, Rozières A, et al. Effector and regulatory mechanisms in allergic contact dermatitis. Allergy. 2009;64:1699-1714.
- Sommer LL, Hejazi EZ, Heymann WR. An acute linear pruritic eruption following allergic contact dermatitis. J Clin Aesthet Dermatol. 2014;7:42-44.
- Rietschel RL, Fowler JF. Plastics, adhesives, and synthetic resins. In: Rietschek RL, Fowler JF, eds. Fisher’s Contact Dermatitis. Hamilton, BC: Decker Inc; 2008:542-560.
- Kao JS, Fluhr JW, Man M, et al. Short-term glucocorticoid treatment compromises both permeability barrier homeostasis and stratum corneum integrity: inhibition of epidermal lipid synthesis accounts for functional abnormalities. J Invest Dermatol. 2003;120:456-464.
- American Academy of Allergy, Asthma and Immunology; American College of Allergy, Asthma and Immunology. Contact dermatitis: a practice parameter. Ann Allergy Asthma Immunol. 2006;97(3 suppl 2):S1-S38.
- US Food and Drug Administration. Premarket approval (PMA). https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpma/pma.cfm?id=p960052. Accessed March 4, 2020.
- Elmore JM, Smith EA, Kirsch AJ. Sutureless circumcision using 2-octyl cyanoacrylate (Dermabond): appraisal after 18-month experience. Urology. 2007;70:803-806.
- Kilic A, Ozdengil E. Skin graft fixation by applying cyanoacrylate without any complication. Plast Reconstr Surg. 2002;110:370-371.
- Gurnaney H, Kraemer FW, Ganesh A. Dermabond decreases pericatheter local anesthetic leakage after continuous perineural infusions. Anesth Analg. 2011;113:206.
- Carr JA. The intracorporeal use of 2-octyl cyanoacrylate resin to control air leaks after lung resection. Eur J Cardiothorac Surg. 2011;39:579-583.
- Miyano G, Yamataka A, Kato Y, et al. Laparoscopic injection of Dermabond tissue adhesive for the repair of inguinal hernia: short- and long-term follow-up. J Pediatr Surg. 2004;39:1867-1870.
- Paral J, Subrt Z, Lochman P, et al. Suture-free anastomosis of the colon. experimental comparison of two cyanoacrylate adhesives. J Gastrointest Surg. 2011;15:451-459.
- Birch DW, Park A. Octylcyanoacrylate tissue adhesive as an alternative to mechanical fixation of expanded polytetrafluoroethylene prosthesis. Am Surg. 2001;67:974-978.
- Ang ES, Tan KC, Tan LH, et al. 2-octylcyanoacrylate-assisted microvascular anastomosis: comparison with a conventional suture technique in rat femoral arteries. J Reconstr Microsurg. 2001;17:193-201.
- Bruns TB, Worthington JM. Using tissue adhesive for wound repair: a practical guide to Dermabond. Am Fam Physician. 2000;61:1383-1388.
- Nuss D, Kelly RE Jr, Croitoru DP, et al. A 10-year review of a minimally invasive technique for the correction of pectus excavatum. J Pediatr Surg. 1998;33:545-552.
- Hivnor CM, Hudkins ML. Allergic contact dermatitis after postsurgical repair with 2-octylcyanoacrylate. Arch Dermatol. 2008;144:814-815.
- Howard BK, Downey SE. Contact dermatitis from Dermabond. Plast Reconstr Surg. 2010;125:E252-E253.
- Perry AW, Sosin M. Severe allergic reaction to Dermabond. Aesthet Surg J. 2009;29:314-316.
- Sachse MM, Junghans T, Rose C, et al. Allergic contact dermatitis caused by topical 2-octyl-cyanoacrylate. Contact Dermatitis. 2013;68:317-319.
- Fehr BS, Takashima A, Bergstresser PR, et al. T cells reactive to keratinocyte antigens are generated during induction of contact hypersensitivity in mice. a model for autoeczematization in humans? Am J Contact Dermat. 2000;11:145-154.
- Gonzalez-Amaro R, Baranda L, Abud-Mendoza C, et al. Autoeczematization is associated with abnormal immune recognition of autologous skin antigens. J Am Acad Dermatol. 1993;28:56-60.
- Vocanson M, Hennino A, Rozières A, et al. Effector and regulatory mechanisms in allergic contact dermatitis. Allergy. 2009;64:1699-1714.
- Sommer LL, Hejazi EZ, Heymann WR. An acute linear pruritic eruption following allergic contact dermatitis. J Clin Aesthet Dermatol. 2014;7:42-44.
- Rietschel RL, Fowler JF. Plastics, adhesives, and synthetic resins. In: Rietschek RL, Fowler JF, eds. Fisher’s Contact Dermatitis. Hamilton, BC: Decker Inc; 2008:542-560.
- Kao JS, Fluhr JW, Man M, et al. Short-term glucocorticoid treatment compromises both permeability barrier homeostasis and stratum corneum integrity: inhibition of epidermal lipid synthesis accounts for functional abnormalities. J Invest Dermatol. 2003;120:456-464.
- American Academy of Allergy, Asthma and Immunology; American College of Allergy, Asthma and Immunology. Contact dermatitis: a practice parameter. Ann Allergy Asthma Immunol. 2006;97(3 suppl 2):S1-S38.
Practice Points
- 2-Octyl-cyanoacrylate (2-CA) tissue adhesive has been reported to cause contact dermatitis when applied topically for surgical site closure.
- Id reactions resulting from the use of 2-CA tissue adhesive are possible, though less commonly observed.
- Id reactions caused by 2-CA tissue adhesive respond well to treatment with a combination of topical steroids and oral antihistamines. Systemic corticosteroids may be warranted in cases involving greater than 20% body surface area.
Breaking bacterial communication may heal EB wounds
LONDON – Disrupting how microorganisms communicate with each other could be a way to overcome antibiotic resistance and to help heal chronic wounds in patients with epidermolysis bullosa (EB), according to presenters at the EB World Congress, organized by the Dystrophic Epidermolysis Bullosa Association (DEBRA).
The majority of chronic wounds in patients with EB are colonized with microorganisms, with a predominance of Staphylococcus species, said Erik Gerner, an industrial PhD student at Mölnlycke Health Care in Gothenburg, Sweden, and Gothenburg University.
Because of the growing problem of antibiotic resistance, alternative treatments are needed, and one possible alternative for treating infected wounds could be interfering with quorum sensing, the cell-to-cell communication used by bacteria, he said. He is hoping to explore this possibility as a novel treatment strategy for infected wounds.
“Quorum sensing is defined as the ability to detect and respond to population density,” Mr. Gerner said, noting that, when there is a sufficient density of bacteria, “they start to communicate with each other.” This enables them to act as a community and perform actions that they could not do as individual cells. Such actions include forming biofilms, which helps protect bacteria from their environment, such as the immune system. Other actions include collectively switching on the production of virulence factors and becoming resistant to treatments.
“Bacteria use quorum sensing to act collectively,” Mr. Gerner said. “If we could shut down this quorum sensing system, it would be very beneficial … and increase the chances to heal the wound.”
The quorum sensing system is based on the production of signaling molecules called AHL (N-acyl homoserine lactones), which are constantly produced at a low rate. This isn’t a problem until the level of bacteria increases and the level of quorum sensing breaches a threshold, he explained.
There are several benefits of inhibiting bacterial communication through disrupting quorum sensing, namely, “a low risk of resistance,” Mr. Gerner said. There is also potentially less toxin production by bacteria, and this could help the immune system in killing the invading bacteria.
One approach to disrupting quorum testing that Mr. Gerner has been investigating is the use of sodium salicylate (NaSa). So far, preclinical work shows that NaSa can reduce toxin production but not the growth rate of bacteria. The advantage of using NaSa is that it is nontoxic to human dermal fibroblasts, with similar results seen in human keratinocytes and immune cells. His work to date has shown that NaSa reduced activity of NF-kB (a proinflammatory signaling pathway) in differentiated and lipopolysaccharide-stimulated monocytes; NF-kB activated production of proinflammatory cytokines (such as interleukin-1 beta and IL-6) are elevated in EB wounds. “My studies support the bodies of evidence that bacteria use quorum sensing to coordinate … and to produce a large number of toxic factors,” Mr. Gerner concluded. Future studies will look at the potential of NaSa to disrupt this activity.
Skin microbiome of EB wounds
Liat Samuelov, MD, of the department of molecular dermatology at Tel Aviv (Israel) Sourasky Medical Center, presented data on skin microbiome characteristics in eight patients with recessive dystrophic EB (RDEB). This showed that there was reduced bacterial diversity in wounds, and a “progressive development of dysbiosis across different stages of DEB wound formation.”
The skin microbiome has been implicated in several skin diseases, Dr. Samuelov and associates observed in a poster presentation. That includes the autoimmune blistering disease bullous pemphigoid (Exp Dermatol. 2017 Dec;26[12]:1221-7). “Colonization of DEB chronic wounds may lead to systemic infections, result in delayed healing, and possibly be involved in the development of squamous cell carcinoma,” they noted in the poster, “thus accurate delineation of the dysbiotic profile … may point to corrective measures of great therapeutic potential.”
The aim was to see what microorganisms were present in the chronic wounds of the patients. To be included in the study, patients must not have had any antibiotic treatment – oral or topical – in the past 6 months. Samples were taken from an untreated wound, around the wound, and from uninvolved skin, which were compared with samples taken from similar areas in age-matched controls.
Reduced bacterial diversity was observed in RDEB wounds, compared with uninvolved or perilesional areas and the skin of control subjects, Dr. Samuelov said in an oral presentation of the study results. There was increased abundance of Staphylococcus epidermidis and decreased Cutibacterium acnes, which she noted was in contrast to other studies where S. aureus was the most common colonizer in RDEB wounds.
Bacterial composition in each group was calculated using the beta-diversity score, while control samples showed similar microbial composition, the DEB samples had no microbial similarities among different samples. These data “suggest the need to ascertain the potential therapeutic benefit of interventions aimed at restoring normal microbiome composition in DEB,” Dr. Samuelov concluded.
Wound colonization and squamous cell carcinoma
Other research on wound microbiology was presented by Laura E. Levin, MD, a dermatologist at New York–Presbyterian, and associates. “Given the potential role of bacteria-induced inflammation in the development of wound-associated SCC [squamous cell carcinoma] in a subset of patients, we sought to improve our understanding of what microbes colonize and infect the wounds of patients with epidermolysis bullosa,” they explained in their poster.
The researchers, from New York–Presbyterian Morgan Stanley Children’s Hospital and Columbia University Irvine Medical Center, New York, presented data from a retrospective analysis of 739 wound cultures taken between 2001 and 2017 from 158 patients enrolled in the Epidermolysis Bullosa Clinical Characterization and Outcomes Database. In the analysis, just under 70% of patients had DEB, of which 90% were of the RDEB subtype; 13% had EB simplex, 14% had junctional EB, and 3% had an unknown EB subtype.
At least one organism grew in 87% of cultures, with the most common microorganism isolated being Staphylococcus aureus (84% of cultures). Other commonly isolated microbes were Pseudomonas aeruginosa in 35% of cultures, Streptococcus group A in 34% of cultures (of which 22% were Streptococcus pyogenes), Corynebacterium species in 31% of cultures, and Proteus species in 18% of cultures.
“Improved understanding of what microbes are colonizing the wounds of our patients may help improve antibiotic stewardship,” the researchers stated.
Looking at the antibiotic susceptibilities, Dr. Levin and associates found that 68% of 115 cultures were sensitive to methicillin and 60% of 15 cultures were sensitive to mupirocin. “Resistance to many systemic and topical antibiotic agents in EB patients supports surveillance cultures with routine testing for mupirocin susceptibility,” they suggested.
A total of 23 patients developed SCC of whom 10 had cultures that grew S. aureus (90%) and P. aeruginosa (50%), and Proteus species (20%). Among the patients who did not develop SCC, the respective cultures positive for each of those microorganisms were 83%, 34%, and 11%. Perhaps “gram-negative and flagellated organisms may be more common in wounds of patients at risk for SCC,” they observed, adding that further studies were needed to determine if “wound microbiome interventions inhibit the risk of development of SCC and improve outcomes.”
Mr. Gerner’s research is supported by Mölnlycke Health Care. Dr. Samuelov had no disclosures. The work by Dr. Levin and associates is supported by the Pediatric Dermatology Research Alliance, EB Research Partnership, and the Epidermolysis Bullosa Medical Research Foundation.
LONDON – Disrupting how microorganisms communicate with each other could be a way to overcome antibiotic resistance and to help heal chronic wounds in patients with epidermolysis bullosa (EB), according to presenters at the EB World Congress, organized by the Dystrophic Epidermolysis Bullosa Association (DEBRA).
The majority of chronic wounds in patients with EB are colonized with microorganisms, with a predominance of Staphylococcus species, said Erik Gerner, an industrial PhD student at Mölnlycke Health Care in Gothenburg, Sweden, and Gothenburg University.
Because of the growing problem of antibiotic resistance, alternative treatments are needed, and one possible alternative for treating infected wounds could be interfering with quorum sensing, the cell-to-cell communication used by bacteria, he said. He is hoping to explore this possibility as a novel treatment strategy for infected wounds.
“Quorum sensing is defined as the ability to detect and respond to population density,” Mr. Gerner said, noting that, when there is a sufficient density of bacteria, “they start to communicate with each other.” This enables them to act as a community and perform actions that they could not do as individual cells. Such actions include forming biofilms, which helps protect bacteria from their environment, such as the immune system. Other actions include collectively switching on the production of virulence factors and becoming resistant to treatments.
“Bacteria use quorum sensing to act collectively,” Mr. Gerner said. “If we could shut down this quorum sensing system, it would be very beneficial … and increase the chances to heal the wound.”
The quorum sensing system is based on the production of signaling molecules called AHL (N-acyl homoserine lactones), which are constantly produced at a low rate. This isn’t a problem until the level of bacteria increases and the level of quorum sensing breaches a threshold, he explained.
There are several benefits of inhibiting bacterial communication through disrupting quorum sensing, namely, “a low risk of resistance,” Mr. Gerner said. There is also potentially less toxin production by bacteria, and this could help the immune system in killing the invading bacteria.
One approach to disrupting quorum testing that Mr. Gerner has been investigating is the use of sodium salicylate (NaSa). So far, preclinical work shows that NaSa can reduce toxin production but not the growth rate of bacteria. The advantage of using NaSa is that it is nontoxic to human dermal fibroblasts, with similar results seen in human keratinocytes and immune cells. His work to date has shown that NaSa reduced activity of NF-kB (a proinflammatory signaling pathway) in differentiated and lipopolysaccharide-stimulated monocytes; NF-kB activated production of proinflammatory cytokines (such as interleukin-1 beta and IL-6) are elevated in EB wounds. “My studies support the bodies of evidence that bacteria use quorum sensing to coordinate … and to produce a large number of toxic factors,” Mr. Gerner concluded. Future studies will look at the potential of NaSa to disrupt this activity.
Skin microbiome of EB wounds
Liat Samuelov, MD, of the department of molecular dermatology at Tel Aviv (Israel) Sourasky Medical Center, presented data on skin microbiome characteristics in eight patients with recessive dystrophic EB (RDEB). This showed that there was reduced bacterial diversity in wounds, and a “progressive development of dysbiosis across different stages of DEB wound formation.”
The skin microbiome has been implicated in several skin diseases, Dr. Samuelov and associates observed in a poster presentation. That includes the autoimmune blistering disease bullous pemphigoid (Exp Dermatol. 2017 Dec;26[12]:1221-7). “Colonization of DEB chronic wounds may lead to systemic infections, result in delayed healing, and possibly be involved in the development of squamous cell carcinoma,” they noted in the poster, “thus accurate delineation of the dysbiotic profile … may point to corrective measures of great therapeutic potential.”
The aim was to see what microorganisms were present in the chronic wounds of the patients. To be included in the study, patients must not have had any antibiotic treatment – oral or topical – in the past 6 months. Samples were taken from an untreated wound, around the wound, and from uninvolved skin, which were compared with samples taken from similar areas in age-matched controls.
Reduced bacterial diversity was observed in RDEB wounds, compared with uninvolved or perilesional areas and the skin of control subjects, Dr. Samuelov said in an oral presentation of the study results. There was increased abundance of Staphylococcus epidermidis and decreased Cutibacterium acnes, which she noted was in contrast to other studies where S. aureus was the most common colonizer in RDEB wounds.
Bacterial composition in each group was calculated using the beta-diversity score, while control samples showed similar microbial composition, the DEB samples had no microbial similarities among different samples. These data “suggest the need to ascertain the potential therapeutic benefit of interventions aimed at restoring normal microbiome composition in DEB,” Dr. Samuelov concluded.
Wound colonization and squamous cell carcinoma
Other research on wound microbiology was presented by Laura E. Levin, MD, a dermatologist at New York–Presbyterian, and associates. “Given the potential role of bacteria-induced inflammation in the development of wound-associated SCC [squamous cell carcinoma] in a subset of patients, we sought to improve our understanding of what microbes colonize and infect the wounds of patients with epidermolysis bullosa,” they explained in their poster.
The researchers, from New York–Presbyterian Morgan Stanley Children’s Hospital and Columbia University Irvine Medical Center, New York, presented data from a retrospective analysis of 739 wound cultures taken between 2001 and 2017 from 158 patients enrolled in the Epidermolysis Bullosa Clinical Characterization and Outcomes Database. In the analysis, just under 70% of patients had DEB, of which 90% were of the RDEB subtype; 13% had EB simplex, 14% had junctional EB, and 3% had an unknown EB subtype.
At least one organism grew in 87% of cultures, with the most common microorganism isolated being Staphylococcus aureus (84% of cultures). Other commonly isolated microbes were Pseudomonas aeruginosa in 35% of cultures, Streptococcus group A in 34% of cultures (of which 22% were Streptococcus pyogenes), Corynebacterium species in 31% of cultures, and Proteus species in 18% of cultures.
“Improved understanding of what microbes are colonizing the wounds of our patients may help improve antibiotic stewardship,” the researchers stated.
Looking at the antibiotic susceptibilities, Dr. Levin and associates found that 68% of 115 cultures were sensitive to methicillin and 60% of 15 cultures were sensitive to mupirocin. “Resistance to many systemic and topical antibiotic agents in EB patients supports surveillance cultures with routine testing for mupirocin susceptibility,” they suggested.
A total of 23 patients developed SCC of whom 10 had cultures that grew S. aureus (90%) and P. aeruginosa (50%), and Proteus species (20%). Among the patients who did not develop SCC, the respective cultures positive for each of those microorganisms were 83%, 34%, and 11%. Perhaps “gram-negative and flagellated organisms may be more common in wounds of patients at risk for SCC,” they observed, adding that further studies were needed to determine if “wound microbiome interventions inhibit the risk of development of SCC and improve outcomes.”
Mr. Gerner’s research is supported by Mölnlycke Health Care. Dr. Samuelov had no disclosures. The work by Dr. Levin and associates is supported by the Pediatric Dermatology Research Alliance, EB Research Partnership, and the Epidermolysis Bullosa Medical Research Foundation.
LONDON – Disrupting how microorganisms communicate with each other could be a way to overcome antibiotic resistance and to help heal chronic wounds in patients with epidermolysis bullosa (EB), according to presenters at the EB World Congress, organized by the Dystrophic Epidermolysis Bullosa Association (DEBRA).
The majority of chronic wounds in patients with EB are colonized with microorganisms, with a predominance of Staphylococcus species, said Erik Gerner, an industrial PhD student at Mölnlycke Health Care in Gothenburg, Sweden, and Gothenburg University.
Because of the growing problem of antibiotic resistance, alternative treatments are needed, and one possible alternative for treating infected wounds could be interfering with quorum sensing, the cell-to-cell communication used by bacteria, he said. He is hoping to explore this possibility as a novel treatment strategy for infected wounds.
“Quorum sensing is defined as the ability to detect and respond to population density,” Mr. Gerner said, noting that, when there is a sufficient density of bacteria, “they start to communicate with each other.” This enables them to act as a community and perform actions that they could not do as individual cells. Such actions include forming biofilms, which helps protect bacteria from their environment, such as the immune system. Other actions include collectively switching on the production of virulence factors and becoming resistant to treatments.
“Bacteria use quorum sensing to act collectively,” Mr. Gerner said. “If we could shut down this quorum sensing system, it would be very beneficial … and increase the chances to heal the wound.”
The quorum sensing system is based on the production of signaling molecules called AHL (N-acyl homoserine lactones), which are constantly produced at a low rate. This isn’t a problem until the level of bacteria increases and the level of quorum sensing breaches a threshold, he explained.
There are several benefits of inhibiting bacterial communication through disrupting quorum sensing, namely, “a low risk of resistance,” Mr. Gerner said. There is also potentially less toxin production by bacteria, and this could help the immune system in killing the invading bacteria.
One approach to disrupting quorum testing that Mr. Gerner has been investigating is the use of sodium salicylate (NaSa). So far, preclinical work shows that NaSa can reduce toxin production but not the growth rate of bacteria. The advantage of using NaSa is that it is nontoxic to human dermal fibroblasts, with similar results seen in human keratinocytes and immune cells. His work to date has shown that NaSa reduced activity of NF-kB (a proinflammatory signaling pathway) in differentiated and lipopolysaccharide-stimulated monocytes; NF-kB activated production of proinflammatory cytokines (such as interleukin-1 beta and IL-6) are elevated in EB wounds. “My studies support the bodies of evidence that bacteria use quorum sensing to coordinate … and to produce a large number of toxic factors,” Mr. Gerner concluded. Future studies will look at the potential of NaSa to disrupt this activity.
Skin microbiome of EB wounds
Liat Samuelov, MD, of the department of molecular dermatology at Tel Aviv (Israel) Sourasky Medical Center, presented data on skin microbiome characteristics in eight patients with recessive dystrophic EB (RDEB). This showed that there was reduced bacterial diversity in wounds, and a “progressive development of dysbiosis across different stages of DEB wound formation.”
The skin microbiome has been implicated in several skin diseases, Dr. Samuelov and associates observed in a poster presentation. That includes the autoimmune blistering disease bullous pemphigoid (Exp Dermatol. 2017 Dec;26[12]:1221-7). “Colonization of DEB chronic wounds may lead to systemic infections, result in delayed healing, and possibly be involved in the development of squamous cell carcinoma,” they noted in the poster, “thus accurate delineation of the dysbiotic profile … may point to corrective measures of great therapeutic potential.”
The aim was to see what microorganisms were present in the chronic wounds of the patients. To be included in the study, patients must not have had any antibiotic treatment – oral or topical – in the past 6 months. Samples were taken from an untreated wound, around the wound, and from uninvolved skin, which were compared with samples taken from similar areas in age-matched controls.
Reduced bacterial diversity was observed in RDEB wounds, compared with uninvolved or perilesional areas and the skin of control subjects, Dr. Samuelov said in an oral presentation of the study results. There was increased abundance of Staphylococcus epidermidis and decreased Cutibacterium acnes, which she noted was in contrast to other studies where S. aureus was the most common colonizer in RDEB wounds.
Bacterial composition in each group was calculated using the beta-diversity score, while control samples showed similar microbial composition, the DEB samples had no microbial similarities among different samples. These data “suggest the need to ascertain the potential therapeutic benefit of interventions aimed at restoring normal microbiome composition in DEB,” Dr. Samuelov concluded.
Wound colonization and squamous cell carcinoma
Other research on wound microbiology was presented by Laura E. Levin, MD, a dermatologist at New York–Presbyterian, and associates. “Given the potential role of bacteria-induced inflammation in the development of wound-associated SCC [squamous cell carcinoma] in a subset of patients, we sought to improve our understanding of what microbes colonize and infect the wounds of patients with epidermolysis bullosa,” they explained in their poster.
The researchers, from New York–Presbyterian Morgan Stanley Children’s Hospital and Columbia University Irvine Medical Center, New York, presented data from a retrospective analysis of 739 wound cultures taken between 2001 and 2017 from 158 patients enrolled in the Epidermolysis Bullosa Clinical Characterization and Outcomes Database. In the analysis, just under 70% of patients had DEB, of which 90% were of the RDEB subtype; 13% had EB simplex, 14% had junctional EB, and 3% had an unknown EB subtype.
At least one organism grew in 87% of cultures, with the most common microorganism isolated being Staphylococcus aureus (84% of cultures). Other commonly isolated microbes were Pseudomonas aeruginosa in 35% of cultures, Streptococcus group A in 34% of cultures (of which 22% were Streptococcus pyogenes), Corynebacterium species in 31% of cultures, and Proteus species in 18% of cultures.
“Improved understanding of what microbes are colonizing the wounds of our patients may help improve antibiotic stewardship,” the researchers stated.
Looking at the antibiotic susceptibilities, Dr. Levin and associates found that 68% of 115 cultures were sensitive to methicillin and 60% of 15 cultures were sensitive to mupirocin. “Resistance to many systemic and topical antibiotic agents in EB patients supports surveillance cultures with routine testing for mupirocin susceptibility,” they suggested.
A total of 23 patients developed SCC of whom 10 had cultures that grew S. aureus (90%) and P. aeruginosa (50%), and Proteus species (20%). Among the patients who did not develop SCC, the respective cultures positive for each of those microorganisms were 83%, 34%, and 11%. Perhaps “gram-negative and flagellated organisms may be more common in wounds of patients at risk for SCC,” they observed, adding that further studies were needed to determine if “wound microbiome interventions inhibit the risk of development of SCC and improve outcomes.”
Mr. Gerner’s research is supported by Mölnlycke Health Care. Dr. Samuelov had no disclosures. The work by Dr. Levin and associates is supported by the Pediatric Dermatology Research Alliance, EB Research Partnership, and the Epidermolysis Bullosa Medical Research Foundation.
REPORTING FROM EB 2020
Granulomatous Reaction After Cholla Cactus Spine Injury
Skin injuries caused by spines of various species of cactus are common in the southwestern United States and Mexico and have been described worldwide.1 Effects of injury vary depending on localization, surface extension, and skin conditions (eg, preexisting erosions, ulcerations, sunburns).
Case Report
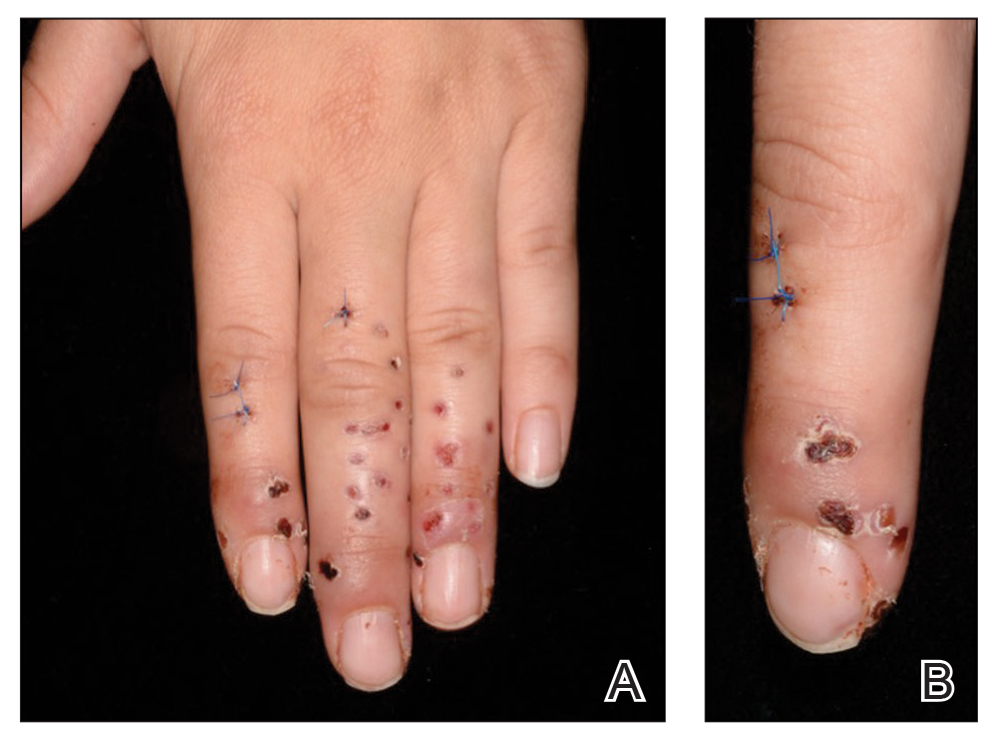
A 22-year-old woman presented to the outpatient department with extremely painful, erythematous papules on the second, third, and fourth fingers of the left hand, as well as diffuse swelling of the entire metacarpophalangeal and interphalangeal joints (Figure 1). She reported accidentally falling on a cholla cactus (genus Cylindropuntia) 2 weeks earlier while walking on a cholla cactus trail during a vacation in California. She reported that the symptoms had worsened over the last week. Class 3 corticosteroid ointments did not provide benefit. The patient had no comorbidities and was allergic to penicillin.

Radiographs of the left hand excluded concomitant fracture. Digital dermoscopy showed multiple white homogeneous areas with a central pustule (Figure 2A). Frequency-domain optical coherence tomography (OCT) displayed round hyperrefractive structures in the dermis suggestive of granulomas, as well as a small needlelike hyperrefractive structure, a foreign body (Figure 2B).
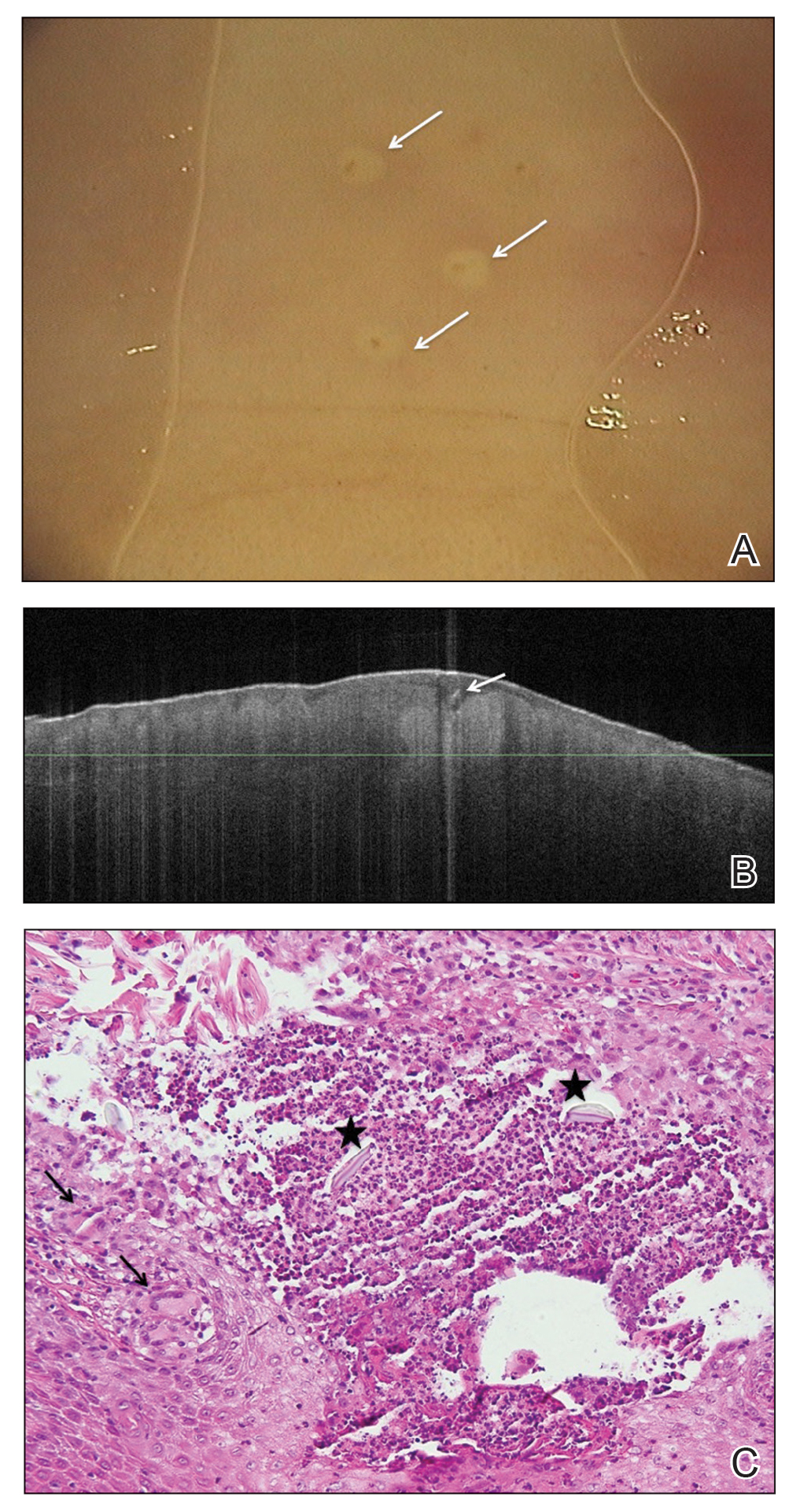
The few visible spines were immediately removed with tweezers; the patient remained symptom free for approximately 2 weeks. Subsequently, extreme pain developed in the left hand; the clinical presentation and pain did not respond to empiric intravenous antibiotic therapy with weight-calculated clarithromycin (500 mg twice daily), systemic analgesia with nonsteroidal anti-inflammatory drugs, and local therapy with antiseptics and class 3 corticosteroid ointment. Four days later, all 27 papules were excised with 3- and 4-mm punch biopsies using digital nerve blocks. Histology showed classic foreign body granulomas with hematoxylin and eosin stain (Figure 2C).
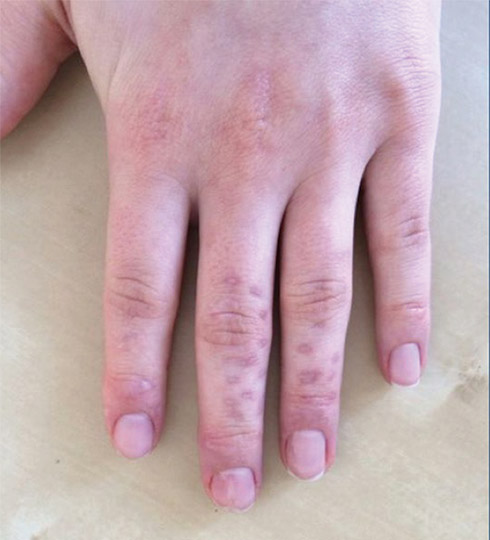
One week later, pain, erythema, and swelling had disappeared; no additional lesions had developed (Figure 3). Follow-up OCT showed no foreign bodies. At 4-week follow-up, the inflammatory component had disappeared, and no granulomas were evident. Six months later, the lesions healed with minimal scarring that could later be treated with fractional laser therapy (Figure 4).
Comment
Pathogenesis and Presentation
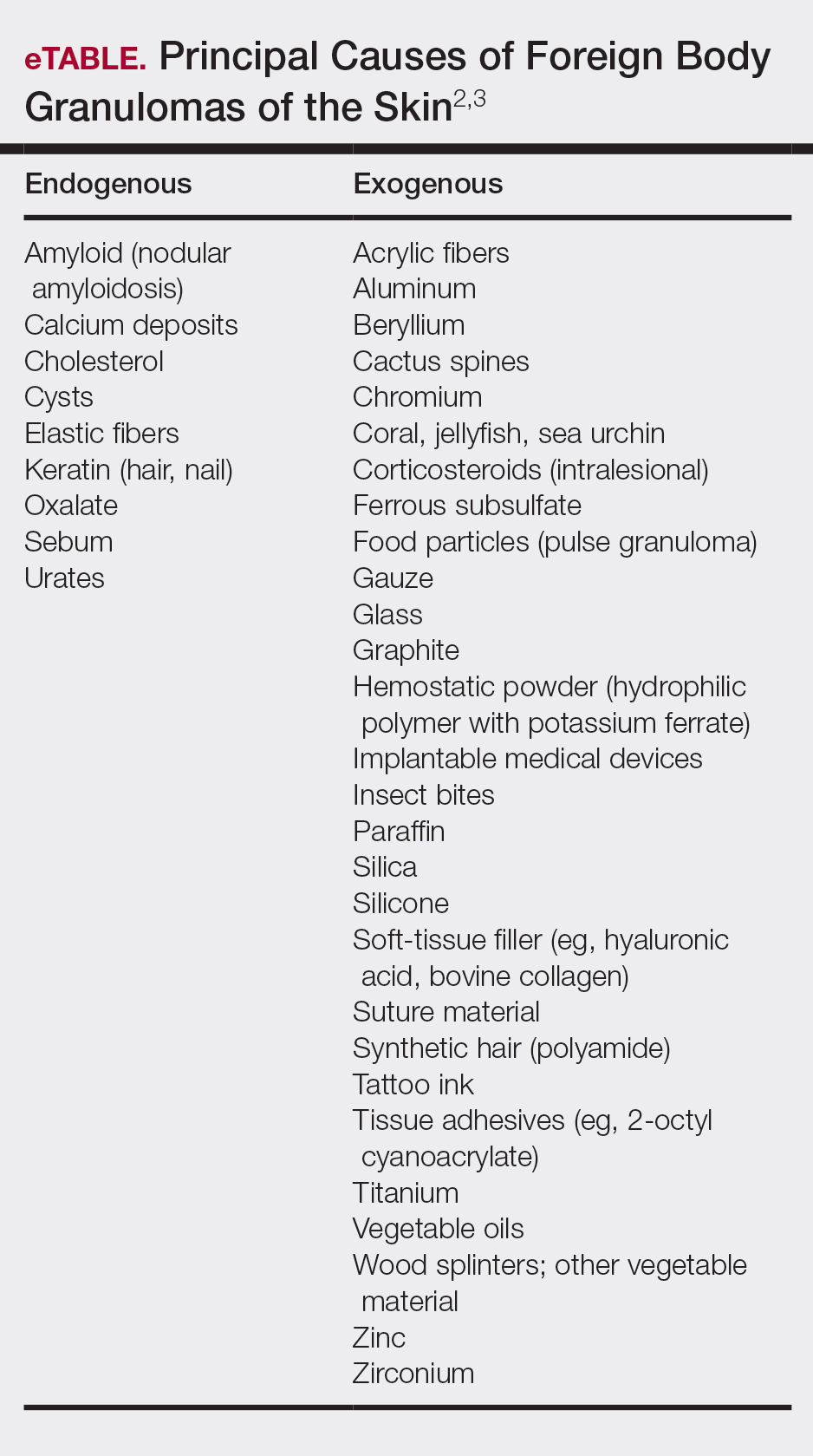
Cactus spines are included in the possible causes of foreign body granulomas of the skin (eTable).2,3 However, granulomatous inflammation after cactus spine injury rarely has been described in the medical literature. In the first known case report in 1955, Winer and Zeilenga4 described a woman who developed multiple hand granulomas that were partially removed by curettage, while the spines underwent slow spontaneous expulsion.
In 1971, Schreiber et al5 hypothesized a type 2 allergic response to cactus spines based on the variability of reactions in different cases. Doctoroff et al6 proposed an unroofing technique based on the removal of spines under microscopy, which brought faster (2–4 months) healing. Madkan et al7 reported that complete response is possible only with punch excision of the largest lesions.
The cholla (Cylindropuntia) cactus has been described as the species most commonly implicated in granulomatous reactions to cactus spines.8,9 Two principal pathogenic mechanisms have been described—foreign body granuloma and allergic reaction to cactus antigens—because not every patient develops granulomatous lesions.
Sequelae
Complications of injury from cactus spines are common, especially when spines are not completely removed, including local inflammation, superinfection, necrosis, allergic reactions, granulomas, scarring, and chronic pain. Rare consequences of cactus injury include bacterial infection with Staphylococcus aureus; Enterobacter species; atypical mycobacteria, including Mycobacterium marinum; Nocardia species; and Clostridium tetani, as well as deep fungal infection, especially in immunocompromised patients.10 In our case, bacterial culture and polymerase chain reaction testing for mycobacteria were negative.
Diagnosis
Cactus spine injuries usually are easy to diagnose based on the clear-cut anamnesis and clinical picture; however, it might be interesting to assess the presence of foreign body granulomas without biopsy. Optical coherence tomography is a noninvasive optical imaging technique based on low-coherence interferometry that uses a low-intensity, 1310-nm infrared laser. Widespread in ophthalmology, OCT has gained importance in dermatologic diagnostics, especially for nonmelanoma skin cancer.11 Moreover, it has demonstrated its usefulness in various dermatologic fields, including granulomatous lesions.12 Further methods include reflectance confocal microscopy, based on a near-infrared laser, and 7.5-MHz ultrasonography. In our experience, however, 7.5-MHz ultrasonography has been ineffective in detecting cactus spines in the current patient as well as others. Preoperative and postoperative monitoring with dermoscopy and OCT helped us evaluate the nature, size, and location of spines and lesions and effective healing.
Treatment
Management strategies are still debated and include watchful waiting, corticosteroid ointment, partial removal of spines, and unroofing.1,2,4-10,13-18 We treated our patient with an innovative radical surgical approach using punch excision for granulomas that developed after cholla cactus spine injury. Our approach resulted in rapid relief of pain and reduced complications, a good aesthetic result, and no recurrence.
- Lindsey D, Lindsey WE. Cactus spine injuries. Am J Emerg Med. 1988;6:362-369.
Molina-Ruiz AM, Requena L. Foreign body granulomas. Dermatol Clin. 2015;33:497-523. - Patterson JW. Weedon’s Skin Pathology. 4th ed. New York, NY: Elsevier; 2016.
- Winer LH, Zeilenga RH. Cactus granulomas of the skin; report of a case. AMA Arch Derm. 1955;72:566-569.
- Schreiber MM, Shapiro SI, Berry CZ. Cactus granulomas of the skin. an allergic phenomenon. Arch Dermatol. 1971;104:374-379.
- Doctoroff A, Vidimos AT, Taylor JS. Cactus skin injuries. Cutis. 2000;65:290-292.
- Madkan VK, Abraham T, Lesher JL Jr. Cactus spine granuloma. Cutis. 2007;79:208-210.
- Spoerke DG, Spoerke SE. Granuloma formation induced by spines of the cactus, Opuntia acanthocarpa. Vet Hum Toxicol. 1991;33:342-344.
- Suzuki H, Baba S. Cactus granuloma of the skin. J Dermatol. 1993;20:424-427.
- Burrell SR, Ostlie DJ, Saubolle M, et al. Apophysomyces elegans infection associated with cactus spine injury in an immunocompetent pediatric patient. Pediatr Infect Dis J. 1998;17:663-664.
- von BraunmT. Optical coherence tomography. Hautarzt. 2015;66:499-503.
- Banzhaf C, Jemec GB. Imaging granulomatous lesions with optical coherence tomography. Case Rep Dermatol. 2012;4:14-18.
- Putnam MH. Simple cactus spine removal. J Pediatr. 1981;98:333.
- Snyder RA, Schwartz RA. Cactus bristle implantation. Report of an unusual case initially seen with rows of yellow hairs. Arch Dermatol. 1983;119:152-154.
- Schunk JE, Corneli HM. Cactus spine removal. J Pediatr. 1987;110:667.
- Gutierrez Ortega MC, Martin Moreno L, Arias Palomo D, et al. Facial granuloma caused by cactus bristles. Med Cutan Ibero Lat Am. 1990;18:197-200.
- Dieter RA Jr, Whitehouse LR, Gulliver R. Cactus spine wounds: a case report and short review of the literature. Wounds. 2017;29:E18-E21.
- O’Neill PJ, Sinha M, McArthur RA, et al. Penetrating cactus spine injury to the mediastinum of a child. J Pediatr Surg. 2008;43:E33-E35.
Skin injuries caused by spines of various species of cactus are common in the southwestern United States and Mexico and have been described worldwide.1 Effects of injury vary depending on localization, surface extension, and skin conditions (eg, preexisting erosions, ulcerations, sunburns).
Case Report
A 22-year-old woman presented to the outpatient department with extremely painful, erythematous papules on the second, third, and fourth fingers of the left hand, as well as diffuse swelling of the entire metacarpophalangeal and interphalangeal joints (Figure 1). She reported accidentally falling on a cholla cactus (genus Cylindropuntia) 2 weeks earlier while walking on a cholla cactus trail during a vacation in California. She reported that the symptoms had worsened over the last week. Class 3 corticosteroid ointments did not provide benefit. The patient had no comorbidities and was allergic to penicillin.

Radiographs of the left hand excluded concomitant fracture. Digital dermoscopy showed multiple white homogeneous areas with a central pustule (Figure 2A). Frequency-domain optical coherence tomography (OCT) displayed round hyperrefractive structures in the dermis suggestive of granulomas, as well as a small needlelike hyperrefractive structure, a foreign body (Figure 2B).

The few visible spines were immediately removed with tweezers; the patient remained symptom free for approximately 2 weeks. Subsequently, extreme pain developed in the left hand; the clinical presentation and pain did not respond to empiric intravenous antibiotic therapy with weight-calculated clarithromycin (500 mg twice daily), systemic analgesia with nonsteroidal anti-inflammatory drugs, and local therapy with antiseptics and class 3 corticosteroid ointment. Four days later, all 27 papules were excised with 3- and 4-mm punch biopsies using digital nerve blocks. Histology showed classic foreign body granulomas with hematoxylin and eosin stain (Figure 2C).
One week later, pain, erythema, and swelling had disappeared; no additional lesions had developed (Figure 3). Follow-up OCT showed no foreign bodies. At 4-week follow-up, the inflammatory component had disappeared, and no granulomas were evident. Six months later, the lesions healed with minimal scarring that could later be treated with fractional laser therapy (Figure 4).


Comment
Pathogenesis and Presentation
Cactus spines are included in the possible causes of foreign body granulomas of the skin (eTable).2,3 However, granulomatous inflammation after cactus spine injury rarely has been described in the medical literature. In the first known case report in 1955, Winer and Zeilenga4 described a woman who developed multiple hand granulomas that were partially removed by curettage, while the spines underwent slow spontaneous expulsion.

In 1971, Schreiber et al5 hypothesized a type 2 allergic response to cactus spines based on the variability of reactions in different cases. Doctoroff et al6 proposed an unroofing technique based on the removal of spines under microscopy, which brought faster (2–4 months) healing. Madkan et al7 reported that complete response is possible only with punch excision of the largest lesions.
The cholla (Cylindropuntia) cactus has been described as the species most commonly implicated in granulomatous reactions to cactus spines.8,9 Two principal pathogenic mechanisms have been described—foreign body granuloma and allergic reaction to cactus antigens—because not every patient develops granulomatous lesions.
Sequelae
Complications of injury from cactus spines are common, especially when spines are not completely removed, including local inflammation, superinfection, necrosis, allergic reactions, granulomas, scarring, and chronic pain. Rare consequences of cactus injury include bacterial infection with Staphylococcus aureus; Enterobacter species; atypical mycobacteria, including Mycobacterium marinum; Nocardia species; and Clostridium tetani, as well as deep fungal infection, especially in immunocompromised patients.10 In our case, bacterial culture and polymerase chain reaction testing for mycobacteria were negative.
Diagnosis
Cactus spine injuries usually are easy to diagnose based on the clear-cut anamnesis and clinical picture; however, it might be interesting to assess the presence of foreign body granulomas without biopsy. Optical coherence tomography is a noninvasive optical imaging technique based on low-coherence interferometry that uses a low-intensity, 1310-nm infrared laser. Widespread in ophthalmology, OCT has gained importance in dermatologic diagnostics, especially for nonmelanoma skin cancer.11 Moreover, it has demonstrated its usefulness in various dermatologic fields, including granulomatous lesions.12 Further methods include reflectance confocal microscopy, based on a near-infrared laser, and 7.5-MHz ultrasonography. In our experience, however, 7.5-MHz ultrasonography has been ineffective in detecting cactus spines in the current patient as well as others. Preoperative and postoperative monitoring with dermoscopy and OCT helped us evaluate the nature, size, and location of spines and lesions and effective healing.
Treatment
Management strategies are still debated and include watchful waiting, corticosteroid ointment, partial removal of spines, and unroofing.1,2,4-10,13-18 We treated our patient with an innovative radical surgical approach using punch excision for granulomas that developed after cholla cactus spine injury. Our approach resulted in rapid relief of pain and reduced complications, a good aesthetic result, and no recurrence.
Skin injuries caused by spines of various species of cactus are common in the southwestern United States and Mexico and have been described worldwide.1 Effects of injury vary depending on localization, surface extension, and skin conditions (eg, preexisting erosions, ulcerations, sunburns).
Case Report
A 22-year-old woman presented to the outpatient department with extremely painful, erythematous papules on the second, third, and fourth fingers of the left hand, as well as diffuse swelling of the entire metacarpophalangeal and interphalangeal joints (Figure 1). She reported accidentally falling on a cholla cactus (genus Cylindropuntia) 2 weeks earlier while walking on a cholla cactus trail during a vacation in California. She reported that the symptoms had worsened over the last week. Class 3 corticosteroid ointments did not provide benefit. The patient had no comorbidities and was allergic to penicillin.

Radiographs of the left hand excluded concomitant fracture. Digital dermoscopy showed multiple white homogeneous areas with a central pustule (Figure 2A). Frequency-domain optical coherence tomography (OCT) displayed round hyperrefractive structures in the dermis suggestive of granulomas, as well as a small needlelike hyperrefractive structure, a foreign body (Figure 2B).

The few visible spines were immediately removed with tweezers; the patient remained symptom free for approximately 2 weeks. Subsequently, extreme pain developed in the left hand; the clinical presentation and pain did not respond to empiric intravenous antibiotic therapy with weight-calculated clarithromycin (500 mg twice daily), systemic analgesia with nonsteroidal anti-inflammatory drugs, and local therapy with antiseptics and class 3 corticosteroid ointment. Four days later, all 27 papules were excised with 3- and 4-mm punch biopsies using digital nerve blocks. Histology showed classic foreign body granulomas with hematoxylin and eosin stain (Figure 2C).
One week later, pain, erythema, and swelling had disappeared; no additional lesions had developed (Figure 3). Follow-up OCT showed no foreign bodies. At 4-week follow-up, the inflammatory component had disappeared, and no granulomas were evident. Six months later, the lesions healed with minimal scarring that could later be treated with fractional laser therapy (Figure 4).


Comment
Pathogenesis and Presentation
Cactus spines are included in the possible causes of foreign body granulomas of the skin (eTable).2,3 However, granulomatous inflammation after cactus spine injury rarely has been described in the medical literature. In the first known case report in 1955, Winer and Zeilenga4 described a woman who developed multiple hand granulomas that were partially removed by curettage, while the spines underwent slow spontaneous expulsion.

In 1971, Schreiber et al5 hypothesized a type 2 allergic response to cactus spines based on the variability of reactions in different cases. Doctoroff et al6 proposed an unroofing technique based on the removal of spines under microscopy, which brought faster (2–4 months) healing. Madkan et al7 reported that complete response is possible only with punch excision of the largest lesions.
The cholla (Cylindropuntia) cactus has been described as the species most commonly implicated in granulomatous reactions to cactus spines.8,9 Two principal pathogenic mechanisms have been described—foreign body granuloma and allergic reaction to cactus antigens—because not every patient develops granulomatous lesions.
Sequelae
Complications of injury from cactus spines are common, especially when spines are not completely removed, including local inflammation, superinfection, necrosis, allergic reactions, granulomas, scarring, and chronic pain. Rare consequences of cactus injury include bacterial infection with Staphylococcus aureus; Enterobacter species; atypical mycobacteria, including Mycobacterium marinum; Nocardia species; and Clostridium tetani, as well as deep fungal infection, especially in immunocompromised patients.10 In our case, bacterial culture and polymerase chain reaction testing for mycobacteria were negative.
Diagnosis
Cactus spine injuries usually are easy to diagnose based on the clear-cut anamnesis and clinical picture; however, it might be interesting to assess the presence of foreign body granulomas without biopsy. Optical coherence tomography is a noninvasive optical imaging technique based on low-coherence interferometry that uses a low-intensity, 1310-nm infrared laser. Widespread in ophthalmology, OCT has gained importance in dermatologic diagnostics, especially for nonmelanoma skin cancer.11 Moreover, it has demonstrated its usefulness in various dermatologic fields, including granulomatous lesions.12 Further methods include reflectance confocal microscopy, based on a near-infrared laser, and 7.5-MHz ultrasonography. In our experience, however, 7.5-MHz ultrasonography has been ineffective in detecting cactus spines in the current patient as well as others. Preoperative and postoperative monitoring with dermoscopy and OCT helped us evaluate the nature, size, and location of spines and lesions and effective healing.
Treatment
Management strategies are still debated and include watchful waiting, corticosteroid ointment, partial removal of spines, and unroofing.1,2,4-10,13-18 We treated our patient with an innovative radical surgical approach using punch excision for granulomas that developed after cholla cactus spine injury. Our approach resulted in rapid relief of pain and reduced complications, a good aesthetic result, and no recurrence.
- Lindsey D, Lindsey WE. Cactus spine injuries. Am J Emerg Med. 1988;6:362-369.
Molina-Ruiz AM, Requena L. Foreign body granulomas. Dermatol Clin. 2015;33:497-523. - Patterson JW. Weedon’s Skin Pathology. 4th ed. New York, NY: Elsevier; 2016.
- Winer LH, Zeilenga RH. Cactus granulomas of the skin; report of a case. AMA Arch Derm. 1955;72:566-569.
- Schreiber MM, Shapiro SI, Berry CZ. Cactus granulomas of the skin. an allergic phenomenon. Arch Dermatol. 1971;104:374-379.
- Doctoroff A, Vidimos AT, Taylor JS. Cactus skin injuries. Cutis. 2000;65:290-292.
- Madkan VK, Abraham T, Lesher JL Jr. Cactus spine granuloma. Cutis. 2007;79:208-210.
- Spoerke DG, Spoerke SE. Granuloma formation induced by spines of the cactus, Opuntia acanthocarpa. Vet Hum Toxicol. 1991;33:342-344.
- Suzuki H, Baba S. Cactus granuloma of the skin. J Dermatol. 1993;20:424-427.
- Burrell SR, Ostlie DJ, Saubolle M, et al. Apophysomyces elegans infection associated with cactus spine injury in an immunocompetent pediatric patient. Pediatr Infect Dis J. 1998;17:663-664.
- von BraunmT. Optical coherence tomography. Hautarzt. 2015;66:499-503.
- Banzhaf C, Jemec GB. Imaging granulomatous lesions with optical coherence tomography. Case Rep Dermatol. 2012;4:14-18.
- Putnam MH. Simple cactus spine removal. J Pediatr. 1981;98:333.
- Snyder RA, Schwartz RA. Cactus bristle implantation. Report of an unusual case initially seen with rows of yellow hairs. Arch Dermatol. 1983;119:152-154.
- Schunk JE, Corneli HM. Cactus spine removal. J Pediatr. 1987;110:667.
- Gutierrez Ortega MC, Martin Moreno L, Arias Palomo D, et al. Facial granuloma caused by cactus bristles. Med Cutan Ibero Lat Am. 1990;18:197-200.
- Dieter RA Jr, Whitehouse LR, Gulliver R. Cactus spine wounds: a case report and short review of the literature. Wounds. 2017;29:E18-E21.
- O’Neill PJ, Sinha M, McArthur RA, et al. Penetrating cactus spine injury to the mediastinum of a child. J Pediatr Surg. 2008;43:E33-E35.
- Lindsey D, Lindsey WE. Cactus spine injuries. Am J Emerg Med. 1988;6:362-369.
Molina-Ruiz AM, Requena L. Foreign body granulomas. Dermatol Clin. 2015;33:497-523. - Patterson JW. Weedon’s Skin Pathology. 4th ed. New York, NY: Elsevier; 2016.
- Winer LH, Zeilenga RH. Cactus granulomas of the skin; report of a case. AMA Arch Derm. 1955;72:566-569.
- Schreiber MM, Shapiro SI, Berry CZ. Cactus granulomas of the skin. an allergic phenomenon. Arch Dermatol. 1971;104:374-379.
- Doctoroff A, Vidimos AT, Taylor JS. Cactus skin injuries. Cutis. 2000;65:290-292.
- Madkan VK, Abraham T, Lesher JL Jr. Cactus spine granuloma. Cutis. 2007;79:208-210.
- Spoerke DG, Spoerke SE. Granuloma formation induced by spines of the cactus, Opuntia acanthocarpa. Vet Hum Toxicol. 1991;33:342-344.
- Suzuki H, Baba S. Cactus granuloma of the skin. J Dermatol. 1993;20:424-427.
- Burrell SR, Ostlie DJ, Saubolle M, et al. Apophysomyces elegans infection associated with cactus spine injury in an immunocompetent pediatric patient. Pediatr Infect Dis J. 1998;17:663-664.
- von BraunmT. Optical coherence tomography. Hautarzt. 2015;66:499-503.
- Banzhaf C, Jemec GB. Imaging granulomatous lesions with optical coherence tomography. Case Rep Dermatol. 2012;4:14-18.
- Putnam MH. Simple cactus spine removal. J Pediatr. 1981;98:333.
- Snyder RA, Schwartz RA. Cactus bristle implantation. Report of an unusual case initially seen with rows of yellow hairs. Arch Dermatol. 1983;119:152-154.
- Schunk JE, Corneli HM. Cactus spine removal. J Pediatr. 1987;110:667.
- Gutierrez Ortega MC, Martin Moreno L, Arias Palomo D, et al. Facial granuloma caused by cactus bristles. Med Cutan Ibero Lat Am. 1990;18:197-200.
- Dieter RA Jr, Whitehouse LR, Gulliver R. Cactus spine wounds: a case report and short review of the literature. Wounds. 2017;29:E18-E21.
- O’Neill PJ, Sinha M, McArthur RA, et al. Penetrating cactus spine injury to the mediastinum of a child. J Pediatr Surg. 2008;43:E33-E35.
Practice Points
- Cactus spine injuries are an important source of morbidity in sports and leisure.
- Even after removal of cactus spines, painful granulomas can develop and persist for a long period of time. Patient education on early treatment can prevent further complications.
- Immediate and complete removal of spines as well as avoidance of bacterial superinfections should be given priority in cactus spine injuries. In case of granulomas, a surgical approach can result in rapid relief of symptoms.
SCC survival remains poor in epidermolysis bullosa
LONDON – Median survival among patients with generalized severe recessive dystrophic epidermolysis bullosa (RDEB-GS) after a first diagnosis of mucocutaneous squamous cell carcinoma (SCC) was 2.4 years in an observational, retrospective study.
The study, conducted at St. Thomas’ Hospital and Great Ormond Street Hospital in London, was a review of all individuals with EB who had developed the skin cancer over a 28-year period, from 1991 to 2019.
A total of 44 subjects were identified who together had 221 primary SCCs. Considering all study subjects, the median age at first diagnosis of SCC was 32.6 years, with a mean of five tumors present. Almost 40% had metastatic tumors, and of the 57% who died during the observation period, 88% of deaths were attributable to the SCC.
“EB-associated SCCs differ from those in the general population,” the study’s investigators wrote in a poster presented at the EB World Congress, organized by the Dystrophic Epidermolysis Bullosa Association (debra). “They affect a younger age group, and there are often multiple primaries,” they added. Furthermore, “they behave aggressively and metastasize early despite being well differentiated.”
Most (31) of the study participants had RDEB-GS and tended to develop their first SCC at a younger age than the group overall, at a median of 29.5 years (compared with 32.6 years for the overall group). The mean number of tumors was 5.8 among those with RDEB-GS, with over half (53.4%) of the SCCs being well differentiated and located on the hands, upper arms, feet, and lower legs. Median survival after a first diagnosis in this group was 2.4 years. The short survival after a first diagnosis of SCC “underscores the poor prognosis in this group,” the researchers wrote.
“As the largest cohort of EB SCC patients with comprehensive data regarding clinical course and management to date, our data reinforce the need for regular clinical surveillance for SCCs in EB patients,” the team concluded. This surveillance should start in adolescence for those with the severe generalized RDEB subtype, they advise, and from the third or fourth decade for other at-risk groups.
These data also highlight “the pressing need for more effective treatments,” the investigators wrote. Most (86.4%) of the SCCs among the patients in the study had been surgically removed by wide local excision, with a few patients undergoing lymph node dissection, radiotherapy, chemotherapy, electrochemotherapy, or receiving targeted cancer therapies such as erlotinib, cetuximab, or cemiplimab.
Surgery may not be an option for many patients, Jemima Mellerio, MD explained in an oral presentation at the meeting. Dr. Mellerio, a consultant dermatologist and chief of St John’s Institute of Dermatology at Guy’s & St. Thomas’ NHS Foundation, London, noted that the location of the tumor was important, as sometimes it was not physically possible to excise it completely.
Guidelines on how to manage SCCs in patients with EB were published a few years ago (Br J Dermatol. 2016;174:56-67) and noted that the clinical detection of SCCs could be difficult because of chronic wound ulceration in these patients. The “possibility of malignancy should be borne in mind, with suspicious lesions biopsied for histological evaluation,” the document states. Evidence for many of the nonsurgical options – radiotherapy, conventional chemotherapy, biologic therapies – was poor, according to the guidelines, and effective nonsurgical options are still desperately needed.
Several avenues of research are being investigated, Dr. Mellerio noted, such as targeting the fibrotic process and perhaps using a micro-RNA inhibitor to stop the upregulation of certain microRNAs in fibroblasts. Targeting inflammatory mechanisms such as thrombospondin 1, which can lead to elevated levels of tumor necrosis factor–beta and contribute to extracellular matrix stiffness, also is under investigation. Raised interleukin-6 may be another target to consider.
Research shows that similar genes are mutated in EB-related and ultraviolet-related SCCs, Dr. Mellerio said. Indeed, mutations in HRAS, NOTCH1, TP53, and CDKN2A have been reported, but mutations in these genes occur much earlier in life in patients with EB. “Something else is going on,” she added, commenting that researchers are looking at apolipoprotein B editing complex (APOBEC) enzymes, which modulate DNA and can cause “particular types of genetic changes in EB cancers.”
One investigator who is studying the genetics of EB SCCs and how APOBEC enzymes might be involved is Andrew South, PhD, an associate professor at Thomas Jefferson University, Philadelphia. APOBEC enzymes are a very prominent source of mutations in RDEB. These mutations are found in 10%-20% of squamous cell carcinomas not associated with RDEB, and 80%-90% of head and neck cancers, he said during a separate talk at the meeting.
Dr. South observed that “RDEB squamous cell carcinoma does not show any particular somatic mutation or upregulation or downregulation of genes that differentiates it from other squamous cell carcinomas, which might be disappointing on the front of it, but actually it does mean that precision therapies that have been developed for other squamous cell carcinomas have application in RDEB.”
RDEB SCC shows the greatest similarity with head and neck SCC, Dr. South said. He also stressed that fibrosis is a major driver of cancer development, SCC tumors in RDEB are homogenous, and that frontline therapy is still unclear.
What is clear, however, is that interdisciplinary management of patients is crucial, said Leena Bruckner-Tuderman, MD, professor and chair of the department of dermatology at the University Medical Center, Albert Ludwig University of Freiburg, Germany.
“In severe RDEB, metastatic SCC is the leading cause of death at a young age. We need monitoring, careful diagnostics, and multidisciplinary treatment,” Dr. Bruckner-Tuderman said. The latter should be delivered by a coordinated team that consists of dermatologists, surgeons, radiologists, oncologists, pathologists, geneticists, and (molecular) tumor boards, she advised.
The study had no commercial funding. Dr. Mellerio disclosed financial relationships with Castle Creek Pharmaceuticals and ProQR Therapeutics, and acted as an unpaid advisor to Helpberby Therapeutics. Dr. South disclosed financial relationships with Krystal Biotech Inc. and Amryt Genetics and has been an advisory board member for Abeona Therapeutics and Sanofi Genzyme. Dr. Bruckner-Tuderman disclosed receiving grants or research support from Constant Pharmaceuticals/Tarix Orphan.
LONDON – Median survival among patients with generalized severe recessive dystrophic epidermolysis bullosa (RDEB-GS) after a first diagnosis of mucocutaneous squamous cell carcinoma (SCC) was 2.4 years in an observational, retrospective study.
The study, conducted at St. Thomas’ Hospital and Great Ormond Street Hospital in London, was a review of all individuals with EB who had developed the skin cancer over a 28-year period, from 1991 to 2019.
A total of 44 subjects were identified who together had 221 primary SCCs. Considering all study subjects, the median age at first diagnosis of SCC was 32.6 years, with a mean of five tumors present. Almost 40% had metastatic tumors, and of the 57% who died during the observation period, 88% of deaths were attributable to the SCC.
“EB-associated SCCs differ from those in the general population,” the study’s investigators wrote in a poster presented at the EB World Congress, organized by the Dystrophic Epidermolysis Bullosa Association (debra). “They affect a younger age group, and there are often multiple primaries,” they added. Furthermore, “they behave aggressively and metastasize early despite being well differentiated.”
Most (31) of the study participants had RDEB-GS and tended to develop their first SCC at a younger age than the group overall, at a median of 29.5 years (compared with 32.6 years for the overall group). The mean number of tumors was 5.8 among those with RDEB-GS, with over half (53.4%) of the SCCs being well differentiated and located on the hands, upper arms, feet, and lower legs. Median survival after a first diagnosis in this group was 2.4 years. The short survival after a first diagnosis of SCC “underscores the poor prognosis in this group,” the researchers wrote.
“As the largest cohort of EB SCC patients with comprehensive data regarding clinical course and management to date, our data reinforce the need for regular clinical surveillance for SCCs in EB patients,” the team concluded. This surveillance should start in adolescence for those with the severe generalized RDEB subtype, they advise, and from the third or fourth decade for other at-risk groups.
These data also highlight “the pressing need for more effective treatments,” the investigators wrote. Most (86.4%) of the SCCs among the patients in the study had been surgically removed by wide local excision, with a few patients undergoing lymph node dissection, radiotherapy, chemotherapy, electrochemotherapy, or receiving targeted cancer therapies such as erlotinib, cetuximab, or cemiplimab.
Surgery may not be an option for many patients, Jemima Mellerio, MD explained in an oral presentation at the meeting. Dr. Mellerio, a consultant dermatologist and chief of St John’s Institute of Dermatology at Guy’s & St. Thomas’ NHS Foundation, London, noted that the location of the tumor was important, as sometimes it was not physically possible to excise it completely.
Guidelines on how to manage SCCs in patients with EB were published a few years ago (Br J Dermatol. 2016;174:56-67) and noted that the clinical detection of SCCs could be difficult because of chronic wound ulceration in these patients. The “possibility of malignancy should be borne in mind, with suspicious lesions biopsied for histological evaluation,” the document states. Evidence for many of the nonsurgical options – radiotherapy, conventional chemotherapy, biologic therapies – was poor, according to the guidelines, and effective nonsurgical options are still desperately needed.
Several avenues of research are being investigated, Dr. Mellerio noted, such as targeting the fibrotic process and perhaps using a micro-RNA inhibitor to stop the upregulation of certain microRNAs in fibroblasts. Targeting inflammatory mechanisms such as thrombospondin 1, which can lead to elevated levels of tumor necrosis factor–beta and contribute to extracellular matrix stiffness, also is under investigation. Raised interleukin-6 may be another target to consider.
Research shows that similar genes are mutated in EB-related and ultraviolet-related SCCs, Dr. Mellerio said. Indeed, mutations in HRAS, NOTCH1, TP53, and CDKN2A have been reported, but mutations in these genes occur much earlier in life in patients with EB. “Something else is going on,” she added, commenting that researchers are looking at apolipoprotein B editing complex (APOBEC) enzymes, which modulate DNA and can cause “particular types of genetic changes in EB cancers.”
One investigator who is studying the genetics of EB SCCs and how APOBEC enzymes might be involved is Andrew South, PhD, an associate professor at Thomas Jefferson University, Philadelphia. APOBEC enzymes are a very prominent source of mutations in RDEB. These mutations are found in 10%-20% of squamous cell carcinomas not associated with RDEB, and 80%-90% of head and neck cancers, he said during a separate talk at the meeting.
Dr. South observed that “RDEB squamous cell carcinoma does not show any particular somatic mutation or upregulation or downregulation of genes that differentiates it from other squamous cell carcinomas, which might be disappointing on the front of it, but actually it does mean that precision therapies that have been developed for other squamous cell carcinomas have application in RDEB.”
RDEB SCC shows the greatest similarity with head and neck SCC, Dr. South said. He also stressed that fibrosis is a major driver of cancer development, SCC tumors in RDEB are homogenous, and that frontline therapy is still unclear.
What is clear, however, is that interdisciplinary management of patients is crucial, said Leena Bruckner-Tuderman, MD, professor and chair of the department of dermatology at the University Medical Center, Albert Ludwig University of Freiburg, Germany.
“In severe RDEB, metastatic SCC is the leading cause of death at a young age. We need monitoring, careful diagnostics, and multidisciplinary treatment,” Dr. Bruckner-Tuderman said. The latter should be delivered by a coordinated team that consists of dermatologists, surgeons, radiologists, oncologists, pathologists, geneticists, and (molecular) tumor boards, she advised.
The study had no commercial funding. Dr. Mellerio disclosed financial relationships with Castle Creek Pharmaceuticals and ProQR Therapeutics, and acted as an unpaid advisor to Helpberby Therapeutics. Dr. South disclosed financial relationships with Krystal Biotech Inc. and Amryt Genetics and has been an advisory board member for Abeona Therapeutics and Sanofi Genzyme. Dr. Bruckner-Tuderman disclosed receiving grants or research support from Constant Pharmaceuticals/Tarix Orphan.
LONDON – Median survival among patients with generalized severe recessive dystrophic epidermolysis bullosa (RDEB-GS) after a first diagnosis of mucocutaneous squamous cell carcinoma (SCC) was 2.4 years in an observational, retrospective study.
The study, conducted at St. Thomas’ Hospital and Great Ormond Street Hospital in London, was a review of all individuals with EB who had developed the skin cancer over a 28-year period, from 1991 to 2019.
A total of 44 subjects were identified who together had 221 primary SCCs. Considering all study subjects, the median age at first diagnosis of SCC was 32.6 years, with a mean of five tumors present. Almost 40% had metastatic tumors, and of the 57% who died during the observation period, 88% of deaths were attributable to the SCC.
“EB-associated SCCs differ from those in the general population,” the study’s investigators wrote in a poster presented at the EB World Congress, organized by the Dystrophic Epidermolysis Bullosa Association (debra). “They affect a younger age group, and there are often multiple primaries,” they added. Furthermore, “they behave aggressively and metastasize early despite being well differentiated.”
Most (31) of the study participants had RDEB-GS and tended to develop their first SCC at a younger age than the group overall, at a median of 29.5 years (compared with 32.6 years for the overall group). The mean number of tumors was 5.8 among those with RDEB-GS, with over half (53.4%) of the SCCs being well differentiated and located on the hands, upper arms, feet, and lower legs. Median survival after a first diagnosis in this group was 2.4 years. The short survival after a first diagnosis of SCC “underscores the poor prognosis in this group,” the researchers wrote.
“As the largest cohort of EB SCC patients with comprehensive data regarding clinical course and management to date, our data reinforce the need for regular clinical surveillance for SCCs in EB patients,” the team concluded. This surveillance should start in adolescence for those with the severe generalized RDEB subtype, they advise, and from the third or fourth decade for other at-risk groups.
These data also highlight “the pressing need for more effective treatments,” the investigators wrote. Most (86.4%) of the SCCs among the patients in the study had been surgically removed by wide local excision, with a few patients undergoing lymph node dissection, radiotherapy, chemotherapy, electrochemotherapy, or receiving targeted cancer therapies such as erlotinib, cetuximab, or cemiplimab.
Surgery may not be an option for many patients, Jemima Mellerio, MD explained in an oral presentation at the meeting. Dr. Mellerio, a consultant dermatologist and chief of St John’s Institute of Dermatology at Guy’s & St. Thomas’ NHS Foundation, London, noted that the location of the tumor was important, as sometimes it was not physically possible to excise it completely.
Guidelines on how to manage SCCs in patients with EB were published a few years ago (Br J Dermatol. 2016;174:56-67) and noted that the clinical detection of SCCs could be difficult because of chronic wound ulceration in these patients. The “possibility of malignancy should be borne in mind, with suspicious lesions biopsied for histological evaluation,” the document states. Evidence for many of the nonsurgical options – radiotherapy, conventional chemotherapy, biologic therapies – was poor, according to the guidelines, and effective nonsurgical options are still desperately needed.
Several avenues of research are being investigated, Dr. Mellerio noted, such as targeting the fibrotic process and perhaps using a micro-RNA inhibitor to stop the upregulation of certain microRNAs in fibroblasts. Targeting inflammatory mechanisms such as thrombospondin 1, which can lead to elevated levels of tumor necrosis factor–beta and contribute to extracellular matrix stiffness, also is under investigation. Raised interleukin-6 may be another target to consider.
Research shows that similar genes are mutated in EB-related and ultraviolet-related SCCs, Dr. Mellerio said. Indeed, mutations in HRAS, NOTCH1, TP53, and CDKN2A have been reported, but mutations in these genes occur much earlier in life in patients with EB. “Something else is going on,” she added, commenting that researchers are looking at apolipoprotein B editing complex (APOBEC) enzymes, which modulate DNA and can cause “particular types of genetic changes in EB cancers.”
One investigator who is studying the genetics of EB SCCs and how APOBEC enzymes might be involved is Andrew South, PhD, an associate professor at Thomas Jefferson University, Philadelphia. APOBEC enzymes are a very prominent source of mutations in RDEB. These mutations are found in 10%-20% of squamous cell carcinomas not associated with RDEB, and 80%-90% of head and neck cancers, he said during a separate talk at the meeting.
Dr. South observed that “RDEB squamous cell carcinoma does not show any particular somatic mutation or upregulation or downregulation of genes that differentiates it from other squamous cell carcinomas, which might be disappointing on the front of it, but actually it does mean that precision therapies that have been developed for other squamous cell carcinomas have application in RDEB.”
RDEB SCC shows the greatest similarity with head and neck SCC, Dr. South said. He also stressed that fibrosis is a major driver of cancer development, SCC tumors in RDEB are homogenous, and that frontline therapy is still unclear.
What is clear, however, is that interdisciplinary management of patients is crucial, said Leena Bruckner-Tuderman, MD, professor and chair of the department of dermatology at the University Medical Center, Albert Ludwig University of Freiburg, Germany.
“In severe RDEB, metastatic SCC is the leading cause of death at a young age. We need monitoring, careful diagnostics, and multidisciplinary treatment,” Dr. Bruckner-Tuderman said. The latter should be delivered by a coordinated team that consists of dermatologists, surgeons, radiologists, oncologists, pathologists, geneticists, and (molecular) tumor boards, she advised.
The study had no commercial funding. Dr. Mellerio disclosed financial relationships with Castle Creek Pharmaceuticals and ProQR Therapeutics, and acted as an unpaid advisor to Helpberby Therapeutics. Dr. South disclosed financial relationships with Krystal Biotech Inc. and Amryt Genetics and has been an advisory board member for Abeona Therapeutics and Sanofi Genzyme. Dr. Bruckner-Tuderman disclosed receiving grants or research support from Constant Pharmaceuticals/Tarix Orphan.
REPORTING FROM EB 2020
Epidermolysis bullosa classification criteria refined and ready
LONDON – have come in understanding this debilitating group of genetic skin diseases, but also how far there is still to go towards improving the management of those affected.
Previous criteria issued in 2014 represented “important progress” and “built on the achievements of several generations of physicians and researchers who described the phenotypes, the level of skin cleavage, developed and characterized antibodies, and discovered EB-associated genes,” Cristina Has, MD, said at the EB World Congress, organized by the Dystrophic Epidermolysis Bullosa Association (DEBRA).
Dr. Has, a senior dermatologist and professor of experimental dermatology at the University of Freiburg (Germany), observed that prior criteria had “introduced genetic and molecular data in a so-called onion-skin classification of EB, and removed most of the eponyms,” which had been maintained in the latest update.
“What is new, and probably the most important change, is making the distinction between classical EB and other disorders with skin fragility,” she said, noting that the revised classification criteria for EB included minor changes to the nomenclature of EB. Six new EB subtypes and genes have also been added, and there are new sections on genotype/phenotype correlations, disease modifying factors, and the natural history of EB. Furthermore, supporting information included a concise description of clinical and genetic features of all EB types and subtypes.
The updated criteria are the result of an expert meeting held in April 2019 and have been accepted for publication. The expert panel that developed the criteria think that the revised classification criteria will be “useful and, we hope, inspiring and motivating for the young generation of dermatologists, pediatricians, and for the researchers who work in this field,” Dr. Has said.
“The term EB has been used in the last years for many new disorders, and this is the reason why we thought we have to somehow control this, and to make the distinction between classical epidermolysis bullosa due to defects at the dermal junction and other disorders with skin fragility where the anomalies occur within other layers of the epidermis or in the dermis,” Dr. Has explained.
There are still 4 main types of classical EB: EB simplex (EBS), dystrophic EB (DEB), junctional EB, and Kindler EB, but there are now 34 subtypes, slightly fewer than before. The updated criteria distinguish between the types and subtypes according to the level of skin cleavage, the inheritance pattern, the mutated gene, and the targeted protein, Dr. Has said.
As for peeling disorders, these have been classified as being erosive or hyperkeratotic, or as affecting the connective tissue with skin blistering. Similar to classical EB, these disorders are associated with fragility of the skin and mucosa and share some pathogenetic mechanisms. Moreover, as “the suffering of the patient is similar,” Dr. Has said, “we’d like to consider them under the umbrella of EB.” Most of the disorders she listed were inherited via an autosomal recessive mechanism, with intraepidermal disorders inherited via an autosomal dominant mechanism. New genes are being identified the time, she added, so these groupings will no doubt be subject to future revisions.
Minor changes to nomenclature were made to avoid confusion among clinicians and those living with the condition. As such, Kindler EB replaces Kindler syndrome, names of some subtypes were simplified, and a new “self-improving” type of DEB was introduced to replace the term “transient dermolysis of the newborn.” Altogether, there are now 11 subtypes of DEB. A distinction was also made between syndromic and nonsyndromic EB. “We all know that EB can be a systemic disorder with secondary manifestations within different organs,” Dr. Has told conference attendees. Anemia and failure to thrive can be associated, but it still remains a nonsyndromic disorder, she said. By contrast, “syndromic EB is due to genetic defects, which are also expressed in other organs than the skin or mucosal membranes, and lead to primary extracutaneous manifestations, such as cardiomyopathy, nephropathy, and so on.”
There are fewer subtypes of EBS and “we think they are better defined,” Dr. Has stated. “EB simplex is the most heterogenous EB type, clinically and genetically, and includes several syndromic disorders,” and the new classification criteria should be useful in helping categorize individuals with EBS and thus help target their management.
One of the six new subtypes of EB included in the revised classification criteria is “syndromic EBS with cardiomyopathy” caused by the KLH24 mutation. This gene was discovered in 2016 and more than 40 cases have so far been identified, 50% of which have been sporadic de novo mutations.
Other new EB subtypes are:
- “EBS with localized nephropathy” caused by a mutation in the CD151 gene.
- An autosomal recessive EBS linked to the KRT5 gene.
- A new phenotype that manifests with oral mucosal blisters linked to the DSG3 gene. (Although only a single case has been reported to date, it was felt worthy of inclusion.)
- Another linked to DSG3 that leads to skin fragility and hypertrichosis.
- A new dystrophic EB subtype linked to mutations in the PLOD3 gene.
In an interview, Dr. Has reiterated the importance of keeping classification criteria updated in line with current research findings. She emphasized that there were many types of EB and how important it was to refine how these were classified based on the underlying genetics.
“We brought much more genetic data into the paper, because we are in the era of personalized medicine,” she said. “There are specific therapies for mutations and for different subtypes and that’s why we think that, step by step, we have to bring in more and more data into the classification.”
There are many people with EBS, she observed, and while these individuals may not have such a dramatic clinical presentation as those with recessive DEB, for example, the effect of the condition on their daily lives is no less. “These people are active, they have jobs, they have to work, and they have pain, they have blister,” Dr. Has said.
While the criteria are intended only for classification of EB, they might help in practice. Dr. Has gave an anecdotal example of a woman that has been misdiagnosed as having a type of DEB with a high risk of squamous cell carcinoma but in fact had a different form of EB with no risk of developing SCC. “That’s why criteria are important,” she said.
Dr. Has had no conflicts of interest to disclose.
LONDON – have come in understanding this debilitating group of genetic skin diseases, but also how far there is still to go towards improving the management of those affected.
Previous criteria issued in 2014 represented “important progress” and “built on the achievements of several generations of physicians and researchers who described the phenotypes, the level of skin cleavage, developed and characterized antibodies, and discovered EB-associated genes,” Cristina Has, MD, said at the EB World Congress, organized by the Dystrophic Epidermolysis Bullosa Association (DEBRA).
Dr. Has, a senior dermatologist and professor of experimental dermatology at the University of Freiburg (Germany), observed that prior criteria had “introduced genetic and molecular data in a so-called onion-skin classification of EB, and removed most of the eponyms,” which had been maintained in the latest update.
“What is new, and probably the most important change, is making the distinction between classical EB and other disorders with skin fragility,” she said, noting that the revised classification criteria for EB included minor changes to the nomenclature of EB. Six new EB subtypes and genes have also been added, and there are new sections on genotype/phenotype correlations, disease modifying factors, and the natural history of EB. Furthermore, supporting information included a concise description of clinical and genetic features of all EB types and subtypes.
The updated criteria are the result of an expert meeting held in April 2019 and have been accepted for publication. The expert panel that developed the criteria think that the revised classification criteria will be “useful and, we hope, inspiring and motivating for the young generation of dermatologists, pediatricians, and for the researchers who work in this field,” Dr. Has said.
“The term EB has been used in the last years for many new disorders, and this is the reason why we thought we have to somehow control this, and to make the distinction between classical epidermolysis bullosa due to defects at the dermal junction and other disorders with skin fragility where the anomalies occur within other layers of the epidermis or in the dermis,” Dr. Has explained.
There are still 4 main types of classical EB: EB simplex (EBS), dystrophic EB (DEB), junctional EB, and Kindler EB, but there are now 34 subtypes, slightly fewer than before. The updated criteria distinguish between the types and subtypes according to the level of skin cleavage, the inheritance pattern, the mutated gene, and the targeted protein, Dr. Has said.
As for peeling disorders, these have been classified as being erosive or hyperkeratotic, or as affecting the connective tissue with skin blistering. Similar to classical EB, these disorders are associated with fragility of the skin and mucosa and share some pathogenetic mechanisms. Moreover, as “the suffering of the patient is similar,” Dr. Has said, “we’d like to consider them under the umbrella of EB.” Most of the disorders she listed were inherited via an autosomal recessive mechanism, with intraepidermal disorders inherited via an autosomal dominant mechanism. New genes are being identified the time, she added, so these groupings will no doubt be subject to future revisions.
Minor changes to nomenclature were made to avoid confusion among clinicians and those living with the condition. As such, Kindler EB replaces Kindler syndrome, names of some subtypes were simplified, and a new “self-improving” type of DEB was introduced to replace the term “transient dermolysis of the newborn.” Altogether, there are now 11 subtypes of DEB. A distinction was also made between syndromic and nonsyndromic EB. “We all know that EB can be a systemic disorder with secondary manifestations within different organs,” Dr. Has told conference attendees. Anemia and failure to thrive can be associated, but it still remains a nonsyndromic disorder, she said. By contrast, “syndromic EB is due to genetic defects, which are also expressed in other organs than the skin or mucosal membranes, and lead to primary extracutaneous manifestations, such as cardiomyopathy, nephropathy, and so on.”
There are fewer subtypes of EBS and “we think they are better defined,” Dr. Has stated. “EB simplex is the most heterogenous EB type, clinically and genetically, and includes several syndromic disorders,” and the new classification criteria should be useful in helping categorize individuals with EBS and thus help target their management.
One of the six new subtypes of EB included in the revised classification criteria is “syndromic EBS with cardiomyopathy” caused by the KLH24 mutation. This gene was discovered in 2016 and more than 40 cases have so far been identified, 50% of which have been sporadic de novo mutations.
Other new EB subtypes are:
- “EBS with localized nephropathy” caused by a mutation in the CD151 gene.
- An autosomal recessive EBS linked to the KRT5 gene.
- A new phenotype that manifests with oral mucosal blisters linked to the DSG3 gene. (Although only a single case has been reported to date, it was felt worthy of inclusion.)
- Another linked to DSG3 that leads to skin fragility and hypertrichosis.
- A new dystrophic EB subtype linked to mutations in the PLOD3 gene.
In an interview, Dr. Has reiterated the importance of keeping classification criteria updated in line with current research findings. She emphasized that there were many types of EB and how important it was to refine how these were classified based on the underlying genetics.
“We brought much more genetic data into the paper, because we are in the era of personalized medicine,” she said. “There are specific therapies for mutations and for different subtypes and that’s why we think that, step by step, we have to bring in more and more data into the classification.”
There are many people with EBS, she observed, and while these individuals may not have such a dramatic clinical presentation as those with recessive DEB, for example, the effect of the condition on their daily lives is no less. “These people are active, they have jobs, they have to work, and they have pain, they have blister,” Dr. Has said.
While the criteria are intended only for classification of EB, they might help in practice. Dr. Has gave an anecdotal example of a woman that has been misdiagnosed as having a type of DEB with a high risk of squamous cell carcinoma but in fact had a different form of EB with no risk of developing SCC. “That’s why criteria are important,” she said.
Dr. Has had no conflicts of interest to disclose.
LONDON – have come in understanding this debilitating group of genetic skin diseases, but also how far there is still to go towards improving the management of those affected.
Previous criteria issued in 2014 represented “important progress” and “built on the achievements of several generations of physicians and researchers who described the phenotypes, the level of skin cleavage, developed and characterized antibodies, and discovered EB-associated genes,” Cristina Has, MD, said at the EB World Congress, organized by the Dystrophic Epidermolysis Bullosa Association (DEBRA).
Dr. Has, a senior dermatologist and professor of experimental dermatology at the University of Freiburg (Germany), observed that prior criteria had “introduced genetic and molecular data in a so-called onion-skin classification of EB, and removed most of the eponyms,” which had been maintained in the latest update.
“What is new, and probably the most important change, is making the distinction between classical EB and other disorders with skin fragility,” she said, noting that the revised classification criteria for EB included minor changes to the nomenclature of EB. Six new EB subtypes and genes have also been added, and there are new sections on genotype/phenotype correlations, disease modifying factors, and the natural history of EB. Furthermore, supporting information included a concise description of clinical and genetic features of all EB types and subtypes.
The updated criteria are the result of an expert meeting held in April 2019 and have been accepted for publication. The expert panel that developed the criteria think that the revised classification criteria will be “useful and, we hope, inspiring and motivating for the young generation of dermatologists, pediatricians, and for the researchers who work in this field,” Dr. Has said.
“The term EB has been used in the last years for many new disorders, and this is the reason why we thought we have to somehow control this, and to make the distinction between classical epidermolysis bullosa due to defects at the dermal junction and other disorders with skin fragility where the anomalies occur within other layers of the epidermis or in the dermis,” Dr. Has explained.
There are still 4 main types of classical EB: EB simplex (EBS), dystrophic EB (DEB), junctional EB, and Kindler EB, but there are now 34 subtypes, slightly fewer than before. The updated criteria distinguish between the types and subtypes according to the level of skin cleavage, the inheritance pattern, the mutated gene, and the targeted protein, Dr. Has said.
As for peeling disorders, these have been classified as being erosive or hyperkeratotic, or as affecting the connective tissue with skin blistering. Similar to classical EB, these disorders are associated with fragility of the skin and mucosa and share some pathogenetic mechanisms. Moreover, as “the suffering of the patient is similar,” Dr. Has said, “we’d like to consider them under the umbrella of EB.” Most of the disorders she listed were inherited via an autosomal recessive mechanism, with intraepidermal disorders inherited via an autosomal dominant mechanism. New genes are being identified the time, she added, so these groupings will no doubt be subject to future revisions.
Minor changes to nomenclature were made to avoid confusion among clinicians and those living with the condition. As such, Kindler EB replaces Kindler syndrome, names of some subtypes were simplified, and a new “self-improving” type of DEB was introduced to replace the term “transient dermolysis of the newborn.” Altogether, there are now 11 subtypes of DEB. A distinction was also made between syndromic and nonsyndromic EB. “We all know that EB can be a systemic disorder with secondary manifestations within different organs,” Dr. Has told conference attendees. Anemia and failure to thrive can be associated, but it still remains a nonsyndromic disorder, she said. By contrast, “syndromic EB is due to genetic defects, which are also expressed in other organs than the skin or mucosal membranes, and lead to primary extracutaneous manifestations, such as cardiomyopathy, nephropathy, and so on.”
There are fewer subtypes of EBS and “we think they are better defined,” Dr. Has stated. “EB simplex is the most heterogenous EB type, clinically and genetically, and includes several syndromic disorders,” and the new classification criteria should be useful in helping categorize individuals with EBS and thus help target their management.
One of the six new subtypes of EB included in the revised classification criteria is “syndromic EBS with cardiomyopathy” caused by the KLH24 mutation. This gene was discovered in 2016 and more than 40 cases have so far been identified, 50% of which have been sporadic de novo mutations.
Other new EB subtypes are:
- “EBS with localized nephropathy” caused by a mutation in the CD151 gene.
- An autosomal recessive EBS linked to the KRT5 gene.
- A new phenotype that manifests with oral mucosal blisters linked to the DSG3 gene. (Although only a single case has been reported to date, it was felt worthy of inclusion.)
- Another linked to DSG3 that leads to skin fragility and hypertrichosis.
- A new dystrophic EB subtype linked to mutations in the PLOD3 gene.
In an interview, Dr. Has reiterated the importance of keeping classification criteria updated in line with current research findings. She emphasized that there were many types of EB and how important it was to refine how these were classified based on the underlying genetics.
“We brought much more genetic data into the paper, because we are in the era of personalized medicine,” she said. “There are specific therapies for mutations and for different subtypes and that’s why we think that, step by step, we have to bring in more and more data into the classification.”
There are many people with EBS, she observed, and while these individuals may not have such a dramatic clinical presentation as those with recessive DEB, for example, the effect of the condition on their daily lives is no less. “These people are active, they have jobs, they have to work, and they have pain, they have blister,” Dr. Has said.
While the criteria are intended only for classification of EB, they might help in practice. Dr. Has gave an anecdotal example of a woman that has been misdiagnosed as having a type of DEB with a high risk of squamous cell carcinoma but in fact had a different form of EB with no risk of developing SCC. “That’s why criteria are important,” she said.
Dr. Has had no conflicts of interest to disclose.
REPORTING FROM EB 2020
Psoriasis ointment helped with itch, healing in phase 2 EB study
LONDON – , in a small, placebo-controlled, phase 2 study.
More importantly, use of the ointment promoted wound healing in those with the severe skin-blistering condition. Indeed, compared with placebo, a greater reduction in wound size was observed after 2 weeks when the ointment was applied (a mean reduction of 65.5% vs. 88.4%; P less than .006). However, at 1 month, no significant differences were seen in the size of the wounds between the two treatment arms.
“Calcipotriol is a vitamin D analog and it is well known that vitamin D is a very critical factor for skin homeostasis and proper wound healing,” Christina Guttmann-Gruber, PhD, said at the EB World Congress, organized by the Dystrophic Epidermolysis Bullosa Association (DEBRA). Dr. Guttmann-Gruber, a group lead researcher for EB House Austria, which is based at the Salzburg (Austria) University Clinic for Dermatology, noted that vitamin D also helps with tissue repair and immune modulation, and enhances local antimicrobial activity.
During an oral poster presentation at the meeting, Dr. Guttmann-Gruber explained that in previous in vitro studies, it was found that low concentrations (100 nmol) of calcipotriol inhibited proliferation of RDEB tumor cells (Sci Rep. 2018 Sep 7;8:13430). Calcipotriol (also known as calcipotriene) also was found to improve the expression of antimicrobial peptides and promote wound closure. “Therefore, we thought that applying calcipotriol at the site of injury, on chronic wounds prone to superinfection where it is needed, might be beneficial for our patients.”
She and her associates designed a two-arm, randomized, double-blind crossover study to assess the effects of an existing calcipotriol-containing ointment on wound healing in patients with RDEB. The ointment used in the study is approved for treating psoriasis but was adapted by the in-house pharmacy team to reduce the concentration of calcipotriol to about 0.05 mcg/g, or around 121 nmol. The reason for the reduction was that, at higher doses, keratinocyte proliferation was reduced, which would be detrimental in RDEB patients.
Nine patients were included in the study and were randomized to either apply 1 g of the active or placebo ointment to each of two designated wounds, of at least 6 cm2 in size, every day for 4 weeks. A 2-month washout period then followed before the groups switched to use the other ointment for 1 month. Six out of the nine patients completed both treatment phases. The reasons for the patients not completing both intervention phases were not related to the drug.
Calcipotriol treatment resulted in a significant and steady reduction in itch over the entire course of treatment, which was not seen among those on placebo, Dr. Guttmann-Gruber observed. The reduction in itch was “striking,” but only while the treatment was being used, she said. Results for pain were less clear, with a significant reduction in pain after 2 weeks seen only in the placebo group, while both treatments reduced pain to the same degree by 1 month.
No serious adverse events were observed at any time point and topical use of the low-dose calcipotriol did not significantly change serum levels of calcium or vitamin D in the two patients in which this was studied, Dr. Guttmann-Gruber said.
“This is an approved drug; it’s used in psoriasis, but at a very high concentration. We were able to use it off label and make a diluted version,” she observed. “Any pharmacy can do it.” Although it was applied topically, it could be done by applying it to the dressing rather directly onto the wounded skin, she said.
Data on the skin microbiome response to treatment were also collected but were not available to analyze in time for presentation, but it appeared that there was improvement with the low-dose calcipotriol treatment, Dr. Guttmann-Gruber said. “When the wounds are healing, the microbial flora is improving.”
The next step will probably be to plan a multicenter trial of this treatment, Dr. Guttmann-Gruber said in an interview. The questions is whether such a trial would get the financial backing it needed, but if an orphan drug designation could be obtained for calcipotriol for EB, then it would be possible to conduct such a trial.
The study was funded by DEBRA Austria. The presenting author, Dr. Guttmann-Gruber, had no conflicts of interest to disclose.
SOURCE: Guttmann-Gruber C et al. EB World Congress 2020. Poster 34.
LONDON – , in a small, placebo-controlled, phase 2 study.
More importantly, use of the ointment promoted wound healing in those with the severe skin-blistering condition. Indeed, compared with placebo, a greater reduction in wound size was observed after 2 weeks when the ointment was applied (a mean reduction of 65.5% vs. 88.4%; P less than .006). However, at 1 month, no significant differences were seen in the size of the wounds between the two treatment arms.
“Calcipotriol is a vitamin D analog and it is well known that vitamin D is a very critical factor for skin homeostasis and proper wound healing,” Christina Guttmann-Gruber, PhD, said at the EB World Congress, organized by the Dystrophic Epidermolysis Bullosa Association (DEBRA). Dr. Guttmann-Gruber, a group lead researcher for EB House Austria, which is based at the Salzburg (Austria) University Clinic for Dermatology, noted that vitamin D also helps with tissue repair and immune modulation, and enhances local antimicrobial activity.
During an oral poster presentation at the meeting, Dr. Guttmann-Gruber explained that in previous in vitro studies, it was found that low concentrations (100 nmol) of calcipotriol inhibited proliferation of RDEB tumor cells (Sci Rep. 2018 Sep 7;8:13430). Calcipotriol (also known as calcipotriene) also was found to improve the expression of antimicrobial peptides and promote wound closure. “Therefore, we thought that applying calcipotriol at the site of injury, on chronic wounds prone to superinfection where it is needed, might be beneficial for our patients.”
She and her associates designed a two-arm, randomized, double-blind crossover study to assess the effects of an existing calcipotriol-containing ointment on wound healing in patients with RDEB. The ointment used in the study is approved for treating psoriasis but was adapted by the in-house pharmacy team to reduce the concentration of calcipotriol to about 0.05 mcg/g, or around 121 nmol. The reason for the reduction was that, at higher doses, keratinocyte proliferation was reduced, which would be detrimental in RDEB patients.
Nine patients were included in the study and were randomized to either apply 1 g of the active or placebo ointment to each of two designated wounds, of at least 6 cm2 in size, every day for 4 weeks. A 2-month washout period then followed before the groups switched to use the other ointment for 1 month. Six out of the nine patients completed both treatment phases. The reasons for the patients not completing both intervention phases were not related to the drug.
Calcipotriol treatment resulted in a significant and steady reduction in itch over the entire course of treatment, which was not seen among those on placebo, Dr. Guttmann-Gruber observed. The reduction in itch was “striking,” but only while the treatment was being used, she said. Results for pain were less clear, with a significant reduction in pain after 2 weeks seen only in the placebo group, while both treatments reduced pain to the same degree by 1 month.
No serious adverse events were observed at any time point and topical use of the low-dose calcipotriol did not significantly change serum levels of calcium or vitamin D in the two patients in which this was studied, Dr. Guttmann-Gruber said.
“This is an approved drug; it’s used in psoriasis, but at a very high concentration. We were able to use it off label and make a diluted version,” she observed. “Any pharmacy can do it.” Although it was applied topically, it could be done by applying it to the dressing rather directly onto the wounded skin, she said.
Data on the skin microbiome response to treatment were also collected but were not available to analyze in time for presentation, but it appeared that there was improvement with the low-dose calcipotriol treatment, Dr. Guttmann-Gruber said. “When the wounds are healing, the microbial flora is improving.”
The next step will probably be to plan a multicenter trial of this treatment, Dr. Guttmann-Gruber said in an interview. The questions is whether such a trial would get the financial backing it needed, but if an orphan drug designation could be obtained for calcipotriol for EB, then it would be possible to conduct such a trial.
The study was funded by DEBRA Austria. The presenting author, Dr. Guttmann-Gruber, had no conflicts of interest to disclose.
SOURCE: Guttmann-Gruber C et al. EB World Congress 2020. Poster 34.
LONDON – , in a small, placebo-controlled, phase 2 study.
More importantly, use of the ointment promoted wound healing in those with the severe skin-blistering condition. Indeed, compared with placebo, a greater reduction in wound size was observed after 2 weeks when the ointment was applied (a mean reduction of 65.5% vs. 88.4%; P less than .006). However, at 1 month, no significant differences were seen in the size of the wounds between the two treatment arms.
“Calcipotriol is a vitamin D analog and it is well known that vitamin D is a very critical factor for skin homeostasis and proper wound healing,” Christina Guttmann-Gruber, PhD, said at the EB World Congress, organized by the Dystrophic Epidermolysis Bullosa Association (DEBRA). Dr. Guttmann-Gruber, a group lead researcher for EB House Austria, which is based at the Salzburg (Austria) University Clinic for Dermatology, noted that vitamin D also helps with tissue repair and immune modulation, and enhances local antimicrobial activity.
During an oral poster presentation at the meeting, Dr. Guttmann-Gruber explained that in previous in vitro studies, it was found that low concentrations (100 nmol) of calcipotriol inhibited proliferation of RDEB tumor cells (Sci Rep. 2018 Sep 7;8:13430). Calcipotriol (also known as calcipotriene) also was found to improve the expression of antimicrobial peptides and promote wound closure. “Therefore, we thought that applying calcipotriol at the site of injury, on chronic wounds prone to superinfection where it is needed, might be beneficial for our patients.”
She and her associates designed a two-arm, randomized, double-blind crossover study to assess the effects of an existing calcipotriol-containing ointment on wound healing in patients with RDEB. The ointment used in the study is approved for treating psoriasis but was adapted by the in-house pharmacy team to reduce the concentration of calcipotriol to about 0.05 mcg/g, or around 121 nmol. The reason for the reduction was that, at higher doses, keratinocyte proliferation was reduced, which would be detrimental in RDEB patients.
Nine patients were included in the study and were randomized to either apply 1 g of the active or placebo ointment to each of two designated wounds, of at least 6 cm2 in size, every day for 4 weeks. A 2-month washout period then followed before the groups switched to use the other ointment for 1 month. Six out of the nine patients completed both treatment phases. The reasons for the patients not completing both intervention phases were not related to the drug.
Calcipotriol treatment resulted in a significant and steady reduction in itch over the entire course of treatment, which was not seen among those on placebo, Dr. Guttmann-Gruber observed. The reduction in itch was “striking,” but only while the treatment was being used, she said. Results for pain were less clear, with a significant reduction in pain after 2 weeks seen only in the placebo group, while both treatments reduced pain to the same degree by 1 month.
No serious adverse events were observed at any time point and topical use of the low-dose calcipotriol did not significantly change serum levels of calcium or vitamin D in the two patients in which this was studied, Dr. Guttmann-Gruber said.
“This is an approved drug; it’s used in psoriasis, but at a very high concentration. We were able to use it off label and make a diluted version,” she observed. “Any pharmacy can do it.” Although it was applied topically, it could be done by applying it to the dressing rather directly onto the wounded skin, she said.
Data on the skin microbiome response to treatment were also collected but were not available to analyze in time for presentation, but it appeared that there was improvement with the low-dose calcipotriol treatment, Dr. Guttmann-Gruber said. “When the wounds are healing, the microbial flora is improving.”
The next step will probably be to plan a multicenter trial of this treatment, Dr. Guttmann-Gruber said in an interview. The questions is whether such a trial would get the financial backing it needed, but if an orphan drug designation could be obtained for calcipotriol for EB, then it would be possible to conduct such a trial.
The study was funded by DEBRA Austria. The presenting author, Dr. Guttmann-Gruber, had no conflicts of interest to disclose.
SOURCE: Guttmann-Gruber C et al. EB World Congress 2020. Poster 34.
REPORTING FROM EB 2020
Losartan showing promise in pediatric epidermolysis bullosa trial
LONDON – Treatment with the in an early clinical study.
In the ongoing phase 1/2 REFLECT (Recessive dystrophic EB: Mechanisms of fibrosis and its prevention with Losartan in vivo) trial, involving 29 children, no severe complications have been noted so far, according to one of the study investigators, Dimitra Kiritsi, MD, of the University of Freiburg, Germany. At the EB World Congress, organized by the Dystrophic Epidermolysis Bullosa Association (DEBRA), she presented interim data on 18 patients in the trial, emphasizing that the primary aim of the trial was to evaluate the safety of this treatment approach.
Over the 2 years the trial has been underway, 65 adverse events have been reported, of which 4 have been severe. Two of these were bacterial infections that required hospital treatment and the other two were a reduction in the general health condition of the child.
Losartan is an angiotensin-II receptor blocker (ARB) that has been in clinical use for more than 25 years in adults and 15 years in children over the age of 6 years.
The drug may be used for treating recessive dystrophic EB (RDEB) in the future, Dr. Kiritsi said, because it attenuates tumor necrosis factor–beta (TGF-beta) signaling, which is thought to be involved in the fibrotic process. So while it may not target the genetic defect, it could help ameliorate the effects of the disease.
The precursor to REFLECT was a study performed in a mouse disease model of EB (EMBO Mol Med. 2015;7:1211-28) where a reduction in fibrotic scarring was seen with losartan with “remarkable effects” on “mitten” deformity, Dr. Kiritsi said. The results of that study suggested that the earlier treatment with losartan was started in the course of the disease, the better the effect, she added. (Mitten deformity is the result of fused skin between the fingers or toes, and the subsequent buildup of fibrotic tissue causes the hand or foot to contract.)
REFLECT is an investigator-initiated trial that started in 2017 and is being funded by DEBRA International. It is a dual-center, nonrandomized, single-arm study in which children aged 3-16 years with RDEB are treated with losartan for 10 months, with follow-up at 3 months.
Various secondary endpoints were included to look for the first signs of any efficacy: the Physician’s Global Assessment (PGA), the Birmingham Epidermolysis Bullosa Severity Score (BEBS), the Epidermolysis Bullosa Disease Activity and Scarring Index (EBDASI), the Itch Assessment Scale for the Pediatric Burn Patients, and two quality of life indices: the Quality of Life in EB (QOLEB) questionnaire and the Children’s Dermatology Life Quality Index (CDLQI).
Dr. Kiritsi highlighted a few of the secondary endpoint findings, saying that reduced BEBS scores showed there was “amelioration of the patients’ phenotype” and that EBDASI scores also decreased, with “nearly 60% of the patients having significant improvement of their skin disease.” Importantly, itch improved in most of the patients, she said. Reductions in CDLQI were observed, “meaning that quality of life was significantly better at the end of the trial.” There were also decreases in inflammatory markers, such as C-reactive protein, interleukin-6, and TNF-alpha.
Although there is no validated tool available to assess hand function, Dr. Kiritsi and her team used their own morphometric scoring instrument to measure how far the hand could stretch; their evaluations suggested that this measure improved – or at least did not worsen – with losartan treatment, she noted.
A larger, randomized trial is needed to confirm if there is any benefit of losartan, but first, a new, easy-to-swallow losartan formulation needs to be developed specifically for EB in the pediatric population, Dr. Kiritsi said. Although a pediatric suspension of losartan was previously available, it is no longer on the market, so the next step is to develop a formulation that could be used in a pivotal clinical trial, she noted.
“Losartan faces fewer technical hurdles compared to other novel treatments as it is an established medicine,” Dr. Kiritsi and associates observed in a poster presentation. There are still economic hurdles, however, since “with losartan patents expired, companies cannot expect to recoup an investment into clinical studies” and alternative funding sources are needed.
In 2019, losartan was granted an orphan drug designation for the treatment of EB from both the Food and Drug Administration and the European Medicines Agency, but its use remains off label in children. “We decided to treat children,” Dr. Kiritsi said, “because we wanted to start as early as possible. If you already have mitten deformities, these cannot be reversed.”
DEBRA International funded the study. Dr. Kiritsi received research support from Rheacell GmbH and honoraria or consultation fees from Amryt Pharma and Rheacell GmbH. She has received other support from DEBRA International, EB Research Partnership, Fritz Thyssen Stiftung, German Research Foundation (funding of research projects), and 3R Pharma Consulting and Midas Pharma GmbH (consultation for losartan new drug formulation).
SOURCE: Kiritsi D et al. EB 2020. Poster 47.
LONDON – Treatment with the in an early clinical study.
In the ongoing phase 1/2 REFLECT (Recessive dystrophic EB: Mechanisms of fibrosis and its prevention with Losartan in vivo) trial, involving 29 children, no severe complications have been noted so far, according to one of the study investigators, Dimitra Kiritsi, MD, of the University of Freiburg, Germany. At the EB World Congress, organized by the Dystrophic Epidermolysis Bullosa Association (DEBRA), she presented interim data on 18 patients in the trial, emphasizing that the primary aim of the trial was to evaluate the safety of this treatment approach.
Over the 2 years the trial has been underway, 65 adverse events have been reported, of which 4 have been severe. Two of these were bacterial infections that required hospital treatment and the other two were a reduction in the general health condition of the child.
Losartan is an angiotensin-II receptor blocker (ARB) that has been in clinical use for more than 25 years in adults and 15 years in children over the age of 6 years.
The drug may be used for treating recessive dystrophic EB (RDEB) in the future, Dr. Kiritsi said, because it attenuates tumor necrosis factor–beta (TGF-beta) signaling, which is thought to be involved in the fibrotic process. So while it may not target the genetic defect, it could help ameliorate the effects of the disease.
The precursor to REFLECT was a study performed in a mouse disease model of EB (EMBO Mol Med. 2015;7:1211-28) where a reduction in fibrotic scarring was seen with losartan with “remarkable effects” on “mitten” deformity, Dr. Kiritsi said. The results of that study suggested that the earlier treatment with losartan was started in the course of the disease, the better the effect, she added. (Mitten deformity is the result of fused skin between the fingers or toes, and the subsequent buildup of fibrotic tissue causes the hand or foot to contract.)
REFLECT is an investigator-initiated trial that started in 2017 and is being funded by DEBRA International. It is a dual-center, nonrandomized, single-arm study in which children aged 3-16 years with RDEB are treated with losartan for 10 months, with follow-up at 3 months.
Various secondary endpoints were included to look for the first signs of any efficacy: the Physician’s Global Assessment (PGA), the Birmingham Epidermolysis Bullosa Severity Score (BEBS), the Epidermolysis Bullosa Disease Activity and Scarring Index (EBDASI), the Itch Assessment Scale for the Pediatric Burn Patients, and two quality of life indices: the Quality of Life in EB (QOLEB) questionnaire and the Children’s Dermatology Life Quality Index (CDLQI).
Dr. Kiritsi highlighted a few of the secondary endpoint findings, saying that reduced BEBS scores showed there was “amelioration of the patients’ phenotype” and that EBDASI scores also decreased, with “nearly 60% of the patients having significant improvement of their skin disease.” Importantly, itch improved in most of the patients, she said. Reductions in CDLQI were observed, “meaning that quality of life was significantly better at the end of the trial.” There were also decreases in inflammatory markers, such as C-reactive protein, interleukin-6, and TNF-alpha.
Although there is no validated tool available to assess hand function, Dr. Kiritsi and her team used their own morphometric scoring instrument to measure how far the hand could stretch; their evaluations suggested that this measure improved – or at least did not worsen – with losartan treatment, she noted.
A larger, randomized trial is needed to confirm if there is any benefit of losartan, but first, a new, easy-to-swallow losartan formulation needs to be developed specifically for EB in the pediatric population, Dr. Kiritsi said. Although a pediatric suspension of losartan was previously available, it is no longer on the market, so the next step is to develop a formulation that could be used in a pivotal clinical trial, she noted.
“Losartan faces fewer technical hurdles compared to other novel treatments as it is an established medicine,” Dr. Kiritsi and associates observed in a poster presentation. There are still economic hurdles, however, since “with losartan patents expired, companies cannot expect to recoup an investment into clinical studies” and alternative funding sources are needed.
In 2019, losartan was granted an orphan drug designation for the treatment of EB from both the Food and Drug Administration and the European Medicines Agency, but its use remains off label in children. “We decided to treat children,” Dr. Kiritsi said, “because we wanted to start as early as possible. If you already have mitten deformities, these cannot be reversed.”
DEBRA International funded the study. Dr. Kiritsi received research support from Rheacell GmbH and honoraria or consultation fees from Amryt Pharma and Rheacell GmbH. She has received other support from DEBRA International, EB Research Partnership, Fritz Thyssen Stiftung, German Research Foundation (funding of research projects), and 3R Pharma Consulting and Midas Pharma GmbH (consultation for losartan new drug formulation).
SOURCE: Kiritsi D et al. EB 2020. Poster 47.
LONDON – Treatment with the in an early clinical study.
In the ongoing phase 1/2 REFLECT (Recessive dystrophic EB: Mechanisms of fibrosis and its prevention with Losartan in vivo) trial, involving 29 children, no severe complications have been noted so far, according to one of the study investigators, Dimitra Kiritsi, MD, of the University of Freiburg, Germany. At the EB World Congress, organized by the Dystrophic Epidermolysis Bullosa Association (DEBRA), she presented interim data on 18 patients in the trial, emphasizing that the primary aim of the trial was to evaluate the safety of this treatment approach.
Over the 2 years the trial has been underway, 65 adverse events have been reported, of which 4 have been severe. Two of these were bacterial infections that required hospital treatment and the other two were a reduction in the general health condition of the child.
Losartan is an angiotensin-II receptor blocker (ARB) that has been in clinical use for more than 25 years in adults and 15 years in children over the age of 6 years.
The drug may be used for treating recessive dystrophic EB (RDEB) in the future, Dr. Kiritsi said, because it attenuates tumor necrosis factor–beta (TGF-beta) signaling, which is thought to be involved in the fibrotic process. So while it may not target the genetic defect, it could help ameliorate the effects of the disease.
The precursor to REFLECT was a study performed in a mouse disease model of EB (EMBO Mol Med. 2015;7:1211-28) where a reduction in fibrotic scarring was seen with losartan with “remarkable effects” on “mitten” deformity, Dr. Kiritsi said. The results of that study suggested that the earlier treatment with losartan was started in the course of the disease, the better the effect, she added. (Mitten deformity is the result of fused skin between the fingers or toes, and the subsequent buildup of fibrotic tissue causes the hand or foot to contract.)
REFLECT is an investigator-initiated trial that started in 2017 and is being funded by DEBRA International. It is a dual-center, nonrandomized, single-arm study in which children aged 3-16 years with RDEB are treated with losartan for 10 months, with follow-up at 3 months.
Various secondary endpoints were included to look for the first signs of any efficacy: the Physician’s Global Assessment (PGA), the Birmingham Epidermolysis Bullosa Severity Score (BEBS), the Epidermolysis Bullosa Disease Activity and Scarring Index (EBDASI), the Itch Assessment Scale for the Pediatric Burn Patients, and two quality of life indices: the Quality of Life in EB (QOLEB) questionnaire and the Children’s Dermatology Life Quality Index (CDLQI).
Dr. Kiritsi highlighted a few of the secondary endpoint findings, saying that reduced BEBS scores showed there was “amelioration of the patients’ phenotype” and that EBDASI scores also decreased, with “nearly 60% of the patients having significant improvement of their skin disease.” Importantly, itch improved in most of the patients, she said. Reductions in CDLQI were observed, “meaning that quality of life was significantly better at the end of the trial.” There were also decreases in inflammatory markers, such as C-reactive protein, interleukin-6, and TNF-alpha.
Although there is no validated tool available to assess hand function, Dr. Kiritsi and her team used their own morphometric scoring instrument to measure how far the hand could stretch; their evaluations suggested that this measure improved – or at least did not worsen – with losartan treatment, she noted.
A larger, randomized trial is needed to confirm if there is any benefit of losartan, but first, a new, easy-to-swallow losartan formulation needs to be developed specifically for EB in the pediatric population, Dr. Kiritsi said. Although a pediatric suspension of losartan was previously available, it is no longer on the market, so the next step is to develop a formulation that could be used in a pivotal clinical trial, she noted.
“Losartan faces fewer technical hurdles compared to other novel treatments as it is an established medicine,” Dr. Kiritsi and associates observed in a poster presentation. There are still economic hurdles, however, since “with losartan patents expired, companies cannot expect to recoup an investment into clinical studies” and alternative funding sources are needed.
In 2019, losartan was granted an orphan drug designation for the treatment of EB from both the Food and Drug Administration and the European Medicines Agency, but its use remains off label in children. “We decided to treat children,” Dr. Kiritsi said, “because we wanted to start as early as possible. If you already have mitten deformities, these cannot be reversed.”
DEBRA International funded the study. Dr. Kiritsi received research support from Rheacell GmbH and honoraria or consultation fees from Amryt Pharma and Rheacell GmbH. She has received other support from DEBRA International, EB Research Partnership, Fritz Thyssen Stiftung, German Research Foundation (funding of research projects), and 3R Pharma Consulting and Midas Pharma GmbH (consultation for losartan new drug formulation).
SOURCE: Kiritsi D et al. EB 2020. Poster 47.
REPORTING FROM EB 2020
High cost of wound dressings for epidermolysis bullosa highlighted
LONDON – More than £2.8 million (RDEB), according to a report at the EB World Congress, organized by the Dystrophic Epidermolysis Bullosa Association (DEBRA).
Results from the Prospective Epidermolysis Bullosa Longitudinal Evaluation Study (PEBLES), which is looking at the natural history of RDEB, showed that wound dressing and bandage costs were highest for study participants with the generalized severe (RDEB-GS) subtype, at just over £85,156 (about $112,450) per patient annually. Respective yearly costs for the generalized intermediate (RDEB-GI) and inversa (RDEB-INV) subtypes were £10,112 (about $13,350) and £1,699 (about $2,240) per patient.
Looking at the costs associated with EB is important, said one of the lead investigators for PEBLES, Jemima Mellerio, MD, FRCP, consultant dermatologist at St John’s Institute of Dermatology, at Guy’s & St. Thomas’ NHS Foundation Trust, London.
“If we are going to justify the kind of expenditure [associated with new treatments], we need to know that what we are treating is already a significant burden on our health care systems,” Dr. Mellerio said.
PEBLES is an ongoing London-based registry study that is enrolling patients with all subtypes of RDEB. Data are collected via a tablet device and include demographic data, information on clinical features, results of skin biopsies and genetic tests, and laboratory findings, as well as objective disease severity and subjective patient-orientated outcome scores.
So far, 60 patients – 49 adults and 11 children – have been enrolled in PEBLES since November 2014: 26 with RDEB-GS, 23 with RDEB-GI, 9 with RDEB-INV, and 2 with the pruriginosa RDEB subtype (RDEB-PR).
Most of the participants (71%) changed all their wound dressings at one time, patching up when required. Fourteen of 49 participants had paid people to help them change their dressings and when the total cost of combined wound dressings and paid care was taken into consideration, the mean annual cost per patient was around £2,500 (about $3,300) for RDEB-INV, £10,375 (about $13,700) per patient for RDEB-GS, and a staggering £98,000 (about $129,000) per patient for RDEB-GS. The total annual cost of dressings and associated care was an estimated £3,184,229 (about $4.2 million).
In addition to data on the cost of wound dressings, data on itch and pain and quality of life were presented at the EB World Congress and discussed by Dr. Mellerio.
A total of 42 participants older than 8 years of age had itch measured via the Leuven Itch Scale, she reported, noting that itch was a consistent symptom across all subtypes of RDEB. Itch is important as it not only causes problems with skin lesions and healing, but also significantly affects sleep and has a negative impact on patients’ mood, she emphasized.
Despite experiencing itch, more than half (58%) of participants were not using any kind of treatment for itch. This “likely reflects the lack of effectiveness of current medication for this debilitating symptom,” Dr. Mellerio and associates noted in one of their poster presentations of PEBLES data.
When treatment was used for itch, it consisted mainly of antihistamines (19% of patients), emollients (19%), or a combination of both (4%). However, treatment was generally “not very good,” with a satisfaction score of just 5 on a scale of 10, Dr. Mellerio pointed out. Participants “reported frustration with the lack of effective treatment for itch,” she said.
Itch was associated with disturbed sleep 1-3 nights per week in 20%-40% of participants, and every night in 20%-30%.
Pain was found to be a significant problem, with a median level of background pain scored as 4 on a 10-cm visual analog scale and a higher level (6) when associated with dressing changes.
Data on how RDEB affected quality of life were reported for 39 adults completing the 17-item Quality of Life in EB Questionnaire (QOLEB) and eight children who were able to complete the Pediatric Quality of Life Inventory (PedsQL) with the aid of their parents.
Dr. Mellerio reported that adults with RDEB-GS had an overall QOLEB score of 24 out of 50, an indication that their condition had a severe impact on their quality of life. The effect on quality of life was greater in terms of their physical functioning than emotional well-being, with respective scores of 19 out of 36, and 5 out of a possible 15. Less impact on quality of life was reported by participants with other RDEB subtypes.
PedsQL scores for the children indicated there might be a lesser effect of physical functioning on quality of life but a greater effect of emotional well-being on quality of life, but the numbers were small. “Interestingly, parents tended to rate their children’s impact on quality of life much higher than the children themselves,” Dr. Mellerio said.
The point of PEBLES is to start to understand the natural history of RDEB and to identify endpoints that might help in clinical trials of potential new treatments. Discussing the next steps for PEBLES, Dr. Mellerio said the aim was to recruit more pediatric patients and look at other data sets, such as bone health. The PEBLES team also hopes to extend recruitment to include other United Kingdom, and ultimately international, EB centers and, perhaps eventually to start to include other types of EB, such as EB simplex.
PEBLES is funded by DEBRA UK. Dr. Mellerio is a PEBLES investigator but had no conflicts of interest to disclose.
SOURCE: Mellerio JE et al. EB 2020. Pillay EI et al. Poster 77; Jeffs E et al. Poster 74; Jeffs et al. Poster 75. https://ebworldcongress.org/.
LONDON – More than £2.8 million (RDEB), according to a report at the EB World Congress, organized by the Dystrophic Epidermolysis Bullosa Association (DEBRA).
Results from the Prospective Epidermolysis Bullosa Longitudinal Evaluation Study (PEBLES), which is looking at the natural history of RDEB, showed that wound dressing and bandage costs were highest for study participants with the generalized severe (RDEB-GS) subtype, at just over £85,156 (about $112,450) per patient annually. Respective yearly costs for the generalized intermediate (RDEB-GI) and inversa (RDEB-INV) subtypes were £10,112 (about $13,350) and £1,699 (about $2,240) per patient.
Looking at the costs associated with EB is important, said one of the lead investigators for PEBLES, Jemima Mellerio, MD, FRCP, consultant dermatologist at St John’s Institute of Dermatology, at Guy’s & St. Thomas’ NHS Foundation Trust, London.
“If we are going to justify the kind of expenditure [associated with new treatments], we need to know that what we are treating is already a significant burden on our health care systems,” Dr. Mellerio said.
PEBLES is an ongoing London-based registry study that is enrolling patients with all subtypes of RDEB. Data are collected via a tablet device and include demographic data, information on clinical features, results of skin biopsies and genetic tests, and laboratory findings, as well as objective disease severity and subjective patient-orientated outcome scores.
So far, 60 patients – 49 adults and 11 children – have been enrolled in PEBLES since November 2014: 26 with RDEB-GS, 23 with RDEB-GI, 9 with RDEB-INV, and 2 with the pruriginosa RDEB subtype (RDEB-PR).
Most of the participants (71%) changed all their wound dressings at one time, patching up when required. Fourteen of 49 participants had paid people to help them change their dressings and when the total cost of combined wound dressings and paid care was taken into consideration, the mean annual cost per patient was around £2,500 (about $3,300) for RDEB-INV, £10,375 (about $13,700) per patient for RDEB-GS, and a staggering £98,000 (about $129,000) per patient for RDEB-GS. The total annual cost of dressings and associated care was an estimated £3,184,229 (about $4.2 million).
In addition to data on the cost of wound dressings, data on itch and pain and quality of life were presented at the EB World Congress and discussed by Dr. Mellerio.
A total of 42 participants older than 8 years of age had itch measured via the Leuven Itch Scale, she reported, noting that itch was a consistent symptom across all subtypes of RDEB. Itch is important as it not only causes problems with skin lesions and healing, but also significantly affects sleep and has a negative impact on patients’ mood, she emphasized.
Despite experiencing itch, more than half (58%) of participants were not using any kind of treatment for itch. This “likely reflects the lack of effectiveness of current medication for this debilitating symptom,” Dr. Mellerio and associates noted in one of their poster presentations of PEBLES data.
When treatment was used for itch, it consisted mainly of antihistamines (19% of patients), emollients (19%), or a combination of both (4%). However, treatment was generally “not very good,” with a satisfaction score of just 5 on a scale of 10, Dr. Mellerio pointed out. Participants “reported frustration with the lack of effective treatment for itch,” she said.
Itch was associated with disturbed sleep 1-3 nights per week in 20%-40% of participants, and every night in 20%-30%.
Pain was found to be a significant problem, with a median level of background pain scored as 4 on a 10-cm visual analog scale and a higher level (6) when associated with dressing changes.
Data on how RDEB affected quality of life were reported for 39 adults completing the 17-item Quality of Life in EB Questionnaire (QOLEB) and eight children who were able to complete the Pediatric Quality of Life Inventory (PedsQL) with the aid of their parents.
Dr. Mellerio reported that adults with RDEB-GS had an overall QOLEB score of 24 out of 50, an indication that their condition had a severe impact on their quality of life. The effect on quality of life was greater in terms of their physical functioning than emotional well-being, with respective scores of 19 out of 36, and 5 out of a possible 15. Less impact on quality of life was reported by participants with other RDEB subtypes.
PedsQL scores for the children indicated there might be a lesser effect of physical functioning on quality of life but a greater effect of emotional well-being on quality of life, but the numbers were small. “Interestingly, parents tended to rate their children’s impact on quality of life much higher than the children themselves,” Dr. Mellerio said.
The point of PEBLES is to start to understand the natural history of RDEB and to identify endpoints that might help in clinical trials of potential new treatments. Discussing the next steps for PEBLES, Dr. Mellerio said the aim was to recruit more pediatric patients and look at other data sets, such as bone health. The PEBLES team also hopes to extend recruitment to include other United Kingdom, and ultimately international, EB centers and, perhaps eventually to start to include other types of EB, such as EB simplex.
PEBLES is funded by DEBRA UK. Dr. Mellerio is a PEBLES investigator but had no conflicts of interest to disclose.
SOURCE: Mellerio JE et al. EB 2020. Pillay EI et al. Poster 77; Jeffs E et al. Poster 74; Jeffs et al. Poster 75. https://ebworldcongress.org/.
LONDON – More than £2.8 million (RDEB), according to a report at the EB World Congress, organized by the Dystrophic Epidermolysis Bullosa Association (DEBRA).
Results from the Prospective Epidermolysis Bullosa Longitudinal Evaluation Study (PEBLES), which is looking at the natural history of RDEB, showed that wound dressing and bandage costs were highest for study participants with the generalized severe (RDEB-GS) subtype, at just over £85,156 (about $112,450) per patient annually. Respective yearly costs for the generalized intermediate (RDEB-GI) and inversa (RDEB-INV) subtypes were £10,112 (about $13,350) and £1,699 (about $2,240) per patient.
Looking at the costs associated with EB is important, said one of the lead investigators for PEBLES, Jemima Mellerio, MD, FRCP, consultant dermatologist at St John’s Institute of Dermatology, at Guy’s & St. Thomas’ NHS Foundation Trust, London.
“If we are going to justify the kind of expenditure [associated with new treatments], we need to know that what we are treating is already a significant burden on our health care systems,” Dr. Mellerio said.
PEBLES is an ongoing London-based registry study that is enrolling patients with all subtypes of RDEB. Data are collected via a tablet device and include demographic data, information on clinical features, results of skin biopsies and genetic tests, and laboratory findings, as well as objective disease severity and subjective patient-orientated outcome scores.
So far, 60 patients – 49 adults and 11 children – have been enrolled in PEBLES since November 2014: 26 with RDEB-GS, 23 with RDEB-GI, 9 with RDEB-INV, and 2 with the pruriginosa RDEB subtype (RDEB-PR).
Most of the participants (71%) changed all their wound dressings at one time, patching up when required. Fourteen of 49 participants had paid people to help them change their dressings and when the total cost of combined wound dressings and paid care was taken into consideration, the mean annual cost per patient was around £2,500 (about $3,300) for RDEB-INV, £10,375 (about $13,700) per patient for RDEB-GS, and a staggering £98,000 (about $129,000) per patient for RDEB-GS. The total annual cost of dressings and associated care was an estimated £3,184,229 (about $4.2 million).
In addition to data on the cost of wound dressings, data on itch and pain and quality of life were presented at the EB World Congress and discussed by Dr. Mellerio.
A total of 42 participants older than 8 years of age had itch measured via the Leuven Itch Scale, she reported, noting that itch was a consistent symptom across all subtypes of RDEB. Itch is important as it not only causes problems with skin lesions and healing, but also significantly affects sleep and has a negative impact on patients’ mood, she emphasized.
Despite experiencing itch, more than half (58%) of participants were not using any kind of treatment for itch. This “likely reflects the lack of effectiveness of current medication for this debilitating symptom,” Dr. Mellerio and associates noted in one of their poster presentations of PEBLES data.
When treatment was used for itch, it consisted mainly of antihistamines (19% of patients), emollients (19%), or a combination of both (4%). However, treatment was generally “not very good,” with a satisfaction score of just 5 on a scale of 10, Dr. Mellerio pointed out. Participants “reported frustration with the lack of effective treatment for itch,” she said.
Itch was associated with disturbed sleep 1-3 nights per week in 20%-40% of participants, and every night in 20%-30%.
Pain was found to be a significant problem, with a median level of background pain scored as 4 on a 10-cm visual analog scale and a higher level (6) when associated with dressing changes.
Data on how RDEB affected quality of life were reported for 39 adults completing the 17-item Quality of Life in EB Questionnaire (QOLEB) and eight children who were able to complete the Pediatric Quality of Life Inventory (PedsQL) with the aid of their parents.
Dr. Mellerio reported that adults with RDEB-GS had an overall QOLEB score of 24 out of 50, an indication that their condition had a severe impact on their quality of life. The effect on quality of life was greater in terms of their physical functioning than emotional well-being, with respective scores of 19 out of 36, and 5 out of a possible 15. Less impact on quality of life was reported by participants with other RDEB subtypes.
PedsQL scores for the children indicated there might be a lesser effect of physical functioning on quality of life but a greater effect of emotional well-being on quality of life, but the numbers were small. “Interestingly, parents tended to rate their children’s impact on quality of life much higher than the children themselves,” Dr. Mellerio said.
The point of PEBLES is to start to understand the natural history of RDEB and to identify endpoints that might help in clinical trials of potential new treatments. Discussing the next steps for PEBLES, Dr. Mellerio said the aim was to recruit more pediatric patients and look at other data sets, such as bone health. The PEBLES team also hopes to extend recruitment to include other United Kingdom, and ultimately international, EB centers and, perhaps eventually to start to include other types of EB, such as EB simplex.
PEBLES is funded by DEBRA UK. Dr. Mellerio is a PEBLES investigator but had no conflicts of interest to disclose.
SOURCE: Mellerio JE et al. EB 2020. Pillay EI et al. Poster 77; Jeffs E et al. Poster 74; Jeffs et al. Poster 75. https://ebworldcongress.org/.
REPORTING FROM EB 2020
Most epidermolysis bullosa patients turn to topical antimicrobials
Most patients with epidermolysis bullosa who use topical products choose antimicrobials, according to data from a survey of 202 children and adults.
Management of epidermolysis bullosa (EB) involves a combination of skin protection and infection management, but patient home care practices have not been well studied, wrote Leila Shayegan of Columbia University, New York, and colleagues.
In a study published in Pediatric Dermatology, the researchers surveyed 202 patients who were enrolled in the Epidermolysis Bullosa Clinical Characterization and Outcomes Database during 2017. The patients ranged in age from 1 month to 62 years with an average age of 11 years; 52% were female. The patients represented a range of EB subtypes, including 130 patients with dystrophic EB, 51 patients with EB simplex, 21 with junctional EB, and 3 patients each with Kindler syndrome and unspecified subtypes.
Overall, most of the patients reported cleaning their skin either every day (37%) or every other day (32%). Of the 188 patients who reported using topical products on their wounds, 131 (70%) said they used at least one antimicrobial product, while 125 patients (66%) reported using at least one emollient; 32 (17%) used emollients only, and 21(11%) reported no use of topical products.
The most popular topical antibiotics were mupirocin (31%) and bacitracin (31%). In addition, 14% of respondents used silver-containing products, and 16% used medical-grade honey. Roughly half (51%) of patients who reported use of at least one antimicrobial product used two or more different antimicrobial products.
A total of 38% of patients used only water for cleansing. Of the 131 patients who reported using additives in their cleansing water, 57% added salt, 54% added bleach, 27% added vinegar, and 26% reported “other” additive use, which could include Epsom salt, baking soda, oatmeal, or essential oils, the researchers said. The concentrations of these additives ranged from barely effective 0.002% sodium hypochlorite and 0.002% acetic acid solutions to potentially cytotoxic solutions of 0.09% sodium hypochlorite and 0.156% acetic acid.
“Although the survey was not designed to correlate skin care practices with wound culture results and resistance patterns, widespread use of topical antimicrobials described among EB patients highlights the need for increased emphasis on antibiotic stewardship,” the researchers noted. They added that health care providers should educate patients and families not only about mindful use of antibiotics, but also appropriate concentrations of cleansing additives.
“Optimizing EB patient home skin care routines, along with future longitudinal studies on the impact of EB skin care interventions on microbial resistance patterns, wound healing and [squamous cell carcinoma] risk are necessary to improve outcomes for patients with EB,” they emphasized.
The Epidermolysis Bullosa Clinical Characterization and Outcomes Database used in the study is funded by the Epidermolysis Bullosa Research Partnership and the Epidermolysis Bullosa Medical Research Foundation. Ms. Shayegan had no financial conflicts to disclose. Several coauthors disclosed relationships with multiple companies including Abeona Therapeutics, Castle Creek Pharmaceuticals, Fibrocell Science, ProQR, and Scioderm.
SOURCE: Shayegan L et al. Pediatr Dermatol. 2020. doi: 10.1111/pde.14102.
Most patients with epidermolysis bullosa who use topical products choose antimicrobials, according to data from a survey of 202 children and adults.
Management of epidermolysis bullosa (EB) involves a combination of skin protection and infection management, but patient home care practices have not been well studied, wrote Leila Shayegan of Columbia University, New York, and colleagues.
In a study published in Pediatric Dermatology, the researchers surveyed 202 patients who were enrolled in the Epidermolysis Bullosa Clinical Characterization and Outcomes Database during 2017. The patients ranged in age from 1 month to 62 years with an average age of 11 years; 52% were female. The patients represented a range of EB subtypes, including 130 patients with dystrophic EB, 51 patients with EB simplex, 21 with junctional EB, and 3 patients each with Kindler syndrome and unspecified subtypes.
Overall, most of the patients reported cleaning their skin either every day (37%) or every other day (32%). Of the 188 patients who reported using topical products on their wounds, 131 (70%) said they used at least one antimicrobial product, while 125 patients (66%) reported using at least one emollient; 32 (17%) used emollients only, and 21(11%) reported no use of topical products.
The most popular topical antibiotics were mupirocin (31%) and bacitracin (31%). In addition, 14% of respondents used silver-containing products, and 16% used medical-grade honey. Roughly half (51%) of patients who reported use of at least one antimicrobial product used two or more different antimicrobial products.
A total of 38% of patients used only water for cleansing. Of the 131 patients who reported using additives in their cleansing water, 57% added salt, 54% added bleach, 27% added vinegar, and 26% reported “other” additive use, which could include Epsom salt, baking soda, oatmeal, or essential oils, the researchers said. The concentrations of these additives ranged from barely effective 0.002% sodium hypochlorite and 0.002% acetic acid solutions to potentially cytotoxic solutions of 0.09% sodium hypochlorite and 0.156% acetic acid.
“Although the survey was not designed to correlate skin care practices with wound culture results and resistance patterns, widespread use of topical antimicrobials described among EB patients highlights the need for increased emphasis on antibiotic stewardship,” the researchers noted. They added that health care providers should educate patients and families not only about mindful use of antibiotics, but also appropriate concentrations of cleansing additives.
“Optimizing EB patient home skin care routines, along with future longitudinal studies on the impact of EB skin care interventions on microbial resistance patterns, wound healing and [squamous cell carcinoma] risk are necessary to improve outcomes for patients with EB,” they emphasized.
The Epidermolysis Bullosa Clinical Characterization and Outcomes Database used in the study is funded by the Epidermolysis Bullosa Research Partnership and the Epidermolysis Bullosa Medical Research Foundation. Ms. Shayegan had no financial conflicts to disclose. Several coauthors disclosed relationships with multiple companies including Abeona Therapeutics, Castle Creek Pharmaceuticals, Fibrocell Science, ProQR, and Scioderm.
SOURCE: Shayegan L et al. Pediatr Dermatol. 2020. doi: 10.1111/pde.14102.
Most patients with epidermolysis bullosa who use topical products choose antimicrobials, according to data from a survey of 202 children and adults.
Management of epidermolysis bullosa (EB) involves a combination of skin protection and infection management, but patient home care practices have not been well studied, wrote Leila Shayegan of Columbia University, New York, and colleagues.
In a study published in Pediatric Dermatology, the researchers surveyed 202 patients who were enrolled in the Epidermolysis Bullosa Clinical Characterization and Outcomes Database during 2017. The patients ranged in age from 1 month to 62 years with an average age of 11 years; 52% were female. The patients represented a range of EB subtypes, including 130 patients with dystrophic EB, 51 patients with EB simplex, 21 with junctional EB, and 3 patients each with Kindler syndrome and unspecified subtypes.
Overall, most of the patients reported cleaning their skin either every day (37%) or every other day (32%). Of the 188 patients who reported using topical products on their wounds, 131 (70%) said they used at least one antimicrobial product, while 125 patients (66%) reported using at least one emollient; 32 (17%) used emollients only, and 21(11%) reported no use of topical products.
The most popular topical antibiotics were mupirocin (31%) and bacitracin (31%). In addition, 14% of respondents used silver-containing products, and 16% used medical-grade honey. Roughly half (51%) of patients who reported use of at least one antimicrobial product used two or more different antimicrobial products.
A total of 38% of patients used only water for cleansing. Of the 131 patients who reported using additives in their cleansing water, 57% added salt, 54% added bleach, 27% added vinegar, and 26% reported “other” additive use, which could include Epsom salt, baking soda, oatmeal, or essential oils, the researchers said. The concentrations of these additives ranged from barely effective 0.002% sodium hypochlorite and 0.002% acetic acid solutions to potentially cytotoxic solutions of 0.09% sodium hypochlorite and 0.156% acetic acid.
“Although the survey was not designed to correlate skin care practices with wound culture results and resistance patterns, widespread use of topical antimicrobials described among EB patients highlights the need for increased emphasis on antibiotic stewardship,” the researchers noted. They added that health care providers should educate patients and families not only about mindful use of antibiotics, but also appropriate concentrations of cleansing additives.
“Optimizing EB patient home skin care routines, along with future longitudinal studies on the impact of EB skin care interventions on microbial resistance patterns, wound healing and [squamous cell carcinoma] risk are necessary to improve outcomes for patients with EB,” they emphasized.
The Epidermolysis Bullosa Clinical Characterization and Outcomes Database used in the study is funded by the Epidermolysis Bullosa Research Partnership and the Epidermolysis Bullosa Medical Research Foundation. Ms. Shayegan had no financial conflicts to disclose. Several coauthors disclosed relationships with multiple companies including Abeona Therapeutics, Castle Creek Pharmaceuticals, Fibrocell Science, ProQR, and Scioderm.
SOURCE: Shayegan L et al. Pediatr Dermatol. 2020. doi: 10.1111/pde.14102.
FROM PEDIATRIC DERMATOLOGY
Cartilage Sutures for a Large Nasal Defect
Practice Gap
A 69-year-old man underwent staged excision for an invasive melanoma (0.4-mm Breslow depth; stage Ia) of the right dorsal nose. Two stages were required to achieve clear margins, leaving a 3.0×2.5-cm defect involving the nasal dorsum, right nasal sidewall, and nasal supratip (Figure 1). He declined any multistage repair and preferred a full-thickness skin graft (FTSG) over any interpolation flap.
Given the size of our patient’s defect, primary repair was not possible and second intention healing may have resulted in a suboptimal cosmetic outcome, potential alar distortion, and prolonged healing. No single local flap, such as the dorsal nasal rotation flap, crescentic advancement flap, bilobed flap, and Rintala flap, would have provided adequate coverage. A FTSG of the entire defect would not have been an ideal tissue match, and given the limited surrounding laxity, a Burow FTSG would have required the linear repair to extend well into the forehead with a questionable cosmetic outcome.
The Technique
We opted to repair the defect using a combination of local flaps for a single-stage repair. Using the right cheek reservoir, a crescentic advancement flap was performed to restore the right nasal sidewall as best as possible with a standing cone taken superiorly. To execute this flap, an incision was made extending from the alar sulcus into the nasolabial fold while preserving the apical triangle of the upper cutaneous lip. The flap was elevated submuscularly on the nose, and broad undermining was performed in the subcutaneous plane of the medial cheek. A crescentic redundancy above the alar sulcus was excised, and periosteal tacking sutures were placed to both help advance the flap and to recreate the nasofacial sulcus.1
Next, a nasal tip spiral/rotation flap was designed to restore the remaining nasal defect.2 An incision was made at the right inferiormost aspect of the defect and extended along the inferior border of the nasal tip as it crossed the midline to the left side of the nose. After incising and elevating the flap in the submuscular plane, there was not enough of a tissue reservoir to cover the entire remaining nasal defect.
To resolve this intraoperative conundrum, simple interrupted sutures were placed into the nasal cartilage at midline to narrow the structure of the nose (Figure 2). Three 4-0 polyglactin 910 sutures were placed beginning with the upper lateral cartilages and extending inferiorly to the lower lateral cartilages. Narrowing the nasal cartilages allowed for a smaller residual defect. The nasal tip rotation flap was then spiraled into place with adequate coverage. Some of the flap tip was trimmed after the superior aspect of the rotation flap was sutured to the inferior edge of the crescentic advancement flap. The immediate postoperative appearance is shown in Figure 3.
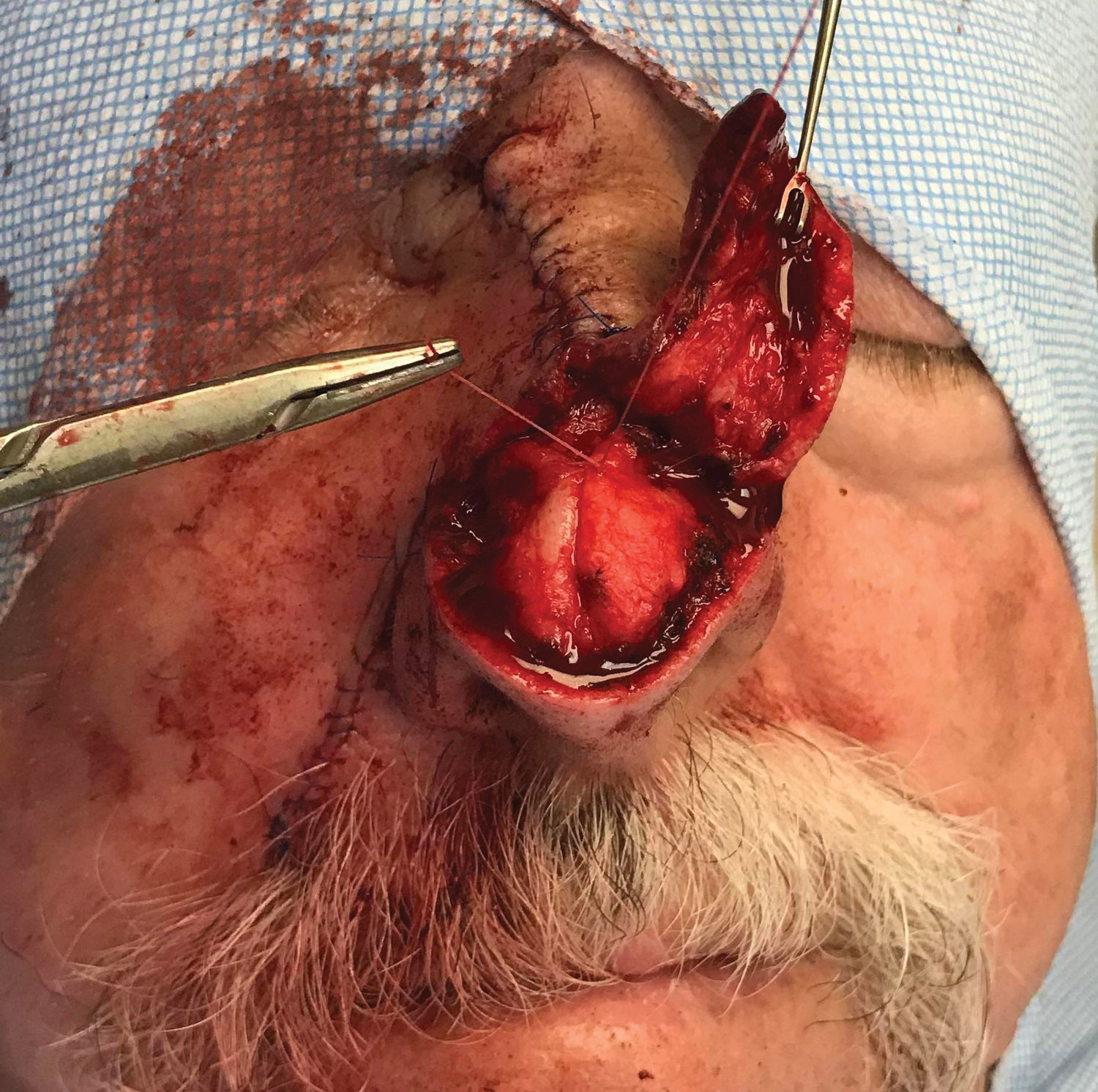
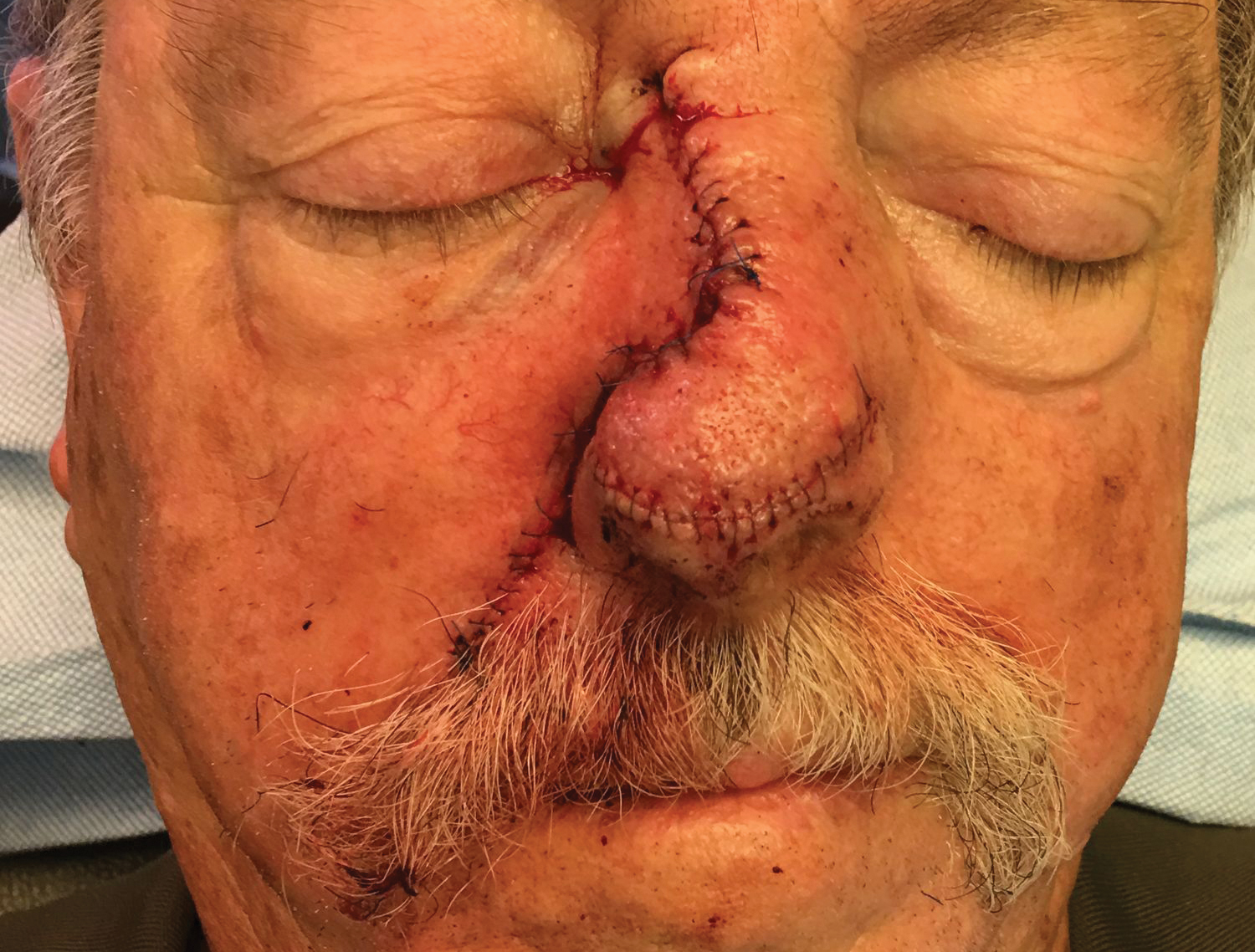
At 4-month follow-up, intralesional triamcinolone was injected into the slight induration at the right nasal tip. At 7-month follow-up, the patient was pleased with the cosmetic and functional result (Figure 4).
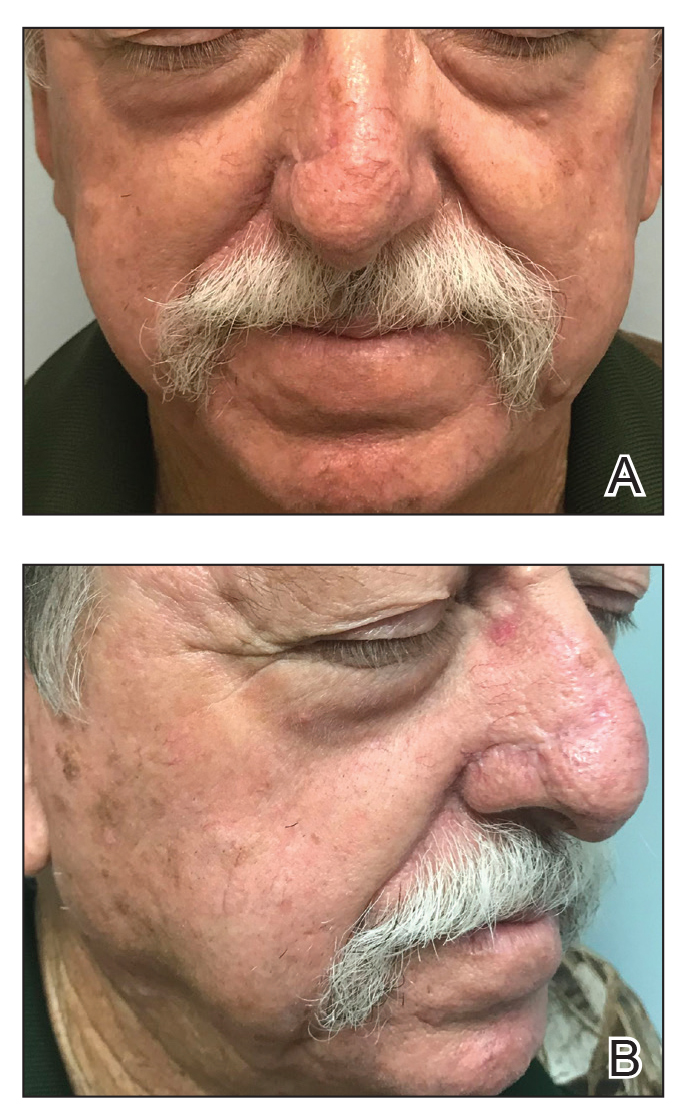
Practice Implications
Cartilage sutures highlight an underutilized technique in nasal reconstruction, with few cases reported
A combination of local flaps may be used to repair large nasal defects involving multiple subunits, especially in patients who decline multistage reconstruction. A nasal tip rotation/spiral flap can be considered for the appropriate nasal tip defect. Suturing the nasal cartilage with either permanent or long-lasting suture can narrow the cartilage and facilitate flap coverage for nasal defects while also improving the appearance of patients with wide prominent lower noses.
- Smith JM, Orseth ML, Nijhawan RI. Reconstruction of large nasal dorsum defects. Dermatol Surg. 2018;44:1607-1610.
- Snow SN. Rotation flaps to reconstruct nasal tip defects following Mohs surgery. Dermatol Surg. 1997;23:916-919.
- Malone CH, Hays JP, Tausend WE, et al. Interdomal sutures for nasal tip refinement and reduced wound size. J Am Acad Dermatol. 2017;77:E107-E108.
- Pelster MW, Behshad R, Maher IA. Large nasal tip defects-utilization of interdomal sutures before Burow’s graft for optimization of nasal contour. Dermatol Surg. 2019;45:743-746.
- Gruber RP, Chang E, Buchanan E. Suture techniques in rhinoplasty. Clin Plast Surg. 2010;37:231-243.
Practice Gap
A 69-year-old man underwent staged excision for an invasive melanoma (0.4-mm Breslow depth; stage Ia) of the right dorsal nose. Two stages were required to achieve clear margins, leaving a 3.0×2.5-cm defect involving the nasal dorsum, right nasal sidewall, and nasal supratip (Figure 1). He declined any multistage repair and preferred a full-thickness skin graft (FTSG) over any interpolation flap.

Given the size of our patient’s defect, primary repair was not possible and second intention healing may have resulted in a suboptimal cosmetic outcome, potential alar distortion, and prolonged healing. No single local flap, such as the dorsal nasal rotation flap, crescentic advancement flap, bilobed flap, and Rintala flap, would have provided adequate coverage. A FTSG of the entire defect would not have been an ideal tissue match, and given the limited surrounding laxity, a Burow FTSG would have required the linear repair to extend well into the forehead with a questionable cosmetic outcome.
The Technique
We opted to repair the defect using a combination of local flaps for a single-stage repair. Using the right cheek reservoir, a crescentic advancement flap was performed to restore the right nasal sidewall as best as possible with a standing cone taken superiorly. To execute this flap, an incision was made extending from the alar sulcus into the nasolabial fold while preserving the apical triangle of the upper cutaneous lip. The flap was elevated submuscularly on the nose, and broad undermining was performed in the subcutaneous plane of the medial cheek. A crescentic redundancy above the alar sulcus was excised, and periosteal tacking sutures were placed to both help advance the flap and to recreate the nasofacial sulcus.1
Next, a nasal tip spiral/rotation flap was designed to restore the remaining nasal defect.2 An incision was made at the right inferiormost aspect of the defect and extended along the inferior border of the nasal tip as it crossed the midline to the left side of the nose. After incising and elevating the flap in the submuscular plane, there was not enough of a tissue reservoir to cover the entire remaining nasal defect.
To resolve this intraoperative conundrum, simple interrupted sutures were placed into the nasal cartilage at midline to narrow the structure of the nose (Figure 2). Three 4-0 polyglactin 910 sutures were placed beginning with the upper lateral cartilages and extending inferiorly to the lower lateral cartilages. Narrowing the nasal cartilages allowed for a smaller residual defect. The nasal tip rotation flap was then spiraled into place with adequate coverage. Some of the flap tip was trimmed after the superior aspect of the rotation flap was sutured to the inferior edge of the crescentic advancement flap. The immediate postoperative appearance is shown in Figure 3.


At 4-month follow-up, intralesional triamcinolone was injected into the slight induration at the right nasal tip. At 7-month follow-up, the patient was pleased with the cosmetic and functional result (Figure 4).

Practice Implications
Cartilage sutures highlight an underutilized technique in nasal reconstruction, with few cases reported
A combination of local flaps may be used to repair large nasal defects involving multiple subunits, especially in patients who decline multistage reconstruction. A nasal tip rotation/spiral flap can be considered for the appropriate nasal tip defect. Suturing the nasal cartilage with either permanent or long-lasting suture can narrow the cartilage and facilitate flap coverage for nasal defects while also improving the appearance of patients with wide prominent lower noses.
Practice Gap
A 69-year-old man underwent staged excision for an invasive melanoma (0.4-mm Breslow depth; stage Ia) of the right dorsal nose. Two stages were required to achieve clear margins, leaving a 3.0×2.5-cm defect involving the nasal dorsum, right nasal sidewall, and nasal supratip (Figure 1). He declined any multistage repair and preferred a full-thickness skin graft (FTSG) over any interpolation flap.

Given the size of our patient’s defect, primary repair was not possible and second intention healing may have resulted in a suboptimal cosmetic outcome, potential alar distortion, and prolonged healing. No single local flap, such as the dorsal nasal rotation flap, crescentic advancement flap, bilobed flap, and Rintala flap, would have provided adequate coverage. A FTSG of the entire defect would not have been an ideal tissue match, and given the limited surrounding laxity, a Burow FTSG would have required the linear repair to extend well into the forehead with a questionable cosmetic outcome.
The Technique
We opted to repair the defect using a combination of local flaps for a single-stage repair. Using the right cheek reservoir, a crescentic advancement flap was performed to restore the right nasal sidewall as best as possible with a standing cone taken superiorly. To execute this flap, an incision was made extending from the alar sulcus into the nasolabial fold while preserving the apical triangle of the upper cutaneous lip. The flap was elevated submuscularly on the nose, and broad undermining was performed in the subcutaneous plane of the medial cheek. A crescentic redundancy above the alar sulcus was excised, and periosteal tacking sutures were placed to both help advance the flap and to recreate the nasofacial sulcus.1
Next, a nasal tip spiral/rotation flap was designed to restore the remaining nasal defect.2 An incision was made at the right inferiormost aspect of the defect and extended along the inferior border of the nasal tip as it crossed the midline to the left side of the nose. After incising and elevating the flap in the submuscular plane, there was not enough of a tissue reservoir to cover the entire remaining nasal defect.
To resolve this intraoperative conundrum, simple interrupted sutures were placed into the nasal cartilage at midline to narrow the structure of the nose (Figure 2). Three 4-0 polyglactin 910 sutures were placed beginning with the upper lateral cartilages and extending inferiorly to the lower lateral cartilages. Narrowing the nasal cartilages allowed for a smaller residual defect. The nasal tip rotation flap was then spiraled into place with adequate coverage. Some of the flap tip was trimmed after the superior aspect of the rotation flap was sutured to the inferior edge of the crescentic advancement flap. The immediate postoperative appearance is shown in Figure 3.


At 4-month follow-up, intralesional triamcinolone was injected into the slight induration at the right nasal tip. At 7-month follow-up, the patient was pleased with the cosmetic and functional result (Figure 4).

Practice Implications
Cartilage sutures highlight an underutilized technique in nasal reconstruction, with few cases reported
A combination of local flaps may be used to repair large nasal defects involving multiple subunits, especially in patients who decline multistage reconstruction. A nasal tip rotation/spiral flap can be considered for the appropriate nasal tip defect. Suturing the nasal cartilage with either permanent or long-lasting suture can narrow the cartilage and facilitate flap coverage for nasal defects while also improving the appearance of patients with wide prominent lower noses.
- Smith JM, Orseth ML, Nijhawan RI. Reconstruction of large nasal dorsum defects. Dermatol Surg. 2018;44:1607-1610.
- Snow SN. Rotation flaps to reconstruct nasal tip defects following Mohs surgery. Dermatol Surg. 1997;23:916-919.
- Malone CH, Hays JP, Tausend WE, et al. Interdomal sutures for nasal tip refinement and reduced wound size. J Am Acad Dermatol. 2017;77:E107-E108.
- Pelster MW, Behshad R, Maher IA. Large nasal tip defects-utilization of interdomal sutures before Burow’s graft for optimization of nasal contour. Dermatol Surg. 2019;45:743-746.
- Gruber RP, Chang E, Buchanan E. Suture techniques in rhinoplasty. Clin Plast Surg. 2010;37:231-243.
- Smith JM, Orseth ML, Nijhawan RI. Reconstruction of large nasal dorsum defects. Dermatol Surg. 2018;44:1607-1610.
- Snow SN. Rotation flaps to reconstruct nasal tip defects following Mohs surgery. Dermatol Surg. 1997;23:916-919.
- Malone CH, Hays JP, Tausend WE, et al. Interdomal sutures for nasal tip refinement and reduced wound size. J Am Acad Dermatol. 2017;77:E107-E108.
- Pelster MW, Behshad R, Maher IA. Large nasal tip defects-utilization of interdomal sutures before Burow’s graft for optimization of nasal contour. Dermatol Surg. 2019;45:743-746.
- Gruber RP, Chang E, Buchanan E. Suture techniques in rhinoplasty. Clin Plast Surg. 2010;37:231-243.