User login
For skin rejuvenation, pick the right light-based technique
LAS VEGAS – Technologies to address the many skin changes associated with photoaging run the gamut from nonablative to ablative – and choosing the right technology for a given patient is a balancing act, according to Dr. Mathew Avram.
“Basically, it’s a trade-off between increased efficacy and increased side effects and downtime,” Dr. Avram said at the Skin Disease Education Foundation’s annual Las Vegas dermatology seminar.
Dr. Avram, director of the Massachusetts General Hospital dermatology, laser, and cosmetic center, Boston, said that traditional resurfacing remains the preferred method for repairing photodamaged skin. “One treatment provides reproducible, excellent results,” but at the cost of a significant amount of wound care and prolonged downtime. Potential adverse effects also include clear lines of demarcation with improper feathering, prolonged erythema, permanent hypopigmentation, infection, and scarring, he added.
For these reasons, the popularity of traditional resurfacing to treat photodamaged skin has declined over time as nonablative and intermediate techniques have arisen.
Intense pulsed light (IPL) is a nonlaser, nonablative technique that can produce overall skin rejuvenation, a “side effect” that patients often notice after they receive IPL for vascular lesions or hyperpigmentation. The benefits tend to be modest, even after several treatments, so this is not usually performed as a standalone technique for skin laxity or rhytides.
Nonablative fractional lasers act by creating thousands of microscopic wounds that are “columns of thermal coagulation,” leaving an intact stratum corneum, said Dr. Avram, who is also faculty director for laser and cosmetic training, Harvard Medical School, Boston.
The most common reason for treatment with a nonablative laser is mild to moderate wrinkles, though it can also be used for various pigmentation issues, as well as acne, surgical, and burn scars.
Although side effects are typically minor, the devices can be used off face, and there’s no worry about lines of demarcation; improvement is also more modest and will happen only with multiple treatments. “Fractional treatments give you fractional results,” Dr. Avram commented.
“You want to think about your treatment in terms of the pathology of what you’re treating,” he added, noting that fractional resurfacing can target both epidermal and dermal conditions. Examples of epidermal conditions that benefit from superficial treatment include dermatoheliosis, lentigines, and melasma.
Acne scars and deep wrinkles should receive deeper treatment in order to create thermal damage to the dermis proper, which, over a period of months, will trigger collagen remodeling and new collagen formation. Pulse energy will determine the depth and width of the zone of coagulation in the skin, so higher energy should be used with deeper skin pathology, Dr. Avram said.
Dermatologists also need to consider density of treatment – what percent of the skin is treated. “These devices go from about 5% to about 48% of skin coverage,” and increased density is really what increases the intensity of treatment, he noted. High-density treatment will result in more redness and swelling and has a greater potential for hyperpigmentation, but will not necessarily yield increased benefit in treatment outcomes, he added.
Pretreatment considerations should include assessing the patient’s expectations and availability for downtime post procedure. Treatment planning should also consider the patient’s skin type and probability for sun exposure. Patients should know they will see only partial improvement in wrinkles, scars, and pigmentation, and should expect some discomfort during the procedure and some side effects and healing time post procedure. Patients with a history of herpes labialis should receive valacyclovir 500 mg twice daily, beginning the day before treatment and continuing for 5-7 days, Dr. Avram said.
Cold-air cooling makes the treatment both safer and more comfortable, he pointed out. Topical compounded anesthesia including higher percentages of lidocaine and tetracaine can also be effective. Though local injected anesthesia can be effective in focal treatment of scars, the injection should be deep, and treatment should not begin immediately. This allows the lidocaine to disperse, minimizing the risk of ulceration from the thermal instability that a depot of lidocaine could cause, he said.
Fractional lasers can also be effective for treating background dermatoheliosis, as opposed to targeted treatment of individual lentigines. “Fractional is going to be great for clearing background damage,” Dr. Avram said.
A hybrid technique called ablative fractional photothermolysis creates tiny columns of ablation, while leaving the surrounding untreated tissue available as a reservoir for rapid healing. This technique will improve the more severe wrinkles that nonablative treatments don’t help, with less downtime than a fully ablative technique. Interestingly, patients have less postprocedure pain with this technique than with nonablative techniques, probably because the ablated channels offer a way for heat to escape the skin, he said
Dr. Avram disclosed that he is a paid consultant or has received honoraria from Invasix, Zeltiq Aesthetics, Galderma, and Kythera Biopharmaceuticals; that he is on the scientific advisory board of Cytrellis and Zeltiq; that he has intellectual property and royalty interests with Cytrellis; and that he has received research equipment from Cutera.
SDEF and this news organization are owned by the same parent company.
On Twitter @karioakes
LAS VEGAS – Technologies to address the many skin changes associated with photoaging run the gamut from nonablative to ablative – and choosing the right technology for a given patient is a balancing act, according to Dr. Mathew Avram.
“Basically, it’s a trade-off between increased efficacy and increased side effects and downtime,” Dr. Avram said at the Skin Disease Education Foundation’s annual Las Vegas dermatology seminar.
Dr. Avram, director of the Massachusetts General Hospital dermatology, laser, and cosmetic center, Boston, said that traditional resurfacing remains the preferred method for repairing photodamaged skin. “One treatment provides reproducible, excellent results,” but at the cost of a significant amount of wound care and prolonged downtime. Potential adverse effects also include clear lines of demarcation with improper feathering, prolonged erythema, permanent hypopigmentation, infection, and scarring, he added.
For these reasons, the popularity of traditional resurfacing to treat photodamaged skin has declined over time as nonablative and intermediate techniques have arisen.
Intense pulsed light (IPL) is a nonlaser, nonablative technique that can produce overall skin rejuvenation, a “side effect” that patients often notice after they receive IPL for vascular lesions or hyperpigmentation. The benefits tend to be modest, even after several treatments, so this is not usually performed as a standalone technique for skin laxity or rhytides.
Nonablative fractional lasers act by creating thousands of microscopic wounds that are “columns of thermal coagulation,” leaving an intact stratum corneum, said Dr. Avram, who is also faculty director for laser and cosmetic training, Harvard Medical School, Boston.
The most common reason for treatment with a nonablative laser is mild to moderate wrinkles, though it can also be used for various pigmentation issues, as well as acne, surgical, and burn scars.
Although side effects are typically minor, the devices can be used off face, and there’s no worry about lines of demarcation; improvement is also more modest and will happen only with multiple treatments. “Fractional treatments give you fractional results,” Dr. Avram commented.
“You want to think about your treatment in terms of the pathology of what you’re treating,” he added, noting that fractional resurfacing can target both epidermal and dermal conditions. Examples of epidermal conditions that benefit from superficial treatment include dermatoheliosis, lentigines, and melasma.
Acne scars and deep wrinkles should receive deeper treatment in order to create thermal damage to the dermis proper, which, over a period of months, will trigger collagen remodeling and new collagen formation. Pulse energy will determine the depth and width of the zone of coagulation in the skin, so higher energy should be used with deeper skin pathology, Dr. Avram said.
Dermatologists also need to consider density of treatment – what percent of the skin is treated. “These devices go from about 5% to about 48% of skin coverage,” and increased density is really what increases the intensity of treatment, he noted. High-density treatment will result in more redness and swelling and has a greater potential for hyperpigmentation, but will not necessarily yield increased benefit in treatment outcomes, he added.
Pretreatment considerations should include assessing the patient’s expectations and availability for downtime post procedure. Treatment planning should also consider the patient’s skin type and probability for sun exposure. Patients should know they will see only partial improvement in wrinkles, scars, and pigmentation, and should expect some discomfort during the procedure and some side effects and healing time post procedure. Patients with a history of herpes labialis should receive valacyclovir 500 mg twice daily, beginning the day before treatment and continuing for 5-7 days, Dr. Avram said.
Cold-air cooling makes the treatment both safer and more comfortable, he pointed out. Topical compounded anesthesia including higher percentages of lidocaine and tetracaine can also be effective. Though local injected anesthesia can be effective in focal treatment of scars, the injection should be deep, and treatment should not begin immediately. This allows the lidocaine to disperse, minimizing the risk of ulceration from the thermal instability that a depot of lidocaine could cause, he said.
Fractional lasers can also be effective for treating background dermatoheliosis, as opposed to targeted treatment of individual lentigines. “Fractional is going to be great for clearing background damage,” Dr. Avram said.
A hybrid technique called ablative fractional photothermolysis creates tiny columns of ablation, while leaving the surrounding untreated tissue available as a reservoir for rapid healing. This technique will improve the more severe wrinkles that nonablative treatments don’t help, with less downtime than a fully ablative technique. Interestingly, patients have less postprocedure pain with this technique than with nonablative techniques, probably because the ablated channels offer a way for heat to escape the skin, he said
Dr. Avram disclosed that he is a paid consultant or has received honoraria from Invasix, Zeltiq Aesthetics, Galderma, and Kythera Biopharmaceuticals; that he is on the scientific advisory board of Cytrellis and Zeltiq; that he has intellectual property and royalty interests with Cytrellis; and that he has received research equipment from Cutera.
SDEF and this news organization are owned by the same parent company.
On Twitter @karioakes
LAS VEGAS – Technologies to address the many skin changes associated with photoaging run the gamut from nonablative to ablative – and choosing the right technology for a given patient is a balancing act, according to Dr. Mathew Avram.
“Basically, it’s a trade-off between increased efficacy and increased side effects and downtime,” Dr. Avram said at the Skin Disease Education Foundation’s annual Las Vegas dermatology seminar.
Dr. Avram, director of the Massachusetts General Hospital dermatology, laser, and cosmetic center, Boston, said that traditional resurfacing remains the preferred method for repairing photodamaged skin. “One treatment provides reproducible, excellent results,” but at the cost of a significant amount of wound care and prolonged downtime. Potential adverse effects also include clear lines of demarcation with improper feathering, prolonged erythema, permanent hypopigmentation, infection, and scarring, he added.
For these reasons, the popularity of traditional resurfacing to treat photodamaged skin has declined over time as nonablative and intermediate techniques have arisen.
Intense pulsed light (IPL) is a nonlaser, nonablative technique that can produce overall skin rejuvenation, a “side effect” that patients often notice after they receive IPL for vascular lesions or hyperpigmentation. The benefits tend to be modest, even after several treatments, so this is not usually performed as a standalone technique for skin laxity or rhytides.
Nonablative fractional lasers act by creating thousands of microscopic wounds that are “columns of thermal coagulation,” leaving an intact stratum corneum, said Dr. Avram, who is also faculty director for laser and cosmetic training, Harvard Medical School, Boston.
The most common reason for treatment with a nonablative laser is mild to moderate wrinkles, though it can also be used for various pigmentation issues, as well as acne, surgical, and burn scars.
Although side effects are typically minor, the devices can be used off face, and there’s no worry about lines of demarcation; improvement is also more modest and will happen only with multiple treatments. “Fractional treatments give you fractional results,” Dr. Avram commented.
“You want to think about your treatment in terms of the pathology of what you’re treating,” he added, noting that fractional resurfacing can target both epidermal and dermal conditions. Examples of epidermal conditions that benefit from superficial treatment include dermatoheliosis, lentigines, and melasma.
Acne scars and deep wrinkles should receive deeper treatment in order to create thermal damage to the dermis proper, which, over a period of months, will trigger collagen remodeling and new collagen formation. Pulse energy will determine the depth and width of the zone of coagulation in the skin, so higher energy should be used with deeper skin pathology, Dr. Avram said.
Dermatologists also need to consider density of treatment – what percent of the skin is treated. “These devices go from about 5% to about 48% of skin coverage,” and increased density is really what increases the intensity of treatment, he noted. High-density treatment will result in more redness and swelling and has a greater potential for hyperpigmentation, but will not necessarily yield increased benefit in treatment outcomes, he added.
Pretreatment considerations should include assessing the patient’s expectations and availability for downtime post procedure. Treatment planning should also consider the patient’s skin type and probability for sun exposure. Patients should know they will see only partial improvement in wrinkles, scars, and pigmentation, and should expect some discomfort during the procedure and some side effects and healing time post procedure. Patients with a history of herpes labialis should receive valacyclovir 500 mg twice daily, beginning the day before treatment and continuing for 5-7 days, Dr. Avram said.
Cold-air cooling makes the treatment both safer and more comfortable, he pointed out. Topical compounded anesthesia including higher percentages of lidocaine and tetracaine can also be effective. Though local injected anesthesia can be effective in focal treatment of scars, the injection should be deep, and treatment should not begin immediately. This allows the lidocaine to disperse, minimizing the risk of ulceration from the thermal instability that a depot of lidocaine could cause, he said.
Fractional lasers can also be effective for treating background dermatoheliosis, as opposed to targeted treatment of individual lentigines. “Fractional is going to be great for clearing background damage,” Dr. Avram said.
A hybrid technique called ablative fractional photothermolysis creates tiny columns of ablation, while leaving the surrounding untreated tissue available as a reservoir for rapid healing. This technique will improve the more severe wrinkles that nonablative treatments don’t help, with less downtime than a fully ablative technique. Interestingly, patients have less postprocedure pain with this technique than with nonablative techniques, probably because the ablated channels offer a way for heat to escape the skin, he said
Dr. Avram disclosed that he is a paid consultant or has received honoraria from Invasix, Zeltiq Aesthetics, Galderma, and Kythera Biopharmaceuticals; that he is on the scientific advisory board of Cytrellis and Zeltiq; that he has intellectual property and royalty interests with Cytrellis; and that he has received research equipment from Cutera.
SDEF and this news organization are owned by the same parent company.
On Twitter @karioakes
EXPERT ANALYSIS FROM SDEF LAS VEGAS DERMATOLOGY SEMINAR
Ixazomib approved for multiple myeloma combo treatment
Ixazomib has been approved by the Food and Drug Administration to be used in a combination treatment for patients with multiple myeloma who have received at least one other treatment for their disease, the agency announced on Nov. 20.
Approval was based on a randomized, double-blind clinical trial of 722 patients with multiple myeloma who had received at least one prior treatment. Compared with patients receiving lenalidomide, dexamethasone, and placebo, those receiving lenalidomide, dexamethasone, and ixazomib had a statistically significant improvement in progression-free survival (PFS). The median PFS for the ixazomib arm was 20.6 months, compared with 14.7 months for the placebo, lenalidomide, and dexamethasone arm.
Common adverse reactions occurring more often in ixazomib-treated patients included diarrhea, constipation, thrombocytopenia, peripheral neuropathy, nausea, peripheral edema, vomiting, and back pain.
Secondary outcome measures are still being evaluated for ixazomib in this ongoing trial. Efficacy measures include overall survival, overall response rate, duration of response and time to progression. Secondary safety and quality of life–related measures include tracking adverse events, lab value abnormalities, as well as performance status and global health status.
Ixazomib, marketed as Ninlaro by Takeda Pharmaceuticals, is the first FDA-approved oral proteasome inhibitor. Proteasome inhibitors as a class take advantage of tumor cells’ reliance on proteasomal clearance of unwanted proteins; ixazomib and other proteasome inhibitors have an antiproliferative effect and promote tumor cell apoptosis. The drug was approved early under the FDA’s priority review program.
“The field of cancer treatment, and multiple myeloma in particular, has never seen a watershed moment like this,” Walter M. Capone, president and CEO of the Multiple Myeloma Research Foundation, said in a statement. He noted that on Nov. 16, the FDA approved daratumumab, the first monoclonal antibody approved to treat multiple myeloma.
Clinical trials are currently underway to investigate the use of ixazomib in patients with residual multiple myeloma, as maintenance therapy following autologous and stem cell transplant procedures for multiple myeloma, and in other cancer types.
On Twitter @karioakes
Ixazomib has been approved by the Food and Drug Administration to be used in a combination treatment for patients with multiple myeloma who have received at least one other treatment for their disease, the agency announced on Nov. 20.
Approval was based on a randomized, double-blind clinical trial of 722 patients with multiple myeloma who had received at least one prior treatment. Compared with patients receiving lenalidomide, dexamethasone, and placebo, those receiving lenalidomide, dexamethasone, and ixazomib had a statistically significant improvement in progression-free survival (PFS). The median PFS for the ixazomib arm was 20.6 months, compared with 14.7 months for the placebo, lenalidomide, and dexamethasone arm.
Common adverse reactions occurring more often in ixazomib-treated patients included diarrhea, constipation, thrombocytopenia, peripheral neuropathy, nausea, peripheral edema, vomiting, and back pain.
Secondary outcome measures are still being evaluated for ixazomib in this ongoing trial. Efficacy measures include overall survival, overall response rate, duration of response and time to progression. Secondary safety and quality of life–related measures include tracking adverse events, lab value abnormalities, as well as performance status and global health status.
Ixazomib, marketed as Ninlaro by Takeda Pharmaceuticals, is the first FDA-approved oral proteasome inhibitor. Proteasome inhibitors as a class take advantage of tumor cells’ reliance on proteasomal clearance of unwanted proteins; ixazomib and other proteasome inhibitors have an antiproliferative effect and promote tumor cell apoptosis. The drug was approved early under the FDA’s priority review program.
“The field of cancer treatment, and multiple myeloma in particular, has never seen a watershed moment like this,” Walter M. Capone, president and CEO of the Multiple Myeloma Research Foundation, said in a statement. He noted that on Nov. 16, the FDA approved daratumumab, the first monoclonal antibody approved to treat multiple myeloma.
Clinical trials are currently underway to investigate the use of ixazomib in patients with residual multiple myeloma, as maintenance therapy following autologous and stem cell transplant procedures for multiple myeloma, and in other cancer types.
On Twitter @karioakes
Ixazomib has been approved by the Food and Drug Administration to be used in a combination treatment for patients with multiple myeloma who have received at least one other treatment for their disease, the agency announced on Nov. 20.
Approval was based on a randomized, double-blind clinical trial of 722 patients with multiple myeloma who had received at least one prior treatment. Compared with patients receiving lenalidomide, dexamethasone, and placebo, those receiving lenalidomide, dexamethasone, and ixazomib had a statistically significant improvement in progression-free survival (PFS). The median PFS for the ixazomib arm was 20.6 months, compared with 14.7 months for the placebo, lenalidomide, and dexamethasone arm.
Common adverse reactions occurring more often in ixazomib-treated patients included diarrhea, constipation, thrombocytopenia, peripheral neuropathy, nausea, peripheral edema, vomiting, and back pain.
Secondary outcome measures are still being evaluated for ixazomib in this ongoing trial. Efficacy measures include overall survival, overall response rate, duration of response and time to progression. Secondary safety and quality of life–related measures include tracking adverse events, lab value abnormalities, as well as performance status and global health status.
Ixazomib, marketed as Ninlaro by Takeda Pharmaceuticals, is the first FDA-approved oral proteasome inhibitor. Proteasome inhibitors as a class take advantage of tumor cells’ reliance on proteasomal clearance of unwanted proteins; ixazomib and other proteasome inhibitors have an antiproliferative effect and promote tumor cell apoptosis. The drug was approved early under the FDA’s priority review program.
“The field of cancer treatment, and multiple myeloma in particular, has never seen a watershed moment like this,” Walter M. Capone, president and CEO of the Multiple Myeloma Research Foundation, said in a statement. He noted that on Nov. 16, the FDA approved daratumumab, the first monoclonal antibody approved to treat multiple myeloma.
Clinical trials are currently underway to investigate the use of ixazomib in patients with residual multiple myeloma, as maintenance therapy following autologous and stem cell transplant procedures for multiple myeloma, and in other cancer types.
On Twitter @karioakes
Common sense drives risk factor screening for systemic psoriasis therapy
LAS VEGAS – For a physician starting a patient on a systemic agent to treat psoriasis, deciding on risk factor screening and management strategies can be confusing. Who gets checked for what? Why? How often?
With a dearth of evidence to guide them, physicians should use what they know about psoriasis itself, as well as patient risk factors and the inherent risks of a given systemic therapy to guide screening, according to Dr. Kristina Callis Duffin of the department of dermatology, University of Utah, Salt Lake City.
Speaking at the Skin Disease Education Foundation’s annual Las Vegas dermatology seminar, Dr. Duffin said that in her practice, this means that all patients being considered for systemic therapy get baseline labs as recommended by the drug manufacturer, and risk-focused monitoring for cardiovascular disease, infection, and cancer. Basically, she noted, the strategy is, “How can we be effective at monitoring and surveying for those ‘bad guys’– those complications of therapy?”
“Some of this is really about using common sense and being a good physician,” she added.
To obtain a pertinent history, it’s worthwhile to formulate a directed intake questionnaire, Dr. Duffin said. Screening items should include history of recurrent infections; hepatitis, HIV, and TB status and risk; travel history; cardiovascular history, including lipid status; history of any liver or kidney problems; history and risk of diabetes mellitus; depression; personal or family history of multiple sclerosis; and history of cancer, including skin cancer, and blood disorders.
The social history should include pregnancy status and birth control method for women (as appropriate); and alcohol use for all patients. Patients should be current with age-appropriate recommended immunizations and cancer screenings.
Obtaining baseline lab tests for all patients before they start treatment with a systemic agent affords the opportunity to detect common metabolic abnormalities, such as diabetes, liver or kidney disease, and dyslipidemia, as well as rare but serious blood abnormalities, Dr. Duffin said. This approach respects the fact that “there is no question that cardiovascular risk factors are overrepresented in the moderate to severe psoriasis population.”
Published TB screening guidelines have universally recommended screening before initiating a biologic agent with a tuberculin skin test, unless the patient has had the BCG vaccine or is immunosuppressed. From there, interpretation of the tine test and further screening requires knowing the patient: “It’s all about pretest probability,” said Dr. Duffin, adding that physicians should not be afraid to use infectious disease consultants to help them sort out tricky cases.
In terms of ongoing management, communication and coordination are key. Continue to ask patients about symptoms of psoriatic arthritis, and make sure that you work with the patient’s primary care provider so that cardiovascular risk management, malignancy screening, and immunizations don’t fall through the cracks, she advised.
“Have a high index of suspicion for infection,” including deep fungal infections, she added. Though the literature does not report frequent cases of serious fungal infections, “there’s no question cases are unreported or numbers can be unclear in the registries.” Patients should know early signs of infection and know to seek care right away.
Patients should know to defer live vaccines, and the physician managing the care of psoriasis should be included in the planning and management process for any type of surgery, to oversee biologic administration. While probably slight, the risk of infection should be weighed against the risk of loss of efficacy in psoriasis or psoriatic arthritis treatment, Dr. Duffin said.
The baseline risk of solid tumors is elevated in patients with psoriasis, but “is not definitively elevated in patients on biologics,” she commented. The risk of nonmelanoma skin cancer, however, is definitely elevated for patients on biologic therapies, so an annual skin survey is a must for these patients, she said.
“Should we be discussing immunizations with our patients? Absolutely,”she added. All patients need the influenza vaccine and the pneumococcal vaccine, and for live virus immunizations, the dermatologist should coordinate administration with the primary care physician.
Managing these risks effectively can make a big difference in quality of life for patients who are able to start and stay on systemic therapy, said Dr. Duffin. The many risks of not treating psoriasis appropriately include not only the significant psychosocial impact, but also progression of psoriatic arthritis, progression of skin disease, and increased risk of heart disease and other cardiovascular comorbidities. “Our job is to treat to patient satisfaction and the best possible quality of life. A lot of this is about anticipation and communication,” she said.
Dr. Duffin reported receiving research support from and consulting for Amgen, Eli Lilly, Janssen, Stiefel, AbbVie, Bristol-Myers Squibb, Celgene, Novartis, and XenoPort; consulting and being on the scientific advisory board for Pfizer; and being on the scientific advisory board for Novartis, Eli Lilly, Janssen, Celgene, and XenoPort.
SDEF and this news organization are owned by the same parent company.
On Twitter @karioakes
LAS VEGAS – For a physician starting a patient on a systemic agent to treat psoriasis, deciding on risk factor screening and management strategies can be confusing. Who gets checked for what? Why? How often?
With a dearth of evidence to guide them, physicians should use what they know about psoriasis itself, as well as patient risk factors and the inherent risks of a given systemic therapy to guide screening, according to Dr. Kristina Callis Duffin of the department of dermatology, University of Utah, Salt Lake City.
Speaking at the Skin Disease Education Foundation’s annual Las Vegas dermatology seminar, Dr. Duffin said that in her practice, this means that all patients being considered for systemic therapy get baseline labs as recommended by the drug manufacturer, and risk-focused monitoring for cardiovascular disease, infection, and cancer. Basically, she noted, the strategy is, “How can we be effective at monitoring and surveying for those ‘bad guys’– those complications of therapy?”
“Some of this is really about using common sense and being a good physician,” she added.
To obtain a pertinent history, it’s worthwhile to formulate a directed intake questionnaire, Dr. Duffin said. Screening items should include history of recurrent infections; hepatitis, HIV, and TB status and risk; travel history; cardiovascular history, including lipid status; history of any liver or kidney problems; history and risk of diabetes mellitus; depression; personal or family history of multiple sclerosis; and history of cancer, including skin cancer, and blood disorders.
The social history should include pregnancy status and birth control method for women (as appropriate); and alcohol use for all patients. Patients should be current with age-appropriate recommended immunizations and cancer screenings.
Obtaining baseline lab tests for all patients before they start treatment with a systemic agent affords the opportunity to detect common metabolic abnormalities, such as diabetes, liver or kidney disease, and dyslipidemia, as well as rare but serious blood abnormalities, Dr. Duffin said. This approach respects the fact that “there is no question that cardiovascular risk factors are overrepresented in the moderate to severe psoriasis population.”
Published TB screening guidelines have universally recommended screening before initiating a biologic agent with a tuberculin skin test, unless the patient has had the BCG vaccine or is immunosuppressed. From there, interpretation of the tine test and further screening requires knowing the patient: “It’s all about pretest probability,” said Dr. Duffin, adding that physicians should not be afraid to use infectious disease consultants to help them sort out tricky cases.
In terms of ongoing management, communication and coordination are key. Continue to ask patients about symptoms of psoriatic arthritis, and make sure that you work with the patient’s primary care provider so that cardiovascular risk management, malignancy screening, and immunizations don’t fall through the cracks, she advised.
“Have a high index of suspicion for infection,” including deep fungal infections, she added. Though the literature does not report frequent cases of serious fungal infections, “there’s no question cases are unreported or numbers can be unclear in the registries.” Patients should know early signs of infection and know to seek care right away.
Patients should know to defer live vaccines, and the physician managing the care of psoriasis should be included in the planning and management process for any type of surgery, to oversee biologic administration. While probably slight, the risk of infection should be weighed against the risk of loss of efficacy in psoriasis or psoriatic arthritis treatment, Dr. Duffin said.
The baseline risk of solid tumors is elevated in patients with psoriasis, but “is not definitively elevated in patients on biologics,” she commented. The risk of nonmelanoma skin cancer, however, is definitely elevated for patients on biologic therapies, so an annual skin survey is a must for these patients, she said.
“Should we be discussing immunizations with our patients? Absolutely,”she added. All patients need the influenza vaccine and the pneumococcal vaccine, and for live virus immunizations, the dermatologist should coordinate administration with the primary care physician.
Managing these risks effectively can make a big difference in quality of life for patients who are able to start and stay on systemic therapy, said Dr. Duffin. The many risks of not treating psoriasis appropriately include not only the significant psychosocial impact, but also progression of psoriatic arthritis, progression of skin disease, and increased risk of heart disease and other cardiovascular comorbidities. “Our job is to treat to patient satisfaction and the best possible quality of life. A lot of this is about anticipation and communication,” she said.
Dr. Duffin reported receiving research support from and consulting for Amgen, Eli Lilly, Janssen, Stiefel, AbbVie, Bristol-Myers Squibb, Celgene, Novartis, and XenoPort; consulting and being on the scientific advisory board for Pfizer; and being on the scientific advisory board for Novartis, Eli Lilly, Janssen, Celgene, and XenoPort.
SDEF and this news organization are owned by the same parent company.
On Twitter @karioakes
LAS VEGAS – For a physician starting a patient on a systemic agent to treat psoriasis, deciding on risk factor screening and management strategies can be confusing. Who gets checked for what? Why? How often?
With a dearth of evidence to guide them, physicians should use what they know about psoriasis itself, as well as patient risk factors and the inherent risks of a given systemic therapy to guide screening, according to Dr. Kristina Callis Duffin of the department of dermatology, University of Utah, Salt Lake City.
Speaking at the Skin Disease Education Foundation’s annual Las Vegas dermatology seminar, Dr. Duffin said that in her practice, this means that all patients being considered for systemic therapy get baseline labs as recommended by the drug manufacturer, and risk-focused monitoring for cardiovascular disease, infection, and cancer. Basically, she noted, the strategy is, “How can we be effective at monitoring and surveying for those ‘bad guys’– those complications of therapy?”
“Some of this is really about using common sense and being a good physician,” she added.
To obtain a pertinent history, it’s worthwhile to formulate a directed intake questionnaire, Dr. Duffin said. Screening items should include history of recurrent infections; hepatitis, HIV, and TB status and risk; travel history; cardiovascular history, including lipid status; history of any liver or kidney problems; history and risk of diabetes mellitus; depression; personal or family history of multiple sclerosis; and history of cancer, including skin cancer, and blood disorders.
The social history should include pregnancy status and birth control method for women (as appropriate); and alcohol use for all patients. Patients should be current with age-appropriate recommended immunizations and cancer screenings.
Obtaining baseline lab tests for all patients before they start treatment with a systemic agent affords the opportunity to detect common metabolic abnormalities, such as diabetes, liver or kidney disease, and dyslipidemia, as well as rare but serious blood abnormalities, Dr. Duffin said. This approach respects the fact that “there is no question that cardiovascular risk factors are overrepresented in the moderate to severe psoriasis population.”
Published TB screening guidelines have universally recommended screening before initiating a biologic agent with a tuberculin skin test, unless the patient has had the BCG vaccine or is immunosuppressed. From there, interpretation of the tine test and further screening requires knowing the patient: “It’s all about pretest probability,” said Dr. Duffin, adding that physicians should not be afraid to use infectious disease consultants to help them sort out tricky cases.
In terms of ongoing management, communication and coordination are key. Continue to ask patients about symptoms of psoriatic arthritis, and make sure that you work with the patient’s primary care provider so that cardiovascular risk management, malignancy screening, and immunizations don’t fall through the cracks, she advised.
“Have a high index of suspicion for infection,” including deep fungal infections, she added. Though the literature does not report frequent cases of serious fungal infections, “there’s no question cases are unreported or numbers can be unclear in the registries.” Patients should know early signs of infection and know to seek care right away.
Patients should know to defer live vaccines, and the physician managing the care of psoriasis should be included in the planning and management process for any type of surgery, to oversee biologic administration. While probably slight, the risk of infection should be weighed against the risk of loss of efficacy in psoriasis or psoriatic arthritis treatment, Dr. Duffin said.
The baseline risk of solid tumors is elevated in patients with psoriasis, but “is not definitively elevated in patients on biologics,” she commented. The risk of nonmelanoma skin cancer, however, is definitely elevated for patients on biologic therapies, so an annual skin survey is a must for these patients, she said.
“Should we be discussing immunizations with our patients? Absolutely,”she added. All patients need the influenza vaccine and the pneumococcal vaccine, and for live virus immunizations, the dermatologist should coordinate administration with the primary care physician.
Managing these risks effectively can make a big difference in quality of life for patients who are able to start and stay on systemic therapy, said Dr. Duffin. The many risks of not treating psoriasis appropriately include not only the significant psychosocial impact, but also progression of psoriatic arthritis, progression of skin disease, and increased risk of heart disease and other cardiovascular comorbidities. “Our job is to treat to patient satisfaction and the best possible quality of life. A lot of this is about anticipation and communication,” she said.
Dr. Duffin reported receiving research support from and consulting for Amgen, Eli Lilly, Janssen, Stiefel, AbbVie, Bristol-Myers Squibb, Celgene, Novartis, and XenoPort; consulting and being on the scientific advisory board for Pfizer; and being on the scientific advisory board for Novartis, Eli Lilly, Janssen, Celgene, and XenoPort.
SDEF and this news organization are owned by the same parent company.
On Twitter @karioakes
EXPERT ANALYSIS FROM SDEF LAS VEGAS DERMATOLOGY SEMINAR
Practical tips help quell pseudofolliculitis barbae
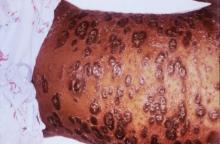
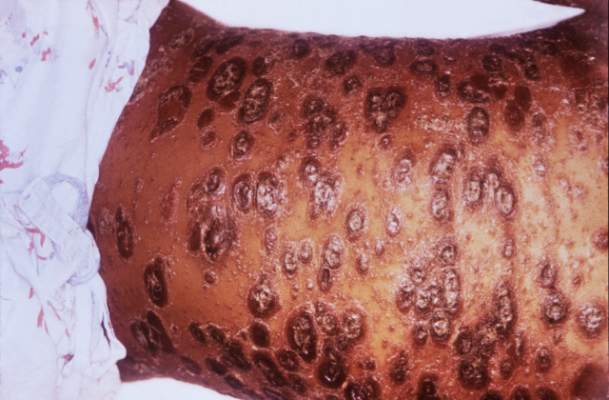
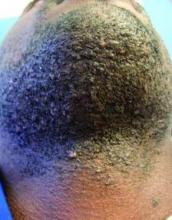
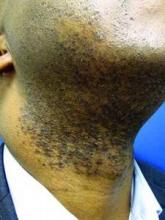
LAS VEGAS – Stubble is okay, and not just because it’s trendy to sport a beard. This was a key message during a presentation on pseudofolliculitis barbae at Skin Disease Education Foundation’s annual Las Vegas dermatology seminar.
Changing up personal grooming habits is an important tactic for men who are plagued with pseudofolliculitis barbae, according to Dr. Andrew F. Alexis, chair of the department of dermatology and director of The Skin of Color Center, Mount Sinai St. Luke’s and Mount Sinai Roosevelt, New York.
This very common skin condition, affecting 45%-83% of men of African ancestry, is best managed by avoiding close shaving and preventing a sharp hair shaft tip. For those who don’t want a full beard for personal or professional reasons, using single blade razors, electric clippers, and even depilatories can help, he said.
All of these techniques prevent curly beard hairs from repenetrating or recurving before emergence – the underpinning of the pathology of pseudofolliculitis barbae. The embedded hairs eventually form a papular or pustular lesion that mimics infectious folliculitis. The inflammatory process can also prompt keloid formation in susceptible individuals.
Providing treatment options is important because the condition can be disfiguring, with such long-term physical sequelae as scarring beard alopecia and postinflammatory hyperpigmentation – changes in appearance that can have a significant psychosocial impact on affected men, Dr. Alexis said.
Therapies are centered around avoiding close shaving and/or preventing a sharp hair shaft tip.
One primary treatment is to stop shaving. “Embedded hairs spontaneously release after about one centimeter of growth,” Dr. Alexis said. This process can take up to 2 months, he said, but military studies dating back to the 1970s showed that the vast majority of pseudofolliculitis barbae cases resolved when service members stopped close shaving practices.
However, many patients want a clean-shaven appearance. “We can work with them to modify their shaving practices. Historically, we have recommended single-blade razors over multiple blade razors” because they shave less closely, he said, pointing out that razor manufacturers have funded studies that challenge this finding.
“Electric clippers are a very good alternative” to razors, Dr. Alexis said. A blade setting that allows at least 0.5-1 mm stubble is desirable.
Chemical depilatories, which act by weakening keratin disulfide bonds, can be effective, since depilated hair does not have a sharp, beveled tip on regrowth and is therefore less likely to repuncture the skin. Patients should be aware, though, that these substances can cause irritant contact dermatitis, he pointed out. Newer formulations are less caustic, but also less efficacious, he said.
In terms of practical tips, shaving technique is important. “Don’t assume the patient knows. There are all sorts of varying techniques out there,” some of which can exacerbate pseudofolliculitis barbae, Dr. Alexis said.
Before shaving, men should wash with a mild cleanser, using a gentle circular technique to free any entrapped hairs, then a moisturizing shaving cream. Razors should be changed every five to seven shaves, and shaving should always be done in the direction of beard growth without pulling on the skin.
Post shave, topical benzoyl peroxide 5%/clindamycin 1% can significantly reduce papules and pustules. Topical retinoids are another effective option. A low-potency steroid can be helpful for inflammatory symptoms.
For cases that just don’t respond to conservative and medical management, laser hair removal is an option. A recent military-funded split-face study found further improvement when topical eflornithine was added to long-pulse Nd:Yag laser therapy, Dr. Alexis said.
Affected individuals may find it difficult to modify shaving practices when uniformed service regulations or office dress codes require men to be close shaven; a note from a physician can be helpful. Dr. Alexis provides patients with a form letter to show their employers, explaining that the patient has a skin disorder that is exacerbated by shaving, and that the patient should be permitted to maintain a well-groomed beard. “I end up writing a lot of these for New York police officers,” he said.
Dr. Alexis disclosed that he has received grants and research support from Allergan and Novartis, and speaker honoraria from Cipla. He has received consulting fees from Aclaris, Allergan, Amgen, Anacor, Bayer, Galderma, Johnson & Johnson, Leo, L’Oreal, Roche, Schick, Suneva, and Valeant.
SDEF and this news organization are owned by the same parent company.
On Twitter @karioakes
LAS VEGAS – Stubble is okay, and not just because it’s trendy to sport a beard. This was a key message during a presentation on pseudofolliculitis barbae at Skin Disease Education Foundation’s annual Las Vegas dermatology seminar.
Changing up personal grooming habits is an important tactic for men who are plagued with pseudofolliculitis barbae, according to Dr. Andrew F. Alexis, chair of the department of dermatology and director of The Skin of Color Center, Mount Sinai St. Luke’s and Mount Sinai Roosevelt, New York.
This very common skin condition, affecting 45%-83% of men of African ancestry, is best managed by avoiding close shaving and preventing a sharp hair shaft tip. For those who don’t want a full beard for personal or professional reasons, using single blade razors, electric clippers, and even depilatories can help, he said.
All of these techniques prevent curly beard hairs from repenetrating or recurving before emergence – the underpinning of the pathology of pseudofolliculitis barbae. The embedded hairs eventually form a papular or pustular lesion that mimics infectious folliculitis. The inflammatory process can also prompt keloid formation in susceptible individuals.
Providing treatment options is important because the condition can be disfiguring, with such long-term physical sequelae as scarring beard alopecia and postinflammatory hyperpigmentation – changes in appearance that can have a significant psychosocial impact on affected men, Dr. Alexis said.
Therapies are centered around avoiding close shaving and/or preventing a sharp hair shaft tip.
One primary treatment is to stop shaving. “Embedded hairs spontaneously release after about one centimeter of growth,” Dr. Alexis said. This process can take up to 2 months, he said, but military studies dating back to the 1970s showed that the vast majority of pseudofolliculitis barbae cases resolved when service members stopped close shaving practices.
However, many patients want a clean-shaven appearance. “We can work with them to modify their shaving practices. Historically, we have recommended single-blade razors over multiple blade razors” because they shave less closely, he said, pointing out that razor manufacturers have funded studies that challenge this finding.
“Electric clippers are a very good alternative” to razors, Dr. Alexis said. A blade setting that allows at least 0.5-1 mm stubble is desirable.
Chemical depilatories, which act by weakening keratin disulfide bonds, can be effective, since depilated hair does not have a sharp, beveled tip on regrowth and is therefore less likely to repuncture the skin. Patients should be aware, though, that these substances can cause irritant contact dermatitis, he pointed out. Newer formulations are less caustic, but also less efficacious, he said.
In terms of practical tips, shaving technique is important. “Don’t assume the patient knows. There are all sorts of varying techniques out there,” some of which can exacerbate pseudofolliculitis barbae, Dr. Alexis said.
Before shaving, men should wash with a mild cleanser, using a gentle circular technique to free any entrapped hairs, then a moisturizing shaving cream. Razors should be changed every five to seven shaves, and shaving should always be done in the direction of beard growth without pulling on the skin.
Post shave, topical benzoyl peroxide 5%/clindamycin 1% can significantly reduce papules and pustules. Topical retinoids are another effective option. A low-potency steroid can be helpful for inflammatory symptoms.
For cases that just don’t respond to conservative and medical management, laser hair removal is an option. A recent military-funded split-face study found further improvement when topical eflornithine was added to long-pulse Nd:Yag laser therapy, Dr. Alexis said.
Affected individuals may find it difficult to modify shaving practices when uniformed service regulations or office dress codes require men to be close shaven; a note from a physician can be helpful. Dr. Alexis provides patients with a form letter to show their employers, explaining that the patient has a skin disorder that is exacerbated by shaving, and that the patient should be permitted to maintain a well-groomed beard. “I end up writing a lot of these for New York police officers,” he said.
Dr. Alexis disclosed that he has received grants and research support from Allergan and Novartis, and speaker honoraria from Cipla. He has received consulting fees from Aclaris, Allergan, Amgen, Anacor, Bayer, Galderma, Johnson & Johnson, Leo, L’Oreal, Roche, Schick, Suneva, and Valeant.
SDEF and this news organization are owned by the same parent company.
On Twitter @karioakes
LAS VEGAS – Stubble is okay, and not just because it’s trendy to sport a beard. This was a key message during a presentation on pseudofolliculitis barbae at Skin Disease Education Foundation’s annual Las Vegas dermatology seminar.
Changing up personal grooming habits is an important tactic for men who are plagued with pseudofolliculitis barbae, according to Dr. Andrew F. Alexis, chair of the department of dermatology and director of The Skin of Color Center, Mount Sinai St. Luke’s and Mount Sinai Roosevelt, New York.
This very common skin condition, affecting 45%-83% of men of African ancestry, is best managed by avoiding close shaving and preventing a sharp hair shaft tip. For those who don’t want a full beard for personal or professional reasons, using single blade razors, electric clippers, and even depilatories can help, he said.
All of these techniques prevent curly beard hairs from repenetrating or recurving before emergence – the underpinning of the pathology of pseudofolliculitis barbae. The embedded hairs eventually form a papular or pustular lesion that mimics infectious folliculitis. The inflammatory process can also prompt keloid formation in susceptible individuals.
Providing treatment options is important because the condition can be disfiguring, with such long-term physical sequelae as scarring beard alopecia and postinflammatory hyperpigmentation – changes in appearance that can have a significant psychosocial impact on affected men, Dr. Alexis said.
Therapies are centered around avoiding close shaving and/or preventing a sharp hair shaft tip.
One primary treatment is to stop shaving. “Embedded hairs spontaneously release after about one centimeter of growth,” Dr. Alexis said. This process can take up to 2 months, he said, but military studies dating back to the 1970s showed that the vast majority of pseudofolliculitis barbae cases resolved when service members stopped close shaving practices.
However, many patients want a clean-shaven appearance. “We can work with them to modify their shaving practices. Historically, we have recommended single-blade razors over multiple blade razors” because they shave less closely, he said, pointing out that razor manufacturers have funded studies that challenge this finding.
“Electric clippers are a very good alternative” to razors, Dr. Alexis said. A blade setting that allows at least 0.5-1 mm stubble is desirable.
Chemical depilatories, which act by weakening keratin disulfide bonds, can be effective, since depilated hair does not have a sharp, beveled tip on regrowth and is therefore less likely to repuncture the skin. Patients should be aware, though, that these substances can cause irritant contact dermatitis, he pointed out. Newer formulations are less caustic, but also less efficacious, he said.
In terms of practical tips, shaving technique is important. “Don’t assume the patient knows. There are all sorts of varying techniques out there,” some of which can exacerbate pseudofolliculitis barbae, Dr. Alexis said.
Before shaving, men should wash with a mild cleanser, using a gentle circular technique to free any entrapped hairs, then a moisturizing shaving cream. Razors should be changed every five to seven shaves, and shaving should always be done in the direction of beard growth without pulling on the skin.
Post shave, topical benzoyl peroxide 5%/clindamycin 1% can significantly reduce papules and pustules. Topical retinoids are another effective option. A low-potency steroid can be helpful for inflammatory symptoms.
For cases that just don’t respond to conservative and medical management, laser hair removal is an option. A recent military-funded split-face study found further improvement when topical eflornithine was added to long-pulse Nd:Yag laser therapy, Dr. Alexis said.
Affected individuals may find it difficult to modify shaving practices when uniformed service regulations or office dress codes require men to be close shaven; a note from a physician can be helpful. Dr. Alexis provides patients with a form letter to show their employers, explaining that the patient has a skin disorder that is exacerbated by shaving, and that the patient should be permitted to maintain a well-groomed beard. “I end up writing a lot of these for New York police officers,” he said.
Dr. Alexis disclosed that he has received grants and research support from Allergan and Novartis, and speaker honoraria from Cipla. He has received consulting fees from Aclaris, Allergan, Amgen, Anacor, Bayer, Galderma, Johnson & Johnson, Leo, L’Oreal, Roche, Schick, Suneva, and Valeant.
SDEF and this news organization are owned by the same parent company.
On Twitter @karioakes
EXPERT ANALYSIS FROM SDEF LAS VEGAS DERMATOLOGY SEMINAR
ASCO: Three-drug combo best for highly emetogenic chemotherapy
Preventing nausea and vomiting from the most emetogenic chemotherapy drugs is best accomplished with a three-drug combination, according to newly updated guidelines from the American Society of Clinical Oncology (ASCO).
The combination should include dexamethasone as well as a 5-hydroxytryptamine-3 (5-HT-3) receptor antagonist and a neurokinin-1 (NK-1) receptor antagonist, said Dr. Paul J. Hesketh and guideline coauthors.
An all-oral regimen that is a treatment option in this setting is the combination of netupitant and palonosetron – NEPA (Akynzeo) – together with dexamethasone, said Dr. Hesketh, an oncologist at Lahey Hospital and Medical Center, Burlington, Mass, and his coauthors.
For this interim update, the guideline committee used clinical trial data to “provide expedited guidance regarding a new agent,” pending a full update of ASCO’s antiemetic guideline (J Clin Oncol. 2015 Nov. 2. doi: 10.1200/JCO.2015.64.3635).
The fixed NEPA combination was approved in October of 2014 by the Food and Drug Administration for treatment of chemotherapy-induced nausea and vomiting (CINV). In clinical trials, NEPA plus dexamethasone was effective for both acute and delayed nausea and vomiting for the majority of individuals receiving moderately or highly emetogenic chemotherapy, and was more effective than just palonosetron combined with dexamethasone.
One phase III trial examined use of NEPA compared with palonosetron alone for individuals with cancer receiving the highly emetogenic combination of anthracycline plus cyclophosphamide. Overall, 74% of the NEPA group vs. 67% of the palonosetron group had a complete response, defined as no vomiting and no need for rescue medications (P less than .001). Another phase III trial found that NEPA’s effectiveness was durable over up to six courses of highly emetogenic chemotherapy, and that the drug was safe over time.
The approved fixed-dose combination of 300 mg of netupitant and 0.5 mg of palonosetron is meant to be taken as a one-capsule dose 1 hour before chemotherapy.
The mechanism of action of NK-1 antagonists, which block substance P from binding to neurokinin, can add a more durable anti-emetic effect and prevent or minimize late-onset CINV. A dose-ranging phase II clinical study of NEPA for CINV in treatment-naive patients receiving cisplatin for solid tumors found that the highest dose of netupitant tested, 300 mg, resulted in the least need for rescue medication, though all doses were significantly more effective in achieving complete response than palonosetron alone, or than ondansetron plus aprepitant.
A real-world consideration for patients and oncologists is that NEPA is an oral medication meant to be taken at home. This means that patients will have to fill – and pay for – NEPA prescriptions. “The out-of-pocket cost will vary by insurance plan, and this point should be discussed with patients,” said Dr. Hesketh and his coauthors.
Full cost analyses are underway; NEPA’s steep cost may be offset, at least in part, by the avoidance of any additional rescue medication for chemotherapy-induced nausea and vomiting.
On Twitter @karioakes
Preventing nausea and vomiting from the most emetogenic chemotherapy drugs is best accomplished with a three-drug combination, according to newly updated guidelines from the American Society of Clinical Oncology (ASCO).
The combination should include dexamethasone as well as a 5-hydroxytryptamine-3 (5-HT-3) receptor antagonist and a neurokinin-1 (NK-1) receptor antagonist, said Dr. Paul J. Hesketh and guideline coauthors.
An all-oral regimen that is a treatment option in this setting is the combination of netupitant and palonosetron – NEPA (Akynzeo) – together with dexamethasone, said Dr. Hesketh, an oncologist at Lahey Hospital and Medical Center, Burlington, Mass, and his coauthors.
For this interim update, the guideline committee used clinical trial data to “provide expedited guidance regarding a new agent,” pending a full update of ASCO’s antiemetic guideline (J Clin Oncol. 2015 Nov. 2. doi: 10.1200/JCO.2015.64.3635).
The fixed NEPA combination was approved in October of 2014 by the Food and Drug Administration for treatment of chemotherapy-induced nausea and vomiting (CINV). In clinical trials, NEPA plus dexamethasone was effective for both acute and delayed nausea and vomiting for the majority of individuals receiving moderately or highly emetogenic chemotherapy, and was more effective than just palonosetron combined with dexamethasone.
One phase III trial examined use of NEPA compared with palonosetron alone for individuals with cancer receiving the highly emetogenic combination of anthracycline plus cyclophosphamide. Overall, 74% of the NEPA group vs. 67% of the palonosetron group had a complete response, defined as no vomiting and no need for rescue medications (P less than .001). Another phase III trial found that NEPA’s effectiveness was durable over up to six courses of highly emetogenic chemotherapy, and that the drug was safe over time.
The approved fixed-dose combination of 300 mg of netupitant and 0.5 mg of palonosetron is meant to be taken as a one-capsule dose 1 hour before chemotherapy.
The mechanism of action of NK-1 antagonists, which block substance P from binding to neurokinin, can add a more durable anti-emetic effect and prevent or minimize late-onset CINV. A dose-ranging phase II clinical study of NEPA for CINV in treatment-naive patients receiving cisplatin for solid tumors found that the highest dose of netupitant tested, 300 mg, resulted in the least need for rescue medication, though all doses were significantly more effective in achieving complete response than palonosetron alone, or than ondansetron plus aprepitant.
A real-world consideration for patients and oncologists is that NEPA is an oral medication meant to be taken at home. This means that patients will have to fill – and pay for – NEPA prescriptions. “The out-of-pocket cost will vary by insurance plan, and this point should be discussed with patients,” said Dr. Hesketh and his coauthors.
Full cost analyses are underway; NEPA’s steep cost may be offset, at least in part, by the avoidance of any additional rescue medication for chemotherapy-induced nausea and vomiting.
On Twitter @karioakes
Preventing nausea and vomiting from the most emetogenic chemotherapy drugs is best accomplished with a three-drug combination, according to newly updated guidelines from the American Society of Clinical Oncology (ASCO).
The combination should include dexamethasone as well as a 5-hydroxytryptamine-3 (5-HT-3) receptor antagonist and a neurokinin-1 (NK-1) receptor antagonist, said Dr. Paul J. Hesketh and guideline coauthors.
An all-oral regimen that is a treatment option in this setting is the combination of netupitant and palonosetron – NEPA (Akynzeo) – together with dexamethasone, said Dr. Hesketh, an oncologist at Lahey Hospital and Medical Center, Burlington, Mass, and his coauthors.
For this interim update, the guideline committee used clinical trial data to “provide expedited guidance regarding a new agent,” pending a full update of ASCO’s antiemetic guideline (J Clin Oncol. 2015 Nov. 2. doi: 10.1200/JCO.2015.64.3635).
The fixed NEPA combination was approved in October of 2014 by the Food and Drug Administration for treatment of chemotherapy-induced nausea and vomiting (CINV). In clinical trials, NEPA plus dexamethasone was effective for both acute and delayed nausea and vomiting for the majority of individuals receiving moderately or highly emetogenic chemotherapy, and was more effective than just palonosetron combined with dexamethasone.
One phase III trial examined use of NEPA compared with palonosetron alone for individuals with cancer receiving the highly emetogenic combination of anthracycline plus cyclophosphamide. Overall, 74% of the NEPA group vs. 67% of the palonosetron group had a complete response, defined as no vomiting and no need for rescue medications (P less than .001). Another phase III trial found that NEPA’s effectiveness was durable over up to six courses of highly emetogenic chemotherapy, and that the drug was safe over time.
The approved fixed-dose combination of 300 mg of netupitant and 0.5 mg of palonosetron is meant to be taken as a one-capsule dose 1 hour before chemotherapy.
The mechanism of action of NK-1 antagonists, which block substance P from binding to neurokinin, can add a more durable anti-emetic effect and prevent or minimize late-onset CINV. A dose-ranging phase II clinical study of NEPA for CINV in treatment-naive patients receiving cisplatin for solid tumors found that the highest dose of netupitant tested, 300 mg, resulted in the least need for rescue medication, though all doses were significantly more effective in achieving complete response than palonosetron alone, or than ondansetron plus aprepitant.
A real-world consideration for patients and oncologists is that NEPA is an oral medication meant to be taken at home. This means that patients will have to fill – and pay for – NEPA prescriptions. “The out-of-pocket cost will vary by insurance plan, and this point should be discussed with patients,” said Dr. Hesketh and his coauthors.
Full cost analyses are underway; NEPA’s steep cost may be offset, at least in part, by the avoidance of any additional rescue medication for chemotherapy-induced nausea and vomiting.
On Twitter @karioakes
FROM THE JOURNAL OF CLINICAL ONCOLOGY
Key clinical point: A neurokinin-1 (NK-1) receptor antagonist, a 5-hydroxytyptamine-3 (5-HT-3) receptor antagonist, and dexamethasone are recommended to prevent nausea and vomiting from the most emetogenic chemotherapies.
Major finding: All patients receiving highly emetogenic chemotherapy should be offered the combination of an NK-1 receptor antagonist, a 5-HT-3 receptor antagonist, and dexamethasone; an all-oral combination of netupitant and palonosetron is a treatment option.
Data source: An interim update of emesis treatment guidelines from the American Society of Clinical Oncologists.
Disclosures: Dr. Hesketh reported no conflicts of interest. Several coauthors reported ties with pharmaceutical companies. No authors reported ties with Eisai or Helsinn Healthcare SA, marketer and license holder, respectively, of Akynzeo.
Cobimetinib approved as add-on to vemurafenib for advanced melanoma
The Food and Drug Administration has approved the once-daily oral MEK inhibitor cobimetinib in combination with vemurafenib to treat patients with advanced melanoma whose tumors express the BRAF V600E or V600K mutation.
“Today’s approval provides a new targeted treatment that, when added to vemurafenib, demonstrates greater benefit than vemurafenib alone in patients with BRAF mutation–positive melanoma,” said Dr. Richard Pazdur, director of the Office of Hematology and Oncology Products in the FDA’s Center for Drug Evaluation and Research, in a press release announcing the approval.
In a pivotal clinical trial, treatment-naive individuals with unresectable, locally advanced or metastatic melanoma whose tumors were positive for BRAF V600E or V600K mutations were randomized to receive the BRAF inhibitor vemurafenib plus placebo (n = 248) or to receive vemurafenib with cobimetinib (n = 247). At the time of data cut-off in May, 2014, median progression-free survival (PFS) was significantly longer for the cobimetinib group (9.9 vs. 6.2 months, hazard ratio, 0.51; P less than .001); PFS was the primary outcome measure.
Of the secondary outcome measures, objective response was seen in two of three patients on cobimetinib, compared with fewer than half of the placebo arm patients (68% vs. 45%; P less than .001). Another secondary outcome measure, overall survival, could not be calculated because too few events had occurred at the end of data collection.
More grade 3 or higher adverse events occurred for patients in the cobimetinib arm than the placebo arm (65% vs. 59%), but the difference was not statistically significant. Patients taking cobimetinib were no more likely than those taking placebo to discontinue taking the study drug.
Side effects most commonly associated with the combination of vemurafenib and cobimetinib included diarrhea, photosensitivity, nausea, fever, and vomiting.
Individuals taking both a BRAF inhibitor and a MEK inhibitor were less likely to develop nonmelanoma secondary skin cancers, a complication that affects about 25% of those taking vemurafenib alone.
Vemurafenib blocks BRAF, part of a molecular signaling pathway implicated in melanoma tumor cell growth and division. Cobimetinib can help delay tumor resistance to vemurafenib by targeting MEK, a gene in the same signaling pathway.
The senior investigator of the pivotal clinical trial, Dr. Antoni Ribas, said in a press release, “Today’s approval is a significant advance in the treatment of metastatic melanoma.
“For patients with a BRAF-mutated melanoma, the combination has higher activity to shrink their tumors, and with less side effects than the drugs on their own,” said Dr. Ribas, a researcher at the University of California, Los Angeles, Jonsson Comprehensive Cancer Center.
The FDA reviewed cobimetinib under its priority review program and gave the drug an orphan drug designation.
Cobimetinib (Cotellic) and vemurafinib (Zelboraf) are both marketed by Genentech.
On Twitter @karioakes
This article was updated November 16, 2015.
The Food and Drug Administration has approved the once-daily oral MEK inhibitor cobimetinib in combination with vemurafenib to treat patients with advanced melanoma whose tumors express the BRAF V600E or V600K mutation.
“Today’s approval provides a new targeted treatment that, when added to vemurafenib, demonstrates greater benefit than vemurafenib alone in patients with BRAF mutation–positive melanoma,” said Dr. Richard Pazdur, director of the Office of Hematology and Oncology Products in the FDA’s Center for Drug Evaluation and Research, in a press release announcing the approval.
In a pivotal clinical trial, treatment-naive individuals with unresectable, locally advanced or metastatic melanoma whose tumors were positive for BRAF V600E or V600K mutations were randomized to receive the BRAF inhibitor vemurafenib plus placebo (n = 248) or to receive vemurafenib with cobimetinib (n = 247). At the time of data cut-off in May, 2014, median progression-free survival (PFS) was significantly longer for the cobimetinib group (9.9 vs. 6.2 months, hazard ratio, 0.51; P less than .001); PFS was the primary outcome measure.
Of the secondary outcome measures, objective response was seen in two of three patients on cobimetinib, compared with fewer than half of the placebo arm patients (68% vs. 45%; P less than .001). Another secondary outcome measure, overall survival, could not be calculated because too few events had occurred at the end of data collection.
More grade 3 or higher adverse events occurred for patients in the cobimetinib arm than the placebo arm (65% vs. 59%), but the difference was not statistically significant. Patients taking cobimetinib were no more likely than those taking placebo to discontinue taking the study drug.
Side effects most commonly associated with the combination of vemurafenib and cobimetinib included diarrhea, photosensitivity, nausea, fever, and vomiting.
Individuals taking both a BRAF inhibitor and a MEK inhibitor were less likely to develop nonmelanoma secondary skin cancers, a complication that affects about 25% of those taking vemurafenib alone.
Vemurafenib blocks BRAF, part of a molecular signaling pathway implicated in melanoma tumor cell growth and division. Cobimetinib can help delay tumor resistance to vemurafenib by targeting MEK, a gene in the same signaling pathway.
The senior investigator of the pivotal clinical trial, Dr. Antoni Ribas, said in a press release, “Today’s approval is a significant advance in the treatment of metastatic melanoma.
“For patients with a BRAF-mutated melanoma, the combination has higher activity to shrink their tumors, and with less side effects than the drugs on their own,” said Dr. Ribas, a researcher at the University of California, Los Angeles, Jonsson Comprehensive Cancer Center.
The FDA reviewed cobimetinib under its priority review program and gave the drug an orphan drug designation.
Cobimetinib (Cotellic) and vemurafinib (Zelboraf) are both marketed by Genentech.
On Twitter @karioakes
This article was updated November 16, 2015.
The Food and Drug Administration has approved the once-daily oral MEK inhibitor cobimetinib in combination with vemurafenib to treat patients with advanced melanoma whose tumors express the BRAF V600E or V600K mutation.
“Today’s approval provides a new targeted treatment that, when added to vemurafenib, demonstrates greater benefit than vemurafenib alone in patients with BRAF mutation–positive melanoma,” said Dr. Richard Pazdur, director of the Office of Hematology and Oncology Products in the FDA’s Center for Drug Evaluation and Research, in a press release announcing the approval.
In a pivotal clinical trial, treatment-naive individuals with unresectable, locally advanced or metastatic melanoma whose tumors were positive for BRAF V600E or V600K mutations were randomized to receive the BRAF inhibitor vemurafenib plus placebo (n = 248) or to receive vemurafenib with cobimetinib (n = 247). At the time of data cut-off in May, 2014, median progression-free survival (PFS) was significantly longer for the cobimetinib group (9.9 vs. 6.2 months, hazard ratio, 0.51; P less than .001); PFS was the primary outcome measure.
Of the secondary outcome measures, objective response was seen in two of three patients on cobimetinib, compared with fewer than half of the placebo arm patients (68% vs. 45%; P less than .001). Another secondary outcome measure, overall survival, could not be calculated because too few events had occurred at the end of data collection.
More grade 3 or higher adverse events occurred for patients in the cobimetinib arm than the placebo arm (65% vs. 59%), but the difference was not statistically significant. Patients taking cobimetinib were no more likely than those taking placebo to discontinue taking the study drug.
Side effects most commonly associated with the combination of vemurafenib and cobimetinib included diarrhea, photosensitivity, nausea, fever, and vomiting.
Individuals taking both a BRAF inhibitor and a MEK inhibitor were less likely to develop nonmelanoma secondary skin cancers, a complication that affects about 25% of those taking vemurafenib alone.
Vemurafenib blocks BRAF, part of a molecular signaling pathway implicated in melanoma tumor cell growth and division. Cobimetinib can help delay tumor resistance to vemurafenib by targeting MEK, a gene in the same signaling pathway.
The senior investigator of the pivotal clinical trial, Dr. Antoni Ribas, said in a press release, “Today’s approval is a significant advance in the treatment of metastatic melanoma.
“For patients with a BRAF-mutated melanoma, the combination has higher activity to shrink their tumors, and with less side effects than the drugs on their own,” said Dr. Ribas, a researcher at the University of California, Los Angeles, Jonsson Comprehensive Cancer Center.
The FDA reviewed cobimetinib under its priority review program and gave the drug an orphan drug designation.
Cobimetinib (Cotellic) and vemurafinib (Zelboraf) are both marketed by Genentech.
On Twitter @karioakes
This article was updated November 16, 2015.
Flibanserin debuts with strings attached
Physicians who treat premenopausal women with hypoactive sexual desire disorder now have a Food and Drug Administration–approved option in flibanserin. But the centrally acting daily medication comes with certain conditions: prescribers and dispensing pharmacies are required to complete a certification process, and patients must sign an agreement not to drink alcohol while taking the drug.
How will these restrictions affect real-world practice? The jury’s still out, but some physicians are seeing patients balk at becoming teetotalers to try flibanserin. And while some herald the new drug option to treat women with sexual problems, others remain critical of the drug and the prescriber certification process.
Sprout Pharmaceuticals, which markets flibanserin as Addyi, launched the certification program on Sept. 21. The program involves viewing 13 slides, completing a four-question multiple choice knowledge assessment, and submitting a two-page enrollment form.
Sprout CEO Cindy Whitehead said the company worked closely with the FDA to create a “meaningful” Risk Evaluation and Mitigation Strategy (REMS) program. The program focuses on ensuring physicians understand the safety profile of the drug, she said.
When the FDA approved flibanserin in August 2015, the agency required a REMS program primarily focused around the risk of syncope and hypotension when flibanserin is taken with alcohol. The REMS was required because clinical trials showed that flibanserin has an increased risk for syncope and hypotension with alcohol and moderate or strong CYP3A4 inhibitors, which include proton pump inhibitors, selective serotonin reuptake inhibitors, benzodiazepines, and antifungals.
“So far, the response from health care providers and physicians continues to grow every day,” Ms. Whitehead said. “Thousands of prescribers and pharmacies have completed the certification process which continues to show the need for physicians to have a medical treatment option in their toolkit.”
Dr. Patrick J. Woodman has completed the REMS process for flibanserin but has yet to prescribe the drug. He estimated that the entire certification process took him less than a half hour.
The FDA “is being cautious in general” by instituting the REMS program, said Dr. Woodman, a urogynecologist and clinical professor at Michigan State University in East Lansing. He noted that the clinical trials involving flibanserin use had participants drinking a considerable amount of alcohol over a short time period. “It was interesting to see that the side effects of flibanserin with alcohol are dose related,” he said.
For some women, recognition of the social lubricant effect of a glass of wine may offset their desire to try flibanserin, Dr. Woodman said. The REMS program requires patients to sign an agreement stating that “I understand that I must not drink alcohol while taking Addyi.”
“I haven’t had a lot of women beating down my door about it,” Dr. Woodman said. However, “for a select population of women who have not had success with other approaches, I think this is a good option.”
Dr. Lisa Larkin, an internist and director of the University of Cincinnati Women’s Center, also treats women with sexual problems. She said she’s happy to have an approved drug to add to the discussion of treatment options. Before flibanserin’s approval, the only pharmaceutical options for low female sexual desire involved off-label use, said Dr. Larkin, who is scientific cochair of Even the Score, a coalition that advocates for more options in female sexual dysfunction treatment.
But Dr. Larkin said she’s been very clear with patients that “while we try flibanserin, there is no alcohol.”
Her approach is to advise patients to try flibanserin for a period of 8-12 weeks and then evaluate if the medication is working for them and if the lifestyle trade-off is worthwhile. The notion of complete abstinence from alcohol has been off-putting for patients, even those who are not particularly heavy drinkers, she said. So far, none of her patients have opted to try flibanserin so she has yet to prescribe it.
Another gynecologist puts it this way on social media: “Want to take flibanserin (Addyi) for low sex drive? You can’t drink alcohol. Ever.” On her personal blog, Dr. Jen Gunter, an ob.gyn. and pain medicine physician, also faults the REMS program both for its brevity and a lack of detail.
“The REMS did not mention how to screen, or limiting prescribing for 8 weeks to assess efficacy, or interactions with other drugs,” Dr. Gunter wrote.
Leonore Tiefer, Ph.D., of the department of psychiatry at New York University and a sexologist is also critical of flibanserin’s approval and of the REMS process.
“The training doesn’t even ask the prescriber to talk with the patient about sexuality,” said Dr. Tiefer, pointing out that there are many effective, nonpharmaceutical interventions for sexual problems. “The general assumption is that everybody knows enough about sex.”
But Dr. Tiefer said that in her experience women need more information on the importance of communication, foreplay, and relaxation in a satisfying sex life. “If these things are common sense, then it’s common sense that nobody uses.”
The flibanserin certification materials are available online at www.Addyi.com. To complete the certification process, prescribers and pharmacists may fax the completed knowledge assessment and enrollment forms to 844-694-3373 or email scanned copies to [email protected].
On Twitter @karioakes
Physicians who treat premenopausal women with hypoactive sexual desire disorder now have a Food and Drug Administration–approved option in flibanserin. But the centrally acting daily medication comes with certain conditions: prescribers and dispensing pharmacies are required to complete a certification process, and patients must sign an agreement not to drink alcohol while taking the drug.
How will these restrictions affect real-world practice? The jury’s still out, but some physicians are seeing patients balk at becoming teetotalers to try flibanserin. And while some herald the new drug option to treat women with sexual problems, others remain critical of the drug and the prescriber certification process.
Sprout Pharmaceuticals, which markets flibanserin as Addyi, launched the certification program on Sept. 21. The program involves viewing 13 slides, completing a four-question multiple choice knowledge assessment, and submitting a two-page enrollment form.
Sprout CEO Cindy Whitehead said the company worked closely with the FDA to create a “meaningful” Risk Evaluation and Mitigation Strategy (REMS) program. The program focuses on ensuring physicians understand the safety profile of the drug, she said.
When the FDA approved flibanserin in August 2015, the agency required a REMS program primarily focused around the risk of syncope and hypotension when flibanserin is taken with alcohol. The REMS was required because clinical trials showed that flibanserin has an increased risk for syncope and hypotension with alcohol and moderate or strong CYP3A4 inhibitors, which include proton pump inhibitors, selective serotonin reuptake inhibitors, benzodiazepines, and antifungals.
“So far, the response from health care providers and physicians continues to grow every day,” Ms. Whitehead said. “Thousands of prescribers and pharmacies have completed the certification process which continues to show the need for physicians to have a medical treatment option in their toolkit.”
Dr. Patrick J. Woodman has completed the REMS process for flibanserin but has yet to prescribe the drug. He estimated that the entire certification process took him less than a half hour.
The FDA “is being cautious in general” by instituting the REMS program, said Dr. Woodman, a urogynecologist and clinical professor at Michigan State University in East Lansing. He noted that the clinical trials involving flibanserin use had participants drinking a considerable amount of alcohol over a short time period. “It was interesting to see that the side effects of flibanserin with alcohol are dose related,” he said.
For some women, recognition of the social lubricant effect of a glass of wine may offset their desire to try flibanserin, Dr. Woodman said. The REMS program requires patients to sign an agreement stating that “I understand that I must not drink alcohol while taking Addyi.”
“I haven’t had a lot of women beating down my door about it,” Dr. Woodman said. However, “for a select population of women who have not had success with other approaches, I think this is a good option.”
Dr. Lisa Larkin, an internist and director of the University of Cincinnati Women’s Center, also treats women with sexual problems. She said she’s happy to have an approved drug to add to the discussion of treatment options. Before flibanserin’s approval, the only pharmaceutical options for low female sexual desire involved off-label use, said Dr. Larkin, who is scientific cochair of Even the Score, a coalition that advocates for more options in female sexual dysfunction treatment.
But Dr. Larkin said she’s been very clear with patients that “while we try flibanserin, there is no alcohol.”
Her approach is to advise patients to try flibanserin for a period of 8-12 weeks and then evaluate if the medication is working for them and if the lifestyle trade-off is worthwhile. The notion of complete abstinence from alcohol has been off-putting for patients, even those who are not particularly heavy drinkers, she said. So far, none of her patients have opted to try flibanserin so she has yet to prescribe it.
Another gynecologist puts it this way on social media: “Want to take flibanserin (Addyi) for low sex drive? You can’t drink alcohol. Ever.” On her personal blog, Dr. Jen Gunter, an ob.gyn. and pain medicine physician, also faults the REMS program both for its brevity and a lack of detail.
“The REMS did not mention how to screen, or limiting prescribing for 8 weeks to assess efficacy, or interactions with other drugs,” Dr. Gunter wrote.
Leonore Tiefer, Ph.D., of the department of psychiatry at New York University and a sexologist is also critical of flibanserin’s approval and of the REMS process.
“The training doesn’t even ask the prescriber to talk with the patient about sexuality,” said Dr. Tiefer, pointing out that there are many effective, nonpharmaceutical interventions for sexual problems. “The general assumption is that everybody knows enough about sex.”
But Dr. Tiefer said that in her experience women need more information on the importance of communication, foreplay, and relaxation in a satisfying sex life. “If these things are common sense, then it’s common sense that nobody uses.”
The flibanserin certification materials are available online at www.Addyi.com. To complete the certification process, prescribers and pharmacists may fax the completed knowledge assessment and enrollment forms to 844-694-3373 or email scanned copies to [email protected].
On Twitter @karioakes
Physicians who treat premenopausal women with hypoactive sexual desire disorder now have a Food and Drug Administration–approved option in flibanserin. But the centrally acting daily medication comes with certain conditions: prescribers and dispensing pharmacies are required to complete a certification process, and patients must sign an agreement not to drink alcohol while taking the drug.
How will these restrictions affect real-world practice? The jury’s still out, but some physicians are seeing patients balk at becoming teetotalers to try flibanserin. And while some herald the new drug option to treat women with sexual problems, others remain critical of the drug and the prescriber certification process.
Sprout Pharmaceuticals, which markets flibanserin as Addyi, launched the certification program on Sept. 21. The program involves viewing 13 slides, completing a four-question multiple choice knowledge assessment, and submitting a two-page enrollment form.
Sprout CEO Cindy Whitehead said the company worked closely with the FDA to create a “meaningful” Risk Evaluation and Mitigation Strategy (REMS) program. The program focuses on ensuring physicians understand the safety profile of the drug, she said.
When the FDA approved flibanserin in August 2015, the agency required a REMS program primarily focused around the risk of syncope and hypotension when flibanserin is taken with alcohol. The REMS was required because clinical trials showed that flibanserin has an increased risk for syncope and hypotension with alcohol and moderate or strong CYP3A4 inhibitors, which include proton pump inhibitors, selective serotonin reuptake inhibitors, benzodiazepines, and antifungals.
“So far, the response from health care providers and physicians continues to grow every day,” Ms. Whitehead said. “Thousands of prescribers and pharmacies have completed the certification process which continues to show the need for physicians to have a medical treatment option in their toolkit.”
Dr. Patrick J. Woodman has completed the REMS process for flibanserin but has yet to prescribe the drug. He estimated that the entire certification process took him less than a half hour.
The FDA “is being cautious in general” by instituting the REMS program, said Dr. Woodman, a urogynecologist and clinical professor at Michigan State University in East Lansing. He noted that the clinical trials involving flibanserin use had participants drinking a considerable amount of alcohol over a short time period. “It was interesting to see that the side effects of flibanserin with alcohol are dose related,” he said.
For some women, recognition of the social lubricant effect of a glass of wine may offset their desire to try flibanserin, Dr. Woodman said. The REMS program requires patients to sign an agreement stating that “I understand that I must not drink alcohol while taking Addyi.”
“I haven’t had a lot of women beating down my door about it,” Dr. Woodman said. However, “for a select population of women who have not had success with other approaches, I think this is a good option.”
Dr. Lisa Larkin, an internist and director of the University of Cincinnati Women’s Center, also treats women with sexual problems. She said she’s happy to have an approved drug to add to the discussion of treatment options. Before flibanserin’s approval, the only pharmaceutical options for low female sexual desire involved off-label use, said Dr. Larkin, who is scientific cochair of Even the Score, a coalition that advocates for more options in female sexual dysfunction treatment.
But Dr. Larkin said she’s been very clear with patients that “while we try flibanserin, there is no alcohol.”
Her approach is to advise patients to try flibanserin for a period of 8-12 weeks and then evaluate if the medication is working for them and if the lifestyle trade-off is worthwhile. The notion of complete abstinence from alcohol has been off-putting for patients, even those who are not particularly heavy drinkers, she said. So far, none of her patients have opted to try flibanserin so she has yet to prescribe it.
Another gynecologist puts it this way on social media: “Want to take flibanserin (Addyi) for low sex drive? You can’t drink alcohol. Ever.” On her personal blog, Dr. Jen Gunter, an ob.gyn. and pain medicine physician, also faults the REMS program both for its brevity and a lack of detail.
“The REMS did not mention how to screen, or limiting prescribing for 8 weeks to assess efficacy, or interactions with other drugs,” Dr. Gunter wrote.
Leonore Tiefer, Ph.D., of the department of psychiatry at New York University and a sexologist is also critical of flibanserin’s approval and of the REMS process.
“The training doesn’t even ask the prescriber to talk with the patient about sexuality,” said Dr. Tiefer, pointing out that there are many effective, nonpharmaceutical interventions for sexual problems. “The general assumption is that everybody knows enough about sex.”
But Dr. Tiefer said that in her experience women need more information on the importance of communication, foreplay, and relaxation in a satisfying sex life. “If these things are common sense, then it’s common sense that nobody uses.”
The flibanserin certification materials are available online at www.Addyi.com. To complete the certification process, prescribers and pharmacists may fax the completed knowledge assessment and enrollment forms to 844-694-3373 or email scanned copies to [email protected].
On Twitter @karioakes
Topicals, PDT offer field treatment options for actinic keratoses
LAS VEGAS – The addition of field treatment to targeted lesion treatment may offer patients the best outcomes when addressing actinic keratoses (AKs), said Dr. David M. Pariser, professor of dermatology at Eastern Virginia Medical School, Norfolk, Virginia.
Speaking at the Skin Disease Education Foundation’s annual Las Vegas dermatology seminar, Dr. Pariser said that the rationale for treating AKs stems from the fact that not all AKs will transform to squamous cell carcinoma (SCC). “We don’t know which ones will and which ones won’t.” Though treatment can be guided by a shared decision-making approach, a personal history of multiple skin cancers is an obvious reason for concern, as is any drug-related or disease-related immunosuppression, he added.
Available treatments for AKs include cryosurgery, curettage, and electrodessication; photodynamic therapy (PDT); and topical agents, including 5-fluorouracil (5-FU), diclofenac, imiquimod, and ingenol mebutate.
“Cryosurgery is what most of us do for most AKs,” said Dr. Pariser, observing that this is a bread-and-butter procedure that is part of the backbone of most dermatology practices. Cryosurgery, curettage, and electrodessication are all used to treat discrete lesions, especially for higher-grade AKs.
However, Dr. Pariser noted that many AKs occur in a “field of cancerization.” This is a larger area of photodamaged skin; the skin may not appear grossly abnormal, but deranged areas will be diffusely evident with fluorescence detection, “indicating that all of this area was damaged,” he said.
PDT and topical agents effectively provide field treatment, and do not just address discrete lesions, although field treatment is technically an off-label use for these modalities, he added.
Lower concentrations and shorter courses of topical agents may be effective and better tolerated by patients, Dr. Pariser said. In clinical trials, for example, the efficacy of imiquimod 2.5% and 3.75% has been comparable to the stronger 5% formulation. Treatment cycles lasting 2 rather than 3 weeks have also shown similar efficacy. The greater palatability of these regimens to patients may increase real-world adherence as well.
Cure rates for PDT range from 67% to 92% for both the blue light–aminolevulinic acid modality and the red light–methyl aminolevulinate more commonly used in Europe. These modalities also treat a broad field, offer cure rates comparable to other topical modalities, and result in less scarring than destructive modalities, Dr. Pariser said.
The pain of PDT may be understandably off-putting for some patients, he commented. In the United States, investigators are experimenting with shorter incubation times for the ALA topical solutions. One study found comparable reductions in the number of AKs by the end of the study period for 1 versus 2 or 3 hours of incubation, and a 2-hour incubation period with spot treatment only was also similarly effective. Clearance rates ranged from about 70%-80%, and all were highly statistically significant, compared with the vehicle-only arm. However, “a second treatment at week 8 seems to be necessary for improved efficacy,” Dr. Pariser said.
Another treatment strategy currently being investigated is “painless PDT,” where the agent is applied and the field is illuminated immediately. Photo-bleaching degrades the protoporphyrins as they are produced, dramatically reducing patient discomfort. Efficacy is promising, he added.
In Europe, “daylight PDT” is being explored – the photosensitizing agent is applied, and the patient simply goes about his or her daily business in normal daylight. This is a nearly painless therapy and shows promise for efficacy. However, said Dr. Pariser, the realities of practice economics make it very unlikely that daylight PDT will catch on in the United States, since the daylight exposure does not represent a billable procedure.
Dr. Pariser reiterated that regardless of the modality, “field therapy has better long-term efficacy rates and better sustained clearance no matter which one of the agents we use, and combination therapy [of lesional and field treatment] seems to be the most effective.”
Dr. Pariser disclosed that he is an investigator and consultant for DUSA Pharmaceuticals, Photocure, and LEO Pharma, and that he discussed off-label uses of drugs and devices.
SDEF and this news organization are owned by the same parent company.
On Twitter @karioakes
LAS VEGAS – The addition of field treatment to targeted lesion treatment may offer patients the best outcomes when addressing actinic keratoses (AKs), said Dr. David M. Pariser, professor of dermatology at Eastern Virginia Medical School, Norfolk, Virginia.
Speaking at the Skin Disease Education Foundation’s annual Las Vegas dermatology seminar, Dr. Pariser said that the rationale for treating AKs stems from the fact that not all AKs will transform to squamous cell carcinoma (SCC). “We don’t know which ones will and which ones won’t.” Though treatment can be guided by a shared decision-making approach, a personal history of multiple skin cancers is an obvious reason for concern, as is any drug-related or disease-related immunosuppression, he added.
Available treatments for AKs include cryosurgery, curettage, and electrodessication; photodynamic therapy (PDT); and topical agents, including 5-fluorouracil (5-FU), diclofenac, imiquimod, and ingenol mebutate.
“Cryosurgery is what most of us do for most AKs,” said Dr. Pariser, observing that this is a bread-and-butter procedure that is part of the backbone of most dermatology practices. Cryosurgery, curettage, and electrodessication are all used to treat discrete lesions, especially for higher-grade AKs.
However, Dr. Pariser noted that many AKs occur in a “field of cancerization.” This is a larger area of photodamaged skin; the skin may not appear grossly abnormal, but deranged areas will be diffusely evident with fluorescence detection, “indicating that all of this area was damaged,” he said.
PDT and topical agents effectively provide field treatment, and do not just address discrete lesions, although field treatment is technically an off-label use for these modalities, he added.
Lower concentrations and shorter courses of topical agents may be effective and better tolerated by patients, Dr. Pariser said. In clinical trials, for example, the efficacy of imiquimod 2.5% and 3.75% has been comparable to the stronger 5% formulation. Treatment cycles lasting 2 rather than 3 weeks have also shown similar efficacy. The greater palatability of these regimens to patients may increase real-world adherence as well.
Cure rates for PDT range from 67% to 92% for both the blue light–aminolevulinic acid modality and the red light–methyl aminolevulinate more commonly used in Europe. These modalities also treat a broad field, offer cure rates comparable to other topical modalities, and result in less scarring than destructive modalities, Dr. Pariser said.
The pain of PDT may be understandably off-putting for some patients, he commented. In the United States, investigators are experimenting with shorter incubation times for the ALA topical solutions. One study found comparable reductions in the number of AKs by the end of the study period for 1 versus 2 or 3 hours of incubation, and a 2-hour incubation period with spot treatment only was also similarly effective. Clearance rates ranged from about 70%-80%, and all were highly statistically significant, compared with the vehicle-only arm. However, “a second treatment at week 8 seems to be necessary for improved efficacy,” Dr. Pariser said.
Another treatment strategy currently being investigated is “painless PDT,” where the agent is applied and the field is illuminated immediately. Photo-bleaching degrades the protoporphyrins as they are produced, dramatically reducing patient discomfort. Efficacy is promising, he added.
In Europe, “daylight PDT” is being explored – the photosensitizing agent is applied, and the patient simply goes about his or her daily business in normal daylight. This is a nearly painless therapy and shows promise for efficacy. However, said Dr. Pariser, the realities of practice economics make it very unlikely that daylight PDT will catch on in the United States, since the daylight exposure does not represent a billable procedure.
Dr. Pariser reiterated that regardless of the modality, “field therapy has better long-term efficacy rates and better sustained clearance no matter which one of the agents we use, and combination therapy [of lesional and field treatment] seems to be the most effective.”
Dr. Pariser disclosed that he is an investigator and consultant for DUSA Pharmaceuticals, Photocure, and LEO Pharma, and that he discussed off-label uses of drugs and devices.
SDEF and this news organization are owned by the same parent company.
On Twitter @karioakes
LAS VEGAS – The addition of field treatment to targeted lesion treatment may offer patients the best outcomes when addressing actinic keratoses (AKs), said Dr. David M. Pariser, professor of dermatology at Eastern Virginia Medical School, Norfolk, Virginia.
Speaking at the Skin Disease Education Foundation’s annual Las Vegas dermatology seminar, Dr. Pariser said that the rationale for treating AKs stems from the fact that not all AKs will transform to squamous cell carcinoma (SCC). “We don’t know which ones will and which ones won’t.” Though treatment can be guided by a shared decision-making approach, a personal history of multiple skin cancers is an obvious reason for concern, as is any drug-related or disease-related immunosuppression, he added.
Available treatments for AKs include cryosurgery, curettage, and electrodessication; photodynamic therapy (PDT); and topical agents, including 5-fluorouracil (5-FU), diclofenac, imiquimod, and ingenol mebutate.
“Cryosurgery is what most of us do for most AKs,” said Dr. Pariser, observing that this is a bread-and-butter procedure that is part of the backbone of most dermatology practices. Cryosurgery, curettage, and electrodessication are all used to treat discrete lesions, especially for higher-grade AKs.
However, Dr. Pariser noted that many AKs occur in a “field of cancerization.” This is a larger area of photodamaged skin; the skin may not appear grossly abnormal, but deranged areas will be diffusely evident with fluorescence detection, “indicating that all of this area was damaged,” he said.
PDT and topical agents effectively provide field treatment, and do not just address discrete lesions, although field treatment is technically an off-label use for these modalities, he added.
Lower concentrations and shorter courses of topical agents may be effective and better tolerated by patients, Dr. Pariser said. In clinical trials, for example, the efficacy of imiquimod 2.5% and 3.75% has been comparable to the stronger 5% formulation. Treatment cycles lasting 2 rather than 3 weeks have also shown similar efficacy. The greater palatability of these regimens to patients may increase real-world adherence as well.
Cure rates for PDT range from 67% to 92% for both the blue light–aminolevulinic acid modality and the red light–methyl aminolevulinate more commonly used in Europe. These modalities also treat a broad field, offer cure rates comparable to other topical modalities, and result in less scarring than destructive modalities, Dr. Pariser said.
The pain of PDT may be understandably off-putting for some patients, he commented. In the United States, investigators are experimenting with shorter incubation times for the ALA topical solutions. One study found comparable reductions in the number of AKs by the end of the study period for 1 versus 2 or 3 hours of incubation, and a 2-hour incubation period with spot treatment only was also similarly effective. Clearance rates ranged from about 70%-80%, and all were highly statistically significant, compared with the vehicle-only arm. However, “a second treatment at week 8 seems to be necessary for improved efficacy,” Dr. Pariser said.
Another treatment strategy currently being investigated is “painless PDT,” where the agent is applied and the field is illuminated immediately. Photo-bleaching degrades the protoporphyrins as they are produced, dramatically reducing patient discomfort. Efficacy is promising, he added.
In Europe, “daylight PDT” is being explored – the photosensitizing agent is applied, and the patient simply goes about his or her daily business in normal daylight. This is a nearly painless therapy and shows promise for efficacy. However, said Dr. Pariser, the realities of practice economics make it very unlikely that daylight PDT will catch on in the United States, since the daylight exposure does not represent a billable procedure.
Dr. Pariser reiterated that regardless of the modality, “field therapy has better long-term efficacy rates and better sustained clearance no matter which one of the agents we use, and combination therapy [of lesional and field treatment] seems to be the most effective.”
Dr. Pariser disclosed that he is an investigator and consultant for DUSA Pharmaceuticals, Photocure, and LEO Pharma, and that he discussed off-label uses of drugs and devices.
SDEF and this news organization are owned by the same parent company.
On Twitter @karioakes
EXPERT ANALYSIS FROM SDEF LAS VEGAS DERMATOLOGY SEMINAR
New low-dose antibiotics, topicals offer options for acne
LAS VEGAS – New acne treatment strategies that address the issue of antibiotic resistance include subantimicrobial dosing; new, narrower-spectrum antibiotics; and topical use of tetracycline-family antibiotics, according to Dr. Linda Stein Gold, a dermatologist at Henry Ford Hospital, Detroit.
Oral antibiotics have long been a mainstay of acne treatment, but long-term use of low-dose antibiotics may be contributing to the global crisis of antibiotic resistance. At least 2 million people become infected with resistant bacteria yearly in the United States alone, and at least 23,000 people die yearly from these infections, she noted.
“In dermatology we use antibiotics quite a bit, and we want to make sure when we’re utilizing drugs, we’re utilizing them in the best possible way,” Dr. Stein Gold said at the Skin Disease Education Foundation’s annual Las Vegas dermatology seminar. Finding the right antibiotic dose for effective treatment of acne can be a challenge, she noted. “Is more better? Is too little bad?”
In a review of new treatment strategies that address these concerns without compromising efficacy, Dr. Stein Gold said that the rationale for using subantimicrobial antibiotic dosing comes from the anti-inflammatory effect seen with many antibiotics, even with doses lower than needed for antimicrobial action.
For example, a study of a subantimicrobial-dose of doxycycline found that when adults with moderate acne were treated with the antibiotic (20 mg, twice daily) for 6 months, their acne significantly improved. The number of comedones, inflammatory lesions, and noninflammatory lesions improved significantly compared with those on placebo (Arch Dermatol. 2003 Apr; 139:459-64).
In another head-to-head trial that compared low-dose modified-release doxycycline with placebo or 100 mg of doxycycline, the lower dose outperformed both placebo and full-strength antibiotics. No resistant organisms were found among skin flora in the subjects, and the microbiota of the patients’ skin did not change significantly during the study period, she said.
Dr. Stein Gold’s work also suggests that systemic antibiotics may not be necessary for all patients with acne: In a study, after 12 weeks of treatment, adapalene plus benzoyl peroxide, in combination with doxycycline, resulted in significantly more patients with clear or almost-clear skin than with vehicle alone plus doxycycline. “Antibiotics are not always the golden nugget in the treatment of acne,” she commented.
Another tactic is to treat with antibiotics for a period of 3-6 months along with potent topicals, to get skin clear or almost clear, then discontinue the antibiotic and continue topical treatment. Many patients will be able to maintain clear skin on this regime, she noted.
A new tetracycline-family antibiotic, sarecycline, is in phase III trials for acne vulgaris and in phase II trials for acne rosacea. Sarecycline, “compared with existing tetracycline antibiotics, showed improved anti-inflammatory properties and a narrower spectrum of activity,” Dr. Stein Gold said.
A topical minocycline in a foam formulation shows promising results for tolerability and efficacy in phase II trials for moderate and severe acne, she added. Dapsone as a 7.5% topical gel formulation is in phase III clinical trials as well.
Another antibiotic with a long history of systemic use for acne, clindamycin, is also showing promising results in combination with benzoyl peroxide (1.2%/3.75% gel). A 12-week double-blind study of the combination, compared with vehicle alone for individuals with moderate or severe acne, showed significant improvement in comedonal and inflammatory lesions, as well as overall global improvement in severity, for the treatment arm, she said (J Drugs Dermatol. 2014 Sep;13[9]:1083-9).
Dr. Stein Gold reports being a consultant and investigator for Galderma, Stiefel Laboratories, and Allergan; a consultant and speaker for Valeant; a speaker for Ranbaxy Laboratories, Promius Pharma, and Actavis; and a medical/legal consultant for Roche.
SDEF and this news organization are owned by the same parent company.
On Twitter @karioakes
LAS VEGAS – New acne treatment strategies that address the issue of antibiotic resistance include subantimicrobial dosing; new, narrower-spectrum antibiotics; and topical use of tetracycline-family antibiotics, according to Dr. Linda Stein Gold, a dermatologist at Henry Ford Hospital, Detroit.
Oral antibiotics have long been a mainstay of acne treatment, but long-term use of low-dose antibiotics may be contributing to the global crisis of antibiotic resistance. At least 2 million people become infected with resistant bacteria yearly in the United States alone, and at least 23,000 people die yearly from these infections, she noted.
“In dermatology we use antibiotics quite a bit, and we want to make sure when we’re utilizing drugs, we’re utilizing them in the best possible way,” Dr. Stein Gold said at the Skin Disease Education Foundation’s annual Las Vegas dermatology seminar. Finding the right antibiotic dose for effective treatment of acne can be a challenge, she noted. “Is more better? Is too little bad?”
In a review of new treatment strategies that address these concerns without compromising efficacy, Dr. Stein Gold said that the rationale for using subantimicrobial antibiotic dosing comes from the anti-inflammatory effect seen with many antibiotics, even with doses lower than needed for antimicrobial action.
For example, a study of a subantimicrobial-dose of doxycycline found that when adults with moderate acne were treated with the antibiotic (20 mg, twice daily) for 6 months, their acne significantly improved. The number of comedones, inflammatory lesions, and noninflammatory lesions improved significantly compared with those on placebo (Arch Dermatol. 2003 Apr; 139:459-64).
In another head-to-head trial that compared low-dose modified-release doxycycline with placebo or 100 mg of doxycycline, the lower dose outperformed both placebo and full-strength antibiotics. No resistant organisms were found among skin flora in the subjects, and the microbiota of the patients’ skin did not change significantly during the study period, she said.
Dr. Stein Gold’s work also suggests that systemic antibiotics may not be necessary for all patients with acne: In a study, after 12 weeks of treatment, adapalene plus benzoyl peroxide, in combination with doxycycline, resulted in significantly more patients with clear or almost-clear skin than with vehicle alone plus doxycycline. “Antibiotics are not always the golden nugget in the treatment of acne,” she commented.
Another tactic is to treat with antibiotics for a period of 3-6 months along with potent topicals, to get skin clear or almost clear, then discontinue the antibiotic and continue topical treatment. Many patients will be able to maintain clear skin on this regime, she noted.
A new tetracycline-family antibiotic, sarecycline, is in phase III trials for acne vulgaris and in phase II trials for acne rosacea. Sarecycline, “compared with existing tetracycline antibiotics, showed improved anti-inflammatory properties and a narrower spectrum of activity,” Dr. Stein Gold said.
A topical minocycline in a foam formulation shows promising results for tolerability and efficacy in phase II trials for moderate and severe acne, she added. Dapsone as a 7.5% topical gel formulation is in phase III clinical trials as well.
Another antibiotic with a long history of systemic use for acne, clindamycin, is also showing promising results in combination with benzoyl peroxide (1.2%/3.75% gel). A 12-week double-blind study of the combination, compared with vehicle alone for individuals with moderate or severe acne, showed significant improvement in comedonal and inflammatory lesions, as well as overall global improvement in severity, for the treatment arm, she said (J Drugs Dermatol. 2014 Sep;13[9]:1083-9).
Dr. Stein Gold reports being a consultant and investigator for Galderma, Stiefel Laboratories, and Allergan; a consultant and speaker for Valeant; a speaker for Ranbaxy Laboratories, Promius Pharma, and Actavis; and a medical/legal consultant for Roche.
SDEF and this news organization are owned by the same parent company.
On Twitter @karioakes
LAS VEGAS – New acne treatment strategies that address the issue of antibiotic resistance include subantimicrobial dosing; new, narrower-spectrum antibiotics; and topical use of tetracycline-family antibiotics, according to Dr. Linda Stein Gold, a dermatologist at Henry Ford Hospital, Detroit.
Oral antibiotics have long been a mainstay of acne treatment, but long-term use of low-dose antibiotics may be contributing to the global crisis of antibiotic resistance. At least 2 million people become infected with resistant bacteria yearly in the United States alone, and at least 23,000 people die yearly from these infections, she noted.
“In dermatology we use antibiotics quite a bit, and we want to make sure when we’re utilizing drugs, we’re utilizing them in the best possible way,” Dr. Stein Gold said at the Skin Disease Education Foundation’s annual Las Vegas dermatology seminar. Finding the right antibiotic dose for effective treatment of acne can be a challenge, she noted. “Is more better? Is too little bad?”
In a review of new treatment strategies that address these concerns without compromising efficacy, Dr. Stein Gold said that the rationale for using subantimicrobial antibiotic dosing comes from the anti-inflammatory effect seen with many antibiotics, even with doses lower than needed for antimicrobial action.
For example, a study of a subantimicrobial-dose of doxycycline found that when adults with moderate acne were treated with the antibiotic (20 mg, twice daily) for 6 months, their acne significantly improved. The number of comedones, inflammatory lesions, and noninflammatory lesions improved significantly compared with those on placebo (Arch Dermatol. 2003 Apr; 139:459-64).
In another head-to-head trial that compared low-dose modified-release doxycycline with placebo or 100 mg of doxycycline, the lower dose outperformed both placebo and full-strength antibiotics. No resistant organisms were found among skin flora in the subjects, and the microbiota of the patients’ skin did not change significantly during the study period, she said.
Dr. Stein Gold’s work also suggests that systemic antibiotics may not be necessary for all patients with acne: In a study, after 12 weeks of treatment, adapalene plus benzoyl peroxide, in combination with doxycycline, resulted in significantly more patients with clear or almost-clear skin than with vehicle alone plus doxycycline. “Antibiotics are not always the golden nugget in the treatment of acne,” she commented.
Another tactic is to treat with antibiotics for a period of 3-6 months along with potent topicals, to get skin clear or almost clear, then discontinue the antibiotic and continue topical treatment. Many patients will be able to maintain clear skin on this regime, she noted.
A new tetracycline-family antibiotic, sarecycline, is in phase III trials for acne vulgaris and in phase II trials for acne rosacea. Sarecycline, “compared with existing tetracycline antibiotics, showed improved anti-inflammatory properties and a narrower spectrum of activity,” Dr. Stein Gold said.
A topical minocycline in a foam formulation shows promising results for tolerability and efficacy in phase II trials for moderate and severe acne, she added. Dapsone as a 7.5% topical gel formulation is in phase III clinical trials as well.
Another antibiotic with a long history of systemic use for acne, clindamycin, is also showing promising results in combination with benzoyl peroxide (1.2%/3.75% gel). A 12-week double-blind study of the combination, compared with vehicle alone for individuals with moderate or severe acne, showed significant improvement in comedonal and inflammatory lesions, as well as overall global improvement in severity, for the treatment arm, she said (J Drugs Dermatol. 2014 Sep;13[9]:1083-9).
Dr. Stein Gold reports being a consultant and investigator for Galderma, Stiefel Laboratories, and Allergan; a consultant and speaker for Valeant; a speaker for Ranbaxy Laboratories, Promius Pharma, and Actavis; and a medical/legal consultant for Roche.
SDEF and this news organization are owned by the same parent company.
On Twitter @karioakes
EXPERT ANALYSIS FROM SDEF LAS VEGAS DERMATOLOGY SEMINAR
Busting eczema treatment myths: applying the evidence
LAS VEGAS – In the treatment of eczema, the gap between evidence and practice can be broad.
Dermatologists who treat atopic dermatitis confront many challenges – patients may be severely atopic, have a hard time being compliant with therapies, and have frequent recurrences, Dr. Robert Sidbury said at the Skin Disease Education Foundation’s annual Las Vegas dermatology seminar. “It just is not easy,” he said.
However, even for challenging patients, physicians should be mindful of evidence-supported treatments and be attentive to practice gaps, said Dr. Sidbury, chief of the division of dermatology at the University of Washington’s Seattle Children’s Hospital.
Though the field is rapidly changing, the American Academy of Dermatology has issued practice guidelines that can help guide clinical treatment decisions, said Dr. Sidbury, who helped develop the AAD guidelines. He noted that prescribers may eventually feel the pain of the practice gap if AMA-driven performance measures are enforced.
At the meeting, Dr. Sidbury discussed areas in which many clinicians may have a practice gap in the treatment of eczema. Topping the list of non–evidence-based eczema care are overuse of steroids, oral antibiotics, and nonsedating antihistamines.
Practice gap: Many clinicians believe that topical steroids are more effective for atopic dermatitis when used twice daily.
Reality: “Randomized, controlled trials and a systematic review suggest that there is no benefit to twice-daily use of steroids,” over once-daily use, Dr. Sidbury said, though he admitted that he’s having a hard time breaking his own longstanding practice habit of prescribing twice- rather than once-daily dosing of topical corticosteroids. He pointed out that Dr. Hywel Williams, the director of the University of Nottingham’s Center of Evidence-Based Dermatology, England, calls this the “lowest-hanging fruit” in terms of cost savings, safety, and convenience to patients.
Practice gap: Unnecessary skin cultures can lead to overuse of systemic antibiotics in atopic dermatitis.
Reality: Colonization with Staphylococcus aureus occurs in more than 90% of adults with atopic dermatitis, but the vast majority of these patients are not infected. “Except for bleach baths in concert with intranasal mupirocin, no topical antistaphylococcal treatment has been shown to be clinically helpful in patients with atopic dermatitis,” Dr. Sidbury said.
“How do we know when eczema is infected? We know it when we see it. You are best served by using your clinical gestalt,” he added. Eczematous skin presents along a continuum, ranging from erythema, scaling, and crusting, to a frankly purulent appearance with clear infection, and clinical presentation and judgment should guide treatment. Barring frank infection, the evidence doesn’t support use of systemic antibiotics (Br J Dermatol. 2010 Jul;163 [1]:12-26).
Practice gap: Many patients with atopic dermatitis receive nonsedating antihistamines for itching.
Reality: There is no evidence that nonsedating antihistamines are beneficial unless the patient has concurrent rhinoconjunctivitis, so data don’t support their use “for the actual itch of eczema,” Dr. Sidbury said. In fact, the AMA-sponsored Physician Consortium for Performance Improvement nearly passed an overuse measure that would have penalized the prescription of nonsedating antihistamines in this setting, he said.
Practice gap: Systemic immunomodulatory therapy is used for pediatric atopic dermatitis, despite the lack of data that provide clear guidance.
Reality: The landscape here is a little more complicated, according to Dr. Sidbury. Among the systemic immunosuppressants, cyclosporine, methotrexate, azathioprine, and mycophenolate have the most evidence backing their use. However, there remains a lack of comparative studies and a lack of studies evaluating these therapies in the pediatric population, he said.
In general, Dr. Sidbury said that systemic therapy for atopic dermatitis is indicated only when control is inadequate despite truly optimized topical care, and the condition is having a “significant negative physical, psychological, or social impact” on the patient. Before beginning these potent systemic therapies, it’s important to assess that the patient and family are truly adherent to the topical treatment regime, and that adjunctive treatments like wet wraps and strict allergen avoidance are being followed. The Food and Drug Administration is beginning to include pediatric patients in clinical trials of systemic therapy for atopic dermatitis, so the quality of data should improve.
If systemic therapy is initiated, some clinical pearls can guide use, he said. Cyclosporine has the quickest onset of action and can be dosed at 3-6 mg/kg per day, divided into twice daily dosing. The maximum dose is 300 mg/day, and the microemulsion form is preferred. Overall, mycophenolate is the best-tolerated immunosuppressive. If methotrexate is chosen, it should be dosed at 0.2-0.7 mg/kg per week; liver function should be checked at 5-7 days after dosing, since methotrexate can cause a transient transaminitis. There are no standard recommendations to guide if or when to do liver biopsy recommendations in children receiving methotrexate. (J Am Acad Dermatol. 2014 Aug;71[2]:327-49).
Practice gap: Eczema appointments may only last 10-20 minutes.
Reality: “Proper eczema education takes much longer,” Dr. Sidbury said. Among patients and clinicians, there is still “rampant misinformation,” with persistence of fundamental knowledge gaps. “Studies repeatedly validate the role of education” in providing optimal care for eczema sufferers and their families, he emphasized (Pediatr Allergy Immunol. 2015 Jan 23. doi: 10.1111/pai.12338).
How is a busy physician to integrate all of these recommendations into practice and make sure patients are getting proper education? “Don’t reinvent the wheel – Google it!” Dr. Sidbury said. Resources from the National Eczema Association, among others, can help guide care. Eczema action plans that can be downloaded provide a roadmap for shared decision making; food allergy clinical practice guidelines help patients avoid allergens, and patients can further their knowledge when directed to reputable websites like the National Eczema Association and resources like www.eczemacenter.org, at Rady Children’s Hospital–San Diego.
Dr. Sidbury disclosed that he was a site principal investigator for an Anacor Pharmaceuticals–sponsored trial of a new topical anti-inflammatory agent for allergic dermatitis, and that he is on the Scientific Advisory Committee of the National Eczema Association.
SDEF and this news organization are owned by the same parent company.
On Twitter @karioakes
LAS VEGAS – In the treatment of eczema, the gap between evidence and practice can be broad.
Dermatologists who treat atopic dermatitis confront many challenges – patients may be severely atopic, have a hard time being compliant with therapies, and have frequent recurrences, Dr. Robert Sidbury said at the Skin Disease Education Foundation’s annual Las Vegas dermatology seminar. “It just is not easy,” he said.
However, even for challenging patients, physicians should be mindful of evidence-supported treatments and be attentive to practice gaps, said Dr. Sidbury, chief of the division of dermatology at the University of Washington’s Seattle Children’s Hospital.
Though the field is rapidly changing, the American Academy of Dermatology has issued practice guidelines that can help guide clinical treatment decisions, said Dr. Sidbury, who helped develop the AAD guidelines. He noted that prescribers may eventually feel the pain of the practice gap if AMA-driven performance measures are enforced.
At the meeting, Dr. Sidbury discussed areas in which many clinicians may have a practice gap in the treatment of eczema. Topping the list of non–evidence-based eczema care are overuse of steroids, oral antibiotics, and nonsedating antihistamines.
Practice gap: Many clinicians believe that topical steroids are more effective for atopic dermatitis when used twice daily.
Reality: “Randomized, controlled trials and a systematic review suggest that there is no benefit to twice-daily use of steroids,” over once-daily use, Dr. Sidbury said, though he admitted that he’s having a hard time breaking his own longstanding practice habit of prescribing twice- rather than once-daily dosing of topical corticosteroids. He pointed out that Dr. Hywel Williams, the director of the University of Nottingham’s Center of Evidence-Based Dermatology, England, calls this the “lowest-hanging fruit” in terms of cost savings, safety, and convenience to patients.
Practice gap: Unnecessary skin cultures can lead to overuse of systemic antibiotics in atopic dermatitis.
Reality: Colonization with Staphylococcus aureus occurs in more than 90% of adults with atopic dermatitis, but the vast majority of these patients are not infected. “Except for bleach baths in concert with intranasal mupirocin, no topical antistaphylococcal treatment has been shown to be clinically helpful in patients with atopic dermatitis,” Dr. Sidbury said.
“How do we know when eczema is infected? We know it when we see it. You are best served by using your clinical gestalt,” he added. Eczematous skin presents along a continuum, ranging from erythema, scaling, and crusting, to a frankly purulent appearance with clear infection, and clinical presentation and judgment should guide treatment. Barring frank infection, the evidence doesn’t support use of systemic antibiotics (Br J Dermatol. 2010 Jul;163 [1]:12-26).
Practice gap: Many patients with atopic dermatitis receive nonsedating antihistamines for itching.
Reality: There is no evidence that nonsedating antihistamines are beneficial unless the patient has concurrent rhinoconjunctivitis, so data don’t support their use “for the actual itch of eczema,” Dr. Sidbury said. In fact, the AMA-sponsored Physician Consortium for Performance Improvement nearly passed an overuse measure that would have penalized the prescription of nonsedating antihistamines in this setting, he said.
Practice gap: Systemic immunomodulatory therapy is used for pediatric atopic dermatitis, despite the lack of data that provide clear guidance.
Reality: The landscape here is a little more complicated, according to Dr. Sidbury. Among the systemic immunosuppressants, cyclosporine, methotrexate, azathioprine, and mycophenolate have the most evidence backing their use. However, there remains a lack of comparative studies and a lack of studies evaluating these therapies in the pediatric population, he said.
In general, Dr. Sidbury said that systemic therapy for atopic dermatitis is indicated only when control is inadequate despite truly optimized topical care, and the condition is having a “significant negative physical, psychological, or social impact” on the patient. Before beginning these potent systemic therapies, it’s important to assess that the patient and family are truly adherent to the topical treatment regime, and that adjunctive treatments like wet wraps and strict allergen avoidance are being followed. The Food and Drug Administration is beginning to include pediatric patients in clinical trials of systemic therapy for atopic dermatitis, so the quality of data should improve.
If systemic therapy is initiated, some clinical pearls can guide use, he said. Cyclosporine has the quickest onset of action and can be dosed at 3-6 mg/kg per day, divided into twice daily dosing. The maximum dose is 300 mg/day, and the microemulsion form is preferred. Overall, mycophenolate is the best-tolerated immunosuppressive. If methotrexate is chosen, it should be dosed at 0.2-0.7 mg/kg per week; liver function should be checked at 5-7 days after dosing, since methotrexate can cause a transient transaminitis. There are no standard recommendations to guide if or when to do liver biopsy recommendations in children receiving methotrexate. (J Am Acad Dermatol. 2014 Aug;71[2]:327-49).
Practice gap: Eczema appointments may only last 10-20 minutes.
Reality: “Proper eczema education takes much longer,” Dr. Sidbury said. Among patients and clinicians, there is still “rampant misinformation,” with persistence of fundamental knowledge gaps. “Studies repeatedly validate the role of education” in providing optimal care for eczema sufferers and their families, he emphasized (Pediatr Allergy Immunol. 2015 Jan 23. doi: 10.1111/pai.12338).
How is a busy physician to integrate all of these recommendations into practice and make sure patients are getting proper education? “Don’t reinvent the wheel – Google it!” Dr. Sidbury said. Resources from the National Eczema Association, among others, can help guide care. Eczema action plans that can be downloaded provide a roadmap for shared decision making; food allergy clinical practice guidelines help patients avoid allergens, and patients can further their knowledge when directed to reputable websites like the National Eczema Association and resources like www.eczemacenter.org, at Rady Children’s Hospital–San Diego.
Dr. Sidbury disclosed that he was a site principal investigator for an Anacor Pharmaceuticals–sponsored trial of a new topical anti-inflammatory agent for allergic dermatitis, and that he is on the Scientific Advisory Committee of the National Eczema Association.
SDEF and this news organization are owned by the same parent company.
On Twitter @karioakes
LAS VEGAS – In the treatment of eczema, the gap between evidence and practice can be broad.
Dermatologists who treat atopic dermatitis confront many challenges – patients may be severely atopic, have a hard time being compliant with therapies, and have frequent recurrences, Dr. Robert Sidbury said at the Skin Disease Education Foundation’s annual Las Vegas dermatology seminar. “It just is not easy,” he said.
However, even for challenging patients, physicians should be mindful of evidence-supported treatments and be attentive to practice gaps, said Dr. Sidbury, chief of the division of dermatology at the University of Washington’s Seattle Children’s Hospital.
Though the field is rapidly changing, the American Academy of Dermatology has issued practice guidelines that can help guide clinical treatment decisions, said Dr. Sidbury, who helped develop the AAD guidelines. He noted that prescribers may eventually feel the pain of the practice gap if AMA-driven performance measures are enforced.
At the meeting, Dr. Sidbury discussed areas in which many clinicians may have a practice gap in the treatment of eczema. Topping the list of non–evidence-based eczema care are overuse of steroids, oral antibiotics, and nonsedating antihistamines.
Practice gap: Many clinicians believe that topical steroids are more effective for atopic dermatitis when used twice daily.
Reality: “Randomized, controlled trials and a systematic review suggest that there is no benefit to twice-daily use of steroids,” over once-daily use, Dr. Sidbury said, though he admitted that he’s having a hard time breaking his own longstanding practice habit of prescribing twice- rather than once-daily dosing of topical corticosteroids. He pointed out that Dr. Hywel Williams, the director of the University of Nottingham’s Center of Evidence-Based Dermatology, England, calls this the “lowest-hanging fruit” in terms of cost savings, safety, and convenience to patients.
Practice gap: Unnecessary skin cultures can lead to overuse of systemic antibiotics in atopic dermatitis.
Reality: Colonization with Staphylococcus aureus occurs in more than 90% of adults with atopic dermatitis, but the vast majority of these patients are not infected. “Except for bleach baths in concert with intranasal mupirocin, no topical antistaphylococcal treatment has been shown to be clinically helpful in patients with atopic dermatitis,” Dr. Sidbury said.
“How do we know when eczema is infected? We know it when we see it. You are best served by using your clinical gestalt,” he added. Eczematous skin presents along a continuum, ranging from erythema, scaling, and crusting, to a frankly purulent appearance with clear infection, and clinical presentation and judgment should guide treatment. Barring frank infection, the evidence doesn’t support use of systemic antibiotics (Br J Dermatol. 2010 Jul;163 [1]:12-26).
Practice gap: Many patients with atopic dermatitis receive nonsedating antihistamines for itching.
Reality: There is no evidence that nonsedating antihistamines are beneficial unless the patient has concurrent rhinoconjunctivitis, so data don’t support their use “for the actual itch of eczema,” Dr. Sidbury said. In fact, the AMA-sponsored Physician Consortium for Performance Improvement nearly passed an overuse measure that would have penalized the prescription of nonsedating antihistamines in this setting, he said.
Practice gap: Systemic immunomodulatory therapy is used for pediatric atopic dermatitis, despite the lack of data that provide clear guidance.
Reality: The landscape here is a little more complicated, according to Dr. Sidbury. Among the systemic immunosuppressants, cyclosporine, methotrexate, azathioprine, and mycophenolate have the most evidence backing their use. However, there remains a lack of comparative studies and a lack of studies evaluating these therapies in the pediatric population, he said.
In general, Dr. Sidbury said that systemic therapy for atopic dermatitis is indicated only when control is inadequate despite truly optimized topical care, and the condition is having a “significant negative physical, psychological, or social impact” on the patient. Before beginning these potent systemic therapies, it’s important to assess that the patient and family are truly adherent to the topical treatment regime, and that adjunctive treatments like wet wraps and strict allergen avoidance are being followed. The Food and Drug Administration is beginning to include pediatric patients in clinical trials of systemic therapy for atopic dermatitis, so the quality of data should improve.
If systemic therapy is initiated, some clinical pearls can guide use, he said. Cyclosporine has the quickest onset of action and can be dosed at 3-6 mg/kg per day, divided into twice daily dosing. The maximum dose is 300 mg/day, and the microemulsion form is preferred. Overall, mycophenolate is the best-tolerated immunosuppressive. If methotrexate is chosen, it should be dosed at 0.2-0.7 mg/kg per week; liver function should be checked at 5-7 days after dosing, since methotrexate can cause a transient transaminitis. There are no standard recommendations to guide if or when to do liver biopsy recommendations in children receiving methotrexate. (J Am Acad Dermatol. 2014 Aug;71[2]:327-49).
Practice gap: Eczema appointments may only last 10-20 minutes.
Reality: “Proper eczema education takes much longer,” Dr. Sidbury said. Among patients and clinicians, there is still “rampant misinformation,” with persistence of fundamental knowledge gaps. “Studies repeatedly validate the role of education” in providing optimal care for eczema sufferers and their families, he emphasized (Pediatr Allergy Immunol. 2015 Jan 23. doi: 10.1111/pai.12338).
How is a busy physician to integrate all of these recommendations into practice and make sure patients are getting proper education? “Don’t reinvent the wheel – Google it!” Dr. Sidbury said. Resources from the National Eczema Association, among others, can help guide care. Eczema action plans that can be downloaded provide a roadmap for shared decision making; food allergy clinical practice guidelines help patients avoid allergens, and patients can further their knowledge when directed to reputable websites like the National Eczema Association and resources like www.eczemacenter.org, at Rady Children’s Hospital–San Diego.
Dr. Sidbury disclosed that he was a site principal investigator for an Anacor Pharmaceuticals–sponsored trial of a new topical anti-inflammatory agent for allergic dermatitis, and that he is on the Scientific Advisory Committee of the National Eczema Association.
SDEF and this news organization are owned by the same parent company.
On Twitter @karioakes
EXPERT ANALYSIS FROM SDEF LAS VEGAS DERMATOLOGY SEMINAR