User login
A New Technique for Obtaining Bone Graft in Cases of Distal Femur Nonunion: Passing a Reamer/Irrigator/Aspirator Retrograde Through the Nonunion Site
Bone grafting is the main method of treating nonunions.1 The multiple bone graft options available include autogenous bone grafts, allogenic bone grafts, and synthetic bone graft substitutes.2,3 Autogenous bone graft has long been considered the gold standard, as it reduces the risk of infection and eliminates the risk of immune rejection associated with allograft; in addition, autograft has the optimal combination of osteogenic, osteoinductive, and osteoconductive properties.2,4,5 Iliac crest bone graft (ICBG), though the most commonly used autogenous bone graft source, has been associated with infection, hematoma, poor cosmetic outcomes, hernia, neurovascular insults, and chronic persistent pain.6,7 Intramedullary bone graft harvest performed with the Reamer/Irrigator/Aspirator (RIA) system (DePuy Synthes) is a novel technique that allows for simultaneous débridement and collection of bone graft, protects against thermal necrosis and extravasation of marrow contents, and maintains biomechanical strength for weight-bearing.3,4,8,9 Furthermore, RIA aspirate is a rich source of autologous bone graft and provides equal or superior amounts of graft in comparison with ICBG.5-7,10-12
In some cases, RIA is associated with the complication of host bone fracture.4,6,7,11,12 In addition, introducing the reamer may contribute to pain at its entry site and may require violation of local soft-tissue attachments at the hip or knees.4,7,13 In this study, we assessed the possibility of using a new RIA technique to eliminate these adverse effects. We hypothesized that distal femoral nonunions could be successfully treated with the RIA passed retrograde through the nonunion site. This technique may obviate the need for a secondary surgical site (required in traditional intramedullary bone graft harvest), minimize the potential entry-site tissue (eg, hip abductor) damage encountered with the antegrade technique, and yield harvested bone graft in quantities similar to those obtained with the standard technique.
After obtaining Institutional Review Board approval for this study, we retrospectively reviewed the medical records of all patients with a distal femur nonunion treated with autogenous bone grafting between 2009 and 2013. Identified patients had undergone a novel intramedullary harvest technique that involved passing an RIA retrograde through the nonunion site. Data (patient demographics, volume of graft obtained, perioperative complications, postoperative clinical course) were extracted from the medical records. Before data collection, all patients provided written informed consent for print and electronic publication of their case reports.
Technique
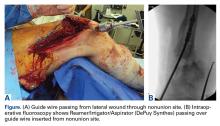
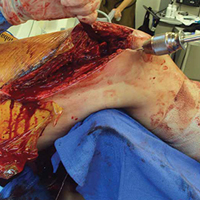
The patient was laid supine on a radiolucent table, and the affected extremity was prepared and draped free. A standard lateral incision previously used for the index procedure was employed. After implant removal, a rongeur, curette, and/or high-speed burr was used to débride the distal femur nonunion of all fibrous tissue. After mobilization and preparation of the distal femoral nonunion, varus angulation was accentuated with delivery of the proximal and distal segments of the nonunion into the wound (Figure A).
Six patients underwent 7 separate procedures for distal femoral nonunion. Of these patients, 5 underwent retrograde RIA through the nonunion site, as described above; the sixth underwent antegrade RIA in the traditional fashion and was therefore excluded. One of the 5 patients underwent another bone grafting procedure after the initial retrograde RIA treatment through the nonunion site. Several outcomes were measured: ability to obtain graft, volume of graft obtained, perioperative complications, and feasibility of the procedure.
Mean age of the 5 patients was 40.4 years (range, 22-66 years). Mean reamer size was 13.4 mm (mode, 14 mm), producing an average bone graft volume of 33 mL. There were no intraoperative or postoperative fractures. In 1 case, the reamer shaft broke during insertion and was retrieved with no retained hardware; passage was made with a new reamer shaft. No patient experienced additional pain or discomfort, as there was no separate entry site for the RIA.
Discussion
Bone grafting for nonunion is one of the most commonly performed procedures in orthopedic trauma surgery. Use of an intramedullary harvest system has become increasingly popular relative to alternative techniques. The RIA system is associated with less donor-site pain and provides relatively more bone graft volume in comparison with ICBG harvest.6,7,10,13 Conversely, intramedullary bone graft harvest may be associated with higher risk of host bone fractures, occurring either during surgery (technical error being the cause) or afterward (a result of patient noncompliance or overaggressive reaming).6,7,11,12 Multiple methods of reducing the risk of iatrogenic fracture caused by technical error of eccentric reaming have been described, including appropriate guide wire placement aided by frequent use of fluoroscopy in 2 planes.4 Despite these potential complications and improved donor-site pain complaints in comparison with ICBG harvest, traditional RIA harvest is still associated with pain at the entry site.4,7,13
In this study, we introduced a novel RIA technique for distal femur nonunion. This technique reduces the complications and adverse effects associated with RIA. It removes the added pain and discomfort associated with a separate entry site. As the reamer is introduced into the medullary canal through the femoral nonunion site, and proximal harvest is limited to the subtrochanteric region, the technique also avoids the complications associated with eccentric reaming of the distal and proximal femur, which may contribute to secondary fracture.6,7,11,12Although the proposed technique is practical, it may present some technical difficulties. First, failed fixation hardware must be removed, and by necessity some stripping of soft tissues is required. These actions are unavoidable, as hardware revision is inherent in the treatment of nonunion. During the procedure, the focus should be on minimizing the insult to bony healing. The nonunion also needs to be completely mobilized to allow adequate angulation, guide wire passage, and sequential reaming. The dual vascular insult of intramedullary reaming combined with the soft-tissue débridement and detachment required for hardware removal and mobilization can be concerning for devascularization of the fracture fragment. However, animal studies have suggested reaming does not affect metaphyseal blood flow; it affects only diaphyseal bone.6,14 The metaphyseal/diaphyseal location of these distal femur nonunions is thought to provide at least partial sparing from the endosteal injury that the RIA may cause. Another difficulty is that the angle of passage of the wire requires a relatively steeper curve to be able to pass beyond the medial distal femoral wall and proceed more proximally. Strong manipulation of the segment is required, which in 1 case caused the reamer shaft to break. This complication had minimal sequelae; the shaft was easily retrieved by withdrawing the ball-tipped guide wire. In addition, strong manipulation of the segment can lead to asymmetric medial reaming or fracture—an outcome easily avoided with a small bend in the distal tip of the guide wire and frequent use of fluoroscopy. In all cases in this series, we achieved proximal passage of the wire and the reamer.
Most RIA bone graft is harvested by reaming the medullary canal at the midshaft of the femur. Passing from the distal femoral nonunion precludes obtaining only a small source of potential distal femoral bone graft, though this metaphyseal bone typically is not used for fear of eccentric reaming and secondary fracture.6,7,11,12 The amount of bone graft obtained from selected patients who undergo retrograde RIA passage through the nonunion site should be similar to the amount obtained with the traditional antegrade method. Our newly proposed technique provided an average bone graft volume of 33 mL, which compares favorably with that reported in the literature for the traditional RIA technique.1,5,6,13,15,16
Conclusion
In distal femoral cases, retrograde passage of the RIA through the nonunion site is technically feasible and has reproducible yields of intramedullary bone graft. Adequate mobilization of the nonunion is a prerequisite for reamer harvest. However, this technique obviates the need for an additional entry point. Furthermore, the technique may limit the perioperative fracture risk previously seen with eccentric reaming of the distal and proximal femur using traditional intramedullary harvest.
Am J Orthop. 2016;45(7):E493-E496. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. Conway JD. Autograft and nonunions: morbidity with intramedullary bone graft versus iliac crest bone graft. Orthop Clin North Am. 2010;41(1):75-84.
2. Schmidmaier G, Herrmann S, Green J, et al. Quantitative assessment of growth factors in reaming aspirate, iliac crest, and platelet preparation. Bone. 2006;39(5):1156-1163.
3. Miller MA, Ivkovic A, Porter R, et al. Autologous bone grafting on steroids: preliminary clinical results. A novel treatment for nonunions and segmental bone defects. Int Orthop. 2011;35(4):599-605.
4. Qvick LM, Ritter CA, Mutty CE, Rohrbacher BJ, Buyea CM, Anders MJ. Donor site morbidity with Reamer-Irrigator-Aspirator (RIA) use for autogenous bone graft harvesting in a single centre 204 case series. Injury. 2013;44(10):1263-1269.
5. Kanakaris NK, Morell D, Gudipati S, Britten S, Giannoudis PV. Reaming Irrigator Aspirator system: early experience of its multipurpose use. Injury. 2011;42(suppl 4):S28-S34.
6. Dimitriou R, Mataliotakis GI, Angoules AG, Kanakaris NK, Giannoudis PV. Complications following autologous bone graft harvesting from the iliac crest and using the RIA: a systematic review. Injury. 2011;42(suppl 2):S3-S15.
7. Belthur MV, Conway JD, Jindal G, Ranade A, Herzenberg JE. Bone graft harvest using a new intramedullary system. Clin Orthop Relat Res. 2008;466(12):2973-2980.
8. Seagrave RA, Sojka J, Goodyear A, Munns SW. Utilizing Reamer Irrigator Aspirator (RIA) autograft for opening wedge high tibial osteotomy: a new surgical technique and report of three cases. Int J Surg Case Rep. 2014;5(1):37-42.
9. Finnan RP, Prayson MJ, Goswami T, Miller D. Use of the Reamer-Irrigator-Aspirator for bone graft harvest: a mechanical comparison of three starting points in cadaveric femurs. J Orthop Trauma. 2010;24(1):36-41.
10. Masquelet AC, Benko PE, Mathevon H, Hannouche D, Obert L; French Society of Orthopaedics and Traumatic Surgery (SoFCOT). Harvest of cortico-cancellous intramedullary femoral bone graft using the Reamer-Irrigator-Aspirator (RIA). Orthop Traumatol Surg Res. 2012;98(2):227-232.
11. Quintero AJ, Tarkin IS, Pape HC. Technical tricks when using the Reamer Irrigator Aspirator technique for autologous bone graft harvesting. J Orthop Trauma. 2010;24(1):42-45.
12. Cox G, Jones E, McGonagle D, Giannoudis PV. Reamer-Irrigator-Aspirator indications and clinical results: a systematic review. Int Orthop. 2011;35(7):951-956.
13. Dawson J, Kiner D, Gardner W 2nd, Swafford R, Nowotarski PJ. The Reamer-Irrigator-Aspirator as a device for harvesting bone graft compared with iliac crest bone graft: union rates and complications. J Orthop Trauma. 2014;28(10):584-590.
14. ElMaraghy AW, Humeniuk B, Anderson GI, Schemitsch EH, Richards RR. Femoral bone blood flow after reaming and intramedullary canal preparation: a canine study using laser Doppler flowmetry. J Arthroplasty. 1999;14(2):220-226.
15. Finkemeier CG, Neiman R, Hallare D. RIA: one community’s experience. Orthop Clin North Am. 2010;41(1):99-103.
16. Myeroff C, Archdeacon M. Autogenous bone graft: donor sites and techniques. J Bone Joint Surg Am. 2011;93(23):2227-2236.
Bone grafting is the main method of treating nonunions.1 The multiple bone graft options available include autogenous bone grafts, allogenic bone grafts, and synthetic bone graft substitutes.2,3 Autogenous bone graft has long been considered the gold standard, as it reduces the risk of infection and eliminates the risk of immune rejection associated with allograft; in addition, autograft has the optimal combination of osteogenic, osteoinductive, and osteoconductive properties.2,4,5 Iliac crest bone graft (ICBG), though the most commonly used autogenous bone graft source, has been associated with infection, hematoma, poor cosmetic outcomes, hernia, neurovascular insults, and chronic persistent pain.6,7 Intramedullary bone graft harvest performed with the Reamer/Irrigator/Aspirator (RIA) system (DePuy Synthes) is a novel technique that allows for simultaneous débridement and collection of bone graft, protects against thermal necrosis and extravasation of marrow contents, and maintains biomechanical strength for weight-bearing.3,4,8,9 Furthermore, RIA aspirate is a rich source of autologous bone graft and provides equal or superior amounts of graft in comparison with ICBG.5-7,10-12
In some cases, RIA is associated with the complication of host bone fracture.4,6,7,11,12 In addition, introducing the reamer may contribute to pain at its entry site and may require violation of local soft-tissue attachments at the hip or knees.4,7,13 In this study, we assessed the possibility of using a new RIA technique to eliminate these adverse effects. We hypothesized that distal femoral nonunions could be successfully treated with the RIA passed retrograde through the nonunion site. This technique may obviate the need for a secondary surgical site (required in traditional intramedullary bone graft harvest), minimize the potential entry-site tissue (eg, hip abductor) damage encountered with the antegrade technique, and yield harvested bone graft in quantities similar to those obtained with the standard technique.
After obtaining Institutional Review Board approval for this study, we retrospectively reviewed the medical records of all patients with a distal femur nonunion treated with autogenous bone grafting between 2009 and 2013. Identified patients had undergone a novel intramedullary harvest technique that involved passing an RIA retrograde through the nonunion site. Data (patient demographics, volume of graft obtained, perioperative complications, postoperative clinical course) were extracted from the medical records. Before data collection, all patients provided written informed consent for print and electronic publication of their case reports.
Technique
The patient was laid supine on a radiolucent table, and the affected extremity was prepared and draped free. A standard lateral incision previously used for the index procedure was employed. After implant removal, a rongeur, curette, and/or high-speed burr was used to débride the distal femur nonunion of all fibrous tissue. After mobilization and preparation of the distal femoral nonunion, varus angulation was accentuated with delivery of the proximal and distal segments of the nonunion into the wound (Figure A).
Six patients underwent 7 separate procedures for distal femoral nonunion. Of these patients, 5 underwent retrograde RIA through the nonunion site, as described above; the sixth underwent antegrade RIA in the traditional fashion and was therefore excluded. One of the 5 patients underwent another bone grafting procedure after the initial retrograde RIA treatment through the nonunion site. Several outcomes were measured: ability to obtain graft, volume of graft obtained, perioperative complications, and feasibility of the procedure.
Mean age of the 5 patients was 40.4 years (range, 22-66 years). Mean reamer size was 13.4 mm (mode, 14 mm), producing an average bone graft volume of 33 mL. There were no intraoperative or postoperative fractures. In 1 case, the reamer shaft broke during insertion and was retrieved with no retained hardware; passage was made with a new reamer shaft. No patient experienced additional pain or discomfort, as there was no separate entry site for the RIA.
Discussion
Bone grafting for nonunion is one of the most commonly performed procedures in orthopedic trauma surgery. Use of an intramedullary harvest system has become increasingly popular relative to alternative techniques. The RIA system is associated with less donor-site pain and provides relatively more bone graft volume in comparison with ICBG harvest.6,7,10,13 Conversely, intramedullary bone graft harvest may be associated with higher risk of host bone fractures, occurring either during surgery (technical error being the cause) or afterward (a result of patient noncompliance or overaggressive reaming).6,7,11,12 Multiple methods of reducing the risk of iatrogenic fracture caused by technical error of eccentric reaming have been described, including appropriate guide wire placement aided by frequent use of fluoroscopy in 2 planes.4 Despite these potential complications and improved donor-site pain complaints in comparison with ICBG harvest, traditional RIA harvest is still associated with pain at the entry site.4,7,13
In this study, we introduced a novel RIA technique for distal femur nonunion. This technique reduces the complications and adverse effects associated with RIA. It removes the added pain and discomfort associated with a separate entry site. As the reamer is introduced into the medullary canal through the femoral nonunion site, and proximal harvest is limited to the subtrochanteric region, the technique also avoids the complications associated with eccentric reaming of the distal and proximal femur, which may contribute to secondary fracture.6,7,11,12Although the proposed technique is practical, it may present some technical difficulties. First, failed fixation hardware must be removed, and by necessity some stripping of soft tissues is required. These actions are unavoidable, as hardware revision is inherent in the treatment of nonunion. During the procedure, the focus should be on minimizing the insult to bony healing. The nonunion also needs to be completely mobilized to allow adequate angulation, guide wire passage, and sequential reaming. The dual vascular insult of intramedullary reaming combined with the soft-tissue débridement and detachment required for hardware removal and mobilization can be concerning for devascularization of the fracture fragment. However, animal studies have suggested reaming does not affect metaphyseal blood flow; it affects only diaphyseal bone.6,14 The metaphyseal/diaphyseal location of these distal femur nonunions is thought to provide at least partial sparing from the endosteal injury that the RIA may cause. Another difficulty is that the angle of passage of the wire requires a relatively steeper curve to be able to pass beyond the medial distal femoral wall and proceed more proximally. Strong manipulation of the segment is required, which in 1 case caused the reamer shaft to break. This complication had minimal sequelae; the shaft was easily retrieved by withdrawing the ball-tipped guide wire. In addition, strong manipulation of the segment can lead to asymmetric medial reaming or fracture—an outcome easily avoided with a small bend in the distal tip of the guide wire and frequent use of fluoroscopy. In all cases in this series, we achieved proximal passage of the wire and the reamer.
Most RIA bone graft is harvested by reaming the medullary canal at the midshaft of the femur. Passing from the distal femoral nonunion precludes obtaining only a small source of potential distal femoral bone graft, though this metaphyseal bone typically is not used for fear of eccentric reaming and secondary fracture.6,7,11,12 The amount of bone graft obtained from selected patients who undergo retrograde RIA passage through the nonunion site should be similar to the amount obtained with the traditional antegrade method. Our newly proposed technique provided an average bone graft volume of 33 mL, which compares favorably with that reported in the literature for the traditional RIA technique.1,5,6,13,15,16
Conclusion
In distal femoral cases, retrograde passage of the RIA through the nonunion site is technically feasible and has reproducible yields of intramedullary bone graft. Adequate mobilization of the nonunion is a prerequisite for reamer harvest. However, this technique obviates the need for an additional entry point. Furthermore, the technique may limit the perioperative fracture risk previously seen with eccentric reaming of the distal and proximal femur using traditional intramedullary harvest.
Am J Orthop. 2016;45(7):E493-E496. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
Bone grafting is the main method of treating nonunions.1 The multiple bone graft options available include autogenous bone grafts, allogenic bone grafts, and synthetic bone graft substitutes.2,3 Autogenous bone graft has long been considered the gold standard, as it reduces the risk of infection and eliminates the risk of immune rejection associated with allograft; in addition, autograft has the optimal combination of osteogenic, osteoinductive, and osteoconductive properties.2,4,5 Iliac crest bone graft (ICBG), though the most commonly used autogenous bone graft source, has been associated with infection, hematoma, poor cosmetic outcomes, hernia, neurovascular insults, and chronic persistent pain.6,7 Intramedullary bone graft harvest performed with the Reamer/Irrigator/Aspirator (RIA) system (DePuy Synthes) is a novel technique that allows for simultaneous débridement and collection of bone graft, protects against thermal necrosis and extravasation of marrow contents, and maintains biomechanical strength for weight-bearing.3,4,8,9 Furthermore, RIA aspirate is a rich source of autologous bone graft and provides equal or superior amounts of graft in comparison with ICBG.5-7,10-12
In some cases, RIA is associated with the complication of host bone fracture.4,6,7,11,12 In addition, introducing the reamer may contribute to pain at its entry site and may require violation of local soft-tissue attachments at the hip or knees.4,7,13 In this study, we assessed the possibility of using a new RIA technique to eliminate these adverse effects. We hypothesized that distal femoral nonunions could be successfully treated with the RIA passed retrograde through the nonunion site. This technique may obviate the need for a secondary surgical site (required in traditional intramedullary bone graft harvest), minimize the potential entry-site tissue (eg, hip abductor) damage encountered with the antegrade technique, and yield harvested bone graft in quantities similar to those obtained with the standard technique.
After obtaining Institutional Review Board approval for this study, we retrospectively reviewed the medical records of all patients with a distal femur nonunion treated with autogenous bone grafting between 2009 and 2013. Identified patients had undergone a novel intramedullary harvest technique that involved passing an RIA retrograde through the nonunion site. Data (patient demographics, volume of graft obtained, perioperative complications, postoperative clinical course) were extracted from the medical records. Before data collection, all patients provided written informed consent for print and electronic publication of their case reports.
Technique
The patient was laid supine on a radiolucent table, and the affected extremity was prepared and draped free. A standard lateral incision previously used for the index procedure was employed. After implant removal, a rongeur, curette, and/or high-speed burr was used to débride the distal femur nonunion of all fibrous tissue. After mobilization and preparation of the distal femoral nonunion, varus angulation was accentuated with delivery of the proximal and distal segments of the nonunion into the wound (Figure A).
Six patients underwent 7 separate procedures for distal femoral nonunion. Of these patients, 5 underwent retrograde RIA through the nonunion site, as described above; the sixth underwent antegrade RIA in the traditional fashion and was therefore excluded. One of the 5 patients underwent another bone grafting procedure after the initial retrograde RIA treatment through the nonunion site. Several outcomes were measured: ability to obtain graft, volume of graft obtained, perioperative complications, and feasibility of the procedure.
Mean age of the 5 patients was 40.4 years (range, 22-66 years). Mean reamer size was 13.4 mm (mode, 14 mm), producing an average bone graft volume of 33 mL. There were no intraoperative or postoperative fractures. In 1 case, the reamer shaft broke during insertion and was retrieved with no retained hardware; passage was made with a new reamer shaft. No patient experienced additional pain or discomfort, as there was no separate entry site for the RIA.
Discussion
Bone grafting for nonunion is one of the most commonly performed procedures in orthopedic trauma surgery. Use of an intramedullary harvest system has become increasingly popular relative to alternative techniques. The RIA system is associated with less donor-site pain and provides relatively more bone graft volume in comparison with ICBG harvest.6,7,10,13 Conversely, intramedullary bone graft harvest may be associated with higher risk of host bone fractures, occurring either during surgery (technical error being the cause) or afterward (a result of patient noncompliance or overaggressive reaming).6,7,11,12 Multiple methods of reducing the risk of iatrogenic fracture caused by technical error of eccentric reaming have been described, including appropriate guide wire placement aided by frequent use of fluoroscopy in 2 planes.4 Despite these potential complications and improved donor-site pain complaints in comparison with ICBG harvest, traditional RIA harvest is still associated with pain at the entry site.4,7,13
In this study, we introduced a novel RIA technique for distal femur nonunion. This technique reduces the complications and adverse effects associated with RIA. It removes the added pain and discomfort associated with a separate entry site. As the reamer is introduced into the medullary canal through the femoral nonunion site, and proximal harvest is limited to the subtrochanteric region, the technique also avoids the complications associated with eccentric reaming of the distal and proximal femur, which may contribute to secondary fracture.6,7,11,12Although the proposed technique is practical, it may present some technical difficulties. First, failed fixation hardware must be removed, and by necessity some stripping of soft tissues is required. These actions are unavoidable, as hardware revision is inherent in the treatment of nonunion. During the procedure, the focus should be on minimizing the insult to bony healing. The nonunion also needs to be completely mobilized to allow adequate angulation, guide wire passage, and sequential reaming. The dual vascular insult of intramedullary reaming combined with the soft-tissue débridement and detachment required for hardware removal and mobilization can be concerning for devascularization of the fracture fragment. However, animal studies have suggested reaming does not affect metaphyseal blood flow; it affects only diaphyseal bone.6,14 The metaphyseal/diaphyseal location of these distal femur nonunions is thought to provide at least partial sparing from the endosteal injury that the RIA may cause. Another difficulty is that the angle of passage of the wire requires a relatively steeper curve to be able to pass beyond the medial distal femoral wall and proceed more proximally. Strong manipulation of the segment is required, which in 1 case caused the reamer shaft to break. This complication had minimal sequelae; the shaft was easily retrieved by withdrawing the ball-tipped guide wire. In addition, strong manipulation of the segment can lead to asymmetric medial reaming or fracture—an outcome easily avoided with a small bend in the distal tip of the guide wire and frequent use of fluoroscopy. In all cases in this series, we achieved proximal passage of the wire and the reamer.
Most RIA bone graft is harvested by reaming the medullary canal at the midshaft of the femur. Passing from the distal femoral nonunion precludes obtaining only a small source of potential distal femoral bone graft, though this metaphyseal bone typically is not used for fear of eccentric reaming and secondary fracture.6,7,11,12 The amount of bone graft obtained from selected patients who undergo retrograde RIA passage through the nonunion site should be similar to the amount obtained with the traditional antegrade method. Our newly proposed technique provided an average bone graft volume of 33 mL, which compares favorably with that reported in the literature for the traditional RIA technique.1,5,6,13,15,16
Conclusion
In distal femoral cases, retrograde passage of the RIA through the nonunion site is technically feasible and has reproducible yields of intramedullary bone graft. Adequate mobilization of the nonunion is a prerequisite for reamer harvest. However, this technique obviates the need for an additional entry point. Furthermore, the technique may limit the perioperative fracture risk previously seen with eccentric reaming of the distal and proximal femur using traditional intramedullary harvest.
Am J Orthop. 2016;45(7):E493-E496. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. Conway JD. Autograft and nonunions: morbidity with intramedullary bone graft versus iliac crest bone graft. Orthop Clin North Am. 2010;41(1):75-84.
2. Schmidmaier G, Herrmann S, Green J, et al. Quantitative assessment of growth factors in reaming aspirate, iliac crest, and platelet preparation. Bone. 2006;39(5):1156-1163.
3. Miller MA, Ivkovic A, Porter R, et al. Autologous bone grafting on steroids: preliminary clinical results. A novel treatment for nonunions and segmental bone defects. Int Orthop. 2011;35(4):599-605.
4. Qvick LM, Ritter CA, Mutty CE, Rohrbacher BJ, Buyea CM, Anders MJ. Donor site morbidity with Reamer-Irrigator-Aspirator (RIA) use for autogenous bone graft harvesting in a single centre 204 case series. Injury. 2013;44(10):1263-1269.
5. Kanakaris NK, Morell D, Gudipati S, Britten S, Giannoudis PV. Reaming Irrigator Aspirator system: early experience of its multipurpose use. Injury. 2011;42(suppl 4):S28-S34.
6. Dimitriou R, Mataliotakis GI, Angoules AG, Kanakaris NK, Giannoudis PV. Complications following autologous bone graft harvesting from the iliac crest and using the RIA: a systematic review. Injury. 2011;42(suppl 2):S3-S15.
7. Belthur MV, Conway JD, Jindal G, Ranade A, Herzenberg JE. Bone graft harvest using a new intramedullary system. Clin Orthop Relat Res. 2008;466(12):2973-2980.
8. Seagrave RA, Sojka J, Goodyear A, Munns SW. Utilizing Reamer Irrigator Aspirator (RIA) autograft for opening wedge high tibial osteotomy: a new surgical technique and report of three cases. Int J Surg Case Rep. 2014;5(1):37-42.
9. Finnan RP, Prayson MJ, Goswami T, Miller D. Use of the Reamer-Irrigator-Aspirator for bone graft harvest: a mechanical comparison of three starting points in cadaveric femurs. J Orthop Trauma. 2010;24(1):36-41.
10. Masquelet AC, Benko PE, Mathevon H, Hannouche D, Obert L; French Society of Orthopaedics and Traumatic Surgery (SoFCOT). Harvest of cortico-cancellous intramedullary femoral bone graft using the Reamer-Irrigator-Aspirator (RIA). Orthop Traumatol Surg Res. 2012;98(2):227-232.
11. Quintero AJ, Tarkin IS, Pape HC. Technical tricks when using the Reamer Irrigator Aspirator technique for autologous bone graft harvesting. J Orthop Trauma. 2010;24(1):42-45.
12. Cox G, Jones E, McGonagle D, Giannoudis PV. Reamer-Irrigator-Aspirator indications and clinical results: a systematic review. Int Orthop. 2011;35(7):951-956.
13. Dawson J, Kiner D, Gardner W 2nd, Swafford R, Nowotarski PJ. The Reamer-Irrigator-Aspirator as a device for harvesting bone graft compared with iliac crest bone graft: union rates and complications. J Orthop Trauma. 2014;28(10):584-590.
14. ElMaraghy AW, Humeniuk B, Anderson GI, Schemitsch EH, Richards RR. Femoral bone blood flow after reaming and intramedullary canal preparation: a canine study using laser Doppler flowmetry. J Arthroplasty. 1999;14(2):220-226.
15. Finkemeier CG, Neiman R, Hallare D. RIA: one community’s experience. Orthop Clin North Am. 2010;41(1):99-103.
16. Myeroff C, Archdeacon M. Autogenous bone graft: donor sites and techniques. J Bone Joint Surg Am. 2011;93(23):2227-2236.
1. Conway JD. Autograft and nonunions: morbidity with intramedullary bone graft versus iliac crest bone graft. Orthop Clin North Am. 2010;41(1):75-84.
2. Schmidmaier G, Herrmann S, Green J, et al. Quantitative assessment of growth factors in reaming aspirate, iliac crest, and platelet preparation. Bone. 2006;39(5):1156-1163.
3. Miller MA, Ivkovic A, Porter R, et al. Autologous bone grafting on steroids: preliminary clinical results. A novel treatment for nonunions and segmental bone defects. Int Orthop. 2011;35(4):599-605.
4. Qvick LM, Ritter CA, Mutty CE, Rohrbacher BJ, Buyea CM, Anders MJ. Donor site morbidity with Reamer-Irrigator-Aspirator (RIA) use for autogenous bone graft harvesting in a single centre 204 case series. Injury. 2013;44(10):1263-1269.
5. Kanakaris NK, Morell D, Gudipati S, Britten S, Giannoudis PV. Reaming Irrigator Aspirator system: early experience of its multipurpose use. Injury. 2011;42(suppl 4):S28-S34.
6. Dimitriou R, Mataliotakis GI, Angoules AG, Kanakaris NK, Giannoudis PV. Complications following autologous bone graft harvesting from the iliac crest and using the RIA: a systematic review. Injury. 2011;42(suppl 2):S3-S15.
7. Belthur MV, Conway JD, Jindal G, Ranade A, Herzenberg JE. Bone graft harvest using a new intramedullary system. Clin Orthop Relat Res. 2008;466(12):2973-2980.
8. Seagrave RA, Sojka J, Goodyear A, Munns SW. Utilizing Reamer Irrigator Aspirator (RIA) autograft for opening wedge high tibial osteotomy: a new surgical technique and report of three cases. Int J Surg Case Rep. 2014;5(1):37-42.
9. Finnan RP, Prayson MJ, Goswami T, Miller D. Use of the Reamer-Irrigator-Aspirator for bone graft harvest: a mechanical comparison of three starting points in cadaveric femurs. J Orthop Trauma. 2010;24(1):36-41.
10. Masquelet AC, Benko PE, Mathevon H, Hannouche D, Obert L; French Society of Orthopaedics and Traumatic Surgery (SoFCOT). Harvest of cortico-cancellous intramedullary femoral bone graft using the Reamer-Irrigator-Aspirator (RIA). Orthop Traumatol Surg Res. 2012;98(2):227-232.
11. Quintero AJ, Tarkin IS, Pape HC. Technical tricks when using the Reamer Irrigator Aspirator technique for autologous bone graft harvesting. J Orthop Trauma. 2010;24(1):42-45.
12. Cox G, Jones E, McGonagle D, Giannoudis PV. Reamer-Irrigator-Aspirator indications and clinical results: a systematic review. Int Orthop. 2011;35(7):951-956.
13. Dawson J, Kiner D, Gardner W 2nd, Swafford R, Nowotarski PJ. The Reamer-Irrigator-Aspirator as a device for harvesting bone graft compared with iliac crest bone graft: union rates and complications. J Orthop Trauma. 2014;28(10):584-590.
14. ElMaraghy AW, Humeniuk B, Anderson GI, Schemitsch EH, Richards RR. Femoral bone blood flow after reaming and intramedullary canal preparation: a canine study using laser Doppler flowmetry. J Arthroplasty. 1999;14(2):220-226.
15. Finkemeier CG, Neiman R, Hallare D. RIA: one community’s experience. Orthop Clin North Am. 2010;41(1):99-103.
16. Myeroff C, Archdeacon M. Autogenous bone graft: donor sites and techniques. J Bone Joint Surg Am. 2011;93(23):2227-2236.
This Month in CHEST: Editor’s Picks
Oral Macrolide Therapy Following Short-term Combination Antibiotic Treatment of Mycobacterium massiliense Lung Disease. By Dr. Won-Jung Koh, et al.
Impact of Acute Changes in CPAP Flow Route in Sleep Apnea Treatment. By Dr. R. G. Andrade, et al.
Endobronchial Ultrasound: Clinical Uses and Professional Reimbursements. By Dr. T. R. Gildea and Dr. K. Nicolacakis.
Chronic Cough Due to Gastroesophageal Reflux in Adults: CHEST Guideline and Expert Panel Report. By Dr. P. J. Kahrilas, et al., on behalf of the CHEST Expert Cough Panel.
Oral Macrolide Therapy Following Short-term Combination Antibiotic Treatment of Mycobacterium massiliense Lung Disease. By Dr. Won-Jung Koh, et al.
Impact of Acute Changes in CPAP Flow Route in Sleep Apnea Treatment. By Dr. R. G. Andrade, et al.
Endobronchial Ultrasound: Clinical Uses and Professional Reimbursements. By Dr. T. R. Gildea and Dr. K. Nicolacakis.
Chronic Cough Due to Gastroesophageal Reflux in Adults: CHEST Guideline and Expert Panel Report. By Dr. P. J. Kahrilas, et al., on behalf of the CHEST Expert Cough Panel.
Oral Macrolide Therapy Following Short-term Combination Antibiotic Treatment of Mycobacterium massiliense Lung Disease. By Dr. Won-Jung Koh, et al.
Impact of Acute Changes in CPAP Flow Route in Sleep Apnea Treatment. By Dr. R. G. Andrade, et al.
Endobronchial Ultrasound: Clinical Uses and Professional Reimbursements. By Dr. T. R. Gildea and Dr. K. Nicolacakis.
Chronic Cough Due to Gastroesophageal Reflux in Adults: CHEST Guideline and Expert Panel Report. By Dr. P. J. Kahrilas, et al., on behalf of the CHEST Expert Cough Panel.
ABIM Pulmonary Medicine Board urges participation in survey
The American Board of Internal Medicine (ABIM) has emailed diplomates a survey regarding the blueprint for the Maintenance of Certification (MOC) pulmonary exam.
This survey relates to the content of the exam, as opposed to a prior survey that asked diplomates for their opinion about new proposals for 2- and 5-year cycles for the exam.
Participating in the survey gives diplomates a voice in determining the content of the MOC exam for pulmonary medicine. If enough individuals participate in the survey and the data support changing the distribution of exam content, it is very likely that ABIM will make improvements to the MOC exam.
The figure below illustrates the information provided by diplomates that ABIM used to help them decide the exam content for the Hospital Medicine exam.
Diplomates can find the survey when they log into their respective homepages on the ABIM website at www.abim.org. The survey does not need to be completed in one sitting, but rather can be done one section at a time. It takes approximately 15 minutes to finish each section.
A link to the survey is located in the My Reminders tab.
This is a great opportunity for individuals to make their voices heard.
The American Board of Internal Medicine (ABIM) has emailed diplomates a survey regarding the blueprint for the Maintenance of Certification (MOC) pulmonary exam.
This survey relates to the content of the exam, as opposed to a prior survey that asked diplomates for their opinion about new proposals for 2- and 5-year cycles for the exam.
Participating in the survey gives diplomates a voice in determining the content of the MOC exam for pulmonary medicine. If enough individuals participate in the survey and the data support changing the distribution of exam content, it is very likely that ABIM will make improvements to the MOC exam.
The figure below illustrates the information provided by diplomates that ABIM used to help them decide the exam content for the Hospital Medicine exam.
Diplomates can find the survey when they log into their respective homepages on the ABIM website at www.abim.org. The survey does not need to be completed in one sitting, but rather can be done one section at a time. It takes approximately 15 minutes to finish each section.
A link to the survey is located in the My Reminders tab.
This is a great opportunity for individuals to make their voices heard.
The American Board of Internal Medicine (ABIM) has emailed diplomates a survey regarding the blueprint for the Maintenance of Certification (MOC) pulmonary exam.
This survey relates to the content of the exam, as opposed to a prior survey that asked diplomates for their opinion about new proposals for 2- and 5-year cycles for the exam.
Participating in the survey gives diplomates a voice in determining the content of the MOC exam for pulmonary medicine. If enough individuals participate in the survey and the data support changing the distribution of exam content, it is very likely that ABIM will make improvements to the MOC exam.
The figure below illustrates the information provided by diplomates that ABIM used to help them decide the exam content for the Hospital Medicine exam.
Diplomates can find the survey when they log into their respective homepages on the ABIM website at www.abim.org. The survey does not need to be completed in one sitting, but rather can be done one section at a time. It takes approximately 15 minutes to finish each section.
A link to the survey is located in the My Reminders tab.
This is a great opportunity for individuals to make their voices heard.
Emergency Imaging: Facial Trauma After a Fall
An 89-year-old man presented to the ED with facial trauma due to a mechanical fall after losing his balance on uneven pavement and hitting the right side of his face. Physical examination revealed an ecchymosis inferior to the right eye and tenderness to palpation at the right maxilla and bilateral nasolabial folds. Maxillofacial computed tomography (CT) was ordered for further evaluation; representative images are presented above (Figure 1a and 1b).

What is the diagnosis?
Answer
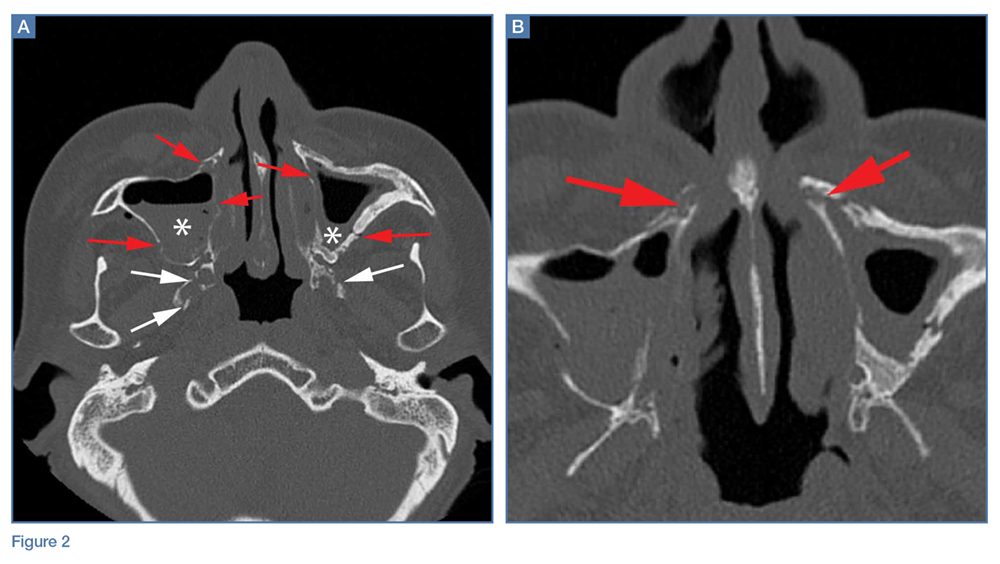
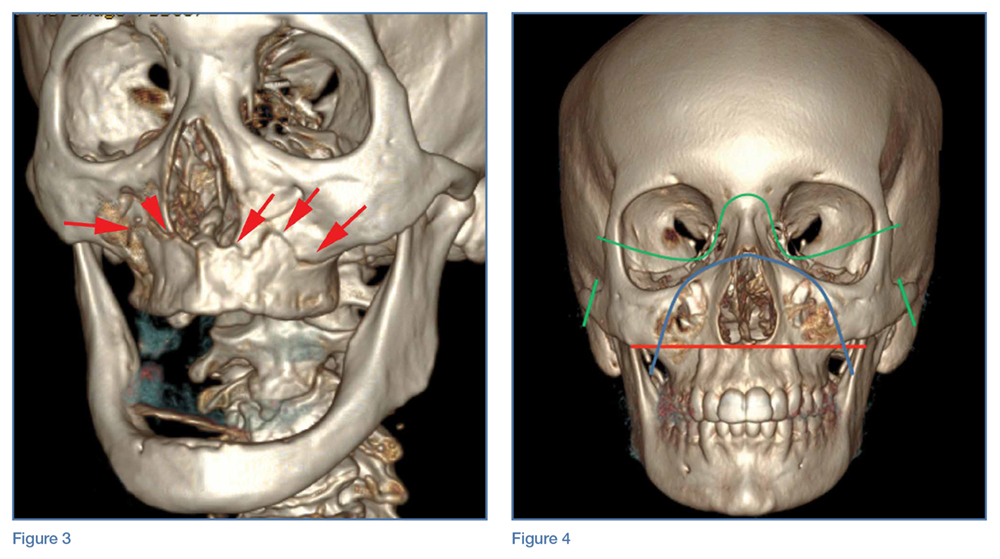
A noncontrast CT of the maxillofacial bones demonstrated acute fractures through the bilateral pterygoid plates (white arrows, Figure 2a). The fractures extended through the medial and lateral walls of the bilateral maxillary sinuses (red arrows, Figure 2a), and propagated to the frontal processes of the maxilla (red arrows, Figure 2b), extending toward the alveolar process, indicating involvement of the anterolateral margin of the nasal fossa. The full extent of the fracture is best seen on a 3D-reconstructed image (red arrows, Figure 3). Additional images (not presented here) confirmed no fracture involvement of the orbital floors, nasal bones, or zygomatic arches. Expected posttraumatic hemorrhage was appreciated within the maxillary sinuses (white asterisks, Figure 2a).
Le Fort Fractures
The findings described above are characteristic of a Le Fort I fracture pattern. Initially described in 1901 by René Le Fort, a French surgeon, the Le Fort classification system details somewhat predictable midface fracture patterns resulting in various degrees of craniofacial disassociation.1 Using weights that were dropped on cadaveric heads, Le Fort discovered that the pterygoid plates must be disrupted in order for the midface facial bones to separate from the skull base. As such, when diagnosing a Le Fort fracture, fracture of the pterygoid plate must be present, regardless of the fracture type (Le Fort I, II, and III).2
Le Fort I Fracture. This fracture pattern (red line, Figure 4) is referred to as a “floating palate” and involves separation of the hard palate from the skull base via fracture extension from the pterygoid plates into the maxillary sinus walls, as demonstrated in this case. The key distinguisher of the Le Fort I pattern is involvement of the anterolateral margin of the nasal fossa.2
Le Fort II Fracture. This fracture pattern (blue line, Figure 4) describes a “floating maxilla” wherein the pterygoid plate fractures are met with a pyramidal-type fracture pattern of the midface. The maxillary teeth form the base of the pyramid, and the fracture extends superiorly through the infraorbital rims bilaterally and toward the nasofrontal suture.2,3 Le Fort II fractures result in the maxilla floating freely from the rest of the midface and skull base.
Le Fort III Fracture. This fracture pattern (green lines, Figure 4) describes a “floating face” with complete craniofacial disjunction resulting from fracture of the pterygoid plates, nasofrontal suture, maxillofrontal suture, orbital wall, and zygomatic arch/zygomaticofrontal suture.2,3
It is important to note that midface trauma represents a complex spectrum of injuries, and Le Fort fractures only account for a small percentage of facial bone fractures that present through Level 1 trauma centers.2 Le Fort fracture patterns can coexist with other fracture patterns and also can be seen in combination with each other. For example, one side of the face may demonstrate a Le Fort II pattern while the other side concurrently demonstrates a Le Fort III pattern. Though not robust enough for complete description of and surgical planning for facial fractures, this classification system is a succinct and well-accepted means of describing major fracture planes.
1. Le Fort R. Etude experimentale sur les fractures de la machoire superieure. Rev Chir. 1901;23:208-227, 360-379, 479-507.
2. Rhea JT, Novelline RA. How to simplify the CT diagnosis of Le Fort fractures. AJR Am J Roentgenol. 2005;184(5):1700-1705.
3. Hopper RA, Salemy S, Sze RW. Diagnosis of midface fractures with CT: what the surgeon needs to know. Radiographics. 2006;26(3):783-793.
An 89-year-old man presented to the ED with facial trauma due to a mechanical fall after losing his balance on uneven pavement and hitting the right side of his face. Physical examination revealed an ecchymosis inferior to the right eye and tenderness to palpation at the right maxilla and bilateral nasolabial folds. Maxillofacial computed tomography (CT) was ordered for further evaluation; representative images are presented above (Figure 1a and 1b).

What is the diagnosis?
Answer
A noncontrast CT of the maxillofacial bones demonstrated acute fractures through the bilateral pterygoid plates (white arrows, Figure 2a). The fractures extended through the medial and lateral walls of the bilateral maxillary sinuses (red arrows, Figure 2a), and propagated to the frontal processes of the maxilla (red arrows, Figure 2b), extending toward the alveolar process, indicating involvement of the anterolateral margin of the nasal fossa. The full extent of the fracture is best seen on a 3D-reconstructed image (red arrows, Figure 3). Additional images (not presented here) confirmed no fracture involvement of the orbital floors, nasal bones, or zygomatic arches. Expected posttraumatic hemorrhage was appreciated within the maxillary sinuses (white asterisks, Figure 2a).


Le Fort Fractures
The findings described above are characteristic of a Le Fort I fracture pattern. Initially described in 1901 by René Le Fort, a French surgeon, the Le Fort classification system details somewhat predictable midface fracture patterns resulting in various degrees of craniofacial disassociation.1 Using weights that were dropped on cadaveric heads, Le Fort discovered that the pterygoid plates must be disrupted in order for the midface facial bones to separate from the skull base. As such, when diagnosing a Le Fort fracture, fracture of the pterygoid plate must be present, regardless of the fracture type (Le Fort I, II, and III).2
Le Fort I Fracture. This fracture pattern (red line, Figure 4) is referred to as a “floating palate” and involves separation of the hard palate from the skull base via fracture extension from the pterygoid plates into the maxillary sinus walls, as demonstrated in this case. The key distinguisher of the Le Fort I pattern is involvement of the anterolateral margin of the nasal fossa.2
Le Fort II Fracture. This fracture pattern (blue line, Figure 4) describes a “floating maxilla” wherein the pterygoid plate fractures are met with a pyramidal-type fracture pattern of the midface. The maxillary teeth form the base of the pyramid, and the fracture extends superiorly through the infraorbital rims bilaterally and toward the nasofrontal suture.2,3 Le Fort II fractures result in the maxilla floating freely from the rest of the midface and skull base.
Le Fort III Fracture. This fracture pattern (green lines, Figure 4) describes a “floating face” with complete craniofacial disjunction resulting from fracture of the pterygoid plates, nasofrontal suture, maxillofrontal suture, orbital wall, and zygomatic arch/zygomaticofrontal suture.2,3
It is important to note that midface trauma represents a complex spectrum of injuries, and Le Fort fractures only account for a small percentage of facial bone fractures that present through Level 1 trauma centers.2 Le Fort fracture patterns can coexist with other fracture patterns and also can be seen in combination with each other. For example, one side of the face may demonstrate a Le Fort II pattern while the other side concurrently demonstrates a Le Fort III pattern. Though not robust enough for complete description of and surgical planning for facial fractures, this classification system is a succinct and well-accepted means of describing major fracture planes.
An 89-year-old man presented to the ED with facial trauma due to a mechanical fall after losing his balance on uneven pavement and hitting the right side of his face. Physical examination revealed an ecchymosis inferior to the right eye and tenderness to palpation at the right maxilla and bilateral nasolabial folds. Maxillofacial computed tomography (CT) was ordered for further evaluation; representative images are presented above (Figure 1a and 1b).

What is the diagnosis?
Answer
A noncontrast CT of the maxillofacial bones demonstrated acute fractures through the bilateral pterygoid plates (white arrows, Figure 2a). The fractures extended through the medial and lateral walls of the bilateral maxillary sinuses (red arrows, Figure 2a), and propagated to the frontal processes of the maxilla (red arrows, Figure 2b), extending toward the alveolar process, indicating involvement of the anterolateral margin of the nasal fossa. The full extent of the fracture is best seen on a 3D-reconstructed image (red arrows, Figure 3). Additional images (not presented here) confirmed no fracture involvement of the orbital floors, nasal bones, or zygomatic arches. Expected posttraumatic hemorrhage was appreciated within the maxillary sinuses (white asterisks, Figure 2a).


Le Fort Fractures
The findings described above are characteristic of a Le Fort I fracture pattern. Initially described in 1901 by René Le Fort, a French surgeon, the Le Fort classification system details somewhat predictable midface fracture patterns resulting in various degrees of craniofacial disassociation.1 Using weights that were dropped on cadaveric heads, Le Fort discovered that the pterygoid plates must be disrupted in order for the midface facial bones to separate from the skull base. As such, when diagnosing a Le Fort fracture, fracture of the pterygoid plate must be present, regardless of the fracture type (Le Fort I, II, and III).2
Le Fort I Fracture. This fracture pattern (red line, Figure 4) is referred to as a “floating palate” and involves separation of the hard palate from the skull base via fracture extension from the pterygoid plates into the maxillary sinus walls, as demonstrated in this case. The key distinguisher of the Le Fort I pattern is involvement of the anterolateral margin of the nasal fossa.2
Le Fort II Fracture. This fracture pattern (blue line, Figure 4) describes a “floating maxilla” wherein the pterygoid plate fractures are met with a pyramidal-type fracture pattern of the midface. The maxillary teeth form the base of the pyramid, and the fracture extends superiorly through the infraorbital rims bilaterally and toward the nasofrontal suture.2,3 Le Fort II fractures result in the maxilla floating freely from the rest of the midface and skull base.
Le Fort III Fracture. This fracture pattern (green lines, Figure 4) describes a “floating face” with complete craniofacial disjunction resulting from fracture of the pterygoid plates, nasofrontal suture, maxillofrontal suture, orbital wall, and zygomatic arch/zygomaticofrontal suture.2,3
It is important to note that midface trauma represents a complex spectrum of injuries, and Le Fort fractures only account for a small percentage of facial bone fractures that present through Level 1 trauma centers.2 Le Fort fracture patterns can coexist with other fracture patterns and also can be seen in combination with each other. For example, one side of the face may demonstrate a Le Fort II pattern while the other side concurrently demonstrates a Le Fort III pattern. Though not robust enough for complete description of and surgical planning for facial fractures, this classification system is a succinct and well-accepted means of describing major fracture planes.
1. Le Fort R. Etude experimentale sur les fractures de la machoire superieure. Rev Chir. 1901;23:208-227, 360-379, 479-507.
2. Rhea JT, Novelline RA. How to simplify the CT diagnosis of Le Fort fractures. AJR Am J Roentgenol. 2005;184(5):1700-1705.
3. Hopper RA, Salemy S, Sze RW. Diagnosis of midface fractures with CT: what the surgeon needs to know. Radiographics. 2006;26(3):783-793.
1. Le Fort R. Etude experimentale sur les fractures de la machoire superieure. Rev Chir. 1901;23:208-227, 360-379, 479-507.
2. Rhea JT, Novelline RA. How to simplify the CT diagnosis of Le Fort fractures. AJR Am J Roentgenol. 2005;184(5):1700-1705.
3. Hopper RA, Salemy S, Sze RW. Diagnosis of midface fractures with CT: what the surgeon needs to know. Radiographics. 2006;26(3):783-793.
Malpractice Counsel: Abdominal pain in an elderly patient
Case
An 89-year-old woman presented to the ED with the chief complaints of abdominal pain and nausea with vomiting. The patient stated that several hours prior, she had ingested an expired beverage, which she related to the sudden onset of her symptoms. The patient denied fever, chills, dysuria, or frequency. Her medical history was significant for chronic atrial fibrillation (AF) and congestive heart failure. The patient’s medications included metoprolol and furosemide; she was not on any anticoagulation medication.
On physical examination, the patient appeared her stated age, and was in moderate distress secondary to the abdominal pain. Vital signs were: temperature, 98.8oF; heart rate, 98 beats/min; respiratory rate, 20 breaths/min; and blood pressure, 116/72 mm Hg. Oxygen saturation was 97% on room air. The head, eyes, ears, nose, and throat examination was unremarkable. On lung examination, breath sounds were equal bilaterally with bibasilar rales. The heart rhythm was irregularly irregular without murmurs, rubs, or gallops. The abdomen was soft to palpitation, but diffusely tender, without rebound, guarding, or mass. Rectal examination revealed normal tone and brown stool, and was trace positive for heme.
The emergency physician (EP) ordered an electrocardiogram (ECG), complete blood count, basic metabolic profile (BMP), urinalysis, and lipase test. The patient was administered intravenous (IV) normal saline at 75 cc/h, and morphine 4 mg and ondansetron 4 mg IV for the abdominal pain, nausea, and vomiting. She required several more doses of morphine due to the severity of the pain. The laboratory results included an elevated white blood count of 18.4 x 109/L with a left shift, but normal hemoglobin and hematocrit values. The ECG demonstrated AF with a controlled ventricular rate; there was no evidence of ischemia or injury. The BMP was remarkable for a slightly depressed potassium level (3.3 mEq/L), a decreased serum bicarbonate of 20 mEq/L, and evidence of renal insufficiency with a blood urea nitrogen of 28 mg/dL and a serum creatinine of 1.6 mg/dL. Given the ongoing severe pain, leukocytosis, metabolic acidosis, and lack of clear etiology, the EP ordered a computed tomography (CT) scan of the abdomen and pelvis; no IV contrast was ordered because of the abnormal renal function studies.
The radiologist interpreted the CT scan as essentially normal. The EP admitted the patient to the on-call hospitalist, who consulted both cardiology and gastroenterology services. During the night, the patient complained of increasing abdominal pain, and her abdomen became distended with peritoneal signs. She was taken emergently to the operating room in the early morning hours. A large segment of gangrenous small intestine was found upon exploration. The surgery was discontinued and comfort care measures were instituted. The patient died the following day.
The patient’s family sued the EP and the hospital for failure to make a timely diagnosis of mesenteric ischemia. They further stated that the EP should have ordered a CT angiogram (CTA) of the abdomen and pelvis. The defense argued that a contrast CT scan was contraindicated because of the patient’s poor renal function. A defense verdict was returned at trial.
Discussion
Elderly patients (defined as older than age 65 years) presenting to the ED with abdominal pain remain a diagnostic challenge for even the most seasoned clinician. While elderly patients with a chief complaint of abdominal pain represent only a small percentage of ED patients, approximately 50% to 66% of these patients will require hospitalization, while one-third will require a surgical intervention.1 The seriousness of this complaint in elderly patients is further emphasized by the fact that older patients with abdominal pain have a 6- to 8-fold increase in mortality compared to younger patients.2,3 This can be partially explained by the simple fact that the life-threatening causes of abdominal pain—abdominal aortic aneurysm, mesenteric ischemia, bowel perforation, volvulus, and acute bowel obstruction—occur more frequently (but not exclusively) in elderly patients. Historical risk factors for life-threatening causes of abdominal pain include: age older than 65 years, immunocompromised state, alcohol abuse, cardiovascular (CV) disease (eg, coronary artery disease, hypertension, AF), major comorbidities (eg, cancer, renal failure), and prior surgery or recent gastrointestinal instrumentation.1
The patient in this case had two risk factors for life-threatening causes of lower abdominal pain—age and AF. These are also two of the major risk factors for mesenteric ischemia, which was her ultimate diagnosis.
Acute mesenteric ischemia refers to the sudden onset of small intestinal hypoperfusion, frequently due to acute occlusion (embolism or thrombosis) of an intestinal artery, most commonly the superior mesenteric artery (SMA).4 The SMA supplies the entire small intestine except for the proximal duodenum. Other causes of acute mesenteric ischemia include venous occlusion (thrombosis) and nonocclusive mesenteric ischemia secondary to vasoconstriction from low-cardiac output or use of vasopressors.4
Thromboembolic occlusion of the SMA is the most common cause of acute mesenteric ischemia, accounting for 67% to 95% of cases.4 In addition to AF, the risk of arterial embolism is increased in patients with valvular disease, infective endocarditis, recent myocardial infarction, aortic atherosclerosis, or aortic aneurysm.4 Risk factors for thrombotic arterial occlusion include peripheral artery disease, advanced age, and low-cardiac output states.5
A frequent presentation of embolic mesenteric arterial ischemia, occurring in approximately one-third of cases, is an elderly patient with AF (or other source of embolism) and onset of severe, sudden abdominal pain out of proportion to physical examination. While nausea and vomiting are also common, bloody bowel movements are less frequent in the early course of the disease process.4 A history of a prior embolic event is present in approximately one-third of such patients.
On physical examination, the abdomen may be normal initially, or demonstrate only mild distention and tenderness without peritoneal signs. However, as the ischemia progresses, the abdomen becomes more distended, bowel sounds become absent, and peritoneal signs (ie, guarding and rebound) become apparent.6
The results of laboratory studies can suggest the diagnosis, but none are confirmatory. Laboratory findings may include a marked leukocytosis with left shift, an elevated hematocrit secondary to hemoconcentration, and metabolic acidosis. A helpful clinical pearl is to consider intestinal ischemia in the differential diagnosis of any patient with acute abdominal pain and metabolic acidosis.6 Serum lactate is frequently elevated (73%-94%) but a very nonspecific marker. Similarly, an arterial blood gas analysis may demonstrate metabolic acidosis. More recently, a normal D-dimer result has been used to help exclude the diagnosis of acute intestinal ischemia, since it is elevated in 96% of patients with the disease.6 Similar to lactate, an abnormal D-dimer result has a poor specificity (40%).6 Early in the disease course, nearly all laboratory studies may be normal.
Depending on the severity of the presentation, imaging can help make the definitive diagnosis. For patients with peritonitis or obvious bowel perforation, IV fluid resuscitation, IV antibiotics, and immediate surgical exploration are indicated. Plain radiographs of the abdomen offer little help, as many of the findings early in the disease course are nonspecific, and radiographs can be normal in 25% of cases.6 Ultrasound can identify arterial stenosis or occlusion of the SMA, but is frequently technically limited by the presence of air-filled loops of distended bowel.6 Magnetic resonance angiography has similar sensitivity and specificity as CTA for mesenteric arterial ischemia, and is actually more sensitive than CTA for mesenteric venous thrombosis; it also can be performed in patients with contrast allergy.6 However, CTA is performed more commonly because of its lower cost, greater speed, and wide availability.6 A CTA of the abdomen and pelvis (without oral contrast) is probably the best study for patients in whom mesenteric ischemia is high on the differential diagnosis.6 For patients with a less clear picture and a broader differential diagnosis, a CT scan of the abdomen/pelvis with both IV and oral contrast is preferred.7 Common findings on CT scan with IV/oral contrast in acute mesenteric ischemia include the following: bowel wall thickening, dilatation, stranding, bowel wall attenuation, abnormal enhancement, and pneumatosis. Unfortunately, many of these findings are nonspecific.7
Once the diagnosis of acute mesenteric ischemia is made, patients should be designated “nothing by mouth” and a nasogastric tube placed to decompress the bowel. These patients will require IV fluid resuscitation with normal saline. The amount and rate will depend on their clinical presentation and underlying CV status. Any electrolyte abnormalities should be corrected and broad spectrum IV antibiotics initiated. Vascular surgery or general surgery services should be consulted to determine the optimal management. Most patients with acute intestinal ischemia due to mesenteric arterial occlusion (or venous occlusive or nonocclusive mesenteric ischemia) will be started on anticoagulation, typically IV heparin, unless contraindications are present.6 Surgical treatment options include arterial embolectomy, arterial bypass, arterial stenting, arterial thrombolysis, or intra-arterial vasodilator infusion.
1. Kendall JL, Moreira ME. Evaluation of the adult with abdominal pain in the emergency department. UpToDate Web site. http://www.uptodate.com/contents/evaluation-of-the-adult-with-abdominal-pain-in-the-emergency-department. Updated September 29, 2016. Accessed November 30, 2016.
2. Lewis LM, Banet GA, Blanda M, Hustey FM, Meldon SW, Gerson LW. Etiology and clinical course of abdominal pain in senior patients: a prospective, multicenter study. J Gerontol A Biol Sci Med Sci. 2005;60(8):1071-1076.
3. Sanson TG, O’Keefe KP. Evaluation of abdominal pain in the elderly. Emerg Med Clin North Am. 1996;14(3):615.
4. Tendler DA, Lamont JT, Pearl G. Acute mesenteric arterial occlusion. UpToDate Web site. http://www.uptodate.com/contents/acute-mesenteric-arterial-occlusion. Updated May 27, 2015. Accessed November 30, 2016.
5. McKinsey JF, Gewertz BL. Acute mesenteric ischemia. Surg Clin North Am. 1997;77(2):307-318.
6. Tendler DA, Lamont JT. Overview of intestinal ischemia in adults. UpToDate Web site. http://www.uptodate.com/contents/overview-of-intestinal-ischemia-in-adults. Updated February 23, 2016. Accessed November 30, 2016.
7. Wiesner W. Khurana B, Ji H, Ros PR. CT of acute bowel ischemia. Radiology. 2003;226(3):635-650.
Case
An 89-year-old woman presented to the ED with the chief complaints of abdominal pain and nausea with vomiting. The patient stated that several hours prior, she had ingested an expired beverage, which she related to the sudden onset of her symptoms. The patient denied fever, chills, dysuria, or frequency. Her medical history was significant for chronic atrial fibrillation (AF) and congestive heart failure. The patient’s medications included metoprolol and furosemide; she was not on any anticoagulation medication.
On physical examination, the patient appeared her stated age, and was in moderate distress secondary to the abdominal pain. Vital signs were: temperature, 98.8oF; heart rate, 98 beats/min; respiratory rate, 20 breaths/min; and blood pressure, 116/72 mm Hg. Oxygen saturation was 97% on room air. The head, eyes, ears, nose, and throat examination was unremarkable. On lung examination, breath sounds were equal bilaterally with bibasilar rales. The heart rhythm was irregularly irregular without murmurs, rubs, or gallops. The abdomen was soft to palpitation, but diffusely tender, without rebound, guarding, or mass. Rectal examination revealed normal tone and brown stool, and was trace positive for heme.
The emergency physician (EP) ordered an electrocardiogram (ECG), complete blood count, basic metabolic profile (BMP), urinalysis, and lipase test. The patient was administered intravenous (IV) normal saline at 75 cc/h, and morphine 4 mg and ondansetron 4 mg IV for the abdominal pain, nausea, and vomiting. She required several more doses of morphine due to the severity of the pain. The laboratory results included an elevated white blood count of 18.4 x 109/L with a left shift, but normal hemoglobin and hematocrit values. The ECG demonstrated AF with a controlled ventricular rate; there was no evidence of ischemia or injury. The BMP was remarkable for a slightly depressed potassium level (3.3 mEq/L), a decreased serum bicarbonate of 20 mEq/L, and evidence of renal insufficiency with a blood urea nitrogen of 28 mg/dL and a serum creatinine of 1.6 mg/dL. Given the ongoing severe pain, leukocytosis, metabolic acidosis, and lack of clear etiology, the EP ordered a computed tomography (CT) scan of the abdomen and pelvis; no IV contrast was ordered because of the abnormal renal function studies.
The radiologist interpreted the CT scan as essentially normal. The EP admitted the patient to the on-call hospitalist, who consulted both cardiology and gastroenterology services. During the night, the patient complained of increasing abdominal pain, and her abdomen became distended with peritoneal signs. She was taken emergently to the operating room in the early morning hours. A large segment of gangrenous small intestine was found upon exploration. The surgery was discontinued and comfort care measures were instituted. The patient died the following day.
The patient’s family sued the EP and the hospital for failure to make a timely diagnosis of mesenteric ischemia. They further stated that the EP should have ordered a CT angiogram (CTA) of the abdomen and pelvis. The defense argued that a contrast CT scan was contraindicated because of the patient’s poor renal function. A defense verdict was returned at trial.
Discussion
Elderly patients (defined as older than age 65 years) presenting to the ED with abdominal pain remain a diagnostic challenge for even the most seasoned clinician. While elderly patients with a chief complaint of abdominal pain represent only a small percentage of ED patients, approximately 50% to 66% of these patients will require hospitalization, while one-third will require a surgical intervention.1 The seriousness of this complaint in elderly patients is further emphasized by the fact that older patients with abdominal pain have a 6- to 8-fold increase in mortality compared to younger patients.2,3 This can be partially explained by the simple fact that the life-threatening causes of abdominal pain—abdominal aortic aneurysm, mesenteric ischemia, bowel perforation, volvulus, and acute bowel obstruction—occur more frequently (but not exclusively) in elderly patients. Historical risk factors for life-threatening causes of abdominal pain include: age older than 65 years, immunocompromised state, alcohol abuse, cardiovascular (CV) disease (eg, coronary artery disease, hypertension, AF), major comorbidities (eg, cancer, renal failure), and prior surgery or recent gastrointestinal instrumentation.1
The patient in this case had two risk factors for life-threatening causes of lower abdominal pain—age and AF. These are also two of the major risk factors for mesenteric ischemia, which was her ultimate diagnosis.
Acute mesenteric ischemia refers to the sudden onset of small intestinal hypoperfusion, frequently due to acute occlusion (embolism or thrombosis) of an intestinal artery, most commonly the superior mesenteric artery (SMA).4 The SMA supplies the entire small intestine except for the proximal duodenum. Other causes of acute mesenteric ischemia include venous occlusion (thrombosis) and nonocclusive mesenteric ischemia secondary to vasoconstriction from low-cardiac output or use of vasopressors.4
Thromboembolic occlusion of the SMA is the most common cause of acute mesenteric ischemia, accounting for 67% to 95% of cases.4 In addition to AF, the risk of arterial embolism is increased in patients with valvular disease, infective endocarditis, recent myocardial infarction, aortic atherosclerosis, or aortic aneurysm.4 Risk factors for thrombotic arterial occlusion include peripheral artery disease, advanced age, and low-cardiac output states.5
A frequent presentation of embolic mesenteric arterial ischemia, occurring in approximately one-third of cases, is an elderly patient with AF (or other source of embolism) and onset of severe, sudden abdominal pain out of proportion to physical examination. While nausea and vomiting are also common, bloody bowel movements are less frequent in the early course of the disease process.4 A history of a prior embolic event is present in approximately one-third of such patients.
On physical examination, the abdomen may be normal initially, or demonstrate only mild distention and tenderness without peritoneal signs. However, as the ischemia progresses, the abdomen becomes more distended, bowel sounds become absent, and peritoneal signs (ie, guarding and rebound) become apparent.6
The results of laboratory studies can suggest the diagnosis, but none are confirmatory. Laboratory findings may include a marked leukocytosis with left shift, an elevated hematocrit secondary to hemoconcentration, and metabolic acidosis. A helpful clinical pearl is to consider intestinal ischemia in the differential diagnosis of any patient with acute abdominal pain and metabolic acidosis.6 Serum lactate is frequently elevated (73%-94%) but a very nonspecific marker. Similarly, an arterial blood gas analysis may demonstrate metabolic acidosis. More recently, a normal D-dimer result has been used to help exclude the diagnosis of acute intestinal ischemia, since it is elevated in 96% of patients with the disease.6 Similar to lactate, an abnormal D-dimer result has a poor specificity (40%).6 Early in the disease course, nearly all laboratory studies may be normal.
Depending on the severity of the presentation, imaging can help make the definitive diagnosis. For patients with peritonitis or obvious bowel perforation, IV fluid resuscitation, IV antibiotics, and immediate surgical exploration are indicated. Plain radiographs of the abdomen offer little help, as many of the findings early in the disease course are nonspecific, and radiographs can be normal in 25% of cases.6 Ultrasound can identify arterial stenosis or occlusion of the SMA, but is frequently technically limited by the presence of air-filled loops of distended bowel.6 Magnetic resonance angiography has similar sensitivity and specificity as CTA for mesenteric arterial ischemia, and is actually more sensitive than CTA for mesenteric venous thrombosis; it also can be performed in patients with contrast allergy.6 However, CTA is performed more commonly because of its lower cost, greater speed, and wide availability.6 A CTA of the abdomen and pelvis (without oral contrast) is probably the best study for patients in whom mesenteric ischemia is high on the differential diagnosis.6 For patients with a less clear picture and a broader differential diagnosis, a CT scan of the abdomen/pelvis with both IV and oral contrast is preferred.7 Common findings on CT scan with IV/oral contrast in acute mesenteric ischemia include the following: bowel wall thickening, dilatation, stranding, bowel wall attenuation, abnormal enhancement, and pneumatosis. Unfortunately, many of these findings are nonspecific.7
Once the diagnosis of acute mesenteric ischemia is made, patients should be designated “nothing by mouth” and a nasogastric tube placed to decompress the bowel. These patients will require IV fluid resuscitation with normal saline. The amount and rate will depend on their clinical presentation and underlying CV status. Any electrolyte abnormalities should be corrected and broad spectrum IV antibiotics initiated. Vascular surgery or general surgery services should be consulted to determine the optimal management. Most patients with acute intestinal ischemia due to mesenteric arterial occlusion (or venous occlusive or nonocclusive mesenteric ischemia) will be started on anticoagulation, typically IV heparin, unless contraindications are present.6 Surgical treatment options include arterial embolectomy, arterial bypass, arterial stenting, arterial thrombolysis, or intra-arterial vasodilator infusion.
Case
An 89-year-old woman presented to the ED with the chief complaints of abdominal pain and nausea with vomiting. The patient stated that several hours prior, she had ingested an expired beverage, which she related to the sudden onset of her symptoms. The patient denied fever, chills, dysuria, or frequency. Her medical history was significant for chronic atrial fibrillation (AF) and congestive heart failure. The patient’s medications included metoprolol and furosemide; she was not on any anticoagulation medication.
On physical examination, the patient appeared her stated age, and was in moderate distress secondary to the abdominal pain. Vital signs were: temperature, 98.8oF; heart rate, 98 beats/min; respiratory rate, 20 breaths/min; and blood pressure, 116/72 mm Hg. Oxygen saturation was 97% on room air. The head, eyes, ears, nose, and throat examination was unremarkable. On lung examination, breath sounds were equal bilaterally with bibasilar rales. The heart rhythm was irregularly irregular without murmurs, rubs, or gallops. The abdomen was soft to palpitation, but diffusely tender, without rebound, guarding, or mass. Rectal examination revealed normal tone and brown stool, and was trace positive for heme.
The emergency physician (EP) ordered an electrocardiogram (ECG), complete blood count, basic metabolic profile (BMP), urinalysis, and lipase test. The patient was administered intravenous (IV) normal saline at 75 cc/h, and morphine 4 mg and ondansetron 4 mg IV for the abdominal pain, nausea, and vomiting. She required several more doses of morphine due to the severity of the pain. The laboratory results included an elevated white blood count of 18.4 x 109/L with a left shift, but normal hemoglobin and hematocrit values. The ECG demonstrated AF with a controlled ventricular rate; there was no evidence of ischemia or injury. The BMP was remarkable for a slightly depressed potassium level (3.3 mEq/L), a decreased serum bicarbonate of 20 mEq/L, and evidence of renal insufficiency with a blood urea nitrogen of 28 mg/dL and a serum creatinine of 1.6 mg/dL. Given the ongoing severe pain, leukocytosis, metabolic acidosis, and lack of clear etiology, the EP ordered a computed tomography (CT) scan of the abdomen and pelvis; no IV contrast was ordered because of the abnormal renal function studies.
The radiologist interpreted the CT scan as essentially normal. The EP admitted the patient to the on-call hospitalist, who consulted both cardiology and gastroenterology services. During the night, the patient complained of increasing abdominal pain, and her abdomen became distended with peritoneal signs. She was taken emergently to the operating room in the early morning hours. A large segment of gangrenous small intestine was found upon exploration. The surgery was discontinued and comfort care measures were instituted. The patient died the following day.
The patient’s family sued the EP and the hospital for failure to make a timely diagnosis of mesenteric ischemia. They further stated that the EP should have ordered a CT angiogram (CTA) of the abdomen and pelvis. The defense argued that a contrast CT scan was contraindicated because of the patient’s poor renal function. A defense verdict was returned at trial.
Discussion
Elderly patients (defined as older than age 65 years) presenting to the ED with abdominal pain remain a diagnostic challenge for even the most seasoned clinician. While elderly patients with a chief complaint of abdominal pain represent only a small percentage of ED patients, approximately 50% to 66% of these patients will require hospitalization, while one-third will require a surgical intervention.1 The seriousness of this complaint in elderly patients is further emphasized by the fact that older patients with abdominal pain have a 6- to 8-fold increase in mortality compared to younger patients.2,3 This can be partially explained by the simple fact that the life-threatening causes of abdominal pain—abdominal aortic aneurysm, mesenteric ischemia, bowel perforation, volvulus, and acute bowel obstruction—occur more frequently (but not exclusively) in elderly patients. Historical risk factors for life-threatening causes of abdominal pain include: age older than 65 years, immunocompromised state, alcohol abuse, cardiovascular (CV) disease (eg, coronary artery disease, hypertension, AF), major comorbidities (eg, cancer, renal failure), and prior surgery or recent gastrointestinal instrumentation.1
The patient in this case had two risk factors for life-threatening causes of lower abdominal pain—age and AF. These are also two of the major risk factors for mesenteric ischemia, which was her ultimate diagnosis.
Acute mesenteric ischemia refers to the sudden onset of small intestinal hypoperfusion, frequently due to acute occlusion (embolism or thrombosis) of an intestinal artery, most commonly the superior mesenteric artery (SMA).4 The SMA supplies the entire small intestine except for the proximal duodenum. Other causes of acute mesenteric ischemia include venous occlusion (thrombosis) and nonocclusive mesenteric ischemia secondary to vasoconstriction from low-cardiac output or use of vasopressors.4
Thromboembolic occlusion of the SMA is the most common cause of acute mesenteric ischemia, accounting for 67% to 95% of cases.4 In addition to AF, the risk of arterial embolism is increased in patients with valvular disease, infective endocarditis, recent myocardial infarction, aortic atherosclerosis, or aortic aneurysm.4 Risk factors for thrombotic arterial occlusion include peripheral artery disease, advanced age, and low-cardiac output states.5
A frequent presentation of embolic mesenteric arterial ischemia, occurring in approximately one-third of cases, is an elderly patient with AF (or other source of embolism) and onset of severe, sudden abdominal pain out of proportion to physical examination. While nausea and vomiting are also common, bloody bowel movements are less frequent in the early course of the disease process.4 A history of a prior embolic event is present in approximately one-third of such patients.
On physical examination, the abdomen may be normal initially, or demonstrate only mild distention and tenderness without peritoneal signs. However, as the ischemia progresses, the abdomen becomes more distended, bowel sounds become absent, and peritoneal signs (ie, guarding and rebound) become apparent.6
The results of laboratory studies can suggest the diagnosis, but none are confirmatory. Laboratory findings may include a marked leukocytosis with left shift, an elevated hematocrit secondary to hemoconcentration, and metabolic acidosis. A helpful clinical pearl is to consider intestinal ischemia in the differential diagnosis of any patient with acute abdominal pain and metabolic acidosis.6 Serum lactate is frequently elevated (73%-94%) but a very nonspecific marker. Similarly, an arterial blood gas analysis may demonstrate metabolic acidosis. More recently, a normal D-dimer result has been used to help exclude the diagnosis of acute intestinal ischemia, since it is elevated in 96% of patients with the disease.6 Similar to lactate, an abnormal D-dimer result has a poor specificity (40%).6 Early in the disease course, nearly all laboratory studies may be normal.
Depending on the severity of the presentation, imaging can help make the definitive diagnosis. For patients with peritonitis or obvious bowel perforation, IV fluid resuscitation, IV antibiotics, and immediate surgical exploration are indicated. Plain radiographs of the abdomen offer little help, as many of the findings early in the disease course are nonspecific, and radiographs can be normal in 25% of cases.6 Ultrasound can identify arterial stenosis or occlusion of the SMA, but is frequently technically limited by the presence of air-filled loops of distended bowel.6 Magnetic resonance angiography has similar sensitivity and specificity as CTA for mesenteric arterial ischemia, and is actually more sensitive than CTA for mesenteric venous thrombosis; it also can be performed in patients with contrast allergy.6 However, CTA is performed more commonly because of its lower cost, greater speed, and wide availability.6 A CTA of the abdomen and pelvis (without oral contrast) is probably the best study for patients in whom mesenteric ischemia is high on the differential diagnosis.6 For patients with a less clear picture and a broader differential diagnosis, a CT scan of the abdomen/pelvis with both IV and oral contrast is preferred.7 Common findings on CT scan with IV/oral contrast in acute mesenteric ischemia include the following: bowel wall thickening, dilatation, stranding, bowel wall attenuation, abnormal enhancement, and pneumatosis. Unfortunately, many of these findings are nonspecific.7
Once the diagnosis of acute mesenteric ischemia is made, patients should be designated “nothing by mouth” and a nasogastric tube placed to decompress the bowel. These patients will require IV fluid resuscitation with normal saline. The amount and rate will depend on their clinical presentation and underlying CV status. Any electrolyte abnormalities should be corrected and broad spectrum IV antibiotics initiated. Vascular surgery or general surgery services should be consulted to determine the optimal management. Most patients with acute intestinal ischemia due to mesenteric arterial occlusion (or venous occlusive or nonocclusive mesenteric ischemia) will be started on anticoagulation, typically IV heparin, unless contraindications are present.6 Surgical treatment options include arterial embolectomy, arterial bypass, arterial stenting, arterial thrombolysis, or intra-arterial vasodilator infusion.
1. Kendall JL, Moreira ME. Evaluation of the adult with abdominal pain in the emergency department. UpToDate Web site. http://www.uptodate.com/contents/evaluation-of-the-adult-with-abdominal-pain-in-the-emergency-department. Updated September 29, 2016. Accessed November 30, 2016.
2. Lewis LM, Banet GA, Blanda M, Hustey FM, Meldon SW, Gerson LW. Etiology and clinical course of abdominal pain in senior patients: a prospective, multicenter study. J Gerontol A Biol Sci Med Sci. 2005;60(8):1071-1076.
3. Sanson TG, O’Keefe KP. Evaluation of abdominal pain in the elderly. Emerg Med Clin North Am. 1996;14(3):615.
4. Tendler DA, Lamont JT, Pearl G. Acute mesenteric arterial occlusion. UpToDate Web site. http://www.uptodate.com/contents/acute-mesenteric-arterial-occlusion. Updated May 27, 2015. Accessed November 30, 2016.
5. McKinsey JF, Gewertz BL. Acute mesenteric ischemia. Surg Clin North Am. 1997;77(2):307-318.
6. Tendler DA, Lamont JT. Overview of intestinal ischemia in adults. UpToDate Web site. http://www.uptodate.com/contents/overview-of-intestinal-ischemia-in-adults. Updated February 23, 2016. Accessed November 30, 2016.
7. Wiesner W. Khurana B, Ji H, Ros PR. CT of acute bowel ischemia. Radiology. 2003;226(3):635-650.
1. Kendall JL, Moreira ME. Evaluation of the adult with abdominal pain in the emergency department. UpToDate Web site. http://www.uptodate.com/contents/evaluation-of-the-adult-with-abdominal-pain-in-the-emergency-department. Updated September 29, 2016. Accessed November 30, 2016.
2. Lewis LM, Banet GA, Blanda M, Hustey FM, Meldon SW, Gerson LW. Etiology and clinical course of abdominal pain in senior patients: a prospective, multicenter study. J Gerontol A Biol Sci Med Sci. 2005;60(8):1071-1076.
3. Sanson TG, O’Keefe KP. Evaluation of abdominal pain in the elderly. Emerg Med Clin North Am. 1996;14(3):615.
4. Tendler DA, Lamont JT, Pearl G. Acute mesenteric arterial occlusion. UpToDate Web site. http://www.uptodate.com/contents/acute-mesenteric-arterial-occlusion. Updated May 27, 2015. Accessed November 30, 2016.
5. McKinsey JF, Gewertz BL. Acute mesenteric ischemia. Surg Clin North Am. 1997;77(2):307-318.
6. Tendler DA, Lamont JT. Overview of intestinal ischemia in adults. UpToDate Web site. http://www.uptodate.com/contents/overview-of-intestinal-ischemia-in-adults. Updated February 23, 2016. Accessed November 30, 2016.
7. Wiesner W. Khurana B, Ji H, Ros PR. CT of acute bowel ischemia. Radiology. 2003;226(3):635-650.
Holiday Poisonings
The holiday season, a time of warmth, joy, and good cheer, is upon us. Yet with this most wonderful time of the year comes the possibility of poisoning and hazards in the home. As emergency physicians (EPs), we must ask ourselves: Which holiday items are potentially toxic to our patients? How do we evaluate and manage poisonings that result from exposure to these items? In this article, we review several plants and decorations that are unique to the holiday season. We discuss recommendations for evaluation and management of holiday poisonings that will avoid inappropriate work-ups and interventions while increasing recognition of truly dangerous ingestions, thus help keeping the season safe and merry.
Plants
Plant exposures are the fourth most common cause for calls to poison centers.1 In 2012, US Poison Control Centers reported more than 30,000 toxic plant exposures in children younger than age 5 years.2 Not surprisingly, toxic plant ingestions occur most commonly in early childhood. The highest rate of mortality, however, takes place during the teenaged years, when suicide attempts are common.2 The most common plant ingestions reported in the United States include peace lily, holly, philodendron, and poinsettia.3 These and the other frequently ingested potentially poisonous plants produce very little, if any, toxic effects. Approximately 95% of unintentional potentially toxic plant ingestions reported in the United States are managed safely at home.3
Poinsettia
The poinsettia is a large, prominent plant that was introduced to the United States in 1825 by Joel Poinsett, the US ambassador to Mexico. The poinsettia is one of the most commonly researched plants, and studies show the plant is not actually toxic.4,5 The myth of poinsettia toxicity is a widely held, yet false, belief. The legend involves a young child of an army officer stationed in Hawaii in 1919 who reportedly died after eating poinsettia leaves.4 However, the reality is that the poinsettia plant was not actually involved in the child’s death. In fact, the wild plant involved in this case probably had little resemblance to the popular plant cultivated domestically in North America today.6
A majority of poinsettia exposures will be asymptomatic, or involve simple nausea and vomiting. Krenzelok et al5 reviewed 22,793 cases of poinsettia exposures from 1985 to 1992. Almost all of these exposures (98.9%) were accidental poisonings.5 Not surprisingly, 93.3% of poinsettia exposures involved children; most importantly, 96.1% of these patients did not require treatment at a health care facility,and there were no fatalities.5 Another study could not identify an LD50 (lethal dose, 50%—ie, the amount of an ingested substance that kills 50% of the test sample) in rats.4
The majority of patients presenting to the ED with symptoms from poinsettia exposure will have gastrointestinal (GI) upset. Most patients require only symptomatic care. Those who do present to the ED do not require gastric emptying.
Interestingly, there is a crossreactivity of poinsettia sap in latex-allergic vulnerable patients.6 Poinsettia is part of the same plant family as natural rubber latex, and patients can present with symptoms of contact dermatitis, especially if they have a latex allergy.4 Washing the area thoroughly with soap and water and avoiding future contact is all that is required for most patients with contact dermatitis.
Holly
Holly exposure accounted for the third highest rate of genus-specific human plant exposure calls to poison centers in 2010.4 In the United States, there are two common forms of holly: English holly and American holly. The berries of both varieties contain saponin, a toxin that can cause erythrocyte hemolysis and changes in the permeability of small intestinal mucosal cells.4 Most holly berry ingestions cause minor or no symptoms. The prickly leaves of the holly plant are nontoxic but consumption may result in minor injury. When symptoms do occur, they can include nausea, vomiting, abdominal cramping, and possible dermatitis.5 Mydriasis, hyperthermia, and drowsiness are rare but possible symptoms.4
For symptoms to develop, children need to have eaten only five berries, while adults reportedly must consume at least 20 to 30 berries.4 A study by Wax et al7 done at the University of Rochester reviewed 103 cases of toxic berry ingestion in children aged 9 months to 5 years, with children who swallowed six or fewer berries of holly, yew, or nightshade. Investigators compared home observation alone versus ipecac administration with home observation. Every patient treated with ipecac had emesis at home with increased sedation and diarrhea, while there was no emesis in the group with home observation alone.7 These results suggest the symptoms were due to the ipecac rather than plant toxicity.7 Thus, ipecac is not recommended, and patients should be treated symptomatically.
Bittersweet and Jerusalem Cherry
Bittersweet, also known as the woody nightshade, and Jerusalem cherry, or Christmas orange, are the most dangerous of the holiday plants. While there is little evidence to support serious toxicity to adults, ingestion may be dangerous to children. Bittersweet has purple and yellow flowers, spreading petals, and red, ovoid berries.4 Both plants are part of the genus Solanum. In both plants, the immature fruit is more poisonous than ripened fruit due to the glycoalkaloid solanine via hypothetical alteration of mitochondrial potassium and calcium transport.4 Case reports document the rare anticholinergic effects of these plants, likely due to dulcamarine.4
The largest case series included 319 ingestions of bittersweet or Jerusalem cherry.4 Of these, 295 patients were under age 10 years, and only nine experienced solanine-related symptoms; none required hospitalization.4 The symptoms of ingestion were primarily nausea and vomiting and abdominal cramping, possibly due to anticholinergic effects. Symptoms typically occur several hours after ingestion and may last for days.
Historically, induced emesis was recommended for ingestion in children, but this is no longer recommended. Prolonged observation may be necessary for children in the setting of high likelihood of ingestion. Management includes rehydration with intravenous (IV) fluids, antiemetics, and physostigmine if clinically warranted.4
Mistletoe
Mistletoe, a perennial with white or translucent berries, has traditionally been associated with kissing, fertility, and vitality. The American mistletoe is known as Phoradendron serotinum and the European mistletoe as Viscum album. Both the American and European mistletoe contain the toxalbumins phoratoxin and viscotoxin, which are associated with inhibiting cellular synthesis, thereby affecting cells with rapid turnover, including the GI mucosa.4
After several hours, clinical effects are primarily GI upset with potential sloughing of portions of the intestinal tract.4 Bradycardia, delirium, and hepatic, central nervous system, kidney, and adrenal gland toxicities can also occur.8 The American species has a lower toxicity compared to the European species. Cases involving death likely related to P serotinum usually occur due to excessive, concentrated herbal use, such as brewing mistletoe in tea.9 Placing the plant in hot water may result in larger amounts of ingested toxin. The only two reported deaths from ingestion of mistletoe were in patients who consumed brewed teas.4
A case review of 14 patients with American mistletoe leaf or berry ingestions failed to find any toxic symptoms.4 Krenzelok et al10 compiled the largest case review of 1,754 exposures from 1985 to 1992. In this review, patient outcomes were good. There were no fatalities, and 99% of patients experienced no morbidity. Outcomes were not influenced by GI decontamination.10
Another study by Spiller et al11 described 92 American mistletoe exposures involving ingestions of up to 20 berries and five leaves. In cases where five or more berries were consumed, none of the patients had symptoms.11 Three of the 11 patients (27%) who swallowed one to five leaves developed GI upset. One child had a seizure, likely not related to the mistletoe. The study concluded that severe toxic symptoms are uncommon.11
Management in the ED should involve supportive care for dehydration and vomiting, typically IV rehydration with normal saline or Ringer’s lactate and IV antiemetics. According to multiple case reviews, GI decontamination is not believed to alter patient outcome and is not recommended.4 An observation period of 6 hours is reasonable.4,10,11
Christmas Cactus
Christmas cactus is an old-time favorite. It is made of arching, drooping branches and spineless joints. Christmas cactus is essentially nontoxic, and patients and family can be reassured of its safety.
Holiday Decorations
Artificial Snow
Fake snow sprays, powders, and granules are popular decorative additions used in holiday games and celebrations. The “snow” typically consists of a polymer of sodium polyacrylate, both of which can cause injury to the eyes. Repeatedly inhaling the aerosol spray can cause breathing problems, especially in patients who have asthma or other underlying bronchospastic disease.
Devastating outcomes may occur from ocular alkaline injury. When mixed with water, the fake snow absorbs the water and expands as a gel material that may stick to the ocular surface, resulting in a change in pH and osmolarity.12 A case report by Al-Amry and Al-Ghadeer12 recently described a 7-year-old boy with corneal epitheliopathy due to a chemical burn injury following ocular contact with fake snow.The case was later managed with multiple debridements over 3 days, topical antibiotics, and bandage contact lenses. The child had complete resolution at 1 week follow-up.12
Some fake snow-product sprays contain acetone or methylene chloride, which is harmful when inhaled and can cause nausea, lightheadedness, and headache.13 Methylene chloride can be metabolized to carbon monoxide, but the quantity required for such an exposure is unknown and has not been reported in this context. Emergency physicians should consider ordering carboxyhemoglobin levels in symptomatic patients.
Tinsel
Tinsel, which gets its name from the Old French word “estincele,” translated as sparkle, used to be made of actual silver, and was affordable only for wealthy individuals. However, in the early 1900s, manufacturers began to make tinsel from metals such as aluminum and copper. These materials did not tarnish and could be reused annually. However, during World War I, copper became difficult to buy, while aluminum proved to be flammable and dangerous. Thus, manufacturers began to produce tinsel from lead. Tinsel was made with lead until the 1970s, when the US Food and Drug Administration realized the toxic risks of lead exposure, especially in young children. Today, tinsel is made of plastic; though a poor imitation of the previous tinsel, it is relatively harmless.14
Angel Hair
Angel hair is finely spun glass that can be irritating to the skin, eyes, and throat, especially if swallowed.13 The greatest danger is airway obstruction if a patient attempts to eat the angel hair and it becomes lodged in the oropharynx. For contact irritation, thoroughly washing and irrigating affected areas are recommended.
Snow Globes
Snow globes are popular holiday decorations that are available in a range of sizes. While the majority of globes made in the United States are filled with water, those manufactured overseas many contain a small amount of ethylene glycol (EG) (ie, antifreeze) to prevent freezing and breakage during shipping. Fortunately, the amount of EG is not usually sufficient to cause symptoms if ingested. For globes made in the United States, the water can be contaminated with bacteria, and drinking it can cause GI upset. The snow in these globes is typically made of inert material and does not cause toxicity. If a child does exhibit symptoms after ingesting any portion of a snow globe, parents are advised to call their local poison center.
Ethanol
While alcohol is not unique to the holiday season, its availability and use are more pronounced during this time of year, and the incidence of alcohol poisoning increases during the holiday season. Some traditional holiday drinks containing alcohol, such as egg nog, can entice young children. Children may often imitate adults and drink from partially filled leftover glasses.
Therefore, families with young children must ensure that all alcoholic beverages are placed out of children’s reach.
A common presentation of alcohol poisoning is seen in the child who is brought to the ED by parents concerned because their child is acting strangely. On examination, the child may appear dazed and have tachycardia, tachypnea, and hypotension, depending on the amount of alcohol ingested. Hypoglycemia in an alcohol-intoxicated pediatric patient is a concern, but it appears the effects of alcohol on glucose regulation in infants is unpredictable.15
Intravenous access should be obtained in any patient presenting with altered mental status, and rapid blood glucose level determined. Blood samples should be sent to assess ethanol concentration. Other laboratory and imaging studies should be obtained as clinically indicated, including electrolytes, serum osmolality, acetaminophen level, urine drug screen, X-ray, and computed tomography scan of the head. Treatment of respiratory depression, hypoglycemia, hypovolemia, and hypothermia are the key interventions to ensure good outcomes.16 Supportive care is the mainstay of therapy for pediatric patients, who rarely require thiamine supplementation.16 Medical evaluation is recommended for all symptomatic children; hourly observation for 6 hours is recommended for asymptomatic children.17
Alcohol is also associated with cardiac arrhythmias. Alcohol-induced atrial arrhythmias, most commonly atrial fibrillation (AF), are referred to as “holiday heart syndrome.” This should be considered early in the differential diagnosis of new-onset AF in young adults. Consuming massive quantities of alcohol or binge drinking can also result in metabolic and electrolyte alterations. Treatment includes rehydration with IV fluids, electrolyte replacement, and IV diltiazem or cardioversion for AF with rapid ventricular response.18
Conclusion
During the holiday season, it is easy to overlook the fact that some of the most unsuspecting items in the home can pose real hazards (Table). In addition, many holiday plants are used as table decorations, which can confuse small children, who may assume the colorful berries must be edible if they are on the dining room table.
It is vital that patients, parents, and physicians know what to do when someone ingests a potential toxin. Parents often try to induce vomiting, but ipecac and other forms of gastric emptying are no longer recommended.6 Instead, the recommended action is to separate the patient from the plant, remove plant material that may cause a sensitivity reaction, and consult a poison control center, which can save unnecessary interventions—including an ED visit.6
Fortunately, most holiday toxicities are relatively nonthreatening. Holiday-related toxic ingestions primarily occur in children, and most are asymptomatic, innocuous, and treated with symptomatic care as necessary. The most poisonous holiday-related toxins are bittersweet and Jerusalem cherry. Work-ups for holiday-plant ingestions are usually limited to severe gastroenteritis, which may require IV fluids and evaluation of electrolytes.
Holiday decorations, such as artificial snow and angel hair, present hazards that should be treated on a case-by-case basis. Finally, alcohol intoxication should be considered in the differential diagnosis for pediatric patients presenting with altered mental status, or the otherwise healthy binge drinker who presents with palpitations and new-onset AF.
1. Krenzelok EP, Jacobsen TD, Aronis J. Those pesky berries...are they a source of concern? Vet Hum Toxicol. 1998;40(2):101-103.
2. Martínez Monseny A, Martínez Sánchez L, Margarit Soler A, Trenchs Sainz de la Maza V, Luaces Cubells C. [Poisonous plants: An ongoing problem]. An Pediatr (Barc). 2015;82(5):347-353. doi:10.1016/j.anpedi.2014.08.008.
3. Krenzelok EP, Mrvos R. Friends and foes in the plant world: a profile of plant ingestions and fatalities. Clin Toxicol (Phila). 2011;49(3):142-149. doi:10.3109/15563650.2011.568945.
4. Evens ZN, Stellpflug SJ. Holiday plants with toxic misconceptions. West J Emerg Med. 2012;13(6):538-542. doi:10.5811/westjem.2012.8.12572.
5. Krenzelok E, Jacobsen TD, Aronis JM. Poinsettia exposures have good outcomes…just as we thought. Am J Emerg Med. 1996;14(7):671-674. doi:10.1016/S0735-6757(96)90086-90088.
6. Courtemanche J, Peterson, RG. Beware the mistletoe. CMAJ. 2006;175(12):1523-1524. doi:10.1503/cmaj.061432.
7. Wax PM, Cobaugh DJ, Lawrence RA. Should home ipecac-induced emesis be routinely recommended in the management of toxic berry ingestions? Vet Hum Toxicol. 1999;41(6):394-397.
8. Palmer ME, Betz JM. Plants. In: Nelson LS, Lewin NA, Howland MA, Hoffman RS, Goldfrank LR, Flomenbaum NE, eds. Goldfrank’s Toxicologic Emergencies. 9th ed. New York, NY: McGraw Hill; 2011:1537-1560.
9. Bruneton J. Toxic plants dangerous to humans and animals. Paris, France: Lavoisier Publishing; 1999.10. Krenzelok EP, Jacobsen TD, Aronis J. American mistletoe exposures. Am J Emerg Med. 1997;15(5):516-520.
11. Spiller HA, Willias DB, Gorman SE, Sanftleban J. Retrospective study of mistletoe ingestion. J Toxicol Clin Toxicol. 1996;34(4):405-408.
12. Al-Amry MA, Al-Ghadeer HA. Corneal epithliopathy after trauma by fake snow powder in a 7-year-old child. Middle East Afr J Ophthalmol. 2016;23(3):274-276. doi:10.4103/0974-9233.186157.
13. California Poison Control Center. Winter holiday safety & poison prevention tips. http://www.calpoison.org/public/winter-holidays.html. Accessed October 16, 2016.
14. Romm C. Don’t lick the tinsel. The Atlantic. December 21, 2015. http://www.theatlantic.com/health/archive/2015/12/dont-lick-the-tinsel/421506/. Accessed October 16, 2016.
15. Minera G, Robinson E. Accidental acute alcohol intoxication in infants: review and case report. J Emerg Med. 2014;47(5):524-526.
16. Baum CR. Ethanol intoxication in children: clinical features, evaluation, and management. UpToDate. http://www.uptodate.com/contents/ethanol-intoxication-in-children-clinical-features-evaluation-and-management. Accessed October 16, 2016.
17. Vogel C, Caraccio T, Mofenson H, Hart S. Alcohol intoxication in young children. J Toxicol Clin Toxicol. 1995;33(1):25-33.
18. Carey MG, Al-Zaiti SS, Kozik TM, Pelter M. Holiday heart syndrome. Am J Crit Care. 2014;23(2):171-172.
The holiday season, a time of warmth, joy, and good cheer, is upon us. Yet with this most wonderful time of the year comes the possibility of poisoning and hazards in the home. As emergency physicians (EPs), we must ask ourselves: Which holiday items are potentially toxic to our patients? How do we evaluate and manage poisonings that result from exposure to these items? In this article, we review several plants and decorations that are unique to the holiday season. We discuss recommendations for evaluation and management of holiday poisonings that will avoid inappropriate work-ups and interventions while increasing recognition of truly dangerous ingestions, thus help keeping the season safe and merry.
Plants
Plant exposures are the fourth most common cause for calls to poison centers.1 In 2012, US Poison Control Centers reported more than 30,000 toxic plant exposures in children younger than age 5 years.2 Not surprisingly, toxic plant ingestions occur most commonly in early childhood. The highest rate of mortality, however, takes place during the teenaged years, when suicide attempts are common.2 The most common plant ingestions reported in the United States include peace lily, holly, philodendron, and poinsettia.3 These and the other frequently ingested potentially poisonous plants produce very little, if any, toxic effects. Approximately 95% of unintentional potentially toxic plant ingestions reported in the United States are managed safely at home.3
Poinsettia
The poinsettia is a large, prominent plant that was introduced to the United States in 1825 by Joel Poinsett, the US ambassador to Mexico. The poinsettia is one of the most commonly researched plants, and studies show the plant is not actually toxic.4,5 The myth of poinsettia toxicity is a widely held, yet false, belief. The legend involves a young child of an army officer stationed in Hawaii in 1919 who reportedly died after eating poinsettia leaves.4 However, the reality is that the poinsettia plant was not actually involved in the child’s death. In fact, the wild plant involved in this case probably had little resemblance to the popular plant cultivated domestically in North America today.6
A majority of poinsettia exposures will be asymptomatic, or involve simple nausea and vomiting. Krenzelok et al5 reviewed 22,793 cases of poinsettia exposures from 1985 to 1992. Almost all of these exposures (98.9%) were accidental poisonings.5 Not surprisingly, 93.3% of poinsettia exposures involved children; most importantly, 96.1% of these patients did not require treatment at a health care facility,and there were no fatalities.5 Another study could not identify an LD50 (lethal dose, 50%—ie, the amount of an ingested substance that kills 50% of the test sample) in rats.4
The majority of patients presenting to the ED with symptoms from poinsettia exposure will have gastrointestinal (GI) upset. Most patients require only symptomatic care. Those who do present to the ED do not require gastric emptying.
Interestingly, there is a crossreactivity of poinsettia sap in latex-allergic vulnerable patients.6 Poinsettia is part of the same plant family as natural rubber latex, and patients can present with symptoms of contact dermatitis, especially if they have a latex allergy.4 Washing the area thoroughly with soap and water and avoiding future contact is all that is required for most patients with contact dermatitis.
Holly
Holly exposure accounted for the third highest rate of genus-specific human plant exposure calls to poison centers in 2010.4 In the United States, there are two common forms of holly: English holly and American holly. The berries of both varieties contain saponin, a toxin that can cause erythrocyte hemolysis and changes in the permeability of small intestinal mucosal cells.4 Most holly berry ingestions cause minor or no symptoms. The prickly leaves of the holly plant are nontoxic but consumption may result in minor injury. When symptoms do occur, they can include nausea, vomiting, abdominal cramping, and possible dermatitis.5 Mydriasis, hyperthermia, and drowsiness are rare but possible symptoms.4
For symptoms to develop, children need to have eaten only five berries, while adults reportedly must consume at least 20 to 30 berries.4 A study by Wax et al7 done at the University of Rochester reviewed 103 cases of toxic berry ingestion in children aged 9 months to 5 years, with children who swallowed six or fewer berries of holly, yew, or nightshade. Investigators compared home observation alone versus ipecac administration with home observation. Every patient treated with ipecac had emesis at home with increased sedation and diarrhea, while there was no emesis in the group with home observation alone.7 These results suggest the symptoms were due to the ipecac rather than plant toxicity.7 Thus, ipecac is not recommended, and patients should be treated symptomatically.
Bittersweet and Jerusalem Cherry
Bittersweet, also known as the woody nightshade, and Jerusalem cherry, or Christmas orange, are the most dangerous of the holiday plants. While there is little evidence to support serious toxicity to adults, ingestion may be dangerous to children. Bittersweet has purple and yellow flowers, spreading petals, and red, ovoid berries.4 Both plants are part of the genus Solanum. In both plants, the immature fruit is more poisonous than ripened fruit due to the glycoalkaloid solanine via hypothetical alteration of mitochondrial potassium and calcium transport.4 Case reports document the rare anticholinergic effects of these plants, likely due to dulcamarine.4
The largest case series included 319 ingestions of bittersweet or Jerusalem cherry.4 Of these, 295 patients were under age 10 years, and only nine experienced solanine-related symptoms; none required hospitalization.4 The symptoms of ingestion were primarily nausea and vomiting and abdominal cramping, possibly due to anticholinergic effects. Symptoms typically occur several hours after ingestion and may last for days.
Historically, induced emesis was recommended for ingestion in children, but this is no longer recommended. Prolonged observation may be necessary for children in the setting of high likelihood of ingestion. Management includes rehydration with intravenous (IV) fluids, antiemetics, and physostigmine if clinically warranted.4
Mistletoe
Mistletoe, a perennial with white or translucent berries, has traditionally been associated with kissing, fertility, and vitality. The American mistletoe is known as Phoradendron serotinum and the European mistletoe as Viscum album. Both the American and European mistletoe contain the toxalbumins phoratoxin and viscotoxin, which are associated with inhibiting cellular synthesis, thereby affecting cells with rapid turnover, including the GI mucosa.4
After several hours, clinical effects are primarily GI upset with potential sloughing of portions of the intestinal tract.4 Bradycardia, delirium, and hepatic, central nervous system, kidney, and adrenal gland toxicities can also occur.8 The American species has a lower toxicity compared to the European species. Cases involving death likely related to P serotinum usually occur due to excessive, concentrated herbal use, such as brewing mistletoe in tea.9 Placing the plant in hot water may result in larger amounts of ingested toxin. The only two reported deaths from ingestion of mistletoe were in patients who consumed brewed teas.4
A case review of 14 patients with American mistletoe leaf or berry ingestions failed to find any toxic symptoms.4 Krenzelok et al10 compiled the largest case review of 1,754 exposures from 1985 to 1992. In this review, patient outcomes were good. There were no fatalities, and 99% of patients experienced no morbidity. Outcomes were not influenced by GI decontamination.10
Another study by Spiller et al11 described 92 American mistletoe exposures involving ingestions of up to 20 berries and five leaves. In cases where five or more berries were consumed, none of the patients had symptoms.11 Three of the 11 patients (27%) who swallowed one to five leaves developed GI upset. One child had a seizure, likely not related to the mistletoe. The study concluded that severe toxic symptoms are uncommon.11
Management in the ED should involve supportive care for dehydration and vomiting, typically IV rehydration with normal saline or Ringer’s lactate and IV antiemetics. According to multiple case reviews, GI decontamination is not believed to alter patient outcome and is not recommended.4 An observation period of 6 hours is reasonable.4,10,11
Christmas Cactus
Christmas cactus is an old-time favorite. It is made of arching, drooping branches and spineless joints. Christmas cactus is essentially nontoxic, and patients and family can be reassured of its safety.
Holiday Decorations
Artificial Snow
Fake snow sprays, powders, and granules are popular decorative additions used in holiday games and celebrations. The “snow” typically consists of a polymer of sodium polyacrylate, both of which can cause injury to the eyes. Repeatedly inhaling the aerosol spray can cause breathing problems, especially in patients who have asthma or other underlying bronchospastic disease.
Devastating outcomes may occur from ocular alkaline injury. When mixed with water, the fake snow absorbs the water and expands as a gel material that may stick to the ocular surface, resulting in a change in pH and osmolarity.12 A case report by Al-Amry and Al-Ghadeer12 recently described a 7-year-old boy with corneal epitheliopathy due to a chemical burn injury following ocular contact with fake snow.The case was later managed with multiple debridements over 3 days, topical antibiotics, and bandage contact lenses. The child had complete resolution at 1 week follow-up.12
Some fake snow-product sprays contain acetone or methylene chloride, which is harmful when inhaled and can cause nausea, lightheadedness, and headache.13 Methylene chloride can be metabolized to carbon monoxide, but the quantity required for such an exposure is unknown and has not been reported in this context. Emergency physicians should consider ordering carboxyhemoglobin levels in symptomatic patients.
Tinsel
Tinsel, which gets its name from the Old French word “estincele,” translated as sparkle, used to be made of actual silver, and was affordable only for wealthy individuals. However, in the early 1900s, manufacturers began to make tinsel from metals such as aluminum and copper. These materials did not tarnish and could be reused annually. However, during World War I, copper became difficult to buy, while aluminum proved to be flammable and dangerous. Thus, manufacturers began to produce tinsel from lead. Tinsel was made with lead until the 1970s, when the US Food and Drug Administration realized the toxic risks of lead exposure, especially in young children. Today, tinsel is made of plastic; though a poor imitation of the previous tinsel, it is relatively harmless.14
Angel Hair
Angel hair is finely spun glass that can be irritating to the skin, eyes, and throat, especially if swallowed.13 The greatest danger is airway obstruction if a patient attempts to eat the angel hair and it becomes lodged in the oropharynx. For contact irritation, thoroughly washing and irrigating affected areas are recommended.
Snow Globes
Snow globes are popular holiday decorations that are available in a range of sizes. While the majority of globes made in the United States are filled with water, those manufactured overseas many contain a small amount of ethylene glycol (EG) (ie, antifreeze) to prevent freezing and breakage during shipping. Fortunately, the amount of EG is not usually sufficient to cause symptoms if ingested. For globes made in the United States, the water can be contaminated with bacteria, and drinking it can cause GI upset. The snow in these globes is typically made of inert material and does not cause toxicity. If a child does exhibit symptoms after ingesting any portion of a snow globe, parents are advised to call their local poison center.
Ethanol
While alcohol is not unique to the holiday season, its availability and use are more pronounced during this time of year, and the incidence of alcohol poisoning increases during the holiday season. Some traditional holiday drinks containing alcohol, such as egg nog, can entice young children. Children may often imitate adults and drink from partially filled leftover glasses.
Therefore, families with young children must ensure that all alcoholic beverages are placed out of children’s reach.
A common presentation of alcohol poisoning is seen in the child who is brought to the ED by parents concerned because their child is acting strangely. On examination, the child may appear dazed and have tachycardia, tachypnea, and hypotension, depending on the amount of alcohol ingested. Hypoglycemia in an alcohol-intoxicated pediatric patient is a concern, but it appears the effects of alcohol on glucose regulation in infants is unpredictable.15
Intravenous access should be obtained in any patient presenting with altered mental status, and rapid blood glucose level determined. Blood samples should be sent to assess ethanol concentration. Other laboratory and imaging studies should be obtained as clinically indicated, including electrolytes, serum osmolality, acetaminophen level, urine drug screen, X-ray, and computed tomography scan of the head. Treatment of respiratory depression, hypoglycemia, hypovolemia, and hypothermia are the key interventions to ensure good outcomes.16 Supportive care is the mainstay of therapy for pediatric patients, who rarely require thiamine supplementation.16 Medical evaluation is recommended for all symptomatic children; hourly observation for 6 hours is recommended for asymptomatic children.17
Alcohol is also associated with cardiac arrhythmias. Alcohol-induced atrial arrhythmias, most commonly atrial fibrillation (AF), are referred to as “holiday heart syndrome.” This should be considered early in the differential diagnosis of new-onset AF in young adults. Consuming massive quantities of alcohol or binge drinking can also result in metabolic and electrolyte alterations. Treatment includes rehydration with IV fluids, electrolyte replacement, and IV diltiazem or cardioversion for AF with rapid ventricular response.18
Conclusion
During the holiday season, it is easy to overlook the fact that some of the most unsuspecting items in the home can pose real hazards (Table). In addition, many holiday plants are used as table decorations, which can confuse small children, who may assume the colorful berries must be edible if they are on the dining room table.
It is vital that patients, parents, and physicians know what to do when someone ingests a potential toxin. Parents often try to induce vomiting, but ipecac and other forms of gastric emptying are no longer recommended.6 Instead, the recommended action is to separate the patient from the plant, remove plant material that may cause a sensitivity reaction, and consult a poison control center, which can save unnecessary interventions—including an ED visit.6
Fortunately, most holiday toxicities are relatively nonthreatening. Holiday-related toxic ingestions primarily occur in children, and most are asymptomatic, innocuous, and treated with symptomatic care as necessary. The most poisonous holiday-related toxins are bittersweet and Jerusalem cherry. Work-ups for holiday-plant ingestions are usually limited to severe gastroenteritis, which may require IV fluids and evaluation of electrolytes.
Holiday decorations, such as artificial snow and angel hair, present hazards that should be treated on a case-by-case basis. Finally, alcohol intoxication should be considered in the differential diagnosis for pediatric patients presenting with altered mental status, or the otherwise healthy binge drinker who presents with palpitations and new-onset AF.
The holiday season, a time of warmth, joy, and good cheer, is upon us. Yet with this most wonderful time of the year comes the possibility of poisoning and hazards in the home. As emergency physicians (EPs), we must ask ourselves: Which holiday items are potentially toxic to our patients? How do we evaluate and manage poisonings that result from exposure to these items? In this article, we review several plants and decorations that are unique to the holiday season. We discuss recommendations for evaluation and management of holiday poisonings that will avoid inappropriate work-ups and interventions while increasing recognition of truly dangerous ingestions, thus help keeping the season safe and merry.
Plants
Plant exposures are the fourth most common cause for calls to poison centers.1 In 2012, US Poison Control Centers reported more than 30,000 toxic plant exposures in children younger than age 5 years.2 Not surprisingly, toxic plant ingestions occur most commonly in early childhood. The highest rate of mortality, however, takes place during the teenaged years, when suicide attempts are common.2 The most common plant ingestions reported in the United States include peace lily, holly, philodendron, and poinsettia.3 These and the other frequently ingested potentially poisonous plants produce very little, if any, toxic effects. Approximately 95% of unintentional potentially toxic plant ingestions reported in the United States are managed safely at home.3
Poinsettia
The poinsettia is a large, prominent plant that was introduced to the United States in 1825 by Joel Poinsett, the US ambassador to Mexico. The poinsettia is one of the most commonly researched plants, and studies show the plant is not actually toxic.4,5 The myth of poinsettia toxicity is a widely held, yet false, belief. The legend involves a young child of an army officer stationed in Hawaii in 1919 who reportedly died after eating poinsettia leaves.4 However, the reality is that the poinsettia plant was not actually involved in the child’s death. In fact, the wild plant involved in this case probably had little resemblance to the popular plant cultivated domestically in North America today.6
A majority of poinsettia exposures will be asymptomatic, or involve simple nausea and vomiting. Krenzelok et al5 reviewed 22,793 cases of poinsettia exposures from 1985 to 1992. Almost all of these exposures (98.9%) were accidental poisonings.5 Not surprisingly, 93.3% of poinsettia exposures involved children; most importantly, 96.1% of these patients did not require treatment at a health care facility,and there were no fatalities.5 Another study could not identify an LD50 (lethal dose, 50%—ie, the amount of an ingested substance that kills 50% of the test sample) in rats.4
The majority of patients presenting to the ED with symptoms from poinsettia exposure will have gastrointestinal (GI) upset. Most patients require only symptomatic care. Those who do present to the ED do not require gastric emptying.
Interestingly, there is a crossreactivity of poinsettia sap in latex-allergic vulnerable patients.6 Poinsettia is part of the same plant family as natural rubber latex, and patients can present with symptoms of contact dermatitis, especially if they have a latex allergy.4 Washing the area thoroughly with soap and water and avoiding future contact is all that is required for most patients with contact dermatitis.
Holly
Holly exposure accounted for the third highest rate of genus-specific human plant exposure calls to poison centers in 2010.4 In the United States, there are two common forms of holly: English holly and American holly. The berries of both varieties contain saponin, a toxin that can cause erythrocyte hemolysis and changes in the permeability of small intestinal mucosal cells.4 Most holly berry ingestions cause minor or no symptoms. The prickly leaves of the holly plant are nontoxic but consumption may result in minor injury. When symptoms do occur, they can include nausea, vomiting, abdominal cramping, and possible dermatitis.5 Mydriasis, hyperthermia, and drowsiness are rare but possible symptoms.4
For symptoms to develop, children need to have eaten only five berries, while adults reportedly must consume at least 20 to 30 berries.4 A study by Wax et al7 done at the University of Rochester reviewed 103 cases of toxic berry ingestion in children aged 9 months to 5 years, with children who swallowed six or fewer berries of holly, yew, or nightshade. Investigators compared home observation alone versus ipecac administration with home observation. Every patient treated with ipecac had emesis at home with increased sedation and diarrhea, while there was no emesis in the group with home observation alone.7 These results suggest the symptoms were due to the ipecac rather than plant toxicity.7 Thus, ipecac is not recommended, and patients should be treated symptomatically.
Bittersweet and Jerusalem Cherry
Bittersweet, also known as the woody nightshade, and Jerusalem cherry, or Christmas orange, are the most dangerous of the holiday plants. While there is little evidence to support serious toxicity to adults, ingestion may be dangerous to children. Bittersweet has purple and yellow flowers, spreading petals, and red, ovoid berries.4 Both plants are part of the genus Solanum. In both plants, the immature fruit is more poisonous than ripened fruit due to the glycoalkaloid solanine via hypothetical alteration of mitochondrial potassium and calcium transport.4 Case reports document the rare anticholinergic effects of these plants, likely due to dulcamarine.4
The largest case series included 319 ingestions of bittersweet or Jerusalem cherry.4 Of these, 295 patients were under age 10 years, and only nine experienced solanine-related symptoms; none required hospitalization.4 The symptoms of ingestion were primarily nausea and vomiting and abdominal cramping, possibly due to anticholinergic effects. Symptoms typically occur several hours after ingestion and may last for days.
Historically, induced emesis was recommended for ingestion in children, but this is no longer recommended. Prolonged observation may be necessary for children in the setting of high likelihood of ingestion. Management includes rehydration with intravenous (IV) fluids, antiemetics, and physostigmine if clinically warranted.4
Mistletoe
Mistletoe, a perennial with white or translucent berries, has traditionally been associated with kissing, fertility, and vitality. The American mistletoe is known as Phoradendron serotinum and the European mistletoe as Viscum album. Both the American and European mistletoe contain the toxalbumins phoratoxin and viscotoxin, which are associated with inhibiting cellular synthesis, thereby affecting cells with rapid turnover, including the GI mucosa.4
After several hours, clinical effects are primarily GI upset with potential sloughing of portions of the intestinal tract.4 Bradycardia, delirium, and hepatic, central nervous system, kidney, and adrenal gland toxicities can also occur.8 The American species has a lower toxicity compared to the European species. Cases involving death likely related to P serotinum usually occur due to excessive, concentrated herbal use, such as brewing mistletoe in tea.9 Placing the plant in hot water may result in larger amounts of ingested toxin. The only two reported deaths from ingestion of mistletoe were in patients who consumed brewed teas.4
A case review of 14 patients with American mistletoe leaf or berry ingestions failed to find any toxic symptoms.4 Krenzelok et al10 compiled the largest case review of 1,754 exposures from 1985 to 1992. In this review, patient outcomes were good. There were no fatalities, and 99% of patients experienced no morbidity. Outcomes were not influenced by GI decontamination.10
Another study by Spiller et al11 described 92 American mistletoe exposures involving ingestions of up to 20 berries and five leaves. In cases where five or more berries were consumed, none of the patients had symptoms.11 Three of the 11 patients (27%) who swallowed one to five leaves developed GI upset. One child had a seizure, likely not related to the mistletoe. The study concluded that severe toxic symptoms are uncommon.11
Management in the ED should involve supportive care for dehydration and vomiting, typically IV rehydration with normal saline or Ringer’s lactate and IV antiemetics. According to multiple case reviews, GI decontamination is not believed to alter patient outcome and is not recommended.4 An observation period of 6 hours is reasonable.4,10,11
Christmas Cactus
Christmas cactus is an old-time favorite. It is made of arching, drooping branches and spineless joints. Christmas cactus is essentially nontoxic, and patients and family can be reassured of its safety.
Holiday Decorations
Artificial Snow
Fake snow sprays, powders, and granules are popular decorative additions used in holiday games and celebrations. The “snow” typically consists of a polymer of sodium polyacrylate, both of which can cause injury to the eyes. Repeatedly inhaling the aerosol spray can cause breathing problems, especially in patients who have asthma or other underlying bronchospastic disease.
Devastating outcomes may occur from ocular alkaline injury. When mixed with water, the fake snow absorbs the water and expands as a gel material that may stick to the ocular surface, resulting in a change in pH and osmolarity.12 A case report by Al-Amry and Al-Ghadeer12 recently described a 7-year-old boy with corneal epitheliopathy due to a chemical burn injury following ocular contact with fake snow.The case was later managed with multiple debridements over 3 days, topical antibiotics, and bandage contact lenses. The child had complete resolution at 1 week follow-up.12
Some fake snow-product sprays contain acetone or methylene chloride, which is harmful when inhaled and can cause nausea, lightheadedness, and headache.13 Methylene chloride can be metabolized to carbon monoxide, but the quantity required for such an exposure is unknown and has not been reported in this context. Emergency physicians should consider ordering carboxyhemoglobin levels in symptomatic patients.
Tinsel
Tinsel, which gets its name from the Old French word “estincele,” translated as sparkle, used to be made of actual silver, and was affordable only for wealthy individuals. However, in the early 1900s, manufacturers began to make tinsel from metals such as aluminum and copper. These materials did not tarnish and could be reused annually. However, during World War I, copper became difficult to buy, while aluminum proved to be flammable and dangerous. Thus, manufacturers began to produce tinsel from lead. Tinsel was made with lead until the 1970s, when the US Food and Drug Administration realized the toxic risks of lead exposure, especially in young children. Today, tinsel is made of plastic; though a poor imitation of the previous tinsel, it is relatively harmless.14
Angel Hair
Angel hair is finely spun glass that can be irritating to the skin, eyes, and throat, especially if swallowed.13 The greatest danger is airway obstruction if a patient attempts to eat the angel hair and it becomes lodged in the oropharynx. For contact irritation, thoroughly washing and irrigating affected areas are recommended.
Snow Globes
Snow globes are popular holiday decorations that are available in a range of sizes. While the majority of globes made in the United States are filled with water, those manufactured overseas many contain a small amount of ethylene glycol (EG) (ie, antifreeze) to prevent freezing and breakage during shipping. Fortunately, the amount of EG is not usually sufficient to cause symptoms if ingested. For globes made in the United States, the water can be contaminated with bacteria, and drinking it can cause GI upset. The snow in these globes is typically made of inert material and does not cause toxicity. If a child does exhibit symptoms after ingesting any portion of a snow globe, parents are advised to call their local poison center.
Ethanol
While alcohol is not unique to the holiday season, its availability and use are more pronounced during this time of year, and the incidence of alcohol poisoning increases during the holiday season. Some traditional holiday drinks containing alcohol, such as egg nog, can entice young children. Children may often imitate adults and drink from partially filled leftover glasses.
Therefore, families with young children must ensure that all alcoholic beverages are placed out of children’s reach.
A common presentation of alcohol poisoning is seen in the child who is brought to the ED by parents concerned because their child is acting strangely. On examination, the child may appear dazed and have tachycardia, tachypnea, and hypotension, depending on the amount of alcohol ingested. Hypoglycemia in an alcohol-intoxicated pediatric patient is a concern, but it appears the effects of alcohol on glucose regulation in infants is unpredictable.15
Intravenous access should be obtained in any patient presenting with altered mental status, and rapid blood glucose level determined. Blood samples should be sent to assess ethanol concentration. Other laboratory and imaging studies should be obtained as clinically indicated, including electrolytes, serum osmolality, acetaminophen level, urine drug screen, X-ray, and computed tomography scan of the head. Treatment of respiratory depression, hypoglycemia, hypovolemia, and hypothermia are the key interventions to ensure good outcomes.16 Supportive care is the mainstay of therapy for pediatric patients, who rarely require thiamine supplementation.16 Medical evaluation is recommended for all symptomatic children; hourly observation for 6 hours is recommended for asymptomatic children.17
Alcohol is also associated with cardiac arrhythmias. Alcohol-induced atrial arrhythmias, most commonly atrial fibrillation (AF), are referred to as “holiday heart syndrome.” This should be considered early in the differential diagnosis of new-onset AF in young adults. Consuming massive quantities of alcohol or binge drinking can also result in metabolic and electrolyte alterations. Treatment includes rehydration with IV fluids, electrolyte replacement, and IV diltiazem or cardioversion for AF with rapid ventricular response.18
Conclusion
During the holiday season, it is easy to overlook the fact that some of the most unsuspecting items in the home can pose real hazards (Table). In addition, many holiday plants are used as table decorations, which can confuse small children, who may assume the colorful berries must be edible if they are on the dining room table.
It is vital that patients, parents, and physicians know what to do when someone ingests a potential toxin. Parents often try to induce vomiting, but ipecac and other forms of gastric emptying are no longer recommended.6 Instead, the recommended action is to separate the patient from the plant, remove plant material that may cause a sensitivity reaction, and consult a poison control center, which can save unnecessary interventions—including an ED visit.6
Fortunately, most holiday toxicities are relatively nonthreatening. Holiday-related toxic ingestions primarily occur in children, and most are asymptomatic, innocuous, and treated with symptomatic care as necessary. The most poisonous holiday-related toxins are bittersweet and Jerusalem cherry. Work-ups for holiday-plant ingestions are usually limited to severe gastroenteritis, which may require IV fluids and evaluation of electrolytes.
Holiday decorations, such as artificial snow and angel hair, present hazards that should be treated on a case-by-case basis. Finally, alcohol intoxication should be considered in the differential diagnosis for pediatric patients presenting with altered mental status, or the otherwise healthy binge drinker who presents with palpitations and new-onset AF.
1. Krenzelok EP, Jacobsen TD, Aronis J. Those pesky berries...are they a source of concern? Vet Hum Toxicol. 1998;40(2):101-103.
2. Martínez Monseny A, Martínez Sánchez L, Margarit Soler A, Trenchs Sainz de la Maza V, Luaces Cubells C. [Poisonous plants: An ongoing problem]. An Pediatr (Barc). 2015;82(5):347-353. doi:10.1016/j.anpedi.2014.08.008.
3. Krenzelok EP, Mrvos R. Friends and foes in the plant world: a profile of plant ingestions and fatalities. Clin Toxicol (Phila). 2011;49(3):142-149. doi:10.3109/15563650.2011.568945.
4. Evens ZN, Stellpflug SJ. Holiday plants with toxic misconceptions. West J Emerg Med. 2012;13(6):538-542. doi:10.5811/westjem.2012.8.12572.
5. Krenzelok E, Jacobsen TD, Aronis JM. Poinsettia exposures have good outcomes…just as we thought. Am J Emerg Med. 1996;14(7):671-674. doi:10.1016/S0735-6757(96)90086-90088.
6. Courtemanche J, Peterson, RG. Beware the mistletoe. CMAJ. 2006;175(12):1523-1524. doi:10.1503/cmaj.061432.
7. Wax PM, Cobaugh DJ, Lawrence RA. Should home ipecac-induced emesis be routinely recommended in the management of toxic berry ingestions? Vet Hum Toxicol. 1999;41(6):394-397.
8. Palmer ME, Betz JM. Plants. In: Nelson LS, Lewin NA, Howland MA, Hoffman RS, Goldfrank LR, Flomenbaum NE, eds. Goldfrank’s Toxicologic Emergencies. 9th ed. New York, NY: McGraw Hill; 2011:1537-1560.
9. Bruneton J. Toxic plants dangerous to humans and animals. Paris, France: Lavoisier Publishing; 1999.10. Krenzelok EP, Jacobsen TD, Aronis J. American mistletoe exposures. Am J Emerg Med. 1997;15(5):516-520.
11. Spiller HA, Willias DB, Gorman SE, Sanftleban J. Retrospective study of mistletoe ingestion. J Toxicol Clin Toxicol. 1996;34(4):405-408.
12. Al-Amry MA, Al-Ghadeer HA. Corneal epithliopathy after trauma by fake snow powder in a 7-year-old child. Middle East Afr J Ophthalmol. 2016;23(3):274-276. doi:10.4103/0974-9233.186157.
13. California Poison Control Center. Winter holiday safety & poison prevention tips. http://www.calpoison.org/public/winter-holidays.html. Accessed October 16, 2016.
14. Romm C. Don’t lick the tinsel. The Atlantic. December 21, 2015. http://www.theatlantic.com/health/archive/2015/12/dont-lick-the-tinsel/421506/. Accessed October 16, 2016.
15. Minera G, Robinson E. Accidental acute alcohol intoxication in infants: review and case report. J Emerg Med. 2014;47(5):524-526.
16. Baum CR. Ethanol intoxication in children: clinical features, evaluation, and management. UpToDate. http://www.uptodate.com/contents/ethanol-intoxication-in-children-clinical-features-evaluation-and-management. Accessed October 16, 2016.
17. Vogel C, Caraccio T, Mofenson H, Hart S. Alcohol intoxication in young children. J Toxicol Clin Toxicol. 1995;33(1):25-33.
18. Carey MG, Al-Zaiti SS, Kozik TM, Pelter M. Holiday heart syndrome. Am J Crit Care. 2014;23(2):171-172.
1. Krenzelok EP, Jacobsen TD, Aronis J. Those pesky berries...are they a source of concern? Vet Hum Toxicol. 1998;40(2):101-103.
2. Martínez Monseny A, Martínez Sánchez L, Margarit Soler A, Trenchs Sainz de la Maza V, Luaces Cubells C. [Poisonous plants: An ongoing problem]. An Pediatr (Barc). 2015;82(5):347-353. doi:10.1016/j.anpedi.2014.08.008.
3. Krenzelok EP, Mrvos R. Friends and foes in the plant world: a profile of plant ingestions and fatalities. Clin Toxicol (Phila). 2011;49(3):142-149. doi:10.3109/15563650.2011.568945.
4. Evens ZN, Stellpflug SJ. Holiday plants with toxic misconceptions. West J Emerg Med. 2012;13(6):538-542. doi:10.5811/westjem.2012.8.12572.
5. Krenzelok E, Jacobsen TD, Aronis JM. Poinsettia exposures have good outcomes…just as we thought. Am J Emerg Med. 1996;14(7):671-674. doi:10.1016/S0735-6757(96)90086-90088.
6. Courtemanche J, Peterson, RG. Beware the mistletoe. CMAJ. 2006;175(12):1523-1524. doi:10.1503/cmaj.061432.
7. Wax PM, Cobaugh DJ, Lawrence RA. Should home ipecac-induced emesis be routinely recommended in the management of toxic berry ingestions? Vet Hum Toxicol. 1999;41(6):394-397.
8. Palmer ME, Betz JM. Plants. In: Nelson LS, Lewin NA, Howland MA, Hoffman RS, Goldfrank LR, Flomenbaum NE, eds. Goldfrank’s Toxicologic Emergencies. 9th ed. New York, NY: McGraw Hill; 2011:1537-1560.
9. Bruneton J. Toxic plants dangerous to humans and animals. Paris, France: Lavoisier Publishing; 1999.10. Krenzelok EP, Jacobsen TD, Aronis J. American mistletoe exposures. Am J Emerg Med. 1997;15(5):516-520.
11. Spiller HA, Willias DB, Gorman SE, Sanftleban J. Retrospective study of mistletoe ingestion. J Toxicol Clin Toxicol. 1996;34(4):405-408.
12. Al-Amry MA, Al-Ghadeer HA. Corneal epithliopathy after trauma by fake snow powder in a 7-year-old child. Middle East Afr J Ophthalmol. 2016;23(3):274-276. doi:10.4103/0974-9233.186157.
13. California Poison Control Center. Winter holiday safety & poison prevention tips. http://www.calpoison.org/public/winter-holidays.html. Accessed October 16, 2016.
14. Romm C. Don’t lick the tinsel. The Atlantic. December 21, 2015. http://www.theatlantic.com/health/archive/2015/12/dont-lick-the-tinsel/421506/. Accessed October 16, 2016.
15. Minera G, Robinson E. Accidental acute alcohol intoxication in infants: review and case report. J Emerg Med. 2014;47(5):524-526.
16. Baum CR. Ethanol intoxication in children: clinical features, evaluation, and management. UpToDate. http://www.uptodate.com/contents/ethanol-intoxication-in-children-clinical-features-evaluation-and-management. Accessed October 16, 2016.
17. Vogel C, Caraccio T, Mofenson H, Hart S. Alcohol intoxication in young children. J Toxicol Clin Toxicol. 1995;33(1):25-33.
18. Carey MG, Al-Zaiti SS, Kozik TM, Pelter M. Holiday heart syndrome. Am J Crit Care. 2014;23(2):171-172.
Dung Lung: Reactive Airway Disease Syndrome From Yak-Dung Biomass Fuel Smoke
Case
A 30-year-old man without prior respiratory illness presented with coughing, wheezing, dyspnea on exertion, and decreased exercise tolerance after a 7-hour overnight exposure to yak-dung smoke. This episode took place at 4,240 m elevation in Pheriche village, along the Everest Base Camp trekking route within the Khumbu region of the Nepali Himalayas. Prior to going to bed that evening, the group of five cohabitants had a difficult time igniting the potbelly heating stove filled with yak-dung biomass fuel in the common room. Each time they tried to light it, the fire would smolder and go out within a few minutes, despite the group’s attempts at adjusting the flue and air intake. Eventually, they abandoned further attempts and retired to bed at approximately 9:30
The differential diagnosis included altitude illness, airway mucociliary dysfunction (commonly known as Khumbu cough),1 carbon monoxide (CO) poisoning, acute inhalation injury (AII) resulting in reactive airway disease syndrome (RADS), and high-altitude pulmonary edema (HAPE). Although the group hiked to Kongma La Pass (elevation, 5,545 m), and slept at 5,200 m (960 m higher than their starting point), the patient had not exhibited any symptoms of altitude illness (eg, headache, dizziness, fatigue, sleep disturbances, anorexia, nausea). Auscultation of the patient by the two physicians who accompanied him on the hike noted mild expiratory wheezing without rales or rhonchi, making HAPE unlikely in the differential diagnosis.
Although it is likely the patient had significant CO exposure, he did not display profound symptoms of CO toxicity (eg, light-headedness, headache, vertigo, nausea, or confusion). It is unclear whether the symptoms of decreased exercise tolerance and fatigue were due to CO poisoning as no co-oximeter was available to assess the patient’s CO levels.2,3 Upon return from the trip, pulse oximetry showed the patient to have an oxygen (O2) saturation of 89% on room air, which was within appropriate range for their altitude.
One of the physicians offered the patient an albuterol metered-dose inhaler, which provided profound and immediate relief of his coughing and wheezing. The patient continued to use the albuterol inhaler every 2 to 4 hours over the next 2 days. The dyspnea on exertion and decreased exercise tolerance improved after 24 hours of treatment; the rest of his respiratory symptoms resolved after approximately 5 days at the starting elevation, and he returned to his usual baseline state of health. No follow-up chest X-rays were obtained, and the patient has had no subsequent recurrence of these symptoms despite return to higher altitude in the subsequent year.
Discussion
Nearly one-third to one-half of the world’s population relies on biomass fuels for domestic heating or cooking, with developing countries accounting for 99% of its use.4 These fuels consist of dried dung cakes or patties, agricultural products, coal, and firewood. In the Khumbu region of Nepal above timberline, yak-dung patties are used exclusively for heating and frequently for cooking. Most guesthouses in this region have potbelly-style stoves in the common dining areas, which are fueled by yak dung and ventilated with a chimney.
Pulmonary Pathophysiology of Inhaled Irritants
Biomass fuels are responsible for numerous air pollutants due to incomplete combustion. These fuels suspend particulate matter, CO, nitrogen dioxide, polycyclic aromatic hydrocarbons, and volatile organic compounds, including acetone, methyl ethyl ketone, benzene, formaldehyde, and toluene.5 Compared to other biomass fuel sources, dung-cake combustion results in higher emissions of relatively very small particulate matter with peak concentrations ranging from 0.23 to 0.3 μm in size, which penetrate and affect the distal airway. 6 Their combustion also releases volatile organic compounds and CO.7,8 Aside from indoor air pollution, yak-dung combustion in the Nepali Himalayan valley contributes significantly to the ambient airborne concentrations of lead, copper, aluminum, magnesium, and elemental and organic carbon.9
Emergency physicians (EPs) are often the first-line treating physician for patients exposed not only to biomass fuels, but also home, forest, or occupational fires resulting in smoke inhalation or AII.10 These terms refer to the wide number of substances that may be present in the smoke and collectively affect the patient. Inhaled substances classified as irritants, such as smoke and particulate matter, can harm the epithelium of the respiratory tract, with highly water-soluble or larger particles (>10 μm) mostly affecting the upper airways. These irritants cause symptoms of progressive coughing, and wheezing; or stridor resulting in tracheitis, bronchitis, bronchiolitis, alveolitis, pulmonary edema, and/or airway obstruction. Smaller particles (<2.5 μm) can penetrate further into the lung and affect the distal airway to a greater degree. These particles are able to infiltrate the terminal bronchioles and alveoli, leading to localized inflammatory reaction and bronchospasm.11Smoke may also contain chemical asphyxiants such as CO or hydrogen cyanide, which can be absorbed, leading to systemic toxicity and interfering with O2 delivery or utilization. Importantly, high concentrations of any gas can act as an asphyxiant due to displacement of O2.12 Thermal injuries are also possible from fire and smoke exposure, typically affecting the upper airways. Steam inhalation can even cause irritation and burns below the vocal cords.13
Reactive Airway Disease Syndrome
Reactive airway disease syndrome is a constellation of symptoms presenting similar to asthma with persistent airway reactivity after an AII, and is the most common sequelae of exposure to biomass fuel combustion. This syndrome is not specifically caused by one type of particulate, irritant, or chemical component of the smoke.
Symptoms
Symptoms such as cough, dyspnea, and wheezing may begin minutes after exposure, and can persist for years due to bronchial hyperresponsiveness.14 These chronic symptoms of RADS have been well highlighted by New York Fire Department rescue workers from the World Trade Center collapse, of whom 16% continued to show symptoms of RADS 1 year later.15
Treatment
Bronchodilator therapy is the mainstay of treatment for RADS. Patients who have RADS often respond well to treatment, and show improvement in symptoms and spirometry testing.
Sequelae Associated With Biomass Fuel Exposure
A cross-sectional study showed significant reductions (P < .001) in all pulmonary function testing parameters for cow-dung fuel users compared to those who use modern energy sources: forced expiratory volume in the first second (FEV1), forced vital capacity (FVC), FEV1/FVC ratio, and mid-flow rate between the first 25% and 75% of forced expiratory flow. Linear regression showed a 12.4% reduction in FVC of cow-dung users, and 36% (compared to 20% in modern energy-source users) were noted to have pulmonary infections.16
Due to these emissions, biomass fuel exposure causes high levels of morbidity and mortality in developing countries, with nearly 2 million attributable deaths annually.1 Chronic exposure to biomass fuel emissions can lead to increased risk of diseases, including respiratory problems (eg, pneumonia, tuberculosis and chronic obstructive pulmonary disease, lung cancer, asthma), low birthweight, cataracts, and cardiovascular events.2,17 Women are at higher risk compared to other family members, as they typically spend approximately 3 to 4 hours longer daily in tents,5 and perform the majoring of the cooking duties. For pregnant women, the developing fetus may also be exposed, which can lead to increased rates of fetal demise.18
Conclusion
Our report represents the first reported case of “dung lung” or RADS from yak-dung biomass fuel combustion exposure. In the medical literature, there has been one previous case report of dung lung by Osbern and Crapo19 in 1981 in which the authors described three patients who died from aspiration of liquid manure in a storage facility.Our case highlights the prevalence of biomass fuel combustion in the third world, the dangerous air pollutants from their emissions, and the morbidity associated with improper ventilation of biomass fuel combustion.
1. Rodway GW, Windsor JS. Airway mucociliary function at high altitude. Wilderness Environ Med. 2006;17(4):271-275.
2. Leigh-Smith S. Carbon monoxide poisoning in tents—a review. Wilderness Environ Med. 2004;15(3):157-163.
3. Lipman GL. Carbon monoxide toxicity at high altitude [Commentary]. Wilderness Environ Med. 2006;17(2):144-145.
4. Prasad R, Singh A, Garg R, Giridhar GB. Biomass fuel exposure and respiratory diseases in India. Biosci Trends. 2012;6(5):219-228.
5. Kim KH, Jahan SA, Kabir E. A review of diseases associated with household air pollution due to the use of biomass fuels. J Hazard Mater. 2011;192(2):425-431.
6. Park D, Barabad ML, Lee G, et al. Emission characteristics of particulate matter and volatile organic compounds in cow dung combustion. Environ Sci Technol. 2013;47(22):12952-12957.
7. Venkataraman C, Rao GU. Emission factors of carbon monoxide and size-resolved aerosols from biofuel combustion. Environ Sci Technol. 2001;35(10):2100-2107.
8. Chen PF, Li CL, Kang SC, et al. [Indoor air pollution in the Nam Co and Ando Regions in the Tibetan Plateau]. [Article in Chinese]. Huan Jing Ke Xue. 2011;32(5):1231-1236.
9. avidson CI, Grimm TC, Nasta MA. Airborne lead and other elements derived from local fires in the himalayas. Science. 1981;214(4527):1344-1366.
10. Gorguner M, Akgun M. Acute inhalation injury. Eurasian J Med. 2010;42(1):28-35.
11. Ainslie G. Inhalational injuries produced by smoke and nitrogen dioxide. Respir Med. 1993;87(3):169-174.
12. Glazer CS. Acute inhalational injury. In: Hanley ME, Welsh CH, eds. Current Diagnosis & Treatment in Pulmonary Medicine. International Ed. New York, NY: McGraw Hill; 2003:354-360.
13. Gu TL, Liou SH, Hsu CH, Hsu JC, Wu TN. Acute health hazards of firefighters after fighting a department store fire. Indust Health. 1996;34(1):13-23.
14. Alberts WM, do Picco GA. Reactive airways dysfunction syndrome. Chest. 1996;109(6):1618-1626.
15. Banauch GI, Dhala A, Alleyne D, et al. Bronchial hyperreactivity and other inhalation lung injuries in rescue/recovery workers after the World Trade Center collapse. Crit Care Med. 2005;33(1 Suppl):S102-S106.
16. Sümer H, Turaçlar UT, Onarlioğlu T, Ozdemir L, Zwahlen M. The association of biomass fuel combustion on pulmonary function tests in the adult population of Mid-Anatolia. Soz Praventivmed. 2004;49(4):247-253.
17. Cesaroni G, Forastiere F, Stafoggia M, et al. Long term exposure to ambient air pollution and incidence of acute coronary events: prospective cohort study and meta-analysis in 11 European cohorts from the ESCAPE Project. BMJ. 2014;348:f7412.
18. de Koning HW, Smith KR, Last JM. Biomass fuel combustion and health. Bull World Health Organ. 1985;63(1):11-26.
19. Osbern LN, Crapo RO. Dung lung: a report of toxic exposure to liquid manure. Ann Intern Med. 1981;95(3):312-314.
Case
A 30-year-old man without prior respiratory illness presented with coughing, wheezing, dyspnea on exertion, and decreased exercise tolerance after a 7-hour overnight exposure to yak-dung smoke. This episode took place at 4,240 m elevation in Pheriche village, along the Everest Base Camp trekking route within the Khumbu region of the Nepali Himalayas. Prior to going to bed that evening, the group of five cohabitants had a difficult time igniting the potbelly heating stove filled with yak-dung biomass fuel in the common room. Each time they tried to light it, the fire would smolder and go out within a few minutes, despite the group’s attempts at adjusting the flue and air intake. Eventually, they abandoned further attempts and retired to bed at approximately 9:30
The differential diagnosis included altitude illness, airway mucociliary dysfunction (commonly known as Khumbu cough),1 carbon monoxide (CO) poisoning, acute inhalation injury (AII) resulting in reactive airway disease syndrome (RADS), and high-altitude pulmonary edema (HAPE). Although the group hiked to Kongma La Pass (elevation, 5,545 m), and slept at 5,200 m (960 m higher than their starting point), the patient had not exhibited any symptoms of altitude illness (eg, headache, dizziness, fatigue, sleep disturbances, anorexia, nausea). Auscultation of the patient by the two physicians who accompanied him on the hike noted mild expiratory wheezing without rales or rhonchi, making HAPE unlikely in the differential diagnosis.
Although it is likely the patient had significant CO exposure, he did not display profound symptoms of CO toxicity (eg, light-headedness, headache, vertigo, nausea, or confusion). It is unclear whether the symptoms of decreased exercise tolerance and fatigue were due to CO poisoning as no co-oximeter was available to assess the patient’s CO levels.2,3 Upon return from the trip, pulse oximetry showed the patient to have an oxygen (O2) saturation of 89% on room air, which was within appropriate range for their altitude.
One of the physicians offered the patient an albuterol metered-dose inhaler, which provided profound and immediate relief of his coughing and wheezing. The patient continued to use the albuterol inhaler every 2 to 4 hours over the next 2 days. The dyspnea on exertion and decreased exercise tolerance improved after 24 hours of treatment; the rest of his respiratory symptoms resolved after approximately 5 days at the starting elevation, and he returned to his usual baseline state of health. No follow-up chest X-rays were obtained, and the patient has had no subsequent recurrence of these symptoms despite return to higher altitude in the subsequent year.
Discussion
Nearly one-third to one-half of the world’s population relies on biomass fuels for domestic heating or cooking, with developing countries accounting for 99% of its use.4 These fuels consist of dried dung cakes or patties, agricultural products, coal, and firewood. In the Khumbu region of Nepal above timberline, yak-dung patties are used exclusively for heating and frequently for cooking. Most guesthouses in this region have potbelly-style stoves in the common dining areas, which are fueled by yak dung and ventilated with a chimney.
Pulmonary Pathophysiology of Inhaled Irritants
Biomass fuels are responsible for numerous air pollutants due to incomplete combustion. These fuels suspend particulate matter, CO, nitrogen dioxide, polycyclic aromatic hydrocarbons, and volatile organic compounds, including acetone, methyl ethyl ketone, benzene, formaldehyde, and toluene.5 Compared to other biomass fuel sources, dung-cake combustion results in higher emissions of relatively very small particulate matter with peak concentrations ranging from 0.23 to 0.3 μm in size, which penetrate and affect the distal airway. 6 Their combustion also releases volatile organic compounds and CO.7,8 Aside from indoor air pollution, yak-dung combustion in the Nepali Himalayan valley contributes significantly to the ambient airborne concentrations of lead, copper, aluminum, magnesium, and elemental and organic carbon.9
Emergency physicians (EPs) are often the first-line treating physician for patients exposed not only to biomass fuels, but also home, forest, or occupational fires resulting in smoke inhalation or AII.10 These terms refer to the wide number of substances that may be present in the smoke and collectively affect the patient. Inhaled substances classified as irritants, such as smoke and particulate matter, can harm the epithelium of the respiratory tract, with highly water-soluble or larger particles (>10 μm) mostly affecting the upper airways. These irritants cause symptoms of progressive coughing, and wheezing; or stridor resulting in tracheitis, bronchitis, bronchiolitis, alveolitis, pulmonary edema, and/or airway obstruction. Smaller particles (<2.5 μm) can penetrate further into the lung and affect the distal airway to a greater degree. These particles are able to infiltrate the terminal bronchioles and alveoli, leading to localized inflammatory reaction and bronchospasm.11Smoke may also contain chemical asphyxiants such as CO or hydrogen cyanide, which can be absorbed, leading to systemic toxicity and interfering with O2 delivery or utilization. Importantly, high concentrations of any gas can act as an asphyxiant due to displacement of O2.12 Thermal injuries are also possible from fire and smoke exposure, typically affecting the upper airways. Steam inhalation can even cause irritation and burns below the vocal cords.13
Reactive Airway Disease Syndrome
Reactive airway disease syndrome is a constellation of symptoms presenting similar to asthma with persistent airway reactivity after an AII, and is the most common sequelae of exposure to biomass fuel combustion. This syndrome is not specifically caused by one type of particulate, irritant, or chemical component of the smoke.
Symptoms
Symptoms such as cough, dyspnea, and wheezing may begin minutes after exposure, and can persist for years due to bronchial hyperresponsiveness.14 These chronic symptoms of RADS have been well highlighted by New York Fire Department rescue workers from the World Trade Center collapse, of whom 16% continued to show symptoms of RADS 1 year later.15
Treatment
Bronchodilator therapy is the mainstay of treatment for RADS. Patients who have RADS often respond well to treatment, and show improvement in symptoms and spirometry testing.
Sequelae Associated With Biomass Fuel Exposure
A cross-sectional study showed significant reductions (P < .001) in all pulmonary function testing parameters for cow-dung fuel users compared to those who use modern energy sources: forced expiratory volume in the first second (FEV1), forced vital capacity (FVC), FEV1/FVC ratio, and mid-flow rate between the first 25% and 75% of forced expiratory flow. Linear regression showed a 12.4% reduction in FVC of cow-dung users, and 36% (compared to 20% in modern energy-source users) were noted to have pulmonary infections.16
Due to these emissions, biomass fuel exposure causes high levels of morbidity and mortality in developing countries, with nearly 2 million attributable deaths annually.1 Chronic exposure to biomass fuel emissions can lead to increased risk of diseases, including respiratory problems (eg, pneumonia, tuberculosis and chronic obstructive pulmonary disease, lung cancer, asthma), low birthweight, cataracts, and cardiovascular events.2,17 Women are at higher risk compared to other family members, as they typically spend approximately 3 to 4 hours longer daily in tents,5 and perform the majoring of the cooking duties. For pregnant women, the developing fetus may also be exposed, which can lead to increased rates of fetal demise.18
Conclusion
Our report represents the first reported case of “dung lung” or RADS from yak-dung biomass fuel combustion exposure. In the medical literature, there has been one previous case report of dung lung by Osbern and Crapo19 in 1981 in which the authors described three patients who died from aspiration of liquid manure in a storage facility.Our case highlights the prevalence of biomass fuel combustion in the third world, the dangerous air pollutants from their emissions, and the morbidity associated with improper ventilation of biomass fuel combustion.
Case
A 30-year-old man without prior respiratory illness presented with coughing, wheezing, dyspnea on exertion, and decreased exercise tolerance after a 7-hour overnight exposure to yak-dung smoke. This episode took place at 4,240 m elevation in Pheriche village, along the Everest Base Camp trekking route within the Khumbu region of the Nepali Himalayas. Prior to going to bed that evening, the group of five cohabitants had a difficult time igniting the potbelly heating stove filled with yak-dung biomass fuel in the common room. Each time they tried to light it, the fire would smolder and go out within a few minutes, despite the group’s attempts at adjusting the flue and air intake. Eventually, they abandoned further attempts and retired to bed at approximately 9:30
The differential diagnosis included altitude illness, airway mucociliary dysfunction (commonly known as Khumbu cough),1 carbon monoxide (CO) poisoning, acute inhalation injury (AII) resulting in reactive airway disease syndrome (RADS), and high-altitude pulmonary edema (HAPE). Although the group hiked to Kongma La Pass (elevation, 5,545 m), and slept at 5,200 m (960 m higher than their starting point), the patient had not exhibited any symptoms of altitude illness (eg, headache, dizziness, fatigue, sleep disturbances, anorexia, nausea). Auscultation of the patient by the two physicians who accompanied him on the hike noted mild expiratory wheezing without rales or rhonchi, making HAPE unlikely in the differential diagnosis.
Although it is likely the patient had significant CO exposure, he did not display profound symptoms of CO toxicity (eg, light-headedness, headache, vertigo, nausea, or confusion). It is unclear whether the symptoms of decreased exercise tolerance and fatigue were due to CO poisoning as no co-oximeter was available to assess the patient’s CO levels.2,3 Upon return from the trip, pulse oximetry showed the patient to have an oxygen (O2) saturation of 89% on room air, which was within appropriate range for their altitude.
One of the physicians offered the patient an albuterol metered-dose inhaler, which provided profound and immediate relief of his coughing and wheezing. The patient continued to use the albuterol inhaler every 2 to 4 hours over the next 2 days. The dyspnea on exertion and decreased exercise tolerance improved after 24 hours of treatment; the rest of his respiratory symptoms resolved after approximately 5 days at the starting elevation, and he returned to his usual baseline state of health. No follow-up chest X-rays were obtained, and the patient has had no subsequent recurrence of these symptoms despite return to higher altitude in the subsequent year.
Discussion
Nearly one-third to one-half of the world’s population relies on biomass fuels for domestic heating or cooking, with developing countries accounting for 99% of its use.4 These fuels consist of dried dung cakes or patties, agricultural products, coal, and firewood. In the Khumbu region of Nepal above timberline, yak-dung patties are used exclusively for heating and frequently for cooking. Most guesthouses in this region have potbelly-style stoves in the common dining areas, which are fueled by yak dung and ventilated with a chimney.
Pulmonary Pathophysiology of Inhaled Irritants
Biomass fuels are responsible for numerous air pollutants due to incomplete combustion. These fuels suspend particulate matter, CO, nitrogen dioxide, polycyclic aromatic hydrocarbons, and volatile organic compounds, including acetone, methyl ethyl ketone, benzene, formaldehyde, and toluene.5 Compared to other biomass fuel sources, dung-cake combustion results in higher emissions of relatively very small particulate matter with peak concentrations ranging from 0.23 to 0.3 μm in size, which penetrate and affect the distal airway. 6 Their combustion also releases volatile organic compounds and CO.7,8 Aside from indoor air pollution, yak-dung combustion in the Nepali Himalayan valley contributes significantly to the ambient airborne concentrations of lead, copper, aluminum, magnesium, and elemental and organic carbon.9
Emergency physicians (EPs) are often the first-line treating physician for patients exposed not only to biomass fuels, but also home, forest, or occupational fires resulting in smoke inhalation or AII.10 These terms refer to the wide number of substances that may be present in the smoke and collectively affect the patient. Inhaled substances classified as irritants, such as smoke and particulate matter, can harm the epithelium of the respiratory tract, with highly water-soluble or larger particles (>10 μm) mostly affecting the upper airways. These irritants cause symptoms of progressive coughing, and wheezing; or stridor resulting in tracheitis, bronchitis, bronchiolitis, alveolitis, pulmonary edema, and/or airway obstruction. Smaller particles (<2.5 μm) can penetrate further into the lung and affect the distal airway to a greater degree. These particles are able to infiltrate the terminal bronchioles and alveoli, leading to localized inflammatory reaction and bronchospasm.11Smoke may also contain chemical asphyxiants such as CO or hydrogen cyanide, which can be absorbed, leading to systemic toxicity and interfering with O2 delivery or utilization. Importantly, high concentrations of any gas can act as an asphyxiant due to displacement of O2.12 Thermal injuries are also possible from fire and smoke exposure, typically affecting the upper airways. Steam inhalation can even cause irritation and burns below the vocal cords.13
Reactive Airway Disease Syndrome
Reactive airway disease syndrome is a constellation of symptoms presenting similar to asthma with persistent airway reactivity after an AII, and is the most common sequelae of exposure to biomass fuel combustion. This syndrome is not specifically caused by one type of particulate, irritant, or chemical component of the smoke.
Symptoms
Symptoms such as cough, dyspnea, and wheezing may begin minutes after exposure, and can persist for years due to bronchial hyperresponsiveness.14 These chronic symptoms of RADS have been well highlighted by New York Fire Department rescue workers from the World Trade Center collapse, of whom 16% continued to show symptoms of RADS 1 year later.15
Treatment
Bronchodilator therapy is the mainstay of treatment for RADS. Patients who have RADS often respond well to treatment, and show improvement in symptoms and spirometry testing.
Sequelae Associated With Biomass Fuel Exposure
A cross-sectional study showed significant reductions (P < .001) in all pulmonary function testing parameters for cow-dung fuel users compared to those who use modern energy sources: forced expiratory volume in the first second (FEV1), forced vital capacity (FVC), FEV1/FVC ratio, and mid-flow rate between the first 25% and 75% of forced expiratory flow. Linear regression showed a 12.4% reduction in FVC of cow-dung users, and 36% (compared to 20% in modern energy-source users) were noted to have pulmonary infections.16
Due to these emissions, biomass fuel exposure causes high levels of morbidity and mortality in developing countries, with nearly 2 million attributable deaths annually.1 Chronic exposure to biomass fuel emissions can lead to increased risk of diseases, including respiratory problems (eg, pneumonia, tuberculosis and chronic obstructive pulmonary disease, lung cancer, asthma), low birthweight, cataracts, and cardiovascular events.2,17 Women are at higher risk compared to other family members, as they typically spend approximately 3 to 4 hours longer daily in tents,5 and perform the majoring of the cooking duties. For pregnant women, the developing fetus may also be exposed, which can lead to increased rates of fetal demise.18
Conclusion
Our report represents the first reported case of “dung lung” or RADS from yak-dung biomass fuel combustion exposure. In the medical literature, there has been one previous case report of dung lung by Osbern and Crapo19 in 1981 in which the authors described three patients who died from aspiration of liquid manure in a storage facility.Our case highlights the prevalence of biomass fuel combustion in the third world, the dangerous air pollutants from their emissions, and the morbidity associated with improper ventilation of biomass fuel combustion.
1. Rodway GW, Windsor JS. Airway mucociliary function at high altitude. Wilderness Environ Med. 2006;17(4):271-275.
2. Leigh-Smith S. Carbon monoxide poisoning in tents—a review. Wilderness Environ Med. 2004;15(3):157-163.
3. Lipman GL. Carbon monoxide toxicity at high altitude [Commentary]. Wilderness Environ Med. 2006;17(2):144-145.
4. Prasad R, Singh A, Garg R, Giridhar GB. Biomass fuel exposure and respiratory diseases in India. Biosci Trends. 2012;6(5):219-228.
5. Kim KH, Jahan SA, Kabir E. A review of diseases associated with household air pollution due to the use of biomass fuels. J Hazard Mater. 2011;192(2):425-431.
6. Park D, Barabad ML, Lee G, et al. Emission characteristics of particulate matter and volatile organic compounds in cow dung combustion. Environ Sci Technol. 2013;47(22):12952-12957.
7. Venkataraman C, Rao GU. Emission factors of carbon monoxide and size-resolved aerosols from biofuel combustion. Environ Sci Technol. 2001;35(10):2100-2107.
8. Chen PF, Li CL, Kang SC, et al. [Indoor air pollution in the Nam Co and Ando Regions in the Tibetan Plateau]. [Article in Chinese]. Huan Jing Ke Xue. 2011;32(5):1231-1236.
9. avidson CI, Grimm TC, Nasta MA. Airborne lead and other elements derived from local fires in the himalayas. Science. 1981;214(4527):1344-1366.
10. Gorguner M, Akgun M. Acute inhalation injury. Eurasian J Med. 2010;42(1):28-35.
11. Ainslie G. Inhalational injuries produced by smoke and nitrogen dioxide. Respir Med. 1993;87(3):169-174.
12. Glazer CS. Acute inhalational injury. In: Hanley ME, Welsh CH, eds. Current Diagnosis & Treatment in Pulmonary Medicine. International Ed. New York, NY: McGraw Hill; 2003:354-360.
13. Gu TL, Liou SH, Hsu CH, Hsu JC, Wu TN. Acute health hazards of firefighters after fighting a department store fire. Indust Health. 1996;34(1):13-23.
14. Alberts WM, do Picco GA. Reactive airways dysfunction syndrome. Chest. 1996;109(6):1618-1626.
15. Banauch GI, Dhala A, Alleyne D, et al. Bronchial hyperreactivity and other inhalation lung injuries in rescue/recovery workers after the World Trade Center collapse. Crit Care Med. 2005;33(1 Suppl):S102-S106.
16. Sümer H, Turaçlar UT, Onarlioğlu T, Ozdemir L, Zwahlen M. The association of biomass fuel combustion on pulmonary function tests in the adult population of Mid-Anatolia. Soz Praventivmed. 2004;49(4):247-253.
17. Cesaroni G, Forastiere F, Stafoggia M, et al. Long term exposure to ambient air pollution and incidence of acute coronary events: prospective cohort study and meta-analysis in 11 European cohorts from the ESCAPE Project. BMJ. 2014;348:f7412.
18. de Koning HW, Smith KR, Last JM. Biomass fuel combustion and health. Bull World Health Organ. 1985;63(1):11-26.
19. Osbern LN, Crapo RO. Dung lung: a report of toxic exposure to liquid manure. Ann Intern Med. 1981;95(3):312-314.
1. Rodway GW, Windsor JS. Airway mucociliary function at high altitude. Wilderness Environ Med. 2006;17(4):271-275.
2. Leigh-Smith S. Carbon monoxide poisoning in tents—a review. Wilderness Environ Med. 2004;15(3):157-163.
3. Lipman GL. Carbon monoxide toxicity at high altitude [Commentary]. Wilderness Environ Med. 2006;17(2):144-145.
4. Prasad R, Singh A, Garg R, Giridhar GB. Biomass fuel exposure and respiratory diseases in India. Biosci Trends. 2012;6(5):219-228.
5. Kim KH, Jahan SA, Kabir E. A review of diseases associated with household air pollution due to the use of biomass fuels. J Hazard Mater. 2011;192(2):425-431.
6. Park D, Barabad ML, Lee G, et al. Emission characteristics of particulate matter and volatile organic compounds in cow dung combustion. Environ Sci Technol. 2013;47(22):12952-12957.
7. Venkataraman C, Rao GU. Emission factors of carbon monoxide and size-resolved aerosols from biofuel combustion. Environ Sci Technol. 2001;35(10):2100-2107.
8. Chen PF, Li CL, Kang SC, et al. [Indoor air pollution in the Nam Co and Ando Regions in the Tibetan Plateau]. [Article in Chinese]. Huan Jing Ke Xue. 2011;32(5):1231-1236.
9. avidson CI, Grimm TC, Nasta MA. Airborne lead and other elements derived from local fires in the himalayas. Science. 1981;214(4527):1344-1366.
10. Gorguner M, Akgun M. Acute inhalation injury. Eurasian J Med. 2010;42(1):28-35.
11. Ainslie G. Inhalational injuries produced by smoke and nitrogen dioxide. Respir Med. 1993;87(3):169-174.
12. Glazer CS. Acute inhalational injury. In: Hanley ME, Welsh CH, eds. Current Diagnosis & Treatment in Pulmonary Medicine. International Ed. New York, NY: McGraw Hill; 2003:354-360.
13. Gu TL, Liou SH, Hsu CH, Hsu JC, Wu TN. Acute health hazards of firefighters after fighting a department store fire. Indust Health. 1996;34(1):13-23.
14. Alberts WM, do Picco GA. Reactive airways dysfunction syndrome. Chest. 1996;109(6):1618-1626.
15. Banauch GI, Dhala A, Alleyne D, et al. Bronchial hyperreactivity and other inhalation lung injuries in rescue/recovery workers after the World Trade Center collapse. Crit Care Med. 2005;33(1 Suppl):S102-S106.
16. Sümer H, Turaçlar UT, Onarlioğlu T, Ozdemir L, Zwahlen M. The association of biomass fuel combustion on pulmonary function tests in the adult population of Mid-Anatolia. Soz Praventivmed. 2004;49(4):247-253.
17. Cesaroni G, Forastiere F, Stafoggia M, et al. Long term exposure to ambient air pollution and incidence of acute coronary events: prospective cohort study and meta-analysis in 11 European cohorts from the ESCAPE Project. BMJ. 2014;348:f7412.
18. de Koning HW, Smith KR, Last JM. Biomass fuel combustion and health. Bull World Health Organ. 1985;63(1):11-26.
19. Osbern LN, Crapo RO. Dung lung: a report of toxic exposure to liquid manure. Ann Intern Med. 1981;95(3):312-314.
Patient-Reported Outcome Measures: How Do Digital Tablets Stack Up to Paper Forms? A Randomized, Controlled Study
Over the past several decades, patient-reported outcomes (PROs) have become increasingly important in assessing the quality and effectiveness of medical and surgical care.1,2 The benefit lies in the ability of PROs to characterize the impact of medical interventions on symptoms, function, and other outcomes from the patient’s perspective. Consequently, clinical practices can improve patients’ objective findings (from radiographic and clinical examinations) as well as their preferences in a social-psychological context.2,3 As a patient’s satisfaction with a surgical intervention may not correlate with the surgeon’s objective assessment of outcome, PROs offer unique insight into the patient’s perceptions of well-being.4
Health-related quality-of-life assessments can be made with either general-health or disease-specific instruments. These instruments traditionally are administered with pen and paper—a data collection method with several limitations, chief being the need to manually transfer the data into an electronic medical record, a research database, or both. In addition, administering surveys on paper risks potential disqualification of partially or incorrectly completed surveys. With pen and paper, it is difficult to mandate that every question be answered accurately.
Currently, there is a potential role for electronic medical records and digital tablet devices in survey administration and data collection and storage. Theoretical advantages include direct input of survey data into databases (eliminating manual data entry and associated entry errors), improved accuracy and completion rates, and long-term storage not dependent on paper charts.5To our knowledge, there have been no prospective studies of different orthopedic outcomes collection methods. Some studies have evaluated use of touch-based tablets in data collection. Dy and colleagues6 considered administration of the DASH (Disabilities of the Arm, Shoulder, and Hand) survey on an iPad tablet (Apple Computers) and retrospectively compared the tablet and paper completion rates. The tablet group’s rate (98%) was significantly higher than the paper group’s rate (76%). Aktas and colleagues7 reported a high completion rate for a tablet survey of palliative care outcomes (they did not compare modalities). A handful of other studies have found higher intraclass correlation and validation for digital data collection than for paper collection.7-14 The comparability of the data collected digitally vs on paper was the nidus for our decision to prospectively evaluate the ease and reliability of digital data collection.
We conducted a prospective, randomized study to compare the performance of tablet and paper versions of several general-health and musculoskeletal disease–specific questionnaires. We hypothesized the tablet and paper surveys would have similar completion rates and times.
Methods
This study was approved by our Institutional Review Board. Participants were recruited during their clinic visit to 3 subspecialty orthopedic services (upper extremity, spine, arthroplasty). The questionnaires included basic demographics questions and questions about tablet use (comfort level with computers, measured on a Likert scale ranging from 1 (strongly disagree) to 5 (strongly agree), and ownership of a tablet or smartphone). Also included were European Quality of Life–5 Dimensions (EQ-5D, General Health), a disease questionnaire specific to 1 of the 3 subspecialty services, and a satisfaction survey. Patients were asked to complete the Oswestry Disability Index (ODI) for low-back pain, the Neck Disability Index (NDI) for neck pain, the Hip Disability and Osteoarthritis Outcomes Score (HOOS) for hip pain, the Knee Injury and Osteoarthritis Outcomes Score (KOOS) for knee pain, or the QuickDASH survey for upper extremity complaints (subspecialty-specific). After recruitment, a computer-generated randomization technique was used to randomly assign patients to either a paper or an electronic (iPad) data collection group.15 We included all surveys for which patients had sufficient completion time (no clinic staff interruptions) and excluded surveys marked incomplete (because of interruptions for clinic workflow efficiency). For direct input from tablets and for data storage, we used the Research Electronic Data Capture (REDCap) system hosted at our institution.16 Our staff registered patients as REDCap participants, assigned them to their disease-specific study arms, and gave them tablets to use to complete the surveys.
Patients who were randomly assigned to take the surveys on paper were given a packet that included the demographics survey, the EQ-5D, a disease-specific survey, and a satisfaction survey. Their responses were then manually entered by the investigators into the REDCap system.
Patients who were randomly assigned to take the surveys on tablets used the REDCap survey feature, which allowed them to directly input their responses into the database (Figure).
Our primary outcome measure was survey completion rate. Secondary outcome measures were total time for completion, number of questions left unanswered on incomplete surveys, patient satisfaction with survey length (Likert scale, 1-5), ease of completion (Likert scale, 1-5), ability to comprehend questions (Likert scale, 1-5), and preference for the other survey modality (Appendix).
We used SPSS statistical software (IBM) to analyze our data, t test to compare continuous variables, χ2 test to compare categorical variables, and linear regression to test the relationship between number of questions and completion rate. Statistical significance was set at P < .05.
Results
Of the 510 patients enrolled in the study, 483 completed the initial demographics questionnaire and were included in the analysis. Patients were excluded if they were unable to complete the initial demographics questionnaire because of clinic workflow (eg, immediate need to be seen by physician, need to transfer to radiology for imaging and not being able to revisit the survey). Mean age was 56 years (range, 14-93 years), and 51% of the respondents were female. Fifty percent owned tablets, 70% owned smartphones, and mean (SD) self-rating of computer skills was 3.13 (1.16) (Likert scale, 1-5). There were no significant demographic differences between the tablet and paper groups (Table 1).
For each disease-specific questionnaire, the instrument’s published instructions for calculating scores were followed; these scores were then compared in order to further characterize the groups. There were significant differences in scores on the EQ-5D descriptive questions, a pain visual analog scale (VAS), and the NDI. Mean EQ-5D score was 0.664 for the tablet group and 0.699 for the paper group (P = .041), mean pain VAS score was 62.5 for the tablet group and 71.6 for the paper group (P < .001), and mean NDI score was 42.8 for the tablet group and 32.4 for the paper group (P = .033).
The overall completion rate for all questionnaires was 84.4%. The KOOS completion rate was 83.3% for the tablet group and 54.5% for the paper group (P = .023). Although it was not statistically significant, there was a trend toward higher rates of completing all disease-specific questionnaires in the tablet group relative to the paper group.
Satisfaction regarding the surveys and their modalities was similar between the groups.
Discussion
Electronic data entry has many advantages over traditional paper-based data collection and can be used with PRO surveys to measure response to treatment. Our study evaluated whether completion rates differed between surveys administered on digital tablets and those administered on traditional paper forms in a clinic setting. We selected general-health and disease-specific instruments commonly used to collect PROs from orthopedic patients. Our primary outcome measure was survey completion rate. Secondary outcome measures were total time for completion, number of questions left unanswered on incomplete surveys, patient satisfaction, and survey preferences.
In this study, our tablet and paper groups had similar overall survey completion rates, which suggests digital tablet-based data collection is noninferior to traditional pen-and-paper data collection with respect to patient response rate in the clinical setting. It is worth emphasizing that the tablet surveys were made to resemble and function as much as possible like the paper surveys. For example, patients were allowed to select multiple answers as well as advance without answering a question. Paper surveys were mimicked so we could study inherent differences in patient responsiveness without adding digital features to prevent patients from selecting multiple answers or skipping questions. We postulate that adding these digital features could have introduced a significant difference in patient responsiveness.
Time for survey completion was not significantly different between the tablet and paper groups, demonstrating that data can be digitally collected and the aforementioned advantages realized without significant delay or clinic workflow disruption. In the future, patients may be able to complete their forms digitally, on their own devices, before arriving for their clinic visits—resulting in improved clinic workflow and data collection efficiency.
Scores computed for the health-related quality-of-life questionnaires were not significantly different between the tablet and paper groups, except for EQ-5D and NDI. Although statistically significant, the 0.035 difference between the groups’ EQ-5D scores (0.664, 0.699) is not clinically significant. (Pickard and colleagues17 established that 0.06 is the clinically significant difference between EQ-5D scores in the United States.) If there were any clinical difference in the present study, our paper group patients appeared to be in better health than our tablet group patients.
Patients’ motivation to complete surveys often plays a large role in meaningful rates of completion. On our subjective satisfaction survey, a larger percentage of patients reported they would prefer to use a tablet for future surveys (Table 4). This finding may be driven by the novelty or ease of using a popular device. Nevertheless, we think it is worthwhile to heed patient preferences, as they may point to more successful data collection and compliance.
Several other studies have compared electronic and paper data capture.6,7,9-14,18-22 Dy and colleagues6 reported on administering the DASH survey on an iPad tablet using REDCap in an outpatient setting. They found that the percentage of surveys that could be scored (<3 questions left unanswered) was significantly higher for their tablet group (98%) than their paper group (76%). The larger difference in survey completion rates in their study (vs ours) may be attributable to their use of DASH, which has more survey items (compared with QuickDASH, the instrument we used) and thus may be more sensitive to detecting differences, at the risk of increasing the burden on survey takers.23 Aktas and colleagues7 conducted a similar but smaller study of completion rates, completion times, and overall practicality of using digital tablets to collect PROs in a palliative care clinic (they did not compare tablet and paper modalities). Marsh and colleagues,12 who studied the agreement between data collected on electronic and paper versions of the WOMAC (Western Ontario and McMaster Universities) Osteoarthritis Index and the SF-12 (12-item Short Form Health Survey, Version 2) after total hip and total knee arthroplasty, found a high intraclass correlation coefficient between the 2 methods. Griffiths-Jones and colleagues11 also found a high degree of agreement between patient data collected on digital and paper surveys. In a similar study, Fanning and McAuley10 compared digital tablet and paper survey administration in an older population and found a higher percentage of preference for tablets, with ease of use and anxiety during survey completion correlating with preference. These findings mirror ours, even with our inclusion of patients in a broader age range.
Strengths of our study included its overall cohort size and the variety of measurement instruments used. In addition, we measured time for survey completion to assess the practicality of tablet-based data collection and refrained from using digital features that could have artificially improved the completion rate for this survey modality.
Our study had a few limitations. First, we recruited unequal numbers of patients from the different subspecialties—a result of each subspecialty having a different number of attending physicians and a different patient volume. Given randomization and use of similar patients across the study arms, however, this likely did not present any significant bias. Second, each patient completed a tablet survey or a paper survey but not both, and therefore we could not compare a patient’s performance on the 2 modalities. However, the burden of completing the same survey more than once likely would have lowered our participation rate and introduced additional biases we wanted to avoid. Third, despite our attempt to mimic the look of a paper survey, the tablet’s user interface presented several potential difficulties. For example, its small text and small answer buttons may have been limiting for patients with poor vision. These design features emphasize the importance of having a user interface that can be adapted to the individual, regardless of handicap. Indeed, adaptability is a potential strength of digital interfaces. For adaptability, an interface designer can use large, scalable text and add audio prompts and other features.
Our findings can be useful in evaluating patient responsiveness to surveys administered on digital tablets in an outpatient clinic setting. In this prospective, randomized study, we found that, for survey completion, use of a tablet device did not require more time than use of a paper form. In addition, the administration modalities had similar completion and error rates for a variety of orthopedic outcomes surveys. We did not activate digital features that would have given unfair advantage to the digital data collection modality. We also found a strong preference for use of technology in PRO data collection, and this may help improve collection rates. Last, though optimizing the flow of patients in our clinic was not a strict research metric, we prioritized making sure patients were not spending any more time completing these surveys than in the past. Given the potential benefits of digital surveys—immediate and accurate transfer of collected data into multiple databases, including the patient’s electronic medical record—our experience supports continuing validation of these instruments for potential wider use.
Am J Orthop. 2016;45(7):E451-E457. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. Howie L, Hirsch B, Locklear T, Abernethy AP. Assessing the value of patient-generated data to comparative effectiveness research. Health Aff (Millwood). 2014;33(7):1220-1228.
2. Higginson IJ, Carr AJ. Measuring quality of life: using quality of life measures in the clinical setting. BMJ. 2001;322(7297):1297-1300.
3. Revicki D, Hays RD, Cella D, Sloan J. Recommended methods for determining responsiveness and minimally important differences for patient-reported outcomes. J Clin Epidemiol. 2008;61(2):102-109.
4. Guyatt GH, Feeny DH, Patrick DL. Measuring health-related quality of life. Ann Intern Med. 1993;118(8):622-629.
5. Paudel D, Ahmed M, Pradhan A, Lal Dangol R. Successful use of tablet personal computers and wireless technologies for the 2011 Nepal Demographic and Health Survey. Glob Heal Sci Pract. 2013;1(2):277-284.
6. Dy CJ, Schmicker T, Tran Q, Chadwick B, Daluiski A. The use of a tablet computer to complete the DASH questionnaire. J Hand Surg Am. 2012;37(12):2589-2594.
7. Aktas A, Hullihen B, Shrotriya S, Thomas S, Walsh D, Estfan B. Connected health: cancer symptom and quality-of-life assessment using a tablet computer: a pilot study. Am J Hosp Palliat Care. 2015;32(2):189-197.
8. Basnov M, Kongsved SM, Bech P, Hjollund NH. Reliability of Short Form-36 in an internet- and a pen-and-paper version. Inform Health Soc Care. 2009;34(1):53-58.
9. Bellamy N, Wilson C, Hendrikz J, et al; EDC Study Group. Osteoarthritis Index delivered by mobile phone (m-WOMAC) is valid, reliable, and responsive. J Clin Epidemiol. 2011;64(2):182-190.
10. Fanning J, McAuley E. A comparison of tablet computer and paper-based questionnaires in healthy aging research. JMIR Res Protoc. 2014;3(3):e38.
11. Griffiths-Jones W, Norton MR, Fern ED, Williams DH. The equivalence of remote electronic and paper patient reported outcome (PRO) collection. J Arthroplasty. 2014;29(11):2136-2139.
12. Marsh JD, Bryant DM, Macdonald SJ, Naudie DD. Patients respond similarly to paper and electronic versions of the WOMAC and SF-12 following total joint arthroplasty. J Arthroplasty. 2014;29(4):670-673.
13. Olajos-Clow J, Minard J, Szpiro K, et al. Validation of an electronic version of the Mini Asthma Quality of Life Questionnaire. Respir Med. 2010;104(5):658-667.
14. Shervin N, Dorrwachter J, Bragdon CR, Shervin D, Zurakowski D, Malchau H. Comparison of paper and computer-based questionnaire modes for measuring health outcomes in patients undergoing total hip arthroplasty. J Bone Joint Surg Am. 2011;93(3):285-293.
15. Suresh K. An overview of randomization techniques: an unbiased assessment of outcome in clinical research. J Hum Reprod Sci. 2011;4(1):8-11.
16. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377-381.
17. Pickard AS, Neary MP, Cella D. Estimation of minimally important differences in EQ-5D utility and VAS scores in cancer. Health Qual Life Outcomes. 2007;5:70.
18. Abdel Messih M, Naylor JM, Descallar J, Manickam A, Mittal R, Harris IA. Mail versus telephone administration of the Oxford Knee and Hip Scores. J Arthroplasty. 2014;29(3):491-494.
19. Kongsved SM, Basnov M, Holm-Christensen K, Hjollund NH. Response rate and completeness of questionnaires: a randomized study of internet versus paper-and-pencil versions. J Med Internet Res. 2007;9(3):e25.
20. Theiler R, Bischoff-Ferrari HA, Good M, Bellamy N. Responsiveness of the electronic touch screen WOMAC 3.1 OA Index in a short term clinical trial with rofecoxib. Osteoarthritis Cartilage. 2004;12(11):912-916.
21. Ryan JM, Corry JR, Attewell R, Smithson MJ. A comparison of an electronic version of the SF-36 General Health Questionnaire to the standard paper version. Qual Life Res. 2002;11(1):19-26.
22. Wilson AS, Kitas GD, Carruthers DM, et al. Computerized information-gathering in specialist rheumatology clinics: an initial evaluation of an electronic version of the Short Form 36. Rheumatology. 2002;41(3):268-273.
23. Angst F, Goldhahn J, Drerup S, Flury M, Schwyzer HK, Simmen BR. How sharp is the short QuickDASH? A refined content and validity analysis of the Short Form of the Disabilities of the Shoulder, Arm and Hand questionnaire in the strata of symptoms and function and specific joint conditions. Qual Life Res. 2009;18(8):1043-1051.
Over the past several decades, patient-reported outcomes (PROs) have become increasingly important in assessing the quality and effectiveness of medical and surgical care.1,2 The benefit lies in the ability of PROs to characterize the impact of medical interventions on symptoms, function, and other outcomes from the patient’s perspective. Consequently, clinical practices can improve patients’ objective findings (from radiographic and clinical examinations) as well as their preferences in a social-psychological context.2,3 As a patient’s satisfaction with a surgical intervention may not correlate with the surgeon’s objective assessment of outcome, PROs offer unique insight into the patient’s perceptions of well-being.4
Health-related quality-of-life assessments can be made with either general-health or disease-specific instruments. These instruments traditionally are administered with pen and paper—a data collection method with several limitations, chief being the need to manually transfer the data into an electronic medical record, a research database, or both. In addition, administering surveys on paper risks potential disqualification of partially or incorrectly completed surveys. With pen and paper, it is difficult to mandate that every question be answered accurately.
Currently, there is a potential role for electronic medical records and digital tablet devices in survey administration and data collection and storage. Theoretical advantages include direct input of survey data into databases (eliminating manual data entry and associated entry errors), improved accuracy and completion rates, and long-term storage not dependent on paper charts.5To our knowledge, there have been no prospective studies of different orthopedic outcomes collection methods. Some studies have evaluated use of touch-based tablets in data collection. Dy and colleagues6 considered administration of the DASH (Disabilities of the Arm, Shoulder, and Hand) survey on an iPad tablet (Apple Computers) and retrospectively compared the tablet and paper completion rates. The tablet group’s rate (98%) was significantly higher than the paper group’s rate (76%). Aktas and colleagues7 reported a high completion rate for a tablet survey of palliative care outcomes (they did not compare modalities). A handful of other studies have found higher intraclass correlation and validation for digital data collection than for paper collection.7-14 The comparability of the data collected digitally vs on paper was the nidus for our decision to prospectively evaluate the ease and reliability of digital data collection.
We conducted a prospective, randomized study to compare the performance of tablet and paper versions of several general-health and musculoskeletal disease–specific questionnaires. We hypothesized the tablet and paper surveys would have similar completion rates and times.
Methods
This study was approved by our Institutional Review Board. Participants were recruited during their clinic visit to 3 subspecialty orthopedic services (upper extremity, spine, arthroplasty). The questionnaires included basic demographics questions and questions about tablet use (comfort level with computers, measured on a Likert scale ranging from 1 (strongly disagree) to 5 (strongly agree), and ownership of a tablet or smartphone). Also included were European Quality of Life–5 Dimensions (EQ-5D, General Health), a disease questionnaire specific to 1 of the 3 subspecialty services, and a satisfaction survey. Patients were asked to complete the Oswestry Disability Index (ODI) for low-back pain, the Neck Disability Index (NDI) for neck pain, the Hip Disability and Osteoarthritis Outcomes Score (HOOS) for hip pain, the Knee Injury and Osteoarthritis Outcomes Score (KOOS) for knee pain, or the QuickDASH survey for upper extremity complaints (subspecialty-specific). After recruitment, a computer-generated randomization technique was used to randomly assign patients to either a paper or an electronic (iPad) data collection group.15 We included all surveys for which patients had sufficient completion time (no clinic staff interruptions) and excluded surveys marked incomplete (because of interruptions for clinic workflow efficiency). For direct input from tablets and for data storage, we used the Research Electronic Data Capture (REDCap) system hosted at our institution.16 Our staff registered patients as REDCap participants, assigned them to their disease-specific study arms, and gave them tablets to use to complete the surveys.
Patients who were randomly assigned to take the surveys on paper were given a packet that included the demographics survey, the EQ-5D, a disease-specific survey, and a satisfaction survey. Their responses were then manually entered by the investigators into the REDCap system.
Patients who were randomly assigned to take the surveys on tablets used the REDCap survey feature, which allowed them to directly input their responses into the database (Figure).
Our primary outcome measure was survey completion rate. Secondary outcome measures were total time for completion, number of questions left unanswered on incomplete surveys, patient satisfaction with survey length (Likert scale, 1-5), ease of completion (Likert scale, 1-5), ability to comprehend questions (Likert scale, 1-5), and preference for the other survey modality (Appendix).
We used SPSS statistical software (IBM) to analyze our data, t test to compare continuous variables, χ2 test to compare categorical variables, and linear regression to test the relationship between number of questions and completion rate. Statistical significance was set at P < .05.
Results
Of the 510 patients enrolled in the study, 483 completed the initial demographics questionnaire and were included in the analysis. Patients were excluded if they were unable to complete the initial demographics questionnaire because of clinic workflow (eg, immediate need to be seen by physician, need to transfer to radiology for imaging and not being able to revisit the survey). Mean age was 56 years (range, 14-93 years), and 51% of the respondents were female. Fifty percent owned tablets, 70% owned smartphones, and mean (SD) self-rating of computer skills was 3.13 (1.16) (Likert scale, 1-5). There were no significant demographic differences between the tablet and paper groups (Table 1).
For each disease-specific questionnaire, the instrument’s published instructions for calculating scores were followed; these scores were then compared in order to further characterize the groups. There were significant differences in scores on the EQ-5D descriptive questions, a pain visual analog scale (VAS), and the NDI. Mean EQ-5D score was 0.664 for the tablet group and 0.699 for the paper group (P = .041), mean pain VAS score was 62.5 for the tablet group and 71.6 for the paper group (P < .001), and mean NDI score was 42.8 for the tablet group and 32.4 for the paper group (P = .033).
The overall completion rate for all questionnaires was 84.4%. The KOOS completion rate was 83.3% for the tablet group and 54.5% for the paper group (P = .023). Although it was not statistically significant, there was a trend toward higher rates of completing all disease-specific questionnaires in the tablet group relative to the paper group.
Satisfaction regarding the surveys and their modalities was similar between the groups.
Discussion
Electronic data entry has many advantages over traditional paper-based data collection and can be used with PRO surveys to measure response to treatment. Our study evaluated whether completion rates differed between surveys administered on digital tablets and those administered on traditional paper forms in a clinic setting. We selected general-health and disease-specific instruments commonly used to collect PROs from orthopedic patients. Our primary outcome measure was survey completion rate. Secondary outcome measures were total time for completion, number of questions left unanswered on incomplete surveys, patient satisfaction, and survey preferences.
In this study, our tablet and paper groups had similar overall survey completion rates, which suggests digital tablet-based data collection is noninferior to traditional pen-and-paper data collection with respect to patient response rate in the clinical setting. It is worth emphasizing that the tablet surveys were made to resemble and function as much as possible like the paper surveys. For example, patients were allowed to select multiple answers as well as advance without answering a question. Paper surveys were mimicked so we could study inherent differences in patient responsiveness without adding digital features to prevent patients from selecting multiple answers or skipping questions. We postulate that adding these digital features could have introduced a significant difference in patient responsiveness.
Time for survey completion was not significantly different between the tablet and paper groups, demonstrating that data can be digitally collected and the aforementioned advantages realized without significant delay or clinic workflow disruption. In the future, patients may be able to complete their forms digitally, on their own devices, before arriving for their clinic visits—resulting in improved clinic workflow and data collection efficiency.
Scores computed for the health-related quality-of-life questionnaires were not significantly different between the tablet and paper groups, except for EQ-5D and NDI. Although statistically significant, the 0.035 difference between the groups’ EQ-5D scores (0.664, 0.699) is not clinically significant. (Pickard and colleagues17 established that 0.06 is the clinically significant difference between EQ-5D scores in the United States.) If there were any clinical difference in the present study, our paper group patients appeared to be in better health than our tablet group patients.
Patients’ motivation to complete surveys often plays a large role in meaningful rates of completion. On our subjective satisfaction survey, a larger percentage of patients reported they would prefer to use a tablet for future surveys (Table 4). This finding may be driven by the novelty or ease of using a popular device. Nevertheless, we think it is worthwhile to heed patient preferences, as they may point to more successful data collection and compliance.
Several other studies have compared electronic and paper data capture.6,7,9-14,18-22 Dy and colleagues6 reported on administering the DASH survey on an iPad tablet using REDCap in an outpatient setting. They found that the percentage of surveys that could be scored (<3 questions left unanswered) was significantly higher for their tablet group (98%) than their paper group (76%). The larger difference in survey completion rates in their study (vs ours) may be attributable to their use of DASH, which has more survey items (compared with QuickDASH, the instrument we used) and thus may be more sensitive to detecting differences, at the risk of increasing the burden on survey takers.23 Aktas and colleagues7 conducted a similar but smaller study of completion rates, completion times, and overall practicality of using digital tablets to collect PROs in a palliative care clinic (they did not compare tablet and paper modalities). Marsh and colleagues,12 who studied the agreement between data collected on electronic and paper versions of the WOMAC (Western Ontario and McMaster Universities) Osteoarthritis Index and the SF-12 (12-item Short Form Health Survey, Version 2) after total hip and total knee arthroplasty, found a high intraclass correlation coefficient between the 2 methods. Griffiths-Jones and colleagues11 also found a high degree of agreement between patient data collected on digital and paper surveys. In a similar study, Fanning and McAuley10 compared digital tablet and paper survey administration in an older population and found a higher percentage of preference for tablets, with ease of use and anxiety during survey completion correlating with preference. These findings mirror ours, even with our inclusion of patients in a broader age range.
Strengths of our study included its overall cohort size and the variety of measurement instruments used. In addition, we measured time for survey completion to assess the practicality of tablet-based data collection and refrained from using digital features that could have artificially improved the completion rate for this survey modality.
Our study had a few limitations. First, we recruited unequal numbers of patients from the different subspecialties—a result of each subspecialty having a different number of attending physicians and a different patient volume. Given randomization and use of similar patients across the study arms, however, this likely did not present any significant bias. Second, each patient completed a tablet survey or a paper survey but not both, and therefore we could not compare a patient’s performance on the 2 modalities. However, the burden of completing the same survey more than once likely would have lowered our participation rate and introduced additional biases we wanted to avoid. Third, despite our attempt to mimic the look of a paper survey, the tablet’s user interface presented several potential difficulties. For example, its small text and small answer buttons may have been limiting for patients with poor vision. These design features emphasize the importance of having a user interface that can be adapted to the individual, regardless of handicap. Indeed, adaptability is a potential strength of digital interfaces. For adaptability, an interface designer can use large, scalable text and add audio prompts and other features.
Our findings can be useful in evaluating patient responsiveness to surveys administered on digital tablets in an outpatient clinic setting. In this prospective, randomized study, we found that, for survey completion, use of a tablet device did not require more time than use of a paper form. In addition, the administration modalities had similar completion and error rates for a variety of orthopedic outcomes surveys. We did not activate digital features that would have given unfair advantage to the digital data collection modality. We also found a strong preference for use of technology in PRO data collection, and this may help improve collection rates. Last, though optimizing the flow of patients in our clinic was not a strict research metric, we prioritized making sure patients were not spending any more time completing these surveys than in the past. Given the potential benefits of digital surveys—immediate and accurate transfer of collected data into multiple databases, including the patient’s electronic medical record—our experience supports continuing validation of these instruments for potential wider use.
Am J Orthop. 2016;45(7):E451-E457. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
Over the past several decades, patient-reported outcomes (PROs) have become increasingly important in assessing the quality and effectiveness of medical and surgical care.1,2 The benefit lies in the ability of PROs to characterize the impact of medical interventions on symptoms, function, and other outcomes from the patient’s perspective. Consequently, clinical practices can improve patients’ objective findings (from radiographic and clinical examinations) as well as their preferences in a social-psychological context.2,3 As a patient’s satisfaction with a surgical intervention may not correlate with the surgeon’s objective assessment of outcome, PROs offer unique insight into the patient’s perceptions of well-being.4
Health-related quality-of-life assessments can be made with either general-health or disease-specific instruments. These instruments traditionally are administered with pen and paper—a data collection method with several limitations, chief being the need to manually transfer the data into an electronic medical record, a research database, or both. In addition, administering surveys on paper risks potential disqualification of partially or incorrectly completed surveys. With pen and paper, it is difficult to mandate that every question be answered accurately.
Currently, there is a potential role for electronic medical records and digital tablet devices in survey administration and data collection and storage. Theoretical advantages include direct input of survey data into databases (eliminating manual data entry and associated entry errors), improved accuracy and completion rates, and long-term storage not dependent on paper charts.5To our knowledge, there have been no prospective studies of different orthopedic outcomes collection methods. Some studies have evaluated use of touch-based tablets in data collection. Dy and colleagues6 considered administration of the DASH (Disabilities of the Arm, Shoulder, and Hand) survey on an iPad tablet (Apple Computers) and retrospectively compared the tablet and paper completion rates. The tablet group’s rate (98%) was significantly higher than the paper group’s rate (76%). Aktas and colleagues7 reported a high completion rate for a tablet survey of palliative care outcomes (they did not compare modalities). A handful of other studies have found higher intraclass correlation and validation for digital data collection than for paper collection.7-14 The comparability of the data collected digitally vs on paper was the nidus for our decision to prospectively evaluate the ease and reliability of digital data collection.
We conducted a prospective, randomized study to compare the performance of tablet and paper versions of several general-health and musculoskeletal disease–specific questionnaires. We hypothesized the tablet and paper surveys would have similar completion rates and times.
Methods
This study was approved by our Institutional Review Board. Participants were recruited during their clinic visit to 3 subspecialty orthopedic services (upper extremity, spine, arthroplasty). The questionnaires included basic demographics questions and questions about tablet use (comfort level with computers, measured on a Likert scale ranging from 1 (strongly disagree) to 5 (strongly agree), and ownership of a tablet or smartphone). Also included were European Quality of Life–5 Dimensions (EQ-5D, General Health), a disease questionnaire specific to 1 of the 3 subspecialty services, and a satisfaction survey. Patients were asked to complete the Oswestry Disability Index (ODI) for low-back pain, the Neck Disability Index (NDI) for neck pain, the Hip Disability and Osteoarthritis Outcomes Score (HOOS) for hip pain, the Knee Injury and Osteoarthritis Outcomes Score (KOOS) for knee pain, or the QuickDASH survey for upper extremity complaints (subspecialty-specific). After recruitment, a computer-generated randomization technique was used to randomly assign patients to either a paper or an electronic (iPad) data collection group.15 We included all surveys for which patients had sufficient completion time (no clinic staff interruptions) and excluded surveys marked incomplete (because of interruptions for clinic workflow efficiency). For direct input from tablets and for data storage, we used the Research Electronic Data Capture (REDCap) system hosted at our institution.16 Our staff registered patients as REDCap participants, assigned them to their disease-specific study arms, and gave them tablets to use to complete the surveys.
Patients who were randomly assigned to take the surveys on paper were given a packet that included the demographics survey, the EQ-5D, a disease-specific survey, and a satisfaction survey. Their responses were then manually entered by the investigators into the REDCap system.
Patients who were randomly assigned to take the surveys on tablets used the REDCap survey feature, which allowed them to directly input their responses into the database (Figure).
Our primary outcome measure was survey completion rate. Secondary outcome measures were total time for completion, number of questions left unanswered on incomplete surveys, patient satisfaction with survey length (Likert scale, 1-5), ease of completion (Likert scale, 1-5), ability to comprehend questions (Likert scale, 1-5), and preference for the other survey modality (Appendix).
We used SPSS statistical software (IBM) to analyze our data, t test to compare continuous variables, χ2 test to compare categorical variables, and linear regression to test the relationship between number of questions and completion rate. Statistical significance was set at P < .05.
Results
Of the 510 patients enrolled in the study, 483 completed the initial demographics questionnaire and were included in the analysis. Patients were excluded if they were unable to complete the initial demographics questionnaire because of clinic workflow (eg, immediate need to be seen by physician, need to transfer to radiology for imaging and not being able to revisit the survey). Mean age was 56 years (range, 14-93 years), and 51% of the respondents were female. Fifty percent owned tablets, 70% owned smartphones, and mean (SD) self-rating of computer skills was 3.13 (1.16) (Likert scale, 1-5). There were no significant demographic differences between the tablet and paper groups (Table 1).
For each disease-specific questionnaire, the instrument’s published instructions for calculating scores were followed; these scores were then compared in order to further characterize the groups. There were significant differences in scores on the EQ-5D descriptive questions, a pain visual analog scale (VAS), and the NDI. Mean EQ-5D score was 0.664 for the tablet group and 0.699 for the paper group (P = .041), mean pain VAS score was 62.5 for the tablet group and 71.6 for the paper group (P < .001), and mean NDI score was 42.8 for the tablet group and 32.4 for the paper group (P = .033).
The overall completion rate for all questionnaires was 84.4%. The KOOS completion rate was 83.3% for the tablet group and 54.5% for the paper group (P = .023). Although it was not statistically significant, there was a trend toward higher rates of completing all disease-specific questionnaires in the tablet group relative to the paper group.
Satisfaction regarding the surveys and their modalities was similar between the groups.
Discussion
Electronic data entry has many advantages over traditional paper-based data collection and can be used with PRO surveys to measure response to treatment. Our study evaluated whether completion rates differed between surveys administered on digital tablets and those administered on traditional paper forms in a clinic setting. We selected general-health and disease-specific instruments commonly used to collect PROs from orthopedic patients. Our primary outcome measure was survey completion rate. Secondary outcome measures were total time for completion, number of questions left unanswered on incomplete surveys, patient satisfaction, and survey preferences.
In this study, our tablet and paper groups had similar overall survey completion rates, which suggests digital tablet-based data collection is noninferior to traditional pen-and-paper data collection with respect to patient response rate in the clinical setting. It is worth emphasizing that the tablet surveys were made to resemble and function as much as possible like the paper surveys. For example, patients were allowed to select multiple answers as well as advance without answering a question. Paper surveys were mimicked so we could study inherent differences in patient responsiveness without adding digital features to prevent patients from selecting multiple answers or skipping questions. We postulate that adding these digital features could have introduced a significant difference in patient responsiveness.
Time for survey completion was not significantly different between the tablet and paper groups, demonstrating that data can be digitally collected and the aforementioned advantages realized without significant delay or clinic workflow disruption. In the future, patients may be able to complete their forms digitally, on their own devices, before arriving for their clinic visits—resulting in improved clinic workflow and data collection efficiency.
Scores computed for the health-related quality-of-life questionnaires were not significantly different between the tablet and paper groups, except for EQ-5D and NDI. Although statistically significant, the 0.035 difference between the groups’ EQ-5D scores (0.664, 0.699) is not clinically significant. (Pickard and colleagues17 established that 0.06 is the clinically significant difference between EQ-5D scores in the United States.) If there were any clinical difference in the present study, our paper group patients appeared to be in better health than our tablet group patients.
Patients’ motivation to complete surveys often plays a large role in meaningful rates of completion. On our subjective satisfaction survey, a larger percentage of patients reported they would prefer to use a tablet for future surveys (Table 4). This finding may be driven by the novelty or ease of using a popular device. Nevertheless, we think it is worthwhile to heed patient preferences, as they may point to more successful data collection and compliance.
Several other studies have compared electronic and paper data capture.6,7,9-14,18-22 Dy and colleagues6 reported on administering the DASH survey on an iPad tablet using REDCap in an outpatient setting. They found that the percentage of surveys that could be scored (<3 questions left unanswered) was significantly higher for their tablet group (98%) than their paper group (76%). The larger difference in survey completion rates in their study (vs ours) may be attributable to their use of DASH, which has more survey items (compared with QuickDASH, the instrument we used) and thus may be more sensitive to detecting differences, at the risk of increasing the burden on survey takers.23 Aktas and colleagues7 conducted a similar but smaller study of completion rates, completion times, and overall practicality of using digital tablets to collect PROs in a palliative care clinic (they did not compare tablet and paper modalities). Marsh and colleagues,12 who studied the agreement between data collected on electronic and paper versions of the WOMAC (Western Ontario and McMaster Universities) Osteoarthritis Index and the SF-12 (12-item Short Form Health Survey, Version 2) after total hip and total knee arthroplasty, found a high intraclass correlation coefficient between the 2 methods. Griffiths-Jones and colleagues11 also found a high degree of agreement between patient data collected on digital and paper surveys. In a similar study, Fanning and McAuley10 compared digital tablet and paper survey administration in an older population and found a higher percentage of preference for tablets, with ease of use and anxiety during survey completion correlating with preference. These findings mirror ours, even with our inclusion of patients in a broader age range.
Strengths of our study included its overall cohort size and the variety of measurement instruments used. In addition, we measured time for survey completion to assess the practicality of tablet-based data collection and refrained from using digital features that could have artificially improved the completion rate for this survey modality.
Our study had a few limitations. First, we recruited unequal numbers of patients from the different subspecialties—a result of each subspecialty having a different number of attending physicians and a different patient volume. Given randomization and use of similar patients across the study arms, however, this likely did not present any significant bias. Second, each patient completed a tablet survey or a paper survey but not both, and therefore we could not compare a patient’s performance on the 2 modalities. However, the burden of completing the same survey more than once likely would have lowered our participation rate and introduced additional biases we wanted to avoid. Third, despite our attempt to mimic the look of a paper survey, the tablet’s user interface presented several potential difficulties. For example, its small text and small answer buttons may have been limiting for patients with poor vision. These design features emphasize the importance of having a user interface that can be adapted to the individual, regardless of handicap. Indeed, adaptability is a potential strength of digital interfaces. For adaptability, an interface designer can use large, scalable text and add audio prompts and other features.
Our findings can be useful in evaluating patient responsiveness to surveys administered on digital tablets in an outpatient clinic setting. In this prospective, randomized study, we found that, for survey completion, use of a tablet device did not require more time than use of a paper form. In addition, the administration modalities had similar completion and error rates for a variety of orthopedic outcomes surveys. We did not activate digital features that would have given unfair advantage to the digital data collection modality. We also found a strong preference for use of technology in PRO data collection, and this may help improve collection rates. Last, though optimizing the flow of patients in our clinic was not a strict research metric, we prioritized making sure patients were not spending any more time completing these surveys than in the past. Given the potential benefits of digital surveys—immediate and accurate transfer of collected data into multiple databases, including the patient’s electronic medical record—our experience supports continuing validation of these instruments for potential wider use.
Am J Orthop. 2016;45(7):E451-E457. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. Howie L, Hirsch B, Locklear T, Abernethy AP. Assessing the value of patient-generated data to comparative effectiveness research. Health Aff (Millwood). 2014;33(7):1220-1228.
2. Higginson IJ, Carr AJ. Measuring quality of life: using quality of life measures in the clinical setting. BMJ. 2001;322(7297):1297-1300.
3. Revicki D, Hays RD, Cella D, Sloan J. Recommended methods for determining responsiveness and minimally important differences for patient-reported outcomes. J Clin Epidemiol. 2008;61(2):102-109.
4. Guyatt GH, Feeny DH, Patrick DL. Measuring health-related quality of life. Ann Intern Med. 1993;118(8):622-629.
5. Paudel D, Ahmed M, Pradhan A, Lal Dangol R. Successful use of tablet personal computers and wireless technologies for the 2011 Nepal Demographic and Health Survey. Glob Heal Sci Pract. 2013;1(2):277-284.
6. Dy CJ, Schmicker T, Tran Q, Chadwick B, Daluiski A. The use of a tablet computer to complete the DASH questionnaire. J Hand Surg Am. 2012;37(12):2589-2594.
7. Aktas A, Hullihen B, Shrotriya S, Thomas S, Walsh D, Estfan B. Connected health: cancer symptom and quality-of-life assessment using a tablet computer: a pilot study. Am J Hosp Palliat Care. 2015;32(2):189-197.
8. Basnov M, Kongsved SM, Bech P, Hjollund NH. Reliability of Short Form-36 in an internet- and a pen-and-paper version. Inform Health Soc Care. 2009;34(1):53-58.
9. Bellamy N, Wilson C, Hendrikz J, et al; EDC Study Group. Osteoarthritis Index delivered by mobile phone (m-WOMAC) is valid, reliable, and responsive. J Clin Epidemiol. 2011;64(2):182-190.
10. Fanning J, McAuley E. A comparison of tablet computer and paper-based questionnaires in healthy aging research. JMIR Res Protoc. 2014;3(3):e38.
11. Griffiths-Jones W, Norton MR, Fern ED, Williams DH. The equivalence of remote electronic and paper patient reported outcome (PRO) collection. J Arthroplasty. 2014;29(11):2136-2139.
12. Marsh JD, Bryant DM, Macdonald SJ, Naudie DD. Patients respond similarly to paper and electronic versions of the WOMAC and SF-12 following total joint arthroplasty. J Arthroplasty. 2014;29(4):670-673.
13. Olajos-Clow J, Minard J, Szpiro K, et al. Validation of an electronic version of the Mini Asthma Quality of Life Questionnaire. Respir Med. 2010;104(5):658-667.
14. Shervin N, Dorrwachter J, Bragdon CR, Shervin D, Zurakowski D, Malchau H. Comparison of paper and computer-based questionnaire modes for measuring health outcomes in patients undergoing total hip arthroplasty. J Bone Joint Surg Am. 2011;93(3):285-293.
15. Suresh K. An overview of randomization techniques: an unbiased assessment of outcome in clinical research. J Hum Reprod Sci. 2011;4(1):8-11.
16. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377-381.
17. Pickard AS, Neary MP, Cella D. Estimation of minimally important differences in EQ-5D utility and VAS scores in cancer. Health Qual Life Outcomes. 2007;5:70.
18. Abdel Messih M, Naylor JM, Descallar J, Manickam A, Mittal R, Harris IA. Mail versus telephone administration of the Oxford Knee and Hip Scores. J Arthroplasty. 2014;29(3):491-494.
19. Kongsved SM, Basnov M, Holm-Christensen K, Hjollund NH. Response rate and completeness of questionnaires: a randomized study of internet versus paper-and-pencil versions. J Med Internet Res. 2007;9(3):e25.
20. Theiler R, Bischoff-Ferrari HA, Good M, Bellamy N. Responsiveness of the electronic touch screen WOMAC 3.1 OA Index in a short term clinical trial with rofecoxib. Osteoarthritis Cartilage. 2004;12(11):912-916.
21. Ryan JM, Corry JR, Attewell R, Smithson MJ. A comparison of an electronic version of the SF-36 General Health Questionnaire to the standard paper version. Qual Life Res. 2002;11(1):19-26.
22. Wilson AS, Kitas GD, Carruthers DM, et al. Computerized information-gathering in specialist rheumatology clinics: an initial evaluation of an electronic version of the Short Form 36. Rheumatology. 2002;41(3):268-273.
23. Angst F, Goldhahn J, Drerup S, Flury M, Schwyzer HK, Simmen BR. How sharp is the short QuickDASH? A refined content and validity analysis of the Short Form of the Disabilities of the Shoulder, Arm and Hand questionnaire in the strata of symptoms and function and specific joint conditions. Qual Life Res. 2009;18(8):1043-1051.
1. Howie L, Hirsch B, Locklear T, Abernethy AP. Assessing the value of patient-generated data to comparative effectiveness research. Health Aff (Millwood). 2014;33(7):1220-1228.
2. Higginson IJ, Carr AJ. Measuring quality of life: using quality of life measures in the clinical setting. BMJ. 2001;322(7297):1297-1300.
3. Revicki D, Hays RD, Cella D, Sloan J. Recommended methods for determining responsiveness and minimally important differences for patient-reported outcomes. J Clin Epidemiol. 2008;61(2):102-109.
4. Guyatt GH, Feeny DH, Patrick DL. Measuring health-related quality of life. Ann Intern Med. 1993;118(8):622-629.
5. Paudel D, Ahmed M, Pradhan A, Lal Dangol R. Successful use of tablet personal computers and wireless technologies for the 2011 Nepal Demographic and Health Survey. Glob Heal Sci Pract. 2013;1(2):277-284.
6. Dy CJ, Schmicker T, Tran Q, Chadwick B, Daluiski A. The use of a tablet computer to complete the DASH questionnaire. J Hand Surg Am. 2012;37(12):2589-2594.
7. Aktas A, Hullihen B, Shrotriya S, Thomas S, Walsh D, Estfan B. Connected health: cancer symptom and quality-of-life assessment using a tablet computer: a pilot study. Am J Hosp Palliat Care. 2015;32(2):189-197.
8. Basnov M, Kongsved SM, Bech P, Hjollund NH. Reliability of Short Form-36 in an internet- and a pen-and-paper version. Inform Health Soc Care. 2009;34(1):53-58.
9. Bellamy N, Wilson C, Hendrikz J, et al; EDC Study Group. Osteoarthritis Index delivered by mobile phone (m-WOMAC) is valid, reliable, and responsive. J Clin Epidemiol. 2011;64(2):182-190.
10. Fanning J, McAuley E. A comparison of tablet computer and paper-based questionnaires in healthy aging research. JMIR Res Protoc. 2014;3(3):e38.
11. Griffiths-Jones W, Norton MR, Fern ED, Williams DH. The equivalence of remote electronic and paper patient reported outcome (PRO) collection. J Arthroplasty. 2014;29(11):2136-2139.
12. Marsh JD, Bryant DM, Macdonald SJ, Naudie DD. Patients respond similarly to paper and electronic versions of the WOMAC and SF-12 following total joint arthroplasty. J Arthroplasty. 2014;29(4):670-673.
13. Olajos-Clow J, Minard J, Szpiro K, et al. Validation of an electronic version of the Mini Asthma Quality of Life Questionnaire. Respir Med. 2010;104(5):658-667.
14. Shervin N, Dorrwachter J, Bragdon CR, Shervin D, Zurakowski D, Malchau H. Comparison of paper and computer-based questionnaire modes for measuring health outcomes in patients undergoing total hip arthroplasty. J Bone Joint Surg Am. 2011;93(3):285-293.
15. Suresh K. An overview of randomization techniques: an unbiased assessment of outcome in clinical research. J Hum Reprod Sci. 2011;4(1):8-11.
16. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377-381.
17. Pickard AS, Neary MP, Cella D. Estimation of minimally important differences in EQ-5D utility and VAS scores in cancer. Health Qual Life Outcomes. 2007;5:70.
18. Abdel Messih M, Naylor JM, Descallar J, Manickam A, Mittal R, Harris IA. Mail versus telephone administration of the Oxford Knee and Hip Scores. J Arthroplasty. 2014;29(3):491-494.
19. Kongsved SM, Basnov M, Holm-Christensen K, Hjollund NH. Response rate and completeness of questionnaires: a randomized study of internet versus paper-and-pencil versions. J Med Internet Res. 2007;9(3):e25.
20. Theiler R, Bischoff-Ferrari HA, Good M, Bellamy N. Responsiveness of the electronic touch screen WOMAC 3.1 OA Index in a short term clinical trial with rofecoxib. Osteoarthritis Cartilage. 2004;12(11):912-916.
21. Ryan JM, Corry JR, Attewell R, Smithson MJ. A comparison of an electronic version of the SF-36 General Health Questionnaire to the standard paper version. Qual Life Res. 2002;11(1):19-26.
22. Wilson AS, Kitas GD, Carruthers DM, et al. Computerized information-gathering in specialist rheumatology clinics: an initial evaluation of an electronic version of the Short Form 36. Rheumatology. 2002;41(3):268-273.
23. Angst F, Goldhahn J, Drerup S, Flury M, Schwyzer HK, Simmen BR. How sharp is the short QuickDASH? A refined content and validity analysis of the Short Form of the Disabilities of the Shoulder, Arm and Hand questionnaire in the strata of symptoms and function and specific joint conditions. Qual Life Res. 2009;18(8):1043-1051.
Suicidal and asking for money for food
CASE Suicidal and hungry
Mr. L, age 59, attempts suicide by taking approximately 20 acetaminophen tablets of unknown dosage. He immediately comes to the emergency department where blood work reveals a 4-hour acetaminophen level of 94.8 μg/mL (therapeutic range, 10 to 30 μg/mL; toxic range, >150 μg/mL); administration of N-acetylcysteine is unnecessary. Mr. L is admitted to general medical services for monitoring and is transferred to our unit for psychiatric evaluation and management.
During our initial interview, Mr. L, who has a developmental disability, is grossly oriented and generally cooperative, reporting depressed mood with an irritable affect. He is preoccupied with having limited funds and repeatedly states he is worried that he can’t buy food, but says that the hospital could help by providing for him. Mr. L states that his depressed mood is directly related to his financial situation and, that if he had more money, he would not be suicidal. He cites worsening visual impairment that requires surgery as an additional stressor.
On several occasions, Mr. L states that the only way to help him is to give him $600 so that he can buy food and pay for medical treatment. Mr. L says he does not feel supported by his family, despite having a sister who lives nearby.
What would you include in the differential diagnosis for Mr. L?
a) major depressive disorder (MDD)
b) depression secondary to a medical condition
c) neurocognitive disorder
d) adjustment disorder with depressive features
e) factitious disorder
The authors’ observations
Our differential diagnosis included MDD, adjustment disorder, neurocognitive disorder, and factitious disorder. He did not meet criteria for MDD because he did not have excessive guilt, loss of interest, change in sleep or appetite, psychomotor dysregulation, or change in energy level. Although suicidal behavior could indicate MDD, the fact that he immediately walked to the hospital after taking an excessive amount of acetaminophen suggests that he did not want to die. Further, he attributed his suicidal thoughts to environmental stressors. Similarly, we ruled out adjustment disorder because he had no reported or observed changes in mood or anxiety. Although financial difficulties might have overwhelmed his limited coping abilities, he took too much acetaminophen to ensure that he was hospitalized. His motivation for seeking hospitalization ruled out factitious disorder. Mr. L has a developmental disability, but information obtained from collateral sources ruled out an acute change to cognitive functioning.
HISTORY Repeated admissions
Mr. L has a history of a psychiatric hospitalization 3 weeks prior to this admission. He presented to an emergency department stating that his blood glucose was low. Mr. L was noted to be confused and anxious and said he was convinced he was going to die. At that time, his thought content was hyper-religious and he claimed he could hear the devil. Mr. L was hospitalized and started on low-dosage risperidone. At discharge, he declined referral for outpatient mental health treatment because he denied having a mental illness. However, he was amenable to follow up at a wellness clinic.
Mr. L has worked at a local supermarket for 19 years and has lived independently throughout his adult life. After he returned to the community, he was repeatedly absent from work, which further exacerbated his financial strain. He attended a follow-up outpatient appointment but reported, “They didn’t help me,” although it was unclear what he meant.
Between admissions to our hospital, Mr. L had 2 visits to an emergency department, the first time saying he felt depressed and the second reporting he attempted suicide by taking 5 acetaminophen tablets. On both occasions he requested placement in a residential facility but was discharged home after an initial assessment. Emergency room records indicated that Mr. L stated, “If you cannot give me money for food, then there is no use and I would rather die.”
What is the most likely DSM-5 diagnosis for Mr. L?
a) schizophrenia
b) malingering
c) brief psychotic disorder
d) dependent personality disorder
The authors’ observations
Malingering in DSM-5 is defined as the “intentional production of false or grossly exaggerated physical or psychological symptoms, motivated by external incentives.”1 These external incentives include financial compensation, avoiding military duties, evading criminal charges, and avoiding work, and are collectively considered as secondary gain. Although not considered a diagnosis in the strictest sense, clinicians must differentiate malingering from other psychiatric disorders. In the literature, case reports describe patients who feigned an array of symptoms including those of posttraumatic stress disorder, paraphilias, cognitive dysfunction, depression, anxiety, and psychosis.2-5
In Mr. L’s case, malingering presented as suicidal behavior with an inadvertently high fatality risk. Notably, Mr. L came to an emergency room a few days before this admission after swallowing 5 acetaminophen tablets in a suicide attempt, which did not lead to a medical or psychiatric hospitalization. In an attempt to ensure admission, Mr. L then took a potentially lethal dose of 20 acetaminophen tablets. In our assessment and according to his statements, the primary motivation for the suicide attempt was to obtain reliable food and housing. Mr. L’s developmental disability might have contributed to a relative lack of understanding of the consequences of his actions. In addition, poor overall communication and coping skills led to an exaggerated response to psychosocial stressors.
Malingering and suicide attempts
Few studies have investigated malingering in regards to suicide and other psychiatric emergencies. In a study of 227 consecutive psychiatric emergencies assessed for evidence of malingering, 13% were thought to be feigned or exaggerated.6 Interestingly, the most commonly reported secondary gain was food and shelter, similar to Mr. L. This study did not report the types of psychiatric emergencies, therefore suicidal actions associated with malingering could not be evaluated.
In another study, 40 patients hospitalized for suicidal ideation (n = 29, 72%) or suicidal gestures (n = 11, 28%) in a large, urban tertiary care center were evaluated for malingering by anonymous report of feigned or exaggerated symptoms.7 Most of these patients were diagnosed with a mood disorder (28%) and/or an adjustment disorder (53%). Four (10%) admitted to malingering. Among the malingerers, reasons for feigning illness included:
- wanting to be hospitalized
- wanting to make someone angry or feel sorry
- gaining access to detoxification programs
- getting treatment for emotional problems.
Interestingly, an analysis of demographic factors associated with malingering reveals an association with suicide attempts but not persistent suicidal ideations. This could be because of selection bias; patients who reported a suicide attempt might be more likely to be hospitalized.
A follow-up study8 evaluated 50 additional consecutive psychiatric inpatients admitted to the same tertiary care hospital for suicide risk. Unlike the previous study, a larger proportion of these patients had made a suicide attempt (n = 21, 42%) and a greater number had made a previous suicide attempt (n = 33, 66%). Primary mood disorders comprised most of the psychiatric diagnoses (n = 28, 56%). In this study, the exact nature of the suicide gestures was not documented, leaving open the question of lethality of the attempts. These studies do not suggest that those who malinger are not at risk for suicide, only that these patients tend to exaggerate the severity of their ideations or behaviors.
OUTCOME Reluctantly discharged
We contact Mr. L’s siblings, who offer to provide temporary housing and financial support and assist him with medical needs. This abated Mr. L’s suicidal ideation; however, he wishes to remain in the hospital with the goals of obtaining eyeglasses and dentures. We explain that psychiatric hospitalization is no longer indicated and he is discharged.
Which of the following is the most effective management strategy for malingering?
a) direct confrontation of the malingering patient
b) immediate discharge once malingering is identified
c) evaluation for possible comorbid psychiatric conditions
d) neuropsychiatric consultation
The authors’ observations
The challenges of treating patients who malinger include clinician uncertainty in making the diagnosis and high variability in occurrence across settings (Table 1). Current estimates indicate that 4% to 8% of medical and psychiatric cases not involved in litigation or compensation have an element of feigned symptoms.3,9 The rate could be higher in specific circumstances such as medicolegal disputes and criminal cases.10
The societal impact of malingering is significant. Therefore, identifying these patients is an important clinical intervention that can have a wide impact.11 However, it is also important to acknowledge that genuine psychiatric illness could be comorbid with malingering. Although differentiating a patient’s true from feigned symptoms can be difficult, it is critical to carefully evaluate the patient in order to provide the best treatment.
It seems that physicians can detect malingering, but documentation often is not provided. In the Rissmiller et al study,7 all 4 cases of malingering were identified retrospectively by study psychiatrists; however, none of their medical records included documentation of malingering, a finding also reported in the Yates et al study.6 Also concerning, the clinicians suspected malingering in some patients who were not feigning symptoms, suggesting that a relatively high threshold is necessary for making the diagnosis.
How to help patients who malinger
Identifying malingering in patients with obvious secondary gain is important to prevent exposure to potential adverse effects of medication and unnecessary use of medical resources. In addition, obtaining collateral information, records from previous admissions or outpatient treatment, and psychological testing adds to the body of evidence suggesting malingering. We also recommend a comprehensive psychosocial evaluation to identify the presence of secondary gain.
Management of malingering (Table 2) includes building a strong therapeutic alliance, exploring reasons for feigning symptoms, open discussion of inciting external factors such as interpersonal conflict or difficulties at work, and/or confrontation.10 In addition, supportive psychotherapy might help strengthen coping mechanisms and problem solving strategies, thereby removing the need for secondary gain.12 Additionally, face-saving mechanisms that allow the patient to discard their feigned symptoms, or enable the person to alter his (her) history, could be to his benefit. Lastly, and importantly, clinicians should focus efforts on ruling out or effectively treating comorbid psychiatric conditions.
From a risk management standpoint, include all available data to support the malingering diagnosis in your progress notes and discharge summaries. A clinician seeking to discharge a patient suspected of malingering who is still endorsing suicidal or homicidal intent will benefit from administrative review, including legal counsel to mitigate risk, and be more confident discharging somebody assessed to be malingering.
We recognize that certain patients could trigger countertransference reactions that impel clinicians to take on a significant caretaking role. Patients skillful at deception could manifest a desire to rescue or save them. In these instances, clinicians should examine why and how these feelings have come about, particularly if there is evidence that the individual could be attempting to use the interaction to achieve secondary gain. Awareness of these feelings could help with the diagnostic formulation. Moreover, a clinician who has such strong feelings might be tempted to abet a patient in achieving the secondary gain, or protect him (her) from the natural consequences of individual’s deception (eg, not discharging a hospitalized patient). This is counter-therapeutic and reinforces maladaptive behaviors and coping processes.13
1. Diagnostic and statistical manual of mental disorders, 5th ed. Washington DC: American Psychiatric Association; 2013.
2. Fedoroff JP, Hanson A, McGuire M, et al. Simulated paraphilias: a preliminary study of patients who imitate or exaggerate paraphilic symptoms and behaviors. J Forensic Sci. 1992;37(3):902-911.
3. Mittenberg W, Patton C, Canyock EM, et al. Base rates of malingering and symptom exaggeration. J Clin Exp Neuropsychol. 2002;24(8):1094-1102.
4. Waite S, Geddes A. Malingered psychosis leading to involuntary psychiatric hospitalization. Australas Psychiatry. 2006;14(4):419-421.
5. Hall RC, Hall RC. Malingering of PTSD: forensic and diagnostic consideration, characteristics of malingerers and clinical presentations. Gen Hosp Psychiatry. 2006;28(6):525-535.
6. Yates BD, Nordquist CR, Shultz-Ross RA. Feigned psychiatric symptoms in the emergency room. Psychiatr Serv. 1996;47(9):998-1000.
7. Rissmiller DJ, Wayslow A, Madison H, et al. Prevalence of malingering in inpatient suicidal ideators and attempters. Crisis. 1998;19(2):62-66.
8. Rissmiller D, Steer RA, Friedman M, et al. Prevalence of malingering in suicidal psychiatric inpatients: a replication. Psychol Rep. 1999;84(3 pt 1):726-730.
9. Sullivan K, Lange RT, Dawes S. Methods of detecting malingering and estimated symptom exaggeration base rates in Australia. Journal of Forensic Neuropsychology. 2007;4(4):49-70.
10. Bass C, Halligan P. Factitious disorders and malingering: challenges for clinical assessment and management. Lancet. 2014;383(9926):1422-1432.
11. Chafetz M, Underhill J. Estimated costs of malingered disability. Arch Clin Neuropsychol. 2013;28(7):633-639.
12. Peebles R, Sabel
13. Malone RD, Lange CL. A clinical approach to the malingering patient. J Am Acad Psychoanal Dyn Psychiatry. 2007;35(1):13-21.
CASE Suicidal and hungry
Mr. L, age 59, attempts suicide by taking approximately 20 acetaminophen tablets of unknown dosage. He immediately comes to the emergency department where blood work reveals a 4-hour acetaminophen level of 94.8 μg/mL (therapeutic range, 10 to 30 μg/mL; toxic range, >150 μg/mL); administration of N-acetylcysteine is unnecessary. Mr. L is admitted to general medical services for monitoring and is transferred to our unit for psychiatric evaluation and management.
During our initial interview, Mr. L, who has a developmental disability, is grossly oriented and generally cooperative, reporting depressed mood with an irritable affect. He is preoccupied with having limited funds and repeatedly states he is worried that he can’t buy food, but says that the hospital could help by providing for him. Mr. L states that his depressed mood is directly related to his financial situation and, that if he had more money, he would not be suicidal. He cites worsening visual impairment that requires surgery as an additional stressor.
On several occasions, Mr. L states that the only way to help him is to give him $600 so that he can buy food and pay for medical treatment. Mr. L says he does not feel supported by his family, despite having a sister who lives nearby.
What would you include in the differential diagnosis for Mr. L?
a) major depressive disorder (MDD)
b) depression secondary to a medical condition
c) neurocognitive disorder
d) adjustment disorder with depressive features
e) factitious disorder
The authors’ observations
Our differential diagnosis included MDD, adjustment disorder, neurocognitive disorder, and factitious disorder. He did not meet criteria for MDD because he did not have excessive guilt, loss of interest, change in sleep or appetite, psychomotor dysregulation, or change in energy level. Although suicidal behavior could indicate MDD, the fact that he immediately walked to the hospital after taking an excessive amount of acetaminophen suggests that he did not want to die. Further, he attributed his suicidal thoughts to environmental stressors. Similarly, we ruled out adjustment disorder because he had no reported or observed changes in mood or anxiety. Although financial difficulties might have overwhelmed his limited coping abilities, he took too much acetaminophen to ensure that he was hospitalized. His motivation for seeking hospitalization ruled out factitious disorder. Mr. L has a developmental disability, but information obtained from collateral sources ruled out an acute change to cognitive functioning.
HISTORY Repeated admissions
Mr. L has a history of a psychiatric hospitalization 3 weeks prior to this admission. He presented to an emergency department stating that his blood glucose was low. Mr. L was noted to be confused and anxious and said he was convinced he was going to die. At that time, his thought content was hyper-religious and he claimed he could hear the devil. Mr. L was hospitalized and started on low-dosage risperidone. At discharge, he declined referral for outpatient mental health treatment because he denied having a mental illness. However, he was amenable to follow up at a wellness clinic.
Mr. L has worked at a local supermarket for 19 years and has lived independently throughout his adult life. After he returned to the community, he was repeatedly absent from work, which further exacerbated his financial strain. He attended a follow-up outpatient appointment but reported, “They didn’t help me,” although it was unclear what he meant.
Between admissions to our hospital, Mr. L had 2 visits to an emergency department, the first time saying he felt depressed and the second reporting he attempted suicide by taking 5 acetaminophen tablets. On both occasions he requested placement in a residential facility but was discharged home after an initial assessment. Emergency room records indicated that Mr. L stated, “If you cannot give me money for food, then there is no use and I would rather die.”
What is the most likely DSM-5 diagnosis for Mr. L?
a) schizophrenia
b) malingering
c) brief psychotic disorder
d) dependent personality disorder
The authors’ observations
Malingering in DSM-5 is defined as the “intentional production of false or grossly exaggerated physical or psychological symptoms, motivated by external incentives.”1 These external incentives include financial compensation, avoiding military duties, evading criminal charges, and avoiding work, and are collectively considered as secondary gain. Although not considered a diagnosis in the strictest sense, clinicians must differentiate malingering from other psychiatric disorders. In the literature, case reports describe patients who feigned an array of symptoms including those of posttraumatic stress disorder, paraphilias, cognitive dysfunction, depression, anxiety, and psychosis.2-5
In Mr. L’s case, malingering presented as suicidal behavior with an inadvertently high fatality risk. Notably, Mr. L came to an emergency room a few days before this admission after swallowing 5 acetaminophen tablets in a suicide attempt, which did not lead to a medical or psychiatric hospitalization. In an attempt to ensure admission, Mr. L then took a potentially lethal dose of 20 acetaminophen tablets. In our assessment and according to his statements, the primary motivation for the suicide attempt was to obtain reliable food and housing. Mr. L’s developmental disability might have contributed to a relative lack of understanding of the consequences of his actions. In addition, poor overall communication and coping skills led to an exaggerated response to psychosocial stressors.
Malingering and suicide attempts
Few studies have investigated malingering in regards to suicide and other psychiatric emergencies. In a study of 227 consecutive psychiatric emergencies assessed for evidence of malingering, 13% were thought to be feigned or exaggerated.6 Interestingly, the most commonly reported secondary gain was food and shelter, similar to Mr. L. This study did not report the types of psychiatric emergencies, therefore suicidal actions associated with malingering could not be evaluated.
In another study, 40 patients hospitalized for suicidal ideation (n = 29, 72%) or suicidal gestures (n = 11, 28%) in a large, urban tertiary care center were evaluated for malingering by anonymous report of feigned or exaggerated symptoms.7 Most of these patients were diagnosed with a mood disorder (28%) and/or an adjustment disorder (53%). Four (10%) admitted to malingering. Among the malingerers, reasons for feigning illness included:
- wanting to be hospitalized
- wanting to make someone angry or feel sorry
- gaining access to detoxification programs
- getting treatment for emotional problems.
Interestingly, an analysis of demographic factors associated with malingering reveals an association with suicide attempts but not persistent suicidal ideations. This could be because of selection bias; patients who reported a suicide attempt might be more likely to be hospitalized.
A follow-up study8 evaluated 50 additional consecutive psychiatric inpatients admitted to the same tertiary care hospital for suicide risk. Unlike the previous study, a larger proportion of these patients had made a suicide attempt (n = 21, 42%) and a greater number had made a previous suicide attempt (n = 33, 66%). Primary mood disorders comprised most of the psychiatric diagnoses (n = 28, 56%). In this study, the exact nature of the suicide gestures was not documented, leaving open the question of lethality of the attempts. These studies do not suggest that those who malinger are not at risk for suicide, only that these patients tend to exaggerate the severity of their ideations or behaviors.
OUTCOME Reluctantly discharged
We contact Mr. L’s siblings, who offer to provide temporary housing and financial support and assist him with medical needs. This abated Mr. L’s suicidal ideation; however, he wishes to remain in the hospital with the goals of obtaining eyeglasses and dentures. We explain that psychiatric hospitalization is no longer indicated and he is discharged.
Which of the following is the most effective management strategy for malingering?
a) direct confrontation of the malingering patient
b) immediate discharge once malingering is identified
c) evaluation for possible comorbid psychiatric conditions
d) neuropsychiatric consultation
The authors’ observations
The challenges of treating patients who malinger include clinician uncertainty in making the diagnosis and high variability in occurrence across settings (Table 1). Current estimates indicate that 4% to 8% of medical and psychiatric cases not involved in litigation or compensation have an element of feigned symptoms.3,9 The rate could be higher in specific circumstances such as medicolegal disputes and criminal cases.10
The societal impact of malingering is significant. Therefore, identifying these patients is an important clinical intervention that can have a wide impact.11 However, it is also important to acknowledge that genuine psychiatric illness could be comorbid with malingering. Although differentiating a patient’s true from feigned symptoms can be difficult, it is critical to carefully evaluate the patient in order to provide the best treatment.
It seems that physicians can detect malingering, but documentation often is not provided. In the Rissmiller et al study,7 all 4 cases of malingering were identified retrospectively by study psychiatrists; however, none of their medical records included documentation of malingering, a finding also reported in the Yates et al study.6 Also concerning, the clinicians suspected malingering in some patients who were not feigning symptoms, suggesting that a relatively high threshold is necessary for making the diagnosis.
How to help patients who malinger
Identifying malingering in patients with obvious secondary gain is important to prevent exposure to potential adverse effects of medication and unnecessary use of medical resources. In addition, obtaining collateral information, records from previous admissions or outpatient treatment, and psychological testing adds to the body of evidence suggesting malingering. We also recommend a comprehensive psychosocial evaluation to identify the presence of secondary gain.
Management of malingering (Table 2) includes building a strong therapeutic alliance, exploring reasons for feigning symptoms, open discussion of inciting external factors such as interpersonal conflict or difficulties at work, and/or confrontation.10 In addition, supportive psychotherapy might help strengthen coping mechanisms and problem solving strategies, thereby removing the need for secondary gain.12 Additionally, face-saving mechanisms that allow the patient to discard their feigned symptoms, or enable the person to alter his (her) history, could be to his benefit. Lastly, and importantly, clinicians should focus efforts on ruling out or effectively treating comorbid psychiatric conditions.
From a risk management standpoint, include all available data to support the malingering diagnosis in your progress notes and discharge summaries. A clinician seeking to discharge a patient suspected of malingering who is still endorsing suicidal or homicidal intent will benefit from administrative review, including legal counsel to mitigate risk, and be more confident discharging somebody assessed to be malingering.
We recognize that certain patients could trigger countertransference reactions that impel clinicians to take on a significant caretaking role. Patients skillful at deception could manifest a desire to rescue or save them. In these instances, clinicians should examine why and how these feelings have come about, particularly if there is evidence that the individual could be attempting to use the interaction to achieve secondary gain. Awareness of these feelings could help with the diagnostic formulation. Moreover, a clinician who has such strong feelings might be tempted to abet a patient in achieving the secondary gain, or protect him (her) from the natural consequences of individual’s deception (eg, not discharging a hospitalized patient). This is counter-therapeutic and reinforces maladaptive behaviors and coping processes.13
CASE Suicidal and hungry
Mr. L, age 59, attempts suicide by taking approximately 20 acetaminophen tablets of unknown dosage. He immediately comes to the emergency department where blood work reveals a 4-hour acetaminophen level of 94.8 μg/mL (therapeutic range, 10 to 30 μg/mL; toxic range, >150 μg/mL); administration of N-acetylcysteine is unnecessary. Mr. L is admitted to general medical services for monitoring and is transferred to our unit for psychiatric evaluation and management.
During our initial interview, Mr. L, who has a developmental disability, is grossly oriented and generally cooperative, reporting depressed mood with an irritable affect. He is preoccupied with having limited funds and repeatedly states he is worried that he can’t buy food, but says that the hospital could help by providing for him. Mr. L states that his depressed mood is directly related to his financial situation and, that if he had more money, he would not be suicidal. He cites worsening visual impairment that requires surgery as an additional stressor.
On several occasions, Mr. L states that the only way to help him is to give him $600 so that he can buy food and pay for medical treatment. Mr. L says he does not feel supported by his family, despite having a sister who lives nearby.
What would you include in the differential diagnosis for Mr. L?
a) major depressive disorder (MDD)
b) depression secondary to a medical condition
c) neurocognitive disorder
d) adjustment disorder with depressive features
e) factitious disorder
The authors’ observations
Our differential diagnosis included MDD, adjustment disorder, neurocognitive disorder, and factitious disorder. He did not meet criteria for MDD because he did not have excessive guilt, loss of interest, change in sleep or appetite, psychomotor dysregulation, or change in energy level. Although suicidal behavior could indicate MDD, the fact that he immediately walked to the hospital after taking an excessive amount of acetaminophen suggests that he did not want to die. Further, he attributed his suicidal thoughts to environmental stressors. Similarly, we ruled out adjustment disorder because he had no reported or observed changes in mood or anxiety. Although financial difficulties might have overwhelmed his limited coping abilities, he took too much acetaminophen to ensure that he was hospitalized. His motivation for seeking hospitalization ruled out factitious disorder. Mr. L has a developmental disability, but information obtained from collateral sources ruled out an acute change to cognitive functioning.
HISTORY Repeated admissions
Mr. L has a history of a psychiatric hospitalization 3 weeks prior to this admission. He presented to an emergency department stating that his blood glucose was low. Mr. L was noted to be confused and anxious and said he was convinced he was going to die. At that time, his thought content was hyper-religious and he claimed he could hear the devil. Mr. L was hospitalized and started on low-dosage risperidone. At discharge, he declined referral for outpatient mental health treatment because he denied having a mental illness. However, he was amenable to follow up at a wellness clinic.
Mr. L has worked at a local supermarket for 19 years and has lived independently throughout his adult life. After he returned to the community, he was repeatedly absent from work, which further exacerbated his financial strain. He attended a follow-up outpatient appointment but reported, “They didn’t help me,” although it was unclear what he meant.
Between admissions to our hospital, Mr. L had 2 visits to an emergency department, the first time saying he felt depressed and the second reporting he attempted suicide by taking 5 acetaminophen tablets. On both occasions he requested placement in a residential facility but was discharged home after an initial assessment. Emergency room records indicated that Mr. L stated, “If you cannot give me money for food, then there is no use and I would rather die.”
What is the most likely DSM-5 diagnosis for Mr. L?
a) schizophrenia
b) malingering
c) brief psychotic disorder
d) dependent personality disorder
The authors’ observations
Malingering in DSM-5 is defined as the “intentional production of false or grossly exaggerated physical or psychological symptoms, motivated by external incentives.”1 These external incentives include financial compensation, avoiding military duties, evading criminal charges, and avoiding work, and are collectively considered as secondary gain. Although not considered a diagnosis in the strictest sense, clinicians must differentiate malingering from other psychiatric disorders. In the literature, case reports describe patients who feigned an array of symptoms including those of posttraumatic stress disorder, paraphilias, cognitive dysfunction, depression, anxiety, and psychosis.2-5
In Mr. L’s case, malingering presented as suicidal behavior with an inadvertently high fatality risk. Notably, Mr. L came to an emergency room a few days before this admission after swallowing 5 acetaminophen tablets in a suicide attempt, which did not lead to a medical or psychiatric hospitalization. In an attempt to ensure admission, Mr. L then took a potentially lethal dose of 20 acetaminophen tablets. In our assessment and according to his statements, the primary motivation for the suicide attempt was to obtain reliable food and housing. Mr. L’s developmental disability might have contributed to a relative lack of understanding of the consequences of his actions. In addition, poor overall communication and coping skills led to an exaggerated response to psychosocial stressors.
Malingering and suicide attempts
Few studies have investigated malingering in regards to suicide and other psychiatric emergencies. In a study of 227 consecutive psychiatric emergencies assessed for evidence of malingering, 13% were thought to be feigned or exaggerated.6 Interestingly, the most commonly reported secondary gain was food and shelter, similar to Mr. L. This study did not report the types of psychiatric emergencies, therefore suicidal actions associated with malingering could not be evaluated.
In another study, 40 patients hospitalized for suicidal ideation (n = 29, 72%) or suicidal gestures (n = 11, 28%) in a large, urban tertiary care center were evaluated for malingering by anonymous report of feigned or exaggerated symptoms.7 Most of these patients were diagnosed with a mood disorder (28%) and/or an adjustment disorder (53%). Four (10%) admitted to malingering. Among the malingerers, reasons for feigning illness included:
- wanting to be hospitalized
- wanting to make someone angry or feel sorry
- gaining access to detoxification programs
- getting treatment for emotional problems.
Interestingly, an analysis of demographic factors associated with malingering reveals an association with suicide attempts but not persistent suicidal ideations. This could be because of selection bias; patients who reported a suicide attempt might be more likely to be hospitalized.
A follow-up study8 evaluated 50 additional consecutive psychiatric inpatients admitted to the same tertiary care hospital for suicide risk. Unlike the previous study, a larger proportion of these patients had made a suicide attempt (n = 21, 42%) and a greater number had made a previous suicide attempt (n = 33, 66%). Primary mood disorders comprised most of the psychiatric diagnoses (n = 28, 56%). In this study, the exact nature of the suicide gestures was not documented, leaving open the question of lethality of the attempts. These studies do not suggest that those who malinger are not at risk for suicide, only that these patients tend to exaggerate the severity of their ideations or behaviors.
OUTCOME Reluctantly discharged
We contact Mr. L’s siblings, who offer to provide temporary housing and financial support and assist him with medical needs. This abated Mr. L’s suicidal ideation; however, he wishes to remain in the hospital with the goals of obtaining eyeglasses and dentures. We explain that psychiatric hospitalization is no longer indicated and he is discharged.
Which of the following is the most effective management strategy for malingering?
a) direct confrontation of the malingering patient
b) immediate discharge once malingering is identified
c) evaluation for possible comorbid psychiatric conditions
d) neuropsychiatric consultation
The authors’ observations
The challenges of treating patients who malinger include clinician uncertainty in making the diagnosis and high variability in occurrence across settings (Table 1). Current estimates indicate that 4% to 8% of medical and psychiatric cases not involved in litigation or compensation have an element of feigned symptoms.3,9 The rate could be higher in specific circumstances such as medicolegal disputes and criminal cases.10
The societal impact of malingering is significant. Therefore, identifying these patients is an important clinical intervention that can have a wide impact.11 However, it is also important to acknowledge that genuine psychiatric illness could be comorbid with malingering. Although differentiating a patient’s true from feigned symptoms can be difficult, it is critical to carefully evaluate the patient in order to provide the best treatment.
It seems that physicians can detect malingering, but documentation often is not provided. In the Rissmiller et al study,7 all 4 cases of malingering were identified retrospectively by study psychiatrists; however, none of their medical records included documentation of malingering, a finding also reported in the Yates et al study.6 Also concerning, the clinicians suspected malingering in some patients who were not feigning symptoms, suggesting that a relatively high threshold is necessary for making the diagnosis.
How to help patients who malinger
Identifying malingering in patients with obvious secondary gain is important to prevent exposure to potential adverse effects of medication and unnecessary use of medical resources. In addition, obtaining collateral information, records from previous admissions or outpatient treatment, and psychological testing adds to the body of evidence suggesting malingering. We also recommend a comprehensive psychosocial evaluation to identify the presence of secondary gain.
Management of malingering (Table 2) includes building a strong therapeutic alliance, exploring reasons for feigning symptoms, open discussion of inciting external factors such as interpersonal conflict or difficulties at work, and/or confrontation.10 In addition, supportive psychotherapy might help strengthen coping mechanisms and problem solving strategies, thereby removing the need for secondary gain.12 Additionally, face-saving mechanisms that allow the patient to discard their feigned symptoms, or enable the person to alter his (her) history, could be to his benefit. Lastly, and importantly, clinicians should focus efforts on ruling out or effectively treating comorbid psychiatric conditions.
From a risk management standpoint, include all available data to support the malingering diagnosis in your progress notes and discharge summaries. A clinician seeking to discharge a patient suspected of malingering who is still endorsing suicidal or homicidal intent will benefit from administrative review, including legal counsel to mitigate risk, and be more confident discharging somebody assessed to be malingering.
We recognize that certain patients could trigger countertransference reactions that impel clinicians to take on a significant caretaking role. Patients skillful at deception could manifest a desire to rescue or save them. In these instances, clinicians should examine why and how these feelings have come about, particularly if there is evidence that the individual could be attempting to use the interaction to achieve secondary gain. Awareness of these feelings could help with the diagnostic formulation. Moreover, a clinician who has such strong feelings might be tempted to abet a patient in achieving the secondary gain, or protect him (her) from the natural consequences of individual’s deception (eg, not discharging a hospitalized patient). This is counter-therapeutic and reinforces maladaptive behaviors and coping processes.13
1. Diagnostic and statistical manual of mental disorders, 5th ed. Washington DC: American Psychiatric Association; 2013.
2. Fedoroff JP, Hanson A, McGuire M, et al. Simulated paraphilias: a preliminary study of patients who imitate or exaggerate paraphilic symptoms and behaviors. J Forensic Sci. 1992;37(3):902-911.
3. Mittenberg W, Patton C, Canyock EM, et al. Base rates of malingering and symptom exaggeration. J Clin Exp Neuropsychol. 2002;24(8):1094-1102.
4. Waite S, Geddes A. Malingered psychosis leading to involuntary psychiatric hospitalization. Australas Psychiatry. 2006;14(4):419-421.
5. Hall RC, Hall RC. Malingering of PTSD: forensic and diagnostic consideration, characteristics of malingerers and clinical presentations. Gen Hosp Psychiatry. 2006;28(6):525-535.
6. Yates BD, Nordquist CR, Shultz-Ross RA. Feigned psychiatric symptoms in the emergency room. Psychiatr Serv. 1996;47(9):998-1000.
7. Rissmiller DJ, Wayslow A, Madison H, et al. Prevalence of malingering in inpatient suicidal ideators and attempters. Crisis. 1998;19(2):62-66.
8. Rissmiller D, Steer RA, Friedman M, et al. Prevalence of malingering in suicidal psychiatric inpatients: a replication. Psychol Rep. 1999;84(3 pt 1):726-730.
9. Sullivan K, Lange RT, Dawes S. Methods of detecting malingering and estimated symptom exaggeration base rates in Australia. Journal of Forensic Neuropsychology. 2007;4(4):49-70.
10. Bass C, Halligan P. Factitious disorders and malingering: challenges for clinical assessment and management. Lancet. 2014;383(9926):1422-1432.
11. Chafetz M, Underhill J. Estimated costs of malingered disability. Arch Clin Neuropsychol. 2013;28(7):633-639.
12. Peebles R, Sabel
13. Malone RD, Lange CL. A clinical approach to the malingering patient. J Am Acad Psychoanal Dyn Psychiatry. 2007;35(1):13-21.
1. Diagnostic and statistical manual of mental disorders, 5th ed. Washington DC: American Psychiatric Association; 2013.
2. Fedoroff JP, Hanson A, McGuire M, et al. Simulated paraphilias: a preliminary study of patients who imitate or exaggerate paraphilic symptoms and behaviors. J Forensic Sci. 1992;37(3):902-911.
3. Mittenberg W, Patton C, Canyock EM, et al. Base rates of malingering and symptom exaggeration. J Clin Exp Neuropsychol. 2002;24(8):1094-1102.
4. Waite S, Geddes A. Malingered psychosis leading to involuntary psychiatric hospitalization. Australas Psychiatry. 2006;14(4):419-421.
5. Hall RC, Hall RC. Malingering of PTSD: forensic and diagnostic consideration, characteristics of malingerers and clinical presentations. Gen Hosp Psychiatry. 2006;28(6):525-535.
6. Yates BD, Nordquist CR, Shultz-Ross RA. Feigned psychiatric symptoms in the emergency room. Psychiatr Serv. 1996;47(9):998-1000.
7. Rissmiller DJ, Wayslow A, Madison H, et al. Prevalence of malingering in inpatient suicidal ideators and attempters. Crisis. 1998;19(2):62-66.
8. Rissmiller D, Steer RA, Friedman M, et al. Prevalence of malingering in suicidal psychiatric inpatients: a replication. Psychol Rep. 1999;84(3 pt 1):726-730.
9. Sullivan K, Lange RT, Dawes S. Methods of detecting malingering and estimated symptom exaggeration base rates in Australia. Journal of Forensic Neuropsychology. 2007;4(4):49-70.
10. Bass C, Halligan P. Factitious disorders and malingering: challenges for clinical assessment and management. Lancet. 2014;383(9926):1422-1432.
11. Chafetz M, Underhill J. Estimated costs of malingered disability. Arch Clin Neuropsychol. 2013;28(7):633-639.
12. Peebles R, Sabel
13. Malone RD, Lange CL. A clinical approach to the malingering patient. J Am Acad Psychoanal Dyn Psychiatry. 2007;35(1):13-21.