User login
Potential Impact of USPS Mail Delivery Delays on Colorectal Cancer Screening Programs
Colorectal cancer (CRC) is the second leading cause of cancer deaths in the United States.1 In 2022, there were an estimated 151,030 new CRC cases and 52,580 deaths.1 Options for CRC screening of patients at average risk include stool tests (annual fecal immunochemical test [FIT], annual guaiac-based fecal occult blood test, or stool FIT-DNA test every 1 to 3 years), colonoscopies every 10 years, flexible sigmoidoscopies every 5 years (or every 10 years with annual FIT), and computed tomography (CT) colonography every 5 years.2 Many health care systems use annual FIT for patients at average risk. Compared with guaiac-based fecal occult blood testing, FIT does not require dietary or medication modifications and yields greater sensitivity and patient participation.3
The COVID-19 pandemic and staffing issues have caused a scheduling backlog for screening, diagnostic, and surveillance endoscopies at some medical centers. As a result, FIT has become the primary means of CRC screening at these institutions. FIT kits for home use are typically distributed to eligible patients at an office visit or by mail, and patients are then instructed to mail the kits back to the laboratory. For the test to be as sensitive as possible, FIT kit manufacturers advise laboratory analysis within 14 to 15 days of collection, if stored at ambient temperature, and to reject the sample if it does not meet testing criteria for stability. Delayed FIT sample analysis has been associated with higher false-negative rates because of hemoglobin degradation.4 FIT sample exposure to high ambient temperatures also has been linked to decreased sensitivity for detecting CRC.5
US Postal Service (USPS) mail delivery delays have plagued many areas of the country. A variety of factors, including the COVID-19 pandemic, understaffing, changes in USPS policies, closure of post offices, and changes in mail delivery standards, may also be contributory causes. According to the USPS website, delivery standard for first-class mail is 1 to 5 days, but this is not guaranteed.6
The Jesse Brown Veterans Affairs Medical Center (JBVAMC) laboratory in Chicago has reported receiving FIT kit envelopes in batches by the USPS, with some prepaid first-class business reply envelopes delivered up to 60 days after the time of sample collection. Polymedco, a company that assists US Department of Veterans Affairs (VA) medical centers with logistics of FIT programs for CRC screening, reports that USPS batching of FIT kits leading to delayed delivery has been a periodic problem for medical centers around the country. Polymedco staff remind USPS staff about 4 points when they encounter this issue: Mailers are first-class mail; mailers contain a human biologic specimen that has limited viability; the biological sample used for detecting cancer is time sensitive; and delays in delivery by holding/batching kits could impact morbidity and mortality. Reviewing these key points with local USPS staff usually helps, however, batching and delayed delivery of the FIT kits can sometimes recur with USPS staffing turnover.
Tracking and identifying when a patient receives the FIT kit is difficult. Patients are instructed to write the date of collection on the kit, so the receiving laboratory knows whether the sample can be reliably analyzed. When patients are notified about delayed delivery of their sample, a staff member asks if they postponed dropping the kit in the mail. Most patients report mailing the sample within 1 to 2 days of collection. Tracking and dating each step of FIT kit events is not feasible with a mass mailing campaign. In our experience, most patients write the date of collection on the kit. If a collection date is not provided, the laboratory will call the patient to confirm a date. Cheng and colleagues reviewed the causes for FIT specimen rejection in a laboratory analyzing specimens for VA patients and found that 14% of submitted samples were rejected because the specimen was received > 14 days after collection, and 6% because the patient did not record the collection date. With a series of interventions aimed at reminding patients and improving laboratory procedures, rates of rejection for these 2 causes were reduced to < 4%.7 USPS delays were not identified as a factor or tracked in this study.
It is unclear why the USPS sometimes holds FIT kits at their facilities and then delivers large bins of them at the same time. Because FIT kits should be analyzed within 14 to 15 days of sample collection to assure reliable results, mail delivery delays can result in increased sample rejection. Based on the JBVAMC experience, up to 30% of submitted samples might need to be discarded when batched delivery takes place. In these cases, patients need to be contacted, informed of the problem, and asked to submit new kits. Understandably, patients are reluctant to repeat this type of testing, and we are concerned this could lead to reduced rates of CRC screening in affected communities.
As an alternative to discarding delayed samples, laboratories could report the results of delayed FIT kits with an added comment that “negative test results may be less reliable due to delayed processing,” but this approach would raise quality and medicolegal concerns. Clinicians have reached out to local USPS supervisory personnel with mixed results. Sometimes batching and delayed deliveries stop for a few months, only to resume without warning. Dropping off the sample directly at the laboratory is not a realistic option for most patients. Some patients can be convinced to submit another sample, some elect to switch to other CRC screening strategies, while others, unfortunately, decline further screening efforts.
Laboratory staff can be overwhelmed with having to process hundreds of samples in a short time frame, especially because there is no way of knowing when USPS will make a batched delivery. Laboratory capacities can limit staff at some facilities to performing analysis of only 10 tests at a time. The FIT kits should be delivered on a rolling basis and without delay so that the samples can be reliably analyzed with a predictable workload for the laboratory personnel and without unexpected surges.
When health care facilities identify delayed mail delivery of FIT kits via USPS, laboratories should first ensure that the correct postage rates are used on the prepaid envelopes and that their USPS accounts are properly funded, so that insufficient funds are not contributing to delayed deliveries. Stakeholders should then reach out to local USPS supervisory staff and request that the practice of batching the delivery of FIT kits be stopped. Educating USPS supervisory staff about concerns related to decreased test reliability associated with delayed mail delivery can be a persuasive argument. Adding additional language to the preprinted envelopes, such as “time sensitive,” may also be helpful. Unfortunately, the JBVAMC experience has been that the problem initially gets better after contacting the USPS, only to unexpectedly resurface months later. This cycle has been repeated several times in the past 2 years at JBVAMC.
All clinicians involved in CRC screening and treatment at institutions that use FIT kits need to be aware of the impact that local USPS delays can have on the reliability of these results. Health care systems should be prepared to implement mitigation strategies if they encounter significant delays with mail delivery. If delays cannot be reliably resolved by working with the local USPS staff, consider involving national USPS oversight bodies. And if the problems persist despite an attempt to work with the USPS, some institutions might find it feasible to offer drop boxes at their clinics and instruct patients to drop off FIT kits immediately following collection, in lieu of mailing them. Switching to private carriers is not a cost-effective alternative for most health care systems, and some may exclude rural areas. Depending on the local availability and capacity of endoscopists, some clinicians might prioritize referring patients for screening colonoscopies or screening flexible sigmoidoscopies, and might deemphasize FIT kits as a preferred option for CRC screening. CT colonography is an alternative screening method that is not as widely offered, nor as widely accepted at this time.
Conclusions
CRC screening is an essential part of preventive medicine, and the percentage of eligible patients screened is a well-established quality metric in primary care settings. Health care systems, clinicians, and laboratories must be vigilant to ensure that USPS delays in delivering FIT kits do not negatively impact their CRC screening programs. Facilities should actively monitor for delays in the return of FIT kits.
Despite the widespread use of mail-order pharmacies and the use of mail to communicate notifications about test results and follow-up appointments, unreliable or delayed mail delivery traditionally has not been considered a social determinant of health.8 This article highlights the impact delayed mail delivery can have on health outcomes. Disadvantaged communities in inner cities and rural areas have been disproportionately affected by the worsening performance of the USPS over the past few years.9 This represents an underappreciated public health concern in need of a sustainable solution.
1. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022;72(1):7-33. doi:10.3322/caac.21708
2. Centers for Disease Control and Prevention. Colorectal cancer screening tests. Updated February 23, 2023. Accessed March 14, 2024. https://www.cdc.gov/cancer/colorectal/basic_info/screening/tests.htm
3. van Rossum LG, van Rijn AF, Laheij RJ, et al. Random comparison of guaiac and immunochemical fecal occult blood tests for colorectal cancer in a screening population. Gastroenterology. 2008;135(1):82-90. doi:10.1053/j.gastro.2008.03.040
4. van Rossum LG, van Rijn AF, van Oijen MG, et al. False negative fecal occult blood tests due to delayed sample return in colorectal cancer screening. Int J Cancer. 2009;125(4):746-750. doi:10.1002/ijc.24458
5. Doubeni CA, Jensen CD, Fedewa SA, et al. Fecal immunochemical test (FIT) for colon cancer screening: variable performance with ambient temperature. J Am Board Fam Med. 2016;29(6):672-681. doi:10.3122/jabfm.2016.06.160060
6. United States Postal Service. Shipping and mailing with USPS. Accessed March 14, 2024. https://www.usps.com/ship
7. Cheng C, Ganz DA, Chang ET, Huynh A, De Peralta S. Reducing rejected fecal immunochemical tests received in the laboratory for colorectal cancer screening. J Healthc Qual. 2019;41(2):75-82.doi:10.1097/JHQ.0000000000000181
8. Hussaini SMQ, Alexander GC. The United States Postal Service: an essential public health agency? J Gen Intern Med. 2020;35(12):3699-3701. doi:10.1007/s11606-020-06275-2
9. Hampton DJ. Colorado mountain towns are plagued by post office delays as residents wait weeks for medication and retirement checks. NBC News. February 25, 2023. Accessed March 14, 2024. https://www.nbcnews.com/news/us-news/colo-mountain-towns-are-plagued-post-office-delays-residents-wait-week-rcna72085
Colorectal cancer (CRC) is the second leading cause of cancer deaths in the United States.1 In 2022, there were an estimated 151,030 new CRC cases and 52,580 deaths.1 Options for CRC screening of patients at average risk include stool tests (annual fecal immunochemical test [FIT], annual guaiac-based fecal occult blood test, or stool FIT-DNA test every 1 to 3 years), colonoscopies every 10 years, flexible sigmoidoscopies every 5 years (or every 10 years with annual FIT), and computed tomography (CT) colonography every 5 years.2 Many health care systems use annual FIT for patients at average risk. Compared with guaiac-based fecal occult blood testing, FIT does not require dietary or medication modifications and yields greater sensitivity and patient participation.3
The COVID-19 pandemic and staffing issues have caused a scheduling backlog for screening, diagnostic, and surveillance endoscopies at some medical centers. As a result, FIT has become the primary means of CRC screening at these institutions. FIT kits for home use are typically distributed to eligible patients at an office visit or by mail, and patients are then instructed to mail the kits back to the laboratory. For the test to be as sensitive as possible, FIT kit manufacturers advise laboratory analysis within 14 to 15 days of collection, if stored at ambient temperature, and to reject the sample if it does not meet testing criteria for stability. Delayed FIT sample analysis has been associated with higher false-negative rates because of hemoglobin degradation.4 FIT sample exposure to high ambient temperatures also has been linked to decreased sensitivity for detecting CRC.5
US Postal Service (USPS) mail delivery delays have plagued many areas of the country. A variety of factors, including the COVID-19 pandemic, understaffing, changes in USPS policies, closure of post offices, and changes in mail delivery standards, may also be contributory causes. According to the USPS website, delivery standard for first-class mail is 1 to 5 days, but this is not guaranteed.6
The Jesse Brown Veterans Affairs Medical Center (JBVAMC) laboratory in Chicago has reported receiving FIT kit envelopes in batches by the USPS, with some prepaid first-class business reply envelopes delivered up to 60 days after the time of sample collection. Polymedco, a company that assists US Department of Veterans Affairs (VA) medical centers with logistics of FIT programs for CRC screening, reports that USPS batching of FIT kits leading to delayed delivery has been a periodic problem for medical centers around the country. Polymedco staff remind USPS staff about 4 points when they encounter this issue: Mailers are first-class mail; mailers contain a human biologic specimen that has limited viability; the biological sample used for detecting cancer is time sensitive; and delays in delivery by holding/batching kits could impact morbidity and mortality. Reviewing these key points with local USPS staff usually helps, however, batching and delayed delivery of the FIT kits can sometimes recur with USPS staffing turnover.
Tracking and identifying when a patient receives the FIT kit is difficult. Patients are instructed to write the date of collection on the kit, so the receiving laboratory knows whether the sample can be reliably analyzed. When patients are notified about delayed delivery of their sample, a staff member asks if they postponed dropping the kit in the mail. Most patients report mailing the sample within 1 to 2 days of collection. Tracking and dating each step of FIT kit events is not feasible with a mass mailing campaign. In our experience, most patients write the date of collection on the kit. If a collection date is not provided, the laboratory will call the patient to confirm a date. Cheng and colleagues reviewed the causes for FIT specimen rejection in a laboratory analyzing specimens for VA patients and found that 14% of submitted samples were rejected because the specimen was received > 14 days after collection, and 6% because the patient did not record the collection date. With a series of interventions aimed at reminding patients and improving laboratory procedures, rates of rejection for these 2 causes were reduced to < 4%.7 USPS delays were not identified as a factor or tracked in this study.
It is unclear why the USPS sometimes holds FIT kits at their facilities and then delivers large bins of them at the same time. Because FIT kits should be analyzed within 14 to 15 days of sample collection to assure reliable results, mail delivery delays can result in increased sample rejection. Based on the JBVAMC experience, up to 30% of submitted samples might need to be discarded when batched delivery takes place. In these cases, patients need to be contacted, informed of the problem, and asked to submit new kits. Understandably, patients are reluctant to repeat this type of testing, and we are concerned this could lead to reduced rates of CRC screening in affected communities.
As an alternative to discarding delayed samples, laboratories could report the results of delayed FIT kits with an added comment that “negative test results may be less reliable due to delayed processing,” but this approach would raise quality and medicolegal concerns. Clinicians have reached out to local USPS supervisory personnel with mixed results. Sometimes batching and delayed deliveries stop for a few months, only to resume without warning. Dropping off the sample directly at the laboratory is not a realistic option for most patients. Some patients can be convinced to submit another sample, some elect to switch to other CRC screening strategies, while others, unfortunately, decline further screening efforts.
Laboratory staff can be overwhelmed with having to process hundreds of samples in a short time frame, especially because there is no way of knowing when USPS will make a batched delivery. Laboratory capacities can limit staff at some facilities to performing analysis of only 10 tests at a time. The FIT kits should be delivered on a rolling basis and without delay so that the samples can be reliably analyzed with a predictable workload for the laboratory personnel and without unexpected surges.
When health care facilities identify delayed mail delivery of FIT kits via USPS, laboratories should first ensure that the correct postage rates are used on the prepaid envelopes and that their USPS accounts are properly funded, so that insufficient funds are not contributing to delayed deliveries. Stakeholders should then reach out to local USPS supervisory staff and request that the practice of batching the delivery of FIT kits be stopped. Educating USPS supervisory staff about concerns related to decreased test reliability associated with delayed mail delivery can be a persuasive argument. Adding additional language to the preprinted envelopes, such as “time sensitive,” may also be helpful. Unfortunately, the JBVAMC experience has been that the problem initially gets better after contacting the USPS, only to unexpectedly resurface months later. This cycle has been repeated several times in the past 2 years at JBVAMC.
All clinicians involved in CRC screening and treatment at institutions that use FIT kits need to be aware of the impact that local USPS delays can have on the reliability of these results. Health care systems should be prepared to implement mitigation strategies if they encounter significant delays with mail delivery. If delays cannot be reliably resolved by working with the local USPS staff, consider involving national USPS oversight bodies. And if the problems persist despite an attempt to work with the USPS, some institutions might find it feasible to offer drop boxes at their clinics and instruct patients to drop off FIT kits immediately following collection, in lieu of mailing them. Switching to private carriers is not a cost-effective alternative for most health care systems, and some may exclude rural areas. Depending on the local availability and capacity of endoscopists, some clinicians might prioritize referring patients for screening colonoscopies or screening flexible sigmoidoscopies, and might deemphasize FIT kits as a preferred option for CRC screening. CT colonography is an alternative screening method that is not as widely offered, nor as widely accepted at this time.
Conclusions
CRC screening is an essential part of preventive medicine, and the percentage of eligible patients screened is a well-established quality metric in primary care settings. Health care systems, clinicians, and laboratories must be vigilant to ensure that USPS delays in delivering FIT kits do not negatively impact their CRC screening programs. Facilities should actively monitor for delays in the return of FIT kits.
Despite the widespread use of mail-order pharmacies and the use of mail to communicate notifications about test results and follow-up appointments, unreliable or delayed mail delivery traditionally has not been considered a social determinant of health.8 This article highlights the impact delayed mail delivery can have on health outcomes. Disadvantaged communities in inner cities and rural areas have been disproportionately affected by the worsening performance of the USPS over the past few years.9 This represents an underappreciated public health concern in need of a sustainable solution.
Colorectal cancer (CRC) is the second leading cause of cancer deaths in the United States.1 In 2022, there were an estimated 151,030 new CRC cases and 52,580 deaths.1 Options for CRC screening of patients at average risk include stool tests (annual fecal immunochemical test [FIT], annual guaiac-based fecal occult blood test, or stool FIT-DNA test every 1 to 3 years), colonoscopies every 10 years, flexible sigmoidoscopies every 5 years (or every 10 years with annual FIT), and computed tomography (CT) colonography every 5 years.2 Many health care systems use annual FIT for patients at average risk. Compared with guaiac-based fecal occult blood testing, FIT does not require dietary or medication modifications and yields greater sensitivity and patient participation.3
The COVID-19 pandemic and staffing issues have caused a scheduling backlog for screening, diagnostic, and surveillance endoscopies at some medical centers. As a result, FIT has become the primary means of CRC screening at these institutions. FIT kits for home use are typically distributed to eligible patients at an office visit or by mail, and patients are then instructed to mail the kits back to the laboratory. For the test to be as sensitive as possible, FIT kit manufacturers advise laboratory analysis within 14 to 15 days of collection, if stored at ambient temperature, and to reject the sample if it does not meet testing criteria for stability. Delayed FIT sample analysis has been associated with higher false-negative rates because of hemoglobin degradation.4 FIT sample exposure to high ambient temperatures also has been linked to decreased sensitivity for detecting CRC.5
US Postal Service (USPS) mail delivery delays have plagued many areas of the country. A variety of factors, including the COVID-19 pandemic, understaffing, changes in USPS policies, closure of post offices, and changes in mail delivery standards, may also be contributory causes. According to the USPS website, delivery standard for first-class mail is 1 to 5 days, but this is not guaranteed.6
The Jesse Brown Veterans Affairs Medical Center (JBVAMC) laboratory in Chicago has reported receiving FIT kit envelopes in batches by the USPS, with some prepaid first-class business reply envelopes delivered up to 60 days after the time of sample collection. Polymedco, a company that assists US Department of Veterans Affairs (VA) medical centers with logistics of FIT programs for CRC screening, reports that USPS batching of FIT kits leading to delayed delivery has been a periodic problem for medical centers around the country. Polymedco staff remind USPS staff about 4 points when they encounter this issue: Mailers are first-class mail; mailers contain a human biologic specimen that has limited viability; the biological sample used for detecting cancer is time sensitive; and delays in delivery by holding/batching kits could impact morbidity and mortality. Reviewing these key points with local USPS staff usually helps, however, batching and delayed delivery of the FIT kits can sometimes recur with USPS staffing turnover.
Tracking and identifying when a patient receives the FIT kit is difficult. Patients are instructed to write the date of collection on the kit, so the receiving laboratory knows whether the sample can be reliably analyzed. When patients are notified about delayed delivery of their sample, a staff member asks if they postponed dropping the kit in the mail. Most patients report mailing the sample within 1 to 2 days of collection. Tracking and dating each step of FIT kit events is not feasible with a mass mailing campaign. In our experience, most patients write the date of collection on the kit. If a collection date is not provided, the laboratory will call the patient to confirm a date. Cheng and colleagues reviewed the causes for FIT specimen rejection in a laboratory analyzing specimens for VA patients and found that 14% of submitted samples were rejected because the specimen was received > 14 days after collection, and 6% because the patient did not record the collection date. With a series of interventions aimed at reminding patients and improving laboratory procedures, rates of rejection for these 2 causes were reduced to < 4%.7 USPS delays were not identified as a factor or tracked in this study.
It is unclear why the USPS sometimes holds FIT kits at their facilities and then delivers large bins of them at the same time. Because FIT kits should be analyzed within 14 to 15 days of sample collection to assure reliable results, mail delivery delays can result in increased sample rejection. Based on the JBVAMC experience, up to 30% of submitted samples might need to be discarded when batched delivery takes place. In these cases, patients need to be contacted, informed of the problem, and asked to submit new kits. Understandably, patients are reluctant to repeat this type of testing, and we are concerned this could lead to reduced rates of CRC screening in affected communities.
As an alternative to discarding delayed samples, laboratories could report the results of delayed FIT kits with an added comment that “negative test results may be less reliable due to delayed processing,” but this approach would raise quality and medicolegal concerns. Clinicians have reached out to local USPS supervisory personnel with mixed results. Sometimes batching and delayed deliveries stop for a few months, only to resume without warning. Dropping off the sample directly at the laboratory is not a realistic option for most patients. Some patients can be convinced to submit another sample, some elect to switch to other CRC screening strategies, while others, unfortunately, decline further screening efforts.
Laboratory staff can be overwhelmed with having to process hundreds of samples in a short time frame, especially because there is no way of knowing when USPS will make a batched delivery. Laboratory capacities can limit staff at some facilities to performing analysis of only 10 tests at a time. The FIT kits should be delivered on a rolling basis and without delay so that the samples can be reliably analyzed with a predictable workload for the laboratory personnel and without unexpected surges.
When health care facilities identify delayed mail delivery of FIT kits via USPS, laboratories should first ensure that the correct postage rates are used on the prepaid envelopes and that their USPS accounts are properly funded, so that insufficient funds are not contributing to delayed deliveries. Stakeholders should then reach out to local USPS supervisory staff and request that the practice of batching the delivery of FIT kits be stopped. Educating USPS supervisory staff about concerns related to decreased test reliability associated with delayed mail delivery can be a persuasive argument. Adding additional language to the preprinted envelopes, such as “time sensitive,” may also be helpful. Unfortunately, the JBVAMC experience has been that the problem initially gets better after contacting the USPS, only to unexpectedly resurface months later. This cycle has been repeated several times in the past 2 years at JBVAMC.
All clinicians involved in CRC screening and treatment at institutions that use FIT kits need to be aware of the impact that local USPS delays can have on the reliability of these results. Health care systems should be prepared to implement mitigation strategies if they encounter significant delays with mail delivery. If delays cannot be reliably resolved by working with the local USPS staff, consider involving national USPS oversight bodies. And if the problems persist despite an attempt to work with the USPS, some institutions might find it feasible to offer drop boxes at their clinics and instruct patients to drop off FIT kits immediately following collection, in lieu of mailing them. Switching to private carriers is not a cost-effective alternative for most health care systems, and some may exclude rural areas. Depending on the local availability and capacity of endoscopists, some clinicians might prioritize referring patients for screening colonoscopies or screening flexible sigmoidoscopies, and might deemphasize FIT kits as a preferred option for CRC screening. CT colonography is an alternative screening method that is not as widely offered, nor as widely accepted at this time.
Conclusions
CRC screening is an essential part of preventive medicine, and the percentage of eligible patients screened is a well-established quality metric in primary care settings. Health care systems, clinicians, and laboratories must be vigilant to ensure that USPS delays in delivering FIT kits do not negatively impact their CRC screening programs. Facilities should actively monitor for delays in the return of FIT kits.
Despite the widespread use of mail-order pharmacies and the use of mail to communicate notifications about test results and follow-up appointments, unreliable or delayed mail delivery traditionally has not been considered a social determinant of health.8 This article highlights the impact delayed mail delivery can have on health outcomes. Disadvantaged communities in inner cities and rural areas have been disproportionately affected by the worsening performance of the USPS over the past few years.9 This represents an underappreciated public health concern in need of a sustainable solution.
1. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022;72(1):7-33. doi:10.3322/caac.21708
2. Centers for Disease Control and Prevention. Colorectal cancer screening tests. Updated February 23, 2023. Accessed March 14, 2024. https://www.cdc.gov/cancer/colorectal/basic_info/screening/tests.htm
3. van Rossum LG, van Rijn AF, Laheij RJ, et al. Random comparison of guaiac and immunochemical fecal occult blood tests for colorectal cancer in a screening population. Gastroenterology. 2008;135(1):82-90. doi:10.1053/j.gastro.2008.03.040
4. van Rossum LG, van Rijn AF, van Oijen MG, et al. False negative fecal occult blood tests due to delayed sample return in colorectal cancer screening. Int J Cancer. 2009;125(4):746-750. doi:10.1002/ijc.24458
5. Doubeni CA, Jensen CD, Fedewa SA, et al. Fecal immunochemical test (FIT) for colon cancer screening: variable performance with ambient temperature. J Am Board Fam Med. 2016;29(6):672-681. doi:10.3122/jabfm.2016.06.160060
6. United States Postal Service. Shipping and mailing with USPS. Accessed March 14, 2024. https://www.usps.com/ship
7. Cheng C, Ganz DA, Chang ET, Huynh A, De Peralta S. Reducing rejected fecal immunochemical tests received in the laboratory for colorectal cancer screening. J Healthc Qual. 2019;41(2):75-82.doi:10.1097/JHQ.0000000000000181
8. Hussaini SMQ, Alexander GC. The United States Postal Service: an essential public health agency? J Gen Intern Med. 2020;35(12):3699-3701. doi:10.1007/s11606-020-06275-2
9. Hampton DJ. Colorado mountain towns are plagued by post office delays as residents wait weeks for medication and retirement checks. NBC News. February 25, 2023. Accessed March 14, 2024. https://www.nbcnews.com/news/us-news/colo-mountain-towns-are-plagued-post-office-delays-residents-wait-week-rcna72085
1. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022;72(1):7-33. doi:10.3322/caac.21708
2. Centers for Disease Control and Prevention. Colorectal cancer screening tests. Updated February 23, 2023. Accessed March 14, 2024. https://www.cdc.gov/cancer/colorectal/basic_info/screening/tests.htm
3. van Rossum LG, van Rijn AF, Laheij RJ, et al. Random comparison of guaiac and immunochemical fecal occult blood tests for colorectal cancer in a screening population. Gastroenterology. 2008;135(1):82-90. doi:10.1053/j.gastro.2008.03.040
4. van Rossum LG, van Rijn AF, van Oijen MG, et al. False negative fecal occult blood tests due to delayed sample return in colorectal cancer screening. Int J Cancer. 2009;125(4):746-750. doi:10.1002/ijc.24458
5. Doubeni CA, Jensen CD, Fedewa SA, et al. Fecal immunochemical test (FIT) for colon cancer screening: variable performance with ambient temperature. J Am Board Fam Med. 2016;29(6):672-681. doi:10.3122/jabfm.2016.06.160060
6. United States Postal Service. Shipping and mailing with USPS. Accessed March 14, 2024. https://www.usps.com/ship
7. Cheng C, Ganz DA, Chang ET, Huynh A, De Peralta S. Reducing rejected fecal immunochemical tests received in the laboratory for colorectal cancer screening. J Healthc Qual. 2019;41(2):75-82.doi:10.1097/JHQ.0000000000000181
8. Hussaini SMQ, Alexander GC. The United States Postal Service: an essential public health agency? J Gen Intern Med. 2020;35(12):3699-3701. doi:10.1007/s11606-020-06275-2
9. Hampton DJ. Colorado mountain towns are plagued by post office delays as residents wait weeks for medication and retirement checks. NBC News. February 25, 2023. Accessed March 14, 2024. https://www.nbcnews.com/news/us-news/colo-mountain-towns-are-plagued-post-office-delays-residents-wait-week-rcna72085
Commentary: Diet and Lifestyle in Migraine, May 2024
Migraine and other headache types are common ailments, and there are many stereotypes and stigmas associated with these conditions. One of the prevailing beliefs about headaches and migraines is that they are linked with internalizing mental health conditions — anxiety and depression. These associations can affect pediatric migraine patients and their parents in complicated ways, potentially hindering adequate diagnosis and treatment. Results of a recent prospective study, published in the journal Headache, provided results that challenge the widespread belief that people who have migraines have a higher-than-average rate of internalizing mental health disorders. The authors provided a discussion and data to explain that their initial hypothesis of a relationship between migraine and mental health was disproven. The study included 123 participants age 8-18 years who had been previously diagnosed with migraine. The patients, who were seen in a pediatric neurology clinic, completed headache questionnaires and validated measures of anxiety and depressive symptoms. The final analysis showed no significant association between migraines or headaches with anxiety or depression.
Why does this matter? Stigma can prevent patients and parents from seeking care if parents feel that they will be judged as bad parents for contributing to their children's anxiety, depression, headaches, and migraines. In fact, beyond mental health stigma, children who have migraine can be blamed for having an unhealthy lifestyle.[1] While advice to get enough sleep, eat healthy, and stay active is worthwhile, there can be an implication that pediatric migraine patients are causing their migraines by living an unhealthy lifestyle.[1] Additionally, the implication that parents are not properly taking care of their children's health can inhibit an accurate symptom history. Releasing pediatric migraine patients and their parents from myths about migraines and headaches can be a beneficial component of doctor-patient communication regarding migraine care.
It is possible that dietary adjustments or supplements could help improve migraine frequency and severity. Maintaining a healthy diet is a frequent recommendation for people who have headaches, but it can be frustrating for patients to receive general recommendations to follow a healthy lifestyle. Specific direction regarding which foods to avoid and which foods to add to a diet can be helpful for patients as they try to navigate the challenge of adopting migraine-friendly lifestyle changes.
Eicosapentaenoic acid (EPA) is one of the omega-3 fatty acids. A recent study, with results published in Brain, Behavior, and Immunity, examined the effects of EPA on migraines. The 12-week randomized, double-blind, placebo-controlled trial included 70 participants who had been diagnosed with episodic migraine. Participants were randomly assigned to either EPA (2 g fish oil with 1.8 g of EPA/day) or placebo (2 g soybean oil/day). Migraine frequency and severity were assessed using standardized scales. According to the authors, the high-dose-EPA group had significantly reduced migraine frequency and severity, fewer number of days using acute treatment, reduced migraine-associated disability, improved anxiety and depression, and improved quality of life in comparison to the placebo group. The EPA group did not experience notable adverse events. To provide a sense of scale regarding dietary EPA, 3 oz of cooked wild salmon has 0.35 g of EPA, 3 oz of cooked shrimp has 0.2 g of EPA, and 3 oz of light canned tuna has 0.02 g of EPA.[2] Thus, it's important to note that the amount of EPA used in this study was higher than what would be expected of dietary EPA.
An observational prospective study published in Scientific Reports examined the effects of dietary phytochemical index (DPI) on migraine. DPI is defined as the proportion of daily energy intake derived from foods rich in phytochemicals. Consumption of phytochemical-rich foods has been associated with cardiovascular and metabolic diseases prevention in various populations. These foods include fruits, vegetables, whole grains, seeds, nuts, and legumes. The study included 265 adults age 20-50 who had a diagnosis of migraine. Participants were asked to fill out a questionnaire, which was used to evaluate their diet in the preceding year, and they were asked to complete a diary to track their migraine symptoms. The results showed an inverse relationship between DPI index and migraine frequency. Participants who had the highest DPI had the lowest migraine frequency.[3] While the authors found the results to be statistically significant, they did not point to a cause and effect. Migraine-associated symptoms such as nausea can have an effect on dietary choices, so patients who experience migraine symptoms may avoid certain foods before, during, or after a migraine episode. They also may consistently avoid foods that they have experienced as migraine triggers.
Diet and lifestyle can have an effect on migraine frequency, severity, and overall migraine-associated quality of life. Beyond general recommendations, however, it is not yet well established which foods or supplements could potentially help alleviate migraines. Advice to maintain a healthy lifestyle is definitely worthwhile for migraine patients, but it is important to avoid conveying blame or stigma when it comes to communication about the effect of lifestyle on migraine. This is especially important for pediatric migraine patients because the stigma extends beyond children to parents and could potentially interfere with clear communication and adequate care.
Additional References
1. Gelfand AA, Irwin SL. Lifestyle advice for pediatric migraine: Blaming the patient, or evidence based? Semin Neurol. 2020;40:277-285. doi: 10.1055/s-0040-1708868 Source
2. National Institutes of Health. Office of Dietary Supplements. Omega-3 fatty acids. Updated February 15, 2023. Source
3. Hamedi-Shahraki S, Jowshan M-R, Zolghadrpour M-A, et al. Dietary phytochemical index is favorably associated with oxidative stress status and cardiovascular risk factors in adults with obesity. Sci Rep. 2023;13:7035. doi: 10.1038/s41598-023-34064-4 Source
Migraine and other headache types are common ailments, and there are many stereotypes and stigmas associated with these conditions. One of the prevailing beliefs about headaches and migraines is that they are linked with internalizing mental health conditions — anxiety and depression. These associations can affect pediatric migraine patients and their parents in complicated ways, potentially hindering adequate diagnosis and treatment. Results of a recent prospective study, published in the journal Headache, provided results that challenge the widespread belief that people who have migraines have a higher-than-average rate of internalizing mental health disorders. The authors provided a discussion and data to explain that their initial hypothesis of a relationship between migraine and mental health was disproven. The study included 123 participants age 8-18 years who had been previously diagnosed with migraine. The patients, who were seen in a pediatric neurology clinic, completed headache questionnaires and validated measures of anxiety and depressive symptoms. The final analysis showed no significant association between migraines or headaches with anxiety or depression.
Why does this matter? Stigma can prevent patients and parents from seeking care if parents feel that they will be judged as bad parents for contributing to their children's anxiety, depression, headaches, and migraines. In fact, beyond mental health stigma, children who have migraine can be blamed for having an unhealthy lifestyle.[1] While advice to get enough sleep, eat healthy, and stay active is worthwhile, there can be an implication that pediatric migraine patients are causing their migraines by living an unhealthy lifestyle.[1] Additionally, the implication that parents are not properly taking care of their children's health can inhibit an accurate symptom history. Releasing pediatric migraine patients and their parents from myths about migraines and headaches can be a beneficial component of doctor-patient communication regarding migraine care.
It is possible that dietary adjustments or supplements could help improve migraine frequency and severity. Maintaining a healthy diet is a frequent recommendation for people who have headaches, but it can be frustrating for patients to receive general recommendations to follow a healthy lifestyle. Specific direction regarding which foods to avoid and which foods to add to a diet can be helpful for patients as they try to navigate the challenge of adopting migraine-friendly lifestyle changes.
Eicosapentaenoic acid (EPA) is one of the omega-3 fatty acids. A recent study, with results published in Brain, Behavior, and Immunity, examined the effects of EPA on migraines. The 12-week randomized, double-blind, placebo-controlled trial included 70 participants who had been diagnosed with episodic migraine. Participants were randomly assigned to either EPA (2 g fish oil with 1.8 g of EPA/day) or placebo (2 g soybean oil/day). Migraine frequency and severity were assessed using standardized scales. According to the authors, the high-dose-EPA group had significantly reduced migraine frequency and severity, fewer number of days using acute treatment, reduced migraine-associated disability, improved anxiety and depression, and improved quality of life in comparison to the placebo group. The EPA group did not experience notable adverse events. To provide a sense of scale regarding dietary EPA, 3 oz of cooked wild salmon has 0.35 g of EPA, 3 oz of cooked shrimp has 0.2 g of EPA, and 3 oz of light canned tuna has 0.02 g of EPA.[2] Thus, it's important to note that the amount of EPA used in this study was higher than what would be expected of dietary EPA.
An observational prospective study published in Scientific Reports examined the effects of dietary phytochemical index (DPI) on migraine. DPI is defined as the proportion of daily energy intake derived from foods rich in phytochemicals. Consumption of phytochemical-rich foods has been associated with cardiovascular and metabolic diseases prevention in various populations. These foods include fruits, vegetables, whole grains, seeds, nuts, and legumes. The study included 265 adults age 20-50 who had a diagnosis of migraine. Participants were asked to fill out a questionnaire, which was used to evaluate their diet in the preceding year, and they were asked to complete a diary to track their migraine symptoms. The results showed an inverse relationship between DPI index and migraine frequency. Participants who had the highest DPI had the lowest migraine frequency.[3] While the authors found the results to be statistically significant, they did not point to a cause and effect. Migraine-associated symptoms such as nausea can have an effect on dietary choices, so patients who experience migraine symptoms may avoid certain foods before, during, or after a migraine episode. They also may consistently avoid foods that they have experienced as migraine triggers.
Diet and lifestyle can have an effect on migraine frequency, severity, and overall migraine-associated quality of life. Beyond general recommendations, however, it is not yet well established which foods or supplements could potentially help alleviate migraines. Advice to maintain a healthy lifestyle is definitely worthwhile for migraine patients, but it is important to avoid conveying blame or stigma when it comes to communication about the effect of lifestyle on migraine. This is especially important for pediatric migraine patients because the stigma extends beyond children to parents and could potentially interfere with clear communication and adequate care.
Additional References
1. Gelfand AA, Irwin SL. Lifestyle advice for pediatric migraine: Blaming the patient, or evidence based? Semin Neurol. 2020;40:277-285. doi: 10.1055/s-0040-1708868 Source
2. National Institutes of Health. Office of Dietary Supplements. Omega-3 fatty acids. Updated February 15, 2023. Source
3. Hamedi-Shahraki S, Jowshan M-R, Zolghadrpour M-A, et al. Dietary phytochemical index is favorably associated with oxidative stress status and cardiovascular risk factors in adults with obesity. Sci Rep. 2023;13:7035. doi: 10.1038/s41598-023-34064-4 Source
Migraine and other headache types are common ailments, and there are many stereotypes and stigmas associated with these conditions. One of the prevailing beliefs about headaches and migraines is that they are linked with internalizing mental health conditions — anxiety and depression. These associations can affect pediatric migraine patients and their parents in complicated ways, potentially hindering adequate diagnosis and treatment. Results of a recent prospective study, published in the journal Headache, provided results that challenge the widespread belief that people who have migraines have a higher-than-average rate of internalizing mental health disorders. The authors provided a discussion and data to explain that their initial hypothesis of a relationship between migraine and mental health was disproven. The study included 123 participants age 8-18 years who had been previously diagnosed with migraine. The patients, who were seen in a pediatric neurology clinic, completed headache questionnaires and validated measures of anxiety and depressive symptoms. The final analysis showed no significant association between migraines or headaches with anxiety or depression.
Why does this matter? Stigma can prevent patients and parents from seeking care if parents feel that they will be judged as bad parents for contributing to their children's anxiety, depression, headaches, and migraines. In fact, beyond mental health stigma, children who have migraine can be blamed for having an unhealthy lifestyle.[1] While advice to get enough sleep, eat healthy, and stay active is worthwhile, there can be an implication that pediatric migraine patients are causing their migraines by living an unhealthy lifestyle.[1] Additionally, the implication that parents are not properly taking care of their children's health can inhibit an accurate symptom history. Releasing pediatric migraine patients and their parents from myths about migraines and headaches can be a beneficial component of doctor-patient communication regarding migraine care.
It is possible that dietary adjustments or supplements could help improve migraine frequency and severity. Maintaining a healthy diet is a frequent recommendation for people who have headaches, but it can be frustrating for patients to receive general recommendations to follow a healthy lifestyle. Specific direction regarding which foods to avoid and which foods to add to a diet can be helpful for patients as they try to navigate the challenge of adopting migraine-friendly lifestyle changes.
Eicosapentaenoic acid (EPA) is one of the omega-3 fatty acids. A recent study, with results published in Brain, Behavior, and Immunity, examined the effects of EPA on migraines. The 12-week randomized, double-blind, placebo-controlled trial included 70 participants who had been diagnosed with episodic migraine. Participants were randomly assigned to either EPA (2 g fish oil with 1.8 g of EPA/day) or placebo (2 g soybean oil/day). Migraine frequency and severity were assessed using standardized scales. According to the authors, the high-dose-EPA group had significantly reduced migraine frequency and severity, fewer number of days using acute treatment, reduced migraine-associated disability, improved anxiety and depression, and improved quality of life in comparison to the placebo group. The EPA group did not experience notable adverse events. To provide a sense of scale regarding dietary EPA, 3 oz of cooked wild salmon has 0.35 g of EPA, 3 oz of cooked shrimp has 0.2 g of EPA, and 3 oz of light canned tuna has 0.02 g of EPA.[2] Thus, it's important to note that the amount of EPA used in this study was higher than what would be expected of dietary EPA.
An observational prospective study published in Scientific Reports examined the effects of dietary phytochemical index (DPI) on migraine. DPI is defined as the proportion of daily energy intake derived from foods rich in phytochemicals. Consumption of phytochemical-rich foods has been associated with cardiovascular and metabolic diseases prevention in various populations. These foods include fruits, vegetables, whole grains, seeds, nuts, and legumes. The study included 265 adults age 20-50 who had a diagnosis of migraine. Participants were asked to fill out a questionnaire, which was used to evaluate their diet in the preceding year, and they were asked to complete a diary to track their migraine symptoms. The results showed an inverse relationship between DPI index and migraine frequency. Participants who had the highest DPI had the lowest migraine frequency.[3] While the authors found the results to be statistically significant, they did not point to a cause and effect. Migraine-associated symptoms such as nausea can have an effect on dietary choices, so patients who experience migraine symptoms may avoid certain foods before, during, or after a migraine episode. They also may consistently avoid foods that they have experienced as migraine triggers.
Diet and lifestyle can have an effect on migraine frequency, severity, and overall migraine-associated quality of life. Beyond general recommendations, however, it is not yet well established which foods or supplements could potentially help alleviate migraines. Advice to maintain a healthy lifestyle is definitely worthwhile for migraine patients, but it is important to avoid conveying blame or stigma when it comes to communication about the effect of lifestyle on migraine. This is especially important for pediatric migraine patients because the stigma extends beyond children to parents and could potentially interfere with clear communication and adequate care.
Additional References
1. Gelfand AA, Irwin SL. Lifestyle advice for pediatric migraine: Blaming the patient, or evidence based? Semin Neurol. 2020;40:277-285. doi: 10.1055/s-0040-1708868 Source
2. National Institutes of Health. Office of Dietary Supplements. Omega-3 fatty acids. Updated February 15, 2023. Source
3. Hamedi-Shahraki S, Jowshan M-R, Zolghadrpour M-A, et al. Dietary phytochemical index is favorably associated with oxidative stress status and cardiovascular risk factors in adults with obesity. Sci Rep. 2023;13:7035. doi: 10.1038/s41598-023-34064-4 Source
Risk for COVID-19 Infection in Patients With Vitiligo
To the Editor:
Vitiligo is a depigmentation disorder that results from the loss of melanocytes in the epidermis.1 The most widely accepted pathophysiology for melanocyte destruction in vitiligo is an autoimmune process involving dysregulated cytokine production and autoreactive T-cell activation.1 Individuals with cutaneous autoinflammatory conditions currently are vital patient populations warranting research, as their susceptibility to COVID-19 infection may differ from the general population. We previously found a small increased risk for COVID-19 infection in patients with psoriasis,2 which suggests that other dermatologic conditions also may impact COVID-19 risk. The risk for COVID-19 infection in patients with vitiligo remains largely unknown. In this retrospective cohort study, we investigated the risk for COVID-19 infection in patients with vitiligo compared with those without vitiligo utilizing claims data from the COVID-19 Research Database (https://covid19researchdatabase.org/).
Claims were evaluated for patients aged 3 years and older with a vitiligo diagnosis (International Classification of Diseases, Tenth Revision [ICD-10] code L80) that was made between January 1, 2016, and January 1, 2020. Individuals without a vitiligo diagnosis during the same period were placed (4:1 ratio) in the control group and were matched with study group patients for age and sex. All comorbidity variables and vitiligo diagnoses were extracted from ICD-10 codes that were given prior to a diagnosis of COVID-19. We then constructed multivariable logistic regression models adjusting for measured confounders to evaluate if vitiligo was associated with higher risk for COVID-19 infection after January 1, 2020.
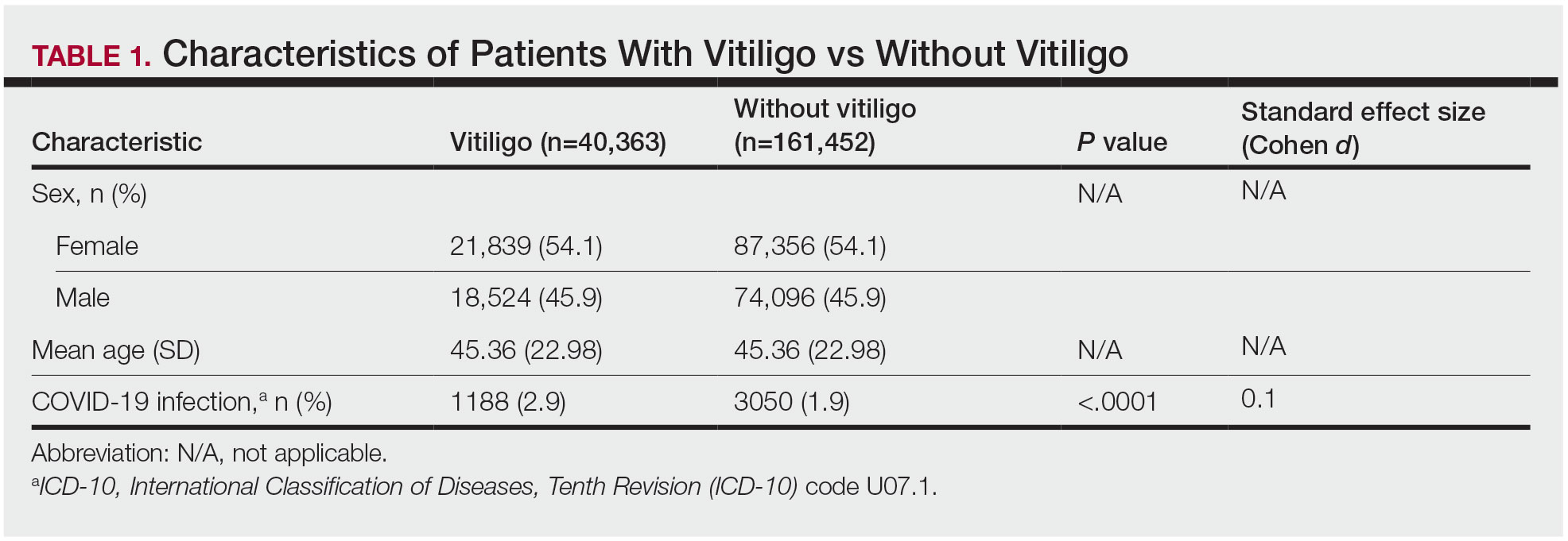
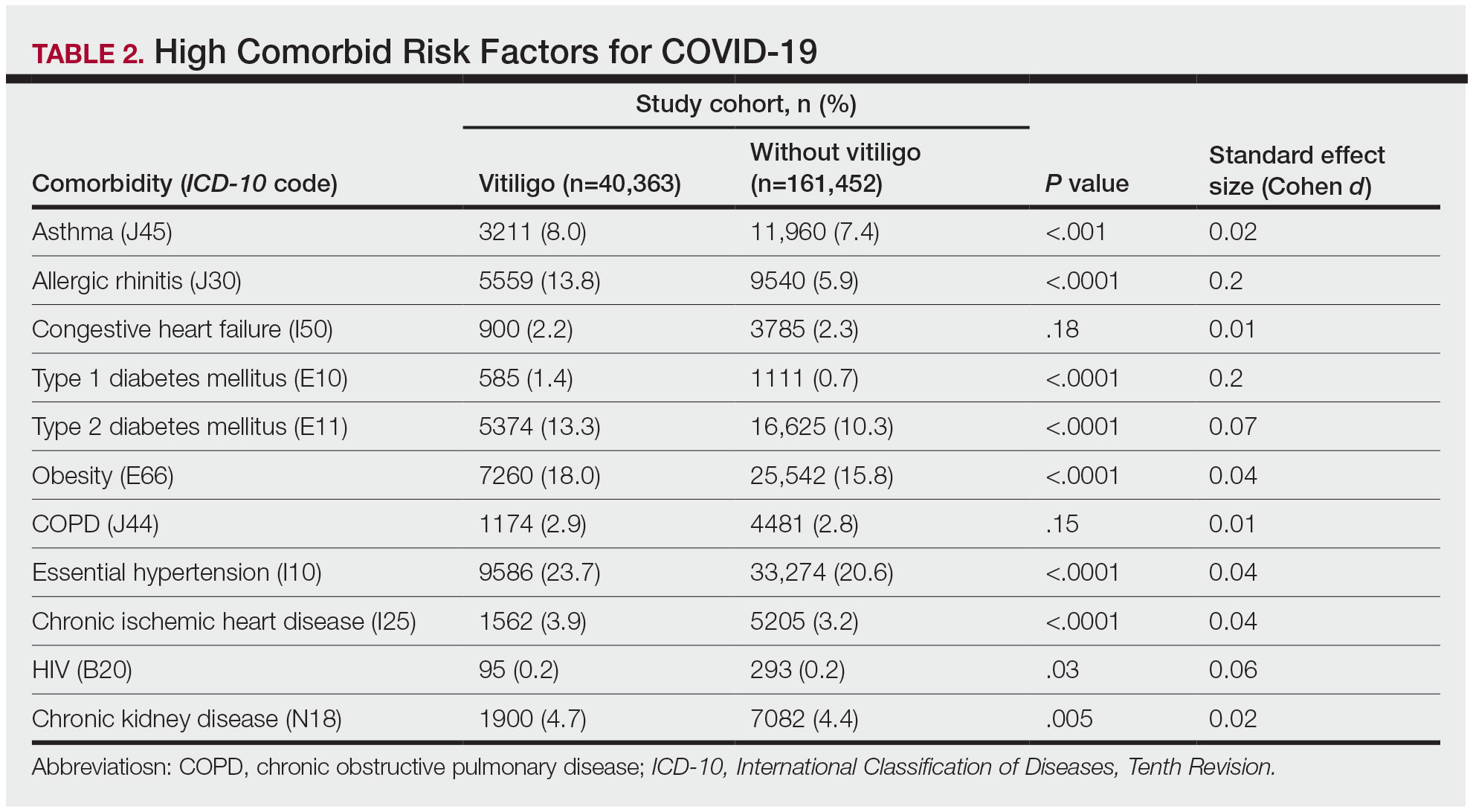
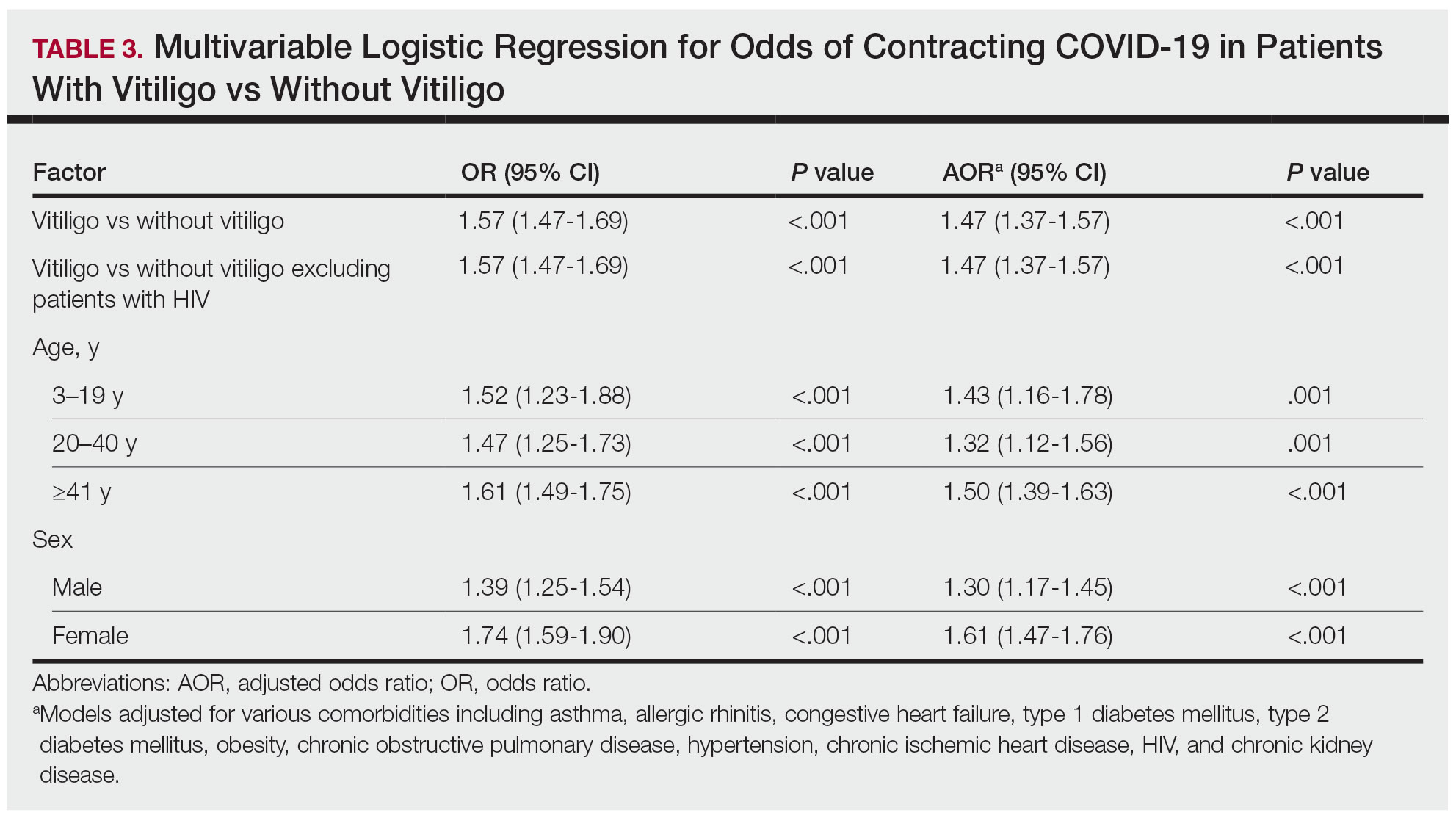
The vitiligo and nonvitiligo cohorts included 40,363 and 161,452 patients, respectively (Table 1). Logistic regression analysis with adjustment for confounding variables, including high comorbid risk factors (Table 2) revealed that patients with a diagnosis of vitiligo had significantly increased odds of COVID-19 infection compared with patients without vitiligo (adjusted odds ratio [AOR], 1.47; 95% CI, 1.37-1.57; P<.001)(Table 3). Additionally, subgroup logistic analyses for sex, age, and exclusion of patients who were HIV positive revealed that females with vitiligo had higher odds of contracting COVID-19 than males with vitiligo (Table 3).
Our results showed that patients with vitiligo had a higher relative risk for contracting COVID-19 than individuals without vitiligo. It has been reported that the prevalence of COVID-19 is higher among patients with autoimmune diseases compared to the general population.3 Additionally, a handful of vitiligo patients are managed with immunosuppressive agents that may further weaken their immune response.1 Moreover, survey results from dermatologists managing vitiligo patients revealed that physicians were fairly comfortable prescribing immunosuppressants and encouraging in-office phototherapy during the COVID-19 pandemic.4 As a result, more patients may have been attending in-office visits for their phototherapy, which may have increased their risk for COVID-19. Although these factors play a role in COVID-19 infection rates, the underlying immune dysregulation in vitiligo in relation to COVID-19 remains unknown and should be further explored.
Our findings are limited by the use of ICD-10 codes, the inability to control for all potential confounding variables, the lack of data regarding the stage of vitiligo, and the absence of data for undiagnosed COVID-19 infections. In addition, patients with vitiligo may be more likely to seek care, potentially increasing their rates of COVID-19 testing. The inability to identify the stage of vitiligo during enrollment in the database may have altered our results, as individuals with active disease have increased levels of IFN-γ. Increased secretion of IFN-γ also potentially helps in the clearance of COVID-19 infection.1 Future studies should investigate this relationship via planned COVID-19 testing, identification of vitiligo stage, and controlling for other associated comorbidities.
- Rashighi M, Harris JE. Vitiligo pathogenesis and emerging treatments. Dermatol Clin. 2017;35:257-265. doi:10.1016/j.det.2016.11.014
- Wu JJ, Liu J, Thatiparthi A, et al. The risk of COVID-19 in patients with psoriasis—a retrospective cohort study [published online September 20, 2022]. J Am Acad Dermatol. doi:10.1016/j.jaad.2022.07.040
- Zhong J, Shen G, Yang H, et al. COVID-19 in patients with rheumatic disease in Hubei province, China: a multicentre retrospective observational study. Lancet Rheumatol. 2020;2:E557-E564. doi:10.1016/S2665-9913(20)30227-7
- Chatterjee M, Das A. Management of vitiligo amidst the COVID-19 pandemic: a survey and resulting consensus. Indian J Dermatol. 2021;66:479-483. doi:10.4103/ijd.ijd_859_20
To the Editor:
Vitiligo is a depigmentation disorder that results from the loss of melanocytes in the epidermis.1 The most widely accepted pathophysiology for melanocyte destruction in vitiligo is an autoimmune process involving dysregulated cytokine production and autoreactive T-cell activation.1 Individuals with cutaneous autoinflammatory conditions currently are vital patient populations warranting research, as their susceptibility to COVID-19 infection may differ from the general population. We previously found a small increased risk for COVID-19 infection in patients with psoriasis,2 which suggests that other dermatologic conditions also may impact COVID-19 risk. The risk for COVID-19 infection in patients with vitiligo remains largely unknown. In this retrospective cohort study, we investigated the risk for COVID-19 infection in patients with vitiligo compared with those without vitiligo utilizing claims data from the COVID-19 Research Database (https://covid19researchdatabase.org/).
Claims were evaluated for patients aged 3 years and older with a vitiligo diagnosis (International Classification of Diseases, Tenth Revision [ICD-10] code L80) that was made between January 1, 2016, and January 1, 2020. Individuals without a vitiligo diagnosis during the same period were placed (4:1 ratio) in the control group and were matched with study group patients for age and sex. All comorbidity variables and vitiligo diagnoses were extracted from ICD-10 codes that were given prior to a diagnosis of COVID-19. We then constructed multivariable logistic regression models adjusting for measured confounders to evaluate if vitiligo was associated with higher risk for COVID-19 infection after January 1, 2020.
The vitiligo and nonvitiligo cohorts included 40,363 and 161,452 patients, respectively (Table 1). Logistic regression analysis with adjustment for confounding variables, including high comorbid risk factors (Table 2) revealed that patients with a diagnosis of vitiligo had significantly increased odds of COVID-19 infection compared with patients without vitiligo (adjusted odds ratio [AOR], 1.47; 95% CI, 1.37-1.57; P<.001)(Table 3). Additionally, subgroup logistic analyses for sex, age, and exclusion of patients who were HIV positive revealed that females with vitiligo had higher odds of contracting COVID-19 than males with vitiligo (Table 3).

Our results showed that patients with vitiligo had a higher relative risk for contracting COVID-19 than individuals without vitiligo. It has been reported that the prevalence of COVID-19 is higher among patients with autoimmune diseases compared to the general population.3 Additionally, a handful of vitiligo patients are managed with immunosuppressive agents that may further weaken their immune response.1 Moreover, survey results from dermatologists managing vitiligo patients revealed that physicians were fairly comfortable prescribing immunosuppressants and encouraging in-office phototherapy during the COVID-19 pandemic.4 As a result, more patients may have been attending in-office visits for their phototherapy, which may have increased their risk for COVID-19. Although these factors play a role in COVID-19 infection rates, the underlying immune dysregulation in vitiligo in relation to COVID-19 remains unknown and should be further explored.

Our findings are limited by the use of ICD-10 codes, the inability to control for all potential confounding variables, the lack of data regarding the stage of vitiligo, and the absence of data for undiagnosed COVID-19 infections. In addition, patients with vitiligo may be more likely to seek care, potentially increasing their rates of COVID-19 testing. The inability to identify the stage of vitiligo during enrollment in the database may have altered our results, as individuals with active disease have increased levels of IFN-γ. Increased secretion of IFN-γ also potentially helps in the clearance of COVID-19 infection.1 Future studies should investigate this relationship via planned COVID-19 testing, identification of vitiligo stage, and controlling for other associated comorbidities.

To the Editor:
Vitiligo is a depigmentation disorder that results from the loss of melanocytes in the epidermis.1 The most widely accepted pathophysiology for melanocyte destruction in vitiligo is an autoimmune process involving dysregulated cytokine production and autoreactive T-cell activation.1 Individuals with cutaneous autoinflammatory conditions currently are vital patient populations warranting research, as their susceptibility to COVID-19 infection may differ from the general population. We previously found a small increased risk for COVID-19 infection in patients with psoriasis,2 which suggests that other dermatologic conditions also may impact COVID-19 risk. The risk for COVID-19 infection in patients with vitiligo remains largely unknown. In this retrospective cohort study, we investigated the risk for COVID-19 infection in patients with vitiligo compared with those without vitiligo utilizing claims data from the COVID-19 Research Database (https://covid19researchdatabase.org/).
Claims were evaluated for patients aged 3 years and older with a vitiligo diagnosis (International Classification of Diseases, Tenth Revision [ICD-10] code L80) that was made between January 1, 2016, and January 1, 2020. Individuals without a vitiligo diagnosis during the same period were placed (4:1 ratio) in the control group and were matched with study group patients for age and sex. All comorbidity variables and vitiligo diagnoses were extracted from ICD-10 codes that were given prior to a diagnosis of COVID-19. We then constructed multivariable logistic regression models adjusting for measured confounders to evaluate if vitiligo was associated with higher risk for COVID-19 infection after January 1, 2020.
The vitiligo and nonvitiligo cohorts included 40,363 and 161,452 patients, respectively (Table 1). Logistic regression analysis with adjustment for confounding variables, including high comorbid risk factors (Table 2) revealed that patients with a diagnosis of vitiligo had significantly increased odds of COVID-19 infection compared with patients without vitiligo (adjusted odds ratio [AOR], 1.47; 95% CI, 1.37-1.57; P<.001)(Table 3). Additionally, subgroup logistic analyses for sex, age, and exclusion of patients who were HIV positive revealed that females with vitiligo had higher odds of contracting COVID-19 than males with vitiligo (Table 3).

Our results showed that patients with vitiligo had a higher relative risk for contracting COVID-19 than individuals without vitiligo. It has been reported that the prevalence of COVID-19 is higher among patients with autoimmune diseases compared to the general population.3 Additionally, a handful of vitiligo patients are managed with immunosuppressive agents that may further weaken their immune response.1 Moreover, survey results from dermatologists managing vitiligo patients revealed that physicians were fairly comfortable prescribing immunosuppressants and encouraging in-office phototherapy during the COVID-19 pandemic.4 As a result, more patients may have been attending in-office visits for their phototherapy, which may have increased their risk for COVID-19. Although these factors play a role in COVID-19 infection rates, the underlying immune dysregulation in vitiligo in relation to COVID-19 remains unknown and should be further explored.

Our findings are limited by the use of ICD-10 codes, the inability to control for all potential confounding variables, the lack of data regarding the stage of vitiligo, and the absence of data for undiagnosed COVID-19 infections. In addition, patients with vitiligo may be more likely to seek care, potentially increasing their rates of COVID-19 testing. The inability to identify the stage of vitiligo during enrollment in the database may have altered our results, as individuals with active disease have increased levels of IFN-γ. Increased secretion of IFN-γ also potentially helps in the clearance of COVID-19 infection.1 Future studies should investigate this relationship via planned COVID-19 testing, identification of vitiligo stage, and controlling for other associated comorbidities.

- Rashighi M, Harris JE. Vitiligo pathogenesis and emerging treatments. Dermatol Clin. 2017;35:257-265. doi:10.1016/j.det.2016.11.014
- Wu JJ, Liu J, Thatiparthi A, et al. The risk of COVID-19 in patients with psoriasis—a retrospective cohort study [published online September 20, 2022]. J Am Acad Dermatol. doi:10.1016/j.jaad.2022.07.040
- Zhong J, Shen G, Yang H, et al. COVID-19 in patients with rheumatic disease in Hubei province, China: a multicentre retrospective observational study. Lancet Rheumatol. 2020;2:E557-E564. doi:10.1016/S2665-9913(20)30227-7
- Chatterjee M, Das A. Management of vitiligo amidst the COVID-19 pandemic: a survey and resulting consensus. Indian J Dermatol. 2021;66:479-483. doi:10.4103/ijd.ijd_859_20
- Rashighi M, Harris JE. Vitiligo pathogenesis and emerging treatments. Dermatol Clin. 2017;35:257-265. doi:10.1016/j.det.2016.11.014
- Wu JJ, Liu J, Thatiparthi A, et al. The risk of COVID-19 in patients with psoriasis—a retrospective cohort study [published online September 20, 2022]. J Am Acad Dermatol. doi:10.1016/j.jaad.2022.07.040
- Zhong J, Shen G, Yang H, et al. COVID-19 in patients with rheumatic disease in Hubei province, China: a multicentre retrospective observational study. Lancet Rheumatol. 2020;2:E557-E564. doi:10.1016/S2665-9913(20)30227-7
- Chatterjee M, Das A. Management of vitiligo amidst the COVID-19 pandemic: a survey and resulting consensus. Indian J Dermatol. 2021;66:479-483. doi:10.4103/ijd.ijd_859_20
Practice Points
- The underlying autoimmune process in vitiligo can result in various changes to the immune system.
- A diagnosis of vitiligo may alter the body’s immune response to COVID-19 infection.
Erythrodermic Pityriasis Rubra Pilaris Following COVID-19 Vaccination
To the Editor:
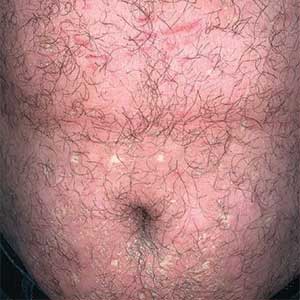
A 32-year-old man presented to our clinic with acute-onset erythroderma associated with severe itching of 1 month’s duration. The patient developed the eruption after receiving the second dose of the Sinopharm BBIBP COVID-19 vaccine (BBIBP-CorV) 2 weeks prior to presentation. His medical history was unremarkable. There was no personal or family history of skin disease and no history of drug intake. Physical examination revealed
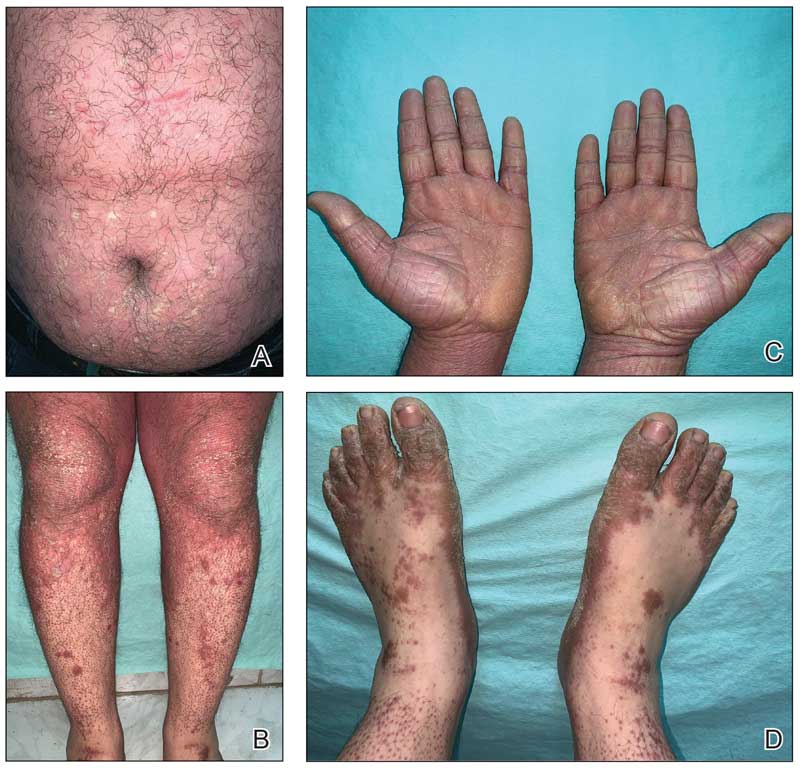
Histopathology of two 4-mm punch biopsies of the skin on the trunk and lower limb showed
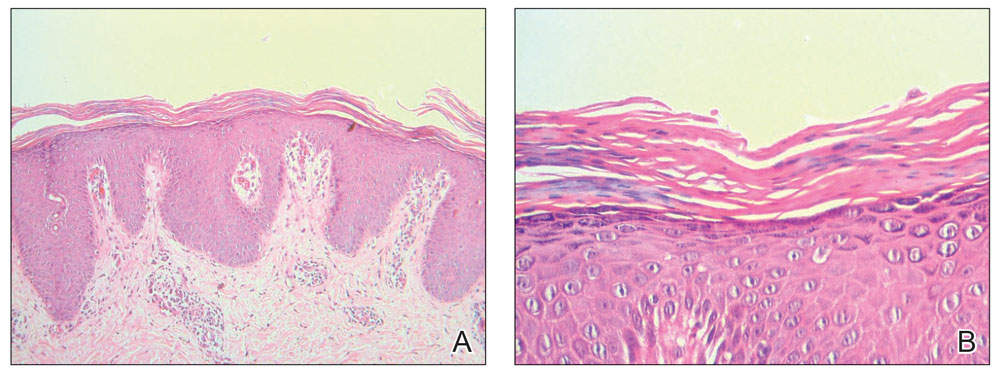
Pityriasis rubra pilaris is a rare papulosquamous skin disease of unknown etiology with several theories including genetic factors, aberrant metabolism of vitamin A, infection, drug reaction, autoimmune disease, and malignancy.1 Clinically, there are 6 types of PRP: type I (classical adult), type II (atypical adult), type III (classical juvenile), type IV (circumscribed juvenile), type V (atypical juvenile), and type VI (HIV associated). Classic features include orange-red keratotic follicular papules that coalesce into plaques with characteristic islands of sparing.1
Pityriasis rubra pilaris is a rare sequela following administration of certain vaccines, including diphtheria, pertussis, and tetanus; measles-mumps-rubella; and polio vaccines.2,3 Among the various skin reactions that have been reported following COVID-19 vaccination, PRP has been reported in 19 patients: 7 (36.8%) after AstraZeneca vaccination, 3 (15.8%) after CoronaVac, 3 (15.8%) after Moderna, 5 (26.3%) after Pfizer-BioNTech,4 and 1 (5.3%) after Sinopharm.5 Our patient represents an additional case of a reaction after the Sinopharm vaccine. The condition developed after the first dose of vaccine in 11 patients, after the second dose in 6 patients, and after the third dose in 2 patients.
Other papulosquamous skin reactions have been reported after
Pityriasis rubra pilaris can be self-limited in some cases and may not require treatment. Topical therapies such as keratolytics, emollients, and vitamin D may be utilized, especially for localized disease. Systemic therapy may be needed for refractory cases, including retinoids or immunosuppressive medications such as methotrexate, which is considered a second-line treatment for refractory PRP (after retinoids) and was used in our case. Azathioprine and cyclosporine also may be used. Phototherapy may play a role in PRP treatment, but the response is variable.7
Pityriasis rubra pilaris should be added to the list of cutaneous adverse reactions that can occur following vaccination with the Sinopharm BBIBP-CorV vaccine. Dermatologists must be aware of the possibility of vaccine-induced PRP, especially in de novo cases.
- Wang D, Chong VC-L, Chong W-S, et al. A review on pityriasis rubra pilaris. Am J Clin Dermatol. 2018;19:377-390. doi:10.1007/s40257-017-0338-1
- Mohamed M, Belhadjali H, Hammedi F, et al. Pityriasis rubra pilaris occurring after vaccination with diphtheria-pertussis-tetanus and oral poliovirus vaccines [letter]. Indian J Dermatol Venereol Leprol. 2015;81:618-620. doi:10.4103/0378-6323.168326
- Naciri Bennani B, Cheikh Rouhou H, Waton J, et al. Pityriasis rubra pilaris after vaccination. Ann Dermatol Venereol. 2011;138:753-756. doi:10.1016/j.annder.2011.01.049
- Liu YA, Dai J, Nagarajan P, et al. Pityriasis rubra pilaris after Moderna COVID-19 vaccination: a case report and literature review. Am J Dermatopathol. 2023;45:185-188. doi:10.1097/DAD.0000000000002369.
- Samarasinghe KH, Janani T, Gunasekera CN. Pityriasis rubra pilaris like eruption following Sinopharm-SARS COVID-19 vaccine. Sri Lanka J Dermatol. 2021;22:99-100.
- Shakoei S, Kalantari Y, Nasimi M, et al. Cutaneous manifestations following COVID-19 vaccination: a report of 25 cases. Dermatol Ther. 2022;35:E15651. doi:10.1111/dth.15651
- Moretta G, De Luca EV, Di Stefani A. Management of refractory pityriasis rubra pilaris: challenges and solutions. Clin Cosmet Investig Dermatol. 2017;10:451-457. doi:10.2147/CCID.S124351.
To the Editor:
A 32-year-old man presented to our clinic with acute-onset erythroderma associated with severe itching of 1 month’s duration. The patient developed the eruption after receiving the second dose of the Sinopharm BBIBP COVID-19 vaccine (BBIBP-CorV) 2 weeks prior to presentation. His medical history was unremarkable. There was no personal or family history of skin disease and no history of drug intake. Physical examination revealed

Histopathology of two 4-mm punch biopsies of the skin on the trunk and lower limb showed

Pityriasis rubra pilaris is a rare papulosquamous skin disease of unknown etiology with several theories including genetic factors, aberrant metabolism of vitamin A, infection, drug reaction, autoimmune disease, and malignancy.1 Clinically, there are 6 types of PRP: type I (classical adult), type II (atypical adult), type III (classical juvenile), type IV (circumscribed juvenile), type V (atypical juvenile), and type VI (HIV associated). Classic features include orange-red keratotic follicular papules that coalesce into plaques with characteristic islands of sparing.1
Pityriasis rubra pilaris is a rare sequela following administration of certain vaccines, including diphtheria, pertussis, and tetanus; measles-mumps-rubella; and polio vaccines.2,3 Among the various skin reactions that have been reported following COVID-19 vaccination, PRP has been reported in 19 patients: 7 (36.8%) after AstraZeneca vaccination, 3 (15.8%) after CoronaVac, 3 (15.8%) after Moderna, 5 (26.3%) after Pfizer-BioNTech,4 and 1 (5.3%) after Sinopharm.5 Our patient represents an additional case of a reaction after the Sinopharm vaccine. The condition developed after the first dose of vaccine in 11 patients, after the second dose in 6 patients, and after the third dose in 2 patients.
Other papulosquamous skin reactions have been reported after
Pityriasis rubra pilaris can be self-limited in some cases and may not require treatment. Topical therapies such as keratolytics, emollients, and vitamin D may be utilized, especially for localized disease. Systemic therapy may be needed for refractory cases, including retinoids or immunosuppressive medications such as methotrexate, which is considered a second-line treatment for refractory PRP (after retinoids) and was used in our case. Azathioprine and cyclosporine also may be used. Phototherapy may play a role in PRP treatment, but the response is variable.7
Pityriasis rubra pilaris should be added to the list of cutaneous adverse reactions that can occur following vaccination with the Sinopharm BBIBP-CorV vaccine. Dermatologists must be aware of the possibility of vaccine-induced PRP, especially in de novo cases.
To the Editor:
A 32-year-old man presented to our clinic with acute-onset erythroderma associated with severe itching of 1 month’s duration. The patient developed the eruption after receiving the second dose of the Sinopharm BBIBP COVID-19 vaccine (BBIBP-CorV) 2 weeks prior to presentation. His medical history was unremarkable. There was no personal or family history of skin disease and no history of drug intake. Physical examination revealed

Histopathology of two 4-mm punch biopsies of the skin on the trunk and lower limb showed

Pityriasis rubra pilaris is a rare papulosquamous skin disease of unknown etiology with several theories including genetic factors, aberrant metabolism of vitamin A, infection, drug reaction, autoimmune disease, and malignancy.1 Clinically, there are 6 types of PRP: type I (classical adult), type II (atypical adult), type III (classical juvenile), type IV (circumscribed juvenile), type V (atypical juvenile), and type VI (HIV associated). Classic features include orange-red keratotic follicular papules that coalesce into plaques with characteristic islands of sparing.1
Pityriasis rubra pilaris is a rare sequela following administration of certain vaccines, including diphtheria, pertussis, and tetanus; measles-mumps-rubella; and polio vaccines.2,3 Among the various skin reactions that have been reported following COVID-19 vaccination, PRP has been reported in 19 patients: 7 (36.8%) after AstraZeneca vaccination, 3 (15.8%) after CoronaVac, 3 (15.8%) after Moderna, 5 (26.3%) after Pfizer-BioNTech,4 and 1 (5.3%) after Sinopharm.5 Our patient represents an additional case of a reaction after the Sinopharm vaccine. The condition developed after the first dose of vaccine in 11 patients, after the second dose in 6 patients, and after the third dose in 2 patients.
Other papulosquamous skin reactions have been reported after
Pityriasis rubra pilaris can be self-limited in some cases and may not require treatment. Topical therapies such as keratolytics, emollients, and vitamin D may be utilized, especially for localized disease. Systemic therapy may be needed for refractory cases, including retinoids or immunosuppressive medications such as methotrexate, which is considered a second-line treatment for refractory PRP (after retinoids) and was used in our case. Azathioprine and cyclosporine also may be used. Phototherapy may play a role in PRP treatment, but the response is variable.7
Pityriasis rubra pilaris should be added to the list of cutaneous adverse reactions that can occur following vaccination with the Sinopharm BBIBP-CorV vaccine. Dermatologists must be aware of the possibility of vaccine-induced PRP, especially in de novo cases.
- Wang D, Chong VC-L, Chong W-S, et al. A review on pityriasis rubra pilaris. Am J Clin Dermatol. 2018;19:377-390. doi:10.1007/s40257-017-0338-1
- Mohamed M, Belhadjali H, Hammedi F, et al. Pityriasis rubra pilaris occurring after vaccination with diphtheria-pertussis-tetanus and oral poliovirus vaccines [letter]. Indian J Dermatol Venereol Leprol. 2015;81:618-620. doi:10.4103/0378-6323.168326
- Naciri Bennani B, Cheikh Rouhou H, Waton J, et al. Pityriasis rubra pilaris after vaccination. Ann Dermatol Venereol. 2011;138:753-756. doi:10.1016/j.annder.2011.01.049
- Liu YA, Dai J, Nagarajan P, et al. Pityriasis rubra pilaris after Moderna COVID-19 vaccination: a case report and literature review. Am J Dermatopathol. 2023;45:185-188. doi:10.1097/DAD.0000000000002369.
- Samarasinghe KH, Janani T, Gunasekera CN. Pityriasis rubra pilaris like eruption following Sinopharm-SARS COVID-19 vaccine. Sri Lanka J Dermatol. 2021;22:99-100.
- Shakoei S, Kalantari Y, Nasimi M, et al. Cutaneous manifestations following COVID-19 vaccination: a report of 25 cases. Dermatol Ther. 2022;35:E15651. doi:10.1111/dth.15651
- Moretta G, De Luca EV, Di Stefani A. Management of refractory pityriasis rubra pilaris: challenges and solutions. Clin Cosmet Investig Dermatol. 2017;10:451-457. doi:10.2147/CCID.S124351.
- Wang D, Chong VC-L, Chong W-S, et al. A review on pityriasis rubra pilaris. Am J Clin Dermatol. 2018;19:377-390. doi:10.1007/s40257-017-0338-1
- Mohamed M, Belhadjali H, Hammedi F, et al. Pityriasis rubra pilaris occurring after vaccination with diphtheria-pertussis-tetanus and oral poliovirus vaccines [letter]. Indian J Dermatol Venereol Leprol. 2015;81:618-620. doi:10.4103/0378-6323.168326
- Naciri Bennani B, Cheikh Rouhou H, Waton J, et al. Pityriasis rubra pilaris after vaccination. Ann Dermatol Venereol. 2011;138:753-756. doi:10.1016/j.annder.2011.01.049
- Liu YA, Dai J, Nagarajan P, et al. Pityriasis rubra pilaris after Moderna COVID-19 vaccination: a case report and literature review. Am J Dermatopathol. 2023;45:185-188. doi:10.1097/DAD.0000000000002369.
- Samarasinghe KH, Janani T, Gunasekera CN. Pityriasis rubra pilaris like eruption following Sinopharm-SARS COVID-19 vaccine. Sri Lanka J Dermatol. 2021;22:99-100.
- Shakoei S, Kalantari Y, Nasimi M, et al. Cutaneous manifestations following COVID-19 vaccination: a report of 25 cases. Dermatol Ther. 2022;35:E15651. doi:10.1111/dth.15651
- Moretta G, De Luca EV, Di Stefani A. Management of refractory pityriasis rubra pilaris: challenges and solutions. Clin Cosmet Investig Dermatol. 2017;10:451-457. doi:10.2147/CCID.S124351.
Practice Points
- Dermatologists must be aware of the possibility of COVID-19 vaccine–induced pityriasis rubra pilaris (PRP), especially in de novo cases.
- Management of these cases usually follows similar standards for PRP cases.
Commentary: Comparisons Among PsA Therapies, May 2024
Bimekizumab is a novel biologic therapy that inhibits interleukin (IL)–17A and IL-17F and is efficacious in the treatment of psoriasis, PsA, and axial spondyloarthritis. In the absence of a formal head-to-head study, matching-adjusted indirect comparison is a method to evaluate comparative effectiveness. Warren and colleagues ran a study that included biological DMARD-naive patients and patients with inadequate response to tumor necrosis factor inhibitors (TNFi-IR) with PsA who received bimekizumab (160 mg every 4 weeks; 431 and 267 patients, respectively) and guselkumab (100 mg every 4 weeks or every 8 weeks; 495 and 189 patients, respectively). They demonstrate that in biological DMARD-naive patients, bimekizumab was associated with a greater likelihood of achieving ≥70% improvement in American College of Rheumatology (ACR) response and minimal disease activity outcome at week 52 compared with guselkumab. Similar outcomes were observed in the TNFi-IR subgroup. Thus, bimekizumab may be more effective than guselkumab in PsA. Formal head-to-head studies comparing bimekizumab vs guselkumab are required.
With the availability of multiple targeted therapies for PsA, choosing the most effective and safe drug for a patient is difficult, especially in the absence of many head-to-head clinical trials. To help address this problem, Lin and Ren conducted a network meta-analysis of head-to-head active comparison studies in PsA. They included 17 studies in their analysis and demonstrated that Janus kinase inhibitors had the highest probability of achieving ACR 20/50/70 response. Treatment with IL-17A inhibitors was more likely than TNFi therapy to lead to resolution of enthesitis and dactylitis and achieving combined ACR 50 and Psoriasis Area Severity Index 100 response. Patients receiving phosphodiesterase 4 inhibitors were least likely to have adverse events. They conclude that when both efficacy and safety are considered, IL-17A inhibitors may be the better agent for initial therapy for PsA. IL-17A inhibitors are indeed safe and efficacious in PsA; more direct head-to-head comparisons as well as strategy trials are required to determine choice of first and subsequent therapy in PsA.
Infections are the most important adverse effects of targeted therapies. The risk for infection in PsA in real-world settings is not well known. In a cohort study that included 12,071 patients with PsA from the French national health insurance database who were new users of targeted therapies (adalimumab, etanercept, golimumab, certolizumab pegol, infliximab, secukinumab, ixekizumab, ustekinumab, and tofacitinib), Bastard and colleagues demonstrated that the incidence of serious infections in users of targeted therapies was 17.0 per 1000 person-years. Compared with new users of adalimumab, the risk for serious infections was significantly lower in new users of etanercept (weighted hazard ratio [wHR] 0.72; 95% CI 0.53-0.97) and ustekinumab (wHR 0.57; 95% CI 0.35-0.93). Thus, the overall risk for serious infections is low, with etanercept and ustekinumab being safer treatment options than adalimumab.
Bimekizumab is a novel biologic therapy that inhibits interleukin (IL)–17A and IL-17F and is efficacious in the treatment of psoriasis, PsA, and axial spondyloarthritis. In the absence of a formal head-to-head study, matching-adjusted indirect comparison is a method to evaluate comparative effectiveness. Warren and colleagues ran a study that included biological DMARD-naive patients and patients with inadequate response to tumor necrosis factor inhibitors (TNFi-IR) with PsA who received bimekizumab (160 mg every 4 weeks; 431 and 267 patients, respectively) and guselkumab (100 mg every 4 weeks or every 8 weeks; 495 and 189 patients, respectively). They demonstrate that in biological DMARD-naive patients, bimekizumab was associated with a greater likelihood of achieving ≥70% improvement in American College of Rheumatology (ACR) response and minimal disease activity outcome at week 52 compared with guselkumab. Similar outcomes were observed in the TNFi-IR subgroup. Thus, bimekizumab may be more effective than guselkumab in PsA. Formal head-to-head studies comparing bimekizumab vs guselkumab are required.
With the availability of multiple targeted therapies for PsA, choosing the most effective and safe drug for a patient is difficult, especially in the absence of many head-to-head clinical trials. To help address this problem, Lin and Ren conducted a network meta-analysis of head-to-head active comparison studies in PsA. They included 17 studies in their analysis and demonstrated that Janus kinase inhibitors had the highest probability of achieving ACR 20/50/70 response. Treatment with IL-17A inhibitors was more likely than TNFi therapy to lead to resolution of enthesitis and dactylitis and achieving combined ACR 50 and Psoriasis Area Severity Index 100 response. Patients receiving phosphodiesterase 4 inhibitors were least likely to have adverse events. They conclude that when both efficacy and safety are considered, IL-17A inhibitors may be the better agent for initial therapy for PsA. IL-17A inhibitors are indeed safe and efficacious in PsA; more direct head-to-head comparisons as well as strategy trials are required to determine choice of first and subsequent therapy in PsA.
Infections are the most important adverse effects of targeted therapies. The risk for infection in PsA in real-world settings is not well known. In a cohort study that included 12,071 patients with PsA from the French national health insurance database who were new users of targeted therapies (adalimumab, etanercept, golimumab, certolizumab pegol, infliximab, secukinumab, ixekizumab, ustekinumab, and tofacitinib), Bastard and colleagues demonstrated that the incidence of serious infections in users of targeted therapies was 17.0 per 1000 person-years. Compared with new users of adalimumab, the risk for serious infections was significantly lower in new users of etanercept (weighted hazard ratio [wHR] 0.72; 95% CI 0.53-0.97) and ustekinumab (wHR 0.57; 95% CI 0.35-0.93). Thus, the overall risk for serious infections is low, with etanercept and ustekinumab being safer treatment options than adalimumab.
Bimekizumab is a novel biologic therapy that inhibits interleukin (IL)–17A and IL-17F and is efficacious in the treatment of psoriasis, PsA, and axial spondyloarthritis. In the absence of a formal head-to-head study, matching-adjusted indirect comparison is a method to evaluate comparative effectiveness. Warren and colleagues ran a study that included biological DMARD-naive patients and patients with inadequate response to tumor necrosis factor inhibitors (TNFi-IR) with PsA who received bimekizumab (160 mg every 4 weeks; 431 and 267 patients, respectively) and guselkumab (100 mg every 4 weeks or every 8 weeks; 495 and 189 patients, respectively). They demonstrate that in biological DMARD-naive patients, bimekizumab was associated with a greater likelihood of achieving ≥70% improvement in American College of Rheumatology (ACR) response and minimal disease activity outcome at week 52 compared with guselkumab. Similar outcomes were observed in the TNFi-IR subgroup. Thus, bimekizumab may be more effective than guselkumab in PsA. Formal head-to-head studies comparing bimekizumab vs guselkumab are required.
With the availability of multiple targeted therapies for PsA, choosing the most effective and safe drug for a patient is difficult, especially in the absence of many head-to-head clinical trials. To help address this problem, Lin and Ren conducted a network meta-analysis of head-to-head active comparison studies in PsA. They included 17 studies in their analysis and demonstrated that Janus kinase inhibitors had the highest probability of achieving ACR 20/50/70 response. Treatment with IL-17A inhibitors was more likely than TNFi therapy to lead to resolution of enthesitis and dactylitis and achieving combined ACR 50 and Psoriasis Area Severity Index 100 response. Patients receiving phosphodiesterase 4 inhibitors were least likely to have adverse events. They conclude that when both efficacy and safety are considered, IL-17A inhibitors may be the better agent for initial therapy for PsA. IL-17A inhibitors are indeed safe and efficacious in PsA; more direct head-to-head comparisons as well as strategy trials are required to determine choice of first and subsequent therapy in PsA.
Infections are the most important adverse effects of targeted therapies. The risk for infection in PsA in real-world settings is not well known. In a cohort study that included 12,071 patients with PsA from the French national health insurance database who were new users of targeted therapies (adalimumab, etanercept, golimumab, certolizumab pegol, infliximab, secukinumab, ixekizumab, ustekinumab, and tofacitinib), Bastard and colleagues demonstrated that the incidence of serious infections in users of targeted therapies was 17.0 per 1000 person-years. Compared with new users of adalimumab, the risk for serious infections was significantly lower in new users of etanercept (weighted hazard ratio [wHR] 0.72; 95% CI 0.53-0.97) and ustekinumab (wHR 0.57; 95% CI 0.35-0.93). Thus, the overall risk for serious infections is low, with etanercept and ustekinumab being safer treatment options than adalimumab.
Commentary: Studies Often Do Not Answer Clinical Questions in AD, May 2024
In "Atopic Dermatitis in Early Childhood and Risk of Inflammatory Bowel Disease: A Scandinavian Birth Cohort Study," Lerchova and colleagues found a statistically significant increased risk for inflammatory bowel disease (IBD) in children with atopic dermatitis. The study had a large patient population, giving it the power to identify very small differences. The researchers found increased risks for IBD, Crohn's disease, and ulcerative colitis (UC) in children with atopic dermatitis; UC had the greatest relative risk. But I don't think this risk was clinically meaningful. About 2 in every 1000 children with atopic dermatitis had UC, whereas about 1 in every 1000 children without atopic dermatitis had UC. Even if the increased absolute risk of 1 in 1000 children was due to atopic dermatitis and not to other factors, I don't think it justifies the authors' conclusion that "these findings might be useful in identifying at-risk individuals for IBD."
Sometimes reviewing articles makes me feel like a crotchety old man. A study by Guttman-Yassky and colleagues, "Targeting IL-13 With Tralokinumab Normalizes Type 2 Inflammation in Atopic Dermatitis Both Early and at 2 Years," didn't seem to test any specific hypothesis. The researchers just looked at a variety of inflammation markers in patients with atopic dermatitis treated with tralokinumab, an interleukin-13 (IL-13) antagonist. In these patients, as expected, the atopic dermatitis improved; so did the inflammatory markers. Did we learn anything clinically useful? I don't think so. We already know that IL-13 is important in atopic dermatitis because when we block IL-13, atopic dermatitis improves.
Vitamin D supplementation doesn't appear to improve atopic dermatitis, as reported by Borzutzky and colleagues in "Effect of Weekly Vitamin D Supplementation on the Severity of Atopic Dermatitis and Type 2 Immunity Biomarkers in Children: A Randomized Controlled Trial." A group of 101 children with atopic dermatitis were randomly assigned to receive oral vitamin D supplementation or placebo. The two groups improved to a similar extent. If you know me, you know I'm wondering whether they took the medication. It appears that they did, because at baseline most of the children were vitamin D deficient, and vitamin D levels improved greatly in the group treated with vitamin D but not in the placebo group.
Journals such as the Journal of the American Academy of Dermatology should require articles to report absolute risk. In "Risk of Lymphoma in Patients With Atopic Dermatitis: A Case-Control Study in the All of Us Database," Powers and colleagues tell us that atopic dermatitis is associated with a statistically significantly increased risk for lymphoma. This means that increased risk wasn't likely due to chance alone. The article says nothing, as far as I could tell, about how big the risk is. Does everyone get lymphoma? Or is it a one in a million risk? Without knowing the absolute risk, the relative risk doesn't tell us whether there is a clinically meaningful increased risk or not. I suspect the increased risk is small. If the incidence of lymphoma is about 2 in 10,000 and peripheral T-cell lymphomas (PTCL) account for 10% of those, even a fourfold increase in the risk for PTCL (the form of lymphoma with the highest relative risk) would not amount to much.
Traidl and colleagues report in "Treatment of Moderate-to-Severe Atopic Dermatitis With Baricitinib: Results From an Interim Analysis of the TREATgermany Registry" that the Janus kinase inhibitor baricitinib makes atopic dermatitis better.
In "Dupilumab Therapy for Atopic Dermatitis Is Associated With Increased Risk of Cutaneous T Cell Lymphoma," Hasan and colleagues report that "it requires 738 prescriptions of dupilumab to produce one case of CTCL [cutaneous T-cell lymphoma]." It seems that this finding could easily be due to 1 in 738 people with a rash thought to be severe atopic dermatitis needing dupilumab having CTCL, not atopic dermatitis, to begin with. If we were to wonder whether dupilumab causes CTCL, perhaps it would be better to study asthma patients treated with or without dupilumab.
In "Atopic Dermatitis in Early Childhood and Risk of Inflammatory Bowel Disease: A Scandinavian Birth Cohort Study," Lerchova and colleagues found a statistically significant increased risk for inflammatory bowel disease (IBD) in children with atopic dermatitis. The study had a large patient population, giving it the power to identify very small differences. The researchers found increased risks for IBD, Crohn's disease, and ulcerative colitis (UC) in children with atopic dermatitis; UC had the greatest relative risk. But I don't think this risk was clinically meaningful. About 2 in every 1000 children with atopic dermatitis had UC, whereas about 1 in every 1000 children without atopic dermatitis had UC. Even if the increased absolute risk of 1 in 1000 children was due to atopic dermatitis and not to other factors, I don't think it justifies the authors' conclusion that "these findings might be useful in identifying at-risk individuals for IBD."
Sometimes reviewing articles makes me feel like a crotchety old man. A study by Guttman-Yassky and colleagues, "Targeting IL-13 With Tralokinumab Normalizes Type 2 Inflammation in Atopic Dermatitis Both Early and at 2 Years," didn't seem to test any specific hypothesis. The researchers just looked at a variety of inflammation markers in patients with atopic dermatitis treated with tralokinumab, an interleukin-13 (IL-13) antagonist. In these patients, as expected, the atopic dermatitis improved; so did the inflammatory markers. Did we learn anything clinically useful? I don't think so. We already know that IL-13 is important in atopic dermatitis because when we block IL-13, atopic dermatitis improves.
Vitamin D supplementation doesn't appear to improve atopic dermatitis, as reported by Borzutzky and colleagues in "Effect of Weekly Vitamin D Supplementation on the Severity of Atopic Dermatitis and Type 2 Immunity Biomarkers in Children: A Randomized Controlled Trial." A group of 101 children with atopic dermatitis were randomly assigned to receive oral vitamin D supplementation or placebo. The two groups improved to a similar extent. If you know me, you know I'm wondering whether they took the medication. It appears that they did, because at baseline most of the children were vitamin D deficient, and vitamin D levels improved greatly in the group treated with vitamin D but not in the placebo group.
Journals such as the Journal of the American Academy of Dermatology should require articles to report absolute risk. In "Risk of Lymphoma in Patients With Atopic Dermatitis: A Case-Control Study in the All of Us Database," Powers and colleagues tell us that atopic dermatitis is associated with a statistically significantly increased risk for lymphoma. This means that increased risk wasn't likely due to chance alone. The article says nothing, as far as I could tell, about how big the risk is. Does everyone get lymphoma? Or is it a one in a million risk? Without knowing the absolute risk, the relative risk doesn't tell us whether there is a clinically meaningful increased risk or not. I suspect the increased risk is small. If the incidence of lymphoma is about 2 in 10,000 and peripheral T-cell lymphomas (PTCL) account for 10% of those, even a fourfold increase in the risk for PTCL (the form of lymphoma with the highest relative risk) would not amount to much.
Traidl and colleagues report in "Treatment of Moderate-to-Severe Atopic Dermatitis With Baricitinib: Results From an Interim Analysis of the TREATgermany Registry" that the Janus kinase inhibitor baricitinib makes atopic dermatitis better.
In "Dupilumab Therapy for Atopic Dermatitis Is Associated With Increased Risk of Cutaneous T Cell Lymphoma," Hasan and colleagues report that "it requires 738 prescriptions of dupilumab to produce one case of CTCL [cutaneous T-cell lymphoma]." It seems that this finding could easily be due to 1 in 738 people with a rash thought to be severe atopic dermatitis needing dupilumab having CTCL, not atopic dermatitis, to begin with. If we were to wonder whether dupilumab causes CTCL, perhaps it would be better to study asthma patients treated with or without dupilumab.
In "Atopic Dermatitis in Early Childhood and Risk of Inflammatory Bowel Disease: A Scandinavian Birth Cohort Study," Lerchova and colleagues found a statistically significant increased risk for inflammatory bowel disease (IBD) in children with atopic dermatitis. The study had a large patient population, giving it the power to identify very small differences. The researchers found increased risks for IBD, Crohn's disease, and ulcerative colitis (UC) in children with atopic dermatitis; UC had the greatest relative risk. But I don't think this risk was clinically meaningful. About 2 in every 1000 children with atopic dermatitis had UC, whereas about 1 in every 1000 children without atopic dermatitis had UC. Even if the increased absolute risk of 1 in 1000 children was due to atopic dermatitis and not to other factors, I don't think it justifies the authors' conclusion that "these findings might be useful in identifying at-risk individuals for IBD."
Sometimes reviewing articles makes me feel like a crotchety old man. A study by Guttman-Yassky and colleagues, "Targeting IL-13 With Tralokinumab Normalizes Type 2 Inflammation in Atopic Dermatitis Both Early and at 2 Years," didn't seem to test any specific hypothesis. The researchers just looked at a variety of inflammation markers in patients with atopic dermatitis treated with tralokinumab, an interleukin-13 (IL-13) antagonist. In these patients, as expected, the atopic dermatitis improved; so did the inflammatory markers. Did we learn anything clinically useful? I don't think so. We already know that IL-13 is important in atopic dermatitis because when we block IL-13, atopic dermatitis improves.
Vitamin D supplementation doesn't appear to improve atopic dermatitis, as reported by Borzutzky and colleagues in "Effect of Weekly Vitamin D Supplementation on the Severity of Atopic Dermatitis and Type 2 Immunity Biomarkers in Children: A Randomized Controlled Trial." A group of 101 children with atopic dermatitis were randomly assigned to receive oral vitamin D supplementation or placebo. The two groups improved to a similar extent. If you know me, you know I'm wondering whether they took the medication. It appears that they did, because at baseline most of the children were vitamin D deficient, and vitamin D levels improved greatly in the group treated with vitamin D but not in the placebo group.
Journals such as the Journal of the American Academy of Dermatology should require articles to report absolute risk. In "Risk of Lymphoma in Patients With Atopic Dermatitis: A Case-Control Study in the All of Us Database," Powers and colleagues tell us that atopic dermatitis is associated with a statistically significantly increased risk for lymphoma. This means that increased risk wasn't likely due to chance alone. The article says nothing, as far as I could tell, about how big the risk is. Does everyone get lymphoma? Or is it a one in a million risk? Without knowing the absolute risk, the relative risk doesn't tell us whether there is a clinically meaningful increased risk or not. I suspect the increased risk is small. If the incidence of lymphoma is about 2 in 10,000 and peripheral T-cell lymphomas (PTCL) account for 10% of those, even a fourfold increase in the risk for PTCL (the form of lymphoma with the highest relative risk) would not amount to much.
Traidl and colleagues report in "Treatment of Moderate-to-Severe Atopic Dermatitis With Baricitinib: Results From an Interim Analysis of the TREATgermany Registry" that the Janus kinase inhibitor baricitinib makes atopic dermatitis better.
In "Dupilumab Therapy for Atopic Dermatitis Is Associated With Increased Risk of Cutaneous T Cell Lymphoma," Hasan and colleagues report that "it requires 738 prescriptions of dupilumab to produce one case of CTCL [cutaneous T-cell lymphoma]." It seems that this finding could easily be due to 1 in 738 people with a rash thought to be severe atopic dermatitis needing dupilumab having CTCL, not atopic dermatitis, to begin with. If we were to wonder whether dupilumab causes CTCL, perhaps it would be better to study asthma patients treated with or without dupilumab.
Dermatologic Care for Refugees: Effective Management of Scabies and Pediculosis
Approximately 108 million individuals have been forcibly displaced across the globe as of 2022, 35 million of whom are formally designated as refugees.1,2 The United States has coordinated resettlement of more refugee populations than any other country; the most common countries of origin are the Democratic Republic of the Congo, Syria, Afghanistan, and Myanmar.3 In 2021, policy to increase the number of refugees resettled in the United States by more than 700% (from 15,000 up to 125,000) was established; since enactment, the United States has seen more than double the refugee arrivals in 2023 than the prior year, making medical care for this population increasingly relevant for the dermatologist.4
Understanding how to care for this population begins with an accurate understanding of the term refugee. The United Nations defines a refugee as a person who is unwilling or unable to return to their country of nationality because of persecution or well-founded fear of persecution due to race, religion, nationality, membership in a particular social group, or political opinion. This term grants a protected status under international law and encompasses access to travel assistance, housing, cultural orientation, and medical evaluation upon resettlement.5,6
The burden of treatable dermatologic conditions in refugee populations ranges from 19% to 96% in the literature7,8 and varies from inflammatory disorders to infectious and parasitic diseases.9 In one study of 6899 displaced individuals in Greece, the prevalence of dermatologic conditions was higher than traumatic injury, cardiac disease, psychological conditions, and dental disease.10
When outlining differential diagnoses for parasitic infestations of the skin that affect refugee populations, helpful considerations include the individual’s country of origin, route traveled, and method of travel.11 Parasitic infestations specifically are more common in refugee populations when there are barriers to basic hygiene, crowded living or travel conditions, or lack of access to health care, which they may experience at any point in their home country, during travel, or in resettlement housing.8
Even with limited examination and diagnostic resources, the skin is the most accessible first indication of patients’ overall well-being and often provides simple diagnostic clues—in combination with contextualization of the patient’s unique circumstances—necessary for successful diagnosis and treatment of scabies and pediculosis.12 The dermatologist working with refugee populations may be the first set of eyes available and trained to discern skin infestations and therefore has the potential to improve overall outcomes.
Some parasitic infestations in refugee populations may fall under the category of neglected tropical diseases, including scabies, ascariasis, trypanosomiasis, leishmaniasis, and schistosomiasis; they affect an estimated 1 billion individuals across the globe but historically have been underrepresented in the literature and in health policy due in part to limited access to care.13 This review will focus on infestations by the scabies mite (Sarcoptes scabiei var hominis) and the human louse, as these frequently are encountered, easily diagnosed, and treatable by trained clinicians, even in resource-limited settings.
Scabies
Scabies is a parasitic skin infestation caused by the 8-legged mite Sarcoptes scabiei var hominis. The female mite begins the infestation process via penetration of the epidermis, particularly the stratum corneum, and commences laying eggs (Figure 1). The subsequent larvae emerge 48 to 72 hours later and remain burrowed in the epidermis. The larvae mature over the next 10 to 14 days and continue the reproductive cycle.14,15 Symptoms of infestation occurs due to a hypersensitivity reaction to the mite and its by-products.16 Transmission of the mite primarily occurs via direct (skin-to-skin) contact with infected individuals or environmental surfaces for 24 to36 hours in specific conditions, though the latter source has been debated in the literature.
The method of transmission is particularly important when considering care for refugee populations. Scabies is found most often in those living in or traveling from tropical regions including East Asia, Southeast Asia, Oceania, and Latin America.17 In displaced or refugee populations, a lack of access to basic hygiene, extended travel in close quarters, and suboptimal health care access all may lead to an increased incidence of untreated scabies infestations.18 Scabies is more prevalent in children, with increased potential for secondary bacterial infections with Streptococcus and Staphylococcus species due to excoriation in unsanitary conditions. Secondary infection with Streptococcus pyogenes can lead to acute poststreptococcal glomerulonephritis, which accounts for a large burden of chronic kidney disease in affected populations.19 However, scabies may be found in any population, regardless of hygiene or health care access. Treating health care providers should keep a broad differential.
Presentation—The latency of scabies symptoms is 2 to 6 weeks in a primary outbreak and may be as short as 1 to 3 days with re-infestation, following the course of delayed-type hypersensitivity.20 The initial hallmark symptom is pruritus with increased severity in the evening. Visible lesions, excoriations, and burrows associated with scattered vesicles or pustules may be seen over the web spaces of the hands and feet, volar surfaces of the wrists, axillae, waist, genitalia, inner thighs, or buttocks.19 Chronic infestation often manifests with genital nodules. In populations with limited access to health care, there are reports of a sensitization phenomenon in which the individual may become less symptomatic after 4 to 6 weeks and yet be a potential carrier of the mite.21
Those with compromised immune function, such as individuals living with HIV or severe malnutrition, may present with crusted scabies, a variant that manifests as widespread hyperkeratotic scaling with more pronounced involvement of the head, neck, and acral areas. In contrast to classic scabies, crusted scabies is associated with minimal pruritus.22
Diagnosis—The diagnosis of scabies is largely clinical with confirmation through skin scrapings. The International Alliance for Control of Scabies has established diagnostic criteria that include a combination of clinical findings, history, and visualization of mites.23 A dermatologist working with refugee populations may employ any combination of history (eg, nocturnal itch, exposure to an affected individual) or clinical findings along with a high degree of suspicion in those with elevated risk. Visualization of mites is helpful to confirm the diagnosis and may be completed with the application of mineral oil at the terminal end of a burrow, skin scraping with a surgical blade or needle, and examination under light microscopy.
Treatment—First-line treatment for scabies consists of application of permethrin cream 5% on the skin of the neck to the soles of the feet, which is to be left on for 8 to 14 hours followed by rinsing. Re-application is recommended in 1 to 2 weeks. Oral ivermectin is a reasonable alternative to permethrin cream due to its low cost and easy administration in large affected groups. It is not labeled for use in pregnant women or children weighing less than 15 kg but has no selective fetal toxicity. Treatment of scabies with ivermectin has the benefit of treating many other parasitic infections. Both medications are on the World Health Organization Model List of Essential Medications and are widely available for treating providers, even in resource-limited settings.24
Much of the world still uses benzyl benzoate or precipitated sulfur ointment to treat scabies, and some botanicals used in folk medicine have genuine antiscabetic properties. Pruritus may persist for 1 to 4 weeks following treatment and does not indicate treatment failure. Topical camphor and menthol preparations, low-potency topical corticosteroids, or emollients all may be employed for relief.25 Sarna is a Spanish term for scabies and has become the proprietary name for topical antipruritic agents. Additional methods of treatment and prevention include washing clothes and linens in hot water and drying on high heat. If machine washing is not available, clothing and linens may be sealed in a plastic bag for 72 hours.
Pediculosis
Pediculosis is an infestation caused by the ectoparasite Pediculus humanus, an obligate, sesame seed–sized louse that feeds exclusively on the blood of its host (Figure 2).26 Of the lice species, 2 require humans as hosts; one is P humanus and the other is Pthirus pubis (pubic lice). Pediculus humanus may be further classified into morphologies based largely on the affected area: body (P humanus corporis) or head (P humanus capitis), both of which will be discussed.27
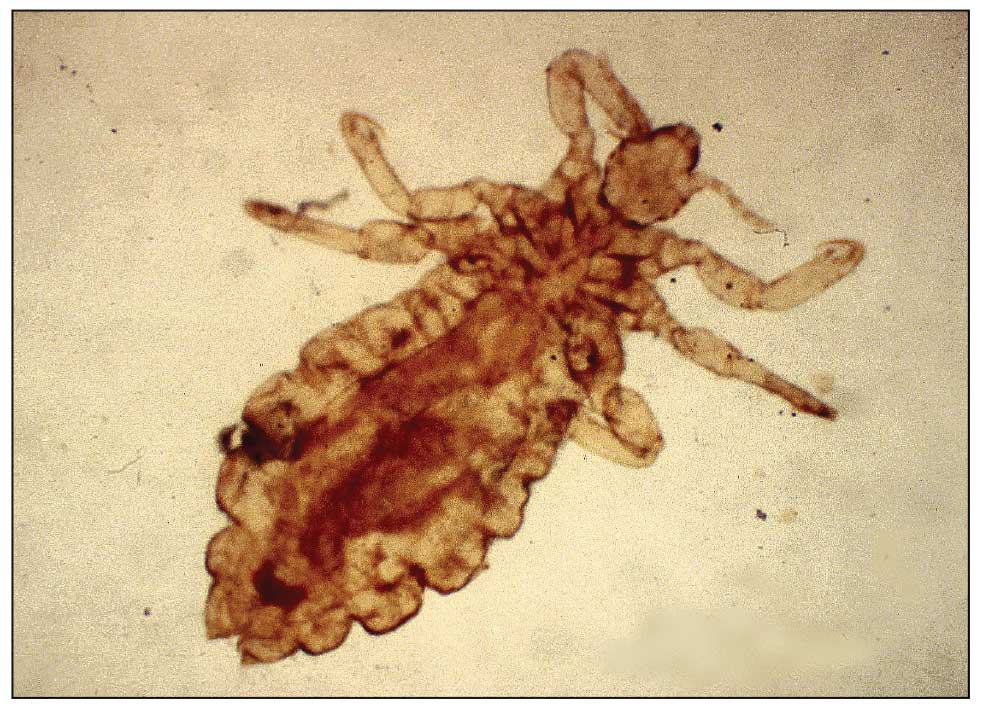
Lice primarily attach to clothing and hair shafts, then transfer to the skin for blood feeds. Females lay eggs that hatch 6 to 10 days later, subsequently maturing into adults. The lifespan of these parasites with regular access to a host is 1 to 3 months for head lice and 18 days for body lice vs only 3 to 5 days without a host.28 Transmission of P humanus capitis primarily occurs via direct contact with affected individuals, either head-to-head contact or sharing of items such as brushes and headscarves; P humanus corporis also may be transmitted via direct contact with affected individuals or clothing.
Pediculosis is an important infestation to consider when providing care for refugee populations. Risk factors include lack of access to basic hygiene, including regular bathing or laundering of clothing, and crowded conditions that make direct person-to-person contact with affected individuals more likely.29 Body lice are associated more often with domestic turbulence and displaced populations30 in comparison to head lice, which have broad demographic variables, most often affecting females and children.28 Fatty acids in adult male sebum make the scalp less hospitable to lice.
Presentation—The most common clinical manifestation of pediculosis is pruritus. Cutaneous findings can include papules, wheals, or hemorrhagic puncta secondary to the louse bite. Due to the Tyndall effect of deep hemosiderin pigment, blue-grey macules termed maculae ceruleae (Figure 3) also may be present in chronic infestations of pediculosis pubis, in contrast to pediculosis capitis or corporis.31 Body louse infestation is associated with a general pruritus concentrated on the neck, shoulders, and waist—areas where clothing makes the most direct contact. Lesions may be visible and include eczematous patches with excoriation and possible secondary bacterial infection. Chronic infestation may exhibit lichenification or hyperpigmentation in associated areas. Head lice most often manifest with localized scalp pruritus and associated excoriation and cervical or occipital lymphadenopathy.32
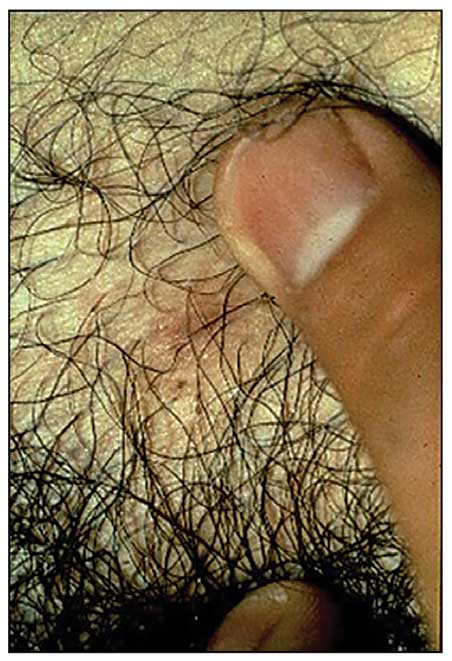
Diagnosis—The diagnosis of pediculosis is clinical, with confirmation requiring direct examination of the insect or nits (the egg case of the parasite)(Figure 4). Body lice and associated nits can be visualized on clothing seams near areas of highest body temperature, particularly the waistband. Head lice may be visualized crawling on hair shafts or on a louse comb. Nits are firmly attached to hair shafts and are visible to the naked eye, whereas pseudonits slide freely along the hair shaft and are not a manifestation of louse infestation (Figure 5).31
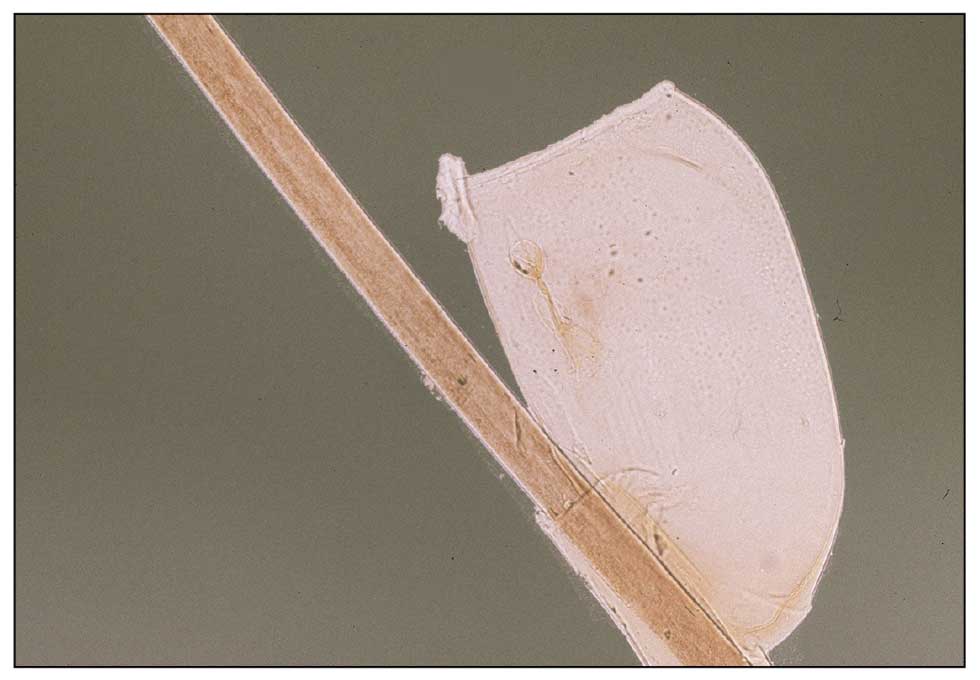
Treatment—Treatment varies by affected area. Pediculosis corporis may be treated with permethrin cream 5% applied to the entire body and left on for 8 to 10 hours, but this may not be necessary if facilities are available to wash and dry clothing.33 The use of oral ivermectin and permethrin-impregnated underwear both have been proposed.34,35 Treatment of pediculosis capitis may be accomplished with a variety of topical pediculicides including permethrin, pyrethrum with piperonyl butoxide, dimethicone, malathion, benzyl alcohol, spinosad, and topical ivermectin.22 Topical corticosteroids or emollients may be employed for residual pruritus.
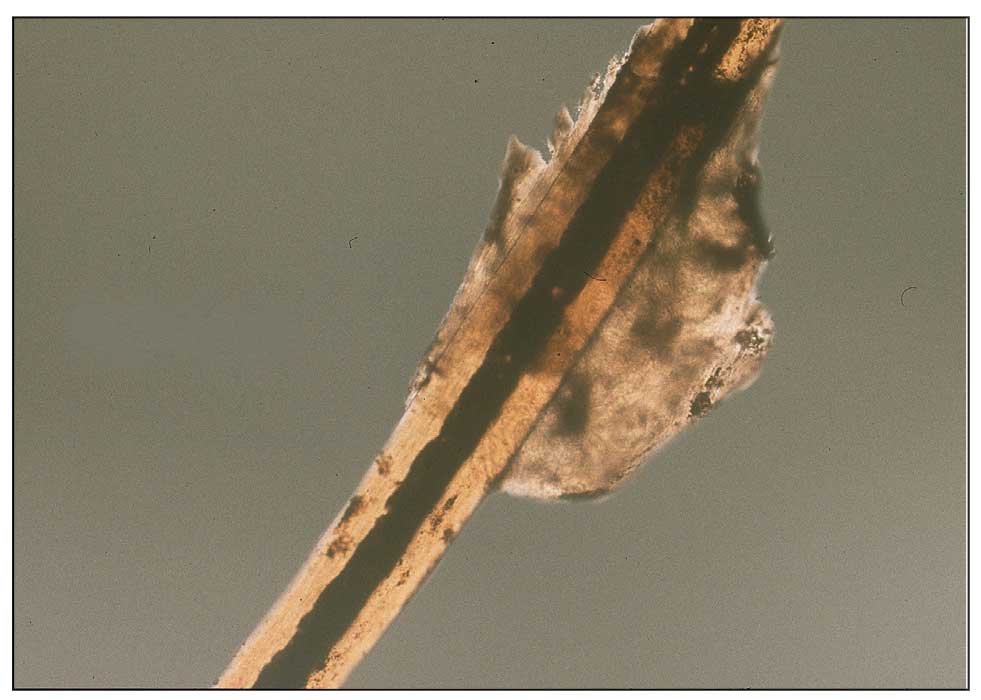
Equally important is environmental elimination of infestation. Clothing should be discarded if possible or washed and dried using high heat. If neither approach is possible or appropriate, clothing may be sealed in a plastic bag for 2 weeks or treated with a pediculicide. Nit combing is an important adjunct in the treatment of pediculosis capitis.36 It is important to encourage return to work and/or school immediately after treatment. “No nit” policies are more harmful to education than helpful for prevention of investation.37
Pediculosis corporis may transmit infectious agents including Bartonella quintana, (trench fever, endocarditis, bacillary angiomatosis), Borrelia recurrentis (louse-borne relapsing fever), and Rickettsia prowazekii (epidemic typhus).31,38,39 Additionally, severe pediculosis infestations have the potential to cause chronic blood loss in affected populations. In a study of patients with active pediculosis infestation, mean hemoglobin values were found to be 2.5 g/dL lower than a matched population without infestation.40 It is important to consider pediculosis as a risk for iron-deficiency anemia in populations who are known to lack access to regular medical evaluation.41
Future Considerations
Increased access to tools and education for clinicians treating refugee populations is key to reducing the burden of parasitic skin disease and related morbidity and mortality in vulnerable groups both domestically and globally. One such tool, the Skin NTDs App, was launched by the World Health Organization in 2020. It is available for free for Android and iOS devices to assist clinicians in the field with the diagnosis and treatment of neglected tropical diseases—including scabies—that may affect refugee populations.42
Additionally, to both improve access and limit preventable sequelae, future investigations into appropriate models of community-based care are paramount. The model of community-based care is centered on the idea of care provision that prioritizes safety, accessibility, affordability, and acceptability in an environment closest to vulnerable populations. The largest dermatologic society, the International League of Dermatological Societies, formed a Migrant Health Dermatology Working Group that prioritizes understanding and improving care for refugee and migrant populations; this group hosted a summit in 2022, bringing together international subject matter leaders to discuss such models of care and set goals for the creation of tool kits for patients, frontline health care workers, and dermatologists.43
Conclusion
Improvement in dermatologic care of refugee populations includes provision of culturally and linguistically appropriate care by trained clinicians, adequate access to the most essential medications, and basic physical or legal access to health care systems in general.8,11,44 Parasitic infestations have the potential to remain asymptomatic for extended periods of time and result in spread to potentially nonendemic regions of resettlement.45 Additionally, the psychosocial well-being of refugee populations upon resettlement may be negatively affected by stigma of disease processes such as scabies and pediculosis, leading to additional barriers to successful re-entry into the patient’s new environment.46 Therefore, proper screening, diagnosis, and treatment of the most common parasitic infestations in this population have great potential to improve outcomes for large groups across the globe.
- Monin K, Batalova J, Lai T. Refugees and Asylees in the United States. Migration Information Source. Published May 13, 2021. Accessed April 4, 2024. https://www.migrationpolicy.org/article/refugees-and-asylees-united-states-2021
- UNHCR. Figures at a Glance. UNHCR USA. Update June 14, 2023. Accessed April 4, 2024. https://www.unhcr.org/en-us/figures-at-a-glance.html
- UNHCR. Refugee resettlement facts. Published October 2023. Accessed April 8, 2024. https://www.unhcr.org/us/media/refugee-resettlement-facts
- US Department of State. Report to Congress on Proposed Refugee Admissions for Fiscal Year 2024. Published November 3, 2023. Accessed April 8, 2024. https://www.state.gov/report-to-congress-on-proposed-refugee-admissions-for-fiscal-year-2024/
- UNHCR. Compact for Migration: Definitions. United Nations. Accessed April 4, 2024. https://refugeesmigrants.un.org/definitions
- United Nations High Commissioner for Refugees (UNHCR). Convention and Protocol Relating to the Status of Refugees. Published December 2010. Accessed January 11, 2024. https://www.unhcr.org/us/media/convention-and-protocol-relating-status-refugees
- Kibar Öztürk M. Skin diseases in rural Nyala, Sudan (in a rural hospital, in 12 orphanages, and in two refugee camps). Int J Dermatol. 2019;58:1341-1349. doi:10.1111/ijd.14619
- Padovese V, Knapp A. Challenges of managing skin diseases in refugees and migrants. Dermatol Clin. 2021;39:101-115. doi:10.1016/j.det.2020.08.010
- Saikal SL, Ge L, Mir A, et al. Skin disease profile of Syrian refugees in Jordan: a field-mission assessment. J Eur Acad Dermatol Venereol. 2020;34:419-425. doi:10.1111/jdv.15909
- Eonomopoulou A, Pavli A, Stasinopoulou P, et al. Migrant screening: lessons learned from the migrant holding level at the Greek-Turkish borders. J Infect Public Health. 2017;10:177-184. doi:10.1016/j.jiph.2016.04.012
- Marano N, Angelo KM, Merrill RD, et al. Expanding travel medicine in the 21st century to address the health needs of the world’s migrants.J Travel Med. 2018;25. doi:10.1093/jtm/tay067
- Hay RJ, Asiedu K. Skin-related neglected tropical diseases (skin NTDs)—a new challenge. Trop Med Infect Dis. 2018;4. doi:10.3390/tropicalmed4010004
- NIAID. Neglected tropical diseases. Updated July 11, 2016. Accessed April 4, 2024. https://www.niaid.nih.gov/research/neglected-tropical-diseases
- Arlian LG, Morgan MS. A review of Sarcoptes scabiei: past, present and future. Parasit Vectors. 2017;10:297. doi:10.1186/s13071-017-2234-1
- Arlian LG, Runyan RA, Achar S, et al. Survival and infectivity of Sarcoptes scabiei var. canis and var. hominis. J Am Acad Dermatol. 1984;11(2 pt 1):210-215. doi:10.1016/s0190-9622(84)70151-4
- Chandler DJ, Fuller LC. A review of scabies: an infestation more than skin deep. Dermatology. 2019;235:79-90. doi:10.1159/000495290
- Karimkhani C, Colombara DV, Drucker AM, et al. The global burden of scabies: a cross-sectional analysis from the Global Burden of Disease Study 2015. Lancet Infect Dis. 2017;17:1247-1254. doi:10.1016/S1473-3099(17)30483-8
- Romani L, Steer AC, Whitfeld MJ, et al. Prevalence of scabies and impetigo worldwide: a systematic review. Lancet Infect Dis. 2015;15:960-967. doi:10.1016/S1473-3099(15)00132-2
- Thomas C, Coates SJ, Engelman D, et al. Ectoparasites: scabies. J Am Acad Dermatol. 2020;82:533-548. doi:10.1016/j.jaad.2019.05.109
- Mellanby K, Johnson CG, Bartley WC. Treatment of scabies. Br Med J. 1942;2:1-4. doi:10.1136/bmj.2.4252.1
- Walton SF. The immunology of susceptibility and resistance to scabies. Parasit Immunol. 2010;32:532-540. doi:10.1111/j.1365-3024.2010.01218.x
- Coates SJ, Thomas C, Chosidow O, et al. Ectoparasites: pediculosis and tungiasis. J Am Acad Dermatol. 2020;82:551-569. doi:10.1016/j.jaad.2019.05.110
- Engelman D, Fuller LC, Steer AC; International Alliance for the Control of Scabies Delphi p. Consensus criteria for the diagnosis of scabies: a Delphi study of international experts. PLoS Negl Trop Dis. 2018;12:E0006549. doi:10.1371/journal.pntd.0006549
- World Health Organization. WHO Model Lists of Essential Medicines—23rd list, 2023. Updated July 26, 2023. Accessed April 8, 2024. https://www.who.int/publications/i/item/WHO-MHP-HPS-EML-2023.02
- Salavastru CM, Chosidow O, Boffa MJ, et al. European guideline for the management of scabies. J Eur Acad Dermatol Venereol. 2017;31:1248-1253. doi:10.1111/jdv.14351
- Badiaga S, Brouqui P. Human louse-transmitted infectious diseases. Clin Microbiol Infect. 2012;18:332-337. doi:10.1111/j.1469-0691.2012.03778.x
- Leo NP, Campbell NJH, Yang X, et al. Evidence from mitochondrial DNA that head lice and body lice of humans (Phthiraptera: Pediculidae) are conspecific. J Med Entomol. 2002;39:662-666. doi:10.1603/0022-2585-39.4.662
- Chosidow O. Scabies and pediculosis. Lancet. 2000;355:819-826. doi:10.1016/S0140-6736(99)09458-1
- Arnaud A, Chosidow O, Détrez M-A, et al. Prevalences of scabies and pediculosis corporis among homeless people in the Paris region: results from two randomized cross-sectional surveys (HYTPEAC study). Br J Dermatol. 2016;174:104-112. doi:10.1111/bjd.14226
- Brouqui P. Arthropod-borne diseases associated with political and social disorder. Annu Rev Entomol. 2011;56:357-374. doi:10.1146/annurev-ento-120709-144739
- Ko CJ, Elston DM. Pediculosis. J Am Acad Dermatol. 2004;50:1-12. doi:10.1016/S0190-9622(03)02729-4
- Bloomfield D. Head lice. Pediatr Rev. 2002;23:34-35; discussion 34-35. doi:10.1542/pir.23-1-34
- Stone SP GJ, Bacelieri RE. Scabies, other mites, and pediculosis. In: Wolf K GL, Katz SI, et al (eds). Fitzpatrick’s Dermatology in General Medicine. McGraw Hill; 2008:2029.
- Foucault C, Ranque S, Badiaga S, et al. Oral ivermectin in the treatment of body lice. J Infect Dis. 2006;193:474-476. doi:10.1086/499279
- Benkouiten S, Drali R, Badiaga S, et al. Effect of permethrin-impregnated underwear on body lice in sheltered homeless persons: a randomized controlled trial. JAMA Dermatol. 2014;150:273-279. doi:10.1001/jamadermatol.2013.6398
- CDC. Parasites: Treatment. Updated October 15, 2019. Accessed April 4, 2024. https://www.cdc.gov/parasites/lice/head/treatment.html
- Devore CD, Schutze GE; Council on School Health and Committee on Infectious Diseases, American Academy of Pediatrics. Head lice. Pediatrics. 2015;135:e1355-e1365. doi:10.1542/peds.2015-0746
- Ohl ME, Spach DH. Bartonella quintana and urban trench fever. Clin Infect Dis. 2000;31:131-135. doi:10.1086/313890
- Drali R, Sangaré AK, Boutellis A, et al. Bartonella quintana in body lice from scalp hair of homeless persons, France. Emerg Infect Dis. 2014;20:907-908. doi:10.3201/eid2005.131242
- Rudd N, Zakaria A, Kohn MA, et al. Association of body lice infestation with hemoglobin values in hospitalized dermatology patients. JAMA Dermatol. 2022;158:691-693. doi:10.1001/jamadermatol.2022.0818
- Guss DA, Koenig M, Castillo EM. Severe iron deficiency anemia and lice infestation. J Emergency Med. 2011;41:362-365. doi:10.1016/j.jemermed.2010.05.030
- Neglected tropical diseases of the skin: WHO launches mobile application to facilitate diagnosis. News release. World Health Organization; July 16, 2020. Accessed April 4, 2024. https://www.who.int/news/item/16-07-2020-neglected-tropical-diseases-of-the-skin-who-launches-mobile-application-to-facilitate-diagnosis
- Padovese V, Fuller LC, Griffiths CEM, et al; Migrant Health Dermatology Working Group of the International Foundation for Dermatology. Migrant skin health: perspectives from the Migrant Health Summit, Malta, 2022. Br J Dermatology. 2023;188:553-554. doi:10.1093/bjd/ljad001
- Knapp AP, Rehmus W, Chang AY. Skin diseases in displaced populations: a review of contributing factors, challenges, and approaches to care. Int J Dermatol. 2020;59:1299-1311. doi:10.1111/ijd.15063
- Norman FF, Comeche B, Chamorro S, et al. Overcoming challenges in the diagnosis and treatment of parasitic infectious diseases in migrants. Expert Rev Anti-infective Therapy. 2020;18:127-143. doi:10.1080/14787210.2020.1713099
- Skin NTDs: prioritizing integrated approaches to reduce suffering, psychosocial impact and stigmatization. News release. World Health Organization; October 29, 2020. Accessed April 4, 2024. https://www.who.int/news/item/29-10-2020-skin-ntds-prioritizing-integrated-approaches-to-reduce-suffering-psychosocial-impact-and-stigmatization
Approximately 108 million individuals have been forcibly displaced across the globe as of 2022, 35 million of whom are formally designated as refugees.1,2 The United States has coordinated resettlement of more refugee populations than any other country; the most common countries of origin are the Democratic Republic of the Congo, Syria, Afghanistan, and Myanmar.3 In 2021, policy to increase the number of refugees resettled in the United States by more than 700% (from 15,000 up to 125,000) was established; since enactment, the United States has seen more than double the refugee arrivals in 2023 than the prior year, making medical care for this population increasingly relevant for the dermatologist.4
Understanding how to care for this population begins with an accurate understanding of the term refugee. The United Nations defines a refugee as a person who is unwilling or unable to return to their country of nationality because of persecution or well-founded fear of persecution due to race, religion, nationality, membership in a particular social group, or political opinion. This term grants a protected status under international law and encompasses access to travel assistance, housing, cultural orientation, and medical evaluation upon resettlement.5,6
The burden of treatable dermatologic conditions in refugee populations ranges from 19% to 96% in the literature7,8 and varies from inflammatory disorders to infectious and parasitic diseases.9 In one study of 6899 displaced individuals in Greece, the prevalence of dermatologic conditions was higher than traumatic injury, cardiac disease, psychological conditions, and dental disease.10
When outlining differential diagnoses for parasitic infestations of the skin that affect refugee populations, helpful considerations include the individual’s country of origin, route traveled, and method of travel.11 Parasitic infestations specifically are more common in refugee populations when there are barriers to basic hygiene, crowded living or travel conditions, or lack of access to health care, which they may experience at any point in their home country, during travel, or in resettlement housing.8
Even with limited examination and diagnostic resources, the skin is the most accessible first indication of patients’ overall well-being and often provides simple diagnostic clues—in combination with contextualization of the patient’s unique circumstances—necessary for successful diagnosis and treatment of scabies and pediculosis.12 The dermatologist working with refugee populations may be the first set of eyes available and trained to discern skin infestations and therefore has the potential to improve overall outcomes.
Some parasitic infestations in refugee populations may fall under the category of neglected tropical diseases, including scabies, ascariasis, trypanosomiasis, leishmaniasis, and schistosomiasis; they affect an estimated 1 billion individuals across the globe but historically have been underrepresented in the literature and in health policy due in part to limited access to care.13 This review will focus on infestations by the scabies mite (Sarcoptes scabiei var hominis) and the human louse, as these frequently are encountered, easily diagnosed, and treatable by trained clinicians, even in resource-limited settings.
Scabies
Scabies is a parasitic skin infestation caused by the 8-legged mite Sarcoptes scabiei var hominis. The female mite begins the infestation process via penetration of the epidermis, particularly the stratum corneum, and commences laying eggs (Figure 1). The subsequent larvae emerge 48 to 72 hours later and remain burrowed in the epidermis. The larvae mature over the next 10 to 14 days and continue the reproductive cycle.14,15 Symptoms of infestation occurs due to a hypersensitivity reaction to the mite and its by-products.16 Transmission of the mite primarily occurs via direct (skin-to-skin) contact with infected individuals or environmental surfaces for 24 to36 hours in specific conditions, though the latter source has been debated in the literature.

The method of transmission is particularly important when considering care for refugee populations. Scabies is found most often in those living in or traveling from tropical regions including East Asia, Southeast Asia, Oceania, and Latin America.17 In displaced or refugee populations, a lack of access to basic hygiene, extended travel in close quarters, and suboptimal health care access all may lead to an increased incidence of untreated scabies infestations.18 Scabies is more prevalent in children, with increased potential for secondary bacterial infections with Streptococcus and Staphylococcus species due to excoriation in unsanitary conditions. Secondary infection with Streptococcus pyogenes can lead to acute poststreptococcal glomerulonephritis, which accounts for a large burden of chronic kidney disease in affected populations.19 However, scabies may be found in any population, regardless of hygiene or health care access. Treating health care providers should keep a broad differential.
Presentation—The latency of scabies symptoms is 2 to 6 weeks in a primary outbreak and may be as short as 1 to 3 days with re-infestation, following the course of delayed-type hypersensitivity.20 The initial hallmark symptom is pruritus with increased severity in the evening. Visible lesions, excoriations, and burrows associated with scattered vesicles or pustules may be seen over the web spaces of the hands and feet, volar surfaces of the wrists, axillae, waist, genitalia, inner thighs, or buttocks.19 Chronic infestation often manifests with genital nodules. In populations with limited access to health care, there are reports of a sensitization phenomenon in which the individual may become less symptomatic after 4 to 6 weeks and yet be a potential carrier of the mite.21
Those with compromised immune function, such as individuals living with HIV or severe malnutrition, may present with crusted scabies, a variant that manifests as widespread hyperkeratotic scaling with more pronounced involvement of the head, neck, and acral areas. In contrast to classic scabies, crusted scabies is associated with minimal pruritus.22
Diagnosis—The diagnosis of scabies is largely clinical with confirmation through skin scrapings. The International Alliance for Control of Scabies has established diagnostic criteria that include a combination of clinical findings, history, and visualization of mites.23 A dermatologist working with refugee populations may employ any combination of history (eg, nocturnal itch, exposure to an affected individual) or clinical findings along with a high degree of suspicion in those with elevated risk. Visualization of mites is helpful to confirm the diagnosis and may be completed with the application of mineral oil at the terminal end of a burrow, skin scraping with a surgical blade or needle, and examination under light microscopy.
Treatment—First-line treatment for scabies consists of application of permethrin cream 5% on the skin of the neck to the soles of the feet, which is to be left on for 8 to 14 hours followed by rinsing. Re-application is recommended in 1 to 2 weeks. Oral ivermectin is a reasonable alternative to permethrin cream due to its low cost and easy administration in large affected groups. It is not labeled for use in pregnant women or children weighing less than 15 kg but has no selective fetal toxicity. Treatment of scabies with ivermectin has the benefit of treating many other parasitic infections. Both medications are on the World Health Organization Model List of Essential Medications and are widely available for treating providers, even in resource-limited settings.24
Much of the world still uses benzyl benzoate or precipitated sulfur ointment to treat scabies, and some botanicals used in folk medicine have genuine antiscabetic properties. Pruritus may persist for 1 to 4 weeks following treatment and does not indicate treatment failure. Topical camphor and menthol preparations, low-potency topical corticosteroids, or emollients all may be employed for relief.25 Sarna is a Spanish term for scabies and has become the proprietary name for topical antipruritic agents. Additional methods of treatment and prevention include washing clothes and linens in hot water and drying on high heat. If machine washing is not available, clothing and linens may be sealed in a plastic bag for 72 hours.
Pediculosis
Pediculosis is an infestation caused by the ectoparasite Pediculus humanus, an obligate, sesame seed–sized louse that feeds exclusively on the blood of its host (Figure 2).26 Of the lice species, 2 require humans as hosts; one is P humanus and the other is Pthirus pubis (pubic lice). Pediculus humanus may be further classified into morphologies based largely on the affected area: body (P humanus corporis) or head (P humanus capitis), both of which will be discussed.27

Lice primarily attach to clothing and hair shafts, then transfer to the skin for blood feeds. Females lay eggs that hatch 6 to 10 days later, subsequently maturing into adults. The lifespan of these parasites with regular access to a host is 1 to 3 months for head lice and 18 days for body lice vs only 3 to 5 days without a host.28 Transmission of P humanus capitis primarily occurs via direct contact with affected individuals, either head-to-head contact or sharing of items such as brushes and headscarves; P humanus corporis also may be transmitted via direct contact with affected individuals or clothing.
Pediculosis is an important infestation to consider when providing care for refugee populations. Risk factors include lack of access to basic hygiene, including regular bathing or laundering of clothing, and crowded conditions that make direct person-to-person contact with affected individuals more likely.29 Body lice are associated more often with domestic turbulence and displaced populations30 in comparison to head lice, which have broad demographic variables, most often affecting females and children.28 Fatty acids in adult male sebum make the scalp less hospitable to lice.
Presentation—The most common clinical manifestation of pediculosis is pruritus. Cutaneous findings can include papules, wheals, or hemorrhagic puncta secondary to the louse bite. Due to the Tyndall effect of deep hemosiderin pigment, blue-grey macules termed maculae ceruleae (Figure 3) also may be present in chronic infestations of pediculosis pubis, in contrast to pediculosis capitis or corporis.31 Body louse infestation is associated with a general pruritus concentrated on the neck, shoulders, and waist—areas where clothing makes the most direct contact. Lesions may be visible and include eczematous patches with excoriation and possible secondary bacterial infection. Chronic infestation may exhibit lichenification or hyperpigmentation in associated areas. Head lice most often manifest with localized scalp pruritus and associated excoriation and cervical or occipital lymphadenopathy.32

Diagnosis—The diagnosis of pediculosis is clinical, with confirmation requiring direct examination of the insect or nits (the egg case of the parasite)(Figure 4). Body lice and associated nits can be visualized on clothing seams near areas of highest body temperature, particularly the waistband. Head lice may be visualized crawling on hair shafts or on a louse comb. Nits are firmly attached to hair shafts and are visible to the naked eye, whereas pseudonits slide freely along the hair shaft and are not a manifestation of louse infestation (Figure 5).31

Treatment—Treatment varies by affected area. Pediculosis corporis may be treated with permethrin cream 5% applied to the entire body and left on for 8 to 10 hours, but this may not be necessary if facilities are available to wash and dry clothing.33 The use of oral ivermectin and permethrin-impregnated underwear both have been proposed.34,35 Treatment of pediculosis capitis may be accomplished with a variety of topical pediculicides including permethrin, pyrethrum with piperonyl butoxide, dimethicone, malathion, benzyl alcohol, spinosad, and topical ivermectin.22 Topical corticosteroids or emollients may be employed for residual pruritus.

Equally important is environmental elimination of infestation. Clothing should be discarded if possible or washed and dried using high heat. If neither approach is possible or appropriate, clothing may be sealed in a plastic bag for 2 weeks or treated with a pediculicide. Nit combing is an important adjunct in the treatment of pediculosis capitis.36 It is important to encourage return to work and/or school immediately after treatment. “No nit” policies are more harmful to education than helpful for prevention of investation.37
Pediculosis corporis may transmit infectious agents including Bartonella quintana, (trench fever, endocarditis, bacillary angiomatosis), Borrelia recurrentis (louse-borne relapsing fever), and Rickettsia prowazekii (epidemic typhus).31,38,39 Additionally, severe pediculosis infestations have the potential to cause chronic blood loss in affected populations. In a study of patients with active pediculosis infestation, mean hemoglobin values were found to be 2.5 g/dL lower than a matched population without infestation.40 It is important to consider pediculosis as a risk for iron-deficiency anemia in populations who are known to lack access to regular medical evaluation.41
Future Considerations
Increased access to tools and education for clinicians treating refugee populations is key to reducing the burden of parasitic skin disease and related morbidity and mortality in vulnerable groups both domestically and globally. One such tool, the Skin NTDs App, was launched by the World Health Organization in 2020. It is available for free for Android and iOS devices to assist clinicians in the field with the diagnosis and treatment of neglected tropical diseases—including scabies—that may affect refugee populations.42
Additionally, to both improve access and limit preventable sequelae, future investigations into appropriate models of community-based care are paramount. The model of community-based care is centered on the idea of care provision that prioritizes safety, accessibility, affordability, and acceptability in an environment closest to vulnerable populations. The largest dermatologic society, the International League of Dermatological Societies, formed a Migrant Health Dermatology Working Group that prioritizes understanding and improving care for refugee and migrant populations; this group hosted a summit in 2022, bringing together international subject matter leaders to discuss such models of care and set goals for the creation of tool kits for patients, frontline health care workers, and dermatologists.43
Conclusion
Improvement in dermatologic care of refugee populations includes provision of culturally and linguistically appropriate care by trained clinicians, adequate access to the most essential medications, and basic physical or legal access to health care systems in general.8,11,44 Parasitic infestations have the potential to remain asymptomatic for extended periods of time and result in spread to potentially nonendemic regions of resettlement.45 Additionally, the psychosocial well-being of refugee populations upon resettlement may be negatively affected by stigma of disease processes such as scabies and pediculosis, leading to additional barriers to successful re-entry into the patient’s new environment.46 Therefore, proper screening, diagnosis, and treatment of the most common parasitic infestations in this population have great potential to improve outcomes for large groups across the globe.
Approximately 108 million individuals have been forcibly displaced across the globe as of 2022, 35 million of whom are formally designated as refugees.1,2 The United States has coordinated resettlement of more refugee populations than any other country; the most common countries of origin are the Democratic Republic of the Congo, Syria, Afghanistan, and Myanmar.3 In 2021, policy to increase the number of refugees resettled in the United States by more than 700% (from 15,000 up to 125,000) was established; since enactment, the United States has seen more than double the refugee arrivals in 2023 than the prior year, making medical care for this population increasingly relevant for the dermatologist.4
Understanding how to care for this population begins with an accurate understanding of the term refugee. The United Nations defines a refugee as a person who is unwilling or unable to return to their country of nationality because of persecution or well-founded fear of persecution due to race, religion, nationality, membership in a particular social group, or political opinion. This term grants a protected status under international law and encompasses access to travel assistance, housing, cultural orientation, and medical evaluation upon resettlement.5,6
The burden of treatable dermatologic conditions in refugee populations ranges from 19% to 96% in the literature7,8 and varies from inflammatory disorders to infectious and parasitic diseases.9 In one study of 6899 displaced individuals in Greece, the prevalence of dermatologic conditions was higher than traumatic injury, cardiac disease, psychological conditions, and dental disease.10
When outlining differential diagnoses for parasitic infestations of the skin that affect refugee populations, helpful considerations include the individual’s country of origin, route traveled, and method of travel.11 Parasitic infestations specifically are more common in refugee populations when there are barriers to basic hygiene, crowded living or travel conditions, or lack of access to health care, which they may experience at any point in their home country, during travel, or in resettlement housing.8
Even with limited examination and diagnostic resources, the skin is the most accessible first indication of patients’ overall well-being and often provides simple diagnostic clues—in combination with contextualization of the patient’s unique circumstances—necessary for successful diagnosis and treatment of scabies and pediculosis.12 The dermatologist working with refugee populations may be the first set of eyes available and trained to discern skin infestations and therefore has the potential to improve overall outcomes.
Some parasitic infestations in refugee populations may fall under the category of neglected tropical diseases, including scabies, ascariasis, trypanosomiasis, leishmaniasis, and schistosomiasis; they affect an estimated 1 billion individuals across the globe but historically have been underrepresented in the literature and in health policy due in part to limited access to care.13 This review will focus on infestations by the scabies mite (Sarcoptes scabiei var hominis) and the human louse, as these frequently are encountered, easily diagnosed, and treatable by trained clinicians, even in resource-limited settings.
Scabies
Scabies is a parasitic skin infestation caused by the 8-legged mite Sarcoptes scabiei var hominis. The female mite begins the infestation process via penetration of the epidermis, particularly the stratum corneum, and commences laying eggs (Figure 1). The subsequent larvae emerge 48 to 72 hours later and remain burrowed in the epidermis. The larvae mature over the next 10 to 14 days and continue the reproductive cycle.14,15 Symptoms of infestation occurs due to a hypersensitivity reaction to the mite and its by-products.16 Transmission of the mite primarily occurs via direct (skin-to-skin) contact with infected individuals or environmental surfaces for 24 to36 hours in specific conditions, though the latter source has been debated in the literature.

The method of transmission is particularly important when considering care for refugee populations. Scabies is found most often in those living in or traveling from tropical regions including East Asia, Southeast Asia, Oceania, and Latin America.17 In displaced or refugee populations, a lack of access to basic hygiene, extended travel in close quarters, and suboptimal health care access all may lead to an increased incidence of untreated scabies infestations.18 Scabies is more prevalent in children, with increased potential for secondary bacterial infections with Streptococcus and Staphylococcus species due to excoriation in unsanitary conditions. Secondary infection with Streptococcus pyogenes can lead to acute poststreptococcal glomerulonephritis, which accounts for a large burden of chronic kidney disease in affected populations.19 However, scabies may be found in any population, regardless of hygiene or health care access. Treating health care providers should keep a broad differential.
Presentation—The latency of scabies symptoms is 2 to 6 weeks in a primary outbreak and may be as short as 1 to 3 days with re-infestation, following the course of delayed-type hypersensitivity.20 The initial hallmark symptom is pruritus with increased severity in the evening. Visible lesions, excoriations, and burrows associated with scattered vesicles or pustules may be seen over the web spaces of the hands and feet, volar surfaces of the wrists, axillae, waist, genitalia, inner thighs, or buttocks.19 Chronic infestation often manifests with genital nodules. In populations with limited access to health care, there are reports of a sensitization phenomenon in which the individual may become less symptomatic after 4 to 6 weeks and yet be a potential carrier of the mite.21
Those with compromised immune function, such as individuals living with HIV or severe malnutrition, may present with crusted scabies, a variant that manifests as widespread hyperkeratotic scaling with more pronounced involvement of the head, neck, and acral areas. In contrast to classic scabies, crusted scabies is associated with minimal pruritus.22
Diagnosis—The diagnosis of scabies is largely clinical with confirmation through skin scrapings. The International Alliance for Control of Scabies has established diagnostic criteria that include a combination of clinical findings, history, and visualization of mites.23 A dermatologist working with refugee populations may employ any combination of history (eg, nocturnal itch, exposure to an affected individual) or clinical findings along with a high degree of suspicion in those with elevated risk. Visualization of mites is helpful to confirm the diagnosis and may be completed with the application of mineral oil at the terminal end of a burrow, skin scraping with a surgical blade or needle, and examination under light microscopy.
Treatment—First-line treatment for scabies consists of application of permethrin cream 5% on the skin of the neck to the soles of the feet, which is to be left on for 8 to 14 hours followed by rinsing. Re-application is recommended in 1 to 2 weeks. Oral ivermectin is a reasonable alternative to permethrin cream due to its low cost and easy administration in large affected groups. It is not labeled for use in pregnant women or children weighing less than 15 kg but has no selective fetal toxicity. Treatment of scabies with ivermectin has the benefit of treating many other parasitic infections. Both medications are on the World Health Organization Model List of Essential Medications and are widely available for treating providers, even in resource-limited settings.24
Much of the world still uses benzyl benzoate or precipitated sulfur ointment to treat scabies, and some botanicals used in folk medicine have genuine antiscabetic properties. Pruritus may persist for 1 to 4 weeks following treatment and does not indicate treatment failure. Topical camphor and menthol preparations, low-potency topical corticosteroids, or emollients all may be employed for relief.25 Sarna is a Spanish term for scabies and has become the proprietary name for topical antipruritic agents. Additional methods of treatment and prevention include washing clothes and linens in hot water and drying on high heat. If machine washing is not available, clothing and linens may be sealed in a plastic bag for 72 hours.
Pediculosis
Pediculosis is an infestation caused by the ectoparasite Pediculus humanus, an obligate, sesame seed–sized louse that feeds exclusively on the blood of its host (Figure 2).26 Of the lice species, 2 require humans as hosts; one is P humanus and the other is Pthirus pubis (pubic lice). Pediculus humanus may be further classified into morphologies based largely on the affected area: body (P humanus corporis) or head (P humanus capitis), both of which will be discussed.27

Lice primarily attach to clothing and hair shafts, then transfer to the skin for blood feeds. Females lay eggs that hatch 6 to 10 days later, subsequently maturing into adults. The lifespan of these parasites with regular access to a host is 1 to 3 months for head lice and 18 days for body lice vs only 3 to 5 days without a host.28 Transmission of P humanus capitis primarily occurs via direct contact with affected individuals, either head-to-head contact or sharing of items such as brushes and headscarves; P humanus corporis also may be transmitted via direct contact with affected individuals or clothing.
Pediculosis is an important infestation to consider when providing care for refugee populations. Risk factors include lack of access to basic hygiene, including regular bathing or laundering of clothing, and crowded conditions that make direct person-to-person contact with affected individuals more likely.29 Body lice are associated more often with domestic turbulence and displaced populations30 in comparison to head lice, which have broad demographic variables, most often affecting females and children.28 Fatty acids in adult male sebum make the scalp less hospitable to lice.
Presentation—The most common clinical manifestation of pediculosis is pruritus. Cutaneous findings can include papules, wheals, or hemorrhagic puncta secondary to the louse bite. Due to the Tyndall effect of deep hemosiderin pigment, blue-grey macules termed maculae ceruleae (Figure 3) also may be present in chronic infestations of pediculosis pubis, in contrast to pediculosis capitis or corporis.31 Body louse infestation is associated with a general pruritus concentrated on the neck, shoulders, and waist—areas where clothing makes the most direct contact. Lesions may be visible and include eczematous patches with excoriation and possible secondary bacterial infection. Chronic infestation may exhibit lichenification or hyperpigmentation in associated areas. Head lice most often manifest with localized scalp pruritus and associated excoriation and cervical or occipital lymphadenopathy.32

Diagnosis—The diagnosis of pediculosis is clinical, with confirmation requiring direct examination of the insect or nits (the egg case of the parasite)(Figure 4). Body lice and associated nits can be visualized on clothing seams near areas of highest body temperature, particularly the waistband. Head lice may be visualized crawling on hair shafts or on a louse comb. Nits are firmly attached to hair shafts and are visible to the naked eye, whereas pseudonits slide freely along the hair shaft and are not a manifestation of louse infestation (Figure 5).31

Treatment—Treatment varies by affected area. Pediculosis corporis may be treated with permethrin cream 5% applied to the entire body and left on for 8 to 10 hours, but this may not be necessary if facilities are available to wash and dry clothing.33 The use of oral ivermectin and permethrin-impregnated underwear both have been proposed.34,35 Treatment of pediculosis capitis may be accomplished with a variety of topical pediculicides including permethrin, pyrethrum with piperonyl butoxide, dimethicone, malathion, benzyl alcohol, spinosad, and topical ivermectin.22 Topical corticosteroids or emollients may be employed for residual pruritus.

Equally important is environmental elimination of infestation. Clothing should be discarded if possible or washed and dried using high heat. If neither approach is possible or appropriate, clothing may be sealed in a plastic bag for 2 weeks or treated with a pediculicide. Nit combing is an important adjunct in the treatment of pediculosis capitis.36 It is important to encourage return to work and/or school immediately after treatment. “No nit” policies are more harmful to education than helpful for prevention of investation.37
Pediculosis corporis may transmit infectious agents including Bartonella quintana, (trench fever, endocarditis, bacillary angiomatosis), Borrelia recurrentis (louse-borne relapsing fever), and Rickettsia prowazekii (epidemic typhus).31,38,39 Additionally, severe pediculosis infestations have the potential to cause chronic blood loss in affected populations. In a study of patients with active pediculosis infestation, mean hemoglobin values were found to be 2.5 g/dL lower than a matched population without infestation.40 It is important to consider pediculosis as a risk for iron-deficiency anemia in populations who are known to lack access to regular medical evaluation.41
Future Considerations
Increased access to tools and education for clinicians treating refugee populations is key to reducing the burden of parasitic skin disease and related morbidity and mortality in vulnerable groups both domestically and globally. One such tool, the Skin NTDs App, was launched by the World Health Organization in 2020. It is available for free for Android and iOS devices to assist clinicians in the field with the diagnosis and treatment of neglected tropical diseases—including scabies—that may affect refugee populations.42
Additionally, to both improve access and limit preventable sequelae, future investigations into appropriate models of community-based care are paramount. The model of community-based care is centered on the idea of care provision that prioritizes safety, accessibility, affordability, and acceptability in an environment closest to vulnerable populations. The largest dermatologic society, the International League of Dermatological Societies, formed a Migrant Health Dermatology Working Group that prioritizes understanding and improving care for refugee and migrant populations; this group hosted a summit in 2022, bringing together international subject matter leaders to discuss such models of care and set goals for the creation of tool kits for patients, frontline health care workers, and dermatologists.43
Conclusion
Improvement in dermatologic care of refugee populations includes provision of culturally and linguistically appropriate care by trained clinicians, adequate access to the most essential medications, and basic physical or legal access to health care systems in general.8,11,44 Parasitic infestations have the potential to remain asymptomatic for extended periods of time and result in spread to potentially nonendemic regions of resettlement.45 Additionally, the psychosocial well-being of refugee populations upon resettlement may be negatively affected by stigma of disease processes such as scabies and pediculosis, leading to additional barriers to successful re-entry into the patient’s new environment.46 Therefore, proper screening, diagnosis, and treatment of the most common parasitic infestations in this population have great potential to improve outcomes for large groups across the globe.
- Monin K, Batalova J, Lai T. Refugees and Asylees in the United States. Migration Information Source. Published May 13, 2021. Accessed April 4, 2024. https://www.migrationpolicy.org/article/refugees-and-asylees-united-states-2021
- UNHCR. Figures at a Glance. UNHCR USA. Update June 14, 2023. Accessed April 4, 2024. https://www.unhcr.org/en-us/figures-at-a-glance.html
- UNHCR. Refugee resettlement facts. Published October 2023. Accessed April 8, 2024. https://www.unhcr.org/us/media/refugee-resettlement-facts
- US Department of State. Report to Congress on Proposed Refugee Admissions for Fiscal Year 2024. Published November 3, 2023. Accessed April 8, 2024. https://www.state.gov/report-to-congress-on-proposed-refugee-admissions-for-fiscal-year-2024/
- UNHCR. Compact for Migration: Definitions. United Nations. Accessed April 4, 2024. https://refugeesmigrants.un.org/definitions
- United Nations High Commissioner for Refugees (UNHCR). Convention and Protocol Relating to the Status of Refugees. Published December 2010. Accessed January 11, 2024. https://www.unhcr.org/us/media/convention-and-protocol-relating-status-refugees
- Kibar Öztürk M. Skin diseases in rural Nyala, Sudan (in a rural hospital, in 12 orphanages, and in two refugee camps). Int J Dermatol. 2019;58:1341-1349. doi:10.1111/ijd.14619
- Padovese V, Knapp A. Challenges of managing skin diseases in refugees and migrants. Dermatol Clin. 2021;39:101-115. doi:10.1016/j.det.2020.08.010
- Saikal SL, Ge L, Mir A, et al. Skin disease profile of Syrian refugees in Jordan: a field-mission assessment. J Eur Acad Dermatol Venereol. 2020;34:419-425. doi:10.1111/jdv.15909
- Eonomopoulou A, Pavli A, Stasinopoulou P, et al. Migrant screening: lessons learned from the migrant holding level at the Greek-Turkish borders. J Infect Public Health. 2017;10:177-184. doi:10.1016/j.jiph.2016.04.012
- Marano N, Angelo KM, Merrill RD, et al. Expanding travel medicine in the 21st century to address the health needs of the world’s migrants.J Travel Med. 2018;25. doi:10.1093/jtm/tay067
- Hay RJ, Asiedu K. Skin-related neglected tropical diseases (skin NTDs)—a new challenge. Trop Med Infect Dis. 2018;4. doi:10.3390/tropicalmed4010004
- NIAID. Neglected tropical diseases. Updated July 11, 2016. Accessed April 4, 2024. https://www.niaid.nih.gov/research/neglected-tropical-diseases
- Arlian LG, Morgan MS. A review of Sarcoptes scabiei: past, present and future. Parasit Vectors. 2017;10:297. doi:10.1186/s13071-017-2234-1
- Arlian LG, Runyan RA, Achar S, et al. Survival and infectivity of Sarcoptes scabiei var. canis and var. hominis. J Am Acad Dermatol. 1984;11(2 pt 1):210-215. doi:10.1016/s0190-9622(84)70151-4
- Chandler DJ, Fuller LC. A review of scabies: an infestation more than skin deep. Dermatology. 2019;235:79-90. doi:10.1159/000495290
- Karimkhani C, Colombara DV, Drucker AM, et al. The global burden of scabies: a cross-sectional analysis from the Global Burden of Disease Study 2015. Lancet Infect Dis. 2017;17:1247-1254. doi:10.1016/S1473-3099(17)30483-8
- Romani L, Steer AC, Whitfeld MJ, et al. Prevalence of scabies and impetigo worldwide: a systematic review. Lancet Infect Dis. 2015;15:960-967. doi:10.1016/S1473-3099(15)00132-2
- Thomas C, Coates SJ, Engelman D, et al. Ectoparasites: scabies. J Am Acad Dermatol. 2020;82:533-548. doi:10.1016/j.jaad.2019.05.109
- Mellanby K, Johnson CG, Bartley WC. Treatment of scabies. Br Med J. 1942;2:1-4. doi:10.1136/bmj.2.4252.1
- Walton SF. The immunology of susceptibility and resistance to scabies. Parasit Immunol. 2010;32:532-540. doi:10.1111/j.1365-3024.2010.01218.x
- Coates SJ, Thomas C, Chosidow O, et al. Ectoparasites: pediculosis and tungiasis. J Am Acad Dermatol. 2020;82:551-569. doi:10.1016/j.jaad.2019.05.110
- Engelman D, Fuller LC, Steer AC; International Alliance for the Control of Scabies Delphi p. Consensus criteria for the diagnosis of scabies: a Delphi study of international experts. PLoS Negl Trop Dis. 2018;12:E0006549. doi:10.1371/journal.pntd.0006549
- World Health Organization. WHO Model Lists of Essential Medicines—23rd list, 2023. Updated July 26, 2023. Accessed April 8, 2024. https://www.who.int/publications/i/item/WHO-MHP-HPS-EML-2023.02
- Salavastru CM, Chosidow O, Boffa MJ, et al. European guideline for the management of scabies. J Eur Acad Dermatol Venereol. 2017;31:1248-1253. doi:10.1111/jdv.14351
- Badiaga S, Brouqui P. Human louse-transmitted infectious diseases. Clin Microbiol Infect. 2012;18:332-337. doi:10.1111/j.1469-0691.2012.03778.x
- Leo NP, Campbell NJH, Yang X, et al. Evidence from mitochondrial DNA that head lice and body lice of humans (Phthiraptera: Pediculidae) are conspecific. J Med Entomol. 2002;39:662-666. doi:10.1603/0022-2585-39.4.662
- Chosidow O. Scabies and pediculosis. Lancet. 2000;355:819-826. doi:10.1016/S0140-6736(99)09458-1
- Arnaud A, Chosidow O, Détrez M-A, et al. Prevalences of scabies and pediculosis corporis among homeless people in the Paris region: results from two randomized cross-sectional surveys (HYTPEAC study). Br J Dermatol. 2016;174:104-112. doi:10.1111/bjd.14226
- Brouqui P. Arthropod-borne diseases associated with political and social disorder. Annu Rev Entomol. 2011;56:357-374. doi:10.1146/annurev-ento-120709-144739
- Ko CJ, Elston DM. Pediculosis. J Am Acad Dermatol. 2004;50:1-12. doi:10.1016/S0190-9622(03)02729-4
- Bloomfield D. Head lice. Pediatr Rev. 2002;23:34-35; discussion 34-35. doi:10.1542/pir.23-1-34
- Stone SP GJ, Bacelieri RE. Scabies, other mites, and pediculosis. In: Wolf K GL, Katz SI, et al (eds). Fitzpatrick’s Dermatology in General Medicine. McGraw Hill; 2008:2029.
- Foucault C, Ranque S, Badiaga S, et al. Oral ivermectin in the treatment of body lice. J Infect Dis. 2006;193:474-476. doi:10.1086/499279
- Benkouiten S, Drali R, Badiaga S, et al. Effect of permethrin-impregnated underwear on body lice in sheltered homeless persons: a randomized controlled trial. JAMA Dermatol. 2014;150:273-279. doi:10.1001/jamadermatol.2013.6398
- CDC. Parasites: Treatment. Updated October 15, 2019. Accessed April 4, 2024. https://www.cdc.gov/parasites/lice/head/treatment.html
- Devore CD, Schutze GE; Council on School Health and Committee on Infectious Diseases, American Academy of Pediatrics. Head lice. Pediatrics. 2015;135:e1355-e1365. doi:10.1542/peds.2015-0746
- Ohl ME, Spach DH. Bartonella quintana and urban trench fever. Clin Infect Dis. 2000;31:131-135. doi:10.1086/313890
- Drali R, Sangaré AK, Boutellis A, et al. Bartonella quintana in body lice from scalp hair of homeless persons, France. Emerg Infect Dis. 2014;20:907-908. doi:10.3201/eid2005.131242
- Rudd N, Zakaria A, Kohn MA, et al. Association of body lice infestation with hemoglobin values in hospitalized dermatology patients. JAMA Dermatol. 2022;158:691-693. doi:10.1001/jamadermatol.2022.0818
- Guss DA, Koenig M, Castillo EM. Severe iron deficiency anemia and lice infestation. J Emergency Med. 2011;41:362-365. doi:10.1016/j.jemermed.2010.05.030
- Neglected tropical diseases of the skin: WHO launches mobile application to facilitate diagnosis. News release. World Health Organization; July 16, 2020. Accessed April 4, 2024. https://www.who.int/news/item/16-07-2020-neglected-tropical-diseases-of-the-skin-who-launches-mobile-application-to-facilitate-diagnosis
- Padovese V, Fuller LC, Griffiths CEM, et al; Migrant Health Dermatology Working Group of the International Foundation for Dermatology. Migrant skin health: perspectives from the Migrant Health Summit, Malta, 2022. Br J Dermatology. 2023;188:553-554. doi:10.1093/bjd/ljad001
- Knapp AP, Rehmus W, Chang AY. Skin diseases in displaced populations: a review of contributing factors, challenges, and approaches to care. Int J Dermatol. 2020;59:1299-1311. doi:10.1111/ijd.15063
- Norman FF, Comeche B, Chamorro S, et al. Overcoming challenges in the diagnosis and treatment of parasitic infectious diseases in migrants. Expert Rev Anti-infective Therapy. 2020;18:127-143. doi:10.1080/14787210.2020.1713099
- Skin NTDs: prioritizing integrated approaches to reduce suffering, psychosocial impact and stigmatization. News release. World Health Organization; October 29, 2020. Accessed April 4, 2024. https://www.who.int/news/item/29-10-2020-skin-ntds-prioritizing-integrated-approaches-to-reduce-suffering-psychosocial-impact-and-stigmatization
- Monin K, Batalova J, Lai T. Refugees and Asylees in the United States. Migration Information Source. Published May 13, 2021. Accessed April 4, 2024. https://www.migrationpolicy.org/article/refugees-and-asylees-united-states-2021
- UNHCR. Figures at a Glance. UNHCR USA. Update June 14, 2023. Accessed April 4, 2024. https://www.unhcr.org/en-us/figures-at-a-glance.html
- UNHCR. Refugee resettlement facts. Published October 2023. Accessed April 8, 2024. https://www.unhcr.org/us/media/refugee-resettlement-facts
- US Department of State. Report to Congress on Proposed Refugee Admissions for Fiscal Year 2024. Published November 3, 2023. Accessed April 8, 2024. https://www.state.gov/report-to-congress-on-proposed-refugee-admissions-for-fiscal-year-2024/
- UNHCR. Compact for Migration: Definitions. United Nations. Accessed April 4, 2024. https://refugeesmigrants.un.org/definitions
- United Nations High Commissioner for Refugees (UNHCR). Convention and Protocol Relating to the Status of Refugees. Published December 2010. Accessed January 11, 2024. https://www.unhcr.org/us/media/convention-and-protocol-relating-status-refugees
- Kibar Öztürk M. Skin diseases in rural Nyala, Sudan (in a rural hospital, in 12 orphanages, and in two refugee camps). Int J Dermatol. 2019;58:1341-1349. doi:10.1111/ijd.14619
- Padovese V, Knapp A. Challenges of managing skin diseases in refugees and migrants. Dermatol Clin. 2021;39:101-115. doi:10.1016/j.det.2020.08.010
- Saikal SL, Ge L, Mir A, et al. Skin disease profile of Syrian refugees in Jordan: a field-mission assessment. J Eur Acad Dermatol Venereol. 2020;34:419-425. doi:10.1111/jdv.15909
- Eonomopoulou A, Pavli A, Stasinopoulou P, et al. Migrant screening: lessons learned from the migrant holding level at the Greek-Turkish borders. J Infect Public Health. 2017;10:177-184. doi:10.1016/j.jiph.2016.04.012
- Marano N, Angelo KM, Merrill RD, et al. Expanding travel medicine in the 21st century to address the health needs of the world’s migrants.J Travel Med. 2018;25. doi:10.1093/jtm/tay067
- Hay RJ, Asiedu K. Skin-related neglected tropical diseases (skin NTDs)—a new challenge. Trop Med Infect Dis. 2018;4. doi:10.3390/tropicalmed4010004
- NIAID. Neglected tropical diseases. Updated July 11, 2016. Accessed April 4, 2024. https://www.niaid.nih.gov/research/neglected-tropical-diseases
- Arlian LG, Morgan MS. A review of Sarcoptes scabiei: past, present and future. Parasit Vectors. 2017;10:297. doi:10.1186/s13071-017-2234-1
- Arlian LG, Runyan RA, Achar S, et al. Survival and infectivity of Sarcoptes scabiei var. canis and var. hominis. J Am Acad Dermatol. 1984;11(2 pt 1):210-215. doi:10.1016/s0190-9622(84)70151-4
- Chandler DJ, Fuller LC. A review of scabies: an infestation more than skin deep. Dermatology. 2019;235:79-90. doi:10.1159/000495290
- Karimkhani C, Colombara DV, Drucker AM, et al. The global burden of scabies: a cross-sectional analysis from the Global Burden of Disease Study 2015. Lancet Infect Dis. 2017;17:1247-1254. doi:10.1016/S1473-3099(17)30483-8
- Romani L, Steer AC, Whitfeld MJ, et al. Prevalence of scabies and impetigo worldwide: a systematic review. Lancet Infect Dis. 2015;15:960-967. doi:10.1016/S1473-3099(15)00132-2
- Thomas C, Coates SJ, Engelman D, et al. Ectoparasites: scabies. J Am Acad Dermatol. 2020;82:533-548. doi:10.1016/j.jaad.2019.05.109
- Mellanby K, Johnson CG, Bartley WC. Treatment of scabies. Br Med J. 1942;2:1-4. doi:10.1136/bmj.2.4252.1
- Walton SF. The immunology of susceptibility and resistance to scabies. Parasit Immunol. 2010;32:532-540. doi:10.1111/j.1365-3024.2010.01218.x
- Coates SJ, Thomas C, Chosidow O, et al. Ectoparasites: pediculosis and tungiasis. J Am Acad Dermatol. 2020;82:551-569. doi:10.1016/j.jaad.2019.05.110
- Engelman D, Fuller LC, Steer AC; International Alliance for the Control of Scabies Delphi p. Consensus criteria for the diagnosis of scabies: a Delphi study of international experts. PLoS Negl Trop Dis. 2018;12:E0006549. doi:10.1371/journal.pntd.0006549
- World Health Organization. WHO Model Lists of Essential Medicines—23rd list, 2023. Updated July 26, 2023. Accessed April 8, 2024. https://www.who.int/publications/i/item/WHO-MHP-HPS-EML-2023.02
- Salavastru CM, Chosidow O, Boffa MJ, et al. European guideline for the management of scabies. J Eur Acad Dermatol Venereol. 2017;31:1248-1253. doi:10.1111/jdv.14351
- Badiaga S, Brouqui P. Human louse-transmitted infectious diseases. Clin Microbiol Infect. 2012;18:332-337. doi:10.1111/j.1469-0691.2012.03778.x
- Leo NP, Campbell NJH, Yang X, et al. Evidence from mitochondrial DNA that head lice and body lice of humans (Phthiraptera: Pediculidae) are conspecific. J Med Entomol. 2002;39:662-666. doi:10.1603/0022-2585-39.4.662
- Chosidow O. Scabies and pediculosis. Lancet. 2000;355:819-826. doi:10.1016/S0140-6736(99)09458-1
- Arnaud A, Chosidow O, Détrez M-A, et al. Prevalences of scabies and pediculosis corporis among homeless people in the Paris region: results from two randomized cross-sectional surveys (HYTPEAC study). Br J Dermatol. 2016;174:104-112. doi:10.1111/bjd.14226
- Brouqui P. Arthropod-borne diseases associated with political and social disorder. Annu Rev Entomol. 2011;56:357-374. doi:10.1146/annurev-ento-120709-144739
- Ko CJ, Elston DM. Pediculosis. J Am Acad Dermatol. 2004;50:1-12. doi:10.1016/S0190-9622(03)02729-4
- Bloomfield D. Head lice. Pediatr Rev. 2002;23:34-35; discussion 34-35. doi:10.1542/pir.23-1-34
- Stone SP GJ, Bacelieri RE. Scabies, other mites, and pediculosis. In: Wolf K GL, Katz SI, et al (eds). Fitzpatrick’s Dermatology in General Medicine. McGraw Hill; 2008:2029.
- Foucault C, Ranque S, Badiaga S, et al. Oral ivermectin in the treatment of body lice. J Infect Dis. 2006;193:474-476. doi:10.1086/499279
- Benkouiten S, Drali R, Badiaga S, et al. Effect of permethrin-impregnated underwear on body lice in sheltered homeless persons: a randomized controlled trial. JAMA Dermatol. 2014;150:273-279. doi:10.1001/jamadermatol.2013.6398
- CDC. Parasites: Treatment. Updated October 15, 2019. Accessed April 4, 2024. https://www.cdc.gov/parasites/lice/head/treatment.html
- Devore CD, Schutze GE; Council on School Health and Committee on Infectious Diseases, American Academy of Pediatrics. Head lice. Pediatrics. 2015;135:e1355-e1365. doi:10.1542/peds.2015-0746
- Ohl ME, Spach DH. Bartonella quintana and urban trench fever. Clin Infect Dis. 2000;31:131-135. doi:10.1086/313890
- Drali R, Sangaré AK, Boutellis A, et al. Bartonella quintana in body lice from scalp hair of homeless persons, France. Emerg Infect Dis. 2014;20:907-908. doi:10.3201/eid2005.131242
- Rudd N, Zakaria A, Kohn MA, et al. Association of body lice infestation with hemoglobin values in hospitalized dermatology patients. JAMA Dermatol. 2022;158:691-693. doi:10.1001/jamadermatol.2022.0818
- Guss DA, Koenig M, Castillo EM. Severe iron deficiency anemia and lice infestation. J Emergency Med. 2011;41:362-365. doi:10.1016/j.jemermed.2010.05.030
- Neglected tropical diseases of the skin: WHO launches mobile application to facilitate diagnosis. News release. World Health Organization; July 16, 2020. Accessed April 4, 2024. https://www.who.int/news/item/16-07-2020-neglected-tropical-diseases-of-the-skin-who-launches-mobile-application-to-facilitate-diagnosis
- Padovese V, Fuller LC, Griffiths CEM, et al; Migrant Health Dermatology Working Group of the International Foundation for Dermatology. Migrant skin health: perspectives from the Migrant Health Summit, Malta, 2022. Br J Dermatology. 2023;188:553-554. doi:10.1093/bjd/ljad001
- Knapp AP, Rehmus W, Chang AY. Skin diseases in displaced populations: a review of contributing factors, challenges, and approaches to care. Int J Dermatol. 2020;59:1299-1311. doi:10.1111/ijd.15063
- Norman FF, Comeche B, Chamorro S, et al. Overcoming challenges in the diagnosis and treatment of parasitic infectious diseases in migrants. Expert Rev Anti-infective Therapy. 2020;18:127-143. doi:10.1080/14787210.2020.1713099
- Skin NTDs: prioritizing integrated approaches to reduce suffering, psychosocial impact and stigmatization. News release. World Health Organization; October 29, 2020. Accessed April 4, 2024. https://www.who.int/news/item/29-10-2020-skin-ntds-prioritizing-integrated-approaches-to-reduce-suffering-psychosocial-impact-and-stigmatization
Practice Points
- War and natural disasters displace populations and disrupt infrastructure and access to medical care.
- Infestations and cutaneous infections are common among refugee populations, and impetigo often is a sign of underlying scabies infestation.
- Body lice are important disease vectors inrefugee populations.
ADHD Behavioral Patterns Linked to Prurigo Nodularis Development in Children With Atopic Dermatitis
Key clinical point: Specific behavioral patterns of attention-deficit/hyperactivity disorder, such as impulsivity and hyperactivity, were associated with the development of prurigo nodularis (PN) in children with atopic dermatitis (AD), regardless of AD severity.
Major finding: Among children with AD, the impulsivity/hyperactivity score was significantly higher in those with vs without PN (5.5 ± 4.2 vs 2.9 ± 2.9; P = .038); no significant differences were observed in Eczema Area Severity Index scores, itch numeric rating scale scores, or other AD outcomes in children with vs without PN (P > .05).
Study details: This cross-sectional study included 39 children with AD who did (n = 21) or did not (n = 18) have PN.
Disclosures: This study did not receive any funding. The authors declared no conflicts of interest.
Source: Kim Y, Lee J, Shin K, et al. Association between prurigo nodularis and behavioural patterns of attention-deficit/hyperactivity disorder in children with atopic dermatitis: A cross-sectional study. J Eur Acad Dermatol Venereol. 2024(Mar 27). doi: 10.1111/jdv.19967 Source
Key clinical point: Specific behavioral patterns of attention-deficit/hyperactivity disorder, such as impulsivity and hyperactivity, were associated with the development of prurigo nodularis (PN) in children with atopic dermatitis (AD), regardless of AD severity.
Major finding: Among children with AD, the impulsivity/hyperactivity score was significantly higher in those with vs without PN (5.5 ± 4.2 vs 2.9 ± 2.9; P = .038); no significant differences were observed in Eczema Area Severity Index scores, itch numeric rating scale scores, or other AD outcomes in children with vs without PN (P > .05).
Study details: This cross-sectional study included 39 children with AD who did (n = 21) or did not (n = 18) have PN.
Disclosures: This study did not receive any funding. The authors declared no conflicts of interest.
Source: Kim Y, Lee J, Shin K, et al. Association between prurigo nodularis and behavioural patterns of attention-deficit/hyperactivity disorder in children with atopic dermatitis: A cross-sectional study. J Eur Acad Dermatol Venereol. 2024(Mar 27). doi: 10.1111/jdv.19967 Source
Key clinical point: Specific behavioral patterns of attention-deficit/hyperactivity disorder, such as impulsivity and hyperactivity, were associated with the development of prurigo nodularis (PN) in children with atopic dermatitis (AD), regardless of AD severity.
Major finding: Among children with AD, the impulsivity/hyperactivity score was significantly higher in those with vs without PN (5.5 ± 4.2 vs 2.9 ± 2.9; P = .038); no significant differences were observed in Eczema Area Severity Index scores, itch numeric rating scale scores, or other AD outcomes in children with vs without PN (P > .05).
Study details: This cross-sectional study included 39 children with AD who did (n = 21) or did not (n = 18) have PN.
Disclosures: This study did not receive any funding. The authors declared no conflicts of interest.
Source: Kim Y, Lee J, Shin K, et al. Association between prurigo nodularis and behavioural patterns of attention-deficit/hyperactivity disorder in children with atopic dermatitis: A cross-sectional study. J Eur Acad Dermatol Venereol. 2024(Mar 27). doi: 10.1111/jdv.19967 Source
Upadacitinib Improved Patient-Reported Outcomes in Atopic Dermatitis
Key clinical point: Upadacitinib treatment rapidly and sustainably improved multiple patient-reported outcomes, including itch, in adults and adolescents with moderate to severe atopic dermatitis (AD).
Major finding: At week 1, more than 10% and 15% of patients receiving 15 and 30 mg upadacitinib, respectively, experienced improvements in itch; thereafter, response rates increased steadily and sustainably through week 52. Similar improvements were observed for pain and other skin symptoms.
Study details: This pooled analysis included 1609 adults and adolescents with moderate to severe AD from the phase 3 Measure Up 1 and Measure Up 2 studies who had received upadacitinib (15 mg n = 557; 30 mg n = 567) or placebo (followed by upadacitinib 15 or 30 mg after 16 weeks; n = 485).
Disclosures: This study was funded by AbbVie, Inc. Eight authors declared being employees of or holding stock or stock options in AbbVie. The other authors declared serving as speakers for, receiving consulting fees, or having other ties with various sources, including AbbVie.
Source: Silverberg JI, Gooderham MJ, Paller AS, et al. Early and sustained improvements in symptoms and quality of life with upadacitinib in adults and adolescents with moderate-to-severe atopic dermatitis: 52-week results from two phase III randomized clinical trials (Measure Up 1 and Measure Up 2). Am J Clin Dermatol. 2024 (Mar 25). doi: 10.1007/s40257-024-00853-4 Source
Key clinical point: Upadacitinib treatment rapidly and sustainably improved multiple patient-reported outcomes, including itch, in adults and adolescents with moderate to severe atopic dermatitis (AD).
Major finding: At week 1, more than 10% and 15% of patients receiving 15 and 30 mg upadacitinib, respectively, experienced improvements in itch; thereafter, response rates increased steadily and sustainably through week 52. Similar improvements were observed for pain and other skin symptoms.
Study details: This pooled analysis included 1609 adults and adolescents with moderate to severe AD from the phase 3 Measure Up 1 and Measure Up 2 studies who had received upadacitinib (15 mg n = 557; 30 mg n = 567) or placebo (followed by upadacitinib 15 or 30 mg after 16 weeks; n = 485).
Disclosures: This study was funded by AbbVie, Inc. Eight authors declared being employees of or holding stock or stock options in AbbVie. The other authors declared serving as speakers for, receiving consulting fees, or having other ties with various sources, including AbbVie.
Source: Silverberg JI, Gooderham MJ, Paller AS, et al. Early and sustained improvements in symptoms and quality of life with upadacitinib in adults and adolescents with moderate-to-severe atopic dermatitis: 52-week results from two phase III randomized clinical trials (Measure Up 1 and Measure Up 2). Am J Clin Dermatol. 2024 (Mar 25). doi: 10.1007/s40257-024-00853-4 Source
Key clinical point: Upadacitinib treatment rapidly and sustainably improved multiple patient-reported outcomes, including itch, in adults and adolescents with moderate to severe atopic dermatitis (AD).
Major finding: At week 1, more than 10% and 15% of patients receiving 15 and 30 mg upadacitinib, respectively, experienced improvements in itch; thereafter, response rates increased steadily and sustainably through week 52. Similar improvements were observed for pain and other skin symptoms.
Study details: This pooled analysis included 1609 adults and adolescents with moderate to severe AD from the phase 3 Measure Up 1 and Measure Up 2 studies who had received upadacitinib (15 mg n = 557; 30 mg n = 567) or placebo (followed by upadacitinib 15 or 30 mg after 16 weeks; n = 485).
Disclosures: This study was funded by AbbVie, Inc. Eight authors declared being employees of or holding stock or stock options in AbbVie. The other authors declared serving as speakers for, receiving consulting fees, or having other ties with various sources, including AbbVie.
Source: Silverberg JI, Gooderham MJ, Paller AS, et al. Early and sustained improvements in symptoms and quality of life with upadacitinib in adults and adolescents with moderate-to-severe atopic dermatitis: 52-week results from two phase III randomized clinical trials (Measure Up 1 and Measure Up 2). Am J Clin Dermatol. 2024 (Mar 25). doi: 10.1007/s40257-024-00853-4 Source
Dupilumab Treatment for Atopic Dermatitis Increases Risk for Cutaneous T-Cell Lymphoma
Key clinical point: Patients with atopic dermatitis (AD) treated with dupilumab have an increased risk for cutaneous T-cell lymphoma (CTCL) compared with those not treated with dupilumab.
Major finding: Patients with AD who did vs did not receive dupilumab had a significantly higher risk of developing CTCL (odds ratio [OR] 4.1003; 95% CI 2.055-8.192). The risk for CTCL persisted in those with no prior exposure to disease-modifying antirheumatic drugs (OR 3.202; 95% CI 1.573-6.514).
Study details: This retrospective cohort study included patients with AD who did (n = 22,888) or did not (n = 22,871) receive dupilumab treatment and did not have a preexisting diagnosis for CTCL, Hodgkin lymphoma, non-Hodgkin lymphoma, nonfollicular lymphoma, leukemia, malignant melanoma, squamous cell carcinoma, or basal cell carcinoma.
Disclosures: This study did not receive any funding. The authors declared no conflicts of interest.
Source: Hasan I, Parsons L, Duran S, Zinn Z. Dupilumab therapy for atopic dermatitis is associated with increased risk of cutaneous T cell lymphoma: A retrospective cohort study. J Am Acad Dermatol. 2024 (Apr 6). doi: 10.1016/j.jaad.2024.03.039 Source
Key clinical point: Patients with atopic dermatitis (AD) treated with dupilumab have an increased risk for cutaneous T-cell lymphoma (CTCL) compared with those not treated with dupilumab.
Major finding: Patients with AD who did vs did not receive dupilumab had a significantly higher risk of developing CTCL (odds ratio [OR] 4.1003; 95% CI 2.055-8.192). The risk for CTCL persisted in those with no prior exposure to disease-modifying antirheumatic drugs (OR 3.202; 95% CI 1.573-6.514).
Study details: This retrospective cohort study included patients with AD who did (n = 22,888) or did not (n = 22,871) receive dupilumab treatment and did not have a preexisting diagnosis for CTCL, Hodgkin lymphoma, non-Hodgkin lymphoma, nonfollicular lymphoma, leukemia, malignant melanoma, squamous cell carcinoma, or basal cell carcinoma.
Disclosures: This study did not receive any funding. The authors declared no conflicts of interest.
Source: Hasan I, Parsons L, Duran S, Zinn Z. Dupilumab therapy for atopic dermatitis is associated with increased risk of cutaneous T cell lymphoma: A retrospective cohort study. J Am Acad Dermatol. 2024 (Apr 6). doi: 10.1016/j.jaad.2024.03.039 Source
Key clinical point: Patients with atopic dermatitis (AD) treated with dupilumab have an increased risk for cutaneous T-cell lymphoma (CTCL) compared with those not treated with dupilumab.
Major finding: Patients with AD who did vs did not receive dupilumab had a significantly higher risk of developing CTCL (odds ratio [OR] 4.1003; 95% CI 2.055-8.192). The risk for CTCL persisted in those with no prior exposure to disease-modifying antirheumatic drugs (OR 3.202; 95% CI 1.573-6.514).
Study details: This retrospective cohort study included patients with AD who did (n = 22,888) or did not (n = 22,871) receive dupilumab treatment and did not have a preexisting diagnosis for CTCL, Hodgkin lymphoma, non-Hodgkin lymphoma, nonfollicular lymphoma, leukemia, malignant melanoma, squamous cell carcinoma, or basal cell carcinoma.
Disclosures: This study did not receive any funding. The authors declared no conflicts of interest.
Source: Hasan I, Parsons L, Duran S, Zinn Z. Dupilumab therapy for atopic dermatitis is associated with increased risk of cutaneous T cell lymphoma: A retrospective cohort study. J Am Acad Dermatol. 2024 (Apr 6). doi: 10.1016/j.jaad.2024.03.039 Source