User login
Using Active Surveillance to Identify Monoclonal Antibody Candidates Among COVID-19–Positive Veterans in the Atlanta VA Health Care System
Early in the COVID-19 pandemic, monoclonal antibody (Mab) therapy was the only outpatient therapy for patients with COVID-19 experiencing mild-to-moderate symptoms. The Blocking Viral Attachment and Cell Entry with SARS-CoV-2 Neutralizing Antibodies (BLAZE-1) and the REGN-COV2 (Regeneron) clinical trials found participants treated with Mab had a shorter duration of symptoms and fewer hospitalizations compared with those receiving placebo.1,2 Mab therapy was most efficacious early in the disease course, and the initial US Food and Drug Administration (FDA) Emergency Use Authorization (EUA) of Mab therapies required use within 10 days of symptom onset.3
The impact of the COVID-19 pandemic has been felt disproportionately among marginalized racial and ethnic groups in the US. The COVID-19 Associated Hospitalization Surveillance Network found that non-Hispanic Black persons have significantly higher rates of hospitalization and death by COVID-19 compared with White persons.4-7 However, marginalized groups are underrepresented in the receipt of therapeutic agents for COVID-19. From March 2020 through August 2021, the mean monthly Mab use among Black patients (2.8%) was lower compared with White patients (4.0%), and Black patients received Mab 22.4% less often than White patients.7
The Mab clinical trials BLAZE-1 and REGN-COV2 study populations consisted of > 80% White participants.1,2 Receipt of COVID-19 outpatient treatments may not align with the disease burden in marginalized racial and ethnic groups, leading to health disparities. Although not exhaustive, reasons for these disparities include patient, health care practitioner, and systems-level issues: patient awareness, trust, and engagement with the health care system; health care practitioner awareness and advocacy to pursue COVID-19 treatment for the patient; and health care capacity to provide the medication and service.7
Here, we describe a novel, quality improvement initiative at the Atlanta Veterans Affairs Health Care System (AVAHCS) in Georgia that paired a proactive laboratory-based surveillance strategy to identify and engage veterans for Mab. By centralizing the surveillance and outreach process, we sought to reduce barriers to the Mab referral process and optimize access to life-saving medication.
Implementation
AVAHCS serves a diverse population of more than 129,000 (50.8% non-Hispanic Black veterans, 37.5% White veterans, and 11.7% of other races) at a main medical campus and 18 surrounding community-based outpatient clinics. From December 28, 2020, to August 31, 2021, veterans with a positive COVID-19 nasopharyngeal polymerase chain reaction (PCR) test at AVAHCS were screened daily. A central Mab team consisting of infectious disease (ID) clinical pharmacists and physicians reviewed daily lists of positive laboratory results and identified high-risk individuals for Mab eligibility, using the FDA EUA inclusion criteria. Eligible patients were called by a Mab team member to discuss Mab treatment, provide anticipatory guidance, obtain verbal consent, and schedule the infusion. Conventional referrals from non-Mab team members (eg, primary care physicians) were also accepted into the screening process and underwent the same procedures and risk prioritization strategy as those identified by the Mab team.
Clinic resources allowed for 1 to 2 patients per day to be given Mab, increasing to a maximum of 5 patients per day during the COVID-19 Delta variant surge. We followed our best clinical judgment in prioritizing patient selection, and we aligned our practice with the standards of our affiliated partner, Emory University. In circumstances where patients who were Mab-eligible outnumbered infusion availability, patients were prioritized using the Veterans Health Administration (VHA) COVID-19 (VACO) Index for 30-day COVID-19 mortality.8 As COVID-19 variants developed resistance to the recommended Mab infusions, bamlanivimab, bamlanivimab-etesevimab, or casirivimab-imdevimab, local protocols adapted to EUA revisions. The Mab team also adopted FDA eligibility criteria revisions as they were available.9,10
We describe the outcomes of our centralized screening process for Mab therapy, as measured by screening, uptake, and time to receipt of Mab from screening. We also describe the demographic and clinical characteristics of Mab recipients. Clinical outcomes include postinfusion adverse events (AEs) at day 1 and day 7, emergency department (ED) visits, inpatient hospitalization, and death.
Results
The Mab team screened 2028 veterans who were COVID-19 positive between December 28, 2020, and August 31, 2021, and identified 289 veterans (14%) who met the EUA criteria. One hundred thirty-two veterans (46%) completed Mab infusion, and of the remaining 145 veterans, 124 (86%) declined treatment, and 21 (14%) veterans did not complete Mab infusion largely due to not keeping the appointment. The Mab team active surveillance strategy identified 101 of 132 infusion candidates (77%); 82% had outpatient Mab infusion.
The mean age of veterans who received Mab was 55 years (range, 29-90), and 75% of veterans were aged ≥ 65 years; most were male (84%) and 86 (65%) identified as non-Hispanic Black individuals (Table 1).
Postinfusion AEs reported at day 1 and day 7 occurred for 38 veterans (29%) and 11 veterans (8%), respectively. Sixteen patients (12%) had postinfusion ED visit, and 12 patients (9%) required hospitalization. Eleven of the 12 hospitalized patients (92%) had worsening respiratory symptoms. No deaths occurred in the 132 patients who received Mab.
Discussion
This novel initiative to optimize access to outpatient COVID-19 treatment demonstrated how the Mab team proactively screened and reached out to eligible veterans with COVID-19 promptly. This approach removed layers in the traditional referral process that could be barriers to accessing care. More than three-quarters of patients who received Mab were identified through this strategy, and the uptake was high at 46%. Conventional passive referrals were suboptimal for identifying candidates, which was also the case at a neighboring institution.
In an Emory University study, referrals to the Mab clinic were made through a traditional, decentralized referral system and resulted in a lower uptake of Mab treatment (4.6%).11 One of the key advantages of the AVAHCS program was that we were able to provide individual education about COVID-19 and counsel on the benefits and risks of therapy. Having a structured, telehealth follow-up plan provided additional reassurance and support to the patient. These personalized patient connections likely helped increase acceptance of the Mab therapy.
Our surveillance and outreach strategy had high uptake among Black patients (65%), which exceeded the proportion of AVAHCS Black veterans (54%).12 In the Emory study, just 30% of the participants were Black patients.11 In a study of bamlanivimab use in Chicago, Black individuals represented just 11% of the study population. White patients were more likely to receive bamlanivimab compared with others races, and the likelihood of receiving bamlanivimab was significantly worse for Black patients (odds ratio, 0.28) compared with White patients.13 These studies highlight the disparity in COVID-19 outpatient treatment that does not reflect the racial and minority group representation of the community at large.
Limitations
The VHA medication allocation system at times created a significant mismatch in supply and demand, which significantly limited the AVAHCS Mab program. VHA facilities nationwide with Mab programs received discrete allocations through the US Department of Health and Human Services via VHA pharmacy benefits management services. Despite our large catchment, AVAHCS was allocated 6 or fewer doses of Mab per week during the evaluated period.
Without formal national guidance in the early period of Mab, the AVAHCS Mab team conferred with Emory University Mab clinicians as well as at other VHA facilities in the country to develop an optimal approach to resource allocation. The Mab team considered all EUA criteria to be as inclusive as possible. However, during times of high demand, our utilitarian approach tried to identify the highest-risk patients who would benefit the most from Mab. The VACO index was validated in early 2021, which facilitated decision making when demand was greater than supply. One limitation of the VACO index is its exclusion of several original Mab EUA criteria, including weight, hypertension, and nonmalignancy-related immunosuppression, into its algorithm.3,8
Conclusions
Through proactive screening and direct outreach to patients, the AVAHCS was able to achieve timely administration of Mab infusion that was well within the initial EUA time frame of 10 days and comparable with the time frame in the REGN-COV2 and BLAZE-1 trials. Improving access to resources by changing the referral structure helped engage veterans who may have otherwise missed the time frame for Mab therapy. The experience of the Mab infusion program at the AVAHCS provided valuable insight into how a health care system could effectively screen a large population and distribute the limited resource of Mab therapy in a timely and proportionate fashion among its represented demographic groups.
Acknowledgments
The authors acknowledge the Veterans Health Administration VISN 7 Clinical Resource Hub and Tele Primary Care group for their support.
1. Chen P, Nirula A, Heller B, et al; BLAZE-1 Investigators. SARS-CoV-2 neutralizing antibody LY-CoV555 in outpatients with COVID-19. N Engl J Med. 2021;384(3):229-237. doi:10.1056/NEJMoa2029849
2. Weinreich DM, Sivapalasingam S, Norton T, et al; Trial Investigators. REGN-COV2, a neutralizing antibody cocktail, in outpatients with COVID-19. N Engl J Med. 2021;384(3):238-251. doi:10.1056/NEJMoa2035002
3. US Food and Drug Administration. Fact sheet for health care providers, emergency use authorization (EUA) of bamlanivimab and etesevimab. Accessed August 6, 2023. https://www.fda.gov/media/145802/download
4. Centers for Disease Control and Prevention. Coronavirus disease 2019 (COVID-19)-associated hospitalization surveillance network (COVID-net). Updated March 24, 2023. Accessed August 6, 2023. https://www.cdc.gov/coronavirus/2019-ncov/covid-data/covid-net/purpose-methods.html
5. Centers for Disease Control and Prevention, National Center for Health Statistics. Provisional COVID-19 deaths: distribution of deaths by race and Hispanic origin. Updated July 26, 2023. Accessed August 8, 2023. https://data.cdc.gov/NCHS/Provisional-COVID-19-Deaths-Distribution-of-Deaths/pj7m-y5uh
6. Price-Haywood EG, Burton J, Fort D, Seoane L. Hospitalization and mortality among Black patients and White patients with COVID-19. N Engl J Med. 2020;382(26):2534-2543. doi:10.1056NEJMsa2011686
7. Wiltz JL, Feehan AK, Mollinari AM, et al. Racial and ethnic disparities in receipt of medications for treatment of COVID-19 - United States, March 2020-August 2021. MMWR Morb Mortal Wkly Rep. 2022;71(3):96-102. doi:10.15585/mmwr.mm7103e1
8. King JT Jr, Yoon JS, Rentsch CT, et al. Development and validation of a 30-day mortality index based on pre-existing medical administrative data from 13,323 COVID-19 patients: the Veterans Health Administration COVID-19 (VACO) Index. PLoS One. 2020;15(11):e0241825. doi:10.1371/journal.pone.0241825
9. US Food and Drug Administration, Office of Media Affairs. Coronavirus (COVID-19) update: FDA revokes emergency use authorization for monoclonal antibody bamlanivimab. Accessed August 8, 2023. https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-revokes-emergency-use-authorization-monoclonal-antibody-bamlanivimab
10. National Institutes of Health. Information on COVID-19 treatment, prevention and research. Accessed August 8, 2023. https://www.covid19treatmentguidelines.nih.gov
11. Anderson B, Smith Z, Edupuganti S, Yan X, Masi CM, Wu HM. Effect of monoclonal antibody treatment on clinical outcomes in ambulatory patients with coronavirus disease 2019. Open Forum Infect Dis. 2021;8(7):ofab315. Published 2021 Jun 12. doi:10.1093/ofid/ofab315
12. United States Census Bureau. Quick facts: DeKalb County, Georgia. Updated July 1, 2022. Accessed August 8, 2023. www.census.gov/quickfacts/dekalbcountygeorgia
13. Kumar R, Wu EL, Stosor V, et al. Real-world experience of bamlanivimab for coronavirus disease 2019 (COVID-19): a case-control study. Clin Infect Dis. 2022;74(1):24-31. doi:10.1093/cid/ciab305
Early in the COVID-19 pandemic, monoclonal antibody (Mab) therapy was the only outpatient therapy for patients with COVID-19 experiencing mild-to-moderate symptoms. The Blocking Viral Attachment and Cell Entry with SARS-CoV-2 Neutralizing Antibodies (BLAZE-1) and the REGN-COV2 (Regeneron) clinical trials found participants treated with Mab had a shorter duration of symptoms and fewer hospitalizations compared with those receiving placebo.1,2 Mab therapy was most efficacious early in the disease course, and the initial US Food and Drug Administration (FDA) Emergency Use Authorization (EUA) of Mab therapies required use within 10 days of symptom onset.3
The impact of the COVID-19 pandemic has been felt disproportionately among marginalized racial and ethnic groups in the US. The COVID-19 Associated Hospitalization Surveillance Network found that non-Hispanic Black persons have significantly higher rates of hospitalization and death by COVID-19 compared with White persons.4-7 However, marginalized groups are underrepresented in the receipt of therapeutic agents for COVID-19. From March 2020 through August 2021, the mean monthly Mab use among Black patients (2.8%) was lower compared with White patients (4.0%), and Black patients received Mab 22.4% less often than White patients.7
The Mab clinical trials BLAZE-1 and REGN-COV2 study populations consisted of > 80% White participants.1,2 Receipt of COVID-19 outpatient treatments may not align with the disease burden in marginalized racial and ethnic groups, leading to health disparities. Although not exhaustive, reasons for these disparities include patient, health care practitioner, and systems-level issues: patient awareness, trust, and engagement with the health care system; health care practitioner awareness and advocacy to pursue COVID-19 treatment for the patient; and health care capacity to provide the medication and service.7
Here, we describe a novel, quality improvement initiative at the Atlanta Veterans Affairs Health Care System (AVAHCS) in Georgia that paired a proactive laboratory-based surveillance strategy to identify and engage veterans for Mab. By centralizing the surveillance and outreach process, we sought to reduce barriers to the Mab referral process and optimize access to life-saving medication.
Implementation
AVAHCS serves a diverse population of more than 129,000 (50.8% non-Hispanic Black veterans, 37.5% White veterans, and 11.7% of other races) at a main medical campus and 18 surrounding community-based outpatient clinics. From December 28, 2020, to August 31, 2021, veterans with a positive COVID-19 nasopharyngeal polymerase chain reaction (PCR) test at AVAHCS were screened daily. A central Mab team consisting of infectious disease (ID) clinical pharmacists and physicians reviewed daily lists of positive laboratory results and identified high-risk individuals for Mab eligibility, using the FDA EUA inclusion criteria. Eligible patients were called by a Mab team member to discuss Mab treatment, provide anticipatory guidance, obtain verbal consent, and schedule the infusion. Conventional referrals from non-Mab team members (eg, primary care physicians) were also accepted into the screening process and underwent the same procedures and risk prioritization strategy as those identified by the Mab team.
Clinic resources allowed for 1 to 2 patients per day to be given Mab, increasing to a maximum of 5 patients per day during the COVID-19 Delta variant surge. We followed our best clinical judgment in prioritizing patient selection, and we aligned our practice with the standards of our affiliated partner, Emory University. In circumstances where patients who were Mab-eligible outnumbered infusion availability, patients were prioritized using the Veterans Health Administration (VHA) COVID-19 (VACO) Index for 30-day COVID-19 mortality.8 As COVID-19 variants developed resistance to the recommended Mab infusions, bamlanivimab, bamlanivimab-etesevimab, or casirivimab-imdevimab, local protocols adapted to EUA revisions. The Mab team also adopted FDA eligibility criteria revisions as they were available.9,10
We describe the outcomes of our centralized screening process for Mab therapy, as measured by screening, uptake, and time to receipt of Mab from screening. We also describe the demographic and clinical characteristics of Mab recipients. Clinical outcomes include postinfusion adverse events (AEs) at day 1 and day 7, emergency department (ED) visits, inpatient hospitalization, and death.
Results
The Mab team screened 2028 veterans who were COVID-19 positive between December 28, 2020, and August 31, 2021, and identified 289 veterans (14%) who met the EUA criteria. One hundred thirty-two veterans (46%) completed Mab infusion, and of the remaining 145 veterans, 124 (86%) declined treatment, and 21 (14%) veterans did not complete Mab infusion largely due to not keeping the appointment. The Mab team active surveillance strategy identified 101 of 132 infusion candidates (77%); 82% had outpatient Mab infusion.
The mean age of veterans who received Mab was 55 years (range, 29-90), and 75% of veterans were aged ≥ 65 years; most were male (84%) and 86 (65%) identified as non-Hispanic Black individuals (Table 1).
Postinfusion AEs reported at day 1 and day 7 occurred for 38 veterans (29%) and 11 veterans (8%), respectively. Sixteen patients (12%) had postinfusion ED visit, and 12 patients (9%) required hospitalization. Eleven of the 12 hospitalized patients (92%) had worsening respiratory symptoms. No deaths occurred in the 132 patients who received Mab.
Discussion
This novel initiative to optimize access to outpatient COVID-19 treatment demonstrated how the Mab team proactively screened and reached out to eligible veterans with COVID-19 promptly. This approach removed layers in the traditional referral process that could be barriers to accessing care. More than three-quarters of patients who received Mab were identified through this strategy, and the uptake was high at 46%. Conventional passive referrals were suboptimal for identifying candidates, which was also the case at a neighboring institution.
In an Emory University study, referrals to the Mab clinic were made through a traditional, decentralized referral system and resulted in a lower uptake of Mab treatment (4.6%).11 One of the key advantages of the AVAHCS program was that we were able to provide individual education about COVID-19 and counsel on the benefits and risks of therapy. Having a structured, telehealth follow-up plan provided additional reassurance and support to the patient. These personalized patient connections likely helped increase acceptance of the Mab therapy.
Our surveillance and outreach strategy had high uptake among Black patients (65%), which exceeded the proportion of AVAHCS Black veterans (54%).12 In the Emory study, just 30% of the participants were Black patients.11 In a study of bamlanivimab use in Chicago, Black individuals represented just 11% of the study population. White patients were more likely to receive bamlanivimab compared with others races, and the likelihood of receiving bamlanivimab was significantly worse for Black patients (odds ratio, 0.28) compared with White patients.13 These studies highlight the disparity in COVID-19 outpatient treatment that does not reflect the racial and minority group representation of the community at large.
Limitations
The VHA medication allocation system at times created a significant mismatch in supply and demand, which significantly limited the AVAHCS Mab program. VHA facilities nationwide with Mab programs received discrete allocations through the US Department of Health and Human Services via VHA pharmacy benefits management services. Despite our large catchment, AVAHCS was allocated 6 or fewer doses of Mab per week during the evaluated period.
Without formal national guidance in the early period of Mab, the AVAHCS Mab team conferred with Emory University Mab clinicians as well as at other VHA facilities in the country to develop an optimal approach to resource allocation. The Mab team considered all EUA criteria to be as inclusive as possible. However, during times of high demand, our utilitarian approach tried to identify the highest-risk patients who would benefit the most from Mab. The VACO index was validated in early 2021, which facilitated decision making when demand was greater than supply. One limitation of the VACO index is its exclusion of several original Mab EUA criteria, including weight, hypertension, and nonmalignancy-related immunosuppression, into its algorithm.3,8
Conclusions
Through proactive screening and direct outreach to patients, the AVAHCS was able to achieve timely administration of Mab infusion that was well within the initial EUA time frame of 10 days and comparable with the time frame in the REGN-COV2 and BLAZE-1 trials. Improving access to resources by changing the referral structure helped engage veterans who may have otherwise missed the time frame for Mab therapy. The experience of the Mab infusion program at the AVAHCS provided valuable insight into how a health care system could effectively screen a large population and distribute the limited resource of Mab therapy in a timely and proportionate fashion among its represented demographic groups.
Acknowledgments
The authors acknowledge the Veterans Health Administration VISN 7 Clinical Resource Hub and Tele Primary Care group for their support.
Early in the COVID-19 pandemic, monoclonal antibody (Mab) therapy was the only outpatient therapy for patients with COVID-19 experiencing mild-to-moderate symptoms. The Blocking Viral Attachment and Cell Entry with SARS-CoV-2 Neutralizing Antibodies (BLAZE-1) and the REGN-COV2 (Regeneron) clinical trials found participants treated with Mab had a shorter duration of symptoms and fewer hospitalizations compared with those receiving placebo.1,2 Mab therapy was most efficacious early in the disease course, and the initial US Food and Drug Administration (FDA) Emergency Use Authorization (EUA) of Mab therapies required use within 10 days of symptom onset.3
The impact of the COVID-19 pandemic has been felt disproportionately among marginalized racial and ethnic groups in the US. The COVID-19 Associated Hospitalization Surveillance Network found that non-Hispanic Black persons have significantly higher rates of hospitalization and death by COVID-19 compared with White persons.4-7 However, marginalized groups are underrepresented in the receipt of therapeutic agents for COVID-19. From March 2020 through August 2021, the mean monthly Mab use among Black patients (2.8%) was lower compared with White patients (4.0%), and Black patients received Mab 22.4% less often than White patients.7
The Mab clinical trials BLAZE-1 and REGN-COV2 study populations consisted of > 80% White participants.1,2 Receipt of COVID-19 outpatient treatments may not align with the disease burden in marginalized racial and ethnic groups, leading to health disparities. Although not exhaustive, reasons for these disparities include patient, health care practitioner, and systems-level issues: patient awareness, trust, and engagement with the health care system; health care practitioner awareness and advocacy to pursue COVID-19 treatment for the patient; and health care capacity to provide the medication and service.7
Here, we describe a novel, quality improvement initiative at the Atlanta Veterans Affairs Health Care System (AVAHCS) in Georgia that paired a proactive laboratory-based surveillance strategy to identify and engage veterans for Mab. By centralizing the surveillance and outreach process, we sought to reduce barriers to the Mab referral process and optimize access to life-saving medication.
Implementation
AVAHCS serves a diverse population of more than 129,000 (50.8% non-Hispanic Black veterans, 37.5% White veterans, and 11.7% of other races) at a main medical campus and 18 surrounding community-based outpatient clinics. From December 28, 2020, to August 31, 2021, veterans with a positive COVID-19 nasopharyngeal polymerase chain reaction (PCR) test at AVAHCS were screened daily. A central Mab team consisting of infectious disease (ID) clinical pharmacists and physicians reviewed daily lists of positive laboratory results and identified high-risk individuals for Mab eligibility, using the FDA EUA inclusion criteria. Eligible patients were called by a Mab team member to discuss Mab treatment, provide anticipatory guidance, obtain verbal consent, and schedule the infusion. Conventional referrals from non-Mab team members (eg, primary care physicians) were also accepted into the screening process and underwent the same procedures and risk prioritization strategy as those identified by the Mab team.
Clinic resources allowed for 1 to 2 patients per day to be given Mab, increasing to a maximum of 5 patients per day during the COVID-19 Delta variant surge. We followed our best clinical judgment in prioritizing patient selection, and we aligned our practice with the standards of our affiliated partner, Emory University. In circumstances where patients who were Mab-eligible outnumbered infusion availability, patients were prioritized using the Veterans Health Administration (VHA) COVID-19 (VACO) Index for 30-day COVID-19 mortality.8 As COVID-19 variants developed resistance to the recommended Mab infusions, bamlanivimab, bamlanivimab-etesevimab, or casirivimab-imdevimab, local protocols adapted to EUA revisions. The Mab team also adopted FDA eligibility criteria revisions as they were available.9,10
We describe the outcomes of our centralized screening process for Mab therapy, as measured by screening, uptake, and time to receipt of Mab from screening. We also describe the demographic and clinical characteristics of Mab recipients. Clinical outcomes include postinfusion adverse events (AEs) at day 1 and day 7, emergency department (ED) visits, inpatient hospitalization, and death.
Results
The Mab team screened 2028 veterans who were COVID-19 positive between December 28, 2020, and August 31, 2021, and identified 289 veterans (14%) who met the EUA criteria. One hundred thirty-two veterans (46%) completed Mab infusion, and of the remaining 145 veterans, 124 (86%) declined treatment, and 21 (14%) veterans did not complete Mab infusion largely due to not keeping the appointment. The Mab team active surveillance strategy identified 101 of 132 infusion candidates (77%); 82% had outpatient Mab infusion.
The mean age of veterans who received Mab was 55 years (range, 29-90), and 75% of veterans were aged ≥ 65 years; most were male (84%) and 86 (65%) identified as non-Hispanic Black individuals (Table 1).
Postinfusion AEs reported at day 1 and day 7 occurred for 38 veterans (29%) and 11 veterans (8%), respectively. Sixteen patients (12%) had postinfusion ED visit, and 12 patients (9%) required hospitalization. Eleven of the 12 hospitalized patients (92%) had worsening respiratory symptoms. No deaths occurred in the 132 patients who received Mab.
Discussion
This novel initiative to optimize access to outpatient COVID-19 treatment demonstrated how the Mab team proactively screened and reached out to eligible veterans with COVID-19 promptly. This approach removed layers in the traditional referral process that could be barriers to accessing care. More than three-quarters of patients who received Mab were identified through this strategy, and the uptake was high at 46%. Conventional passive referrals were suboptimal for identifying candidates, which was also the case at a neighboring institution.
In an Emory University study, referrals to the Mab clinic were made through a traditional, decentralized referral system and resulted in a lower uptake of Mab treatment (4.6%).11 One of the key advantages of the AVAHCS program was that we were able to provide individual education about COVID-19 and counsel on the benefits and risks of therapy. Having a structured, telehealth follow-up plan provided additional reassurance and support to the patient. These personalized patient connections likely helped increase acceptance of the Mab therapy.
Our surveillance and outreach strategy had high uptake among Black patients (65%), which exceeded the proportion of AVAHCS Black veterans (54%).12 In the Emory study, just 30% of the participants were Black patients.11 In a study of bamlanivimab use in Chicago, Black individuals represented just 11% of the study population. White patients were more likely to receive bamlanivimab compared with others races, and the likelihood of receiving bamlanivimab was significantly worse for Black patients (odds ratio, 0.28) compared with White patients.13 These studies highlight the disparity in COVID-19 outpatient treatment that does not reflect the racial and minority group representation of the community at large.
Limitations
The VHA medication allocation system at times created a significant mismatch in supply and demand, which significantly limited the AVAHCS Mab program. VHA facilities nationwide with Mab programs received discrete allocations through the US Department of Health and Human Services via VHA pharmacy benefits management services. Despite our large catchment, AVAHCS was allocated 6 or fewer doses of Mab per week during the evaluated period.
Without formal national guidance in the early period of Mab, the AVAHCS Mab team conferred with Emory University Mab clinicians as well as at other VHA facilities in the country to develop an optimal approach to resource allocation. The Mab team considered all EUA criteria to be as inclusive as possible. However, during times of high demand, our utilitarian approach tried to identify the highest-risk patients who would benefit the most from Mab. The VACO index was validated in early 2021, which facilitated decision making when demand was greater than supply. One limitation of the VACO index is its exclusion of several original Mab EUA criteria, including weight, hypertension, and nonmalignancy-related immunosuppression, into its algorithm.3,8
Conclusions
Through proactive screening and direct outreach to patients, the AVAHCS was able to achieve timely administration of Mab infusion that was well within the initial EUA time frame of 10 days and comparable with the time frame in the REGN-COV2 and BLAZE-1 trials. Improving access to resources by changing the referral structure helped engage veterans who may have otherwise missed the time frame for Mab therapy. The experience of the Mab infusion program at the AVAHCS provided valuable insight into how a health care system could effectively screen a large population and distribute the limited resource of Mab therapy in a timely and proportionate fashion among its represented demographic groups.
Acknowledgments
The authors acknowledge the Veterans Health Administration VISN 7 Clinical Resource Hub and Tele Primary Care group for their support.
1. Chen P, Nirula A, Heller B, et al; BLAZE-1 Investigators. SARS-CoV-2 neutralizing antibody LY-CoV555 in outpatients with COVID-19. N Engl J Med. 2021;384(3):229-237. doi:10.1056/NEJMoa2029849
2. Weinreich DM, Sivapalasingam S, Norton T, et al; Trial Investigators. REGN-COV2, a neutralizing antibody cocktail, in outpatients with COVID-19. N Engl J Med. 2021;384(3):238-251. doi:10.1056/NEJMoa2035002
3. US Food and Drug Administration. Fact sheet for health care providers, emergency use authorization (EUA) of bamlanivimab and etesevimab. Accessed August 6, 2023. https://www.fda.gov/media/145802/download
4. Centers for Disease Control and Prevention. Coronavirus disease 2019 (COVID-19)-associated hospitalization surveillance network (COVID-net). Updated March 24, 2023. Accessed August 6, 2023. https://www.cdc.gov/coronavirus/2019-ncov/covid-data/covid-net/purpose-methods.html
5. Centers for Disease Control and Prevention, National Center for Health Statistics. Provisional COVID-19 deaths: distribution of deaths by race and Hispanic origin. Updated July 26, 2023. Accessed August 8, 2023. https://data.cdc.gov/NCHS/Provisional-COVID-19-Deaths-Distribution-of-Deaths/pj7m-y5uh
6. Price-Haywood EG, Burton J, Fort D, Seoane L. Hospitalization and mortality among Black patients and White patients with COVID-19. N Engl J Med. 2020;382(26):2534-2543. doi:10.1056NEJMsa2011686
7. Wiltz JL, Feehan AK, Mollinari AM, et al. Racial and ethnic disparities in receipt of medications for treatment of COVID-19 - United States, March 2020-August 2021. MMWR Morb Mortal Wkly Rep. 2022;71(3):96-102. doi:10.15585/mmwr.mm7103e1
8. King JT Jr, Yoon JS, Rentsch CT, et al. Development and validation of a 30-day mortality index based on pre-existing medical administrative data from 13,323 COVID-19 patients: the Veterans Health Administration COVID-19 (VACO) Index. PLoS One. 2020;15(11):e0241825. doi:10.1371/journal.pone.0241825
9. US Food and Drug Administration, Office of Media Affairs. Coronavirus (COVID-19) update: FDA revokes emergency use authorization for monoclonal antibody bamlanivimab. Accessed August 8, 2023. https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-revokes-emergency-use-authorization-monoclonal-antibody-bamlanivimab
10. National Institutes of Health. Information on COVID-19 treatment, prevention and research. Accessed August 8, 2023. https://www.covid19treatmentguidelines.nih.gov
11. Anderson B, Smith Z, Edupuganti S, Yan X, Masi CM, Wu HM. Effect of monoclonal antibody treatment on clinical outcomes in ambulatory patients with coronavirus disease 2019. Open Forum Infect Dis. 2021;8(7):ofab315. Published 2021 Jun 12. doi:10.1093/ofid/ofab315
12. United States Census Bureau. Quick facts: DeKalb County, Georgia. Updated July 1, 2022. Accessed August 8, 2023. www.census.gov/quickfacts/dekalbcountygeorgia
13. Kumar R, Wu EL, Stosor V, et al. Real-world experience of bamlanivimab for coronavirus disease 2019 (COVID-19): a case-control study. Clin Infect Dis. 2022;74(1):24-31. doi:10.1093/cid/ciab305
1. Chen P, Nirula A, Heller B, et al; BLAZE-1 Investigators. SARS-CoV-2 neutralizing antibody LY-CoV555 in outpatients with COVID-19. N Engl J Med. 2021;384(3):229-237. doi:10.1056/NEJMoa2029849
2. Weinreich DM, Sivapalasingam S, Norton T, et al; Trial Investigators. REGN-COV2, a neutralizing antibody cocktail, in outpatients with COVID-19. N Engl J Med. 2021;384(3):238-251. doi:10.1056/NEJMoa2035002
3. US Food and Drug Administration. Fact sheet for health care providers, emergency use authorization (EUA) of bamlanivimab and etesevimab. Accessed August 6, 2023. https://www.fda.gov/media/145802/download
4. Centers for Disease Control and Prevention. Coronavirus disease 2019 (COVID-19)-associated hospitalization surveillance network (COVID-net). Updated March 24, 2023. Accessed August 6, 2023. https://www.cdc.gov/coronavirus/2019-ncov/covid-data/covid-net/purpose-methods.html
5. Centers for Disease Control and Prevention, National Center for Health Statistics. Provisional COVID-19 deaths: distribution of deaths by race and Hispanic origin. Updated July 26, 2023. Accessed August 8, 2023. https://data.cdc.gov/NCHS/Provisional-COVID-19-Deaths-Distribution-of-Deaths/pj7m-y5uh
6. Price-Haywood EG, Burton J, Fort D, Seoane L. Hospitalization and mortality among Black patients and White patients with COVID-19. N Engl J Med. 2020;382(26):2534-2543. doi:10.1056NEJMsa2011686
7. Wiltz JL, Feehan AK, Mollinari AM, et al. Racial and ethnic disparities in receipt of medications for treatment of COVID-19 - United States, March 2020-August 2021. MMWR Morb Mortal Wkly Rep. 2022;71(3):96-102. doi:10.15585/mmwr.mm7103e1
8. King JT Jr, Yoon JS, Rentsch CT, et al. Development and validation of a 30-day mortality index based on pre-existing medical administrative data from 13,323 COVID-19 patients: the Veterans Health Administration COVID-19 (VACO) Index. PLoS One. 2020;15(11):e0241825. doi:10.1371/journal.pone.0241825
9. US Food and Drug Administration, Office of Media Affairs. Coronavirus (COVID-19) update: FDA revokes emergency use authorization for monoclonal antibody bamlanivimab. Accessed August 8, 2023. https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-revokes-emergency-use-authorization-monoclonal-antibody-bamlanivimab
10. National Institutes of Health. Information on COVID-19 treatment, prevention and research. Accessed August 8, 2023. https://www.covid19treatmentguidelines.nih.gov
11. Anderson B, Smith Z, Edupuganti S, Yan X, Masi CM, Wu HM. Effect of monoclonal antibody treatment on clinical outcomes in ambulatory patients with coronavirus disease 2019. Open Forum Infect Dis. 2021;8(7):ofab315. Published 2021 Jun 12. doi:10.1093/ofid/ofab315
12. United States Census Bureau. Quick facts: DeKalb County, Georgia. Updated July 1, 2022. Accessed August 8, 2023. www.census.gov/quickfacts/dekalbcountygeorgia
13. Kumar R, Wu EL, Stosor V, et al. Real-world experience of bamlanivimab for coronavirus disease 2019 (COVID-19): a case-control study. Clin Infect Dis. 2022;74(1):24-31. doi:10.1093/cid/ciab305
Atopic dermatitis tied to a higher risk for inflammatory bowel disease in children and adults
Key clinical point: Children and adults with atopic dermatitis (AD) have a significantly increased risk of developing inflammatory bowel disease (IBD), including Crohn’s disease (CD).
Major finding: Children with vs without AD had a significantly higher risk for IBD (adjusted HR [aHR] 1.44; 95% CI 1.31-1.58) and CD (aHR 1.74; 95% CI 1.54-1.97), but the risk for ulcerative colitis (UC; aHR 1.65; 95% CI 1.02-2.67) was higher only in children with severe AD. Adults with vs without AD had a significantly increased risk for IBD (aHR 1.34; 95% CI 1.27-1.40), CD (aHR 1.36; 95% CI 1.26-1.47), and UC (aHR 1.32; 95% CI 1.24-1.41).
Study details: This population-based cohort study matched children (n = 409,431; age < 18 years) and adults (n = 625,083) with AD with control children (n = 1,809,029) and adults (n = 2,678,888) without AD, respectively.
Disclosures: This study was supported by a contract from Pfizer Inc. Five authors declared receiving grants, personal fees, and fellowship funding from various sources, including Pfizer Inc.
Source: Chiesa Fuxench ZC et al. Risk of inflammatory bowel disease in patients with atopic dermatitis. JAMA Dermatol. 2023 (Aug 30). doi: 10.1001/jamadermatol.2023.2875
Key clinical point: Children and adults with atopic dermatitis (AD) have a significantly increased risk of developing inflammatory bowel disease (IBD), including Crohn’s disease (CD).
Major finding: Children with vs without AD had a significantly higher risk for IBD (adjusted HR [aHR] 1.44; 95% CI 1.31-1.58) and CD (aHR 1.74; 95% CI 1.54-1.97), but the risk for ulcerative colitis (UC; aHR 1.65; 95% CI 1.02-2.67) was higher only in children with severe AD. Adults with vs without AD had a significantly increased risk for IBD (aHR 1.34; 95% CI 1.27-1.40), CD (aHR 1.36; 95% CI 1.26-1.47), and UC (aHR 1.32; 95% CI 1.24-1.41).
Study details: This population-based cohort study matched children (n = 409,431; age < 18 years) and adults (n = 625,083) with AD with control children (n = 1,809,029) and adults (n = 2,678,888) without AD, respectively.
Disclosures: This study was supported by a contract from Pfizer Inc. Five authors declared receiving grants, personal fees, and fellowship funding from various sources, including Pfizer Inc.
Source: Chiesa Fuxench ZC et al. Risk of inflammatory bowel disease in patients with atopic dermatitis. JAMA Dermatol. 2023 (Aug 30). doi: 10.1001/jamadermatol.2023.2875
Key clinical point: Children and adults with atopic dermatitis (AD) have a significantly increased risk of developing inflammatory bowel disease (IBD), including Crohn’s disease (CD).
Major finding: Children with vs without AD had a significantly higher risk for IBD (adjusted HR [aHR] 1.44; 95% CI 1.31-1.58) and CD (aHR 1.74; 95% CI 1.54-1.97), but the risk for ulcerative colitis (UC; aHR 1.65; 95% CI 1.02-2.67) was higher only in children with severe AD. Adults with vs without AD had a significantly increased risk for IBD (aHR 1.34; 95% CI 1.27-1.40), CD (aHR 1.36; 95% CI 1.26-1.47), and UC (aHR 1.32; 95% CI 1.24-1.41).
Study details: This population-based cohort study matched children (n = 409,431; age < 18 years) and adults (n = 625,083) with AD with control children (n = 1,809,029) and adults (n = 2,678,888) without AD, respectively.
Disclosures: This study was supported by a contract from Pfizer Inc. Five authors declared receiving grants, personal fees, and fellowship funding from various sources, including Pfizer Inc.
Source: Chiesa Fuxench ZC et al. Risk of inflammatory bowel disease in patients with atopic dermatitis. JAMA Dermatol. 2023 (Aug 30). doi: 10.1001/jamadermatol.2023.2875
Dupilumab improves sleep outcomes in atopic dermatitis
Key clinical point: Dupilumab significantly improved the overall sleep continuity and quality, itch, and other signs and symptoms of atopic dermatitis (AD) in patients with moderate-to-severe AD.
Major finding: At week 12, dupilumab vs placebo led to a significant improvement in the sleep Numeric Rating Scale (NRS), peak pruritus NRS, SCORing Atopic Dermatitis (SCORAD), SCORAD sleep visual analog scale, Eczema Area and Severity Index, Epworth Sleepiness Scale, and sleep-related impairment T-scores (all P < .001) and a lower overall treatment-emergent adverse event rate (56.7% vs 67.2%).
Study details: Findings are from the prospective phase 4 DUPISTAD study including patients with moderate-to-severe AD who were randomly assigned to receive 300 mg dupilumab every 2 weeks (n = 127) or placebo (n = 61).
Disclosures: This study was sponsored by Sanofi and Regeneron Pharmaceuticals Inc. Three authors declared being employees of and holding stocks or stock options in Sanofi or Regeneron. Other authors declared receiving grant support, travel grants, or speaker fees from or serving as principal investigators, advisory board members, or consultants for various sources.
Source: Merola JF et al. Dupilumab significantly improves sleep in adults with atopic dermatitis: Results from the 12-week placebo-controlled period of the 24-week phase 4 randomized double-blinded placebo-controlled DUPISTAD study. Br J Dermatol. 2023 (Aug 10). doi: 10.1093/bjd/ljad284
Key clinical point: Dupilumab significantly improved the overall sleep continuity and quality, itch, and other signs and symptoms of atopic dermatitis (AD) in patients with moderate-to-severe AD.
Major finding: At week 12, dupilumab vs placebo led to a significant improvement in the sleep Numeric Rating Scale (NRS), peak pruritus NRS, SCORing Atopic Dermatitis (SCORAD), SCORAD sleep visual analog scale, Eczema Area and Severity Index, Epworth Sleepiness Scale, and sleep-related impairment T-scores (all P < .001) and a lower overall treatment-emergent adverse event rate (56.7% vs 67.2%).
Study details: Findings are from the prospective phase 4 DUPISTAD study including patients with moderate-to-severe AD who were randomly assigned to receive 300 mg dupilumab every 2 weeks (n = 127) or placebo (n = 61).
Disclosures: This study was sponsored by Sanofi and Regeneron Pharmaceuticals Inc. Three authors declared being employees of and holding stocks or stock options in Sanofi or Regeneron. Other authors declared receiving grant support, travel grants, or speaker fees from or serving as principal investigators, advisory board members, or consultants for various sources.
Source: Merola JF et al. Dupilumab significantly improves sleep in adults with atopic dermatitis: Results from the 12-week placebo-controlled period of the 24-week phase 4 randomized double-blinded placebo-controlled DUPISTAD study. Br J Dermatol. 2023 (Aug 10). doi: 10.1093/bjd/ljad284
Key clinical point: Dupilumab significantly improved the overall sleep continuity and quality, itch, and other signs and symptoms of atopic dermatitis (AD) in patients with moderate-to-severe AD.
Major finding: At week 12, dupilumab vs placebo led to a significant improvement in the sleep Numeric Rating Scale (NRS), peak pruritus NRS, SCORing Atopic Dermatitis (SCORAD), SCORAD sleep visual analog scale, Eczema Area and Severity Index, Epworth Sleepiness Scale, and sleep-related impairment T-scores (all P < .001) and a lower overall treatment-emergent adverse event rate (56.7% vs 67.2%).
Study details: Findings are from the prospective phase 4 DUPISTAD study including patients with moderate-to-severe AD who were randomly assigned to receive 300 mg dupilumab every 2 weeks (n = 127) or placebo (n = 61).
Disclosures: This study was sponsored by Sanofi and Regeneron Pharmaceuticals Inc. Three authors declared being employees of and holding stocks or stock options in Sanofi or Regeneron. Other authors declared receiving grant support, travel grants, or speaker fees from or serving as principal investigators, advisory board members, or consultants for various sources.
Source: Merola JF et al. Dupilumab significantly improves sleep in adults with atopic dermatitis: Results from the 12-week placebo-controlled period of the 24-week phase 4 randomized double-blinded placebo-controlled DUPISTAD study. Br J Dermatol. 2023 (Aug 10). doi: 10.1093/bjd/ljad284
Rifampin for Prosthetic Joint Infections: Lessons Learned Over 20 Years at a VA Medical Center
Orthopedic implants are frequently used to repair fractures and replace joints. The number of total joint replacements is high, with > 1 million total hip (THA) and total knee (TKA) arthroplasties performed in the United States each year.1 While most joint arthroplasties are successful and significantly improve patient quality of life, a small proportion become infected.2 Prosthetic joint infection (PJI) causes substantial morbidity and mortality, particularly among older patients, and is difficult and costly to treat.3
The historic gold standard treatment for PJI is a 2-stage replacement, wherein the prosthesis is removed in one procedure and a new prosthesis is implanted in a second procedure after an extended course of antibiotics. This approach requires the patient to undergo 2 major procedures and spend considerable time without a functioning prosthesis, contributing to immobility and deconditioning. This option is difficult for frail or older patients and is associated with high medical costs.4
In 1998, a novel method of treatment known as debridement, antibiotics, and implant retention (DAIR) was evaluated in a small, randomized controlled trial.5 This study used a unique antimicrobial approach: the administration of ciprofloxacin plus either rifampin or placebo for 3 to 6 months, combined with a single surgical debridement. Eliminating a second surgical procedure and largely relying on oral antimicrobials reduces surgical risks and decreases costs.4 Current guidelines endorse DAIR with rifampin and a second antibiotic for patients diagnosed with PJI within about 30 days of prosthesis implantation who have a well-fixed implant without evidence of a sinus tract.6 Clinical trial data demonstrate that this approach is > 90% effective in patients with a well-fixed prosthesis and acute staphylococcal PJI.3,7
Thus far, clinical trials examining this approach have been small and did not include veterans who are typically older and have more comorbidities.8 The Minneapolis Veterans Affairs Health Care System (MVAHCS) infectious disease section has implemented the rifampin-based DAIR approach for orthopedic device-related infections since this approach was first described in 1998 but has not systematically evaluated its effectiveness or whether there are areas for improvement.
METHODS
We conducted a retrospective analysis of patients who underwent DAIR combined with a rifampin-containing regimen at the MVAHCS from January 1, 2001, through June 30, 2021. Inclusion required a diagnosis of orthopedic device-related infection and treatment with DAIR followed by antimicrobial therapy that included rifampin for 1 to 6 months. PJI was defined by meeting ≥ 1 of the following criteria: (1) isolation of the same microorganism from ≥ 2 cultures from joint aspirates or intraoperative tissue specimens; (2) purulence surrounding the prosthesis at the time of surgery; (3) acute inflammation consistent with infection on histopathological examination or periprosthetic tissue; or (4) presence of a sinus tract communicating with the prosthesis.
All cases of orthopedic device infection managed with DAIR and rifampin were included, regardless of implant stability, age of the implant at the time of symptom onset, presence of a sinus tract, or infecting microorganism. Exclusion criteria included patients who started or finished PJI treatment at another facility, were lost to follow-up, discontinued rifampin, died within 1 year of completing antibiotic therapy due to reasons unrelated to treatment failure, received rifampin for < 50% of their antimicrobial treatment course, had complete hardware removal, or had < 1 year between the completion of antimicrobial therapy and the time of data collection.
Management of DAIR procedures at the MVAHCS involves evaluating the fixation of the prosthesis, tissue sampling for microbiological analysis, and thorough debridement of infected tissue. Following debridement, a course of IV antibiotics is administered before initiating oral antibiotic therapy. To protect against resistance, rifampin is combined with another antibiotic typically from the fluoroquinolone, tetracycline, or cephalosporin class. Current guidelines suggest 3 and 6 months of oral antibiotics for prosthetic hip and knee infections, respectively.6
Treatment Outcomes
The primary outcome was treatment success, defined as meeting all of the following: (1) lack of clinical signs and symptoms of infection; (2) absence of radiological signs of loosening or infection within 1 year after the conclusion of treatment; and (3) absence of additional PJI treatment interventions for the prosthesis of concern within 1 year after completing the original antibiotic treatment.
Treatment failure was defined as meeting any of the following: (1) recurrence of PJI (original strain or different microorganism) within 1 year after the completion of antibiotic therapy; (2) death attributed to PJI anytime after the initial debridement; (3) removal of the prosthetic joint within 1 year after the completion of antibiotic therapy; or (4) long-term antibiotic use to suppress the PJI after the completion of the initial antibiotic therapy.
Statistical Analysis
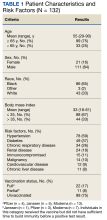
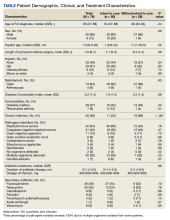
Descriptive statistics were used to define the baseline characteristics of patients receiving rifampin therapy for orthopedic implant infections at the MVAHCS. Variables analyzed were age, sex, race and ethnicity, type of implant, age of implant, duration of symptoms, comorbidities (diabetes and rheumatoid arthritis), and presence of chronic infection. Patients were classified as having a chronic infection if they received previous infection treatment (antibiotics or surgery) for the orthopedic device in question. We created this category because patients with persistent infection after a medical or surgical attempt at treatment are likely to have a higher probability of treatment failure compared with those with no prior therapy. Charlson Comorbidity Index was calculated using clinical information present at the onset of infection.9 Fisher exact test was used to assess differences between categorical variables, and an independent t test was used to assess differences in continuous variables. P < .05 indicated statistical significance.
To assess the ability of a rifampin-based regimen to achieve a cure of PJI, we grouped participants into 2 categories: those with an intent to cure strategy and those without intent to cure based on documentation in the electronic health record (EHR). Participants who were prescribed rifampin with the documented goal of prosthesis retention with no further suppressive antibiotics were included in the intent-to-cure group, the primary focus of this study. Those excluded from the intent-to-cure group were given rifampin and another antibiotic, but there was a documented plan of either ongoing chronic suppression or eventual explantation; these participants were placed in the without-intent-to-cure group. Analysis of treatment success and failure was limited to the intent-to-cure group, whereas both groups were included for assessment of adverse effects (AEs) and treatment duration. This project was reviewed by the MVAHCS Institutional Review Board and determined to be a quality improvement initiative and to not meet the definition of research, and as such did not require review; it was reviewed and approved by the MVAHCS Research and Development Committee.
RESULTS
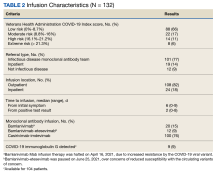
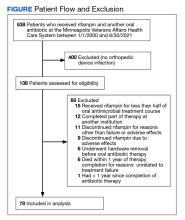
A total of 538 patients were identified who simultaneously received rifampin and another oral antibiotic between January 1, 2000, and June 30, 2021.
Forty-two participants (54%) had Staphylococcus aureus and 31 participants (40%) had coagulase-negative staphylococci infections, while 11 gram-negative organisms (14%) and 6 gram-positive anaerobic cocci (8%) infections were noted. Cutibacterium acnes and Streptococcus agalactiae were each found in 3 participants (4% of), and diphtheroids (not further identified) was found on 2 participants (3%). Candida albicans was identified in a single participant (1%), along with coagulase-negative staphylococci, and 2 participants (3%) had no identified organisms. There were multiple organisms isolated from 20 patients (26%).
Fifty participants had clear documentation in their EHR that cure of infection was the goal, meeting the criteria for the intent-to-cure group. The remaining 28 participants were placed in the without-intent-to-cure group. Success and failure rates were only measured in the intent-to-cure group, as by definition the without-intent-to-cure group patients would meet the criteria for failure (removal of prosthesis or long-term antibiotic use). The without-intent-to-cure group had a higher median age than the intent-to-cure group (69 years vs 64 years, P = .24) and a higher proportion of male participants (96% vs 80%, P = .09). The median (IQR) implant age of 11 months (1.0-50.5) in the without-intent-to-cure group was also higher than the median implant age of 1 month (0.6-22.0) in the primary group (P = .22). In the without-intent-to-cure group, 19 participants (68%) had a chronic infection, compared with 11 (22%) in the intent-to-cure group (P < .001).
The mean (SD) Charlson Comorbidity Index in the without-intent-to-cure group was 2.5 (1.3) compared with 1.9 (1.4) in the intent-to-cure group (P = .09). There was no significant difference in the type of implant or microbiology of the infecting organism between the 2 groups, although it should be noted that in the intent-to-cure group, 48 patients (96%) had Staphylococcus aureus or coagulase-negative staphylococci isolated.
The median (IQR) dosage of rifampin was 600 mg (300-900). The secondary oral antibiotics used most often were 36 fluoroquinolones (46%) followed by 20 tetracyclines (26%), 6 cephalosporins (8%), and 6 penicillins (8%). Additionally, 6 participants (8%) received IV vancomycin, and 1 participant (1%) was given an oral antifungal in addition to a fluoroquinolone because cultures revealed bacterial and fungal growth. The median (IQR) duration of antimicrobial therapy was 3 months (1.4-3.0). The mean (SD) duration of antimicrobial therapy was 3.6 (2.4) months for TKA infections and 2.4 (0.9) months for THA infections.
Clinical Outcome
Forty-one intent-to-cure group participants (82%) experienced treatment success. We further subdivided the intent-to-cure group by implant age. Participants whose implant was < 2 months old had a success rate of 93%, whereas patients whose implant was older had a success rate of 65% (P = .02).
Secondary Outcomes
The median (IQR) duration of antimicrobial treatment was 3 months (1.4-3.0) for the 38 patients with TKA-related infections and 3 months (1.4-6.0) for the 29 patients with THA infections. AEs were recorded in 24 (31%) of all study participants. Of those with AEs, the average number reported per patient was 1.6. Diarrhea, gastric upset, and nausea were each reported 7 times, accounting for 87% of all recorded AEs. Five participants reported having a rash while on antibiotics, and 2 experienced dysgeusia. One participant reported developing a yeast infection and another experienced vaginitis.
DISCUSSION
Among patients with orthopedic implant infections treated with intent to cure using a rifampin-containing antibiotic regimen at the MVAHCS, 82% had clinical success. Although this is lower than the success rates reported in clinical trials, this is not entirely unexpected.5,7 In most clinical trials studying DAIR and rifampin for PJI, patients are excluded if they do not have an acute staphylococcal infection in the setting of a well-fixed prosthesis without evidence of a sinus tract. Such exclusion criteria were not present in our retrospective study, which was designed to evaluate the real-world practice patterns at this facility. The population at the US Department of Veterans Affairs (VA) is older, more frail, and with more comorbid conditions than populations in prior studies. It is possible that patients with characteristics that would have caused them to be excluded from a clinical trial would be less likely to receive rifampin therapy with the intent to cure. This is suggested by the significantly higher prevalence of chronic infections (68%) in the without-intent-to-cure group compared with 22% in the intent-to-cure group. However, there were reasonably high proportions of participants included in the intent-to-cure group who did have conditions that would have led to their exclusion from prior trials, such as chronic infection (22%) and implant age ≥ 2 months (40%).
When evaluating participants by the age of their implant, treatment success rose to 93% for patients with implants < 2 months old compared with 65% for patients with older implants. This suggests that participants with a newer implant or more recent infection have a greater likelihood of successful treatment, which is consistent with the results of previous clinical trials.5,10 Considering how difficult multiple surgeries can be for older adult patients with comorbidities, we suggest that DAIR with a rifampin-containing regimen be considered as the primary treatment option for early PJIs at the MVAHCS. We also note inconsistent adherence to IDSA treatment guidelines on rifampin therapy, in that patients without intent to cure were prescribed a regimen including rifampin. This may reflect appropriate variability in the care of individual patients but may also offer an opportunity to change processes to improve care.
Limitations
Our analysis has limitations. As with any retrospective study evaluating the efficacy of a specific antibiotic, we were not able to attribute specific outcomes to the antibiotic of interest. Since the choice of antibiotics was left to the treating health care practitioner, therapy was not standardized, and because this was a retrospective study, causal relationships could not be inferred. Our analysis was also limited by the lack of intent to cure in 28 participants (36%), which could be an indication of practitioner bias in therapy selection or characteristic differences between the 2 groups. We looked for signs of infection failure 1 year after the completion of antimicrobial therapy, but longer follow-up could have led to higher rates of failure. Also, while participants’ infections were considered cured if they never sought further medical care for the infection at the MVAHCS, it is possible that patients could have sought care at another facility. We note that 9 patients were excluded because they were unable to complete a treatment course due to rifampin AEs, meaning that the success rates reported here reflect the success that may be expected if a patient can tolerate and complete a rifampin-based regimen. This study was conducted in a single VA hospital and may not be generalizable to nonveterans or veterans seeking care at other facilities.
Conclusions
DAIR followed by a short course of IV antibiotics and an oral regimen including rifampin and another antimicrobial is a reasonable option for veterans with acute staphylococcal orthopedic device infections at the MVAHCS. Patients with a well-placed prosthesis and an acute infection seem especially well suited for this treatment, and treatment with intent to cure should be pursued in patients who meet the criteria for rifampin therapy.
Acknowledgments
We thank Erik Stensgard, PharmD, for assistance in compiling the list of patients receiving rifampin and another antimicrobial.
1. Maradit Kremers H, Larson DR, Crowson CS, et al. Prevalence of total hip and knee replacement in the United States. J Bone Joint Surg Am. 2015;97(17):1386-1397. doi:10.2106/JBJS.N.01141
2. Kapadia BH, Berg RA, Daley JA, Fritz J, Bhave A, Mont MA. Periprosthetic joint infection. Lancet. 2016;387(10016):386-394. doi:10.1016/S0140-6736(14)61798-0
3. Zhan C, Kaczmarek R, Loyo-Berrios N, Sangl J, Bright RA. Incidence and short-term outcomes of primary and revision hip replacement in the United States. J Bone Joint Surg Am. 2007;89(3):526-533. doi:10.2106/JBJS.F.00952
4. Fisman DN, Reilly DT, Karchmer AW, Goldie SJ. Clinical effectiveness and cost-effectiveness of 2 management strategies for infected total hip arthroplasty in the elderly. Clin Infect Dis. 2001;32(3):419-430. doi:10.1086/318502
5. Zimmerli W, Widmer AF, Blatter M, Frei R, Ochsner PE. Role of rifampin for treatment of orthopedic implant-related staphylococcal infections: a randomized controlled trial. Foreign-Body Infection (FBI) Study Group. JAMA. 1998;279(19):1537-1541. doi:10.1001/jama.279.19.1537
6. Osmon DR, Berbari EF, Berendt AR, et al. Diagnosis and management of prosthetic joint infection: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis. 2013;56(1):e1-e25. doi:10.1093/cid/cis803
7. Lora-Tamayo J, Euba G, Cobo J, et al. Short- versus long-duration levofloxacin plus rifampicin for acute staphylococcal prosthetic joint infection managed with implant retention: a randomised clinical trial. Int J Antimicrob Agents. 2016;48(3):310-316. doi:10.1016/j.ijantimicag.2016.05.021
8. Agha Z, Lofgren RP, VanRuiswyk JV, Layde PM. Are patients at Veterans Affairs medical centers sicker? A comparative analysis of health status and medical resource use. Arch Intern Med. 2000;160(21):3252-3257. doi:10.1001/archinte.160.21.3252
9. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373-383. doi:10.1016/0021-9681(87)90171-8
10. Vilchez F, Martínez-Pastor JC, García-Ramiro S, et al. Outcome and predictors of treatment failure in early post-surgical prosthetic joint infections due to Staphylococcus aureus treated with debridement. Clin Microbiol Infect. 2011;17(3):439-444. doi:10.1111/j.1469-0691.2010.03244.x
Orthopedic implants are frequently used to repair fractures and replace joints. The number of total joint replacements is high, with > 1 million total hip (THA) and total knee (TKA) arthroplasties performed in the United States each year.1 While most joint arthroplasties are successful and significantly improve patient quality of life, a small proportion become infected.2 Prosthetic joint infection (PJI) causes substantial morbidity and mortality, particularly among older patients, and is difficult and costly to treat.3
The historic gold standard treatment for PJI is a 2-stage replacement, wherein the prosthesis is removed in one procedure and a new prosthesis is implanted in a second procedure after an extended course of antibiotics. This approach requires the patient to undergo 2 major procedures and spend considerable time without a functioning prosthesis, contributing to immobility and deconditioning. This option is difficult for frail or older patients and is associated with high medical costs.4
In 1998, a novel method of treatment known as debridement, antibiotics, and implant retention (DAIR) was evaluated in a small, randomized controlled trial.5 This study used a unique antimicrobial approach: the administration of ciprofloxacin plus either rifampin or placebo for 3 to 6 months, combined with a single surgical debridement. Eliminating a second surgical procedure and largely relying on oral antimicrobials reduces surgical risks and decreases costs.4 Current guidelines endorse DAIR with rifampin and a second antibiotic for patients diagnosed with PJI within about 30 days of prosthesis implantation who have a well-fixed implant without evidence of a sinus tract.6 Clinical trial data demonstrate that this approach is > 90% effective in patients with a well-fixed prosthesis and acute staphylococcal PJI.3,7
Thus far, clinical trials examining this approach have been small and did not include veterans who are typically older and have more comorbidities.8 The Minneapolis Veterans Affairs Health Care System (MVAHCS) infectious disease section has implemented the rifampin-based DAIR approach for orthopedic device-related infections since this approach was first described in 1998 but has not systematically evaluated its effectiveness or whether there are areas for improvement.
METHODS
We conducted a retrospective analysis of patients who underwent DAIR combined with a rifampin-containing regimen at the MVAHCS from January 1, 2001, through June 30, 2021. Inclusion required a diagnosis of orthopedic device-related infection and treatment with DAIR followed by antimicrobial therapy that included rifampin for 1 to 6 months. PJI was defined by meeting ≥ 1 of the following criteria: (1) isolation of the same microorganism from ≥ 2 cultures from joint aspirates or intraoperative tissue specimens; (2) purulence surrounding the prosthesis at the time of surgery; (3) acute inflammation consistent with infection on histopathological examination or periprosthetic tissue; or (4) presence of a sinus tract communicating with the prosthesis.
All cases of orthopedic device infection managed with DAIR and rifampin were included, regardless of implant stability, age of the implant at the time of symptom onset, presence of a sinus tract, or infecting microorganism. Exclusion criteria included patients who started or finished PJI treatment at another facility, were lost to follow-up, discontinued rifampin, died within 1 year of completing antibiotic therapy due to reasons unrelated to treatment failure, received rifampin for < 50% of their antimicrobial treatment course, had complete hardware removal, or had < 1 year between the completion of antimicrobial therapy and the time of data collection.
Management of DAIR procedures at the MVAHCS involves evaluating the fixation of the prosthesis, tissue sampling for microbiological analysis, and thorough debridement of infected tissue. Following debridement, a course of IV antibiotics is administered before initiating oral antibiotic therapy. To protect against resistance, rifampin is combined with another antibiotic typically from the fluoroquinolone, tetracycline, or cephalosporin class. Current guidelines suggest 3 and 6 months of oral antibiotics for prosthetic hip and knee infections, respectively.6
Treatment Outcomes
The primary outcome was treatment success, defined as meeting all of the following: (1) lack of clinical signs and symptoms of infection; (2) absence of radiological signs of loosening or infection within 1 year after the conclusion of treatment; and (3) absence of additional PJI treatment interventions for the prosthesis of concern within 1 year after completing the original antibiotic treatment.
Treatment failure was defined as meeting any of the following: (1) recurrence of PJI (original strain or different microorganism) within 1 year after the completion of antibiotic therapy; (2) death attributed to PJI anytime after the initial debridement; (3) removal of the prosthetic joint within 1 year after the completion of antibiotic therapy; or (4) long-term antibiotic use to suppress the PJI after the completion of the initial antibiotic therapy.
Statistical Analysis
Descriptive statistics were used to define the baseline characteristics of patients receiving rifampin therapy for orthopedic implant infections at the MVAHCS. Variables analyzed were age, sex, race and ethnicity, type of implant, age of implant, duration of symptoms, comorbidities (diabetes and rheumatoid arthritis), and presence of chronic infection. Patients were classified as having a chronic infection if they received previous infection treatment (antibiotics or surgery) for the orthopedic device in question. We created this category because patients with persistent infection after a medical or surgical attempt at treatment are likely to have a higher probability of treatment failure compared with those with no prior therapy. Charlson Comorbidity Index was calculated using clinical information present at the onset of infection.9 Fisher exact test was used to assess differences between categorical variables, and an independent t test was used to assess differences in continuous variables. P < .05 indicated statistical significance.
To assess the ability of a rifampin-based regimen to achieve a cure of PJI, we grouped participants into 2 categories: those with an intent to cure strategy and those without intent to cure based on documentation in the electronic health record (EHR). Participants who were prescribed rifampin with the documented goal of prosthesis retention with no further suppressive antibiotics were included in the intent-to-cure group, the primary focus of this study. Those excluded from the intent-to-cure group were given rifampin and another antibiotic, but there was a documented plan of either ongoing chronic suppression or eventual explantation; these participants were placed in the without-intent-to-cure group. Analysis of treatment success and failure was limited to the intent-to-cure group, whereas both groups were included for assessment of adverse effects (AEs) and treatment duration. This project was reviewed by the MVAHCS Institutional Review Board and determined to be a quality improvement initiative and to not meet the definition of research, and as such did not require review; it was reviewed and approved by the MVAHCS Research and Development Committee.
RESULTS
A total of 538 patients were identified who simultaneously received rifampin and another oral antibiotic between January 1, 2000, and June 30, 2021.
Forty-two participants (54%) had Staphylococcus aureus and 31 participants (40%) had coagulase-negative staphylococci infections, while 11 gram-negative organisms (14%) and 6 gram-positive anaerobic cocci (8%) infections were noted. Cutibacterium acnes and Streptococcus agalactiae were each found in 3 participants (4% of), and diphtheroids (not further identified) was found on 2 participants (3%). Candida albicans was identified in a single participant (1%), along with coagulase-negative staphylococci, and 2 participants (3%) had no identified organisms. There were multiple organisms isolated from 20 patients (26%).
Fifty participants had clear documentation in their EHR that cure of infection was the goal, meeting the criteria for the intent-to-cure group. The remaining 28 participants were placed in the without-intent-to-cure group. Success and failure rates were only measured in the intent-to-cure group, as by definition the without-intent-to-cure group patients would meet the criteria for failure (removal of prosthesis or long-term antibiotic use). The without-intent-to-cure group had a higher median age than the intent-to-cure group (69 years vs 64 years, P = .24) and a higher proportion of male participants (96% vs 80%, P = .09). The median (IQR) implant age of 11 months (1.0-50.5) in the without-intent-to-cure group was also higher than the median implant age of 1 month (0.6-22.0) in the primary group (P = .22). In the without-intent-to-cure group, 19 participants (68%) had a chronic infection, compared with 11 (22%) in the intent-to-cure group (P < .001).
The mean (SD) Charlson Comorbidity Index in the without-intent-to-cure group was 2.5 (1.3) compared with 1.9 (1.4) in the intent-to-cure group (P = .09). There was no significant difference in the type of implant or microbiology of the infecting organism between the 2 groups, although it should be noted that in the intent-to-cure group, 48 patients (96%) had Staphylococcus aureus or coagulase-negative staphylococci isolated.
The median (IQR) dosage of rifampin was 600 mg (300-900). The secondary oral antibiotics used most often were 36 fluoroquinolones (46%) followed by 20 tetracyclines (26%), 6 cephalosporins (8%), and 6 penicillins (8%). Additionally, 6 participants (8%) received IV vancomycin, and 1 participant (1%) was given an oral antifungal in addition to a fluoroquinolone because cultures revealed bacterial and fungal growth. The median (IQR) duration of antimicrobial therapy was 3 months (1.4-3.0). The mean (SD) duration of antimicrobial therapy was 3.6 (2.4) months for TKA infections and 2.4 (0.9) months for THA infections.
Clinical Outcome
Forty-one intent-to-cure group participants (82%) experienced treatment success. We further subdivided the intent-to-cure group by implant age. Participants whose implant was < 2 months old had a success rate of 93%, whereas patients whose implant was older had a success rate of 65% (P = .02).
Secondary Outcomes
The median (IQR) duration of antimicrobial treatment was 3 months (1.4-3.0) for the 38 patients with TKA-related infections and 3 months (1.4-6.0) for the 29 patients with THA infections. AEs were recorded in 24 (31%) of all study participants. Of those with AEs, the average number reported per patient was 1.6. Diarrhea, gastric upset, and nausea were each reported 7 times, accounting for 87% of all recorded AEs. Five participants reported having a rash while on antibiotics, and 2 experienced dysgeusia. One participant reported developing a yeast infection and another experienced vaginitis.
DISCUSSION
Among patients with orthopedic implant infections treated with intent to cure using a rifampin-containing antibiotic regimen at the MVAHCS, 82% had clinical success. Although this is lower than the success rates reported in clinical trials, this is not entirely unexpected.5,7 In most clinical trials studying DAIR and rifampin for PJI, patients are excluded if they do not have an acute staphylococcal infection in the setting of a well-fixed prosthesis without evidence of a sinus tract. Such exclusion criteria were not present in our retrospective study, which was designed to evaluate the real-world practice patterns at this facility. The population at the US Department of Veterans Affairs (VA) is older, more frail, and with more comorbid conditions than populations in prior studies. It is possible that patients with characteristics that would have caused them to be excluded from a clinical trial would be less likely to receive rifampin therapy with the intent to cure. This is suggested by the significantly higher prevalence of chronic infections (68%) in the without-intent-to-cure group compared with 22% in the intent-to-cure group. However, there were reasonably high proportions of participants included in the intent-to-cure group who did have conditions that would have led to their exclusion from prior trials, such as chronic infection (22%) and implant age ≥ 2 months (40%).
When evaluating participants by the age of their implant, treatment success rose to 93% for patients with implants < 2 months old compared with 65% for patients with older implants. This suggests that participants with a newer implant or more recent infection have a greater likelihood of successful treatment, which is consistent with the results of previous clinical trials.5,10 Considering how difficult multiple surgeries can be for older adult patients with comorbidities, we suggest that DAIR with a rifampin-containing regimen be considered as the primary treatment option for early PJIs at the MVAHCS. We also note inconsistent adherence to IDSA treatment guidelines on rifampin therapy, in that patients without intent to cure were prescribed a regimen including rifampin. This may reflect appropriate variability in the care of individual patients but may also offer an opportunity to change processes to improve care.
Limitations
Our analysis has limitations. As with any retrospective study evaluating the efficacy of a specific antibiotic, we were not able to attribute specific outcomes to the antibiotic of interest. Since the choice of antibiotics was left to the treating health care practitioner, therapy was not standardized, and because this was a retrospective study, causal relationships could not be inferred. Our analysis was also limited by the lack of intent to cure in 28 participants (36%), which could be an indication of practitioner bias in therapy selection or characteristic differences between the 2 groups. We looked for signs of infection failure 1 year after the completion of antimicrobial therapy, but longer follow-up could have led to higher rates of failure. Also, while participants’ infections were considered cured if they never sought further medical care for the infection at the MVAHCS, it is possible that patients could have sought care at another facility. We note that 9 patients were excluded because they were unable to complete a treatment course due to rifampin AEs, meaning that the success rates reported here reflect the success that may be expected if a patient can tolerate and complete a rifampin-based regimen. This study was conducted in a single VA hospital and may not be generalizable to nonveterans or veterans seeking care at other facilities.
Conclusions
DAIR followed by a short course of IV antibiotics and an oral regimen including rifampin and another antimicrobial is a reasonable option for veterans with acute staphylococcal orthopedic device infections at the MVAHCS. Patients with a well-placed prosthesis and an acute infection seem especially well suited for this treatment, and treatment with intent to cure should be pursued in patients who meet the criteria for rifampin therapy.
Acknowledgments
We thank Erik Stensgard, PharmD, for assistance in compiling the list of patients receiving rifampin and another antimicrobial.
Orthopedic implants are frequently used to repair fractures and replace joints. The number of total joint replacements is high, with > 1 million total hip (THA) and total knee (TKA) arthroplasties performed in the United States each year.1 While most joint arthroplasties are successful and significantly improve patient quality of life, a small proportion become infected.2 Prosthetic joint infection (PJI) causes substantial morbidity and mortality, particularly among older patients, and is difficult and costly to treat.3
The historic gold standard treatment for PJI is a 2-stage replacement, wherein the prosthesis is removed in one procedure and a new prosthesis is implanted in a second procedure after an extended course of antibiotics. This approach requires the patient to undergo 2 major procedures and spend considerable time without a functioning prosthesis, contributing to immobility and deconditioning. This option is difficult for frail or older patients and is associated with high medical costs.4
In 1998, a novel method of treatment known as debridement, antibiotics, and implant retention (DAIR) was evaluated in a small, randomized controlled trial.5 This study used a unique antimicrobial approach: the administration of ciprofloxacin plus either rifampin or placebo for 3 to 6 months, combined with a single surgical debridement. Eliminating a second surgical procedure and largely relying on oral antimicrobials reduces surgical risks and decreases costs.4 Current guidelines endorse DAIR with rifampin and a second antibiotic for patients diagnosed with PJI within about 30 days of prosthesis implantation who have a well-fixed implant without evidence of a sinus tract.6 Clinical trial data demonstrate that this approach is > 90% effective in patients with a well-fixed prosthesis and acute staphylococcal PJI.3,7
Thus far, clinical trials examining this approach have been small and did not include veterans who are typically older and have more comorbidities.8 The Minneapolis Veterans Affairs Health Care System (MVAHCS) infectious disease section has implemented the rifampin-based DAIR approach for orthopedic device-related infections since this approach was first described in 1998 but has not systematically evaluated its effectiveness or whether there are areas for improvement.
METHODS
We conducted a retrospective analysis of patients who underwent DAIR combined with a rifampin-containing regimen at the MVAHCS from January 1, 2001, through June 30, 2021. Inclusion required a diagnosis of orthopedic device-related infection and treatment with DAIR followed by antimicrobial therapy that included rifampin for 1 to 6 months. PJI was defined by meeting ≥ 1 of the following criteria: (1) isolation of the same microorganism from ≥ 2 cultures from joint aspirates or intraoperative tissue specimens; (2) purulence surrounding the prosthesis at the time of surgery; (3) acute inflammation consistent with infection on histopathological examination or periprosthetic tissue; or (4) presence of a sinus tract communicating with the prosthesis.
All cases of orthopedic device infection managed with DAIR and rifampin were included, regardless of implant stability, age of the implant at the time of symptom onset, presence of a sinus tract, or infecting microorganism. Exclusion criteria included patients who started or finished PJI treatment at another facility, were lost to follow-up, discontinued rifampin, died within 1 year of completing antibiotic therapy due to reasons unrelated to treatment failure, received rifampin for < 50% of their antimicrobial treatment course, had complete hardware removal, or had < 1 year between the completion of antimicrobial therapy and the time of data collection.
Management of DAIR procedures at the MVAHCS involves evaluating the fixation of the prosthesis, tissue sampling for microbiological analysis, and thorough debridement of infected tissue. Following debridement, a course of IV antibiotics is administered before initiating oral antibiotic therapy. To protect against resistance, rifampin is combined with another antibiotic typically from the fluoroquinolone, tetracycline, or cephalosporin class. Current guidelines suggest 3 and 6 months of oral antibiotics for prosthetic hip and knee infections, respectively.6
Treatment Outcomes
The primary outcome was treatment success, defined as meeting all of the following: (1) lack of clinical signs and symptoms of infection; (2) absence of radiological signs of loosening or infection within 1 year after the conclusion of treatment; and (3) absence of additional PJI treatment interventions for the prosthesis of concern within 1 year after completing the original antibiotic treatment.
Treatment failure was defined as meeting any of the following: (1) recurrence of PJI (original strain or different microorganism) within 1 year after the completion of antibiotic therapy; (2) death attributed to PJI anytime after the initial debridement; (3) removal of the prosthetic joint within 1 year after the completion of antibiotic therapy; or (4) long-term antibiotic use to suppress the PJI after the completion of the initial antibiotic therapy.
Statistical Analysis
Descriptive statistics were used to define the baseline characteristics of patients receiving rifampin therapy for orthopedic implant infections at the MVAHCS. Variables analyzed were age, sex, race and ethnicity, type of implant, age of implant, duration of symptoms, comorbidities (diabetes and rheumatoid arthritis), and presence of chronic infection. Patients were classified as having a chronic infection if they received previous infection treatment (antibiotics or surgery) for the orthopedic device in question. We created this category because patients with persistent infection after a medical or surgical attempt at treatment are likely to have a higher probability of treatment failure compared with those with no prior therapy. Charlson Comorbidity Index was calculated using clinical information present at the onset of infection.9 Fisher exact test was used to assess differences between categorical variables, and an independent t test was used to assess differences in continuous variables. P < .05 indicated statistical significance.
To assess the ability of a rifampin-based regimen to achieve a cure of PJI, we grouped participants into 2 categories: those with an intent to cure strategy and those without intent to cure based on documentation in the electronic health record (EHR). Participants who were prescribed rifampin with the documented goal of prosthesis retention with no further suppressive antibiotics were included in the intent-to-cure group, the primary focus of this study. Those excluded from the intent-to-cure group were given rifampin and another antibiotic, but there was a documented plan of either ongoing chronic suppression or eventual explantation; these participants were placed in the without-intent-to-cure group. Analysis of treatment success and failure was limited to the intent-to-cure group, whereas both groups were included for assessment of adverse effects (AEs) and treatment duration. This project was reviewed by the MVAHCS Institutional Review Board and determined to be a quality improvement initiative and to not meet the definition of research, and as such did not require review; it was reviewed and approved by the MVAHCS Research and Development Committee.
RESULTS
A total of 538 patients were identified who simultaneously received rifampin and another oral antibiotic between January 1, 2000, and June 30, 2021.
Forty-two participants (54%) had Staphylococcus aureus and 31 participants (40%) had coagulase-negative staphylococci infections, while 11 gram-negative organisms (14%) and 6 gram-positive anaerobic cocci (8%) infections were noted. Cutibacterium acnes and Streptococcus agalactiae were each found in 3 participants (4% of), and diphtheroids (not further identified) was found on 2 participants (3%). Candida albicans was identified in a single participant (1%), along with coagulase-negative staphylococci, and 2 participants (3%) had no identified organisms. There were multiple organisms isolated from 20 patients (26%).
Fifty participants had clear documentation in their EHR that cure of infection was the goal, meeting the criteria for the intent-to-cure group. The remaining 28 participants were placed in the without-intent-to-cure group. Success and failure rates were only measured in the intent-to-cure group, as by definition the without-intent-to-cure group patients would meet the criteria for failure (removal of prosthesis or long-term antibiotic use). The without-intent-to-cure group had a higher median age than the intent-to-cure group (69 years vs 64 years, P = .24) and a higher proportion of male participants (96% vs 80%, P = .09). The median (IQR) implant age of 11 months (1.0-50.5) in the without-intent-to-cure group was also higher than the median implant age of 1 month (0.6-22.0) in the primary group (P = .22). In the without-intent-to-cure group, 19 participants (68%) had a chronic infection, compared with 11 (22%) in the intent-to-cure group (P < .001).
The mean (SD) Charlson Comorbidity Index in the without-intent-to-cure group was 2.5 (1.3) compared with 1.9 (1.4) in the intent-to-cure group (P = .09). There was no significant difference in the type of implant or microbiology of the infecting organism between the 2 groups, although it should be noted that in the intent-to-cure group, 48 patients (96%) had Staphylococcus aureus or coagulase-negative staphylococci isolated.
The median (IQR) dosage of rifampin was 600 mg (300-900). The secondary oral antibiotics used most often were 36 fluoroquinolones (46%) followed by 20 tetracyclines (26%), 6 cephalosporins (8%), and 6 penicillins (8%). Additionally, 6 participants (8%) received IV vancomycin, and 1 participant (1%) was given an oral antifungal in addition to a fluoroquinolone because cultures revealed bacterial and fungal growth. The median (IQR) duration of antimicrobial therapy was 3 months (1.4-3.0). The mean (SD) duration of antimicrobial therapy was 3.6 (2.4) months for TKA infections and 2.4 (0.9) months for THA infections.
Clinical Outcome
Forty-one intent-to-cure group participants (82%) experienced treatment success. We further subdivided the intent-to-cure group by implant age. Participants whose implant was < 2 months old had a success rate of 93%, whereas patients whose implant was older had a success rate of 65% (P = .02).
Secondary Outcomes
The median (IQR) duration of antimicrobial treatment was 3 months (1.4-3.0) for the 38 patients with TKA-related infections and 3 months (1.4-6.0) for the 29 patients with THA infections. AEs were recorded in 24 (31%) of all study participants. Of those with AEs, the average number reported per patient was 1.6. Diarrhea, gastric upset, and nausea were each reported 7 times, accounting for 87% of all recorded AEs. Five participants reported having a rash while on antibiotics, and 2 experienced dysgeusia. One participant reported developing a yeast infection and another experienced vaginitis.
DISCUSSION
Among patients with orthopedic implant infections treated with intent to cure using a rifampin-containing antibiotic regimen at the MVAHCS, 82% had clinical success. Although this is lower than the success rates reported in clinical trials, this is not entirely unexpected.5,7 In most clinical trials studying DAIR and rifampin for PJI, patients are excluded if they do not have an acute staphylococcal infection in the setting of a well-fixed prosthesis without evidence of a sinus tract. Such exclusion criteria were not present in our retrospective study, which was designed to evaluate the real-world practice patterns at this facility. The population at the US Department of Veterans Affairs (VA) is older, more frail, and with more comorbid conditions than populations in prior studies. It is possible that patients with characteristics that would have caused them to be excluded from a clinical trial would be less likely to receive rifampin therapy with the intent to cure. This is suggested by the significantly higher prevalence of chronic infections (68%) in the without-intent-to-cure group compared with 22% in the intent-to-cure group. However, there were reasonably high proportions of participants included in the intent-to-cure group who did have conditions that would have led to their exclusion from prior trials, such as chronic infection (22%) and implant age ≥ 2 months (40%).
When evaluating participants by the age of their implant, treatment success rose to 93% for patients with implants < 2 months old compared with 65% for patients with older implants. This suggests that participants with a newer implant or more recent infection have a greater likelihood of successful treatment, which is consistent with the results of previous clinical trials.5,10 Considering how difficult multiple surgeries can be for older adult patients with comorbidities, we suggest that DAIR with a rifampin-containing regimen be considered as the primary treatment option for early PJIs at the MVAHCS. We also note inconsistent adherence to IDSA treatment guidelines on rifampin therapy, in that patients without intent to cure were prescribed a regimen including rifampin. This may reflect appropriate variability in the care of individual patients but may also offer an opportunity to change processes to improve care.
Limitations
Our analysis has limitations. As with any retrospective study evaluating the efficacy of a specific antibiotic, we were not able to attribute specific outcomes to the antibiotic of interest. Since the choice of antibiotics was left to the treating health care practitioner, therapy was not standardized, and because this was a retrospective study, causal relationships could not be inferred. Our analysis was also limited by the lack of intent to cure in 28 participants (36%), which could be an indication of practitioner bias in therapy selection or characteristic differences between the 2 groups. We looked for signs of infection failure 1 year after the completion of antimicrobial therapy, but longer follow-up could have led to higher rates of failure. Also, while participants’ infections were considered cured if they never sought further medical care for the infection at the MVAHCS, it is possible that patients could have sought care at another facility. We note that 9 patients were excluded because they were unable to complete a treatment course due to rifampin AEs, meaning that the success rates reported here reflect the success that may be expected if a patient can tolerate and complete a rifampin-based regimen. This study was conducted in a single VA hospital and may not be generalizable to nonveterans or veterans seeking care at other facilities.
Conclusions
DAIR followed by a short course of IV antibiotics and an oral regimen including rifampin and another antimicrobial is a reasonable option for veterans with acute staphylococcal orthopedic device infections at the MVAHCS. Patients with a well-placed prosthesis and an acute infection seem especially well suited for this treatment, and treatment with intent to cure should be pursued in patients who meet the criteria for rifampin therapy.
Acknowledgments
We thank Erik Stensgard, PharmD, for assistance in compiling the list of patients receiving rifampin and another antimicrobial.
1. Maradit Kremers H, Larson DR, Crowson CS, et al. Prevalence of total hip and knee replacement in the United States. J Bone Joint Surg Am. 2015;97(17):1386-1397. doi:10.2106/JBJS.N.01141
2. Kapadia BH, Berg RA, Daley JA, Fritz J, Bhave A, Mont MA. Periprosthetic joint infection. Lancet. 2016;387(10016):386-394. doi:10.1016/S0140-6736(14)61798-0
3. Zhan C, Kaczmarek R, Loyo-Berrios N, Sangl J, Bright RA. Incidence and short-term outcomes of primary and revision hip replacement in the United States. J Bone Joint Surg Am. 2007;89(3):526-533. doi:10.2106/JBJS.F.00952
4. Fisman DN, Reilly DT, Karchmer AW, Goldie SJ. Clinical effectiveness and cost-effectiveness of 2 management strategies for infected total hip arthroplasty in the elderly. Clin Infect Dis. 2001;32(3):419-430. doi:10.1086/318502
5. Zimmerli W, Widmer AF, Blatter M, Frei R, Ochsner PE. Role of rifampin for treatment of orthopedic implant-related staphylococcal infections: a randomized controlled trial. Foreign-Body Infection (FBI) Study Group. JAMA. 1998;279(19):1537-1541. doi:10.1001/jama.279.19.1537
6. Osmon DR, Berbari EF, Berendt AR, et al. Diagnosis and management of prosthetic joint infection: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis. 2013;56(1):e1-e25. doi:10.1093/cid/cis803
7. Lora-Tamayo J, Euba G, Cobo J, et al. Short- versus long-duration levofloxacin plus rifampicin for acute staphylococcal prosthetic joint infection managed with implant retention: a randomised clinical trial. Int J Antimicrob Agents. 2016;48(3):310-316. doi:10.1016/j.ijantimicag.2016.05.021
8. Agha Z, Lofgren RP, VanRuiswyk JV, Layde PM. Are patients at Veterans Affairs medical centers sicker? A comparative analysis of health status and medical resource use. Arch Intern Med. 2000;160(21):3252-3257. doi:10.1001/archinte.160.21.3252
9. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373-383. doi:10.1016/0021-9681(87)90171-8
10. Vilchez F, Martínez-Pastor JC, García-Ramiro S, et al. Outcome and predictors of treatment failure in early post-surgical prosthetic joint infections due to Staphylococcus aureus treated with debridement. Clin Microbiol Infect. 2011;17(3):439-444. doi:10.1111/j.1469-0691.2010.03244.x
1. Maradit Kremers H, Larson DR, Crowson CS, et al. Prevalence of total hip and knee replacement in the United States. J Bone Joint Surg Am. 2015;97(17):1386-1397. doi:10.2106/JBJS.N.01141
2. Kapadia BH, Berg RA, Daley JA, Fritz J, Bhave A, Mont MA. Periprosthetic joint infection. Lancet. 2016;387(10016):386-394. doi:10.1016/S0140-6736(14)61798-0
3. Zhan C, Kaczmarek R, Loyo-Berrios N, Sangl J, Bright RA. Incidence and short-term outcomes of primary and revision hip replacement in the United States. J Bone Joint Surg Am. 2007;89(3):526-533. doi:10.2106/JBJS.F.00952
4. Fisman DN, Reilly DT, Karchmer AW, Goldie SJ. Clinical effectiveness and cost-effectiveness of 2 management strategies for infected total hip arthroplasty in the elderly. Clin Infect Dis. 2001;32(3):419-430. doi:10.1086/318502
5. Zimmerli W, Widmer AF, Blatter M, Frei R, Ochsner PE. Role of rifampin for treatment of orthopedic implant-related staphylococcal infections: a randomized controlled trial. Foreign-Body Infection (FBI) Study Group. JAMA. 1998;279(19):1537-1541. doi:10.1001/jama.279.19.1537
6. Osmon DR, Berbari EF, Berendt AR, et al. Diagnosis and management of prosthetic joint infection: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis. 2013;56(1):e1-e25. doi:10.1093/cid/cis803
7. Lora-Tamayo J, Euba G, Cobo J, et al. Short- versus long-duration levofloxacin plus rifampicin for acute staphylococcal prosthetic joint infection managed with implant retention: a randomised clinical trial. Int J Antimicrob Agents. 2016;48(3):310-316. doi:10.1016/j.ijantimicag.2016.05.021
8. Agha Z, Lofgren RP, VanRuiswyk JV, Layde PM. Are patients at Veterans Affairs medical centers sicker? A comparative analysis of health status and medical resource use. Arch Intern Med. 2000;160(21):3252-3257. doi:10.1001/archinte.160.21.3252
9. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373-383. doi:10.1016/0021-9681(87)90171-8
10. Vilchez F, Martínez-Pastor JC, García-Ramiro S, et al. Outcome and predictors of treatment failure in early post-surgical prosthetic joint infections due to Staphylococcus aureus treated with debridement. Clin Microbiol Infect. 2011;17(3):439-444. doi:10.1111/j.1469-0691.2010.03244.x
Salute to Service Dogs
The psychological and moral comfort of a presence at once humble and understanding—this is the greatest benefit that the dog has bestowed upon man. Percy Bysshe Shelley
The nature of their special training to perform specific tasks for the safety and well-being of veterans distinguishes service dogs from pets or emotional support animals. Most of us recognize the happiness and meaning animals bring to our lives. What we may not appreciate is the impressive contribution service dogs make to the health and rehabilitation of those who have served their country. Veteran patients with neurologic conditions such as seizures know the difference a service dog trained to warn them of an emerging seizure makes for their freedom of movement and peace of mind. Veterans with diabetes have described times when their service dogs sought help before they realized their blood sugar was dangerously low.
Patients, friends, and neighbors who have been paired with service dogs describe ways their new companion helped them transition from a life in which even surviving was a struggle to one of holistic thriving. A Vietnam veteran neighbor with a significant tremor due to Parkinson disease benefitted from the ability of his dog to fetch and bring, retrieve, and carry. His dog has learned to hold essential items still, which would otherwise be too shaky in human hands, enabling the veteran to open his closet door and dress independently each morning. One veteran classmate avoided all forms of public transportation due to memories of a traumatic mobile-based mass evacuation she assisted with during her military service. She dreaded her long, inconvenient daily drive back and forth to work. She was then partnered with a large dog breed that was trained to stand a short distance from her to protect her sense of space and open air. The dog would stretch out his body to claim more space for her among crowds. This veteran started to ride the bus to campus each morning and appreciated the interaction with other riders as well as the saved travel time, mileage, gasoline expense, and parking stress.
A veteran brought his sweet retriever to the neighborhood weekly “Paws-itive Reading” program for children in the local public library. When MW’s daughter was busy reading to his furry friend, the veteran shared that for at least 5 years after his combat tours, he rarely left his window-shuttered home. His dog’s steady comfort re-established his ability to participate in his community. He now generously shares his dog’s patient affection with children learning to read.
MW recently witnessed the profound and protective presence of a service dog in comforting a veteran during a posttraumatic stress disorder (PTSD)–related crisis. The service dog offered a lean and reassuring paw pressure on the veteran’s shoulder if he was reexperiencing trauma. The dog’s steady breathing and familiar warmth helped to reorient their human companion to the safety of his present physical surroundings. Bearing witness to the dog’s trained interaction with the veteran left MW speechless. The trust between them was therapeutic in a way that transcended her ability to articulate what she experienced. This compelled MW to investigate whether this was a rare relationship or whether there was existing data on the impact of trained service dogs and PTSD.
Service dog placement with veterans with PTSD has been shown to have a positive influence on both physiological (arousal-related functioning and cortisol awakening response) and psychosocial well-being, including decreased isolation and increased physical activity.1,2 Veterans with PTSD paired with service dogs showed significantly fewer PTSD-related symptoms, better sleep quality, and improved well-being, compared with those with just a pet.3 A recent meta-analysis revealed that veteran partnerships with a service dog had a clinically meaningful, significant, and large effect on PTSD severity scores (P < .001).2 The mechanism for impact is thought to be not only the dog’s working role but also the transcendent loyalty of the canine-veteran bond.
Many accredited dog training programs describe a certain reciprocity to the dog-human relationship. Some use rescue puppies to give the dogs a new life and purpose. Dogs who have undergone challenges often need patience, time, safe relationships, and trustworthy new experiences to maximize their potential. Reciprocally, trained service dogs have the potential to foster access to these same emotional, relational, existential, and physical safety needs for veterans exposed to trauma.
Recent legislation has made progress in recognizing the role of service dogs for veterans and improving access. The Puppies Assisting Wounded Servicemembers (PAWS) for Veterans Therapy Act (38 USC §1705, 1714) was passed in 2021. The PAWS Act implemented a policy and 5-year pilot program to connect trained canines to eligible veterans diagnosed with PTSD as an element of an integrative health program, regardless of whether the veteran has a mobility impairment. The PAWS Act gives federal funding to accredited dog training organizations to help pair eligible veterans with service dogs by covering veteran travel expenses for the training, training program participation, and relevant veterinary expenses. The bipartisan Service Dogs Assisting Veterans Act (SAVES) Act was introduced this summer to award grants to nonprofit organizations Assistance Dog International has accredited to help these groups provide service dogs to veterans.
The US Department of Veterans Affairs (VA) has made strides in welcoming service dogs. Trained service dogs of all breeds under the control of a human companion are now allowed in VA facilities other than in areas where safety and infection control standards would be compromised (ie, sterile equipment rooms).4 A prescribing clinician can now evaluate eligible veterans to determine their ability, resources, and goals for having a service dog.5 An assessment of the veteran’s ability to care for a dog and education on expectations for the partnership is critical to success and animal welfare. Those veterans approved for a service dog are then referred to accredited agencies. The VA Veterinary Health Insurance Benefit includes aspects of coverage for the veteran to attend service dog training, veterinary wellness (preventive care, immunizations, dental cleanings, certain prescriptions, etc), and care for the dog’s illnesses when treatment enables the dog to perform duties in service to the veteran.6
The productive purpose and friendship of a service dog become a formidable force in a veteran’s life. Veterans spent an average of 82% of their time with service dogs (assessed via Bluetooth proximity between collar and smartphone).7 Human partners of veterans with service dogs may experience improved quality of life and relationship functioning with the inclusion of a service dog in the family unit.8 Veterans depict increased community engagement, social connectedness, and personal confidence as a result of the canine companionship.9,10 Veterans with service dogs often speak of the ways the dog’s presence transformed their lives and many speak of the dog literally saving their lives.11 Meta-analyses showed improved mental health treatment engagement, medication adherence, and decreased suicidality.2,12
A story was recently shared with us about the compassion and competence of VA staff in a perioperative unit. A veteran was scheduled for a life-altering surgery and yet was anxious about entering the room for his scheduled pre-anesthesia check-in, knowing his service dog could not accompany him through the entire procedure. The staff recognized that the veteran was increasingly nervous and even started to question whether he would stay for the scheduled procedure they deemed would benefit his health. The perioperative team then proactively worked together to safely walk the veteran through the preparation processes in a sterile setting while keeping the dog within sight of the veteran. They then ensured that the veteran’s service dog was by his side early in the recovery room so that the veteran woke to wags and licks. In these months of canine recognition, we honor the ways the VA has fostered companionship and courage in veterans’ lives through the inclusion of service dogs in so many aspects of their care and life.
1. Rodriguez KE, Bryce CI, Granger DA, O’Haire ME. The effect of a service dog on salivary cortisol awakening response in a military population with posttraumatic stress disorder (PTSD). Psychoneuroendocrinology. 2018;98:202-210. doi:10.1016/j.psyneuen.2018.04.026
2. Leighton SC, Nieforth LO, O’Haire ME. Assistance dogs for military veterans with PTSD: A systematic review, meta-analysis, and meta-synthesis. PloS One. 2022;17(9):e0274960. doi:10.1371/journal.pone.0274960
3. Van Houtert EAE, Rodenburg TB, Vermetten E, Endenburg N. The impact of service dogs on military veterans and (ex) first aid responders with post-traumatic stress disorder. Front Psychiatry. 2022;13:834291. doi:10.3389/fpsyt.2022.834291
4. VHA Directive 1188(1). Animals on Veterans Health Administration (VHA) Property. Veterans Health Administration. August 26, 2015. Amended April 25, 2019. https://www.va.gov/vhapublications/ViewPublication.asp?pub_ID=3138
5. Veteran Affairs Rehabilitation and Prosthetic Services. Service Dog Veterinary Health Benefit. US Department of Veterans Affairs. Updated September 19, 2019. Accessed August 21, 2023. https://www.prosthetics.va.gov/serviceandguidedogs.asp
6. Service Dog Veterinary Health Insurance (VHIB) Benefit Rules. US Department of Veterans Affairs Rehabilitation and Prosthetic Services. Updated December 2022. Accessed August 21, 2023. https://www.prosthetics.va.gov/factsheet/PSAS-FactSheet-ServiceDogs.pdf
7. Jensen CL, Rodriguez KE, MacLean EL, Abdul Wahab AH, Sabbaghi A, O’Haire ME. Characterizing veteran and PTSD service dog teams: exploring potential mechanisms of symptom change and canine predictors of efficacy. PloS One. 2022;17(7):e0269186. doi:10.1371/journal.pone.0269186
8. McCall CE, Rodriguez KE, Wadsworth SMM, Meis LA, O’Haire ME. “A Part of Our Family”? Effects of psychiatric service dogs on quality of life and relationship functioning in military-connected couples. Mil Behav Health. 2020;8(4):410-423. doi:10.1080/21635781.2020.1825243
9. Krause-Parello CA, Sarni S, Padden E. Military veterans and canine assistance for post-traumatic stress disorder: a narrative review of the literature. Nurse Educ Today. 2016;47:43-50. doi:10.1016/j.nedt.2016.04.020
10. Van Houtert EAE, Endenburg N, Wijnker JJ, Rodenburg B, Vermetten E. The study of service dogs for veterans with post-traumatic stress disorder: a scoping literature review. Eur J Psychotraumatol. 2018;9(suppl 3):1503523. doi:10.1080/20008198.2018.1503523
11. Sherman M, Hutchinson AD, Bowen H, Iannos M, Van Hooff M. Effectiveness of Operation K9 assistance dogs on suicidality in Australian veterans with PTSD: a 12-month mixed-methods follow-up study. Int J Environ Res Public Health. 2023;20(4):3607. Published 2023 Feb 17. doi:10.3390/ijerph20043607
12. Richerson JT, Saunders GH, Skelton K, et al. A randomized trial of differential effectiveness of service dog pairing to improve quality of life for veterans with PTSD. Office of Research and Development, Veterans Health Administration. 2020:186. https://www.research.va.gov/REPORT-Study-of-Costs-and-Benefits-Associated-with-the-Use-of-Service-Dogs-Monograph1.pdf
The psychological and moral comfort of a presence at once humble and understanding—this is the greatest benefit that the dog has bestowed upon man. Percy Bysshe Shelley
The nature of their special training to perform specific tasks for the safety and well-being of veterans distinguishes service dogs from pets or emotional support animals. Most of us recognize the happiness and meaning animals bring to our lives. What we may not appreciate is the impressive contribution service dogs make to the health and rehabilitation of those who have served their country. Veteran patients with neurologic conditions such as seizures know the difference a service dog trained to warn them of an emerging seizure makes for their freedom of movement and peace of mind. Veterans with diabetes have described times when their service dogs sought help before they realized their blood sugar was dangerously low.
Patients, friends, and neighbors who have been paired with service dogs describe ways their new companion helped them transition from a life in which even surviving was a struggle to one of holistic thriving. A Vietnam veteran neighbor with a significant tremor due to Parkinson disease benefitted from the ability of his dog to fetch and bring, retrieve, and carry. His dog has learned to hold essential items still, which would otherwise be too shaky in human hands, enabling the veteran to open his closet door and dress independently each morning. One veteran classmate avoided all forms of public transportation due to memories of a traumatic mobile-based mass evacuation she assisted with during her military service. She dreaded her long, inconvenient daily drive back and forth to work. She was then partnered with a large dog breed that was trained to stand a short distance from her to protect her sense of space and open air. The dog would stretch out his body to claim more space for her among crowds. This veteran started to ride the bus to campus each morning and appreciated the interaction with other riders as well as the saved travel time, mileage, gasoline expense, and parking stress.
A veteran brought his sweet retriever to the neighborhood weekly “Paws-itive Reading” program for children in the local public library. When MW’s daughter was busy reading to his furry friend, the veteran shared that for at least 5 years after his combat tours, he rarely left his window-shuttered home. His dog’s steady comfort re-established his ability to participate in his community. He now generously shares his dog’s patient affection with children learning to read.
MW recently witnessed the profound and protective presence of a service dog in comforting a veteran during a posttraumatic stress disorder (PTSD)–related crisis. The service dog offered a lean and reassuring paw pressure on the veteran’s shoulder if he was reexperiencing trauma. The dog’s steady breathing and familiar warmth helped to reorient their human companion to the safety of his present physical surroundings. Bearing witness to the dog’s trained interaction with the veteran left MW speechless. The trust between them was therapeutic in a way that transcended her ability to articulate what she experienced. This compelled MW to investigate whether this was a rare relationship or whether there was existing data on the impact of trained service dogs and PTSD.
Service dog placement with veterans with PTSD has been shown to have a positive influence on both physiological (arousal-related functioning and cortisol awakening response) and psychosocial well-being, including decreased isolation and increased physical activity.1,2 Veterans with PTSD paired with service dogs showed significantly fewer PTSD-related symptoms, better sleep quality, and improved well-being, compared with those with just a pet.3 A recent meta-analysis revealed that veteran partnerships with a service dog had a clinically meaningful, significant, and large effect on PTSD severity scores (P < .001).2 The mechanism for impact is thought to be not only the dog’s working role but also the transcendent loyalty of the canine-veteran bond.
Many accredited dog training programs describe a certain reciprocity to the dog-human relationship. Some use rescue puppies to give the dogs a new life and purpose. Dogs who have undergone challenges often need patience, time, safe relationships, and trustworthy new experiences to maximize their potential. Reciprocally, trained service dogs have the potential to foster access to these same emotional, relational, existential, and physical safety needs for veterans exposed to trauma.
Recent legislation has made progress in recognizing the role of service dogs for veterans and improving access. The Puppies Assisting Wounded Servicemembers (PAWS) for Veterans Therapy Act (38 USC §1705, 1714) was passed in 2021. The PAWS Act implemented a policy and 5-year pilot program to connect trained canines to eligible veterans diagnosed with PTSD as an element of an integrative health program, regardless of whether the veteran has a mobility impairment. The PAWS Act gives federal funding to accredited dog training organizations to help pair eligible veterans with service dogs by covering veteran travel expenses for the training, training program participation, and relevant veterinary expenses. The bipartisan Service Dogs Assisting Veterans Act (SAVES) Act was introduced this summer to award grants to nonprofit organizations Assistance Dog International has accredited to help these groups provide service dogs to veterans.
The US Department of Veterans Affairs (VA) has made strides in welcoming service dogs. Trained service dogs of all breeds under the control of a human companion are now allowed in VA facilities other than in areas where safety and infection control standards would be compromised (ie, sterile equipment rooms).4 A prescribing clinician can now evaluate eligible veterans to determine their ability, resources, and goals for having a service dog.5 An assessment of the veteran’s ability to care for a dog and education on expectations for the partnership is critical to success and animal welfare. Those veterans approved for a service dog are then referred to accredited agencies. The VA Veterinary Health Insurance Benefit includes aspects of coverage for the veteran to attend service dog training, veterinary wellness (preventive care, immunizations, dental cleanings, certain prescriptions, etc), and care for the dog’s illnesses when treatment enables the dog to perform duties in service to the veteran.6
The productive purpose and friendship of a service dog become a formidable force in a veteran’s life. Veterans spent an average of 82% of their time with service dogs (assessed via Bluetooth proximity between collar and smartphone).7 Human partners of veterans with service dogs may experience improved quality of life and relationship functioning with the inclusion of a service dog in the family unit.8 Veterans depict increased community engagement, social connectedness, and personal confidence as a result of the canine companionship.9,10 Veterans with service dogs often speak of the ways the dog’s presence transformed their lives and many speak of the dog literally saving their lives.11 Meta-analyses showed improved mental health treatment engagement, medication adherence, and decreased suicidality.2,12
A story was recently shared with us about the compassion and competence of VA staff in a perioperative unit. A veteran was scheduled for a life-altering surgery and yet was anxious about entering the room for his scheduled pre-anesthesia check-in, knowing his service dog could not accompany him through the entire procedure. The staff recognized that the veteran was increasingly nervous and even started to question whether he would stay for the scheduled procedure they deemed would benefit his health. The perioperative team then proactively worked together to safely walk the veteran through the preparation processes in a sterile setting while keeping the dog within sight of the veteran. They then ensured that the veteran’s service dog was by his side early in the recovery room so that the veteran woke to wags and licks. In these months of canine recognition, we honor the ways the VA has fostered companionship and courage in veterans’ lives through the inclusion of service dogs in so many aspects of their care and life.
The psychological and moral comfort of a presence at once humble and understanding—this is the greatest benefit that the dog has bestowed upon man. Percy Bysshe Shelley
The nature of their special training to perform specific tasks for the safety and well-being of veterans distinguishes service dogs from pets or emotional support animals. Most of us recognize the happiness and meaning animals bring to our lives. What we may not appreciate is the impressive contribution service dogs make to the health and rehabilitation of those who have served their country. Veteran patients with neurologic conditions such as seizures know the difference a service dog trained to warn them of an emerging seizure makes for their freedom of movement and peace of mind. Veterans with diabetes have described times when their service dogs sought help before they realized their blood sugar was dangerously low.
Patients, friends, and neighbors who have been paired with service dogs describe ways their new companion helped them transition from a life in which even surviving was a struggle to one of holistic thriving. A Vietnam veteran neighbor with a significant tremor due to Parkinson disease benefitted from the ability of his dog to fetch and bring, retrieve, and carry. His dog has learned to hold essential items still, which would otherwise be too shaky in human hands, enabling the veteran to open his closet door and dress independently each morning. One veteran classmate avoided all forms of public transportation due to memories of a traumatic mobile-based mass evacuation she assisted with during her military service. She dreaded her long, inconvenient daily drive back and forth to work. She was then partnered with a large dog breed that was trained to stand a short distance from her to protect her sense of space and open air. The dog would stretch out his body to claim more space for her among crowds. This veteran started to ride the bus to campus each morning and appreciated the interaction with other riders as well as the saved travel time, mileage, gasoline expense, and parking stress.
A veteran brought his sweet retriever to the neighborhood weekly “Paws-itive Reading” program for children in the local public library. When MW’s daughter was busy reading to his furry friend, the veteran shared that for at least 5 years after his combat tours, he rarely left his window-shuttered home. His dog’s steady comfort re-established his ability to participate in his community. He now generously shares his dog’s patient affection with children learning to read.
MW recently witnessed the profound and protective presence of a service dog in comforting a veteran during a posttraumatic stress disorder (PTSD)–related crisis. The service dog offered a lean and reassuring paw pressure on the veteran’s shoulder if he was reexperiencing trauma. The dog’s steady breathing and familiar warmth helped to reorient their human companion to the safety of his present physical surroundings. Bearing witness to the dog’s trained interaction with the veteran left MW speechless. The trust between them was therapeutic in a way that transcended her ability to articulate what she experienced. This compelled MW to investigate whether this was a rare relationship or whether there was existing data on the impact of trained service dogs and PTSD.
Service dog placement with veterans with PTSD has been shown to have a positive influence on both physiological (arousal-related functioning and cortisol awakening response) and psychosocial well-being, including decreased isolation and increased physical activity.1,2 Veterans with PTSD paired with service dogs showed significantly fewer PTSD-related symptoms, better sleep quality, and improved well-being, compared with those with just a pet.3 A recent meta-analysis revealed that veteran partnerships with a service dog had a clinically meaningful, significant, and large effect on PTSD severity scores (P < .001).2 The mechanism for impact is thought to be not only the dog’s working role but also the transcendent loyalty of the canine-veteran bond.
Many accredited dog training programs describe a certain reciprocity to the dog-human relationship. Some use rescue puppies to give the dogs a new life and purpose. Dogs who have undergone challenges often need patience, time, safe relationships, and trustworthy new experiences to maximize their potential. Reciprocally, trained service dogs have the potential to foster access to these same emotional, relational, existential, and physical safety needs for veterans exposed to trauma.
Recent legislation has made progress in recognizing the role of service dogs for veterans and improving access. The Puppies Assisting Wounded Servicemembers (PAWS) for Veterans Therapy Act (38 USC §1705, 1714) was passed in 2021. The PAWS Act implemented a policy and 5-year pilot program to connect trained canines to eligible veterans diagnosed with PTSD as an element of an integrative health program, regardless of whether the veteran has a mobility impairment. The PAWS Act gives federal funding to accredited dog training organizations to help pair eligible veterans with service dogs by covering veteran travel expenses for the training, training program participation, and relevant veterinary expenses. The bipartisan Service Dogs Assisting Veterans Act (SAVES) Act was introduced this summer to award grants to nonprofit organizations Assistance Dog International has accredited to help these groups provide service dogs to veterans.
The US Department of Veterans Affairs (VA) has made strides in welcoming service dogs. Trained service dogs of all breeds under the control of a human companion are now allowed in VA facilities other than in areas where safety and infection control standards would be compromised (ie, sterile equipment rooms).4 A prescribing clinician can now evaluate eligible veterans to determine their ability, resources, and goals for having a service dog.5 An assessment of the veteran’s ability to care for a dog and education on expectations for the partnership is critical to success and animal welfare. Those veterans approved for a service dog are then referred to accredited agencies. The VA Veterinary Health Insurance Benefit includes aspects of coverage for the veteran to attend service dog training, veterinary wellness (preventive care, immunizations, dental cleanings, certain prescriptions, etc), and care for the dog’s illnesses when treatment enables the dog to perform duties in service to the veteran.6
The productive purpose and friendship of a service dog become a formidable force in a veteran’s life. Veterans spent an average of 82% of their time with service dogs (assessed via Bluetooth proximity between collar and smartphone).7 Human partners of veterans with service dogs may experience improved quality of life and relationship functioning with the inclusion of a service dog in the family unit.8 Veterans depict increased community engagement, social connectedness, and personal confidence as a result of the canine companionship.9,10 Veterans with service dogs often speak of the ways the dog’s presence transformed their lives and many speak of the dog literally saving their lives.11 Meta-analyses showed improved mental health treatment engagement, medication adherence, and decreased suicidality.2,12
A story was recently shared with us about the compassion and competence of VA staff in a perioperative unit. A veteran was scheduled for a life-altering surgery and yet was anxious about entering the room for his scheduled pre-anesthesia check-in, knowing his service dog could not accompany him through the entire procedure. The staff recognized that the veteran was increasingly nervous and even started to question whether he would stay for the scheduled procedure they deemed would benefit his health. The perioperative team then proactively worked together to safely walk the veteran through the preparation processes in a sterile setting while keeping the dog within sight of the veteran. They then ensured that the veteran’s service dog was by his side early in the recovery room so that the veteran woke to wags and licks. In these months of canine recognition, we honor the ways the VA has fostered companionship and courage in veterans’ lives through the inclusion of service dogs in so many aspects of their care and life.
1. Rodriguez KE, Bryce CI, Granger DA, O’Haire ME. The effect of a service dog on salivary cortisol awakening response in a military population with posttraumatic stress disorder (PTSD). Psychoneuroendocrinology. 2018;98:202-210. doi:10.1016/j.psyneuen.2018.04.026
2. Leighton SC, Nieforth LO, O’Haire ME. Assistance dogs for military veterans with PTSD: A systematic review, meta-analysis, and meta-synthesis. PloS One. 2022;17(9):e0274960. doi:10.1371/journal.pone.0274960
3. Van Houtert EAE, Rodenburg TB, Vermetten E, Endenburg N. The impact of service dogs on military veterans and (ex) first aid responders with post-traumatic stress disorder. Front Psychiatry. 2022;13:834291. doi:10.3389/fpsyt.2022.834291
4. VHA Directive 1188(1). Animals on Veterans Health Administration (VHA) Property. Veterans Health Administration. August 26, 2015. Amended April 25, 2019. https://www.va.gov/vhapublications/ViewPublication.asp?pub_ID=3138
5. Veteran Affairs Rehabilitation and Prosthetic Services. Service Dog Veterinary Health Benefit. US Department of Veterans Affairs. Updated September 19, 2019. Accessed August 21, 2023. https://www.prosthetics.va.gov/serviceandguidedogs.asp
6. Service Dog Veterinary Health Insurance (VHIB) Benefit Rules. US Department of Veterans Affairs Rehabilitation and Prosthetic Services. Updated December 2022. Accessed August 21, 2023. https://www.prosthetics.va.gov/factsheet/PSAS-FactSheet-ServiceDogs.pdf
7. Jensen CL, Rodriguez KE, MacLean EL, Abdul Wahab AH, Sabbaghi A, O’Haire ME. Characterizing veteran and PTSD service dog teams: exploring potential mechanisms of symptom change and canine predictors of efficacy. PloS One. 2022;17(7):e0269186. doi:10.1371/journal.pone.0269186
8. McCall CE, Rodriguez KE, Wadsworth SMM, Meis LA, O’Haire ME. “A Part of Our Family”? Effects of psychiatric service dogs on quality of life and relationship functioning in military-connected couples. Mil Behav Health. 2020;8(4):410-423. doi:10.1080/21635781.2020.1825243
9. Krause-Parello CA, Sarni S, Padden E. Military veterans and canine assistance for post-traumatic stress disorder: a narrative review of the literature. Nurse Educ Today. 2016;47:43-50. doi:10.1016/j.nedt.2016.04.020
10. Van Houtert EAE, Endenburg N, Wijnker JJ, Rodenburg B, Vermetten E. The study of service dogs for veterans with post-traumatic stress disorder: a scoping literature review. Eur J Psychotraumatol. 2018;9(suppl 3):1503523. doi:10.1080/20008198.2018.1503523
11. Sherman M, Hutchinson AD, Bowen H, Iannos M, Van Hooff M. Effectiveness of Operation K9 assistance dogs on suicidality in Australian veterans with PTSD: a 12-month mixed-methods follow-up study. Int J Environ Res Public Health. 2023;20(4):3607. Published 2023 Feb 17. doi:10.3390/ijerph20043607
12. Richerson JT, Saunders GH, Skelton K, et al. A randomized trial of differential effectiveness of service dog pairing to improve quality of life for veterans with PTSD. Office of Research and Development, Veterans Health Administration. 2020:186. https://www.research.va.gov/REPORT-Study-of-Costs-and-Benefits-Associated-with-the-Use-of-Service-Dogs-Monograph1.pdf
1. Rodriguez KE, Bryce CI, Granger DA, O’Haire ME. The effect of a service dog on salivary cortisol awakening response in a military population with posttraumatic stress disorder (PTSD). Psychoneuroendocrinology. 2018;98:202-210. doi:10.1016/j.psyneuen.2018.04.026
2. Leighton SC, Nieforth LO, O’Haire ME. Assistance dogs for military veterans with PTSD: A systematic review, meta-analysis, and meta-synthesis. PloS One. 2022;17(9):e0274960. doi:10.1371/journal.pone.0274960
3. Van Houtert EAE, Rodenburg TB, Vermetten E, Endenburg N. The impact of service dogs on military veterans and (ex) first aid responders with post-traumatic stress disorder. Front Psychiatry. 2022;13:834291. doi:10.3389/fpsyt.2022.834291
4. VHA Directive 1188(1). Animals on Veterans Health Administration (VHA) Property. Veterans Health Administration. August 26, 2015. Amended April 25, 2019. https://www.va.gov/vhapublications/ViewPublication.asp?pub_ID=3138
5. Veteran Affairs Rehabilitation and Prosthetic Services. Service Dog Veterinary Health Benefit. US Department of Veterans Affairs. Updated September 19, 2019. Accessed August 21, 2023. https://www.prosthetics.va.gov/serviceandguidedogs.asp
6. Service Dog Veterinary Health Insurance (VHIB) Benefit Rules. US Department of Veterans Affairs Rehabilitation and Prosthetic Services. Updated December 2022. Accessed August 21, 2023. https://www.prosthetics.va.gov/factsheet/PSAS-FactSheet-ServiceDogs.pdf
7. Jensen CL, Rodriguez KE, MacLean EL, Abdul Wahab AH, Sabbaghi A, O’Haire ME. Characterizing veteran and PTSD service dog teams: exploring potential mechanisms of symptom change and canine predictors of efficacy. PloS One. 2022;17(7):e0269186. doi:10.1371/journal.pone.0269186
8. McCall CE, Rodriguez KE, Wadsworth SMM, Meis LA, O’Haire ME. “A Part of Our Family”? Effects of psychiatric service dogs on quality of life and relationship functioning in military-connected couples. Mil Behav Health. 2020;8(4):410-423. doi:10.1080/21635781.2020.1825243
9. Krause-Parello CA, Sarni S, Padden E. Military veterans and canine assistance for post-traumatic stress disorder: a narrative review of the literature. Nurse Educ Today. 2016;47:43-50. doi:10.1016/j.nedt.2016.04.020
10. Van Houtert EAE, Endenburg N, Wijnker JJ, Rodenburg B, Vermetten E. The study of service dogs for veterans with post-traumatic stress disorder: a scoping literature review. Eur J Psychotraumatol. 2018;9(suppl 3):1503523. doi:10.1080/20008198.2018.1503523
11. Sherman M, Hutchinson AD, Bowen H, Iannos M, Van Hooff M. Effectiveness of Operation K9 assistance dogs on suicidality in Australian veterans with PTSD: a 12-month mixed-methods follow-up study. Int J Environ Res Public Health. 2023;20(4):3607. Published 2023 Feb 17. doi:10.3390/ijerph20043607
12. Richerson JT, Saunders GH, Skelton K, et al. A randomized trial of differential effectiveness of service dog pairing to improve quality of life for veterans with PTSD. Office of Research and Development, Veterans Health Administration. 2020:186. https://www.research.va.gov/REPORT-Study-of-Costs-and-Benefits-Associated-with-the-Use-of-Service-Dogs-Monograph1.pdf
CDK4/6i can replace chemotherapy in ER+/HER2− advanced BC with impending or established visceral crisis
Key clinical point: Compared with paclitaxel chemotherapy, treatment with first-line cyclin-dependent kinase 4/6 inhibitors (CDK4/6i) demonstrated better survival outcomes and a similar speed of improvement in patients with estrogen receptor-positive (ER+)/human epidermal growth factor receptor 2-negative (HER2−) advanced breast cancer (BC) who had a visceral crisis (VC) or impending VC (IVC).
Major finding: CDK4/6i vs paclitaxel improved time-to-treatment failure (hazard ratio [HR] 0.33; P = .0002), progression-free survival (HR 0.38; P = .002), and overall survival (HR 0.37; P = .002) outcomes. The median time to first improvement in IVC/VC was comparable between the treatment groups (P = .773).
Study details: Findings are from a retrospective study including 59 patients with ER+/HER2− advanced BC who had either VC or IVC, of whom 27 patients received first-line treatment with CDK4/6i + endocrine therapy and 32 patients who were treated with weekly paclitaxel.
Disclosures: This study did not receive any funding. Two authors declared having joint working agreements with or receiving honoraria, conference fees, travel expenses, or research funding from various sources.
Source: Behrouzi R et al. CDK4/6 inhibitors versus weekly paclitaxel for treatment of ER+/HER2− advanced breast cancer with impending or established visceral crisis. Breast Cancer Res Treat. 2023 (Aug 16). doi: 10.1007/s10549-023-07035-6
Key clinical point: Compared with paclitaxel chemotherapy, treatment with first-line cyclin-dependent kinase 4/6 inhibitors (CDK4/6i) demonstrated better survival outcomes and a similar speed of improvement in patients with estrogen receptor-positive (ER+)/human epidermal growth factor receptor 2-negative (HER2−) advanced breast cancer (BC) who had a visceral crisis (VC) or impending VC (IVC).
Major finding: CDK4/6i vs paclitaxel improved time-to-treatment failure (hazard ratio [HR] 0.33; P = .0002), progression-free survival (HR 0.38; P = .002), and overall survival (HR 0.37; P = .002) outcomes. The median time to first improvement in IVC/VC was comparable between the treatment groups (P = .773).
Study details: Findings are from a retrospective study including 59 patients with ER+/HER2− advanced BC who had either VC or IVC, of whom 27 patients received first-line treatment with CDK4/6i + endocrine therapy and 32 patients who were treated with weekly paclitaxel.
Disclosures: This study did not receive any funding. Two authors declared having joint working agreements with or receiving honoraria, conference fees, travel expenses, or research funding from various sources.
Source: Behrouzi R et al. CDK4/6 inhibitors versus weekly paclitaxel for treatment of ER+/HER2− advanced breast cancer with impending or established visceral crisis. Breast Cancer Res Treat. 2023 (Aug 16). doi: 10.1007/s10549-023-07035-6
Key clinical point: Compared with paclitaxel chemotherapy, treatment with first-line cyclin-dependent kinase 4/6 inhibitors (CDK4/6i) demonstrated better survival outcomes and a similar speed of improvement in patients with estrogen receptor-positive (ER+)/human epidermal growth factor receptor 2-negative (HER2−) advanced breast cancer (BC) who had a visceral crisis (VC) or impending VC (IVC).
Major finding: CDK4/6i vs paclitaxel improved time-to-treatment failure (hazard ratio [HR] 0.33; P = .0002), progression-free survival (HR 0.38; P = .002), and overall survival (HR 0.37; P = .002) outcomes. The median time to first improvement in IVC/VC was comparable between the treatment groups (P = .773).
Study details: Findings are from a retrospective study including 59 patients with ER+/HER2− advanced BC who had either VC or IVC, of whom 27 patients received first-line treatment with CDK4/6i + endocrine therapy and 32 patients who were treated with weekly paclitaxel.
Disclosures: This study did not receive any funding. Two authors declared having joint working agreements with or receiving honoraria, conference fees, travel expenses, or research funding from various sources.
Source: Behrouzi R et al. CDK4/6 inhibitors versus weekly paclitaxel for treatment of ER+/HER2− advanced breast cancer with impending or established visceral crisis. Breast Cancer Res Treat. 2023 (Aug 16). doi: 10.1007/s10549-023-07035-6
Meta-analysis indicates an elevated risk for type 2 diabetes in breast cancer survivors
Key clinical point: Patients who survive breast cancer (BC) may have an elevated risk of developing type 2 diabetes mellitus (T2D), especially after receiving tamoxifen therapy.
Major finding: The risk for incident T2D was elevated in patients with BC (effect estimate [EE] 1.23; 95% CI 1.13-1.33), particularly those who received endocrine therapy (EE 1.23; 95% CI 1.16-1.32), compared with individuals without BC. Moreover, the risk of developing T2D was higher among patients with BC who did vs did not receive tamoxifen (EE 1.28; 95% CI 1.18-1.38).
Study details: Findings are from a meta-analysis of 15 observational studies.
Disclosures: This study was funded by the Novo Nordisk Foundation and other sources. The authors declared no conflicts of interest.
Source: Jordt N et al. Breast cancer and incidence of type 2 diabetes mellitus: A systematic review and meta-analysis. Breast Cancer Res Treat. 2023 (Sep 1). doi: 10.1007/s10549-023-07043-6
Key clinical point: Patients who survive breast cancer (BC) may have an elevated risk of developing type 2 diabetes mellitus (T2D), especially after receiving tamoxifen therapy.
Major finding: The risk for incident T2D was elevated in patients with BC (effect estimate [EE] 1.23; 95% CI 1.13-1.33), particularly those who received endocrine therapy (EE 1.23; 95% CI 1.16-1.32), compared with individuals without BC. Moreover, the risk of developing T2D was higher among patients with BC who did vs did not receive tamoxifen (EE 1.28; 95% CI 1.18-1.38).
Study details: Findings are from a meta-analysis of 15 observational studies.
Disclosures: This study was funded by the Novo Nordisk Foundation and other sources. The authors declared no conflicts of interest.
Source: Jordt N et al. Breast cancer and incidence of type 2 diabetes mellitus: A systematic review and meta-analysis. Breast Cancer Res Treat. 2023 (Sep 1). doi: 10.1007/s10549-023-07043-6
Key clinical point: Patients who survive breast cancer (BC) may have an elevated risk of developing type 2 diabetes mellitus (T2D), especially after receiving tamoxifen therapy.
Major finding: The risk for incident T2D was elevated in patients with BC (effect estimate [EE] 1.23; 95% CI 1.13-1.33), particularly those who received endocrine therapy (EE 1.23; 95% CI 1.16-1.32), compared with individuals without BC. Moreover, the risk of developing T2D was higher among patients with BC who did vs did not receive tamoxifen (EE 1.28; 95% CI 1.18-1.38).
Study details: Findings are from a meta-analysis of 15 observational studies.
Disclosures: This study was funded by the Novo Nordisk Foundation and other sources. The authors declared no conflicts of interest.
Source: Jordt N et al. Breast cancer and incidence of type 2 diabetes mellitus: A systematic review and meta-analysis. Breast Cancer Res Treat. 2023 (Sep 1). doi: 10.1007/s10549-023-07043-6
Predictors of 4 or more axillary lymph node metastases in clinically node-negative BC patients
Key clinical point: Certain preoperative clinicopathological factors can predict the presence of ≥4 pathologically positive lymph nodes in postmenopausal women with clinically node-negative (cN0) breast cancer (BC) who underwent sentinel lymph node biopsy (SLNB) with or without completion axillary lymph node dissection (cALND).
Major finding: Only 2.5% of the evaluated patients reported having ≥4 positive lymph nodes, with the factors serving as independent predictors of ≥4 positive nodes being larger tumor size (odds ratio [OR] 1.42; P < .0001), invasive lobular carcinoma (ILC) or mixed invasive ductal carcinoma/ILC histology (OR 3.03 or 1.99, respectively; P = .008), multifocality (OR 3.58; P < .0001), and the presence of lymphovascular invasion (OR 4.77; P < .0001).
Study details: This retrospective review included 2532 postmenopausal women with cN0 BC who underwent SLNB, of whom 24.3% underwent cALND.
Disclosures: This study was supported by an US National Institutes of Health/National Cancer Institute Cancer Center Support Grant. Some authors declared serving on medical or scientific advisory boards of, receiving research funding or support for clinical trials from, or having other ties with various sources.
Source: Farley C et al. To dissect or not to dissect: Can we predict the presence of four or more axillary lymph node metastases in postmenopausal women with clinically node-negative breast cancer? Ann Surg Oncol. 2023 (Sep 5). doi: 10.1245/s10434-023-14245-1
Key clinical point: Certain preoperative clinicopathological factors can predict the presence of ≥4 pathologically positive lymph nodes in postmenopausal women with clinically node-negative (cN0) breast cancer (BC) who underwent sentinel lymph node biopsy (SLNB) with or without completion axillary lymph node dissection (cALND).
Major finding: Only 2.5% of the evaluated patients reported having ≥4 positive lymph nodes, with the factors serving as independent predictors of ≥4 positive nodes being larger tumor size (odds ratio [OR] 1.42; P < .0001), invasive lobular carcinoma (ILC) or mixed invasive ductal carcinoma/ILC histology (OR 3.03 or 1.99, respectively; P = .008), multifocality (OR 3.58; P < .0001), and the presence of lymphovascular invasion (OR 4.77; P < .0001).
Study details: This retrospective review included 2532 postmenopausal women with cN0 BC who underwent SLNB, of whom 24.3% underwent cALND.
Disclosures: This study was supported by an US National Institutes of Health/National Cancer Institute Cancer Center Support Grant. Some authors declared serving on medical or scientific advisory boards of, receiving research funding or support for clinical trials from, or having other ties with various sources.
Source: Farley C et al. To dissect or not to dissect: Can we predict the presence of four or more axillary lymph node metastases in postmenopausal women with clinically node-negative breast cancer? Ann Surg Oncol. 2023 (Sep 5). doi: 10.1245/s10434-023-14245-1
Key clinical point: Certain preoperative clinicopathological factors can predict the presence of ≥4 pathologically positive lymph nodes in postmenopausal women with clinically node-negative (cN0) breast cancer (BC) who underwent sentinel lymph node biopsy (SLNB) with or without completion axillary lymph node dissection (cALND).
Major finding: Only 2.5% of the evaluated patients reported having ≥4 positive lymph nodes, with the factors serving as independent predictors of ≥4 positive nodes being larger tumor size (odds ratio [OR] 1.42; P < .0001), invasive lobular carcinoma (ILC) or mixed invasive ductal carcinoma/ILC histology (OR 3.03 or 1.99, respectively; P = .008), multifocality (OR 3.58; P < .0001), and the presence of lymphovascular invasion (OR 4.77; P < .0001).
Study details: This retrospective review included 2532 postmenopausal women with cN0 BC who underwent SLNB, of whom 24.3% underwent cALND.
Disclosures: This study was supported by an US National Institutes of Health/National Cancer Institute Cancer Center Support Grant. Some authors declared serving on medical or scientific advisory boards of, receiving research funding or support for clinical trials from, or having other ties with various sources.
Source: Farley C et al. To dissect or not to dissect: Can we predict the presence of four or more axillary lymph node metastases in postmenopausal women with clinically node-negative breast cancer? Ann Surg Oncol. 2023 (Sep 5). doi: 10.1245/s10434-023-14245-1
Surgical complications likely in obese patients undergoing mastectomy with immediate reconstruction
Key clinical point: Patients with obesity and stages I-III breast cancer (BC) who undergo mastectomy with immediate reconstruction have a higher likelihood of experiencing surgical complications than patients without obesity.
Major finding: Compared with patients who underwent lumpectomy, the risk for surgical complications was significantly higher in those who underwent mastectomy with (odds ratio [OR] 7.45; P < .0001) or without (OR 3.15; P = .002) immediate reconstruction. Moreover, obesity vs non-obesity was associated with worse surgical complications among patients undergoing mastectomy with reconstruction (OR 2.25; P = .02).
Study details: Findings are from a retrospective study including 692 patients with stages I-III BC who underwent surgery and received body composition measurements using bioelectrical impedance spectrometry.
Disclosures: This study did not disclose the funding source. SA Valente and HCF Moore declared serving as consultants for, receiving fees or grants from, or having contracts with various sources.
Source: Aleixo GFP et al. Association of body composition and surgical outcomes in patients with early-stage breast cancer. Breast Cancer Res Treat. 2023 (Aug 28). doi: 10.1007/s10549-023-07060-5
Key clinical point: Patients with obesity and stages I-III breast cancer (BC) who undergo mastectomy with immediate reconstruction have a higher likelihood of experiencing surgical complications than patients without obesity.
Major finding: Compared with patients who underwent lumpectomy, the risk for surgical complications was significantly higher in those who underwent mastectomy with (odds ratio [OR] 7.45; P < .0001) or without (OR 3.15; P = .002) immediate reconstruction. Moreover, obesity vs non-obesity was associated with worse surgical complications among patients undergoing mastectomy with reconstruction (OR 2.25; P = .02).
Study details: Findings are from a retrospective study including 692 patients with stages I-III BC who underwent surgery and received body composition measurements using bioelectrical impedance spectrometry.
Disclosures: This study did not disclose the funding source. SA Valente and HCF Moore declared serving as consultants for, receiving fees or grants from, or having contracts with various sources.
Source: Aleixo GFP et al. Association of body composition and surgical outcomes in patients with early-stage breast cancer. Breast Cancer Res Treat. 2023 (Aug 28). doi: 10.1007/s10549-023-07060-5
Key clinical point: Patients with obesity and stages I-III breast cancer (BC) who undergo mastectomy with immediate reconstruction have a higher likelihood of experiencing surgical complications than patients without obesity.
Major finding: Compared with patients who underwent lumpectomy, the risk for surgical complications was significantly higher in those who underwent mastectomy with (odds ratio [OR] 7.45; P < .0001) or without (OR 3.15; P = .002) immediate reconstruction. Moreover, obesity vs non-obesity was associated with worse surgical complications among patients undergoing mastectomy with reconstruction (OR 2.25; P = .02).
Study details: Findings are from a retrospective study including 692 patients with stages I-III BC who underwent surgery and received body composition measurements using bioelectrical impedance spectrometry.
Disclosures: This study did not disclose the funding source. SA Valente and HCF Moore declared serving as consultants for, receiving fees or grants from, or having contracts with various sources.
Source: Aleixo GFP et al. Association of body composition and surgical outcomes in patients with early-stage breast cancer. Breast Cancer Res Treat. 2023 (Aug 28). doi: 10.1007/s10549-023-07060-5
Home-based exercise and nutritional intervention may benefit patients with newly diagnosed BC
Key clinical point: Home-based exercise and nutritional intervention vs usual care (UC) routine improved the physical activity levels, diet quality, and pathological complete response (pCR) rates in patients who initiated chemotherapy after a breast cancer (BC) diagnosis.
Major finding: Women in the intervention vs UC group reported greater improvements in physical activity (143.4 vs 47.7 minutes/week; P < .001) and a higher intake of fruits, vegetables, and dietary fiber (P ≤ .01) along with increased pCR rates (53% vs 28%; P = .037).
Study details: Findings are from the Lifestyle, Exercise, and Nutrition Early After Breast Cancer study including 173 women with newly diagnosed stages I-III breast cancer who initiated chemotherapy and were randomly assigned to undergo either a UC routine or a home-based exercise and nutritional intervention.
Disclosures: This study was financially supported by the corresponding author ML Irwin. Some authors declared serving in the speakers’ bureau of or in consulting, advisory, or leadership roles in or receiving honoraria from various sources.
Source: Sanft T et al. Randomized trial of exercise and nutrition on chemotherapy completion and pathologic complete response in women with breast cancer: The Lifestyle, Exercise, and Nutrition Early After Diagnosis study. J Clin Oncol. 2023 (Sep 1). doi: 10.1200/JCO.23.00871
Key clinical point: Home-based exercise and nutritional intervention vs usual care (UC) routine improved the physical activity levels, diet quality, and pathological complete response (pCR) rates in patients who initiated chemotherapy after a breast cancer (BC) diagnosis.
Major finding: Women in the intervention vs UC group reported greater improvements in physical activity (143.4 vs 47.7 minutes/week; P < .001) and a higher intake of fruits, vegetables, and dietary fiber (P ≤ .01) along with increased pCR rates (53% vs 28%; P = .037).
Study details: Findings are from the Lifestyle, Exercise, and Nutrition Early After Breast Cancer study including 173 women with newly diagnosed stages I-III breast cancer who initiated chemotherapy and were randomly assigned to undergo either a UC routine or a home-based exercise and nutritional intervention.
Disclosures: This study was financially supported by the corresponding author ML Irwin. Some authors declared serving in the speakers’ bureau of or in consulting, advisory, or leadership roles in or receiving honoraria from various sources.
Source: Sanft T et al. Randomized trial of exercise and nutrition on chemotherapy completion and pathologic complete response in women with breast cancer: The Lifestyle, Exercise, and Nutrition Early After Diagnosis study. J Clin Oncol. 2023 (Sep 1). doi: 10.1200/JCO.23.00871
Key clinical point: Home-based exercise and nutritional intervention vs usual care (UC) routine improved the physical activity levels, diet quality, and pathological complete response (pCR) rates in patients who initiated chemotherapy after a breast cancer (BC) diagnosis.
Major finding: Women in the intervention vs UC group reported greater improvements in physical activity (143.4 vs 47.7 minutes/week; P < .001) and a higher intake of fruits, vegetables, and dietary fiber (P ≤ .01) along with increased pCR rates (53% vs 28%; P = .037).
Study details: Findings are from the Lifestyle, Exercise, and Nutrition Early After Breast Cancer study including 173 women with newly diagnosed stages I-III breast cancer who initiated chemotherapy and were randomly assigned to undergo either a UC routine or a home-based exercise and nutritional intervention.
Disclosures: This study was financially supported by the corresponding author ML Irwin. Some authors declared serving in the speakers’ bureau of or in consulting, advisory, or leadership roles in or receiving honoraria from various sources.
Source: Sanft T et al. Randomized trial of exercise and nutrition on chemotherapy completion and pathologic complete response in women with breast cancer: The Lifestyle, Exercise, and Nutrition Early After Diagnosis study. J Clin Oncol. 2023 (Sep 1). doi: 10.1200/JCO.23.00871