User login
Melanoma incidence drops for U.S. children and teens
The incidence of melanoma among American children and teens decreased by approximately 12% from 2004 to 2010, with the decline most notable for adolescents. The findings were published online in the Journal of Pediatrics.
In a review of data from the period of 2000-2010, Dr. Laura Campbell of Stanford (Calif.) University and her colleagues at Case Western Reserve University in Cleveland found an overall reduction in melanoma diagnoses of 11.58% per year for the period of 2004-2010 (J. Pediatr. 2015 [doi: 10.1016/j.jpeds. 2015.02.050]).
The study was conducted at Case Western Reserve University, and the researchers used the National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER-18) registry to examine trends in the incidence of pediatric melanoma.
Of note, the number of new melanoma cases decreased significantly (approximately 11%) among 15- to19-year-olds between 2003 and 2010. In addition, the overall incidence of melanoma decreased significantly (7%) among boys between 2000 and 2010.
The data revealed significant decreases for the number of new cases of melanoma on the trunk (15% per year from 2004 to 2010) and upper extremities (5% from 2000 to 2010).
Dr. Campbell and her colleagues determined that a melanoma diagnosis was equally likely for male and female patients, and was more common in older than in younger patients. White patients had by far the greatest incidence of melanoma, with 97% of the overall diagnoses; 90% of the cases were in non-Hispanic whites. Superficial spreading melanoma was the most common type of melanoma, at 31%, though nodular histology was seen almost as frequently in the 0- to 9-year-olds. This younger group was more likely to have thicker tumors, ulceration, lymph node involvement, and distant metastases.
Drawing on this large registry allowed researchers more confidence that they were identifying true trends in melanoma incidence, Dr. Campbell noted.
The reasons for this decrease, which stands in contrast to earlier data showing increased incidence rates of pediatric melanoma, were not examined in this study. However, Dr. Campbell drew on these earlier studies, as well as some international studies, to identify the potential contribution of public health campaigns advocating sun protection. These campaigns began in the 1990s in the United States, and would have benefited the 15- to 19-year olds in the SEER-18 data, in whom melanoma incidence decreased beginning in 2003. Some Swedish and Australian studies showing decreased melanoma cases were confounded by an immigration-driven decrease in the highest risk light-skinned population, noted Dr. Campbell; however, the quality of the SEER-18 data allowed researchers to account for this variable, she said.
Although the widespread adoption of sun-protective behaviors (wearing hats and protective clothing, using sunscreen appropriately, and avoiding midday sun exposure) may have accounted for some of the reduction in pediatric melanomas, other societal changes may have been at play.
“We hypothesize that there has been a shift in youth participating increasingly in indoor activities, such as television/electronic devices, which may be decreasing their UVR exposure,” Dr. Campbell said.
The incidence of melanoma among American children and teens decreased by approximately 12% from 2004 to 2010, with the decline most notable for adolescents. The findings were published online in the Journal of Pediatrics.
In a review of data from the period of 2000-2010, Dr. Laura Campbell of Stanford (Calif.) University and her colleagues at Case Western Reserve University in Cleveland found an overall reduction in melanoma diagnoses of 11.58% per year for the period of 2004-2010 (J. Pediatr. 2015 [doi: 10.1016/j.jpeds. 2015.02.050]).
The study was conducted at Case Western Reserve University, and the researchers used the National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER-18) registry to examine trends in the incidence of pediatric melanoma.
Of note, the number of new melanoma cases decreased significantly (approximately 11%) among 15- to19-year-olds between 2003 and 2010. In addition, the overall incidence of melanoma decreased significantly (7%) among boys between 2000 and 2010.
The data revealed significant decreases for the number of new cases of melanoma on the trunk (15% per year from 2004 to 2010) and upper extremities (5% from 2000 to 2010).
Dr. Campbell and her colleagues determined that a melanoma diagnosis was equally likely for male and female patients, and was more common in older than in younger patients. White patients had by far the greatest incidence of melanoma, with 97% of the overall diagnoses; 90% of the cases were in non-Hispanic whites. Superficial spreading melanoma was the most common type of melanoma, at 31%, though nodular histology was seen almost as frequently in the 0- to 9-year-olds. This younger group was more likely to have thicker tumors, ulceration, lymph node involvement, and distant metastases.
Drawing on this large registry allowed researchers more confidence that they were identifying true trends in melanoma incidence, Dr. Campbell noted.
The reasons for this decrease, which stands in contrast to earlier data showing increased incidence rates of pediatric melanoma, were not examined in this study. However, Dr. Campbell drew on these earlier studies, as well as some international studies, to identify the potential contribution of public health campaigns advocating sun protection. These campaigns began in the 1990s in the United States, and would have benefited the 15- to 19-year olds in the SEER-18 data, in whom melanoma incidence decreased beginning in 2003. Some Swedish and Australian studies showing decreased melanoma cases were confounded by an immigration-driven decrease in the highest risk light-skinned population, noted Dr. Campbell; however, the quality of the SEER-18 data allowed researchers to account for this variable, she said.
Although the widespread adoption of sun-protective behaviors (wearing hats and protective clothing, using sunscreen appropriately, and avoiding midday sun exposure) may have accounted for some of the reduction in pediatric melanomas, other societal changes may have been at play.
“We hypothesize that there has been a shift in youth participating increasingly in indoor activities, such as television/electronic devices, which may be decreasing their UVR exposure,” Dr. Campbell said.
The incidence of melanoma among American children and teens decreased by approximately 12% from 2004 to 2010, with the decline most notable for adolescents. The findings were published online in the Journal of Pediatrics.
In a review of data from the period of 2000-2010, Dr. Laura Campbell of Stanford (Calif.) University and her colleagues at Case Western Reserve University in Cleveland found an overall reduction in melanoma diagnoses of 11.58% per year for the period of 2004-2010 (J. Pediatr. 2015 [doi: 10.1016/j.jpeds. 2015.02.050]).
The study was conducted at Case Western Reserve University, and the researchers used the National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER-18) registry to examine trends in the incidence of pediatric melanoma.
Of note, the number of new melanoma cases decreased significantly (approximately 11%) among 15- to19-year-olds between 2003 and 2010. In addition, the overall incidence of melanoma decreased significantly (7%) among boys between 2000 and 2010.
The data revealed significant decreases for the number of new cases of melanoma on the trunk (15% per year from 2004 to 2010) and upper extremities (5% from 2000 to 2010).
Dr. Campbell and her colleagues determined that a melanoma diagnosis was equally likely for male and female patients, and was more common in older than in younger patients. White patients had by far the greatest incidence of melanoma, with 97% of the overall diagnoses; 90% of the cases were in non-Hispanic whites. Superficial spreading melanoma was the most common type of melanoma, at 31%, though nodular histology was seen almost as frequently in the 0- to 9-year-olds. This younger group was more likely to have thicker tumors, ulceration, lymph node involvement, and distant metastases.
Drawing on this large registry allowed researchers more confidence that they were identifying true trends in melanoma incidence, Dr. Campbell noted.
The reasons for this decrease, which stands in contrast to earlier data showing increased incidence rates of pediatric melanoma, were not examined in this study. However, Dr. Campbell drew on these earlier studies, as well as some international studies, to identify the potential contribution of public health campaigns advocating sun protection. These campaigns began in the 1990s in the United States, and would have benefited the 15- to 19-year olds in the SEER-18 data, in whom melanoma incidence decreased beginning in 2003. Some Swedish and Australian studies showing decreased melanoma cases were confounded by an immigration-driven decrease in the highest risk light-skinned population, noted Dr. Campbell; however, the quality of the SEER-18 data allowed researchers to account for this variable, she said.
Although the widespread adoption of sun-protective behaviors (wearing hats and protective clothing, using sunscreen appropriately, and avoiding midday sun exposure) may have accounted for some of the reduction in pediatric melanomas, other societal changes may have been at play.
“We hypothesize that there has been a shift in youth participating increasingly in indoor activities, such as television/electronic devices, which may be decreasing their UVR exposure,” Dr. Campbell said.
FROM THE JOURNAL OF PEDIATRICS
Key clinical point: The overall incidence of melanoma in American children and teens decreased from 2004 to 2010.
Major finding: Researchers identified 1,185 patients younger than 20 years of age with melanoma diagnoses during the period of 2000-2010, and noted a significant decrease of 11.58% per year in melanoma diagnoses from 2004 to 2010.
Data source: The National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER-18) registry for 2000-2010.
Disclosures: The authors reported no conflicts of interest.
Gout increases risk of vascular disease, especially for women
Gout’s association with a host of vascular events was confirmed in a new study that explored the links between the inflammatory condition and coronary artery disease, peripheral vascular disease, and cerebrovascular events.
Though both men and women with gout were at increased risk for vascular events overall, the association appeared strongest for women. Dr. Lorna Clarson of Keele (England) University and her associates drew these conclusions from a retrospective cohort study of men and women with an incident diagnosis of gout (Ann. Rheum. Dis. 2015;74:642-7).
Gout, caused by the deposition of uric acid crystals in joints, is characterized by acute flares of intensely painful and inflamed joints. However, the state of hyperuricemia that predisposes patients to acute attacks of gout may precede the first attack by years, and may persist between flares. The proinflammatory course of the natural history of gout has increasingly been recognized as a potential contributor to vascular disease.
The precise mechanism by which gout may increase vascular risk has not been identified. Dr. Clarson and associates noted that in addition to the acute and chronic inflammation associated with gout and hyperuricemia, serum uric acid may have a more direct effect on vascular health, as urate crystal deposition on vessel walls may promote vascular damage.
To clarify gout’s impact on vascular risk, Dr. Clarson and her associates used the Clinical Practice Datalink, a large United Kingdom health database, to compare 8,366 patients with gout to 39,766 age- and sex-matched controls. None of those studied had a baseline history of vascular disease, and all were aged 50 or older.
Careful accounting for covariates was accomplished by multivariate analysis that took into account sex, age, body mass index, tobacco and alcohol consumption, statin or aspirin use, and any history of hypertension, dyslipidemia, or chronic kidney disease. In addition, the study employed the composite Charlson Comorbidity Index, which weights 19 comorbid conditions – including diabetes – to arrive at a single score that captures many risk factors. Patients in the cohort were tracked until their first vascular event, or until death or loss to follow-up. Patient data collection was censored at 10 years from baseline or at the end of study data collection, whichever came first.
To assess the incidence of vascular events, the study noted the first recording in the medical record of any events signaling vascular disease. These included angina or myocardial infarction, transient ischemic attack and stroke, and a range of diagnoses associated with peripheral vascular disease.
Final analysis after accounting for the many covariates tracked in the study showed increased risk for vascular events for those with gout, with a definite difference between the sexes. For men, gout predicted an increased risk of any vascular event (hazard ratio, 1.06; 95% confidence interval, 1.01–1.12) and of coronary heart disease and peripheral vascular disease. For women, gout predicted an increased risk of all vascular events (HR, 1.25; 95% CI, 1.15-1.35) except myocardial infarction and cerebrovascular disease overall. Further, the degree of increased risk of vascular events was greater for women than for men with gout (P< .001 for intersex difference).
Dr. Clarson and her associates proposed that the higher risk for vascular events among women with gout may arise from the longer exposure to elevated serum uric acid, since women have a longer prodrome before first gout attack, though they recommend further study to elucidate the mechanism.
Noting that “clinical management of gout in primary care is suboptimal,” Dr. Clarson and her colleagues urged greater attention to screening for vascular risk in those diagnosed with gout; these individuals comprise a significant population of over 8 million people in the United States. International guidelines recommend screening for cardiovascular risk when gout is diagnosed, but only one in four gout patients are so evaluated.
Regarding the sex differences unearthed in their study, Dr. Clarson and her associates observed that “both gout and vascular disease have historically been considered diseases of men ... [M]ore attention should be paid to prompt and reliable diagnosis of gout, followed by optimal management in female patients, including serious consideration of vascular risk reduction.”
The United Kingdom’s National School for Primary Research funded the study. The authors reported no relevant disclosures.
The significant strengths of Dr. Clarson and colleagues’ study of the association between gout and vascular disease are its large sample size, its accounting for many potentially confounding risk factors, and its exclusion of those with known antecedent heart disease. It is limited by its lack of validation of gout and cardiac diagnoses and the self-selection bias inherent in using primary care registries, rather than a true population-based cohort.
For individuals without gout, women’s overall vascular risk is lower, but the lack of a difference in cardiovascular disease risk in men and women with gout means that we do not yet understand why women’s risk rises more with the disease. It is possible that systemic inflammation induced by gout in women is more atherogenic than that in men, which necessitates studies to determine whether there are sex-based differences in the pathogenesis of gout-associated heart disease.

|
Dr. Jasvinder Singh |
Patients older than 35 or 40 with gout should be screened and followed with lipid profiles, hemoglobin A1c levels, blood pressure levels, and smoking status, and should undergo an assessment of other lifestyle factors that may impact cardiovascular risk.
Recognizing gout’s contribution to cardiac risk, and managing both the disease and associated risk factors, will be a key task for primary care doctors, rheumatologists, and cardiologists going forward.
Dr. Jasvinder Singh is a professor of medicine in the division of clinical immunology and rheumatology at the University of Alabama, Birmingham. These comments are summarized from his editorial accompanying Dr. Clarson’s report (Ann. Rheum. Dis. 2015;74:631-4). He reported receiving research and travel grants from Takeda and Savient, and consultant fees from Takeda, Savient, Regeneron, and Allergan.
The significant strengths of Dr. Clarson and colleagues’ study of the association between gout and vascular disease are its large sample size, its accounting for many potentially confounding risk factors, and its exclusion of those with known antecedent heart disease. It is limited by its lack of validation of gout and cardiac diagnoses and the self-selection bias inherent in using primary care registries, rather than a true population-based cohort.
For individuals without gout, women’s overall vascular risk is lower, but the lack of a difference in cardiovascular disease risk in men and women with gout means that we do not yet understand why women’s risk rises more with the disease. It is possible that systemic inflammation induced by gout in women is more atherogenic than that in men, which necessitates studies to determine whether there are sex-based differences in the pathogenesis of gout-associated heart disease.

|
Dr. Jasvinder Singh |
Patients older than 35 or 40 with gout should be screened and followed with lipid profiles, hemoglobin A1c levels, blood pressure levels, and smoking status, and should undergo an assessment of other lifestyle factors that may impact cardiovascular risk.
Recognizing gout’s contribution to cardiac risk, and managing both the disease and associated risk factors, will be a key task for primary care doctors, rheumatologists, and cardiologists going forward.
Dr. Jasvinder Singh is a professor of medicine in the division of clinical immunology and rheumatology at the University of Alabama, Birmingham. These comments are summarized from his editorial accompanying Dr. Clarson’s report (Ann. Rheum. Dis. 2015;74:631-4). He reported receiving research and travel grants from Takeda and Savient, and consultant fees from Takeda, Savient, Regeneron, and Allergan.
The significant strengths of Dr. Clarson and colleagues’ study of the association between gout and vascular disease are its large sample size, its accounting for many potentially confounding risk factors, and its exclusion of those with known antecedent heart disease. It is limited by its lack of validation of gout and cardiac diagnoses and the self-selection bias inherent in using primary care registries, rather than a true population-based cohort.
For individuals without gout, women’s overall vascular risk is lower, but the lack of a difference in cardiovascular disease risk in men and women with gout means that we do not yet understand why women’s risk rises more with the disease. It is possible that systemic inflammation induced by gout in women is more atherogenic than that in men, which necessitates studies to determine whether there are sex-based differences in the pathogenesis of gout-associated heart disease.

|
Dr. Jasvinder Singh |
Patients older than 35 or 40 with gout should be screened and followed with lipid profiles, hemoglobin A1c levels, blood pressure levels, and smoking status, and should undergo an assessment of other lifestyle factors that may impact cardiovascular risk.
Recognizing gout’s contribution to cardiac risk, and managing both the disease and associated risk factors, will be a key task for primary care doctors, rheumatologists, and cardiologists going forward.
Dr. Jasvinder Singh is a professor of medicine in the division of clinical immunology and rheumatology at the University of Alabama, Birmingham. These comments are summarized from his editorial accompanying Dr. Clarson’s report (Ann. Rheum. Dis. 2015;74:631-4). He reported receiving research and travel grants from Takeda and Savient, and consultant fees from Takeda, Savient, Regeneron, and Allergan.
Gout’s association with a host of vascular events was confirmed in a new study that explored the links between the inflammatory condition and coronary artery disease, peripheral vascular disease, and cerebrovascular events.
Though both men and women with gout were at increased risk for vascular events overall, the association appeared strongest for women. Dr. Lorna Clarson of Keele (England) University and her associates drew these conclusions from a retrospective cohort study of men and women with an incident diagnosis of gout (Ann. Rheum. Dis. 2015;74:642-7).
Gout, caused by the deposition of uric acid crystals in joints, is characterized by acute flares of intensely painful and inflamed joints. However, the state of hyperuricemia that predisposes patients to acute attacks of gout may precede the first attack by years, and may persist between flares. The proinflammatory course of the natural history of gout has increasingly been recognized as a potential contributor to vascular disease.
The precise mechanism by which gout may increase vascular risk has not been identified. Dr. Clarson and associates noted that in addition to the acute and chronic inflammation associated with gout and hyperuricemia, serum uric acid may have a more direct effect on vascular health, as urate crystal deposition on vessel walls may promote vascular damage.
To clarify gout’s impact on vascular risk, Dr. Clarson and her associates used the Clinical Practice Datalink, a large United Kingdom health database, to compare 8,366 patients with gout to 39,766 age- and sex-matched controls. None of those studied had a baseline history of vascular disease, and all were aged 50 or older.
Careful accounting for covariates was accomplished by multivariate analysis that took into account sex, age, body mass index, tobacco and alcohol consumption, statin or aspirin use, and any history of hypertension, dyslipidemia, or chronic kidney disease. In addition, the study employed the composite Charlson Comorbidity Index, which weights 19 comorbid conditions – including diabetes – to arrive at a single score that captures many risk factors. Patients in the cohort were tracked until their first vascular event, or until death or loss to follow-up. Patient data collection was censored at 10 years from baseline or at the end of study data collection, whichever came first.
To assess the incidence of vascular events, the study noted the first recording in the medical record of any events signaling vascular disease. These included angina or myocardial infarction, transient ischemic attack and stroke, and a range of diagnoses associated with peripheral vascular disease.
Final analysis after accounting for the many covariates tracked in the study showed increased risk for vascular events for those with gout, with a definite difference between the sexes. For men, gout predicted an increased risk of any vascular event (hazard ratio, 1.06; 95% confidence interval, 1.01–1.12) and of coronary heart disease and peripheral vascular disease. For women, gout predicted an increased risk of all vascular events (HR, 1.25; 95% CI, 1.15-1.35) except myocardial infarction and cerebrovascular disease overall. Further, the degree of increased risk of vascular events was greater for women than for men with gout (P< .001 for intersex difference).
Dr. Clarson and her associates proposed that the higher risk for vascular events among women with gout may arise from the longer exposure to elevated serum uric acid, since women have a longer prodrome before first gout attack, though they recommend further study to elucidate the mechanism.
Noting that “clinical management of gout in primary care is suboptimal,” Dr. Clarson and her colleagues urged greater attention to screening for vascular risk in those diagnosed with gout; these individuals comprise a significant population of over 8 million people in the United States. International guidelines recommend screening for cardiovascular risk when gout is diagnosed, but only one in four gout patients are so evaluated.
Regarding the sex differences unearthed in their study, Dr. Clarson and her associates observed that “both gout and vascular disease have historically been considered diseases of men ... [M]ore attention should be paid to prompt and reliable diagnosis of gout, followed by optimal management in female patients, including serious consideration of vascular risk reduction.”
The United Kingdom’s National School for Primary Research funded the study. The authors reported no relevant disclosures.
Gout’s association with a host of vascular events was confirmed in a new study that explored the links between the inflammatory condition and coronary artery disease, peripheral vascular disease, and cerebrovascular events.
Though both men and women with gout were at increased risk for vascular events overall, the association appeared strongest for women. Dr. Lorna Clarson of Keele (England) University and her associates drew these conclusions from a retrospective cohort study of men and women with an incident diagnosis of gout (Ann. Rheum. Dis. 2015;74:642-7).
Gout, caused by the deposition of uric acid crystals in joints, is characterized by acute flares of intensely painful and inflamed joints. However, the state of hyperuricemia that predisposes patients to acute attacks of gout may precede the first attack by years, and may persist between flares. The proinflammatory course of the natural history of gout has increasingly been recognized as a potential contributor to vascular disease.
The precise mechanism by which gout may increase vascular risk has not been identified. Dr. Clarson and associates noted that in addition to the acute and chronic inflammation associated with gout and hyperuricemia, serum uric acid may have a more direct effect on vascular health, as urate crystal deposition on vessel walls may promote vascular damage.
To clarify gout’s impact on vascular risk, Dr. Clarson and her associates used the Clinical Practice Datalink, a large United Kingdom health database, to compare 8,366 patients with gout to 39,766 age- and sex-matched controls. None of those studied had a baseline history of vascular disease, and all were aged 50 or older.
Careful accounting for covariates was accomplished by multivariate analysis that took into account sex, age, body mass index, tobacco and alcohol consumption, statin or aspirin use, and any history of hypertension, dyslipidemia, or chronic kidney disease. In addition, the study employed the composite Charlson Comorbidity Index, which weights 19 comorbid conditions – including diabetes – to arrive at a single score that captures many risk factors. Patients in the cohort were tracked until their first vascular event, or until death or loss to follow-up. Patient data collection was censored at 10 years from baseline or at the end of study data collection, whichever came first.
To assess the incidence of vascular events, the study noted the first recording in the medical record of any events signaling vascular disease. These included angina or myocardial infarction, transient ischemic attack and stroke, and a range of diagnoses associated with peripheral vascular disease.
Final analysis after accounting for the many covariates tracked in the study showed increased risk for vascular events for those with gout, with a definite difference between the sexes. For men, gout predicted an increased risk of any vascular event (hazard ratio, 1.06; 95% confidence interval, 1.01–1.12) and of coronary heart disease and peripheral vascular disease. For women, gout predicted an increased risk of all vascular events (HR, 1.25; 95% CI, 1.15-1.35) except myocardial infarction and cerebrovascular disease overall. Further, the degree of increased risk of vascular events was greater for women than for men with gout (P< .001 for intersex difference).
Dr. Clarson and her associates proposed that the higher risk for vascular events among women with gout may arise from the longer exposure to elevated serum uric acid, since women have a longer prodrome before first gout attack, though they recommend further study to elucidate the mechanism.
Noting that “clinical management of gout in primary care is suboptimal,” Dr. Clarson and her colleagues urged greater attention to screening for vascular risk in those diagnosed with gout; these individuals comprise a significant population of over 8 million people in the United States. International guidelines recommend screening for cardiovascular risk when gout is diagnosed, but only one in four gout patients are so evaluated.
Regarding the sex differences unearthed in their study, Dr. Clarson and her associates observed that “both gout and vascular disease have historically been considered diseases of men ... [M]ore attention should be paid to prompt and reliable diagnosis of gout, followed by optimal management in female patients, including serious consideration of vascular risk reduction.”
The United Kingdom’s National School for Primary Research funded the study. The authors reported no relevant disclosures.
FROM ANNALS OF THE RHEUMATIC DISEASES
Key clinical point: Gout increased the risk of a variety of vascular events, with women at greatest risk.
Major finding: Men with gout were at increased risk of vascular events with an adjusted hazard ratio (HR) of 1.06 overall; women’s risk was greater, with an overall adjusted HR of 1.25.
Data source: Comparison of 8,836 patients with gout to 39,766 matched controls without gout, from the United Kingdom’s Clinical Practice Research Datalink.
Disclosures: The United Kingdom’s National School for Primary Research funded the study. The authors reported no relevant disclosures.
FDA issues new industry guidance for abuse-deterrent opioids
Clinicians may soon have a wider array of medicines that achieve effective pain management for those with legitimate need, but that also address the current public health crisis of prescription opioid diversion and abuse.
The FDA is leading that effort with its newly available document guiding industry in the evaluation and labeling of abuse-deterrent opioids, Dr. Douglas Throckmorton, deputy director of regulatory programs for the U.S. Food and Drug Administration’s Center for Drug Evaluation and Research, announced in a telebriefing held on April 1.
Entitled “Abuse-Deterrent Opioids – Evaluation and Labeling,” this FDA document provides guidance and recommendations for the pharmacy industry. While the document does not have immediate impact on prescribing practices, prescribers can expect to see development of a wider array of products using a variety of strategies to deter abuse, thanks to the clarification provided by this document and the expedited review of abuse-deterrent opioid formulations offered by the FDA.
A particular area of interest has been novel naloxone-containing opioids. Naloxone, an opioid receptor antagonist, quickly and powerfully reverses the effect of opioids and is particularly effective at reversing respiratory depression, a common proximate cause of death in opioid overdose.
Other chemical or physical formulations, the inclusion of aversive substances, and the use of abuse-resistant delivery systems are strategies encouraged by the draft document.
Ensuring the availability of more opioids with abuse-deterrent formulations is an important public health measure to stem opioid abuse, and the FDA is but one of several federal agencies working on this problem. The guidance document was issued to provide industry with the FDA’s current thinking regarding the studies that should be conducted, how drugs should be evaluated, and what labeling claims may be approved, said Dr. Throckmorton.
The FDA is focused on supporting the development of these medications to balance the need of those in pain while reducing abuse. To that end, applicants can also expect an expedited review process. “Tackling the opioid epidemic is a high priority for the FDA,” noted Dr. Throckmorton.
Further guidance regarding generic opioids with abuse-deterrent properties is expected soon. Dr. Sharon Hertz, acting director of CDER’s Division of Anesthesia, Analgesia, and Addiction Products, noted that current FDA and state laws still govern substitutability of generic opioids.
Today’s telebriefing caps off a period of brisk activity. An October 2014 public meeting regarding a draft guidance document was followed by a public comment period. To date, the FDA has met with more than 30 manufacturers interested in producing abuse-deterrent opioid formulations, noted Dr. Throckmorton.
Clinicians may soon have a wider array of medicines that achieve effective pain management for those with legitimate need, but that also address the current public health crisis of prescription opioid diversion and abuse.
The FDA is leading that effort with its newly available document guiding industry in the evaluation and labeling of abuse-deterrent opioids, Dr. Douglas Throckmorton, deputy director of regulatory programs for the U.S. Food and Drug Administration’s Center for Drug Evaluation and Research, announced in a telebriefing held on April 1.
Entitled “Abuse-Deterrent Opioids – Evaluation and Labeling,” this FDA document provides guidance and recommendations for the pharmacy industry. While the document does not have immediate impact on prescribing practices, prescribers can expect to see development of a wider array of products using a variety of strategies to deter abuse, thanks to the clarification provided by this document and the expedited review of abuse-deterrent opioid formulations offered by the FDA.
A particular area of interest has been novel naloxone-containing opioids. Naloxone, an opioid receptor antagonist, quickly and powerfully reverses the effect of opioids and is particularly effective at reversing respiratory depression, a common proximate cause of death in opioid overdose.
Other chemical or physical formulations, the inclusion of aversive substances, and the use of abuse-resistant delivery systems are strategies encouraged by the draft document.
Ensuring the availability of more opioids with abuse-deterrent formulations is an important public health measure to stem opioid abuse, and the FDA is but one of several federal agencies working on this problem. The guidance document was issued to provide industry with the FDA’s current thinking regarding the studies that should be conducted, how drugs should be evaluated, and what labeling claims may be approved, said Dr. Throckmorton.
The FDA is focused on supporting the development of these medications to balance the need of those in pain while reducing abuse. To that end, applicants can also expect an expedited review process. “Tackling the opioid epidemic is a high priority for the FDA,” noted Dr. Throckmorton.
Further guidance regarding generic opioids with abuse-deterrent properties is expected soon. Dr. Sharon Hertz, acting director of CDER’s Division of Anesthesia, Analgesia, and Addiction Products, noted that current FDA and state laws still govern substitutability of generic opioids.
Today’s telebriefing caps off a period of brisk activity. An October 2014 public meeting regarding a draft guidance document was followed by a public comment period. To date, the FDA has met with more than 30 manufacturers interested in producing abuse-deterrent opioid formulations, noted Dr. Throckmorton.
Clinicians may soon have a wider array of medicines that achieve effective pain management for those with legitimate need, but that also address the current public health crisis of prescription opioid diversion and abuse.
The FDA is leading that effort with its newly available document guiding industry in the evaluation and labeling of abuse-deterrent opioids, Dr. Douglas Throckmorton, deputy director of regulatory programs for the U.S. Food and Drug Administration’s Center for Drug Evaluation and Research, announced in a telebriefing held on April 1.
Entitled “Abuse-Deterrent Opioids – Evaluation and Labeling,” this FDA document provides guidance and recommendations for the pharmacy industry. While the document does not have immediate impact on prescribing practices, prescribers can expect to see development of a wider array of products using a variety of strategies to deter abuse, thanks to the clarification provided by this document and the expedited review of abuse-deterrent opioid formulations offered by the FDA.
A particular area of interest has been novel naloxone-containing opioids. Naloxone, an opioid receptor antagonist, quickly and powerfully reverses the effect of opioids and is particularly effective at reversing respiratory depression, a common proximate cause of death in opioid overdose.
Other chemical or physical formulations, the inclusion of aversive substances, and the use of abuse-resistant delivery systems are strategies encouraged by the draft document.
Ensuring the availability of more opioids with abuse-deterrent formulations is an important public health measure to stem opioid abuse, and the FDA is but one of several federal agencies working on this problem. The guidance document was issued to provide industry with the FDA’s current thinking regarding the studies that should be conducted, how drugs should be evaluated, and what labeling claims may be approved, said Dr. Throckmorton.
The FDA is focused on supporting the development of these medications to balance the need of those in pain while reducing abuse. To that end, applicants can also expect an expedited review process. “Tackling the opioid epidemic is a high priority for the FDA,” noted Dr. Throckmorton.
Further guidance regarding generic opioids with abuse-deterrent properties is expected soon. Dr. Sharon Hertz, acting director of CDER’s Division of Anesthesia, Analgesia, and Addiction Products, noted that current FDA and state laws still govern substitutability of generic opioids.
Today’s telebriefing caps off a period of brisk activity. An October 2014 public meeting regarding a draft guidance document was followed by a public comment period. To date, the FDA has met with more than 30 manufacturers interested in producing abuse-deterrent opioid formulations, noted Dr. Throckmorton.
FROM AN FDA TELEBRIEFING
New hepatitis treatment cost effective for some patient types
For individuals infected with hepatitis C virus (HCV), new regimens are highly effective, but also very expensive, at approximately $28,000 for 4 weeks of treatment. However, the treatment is cost effective for HCV patients with cirrhosis and for those without cirrhosis who have failed other treatments, according to a new study.
In an analysis of the cost effectiveness of sofosbuvir-based treatments for patients with HCV genotypes 2 and 3, Dr. Benjamin Linas of Boston Medical Center reported that when compared with pegylated interferon and ribavirin therapy, treatment regimens based on the new direct-acting antiviral are only cost effective for select groups (Ann. Intern Med, [doi:10.7326/M15-0674]).
Cure rates with sofosbuvir are higher than are rates with the previous standard of care, and sustained virologic response (SVR) “is associated with a greatly reduced lifetime risk for liver-related morbidity and mortality,” noted Dr. Linas and his colleagues.
Using sophisticated statistical modeling to compare HCV treatment with pegylated interferon and ribavirin – the previous standard of care – to the newly Food and Drug Administration–approved sofosbuvir-ribavirin regimen, Dr. Linas explored clinical outcomes and costs for several different patient groups, including treatment-naive and treatment-experienced HCV genotype 2 and 3 patients with and without cirrhosis.
Clinical outcomes, modeled from clinical trial and observational cohort data, were expressed as life expectancy in quality-adjusted life-years (QALYs) and lifetime medical costs. Investigators then calculated the incremental cost-effectiveness ratio (ICER) for each treatment strategy, dividing any additional cost for a more expensive treatment by the QALYs gained from this regimen.
Using the commonly accepted ICER threshold of $100,000 per QALY, Dr. Linas and colleagues concluded that sofosbuvir-based HCV therapy for treatment-naive patients without cirrhosis was not cost effective, with ICERs of $238,000-$266,000. Interferon-based treatments, they noted, still achieve an approximately 80% rate of cure in this population.
In the constrained resources of the real world, Dr. Linas and his associates noted that this analysis is important. “Treatment strategies that do not use limited resources where they are likely to have the greatest impact may result in unequal access to interferon-free regimens, thereby limiting the population-level benefits of new HCV treatments,” they said.
AGA Note
Review the AGA Hepatitis C Clinical Service Line to learn more about managing the disease.
In an editorial accompanying Dr. Linas’ study, Dr. Etzion and Dr. Ghany placed this analysis of the cost-effectiveness of sofosbuvir in the context of payer and clinician priorities. They noted that “from the patient and physician perspective, the benefits of new treatment are evident.” For payers, however, resources are constrained and tough decisions will have to be made.
This cost-effectiveness analysis is needed to inform resource allocation decisions, since the cost of using direct-acting antivirals to treat all those infected in the United States alone would exceed $300 billion. In that context, the editorial noted, quality-of-life assessments become important. HCV infection causes relatively little diminution of quality of life until the stage of cirrhosis is reached; in this analysis, therefore, interferon-based regimens are still a reasonable choice for treatment-naive HCV patients without cirrhosis.
Though Dr. Linas’ study also models incremental cost-effectiveness ratios for various lower price points for sofosbuvir, the editorial authors point out that the price point for the public’s willingness to eradicate HCV has not been established.
Dr. Ohad Etzion and Dr. Marc G. Ghany are at the National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, Bethesda, Md. Dr. Ghany reported nonfinancial support from Bristol Meyers Squibb.
In an editorial accompanying Dr. Linas’ study, Dr. Etzion and Dr. Ghany placed this analysis of the cost-effectiveness of sofosbuvir in the context of payer and clinician priorities. They noted that “from the patient and physician perspective, the benefits of new treatment are evident.” For payers, however, resources are constrained and tough decisions will have to be made.
This cost-effectiveness analysis is needed to inform resource allocation decisions, since the cost of using direct-acting antivirals to treat all those infected in the United States alone would exceed $300 billion. In that context, the editorial noted, quality-of-life assessments become important. HCV infection causes relatively little diminution of quality of life until the stage of cirrhosis is reached; in this analysis, therefore, interferon-based regimens are still a reasonable choice for treatment-naive HCV patients without cirrhosis.
Though Dr. Linas’ study also models incremental cost-effectiveness ratios for various lower price points for sofosbuvir, the editorial authors point out that the price point for the public’s willingness to eradicate HCV has not been established.
Dr. Ohad Etzion and Dr. Marc G. Ghany are at the National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, Bethesda, Md. Dr. Ghany reported nonfinancial support from Bristol Meyers Squibb.
In an editorial accompanying Dr. Linas’ study, Dr. Etzion and Dr. Ghany placed this analysis of the cost-effectiveness of sofosbuvir in the context of payer and clinician priorities. They noted that “from the patient and physician perspective, the benefits of new treatment are evident.” For payers, however, resources are constrained and tough decisions will have to be made.
This cost-effectiveness analysis is needed to inform resource allocation decisions, since the cost of using direct-acting antivirals to treat all those infected in the United States alone would exceed $300 billion. In that context, the editorial noted, quality-of-life assessments become important. HCV infection causes relatively little diminution of quality of life until the stage of cirrhosis is reached; in this analysis, therefore, interferon-based regimens are still a reasonable choice for treatment-naive HCV patients without cirrhosis.
Though Dr. Linas’ study also models incremental cost-effectiveness ratios for various lower price points for sofosbuvir, the editorial authors point out that the price point for the public’s willingness to eradicate HCV has not been established.
Dr. Ohad Etzion and Dr. Marc G. Ghany are at the National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, Bethesda, Md. Dr. Ghany reported nonfinancial support from Bristol Meyers Squibb.
For individuals infected with hepatitis C virus (HCV), new regimens are highly effective, but also very expensive, at approximately $28,000 for 4 weeks of treatment. However, the treatment is cost effective for HCV patients with cirrhosis and for those without cirrhosis who have failed other treatments, according to a new study.
In an analysis of the cost effectiveness of sofosbuvir-based treatments for patients with HCV genotypes 2 and 3, Dr. Benjamin Linas of Boston Medical Center reported that when compared with pegylated interferon and ribavirin therapy, treatment regimens based on the new direct-acting antiviral are only cost effective for select groups (Ann. Intern Med, [doi:10.7326/M15-0674]).
Cure rates with sofosbuvir are higher than are rates with the previous standard of care, and sustained virologic response (SVR) “is associated with a greatly reduced lifetime risk for liver-related morbidity and mortality,” noted Dr. Linas and his colleagues.
Using sophisticated statistical modeling to compare HCV treatment with pegylated interferon and ribavirin – the previous standard of care – to the newly Food and Drug Administration–approved sofosbuvir-ribavirin regimen, Dr. Linas explored clinical outcomes and costs for several different patient groups, including treatment-naive and treatment-experienced HCV genotype 2 and 3 patients with and without cirrhosis.
Clinical outcomes, modeled from clinical trial and observational cohort data, were expressed as life expectancy in quality-adjusted life-years (QALYs) and lifetime medical costs. Investigators then calculated the incremental cost-effectiveness ratio (ICER) for each treatment strategy, dividing any additional cost for a more expensive treatment by the QALYs gained from this regimen.
Using the commonly accepted ICER threshold of $100,000 per QALY, Dr. Linas and colleagues concluded that sofosbuvir-based HCV therapy for treatment-naive patients without cirrhosis was not cost effective, with ICERs of $238,000-$266,000. Interferon-based treatments, they noted, still achieve an approximately 80% rate of cure in this population.
In the constrained resources of the real world, Dr. Linas and his associates noted that this analysis is important. “Treatment strategies that do not use limited resources where they are likely to have the greatest impact may result in unequal access to interferon-free regimens, thereby limiting the population-level benefits of new HCV treatments,” they said.
AGA Note
Review the AGA Hepatitis C Clinical Service Line to learn more about managing the disease.
For individuals infected with hepatitis C virus (HCV), new regimens are highly effective, but also very expensive, at approximately $28,000 for 4 weeks of treatment. However, the treatment is cost effective for HCV patients with cirrhosis and for those without cirrhosis who have failed other treatments, according to a new study.
In an analysis of the cost effectiveness of sofosbuvir-based treatments for patients with HCV genotypes 2 and 3, Dr. Benjamin Linas of Boston Medical Center reported that when compared with pegylated interferon and ribavirin therapy, treatment regimens based on the new direct-acting antiviral are only cost effective for select groups (Ann. Intern Med, [doi:10.7326/M15-0674]).
Cure rates with sofosbuvir are higher than are rates with the previous standard of care, and sustained virologic response (SVR) “is associated with a greatly reduced lifetime risk for liver-related morbidity and mortality,” noted Dr. Linas and his colleagues.
Using sophisticated statistical modeling to compare HCV treatment with pegylated interferon and ribavirin – the previous standard of care – to the newly Food and Drug Administration–approved sofosbuvir-ribavirin regimen, Dr. Linas explored clinical outcomes and costs for several different patient groups, including treatment-naive and treatment-experienced HCV genotype 2 and 3 patients with and without cirrhosis.
Clinical outcomes, modeled from clinical trial and observational cohort data, were expressed as life expectancy in quality-adjusted life-years (QALYs) and lifetime medical costs. Investigators then calculated the incremental cost-effectiveness ratio (ICER) for each treatment strategy, dividing any additional cost for a more expensive treatment by the QALYs gained from this regimen.
Using the commonly accepted ICER threshold of $100,000 per QALY, Dr. Linas and colleagues concluded that sofosbuvir-based HCV therapy for treatment-naive patients without cirrhosis was not cost effective, with ICERs of $238,000-$266,000. Interferon-based treatments, they noted, still achieve an approximately 80% rate of cure in this population.
In the constrained resources of the real world, Dr. Linas and his associates noted that this analysis is important. “Treatment strategies that do not use limited resources where they are likely to have the greatest impact may result in unequal access to interferon-free regimens, thereby limiting the population-level benefits of new HCV treatments,” they said.
AGA Note
Review the AGA Hepatitis C Clinical Service Line to learn more about managing the disease.
FROM ANNALS OF INTERNAL MEDICINE
Key clinical point: New treatment for hepatitis C virus (HCV) is cost effective for patients with cirrhosis and for those without cirrhosis who have failed other treatments.
Major finding: Incremental cost-effectiveness ratios for sofosbuvir-based HCV treatment ranged from $238,000 to $266,000 for treatment-naive noncirrhotic patients, exceeding the $100,000 per quality-adjusted life-year cost-effectiveness threshold.
Data source: Application of the Hepatitis C Cost-Effectiveness model to clinical trial and observational cohort data.
Disclosures: The National Institute on Drug Abuse and the National Institute of Allergy and Infectious Disease provided funding. Dr. Weinstein reported receiving personal compensation from OptumInsight; Dr. Kim reported receiving grants and personal compensation from Gilead Sciences, AbbVie Pharmaceuticals, and Bristol-Myers Squibb. The remaining authors reported no relevant disclosures.
New Hepatitis Treatment Cost Effective for Some Patient Types
For individuals infected with hepatitis C virus (HCV), new regimens are highly effective, but also very expensive, at approximately $28,000 for 4 weeks of treatment. However, the treatment is cost effective for HCV patients with cirrhosis and for those without cirrhosis who have failed other treatments, according to a new study.
In an analysis of the cost effectiveness of sofosbuvir-based treatments for patients with HCV genotypes 2 and 3, Dr. Benjamin Linas of Boston Medical Center reported that when compared with pegylated interferon and ribavirin therapy, treatment regimens based on the new direct-acting antiviral are only cost effective for select groups (Ann. Intern Med, [doi:10.7326/M15-0674]).
Cure rates with sofosbuvir are higher than are rates with the previous standard of care, and sustained virologic response (SVR) “is associated with a greatly reduced lifetime risk for liver-related morbidity and mortality,” noted Dr. Linas and his colleagues.
Using sophisticated statistical modeling to compare HCV treatment with pegylated interferon and ribavirin – the previous standard of care – to the newly Food and Drug Administration–approved sofosbuvir-ribavirin regimen, Dr. Linas explored clinical outcomes and costs for several different patient groups, including treatment-naive and treatment-experienced HCV genotype 2 and 3 patients with and without cirrhosis.
Clinical outcomes, modeled from clinical trial and observational cohort data, were expressed as life expectancy in quality-adjusted life-years (QALYs) and lifetime medical costs. Investigators then calculated the incremental cost-effectiveness ratio (ICER) for each treatment strategy, dividing any additional cost for a more expensive treatment by the QALYs gained from this regimen.
Using the commonly accepted ICER threshold of $100,000 per QALY, Dr. Linas and colleagues concluded that sofosbuvir-based HCV therapy for treatment-naive patients without cirrhosis was not cost effective, with ICERs of $238,000-$266,000. Interferon-based treatments, they noted, still achieve an approximately 80% rate of cure in this population.
In the constrained resources of the real world, Dr. Linas and his associates noted that this analysis is important. “Treatment strategies that do not use limited resources where they are likely to have the greatest impact may result in unequal access to interferon-free regimens, thereby limiting the population-level benefits of new HCV treatments,” they said.
In an editorial accompanying Dr. Linas’ study, Dr. Etzion and Dr. Ghany placed this analysis of the cost-effectiveness of sofosbuvir in the context of payer and clinician priorities. They noted that “from the patient and physician perspective, the benefits of new treatment are evident.” For payers, however, resources are constrained and tough decisions will have to be made.
This cost-effectiveness analysis is needed to inform resource allocation decisions, since the cost of using direct-acting antivirals to treat all those infected in the United States alone would exceed $300 billion. In that context, the editorial noted, quality-of-life assessments become important. HCV infection causes relatively little diminution of quality of life until the stage of cirrhosis is reached; in this analysis, therefore, interferon-based regimens are still a reasonable choice for treatment-naive HCV patients without cirrhosis.
Though Dr. Linas’ study also models incremental cost-effectiveness ratios for various lower price points for sofosbuvir, the editorial authors point out that the price point for the public’s willingness to eradicate HCV has not been established.
Dr. Ohad Etzion and Dr. Marc G. Ghany are at the National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, Bethesda, Md. Dr. Ghany reported nonfinancial support from Bristol Meyers Squibb.
In an editorial accompanying Dr. Linas’ study, Dr. Etzion and Dr. Ghany placed this analysis of the cost-effectiveness of sofosbuvir in the context of payer and clinician priorities. They noted that “from the patient and physician perspective, the benefits of new treatment are evident.” For payers, however, resources are constrained and tough decisions will have to be made.
This cost-effectiveness analysis is needed to inform resource allocation decisions, since the cost of using direct-acting antivirals to treat all those infected in the United States alone would exceed $300 billion. In that context, the editorial noted, quality-of-life assessments become important. HCV infection causes relatively little diminution of quality of life until the stage of cirrhosis is reached; in this analysis, therefore, interferon-based regimens are still a reasonable choice for treatment-naive HCV patients without cirrhosis.
Though Dr. Linas’ study also models incremental cost-effectiveness ratios for various lower price points for sofosbuvir, the editorial authors point out that the price point for the public’s willingness to eradicate HCV has not been established.
Dr. Ohad Etzion and Dr. Marc G. Ghany are at the National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, Bethesda, Md. Dr. Ghany reported nonfinancial support from Bristol Meyers Squibb.
In an editorial accompanying Dr. Linas’ study, Dr. Etzion and Dr. Ghany placed this analysis of the cost-effectiveness of sofosbuvir in the context of payer and clinician priorities. They noted that “from the patient and physician perspective, the benefits of new treatment are evident.” For payers, however, resources are constrained and tough decisions will have to be made.
This cost-effectiveness analysis is needed to inform resource allocation decisions, since the cost of using direct-acting antivirals to treat all those infected in the United States alone would exceed $300 billion. In that context, the editorial noted, quality-of-life assessments become important. HCV infection causes relatively little diminution of quality of life until the stage of cirrhosis is reached; in this analysis, therefore, interferon-based regimens are still a reasonable choice for treatment-naive HCV patients without cirrhosis.
Though Dr. Linas’ study also models incremental cost-effectiveness ratios for various lower price points for sofosbuvir, the editorial authors point out that the price point for the public’s willingness to eradicate HCV has not been established.
Dr. Ohad Etzion and Dr. Marc G. Ghany are at the National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, Bethesda, Md. Dr. Ghany reported nonfinancial support from Bristol Meyers Squibb.
For individuals infected with hepatitis C virus (HCV), new regimens are highly effective, but also very expensive, at approximately $28,000 for 4 weeks of treatment. However, the treatment is cost effective for HCV patients with cirrhosis and for those without cirrhosis who have failed other treatments, according to a new study.
In an analysis of the cost effectiveness of sofosbuvir-based treatments for patients with HCV genotypes 2 and 3, Dr. Benjamin Linas of Boston Medical Center reported that when compared with pegylated interferon and ribavirin therapy, treatment regimens based on the new direct-acting antiviral are only cost effective for select groups (Ann. Intern Med, [doi:10.7326/M15-0674]).
Cure rates with sofosbuvir are higher than are rates with the previous standard of care, and sustained virologic response (SVR) “is associated with a greatly reduced lifetime risk for liver-related morbidity and mortality,” noted Dr. Linas and his colleagues.
Using sophisticated statistical modeling to compare HCV treatment with pegylated interferon and ribavirin – the previous standard of care – to the newly Food and Drug Administration–approved sofosbuvir-ribavirin regimen, Dr. Linas explored clinical outcomes and costs for several different patient groups, including treatment-naive and treatment-experienced HCV genotype 2 and 3 patients with and without cirrhosis.
Clinical outcomes, modeled from clinical trial and observational cohort data, were expressed as life expectancy in quality-adjusted life-years (QALYs) and lifetime medical costs. Investigators then calculated the incremental cost-effectiveness ratio (ICER) for each treatment strategy, dividing any additional cost for a more expensive treatment by the QALYs gained from this regimen.
Using the commonly accepted ICER threshold of $100,000 per QALY, Dr. Linas and colleagues concluded that sofosbuvir-based HCV therapy for treatment-naive patients without cirrhosis was not cost effective, with ICERs of $238,000-$266,000. Interferon-based treatments, they noted, still achieve an approximately 80% rate of cure in this population.
In the constrained resources of the real world, Dr. Linas and his associates noted that this analysis is important. “Treatment strategies that do not use limited resources where they are likely to have the greatest impact may result in unequal access to interferon-free regimens, thereby limiting the population-level benefits of new HCV treatments,” they said.
For individuals infected with hepatitis C virus (HCV), new regimens are highly effective, but also very expensive, at approximately $28,000 for 4 weeks of treatment. However, the treatment is cost effective for HCV patients with cirrhosis and for those without cirrhosis who have failed other treatments, according to a new study.
In an analysis of the cost effectiveness of sofosbuvir-based treatments for patients with HCV genotypes 2 and 3, Dr. Benjamin Linas of Boston Medical Center reported that when compared with pegylated interferon and ribavirin therapy, treatment regimens based on the new direct-acting antiviral are only cost effective for select groups (Ann. Intern Med, [doi:10.7326/M15-0674]).
Cure rates with sofosbuvir are higher than are rates with the previous standard of care, and sustained virologic response (SVR) “is associated with a greatly reduced lifetime risk for liver-related morbidity and mortality,” noted Dr. Linas and his colleagues.
Using sophisticated statistical modeling to compare HCV treatment with pegylated interferon and ribavirin – the previous standard of care – to the newly Food and Drug Administration–approved sofosbuvir-ribavirin regimen, Dr. Linas explored clinical outcomes and costs for several different patient groups, including treatment-naive and treatment-experienced HCV genotype 2 and 3 patients with and without cirrhosis.
Clinical outcomes, modeled from clinical trial and observational cohort data, were expressed as life expectancy in quality-adjusted life-years (QALYs) and lifetime medical costs. Investigators then calculated the incremental cost-effectiveness ratio (ICER) for each treatment strategy, dividing any additional cost for a more expensive treatment by the QALYs gained from this regimen.
Using the commonly accepted ICER threshold of $100,000 per QALY, Dr. Linas and colleagues concluded that sofosbuvir-based HCV therapy for treatment-naive patients without cirrhosis was not cost effective, with ICERs of $238,000-$266,000. Interferon-based treatments, they noted, still achieve an approximately 80% rate of cure in this population.
In the constrained resources of the real world, Dr. Linas and his associates noted that this analysis is important. “Treatment strategies that do not use limited resources where they are likely to have the greatest impact may result in unequal access to interferon-free regimens, thereby limiting the population-level benefits of new HCV treatments,” they said.
FROM ANNALS OF INTERNAL MEDICINE
New hepatitis treatment cost effective for some patient types
For individuals infected with hepatitis C virus (HCV), new regimens are highly effective, but also very expensive, at approximately $28,000 for 4 weeks of treatment. However, the treatment is cost effective for HCV patients with cirrhosis and for those without cirrhosis who have failed other treatments, according to a new study.
In an analysis of the cost effectiveness of sofosbuvir-based treatments for patients with HCV genotypes 2 and 3, Dr. Benjamin Linas of Boston Medical Center reported that when compared with pegylated interferon and ribavirin therapy, treatment regimens based on the new direct-acting antiviral are only cost effective for select groups (Ann. Intern Med, [doi:10.7326/M15-0674]).
Cure rates with sofosbuvir are higher than are rates with the previous standard of care, and sustained virologic response (SVR) “is associated with a greatly reduced lifetime risk for liver-related morbidity and mortality,” noted Dr. Linas and his colleagues.
Using sophisticated statistical modeling to compare HCV treatment with pegylated interferon and ribavirin – the previous standard of care – to the newly Food and Drug Administration–approved sofosbuvir-ribavirin regimen, Dr. Linas explored clinical outcomes and costs for several different patient groups, including treatment-naive and treatment-experienced HCV genotype 2 and 3 patients with and without cirrhosis.
Clinical outcomes, modeled from clinical trial and observational cohort data, were expressed as life expectancy in quality-adjusted life-years (QALYs) and lifetime medical costs. Investigators then calculated the incremental cost-effectiveness ratio (ICER) for each treatment strategy, dividing any additional cost for a more expensive treatment by the QALYs gained from this regimen.
Using the commonly accepted ICER threshold of $100,000 per QALY, Dr. Linas and colleagues concluded that sofosbuvir-based HCV therapy for treatment-naive patients without cirrhosis was not cost effective, with ICERs of $238,000-$266,000. Interferon-based treatments, they noted, still achieve an approximately 80% rate of cure in this population.
In the constrained resources of the real world, Dr. Linas and his associates noted that this analysis is important. “Treatment strategies that do not use limited resources where they are likely to have the greatest impact may result in unequal access to interferon-free regimens, thereby limiting the population-level benefits of new HCV treatments,” they said.
In an editorial accompanying Dr. Linas’ study, Dr. Etzion and Dr. Ghany placed this analysis of the cost-effectiveness of sofosbuvir in the context of payer and clinician priorities. They noted that “from the patient and physician perspective, the benefits of new treatment are evident.” For payers, however, resources are constrained and tough decisions will have to be made.
This cost-effectiveness analysis is needed to inform resource allocation decisions, since the cost of using direct-acting antivirals to treat all those infected in the United States alone would exceed $300 billion. In that context, the editorial noted, quality-of-life assessments become important. HCV infection causes relatively little diminution of quality of life until the stage of cirrhosis is reached; in this analysis, therefore, interferon-based regimens are still a reasonable choice for treatment-naive HCV patients without cirrhosis.
Though Dr. Linas’ study also models incremental cost-effectiveness ratios for various lower price points for sofosbuvir, the editorial authors point out that the price point for the public’s willingness to eradicate HCV has not been established.
Dr. Ohad Etzion and Dr. Marc G. Ghany are at the National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, Bethesda, Md. Dr. Ghany reported nonfinancial support from Bristol Meyers Squibb.
In an editorial accompanying Dr. Linas’ study, Dr. Etzion and Dr. Ghany placed this analysis of the cost-effectiveness of sofosbuvir in the context of payer and clinician priorities. They noted that “from the patient and physician perspective, the benefits of new treatment are evident.” For payers, however, resources are constrained and tough decisions will have to be made.
This cost-effectiveness analysis is needed to inform resource allocation decisions, since the cost of using direct-acting antivirals to treat all those infected in the United States alone would exceed $300 billion. In that context, the editorial noted, quality-of-life assessments become important. HCV infection causes relatively little diminution of quality of life until the stage of cirrhosis is reached; in this analysis, therefore, interferon-based regimens are still a reasonable choice for treatment-naive HCV patients without cirrhosis.
Though Dr. Linas’ study also models incremental cost-effectiveness ratios for various lower price points for sofosbuvir, the editorial authors point out that the price point for the public’s willingness to eradicate HCV has not been established.
Dr. Ohad Etzion and Dr. Marc G. Ghany are at the National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, Bethesda, Md. Dr. Ghany reported nonfinancial support from Bristol Meyers Squibb.
In an editorial accompanying Dr. Linas’ study, Dr. Etzion and Dr. Ghany placed this analysis of the cost-effectiveness of sofosbuvir in the context of payer and clinician priorities. They noted that “from the patient and physician perspective, the benefits of new treatment are evident.” For payers, however, resources are constrained and tough decisions will have to be made.
This cost-effectiveness analysis is needed to inform resource allocation decisions, since the cost of using direct-acting antivirals to treat all those infected in the United States alone would exceed $300 billion. In that context, the editorial noted, quality-of-life assessments become important. HCV infection causes relatively little diminution of quality of life until the stage of cirrhosis is reached; in this analysis, therefore, interferon-based regimens are still a reasonable choice for treatment-naive HCV patients without cirrhosis.
Though Dr. Linas’ study also models incremental cost-effectiveness ratios for various lower price points for sofosbuvir, the editorial authors point out that the price point for the public’s willingness to eradicate HCV has not been established.
Dr. Ohad Etzion and Dr. Marc G. Ghany are at the National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, Bethesda, Md. Dr. Ghany reported nonfinancial support from Bristol Meyers Squibb.
For individuals infected with hepatitis C virus (HCV), new regimens are highly effective, but also very expensive, at approximately $28,000 for 4 weeks of treatment. However, the treatment is cost effective for HCV patients with cirrhosis and for those without cirrhosis who have failed other treatments, according to a new study.
In an analysis of the cost effectiveness of sofosbuvir-based treatments for patients with HCV genotypes 2 and 3, Dr. Benjamin Linas of Boston Medical Center reported that when compared with pegylated interferon and ribavirin therapy, treatment regimens based on the new direct-acting antiviral are only cost effective for select groups (Ann. Intern Med, [doi:10.7326/M15-0674]).
Cure rates with sofosbuvir are higher than are rates with the previous standard of care, and sustained virologic response (SVR) “is associated with a greatly reduced lifetime risk for liver-related morbidity and mortality,” noted Dr. Linas and his colleagues.
Using sophisticated statistical modeling to compare HCV treatment with pegylated interferon and ribavirin – the previous standard of care – to the newly Food and Drug Administration–approved sofosbuvir-ribavirin regimen, Dr. Linas explored clinical outcomes and costs for several different patient groups, including treatment-naive and treatment-experienced HCV genotype 2 and 3 patients with and without cirrhosis.
Clinical outcomes, modeled from clinical trial and observational cohort data, were expressed as life expectancy in quality-adjusted life-years (QALYs) and lifetime medical costs. Investigators then calculated the incremental cost-effectiveness ratio (ICER) for each treatment strategy, dividing any additional cost for a more expensive treatment by the QALYs gained from this regimen.
Using the commonly accepted ICER threshold of $100,000 per QALY, Dr. Linas and colleagues concluded that sofosbuvir-based HCV therapy for treatment-naive patients without cirrhosis was not cost effective, with ICERs of $238,000-$266,000. Interferon-based treatments, they noted, still achieve an approximately 80% rate of cure in this population.
In the constrained resources of the real world, Dr. Linas and his associates noted that this analysis is important. “Treatment strategies that do not use limited resources where they are likely to have the greatest impact may result in unequal access to interferon-free regimens, thereby limiting the population-level benefits of new HCV treatments,” they said.
For individuals infected with hepatitis C virus (HCV), new regimens are highly effective, but also very expensive, at approximately $28,000 for 4 weeks of treatment. However, the treatment is cost effective for HCV patients with cirrhosis and for those without cirrhosis who have failed other treatments, according to a new study.
In an analysis of the cost effectiveness of sofosbuvir-based treatments for patients with HCV genotypes 2 and 3, Dr. Benjamin Linas of Boston Medical Center reported that when compared with pegylated interferon and ribavirin therapy, treatment regimens based on the new direct-acting antiviral are only cost effective for select groups (Ann. Intern Med, [doi:10.7326/M15-0674]).
Cure rates with sofosbuvir are higher than are rates with the previous standard of care, and sustained virologic response (SVR) “is associated with a greatly reduced lifetime risk for liver-related morbidity and mortality,” noted Dr. Linas and his colleagues.
Using sophisticated statistical modeling to compare HCV treatment with pegylated interferon and ribavirin – the previous standard of care – to the newly Food and Drug Administration–approved sofosbuvir-ribavirin regimen, Dr. Linas explored clinical outcomes and costs for several different patient groups, including treatment-naive and treatment-experienced HCV genotype 2 and 3 patients with and without cirrhosis.
Clinical outcomes, modeled from clinical trial and observational cohort data, were expressed as life expectancy in quality-adjusted life-years (QALYs) and lifetime medical costs. Investigators then calculated the incremental cost-effectiveness ratio (ICER) for each treatment strategy, dividing any additional cost for a more expensive treatment by the QALYs gained from this regimen.
Using the commonly accepted ICER threshold of $100,000 per QALY, Dr. Linas and colleagues concluded that sofosbuvir-based HCV therapy for treatment-naive patients without cirrhosis was not cost effective, with ICERs of $238,000-$266,000. Interferon-based treatments, they noted, still achieve an approximately 80% rate of cure in this population.
In the constrained resources of the real world, Dr. Linas and his associates noted that this analysis is important. “Treatment strategies that do not use limited resources where they are likely to have the greatest impact may result in unequal access to interferon-free regimens, thereby limiting the population-level benefits of new HCV treatments,” they said.
FROM ANNALS OF INTERNAL MEDICINE
Key clinical point: New treatment for hepatitis C virus (HCV) is cost effective for patients with cirrhosis and for those without cirrhosis who have failed other treatments.
Major finding: Incremental cost-effectiveness ratios for sofosbuvir-based HCV treatment ranged from $238,000 to $266,000 for treatment-naive noncirrhotic patients, exceeding the $100,000 per quality-adjusted life-year cost-effectiveness threshold.
Data source: Application of the Hepatitis C Cost-Effectiveness model to clinical trial and observational cohort data.
Disclosures: The National Institute on Drug Abuse and the National Institute of Allergy and Infectious Disease provided funding. Dr. Weinstein reported receiving personal compensation from OptumInsight; Dr. Kim reported receiving grants and personal compensation from Gilead Sciences, AbbVie Pharmaceuticals, and Bristol-Myers Squibb. The remaining authors reported no relevant disclosures.
Universal bisphosphonates after wrist fracture prevent hip fractures but at a cost
Giving bone-strengthening medication routinely to all elderly women who sustain a wrist fracture would reduce subsequent hip fractures by about a quarter, but at a cost of over $200,000 per prevented fracture. Additionally, expanded bisphosphonate use could cause nearly 20,000 more atypical femur fractures in this population.
Dr. Suneel Bhat and his associates at Thomas Jefferson University, Philadelphia, used sophisticated modeling techniques to project costs and consequences of wider prescribing of bisphosphonates for bone fragility in women aged 65 years and older. Distal radius fracture is known to be associated with osteoporosis in women of this age, who are then at increased risk of subsequent fracture. Dr. Bhat presented his findings on March 24 at the annual meeting of the American Academy of Orthopaedic Surgeons in Las Vegas.
Study authors drew from the medical literature and publicly available Medicare data to obtain fracture incidence data and cost information for statistical modeling. Bhat and colleagues obtained age-specific incidence of distal radius fractures among women aged 65 and older, as well as rates of hip fracture following wrist fractures, both for those who did and did not receive the bisphosphonate risendronate. Atypical femur fracture is a known complication of bisphosphonate treatment for some patients; the risk of this complication and medication costs were drawn from the literature.
To assess the direct costs of hip fracture treatment, investigators used Medicare reimbursement data to price treatment components, including inpatient care as well as surgical and anesthesia services.
From these data, investigators used a modified Monte Carlo technique to obtain a cost and incidence model. This model predicted that 357,656 lifetime subsequent hip fractures would occur in elderly women with wrist fracture; this number would drop to 262,767 with universal bisphosphonate treatment. The cost for this savings, an aggregate $19.5 billion in drug costs, meant that each fracture prevented would cost $205,534. An additional 19,464 patients would sustain atypical femur fracture from risendronate treatment.
Average risendronate costs were estimated at $1,485/patient-year. This figure would have to fall to just $70/patient yearly to make risendronate treatment cost effective, Dr. Bhat calculated.
Giving bone-strengthening medication routinely to all elderly women who sustain a wrist fracture would reduce subsequent hip fractures by about a quarter, but at a cost of over $200,000 per prevented fracture. Additionally, expanded bisphosphonate use could cause nearly 20,000 more atypical femur fractures in this population.
Dr. Suneel Bhat and his associates at Thomas Jefferson University, Philadelphia, used sophisticated modeling techniques to project costs and consequences of wider prescribing of bisphosphonates for bone fragility in women aged 65 years and older. Distal radius fracture is known to be associated with osteoporosis in women of this age, who are then at increased risk of subsequent fracture. Dr. Bhat presented his findings on March 24 at the annual meeting of the American Academy of Orthopaedic Surgeons in Las Vegas.
Study authors drew from the medical literature and publicly available Medicare data to obtain fracture incidence data and cost information for statistical modeling. Bhat and colleagues obtained age-specific incidence of distal radius fractures among women aged 65 and older, as well as rates of hip fracture following wrist fractures, both for those who did and did not receive the bisphosphonate risendronate. Atypical femur fracture is a known complication of bisphosphonate treatment for some patients; the risk of this complication and medication costs were drawn from the literature.
To assess the direct costs of hip fracture treatment, investigators used Medicare reimbursement data to price treatment components, including inpatient care as well as surgical and anesthesia services.
From these data, investigators used a modified Monte Carlo technique to obtain a cost and incidence model. This model predicted that 357,656 lifetime subsequent hip fractures would occur in elderly women with wrist fracture; this number would drop to 262,767 with universal bisphosphonate treatment. The cost for this savings, an aggregate $19.5 billion in drug costs, meant that each fracture prevented would cost $205,534. An additional 19,464 patients would sustain atypical femur fracture from risendronate treatment.
Average risendronate costs were estimated at $1,485/patient-year. This figure would have to fall to just $70/patient yearly to make risendronate treatment cost effective, Dr. Bhat calculated.
Giving bone-strengthening medication routinely to all elderly women who sustain a wrist fracture would reduce subsequent hip fractures by about a quarter, but at a cost of over $200,000 per prevented fracture. Additionally, expanded bisphosphonate use could cause nearly 20,000 more atypical femur fractures in this population.
Dr. Suneel Bhat and his associates at Thomas Jefferson University, Philadelphia, used sophisticated modeling techniques to project costs and consequences of wider prescribing of bisphosphonates for bone fragility in women aged 65 years and older. Distal radius fracture is known to be associated with osteoporosis in women of this age, who are then at increased risk of subsequent fracture. Dr. Bhat presented his findings on March 24 at the annual meeting of the American Academy of Orthopaedic Surgeons in Las Vegas.
Study authors drew from the medical literature and publicly available Medicare data to obtain fracture incidence data and cost information for statistical modeling. Bhat and colleagues obtained age-specific incidence of distal radius fractures among women aged 65 and older, as well as rates of hip fracture following wrist fractures, both for those who did and did not receive the bisphosphonate risendronate. Atypical femur fracture is a known complication of bisphosphonate treatment for some patients; the risk of this complication and medication costs were drawn from the literature.
To assess the direct costs of hip fracture treatment, investigators used Medicare reimbursement data to price treatment components, including inpatient care as well as surgical and anesthesia services.
From these data, investigators used a modified Monte Carlo technique to obtain a cost and incidence model. This model predicted that 357,656 lifetime subsequent hip fractures would occur in elderly women with wrist fracture; this number would drop to 262,767 with universal bisphosphonate treatment. The cost for this savings, an aggregate $19.5 billion in drug costs, meant that each fracture prevented would cost $205,534. An additional 19,464 patients would sustain atypical femur fracture from risendronate treatment.
Average risendronate costs were estimated at $1,485/patient-year. This figure would have to fall to just $70/patient yearly to make risendronate treatment cost effective, Dr. Bhat calculated.
FROM AAOS 2015
Key clinical point: Savings from prevention of hip fractures with universal bisphosphonates after distal radius fractures in elderly women would be outweighed by drug costs.
Major findings: If all women 65 years of age and older who have had a distal radius fracture went on to receive bisphosphonate treatment, 94,888 lifetime hip fractures would be avoided, but at a cost of $205,534 per averted fracture, and with an additional 19,464 atypical femur fractures.
Data source: Modeling based on fracture incidence data and medication, surgical, and aftercare cost data drawn from literature review and publicly available Medicare databases.
Disclosures: Dr. Asif Ilyas reported publishing royalties and financial or material support from Jaypee Medical Publishers and is on the editorial or governing board of Orthopedic Clinics of North America. The other authors reported that they had no conflicts of interest.
Home-administered biologics for RA more common with Medicare Part D subsidies
For Medicare beneficiaries with rheumatoid arthritis, out-of-pocket costs are closely linked to whether a patient is more likely to receive facility- or home-administered biologic treatment, according to a retrospective analysis of how patient cost sharing affects utilization and spending patterns.
Medicare Part D beneficiaries with rheumatoid arthritis (RA) who receive a low-income subsidy (LIS) are more likely to receive biologic medication that can be self-injected at home, the study found, while those without subsidies face greater out-of-pocket costs for outpatient medications and are more likely to receive facility-administered drugs. Lead study author Dr. Jinoos Yazdany noted, “Our findings shed light on the complexity of current Medicare drug coverage policies for biologic DMARDs.”
Dr. Yazdany and her associates at the University of California, San Francisco, used 2009 Medicare claims to obtain a random nationwide sample of 5% of all Medicare beneficiaries with RA who were dispensed at least one RA medication. Of the 6,932 beneficiaries identified, 1,812 (26.1%) were dispensed a biologic disease-modifying antirheumatic drug (DMARD). Utilization patterns of the remainder, who received a nonbiologic DMARD, were used for comparison. In 2009, the biologic DMARDs infliximab, abatacept, and rituximab were all facility-administered medications reimbursable under Medicare Part B, while etanercept, adalimumab, and anakinra were available for home self-injection and were therefore Part D drugs (Arthritis Care Res. 2015 March 16 [doi:10.1002/acr.22580]).
Examining biologic DMARD use and costs, the study calculated costs to Medicare for both home- and facility-administered medications. Part D dispensing data were used to calculate costs to patients by determining the actual out-of-pocket dollar costs for beneficiaries. This information was not available for Part B events, so investigators used a range from 0%-20% cost-sharing to estimate patient costs.
Patient costs for Medicare Part D were further divided by LIS status, since LIS confers minimal cost sharing for outpatient drugs. By contrast, nonsubsidized Part D recipients incur variable costs; the current Medicare Part D coverage gap – also known as the doughnut hole – means that up to 100% of the cost of medications may be borne by the patient at certain points in the coverage cycle.
Multinomial regression analysis showed that those receiving a facility-administered biologic DMARD under Part B were significantly less likely than those receiving nonbiologic DMARDs to have a LIS (relative risk, 0.58; 95% confidence interval, 0.48-0.69). Recipients of self-injected DMARDs under Part D, in contrast, were much more likely to have a LIS than were those in the nonbiologic DMARD group (RR, 2.98; 95% CI, 2.50-3.56). Annual Part D out-of-pocket costs for those receiving biologics with no LIS were a mean $3,751, compared with a mean $72 for the LIS Part D group. However, beneficiaries with no LIS paid a maximum of $2,584 out of pocket for biologic DMARDs through Part B (assuming 20% supplemental insurance coverage).
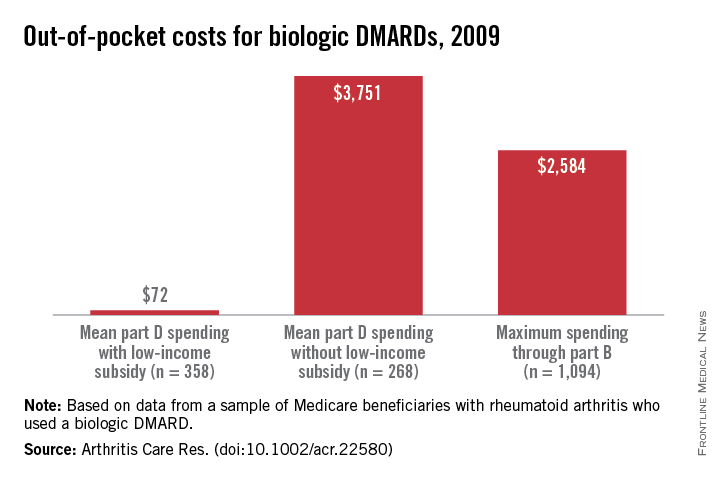
Since the Affordable Care Act will reform Part D to help close the coverage gap, the study also projected how ACA reform, once adopted, would affect cost sharing and use patterns. Dr. Yazdany and her colleagues projected out-of-pocket biologic DMARD costs into full reform implementation in the year 2020, calculating that nonsubsidized out-of-pocket expenses would still be $2,588. The authors noted that “patient cost sharing is an important determinant of drug choice in Medicare’s part B versus part D programs ... the current system risks significant financial burden to many patients.”
When asked to comment on the study, Kaleb Michaud, Ph.D., assistant professor of rheumatology at the University of Nebraska Medical Center, Omaha, and codirector of the National Data Bank for Rheumatic Diseases, noted that this well-constructed study made very good use of Medicare data to point to a fundamental problem with reimbursement for rheumatic disease medications. “Rather than asking, ‘What’s the best drug for this patient?’ we find ourselves asking, ‘What drug can this patient afford?’ ” he said.
The study was funded by the National Institutes of Health, a Research Allocation and Evaluation Award from the University of California, San Francisco, and the Rosaline Russell Medical Research Center for Arthritis. The authors reported that they had no conflicts of interest.
For Medicare beneficiaries with rheumatoid arthritis, out-of-pocket costs are closely linked to whether a patient is more likely to receive facility- or home-administered biologic treatment, according to a retrospective analysis of how patient cost sharing affects utilization and spending patterns.
Medicare Part D beneficiaries with rheumatoid arthritis (RA) who receive a low-income subsidy (LIS) are more likely to receive biologic medication that can be self-injected at home, the study found, while those without subsidies face greater out-of-pocket costs for outpatient medications and are more likely to receive facility-administered drugs. Lead study author Dr. Jinoos Yazdany noted, “Our findings shed light on the complexity of current Medicare drug coverage policies for biologic DMARDs.”
Dr. Yazdany and her associates at the University of California, San Francisco, used 2009 Medicare claims to obtain a random nationwide sample of 5% of all Medicare beneficiaries with RA who were dispensed at least one RA medication. Of the 6,932 beneficiaries identified, 1,812 (26.1%) were dispensed a biologic disease-modifying antirheumatic drug (DMARD). Utilization patterns of the remainder, who received a nonbiologic DMARD, were used for comparison. In 2009, the biologic DMARDs infliximab, abatacept, and rituximab were all facility-administered medications reimbursable under Medicare Part B, while etanercept, adalimumab, and anakinra were available for home self-injection and were therefore Part D drugs (Arthritis Care Res. 2015 March 16 [doi:10.1002/acr.22580]).
Examining biologic DMARD use and costs, the study calculated costs to Medicare for both home- and facility-administered medications. Part D dispensing data were used to calculate costs to patients by determining the actual out-of-pocket dollar costs for beneficiaries. This information was not available for Part B events, so investigators used a range from 0%-20% cost-sharing to estimate patient costs.
Patient costs for Medicare Part D were further divided by LIS status, since LIS confers minimal cost sharing for outpatient drugs. By contrast, nonsubsidized Part D recipients incur variable costs; the current Medicare Part D coverage gap – also known as the doughnut hole – means that up to 100% of the cost of medications may be borne by the patient at certain points in the coverage cycle.
Multinomial regression analysis showed that those receiving a facility-administered biologic DMARD under Part B were significantly less likely than those receiving nonbiologic DMARDs to have a LIS (relative risk, 0.58; 95% confidence interval, 0.48-0.69). Recipients of self-injected DMARDs under Part D, in contrast, were much more likely to have a LIS than were those in the nonbiologic DMARD group (RR, 2.98; 95% CI, 2.50-3.56). Annual Part D out-of-pocket costs for those receiving biologics with no LIS were a mean $3,751, compared with a mean $72 for the LIS Part D group. However, beneficiaries with no LIS paid a maximum of $2,584 out of pocket for biologic DMARDs through Part B (assuming 20% supplemental insurance coverage).

Since the Affordable Care Act will reform Part D to help close the coverage gap, the study also projected how ACA reform, once adopted, would affect cost sharing and use patterns. Dr. Yazdany and her colleagues projected out-of-pocket biologic DMARD costs into full reform implementation in the year 2020, calculating that nonsubsidized out-of-pocket expenses would still be $2,588. The authors noted that “patient cost sharing is an important determinant of drug choice in Medicare’s part B versus part D programs ... the current system risks significant financial burden to many patients.”
When asked to comment on the study, Kaleb Michaud, Ph.D., assistant professor of rheumatology at the University of Nebraska Medical Center, Omaha, and codirector of the National Data Bank for Rheumatic Diseases, noted that this well-constructed study made very good use of Medicare data to point to a fundamental problem with reimbursement for rheumatic disease medications. “Rather than asking, ‘What’s the best drug for this patient?’ we find ourselves asking, ‘What drug can this patient afford?’ ” he said.
The study was funded by the National Institutes of Health, a Research Allocation and Evaluation Award from the University of California, San Francisco, and the Rosaline Russell Medical Research Center for Arthritis. The authors reported that they had no conflicts of interest.
For Medicare beneficiaries with rheumatoid arthritis, out-of-pocket costs are closely linked to whether a patient is more likely to receive facility- or home-administered biologic treatment, according to a retrospective analysis of how patient cost sharing affects utilization and spending patterns.
Medicare Part D beneficiaries with rheumatoid arthritis (RA) who receive a low-income subsidy (LIS) are more likely to receive biologic medication that can be self-injected at home, the study found, while those without subsidies face greater out-of-pocket costs for outpatient medications and are more likely to receive facility-administered drugs. Lead study author Dr. Jinoos Yazdany noted, “Our findings shed light on the complexity of current Medicare drug coverage policies for biologic DMARDs.”
Dr. Yazdany and her associates at the University of California, San Francisco, used 2009 Medicare claims to obtain a random nationwide sample of 5% of all Medicare beneficiaries with RA who were dispensed at least one RA medication. Of the 6,932 beneficiaries identified, 1,812 (26.1%) were dispensed a biologic disease-modifying antirheumatic drug (DMARD). Utilization patterns of the remainder, who received a nonbiologic DMARD, were used for comparison. In 2009, the biologic DMARDs infliximab, abatacept, and rituximab were all facility-administered medications reimbursable under Medicare Part B, while etanercept, adalimumab, and anakinra were available for home self-injection and were therefore Part D drugs (Arthritis Care Res. 2015 March 16 [doi:10.1002/acr.22580]).
Examining biologic DMARD use and costs, the study calculated costs to Medicare for both home- and facility-administered medications. Part D dispensing data were used to calculate costs to patients by determining the actual out-of-pocket dollar costs for beneficiaries. This information was not available for Part B events, so investigators used a range from 0%-20% cost-sharing to estimate patient costs.
Patient costs for Medicare Part D were further divided by LIS status, since LIS confers minimal cost sharing for outpatient drugs. By contrast, nonsubsidized Part D recipients incur variable costs; the current Medicare Part D coverage gap – also known as the doughnut hole – means that up to 100% of the cost of medications may be borne by the patient at certain points in the coverage cycle.
Multinomial regression analysis showed that those receiving a facility-administered biologic DMARD under Part B were significantly less likely than those receiving nonbiologic DMARDs to have a LIS (relative risk, 0.58; 95% confidence interval, 0.48-0.69). Recipients of self-injected DMARDs under Part D, in contrast, were much more likely to have a LIS than were those in the nonbiologic DMARD group (RR, 2.98; 95% CI, 2.50-3.56). Annual Part D out-of-pocket costs for those receiving biologics with no LIS were a mean $3,751, compared with a mean $72 for the LIS Part D group. However, beneficiaries with no LIS paid a maximum of $2,584 out of pocket for biologic DMARDs through Part B (assuming 20% supplemental insurance coverage).

Since the Affordable Care Act will reform Part D to help close the coverage gap, the study also projected how ACA reform, once adopted, would affect cost sharing and use patterns. Dr. Yazdany and her colleagues projected out-of-pocket biologic DMARD costs into full reform implementation in the year 2020, calculating that nonsubsidized out-of-pocket expenses would still be $2,588. The authors noted that “patient cost sharing is an important determinant of drug choice in Medicare’s part B versus part D programs ... the current system risks significant financial burden to many patients.”
When asked to comment on the study, Kaleb Michaud, Ph.D., assistant professor of rheumatology at the University of Nebraska Medical Center, Omaha, and codirector of the National Data Bank for Rheumatic Diseases, noted that this well-constructed study made very good use of Medicare data to point to a fundamental problem with reimbursement for rheumatic disease medications. “Rather than asking, ‘What’s the best drug for this patient?’ we find ourselves asking, ‘What drug can this patient afford?’ ” he said.
The study was funded by the National Institutes of Health, a Research Allocation and Evaluation Award from the University of California, San Francisco, and the Rosaline Russell Medical Research Center for Arthritis. The authors reported that they had no conflicts of interest.
FROM ARTHRITIS CARE & RESEARCH
Key clinical point: Medicare beneficiaries who receive Part D low-income subsidies (LIS) are more likely to receive home-administered biologics for rheumatoid arthritis.
Major finding: LIS Medicare beneficiaries were more likely to receive Part D–reimbursable biologics (RR 2.98, 95% CI, 2.50-3.56); out of pocket Part D costs were lower for LIS beneficiaries ($72) than for those not on subsidies ($3,751).
Data source: Retrospective, nationwide analysis of a random 5% sample of 2009 Medicare beneficiaries with RA and receiving biologic medication.
Disclosures: The study was funded by the National Institutes of Health, a Research Allocation and Evaluation Award from the University of California, San Francisco, and the Rosaline Russell Medical Research Center for Arthritis. The authors reported that they had no conflicts of interest.
Midlife Blood Pressure Patterns Predict CVD, Mortality Risk
BALTIMORE – Distinct patterns of blood pressure changes in midlife are associated with varying degrees of risk for cardiovascular disease and death, according to a multisite study.
These patterns of change contributed to risk for both CVD and mortality, separate from the known association of absolute elevation of systolic blood pressure with CVD and death. Natalia Petruski-Ivleva of the University of North Carolina, Chapel Hill, and colleagues identified SBP patterns emerging from the biracial, multisite Atherosclerosis Risk in Communities (ARIC) study and presented the results at the American Heart Association Epidemiology and Prevention, Lifestyle and Cardiometabolic Health 2015 Scientific Sessions.
The study included 9,882 patients from the ARIC population who had recorded BPs at four study visits between 1987 and 1998, and whose outcomes were tracked over the period from 1987 to 2011. Median follow-up after the last study visit was 13.2 years. Participants were grouped by patterns of change in SBP over time using a latent class growth model; this analytic model allowed for the discovery of similar groups of cases within the data.
Results were adjusted for age and demographic characteristics, as well as for self-reported hypertension medication use. In addition to all-cause mortality, outcomes included coronary heart disease, heart failure, and stroke.
In all, six distinct patterns emerged of change in SBP over the study visit period, with three groups having SBPs consistently below the threshold of 140 mm Hg, and three other groups showing varying patterns of elevation. About 84% of participants fell into one of the three groups that showed parallel patterns of SBP change, with pressures slowly rising over time, but never exceeding 140 mm Hg. The remainder of participants were grouped into three other patterns. One showed a steep increase over time from an initial SBP just under 140 mm Hg to a final reading just over 160 mm Hg; a second showed high and sustained SBPs of more than 160 mm Hg at all study visits. Finally, some participants had initially elevated SBPs that fell to the normal range by the end of the study.
Overall, analysis showed a gradient of risk, with lower SBP associated with lower risk of CVD and death. This was true even for those participants in the first three groups, whose SBPs stayed below 140 mm Hg throughout. Notably, the pattern of steep increase of already elevated SBP, as well as that of sustained elevated blood pressures, were both associated with the highest all-cause mortality. Reducing SBP to less than 140 mm Hg was not associated with reduced risk of CHD in the final group.
The three higher-risk groups were more likely to be obese, black, on hypertension medication, and have diabetes. They were also older on average than participants with the three nonelevated patterns.
These patterns of change, said Ms. Petruski-Ivleva, “contribute varying amounts of risk of CVD and mortality in addition to the risk imparted by the absolute SBP level.” Though the clinical significance of these patterns of blood pressure change needs more examination, she said that the results underscore the cumulative impact of elevated SBP through midlife.
BALTIMORE – Distinct patterns of blood pressure changes in midlife are associated with varying degrees of risk for cardiovascular disease and death, according to a multisite study.
These patterns of change contributed to risk for both CVD and mortality, separate from the known association of absolute elevation of systolic blood pressure with CVD and death. Natalia Petruski-Ivleva of the University of North Carolina, Chapel Hill, and colleagues identified SBP patterns emerging from the biracial, multisite Atherosclerosis Risk in Communities (ARIC) study and presented the results at the American Heart Association Epidemiology and Prevention, Lifestyle and Cardiometabolic Health 2015 Scientific Sessions.
The study included 9,882 patients from the ARIC population who had recorded BPs at four study visits between 1987 and 1998, and whose outcomes were tracked over the period from 1987 to 2011. Median follow-up after the last study visit was 13.2 years. Participants were grouped by patterns of change in SBP over time using a latent class growth model; this analytic model allowed for the discovery of similar groups of cases within the data.
Results were adjusted for age and demographic characteristics, as well as for self-reported hypertension medication use. In addition to all-cause mortality, outcomes included coronary heart disease, heart failure, and stroke.
In all, six distinct patterns emerged of change in SBP over the study visit period, with three groups having SBPs consistently below the threshold of 140 mm Hg, and three other groups showing varying patterns of elevation. About 84% of participants fell into one of the three groups that showed parallel patterns of SBP change, with pressures slowly rising over time, but never exceeding 140 mm Hg. The remainder of participants were grouped into three other patterns. One showed a steep increase over time from an initial SBP just under 140 mm Hg to a final reading just over 160 mm Hg; a second showed high and sustained SBPs of more than 160 mm Hg at all study visits. Finally, some participants had initially elevated SBPs that fell to the normal range by the end of the study.
Overall, analysis showed a gradient of risk, with lower SBP associated with lower risk of CVD and death. This was true even for those participants in the first three groups, whose SBPs stayed below 140 mm Hg throughout. Notably, the pattern of steep increase of already elevated SBP, as well as that of sustained elevated blood pressures, were both associated with the highest all-cause mortality. Reducing SBP to less than 140 mm Hg was not associated with reduced risk of CHD in the final group.
The three higher-risk groups were more likely to be obese, black, on hypertension medication, and have diabetes. They were also older on average than participants with the three nonelevated patterns.
These patterns of change, said Ms. Petruski-Ivleva, “contribute varying amounts of risk of CVD and mortality in addition to the risk imparted by the absolute SBP level.” Though the clinical significance of these patterns of blood pressure change needs more examination, she said that the results underscore the cumulative impact of elevated SBP through midlife.
BALTIMORE – Distinct patterns of blood pressure changes in midlife are associated with varying degrees of risk for cardiovascular disease and death, according to a multisite study.
These patterns of change contributed to risk for both CVD and mortality, separate from the known association of absolute elevation of systolic blood pressure with CVD and death. Natalia Petruski-Ivleva of the University of North Carolina, Chapel Hill, and colleagues identified SBP patterns emerging from the biracial, multisite Atherosclerosis Risk in Communities (ARIC) study and presented the results at the American Heart Association Epidemiology and Prevention, Lifestyle and Cardiometabolic Health 2015 Scientific Sessions.
The study included 9,882 patients from the ARIC population who had recorded BPs at four study visits between 1987 and 1998, and whose outcomes were tracked over the period from 1987 to 2011. Median follow-up after the last study visit was 13.2 years. Participants were grouped by patterns of change in SBP over time using a latent class growth model; this analytic model allowed for the discovery of similar groups of cases within the data.
Results were adjusted for age and demographic characteristics, as well as for self-reported hypertension medication use. In addition to all-cause mortality, outcomes included coronary heart disease, heart failure, and stroke.
In all, six distinct patterns emerged of change in SBP over the study visit period, with three groups having SBPs consistently below the threshold of 140 mm Hg, and three other groups showing varying patterns of elevation. About 84% of participants fell into one of the three groups that showed parallel patterns of SBP change, with pressures slowly rising over time, but never exceeding 140 mm Hg. The remainder of participants were grouped into three other patterns. One showed a steep increase over time from an initial SBP just under 140 mm Hg to a final reading just over 160 mm Hg; a second showed high and sustained SBPs of more than 160 mm Hg at all study visits. Finally, some participants had initially elevated SBPs that fell to the normal range by the end of the study.
Overall, analysis showed a gradient of risk, with lower SBP associated with lower risk of CVD and death. This was true even for those participants in the first three groups, whose SBPs stayed below 140 mm Hg throughout. Notably, the pattern of steep increase of already elevated SBP, as well as that of sustained elevated blood pressures, were both associated with the highest all-cause mortality. Reducing SBP to less than 140 mm Hg was not associated with reduced risk of CHD in the final group.
The three higher-risk groups were more likely to be obese, black, on hypertension medication, and have diabetes. They were also older on average than participants with the three nonelevated patterns.
These patterns of change, said Ms. Petruski-Ivleva, “contribute varying amounts of risk of CVD and mortality in addition to the risk imparted by the absolute SBP level.” Though the clinical significance of these patterns of blood pressure change needs more examination, she said that the results underscore the cumulative impact of elevated SBP through midlife.
Obeticholic acid for NASH improves markers and fibrosis
For patients with nonalcoholic steatohepatitis (NASH), a bile acid derivative that acts on a nuclear receptor reduced liver fat and fibrosis, but patients taking the drug also had worsening in serum lipid values and increased insulin resistance.
The lipophilic bile acid obeticholic acid showed promise in the FLINT trial (Farnesoid X Receptor Ligand Obeticholic Acid in NASH Treatment) for NASH patients whose liver disease improved but did not resolve.
Dr. Brent Neuschwander-Tetri of Saint Louis University, and coinvestigators in the National Institute of Diabetes and Digestive and Kidney Diseases’ NASH Clinical Research Network, conducted the multicenter, randomized, placebo-controlled study. The findings were published in the Lancet.
Obeticholic acid, a farnesoid X nuclear receptor agonist, works at a site whose activation promotes increased insulin sensitivity and decreased serum triglycerides. Serum lipids may also be improved by increased peripheral clearance of VLDL, increased reverse cholesterol transport, and reduced lipid synthesis in the liver. Currently, the only other treatments for NASH, aside from diet and lifestyle modifications, are thiazolidinediones and vitamin E. Long-term safety and efficacy of these medications are unknown, especially in NASH patients with diabetes (Lancet 2015;385:956-65 [doi:10.1016/S0140-6736(14)61933-4]).
Study participants were adults with biopsy-confirmed NASH or borderline NASH with histologic steatosis, ballooning, and lobular inflammation, but without cirrhosis. The study excluded heavy consumers of alcohol. Baseline and final assessments included liver biopsy and demographic and anthropometric information, as well as metabolic and lipid markers and liver enzymes.
FLINT’s primary outcome measure was improved liver histology, defined as a decrease of two or more points on a nonalcoholic fatty liver disease (NAFLD) pathology scoring system, without a worsening of liver fibrosis. Resolution of NASH was a secondary outcome, as were changes in liver enzymes, body measurements, and homeostasis model of assessment of insulin resistance (HOMA-IR) levels.
The study’s Data Safety Monitoring Board recommended stopping biopsies after about half the patients had received biopsies and the primary histologic outcome measure was met, in order to avoid unnecessary patient risk.
An intention-to-treat analysis found that 50 (45%) of 110 patients receiving obeticholic acid had improved liver histology, compared to 23 (21%) of 109 patients receiving placebo (relative risk 1.9, 95% CI 1.3-2.8, P = .0002). This difference did not change with prespecified subgroup analyses. Serum AST and ALT measures decreased significantly for the treatment group. The medication was generally well tolerated, though pruritis developed in 33 (23%) of 141 patients in the intervention group and 9 (6%) of the placebo group.
Total serum cholesterol and LDL cholesterol, however, increased for the obeticholic acid group, while HDL cholesterol decreased. Insulin resistance as measured by HOMA-IR increased slightly for the treatment group, an unexpected effect. Study authors noted that this class of medication’s effect on cholesterol transport is complex. “Future studies of farnesoid X nuclear receptor agonists,” they noted, “will need to address the consequences of these changes on cardiovascular outcomes.”
Dr. Neuschwander-Tetri disclosed ties with Genentech/Roche, Nimbus Discovery, Boehringer Ingelheim, and Bristol-Myers Squibb. Several coinvestigators disclosed ties with various companies, including Intercept Pharmaceuticals, which provided partial funding for the trial under a Collaborative Research and Development Agreement with the National Institute of Diabetes and Digestive and Kidney Diseases.
For patients with nonalcoholic steatohepatitis (NASH), a bile acid derivative that acts on a nuclear receptor reduced liver fat and fibrosis, but patients taking the drug also had worsening in serum lipid values and increased insulin resistance.
The lipophilic bile acid obeticholic acid showed promise in the FLINT trial (Farnesoid X Receptor Ligand Obeticholic Acid in NASH Treatment) for NASH patients whose liver disease improved but did not resolve.
Dr. Brent Neuschwander-Tetri of Saint Louis University, and coinvestigators in the National Institute of Diabetes and Digestive and Kidney Diseases’ NASH Clinical Research Network, conducted the multicenter, randomized, placebo-controlled study. The findings were published in the Lancet.
Obeticholic acid, a farnesoid X nuclear receptor agonist, works at a site whose activation promotes increased insulin sensitivity and decreased serum triglycerides. Serum lipids may also be improved by increased peripheral clearance of VLDL, increased reverse cholesterol transport, and reduced lipid synthesis in the liver. Currently, the only other treatments for NASH, aside from diet and lifestyle modifications, are thiazolidinediones and vitamin E. Long-term safety and efficacy of these medications are unknown, especially in NASH patients with diabetes (Lancet 2015;385:956-65 [doi:10.1016/S0140-6736(14)61933-4]).
Study participants were adults with biopsy-confirmed NASH or borderline NASH with histologic steatosis, ballooning, and lobular inflammation, but without cirrhosis. The study excluded heavy consumers of alcohol. Baseline and final assessments included liver biopsy and demographic and anthropometric information, as well as metabolic and lipid markers and liver enzymes.
FLINT’s primary outcome measure was improved liver histology, defined as a decrease of two or more points on a nonalcoholic fatty liver disease (NAFLD) pathology scoring system, without a worsening of liver fibrosis. Resolution of NASH was a secondary outcome, as were changes in liver enzymes, body measurements, and homeostasis model of assessment of insulin resistance (HOMA-IR) levels.
The study’s Data Safety Monitoring Board recommended stopping biopsies after about half the patients had received biopsies and the primary histologic outcome measure was met, in order to avoid unnecessary patient risk.
An intention-to-treat analysis found that 50 (45%) of 110 patients receiving obeticholic acid had improved liver histology, compared to 23 (21%) of 109 patients receiving placebo (relative risk 1.9, 95% CI 1.3-2.8, P = .0002). This difference did not change with prespecified subgroup analyses. Serum AST and ALT measures decreased significantly for the treatment group. The medication was generally well tolerated, though pruritis developed in 33 (23%) of 141 patients in the intervention group and 9 (6%) of the placebo group.
Total serum cholesterol and LDL cholesterol, however, increased for the obeticholic acid group, while HDL cholesterol decreased. Insulin resistance as measured by HOMA-IR increased slightly for the treatment group, an unexpected effect. Study authors noted that this class of medication’s effect on cholesterol transport is complex. “Future studies of farnesoid X nuclear receptor agonists,” they noted, “will need to address the consequences of these changes on cardiovascular outcomes.”
Dr. Neuschwander-Tetri disclosed ties with Genentech/Roche, Nimbus Discovery, Boehringer Ingelheim, and Bristol-Myers Squibb. Several coinvestigators disclosed ties with various companies, including Intercept Pharmaceuticals, which provided partial funding for the trial under a Collaborative Research and Development Agreement with the National Institute of Diabetes and Digestive and Kidney Diseases.
For patients with nonalcoholic steatohepatitis (NASH), a bile acid derivative that acts on a nuclear receptor reduced liver fat and fibrosis, but patients taking the drug also had worsening in serum lipid values and increased insulin resistance.
The lipophilic bile acid obeticholic acid showed promise in the FLINT trial (Farnesoid X Receptor Ligand Obeticholic Acid in NASH Treatment) for NASH patients whose liver disease improved but did not resolve.
Dr. Brent Neuschwander-Tetri of Saint Louis University, and coinvestigators in the National Institute of Diabetes and Digestive and Kidney Diseases’ NASH Clinical Research Network, conducted the multicenter, randomized, placebo-controlled study. The findings were published in the Lancet.
Obeticholic acid, a farnesoid X nuclear receptor agonist, works at a site whose activation promotes increased insulin sensitivity and decreased serum triglycerides. Serum lipids may also be improved by increased peripheral clearance of VLDL, increased reverse cholesterol transport, and reduced lipid synthesis in the liver. Currently, the only other treatments for NASH, aside from diet and lifestyle modifications, are thiazolidinediones and vitamin E. Long-term safety and efficacy of these medications are unknown, especially in NASH patients with diabetes (Lancet 2015;385:956-65 [doi:10.1016/S0140-6736(14)61933-4]).
Study participants were adults with biopsy-confirmed NASH or borderline NASH with histologic steatosis, ballooning, and lobular inflammation, but without cirrhosis. The study excluded heavy consumers of alcohol. Baseline and final assessments included liver biopsy and demographic and anthropometric information, as well as metabolic and lipid markers and liver enzymes.
FLINT’s primary outcome measure was improved liver histology, defined as a decrease of two or more points on a nonalcoholic fatty liver disease (NAFLD) pathology scoring system, without a worsening of liver fibrosis. Resolution of NASH was a secondary outcome, as were changes in liver enzymes, body measurements, and homeostasis model of assessment of insulin resistance (HOMA-IR) levels.
The study’s Data Safety Monitoring Board recommended stopping biopsies after about half the patients had received biopsies and the primary histologic outcome measure was met, in order to avoid unnecessary patient risk.
An intention-to-treat analysis found that 50 (45%) of 110 patients receiving obeticholic acid had improved liver histology, compared to 23 (21%) of 109 patients receiving placebo (relative risk 1.9, 95% CI 1.3-2.8, P = .0002). This difference did not change with prespecified subgroup analyses. Serum AST and ALT measures decreased significantly for the treatment group. The medication was generally well tolerated, though pruritis developed in 33 (23%) of 141 patients in the intervention group and 9 (6%) of the placebo group.
Total serum cholesterol and LDL cholesterol, however, increased for the obeticholic acid group, while HDL cholesterol decreased. Insulin resistance as measured by HOMA-IR increased slightly for the treatment group, an unexpected effect. Study authors noted that this class of medication’s effect on cholesterol transport is complex. “Future studies of farnesoid X nuclear receptor agonists,” they noted, “will need to address the consequences of these changes on cardiovascular outcomes.”
Dr. Neuschwander-Tetri disclosed ties with Genentech/Roche, Nimbus Discovery, Boehringer Ingelheim, and Bristol-Myers Squibb. Several coinvestigators disclosed ties with various companies, including Intercept Pharmaceuticals, which provided partial funding for the trial under a Collaborative Research and Development Agreement with the National Institute of Diabetes and Digestive and Kidney Diseases.
FROM THE LANCET
Key clinical point: The farnesoid X nuclear receptor agonist obeticholic acid improved clinical markers of nonalcoholic steatohepatitis (NASH) but increased serum cholesterol.
Major finding: Of patients assigned to receive liver biopsies, 50/110 (45%) receiving obeticholic acid showed improvement in liver histology compared with 23/109 (21%) in the placebo group (P = .0002).
Data source: The Farnesoid X Receptor Ligand Obeticholic Acid in NASH Treatment (FLINT) study, a 72 week multicenter, double-blind, placebo-controlled trial of obeticholic acid for treatment of 283 patients with noncirrhotic NASH.
Disclosures: Dr. Neuschwander-Tetri disclosed ties with Genentech/Roche, Nimbus Discovery, Boehringer Ingelheim, and Bristol-Myers Squibb. Several coinvestigators disclosed ties with various companies, including Intercept Pharmaceuticals, which provided partial funding for the trial under a Collaborative Research and Development Agreement with the National Institute of Diabetes and Digestive and Kidney Diseases.








