User login
Five personal finance questions for the young GI
While this article will get you started, these are complex topics, and each could warrant several standalone articles. I strongly encourage you to develop some basic understanding of personal finance through books, websites, and podcasts. If you can manage Barrett’s esophagus, Crohn’s, and cirrhosis, you can understand the basics of personal finance.
1. What should I do about my student loans? Go for public service loan forgiveness or pay them off?
The first step is knowing your debt burden, knowing your options, and developing a plan to pay off student loans. Public service loan forgiveness (PSLF) can be a good option in many situations. For borrowers staying in academic or other 501(c)(3) positions, PSLF is often an obvious move. Importantly, a fall 2022 statement by the U.S. Department of Education clarified that physicians working as contractors for nonprofit hospitals in California and Texas may now qualify for PSLF.1,2
For trainees debating an academic/501(c)(3) position vs. private practice, I would generally not advise making a career choice based purely on PSLF eligibility. However, borrowers with very high federal student loan burdens (e.g., debt to income ratio of > 2:1), or who are very close to the PSLF 10-year requirement may want to consider choosing a qualifying position for a few years to receive PSLF student loan forgiveness. Please see TNG’s 2020 article3 for a deeper discussion. Consultation with a company specializing in student loan advice for physicians may be well worth the upfront cost.
2. Do I need disability insurance? What should I look for?
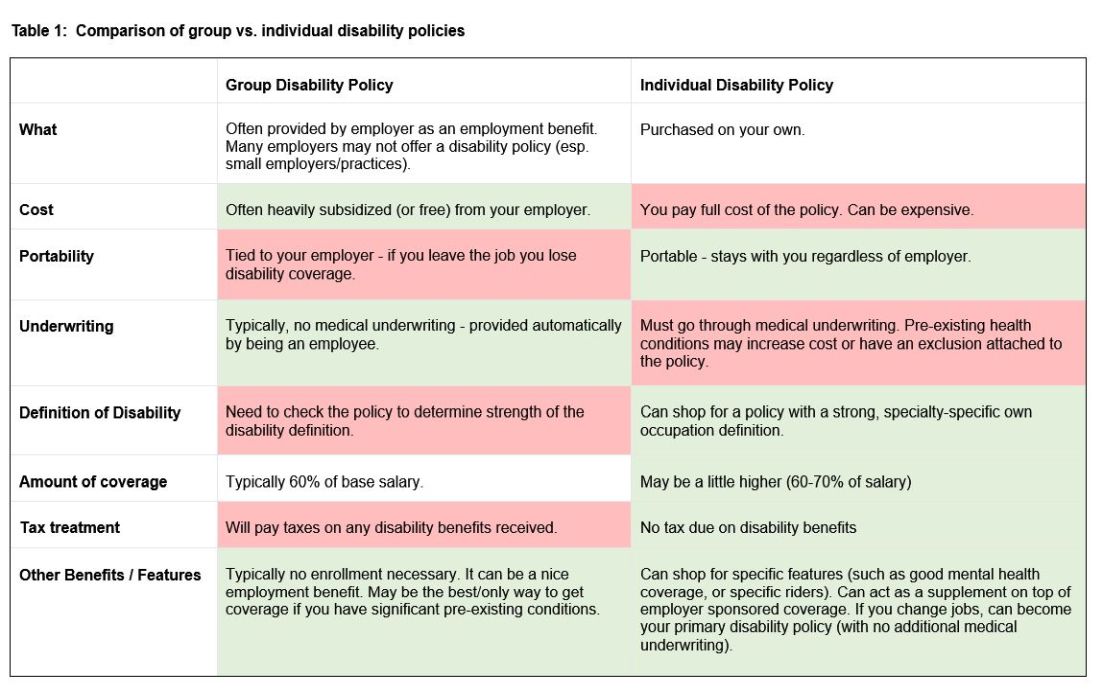
I would strongly advise getting disability insurance as soon as possible (including while in training). While disability insurance is not cheap, it is one of the first steps you should take and one of the most important ways to protect your financial future. It is essential to look for a specialty-specific own occupation policy. Such a policy will provide disability payments if you are no longer able to work as a gastroenterologist/hepatologist (including an injury which prevents you from doing endoscopies).
There are two major types of disability policies: group policies and individual policies. See table 1 for a detailed comparison.
Your hospital/employer may provide a group policy at a heavily subsidized rate. Alternatively, you can purchase an individual disability policy, which is independent of your employer and will stay with you even if you change jobs. Currently, the only companies providing high quality own-occupation policies for physicians are Mass Mutual, Principal, Guardian, The Standard, and Ameritas. Because disability insurance is complicated, it is highly advisable to work with an agent experienced in physician disability policies.
Importantly, even if you have a group disability policy, you can purchase an individual policy as a supplement to provide extra coverage. If you leave employers, the individual policy can then become your primary disability policy without any additional medical underwriting.
3. Do I need life insurance? What type should I get?
If anyone is dependent on your income (partner, child, etc.), you should have life insurance. Moreover, if you expect to have dependents in the near future (e.g., children), you could consider getting life insurance now while you are younger and healthier. For a young GI with multiple financial obligations, term life insurance is generally the right product. Term life insurance is a straightforward, affordable product that can be purchased from multiple high-quality insurance carriers. There are two major considerations: The amount of coverage ($2 million, $3 million, etc.) and the length of coverage (20 years, 30 years, etc.). To estimate the appropriate amount of coverage, start with your expected annual household living expenses, and multiply by 25-30. While this is a rule of thumb, it will get you in the ballpark. For many young physicians, a $2-$5 million policy with 20- to 30-year coverage is reasonable.
Many financial advisers may suggest whole life insurance policies. These are typically not the ideal policy for young GIs who are just starting their careers. While whole life insurance may be the right choice in select cases, term life insurance will be the best product for most of TNG’s audience. As an example, a $3 million, 25-year term policy for a healthy, nonsmoking 35-year-old male would cost approximately $175 per month. A similar $3 million whole life policy could cost $2,000 per month or more.
4. What do I need to know about retirement accounts and investing?
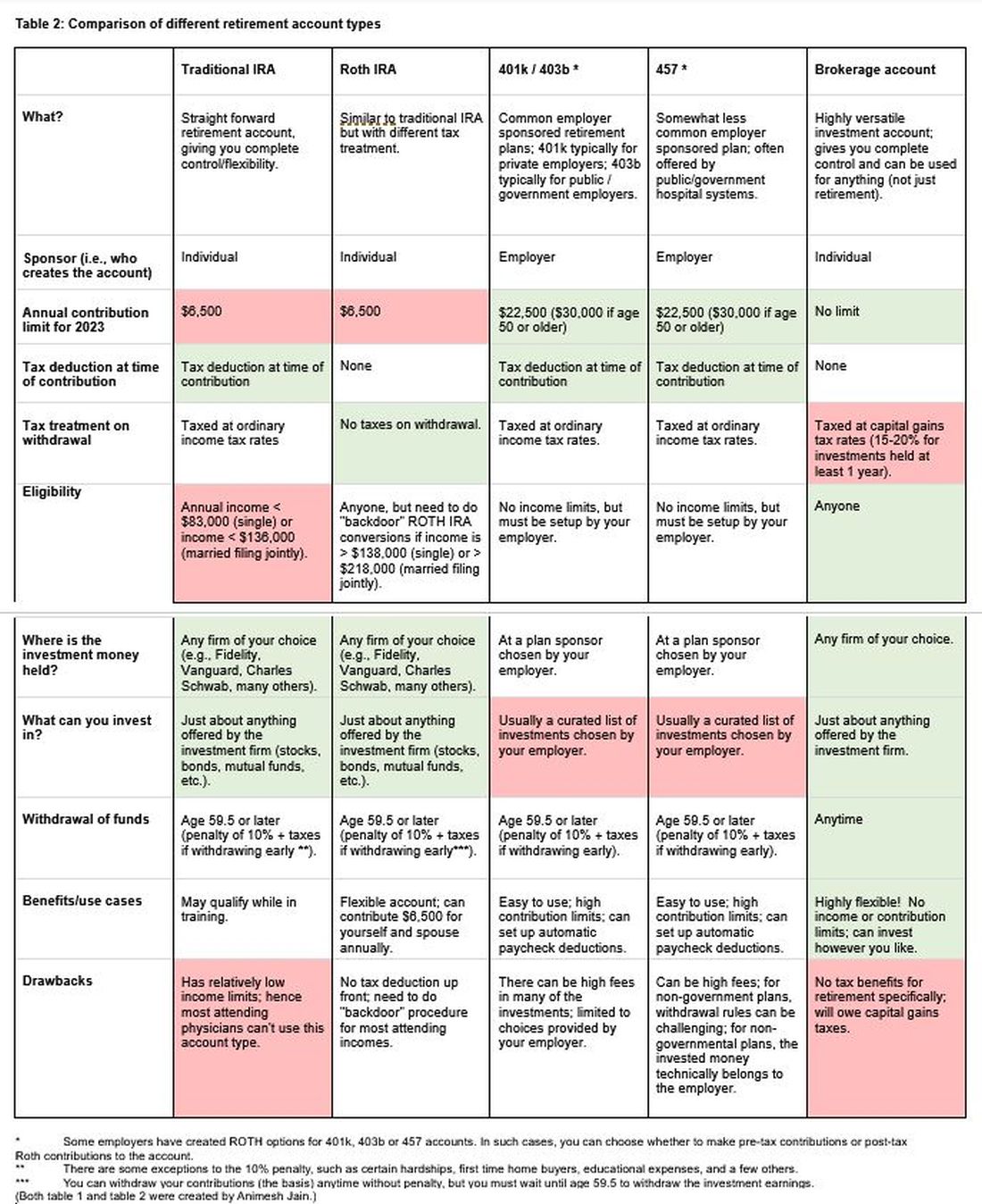
The alphabet soup of retirement accounts can be confusing – IRA, 401k, 457. Retirement accounts provide a tax break to incentivize saving for retirement. Traditional (“non-Roth”) accounts provide a tax break today, but you will pay taxes when withdrawing the money in retirement. Roth accounts provide no tax break now but provide tax-free growth for decades, and no taxes are due when withdrawing money. See table 2 for a detailed comparison of retirement accounts.
Once you place money into a retirement account, you will need to choose specific investments to grow your money. The two most common asset classes are stocks and bonds, though there are many other reasonable assets, such as real estate, commodities, and alternative currencies. It is generally recommended to have a higher proportion of stock-based investments early on (60%-90%) and then increase the ratio of bonds closer to retirement. Using low cost, passive index funds (or exchange traded funds) is a good way to get stock exposure. Target date retirement funds can be a nice tool for beginning investors since they will automatically adjust the stock/bond ratio for you.
Calculating the amount needed for retirement is beyond the scope of this article. However, saving at least 20% of your gross income specifically for retirement is a good starting point and should set you up for a reasonable retirement in about 30 years. For the average GI physician, this would mean saving $4,000 or more per month for retirement. If you aim to retire earlier, consider investing a higher percentage.
5. What do I need to know about buying a house?
The first question to ask is whether it makes sense to rent or buy a house. This is a personal and lifestyle decision, not just a financial decision. Today’s market is difficult with both high home prices and high rent costs. If there is a reasonable chance that you will be moving within 3-5 years, I would consider not buying until your long-term plans are more stable. Moreover, a high proportion of physicians change jobs.4,5,6 If you are just starting a new job, it is often wise to wait at least 6-12 months before buying a house to ensure the new job is a good fit. If you are in a stable long-term situation, it may be reasonable to buy a house. While it is commonly believed that buying a house is a “good financial move,” there are many hidden costs to home ownership, including big ticket repairs, property taxes, and real estate fees when selling a home.
First-time physician home buyers can often secure a physician mortgage with competitive interest rates and a low down payment of 0%-10% instead of the traditional 20% down payment. Moreover, a good physician mortgage should not have private mortgage insurance (PMI). Given the variation between mortgage companies, my most important piece of advice is to shop around for a good mortgage. An independent mortgage broker can be very valuable.
Dr. Jain is associate professor of medicine in the division of gastroenterology and hepatology, University of North Carolina School of Medicine, Chapel Hill. He has no conflicts of interest. The information in this article is meant for general educational purposes only. For individualized personal finance advice, please seek your own financial advisor, tax accountant, insurance broker, attorney, or other financial professional. Follow Dr. Jain @AJainMD on X.
References
1. Future of PSLF Fact Sheet
2. The Loophole That Can Get Thousands of Doctors into PSLF
3. Student loan management: An introduction for the young gastroenterologist
4. Study Shows First Job after Medical Residency Often Doesn’t Last
5. More physicians want to leave their jobs as pay rates fall, survey finds
6. Physician turnover rates are climbing as they clamor for better work-life balance
While this article will get you started, these are complex topics, and each could warrant several standalone articles. I strongly encourage you to develop some basic understanding of personal finance through books, websites, and podcasts. If you can manage Barrett’s esophagus, Crohn’s, and cirrhosis, you can understand the basics of personal finance.
1. What should I do about my student loans? Go for public service loan forgiveness or pay them off?
The first step is knowing your debt burden, knowing your options, and developing a plan to pay off student loans. Public service loan forgiveness (PSLF) can be a good option in many situations. For borrowers staying in academic or other 501(c)(3) positions, PSLF is often an obvious move. Importantly, a fall 2022 statement by the U.S. Department of Education clarified that physicians working as contractors for nonprofit hospitals in California and Texas may now qualify for PSLF.1,2
For trainees debating an academic/501(c)(3) position vs. private practice, I would generally not advise making a career choice based purely on PSLF eligibility. However, borrowers with very high federal student loan burdens (e.g., debt to income ratio of > 2:1), or who are very close to the PSLF 10-year requirement may want to consider choosing a qualifying position for a few years to receive PSLF student loan forgiveness. Please see TNG’s 2020 article3 for a deeper discussion. Consultation with a company specializing in student loan advice for physicians may be well worth the upfront cost.
2. Do I need disability insurance? What should I look for?
I would strongly advise getting disability insurance as soon as possible (including while in training). While disability insurance is not cheap, it is one of the first steps you should take and one of the most important ways to protect your financial future. It is essential to look for a specialty-specific own occupation policy. Such a policy will provide disability payments if you are no longer able to work as a gastroenterologist/hepatologist (including an injury which prevents you from doing endoscopies).
There are two major types of disability policies: group policies and individual policies. See table 1 for a detailed comparison.
Your hospital/employer may provide a group policy at a heavily subsidized rate. Alternatively, you can purchase an individual disability policy, which is independent of your employer and will stay with you even if you change jobs. Currently, the only companies providing high quality own-occupation policies for physicians are Mass Mutual, Principal, Guardian, The Standard, and Ameritas. Because disability insurance is complicated, it is highly advisable to work with an agent experienced in physician disability policies.
Importantly, even if you have a group disability policy, you can purchase an individual policy as a supplement to provide extra coverage. If you leave employers, the individual policy can then become your primary disability policy without any additional medical underwriting.
3. Do I need life insurance? What type should I get?
If anyone is dependent on your income (partner, child, etc.), you should have life insurance. Moreover, if you expect to have dependents in the near future (e.g., children), you could consider getting life insurance now while you are younger and healthier. For a young GI with multiple financial obligations, term life insurance is generally the right product. Term life insurance is a straightforward, affordable product that can be purchased from multiple high-quality insurance carriers. There are two major considerations: The amount of coverage ($2 million, $3 million, etc.) and the length of coverage (20 years, 30 years, etc.). To estimate the appropriate amount of coverage, start with your expected annual household living expenses, and multiply by 25-30. While this is a rule of thumb, it will get you in the ballpark. For many young physicians, a $2-$5 million policy with 20- to 30-year coverage is reasonable.
Many financial advisers may suggest whole life insurance policies. These are typically not the ideal policy for young GIs who are just starting their careers. While whole life insurance may be the right choice in select cases, term life insurance will be the best product for most of TNG’s audience. As an example, a $3 million, 25-year term policy for a healthy, nonsmoking 35-year-old male would cost approximately $175 per month. A similar $3 million whole life policy could cost $2,000 per month or more.
4. What do I need to know about retirement accounts and investing?
The alphabet soup of retirement accounts can be confusing – IRA, 401k, 457. Retirement accounts provide a tax break to incentivize saving for retirement. Traditional (“non-Roth”) accounts provide a tax break today, but you will pay taxes when withdrawing the money in retirement. Roth accounts provide no tax break now but provide tax-free growth for decades, and no taxes are due when withdrawing money. See table 2 for a detailed comparison of retirement accounts.
Once you place money into a retirement account, you will need to choose specific investments to grow your money. The two most common asset classes are stocks and bonds, though there are many other reasonable assets, such as real estate, commodities, and alternative currencies. It is generally recommended to have a higher proportion of stock-based investments early on (60%-90%) and then increase the ratio of bonds closer to retirement. Using low cost, passive index funds (or exchange traded funds) is a good way to get stock exposure. Target date retirement funds can be a nice tool for beginning investors since they will automatically adjust the stock/bond ratio for you.
Calculating the amount needed for retirement is beyond the scope of this article. However, saving at least 20% of your gross income specifically for retirement is a good starting point and should set you up for a reasonable retirement in about 30 years. For the average GI physician, this would mean saving $4,000 or more per month for retirement. If you aim to retire earlier, consider investing a higher percentage.
5. What do I need to know about buying a house?
The first question to ask is whether it makes sense to rent or buy a house. This is a personal and lifestyle decision, not just a financial decision. Today’s market is difficult with both high home prices and high rent costs. If there is a reasonable chance that you will be moving within 3-5 years, I would consider not buying until your long-term plans are more stable. Moreover, a high proportion of physicians change jobs.4,5,6 If you are just starting a new job, it is often wise to wait at least 6-12 months before buying a house to ensure the new job is a good fit. If you are in a stable long-term situation, it may be reasonable to buy a house. While it is commonly believed that buying a house is a “good financial move,” there are many hidden costs to home ownership, including big ticket repairs, property taxes, and real estate fees when selling a home.
First-time physician home buyers can often secure a physician mortgage with competitive interest rates and a low down payment of 0%-10% instead of the traditional 20% down payment. Moreover, a good physician mortgage should not have private mortgage insurance (PMI). Given the variation between mortgage companies, my most important piece of advice is to shop around for a good mortgage. An independent mortgage broker can be very valuable.
Dr. Jain is associate professor of medicine in the division of gastroenterology and hepatology, University of North Carolina School of Medicine, Chapel Hill. He has no conflicts of interest. The information in this article is meant for general educational purposes only. For individualized personal finance advice, please seek your own financial advisor, tax accountant, insurance broker, attorney, or other financial professional. Follow Dr. Jain @AJainMD on X.
References
1. Future of PSLF Fact Sheet
2. The Loophole That Can Get Thousands of Doctors into PSLF
3. Student loan management: An introduction for the young gastroenterologist
4. Study Shows First Job after Medical Residency Often Doesn’t Last
5. More physicians want to leave their jobs as pay rates fall, survey finds
6. Physician turnover rates are climbing as they clamor for better work-life balance
While this article will get you started, these are complex topics, and each could warrant several standalone articles. I strongly encourage you to develop some basic understanding of personal finance through books, websites, and podcasts. If you can manage Barrett’s esophagus, Crohn’s, and cirrhosis, you can understand the basics of personal finance.
1. What should I do about my student loans? Go for public service loan forgiveness or pay them off?
The first step is knowing your debt burden, knowing your options, and developing a plan to pay off student loans. Public service loan forgiveness (PSLF) can be a good option in many situations. For borrowers staying in academic or other 501(c)(3) positions, PSLF is often an obvious move. Importantly, a fall 2022 statement by the U.S. Department of Education clarified that physicians working as contractors for nonprofit hospitals in California and Texas may now qualify for PSLF.1,2
For trainees debating an academic/501(c)(3) position vs. private practice, I would generally not advise making a career choice based purely on PSLF eligibility. However, borrowers with very high federal student loan burdens (e.g., debt to income ratio of > 2:1), or who are very close to the PSLF 10-year requirement may want to consider choosing a qualifying position for a few years to receive PSLF student loan forgiveness. Please see TNG’s 2020 article3 for a deeper discussion. Consultation with a company specializing in student loan advice for physicians may be well worth the upfront cost.
2. Do I need disability insurance? What should I look for?
I would strongly advise getting disability insurance as soon as possible (including while in training). While disability insurance is not cheap, it is one of the first steps you should take and one of the most important ways to protect your financial future. It is essential to look for a specialty-specific own occupation policy. Such a policy will provide disability payments if you are no longer able to work as a gastroenterologist/hepatologist (including an injury which prevents you from doing endoscopies).
There are two major types of disability policies: group policies and individual policies. See table 1 for a detailed comparison.
Your hospital/employer may provide a group policy at a heavily subsidized rate. Alternatively, you can purchase an individual disability policy, which is independent of your employer and will stay with you even if you change jobs. Currently, the only companies providing high quality own-occupation policies for physicians are Mass Mutual, Principal, Guardian, The Standard, and Ameritas. Because disability insurance is complicated, it is highly advisable to work with an agent experienced in physician disability policies.
Importantly, even if you have a group disability policy, you can purchase an individual policy as a supplement to provide extra coverage. If you leave employers, the individual policy can then become your primary disability policy without any additional medical underwriting.
3. Do I need life insurance? What type should I get?
If anyone is dependent on your income (partner, child, etc.), you should have life insurance. Moreover, if you expect to have dependents in the near future (e.g., children), you could consider getting life insurance now while you are younger and healthier. For a young GI with multiple financial obligations, term life insurance is generally the right product. Term life insurance is a straightforward, affordable product that can be purchased from multiple high-quality insurance carriers. There are two major considerations: The amount of coverage ($2 million, $3 million, etc.) and the length of coverage (20 years, 30 years, etc.). To estimate the appropriate amount of coverage, start with your expected annual household living expenses, and multiply by 25-30. While this is a rule of thumb, it will get you in the ballpark. For many young physicians, a $2-$5 million policy with 20- to 30-year coverage is reasonable.
Many financial advisers may suggest whole life insurance policies. These are typically not the ideal policy for young GIs who are just starting their careers. While whole life insurance may be the right choice in select cases, term life insurance will be the best product for most of TNG’s audience. As an example, a $3 million, 25-year term policy for a healthy, nonsmoking 35-year-old male would cost approximately $175 per month. A similar $3 million whole life policy could cost $2,000 per month or more.
4. What do I need to know about retirement accounts and investing?
The alphabet soup of retirement accounts can be confusing – IRA, 401k, 457. Retirement accounts provide a tax break to incentivize saving for retirement. Traditional (“non-Roth”) accounts provide a tax break today, but you will pay taxes when withdrawing the money in retirement. Roth accounts provide no tax break now but provide tax-free growth for decades, and no taxes are due when withdrawing money. See table 2 for a detailed comparison of retirement accounts.
Once you place money into a retirement account, you will need to choose specific investments to grow your money. The two most common asset classes are stocks and bonds, though there are many other reasonable assets, such as real estate, commodities, and alternative currencies. It is generally recommended to have a higher proportion of stock-based investments early on (60%-90%) and then increase the ratio of bonds closer to retirement. Using low cost, passive index funds (or exchange traded funds) is a good way to get stock exposure. Target date retirement funds can be a nice tool for beginning investors since they will automatically adjust the stock/bond ratio for you.
Calculating the amount needed for retirement is beyond the scope of this article. However, saving at least 20% of your gross income specifically for retirement is a good starting point and should set you up for a reasonable retirement in about 30 years. For the average GI physician, this would mean saving $4,000 or more per month for retirement. If you aim to retire earlier, consider investing a higher percentage.
5. What do I need to know about buying a house?
The first question to ask is whether it makes sense to rent or buy a house. This is a personal and lifestyle decision, not just a financial decision. Today’s market is difficult with both high home prices and high rent costs. If there is a reasonable chance that you will be moving within 3-5 years, I would consider not buying until your long-term plans are more stable. Moreover, a high proportion of physicians change jobs.4,5,6 If you are just starting a new job, it is often wise to wait at least 6-12 months before buying a house to ensure the new job is a good fit. If you are in a stable long-term situation, it may be reasonable to buy a house. While it is commonly believed that buying a house is a “good financial move,” there are many hidden costs to home ownership, including big ticket repairs, property taxes, and real estate fees when selling a home.
First-time physician home buyers can often secure a physician mortgage with competitive interest rates and a low down payment of 0%-10% instead of the traditional 20% down payment. Moreover, a good physician mortgage should not have private mortgage insurance (PMI). Given the variation between mortgage companies, my most important piece of advice is to shop around for a good mortgage. An independent mortgage broker can be very valuable.
Dr. Jain is associate professor of medicine in the division of gastroenterology and hepatology, University of North Carolina School of Medicine, Chapel Hill. He has no conflicts of interest. The information in this article is meant for general educational purposes only. For individualized personal finance advice, please seek your own financial advisor, tax accountant, insurance broker, attorney, or other financial professional. Follow Dr. Jain @AJainMD on X.
References
1. Future of PSLF Fact Sheet
2. The Loophole That Can Get Thousands of Doctors into PSLF
3. Student loan management: An introduction for the young gastroenterologist
4. Study Shows First Job after Medical Residency Often Doesn’t Last
5. More physicians want to leave their jobs as pay rates fall, survey finds
6. Physician turnover rates are climbing as they clamor for better work-life balance
How to think about second-line therapy in NSCLC
This transcript has been edited for clarity.
I’ve been thinking lately about treatments after initial therapy for non–small cell lung cancers, what people often call second-line therapy.
I think the first thought is that, for all the regimens that are available and tested, the results are clearly not as good as seen with first-line therapy. I’ll get into some specifics in a second. That being the case, it’s really important to make the best choice for first-line therapy.
The second thing that is absolutely critical is to very carefully assess when that first-line therapy has stopped working and whether there is a need for a new systemic therapy. We very often have these situations where there is an oligoprogression, and by treating a single symptomatic lesion, you may get the patient in a very good place and may continue initial therapy. Very often, there is inconsequential growth of the cancer.
For example, if there is a 21% increase in the size of a primary tumor that is not associated with any symptoms in a person who is living their life and is not having any severe side effects, you have to think long and hard about changing that therapy. I wouldn’t even give a consolidative therapy there if they’re really doing well. Obviously, consolidative therapies are a new therapy, and they have their side effects with them as well.
With second-line therapy, sadly, none of them have a huge benefit anywhere near what we see in first line. All the rates of response are well under 50%. Just getting into it, you’re not going to shrink the cancer by more than 30% in the majority of patients, so you have to think long and hard about making that switch.
Second, our standard still remains docetaxel, and the numbers on docetaxel are really not great. It’s about a 15% rate of response and a median survival of about 5 months. Now, by adding other RET drugs to docetaxel, you can achieve better results. By adding ramucirumab, for example, the response rate just about doubles and the duration of response and progression-free survival both go up by a few months.
For patients who have KRAS G12C, in the randomized trial that has been done so far, over docetaxel, you get, again, a doubling of response. For patients where response is important, you really double that response rate, but also you get an improvement in median progression-free survival by, again, 2-3 months. There is benefit there in terms of response and progression-free survival; however, it’s not huge.
Please remember, if you’re choosing to use docetaxel, to think about using alternative dosages and schedules. When you look at the course of a person treated with docetaxel over, let’s say, a 6-month period, you often see that doses are held. When you look at the total dose, it’s very similar to an every-2-week dose of a lower amount. I routinely give a 60-mg flat dose every 2 weeks.
I urge you to look at the progress of one of your patients over a 6-month period who was given the 75-mg dose. Many of those doses end up getting held. When all is said and done, you give a lower dose over that whole time from that 75-mg dose. Giving 35 mg/m2 or a 60-mg flat dose every 2 weeks, you end up getting almost exactly the same amount of docetaxel. There’s really no convincing evidence that the higher dose is better. It’s clearly harder on the patient.
I’ve shared some thoughts about second-line therapy. We really have to do better. Please make sure that your first-line therapy is the best you can give. Make sure you’ve gotten everything out of that first-line therapy and that it will be continued as long as possible, as long as you and the patient have concluded that there’s benefit. When you do switch, try to give the most effective regimen that you have, which would be docetaxel with ramucirumab, or for patients with KRAS G12C, giving adagrasib or sotorasib at this point.
Dr. Kris is chief of the thoracic oncology service and the William and Joy Ruane Chair in Thoracic Oncology at Memorial Sloan Kettering Cancer Center in New York. He reported conflicts of interest with AstraZeneca, Roche/Genentech, Ariad Pharmaceuticals, Pfizer, and PUMA.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
I’ve been thinking lately about treatments after initial therapy for non–small cell lung cancers, what people often call second-line therapy.
I think the first thought is that, for all the regimens that are available and tested, the results are clearly not as good as seen with first-line therapy. I’ll get into some specifics in a second. That being the case, it’s really important to make the best choice for first-line therapy.
The second thing that is absolutely critical is to very carefully assess when that first-line therapy has stopped working and whether there is a need for a new systemic therapy. We very often have these situations where there is an oligoprogression, and by treating a single symptomatic lesion, you may get the patient in a very good place and may continue initial therapy. Very often, there is inconsequential growth of the cancer.
For example, if there is a 21% increase in the size of a primary tumor that is not associated with any symptoms in a person who is living their life and is not having any severe side effects, you have to think long and hard about changing that therapy. I wouldn’t even give a consolidative therapy there if they’re really doing well. Obviously, consolidative therapies are a new therapy, and they have their side effects with them as well.
With second-line therapy, sadly, none of them have a huge benefit anywhere near what we see in first line. All the rates of response are well under 50%. Just getting into it, you’re not going to shrink the cancer by more than 30% in the majority of patients, so you have to think long and hard about making that switch.
Second, our standard still remains docetaxel, and the numbers on docetaxel are really not great. It’s about a 15% rate of response and a median survival of about 5 months. Now, by adding other RET drugs to docetaxel, you can achieve better results. By adding ramucirumab, for example, the response rate just about doubles and the duration of response and progression-free survival both go up by a few months.
For patients who have KRAS G12C, in the randomized trial that has been done so far, over docetaxel, you get, again, a doubling of response. For patients where response is important, you really double that response rate, but also you get an improvement in median progression-free survival by, again, 2-3 months. There is benefit there in terms of response and progression-free survival; however, it’s not huge.
Please remember, if you’re choosing to use docetaxel, to think about using alternative dosages and schedules. When you look at the course of a person treated with docetaxel over, let’s say, a 6-month period, you often see that doses are held. When you look at the total dose, it’s very similar to an every-2-week dose of a lower amount. I routinely give a 60-mg flat dose every 2 weeks.
I urge you to look at the progress of one of your patients over a 6-month period who was given the 75-mg dose. Many of those doses end up getting held. When all is said and done, you give a lower dose over that whole time from that 75-mg dose. Giving 35 mg/m2 or a 60-mg flat dose every 2 weeks, you end up getting almost exactly the same amount of docetaxel. There’s really no convincing evidence that the higher dose is better. It’s clearly harder on the patient.
I’ve shared some thoughts about second-line therapy. We really have to do better. Please make sure that your first-line therapy is the best you can give. Make sure you’ve gotten everything out of that first-line therapy and that it will be continued as long as possible, as long as you and the patient have concluded that there’s benefit. When you do switch, try to give the most effective regimen that you have, which would be docetaxel with ramucirumab, or for patients with KRAS G12C, giving adagrasib or sotorasib at this point.
Dr. Kris is chief of the thoracic oncology service and the William and Joy Ruane Chair in Thoracic Oncology at Memorial Sloan Kettering Cancer Center in New York. He reported conflicts of interest with AstraZeneca, Roche/Genentech, Ariad Pharmaceuticals, Pfizer, and PUMA.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
I’ve been thinking lately about treatments after initial therapy for non–small cell lung cancers, what people often call second-line therapy.
I think the first thought is that, for all the regimens that are available and tested, the results are clearly not as good as seen with first-line therapy. I’ll get into some specifics in a second. That being the case, it’s really important to make the best choice for first-line therapy.
The second thing that is absolutely critical is to very carefully assess when that first-line therapy has stopped working and whether there is a need for a new systemic therapy. We very often have these situations where there is an oligoprogression, and by treating a single symptomatic lesion, you may get the patient in a very good place and may continue initial therapy. Very often, there is inconsequential growth of the cancer.
For example, if there is a 21% increase in the size of a primary tumor that is not associated with any symptoms in a person who is living their life and is not having any severe side effects, you have to think long and hard about changing that therapy. I wouldn’t even give a consolidative therapy there if they’re really doing well. Obviously, consolidative therapies are a new therapy, and they have their side effects with them as well.
With second-line therapy, sadly, none of them have a huge benefit anywhere near what we see in first line. All the rates of response are well under 50%. Just getting into it, you’re not going to shrink the cancer by more than 30% in the majority of patients, so you have to think long and hard about making that switch.
Second, our standard still remains docetaxel, and the numbers on docetaxel are really not great. It’s about a 15% rate of response and a median survival of about 5 months. Now, by adding other RET drugs to docetaxel, you can achieve better results. By adding ramucirumab, for example, the response rate just about doubles and the duration of response and progression-free survival both go up by a few months.
For patients who have KRAS G12C, in the randomized trial that has been done so far, over docetaxel, you get, again, a doubling of response. For patients where response is important, you really double that response rate, but also you get an improvement in median progression-free survival by, again, 2-3 months. There is benefit there in terms of response and progression-free survival; however, it’s not huge.
Please remember, if you’re choosing to use docetaxel, to think about using alternative dosages and schedules. When you look at the course of a person treated with docetaxel over, let’s say, a 6-month period, you often see that doses are held. When you look at the total dose, it’s very similar to an every-2-week dose of a lower amount. I routinely give a 60-mg flat dose every 2 weeks.
I urge you to look at the progress of one of your patients over a 6-month period who was given the 75-mg dose. Many of those doses end up getting held. When all is said and done, you give a lower dose over that whole time from that 75-mg dose. Giving 35 mg/m2 or a 60-mg flat dose every 2 weeks, you end up getting almost exactly the same amount of docetaxel. There’s really no convincing evidence that the higher dose is better. It’s clearly harder on the patient.
I’ve shared some thoughts about second-line therapy. We really have to do better. Please make sure that your first-line therapy is the best you can give. Make sure you’ve gotten everything out of that first-line therapy and that it will be continued as long as possible, as long as you and the patient have concluded that there’s benefit. When you do switch, try to give the most effective regimen that you have, which would be docetaxel with ramucirumab, or for patients with KRAS G12C, giving adagrasib or sotorasib at this point.
Dr. Kris is chief of the thoracic oncology service and the William and Joy Ruane Chair in Thoracic Oncology at Memorial Sloan Kettering Cancer Center in New York. He reported conflicts of interest with AstraZeneca, Roche/Genentech, Ariad Pharmaceuticals, Pfizer, and PUMA.
A version of this article first appeared on Medscape.com.
Perinatal depression rarely stands alone
Mental health conditions are the leading cause of pregnancy-related death in Illinois (40%) and across the United States (21%).1,2 Funding bodies, such as the Agency for Healthcare Research and Quality3 and the Health Resources and Service Administration,4 have spotlights on improving screening and access to care for depression and substance use disorders (SUDs). However, the needs of individuals with multiple mental health conditions still often go unrecognized and unaddressed in perinatal health settings.
The U.S. Preventive Services Task Force recommends that all adults be screened for depression, alcohol use, and drug use, and will be recommending screening for anxiety.5,6 The American College of Obstetrics and Gynecology recommends screening for perinatal mental health conditions including depression, anxiety, bipolar disorder, acute postpartum psychosis, and suicidality; however, despite these recommendations, screening and treatment for comorbid mental health disorders during pregnancy and the postpartum is not standard practice.7
Addressing perinatal mental health is critical because untreated mental health conditions during the perinatal period can cause long-term adverse psychiatric and medical outcomes for the birthing person, the baby, and the family.8 This commentary highlights the importance of recognizing and screening for perinatal mental health comorbidities, improving referral rates for mental health treatment, and raising awareness of the importance of addressing rural perinatal mental health.
Perinatal mental health comorbidities
Major depressive disorder is the most common mental health condition during the perinatal period9 and is often comorbid.10-12 In “Perinatal mental health in low-income urban and rural patients: The importance of screening for comorbidities,” Craemer et al.13 reported that nearly half of the perinatal patients who screened positive for MDD also screened positive for at least one other mental health condition, among them general anxiety disorder (GAD), SUD, posttraumatic stress disorder (PTSD), and suicidality.
Many (9%) of the perinatal patients with MDD had a severe comorbidity profile characterized by four diagnoses – MDD, GAD, SUD, and PTSD. In routine medical care these comorbidities often go undetected even though the risk to mothers and babies increases with more severe mental health symptoms.8
The high frequency of perinatal mental health comorbidities Craemer et al.13 found demonstrates a compelling need for comorbid mental health screening during the perinatal period, particularly for low-income Black, Hispanic, and rural birthing persons. Positive screens for perinatal mental health disorders may reflect the onset of these disorders in pregnancy or the postpartum, or preexisting disorders that have gone undetected or untreated before pregnancy.
For many patients, the perinatal period is the first time they are screened for any mental health disorder; typically, they are screened solely for depression. Screening alone can have a positive impact on perinatal mental health. In fact, the USPSTF found that programs to screen perinatal patients, with or without treatment-related support, resulted in a 2%-9% absolute reduction in depression prevalence.14 However, screening for MDD is too infrequent for many reasons, including the logistics of integrating screening into the clinic workflow and limited provider availability, time, and training in mental health.
We recommend screening perinatal patients for mental health comorbidities. This recommendation may seem impractical given the lack of screening tools for comorbid mental health conditions; however, the Computerized Adaptive Test for Mental Health (CAT-MH), the validated tool15-17 used in this study, is an ideal option. CAT-MH is uniquely capable of screening for MDD, GAD, PTSD, SUD, and suicidality in one platform and is routinely used in diverse settings including the Veterans Administration,18 foster care,19 and universities.20 The main limitation of this more comprehensive screening is that it takes about 10 minutes per patient. However, CAT-MH is self-administered and can be done in the waiting room or on a mobile device prior to a clinic visit.
CAT-MH can also be easily integrated into clinical workflow when added to the Electronic Medical Record21, and is a more comprehensive tool than existing perinatal depression tools such as the Perinatal Health Questionaire-9 (PHQ-9) and Edinburgh Perinatal Depression Scale (EPDS).22 Another limitation is cost – currently $5.00 per assessment – however, this is less than routine blood work.23 If CAT-MH is not an option, we recommend a stepped approach of screening for GAD when perinatal patients screen positive for MDD, as this is the most common comorbidity profile. The GAD-7 is a free and widely available tool.24
Barriers to care
In Craemer et al,13 nearly two-thirds (64.9%) of perinatal patients with a positive screen did not receive a referral to follow-up care or a medication prescription. These low referral rates may reflect a variety of widely recognized barriers to care, including lack of referral options, provider and/or patient reluctance to pursue referrals, barriers to insurance coverage, or inadequate behavioral health infrastructure to ensure referral and diagnostic follow-up.
Further, rural residing perinatal patients are an underserved population that need more resources and screening. Despite an on-site behavioral specialist at the rural clinic, Craemer et al13 found a stark disparity in referral rates: referrals to treatment for a positive diagnosis was over two times less at the rural clinic (23.9%), compared with the urban clinics (51.6%). The most common treatment offered at the rural clinic was a prescription for medication (17.4%), while referral to follow-up care was the most common at the urban clinics (35.5%). Rural areas not only have a shortage of health care providers, but community members seeking mental health care often encounter greater stigma, compared with urban residents.25,26
These data highlight an unmet need for referrals to treatment for patients in rural communities, particularly in Illinois where the pregnancy-related mortality ratio attributable to mental health conditions is three times greater in rural areas, compared with those residing in urban Cook County (Chicago).2 Increasing access and availability to mental health treatment and prevention resources in Illinois, especially in rural areas, is an opportunity to prevent pregnancy-related mortality attributable to mental health conditions.
Overall, there is a critical need for screening for perinatal mental health comorbidities, increased attention to low rates of referral to mental health treatment, and investing in rural perinatal mental health. Addressing perinatal mental health disorders is key to decreasing the burden of maternal mortality, particularly in Illinois.
Ms. Craemer and Ms. Sayah are senior research specialists at the Center for Research on Women & Gender, University of Illinois at Chicago. Dr. Duffecy is a professor of clinical psychiatry at the University of Illinois at Chicago. Dr. Geller is a professor of obstetrics & gynecology and director of the Center for Research on Women & Gender, University of Illinois at Chicago. Dr. Maki is a professor of psychiatry, psychology, and obstetrics & gynecology at the University of Illinois at Chicago.
References
1. Trost S et al. Pregnancy-related deaths: Data from maternal mortality review committees in 36 states, 2017-2019. Atlanta: Centers for Disease Control and Prevention, U.S. Department of Health & Human Services, 2022.
2. Illinois Department of Public Health. Illinois maternal morbidity and mortality report 2016-2017. 2021.
3. AHRQ. Funding opportunities to address opioid and other substance use disorders. Updated 2023.
4. HRSA. Screening and treatment for maternal mental health and substance use disorders.
5. U.S. Preventive Services Task Force. Recommendations for primary care practice. Accessed May 26, 2023.
6. U.S. Preventive Services Task Force. Draft recommendation statement: Anxiety in adults: Screening. 2022.
7. ACOG. Screening and diagnosis of mental health conditions during pregnancy and postpartum. Clinical Practice Guideline. Number 4. 2023 June.
8. Meltzer-Brody S and Stuebe A. The long-term psychiatric and medical prognosis of perinatal mental illness. Best Pract Res Clin Obstet Gynaecol. 2014 Jan. doi: 10.1016/j.bpobgyn.2013.08.009.
9. Van Niel MS and Payne JL. Perinatal depression: A review. Cleve Clin J Med. 2020 May. doi: 10.3949/ccjm.87a.19054.
10. Wisner KL et al. Onset timing, thoughts of self-harm, and diagnoses in postpartum women with screen-positive depression findings. 2013 May. doi: 10.1001/jamapsychiatry.2013.87.
11. Falah-Hassani K et al. The prevalence of antenatal and postnatal co-morbid anxiety and depression: A meta-analysis. Psychol Med. 2017 Sep. doi: 10.1017/S0033291717000617.
12. Pentecost R et al. Scoping review of the associations between perinatal substance use and perinatal depression and anxiety. J Obstet Gynecol Neonatal Nurs. 2021 Jul. doi: 10.1016/j.jogn.2021.02.008.
13. Craemer KA et al. Perinatal mental health in low-income urban and rural patients: The importance of screening for comorbidities. Gen Hosp Psychiatry. 2023 Jul-Aug. doi: 10.1016/j.genhosppsych.2023.05.007.
14. O’Connor E et al. Primary care screening for and treatment of depression in pregnant and postpartum women: Evidence report and systematic review for the U.S. Preventive Services Task Force. JAMA. 2016 Jan 26. doi: 10.1001/jama.2015.18948.
15. Kozhimannil KB et al. Racial and ethnic disparities in postpartum depression care among low-income women. Psychiatr Serv. 2011 Jun. doi: 10.1176/ps.62.6.pss6206_0619.
16. Wenzel ES et al. Depression and anxiety symptoms across pregnancy and the postpartum in low-income Black and Latina women. Arch Womens Ment Health. 2021 Dec. doi: 10.1007/s00737-021-01139-y.
17. Gibbons RD et al. Development of a computerized adaptive substance use disorder scale for screening and measurement: The CAT‐SUD. Addiction. 2020 Jul. doi: 10.1111/add.14938.
18. Brenner LA et al. Validation of a computerized adaptive test suicide scale (CAT-SS) among united states military veterans. PloS One. 2022 Jan 21. doi: 10.1371/journal.pone.0261920.
19. The Center for State Child Welfare Data. Using technology to diagnose and report on behavioral health challenges facing foster youth. 2018.
20. Kim JJ et al. The experience of depression, anxiety, and mania among perinatal women. Arch Womens Ment Health. 2016 Oct. doi: 10.1007/s00737-016-0632-6.
21. Tepper MC et al. Toward population health: Using a learning behavioral health system and measurement-based care to improve access, care, outcomes, and disparities. Community Ment Health J. 2022 Nov. doi: 10.1007/s10597-022-00957-3.
22. Wenzel E et al. Using computerised adaptive tests to screen for perinatal depression in underserved women of colour. Evid Based Ment Health. 2022 Feb. doi: 10.1136/ebmental-2021-300262.
23. Sanger-Katz M. They want it to be secret: How a common blood test can cost $11 or almost $1,000. New York Times. 2019 Apr 19.
24. Spitzer RL et al. A brief measure for assessing generalized anxiety disorder: The GAD-7. Arch Intern Med. 2006 May 22. doi: 10.1001/archinte.166.10.1092.
25. Mollard E et al. An integrative review of postpartum depression in rural US communities. Arch Psychiatr Nurs. 2016 Jun. doi: 10.1016/j.apnu.2015.12.003.
26. Anglim AJ and Radke SM. Rural maternal health care outcomes, drivers, and patient perspectives. Clin Obstet Gynecol. 2022 Dec 1. doi: 10.1097/GRF.0000000000000753.
Mental health conditions are the leading cause of pregnancy-related death in Illinois (40%) and across the United States (21%).1,2 Funding bodies, such as the Agency for Healthcare Research and Quality3 and the Health Resources and Service Administration,4 have spotlights on improving screening and access to care for depression and substance use disorders (SUDs). However, the needs of individuals with multiple mental health conditions still often go unrecognized and unaddressed in perinatal health settings.
The U.S. Preventive Services Task Force recommends that all adults be screened for depression, alcohol use, and drug use, and will be recommending screening for anxiety.5,6 The American College of Obstetrics and Gynecology recommends screening for perinatal mental health conditions including depression, anxiety, bipolar disorder, acute postpartum psychosis, and suicidality; however, despite these recommendations, screening and treatment for comorbid mental health disorders during pregnancy and the postpartum is not standard practice.7
Addressing perinatal mental health is critical because untreated mental health conditions during the perinatal period can cause long-term adverse psychiatric and medical outcomes for the birthing person, the baby, and the family.8 This commentary highlights the importance of recognizing and screening for perinatal mental health comorbidities, improving referral rates for mental health treatment, and raising awareness of the importance of addressing rural perinatal mental health.
Perinatal mental health comorbidities
Major depressive disorder is the most common mental health condition during the perinatal period9 and is often comorbid.10-12 In “Perinatal mental health in low-income urban and rural patients: The importance of screening for comorbidities,” Craemer et al.13 reported that nearly half of the perinatal patients who screened positive for MDD also screened positive for at least one other mental health condition, among them general anxiety disorder (GAD), SUD, posttraumatic stress disorder (PTSD), and suicidality.
Many (9%) of the perinatal patients with MDD had a severe comorbidity profile characterized by four diagnoses – MDD, GAD, SUD, and PTSD. In routine medical care these comorbidities often go undetected even though the risk to mothers and babies increases with more severe mental health symptoms.8
The high frequency of perinatal mental health comorbidities Craemer et al.13 found demonstrates a compelling need for comorbid mental health screening during the perinatal period, particularly for low-income Black, Hispanic, and rural birthing persons. Positive screens for perinatal mental health disorders may reflect the onset of these disorders in pregnancy or the postpartum, or preexisting disorders that have gone undetected or untreated before pregnancy.
For many patients, the perinatal period is the first time they are screened for any mental health disorder; typically, they are screened solely for depression. Screening alone can have a positive impact on perinatal mental health. In fact, the USPSTF found that programs to screen perinatal patients, with or without treatment-related support, resulted in a 2%-9% absolute reduction in depression prevalence.14 However, screening for MDD is too infrequent for many reasons, including the logistics of integrating screening into the clinic workflow and limited provider availability, time, and training in mental health.
We recommend screening perinatal patients for mental health comorbidities. This recommendation may seem impractical given the lack of screening tools for comorbid mental health conditions; however, the Computerized Adaptive Test for Mental Health (CAT-MH), the validated tool15-17 used in this study, is an ideal option. CAT-MH is uniquely capable of screening for MDD, GAD, PTSD, SUD, and suicidality in one platform and is routinely used in diverse settings including the Veterans Administration,18 foster care,19 and universities.20 The main limitation of this more comprehensive screening is that it takes about 10 minutes per patient. However, CAT-MH is self-administered and can be done in the waiting room or on a mobile device prior to a clinic visit.
CAT-MH can also be easily integrated into clinical workflow when added to the Electronic Medical Record21, and is a more comprehensive tool than existing perinatal depression tools such as the Perinatal Health Questionaire-9 (PHQ-9) and Edinburgh Perinatal Depression Scale (EPDS).22 Another limitation is cost – currently $5.00 per assessment – however, this is less than routine blood work.23 If CAT-MH is not an option, we recommend a stepped approach of screening for GAD when perinatal patients screen positive for MDD, as this is the most common comorbidity profile. The GAD-7 is a free and widely available tool.24
Barriers to care
In Craemer et al,13 nearly two-thirds (64.9%) of perinatal patients with a positive screen did not receive a referral to follow-up care or a medication prescription. These low referral rates may reflect a variety of widely recognized barriers to care, including lack of referral options, provider and/or patient reluctance to pursue referrals, barriers to insurance coverage, or inadequate behavioral health infrastructure to ensure referral and diagnostic follow-up.
Further, rural residing perinatal patients are an underserved population that need more resources and screening. Despite an on-site behavioral specialist at the rural clinic, Craemer et al13 found a stark disparity in referral rates: referrals to treatment for a positive diagnosis was over two times less at the rural clinic (23.9%), compared with the urban clinics (51.6%). The most common treatment offered at the rural clinic was a prescription for medication (17.4%), while referral to follow-up care was the most common at the urban clinics (35.5%). Rural areas not only have a shortage of health care providers, but community members seeking mental health care often encounter greater stigma, compared with urban residents.25,26
These data highlight an unmet need for referrals to treatment for patients in rural communities, particularly in Illinois where the pregnancy-related mortality ratio attributable to mental health conditions is three times greater in rural areas, compared with those residing in urban Cook County (Chicago).2 Increasing access and availability to mental health treatment and prevention resources in Illinois, especially in rural areas, is an opportunity to prevent pregnancy-related mortality attributable to mental health conditions.
Overall, there is a critical need for screening for perinatal mental health comorbidities, increased attention to low rates of referral to mental health treatment, and investing in rural perinatal mental health. Addressing perinatal mental health disorders is key to decreasing the burden of maternal mortality, particularly in Illinois.
Ms. Craemer and Ms. Sayah are senior research specialists at the Center for Research on Women & Gender, University of Illinois at Chicago. Dr. Duffecy is a professor of clinical psychiatry at the University of Illinois at Chicago. Dr. Geller is a professor of obstetrics & gynecology and director of the Center for Research on Women & Gender, University of Illinois at Chicago. Dr. Maki is a professor of psychiatry, psychology, and obstetrics & gynecology at the University of Illinois at Chicago.
References
1. Trost S et al. Pregnancy-related deaths: Data from maternal mortality review committees in 36 states, 2017-2019. Atlanta: Centers for Disease Control and Prevention, U.S. Department of Health & Human Services, 2022.
2. Illinois Department of Public Health. Illinois maternal morbidity and mortality report 2016-2017. 2021.
3. AHRQ. Funding opportunities to address opioid and other substance use disorders. Updated 2023.
4. HRSA. Screening and treatment for maternal mental health and substance use disorders.
5. U.S. Preventive Services Task Force. Recommendations for primary care practice. Accessed May 26, 2023.
6. U.S. Preventive Services Task Force. Draft recommendation statement: Anxiety in adults: Screening. 2022.
7. ACOG. Screening and diagnosis of mental health conditions during pregnancy and postpartum. Clinical Practice Guideline. Number 4. 2023 June.
8. Meltzer-Brody S and Stuebe A. The long-term psychiatric and medical prognosis of perinatal mental illness. Best Pract Res Clin Obstet Gynaecol. 2014 Jan. doi: 10.1016/j.bpobgyn.2013.08.009.
9. Van Niel MS and Payne JL. Perinatal depression: A review. Cleve Clin J Med. 2020 May. doi: 10.3949/ccjm.87a.19054.
10. Wisner KL et al. Onset timing, thoughts of self-harm, and diagnoses in postpartum women with screen-positive depression findings. 2013 May. doi: 10.1001/jamapsychiatry.2013.87.
11. Falah-Hassani K et al. The prevalence of antenatal and postnatal co-morbid anxiety and depression: A meta-analysis. Psychol Med. 2017 Sep. doi: 10.1017/S0033291717000617.
12. Pentecost R et al. Scoping review of the associations between perinatal substance use and perinatal depression and anxiety. J Obstet Gynecol Neonatal Nurs. 2021 Jul. doi: 10.1016/j.jogn.2021.02.008.
13. Craemer KA et al. Perinatal mental health in low-income urban and rural patients: The importance of screening for comorbidities. Gen Hosp Psychiatry. 2023 Jul-Aug. doi: 10.1016/j.genhosppsych.2023.05.007.
14. O’Connor E et al. Primary care screening for and treatment of depression in pregnant and postpartum women: Evidence report and systematic review for the U.S. Preventive Services Task Force. JAMA. 2016 Jan 26. doi: 10.1001/jama.2015.18948.
15. Kozhimannil KB et al. Racial and ethnic disparities in postpartum depression care among low-income women. Psychiatr Serv. 2011 Jun. doi: 10.1176/ps.62.6.pss6206_0619.
16. Wenzel ES et al. Depression and anxiety symptoms across pregnancy and the postpartum in low-income Black and Latina women. Arch Womens Ment Health. 2021 Dec. doi: 10.1007/s00737-021-01139-y.
17. Gibbons RD et al. Development of a computerized adaptive substance use disorder scale for screening and measurement: The CAT‐SUD. Addiction. 2020 Jul. doi: 10.1111/add.14938.
18. Brenner LA et al. Validation of a computerized adaptive test suicide scale (CAT-SS) among united states military veterans. PloS One. 2022 Jan 21. doi: 10.1371/journal.pone.0261920.
19. The Center for State Child Welfare Data. Using technology to diagnose and report on behavioral health challenges facing foster youth. 2018.
20. Kim JJ et al. The experience of depression, anxiety, and mania among perinatal women. Arch Womens Ment Health. 2016 Oct. doi: 10.1007/s00737-016-0632-6.
21. Tepper MC et al. Toward population health: Using a learning behavioral health system and measurement-based care to improve access, care, outcomes, and disparities. Community Ment Health J. 2022 Nov. doi: 10.1007/s10597-022-00957-3.
22. Wenzel E et al. Using computerised adaptive tests to screen for perinatal depression in underserved women of colour. Evid Based Ment Health. 2022 Feb. doi: 10.1136/ebmental-2021-300262.
23. Sanger-Katz M. They want it to be secret: How a common blood test can cost $11 or almost $1,000. New York Times. 2019 Apr 19.
24. Spitzer RL et al. A brief measure for assessing generalized anxiety disorder: The GAD-7. Arch Intern Med. 2006 May 22. doi: 10.1001/archinte.166.10.1092.
25. Mollard E et al. An integrative review of postpartum depression in rural US communities. Arch Psychiatr Nurs. 2016 Jun. doi: 10.1016/j.apnu.2015.12.003.
26. Anglim AJ and Radke SM. Rural maternal health care outcomes, drivers, and patient perspectives. Clin Obstet Gynecol. 2022 Dec 1. doi: 10.1097/GRF.0000000000000753.
Mental health conditions are the leading cause of pregnancy-related death in Illinois (40%) and across the United States (21%).1,2 Funding bodies, such as the Agency for Healthcare Research and Quality3 and the Health Resources and Service Administration,4 have spotlights on improving screening and access to care for depression and substance use disorders (SUDs). However, the needs of individuals with multiple mental health conditions still often go unrecognized and unaddressed in perinatal health settings.
The U.S. Preventive Services Task Force recommends that all adults be screened for depression, alcohol use, and drug use, and will be recommending screening for anxiety.5,6 The American College of Obstetrics and Gynecology recommends screening for perinatal mental health conditions including depression, anxiety, bipolar disorder, acute postpartum psychosis, and suicidality; however, despite these recommendations, screening and treatment for comorbid mental health disorders during pregnancy and the postpartum is not standard practice.7
Addressing perinatal mental health is critical because untreated mental health conditions during the perinatal period can cause long-term adverse psychiatric and medical outcomes for the birthing person, the baby, and the family.8 This commentary highlights the importance of recognizing and screening for perinatal mental health comorbidities, improving referral rates for mental health treatment, and raising awareness of the importance of addressing rural perinatal mental health.
Perinatal mental health comorbidities
Major depressive disorder is the most common mental health condition during the perinatal period9 and is often comorbid.10-12 In “Perinatal mental health in low-income urban and rural patients: The importance of screening for comorbidities,” Craemer et al.13 reported that nearly half of the perinatal patients who screened positive for MDD also screened positive for at least one other mental health condition, among them general anxiety disorder (GAD), SUD, posttraumatic stress disorder (PTSD), and suicidality.
Many (9%) of the perinatal patients with MDD had a severe comorbidity profile characterized by four diagnoses – MDD, GAD, SUD, and PTSD. In routine medical care these comorbidities often go undetected even though the risk to mothers and babies increases with more severe mental health symptoms.8
The high frequency of perinatal mental health comorbidities Craemer et al.13 found demonstrates a compelling need for comorbid mental health screening during the perinatal period, particularly for low-income Black, Hispanic, and rural birthing persons. Positive screens for perinatal mental health disorders may reflect the onset of these disorders in pregnancy or the postpartum, or preexisting disorders that have gone undetected or untreated before pregnancy.
For many patients, the perinatal period is the first time they are screened for any mental health disorder; typically, they are screened solely for depression. Screening alone can have a positive impact on perinatal mental health. In fact, the USPSTF found that programs to screen perinatal patients, with or without treatment-related support, resulted in a 2%-9% absolute reduction in depression prevalence.14 However, screening for MDD is too infrequent for many reasons, including the logistics of integrating screening into the clinic workflow and limited provider availability, time, and training in mental health.
We recommend screening perinatal patients for mental health comorbidities. This recommendation may seem impractical given the lack of screening tools for comorbid mental health conditions; however, the Computerized Adaptive Test for Mental Health (CAT-MH), the validated tool15-17 used in this study, is an ideal option. CAT-MH is uniquely capable of screening for MDD, GAD, PTSD, SUD, and suicidality in one platform and is routinely used in diverse settings including the Veterans Administration,18 foster care,19 and universities.20 The main limitation of this more comprehensive screening is that it takes about 10 minutes per patient. However, CAT-MH is self-administered and can be done in the waiting room or on a mobile device prior to a clinic visit.
CAT-MH can also be easily integrated into clinical workflow when added to the Electronic Medical Record21, and is a more comprehensive tool than existing perinatal depression tools such as the Perinatal Health Questionaire-9 (PHQ-9) and Edinburgh Perinatal Depression Scale (EPDS).22 Another limitation is cost – currently $5.00 per assessment – however, this is less than routine blood work.23 If CAT-MH is not an option, we recommend a stepped approach of screening for GAD when perinatal patients screen positive for MDD, as this is the most common comorbidity profile. The GAD-7 is a free and widely available tool.24
Barriers to care
In Craemer et al,13 nearly two-thirds (64.9%) of perinatal patients with a positive screen did not receive a referral to follow-up care or a medication prescription. These low referral rates may reflect a variety of widely recognized barriers to care, including lack of referral options, provider and/or patient reluctance to pursue referrals, barriers to insurance coverage, or inadequate behavioral health infrastructure to ensure referral and diagnostic follow-up.
Further, rural residing perinatal patients are an underserved population that need more resources and screening. Despite an on-site behavioral specialist at the rural clinic, Craemer et al13 found a stark disparity in referral rates: referrals to treatment for a positive diagnosis was over two times less at the rural clinic (23.9%), compared with the urban clinics (51.6%). The most common treatment offered at the rural clinic was a prescription for medication (17.4%), while referral to follow-up care was the most common at the urban clinics (35.5%). Rural areas not only have a shortage of health care providers, but community members seeking mental health care often encounter greater stigma, compared with urban residents.25,26
These data highlight an unmet need for referrals to treatment for patients in rural communities, particularly in Illinois where the pregnancy-related mortality ratio attributable to mental health conditions is three times greater in rural areas, compared with those residing in urban Cook County (Chicago).2 Increasing access and availability to mental health treatment and prevention resources in Illinois, especially in rural areas, is an opportunity to prevent pregnancy-related mortality attributable to mental health conditions.
Overall, there is a critical need for screening for perinatal mental health comorbidities, increased attention to low rates of referral to mental health treatment, and investing in rural perinatal mental health. Addressing perinatal mental health disorders is key to decreasing the burden of maternal mortality, particularly in Illinois.
Ms. Craemer and Ms. Sayah are senior research specialists at the Center for Research on Women & Gender, University of Illinois at Chicago. Dr. Duffecy is a professor of clinical psychiatry at the University of Illinois at Chicago. Dr. Geller is a professor of obstetrics & gynecology and director of the Center for Research on Women & Gender, University of Illinois at Chicago. Dr. Maki is a professor of psychiatry, psychology, and obstetrics & gynecology at the University of Illinois at Chicago.
References
1. Trost S et al. Pregnancy-related deaths: Data from maternal mortality review committees in 36 states, 2017-2019. Atlanta: Centers for Disease Control and Prevention, U.S. Department of Health & Human Services, 2022.
2. Illinois Department of Public Health. Illinois maternal morbidity and mortality report 2016-2017. 2021.
3. AHRQ. Funding opportunities to address opioid and other substance use disorders. Updated 2023.
4. HRSA. Screening and treatment for maternal mental health and substance use disorders.
5. U.S. Preventive Services Task Force. Recommendations for primary care practice. Accessed May 26, 2023.
6. U.S. Preventive Services Task Force. Draft recommendation statement: Anxiety in adults: Screening. 2022.
7. ACOG. Screening and diagnosis of mental health conditions during pregnancy and postpartum. Clinical Practice Guideline. Number 4. 2023 June.
8. Meltzer-Brody S and Stuebe A. The long-term psychiatric and medical prognosis of perinatal mental illness. Best Pract Res Clin Obstet Gynaecol. 2014 Jan. doi: 10.1016/j.bpobgyn.2013.08.009.
9. Van Niel MS and Payne JL. Perinatal depression: A review. Cleve Clin J Med. 2020 May. doi: 10.3949/ccjm.87a.19054.
10. Wisner KL et al. Onset timing, thoughts of self-harm, and diagnoses in postpartum women with screen-positive depression findings. 2013 May. doi: 10.1001/jamapsychiatry.2013.87.
11. Falah-Hassani K et al. The prevalence of antenatal and postnatal co-morbid anxiety and depression: A meta-analysis. Psychol Med. 2017 Sep. doi: 10.1017/S0033291717000617.
12. Pentecost R et al. Scoping review of the associations between perinatal substance use and perinatal depression and anxiety. J Obstet Gynecol Neonatal Nurs. 2021 Jul. doi: 10.1016/j.jogn.2021.02.008.
13. Craemer KA et al. Perinatal mental health in low-income urban and rural patients: The importance of screening for comorbidities. Gen Hosp Psychiatry. 2023 Jul-Aug. doi: 10.1016/j.genhosppsych.2023.05.007.
14. O’Connor E et al. Primary care screening for and treatment of depression in pregnant and postpartum women: Evidence report and systematic review for the U.S. Preventive Services Task Force. JAMA. 2016 Jan 26. doi: 10.1001/jama.2015.18948.
15. Kozhimannil KB et al. Racial and ethnic disparities in postpartum depression care among low-income women. Psychiatr Serv. 2011 Jun. doi: 10.1176/ps.62.6.pss6206_0619.
16. Wenzel ES et al. Depression and anxiety symptoms across pregnancy and the postpartum in low-income Black and Latina women. Arch Womens Ment Health. 2021 Dec. doi: 10.1007/s00737-021-01139-y.
17. Gibbons RD et al. Development of a computerized adaptive substance use disorder scale for screening and measurement: The CAT‐SUD. Addiction. 2020 Jul. doi: 10.1111/add.14938.
18. Brenner LA et al. Validation of a computerized adaptive test suicide scale (CAT-SS) among united states military veterans. PloS One. 2022 Jan 21. doi: 10.1371/journal.pone.0261920.
19. The Center for State Child Welfare Data. Using technology to diagnose and report on behavioral health challenges facing foster youth. 2018.
20. Kim JJ et al. The experience of depression, anxiety, and mania among perinatal women. Arch Womens Ment Health. 2016 Oct. doi: 10.1007/s00737-016-0632-6.
21. Tepper MC et al. Toward population health: Using a learning behavioral health system and measurement-based care to improve access, care, outcomes, and disparities. Community Ment Health J. 2022 Nov. doi: 10.1007/s10597-022-00957-3.
22. Wenzel E et al. Using computerised adaptive tests to screen for perinatal depression in underserved women of colour. Evid Based Ment Health. 2022 Feb. doi: 10.1136/ebmental-2021-300262.
23. Sanger-Katz M. They want it to be secret: How a common blood test can cost $11 or almost $1,000. New York Times. 2019 Apr 19.
24. Spitzer RL et al. A brief measure for assessing generalized anxiety disorder: The GAD-7. Arch Intern Med. 2006 May 22. doi: 10.1001/archinte.166.10.1092.
25. Mollard E et al. An integrative review of postpartum depression in rural US communities. Arch Psychiatr Nurs. 2016 Jun. doi: 10.1016/j.apnu.2015.12.003.
26. Anglim AJ and Radke SM. Rural maternal health care outcomes, drivers, and patient perspectives. Clin Obstet Gynecol. 2022 Dec 1. doi: 10.1097/GRF.0000000000000753.
Addressing supply-demand mismatch in GI
Impacts of this supply-demand mismatch are felt daily in our GI practices as we strive to expand access in our clinics and endoscopy suites, particularly in rural and urban underserved communities. In gastroenterology, increased demand for care has been driven by a perfect storm of population growth, increased patient awareness of GI health, and rising incidence of digestive diseases.
Between 2019 and 2034, the U.S. population is expected to grow by 10.6%, while the population aged 65 and older expands by over 42%. Recent increases in the CRC screening–eligible population also have contributed to unprecedented demand for GI care. Furthermore, care delivery has become more complex and time-consuming with the evolution of personalized medicine and high prevalence of comorbid conditions. At the same time, we are faced with a dwindling supply of gastroenterology providers. In 2021, there were 15,678 practicing gastroenterologists in the U.S., over half of whom were 55 years or older. This translates to 1 gastroenterologist per 20,830 people captured in the U.S. Census.
Addressing this striking supply-demand mismatch in GI requires a multi-pronged approach that addresses its complex drivers. First and foremost, we must expand the number of GI fellowship training slots to boost our pipeline. There are approximately 1,840 GI fellows currently in training, a third of whom enter the workforce each year. While the number of GI fellowship slots in the GI fellowship match has slowly increased over time (from 525 available slots across 199 programs in 2019 to 657 slots across 230 programs in 2023), this incremental growth is dwarfed by overall need. Continued advocacy for increased funding to support expansion of training slots is necessary to further move the needle – such lobbying recently led to the addition of 1,000 new Medicare-supported graduate medical education positions across specialties over a 5-year period starting in 2020, illustrating that change is possible. At the same time, we must address the factors that are causing gastroenterologists to leave the workforce prematurely through early retirement or part-time work by investing in innovative solutions to address burnout, reduce administrative burdens, enhance the efficiency of care delivery, and maintain financial viability. By investing in our physician workforce and its sustainability, we can ensure that our profession is better prepared to meet the needs of our growing and increasingly complex patient population now and in the future.
We hope you enjoy the November issue of GI & Hepatology News and have a wonderful Thanksgiving.
Megan A. Adams, MD, JD, MSc
Editor-in-Chief
Impacts of this supply-demand mismatch are felt daily in our GI practices as we strive to expand access in our clinics and endoscopy suites, particularly in rural and urban underserved communities. In gastroenterology, increased demand for care has been driven by a perfect storm of population growth, increased patient awareness of GI health, and rising incidence of digestive diseases.
Between 2019 and 2034, the U.S. population is expected to grow by 10.6%, while the population aged 65 and older expands by over 42%. Recent increases in the CRC screening–eligible population also have contributed to unprecedented demand for GI care. Furthermore, care delivery has become more complex and time-consuming with the evolution of personalized medicine and high prevalence of comorbid conditions. At the same time, we are faced with a dwindling supply of gastroenterology providers. In 2021, there were 15,678 practicing gastroenterologists in the U.S., over half of whom were 55 years or older. This translates to 1 gastroenterologist per 20,830 people captured in the U.S. Census.
Addressing this striking supply-demand mismatch in GI requires a multi-pronged approach that addresses its complex drivers. First and foremost, we must expand the number of GI fellowship training slots to boost our pipeline. There are approximately 1,840 GI fellows currently in training, a third of whom enter the workforce each year. While the number of GI fellowship slots in the GI fellowship match has slowly increased over time (from 525 available slots across 199 programs in 2019 to 657 slots across 230 programs in 2023), this incremental growth is dwarfed by overall need. Continued advocacy for increased funding to support expansion of training slots is necessary to further move the needle – such lobbying recently led to the addition of 1,000 new Medicare-supported graduate medical education positions across specialties over a 5-year period starting in 2020, illustrating that change is possible. At the same time, we must address the factors that are causing gastroenterologists to leave the workforce prematurely through early retirement or part-time work by investing in innovative solutions to address burnout, reduce administrative burdens, enhance the efficiency of care delivery, and maintain financial viability. By investing in our physician workforce and its sustainability, we can ensure that our profession is better prepared to meet the needs of our growing and increasingly complex patient population now and in the future.
We hope you enjoy the November issue of GI & Hepatology News and have a wonderful Thanksgiving.
Megan A. Adams, MD, JD, MSc
Editor-in-Chief
Impacts of this supply-demand mismatch are felt daily in our GI practices as we strive to expand access in our clinics and endoscopy suites, particularly in rural and urban underserved communities. In gastroenterology, increased demand for care has been driven by a perfect storm of population growth, increased patient awareness of GI health, and rising incidence of digestive diseases.
Between 2019 and 2034, the U.S. population is expected to grow by 10.6%, while the population aged 65 and older expands by over 42%. Recent increases in the CRC screening–eligible population also have contributed to unprecedented demand for GI care. Furthermore, care delivery has become more complex and time-consuming with the evolution of personalized medicine and high prevalence of comorbid conditions. At the same time, we are faced with a dwindling supply of gastroenterology providers. In 2021, there were 15,678 practicing gastroenterologists in the U.S., over half of whom were 55 years or older. This translates to 1 gastroenterologist per 20,830 people captured in the U.S. Census.
Addressing this striking supply-demand mismatch in GI requires a multi-pronged approach that addresses its complex drivers. First and foremost, we must expand the number of GI fellowship training slots to boost our pipeline. There are approximately 1,840 GI fellows currently in training, a third of whom enter the workforce each year. While the number of GI fellowship slots in the GI fellowship match has slowly increased over time (from 525 available slots across 199 programs in 2019 to 657 slots across 230 programs in 2023), this incremental growth is dwarfed by overall need. Continued advocacy for increased funding to support expansion of training slots is necessary to further move the needle – such lobbying recently led to the addition of 1,000 new Medicare-supported graduate medical education positions across specialties over a 5-year period starting in 2020, illustrating that change is possible. At the same time, we must address the factors that are causing gastroenterologists to leave the workforce prematurely through early retirement or part-time work by investing in innovative solutions to address burnout, reduce administrative burdens, enhance the efficiency of care delivery, and maintain financial viability. By investing in our physician workforce and its sustainability, we can ensure that our profession is better prepared to meet the needs of our growing and increasingly complex patient population now and in the future.
We hope you enjoy the November issue of GI & Hepatology News and have a wonderful Thanksgiving.
Megan A. Adams, MD, JD, MSc
Editor-in-Chief
Selecting therapies in moderate to severe inflammatory bowel disease: Key factors in decision making
Despite new advances in treatment, head to head clinical trials, which are considered the gold standard when comparing therapies, remain limited. Other comparative effectiveness studies and network meta-analyses are the currently available substitutes to guide decision making.1
While efficacy is often considered first when choosing a drug, other critical factors play a role in tailoring a treatment plan. This article focuses on key considerations to help guide clinical decision making when treating patients with moderate to severe IBD (Figure 1).

Disease activity versus severity
Both disease activity and disease severity should be considered when evaluating a patient for treatment. Disease activity is a cross-sectional view of one’s signs and symptoms which can vary visit to visit. Standardized indices measure disease activity in both Crohn’s disease (CD) and ulcerative colitis (UC).2,3 Disease severity encompasses the overall prognosis of disease over time and includes factors such as the presence or absence of high risk features, prior medication exposure, history of surgery, hospitalizations and the impact on quality of life.4
To prevent disease complications, the goals of treatment should be aimed at both reducing active symptoms (disease activity) but also healing mucosal inflammation, preventing disease progression (disease severity) and downstream sequelae including cancer, hospitalization or surgery.5 Determining the best treatment option takes disease activity and severity into account, in addition to the other key factors listed below (Figure 2).
Extraintestinal manifestations
Inflammation of organs outside of the gastrointestinal tract is common and can occur in up to 50% of patients with IBD.6 The most prevalent extraintestinal manifestations (EIMs) involve the skin and joints, which will be the primary focus in this article. We will also focus on treatment options with the most evidence supporting their use. Peripheral arthritis is often associated with intestinal inflammation, and treatment of underlying IBD can simultaneously improve joint symptoms. Conversely, axial spondyloarthritis does not commonly parallel intestinal inflammation. Anti–tumor necrosis factor (TNF) agents including infliximab and adalimumab are effective for the treatment of both peripheral and axial disease.6
Ustekinumab, an interleukin (IL)-12/23 inhibitor, may be effective for peripheral arthritis, however is ineffective for the treatment of axial spondyloarthritis.6 Janus kinase (JAK) inhibitors which include tofacitinib and upadacitinib are oral small molecules used to treat peripheral and axial spondyloarthritis and have more recently been approved for moderate to severe IBD.6,7
Erythema nodosum (EN) and pyoderma gangrenosum (PG) are skin manifestations seen in patients with IBD. EN appears as subcutaneous nodules and parallels intestinal inflammation, while PG consists of violaceous, ulcerated plaques, and presents with more significant pain. Anti-TNFs are effective for both EN and PG, with infliximab being the only biologic studied in a randomized control trial of patients with PG.8 In addition, small case reports have described some benefit from ustekinumab and upadacitinib in the treatment of PG.9,10
Safety
The safety of IBD therapies is a key consideration and often the most important factor to patients when choosing a treatment option. It is important to note that untreated disease is associated with significant morbidity, and should be weighed when discussing risks of medications with patients. In general, anti-TNFs and JAK inhibitors may be associated with an increased risk of infection and malignancy, while ustekinumab, vedolizumab, risankizumab and ozanimod offer a more favorable safety profile.11 In large registries and observational studies, infliximab was associated with up to a two times greater risk of serious infection as compared to nonbiologic medications, with the most common infections being pneumonia, sepsis and herpes zoster.12 JAK inhibitors are associated with an increased risk of herpes zoster infection, with a dose dependent effect seen in the maintenance clinical trials with tofacitinib.7
Ozanimod may be associated with atrioventricular conduction delays and bradycardia, however long-term safety data has reported a low incidence of serious cardiac related adverse events.13 Overall, though risks of infection may vary with different therapies, other consistent risk factors associated with greater rates of serious infection include prolonged corticosteroid use, combination therapy with thiopurines, and disease severity. Anti-TNFs have also been associated with a somewhat increased risk of lymphoma, increased when used in combination with thiopurines. Reassuringly, however, in patients with a prior history of cancer, anti-TNFs and non-TNF biologics have not been found to increase the risk of new or recurrent cancer.14
Ultimately, in patients with a prior history of cancer, the choice of biologic or small molecule should be made in collaboration with a patient’s oncologist.
Anti-TNF exposure
Anti-TNFs were the first available biologics for the treatment of IBD. After the approval of vedolizumab in 2014, the first non-TNF biologic, many patients enrolled in clinical trials thereafter had already tried and failed anti-TNFs. In general, exposure to anti-TNFs may reduce the efficacy of a future biologic. In patients treated with vedolizumab, endoscopic and clinical outcomes were negatively impacted by prior anti-TNF exposure.15 However, in VARSITY, a head-to-head clinical trial where 20% of patients with UC were previously exposed to anti-TNFs other than adalimumab, vedolizumab had significantly higher rates of clinical remission and endoscopic improvement compared to adalimumab.16 Clinical remission rates with tofacitinib were not impacted by exposure to anti-TNF treatment, and similar findings were observed with ustekinumab.7,17 Risankizumab, a newly approved selective anti-IL23, also does not appear to be impacted by prior anti-TNF exposure by demonstrating similar rates of clinical remission regardless of biologic exposure status.18 Therefore, in patients with prior history of anti-TNF use, consideration of ustekinumab, risankizumab or JAK inhibitors as second line agents may be more favorable as compared to vedolizumab.
Perianal fistulizing disease
Perianal fistulizing disease can affect up to one-third of patients with CD and significantly impact a patient’s quality of life.19 The most robust data for the treatment of perianal fistulizing disease includes the use of infliximab with up to one-third of patients on maintenance therapy achieving complete resolution of fistula drainage. While no head-to-head trials compare combination therapy with infliximab plus immunomodulators versus infliximab alone for this indication specifically, one observational study demonstrated higher rates of fistula closure with combination therapy as compared to infliximab mono-therapy.19 In a post hoc analysis, higher infliximab concentrations at week 14 were associated with greater fistula response and remission rates.20 In patients with perianal disease, ustekinumab and vedolizumab may also be an effective treatment option by promoting resolution of fistula drainage.21
More recently, emerging data demonstrate that upadacitinib may be an excellent option as a second-line treatment for perianal disease in patients who have failed anti-TNF therapy. Use of upadacitinib was associated with greater rates of complete resolution of fistula drainage and higher rates of external fistula closure (Figure 2).22 Lastly, as an alternative to medical therapy, mesenchymal stem cell therapy has also shown to improve fistula drainage and improve external fistula openings in patients with CD.23 Stem cell therapy is only available through clinical trials at this time.
Patient preferences
Overall, data are lacking for evaluating patient preferences in treatment options for IBD especially with the recent increase in therapeutic options. One survey demonstrated that patient preferences were most impacted by the possibility of improving abdominal pain, with patients accepting additional risk of treatment side effects in order to reduce their abdominal pain.24 An oral route of administration and improving fatigue and bowel urgency were similarly important to patients. Patient preferences can also be highly variable with some valuing avoidance of corticosteroid use while others valuing avoidance of symptoms or risks of medication side effects and surgery. It is important to tailor the discussion on treatment strategies to each individual patient and inquire about the patient’s lifestyle, medical history, and value system, which may impact their treatment preferences utilizing shared decision making.
Access to treatment including the role of social determinants of health
The expanded therapeutic armamentarium has the potential to help patients achieve the current goals of care in IBD. However, these medications are not available to all patients due to numerous barriers including step therapy payer policies, prohibitive costs, insurance prior authorizations, and the role of social determinants of health and proximity to IBD expertise.25 While clinicians work with patients to determine the best treatment option, more often than not, the decision lies with the insurance payer. Step therapy is the protocol used by insurance companies that requires patients to try a lower-cost medication and fail to respond before they approve the originally requested treatment. This can lead to treatment delays, progression of disease, and disease complications. The option to incorporate the use of biosimilars, currently available for anti-TNFs, and other biologics in the near future, will reduce cost and potentially increase access.26 Additionally, working with a clinical pharmacist to navigate access and utilize patient assistance programs may help overcome cost related barriers to treatment and prevent delays in care.
Socioeconomic status has been shown to impact IBD disease outcomes, and compliance rates in treatment vary depending on race and ethnicity.27 Certain racial and ethnic groups remain vulnerable and may require additional support to achieve treatment goals. For example, disparities in health literacy in patients with IBD have been demonstrated with older black men at risk.28 Additionally, the patient’s proximity to their health care facility may impact treatment options. Most IBD centers are located in metropolitan areas and numerous “IBD deserts” exist, potentially limiting therapies for patients from more remote/rural settings.29 Access to treatment and the interplay of social determinants of health can have a large role in therapy selection.
Special considerations: Pregnancy and older adults
Certain patient populations warrant special consideration when approaching treatment strategies. Pregnancy in IBD will not be addressed in full depth in this article, however a key takeaway is that planning is critical and providers should emphasize the importance of steroid-free clinical remission for at least 3 months before conception.30 Additionally, biologic use during pregnancy has not been shown to increase adverse fetal outcomes, thus should be continued to minimize disease flare. Newer novel small molecules are generally avoided during pregnancy due to limited available safety data.
Older adults are the largest growing patient population with IBD. Frailty, or a state of decreased reserve, is more commonly observed in older patients and has been shown to increase adverse events including hospitalization and mortaility.31 Ultimately reducing polypharmacy, ensuring adequate nutrition, minimizing corticosteroid exposure and avoiding undertreatment of active IBD are all key in optimizing outcomes in an older patient with IBD.
Conclusion
When discussing treatment options with patients with IBD, it is important to individualize care and share the decision-making process with patients. Goals include improving symptoms and quality of life while working to achieve the goal of healing intestinal inflammation. In summary, this article can serve as a guide to clinicians for key factors in decision making when selecting therapies in moderate to severe IBD.
Dr. Holmer is a gastroenterologist with NYU Langone Health specializing in inflammatory bowel disease. Dr. Chang is director of clinical operations for the NYU Langone Health Inflammatory Bowel Disease Center. Dr. Malter is director of education for the Inflammatory Bowel Disease Center at NYU Langone Health and director of the inflammatory bowel disease program at Bellevue Hospital Center. Follow Dr. Holmer on X (formerly Twitter) at @HolmerMd and Dr. Chang @shannonchangmd. Dr. Holmer disclosed affiliations with Pfizer, Bristol Myers Squibb, and AvevoRx. Dr. Chang disclosed affiliations with Pfizer and Bristol Myers Squibb. Dr. Malter disclosed receiving educational grants form Abbvie, Janssen, Pfizer and Takeda, and serving on the advisory boards of AbbVie, Bristol Myers Squibb, Celltrion, Janssen, Merck, and Takeda.
References
1. Chang S et al. Am J Gastroenterol. 2023 Aug 24. doi: 10.14309/ajg.0000000000002485.
2. Harvey RF et al. The Lancet. 1980;1:514.
3. Lewis JD et al. Inflammatory Bowel Diseases. 2008;14:1660-1666.
4. Siegel CA et al. Gut. 2018;67(2):244-54.
5. Peyrin-Biroulet L et al. Am J Gastroenterol. 2015;110:1324-38
6. Rogler G et al. Gastroenterology. 2021;161:1118-32.
7. Sandborn WJ et al. N Engl J Med. 2017;376:1723-36.
8. Brooklyn TN et al. Gut. 2006;55:505-9.
9. Fahmy M et al. Am J Gastroenterol. 2012;107:794-5.
10. Van Eycken L et al. JAAD Case Rep. 2023;37:89-91.
11. Lasa JS et al. Lancet Gastroenterol Hepatol. 2022;7:161-70.
12. Lichtenstein GR et al. Inflamm Bowel Dis. 2018;24:490-501.
13. Long MD et al. Gastroenterology. 2022;162:S-5-S-6.
14. Holmer AK et al. Clin Gastroenterol Hepatol.2023;21:1598-1606.e5.
15. Sands BE et al. Gastroenterology. 2014;147:618-27.e3.
16. Sands BE et al. N Engl J Med. 2019;381:1215-26.
17. Sands BE et al. N Engl J Med. 2019;381:1201-14.
18. D’Haens G et al. Lancet. 2022;399:2015-30.
19. Bouguen G et al. Clin Gastroenterol Hepatol. 2013;11:975-81.e1-4.
20. Papamichael K et al. Am J Gastroenterol. 2021;116:1007-14.
21. Shehab M et al. Inflamm Bowel Dis. 2023;29:367-75.
22. Colombel JF et al. J Crohns Colitis. 2023;17:i620-i623.
23. Garcia-Olmo D et al. Dis Colon Rectum. 2022;65:713-20.
24. Louis E et al. J Crohns Colitis. 2023;17:231-9.
25. Rubin DT et al. Inflamm Bowel Dis. 2017;23:224-32.
26. Gulacsi L et al. Curr Med Chem. 2019;26:259-69.
27. Cai Q et al. BMC Gastroenterol. 2022;22:545.
28. Dos Santos Marques IC et al. Crohns Colitis 360. 2020 Oct;2(4):otaa076.
29. Deepak P et al. Gastroenterology. 2023;165:11-15.
30. Mahadevan U et al. Gastroenterology. 2019;156:1508-24.
31. Faye AS et al. Inflamm Bowel Dis. 2022;28:126-32.
32. Berinstein JA et al. Clin Gastroenterol Hepatol. 2021;19:2112-20.e1.
33. Levine J et al. Gastroenterology. 2023;164:S103-S104.
Despite new advances in treatment, head to head clinical trials, which are considered the gold standard when comparing therapies, remain limited. Other comparative effectiveness studies and network meta-analyses are the currently available substitutes to guide decision making.1
While efficacy is often considered first when choosing a drug, other critical factors play a role in tailoring a treatment plan. This article focuses on key considerations to help guide clinical decision making when treating patients with moderate to severe IBD (Figure 1).

Disease activity versus severity
Both disease activity and disease severity should be considered when evaluating a patient for treatment. Disease activity is a cross-sectional view of one’s signs and symptoms which can vary visit to visit. Standardized indices measure disease activity in both Crohn’s disease (CD) and ulcerative colitis (UC).2,3 Disease severity encompasses the overall prognosis of disease over time and includes factors such as the presence or absence of high risk features, prior medication exposure, history of surgery, hospitalizations and the impact on quality of life.4
To prevent disease complications, the goals of treatment should be aimed at both reducing active symptoms (disease activity) but also healing mucosal inflammation, preventing disease progression (disease severity) and downstream sequelae including cancer, hospitalization or surgery.5 Determining the best treatment option takes disease activity and severity into account, in addition to the other key factors listed below (Figure 2).

Extraintestinal manifestations
Inflammation of organs outside of the gastrointestinal tract is common and can occur in up to 50% of patients with IBD.6 The most prevalent extraintestinal manifestations (EIMs) involve the skin and joints, which will be the primary focus in this article. We will also focus on treatment options with the most evidence supporting their use. Peripheral arthritis is often associated with intestinal inflammation, and treatment of underlying IBD can simultaneously improve joint symptoms. Conversely, axial spondyloarthritis does not commonly parallel intestinal inflammation. Anti–tumor necrosis factor (TNF) agents including infliximab and adalimumab are effective for the treatment of both peripheral and axial disease.6
Ustekinumab, an interleukin (IL)-12/23 inhibitor, may be effective for peripheral arthritis, however is ineffective for the treatment of axial spondyloarthritis.6 Janus kinase (JAK) inhibitors which include tofacitinib and upadacitinib are oral small molecules used to treat peripheral and axial spondyloarthritis and have more recently been approved for moderate to severe IBD.6,7
Erythema nodosum (EN) and pyoderma gangrenosum (PG) are skin manifestations seen in patients with IBD. EN appears as subcutaneous nodules and parallels intestinal inflammation, while PG consists of violaceous, ulcerated plaques, and presents with more significant pain. Anti-TNFs are effective for both EN and PG, with infliximab being the only biologic studied in a randomized control trial of patients with PG.8 In addition, small case reports have described some benefit from ustekinumab and upadacitinib in the treatment of PG.9,10
Safety
The safety of IBD therapies is a key consideration and often the most important factor to patients when choosing a treatment option. It is important to note that untreated disease is associated with significant morbidity, and should be weighed when discussing risks of medications with patients. In general, anti-TNFs and JAK inhibitors may be associated with an increased risk of infection and malignancy, while ustekinumab, vedolizumab, risankizumab and ozanimod offer a more favorable safety profile.11 In large registries and observational studies, infliximab was associated with up to a two times greater risk of serious infection as compared to nonbiologic medications, with the most common infections being pneumonia, sepsis and herpes zoster.12 JAK inhibitors are associated with an increased risk of herpes zoster infection, with a dose dependent effect seen in the maintenance clinical trials with tofacitinib.7
Ozanimod may be associated with atrioventricular conduction delays and bradycardia, however long-term safety data has reported a low incidence of serious cardiac related adverse events.13 Overall, though risks of infection may vary with different therapies, other consistent risk factors associated with greater rates of serious infection include prolonged corticosteroid use, combination therapy with thiopurines, and disease severity. Anti-TNFs have also been associated with a somewhat increased risk of lymphoma, increased when used in combination with thiopurines. Reassuringly, however, in patients with a prior history of cancer, anti-TNFs and non-TNF biologics have not been found to increase the risk of new or recurrent cancer.14
Ultimately, in patients with a prior history of cancer, the choice of biologic or small molecule should be made in collaboration with a patient’s oncologist.
Anti-TNF exposure
Anti-TNFs were the first available biologics for the treatment of IBD. After the approval of vedolizumab in 2014, the first non-TNF biologic, many patients enrolled in clinical trials thereafter had already tried and failed anti-TNFs. In general, exposure to anti-TNFs may reduce the efficacy of a future biologic. In patients treated with vedolizumab, endoscopic and clinical outcomes were negatively impacted by prior anti-TNF exposure.15 However, in VARSITY, a head-to-head clinical trial where 20% of patients with UC were previously exposed to anti-TNFs other than adalimumab, vedolizumab had significantly higher rates of clinical remission and endoscopic improvement compared to adalimumab.16 Clinical remission rates with tofacitinib were not impacted by exposure to anti-TNF treatment, and similar findings were observed with ustekinumab.7,17 Risankizumab, a newly approved selective anti-IL23, also does not appear to be impacted by prior anti-TNF exposure by demonstrating similar rates of clinical remission regardless of biologic exposure status.18 Therefore, in patients with prior history of anti-TNF use, consideration of ustekinumab, risankizumab or JAK inhibitors as second line agents may be more favorable as compared to vedolizumab.
Perianal fistulizing disease
Perianal fistulizing disease can affect up to one-third of patients with CD and significantly impact a patient’s quality of life.19 The most robust data for the treatment of perianal fistulizing disease includes the use of infliximab with up to one-third of patients on maintenance therapy achieving complete resolution of fistula drainage. While no head-to-head trials compare combination therapy with infliximab plus immunomodulators versus infliximab alone for this indication specifically, one observational study demonstrated higher rates of fistula closure with combination therapy as compared to infliximab mono-therapy.19 In a post hoc analysis, higher infliximab concentrations at week 14 were associated with greater fistula response and remission rates.20 In patients with perianal disease, ustekinumab and vedolizumab may also be an effective treatment option by promoting resolution of fistula drainage.21
More recently, emerging data demonstrate that upadacitinib may be an excellent option as a second-line treatment for perianal disease in patients who have failed anti-TNF therapy. Use of upadacitinib was associated with greater rates of complete resolution of fistula drainage and higher rates of external fistula closure (Figure 2).22 Lastly, as an alternative to medical therapy, mesenchymal stem cell therapy has also shown to improve fistula drainage and improve external fistula openings in patients with CD.23 Stem cell therapy is only available through clinical trials at this time.
Patient preferences
Overall, data are lacking for evaluating patient preferences in treatment options for IBD especially with the recent increase in therapeutic options. One survey demonstrated that patient preferences were most impacted by the possibility of improving abdominal pain, with patients accepting additional risk of treatment side effects in order to reduce their abdominal pain.24 An oral route of administration and improving fatigue and bowel urgency were similarly important to patients. Patient preferences can also be highly variable with some valuing avoidance of corticosteroid use while others valuing avoidance of symptoms or risks of medication side effects and surgery. It is important to tailor the discussion on treatment strategies to each individual patient and inquire about the patient’s lifestyle, medical history, and value system, which may impact their treatment preferences utilizing shared decision making.
Access to treatment including the role of social determinants of health
The expanded therapeutic armamentarium has the potential to help patients achieve the current goals of care in IBD. However, these medications are not available to all patients due to numerous barriers including step therapy payer policies, prohibitive costs, insurance prior authorizations, and the role of social determinants of health and proximity to IBD expertise.25 While clinicians work with patients to determine the best treatment option, more often than not, the decision lies with the insurance payer. Step therapy is the protocol used by insurance companies that requires patients to try a lower-cost medication and fail to respond before they approve the originally requested treatment. This can lead to treatment delays, progression of disease, and disease complications. The option to incorporate the use of biosimilars, currently available for anti-TNFs, and other biologics in the near future, will reduce cost and potentially increase access.26 Additionally, working with a clinical pharmacist to navigate access and utilize patient assistance programs may help overcome cost related barriers to treatment and prevent delays in care.
Socioeconomic status has been shown to impact IBD disease outcomes, and compliance rates in treatment vary depending on race and ethnicity.27 Certain racial and ethnic groups remain vulnerable and may require additional support to achieve treatment goals. For example, disparities in health literacy in patients with IBD have been demonstrated with older black men at risk.28 Additionally, the patient’s proximity to their health care facility may impact treatment options. Most IBD centers are located in metropolitan areas and numerous “IBD deserts” exist, potentially limiting therapies for patients from more remote/rural settings.29 Access to treatment and the interplay of social determinants of health can have a large role in therapy selection.
Special considerations: Pregnancy and older adults
Certain patient populations warrant special consideration when approaching treatment strategies. Pregnancy in IBD will not be addressed in full depth in this article, however a key takeaway is that planning is critical and providers should emphasize the importance of steroid-free clinical remission for at least 3 months before conception.30 Additionally, biologic use during pregnancy has not been shown to increase adverse fetal outcomes, thus should be continued to minimize disease flare. Newer novel small molecules are generally avoided during pregnancy due to limited available safety data.
Older adults are the largest growing patient population with IBD. Frailty, or a state of decreased reserve, is more commonly observed in older patients and has been shown to increase adverse events including hospitalization and mortaility.31 Ultimately reducing polypharmacy, ensuring adequate nutrition, minimizing corticosteroid exposure and avoiding undertreatment of active IBD are all key in optimizing outcomes in an older patient with IBD.
Conclusion
When discussing treatment options with patients with IBD, it is important to individualize care and share the decision-making process with patients. Goals include improving symptoms and quality of life while working to achieve the goal of healing intestinal inflammation. In summary, this article can serve as a guide to clinicians for key factors in decision making when selecting therapies in moderate to severe IBD.
Dr. Holmer is a gastroenterologist with NYU Langone Health specializing in inflammatory bowel disease. Dr. Chang is director of clinical operations for the NYU Langone Health Inflammatory Bowel Disease Center. Dr. Malter is director of education for the Inflammatory Bowel Disease Center at NYU Langone Health and director of the inflammatory bowel disease program at Bellevue Hospital Center. Follow Dr. Holmer on X (formerly Twitter) at @HolmerMd and Dr. Chang @shannonchangmd. Dr. Holmer disclosed affiliations with Pfizer, Bristol Myers Squibb, and AvevoRx. Dr. Chang disclosed affiliations with Pfizer and Bristol Myers Squibb. Dr. Malter disclosed receiving educational grants form Abbvie, Janssen, Pfizer and Takeda, and serving on the advisory boards of AbbVie, Bristol Myers Squibb, Celltrion, Janssen, Merck, and Takeda.
References
1. Chang S et al. Am J Gastroenterol. 2023 Aug 24. doi: 10.14309/ajg.0000000000002485.
2. Harvey RF et al. The Lancet. 1980;1:514.
3. Lewis JD et al. Inflammatory Bowel Diseases. 2008;14:1660-1666.
4. Siegel CA et al. Gut. 2018;67(2):244-54.
5. Peyrin-Biroulet L et al. Am J Gastroenterol. 2015;110:1324-38
6. Rogler G et al. Gastroenterology. 2021;161:1118-32.
7. Sandborn WJ et al. N Engl J Med. 2017;376:1723-36.
8. Brooklyn TN et al. Gut. 2006;55:505-9.
9. Fahmy M et al. Am J Gastroenterol. 2012;107:794-5.
10. Van Eycken L et al. JAAD Case Rep. 2023;37:89-91.
11. Lasa JS et al. Lancet Gastroenterol Hepatol. 2022;7:161-70.
12. Lichtenstein GR et al. Inflamm Bowel Dis. 2018;24:490-501.
13. Long MD et al. Gastroenterology. 2022;162:S-5-S-6.
14. Holmer AK et al. Clin Gastroenterol Hepatol.2023;21:1598-1606.e5.
15. Sands BE et al. Gastroenterology. 2014;147:618-27.e3.
16. Sands BE et al. N Engl J Med. 2019;381:1215-26.
17. Sands BE et al. N Engl J Med. 2019;381:1201-14.
18. D’Haens G et al. Lancet. 2022;399:2015-30.
19. Bouguen G et al. Clin Gastroenterol Hepatol. 2013;11:975-81.e1-4.
20. Papamichael K et al. Am J Gastroenterol. 2021;116:1007-14.
21. Shehab M et al. Inflamm Bowel Dis. 2023;29:367-75.
22. Colombel JF et al. J Crohns Colitis. 2023;17:i620-i623.
23. Garcia-Olmo D et al. Dis Colon Rectum. 2022;65:713-20.
24. Louis E et al. J Crohns Colitis. 2023;17:231-9.
25. Rubin DT et al. Inflamm Bowel Dis. 2017;23:224-32.
26. Gulacsi L et al. Curr Med Chem. 2019;26:259-69.
27. Cai Q et al. BMC Gastroenterol. 2022;22:545.
28. Dos Santos Marques IC et al. Crohns Colitis 360. 2020 Oct;2(4):otaa076.
29. Deepak P et al. Gastroenterology. 2023;165:11-15.
30. Mahadevan U et al. Gastroenterology. 2019;156:1508-24.
31. Faye AS et al. Inflamm Bowel Dis. 2022;28:126-32.
32. Berinstein JA et al. Clin Gastroenterol Hepatol. 2021;19:2112-20.e1.
33. Levine J et al. Gastroenterology. 2023;164:S103-S104.
Despite new advances in treatment, head to head clinical trials, which are considered the gold standard when comparing therapies, remain limited. Other comparative effectiveness studies and network meta-analyses are the currently available substitutes to guide decision making.1
While efficacy is often considered first when choosing a drug, other critical factors play a role in tailoring a treatment plan. This article focuses on key considerations to help guide clinical decision making when treating patients with moderate to severe IBD (Figure 1).

Disease activity versus severity
Both disease activity and disease severity should be considered when evaluating a patient for treatment. Disease activity is a cross-sectional view of one’s signs and symptoms which can vary visit to visit. Standardized indices measure disease activity in both Crohn’s disease (CD) and ulcerative colitis (UC).2,3 Disease severity encompasses the overall prognosis of disease over time and includes factors such as the presence or absence of high risk features, prior medication exposure, history of surgery, hospitalizations and the impact on quality of life.4
To prevent disease complications, the goals of treatment should be aimed at both reducing active symptoms (disease activity) but also healing mucosal inflammation, preventing disease progression (disease severity) and downstream sequelae including cancer, hospitalization or surgery.5 Determining the best treatment option takes disease activity and severity into account, in addition to the other key factors listed below (Figure 2).

Extraintestinal manifestations
Inflammation of organs outside of the gastrointestinal tract is common and can occur in up to 50% of patients with IBD.6 The most prevalent extraintestinal manifestations (EIMs) involve the skin and joints, which will be the primary focus in this article. We will also focus on treatment options with the most evidence supporting their use. Peripheral arthritis is often associated with intestinal inflammation, and treatment of underlying IBD can simultaneously improve joint symptoms. Conversely, axial spondyloarthritis does not commonly parallel intestinal inflammation. Anti–tumor necrosis factor (TNF) agents including infliximab and adalimumab are effective for the treatment of both peripheral and axial disease.6
Ustekinumab, an interleukin (IL)-12/23 inhibitor, may be effective for peripheral arthritis, however is ineffective for the treatment of axial spondyloarthritis.6 Janus kinase (JAK) inhibitors which include tofacitinib and upadacitinib are oral small molecules used to treat peripheral and axial spondyloarthritis and have more recently been approved for moderate to severe IBD.6,7
Erythema nodosum (EN) and pyoderma gangrenosum (PG) are skin manifestations seen in patients with IBD. EN appears as subcutaneous nodules and parallels intestinal inflammation, while PG consists of violaceous, ulcerated plaques, and presents with more significant pain. Anti-TNFs are effective for both EN and PG, with infliximab being the only biologic studied in a randomized control trial of patients with PG.8 In addition, small case reports have described some benefit from ustekinumab and upadacitinib in the treatment of PG.9,10
Safety
The safety of IBD therapies is a key consideration and often the most important factor to patients when choosing a treatment option. It is important to note that untreated disease is associated with significant morbidity, and should be weighed when discussing risks of medications with patients. In general, anti-TNFs and JAK inhibitors may be associated with an increased risk of infection and malignancy, while ustekinumab, vedolizumab, risankizumab and ozanimod offer a more favorable safety profile.11 In large registries and observational studies, infliximab was associated with up to a two times greater risk of serious infection as compared to nonbiologic medications, with the most common infections being pneumonia, sepsis and herpes zoster.12 JAK inhibitors are associated with an increased risk of herpes zoster infection, with a dose dependent effect seen in the maintenance clinical trials with tofacitinib.7
Ozanimod may be associated with atrioventricular conduction delays and bradycardia, however long-term safety data has reported a low incidence of serious cardiac related adverse events.13 Overall, though risks of infection may vary with different therapies, other consistent risk factors associated with greater rates of serious infection include prolonged corticosteroid use, combination therapy with thiopurines, and disease severity. Anti-TNFs have also been associated with a somewhat increased risk of lymphoma, increased when used in combination with thiopurines. Reassuringly, however, in patients with a prior history of cancer, anti-TNFs and non-TNF biologics have not been found to increase the risk of new or recurrent cancer.14
Ultimately, in patients with a prior history of cancer, the choice of biologic or small molecule should be made in collaboration with a patient’s oncologist.
Anti-TNF exposure
Anti-TNFs were the first available biologics for the treatment of IBD. After the approval of vedolizumab in 2014, the first non-TNF biologic, many patients enrolled in clinical trials thereafter had already tried and failed anti-TNFs. In general, exposure to anti-TNFs may reduce the efficacy of a future biologic. In patients treated with vedolizumab, endoscopic and clinical outcomes were negatively impacted by prior anti-TNF exposure.15 However, in VARSITY, a head-to-head clinical trial where 20% of patients with UC were previously exposed to anti-TNFs other than adalimumab, vedolizumab had significantly higher rates of clinical remission and endoscopic improvement compared to adalimumab.16 Clinical remission rates with tofacitinib were not impacted by exposure to anti-TNF treatment, and similar findings were observed with ustekinumab.7,17 Risankizumab, a newly approved selective anti-IL23, also does not appear to be impacted by prior anti-TNF exposure by demonstrating similar rates of clinical remission regardless of biologic exposure status.18 Therefore, in patients with prior history of anti-TNF use, consideration of ustekinumab, risankizumab or JAK inhibitors as second line agents may be more favorable as compared to vedolizumab.
Perianal fistulizing disease
Perianal fistulizing disease can affect up to one-third of patients with CD and significantly impact a patient’s quality of life.19 The most robust data for the treatment of perianal fistulizing disease includes the use of infliximab with up to one-third of patients on maintenance therapy achieving complete resolution of fistula drainage. While no head-to-head trials compare combination therapy with infliximab plus immunomodulators versus infliximab alone for this indication specifically, one observational study demonstrated higher rates of fistula closure with combination therapy as compared to infliximab mono-therapy.19 In a post hoc analysis, higher infliximab concentrations at week 14 were associated with greater fistula response and remission rates.20 In patients with perianal disease, ustekinumab and vedolizumab may also be an effective treatment option by promoting resolution of fistula drainage.21
More recently, emerging data demonstrate that upadacitinib may be an excellent option as a second-line treatment for perianal disease in patients who have failed anti-TNF therapy. Use of upadacitinib was associated with greater rates of complete resolution of fistula drainage and higher rates of external fistula closure (Figure 2).22 Lastly, as an alternative to medical therapy, mesenchymal stem cell therapy has also shown to improve fistula drainage and improve external fistula openings in patients with CD.23 Stem cell therapy is only available through clinical trials at this time.
Patient preferences
Overall, data are lacking for evaluating patient preferences in treatment options for IBD especially with the recent increase in therapeutic options. One survey demonstrated that patient preferences were most impacted by the possibility of improving abdominal pain, with patients accepting additional risk of treatment side effects in order to reduce their abdominal pain.24 An oral route of administration and improving fatigue and bowel urgency were similarly important to patients. Patient preferences can also be highly variable with some valuing avoidance of corticosteroid use while others valuing avoidance of symptoms or risks of medication side effects and surgery. It is important to tailor the discussion on treatment strategies to each individual patient and inquire about the patient’s lifestyle, medical history, and value system, which may impact their treatment preferences utilizing shared decision making.
Access to treatment including the role of social determinants of health
The expanded therapeutic armamentarium has the potential to help patients achieve the current goals of care in IBD. However, these medications are not available to all patients due to numerous barriers including step therapy payer policies, prohibitive costs, insurance prior authorizations, and the role of social determinants of health and proximity to IBD expertise.25 While clinicians work with patients to determine the best treatment option, more often than not, the decision lies with the insurance payer. Step therapy is the protocol used by insurance companies that requires patients to try a lower-cost medication and fail to respond before they approve the originally requested treatment. This can lead to treatment delays, progression of disease, and disease complications. The option to incorporate the use of biosimilars, currently available for anti-TNFs, and other biologics in the near future, will reduce cost and potentially increase access.26 Additionally, working with a clinical pharmacist to navigate access and utilize patient assistance programs may help overcome cost related barriers to treatment and prevent delays in care.
Socioeconomic status has been shown to impact IBD disease outcomes, and compliance rates in treatment vary depending on race and ethnicity.27 Certain racial and ethnic groups remain vulnerable and may require additional support to achieve treatment goals. For example, disparities in health literacy in patients with IBD have been demonstrated with older black men at risk.28 Additionally, the patient’s proximity to their health care facility may impact treatment options. Most IBD centers are located in metropolitan areas and numerous “IBD deserts” exist, potentially limiting therapies for patients from more remote/rural settings.29 Access to treatment and the interplay of social determinants of health can have a large role in therapy selection.
Special considerations: Pregnancy and older adults
Certain patient populations warrant special consideration when approaching treatment strategies. Pregnancy in IBD will not be addressed in full depth in this article, however a key takeaway is that planning is critical and providers should emphasize the importance of steroid-free clinical remission for at least 3 months before conception.30 Additionally, biologic use during pregnancy has not been shown to increase adverse fetal outcomes, thus should be continued to minimize disease flare. Newer novel small molecules are generally avoided during pregnancy due to limited available safety data.
Older adults are the largest growing patient population with IBD. Frailty, or a state of decreased reserve, is more commonly observed in older patients and has been shown to increase adverse events including hospitalization and mortaility.31 Ultimately reducing polypharmacy, ensuring adequate nutrition, minimizing corticosteroid exposure and avoiding undertreatment of active IBD are all key in optimizing outcomes in an older patient with IBD.
Conclusion
When discussing treatment options with patients with IBD, it is important to individualize care and share the decision-making process with patients. Goals include improving symptoms and quality of life while working to achieve the goal of healing intestinal inflammation. In summary, this article can serve as a guide to clinicians for key factors in decision making when selecting therapies in moderate to severe IBD.
Dr. Holmer is a gastroenterologist with NYU Langone Health specializing in inflammatory bowel disease. Dr. Chang is director of clinical operations for the NYU Langone Health Inflammatory Bowel Disease Center. Dr. Malter is director of education for the Inflammatory Bowel Disease Center at NYU Langone Health and director of the inflammatory bowel disease program at Bellevue Hospital Center. Follow Dr. Holmer on X (formerly Twitter) at @HolmerMd and Dr. Chang @shannonchangmd. Dr. Holmer disclosed affiliations with Pfizer, Bristol Myers Squibb, and AvevoRx. Dr. Chang disclosed affiliations with Pfizer and Bristol Myers Squibb. Dr. Malter disclosed receiving educational grants form Abbvie, Janssen, Pfizer and Takeda, and serving on the advisory boards of AbbVie, Bristol Myers Squibb, Celltrion, Janssen, Merck, and Takeda.
References
1. Chang S et al. Am J Gastroenterol. 2023 Aug 24. doi: 10.14309/ajg.0000000000002485.
2. Harvey RF et al. The Lancet. 1980;1:514.
3. Lewis JD et al. Inflammatory Bowel Diseases. 2008;14:1660-1666.
4. Siegel CA et al. Gut. 2018;67(2):244-54.
5. Peyrin-Biroulet L et al. Am J Gastroenterol. 2015;110:1324-38
6. Rogler G et al. Gastroenterology. 2021;161:1118-32.
7. Sandborn WJ et al. N Engl J Med. 2017;376:1723-36.
8. Brooklyn TN et al. Gut. 2006;55:505-9.
9. Fahmy M et al. Am J Gastroenterol. 2012;107:794-5.
10. Van Eycken L et al. JAAD Case Rep. 2023;37:89-91.
11. Lasa JS et al. Lancet Gastroenterol Hepatol. 2022;7:161-70.
12. Lichtenstein GR et al. Inflamm Bowel Dis. 2018;24:490-501.
13. Long MD et al. Gastroenterology. 2022;162:S-5-S-6.
14. Holmer AK et al. Clin Gastroenterol Hepatol.2023;21:1598-1606.e5.
15. Sands BE et al. Gastroenterology. 2014;147:618-27.e3.
16. Sands BE et al. N Engl J Med. 2019;381:1215-26.
17. Sands BE et al. N Engl J Med. 2019;381:1201-14.
18. D’Haens G et al. Lancet. 2022;399:2015-30.
19. Bouguen G et al. Clin Gastroenterol Hepatol. 2013;11:975-81.e1-4.
20. Papamichael K et al. Am J Gastroenterol. 2021;116:1007-14.
21. Shehab M et al. Inflamm Bowel Dis. 2023;29:367-75.
22. Colombel JF et al. J Crohns Colitis. 2023;17:i620-i623.
23. Garcia-Olmo D et al. Dis Colon Rectum. 2022;65:713-20.
24. Louis E et al. J Crohns Colitis. 2023;17:231-9.
25. Rubin DT et al. Inflamm Bowel Dis. 2017;23:224-32.
26. Gulacsi L et al. Curr Med Chem. 2019;26:259-69.
27. Cai Q et al. BMC Gastroenterol. 2022;22:545.
28. Dos Santos Marques IC et al. Crohns Colitis 360. 2020 Oct;2(4):otaa076.
29. Deepak P et al. Gastroenterology. 2023;165:11-15.
30. Mahadevan U et al. Gastroenterology. 2019;156:1508-24.
31. Faye AS et al. Inflamm Bowel Dis. 2022;28:126-32.
32. Berinstein JA et al. Clin Gastroenterol Hepatol. 2021;19:2112-20.e1.
33. Levine J et al. Gastroenterology. 2023;164:S103-S104.
Cysteamine and melasma
Most subjects covered in this column are botanical ingredients used for multiple conditions in topical skin care. The focus this month, though, is a natural agent garnering attention primarily for one indication. Present in many mammals and in various cells in the human body (and particularly highly concentrated in human milk), cysteamine is a stable aminothiol that acts as an antioxidant as a result of the degradation of coenzyme A and is known to play a protective function.1 Melasma, an acquired recurrent, chronic hyperpigmentary disorder, continues to be a treatment challenge and is often psychologically troublesome for those affected, approximately 90% of whom are women.2 Individuals with Fitzpatrick skin types IV and V who reside in regions where UV exposure is likely are particularly prominent among those with melasma.2 While triple combination therapy (also known as Kligman’s formula) continues to be the modern gold standard of care for melasma (over the last 30 years),3 cysteamine, a nonmelanocytotoxic molecule, is considered viable for long-term use and safer than the long-time skin-lightening gold standard over several decades, hydroquinone (HQ), which is associated with safety concerns.4.
Recent history and the 2015 study
Prior to 2015, the quick oxidation and malodorous nature of cysteamine rendered it unsuitable for use as a topical agent. However, stabilization efforts resulted in a product that first began to show efficacy that year.5
Mansouri et al. conducted a randomized, double-blind, placebo-controlled trial to assess the efficacy of topical cysteamine 5% to treat epidermal melasma in 2015. Over 4 months, 50 volunteers (25 in each group) applied either cysteamine cream or placebo on lesions once nightly. The mean differences at baseline between pigmented and normal skin were 75.2 ± 37 in the cysteamine group and 68.9 ± 31 in the placebo group. Statistically significant differences between the groups were identified at the 2- and 4-month points. At 2 months, the mean differences were 39.7 ± 16.6 in the cysteamine group and 63.8 ± 28.6 in the placebo group; at 4 months, the respective differences were 26.2 ± 16 and 60.7 ± 27.3. Melasma area severity index (MASI) scores were significantly lower in the cysteamine group compared with the placebo group at the end of the study, and investigator global assessment scores and patient questionnaire results revealed substantial comparative efficacy of cysteamine cream.6 Topical cysteamine has also demonstrated notable efficacy in treating senile lentigines, which typically do not respond to topical depigmenting products.5
Farshi et al. used Dermacatch as a novel measurement tool to ascertain the efficacy of cysteamine cream for treating epidermal melasma in a 2018 report of a randomized, double-blind, placebo-controlled study with 40 patients. During the 4-month trial, cysteamine cream or placebo was applied nightly before sleep. Investigators measured treatment efficacy through Dermacatch, and Mexameter skin colorimetry, MASI scores, investigator global assessments, and patient questionnaires at baseline, 2 months, and 4 months. Through all measurement methods, cysteamine was found to reduce melanin content of melasma lesions, with Dermacatch performing reliably and comparably to Mexameter.7 Since then, cysteamine has been compared to several first-line melasma therapies.
Reviews
A 2019 systematic review by Austin et al. of randomized controlled trials (RCTs) on topical treatments for melasma identified 35 original RCTs evaluating a wide range of approximately 20 agents. They identified cysteamine, triple combination therapy, and tranexamic acid as the products netting the most robust recommendations. The researchers characterized cysteamine as conferring strong efficacy and reported anticancer activity while triple combination therapy poses the potential risk of ochronosis and tranexamic acid may present the risk for thrombosis. They concluded that more research is necessary, though, to establish the proper concentration and optimal formulation of cysteamine as a frontline therapy.8
More reviews have since been published to further clarify where cysteamine stands among the optimal treatments for melasma. In a May 2022 systematic PubMed review of topical agents used to treat melasma, González-Molina et al. identified 80 papers meeting inclusion criteria (double or single blinded, prospective, controlled or RCTs, reviews of literature, and meta-analysis studies), with tranexamic acid and cysteamine among the novel well-tolerated agents. Cysteamine was not associated with any severe adverse effects and is recommended as an adjuvant and maintenance therapy.3
A September 2022 review by Niazi et al. found that while the signaling mechanisms through which cysteamine suppresses melasma are not well understood, the topical application of cysteamine cream is seen as safe and effective alone or in combination with other products to treat melasma.2
A systematic review and meta-analysis reported by Gomes dos Santos-Neto et al. at the end of 2022 considered the efficacy of depigmenting formulations containing 5% cysteamine for treating melasma. The meta-analysis covered six studies, with 120 melasma patients treated. The conclusion was that 5% cysteamine was effective with adverse effects unlikely.9
Cysteamine vs. hydroquinone
In 2020, Lima et al. reported the results of a quasi-randomized, multicenter, evaluator-blinded comparative study of topical 0.56% cysteamine and 4% HQ in 40 women with facial melasma. (Note that this study originally claimed a 5% cysteamine concentration, but a letter to the editor of the International Journal of Dermatology in 2020 disputed this and proved it was 0.56%) For 120 days, volunteers applied either 0.56% cysteamine or 4% HQ nightly. Tinted sunscreen (SPF 50; PPD 19) use was required for all participants. There were no differences in colorimetric evaluations between the groups, both of which showed progressive depigmenting, or in photographic assessments. The HQ group demonstrated greater mean decreases in modified melasma area severity index (mMASI) scores (41% for HQ and 24% for cysteamine at 60 days; 53% for HQ and 38% for cysteamine at 120 days). The investigators observed that while cysteamine was safe, well tolerated, and effective, it was outperformed by HQ in terms of mMASI and melasma quality of life (MELASQoL) scores.10
Early the next year, results of a randomized, double-blind, single-center study in 20 women, conducted by Nguyen et al. comparing the efficacy of cysteamine cream with HQ for melasma treatment were published. Participants were given either treatment over 16 weeks. Ultimately, five volunteers in the cysteamine group and nine in the HQ group completed the study. There was no statistically significant difference in mMASI scores between the groups. In this notably small study, HQ was tolerated better. The researchers concluded that their findings supported the argument of comparable efficacy between cysteamine and HQ, with further studies needed to establish whether cysteamine would be an appropriate alternative to HQ.11 Notably, HQ was banned by the Food and Drug Administration in 2020 in over-the-counter products.
Cysteamine vs. Kligman’s formula
Early in 2021, Karrabi et al. published the results of a randomized, double-blind clinical trial of 50 subjects with epidermal melasma to compare cysteamine 5% with Modified Kligman’s formula. Over 4 months, participants applied once daily either cysteamine cream 5% (15 minutes exposure) or the Modified Kligman’s formula (4% hydroquinone, 0.05% retinoic acid and 0.1% betamethasone) for whole night exposure. At 2 and 4 months, a statistically significant difference in mMASI score was noted, with the percentage decline in mMASI score nearly 9% higher in the cysteamine group. The investigators concluded that cysteamine 5% demonstrated greater efficacy than the Modified Kligman’s formula and was also better tolerated.12
Cysteamine vs. tranexamic acid
Later that year, Karrabi et al. published the results of a single-blind, randomized clinical trial assessing the efficacy of tranexamic acid mesotherapy compared with cysteamine 5% cream in 54 melasma patients. For 4 consecutive months, the cysteamine 5% cream group applied the cream on lesions 30 minutes before going to sleep. Every 4 weeks until 2 months, a physician performed tranexamic acid mesotherapy (0.05 mL; 4 mg/mL) on individuals in the tranexamic acid group. The researchers concluded, after measurements using both a Dermacatch device and the mMASI, that neither treatment was significantly better than the other but fewer complications were observed in the cysteamine group.13
Safety
In 2022, Sepaskhah et al. assessed the effects of a cysteamine 5% cream and compared it with HQ 4%/ascorbic acid 3% cream for epidermal melasma in a single-blind, randomized controlled trial. Sixty-five of 80 patients completed the study. The difference in mMASI scores after 4 months was not significant between the groups nor was the improvement in quality of life, but the melanin index was significantly lower in the HQ/ascorbic acid group compared with the less substantial reduction for the cysteamine group. Nevertheless, the researchers concluded that cysteamine is a safe and suitable substitute for HQ/ascorbic acid.4
Conclusion
In the last decade, cysteamine has been established as a potent depigmenting agent. Its suitability and desirability as a top consideration for melasma treatment also appears to be compelling. More RCTs comparing cysteamine and other topline therapies are warranted, but current evidence shows that cysteamine is an effective and safe therapy for melasma.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur in Miami. She founded the division of cosmetic dermatology at the University of Miami in 1997. The third edition of her bestselling textbook, “Cosmetic Dermatology,” was published in 2022. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Johnson & Johnson, and Burt’s Bees. She is the CEO of Skin Type Solutions Inc., a SaaS company used to generate skin care routines in office and as an ecommerce solution. Write to her at [email protected].
References
1. Konar MC et al. J Trop Pediatr. 2020 Apr 1;66(2):129-35.
2. Niazi S et al. J Cosmet Dermatol. 2022 Sep;21(9):3867-75.
3. González-Molina V et al. J Clin Aesthet Dermatol. 2022 May;15(5):19-28.
4. Sepaskhah M et al. J Cosmet Dermatol. 2022 Jul;21(7):2871-8.
5. Desai S et al. J Drugs Dermatol. 2021 Dec 1;20(12):1276-9.
6. Mansouri P et al. Br J Dermatol. 2015 Jul;173(1):209-17.
7. Farshi S et al. J Dermatolog Treat. 2018 Mar;29(2):182-9.
8. Austin E et al. J Drugs Dermatol. 2019 Nov 1;18(11):S1545961619P1156X.
9. Gomes dos Santos-Neto A et al. Dermatol Ther. 2022 Dec;35(12):e15961.
10. Lima PB et al. Int J Dermatol. 2020 Dec;59(12):1531-6.
11. Nguyen J et al. Australas J Dermatol. 2021 Feb;62(1):e41-e46.
12. Karrabi M et al. Skin Res Technol. 2021 Jan;27(1):24-31.
13. Karrabi M et al. Arch Dermatol Res. 2021 Sep;313(7):539-47.
Most subjects covered in this column are botanical ingredients used for multiple conditions in topical skin care. The focus this month, though, is a natural agent garnering attention primarily for one indication. Present in many mammals and in various cells in the human body (and particularly highly concentrated in human milk), cysteamine is a stable aminothiol that acts as an antioxidant as a result of the degradation of coenzyme A and is known to play a protective function.1 Melasma, an acquired recurrent, chronic hyperpigmentary disorder, continues to be a treatment challenge and is often psychologically troublesome for those affected, approximately 90% of whom are women.2 Individuals with Fitzpatrick skin types IV and V who reside in regions where UV exposure is likely are particularly prominent among those with melasma.2 While triple combination therapy (also known as Kligman’s formula) continues to be the modern gold standard of care for melasma (over the last 30 years),3 cysteamine, a nonmelanocytotoxic molecule, is considered viable for long-term use and safer than the long-time skin-lightening gold standard over several decades, hydroquinone (HQ), which is associated with safety concerns.4.
Recent history and the 2015 study
Prior to 2015, the quick oxidation and malodorous nature of cysteamine rendered it unsuitable for use as a topical agent. However, stabilization efforts resulted in a product that first began to show efficacy that year.5
Mansouri et al. conducted a randomized, double-blind, placebo-controlled trial to assess the efficacy of topical cysteamine 5% to treat epidermal melasma in 2015. Over 4 months, 50 volunteers (25 in each group) applied either cysteamine cream or placebo on lesions once nightly. The mean differences at baseline between pigmented and normal skin were 75.2 ± 37 in the cysteamine group and 68.9 ± 31 in the placebo group. Statistically significant differences between the groups were identified at the 2- and 4-month points. At 2 months, the mean differences were 39.7 ± 16.6 in the cysteamine group and 63.8 ± 28.6 in the placebo group; at 4 months, the respective differences were 26.2 ± 16 and 60.7 ± 27.3. Melasma area severity index (MASI) scores were significantly lower in the cysteamine group compared with the placebo group at the end of the study, and investigator global assessment scores and patient questionnaire results revealed substantial comparative efficacy of cysteamine cream.6 Topical cysteamine has also demonstrated notable efficacy in treating senile lentigines, which typically do not respond to topical depigmenting products.5
Farshi et al. used Dermacatch as a novel measurement tool to ascertain the efficacy of cysteamine cream for treating epidermal melasma in a 2018 report of a randomized, double-blind, placebo-controlled study with 40 patients. During the 4-month trial, cysteamine cream or placebo was applied nightly before sleep. Investigators measured treatment efficacy through Dermacatch, and Mexameter skin colorimetry, MASI scores, investigator global assessments, and patient questionnaires at baseline, 2 months, and 4 months. Through all measurement methods, cysteamine was found to reduce melanin content of melasma lesions, with Dermacatch performing reliably and comparably to Mexameter.7 Since then, cysteamine has been compared to several first-line melasma therapies.
Reviews
A 2019 systematic review by Austin et al. of randomized controlled trials (RCTs) on topical treatments for melasma identified 35 original RCTs evaluating a wide range of approximately 20 agents. They identified cysteamine, triple combination therapy, and tranexamic acid as the products netting the most robust recommendations. The researchers characterized cysteamine as conferring strong efficacy and reported anticancer activity while triple combination therapy poses the potential risk of ochronosis and tranexamic acid may present the risk for thrombosis. They concluded that more research is necessary, though, to establish the proper concentration and optimal formulation of cysteamine as a frontline therapy.8
More reviews have since been published to further clarify where cysteamine stands among the optimal treatments for melasma. In a May 2022 systematic PubMed review of topical agents used to treat melasma, González-Molina et al. identified 80 papers meeting inclusion criteria (double or single blinded, prospective, controlled or RCTs, reviews of literature, and meta-analysis studies), with tranexamic acid and cysteamine among the novel well-tolerated agents. Cysteamine was not associated with any severe adverse effects and is recommended as an adjuvant and maintenance therapy.3
A September 2022 review by Niazi et al. found that while the signaling mechanisms through which cysteamine suppresses melasma are not well understood, the topical application of cysteamine cream is seen as safe and effective alone or in combination with other products to treat melasma.2
A systematic review and meta-analysis reported by Gomes dos Santos-Neto et al. at the end of 2022 considered the efficacy of depigmenting formulations containing 5% cysteamine for treating melasma. The meta-analysis covered six studies, with 120 melasma patients treated. The conclusion was that 5% cysteamine was effective with adverse effects unlikely.9
Cysteamine vs. hydroquinone
In 2020, Lima et al. reported the results of a quasi-randomized, multicenter, evaluator-blinded comparative study of topical 0.56% cysteamine and 4% HQ in 40 women with facial melasma. (Note that this study originally claimed a 5% cysteamine concentration, but a letter to the editor of the International Journal of Dermatology in 2020 disputed this and proved it was 0.56%) For 120 days, volunteers applied either 0.56% cysteamine or 4% HQ nightly. Tinted sunscreen (SPF 50; PPD 19) use was required for all participants. There were no differences in colorimetric evaluations between the groups, both of which showed progressive depigmenting, or in photographic assessments. The HQ group demonstrated greater mean decreases in modified melasma area severity index (mMASI) scores (41% for HQ and 24% for cysteamine at 60 days; 53% for HQ and 38% for cysteamine at 120 days). The investigators observed that while cysteamine was safe, well tolerated, and effective, it was outperformed by HQ in terms of mMASI and melasma quality of life (MELASQoL) scores.10
Early the next year, results of a randomized, double-blind, single-center study in 20 women, conducted by Nguyen et al. comparing the efficacy of cysteamine cream with HQ for melasma treatment were published. Participants were given either treatment over 16 weeks. Ultimately, five volunteers in the cysteamine group and nine in the HQ group completed the study. There was no statistically significant difference in mMASI scores between the groups. In this notably small study, HQ was tolerated better. The researchers concluded that their findings supported the argument of comparable efficacy between cysteamine and HQ, with further studies needed to establish whether cysteamine would be an appropriate alternative to HQ.11 Notably, HQ was banned by the Food and Drug Administration in 2020 in over-the-counter products.
Cysteamine vs. Kligman’s formula
Early in 2021, Karrabi et al. published the results of a randomized, double-blind clinical trial of 50 subjects with epidermal melasma to compare cysteamine 5% with Modified Kligman’s formula. Over 4 months, participants applied once daily either cysteamine cream 5% (15 minutes exposure) or the Modified Kligman’s formula (4% hydroquinone, 0.05% retinoic acid and 0.1% betamethasone) for whole night exposure. At 2 and 4 months, a statistically significant difference in mMASI score was noted, with the percentage decline in mMASI score nearly 9% higher in the cysteamine group. The investigators concluded that cysteamine 5% demonstrated greater efficacy than the Modified Kligman’s formula and was also better tolerated.12
Cysteamine vs. tranexamic acid
Later that year, Karrabi et al. published the results of a single-blind, randomized clinical trial assessing the efficacy of tranexamic acid mesotherapy compared with cysteamine 5% cream in 54 melasma patients. For 4 consecutive months, the cysteamine 5% cream group applied the cream on lesions 30 minutes before going to sleep. Every 4 weeks until 2 months, a physician performed tranexamic acid mesotherapy (0.05 mL; 4 mg/mL) on individuals in the tranexamic acid group. The researchers concluded, after measurements using both a Dermacatch device and the mMASI, that neither treatment was significantly better than the other but fewer complications were observed in the cysteamine group.13
Safety
In 2022, Sepaskhah et al. assessed the effects of a cysteamine 5% cream and compared it with HQ 4%/ascorbic acid 3% cream for epidermal melasma in a single-blind, randomized controlled trial. Sixty-five of 80 patients completed the study. The difference in mMASI scores after 4 months was not significant between the groups nor was the improvement in quality of life, but the melanin index was significantly lower in the HQ/ascorbic acid group compared with the less substantial reduction for the cysteamine group. Nevertheless, the researchers concluded that cysteamine is a safe and suitable substitute for HQ/ascorbic acid.4
Conclusion
In the last decade, cysteamine has been established as a potent depigmenting agent. Its suitability and desirability as a top consideration for melasma treatment also appears to be compelling. More RCTs comparing cysteamine and other topline therapies are warranted, but current evidence shows that cysteamine is an effective and safe therapy for melasma.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur in Miami. She founded the division of cosmetic dermatology at the University of Miami in 1997. The third edition of her bestselling textbook, “Cosmetic Dermatology,” was published in 2022. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Johnson & Johnson, and Burt’s Bees. She is the CEO of Skin Type Solutions Inc., a SaaS company used to generate skin care routines in office and as an ecommerce solution. Write to her at [email protected].
References
1. Konar MC et al. J Trop Pediatr. 2020 Apr 1;66(2):129-35.
2. Niazi S et al. J Cosmet Dermatol. 2022 Sep;21(9):3867-75.
3. González-Molina V et al. J Clin Aesthet Dermatol. 2022 May;15(5):19-28.
4. Sepaskhah M et al. J Cosmet Dermatol. 2022 Jul;21(7):2871-8.
5. Desai S et al. J Drugs Dermatol. 2021 Dec 1;20(12):1276-9.
6. Mansouri P et al. Br J Dermatol. 2015 Jul;173(1):209-17.
7. Farshi S et al. J Dermatolog Treat. 2018 Mar;29(2):182-9.
8. Austin E et al. J Drugs Dermatol. 2019 Nov 1;18(11):S1545961619P1156X.
9. Gomes dos Santos-Neto A et al. Dermatol Ther. 2022 Dec;35(12):e15961.
10. Lima PB et al. Int J Dermatol. 2020 Dec;59(12):1531-6.
11. Nguyen J et al. Australas J Dermatol. 2021 Feb;62(1):e41-e46.
12. Karrabi M et al. Skin Res Technol. 2021 Jan;27(1):24-31.
13. Karrabi M et al. Arch Dermatol Res. 2021 Sep;313(7):539-47.
Most subjects covered in this column are botanical ingredients used for multiple conditions in topical skin care. The focus this month, though, is a natural agent garnering attention primarily for one indication. Present in many mammals and in various cells in the human body (and particularly highly concentrated in human milk), cysteamine is a stable aminothiol that acts as an antioxidant as a result of the degradation of coenzyme A and is known to play a protective function.1 Melasma, an acquired recurrent, chronic hyperpigmentary disorder, continues to be a treatment challenge and is often psychologically troublesome for those affected, approximately 90% of whom are women.2 Individuals with Fitzpatrick skin types IV and V who reside in regions where UV exposure is likely are particularly prominent among those with melasma.2 While triple combination therapy (also known as Kligman’s formula) continues to be the modern gold standard of care for melasma (over the last 30 years),3 cysteamine, a nonmelanocytotoxic molecule, is considered viable for long-term use and safer than the long-time skin-lightening gold standard over several decades, hydroquinone (HQ), which is associated with safety concerns.4.
Recent history and the 2015 study
Prior to 2015, the quick oxidation and malodorous nature of cysteamine rendered it unsuitable for use as a topical agent. However, stabilization efforts resulted in a product that first began to show efficacy that year.5
Mansouri et al. conducted a randomized, double-blind, placebo-controlled trial to assess the efficacy of topical cysteamine 5% to treat epidermal melasma in 2015. Over 4 months, 50 volunteers (25 in each group) applied either cysteamine cream or placebo on lesions once nightly. The mean differences at baseline between pigmented and normal skin were 75.2 ± 37 in the cysteamine group and 68.9 ± 31 in the placebo group. Statistically significant differences between the groups were identified at the 2- and 4-month points. At 2 months, the mean differences were 39.7 ± 16.6 in the cysteamine group and 63.8 ± 28.6 in the placebo group; at 4 months, the respective differences were 26.2 ± 16 and 60.7 ± 27.3. Melasma area severity index (MASI) scores were significantly lower in the cysteamine group compared with the placebo group at the end of the study, and investigator global assessment scores and patient questionnaire results revealed substantial comparative efficacy of cysteamine cream.6 Topical cysteamine has also demonstrated notable efficacy in treating senile lentigines, which typically do not respond to topical depigmenting products.5
Farshi et al. used Dermacatch as a novel measurement tool to ascertain the efficacy of cysteamine cream for treating epidermal melasma in a 2018 report of a randomized, double-blind, placebo-controlled study with 40 patients. During the 4-month trial, cysteamine cream or placebo was applied nightly before sleep. Investigators measured treatment efficacy through Dermacatch, and Mexameter skin colorimetry, MASI scores, investigator global assessments, and patient questionnaires at baseline, 2 months, and 4 months. Through all measurement methods, cysteamine was found to reduce melanin content of melasma lesions, with Dermacatch performing reliably and comparably to Mexameter.7 Since then, cysteamine has been compared to several first-line melasma therapies.
Reviews
A 2019 systematic review by Austin et al. of randomized controlled trials (RCTs) on topical treatments for melasma identified 35 original RCTs evaluating a wide range of approximately 20 agents. They identified cysteamine, triple combination therapy, and tranexamic acid as the products netting the most robust recommendations. The researchers characterized cysteamine as conferring strong efficacy and reported anticancer activity while triple combination therapy poses the potential risk of ochronosis and tranexamic acid may present the risk for thrombosis. They concluded that more research is necessary, though, to establish the proper concentration and optimal formulation of cysteamine as a frontline therapy.8
More reviews have since been published to further clarify where cysteamine stands among the optimal treatments for melasma. In a May 2022 systematic PubMed review of topical agents used to treat melasma, González-Molina et al. identified 80 papers meeting inclusion criteria (double or single blinded, prospective, controlled or RCTs, reviews of literature, and meta-analysis studies), with tranexamic acid and cysteamine among the novel well-tolerated agents. Cysteamine was not associated with any severe adverse effects and is recommended as an adjuvant and maintenance therapy.3
A September 2022 review by Niazi et al. found that while the signaling mechanisms through which cysteamine suppresses melasma are not well understood, the topical application of cysteamine cream is seen as safe and effective alone or in combination with other products to treat melasma.2
A systematic review and meta-analysis reported by Gomes dos Santos-Neto et al. at the end of 2022 considered the efficacy of depigmenting formulations containing 5% cysteamine for treating melasma. The meta-analysis covered six studies, with 120 melasma patients treated. The conclusion was that 5% cysteamine was effective with adverse effects unlikely.9
Cysteamine vs. hydroquinone
In 2020, Lima et al. reported the results of a quasi-randomized, multicenter, evaluator-blinded comparative study of topical 0.56% cysteamine and 4% HQ in 40 women with facial melasma. (Note that this study originally claimed a 5% cysteamine concentration, but a letter to the editor of the International Journal of Dermatology in 2020 disputed this and proved it was 0.56%) For 120 days, volunteers applied either 0.56% cysteamine or 4% HQ nightly. Tinted sunscreen (SPF 50; PPD 19) use was required for all participants. There were no differences in colorimetric evaluations between the groups, both of which showed progressive depigmenting, or in photographic assessments. The HQ group demonstrated greater mean decreases in modified melasma area severity index (mMASI) scores (41% for HQ and 24% for cysteamine at 60 days; 53% for HQ and 38% for cysteamine at 120 days). The investigators observed that while cysteamine was safe, well tolerated, and effective, it was outperformed by HQ in terms of mMASI and melasma quality of life (MELASQoL) scores.10
Early the next year, results of a randomized, double-blind, single-center study in 20 women, conducted by Nguyen et al. comparing the efficacy of cysteamine cream with HQ for melasma treatment were published. Participants were given either treatment over 16 weeks. Ultimately, five volunteers in the cysteamine group and nine in the HQ group completed the study. There was no statistically significant difference in mMASI scores between the groups. In this notably small study, HQ was tolerated better. The researchers concluded that their findings supported the argument of comparable efficacy between cysteamine and HQ, with further studies needed to establish whether cysteamine would be an appropriate alternative to HQ.11 Notably, HQ was banned by the Food and Drug Administration in 2020 in over-the-counter products.
Cysteamine vs. Kligman’s formula
Early in 2021, Karrabi et al. published the results of a randomized, double-blind clinical trial of 50 subjects with epidermal melasma to compare cysteamine 5% with Modified Kligman’s formula. Over 4 months, participants applied once daily either cysteamine cream 5% (15 minutes exposure) or the Modified Kligman’s formula (4% hydroquinone, 0.05% retinoic acid and 0.1% betamethasone) for whole night exposure. At 2 and 4 months, a statistically significant difference in mMASI score was noted, with the percentage decline in mMASI score nearly 9% higher in the cysteamine group. The investigators concluded that cysteamine 5% demonstrated greater efficacy than the Modified Kligman’s formula and was also better tolerated.12
Cysteamine vs. tranexamic acid
Later that year, Karrabi et al. published the results of a single-blind, randomized clinical trial assessing the efficacy of tranexamic acid mesotherapy compared with cysteamine 5% cream in 54 melasma patients. For 4 consecutive months, the cysteamine 5% cream group applied the cream on lesions 30 minutes before going to sleep. Every 4 weeks until 2 months, a physician performed tranexamic acid mesotherapy (0.05 mL; 4 mg/mL) on individuals in the tranexamic acid group. The researchers concluded, after measurements using both a Dermacatch device and the mMASI, that neither treatment was significantly better than the other but fewer complications were observed in the cysteamine group.13
Safety
In 2022, Sepaskhah et al. assessed the effects of a cysteamine 5% cream and compared it with HQ 4%/ascorbic acid 3% cream for epidermal melasma in a single-blind, randomized controlled trial. Sixty-five of 80 patients completed the study. The difference in mMASI scores after 4 months was not significant between the groups nor was the improvement in quality of life, but the melanin index was significantly lower in the HQ/ascorbic acid group compared with the less substantial reduction for the cysteamine group. Nevertheless, the researchers concluded that cysteamine is a safe and suitable substitute for HQ/ascorbic acid.4
Conclusion
In the last decade, cysteamine has been established as a potent depigmenting agent. Its suitability and desirability as a top consideration for melasma treatment also appears to be compelling. More RCTs comparing cysteamine and other topline therapies are warranted, but current evidence shows that cysteamine is an effective and safe therapy for melasma.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur in Miami. She founded the division of cosmetic dermatology at the University of Miami in 1997. The third edition of her bestselling textbook, “Cosmetic Dermatology,” was published in 2022. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Johnson & Johnson, and Burt’s Bees. She is the CEO of Skin Type Solutions Inc., a SaaS company used to generate skin care routines in office and as an ecommerce solution. Write to her at [email protected].
References
1. Konar MC et al. J Trop Pediatr. 2020 Apr 1;66(2):129-35.
2. Niazi S et al. J Cosmet Dermatol. 2022 Sep;21(9):3867-75.
3. González-Molina V et al. J Clin Aesthet Dermatol. 2022 May;15(5):19-28.
4. Sepaskhah M et al. J Cosmet Dermatol. 2022 Jul;21(7):2871-8.
5. Desai S et al. J Drugs Dermatol. 2021 Dec 1;20(12):1276-9.
6. Mansouri P et al. Br J Dermatol. 2015 Jul;173(1):209-17.
7. Farshi S et al. J Dermatolog Treat. 2018 Mar;29(2):182-9.
8. Austin E et al. J Drugs Dermatol. 2019 Nov 1;18(11):S1545961619P1156X.
9. Gomes dos Santos-Neto A et al. Dermatol Ther. 2022 Dec;35(12):e15961.
10. Lima PB et al. Int J Dermatol. 2020 Dec;59(12):1531-6.
11. Nguyen J et al. Australas J Dermatol. 2021 Feb;62(1):e41-e46.
12. Karrabi M et al. Skin Res Technol. 2021 Jan;27(1):24-31.
13. Karrabi M et al. Arch Dermatol Res. 2021 Sep;313(7):539-47.
Breastfeeding and colorectal cancer
I, like every pediatrician I know, believe that breast milk is the best nutrition for human newborns. Its balance of nutritive elements and its role in preventing of a wide range of illnesses are so great that we are still learning the extent of their magnitude. It just makes sense that a mother’s milk is most well suited for her baby.
I am a bit less unambiguous about breastfeeding. By that I mean the process of providing breast milk to an infant directly from its mother’s breast. Before you yank my AAP membership card, let me make it clear that I think every woman should consider breastfeeding her infant. But we must accept that in a few situations, even with help from caring and enlightened health care providers and family members, breastfeeding doesn’t work as well as we would have hoped. Fortunately, there are alternatives.
My reservations about the process are few, and until recently I have had an unwaveringly positive attitude toward the safety of breast milk. The cause of my little bit of uncertainty arrived in a recent study by two researchers at the Dana Farber Institute in Boston, in which the A younger cohort within that larger group had a dramatic 40% increased risk of developing high-risk cancer before reaching age 55.
The population the investigators studied came from the large Nurses’ Health Study II, a well-known repository of longitudinal health data. The researchers reported that they included biometric data and a large collection of lifestyle factors including smoking, alcohol intake, and diet in their calculations. However, breastfeeding continued to register the highest association. Interestingly, the investigators found that women who were breastfed for 9 months or longer had twice the risk of colorectal cancer as those who breastfed for from 4 to 8 months.
The study population was all women and predominantly white. However, in the general population it is the non-Hispanic white population that is experiencing the greatest increase in incidence. Of course, the study could not answer whether this association with breastfeeding also existed in minority populations.
The researchers suspect that what they are seeing is a reflection of the Westernization of the American lifestyle. One of the researchers is interested in the gut biome of infants and plans to further the investigation in that direction. Could some substance from the environment be concentrating in breast milk? Or is something missing in breast milk? She points out that, while formulas are generally fortified with vitamin D, breast milk is not.
As concerning as the results of this study may sound, the authors are very careful to urge mothers to continue to breastfeed and choose it as their first choice for feeding their babies. I have been pleasantly surprised that this study has not gotten widespread media attention because bad news travels fast. I have chosen to share it with you because at some point you may begin getting questions from concerned parents.
While apparently well done, this study is just the beginning. Like any good research, it poses more questions than it answers. For us as pediatricians it means we should continue to recommend breast milk as the first food. But, we must stay alert as further research looks deeper into this association.
We should also take advantage of our special access to young parents, a demographic that less frequently sees a physician for preventive care. For whatever reason colorectal cancer is occurring at younger ages. When we have the opportunity we should be reminding 40-year-olds not to wait until age 50 to screen for colorectal cancer, particularly if they have a family history of the disease.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
I, like every pediatrician I know, believe that breast milk is the best nutrition for human newborns. Its balance of nutritive elements and its role in preventing of a wide range of illnesses are so great that we are still learning the extent of their magnitude. It just makes sense that a mother’s milk is most well suited for her baby.
I am a bit less unambiguous about breastfeeding. By that I mean the process of providing breast milk to an infant directly from its mother’s breast. Before you yank my AAP membership card, let me make it clear that I think every woman should consider breastfeeding her infant. But we must accept that in a few situations, even with help from caring and enlightened health care providers and family members, breastfeeding doesn’t work as well as we would have hoped. Fortunately, there are alternatives.
My reservations about the process are few, and until recently I have had an unwaveringly positive attitude toward the safety of breast milk. The cause of my little bit of uncertainty arrived in a recent study by two researchers at the Dana Farber Institute in Boston, in which the A younger cohort within that larger group had a dramatic 40% increased risk of developing high-risk cancer before reaching age 55.
The population the investigators studied came from the large Nurses’ Health Study II, a well-known repository of longitudinal health data. The researchers reported that they included biometric data and a large collection of lifestyle factors including smoking, alcohol intake, and diet in their calculations. However, breastfeeding continued to register the highest association. Interestingly, the investigators found that women who were breastfed for 9 months or longer had twice the risk of colorectal cancer as those who breastfed for from 4 to 8 months.
The study population was all women and predominantly white. However, in the general population it is the non-Hispanic white population that is experiencing the greatest increase in incidence. Of course, the study could not answer whether this association with breastfeeding also existed in minority populations.
The researchers suspect that what they are seeing is a reflection of the Westernization of the American lifestyle. One of the researchers is interested in the gut biome of infants and plans to further the investigation in that direction. Could some substance from the environment be concentrating in breast milk? Or is something missing in breast milk? She points out that, while formulas are generally fortified with vitamin D, breast milk is not.
As concerning as the results of this study may sound, the authors are very careful to urge mothers to continue to breastfeed and choose it as their first choice for feeding their babies. I have been pleasantly surprised that this study has not gotten widespread media attention because bad news travels fast. I have chosen to share it with you because at some point you may begin getting questions from concerned parents.
While apparently well done, this study is just the beginning. Like any good research, it poses more questions than it answers. For us as pediatricians it means we should continue to recommend breast milk as the first food. But, we must stay alert as further research looks deeper into this association.
We should also take advantage of our special access to young parents, a demographic that less frequently sees a physician for preventive care. For whatever reason colorectal cancer is occurring at younger ages. When we have the opportunity we should be reminding 40-year-olds not to wait until age 50 to screen for colorectal cancer, particularly if they have a family history of the disease.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
I, like every pediatrician I know, believe that breast milk is the best nutrition for human newborns. Its balance of nutritive elements and its role in preventing of a wide range of illnesses are so great that we are still learning the extent of their magnitude. It just makes sense that a mother’s milk is most well suited for her baby.
I am a bit less unambiguous about breastfeeding. By that I mean the process of providing breast milk to an infant directly from its mother’s breast. Before you yank my AAP membership card, let me make it clear that I think every woman should consider breastfeeding her infant. But we must accept that in a few situations, even with help from caring and enlightened health care providers and family members, breastfeeding doesn’t work as well as we would have hoped. Fortunately, there are alternatives.
My reservations about the process are few, and until recently I have had an unwaveringly positive attitude toward the safety of breast milk. The cause of my little bit of uncertainty arrived in a recent study by two researchers at the Dana Farber Institute in Boston, in which the A younger cohort within that larger group had a dramatic 40% increased risk of developing high-risk cancer before reaching age 55.
The population the investigators studied came from the large Nurses’ Health Study II, a well-known repository of longitudinal health data. The researchers reported that they included biometric data and a large collection of lifestyle factors including smoking, alcohol intake, and diet in their calculations. However, breastfeeding continued to register the highest association. Interestingly, the investigators found that women who were breastfed for 9 months or longer had twice the risk of colorectal cancer as those who breastfed for from 4 to 8 months.
The study population was all women and predominantly white. However, in the general population it is the non-Hispanic white population that is experiencing the greatest increase in incidence. Of course, the study could not answer whether this association with breastfeeding also existed in minority populations.
The researchers suspect that what they are seeing is a reflection of the Westernization of the American lifestyle. One of the researchers is interested in the gut biome of infants and plans to further the investigation in that direction. Could some substance from the environment be concentrating in breast milk? Or is something missing in breast milk? She points out that, while formulas are generally fortified with vitamin D, breast milk is not.
As concerning as the results of this study may sound, the authors are very careful to urge mothers to continue to breastfeed and choose it as their first choice for feeding their babies. I have been pleasantly surprised that this study has not gotten widespread media attention because bad news travels fast. I have chosen to share it with you because at some point you may begin getting questions from concerned parents.
While apparently well done, this study is just the beginning. Like any good research, it poses more questions than it answers. For us as pediatricians it means we should continue to recommend breast milk as the first food. But, we must stay alert as further research looks deeper into this association.
We should also take advantage of our special access to young parents, a demographic that less frequently sees a physician for preventive care. For whatever reason colorectal cancer is occurring at younger ages. When we have the opportunity we should be reminding 40-year-olds not to wait until age 50 to screen for colorectal cancer, particularly if they have a family history of the disease.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
GI symptoms during menopause deserve attention
This transcript has been edited for clarity.
Welcome back to another GI Common Concerns.
Today, I want to highlight some information about menopause.
Approximately 1.5 million women in the United States per year enter into menopause. Hysterectomy is also one of the most common surgeries for women worldwide, with an estimated 20%-40% undergoing this procedure by the age of 60.
Therefore, whether it’s because of biologic onset with age or surgical induction, menopause is a very common condition, and it’s important that we understand its symptoms and the latest information around it.
Impact on GI motility
One of the clearest functional symptoms to be aware of with menopause relates to alterations in hormonal balance. This has an impact on gastrointestinal (GI) motility by increasing abdominal muscle stimulation related to different patterns of secretion and can result in a number of symptomatic changes.
One such change that can occur is food intolerance. It is believed that menopause-associated food intolerance has multiple possible causes and may be related more to alterations to the microbiome, which can be contributed to by diet, activity, sleep cycle, and other factors.
When food intolerances are triggered in the perimenopausal or menopausal patient, it may lead you to recommend the well-established FODMAP diet, which is known to reduce symptoms. But the answer for every patient is not simply placing them on a FODMAP diet and telling them they have irritable bowel syndrome.
Other approaches can be considered for addressing food intolerance in these patients. The data are quite strong that adjunctive use of a dietitian is tremendously helpful in this particular population.
When it comes to menopausal patients, however, we need to consider other changes in their activity or adverse contributors to their mental health, such as stress or anxiety. These all contribute to more of a multifactorial composite in this population, for which irritable bowel syndrome serves as a similar example.
This means that we may need to expand our horizons rather than to focus on solely on antispasmodic or diet-related interventions.
Instead, we can start to consider more of a multidimensional treatment approach consisting of education, relaxation, cognitive-behavioral therapy, and physical activity. Certainly, there are now behavioral interventions using Internet-based digital formats to increase the acceptability and sustainability among patients.
Choosing such a multidisciplinary approach can be quite helpful.
The metabolic consequences of altering hormonal balance
Recent data from a rat model study investigated the metabolic impact of changing hormonal balance.
Investigators looked at ovariectomized rats and found that there was a biologic change in the diversity of the general GI biome. There were also noteworthy associations with weight fluctuations and dramatic changes in the spatial memory and cognitive performance characteristics of these rats, which was subsequently improved by supplemental estrogen.
This indicates that we may be able to remediate these effects with the similar use of supplemental hormone replacement treatments.
Another recent study looked at nonalcoholic fatty liver disease, which is very common in the general population and has a > 20% worldwide prevalence in postmenopausal women. Albeit small in numbers, this was a very interesting study.
Investigators looked at the delivery method for menopausal hormone therapy, which was transdermal for 75 patients and oral for 293 patients. Then, they looked at ultrasound definition of nonalcoholic fatty liver disease after 1 year as the endpoint. They found an approximate 7% reduction in the patients who received the transdermal administration compared with a 4% increase in the patients who received it orally.
Again, we have to remember this is a relatively small study, but the results indicate that the route of estrogen administration may be an important consideration in nonalcoholic fatty liver disease.
Sleep disturbances: fragmentation, duration, and quality
Sleep is something that’s near and dear to my heart and is the focus of a lot of our research.
Sleep disturbances are really part and parcel of menopause and are observed with hormonal imbalances and temperature intolerances. Disturbances such as sleep fragmentation, shorter sleep duration, and poorer sleep quality have a dramatic effect not only on the biome but also on sensory thresholds.
Therefore, as we start to look at mitigating strategies here, we need to focus on sleep and ask the right questions.
In my own practice, I try not to just ask, “How did you sleep last night?” That’s because sleep can be somewhat amnestic. You may have a cognitive awakening or a noncognitive awakening but still have experienced fragmentation.
As a result, my focus is on next-day function. I ask my patients, “When you get up in the morning, are you refreshed? Do you have the ability to perform daytime activities? Do you experience early fatigue or cognitive changes that occur?”
These questions can provide good insights into the sleep efficiency of the previous night.
The effect of the microbiome on osteoporosis
One final topic I found very interesting pertains to the effects of menopause on osteoporosis.
We certainly know that postmenopausal women have a very high prevalence of osteopenia, and that osteoporosis is a progression of that, as well as that increased bone-related disease affects fractures and related morbidity and mortality.
However, there’s accumulating evidence on the osteoporotic effects of biomarker changes in menopause, which shows that the biome regulates the pathophysiologic process of at least a large degree of osteoporosis.
This starts to make sense when you look at the pro-inflammatory factors that increase with changes in biome diversity, in particular tumor necrosis factor alpha (which is something we also see in inflammatory bowel disease), interleukin-1, and increased activated osteoclasts.
Therefore, when it comes to decreasing bone loss among patients who are perimenopausal or postmenopausal, we don’t yet have a clear answer. Hormone therapy, diet, activity, vitamin D supplementation, and other things may positively change the biome. They are worthy topics for patients to bring up with their ob.gyns. or primary care doctors.
Although it may be a little bit outside the scope of gastroenterology, in my opinion there are a number of new findings relating to menopause that we as a field need to be more proactive in addressing.
Ask the right questions when these people come in to you, irrespective of why they’re there. Start to ask about the quality of their sleep. What are their other functional symptoms? What are their other potential osteoporosis-related risks?
We must do a better job about individualizing care. Rather than treating patients as disease states, we must start to do specific patient-focused care.
I hope this gives you some provocative thoughts when you have your next session with a patient in the perimenopausal or menopausal state. There are lots of things that we continue to learn.
Dr. Johnson is professor of medicine and chief of gastroenterology at Eastern Virginia Medical School in Norfolk, Va., and a past president of the American College of Gastroenterology. He serves as an adviser to ISOThrive and Johnson & Johnson.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
Welcome back to another GI Common Concerns.
Today, I want to highlight some information about menopause.
Approximately 1.5 million women in the United States per year enter into menopause. Hysterectomy is also one of the most common surgeries for women worldwide, with an estimated 20%-40% undergoing this procedure by the age of 60.
Therefore, whether it’s because of biologic onset with age or surgical induction, menopause is a very common condition, and it’s important that we understand its symptoms and the latest information around it.
Impact on GI motility
One of the clearest functional symptoms to be aware of with menopause relates to alterations in hormonal balance. This has an impact on gastrointestinal (GI) motility by increasing abdominal muscle stimulation related to different patterns of secretion and can result in a number of symptomatic changes.
One such change that can occur is food intolerance. It is believed that menopause-associated food intolerance has multiple possible causes and may be related more to alterations to the microbiome, which can be contributed to by diet, activity, sleep cycle, and other factors.
When food intolerances are triggered in the perimenopausal or menopausal patient, it may lead you to recommend the well-established FODMAP diet, which is known to reduce symptoms. But the answer for every patient is not simply placing them on a FODMAP diet and telling them they have irritable bowel syndrome.
Other approaches can be considered for addressing food intolerance in these patients. The data are quite strong that adjunctive use of a dietitian is tremendously helpful in this particular population.
When it comes to menopausal patients, however, we need to consider other changes in their activity or adverse contributors to their mental health, such as stress or anxiety. These all contribute to more of a multifactorial composite in this population, for which irritable bowel syndrome serves as a similar example.
This means that we may need to expand our horizons rather than to focus on solely on antispasmodic or diet-related interventions.
Instead, we can start to consider more of a multidimensional treatment approach consisting of education, relaxation, cognitive-behavioral therapy, and physical activity. Certainly, there are now behavioral interventions using Internet-based digital formats to increase the acceptability and sustainability among patients.
Choosing such a multidisciplinary approach can be quite helpful.
The metabolic consequences of altering hormonal balance
Recent data from a rat model study investigated the metabolic impact of changing hormonal balance.
Investigators looked at ovariectomized rats and found that there was a biologic change in the diversity of the general GI biome. There were also noteworthy associations with weight fluctuations and dramatic changes in the spatial memory and cognitive performance characteristics of these rats, which was subsequently improved by supplemental estrogen.
This indicates that we may be able to remediate these effects with the similar use of supplemental hormone replacement treatments.
Another recent study looked at nonalcoholic fatty liver disease, which is very common in the general population and has a > 20% worldwide prevalence in postmenopausal women. Albeit small in numbers, this was a very interesting study.
Investigators looked at the delivery method for menopausal hormone therapy, which was transdermal for 75 patients and oral for 293 patients. Then, they looked at ultrasound definition of nonalcoholic fatty liver disease after 1 year as the endpoint. They found an approximate 7% reduction in the patients who received the transdermal administration compared with a 4% increase in the patients who received it orally.
Again, we have to remember this is a relatively small study, but the results indicate that the route of estrogen administration may be an important consideration in nonalcoholic fatty liver disease.
Sleep disturbances: fragmentation, duration, and quality
Sleep is something that’s near and dear to my heart and is the focus of a lot of our research.
Sleep disturbances are really part and parcel of menopause and are observed with hormonal imbalances and temperature intolerances. Disturbances such as sleep fragmentation, shorter sleep duration, and poorer sleep quality have a dramatic effect not only on the biome but also on sensory thresholds.
Therefore, as we start to look at mitigating strategies here, we need to focus on sleep and ask the right questions.
In my own practice, I try not to just ask, “How did you sleep last night?” That’s because sleep can be somewhat amnestic. You may have a cognitive awakening or a noncognitive awakening but still have experienced fragmentation.
As a result, my focus is on next-day function. I ask my patients, “When you get up in the morning, are you refreshed? Do you have the ability to perform daytime activities? Do you experience early fatigue or cognitive changes that occur?”
These questions can provide good insights into the sleep efficiency of the previous night.
The effect of the microbiome on osteoporosis
One final topic I found very interesting pertains to the effects of menopause on osteoporosis.
We certainly know that postmenopausal women have a very high prevalence of osteopenia, and that osteoporosis is a progression of that, as well as that increased bone-related disease affects fractures and related morbidity and mortality.
However, there’s accumulating evidence on the osteoporotic effects of biomarker changes in menopause, which shows that the biome regulates the pathophysiologic process of at least a large degree of osteoporosis.
This starts to make sense when you look at the pro-inflammatory factors that increase with changes in biome diversity, in particular tumor necrosis factor alpha (which is something we also see in inflammatory bowel disease), interleukin-1, and increased activated osteoclasts.
Therefore, when it comes to decreasing bone loss among patients who are perimenopausal or postmenopausal, we don’t yet have a clear answer. Hormone therapy, diet, activity, vitamin D supplementation, and other things may positively change the biome. They are worthy topics for patients to bring up with their ob.gyns. or primary care doctors.
Although it may be a little bit outside the scope of gastroenterology, in my opinion there are a number of new findings relating to menopause that we as a field need to be more proactive in addressing.
Ask the right questions when these people come in to you, irrespective of why they’re there. Start to ask about the quality of their sleep. What are their other functional symptoms? What are their other potential osteoporosis-related risks?
We must do a better job about individualizing care. Rather than treating patients as disease states, we must start to do specific patient-focused care.
I hope this gives you some provocative thoughts when you have your next session with a patient in the perimenopausal or menopausal state. There are lots of things that we continue to learn.
Dr. Johnson is professor of medicine and chief of gastroenterology at Eastern Virginia Medical School in Norfolk, Va., and a past president of the American College of Gastroenterology. He serves as an adviser to ISOThrive and Johnson & Johnson.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
Welcome back to another GI Common Concerns.
Today, I want to highlight some information about menopause.
Approximately 1.5 million women in the United States per year enter into menopause. Hysterectomy is also one of the most common surgeries for women worldwide, with an estimated 20%-40% undergoing this procedure by the age of 60.
Therefore, whether it’s because of biologic onset with age or surgical induction, menopause is a very common condition, and it’s important that we understand its symptoms and the latest information around it.
Impact on GI motility
One of the clearest functional symptoms to be aware of with menopause relates to alterations in hormonal balance. This has an impact on gastrointestinal (GI) motility by increasing abdominal muscle stimulation related to different patterns of secretion and can result in a number of symptomatic changes.
One such change that can occur is food intolerance. It is believed that menopause-associated food intolerance has multiple possible causes and may be related more to alterations to the microbiome, which can be contributed to by diet, activity, sleep cycle, and other factors.
When food intolerances are triggered in the perimenopausal or menopausal patient, it may lead you to recommend the well-established FODMAP diet, which is known to reduce symptoms. But the answer for every patient is not simply placing them on a FODMAP diet and telling them they have irritable bowel syndrome.
Other approaches can be considered for addressing food intolerance in these patients. The data are quite strong that adjunctive use of a dietitian is tremendously helpful in this particular population.
When it comes to menopausal patients, however, we need to consider other changes in their activity or adverse contributors to their mental health, such as stress or anxiety. These all contribute to more of a multifactorial composite in this population, for which irritable bowel syndrome serves as a similar example.
This means that we may need to expand our horizons rather than to focus on solely on antispasmodic or diet-related interventions.
Instead, we can start to consider more of a multidimensional treatment approach consisting of education, relaxation, cognitive-behavioral therapy, and physical activity. Certainly, there are now behavioral interventions using Internet-based digital formats to increase the acceptability and sustainability among patients.
Choosing such a multidisciplinary approach can be quite helpful.
The metabolic consequences of altering hormonal balance
Recent data from a rat model study investigated the metabolic impact of changing hormonal balance.
Investigators looked at ovariectomized rats and found that there was a biologic change in the diversity of the general GI biome. There were also noteworthy associations with weight fluctuations and dramatic changes in the spatial memory and cognitive performance characteristics of these rats, which was subsequently improved by supplemental estrogen.
This indicates that we may be able to remediate these effects with the similar use of supplemental hormone replacement treatments.
Another recent study looked at nonalcoholic fatty liver disease, which is very common in the general population and has a > 20% worldwide prevalence in postmenopausal women. Albeit small in numbers, this was a very interesting study.
Investigators looked at the delivery method for menopausal hormone therapy, which was transdermal for 75 patients and oral for 293 patients. Then, they looked at ultrasound definition of nonalcoholic fatty liver disease after 1 year as the endpoint. They found an approximate 7% reduction in the patients who received the transdermal administration compared with a 4% increase in the patients who received it orally.
Again, we have to remember this is a relatively small study, but the results indicate that the route of estrogen administration may be an important consideration in nonalcoholic fatty liver disease.
Sleep disturbances: fragmentation, duration, and quality
Sleep is something that’s near and dear to my heart and is the focus of a lot of our research.
Sleep disturbances are really part and parcel of menopause and are observed with hormonal imbalances and temperature intolerances. Disturbances such as sleep fragmentation, shorter sleep duration, and poorer sleep quality have a dramatic effect not only on the biome but also on sensory thresholds.
Therefore, as we start to look at mitigating strategies here, we need to focus on sleep and ask the right questions.
In my own practice, I try not to just ask, “How did you sleep last night?” That’s because sleep can be somewhat amnestic. You may have a cognitive awakening or a noncognitive awakening but still have experienced fragmentation.
As a result, my focus is on next-day function. I ask my patients, “When you get up in the morning, are you refreshed? Do you have the ability to perform daytime activities? Do you experience early fatigue or cognitive changes that occur?”
These questions can provide good insights into the sleep efficiency of the previous night.
The effect of the microbiome on osteoporosis
One final topic I found very interesting pertains to the effects of menopause on osteoporosis.
We certainly know that postmenopausal women have a very high prevalence of osteopenia, and that osteoporosis is a progression of that, as well as that increased bone-related disease affects fractures and related morbidity and mortality.
However, there’s accumulating evidence on the osteoporotic effects of biomarker changes in menopause, which shows that the biome regulates the pathophysiologic process of at least a large degree of osteoporosis.
This starts to make sense when you look at the pro-inflammatory factors that increase with changes in biome diversity, in particular tumor necrosis factor alpha (which is something we also see in inflammatory bowel disease), interleukin-1, and increased activated osteoclasts.
Therefore, when it comes to decreasing bone loss among patients who are perimenopausal or postmenopausal, we don’t yet have a clear answer. Hormone therapy, diet, activity, vitamin D supplementation, and other things may positively change the biome. They are worthy topics for patients to bring up with their ob.gyns. or primary care doctors.
Although it may be a little bit outside the scope of gastroenterology, in my opinion there are a number of new findings relating to menopause that we as a field need to be more proactive in addressing.
Ask the right questions when these people come in to you, irrespective of why they’re there. Start to ask about the quality of their sleep. What are their other functional symptoms? What are their other potential osteoporosis-related risks?
We must do a better job about individualizing care. Rather than treating patients as disease states, we must start to do specific patient-focused care.
I hope this gives you some provocative thoughts when you have your next session with a patient in the perimenopausal or menopausal state. There are lots of things that we continue to learn.
Dr. Johnson is professor of medicine and chief of gastroenterology at Eastern Virginia Medical School in Norfolk, Va., and a past president of the American College of Gastroenterology. He serves as an adviser to ISOThrive and Johnson & Johnson.
A version of this article first appeared on Medscape.com.
Thinking about masks
I have a cold.
This is nothing new. Like most of us, I’ve probably gotten two or three a year for most of my life. I load up on Tylenol, Sudafed, cough syrup, and ginger ale (I’m not a chicken soup person), and I power through.
I may be sick, but there are patients to see. For better or worse, the idea of calling in sick never seems to apply to the health care profession. So I put on a mask to protect my patients and go ahead with my day.
But, as I blow my nose and accept my fate for the next week, I realize that I haven’t been sick with anything since 2019. Really.
I have no idea how many times in the last week I’ve told someone “I’d forgotten how much I hated being sick.” Certainly there are far worse things to have (colds are high on the “annoying” but low on the “serious” scales), but it’s odd to find myself back in the familiar pattern of coughing, sneezing, and low-grade fever that used to be a semi-annual occurrence.
So I look at myself in the mirror and wonder if the masks were that bad an idea? Certainly I have my share of patients, usually with immune diseases, who still wear them, and I see people at the store doing the same. There are countries where it was common to have them on even before the pandemic, though that was more for pollution.
I’m still pretty careful about hand washing, but that’s the nature of my job, anyway.
I keep coming back to the mask, though. Obviously, nothing is 100% successful, but certainly it puts a respiratory filter of sorts between us and the world (and vice versa). We use them in surgery and isolation rooms. It’s probably not the only reason I went 4 years without a cold, but it likely helped.
On the other hand, it has its drawbacks. A lot of my patients have hearing issues, and the mask doesn’t improve that. It also limits communication by facial expression, which is always important. It fogs up my classes (during the pandemic it became quite clear that any mask that claimed to be fog-free was lying).
I’m not saying everyone should wear them. This is up to me, that’s up to them.
But, for myself, it’s something to think about.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
I have a cold.
This is nothing new. Like most of us, I’ve probably gotten two or three a year for most of my life. I load up on Tylenol, Sudafed, cough syrup, and ginger ale (I’m not a chicken soup person), and I power through.
I may be sick, but there are patients to see. For better or worse, the idea of calling in sick never seems to apply to the health care profession. So I put on a mask to protect my patients and go ahead with my day.
But, as I blow my nose and accept my fate for the next week, I realize that I haven’t been sick with anything since 2019. Really.
I have no idea how many times in the last week I’ve told someone “I’d forgotten how much I hated being sick.” Certainly there are far worse things to have (colds are high on the “annoying” but low on the “serious” scales), but it’s odd to find myself back in the familiar pattern of coughing, sneezing, and low-grade fever that used to be a semi-annual occurrence.
So I look at myself in the mirror and wonder if the masks were that bad an idea? Certainly I have my share of patients, usually with immune diseases, who still wear them, and I see people at the store doing the same. There are countries where it was common to have them on even before the pandemic, though that was more for pollution.
I’m still pretty careful about hand washing, but that’s the nature of my job, anyway.
I keep coming back to the mask, though. Obviously, nothing is 100% successful, but certainly it puts a respiratory filter of sorts between us and the world (and vice versa). We use them in surgery and isolation rooms. It’s probably not the only reason I went 4 years without a cold, but it likely helped.
On the other hand, it has its drawbacks. A lot of my patients have hearing issues, and the mask doesn’t improve that. It also limits communication by facial expression, which is always important. It fogs up my classes (during the pandemic it became quite clear that any mask that claimed to be fog-free was lying).
I’m not saying everyone should wear them. This is up to me, that’s up to them.
But, for myself, it’s something to think about.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
I have a cold.
This is nothing new. Like most of us, I’ve probably gotten two or three a year for most of my life. I load up on Tylenol, Sudafed, cough syrup, and ginger ale (I’m not a chicken soup person), and I power through.
I may be sick, but there are patients to see. For better or worse, the idea of calling in sick never seems to apply to the health care profession. So I put on a mask to protect my patients and go ahead with my day.
But, as I blow my nose and accept my fate for the next week, I realize that I haven’t been sick with anything since 2019. Really.
I have no idea how many times in the last week I’ve told someone “I’d forgotten how much I hated being sick.” Certainly there are far worse things to have (colds are high on the “annoying” but low on the “serious” scales), but it’s odd to find myself back in the familiar pattern of coughing, sneezing, and low-grade fever that used to be a semi-annual occurrence.
So I look at myself in the mirror and wonder if the masks were that bad an idea? Certainly I have my share of patients, usually with immune diseases, who still wear them, and I see people at the store doing the same. There are countries where it was common to have them on even before the pandemic, though that was more for pollution.
I’m still pretty careful about hand washing, but that’s the nature of my job, anyway.
I keep coming back to the mask, though. Obviously, nothing is 100% successful, but certainly it puts a respiratory filter of sorts between us and the world (and vice versa). We use them in surgery and isolation rooms. It’s probably not the only reason I went 4 years without a cold, but it likely helped.
On the other hand, it has its drawbacks. A lot of my patients have hearing issues, and the mask doesn’t improve that. It also limits communication by facial expression, which is always important. It fogs up my classes (during the pandemic it became quite clear that any mask that claimed to be fog-free was lying).
I’m not saying everyone should wear them. This is up to me, that’s up to them.
But, for myself, it’s something to think about.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
Heart rate variability: Are we ignoring a harbinger of health?
A very long time ago, when I ran clinical labs, one of the most ordered tests was the “sed rate” (aka ESR, the erythrocyte sedimentation rate). Easy, quick, and low cost, with high sensitivity but very low specificity. If the sed rate was normal, the patient probably did not have an infectious or inflammatory disease. If it was elevated, they probably did, but no telling what. Later, the C-reactive protein (CRP) test came into common use. Same general inferences: If the CRP was low, the patient was unlikely to have an inflammatory process; if high, they were sick, but we didn’t know what with.
Could the heart rate variability (HRV) score come to be thought of similarly? Much as the sed rate and CRP are sensitivity indicators of infectious or inflammatory diseases, might the HRV score be a sensitivity indicator for nervous system (central and autonomic) and cardiovascular (especially heart rhythm) malfunctions?
A substantial and relatively old body of heart rhythm literature ties HRV alterations to posttraumatic stress disorder, physician occupational stress, sleep disorders, depression, autonomic nervous system derangements, various cardiac arrhythmias, fatigue, overexertion, medications, and age itself.
More than 100 million Americans are now believed to use smartwatches or personal fitness monitors. Some 30%-40% of these devices measure HRV. So what? Credible research about this huge mass of accumulating data from “wearables” is lacking.
What is HRV?
HRV is the variation in time between each heartbeat, in milliseconds. HRV is influenced by the autonomic nervous system, perhaps reflecting sympathetic-parasympathetic balance. Some devices measure HRV 24/7. My Fitbit Inspire 2 reports only nighttime measures during 3 hours of sustained sleep. Most trackers report averages; some calculate the root mean squares; others calculate standard deviations. All fitness trackers warn not to use the data for medical purposes.
Normal values (reference ranges) for HRV begin at an average of 100 msec in the first decade of life and decline by approximately 10 msec per decade lived. At age 30-40, the average is 70 msec; age 60-70, it’s 40 msec; and at age 90-100, it’s 10 msec.
As a long-time lab guy, I used to teach proper use of lab tests. Fitness trackers are “lab tests” of a sort. We taught never to do a lab test unless you know what you are going to do with the result, no matter what it is. We also taught “never do anything just because you can.” Curiosity, we know, is a frequent driver of lab test ordering.
That underlying philosophy gives me a hard time when it comes to wearables. I have been enamored of watching my step count, active zone minutes, resting heart rate, active heart rate, various sleep scores, and breathing rate (and, of course, a manually entered early morning daily body weight) for several years. I even check my “readiness score” (a calculation using resting heart rate, recent sleep, recent active zone minutes, and perhaps HRV) each morning and adjust my behaviors accordingly.
Why monitor HRV?
But what should we do with HRV scores? Ignore them? Try to understand them, perhaps as a screening tool? Or monitor HRV for consistency or change? “Monitoring” is a proper and common use of lab tests.
Some say we should improve the HRV score by managing stress, getting regular exercise, eating a healthy diet, getting enough sleep, and not smoking or consuming excess alcohol. Duh! I do all of that anyway.
The claims that HRV is a “simple but powerful tool that can be used to track overall health and well-being” might turn out to be true. Proper study and sharing of data will enable that determination.
To advance understanding, I offer an n-of-1, a real-world personal anecdote about HRV.
I did not request the HRV function on my Fitbit Inspire 2. It simply appeared, and I ignored it for some time.
A year or two ago, I started noticing my HRV score every morning. Initially, I did not like to see my “low” score, until I learned that the reference range was dramatically affected by age and I was in my late 80s at the time. The vast majority of my HRV readings were in the range of 17 msec to 27 msec.
Last week, I was administered the new Moderna COVID-19 Spikevax vaccine and the old folks’ influenza vaccine simultaneously. In my case, side effects from each vaccine have been modest in the past, but I never previously had both administered at the same time. My immune response was, shall we say, robust. Chills, muscle aches, headache, fatigue, deltoid swelling, fitful sleep, and increased resting heart rate.
My nightly average HRV had been running between 17 msec and 35 msec for many months. WHOA! After the shots, my overnight HRV score plummeted from 24 msec to 10 msec, my lowest ever. Instant worry. The next day, it rebounded to 28 msec, and it has been in the high teens or low 20s since then.
Off to PubMed. A recent study of HRV on the second and 10th days after administering the Pfizer mRNA vaccine to 75 healthy volunteers found that the HRV on day 2 was dramatically lower than prevaccination levels and by day 10, it had returned to prevaccination levels. Some comfort there.
Another review article has reported a rapid fall and rapid rebound of HRV after COVID-19 vaccination. A 2010 report demonstrated a significant but not dramatic short-term lowering of HRV after influenza A vaccination and correlated it with CRP changes.
Some believe that the decline in HRV after vaccination reflects an increased immune response and sympathetic nervous activity.
I don’t plan to receive my flu and COVID vaccines on the same day again.
So, I went back to review what happened to my HRV when I had COVID in 2023. My HRV was 14 msec and 12 msec on the first 2 days of symptoms, and then returned to the 20 msec range.
I received the RSV vaccine this year without adverse effects, and my HRV scores were 29 msec, 33 msec, and 32 msec on the first 3 days after vaccination. Finally, after receiving a pneumococcal vaccine in 2023, I had no adverse effects, and my HRV scores on the 5 days after vaccination were indeterminate: 19 msec, 14 msec, 18 msec, 13 msec, and 17 msec.
Of course, correlation is not causation. Cause and effect remain undetermined. But I find these observations interesting for a potentially useful screening test.
George D. Lundberg, MD, is the Editor in Chief of Cancer Commons.
A version of this article first appeared on Medscape.com.
A very long time ago, when I ran clinical labs, one of the most ordered tests was the “sed rate” (aka ESR, the erythrocyte sedimentation rate). Easy, quick, and low cost, with high sensitivity but very low specificity. If the sed rate was normal, the patient probably did not have an infectious or inflammatory disease. If it was elevated, they probably did, but no telling what. Later, the C-reactive protein (CRP) test came into common use. Same general inferences: If the CRP was low, the patient was unlikely to have an inflammatory process; if high, they were sick, but we didn’t know what with.
Could the heart rate variability (HRV) score come to be thought of similarly? Much as the sed rate and CRP are sensitivity indicators of infectious or inflammatory diseases, might the HRV score be a sensitivity indicator for nervous system (central and autonomic) and cardiovascular (especially heart rhythm) malfunctions?
A substantial and relatively old body of heart rhythm literature ties HRV alterations to posttraumatic stress disorder, physician occupational stress, sleep disorders, depression, autonomic nervous system derangements, various cardiac arrhythmias, fatigue, overexertion, medications, and age itself.
More than 100 million Americans are now believed to use smartwatches or personal fitness monitors. Some 30%-40% of these devices measure HRV. So what? Credible research about this huge mass of accumulating data from “wearables” is lacking.
What is HRV?
HRV is the variation in time between each heartbeat, in milliseconds. HRV is influenced by the autonomic nervous system, perhaps reflecting sympathetic-parasympathetic balance. Some devices measure HRV 24/7. My Fitbit Inspire 2 reports only nighttime measures during 3 hours of sustained sleep. Most trackers report averages; some calculate the root mean squares; others calculate standard deviations. All fitness trackers warn not to use the data for medical purposes.
Normal values (reference ranges) for HRV begin at an average of 100 msec in the first decade of life and decline by approximately 10 msec per decade lived. At age 30-40, the average is 70 msec; age 60-70, it’s 40 msec; and at age 90-100, it’s 10 msec.
As a long-time lab guy, I used to teach proper use of lab tests. Fitness trackers are “lab tests” of a sort. We taught never to do a lab test unless you know what you are going to do with the result, no matter what it is. We also taught “never do anything just because you can.” Curiosity, we know, is a frequent driver of lab test ordering.
That underlying philosophy gives me a hard time when it comes to wearables. I have been enamored of watching my step count, active zone minutes, resting heart rate, active heart rate, various sleep scores, and breathing rate (and, of course, a manually entered early morning daily body weight) for several years. I even check my “readiness score” (a calculation using resting heart rate, recent sleep, recent active zone minutes, and perhaps HRV) each morning and adjust my behaviors accordingly.
Why monitor HRV?
But what should we do with HRV scores? Ignore them? Try to understand them, perhaps as a screening tool? Or monitor HRV for consistency or change? “Monitoring” is a proper and common use of lab tests.
Some say we should improve the HRV score by managing stress, getting regular exercise, eating a healthy diet, getting enough sleep, and not smoking or consuming excess alcohol. Duh! I do all of that anyway.
The claims that HRV is a “simple but powerful tool that can be used to track overall health and well-being” might turn out to be true. Proper study and sharing of data will enable that determination.
To advance understanding, I offer an n-of-1, a real-world personal anecdote about HRV.
I did not request the HRV function on my Fitbit Inspire 2. It simply appeared, and I ignored it for some time.
A year or two ago, I started noticing my HRV score every morning. Initially, I did not like to see my “low” score, until I learned that the reference range was dramatically affected by age and I was in my late 80s at the time. The vast majority of my HRV readings were in the range of 17 msec to 27 msec.
Last week, I was administered the new Moderna COVID-19 Spikevax vaccine and the old folks’ influenza vaccine simultaneously. In my case, side effects from each vaccine have been modest in the past, but I never previously had both administered at the same time. My immune response was, shall we say, robust. Chills, muscle aches, headache, fatigue, deltoid swelling, fitful sleep, and increased resting heart rate.
My nightly average HRV had been running between 17 msec and 35 msec for many months. WHOA! After the shots, my overnight HRV score plummeted from 24 msec to 10 msec, my lowest ever. Instant worry. The next day, it rebounded to 28 msec, and it has been in the high teens or low 20s since then.
Off to PubMed. A recent study of HRV on the second and 10th days after administering the Pfizer mRNA vaccine to 75 healthy volunteers found that the HRV on day 2 was dramatically lower than prevaccination levels and by day 10, it had returned to prevaccination levels. Some comfort there.
Another review article has reported a rapid fall and rapid rebound of HRV after COVID-19 vaccination. A 2010 report demonstrated a significant but not dramatic short-term lowering of HRV after influenza A vaccination and correlated it with CRP changes.
Some believe that the decline in HRV after vaccination reflects an increased immune response and sympathetic nervous activity.
I don’t plan to receive my flu and COVID vaccines on the same day again.
So, I went back to review what happened to my HRV when I had COVID in 2023. My HRV was 14 msec and 12 msec on the first 2 days of symptoms, and then returned to the 20 msec range.
I received the RSV vaccine this year without adverse effects, and my HRV scores were 29 msec, 33 msec, and 32 msec on the first 3 days after vaccination. Finally, after receiving a pneumococcal vaccine in 2023, I had no adverse effects, and my HRV scores on the 5 days after vaccination were indeterminate: 19 msec, 14 msec, 18 msec, 13 msec, and 17 msec.
Of course, correlation is not causation. Cause and effect remain undetermined. But I find these observations interesting for a potentially useful screening test.
George D. Lundberg, MD, is the Editor in Chief of Cancer Commons.
A version of this article first appeared on Medscape.com.
A very long time ago, when I ran clinical labs, one of the most ordered tests was the “sed rate” (aka ESR, the erythrocyte sedimentation rate). Easy, quick, and low cost, with high sensitivity but very low specificity. If the sed rate was normal, the patient probably did not have an infectious or inflammatory disease. If it was elevated, they probably did, but no telling what. Later, the C-reactive protein (CRP) test came into common use. Same general inferences: If the CRP was low, the patient was unlikely to have an inflammatory process; if high, they were sick, but we didn’t know what with.
Could the heart rate variability (HRV) score come to be thought of similarly? Much as the sed rate and CRP are sensitivity indicators of infectious or inflammatory diseases, might the HRV score be a sensitivity indicator for nervous system (central and autonomic) and cardiovascular (especially heart rhythm) malfunctions?
A substantial and relatively old body of heart rhythm literature ties HRV alterations to posttraumatic stress disorder, physician occupational stress, sleep disorders, depression, autonomic nervous system derangements, various cardiac arrhythmias, fatigue, overexertion, medications, and age itself.
More than 100 million Americans are now believed to use smartwatches or personal fitness monitors. Some 30%-40% of these devices measure HRV. So what? Credible research about this huge mass of accumulating data from “wearables” is lacking.
What is HRV?
HRV is the variation in time between each heartbeat, in milliseconds. HRV is influenced by the autonomic nervous system, perhaps reflecting sympathetic-parasympathetic balance. Some devices measure HRV 24/7. My Fitbit Inspire 2 reports only nighttime measures during 3 hours of sustained sleep. Most trackers report averages; some calculate the root mean squares; others calculate standard deviations. All fitness trackers warn not to use the data for medical purposes.
Normal values (reference ranges) for HRV begin at an average of 100 msec in the first decade of life and decline by approximately 10 msec per decade lived. At age 30-40, the average is 70 msec; age 60-70, it’s 40 msec; and at age 90-100, it’s 10 msec.
As a long-time lab guy, I used to teach proper use of lab tests. Fitness trackers are “lab tests” of a sort. We taught never to do a lab test unless you know what you are going to do with the result, no matter what it is. We also taught “never do anything just because you can.” Curiosity, we know, is a frequent driver of lab test ordering.
That underlying philosophy gives me a hard time when it comes to wearables. I have been enamored of watching my step count, active zone minutes, resting heart rate, active heart rate, various sleep scores, and breathing rate (and, of course, a manually entered early morning daily body weight) for several years. I even check my “readiness score” (a calculation using resting heart rate, recent sleep, recent active zone minutes, and perhaps HRV) each morning and adjust my behaviors accordingly.
Why monitor HRV?
But what should we do with HRV scores? Ignore them? Try to understand them, perhaps as a screening tool? Or monitor HRV for consistency or change? “Monitoring” is a proper and common use of lab tests.
Some say we should improve the HRV score by managing stress, getting regular exercise, eating a healthy diet, getting enough sleep, and not smoking or consuming excess alcohol. Duh! I do all of that anyway.
The claims that HRV is a “simple but powerful tool that can be used to track overall health and well-being” might turn out to be true. Proper study and sharing of data will enable that determination.
To advance understanding, I offer an n-of-1, a real-world personal anecdote about HRV.
I did not request the HRV function on my Fitbit Inspire 2. It simply appeared, and I ignored it for some time.
A year or two ago, I started noticing my HRV score every morning. Initially, I did not like to see my “low” score, until I learned that the reference range was dramatically affected by age and I was in my late 80s at the time. The vast majority of my HRV readings were in the range of 17 msec to 27 msec.
Last week, I was administered the new Moderna COVID-19 Spikevax vaccine and the old folks’ influenza vaccine simultaneously. In my case, side effects from each vaccine have been modest in the past, but I never previously had both administered at the same time. My immune response was, shall we say, robust. Chills, muscle aches, headache, fatigue, deltoid swelling, fitful sleep, and increased resting heart rate.
My nightly average HRV had been running between 17 msec and 35 msec for many months. WHOA! After the shots, my overnight HRV score plummeted from 24 msec to 10 msec, my lowest ever. Instant worry. The next day, it rebounded to 28 msec, and it has been in the high teens or low 20s since then.
Off to PubMed. A recent study of HRV on the second and 10th days after administering the Pfizer mRNA vaccine to 75 healthy volunteers found that the HRV on day 2 was dramatically lower than prevaccination levels and by day 10, it had returned to prevaccination levels. Some comfort there.
Another review article has reported a rapid fall and rapid rebound of HRV after COVID-19 vaccination. A 2010 report demonstrated a significant but not dramatic short-term lowering of HRV after influenza A vaccination and correlated it with CRP changes.
Some believe that the decline in HRV after vaccination reflects an increased immune response and sympathetic nervous activity.
I don’t plan to receive my flu and COVID vaccines on the same day again.
So, I went back to review what happened to my HRV when I had COVID in 2023. My HRV was 14 msec and 12 msec on the first 2 days of symptoms, and then returned to the 20 msec range.
I received the RSV vaccine this year without adverse effects, and my HRV scores were 29 msec, 33 msec, and 32 msec on the first 3 days after vaccination. Finally, after receiving a pneumococcal vaccine in 2023, I had no adverse effects, and my HRV scores on the 5 days after vaccination were indeterminate: 19 msec, 14 msec, 18 msec, 13 msec, and 17 msec.
Of course, correlation is not causation. Cause and effect remain undetermined. But I find these observations interesting for a potentially useful screening test.
George D. Lundberg, MD, is the Editor in Chief of Cancer Commons.
A version of this article first appeared on Medscape.com.