User login
Lucas Franki is an associate editor for MDedge News, and has been with the company since 2014. He has a BA in English from Penn State University and is an Eagle Scout.
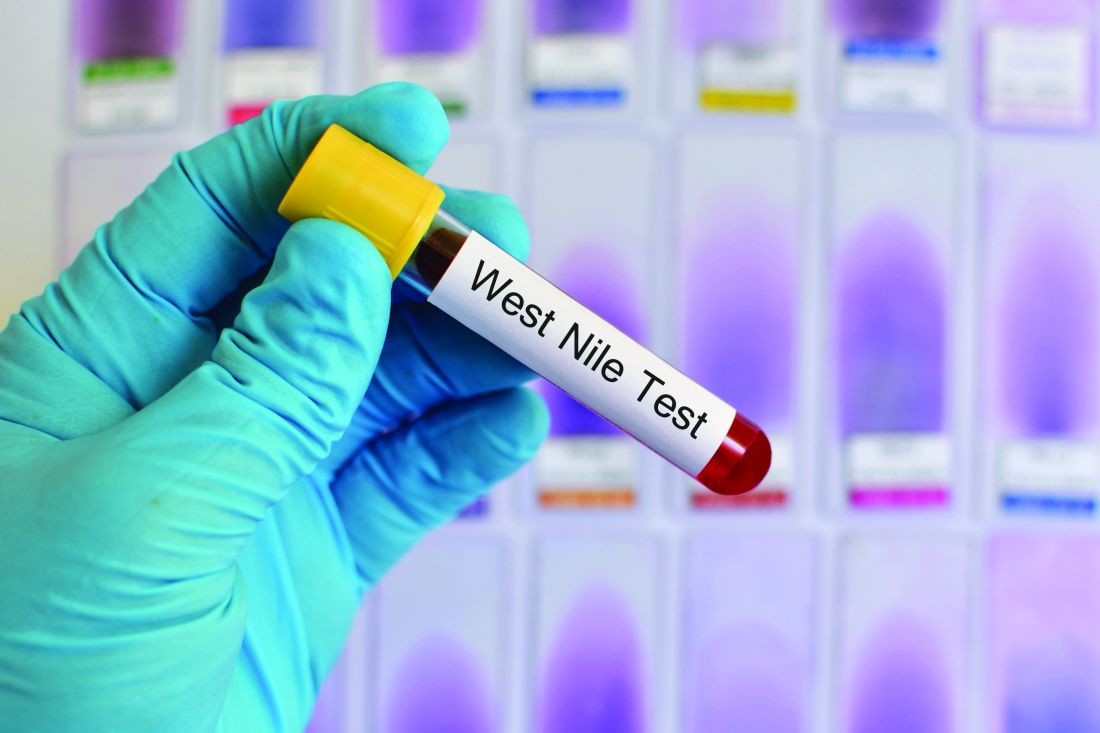
West Nile virus accounted for 95% of domestic arboviral disease in 2015
West Nile virus was the most common cause of domestically acquired arboviral disease in the United States in 2015, according to a report from the Centers for Disease Control and Prevention.
A total of 2,282 cases of arboviral disease were reported to the CDC in 2015. Of those, 2,175 cases were caused by the West Nile virus. Of the patients with WNV, 1,616 were hospitalized because of the disease, and 146 died. Neuroinvasive WNV, which occurred in 1,455 cases, accounted for 1,382 of 1,616 WNV hospitalizations and 142 of 146 deaths.
Of the 107 non-WNV arbovirus cases reported to the CDC, 55 were La Crosse virus, 23 were St. Louis encephalitis, 11 were Jamestown Canyon virus, 7 were Powassan virus, and 6 were eastern equine encephalitis. In addition to La Crosse and Jamestown Canyon, 4 cases of additional California serogroup viruses were reported, as was 1 case of Cache Valley virus.
“Health care providers should consider arboviral infections in the differential diagnosis of cases of aseptic meningitis and encephalitis, obtain appropriate specimens for laboratory testing, and promptly report cases to public health authorities. Because human vaccines against domestic arboviruses are not available, prevention depends on community and household efforts to reduce vector populations, personal protective measures to decrease exposure to mosquitoes and ticks, and screening of blood donors,” the CDC investigators concluded.
Find the full report in the MMWR (doi: 10.15585/mmwr.mm6602a3).
West Nile virus was the most common cause of domestically acquired arboviral disease in the United States in 2015, according to a report from the Centers for Disease Control and Prevention.
A total of 2,282 cases of arboviral disease were reported to the CDC in 2015. Of those, 2,175 cases were caused by the West Nile virus. Of the patients with WNV, 1,616 were hospitalized because of the disease, and 146 died. Neuroinvasive WNV, which occurred in 1,455 cases, accounted for 1,382 of 1,616 WNV hospitalizations and 142 of 146 deaths.
Of the 107 non-WNV arbovirus cases reported to the CDC, 55 were La Crosse virus, 23 were St. Louis encephalitis, 11 were Jamestown Canyon virus, 7 were Powassan virus, and 6 were eastern equine encephalitis. In addition to La Crosse and Jamestown Canyon, 4 cases of additional California serogroup viruses were reported, as was 1 case of Cache Valley virus.
“Health care providers should consider arboviral infections in the differential diagnosis of cases of aseptic meningitis and encephalitis, obtain appropriate specimens for laboratory testing, and promptly report cases to public health authorities. Because human vaccines against domestic arboviruses are not available, prevention depends on community and household efforts to reduce vector populations, personal protective measures to decrease exposure to mosquitoes and ticks, and screening of blood donors,” the CDC investigators concluded.
Find the full report in the MMWR (doi: 10.15585/mmwr.mm6602a3).
West Nile virus was the most common cause of domestically acquired arboviral disease in the United States in 2015, according to a report from the Centers for Disease Control and Prevention.
A total of 2,282 cases of arboviral disease were reported to the CDC in 2015. Of those, 2,175 cases were caused by the West Nile virus. Of the patients with WNV, 1,616 were hospitalized because of the disease, and 146 died. Neuroinvasive WNV, which occurred in 1,455 cases, accounted for 1,382 of 1,616 WNV hospitalizations and 142 of 146 deaths.
Of the 107 non-WNV arbovirus cases reported to the CDC, 55 were La Crosse virus, 23 were St. Louis encephalitis, 11 were Jamestown Canyon virus, 7 were Powassan virus, and 6 were eastern equine encephalitis. In addition to La Crosse and Jamestown Canyon, 4 cases of additional California serogroup viruses were reported, as was 1 case of Cache Valley virus.
“Health care providers should consider arboviral infections in the differential diagnosis of cases of aseptic meningitis and encephalitis, obtain appropriate specimens for laboratory testing, and promptly report cases to public health authorities. Because human vaccines against domestic arboviruses are not available, prevention depends on community and household efforts to reduce vector populations, personal protective measures to decrease exposure to mosquitoes and ticks, and screening of blood donors,” the CDC investigators concluded.
Find the full report in the MMWR (doi: 10.15585/mmwr.mm6602a3).
FROM MORBIDITY AND MORTALITY REPORT
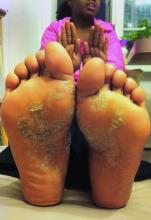
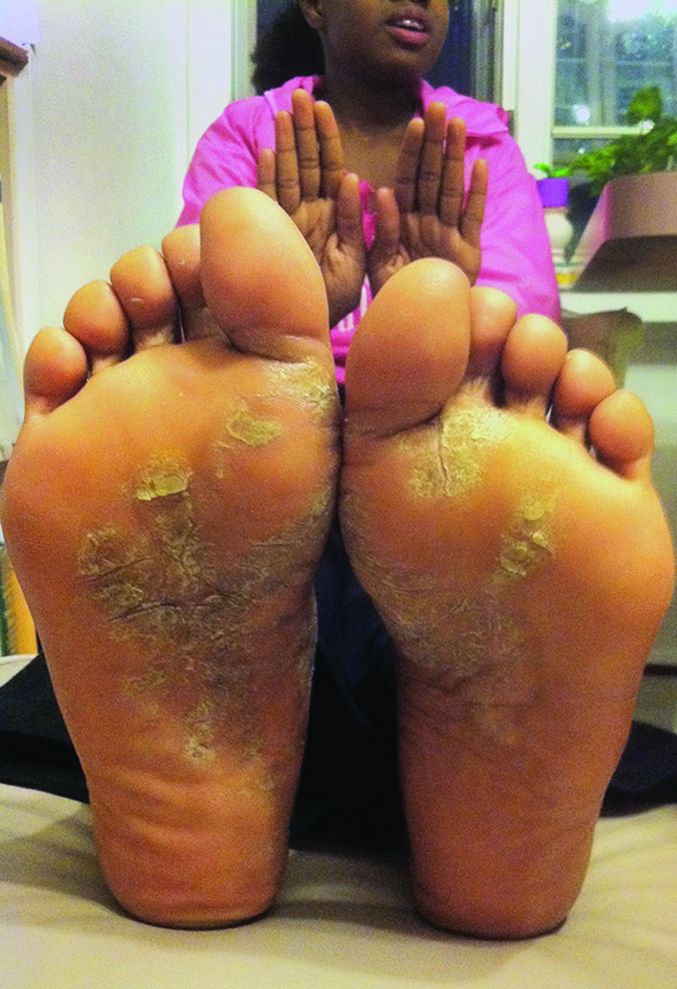
Childhood psoriasis negatively impacts parental QOL
Parents of children with psoriasis experience significant negative quality-of-life issues, according to Dr. Megha Tollefson and her associates.
In interviews with 31 parents of children with psoriasis, four themes impacting quality of life emerged. Parental health and self-care were negatively affected, with 52% reporting affected sleep patterns and 35% reporting difficulty in maintaining self-care. Mental health issues were common as well, with 65% of parents reporting concern over their child’s condition and nearly half of parents reporting excess stress and sadness, anxiety, or depression.
Family and social functions were adversely affected as well, with parents commonly citing financial difficulty, tension with their spouse or significant other, difficulty in other relationships, a lack of awareness regarding psoriasis, and the burden of care. The final theme highlighted was the sacrifice of personal well-being and life pursuits, with 29% of parents reporting career impacts, 65% reporting a need for special accommodation for their child, and 48% reporting a feeling of having no time.
“The results of this study are a testament to the pervasiveness of childhood psoriasis in a parent’s life. Development of support strategies is recommended for children with psoriasis and their families,” Dr. Tollefson and her coauthors at the Mayo Clinic in Rochester, Minn., concluded.
Find the full study in the Journal of the American Academy of Dermatology (doi:10.1016/j.jaad.2016.09.014).
Parents of children with psoriasis experience significant negative quality-of-life issues, according to Dr. Megha Tollefson and her associates.
In interviews with 31 parents of children with psoriasis, four themes impacting quality of life emerged. Parental health and self-care were negatively affected, with 52% reporting affected sleep patterns and 35% reporting difficulty in maintaining self-care. Mental health issues were common as well, with 65% of parents reporting concern over their child’s condition and nearly half of parents reporting excess stress and sadness, anxiety, or depression.
Family and social functions were adversely affected as well, with parents commonly citing financial difficulty, tension with their spouse or significant other, difficulty in other relationships, a lack of awareness regarding psoriasis, and the burden of care. The final theme highlighted was the sacrifice of personal well-being and life pursuits, with 29% of parents reporting career impacts, 65% reporting a need for special accommodation for their child, and 48% reporting a feeling of having no time.
“The results of this study are a testament to the pervasiveness of childhood psoriasis in a parent’s life. Development of support strategies is recommended for children with psoriasis and their families,” Dr. Tollefson and her coauthors at the Mayo Clinic in Rochester, Minn., concluded.
Find the full study in the Journal of the American Academy of Dermatology (doi:10.1016/j.jaad.2016.09.014).
Parents of children with psoriasis experience significant negative quality-of-life issues, according to Dr. Megha Tollefson and her associates.
In interviews with 31 parents of children with psoriasis, four themes impacting quality of life emerged. Parental health and self-care were negatively affected, with 52% reporting affected sleep patterns and 35% reporting difficulty in maintaining self-care. Mental health issues were common as well, with 65% of parents reporting concern over their child’s condition and nearly half of parents reporting excess stress and sadness, anxiety, or depression.
Family and social functions were adversely affected as well, with parents commonly citing financial difficulty, tension with their spouse or significant other, difficulty in other relationships, a lack of awareness regarding psoriasis, and the burden of care. The final theme highlighted was the sacrifice of personal well-being and life pursuits, with 29% of parents reporting career impacts, 65% reporting a need for special accommodation for their child, and 48% reporting a feeling of having no time.
“The results of this study are a testament to the pervasiveness of childhood psoriasis in a parent’s life. Development of support strategies is recommended for children with psoriasis and their families,” Dr. Tollefson and her coauthors at the Mayo Clinic in Rochester, Minn., concluded.
Find the full study in the Journal of the American Academy of Dermatology (doi:10.1016/j.jaad.2016.09.014).
FROM THE JOURNAL OF THE AMERICAN ACADEMY OF DERMATOLOGY
Adding entacapone to intestinal gel could lower levodopa effective dose
The addition of the catechol-O-methyltransferase inhibitor entacapone to levodopa-carbidopa intestinal gel provided adequate levodopa exposure at a lower dose than with levodopa-carbidopa intestinal gel alone in a small, open-label randomized, crossover study of patients with advanced Parkinson’s disease.
Over 2 consecutive days, Marina Senek and her associates randomized 11 patients who were on stable levodopa-carbidopa intestinal gel (LCIG; Duodopa) therapy to receive 14-hour infusions of new LCIG formulation with entacapone (LECIG) followed by LCIG alone the next day or vice versa. Five patients received LECIG morning doses that corresponded to 80% of their individual morning dose of LCIG and six received 90% of their individual morning dose. They received maintenance doses that were equal to 80% of the LCIG maintenance dose and extra doses that were equal to 80% of extra LCIG doses.
Dose-adjusted levodopa exposure was significantly higher during LECIG treatment, with 9 of 11 patients exceeding the 20% increase in levodopa exposure targeted. Systemic levodopa exposure did not differ significantly between treatment types.
Sixteen adverse events were reported: 6 by two patients during LCIG treatment, and 10 by five patients during LECIG treatment. Headache was the most common adverse event, experienced by one patient in the LCIG group and by three in the LECIG group. Other adverse events related to treatment were nausea, diarrhea, and dizziness. No serious adverse events were reported.
By blocking the second-largest metabolic pathway for levodopa, the administration of entacapone leads to less levodopa conversion to 3-O-methyldopa and thereby increases the levodopa plasma concentration, the investigators said.
“Because of the short treatment time, conclusions have to be drawn with caution, and long-term comparative efficacy studies are needed to confirm the results and investigate the possible long-term side effects with the addition of entacapone,” the investigators noted.
Read the full study in Movement Disorders (doi: 10.1002/mds.26855).
The addition of the catechol-O-methyltransferase inhibitor entacapone to levodopa-carbidopa intestinal gel provided adequate levodopa exposure at a lower dose than with levodopa-carbidopa intestinal gel alone in a small, open-label randomized, crossover study of patients with advanced Parkinson’s disease.
Over 2 consecutive days, Marina Senek and her associates randomized 11 patients who were on stable levodopa-carbidopa intestinal gel (LCIG; Duodopa) therapy to receive 14-hour infusions of new LCIG formulation with entacapone (LECIG) followed by LCIG alone the next day or vice versa. Five patients received LECIG morning doses that corresponded to 80% of their individual morning dose of LCIG and six received 90% of their individual morning dose. They received maintenance doses that were equal to 80% of the LCIG maintenance dose and extra doses that were equal to 80% of extra LCIG doses.
Dose-adjusted levodopa exposure was significantly higher during LECIG treatment, with 9 of 11 patients exceeding the 20% increase in levodopa exposure targeted. Systemic levodopa exposure did not differ significantly between treatment types.
Sixteen adverse events were reported: 6 by two patients during LCIG treatment, and 10 by five patients during LECIG treatment. Headache was the most common adverse event, experienced by one patient in the LCIG group and by three in the LECIG group. Other adverse events related to treatment were nausea, diarrhea, and dizziness. No serious adverse events were reported.
By blocking the second-largest metabolic pathway for levodopa, the administration of entacapone leads to less levodopa conversion to 3-O-methyldopa and thereby increases the levodopa plasma concentration, the investigators said.
“Because of the short treatment time, conclusions have to be drawn with caution, and long-term comparative efficacy studies are needed to confirm the results and investigate the possible long-term side effects with the addition of entacapone,” the investigators noted.
Read the full study in Movement Disorders (doi: 10.1002/mds.26855).
The addition of the catechol-O-methyltransferase inhibitor entacapone to levodopa-carbidopa intestinal gel provided adequate levodopa exposure at a lower dose than with levodopa-carbidopa intestinal gel alone in a small, open-label randomized, crossover study of patients with advanced Parkinson’s disease.
Over 2 consecutive days, Marina Senek and her associates randomized 11 patients who were on stable levodopa-carbidopa intestinal gel (LCIG; Duodopa) therapy to receive 14-hour infusions of new LCIG formulation with entacapone (LECIG) followed by LCIG alone the next day or vice versa. Five patients received LECIG morning doses that corresponded to 80% of their individual morning dose of LCIG and six received 90% of their individual morning dose. They received maintenance doses that were equal to 80% of the LCIG maintenance dose and extra doses that were equal to 80% of extra LCIG doses.
Dose-adjusted levodopa exposure was significantly higher during LECIG treatment, with 9 of 11 patients exceeding the 20% increase in levodopa exposure targeted. Systemic levodopa exposure did not differ significantly between treatment types.
Sixteen adverse events were reported: 6 by two patients during LCIG treatment, and 10 by five patients during LECIG treatment. Headache was the most common adverse event, experienced by one patient in the LCIG group and by three in the LECIG group. Other adverse events related to treatment were nausea, diarrhea, and dizziness. No serious adverse events were reported.
By blocking the second-largest metabolic pathway for levodopa, the administration of entacapone leads to less levodopa conversion to 3-O-methyldopa and thereby increases the levodopa plasma concentration, the investigators said.
“Because of the short treatment time, conclusions have to be drawn with caution, and long-term comparative efficacy studies are needed to confirm the results and investigate the possible long-term side effects with the addition of entacapone,” the investigators noted.
Read the full study in Movement Disorders (doi: 10.1002/mds.26855).
FROM MOVEMENT DISORDERS
FDA warns of false-positive results with Zika IgM test
, according to a safety alert issued by the Food and Drug Administration.
The ZIKV Detect IgM Capture ELISA test is the first commercially available Zika serological IgM test – it was approved by the FDA in August 2016 and is used by several commercial laboratories. The test reports only presumptive positive results and a sample has to be sent to the Centers for Disease Control and Prevention for confirmation. Final results can take up to a month to be delivered. In most instances, the preliminary test results have matched the confirmed sample results.
The FDA recommends that health care providers inform patients that presumptive positive results need to be confirmed and that they not rely on positive IgM test results as the sole basis of patient management. If a patient is pregnant, the FDA recommends contacting the laboratory to expedite the confirmation testing.
FDA officials are working with LabCorp and ZIKV Detect manufacturer InBios International to determine if the false-positive results are related to problems with the test or the commercial testing facility.
Find the full safety alert on the FDA website.
, according to a safety alert issued by the Food and Drug Administration.
The ZIKV Detect IgM Capture ELISA test is the first commercially available Zika serological IgM test – it was approved by the FDA in August 2016 and is used by several commercial laboratories. The test reports only presumptive positive results and a sample has to be sent to the Centers for Disease Control and Prevention for confirmation. Final results can take up to a month to be delivered. In most instances, the preliminary test results have matched the confirmed sample results.
The FDA recommends that health care providers inform patients that presumptive positive results need to be confirmed and that they not rely on positive IgM test results as the sole basis of patient management. If a patient is pregnant, the FDA recommends contacting the laboratory to expedite the confirmation testing.
FDA officials are working with LabCorp and ZIKV Detect manufacturer InBios International to determine if the false-positive results are related to problems with the test or the commercial testing facility.
Find the full safety alert on the FDA website.
, according to a safety alert issued by the Food and Drug Administration.
The ZIKV Detect IgM Capture ELISA test is the first commercially available Zika serological IgM test – it was approved by the FDA in August 2016 and is used by several commercial laboratories. The test reports only presumptive positive results and a sample has to be sent to the Centers for Disease Control and Prevention for confirmation. Final results can take up to a month to be delivered. In most instances, the preliminary test results have matched the confirmed sample results.
The FDA recommends that health care providers inform patients that presumptive positive results need to be confirmed and that they not rely on positive IgM test results as the sole basis of patient management. If a patient is pregnant, the FDA recommends contacting the laboratory to expedite the confirmation testing.
FDA officials are working with LabCorp and ZIKV Detect manufacturer InBios International to determine if the false-positive results are related to problems with the test or the commercial testing facility.
Find the full safety alert on the FDA website.
History of complex regional pain syndrome increases risk of secondary CRPS
Secondary complex regional pain syndrome is significantly more likely in people currently experiencing CRPS in an unrelated extremity than in the general population, according to Ellen Satteson, MD, and her associates.
In a study of 93 patients with CRPS, 20.4% developed secondary CRPS in another extremity. Twenty patients in the primary CRPS group experienced a secondary inciting event. Of this group, 75% developed secondary CRPS. CRPS in all four extremities occurred in six patients, of whom five had inciting events for each extremity.
The odds ratio for secondary CRPS in the study group compared to the general population was found to be 1,069.6, while the OR for CRPS after an inciting event compared to the general population was 11.7.
“An odds ratio of over 1,000 when comparing the reported population incidence of CRPS to the rate of secondary CRPS documented in this study strongly suggests that patients with a history of CRPS may be at considerable risk of developing secondary CRPS ... Additional, prospective studies with standardized follow-up to assess for subsequent injuries and secondary CRPS, however, are needed to better elucidate the significance of this risk,” the investigators noted.
Find the study in the Scandinavian Journal of Pain (doi: 10.1016/j.sjpain.2016.10.005).
Secondary complex regional pain syndrome is significantly more likely in people currently experiencing CRPS in an unrelated extremity than in the general population, according to Ellen Satteson, MD, and her associates.
In a study of 93 patients with CRPS, 20.4% developed secondary CRPS in another extremity. Twenty patients in the primary CRPS group experienced a secondary inciting event. Of this group, 75% developed secondary CRPS. CRPS in all four extremities occurred in six patients, of whom five had inciting events for each extremity.
The odds ratio for secondary CRPS in the study group compared to the general population was found to be 1,069.6, while the OR for CRPS after an inciting event compared to the general population was 11.7.
“An odds ratio of over 1,000 when comparing the reported population incidence of CRPS to the rate of secondary CRPS documented in this study strongly suggests that patients with a history of CRPS may be at considerable risk of developing secondary CRPS ... Additional, prospective studies with standardized follow-up to assess for subsequent injuries and secondary CRPS, however, are needed to better elucidate the significance of this risk,” the investigators noted.
Find the study in the Scandinavian Journal of Pain (doi: 10.1016/j.sjpain.2016.10.005).
Secondary complex regional pain syndrome is significantly more likely in people currently experiencing CRPS in an unrelated extremity than in the general population, according to Ellen Satteson, MD, and her associates.
In a study of 93 patients with CRPS, 20.4% developed secondary CRPS in another extremity. Twenty patients in the primary CRPS group experienced a secondary inciting event. Of this group, 75% developed secondary CRPS. CRPS in all four extremities occurred in six patients, of whom five had inciting events for each extremity.
The odds ratio for secondary CRPS in the study group compared to the general population was found to be 1,069.6, while the OR for CRPS after an inciting event compared to the general population was 11.7.
“An odds ratio of over 1,000 when comparing the reported population incidence of CRPS to the rate of secondary CRPS documented in this study strongly suggests that patients with a history of CRPS may be at considerable risk of developing secondary CRPS ... Additional, prospective studies with standardized follow-up to assess for subsequent injuries and secondary CRPS, however, are needed to better elucidate the significance of this risk,” the investigators noted.
Find the study in the Scandinavian Journal of Pain (doi: 10.1016/j.sjpain.2016.10.005).
FROM THE SCANDINAVIAN JOURNAL OF PAIN
Mass spectrometry of gastric aspirates can predict RDS in premature infants
A mass spectrometry test was able to rapidly measure lung maturity in premature infants at risk for respiratory distress syndrome (RDS), according to Henrik Verder, MD, of Holbaek (Denmark) University Hospital, and his associates.
Samples of gastric aspirates were taken from 136 infants with gestation periods of 24-31 weeks, and analyzed with mass spectrometry. Of this group, 61 developed RDS, and 7 died before the end of the study period. With a lecithin/sphingomyelin (L/S) cut-off ratio of 2.2, sensitivity of the mass spectrometry test was 92%, specificity was 73%, positive predictive value was 74%, and negative predictive was value of 92%. Sensitivity was high for all gestational age groups, the investigators noted.
Oropharyngeal secretions were sampled from an additional group of 59 infants and analyzed using spectrometry, with an L/S cut-off value of 3.7; however sensitivity and specificity were lower than for gastric aspirates.
“This test could help identify which infants will benefit from very early surfactant treatment, with the potential to significantly improve clinical outcomes resulting in less severe RDS, less need of mechanical ventilation and oxygen and less severe bronchopulmonary dysplasia,” the investigators concluded.
Find the study in Acta Paediatrica (2016. doi: 10.1111/apa.13683)
A mass spectrometry test was able to rapidly measure lung maturity in premature infants at risk for respiratory distress syndrome (RDS), according to Henrik Verder, MD, of Holbaek (Denmark) University Hospital, and his associates.
Samples of gastric aspirates were taken from 136 infants with gestation periods of 24-31 weeks, and analyzed with mass spectrometry. Of this group, 61 developed RDS, and 7 died before the end of the study period. With a lecithin/sphingomyelin (L/S) cut-off ratio of 2.2, sensitivity of the mass spectrometry test was 92%, specificity was 73%, positive predictive value was 74%, and negative predictive was value of 92%. Sensitivity was high for all gestational age groups, the investigators noted.
Oropharyngeal secretions were sampled from an additional group of 59 infants and analyzed using spectrometry, with an L/S cut-off value of 3.7; however sensitivity and specificity were lower than for gastric aspirates.
“This test could help identify which infants will benefit from very early surfactant treatment, with the potential to significantly improve clinical outcomes resulting in less severe RDS, less need of mechanical ventilation and oxygen and less severe bronchopulmonary dysplasia,” the investigators concluded.
Find the study in Acta Paediatrica (2016. doi: 10.1111/apa.13683)
A mass spectrometry test was able to rapidly measure lung maturity in premature infants at risk for respiratory distress syndrome (RDS), according to Henrik Verder, MD, of Holbaek (Denmark) University Hospital, and his associates.
Samples of gastric aspirates were taken from 136 infants with gestation periods of 24-31 weeks, and analyzed with mass spectrometry. Of this group, 61 developed RDS, and 7 died before the end of the study period. With a lecithin/sphingomyelin (L/S) cut-off ratio of 2.2, sensitivity of the mass spectrometry test was 92%, specificity was 73%, positive predictive value was 74%, and negative predictive was value of 92%. Sensitivity was high for all gestational age groups, the investigators noted.
Oropharyngeal secretions were sampled from an additional group of 59 infants and analyzed using spectrometry, with an L/S cut-off value of 3.7; however sensitivity and specificity were lower than for gastric aspirates.
“This test could help identify which infants will benefit from very early surfactant treatment, with the potential to significantly improve clinical outcomes resulting in less severe RDS, less need of mechanical ventilation and oxygen and less severe bronchopulmonary dysplasia,” the investigators concluded.
Find the study in Acta Paediatrica (2016. doi: 10.1111/apa.13683)
Partnerships with pediatric tertiary care centers improve community ED asthma treatment
Partnerships between community emergency departments and pediatric tertiary care centers are feasible and improve care of pediatric asthma, according to Theresa A. Walls, MD, of the Children’s National Health Systems, Washington, D.C., and her associates.
A total of 724 asthma patients aged 2-17 years were included in the study. Of this group, 289 (40%) were treated at the community ED before the pediatric tertiary care center intervention and 435 (60%) were treated after the intervention. Treatment with steroids was significantly increased post intervention, with 76% of patients receiving steroids, compared with 60% of patients before the intervention.
“Because the overwhelming majority of pediatric emergency visits occur in community EDs, partnerships with these EDs can broaden the impact of quality improvement activities and should be part of future quality improvement efforts,” the investigators concluded.
Find the full study in Pediatrics (2016. doi: 10.1542/peds.2016-0088).
Dr. Walls and her group developed a quality improvement (QI) initiative with a community emergency department. One important part of the study was the use of an asthma score, which helped determine steps for ED therapy.
Dr. Walls and her group developed a quality improvement (QI) initiative with a community emergency department. One important part of the study was the use of an asthma score, which helped determine steps for ED therapy.
Dr. Walls and her group developed a quality improvement (QI) initiative with a community emergency department. One important part of the study was the use of an asthma score, which helped determine steps for ED therapy.
Partnerships between community emergency departments and pediatric tertiary care centers are feasible and improve care of pediatric asthma, according to Theresa A. Walls, MD, of the Children’s National Health Systems, Washington, D.C., and her associates.
A total of 724 asthma patients aged 2-17 years were included in the study. Of this group, 289 (40%) were treated at the community ED before the pediatric tertiary care center intervention and 435 (60%) were treated after the intervention. Treatment with steroids was significantly increased post intervention, with 76% of patients receiving steroids, compared with 60% of patients before the intervention.
“Because the overwhelming majority of pediatric emergency visits occur in community EDs, partnerships with these EDs can broaden the impact of quality improvement activities and should be part of future quality improvement efforts,” the investigators concluded.
Find the full study in Pediatrics (2016. doi: 10.1542/peds.2016-0088).
Partnerships between community emergency departments and pediatric tertiary care centers are feasible and improve care of pediatric asthma, according to Theresa A. Walls, MD, of the Children’s National Health Systems, Washington, D.C., and her associates.
A total of 724 asthma patients aged 2-17 years were included in the study. Of this group, 289 (40%) were treated at the community ED before the pediatric tertiary care center intervention and 435 (60%) were treated after the intervention. Treatment with steroids was significantly increased post intervention, with 76% of patients receiving steroids, compared with 60% of patients before the intervention.
“Because the overwhelming majority of pediatric emergency visits occur in community EDs, partnerships with these EDs can broaden the impact of quality improvement activities and should be part of future quality improvement efforts,” the investigators concluded.
Find the full study in Pediatrics (2016. doi: 10.1542/peds.2016-0088).
FROM PEDIATRICS
Nomogram predicts conversion of first demyelinating event to definite MS
Baseline and follow-up data from a prospective, multinational cohort of more than 3,000 patients with clinically isolated events allowed researchers to develop an accurate prognostic nomogram to calculate an individual’s risk for conversion to clinically definite multiple sclerosis at 12 months.
“Identification of patient, disease, and examination factors associated with higher probability of second attack in clinical practice may enable clinicians to flag patients that could benefit from more intensive follow-up and consideration of early DMD [disease-modifying drug] treatment intervention, facilitating more favorable patient outcomes,” wrote Tim Spelman, PhD, and his associates.
Risk of relapse was decreased when clinically isolated syndrome was diagnosed later, with an adjusted hazard ratio of 0.9 for every 5 additional years of age. Other risk factors for earlier relapse include higher Expanded Disability Status Scale score at the onset of the first demyelinating event, DMD exposure prior to the first attack, multiple brain and spinal MRI criteria, and oligoclonal bands.
“These results corroborate and extend prior, albeit smaller, studies observing similar sets of predictors of clinical conversion probability,” Dr. Spelman and his coauthors wrote.
The predictive nomogram had a concordance index of 0.81 between the 12-month estimated and observed conversion probabilities.
“While our own internal validation suggested good performance, both an additional training-validation approach and an external validation through the application of the nomogram to a separate MS data set or population are required to confirm the generalizability of the nomogram,” they wrote.
Read the full study in Multiple Sclerosis Journal (2016 Nov 24. doi: 10.1177/1352458516679893).
Baseline and follow-up data from a prospective, multinational cohort of more than 3,000 patients with clinically isolated events allowed researchers to develop an accurate prognostic nomogram to calculate an individual’s risk for conversion to clinically definite multiple sclerosis at 12 months.
“Identification of patient, disease, and examination factors associated with higher probability of second attack in clinical practice may enable clinicians to flag patients that could benefit from more intensive follow-up and consideration of early DMD [disease-modifying drug] treatment intervention, facilitating more favorable patient outcomes,” wrote Tim Spelman, PhD, and his associates.
Risk of relapse was decreased when clinically isolated syndrome was diagnosed later, with an adjusted hazard ratio of 0.9 for every 5 additional years of age. Other risk factors for earlier relapse include higher Expanded Disability Status Scale score at the onset of the first demyelinating event, DMD exposure prior to the first attack, multiple brain and spinal MRI criteria, and oligoclonal bands.
“These results corroborate and extend prior, albeit smaller, studies observing similar sets of predictors of clinical conversion probability,” Dr. Spelman and his coauthors wrote.
The predictive nomogram had a concordance index of 0.81 between the 12-month estimated and observed conversion probabilities.
“While our own internal validation suggested good performance, both an additional training-validation approach and an external validation through the application of the nomogram to a separate MS data set or population are required to confirm the generalizability of the nomogram,” they wrote.
Read the full study in Multiple Sclerosis Journal (2016 Nov 24. doi: 10.1177/1352458516679893).
Baseline and follow-up data from a prospective, multinational cohort of more than 3,000 patients with clinically isolated events allowed researchers to develop an accurate prognostic nomogram to calculate an individual’s risk for conversion to clinically definite multiple sclerosis at 12 months.
“Identification of patient, disease, and examination factors associated with higher probability of second attack in clinical practice may enable clinicians to flag patients that could benefit from more intensive follow-up and consideration of early DMD [disease-modifying drug] treatment intervention, facilitating more favorable patient outcomes,” wrote Tim Spelman, PhD, and his associates.
Risk of relapse was decreased when clinically isolated syndrome was diagnosed later, with an adjusted hazard ratio of 0.9 for every 5 additional years of age. Other risk factors for earlier relapse include higher Expanded Disability Status Scale score at the onset of the first demyelinating event, DMD exposure prior to the first attack, multiple brain and spinal MRI criteria, and oligoclonal bands.
“These results corroborate and extend prior, albeit smaller, studies observing similar sets of predictors of clinical conversion probability,” Dr. Spelman and his coauthors wrote.
The predictive nomogram had a concordance index of 0.81 between the 12-month estimated and observed conversion probabilities.
“While our own internal validation suggested good performance, both an additional training-validation approach and an external validation through the application of the nomogram to a separate MS data set or population are required to confirm the generalizability of the nomogram,” they wrote.
Read the full study in Multiple Sclerosis Journal (2016 Nov 24. doi: 10.1177/1352458516679893).
FROM MULTIPLE SCLEROSIS JOURNAL
Parents look online for atopic dermatitis advice
Many caregivers of children with atopic dermatitis do not receive clear instructions on how to properly use topical corticosteroids and seek advice on online forums, according to Emma Teasdale, PhD, and her associates.
The investigators analyzed 27 forum discussions involving 95 participants from 2003 to 2015 and found that parents expressed a range of beliefs regarding the use of topical corticosteroids. Some parents expressed positive views, but many were cautious and perceived topical corticosteroids as unnatural or too strong. Notably, parents said they believed that topical corticosteroids thinned or weakened the skin.
Parents also expressed uncertainty over how to use topical corticosteroids. Common questions involved duration of use, where and when to apply, and confusion over the strength of different preparations. Parents also noted that they received conflicting physician instructions regarding duration, dosage, tapering, and safety.
“Given the prevalence of concerns about potential adverse effects of topical-corticosteroids, it would seem prudent to signpost parents/carers towards convenient, consistent, evidence-based information to ensure that the potential negative impacts of seeking (unsubstantiated) medical advice online are minimized. In the absence of such information they are likely to turn to online discussion forums as their sole resource where, although much useful support and advice can be found, some is of questionable validity,” the investigators concluded.
Find the full study in the British Journal of Dermatology (doi: 10.1111/bjd.15130).
Many caregivers of children with atopic dermatitis do not receive clear instructions on how to properly use topical corticosteroids and seek advice on online forums, according to Emma Teasdale, PhD, and her associates.
The investigators analyzed 27 forum discussions involving 95 participants from 2003 to 2015 and found that parents expressed a range of beliefs regarding the use of topical corticosteroids. Some parents expressed positive views, but many were cautious and perceived topical corticosteroids as unnatural or too strong. Notably, parents said they believed that topical corticosteroids thinned or weakened the skin.
Parents also expressed uncertainty over how to use topical corticosteroids. Common questions involved duration of use, where and when to apply, and confusion over the strength of different preparations. Parents also noted that they received conflicting physician instructions regarding duration, dosage, tapering, and safety.
“Given the prevalence of concerns about potential adverse effects of topical-corticosteroids, it would seem prudent to signpost parents/carers towards convenient, consistent, evidence-based information to ensure that the potential negative impacts of seeking (unsubstantiated) medical advice online are minimized. In the absence of such information they are likely to turn to online discussion forums as their sole resource where, although much useful support and advice can be found, some is of questionable validity,” the investigators concluded.
Find the full study in the British Journal of Dermatology (doi: 10.1111/bjd.15130).
Many caregivers of children with atopic dermatitis do not receive clear instructions on how to properly use topical corticosteroids and seek advice on online forums, according to Emma Teasdale, PhD, and her associates.
The investigators analyzed 27 forum discussions involving 95 participants from 2003 to 2015 and found that parents expressed a range of beliefs regarding the use of topical corticosteroids. Some parents expressed positive views, but many were cautious and perceived topical corticosteroids as unnatural or too strong. Notably, parents said they believed that topical corticosteroids thinned or weakened the skin.
Parents also expressed uncertainty over how to use topical corticosteroids. Common questions involved duration of use, where and when to apply, and confusion over the strength of different preparations. Parents also noted that they received conflicting physician instructions regarding duration, dosage, tapering, and safety.
“Given the prevalence of concerns about potential adverse effects of topical-corticosteroids, it would seem prudent to signpost parents/carers towards convenient, consistent, evidence-based information to ensure that the potential negative impacts of seeking (unsubstantiated) medical advice online are minimized. In the absence of such information they are likely to turn to online discussion forums as their sole resource where, although much useful support and advice can be found, some is of questionable validity,” the investigators concluded.
Find the full study in the British Journal of Dermatology (doi: 10.1111/bjd.15130).
FROM THE BRITISH JOURNAL OF DERMATOLOGY
Autism risk not increased by maternal influenza infection during pregnancy
Maternal influenza infection during pregnancy does not increase the risk for autism spectrum disorder (ASD) in children, according to Ousseny Zerbo, PhD, and associates.
In a study of 196,929 mother-child pairs (the children were born at Kaiser Permanente Northern California between Jan. 1, 2000, and Dec. 31, 2010), 1.6% of the children were diagnosed with ASD. Influenza was diagnosed in 0.7% of mothers during their pregnancy, and 23% received an influenza vaccination during pregnancy.
Overall, maternal influenza vaccination did not effect likelihood of ASD diagnosis, with 1.7% of children in this group receiving an ASD diagnosis. A small association between ASD diagnosis and maternal influenza vaccination, however, was seen in the first trimester of pregnancy, with an adjusted hazard ratio of 1.2, translating to a potential extra 4 cases of autism per 1,000 births. But further analysis suggested that this could be caused by bias and chance, and “the association was insignificant after statistical correction for multiple comparisons,” the investigators said.
“While we do not advocate changes in vaccine policy or practice, we believe that additional studies are warranted to further evaluate any potential associations between first-trimester maternal influenza vaccination and autism,” the investigators concluded.
Find the full study in JAMA Pediatrics (doi: 10.1001/jamapediatrics.2016.3609).
Maternal influenza infection during pregnancy does not increase the risk for autism spectrum disorder (ASD) in children, according to Ousseny Zerbo, PhD, and associates.
In a study of 196,929 mother-child pairs (the children were born at Kaiser Permanente Northern California between Jan. 1, 2000, and Dec. 31, 2010), 1.6% of the children were diagnosed with ASD. Influenza was diagnosed in 0.7% of mothers during their pregnancy, and 23% received an influenza vaccination during pregnancy.
Overall, maternal influenza vaccination did not effect likelihood of ASD diagnosis, with 1.7% of children in this group receiving an ASD diagnosis. A small association between ASD diagnosis and maternal influenza vaccination, however, was seen in the first trimester of pregnancy, with an adjusted hazard ratio of 1.2, translating to a potential extra 4 cases of autism per 1,000 births. But further analysis suggested that this could be caused by bias and chance, and “the association was insignificant after statistical correction for multiple comparisons,” the investigators said.
“While we do not advocate changes in vaccine policy or practice, we believe that additional studies are warranted to further evaluate any potential associations between first-trimester maternal influenza vaccination and autism,” the investigators concluded.
Find the full study in JAMA Pediatrics (doi: 10.1001/jamapediatrics.2016.3609).
Maternal influenza infection during pregnancy does not increase the risk for autism spectrum disorder (ASD) in children, according to Ousseny Zerbo, PhD, and associates.
In a study of 196,929 mother-child pairs (the children were born at Kaiser Permanente Northern California between Jan. 1, 2000, and Dec. 31, 2010), 1.6% of the children were diagnosed with ASD. Influenza was diagnosed in 0.7% of mothers during their pregnancy, and 23% received an influenza vaccination during pregnancy.
Overall, maternal influenza vaccination did not effect likelihood of ASD diagnosis, with 1.7% of children in this group receiving an ASD diagnosis. A small association between ASD diagnosis and maternal influenza vaccination, however, was seen in the first trimester of pregnancy, with an adjusted hazard ratio of 1.2, translating to a potential extra 4 cases of autism per 1,000 births. But further analysis suggested that this could be caused by bias and chance, and “the association was insignificant after statistical correction for multiple comparisons,” the investigators said.
“While we do not advocate changes in vaccine policy or practice, we believe that additional studies are warranted to further evaluate any potential associations between first-trimester maternal influenza vaccination and autism,” the investigators concluded.
Find the full study in JAMA Pediatrics (doi: 10.1001/jamapediatrics.2016.3609).
FROM JAMA PEDIATRICS