User login
Nano-antifungal shows promise as topical treatment
Nanotechnology may help control the fungal infections that can lead to mortality in burn patients, early research suggests.
Nanoparticle encapsulated amphotericin b reduced fungal activity by 80%-95% compared with controls in a burn wound model. The findings were published online June 12 in Nanomedicine: Nanotechnology, Biology, and Medicine (doi:10.1016/j.nano.2013.06.002).
"Infection and sepsis persist as frequent causes of morbidity and mortality for burn victims due to extensive compromise of the skin and contiguous tissue that serve as a protective barrier against microbial invasion," wrote David A. Sanchez and his colleagues at the Albert Einstein College of Medicine, New York.
Currently available antifungals such as amphotericin b (AmB) are associated with liver toxicity at high doses, but the researchers proposed that encapsulating the drug would allow for topical use.
In this study, nanoparticle encapsulated AmB (AmB-np) significantly reduced fungal biofilm activity in a burn tissue model compared with untreated control areas over an exposure period of 24 hours, and a significant reduction in fungal activity compared with controls remained evident after 15 days of treatment. Fungal activity was measured via CFU assay to test for Candida spp strains including C. albicans, C. glabrata, and C. parapsilosis.
The researchers also compared AmB-np to solubilized AmB (AmB-sol) and found similar antifungal efficacy. The data substantiate the hypothesis "that encapsulated AmB is actively being liberated from the nanoparticles," they noted.
The burn tissue was assessed at 7 and 11 days from baseline wounding. AmB-np and AmB-sol were similarly effective and were associated with "decreased peripheral wound erythema, tissue induration and edema, and necrotic crusting in comparison to infected controls," the researchers said. However, histology data showed "more advanced re-epithelialization, organized dermal proliferation and appropriate/expected dermal remodeling as compared to the AmB-sol and untreated infected control," they added.
Toxicity concerns remain a controversy associated with nanoparticle application, the researchers noted. However, AmB-np showed no significant difference compared with nontreated and solubilized groups in an animal model. "Moreover, our histological studies show enhanced tissue healing in the AmB-np animals compared to the other groups," the researchers noted.
The findings were limited by the differences between a murine model and human skin, and by the absence of blank nanoparticles as controls. But the results suggest that AmB-np has clinical potential as a topical antifungal because of its "uncompromised antimycotic action" against multiple fungal strains, and its successful delivery in an in vivo burn wound model, the researchers said.
Mr. Sanchez and his colleagues had no financial conflicts to disclose.
On Twitter: @hsplete
Nanotechnology may help control the fungal infections that can lead to mortality in burn patients, early research suggests.
Nanoparticle encapsulated amphotericin b reduced fungal activity by 80%-95% compared with controls in a burn wound model. The findings were published online June 12 in Nanomedicine: Nanotechnology, Biology, and Medicine (doi:10.1016/j.nano.2013.06.002).
"Infection and sepsis persist as frequent causes of morbidity and mortality for burn victims due to extensive compromise of the skin and contiguous tissue that serve as a protective barrier against microbial invasion," wrote David A. Sanchez and his colleagues at the Albert Einstein College of Medicine, New York.
Currently available antifungals such as amphotericin b (AmB) are associated with liver toxicity at high doses, but the researchers proposed that encapsulating the drug would allow for topical use.
In this study, nanoparticle encapsulated AmB (AmB-np) significantly reduced fungal biofilm activity in a burn tissue model compared with untreated control areas over an exposure period of 24 hours, and a significant reduction in fungal activity compared with controls remained evident after 15 days of treatment. Fungal activity was measured via CFU assay to test for Candida spp strains including C. albicans, C. glabrata, and C. parapsilosis.
The researchers also compared AmB-np to solubilized AmB (AmB-sol) and found similar antifungal efficacy. The data substantiate the hypothesis "that encapsulated AmB is actively being liberated from the nanoparticles," they noted.
The burn tissue was assessed at 7 and 11 days from baseline wounding. AmB-np and AmB-sol were similarly effective and were associated with "decreased peripheral wound erythema, tissue induration and edema, and necrotic crusting in comparison to infected controls," the researchers said. However, histology data showed "more advanced re-epithelialization, organized dermal proliferation and appropriate/expected dermal remodeling as compared to the AmB-sol and untreated infected control," they added.
Toxicity concerns remain a controversy associated with nanoparticle application, the researchers noted. However, AmB-np showed no significant difference compared with nontreated and solubilized groups in an animal model. "Moreover, our histological studies show enhanced tissue healing in the AmB-np animals compared to the other groups," the researchers noted.
The findings were limited by the differences between a murine model and human skin, and by the absence of blank nanoparticles as controls. But the results suggest that AmB-np has clinical potential as a topical antifungal because of its "uncompromised antimycotic action" against multiple fungal strains, and its successful delivery in an in vivo burn wound model, the researchers said.
Mr. Sanchez and his colleagues had no financial conflicts to disclose.
On Twitter: @hsplete
Nanotechnology may help control the fungal infections that can lead to mortality in burn patients, early research suggests.
Nanoparticle encapsulated amphotericin b reduced fungal activity by 80%-95% compared with controls in a burn wound model. The findings were published online June 12 in Nanomedicine: Nanotechnology, Biology, and Medicine (doi:10.1016/j.nano.2013.06.002).
"Infection and sepsis persist as frequent causes of morbidity and mortality for burn victims due to extensive compromise of the skin and contiguous tissue that serve as a protective barrier against microbial invasion," wrote David A. Sanchez and his colleagues at the Albert Einstein College of Medicine, New York.
Currently available antifungals such as amphotericin b (AmB) are associated with liver toxicity at high doses, but the researchers proposed that encapsulating the drug would allow for topical use.
In this study, nanoparticle encapsulated AmB (AmB-np) significantly reduced fungal biofilm activity in a burn tissue model compared with untreated control areas over an exposure period of 24 hours, and a significant reduction in fungal activity compared with controls remained evident after 15 days of treatment. Fungal activity was measured via CFU assay to test for Candida spp strains including C. albicans, C. glabrata, and C. parapsilosis.
The researchers also compared AmB-np to solubilized AmB (AmB-sol) and found similar antifungal efficacy. The data substantiate the hypothesis "that encapsulated AmB is actively being liberated from the nanoparticles," they noted.
The burn tissue was assessed at 7 and 11 days from baseline wounding. AmB-np and AmB-sol were similarly effective and were associated with "decreased peripheral wound erythema, tissue induration and edema, and necrotic crusting in comparison to infected controls," the researchers said. However, histology data showed "more advanced re-epithelialization, organized dermal proliferation and appropriate/expected dermal remodeling as compared to the AmB-sol and untreated infected control," they added.
Toxicity concerns remain a controversy associated with nanoparticle application, the researchers noted. However, AmB-np showed no significant difference compared with nontreated and solubilized groups in an animal model. "Moreover, our histological studies show enhanced tissue healing in the AmB-np animals compared to the other groups," the researchers noted.
The findings were limited by the differences between a murine model and human skin, and by the absence of blank nanoparticles as controls. But the results suggest that AmB-np has clinical potential as a topical antifungal because of its "uncompromised antimycotic action" against multiple fungal strains, and its successful delivery in an in vivo burn wound model, the researchers said.
Mr. Sanchez and his colleagues had no financial conflicts to disclose.
On Twitter: @hsplete
FROM NANOMEDICINE: NANOTECHNOLOGY, BIOLOGY AND MEDICINE
Pulley stitch: A go-to for defects under tension
EDITOR'S NOTE: August 26, 2013: This article has been amended since it was first published to make it clear that Dr. Kelley Pagliai Redbord's description of the pulley stitch procedure was taken directly from an article published by Dr. Cyndi Yag-Howard in Dermatologic Surgery (Dermatol. Surg.2011;37:1503-5). In her presentation, Dr. Redbord credited Dr. Yag-Howard and her article. However, this credit and attribution to Dr. Yag-Howard was not included in the article published.
WASHINGTON – The pulley stitch "is my go-to stitch for defects under tension," said Dr. Kelley Pagliai Redbord.
The pulley stitch allows for considerable reduction in the surface area of a large defect that can’t be closed by side-to-side stitches alone, making it an excellent choice for use on the scalp and legs, Dr. Redbord said at the Atlantic Dermatological Conference.
"When the tension across the wound is decreased, buried dermal sutures can be placed more easily and accurately," she said. "I use it a lot as an intraoperative tissue expander."
Dr. Redbord said that her description of the pulley stitch was taken from an article by Dr. Cyndi Yag-Howard in Dermatologic Surgery (Dermatol. Surg. 2011; 37:1503-5).*
The pulley stitch can serve as a temporary suture that can be left in place or removed, said Dr. Redbord, a dermatologist in group practice in Rockville, Md.
The technique follows a far-near-near-far pattern, starting the stitch 8 mm from the wound edge (far), then bringing it to the opposite side just 4 mm from the wound edge (near). Dr. Redbord then reenters the stitch 4 mm from the wound edge on the initial side (near), and makes another pass to the opposite side 8 mm from the wound edge (far).
Multiple passes through the tissue create resistance that keeps the suture from slipping. "The loops of the stitch are placed at an oblique angle so that the inner and outer loops are offset and do not override each other," she noted. This technique minimizes potential skin damage from pressure necrosis caused by overriding loop sutures. The pulley stitch has a 2:1 mechanical advantage over an interrupted suture, and the additional friction of a second loop prevents the knot from slipping.
A modification of the pulley stitch is to loop the suture through an external loop on the opposite side of the incision, and pull across. "This new loop functions as a pulley and directs the tension away from the other strands," she said.
Another stitch with excellent eversion, in which the pulley stitch plays a key role, is the subcutaneous inverted cross mattress stitch (SICM). The SCIM is entirely subcutaneous, and combines the buried vertical mattress stitch and the buried pulley stitch.
The SCIM "uses the buried vertical mattress’s ability to evert wound edges and combines it with the pulley stitch’s ability to decrease tension at the wound edge," she said.
The four-step process is as follows:
• 1. Insert the needle into the dermis 3-5 mm lateral to the wound edge. Advance the needle into the upper reticular dermis, and then curve down to exit through the lower reticular dermis.
• 2. Insert the needle into the opposite edge of the wound at the lower reticular dermis and advance into the upper reticular dermis, then curve down and exit intradermally.
• 3. Insert the needle across the defect using an intradermal approach 1-2 mm lateral to the initial needle insertion point. Then, create a second buried vertical mattress stitch.
• 4. Pull the two stitches to close, which "creates a pulley effect with minimal recoil, and tie off," Dr. Redbord said.
"The pulley system locks the wound edges so that a knot can be tied without slipping," she added.
Dr. Redbord said she had no relevant financial disclosures.
EDITOR'S NOTE: August 26, 2013: This article has been amended since it was first published to make it clear that Dr. Kelley Pagliai Redbord's description of the pulley stitch procedure was taken directly from an article published by Dr. Cyndi Yag-Howard in Dermatologic Surgery (Dermatol. Surg.2011;37:1503-5). In her presentation, Dr. Redbord credited Dr. Yag-Howard and her article. However, this credit and attribution to Dr. Yag-Howard was not included in the article published.
WASHINGTON – The pulley stitch "is my go-to stitch for defects under tension," said Dr. Kelley Pagliai Redbord.
The pulley stitch allows for considerable reduction in the surface area of a large defect that can’t be closed by side-to-side stitches alone, making it an excellent choice for use on the scalp and legs, Dr. Redbord said at the Atlantic Dermatological Conference.
"When the tension across the wound is decreased, buried dermal sutures can be placed more easily and accurately," she said. "I use it a lot as an intraoperative tissue expander."
Dr. Redbord said that her description of the pulley stitch was taken from an article by Dr. Cyndi Yag-Howard in Dermatologic Surgery (Dermatol. Surg. 2011; 37:1503-5).*
The pulley stitch can serve as a temporary suture that can be left in place or removed, said Dr. Redbord, a dermatologist in group practice in Rockville, Md.
The technique follows a far-near-near-far pattern, starting the stitch 8 mm from the wound edge (far), then bringing it to the opposite side just 4 mm from the wound edge (near). Dr. Redbord then reenters the stitch 4 mm from the wound edge on the initial side (near), and makes another pass to the opposite side 8 mm from the wound edge (far).
Multiple passes through the tissue create resistance that keeps the suture from slipping. "The loops of the stitch are placed at an oblique angle so that the inner and outer loops are offset and do not override each other," she noted. This technique minimizes potential skin damage from pressure necrosis caused by overriding loop sutures. The pulley stitch has a 2:1 mechanical advantage over an interrupted suture, and the additional friction of a second loop prevents the knot from slipping.
A modification of the pulley stitch is to loop the suture through an external loop on the opposite side of the incision, and pull across. "This new loop functions as a pulley and directs the tension away from the other strands," she said.
Another stitch with excellent eversion, in which the pulley stitch plays a key role, is the subcutaneous inverted cross mattress stitch (SICM). The SCIM is entirely subcutaneous, and combines the buried vertical mattress stitch and the buried pulley stitch.
The SCIM "uses the buried vertical mattress’s ability to evert wound edges and combines it with the pulley stitch’s ability to decrease tension at the wound edge," she said.
The four-step process is as follows:
• 1. Insert the needle into the dermis 3-5 mm lateral to the wound edge. Advance the needle into the upper reticular dermis, and then curve down to exit through the lower reticular dermis.
• 2. Insert the needle into the opposite edge of the wound at the lower reticular dermis and advance into the upper reticular dermis, then curve down and exit intradermally.
• 3. Insert the needle across the defect using an intradermal approach 1-2 mm lateral to the initial needle insertion point. Then, create a second buried vertical mattress stitch.
• 4. Pull the two stitches to close, which "creates a pulley effect with minimal recoil, and tie off," Dr. Redbord said.
"The pulley system locks the wound edges so that a knot can be tied without slipping," she added.
Dr. Redbord said she had no relevant financial disclosures.
EDITOR'S NOTE: August 26, 2013: This article has been amended since it was first published to make it clear that Dr. Kelley Pagliai Redbord's description of the pulley stitch procedure was taken directly from an article published by Dr. Cyndi Yag-Howard in Dermatologic Surgery (Dermatol. Surg.2011;37:1503-5). In her presentation, Dr. Redbord credited Dr. Yag-Howard and her article. However, this credit and attribution to Dr. Yag-Howard was not included in the article published.
WASHINGTON – The pulley stitch "is my go-to stitch for defects under tension," said Dr. Kelley Pagliai Redbord.
The pulley stitch allows for considerable reduction in the surface area of a large defect that can’t be closed by side-to-side stitches alone, making it an excellent choice for use on the scalp and legs, Dr. Redbord said at the Atlantic Dermatological Conference.
"When the tension across the wound is decreased, buried dermal sutures can be placed more easily and accurately," she said. "I use it a lot as an intraoperative tissue expander."
Dr. Redbord said that her description of the pulley stitch was taken from an article by Dr. Cyndi Yag-Howard in Dermatologic Surgery (Dermatol. Surg. 2011; 37:1503-5).*
The pulley stitch can serve as a temporary suture that can be left in place or removed, said Dr. Redbord, a dermatologist in group practice in Rockville, Md.
The technique follows a far-near-near-far pattern, starting the stitch 8 mm from the wound edge (far), then bringing it to the opposite side just 4 mm from the wound edge (near). Dr. Redbord then reenters the stitch 4 mm from the wound edge on the initial side (near), and makes another pass to the opposite side 8 mm from the wound edge (far).
Multiple passes through the tissue create resistance that keeps the suture from slipping. "The loops of the stitch are placed at an oblique angle so that the inner and outer loops are offset and do not override each other," she noted. This technique minimizes potential skin damage from pressure necrosis caused by overriding loop sutures. The pulley stitch has a 2:1 mechanical advantage over an interrupted suture, and the additional friction of a second loop prevents the knot from slipping.
A modification of the pulley stitch is to loop the suture through an external loop on the opposite side of the incision, and pull across. "This new loop functions as a pulley and directs the tension away from the other strands," she said.
Another stitch with excellent eversion, in which the pulley stitch plays a key role, is the subcutaneous inverted cross mattress stitch (SICM). The SCIM is entirely subcutaneous, and combines the buried vertical mattress stitch and the buried pulley stitch.
The SCIM "uses the buried vertical mattress’s ability to evert wound edges and combines it with the pulley stitch’s ability to decrease tension at the wound edge," she said.
The four-step process is as follows:
• 1. Insert the needle into the dermis 3-5 mm lateral to the wound edge. Advance the needle into the upper reticular dermis, and then curve down to exit through the lower reticular dermis.
• 2. Insert the needle into the opposite edge of the wound at the lower reticular dermis and advance into the upper reticular dermis, then curve down and exit intradermally.
• 3. Insert the needle across the defect using an intradermal approach 1-2 mm lateral to the initial needle insertion point. Then, create a second buried vertical mattress stitch.
• 4. Pull the two stitches to close, which "creates a pulley effect with minimal recoil, and tie off," Dr. Redbord said.
"The pulley system locks the wound edges so that a knot can be tied without slipping," she added.
Dr. Redbord said she had no relevant financial disclosures.
EXPERT ANALYSIS FROM THE ATLANTIC DERMATOLOGICAL CONFERENCE
Major finding: Key numerical finding (e.g., number needed to treat to prevent one death/event; number lived or died as result of intervention). Maximum 10 words/1 sentence.
Data source: Include type of study (e.g., randomized, placebo controlled trial; retrospective case-control study). Include number in the study.
Disclosures: Sponsor of study, funding source, relevant disclosures. If author has no relevant disclosures, "Dr. X reported having no financial disclosures." If necessary, "Meeting Y did not require reports of financial disclosures." Check meeting website because many list disclosures. Written in sentence form.
The diabetic foot: Intervene for vascular disease
MIAMI BEACH – The extent of vascular disease, not the presence of diabetes or the cause of the ulceration, should drive the decision to intervene in a patient with a "diabetic foot," according to Dr. Joshua Beckman.
"When do I intervene on a diabetic foot? When I intervene on all feet: when there’s inadequate blood flow to heal the lesion," he said at the International Symposium on Endovascular Therapy 2013.
When lesions are detected in diabetic patients with PAD, assess for ankle pressures and determine ankle brachial index regardless of whether the lesion is neuropathic, he advised, explaining that, "even if you have a neuropathic initiation for your ulcer, if you have a low perfusion pressure, particularly under 50 mmHg, you’re not going to heal that lesion no matter how it was started."
"I always assess patients, whether I think the lesion is a neuropathic one or not, for the ankle pressures and do ankle brachial index and, assuming it’s low, I refer for revascularization based on the same criteria I would everybody else," said Dr. Beckman of Harvard Medical School, Boston.
That said, up to one-third of patients with diabetes have peripheral artery disease, and its presence is an independent predictor of adverse outcomes. "In fact, the combination of PAD and diabetes represents the group of people in the United States that has the most amputations, and their risk may be 20-fold that of people without both PAD and diabetes," he noted.
Indeed, the risk of amputation is much greater in diabetic patients, regardless of the care they receive, Dr. Beckman said, citing a study of 136 lower-extremity angiograms in patients with PAD. The odds ratios for amputation and death at 4.5 years’ follow-up were 5.4 for those with diabetes and 3.1 for those without diabetes (Diabetes Care 2001;24:1433-7).
Additionally, in a study of 1,244 men with claudication, the risk of ischemic ulceration was 4-fold higher in diabetic patients than in nondiabetic patients. Ankle brachial indices ranged from 0.9 to 0.1, and there was no point at which a diabetic patient was not worse off than a nondiabetic patient, he said. The risk in a diabetic patient with an ankle brachial index of 0.9 was equal to the risk in a nondiabetic patient with an ankle brachial index of 0.1 (J. Vasc. Surg. 2001;34:962-70).
Diabetic patients also can have a number of other initiators of diabetic foot ulceration, including neuropathy, bone deformity, callus, penetrating injury, and ill-fitting shoes. In combination, these factors can be especially problematic, he said.
Also, the extensor muscle abnormality that can result from motor neuropathy can change foot morphology, thereby increasing pressure on areas such as the ball of the foot, where ulcers are common.
Furthermore, a patient with neuropathy can walk around for a long time without recognizing a problem that an individual with normal nerve function wouldn’t tolerate. "A diabetic patient can have a bad shoe that rubs all day and not appreciate it," Dr. Beckman said.
With PAD, there are a "whole host of issues we never deal with in the nondiabetic patient that really accelerate the pathway toward ischemic ulceration and critical limb ischemia," he said. "Diabetes is a risk multiplier. It makes things worse, and it’s far more involved in the initiation [of ulceration] than in the decision whether to fix somebody."
Further underscoring the importance of such follow-up is a study of 533 diabetic patients hospitalized for critical limb ischemia. Nearly 50% of them developed critical limb ischemia in the contralateral limb at 6-year follow-up (Diabetes Res. Clin. Prac. 2007;77:445-50).
"So this is a group of people who need not only exquisite care, but exquisite surveillance, because it is very likely that, over time, they will come back to your office with the same problem in the other foot. I’m not recommending intervention or surgery in the asymptomatic foot, but I am suggesting you should probably never let them go, and have routine follow-ups where you look at their feet every time," he said.
Dr. Beckman reported having no disclosures relevant to his talk.
MIAMI BEACH – The extent of vascular disease, not the presence of diabetes or the cause of the ulceration, should drive the decision to intervene in a patient with a "diabetic foot," according to Dr. Joshua Beckman.
"When do I intervene on a diabetic foot? When I intervene on all feet: when there’s inadequate blood flow to heal the lesion," he said at the International Symposium on Endovascular Therapy 2013.
When lesions are detected in diabetic patients with PAD, assess for ankle pressures and determine ankle brachial index regardless of whether the lesion is neuropathic, he advised, explaining that, "even if you have a neuropathic initiation for your ulcer, if you have a low perfusion pressure, particularly under 50 mmHg, you’re not going to heal that lesion no matter how it was started."
"I always assess patients, whether I think the lesion is a neuropathic one or not, for the ankle pressures and do ankle brachial index and, assuming it’s low, I refer for revascularization based on the same criteria I would everybody else," said Dr. Beckman of Harvard Medical School, Boston.
That said, up to one-third of patients with diabetes have peripheral artery disease, and its presence is an independent predictor of adverse outcomes. "In fact, the combination of PAD and diabetes represents the group of people in the United States that has the most amputations, and their risk may be 20-fold that of people without both PAD and diabetes," he noted.
Indeed, the risk of amputation is much greater in diabetic patients, regardless of the care they receive, Dr. Beckman said, citing a study of 136 lower-extremity angiograms in patients with PAD. The odds ratios for amputation and death at 4.5 years’ follow-up were 5.4 for those with diabetes and 3.1 for those without diabetes (Diabetes Care 2001;24:1433-7).
Additionally, in a study of 1,244 men with claudication, the risk of ischemic ulceration was 4-fold higher in diabetic patients than in nondiabetic patients. Ankle brachial indices ranged from 0.9 to 0.1, and there was no point at which a diabetic patient was not worse off than a nondiabetic patient, he said. The risk in a diabetic patient with an ankle brachial index of 0.9 was equal to the risk in a nondiabetic patient with an ankle brachial index of 0.1 (J. Vasc. Surg. 2001;34:962-70).
Diabetic patients also can have a number of other initiators of diabetic foot ulceration, including neuropathy, bone deformity, callus, penetrating injury, and ill-fitting shoes. In combination, these factors can be especially problematic, he said.
Also, the extensor muscle abnormality that can result from motor neuropathy can change foot morphology, thereby increasing pressure on areas such as the ball of the foot, where ulcers are common.
Furthermore, a patient with neuropathy can walk around for a long time without recognizing a problem that an individual with normal nerve function wouldn’t tolerate. "A diabetic patient can have a bad shoe that rubs all day and not appreciate it," Dr. Beckman said.
With PAD, there are a "whole host of issues we never deal with in the nondiabetic patient that really accelerate the pathway toward ischemic ulceration and critical limb ischemia," he said. "Diabetes is a risk multiplier. It makes things worse, and it’s far more involved in the initiation [of ulceration] than in the decision whether to fix somebody."
Further underscoring the importance of such follow-up is a study of 533 diabetic patients hospitalized for critical limb ischemia. Nearly 50% of them developed critical limb ischemia in the contralateral limb at 6-year follow-up (Diabetes Res. Clin. Prac. 2007;77:445-50).
"So this is a group of people who need not only exquisite care, but exquisite surveillance, because it is very likely that, over time, they will come back to your office with the same problem in the other foot. I’m not recommending intervention or surgery in the asymptomatic foot, but I am suggesting you should probably never let them go, and have routine follow-ups where you look at their feet every time," he said.
Dr. Beckman reported having no disclosures relevant to his talk.
MIAMI BEACH – The extent of vascular disease, not the presence of diabetes or the cause of the ulceration, should drive the decision to intervene in a patient with a "diabetic foot," according to Dr. Joshua Beckman.
"When do I intervene on a diabetic foot? When I intervene on all feet: when there’s inadequate blood flow to heal the lesion," he said at the International Symposium on Endovascular Therapy 2013.
When lesions are detected in diabetic patients with PAD, assess for ankle pressures and determine ankle brachial index regardless of whether the lesion is neuropathic, he advised, explaining that, "even if you have a neuropathic initiation for your ulcer, if you have a low perfusion pressure, particularly under 50 mmHg, you’re not going to heal that lesion no matter how it was started."
"I always assess patients, whether I think the lesion is a neuropathic one or not, for the ankle pressures and do ankle brachial index and, assuming it’s low, I refer for revascularization based on the same criteria I would everybody else," said Dr. Beckman of Harvard Medical School, Boston.
That said, up to one-third of patients with diabetes have peripheral artery disease, and its presence is an independent predictor of adverse outcomes. "In fact, the combination of PAD and diabetes represents the group of people in the United States that has the most amputations, and their risk may be 20-fold that of people without both PAD and diabetes," he noted.
Indeed, the risk of amputation is much greater in diabetic patients, regardless of the care they receive, Dr. Beckman said, citing a study of 136 lower-extremity angiograms in patients with PAD. The odds ratios for amputation and death at 4.5 years’ follow-up were 5.4 for those with diabetes and 3.1 for those without diabetes (Diabetes Care 2001;24:1433-7).
Additionally, in a study of 1,244 men with claudication, the risk of ischemic ulceration was 4-fold higher in diabetic patients than in nondiabetic patients. Ankle brachial indices ranged from 0.9 to 0.1, and there was no point at which a diabetic patient was not worse off than a nondiabetic patient, he said. The risk in a diabetic patient with an ankle brachial index of 0.9 was equal to the risk in a nondiabetic patient with an ankle brachial index of 0.1 (J. Vasc. Surg. 2001;34:962-70).
Diabetic patients also can have a number of other initiators of diabetic foot ulceration, including neuropathy, bone deformity, callus, penetrating injury, and ill-fitting shoes. In combination, these factors can be especially problematic, he said.
Also, the extensor muscle abnormality that can result from motor neuropathy can change foot morphology, thereby increasing pressure on areas such as the ball of the foot, where ulcers are common.
Furthermore, a patient with neuropathy can walk around for a long time without recognizing a problem that an individual with normal nerve function wouldn’t tolerate. "A diabetic patient can have a bad shoe that rubs all day and not appreciate it," Dr. Beckman said.
With PAD, there are a "whole host of issues we never deal with in the nondiabetic patient that really accelerate the pathway toward ischemic ulceration and critical limb ischemia," he said. "Diabetes is a risk multiplier. It makes things worse, and it’s far more involved in the initiation [of ulceration] than in the decision whether to fix somebody."
Further underscoring the importance of such follow-up is a study of 533 diabetic patients hospitalized for critical limb ischemia. Nearly 50% of them developed critical limb ischemia in the contralateral limb at 6-year follow-up (Diabetes Res. Clin. Prac. 2007;77:445-50).
"So this is a group of people who need not only exquisite care, but exquisite surveillance, because it is very likely that, over time, they will come back to your office with the same problem in the other foot. I’m not recommending intervention or surgery in the asymptomatic foot, but I am suggesting you should probably never let them go, and have routine follow-ups where you look at their feet every time," he said.
Dr. Beckman reported having no disclosures relevant to his talk.
EXPERT ANALYSIS FROM ISET 2013
Hypercoagulability occurred despite VTE prophylaxis in burn patients
SCOTTSDALE, ARIZ. – Despite thromboprophylaxis, burn patients become hypercoagulable during recovery, putting them at increased risk of venous thromboembolism, a small, prospective study has shown.
"The hypercoagulable state is likely multifactorial, and we believe that additional prophylaxis and monitoring may be needed," Dr. Robert Van Haren said at the annual scientific assembly of the Eastern Association for the Surgery of Trauma.
Hypercoagulability has long been known to contribute to venous thromboembolism (VTE), but it’s only more recently that the increased incidence of thromboembolic complications in burn patients has been appreciated.
A study using duplex ultrasound screening reported that 23% of burn patients developed deep venous thrombosis at an average of 6.7 days after admission (J. Burn Care Rehabil. 2002;23:439-43).
More recent work reports that such in-hospital risk factors as number of operations, pneumonia, and central venous access are significantly associated with VTE after thermal injury (J. Burn Care Res. 2012;33:84-8).
Dr. Van Haren and his colleagues at the University of Miami used thromboelastography (TEG) and coagulation tests to analyze blood samples drawn from indwelling catheters upon admission and at 1 week in 24 patients. All patients were placed on unfractionated or low-molecular-weight heparin at admission.
Their median age was 49 years, 88% were male, and the median total body surface area burned was 29%. Inhalation injuries also were present in 17%.
TEG values were within normal limits at admission for clotting time (R = 11.5 minutes), initial clot formation (K = 2.8 minutes), clot kinetics (alpha angle = 54.6 degrees), and clot strength (maximum amplitude = 62.5 mm).
Repeat TEG at 1 week in 16 patients who remained hospitalized revealed significantly decreased R (8.3 minutes) and K (2.0 minutes) times, and elevated alpha angle (65.5 degrees) and maximum amplitude (73.1 mm; all statistically significant, P less than .05).
"All of these changes demonstrate that these patients became more hypercoagulable at week 1," said Dr. Van Haren, a 4th-year general surgery resident.
Coagulation tests were generally supportive of TEG findings. From admission to week 1, significant decreases were observed in median prothrombin time (17.4 seconds vs. 15.7 seconds; P = .013) and international normalized ratio (1.47 vs. 1.28; P = .013). At the same time, significant elevations occurred in protein C activity (75% vs. 93%; P = .017), protein S activity (69% vs. 76%; P = .030), antithrombin III (62% vs. 88%; P = .005), and fibrinogen (524 mg/dL vs. 676 mg/dL; P = .047).
The changes suggest a procoagulant state, and cannot be attributed to hemoconcentration, as fluid balance was more positive and hematocrit was lower on repeat samples, Dr. Van Haren reported.
The only in-hospital risk factor significant for hypercoagulability at 1 week was a pre-TEG operation, he said. Men, however, were more likely to be hypercoagulable than women.
Two patients, both male, developed a deep venous thrombosis. Contrary to previous studies, the only predictive markers of VTE were decreased partial thromboplastin time and fibrinogen and elevated prothrombin fragment 1 + 2.
During the discussion period after Dr. Van Haren’s presentation, audience members asked why hypercoagulability was evaluated at 1 week, since hypercoagulability has been shown to develop quite rapidly in trauma patients. Other questions addressed whether clinicians should use the data to increase thromboprophylaxis or begin dosing based on body mass index in burn patients.
Dr. Van Haren said the 1-week time point was somewhat arbitrary, and plans to look at earlier time periods in another cohort to determine exactly when the transition to hypercoagulability occurs. "I think the most interesting thing would be to see if you can guide your thromboprophylaxis based on TEG, and use it to titrate your dose to see if it will result in decreases in VTE rates."
The study was funded in part by grants from the Office of Naval Research and U.S. Army. Dr. Van Haren and his coauthors reported no relevant financial conflicts.
SCOTTSDALE, ARIZ. – Despite thromboprophylaxis, burn patients become hypercoagulable during recovery, putting them at increased risk of venous thromboembolism, a small, prospective study has shown.
"The hypercoagulable state is likely multifactorial, and we believe that additional prophylaxis and monitoring may be needed," Dr. Robert Van Haren said at the annual scientific assembly of the Eastern Association for the Surgery of Trauma.
Hypercoagulability has long been known to contribute to venous thromboembolism (VTE), but it’s only more recently that the increased incidence of thromboembolic complications in burn patients has been appreciated.
A study using duplex ultrasound screening reported that 23% of burn patients developed deep venous thrombosis at an average of 6.7 days after admission (J. Burn Care Rehabil. 2002;23:439-43).
More recent work reports that such in-hospital risk factors as number of operations, pneumonia, and central venous access are significantly associated with VTE after thermal injury (J. Burn Care Res. 2012;33:84-8).
Dr. Van Haren and his colleagues at the University of Miami used thromboelastography (TEG) and coagulation tests to analyze blood samples drawn from indwelling catheters upon admission and at 1 week in 24 patients. All patients were placed on unfractionated or low-molecular-weight heparin at admission.
Their median age was 49 years, 88% were male, and the median total body surface area burned was 29%. Inhalation injuries also were present in 17%.
TEG values were within normal limits at admission for clotting time (R = 11.5 minutes), initial clot formation (K = 2.8 minutes), clot kinetics (alpha angle = 54.6 degrees), and clot strength (maximum amplitude = 62.5 mm).
Repeat TEG at 1 week in 16 patients who remained hospitalized revealed significantly decreased R (8.3 minutes) and K (2.0 minutes) times, and elevated alpha angle (65.5 degrees) and maximum amplitude (73.1 mm; all statistically significant, P less than .05).
"All of these changes demonstrate that these patients became more hypercoagulable at week 1," said Dr. Van Haren, a 4th-year general surgery resident.
Coagulation tests were generally supportive of TEG findings. From admission to week 1, significant decreases were observed in median prothrombin time (17.4 seconds vs. 15.7 seconds; P = .013) and international normalized ratio (1.47 vs. 1.28; P = .013). At the same time, significant elevations occurred in protein C activity (75% vs. 93%; P = .017), protein S activity (69% vs. 76%; P = .030), antithrombin III (62% vs. 88%; P = .005), and fibrinogen (524 mg/dL vs. 676 mg/dL; P = .047).
The changes suggest a procoagulant state, and cannot be attributed to hemoconcentration, as fluid balance was more positive and hematocrit was lower on repeat samples, Dr. Van Haren reported.
The only in-hospital risk factor significant for hypercoagulability at 1 week was a pre-TEG operation, he said. Men, however, were more likely to be hypercoagulable than women.
Two patients, both male, developed a deep venous thrombosis. Contrary to previous studies, the only predictive markers of VTE were decreased partial thromboplastin time and fibrinogen and elevated prothrombin fragment 1 + 2.
During the discussion period after Dr. Van Haren’s presentation, audience members asked why hypercoagulability was evaluated at 1 week, since hypercoagulability has been shown to develop quite rapidly in trauma patients. Other questions addressed whether clinicians should use the data to increase thromboprophylaxis or begin dosing based on body mass index in burn patients.
Dr. Van Haren said the 1-week time point was somewhat arbitrary, and plans to look at earlier time periods in another cohort to determine exactly when the transition to hypercoagulability occurs. "I think the most interesting thing would be to see if you can guide your thromboprophylaxis based on TEG, and use it to titrate your dose to see if it will result in decreases in VTE rates."
The study was funded in part by grants from the Office of Naval Research and U.S. Army. Dr. Van Haren and his coauthors reported no relevant financial conflicts.
SCOTTSDALE, ARIZ. – Despite thromboprophylaxis, burn patients become hypercoagulable during recovery, putting them at increased risk of venous thromboembolism, a small, prospective study has shown.
"The hypercoagulable state is likely multifactorial, and we believe that additional prophylaxis and monitoring may be needed," Dr. Robert Van Haren said at the annual scientific assembly of the Eastern Association for the Surgery of Trauma.
Hypercoagulability has long been known to contribute to venous thromboembolism (VTE), but it’s only more recently that the increased incidence of thromboembolic complications in burn patients has been appreciated.
A study using duplex ultrasound screening reported that 23% of burn patients developed deep venous thrombosis at an average of 6.7 days after admission (J. Burn Care Rehabil. 2002;23:439-43).
More recent work reports that such in-hospital risk factors as number of operations, pneumonia, and central venous access are significantly associated with VTE after thermal injury (J. Burn Care Res. 2012;33:84-8).
Dr. Van Haren and his colleagues at the University of Miami used thromboelastography (TEG) and coagulation tests to analyze blood samples drawn from indwelling catheters upon admission and at 1 week in 24 patients. All patients were placed on unfractionated or low-molecular-weight heparin at admission.
Their median age was 49 years, 88% were male, and the median total body surface area burned was 29%. Inhalation injuries also were present in 17%.
TEG values were within normal limits at admission for clotting time (R = 11.5 minutes), initial clot formation (K = 2.8 minutes), clot kinetics (alpha angle = 54.6 degrees), and clot strength (maximum amplitude = 62.5 mm).
Repeat TEG at 1 week in 16 patients who remained hospitalized revealed significantly decreased R (8.3 minutes) and K (2.0 minutes) times, and elevated alpha angle (65.5 degrees) and maximum amplitude (73.1 mm; all statistically significant, P less than .05).
"All of these changes demonstrate that these patients became more hypercoagulable at week 1," said Dr. Van Haren, a 4th-year general surgery resident.
Coagulation tests were generally supportive of TEG findings. From admission to week 1, significant decreases were observed in median prothrombin time (17.4 seconds vs. 15.7 seconds; P = .013) and international normalized ratio (1.47 vs. 1.28; P = .013). At the same time, significant elevations occurred in protein C activity (75% vs. 93%; P = .017), protein S activity (69% vs. 76%; P = .030), antithrombin III (62% vs. 88%; P = .005), and fibrinogen (524 mg/dL vs. 676 mg/dL; P = .047).
The changes suggest a procoagulant state, and cannot be attributed to hemoconcentration, as fluid balance was more positive and hematocrit was lower on repeat samples, Dr. Van Haren reported.
The only in-hospital risk factor significant for hypercoagulability at 1 week was a pre-TEG operation, he said. Men, however, were more likely to be hypercoagulable than women.
Two patients, both male, developed a deep venous thrombosis. Contrary to previous studies, the only predictive markers of VTE were decreased partial thromboplastin time and fibrinogen and elevated prothrombin fragment 1 + 2.
During the discussion period after Dr. Van Haren’s presentation, audience members asked why hypercoagulability was evaluated at 1 week, since hypercoagulability has been shown to develop quite rapidly in trauma patients. Other questions addressed whether clinicians should use the data to increase thromboprophylaxis or begin dosing based on body mass index in burn patients.
Dr. Van Haren said the 1-week time point was somewhat arbitrary, and plans to look at earlier time periods in another cohort to determine exactly when the transition to hypercoagulability occurs. "I think the most interesting thing would be to see if you can guide your thromboprophylaxis based on TEG, and use it to titrate your dose to see if it will result in decreases in VTE rates."
The study was funded in part by grants from the Office of Naval Research and U.S. Army. Dr. Van Haren and his coauthors reported no relevant financial conflicts.
AT THE EAST SCIENTIFIC ASSEMBLY
Major Finding: Repeat TEG at 1 week in 16 hospitalized burn patients revealed significantly decreased clotting (8.3 minutes) and initial clot formation (2.0 minutes) times, and elevated alpha angle (65.5 degrees) and maximum amplitude (73.1 mm; all statistically significant, P less than .05).
Data Source: A prospective study of 24 patients with thermal injuries.
Disclosures: The study was funded in part by grants from the Office of Naval Research and U.S. Army. Dr. Van Haren and his coauthors reported no relevant financial conflicts.
Dermatologic surgery checklist improves patient safety
Office-based dermatologic surgery should always start with a detailed surgery checklist that covers everything a physician needs to ensure a seamless procedure, according to Dr. Roger I. Ceilley.
The focus is on documentation and, ultimately, safety. "Document, document, document," he said during a talk at the Hawaii Dermatology Seminar sponsored by Global Academy for Medical Education/Skin Disease Education Foundation.
A sample checklist that he shared included the following items:
• Referring physician.
• Who did the biopsy?
• Sign consent.
• Circle surgery site with patient verification.
• Verify and record pacemaker/defibrillator or other electronic implants.
• Review allergies to any anesthesia/antibiotics/latex/bandages.
• Check and record anticoagulants.
• Prophylactic antibiotics needed? If so, why?
• Record blood pressure and pulse – notify provider if elevated or too low.
• Check for special health concerns such as diabetes, etc.
• Buffered and unbuffered anesthesia on the field.
• Mapping card.
• Verify pathology report.
• Photo.
• Miscellaneous patient concerns.
Using such a checklist will ensure that all aspects of the pending procedure have been documented and discussed with the patient and also that the clinical assistant and physician are "on the same page" as to what will be happening before the procedure begins, said Dr. Ceilley of the University of Iowa, Iowa City.
Preoperative photography is a particularly important item on this list, as it can help to prevent wrong-site surgery, he noted, adding that it is also imperative to site confirm with the patient (one of the steps on the checklist) prior to beginning the procedure.
This information should be readily available because of the proper prior documentation. An accurate diagram and measurement also will help, he said.
Dr. Ceilley covered several other topics:
• Surgical equipment needs. A properly preselected, prewrapped, autoclaved pack of surgical instruments is a necessity; it should include a curette, forceps, scalpel blade holder, needle driver, hemostat, iris scissors, and straight scissor, he said.
The surgical tray also should include readily available gauze, cotton swabs, and extra anesthesia, and the equipment should be arranged on a tray in a standard fashion and kept organized during the procedure, with the sharps placed consistently in the same area on the tray.
• Local anesthesia. Dr. Ceilley described alternatives, including diphenhydramine and bacteriostatic saline, for the very rare patient with anesthesia allergies and provided a number of pearls for using local anesthesia. He discussed the use of topical versus subcutaneous lidocaine, the use of ice or alternate refrigerants, and the benefits of rubbing the area after infiltration.
He also noted that a number of other nonpharmacologic measures – including "talk-esthesia" (use of conversation to keep the patient’s mind busy and distracted from some of the more invasive aspects of the procedure), ice packs, accupressure, headphones, and even stuffed animals – that can provide pain relief or comfort for surgical patients.
• Sutureless closures. Among the options for sutureless closures are staples, steri-strips/paper tape, and Dermabond or other tissue glues. Tapes and glues are best for low-tension wounds, he noted.
• Closing tight wound defects. Pinching and stretching the wound can help with closure of tight wounds, as can the use of antitension clamps, he said, adding that a temporary horizontal mattress or pulley stitch also may help stretch tissue and facilitate closure.
• Hemostasis. Stretching, pinching, and applying ring pressure can help with hemostasis, he said.
• Surgical wound dressing. Keep a dressing tray handy and "do them with pride," Dr. Ceilley said of wound dressings.
"You will want to have on-hand items that you have learned are the most useful for wound dressing. These should include tan/skin-colored tapes, mupirocin or petrolatum, Coban (3M), Hypafix, and steri-strips, to name a few. Only use the minimum necessary for proper dressing of any item to ensure the least visibly noticeable appearance for your patient," he said.
Also, provide patients with supplies for dressing changes if possible, or with information on where to obtain the appropriate supplies, and include detailed wound care instructions, he said.
• Postoperative care. In addition to information on wound care and dressing, also provide patients with handouts on various aspects of postoperative care, including information on resuming activities and warnings about swelling, hematoma, drainage, and infection, he advised.
"I can’t overemphasize the importance of attention to detail in all aspects of dermatologic surgery, from evaluation and explanation to procedure, dressing, and postoperative care. The final results bear your signature and enhance or detract from your reputation and that of all dermatologic surgeons," he said.
Dr. Ceilley reported having no relevant disclosures. SDEF and this news organization are owned by the same parent company.
*This story was updated March 1, 2013.
Office-based dermatologic surgery should always start with a detailed surgery checklist that covers everything a physician needs to ensure a seamless procedure, according to Dr. Roger I. Ceilley.
The focus is on documentation and, ultimately, safety. "Document, document, document," he said during a talk at the Hawaii Dermatology Seminar sponsored by Global Academy for Medical Education/Skin Disease Education Foundation.
A sample checklist that he shared included the following items:
• Referring physician.
• Who did the biopsy?
• Sign consent.
• Circle surgery site with patient verification.
• Verify and record pacemaker/defibrillator or other electronic implants.
• Review allergies to any anesthesia/antibiotics/latex/bandages.
• Check and record anticoagulants.
• Prophylactic antibiotics needed? If so, why?
• Record blood pressure and pulse – notify provider if elevated or too low.
• Check for special health concerns such as diabetes, etc.
• Buffered and unbuffered anesthesia on the field.
• Mapping card.
• Verify pathology report.
• Photo.
• Miscellaneous patient concerns.
Using such a checklist will ensure that all aspects of the pending procedure have been documented and discussed with the patient and also that the clinical assistant and physician are "on the same page" as to what will be happening before the procedure begins, said Dr. Ceilley of the University of Iowa, Iowa City.
Preoperative photography is a particularly important item on this list, as it can help to prevent wrong-site surgery, he noted, adding that it is also imperative to site confirm with the patient (one of the steps on the checklist) prior to beginning the procedure.
This information should be readily available because of the proper prior documentation. An accurate diagram and measurement also will help, he said.
Dr. Ceilley covered several other topics:
• Surgical equipment needs. A properly preselected, prewrapped, autoclaved pack of surgical instruments is a necessity; it should include a curette, forceps, scalpel blade holder, needle driver, hemostat, iris scissors, and straight scissor, he said.
The surgical tray also should include readily available gauze, cotton swabs, and extra anesthesia, and the equipment should be arranged on a tray in a standard fashion and kept organized during the procedure, with the sharps placed consistently in the same area on the tray.
• Local anesthesia. Dr. Ceilley described alternatives, including diphenhydramine and bacteriostatic saline, for the very rare patient with anesthesia allergies and provided a number of pearls for using local anesthesia. He discussed the use of topical versus subcutaneous lidocaine, the use of ice or alternate refrigerants, and the benefits of rubbing the area after infiltration.
He also noted that a number of other nonpharmacologic measures – including "talk-esthesia" (use of conversation to keep the patient’s mind busy and distracted from some of the more invasive aspects of the procedure), ice packs, accupressure, headphones, and even stuffed animals – that can provide pain relief or comfort for surgical patients.
• Sutureless closures. Among the options for sutureless closures are staples, steri-strips/paper tape, and Dermabond or other tissue glues. Tapes and glues are best for low-tension wounds, he noted.
• Closing tight wound defects. Pinching and stretching the wound can help with closure of tight wounds, as can the use of antitension clamps, he said, adding that a temporary horizontal mattress or pulley stitch also may help stretch tissue and facilitate closure.
• Hemostasis. Stretching, pinching, and applying ring pressure can help with hemostasis, he said.
• Surgical wound dressing. Keep a dressing tray handy and "do them with pride," Dr. Ceilley said of wound dressings.
"You will want to have on-hand items that you have learned are the most useful for wound dressing. These should include tan/skin-colored tapes, mupirocin or petrolatum, Coban (3M), Hypafix, and steri-strips, to name a few. Only use the minimum necessary for proper dressing of any item to ensure the least visibly noticeable appearance for your patient," he said.
Also, provide patients with supplies for dressing changes if possible, or with information on where to obtain the appropriate supplies, and include detailed wound care instructions, he said.
• Postoperative care. In addition to information on wound care and dressing, also provide patients with handouts on various aspects of postoperative care, including information on resuming activities and warnings about swelling, hematoma, drainage, and infection, he advised.
"I can’t overemphasize the importance of attention to detail in all aspects of dermatologic surgery, from evaluation and explanation to procedure, dressing, and postoperative care. The final results bear your signature and enhance or detract from your reputation and that of all dermatologic surgeons," he said.
Dr. Ceilley reported having no relevant disclosures. SDEF and this news organization are owned by the same parent company.
*This story was updated March 1, 2013.
Office-based dermatologic surgery should always start with a detailed surgery checklist that covers everything a physician needs to ensure a seamless procedure, according to Dr. Roger I. Ceilley.
The focus is on documentation and, ultimately, safety. "Document, document, document," he said during a talk at the Hawaii Dermatology Seminar sponsored by Global Academy for Medical Education/Skin Disease Education Foundation.
A sample checklist that he shared included the following items:
• Referring physician.
• Who did the biopsy?
• Sign consent.
• Circle surgery site with patient verification.
• Verify and record pacemaker/defibrillator or other electronic implants.
• Review allergies to any anesthesia/antibiotics/latex/bandages.
• Check and record anticoagulants.
• Prophylactic antibiotics needed? If so, why?
• Record blood pressure and pulse – notify provider if elevated or too low.
• Check for special health concerns such as diabetes, etc.
• Buffered and unbuffered anesthesia on the field.
• Mapping card.
• Verify pathology report.
• Photo.
• Miscellaneous patient concerns.
Using such a checklist will ensure that all aspects of the pending procedure have been documented and discussed with the patient and also that the clinical assistant and physician are "on the same page" as to what will be happening before the procedure begins, said Dr. Ceilley of the University of Iowa, Iowa City.
Preoperative photography is a particularly important item on this list, as it can help to prevent wrong-site surgery, he noted, adding that it is also imperative to site confirm with the patient (one of the steps on the checklist) prior to beginning the procedure.
This information should be readily available because of the proper prior documentation. An accurate diagram and measurement also will help, he said.
Dr. Ceilley covered several other topics:
• Surgical equipment needs. A properly preselected, prewrapped, autoclaved pack of surgical instruments is a necessity; it should include a curette, forceps, scalpel blade holder, needle driver, hemostat, iris scissors, and straight scissor, he said.
The surgical tray also should include readily available gauze, cotton swabs, and extra anesthesia, and the equipment should be arranged on a tray in a standard fashion and kept organized during the procedure, with the sharps placed consistently in the same area on the tray.
• Local anesthesia. Dr. Ceilley described alternatives, including diphenhydramine and bacteriostatic saline, for the very rare patient with anesthesia allergies and provided a number of pearls for using local anesthesia. He discussed the use of topical versus subcutaneous lidocaine, the use of ice or alternate refrigerants, and the benefits of rubbing the area after infiltration.
He also noted that a number of other nonpharmacologic measures – including "talk-esthesia" (use of conversation to keep the patient’s mind busy and distracted from some of the more invasive aspects of the procedure), ice packs, accupressure, headphones, and even stuffed animals – that can provide pain relief or comfort for surgical patients.
• Sutureless closures. Among the options for sutureless closures are staples, steri-strips/paper tape, and Dermabond or other tissue glues. Tapes and glues are best for low-tension wounds, he noted.
• Closing tight wound defects. Pinching and stretching the wound can help with closure of tight wounds, as can the use of antitension clamps, he said, adding that a temporary horizontal mattress or pulley stitch also may help stretch tissue and facilitate closure.
• Hemostasis. Stretching, pinching, and applying ring pressure can help with hemostasis, he said.
• Surgical wound dressing. Keep a dressing tray handy and "do them with pride," Dr. Ceilley said of wound dressings.
"You will want to have on-hand items that you have learned are the most useful for wound dressing. These should include tan/skin-colored tapes, mupirocin or petrolatum, Coban (3M), Hypafix, and steri-strips, to name a few. Only use the minimum necessary for proper dressing of any item to ensure the least visibly noticeable appearance for your patient," he said.
Also, provide patients with supplies for dressing changes if possible, or with information on where to obtain the appropriate supplies, and include detailed wound care instructions, he said.
• Postoperative care. In addition to information on wound care and dressing, also provide patients with handouts on various aspects of postoperative care, including information on resuming activities and warnings about swelling, hematoma, drainage, and infection, he advised.
"I can’t overemphasize the importance of attention to detail in all aspects of dermatologic surgery, from evaluation and explanation to procedure, dressing, and postoperative care. The final results bear your signature and enhance or detract from your reputation and that of all dermatologic surgeons," he said.
Dr. Ceilley reported having no relevant disclosures. SDEF and this news organization are owned by the same parent company.
*This story was updated March 1, 2013.
EXPERT ANALYSIS FROM THE HAWAII DERMATOLOGY SEMINAR SPONSORED BY SKIN DISEASE EDUCATION FOUNDATION (SDEF)
Livedoid Vasculopathy: Review of Pathogenesis, Clinical Presentation, Diagnostic Workup, and Treatment
Hydrocolloid Outdoes Gauze as Wound Dressing
Wide variation exists in the local treatment of donor site wounds after split-skin grafting, ranging from classic gauze dressings to modern silicone dressings.
In a multicenter randomized trial of 289 patients, hydrocolloid dressings led to a shorter healing time than other commonly used dressing materials for donor site wounds (DSWs) after split-skin grafting. Standard gauze dressings were found to increase the risk of infection, Dr. Fleur E. Brölmann said in presenting the study results at the Veith symposium on vascular medicine sponsored by the Cleveland Clinic.
A 14-center, six-armed, randomized clinical trial (stratified per center) compared six wound dressing materials in adults with DSWs larger than 10 cm2 for any indication. Primary outcomes were complete re-epithelialization and pain using a visual analog scale (VAS; 4 weeks). Secondary outcomes included itching (VAS; 4 weeks), adverse events, and scarring after 12 weeks as measured using the Patient and Observer Scar Assessment Scale (POSAS), said Dr. Brölmann, who is a Ph.D. candidate at the Academic Medical Center in Amsterdam.
Between October 2009 and December 2011, patients were randomized to either alginate (45 patients), film (49), gauze (50), hydrocolloid (47), hydrofiber (47), or silicone (48).
Time to complete re-epithelialization using hydrocolloid dressings (median 16 days) was 7 days shorter than with any other dressing, a significant difference, she said in her presentation titled "How To Treat Donor Site Wounds: An Evidence Based Approach."
Overall pain scores were low and slightly but significantly lower using film dressings. The infection rate among patients treated with gauzes was twice as high as in those receiving other dressings (18% vs. 9%; relative risk, 2.39; 95% confidence interval, 1.14-5.01). Patients receiving films were least satisfied with overall scar quality.
"This trial shows that hydrocolloid dressings led to the shortest healing time of DSWs among the dressings investigated, while gauzes should be avoided due to increased risk of infection," Dr. Brölmann said. Based on these results, hydrocolloid is the first-choice dressing for DSWs, and should be the standard of care, she added.
Wide variation exists in the local treatment of donor site wounds after split-skin grafting, ranging from classic gauze dressings to modern silicone dressings.
In a multicenter randomized trial of 289 patients, hydrocolloid dressings led to a shorter healing time than other commonly used dressing materials for donor site wounds (DSWs) after split-skin grafting. Standard gauze dressings were found to increase the risk of infection, Dr. Fleur E. Brölmann said in presenting the study results at the Veith symposium on vascular medicine sponsored by the Cleveland Clinic.
A 14-center, six-armed, randomized clinical trial (stratified per center) compared six wound dressing materials in adults with DSWs larger than 10 cm2 for any indication. Primary outcomes were complete re-epithelialization and pain using a visual analog scale (VAS; 4 weeks). Secondary outcomes included itching (VAS; 4 weeks), adverse events, and scarring after 12 weeks as measured using the Patient and Observer Scar Assessment Scale (POSAS), said Dr. Brölmann, who is a Ph.D. candidate at the Academic Medical Center in Amsterdam.
Between October 2009 and December 2011, patients were randomized to either alginate (45 patients), film (49), gauze (50), hydrocolloid (47), hydrofiber (47), or silicone (48).
Time to complete re-epithelialization using hydrocolloid dressings (median 16 days) was 7 days shorter than with any other dressing, a significant difference, she said in her presentation titled "How To Treat Donor Site Wounds: An Evidence Based Approach."
Overall pain scores were low and slightly but significantly lower using film dressings. The infection rate among patients treated with gauzes was twice as high as in those receiving other dressings (18% vs. 9%; relative risk, 2.39; 95% confidence interval, 1.14-5.01). Patients receiving films were least satisfied with overall scar quality.
"This trial shows that hydrocolloid dressings led to the shortest healing time of DSWs among the dressings investigated, while gauzes should be avoided due to increased risk of infection," Dr. Brölmann said. Based on these results, hydrocolloid is the first-choice dressing for DSWs, and should be the standard of care, she added.
Wide variation exists in the local treatment of donor site wounds after split-skin grafting, ranging from classic gauze dressings to modern silicone dressings.
In a multicenter randomized trial of 289 patients, hydrocolloid dressings led to a shorter healing time than other commonly used dressing materials for donor site wounds (DSWs) after split-skin grafting. Standard gauze dressings were found to increase the risk of infection, Dr. Fleur E. Brölmann said in presenting the study results at the Veith symposium on vascular medicine sponsored by the Cleveland Clinic.
A 14-center, six-armed, randomized clinical trial (stratified per center) compared six wound dressing materials in adults with DSWs larger than 10 cm2 for any indication. Primary outcomes were complete re-epithelialization and pain using a visual analog scale (VAS; 4 weeks). Secondary outcomes included itching (VAS; 4 weeks), adverse events, and scarring after 12 weeks as measured using the Patient and Observer Scar Assessment Scale (POSAS), said Dr. Brölmann, who is a Ph.D. candidate at the Academic Medical Center in Amsterdam.
Between October 2009 and December 2011, patients were randomized to either alginate (45 patients), film (49), gauze (50), hydrocolloid (47), hydrofiber (47), or silicone (48).
Time to complete re-epithelialization using hydrocolloid dressings (median 16 days) was 7 days shorter than with any other dressing, a significant difference, she said in her presentation titled "How To Treat Donor Site Wounds: An Evidence Based Approach."
Overall pain scores were low and slightly but significantly lower using film dressings. The infection rate among patients treated with gauzes was twice as high as in those receiving other dressings (18% vs. 9%; relative risk, 2.39; 95% confidence interval, 1.14-5.01). Patients receiving films were least satisfied with overall scar quality.
"This trial shows that hydrocolloid dressings led to the shortest healing time of DSWs among the dressings investigated, while gauzes should be avoided due to increased risk of infection," Dr. Brölmann said. Based on these results, hydrocolloid is the first-choice dressing for DSWs, and should be the standard of care, she added.
FROM THE VEITH SYMPOSIUM
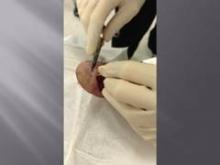
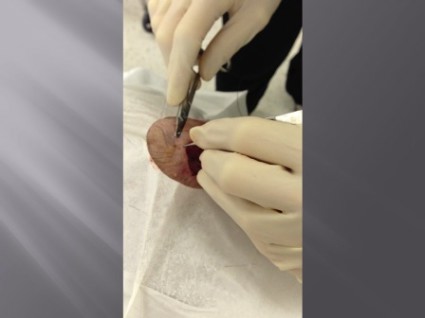
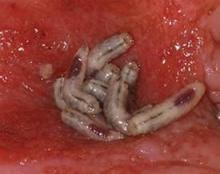
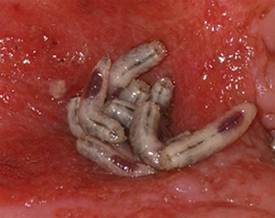
Maggots Prove Wound-Cleaning Worth
PRAGUE – Maggot debridement of wounds proved significantly faster, less painful, and less labor intensive than surgical debridement and conventional dressings in a randomized, multicenter clinical trial.
"There was quite an amazing debridement after 7 days of maggot therapy," Dr. Kristina Opletalova said of the phase III study findings presented at the annual congress of the European Academy of Dermatology and Venereology.
She reported on 119 patients hospitalized for 2 weeks for treatment of nonhealing sloughy wounds, most of which were venous ulcers on the lower limbs. Participants were randomized to maggot therapy or to thrice-weekly surgical debridement with topical anesthesia and conventional dressings.
The maggot therapy was administered via a novel delivery system: 80 maggots of Lucilia sericata were bagged in a special two-layer dressing, known as a Vitapad (BioMonde Laboratories), which allowed the critters to move and feed on the wound surface and kept them from escaping. The dressing was changed twice weekly.
Patients were blindfolded for all dressing changes so they didn’t know which study arm they were in. Wound sloughing was analyzed using computerized planimetry software, and other end points were assessed by an investigator blinded to treatment arm.
After 7 days of therapy, the wound status was significantly better in the maggot debridement group. The mean percentage of slough in wounds was 54.5%, compared with 66.5% in controls, but the significantly faster debridement didn’t boost the final healing rate. The day 15 percentage of slough in the wounds didn’t differ significantly between the two groups: 55.4% with maggot therapy and 53.8% in controls.
"So we think maggot debridement therapy should be stopped after 1 week and other types of dressings should then be used," said Dr. Opletalova, a dermatologist at the University of Caen (France).
Pain scores, which were assessed on a weekly basis, were similarly low in the two groups, but it must be noted that the control group received topical anesthetic and the maggot therapy group did not. Nursing time was four-fold greater in the control group. The study didn’t include a formal cost analysis, but the markedly reduced nursing time in the maggot therapy group is likely to spell cost savings, she said.
Maggot therapy was well tolerated. Patients expressed no reticence about it. A similar number of patients in both study arms reported a crawling sensation on their wounds.
Dr. Opletalova said that maggot therapy is likely to be particularly useful in patients with wounds that need rapid debridement, such as those with diabetes, or to prepare a wound for skin grafting or when there is an increased risk for infection.
Further details of the recently published randomized trial can be found in the Archives of Dermatology (2012;148:432-8).
The study was supported by university hospital research funding and by a grant from the French Society of Dermatology. Dr. Opletalova reported having no financial conflicts.
PRAGUE – Maggot debridement of wounds proved significantly faster, less painful, and less labor intensive than surgical debridement and conventional dressings in a randomized, multicenter clinical trial.
"There was quite an amazing debridement after 7 days of maggot therapy," Dr. Kristina Opletalova said of the phase III study findings presented at the annual congress of the European Academy of Dermatology and Venereology.
She reported on 119 patients hospitalized for 2 weeks for treatment of nonhealing sloughy wounds, most of which were venous ulcers on the lower limbs. Participants were randomized to maggot therapy or to thrice-weekly surgical debridement with topical anesthesia and conventional dressings.
The maggot therapy was administered via a novel delivery system: 80 maggots of Lucilia sericata were bagged in a special two-layer dressing, known as a Vitapad (BioMonde Laboratories), which allowed the critters to move and feed on the wound surface and kept them from escaping. The dressing was changed twice weekly.
Patients were blindfolded for all dressing changes so they didn’t know which study arm they were in. Wound sloughing was analyzed using computerized planimetry software, and other end points were assessed by an investigator blinded to treatment arm.
After 7 days of therapy, the wound status was significantly better in the maggot debridement group. The mean percentage of slough in wounds was 54.5%, compared with 66.5% in controls, but the significantly faster debridement didn’t boost the final healing rate. The day 15 percentage of slough in the wounds didn’t differ significantly between the two groups: 55.4% with maggot therapy and 53.8% in controls.
"So we think maggot debridement therapy should be stopped after 1 week and other types of dressings should then be used," said Dr. Opletalova, a dermatologist at the University of Caen (France).
Pain scores, which were assessed on a weekly basis, were similarly low in the two groups, but it must be noted that the control group received topical anesthetic and the maggot therapy group did not. Nursing time was four-fold greater in the control group. The study didn’t include a formal cost analysis, but the markedly reduced nursing time in the maggot therapy group is likely to spell cost savings, she said.
Maggot therapy was well tolerated. Patients expressed no reticence about it. A similar number of patients in both study arms reported a crawling sensation on their wounds.
Dr. Opletalova said that maggot therapy is likely to be particularly useful in patients with wounds that need rapid debridement, such as those with diabetes, or to prepare a wound for skin grafting or when there is an increased risk for infection.
Further details of the recently published randomized trial can be found in the Archives of Dermatology (2012;148:432-8).
The study was supported by university hospital research funding and by a grant from the French Society of Dermatology. Dr. Opletalova reported having no financial conflicts.
PRAGUE – Maggot debridement of wounds proved significantly faster, less painful, and less labor intensive than surgical debridement and conventional dressings in a randomized, multicenter clinical trial.
"There was quite an amazing debridement after 7 days of maggot therapy," Dr. Kristina Opletalova said of the phase III study findings presented at the annual congress of the European Academy of Dermatology and Venereology.
She reported on 119 patients hospitalized for 2 weeks for treatment of nonhealing sloughy wounds, most of which were venous ulcers on the lower limbs. Participants were randomized to maggot therapy or to thrice-weekly surgical debridement with topical anesthesia and conventional dressings.
The maggot therapy was administered via a novel delivery system: 80 maggots of Lucilia sericata were bagged in a special two-layer dressing, known as a Vitapad (BioMonde Laboratories), which allowed the critters to move and feed on the wound surface and kept them from escaping. The dressing was changed twice weekly.
Patients were blindfolded for all dressing changes so they didn’t know which study arm they were in. Wound sloughing was analyzed using computerized planimetry software, and other end points were assessed by an investigator blinded to treatment arm.
After 7 days of therapy, the wound status was significantly better in the maggot debridement group. The mean percentage of slough in wounds was 54.5%, compared with 66.5% in controls, but the significantly faster debridement didn’t boost the final healing rate. The day 15 percentage of slough in the wounds didn’t differ significantly between the two groups: 55.4% with maggot therapy and 53.8% in controls.
"So we think maggot debridement therapy should be stopped after 1 week and other types of dressings should then be used," said Dr. Opletalova, a dermatologist at the University of Caen (France).
Pain scores, which were assessed on a weekly basis, were similarly low in the two groups, but it must be noted that the control group received topical anesthetic and the maggot therapy group did not. Nursing time was four-fold greater in the control group. The study didn’t include a formal cost analysis, but the markedly reduced nursing time in the maggot therapy group is likely to spell cost savings, she said.
Maggot therapy was well tolerated. Patients expressed no reticence about it. A similar number of patients in both study arms reported a crawling sensation on their wounds.
Dr. Opletalova said that maggot therapy is likely to be particularly useful in patients with wounds that need rapid debridement, such as those with diabetes, or to prepare a wound for skin grafting or when there is an increased risk for infection.
Further details of the recently published randomized trial can be found in the Archives of Dermatology (2012;148:432-8).
The study was supported by university hospital research funding and by a grant from the French Society of Dermatology. Dr. Opletalova reported having no financial conflicts.
AT THE ANNUAL CONGRESS OF THE EUROPEAN ACADEMY OF DERMATOLOGY AND VENEREOLOGY
Major Finding: After 7 days of therapy, the mean percentage of slough in wounds was 54.5% in the maggot debridement group, compared with 66.5% in the control group.
Data Source: The phase-III findings come from a randomized, multicenter, blinded, prospective clinical trial involving 119 hospitalized patients.
Disclosures: The study was supported by university research funding and by a grant from the French Society of Dermatology. Dr. Opletalova reported having no financial conflicts.
Medicare Okays Wound Plasma Gel for Clinical Trial Patients
The Centers for Medicare and Medicaid Services has announced coverage of platelet-rich plasma gel for chronic wounds, but only for patients who are enrolled in clinical trials that meet the agency’s criteria.
"The available evidence does not permit us to conclude that use of autologous PRP improves health outcomes in beneficiaries with chronic diabetic, pressure, and/or venous wounds," the agency wrote in its decision statement Therefore, trials are needed to prove effectiveness.
The decision comes after CMS rejection of coverage since 1992, according to Cytomedix of Gaithersburg, Md., which manufactures a device called AutoloGel that produces PRP from a patient’s own blood. "This determination reverses a nearly 20-year non-coverage determination for PRP and provides for an appropriate research study with practical study designs we are confident will demonstrate that patients treated with our autologous PRP product, the AutoloGel System, experience clinically significant health outcomes," Martin P. Rosendale, CEO of Cytomedix, said in a statement.
Cytomedix submitted another request in October 2011 for CMS review of its PRP therapy, which was approved under the coverage with evidence development (CED) program.
Under the program, services, equipment, and supplies are covered through Medicare and Medicaid while a product is further investigated. CMS noted in its decision that it "is evaluating PRP as a service, and not a specific system for administrating PRP."
In the PRP process, autologous blood is centrifuged to produce a concentrate of platelets and plasma proteins. Individual growth factors are not identified or separated, but additives are used to change the consistency.
Autologous PRP has had many uses over the years, including use as an adhesive in plastic surgery and as filler for acute wounds. It has also been used to treat amateur and professional athletes – such as golfer Tiger Woods, basketball player Kobe Bryant, and baseball star Alex Rodriguez – to help heal tendons and ligaments, and is growing in popularity in the sports world, according to recent consumer news reports.
According to the CMS, to continue being covered for wound healing PRP therapy must show complete wound healing in patients and a reduction of wound size or healing trajectory. It also must help a patient’s ability to return to previous function and normal activities. The studies have to be prospective and enroll Medicare beneficiaries with chronic nonhealing diabetic, pressure, and/or venous wounds who are receiving optimal care, in addition to the therapy.
The agency must receive all pertinent data by August 2014.
Cytomedix said that it has been in talks with CMS about trials. "We look forward to continuing to work with CMS in the coming weeks in further defining the specifics of the protocols for the research studies and clinical questions to be answered through the CED program," said Mr. Rosendale.
The Centers for Medicare and Medicaid Services has announced coverage of platelet-rich plasma gel for chronic wounds, but only for patients who are enrolled in clinical trials that meet the agency’s criteria.
"The available evidence does not permit us to conclude that use of autologous PRP improves health outcomes in beneficiaries with chronic diabetic, pressure, and/or venous wounds," the agency wrote in its decision statement Therefore, trials are needed to prove effectiveness.
The decision comes after CMS rejection of coverage since 1992, according to Cytomedix of Gaithersburg, Md., which manufactures a device called AutoloGel that produces PRP from a patient’s own blood. "This determination reverses a nearly 20-year non-coverage determination for PRP and provides for an appropriate research study with practical study designs we are confident will demonstrate that patients treated with our autologous PRP product, the AutoloGel System, experience clinically significant health outcomes," Martin P. Rosendale, CEO of Cytomedix, said in a statement.
Cytomedix submitted another request in October 2011 for CMS review of its PRP therapy, which was approved under the coverage with evidence development (CED) program.
Under the program, services, equipment, and supplies are covered through Medicare and Medicaid while a product is further investigated. CMS noted in its decision that it "is evaluating PRP as a service, and not a specific system for administrating PRP."
In the PRP process, autologous blood is centrifuged to produce a concentrate of platelets and plasma proteins. Individual growth factors are not identified or separated, but additives are used to change the consistency.
Autologous PRP has had many uses over the years, including use as an adhesive in plastic surgery and as filler for acute wounds. It has also been used to treat amateur and professional athletes – such as golfer Tiger Woods, basketball player Kobe Bryant, and baseball star Alex Rodriguez – to help heal tendons and ligaments, and is growing in popularity in the sports world, according to recent consumer news reports.
According to the CMS, to continue being covered for wound healing PRP therapy must show complete wound healing in patients and a reduction of wound size or healing trajectory. It also must help a patient’s ability to return to previous function and normal activities. The studies have to be prospective and enroll Medicare beneficiaries with chronic nonhealing diabetic, pressure, and/or venous wounds who are receiving optimal care, in addition to the therapy.
The agency must receive all pertinent data by August 2014.
Cytomedix said that it has been in talks with CMS about trials. "We look forward to continuing to work with CMS in the coming weeks in further defining the specifics of the protocols for the research studies and clinical questions to be answered through the CED program," said Mr. Rosendale.
The Centers for Medicare and Medicaid Services has announced coverage of platelet-rich plasma gel for chronic wounds, but only for patients who are enrolled in clinical trials that meet the agency’s criteria.
"The available evidence does not permit us to conclude that use of autologous PRP improves health outcomes in beneficiaries with chronic diabetic, pressure, and/or venous wounds," the agency wrote in its decision statement Therefore, trials are needed to prove effectiveness.
The decision comes after CMS rejection of coverage since 1992, according to Cytomedix of Gaithersburg, Md., which manufactures a device called AutoloGel that produces PRP from a patient’s own blood. "This determination reverses a nearly 20-year non-coverage determination for PRP and provides for an appropriate research study with practical study designs we are confident will demonstrate that patients treated with our autologous PRP product, the AutoloGel System, experience clinically significant health outcomes," Martin P. Rosendale, CEO of Cytomedix, said in a statement.
Cytomedix submitted another request in October 2011 for CMS review of its PRP therapy, which was approved under the coverage with evidence development (CED) program.
Under the program, services, equipment, and supplies are covered through Medicare and Medicaid while a product is further investigated. CMS noted in its decision that it "is evaluating PRP as a service, and not a specific system for administrating PRP."
In the PRP process, autologous blood is centrifuged to produce a concentrate of platelets and plasma proteins. Individual growth factors are not identified or separated, but additives are used to change the consistency.
Autologous PRP has had many uses over the years, including use as an adhesive in plastic surgery and as filler for acute wounds. It has also been used to treat amateur and professional athletes – such as golfer Tiger Woods, basketball player Kobe Bryant, and baseball star Alex Rodriguez – to help heal tendons and ligaments, and is growing in popularity in the sports world, according to recent consumer news reports.
According to the CMS, to continue being covered for wound healing PRP therapy must show complete wound healing in patients and a reduction of wound size or healing trajectory. It also must help a patient’s ability to return to previous function and normal activities. The studies have to be prospective and enroll Medicare beneficiaries with chronic nonhealing diabetic, pressure, and/or venous wounds who are receiving optimal care, in addition to the therapy.
The agency must receive all pertinent data by August 2014.
Cytomedix said that it has been in talks with CMS about trials. "We look forward to continuing to work with CMS in the coming weeks in further defining the specifics of the protocols for the research studies and clinical questions to be answered through the CED program," said Mr. Rosendale.
Spray-On Cells Promote Venous Ulcer Healing
A spray formulation of allogeneic neonatal keratinocytes and fibroblasts significantly improved wound healing in a multicenter, randomized, placebo-controlled phase II study of more than 200 patients with venous leg ulcers.
The mean reduction in wound area was significantly greater among 177 patients who received 12 weeks of active treatment with the novel cell therapy, known as HP802-247, than among 50 patients who were treated with application vehicle of a modified fibrin spray alone. HP802-247 consists of cryopreserved, allogenic, growth-arrested fibroblasts and keratinocytes derived from neonatal foreskin. The largest improvement (16% on average over placebo) was seen in 44 patients assigned to treatment with 5.0x106 cells per mL every 14 days, Dr. Robert S. Kirsner of the University of Miami and his colleagues reported. The study was published online in the Aug. 3 issue of The Lancet.
Reduction in wound area was between 8% and 12% over that seen with placebo in 45 patients treated with 5.0x106 cells per mL every 7 days, 44 patients treated with 5.0x106 cells per mL every 14 days, and 43 patients treated with 0.5x106 cells per mL every 7 days, the investigators said (Lancet 2012 Aug. 3 [doi:10.1016/S0140-6736(12)60644-8]).
The study investigators included vascular surgeons, dermatologists, and podiatrists at universities and in private practice. All patients were treated with standard compression bandaging as well as the study treatment.
"Wound area began to decrease rapidly after initiation of treatment. After one application of the 0.5x106/mL every 14 days dose, the mean reduction in wound area was 40%, compared with 23% for the vehicle group. This effect persisted with the maximum difference in mean reduction between the group assigned [0.5x106 cells per mL] every 14 days and the vehicle group occurring at week 7 of treatment (87% vs. 65%)," they wrote.
At week 12 of treatment, the mean wound area in the group treated with 0.5x106 cells per mL every 14 days was reduced by 91%, compared with 80% with vehicle, and for the other treatment groups, the mean wound area was reduced by between 84% and 87%.
"Overall, patients treated with cells had higher proportions of healed wounds than did those assigned vehicle alone, but only the group assigned [0.5x106 cells per mL] every 14 days differed significantly compared with control," they noted.
Adverse events were similar across all groups, and most were nonserious, mild to moderate events that resolved. Only new skin ulcers and cellulitis occurred at a frequency of greater than 5%. Immunotoxicity testing showed that there was no treatment-induced alloantibody formation or induction of autoimmunity.
Included in this study were adult outpatients enrolled from 28 centers in the United States and Canada between 2009 and 2011. All had venous reflux confirmed by duplex Doppler ultrasonography, and up to three venous leg ulcers. Those assigned to the placebo group received vehicle alone applied every 7 days for the 12 weeks of the trial.
The findings may have important implications for the treatment of venous leg ulcers, which affect an estimated 1.65% to 1.74% of adults aged 65 years and older. Standard treatment with infection control, primary dressings, and high-strength compressions heal between 30% and 75% of such ulcers, compared with the 84%-91% healing seen with HP802-247 in this study, with the remainder becoming "chronic, with unresolved inflammatory processes within the extracellular matrix of the wound bed and dysfunction of wound fibroblasts and keratinocytes," the investigators noted.
"The results are sufficiently promising that larger randomized trials comparing HP802-247 to standard treatment for venous leg ulcers are now warranted," they concluded.
The researchers reported that their study was limited by the wound upper end boundary of 12 cm3, which represents 80% of all venous leg ulcers, but a smaller proportion of chronic venous ulcers.
The research trial is registered with ClinicalTrials.gov as NCT00852995.
Dr. Kirsner received consulting fees for his assistance with protocol design and data interpretation. Two of his coauthors also received such consulting fees, and other authors on the study disclosed that they are employed by Healthpoint, and/or hold adjunct academic appointments at the University of North Texas Health Sciences Center in Fort Worth, Tex.
The findings of this "well-done clinical trial" highlight the potential of cell-based therapies not only for venous leg ulcer treatment, but for the treatment of other chronic wounds as well, Dr. Matthias Augustin and Dr. Wolfgang Vanscheidt wrote in an accompanying editorial.
The authors have "clearly shown that a specific cell therapy for venous leg ulcers can lead to a significantly higher healing rate than for vehicle alone in hard-to-treat wounds for which compression treatment has been applied unsuccessfully," they noted, adding that "the benefits identified by Kirsner and colleagues could well be applicable in other chronic wounds such as ischemic and diabetic foot ulcers."
Dr. Augustin and Dr. Vanscheidt stressed the importance of gaining insight into the mechanism of action to further understand the best potential uses of these types of therapies, and called on the scientific community and industry to investigate the effects of tissue and cell therapy products in patients with mixed arterial-venous leg ulcers in whom revascularization is not possible (Lancet 2012 Aug. 3 [doi:10.1016/S0140-6736(12)61255-0]).
"In this indication, bioengineered tissue and cell therapy products are presumably among the very few methods that could achieve sustained healing. Because of aging of the population in most societies, this fatal combination of venous insufficiency and arterial occlusive disease will become an ever-growing problem. We believe that biotherapy of such ulcers will be a challenge but will also offer hope to wound researchers in the coming decades," they said.
Dr. Augustin and Dr. Vanscheidt are with the Institute for Health Services Research in Dermatology and Nursing and Comprehensive Wound Center, University Medical Center Hamburg (Germany), and Freiburg, Germany, respectively. They reported having no conflicts of interest.
The findings of this "well-done clinical trial" highlight the potential of cell-based therapies not only for venous leg ulcer treatment, but for the treatment of other chronic wounds as well, Dr. Matthias Augustin and Dr. Wolfgang Vanscheidt wrote in an accompanying editorial.
The authors have "clearly shown that a specific cell therapy for venous leg ulcers can lead to a significantly higher healing rate than for vehicle alone in hard-to-treat wounds for which compression treatment has been applied unsuccessfully," they noted, adding that "the benefits identified by Kirsner and colleagues could well be applicable in other chronic wounds such as ischemic and diabetic foot ulcers."
Dr. Augustin and Dr. Vanscheidt stressed the importance of gaining insight into the mechanism of action to further understand the best potential uses of these types of therapies, and called on the scientific community and industry to investigate the effects of tissue and cell therapy products in patients with mixed arterial-venous leg ulcers in whom revascularization is not possible (Lancet 2012 Aug. 3 [doi:10.1016/S0140-6736(12)61255-0]).
"In this indication, bioengineered tissue and cell therapy products are presumably among the very few methods that could achieve sustained healing. Because of aging of the population in most societies, this fatal combination of venous insufficiency and arterial occlusive disease will become an ever-growing problem. We believe that biotherapy of such ulcers will be a challenge but will also offer hope to wound researchers in the coming decades," they said.
Dr. Augustin and Dr. Vanscheidt are with the Institute for Health Services Research in Dermatology and Nursing and Comprehensive Wound Center, University Medical Center Hamburg (Germany), and Freiburg, Germany, respectively. They reported having no conflicts of interest.
The findings of this "well-done clinical trial" highlight the potential of cell-based therapies not only for venous leg ulcer treatment, but for the treatment of other chronic wounds as well, Dr. Matthias Augustin and Dr. Wolfgang Vanscheidt wrote in an accompanying editorial.
The authors have "clearly shown that a specific cell therapy for venous leg ulcers can lead to a significantly higher healing rate than for vehicle alone in hard-to-treat wounds for which compression treatment has been applied unsuccessfully," they noted, adding that "the benefits identified by Kirsner and colleagues could well be applicable in other chronic wounds such as ischemic and diabetic foot ulcers."
Dr. Augustin and Dr. Vanscheidt stressed the importance of gaining insight into the mechanism of action to further understand the best potential uses of these types of therapies, and called on the scientific community and industry to investigate the effects of tissue and cell therapy products in patients with mixed arterial-venous leg ulcers in whom revascularization is not possible (Lancet 2012 Aug. 3 [doi:10.1016/S0140-6736(12)61255-0]).
"In this indication, bioengineered tissue and cell therapy products are presumably among the very few methods that could achieve sustained healing. Because of aging of the population in most societies, this fatal combination of venous insufficiency and arterial occlusive disease will become an ever-growing problem. We believe that biotherapy of such ulcers will be a challenge but will also offer hope to wound researchers in the coming decades," they said.
Dr. Augustin and Dr. Vanscheidt are with the Institute for Health Services Research in Dermatology and Nursing and Comprehensive Wound Center, University Medical Center Hamburg (Germany), and Freiburg, Germany, respectively. They reported having no conflicts of interest.
A spray formulation of allogeneic neonatal keratinocytes and fibroblasts significantly improved wound healing in a multicenter, randomized, placebo-controlled phase II study of more than 200 patients with venous leg ulcers.
The mean reduction in wound area was significantly greater among 177 patients who received 12 weeks of active treatment with the novel cell therapy, known as HP802-247, than among 50 patients who were treated with application vehicle of a modified fibrin spray alone. HP802-247 consists of cryopreserved, allogenic, growth-arrested fibroblasts and keratinocytes derived from neonatal foreskin. The largest improvement (16% on average over placebo) was seen in 44 patients assigned to treatment with 5.0x106 cells per mL every 14 days, Dr. Robert S. Kirsner of the University of Miami and his colleagues reported. The study was published online in the Aug. 3 issue of The Lancet.
Reduction in wound area was between 8% and 12% over that seen with placebo in 45 patients treated with 5.0x106 cells per mL every 7 days, 44 patients treated with 5.0x106 cells per mL every 14 days, and 43 patients treated with 0.5x106 cells per mL every 7 days, the investigators said (Lancet 2012 Aug. 3 [doi:10.1016/S0140-6736(12)60644-8]).
The study investigators included vascular surgeons, dermatologists, and podiatrists at universities and in private practice. All patients were treated with standard compression bandaging as well as the study treatment.
"Wound area began to decrease rapidly after initiation of treatment. After one application of the 0.5x106/mL every 14 days dose, the mean reduction in wound area was 40%, compared with 23% for the vehicle group. This effect persisted with the maximum difference in mean reduction between the group assigned [0.5x106 cells per mL] every 14 days and the vehicle group occurring at week 7 of treatment (87% vs. 65%)," they wrote.
At week 12 of treatment, the mean wound area in the group treated with 0.5x106 cells per mL every 14 days was reduced by 91%, compared with 80% with vehicle, and for the other treatment groups, the mean wound area was reduced by between 84% and 87%.
"Overall, patients treated with cells had higher proportions of healed wounds than did those assigned vehicle alone, but only the group assigned [0.5x106 cells per mL] every 14 days differed significantly compared with control," they noted.
Adverse events were similar across all groups, and most were nonserious, mild to moderate events that resolved. Only new skin ulcers and cellulitis occurred at a frequency of greater than 5%. Immunotoxicity testing showed that there was no treatment-induced alloantibody formation or induction of autoimmunity.
Included in this study were adult outpatients enrolled from 28 centers in the United States and Canada between 2009 and 2011. All had venous reflux confirmed by duplex Doppler ultrasonography, and up to three venous leg ulcers. Those assigned to the placebo group received vehicle alone applied every 7 days for the 12 weeks of the trial.
The findings may have important implications for the treatment of venous leg ulcers, which affect an estimated 1.65% to 1.74% of adults aged 65 years and older. Standard treatment with infection control, primary dressings, and high-strength compressions heal between 30% and 75% of such ulcers, compared with the 84%-91% healing seen with HP802-247 in this study, with the remainder becoming "chronic, with unresolved inflammatory processes within the extracellular matrix of the wound bed and dysfunction of wound fibroblasts and keratinocytes," the investigators noted.
"The results are sufficiently promising that larger randomized trials comparing HP802-247 to standard treatment for venous leg ulcers are now warranted," they concluded.
The researchers reported that their study was limited by the wound upper end boundary of 12 cm3, which represents 80% of all venous leg ulcers, but a smaller proportion of chronic venous ulcers.
The research trial is registered with ClinicalTrials.gov as NCT00852995.
Dr. Kirsner received consulting fees for his assistance with protocol design and data interpretation. Two of his coauthors also received such consulting fees, and other authors on the study disclosed that they are employed by Healthpoint, and/or hold adjunct academic appointments at the University of North Texas Health Sciences Center in Fort Worth, Tex.
A spray formulation of allogeneic neonatal keratinocytes and fibroblasts significantly improved wound healing in a multicenter, randomized, placebo-controlled phase II study of more than 200 patients with venous leg ulcers.
The mean reduction in wound area was significantly greater among 177 patients who received 12 weeks of active treatment with the novel cell therapy, known as HP802-247, than among 50 patients who were treated with application vehicle of a modified fibrin spray alone. HP802-247 consists of cryopreserved, allogenic, growth-arrested fibroblasts and keratinocytes derived from neonatal foreskin. The largest improvement (16% on average over placebo) was seen in 44 patients assigned to treatment with 5.0x106 cells per mL every 14 days, Dr. Robert S. Kirsner of the University of Miami and his colleagues reported. The study was published online in the Aug. 3 issue of The Lancet.
Reduction in wound area was between 8% and 12% over that seen with placebo in 45 patients treated with 5.0x106 cells per mL every 7 days, 44 patients treated with 5.0x106 cells per mL every 14 days, and 43 patients treated with 0.5x106 cells per mL every 7 days, the investigators said (Lancet 2012 Aug. 3 [doi:10.1016/S0140-6736(12)60644-8]).
The study investigators included vascular surgeons, dermatologists, and podiatrists at universities and in private practice. All patients were treated with standard compression bandaging as well as the study treatment.
"Wound area began to decrease rapidly after initiation of treatment. After one application of the 0.5x106/mL every 14 days dose, the mean reduction in wound area was 40%, compared with 23% for the vehicle group. This effect persisted with the maximum difference in mean reduction between the group assigned [0.5x106 cells per mL] every 14 days and the vehicle group occurring at week 7 of treatment (87% vs. 65%)," they wrote.
At week 12 of treatment, the mean wound area in the group treated with 0.5x106 cells per mL every 14 days was reduced by 91%, compared with 80% with vehicle, and for the other treatment groups, the mean wound area was reduced by between 84% and 87%.
"Overall, patients treated with cells had higher proportions of healed wounds than did those assigned vehicle alone, but only the group assigned [0.5x106 cells per mL] every 14 days differed significantly compared with control," they noted.
Adverse events were similar across all groups, and most were nonserious, mild to moderate events that resolved. Only new skin ulcers and cellulitis occurred at a frequency of greater than 5%. Immunotoxicity testing showed that there was no treatment-induced alloantibody formation or induction of autoimmunity.
Included in this study were adult outpatients enrolled from 28 centers in the United States and Canada between 2009 and 2011. All had venous reflux confirmed by duplex Doppler ultrasonography, and up to three venous leg ulcers. Those assigned to the placebo group received vehicle alone applied every 7 days for the 12 weeks of the trial.
The findings may have important implications for the treatment of venous leg ulcers, which affect an estimated 1.65% to 1.74% of adults aged 65 years and older. Standard treatment with infection control, primary dressings, and high-strength compressions heal between 30% and 75% of such ulcers, compared with the 84%-91% healing seen with HP802-247 in this study, with the remainder becoming "chronic, with unresolved inflammatory processes within the extracellular matrix of the wound bed and dysfunction of wound fibroblasts and keratinocytes," the investigators noted.
"The results are sufficiently promising that larger randomized trials comparing HP802-247 to standard treatment for venous leg ulcers are now warranted," they concluded.
The researchers reported that their study was limited by the wound upper end boundary of 12 cm3, which represents 80% of all venous leg ulcers, but a smaller proportion of chronic venous ulcers.
The research trial is registered with ClinicalTrials.gov as NCT00852995.
Dr. Kirsner received consulting fees for his assistance with protocol design and data interpretation. Two of his coauthors also received such consulting fees, and other authors on the study disclosed that they are employed by Healthpoint, and/or hold adjunct academic appointments at the University of North Texas Health Sciences Center in Fort Worth, Tex.
FROM THE LANCET
Major Finding: At week 12 of treatment, the mean wound area in the group treated with 0.5x106 cells per mL every 14 days was reduced by 91%, compared with 80% with vehicle, and for the other treatment groups the mean wound area was reduced by between 84% and 87%.
Data Source: This was a randomized, placebo-controlled phase II trial.
Disclosures: Dr. Kirsner received consulting fees for his assistance with protocol design and data interpretation. Two of his coauthors also received such consulting fees, and other authors on the study disclosed that they are employed by Healthpoint, and/or hold adjunct academic appointments at the University of North Texas Health Sciences Center in Fort Worth, Tex. The authors of the editorial reported having no disclosures.