User login
Moving toward safer morcellation techniques
For minimally invasive surgeons throughout the world, particularly in the United States, as well as the patients we treat, April 17, 2014, is our day of infamy. It was on this day that the Food and Drug Administration recommended against the use of the electronic power morcellator. The basis of the agency’s decision was the concern about inadvertent spread of sarcomatous tissue. Many hospitals, medical centers, and hospital systems subsequently banned the use of power morcellation. With such bans, a subsequent study by Wright et al. noted a decrease in the percentage of both laparoscopic and vaginal hysterectomy (JAMA. 2016 Aug 23-30;316[8]:877-8). This is concerning when you consider that the complication rate for abdominal hysterectomy is around 17%, compared with about 4% for the minimally invasive procedure.
For this edition of the Master Class in Gynecologic Surgery, I have asked Tony Shibley, MD, to describe the PneumoLiner, the first FDA-approved bag for the purpose of contained laparoscopic morcellation. Dr. Shibley, who is in private practice in the Minneapolis area, first came to national attention because of his expertise in single-port surgery. He has been performing power morcellation in a contained system for 5 years and is the thought leader behind the design and creation of the PneumoLiner.
Dr. Miller is clinical associate professor at the University of Illinois at Chicago, and past president of the AAGL and the International Society for Gynecologic Endoscopy. He is a reproductive endocrinologist and minimally invasive gynecologic surgeon in private practice in Naperville and Schaumburg, Ill.; director of minimally invasive gynecologic surgery and the director of the AAGL/SRS fellowship in minimally invasive gynecologic surgery at Advocate Lutheran General Hospital, Park Ridge, Ill.; and the medical editor of this column, Master Class. He reported receiving research funds from Espiner Medical Inc., and being a consultant to Olympus, which manufacturers the PneumoLiner.
For minimally invasive surgeons throughout the world, particularly in the United States, as well as the patients we treat, April 17, 2014, is our day of infamy. It was on this day that the Food and Drug Administration recommended against the use of the electronic power morcellator. The basis of the agency’s decision was the concern about inadvertent spread of sarcomatous tissue. Many hospitals, medical centers, and hospital systems subsequently banned the use of power morcellation. With such bans, a subsequent study by Wright et al. noted a decrease in the percentage of both laparoscopic and vaginal hysterectomy (JAMA. 2016 Aug 23-30;316[8]:877-8). This is concerning when you consider that the complication rate for abdominal hysterectomy is around 17%, compared with about 4% for the minimally invasive procedure.
For this edition of the Master Class in Gynecologic Surgery, I have asked Tony Shibley, MD, to describe the PneumoLiner, the first FDA-approved bag for the purpose of contained laparoscopic morcellation. Dr. Shibley, who is in private practice in the Minneapolis area, first came to national attention because of his expertise in single-port surgery. He has been performing power morcellation in a contained system for 5 years and is the thought leader behind the design and creation of the PneumoLiner.
Dr. Miller is clinical associate professor at the University of Illinois at Chicago, and past president of the AAGL and the International Society for Gynecologic Endoscopy. He is a reproductive endocrinologist and minimally invasive gynecologic surgeon in private practice in Naperville and Schaumburg, Ill.; director of minimally invasive gynecologic surgery and the director of the AAGL/SRS fellowship in minimally invasive gynecologic surgery at Advocate Lutheran General Hospital, Park Ridge, Ill.; and the medical editor of this column, Master Class. He reported receiving research funds from Espiner Medical Inc., and being a consultant to Olympus, which manufacturers the PneumoLiner.
For minimally invasive surgeons throughout the world, particularly in the United States, as well as the patients we treat, April 17, 2014, is our day of infamy. It was on this day that the Food and Drug Administration recommended against the use of the electronic power morcellator. The basis of the agency’s decision was the concern about inadvertent spread of sarcomatous tissue. Many hospitals, medical centers, and hospital systems subsequently banned the use of power morcellation. With such bans, a subsequent study by Wright et al. noted a decrease in the percentage of both laparoscopic and vaginal hysterectomy (JAMA. 2016 Aug 23-30;316[8]:877-8). This is concerning when you consider that the complication rate for abdominal hysterectomy is around 17%, compared with about 4% for the minimally invasive procedure.
For this edition of the Master Class in Gynecologic Surgery, I have asked Tony Shibley, MD, to describe the PneumoLiner, the first FDA-approved bag for the purpose of contained laparoscopic morcellation. Dr. Shibley, who is in private practice in the Minneapolis area, first came to national attention because of his expertise in single-port surgery. He has been performing power morcellation in a contained system for 5 years and is the thought leader behind the design and creation of the PneumoLiner.
Dr. Miller is clinical associate professor at the University of Illinois at Chicago, and past president of the AAGL and the International Society for Gynecologic Endoscopy. He is a reproductive endocrinologist and minimally invasive gynecologic surgeon in private practice in Naperville and Schaumburg, Ill.; director of minimally invasive gynecologic surgery and the director of the AAGL/SRS fellowship in minimally invasive gynecologic surgery at Advocate Lutheran General Hospital, Park Ridge, Ill.; and the medical editor of this column, Master Class. He reported receiving research funds from Espiner Medical Inc., and being a consultant to Olympus, which manufacturers the PneumoLiner.
Diagnosis and treatment of global endometrial ablation failure
One in seven women suffer with abnormal uterine bleeding during their reproductive years, according to Fraser et al. (Exp Rev Obstet Gynecol. 2009;4:179-89). Heavy menstrual bleeding (menorrhagia) is the most common pattern. Global endometrial ablation has become a very popular surgical technique for women complaining of menorrhagia, disinterested in either medical management or definitive therapy – hysterectomy – or where medical management has failed. With proper patient selection, endometrial ablation yields an 80%-90% success rate in reducing heavy menstrual flow and is associated with a 90% patient satisfaction rate (Cochrane Database Syst Rev. 2009 Oct 7;[4]:CD001501).
Literature is replete with conditions believed to increase risk of endometrial ablation failure. This list includes untreated uterine cornua, endometrial regrowth, the presence of submucous leiomyomas or polyps, abnormal uterine cavity, enlarged uterine cavity (width and/or length), endometrial ablation in a young patient, parity of five or greater, unsuspected adhesiolysis, postablation tubal sterilization syndrome, history of dysmenorrhea, smoking, obesity, prior cesarean section, previous gynecologic surgery, and procedure length. Interestingly, type of global endometrial ablation procedure or original bleeding pattern does not influence failure rate.
In this edition of the Master Class in Gynecologic Surgery, Dr. Morris Wortman discusses not only the prevention of endometrial ablation failure, but also how to treat the problem via conservative surgical management.
Dr. Wortman is a clinical associate professor of obstetrics and gynecology at the University of Rochester (N.Y.) and is the director at the Center for Menstrual Disorders and Reproductive Choice, also in Rochester. Dr. Wortman has lectured extensively on endometrial ablation and has authored several scientific articles in peer reviewed journals.
Dr. Miller is clinical associate professor at the University of Illinois at Chicago, and past president of the AAGL and the International Society for Gynecologic Endoscopy. He is a reproductive endocrinologist and minimally invasive gynecologic surgeon in private practice in Naperville and Schaumburg, Ill.; director of minimally invasive gynecologic surgery and the director of the AAGL/SRS fellowship in minimally invasive gynecologic surgery at Advocate Lutheran General Hospital, Park Ridge, Ill.; and the medical editor of this column, Master Class. He reported being a subinvestigator on a study sponsored by Channel Medsystems. Email him at [email protected].
Why failures occur and how to correct them
BY MORRIS WORTMAN, MD
Since the introduction almost 20 years ago of devices for nonresectoscopic – or “global” – endometrial ablation, the procedure has been widely adopted as the treatment of choice for abnormal uterine bleeding that is refractory to medical management.
Between 400,000 and 500,000 endometrial ablations are done in the United States every year in women who have completed childbearing, and it probably won’t be long before the procedure surpasses hysterectomy in prevalence for the management of abnormal bleeding.
In recent years, the literature has begun to address the incidence of these delayed complications and the requirement for subsequent hysterectomy. A 2007 practice bulletin issued by the American College of Obstetricians and Gynecologists stated that hysterectomy rates within 4 years of endometrial ablation are at least 24% (Obstet Gynecol. 2007 May;109[5]:1233-48). And a study published the following year reported that 26% of 3,681 women undergoing EA at Kaiser Permanente facilities in Northern California required hysterectomy within 8 years (Obstet Gynecol. 2008 Dec;112[6]:1214-20).
It appears that the vast majority of what we now refer to as late-onset EA failures – complications attributable to EA that occur beyond a perioperative period of 1 month – will occur within 5 years. Some EA failures have occurred over 5-10 years, however, and in my practice we have seen late-onset complications occurring 17 or more years after the initial ablation.
In our practice, we are successfully managing delayed complications after GEA using ultrasound-guided reoperative hysteroscopy to fully explore the uterine cavity and excise areas of endometrial growth and other disease. In 2014, we published a retrospective review of 50 women whom we treated for delayed complications after a variety of GEA techniques; almost 90% avoided hysterectomy during a mean follow-up period of 18 months (J Minim Invasive Gynecol. 2014 Mar-Apr;21[2]:238-44).
Our experience since then has included reoperative surgery on more than 115 GEA failures. Additionally, we’ve managed 220 patients who have undergone various hysteroscopic and resectoscopic endometrial ablations, some of which date back to the use of the Nd:YAG laser in the late 1980s.
The fact that late-onset EA failures occur does not mean that hysterectomy should routinely be performed as a first-line treatment for intractable uterine bleeding. Overall, there is much more morbidity associated with hysterectomy than with EA.
What failures do suggest is that there are certain risk factors for late-onset EA complications. Our experience in treating women who have experienced late-onset EA failure has provided us with insight into who may be at greatest risk for late-onset EA failure and how patients can best be selected for the procedure. We’ve also learned more about the diagnosis of delayed complications.
Causes of EA failure
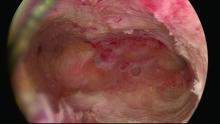
Untreated uterine cornua, and untreated submucous leiomyomas and endometrial polyps, are common causes of EA failure. Among the 50 women included in our retrospective review of ultrasound-guided reoperative hysteroscopy after GEA failure, 44% had intraoperative evidence of untreated cornua and nearly one-fourth had persistent or enlarging submucous leiomyomas.
Contrary to what some believe, most endometrial ablations will not adequately destroy submucous or intramural leiomyomas. Therefore, we recommend that these fibroids be entirely removed immediately before EA.
Moreover, GEA will not always provide adequate thermal destruction to the entire endometrial cavity. The cornua regions are particularly at risk; they are difficult to reach under ideal circumstances, and especially difficult to treat in patients who have a uterine septum or a T-shaped uterus (with the ostia and cornua deeply recessed). We have also seen late-onset EA failures in patients with an extended uterine transverse diameter. The limits of GEA are greatest when a device with a fixed configuration or geometry is used.
A history of abnormal hysteroscopy or other evidence of such anatomic distortions are therefore among the reported risk factors for GEA failure (J Minim Invasive Gynecol. 2015 Mar-Apr;22[3]:323-31). A history of tubal ligation also confers risk; the procedure further increases susceptibility for failure when functioning endometrial tissue remains or regrows at the cornua, because any retrograde menstrual bleeding that occurs will be constrained by the obstructed proximal portion of the fallopian tubes.
Obesity is another risk factor for GEA failure in that the condition increases the risk of endometrial cancer, making the need for reliable biopsies in the case of spotting or other signs or symptoms even more important. On the other hand, obesity may also worsen a patient’s status as a candidate for hysterectomy.
There is much to consider with these patients. For some obese patients, GEA may be less risky than hysterectomy while for others, such as those who also have polycystic ovarian syndrome (in whom the risk for developing endometrial cancer is further increased) the scale may tip in favor of hysterectomy.
Age at the time of the primary GEA may be the single most important risk factor for GEA failure and is an important predictor of success in patient selection. Numerous investigators have shown that women younger than 35 years of age at the time of their EA had a significantly increased risk for hysterectomy, compared with women who were at least 45 years old. The younger the patient, the longer the “bridge” to menopause and the greater the likelihood that bridge will fail.
While age is not necessarily a contraindication, it is worthy of serious consideration. We generally discourage GEA for patients younger than 35. We also advise ensuring that each patient undergoing initial EA is highly self-motivated to have a uterine-sparing procedure; if not, symptoms she may experience later will likely drive her toward hysterectomy anyway.
Additionally, we caution against performing GEA in patients who have chronic pelvic pain; these patients tend to have poorer outcomes with any type of hysteroscopic surgery.
Diagnosing failed EA
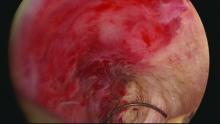
Delayed complications manifest in several ways: Renewed and increasing vaginal bleeding after a period of improvement, cyclic pelvic pain (unilateral, bilateral, or suprapubic), or both bleeding and pain. Some women – likely an underreported number of them – present with postmenopausal bleeding and proceed to have unsuccessful attempts at an endometrial biopsy due to EA-associated endometrial scarring.
The cyclic pelvic pain associated with endometrial persistence or regrowth tends to worsen over time and is often described as sharp or laborlike. In our experience, a description of “laborlike” pain and a history of EA is almost fully predictive of a finding of endometrial growth. Often a hematometra can be demonstrated on transvaginal ultrasound, but this isn’t always the case.
Pain typically precedes bleeding in patients who demonstrate both. In such cases, blood from functioning endometrial tissue or other sources becomes blocked from exiting the uterine cavity by EA-induced intrauterine scarring and contracture. Painful uterine contractions then aim to expel the pooled blood. In other cases of pain – mainly those without significant vaginal bleeding – the pain is often attributed to cornual and central hematometra.
For the majority of EA failures, the diagnosis lies in the history and current symptoms. Unfortunately, the traditional methods of assessing the endometrial cavity have little merit for women presenting with delayed-onset EA complications. A sonographically assisted pelvic examination can be useful in evaluating complications, but the interpretation of ultrasounds in women with a prior EA can be challenging and is often beyond the training of most radiologists and gynecologists.
It is not uncommon for images to be incorrectly interpreted in the emergency department or physicians’ offices as “normal” and for such readings to set off a chain of CT scans, MRIs, laparoscopies, ovarian cystectomies, and other procedures that miss the root causes of pain.
Unfortunately, there is little in the literature that describes and defines ultrasound findings after EA. We do know that sonography should be timed with episodes of pain, and that the absence of a demonstrable hematometra does not exclude a diagnosis of EA failure.
Correcting late-onset failures
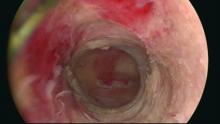
Our office-based operating room is fitted with side-by-side monitors that enable simultaneous sonographic and hysteroscopic views for correction of GEA failures; the rest of the set-up is similar to that of other operative hysteroscopies. However, we do employ a wide variety of resectoscopes with diameters ranging from 13 to 28 Fr. The smaller-diameter scopes are particularly useful for evaluating postmenopausal bleeding in women with a prior EA.
For those inexperienced with ultrasound-guided surgery, the initial resection is often the most challenging. The initial tissue removal is carried out on the thickest observed uterine wall – usually the posterior or anterior wall – and is done with near complete reliance on the ultrasound image. Hysteroscopic visualization is poor at this time because the outflow ports of the continuous flow resectoscope are obstructed by tissue in the narrow tubular cavity.
We then actually remove the resectoscope and clean the outflow ports of clots and debris that may have accumulated. When the scope is reinserted, there is typically sufficient room in the uterine cavity for continuous flow and excellent hysteroscopic visualization.
The sequence of resection from this point on will vary. If we’ve begun on the anterior wall, we’ll move to the posterior and then the two lateral walls to further restore the cavity. Areas of endometrial regrowth will typically be identified at this point and resected. The dissection then will extend upward, usually to within 10 mm of the fundus in the midline as measured by ultrasound. Reconfiguring the loop electrode to a 135- to 160-degree angle can be helpful in the delicate dissection that is required at the fundus.
Once all areas of endometrium have been identified and excised, we will deeply coagulate exposed myometrium with a ball-end electrode. Rarely, we will reach our maximum allowable fluid absorption limit prior to completing the case, a scenario seen in less than 1% of our patients.
In more than 330 reoperative hysteroscopic procedures, we’ve had only one uterine perforation that occurred when we switched ultrasound machines. Very likely, we were too aggressive in removing tissue at the fundus. The patient required a diagnostic laparoscopy but sustained no visceral injury.
Dr. Wortman is a clinical associate professor of obstetrics and gynecology at the University of Rochester (N.Y.) and the director of the Center for Menstrual Disorders and Reproductive Choice in Rochester. He reported having no relevant financial disclosures.
One in seven women suffer with abnormal uterine bleeding during their reproductive years, according to Fraser et al. (Exp Rev Obstet Gynecol. 2009;4:179-89). Heavy menstrual bleeding (menorrhagia) is the most common pattern. Global endometrial ablation has become a very popular surgical technique for women complaining of menorrhagia, disinterested in either medical management or definitive therapy – hysterectomy – or where medical management has failed. With proper patient selection, endometrial ablation yields an 80%-90% success rate in reducing heavy menstrual flow and is associated with a 90% patient satisfaction rate (Cochrane Database Syst Rev. 2009 Oct 7;[4]:CD001501).
Literature is replete with conditions believed to increase risk of endometrial ablation failure. This list includes untreated uterine cornua, endometrial regrowth, the presence of submucous leiomyomas or polyps, abnormal uterine cavity, enlarged uterine cavity (width and/or length), endometrial ablation in a young patient, parity of five or greater, unsuspected adhesiolysis, postablation tubal sterilization syndrome, history of dysmenorrhea, smoking, obesity, prior cesarean section, previous gynecologic surgery, and procedure length. Interestingly, type of global endometrial ablation procedure or original bleeding pattern does not influence failure rate.
In this edition of the Master Class in Gynecologic Surgery, Dr. Morris Wortman discusses not only the prevention of endometrial ablation failure, but also how to treat the problem via conservative surgical management.
Dr. Wortman is a clinical associate professor of obstetrics and gynecology at the University of Rochester (N.Y.) and is the director at the Center for Menstrual Disorders and Reproductive Choice, also in Rochester. Dr. Wortman has lectured extensively on endometrial ablation and has authored several scientific articles in peer reviewed journals.
Dr. Miller is clinical associate professor at the University of Illinois at Chicago, and past president of the AAGL and the International Society for Gynecologic Endoscopy. He is a reproductive endocrinologist and minimally invasive gynecologic surgeon in private practice in Naperville and Schaumburg, Ill.; director of minimally invasive gynecologic surgery and the director of the AAGL/SRS fellowship in minimally invasive gynecologic surgery at Advocate Lutheran General Hospital, Park Ridge, Ill.; and the medical editor of this column, Master Class. He reported being a subinvestigator on a study sponsored by Channel Medsystems. Email him at [email protected].
Why failures occur and how to correct them
BY MORRIS WORTMAN, MD
Since the introduction almost 20 years ago of devices for nonresectoscopic – or “global” – endometrial ablation, the procedure has been widely adopted as the treatment of choice for abnormal uterine bleeding that is refractory to medical management.
Between 400,000 and 500,000 endometrial ablations are done in the United States every year in women who have completed childbearing, and it probably won’t be long before the procedure surpasses hysterectomy in prevalence for the management of abnormal bleeding.
In recent years, the literature has begun to address the incidence of these delayed complications and the requirement for subsequent hysterectomy. A 2007 practice bulletin issued by the American College of Obstetricians and Gynecologists stated that hysterectomy rates within 4 years of endometrial ablation are at least 24% (Obstet Gynecol. 2007 May;109[5]:1233-48). And a study published the following year reported that 26% of 3,681 women undergoing EA at Kaiser Permanente facilities in Northern California required hysterectomy within 8 years (Obstet Gynecol. 2008 Dec;112[6]:1214-20).
It appears that the vast majority of what we now refer to as late-onset EA failures – complications attributable to EA that occur beyond a perioperative period of 1 month – will occur within 5 years. Some EA failures have occurred over 5-10 years, however, and in my practice we have seen late-onset complications occurring 17 or more years after the initial ablation.
In our practice, we are successfully managing delayed complications after GEA using ultrasound-guided reoperative hysteroscopy to fully explore the uterine cavity and excise areas of endometrial growth and other disease. In 2014, we published a retrospective review of 50 women whom we treated for delayed complications after a variety of GEA techniques; almost 90% avoided hysterectomy during a mean follow-up period of 18 months (J Minim Invasive Gynecol. 2014 Mar-Apr;21[2]:238-44).
Our experience since then has included reoperative surgery on more than 115 GEA failures. Additionally, we’ve managed 220 patients who have undergone various hysteroscopic and resectoscopic endometrial ablations, some of which date back to the use of the Nd:YAG laser in the late 1980s.
The fact that late-onset EA failures occur does not mean that hysterectomy should routinely be performed as a first-line treatment for intractable uterine bleeding. Overall, there is much more morbidity associated with hysterectomy than with EA.
What failures do suggest is that there are certain risk factors for late-onset EA complications. Our experience in treating women who have experienced late-onset EA failure has provided us with insight into who may be at greatest risk for late-onset EA failure and how patients can best be selected for the procedure. We’ve also learned more about the diagnosis of delayed complications.
Causes of EA failure
Untreated uterine cornua, and untreated submucous leiomyomas and endometrial polyps, are common causes of EA failure. Among the 50 women included in our retrospective review of ultrasound-guided reoperative hysteroscopy after GEA failure, 44% had intraoperative evidence of untreated cornua and nearly one-fourth had persistent or enlarging submucous leiomyomas.
Contrary to what some believe, most endometrial ablations will not adequately destroy submucous or intramural leiomyomas. Therefore, we recommend that these fibroids be entirely removed immediately before EA.
Moreover, GEA will not always provide adequate thermal destruction to the entire endometrial cavity. The cornua regions are particularly at risk; they are difficult to reach under ideal circumstances, and especially difficult to treat in patients who have a uterine septum or a T-shaped uterus (with the ostia and cornua deeply recessed). We have also seen late-onset EA failures in patients with an extended uterine transverse diameter. The limits of GEA are greatest when a device with a fixed configuration or geometry is used.
A history of abnormal hysteroscopy or other evidence of such anatomic distortions are therefore among the reported risk factors for GEA failure (J Minim Invasive Gynecol. 2015 Mar-Apr;22[3]:323-31). A history of tubal ligation also confers risk; the procedure further increases susceptibility for failure when functioning endometrial tissue remains or regrows at the cornua, because any retrograde menstrual bleeding that occurs will be constrained by the obstructed proximal portion of the fallopian tubes.
Obesity is another risk factor for GEA failure in that the condition increases the risk of endometrial cancer, making the need for reliable biopsies in the case of spotting or other signs or symptoms even more important. On the other hand, obesity may also worsen a patient’s status as a candidate for hysterectomy.
There is much to consider with these patients. For some obese patients, GEA may be less risky than hysterectomy while for others, such as those who also have polycystic ovarian syndrome (in whom the risk for developing endometrial cancer is further increased) the scale may tip in favor of hysterectomy.
Age at the time of the primary GEA may be the single most important risk factor for GEA failure and is an important predictor of success in patient selection. Numerous investigators have shown that women younger than 35 years of age at the time of their EA had a significantly increased risk for hysterectomy, compared with women who were at least 45 years old. The younger the patient, the longer the “bridge” to menopause and the greater the likelihood that bridge will fail.
While age is not necessarily a contraindication, it is worthy of serious consideration. We generally discourage GEA for patients younger than 35. We also advise ensuring that each patient undergoing initial EA is highly self-motivated to have a uterine-sparing procedure; if not, symptoms she may experience later will likely drive her toward hysterectomy anyway.
Additionally, we caution against performing GEA in patients who have chronic pelvic pain; these patients tend to have poorer outcomes with any type of hysteroscopic surgery.
Diagnosing failed EA
Delayed complications manifest in several ways: Renewed and increasing vaginal bleeding after a period of improvement, cyclic pelvic pain (unilateral, bilateral, or suprapubic), or both bleeding and pain. Some women – likely an underreported number of them – present with postmenopausal bleeding and proceed to have unsuccessful attempts at an endometrial biopsy due to EA-associated endometrial scarring.
The cyclic pelvic pain associated with endometrial persistence or regrowth tends to worsen over time and is often described as sharp or laborlike. In our experience, a description of “laborlike” pain and a history of EA is almost fully predictive of a finding of endometrial growth. Often a hematometra can be demonstrated on transvaginal ultrasound, but this isn’t always the case.
Pain typically precedes bleeding in patients who demonstrate both. In such cases, blood from functioning endometrial tissue or other sources becomes blocked from exiting the uterine cavity by EA-induced intrauterine scarring and contracture. Painful uterine contractions then aim to expel the pooled blood. In other cases of pain – mainly those without significant vaginal bleeding – the pain is often attributed to cornual and central hematometra.
For the majority of EA failures, the diagnosis lies in the history and current symptoms. Unfortunately, the traditional methods of assessing the endometrial cavity have little merit for women presenting with delayed-onset EA complications. A sonographically assisted pelvic examination can be useful in evaluating complications, but the interpretation of ultrasounds in women with a prior EA can be challenging and is often beyond the training of most radiologists and gynecologists.
It is not uncommon for images to be incorrectly interpreted in the emergency department or physicians’ offices as “normal” and for such readings to set off a chain of CT scans, MRIs, laparoscopies, ovarian cystectomies, and other procedures that miss the root causes of pain.
Unfortunately, there is little in the literature that describes and defines ultrasound findings after EA. We do know that sonography should be timed with episodes of pain, and that the absence of a demonstrable hematometra does not exclude a diagnosis of EA failure.
Correcting late-onset failures
Our office-based operating room is fitted with side-by-side monitors that enable simultaneous sonographic and hysteroscopic views for correction of GEA failures; the rest of the set-up is similar to that of other operative hysteroscopies. However, we do employ a wide variety of resectoscopes with diameters ranging from 13 to 28 Fr. The smaller-diameter scopes are particularly useful for evaluating postmenopausal bleeding in women with a prior EA.
For those inexperienced with ultrasound-guided surgery, the initial resection is often the most challenging. The initial tissue removal is carried out on the thickest observed uterine wall – usually the posterior or anterior wall – and is done with near complete reliance on the ultrasound image. Hysteroscopic visualization is poor at this time because the outflow ports of the continuous flow resectoscope are obstructed by tissue in the narrow tubular cavity.
We then actually remove the resectoscope and clean the outflow ports of clots and debris that may have accumulated. When the scope is reinserted, there is typically sufficient room in the uterine cavity for continuous flow and excellent hysteroscopic visualization.
The sequence of resection from this point on will vary. If we’ve begun on the anterior wall, we’ll move to the posterior and then the two lateral walls to further restore the cavity. Areas of endometrial regrowth will typically be identified at this point and resected. The dissection then will extend upward, usually to within 10 mm of the fundus in the midline as measured by ultrasound. Reconfiguring the loop electrode to a 135- to 160-degree angle can be helpful in the delicate dissection that is required at the fundus.
Once all areas of endometrium have been identified and excised, we will deeply coagulate exposed myometrium with a ball-end electrode. Rarely, we will reach our maximum allowable fluid absorption limit prior to completing the case, a scenario seen in less than 1% of our patients.
In more than 330 reoperative hysteroscopic procedures, we’ve had only one uterine perforation that occurred when we switched ultrasound machines. Very likely, we were too aggressive in removing tissue at the fundus. The patient required a diagnostic laparoscopy but sustained no visceral injury.
Dr. Wortman is a clinical associate professor of obstetrics and gynecology at the University of Rochester (N.Y.) and the director of the Center for Menstrual Disorders and Reproductive Choice in Rochester. He reported having no relevant financial disclosures.
One in seven women suffer with abnormal uterine bleeding during their reproductive years, according to Fraser et al. (Exp Rev Obstet Gynecol. 2009;4:179-89). Heavy menstrual bleeding (menorrhagia) is the most common pattern. Global endometrial ablation has become a very popular surgical technique for women complaining of menorrhagia, disinterested in either medical management or definitive therapy – hysterectomy – or where medical management has failed. With proper patient selection, endometrial ablation yields an 80%-90% success rate in reducing heavy menstrual flow and is associated with a 90% patient satisfaction rate (Cochrane Database Syst Rev. 2009 Oct 7;[4]:CD001501).
Literature is replete with conditions believed to increase risk of endometrial ablation failure. This list includes untreated uterine cornua, endometrial regrowth, the presence of submucous leiomyomas or polyps, abnormal uterine cavity, enlarged uterine cavity (width and/or length), endometrial ablation in a young patient, parity of five or greater, unsuspected adhesiolysis, postablation tubal sterilization syndrome, history of dysmenorrhea, smoking, obesity, prior cesarean section, previous gynecologic surgery, and procedure length. Interestingly, type of global endometrial ablation procedure or original bleeding pattern does not influence failure rate.
In this edition of the Master Class in Gynecologic Surgery, Dr. Morris Wortman discusses not only the prevention of endometrial ablation failure, but also how to treat the problem via conservative surgical management.
Dr. Wortman is a clinical associate professor of obstetrics and gynecology at the University of Rochester (N.Y.) and is the director at the Center for Menstrual Disorders and Reproductive Choice, also in Rochester. Dr. Wortman has lectured extensively on endometrial ablation and has authored several scientific articles in peer reviewed journals.
Dr. Miller is clinical associate professor at the University of Illinois at Chicago, and past president of the AAGL and the International Society for Gynecologic Endoscopy. He is a reproductive endocrinologist and minimally invasive gynecologic surgeon in private practice in Naperville and Schaumburg, Ill.; director of minimally invasive gynecologic surgery and the director of the AAGL/SRS fellowship in minimally invasive gynecologic surgery at Advocate Lutheran General Hospital, Park Ridge, Ill.; and the medical editor of this column, Master Class. He reported being a subinvestigator on a study sponsored by Channel Medsystems. Email him at [email protected].
Why failures occur and how to correct them
BY MORRIS WORTMAN, MD
Since the introduction almost 20 years ago of devices for nonresectoscopic – or “global” – endometrial ablation, the procedure has been widely adopted as the treatment of choice for abnormal uterine bleeding that is refractory to medical management.
Between 400,000 and 500,000 endometrial ablations are done in the United States every year in women who have completed childbearing, and it probably won’t be long before the procedure surpasses hysterectomy in prevalence for the management of abnormal bleeding.
In recent years, the literature has begun to address the incidence of these delayed complications and the requirement for subsequent hysterectomy. A 2007 practice bulletin issued by the American College of Obstetricians and Gynecologists stated that hysterectomy rates within 4 years of endometrial ablation are at least 24% (Obstet Gynecol. 2007 May;109[5]:1233-48). And a study published the following year reported that 26% of 3,681 women undergoing EA at Kaiser Permanente facilities in Northern California required hysterectomy within 8 years (Obstet Gynecol. 2008 Dec;112[6]:1214-20).
It appears that the vast majority of what we now refer to as late-onset EA failures – complications attributable to EA that occur beyond a perioperative period of 1 month – will occur within 5 years. Some EA failures have occurred over 5-10 years, however, and in my practice we have seen late-onset complications occurring 17 or more years after the initial ablation.
In our practice, we are successfully managing delayed complications after GEA using ultrasound-guided reoperative hysteroscopy to fully explore the uterine cavity and excise areas of endometrial growth and other disease. In 2014, we published a retrospective review of 50 women whom we treated for delayed complications after a variety of GEA techniques; almost 90% avoided hysterectomy during a mean follow-up period of 18 months (J Minim Invasive Gynecol. 2014 Mar-Apr;21[2]:238-44).
Our experience since then has included reoperative surgery on more than 115 GEA failures. Additionally, we’ve managed 220 patients who have undergone various hysteroscopic and resectoscopic endometrial ablations, some of which date back to the use of the Nd:YAG laser in the late 1980s.
The fact that late-onset EA failures occur does not mean that hysterectomy should routinely be performed as a first-line treatment for intractable uterine bleeding. Overall, there is much more morbidity associated with hysterectomy than with EA.
What failures do suggest is that there are certain risk factors for late-onset EA complications. Our experience in treating women who have experienced late-onset EA failure has provided us with insight into who may be at greatest risk for late-onset EA failure and how patients can best be selected for the procedure. We’ve also learned more about the diagnosis of delayed complications.
Causes of EA failure
Untreated uterine cornua, and untreated submucous leiomyomas and endometrial polyps, are common causes of EA failure. Among the 50 women included in our retrospective review of ultrasound-guided reoperative hysteroscopy after GEA failure, 44% had intraoperative evidence of untreated cornua and nearly one-fourth had persistent or enlarging submucous leiomyomas.
Contrary to what some believe, most endometrial ablations will not adequately destroy submucous or intramural leiomyomas. Therefore, we recommend that these fibroids be entirely removed immediately before EA.
Moreover, GEA will not always provide adequate thermal destruction to the entire endometrial cavity. The cornua regions are particularly at risk; they are difficult to reach under ideal circumstances, and especially difficult to treat in patients who have a uterine septum or a T-shaped uterus (with the ostia and cornua deeply recessed). We have also seen late-onset EA failures in patients with an extended uterine transverse diameter. The limits of GEA are greatest when a device with a fixed configuration or geometry is used.
A history of abnormal hysteroscopy or other evidence of such anatomic distortions are therefore among the reported risk factors for GEA failure (J Minim Invasive Gynecol. 2015 Mar-Apr;22[3]:323-31). A history of tubal ligation also confers risk; the procedure further increases susceptibility for failure when functioning endometrial tissue remains or regrows at the cornua, because any retrograde menstrual bleeding that occurs will be constrained by the obstructed proximal portion of the fallopian tubes.
Obesity is another risk factor for GEA failure in that the condition increases the risk of endometrial cancer, making the need for reliable biopsies in the case of spotting or other signs or symptoms even more important. On the other hand, obesity may also worsen a patient’s status as a candidate for hysterectomy.
There is much to consider with these patients. For some obese patients, GEA may be less risky than hysterectomy while for others, such as those who also have polycystic ovarian syndrome (in whom the risk for developing endometrial cancer is further increased) the scale may tip in favor of hysterectomy.
Age at the time of the primary GEA may be the single most important risk factor for GEA failure and is an important predictor of success in patient selection. Numerous investigators have shown that women younger than 35 years of age at the time of their EA had a significantly increased risk for hysterectomy, compared with women who were at least 45 years old. The younger the patient, the longer the “bridge” to menopause and the greater the likelihood that bridge will fail.
While age is not necessarily a contraindication, it is worthy of serious consideration. We generally discourage GEA for patients younger than 35. We also advise ensuring that each patient undergoing initial EA is highly self-motivated to have a uterine-sparing procedure; if not, symptoms she may experience later will likely drive her toward hysterectomy anyway.
Additionally, we caution against performing GEA in patients who have chronic pelvic pain; these patients tend to have poorer outcomes with any type of hysteroscopic surgery.
Diagnosing failed EA
Delayed complications manifest in several ways: Renewed and increasing vaginal bleeding after a period of improvement, cyclic pelvic pain (unilateral, bilateral, or suprapubic), or both bleeding and pain. Some women – likely an underreported number of them – present with postmenopausal bleeding and proceed to have unsuccessful attempts at an endometrial biopsy due to EA-associated endometrial scarring.
The cyclic pelvic pain associated with endometrial persistence or regrowth tends to worsen over time and is often described as sharp or laborlike. In our experience, a description of “laborlike” pain and a history of EA is almost fully predictive of a finding of endometrial growth. Often a hematometra can be demonstrated on transvaginal ultrasound, but this isn’t always the case.
Pain typically precedes bleeding in patients who demonstrate both. In such cases, blood from functioning endometrial tissue or other sources becomes blocked from exiting the uterine cavity by EA-induced intrauterine scarring and contracture. Painful uterine contractions then aim to expel the pooled blood. In other cases of pain – mainly those without significant vaginal bleeding – the pain is often attributed to cornual and central hematometra.
For the majority of EA failures, the diagnosis lies in the history and current symptoms. Unfortunately, the traditional methods of assessing the endometrial cavity have little merit for women presenting with delayed-onset EA complications. A sonographically assisted pelvic examination can be useful in evaluating complications, but the interpretation of ultrasounds in women with a prior EA can be challenging and is often beyond the training of most radiologists and gynecologists.
It is not uncommon for images to be incorrectly interpreted in the emergency department or physicians’ offices as “normal” and for such readings to set off a chain of CT scans, MRIs, laparoscopies, ovarian cystectomies, and other procedures that miss the root causes of pain.
Unfortunately, there is little in the literature that describes and defines ultrasound findings after EA. We do know that sonography should be timed with episodes of pain, and that the absence of a demonstrable hematometra does not exclude a diagnosis of EA failure.
Correcting late-onset failures
Our office-based operating room is fitted with side-by-side monitors that enable simultaneous sonographic and hysteroscopic views for correction of GEA failures; the rest of the set-up is similar to that of other operative hysteroscopies. However, we do employ a wide variety of resectoscopes with diameters ranging from 13 to 28 Fr. The smaller-diameter scopes are particularly useful for evaluating postmenopausal bleeding in women with a prior EA.
For those inexperienced with ultrasound-guided surgery, the initial resection is often the most challenging. The initial tissue removal is carried out on the thickest observed uterine wall – usually the posterior or anterior wall – and is done with near complete reliance on the ultrasound image. Hysteroscopic visualization is poor at this time because the outflow ports of the continuous flow resectoscope are obstructed by tissue in the narrow tubular cavity.
We then actually remove the resectoscope and clean the outflow ports of clots and debris that may have accumulated. When the scope is reinserted, there is typically sufficient room in the uterine cavity for continuous flow and excellent hysteroscopic visualization.
The sequence of resection from this point on will vary. If we’ve begun on the anterior wall, we’ll move to the posterior and then the two lateral walls to further restore the cavity. Areas of endometrial regrowth will typically be identified at this point and resected. The dissection then will extend upward, usually to within 10 mm of the fundus in the midline as measured by ultrasound. Reconfiguring the loop electrode to a 135- to 160-degree angle can be helpful in the delicate dissection that is required at the fundus.
Once all areas of endometrium have been identified and excised, we will deeply coagulate exposed myometrium with a ball-end electrode. Rarely, we will reach our maximum allowable fluid absorption limit prior to completing the case, a scenario seen in less than 1% of our patients.
In more than 330 reoperative hysteroscopic procedures, we’ve had only one uterine perforation that occurred when we switched ultrasound machines. Very likely, we were too aggressive in removing tissue at the fundus. The patient required a diagnostic laparoscopy but sustained no visceral injury.
Dr. Wortman is a clinical associate professor of obstetrics and gynecology at the University of Rochester (N.Y.) and the director of the Center for Menstrual Disorders and Reproductive Choice in Rochester. He reported having no relevant financial disclosures.
VIDEO: The surgical treatment of pelvic congestion
BY CHARLES E. MILLER, MD
Chronic pelvic pain is described as the presence of lower abdominal or pelvic pain for longer than 6 months. It is believed to affect approximately one in six women and 12%-15% of women of reproductive age. The diagnosis and treatment of chronic pelvic pain adds as much as a $2 billion burden to our health system annually.
It was first described clinically in the literature in 1857, while the existence of pelvic varicosities wasn’t documented for nearly another 100 years. Pelvic congestion syndrome (PCS) accounts for 30%-70% of cases presenting with chronic pelvic pain. PCS can be due to pelvic venous insufficiency, characterized by reflux into pelvic veins leading to pelvic varicosities or alternative venous pathways secondary to varicose veins of the leg.
Other etiologies of PCS include nutcracker syndrome (left renal vein compressed between the aorta and the superior mesenteric artery), May-Thurner syndrome (compression of the left common iliac vein by the right common iliac artery) or, less likely, tumor thrombosis of the inferior vena cava, portal vein thrombosis, renal cell carcinoma, left renal thrombosis, or left kidney arterial-venous fistula.
While there appears to be significant literature indicating a long-term success rate of greater than 80% in patients treated by percutaneous endovascular procedures (embolization, stenting), there is far less information on the postsurgical success of blocking the varicose gonadal vein. Nevertheless, our long-term results with gonadal vein clipping is virtually the same as that of our radiological colleagues.
It is a pleasure to welcome Courtney Steller, DO, to this edition of the Master Class in Gynecologic Surgery to discuss the diagnosis and treatment of PCS, with an emphasis on surgical correction.
Dr. Steller is a recent graduate of the AAGL/SRS Fellowship in Minimally Invasive Gynecologic Surgery at Advocate Lutheran General Hospital, Park Ridge, Ill. She is currently in private practice and is an associate at the Family Health Centers of San Diego, Calif.
Dr. Miller is clinical associate professor at the University of Illinois at Chicago, and past president of the AAGL and the International Society for Gynecologic Endoscopy. He is a reproductive endocrinologist and minimally invasive gynecologic surgeon in private practice in Naperville and Schaumburg, Ill.; director of minimally invasive gynecologic surgery and the director of the AAGL/SRS Fellowship in Minimally Invasive Gynecologic Surgery at Advocate Lutheran General Hospital, Park Ridge, Ill.; and the medical editor of this column, Master Class. He reported having no financial disclosures relevant to this column. Email him at [email protected].
Pelvic congestion syndrome: A treatable cause of pain
BY COURTNEY STELLER, DO
Pelvic congestion syndrome is a poorly understood and underdiagnosed disease. Yet, over the last decade, the syndrome has become less controversial as the etiology has become better understood and as the diagnostic approach has become more specific. Through these advances, treatments have also become increasingly more successful.
This is an important shift, because the chronic pelvic pain experienced by patients with pelvic congestion significantly impacts their quality of life and well-being. As the pain persists, it can become exceedingly difficult to manage. Many patients we have ultimately treated for pelvic congestion syndrome have had years of various work-ups, significant diagnostic investigations, and trials of different treatments without having any cause of their pain identified or achieving any lasting symptom relief.
The pelvic pain in patients with pelvic congestion syndrome (PCS) can be noncyclical or cyclical. It is present most of the time but tends to get worse at the end of the day and after long periods of standing and/or sitting. The pain also may worsen with intercourse, largely afterward. The syndrome tends to occur in premenopausal and multiparous women, but it’s important to appreciate that this is not always the case; we have diagnosed and treated PCS in several young, nulliparous patients as well.
Features and diagnosis
PCS is a disorder of pelvic venous circulation that predominantly affects the ovarian veins. It is sometimes referred to as pelvic vein incompetence or pelvic vascular dysfunction. Just as veins in the legs can enlarge and become varicose, the ovarian veins – and sometimes the internal iliac veins – can become incompetent and unable to effectively return blood back to the heart.
Pregnancy may predispose patients to developing the abnormally dilated and refluxing veins that characterize PCS, as the increase in pelvic vein capacity and uterine compression can lead to significant stasis of blood in the pelvis and subsequent damage to the veins and the venous valves. There also is believed to be an estrogen component to the development of PCS, because estrogen is known to act as a vasodilator. Moreover, a congenital absence and incompetence of venous valves in some cases has been reported.
In a recent study looking at pelvic vein incompetence and symptoms of chronic pelvic pain, these women were reported to have a distinctive symptom profile, with the “most notable” features being the presence of dull pelvic pain that radiates to the upper thighs and is aggravated by prolonged standing and walking – symptoms that are similar to the leg symptoms experienced by patients with severe varicose veins (Eur J Obstet Gynecol Reprod Biol. 2016 Jan;196:21-5).
Other investigators have similarly described the pelvic pain related to PCS as a dull ache or heaviness sensation that is most severe at the end of the day and that is lessened with supine positioning (though not necessarily immediately) and often exacerbated with sexual intercourse, especially post coitus. These descriptions are in line with my experience with PCS. There is usually exquisite tenderness on pelvic exam, especially localized to the adnexa. Patients will often have varicose veins on their upper legs or labia.
Interestingly, it has been repeatedly shown that many women have dilated and incompetent pelvic veins without also having such pathognomonic pain. We therefore cannot treat women based solely on the finding of abnormal veins.
On the other hand we must determine which patients with chronic pelvic pain have PCS. The differential diagnosis for PCS includes endometriosis, adenomyosis chronic pelvic inflammatory disease, adhesive disease, adnexal masses, adnexal torsion, and several nongynecologic diseases including interstitial cystitis and irritable bowel syndrome.
Venography has become the gold standard for diagnosing pelvic congestion. The procedure involves catheterization of the ovarian veins through a femoral or jugular approach. In our experience, the common femoral vein is the more frequently used access point. Using a contrast injection, the interventional radiologist can assess the degree of venous dilation and reflux in the pelvis.

There currently is no consensus on a cutoff for vein diameter or on any validated measures for congestion. According to one report on PCS authored by interventional radiologists, the diagnosis of PCS is confirmed with the venographic findings of ovarian vein diameter greater than 6 mm, retrograde ovarian or pelvic venous flow, presence of several tortuous collateral pelvic venous pathways, and delayed or stagnant clearance on contrast (Semin Intervent Radiol. 2008 Dec;25[4]:361-8).
The criteria vary, however. A recent literature review on pelvic congestion syndrome by Chiara Borghi, MD, and Lucio Dell’Atti, MD, states that incompetent pelvic veins are defined as more than 5-10 mm in diameter (Arch Gynecol Obstet. 2016 Feb;293[2]:291-301).
To more accurately diagnose PCS, our patients undergo tilt-table venography. The patient is placed into a reverse-Trendelenburg upright or semi-upright position to potentially exacerbate any venous reflux or dilation.
Other methods of identifying and diagnosing pelvic congestion have included transabdominal and transvaginal ultrasound, CT, and MRI. While CT and MRI both offer an overview of the pelvic vasculature and are helpful for ruling out other causes of chronic pelvic pain, they have low specificity for pelvic varices, according to the Italian review.
Sonography performed in the supine position, on the other hand, appears to be increasingly viewed as an acceptable screening tool for determining which patients may ultimately benefit from venography. It is also important in evaluation to rule out other pathologies not yet excluded. However, it should not be used for diagnosis of PCS.
Treating PCS
There are two main approaches to treating PCS: venous ligation (a gynecologic surgical approach) and percutaneous transcatheter embolization (performed by interventional radiologists).
The literature and evidence base is still in its infancy, but is growing. In our experience, both approaches lead to good resolution of symptoms over time in the majority of patients, and appear superior to the medical therapies that have been proposed for treating PCS, such as progestins and gonadotropin-releasing hormone agonists. Success rates with medical therapy are more variable and appear to be more short lived.
A review published this year on the effectiveness of embolization of pelvic veins for reducing chronic pelvic pain showed that 75% of women undergoing embolization had symptomatic relief that generally increased over time and was sustained. The authors concluded that embolization appears to be effective for the majority of women, and is safe, although they also noted that the quality of the evidence is low (J Vasc Interv Radiol. 2016 Oct;27[10]:1478-86.e8). Their review was based almost entirely on prospective case series.
Dr. Borghi and Dr. Dell’Atti offered a similar assessment of embolization for PCS, stating in their review article that clinical success has been reported in 70%-85% of patients. They also report nearly equivalent success rates of up to 75% with treatment via surgical ligation of ovarian and/or pelvic vasculature. These findings are from mostly observational data and case series.
Decisions about which approach to take should be individualized. If there are no differences with respect to insurance coverage for the patient, then embolization may be the preferred approach because it is the most minimally invasive technique and can potentially be performed at the time of diagnostic venography, negating the need for a second procedure. A skilled interventional radiologist familiar with the disease and the treatment is necessary. Various embolic agents are utilized, including coils, glues, foams, and other agents that cause sclerosis of the abnormal veins.
In other cases, venous ligation is preferred, especially when an additional gynecologic surgery, such as a cystectomy or myomectomy, is required.
Surgical ligation of ovarian veins was initially performed via laparotomy using a traditional retroperitoneal approach. The surgical goal is to isolate the ovarian vein significantly above the pelvic brim and before the vein becomes substantially dilated. Laparotomy therefore requires a vertical mid-line incision to provide adequate access to the appropriate portion of the ovarian vessels, leading to potentially high morbidity and poor cosmesis.
More recently, gynecologic surgeons skilled in laparoscopy have successfully managed PCS transperitoneally. A few small series of bilateral laparoscopic transperitoneal ligation of ovarian veins have been reported, including one by Tigellio Gargiulo, MD, who clipped both veins in their upper third, near their distal ends at the inferior vena cava (right) and the renal vein (left) (J Am Assoc Gynecol Laparosc. 2003 Nov;10[4]:501-4).
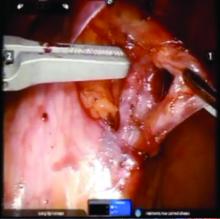
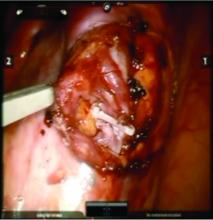
We prefer a robot-assisted laparoscopic approach for most of our patients. Not only does the improved dexterity help while working with sensitive vasculature, but more importantly we are able to use Firefly fluorescence.
The procedure generally is as follows. The uterine adnexa on the affected side is grasped and placed on tension so that the infundibulopelvic (IP) ligament can be visualized as it courses up and above the pelvic brim. The peritoneum immediately over the IP ligament is gently grasped and tented upward, and a small incision is made into the peritoneum, providing access into the retroperitoneum. The ureter should be visualized medial to this dissection.
The peritoneal tissue is then gently dissected off the ovarian vessels. Once the vessels are freed from the peritoneal tissue, the dilated ovarian vein is often clearly visualized. It is important to note that if no venous dilation is seen during laparoscopy, the procedure should not be aborted. Due to the Trendelenburg position that is utilized in gynecologic – and especially laparoscopic – surgery, the venous system sometimes appears falsely “normal” at this time.
Once the ovarian vessels have been isolated, the arteries must be separated from the veins. The adventitial tissue is dissected until the vessels are separated. Great care should be taken to ensure that all movements run parallel to the vessels and not perpendicular, therefore decreasing the risk of bleeding.
This process can be challenging. The surgeon is working with delicate vasculature. Often there are several branches from the vein that have formed due to the abnormal venous system. The best way to approach it is to identify planes and separate those planes in order to isolate individual vessels. If difficulties are still encountered, the surgeon should restart the dissection higher.
Once the dilated ovarian vein is isolated, one to two clips are placed.
Usually the artery is clearly distinct from the vein as it is smaller, more elastic, and can be seen pulsing. However, occasionally it is difficult to distinguish. In these cases, assistance with the da Vinci surgical system is useful: Indocyanine green (ICG) dye can be injected intravenously and visualized with a near-infrared light on the da Vinci platform. The dye is then seen glowing green as it first courses through the artery and then the vein.
For patients who have been found on venography to have bilateral disease, we perform the ligation procedure bilaterally. Once ligation is complete, the more competent collateral veins in the pelvis will assume more of the venous circulation.
In our experience, patients have ultimately noted substantial pain relief after these procedures, both with the endoscopic embolization and the surgical ligation. Patients are counseled that it can take several months to notice a relief in the pain.
In rare cases, pelvic congestion is related to extrinsic compression. For instance, the left renal vein can become compressed between the aorta and the superior mesenteric artery (the nutcracker syndrome), or the left common iliac vein can be compressed between the overlying right internal iliac artery and the underlying vertebral body (May-Thurner syndrome). Both of these conditions can lead to secondary PCS.
Such complex conditions are usually treated by vascular surgeons. May-Thurner syndrome is treated via stenting, while nutcracker syndrome can be treated with stenting or transposition of the renal vein to the distal vena cava.
Dr. Steller is an associate at the Family Health Centers of San Diego. She reported having no relevant financial disclosures.
BY CHARLES E. MILLER, MD
Chronic pelvic pain is described as the presence of lower abdominal or pelvic pain for longer than 6 months. It is believed to affect approximately one in six women and 12%-15% of women of reproductive age. The diagnosis and treatment of chronic pelvic pain adds as much as a $2 billion burden to our health system annually.
It was first described clinically in the literature in 1857, while the existence of pelvic varicosities wasn’t documented for nearly another 100 years. Pelvic congestion syndrome (PCS) accounts for 30%-70% of cases presenting with chronic pelvic pain. PCS can be due to pelvic venous insufficiency, characterized by reflux into pelvic veins leading to pelvic varicosities or alternative venous pathways secondary to varicose veins of the leg.
Other etiologies of PCS include nutcracker syndrome (left renal vein compressed between the aorta and the superior mesenteric artery), May-Thurner syndrome (compression of the left common iliac vein by the right common iliac artery) or, less likely, tumor thrombosis of the inferior vena cava, portal vein thrombosis, renal cell carcinoma, left renal thrombosis, or left kidney arterial-venous fistula.
While there appears to be significant literature indicating a long-term success rate of greater than 80% in patients treated by percutaneous endovascular procedures (embolization, stenting), there is far less information on the postsurgical success of blocking the varicose gonadal vein. Nevertheless, our long-term results with gonadal vein clipping is virtually the same as that of our radiological colleagues.
It is a pleasure to welcome Courtney Steller, DO, to this edition of the Master Class in Gynecologic Surgery to discuss the diagnosis and treatment of PCS, with an emphasis on surgical correction.
Dr. Steller is a recent graduate of the AAGL/SRS Fellowship in Minimally Invasive Gynecologic Surgery at Advocate Lutheran General Hospital, Park Ridge, Ill. She is currently in private practice and is an associate at the Family Health Centers of San Diego, Calif.
Dr. Miller is clinical associate professor at the University of Illinois at Chicago, and past president of the AAGL and the International Society for Gynecologic Endoscopy. He is a reproductive endocrinologist and minimally invasive gynecologic surgeon in private practice in Naperville and Schaumburg, Ill.; director of minimally invasive gynecologic surgery and the director of the AAGL/SRS Fellowship in Minimally Invasive Gynecologic Surgery at Advocate Lutheran General Hospital, Park Ridge, Ill.; and the medical editor of this column, Master Class. He reported having no financial disclosures relevant to this column. Email him at [email protected].
Pelvic congestion syndrome: A treatable cause of pain
BY COURTNEY STELLER, DO
Pelvic congestion syndrome is a poorly understood and underdiagnosed disease. Yet, over the last decade, the syndrome has become less controversial as the etiology has become better understood and as the diagnostic approach has become more specific. Through these advances, treatments have also become increasingly more successful.
This is an important shift, because the chronic pelvic pain experienced by patients with pelvic congestion significantly impacts their quality of life and well-being. As the pain persists, it can become exceedingly difficult to manage. Many patients we have ultimately treated for pelvic congestion syndrome have had years of various work-ups, significant diagnostic investigations, and trials of different treatments without having any cause of their pain identified or achieving any lasting symptom relief.
The pelvic pain in patients with pelvic congestion syndrome (PCS) can be noncyclical or cyclical. It is present most of the time but tends to get worse at the end of the day and after long periods of standing and/or sitting. The pain also may worsen with intercourse, largely afterward. The syndrome tends to occur in premenopausal and multiparous women, but it’s important to appreciate that this is not always the case; we have diagnosed and treated PCS in several young, nulliparous patients as well.
Features and diagnosis
PCS is a disorder of pelvic venous circulation that predominantly affects the ovarian veins. It is sometimes referred to as pelvic vein incompetence or pelvic vascular dysfunction. Just as veins in the legs can enlarge and become varicose, the ovarian veins – and sometimes the internal iliac veins – can become incompetent and unable to effectively return blood back to the heart.
Pregnancy may predispose patients to developing the abnormally dilated and refluxing veins that characterize PCS, as the increase in pelvic vein capacity and uterine compression can lead to significant stasis of blood in the pelvis and subsequent damage to the veins and the venous valves. There also is believed to be an estrogen component to the development of PCS, because estrogen is known to act as a vasodilator. Moreover, a congenital absence and incompetence of venous valves in some cases has been reported.
In a recent study looking at pelvic vein incompetence and symptoms of chronic pelvic pain, these women were reported to have a distinctive symptom profile, with the “most notable” features being the presence of dull pelvic pain that radiates to the upper thighs and is aggravated by prolonged standing and walking – symptoms that are similar to the leg symptoms experienced by patients with severe varicose veins (Eur J Obstet Gynecol Reprod Biol. 2016 Jan;196:21-5).
Other investigators have similarly described the pelvic pain related to PCS as a dull ache or heaviness sensation that is most severe at the end of the day and that is lessened with supine positioning (though not necessarily immediately) and often exacerbated with sexual intercourse, especially post coitus. These descriptions are in line with my experience with PCS. There is usually exquisite tenderness on pelvic exam, especially localized to the adnexa. Patients will often have varicose veins on their upper legs or labia.
Interestingly, it has been repeatedly shown that many women have dilated and incompetent pelvic veins without also having such pathognomonic pain. We therefore cannot treat women based solely on the finding of abnormal veins.
On the other hand we must determine which patients with chronic pelvic pain have PCS. The differential diagnosis for PCS includes endometriosis, adenomyosis chronic pelvic inflammatory disease, adhesive disease, adnexal masses, adnexal torsion, and several nongynecologic diseases including interstitial cystitis and irritable bowel syndrome.
Venography has become the gold standard for diagnosing pelvic congestion. The procedure involves catheterization of the ovarian veins through a femoral or jugular approach. In our experience, the common femoral vein is the more frequently used access point. Using a contrast injection, the interventional radiologist can assess the degree of venous dilation and reflux in the pelvis.

There currently is no consensus on a cutoff for vein diameter or on any validated measures for congestion. According to one report on PCS authored by interventional radiologists, the diagnosis of PCS is confirmed with the venographic findings of ovarian vein diameter greater than 6 mm, retrograde ovarian or pelvic venous flow, presence of several tortuous collateral pelvic venous pathways, and delayed or stagnant clearance on contrast (Semin Intervent Radiol. 2008 Dec;25[4]:361-8).
The criteria vary, however. A recent literature review on pelvic congestion syndrome by Chiara Borghi, MD, and Lucio Dell’Atti, MD, states that incompetent pelvic veins are defined as more than 5-10 mm in diameter (Arch Gynecol Obstet. 2016 Feb;293[2]:291-301).
To more accurately diagnose PCS, our patients undergo tilt-table venography. The patient is placed into a reverse-Trendelenburg upright or semi-upright position to potentially exacerbate any venous reflux or dilation.
Other methods of identifying and diagnosing pelvic congestion have included transabdominal and transvaginal ultrasound, CT, and MRI. While CT and MRI both offer an overview of the pelvic vasculature and are helpful for ruling out other causes of chronic pelvic pain, they have low specificity for pelvic varices, according to the Italian review.
Sonography performed in the supine position, on the other hand, appears to be increasingly viewed as an acceptable screening tool for determining which patients may ultimately benefit from venography. It is also important in evaluation to rule out other pathologies not yet excluded. However, it should not be used for diagnosis of PCS.
Treating PCS
There are two main approaches to treating PCS: venous ligation (a gynecologic surgical approach) and percutaneous transcatheter embolization (performed by interventional radiologists).
The literature and evidence base is still in its infancy, but is growing. In our experience, both approaches lead to good resolution of symptoms over time in the majority of patients, and appear superior to the medical therapies that have been proposed for treating PCS, such as progestins and gonadotropin-releasing hormone agonists. Success rates with medical therapy are more variable and appear to be more short lived.
A review published this year on the effectiveness of embolization of pelvic veins for reducing chronic pelvic pain showed that 75% of women undergoing embolization had symptomatic relief that generally increased over time and was sustained. The authors concluded that embolization appears to be effective for the majority of women, and is safe, although they also noted that the quality of the evidence is low (J Vasc Interv Radiol. 2016 Oct;27[10]:1478-86.e8). Their review was based almost entirely on prospective case series.
Dr. Borghi and Dr. Dell’Atti offered a similar assessment of embolization for PCS, stating in their review article that clinical success has been reported in 70%-85% of patients. They also report nearly equivalent success rates of up to 75% with treatment via surgical ligation of ovarian and/or pelvic vasculature. These findings are from mostly observational data and case series.
Decisions about which approach to take should be individualized. If there are no differences with respect to insurance coverage for the patient, then embolization may be the preferred approach because it is the most minimally invasive technique and can potentially be performed at the time of diagnostic venography, negating the need for a second procedure. A skilled interventional radiologist familiar with the disease and the treatment is necessary. Various embolic agents are utilized, including coils, glues, foams, and other agents that cause sclerosis of the abnormal veins.
In other cases, venous ligation is preferred, especially when an additional gynecologic surgery, such as a cystectomy or myomectomy, is required.
Surgical ligation of ovarian veins was initially performed via laparotomy using a traditional retroperitoneal approach. The surgical goal is to isolate the ovarian vein significantly above the pelvic brim and before the vein becomes substantially dilated. Laparotomy therefore requires a vertical mid-line incision to provide adequate access to the appropriate portion of the ovarian vessels, leading to potentially high morbidity and poor cosmesis.
More recently, gynecologic surgeons skilled in laparoscopy have successfully managed PCS transperitoneally. A few small series of bilateral laparoscopic transperitoneal ligation of ovarian veins have been reported, including one by Tigellio Gargiulo, MD, who clipped both veins in their upper third, near their distal ends at the inferior vena cava (right) and the renal vein (left) (J Am Assoc Gynecol Laparosc. 2003 Nov;10[4]:501-4).
We prefer a robot-assisted laparoscopic approach for most of our patients. Not only does the improved dexterity help while working with sensitive vasculature, but more importantly we are able to use Firefly fluorescence.
The procedure generally is as follows. The uterine adnexa on the affected side is grasped and placed on tension so that the infundibulopelvic (IP) ligament can be visualized as it courses up and above the pelvic brim. The peritoneum immediately over the IP ligament is gently grasped and tented upward, and a small incision is made into the peritoneum, providing access into the retroperitoneum. The ureter should be visualized medial to this dissection.
The peritoneal tissue is then gently dissected off the ovarian vessels. Once the vessels are freed from the peritoneal tissue, the dilated ovarian vein is often clearly visualized. It is important to note that if no venous dilation is seen during laparoscopy, the procedure should not be aborted. Due to the Trendelenburg position that is utilized in gynecologic – and especially laparoscopic – surgery, the venous system sometimes appears falsely “normal” at this time.
Once the ovarian vessels have been isolated, the arteries must be separated from the veins. The adventitial tissue is dissected until the vessels are separated. Great care should be taken to ensure that all movements run parallel to the vessels and not perpendicular, therefore decreasing the risk of bleeding.
This process can be challenging. The surgeon is working with delicate vasculature. Often there are several branches from the vein that have formed due to the abnormal venous system. The best way to approach it is to identify planes and separate those planes in order to isolate individual vessels. If difficulties are still encountered, the surgeon should restart the dissection higher.
Once the dilated ovarian vein is isolated, one to two clips are placed.
Usually the artery is clearly distinct from the vein as it is smaller, more elastic, and can be seen pulsing. However, occasionally it is difficult to distinguish. In these cases, assistance with the da Vinci surgical system is useful: Indocyanine green (ICG) dye can be injected intravenously and visualized with a near-infrared light on the da Vinci platform. The dye is then seen glowing green as it first courses through the artery and then the vein.
For patients who have been found on venography to have bilateral disease, we perform the ligation procedure bilaterally. Once ligation is complete, the more competent collateral veins in the pelvis will assume more of the venous circulation.
In our experience, patients have ultimately noted substantial pain relief after these procedures, both with the endoscopic embolization and the surgical ligation. Patients are counseled that it can take several months to notice a relief in the pain.
In rare cases, pelvic congestion is related to extrinsic compression. For instance, the left renal vein can become compressed between the aorta and the superior mesenteric artery (the nutcracker syndrome), or the left common iliac vein can be compressed between the overlying right internal iliac artery and the underlying vertebral body (May-Thurner syndrome). Both of these conditions can lead to secondary PCS.
Such complex conditions are usually treated by vascular surgeons. May-Thurner syndrome is treated via stenting, while nutcracker syndrome can be treated with stenting or transposition of the renal vein to the distal vena cava.
Dr. Steller is an associate at the Family Health Centers of San Diego. She reported having no relevant financial disclosures.
BY CHARLES E. MILLER, MD
Chronic pelvic pain is described as the presence of lower abdominal or pelvic pain for longer than 6 months. It is believed to affect approximately one in six women and 12%-15% of women of reproductive age. The diagnosis and treatment of chronic pelvic pain adds as much as a $2 billion burden to our health system annually.
It was first described clinically in the literature in 1857, while the existence of pelvic varicosities wasn’t documented for nearly another 100 years. Pelvic congestion syndrome (PCS) accounts for 30%-70% of cases presenting with chronic pelvic pain. PCS can be due to pelvic venous insufficiency, characterized by reflux into pelvic veins leading to pelvic varicosities or alternative venous pathways secondary to varicose veins of the leg.
Other etiologies of PCS include nutcracker syndrome (left renal vein compressed between the aorta and the superior mesenteric artery), May-Thurner syndrome (compression of the left common iliac vein by the right common iliac artery) or, less likely, tumor thrombosis of the inferior vena cava, portal vein thrombosis, renal cell carcinoma, left renal thrombosis, or left kidney arterial-venous fistula.
While there appears to be significant literature indicating a long-term success rate of greater than 80% in patients treated by percutaneous endovascular procedures (embolization, stenting), there is far less information on the postsurgical success of blocking the varicose gonadal vein. Nevertheless, our long-term results with gonadal vein clipping is virtually the same as that of our radiological colleagues.
It is a pleasure to welcome Courtney Steller, DO, to this edition of the Master Class in Gynecologic Surgery to discuss the diagnosis and treatment of PCS, with an emphasis on surgical correction.
Dr. Steller is a recent graduate of the AAGL/SRS Fellowship in Minimally Invasive Gynecologic Surgery at Advocate Lutheran General Hospital, Park Ridge, Ill. She is currently in private practice and is an associate at the Family Health Centers of San Diego, Calif.
Dr. Miller is clinical associate professor at the University of Illinois at Chicago, and past president of the AAGL and the International Society for Gynecologic Endoscopy. He is a reproductive endocrinologist and minimally invasive gynecologic surgeon in private practice in Naperville and Schaumburg, Ill.; director of minimally invasive gynecologic surgery and the director of the AAGL/SRS Fellowship in Minimally Invasive Gynecologic Surgery at Advocate Lutheran General Hospital, Park Ridge, Ill.; and the medical editor of this column, Master Class. He reported having no financial disclosures relevant to this column. Email him at [email protected].
Pelvic congestion syndrome: A treatable cause of pain
BY COURTNEY STELLER, DO
Pelvic congestion syndrome is a poorly understood and underdiagnosed disease. Yet, over the last decade, the syndrome has become less controversial as the etiology has become better understood and as the diagnostic approach has become more specific. Through these advances, treatments have also become increasingly more successful.
This is an important shift, because the chronic pelvic pain experienced by patients with pelvic congestion significantly impacts their quality of life and well-being. As the pain persists, it can become exceedingly difficult to manage. Many patients we have ultimately treated for pelvic congestion syndrome have had years of various work-ups, significant diagnostic investigations, and trials of different treatments without having any cause of their pain identified or achieving any lasting symptom relief.
The pelvic pain in patients with pelvic congestion syndrome (PCS) can be noncyclical or cyclical. It is present most of the time but tends to get worse at the end of the day and after long periods of standing and/or sitting. The pain also may worsen with intercourse, largely afterward. The syndrome tends to occur in premenopausal and multiparous women, but it’s important to appreciate that this is not always the case; we have diagnosed and treated PCS in several young, nulliparous patients as well.
Features and diagnosis
PCS is a disorder of pelvic venous circulation that predominantly affects the ovarian veins. It is sometimes referred to as pelvic vein incompetence or pelvic vascular dysfunction. Just as veins in the legs can enlarge and become varicose, the ovarian veins – and sometimes the internal iliac veins – can become incompetent and unable to effectively return blood back to the heart.
Pregnancy may predispose patients to developing the abnormally dilated and refluxing veins that characterize PCS, as the increase in pelvic vein capacity and uterine compression can lead to significant stasis of blood in the pelvis and subsequent damage to the veins and the venous valves. There also is believed to be an estrogen component to the development of PCS, because estrogen is known to act as a vasodilator. Moreover, a congenital absence and incompetence of venous valves in some cases has been reported.
In a recent study looking at pelvic vein incompetence and symptoms of chronic pelvic pain, these women were reported to have a distinctive symptom profile, with the “most notable” features being the presence of dull pelvic pain that radiates to the upper thighs and is aggravated by prolonged standing and walking – symptoms that are similar to the leg symptoms experienced by patients with severe varicose veins (Eur J Obstet Gynecol Reprod Biol. 2016 Jan;196:21-5).
Other investigators have similarly described the pelvic pain related to PCS as a dull ache or heaviness sensation that is most severe at the end of the day and that is lessened with supine positioning (though not necessarily immediately) and often exacerbated with sexual intercourse, especially post coitus. These descriptions are in line with my experience with PCS. There is usually exquisite tenderness on pelvic exam, especially localized to the adnexa. Patients will often have varicose veins on their upper legs or labia.
Interestingly, it has been repeatedly shown that many women have dilated and incompetent pelvic veins without also having such pathognomonic pain. We therefore cannot treat women based solely on the finding of abnormal veins.
On the other hand we must determine which patients with chronic pelvic pain have PCS. The differential diagnosis for PCS includes endometriosis, adenomyosis chronic pelvic inflammatory disease, adhesive disease, adnexal masses, adnexal torsion, and several nongynecologic diseases including interstitial cystitis and irritable bowel syndrome.
Venography has become the gold standard for diagnosing pelvic congestion. The procedure involves catheterization of the ovarian veins through a femoral or jugular approach. In our experience, the common femoral vein is the more frequently used access point. Using a contrast injection, the interventional radiologist can assess the degree of venous dilation and reflux in the pelvis.

There currently is no consensus on a cutoff for vein diameter or on any validated measures for congestion. According to one report on PCS authored by interventional radiologists, the diagnosis of PCS is confirmed with the venographic findings of ovarian vein diameter greater than 6 mm, retrograde ovarian or pelvic venous flow, presence of several tortuous collateral pelvic venous pathways, and delayed or stagnant clearance on contrast (Semin Intervent Radiol. 2008 Dec;25[4]:361-8).
The criteria vary, however. A recent literature review on pelvic congestion syndrome by Chiara Borghi, MD, and Lucio Dell’Atti, MD, states that incompetent pelvic veins are defined as more than 5-10 mm in diameter (Arch Gynecol Obstet. 2016 Feb;293[2]:291-301).
To more accurately diagnose PCS, our patients undergo tilt-table venography. The patient is placed into a reverse-Trendelenburg upright or semi-upright position to potentially exacerbate any venous reflux or dilation.
Other methods of identifying and diagnosing pelvic congestion have included transabdominal and transvaginal ultrasound, CT, and MRI. While CT and MRI both offer an overview of the pelvic vasculature and are helpful for ruling out other causes of chronic pelvic pain, they have low specificity for pelvic varices, according to the Italian review.
Sonography performed in the supine position, on the other hand, appears to be increasingly viewed as an acceptable screening tool for determining which patients may ultimately benefit from venography. It is also important in evaluation to rule out other pathologies not yet excluded. However, it should not be used for diagnosis of PCS.
Treating PCS
There are two main approaches to treating PCS: venous ligation (a gynecologic surgical approach) and percutaneous transcatheter embolization (performed by interventional radiologists).
The literature and evidence base is still in its infancy, but is growing. In our experience, both approaches lead to good resolution of symptoms over time in the majority of patients, and appear superior to the medical therapies that have been proposed for treating PCS, such as progestins and gonadotropin-releasing hormone agonists. Success rates with medical therapy are more variable and appear to be more short lived.
A review published this year on the effectiveness of embolization of pelvic veins for reducing chronic pelvic pain showed that 75% of women undergoing embolization had symptomatic relief that generally increased over time and was sustained. The authors concluded that embolization appears to be effective for the majority of women, and is safe, although they also noted that the quality of the evidence is low (J Vasc Interv Radiol. 2016 Oct;27[10]:1478-86.e8). Their review was based almost entirely on prospective case series.
Dr. Borghi and Dr. Dell’Atti offered a similar assessment of embolization for PCS, stating in their review article that clinical success has been reported in 70%-85% of patients. They also report nearly equivalent success rates of up to 75% with treatment via surgical ligation of ovarian and/or pelvic vasculature. These findings are from mostly observational data and case series.
Decisions about which approach to take should be individualized. If there are no differences with respect to insurance coverage for the patient, then embolization may be the preferred approach because it is the most minimally invasive technique and can potentially be performed at the time of diagnostic venography, negating the need for a second procedure. A skilled interventional radiologist familiar with the disease and the treatment is necessary. Various embolic agents are utilized, including coils, glues, foams, and other agents that cause sclerosis of the abnormal veins.
In other cases, venous ligation is preferred, especially when an additional gynecologic surgery, such as a cystectomy or myomectomy, is required.
Surgical ligation of ovarian veins was initially performed via laparotomy using a traditional retroperitoneal approach. The surgical goal is to isolate the ovarian vein significantly above the pelvic brim and before the vein becomes substantially dilated. Laparotomy therefore requires a vertical mid-line incision to provide adequate access to the appropriate portion of the ovarian vessels, leading to potentially high morbidity and poor cosmesis.
More recently, gynecologic surgeons skilled in laparoscopy have successfully managed PCS transperitoneally. A few small series of bilateral laparoscopic transperitoneal ligation of ovarian veins have been reported, including one by Tigellio Gargiulo, MD, who clipped both veins in their upper third, near their distal ends at the inferior vena cava (right) and the renal vein (left) (J Am Assoc Gynecol Laparosc. 2003 Nov;10[4]:501-4).
We prefer a robot-assisted laparoscopic approach for most of our patients. Not only does the improved dexterity help while working with sensitive vasculature, but more importantly we are able to use Firefly fluorescence.
The procedure generally is as follows. The uterine adnexa on the affected side is grasped and placed on tension so that the infundibulopelvic (IP) ligament can be visualized as it courses up and above the pelvic brim. The peritoneum immediately over the IP ligament is gently grasped and tented upward, and a small incision is made into the peritoneum, providing access into the retroperitoneum. The ureter should be visualized medial to this dissection.
The peritoneal tissue is then gently dissected off the ovarian vessels. Once the vessels are freed from the peritoneal tissue, the dilated ovarian vein is often clearly visualized. It is important to note that if no venous dilation is seen during laparoscopy, the procedure should not be aborted. Due to the Trendelenburg position that is utilized in gynecologic – and especially laparoscopic – surgery, the venous system sometimes appears falsely “normal” at this time.
Once the ovarian vessels have been isolated, the arteries must be separated from the veins. The adventitial tissue is dissected until the vessels are separated. Great care should be taken to ensure that all movements run parallel to the vessels and not perpendicular, therefore decreasing the risk of bleeding.
This process can be challenging. The surgeon is working with delicate vasculature. Often there are several branches from the vein that have formed due to the abnormal venous system. The best way to approach it is to identify planes and separate those planes in order to isolate individual vessels. If difficulties are still encountered, the surgeon should restart the dissection higher.
Once the dilated ovarian vein is isolated, one to two clips are placed.
Usually the artery is clearly distinct from the vein as it is smaller, more elastic, and can be seen pulsing. However, occasionally it is difficult to distinguish. In these cases, assistance with the da Vinci surgical system is useful: Indocyanine green (ICG) dye can be injected intravenously and visualized with a near-infrared light on the da Vinci platform. The dye is then seen glowing green as it first courses through the artery and then the vein.
For patients who have been found on venography to have bilateral disease, we perform the ligation procedure bilaterally. Once ligation is complete, the more competent collateral veins in the pelvis will assume more of the venous circulation.
In our experience, patients have ultimately noted substantial pain relief after these procedures, both with the endoscopic embolization and the surgical ligation. Patients are counseled that it can take several months to notice a relief in the pain.
In rare cases, pelvic congestion is related to extrinsic compression. For instance, the left renal vein can become compressed between the aorta and the superior mesenteric artery (the nutcracker syndrome), or the left common iliac vein can be compressed between the overlying right internal iliac artery and the underlying vertebral body (May-Thurner syndrome). Both of these conditions can lead to secondary PCS.
Such complex conditions are usually treated by vascular surgeons. May-Thurner syndrome is treated via stenting, while nutcracker syndrome can be treated with stenting or transposition of the renal vein to the distal vena cava.
Dr. Steller is an associate at the Family Health Centers of San Diego. She reported having no relevant financial disclosures.
Taking hysteroscopy to the office
Along with global endometrial ablation, diagnostic and minor operative hysteroscopy are excellent procedures to bring into your office environment. These operations are generally of short duration and provide little risk to the patient. Moreover, reimbursement exceeds that for the hospital setting. A constant revenue stream can be created after an initial moderate expenditure.
The key to a successful office procedure is patient comfort; this begins with minimizing pain and trauma. In our practice, we note decreased pain when performing vaginoscopy and hysteroscopy without the use of a speculum or tenaculum. This is well substantiated in literature by Professor Stefano Bettocchi, who immediately preceded me as president of the International Society for Gynecologic Endoscopy (ISGE).
In this issue of Master Class in Gynecologic Surgery, I have asked my partner, Aarathi Cholkeri-Singh, MD, to discuss vaginoscopy. Dr. Cholkeri-Singh is clinical assistant professor at the University of Illinois at Chicago, lecturer at Rosalind Franklin University of Medicine and Science, and associate director of minimally invasive gynecologic surgery at Advocate Lutheran General Hospital, Park Ridge, Ill.
She also serves as codirector of the AAGL/Society of Reproductive Surgeons fellowship in minimally invasive gynecologic surgery and director of gynecologic surgical education at Advocate Lutheran, and is chair for a postgraduate course on hysteroscopy at the upcoming AAGL 45th Annual Global Congress. Among her publications is a recent review in the Journal of Minimally Invasive Gynecology on hysteroscopy for infertile women (doi:10.1016/j.jmig.2014.12.163).
Dr. Miller is clinical associate professor at the University of Illinois at Chicago, and past president of the AAGL and the International Society for Gynecologic Endoscopy. He is a reproductive endocrinologist and minimally invasive gynecologic surgeon in private practice in Naperville and Schaumburg, Ill.; director of minimally invasive gynecologic surgery and the director of the AAGL/SRS fellowship in minimally invasive gynecologic surgery at Advocate Lutheran General Hospital, Park Ridge, Ill.; and the medical editor of this column, Master Class. He reported having no financial disclosures relevant to this column. Email him at [email protected].
Along with global endometrial ablation, diagnostic and minor operative hysteroscopy are excellent procedures to bring into your office environment. These operations are generally of short duration and provide little risk to the patient. Moreover, reimbursement exceeds that for the hospital setting. A constant revenue stream can be created after an initial moderate expenditure.
The key to a successful office procedure is patient comfort; this begins with minimizing pain and trauma. In our practice, we note decreased pain when performing vaginoscopy and hysteroscopy without the use of a speculum or tenaculum. This is well substantiated in literature by Professor Stefano Bettocchi, who immediately preceded me as president of the International Society for Gynecologic Endoscopy (ISGE).
In this issue of Master Class in Gynecologic Surgery, I have asked my partner, Aarathi Cholkeri-Singh, MD, to discuss vaginoscopy. Dr. Cholkeri-Singh is clinical assistant professor at the University of Illinois at Chicago, lecturer at Rosalind Franklin University of Medicine and Science, and associate director of minimally invasive gynecologic surgery at Advocate Lutheran General Hospital, Park Ridge, Ill.
She also serves as codirector of the AAGL/Society of Reproductive Surgeons fellowship in minimally invasive gynecologic surgery and director of gynecologic surgical education at Advocate Lutheran, and is chair for a postgraduate course on hysteroscopy at the upcoming AAGL 45th Annual Global Congress. Among her publications is a recent review in the Journal of Minimally Invasive Gynecology on hysteroscopy for infertile women (doi:10.1016/j.jmig.2014.12.163).
Dr. Miller is clinical associate professor at the University of Illinois at Chicago, and past president of the AAGL and the International Society for Gynecologic Endoscopy. He is a reproductive endocrinologist and minimally invasive gynecologic surgeon in private practice in Naperville and Schaumburg, Ill.; director of minimally invasive gynecologic surgery and the director of the AAGL/SRS fellowship in minimally invasive gynecologic surgery at Advocate Lutheran General Hospital, Park Ridge, Ill.; and the medical editor of this column, Master Class. He reported having no financial disclosures relevant to this column. Email him at [email protected].
Along with global endometrial ablation, diagnostic and minor operative hysteroscopy are excellent procedures to bring into your office environment. These operations are generally of short duration and provide little risk to the patient. Moreover, reimbursement exceeds that for the hospital setting. A constant revenue stream can be created after an initial moderate expenditure.
The key to a successful office procedure is patient comfort; this begins with minimizing pain and trauma. In our practice, we note decreased pain when performing vaginoscopy and hysteroscopy without the use of a speculum or tenaculum. This is well substantiated in literature by Professor Stefano Bettocchi, who immediately preceded me as president of the International Society for Gynecologic Endoscopy (ISGE).
In this issue of Master Class in Gynecologic Surgery, I have asked my partner, Aarathi Cholkeri-Singh, MD, to discuss vaginoscopy. Dr. Cholkeri-Singh is clinical assistant professor at the University of Illinois at Chicago, lecturer at Rosalind Franklin University of Medicine and Science, and associate director of minimally invasive gynecologic surgery at Advocate Lutheran General Hospital, Park Ridge, Ill.
She also serves as codirector of the AAGL/Society of Reproductive Surgeons fellowship in minimally invasive gynecologic surgery and director of gynecologic surgical education at Advocate Lutheran, and is chair for a postgraduate course on hysteroscopy at the upcoming AAGL 45th Annual Global Congress. Among her publications is a recent review in the Journal of Minimally Invasive Gynecology on hysteroscopy for infertile women (doi:10.1016/j.jmig.2014.12.163).
Dr. Miller is clinical associate professor at the University of Illinois at Chicago, and past president of the AAGL and the International Society for Gynecologic Endoscopy. He is a reproductive endocrinologist and minimally invasive gynecologic surgeon in private practice in Naperville and Schaumburg, Ill.; director of minimally invasive gynecologic surgery and the director of the AAGL/SRS fellowship in minimally invasive gynecologic surgery at Advocate Lutheran General Hospital, Park Ridge, Ill.; and the medical editor of this column, Master Class. He reported having no financial disclosures relevant to this column. Email him at [email protected].
50 years of gynecologic surgery: A large dose of ingenuity, a small dose of controversy
Over the past 50 years, there has been explosive change in gynecologic surgery. Ob.Gyn. News has been at the forefront of capturing and chronicling this paradigm shift in the treatment of the female patient.
Our beginnings
From antiquity, physicians and surgeons have struggled with pelvic prolapse, uterine fibroids, ovarian cysts, urinary incontinence, vesicovaginal fistulas, pelvic pain, and abnormal uterine bleeding. At the time of the first edition of Ob.Gyn. News, it had been less than a century since Thomas Edison invented the light bulb; just over 50 years since Hans Christian Jacobaeus first created air pneumoperitoneum using a trocar, followed by the Nitze cystoscope; about 40 years since Richard Zollikofer created a carbon dioxide pneumoperitoneum; 25 years since F.H. Powers and A.C. Barnes had first described laparoscopic tubal sterilization by cautery; and about 20 years since Raoul Palmer, considered the father of modern laparoscopy, had first described the technique – left upper quadrant entry, testing insufflation, Trendelenburg positioning, and simple laparoscopic instrumentation.
In the 1950s, Hans Frangenheim would bring monopolar electrosurgery to laparoscopy and Harold Hopkins would introduce fiber optics. It was not until 1967 that Patrick Steptoe would publish the first textbook on laparoscopy in the English language.
Although usage as a diagnostic tool and a method of sterilization increased popularity of laparoscopy in the 1960s and early 1970s, there were few advances. In fact, a review of early editions of Ob.Gyn. News during that time period shows that the majority of articles involving laparoscopy dealt with sterilization; including the introduction of clips for tubal sterilization by Jaroslav Hulka in 1972. This did not deter the efforts of Jordan Phillips, who along with Jacques Rioux, Louis Keith, Richard Soderstrom – four early laparoscopists – incorporated a new society, the American Association of Gynecologic Laparoscopists (the AAGL) in 1971.
Simultaneously, in 1979, James Daniell in the United States, Maurice Bruhart in France, and Yona Tadir in Israel were promoting efforts to couple the carbon dioxide laser to the laparoscope to treat pelvic adhesions and endometriosis. Later on, fiber lasers, KTP, Nd:YAG, and Argon lasers would be utilized in our field. Still, only a few extirpative procedures were being performed via a laparoscope route. This included linear salpingostomy for the treatment of ectopic pregnancy, championed by Professor Bruhart and H. Manhes in Europe, and Alan DeCherney in the United States.
During the 1980s, laparoscopic surgery was at its innovative best. Through the pioneering efforts of Professor Kurt Semm and his protégée, Liselotte Mettler, the gynecologic laparoscopist was introduced to endoloops, simple suturing techniques, and mechanical morcellation techniques.
Procedures such as salpingo-oophorectomy, appendectomy, and myomectomy could now be performed via the laparoscope. Dr. Camran Nezhat coupled the carbon dioxide laser, the laparoscope, and the television monitor, coining the term laparoscopy. Most importantly, the laparoscopic surgeon was liberated; he or she could remain upright and perform surgery with both hands. Through the 1980s and 1990s, Dr. Nezhat, Dr. Harry Reich, and other innovators pushed the envelope in increasing the ability to extirpate endometriosis, excise severe pelvic adhesions, and perform discoid and segmental bowel resection.
The day the earth stood still
Every gynecologic laparoscopic surgeon should remember Jan. 26, 1988, as that was the date that Dr. Harry Reich performed the first total laparoscopic hysterectomy. Now, little more than 25 years later, in many parts of the country, a laparoscopic approach to hysterectomy is indeed the most common route. Over the years, with the evolution of instrumentation, including new energy systems (ultrasonic, advanced bipolar) and the introduction of barbed sutures, hysterectomy can now be performed via minilaparoscopy, single-site laparoscopy, robot-assisted, and robotic single site, all of which have been featured in the Ob.Gyn. News’ Master Class in Gynecologic Surgery.
But hysteroscopy came first
Abulkasim utilized a mirror to reflect light into the vaginal vault in 1,000 A.D. In 1806, Philipp Bozzini originated the idea of illuminating body cavities by an external light source. Through a system of mirrors and tubes, candlelight could be reflected into the body. In 1869, D.C. Pantaleoni used a cystoscope developed by Antoine Desormeaux – who has been called the father of endoscopy – to treat endometrial polyps with silver nitrate.
Through the 50 years of Ob.Gyn. News and over the past 12 years of the Master Class in Gynecologic Surgery, our community has been consistently updated as to advances in hysteroscopy, not only to enhance treatment efficacy, but safety as well. This has included such advances as the continuous flow hysteroscope, the Hamou contact hysteroscope, and fluid management systems to enhance visualization.
In 1978, Robert Neuwirth introduced loops to perform hysteroscopic myomectomy. The loop resectoscope was quickly followed by the rollerball to perform endometrial ablation. In the late 1990s, hysteroscopic bipolar cutting loops were introduced. This enabled use of ionic distension media saline, instead of nonionic media, thus decreasing risks related to hyponatremia.
In 2003, Mark Emanuel introduced hysteroscopic morcellation systems, which enabled more gynecologists to perform operative hysteroscopy safely. Resected tissue is removed immediately to allow superior visualization. The flexible hysteroscope coupled with vaginoscopy has enabled hysteroscopy to be done with minimal to no anesthesia in an in-office setting.
With advances in hysteroscopy over the past 35 years, hysteroscopic procedures such as polypectomy, myomectomy, lysis of adhesions, transection of endometriosis, evacuation of retained products of conception, and endometrial ablation/resection have become routine.
And now, the controversy
Since its inception, laparoscopic surgery has not been without controversy. In 1933, Karl Fervers described explosion and flashes of light from a combination of high frequency electric current and oxygen distension gas while performing laparoscopic adhesiolysis with the coagulation probe of the ureterocystoscope.
In the early 1970s, Professor Kurt Semm’s pioneering effort was not rewarded by his department, in Kiel, Germany, which instead recommended he schedule a brain scan and psychological testing.
Nearly 20 years later, in a 1992 edition of Current Science, Professor Semm, along with Alan DeCherney, stated that “over 80% of gynecological operations can now be performed by laparoscopy.” Shortly thereafter, however, Dr. Roy Pitkin, who at the time was president of the American College of Obstetricians and Gynecologists, wrote an editorial in the Journal of Obstetrics and Gynecology – “Operative Laparoscopy: Surgical Advance or Technical Gimmick?” (Obstet Gynecol. 1992 Mar;79[3]:441-2).
Fortunately, 18 years later, with the continued advances in laparoscopic surgery making it less expensive, safer, and more accessible, Dr. Pitkin did retract his statement (Obstet Gynecol. 2010 May;115[5]:890-1).
Currently, the gynecologic community is embroiled in controversies involving the use of the robot to assist in the performance of laparoscopic surgery, the incorporation of synthetic mesh to enhance urogynecologic procedures, the placement of Essure micro-inserts to occlude fallopian tubes, and the use of electronic power morcellation at time of laparoscopic or robot-assisted hysterectomy, myomectomy, or sacrocolpopexy.
After reading the 2013 article by Dr. Jason Wright, published in JAMA, comparing laparoscopic hysterectomy to robotic hysterectomy, no one can deny that the rise in a minimally invasive route to hysterectomy has coincided with the advent of the robot (JAMA. 2013 Feb 20;309[7]:689-98). On the other hand, many detractors, including Dr. James Breeden (past ACOG president 2012-2013), find the higher cost of robotic surgery very problematic. In fact, many of these detractors cite the paucity of data showing a significant advantage to use of robotics.
While certainly cost, more than ever, must be a major consideration, remember that during the 1990s, there were multiple articles in Ob.Gyn. News raising concerns about the cost of laparoscopic hysterectomy. Interestingly, studies over the past decade by Warren and Jonsdottir show a cost savings when hysterectomy is done laparoscopically as opposed to its being done by laparotomy. Thus, it certainly can be anticipated that with more physician experience, improved instrumentation, and robotic industry competition, the overall cost will become more comparable to a laparoscopic route.
In 1995, Ulf Ulmsten first described the use of tension-free tape (TVT) to treat stress urinary incontinence. In 1998, the Food and Drug Administration approved the use of the TVT sling in the United States. Since then, transobturator tension-free vaginal tape (TVT-O) and single incision mini-slings have been introduced. All of these techniques have been shown to be successful and have been well adapted into the armamentarium of physicians treating stress urinary incontinence.
With the success of synthetic mesh for the treatment of stress urinary incontinence, its use was extended to pelvic prolapse. In 2002, the first mesh device with indications for the treatment of pelvic organ prolapse was approved by the FDA. While the erosion rate utilizing synthetic mesh for stress urinary incontinence has been noted to be 2%, rates up to 8.3% have been noted in patients treated for pelvic prolapse.
In 2008, the FDA issued a warning regarding the use of mesh for prolapse and incontinence repair secondary to the sequelae of mesh erosion. Subsequently, in 2011, the concern was limited to vaginal mesh to correct pelvic organ prolapse. Finally, on Jan. 4, 2016, the FDA issued an order to reclassify surgical mesh to repair pelvic organ prolapse from class II, which includes moderate-risk devices, to class III, which includes high-risk devices. Moreover, the FDA issued a second order to manufacturers to submit a premarket approval application to support the safety and effectiveness of synthetic mesh for transvaginal repair of pelvic organ prolapse.
Essure micro-inserts for permanent birth control received initial approval from the FDA in November 2002. Despite the fact that Essure can be easily placed, is highly effective, and has seemingly low complication rates, concerns have been raised by the Facebook group “Essure Problems” and Erin Brockovich, the focus of the 2000 biographical film starring Julia Roberts.
After more than 5,000 women filed grievances with the FDA between November 2002 and May 2015, based on unintended pregnancies, miscarriages, stillbirths, severe pain, and bleeding, the FDA announced in 2016 that it would require a boxed warning label for Essure. The FDA also called upon Bayer, which makes and markets Essure, to conduct surveillance to assess “risks of the device in a real-world environment.” The agency stated it will use the results to “determine what, if any, further actions related to Essure are needed to protect public health.”
While Jan. 26, 1988, is a very special date in minimally invasive gynecologic surgery, April 17, 2014, is a day of infamy for the gynecologic laparoscopist. For on this day, the FDA announced a warning regarding electronic power morcellation. Many hospitals and hospital systems throughout the country issued bans on electronic power morcellation, leading to needless open laparotomy procedures and thus, introducing prolonged recovery times and increased risk.
At a time when the recent introduction of barbed suture had made both closure of the vaginal cuff at time of hysterectomy and repair of the hysterotomy at myomectomy easier and faster, the gynecologic laparoscopist was taking a step backward. The FDA based this decision and a subsequent boxed warning – issued in November 2014 – on a small number of studies showing potential upstaging of leiomyosarcoma post electronic power morcellation. Interestingly, many of the morcellation procedures cited did not use power morcellation. Furthermore, a more comprehensive meta-analysis by Elizabeth A. Pritts and colleagues, showed a far lower risk than suggested by the FDA (Gynecol Surg. 2015;12[3]:165-77).
Recently, an article by William Parker and colleagues recommended that the FDA reverse its position (Obstet Gynecol. 2016 Jan;127[1]:18-22). Many believe that ultimately, the solution will be morcellation in a containment bag, which I and my colleagues have been performing in virtually every power morcellation procedure since May 2014. During this current power morcellation controversy, the Master Class in Gynecologic Surgery has continued to update its readers with three different articles related to the subject.
And in conclusion
Without a doubt, the past 50 years of gynecologic surgery has been a time of unparalleled innovation with occasional controversy thrown in. Ob.Gyn. News and more recently, the Master Class in Gynecologic Surgery, has had a major leadership role in bringing this profound ingenuity to the gynecology community by introducing this explosion of surgical creativity to its readers.
And what will the next 50 years bring? I believe we will continue to see tremendous advancements in minimally invasive gynecologic surgery. There will be a definite impact of costs on the marketplace. Thus, many of the minor minimally invasive procedures currently performed in the hospital or surgery center will be brought into office settings. In addition, secondary to reimbursement, the more complex cases will be carried out by fewer gynecologic surgeons who have undergone more intense training in pelvic surgery and who can perform these cases more efficiently and with fewer complications. Our ability to perform surgery and what type of procedures we do will not only be based on randomized, controlled trials, but big data collection as well.
Dr. Miller is clinical associate professor at the University of Illinois at Chicago, and past president of the AAGL and the International Society for Gynecologic Endoscopy (ISGE). He is a reproductive endocrinologist and minimally invasive gynecologic surgeon in private practice in Naperville and Schaumburg, Ill.; director of minimally invasive gynecologic surgery and the director of the AAGL/Society of Reproductive Surgery fellowship in minimally invasive gynecologic surgery at Advocate Lutheran General Hospital, Park Ridge, Ill.; and the medical editor of this column, Master Class. Dr. Miller is a consultant and is on the speakers bureau for Ethicon.
Over the past 50 years, there has been explosive change in gynecologic surgery. Ob.Gyn. News has been at the forefront of capturing and chronicling this paradigm shift in the treatment of the female patient.
Our beginnings
From antiquity, physicians and surgeons have struggled with pelvic prolapse, uterine fibroids, ovarian cysts, urinary incontinence, vesicovaginal fistulas, pelvic pain, and abnormal uterine bleeding. At the time of the first edition of Ob.Gyn. News, it had been less than a century since Thomas Edison invented the light bulb; just over 50 years since Hans Christian Jacobaeus first created air pneumoperitoneum using a trocar, followed by the Nitze cystoscope; about 40 years since Richard Zollikofer created a carbon dioxide pneumoperitoneum; 25 years since F.H. Powers and A.C. Barnes had first described laparoscopic tubal sterilization by cautery; and about 20 years since Raoul Palmer, considered the father of modern laparoscopy, had first described the technique – left upper quadrant entry, testing insufflation, Trendelenburg positioning, and simple laparoscopic instrumentation.
In the 1950s, Hans Frangenheim would bring monopolar electrosurgery to laparoscopy and Harold Hopkins would introduce fiber optics. It was not until 1967 that Patrick Steptoe would publish the first textbook on laparoscopy in the English language.
Although usage as a diagnostic tool and a method of sterilization increased popularity of laparoscopy in the 1960s and early 1970s, there were few advances. In fact, a review of early editions of Ob.Gyn. News during that time period shows that the majority of articles involving laparoscopy dealt with sterilization; including the introduction of clips for tubal sterilization by Jaroslav Hulka in 1972. This did not deter the efforts of Jordan Phillips, who along with Jacques Rioux, Louis Keith, Richard Soderstrom – four early laparoscopists – incorporated a new society, the American Association of Gynecologic Laparoscopists (the AAGL) in 1971.
Simultaneously, in 1979, James Daniell in the United States, Maurice Bruhart in France, and Yona Tadir in Israel were promoting efforts to couple the carbon dioxide laser to the laparoscope to treat pelvic adhesions and endometriosis. Later on, fiber lasers, KTP, Nd:YAG, and Argon lasers would be utilized in our field. Still, only a few extirpative procedures were being performed via a laparoscope route. This included linear salpingostomy for the treatment of ectopic pregnancy, championed by Professor Bruhart and H. Manhes in Europe, and Alan DeCherney in the United States.
During the 1980s, laparoscopic surgery was at its innovative best. Through the pioneering efforts of Professor Kurt Semm and his protégée, Liselotte Mettler, the gynecologic laparoscopist was introduced to endoloops, simple suturing techniques, and mechanical morcellation techniques.
Procedures such as salpingo-oophorectomy, appendectomy, and myomectomy could now be performed via the laparoscope. Dr. Camran Nezhat coupled the carbon dioxide laser, the laparoscope, and the television monitor, coining the term laparoscopy. Most importantly, the laparoscopic surgeon was liberated; he or she could remain upright and perform surgery with both hands. Through the 1980s and 1990s, Dr. Nezhat, Dr. Harry Reich, and other innovators pushed the envelope in increasing the ability to extirpate endometriosis, excise severe pelvic adhesions, and perform discoid and segmental bowel resection.
The day the earth stood still
Every gynecologic laparoscopic surgeon should remember Jan. 26, 1988, as that was the date that Dr. Harry Reich performed the first total laparoscopic hysterectomy. Now, little more than 25 years later, in many parts of the country, a laparoscopic approach to hysterectomy is indeed the most common route. Over the years, with the evolution of instrumentation, including new energy systems (ultrasonic, advanced bipolar) and the introduction of barbed sutures, hysterectomy can now be performed via minilaparoscopy, single-site laparoscopy, robot-assisted, and robotic single site, all of which have been featured in the Ob.Gyn. News’ Master Class in Gynecologic Surgery.
But hysteroscopy came first
Abulkasim utilized a mirror to reflect light into the vaginal vault in 1,000 A.D. In 1806, Philipp Bozzini originated the idea of illuminating body cavities by an external light source. Through a system of mirrors and tubes, candlelight could be reflected into the body. In 1869, D.C. Pantaleoni used a cystoscope developed by Antoine Desormeaux – who has been called the father of endoscopy – to treat endometrial polyps with silver nitrate.
Through the 50 years of Ob.Gyn. News and over the past 12 years of the Master Class in Gynecologic Surgery, our community has been consistently updated as to advances in hysteroscopy, not only to enhance treatment efficacy, but safety as well. This has included such advances as the continuous flow hysteroscope, the Hamou contact hysteroscope, and fluid management systems to enhance visualization.
In 1978, Robert Neuwirth introduced loops to perform hysteroscopic myomectomy. The loop resectoscope was quickly followed by the rollerball to perform endometrial ablation. In the late 1990s, hysteroscopic bipolar cutting loops were introduced. This enabled use of ionic distension media saline, instead of nonionic media, thus decreasing risks related to hyponatremia.
In 2003, Mark Emanuel introduced hysteroscopic morcellation systems, which enabled more gynecologists to perform operative hysteroscopy safely. Resected tissue is removed immediately to allow superior visualization. The flexible hysteroscope coupled with vaginoscopy has enabled hysteroscopy to be done with minimal to no anesthesia in an in-office setting.
With advances in hysteroscopy over the past 35 years, hysteroscopic procedures such as polypectomy, myomectomy, lysis of adhesions, transection of endometriosis, evacuation of retained products of conception, and endometrial ablation/resection have become routine.
And now, the controversy
Since its inception, laparoscopic surgery has not been without controversy. In 1933, Karl Fervers described explosion and flashes of light from a combination of high frequency electric current and oxygen distension gas while performing laparoscopic adhesiolysis with the coagulation probe of the ureterocystoscope.
In the early 1970s, Professor Kurt Semm’s pioneering effort was not rewarded by his department, in Kiel, Germany, which instead recommended he schedule a brain scan and psychological testing.
Nearly 20 years later, in a 1992 edition of Current Science, Professor Semm, along with Alan DeCherney, stated that “over 80% of gynecological operations can now be performed by laparoscopy.” Shortly thereafter, however, Dr. Roy Pitkin, who at the time was president of the American College of Obstetricians and Gynecologists, wrote an editorial in the Journal of Obstetrics and Gynecology – “Operative Laparoscopy: Surgical Advance or Technical Gimmick?” (Obstet Gynecol. 1992 Mar;79[3]:441-2).
Fortunately, 18 years later, with the continued advances in laparoscopic surgery making it less expensive, safer, and more accessible, Dr. Pitkin did retract his statement (Obstet Gynecol. 2010 May;115[5]:890-1).
Currently, the gynecologic community is embroiled in controversies involving the use of the robot to assist in the performance of laparoscopic surgery, the incorporation of synthetic mesh to enhance urogynecologic procedures, the placement of Essure micro-inserts to occlude fallopian tubes, and the use of electronic power morcellation at time of laparoscopic or robot-assisted hysterectomy, myomectomy, or sacrocolpopexy.
After reading the 2013 article by Dr. Jason Wright, published in JAMA, comparing laparoscopic hysterectomy to robotic hysterectomy, no one can deny that the rise in a minimally invasive route to hysterectomy has coincided with the advent of the robot (JAMA. 2013 Feb 20;309[7]:689-98). On the other hand, many detractors, including Dr. James Breeden (past ACOG president 2012-2013), find the higher cost of robotic surgery very problematic. In fact, many of these detractors cite the paucity of data showing a significant advantage to use of robotics.
While certainly cost, more than ever, must be a major consideration, remember that during the 1990s, there were multiple articles in Ob.Gyn. News raising concerns about the cost of laparoscopic hysterectomy. Interestingly, studies over the past decade by Warren and Jonsdottir show a cost savings when hysterectomy is done laparoscopically as opposed to its being done by laparotomy. Thus, it certainly can be anticipated that with more physician experience, improved instrumentation, and robotic industry competition, the overall cost will become more comparable to a laparoscopic route.
In 1995, Ulf Ulmsten first described the use of tension-free tape (TVT) to treat stress urinary incontinence. In 1998, the Food and Drug Administration approved the use of the TVT sling in the United States. Since then, transobturator tension-free vaginal tape (TVT-O) and single incision mini-slings have been introduced. All of these techniques have been shown to be successful and have been well adapted into the armamentarium of physicians treating stress urinary incontinence.
With the success of synthetic mesh for the treatment of stress urinary incontinence, its use was extended to pelvic prolapse. In 2002, the first mesh device with indications for the treatment of pelvic organ prolapse was approved by the FDA. While the erosion rate utilizing synthetic mesh for stress urinary incontinence has been noted to be 2%, rates up to 8.3% have been noted in patients treated for pelvic prolapse.
In 2008, the FDA issued a warning regarding the use of mesh for prolapse and incontinence repair secondary to the sequelae of mesh erosion. Subsequently, in 2011, the concern was limited to vaginal mesh to correct pelvic organ prolapse. Finally, on Jan. 4, 2016, the FDA issued an order to reclassify surgical mesh to repair pelvic organ prolapse from class II, which includes moderate-risk devices, to class III, which includes high-risk devices. Moreover, the FDA issued a second order to manufacturers to submit a premarket approval application to support the safety and effectiveness of synthetic mesh for transvaginal repair of pelvic organ prolapse.
Essure micro-inserts for permanent birth control received initial approval from the FDA in November 2002. Despite the fact that Essure can be easily placed, is highly effective, and has seemingly low complication rates, concerns have been raised by the Facebook group “Essure Problems” and Erin Brockovich, the focus of the 2000 biographical film starring Julia Roberts.
After more than 5,000 women filed grievances with the FDA between November 2002 and May 2015, based on unintended pregnancies, miscarriages, stillbirths, severe pain, and bleeding, the FDA announced in 2016 that it would require a boxed warning label for Essure. The FDA also called upon Bayer, which makes and markets Essure, to conduct surveillance to assess “risks of the device in a real-world environment.” The agency stated it will use the results to “determine what, if any, further actions related to Essure are needed to protect public health.”
While Jan. 26, 1988, is a very special date in minimally invasive gynecologic surgery, April 17, 2014, is a day of infamy for the gynecologic laparoscopist. For on this day, the FDA announced a warning regarding electronic power morcellation. Many hospitals and hospital systems throughout the country issued bans on electronic power morcellation, leading to needless open laparotomy procedures and thus, introducing prolonged recovery times and increased risk.
At a time when the recent introduction of barbed suture had made both closure of the vaginal cuff at time of hysterectomy and repair of the hysterotomy at myomectomy easier and faster, the gynecologic laparoscopist was taking a step backward. The FDA based this decision and a subsequent boxed warning – issued in November 2014 – on a small number of studies showing potential upstaging of leiomyosarcoma post electronic power morcellation. Interestingly, many of the morcellation procedures cited did not use power morcellation. Furthermore, a more comprehensive meta-analysis by Elizabeth A. Pritts and colleagues, showed a far lower risk than suggested by the FDA (Gynecol Surg. 2015;12[3]:165-77).
Recently, an article by William Parker and colleagues recommended that the FDA reverse its position (Obstet Gynecol. 2016 Jan;127[1]:18-22). Many believe that ultimately, the solution will be morcellation in a containment bag, which I and my colleagues have been performing in virtually every power morcellation procedure since May 2014. During this current power morcellation controversy, the Master Class in Gynecologic Surgery has continued to update its readers with three different articles related to the subject.
And in conclusion
Without a doubt, the past 50 years of gynecologic surgery has been a time of unparalleled innovation with occasional controversy thrown in. Ob.Gyn. News and more recently, the Master Class in Gynecologic Surgery, has had a major leadership role in bringing this profound ingenuity to the gynecology community by introducing this explosion of surgical creativity to its readers.
And what will the next 50 years bring? I believe we will continue to see tremendous advancements in minimally invasive gynecologic surgery. There will be a definite impact of costs on the marketplace. Thus, many of the minor minimally invasive procedures currently performed in the hospital or surgery center will be brought into office settings. In addition, secondary to reimbursement, the more complex cases will be carried out by fewer gynecologic surgeons who have undergone more intense training in pelvic surgery and who can perform these cases more efficiently and with fewer complications. Our ability to perform surgery and what type of procedures we do will not only be based on randomized, controlled trials, but big data collection as well.
Dr. Miller is clinical associate professor at the University of Illinois at Chicago, and past president of the AAGL and the International Society for Gynecologic Endoscopy (ISGE). He is a reproductive endocrinologist and minimally invasive gynecologic surgeon in private practice in Naperville and Schaumburg, Ill.; director of minimally invasive gynecologic surgery and the director of the AAGL/Society of Reproductive Surgery fellowship in minimally invasive gynecologic surgery at Advocate Lutheran General Hospital, Park Ridge, Ill.; and the medical editor of this column, Master Class. Dr. Miller is a consultant and is on the speakers bureau for Ethicon.
Over the past 50 years, there has been explosive change in gynecologic surgery. Ob.Gyn. News has been at the forefront of capturing and chronicling this paradigm shift in the treatment of the female patient.
Our beginnings
From antiquity, physicians and surgeons have struggled with pelvic prolapse, uterine fibroids, ovarian cysts, urinary incontinence, vesicovaginal fistulas, pelvic pain, and abnormal uterine bleeding. At the time of the first edition of Ob.Gyn. News, it had been less than a century since Thomas Edison invented the light bulb; just over 50 years since Hans Christian Jacobaeus first created air pneumoperitoneum using a trocar, followed by the Nitze cystoscope; about 40 years since Richard Zollikofer created a carbon dioxide pneumoperitoneum; 25 years since F.H. Powers and A.C. Barnes had first described laparoscopic tubal sterilization by cautery; and about 20 years since Raoul Palmer, considered the father of modern laparoscopy, had first described the technique – left upper quadrant entry, testing insufflation, Trendelenburg positioning, and simple laparoscopic instrumentation.
In the 1950s, Hans Frangenheim would bring monopolar electrosurgery to laparoscopy and Harold Hopkins would introduce fiber optics. It was not until 1967 that Patrick Steptoe would publish the first textbook on laparoscopy in the English language.
Although usage as a diagnostic tool and a method of sterilization increased popularity of laparoscopy in the 1960s and early 1970s, there were few advances. In fact, a review of early editions of Ob.Gyn. News during that time period shows that the majority of articles involving laparoscopy dealt with sterilization; including the introduction of clips for tubal sterilization by Jaroslav Hulka in 1972. This did not deter the efforts of Jordan Phillips, who along with Jacques Rioux, Louis Keith, Richard Soderstrom – four early laparoscopists – incorporated a new society, the American Association of Gynecologic Laparoscopists (the AAGL) in 1971.
Simultaneously, in 1979, James Daniell in the United States, Maurice Bruhart in France, and Yona Tadir in Israel were promoting efforts to couple the carbon dioxide laser to the laparoscope to treat pelvic adhesions and endometriosis. Later on, fiber lasers, KTP, Nd:YAG, and Argon lasers would be utilized in our field. Still, only a few extirpative procedures were being performed via a laparoscope route. This included linear salpingostomy for the treatment of ectopic pregnancy, championed by Professor Bruhart and H. Manhes in Europe, and Alan DeCherney in the United States.
During the 1980s, laparoscopic surgery was at its innovative best. Through the pioneering efforts of Professor Kurt Semm and his protégée, Liselotte Mettler, the gynecologic laparoscopist was introduced to endoloops, simple suturing techniques, and mechanical morcellation techniques.
Procedures such as salpingo-oophorectomy, appendectomy, and myomectomy could now be performed via the laparoscope. Dr. Camran Nezhat coupled the carbon dioxide laser, the laparoscope, and the television monitor, coining the term laparoscopy. Most importantly, the laparoscopic surgeon was liberated; he or she could remain upright and perform surgery with both hands. Through the 1980s and 1990s, Dr. Nezhat, Dr. Harry Reich, and other innovators pushed the envelope in increasing the ability to extirpate endometriosis, excise severe pelvic adhesions, and perform discoid and segmental bowel resection.
The day the earth stood still
Every gynecologic laparoscopic surgeon should remember Jan. 26, 1988, as that was the date that Dr. Harry Reich performed the first total laparoscopic hysterectomy. Now, little more than 25 years later, in many parts of the country, a laparoscopic approach to hysterectomy is indeed the most common route. Over the years, with the evolution of instrumentation, including new energy systems (ultrasonic, advanced bipolar) and the introduction of barbed sutures, hysterectomy can now be performed via minilaparoscopy, single-site laparoscopy, robot-assisted, and robotic single site, all of which have been featured in the Ob.Gyn. News’ Master Class in Gynecologic Surgery.
But hysteroscopy came first
Abulkasim utilized a mirror to reflect light into the vaginal vault in 1,000 A.D. In 1806, Philipp Bozzini originated the idea of illuminating body cavities by an external light source. Through a system of mirrors and tubes, candlelight could be reflected into the body. In 1869, D.C. Pantaleoni used a cystoscope developed by Antoine Desormeaux – who has been called the father of endoscopy – to treat endometrial polyps with silver nitrate.
Through the 50 years of Ob.Gyn. News and over the past 12 years of the Master Class in Gynecologic Surgery, our community has been consistently updated as to advances in hysteroscopy, not only to enhance treatment efficacy, but safety as well. This has included such advances as the continuous flow hysteroscope, the Hamou contact hysteroscope, and fluid management systems to enhance visualization.
In 1978, Robert Neuwirth introduced loops to perform hysteroscopic myomectomy. The loop resectoscope was quickly followed by the rollerball to perform endometrial ablation. In the late 1990s, hysteroscopic bipolar cutting loops were introduced. This enabled use of ionic distension media saline, instead of nonionic media, thus decreasing risks related to hyponatremia.
In 2003, Mark Emanuel introduced hysteroscopic morcellation systems, which enabled more gynecologists to perform operative hysteroscopy safely. Resected tissue is removed immediately to allow superior visualization. The flexible hysteroscope coupled with vaginoscopy has enabled hysteroscopy to be done with minimal to no anesthesia in an in-office setting.
With advances in hysteroscopy over the past 35 years, hysteroscopic procedures such as polypectomy, myomectomy, lysis of adhesions, transection of endometriosis, evacuation of retained products of conception, and endometrial ablation/resection have become routine.
And now, the controversy
Since its inception, laparoscopic surgery has not been without controversy. In 1933, Karl Fervers described explosion and flashes of light from a combination of high frequency electric current and oxygen distension gas while performing laparoscopic adhesiolysis with the coagulation probe of the ureterocystoscope.
In the early 1970s, Professor Kurt Semm’s pioneering effort was not rewarded by his department, in Kiel, Germany, which instead recommended he schedule a brain scan and psychological testing.
Nearly 20 years later, in a 1992 edition of Current Science, Professor Semm, along with Alan DeCherney, stated that “over 80% of gynecological operations can now be performed by laparoscopy.” Shortly thereafter, however, Dr. Roy Pitkin, who at the time was president of the American College of Obstetricians and Gynecologists, wrote an editorial in the Journal of Obstetrics and Gynecology – “Operative Laparoscopy: Surgical Advance or Technical Gimmick?” (Obstet Gynecol. 1992 Mar;79[3]:441-2).
Fortunately, 18 years later, with the continued advances in laparoscopic surgery making it less expensive, safer, and more accessible, Dr. Pitkin did retract his statement (Obstet Gynecol. 2010 May;115[5]:890-1).
Currently, the gynecologic community is embroiled in controversies involving the use of the robot to assist in the performance of laparoscopic surgery, the incorporation of synthetic mesh to enhance urogynecologic procedures, the placement of Essure micro-inserts to occlude fallopian tubes, and the use of electronic power morcellation at time of laparoscopic or robot-assisted hysterectomy, myomectomy, or sacrocolpopexy.
After reading the 2013 article by Dr. Jason Wright, published in JAMA, comparing laparoscopic hysterectomy to robotic hysterectomy, no one can deny that the rise in a minimally invasive route to hysterectomy has coincided with the advent of the robot (JAMA. 2013 Feb 20;309[7]:689-98). On the other hand, many detractors, including Dr. James Breeden (past ACOG president 2012-2013), find the higher cost of robotic surgery very problematic. In fact, many of these detractors cite the paucity of data showing a significant advantage to use of robotics.
While certainly cost, more than ever, must be a major consideration, remember that during the 1990s, there were multiple articles in Ob.Gyn. News raising concerns about the cost of laparoscopic hysterectomy. Interestingly, studies over the past decade by Warren and Jonsdottir show a cost savings when hysterectomy is done laparoscopically as opposed to its being done by laparotomy. Thus, it certainly can be anticipated that with more physician experience, improved instrumentation, and robotic industry competition, the overall cost will become more comparable to a laparoscopic route.
In 1995, Ulf Ulmsten first described the use of tension-free tape (TVT) to treat stress urinary incontinence. In 1998, the Food and Drug Administration approved the use of the TVT sling in the United States. Since then, transobturator tension-free vaginal tape (TVT-O) and single incision mini-slings have been introduced. All of these techniques have been shown to be successful and have been well adapted into the armamentarium of physicians treating stress urinary incontinence.
With the success of synthetic mesh for the treatment of stress urinary incontinence, its use was extended to pelvic prolapse. In 2002, the first mesh device with indications for the treatment of pelvic organ prolapse was approved by the FDA. While the erosion rate utilizing synthetic mesh for stress urinary incontinence has been noted to be 2%, rates up to 8.3% have been noted in patients treated for pelvic prolapse.
In 2008, the FDA issued a warning regarding the use of mesh for prolapse and incontinence repair secondary to the sequelae of mesh erosion. Subsequently, in 2011, the concern was limited to vaginal mesh to correct pelvic organ prolapse. Finally, on Jan. 4, 2016, the FDA issued an order to reclassify surgical mesh to repair pelvic organ prolapse from class II, which includes moderate-risk devices, to class III, which includes high-risk devices. Moreover, the FDA issued a second order to manufacturers to submit a premarket approval application to support the safety and effectiveness of synthetic mesh for transvaginal repair of pelvic organ prolapse.
Essure micro-inserts for permanent birth control received initial approval from the FDA in November 2002. Despite the fact that Essure can be easily placed, is highly effective, and has seemingly low complication rates, concerns have been raised by the Facebook group “Essure Problems” and Erin Brockovich, the focus of the 2000 biographical film starring Julia Roberts.
After more than 5,000 women filed grievances with the FDA between November 2002 and May 2015, based on unintended pregnancies, miscarriages, stillbirths, severe pain, and bleeding, the FDA announced in 2016 that it would require a boxed warning label for Essure. The FDA also called upon Bayer, which makes and markets Essure, to conduct surveillance to assess “risks of the device in a real-world environment.” The agency stated it will use the results to “determine what, if any, further actions related to Essure are needed to protect public health.”
While Jan. 26, 1988, is a very special date in minimally invasive gynecologic surgery, April 17, 2014, is a day of infamy for the gynecologic laparoscopist. For on this day, the FDA announced a warning regarding electronic power morcellation. Many hospitals and hospital systems throughout the country issued bans on electronic power morcellation, leading to needless open laparotomy procedures and thus, introducing prolonged recovery times and increased risk.
At a time when the recent introduction of barbed suture had made both closure of the vaginal cuff at time of hysterectomy and repair of the hysterotomy at myomectomy easier and faster, the gynecologic laparoscopist was taking a step backward. The FDA based this decision and a subsequent boxed warning – issued in November 2014 – on a small number of studies showing potential upstaging of leiomyosarcoma post electronic power morcellation. Interestingly, many of the morcellation procedures cited did not use power morcellation. Furthermore, a more comprehensive meta-analysis by Elizabeth A. Pritts and colleagues, showed a far lower risk than suggested by the FDA (Gynecol Surg. 2015;12[3]:165-77).
Recently, an article by William Parker and colleagues recommended that the FDA reverse its position (Obstet Gynecol. 2016 Jan;127[1]:18-22). Many believe that ultimately, the solution will be morcellation in a containment bag, which I and my colleagues have been performing in virtually every power morcellation procedure since May 2014. During this current power morcellation controversy, the Master Class in Gynecologic Surgery has continued to update its readers with three different articles related to the subject.
And in conclusion
Without a doubt, the past 50 years of gynecologic surgery has been a time of unparalleled innovation with occasional controversy thrown in. Ob.Gyn. News and more recently, the Master Class in Gynecologic Surgery, has had a major leadership role in bringing this profound ingenuity to the gynecology community by introducing this explosion of surgical creativity to its readers.
And what will the next 50 years bring? I believe we will continue to see tremendous advancements in minimally invasive gynecologic surgery. There will be a definite impact of costs on the marketplace. Thus, many of the minor minimally invasive procedures currently performed in the hospital or surgery center will be brought into office settings. In addition, secondary to reimbursement, the more complex cases will be carried out by fewer gynecologic surgeons who have undergone more intense training in pelvic surgery and who can perform these cases more efficiently and with fewer complications. Our ability to perform surgery and what type of procedures we do will not only be based on randomized, controlled trials, but big data collection as well.
Dr. Miller is clinical associate professor at the University of Illinois at Chicago, and past president of the AAGL and the International Society for Gynecologic Endoscopy (ISGE). He is a reproductive endocrinologist and minimally invasive gynecologic surgeon in private practice in Naperville and Schaumburg, Ill.; director of minimally invasive gynecologic surgery and the director of the AAGL/Society of Reproductive Surgery fellowship in minimally invasive gynecologic surgery at Advocate Lutheran General Hospital, Park Ridge, Ill.; and the medical editor of this column, Master Class. Dr. Miller is a consultant and is on the speakers bureau for Ethicon.
The impact of endometriosis on ovarian cancer
During an ob.gyn. rotation, a medical student quickly learns the risks related to endometriosis; that is, pelvic pain, abnormal uterine bleeding, and infertility. With more experience, the young practitioner realizes the concern of unopposed estrogen therapy in patients with a history of endometriosis (i.e., cancer).
Now, in this excellent discussion by Dr. Farr Nezhat, for the current edition of the Master Class in Gynecologic Surgery, he describes the risk of endometriosis and ovarian cancer. Not only does Dr. Nezhat present data revealing the increased association between ovarian cancer and endometriosis, but he goes on to describe the usual type of epithelial ovarian cancer that is noted in the patient with endometriosis.
Dr. Nezhat describes women who appear to be predisposed to malignant transformation and provides current recommendations to lower the risk of malignancy in patients with endometriosis. This includes complete surgical resection of endometriosis, routine ultrasound/MRI if endometriosis is not resected, suppressive hormonal therapy, and bilateral salpingectomy. Moreover, Dr. Nezhat looks to the future and the possibility of genetic screening tests.
Dr. Nezhat is board certified in gynecologic oncology and is world renowned for his work with advanced laparoscopic and robotic surgery for the treatment of gynecologic cancers and complex benign conditions. He is the director of minimally invasive gynecologic surgery and robotics at Winthrop University Hospital in Mineola, N.Y., and an adjunct professor of obstetrics, gynecology, and reproductive medicine at State University of New York at Stony Brook.
His main areas of interest and research include early detection and treatment of early and advanced ovarian cancer, as well as cancer arising in endometriosis. Dr. Nezhat has authored and coauthored more than 200 medical and scientific manuscripts and book chapters.
Dr. Miller is a clinical associate professor at the University of Illinois at Chicago, and a past president of the AAGL and the International Society for Gynecologic Endoscopy (ISGE). He is a reproductive endocrinologist and minimally invasive gynecologic surgeon in private practice in Naperville and Schaumburg, Ill.; director of minimally invasive gynecologic surgery and the director of the AAGL/Society of Reproductive Surgery fellowship in minimally invasive gynecologic surgery at Advocate Lutheran General Hospital, Park Ridge, Ill.; and the medical editor of this column, Master Class. Dr. Miller reported having no financial disclosures relevant to this column.
During an ob.gyn. rotation, a medical student quickly learns the risks related to endometriosis; that is, pelvic pain, abnormal uterine bleeding, and infertility. With more experience, the young practitioner realizes the concern of unopposed estrogen therapy in patients with a history of endometriosis (i.e., cancer).
Now, in this excellent discussion by Dr. Farr Nezhat, for the current edition of the Master Class in Gynecologic Surgery, he describes the risk of endometriosis and ovarian cancer. Not only does Dr. Nezhat present data revealing the increased association between ovarian cancer and endometriosis, but he goes on to describe the usual type of epithelial ovarian cancer that is noted in the patient with endometriosis.
Dr. Nezhat describes women who appear to be predisposed to malignant transformation and provides current recommendations to lower the risk of malignancy in patients with endometriosis. This includes complete surgical resection of endometriosis, routine ultrasound/MRI if endometriosis is not resected, suppressive hormonal therapy, and bilateral salpingectomy. Moreover, Dr. Nezhat looks to the future and the possibility of genetic screening tests.
Dr. Nezhat is board certified in gynecologic oncology and is world renowned for his work with advanced laparoscopic and robotic surgery for the treatment of gynecologic cancers and complex benign conditions. He is the director of minimally invasive gynecologic surgery and robotics at Winthrop University Hospital in Mineola, N.Y., and an adjunct professor of obstetrics, gynecology, and reproductive medicine at State University of New York at Stony Brook.
His main areas of interest and research include early detection and treatment of early and advanced ovarian cancer, as well as cancer arising in endometriosis. Dr. Nezhat has authored and coauthored more than 200 medical and scientific manuscripts and book chapters.
Dr. Miller is a clinical associate professor at the University of Illinois at Chicago, and a past president of the AAGL and the International Society for Gynecologic Endoscopy (ISGE). He is a reproductive endocrinologist and minimally invasive gynecologic surgeon in private practice in Naperville and Schaumburg, Ill.; director of minimally invasive gynecologic surgery and the director of the AAGL/Society of Reproductive Surgery fellowship in minimally invasive gynecologic surgery at Advocate Lutheran General Hospital, Park Ridge, Ill.; and the medical editor of this column, Master Class. Dr. Miller reported having no financial disclosures relevant to this column.
During an ob.gyn. rotation, a medical student quickly learns the risks related to endometriosis; that is, pelvic pain, abnormal uterine bleeding, and infertility. With more experience, the young practitioner realizes the concern of unopposed estrogen therapy in patients with a history of endometriosis (i.e., cancer).
Now, in this excellent discussion by Dr. Farr Nezhat, for the current edition of the Master Class in Gynecologic Surgery, he describes the risk of endometriosis and ovarian cancer. Not only does Dr. Nezhat present data revealing the increased association between ovarian cancer and endometriosis, but he goes on to describe the usual type of epithelial ovarian cancer that is noted in the patient with endometriosis.
Dr. Nezhat describes women who appear to be predisposed to malignant transformation and provides current recommendations to lower the risk of malignancy in patients with endometriosis. This includes complete surgical resection of endometriosis, routine ultrasound/MRI if endometriosis is not resected, suppressive hormonal therapy, and bilateral salpingectomy. Moreover, Dr. Nezhat looks to the future and the possibility of genetic screening tests.
Dr. Nezhat is board certified in gynecologic oncology and is world renowned for his work with advanced laparoscopic and robotic surgery for the treatment of gynecologic cancers and complex benign conditions. He is the director of minimally invasive gynecologic surgery and robotics at Winthrop University Hospital in Mineola, N.Y., and an adjunct professor of obstetrics, gynecology, and reproductive medicine at State University of New York at Stony Brook.
His main areas of interest and research include early detection and treatment of early and advanced ovarian cancer, as well as cancer arising in endometriosis. Dr. Nezhat has authored and coauthored more than 200 medical and scientific manuscripts and book chapters.
Dr. Miller is a clinical associate professor at the University of Illinois at Chicago, and a past president of the AAGL and the International Society for Gynecologic Endoscopy (ISGE). He is a reproductive endocrinologist and minimally invasive gynecologic surgeon in private practice in Naperville and Schaumburg, Ill.; director of minimally invasive gynecologic surgery and the director of the AAGL/Society of Reproductive Surgery fellowship in minimally invasive gynecologic surgery at Advocate Lutheran General Hospital, Park Ridge, Ill.; and the medical editor of this column, Master Class. Dr. Miller reported having no financial disclosures relevant to this column.
Radiofrequency volumetric thermal ablation for symptomatic uterine fibroids
In 2002, Dr. Bruce B. Lee first described a laparoscopic technique to ablate symptomatic uterine fibroids utilizing radiofrequency under ultrasound guidance. Since this time, several papers have documented the procedure’s feasibility and efficacy, including reduction in menstrual blood loss, fibroid volume decrease, and improvement in quality of life.
In a randomized, prospective, single-center, longitudinal study that compared laparoscopic radiofrequency volumetric thermal ablation (RFVTA) of fibroids with laparoscopic myomectomy, Dr. Sara Y. Brucker and her colleagues concluded that RFVTA resulted in the treatment of more fibroids, a significantly shorter hospital stay, and less intraoperative blood loss than did laparoscopic myomectomy (Int J Gynaecol Obstet. 2014 Jun;125[3]:261-5).
More recently, in the literature and at the 2015 American Association of Gynecologic Laparoscopists (AAGL) Global Congress in November, viable, full-term pregnancies have been reported in patients previously treated for symptomatic fibroids via RFVTA (J Reprod Med. 2015 May-Jun;60[5-6]:194-8).
The system for performing RFVTA of symptomatic fibroids – the Acessa System (Halt Medical) – has continued to improve. Earlier this year, Dr. Donald I. Galen described the use of electromagnetic image guidance, which has been cleared by the Food and Drug Administration and incorporated into the Acessa Guidance System. Dr. Galen’s feasibility study showed that the guidance system enhances the ultrasonic image of Acessa’s handpiece to facilitate accurate tip placement during the targeting and ablation of uterine fibroids (Biomed Eng Online. 2015 Oct 15;14:90).
In this edition of the Master Class in Gynecologic Surgery, Dr. Jay M. Berman discusses the use of RFVTA for the treatment of symptomatic uterine fibroids. Dr. Berman is interim chairman of Wayne State University’s department of obstetrics and gynecology and interim specialist in chief for obstetrics and gynecology at the Detroit Medical Center. He served as a principal investigator of the pivotal trial of Acessa and has reported on reproductive outcomes. Dr. Berman has long been interested in alternatives to hysterectomy for fibroid management and has incorporated RFVTA into his armamentarium of therapies.
Dr. Miller is a clinical associate professor at the University of Illinois at Chicago, and a past president of the AAGL and the International Society for Gynecologic Endoscopy (ISGE). He is a reproductive endocrinologist and minimally invasive gynecologic surgeon in private practice in Naperville and Schaumburg, Ill.; director of minimally invasive gynecologic surgery and the director of the AAGL/Society of Reproductive Surgery fellowship in minimally invasive gynecologic surgery at Advocate Lutheran General Hospital, Park Ridge, Ill.; and the medical editor of this column, Master Class. Dr. Miller reported that he is a consultant for Halt Medical Inc., which developed the Acessa System.
In 2002, Dr. Bruce B. Lee first described a laparoscopic technique to ablate symptomatic uterine fibroids utilizing radiofrequency under ultrasound guidance. Since this time, several papers have documented the procedure’s feasibility and efficacy, including reduction in menstrual blood loss, fibroid volume decrease, and improvement in quality of life.
In a randomized, prospective, single-center, longitudinal study that compared laparoscopic radiofrequency volumetric thermal ablation (RFVTA) of fibroids with laparoscopic myomectomy, Dr. Sara Y. Brucker and her colleagues concluded that RFVTA resulted in the treatment of more fibroids, a significantly shorter hospital stay, and less intraoperative blood loss than did laparoscopic myomectomy (Int J Gynaecol Obstet. 2014 Jun;125[3]:261-5).
More recently, in the literature and at the 2015 American Association of Gynecologic Laparoscopists (AAGL) Global Congress in November, viable, full-term pregnancies have been reported in patients previously treated for symptomatic fibroids via RFVTA (J Reprod Med. 2015 May-Jun;60[5-6]:194-8).
The system for performing RFVTA of symptomatic fibroids – the Acessa System (Halt Medical) – has continued to improve. Earlier this year, Dr. Donald I. Galen described the use of electromagnetic image guidance, which has been cleared by the Food and Drug Administration and incorporated into the Acessa Guidance System. Dr. Galen’s feasibility study showed that the guidance system enhances the ultrasonic image of Acessa’s handpiece to facilitate accurate tip placement during the targeting and ablation of uterine fibroids (Biomed Eng Online. 2015 Oct 15;14:90).
In this edition of the Master Class in Gynecologic Surgery, Dr. Jay M. Berman discusses the use of RFVTA for the treatment of symptomatic uterine fibroids. Dr. Berman is interim chairman of Wayne State University’s department of obstetrics and gynecology and interim specialist in chief for obstetrics and gynecology at the Detroit Medical Center. He served as a principal investigator of the pivotal trial of Acessa and has reported on reproductive outcomes. Dr. Berman has long been interested in alternatives to hysterectomy for fibroid management and has incorporated RFVTA into his armamentarium of therapies.
Dr. Miller is a clinical associate professor at the University of Illinois at Chicago, and a past president of the AAGL and the International Society for Gynecologic Endoscopy (ISGE). He is a reproductive endocrinologist and minimally invasive gynecologic surgeon in private practice in Naperville and Schaumburg, Ill.; director of minimally invasive gynecologic surgery and the director of the AAGL/Society of Reproductive Surgery fellowship in minimally invasive gynecologic surgery at Advocate Lutheran General Hospital, Park Ridge, Ill.; and the medical editor of this column, Master Class. Dr. Miller reported that he is a consultant for Halt Medical Inc., which developed the Acessa System.
In 2002, Dr. Bruce B. Lee first described a laparoscopic technique to ablate symptomatic uterine fibroids utilizing radiofrequency under ultrasound guidance. Since this time, several papers have documented the procedure’s feasibility and efficacy, including reduction in menstrual blood loss, fibroid volume decrease, and improvement in quality of life.
In a randomized, prospective, single-center, longitudinal study that compared laparoscopic radiofrequency volumetric thermal ablation (RFVTA) of fibroids with laparoscopic myomectomy, Dr. Sara Y. Brucker and her colleagues concluded that RFVTA resulted in the treatment of more fibroids, a significantly shorter hospital stay, and less intraoperative blood loss than did laparoscopic myomectomy (Int J Gynaecol Obstet. 2014 Jun;125[3]:261-5).
More recently, in the literature and at the 2015 American Association of Gynecologic Laparoscopists (AAGL) Global Congress in November, viable, full-term pregnancies have been reported in patients previously treated for symptomatic fibroids via RFVTA (J Reprod Med. 2015 May-Jun;60[5-6]:194-8).
The system for performing RFVTA of symptomatic fibroids – the Acessa System (Halt Medical) – has continued to improve. Earlier this year, Dr. Donald I. Galen described the use of electromagnetic image guidance, which has been cleared by the Food and Drug Administration and incorporated into the Acessa Guidance System. Dr. Galen’s feasibility study showed that the guidance system enhances the ultrasonic image of Acessa’s handpiece to facilitate accurate tip placement during the targeting and ablation of uterine fibroids (Biomed Eng Online. 2015 Oct 15;14:90).
In this edition of the Master Class in Gynecologic Surgery, Dr. Jay M. Berman discusses the use of RFVTA for the treatment of symptomatic uterine fibroids. Dr. Berman is interim chairman of Wayne State University’s department of obstetrics and gynecology and interim specialist in chief for obstetrics and gynecology at the Detroit Medical Center. He served as a principal investigator of the pivotal trial of Acessa and has reported on reproductive outcomes. Dr. Berman has long been interested in alternatives to hysterectomy for fibroid management and has incorporated RFVTA into his armamentarium of therapies.
Dr. Miller is a clinical associate professor at the University of Illinois at Chicago, and a past president of the AAGL and the International Society for Gynecologic Endoscopy (ISGE). He is a reproductive endocrinologist and minimally invasive gynecologic surgeon in private practice in Naperville and Schaumburg, Ill.; director of minimally invasive gynecologic surgery and the director of the AAGL/Society of Reproductive Surgery fellowship in minimally invasive gynecologic surgery at Advocate Lutheran General Hospital, Park Ridge, Ill.; and the medical editor of this column, Master Class. Dr. Miller reported that he is a consultant for Halt Medical Inc., which developed the Acessa System.
Cystoscopies are us
In 2012, the AAGL issued Guidelines for Intraoperative Cystoscopy in Laparoscopic Hysterectomy (J Minim Invasive Gynecol. 2012 Jul-Aug;19[4]:407-11.). In this AAGL report, a meta-analysis noted 27 published trials comprising 3,643 cases. Laparoscopic hysterectomy was associated with an increased risk of urinary tract injury when compared with abdominal hysterectomy (odds ratio, 2.61; 95% confidence interval, 1.22-5.60), according to the meta-analysis (BMJ. 2005 Jun 25;330[7506]:1478.).
As a result of this meta-analysis, as well as multiple other studies, the AAGL Guidelines Committee noted that “current evidence supports the conclusion that cystoscopic evaluation of the lower urinary tract should be readily available to gynecologic surgeons performing laparoscopic hysterectomy.” The resultant guidelines recommend that “a surgeon with appropriate education, training, and institutional privileges be available without delay to perform the task (cystoscopy).”
Besides the evaluation of the urinary tract for potential injury at hysterectomy, cystoscopy is useful in evaluation of various urogynecologic concerns, potential malignancy, and possible genitourinary fistula.
In this edition of the Master Class in Gynecologic Surgery, I have asked urogynecologist Dr. Neeraj Kohli to discuss the use of cystoscopy in gynecology, as well as to present new instrumentation to aide in the performance of the procedure.
Dr. Kohli is in private practice as medical director of Boston Urogyn in Wellesley, Mass., an ob.gyn. staff member at Brigham Women’s Hospital/Newton Wellesley Hospital, and assistant professor of ob.gyn. at Harvard Medical School in Boston.
Dr. Kohli is a nationally recognized leader in the field of urogynecology and reconstructive pelvic surgery, specializing in the treatment of pelvic prolapse, urinary incontinence, and advanced pelvic surgery. He has authored more than 100 scientific articles, book chapters, research abstracts, clinical presentations and multimedia educational tools.
Dr. Miller is a clinical associate professor at the University of Illinois at Chicago, immediate past president of the International Society for Gynecologic Endoscopy (ISGE), and a past president of the AAGL. He is a reproductive endocrinologist and minimally invasive gynecologic surgeon in private practice in Naperville, Ill., and Schaumburg, Ill.; director of minimally invasive gynecologic surgery and the director of the AAGL/SRS fellowship in minimally invasive gynecologic surgery at Advocate Lutheran General Hospital, Park Ridge, Ill; and the medical editor of this column, Master Class. Dr. Miller reported having no financial disclosures relevant to this Master Class.
In 2012, the AAGL issued Guidelines for Intraoperative Cystoscopy in Laparoscopic Hysterectomy (J Minim Invasive Gynecol. 2012 Jul-Aug;19[4]:407-11.). In this AAGL report, a meta-analysis noted 27 published trials comprising 3,643 cases. Laparoscopic hysterectomy was associated with an increased risk of urinary tract injury when compared with abdominal hysterectomy (odds ratio, 2.61; 95% confidence interval, 1.22-5.60), according to the meta-analysis (BMJ. 2005 Jun 25;330[7506]:1478.).
As a result of this meta-analysis, as well as multiple other studies, the AAGL Guidelines Committee noted that “current evidence supports the conclusion that cystoscopic evaluation of the lower urinary tract should be readily available to gynecologic surgeons performing laparoscopic hysterectomy.” The resultant guidelines recommend that “a surgeon with appropriate education, training, and institutional privileges be available without delay to perform the task (cystoscopy).”
Besides the evaluation of the urinary tract for potential injury at hysterectomy, cystoscopy is useful in evaluation of various urogynecologic concerns, potential malignancy, and possible genitourinary fistula.
In this edition of the Master Class in Gynecologic Surgery, I have asked urogynecologist Dr. Neeraj Kohli to discuss the use of cystoscopy in gynecology, as well as to present new instrumentation to aide in the performance of the procedure.
Dr. Kohli is in private practice as medical director of Boston Urogyn in Wellesley, Mass., an ob.gyn. staff member at Brigham Women’s Hospital/Newton Wellesley Hospital, and assistant professor of ob.gyn. at Harvard Medical School in Boston.
Dr. Kohli is a nationally recognized leader in the field of urogynecology and reconstructive pelvic surgery, specializing in the treatment of pelvic prolapse, urinary incontinence, and advanced pelvic surgery. He has authored more than 100 scientific articles, book chapters, research abstracts, clinical presentations and multimedia educational tools.
Dr. Miller is a clinical associate professor at the University of Illinois at Chicago, immediate past president of the International Society for Gynecologic Endoscopy (ISGE), and a past president of the AAGL. He is a reproductive endocrinologist and minimally invasive gynecologic surgeon in private practice in Naperville, Ill., and Schaumburg, Ill.; director of minimally invasive gynecologic surgery and the director of the AAGL/SRS fellowship in minimally invasive gynecologic surgery at Advocate Lutheran General Hospital, Park Ridge, Ill; and the medical editor of this column, Master Class. Dr. Miller reported having no financial disclosures relevant to this Master Class.
In 2012, the AAGL issued Guidelines for Intraoperative Cystoscopy in Laparoscopic Hysterectomy (J Minim Invasive Gynecol. 2012 Jul-Aug;19[4]:407-11.). In this AAGL report, a meta-analysis noted 27 published trials comprising 3,643 cases. Laparoscopic hysterectomy was associated with an increased risk of urinary tract injury when compared with abdominal hysterectomy (odds ratio, 2.61; 95% confidence interval, 1.22-5.60), according to the meta-analysis (BMJ. 2005 Jun 25;330[7506]:1478.).
As a result of this meta-analysis, as well as multiple other studies, the AAGL Guidelines Committee noted that “current evidence supports the conclusion that cystoscopic evaluation of the lower urinary tract should be readily available to gynecologic surgeons performing laparoscopic hysterectomy.” The resultant guidelines recommend that “a surgeon with appropriate education, training, and institutional privileges be available without delay to perform the task (cystoscopy).”
Besides the evaluation of the urinary tract for potential injury at hysterectomy, cystoscopy is useful in evaluation of various urogynecologic concerns, potential malignancy, and possible genitourinary fistula.
In this edition of the Master Class in Gynecologic Surgery, I have asked urogynecologist Dr. Neeraj Kohli to discuss the use of cystoscopy in gynecology, as well as to present new instrumentation to aide in the performance of the procedure.
Dr. Kohli is in private practice as medical director of Boston Urogyn in Wellesley, Mass., an ob.gyn. staff member at Brigham Women’s Hospital/Newton Wellesley Hospital, and assistant professor of ob.gyn. at Harvard Medical School in Boston.
Dr. Kohli is a nationally recognized leader in the field of urogynecology and reconstructive pelvic surgery, specializing in the treatment of pelvic prolapse, urinary incontinence, and advanced pelvic surgery. He has authored more than 100 scientific articles, book chapters, research abstracts, clinical presentations and multimedia educational tools.
Dr. Miller is a clinical associate professor at the University of Illinois at Chicago, immediate past president of the International Society for Gynecologic Endoscopy (ISGE), and a past president of the AAGL. He is a reproductive endocrinologist and minimally invasive gynecologic surgeon in private practice in Naperville, Ill., and Schaumburg, Ill.; director of minimally invasive gynecologic surgery and the director of the AAGL/SRS fellowship in minimally invasive gynecologic surgery at Advocate Lutheran General Hospital, Park Ridge, Ill; and the medical editor of this column, Master Class. Dr. Miller reported having no financial disclosures relevant to this Master Class.
Keeping laparoscopy safe for the obese patient
As I was writing my introduction to this current edition of the Master Class in Gynecologic Surgery, focusing on minimally invasive surgery for the obese female patient, I was listening to Chuck Todd, host of “Meet the Press.” Instantaneously, television and my thoughts became one; in the last segment of the program, Mr. Todd discussed what he was able to consume for $50 at the Iowa State Fair. I learned that his diet that day consisted of a pork chop on a stick, mac and cheese, a bacon-wrapped corn dog, cheese on a stick with jalapeños, a deep-fried Twinkie, and even fried apple pie with bacon. While Mr. Todd is thin and healthy, the array of foods at the fair reflects our nation’s penchant toward fast food that is fat laden and fried. Though our county is not alone in the world, obesity has reached epidemic proportion in the United States.
According to a May 2015 Department of Health & Human Services report on the health status of the nation, 69% of adults in the United States are overweight and 35% are obese. As a result, the minimally invasive gynecologic surgeon is dealing with an increasing population of women with comorbidities related to their obesity that can confound surgery outcomes. Moreover, anatomic landmarks that the young medical student learns in his or her first anatomy classes are modified due to the size of panniculus and the migration of the umbilicus relative to the bifurcation of the aorta.
I asked Dr. Amina Ahmed to join me in discussing the management of the obese patient undergoing minimally invasive gynecologic surgery. After completing her fellowship in gynecologic oncology, Dr. Ahmed has been on staff at both the University of Iowa Hospitals and Clinics, Iowa City, and Advocate Lutheran General Hospital, Park Ridge, Ill. She will soon join the gynecologic oncology faculty at Rush University Medical Center, Chicago. Given the increased rate of obesity in both Chicago and Iowa, Dr. Ahmed has become an expert in this area in a short period of time.
Dr. Miller is a clinical associate professor at the University of Illinois at Chicago, immediate past president of the International Society for Gynecologic Endoscopy (ISGE), and a past president of the AAGL. He is a reproductive endocrinologist and minimally invasive gynecologic surgeon in private practice in Naperville, Ill., and Schaumburg, Ill.; director of minimally invasive gynecologic surgery and the director of the AAGL/SRS fellowship in minimally invasive gynecologic surgery at Advocate Lutheran General Hospital, Park Ridge, Ill; and the medical editor of this column, Master Class. Dr. Miller disclosed that he is a consultant and on the speakers bureau for Ethicon and Intuitive Surgical, and is a consultant for Covidien.
As I was writing my introduction to this current edition of the Master Class in Gynecologic Surgery, focusing on minimally invasive surgery for the obese female patient, I was listening to Chuck Todd, host of “Meet the Press.” Instantaneously, television and my thoughts became one; in the last segment of the program, Mr. Todd discussed what he was able to consume for $50 at the Iowa State Fair. I learned that his diet that day consisted of a pork chop on a stick, mac and cheese, a bacon-wrapped corn dog, cheese on a stick with jalapeños, a deep-fried Twinkie, and even fried apple pie with bacon. While Mr. Todd is thin and healthy, the array of foods at the fair reflects our nation’s penchant toward fast food that is fat laden and fried. Though our county is not alone in the world, obesity has reached epidemic proportion in the United States.
According to a May 2015 Department of Health & Human Services report on the health status of the nation, 69% of adults in the United States are overweight and 35% are obese. As a result, the minimally invasive gynecologic surgeon is dealing with an increasing population of women with comorbidities related to their obesity that can confound surgery outcomes. Moreover, anatomic landmarks that the young medical student learns in his or her first anatomy classes are modified due to the size of panniculus and the migration of the umbilicus relative to the bifurcation of the aorta.
I asked Dr. Amina Ahmed to join me in discussing the management of the obese patient undergoing minimally invasive gynecologic surgery. After completing her fellowship in gynecologic oncology, Dr. Ahmed has been on staff at both the University of Iowa Hospitals and Clinics, Iowa City, and Advocate Lutheran General Hospital, Park Ridge, Ill. She will soon join the gynecologic oncology faculty at Rush University Medical Center, Chicago. Given the increased rate of obesity in both Chicago and Iowa, Dr. Ahmed has become an expert in this area in a short period of time.
Dr. Miller is a clinical associate professor at the University of Illinois at Chicago, immediate past president of the International Society for Gynecologic Endoscopy (ISGE), and a past president of the AAGL. He is a reproductive endocrinologist and minimally invasive gynecologic surgeon in private practice in Naperville, Ill., and Schaumburg, Ill.; director of minimally invasive gynecologic surgery and the director of the AAGL/SRS fellowship in minimally invasive gynecologic surgery at Advocate Lutheran General Hospital, Park Ridge, Ill; and the medical editor of this column, Master Class. Dr. Miller disclosed that he is a consultant and on the speakers bureau for Ethicon and Intuitive Surgical, and is a consultant for Covidien.
As I was writing my introduction to this current edition of the Master Class in Gynecologic Surgery, focusing on minimally invasive surgery for the obese female patient, I was listening to Chuck Todd, host of “Meet the Press.” Instantaneously, television and my thoughts became one; in the last segment of the program, Mr. Todd discussed what he was able to consume for $50 at the Iowa State Fair. I learned that his diet that day consisted of a pork chop on a stick, mac and cheese, a bacon-wrapped corn dog, cheese on a stick with jalapeños, a deep-fried Twinkie, and even fried apple pie with bacon. While Mr. Todd is thin and healthy, the array of foods at the fair reflects our nation’s penchant toward fast food that is fat laden and fried. Though our county is not alone in the world, obesity has reached epidemic proportion in the United States.
According to a May 2015 Department of Health & Human Services report on the health status of the nation, 69% of adults in the United States are overweight and 35% are obese. As a result, the minimally invasive gynecologic surgeon is dealing with an increasing population of women with comorbidities related to their obesity that can confound surgery outcomes. Moreover, anatomic landmarks that the young medical student learns in his or her first anatomy classes are modified due to the size of panniculus and the migration of the umbilicus relative to the bifurcation of the aorta.
I asked Dr. Amina Ahmed to join me in discussing the management of the obese patient undergoing minimally invasive gynecologic surgery. After completing her fellowship in gynecologic oncology, Dr. Ahmed has been on staff at both the University of Iowa Hospitals and Clinics, Iowa City, and Advocate Lutheran General Hospital, Park Ridge, Ill. She will soon join the gynecologic oncology faculty at Rush University Medical Center, Chicago. Given the increased rate of obesity in both Chicago and Iowa, Dr. Ahmed has become an expert in this area in a short period of time.
Dr. Miller is a clinical associate professor at the University of Illinois at Chicago, immediate past president of the International Society for Gynecologic Endoscopy (ISGE), and a past president of the AAGL. He is a reproductive endocrinologist and minimally invasive gynecologic surgeon in private practice in Naperville, Ill., and Schaumburg, Ill.; director of minimally invasive gynecologic surgery and the director of the AAGL/SRS fellowship in minimally invasive gynecologic surgery at Advocate Lutheran General Hospital, Park Ridge, Ill; and the medical editor of this column, Master Class. Dr. Miller disclosed that he is a consultant and on the speakers bureau for Ethicon and Intuitive Surgical, and is a consultant for Covidien.
Putting isthmocele into perspective
With the increase in cesarean sections worldwide, it is imperative that physicians properly inform their patients as to potential procedure risks. One potential postcesarean section problem that is receiving increasing attention is the isthmocele or niche.
Defined as an anechoic area in the cesarean section scar, it has been noted to occur in 24%-69% of women undergoing transvaginal sonography, and 56%-78% of women evaluated with transvaginal saline infused sonogram. While most cesarean section defects are asymptomatic, the isthmocele has been noted to be associated with abnormal uterine bleeding, including prolonged menstruation or postmenopausal spotting, and fertility concerns (BJOG. 2014;121:145-56).
Interestingly, it has been 40 years since Stewart, et al. first reported the relationship of abnormal uterine bleeding and cesarean section (Br. J. Gynaecol. 1975;82:682-6). Bloody fluid can be generated at the isthmocele site, which travels up the endometrial canal, thus impacting implantation. The niche can also be the site of ectopic pregnancy implantation.
In this edition of Master Class in gynecologic surgery, I have asked my newest partner, Dr. Kirsten Sasaki, to share our views on this increasingly important subject. Dr. Sasaki completed her internship and residency at Tufts Medical Center, Boston, where she was awarded the Outstanding Chief Resident Clinician Award. Dr. Sasaki then went on to become our second fellow in the Fellowship in Minimally Invasive Gynecologic Surgery in affiliation with AAGL and SRS at Advocate Lutheran General Hospital, Park Ridge, Ill. Once again, Dr. Sasaki was singled out for her excellent teaching and research capabilities. Ultimately however, it was her tremendous surgical skills and surgical sense that led Dr. Aarathi Cholkeri-Singh and I to invite her into our practice.
Dr. Miller is clinical associate professor at the University of Illinois at Chicago, immediate past president of the International Society for Gynecologic Endoscopy (ISGE), and a past president of the AAGL. He is a reproductive endocrinologist and minimally invasive gynecologic surgeon in private practice in Naperville, Ill., and Schaumburg, Ill.; the director of minimally invasive gynecologic surgery and the director of the AAGL/SRS fellowship in minimally invasive gynecologic surgery at Advocate Lutheran General Hospital, Park Ridge, Ill.; and the medical editor of this column, Master Class. Dr. Miller is a consultant and on the speakers bureau for Ethicon. He is also a consultant, on the speakers bureau, and has received grant and research support from Intuitive Surgical.
With the increase in cesarean sections worldwide, it is imperative that physicians properly inform their patients as to potential procedure risks. One potential postcesarean section problem that is receiving increasing attention is the isthmocele or niche.
Defined as an anechoic area in the cesarean section scar, it has been noted to occur in 24%-69% of women undergoing transvaginal sonography, and 56%-78% of women evaluated with transvaginal saline infused sonogram. While most cesarean section defects are asymptomatic, the isthmocele has been noted to be associated with abnormal uterine bleeding, including prolonged menstruation or postmenopausal spotting, and fertility concerns (BJOG. 2014;121:145-56).
Interestingly, it has been 40 years since Stewart, et al. first reported the relationship of abnormal uterine bleeding and cesarean section (Br. J. Gynaecol. 1975;82:682-6). Bloody fluid can be generated at the isthmocele site, which travels up the endometrial canal, thus impacting implantation. The niche can also be the site of ectopic pregnancy implantation.
In this edition of Master Class in gynecologic surgery, I have asked my newest partner, Dr. Kirsten Sasaki, to share our views on this increasingly important subject. Dr. Sasaki completed her internship and residency at Tufts Medical Center, Boston, where she was awarded the Outstanding Chief Resident Clinician Award. Dr. Sasaki then went on to become our second fellow in the Fellowship in Minimally Invasive Gynecologic Surgery in affiliation with AAGL and SRS at Advocate Lutheran General Hospital, Park Ridge, Ill. Once again, Dr. Sasaki was singled out for her excellent teaching and research capabilities. Ultimately however, it was her tremendous surgical skills and surgical sense that led Dr. Aarathi Cholkeri-Singh and I to invite her into our practice.
Dr. Miller is clinical associate professor at the University of Illinois at Chicago, immediate past president of the International Society for Gynecologic Endoscopy (ISGE), and a past president of the AAGL. He is a reproductive endocrinologist and minimally invasive gynecologic surgeon in private practice in Naperville, Ill., and Schaumburg, Ill.; the director of minimally invasive gynecologic surgery and the director of the AAGL/SRS fellowship in minimally invasive gynecologic surgery at Advocate Lutheran General Hospital, Park Ridge, Ill.; and the medical editor of this column, Master Class. Dr. Miller is a consultant and on the speakers bureau for Ethicon. He is also a consultant, on the speakers bureau, and has received grant and research support from Intuitive Surgical.
With the increase in cesarean sections worldwide, it is imperative that physicians properly inform their patients as to potential procedure risks. One potential postcesarean section problem that is receiving increasing attention is the isthmocele or niche.
Defined as an anechoic area in the cesarean section scar, it has been noted to occur in 24%-69% of women undergoing transvaginal sonography, and 56%-78% of women evaluated with transvaginal saline infused sonogram. While most cesarean section defects are asymptomatic, the isthmocele has been noted to be associated with abnormal uterine bleeding, including prolonged menstruation or postmenopausal spotting, and fertility concerns (BJOG. 2014;121:145-56).
Interestingly, it has been 40 years since Stewart, et al. first reported the relationship of abnormal uterine bleeding and cesarean section (Br. J. Gynaecol. 1975;82:682-6). Bloody fluid can be generated at the isthmocele site, which travels up the endometrial canal, thus impacting implantation. The niche can also be the site of ectopic pregnancy implantation.
In this edition of Master Class in gynecologic surgery, I have asked my newest partner, Dr. Kirsten Sasaki, to share our views on this increasingly important subject. Dr. Sasaki completed her internship and residency at Tufts Medical Center, Boston, where she was awarded the Outstanding Chief Resident Clinician Award. Dr. Sasaki then went on to become our second fellow in the Fellowship in Minimally Invasive Gynecologic Surgery in affiliation with AAGL and SRS at Advocate Lutheran General Hospital, Park Ridge, Ill. Once again, Dr. Sasaki was singled out for her excellent teaching and research capabilities. Ultimately however, it was her tremendous surgical skills and surgical sense that led Dr. Aarathi Cholkeri-Singh and I to invite her into our practice.
Dr. Miller is clinical associate professor at the University of Illinois at Chicago, immediate past president of the International Society for Gynecologic Endoscopy (ISGE), and a past president of the AAGL. He is a reproductive endocrinologist and minimally invasive gynecologic surgeon in private practice in Naperville, Ill., and Schaumburg, Ill.; the director of minimally invasive gynecologic surgery and the director of the AAGL/SRS fellowship in minimally invasive gynecologic surgery at Advocate Lutheran General Hospital, Park Ridge, Ill.; and the medical editor of this column, Master Class. Dr. Miller is a consultant and on the speakers bureau for Ethicon. He is also a consultant, on the speakers bureau, and has received grant and research support from Intuitive Surgical.