User login
Official Newspaper of the American College of Surgeons
Drug-coated balloons: The future of hemodialysis access?
CHICAGO – Drug-coated balloons show promise of being a long-sought major advance in the endovascular treatment of stenotic arteriovenous fistulae and grafts for hemodialysis access, Syed M. Hussain, MD, said at a symposium on vascular surgery sponsored by Northwestern University.
Something significantly better than today’s standard treatment options is needed, according to Dr. Hussain. Medicare pays out more than $50 billion annually for the treatment of patients with end-stage renal disease, and a hefty chunk of that money goes for oft-repeated procedures aimed at preserving the patency of the access sites.
“Primary patency rates leave much room for improvement,” observed Dr. Hussain, a vascular surgeon at the Christie Clinic in Champaign, Ill.
Indeed, the 50% primary patency rate at 6 months that was optimistically declared a “reasonable goal” in the 2006 Kidney Disease Outcomes Quality Initiative is actually far-fetched using the conventional tools.
“That 50% patency at 6 months would be a tall order to try to meet. Anybody in this room that does fistulography and angioplasty knows the numbers are actually a lot lower than 50%,” said Dr. Hussain.
Plain old balloon angioplasty, the standard first-line intervention for stenotic hemodialysis access sites, has a 6-month patency rate of about 30%. Bare metal stents push the rate up to about 39%. Covered stent grafts are the most effective of the conventional treatment modalities, with a 6-month patency of 51%-53%; however, they are widely considered too expensive for routine use.
Drug-coated balloons (DCBs) have been available for close to 3 years for treatment of lower extremity peripheral artery disease, where they have achieved considerable success. The Food and Drug Administration has approved three commercially available DCBs for this purpose: Bard’s Lutonix 035 AV, Medtronic’s IN.PACT Admiral, and most recently the Stellarex DCB.
In addition, the Lutonix DCB is approved for treatment of dysfunctional or stenotic arteriovenous (AV) fistulae on the strength of the positive results of the first prospective randomized multicenter trial of a DCB versus balloon angioplasty for AV access stenosis as reported at a conference in Leipzig, Germany, in 2017 and summarized by Dr. Hussain.
The pathophysiology of arterial atherosclerotic stenosis is very different from the stenosis that plagues AV access for dialysis. Arterial atherosclerotic stenosis is due to neointimal hyperplasia caused by inflammation and barotrauma secondary to angioplasty. In contrast, the neointimal hyperplasia in AV access stenosis is due to smooth muscle cell proliferation in response to nonphysiologic blood flow dynamics and shear forces between a high-pressure arterial system and the low-pressure venous system to which it has been connected, with resultant stenosis at the venous outflow anastomosis and often at the cephalic arch, Dr. Hussain explained.
Other contributors to the high rate of early stenosis in AV fistulae and grafts include traumatic balloon dilation, uremia, and repetitive traumatic needle insertion.
The breakthrough for DCBs as a potential game changer in dialysis access stenosis came with the discovery that venous smooth muscle cells are much more sensitive to paclitaxel and other antiproliferative drugs than are arterial smooth muscle cells. All three commercially available DCBs utilize paclitaxel as their active agent.
Multiple small single-center studies involving off-label use of the DCBs for dialysis access stenosis strongly suggested 6-month patency rates were higher than with balloon angioplasty. Then came the core lab-adjudicated Lutonix multicenter trial, in which 285 hemodialysis patients at 23 sites were randomized to the DCB or balloon angioplasty. Participants had to have a target lesion less than 10 cm long and had to undergo successful predilatation with high-pressure balloon angioplasty.
“The key thing to remember when we talk about dialysis grafts or fistulae is that we have to look at patency in periods of months. We can’t look at years because it’s pretty unusual to see a fistula stay open that long. So most of the time we’re trying to achieve extra months on these types of circuits,” noted Dr. Hussain.
That being said, the 8-month target lesion primary patency rate was 61.6% in the Lutonix DCB group, compared with 49.4% for percutaneous angioplasty, a statistically significant and clinically meaningful difference. Moreover, 66 interventions were required to maintain target lesion patency during that time frame in the DCB group, versus 94 in the angioplasty group; that translated to a 30% reduction in repeat interventions.
“This clearly has the potential to save a lot of money for the health care system,” he said.
The two forms of treatment were equally safe.
The expanded indication for the Lutonix DCB that resulted from this large randomized trial has triggered considerable research interest in DCBs for AV access stenosis around the world. Major ongoing randomized trials include the PAVE trial in the United Kingdom, the Spanish FISBOL trial, the APERTO trial in the Netherlands, and an Israeli randomized trial restricted to patients with cephalic arch stenosis.
Dr. Hussain is particularly excited about the ongoing 330-patient, prospective, multicenter, single-blinded clinical trial of the IN.PACT Admiral DCB versus plain balloon angioplasty. The Medtronic DCB employs a higher dosage of paclitaxel: 3.5 mcg/mm2, compared with 2.0 mcg/mm2 for the Lutonix DCB. Also, due to differences in the excipients used in the two DCBs, the paclitaxel from the IN.PACT device stays in the media of blood vessels for up to 180 days, compared with 60 days following drug delivery with the Lutonix balloon. Whether this longer period of close range antiproliferative activity will translate into a higher patency rate remains to be seen.
Dr. Hussain reported having no financial conflicts of interest regarding his presentation.
Costs associated with the management of patients with chronic kidney disease, particularly those with end-stage renal disease requiring hemodialysis, is a huge component of the Medicare budget. The maintenance of functional vascular access in these patients remains an ongoing challenge and reduction of costs related to access failure is critical to the continued funding of the program. Traditional methods of maintaining access patency such as balloon angioplasty and bare metal stents have poor long-term outcomes, and moderate improvement is seen with the use of covered stents.
Dr. Hussain reviews the current status of drug-coated balloons (DCB) in the endovascular treatment of dysfunctional hemodialysis fistulas and grafts. Safety and efficacy data from a prospective randomized multicenter trial comparing the Bard Lutonix DCB and plain old balloon angioplasty demonstrated a significant improvement in primary patency and a 30% reduction in repeat interventions with the DCB. This led to FDA approval for the Lutonix DCB in the treatment of dysfunctional or stenotic arteriovenous fistulas. Encouraged by these results, researchers conducting ongoing international randomized trials are attempting to clarify the potential expanded indications for DCB in access stenosis. Of particular interest is the ongoing 330-patient prospective, multicenter IN-PACT trial comparing Admiral DCB to balloon angioplasty in failing arteriovenous fistulas. Both the Admiral DCB and Lutonix DCB utilize paclitaxel as the antiproliferative agent. Dr. Hussain describes the increased sensitivity of venous smooth muscle cells to paclitaxel and other antiproliferative drugs when compared with arterial smooth muscle cells. This exciting finding may explain the improved outcomes in the treatment of dialysis access lesions where pathology frequently occurs at the venous anastomosis or in the venous conduit.
Although early results with the use of DCBs are promising, ongoing clinical trials and careful analysis of data and cost effectiveness are critical to optimize outcomes in treating dialysis access dysfunction. Dr. Hussain appropriately expresses cautious optimism regarding the future of hemodialysis access with this new tool available to interventionists treating these complex patients.
Larry A. Scher, MD, is a vascular surgeon at Montefiore Einstein Center for Heart and Vascular Care, New York, and an associate medical editor for Vascular Specialist.
Costs associated with the management of patients with chronic kidney disease, particularly those with end-stage renal disease requiring hemodialysis, is a huge component of the Medicare budget. The maintenance of functional vascular access in these patients remains an ongoing challenge and reduction of costs related to access failure is critical to the continued funding of the program. Traditional methods of maintaining access patency such as balloon angioplasty and bare metal stents have poor long-term outcomes, and moderate improvement is seen with the use of covered stents.
Dr. Hussain reviews the current status of drug-coated balloons (DCB) in the endovascular treatment of dysfunctional hemodialysis fistulas and grafts. Safety and efficacy data from a prospective randomized multicenter trial comparing the Bard Lutonix DCB and plain old balloon angioplasty demonstrated a significant improvement in primary patency and a 30% reduction in repeat interventions with the DCB. This led to FDA approval for the Lutonix DCB in the treatment of dysfunctional or stenotic arteriovenous fistulas. Encouraged by these results, researchers conducting ongoing international randomized trials are attempting to clarify the potential expanded indications for DCB in access stenosis. Of particular interest is the ongoing 330-patient prospective, multicenter IN-PACT trial comparing Admiral DCB to balloon angioplasty in failing arteriovenous fistulas. Both the Admiral DCB and Lutonix DCB utilize paclitaxel as the antiproliferative agent. Dr. Hussain describes the increased sensitivity of venous smooth muscle cells to paclitaxel and other antiproliferative drugs when compared with arterial smooth muscle cells. This exciting finding may explain the improved outcomes in the treatment of dialysis access lesions where pathology frequently occurs at the venous anastomosis or in the venous conduit.
Although early results with the use of DCBs are promising, ongoing clinical trials and careful analysis of data and cost effectiveness are critical to optimize outcomes in treating dialysis access dysfunction. Dr. Hussain appropriately expresses cautious optimism regarding the future of hemodialysis access with this new tool available to interventionists treating these complex patients.
Larry A. Scher, MD, is a vascular surgeon at Montefiore Einstein Center for Heart and Vascular Care, New York, and an associate medical editor for Vascular Specialist.
Costs associated with the management of patients with chronic kidney disease, particularly those with end-stage renal disease requiring hemodialysis, is a huge component of the Medicare budget. The maintenance of functional vascular access in these patients remains an ongoing challenge and reduction of costs related to access failure is critical to the continued funding of the program. Traditional methods of maintaining access patency such as balloon angioplasty and bare metal stents have poor long-term outcomes, and moderate improvement is seen with the use of covered stents.
Dr. Hussain reviews the current status of drug-coated balloons (DCB) in the endovascular treatment of dysfunctional hemodialysis fistulas and grafts. Safety and efficacy data from a prospective randomized multicenter trial comparing the Bard Lutonix DCB and plain old balloon angioplasty demonstrated a significant improvement in primary patency and a 30% reduction in repeat interventions with the DCB. This led to FDA approval for the Lutonix DCB in the treatment of dysfunctional or stenotic arteriovenous fistulas. Encouraged by these results, researchers conducting ongoing international randomized trials are attempting to clarify the potential expanded indications for DCB in access stenosis. Of particular interest is the ongoing 330-patient prospective, multicenter IN-PACT trial comparing Admiral DCB to balloon angioplasty in failing arteriovenous fistulas. Both the Admiral DCB and Lutonix DCB utilize paclitaxel as the antiproliferative agent. Dr. Hussain describes the increased sensitivity of venous smooth muscle cells to paclitaxel and other antiproliferative drugs when compared with arterial smooth muscle cells. This exciting finding may explain the improved outcomes in the treatment of dialysis access lesions where pathology frequently occurs at the venous anastomosis or in the venous conduit.
Although early results with the use of DCBs are promising, ongoing clinical trials and careful analysis of data and cost effectiveness are critical to optimize outcomes in treating dialysis access dysfunction. Dr. Hussain appropriately expresses cautious optimism regarding the future of hemodialysis access with this new tool available to interventionists treating these complex patients.
Larry A. Scher, MD, is a vascular surgeon at Montefiore Einstein Center for Heart and Vascular Care, New York, and an associate medical editor for Vascular Specialist.
CHICAGO – Drug-coated balloons show promise of being a long-sought major advance in the endovascular treatment of stenotic arteriovenous fistulae and grafts for hemodialysis access, Syed M. Hussain, MD, said at a symposium on vascular surgery sponsored by Northwestern University.
Something significantly better than today’s standard treatment options is needed, according to Dr. Hussain. Medicare pays out more than $50 billion annually for the treatment of patients with end-stage renal disease, and a hefty chunk of that money goes for oft-repeated procedures aimed at preserving the patency of the access sites.
“Primary patency rates leave much room for improvement,” observed Dr. Hussain, a vascular surgeon at the Christie Clinic in Champaign, Ill.
Indeed, the 50% primary patency rate at 6 months that was optimistically declared a “reasonable goal” in the 2006 Kidney Disease Outcomes Quality Initiative is actually far-fetched using the conventional tools.
“That 50% patency at 6 months would be a tall order to try to meet. Anybody in this room that does fistulography and angioplasty knows the numbers are actually a lot lower than 50%,” said Dr. Hussain.
Plain old balloon angioplasty, the standard first-line intervention for stenotic hemodialysis access sites, has a 6-month patency rate of about 30%. Bare metal stents push the rate up to about 39%. Covered stent grafts are the most effective of the conventional treatment modalities, with a 6-month patency of 51%-53%; however, they are widely considered too expensive for routine use.
Drug-coated balloons (DCBs) have been available for close to 3 years for treatment of lower extremity peripheral artery disease, where they have achieved considerable success. The Food and Drug Administration has approved three commercially available DCBs for this purpose: Bard’s Lutonix 035 AV, Medtronic’s IN.PACT Admiral, and most recently the Stellarex DCB.
In addition, the Lutonix DCB is approved for treatment of dysfunctional or stenotic arteriovenous (AV) fistulae on the strength of the positive results of the first prospective randomized multicenter trial of a DCB versus balloon angioplasty for AV access stenosis as reported at a conference in Leipzig, Germany, in 2017 and summarized by Dr. Hussain.
The pathophysiology of arterial atherosclerotic stenosis is very different from the stenosis that plagues AV access for dialysis. Arterial atherosclerotic stenosis is due to neointimal hyperplasia caused by inflammation and barotrauma secondary to angioplasty. In contrast, the neointimal hyperplasia in AV access stenosis is due to smooth muscle cell proliferation in response to nonphysiologic blood flow dynamics and shear forces between a high-pressure arterial system and the low-pressure venous system to which it has been connected, with resultant stenosis at the venous outflow anastomosis and often at the cephalic arch, Dr. Hussain explained.
Other contributors to the high rate of early stenosis in AV fistulae and grafts include traumatic balloon dilation, uremia, and repetitive traumatic needle insertion.
The breakthrough for DCBs as a potential game changer in dialysis access stenosis came with the discovery that venous smooth muscle cells are much more sensitive to paclitaxel and other antiproliferative drugs than are arterial smooth muscle cells. All three commercially available DCBs utilize paclitaxel as their active agent.
Multiple small single-center studies involving off-label use of the DCBs for dialysis access stenosis strongly suggested 6-month patency rates were higher than with balloon angioplasty. Then came the core lab-adjudicated Lutonix multicenter trial, in which 285 hemodialysis patients at 23 sites were randomized to the DCB or balloon angioplasty. Participants had to have a target lesion less than 10 cm long and had to undergo successful predilatation with high-pressure balloon angioplasty.
“The key thing to remember when we talk about dialysis grafts or fistulae is that we have to look at patency in periods of months. We can’t look at years because it’s pretty unusual to see a fistula stay open that long. So most of the time we’re trying to achieve extra months on these types of circuits,” noted Dr. Hussain.
That being said, the 8-month target lesion primary patency rate was 61.6% in the Lutonix DCB group, compared with 49.4% for percutaneous angioplasty, a statistically significant and clinically meaningful difference. Moreover, 66 interventions were required to maintain target lesion patency during that time frame in the DCB group, versus 94 in the angioplasty group; that translated to a 30% reduction in repeat interventions.
“This clearly has the potential to save a lot of money for the health care system,” he said.
The two forms of treatment were equally safe.
The expanded indication for the Lutonix DCB that resulted from this large randomized trial has triggered considerable research interest in DCBs for AV access stenosis around the world. Major ongoing randomized trials include the PAVE trial in the United Kingdom, the Spanish FISBOL trial, the APERTO trial in the Netherlands, and an Israeli randomized trial restricted to patients with cephalic arch stenosis.
Dr. Hussain is particularly excited about the ongoing 330-patient, prospective, multicenter, single-blinded clinical trial of the IN.PACT Admiral DCB versus plain balloon angioplasty. The Medtronic DCB employs a higher dosage of paclitaxel: 3.5 mcg/mm2, compared with 2.0 mcg/mm2 for the Lutonix DCB. Also, due to differences in the excipients used in the two DCBs, the paclitaxel from the IN.PACT device stays in the media of blood vessels for up to 180 days, compared with 60 days following drug delivery with the Lutonix balloon. Whether this longer period of close range antiproliferative activity will translate into a higher patency rate remains to be seen.
Dr. Hussain reported having no financial conflicts of interest regarding his presentation.
CHICAGO – Drug-coated balloons show promise of being a long-sought major advance in the endovascular treatment of stenotic arteriovenous fistulae and grafts for hemodialysis access, Syed M. Hussain, MD, said at a symposium on vascular surgery sponsored by Northwestern University.
Something significantly better than today’s standard treatment options is needed, according to Dr. Hussain. Medicare pays out more than $50 billion annually for the treatment of patients with end-stage renal disease, and a hefty chunk of that money goes for oft-repeated procedures aimed at preserving the patency of the access sites.
“Primary patency rates leave much room for improvement,” observed Dr. Hussain, a vascular surgeon at the Christie Clinic in Champaign, Ill.
Indeed, the 50% primary patency rate at 6 months that was optimistically declared a “reasonable goal” in the 2006 Kidney Disease Outcomes Quality Initiative is actually far-fetched using the conventional tools.
“That 50% patency at 6 months would be a tall order to try to meet. Anybody in this room that does fistulography and angioplasty knows the numbers are actually a lot lower than 50%,” said Dr. Hussain.
Plain old balloon angioplasty, the standard first-line intervention for stenotic hemodialysis access sites, has a 6-month patency rate of about 30%. Bare metal stents push the rate up to about 39%. Covered stent grafts are the most effective of the conventional treatment modalities, with a 6-month patency of 51%-53%; however, they are widely considered too expensive for routine use.
Drug-coated balloons (DCBs) have been available for close to 3 years for treatment of lower extremity peripheral artery disease, where they have achieved considerable success. The Food and Drug Administration has approved three commercially available DCBs for this purpose: Bard’s Lutonix 035 AV, Medtronic’s IN.PACT Admiral, and most recently the Stellarex DCB.
In addition, the Lutonix DCB is approved for treatment of dysfunctional or stenotic arteriovenous (AV) fistulae on the strength of the positive results of the first prospective randomized multicenter trial of a DCB versus balloon angioplasty for AV access stenosis as reported at a conference in Leipzig, Germany, in 2017 and summarized by Dr. Hussain.
The pathophysiology of arterial atherosclerotic stenosis is very different from the stenosis that plagues AV access for dialysis. Arterial atherosclerotic stenosis is due to neointimal hyperplasia caused by inflammation and barotrauma secondary to angioplasty. In contrast, the neointimal hyperplasia in AV access stenosis is due to smooth muscle cell proliferation in response to nonphysiologic blood flow dynamics and shear forces between a high-pressure arterial system and the low-pressure venous system to which it has been connected, with resultant stenosis at the venous outflow anastomosis and often at the cephalic arch, Dr. Hussain explained.
Other contributors to the high rate of early stenosis in AV fistulae and grafts include traumatic balloon dilation, uremia, and repetitive traumatic needle insertion.
The breakthrough for DCBs as a potential game changer in dialysis access stenosis came with the discovery that venous smooth muscle cells are much more sensitive to paclitaxel and other antiproliferative drugs than are arterial smooth muscle cells. All three commercially available DCBs utilize paclitaxel as their active agent.
Multiple small single-center studies involving off-label use of the DCBs for dialysis access stenosis strongly suggested 6-month patency rates were higher than with balloon angioplasty. Then came the core lab-adjudicated Lutonix multicenter trial, in which 285 hemodialysis patients at 23 sites were randomized to the DCB or balloon angioplasty. Participants had to have a target lesion less than 10 cm long and had to undergo successful predilatation with high-pressure balloon angioplasty.
“The key thing to remember when we talk about dialysis grafts or fistulae is that we have to look at patency in periods of months. We can’t look at years because it’s pretty unusual to see a fistula stay open that long. So most of the time we’re trying to achieve extra months on these types of circuits,” noted Dr. Hussain.
That being said, the 8-month target lesion primary patency rate was 61.6% in the Lutonix DCB group, compared with 49.4% for percutaneous angioplasty, a statistically significant and clinically meaningful difference. Moreover, 66 interventions were required to maintain target lesion patency during that time frame in the DCB group, versus 94 in the angioplasty group; that translated to a 30% reduction in repeat interventions.
“This clearly has the potential to save a lot of money for the health care system,” he said.
The two forms of treatment were equally safe.
The expanded indication for the Lutonix DCB that resulted from this large randomized trial has triggered considerable research interest in DCBs for AV access stenosis around the world. Major ongoing randomized trials include the PAVE trial in the United Kingdom, the Spanish FISBOL trial, the APERTO trial in the Netherlands, and an Israeli randomized trial restricted to patients with cephalic arch stenosis.
Dr. Hussain is particularly excited about the ongoing 330-patient, prospective, multicenter, single-blinded clinical trial of the IN.PACT Admiral DCB versus plain balloon angioplasty. The Medtronic DCB employs a higher dosage of paclitaxel: 3.5 mcg/mm2, compared with 2.0 mcg/mm2 for the Lutonix DCB. Also, due to differences in the excipients used in the two DCBs, the paclitaxel from the IN.PACT device stays in the media of blood vessels for up to 180 days, compared with 60 days following drug delivery with the Lutonix balloon. Whether this longer period of close range antiproliferative activity will translate into a higher patency rate remains to be seen.
Dr. Hussain reported having no financial conflicts of interest regarding his presentation.
EXPERT ANALYSIS FROM THE NORTHWESTERN VASCULAR SYMPOSIUM
Colorectal surgeons see barriers to optimal palliative care
While to its optimal implementation at the patient, family, and clinician level, results of a recent survey suggest.
“We found that surgeons reported the most important barriers to be their own,” said Pasithorn A. Suwanabol, MD, division of colorectal surgery, University of Michigan, Ann Arbor, and coauthors of a report on the survey.
More than three-quarters of surgeons said they had no formal education in palliative care, and a substantial number specifically noted inadequate training in both communication and techniques to forgo life-sustaining measures, according to the report, published in the Journal of Palliative Medicine.
Dr. Suwanabol and her colleagues sought surgeon perspectives on palliative care for stage IV colorectal cancer in part because palliative care is often not integrated with cancer treatment in these potentially incurable patients.
“Compared with patients treated by primary providers, surgical patients with terminal diseases are significantly less likely to receive palliative or end-of-life care,” they said in the report.
They conducted a mixed methods study including members of a surgical society (American Society of Colon and Rectal Surgeons) who were asked to submit Internet responses to a validated survey. This mixed methods study is believed to be the first to characterize surgeons’ perceived barriers to optimal palliative and end-of life care for patients with advanced colorectal cancer, Dr. Suwanabol and her colleagues noted.
A total of 131 surgeons responded to the survey, for a response rate of 16.5%. The majority of surgeons responding (76.1%) said they did not have any formal palliative care training, while 42.7% said they lacked specific education in communication and 37.9% lacked training in techniques to forgo life-sustaining measures.
Many patients with stage IV colorectal cancer are candidates for palliative care. “Specialty palliative care should be offered to patients who have more complex issues such as refractory pain, depression and anxiety, and existential distress. In addition, specialty palliative care may be helpful in those with challenging family dynamics and/or conflict between family members and/or members of the care team. Patients with stage IV colon cancer may not be candidates for curative therapies and a palliative approach allows the surgeon and patient to carefully weigh the risk and benefits of each therapy in the context of the overall goals of the patient,” Dr. Suwanabol said in an interview.
Among the survey responders, 61.8% said discussion of palliation was limited because of patients and families who had unrealistic expectations and demanded aggressive interventions.
One such recollection in the report reads as follows: ‘‘Patient with poorly responding stage IV colon cancer in multisystem organ failure getting same chemo that already failed ... family wanted everything done…’’
The report includes a number of other reflections from surgeons that suggest a level of anxiety, frustration, disappointment, or uncertainty regarding palliative care.
“I once operated on a young man with carcinomatosis, implants everywhere in the abdominal cavity,” one surgeon said in his survey response. “(He) went to another major center where they reoperated … I still have doubts about whether or not (a) I didn’t do enough for the patient or (b) the other center did too much.’’
Another surgeon reflected on the uncertainty of prognostication in some cases of advanced colon cancer. “I had a patient in the ICU with florid sepsis and multisystem organ failure. The entire care team began to wonder how long we should continue to press on with a patient who clearly could not survive. Days later he is awake and alert, off pressors, on trach collar, and fully communicative. Sometimes even experienced clinicians cannot predict when a patient will die or recover.”
Communication barriers remain. “A number of surgeons reported not knowing how to discuss this with families knowing that there is a stigma associated with palliative care – how to convey that they may pursue continued treatment in conjunction with palliative care,” said Dr. Suwanabol.
Surgeons adept at incorporating palliative care into their treatment plans continue to see the patients. “Even if a patient is not pursuing curative treatment, we do not want the patient to lose hope or feel abandoned by us, and I continue seeing my patients in follow-up until they feel overburdened by their clinic visits and choose not to come,” said Dr. Suwanabol.
In the absence of required palliative care training in medical schools or residencies, alternative approaches to achieving competency could include the American College of Surgeons Palliative Surgical Care Course, mentorships or collaborations with local palliative care specialists, or structured curricula implemented by experienced faculty, they added.
Dr. Suwanabol and her coauthors reported that they had no competing financial interests related to their study.
SOURCE: Suwanabol PA, et al. J Palliat Med. 2018 Mar 13. doi: 10.1089/jpm.2017.0470.
While to its optimal implementation at the patient, family, and clinician level, results of a recent survey suggest.
“We found that surgeons reported the most important barriers to be their own,” said Pasithorn A. Suwanabol, MD, division of colorectal surgery, University of Michigan, Ann Arbor, and coauthors of a report on the survey.
More than three-quarters of surgeons said they had no formal education in palliative care, and a substantial number specifically noted inadequate training in both communication and techniques to forgo life-sustaining measures, according to the report, published in the Journal of Palliative Medicine.
Dr. Suwanabol and her colleagues sought surgeon perspectives on palliative care for stage IV colorectal cancer in part because palliative care is often not integrated with cancer treatment in these potentially incurable patients.
“Compared with patients treated by primary providers, surgical patients with terminal diseases are significantly less likely to receive palliative or end-of-life care,” they said in the report.
They conducted a mixed methods study including members of a surgical society (American Society of Colon and Rectal Surgeons) who were asked to submit Internet responses to a validated survey. This mixed methods study is believed to be the first to characterize surgeons’ perceived barriers to optimal palliative and end-of life care for patients with advanced colorectal cancer, Dr. Suwanabol and her colleagues noted.
A total of 131 surgeons responded to the survey, for a response rate of 16.5%. The majority of surgeons responding (76.1%) said they did not have any formal palliative care training, while 42.7% said they lacked specific education in communication and 37.9% lacked training in techniques to forgo life-sustaining measures.
Many patients with stage IV colorectal cancer are candidates for palliative care. “Specialty palliative care should be offered to patients who have more complex issues such as refractory pain, depression and anxiety, and existential distress. In addition, specialty palliative care may be helpful in those with challenging family dynamics and/or conflict between family members and/or members of the care team. Patients with stage IV colon cancer may not be candidates for curative therapies and a palliative approach allows the surgeon and patient to carefully weigh the risk and benefits of each therapy in the context of the overall goals of the patient,” Dr. Suwanabol said in an interview.
Among the survey responders, 61.8% said discussion of palliation was limited because of patients and families who had unrealistic expectations and demanded aggressive interventions.
One such recollection in the report reads as follows: ‘‘Patient with poorly responding stage IV colon cancer in multisystem organ failure getting same chemo that already failed ... family wanted everything done…’’
The report includes a number of other reflections from surgeons that suggest a level of anxiety, frustration, disappointment, or uncertainty regarding palliative care.
“I once operated on a young man with carcinomatosis, implants everywhere in the abdominal cavity,” one surgeon said in his survey response. “(He) went to another major center where they reoperated … I still have doubts about whether or not (a) I didn’t do enough for the patient or (b) the other center did too much.’’
Another surgeon reflected on the uncertainty of prognostication in some cases of advanced colon cancer. “I had a patient in the ICU with florid sepsis and multisystem organ failure. The entire care team began to wonder how long we should continue to press on with a patient who clearly could not survive. Days later he is awake and alert, off pressors, on trach collar, and fully communicative. Sometimes even experienced clinicians cannot predict when a patient will die or recover.”
Communication barriers remain. “A number of surgeons reported not knowing how to discuss this with families knowing that there is a stigma associated with palliative care – how to convey that they may pursue continued treatment in conjunction with palliative care,” said Dr. Suwanabol.
Surgeons adept at incorporating palliative care into their treatment plans continue to see the patients. “Even if a patient is not pursuing curative treatment, we do not want the patient to lose hope or feel abandoned by us, and I continue seeing my patients in follow-up until they feel overburdened by their clinic visits and choose not to come,” said Dr. Suwanabol.
In the absence of required palliative care training in medical schools or residencies, alternative approaches to achieving competency could include the American College of Surgeons Palliative Surgical Care Course, mentorships or collaborations with local palliative care specialists, or structured curricula implemented by experienced faculty, they added.
Dr. Suwanabol and her coauthors reported that they had no competing financial interests related to their study.
SOURCE: Suwanabol PA, et al. J Palliat Med. 2018 Mar 13. doi: 10.1089/jpm.2017.0470.
While to its optimal implementation at the patient, family, and clinician level, results of a recent survey suggest.
“We found that surgeons reported the most important barriers to be their own,” said Pasithorn A. Suwanabol, MD, division of colorectal surgery, University of Michigan, Ann Arbor, and coauthors of a report on the survey.
More than three-quarters of surgeons said they had no formal education in palliative care, and a substantial number specifically noted inadequate training in both communication and techniques to forgo life-sustaining measures, according to the report, published in the Journal of Palliative Medicine.
Dr. Suwanabol and her colleagues sought surgeon perspectives on palliative care for stage IV colorectal cancer in part because palliative care is often not integrated with cancer treatment in these potentially incurable patients.
“Compared with patients treated by primary providers, surgical patients with terminal diseases are significantly less likely to receive palliative or end-of-life care,” they said in the report.
They conducted a mixed methods study including members of a surgical society (American Society of Colon and Rectal Surgeons) who were asked to submit Internet responses to a validated survey. This mixed methods study is believed to be the first to characterize surgeons’ perceived barriers to optimal palliative and end-of life care for patients with advanced colorectal cancer, Dr. Suwanabol and her colleagues noted.
A total of 131 surgeons responded to the survey, for a response rate of 16.5%. The majority of surgeons responding (76.1%) said they did not have any formal palliative care training, while 42.7% said they lacked specific education in communication and 37.9% lacked training in techniques to forgo life-sustaining measures.
Many patients with stage IV colorectal cancer are candidates for palliative care. “Specialty palliative care should be offered to patients who have more complex issues such as refractory pain, depression and anxiety, and existential distress. In addition, specialty palliative care may be helpful in those with challenging family dynamics and/or conflict between family members and/or members of the care team. Patients with stage IV colon cancer may not be candidates for curative therapies and a palliative approach allows the surgeon and patient to carefully weigh the risk and benefits of each therapy in the context of the overall goals of the patient,” Dr. Suwanabol said in an interview.
Among the survey responders, 61.8% said discussion of palliation was limited because of patients and families who had unrealistic expectations and demanded aggressive interventions.
One such recollection in the report reads as follows: ‘‘Patient with poorly responding stage IV colon cancer in multisystem organ failure getting same chemo that already failed ... family wanted everything done…’’
The report includes a number of other reflections from surgeons that suggest a level of anxiety, frustration, disappointment, or uncertainty regarding palliative care.
“I once operated on a young man with carcinomatosis, implants everywhere in the abdominal cavity,” one surgeon said in his survey response. “(He) went to another major center where they reoperated … I still have doubts about whether or not (a) I didn’t do enough for the patient or (b) the other center did too much.’’
Another surgeon reflected on the uncertainty of prognostication in some cases of advanced colon cancer. “I had a patient in the ICU with florid sepsis and multisystem organ failure. The entire care team began to wonder how long we should continue to press on with a patient who clearly could not survive. Days later he is awake and alert, off pressors, on trach collar, and fully communicative. Sometimes even experienced clinicians cannot predict when a patient will die or recover.”
Communication barriers remain. “A number of surgeons reported not knowing how to discuss this with families knowing that there is a stigma associated with palliative care – how to convey that they may pursue continued treatment in conjunction with palliative care,” said Dr. Suwanabol.
Surgeons adept at incorporating palliative care into their treatment plans continue to see the patients. “Even if a patient is not pursuing curative treatment, we do not want the patient to lose hope or feel abandoned by us, and I continue seeing my patients in follow-up until they feel overburdened by their clinic visits and choose not to come,” said Dr. Suwanabol.
In the absence of required palliative care training in medical schools or residencies, alternative approaches to achieving competency could include the American College of Surgeons Palliative Surgical Care Course, mentorships or collaborations with local palliative care specialists, or structured curricula implemented by experienced faculty, they added.
Dr. Suwanabol and her coauthors reported that they had no competing financial interests related to their study.
SOURCE: Suwanabol PA, et al. J Palliat Med. 2018 Mar 13. doi: 10.1089/jpm.2017.0470.
FROM THE JOURNAL OF PALLIATIVE MEDICINE
Key clinical point: Surgeons valued palliative and end-of-life care for patients with stage IV colorectal cancer, but reported multiple barriers to implementation.
Major finding: More than three-quarters of surgeons (76.1%) reported no formal education in palliative care, and 61.8% said patients and families had unrealistic expectations.
Study details: A mixed methods study including 131 members of a surgical society (American Society of Colon and Rectal Surgeons) who submitted Internet responses to a validated survey.
Disclosures: The authors declared that no competing interests relative to this report exist.
Source: Suwanabol PA et al. J Palliat Med. 2018 Mar 13. doi: 10.1089/jpm.2017.0470.
From the Washington Office: Upcoming Leadership and Advocacy Summit
The seventh annual American College of Surgeons Leadership and Advocacy Summit will be held May 19-22 at the Renaissance Washington, DC Downtown Hotel.
The event will kick off with the Leadership portion on Saturday evening, May 19, with a Welcome Reception open to all registrants and continues with a full-day agenda on Sunday, May 20. The program on Sunday includes sessions addressing important topics such as mentoring for a career in surgical leadership, ethics in surgical leadership, leading in times of crisis, change management, managing complex teams, and more.
1) “Understanding Strategic Advocacy” presented by staff of the Washington office
2) “Regulatory Reform: Past, Present, and Patient-Focused” featuring staff from the Centers for Medicare and Medicaid Services.
3) A historical perspective on health care reform entitled, “Health Care Reform, Then and Now,” presented by long-time Health Affairs columnist, Professor Timothy S. Jost.
4) “The Opioid Epidemic: Long-term Solutions for Sustained Success” featuring staff from the Food and Drug Administration and the Drug Enforcement Administration.
5) A luncheon, sponsored by the ACSPA-SurgeonsPAC, where attendees will hear remarks on the upcoming mid-term elections from the Executive Directors of both the Democratic Congressional Campaign Committee (DCCC) and the National Republican Congressional Committee (NRCC).
The day will also include issue briefings and specific “asks” on topics in preparation for Hill visits. Specifically, attendees will be briefed on the Pandemic and All-Hazards Preparedness Act (PAHPA), the Standardizing Electronic Prior Authorization for Safe Prescribing Act, the Ensuring Access to General Surgery Act, the Removing Barriers to Colorectal Screening Act, the Childhood Cancer STAR Act, and funding for the CDC to conduct research on firearm injury prevention. Following this training, Fellows will be very well prepared to discuss the issues the following day on Capitol Hill.
Pending last minute conflicts, several Members of Congress are also scheduled to address the group, including a member of leadership from the House of Representatives. Monday’s activities will conclude with an evening reception for 2018 SurgeonsPAC members at the historic Willard InterContinental Hotel. On Tuesday, May 22, attendees will then apply the knowledge and skill gained from Monday’s sessions during meetings with their individual Members of Congress and their staff on Capitol Hill.
As I write, nearly three weeks prior to the event, attendance is already projected to be at record levels. We look forward to welcoming all those already registered to DC for this exciting, informative and important event. Though by press time pre-registration will have closed, on-site registration will be available if you would be able to join us.
For questions regarding the Leadership Summit please contact Brian Frankel at [email protected], or 312-202-5361. For questions regarding the Advocacy Summit please contact Michael Carmody at [email protected], or 202-672-1511.
Until next month ….
The seventh annual American College of Surgeons Leadership and Advocacy Summit will be held May 19-22 at the Renaissance Washington, DC Downtown Hotel.
The event will kick off with the Leadership portion on Saturday evening, May 19, with a Welcome Reception open to all registrants and continues with a full-day agenda on Sunday, May 20. The program on Sunday includes sessions addressing important topics such as mentoring for a career in surgical leadership, ethics in surgical leadership, leading in times of crisis, change management, managing complex teams, and more.
1) “Understanding Strategic Advocacy” presented by staff of the Washington office
2) “Regulatory Reform: Past, Present, and Patient-Focused” featuring staff from the Centers for Medicare and Medicaid Services.
3) A historical perspective on health care reform entitled, “Health Care Reform, Then and Now,” presented by long-time Health Affairs columnist, Professor Timothy S. Jost.
4) “The Opioid Epidemic: Long-term Solutions for Sustained Success” featuring staff from the Food and Drug Administration and the Drug Enforcement Administration.
5) A luncheon, sponsored by the ACSPA-SurgeonsPAC, where attendees will hear remarks on the upcoming mid-term elections from the Executive Directors of both the Democratic Congressional Campaign Committee (DCCC) and the National Republican Congressional Committee (NRCC).
The day will also include issue briefings and specific “asks” on topics in preparation for Hill visits. Specifically, attendees will be briefed on the Pandemic and All-Hazards Preparedness Act (PAHPA), the Standardizing Electronic Prior Authorization for Safe Prescribing Act, the Ensuring Access to General Surgery Act, the Removing Barriers to Colorectal Screening Act, the Childhood Cancer STAR Act, and funding for the CDC to conduct research on firearm injury prevention. Following this training, Fellows will be very well prepared to discuss the issues the following day on Capitol Hill.
Pending last minute conflicts, several Members of Congress are also scheduled to address the group, including a member of leadership from the House of Representatives. Monday’s activities will conclude with an evening reception for 2018 SurgeonsPAC members at the historic Willard InterContinental Hotel. On Tuesday, May 22, attendees will then apply the knowledge and skill gained from Monday’s sessions during meetings with their individual Members of Congress and their staff on Capitol Hill.
As I write, nearly three weeks prior to the event, attendance is already projected to be at record levels. We look forward to welcoming all those already registered to DC for this exciting, informative and important event. Though by press time pre-registration will have closed, on-site registration will be available if you would be able to join us.
For questions regarding the Leadership Summit please contact Brian Frankel at [email protected], or 312-202-5361. For questions regarding the Advocacy Summit please contact Michael Carmody at [email protected], or 202-672-1511.
Until next month ….
The seventh annual American College of Surgeons Leadership and Advocacy Summit will be held May 19-22 at the Renaissance Washington, DC Downtown Hotel.
The event will kick off with the Leadership portion on Saturday evening, May 19, with a Welcome Reception open to all registrants and continues with a full-day agenda on Sunday, May 20. The program on Sunday includes sessions addressing important topics such as mentoring for a career in surgical leadership, ethics in surgical leadership, leading in times of crisis, change management, managing complex teams, and more.
1) “Understanding Strategic Advocacy” presented by staff of the Washington office
2) “Regulatory Reform: Past, Present, and Patient-Focused” featuring staff from the Centers for Medicare and Medicaid Services.
3) A historical perspective on health care reform entitled, “Health Care Reform, Then and Now,” presented by long-time Health Affairs columnist, Professor Timothy S. Jost.
4) “The Opioid Epidemic: Long-term Solutions for Sustained Success” featuring staff from the Food and Drug Administration and the Drug Enforcement Administration.
5) A luncheon, sponsored by the ACSPA-SurgeonsPAC, where attendees will hear remarks on the upcoming mid-term elections from the Executive Directors of both the Democratic Congressional Campaign Committee (DCCC) and the National Republican Congressional Committee (NRCC).
The day will also include issue briefings and specific “asks” on topics in preparation for Hill visits. Specifically, attendees will be briefed on the Pandemic and All-Hazards Preparedness Act (PAHPA), the Standardizing Electronic Prior Authorization for Safe Prescribing Act, the Ensuring Access to General Surgery Act, the Removing Barriers to Colorectal Screening Act, the Childhood Cancer STAR Act, and funding for the CDC to conduct research on firearm injury prevention. Following this training, Fellows will be very well prepared to discuss the issues the following day on Capitol Hill.
Pending last minute conflicts, several Members of Congress are also scheduled to address the group, including a member of leadership from the House of Representatives. Monday’s activities will conclude with an evening reception for 2018 SurgeonsPAC members at the historic Willard InterContinental Hotel. On Tuesday, May 22, attendees will then apply the knowledge and skill gained from Monday’s sessions during meetings with their individual Members of Congress and their staff on Capitol Hill.
As I write, nearly three weeks prior to the event, attendance is already projected to be at record levels. We look forward to welcoming all those already registered to DC for this exciting, informative and important event. Though by press time pre-registration will have closed, on-site registration will be available if you would be able to join us.
For questions regarding the Leadership Summit please contact Brian Frankel at [email protected], or 312-202-5361. For questions regarding the Advocacy Summit please contact Michael Carmody at [email protected], or 202-672-1511.
Until next month ….
What is an old doctor to do?
I was in Miami recently to give a talk on diabetes when a physician, Pablo Michel, MD, asked me whether we could address an issue that’s important to him and many of his colleagues. His question was, Do we have any suggestions about how to help “older doctors” such as himself deal with electronic health records?
One of the problems with his question was that he didn’t really look “old”; he looked like he was about 50 years of age and in good shape. This physician had come on a Saturday morning to spend 4 hours learning about diabetes, which made it clear that he cared about his patients, his craft, and staying current with the medical literature.
I was struck by his questions, as well as his concern for both the quality of care for his patients and the issues he and his colleagues were facing. And it is not just him. Increased computerization of practices has been listed among the top five causes of physician burnout.1
A recent article in Annals of Internal Medicine showed that physicians spent only a quarter of their total time directly talking with patients and 50% of their time on EHR and other administrative tasks.2 It is likely that, among older physicians, the EHR takes proportionally more time and is an even larger cause of burnout. Given the importance of EHR, it seems time to revisit both the dilemma of, and propose some solutions for, this common problem.
One of the core issues for many older physicians is an inability to type. If you don’t type well, then entering a patient’s history or documenting the assessment and plan is unduly burdensome. Ten years ago, we might have suggested learning to type, which was an unrealistic recommendation then and, fortunately, is unnecessary now.
Now, solutions ranging from medical scribes to voice recognition have become commonplace. Voice recognition technology has advanced incredibly over the past 10 years, so much so that it is used now in our everyday life. The most well-known voice technology in everyday life might be Siri, Apple’s voice technology. It is easy now to dictate texts and to look up information. Similar voice technologies are available with the Amazon Echo and Google Assistant.
We now also have the advantage of well-developed medical voice recognition technology that can be used with most EHRs. Although some doctors say that the software is expensive, it can cost about $1,500 for the software and another $200-$300 for a good microphone, as well as the time to train on the software. But that expense needs to be weighed against the lost productivity of not using such software. A common complaint we hear from older doctors is that they are spending 1 to 2 hours a night completing charts. If voice recognition software could shave off half that time, decrease stress, and increase satisfaction, then it would pay for itself in 2 weeks.
Another issue is that, because the EHR enables so many things to be done from the EHR platform, many doctors find themselves doing all the work. It is important to work as a team and let each member of the team contribute to making the process more efficient. It turns out that this usually ends up being satisfying for everyone who contributes to patient care. It requires standing back from the process periodically and thinking about areas of inefficiency and how things can be done better.
One clear example is medication reconciliation: A nurse or clinical pharmacist can go over medicines with patients, and while the physician still needs to review the medications, it takes much less time to review medications than it does to enter each medication with the correct dose. Nurses also can help with preventive health initiatives. Performing recommended preventive health activities ranging from hepatitis C screening to colonoscopy can be greatly facilitated by the participation of nursing staff, and their participation will free up doctors so they can have more time to focus on diagnosis and treatment. Teamwork is critical.
Finally, if you don’t know something that is important to your practice – learn it! We are accustomed to going to CME conferences and spending our time learning about diseases like diabetes, asthma, and COPD. Each of these disease accounts for 5%-10% of the patients we see in our practice, and it is critically important to stay current and learn about them. We use our EHR for 100% of the patients we see; therefore, we should allocate time to learning about how to navigate the EHR and work more efficiently with it.
These issues are real, and the processes continue to change, but by standing back and acknowledging the challenges, we can thoughtfully construct an approach to maximize our ability to continue to have productive, gratifying careers while helping our patients.
Dr. Skolnik is a professor of family and community medicine at Jefferson Medical College, Philadelphia, and an associate director of the family medicine residency program at Abington (Pa.) Jefferson Health. Dr. Notte is a family physician and associate chief medical information officer for Abington Jefferson Health. Follow him on twitter @doctornotte.
References
1. Medscape Physician Lifestyle Report 2015. Accessed April 27, 2018. https://www.medscape.com/slideshow/lifestyle-2015-overview-6006535#1.
2. Sinsky C et al. Ann Intern Med. 2016;165(11):753-60.
I was in Miami recently to give a talk on diabetes when a physician, Pablo Michel, MD, asked me whether we could address an issue that’s important to him and many of his colleagues. His question was, Do we have any suggestions about how to help “older doctors” such as himself deal with electronic health records?
One of the problems with his question was that he didn’t really look “old”; he looked like he was about 50 years of age and in good shape. This physician had come on a Saturday morning to spend 4 hours learning about diabetes, which made it clear that he cared about his patients, his craft, and staying current with the medical literature.
I was struck by his questions, as well as his concern for both the quality of care for his patients and the issues he and his colleagues were facing. And it is not just him. Increased computerization of practices has been listed among the top five causes of physician burnout.1
A recent article in Annals of Internal Medicine showed that physicians spent only a quarter of their total time directly talking with patients and 50% of their time on EHR and other administrative tasks.2 It is likely that, among older physicians, the EHR takes proportionally more time and is an even larger cause of burnout. Given the importance of EHR, it seems time to revisit both the dilemma of, and propose some solutions for, this common problem.
One of the core issues for many older physicians is an inability to type. If you don’t type well, then entering a patient’s history or documenting the assessment and plan is unduly burdensome. Ten years ago, we might have suggested learning to type, which was an unrealistic recommendation then and, fortunately, is unnecessary now.
Now, solutions ranging from medical scribes to voice recognition have become commonplace. Voice recognition technology has advanced incredibly over the past 10 years, so much so that it is used now in our everyday life. The most well-known voice technology in everyday life might be Siri, Apple’s voice technology. It is easy now to dictate texts and to look up information. Similar voice technologies are available with the Amazon Echo and Google Assistant.
We now also have the advantage of well-developed medical voice recognition technology that can be used with most EHRs. Although some doctors say that the software is expensive, it can cost about $1,500 for the software and another $200-$300 for a good microphone, as well as the time to train on the software. But that expense needs to be weighed against the lost productivity of not using such software. A common complaint we hear from older doctors is that they are spending 1 to 2 hours a night completing charts. If voice recognition software could shave off half that time, decrease stress, and increase satisfaction, then it would pay for itself in 2 weeks.
Another issue is that, because the EHR enables so many things to be done from the EHR platform, many doctors find themselves doing all the work. It is important to work as a team and let each member of the team contribute to making the process more efficient. It turns out that this usually ends up being satisfying for everyone who contributes to patient care. It requires standing back from the process periodically and thinking about areas of inefficiency and how things can be done better.
One clear example is medication reconciliation: A nurse or clinical pharmacist can go over medicines with patients, and while the physician still needs to review the medications, it takes much less time to review medications than it does to enter each medication with the correct dose. Nurses also can help with preventive health initiatives. Performing recommended preventive health activities ranging from hepatitis C screening to colonoscopy can be greatly facilitated by the participation of nursing staff, and their participation will free up doctors so they can have more time to focus on diagnosis and treatment. Teamwork is critical.
Finally, if you don’t know something that is important to your practice – learn it! We are accustomed to going to CME conferences and spending our time learning about diseases like diabetes, asthma, and COPD. Each of these disease accounts for 5%-10% of the patients we see in our practice, and it is critically important to stay current and learn about them. We use our EHR for 100% of the patients we see; therefore, we should allocate time to learning about how to navigate the EHR and work more efficiently with it.
These issues are real, and the processes continue to change, but by standing back and acknowledging the challenges, we can thoughtfully construct an approach to maximize our ability to continue to have productive, gratifying careers while helping our patients.
Dr. Skolnik is a professor of family and community medicine at Jefferson Medical College, Philadelphia, and an associate director of the family medicine residency program at Abington (Pa.) Jefferson Health. Dr. Notte is a family physician and associate chief medical information officer for Abington Jefferson Health. Follow him on twitter @doctornotte.
References
1. Medscape Physician Lifestyle Report 2015. Accessed April 27, 2018. https://www.medscape.com/slideshow/lifestyle-2015-overview-6006535#1.
2. Sinsky C et al. Ann Intern Med. 2016;165(11):753-60.
I was in Miami recently to give a talk on diabetes when a physician, Pablo Michel, MD, asked me whether we could address an issue that’s important to him and many of his colleagues. His question was, Do we have any suggestions about how to help “older doctors” such as himself deal with electronic health records?
One of the problems with his question was that he didn’t really look “old”; he looked like he was about 50 years of age and in good shape. This physician had come on a Saturday morning to spend 4 hours learning about diabetes, which made it clear that he cared about his patients, his craft, and staying current with the medical literature.
I was struck by his questions, as well as his concern for both the quality of care for his patients and the issues he and his colleagues were facing. And it is not just him. Increased computerization of practices has been listed among the top five causes of physician burnout.1
A recent article in Annals of Internal Medicine showed that physicians spent only a quarter of their total time directly talking with patients and 50% of their time on EHR and other administrative tasks.2 It is likely that, among older physicians, the EHR takes proportionally more time and is an even larger cause of burnout. Given the importance of EHR, it seems time to revisit both the dilemma of, and propose some solutions for, this common problem.
One of the core issues for many older physicians is an inability to type. If you don’t type well, then entering a patient’s history or documenting the assessment and plan is unduly burdensome. Ten years ago, we might have suggested learning to type, which was an unrealistic recommendation then and, fortunately, is unnecessary now.
Now, solutions ranging from medical scribes to voice recognition have become commonplace. Voice recognition technology has advanced incredibly over the past 10 years, so much so that it is used now in our everyday life. The most well-known voice technology in everyday life might be Siri, Apple’s voice technology. It is easy now to dictate texts and to look up information. Similar voice technologies are available with the Amazon Echo and Google Assistant.
We now also have the advantage of well-developed medical voice recognition technology that can be used with most EHRs. Although some doctors say that the software is expensive, it can cost about $1,500 for the software and another $200-$300 for a good microphone, as well as the time to train on the software. But that expense needs to be weighed against the lost productivity of not using such software. A common complaint we hear from older doctors is that they are spending 1 to 2 hours a night completing charts. If voice recognition software could shave off half that time, decrease stress, and increase satisfaction, then it would pay for itself in 2 weeks.
Another issue is that, because the EHR enables so many things to be done from the EHR platform, many doctors find themselves doing all the work. It is important to work as a team and let each member of the team contribute to making the process more efficient. It turns out that this usually ends up being satisfying for everyone who contributes to patient care. It requires standing back from the process periodically and thinking about areas of inefficiency and how things can be done better.
One clear example is medication reconciliation: A nurse or clinical pharmacist can go over medicines with patients, and while the physician still needs to review the medications, it takes much less time to review medications than it does to enter each medication with the correct dose. Nurses also can help with preventive health initiatives. Performing recommended preventive health activities ranging from hepatitis C screening to colonoscopy can be greatly facilitated by the participation of nursing staff, and their participation will free up doctors so they can have more time to focus on diagnosis and treatment. Teamwork is critical.
Finally, if you don’t know something that is important to your practice – learn it! We are accustomed to going to CME conferences and spending our time learning about diseases like diabetes, asthma, and COPD. Each of these disease accounts for 5%-10% of the patients we see in our practice, and it is critically important to stay current and learn about them. We use our EHR for 100% of the patients we see; therefore, we should allocate time to learning about how to navigate the EHR and work more efficiently with it.
These issues are real, and the processes continue to change, but by standing back and acknowledging the challenges, we can thoughtfully construct an approach to maximize our ability to continue to have productive, gratifying careers while helping our patients.
Dr. Skolnik is a professor of family and community medicine at Jefferson Medical College, Philadelphia, and an associate director of the family medicine residency program at Abington (Pa.) Jefferson Health. Dr. Notte is a family physician and associate chief medical information officer for Abington Jefferson Health. Follow him on twitter @doctornotte.
References
1. Medscape Physician Lifestyle Report 2015. Accessed April 27, 2018. https://www.medscape.com/slideshow/lifestyle-2015-overview-6006535#1.
2. Sinsky C et al. Ann Intern Med. 2016;165(11):753-60.
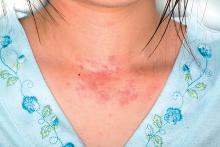
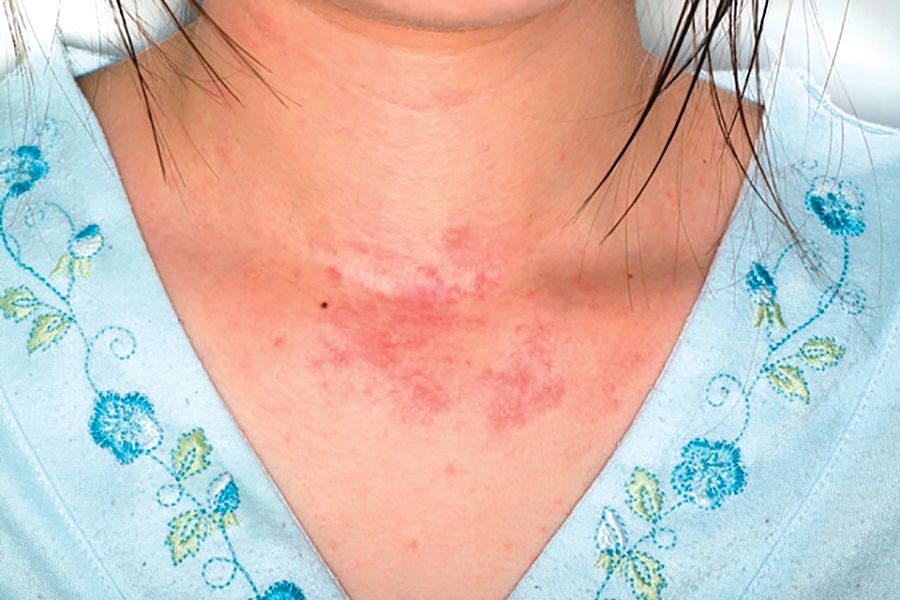
Allergy, eczema common after pediatric solid organ transplantation
A total of 34% of children who underwent solid organ transplantation subsequently developed eczema, food allergy, rhinitis, eosinophilic gastrointestinal disease, or asthma, according to the results of a single-center retrospective cohort study.
Another 6.6% of patients developed autoimmunity, usually autoimmune cytopenia, inflammatory bowel disease, or vasculitis, wrote Nufar Marcus, MD, of the University of Toronto, and her associates.
Posttransplant allergy, autoimmunity, and immune-mediated disorders (PTAA) likely share a common pathogenesis “and may represent a unique state of post-transplant immune-dysregulation,” they wrote. The report was published in the Journal of Pediatrics.
The study included 273 children who underwent solid organ transplantation and were followed for a median 3.6 years (range, 1.7-6.3 years). None had immune-mediated conditions or allergies diagnosed at baseline. Posttransplantation allergies most commonly included eczema (51%), asthma (32%), food allergy (25%, including 5% with associated anaphylaxis), rhinitis (17%), and eosinophilic esophagitis, gastritis, or enteritis (13%).
Although only 31% of patients had information available on family history of allergy, those with a positive family history of allergy had a fivefold greater odds of posttransplantation PTAA, compared with other patients. Other risk factors for PTAA included female sex, young age at transplantation, eosinophilia, and a positive test for Epstein-Barr virus after transplantation, Dr. Marcus and associates said.
“The association of blood eosinophilia and PTAA reached statistical significance only when the transplant recipient was at least 6 months of age, demonstrating the nonspecific nature of abnormally high eosinophil counts during the first months of life,” they noted. The longer patients had eosinophilia after transplantation, the more likely they were to develop PTAA, “suggest[ing] a potential detrimental effect of prolonged activation of the eosinophilic-associated immune arms.”
Factors that appeared unlinked with PTAA included acute organ rejection, duration of posttransplantation steroidal treatment, organ type (living versus cadaveric), donor/recipient blood type and compatibility, infections besides Epstein-Barr virus, and posttransplant lymphoproliferative disease. “The specific type of post-transplantation immunosuppression regimen was neither associated nor protective of PTAA,” the investigators wrote. “However, a significant limitation was our inability to assess the effect of tacrolimus, as nearly all the cohort (97.8%) was treated with this medication.”
Ashley’s Angels fund provided support. The researchers reported having no conflicts of interest.
SOURCE: Marcus N et al. J Pediatr. 2018;196:154-60.
The study is one of several to highlight the occurrence of atopy and allergy following solid organ transplantation in children, Helen M. Evans, MBChB, wrote in an editorial accompanying the report by Marcus et al.
This report differed because it studied the differences in rates of atopy and allergy between transplanted solid organ groups. These occurred in 41% and 40% of liver and heart recipients, respectively, but in only 4% of kidney recipients. Atopy or allergy developed in 57% of multivisceral transplant patients, but the number of patients was very small (n = 7). The majority of the conditions developed within 1 year of transplantation.
The recent spike in these reports could signify better recognition of the problem or “the widespread switch of primary immunosuppression from cyclosporine to tacrolimus over the last few decades,” wrote Dr. Evans.
Most of these reports have been single-center retrospective studies, which are subject to inconsistent case definitions and recall bias, she noted. “The time is right for well-conducted multicenter prospective studies to better inform the true extent of these conditions after solid organ transplantation.”
In the meantime, transplantation centers should routinely track de novo eczema, allergy, and eosinophilic gastrointestinal disease in children being assessed for solid organ transplantation, and should take “rigorous” personal and family histories, said Dr. Evans. Ultimately, this work will help “minimize the risk of children developing these conditions” and “effectively treat them in the setting of immunosuppression after transplantation.”
Dr. Evans is a pediatric gastroenterologist at Starship Child Health in Aukland, New Zealand. She reported having no conflicts of interest. These comments summarize her editorial ( J Pediatr. 2018;196:10-11 ).
The study is one of several to highlight the occurrence of atopy and allergy following solid organ transplantation in children, Helen M. Evans, MBChB, wrote in an editorial accompanying the report by Marcus et al.
This report differed because it studied the differences in rates of atopy and allergy between transplanted solid organ groups. These occurred in 41% and 40% of liver and heart recipients, respectively, but in only 4% of kidney recipients. Atopy or allergy developed in 57% of multivisceral transplant patients, but the number of patients was very small (n = 7). The majority of the conditions developed within 1 year of transplantation.
The recent spike in these reports could signify better recognition of the problem or “the widespread switch of primary immunosuppression from cyclosporine to tacrolimus over the last few decades,” wrote Dr. Evans.
Most of these reports have been single-center retrospective studies, which are subject to inconsistent case definitions and recall bias, she noted. “The time is right for well-conducted multicenter prospective studies to better inform the true extent of these conditions after solid organ transplantation.”
In the meantime, transplantation centers should routinely track de novo eczema, allergy, and eosinophilic gastrointestinal disease in children being assessed for solid organ transplantation, and should take “rigorous” personal and family histories, said Dr. Evans. Ultimately, this work will help “minimize the risk of children developing these conditions” and “effectively treat them in the setting of immunosuppression after transplantation.”
Dr. Evans is a pediatric gastroenterologist at Starship Child Health in Aukland, New Zealand. She reported having no conflicts of interest. These comments summarize her editorial ( J Pediatr. 2018;196:10-11 ).
The study is one of several to highlight the occurrence of atopy and allergy following solid organ transplantation in children, Helen M. Evans, MBChB, wrote in an editorial accompanying the report by Marcus et al.
This report differed because it studied the differences in rates of atopy and allergy between transplanted solid organ groups. These occurred in 41% and 40% of liver and heart recipients, respectively, but in only 4% of kidney recipients. Atopy or allergy developed in 57% of multivisceral transplant patients, but the number of patients was very small (n = 7). The majority of the conditions developed within 1 year of transplantation.
The recent spike in these reports could signify better recognition of the problem or “the widespread switch of primary immunosuppression from cyclosporine to tacrolimus over the last few decades,” wrote Dr. Evans.
Most of these reports have been single-center retrospective studies, which are subject to inconsistent case definitions and recall bias, she noted. “The time is right for well-conducted multicenter prospective studies to better inform the true extent of these conditions after solid organ transplantation.”
In the meantime, transplantation centers should routinely track de novo eczema, allergy, and eosinophilic gastrointestinal disease in children being assessed for solid organ transplantation, and should take “rigorous” personal and family histories, said Dr. Evans. Ultimately, this work will help “minimize the risk of children developing these conditions” and “effectively treat them in the setting of immunosuppression after transplantation.”
Dr. Evans is a pediatric gastroenterologist at Starship Child Health in Aukland, New Zealand. She reported having no conflicts of interest. These comments summarize her editorial ( J Pediatr. 2018;196:10-11 ).
A total of 34% of children who underwent solid organ transplantation subsequently developed eczema, food allergy, rhinitis, eosinophilic gastrointestinal disease, or asthma, according to the results of a single-center retrospective cohort study.
Another 6.6% of patients developed autoimmunity, usually autoimmune cytopenia, inflammatory bowel disease, or vasculitis, wrote Nufar Marcus, MD, of the University of Toronto, and her associates.
Posttransplant allergy, autoimmunity, and immune-mediated disorders (PTAA) likely share a common pathogenesis “and may represent a unique state of post-transplant immune-dysregulation,” they wrote. The report was published in the Journal of Pediatrics.
The study included 273 children who underwent solid organ transplantation and were followed for a median 3.6 years (range, 1.7-6.3 years). None had immune-mediated conditions or allergies diagnosed at baseline. Posttransplantation allergies most commonly included eczema (51%), asthma (32%), food allergy (25%, including 5% with associated anaphylaxis), rhinitis (17%), and eosinophilic esophagitis, gastritis, or enteritis (13%).
Although only 31% of patients had information available on family history of allergy, those with a positive family history of allergy had a fivefold greater odds of posttransplantation PTAA, compared with other patients. Other risk factors for PTAA included female sex, young age at transplantation, eosinophilia, and a positive test for Epstein-Barr virus after transplantation, Dr. Marcus and associates said.
“The association of blood eosinophilia and PTAA reached statistical significance only when the transplant recipient was at least 6 months of age, demonstrating the nonspecific nature of abnormally high eosinophil counts during the first months of life,” they noted. The longer patients had eosinophilia after transplantation, the more likely they were to develop PTAA, “suggest[ing] a potential detrimental effect of prolonged activation of the eosinophilic-associated immune arms.”
Factors that appeared unlinked with PTAA included acute organ rejection, duration of posttransplantation steroidal treatment, organ type (living versus cadaveric), donor/recipient blood type and compatibility, infections besides Epstein-Barr virus, and posttransplant lymphoproliferative disease. “The specific type of post-transplantation immunosuppression regimen was neither associated nor protective of PTAA,” the investigators wrote. “However, a significant limitation was our inability to assess the effect of tacrolimus, as nearly all the cohort (97.8%) was treated with this medication.”
Ashley’s Angels fund provided support. The researchers reported having no conflicts of interest.
SOURCE: Marcus N et al. J Pediatr. 2018;196:154-60.
A total of 34% of children who underwent solid organ transplantation subsequently developed eczema, food allergy, rhinitis, eosinophilic gastrointestinal disease, or asthma, according to the results of a single-center retrospective cohort study.
Another 6.6% of patients developed autoimmunity, usually autoimmune cytopenia, inflammatory bowel disease, or vasculitis, wrote Nufar Marcus, MD, of the University of Toronto, and her associates.
Posttransplant allergy, autoimmunity, and immune-mediated disorders (PTAA) likely share a common pathogenesis “and may represent a unique state of post-transplant immune-dysregulation,” they wrote. The report was published in the Journal of Pediatrics.
The study included 273 children who underwent solid organ transplantation and were followed for a median 3.6 years (range, 1.7-6.3 years). None had immune-mediated conditions or allergies diagnosed at baseline. Posttransplantation allergies most commonly included eczema (51%), asthma (32%), food allergy (25%, including 5% with associated anaphylaxis), rhinitis (17%), and eosinophilic esophagitis, gastritis, or enteritis (13%).
Although only 31% of patients had information available on family history of allergy, those with a positive family history of allergy had a fivefold greater odds of posttransplantation PTAA, compared with other patients. Other risk factors for PTAA included female sex, young age at transplantation, eosinophilia, and a positive test for Epstein-Barr virus after transplantation, Dr. Marcus and associates said.
“The association of blood eosinophilia and PTAA reached statistical significance only when the transplant recipient was at least 6 months of age, demonstrating the nonspecific nature of abnormally high eosinophil counts during the first months of life,” they noted. The longer patients had eosinophilia after transplantation, the more likely they were to develop PTAA, “suggest[ing] a potential detrimental effect of prolonged activation of the eosinophilic-associated immune arms.”
Factors that appeared unlinked with PTAA included acute organ rejection, duration of posttransplantation steroidal treatment, organ type (living versus cadaveric), donor/recipient blood type and compatibility, infections besides Epstein-Barr virus, and posttransplant lymphoproliferative disease. “The specific type of post-transplantation immunosuppression regimen was neither associated nor protective of PTAA,” the investigators wrote. “However, a significant limitation was our inability to assess the effect of tacrolimus, as nearly all the cohort (97.8%) was treated with this medication.”
Ashley’s Angels fund provided support. The researchers reported having no conflicts of interest.
SOURCE: Marcus N et al. J Pediatr. 2018;196:154-60.
FROM JOURNAL OF PEDIATRICS
Key clinical point: Children undergoing solid organ transplantation often developed allergy or autoimmunity.
Major finding: Study details: Single-center retrospective cross-sectional study of 273 patients aged 18 and under who underwent solid organ transplantation followed for a median 3.6 years.
Disclosures: Ashley’s Angels fund provided support. The researchers reported having no conflicts of interest.
Source: Marcus N et al. J Pediatr. 2018;196:154-60.
CMS will release Medicare Advantage claims data to researchers
WASHINGTON – Centers for Medicare & Medicaid Services Administrator Seema Verma announced.
“We recognize that the Medicare Advantage data are not perfect, but we have determined that the quality of the available data is adequate enough to support research,” Ms. Verma told attendees April 26 at an annual conference on health data and innovation.
CMS is starting with the Medicare managed care plans’ encounter data from 2015, and Ms. Verma said the data will be updated annually.
In addition, she announced that in 2019 CMS will make Medicaid and Children’s Health Insurance Program data available. That will give researchers access to data from another 70 million patients.
Ms. Verma noted that the Medicaid population includes a range of people, including people with disabilities, pregnant women, children, and low-income adults. Those low-income adults “often experience multiple health issues and face challenges managing their care,” Ms. Verma noted. “Our hope is that these data will be used for critical research on this vulnerable population.”
CMS also will look to the health information technology developer community to create open application program interface tools “to modernize how we share data with our partners,” she said. That push is part of the overall MyHealthEData initiative to improve patient access to their health data and become more informed about their own health care.
“Who knows what knowledge, treatments, and cures are hidden in the reams of CMS data?” Ms. Verma said. “Help us use it securely. After all, this is knowledge that could change the life of a patient or the trajectory of a health care system.”
WASHINGTON – Centers for Medicare & Medicaid Services Administrator Seema Verma announced.
“We recognize that the Medicare Advantage data are not perfect, but we have determined that the quality of the available data is adequate enough to support research,” Ms. Verma told attendees April 26 at an annual conference on health data and innovation.
CMS is starting with the Medicare managed care plans’ encounter data from 2015, and Ms. Verma said the data will be updated annually.
In addition, she announced that in 2019 CMS will make Medicaid and Children’s Health Insurance Program data available. That will give researchers access to data from another 70 million patients.
Ms. Verma noted that the Medicaid population includes a range of people, including people with disabilities, pregnant women, children, and low-income adults. Those low-income adults “often experience multiple health issues and face challenges managing their care,” Ms. Verma noted. “Our hope is that these data will be used for critical research on this vulnerable population.”
CMS also will look to the health information technology developer community to create open application program interface tools “to modernize how we share data with our partners,” she said. That push is part of the overall MyHealthEData initiative to improve patient access to their health data and become more informed about their own health care.
“Who knows what knowledge, treatments, and cures are hidden in the reams of CMS data?” Ms. Verma said. “Help us use it securely. After all, this is knowledge that could change the life of a patient or the trajectory of a health care system.”
WASHINGTON – Centers for Medicare & Medicaid Services Administrator Seema Verma announced.
“We recognize that the Medicare Advantage data are not perfect, but we have determined that the quality of the available data is adequate enough to support research,” Ms. Verma told attendees April 26 at an annual conference on health data and innovation.
CMS is starting with the Medicare managed care plans’ encounter data from 2015, and Ms. Verma said the data will be updated annually.
In addition, she announced that in 2019 CMS will make Medicaid and Children’s Health Insurance Program data available. That will give researchers access to data from another 70 million patients.
Ms. Verma noted that the Medicaid population includes a range of people, including people with disabilities, pregnant women, children, and low-income adults. Those low-income adults “often experience multiple health issues and face challenges managing their care,” Ms. Verma noted. “Our hope is that these data will be used for critical research on this vulnerable population.”
CMS also will look to the health information technology developer community to create open application program interface tools “to modernize how we share data with our partners,” she said. That push is part of the overall MyHealthEData initiative to improve patient access to their health data and become more informed about their own health care.
“Who knows what knowledge, treatments, and cures are hidden in the reams of CMS data?” Ms. Verma said. “Help us use it securely. After all, this is knowledge that could change the life of a patient or the trajectory of a health care system.”
REPORTING FROM HEALTH DATAPALOOZA 2018
Leg lymphedema after gynecologic lymphadenectomy exceeds expectations
NEW ORLEANS – Leg lymphedema occurred in 19%-40% of women with a gynecologic cancer who underwent surgery with lymphadenectomy in a prospective study of 821 U.S. patients.
The incidence of lymphedema of the lower extremity (LLE) during 2 years of follow-up was 18% among 672 endometrial cancer patients, 25% among 124 cervical cancer patients, and 40% among 24 vulvar cancer patients, Jay W. Carlson, DO, said at the annual meeting of the Society of Gynecologic Oncology.
Although the study followed patients for 2 years after surgery, 84% of the LLE events occurred within the first 6 months after surgery, and 95% within the first 12 months. The robust incidence rates documented in this study contrasted with a general perception that LLE is relatively uncommon, leading Dr. Carlson to note that the new data show “the incidence of LLE is under recognized.” The findings also bucked conventional wisdom by showing no link between the incidence of LLE and number of lymph nodes dissected or with use of radiation treatment, said Dr. Carlson, a gynecologic oncologist at Mercy Clinic Women’s Oncology in Springfield, Mo.
To better define the incidence of LLE after lymphadenectomy for gynecologic cancers, the Gynecologic Oncology Group organized the Lymphedema and Gynecologic Cancer (LEG) study, run at more than 70 U.S. centers during June 2012–November 2014. The study enrolled patients scheduled for surgery to treat endometrial, cervical, or vulvar cancer, and applied systematic leg measurement to patients just before and at several prespecified times following surgery through 2 years of follow-up.
The study began with a total of 1,054 patients, but the final analysis that Dr. Carlson presented excluded patients who did not actually undergo lymphadenectomy during their surgery, did not have leg volume data available both before and after their surgery, or had a comorbidity or change in body mass that could have caused the change in leg size. The researchers also required patients identified with LLE to have completed the Gynecologic Cancer Lymphedema Questionnaire (Gynecol Oncol. 2010 May;117[2]:317-23) and tallied a score of at least 4, and to have at least a 10% increase in leg volume at the time of diagnosis, compared with the presurgical volume.
The exclusions yielded a total of 672 patients with endometrial cancer, including 127 who developed LLE (19%); 124 patients with cervical cancer, including 31 who developed LLE (25%); and 25 patients with vulvar cancer, including 10 who developed LLE (40%), Dr. Carlson reported.
Analysis of the patients who developed LLE showed no significant association with type of surgery (open, robotic, or laparoscopic), and no significant associations with several patient-specific factors including age, race, cancer stage, surgical blood loss, or serum albumin, he said.
[email protected]
On Twitter @mitchelzoler
SOURCE: Carlson J et al. SGO 2018, Abstract 11.
NEW ORLEANS – Leg lymphedema occurred in 19%-40% of women with a gynecologic cancer who underwent surgery with lymphadenectomy in a prospective study of 821 U.S. patients.
The incidence of lymphedema of the lower extremity (LLE) during 2 years of follow-up was 18% among 672 endometrial cancer patients, 25% among 124 cervical cancer patients, and 40% among 24 vulvar cancer patients, Jay W. Carlson, DO, said at the annual meeting of the Society of Gynecologic Oncology.
Although the study followed patients for 2 years after surgery, 84% of the LLE events occurred within the first 6 months after surgery, and 95% within the first 12 months. The robust incidence rates documented in this study contrasted with a general perception that LLE is relatively uncommon, leading Dr. Carlson to note that the new data show “the incidence of LLE is under recognized.” The findings also bucked conventional wisdom by showing no link between the incidence of LLE and number of lymph nodes dissected or with use of radiation treatment, said Dr. Carlson, a gynecologic oncologist at Mercy Clinic Women’s Oncology in Springfield, Mo.
To better define the incidence of LLE after lymphadenectomy for gynecologic cancers, the Gynecologic Oncology Group organized the Lymphedema and Gynecologic Cancer (LEG) study, run at more than 70 U.S. centers during June 2012–November 2014. The study enrolled patients scheduled for surgery to treat endometrial, cervical, or vulvar cancer, and applied systematic leg measurement to patients just before and at several prespecified times following surgery through 2 years of follow-up.
The study began with a total of 1,054 patients, but the final analysis that Dr. Carlson presented excluded patients who did not actually undergo lymphadenectomy during their surgery, did not have leg volume data available both before and after their surgery, or had a comorbidity or change in body mass that could have caused the change in leg size. The researchers also required patients identified with LLE to have completed the Gynecologic Cancer Lymphedema Questionnaire (Gynecol Oncol. 2010 May;117[2]:317-23) and tallied a score of at least 4, and to have at least a 10% increase in leg volume at the time of diagnosis, compared with the presurgical volume.
The exclusions yielded a total of 672 patients with endometrial cancer, including 127 who developed LLE (19%); 124 patients with cervical cancer, including 31 who developed LLE (25%); and 25 patients with vulvar cancer, including 10 who developed LLE (40%), Dr. Carlson reported.
Analysis of the patients who developed LLE showed no significant association with type of surgery (open, robotic, or laparoscopic), and no significant associations with several patient-specific factors including age, race, cancer stage, surgical blood loss, or serum albumin, he said.
[email protected]
On Twitter @mitchelzoler
SOURCE: Carlson J et al. SGO 2018, Abstract 11.
NEW ORLEANS – Leg lymphedema occurred in 19%-40% of women with a gynecologic cancer who underwent surgery with lymphadenectomy in a prospective study of 821 U.S. patients.
The incidence of lymphedema of the lower extremity (LLE) during 2 years of follow-up was 18% among 672 endometrial cancer patients, 25% among 124 cervical cancer patients, and 40% among 24 vulvar cancer patients, Jay W. Carlson, DO, said at the annual meeting of the Society of Gynecologic Oncology.
Although the study followed patients for 2 years after surgery, 84% of the LLE events occurred within the first 6 months after surgery, and 95% within the first 12 months. The robust incidence rates documented in this study contrasted with a general perception that LLE is relatively uncommon, leading Dr. Carlson to note that the new data show “the incidence of LLE is under recognized.” The findings also bucked conventional wisdom by showing no link between the incidence of LLE and number of lymph nodes dissected or with use of radiation treatment, said Dr. Carlson, a gynecologic oncologist at Mercy Clinic Women’s Oncology in Springfield, Mo.
To better define the incidence of LLE after lymphadenectomy for gynecologic cancers, the Gynecologic Oncology Group organized the Lymphedema and Gynecologic Cancer (LEG) study, run at more than 70 U.S. centers during June 2012–November 2014. The study enrolled patients scheduled for surgery to treat endometrial, cervical, or vulvar cancer, and applied systematic leg measurement to patients just before and at several prespecified times following surgery through 2 years of follow-up.
The study began with a total of 1,054 patients, but the final analysis that Dr. Carlson presented excluded patients who did not actually undergo lymphadenectomy during their surgery, did not have leg volume data available both before and after their surgery, or had a comorbidity or change in body mass that could have caused the change in leg size. The researchers also required patients identified with LLE to have completed the Gynecologic Cancer Lymphedema Questionnaire (Gynecol Oncol. 2010 May;117[2]:317-23) and tallied a score of at least 4, and to have at least a 10% increase in leg volume at the time of diagnosis, compared with the presurgical volume.
The exclusions yielded a total of 672 patients with endometrial cancer, including 127 who developed LLE (19%); 124 patients with cervical cancer, including 31 who developed LLE (25%); and 25 patients with vulvar cancer, including 10 who developed LLE (40%), Dr. Carlson reported.
Analysis of the patients who developed LLE showed no significant association with type of surgery (open, robotic, or laparoscopic), and no significant associations with several patient-specific factors including age, race, cancer stage, surgical blood loss, or serum albumin, he said.
[email protected]
On Twitter @mitchelzoler
SOURCE: Carlson J et al. SGO 2018, Abstract 11.
REPORTING FROM SGO 2018
Key clinical point: Leg lymphedema is common following lymphadenectomy for a gynecologic cancer.
Major finding: Leg lymphedema incidence was 19%-40% during 2-year follow-up after lymphadenectomy during gynecologic cancer surgery.
Study details: LEG, a multicenter, U.S. prospective study with 821 gynecologic cancer patients in the final analysis.
Disclosures: LEG had no commercial funding. Dr. Carlson had no disclosures.
Source: Carlson J et al. SGO 2018, Abstract 11.
Female physicians face enduring wage gap
Male physicians make more money than female physicians, and that seems to be a rule with few exceptions. Among the 50 largest metro areas, there were none where women earn as much as men, according to a new survey by the medical social network Doximity.
The metro area that comes the closest is Las Vegas, where female physicians earned 20% less – that works out to $73,654 – than their male counterparts in 2017. Rochester, N.Y., had the smallest gap in terms of dollars ($68,758) and the second-smallest percent difference (21%), Doximity said in its 2018 Physician Compensation Report.
A quick look at the 2016 data shows that the wage gap between female and male physicians increased from 26.5% to 27.7% in 2017, going from more than $92,000 to $105,000. “Medicine is a highly trained field, and as such, one might expect the gender wage gap to be less prominent here than in other industries. However, the gap endures, despite the level of education required to practice medicine and market forces suggesting that this gap should shrink,” Doximity said.
Male physicians make more money than female physicians, and that seems to be a rule with few exceptions. Among the 50 largest metro areas, there were none where women earn as much as men, according to a new survey by the medical social network Doximity.
The metro area that comes the closest is Las Vegas, where female physicians earned 20% less – that works out to $73,654 – than their male counterparts in 2017. Rochester, N.Y., had the smallest gap in terms of dollars ($68,758) and the second-smallest percent difference (21%), Doximity said in its 2018 Physician Compensation Report.
A quick look at the 2016 data shows that the wage gap between female and male physicians increased from 26.5% to 27.7% in 2017, going from more than $92,000 to $105,000. “Medicine is a highly trained field, and as such, one might expect the gender wage gap to be less prominent here than in other industries. However, the gap endures, despite the level of education required to practice medicine and market forces suggesting that this gap should shrink,” Doximity said.
Male physicians make more money than female physicians, and that seems to be a rule with few exceptions. Among the 50 largest metro areas, there were none where women earn as much as men, according to a new survey by the medical social network Doximity.
The metro area that comes the closest is Las Vegas, where female physicians earned 20% less – that works out to $73,654 – than their male counterparts in 2017. Rochester, N.Y., had the smallest gap in terms of dollars ($68,758) and the second-smallest percent difference (21%), Doximity said in its 2018 Physician Compensation Report.
A quick look at the 2016 data shows that the wage gap between female and male physicians increased from 26.5% to 27.7% in 2017, going from more than $92,000 to $105,000. “Medicine is a highly trained field, and as such, one might expect the gender wage gap to be less prominent here than in other industries. However, the gap endures, despite the level of education required to practice medicine and market forces suggesting that this gap should shrink,” Doximity said.
Piperacillin-tazobactam tripled risk of death for patients with cephalosporin-resistant septicemia
MADRID – A study designed to test the benefit of piperacillin-tazobactam in cephalosporin-resistant bloodstream infections has showed just the opposite: The combination can be fatal for these patients, conferring a threefold increased risk of death compared with meropenem.
The piperacillin-tazobactam combination (PTZ) was associated with a significantly higher 30-day mortality than that of meropenem (12.3% vs. 3.7%; RR 3.4), Patrick Harris, MD, said at the European Society of Clinical Microbiology and Infectious Diseases annual congress.
The number needed to harm with PTZ treatment was 12, said Dr. Harris of the University of Queensland, Australia.
“This was really not the result we wanted. We were expecting to show noninferiority, but the answer we did get was quite compelling. We have to say that in patients with these kinds of bloodstream infections, the use of piperacillin-tazobactam is definitely not supported.”
The signal came on strongly and quickly in the 32-country MERINO trial, he said. An independent data safety monitoring board stopped the study at 75% recruitment after reviewing the alarming interim results last summer.
The trial was designed to test a seemingly sound hypothesis. PTZ is an effective weapon against increasingly extended spectrum beta-lactamase–producing (ESBL) Escherichia coli and Klebsiella infections. These have long been treated with carbapenems, including meropenem, but the widespread global use of that class is putting heavy environmental pressure on these bacteria and creating carbapenem resistance, Dr. Harris said.
“Carbapenems have for many years been the top therapy for these infections, but it may well be a strong selection driver for carbapenem resistance in Gram negative bacilli. We should be thinking about carbapenem-sparing therapy, and it seemed that piperacillin-tazobactam could be useful here.”
Some observational studies do suggest a use for it in this setting but the combination had never been formally investigated. MERINO was designed to do so; investigators hoped to show that PTZ would be noninferior to meropenem in patients with septicemias caused by ESBL E. coli and K. pneumoniae.
The enrollment target for MERINO was 454 patients. Between 2014 and 2017, the study enrolled 391, of whom 379 were included in the final analysis. Patients had to start treatment with the study drugs within 72 hours of confirmatory blood culture. Both arms underwent 4 days of treatment with either PTZ 4.5 g every 6 hours or meropenem 1 g every 8 hours.
The study’s primary outcome was 30-day all-cause mortality. Secondary outcomes were days to clinical and microbiological resolution, clinical and microbiological success at day 4, relapsing septicemia or secondary infection with a PTZ- or meropenem-resistant organism, or Clostridium difficile infection.
The mean age of the patients was 66 years. Most (86%) were infected with resistant strains of E. coli; the rest had K. pneumoniae. About 60% of the infections were acquired in a health care or hospital setting, and about 50% originated in the urinary tract. APACHE II scores were different between the meropenem and PTZ groups (21 vs. 17.9). More patients in PTZ arm had immune compromise (27% vs. 21%).
By 30 days, 23 of those randomized to the combination therapy (12.3%) and seven (3.7%) of those randomized to meropenem had died – a significant 8.6% difference. This translated to more than a threefold increase in the risk of death for those taking the combination (RR 3.4; P = .002). The number needed to harm was just 12.
All of the secondary endpoints also favored meropenem, although the differences were not statistically significant. Patients taking meropenem experienced clinical and microbiological improvement a mean of 1 day sooner (2 vs. 3 days). Microbiological relapse occurred in 2% of those taking meropenem compared with 4.8% of those taking PTZ. The meropenem group was also less likely to develop a multidrug resistant organism or C. difficile infection (4.2% vs. 8%).
The investigators performed several subgroup analyses looking for other trends in 30-day mortality. The difference remained significant no matter how the groups were analyzed.
“Patients with urinary tract infections had a slightly lower risk of mortality, but even after adjusting for risk in several multivariate regression models, the increased risk of 30-day mortality remained,” Dr. Harris said.
The Australasian Society for Antimicrobials and the International Society for Chemotherapy funded the work. Dr. Harris reported having no financial declarations.
MADRID – A study designed to test the benefit of piperacillin-tazobactam in cephalosporin-resistant bloodstream infections has showed just the opposite: The combination can be fatal for these patients, conferring a threefold increased risk of death compared with meropenem.
The piperacillin-tazobactam combination (PTZ) was associated with a significantly higher 30-day mortality than that of meropenem (12.3% vs. 3.7%; RR 3.4), Patrick Harris, MD, said at the European Society of Clinical Microbiology and Infectious Diseases annual congress.
The number needed to harm with PTZ treatment was 12, said Dr. Harris of the University of Queensland, Australia.
“This was really not the result we wanted. We were expecting to show noninferiority, but the answer we did get was quite compelling. We have to say that in patients with these kinds of bloodstream infections, the use of piperacillin-tazobactam is definitely not supported.”
The signal came on strongly and quickly in the 32-country MERINO trial, he said. An independent data safety monitoring board stopped the study at 75% recruitment after reviewing the alarming interim results last summer.
The trial was designed to test a seemingly sound hypothesis. PTZ is an effective weapon against increasingly extended spectrum beta-lactamase–producing (ESBL) Escherichia coli and Klebsiella infections. These have long been treated with carbapenems, including meropenem, but the widespread global use of that class is putting heavy environmental pressure on these bacteria and creating carbapenem resistance, Dr. Harris said.
“Carbapenems have for many years been the top therapy for these infections, but it may well be a strong selection driver for carbapenem resistance in Gram negative bacilli. We should be thinking about carbapenem-sparing therapy, and it seemed that piperacillin-tazobactam could be useful here.”
Some observational studies do suggest a use for it in this setting but the combination had never been formally investigated. MERINO was designed to do so; investigators hoped to show that PTZ would be noninferior to meropenem in patients with septicemias caused by ESBL E. coli and K. pneumoniae.
The enrollment target for MERINO was 454 patients. Between 2014 and 2017, the study enrolled 391, of whom 379 were included in the final analysis. Patients had to start treatment with the study drugs within 72 hours of confirmatory blood culture. Both arms underwent 4 days of treatment with either PTZ 4.5 g every 6 hours or meropenem 1 g every 8 hours.
The study’s primary outcome was 30-day all-cause mortality. Secondary outcomes were days to clinical and microbiological resolution, clinical and microbiological success at day 4, relapsing septicemia or secondary infection with a PTZ- or meropenem-resistant organism, or Clostridium difficile infection.
The mean age of the patients was 66 years. Most (86%) were infected with resistant strains of E. coli; the rest had K. pneumoniae. About 60% of the infections were acquired in a health care or hospital setting, and about 50% originated in the urinary tract. APACHE II scores were different between the meropenem and PTZ groups (21 vs. 17.9). More patients in PTZ arm had immune compromise (27% vs. 21%).
By 30 days, 23 of those randomized to the combination therapy (12.3%) and seven (3.7%) of those randomized to meropenem had died – a significant 8.6% difference. This translated to more than a threefold increase in the risk of death for those taking the combination (RR 3.4; P = .002). The number needed to harm was just 12.
All of the secondary endpoints also favored meropenem, although the differences were not statistically significant. Patients taking meropenem experienced clinical and microbiological improvement a mean of 1 day sooner (2 vs. 3 days). Microbiological relapse occurred in 2% of those taking meropenem compared with 4.8% of those taking PTZ. The meropenem group was also less likely to develop a multidrug resistant organism or C. difficile infection (4.2% vs. 8%).
The investigators performed several subgroup analyses looking for other trends in 30-day mortality. The difference remained significant no matter how the groups were analyzed.
“Patients with urinary tract infections had a slightly lower risk of mortality, but even after adjusting for risk in several multivariate regression models, the increased risk of 30-day mortality remained,” Dr. Harris said.
The Australasian Society for Antimicrobials and the International Society for Chemotherapy funded the work. Dr. Harris reported having no financial declarations.
MADRID – A study designed to test the benefit of piperacillin-tazobactam in cephalosporin-resistant bloodstream infections has showed just the opposite: The combination can be fatal for these patients, conferring a threefold increased risk of death compared with meropenem.
The piperacillin-tazobactam combination (PTZ) was associated with a significantly higher 30-day mortality than that of meropenem (12.3% vs. 3.7%; RR 3.4), Patrick Harris, MD, said at the European Society of Clinical Microbiology and Infectious Diseases annual congress.
The number needed to harm with PTZ treatment was 12, said Dr. Harris of the University of Queensland, Australia.
“This was really not the result we wanted. We were expecting to show noninferiority, but the answer we did get was quite compelling. We have to say that in patients with these kinds of bloodstream infections, the use of piperacillin-tazobactam is definitely not supported.”
The signal came on strongly and quickly in the 32-country MERINO trial, he said. An independent data safety monitoring board stopped the study at 75% recruitment after reviewing the alarming interim results last summer.
The trial was designed to test a seemingly sound hypothesis. PTZ is an effective weapon against increasingly extended spectrum beta-lactamase–producing (ESBL) Escherichia coli and Klebsiella infections. These have long been treated with carbapenems, including meropenem, but the widespread global use of that class is putting heavy environmental pressure on these bacteria and creating carbapenem resistance, Dr. Harris said.
“Carbapenems have for many years been the top therapy for these infections, but it may well be a strong selection driver for carbapenem resistance in Gram negative bacilli. We should be thinking about carbapenem-sparing therapy, and it seemed that piperacillin-tazobactam could be useful here.”
Some observational studies do suggest a use for it in this setting but the combination had never been formally investigated. MERINO was designed to do so; investigators hoped to show that PTZ would be noninferior to meropenem in patients with septicemias caused by ESBL E. coli and K. pneumoniae.
The enrollment target for MERINO was 454 patients. Between 2014 and 2017, the study enrolled 391, of whom 379 were included in the final analysis. Patients had to start treatment with the study drugs within 72 hours of confirmatory blood culture. Both arms underwent 4 days of treatment with either PTZ 4.5 g every 6 hours or meropenem 1 g every 8 hours.
The study’s primary outcome was 30-day all-cause mortality. Secondary outcomes were days to clinical and microbiological resolution, clinical and microbiological success at day 4, relapsing septicemia or secondary infection with a PTZ- or meropenem-resistant organism, or Clostridium difficile infection.
The mean age of the patients was 66 years. Most (86%) were infected with resistant strains of E. coli; the rest had K. pneumoniae. About 60% of the infections were acquired in a health care or hospital setting, and about 50% originated in the urinary tract. APACHE II scores were different between the meropenem and PTZ groups (21 vs. 17.9). More patients in PTZ arm had immune compromise (27% vs. 21%).
By 30 days, 23 of those randomized to the combination therapy (12.3%) and seven (3.7%) of those randomized to meropenem had died – a significant 8.6% difference. This translated to more than a threefold increase in the risk of death for those taking the combination (RR 3.4; P = .002). The number needed to harm was just 12.
All of the secondary endpoints also favored meropenem, although the differences were not statistically significant. Patients taking meropenem experienced clinical and microbiological improvement a mean of 1 day sooner (2 vs. 3 days). Microbiological relapse occurred in 2% of those taking meropenem compared with 4.8% of those taking PTZ. The meropenem group was also less likely to develop a multidrug resistant organism or C. difficile infection (4.2% vs. 8%).
The investigators performed several subgroup analyses looking for other trends in 30-day mortality. The difference remained significant no matter how the groups were analyzed.
“Patients with urinary tract infections had a slightly lower risk of mortality, but even after adjusting for risk in several multivariate regression models, the increased risk of 30-day mortality remained,” Dr. Harris said.
The Australasian Society for Antimicrobials and the International Society for Chemotherapy funded the work. Dr. Harris reported having no financial declarations.
REPORTING FROM ECCMID 2018
Key clinical point: PTZ more than tripled the risk of 30-day mortality in patients with cephalosporin-resistant bloodstream infections.
Major finding: Compared with meropenem, PZT increased the risk of death by 3.4, with a number needed to harm of 12.
Study details: The study randomized 391 patients.
Disclosures: The Australasian Society for Antimicrobials and the International Society for Chemotherapy funded the work. Dr. Harris has no financial declarations.
Source: Harris et al. ECCMID 2018, abstract O1121.
What’s in a name? Get ready for the feds’ Promoting Interoperability program
The federal EHR Incentive Program – a program most doctors love to hate – is getting a new name to better reflect a focus on interoperability and improved patient access to their health care data, the Centers for Medicare & Medicaid Services announced.
For clinicians who participate in fee-for-service Medicare, eligible hospitals, and critical access hospitals, the new name of the program will be the Promoting Interoperability Program. For those participating in the Merit-Based Incentive Payment System (MIPS) track of the Quality Payment Program (QPP), the Advancing Care Information performance category will be rebranded the Promoting Interoperability performance category.
The first steps of these changes were announced April 25 in the 2019 proposed rule for the inpatient prospective payment system and the Long-Term Care Hospital Prospective Payment System.
CMS notes in the proposed rule that the name EHR Incentive Program “does not adequately reflect the current status of the programs, as the incentive payments under Medicare generally have ended.” Eligible medical professionals and hospitals have received Medicare bonuses for adopting and demonstrating meaningful use of EHRs. Penalties for not doing so are still in place.
The federal EHR Incentive Program – a program most doctors love to hate – is getting a new name to better reflect a focus on interoperability and improved patient access to their health care data, the Centers for Medicare & Medicaid Services announced.
For clinicians who participate in fee-for-service Medicare, eligible hospitals, and critical access hospitals, the new name of the program will be the Promoting Interoperability Program. For those participating in the Merit-Based Incentive Payment System (MIPS) track of the Quality Payment Program (QPP), the Advancing Care Information performance category will be rebranded the Promoting Interoperability performance category.
The first steps of these changes were announced April 25 in the 2019 proposed rule for the inpatient prospective payment system and the Long-Term Care Hospital Prospective Payment System.
CMS notes in the proposed rule that the name EHR Incentive Program “does not adequately reflect the current status of the programs, as the incentive payments under Medicare generally have ended.” Eligible medical professionals and hospitals have received Medicare bonuses for adopting and demonstrating meaningful use of EHRs. Penalties for not doing so are still in place.
The federal EHR Incentive Program – a program most doctors love to hate – is getting a new name to better reflect a focus on interoperability and improved patient access to their health care data, the Centers for Medicare & Medicaid Services announced.
For clinicians who participate in fee-for-service Medicare, eligible hospitals, and critical access hospitals, the new name of the program will be the Promoting Interoperability Program. For those participating in the Merit-Based Incentive Payment System (MIPS) track of the Quality Payment Program (QPP), the Advancing Care Information performance category will be rebranded the Promoting Interoperability performance category.
The first steps of these changes were announced April 25 in the 2019 proposed rule for the inpatient prospective payment system and the Long-Term Care Hospital Prospective Payment System.
CMS notes in the proposed rule that the name EHR Incentive Program “does not adequately reflect the current status of the programs, as the incentive payments under Medicare generally have ended.” Eligible medical professionals and hospitals have received Medicare bonuses for adopting and demonstrating meaningful use of EHRs. Penalties for not doing so are still in place.