User login
Mystery Burns and Nocturnal Seizure Safety
Patients with seizures are placed at an increased risk for sustaining burn injuries, which may occur during common daily activities such as cooking, showering, and using heaters.1 Although patients are warned of the risks of injury at the time of their epilepsy diagnosis, patients still experience injuries that commonly occur during the seizure or the postictal phase. In a study of 134 patients with epilepsy, only 38% recalled being burned during a seizure, with approximately 9% being burned multiple times.2 Another study investigated the circumstances resulting in burns in this patient population and found that cooking on a stove was the most common cause, followed by hot water while showering and exposed room heaters.1 Another study found that the majority of burns in seizure patients were from spilled hot drinks.3
We report 2 patients who presented to the dermatology clinic with second-degree burns following nocturnal seizures. In both cases, the patients were sleeping next to exposed heaters, which led to burn injuries from seizures that occurred in the night.
Case Reports
Patient 1
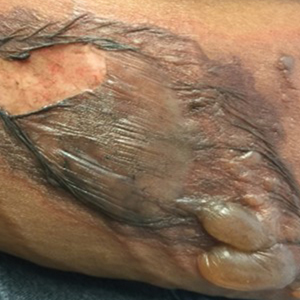
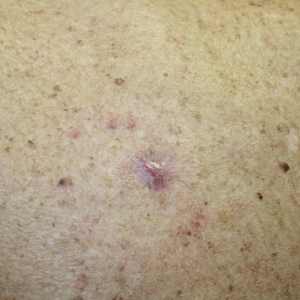
A 30-year-old woman with a history of a seizure disorder presented with painful second-degree blistering burns along the left arm and flank (Figure 1). One day prior to presentation, she had woken up to find these lesions and visited the emergency department where she was prescribed silver sulfadiazine cream to prevent infection of the wound site and was referred to our dermatology clinic. Initially, the patient had difficulty pinpointing the source of the burn lesions and thought that it may have been due to sleeping with her cell phone, but she later realized that they were due to the space heater placed next to her bed. Because of the unclear etiology at the initial presentation, a skin biopsy of a lesion was taken while she was at the clinic.
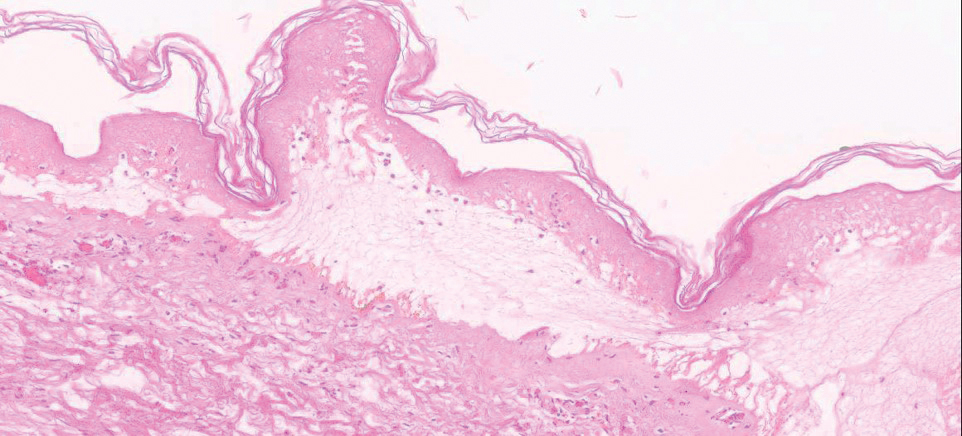
Biopsy of the lesions exhibited separation of the epidermal and dermal layers (Figure 2). Thermal damage was seen extending into the dermal layers with notable edema present. A few inflammatory cells, neutrophils, and monocytes were noted in the biopsy. The initial pathology results showed the epidermis was necrotic with edema, spongiform vesicles, and few neutrophils. The histologic findings aligned with the timeline of the injury occurring 2 days prior to the biopsy. She was treated supportively using mupirocin ointment to prevent secondary infection.
Case 2
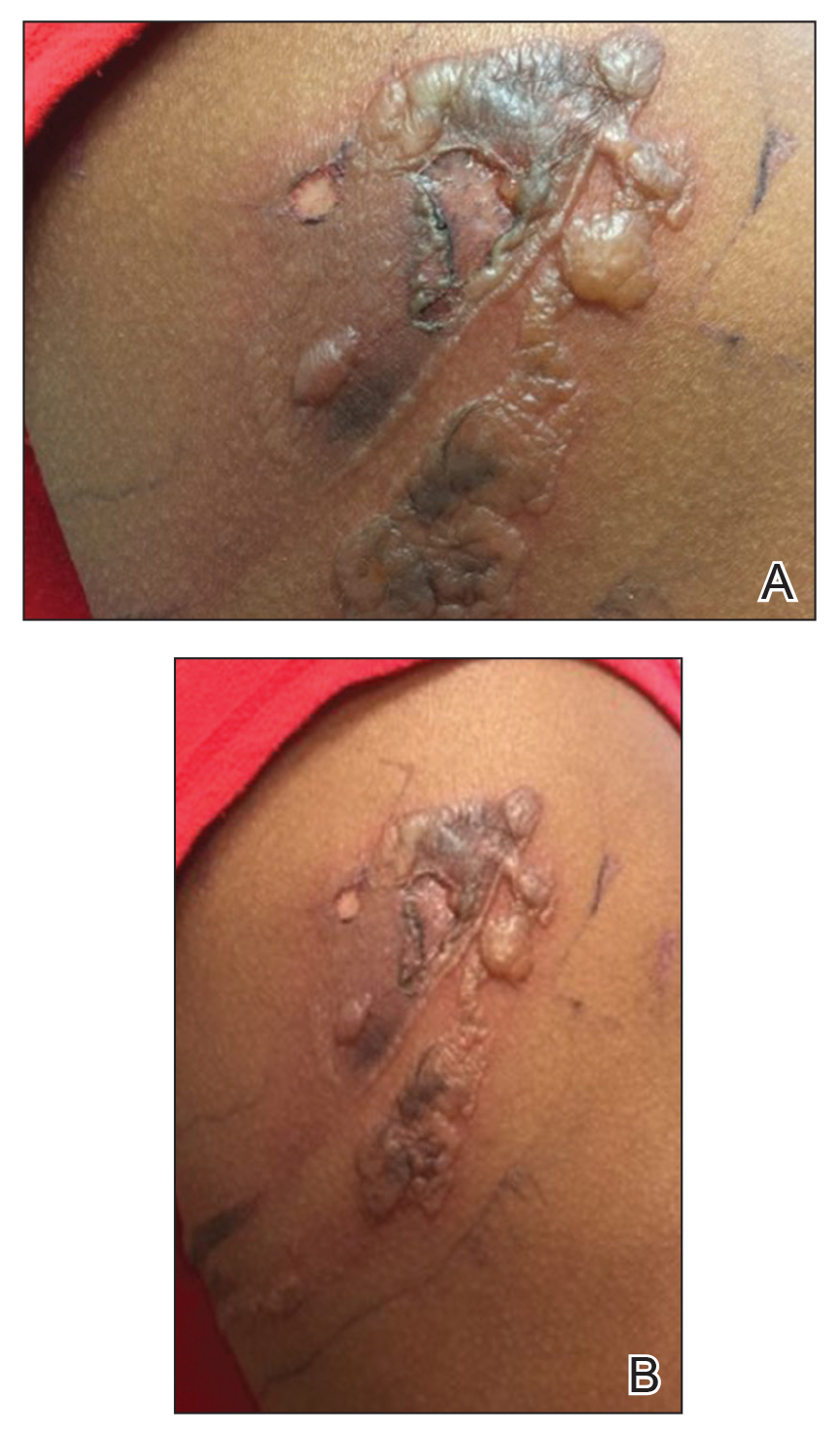
A 27-year-old woman with a history of epilepsy presented to the dermatology clinic with painful blistering lesions along the right upper arm (Figure 3). She was found to have notable second-degree burns along the right arm. She reported placing her bed near a baseboard heater to stay warm overnight. She noticed the painful lesions after waking up next to the heater following a suspected seizure. She was treated supportively using mupirocin ointment to prevent secondary infection.
Comment
Classification of Burns and Damage
According to the World Health Organization, nonfatal burn injuries are a leading cause of morbidity and occur mainly in the home and workplace.4 There are many types of burns: radiation, electrical, chemical, friction, and thermal. The most common type of burns are thermal burns,4 which can be further subdivided into wet and dry. Both of our patients experienced dry thermal burns.
Based on the skin tissue layers involved in the thermal damage, burn wounds are further divided into first-degree burns, superficial second-degree burns, deep second-degree burns, and third-degree burns.5 These classifications each have characteristic gross features. Based on these criteria, our patients both presented with blistering and ruptured bullae and no eschar formation, which is classified as second-degree superficial burns.
Following thermal insult to the skin, 3 zones are formed. The central zone consists of irreparable damage referred to as the zone of coagulation. The zone of stasis lies between the completely damaged central region and the outermost regions of the burn lesion, and it receives slightly less blood flow. This area can fully recover after complete perfusion is returned early in the healing process. The outermost zone of hyperemia can fully recover and is an area marked by intense vasodilation from inflammatory reactions.5
Wound Healing
During the healing process, metabolic activity is remarkably increased, which leads to formation of
Burns in Patients With Seizure Disorders
Burns pose a serious risk to patients with seizure disorders that often is underappreciated by patients and health care providers. Although many burns are first-degree burns, up to 10% of burns require medical attention.1 In the initial phase following a thermal insult, the skin’s microflora is killed off, but within a week the sterile skin can become infected.5 The most common microbial invasions seen in blistering wounds are due to Pseudomonas aeruginosa and Staphylococcus aureus.8 With larger burns associated with immunocompromising factors such as diabetes mellitus or older age, patients are at an increased risk for becoming septic. Prior to the period of infection, the damage caused by the heat leads to vasodilation of the microvasculature surrounding the injured area. In addition, release of cytokines leads to migration of inflammatory cells. With the vasodilation of vasculature, proteinaceous fluids from the intravascular space can collect between the dead epidermal and dermal layers to form blisters.5 In larger burns, the fluid shifts will lead to severe oncotic pressure decreases intravascularly and can lead to hypotensive shock.6 When burns have a more severe global effect, aggressive resuscitation and vasopressors are required to maintain perfusion of vital organs.
Both of our patients experienced painful lesions, but they were fortunate to have factors of youth, superficial damage, and low total body surface area burns for a smaller risk for infection, fluid loss, and severely disfiguring scars.8 Because the duration of the postictal phase can vary, there is potential for more severe burns that can leave a lifelong reminder of the event. Depending on the skin type and the depth of the thermal insult, evidence of injury may last many years in the form of hypertrophic scars, contractures, and changes in skin pigmentation.5 At distances 30 cm or less from the standard blow-dryer, it takes 2 minutes to cause cell death.9 In comparison to a heat source that is meant to provide warmth to a room, there is a notable difference in potential for severe burns with the standard heater vs the standard blow-dryer.
Along with the physical pain, the visual reminders of the injurious event can have notable psychological effects. Scars can decrease self-esteem and lead to depression, anxiety, body image problems, and sexuality issues.10
Given the immense risks associated with burn injuries and the many unfortunate outcomes, emphasis should be placed on patient education regarding safety precautions with seizure disorders. In one study, it was found that only 5% of patients recall receiving a warning about the risk for burn injuries with seizures.2 It is important for patients and physicians to develop a written comprehensive safety plan that addresses the risks for daily activities during the day and night. Although patients may not remember being told about the risks, a written safety plan likely will increase patient awareness and reduce avoidable injuries. In addition to written safety plans, prior recommendations for reducing burn injuries in seizure patients include the use of fire and heater guards as well as flame-retardant clothing and blankets.11
- Spitz MC, Towbin JA, Shantz D, et al. Risk factors for burns as a consequence of seizures in persons with epilepsy. Epilepsia. 1994;35:764-767.
- Hampton KK, Peatfield RC, Pullar T, et al. Burns because of epilepsy. Br Med J (Clin Res Ed). 1988;296:1659-1660.
- Kinton L, Duncan JS. Frequency, causes, and consequences of burns in patients with epilepsy. J Neurol Neurosurg Psychiatry. 1998;65:404-405.
- World Health Organization. Burns. http://www.who.int/news-room/fact-sheets/detail/burns. Published March 6, 2018. Accessed December 13, 2019.
- Tiwari VK. Burn wound: how it differs from other wounds? Indian J Plast Surg. 2012;45:364-373.
- Nielson CB, Duethman NC, Howard JM, et al. Burns: pathophysiology of systemic complications and current management. J Burn Care Res. 2017;38:E469-E481.
- Travers JB, Murphy RC, Johnson CA, et al. Identification and pharmacological characterization of platelet-activating factor and related 1-palmitoyl species found in human inflammatory blistering diseases. Prostaglandins Other Lipid Mediat. 1998;5:305-324.
- Church D, Elsayed S, Reid O, et al. Burn wound infections. Clin Microbiol Rev. 2006;19:403-434.
- Aslam A, Khoo CT. No sense; no sensibility—a tale of two adult hair-drier burns. Burns. 1997;23:454-457.
- Van Loey NE, Van Son MJ. Psychopathology and psychological problems in patients with burn scars: epidemiology and management. Am J Clin Dermatol. 2003;4:245-272.
- Josty IC, Narayanan V, Dickson WA. Burns in patients with epilepsy: changes in epidemiology and implications for burn treatment and prevention. Epilepsia. 2000;41:453-456.
Patients with seizures are placed at an increased risk for sustaining burn injuries, which may occur during common daily activities such as cooking, showering, and using heaters.1 Although patients are warned of the risks of injury at the time of their epilepsy diagnosis, patients still experience injuries that commonly occur during the seizure or the postictal phase. In a study of 134 patients with epilepsy, only 38% recalled being burned during a seizure, with approximately 9% being burned multiple times.2 Another study investigated the circumstances resulting in burns in this patient population and found that cooking on a stove was the most common cause, followed by hot water while showering and exposed room heaters.1 Another study found that the majority of burns in seizure patients were from spilled hot drinks.3
We report 2 patients who presented to the dermatology clinic with second-degree burns following nocturnal seizures. In both cases, the patients were sleeping next to exposed heaters, which led to burn injuries from seizures that occurred in the night.
Case Reports
Patient 1
A 30-year-old woman with a history of a seizure disorder presented with painful second-degree blistering burns along the left arm and flank (Figure 1). One day prior to presentation, she had woken up to find these lesions and visited the emergency department where she was prescribed silver sulfadiazine cream to prevent infection of the wound site and was referred to our dermatology clinic. Initially, the patient had difficulty pinpointing the source of the burn lesions and thought that it may have been due to sleeping with her cell phone, but she later realized that they were due to the space heater placed next to her bed. Because of the unclear etiology at the initial presentation, a skin biopsy of a lesion was taken while she was at the clinic.

Biopsy of the lesions exhibited separation of the epidermal and dermal layers (Figure 2). Thermal damage was seen extending into the dermal layers with notable edema present. A few inflammatory cells, neutrophils, and monocytes were noted in the biopsy. The initial pathology results showed the epidermis was necrotic with edema, spongiform vesicles, and few neutrophils. The histologic findings aligned with the timeline of the injury occurring 2 days prior to the biopsy. She was treated supportively using mupirocin ointment to prevent secondary infection.

Case 2
A 27-year-old woman with a history of epilepsy presented to the dermatology clinic with painful blistering lesions along the right upper arm (Figure 3). She was found to have notable second-degree burns along the right arm. She reported placing her bed near a baseboard heater to stay warm overnight. She noticed the painful lesions after waking up next to the heater following a suspected seizure. She was treated supportively using mupirocin ointment to prevent secondary infection.

Comment
Classification of Burns and Damage
According to the World Health Organization, nonfatal burn injuries are a leading cause of morbidity and occur mainly in the home and workplace.4 There are many types of burns: radiation, electrical, chemical, friction, and thermal. The most common type of burns are thermal burns,4 which can be further subdivided into wet and dry. Both of our patients experienced dry thermal burns.
Based on the skin tissue layers involved in the thermal damage, burn wounds are further divided into first-degree burns, superficial second-degree burns, deep second-degree burns, and third-degree burns.5 These classifications each have characteristic gross features. Based on these criteria, our patients both presented with blistering and ruptured bullae and no eschar formation, which is classified as second-degree superficial burns.
Following thermal insult to the skin, 3 zones are formed. The central zone consists of irreparable damage referred to as the zone of coagulation. The zone of stasis lies between the completely damaged central region and the outermost regions of the burn lesion, and it receives slightly less blood flow. This area can fully recover after complete perfusion is returned early in the healing process. The outermost zone of hyperemia can fully recover and is an area marked by intense vasodilation from inflammatory reactions.5
Wound Healing
During the healing process, metabolic activity is remarkably increased, which leads to formation of
Burns in Patients With Seizure Disorders
Burns pose a serious risk to patients with seizure disorders that often is underappreciated by patients and health care providers. Although many burns are first-degree burns, up to 10% of burns require medical attention.1 In the initial phase following a thermal insult, the skin’s microflora is killed off, but within a week the sterile skin can become infected.5 The most common microbial invasions seen in blistering wounds are due to Pseudomonas aeruginosa and Staphylococcus aureus.8 With larger burns associated with immunocompromising factors such as diabetes mellitus or older age, patients are at an increased risk for becoming septic. Prior to the period of infection, the damage caused by the heat leads to vasodilation of the microvasculature surrounding the injured area. In addition, release of cytokines leads to migration of inflammatory cells. With the vasodilation of vasculature, proteinaceous fluids from the intravascular space can collect between the dead epidermal and dermal layers to form blisters.5 In larger burns, the fluid shifts will lead to severe oncotic pressure decreases intravascularly and can lead to hypotensive shock.6 When burns have a more severe global effect, aggressive resuscitation and vasopressors are required to maintain perfusion of vital organs.
Both of our patients experienced painful lesions, but they were fortunate to have factors of youth, superficial damage, and low total body surface area burns for a smaller risk for infection, fluid loss, and severely disfiguring scars.8 Because the duration of the postictal phase can vary, there is potential for more severe burns that can leave a lifelong reminder of the event. Depending on the skin type and the depth of the thermal insult, evidence of injury may last many years in the form of hypertrophic scars, contractures, and changes in skin pigmentation.5 At distances 30 cm or less from the standard blow-dryer, it takes 2 minutes to cause cell death.9 In comparison to a heat source that is meant to provide warmth to a room, there is a notable difference in potential for severe burns with the standard heater vs the standard blow-dryer.
Along with the physical pain, the visual reminders of the injurious event can have notable psychological effects. Scars can decrease self-esteem and lead to depression, anxiety, body image problems, and sexuality issues.10
Given the immense risks associated with burn injuries and the many unfortunate outcomes, emphasis should be placed on patient education regarding safety precautions with seizure disorders. In one study, it was found that only 5% of patients recall receiving a warning about the risk for burn injuries with seizures.2 It is important for patients and physicians to develop a written comprehensive safety plan that addresses the risks for daily activities during the day and night. Although patients may not remember being told about the risks, a written safety plan likely will increase patient awareness and reduce avoidable injuries. In addition to written safety plans, prior recommendations for reducing burn injuries in seizure patients include the use of fire and heater guards as well as flame-retardant clothing and blankets.11
Patients with seizures are placed at an increased risk for sustaining burn injuries, which may occur during common daily activities such as cooking, showering, and using heaters.1 Although patients are warned of the risks of injury at the time of their epilepsy diagnosis, patients still experience injuries that commonly occur during the seizure or the postictal phase. In a study of 134 patients with epilepsy, only 38% recalled being burned during a seizure, with approximately 9% being burned multiple times.2 Another study investigated the circumstances resulting in burns in this patient population and found that cooking on a stove was the most common cause, followed by hot water while showering and exposed room heaters.1 Another study found that the majority of burns in seizure patients were from spilled hot drinks.3
We report 2 patients who presented to the dermatology clinic with second-degree burns following nocturnal seizures. In both cases, the patients were sleeping next to exposed heaters, which led to burn injuries from seizures that occurred in the night.
Case Reports
Patient 1
A 30-year-old woman with a history of a seizure disorder presented with painful second-degree blistering burns along the left arm and flank (Figure 1). One day prior to presentation, she had woken up to find these lesions and visited the emergency department where she was prescribed silver sulfadiazine cream to prevent infection of the wound site and was referred to our dermatology clinic. Initially, the patient had difficulty pinpointing the source of the burn lesions and thought that it may have been due to sleeping with her cell phone, but she later realized that they were due to the space heater placed next to her bed. Because of the unclear etiology at the initial presentation, a skin biopsy of a lesion was taken while she was at the clinic.

Biopsy of the lesions exhibited separation of the epidermal and dermal layers (Figure 2). Thermal damage was seen extending into the dermal layers with notable edema present. A few inflammatory cells, neutrophils, and monocytes were noted in the biopsy. The initial pathology results showed the epidermis was necrotic with edema, spongiform vesicles, and few neutrophils. The histologic findings aligned with the timeline of the injury occurring 2 days prior to the biopsy. She was treated supportively using mupirocin ointment to prevent secondary infection.

Case 2
A 27-year-old woman with a history of epilepsy presented to the dermatology clinic with painful blistering lesions along the right upper arm (Figure 3). She was found to have notable second-degree burns along the right arm. She reported placing her bed near a baseboard heater to stay warm overnight. She noticed the painful lesions after waking up next to the heater following a suspected seizure. She was treated supportively using mupirocin ointment to prevent secondary infection.

Comment
Classification of Burns and Damage
According to the World Health Organization, nonfatal burn injuries are a leading cause of morbidity and occur mainly in the home and workplace.4 There are many types of burns: radiation, electrical, chemical, friction, and thermal. The most common type of burns are thermal burns,4 which can be further subdivided into wet and dry. Both of our patients experienced dry thermal burns.
Based on the skin tissue layers involved in the thermal damage, burn wounds are further divided into first-degree burns, superficial second-degree burns, deep second-degree burns, and third-degree burns.5 These classifications each have characteristic gross features. Based on these criteria, our patients both presented with blistering and ruptured bullae and no eschar formation, which is classified as second-degree superficial burns.
Following thermal insult to the skin, 3 zones are formed. The central zone consists of irreparable damage referred to as the zone of coagulation. The zone of stasis lies between the completely damaged central region and the outermost regions of the burn lesion, and it receives slightly less blood flow. This area can fully recover after complete perfusion is returned early in the healing process. The outermost zone of hyperemia can fully recover and is an area marked by intense vasodilation from inflammatory reactions.5
Wound Healing
During the healing process, metabolic activity is remarkably increased, which leads to formation of
Burns in Patients With Seizure Disorders
Burns pose a serious risk to patients with seizure disorders that often is underappreciated by patients and health care providers. Although many burns are first-degree burns, up to 10% of burns require medical attention.1 In the initial phase following a thermal insult, the skin’s microflora is killed off, but within a week the sterile skin can become infected.5 The most common microbial invasions seen in blistering wounds are due to Pseudomonas aeruginosa and Staphylococcus aureus.8 With larger burns associated with immunocompromising factors such as diabetes mellitus or older age, patients are at an increased risk for becoming septic. Prior to the period of infection, the damage caused by the heat leads to vasodilation of the microvasculature surrounding the injured area. In addition, release of cytokines leads to migration of inflammatory cells. With the vasodilation of vasculature, proteinaceous fluids from the intravascular space can collect between the dead epidermal and dermal layers to form blisters.5 In larger burns, the fluid shifts will lead to severe oncotic pressure decreases intravascularly and can lead to hypotensive shock.6 When burns have a more severe global effect, aggressive resuscitation and vasopressors are required to maintain perfusion of vital organs.
Both of our patients experienced painful lesions, but they were fortunate to have factors of youth, superficial damage, and low total body surface area burns for a smaller risk for infection, fluid loss, and severely disfiguring scars.8 Because the duration of the postictal phase can vary, there is potential for more severe burns that can leave a lifelong reminder of the event. Depending on the skin type and the depth of the thermal insult, evidence of injury may last many years in the form of hypertrophic scars, contractures, and changes in skin pigmentation.5 At distances 30 cm or less from the standard blow-dryer, it takes 2 minutes to cause cell death.9 In comparison to a heat source that is meant to provide warmth to a room, there is a notable difference in potential for severe burns with the standard heater vs the standard blow-dryer.
Along with the physical pain, the visual reminders of the injurious event can have notable psychological effects. Scars can decrease self-esteem and lead to depression, anxiety, body image problems, and sexuality issues.10
Given the immense risks associated with burn injuries and the many unfortunate outcomes, emphasis should be placed on patient education regarding safety precautions with seizure disorders. In one study, it was found that only 5% of patients recall receiving a warning about the risk for burn injuries with seizures.2 It is important for patients and physicians to develop a written comprehensive safety plan that addresses the risks for daily activities during the day and night. Although patients may not remember being told about the risks, a written safety plan likely will increase patient awareness and reduce avoidable injuries. In addition to written safety plans, prior recommendations for reducing burn injuries in seizure patients include the use of fire and heater guards as well as flame-retardant clothing and blankets.11
- Spitz MC, Towbin JA, Shantz D, et al. Risk factors for burns as a consequence of seizures in persons with epilepsy. Epilepsia. 1994;35:764-767.
- Hampton KK, Peatfield RC, Pullar T, et al. Burns because of epilepsy. Br Med J (Clin Res Ed). 1988;296:1659-1660.
- Kinton L, Duncan JS. Frequency, causes, and consequences of burns in patients with epilepsy. J Neurol Neurosurg Psychiatry. 1998;65:404-405.
- World Health Organization. Burns. http://www.who.int/news-room/fact-sheets/detail/burns. Published March 6, 2018. Accessed December 13, 2019.
- Tiwari VK. Burn wound: how it differs from other wounds? Indian J Plast Surg. 2012;45:364-373.
- Nielson CB, Duethman NC, Howard JM, et al. Burns: pathophysiology of systemic complications and current management. J Burn Care Res. 2017;38:E469-E481.
- Travers JB, Murphy RC, Johnson CA, et al. Identification and pharmacological characterization of platelet-activating factor and related 1-palmitoyl species found in human inflammatory blistering diseases. Prostaglandins Other Lipid Mediat. 1998;5:305-324.
- Church D, Elsayed S, Reid O, et al. Burn wound infections. Clin Microbiol Rev. 2006;19:403-434.
- Aslam A, Khoo CT. No sense; no sensibility—a tale of two adult hair-drier burns. Burns. 1997;23:454-457.
- Van Loey NE, Van Son MJ. Psychopathology and psychological problems in patients with burn scars: epidemiology and management. Am J Clin Dermatol. 2003;4:245-272.
- Josty IC, Narayanan V, Dickson WA. Burns in patients with epilepsy: changes in epidemiology and implications for burn treatment and prevention. Epilepsia. 2000;41:453-456.
- Spitz MC, Towbin JA, Shantz D, et al. Risk factors for burns as a consequence of seizures in persons with epilepsy. Epilepsia. 1994;35:764-767.
- Hampton KK, Peatfield RC, Pullar T, et al. Burns because of epilepsy. Br Med J (Clin Res Ed). 1988;296:1659-1660.
- Kinton L, Duncan JS. Frequency, causes, and consequences of burns in patients with epilepsy. J Neurol Neurosurg Psychiatry. 1998;65:404-405.
- World Health Organization. Burns. http://www.who.int/news-room/fact-sheets/detail/burns. Published March 6, 2018. Accessed December 13, 2019.
- Tiwari VK. Burn wound: how it differs from other wounds? Indian J Plast Surg. 2012;45:364-373.
- Nielson CB, Duethman NC, Howard JM, et al. Burns: pathophysiology of systemic complications and current management. J Burn Care Res. 2017;38:E469-E481.
- Travers JB, Murphy RC, Johnson CA, et al. Identification and pharmacological characterization of platelet-activating factor and related 1-palmitoyl species found in human inflammatory blistering diseases. Prostaglandins Other Lipid Mediat. 1998;5:305-324.
- Church D, Elsayed S, Reid O, et al. Burn wound infections. Clin Microbiol Rev. 2006;19:403-434.
- Aslam A, Khoo CT. No sense; no sensibility—a tale of two adult hair-drier burns. Burns. 1997;23:454-457.
- Van Loey NE, Van Son MJ. Psychopathology and psychological problems in patients with burn scars: epidemiology and management. Am J Clin Dermatol. 2003;4:245-272.
- Josty IC, Narayanan V, Dickson WA. Burns in patients with epilepsy: changes in epidemiology and implications for burn treatment and prevention. Epilepsia. 2000;41:453-456.
Practice Points
- Burns and scars from burns can lead to both life-threatening consequences and lifelong psychological effects.
- Many epileptic patients who present with thermal burn injuries do not remember getting burned.
- Clinicians should be aware of all the potential dangers that patients with epilepsy may encounter both during the day and night.
Hyperbaric Oxygen Therapy in Dermatology
Hyperbaric oxygen therapy (HOT) is a treatment modality dating to 1861 in the United States.1 Today, there are 14 indications2 for HOT (Table), issued by the Undersea & Hyperbaric Medical Society, which also administers an accreditation program for facilities providing HOT.3 The 14 indications also are relevant because it is unlikely that HOT will be covered by insurance for unapproved indications.4

Although HOT is not commonly seen as a first-line intervention in dermatology, there are scenarios in which it can be used to good effect: compromised grafts and flaps; poorly healing ulceration related to vasculitis and autoimmune disorders; and possibly for vascular compromise, including cutaneous ischemia caused by fillers. We review its indications, dermatologic applications, and potential complications.
Overview of HOT
Hyperbaric oxygen therapy involves sitting or lying in a special chamber that allows for controlled levels of oxygen (O2) at increased atmospheric pressure, which specifically involves breathing near 100% O2 while inside a monoplace or multiplace chamber5 that is pressurized to greater than sea level pressure (≥1.4 atmosphere absolute).2
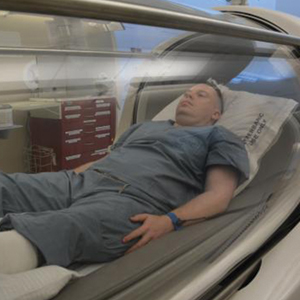
A monoplace chamber is designed to treat a single person (Figure 1); a multiplace chamber (Figure 2) accommodates as many as 5 to 25 patients.5,6 The chambers also accommodate hospital beds and medical attendants, if needed. Hyperbaric O2 is inhaled through a mask, a tight-fitting hood, or an endotracheal tube, depending on the patient’s status.7 Treatment ranges from only 1 or 2 iterations for acute conditions to 30 sessions or more for chronic conditions. Individual sessions last 45 minutes to 5 hours; 120 minutes is considered a safe maximum duration.7 A television often is provided to help the patient pass the time.8

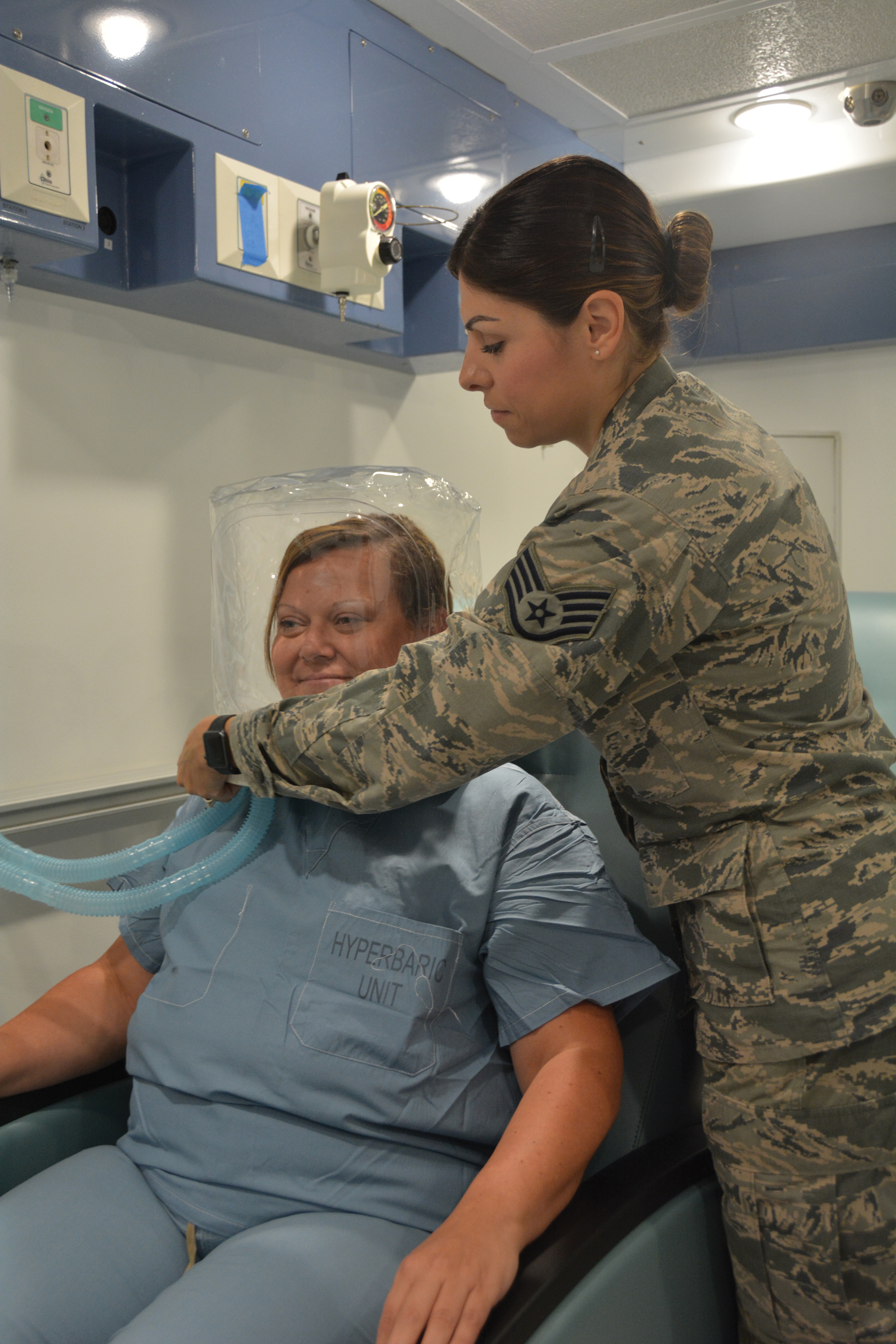
Long-standing Use in Decompression Sickness
Hyperbaric oxygen therapy is best known for its effectiveness in treating decompression sickness (DCS) and carbon monoxide poisoning. Decompression sickness involves liberation of free gas from tissue, in the form of bubbles, when a person experiences a relative decrease in atmospheric pressure, which results in an imbalance in the sum of gas tensions in tissue compared to ambient pressure.
Decompression sickness has special military significance because it can affect divers and pilots, particularly those flying at high altitude. Over the course of 12 years, approximately 50 pilot trainees at an Air Force training site in Colorado required HOT when ground-level O2 failed to resolve their DCS symptoms.10
Symptoms of DCS range from musculoskeletal pain to severe neurologic and pulmonary complications. First-line therapy for DCS is 100% O2 at ground level. When symptoms are severe or persistent, HOT is the treatment of choice. It works by decreasing the volume of air bubbles (as predicted by Boyle’s Law), providing oxygenation to hypoxic tissue and mitigating inflammatory responses implicated in tissue injury9; HOT can be considered salvage treatment for rare, severe, or unresponsive complications of DCS during common activities such as diving and flying.
The emergent nature of DCS often necessitates an on-call, on-site HOT facility or contracted community services. Although DCS is a rare complication, it can be devastating, as was the case for a military pilot flying an ultrahigh altitude reconnaissance aircraft.11 He developed a near fatal case of neurologic DCS during a military mission and required treatment with emergent HOT. Although his symptoms were reduced with therapy, he has persistent cognitive deficits.11
Other Indications
Dermatologic Flaps and Grafts
Although less commonly discussed in dermatologic literature, the use of HOT in compromised grafts and flaps has been addressed in the plastic surgery literature. In a large multicenter study, researchers evaluated 20,821 Mohs micrographic surgery procedures and reported 149 adverse events, of which 20.1% were dehiscence and partial or full necrosis.12 These complications, though rare, are potentially devastating, particularly in cosmetically sensitive locations such as the face. Traditional care for compromised grafts and flaps includes local wound care, surgical debridement, and additional reconstructive procedures. These interventions can be expensive and uncomfortable for patients and carry risk for further morbidity.13
Grafts become compromised when their metabolic demand outpaces the ability of the recipient bed due to characteristics of the graft or the recipient bed or both. Flaps carry their own blood supply, which can be compromised if the flap is too long or too large for the pedicle, there is notable tension on the wound, or blood flow is mechanically obstructed by kinking or twisting. Under these conditions, HOT can be beneficial, as O2 dissolves in plasma, thus improving the O2 tissue cellular diffusion gradient.7 An increased level of systemic O2 promotes wound healing and graft or flap survival by improving fibroblast function, blood flow, and vascularity, and by mitigating ischemia-reperfusion injury.13
Radiation-Induced Ulceration
Radionecrosis, a complication of radiotherapy, is caused by progressive obliterating endarteritis with resultant vascular stenosis and fibroatrophy, which eventually cause stromal fibrosis.15 In a study that looked at 1267 nonmelanoma skin cancers that had been treated with radiotherapy, the ulceration rate was 6.3%. Most of the ulcerated lesions were treatable conservatively, but some were more treatment resistant.16 Hampson et al17 reported on 58 patients with cutaneous wounds due to soft-tissue radionecrosis who were treated with HOT as part of a larger observational case series in which investigators looked at multiple types of radionecrosis. They found that 76% of these patients improved: 26% showed complete resolution and the remaining 50% had 50% to 90% improvement.17
Vasculitis or Autoimmune Ulceration
Vasculitis and vasculopathy can occur independent of, or in association with, connective tissue disease and can result in chronic ulceration. At our institution, a patient with antimelanoma differentiation-associated protein 5 dermatomyositis who had refractory digital ulcerations despite intensive systemic therapy had an excellent response to HOT; ulcerations resolved after 37 treatments.18
Efrati et al19 reported on 35 patients who had chronic nonhealing vasculitic ulcerations despite immunosuppression medication who were treated with HOT. Twenty-eight patients completely healed, 4 had partial healing, and 3 had no improvement.
Mirasoglu et al20 reported on a case series of 6 systemic sclerosis patients who had ulcerations that persisted despite other treatments. After initiation of HOT, 4 patients experienced complete response and 2 experienced partial response, which is notable because such ulcerations are often extremely difficult to treat and have usually failed multiple therapies before being addressed with HOT.
Cutaneous Vascular Compromise
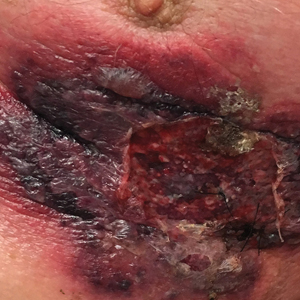
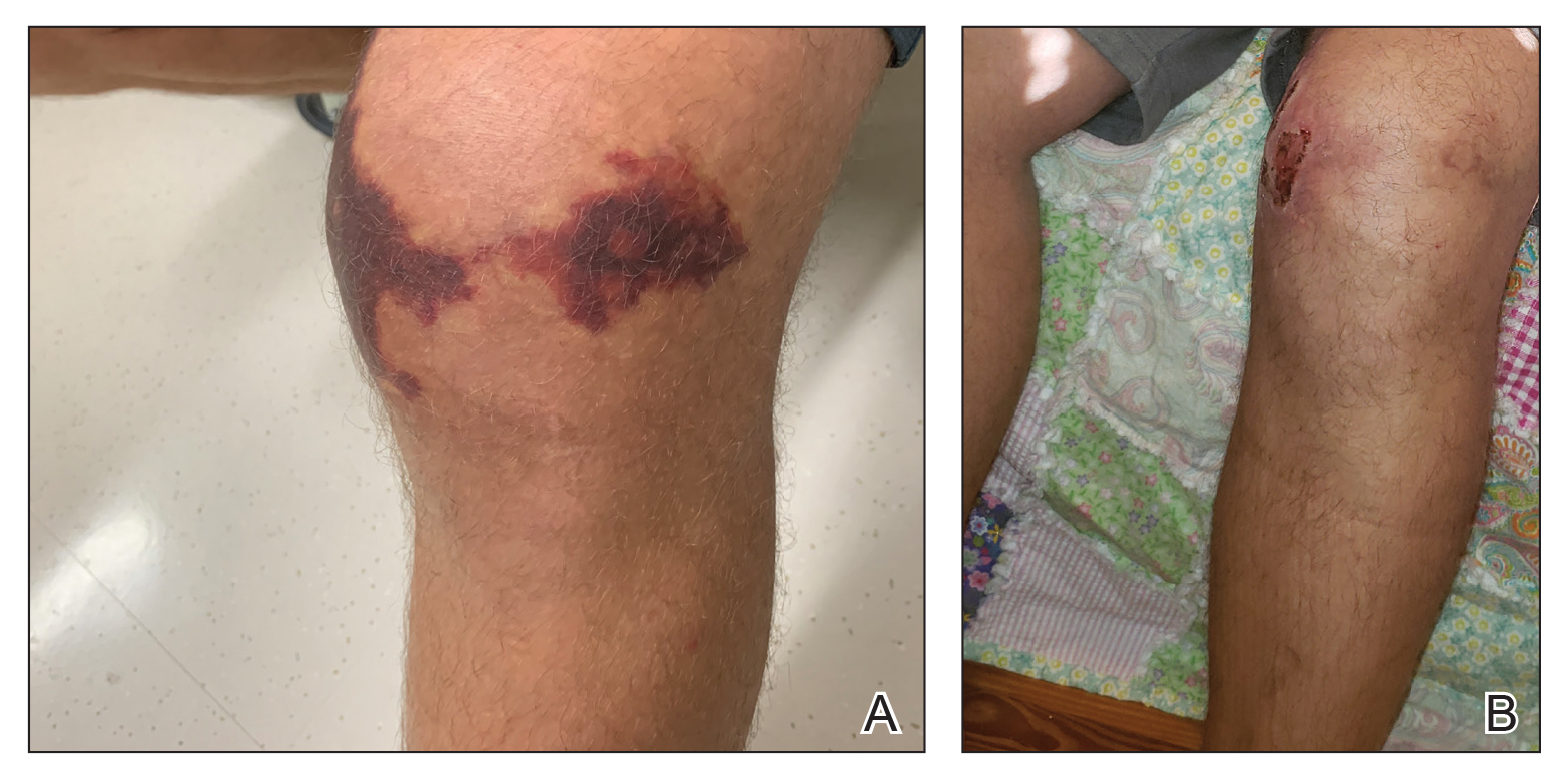
At our institution, a 36-year-old man was referred to the dermatology clinic 2 days after undergoing embolization of a symptomatic arteriovenous malformation in the right knee (Figure 3A). The procedure was complicated by cutaneous purpura concerning for necrosis, a known complication of this procedure. We referred the patient for evaluation to consider HOT. Although he was outside the ideal window for starting treatment, HOT was initiated. With a late start in treatment, areas of skin had already progressed to full necrosis, which did not respond to treatment; however, contiguous areas that initially looked very similar clinically did respond to treatment (Figure 3B). This case suggests a penumbralike effect in which vulnerable tissue that would most likely have been lost was salvaged by HOT.
Ischemia
Hyperbaric oxygen therapy has been used to treat ischemia caused by injection of cosmetic filler. Henderson et al21 described a 37-year-old woman who experienced occlusion of the left superficial temporal artery while self-injecting a hyaluronic acid filler around the temples. The problem was complicated by left-sided hearing loss, cutaneous blanching of the left face, and pain. She was treated with enoxaparin, aspirin, dexamethasone, antibiotics, and intradermal lidocaine. Additionally, she was urgently referred to a HOT facility and was treated with 6 HOT treatments in 3 days, with the first treatment provided 15 hours after the initial insult. The patient showed a decrease in ischemic discoloration over the course of the treatment. Eventually, her hearing returned to baseline and she achieved an acceptable cosmetic outcome.21
Uittenbogaard et al22 reported the treatment of a patient who experienced dermal ischemia after receiving calcium hydroxylapatite at an aesthetic clinic. She did not improve with standard treatment but subsequently experienced resolution of symptoms after treatment with HOT. She had an excellent cosmetic outcome at 6-month follow-up.22
Complications and a Contraindication
Hyperbaric oxygen therapy generally is safe, but there is potential for complications.
Fire
This rare risk has a catastrophic outcome.23 Standards for fire prevention in hyperbaric facilities are issued by the National Fire Protection Association, covering construction and building materials, lighting, electrical wiring, exposure to flammable materials, and other possible ignition sources.24
Middle Ear Barotrauma
The incidence of the most common adverse effect of HOT is reported at 2% to 30%.7,25 Middle ear barotrauma occurs most commonly during the compression phase of treatment. It is more common in patients treated in a monoplace chamber because they are kept supine and are less able to regulate middle ear pressure.26 Symptoms of middle ear barotrauma can be relieved by teaching patients autoinflation technique, such as the Valsalva maneuver, or by placing tympanoplasty tubes.27
Reversible Myopia
Caused by direct O2 toxicity to the lens, this complication can last for weeks, though it eventually resolves spontaneously. Reversible myopia has been reported to be at least as common as middle ear barotrauma.27
Other Complications
Central nervous system complications, such as seizures, and pulmonary O2 toxicity are rare, more serious complications.27
Untreated Pneumothorax
The only absolute contraindication to HOT, pneumothorax can decompensate during HOT if left untreated. However, HOT can proceed once pneumothorax is addressed.7
Conclusion
Hyperbaric O2 therapy can make a positive contribution to the dermatologic therapeutic armamentarium, in specific patients, for impending graft or flap failure, chronic wounds and ulcerations, and cutaneous vascular compromise. Although HOT is not a commonly needed treatment in dermatology, it is important to be aware of its potential because delay in treatment can decrease its effectiveness. It is recommended that dermatologists locate the nearest HOT facility and become familiar with its capabilities.
- Carney AY. Hyperbaric oxygen therapy: an introduction. Crit Care Nurs Q. 2013;36:274-279.
- Weaver LK, ed. Hyperbaric Oxygen Therapy Indications: The Hyperbaric Oxygen Therapy Committee Report. 13th ed. Undersea and Hyperbaric Medical Society. 2014.https://www.uhms.
org/images/indications/UHMS_HBO2_Indications
_13th_Ed._Front_Matter__References.pdf. Accessed December 18, 2019. - Undersea & Hyperbaric Medical Society. UHMS Hyperbaric Facility Accreditation Program. https://www.uhms.org/about/accreditation/accreditation-for-hyperbaric-medicine.html. Accessed December 18, 2019.
- Hyperbaric oxygen (HBO) therapy. US Centers for Medicare & Medicaid Services. https://www.medicare.gov/coverage/hyperbaric-oxygen-hbo-therapy. Accessed December 18, 2019.
- Gracia L, Perez-Vidal C, de Paco JM, et al. Identification and control of a multiplace hyperbaric chamber. PLoS One. 2018;13:e0200407.
- Monoplace vs multiplace hyperbaric chamber. CutisCare. https://cutiscareusa.com/hyperbaric-oxygen-therapy/monoplace-vs-multiplace-hyperbaric-chamber/. Published August 31, 2018. Accessed December 18, 2019.
- Leach RM, Rees PJ, Wilmshurst PP. Hyperbaric oxygen therapy. BMJ. 1998;317:1140-1143.
- Health Quality Ontario. Hyperbaric oxygen therapy for the treatment of diabetic foot ulcers: a health technology assessment. Ont Health Technol Assess Ser. 2017;17:1-142.
- Vann RD, Butler FK, Mitchell SJ, et al. Decompression illness. Lancet. 2011;377:153-164.
- Rhodes WC, Hertner G, Price R, et al. Treating decompression sickness: military flight simulation site-community hospital partnership. Mil Med. 2017;182:e1718-e1721.
- Jersey SL, Baril RT, McCarty RD, et al. Severe neurological decompression sickness in a U-2 pilot. Aviat Space Environ Med. 2010;81:64-68.
- Alam M, Ibrahim O, Nodzenski M, et al. Adverse events associated with Mohs micrographic surgery: multicenter prospective cohort study of 20,821 cases at 23 centers. JAMA Dermatol. 2013;149:1378-1385.
- Francis A, Baynosa RC. Hyperbaric oxygen therapy for the compromised graft or flap. Adv Wound Care (New Rochelle). 2017;6:23-32.
- Bowersox JC, Strauss MB, Hart GB. Clinical experience with hyperbaric oxygen therapy in the salvage of ischemic skin flaps and grafts. J Hyperb Med. 1986;1:141-149.
- Fernández Canedo I, Padilla España L, Francisco Millán Cayetano J, et al. Hyperbaric oxygen therapy: an alternative treatment for radiation-induced cutaneous ulcers. Australas J Dermatol. 2018;59:e203-e207.
- Schulte KW, Lippold A, Auras C, et al. Soft x-ray therapy for cutaneous basal cell and squamous cell carcinomas. J Am Acad Dermatol. 2005;53:993-1001.
- Hampson NB, Holm JR, Wreford-Brown CE, et al. Prospective assessment of outcomes in 411 patients treated with hyperbaric oxygen for chronic radiation tissue injury. Cancer. 2012;118:3860-3868.
- Jeter J, Wolf EG, Richards M, et al. Successful treatment of anti-MDA5 dermatomyositis associated cutaneous digital pulp ulcerations with hyperbaric oxygen therapy [published online August 21, 2019]. J Clin Rheumatol. doi:10.1097/RHU.0000000000001114.
- Efrati S, Bergan J, Fishlev G, et al. Hyperbaric oxygen therapy for nonhealing vasculitic ulcers. Clin Exp Dermatol. 2007;32:12-17.
- Mirasoglu B, Bagli BS, Aktas S. Hyperbaric oxygen therapy for chronic ulcers in systemic sclerosis—case series. Int J Dermatol. 2017;56:636-640.
- Henderson R, Reilly DA, Cooper JS. Hyperbaric oxygen for ischemia due to injection of cosmetic fillers: case report and issues. Plast Reconstr Surg Glob Open. 2018;6:e1618.
- Uittenbogaard D, Lansdorp CA, Bauland CG, et al. Hyperbaric oxygen therapy for dermal ischemia after dermal filler injection with calcium hydroxylapatite: a case report. Undersea Hyperb Med. 2019;46:207-210.
- Schorow S. The air in there. NFPA Journal. January 3, 2017. https://www.nfpa.org/News-and-Research/Publications-and-media/NFPA-Journal/2017/January-February-2017/Features/Hyperbaric-chambers. Accessed December 18, 2019.
- National Fire Protection Association. NFPA 99: Health Care Facilities Code 2018. https://www.nfpa.org/codes-and-standards/all-codes-and-standards/list-of-codes-and-standards/detail?code=99. Accessed December 18, 2019.
- Blanshard J, Toma A, Bryson P, et al. Middle ear barotrauma in patients undergoing hyperbaric oxygen therapy. Clin Otolaryngol. 1996;21:400-403.
- Lima MA, Farage L, Cury MC, et al. Update on middle ear barotrauma after hyperbaric oxygen therapy—insights on pathophysiology. Int Arch Otorhinolaryngol. 2014;18:204-209.
- Heyboer M, Sharma D, Santiago W, et al. Hyperbaric oxygen therapy: side effects defined and quantified. Adv Wound Care (New Rochelle). 2017;6:210-224.
Hyperbaric oxygen therapy (HOT) is a treatment modality dating to 1861 in the United States.1 Today, there are 14 indications2 for HOT (Table), issued by the Undersea & Hyperbaric Medical Society, which also administers an accreditation program for facilities providing HOT.3 The 14 indications also are relevant because it is unlikely that HOT will be covered by insurance for unapproved indications.4

Although HOT is not commonly seen as a first-line intervention in dermatology, there are scenarios in which it can be used to good effect: compromised grafts and flaps; poorly healing ulceration related to vasculitis and autoimmune disorders; and possibly for vascular compromise, including cutaneous ischemia caused by fillers. We review its indications, dermatologic applications, and potential complications.
Overview of HOT
Hyperbaric oxygen therapy involves sitting or lying in a special chamber that allows for controlled levels of oxygen (O2) at increased atmospheric pressure, which specifically involves breathing near 100% O2 while inside a monoplace or multiplace chamber5 that is pressurized to greater than sea level pressure (≥1.4 atmosphere absolute).2
A monoplace chamber is designed to treat a single person (Figure 1); a multiplace chamber (Figure 2) accommodates as many as 5 to 25 patients.5,6 The chambers also accommodate hospital beds and medical attendants, if needed. Hyperbaric O2 is inhaled through a mask, a tight-fitting hood, or an endotracheal tube, depending on the patient’s status.7 Treatment ranges from only 1 or 2 iterations for acute conditions to 30 sessions or more for chronic conditions. Individual sessions last 45 minutes to 5 hours; 120 minutes is considered a safe maximum duration.7 A television often is provided to help the patient pass the time.8


Long-standing Use in Decompression Sickness
Hyperbaric oxygen therapy is best known for its effectiveness in treating decompression sickness (DCS) and carbon monoxide poisoning. Decompression sickness involves liberation of free gas from tissue, in the form of bubbles, when a person experiences a relative decrease in atmospheric pressure, which results in an imbalance in the sum of gas tensions in tissue compared to ambient pressure.
Decompression sickness has special military significance because it can affect divers and pilots, particularly those flying at high altitude. Over the course of 12 years, approximately 50 pilot trainees at an Air Force training site in Colorado required HOT when ground-level O2 failed to resolve their DCS symptoms.10
Symptoms of DCS range from musculoskeletal pain to severe neurologic and pulmonary complications. First-line therapy for DCS is 100% O2 at ground level. When symptoms are severe or persistent, HOT is the treatment of choice. It works by decreasing the volume of air bubbles (as predicted by Boyle’s Law), providing oxygenation to hypoxic tissue and mitigating inflammatory responses implicated in tissue injury9; HOT can be considered salvage treatment for rare, severe, or unresponsive complications of DCS during common activities such as diving and flying.
The emergent nature of DCS often necessitates an on-call, on-site HOT facility or contracted community services. Although DCS is a rare complication, it can be devastating, as was the case for a military pilot flying an ultrahigh altitude reconnaissance aircraft.11 He developed a near fatal case of neurologic DCS during a military mission and required treatment with emergent HOT. Although his symptoms were reduced with therapy, he has persistent cognitive deficits.11
Other Indications
Dermatologic Flaps and Grafts
Although less commonly discussed in dermatologic literature, the use of HOT in compromised grafts and flaps has been addressed in the plastic surgery literature. In a large multicenter study, researchers evaluated 20,821 Mohs micrographic surgery procedures and reported 149 adverse events, of which 20.1% were dehiscence and partial or full necrosis.12 These complications, though rare, are potentially devastating, particularly in cosmetically sensitive locations such as the face. Traditional care for compromised grafts and flaps includes local wound care, surgical debridement, and additional reconstructive procedures. These interventions can be expensive and uncomfortable for patients and carry risk for further morbidity.13
Grafts become compromised when their metabolic demand outpaces the ability of the recipient bed due to characteristics of the graft or the recipient bed or both. Flaps carry their own blood supply, which can be compromised if the flap is too long or too large for the pedicle, there is notable tension on the wound, or blood flow is mechanically obstructed by kinking or twisting. Under these conditions, HOT can be beneficial, as O2 dissolves in plasma, thus improving the O2 tissue cellular diffusion gradient.7 An increased level of systemic O2 promotes wound healing and graft or flap survival by improving fibroblast function, blood flow, and vascularity, and by mitigating ischemia-reperfusion injury.13
Radiation-Induced Ulceration
Radionecrosis, a complication of radiotherapy, is caused by progressive obliterating endarteritis with resultant vascular stenosis and fibroatrophy, which eventually cause stromal fibrosis.15 In a study that looked at 1267 nonmelanoma skin cancers that had been treated with radiotherapy, the ulceration rate was 6.3%. Most of the ulcerated lesions were treatable conservatively, but some were more treatment resistant.16 Hampson et al17 reported on 58 patients with cutaneous wounds due to soft-tissue radionecrosis who were treated with HOT as part of a larger observational case series in which investigators looked at multiple types of radionecrosis. They found that 76% of these patients improved: 26% showed complete resolution and the remaining 50% had 50% to 90% improvement.17
Vasculitis or Autoimmune Ulceration
Vasculitis and vasculopathy can occur independent of, or in association with, connective tissue disease and can result in chronic ulceration. At our institution, a patient with antimelanoma differentiation-associated protein 5 dermatomyositis who had refractory digital ulcerations despite intensive systemic therapy had an excellent response to HOT; ulcerations resolved after 37 treatments.18
Efrati et al19 reported on 35 patients who had chronic nonhealing vasculitic ulcerations despite immunosuppression medication who were treated with HOT. Twenty-eight patients completely healed, 4 had partial healing, and 3 had no improvement.
Mirasoglu et al20 reported on a case series of 6 systemic sclerosis patients who had ulcerations that persisted despite other treatments. After initiation of HOT, 4 patients experienced complete response and 2 experienced partial response, which is notable because such ulcerations are often extremely difficult to treat and have usually failed multiple therapies before being addressed with HOT.
Cutaneous Vascular Compromise
At our institution, a 36-year-old man was referred to the dermatology clinic 2 days after undergoing embolization of a symptomatic arteriovenous malformation in the right knee (Figure 3A). The procedure was complicated by cutaneous purpura concerning for necrosis, a known complication of this procedure. We referred the patient for evaluation to consider HOT. Although he was outside the ideal window for starting treatment, HOT was initiated. With a late start in treatment, areas of skin had already progressed to full necrosis, which did not respond to treatment; however, contiguous areas that initially looked very similar clinically did respond to treatment (Figure 3B). This case suggests a penumbralike effect in which vulnerable tissue that would most likely have been lost was salvaged by HOT.

Ischemia
Hyperbaric oxygen therapy has been used to treat ischemia caused by injection of cosmetic filler. Henderson et al21 described a 37-year-old woman who experienced occlusion of the left superficial temporal artery while self-injecting a hyaluronic acid filler around the temples. The problem was complicated by left-sided hearing loss, cutaneous blanching of the left face, and pain. She was treated with enoxaparin, aspirin, dexamethasone, antibiotics, and intradermal lidocaine. Additionally, she was urgently referred to a HOT facility and was treated with 6 HOT treatments in 3 days, with the first treatment provided 15 hours after the initial insult. The patient showed a decrease in ischemic discoloration over the course of the treatment. Eventually, her hearing returned to baseline and she achieved an acceptable cosmetic outcome.21
Uittenbogaard et al22 reported the treatment of a patient who experienced dermal ischemia after receiving calcium hydroxylapatite at an aesthetic clinic. She did not improve with standard treatment but subsequently experienced resolution of symptoms after treatment with HOT. She had an excellent cosmetic outcome at 6-month follow-up.22
Complications and a Contraindication
Hyperbaric oxygen therapy generally is safe, but there is potential for complications.
Fire
This rare risk has a catastrophic outcome.23 Standards for fire prevention in hyperbaric facilities are issued by the National Fire Protection Association, covering construction and building materials, lighting, electrical wiring, exposure to flammable materials, and other possible ignition sources.24
Middle Ear Barotrauma
The incidence of the most common adverse effect of HOT is reported at 2% to 30%.7,25 Middle ear barotrauma occurs most commonly during the compression phase of treatment. It is more common in patients treated in a monoplace chamber because they are kept supine and are less able to regulate middle ear pressure.26 Symptoms of middle ear barotrauma can be relieved by teaching patients autoinflation technique, such as the Valsalva maneuver, or by placing tympanoplasty tubes.27
Reversible Myopia
Caused by direct O2 toxicity to the lens, this complication can last for weeks, though it eventually resolves spontaneously. Reversible myopia has been reported to be at least as common as middle ear barotrauma.27
Other Complications
Central nervous system complications, such as seizures, and pulmonary O2 toxicity are rare, more serious complications.27
Untreated Pneumothorax
The only absolute contraindication to HOT, pneumothorax can decompensate during HOT if left untreated. However, HOT can proceed once pneumothorax is addressed.7
Conclusion
Hyperbaric O2 therapy can make a positive contribution to the dermatologic therapeutic armamentarium, in specific patients, for impending graft or flap failure, chronic wounds and ulcerations, and cutaneous vascular compromise. Although HOT is not a commonly needed treatment in dermatology, it is important to be aware of its potential because delay in treatment can decrease its effectiveness. It is recommended that dermatologists locate the nearest HOT facility and become familiar with its capabilities.
Hyperbaric oxygen therapy (HOT) is a treatment modality dating to 1861 in the United States.1 Today, there are 14 indications2 for HOT (Table), issued by the Undersea & Hyperbaric Medical Society, which also administers an accreditation program for facilities providing HOT.3 The 14 indications also are relevant because it is unlikely that HOT will be covered by insurance for unapproved indications.4

Although HOT is not commonly seen as a first-line intervention in dermatology, there are scenarios in which it can be used to good effect: compromised grafts and flaps; poorly healing ulceration related to vasculitis and autoimmune disorders; and possibly for vascular compromise, including cutaneous ischemia caused by fillers. We review its indications, dermatologic applications, and potential complications.
Overview of HOT
Hyperbaric oxygen therapy involves sitting or lying in a special chamber that allows for controlled levels of oxygen (O2) at increased atmospheric pressure, which specifically involves breathing near 100% O2 while inside a monoplace or multiplace chamber5 that is pressurized to greater than sea level pressure (≥1.4 atmosphere absolute).2
A monoplace chamber is designed to treat a single person (Figure 1); a multiplace chamber (Figure 2) accommodates as many as 5 to 25 patients.5,6 The chambers also accommodate hospital beds and medical attendants, if needed. Hyperbaric O2 is inhaled through a mask, a tight-fitting hood, or an endotracheal tube, depending on the patient’s status.7 Treatment ranges from only 1 or 2 iterations for acute conditions to 30 sessions or more for chronic conditions. Individual sessions last 45 minutes to 5 hours; 120 minutes is considered a safe maximum duration.7 A television often is provided to help the patient pass the time.8


Long-standing Use in Decompression Sickness
Hyperbaric oxygen therapy is best known for its effectiveness in treating decompression sickness (DCS) and carbon monoxide poisoning. Decompression sickness involves liberation of free gas from tissue, in the form of bubbles, when a person experiences a relative decrease in atmospheric pressure, which results in an imbalance in the sum of gas tensions in tissue compared to ambient pressure.
Decompression sickness has special military significance because it can affect divers and pilots, particularly those flying at high altitude. Over the course of 12 years, approximately 50 pilot trainees at an Air Force training site in Colorado required HOT when ground-level O2 failed to resolve their DCS symptoms.10
Symptoms of DCS range from musculoskeletal pain to severe neurologic and pulmonary complications. First-line therapy for DCS is 100% O2 at ground level. When symptoms are severe or persistent, HOT is the treatment of choice. It works by decreasing the volume of air bubbles (as predicted by Boyle’s Law), providing oxygenation to hypoxic tissue and mitigating inflammatory responses implicated in tissue injury9; HOT can be considered salvage treatment for rare, severe, or unresponsive complications of DCS during common activities such as diving and flying.
The emergent nature of DCS often necessitates an on-call, on-site HOT facility or contracted community services. Although DCS is a rare complication, it can be devastating, as was the case for a military pilot flying an ultrahigh altitude reconnaissance aircraft.11 He developed a near fatal case of neurologic DCS during a military mission and required treatment with emergent HOT. Although his symptoms were reduced with therapy, he has persistent cognitive deficits.11
Other Indications
Dermatologic Flaps and Grafts
Although less commonly discussed in dermatologic literature, the use of HOT in compromised grafts and flaps has been addressed in the plastic surgery literature. In a large multicenter study, researchers evaluated 20,821 Mohs micrographic surgery procedures and reported 149 adverse events, of which 20.1% were dehiscence and partial or full necrosis.12 These complications, though rare, are potentially devastating, particularly in cosmetically sensitive locations such as the face. Traditional care for compromised grafts and flaps includes local wound care, surgical debridement, and additional reconstructive procedures. These interventions can be expensive and uncomfortable for patients and carry risk for further morbidity.13
Grafts become compromised when their metabolic demand outpaces the ability of the recipient bed due to characteristics of the graft or the recipient bed or both. Flaps carry their own blood supply, which can be compromised if the flap is too long or too large for the pedicle, there is notable tension on the wound, or blood flow is mechanically obstructed by kinking or twisting. Under these conditions, HOT can be beneficial, as O2 dissolves in plasma, thus improving the O2 tissue cellular diffusion gradient.7 An increased level of systemic O2 promotes wound healing and graft or flap survival by improving fibroblast function, blood flow, and vascularity, and by mitigating ischemia-reperfusion injury.13
Radiation-Induced Ulceration
Radionecrosis, a complication of radiotherapy, is caused by progressive obliterating endarteritis with resultant vascular stenosis and fibroatrophy, which eventually cause stromal fibrosis.15 In a study that looked at 1267 nonmelanoma skin cancers that had been treated with radiotherapy, the ulceration rate was 6.3%. Most of the ulcerated lesions were treatable conservatively, but some were more treatment resistant.16 Hampson et al17 reported on 58 patients with cutaneous wounds due to soft-tissue radionecrosis who were treated with HOT as part of a larger observational case series in which investigators looked at multiple types of radionecrosis. They found that 76% of these patients improved: 26% showed complete resolution and the remaining 50% had 50% to 90% improvement.17
Vasculitis or Autoimmune Ulceration
Vasculitis and vasculopathy can occur independent of, or in association with, connective tissue disease and can result in chronic ulceration. At our institution, a patient with antimelanoma differentiation-associated protein 5 dermatomyositis who had refractory digital ulcerations despite intensive systemic therapy had an excellent response to HOT; ulcerations resolved after 37 treatments.18
Efrati et al19 reported on 35 patients who had chronic nonhealing vasculitic ulcerations despite immunosuppression medication who were treated with HOT. Twenty-eight patients completely healed, 4 had partial healing, and 3 had no improvement.
Mirasoglu et al20 reported on a case series of 6 systemic sclerosis patients who had ulcerations that persisted despite other treatments. After initiation of HOT, 4 patients experienced complete response and 2 experienced partial response, which is notable because such ulcerations are often extremely difficult to treat and have usually failed multiple therapies before being addressed with HOT.
Cutaneous Vascular Compromise
At our institution, a 36-year-old man was referred to the dermatology clinic 2 days after undergoing embolization of a symptomatic arteriovenous malformation in the right knee (Figure 3A). The procedure was complicated by cutaneous purpura concerning for necrosis, a known complication of this procedure. We referred the patient for evaluation to consider HOT. Although he was outside the ideal window for starting treatment, HOT was initiated. With a late start in treatment, areas of skin had already progressed to full necrosis, which did not respond to treatment; however, contiguous areas that initially looked very similar clinically did respond to treatment (Figure 3B). This case suggests a penumbralike effect in which vulnerable tissue that would most likely have been lost was salvaged by HOT.

Ischemia
Hyperbaric oxygen therapy has been used to treat ischemia caused by injection of cosmetic filler. Henderson et al21 described a 37-year-old woman who experienced occlusion of the left superficial temporal artery while self-injecting a hyaluronic acid filler around the temples. The problem was complicated by left-sided hearing loss, cutaneous blanching of the left face, and pain. She was treated with enoxaparin, aspirin, dexamethasone, antibiotics, and intradermal lidocaine. Additionally, she was urgently referred to a HOT facility and was treated with 6 HOT treatments in 3 days, with the first treatment provided 15 hours after the initial insult. The patient showed a decrease in ischemic discoloration over the course of the treatment. Eventually, her hearing returned to baseline and she achieved an acceptable cosmetic outcome.21
Uittenbogaard et al22 reported the treatment of a patient who experienced dermal ischemia after receiving calcium hydroxylapatite at an aesthetic clinic. She did not improve with standard treatment but subsequently experienced resolution of symptoms after treatment with HOT. She had an excellent cosmetic outcome at 6-month follow-up.22
Complications and a Contraindication
Hyperbaric oxygen therapy generally is safe, but there is potential for complications.
Fire
This rare risk has a catastrophic outcome.23 Standards for fire prevention in hyperbaric facilities are issued by the National Fire Protection Association, covering construction and building materials, lighting, electrical wiring, exposure to flammable materials, and other possible ignition sources.24
Middle Ear Barotrauma
The incidence of the most common adverse effect of HOT is reported at 2% to 30%.7,25 Middle ear barotrauma occurs most commonly during the compression phase of treatment. It is more common in patients treated in a monoplace chamber because they are kept supine and are less able to regulate middle ear pressure.26 Symptoms of middle ear barotrauma can be relieved by teaching patients autoinflation technique, such as the Valsalva maneuver, or by placing tympanoplasty tubes.27
Reversible Myopia
Caused by direct O2 toxicity to the lens, this complication can last for weeks, though it eventually resolves spontaneously. Reversible myopia has been reported to be at least as common as middle ear barotrauma.27
Other Complications
Central nervous system complications, such as seizures, and pulmonary O2 toxicity are rare, more serious complications.27
Untreated Pneumothorax
The only absolute contraindication to HOT, pneumothorax can decompensate during HOT if left untreated. However, HOT can proceed once pneumothorax is addressed.7
Conclusion
Hyperbaric O2 therapy can make a positive contribution to the dermatologic therapeutic armamentarium, in specific patients, for impending graft or flap failure, chronic wounds and ulcerations, and cutaneous vascular compromise. Although HOT is not a commonly needed treatment in dermatology, it is important to be aware of its potential because delay in treatment can decrease its effectiveness. It is recommended that dermatologists locate the nearest HOT facility and become familiar with its capabilities.
- Carney AY. Hyperbaric oxygen therapy: an introduction. Crit Care Nurs Q. 2013;36:274-279.
- Weaver LK, ed. Hyperbaric Oxygen Therapy Indications: The Hyperbaric Oxygen Therapy Committee Report. 13th ed. Undersea and Hyperbaric Medical Society. 2014.https://www.uhms.
org/images/indications/UHMS_HBO2_Indications
_13th_Ed._Front_Matter__References.pdf. Accessed December 18, 2019. - Undersea & Hyperbaric Medical Society. UHMS Hyperbaric Facility Accreditation Program. https://www.uhms.org/about/accreditation/accreditation-for-hyperbaric-medicine.html. Accessed December 18, 2019.
- Hyperbaric oxygen (HBO) therapy. US Centers for Medicare & Medicaid Services. https://www.medicare.gov/coverage/hyperbaric-oxygen-hbo-therapy. Accessed December 18, 2019.
- Gracia L, Perez-Vidal C, de Paco JM, et al. Identification and control of a multiplace hyperbaric chamber. PLoS One. 2018;13:e0200407.
- Monoplace vs multiplace hyperbaric chamber. CutisCare. https://cutiscareusa.com/hyperbaric-oxygen-therapy/monoplace-vs-multiplace-hyperbaric-chamber/. Published August 31, 2018. Accessed December 18, 2019.
- Leach RM, Rees PJ, Wilmshurst PP. Hyperbaric oxygen therapy. BMJ. 1998;317:1140-1143.
- Health Quality Ontario. Hyperbaric oxygen therapy for the treatment of diabetic foot ulcers: a health technology assessment. Ont Health Technol Assess Ser. 2017;17:1-142.
- Vann RD, Butler FK, Mitchell SJ, et al. Decompression illness. Lancet. 2011;377:153-164.
- Rhodes WC, Hertner G, Price R, et al. Treating decompression sickness: military flight simulation site-community hospital partnership. Mil Med. 2017;182:e1718-e1721.
- Jersey SL, Baril RT, McCarty RD, et al. Severe neurological decompression sickness in a U-2 pilot. Aviat Space Environ Med. 2010;81:64-68.
- Alam M, Ibrahim O, Nodzenski M, et al. Adverse events associated with Mohs micrographic surgery: multicenter prospective cohort study of 20,821 cases at 23 centers. JAMA Dermatol. 2013;149:1378-1385.
- Francis A, Baynosa RC. Hyperbaric oxygen therapy for the compromised graft or flap. Adv Wound Care (New Rochelle). 2017;6:23-32.
- Bowersox JC, Strauss MB, Hart GB. Clinical experience with hyperbaric oxygen therapy in the salvage of ischemic skin flaps and grafts. J Hyperb Med. 1986;1:141-149.
- Fernández Canedo I, Padilla España L, Francisco Millán Cayetano J, et al. Hyperbaric oxygen therapy: an alternative treatment for radiation-induced cutaneous ulcers. Australas J Dermatol. 2018;59:e203-e207.
- Schulte KW, Lippold A, Auras C, et al. Soft x-ray therapy for cutaneous basal cell and squamous cell carcinomas. J Am Acad Dermatol. 2005;53:993-1001.
- Hampson NB, Holm JR, Wreford-Brown CE, et al. Prospective assessment of outcomes in 411 patients treated with hyperbaric oxygen for chronic radiation tissue injury. Cancer. 2012;118:3860-3868.
- Jeter J, Wolf EG, Richards M, et al. Successful treatment of anti-MDA5 dermatomyositis associated cutaneous digital pulp ulcerations with hyperbaric oxygen therapy [published online August 21, 2019]. J Clin Rheumatol. doi:10.1097/RHU.0000000000001114.
- Efrati S, Bergan J, Fishlev G, et al. Hyperbaric oxygen therapy for nonhealing vasculitic ulcers. Clin Exp Dermatol. 2007;32:12-17.
- Mirasoglu B, Bagli BS, Aktas S. Hyperbaric oxygen therapy for chronic ulcers in systemic sclerosis—case series. Int J Dermatol. 2017;56:636-640.
- Henderson R, Reilly DA, Cooper JS. Hyperbaric oxygen for ischemia due to injection of cosmetic fillers: case report and issues. Plast Reconstr Surg Glob Open. 2018;6:e1618.
- Uittenbogaard D, Lansdorp CA, Bauland CG, et al. Hyperbaric oxygen therapy for dermal ischemia after dermal filler injection with calcium hydroxylapatite: a case report. Undersea Hyperb Med. 2019;46:207-210.
- Schorow S. The air in there. NFPA Journal. January 3, 2017. https://www.nfpa.org/News-and-Research/Publications-and-media/NFPA-Journal/2017/January-February-2017/Features/Hyperbaric-chambers. Accessed December 18, 2019.
- National Fire Protection Association. NFPA 99: Health Care Facilities Code 2018. https://www.nfpa.org/codes-and-standards/all-codes-and-standards/list-of-codes-and-standards/detail?code=99. Accessed December 18, 2019.
- Blanshard J, Toma A, Bryson P, et al. Middle ear barotrauma in patients undergoing hyperbaric oxygen therapy. Clin Otolaryngol. 1996;21:400-403.
- Lima MA, Farage L, Cury MC, et al. Update on middle ear barotrauma after hyperbaric oxygen therapy—insights on pathophysiology. Int Arch Otorhinolaryngol. 2014;18:204-209.
- Heyboer M, Sharma D, Santiago W, et al. Hyperbaric oxygen therapy: side effects defined and quantified. Adv Wound Care (New Rochelle). 2017;6:210-224.
- Carney AY. Hyperbaric oxygen therapy: an introduction. Crit Care Nurs Q. 2013;36:274-279.
- Weaver LK, ed. Hyperbaric Oxygen Therapy Indications: The Hyperbaric Oxygen Therapy Committee Report. 13th ed. Undersea and Hyperbaric Medical Society. 2014.https://www.uhms.
org/images/indications/UHMS_HBO2_Indications
_13th_Ed._Front_Matter__References.pdf. Accessed December 18, 2019. - Undersea & Hyperbaric Medical Society. UHMS Hyperbaric Facility Accreditation Program. https://www.uhms.org/about/accreditation/accreditation-for-hyperbaric-medicine.html. Accessed December 18, 2019.
- Hyperbaric oxygen (HBO) therapy. US Centers for Medicare & Medicaid Services. https://www.medicare.gov/coverage/hyperbaric-oxygen-hbo-therapy. Accessed December 18, 2019.
- Gracia L, Perez-Vidal C, de Paco JM, et al. Identification and control of a multiplace hyperbaric chamber. PLoS One. 2018;13:e0200407.
- Monoplace vs multiplace hyperbaric chamber. CutisCare. https://cutiscareusa.com/hyperbaric-oxygen-therapy/monoplace-vs-multiplace-hyperbaric-chamber/. Published August 31, 2018. Accessed December 18, 2019.
- Leach RM, Rees PJ, Wilmshurst PP. Hyperbaric oxygen therapy. BMJ. 1998;317:1140-1143.
- Health Quality Ontario. Hyperbaric oxygen therapy for the treatment of diabetic foot ulcers: a health technology assessment. Ont Health Technol Assess Ser. 2017;17:1-142.
- Vann RD, Butler FK, Mitchell SJ, et al. Decompression illness. Lancet. 2011;377:153-164.
- Rhodes WC, Hertner G, Price R, et al. Treating decompression sickness: military flight simulation site-community hospital partnership. Mil Med. 2017;182:e1718-e1721.
- Jersey SL, Baril RT, McCarty RD, et al. Severe neurological decompression sickness in a U-2 pilot. Aviat Space Environ Med. 2010;81:64-68.
- Alam M, Ibrahim O, Nodzenski M, et al. Adverse events associated with Mohs micrographic surgery: multicenter prospective cohort study of 20,821 cases at 23 centers. JAMA Dermatol. 2013;149:1378-1385.
- Francis A, Baynosa RC. Hyperbaric oxygen therapy for the compromised graft or flap. Adv Wound Care (New Rochelle). 2017;6:23-32.
- Bowersox JC, Strauss MB, Hart GB. Clinical experience with hyperbaric oxygen therapy in the salvage of ischemic skin flaps and grafts. J Hyperb Med. 1986;1:141-149.
- Fernández Canedo I, Padilla España L, Francisco Millán Cayetano J, et al. Hyperbaric oxygen therapy: an alternative treatment for radiation-induced cutaneous ulcers. Australas J Dermatol. 2018;59:e203-e207.
- Schulte KW, Lippold A, Auras C, et al. Soft x-ray therapy for cutaneous basal cell and squamous cell carcinomas. J Am Acad Dermatol. 2005;53:993-1001.
- Hampson NB, Holm JR, Wreford-Brown CE, et al. Prospective assessment of outcomes in 411 patients treated with hyperbaric oxygen for chronic radiation tissue injury. Cancer. 2012;118:3860-3868.
- Jeter J, Wolf EG, Richards M, et al. Successful treatment of anti-MDA5 dermatomyositis associated cutaneous digital pulp ulcerations with hyperbaric oxygen therapy [published online August 21, 2019]. J Clin Rheumatol. doi:10.1097/RHU.0000000000001114.
- Efrati S, Bergan J, Fishlev G, et al. Hyperbaric oxygen therapy for nonhealing vasculitic ulcers. Clin Exp Dermatol. 2007;32:12-17.
- Mirasoglu B, Bagli BS, Aktas S. Hyperbaric oxygen therapy for chronic ulcers in systemic sclerosis—case series. Int J Dermatol. 2017;56:636-640.
- Henderson R, Reilly DA, Cooper JS. Hyperbaric oxygen for ischemia due to injection of cosmetic fillers: case report and issues. Plast Reconstr Surg Glob Open. 2018;6:e1618.
- Uittenbogaard D, Lansdorp CA, Bauland CG, et al. Hyperbaric oxygen therapy for dermal ischemia after dermal filler injection with calcium hydroxylapatite: a case report. Undersea Hyperb Med. 2019;46:207-210.
- Schorow S. The air in there. NFPA Journal. January 3, 2017. https://www.nfpa.org/News-and-Research/Publications-and-media/NFPA-Journal/2017/January-February-2017/Features/Hyperbaric-chambers. Accessed December 18, 2019.
- National Fire Protection Association. NFPA 99: Health Care Facilities Code 2018. https://www.nfpa.org/codes-and-standards/all-codes-and-standards/list-of-codes-and-standards/detail?code=99. Accessed December 18, 2019.
- Blanshard J, Toma A, Bryson P, et al. Middle ear barotrauma in patients undergoing hyperbaric oxygen therapy. Clin Otolaryngol. 1996;21:400-403.
- Lima MA, Farage L, Cury MC, et al. Update on middle ear barotrauma after hyperbaric oxygen therapy—insights on pathophysiology. Int Arch Otorhinolaryngol. 2014;18:204-209.
- Heyboer M, Sharma D, Santiago W, et al. Hyperbaric oxygen therapy: side effects defined and quantified. Adv Wound Care (New Rochelle). 2017;6:210-224.
Practice Points
- Hyperbaric oxygen therapy can be considered for the treatment of failing cutaneous grafts and flaps, chronic ulcerations caused by vasculitis or autoimmune disorders, and vascular compromise, including cutaneous ischemia caused by fillers.
- Hyperbaric oxygen therapy involves 1- to 2-hour treatments, 5 days a week, for as long as 1 month.
- Hyperbaric oxygen therapy is safe and well-tolerated, with few contraindications. The sooner therapy is started, the greater the potential for benefit.
What’s Eating You? Vespids Revisited
Identification
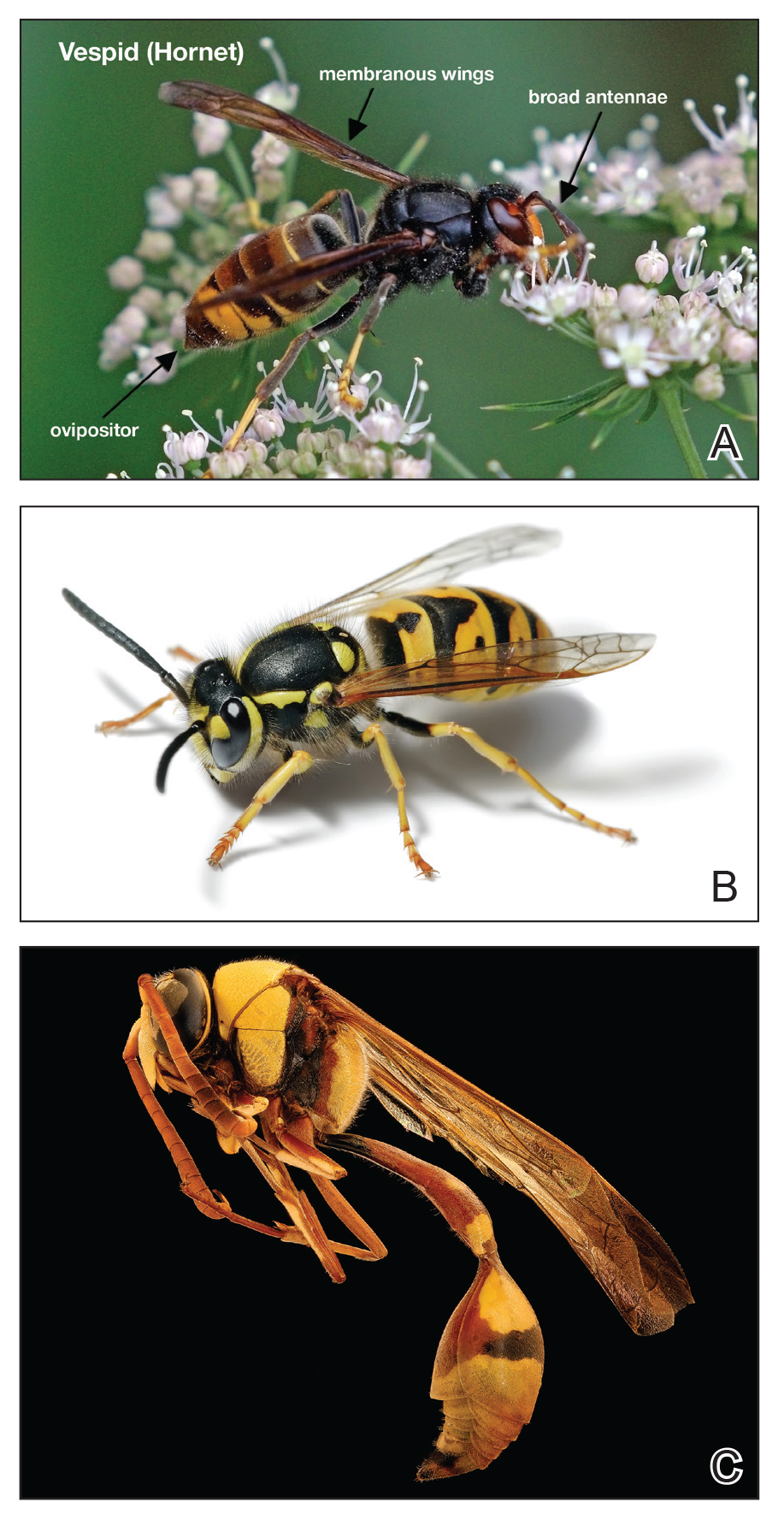
The Hymenoptera order of insects includes Apidae (bees), Vespidae (wasps, yellow jackets, hornets), and Formicidae (fire ants). All 3 of these families of insects inject venom into their prey or as a defense mechanism via ovipositors in their abdomen. Vespids are the most aggressive and are found in each of the United States.1 They have membranous wings, broad antennae, and a nonbarbed stinger (Figure 1).2 The nonbarbed stinger of Vespidae differentiates them from Apidae and allows these insects to sting their prey multiple times. Vespids can build nests in the ground (yellow jackets), trees (hornets), or areas of cover such as window shutters (mud wasps). Because only the queens survive winter, larger populations do not develop until late summer when the most stings take place. Stings most often take place near the nest of the vespid or while the victim is eating outdoors.3
Envenomation
When vespids sting their prey they inject venom via their ovipositors.1 The venom is composed of a mixture of low-molecular-weight proteins, kinins, proteolytic enzymes, lipids, carbohydrates, and high-molecular-weight proteins that act as allergens.1,4,5 The proteolytic enzymes degrade the surrounding tissue, basophils become activated, and histamine is released secondary to mast cell degranulation, which results in vasodilation and an inflammatory response characterized by edema, erythema, warmth, and pain.1 The pain of the sting is immediate and can be intense; almost all victims are acutely aware of the discomforting sensation.4
Management of Reactions
Three types of reactions can be seen after a vespid sting: uncomplicated local reactions, large local reactions, and systemic reactions (SRs). The most common reaction is the self-limiting uncomplicated local reaction that includes a focal area of warmth, edema, erythema, induration, and tenderness.1 Treatment of this kind of reaction is supportive, with ice, nonsteroidal anti-inflammatory drugs, and H1 and H2 blockers being commonly used methods. Large local reactions (Figure 2) are similar to uncomplicated local reactions but are greater than 10 cm in diameter and last longer. The same symptomatic treatment may be used along with possible short (3–5 days) oral glucocorticoid (40–60 mg prednisone) or potent topical steroid administration if symptoms persist. Systemic reactions involve IgE-mediated generalized urticaria, angioedema, face swelling, stridor, bronchospasm, nausea, vomiting, flushing, and respiratory distress.1 Emergency management includes maintenance of airway, breathing, and circulation. Epinephrine injection commonly is employed and should be given via intramuscular injection into the anterolateral thigh; a dose of 0.3 to 0.5 mg can be repeatedly injected every 5 to 15 minutes, as needed.1
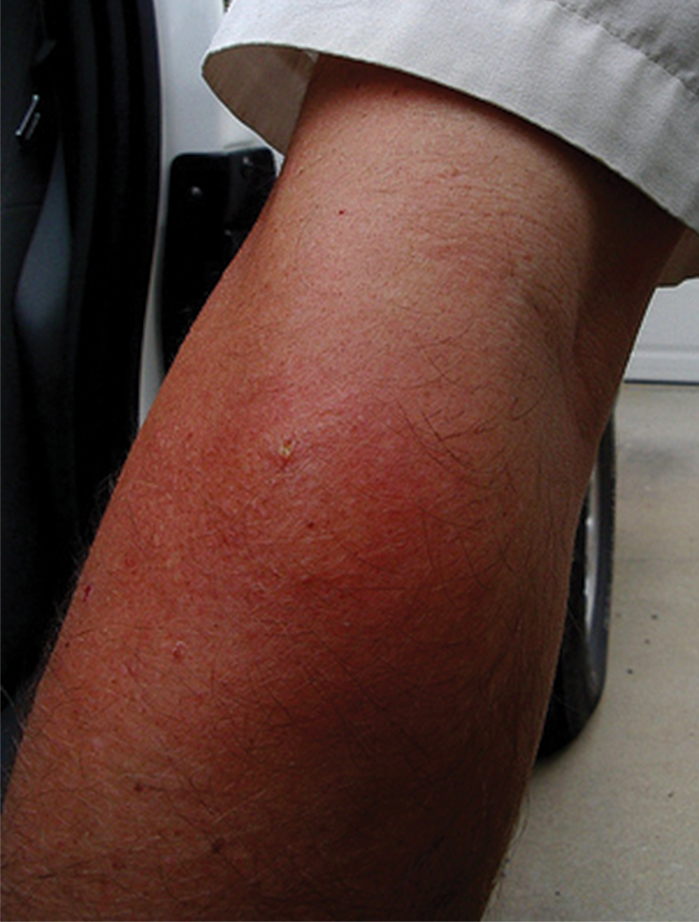
If an individual has an SR, it is recommended to go to an emergency department after stabilization for monitoring. Referral to an allergist for desensitization is appropriate. A radioallergosorbent test to measure allergen-specific IgE can be helpful to confirm an allergy.4 This test also should be done weeks after the incident because during the first few days IgE may be too low to measure. Once the allergy is confirmed, the desensitization with venom immunotherapy (VIT) can begin. Venom immunotherapy is effective and reduces a patient’s risk for recurrent SRs to less than 5% to 20%.6 A 2015 study recommended longer duration of VIT therapy due to risk for repeat SRs after discontinuing therapy. This study concluded that VIT is to be administered for 5 years, unless the patient is at high risk for SRs after VIT therapy—risk factors include older age, cardiopulmonary disease, SR during VIT treatment, mast cell disorders, and elevated serum tryptase—in which case VIT may have to be continued indefinitely. It is recommended that all patients with history of SR carry an epinephrine autoinjector in case of emergency.6
Epidemiologic data show a prevalence of 0.3% to 7.5% for self-reported SRs due to stings, with lower prevalence in children (0.15%–0.3%).4,7 An additional study looking at data from an allergy practice determined 24% of all cases of anaphylaxis were due to insect stings.5
Conclusion
Although many vespid stings can be managed symptomatically, it is imperative for patients and providers to be aware of the possible severe reactions that can take place. It is essential for providers to be aware of how to care for and treat large local reactions and SRs, as symptom recognition and timely treatment can improve patient safety and result in better outcomes.
- Arif F, Williams M. Hymenoptera Stings (Bee, Vespids and Ants). Treasure Island, FL: StatPearls Publishing LLC; 2019. https://www.ncbi.nlm.nih.gov/books/NBK518972/. Updated April 20, 2019. Accessed December 11, 2019.
- Elston, DM. Life-threatening stings, bites, infestations, and parasitic diseases. Clin Dermatol. 2005;23:164-170.
- Ulrich RM, Gabrielle H, Arthur H. Allergic reactions to stinging and biting insects. In: Rich RR, Fleisher T, Shearer W, et al, eds. Clinical Immunology: Principles and Practice. 3rd ed. St. Louis, MO: Mosby/Elsevier; 2008:657-666.
- Biló BM, Rueff F, Mosbech H, et al. Diagnosis of hymenoptera venom allergy. Allergy. 2005;60:1339-1349.
- Schafer T, Przybilla B. IgE antibodies to hymenoptera venoms in the serum are common in the general population and are related to indication of atopy. Allergy. 1996;51:372-377.
- Ulrich MR, Johannes R. When can immunotherapy for insect sting allergy be stopped? J Allergy Clin Immunol. 2015;3:324-328.
- Abrishami MH, Boyd GK, Settipane GA. Prevalence of bee sting allergy in 2010 girl scouts. Acta Allergol. 1971;26:117-120.
Identification
The Hymenoptera order of insects includes Apidae (bees), Vespidae (wasps, yellow jackets, hornets), and Formicidae (fire ants). All 3 of these families of insects inject venom into their prey or as a defense mechanism via ovipositors in their abdomen. Vespids are the most aggressive and are found in each of the United States.1 They have membranous wings, broad antennae, and a nonbarbed stinger (Figure 1).2 The nonbarbed stinger of Vespidae differentiates them from Apidae and allows these insects to sting their prey multiple times. Vespids can build nests in the ground (yellow jackets), trees (hornets), or areas of cover such as window shutters (mud wasps). Because only the queens survive winter, larger populations do not develop until late summer when the most stings take place. Stings most often take place near the nest of the vespid or while the victim is eating outdoors.3

Envenomation
When vespids sting their prey they inject venom via their ovipositors.1 The venom is composed of a mixture of low-molecular-weight proteins, kinins, proteolytic enzymes, lipids, carbohydrates, and high-molecular-weight proteins that act as allergens.1,4,5 The proteolytic enzymes degrade the surrounding tissue, basophils become activated, and histamine is released secondary to mast cell degranulation, which results in vasodilation and an inflammatory response characterized by edema, erythema, warmth, and pain.1 The pain of the sting is immediate and can be intense; almost all victims are acutely aware of the discomforting sensation.4
Management of Reactions
Three types of reactions can be seen after a vespid sting: uncomplicated local reactions, large local reactions, and systemic reactions (SRs). The most common reaction is the self-limiting uncomplicated local reaction that includes a focal area of warmth, edema, erythema, induration, and tenderness.1 Treatment of this kind of reaction is supportive, with ice, nonsteroidal anti-inflammatory drugs, and H1 and H2 blockers being commonly used methods. Large local reactions (Figure 2) are similar to uncomplicated local reactions but are greater than 10 cm in diameter and last longer. The same symptomatic treatment may be used along with possible short (3–5 days) oral glucocorticoid (40–60 mg prednisone) or potent topical steroid administration if symptoms persist. Systemic reactions involve IgE-mediated generalized urticaria, angioedema, face swelling, stridor, bronchospasm, nausea, vomiting, flushing, and respiratory distress.1 Emergency management includes maintenance of airway, breathing, and circulation. Epinephrine injection commonly is employed and should be given via intramuscular injection into the anterolateral thigh; a dose of 0.3 to 0.5 mg can be repeatedly injected every 5 to 15 minutes, as needed.1

If an individual has an SR, it is recommended to go to an emergency department after stabilization for monitoring. Referral to an allergist for desensitization is appropriate. A radioallergosorbent test to measure allergen-specific IgE can be helpful to confirm an allergy.4 This test also should be done weeks after the incident because during the first few days IgE may be too low to measure. Once the allergy is confirmed, the desensitization with venom immunotherapy (VIT) can begin. Venom immunotherapy is effective and reduces a patient’s risk for recurrent SRs to less than 5% to 20%.6 A 2015 study recommended longer duration of VIT therapy due to risk for repeat SRs after discontinuing therapy. This study concluded that VIT is to be administered for 5 years, unless the patient is at high risk for SRs after VIT therapy—risk factors include older age, cardiopulmonary disease, SR during VIT treatment, mast cell disorders, and elevated serum tryptase—in which case VIT may have to be continued indefinitely. It is recommended that all patients with history of SR carry an epinephrine autoinjector in case of emergency.6
Epidemiologic data show a prevalence of 0.3% to 7.5% for self-reported SRs due to stings, with lower prevalence in children (0.15%–0.3%).4,7 An additional study looking at data from an allergy practice determined 24% of all cases of anaphylaxis were due to insect stings.5
Conclusion
Although many vespid stings can be managed symptomatically, it is imperative for patients and providers to be aware of the possible severe reactions that can take place. It is essential for providers to be aware of how to care for and treat large local reactions and SRs, as symptom recognition and timely treatment can improve patient safety and result in better outcomes.
Identification
The Hymenoptera order of insects includes Apidae (bees), Vespidae (wasps, yellow jackets, hornets), and Formicidae (fire ants). All 3 of these families of insects inject venom into their prey or as a defense mechanism via ovipositors in their abdomen. Vespids are the most aggressive and are found in each of the United States.1 They have membranous wings, broad antennae, and a nonbarbed stinger (Figure 1).2 The nonbarbed stinger of Vespidae differentiates them from Apidae and allows these insects to sting their prey multiple times. Vespids can build nests in the ground (yellow jackets), trees (hornets), or areas of cover such as window shutters (mud wasps). Because only the queens survive winter, larger populations do not develop until late summer when the most stings take place. Stings most often take place near the nest of the vespid or while the victim is eating outdoors.3

Envenomation
When vespids sting their prey they inject venom via their ovipositors.1 The venom is composed of a mixture of low-molecular-weight proteins, kinins, proteolytic enzymes, lipids, carbohydrates, and high-molecular-weight proteins that act as allergens.1,4,5 The proteolytic enzymes degrade the surrounding tissue, basophils become activated, and histamine is released secondary to mast cell degranulation, which results in vasodilation and an inflammatory response characterized by edema, erythema, warmth, and pain.1 The pain of the sting is immediate and can be intense; almost all victims are acutely aware of the discomforting sensation.4
Management of Reactions
Three types of reactions can be seen after a vespid sting: uncomplicated local reactions, large local reactions, and systemic reactions (SRs). The most common reaction is the self-limiting uncomplicated local reaction that includes a focal area of warmth, edema, erythema, induration, and tenderness.1 Treatment of this kind of reaction is supportive, with ice, nonsteroidal anti-inflammatory drugs, and H1 and H2 blockers being commonly used methods. Large local reactions (Figure 2) are similar to uncomplicated local reactions but are greater than 10 cm in diameter and last longer. The same symptomatic treatment may be used along with possible short (3–5 days) oral glucocorticoid (40–60 mg prednisone) or potent topical steroid administration if symptoms persist. Systemic reactions involve IgE-mediated generalized urticaria, angioedema, face swelling, stridor, bronchospasm, nausea, vomiting, flushing, and respiratory distress.1 Emergency management includes maintenance of airway, breathing, and circulation. Epinephrine injection commonly is employed and should be given via intramuscular injection into the anterolateral thigh; a dose of 0.3 to 0.5 mg can be repeatedly injected every 5 to 15 minutes, as needed.1

If an individual has an SR, it is recommended to go to an emergency department after stabilization for monitoring. Referral to an allergist for desensitization is appropriate. A radioallergosorbent test to measure allergen-specific IgE can be helpful to confirm an allergy.4 This test also should be done weeks after the incident because during the first few days IgE may be too low to measure. Once the allergy is confirmed, the desensitization with venom immunotherapy (VIT) can begin. Venom immunotherapy is effective and reduces a patient’s risk for recurrent SRs to less than 5% to 20%.6 A 2015 study recommended longer duration of VIT therapy due to risk for repeat SRs after discontinuing therapy. This study concluded that VIT is to be administered for 5 years, unless the patient is at high risk for SRs after VIT therapy—risk factors include older age, cardiopulmonary disease, SR during VIT treatment, mast cell disorders, and elevated serum tryptase—in which case VIT may have to be continued indefinitely. It is recommended that all patients with history of SR carry an epinephrine autoinjector in case of emergency.6
Epidemiologic data show a prevalence of 0.3% to 7.5% for self-reported SRs due to stings, with lower prevalence in children (0.15%–0.3%).4,7 An additional study looking at data from an allergy practice determined 24% of all cases of anaphylaxis were due to insect stings.5
Conclusion
Although many vespid stings can be managed symptomatically, it is imperative for patients and providers to be aware of the possible severe reactions that can take place. It is essential for providers to be aware of how to care for and treat large local reactions and SRs, as symptom recognition and timely treatment can improve patient safety and result in better outcomes.
- Arif F, Williams M. Hymenoptera Stings (Bee, Vespids and Ants). Treasure Island, FL: StatPearls Publishing LLC; 2019. https://www.ncbi.nlm.nih.gov/books/NBK518972/. Updated April 20, 2019. Accessed December 11, 2019.
- Elston, DM. Life-threatening stings, bites, infestations, and parasitic diseases. Clin Dermatol. 2005;23:164-170.
- Ulrich RM, Gabrielle H, Arthur H. Allergic reactions to stinging and biting insects. In: Rich RR, Fleisher T, Shearer W, et al, eds. Clinical Immunology: Principles and Practice. 3rd ed. St. Louis, MO: Mosby/Elsevier; 2008:657-666.
- Biló BM, Rueff F, Mosbech H, et al. Diagnosis of hymenoptera venom allergy. Allergy. 2005;60:1339-1349.
- Schafer T, Przybilla B. IgE antibodies to hymenoptera venoms in the serum are common in the general population and are related to indication of atopy. Allergy. 1996;51:372-377.
- Ulrich MR, Johannes R. When can immunotherapy for insect sting allergy be stopped? J Allergy Clin Immunol. 2015;3:324-328.
- Abrishami MH, Boyd GK, Settipane GA. Prevalence of bee sting allergy in 2010 girl scouts. Acta Allergol. 1971;26:117-120.
- Arif F, Williams M. Hymenoptera Stings (Bee, Vespids and Ants). Treasure Island, FL: StatPearls Publishing LLC; 2019. https://www.ncbi.nlm.nih.gov/books/NBK518972/. Updated April 20, 2019. Accessed December 11, 2019.
- Elston, DM. Life-threatening stings, bites, infestations, and parasitic diseases. Clin Dermatol. 2005;23:164-170.
- Ulrich RM, Gabrielle H, Arthur H. Allergic reactions to stinging and biting insects. In: Rich RR, Fleisher T, Shearer W, et al, eds. Clinical Immunology: Principles and Practice. 3rd ed. St. Louis, MO: Mosby/Elsevier; 2008:657-666.
- Biló BM, Rueff F, Mosbech H, et al. Diagnosis of hymenoptera venom allergy. Allergy. 2005;60:1339-1349.
- Schafer T, Przybilla B. IgE antibodies to hymenoptera venoms in the serum are common in the general population and are related to indication of atopy. Allergy. 1996;51:372-377.
- Ulrich MR, Johannes R. When can immunotherapy for insect sting allergy be stopped? J Allergy Clin Immunol. 2015;3:324-328.
- Abrishami MH, Boyd GK, Settipane GA. Prevalence of bee sting allergy in 2010 girl scouts. Acta Allergol. 1971;26:117-120.
Practice Points
- Most vespid stings can be managed with nonsteroidal anti-inflammatory drugs, ice, and antihistamines.
- For systemic reactions, prompt recognition and initiation of intramuscular epinephrine is recommended.
- In patients with confirmed allergy, recent data now suggest at least 5 years of venom immunotherapy and potentially lifelong for specific patients.
Cynodon dactylon
medicine to treat cutaneous diseases, fevers, and rheumatism,as well as a variety of chronic inflammatory conditions.1,2 The Ayurvedic armamentarium is thought to be the most abundant source of botanically based drugs used to treat wounds.3 Unrelated to health concerns, with the possible exception of allergic reactions, C. dactylon – which originated in Africa, is widely dispersed in Europe, and became an invasive species in locations such as Bermuda – is also used on putting greens on golf courses in subtropical and tropical climates.4 This grass has been shown to be safe and effective for treating induced RA in rats.1,2 Recent findings are encouraging in the area of wound healing.
Chemical constituents
Among the numerous ingredients contained in C. dactylon are proteins, carbohydrates, minerals, terpenoids, vitamin C, palmitic acid, and alkaloids.3 Other key phytoconstituents known to impart beneficial health effects that are present in the plant include flavonoids (such as apigenin and luteolin), carotenoids (such as beta-carotene and neoxanthin), phenolics, phytosterols, glycosides, saponins, and volatile oils.3 Given such components, it should not be surprising that C. dactylon has demonstrated antioxidant activity by scavenging the 2,2-diphenyl-1-picrylhydrazyl radical.3
Wound healing
Given the reputation of C. dactylon as an effective compound used in traditional medicine for wound healing as a hemostatic agent, Biswas et al. set out in 2017 to determine if they could provide scientific validation of the botanical as a viable wound-healing option. The investigators first undertook to compare a 15% ointment of the extract with a placebo control and the standard framycetin on full-thickness punch wounds in Wistar rats. Across all parameters, results for the C. dactylon–treated group far exceeded the control group and were comparable with the framycetin group. Subsequently, in a pilot clinical study, the researchers assessed the botanical ointment in a small cohort (n = 12) of men and women aged 65-75 years (n = 12) with chronic and complicated wounds. Half were treated with a topical C. dactylon ointment and half were treated with a topical framycetin sulfate ointment. Comparable effects were seen across the groups, with significant contraction of wounds and wound area noted, along with significant development of granulation and epithelial tissues. Hematologic parameters indicating improvement were comparable between the groups. The investigators concluded that all patients treated with C. dactylon healed successfully. They added that the antioxidant activity of the constituent phenolic acids and flavonoids in C. dactylon likely play a key role in conferring potent wound-healing effects by promoting collagenesis.3
In 2018, Perumal et al. created a collagen-silica biocomposite enriched with C. dactylon extract and studied its wound-healing potential in vitro and in vivo in comparison with collagen as well as collagen-silica scaffold controls. The investigators found that the stability of the enriched product surpassed that of native collagen by virtue of the intermolecular interactions between the botanical ingredient and collagen. In a full-thickness excision wound model using Wistar rats, the biocomposite was associated with more rapid healing than wounds treated with collagen and the scaffold control.5
Arthritis
In 2009, Sindhu et al. orally administered C. dactylon to rats after intradermally inducing arthritis. The induction produced inflammation, and a marked rise in the levels of inflammatory mediators, C-reactive protein, myeloperoxidase, and nitrite. Resultant oxidative stress was noted with substantial declines in the activity of catalase, superoxide dismutase, and glutathione peroxidase, as well as levels of glutathione, vitamins C and E, and an increase in lipid peroxidation. Administration of C. dactylon yielded substantial changes, with mitigation of the inflammatory response and oxidative stress as well as diminution of the arthritic response nearly to the baseline condition. The investigators concluded that the botanical agent clearly demonstrates potential to protect against arthritis.2
A subsequent study in rats by Bhangale and Acharya supported the use of C. dactylon for RA, as its oral administration was found safe at all dose levels (100, 200 and 400 mg/kg), with 400 mg/kg as the most effective at ameliorating hemoglobin and red blood cell levels and C-reactive protein, as well as lowering tumor necrosis factor–alpha. The authors also noted that the ethanolic extract of C. dactylon contained alkaloids, flavonoids, and glycosides, all of which are known to confer health benefits.1
Allergy
In 2016, López-Matas et al. studied the profiles of sensitization to C. dactylon (as well as Phragmites communis) in subjects sensitized to grasses and evaluated cross-reactivity between these grasses as well as temperate ones. Patients received skin prick tests with a grass mixture, and 24 patients (80%) were found to have had positive results for C. dactylon (and 90% to P. communis). The researchers concluded that sensitization to these species appears to be engendered by allergens other than those present in sweet grasses.6
Mehta et al. reported in 2018 on their investigation of common allergens in Ambala, India, using intradermal tests in patients with asthma, allergic rhinitis, and eczema. The study included 100 patients over an 8-year period, with 197 allergens (50 types of pollen, 19 fungi, 17 insects, 14 types of dust, 6 kinds of animal dander, 7 varieties of fabric and feathers, 82 foods, dust mites, and parthenium) tested. Pollens (51%) were the major allergens, followed by foods (28.9%), insects (26.9%), fungi (12.6%), and dusts (6.7%). C. dactylon (5%) was among two other species ranking fourth among pollen allergens.7
Also that year, Sánchez et al. investigated whether growing conditions (rural vs. urban) might influence the nasal inflammatory response to C. dactylon among patients with allergic rhinitis. They observed that the urban extract provoked larger wheals, and more patients with rhinitis experienced a positive nasal challenge test than those administered the rural extract. The skin and nasal tests did not elicit reactions in healthy controls. The researchers reached the conclusion that growth of C. dactylon in an urban setting can produce alterations in the protein extract, with potential clinical ramifications for patients who experience allergic rhinitis.8
Conclusion
Regular readers of this column know of my interest in botanically sourced topical products. Such ingredients with an extensive history of traditional medical use are particularly compelling. Many of these compounds are found in the modern medical and dermatologic armamentaria. C. dactylon does boast a track record of use in Ayurvedic medicine. However, there is a paucity of modern research at the present time. While there are concerns about its allergenicity, some encouraging results have been seen in relation to RA and wound healing. Much more research is needed, though, before this botanical agent can be included feasibly for standard skin care.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote two textbooks, “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002) and “Cosmeceuticals and Cosmetic Ingredients” (New York: McGraw-Hill, 2014), as well as a New York Times Best Sellers book for consumers, “The Skin Type Solution” (New York: Bantam Dell, 2006). Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Evolus, Galderma, and Revance. She is the founder and CEO of Skin Type Solutions Franchise Systems. Write to her at [email protected].
References
1. Bhangale J, Acharya S. Pers. Indian J Exp Biol. 2014 Mar;52(3):215-22.
2. Sindhu G et al. Immunopharmacol Immunotoxicol. 2009;31(4):647-53.
3. Biswas TK et al. J Ethnopharmacol. 2017 Feb 2;197:128-37.
4. Reasor EH et al. Planta. 2016 Oct;244(4):761-73.
5. Perumal RK et al. Mater Sci Eng C Mater Biol Appl. 2018 Nov 1;92:297-306.
6. López-Matas MA et al. J Investig Allergol Clin Immunol. 2016;26(5):295-303.
7. Mehta D et al. Indian J Dermatol. 2018 Jul-Aug;63(4):311-6.
8. Sánchez J et al. Allergy Rhinol (Providence). 2018 Dec 17;9:2152656718815870.
medicine to treat cutaneous diseases, fevers, and rheumatism,as well as a variety of chronic inflammatory conditions.1,2 The Ayurvedic armamentarium is thought to be the most abundant source of botanically based drugs used to treat wounds.3 Unrelated to health concerns, with the possible exception of allergic reactions, C. dactylon – which originated in Africa, is widely dispersed in Europe, and became an invasive species in locations such as Bermuda – is also used on putting greens on golf courses in subtropical and tropical climates.4 This grass has been shown to be safe and effective for treating induced RA in rats.1,2 Recent findings are encouraging in the area of wound healing.
Chemical constituents
Among the numerous ingredients contained in C. dactylon are proteins, carbohydrates, minerals, terpenoids, vitamin C, palmitic acid, and alkaloids.3 Other key phytoconstituents known to impart beneficial health effects that are present in the plant include flavonoids (such as apigenin and luteolin), carotenoids (such as beta-carotene and neoxanthin), phenolics, phytosterols, glycosides, saponins, and volatile oils.3 Given such components, it should not be surprising that C. dactylon has demonstrated antioxidant activity by scavenging the 2,2-diphenyl-1-picrylhydrazyl radical.3
Wound healing
Given the reputation of C. dactylon as an effective compound used in traditional medicine for wound healing as a hemostatic agent, Biswas et al. set out in 2017 to determine if they could provide scientific validation of the botanical as a viable wound-healing option. The investigators first undertook to compare a 15% ointment of the extract with a placebo control and the standard framycetin on full-thickness punch wounds in Wistar rats. Across all parameters, results for the C. dactylon–treated group far exceeded the control group and were comparable with the framycetin group. Subsequently, in a pilot clinical study, the researchers assessed the botanical ointment in a small cohort (n = 12) of men and women aged 65-75 years (n = 12) with chronic and complicated wounds. Half were treated with a topical C. dactylon ointment and half were treated with a topical framycetin sulfate ointment. Comparable effects were seen across the groups, with significant contraction of wounds and wound area noted, along with significant development of granulation and epithelial tissues. Hematologic parameters indicating improvement were comparable between the groups. The investigators concluded that all patients treated with C. dactylon healed successfully. They added that the antioxidant activity of the constituent phenolic acids and flavonoids in C. dactylon likely play a key role in conferring potent wound-healing effects by promoting collagenesis.3
In 2018, Perumal et al. created a collagen-silica biocomposite enriched with C. dactylon extract and studied its wound-healing potential in vitro and in vivo in comparison with collagen as well as collagen-silica scaffold controls. The investigators found that the stability of the enriched product surpassed that of native collagen by virtue of the intermolecular interactions between the botanical ingredient and collagen. In a full-thickness excision wound model using Wistar rats, the biocomposite was associated with more rapid healing than wounds treated with collagen and the scaffold control.5
Arthritis
In 2009, Sindhu et al. orally administered C. dactylon to rats after intradermally inducing arthritis. The induction produced inflammation, and a marked rise in the levels of inflammatory mediators, C-reactive protein, myeloperoxidase, and nitrite. Resultant oxidative stress was noted with substantial declines in the activity of catalase, superoxide dismutase, and glutathione peroxidase, as well as levels of glutathione, vitamins C and E, and an increase in lipid peroxidation. Administration of C. dactylon yielded substantial changes, with mitigation of the inflammatory response and oxidative stress as well as diminution of the arthritic response nearly to the baseline condition. The investigators concluded that the botanical agent clearly demonstrates potential to protect against arthritis.2
A subsequent study in rats by Bhangale and Acharya supported the use of C. dactylon for RA, as its oral administration was found safe at all dose levels (100, 200 and 400 mg/kg), with 400 mg/kg as the most effective at ameliorating hemoglobin and red blood cell levels and C-reactive protein, as well as lowering tumor necrosis factor–alpha. The authors also noted that the ethanolic extract of C. dactylon contained alkaloids, flavonoids, and glycosides, all of which are known to confer health benefits.1
Allergy
In 2016, López-Matas et al. studied the profiles of sensitization to C. dactylon (as well as Phragmites communis) in subjects sensitized to grasses and evaluated cross-reactivity between these grasses as well as temperate ones. Patients received skin prick tests with a grass mixture, and 24 patients (80%) were found to have had positive results for C. dactylon (and 90% to P. communis). The researchers concluded that sensitization to these species appears to be engendered by allergens other than those present in sweet grasses.6
Mehta et al. reported in 2018 on their investigation of common allergens in Ambala, India, using intradermal tests in patients with asthma, allergic rhinitis, and eczema. The study included 100 patients over an 8-year period, with 197 allergens (50 types of pollen, 19 fungi, 17 insects, 14 types of dust, 6 kinds of animal dander, 7 varieties of fabric and feathers, 82 foods, dust mites, and parthenium) tested. Pollens (51%) were the major allergens, followed by foods (28.9%), insects (26.9%), fungi (12.6%), and dusts (6.7%). C. dactylon (5%) was among two other species ranking fourth among pollen allergens.7
Also that year, Sánchez et al. investigated whether growing conditions (rural vs. urban) might influence the nasal inflammatory response to C. dactylon among patients with allergic rhinitis. They observed that the urban extract provoked larger wheals, and more patients with rhinitis experienced a positive nasal challenge test than those administered the rural extract. The skin and nasal tests did not elicit reactions in healthy controls. The researchers reached the conclusion that growth of C. dactylon in an urban setting can produce alterations in the protein extract, with potential clinical ramifications for patients who experience allergic rhinitis.8
Conclusion
Regular readers of this column know of my interest in botanically sourced topical products. Such ingredients with an extensive history of traditional medical use are particularly compelling. Many of these compounds are found in the modern medical and dermatologic armamentaria. C. dactylon does boast a track record of use in Ayurvedic medicine. However, there is a paucity of modern research at the present time. While there are concerns about its allergenicity, some encouraging results have been seen in relation to RA and wound healing. Much more research is needed, though, before this botanical agent can be included feasibly for standard skin care.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote two textbooks, “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002) and “Cosmeceuticals and Cosmetic Ingredients” (New York: McGraw-Hill, 2014), as well as a New York Times Best Sellers book for consumers, “The Skin Type Solution” (New York: Bantam Dell, 2006). Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Evolus, Galderma, and Revance. She is the founder and CEO of Skin Type Solutions Franchise Systems. Write to her at [email protected].
References
1. Bhangale J, Acharya S. Pers. Indian J Exp Biol. 2014 Mar;52(3):215-22.
2. Sindhu G et al. Immunopharmacol Immunotoxicol. 2009;31(4):647-53.
3. Biswas TK et al. J Ethnopharmacol. 2017 Feb 2;197:128-37.
4. Reasor EH et al. Planta. 2016 Oct;244(4):761-73.
5. Perumal RK et al. Mater Sci Eng C Mater Biol Appl. 2018 Nov 1;92:297-306.
6. López-Matas MA et al. J Investig Allergol Clin Immunol. 2016;26(5):295-303.
7. Mehta D et al. Indian J Dermatol. 2018 Jul-Aug;63(4):311-6.
8. Sánchez J et al. Allergy Rhinol (Providence). 2018 Dec 17;9:2152656718815870.
medicine to treat cutaneous diseases, fevers, and rheumatism,as well as a variety of chronic inflammatory conditions.1,2 The Ayurvedic armamentarium is thought to be the most abundant source of botanically based drugs used to treat wounds.3 Unrelated to health concerns, with the possible exception of allergic reactions, C. dactylon – which originated in Africa, is widely dispersed in Europe, and became an invasive species in locations such as Bermuda – is also used on putting greens on golf courses in subtropical and tropical climates.4 This grass has been shown to be safe and effective for treating induced RA in rats.1,2 Recent findings are encouraging in the area of wound healing.
Chemical constituents
Among the numerous ingredients contained in C. dactylon are proteins, carbohydrates, minerals, terpenoids, vitamin C, palmitic acid, and alkaloids.3 Other key phytoconstituents known to impart beneficial health effects that are present in the plant include flavonoids (such as apigenin and luteolin), carotenoids (such as beta-carotene and neoxanthin), phenolics, phytosterols, glycosides, saponins, and volatile oils.3 Given such components, it should not be surprising that C. dactylon has demonstrated antioxidant activity by scavenging the 2,2-diphenyl-1-picrylhydrazyl radical.3
Wound healing
Given the reputation of C. dactylon as an effective compound used in traditional medicine for wound healing as a hemostatic agent, Biswas et al. set out in 2017 to determine if they could provide scientific validation of the botanical as a viable wound-healing option. The investigators first undertook to compare a 15% ointment of the extract with a placebo control and the standard framycetin on full-thickness punch wounds in Wistar rats. Across all parameters, results for the C. dactylon–treated group far exceeded the control group and were comparable with the framycetin group. Subsequently, in a pilot clinical study, the researchers assessed the botanical ointment in a small cohort (n = 12) of men and women aged 65-75 years (n = 12) with chronic and complicated wounds. Half were treated with a topical C. dactylon ointment and half were treated with a topical framycetin sulfate ointment. Comparable effects were seen across the groups, with significant contraction of wounds and wound area noted, along with significant development of granulation and epithelial tissues. Hematologic parameters indicating improvement were comparable between the groups. The investigators concluded that all patients treated with C. dactylon healed successfully. They added that the antioxidant activity of the constituent phenolic acids and flavonoids in C. dactylon likely play a key role in conferring potent wound-healing effects by promoting collagenesis.3
In 2018, Perumal et al. created a collagen-silica biocomposite enriched with C. dactylon extract and studied its wound-healing potential in vitro and in vivo in comparison with collagen as well as collagen-silica scaffold controls. The investigators found that the stability of the enriched product surpassed that of native collagen by virtue of the intermolecular interactions between the botanical ingredient and collagen. In a full-thickness excision wound model using Wistar rats, the biocomposite was associated with more rapid healing than wounds treated with collagen and the scaffold control.5
Arthritis
In 2009, Sindhu et al. orally administered C. dactylon to rats after intradermally inducing arthritis. The induction produced inflammation, and a marked rise in the levels of inflammatory mediators, C-reactive protein, myeloperoxidase, and nitrite. Resultant oxidative stress was noted with substantial declines in the activity of catalase, superoxide dismutase, and glutathione peroxidase, as well as levels of glutathione, vitamins C and E, and an increase in lipid peroxidation. Administration of C. dactylon yielded substantial changes, with mitigation of the inflammatory response and oxidative stress as well as diminution of the arthritic response nearly to the baseline condition. The investigators concluded that the botanical agent clearly demonstrates potential to protect against arthritis.2
A subsequent study in rats by Bhangale and Acharya supported the use of C. dactylon for RA, as its oral administration was found safe at all dose levels (100, 200 and 400 mg/kg), with 400 mg/kg as the most effective at ameliorating hemoglobin and red blood cell levels and C-reactive protein, as well as lowering tumor necrosis factor–alpha. The authors also noted that the ethanolic extract of C. dactylon contained alkaloids, flavonoids, and glycosides, all of which are known to confer health benefits.1
Allergy
In 2016, López-Matas et al. studied the profiles of sensitization to C. dactylon (as well as Phragmites communis) in subjects sensitized to grasses and evaluated cross-reactivity between these grasses as well as temperate ones. Patients received skin prick tests with a grass mixture, and 24 patients (80%) were found to have had positive results for C. dactylon (and 90% to P. communis). The researchers concluded that sensitization to these species appears to be engendered by allergens other than those present in sweet grasses.6
Mehta et al. reported in 2018 on their investigation of common allergens in Ambala, India, using intradermal tests in patients with asthma, allergic rhinitis, and eczema. The study included 100 patients over an 8-year period, with 197 allergens (50 types of pollen, 19 fungi, 17 insects, 14 types of dust, 6 kinds of animal dander, 7 varieties of fabric and feathers, 82 foods, dust mites, and parthenium) tested. Pollens (51%) were the major allergens, followed by foods (28.9%), insects (26.9%), fungi (12.6%), and dusts (6.7%). C. dactylon (5%) was among two other species ranking fourth among pollen allergens.7
Also that year, Sánchez et al. investigated whether growing conditions (rural vs. urban) might influence the nasal inflammatory response to C. dactylon among patients with allergic rhinitis. They observed that the urban extract provoked larger wheals, and more patients with rhinitis experienced a positive nasal challenge test than those administered the rural extract. The skin and nasal tests did not elicit reactions in healthy controls. The researchers reached the conclusion that growth of C. dactylon in an urban setting can produce alterations in the protein extract, with potential clinical ramifications for patients who experience allergic rhinitis.8
Conclusion
Regular readers of this column know of my interest in botanically sourced topical products. Such ingredients with an extensive history of traditional medical use are particularly compelling. Many of these compounds are found in the modern medical and dermatologic armamentaria. C. dactylon does boast a track record of use in Ayurvedic medicine. However, there is a paucity of modern research at the present time. While there are concerns about its allergenicity, some encouraging results have been seen in relation to RA and wound healing. Much more research is needed, though, before this botanical agent can be included feasibly for standard skin care.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote two textbooks, “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002) and “Cosmeceuticals and Cosmetic Ingredients” (New York: McGraw-Hill, 2014), as well as a New York Times Best Sellers book for consumers, “The Skin Type Solution” (New York: Bantam Dell, 2006). Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Evolus, Galderma, and Revance. She is the founder and CEO of Skin Type Solutions Franchise Systems. Write to her at [email protected].
References
1. Bhangale J, Acharya S. Pers. Indian J Exp Biol. 2014 Mar;52(3):215-22.
2. Sindhu G et al. Immunopharmacol Immunotoxicol. 2009;31(4):647-53.
3. Biswas TK et al. J Ethnopharmacol. 2017 Feb 2;197:128-37.
4. Reasor EH et al. Planta. 2016 Oct;244(4):761-73.
5. Perumal RK et al. Mater Sci Eng C Mater Biol Appl. 2018 Nov 1;92:297-306.
6. López-Matas MA et al. J Investig Allergol Clin Immunol. 2016;26(5):295-303.
7. Mehta D et al. Indian J Dermatol. 2018 Jul-Aug;63(4):311-6.
8. Sánchez J et al. Allergy Rhinol (Providence). 2018 Dec 17;9:2152656718815870.
Hydrogen Peroxide as a Hemostatic Agent During Dermatologic Surgery
The number of skin cancer surgeries continues to rise, especially in the older population, many of whom are on blood thinners. The sequela of bleeding, even in minor cases, is one of the most frequently encountered complications of cutaneous surgery. Surgical site bleeding can increase the risk for infection, skin graft failure, wound dehiscence, and hematoma formation, which may lead to disrupted wound healing and eventual poor scar outcome. Although achieving hemostasis is important, it is recommended to limit certain alternative modalities such as electrosurgery due to the accompanied thermal tissue damage that in turn can prolong healing time, worsen scarring, and increase the risk for infection.1
Practice Gap
Hydrogen peroxide (H2O2) is a common topical antiseptic used to clean wounds by killing pathogens through oxidation burst and local oxygen production.2 It is generally affordable, nonallergenic, and easy to obtain. We describe our positive experience using H2O2 as a hemostatic agent during dermatologic surgery, highlighting the agent’s underutilization as well as the recent literature negating traditional viewpoints that it probably causes tissue necrosis and impaired wound healing through its high oxidative property.
The Technique
Before surgery, the site is prepared with chlorhexidine gluconate. A stack of 4×4-in gauze on the surgical tray is saturated with 3% H2O2 and used by the surgeon and surgical assistant throughout the procedure. We currently use this technique during standard excisions, Mohs micrographic surgery stages, repairs, and dermabrasion. Additionally, as a first measure of hemostasis, we recommend H2O2 soaks immediately postoperatively in patients with active bleeding.
We have been utilizing this technique since H2O2 was described as an intraprocedural hemostatic agent during manual dermabrasion.3 Hydrogen peroxide is known to facilitate hemostasis with several accepted mechanisms that include regulating the contractility and barrier function of endothelial cells, activating latent cell surface tissue factor and platelet aggregation, and stimulating platelet-derived growth factor activation.4 It has been reported that increasing H2O2 levels leads to a dose-response increase in aggregation in the presence of subaggregating amounts of collagen.5 This concept was described in an article that utilizes H2O2 as a way to obtain hemostasis before skin grafting burn patients.6 A PubMed search of articles indexed for MEDLINE using the terms h202, hydrogen peroxide, hemostasis, wound healing, surgery, and wound produced several surgical specialties—neurosurgery, orthopedics, gastroenterology, and maxillofacial surgery—that also utilize H2O2 as a hemostatic agent.7,8 One article described a dual-enzyme H2O2 generation machinery in hydrogels as a novel antimicrobial wound treatment.9
Practice Implications
The use of H2O2 as a topical hemostatic agent during surgery was described in 1984.2 The use of H2O2 is not suggested as a substitute for other strong and well-known hemostatic agents, such as aluminum chloride and ferric subsulfate, but rather as a technique that can be used in conjunction with standard methods of hemostasis and antisepsis. For surgical sites that are intended to be closed, we do not suggest these hemostatic agents, as they are known to be caustic, irritating, and pigmenting. In addition to H2O2’s known hemostatic and antiseptic properties, more recent literature invalidates wound impairment concerns and describes its possible role in signaling effector cells to respond downstream, contributing to tissue formation and remodeling.4 The use of H2O2 in wound and incision care has been controversial and avoided due to described skin irritation and possible premature removal of suture10; however, positive biochemical effects of H2O2 on acute wounds have been reported and dispel arguments that this agent causes tissue damage.4 Contrary to the traditional viewpoint that H2O2 probably impairs tissue through its high oxidative property, a proper level of H2O2 is considered an important requirement for normal wound healing. The report published in 1985 that raised concerns of H2O2 causing impaired wound healing through its effect on fibroblasts has been challenged given that the killed cultured fibroblasts were in an in vitro model and not likely representative of the complexities of a healing wound.10 In our experience, the use of H2O2 has not demonstrated any impairments or delays in wound healing, and we postulate that the exposure to H2O2 as described in our technique is not sufficient to cause notable impairment in fibroblast function in vivo. In addition, the role of H2O2 promoting oxidative stress as well as resolving inflammation may suggest it serves as a bidirectional regulator.
Future Directions
Additional studies are needed to assess this precise balance of H2O2 forming a favorable microenvironment in wounds. Similarly, although we discuss minimal and brief use of H2O2 during a procedure, the lack of data on the role of H2O2 as a prophylactic anti-infective agent for postoperative wound care also may be an area of future exploration.
- Henley J, Brewer JD. Newer hemostatic agents used in the practice of dermatologic surgery. Dermatol Res Pract. 2013;2013:279289.
- Hankin FM, Campbell SE, Goldstein SA, et al. Hydrogen peroxide as a topical hemostatic agent. Clin Orthop Relat Res. 1984;186:244-247.
- Weiss J, Winkleman FJ, Titone A, et al. Evaluation of hydrogen peroxide as an intraprocedural hemostatic agent in manual dermabrasion. Dermatol Surg. 2010;36:1601-1603.
- Zhu G, Wang Q, Lu S, et al. Hydrogen peroxide: a potential wound therapeutic target? Med Princ Pract. 2017;26:301-308.
- Practicò D, Iuliano L, Ghiselli A, et al. Hydrogen peroxide as trigger of platelet aggregation. Haemostasis. 1991;21:169-174.
- Potyondy L, Lottenberg L, Anderson J, et al. The use of hydrogen peroxide for achieving dermal hemostasis after burn excision in a patient with platelet dysfunction. J Burn Care Res. 2006;27:99-101.
- Mawk JR. Hydrogen peroxide for hemostasis. Neurosurgery. 1986;18:827.
- Arakeri G, Brennan PA. Povidone-iodine and hydrogen peroxide mixture soaked gauze pack: a novel hemostatic technique. J Oral Maxillofac Surg. 2013;71:1833.e1-1833.e3.
- Huber D, Tegl G, Mensah A, et al. A dual-enzyme hydrogen peroxide generation machinery in hydrogels supports antimicrobial wound treatment. ACS Appl Mater Interfaces. 2017;9:15307-15316.
- Lineaweaver W, McMorris S, Soucy D, et al. Cellular and bacterial toxicities of topical antimicrobials. Plast Reconstr Surg. 1985;75:394-396.
The number of skin cancer surgeries continues to rise, especially in the older population, many of whom are on blood thinners. The sequela of bleeding, even in minor cases, is one of the most frequently encountered complications of cutaneous surgery. Surgical site bleeding can increase the risk for infection, skin graft failure, wound dehiscence, and hematoma formation, which may lead to disrupted wound healing and eventual poor scar outcome. Although achieving hemostasis is important, it is recommended to limit certain alternative modalities such as electrosurgery due to the accompanied thermal tissue damage that in turn can prolong healing time, worsen scarring, and increase the risk for infection.1
Practice Gap
Hydrogen peroxide (H2O2) is a common topical antiseptic used to clean wounds by killing pathogens through oxidation burst and local oxygen production.2 It is generally affordable, nonallergenic, and easy to obtain. We describe our positive experience using H2O2 as a hemostatic agent during dermatologic surgery, highlighting the agent’s underutilization as well as the recent literature negating traditional viewpoints that it probably causes tissue necrosis and impaired wound healing through its high oxidative property.
The Technique
Before surgery, the site is prepared with chlorhexidine gluconate. A stack of 4×4-in gauze on the surgical tray is saturated with 3% H2O2 and used by the surgeon and surgical assistant throughout the procedure. We currently use this technique during standard excisions, Mohs micrographic surgery stages, repairs, and dermabrasion. Additionally, as a first measure of hemostasis, we recommend H2O2 soaks immediately postoperatively in patients with active bleeding.
We have been utilizing this technique since H2O2 was described as an intraprocedural hemostatic agent during manual dermabrasion.3 Hydrogen peroxide is known to facilitate hemostasis with several accepted mechanisms that include regulating the contractility and barrier function of endothelial cells, activating latent cell surface tissue factor and platelet aggregation, and stimulating platelet-derived growth factor activation.4 It has been reported that increasing H2O2 levels leads to a dose-response increase in aggregation in the presence of subaggregating amounts of collagen.5 This concept was described in an article that utilizes H2O2 as a way to obtain hemostasis before skin grafting burn patients.6 A PubMed search of articles indexed for MEDLINE using the terms h202, hydrogen peroxide, hemostasis, wound healing, surgery, and wound produced several surgical specialties—neurosurgery, orthopedics, gastroenterology, and maxillofacial surgery—that also utilize H2O2 as a hemostatic agent.7,8 One article described a dual-enzyme H2O2 generation machinery in hydrogels as a novel antimicrobial wound treatment.9
Practice Implications
The use of H2O2 as a topical hemostatic agent during surgery was described in 1984.2 The use of H2O2 is not suggested as a substitute for other strong and well-known hemostatic agents, such as aluminum chloride and ferric subsulfate, but rather as a technique that can be used in conjunction with standard methods of hemostasis and antisepsis. For surgical sites that are intended to be closed, we do not suggest these hemostatic agents, as they are known to be caustic, irritating, and pigmenting. In addition to H2O2’s known hemostatic and antiseptic properties, more recent literature invalidates wound impairment concerns and describes its possible role in signaling effector cells to respond downstream, contributing to tissue formation and remodeling.4 The use of H2O2 in wound and incision care has been controversial and avoided due to described skin irritation and possible premature removal of suture10; however, positive biochemical effects of H2O2 on acute wounds have been reported and dispel arguments that this agent causes tissue damage.4 Contrary to the traditional viewpoint that H2O2 probably impairs tissue through its high oxidative property, a proper level of H2O2 is considered an important requirement for normal wound healing. The report published in 1985 that raised concerns of H2O2 causing impaired wound healing through its effect on fibroblasts has been challenged given that the killed cultured fibroblasts were in an in vitro model and not likely representative of the complexities of a healing wound.10 In our experience, the use of H2O2 has not demonstrated any impairments or delays in wound healing, and we postulate that the exposure to H2O2 as described in our technique is not sufficient to cause notable impairment in fibroblast function in vivo. In addition, the role of H2O2 promoting oxidative stress as well as resolving inflammation may suggest it serves as a bidirectional regulator.
Future Directions
Additional studies are needed to assess this precise balance of H2O2 forming a favorable microenvironment in wounds. Similarly, although we discuss minimal and brief use of H2O2 during a procedure, the lack of data on the role of H2O2 as a prophylactic anti-infective agent for postoperative wound care also may be an area of future exploration.
The number of skin cancer surgeries continues to rise, especially in the older population, many of whom are on blood thinners. The sequela of bleeding, even in minor cases, is one of the most frequently encountered complications of cutaneous surgery. Surgical site bleeding can increase the risk for infection, skin graft failure, wound dehiscence, and hematoma formation, which may lead to disrupted wound healing and eventual poor scar outcome. Although achieving hemostasis is important, it is recommended to limit certain alternative modalities such as electrosurgery due to the accompanied thermal tissue damage that in turn can prolong healing time, worsen scarring, and increase the risk for infection.1
Practice Gap
Hydrogen peroxide (H2O2) is a common topical antiseptic used to clean wounds by killing pathogens through oxidation burst and local oxygen production.2 It is generally affordable, nonallergenic, and easy to obtain. We describe our positive experience using H2O2 as a hemostatic agent during dermatologic surgery, highlighting the agent’s underutilization as well as the recent literature negating traditional viewpoints that it probably causes tissue necrosis and impaired wound healing through its high oxidative property.
The Technique
Before surgery, the site is prepared with chlorhexidine gluconate. A stack of 4×4-in gauze on the surgical tray is saturated with 3% H2O2 and used by the surgeon and surgical assistant throughout the procedure. We currently use this technique during standard excisions, Mohs micrographic surgery stages, repairs, and dermabrasion. Additionally, as a first measure of hemostasis, we recommend H2O2 soaks immediately postoperatively in patients with active bleeding.
We have been utilizing this technique since H2O2 was described as an intraprocedural hemostatic agent during manual dermabrasion.3 Hydrogen peroxide is known to facilitate hemostasis with several accepted mechanisms that include regulating the contractility and barrier function of endothelial cells, activating latent cell surface tissue factor and platelet aggregation, and stimulating platelet-derived growth factor activation.4 It has been reported that increasing H2O2 levels leads to a dose-response increase in aggregation in the presence of subaggregating amounts of collagen.5 This concept was described in an article that utilizes H2O2 as a way to obtain hemostasis before skin grafting burn patients.6 A PubMed search of articles indexed for MEDLINE using the terms h202, hydrogen peroxide, hemostasis, wound healing, surgery, and wound produced several surgical specialties—neurosurgery, orthopedics, gastroenterology, and maxillofacial surgery—that also utilize H2O2 as a hemostatic agent.7,8 One article described a dual-enzyme H2O2 generation machinery in hydrogels as a novel antimicrobial wound treatment.9
Practice Implications
The use of H2O2 as a topical hemostatic agent during surgery was described in 1984.2 The use of H2O2 is not suggested as a substitute for other strong and well-known hemostatic agents, such as aluminum chloride and ferric subsulfate, but rather as a technique that can be used in conjunction with standard methods of hemostasis and antisepsis. For surgical sites that are intended to be closed, we do not suggest these hemostatic agents, as they are known to be caustic, irritating, and pigmenting. In addition to H2O2’s known hemostatic and antiseptic properties, more recent literature invalidates wound impairment concerns and describes its possible role in signaling effector cells to respond downstream, contributing to tissue formation and remodeling.4 The use of H2O2 in wound and incision care has been controversial and avoided due to described skin irritation and possible premature removal of suture10; however, positive biochemical effects of H2O2 on acute wounds have been reported and dispel arguments that this agent causes tissue damage.4 Contrary to the traditional viewpoint that H2O2 probably impairs tissue through its high oxidative property, a proper level of H2O2 is considered an important requirement for normal wound healing. The report published in 1985 that raised concerns of H2O2 causing impaired wound healing through its effect on fibroblasts has been challenged given that the killed cultured fibroblasts were in an in vitro model and not likely representative of the complexities of a healing wound.10 In our experience, the use of H2O2 has not demonstrated any impairments or delays in wound healing, and we postulate that the exposure to H2O2 as described in our technique is not sufficient to cause notable impairment in fibroblast function in vivo. In addition, the role of H2O2 promoting oxidative stress as well as resolving inflammation may suggest it serves as a bidirectional regulator.
Future Directions
Additional studies are needed to assess this precise balance of H2O2 forming a favorable microenvironment in wounds. Similarly, although we discuss minimal and brief use of H2O2 during a procedure, the lack of data on the role of H2O2 as a prophylactic anti-infective agent for postoperative wound care also may be an area of future exploration.
- Henley J, Brewer JD. Newer hemostatic agents used in the practice of dermatologic surgery. Dermatol Res Pract. 2013;2013:279289.
- Hankin FM, Campbell SE, Goldstein SA, et al. Hydrogen peroxide as a topical hemostatic agent. Clin Orthop Relat Res. 1984;186:244-247.
- Weiss J, Winkleman FJ, Titone A, et al. Evaluation of hydrogen peroxide as an intraprocedural hemostatic agent in manual dermabrasion. Dermatol Surg. 2010;36:1601-1603.
- Zhu G, Wang Q, Lu S, et al. Hydrogen peroxide: a potential wound therapeutic target? Med Princ Pract. 2017;26:301-308.
- Practicò D, Iuliano L, Ghiselli A, et al. Hydrogen peroxide as trigger of platelet aggregation. Haemostasis. 1991;21:169-174.
- Potyondy L, Lottenberg L, Anderson J, et al. The use of hydrogen peroxide for achieving dermal hemostasis after burn excision in a patient with platelet dysfunction. J Burn Care Res. 2006;27:99-101.
- Mawk JR. Hydrogen peroxide for hemostasis. Neurosurgery. 1986;18:827.
- Arakeri G, Brennan PA. Povidone-iodine and hydrogen peroxide mixture soaked gauze pack: a novel hemostatic technique. J Oral Maxillofac Surg. 2013;71:1833.e1-1833.e3.
- Huber D, Tegl G, Mensah A, et al. A dual-enzyme hydrogen peroxide generation machinery in hydrogels supports antimicrobial wound treatment. ACS Appl Mater Interfaces. 2017;9:15307-15316.
- Lineaweaver W, McMorris S, Soucy D, et al. Cellular and bacterial toxicities of topical antimicrobials. Plast Reconstr Surg. 1985;75:394-396.
- Henley J, Brewer JD. Newer hemostatic agents used in the practice of dermatologic surgery. Dermatol Res Pract. 2013;2013:279289.
- Hankin FM, Campbell SE, Goldstein SA, et al. Hydrogen peroxide as a topical hemostatic agent. Clin Orthop Relat Res. 1984;186:244-247.
- Weiss J, Winkleman FJ, Titone A, et al. Evaluation of hydrogen peroxide as an intraprocedural hemostatic agent in manual dermabrasion. Dermatol Surg. 2010;36:1601-1603.
- Zhu G, Wang Q, Lu S, et al. Hydrogen peroxide: a potential wound therapeutic target? Med Princ Pract. 2017;26:301-308.
- Practicò D, Iuliano L, Ghiselli A, et al. Hydrogen peroxide as trigger of platelet aggregation. Haemostasis. 1991;21:169-174.
- Potyondy L, Lottenberg L, Anderson J, et al. The use of hydrogen peroxide for achieving dermal hemostasis after burn excision in a patient with platelet dysfunction. J Burn Care Res. 2006;27:99-101.
- Mawk JR. Hydrogen peroxide for hemostasis. Neurosurgery. 1986;18:827.
- Arakeri G, Brennan PA. Povidone-iodine and hydrogen peroxide mixture soaked gauze pack: a novel hemostatic technique. J Oral Maxillofac Surg. 2013;71:1833.e1-1833.e3.
- Huber D, Tegl G, Mensah A, et al. A dual-enzyme hydrogen peroxide generation machinery in hydrogels supports antimicrobial wound treatment. ACS Appl Mater Interfaces. 2017;9:15307-15316.
- Lineaweaver W, McMorris S, Soucy D, et al. Cellular and bacterial toxicities of topical antimicrobials. Plast Reconstr Surg. 1985;75:394-396.
Pyoderma Gangrenosum Developing After Chest Tube Placement in a Patient With Chronic Lymphocytic Leukemia
Diagnosis of a neutrophilic dermatosis, such as pyoderma gangrenosum (PG), often is challenging at onset because it can be impossible to distinguish clinically and histopathologically from acute infection in an immunosuppressed patient, necessitating a detailed history as well as correlation pathology with microbial tissue cultures. The dermatologist’s ability to distinguish a neutrophilic dermatosis from active infection is of paramount importance because the decision to treat with surgical debridement, in addition to an antibiotic regimen, can have grave consequences in the misdiagnosed patient.
Pyoderma gangrenosum is a neutrophilic dermatosis histologically characterized by a pandermal neutrophilic infiltrate without evidence of an infectious cause or true vasculitis. It is classically associated with inflammatory bowel disease or an underlying hematologic malignancy. Pyoderma gangrenosum in the setting of chronic lymphocytic leukemia (CLL) is rare, with as few as 4 cases having been described in the literature and only 1 case of PG developing after a surgical procedure.1-4 We present a case of PG occurring at a chest tube site in a patient with CLL. We highlight the challenges and therapeutic importance of arriving at the correct diagnosis.
Case Report
An 87-year-old man with a history of refractory CLL was admitted to the hospital with pneumonia and pleural effusion requiring chest tube placement (left). His most recent therapeutic regimen for CLL was rituximab and bendamustine, which was administered 9 days prior to admission. After removal of the chest tube, an erythematous plaque with central necrosis surrounding the chest tube site developed (Figure 1A). During this time period, the patient had documented intermittent fevers, leukopenia, and neutropenia. Serial blood cultures yielded no growth. Because the patient was on broad-spectrum antibiotic coverage, dermatology was consulted for possible angioinvasive fungal infection.
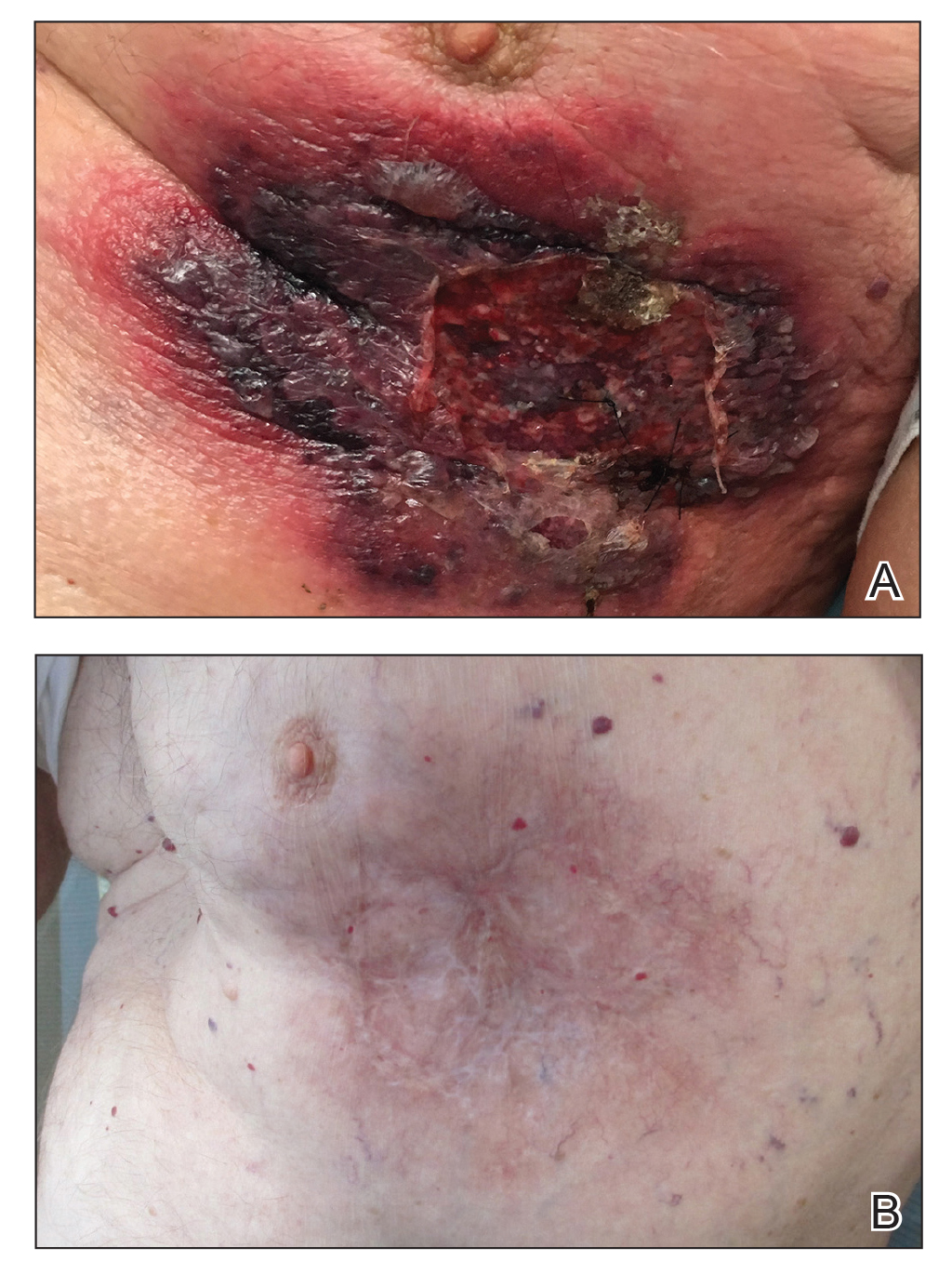
Physical examination revealed an indurated, erythematous-violaceous, targetoid, well-defined, ulcerated plaque with central necrosis on the left side of the chest. Notably, we observed an isolated bulla with an erythematous base within the right antecubital fossa at the site of intravenous placement, suggesting pathergy.
Multiple punch biopsies revealed an ulcer with an underlying dense neutrophilic infiltrate within the dermis and subcutaneous tissues (Figure 2). Grocott-Gomori methenamine-silver, periodic acid–Schiff, and acid-fast bacillus stains were all negative for organisms. Tissue cultures for bacterial, fungal, and acid-fast bacilli revealed no growth. Due to the rapidly expanding nature of the plaque and the possibility of infection despite negative microbial stains and cultures, the patient was scheduled for surgical debridement by the surgical team.
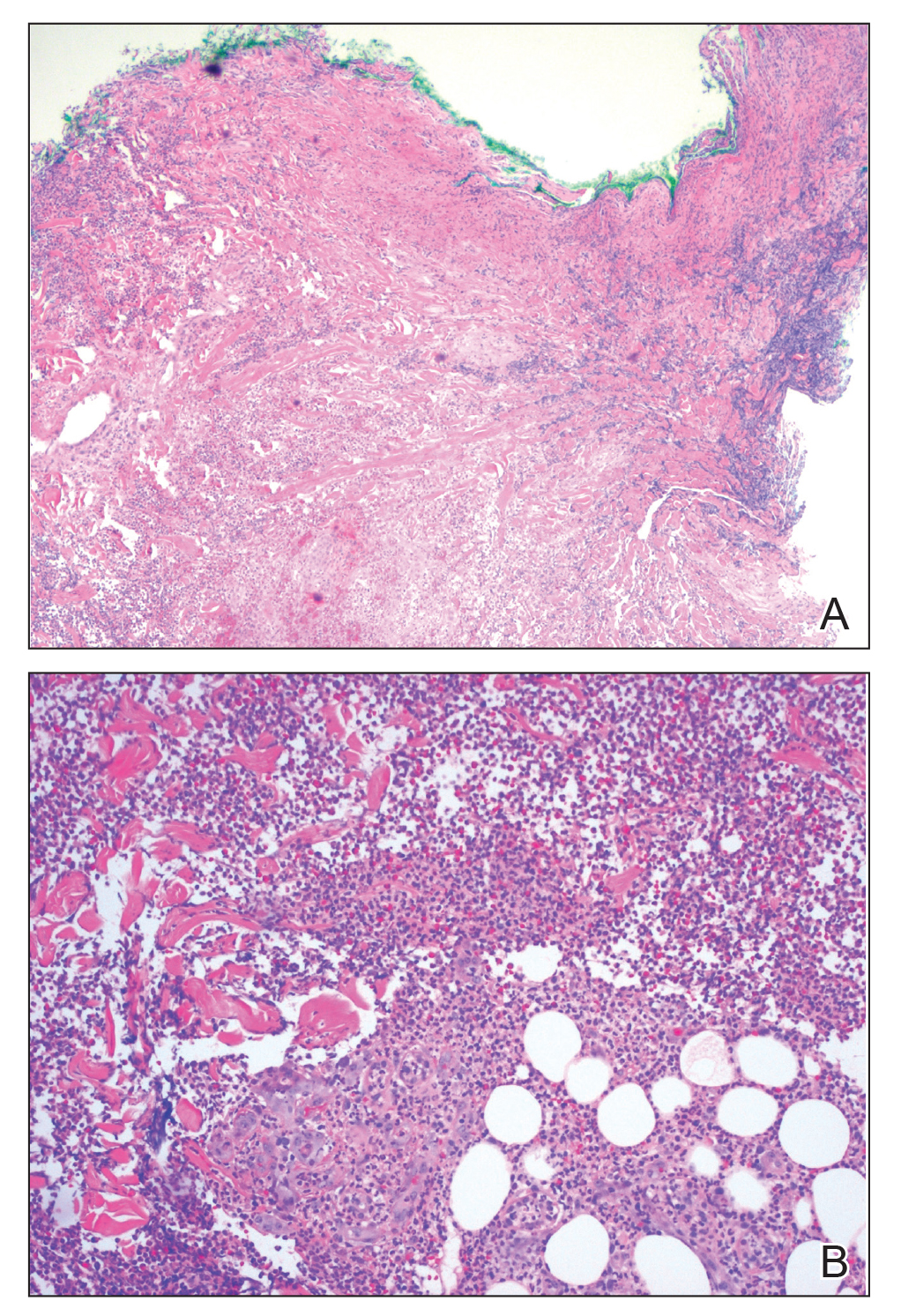
Opportunely, after thoughtful consideration of the clinical history, histopathology, and negative tissue cultures, we made a diagnosis of PG, a condition that would have been further exacerbated by debridement and unimproved with antibiotics. Based on our recommendation, the patient received immunosuppressive treatment with prednisone 60 mg/d and triamcinolone ointment 0.1%. He experienced immediate clinical improvement, allowing him to be discharged to the care of dermatology as an outpatient. He continued to receive a monthly rituximab infusion. We intentionally tapered the patient’s prednisone dosage slowly over 4 months and photodocumented steady improvement with eventual resolution of the PG (Figure 1B).
Comment
Pathogenesis of PG
Pyoderma gangrenosum lies in the spectrum of neutrophilic dermatoses, which are characterized histologically by a pandermal neutrophilic infiltrate without evidence of an infectious cause or true vasculitis. Clinically, PG typically presents as a steadily expanding ulceration with an undermined or slightly raised border, and often is associated with the pathergy phenomenon. Historically, PG is classically linked to inflammatory bowel disease; however, association with underlying malignancy, including acute myelogenous leukemia, chronic myelogenous leukemia, myeloma, and myeloid metaplasia, also has been described.5
Pathogenesis of CLL
Chronic lymphocytic leukemia represents the most prevalent form of leukemia in US adults, with the second highest annual incidence.6 Cutaneous findings are seen in 25% of patients with CLL, varying from leukemia cutis to secondary findings such as vasculitis, purpura, generalized pruritus, exfoliative erythroderma, paraneoplastic pemphigus, infections, and rarely neutrophilic dermatoses.7 According to a PubMed search of articles indexed for MEDLINE using the term pyoderma gangrenosum in CLL, only 4 cases of PG occurring in the setting of CLL exist in the literature, with 1 case demonstrating development after a surgical procedure, making ours the second such case (Table).1-4
Diagnosis
Making the diagnosis of a neutrophilic dermatosis such as PG or Sweet syndrome (SS) in the hospital setting is not only difficult but also imperative, considering that the counterdiagnosis more often is an infectious process. The distinction between individual neutrophilic dermatoses is less crucial at the onset because the initial treatment is the same.
Sweet syndrome is classically the most challenging entity within the spectrum to differentiate from PG. However, our case outlines several key distinguishing features:
• The lesion in classic PG is a rapidly expanding ulceration with undermined borders, whereas SS is less commonly associated with ulceration and instead classically presents with multiple edematous papules that progress to juicy plaques.8
• The pathergy phenomenon has been reported in SS, though it is more commonly associated with PG.9
• In reported cases of SS that were related to cutaneous trauma, lesions developed outside the area of trauma and there was documented leukocytosis and neutrophilia.10-14
• Although leukocytosis is part of the minor diagnostic criteria for SS, it is not required for the diagnosis of PG. Considering that our patient had ulcerated lesions, lesions only at the site of trauma, and leukopenia with intermittent neutropenia, the diagnosis was consistent with PG.
The primary value of early recognition and diagnosis of PG lies in the physician’s ability to distinguish PG from an infectious process, which can be challenging in an immunosuppressed patient with an underlying hematologic malignancy.
Conclusion
This case report represents our experience in arriving at the correct diagnosis of PG in a febrile neutrophilic patient with CLL. In the case of PG in a complicated patient, it is critical to initiate appropriate treatment and avoid inappropriate therapies. Aggressive surgical debridement could have resulted in a fatal outcome for our patient, highlighting the need for dermatologists to raise physician awareness of this challenging disease.
Acknowledgments
The authors acknowledge the contributions of Sarah Shalin, MD, PhD; Nikhil Meena, MD; and Aditya Chada, MD (all from Little Rock, Arkansas), for excellent patient care.
- Solovan C, Smiszek R, Wickenhauser C, et al. Postoperative pyoderma gangrenosum in association with renal cell carcinoma and chronic lymphocytic leukemia. Infect Dis Ther. 2013;2:75-80.
- Sławińska M, Barańska-Rybak W, Sobjanek M, et al. Ibrutinib-induced pyoderma gangrenosum. Pol Arch Med Wewn. 2016;126:710-711.
- Swale VJ, Saha M, Kapur N, et al. Pyoderma gangrenosum outside the context of inflammatory bowel disease treated successfully with infliximab. Clin Exp Dermatol. 2005;30:134-136.
- Wong SM, McComish J, Douglass J, et al. Rare skin manifestations successfully treated with primary B-cell chronic lymphocytic leukemia treatment. J Cutan Pathol. 2016;43:552-555.
- Jockenhöfer F, Herberger K, Schaller J, et al. Tricenter analysis of cofactors and comorbidity in patients with pyoderma gangrenosum. J Dtsch Dermatol Ges. 2016;14:1023-1030.
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7-30. 7.
- Robak E, Robak T. Skin lesions in chronic lymphocytic leukemia. Leuk Lymphoma. 2007;48:855-865.
- Beasley JM, Sluzevich JC. A recurrent vesiculobullous eruption on the head, trunk, and extremities. Bullous Sweet’s syndrome. Int J Dermatol. 2016;55:149-150.
- Awan F, Hamadani M, Devine S. Paraneoplastic Sweet’s syndrome and the pathergy phenomenon. Ann Hematol. 2007;86:613-614.
- de Moya MA, Wong JT, Kroshinsky D, et al. Case records of the Massachusetts General Hospital. Case 28-2012. A 30-year-old woman with shock and abdominal-wall necrosis after cesarean section. N Engl J Med. 2012;367:1046-1057.
- Minocha R, Sebaratnam DF, Choi JY. Sweet’s syndrome following surgery: cutaneous trauma as a possible aetiological co-factor in neutrophilic dermatoses. Australas J Dermatol. 2015;56:
e74-e76. - Phua YS, Al-Ani SA, She RB, et al. Sweet’s syndrome triggered by scalding: a case study and review of the literature. Burns. 2010;36:e49-e52.
- Schwarz RE, Quinn MA, Molina A. Acute postoperative dermatosis at the site of the electrocautery pad: sweet diagnosis of a burning issue. Surg Today. 2000;30:207-209.
- Tan AW, Tan HH, Lim PL. Bullous Sweet’s syndrome following influenza vaccination in a HIV-infected patient. Int J Dermatol. 2006;45:1254-1255.
Diagnosis of a neutrophilic dermatosis, such as pyoderma gangrenosum (PG), often is challenging at onset because it can be impossible to distinguish clinically and histopathologically from acute infection in an immunosuppressed patient, necessitating a detailed history as well as correlation pathology with microbial tissue cultures. The dermatologist’s ability to distinguish a neutrophilic dermatosis from active infection is of paramount importance because the decision to treat with surgical debridement, in addition to an antibiotic regimen, can have grave consequences in the misdiagnosed patient.
Pyoderma gangrenosum is a neutrophilic dermatosis histologically characterized by a pandermal neutrophilic infiltrate without evidence of an infectious cause or true vasculitis. It is classically associated with inflammatory bowel disease or an underlying hematologic malignancy. Pyoderma gangrenosum in the setting of chronic lymphocytic leukemia (CLL) is rare, with as few as 4 cases having been described in the literature and only 1 case of PG developing after a surgical procedure.1-4 We present a case of PG occurring at a chest tube site in a patient with CLL. We highlight the challenges and therapeutic importance of arriving at the correct diagnosis.
Case Report
An 87-year-old man with a history of refractory CLL was admitted to the hospital with pneumonia and pleural effusion requiring chest tube placement (left). His most recent therapeutic regimen for CLL was rituximab and bendamustine, which was administered 9 days prior to admission. After removal of the chest tube, an erythematous plaque with central necrosis surrounding the chest tube site developed (Figure 1A). During this time period, the patient had documented intermittent fevers, leukopenia, and neutropenia. Serial blood cultures yielded no growth. Because the patient was on broad-spectrum antibiotic coverage, dermatology was consulted for possible angioinvasive fungal infection.

Physical examination revealed an indurated, erythematous-violaceous, targetoid, well-defined, ulcerated plaque with central necrosis on the left side of the chest. Notably, we observed an isolated bulla with an erythematous base within the right antecubital fossa at the site of intravenous placement, suggesting pathergy.
Multiple punch biopsies revealed an ulcer with an underlying dense neutrophilic infiltrate within the dermis and subcutaneous tissues (Figure 2). Grocott-Gomori methenamine-silver, periodic acid–Schiff, and acid-fast bacillus stains were all negative for organisms. Tissue cultures for bacterial, fungal, and acid-fast bacilli revealed no growth. Due to the rapidly expanding nature of the plaque and the possibility of infection despite negative microbial stains and cultures, the patient was scheduled for surgical debridement by the surgical team.

Opportunely, after thoughtful consideration of the clinical history, histopathology, and negative tissue cultures, we made a diagnosis of PG, a condition that would have been further exacerbated by debridement and unimproved with antibiotics. Based on our recommendation, the patient received immunosuppressive treatment with prednisone 60 mg/d and triamcinolone ointment 0.1%. He experienced immediate clinical improvement, allowing him to be discharged to the care of dermatology as an outpatient. He continued to receive a monthly rituximab infusion. We intentionally tapered the patient’s prednisone dosage slowly over 4 months and photodocumented steady improvement with eventual resolution of the PG (Figure 1B).
Comment
Pathogenesis of PG
Pyoderma gangrenosum lies in the spectrum of neutrophilic dermatoses, which are characterized histologically by a pandermal neutrophilic infiltrate without evidence of an infectious cause or true vasculitis. Clinically, PG typically presents as a steadily expanding ulceration with an undermined or slightly raised border, and often is associated with the pathergy phenomenon. Historically, PG is classically linked to inflammatory bowel disease; however, association with underlying malignancy, including acute myelogenous leukemia, chronic myelogenous leukemia, myeloma, and myeloid metaplasia, also has been described.5
Pathogenesis of CLL
Chronic lymphocytic leukemia represents the most prevalent form of leukemia in US adults, with the second highest annual incidence.6 Cutaneous findings are seen in 25% of patients with CLL, varying from leukemia cutis to secondary findings such as vasculitis, purpura, generalized pruritus, exfoliative erythroderma, paraneoplastic pemphigus, infections, and rarely neutrophilic dermatoses.7 According to a PubMed search of articles indexed for MEDLINE using the term pyoderma gangrenosum in CLL, only 4 cases of PG occurring in the setting of CLL exist in the literature, with 1 case demonstrating development after a surgical procedure, making ours the second such case (Table).1-4

Diagnosis
Making the diagnosis of a neutrophilic dermatosis such as PG or Sweet syndrome (SS) in the hospital setting is not only difficult but also imperative, considering that the counterdiagnosis more often is an infectious process. The distinction between individual neutrophilic dermatoses is less crucial at the onset because the initial treatment is the same.
Sweet syndrome is classically the most challenging entity within the spectrum to differentiate from PG. However, our case outlines several key distinguishing features:
• The lesion in classic PG is a rapidly expanding ulceration with undermined borders, whereas SS is less commonly associated with ulceration and instead classically presents with multiple edematous papules that progress to juicy plaques.8
• The pathergy phenomenon has been reported in SS, though it is more commonly associated with PG.9
• In reported cases of SS that were related to cutaneous trauma, lesions developed outside the area of trauma and there was documented leukocytosis and neutrophilia.10-14
• Although leukocytosis is part of the minor diagnostic criteria for SS, it is not required for the diagnosis of PG. Considering that our patient had ulcerated lesions, lesions only at the site of trauma, and leukopenia with intermittent neutropenia, the diagnosis was consistent with PG.
The primary value of early recognition and diagnosis of PG lies in the physician’s ability to distinguish PG from an infectious process, which can be challenging in an immunosuppressed patient with an underlying hematologic malignancy.
Conclusion
This case report represents our experience in arriving at the correct diagnosis of PG in a febrile neutrophilic patient with CLL. In the case of PG in a complicated patient, it is critical to initiate appropriate treatment and avoid inappropriate therapies. Aggressive surgical debridement could have resulted in a fatal outcome for our patient, highlighting the need for dermatologists to raise physician awareness of this challenging disease.
Acknowledgments
The authors acknowledge the contributions of Sarah Shalin, MD, PhD; Nikhil Meena, MD; and Aditya Chada, MD (all from Little Rock, Arkansas), for excellent patient care.
Diagnosis of a neutrophilic dermatosis, such as pyoderma gangrenosum (PG), often is challenging at onset because it can be impossible to distinguish clinically and histopathologically from acute infection in an immunosuppressed patient, necessitating a detailed history as well as correlation pathology with microbial tissue cultures. The dermatologist’s ability to distinguish a neutrophilic dermatosis from active infection is of paramount importance because the decision to treat with surgical debridement, in addition to an antibiotic regimen, can have grave consequences in the misdiagnosed patient.
Pyoderma gangrenosum is a neutrophilic dermatosis histologically characterized by a pandermal neutrophilic infiltrate without evidence of an infectious cause or true vasculitis. It is classically associated with inflammatory bowel disease or an underlying hematologic malignancy. Pyoderma gangrenosum in the setting of chronic lymphocytic leukemia (CLL) is rare, with as few as 4 cases having been described in the literature and only 1 case of PG developing after a surgical procedure.1-4 We present a case of PG occurring at a chest tube site in a patient with CLL. We highlight the challenges and therapeutic importance of arriving at the correct diagnosis.
Case Report
An 87-year-old man with a history of refractory CLL was admitted to the hospital with pneumonia and pleural effusion requiring chest tube placement (left). His most recent therapeutic regimen for CLL was rituximab and bendamustine, which was administered 9 days prior to admission. After removal of the chest tube, an erythematous plaque with central necrosis surrounding the chest tube site developed (Figure 1A). During this time period, the patient had documented intermittent fevers, leukopenia, and neutropenia. Serial blood cultures yielded no growth. Because the patient was on broad-spectrum antibiotic coverage, dermatology was consulted for possible angioinvasive fungal infection.

Physical examination revealed an indurated, erythematous-violaceous, targetoid, well-defined, ulcerated plaque with central necrosis on the left side of the chest. Notably, we observed an isolated bulla with an erythematous base within the right antecubital fossa at the site of intravenous placement, suggesting pathergy.
Multiple punch biopsies revealed an ulcer with an underlying dense neutrophilic infiltrate within the dermis and subcutaneous tissues (Figure 2). Grocott-Gomori methenamine-silver, periodic acid–Schiff, and acid-fast bacillus stains were all negative for organisms. Tissue cultures for bacterial, fungal, and acid-fast bacilli revealed no growth. Due to the rapidly expanding nature of the plaque and the possibility of infection despite negative microbial stains and cultures, the patient was scheduled for surgical debridement by the surgical team.

Opportunely, after thoughtful consideration of the clinical history, histopathology, and negative tissue cultures, we made a diagnosis of PG, a condition that would have been further exacerbated by debridement and unimproved with antibiotics. Based on our recommendation, the patient received immunosuppressive treatment with prednisone 60 mg/d and triamcinolone ointment 0.1%. He experienced immediate clinical improvement, allowing him to be discharged to the care of dermatology as an outpatient. He continued to receive a monthly rituximab infusion. We intentionally tapered the patient’s prednisone dosage slowly over 4 months and photodocumented steady improvement with eventual resolution of the PG (Figure 1B).
Comment
Pathogenesis of PG
Pyoderma gangrenosum lies in the spectrum of neutrophilic dermatoses, which are characterized histologically by a pandermal neutrophilic infiltrate without evidence of an infectious cause or true vasculitis. Clinically, PG typically presents as a steadily expanding ulceration with an undermined or slightly raised border, and often is associated with the pathergy phenomenon. Historically, PG is classically linked to inflammatory bowel disease; however, association with underlying malignancy, including acute myelogenous leukemia, chronic myelogenous leukemia, myeloma, and myeloid metaplasia, also has been described.5
Pathogenesis of CLL
Chronic lymphocytic leukemia represents the most prevalent form of leukemia in US adults, with the second highest annual incidence.6 Cutaneous findings are seen in 25% of patients with CLL, varying from leukemia cutis to secondary findings such as vasculitis, purpura, generalized pruritus, exfoliative erythroderma, paraneoplastic pemphigus, infections, and rarely neutrophilic dermatoses.7 According to a PubMed search of articles indexed for MEDLINE using the term pyoderma gangrenosum in CLL, only 4 cases of PG occurring in the setting of CLL exist in the literature, with 1 case demonstrating development after a surgical procedure, making ours the second such case (Table).1-4

Diagnosis
Making the diagnosis of a neutrophilic dermatosis such as PG or Sweet syndrome (SS) in the hospital setting is not only difficult but also imperative, considering that the counterdiagnosis more often is an infectious process. The distinction between individual neutrophilic dermatoses is less crucial at the onset because the initial treatment is the same.
Sweet syndrome is classically the most challenging entity within the spectrum to differentiate from PG. However, our case outlines several key distinguishing features:
• The lesion in classic PG is a rapidly expanding ulceration with undermined borders, whereas SS is less commonly associated with ulceration and instead classically presents with multiple edematous papules that progress to juicy plaques.8
• The pathergy phenomenon has been reported in SS, though it is more commonly associated with PG.9
• In reported cases of SS that were related to cutaneous trauma, lesions developed outside the area of trauma and there was documented leukocytosis and neutrophilia.10-14
• Although leukocytosis is part of the minor diagnostic criteria for SS, it is not required for the diagnosis of PG. Considering that our patient had ulcerated lesions, lesions only at the site of trauma, and leukopenia with intermittent neutropenia, the diagnosis was consistent with PG.
The primary value of early recognition and diagnosis of PG lies in the physician’s ability to distinguish PG from an infectious process, which can be challenging in an immunosuppressed patient with an underlying hematologic malignancy.
Conclusion
This case report represents our experience in arriving at the correct diagnosis of PG in a febrile neutrophilic patient with CLL. In the case of PG in a complicated patient, it is critical to initiate appropriate treatment and avoid inappropriate therapies. Aggressive surgical debridement could have resulted in a fatal outcome for our patient, highlighting the need for dermatologists to raise physician awareness of this challenging disease.
Acknowledgments
The authors acknowledge the contributions of Sarah Shalin, MD, PhD; Nikhil Meena, MD; and Aditya Chada, MD (all from Little Rock, Arkansas), for excellent patient care.
- Solovan C, Smiszek R, Wickenhauser C, et al. Postoperative pyoderma gangrenosum in association with renal cell carcinoma and chronic lymphocytic leukemia. Infect Dis Ther. 2013;2:75-80.
- Sławińska M, Barańska-Rybak W, Sobjanek M, et al. Ibrutinib-induced pyoderma gangrenosum. Pol Arch Med Wewn. 2016;126:710-711.
- Swale VJ, Saha M, Kapur N, et al. Pyoderma gangrenosum outside the context of inflammatory bowel disease treated successfully with infliximab. Clin Exp Dermatol. 2005;30:134-136.
- Wong SM, McComish J, Douglass J, et al. Rare skin manifestations successfully treated with primary B-cell chronic lymphocytic leukemia treatment. J Cutan Pathol. 2016;43:552-555.
- Jockenhöfer F, Herberger K, Schaller J, et al. Tricenter analysis of cofactors and comorbidity in patients with pyoderma gangrenosum. J Dtsch Dermatol Ges. 2016;14:1023-1030.
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7-30. 7.
- Robak E, Robak T. Skin lesions in chronic lymphocytic leukemia. Leuk Lymphoma. 2007;48:855-865.
- Beasley JM, Sluzevich JC. A recurrent vesiculobullous eruption on the head, trunk, and extremities. Bullous Sweet’s syndrome. Int J Dermatol. 2016;55:149-150.
- Awan F, Hamadani M, Devine S. Paraneoplastic Sweet’s syndrome and the pathergy phenomenon. Ann Hematol. 2007;86:613-614.
- de Moya MA, Wong JT, Kroshinsky D, et al. Case records of the Massachusetts General Hospital. Case 28-2012. A 30-year-old woman with shock and abdominal-wall necrosis after cesarean section. N Engl J Med. 2012;367:1046-1057.
- Minocha R, Sebaratnam DF, Choi JY. Sweet’s syndrome following surgery: cutaneous trauma as a possible aetiological co-factor in neutrophilic dermatoses. Australas J Dermatol. 2015;56:
e74-e76. - Phua YS, Al-Ani SA, She RB, et al. Sweet’s syndrome triggered by scalding: a case study and review of the literature. Burns. 2010;36:e49-e52.
- Schwarz RE, Quinn MA, Molina A. Acute postoperative dermatosis at the site of the electrocautery pad: sweet diagnosis of a burning issue. Surg Today. 2000;30:207-209.
- Tan AW, Tan HH, Lim PL. Bullous Sweet’s syndrome following influenza vaccination in a HIV-infected patient. Int J Dermatol. 2006;45:1254-1255.
- Solovan C, Smiszek R, Wickenhauser C, et al. Postoperative pyoderma gangrenosum in association with renal cell carcinoma and chronic lymphocytic leukemia. Infect Dis Ther. 2013;2:75-80.
- Sławińska M, Barańska-Rybak W, Sobjanek M, et al. Ibrutinib-induced pyoderma gangrenosum. Pol Arch Med Wewn. 2016;126:710-711.
- Swale VJ, Saha M, Kapur N, et al. Pyoderma gangrenosum outside the context of inflammatory bowel disease treated successfully with infliximab. Clin Exp Dermatol. 2005;30:134-136.
- Wong SM, McComish J, Douglass J, et al. Rare skin manifestations successfully treated with primary B-cell chronic lymphocytic leukemia treatment. J Cutan Pathol. 2016;43:552-555.
- Jockenhöfer F, Herberger K, Schaller J, et al. Tricenter analysis of cofactors and comorbidity in patients with pyoderma gangrenosum. J Dtsch Dermatol Ges. 2016;14:1023-1030.
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7-30. 7.
- Robak E, Robak T. Skin lesions in chronic lymphocytic leukemia. Leuk Lymphoma. 2007;48:855-865.
- Beasley JM, Sluzevich JC. A recurrent vesiculobullous eruption on the head, trunk, and extremities. Bullous Sweet’s syndrome. Int J Dermatol. 2016;55:149-150.
- Awan F, Hamadani M, Devine S. Paraneoplastic Sweet’s syndrome and the pathergy phenomenon. Ann Hematol. 2007;86:613-614.
- de Moya MA, Wong JT, Kroshinsky D, et al. Case records of the Massachusetts General Hospital. Case 28-2012. A 30-year-old woman with shock and abdominal-wall necrosis after cesarean section. N Engl J Med. 2012;367:1046-1057.
- Minocha R, Sebaratnam DF, Choi JY. Sweet’s syndrome following surgery: cutaneous trauma as a possible aetiological co-factor in neutrophilic dermatoses. Australas J Dermatol. 2015;56:
e74-e76. - Phua YS, Al-Ani SA, She RB, et al. Sweet’s syndrome triggered by scalding: a case study and review of the literature. Burns. 2010;36:e49-e52.
- Schwarz RE, Quinn MA, Molina A. Acute postoperative dermatosis at the site of the electrocautery pad: sweet diagnosis of a burning issue. Surg Today. 2000;30:207-209.
- Tan AW, Tan HH, Lim PL. Bullous Sweet’s syndrome following influenza vaccination in a HIV-infected patient. Int J Dermatol. 2006;45:1254-1255.
Practice Points
- The primary value of early recognition and diagnosis of pyoderma gangrenosum (PG) lies in the physician’s ability to distinguish PG from an infectious process.
- Surgical debridement would further exacerbate PG, making proper diagnosis of a neutrophilic dermatosis of paramount importance to avoid treatments that could have grave consequences in the misdiagnosed patient.
- Cutaneous findings are seen in one-quarter of patients with chronic lymphocytic leukemia.
- Pyoderma gangrenosum is commonly associated with inflammatory bowel disease but also can be seen in many hematologic malignancies. Physicians should be aware of this association to ensure these patients are diagnosed properly.
Reflectance Confocal Microscopy to Facilitate Knifeless Skin Cancer Management
Practice Gap
Management of nonmelanoma skin cancer (NMSC) in elderly patients can cause morbidity because these patients frequently struggle to care for their biopsy sites and experience biopsy- and surgery-related complications. To minimize this treatment-related morbidity, we designed a knifeless treatment approach that employs reflectance confocal microscopy (RCM) in lieu of skin biopsy to establish the diagnosis of NMSC, then uses either intralesional or topical chemotherapy or immunotherapy (as appropriate, depending on depth of invasion) to cure the NMSC. With this approach, the patient is spared both biopsy- and surgery-related difficulties, though both intralesional and topical chemotherapy are accompanied by their own risks for adverse effects.
The Technique
Elderly patients, diabetic patients, and patients with lesions suspicious for NMSC on areas prone to poor wound healing or to notable treatment-related morbidity (eg, lower legs, genitals, the face of younger patients) are offered skin biopsy or RCM; the latter is performed during the appointment by an RC
When resolution is uncertain, RCM is repeated to assess for tumor clearance. Repeat RCM is performed at least 4 weeks after termination of treatment to avoid misinterpretation caused by treatment-related tissue inflammation. Patients who are not cured using this management approach are offered appropriate surgical management.
Practice Implications
Reflectance confocal microscopy has emerged as an effective modality for confirming the diagnosis of NMSC with high sensitivity and specificity.1,2 Emergence of this technology presents an opportunity for improving the way the NMSC is managed because RCM allows dermatologists to confirm the diagnosis of BCC and SCC by interpretation of RCM mosaics rather than by histopathologic examination of biopsied tissue. Our knifeless approach to skin cancer management is especially beneficial when biopsy and dermatologic surgery are likely to confer notable morbidity, such as managing NMSC on the face of a young adult, in the frail elderly population, or in diabetic patients, and when treating sites on the lower extremity prone to poor wound healing.
- Song E, Grant-Kels JM, Swede H, et al. Paired comparison of the sensitivity and specificity of multispectral digital skin lesion analysis and reflectance confocal microscopy in the detection of melanoma in vivo: a cross-sectional study. J Am Acad Dermatol. 2016;75:1187-1192.
- Ferrari B, Salgarelli AC, Mandel VD, et al. Non-melanoma skin cancer of the head and neck: the aid of reflectance confocal microscopy for the accurate diagnosis and management. G Ital Dermatol Venereol. 2017;152:169-177.
Practice Gap
Management of nonmelanoma skin cancer (NMSC) in elderly patients can cause morbidity because these patients frequently struggle to care for their biopsy sites and experience biopsy- and surgery-related complications. To minimize this treatment-related morbidity, we designed a knifeless treatment approach that employs reflectance confocal microscopy (RCM) in lieu of skin biopsy to establish the diagnosis of NMSC, then uses either intralesional or topical chemotherapy or immunotherapy (as appropriate, depending on depth of invasion) to cure the NMSC. With this approach, the patient is spared both biopsy- and surgery-related difficulties, though both intralesional and topical chemotherapy are accompanied by their own risks for adverse effects.
The Technique
Elderly patients, diabetic patients, and patients with lesions suspicious for NMSC on areas prone to poor wound healing or to notable treatment-related morbidity (eg, lower legs, genitals, the face of younger patients) are offered skin biopsy or RCM; the latter is performed during the appointment by an RC
When resolution is uncertain, RCM is repeated to assess for tumor clearance. Repeat RCM is performed at least 4 weeks after termination of treatment to avoid misinterpretation caused by treatment-related tissue inflammation. Patients who are not cured using this management approach are offered appropriate surgical management.
Practice Implications
Reflectance confocal microscopy has emerged as an effective modality for confirming the diagnosis of NMSC with high sensitivity and specificity.1,2 Emergence of this technology presents an opportunity for improving the way the NMSC is managed because RCM allows dermatologists to confirm the diagnosis of BCC and SCC by interpretation of RCM mosaics rather than by histopathologic examination of biopsied tissue. Our knifeless approach to skin cancer management is especially beneficial when biopsy and dermatologic surgery are likely to confer notable morbidity, such as managing NMSC on the face of a young adult, in the frail elderly population, or in diabetic patients, and when treating sites on the lower extremity prone to poor wound healing.
Practice Gap
Management of nonmelanoma skin cancer (NMSC) in elderly patients can cause morbidity because these patients frequently struggle to care for their biopsy sites and experience biopsy- and surgery-related complications. To minimize this treatment-related morbidity, we designed a knifeless treatment approach that employs reflectance confocal microscopy (RCM) in lieu of skin biopsy to establish the diagnosis of NMSC, then uses either intralesional or topical chemotherapy or immunotherapy (as appropriate, depending on depth of invasion) to cure the NMSC. With this approach, the patient is spared both biopsy- and surgery-related difficulties, though both intralesional and topical chemotherapy are accompanied by their own risks for adverse effects.
The Technique
Elderly patients, diabetic patients, and patients with lesions suspicious for NMSC on areas prone to poor wound healing or to notable treatment-related morbidity (eg, lower legs, genitals, the face of younger patients) are offered skin biopsy or RCM; the latter is performed during the appointment by an RC
When resolution is uncertain, RCM is repeated to assess for tumor clearance. Repeat RCM is performed at least 4 weeks after termination of treatment to avoid misinterpretation caused by treatment-related tissue inflammation. Patients who are not cured using this management approach are offered appropriate surgical management.
Practice Implications
Reflectance confocal microscopy has emerged as an effective modality for confirming the diagnosis of NMSC with high sensitivity and specificity.1,2 Emergence of this technology presents an opportunity for improving the way the NMSC is managed because RCM allows dermatologists to confirm the diagnosis of BCC and SCC by interpretation of RCM mosaics rather than by histopathologic examination of biopsied tissue. Our knifeless approach to skin cancer management is especially beneficial when biopsy and dermatologic surgery are likely to confer notable morbidity, such as managing NMSC on the face of a young adult, in the frail elderly population, or in diabetic patients, and when treating sites on the lower extremity prone to poor wound healing.
- Song E, Grant-Kels JM, Swede H, et al. Paired comparison of the sensitivity and specificity of multispectral digital skin lesion analysis and reflectance confocal microscopy in the detection of melanoma in vivo: a cross-sectional study. J Am Acad Dermatol. 2016;75:1187-1192.
- Ferrari B, Salgarelli AC, Mandel VD, et al. Non-melanoma skin cancer of the head and neck: the aid of reflectance confocal microscopy for the accurate diagnosis and management. G Ital Dermatol Venereol. 2017;152:169-177.
- Song E, Grant-Kels JM, Swede H, et al. Paired comparison of the sensitivity and specificity of multispectral digital skin lesion analysis and reflectance confocal microscopy in the detection of melanoma in vivo: a cross-sectional study. J Am Acad Dermatol. 2016;75:1187-1192.
- Ferrari B, Salgarelli AC, Mandel VD, et al. Non-melanoma skin cancer of the head and neck: the aid of reflectance confocal microscopy for the accurate diagnosis and management. G Ital Dermatol Venereol. 2017;152:169-177.
Clinical Pearl: Topical Timolol for Refractory Hypergranulation
Practice Gap
Hypergranulation is a frequent complication of dermatologic surgery, especially when surgical defects are left to heal by secondary intention (eg, after electrodesiccation and curettage). Although management of postoperative hypergranulation with routine wound care, superpotent topical corticosteroids, and/or topical silver nitrate often is effective, refractory cases pose a difficult challenge given the paucity of treatment options. Effective management of these cases is important because hypergranulation can delay wound healing, cause patient discomfort, and lead to poor wound cosmesis.
The Technique
If refractory hypergranulation fails to respond to treatment with routine wound care and topical silver nitrate, we prescribe twice-daily application of timolol maleate ophthalmic gel forming solution 0.5% for up to 14 days or until complete resolution of the hypergranulation is achieved. We counsel patients to continue routine wound care with daily dressing changes in conjunction with topical timolol application.
We initiated treatment with topical timolol in a patient who developed hypergranulation at 2 separate electrodesiccation and curettage sites that was refractory to 6 weeks of routine wound care with white petrolatum under nonadherent sterile gauze dressings and 2 subsequent topical silver nitrate applications (Figure 1). After 2 weeks of treatment with topical timolol, resolution of the hypergranulation and re-epithelialization of the surgical sites was observed (Figure 2). Another patient presented with hypergranulation that developed following a traumatic injury on the left upper arm and had been treated unsuccessfully for several months at a wound care clinic with daily nonadherent sterile gauze dressings and both topical and oral antibiotics (Figure 3A). After treatment for 9 days with topical timolol, resolution of the hypergranulation and re-epithelialization of the surgical sites was observed (Figure 3B).
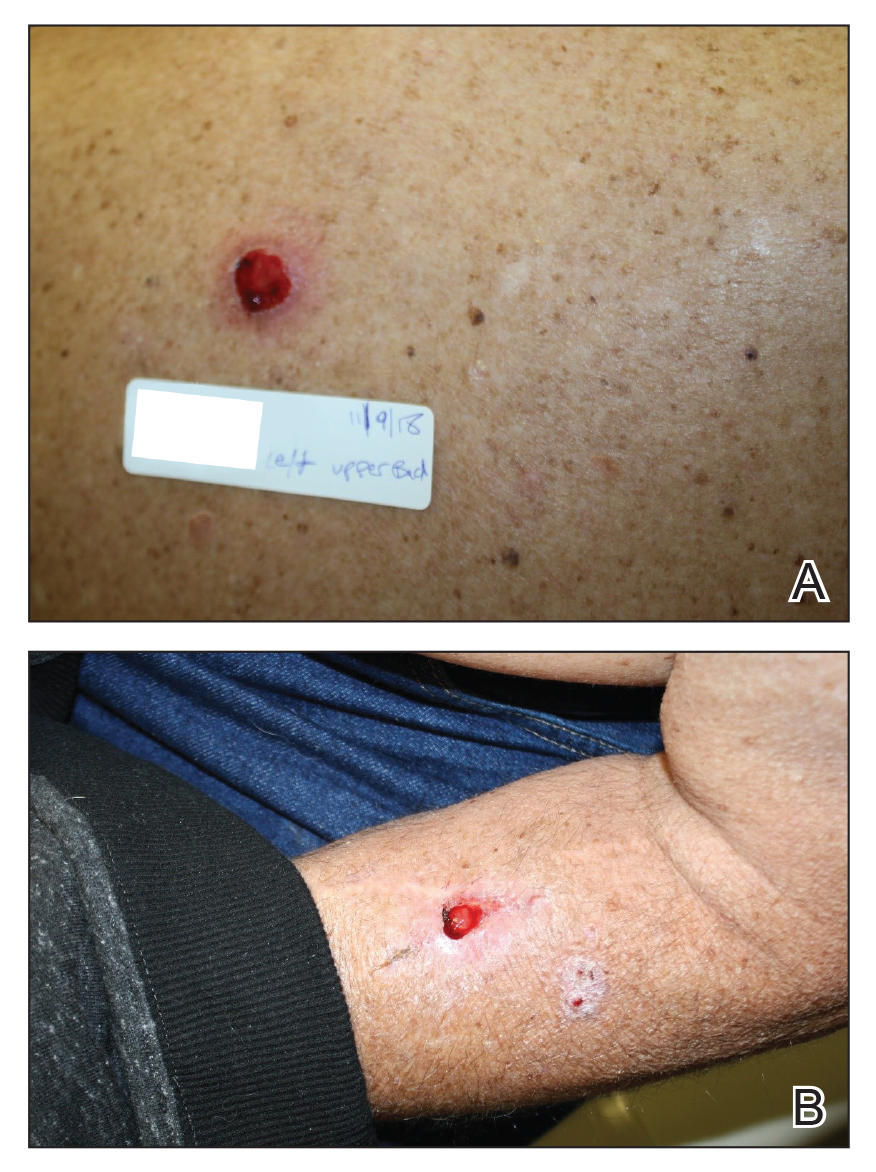
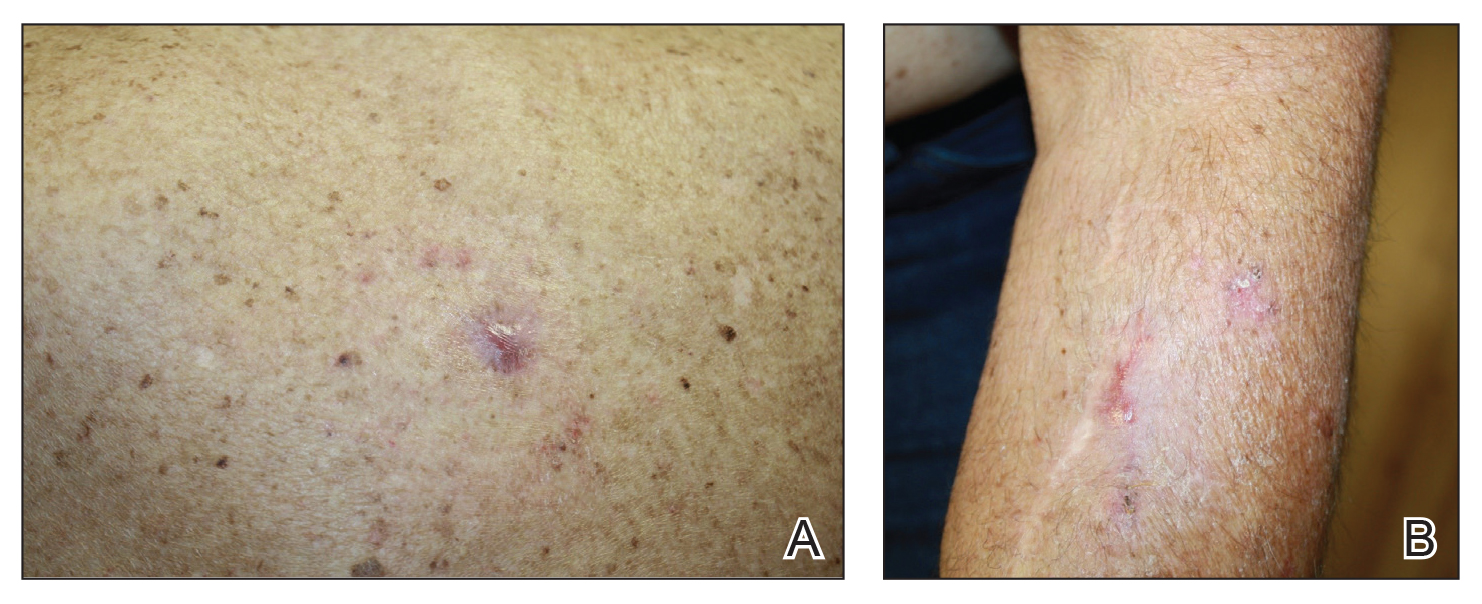
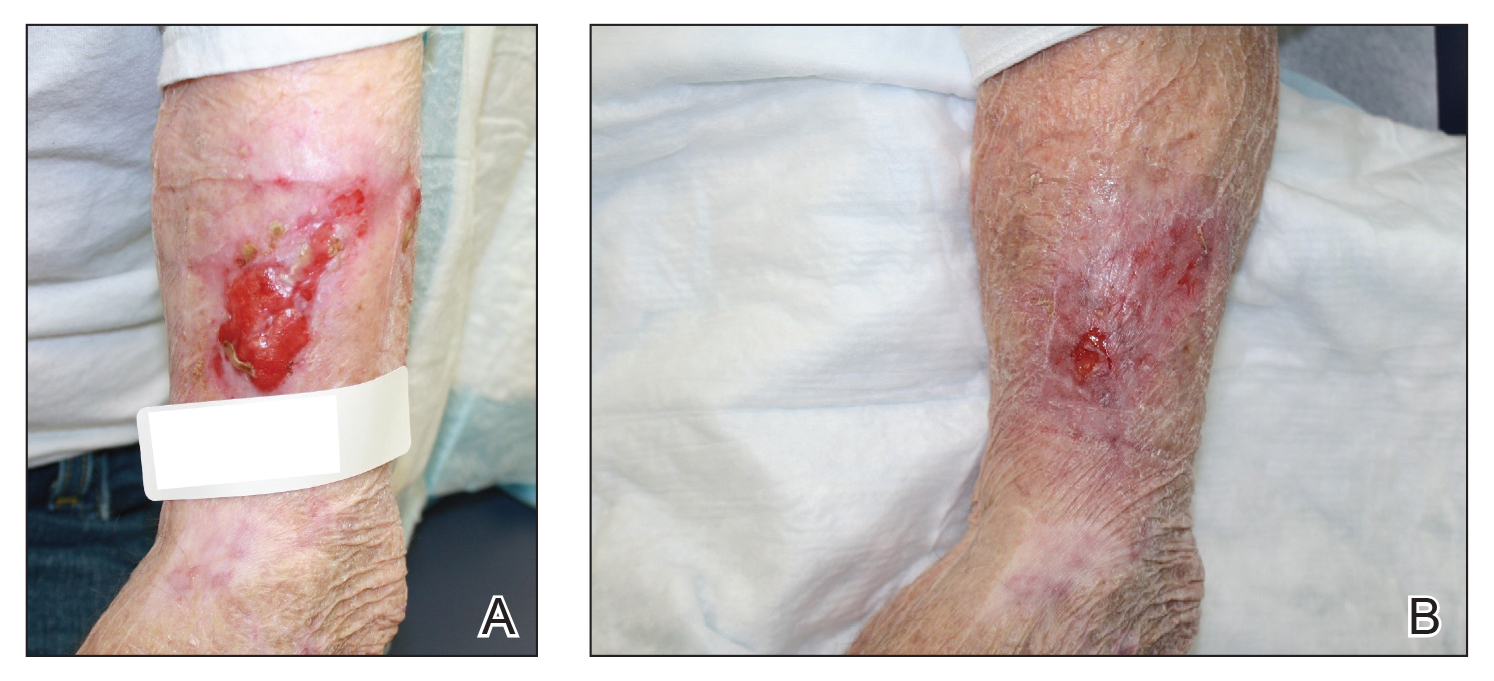
Practice Implications
Beta-blockers are increasingly being used for management of chronic nonhealing wounds since the 1990s when oral administration of propranolol initially was reported to be an effective adjuvant therapy for managing severe burns.1 Since then, topical beta-blockers have been reported to be effective for management of ulcerated hemangiomas, venous stasis ulcers, chronic diabetic ulcers, and chronic nonhealing surgical wounds; however, there are no known reports of using topical beta-blockers for management of hypergranulation.2-5 We found timolol ophthalmic gel to be an excellent second-line therapy for management of postoperative hypergranulation if prior treatment with routine wound care and superpotent topical corticosteroids has failed. To date, we have found no reported adverse effects from the use of topical timolol for this indication that have required discontinuation of the medication. Use of this simple and safe intervention can be effective as a solution to a common postoperative condition.
- Herndon DN, Hart DW, Wolf SE, et al. Reversal of catabolism by beta-blockade after severe burns. N Engl J Med. 2001;345:1223-1229.
- Pope E, Chakkittakandiyil A. Topical timolol gel for infantile hemangiomas: a pilot study. Arch Dermatol. 2010;146:564-565.
- Braun L, Lamel S, Richmond N, et al. Topical timolol for recalcitrant wounds. JAMA Dermatol. 2013;149:1400-1402.
- Thomas B, Kurien J, Jose T, et al. Topical timolol promotes healing of chronic leg ulcer. J Vasc Surg. 2017;5:844-850.
- Tang J, Dosal J, Kirsner RS. Topical timolol for a refractory wound. Dermatol Surg. 2012;38:135-138.
Practice Gap
Hypergranulation is a frequent complication of dermatologic surgery, especially when surgical defects are left to heal by secondary intention (eg, after electrodesiccation and curettage). Although management of postoperative hypergranulation with routine wound care, superpotent topical corticosteroids, and/or topical silver nitrate often is effective, refractory cases pose a difficult challenge given the paucity of treatment options. Effective management of these cases is important because hypergranulation can delay wound healing, cause patient discomfort, and lead to poor wound cosmesis.
The Technique
If refractory hypergranulation fails to respond to treatment with routine wound care and topical silver nitrate, we prescribe twice-daily application of timolol maleate ophthalmic gel forming solution 0.5% for up to 14 days or until complete resolution of the hypergranulation is achieved. We counsel patients to continue routine wound care with daily dressing changes in conjunction with topical timolol application.
We initiated treatment with topical timolol in a patient who developed hypergranulation at 2 separate electrodesiccation and curettage sites that was refractory to 6 weeks of routine wound care with white petrolatum under nonadherent sterile gauze dressings and 2 subsequent topical silver nitrate applications (Figure 1). After 2 weeks of treatment with topical timolol, resolution of the hypergranulation and re-epithelialization of the surgical sites was observed (Figure 2). Another patient presented with hypergranulation that developed following a traumatic injury on the left upper arm and had been treated unsuccessfully for several months at a wound care clinic with daily nonadherent sterile gauze dressings and both topical and oral antibiotics (Figure 3A). After treatment for 9 days with topical timolol, resolution of the hypergranulation and re-epithelialization of the surgical sites was observed (Figure 3B).



Practice Implications
Beta-blockers are increasingly being used for management of chronic nonhealing wounds since the 1990s when oral administration of propranolol initially was reported to be an effective adjuvant therapy for managing severe burns.1 Since then, topical beta-blockers have been reported to be effective for management of ulcerated hemangiomas, venous stasis ulcers, chronic diabetic ulcers, and chronic nonhealing surgical wounds; however, there are no known reports of using topical beta-blockers for management of hypergranulation.2-5 We found timolol ophthalmic gel to be an excellent second-line therapy for management of postoperative hypergranulation if prior treatment with routine wound care and superpotent topical corticosteroids has failed. To date, we have found no reported adverse effects from the use of topical timolol for this indication that have required discontinuation of the medication. Use of this simple and safe intervention can be effective as a solution to a common postoperative condition.
Practice Gap
Hypergranulation is a frequent complication of dermatologic surgery, especially when surgical defects are left to heal by secondary intention (eg, after electrodesiccation and curettage). Although management of postoperative hypergranulation with routine wound care, superpotent topical corticosteroids, and/or topical silver nitrate often is effective, refractory cases pose a difficult challenge given the paucity of treatment options. Effective management of these cases is important because hypergranulation can delay wound healing, cause patient discomfort, and lead to poor wound cosmesis.
The Technique
If refractory hypergranulation fails to respond to treatment with routine wound care and topical silver nitrate, we prescribe twice-daily application of timolol maleate ophthalmic gel forming solution 0.5% for up to 14 days or until complete resolution of the hypergranulation is achieved. We counsel patients to continue routine wound care with daily dressing changes in conjunction with topical timolol application.
We initiated treatment with topical timolol in a patient who developed hypergranulation at 2 separate electrodesiccation and curettage sites that was refractory to 6 weeks of routine wound care with white petrolatum under nonadherent sterile gauze dressings and 2 subsequent topical silver nitrate applications (Figure 1). After 2 weeks of treatment with topical timolol, resolution of the hypergranulation and re-epithelialization of the surgical sites was observed (Figure 2). Another patient presented with hypergranulation that developed following a traumatic injury on the left upper arm and had been treated unsuccessfully for several months at a wound care clinic with daily nonadherent sterile gauze dressings and both topical and oral antibiotics (Figure 3A). After treatment for 9 days with topical timolol, resolution of the hypergranulation and re-epithelialization of the surgical sites was observed (Figure 3B).



Practice Implications
Beta-blockers are increasingly being used for management of chronic nonhealing wounds since the 1990s when oral administration of propranolol initially was reported to be an effective adjuvant therapy for managing severe burns.1 Since then, topical beta-blockers have been reported to be effective for management of ulcerated hemangiomas, venous stasis ulcers, chronic diabetic ulcers, and chronic nonhealing surgical wounds; however, there are no known reports of using topical beta-blockers for management of hypergranulation.2-5 We found timolol ophthalmic gel to be an excellent second-line therapy for management of postoperative hypergranulation if prior treatment with routine wound care and superpotent topical corticosteroids has failed. To date, we have found no reported adverse effects from the use of topical timolol for this indication that have required discontinuation of the medication. Use of this simple and safe intervention can be effective as a solution to a common postoperative condition.
- Herndon DN, Hart DW, Wolf SE, et al. Reversal of catabolism by beta-blockade after severe burns. N Engl J Med. 2001;345:1223-1229.
- Pope E, Chakkittakandiyil A. Topical timolol gel for infantile hemangiomas: a pilot study. Arch Dermatol. 2010;146:564-565.
- Braun L, Lamel S, Richmond N, et al. Topical timolol for recalcitrant wounds. JAMA Dermatol. 2013;149:1400-1402.
- Thomas B, Kurien J, Jose T, et al. Topical timolol promotes healing of chronic leg ulcer. J Vasc Surg. 2017;5:844-850.
- Tang J, Dosal J, Kirsner RS. Topical timolol for a refractory wound. Dermatol Surg. 2012;38:135-138.
- Herndon DN, Hart DW, Wolf SE, et al. Reversal of catabolism by beta-blockade after severe burns. N Engl J Med. 2001;345:1223-1229.
- Pope E, Chakkittakandiyil A. Topical timolol gel for infantile hemangiomas: a pilot study. Arch Dermatol. 2010;146:564-565.
- Braun L, Lamel S, Richmond N, et al. Topical timolol for recalcitrant wounds. JAMA Dermatol. 2013;149:1400-1402.
- Thomas B, Kurien J, Jose T, et al. Topical timolol promotes healing of chronic leg ulcer. J Vasc Surg. 2017;5:844-850.
- Tang J, Dosal J, Kirsner RS. Topical timolol for a refractory wound. Dermatol Surg. 2012;38:135-138.
Collagen powder deemed noninferior to primary closure for punch-biopsy healing
Collagen powder may be noninferior to primary closure for healing punch biopsy–induced wounds and possibly leads to improved early cosmetic outcomes and accelerated wound maturation, according to Azam Qureshi of the University of Maryland, Baltimore, and associates.
In a small pilot study published in Journal of Drugs in Dermatology, eight volunteers (mean age, 37 years) received a 4-mm punch biopsy on each thigh. One wound was managed with primary closure, the other with daily application of collagen powder. The wounds were biopsied at 4 weeks for histopathologic analysis, and the study subjects rated pain, itch, and treatment preferences at 1, 2, 4, 6, and 12 weeks.
The size of wounds treated with collagen was reduced by 28.95% at 1 week, 55.76% at 2 weeks, and 95.94% at 4 weeks; six of the eight collagen-treated wounds were completely healed at 4 weeks. Wound size was reduced by 75.71% 1 week after the second biopsy, much faster than the initial healing. In addition to collagen, one patient required hyfrecation for hemostasis, which did not affect results; three of the eight subjects rated the collagen treatment as “annoying,” but no one rated it as “difficult,” and patients generally regarded collagen treatment as more time consuming.
The histopathologic analysis showed epidermal reepithelialization in collagen-treated wounds and wounds managed with primary closure, with more organized granulation tissue in the collagen-treated wounds. Similar pain and itch ratings were reported between wound types, and both patients and blinded dermatologists observing the study preferred the appearance of collagen-treated wounds.
“Future research elucidating the optimal duration of collagen therapy is needed, as less than 4 weeks may be sufficient. Shortened treatment courses would decrease the cost and effort required by patients. Future studies should also investigate the efficacy of collagen powder in healing larger wounds and in comparison to healing by secondary intention,” the investigators wrote.
CPN Biosciences funded the study. No authors had relevant financial disclosures.
SOURCE: Qureshi A et al. J Drug Dermatol. 2019;18(7):667-73
Collagen powder may be noninferior to primary closure for healing punch biopsy–induced wounds and possibly leads to improved early cosmetic outcomes and accelerated wound maturation, according to Azam Qureshi of the University of Maryland, Baltimore, and associates.
In a small pilot study published in Journal of Drugs in Dermatology, eight volunteers (mean age, 37 years) received a 4-mm punch biopsy on each thigh. One wound was managed with primary closure, the other with daily application of collagen powder. The wounds were biopsied at 4 weeks for histopathologic analysis, and the study subjects rated pain, itch, and treatment preferences at 1, 2, 4, 6, and 12 weeks.
The size of wounds treated with collagen was reduced by 28.95% at 1 week, 55.76% at 2 weeks, and 95.94% at 4 weeks; six of the eight collagen-treated wounds were completely healed at 4 weeks. Wound size was reduced by 75.71% 1 week after the second biopsy, much faster than the initial healing. In addition to collagen, one patient required hyfrecation for hemostasis, which did not affect results; three of the eight subjects rated the collagen treatment as “annoying,” but no one rated it as “difficult,” and patients generally regarded collagen treatment as more time consuming.
The histopathologic analysis showed epidermal reepithelialization in collagen-treated wounds and wounds managed with primary closure, with more organized granulation tissue in the collagen-treated wounds. Similar pain and itch ratings were reported between wound types, and both patients and blinded dermatologists observing the study preferred the appearance of collagen-treated wounds.
“Future research elucidating the optimal duration of collagen therapy is needed, as less than 4 weeks may be sufficient. Shortened treatment courses would decrease the cost and effort required by patients. Future studies should also investigate the efficacy of collagen powder in healing larger wounds and in comparison to healing by secondary intention,” the investigators wrote.
CPN Biosciences funded the study. No authors had relevant financial disclosures.
SOURCE: Qureshi A et al. J Drug Dermatol. 2019;18(7):667-73
Collagen powder may be noninferior to primary closure for healing punch biopsy–induced wounds and possibly leads to improved early cosmetic outcomes and accelerated wound maturation, according to Azam Qureshi of the University of Maryland, Baltimore, and associates.
In a small pilot study published in Journal of Drugs in Dermatology, eight volunteers (mean age, 37 years) received a 4-mm punch biopsy on each thigh. One wound was managed with primary closure, the other with daily application of collagen powder. The wounds were biopsied at 4 weeks for histopathologic analysis, and the study subjects rated pain, itch, and treatment preferences at 1, 2, 4, 6, and 12 weeks.
The size of wounds treated with collagen was reduced by 28.95% at 1 week, 55.76% at 2 weeks, and 95.94% at 4 weeks; six of the eight collagen-treated wounds were completely healed at 4 weeks. Wound size was reduced by 75.71% 1 week after the second biopsy, much faster than the initial healing. In addition to collagen, one patient required hyfrecation for hemostasis, which did not affect results; three of the eight subjects rated the collagen treatment as “annoying,” but no one rated it as “difficult,” and patients generally regarded collagen treatment as more time consuming.
The histopathologic analysis showed epidermal reepithelialization in collagen-treated wounds and wounds managed with primary closure, with more organized granulation tissue in the collagen-treated wounds. Similar pain and itch ratings were reported between wound types, and both patients and blinded dermatologists observing the study preferred the appearance of collagen-treated wounds.
“Future research elucidating the optimal duration of collagen therapy is needed, as less than 4 weeks may be sufficient. Shortened treatment courses would decrease the cost and effort required by patients. Future studies should also investigate the efficacy of collagen powder in healing larger wounds and in comparison to healing by secondary intention,” the investigators wrote.
CPN Biosciences funded the study. No authors had relevant financial disclosures.
SOURCE: Qureshi A et al. J Drug Dermatol. 2019;18(7):667-73
FROM JOURNAL OF DRUGS IN DERMATOLOGY
Cellulitis ranks as top reason for skin-related pediatric inpatient admissions
AUSTIN, TEX. – showed.
“Skin conditions significantly affect pediatric inpatients, and dermatologists ought be accessible for consultation to enhance care and costs,” the study’s first author, Marcus L. Elias, said in an interview prior to the annual meeting of the Society for Pediatric Dermatology.
According to Mr. Elias, who is a 4th-year medical student at Rutgers New Jersey Medical School–Newark, few national studies on skin diseases for pediatric inpatients have been published in the medical literature. Earlier this year, researchers examined inpatient dermatologic conditions in patients aged 18 years and older (J Am Acad Dermatol 2019;80[2]:425-32), but Mr. Elias and associates set out to analyze the burden of inpatient pediatric dermatologic conditions on a national basis. “We wanted to see if the same conditions that were hospitalizing adults were also hospitalizing kids,” he said. “We found that this was indeed the case.”
The researchers queried the National Inpatient Sample database for all cases involving patients aged 18 years and younger during 2001-2013. The search yielded a sample of 16,837,857 patients. From this, the researchers analyzed diagnosis-related groups for dermatologic conditions denoting the principal diagnosis at discharge, which left a final sample of 84,090 patients. Frequency and chi-squared tests were used to analyze categorical variables.
More than half of patients (54%) were male, 36% were white, 48% had Medicaid insurance, and 43% had private insurance. Mr. Elias reported that the median length of stay for patients was 2 days and the median cost of care was $6,289.50 for each case. More than three-quarters of pediatric inpatients with dermatologic diagnoses were treated for “cellulitis” (66,147 cases, or 79%), with most cases involving the legs (16,875 cases, or 20%). Other pediatric inpatients were admitted for “minor skin disorder without complications” (5,458 cases, or 7%), and “minor skin disorder with complications” (2,822 cases, or 3%). A total of 64 patients died during the study period. Of these, 31 cases (50%) involved “skin graft and/or debridement of skin ulcer or cellulitis without complications,” the study found.
“We were surprised that the major cause of mortality for our patients was classified as ‘skin graft and/or debridement of skin ulcer or cellulitis without complications,’ as a similar diagnosis-related groupings exist denoting that complications did arise,” Mr. Elias said. “Still, it is not possible for us to determine if the mortality was from the skin graft/debridement or another cause entirely. It is possible that the procedure was without complications, only to have the patient succumb to an ancillary process.”
He acknowledged certain limitations of the study, including the fact that the function of dermatologic consults for hospitalized patients was not examined. “We also cannot draw conclusions as to whether improved outpatient therapy reduces the need for hospitalization,” he said. Mr. Elias reported having no financial disclosures.
AUSTIN, TEX. – showed.
“Skin conditions significantly affect pediatric inpatients, and dermatologists ought be accessible for consultation to enhance care and costs,” the study’s first author, Marcus L. Elias, said in an interview prior to the annual meeting of the Society for Pediatric Dermatology.
According to Mr. Elias, who is a 4th-year medical student at Rutgers New Jersey Medical School–Newark, few national studies on skin diseases for pediatric inpatients have been published in the medical literature. Earlier this year, researchers examined inpatient dermatologic conditions in patients aged 18 years and older (J Am Acad Dermatol 2019;80[2]:425-32), but Mr. Elias and associates set out to analyze the burden of inpatient pediatric dermatologic conditions on a national basis. “We wanted to see if the same conditions that were hospitalizing adults were also hospitalizing kids,” he said. “We found that this was indeed the case.”
The researchers queried the National Inpatient Sample database for all cases involving patients aged 18 years and younger during 2001-2013. The search yielded a sample of 16,837,857 patients. From this, the researchers analyzed diagnosis-related groups for dermatologic conditions denoting the principal diagnosis at discharge, which left a final sample of 84,090 patients. Frequency and chi-squared tests were used to analyze categorical variables.
More than half of patients (54%) were male, 36% were white, 48% had Medicaid insurance, and 43% had private insurance. Mr. Elias reported that the median length of stay for patients was 2 days and the median cost of care was $6,289.50 for each case. More than three-quarters of pediatric inpatients with dermatologic diagnoses were treated for “cellulitis” (66,147 cases, or 79%), with most cases involving the legs (16,875 cases, or 20%). Other pediatric inpatients were admitted for “minor skin disorder without complications” (5,458 cases, or 7%), and “minor skin disorder with complications” (2,822 cases, or 3%). A total of 64 patients died during the study period. Of these, 31 cases (50%) involved “skin graft and/or debridement of skin ulcer or cellulitis without complications,” the study found.
“We were surprised that the major cause of mortality for our patients was classified as ‘skin graft and/or debridement of skin ulcer or cellulitis without complications,’ as a similar diagnosis-related groupings exist denoting that complications did arise,” Mr. Elias said. “Still, it is not possible for us to determine if the mortality was from the skin graft/debridement or another cause entirely. It is possible that the procedure was without complications, only to have the patient succumb to an ancillary process.”
He acknowledged certain limitations of the study, including the fact that the function of dermatologic consults for hospitalized patients was not examined. “We also cannot draw conclusions as to whether improved outpatient therapy reduces the need for hospitalization,” he said. Mr. Elias reported having no financial disclosures.
AUSTIN, TEX. – showed.
“Skin conditions significantly affect pediatric inpatients, and dermatologists ought be accessible for consultation to enhance care and costs,” the study’s first author, Marcus L. Elias, said in an interview prior to the annual meeting of the Society for Pediatric Dermatology.
According to Mr. Elias, who is a 4th-year medical student at Rutgers New Jersey Medical School–Newark, few national studies on skin diseases for pediatric inpatients have been published in the medical literature. Earlier this year, researchers examined inpatient dermatologic conditions in patients aged 18 years and older (J Am Acad Dermatol 2019;80[2]:425-32), but Mr. Elias and associates set out to analyze the burden of inpatient pediatric dermatologic conditions on a national basis. “We wanted to see if the same conditions that were hospitalizing adults were also hospitalizing kids,” he said. “We found that this was indeed the case.”
The researchers queried the National Inpatient Sample database for all cases involving patients aged 18 years and younger during 2001-2013. The search yielded a sample of 16,837,857 patients. From this, the researchers analyzed diagnosis-related groups for dermatologic conditions denoting the principal diagnosis at discharge, which left a final sample of 84,090 patients. Frequency and chi-squared tests were used to analyze categorical variables.
More than half of patients (54%) were male, 36% were white, 48% had Medicaid insurance, and 43% had private insurance. Mr. Elias reported that the median length of stay for patients was 2 days and the median cost of care was $6,289.50 for each case. More than three-quarters of pediatric inpatients with dermatologic diagnoses were treated for “cellulitis” (66,147 cases, or 79%), with most cases involving the legs (16,875 cases, or 20%). Other pediatric inpatients were admitted for “minor skin disorder without complications” (5,458 cases, or 7%), and “minor skin disorder with complications” (2,822 cases, or 3%). A total of 64 patients died during the study period. Of these, 31 cases (50%) involved “skin graft and/or debridement of skin ulcer or cellulitis without complications,” the study found.
“We were surprised that the major cause of mortality for our patients was classified as ‘skin graft and/or debridement of skin ulcer or cellulitis without complications,’ as a similar diagnosis-related groupings exist denoting that complications did arise,” Mr. Elias said. “Still, it is not possible for us to determine if the mortality was from the skin graft/debridement or another cause entirely. It is possible that the procedure was without complications, only to have the patient succumb to an ancillary process.”
He acknowledged certain limitations of the study, including the fact that the function of dermatologic consults for hospitalized patients was not examined. “We also cannot draw conclusions as to whether improved outpatient therapy reduces the need for hospitalization,” he said. Mr. Elias reported having no financial disclosures.
REPORTING FROM SPD 2019
Key clinical point: Cellulitis is the cause of the majority of skin-related pediatric inpatient admissions in the United States.
Major finding: In all, 79% of pediatric inpatients with dermatologic diagnoses were treated for cellulitis.
Study details: An analysis of data from 84,090 patients younger than age 18 in the National Inpatient Sample.
Disclosures: The researchers reported having no financial disclosures.