User login
Pembrolizumab is the first immune checkpoint inhibitor to receive approval for head and neck cancer
The first immune checkpoint inhibitor was approved for the treatment of head and neck cancer approved in August 2016. Pembrolizumab, which targets the programmed cell death 1 (PD-1) protein, is designed to reinstate the anti-tumor immune response to kill cancer cells and was approved for the treatment of recurrent or metastatic disease that progressed during or after platinum-containing chemotherapy.
Click on the PDF icon at the top of this introduction to read the full article.
The first immune checkpoint inhibitor was approved for the treatment of head and neck cancer approved in August 2016. Pembrolizumab, which targets the programmed cell death 1 (PD-1) protein, is designed to reinstate the anti-tumor immune response to kill cancer cells and was approved for the treatment of recurrent or metastatic disease that progressed during or after platinum-containing chemotherapy.
Click on the PDF icon at the top of this introduction to read the full article.
The first immune checkpoint inhibitor was approved for the treatment of head and neck cancer approved in August 2016. Pembrolizumab, which targets the programmed cell death 1 (PD-1) protein, is designed to reinstate the anti-tumor immune response to kill cancer cells and was approved for the treatment of recurrent or metastatic disease that progressed during or after platinum-containing chemotherapy.
Click on the PDF icon at the top of this introduction to read the full article.
SVS (Specialty of Vascular Surgery): Why, How, and When
The November 2016 issue of Annals of Vascular Surgery was devoted entirely to the history of the American Board of Vascular Surgery (ABVS) and the unsuccessful attempt to establish an independent specialty of Vascular Surgery. The manuscript is methodically detailed by founders of the ABVS, James Stanley, MD, and Frank Veith, MD, and supplemented by commentaries from past board members as well as thought leaders in vascular surgery. In an attempt to maintain neutrality, readers are also provided with many of the documents that were either supportive or contrary to the development of the ABVS. Most senior vascular surgeons will recall the intense discussion and sometimes acrimonious arguments that accompanied the progress of the Board and its failed attempt to be recognized by the American Board of Medical Specialties (ABMS).
Younger vascular surgeons may not realize that the ABVS was ever established. Some may not even realize that, until relatively recently, vascular surgeons were not able to claim board certification even if they had completed a fellowship. Accordingly, as an historical document detailing an important aspect of the evolution of our specialty, this edition of Annals of Vascular surgery is a must read.
Cogent arguments both for and against an independent specialty were made by the leaders of our specialty at the time that the ABVS was being developed. Unfortunately, this did not lead to a uniform policy but rather long-standing, rancorous, and bitter divisions that in all probability prevented the ABVS from being recognized by the ABMS. Despite this failure, the debate around this issue elevated the stature of vascular surgery when the American Board of Surgery conceded that vascular surgeons could now claim “Board certification in vascular surgery” without having to be trained in general surgery. However, all important modifications to the current design of vascular residency and fellowship programs still need to be decided by the American Board of Surgery and its associated Residency Review Committee for Surgery (RRC-S). Further, many hospital administrators subordinate vascular surgery by insisting that vascular surgeons' interests be controlled by general or cardiothoracic surgeons.
Most notably, this issue of Annals reignites fundamental questions that are at the heart of our existence as vascular surgeons. For example, has vascular surgery matured sufficiently to be considered a distinct specialty equivalent to other surgical specialties such as orthopedics, colorectal, urology, and otolaryngology surgery? If so, why did this not occur earlier? Does it warrant becoming independent from the American Board of Surgery such that only vascular surgeons will be in control of training programs, graduate education, and the practice of vascular surgery at universities, hospitals, and community practices? More significantly, why should these institutions, health agencies and the lay public care that there is a separate independent specialty – vascular surgery? The answer to these questions becomes apparent by an analysis of four historic elements that have changed since the ABVS was being formulated.
First, and perhaps most importantly, the argument for an ABVS occurred when vascular surgery had just entered the endovascular revolution. How difficult it must have been for those early vascular surgeons to realize that within a few years perhaps upward of 70%-80% of all procedures would not be performed in a standard operating room but rather an angiography suite, cath lab, or hybrid room? Could they envisage an era where abdominal aneurysms were treated not only without a laparotomy scar but even without a groin incision? That carotid endarterectomy may be replaced by a stent or that varicose veins would be abolished by an outpatient laser procedure? Without such foresight, general surgeons and even those early vascular surgeons had to believe that vascular surgery, as then practiced, required general surgery training.
A second historical reality that impacted the progress of the ABVS was the fragmentation of the governance of vascular surgeons on both a local and national level. Locally, university surgeons, assuming that vascular surgery was an intrinsic part of general surgery, may have been concerned that their leadership roles would be diminished if they were relegated to division heads rather than department chairs. Nationally, there existed three bodies representing vascular surgeons, each with its own leadership and motivations. These were the Society for Vascular Surgery (SVS), the North American chapter of the International Society for Cardiovascular Surgery (NA-ISCVS) which later changed its name to the American Association for Vascular Surgery (AAVS) and the Society for Clinical Vascular Surgery (SCVS).
The SVS at the time was predominantly an academic association with its primary goal being the annual meeting. The SCVS was a casual community of predominantly private practice surgeons. The AAVS was the most representative but it did not have the infrastructure to be a dominant force. Further, there also existed the Association of Program Directors in Vascular Surgery (APDVS). This division was compounded by the formation of the ABVS. Despite three polls of vascular surgeons, the majority of which supported an independent specialty, the divided leadership of these various organizations refused to abide by the voice of their respective memberships. The destructive internecine arguments that developed are detailed in the Annals manuscript, and this disunion of the vascular community and its leadership clearly hampered a collective identity.
Thirdly, the members of the ABVS argued that an independent specialty was necessary in order to train vascular surgeons in the evolving field of endovascular procedures. However, many established leaders balked at this proposal and resisted incorporating such training into their programs. Their refusal to assist in the education of endo-competent vascular surgeons and the development of an independent specialty allowed cardiologists and interventional radiologists to infiltrate the field. Now, the argument for an independent specialty of vascular surgery is not so much with general surgeons but rather with Cardiologists and interventional radiologists.
Fourth, the ABS at the time still considered itself an authoritative Board protective of an all-encompassing General surgery. Its leaders feared that separation of vascular surgery would lead to a stampede with other subspecialties such as pediatric and hand surgery clamoring for independence.
It is not surprising that there was little chance that the ABVS would succeed. However, much can be learned from this historical review that predicts a new initiative in today’s healthcare environment will likely be successful and benefit not only vascular surgeons but also their patients.
In this modern era the practice of vascular surgery involves multiple disciplines and various forms of therapy. As I have frequently claimed, vascular surgeons “operate, medicate, and dilate”. When so much of what vascular surgeons do is beyond the realm of open surgery, wouldn’t most agree that vascular surgery should not be controlled by a governing body, the ABS, whose primary motivation remains operative therapy?
On the other hand, the current ABS recognizes all its subspecialties are similarly morphing away from general surgery and so the ABS is evolving into a Federation of quasi-independent boards. Accordingly, it is likely to be less resistant to a fully independent vascular specialty board existing under its umbrella organization.
Concomitantly, heads of divisions of vascular surgery in universities as well as community practice hospitals can no longer rely on the largesse of chairpersons of general or cardiothoracic surgery since most will not have clinical vascular experience. Accordingly, these vascular surgeons must have complete autonomy with titles and positions elevated to chairs of a department rather than a division.
Vascular surgeons should also acknowledge that they can no longer claim total control of vascular patients. Vascular internists, cardiologists, interventional radiologists and even interventional nephrologists are all involved. An attempt to block further inroads will alienate these other specialties who in turn will attempt to deny us independent specialty designation. We need to work in conjunction, while remaining the only specialty that can offer all forms of therapy. By providing quality care vascular surgeons will gain the respect of government, insurance agencies and our patients and thus support for our independent status.
Although our small specialty of probably no more than 3000 active vascular surgeons is still represented by many differing societies, the SVS has now become the de facto union of vascular surgeons. It has the ability to bring together all factions and it has the finances, the manpower and the organizational structure to represent all vascular surgeons on the national and international level. As such it is already recognized by governmental and commercial agencies as the authoritative voice of vascular surgeons. The SVS, which has built a strong relationship with the APDVS, is also in a strong position to support and facilitate the undergraduate and postgraduate training of vascular surgeons and strengthen all aspects of an independent specialty of vascular surgery. Although there may still be disagreements about whether vascular surgery should be an independent specialty, the SVS should be the organization that serves as “convener” and ultimately implements the decision of the majority of vascular surgeons. It may be appropriate that the SVS Executive Committee authorizes one more survey of its membership to determine whether we continue to seek independent specialty designation and to approve it as a binding membership referendum.
The plusses and minuses should be carefully defined and much thought given to how the questions in the opinion poll are defined. Whatever the results, they should stand, and be implemented.
Finally, as a practicing vascular surgeon and not necessarily in my role as medical editor of the Vascular Specialist I would like to thank Dr. Timothy M. Sullivan and the Annals of Vascular Surgery for publication of this review and Drs. Stanley and Veith for providing us with the gift of historical perspective. Now our goal should not be to repeat history, but rather to learn from our past experiences. I am sure most will commend Drs. Stanley and Veith and all the other vascular surgeons who dedicated so much of their time in the pursuit of an independent vascular specialty.
However, we should not demonize those that held a contrary view, for most were a product of their times. As recent Nobel Laureate Bob Dylan has written, “The times they are a’changing,” and they are changing in our favor. ■
The November 2016 issue of Annals of Vascular Surgery was devoted entirely to the history of the American Board of Vascular Surgery (ABVS) and the unsuccessful attempt to establish an independent specialty of Vascular Surgery. The manuscript is methodically detailed by founders of the ABVS, James Stanley, MD, and Frank Veith, MD, and supplemented by commentaries from past board members as well as thought leaders in vascular surgery. In an attempt to maintain neutrality, readers are also provided with many of the documents that were either supportive or contrary to the development of the ABVS. Most senior vascular surgeons will recall the intense discussion and sometimes acrimonious arguments that accompanied the progress of the Board and its failed attempt to be recognized by the American Board of Medical Specialties (ABMS).
Younger vascular surgeons may not realize that the ABVS was ever established. Some may not even realize that, until relatively recently, vascular surgeons were not able to claim board certification even if they had completed a fellowship. Accordingly, as an historical document detailing an important aspect of the evolution of our specialty, this edition of Annals of Vascular surgery is a must read.
Cogent arguments both for and against an independent specialty were made by the leaders of our specialty at the time that the ABVS was being developed. Unfortunately, this did not lead to a uniform policy but rather long-standing, rancorous, and bitter divisions that in all probability prevented the ABVS from being recognized by the ABMS. Despite this failure, the debate around this issue elevated the stature of vascular surgery when the American Board of Surgery conceded that vascular surgeons could now claim “Board certification in vascular surgery” without having to be trained in general surgery. However, all important modifications to the current design of vascular residency and fellowship programs still need to be decided by the American Board of Surgery and its associated Residency Review Committee for Surgery (RRC-S). Further, many hospital administrators subordinate vascular surgery by insisting that vascular surgeons' interests be controlled by general or cardiothoracic surgeons.
Most notably, this issue of Annals reignites fundamental questions that are at the heart of our existence as vascular surgeons. For example, has vascular surgery matured sufficiently to be considered a distinct specialty equivalent to other surgical specialties such as orthopedics, colorectal, urology, and otolaryngology surgery? If so, why did this not occur earlier? Does it warrant becoming independent from the American Board of Surgery such that only vascular surgeons will be in control of training programs, graduate education, and the practice of vascular surgery at universities, hospitals, and community practices? More significantly, why should these institutions, health agencies and the lay public care that there is a separate independent specialty – vascular surgery? The answer to these questions becomes apparent by an analysis of four historic elements that have changed since the ABVS was being formulated.
First, and perhaps most importantly, the argument for an ABVS occurred when vascular surgery had just entered the endovascular revolution. How difficult it must have been for those early vascular surgeons to realize that within a few years perhaps upward of 70%-80% of all procedures would not be performed in a standard operating room but rather an angiography suite, cath lab, or hybrid room? Could they envisage an era where abdominal aneurysms were treated not only without a laparotomy scar but even without a groin incision? That carotid endarterectomy may be replaced by a stent or that varicose veins would be abolished by an outpatient laser procedure? Without such foresight, general surgeons and even those early vascular surgeons had to believe that vascular surgery, as then practiced, required general surgery training.
A second historical reality that impacted the progress of the ABVS was the fragmentation of the governance of vascular surgeons on both a local and national level. Locally, university surgeons, assuming that vascular surgery was an intrinsic part of general surgery, may have been concerned that their leadership roles would be diminished if they were relegated to division heads rather than department chairs. Nationally, there existed three bodies representing vascular surgeons, each with its own leadership and motivations. These were the Society for Vascular Surgery (SVS), the North American chapter of the International Society for Cardiovascular Surgery (NA-ISCVS) which later changed its name to the American Association for Vascular Surgery (AAVS) and the Society for Clinical Vascular Surgery (SCVS).
The SVS at the time was predominantly an academic association with its primary goal being the annual meeting. The SCVS was a casual community of predominantly private practice surgeons. The AAVS was the most representative but it did not have the infrastructure to be a dominant force. Further, there also existed the Association of Program Directors in Vascular Surgery (APDVS). This division was compounded by the formation of the ABVS. Despite three polls of vascular surgeons, the majority of which supported an independent specialty, the divided leadership of these various organizations refused to abide by the voice of their respective memberships. The destructive internecine arguments that developed are detailed in the Annals manuscript, and this disunion of the vascular community and its leadership clearly hampered a collective identity.
Thirdly, the members of the ABVS argued that an independent specialty was necessary in order to train vascular surgeons in the evolving field of endovascular procedures. However, many established leaders balked at this proposal and resisted incorporating such training into their programs. Their refusal to assist in the education of endo-competent vascular surgeons and the development of an independent specialty allowed cardiologists and interventional radiologists to infiltrate the field. Now, the argument for an independent specialty of vascular surgery is not so much with general surgeons but rather with Cardiologists and interventional radiologists.
Fourth, the ABS at the time still considered itself an authoritative Board protective of an all-encompassing General surgery. Its leaders feared that separation of vascular surgery would lead to a stampede with other subspecialties such as pediatric and hand surgery clamoring for independence.
It is not surprising that there was little chance that the ABVS would succeed. However, much can be learned from this historical review that predicts a new initiative in today’s healthcare environment will likely be successful and benefit not only vascular surgeons but also their patients.
In this modern era the practice of vascular surgery involves multiple disciplines and various forms of therapy. As I have frequently claimed, vascular surgeons “operate, medicate, and dilate”. When so much of what vascular surgeons do is beyond the realm of open surgery, wouldn’t most agree that vascular surgery should not be controlled by a governing body, the ABS, whose primary motivation remains operative therapy?
On the other hand, the current ABS recognizes all its subspecialties are similarly morphing away from general surgery and so the ABS is evolving into a Federation of quasi-independent boards. Accordingly, it is likely to be less resistant to a fully independent vascular specialty board existing under its umbrella organization.
Concomitantly, heads of divisions of vascular surgery in universities as well as community practice hospitals can no longer rely on the largesse of chairpersons of general or cardiothoracic surgery since most will not have clinical vascular experience. Accordingly, these vascular surgeons must have complete autonomy with titles and positions elevated to chairs of a department rather than a division.
Vascular surgeons should also acknowledge that they can no longer claim total control of vascular patients. Vascular internists, cardiologists, interventional radiologists and even interventional nephrologists are all involved. An attempt to block further inroads will alienate these other specialties who in turn will attempt to deny us independent specialty designation. We need to work in conjunction, while remaining the only specialty that can offer all forms of therapy. By providing quality care vascular surgeons will gain the respect of government, insurance agencies and our patients and thus support for our independent status.
Although our small specialty of probably no more than 3000 active vascular surgeons is still represented by many differing societies, the SVS has now become the de facto union of vascular surgeons. It has the ability to bring together all factions and it has the finances, the manpower and the organizational structure to represent all vascular surgeons on the national and international level. As such it is already recognized by governmental and commercial agencies as the authoritative voice of vascular surgeons. The SVS, which has built a strong relationship with the APDVS, is also in a strong position to support and facilitate the undergraduate and postgraduate training of vascular surgeons and strengthen all aspects of an independent specialty of vascular surgery. Although there may still be disagreements about whether vascular surgery should be an independent specialty, the SVS should be the organization that serves as “convener” and ultimately implements the decision of the majority of vascular surgeons. It may be appropriate that the SVS Executive Committee authorizes one more survey of its membership to determine whether we continue to seek independent specialty designation and to approve it as a binding membership referendum.
The plusses and minuses should be carefully defined and much thought given to how the questions in the opinion poll are defined. Whatever the results, they should stand, and be implemented.
Finally, as a practicing vascular surgeon and not necessarily in my role as medical editor of the Vascular Specialist I would like to thank Dr. Timothy M. Sullivan and the Annals of Vascular Surgery for publication of this review and Drs. Stanley and Veith for providing us with the gift of historical perspective. Now our goal should not be to repeat history, but rather to learn from our past experiences. I am sure most will commend Drs. Stanley and Veith and all the other vascular surgeons who dedicated so much of their time in the pursuit of an independent vascular specialty.
However, we should not demonize those that held a contrary view, for most were a product of their times. As recent Nobel Laureate Bob Dylan has written, “The times they are a’changing,” and they are changing in our favor. ■
The November 2016 issue of Annals of Vascular Surgery was devoted entirely to the history of the American Board of Vascular Surgery (ABVS) and the unsuccessful attempt to establish an independent specialty of Vascular Surgery. The manuscript is methodically detailed by founders of the ABVS, James Stanley, MD, and Frank Veith, MD, and supplemented by commentaries from past board members as well as thought leaders in vascular surgery. In an attempt to maintain neutrality, readers are also provided with many of the documents that were either supportive or contrary to the development of the ABVS. Most senior vascular surgeons will recall the intense discussion and sometimes acrimonious arguments that accompanied the progress of the Board and its failed attempt to be recognized by the American Board of Medical Specialties (ABMS).
Younger vascular surgeons may not realize that the ABVS was ever established. Some may not even realize that, until relatively recently, vascular surgeons were not able to claim board certification even if they had completed a fellowship. Accordingly, as an historical document detailing an important aspect of the evolution of our specialty, this edition of Annals of Vascular surgery is a must read.
Cogent arguments both for and against an independent specialty were made by the leaders of our specialty at the time that the ABVS was being developed. Unfortunately, this did not lead to a uniform policy but rather long-standing, rancorous, and bitter divisions that in all probability prevented the ABVS from being recognized by the ABMS. Despite this failure, the debate around this issue elevated the stature of vascular surgery when the American Board of Surgery conceded that vascular surgeons could now claim “Board certification in vascular surgery” without having to be trained in general surgery. However, all important modifications to the current design of vascular residency and fellowship programs still need to be decided by the American Board of Surgery and its associated Residency Review Committee for Surgery (RRC-S). Further, many hospital administrators subordinate vascular surgery by insisting that vascular surgeons' interests be controlled by general or cardiothoracic surgeons.
Most notably, this issue of Annals reignites fundamental questions that are at the heart of our existence as vascular surgeons. For example, has vascular surgery matured sufficiently to be considered a distinct specialty equivalent to other surgical specialties such as orthopedics, colorectal, urology, and otolaryngology surgery? If so, why did this not occur earlier? Does it warrant becoming independent from the American Board of Surgery such that only vascular surgeons will be in control of training programs, graduate education, and the practice of vascular surgery at universities, hospitals, and community practices? More significantly, why should these institutions, health agencies and the lay public care that there is a separate independent specialty – vascular surgery? The answer to these questions becomes apparent by an analysis of four historic elements that have changed since the ABVS was being formulated.
First, and perhaps most importantly, the argument for an ABVS occurred when vascular surgery had just entered the endovascular revolution. How difficult it must have been for those early vascular surgeons to realize that within a few years perhaps upward of 70%-80% of all procedures would not be performed in a standard operating room but rather an angiography suite, cath lab, or hybrid room? Could they envisage an era where abdominal aneurysms were treated not only without a laparotomy scar but even without a groin incision? That carotid endarterectomy may be replaced by a stent or that varicose veins would be abolished by an outpatient laser procedure? Without such foresight, general surgeons and even those early vascular surgeons had to believe that vascular surgery, as then practiced, required general surgery training.
A second historical reality that impacted the progress of the ABVS was the fragmentation of the governance of vascular surgeons on both a local and national level. Locally, university surgeons, assuming that vascular surgery was an intrinsic part of general surgery, may have been concerned that their leadership roles would be diminished if they were relegated to division heads rather than department chairs. Nationally, there existed three bodies representing vascular surgeons, each with its own leadership and motivations. These were the Society for Vascular Surgery (SVS), the North American chapter of the International Society for Cardiovascular Surgery (NA-ISCVS) which later changed its name to the American Association for Vascular Surgery (AAVS) and the Society for Clinical Vascular Surgery (SCVS).
The SVS at the time was predominantly an academic association with its primary goal being the annual meeting. The SCVS was a casual community of predominantly private practice surgeons. The AAVS was the most representative but it did not have the infrastructure to be a dominant force. Further, there also existed the Association of Program Directors in Vascular Surgery (APDVS). This division was compounded by the formation of the ABVS. Despite three polls of vascular surgeons, the majority of which supported an independent specialty, the divided leadership of these various organizations refused to abide by the voice of their respective memberships. The destructive internecine arguments that developed are detailed in the Annals manuscript, and this disunion of the vascular community and its leadership clearly hampered a collective identity.
Thirdly, the members of the ABVS argued that an independent specialty was necessary in order to train vascular surgeons in the evolving field of endovascular procedures. However, many established leaders balked at this proposal and resisted incorporating such training into their programs. Their refusal to assist in the education of endo-competent vascular surgeons and the development of an independent specialty allowed cardiologists and interventional radiologists to infiltrate the field. Now, the argument for an independent specialty of vascular surgery is not so much with general surgeons but rather with Cardiologists and interventional radiologists.
Fourth, the ABS at the time still considered itself an authoritative Board protective of an all-encompassing General surgery. Its leaders feared that separation of vascular surgery would lead to a stampede with other subspecialties such as pediatric and hand surgery clamoring for independence.
It is not surprising that there was little chance that the ABVS would succeed. However, much can be learned from this historical review that predicts a new initiative in today’s healthcare environment will likely be successful and benefit not only vascular surgeons but also their patients.
In this modern era the practice of vascular surgery involves multiple disciplines and various forms of therapy. As I have frequently claimed, vascular surgeons “operate, medicate, and dilate”. When so much of what vascular surgeons do is beyond the realm of open surgery, wouldn’t most agree that vascular surgery should not be controlled by a governing body, the ABS, whose primary motivation remains operative therapy?
On the other hand, the current ABS recognizes all its subspecialties are similarly morphing away from general surgery and so the ABS is evolving into a Federation of quasi-independent boards. Accordingly, it is likely to be less resistant to a fully independent vascular specialty board existing under its umbrella organization.
Concomitantly, heads of divisions of vascular surgery in universities as well as community practice hospitals can no longer rely on the largesse of chairpersons of general or cardiothoracic surgery since most will not have clinical vascular experience. Accordingly, these vascular surgeons must have complete autonomy with titles and positions elevated to chairs of a department rather than a division.
Vascular surgeons should also acknowledge that they can no longer claim total control of vascular patients. Vascular internists, cardiologists, interventional radiologists and even interventional nephrologists are all involved. An attempt to block further inroads will alienate these other specialties who in turn will attempt to deny us independent specialty designation. We need to work in conjunction, while remaining the only specialty that can offer all forms of therapy. By providing quality care vascular surgeons will gain the respect of government, insurance agencies and our patients and thus support for our independent status.
Although our small specialty of probably no more than 3000 active vascular surgeons is still represented by many differing societies, the SVS has now become the de facto union of vascular surgeons. It has the ability to bring together all factions and it has the finances, the manpower and the organizational structure to represent all vascular surgeons on the national and international level. As such it is already recognized by governmental and commercial agencies as the authoritative voice of vascular surgeons. The SVS, which has built a strong relationship with the APDVS, is also in a strong position to support and facilitate the undergraduate and postgraduate training of vascular surgeons and strengthen all aspects of an independent specialty of vascular surgery. Although there may still be disagreements about whether vascular surgery should be an independent specialty, the SVS should be the organization that serves as “convener” and ultimately implements the decision of the majority of vascular surgeons. It may be appropriate that the SVS Executive Committee authorizes one more survey of its membership to determine whether we continue to seek independent specialty designation and to approve it as a binding membership referendum.
The plusses and minuses should be carefully defined and much thought given to how the questions in the opinion poll are defined. Whatever the results, they should stand, and be implemented.
Finally, as a practicing vascular surgeon and not necessarily in my role as medical editor of the Vascular Specialist I would like to thank Dr. Timothy M. Sullivan and the Annals of Vascular Surgery for publication of this review and Drs. Stanley and Veith for providing us with the gift of historical perspective. Now our goal should not be to repeat history, but rather to learn from our past experiences. I am sure most will commend Drs. Stanley and Veith and all the other vascular surgeons who dedicated so much of their time in the pursuit of an independent vascular specialty.
However, we should not demonize those that held a contrary view, for most were a product of their times. As recent Nobel Laureate Bob Dylan has written, “The times they are a’changing,” and they are changing in our favor. ■
Ulnar Collateral Ligament Reconstruction: Current Philosophy in 2016
The ulnar collateral ligament (UCL) is the primary restraint to valgus stress between 20° and 125° of motion.1-5 Overhead athletes, most commonly baseball pitchers, are at risk of developing UCL insufficiency, and dysfunction presents as pain with loss of velocity and control. Some injuries may present acutely while throwing, but many patients, when questioned, report a preceding period of either pain or loss of velocity and control.
Authors have documented a significant rise in elbow injuries in young athletes, especially pitchers.6 Extended seasons, higher pitch counts, year-round pitching, pitching while fatigued, and pitching for multiple teams are risk factors for elbow injuries.7 Pitchers in the southern United States are more likely to undergo UCL reconstruction than those from the northern states.8 Pitchers who also play catcher are at a higher risk due to more total throws than those who pitch and play other positions or pitch only. Throwers with higher velocity are more likely to pitch in showcases, pitch for multiple teams, and pitch with pain and fatigue, and these are all risk factors.6 Also, in one study of youth baseball injuries, individuals in the injured group were found to be taller and heavier than those in the uninjured group.6 Pitch counts, rest from pitching during the off-season, adequate rest, and ensuring pain-free pitching can lessen the risk of injury.6 As expected with the rise in throwing injuries, the rise in medial elbow procedures has risen.9
While throwing, stress across the medial elbow has been measured to be nearly 300 N. A maximum varus force during pitching was measured to be 64 N-m at 95° ± 14°.10 Morrey and An4 determined that the UCL generated 54% of the varus force at 90° of flexion. During active pitching, this value is likely reduced due to simultaneous muscle contraction, but if one assumes the UCL bears 54% of the maximal load, the UCL must be able to withstand 34 N-m. The UCL can withstand a maximum valgus torque between 22.7 and 34 N-m11-13; therefore, during pitching, the UCL is at or above its failure load. After thousands of cycles over many years, one can imagine how the UCL might be injured.
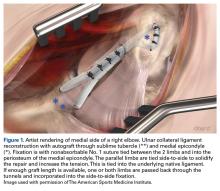
Multiple techniques have been proposed in the surgical treatment of UCL injuries. Jobe14 pioneered UCL reconstruction in 1974 in Tommy John, a Major League Baseball pitcher. John returned to pitch successfully, and both the UCL and the reconstruction are commonly called by his name. Jobe14 reported his technique in 1986, and it has remained, with a few modifications, the primary method for reconstruction of the UCL (Figure 1).
Evaluation
A standard evaluation with physical examination and imaging is completed in all throwers with elbow pain. In our prior study,16 we found that 100% of patients experienced pain during athletic activity and that 96% of throwers complained of pain during late cocking and acceleration phases of the throwing motion. Nearly half reported an acute onset of pain, while 53% were unable to identify a single inciting event. Seventy-five percent of the acute injuries were during competition. Delayed diagnosis was very common, with an average time to diagnosis after onset of symptoms of 6.4 months. Neurologic symptoms were seen in 23% of athletes, most of which were ulnar nerve paresthesias during throwing.16
Physical examination includes inspection for swelling, hand intrinsic atrophy, neurovascular examination, range of motion, shoulder examination, and elbow stress examination. Range of motion at presentation averaged 5° to 135° with 85° of supination and pronation.16 All patients need neurologic evaluation for ulnar nerve dysfunction. Tinel test of the cubital tunnel was positive in 21%.16 Significant ulnar nerve dysfunction, including hand weakness, is much less common but must be well examined and documented. The shoulder must also be evaluated for loss of rotation, which can lead to increased stress on the elbow. An evaluation of mechanics may point out flaws in technique, which may be contributing to elbow stress. The UCL stress examination includes static stress at 30° of flexion, the milking test at 90°, and the moving valgus stress test. The presence of pain directly over the UCL or laxity compared to the uninvolved side is suggestive of UCL injury.
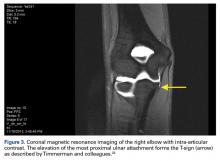
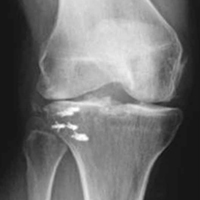
Radiographic evaluation is completed in all patients with concern for UCL injury. Standard x-rays of the elbow, including anteroposterior, medial, and lateral obliques, axial olecranon, and lateral views, are obtained to evaluate bony abnormalities. Fifty-seven percent of our series showed some abnormality, most commonly olecranon osteophyte formation or ectopic calcification within the UCL substance. Stress radiography rarely changed the treatment course and is somewhat difficult to interpret because of the reports documenting normal increased medial elbow opening in the dominant arm of throwing athletes.21 Magnetic resonance imaging (MRI) is obtained very commonly in this patient population, and intra-articular contrast is crucial. Partial, undersurface tears are common, and a contrasted study better demonstrates undersurface tears or avulsions. The T-sign as described by Timmerman and colleagues22 using computed tomography (CT) arthrography shows partial undersurface detachment, which can be difficult to see without intra-articular contrast.22 This finding is very well visualized on MRI arthrogram as well (Figure 3).
Nonoperative Management
Nonoperative treatment is recommended for 3 months prior to performing reconstruction. Patients are given complete rest from throwing, but rehabilitation is initiated immediately. Rehabilitation exercises and nonsteroidal anti-inflammatory medications are prescribed, and activities that place valgus stress across the elbow are avoided. After resolution of symptoms, an interval throwing program is initiated, and the athlete is gradually returned to sport. Unfortunately, due to season-specific schedules and time-sensitive demands in high-level throwers, operative treatment is often chosen without an extended period of conservative treatment.
Platelet-rich plasma (PRP) therapy has recently been shown to improve healing rates and promote healing in partial UCL tears,23 and as orthobiologics are advanced, they will likely play a larger role in the treatment of UCL injuries.
Surgical Technique
At our institution, UCL reconstruction is performed with the modified Jobe technique as described by Azar and colleagues.17 Arthroscopy prior to reconstruction was routinely performed at our institution until we recognized that arthroscopy rarely changed the preoperative plan.16 Currently, the presence of anterior pathology such as loose bodies or osteochondral defect is our only indication for arthroscopy before reconstruction.
Ipsilateral palmaris autograft is our current graft of choice. This must be examined preoperatively because 16% of patients have unilateral absence and 9% have bilateral absence.24 In revision cases or in patients with insufficient or absent palmaris, contralateral palmaris followed by contralateral gracilis tendon is used. The contralateral gracilis is chosen because of ease of setup and position of the surgeon during the harvest. Gracilis tendon is also used in cases with bony involvement of the ligament based on the results from Dugas and colleagues.25 Toe extensors, plantaris, and patellar tendon grafts have also been used. One recent study showed that neither graft choice nor diameter affected resistance to valgus stress, and that all reconstruction types restored strength at 60° to 120° of flexion.26
Ulnar nerve transposition is performed in all cases regardless of the presence of preoperative nerve symptoms. A complete decompression is completed proximally to the Arcade of Struthers and distally to the deep portion of the flexor carpi ulnaris. A single fascial sling of medial intermuscular septum originating from the epicondylar attachment is used to stabilize the nerve without compression. At wound closure, the deep fascia on the posterior skin flap is also sewn into the cubital tunnel to prevent the nerve from subluxating back into the groove. A single suture is placed distally closing the muscle fascia to prevent propagation of the fascial incision, which can lead to herniation. Transposition is necessary because of the ulnar nerve exposure required in the modified Jobe technique to allow elevation of the deep flexor muscle mass for ligament exposure.
The reconstruction is completed as described by Jobe14 but with a few modifications as described by Azar and colleagues17 and slight adaptations implemented since that time. The flexor-pronator mass is retracted laterally instead of detachment or splitting as described by Thompson and colleagues.27 A subcutaneous rather than a submuscular ulnar nerve transposition is used.
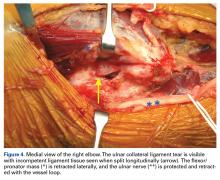
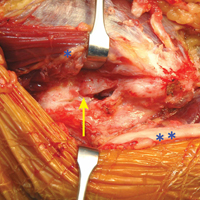
The patient is positioned supine using an arm board. If gracilis tendon is chosen, the contralateral leg is prepped and draped simultaneously. A tourniquet is inflated after exsanguination. A medial approach is performed, and the medial antebrachial nerve is located and protected. The ulnar nerve is then located in the cubital tunnel and mobilized. The neurolysis extends to the deep portion of the flexor carpi ulnaris distally and proximally to the Arcade of Struthers, and the nerve is retracted with a vessel loop. The flexor muscle mass is not elevated from the medial epicondyle; rather, it is retracted anteriorly by small Hohmann retractors. The dissection is carried down to the UCL and found at its attachments to the medial epicondyle and sublime tubercle. If no tear is seen on the superficial surface of the ligament, a longitudinal incision is made through the ligament. Undersurface tears, partial tears, and avulsions can then be identified (Figure 4).
The autologous graft of choice is then harvested. Our technique for palmaris harvest is performed with three 1-cm transverse incisions. The palmaris is palpated and marked with the first incision made near the distal wrist crease, and the second incision is made 3 to 4 cm proximal to the first. The tendon is found in both distal incisions and cut distally with the wrist flexed to maximize tendon length. The tendon is then pulled through the second incision and tensioned to identify the most proximal location the tendon can be palpated. A third incision is made directly over this point and carried down to cut the tendon. This usually provides a graft length of 15 to 20 cm; 13 cm is the minimum graft length to ensure good graft fixation. Muscle is removed from the tendon and each end is secured with a No. 1 nonabsorbable suture in a locking fashion.
If posterior osteophytes are present, they are removed through a posterior, vertical arthrotomy. Over-resection of the olecranon must be avoided, as this can further destabilize the elbow and place increased stress on the reconstruction. Posterior loose bodies can also be removed through this arthrotomy. The arthrotomy is then closed with absorbable suture.
Tunnel placement is critical to success. A 3.2-mm drill bit is used with palmaris grafts and a 4-mm drill bit is used with gracilis grafts. Two convergent tunnels are drilled in the medial epicondyle in a Y fashion and 2 convergent tunnels are drilled at the sublime tubercle in a U or V fashion. After drilling the first tunnel on each side, a hemostat is placed in the tunnel as an aiming point to ensure a complete tunnel is made. The junction is smoothed with a curette, leaving a 5-mm bone bridge between the articular surface and the tunnels. A bent Hewson suture passer is used to pass one end of the graft through the ulna. The 2 limbs of the tendon graft are then passed through the humeral tunnels, creating a figure-of-eight. A varus stress is applied with the elbow at roughly 30° and the 2 limbs are tied together with a No. 1 nonabsorbable suture. If enough graft remains, one or both limbs are passed back through the tunnels and secured again with No. 1 nonabsorbable suture. The 2 limbs are then tied side-to-side, incorporating the native ligament to further secure and tighten the reconstruction.
The ulnar nerve is then secured using a strip of medial intermuscular septum left intact to its insertion at the medial epicondyle. This is attached to the flexor-pronator muscle fascia with a 3-0 nonabsorbable suture. Enough length should be harvested from the septum to ensure there is no compression on the nerve. The deep posterior fascial tissue is then sewn to the periosteum of the medial epicondyle to further prevent subluxation of the nerve back into the groove. The skin is then closed in layered fashion over a superficial drain. The patient is placed in a well-padded posterior splint for 1 week, then the rehabilitation protocol is initiated as discussed below.
Postoperative Rehabilitation
A standardized postoperative 4-phase rehabilitation program for ulnar collateral reconstruction is followed as described by Wilk and colleagues.28-30 The first phase begins immediately after surgery and continues for 4 weeks. During surgery, the patient’s elbow is placed in a compression dressing with a posterior splint to immobilize the elbow in 90° of flexion with wrist motion for 1 week to allow initial healing. Full range of motion of the elbow joint is restored by the end of the fifth to sixth week after surgery.
During phase II (weeks 4-10), a progressive isotonic strengthening program is initiated. Exercises are focused on scapular, rotator cuff, deltoid, and arm musculature. Shoulder range of motion and stretching exercises are performed during this phase and the Thrower’s Ten exercise program is initiated. Any adaptations or strength deficits are addressed during this phase.
During the advanced strengthening phase (phase III), from weeks 10 to 16, a sport-specific exercise/rehabilitation program is initiated. During this phase, stretching and flexibility exercises are performed to enhance strength, power, and endurance. During this phase the patient is placed on the advanced Thrower’s Ten program. Isotonic strengthening exercises are progressed, and at week 12, the athlete is allowed to begin an isotonic lifting program, including bench press, seated rowing, latissimus dorsi pull downs, triceps push downs, and biceps curls. In addition, the athlete performs specific exercises to emphasize sport-specific movements. At week 12, overhead athletes begin a 2-hand plyometric throwing program, and at 14 weeks, a 1
Discussion
Results after ulnar collateral reconstruction have been good. In our series of 743 patients, 83% returned to the same or higher level at an average of 11.6 months.16 There was a 4% major complication rate and 16% minor complication rate. Major complications included medial epicondyle fracture (0.5%), significant ulnar nerve dysfunction (1 patient), rupture of graft (1%), and graft site infection. Sixteen percent of patients had ulnar nerve dysfunction, and 82% of these resolved within 6 weeks. All but 1 patient’s paresthesias resolved within 1 year.16 The 10-year follow-up of this group of patients included 256 patients and was reported by Osbahr and colleagues31 in 2014. Retirement from baseball was due to reasons other than the elbow in 86%, and 98% were still able to throw on at least a recreational level. The overall longevity was 3.6 years, with 2.9 years at pre-injury level or higher. Statistically, pitchers performed at a higher level after reconstruction.31
A recent review by Erickson and colleagues9 showed an overall 82% excellent and 8% good result when evaluating different techniques, including the American Sports Medicine Institute (ASMI) modification of Jobe’s technique, docking technique, and Jobe’s technique. With an overall complication rate of 10% (75% of which was transient ulnar neuritis), the procedure was deemed overall a safe surgical option. Collegiate athletes had the highest return to sport (95%) compared with high school athletes (89%) and professional athletes (86%). The docking technique had the highest rate of return to play (97%) compared with ASMI technique (93%) and Jobe technique (66%).9 Results after repair have not been as good as reconstruction, as reported in 2 studies.16,32 Savoie and colleagues,15 however, reported 93% good/excellent results after primary UCL repair alone.
Another recent review of outcomes showed an overall return to same or higher level was best with docking or modified docking techniques (90.4% and 91.3%, respectively).19 Overall return with modified Jobe technique was 77%.19 O’Brien and colleagues20 performed a review of 33 patients with either modified Jobe or docking technique that showed 81% return to same or higher level with modified Jobe vs 92% with docking technique. The Kerlan-Jobe Orthopaedic Clinic scores were higher in the modified Jobe group (79 vs 74) and the docking technique group returned to play nearly 1 month sooner (12.4 months vs 11.8 months).20 However, comparing different techniques in a heterogenous patient population over 40 years is difficult. Many of the modified Jobe technique cases were performed in the early evolution of the rehabilitation and return-to-play programs. We believe that the current modified Jobe technique has results equal to any other variation.
Despite good results with reconstructions, the recovery is lengthy and most pitchers cannot fully return to competition level for 12 to 18 months. Extensive research has been performed in exploring alternatives to the traditional reconstruction. Advancements in orthobiologics and development of new surgical options seem to provide an alternative to reconstruction, and may allow faster return to competition with less morbidity.
PRP has been at the forefront of orthopedic research for the last 2 decades, mostly focused in tendon and bone healing. Due to the release of many inflammatory mediators, PRP is theorized to initiate a healing response with growth factors that can direct healing towards normal tissue.33 Two main types of PRP are reported based on the presence or absence of leukocytes. PRP has been studied in many applications, but only one clinical study on the UCL has been published to date. Podesta and colleagues23 injected PRP into the elbow of 34 baseball players with MRI-confirmed partial UCL tear. The athletes then underwent a rehabilitation program, which limited stress across the UCL. Type 1A PRP was used (leukocyte-rich, unactivated, 5x or greater platelet concentration33). Athletes were allowed to return to sport based on symptoms and examination findings. Eighty-eight percent returned to same level of play without complaints at average 70 week follow-up, and average return to play ranged from 10 to 15 weeks.23 No specific data were given on the 16 pitchers in the group, but with such a high rate of return, PRP needs to be further evaluated in the treatment of UCL injuries.
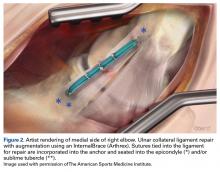
Another recent study from Dugas and colleagues18 presented primary UCL repair using a tape augment (InternalBrace, Arthrex). Nine matched cadaver elbows underwent UCL sectioning and then either modified Jobe reconstruction or primary repair of the UCL with placement of the InternalBrace. The biomechanical data showed the repair with internal brace to have slightly less gap, more stiffness, and higher failure strength, although these findings were not statistically significant.18 This bone-preserving technique with less exposure and healing of the native ligament may be another step towards good results with a quicker return to throwing.
Conclusion
UCL injuries can be disabling in throwers. Reconstruction has afforded throwers a high rate of return to preinjury function or better, and several techniques have been presented that produce acceptable results. Overall complication rates range from 10% to 15%, and the majority of complications are transient ulnar neuropraxias. Orthobiologics and repair with augmentation have more recently offered additional options that may improve success of nonoperative treatment or allow less-invasive surgical treatment. Increased involvement in youth sports and early specialization is driving injury rates in young athletes. The orthopedic community must continue to look for better ways to prevent these injuries and investigate better methods to return athletes to high-level competition.
Am J Orthop. 2016;45(7):E534-E540. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. Fuss FK. The ulnar collateral ligament of the human elbow joint. Anatomy, function and biomechanics. J Anat. 1991;175:203-212.
2. Hotchkiss RN, Weiland AJ. Valgus stability of the elbow. J Orthop Res. 1987;5(3):372-377.
3. Morrey BF. Applied anatomy and biomechanics of the elbow joint. Instr Course Lect. 1986;35:59-68.
4. Morrey BF, An KN. Articular and ligamentous contributions to the stability of the elbow joint. Am J Sports Med. 1983;11(5):315-319.
5. Morrey BF, An KN. Functional anatomy of the ligaments of the elbow. Clin Orthop. 1985;(201):84-90.
6. Olsen SJ 2nd, Fleisig GS, Dun S, Loftice J, Andrews JR. Risk factors for shoulder and elbow injuries in adolescent baseball pitchers. Am J Sports Med. 2006;34(6):905-912.
7. Fleisig GS, Andrews JR. Prevention of elbow injuries in youth baseball pitchers. Sports Health. 2012;4(5):419-424.
8. Zaremski JL, Horodyski M, Donlan RM, Brisbane ST, Farmer KW. Does geographic location matter on the prevalence of ulnar collateral ligament reconstruction in collegiate baseball pitchers? Orthop J Sports Med. 2015;3(11):2325967115616582.
9. Erickson BJ, Nwachukwu BU, Rosas S, et al. Trends in medial ulnar collateral ligament reconstruction in the United States: A retrospective review of a large private-payer database from 2007 to 2011. Am J Sports Med. 2015;43(7):1770-1774.
10. Fleisig GS, Andrews JR, Dillman CJ. Kinetics of baseball pitching with implications about injury mechanism. Am J Sports Med. 1995;23(2):233-239.
11. Dillman CJ, Smutz P, Werner S. Valgus extension overload in baseball pitching. Med Sci Sports Exerc. 1991;23(suppl 4):S135.
12. Hechtman KS, Tjin-A-Tsoi EW, Zvijac JE, Uribe JW, Latta LL. Biomechanics of a less invasive procedure for reconstruction of the ulnar collateral ligament of the elbow. Am J Sports Med. 1998;26(5):620-624.
13. Ahmad CS, Lee TQ, ElAttrache NS. Biomechanical evaluation of a new ulnar collateral ligament reconstruction technique with interference screw fixation. Am J Sports Med. 2003;31(3):332-337.
14. Jobe FW, Stark HE, Lombardo SJ. Reconstruction of the ulnar collateral ligament in athletes. J Bone Joint Surg Am. 1986;68(8):1158-1163.
15. Savoie FH 3rd, Trenhaile SW, Roberts J, Field LD, Ramsey JR. Primary repair of ulnar collateral ligament injuries of the elbow in young athletes: a case series of injuries to the proximal and distal ends of the ligament. Am J Sports Med. 2008;36(6):1066-1072.
16. Cain EL, Andrews JR, Dugas JR, et al. Outcome of ulnar collateral ligament reconstruction of the elbow in 1281 athletes results in 743 athletes with minimum 2-year follow-up. Am J Sports Med. 2010;38(12):2426-2434.
17. Azar FM, Andrews JR, Wilk KE, Groh D. Operative treatment of ulnar collateral ligament injuries of the elbow in athletes. Am J Sports Med. 2000;28(1):16-23.
18. Dugas JR, Walters BL, Beason DP, Fleisig GS, Chronister JE. Biomechanical comparison of ulnar collateral ligament repair with internal bracing versus modified Jobe reconstruction. Am J Sports Med. 2016;44(3):735-741.
19. Watson JN, McQueen P, Hutchinson MR. A systematic review of ulnar collateral ligament reconstruction techniques. Am J Sports Med. 2014;42(10):2510-2516.
20. O’Brien DF, O’Hagan T, Stewart R, et al. Outcomes for ulnar collateral ligament reconstruction: A retrospective review using the KJOC assessment score with two-year follow-up in an overhead throwing population. J Shoulder Elbow Surg. 2015;24(6):934-940.
21. Ellenbecker TS, Mattalino AJ, Elam EA, Caplinger RA. Medial elbow joint laxity in professional baseball pitchers a bilateral comparison using stress radiography. Am J Sports Med. 1998;26(3):420-424.
22. Timmerman LA, Schwartz ML, Andrews JR. Preoperative evaluation of the ulnar collateral ligament by magnetic resonance imaging and computed tomography arthrography evaluation in 25 baseball players with surgical confirmation. Am J Sports Med. 1994;22(1):26-32.
23. Podesta L, Crow SA, Volkmer D, Bert T, Yocum LA. Treatment of partial ulnar collateral ligament tears in the elbow with platelet-rich plasma. Am J Sports Med. 2013;41(7):1689-1694.
24. Thompson NW, Mockford BJ, Cran GW. Absence of the palmaris longus muscle: a population study. Ulster Med J. 2001;70(1):22-24.
25. Dugas JR, Bilotta J, Watts CD, et al. Ulnar collateral ligament reconstruction with gracilis tendon in athletes with intraligamentous bony excision technique and results. Am J Sports Med. 2012;40(7):1578-1582.
26. Dargel J, Küpper F, Wegmann K, Oppermann J, Eysel P, Müller LP. Graft diameter does not influence primary stability of ulnar collateral ligament reconstruction of the elbow. J Orthop Sci. 2015;20(2):307-313.
27. Thompson WH, Jobe FW, Yocum LA, Pink MM. Ulnar collateral ligament reconstruction in athletes: muscle-splitting approach without transposition of the ulnar nerve. J Shoulder Elbow Surg. 2001;10(2):152-157.
28. Wilk KE, Arrigo CA, Andrews JR. Rehabilitation of the elbow in the throwing athlete. J Orthop Sports Phys Ther. 1993;17(6):305-317.
29. Wilk KE, Arrigo CA, Andrews JR, et al. Rehabilitation following elbow surgery in the throwing athlete. Oper Tech Sports Med. 1996;4:114-132.
30. Wilk KE, Arrigo CA, Andrews JR, et al. Preventative and Rehabilitation Exercises for the Shoulder and Elbow. 4th ed. Birmingham, AL: American Sports Medicine Institute; 1996.
31. Osbahr DC, Cain EL, Raines BT, Fortenbaugh D, Dugas JR, Andrews JR. Long-term outcomes after ulnar collateral ligament reconstruction in competitive baseball players minimum 10-year follow-up. Am J Sports Med. 2014;42(6):1333-1342.
32. Conway JE, Jobe FW, Glousman RE, Pink M. Medial instability of the elbow in throwing athletes. Treatment by repair or reconstruction of the ulnar collateral ligament. J Bone Joint Surg Am. 1992;74(1):67-83.
33. Mishra A, Harmon K, Woodall J, Vieira A. Sports medicine applications of platelet rich plasma. Curr Pharm Biotechnol. 2012;13(7):1185-1195.
The ulnar collateral ligament (UCL) is the primary restraint to valgus stress between 20° and 125° of motion.1-5 Overhead athletes, most commonly baseball pitchers, are at risk of developing UCL insufficiency, and dysfunction presents as pain with loss of velocity and control. Some injuries may present acutely while throwing, but many patients, when questioned, report a preceding period of either pain or loss of velocity and control.
Authors have documented a significant rise in elbow injuries in young athletes, especially pitchers.6 Extended seasons, higher pitch counts, year-round pitching, pitching while fatigued, and pitching for multiple teams are risk factors for elbow injuries.7 Pitchers in the southern United States are more likely to undergo UCL reconstruction than those from the northern states.8 Pitchers who also play catcher are at a higher risk due to more total throws than those who pitch and play other positions or pitch only. Throwers with higher velocity are more likely to pitch in showcases, pitch for multiple teams, and pitch with pain and fatigue, and these are all risk factors.6 Also, in one study of youth baseball injuries, individuals in the injured group were found to be taller and heavier than those in the uninjured group.6 Pitch counts, rest from pitching during the off-season, adequate rest, and ensuring pain-free pitching can lessen the risk of injury.6 As expected with the rise in throwing injuries, the rise in medial elbow procedures has risen.9
While throwing, stress across the medial elbow has been measured to be nearly 300 N. A maximum varus force during pitching was measured to be 64 N-m at 95° ± 14°.10 Morrey and An4 determined that the UCL generated 54% of the varus force at 90° of flexion. During active pitching, this value is likely reduced due to simultaneous muscle contraction, but if one assumes the UCL bears 54% of the maximal load, the UCL must be able to withstand 34 N-m. The UCL can withstand a maximum valgus torque between 22.7 and 34 N-m11-13; therefore, during pitching, the UCL is at or above its failure load. After thousands of cycles over many years, one can imagine how the UCL might be injured.
Multiple techniques have been proposed in the surgical treatment of UCL injuries. Jobe14 pioneered UCL reconstruction in 1974 in Tommy John, a Major League Baseball pitcher. John returned to pitch successfully, and both the UCL and the reconstruction are commonly called by his name. Jobe14 reported his technique in 1986, and it has remained, with a few modifications, the primary method for reconstruction of the UCL (Figure 1).
Evaluation
A standard evaluation with physical examination and imaging is completed in all throwers with elbow pain. In our prior study,16 we found that 100% of patients experienced pain during athletic activity and that 96% of throwers complained of pain during late cocking and acceleration phases of the throwing motion. Nearly half reported an acute onset of pain, while 53% were unable to identify a single inciting event. Seventy-five percent of the acute injuries were during competition. Delayed diagnosis was very common, with an average time to diagnosis after onset of symptoms of 6.4 months. Neurologic symptoms were seen in 23% of athletes, most of which were ulnar nerve paresthesias during throwing.16
Physical examination includes inspection for swelling, hand intrinsic atrophy, neurovascular examination, range of motion, shoulder examination, and elbow stress examination. Range of motion at presentation averaged 5° to 135° with 85° of supination and pronation.16 All patients need neurologic evaluation for ulnar nerve dysfunction. Tinel test of the cubital tunnel was positive in 21%.16 Significant ulnar nerve dysfunction, including hand weakness, is much less common but must be well examined and documented. The shoulder must also be evaluated for loss of rotation, which can lead to increased stress on the elbow. An evaluation of mechanics may point out flaws in technique, which may be contributing to elbow stress. The UCL stress examination includes static stress at 30° of flexion, the milking test at 90°, and the moving valgus stress test. The presence of pain directly over the UCL or laxity compared to the uninvolved side is suggestive of UCL injury.
Radiographic evaluation is completed in all patients with concern for UCL injury. Standard x-rays of the elbow, including anteroposterior, medial, and lateral obliques, axial olecranon, and lateral views, are obtained to evaluate bony abnormalities. Fifty-seven percent of our series showed some abnormality, most commonly olecranon osteophyte formation or ectopic calcification within the UCL substance. Stress radiography rarely changed the treatment course and is somewhat difficult to interpret because of the reports documenting normal increased medial elbow opening in the dominant arm of throwing athletes.21 Magnetic resonance imaging (MRI) is obtained very commonly in this patient population, and intra-articular contrast is crucial. Partial, undersurface tears are common, and a contrasted study better demonstrates undersurface tears or avulsions. The T-sign as described by Timmerman and colleagues22 using computed tomography (CT) arthrography shows partial undersurface detachment, which can be difficult to see without intra-articular contrast.22 This finding is very well visualized on MRI arthrogram as well (Figure 3).
Nonoperative Management
Nonoperative treatment is recommended for 3 months prior to performing reconstruction. Patients are given complete rest from throwing, but rehabilitation is initiated immediately. Rehabilitation exercises and nonsteroidal anti-inflammatory medications are prescribed, and activities that place valgus stress across the elbow are avoided. After resolution of symptoms, an interval throwing program is initiated, and the athlete is gradually returned to sport. Unfortunately, due to season-specific schedules and time-sensitive demands in high-level throwers, operative treatment is often chosen without an extended period of conservative treatment.
Platelet-rich plasma (PRP) therapy has recently been shown to improve healing rates and promote healing in partial UCL tears,23 and as orthobiologics are advanced, they will likely play a larger role in the treatment of UCL injuries.
Surgical Technique
At our institution, UCL reconstruction is performed with the modified Jobe technique as described by Azar and colleagues.17 Arthroscopy prior to reconstruction was routinely performed at our institution until we recognized that arthroscopy rarely changed the preoperative plan.16 Currently, the presence of anterior pathology such as loose bodies or osteochondral defect is our only indication for arthroscopy before reconstruction.
Ipsilateral palmaris autograft is our current graft of choice. This must be examined preoperatively because 16% of patients have unilateral absence and 9% have bilateral absence.24 In revision cases or in patients with insufficient or absent palmaris, contralateral palmaris followed by contralateral gracilis tendon is used. The contralateral gracilis is chosen because of ease of setup and position of the surgeon during the harvest. Gracilis tendon is also used in cases with bony involvement of the ligament based on the results from Dugas and colleagues.25 Toe extensors, plantaris, and patellar tendon grafts have also been used. One recent study showed that neither graft choice nor diameter affected resistance to valgus stress, and that all reconstruction types restored strength at 60° to 120° of flexion.26
Ulnar nerve transposition is performed in all cases regardless of the presence of preoperative nerve symptoms. A complete decompression is completed proximally to the Arcade of Struthers and distally to the deep portion of the flexor carpi ulnaris. A single fascial sling of medial intermuscular septum originating from the epicondylar attachment is used to stabilize the nerve without compression. At wound closure, the deep fascia on the posterior skin flap is also sewn into the cubital tunnel to prevent the nerve from subluxating back into the groove. A single suture is placed distally closing the muscle fascia to prevent propagation of the fascial incision, which can lead to herniation. Transposition is necessary because of the ulnar nerve exposure required in the modified Jobe technique to allow elevation of the deep flexor muscle mass for ligament exposure.
The reconstruction is completed as described by Jobe14 but with a few modifications as described by Azar and colleagues17 and slight adaptations implemented since that time. The flexor-pronator mass is retracted laterally instead of detachment or splitting as described by Thompson and colleagues.27 A subcutaneous rather than a submuscular ulnar nerve transposition is used.
The patient is positioned supine using an arm board. If gracilis tendon is chosen, the contralateral leg is prepped and draped simultaneously. A tourniquet is inflated after exsanguination. A medial approach is performed, and the medial antebrachial nerve is located and protected. The ulnar nerve is then located in the cubital tunnel and mobilized. The neurolysis extends to the deep portion of the flexor carpi ulnaris distally and proximally to the Arcade of Struthers, and the nerve is retracted with a vessel loop. The flexor muscle mass is not elevated from the medial epicondyle; rather, it is retracted anteriorly by small Hohmann retractors. The dissection is carried down to the UCL and found at its attachments to the medial epicondyle and sublime tubercle. If no tear is seen on the superficial surface of the ligament, a longitudinal incision is made through the ligament. Undersurface tears, partial tears, and avulsions can then be identified (Figure 4).
The autologous graft of choice is then harvested. Our technique for palmaris harvest is performed with three 1-cm transverse incisions. The palmaris is palpated and marked with the first incision made near the distal wrist crease, and the second incision is made 3 to 4 cm proximal to the first. The tendon is found in both distal incisions and cut distally with the wrist flexed to maximize tendon length. The tendon is then pulled through the second incision and tensioned to identify the most proximal location the tendon can be palpated. A third incision is made directly over this point and carried down to cut the tendon. This usually provides a graft length of 15 to 20 cm; 13 cm is the minimum graft length to ensure good graft fixation. Muscle is removed from the tendon and each end is secured with a No. 1 nonabsorbable suture in a locking fashion.
If posterior osteophytes are present, they are removed through a posterior, vertical arthrotomy. Over-resection of the olecranon must be avoided, as this can further destabilize the elbow and place increased stress on the reconstruction. Posterior loose bodies can also be removed through this arthrotomy. The arthrotomy is then closed with absorbable suture.
Tunnel placement is critical to success. A 3.2-mm drill bit is used with palmaris grafts and a 4-mm drill bit is used with gracilis grafts. Two convergent tunnels are drilled in the medial epicondyle in a Y fashion and 2 convergent tunnels are drilled at the sublime tubercle in a U or V fashion. After drilling the first tunnel on each side, a hemostat is placed in the tunnel as an aiming point to ensure a complete tunnel is made. The junction is smoothed with a curette, leaving a 5-mm bone bridge between the articular surface and the tunnels. A bent Hewson suture passer is used to pass one end of the graft through the ulna. The 2 limbs of the tendon graft are then passed through the humeral tunnels, creating a figure-of-eight. A varus stress is applied with the elbow at roughly 30° and the 2 limbs are tied together with a No. 1 nonabsorbable suture. If enough graft remains, one or both limbs are passed back through the tunnels and secured again with No. 1 nonabsorbable suture. The 2 limbs are then tied side-to-side, incorporating the native ligament to further secure and tighten the reconstruction.
The ulnar nerve is then secured using a strip of medial intermuscular septum left intact to its insertion at the medial epicondyle. This is attached to the flexor-pronator muscle fascia with a 3-0 nonabsorbable suture. Enough length should be harvested from the septum to ensure there is no compression on the nerve. The deep posterior fascial tissue is then sewn to the periosteum of the medial epicondyle to further prevent subluxation of the nerve back into the groove. The skin is then closed in layered fashion over a superficial drain. The patient is placed in a well-padded posterior splint for 1 week, then the rehabilitation protocol is initiated as discussed below.
Postoperative Rehabilitation
A standardized postoperative 4-phase rehabilitation program for ulnar collateral reconstruction is followed as described by Wilk and colleagues.28-30 The first phase begins immediately after surgery and continues for 4 weeks. During surgery, the patient’s elbow is placed in a compression dressing with a posterior splint to immobilize the elbow in 90° of flexion with wrist motion for 1 week to allow initial healing. Full range of motion of the elbow joint is restored by the end of the fifth to sixth week after surgery.
During phase II (weeks 4-10), a progressive isotonic strengthening program is initiated. Exercises are focused on scapular, rotator cuff, deltoid, and arm musculature. Shoulder range of motion and stretching exercises are performed during this phase and the Thrower’s Ten exercise program is initiated. Any adaptations or strength deficits are addressed during this phase.
During the advanced strengthening phase (phase III), from weeks 10 to 16, a sport-specific exercise/rehabilitation program is initiated. During this phase, stretching and flexibility exercises are performed to enhance strength, power, and endurance. During this phase the patient is placed on the advanced Thrower’s Ten program. Isotonic strengthening exercises are progressed, and at week 12, the athlete is allowed to begin an isotonic lifting program, including bench press, seated rowing, latissimus dorsi pull downs, triceps push downs, and biceps curls. In addition, the athlete performs specific exercises to emphasize sport-specific movements. At week 12, overhead athletes begin a 2-hand plyometric throwing program, and at 14 weeks, a 1
Discussion
Results after ulnar collateral reconstruction have been good. In our series of 743 patients, 83% returned to the same or higher level at an average of 11.6 months.16 There was a 4% major complication rate and 16% minor complication rate. Major complications included medial epicondyle fracture (0.5%), significant ulnar nerve dysfunction (1 patient), rupture of graft (1%), and graft site infection. Sixteen percent of patients had ulnar nerve dysfunction, and 82% of these resolved within 6 weeks. All but 1 patient’s paresthesias resolved within 1 year.16 The 10-year follow-up of this group of patients included 256 patients and was reported by Osbahr and colleagues31 in 2014. Retirement from baseball was due to reasons other than the elbow in 86%, and 98% were still able to throw on at least a recreational level. The overall longevity was 3.6 years, with 2.9 years at pre-injury level or higher. Statistically, pitchers performed at a higher level after reconstruction.31
A recent review by Erickson and colleagues9 showed an overall 82% excellent and 8% good result when evaluating different techniques, including the American Sports Medicine Institute (ASMI) modification of Jobe’s technique, docking technique, and Jobe’s technique. With an overall complication rate of 10% (75% of which was transient ulnar neuritis), the procedure was deemed overall a safe surgical option. Collegiate athletes had the highest return to sport (95%) compared with high school athletes (89%) and professional athletes (86%). The docking technique had the highest rate of return to play (97%) compared with ASMI technique (93%) and Jobe technique (66%).9 Results after repair have not been as good as reconstruction, as reported in 2 studies.16,32 Savoie and colleagues,15 however, reported 93% good/excellent results after primary UCL repair alone.
Another recent review of outcomes showed an overall return to same or higher level was best with docking or modified docking techniques (90.4% and 91.3%, respectively).19 Overall return with modified Jobe technique was 77%.19 O’Brien and colleagues20 performed a review of 33 patients with either modified Jobe or docking technique that showed 81% return to same or higher level with modified Jobe vs 92% with docking technique. The Kerlan-Jobe Orthopaedic Clinic scores were higher in the modified Jobe group (79 vs 74) and the docking technique group returned to play nearly 1 month sooner (12.4 months vs 11.8 months).20 However, comparing different techniques in a heterogenous patient population over 40 years is difficult. Many of the modified Jobe technique cases were performed in the early evolution of the rehabilitation and return-to-play programs. We believe that the current modified Jobe technique has results equal to any other variation.
Despite good results with reconstructions, the recovery is lengthy and most pitchers cannot fully return to competition level for 12 to 18 months. Extensive research has been performed in exploring alternatives to the traditional reconstruction. Advancements in orthobiologics and development of new surgical options seem to provide an alternative to reconstruction, and may allow faster return to competition with less morbidity.
PRP has been at the forefront of orthopedic research for the last 2 decades, mostly focused in tendon and bone healing. Due to the release of many inflammatory mediators, PRP is theorized to initiate a healing response with growth factors that can direct healing towards normal tissue.33 Two main types of PRP are reported based on the presence or absence of leukocytes. PRP has been studied in many applications, but only one clinical study on the UCL has been published to date. Podesta and colleagues23 injected PRP into the elbow of 34 baseball players with MRI-confirmed partial UCL tear. The athletes then underwent a rehabilitation program, which limited stress across the UCL. Type 1A PRP was used (leukocyte-rich, unactivated, 5x or greater platelet concentration33). Athletes were allowed to return to sport based on symptoms and examination findings. Eighty-eight percent returned to same level of play without complaints at average 70 week follow-up, and average return to play ranged from 10 to 15 weeks.23 No specific data were given on the 16 pitchers in the group, but with such a high rate of return, PRP needs to be further evaluated in the treatment of UCL injuries.
Another recent study from Dugas and colleagues18 presented primary UCL repair using a tape augment (InternalBrace, Arthrex). Nine matched cadaver elbows underwent UCL sectioning and then either modified Jobe reconstruction or primary repair of the UCL with placement of the InternalBrace. The biomechanical data showed the repair with internal brace to have slightly less gap, more stiffness, and higher failure strength, although these findings were not statistically significant.18 This bone-preserving technique with less exposure and healing of the native ligament may be another step towards good results with a quicker return to throwing.
Conclusion
UCL injuries can be disabling in throwers. Reconstruction has afforded throwers a high rate of return to preinjury function or better, and several techniques have been presented that produce acceptable results. Overall complication rates range from 10% to 15%, and the majority of complications are transient ulnar neuropraxias. Orthobiologics and repair with augmentation have more recently offered additional options that may improve success of nonoperative treatment or allow less-invasive surgical treatment. Increased involvement in youth sports and early specialization is driving injury rates in young athletes. The orthopedic community must continue to look for better ways to prevent these injuries and investigate better methods to return athletes to high-level competition.
Am J Orthop. 2016;45(7):E534-E540. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
The ulnar collateral ligament (UCL) is the primary restraint to valgus stress between 20° and 125° of motion.1-5 Overhead athletes, most commonly baseball pitchers, are at risk of developing UCL insufficiency, and dysfunction presents as pain with loss of velocity and control. Some injuries may present acutely while throwing, but many patients, when questioned, report a preceding period of either pain or loss of velocity and control.
Authors have documented a significant rise in elbow injuries in young athletes, especially pitchers.6 Extended seasons, higher pitch counts, year-round pitching, pitching while fatigued, and pitching for multiple teams are risk factors for elbow injuries.7 Pitchers in the southern United States are more likely to undergo UCL reconstruction than those from the northern states.8 Pitchers who also play catcher are at a higher risk due to more total throws than those who pitch and play other positions or pitch only. Throwers with higher velocity are more likely to pitch in showcases, pitch for multiple teams, and pitch with pain and fatigue, and these are all risk factors.6 Also, in one study of youth baseball injuries, individuals in the injured group were found to be taller and heavier than those in the uninjured group.6 Pitch counts, rest from pitching during the off-season, adequate rest, and ensuring pain-free pitching can lessen the risk of injury.6 As expected with the rise in throwing injuries, the rise in medial elbow procedures has risen.9
While throwing, stress across the medial elbow has been measured to be nearly 300 N. A maximum varus force during pitching was measured to be 64 N-m at 95° ± 14°.10 Morrey and An4 determined that the UCL generated 54% of the varus force at 90° of flexion. During active pitching, this value is likely reduced due to simultaneous muscle contraction, but if one assumes the UCL bears 54% of the maximal load, the UCL must be able to withstand 34 N-m. The UCL can withstand a maximum valgus torque between 22.7 and 34 N-m11-13; therefore, during pitching, the UCL is at or above its failure load. After thousands of cycles over many years, one can imagine how the UCL might be injured.
Multiple techniques have been proposed in the surgical treatment of UCL injuries. Jobe14 pioneered UCL reconstruction in 1974 in Tommy John, a Major League Baseball pitcher. John returned to pitch successfully, and both the UCL and the reconstruction are commonly called by his name. Jobe14 reported his technique in 1986, and it has remained, with a few modifications, the primary method for reconstruction of the UCL (Figure 1).
Evaluation
A standard evaluation with physical examination and imaging is completed in all throwers with elbow pain. In our prior study,16 we found that 100% of patients experienced pain during athletic activity and that 96% of throwers complained of pain during late cocking and acceleration phases of the throwing motion. Nearly half reported an acute onset of pain, while 53% were unable to identify a single inciting event. Seventy-five percent of the acute injuries were during competition. Delayed diagnosis was very common, with an average time to diagnosis after onset of symptoms of 6.4 months. Neurologic symptoms were seen in 23% of athletes, most of which were ulnar nerve paresthesias during throwing.16
Physical examination includes inspection for swelling, hand intrinsic atrophy, neurovascular examination, range of motion, shoulder examination, and elbow stress examination. Range of motion at presentation averaged 5° to 135° with 85° of supination and pronation.16 All patients need neurologic evaluation for ulnar nerve dysfunction. Tinel test of the cubital tunnel was positive in 21%.16 Significant ulnar nerve dysfunction, including hand weakness, is much less common but must be well examined and documented. The shoulder must also be evaluated for loss of rotation, which can lead to increased stress on the elbow. An evaluation of mechanics may point out flaws in technique, which may be contributing to elbow stress. The UCL stress examination includes static stress at 30° of flexion, the milking test at 90°, and the moving valgus stress test. The presence of pain directly over the UCL or laxity compared to the uninvolved side is suggestive of UCL injury.
Radiographic evaluation is completed in all patients with concern for UCL injury. Standard x-rays of the elbow, including anteroposterior, medial, and lateral obliques, axial olecranon, and lateral views, are obtained to evaluate bony abnormalities. Fifty-seven percent of our series showed some abnormality, most commonly olecranon osteophyte formation or ectopic calcification within the UCL substance. Stress radiography rarely changed the treatment course and is somewhat difficult to interpret because of the reports documenting normal increased medial elbow opening in the dominant arm of throwing athletes.21 Magnetic resonance imaging (MRI) is obtained very commonly in this patient population, and intra-articular contrast is crucial. Partial, undersurface tears are common, and a contrasted study better demonstrates undersurface tears or avulsions. The T-sign as described by Timmerman and colleagues22 using computed tomography (CT) arthrography shows partial undersurface detachment, which can be difficult to see without intra-articular contrast.22 This finding is very well visualized on MRI arthrogram as well (Figure 3).
Nonoperative Management
Nonoperative treatment is recommended for 3 months prior to performing reconstruction. Patients are given complete rest from throwing, but rehabilitation is initiated immediately. Rehabilitation exercises and nonsteroidal anti-inflammatory medications are prescribed, and activities that place valgus stress across the elbow are avoided. After resolution of symptoms, an interval throwing program is initiated, and the athlete is gradually returned to sport. Unfortunately, due to season-specific schedules and time-sensitive demands in high-level throwers, operative treatment is often chosen without an extended period of conservative treatment.
Platelet-rich plasma (PRP) therapy has recently been shown to improve healing rates and promote healing in partial UCL tears,23 and as orthobiologics are advanced, they will likely play a larger role in the treatment of UCL injuries.
Surgical Technique
At our institution, UCL reconstruction is performed with the modified Jobe technique as described by Azar and colleagues.17 Arthroscopy prior to reconstruction was routinely performed at our institution until we recognized that arthroscopy rarely changed the preoperative plan.16 Currently, the presence of anterior pathology such as loose bodies or osteochondral defect is our only indication for arthroscopy before reconstruction.
Ipsilateral palmaris autograft is our current graft of choice. This must be examined preoperatively because 16% of patients have unilateral absence and 9% have bilateral absence.24 In revision cases or in patients with insufficient or absent palmaris, contralateral palmaris followed by contralateral gracilis tendon is used. The contralateral gracilis is chosen because of ease of setup and position of the surgeon during the harvest. Gracilis tendon is also used in cases with bony involvement of the ligament based on the results from Dugas and colleagues.25 Toe extensors, plantaris, and patellar tendon grafts have also been used. One recent study showed that neither graft choice nor diameter affected resistance to valgus stress, and that all reconstruction types restored strength at 60° to 120° of flexion.26
Ulnar nerve transposition is performed in all cases regardless of the presence of preoperative nerve symptoms. A complete decompression is completed proximally to the Arcade of Struthers and distally to the deep portion of the flexor carpi ulnaris. A single fascial sling of medial intermuscular septum originating from the epicondylar attachment is used to stabilize the nerve without compression. At wound closure, the deep fascia on the posterior skin flap is also sewn into the cubital tunnel to prevent the nerve from subluxating back into the groove. A single suture is placed distally closing the muscle fascia to prevent propagation of the fascial incision, which can lead to herniation. Transposition is necessary because of the ulnar nerve exposure required in the modified Jobe technique to allow elevation of the deep flexor muscle mass for ligament exposure.
The reconstruction is completed as described by Jobe14 but with a few modifications as described by Azar and colleagues17 and slight adaptations implemented since that time. The flexor-pronator mass is retracted laterally instead of detachment or splitting as described by Thompson and colleagues.27 A subcutaneous rather than a submuscular ulnar nerve transposition is used.
The patient is positioned supine using an arm board. If gracilis tendon is chosen, the contralateral leg is prepped and draped simultaneously. A tourniquet is inflated after exsanguination. A medial approach is performed, and the medial antebrachial nerve is located and protected. The ulnar nerve is then located in the cubital tunnel and mobilized. The neurolysis extends to the deep portion of the flexor carpi ulnaris distally and proximally to the Arcade of Struthers, and the nerve is retracted with a vessel loop. The flexor muscle mass is not elevated from the medial epicondyle; rather, it is retracted anteriorly by small Hohmann retractors. The dissection is carried down to the UCL and found at its attachments to the medial epicondyle and sublime tubercle. If no tear is seen on the superficial surface of the ligament, a longitudinal incision is made through the ligament. Undersurface tears, partial tears, and avulsions can then be identified (Figure 4).
The autologous graft of choice is then harvested. Our technique for palmaris harvest is performed with three 1-cm transverse incisions. The palmaris is palpated and marked with the first incision made near the distal wrist crease, and the second incision is made 3 to 4 cm proximal to the first. The tendon is found in both distal incisions and cut distally with the wrist flexed to maximize tendon length. The tendon is then pulled through the second incision and tensioned to identify the most proximal location the tendon can be palpated. A third incision is made directly over this point and carried down to cut the tendon. This usually provides a graft length of 15 to 20 cm; 13 cm is the minimum graft length to ensure good graft fixation. Muscle is removed from the tendon and each end is secured with a No. 1 nonabsorbable suture in a locking fashion.
If posterior osteophytes are present, they are removed through a posterior, vertical arthrotomy. Over-resection of the olecranon must be avoided, as this can further destabilize the elbow and place increased stress on the reconstruction. Posterior loose bodies can also be removed through this arthrotomy. The arthrotomy is then closed with absorbable suture.
Tunnel placement is critical to success. A 3.2-mm drill bit is used with palmaris grafts and a 4-mm drill bit is used with gracilis grafts. Two convergent tunnels are drilled in the medial epicondyle in a Y fashion and 2 convergent tunnels are drilled at the sublime tubercle in a U or V fashion. After drilling the first tunnel on each side, a hemostat is placed in the tunnel as an aiming point to ensure a complete tunnel is made. The junction is smoothed with a curette, leaving a 5-mm bone bridge between the articular surface and the tunnels. A bent Hewson suture passer is used to pass one end of the graft through the ulna. The 2 limbs of the tendon graft are then passed through the humeral tunnels, creating a figure-of-eight. A varus stress is applied with the elbow at roughly 30° and the 2 limbs are tied together with a No. 1 nonabsorbable suture. If enough graft remains, one or both limbs are passed back through the tunnels and secured again with No. 1 nonabsorbable suture. The 2 limbs are then tied side-to-side, incorporating the native ligament to further secure and tighten the reconstruction.
The ulnar nerve is then secured using a strip of medial intermuscular septum left intact to its insertion at the medial epicondyle. This is attached to the flexor-pronator muscle fascia with a 3-0 nonabsorbable suture. Enough length should be harvested from the septum to ensure there is no compression on the nerve. The deep posterior fascial tissue is then sewn to the periosteum of the medial epicondyle to further prevent subluxation of the nerve back into the groove. The skin is then closed in layered fashion over a superficial drain. The patient is placed in a well-padded posterior splint for 1 week, then the rehabilitation protocol is initiated as discussed below.
Postoperative Rehabilitation
A standardized postoperative 4-phase rehabilitation program for ulnar collateral reconstruction is followed as described by Wilk and colleagues.28-30 The first phase begins immediately after surgery and continues for 4 weeks. During surgery, the patient’s elbow is placed in a compression dressing with a posterior splint to immobilize the elbow in 90° of flexion with wrist motion for 1 week to allow initial healing. Full range of motion of the elbow joint is restored by the end of the fifth to sixth week after surgery.
During phase II (weeks 4-10), a progressive isotonic strengthening program is initiated. Exercises are focused on scapular, rotator cuff, deltoid, and arm musculature. Shoulder range of motion and stretching exercises are performed during this phase and the Thrower’s Ten exercise program is initiated. Any adaptations or strength deficits are addressed during this phase.
During the advanced strengthening phase (phase III), from weeks 10 to 16, a sport-specific exercise/rehabilitation program is initiated. During this phase, stretching and flexibility exercises are performed to enhance strength, power, and endurance. During this phase the patient is placed on the advanced Thrower’s Ten program. Isotonic strengthening exercises are progressed, and at week 12, the athlete is allowed to begin an isotonic lifting program, including bench press, seated rowing, latissimus dorsi pull downs, triceps push downs, and biceps curls. In addition, the athlete performs specific exercises to emphasize sport-specific movements. At week 12, overhead athletes begin a 2-hand plyometric throwing program, and at 14 weeks, a 1
Discussion
Results after ulnar collateral reconstruction have been good. In our series of 743 patients, 83% returned to the same or higher level at an average of 11.6 months.16 There was a 4% major complication rate and 16% minor complication rate. Major complications included medial epicondyle fracture (0.5%), significant ulnar nerve dysfunction (1 patient), rupture of graft (1%), and graft site infection. Sixteen percent of patients had ulnar nerve dysfunction, and 82% of these resolved within 6 weeks. All but 1 patient’s paresthesias resolved within 1 year.16 The 10-year follow-up of this group of patients included 256 patients and was reported by Osbahr and colleagues31 in 2014. Retirement from baseball was due to reasons other than the elbow in 86%, and 98% were still able to throw on at least a recreational level. The overall longevity was 3.6 years, with 2.9 years at pre-injury level or higher. Statistically, pitchers performed at a higher level after reconstruction.31
A recent review by Erickson and colleagues9 showed an overall 82% excellent and 8% good result when evaluating different techniques, including the American Sports Medicine Institute (ASMI) modification of Jobe’s technique, docking technique, and Jobe’s technique. With an overall complication rate of 10% (75% of which was transient ulnar neuritis), the procedure was deemed overall a safe surgical option. Collegiate athletes had the highest return to sport (95%) compared with high school athletes (89%) and professional athletes (86%). The docking technique had the highest rate of return to play (97%) compared with ASMI technique (93%) and Jobe technique (66%).9 Results after repair have not been as good as reconstruction, as reported in 2 studies.16,32 Savoie and colleagues,15 however, reported 93% good/excellent results after primary UCL repair alone.
Another recent review of outcomes showed an overall return to same or higher level was best with docking or modified docking techniques (90.4% and 91.3%, respectively).19 Overall return with modified Jobe technique was 77%.19 O’Brien and colleagues20 performed a review of 33 patients with either modified Jobe or docking technique that showed 81% return to same or higher level with modified Jobe vs 92% with docking technique. The Kerlan-Jobe Orthopaedic Clinic scores were higher in the modified Jobe group (79 vs 74) and the docking technique group returned to play nearly 1 month sooner (12.4 months vs 11.8 months).20 However, comparing different techniques in a heterogenous patient population over 40 years is difficult. Many of the modified Jobe technique cases were performed in the early evolution of the rehabilitation and return-to-play programs. We believe that the current modified Jobe technique has results equal to any other variation.
Despite good results with reconstructions, the recovery is lengthy and most pitchers cannot fully return to competition level for 12 to 18 months. Extensive research has been performed in exploring alternatives to the traditional reconstruction. Advancements in orthobiologics and development of new surgical options seem to provide an alternative to reconstruction, and may allow faster return to competition with less morbidity.
PRP has been at the forefront of orthopedic research for the last 2 decades, mostly focused in tendon and bone healing. Due to the release of many inflammatory mediators, PRP is theorized to initiate a healing response with growth factors that can direct healing towards normal tissue.33 Two main types of PRP are reported based on the presence or absence of leukocytes. PRP has been studied in many applications, but only one clinical study on the UCL has been published to date. Podesta and colleagues23 injected PRP into the elbow of 34 baseball players with MRI-confirmed partial UCL tear. The athletes then underwent a rehabilitation program, which limited stress across the UCL. Type 1A PRP was used (leukocyte-rich, unactivated, 5x or greater platelet concentration33). Athletes were allowed to return to sport based on symptoms and examination findings. Eighty-eight percent returned to same level of play without complaints at average 70 week follow-up, and average return to play ranged from 10 to 15 weeks.23 No specific data were given on the 16 pitchers in the group, but with such a high rate of return, PRP needs to be further evaluated in the treatment of UCL injuries.
Another recent study from Dugas and colleagues18 presented primary UCL repair using a tape augment (InternalBrace, Arthrex). Nine matched cadaver elbows underwent UCL sectioning and then either modified Jobe reconstruction or primary repair of the UCL with placement of the InternalBrace. The biomechanical data showed the repair with internal brace to have slightly less gap, more stiffness, and higher failure strength, although these findings were not statistically significant.18 This bone-preserving technique with less exposure and healing of the native ligament may be another step towards good results with a quicker return to throwing.
Conclusion
UCL injuries can be disabling in throwers. Reconstruction has afforded throwers a high rate of return to preinjury function or better, and several techniques have been presented that produce acceptable results. Overall complication rates range from 10% to 15%, and the majority of complications are transient ulnar neuropraxias. Orthobiologics and repair with augmentation have more recently offered additional options that may improve success of nonoperative treatment or allow less-invasive surgical treatment. Increased involvement in youth sports and early specialization is driving injury rates in young athletes. The orthopedic community must continue to look for better ways to prevent these injuries and investigate better methods to return athletes to high-level competition.
Am J Orthop. 2016;45(7):E534-E540. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. Fuss FK. The ulnar collateral ligament of the human elbow joint. Anatomy, function and biomechanics. J Anat. 1991;175:203-212.
2. Hotchkiss RN, Weiland AJ. Valgus stability of the elbow. J Orthop Res. 1987;5(3):372-377.
3. Morrey BF. Applied anatomy and biomechanics of the elbow joint. Instr Course Lect. 1986;35:59-68.
4. Morrey BF, An KN. Articular and ligamentous contributions to the stability of the elbow joint. Am J Sports Med. 1983;11(5):315-319.
5. Morrey BF, An KN. Functional anatomy of the ligaments of the elbow. Clin Orthop. 1985;(201):84-90.
6. Olsen SJ 2nd, Fleisig GS, Dun S, Loftice J, Andrews JR. Risk factors for shoulder and elbow injuries in adolescent baseball pitchers. Am J Sports Med. 2006;34(6):905-912.
7. Fleisig GS, Andrews JR. Prevention of elbow injuries in youth baseball pitchers. Sports Health. 2012;4(5):419-424.
8. Zaremski JL, Horodyski M, Donlan RM, Brisbane ST, Farmer KW. Does geographic location matter on the prevalence of ulnar collateral ligament reconstruction in collegiate baseball pitchers? Orthop J Sports Med. 2015;3(11):2325967115616582.
9. Erickson BJ, Nwachukwu BU, Rosas S, et al. Trends in medial ulnar collateral ligament reconstruction in the United States: A retrospective review of a large private-payer database from 2007 to 2011. Am J Sports Med. 2015;43(7):1770-1774.
10. Fleisig GS, Andrews JR, Dillman CJ. Kinetics of baseball pitching with implications about injury mechanism. Am J Sports Med. 1995;23(2):233-239.
11. Dillman CJ, Smutz P, Werner S. Valgus extension overload in baseball pitching. Med Sci Sports Exerc. 1991;23(suppl 4):S135.
12. Hechtman KS, Tjin-A-Tsoi EW, Zvijac JE, Uribe JW, Latta LL. Biomechanics of a less invasive procedure for reconstruction of the ulnar collateral ligament of the elbow. Am J Sports Med. 1998;26(5):620-624.
13. Ahmad CS, Lee TQ, ElAttrache NS. Biomechanical evaluation of a new ulnar collateral ligament reconstruction technique with interference screw fixation. Am J Sports Med. 2003;31(3):332-337.
14. Jobe FW, Stark HE, Lombardo SJ. Reconstruction of the ulnar collateral ligament in athletes. J Bone Joint Surg Am. 1986;68(8):1158-1163.
15. Savoie FH 3rd, Trenhaile SW, Roberts J, Field LD, Ramsey JR. Primary repair of ulnar collateral ligament injuries of the elbow in young athletes: a case series of injuries to the proximal and distal ends of the ligament. Am J Sports Med. 2008;36(6):1066-1072.
16. Cain EL, Andrews JR, Dugas JR, et al. Outcome of ulnar collateral ligament reconstruction of the elbow in 1281 athletes results in 743 athletes with minimum 2-year follow-up. Am J Sports Med. 2010;38(12):2426-2434.
17. Azar FM, Andrews JR, Wilk KE, Groh D. Operative treatment of ulnar collateral ligament injuries of the elbow in athletes. Am J Sports Med. 2000;28(1):16-23.
18. Dugas JR, Walters BL, Beason DP, Fleisig GS, Chronister JE. Biomechanical comparison of ulnar collateral ligament repair with internal bracing versus modified Jobe reconstruction. Am J Sports Med. 2016;44(3):735-741.
19. Watson JN, McQueen P, Hutchinson MR. A systematic review of ulnar collateral ligament reconstruction techniques. Am J Sports Med. 2014;42(10):2510-2516.
20. O’Brien DF, O’Hagan T, Stewart R, et al. Outcomes for ulnar collateral ligament reconstruction: A retrospective review using the KJOC assessment score with two-year follow-up in an overhead throwing population. J Shoulder Elbow Surg. 2015;24(6):934-940.
21. Ellenbecker TS, Mattalino AJ, Elam EA, Caplinger RA. Medial elbow joint laxity in professional baseball pitchers a bilateral comparison using stress radiography. Am J Sports Med. 1998;26(3):420-424.
22. Timmerman LA, Schwartz ML, Andrews JR. Preoperative evaluation of the ulnar collateral ligament by magnetic resonance imaging and computed tomography arthrography evaluation in 25 baseball players with surgical confirmation. Am J Sports Med. 1994;22(1):26-32.
23. Podesta L, Crow SA, Volkmer D, Bert T, Yocum LA. Treatment of partial ulnar collateral ligament tears in the elbow with platelet-rich plasma. Am J Sports Med. 2013;41(7):1689-1694.
24. Thompson NW, Mockford BJ, Cran GW. Absence of the palmaris longus muscle: a population study. Ulster Med J. 2001;70(1):22-24.
25. Dugas JR, Bilotta J, Watts CD, et al. Ulnar collateral ligament reconstruction with gracilis tendon in athletes with intraligamentous bony excision technique and results. Am J Sports Med. 2012;40(7):1578-1582.
26. Dargel J, Küpper F, Wegmann K, Oppermann J, Eysel P, Müller LP. Graft diameter does not influence primary stability of ulnar collateral ligament reconstruction of the elbow. J Orthop Sci. 2015;20(2):307-313.
27. Thompson WH, Jobe FW, Yocum LA, Pink MM. Ulnar collateral ligament reconstruction in athletes: muscle-splitting approach without transposition of the ulnar nerve. J Shoulder Elbow Surg. 2001;10(2):152-157.
28. Wilk KE, Arrigo CA, Andrews JR. Rehabilitation of the elbow in the throwing athlete. J Orthop Sports Phys Ther. 1993;17(6):305-317.
29. Wilk KE, Arrigo CA, Andrews JR, et al. Rehabilitation following elbow surgery in the throwing athlete. Oper Tech Sports Med. 1996;4:114-132.
30. Wilk KE, Arrigo CA, Andrews JR, et al. Preventative and Rehabilitation Exercises for the Shoulder and Elbow. 4th ed. Birmingham, AL: American Sports Medicine Institute; 1996.
31. Osbahr DC, Cain EL, Raines BT, Fortenbaugh D, Dugas JR, Andrews JR. Long-term outcomes after ulnar collateral ligament reconstruction in competitive baseball players minimum 10-year follow-up. Am J Sports Med. 2014;42(6):1333-1342.
32. Conway JE, Jobe FW, Glousman RE, Pink M. Medial instability of the elbow in throwing athletes. Treatment by repair or reconstruction of the ulnar collateral ligament. J Bone Joint Surg Am. 1992;74(1):67-83.
33. Mishra A, Harmon K, Woodall J, Vieira A. Sports medicine applications of platelet rich plasma. Curr Pharm Biotechnol. 2012;13(7):1185-1195.
1. Fuss FK. The ulnar collateral ligament of the human elbow joint. Anatomy, function and biomechanics. J Anat. 1991;175:203-212.
2. Hotchkiss RN, Weiland AJ. Valgus stability of the elbow. J Orthop Res. 1987;5(3):372-377.
3. Morrey BF. Applied anatomy and biomechanics of the elbow joint. Instr Course Lect. 1986;35:59-68.
4. Morrey BF, An KN. Articular and ligamentous contributions to the stability of the elbow joint. Am J Sports Med. 1983;11(5):315-319.
5. Morrey BF, An KN. Functional anatomy of the ligaments of the elbow. Clin Orthop. 1985;(201):84-90.
6. Olsen SJ 2nd, Fleisig GS, Dun S, Loftice J, Andrews JR. Risk factors for shoulder and elbow injuries in adolescent baseball pitchers. Am J Sports Med. 2006;34(6):905-912.
7. Fleisig GS, Andrews JR. Prevention of elbow injuries in youth baseball pitchers. Sports Health. 2012;4(5):419-424.
8. Zaremski JL, Horodyski M, Donlan RM, Brisbane ST, Farmer KW. Does geographic location matter on the prevalence of ulnar collateral ligament reconstruction in collegiate baseball pitchers? Orthop J Sports Med. 2015;3(11):2325967115616582.
9. Erickson BJ, Nwachukwu BU, Rosas S, et al. Trends in medial ulnar collateral ligament reconstruction in the United States: A retrospective review of a large private-payer database from 2007 to 2011. Am J Sports Med. 2015;43(7):1770-1774.
10. Fleisig GS, Andrews JR, Dillman CJ. Kinetics of baseball pitching with implications about injury mechanism. Am J Sports Med. 1995;23(2):233-239.
11. Dillman CJ, Smutz P, Werner S. Valgus extension overload in baseball pitching. Med Sci Sports Exerc. 1991;23(suppl 4):S135.
12. Hechtman KS, Tjin-A-Tsoi EW, Zvijac JE, Uribe JW, Latta LL. Biomechanics of a less invasive procedure for reconstruction of the ulnar collateral ligament of the elbow. Am J Sports Med. 1998;26(5):620-624.
13. Ahmad CS, Lee TQ, ElAttrache NS. Biomechanical evaluation of a new ulnar collateral ligament reconstruction technique with interference screw fixation. Am J Sports Med. 2003;31(3):332-337.
14. Jobe FW, Stark HE, Lombardo SJ. Reconstruction of the ulnar collateral ligament in athletes. J Bone Joint Surg Am. 1986;68(8):1158-1163.
15. Savoie FH 3rd, Trenhaile SW, Roberts J, Field LD, Ramsey JR. Primary repair of ulnar collateral ligament injuries of the elbow in young athletes: a case series of injuries to the proximal and distal ends of the ligament. Am J Sports Med. 2008;36(6):1066-1072.
16. Cain EL, Andrews JR, Dugas JR, et al. Outcome of ulnar collateral ligament reconstruction of the elbow in 1281 athletes results in 743 athletes with minimum 2-year follow-up. Am J Sports Med. 2010;38(12):2426-2434.
17. Azar FM, Andrews JR, Wilk KE, Groh D. Operative treatment of ulnar collateral ligament injuries of the elbow in athletes. Am J Sports Med. 2000;28(1):16-23.
18. Dugas JR, Walters BL, Beason DP, Fleisig GS, Chronister JE. Biomechanical comparison of ulnar collateral ligament repair with internal bracing versus modified Jobe reconstruction. Am J Sports Med. 2016;44(3):735-741.
19. Watson JN, McQueen P, Hutchinson MR. A systematic review of ulnar collateral ligament reconstruction techniques. Am J Sports Med. 2014;42(10):2510-2516.
20. O’Brien DF, O’Hagan T, Stewart R, et al. Outcomes for ulnar collateral ligament reconstruction: A retrospective review using the KJOC assessment score with two-year follow-up in an overhead throwing population. J Shoulder Elbow Surg. 2015;24(6):934-940.
21. Ellenbecker TS, Mattalino AJ, Elam EA, Caplinger RA. Medial elbow joint laxity in professional baseball pitchers a bilateral comparison using stress radiography. Am J Sports Med. 1998;26(3):420-424.
22. Timmerman LA, Schwartz ML, Andrews JR. Preoperative evaluation of the ulnar collateral ligament by magnetic resonance imaging and computed tomography arthrography evaluation in 25 baseball players with surgical confirmation. Am J Sports Med. 1994;22(1):26-32.
23. Podesta L, Crow SA, Volkmer D, Bert T, Yocum LA. Treatment of partial ulnar collateral ligament tears in the elbow with platelet-rich plasma. Am J Sports Med. 2013;41(7):1689-1694.
24. Thompson NW, Mockford BJ, Cran GW. Absence of the palmaris longus muscle: a population study. Ulster Med J. 2001;70(1):22-24.
25. Dugas JR, Bilotta J, Watts CD, et al. Ulnar collateral ligament reconstruction with gracilis tendon in athletes with intraligamentous bony excision technique and results. Am J Sports Med. 2012;40(7):1578-1582.
26. Dargel J, Küpper F, Wegmann K, Oppermann J, Eysel P, Müller LP. Graft diameter does not influence primary stability of ulnar collateral ligament reconstruction of the elbow. J Orthop Sci. 2015;20(2):307-313.
27. Thompson WH, Jobe FW, Yocum LA, Pink MM. Ulnar collateral ligament reconstruction in athletes: muscle-splitting approach without transposition of the ulnar nerve. J Shoulder Elbow Surg. 2001;10(2):152-157.
28. Wilk KE, Arrigo CA, Andrews JR. Rehabilitation of the elbow in the throwing athlete. J Orthop Sports Phys Ther. 1993;17(6):305-317.
29. Wilk KE, Arrigo CA, Andrews JR, et al. Rehabilitation following elbow surgery in the throwing athlete. Oper Tech Sports Med. 1996;4:114-132.
30. Wilk KE, Arrigo CA, Andrews JR, et al. Preventative and Rehabilitation Exercises for the Shoulder and Elbow. 4th ed. Birmingham, AL: American Sports Medicine Institute; 1996.
31. Osbahr DC, Cain EL, Raines BT, Fortenbaugh D, Dugas JR, Andrews JR. Long-term outcomes after ulnar collateral ligament reconstruction in competitive baseball players minimum 10-year follow-up. Am J Sports Med. 2014;42(6):1333-1342.
32. Conway JE, Jobe FW, Glousman RE, Pink M. Medial instability of the elbow in throwing athletes. Treatment by repair or reconstruction of the ulnar collateral ligament. J Bone Joint Surg Am. 1992;74(1):67-83.
33. Mishra A, Harmon K, Woodall J, Vieira A. Sports medicine applications of platelet rich plasma. Curr Pharm Biotechnol. 2012;13(7):1185-1195.
Potential Operating Room Fire Hazard of Bone Cement
Approximately 600 cases of operating room (OR) fires are reported annually.1 The incidence of OR fires in the United States equals that of wrong-site surgeries, and 20% of cases have associated morbidity.1,2 The estimated mortality rate is 1 to 2 cases per year.3-5 The most commonly involved anatomical regions are the airway (33%) and the face (28%).4 Most surgical fires are reported in anesthetized patients with open oxygen delivery systems during head, neck, and upper chest surgeries; electrosurgical instruments are the ignition source in 90% of these cases.6 Despite extensive fire safety education and training, complete elimination of OR fires still has not been achieved.
Each fire requires an ignition source, a fuel source, and an oxidizer.7 In the OR, the 2 most common oxidizers are oxygen and nitrous oxide. Head and neck surgeries have a high concentration of these gases near the working field and therefore a higher risk and incidence of fires. Furthermore, surgical drapes and equipment (eg, closed or semi-closed breathing systems, masks) may potentiate this risk by reducing ventilation in areas where gases can accumulate and ignite. Ignition sources provide the energy that starts fires; common sources are electrocautery, lasers, fiber-optic light cords, drills/burrs, and defibrillator paddles. Fires are propagated by fuel sources, which encompass any flammable material, including tracheal tubes, sponges, alcohol-based solutions, hair, gastrointestinal tract gases, gloves, and packaging materials.8 Of note, alcohol-based skin-preparation agents emit flammable vapors that can ignite.9-14 Before draping or exposure to an ignition source, chlorhexidine gluconate-based preparations must be allowed to dry for at least 3 minutes after application to hairless skin and up to 1 hour after application to hair.15 Inadequate drying poses a risk of fire.10We present the case of an OR fire ignited by electrocautery near freshly applied bone cement. No patient information is disclosed in this report.
Case Report
Our patient was evaluated in clinic and scheduled for total knee arthroplasty (TKA). All preoperative safety checklists and time-out procedures were followed and documented at the start of surgery. The TKA was performed with a standard medial patellar arthrotomy. Tourniquet control was used after Esmarch exsanguination. The surgery proceeded uneventfully until just after the bone cement was applied to the tibial surface. The surgeon was using a Bovie to resect residual lateral meniscus tissue when a fire instantaneously erupted within the joint space. Fortunately, the surgeon quickly suffocated the fire with a dry towel. The ignited bone cement was removed, and the patient was examined. There was no injury to surrounding tissue or joint space. Surgery was resumed with application of new bone cement to the tibial surface. The artificial joint was then successfully implanted and the case completed without further incident. The patient was discharged from the hospital and followed up as an outpatient without any postoperative complications.
Discussion
Bone cement, which is commonly used in artificial joint anchoring, craniofacial reconstruction, and vertebroplasty, has liquid and powder components. The liquid monomer methyl methacrylate (MMA) is colorless and flammable and has a distinct odor.16 Exposure to heat or light can prematurely polymerize MMA, requiring the addition of hydroquinone to inhibit the reaction.16 The powder polymethylmethacrylate affords excellent structural support, radiopacity, and facility of use.17 Dibenzoyl peroxide and N,N-dimethyl-p-toluidine are added to the powder to facilitate the polymerization reaction at room temperature (ie, cold curing of cement). Premature application of unpolymerized cement increases the risk of fire from the volatile liquid component.
In the OR, bone cement is prepared by mixing together its powder and liquid components.18 The reaction is exothermic polymerization. The liquid is highly volatile and flammable in both liquid and vapor states.16,19 The vapors are denser than air and can concentrate in poorly ventilated areas. The OR and the application site must be adequately ventilated to eliminate any pockets of vapor accumulation.16 A vacuum mixer can be used to minimize fume exposure, enhance cement strength, and reduce fire risk while combining the 2 components.
MMA’s flash point, the temperature at which the fumes could ignite in the presence of an ignition source, is 10.5ºC. The auto-ignition point, the temperature at which MMA spontaneously combusts, is 421ºC.20 The OR is usually warmer than the flash point temperature, but the electrocautery tip can generate up to 1200ºC of heat.21 Therefore, bone cement is a potential fire hazard, and use of Bovies or other ignition sources in its vicinity must be avoided.
The Table lists the recommended times for preparing various bone cement products.22,23Mix time is the time needed to combine the liquid and powder into a homogenous putty.
For OR fires, the standard guidelines for rapid containment and safety apply. These guidelines are detailed by the American Society of Anesthesiologists.8 Briefly, delivery of all airway gases to the patient is discontinued. Any burning material is removed and extinguished by the OR staff.1 Carbon dioxide fire extinguishers are used to put out any patient fires and minimize the risk of thermal injury. (Water-mist fire extinguishers can contaminate surgical wounds and present an electric shock hazard with surgical devices and should be avoided.24) If a fire occurs in a patient’s airway, the tracheal tube is removed, and airway patency is maintained with use of other invasive or noninvasive techniques. Often, noninvasive positive pressure ventilation without supplemental oxygen is used until the fire is controlled and the patient is safe. Once the patient fire is controlled, ventilation is restarted, and the patient is evacuated from the OR and away from any other hazards, as required. Last, the patient is physically examined for any injuries and treated.24 Specific to TKA, the procedure is resumed after removal of all bone cement, inspection of the operative site, and treatment of any fire-related injuries.
We have reported the case of an OR fire during TKA. Appropriate selection and use of bone cement products, proper assessment of set time, and avoidance of electrocautery near cement application sites may dramatically reduce associated fire risks.
Am J Orthop. 2016;45(7):E512-E514. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. Hart SR, Yajnik A, Ashford J, Springer R, Harvey S. Operating room fire safety. Ochsner J. 2011;11(1):37-42.
2. American Society of Anesthesiologists Task Force on Operating Room Fires; Caplan RA, Barker SJ, Connis RT, et al. Practice advisory for the prevention and management of operating room fires. Anesthesiology. 2008;108(5):786-801.
3. Bruley M. Surgical fires: perioperative communication is essential to prevent this rare but devastating complication. Qual Saf HealthCare. 2004;13(6):467-471.
4. Daane SP, Toth BA. Fire in the operating room: principles and prevention. Plast Reconstr Surg. 2005;115(5):73e-75e.
5. Rinder CS. Fire safety in the operating room. Curr Opin Anaesthesiol. 2008;21(6):790-795.
6. Mathias JM. Fast action, team coordination critical when surgical fires occur. OR Manager. 2013;29(11):9-10.
7. Culp WC Jr, Kimbrough BA, Luna S. Flammability of surgical drapes and materials in varying concentrations of oxygen. Anesthesiology. 2013;119(4):770-776.
8. Apfelbaum JL, Caplan RA, Barker SJ, et al; American Society of Anesthesiologists Task Force on Operating Room Fires. Practice advisory for the prevention and management of operating room fires: an updated report by the American Society of Anesthesiologists Task Force on Operating Room Fires. Anesthesiology. 2013;118(2):271-290.
9. Barker SJ, Polson JS. Fire in the operating room: a case report and laboratory study. Anesth Analg. 2001;93(4):960-965.
10. Fire hazard created by the misuse of DuraPrep solution. Health Devices. 1998;27(11):400-402.
11. Hurt TL, Schweich PJ. Do not get burned: preventing iatrogenic fires and burns in the emergency department. Pediatr Emerg Care. 2003;19(4):255-259.
12. Prasad R, Quezado Z, St Andre A, O’Grady NP. Fires in the operating room and intensive care unit: awareness is the key to prevention. Anesth Analg. 2006;102(1):172-174.
13. Shah SC. Correspondence: operating room flash fire. Anesth Analg. 1974;53(2):288.
14. Tooher R, Maddern GJ, Simpson J. Surgical fires and alcohol-based skin preparations. ANZ J Surg. 2004;74(5):382-385.
15. Using ChloraPrep™ products and the skin prep portfolio. http://www.carefusion.com/medical-products/infection-prevention/skin-preparation/using-chloraprep.aspx. Accessed October 7, 2016.16. DePuy CMW. DePuy Orthopaedic Gentamicin Bone Cements. Blackpool, United Kingdom: DePuy International Ltd; 2008.
17. Dall’Oca C, Maluta T, Cavani F, et al. The biocompatibility of porous vs non-porous bone cements: a new methodological approach. Eur J Histochem. 2014;58(2):2255.
18. Zimmer Biomet. Bone Cement: Biomet Cement and Cementing Systems. http://www.biomet.com/wps/portal/internet/Biomet/Healthcare-Professionals/products/orthopedics. 2014. Accessed October 7, 2016.
19. Sigma-Aldrich. Methyl methacrylate. http://www.sigmaaldrich.com/catalog/product/aldrich/w400201?lang=en®ion=US. Accessed October 7, 2016.
20. DePuy Synthes. Unmedicated bone cements MSDS. Blackpool, United Kingdom: DePuy International Ltd. http://msdsdigital.com/unmedicated-bone-cements-msds. Accessed October 7, 2016.
21. Mir MR, Sun GS, Wang CM. Electrocautery. http://emedicine.medscape.com/article/2111163-overview#showall. Accessed October 7, 2016.
22. DePuy Synthes. Bone cement time setting.
23. Berry DJ, Lieberman JR, eds. Surgery of the Hip. New York, NY: Elsevier; 2011.
24. ECRI Institute. Surgical Fire Prevention. https://www.ecri.org/Accident_Investigation/Pages/Surgical-Fire-Prevention.aspx. 2014. Accessed October 7, 2016.
Approximately 600 cases of operating room (OR) fires are reported annually.1 The incidence of OR fires in the United States equals that of wrong-site surgeries, and 20% of cases have associated morbidity.1,2 The estimated mortality rate is 1 to 2 cases per year.3-5 The most commonly involved anatomical regions are the airway (33%) and the face (28%).4 Most surgical fires are reported in anesthetized patients with open oxygen delivery systems during head, neck, and upper chest surgeries; electrosurgical instruments are the ignition source in 90% of these cases.6 Despite extensive fire safety education and training, complete elimination of OR fires still has not been achieved.
Each fire requires an ignition source, a fuel source, and an oxidizer.7 In the OR, the 2 most common oxidizers are oxygen and nitrous oxide. Head and neck surgeries have a high concentration of these gases near the working field and therefore a higher risk and incidence of fires. Furthermore, surgical drapes and equipment (eg, closed or semi-closed breathing systems, masks) may potentiate this risk by reducing ventilation in areas where gases can accumulate and ignite. Ignition sources provide the energy that starts fires; common sources are electrocautery, lasers, fiber-optic light cords, drills/burrs, and defibrillator paddles. Fires are propagated by fuel sources, which encompass any flammable material, including tracheal tubes, sponges, alcohol-based solutions, hair, gastrointestinal tract gases, gloves, and packaging materials.8 Of note, alcohol-based skin-preparation agents emit flammable vapors that can ignite.9-14 Before draping or exposure to an ignition source, chlorhexidine gluconate-based preparations must be allowed to dry for at least 3 minutes after application to hairless skin and up to 1 hour after application to hair.15 Inadequate drying poses a risk of fire.10We present the case of an OR fire ignited by electrocautery near freshly applied bone cement. No patient information is disclosed in this report.
Case Report
Our patient was evaluated in clinic and scheduled for total knee arthroplasty (TKA). All preoperative safety checklists and time-out procedures were followed and documented at the start of surgery. The TKA was performed with a standard medial patellar arthrotomy. Tourniquet control was used after Esmarch exsanguination. The surgery proceeded uneventfully until just after the bone cement was applied to the tibial surface. The surgeon was using a Bovie to resect residual lateral meniscus tissue when a fire instantaneously erupted within the joint space. Fortunately, the surgeon quickly suffocated the fire with a dry towel. The ignited bone cement was removed, and the patient was examined. There was no injury to surrounding tissue or joint space. Surgery was resumed with application of new bone cement to the tibial surface. The artificial joint was then successfully implanted and the case completed without further incident. The patient was discharged from the hospital and followed up as an outpatient without any postoperative complications.
Discussion
Bone cement, which is commonly used in artificial joint anchoring, craniofacial reconstruction, and vertebroplasty, has liquid and powder components. The liquid monomer methyl methacrylate (MMA) is colorless and flammable and has a distinct odor.16 Exposure to heat or light can prematurely polymerize MMA, requiring the addition of hydroquinone to inhibit the reaction.16 The powder polymethylmethacrylate affords excellent structural support, radiopacity, and facility of use.17 Dibenzoyl peroxide and N,N-dimethyl-p-toluidine are added to the powder to facilitate the polymerization reaction at room temperature (ie, cold curing of cement). Premature application of unpolymerized cement increases the risk of fire from the volatile liquid component.
In the OR, bone cement is prepared by mixing together its powder and liquid components.18 The reaction is exothermic polymerization. The liquid is highly volatile and flammable in both liquid and vapor states.16,19 The vapors are denser than air and can concentrate in poorly ventilated areas. The OR and the application site must be adequately ventilated to eliminate any pockets of vapor accumulation.16 A vacuum mixer can be used to minimize fume exposure, enhance cement strength, and reduce fire risk while combining the 2 components.
MMA’s flash point, the temperature at which the fumes could ignite in the presence of an ignition source, is 10.5ºC. The auto-ignition point, the temperature at which MMA spontaneously combusts, is 421ºC.20 The OR is usually warmer than the flash point temperature, but the electrocautery tip can generate up to 1200ºC of heat.21 Therefore, bone cement is a potential fire hazard, and use of Bovies or other ignition sources in its vicinity must be avoided.
The Table lists the recommended times for preparing various bone cement products.22,23Mix time is the time needed to combine the liquid and powder into a homogenous putty.
For OR fires, the standard guidelines for rapid containment and safety apply. These guidelines are detailed by the American Society of Anesthesiologists.8 Briefly, delivery of all airway gases to the patient is discontinued. Any burning material is removed and extinguished by the OR staff.1 Carbon dioxide fire extinguishers are used to put out any patient fires and minimize the risk of thermal injury. (Water-mist fire extinguishers can contaminate surgical wounds and present an electric shock hazard with surgical devices and should be avoided.24) If a fire occurs in a patient’s airway, the tracheal tube is removed, and airway patency is maintained with use of other invasive or noninvasive techniques. Often, noninvasive positive pressure ventilation without supplemental oxygen is used until the fire is controlled and the patient is safe. Once the patient fire is controlled, ventilation is restarted, and the patient is evacuated from the OR and away from any other hazards, as required. Last, the patient is physically examined for any injuries and treated.24 Specific to TKA, the procedure is resumed after removal of all bone cement, inspection of the operative site, and treatment of any fire-related injuries.
We have reported the case of an OR fire during TKA. Appropriate selection and use of bone cement products, proper assessment of set time, and avoidance of electrocautery near cement application sites may dramatically reduce associated fire risks.
Am J Orthop. 2016;45(7):E512-E514. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
Approximately 600 cases of operating room (OR) fires are reported annually.1 The incidence of OR fires in the United States equals that of wrong-site surgeries, and 20% of cases have associated morbidity.1,2 The estimated mortality rate is 1 to 2 cases per year.3-5 The most commonly involved anatomical regions are the airway (33%) and the face (28%).4 Most surgical fires are reported in anesthetized patients with open oxygen delivery systems during head, neck, and upper chest surgeries; electrosurgical instruments are the ignition source in 90% of these cases.6 Despite extensive fire safety education and training, complete elimination of OR fires still has not been achieved.
Each fire requires an ignition source, a fuel source, and an oxidizer.7 In the OR, the 2 most common oxidizers are oxygen and nitrous oxide. Head and neck surgeries have a high concentration of these gases near the working field and therefore a higher risk and incidence of fires. Furthermore, surgical drapes and equipment (eg, closed or semi-closed breathing systems, masks) may potentiate this risk by reducing ventilation in areas where gases can accumulate and ignite. Ignition sources provide the energy that starts fires; common sources are electrocautery, lasers, fiber-optic light cords, drills/burrs, and defibrillator paddles. Fires are propagated by fuel sources, which encompass any flammable material, including tracheal tubes, sponges, alcohol-based solutions, hair, gastrointestinal tract gases, gloves, and packaging materials.8 Of note, alcohol-based skin-preparation agents emit flammable vapors that can ignite.9-14 Before draping or exposure to an ignition source, chlorhexidine gluconate-based preparations must be allowed to dry for at least 3 minutes after application to hairless skin and up to 1 hour after application to hair.15 Inadequate drying poses a risk of fire.10We present the case of an OR fire ignited by electrocautery near freshly applied bone cement. No patient information is disclosed in this report.
Case Report
Our patient was evaluated in clinic and scheduled for total knee arthroplasty (TKA). All preoperative safety checklists and time-out procedures were followed and documented at the start of surgery. The TKA was performed with a standard medial patellar arthrotomy. Tourniquet control was used after Esmarch exsanguination. The surgery proceeded uneventfully until just after the bone cement was applied to the tibial surface. The surgeon was using a Bovie to resect residual lateral meniscus tissue when a fire instantaneously erupted within the joint space. Fortunately, the surgeon quickly suffocated the fire with a dry towel. The ignited bone cement was removed, and the patient was examined. There was no injury to surrounding tissue or joint space. Surgery was resumed with application of new bone cement to the tibial surface. The artificial joint was then successfully implanted and the case completed without further incident. The patient was discharged from the hospital and followed up as an outpatient without any postoperative complications.
Discussion
Bone cement, which is commonly used in artificial joint anchoring, craniofacial reconstruction, and vertebroplasty, has liquid and powder components. The liquid monomer methyl methacrylate (MMA) is colorless and flammable and has a distinct odor.16 Exposure to heat or light can prematurely polymerize MMA, requiring the addition of hydroquinone to inhibit the reaction.16 The powder polymethylmethacrylate affords excellent structural support, radiopacity, and facility of use.17 Dibenzoyl peroxide and N,N-dimethyl-p-toluidine are added to the powder to facilitate the polymerization reaction at room temperature (ie, cold curing of cement). Premature application of unpolymerized cement increases the risk of fire from the volatile liquid component.
In the OR, bone cement is prepared by mixing together its powder and liquid components.18 The reaction is exothermic polymerization. The liquid is highly volatile and flammable in both liquid and vapor states.16,19 The vapors are denser than air and can concentrate in poorly ventilated areas. The OR and the application site must be adequately ventilated to eliminate any pockets of vapor accumulation.16 A vacuum mixer can be used to minimize fume exposure, enhance cement strength, and reduce fire risk while combining the 2 components.
MMA’s flash point, the temperature at which the fumes could ignite in the presence of an ignition source, is 10.5ºC. The auto-ignition point, the temperature at which MMA spontaneously combusts, is 421ºC.20 The OR is usually warmer than the flash point temperature, but the electrocautery tip can generate up to 1200ºC of heat.21 Therefore, bone cement is a potential fire hazard, and use of Bovies or other ignition sources in its vicinity must be avoided.
The Table lists the recommended times for preparing various bone cement products.22,23Mix time is the time needed to combine the liquid and powder into a homogenous putty.
For OR fires, the standard guidelines for rapid containment and safety apply. These guidelines are detailed by the American Society of Anesthesiologists.8 Briefly, delivery of all airway gases to the patient is discontinued. Any burning material is removed and extinguished by the OR staff.1 Carbon dioxide fire extinguishers are used to put out any patient fires and minimize the risk of thermal injury. (Water-mist fire extinguishers can contaminate surgical wounds and present an electric shock hazard with surgical devices and should be avoided.24) If a fire occurs in a patient’s airway, the tracheal tube is removed, and airway patency is maintained with use of other invasive or noninvasive techniques. Often, noninvasive positive pressure ventilation without supplemental oxygen is used until the fire is controlled and the patient is safe. Once the patient fire is controlled, ventilation is restarted, and the patient is evacuated from the OR and away from any other hazards, as required. Last, the patient is physically examined for any injuries and treated.24 Specific to TKA, the procedure is resumed after removal of all bone cement, inspection of the operative site, and treatment of any fire-related injuries.
We have reported the case of an OR fire during TKA. Appropriate selection and use of bone cement products, proper assessment of set time, and avoidance of electrocautery near cement application sites may dramatically reduce associated fire risks.
Am J Orthop. 2016;45(7):E512-E514. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. Hart SR, Yajnik A, Ashford J, Springer R, Harvey S. Operating room fire safety. Ochsner J. 2011;11(1):37-42.
2. American Society of Anesthesiologists Task Force on Operating Room Fires; Caplan RA, Barker SJ, Connis RT, et al. Practice advisory for the prevention and management of operating room fires. Anesthesiology. 2008;108(5):786-801.
3. Bruley M. Surgical fires: perioperative communication is essential to prevent this rare but devastating complication. Qual Saf HealthCare. 2004;13(6):467-471.
4. Daane SP, Toth BA. Fire in the operating room: principles and prevention. Plast Reconstr Surg. 2005;115(5):73e-75e.
5. Rinder CS. Fire safety in the operating room. Curr Opin Anaesthesiol. 2008;21(6):790-795.
6. Mathias JM. Fast action, team coordination critical when surgical fires occur. OR Manager. 2013;29(11):9-10.
7. Culp WC Jr, Kimbrough BA, Luna S. Flammability of surgical drapes and materials in varying concentrations of oxygen. Anesthesiology. 2013;119(4):770-776.
8. Apfelbaum JL, Caplan RA, Barker SJ, et al; American Society of Anesthesiologists Task Force on Operating Room Fires. Practice advisory for the prevention and management of operating room fires: an updated report by the American Society of Anesthesiologists Task Force on Operating Room Fires. Anesthesiology. 2013;118(2):271-290.
9. Barker SJ, Polson JS. Fire in the operating room: a case report and laboratory study. Anesth Analg. 2001;93(4):960-965.
10. Fire hazard created by the misuse of DuraPrep solution. Health Devices. 1998;27(11):400-402.
11. Hurt TL, Schweich PJ. Do not get burned: preventing iatrogenic fires and burns in the emergency department. Pediatr Emerg Care. 2003;19(4):255-259.
12. Prasad R, Quezado Z, St Andre A, O’Grady NP. Fires in the operating room and intensive care unit: awareness is the key to prevention. Anesth Analg. 2006;102(1):172-174.
13. Shah SC. Correspondence: operating room flash fire. Anesth Analg. 1974;53(2):288.
14. Tooher R, Maddern GJ, Simpson J. Surgical fires and alcohol-based skin preparations. ANZ J Surg. 2004;74(5):382-385.
15. Using ChloraPrep™ products and the skin prep portfolio. http://www.carefusion.com/medical-products/infection-prevention/skin-preparation/using-chloraprep.aspx. Accessed October 7, 2016.16. DePuy CMW. DePuy Orthopaedic Gentamicin Bone Cements. Blackpool, United Kingdom: DePuy International Ltd; 2008.
17. Dall’Oca C, Maluta T, Cavani F, et al. The biocompatibility of porous vs non-porous bone cements: a new methodological approach. Eur J Histochem. 2014;58(2):2255.
18. Zimmer Biomet. Bone Cement: Biomet Cement and Cementing Systems. http://www.biomet.com/wps/portal/internet/Biomet/Healthcare-Professionals/products/orthopedics. 2014. Accessed October 7, 2016.
19. Sigma-Aldrich. Methyl methacrylate. http://www.sigmaaldrich.com/catalog/product/aldrich/w400201?lang=en®ion=US. Accessed October 7, 2016.
20. DePuy Synthes. Unmedicated bone cements MSDS. Blackpool, United Kingdom: DePuy International Ltd. http://msdsdigital.com/unmedicated-bone-cements-msds. Accessed October 7, 2016.
21. Mir MR, Sun GS, Wang CM. Electrocautery. http://emedicine.medscape.com/article/2111163-overview#showall. Accessed October 7, 2016.
22. DePuy Synthes. Bone cement time setting.
23. Berry DJ, Lieberman JR, eds. Surgery of the Hip. New York, NY: Elsevier; 2011.
24. ECRI Institute. Surgical Fire Prevention. https://www.ecri.org/Accident_Investigation/Pages/Surgical-Fire-Prevention.aspx. 2014. Accessed October 7, 2016.
1. Hart SR, Yajnik A, Ashford J, Springer R, Harvey S. Operating room fire safety. Ochsner J. 2011;11(1):37-42.
2. American Society of Anesthesiologists Task Force on Operating Room Fires; Caplan RA, Barker SJ, Connis RT, et al. Practice advisory for the prevention and management of operating room fires. Anesthesiology. 2008;108(5):786-801.
3. Bruley M. Surgical fires: perioperative communication is essential to prevent this rare but devastating complication. Qual Saf HealthCare. 2004;13(6):467-471.
4. Daane SP, Toth BA. Fire in the operating room: principles and prevention. Plast Reconstr Surg. 2005;115(5):73e-75e.
5. Rinder CS. Fire safety in the operating room. Curr Opin Anaesthesiol. 2008;21(6):790-795.
6. Mathias JM. Fast action, team coordination critical when surgical fires occur. OR Manager. 2013;29(11):9-10.
7. Culp WC Jr, Kimbrough BA, Luna S. Flammability of surgical drapes and materials in varying concentrations of oxygen. Anesthesiology. 2013;119(4):770-776.
8. Apfelbaum JL, Caplan RA, Barker SJ, et al; American Society of Anesthesiologists Task Force on Operating Room Fires. Practice advisory for the prevention and management of operating room fires: an updated report by the American Society of Anesthesiologists Task Force on Operating Room Fires. Anesthesiology. 2013;118(2):271-290.
9. Barker SJ, Polson JS. Fire in the operating room: a case report and laboratory study. Anesth Analg. 2001;93(4):960-965.
10. Fire hazard created by the misuse of DuraPrep solution. Health Devices. 1998;27(11):400-402.
11. Hurt TL, Schweich PJ. Do not get burned: preventing iatrogenic fires and burns in the emergency department. Pediatr Emerg Care. 2003;19(4):255-259.
12. Prasad R, Quezado Z, St Andre A, O’Grady NP. Fires in the operating room and intensive care unit: awareness is the key to prevention. Anesth Analg. 2006;102(1):172-174.
13. Shah SC. Correspondence: operating room flash fire. Anesth Analg. 1974;53(2):288.
14. Tooher R, Maddern GJ, Simpson J. Surgical fires and alcohol-based skin preparations. ANZ J Surg. 2004;74(5):382-385.
15. Using ChloraPrep™ products and the skin prep portfolio. http://www.carefusion.com/medical-products/infection-prevention/skin-preparation/using-chloraprep.aspx. Accessed October 7, 2016.16. DePuy CMW. DePuy Orthopaedic Gentamicin Bone Cements. Blackpool, United Kingdom: DePuy International Ltd; 2008.
17. Dall’Oca C, Maluta T, Cavani F, et al. The biocompatibility of porous vs non-porous bone cements: a new methodological approach. Eur J Histochem. 2014;58(2):2255.
18. Zimmer Biomet. Bone Cement: Biomet Cement and Cementing Systems. http://www.biomet.com/wps/portal/internet/Biomet/Healthcare-Professionals/products/orthopedics. 2014. Accessed October 7, 2016.
19. Sigma-Aldrich. Methyl methacrylate. http://www.sigmaaldrich.com/catalog/product/aldrich/w400201?lang=en®ion=US. Accessed October 7, 2016.
20. DePuy Synthes. Unmedicated bone cements MSDS. Blackpool, United Kingdom: DePuy International Ltd. http://msdsdigital.com/unmedicated-bone-cements-msds. Accessed October 7, 2016.
21. Mir MR, Sun GS, Wang CM. Electrocautery. http://emedicine.medscape.com/article/2111163-overview#showall. Accessed October 7, 2016.
22. DePuy Synthes. Bone cement time setting.
23. Berry DJ, Lieberman JR, eds. Surgery of the Hip. New York, NY: Elsevier; 2011.
24. ECRI Institute. Surgical Fire Prevention. https://www.ecri.org/Accident_Investigation/Pages/Surgical-Fire-Prevention.aspx. 2014. Accessed October 7, 2016.
An Update on Management of Syndesmosis Injury: A National US Database Study
Acute ankle injuries are common problems treated by orthopedic surgeons. In the United States, nearly 2 million ankle sprains occur each year,1 and ankle fractures account for 9% to 18% of all fractures treated in emergency departments.2,3 Ankle injuries that involve the syndesmotic ligaments may result in instability and require specific treatment beyond fixation of the malleolar fractures.
The usual mechanism of syndesmotic injury is external rotation of the ankle with hyperdorsiflexion of a pronated or supinated foot.4,5 Syndesmotic injuries are estimated to occur in up to 10% of ankle sprains6 and up to 23% of all ankle fractures.7 Overall US incidence of syndesmotic injury is estimated at 6445 injuries per year.8 Syndesmotic injury occurs in 39% to 45% of supination-external rotation IV ankle fractures.9,10 Pronation-external rotation ankle fractures have the highest rate of syndesmotic injury. Syndesmotic injury may be less common in other types of malleolar fracture, but the exact incidence has not been reliably reported.
Traditionally, isolated nondisplaced syndesmotic injuries are treated nonoperatively, and syndesmotic injuries with concomitant malleolar fractures are treated surgically. Various options are available for syndesmotic fixation. The gold standard is syndesmotic screw placement from the lateral aspect of the fibula through the tibia. Fixation may be achieved with screws in a variety of configurations and formats. However, fixation with two 4.5-mm screws is stronger.11,12 Functional outcomes are similar, regardless of screw material,13-16 number of cortices,17 or number of screws.18 Disadvantages specific to screw fixation include altered ankle biomechanics,19,20 potential for screw breakage,21 and need for implant removal.3Alternatively, suture button fixation is said to be equally as effective as screw fixation in achieving syndesmotic reduction, and their functional outcomes are similar.22,23 The initial cost of suture button fixation is higher than that of screw fixation, but the difference may be offset by potential elimination of a second surgery for syndesmotic screw removal.24 Soft-tissue irritation caused by the suture material and local osteolysis are reported complications of suture button fixation.25-27
Regardless of fixation method used, achieving anatomical reduction of the syndesmosis is considered the most important factor in optimizing functional outcomes.28-31 However, achieving and verifying anatomical reduction of the syndesmosis during surgery can be quite challenging.30,32-34 Various methods of lowering the malreduction risk, including direct visualization of the tibiofibular joint during reduction30,35 and intraoperative 3-dimensional imaging,33,36 have been proposed.
In the study reported here, we used a US insurance database to determine the incidence and rate of syndesmotic stabilization within various ankle injuries and fracture patterns.
Materials and Methods
All data for this study were obtained from a publicly available for-fee healthcare database, the PearlDiver Patient Records Database, which includes procedural volumes and demographic information for patients with International Classification of Diseases, Ninth Revision (ICD-9) diagnoses and procedures or Current Procedural Terminology (CPT) codes. Data for the study were derived from 2 databases within PearlDiver: a private-payer database, which has its largest contribution (>30 million individual patient records for 2007-2011) from United HealthCare, and a Medicare database (>50 million patient records for 2007-2011). Access to the database was granted by PearlDiver Technologies for the purpose of academic research. The database was stored on a password-protected server maintained by PearlDiver.
We searched the database for cases of ankle fracture fixation, including fixation of isolated lateral malleolus (CPT 27792), bimalleolar (CPT 27814), and trimalleolar (CPTs 27822 and 27823) fractures. CPT 27829 was used to search for syndesmotic fixation, and CPT 20680 for implant removal. These codes were used individually and in combination.
Overall procedural volume data are reported as number of patients with the given CPT(s) in the database output and as incidence, calculated as number of patients with the CPT of interest normalized to total number of patients in the database for that particular subgroup. Results of age group and sex analyses are reported as number of patients reported in the database output and as percentage of patients who had the CPT procedure of interest that year. As United HealthCare is the largest contributor to the private-payer portion of the database and is represented most prominently in the southern region, data for the regional analysis are presented only as incidence. This incidence was calculated as number of patients in a particular region and year normalized to total number of patients in the database for that region or year. The regions were Midwest (IA, IL, IN, KS, MI, MN, MO, ND, NE, OH, SD, WI), Northeast (CT, MA, ME, NH, NJ, NY, PA, RI, VT), South (AL, AR, DC, DE, FL, GA, KY, LA, MD, MI, NC, OK, SC, TN, TX, VA, WV), and West (AK, AZ, CA, CO, HI, ID, MT, NM, NV, OR, UT, WA, WY).
Chi-square linear-by-linear association analysis was used to determine the statistical significance of time trends in procedural volume, sex, age group, and region. For all statistical comparisons, P < .05 was considered significant.
Results
Number of open reduction and internal fixation (ORIF) procedures increased for all ankle fracture types over the period 2007 to 2011 (Table 1).
ORIF was performed for an ankle injury in 54,767 patients during the period 2007 to 2011, resulting in a cumulative incidence of 64.2 per 1000 patients (Table 2).
More ankle ORIF procedures were performed in females (33,565) than in males (21,202); incidence of ankle ORIF procedures was higher in females (68.6/1000 patients) than in males (58.4/1000 patients) (Table 2); percentages of bimalleolar and trimalleolar fractures were higher in females (bi, 40.6%; tri, 27.8%) than in males (bi, 34.6%; tri, 15.2%); and percentage of lateral malleolus fractures was higher in males (50.2%) than in females (31.6%).
Incidence of ankle ORIF procedures was similar in the South (69.6/1000 patients), Midwest (69.4/100 patients), and West (65.1/1000 patients) but lower in the Northeast (43.3/1000 patients) (Table 2). Lateral malleolus fractures were the most common ankle fractures in the Midwest (40.7%) and West (41.3%), followed by bimalleolar fractures (Midwest, 36.3%; West 36.0%) and trimalleolar fractures (Midwest, 23.0%; West, 22.7%). Bimalleolar fractures were most common in the Northeast (40.2%) and South (39.8%), followed by lateral malleolus fractures (Northeast, 34.4%; South, 38.0%) and trimalleolar fractures (Northeast, 25.4%; South, 22.3%).
Discussion
The present study found no significant change in number of lateral malleolus, bimalleolar, and trimalleolar ankle fracture ORIF procedures performed over the period 2007 to 2011. However, over the same period, incidence of syndesmosis fixation increased significantly in patients with isolated syndesmotic injuries and in patients with concomitant ankle fracture and syndesmotic injury. The largest percentage change was found in the bimalleolar ORIF group, which showed nearly a doubling of syndesmotic fixation over the 4-year study period, followed by a 38.1% increase in syndesmotic fixation in the trimalleolar ORIF group. Both groups had a syndesmotic fixation percentage change about twice that seen in the isolated lateral malleolus group.
There are several explanations for these trends. First, bimalleolar and trimalleolar fractures are more severe ankle fractures that tend to result from a more forceful mechanism, allowing for a higher rate of syndesmotic injury. Second, these trends likely do not reflect a true increase in the rate of syndesmosis injury but, rather, increased recognition of syndesmotic injury. Third, the data likely reflect a well-established approach to ankle fracture fixation and an increase in thinking that syndesmotic injuries should be stabilized in the setting of ankle fixation.
Incidence of syndesmotic injury as indicated by stabilization procedures can be compared with the data of Vosseller and colleagues,8 who reported an incidence of 6445 syndesmotic injuries per year in the United States. Our data showed fewer syndesmotic injuries, which may be related to use of CPT codes rather than ICD-9 codes for database searches, such that only operative syndesmotic injuries are represented in our data. Population differences between the 2 studies could also account for some of the differences in syndesmotic injury incidence.
We also found a significant change in the rate of hardware removal after syndesmosis ORIF. Across all treatment groups, incidence of screw removal decreased—a trend likely reflecting a change in attitude about the need for routine screw removal. Studies have shown that patients have favorable outcomes in the setting of syndesmotic screw loosening and screw breakage.37 Some authors have suggested that screw breakage or removal could be advantageous, as it allows the syndesmosis to settle into a more anatomical position after imperfect reduction.38 In addition, the trend of decreased syndesmotic screw removal could also have resulted from increased suture button fixation, which may less frequently require implant removal. Regardless, the overall trend is that routine syndesmotic implant removal has become less common.
This study had several limitations. First are the many limitations inherent to all studies that use large administrative databases, such as PearlDiver. The power of analysis depends on data quality; potential sources of error include accuracy of billing codes and physicians’ miscoding or noncoding. Although we tried to accurately represent a large population of interest through use of this database, we cannot be sure that the database represents a true cross-section of the United States. In addition, as we could not determine the method of syndesmotic fixation—the same CPT code is used for both suture button fixation and screw fixation—we could not establish trends for the rate of each method. More research is needed to establish these trends, and this research likely will require analysis of data from a large trauma center or from multiple centers.
Potential regional differences are another limitation. In the PearlDiver database, the South and Midwest are highly represented, the Northeast and West much less so. The South, Midwest, and West (but not the Northeast) had similar overall incidence and subgroup incidence of ankle ORIF. However, any regional differences in the rate of syndesmotic fixation could have skewed our data.
Ankle fractures and associated syndesmotic injuries remain a common problem. Although the prevalence of ankle fracture fixation has been relatively constant, the rate of syndesmosis stabilization has increased significantly. Young adults have the highest incidence of ankle fracture and associated syndesmotic fixation, but more ankle fractures occur in the large and growing elderly population. Increased awareness of syndesmotic injury likely has contributed to the recent rise in syndesmosis fixation seen in the present study. Given this trend, we recommend further analysis of outcome data and to establish treatment guidelines.
Am J Orthop. 2016;45(7):E472-E477. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. Waterman BR, Owens BD, Davey S, Zacchilli MA, Belmont PJ Jr. The epidemiology of ankle sprains in the United States. J Bone Joint Surg Am. 2010;92(13):2279-2284.
2. Court-Brown CM, Caesar B. Epidemiology of adult fractures: a review. Injury. 2006;37(8):691-697.
3. Miller AN, Paul O, Boraiah S, Parker RJ, Helfet DL, Lorich DG. Functional outcomes after syndesmotic screw fixation and removal. J Orthop Trauma. 2010;24(1):12-16.
4. Edwards GS Jr, DeLee JC. Ankle diastasis without fracture. Foot Ankle. 1984;4(6):305-312.
5. Norkus SA, Floyd RT. The anatomy and mechanisms of syndesmotic ankle sprains. J Athl Train. 2001;36(1):68-73.
6. Brosky T, Nyland J, Nitz A, Caborn DN. The ankle ligaments: consideration of syndesmotic injury and implications for rehabilitation. J Orthop Sports Phys Ther. 1995;21(4):197-205.
7. Purvis GD. Displaced, unstable ankle fractures: classification, incidence, and management of a consecutive series. Clin Orthop Relat Res. 1982;(165):91-98.
8. Vosseller JT, Karl JW, Greisberg JK. Incidence of syndesmotic injury. Orthopedics. 2014;37(3):e226-e229.
9. Stark E, Tornetta P 3rd, Creevy WR. Syndesmotic instability in Weber B ankle fractures: a clinical evaluation. J Orthop Trauma. 2007;21(9):643-646.
10. Tornetta P 3rd, Axelrad TW, Sibai TA, Creevy WR. Treatment of the stress positive ligamentous SE4 ankle fracture: incidence of syndesmotic injury and clinical decision making. J Orthop Trauma. 2012;26(11):659-661.
11. Xenos JS, Hopkinson WJ, Mulligan ME, Olson EJ, Popovic NA. The tibiofibular syndesmosis. Evaluation of the ligamentous structures, methods of fixation, and radiographic assessment. J Bone Joint Surg Am. 1995;77(6):847-856.
12. Ebraheim NA, Lu J, Yang H, Mekhail AO, Yeasting RA. Radiographic and CT evaluation of tibiofibular syndesmotic diastasis: a cadaver study. Foot Ankle Int. 1997;18(11):693-698.
13. Ahmad J, Raikin SM, Pour AE, Haytmanek C. Bioabsorbable screw fixation of the syndesmosis in unstable ankle injuries. Foot Ankle Int. 2009;30(2):99-105.
14. Hovis WD, Kaiser BW, Watson JT, Bucholz RW. Treatment of syndesmotic disruptions of the ankle with bioabsorbable screw fixation. J Bone Joint Surg Am. 2002;84(1):26-31.
15. Kaukonen JP, Lamberg T, Korkala O, Pajarinen J. Fixation of syndesmotic ruptures in 38 patients with a malleolar fracture: a randomized study comparing a metallic and a bioabsorbable screw. J Orthop Trauma. 2005;19(6):392-395.
16. Thordarson DB, Samuelson M, Shepherd LE, Merkle PF, Lee J. Bioabsorbable versus stainless steel screw fixation of the syndesmosis in pronation-lateral rotation ankle fractures: a prospective randomized trial. Foot Ankle Int. 2001;22(4):335-338.
17. Moore JA Jr, Shank JR, Morgan SJ, Smith WR. Syndesmosis fixation: a comparison of three and four cortices of screw fixation without hardware removal. Foot Ankle Int. 2006;27(8):567-572.
18. Høiness P, Strømsøe K. Tricortical versus quadricortical syndesmosis fixation in ankle fractures: a prospective, randomized study comparing two methods of syndesmosis fixation. J Orthop Trauma. 2004;18(6):331-337.
19. Huber T, Schmoelz W, Bölderl A. Motion of the fibula relative to the tibia and its alterations with syndesmosis screws: a cadaver study. Foot Ankle Surg. 2012;18(3):203-209.
20. Needleman RL, Skrade DA, Stiehl JB. Effect of the syndesmotic screw on ankle motion. Foot Ankle. 1989;10(1):17-24.
21. Mendelsohn ES, Hoshino CM, Harris TG, Zinar DM. The effect of obesity on early failure after operative syndesmosis injuries. J Orthop Trauma. 2013;27(4):201-206.
22. Schepers T. Acute distal tibiofibular syndesmosis injury: a systematic review of suture-button versus syndesmotic screw repair. Int Orthop. 2012;36(6):1199-1206.
23. Cottom JM, Hyer CF, Philbin TM, Berlet GC. Transosseous fixation of the distal tibiofibular syndesmosis: comparison of an interosseous suture and Endobutton to traditional screw fixation in 50 cases. J Foot Ankle Surg. 2009;48(6):620-630.
24. Thornes B, Shannon F, Guiney AM, Hession P, Masterson E. Suture-button syndesmosis fixation: accelerated rehabilitation and improved outcomes. Clin Orthop Relat Res. 2005;(431):207-212.
25. Willmott HJ, Singh B, David LA. Outcome and complications of treatment of ankle diastasis with tightrope fixation. Injury. 2009;40(11):1204-1206.
26. Qamar F, Kadakia A, Venkateswaran B. An anatomical way of treating ankle syndesmotic injuries. J Foot Ankle Surg. 2011;50(6):762-765.
27. Degroot H, Al-Omari AA, El Ghazaly SA. Outcomes of suture button repair of the distal tibiofibular syndesmosis. Foot Ankle Int. 2011;32(3):250-256.
28. Ramsey PL, Hamilton W. Changes in tibiotalar area of contact caused by lateral talar shift. J Bone Joint Surg Am. 1976;58(3):356-357.
29. Weening B, Bhandari M. Predictors of functional outcome following transsyndesmotic screw fixation of ankle fractures. J Orthop Trauma. 2005;19(2):102-108.
30. Sagi HC, Shah AR, Sanders RW. The functional consequence of syndesmotic joint malreduction at a minimum 2-year follow-up. J Orthop Trauma. 2012;26(7):439-443.
31. Naqvi GA, Cunningham P, Lynch B, Galvin R, Awan N. Fixation of ankle syndesmotic injuries: comparison of tightrope fixation and syndesmotic screw fixation for accuracy of syndesmotic reduction. Am J Sports Med. 2012;40(12):2828-2835.
32. Marmor M, Hansen E, Han HK, Buckley J, Matityahu A. Limitations of standard fluoroscopy in detecting rotational malreduction of the syndesmosis in an ankle fracture model. Foot Ankle Int. 2011;32(6):616-622.
33. Franke J, von Recum J, Suda AJ, Grützner PA, Wendl K. Intraoperative three-dimensional imaging in the treatment of acute unstable syndesmotic injuries. J Bone Joint Surg Am. 2012;94(15):1386-1390.
34. Gardner MJ, Demetrakopoulos D, Briggs SM, Helfet DL, Lorich DG. Malreduction of the tibiofibular syndesmosis in ankle fractures. Foot Ankle Int. 2006;27(10):788-792.
35. Miller AN, Carroll EA, Parker RJ, Boraiah S, Helfet DL, Lorich DG. Direct visualization for syndesmotic stabilization of ankle fractures. Foot Ankle Int. 2009;30(5):419-426.
36. Ruan Z, Luo C, Shi Z, Zhang B, Zeng B, Zhang C. Intraoperative reduction of distal tibiofibular joint aided by three-dimensional fluoroscopy. Technol Health Care. 2011;19(3):161-166.
37. Hamid N, Loeffler BJ, Braddy W, Kellam JF, Cohen BE, Bosse MJ. Outcome after fixation of ankle fractures with an injury to the syndesmosis: the effect of the syndesmosis screw. J Bone Joint Surg Br. 2009;91(8):1069-1073.
38. Song DJ, Lanzi JT, Groth AT, et al. The effect of syndesmosis screw removal on the reduction of the distal tibiofibular joint: a prospective radiographic study. Foot Ankle Int. 2014;35(6):543-548.
Acute ankle injuries are common problems treated by orthopedic surgeons. In the United States, nearly 2 million ankle sprains occur each year,1 and ankle fractures account for 9% to 18% of all fractures treated in emergency departments.2,3 Ankle injuries that involve the syndesmotic ligaments may result in instability and require specific treatment beyond fixation of the malleolar fractures.
The usual mechanism of syndesmotic injury is external rotation of the ankle with hyperdorsiflexion of a pronated or supinated foot.4,5 Syndesmotic injuries are estimated to occur in up to 10% of ankle sprains6 and up to 23% of all ankle fractures.7 Overall US incidence of syndesmotic injury is estimated at 6445 injuries per year.8 Syndesmotic injury occurs in 39% to 45% of supination-external rotation IV ankle fractures.9,10 Pronation-external rotation ankle fractures have the highest rate of syndesmotic injury. Syndesmotic injury may be less common in other types of malleolar fracture, but the exact incidence has not been reliably reported.
Traditionally, isolated nondisplaced syndesmotic injuries are treated nonoperatively, and syndesmotic injuries with concomitant malleolar fractures are treated surgically. Various options are available for syndesmotic fixation. The gold standard is syndesmotic screw placement from the lateral aspect of the fibula through the tibia. Fixation may be achieved with screws in a variety of configurations and formats. However, fixation with two 4.5-mm screws is stronger.11,12 Functional outcomes are similar, regardless of screw material,13-16 number of cortices,17 or number of screws.18 Disadvantages specific to screw fixation include altered ankle biomechanics,19,20 potential for screw breakage,21 and need for implant removal.3Alternatively, suture button fixation is said to be equally as effective as screw fixation in achieving syndesmotic reduction, and their functional outcomes are similar.22,23 The initial cost of suture button fixation is higher than that of screw fixation, but the difference may be offset by potential elimination of a second surgery for syndesmotic screw removal.24 Soft-tissue irritation caused by the suture material and local osteolysis are reported complications of suture button fixation.25-27
Regardless of fixation method used, achieving anatomical reduction of the syndesmosis is considered the most important factor in optimizing functional outcomes.28-31 However, achieving and verifying anatomical reduction of the syndesmosis during surgery can be quite challenging.30,32-34 Various methods of lowering the malreduction risk, including direct visualization of the tibiofibular joint during reduction30,35 and intraoperative 3-dimensional imaging,33,36 have been proposed.
In the study reported here, we used a US insurance database to determine the incidence and rate of syndesmotic stabilization within various ankle injuries and fracture patterns.
Materials and Methods
All data for this study were obtained from a publicly available for-fee healthcare database, the PearlDiver Patient Records Database, which includes procedural volumes and demographic information for patients with International Classification of Diseases, Ninth Revision (ICD-9) diagnoses and procedures or Current Procedural Terminology (CPT) codes. Data for the study were derived from 2 databases within PearlDiver: a private-payer database, which has its largest contribution (>30 million individual patient records for 2007-2011) from United HealthCare, and a Medicare database (>50 million patient records for 2007-2011). Access to the database was granted by PearlDiver Technologies for the purpose of academic research. The database was stored on a password-protected server maintained by PearlDiver.
We searched the database for cases of ankle fracture fixation, including fixation of isolated lateral malleolus (CPT 27792), bimalleolar (CPT 27814), and trimalleolar (CPTs 27822 and 27823) fractures. CPT 27829 was used to search for syndesmotic fixation, and CPT 20680 for implant removal. These codes were used individually and in combination.
Overall procedural volume data are reported as number of patients with the given CPT(s) in the database output and as incidence, calculated as number of patients with the CPT of interest normalized to total number of patients in the database for that particular subgroup. Results of age group and sex analyses are reported as number of patients reported in the database output and as percentage of patients who had the CPT procedure of interest that year. As United HealthCare is the largest contributor to the private-payer portion of the database and is represented most prominently in the southern region, data for the regional analysis are presented only as incidence. This incidence was calculated as number of patients in a particular region and year normalized to total number of patients in the database for that region or year. The regions were Midwest (IA, IL, IN, KS, MI, MN, MO, ND, NE, OH, SD, WI), Northeast (CT, MA, ME, NH, NJ, NY, PA, RI, VT), South (AL, AR, DC, DE, FL, GA, KY, LA, MD, MI, NC, OK, SC, TN, TX, VA, WV), and West (AK, AZ, CA, CO, HI, ID, MT, NM, NV, OR, UT, WA, WY).
Chi-square linear-by-linear association analysis was used to determine the statistical significance of time trends in procedural volume, sex, age group, and region. For all statistical comparisons, P < .05 was considered significant.
Results
Number of open reduction and internal fixation (ORIF) procedures increased for all ankle fracture types over the period 2007 to 2011 (Table 1).
ORIF was performed for an ankle injury in 54,767 patients during the period 2007 to 2011, resulting in a cumulative incidence of 64.2 per 1000 patients (Table 2).
More ankle ORIF procedures were performed in females (33,565) than in males (21,202); incidence of ankle ORIF procedures was higher in females (68.6/1000 patients) than in males (58.4/1000 patients) (Table 2); percentages of bimalleolar and trimalleolar fractures were higher in females (bi, 40.6%; tri, 27.8%) than in males (bi, 34.6%; tri, 15.2%); and percentage of lateral malleolus fractures was higher in males (50.2%) than in females (31.6%).
Incidence of ankle ORIF procedures was similar in the South (69.6/1000 patients), Midwest (69.4/100 patients), and West (65.1/1000 patients) but lower in the Northeast (43.3/1000 patients) (Table 2). Lateral malleolus fractures were the most common ankle fractures in the Midwest (40.7%) and West (41.3%), followed by bimalleolar fractures (Midwest, 36.3%; West 36.0%) and trimalleolar fractures (Midwest, 23.0%; West, 22.7%). Bimalleolar fractures were most common in the Northeast (40.2%) and South (39.8%), followed by lateral malleolus fractures (Northeast, 34.4%; South, 38.0%) and trimalleolar fractures (Northeast, 25.4%; South, 22.3%).
Discussion
The present study found no significant change in number of lateral malleolus, bimalleolar, and trimalleolar ankle fracture ORIF procedures performed over the period 2007 to 2011. However, over the same period, incidence of syndesmosis fixation increased significantly in patients with isolated syndesmotic injuries and in patients with concomitant ankle fracture and syndesmotic injury. The largest percentage change was found in the bimalleolar ORIF group, which showed nearly a doubling of syndesmotic fixation over the 4-year study period, followed by a 38.1% increase in syndesmotic fixation in the trimalleolar ORIF group. Both groups had a syndesmotic fixation percentage change about twice that seen in the isolated lateral malleolus group.
There are several explanations for these trends. First, bimalleolar and trimalleolar fractures are more severe ankle fractures that tend to result from a more forceful mechanism, allowing for a higher rate of syndesmotic injury. Second, these trends likely do not reflect a true increase in the rate of syndesmosis injury but, rather, increased recognition of syndesmotic injury. Third, the data likely reflect a well-established approach to ankle fracture fixation and an increase in thinking that syndesmotic injuries should be stabilized in the setting of ankle fixation.
Incidence of syndesmotic injury as indicated by stabilization procedures can be compared with the data of Vosseller and colleagues,8 who reported an incidence of 6445 syndesmotic injuries per year in the United States. Our data showed fewer syndesmotic injuries, which may be related to use of CPT codes rather than ICD-9 codes for database searches, such that only operative syndesmotic injuries are represented in our data. Population differences between the 2 studies could also account for some of the differences in syndesmotic injury incidence.
We also found a significant change in the rate of hardware removal after syndesmosis ORIF. Across all treatment groups, incidence of screw removal decreased—a trend likely reflecting a change in attitude about the need for routine screw removal. Studies have shown that patients have favorable outcomes in the setting of syndesmotic screw loosening and screw breakage.37 Some authors have suggested that screw breakage or removal could be advantageous, as it allows the syndesmosis to settle into a more anatomical position after imperfect reduction.38 In addition, the trend of decreased syndesmotic screw removal could also have resulted from increased suture button fixation, which may less frequently require implant removal. Regardless, the overall trend is that routine syndesmotic implant removal has become less common.
This study had several limitations. First are the many limitations inherent to all studies that use large administrative databases, such as PearlDiver. The power of analysis depends on data quality; potential sources of error include accuracy of billing codes and physicians’ miscoding or noncoding. Although we tried to accurately represent a large population of interest through use of this database, we cannot be sure that the database represents a true cross-section of the United States. In addition, as we could not determine the method of syndesmotic fixation—the same CPT code is used for both suture button fixation and screw fixation—we could not establish trends for the rate of each method. More research is needed to establish these trends, and this research likely will require analysis of data from a large trauma center or from multiple centers.
Potential regional differences are another limitation. In the PearlDiver database, the South and Midwest are highly represented, the Northeast and West much less so. The South, Midwest, and West (but not the Northeast) had similar overall incidence and subgroup incidence of ankle ORIF. However, any regional differences in the rate of syndesmotic fixation could have skewed our data.
Ankle fractures and associated syndesmotic injuries remain a common problem. Although the prevalence of ankle fracture fixation has been relatively constant, the rate of syndesmosis stabilization has increased significantly. Young adults have the highest incidence of ankle fracture and associated syndesmotic fixation, but more ankle fractures occur in the large and growing elderly population. Increased awareness of syndesmotic injury likely has contributed to the recent rise in syndesmosis fixation seen in the present study. Given this trend, we recommend further analysis of outcome data and to establish treatment guidelines.
Am J Orthop. 2016;45(7):E472-E477. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
Acute ankle injuries are common problems treated by orthopedic surgeons. In the United States, nearly 2 million ankle sprains occur each year,1 and ankle fractures account for 9% to 18% of all fractures treated in emergency departments.2,3 Ankle injuries that involve the syndesmotic ligaments may result in instability and require specific treatment beyond fixation of the malleolar fractures.
The usual mechanism of syndesmotic injury is external rotation of the ankle with hyperdorsiflexion of a pronated or supinated foot.4,5 Syndesmotic injuries are estimated to occur in up to 10% of ankle sprains6 and up to 23% of all ankle fractures.7 Overall US incidence of syndesmotic injury is estimated at 6445 injuries per year.8 Syndesmotic injury occurs in 39% to 45% of supination-external rotation IV ankle fractures.9,10 Pronation-external rotation ankle fractures have the highest rate of syndesmotic injury. Syndesmotic injury may be less common in other types of malleolar fracture, but the exact incidence has not been reliably reported.
Traditionally, isolated nondisplaced syndesmotic injuries are treated nonoperatively, and syndesmotic injuries with concomitant malleolar fractures are treated surgically. Various options are available for syndesmotic fixation. The gold standard is syndesmotic screw placement from the lateral aspect of the fibula through the tibia. Fixation may be achieved with screws in a variety of configurations and formats. However, fixation with two 4.5-mm screws is stronger.11,12 Functional outcomes are similar, regardless of screw material,13-16 number of cortices,17 or number of screws.18 Disadvantages specific to screw fixation include altered ankle biomechanics,19,20 potential for screw breakage,21 and need for implant removal.3Alternatively, suture button fixation is said to be equally as effective as screw fixation in achieving syndesmotic reduction, and their functional outcomes are similar.22,23 The initial cost of suture button fixation is higher than that of screw fixation, but the difference may be offset by potential elimination of a second surgery for syndesmotic screw removal.24 Soft-tissue irritation caused by the suture material and local osteolysis are reported complications of suture button fixation.25-27
Regardless of fixation method used, achieving anatomical reduction of the syndesmosis is considered the most important factor in optimizing functional outcomes.28-31 However, achieving and verifying anatomical reduction of the syndesmosis during surgery can be quite challenging.30,32-34 Various methods of lowering the malreduction risk, including direct visualization of the tibiofibular joint during reduction30,35 and intraoperative 3-dimensional imaging,33,36 have been proposed.
In the study reported here, we used a US insurance database to determine the incidence and rate of syndesmotic stabilization within various ankle injuries and fracture patterns.
Materials and Methods
All data for this study were obtained from a publicly available for-fee healthcare database, the PearlDiver Patient Records Database, which includes procedural volumes and demographic information for patients with International Classification of Diseases, Ninth Revision (ICD-9) diagnoses and procedures or Current Procedural Terminology (CPT) codes. Data for the study were derived from 2 databases within PearlDiver: a private-payer database, which has its largest contribution (>30 million individual patient records for 2007-2011) from United HealthCare, and a Medicare database (>50 million patient records for 2007-2011). Access to the database was granted by PearlDiver Technologies for the purpose of academic research. The database was stored on a password-protected server maintained by PearlDiver.
We searched the database for cases of ankle fracture fixation, including fixation of isolated lateral malleolus (CPT 27792), bimalleolar (CPT 27814), and trimalleolar (CPTs 27822 and 27823) fractures. CPT 27829 was used to search for syndesmotic fixation, and CPT 20680 for implant removal. These codes were used individually and in combination.
Overall procedural volume data are reported as number of patients with the given CPT(s) in the database output and as incidence, calculated as number of patients with the CPT of interest normalized to total number of patients in the database for that particular subgroup. Results of age group and sex analyses are reported as number of patients reported in the database output and as percentage of patients who had the CPT procedure of interest that year. As United HealthCare is the largest contributor to the private-payer portion of the database and is represented most prominently in the southern region, data for the regional analysis are presented only as incidence. This incidence was calculated as number of patients in a particular region and year normalized to total number of patients in the database for that region or year. The regions were Midwest (IA, IL, IN, KS, MI, MN, MO, ND, NE, OH, SD, WI), Northeast (CT, MA, ME, NH, NJ, NY, PA, RI, VT), South (AL, AR, DC, DE, FL, GA, KY, LA, MD, MI, NC, OK, SC, TN, TX, VA, WV), and West (AK, AZ, CA, CO, HI, ID, MT, NM, NV, OR, UT, WA, WY).
Chi-square linear-by-linear association analysis was used to determine the statistical significance of time trends in procedural volume, sex, age group, and region. For all statistical comparisons, P < .05 was considered significant.
Results
Number of open reduction and internal fixation (ORIF) procedures increased for all ankle fracture types over the period 2007 to 2011 (Table 1).
ORIF was performed for an ankle injury in 54,767 patients during the period 2007 to 2011, resulting in a cumulative incidence of 64.2 per 1000 patients (Table 2).
More ankle ORIF procedures were performed in females (33,565) than in males (21,202); incidence of ankle ORIF procedures was higher in females (68.6/1000 patients) than in males (58.4/1000 patients) (Table 2); percentages of bimalleolar and trimalleolar fractures were higher in females (bi, 40.6%; tri, 27.8%) than in males (bi, 34.6%; tri, 15.2%); and percentage of lateral malleolus fractures was higher in males (50.2%) than in females (31.6%).
Incidence of ankle ORIF procedures was similar in the South (69.6/1000 patients), Midwest (69.4/100 patients), and West (65.1/1000 patients) but lower in the Northeast (43.3/1000 patients) (Table 2). Lateral malleolus fractures were the most common ankle fractures in the Midwest (40.7%) and West (41.3%), followed by bimalleolar fractures (Midwest, 36.3%; West 36.0%) and trimalleolar fractures (Midwest, 23.0%; West, 22.7%). Bimalleolar fractures were most common in the Northeast (40.2%) and South (39.8%), followed by lateral malleolus fractures (Northeast, 34.4%; South, 38.0%) and trimalleolar fractures (Northeast, 25.4%; South, 22.3%).
Discussion
The present study found no significant change in number of lateral malleolus, bimalleolar, and trimalleolar ankle fracture ORIF procedures performed over the period 2007 to 2011. However, over the same period, incidence of syndesmosis fixation increased significantly in patients with isolated syndesmotic injuries and in patients with concomitant ankle fracture and syndesmotic injury. The largest percentage change was found in the bimalleolar ORIF group, which showed nearly a doubling of syndesmotic fixation over the 4-year study period, followed by a 38.1% increase in syndesmotic fixation in the trimalleolar ORIF group. Both groups had a syndesmotic fixation percentage change about twice that seen in the isolated lateral malleolus group.
There are several explanations for these trends. First, bimalleolar and trimalleolar fractures are more severe ankle fractures that tend to result from a more forceful mechanism, allowing for a higher rate of syndesmotic injury. Second, these trends likely do not reflect a true increase in the rate of syndesmosis injury but, rather, increased recognition of syndesmotic injury. Third, the data likely reflect a well-established approach to ankle fracture fixation and an increase in thinking that syndesmotic injuries should be stabilized in the setting of ankle fixation.
Incidence of syndesmotic injury as indicated by stabilization procedures can be compared with the data of Vosseller and colleagues,8 who reported an incidence of 6445 syndesmotic injuries per year in the United States. Our data showed fewer syndesmotic injuries, which may be related to use of CPT codes rather than ICD-9 codes for database searches, such that only operative syndesmotic injuries are represented in our data. Population differences between the 2 studies could also account for some of the differences in syndesmotic injury incidence.
We also found a significant change in the rate of hardware removal after syndesmosis ORIF. Across all treatment groups, incidence of screw removal decreased—a trend likely reflecting a change in attitude about the need for routine screw removal. Studies have shown that patients have favorable outcomes in the setting of syndesmotic screw loosening and screw breakage.37 Some authors have suggested that screw breakage or removal could be advantageous, as it allows the syndesmosis to settle into a more anatomical position after imperfect reduction.38 In addition, the trend of decreased syndesmotic screw removal could also have resulted from increased suture button fixation, which may less frequently require implant removal. Regardless, the overall trend is that routine syndesmotic implant removal has become less common.
This study had several limitations. First are the many limitations inherent to all studies that use large administrative databases, such as PearlDiver. The power of analysis depends on data quality; potential sources of error include accuracy of billing codes and physicians’ miscoding or noncoding. Although we tried to accurately represent a large population of interest through use of this database, we cannot be sure that the database represents a true cross-section of the United States. In addition, as we could not determine the method of syndesmotic fixation—the same CPT code is used for both suture button fixation and screw fixation—we could not establish trends for the rate of each method. More research is needed to establish these trends, and this research likely will require analysis of data from a large trauma center or from multiple centers.
Potential regional differences are another limitation. In the PearlDiver database, the South and Midwest are highly represented, the Northeast and West much less so. The South, Midwest, and West (but not the Northeast) had similar overall incidence and subgroup incidence of ankle ORIF. However, any regional differences in the rate of syndesmotic fixation could have skewed our data.
Ankle fractures and associated syndesmotic injuries remain a common problem. Although the prevalence of ankle fracture fixation has been relatively constant, the rate of syndesmosis stabilization has increased significantly. Young adults have the highest incidence of ankle fracture and associated syndesmotic fixation, but more ankle fractures occur in the large and growing elderly population. Increased awareness of syndesmotic injury likely has contributed to the recent rise in syndesmosis fixation seen in the present study. Given this trend, we recommend further analysis of outcome data and to establish treatment guidelines.
Am J Orthop. 2016;45(7):E472-E477. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. Waterman BR, Owens BD, Davey S, Zacchilli MA, Belmont PJ Jr. The epidemiology of ankle sprains in the United States. J Bone Joint Surg Am. 2010;92(13):2279-2284.
2. Court-Brown CM, Caesar B. Epidemiology of adult fractures: a review. Injury. 2006;37(8):691-697.
3. Miller AN, Paul O, Boraiah S, Parker RJ, Helfet DL, Lorich DG. Functional outcomes after syndesmotic screw fixation and removal. J Orthop Trauma. 2010;24(1):12-16.
4. Edwards GS Jr, DeLee JC. Ankle diastasis without fracture. Foot Ankle. 1984;4(6):305-312.
5. Norkus SA, Floyd RT. The anatomy and mechanisms of syndesmotic ankle sprains. J Athl Train. 2001;36(1):68-73.
6. Brosky T, Nyland J, Nitz A, Caborn DN. The ankle ligaments: consideration of syndesmotic injury and implications for rehabilitation. J Orthop Sports Phys Ther. 1995;21(4):197-205.
7. Purvis GD. Displaced, unstable ankle fractures: classification, incidence, and management of a consecutive series. Clin Orthop Relat Res. 1982;(165):91-98.
8. Vosseller JT, Karl JW, Greisberg JK. Incidence of syndesmotic injury. Orthopedics. 2014;37(3):e226-e229.
9. Stark E, Tornetta P 3rd, Creevy WR. Syndesmotic instability in Weber B ankle fractures: a clinical evaluation. J Orthop Trauma. 2007;21(9):643-646.
10. Tornetta P 3rd, Axelrad TW, Sibai TA, Creevy WR. Treatment of the stress positive ligamentous SE4 ankle fracture: incidence of syndesmotic injury and clinical decision making. J Orthop Trauma. 2012;26(11):659-661.
11. Xenos JS, Hopkinson WJ, Mulligan ME, Olson EJ, Popovic NA. The tibiofibular syndesmosis. Evaluation of the ligamentous structures, methods of fixation, and radiographic assessment. J Bone Joint Surg Am. 1995;77(6):847-856.
12. Ebraheim NA, Lu J, Yang H, Mekhail AO, Yeasting RA. Radiographic and CT evaluation of tibiofibular syndesmotic diastasis: a cadaver study. Foot Ankle Int. 1997;18(11):693-698.
13. Ahmad J, Raikin SM, Pour AE, Haytmanek C. Bioabsorbable screw fixation of the syndesmosis in unstable ankle injuries. Foot Ankle Int. 2009;30(2):99-105.
14. Hovis WD, Kaiser BW, Watson JT, Bucholz RW. Treatment of syndesmotic disruptions of the ankle with bioabsorbable screw fixation. J Bone Joint Surg Am. 2002;84(1):26-31.
15. Kaukonen JP, Lamberg T, Korkala O, Pajarinen J. Fixation of syndesmotic ruptures in 38 patients with a malleolar fracture: a randomized study comparing a metallic and a bioabsorbable screw. J Orthop Trauma. 2005;19(6):392-395.
16. Thordarson DB, Samuelson M, Shepherd LE, Merkle PF, Lee J. Bioabsorbable versus stainless steel screw fixation of the syndesmosis in pronation-lateral rotation ankle fractures: a prospective randomized trial. Foot Ankle Int. 2001;22(4):335-338.
17. Moore JA Jr, Shank JR, Morgan SJ, Smith WR. Syndesmosis fixation: a comparison of three and four cortices of screw fixation without hardware removal. Foot Ankle Int. 2006;27(8):567-572.
18. Høiness P, Strømsøe K. Tricortical versus quadricortical syndesmosis fixation in ankle fractures: a prospective, randomized study comparing two methods of syndesmosis fixation. J Orthop Trauma. 2004;18(6):331-337.
19. Huber T, Schmoelz W, Bölderl A. Motion of the fibula relative to the tibia and its alterations with syndesmosis screws: a cadaver study. Foot Ankle Surg. 2012;18(3):203-209.
20. Needleman RL, Skrade DA, Stiehl JB. Effect of the syndesmotic screw on ankle motion. Foot Ankle. 1989;10(1):17-24.
21. Mendelsohn ES, Hoshino CM, Harris TG, Zinar DM. The effect of obesity on early failure after operative syndesmosis injuries. J Orthop Trauma. 2013;27(4):201-206.
22. Schepers T. Acute distal tibiofibular syndesmosis injury: a systematic review of suture-button versus syndesmotic screw repair. Int Orthop. 2012;36(6):1199-1206.
23. Cottom JM, Hyer CF, Philbin TM, Berlet GC. Transosseous fixation of the distal tibiofibular syndesmosis: comparison of an interosseous suture and Endobutton to traditional screw fixation in 50 cases. J Foot Ankle Surg. 2009;48(6):620-630.
24. Thornes B, Shannon F, Guiney AM, Hession P, Masterson E. Suture-button syndesmosis fixation: accelerated rehabilitation and improved outcomes. Clin Orthop Relat Res. 2005;(431):207-212.
25. Willmott HJ, Singh B, David LA. Outcome and complications of treatment of ankle diastasis with tightrope fixation. Injury. 2009;40(11):1204-1206.
26. Qamar F, Kadakia A, Venkateswaran B. An anatomical way of treating ankle syndesmotic injuries. J Foot Ankle Surg. 2011;50(6):762-765.
27. Degroot H, Al-Omari AA, El Ghazaly SA. Outcomes of suture button repair of the distal tibiofibular syndesmosis. Foot Ankle Int. 2011;32(3):250-256.
28. Ramsey PL, Hamilton W. Changes in tibiotalar area of contact caused by lateral talar shift. J Bone Joint Surg Am. 1976;58(3):356-357.
29. Weening B, Bhandari M. Predictors of functional outcome following transsyndesmotic screw fixation of ankle fractures. J Orthop Trauma. 2005;19(2):102-108.
30. Sagi HC, Shah AR, Sanders RW. The functional consequence of syndesmotic joint malreduction at a minimum 2-year follow-up. J Orthop Trauma. 2012;26(7):439-443.
31. Naqvi GA, Cunningham P, Lynch B, Galvin R, Awan N. Fixation of ankle syndesmotic injuries: comparison of tightrope fixation and syndesmotic screw fixation for accuracy of syndesmotic reduction. Am J Sports Med. 2012;40(12):2828-2835.
32. Marmor M, Hansen E, Han HK, Buckley J, Matityahu A. Limitations of standard fluoroscopy in detecting rotational malreduction of the syndesmosis in an ankle fracture model. Foot Ankle Int. 2011;32(6):616-622.
33. Franke J, von Recum J, Suda AJ, Grützner PA, Wendl K. Intraoperative three-dimensional imaging in the treatment of acute unstable syndesmotic injuries. J Bone Joint Surg Am. 2012;94(15):1386-1390.
34. Gardner MJ, Demetrakopoulos D, Briggs SM, Helfet DL, Lorich DG. Malreduction of the tibiofibular syndesmosis in ankle fractures. Foot Ankle Int. 2006;27(10):788-792.
35. Miller AN, Carroll EA, Parker RJ, Boraiah S, Helfet DL, Lorich DG. Direct visualization for syndesmotic stabilization of ankle fractures. Foot Ankle Int. 2009;30(5):419-426.
36. Ruan Z, Luo C, Shi Z, Zhang B, Zeng B, Zhang C. Intraoperative reduction of distal tibiofibular joint aided by three-dimensional fluoroscopy. Technol Health Care. 2011;19(3):161-166.
37. Hamid N, Loeffler BJ, Braddy W, Kellam JF, Cohen BE, Bosse MJ. Outcome after fixation of ankle fractures with an injury to the syndesmosis: the effect of the syndesmosis screw. J Bone Joint Surg Br. 2009;91(8):1069-1073.
38. Song DJ, Lanzi JT, Groth AT, et al. The effect of syndesmosis screw removal on the reduction of the distal tibiofibular joint: a prospective radiographic study. Foot Ankle Int. 2014;35(6):543-548.
1. Waterman BR, Owens BD, Davey S, Zacchilli MA, Belmont PJ Jr. The epidemiology of ankle sprains in the United States. J Bone Joint Surg Am. 2010;92(13):2279-2284.
2. Court-Brown CM, Caesar B. Epidemiology of adult fractures: a review. Injury. 2006;37(8):691-697.
3. Miller AN, Paul O, Boraiah S, Parker RJ, Helfet DL, Lorich DG. Functional outcomes after syndesmotic screw fixation and removal. J Orthop Trauma. 2010;24(1):12-16.
4. Edwards GS Jr, DeLee JC. Ankle diastasis without fracture. Foot Ankle. 1984;4(6):305-312.
5. Norkus SA, Floyd RT. The anatomy and mechanisms of syndesmotic ankle sprains. J Athl Train. 2001;36(1):68-73.
6. Brosky T, Nyland J, Nitz A, Caborn DN. The ankle ligaments: consideration of syndesmotic injury and implications for rehabilitation. J Orthop Sports Phys Ther. 1995;21(4):197-205.
7. Purvis GD. Displaced, unstable ankle fractures: classification, incidence, and management of a consecutive series. Clin Orthop Relat Res. 1982;(165):91-98.
8. Vosseller JT, Karl JW, Greisberg JK. Incidence of syndesmotic injury. Orthopedics. 2014;37(3):e226-e229.
9. Stark E, Tornetta P 3rd, Creevy WR. Syndesmotic instability in Weber B ankle fractures: a clinical evaluation. J Orthop Trauma. 2007;21(9):643-646.
10. Tornetta P 3rd, Axelrad TW, Sibai TA, Creevy WR. Treatment of the stress positive ligamentous SE4 ankle fracture: incidence of syndesmotic injury and clinical decision making. J Orthop Trauma. 2012;26(11):659-661.
11. Xenos JS, Hopkinson WJ, Mulligan ME, Olson EJ, Popovic NA. The tibiofibular syndesmosis. Evaluation of the ligamentous structures, methods of fixation, and radiographic assessment. J Bone Joint Surg Am. 1995;77(6):847-856.
12. Ebraheim NA, Lu J, Yang H, Mekhail AO, Yeasting RA. Radiographic and CT evaluation of tibiofibular syndesmotic diastasis: a cadaver study. Foot Ankle Int. 1997;18(11):693-698.
13. Ahmad J, Raikin SM, Pour AE, Haytmanek C. Bioabsorbable screw fixation of the syndesmosis in unstable ankle injuries. Foot Ankle Int. 2009;30(2):99-105.
14. Hovis WD, Kaiser BW, Watson JT, Bucholz RW. Treatment of syndesmotic disruptions of the ankle with bioabsorbable screw fixation. J Bone Joint Surg Am. 2002;84(1):26-31.
15. Kaukonen JP, Lamberg T, Korkala O, Pajarinen J. Fixation of syndesmotic ruptures in 38 patients with a malleolar fracture: a randomized study comparing a metallic and a bioabsorbable screw. J Orthop Trauma. 2005;19(6):392-395.
16. Thordarson DB, Samuelson M, Shepherd LE, Merkle PF, Lee J. Bioabsorbable versus stainless steel screw fixation of the syndesmosis in pronation-lateral rotation ankle fractures: a prospective randomized trial. Foot Ankle Int. 2001;22(4):335-338.
17. Moore JA Jr, Shank JR, Morgan SJ, Smith WR. Syndesmosis fixation: a comparison of three and four cortices of screw fixation without hardware removal. Foot Ankle Int. 2006;27(8):567-572.
18. Høiness P, Strømsøe K. Tricortical versus quadricortical syndesmosis fixation in ankle fractures: a prospective, randomized study comparing two methods of syndesmosis fixation. J Orthop Trauma. 2004;18(6):331-337.
19. Huber T, Schmoelz W, Bölderl A. Motion of the fibula relative to the tibia and its alterations with syndesmosis screws: a cadaver study. Foot Ankle Surg. 2012;18(3):203-209.
20. Needleman RL, Skrade DA, Stiehl JB. Effect of the syndesmotic screw on ankle motion. Foot Ankle. 1989;10(1):17-24.
21. Mendelsohn ES, Hoshino CM, Harris TG, Zinar DM. The effect of obesity on early failure after operative syndesmosis injuries. J Orthop Trauma. 2013;27(4):201-206.
22. Schepers T. Acute distal tibiofibular syndesmosis injury: a systematic review of suture-button versus syndesmotic screw repair. Int Orthop. 2012;36(6):1199-1206.
23. Cottom JM, Hyer CF, Philbin TM, Berlet GC. Transosseous fixation of the distal tibiofibular syndesmosis: comparison of an interosseous suture and Endobutton to traditional screw fixation in 50 cases. J Foot Ankle Surg. 2009;48(6):620-630.
24. Thornes B, Shannon F, Guiney AM, Hession P, Masterson E. Suture-button syndesmosis fixation: accelerated rehabilitation and improved outcomes. Clin Orthop Relat Res. 2005;(431):207-212.
25. Willmott HJ, Singh B, David LA. Outcome and complications of treatment of ankle diastasis with tightrope fixation. Injury. 2009;40(11):1204-1206.
26. Qamar F, Kadakia A, Venkateswaran B. An anatomical way of treating ankle syndesmotic injuries. J Foot Ankle Surg. 2011;50(6):762-765.
27. Degroot H, Al-Omari AA, El Ghazaly SA. Outcomes of suture button repair of the distal tibiofibular syndesmosis. Foot Ankle Int. 2011;32(3):250-256.
28. Ramsey PL, Hamilton W. Changes in tibiotalar area of contact caused by lateral talar shift. J Bone Joint Surg Am. 1976;58(3):356-357.
29. Weening B, Bhandari M. Predictors of functional outcome following transsyndesmotic screw fixation of ankle fractures. J Orthop Trauma. 2005;19(2):102-108.
30. Sagi HC, Shah AR, Sanders RW. The functional consequence of syndesmotic joint malreduction at a minimum 2-year follow-up. J Orthop Trauma. 2012;26(7):439-443.
31. Naqvi GA, Cunningham P, Lynch B, Galvin R, Awan N. Fixation of ankle syndesmotic injuries: comparison of tightrope fixation and syndesmotic screw fixation for accuracy of syndesmotic reduction. Am J Sports Med. 2012;40(12):2828-2835.
32. Marmor M, Hansen E, Han HK, Buckley J, Matityahu A. Limitations of standard fluoroscopy in detecting rotational malreduction of the syndesmosis in an ankle fracture model. Foot Ankle Int. 2011;32(6):616-622.
33. Franke J, von Recum J, Suda AJ, Grützner PA, Wendl K. Intraoperative three-dimensional imaging in the treatment of acute unstable syndesmotic injuries. J Bone Joint Surg Am. 2012;94(15):1386-1390.
34. Gardner MJ, Demetrakopoulos D, Briggs SM, Helfet DL, Lorich DG. Malreduction of the tibiofibular syndesmosis in ankle fractures. Foot Ankle Int. 2006;27(10):788-792.
35. Miller AN, Carroll EA, Parker RJ, Boraiah S, Helfet DL, Lorich DG. Direct visualization for syndesmotic stabilization of ankle fractures. Foot Ankle Int. 2009;30(5):419-426.
36. Ruan Z, Luo C, Shi Z, Zhang B, Zeng B, Zhang C. Intraoperative reduction of distal tibiofibular joint aided by three-dimensional fluoroscopy. Technol Health Care. 2011;19(3):161-166.
37. Hamid N, Loeffler BJ, Braddy W, Kellam JF, Cohen BE, Bosse MJ. Outcome after fixation of ankle fractures with an injury to the syndesmosis: the effect of the syndesmosis screw. J Bone Joint Surg Br. 2009;91(8):1069-1073.
38. Song DJ, Lanzi JT, Groth AT, et al. The effect of syndesmosis screw removal on the reduction of the distal tibiofibular joint: a prospective radiographic study. Foot Ankle Int. 2014;35(6):543-548.
Hinged-Knee External Fixator Used to Reduce and Maintain Subacute Tibiofemoral Coronal Subluxation
Dislocation of the knee is a severe injury that usually results from high-energy blunt trauma.1 Recognition of knee dislocations has increased with expansion of the definition beyond radiographically confirmed loss of tibiofemoral articulation to include injury of multiple knee ligaments with multidirectional joint instability, or the rupture of the anterior and posterior cruciate ligaments (ACL, PCL) when no gross dislocation can be identified2 (though knee dislocations without rupture of either ligament have been reported3,4). Knee dislocations account for 0.02% to 0.2% of orthopedic injuries.5 These multiligamentous injuries are rare, but their clinical outcomes are often complicated by arthrofibrosis, pain, and instability, as surgeons contend with the competing interests of long-term joint stability and range of motion (ROM).6-9
Whereas treatment standards for acute knee dislocations are becoming clearer, treatment of subacute and chronic tibiofemoral dislocations and subluxations is less defined.5 Success with articulated external fixation originally across the ankle and elbow inspired interest in its use for the knee.10-12 Richter and Lobenhoffer13 and Simonian and colleagues14 were the first to report on the postoperative use of a hinged external fixation device to help maintain the reduction of chronic fixed posterior knee dislocations. The literature has even supported nonoperative reduction of small fixed anterior or posterior (sagittal) subluxations with knee bracing alone.15,16 However, there are no reports on treatment of chronic tibial subluxation in the coronal plane.
We report a case of a hinged-knee external fixator (HEF) used alone to reduce a chronic medial tibia subluxation that presented after initial repair of a knee dislocation sustained in a motor vehicle accident. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 51-year-old healthy woman who was traveling out of state sustained multiple orthopedic injuries in a motor vehicle accident. She had a pelvic fracture, a contralateral femoral shaft fracture, significant multiligamentous damage to the right knee, and a cavitary impaction fracture of the tibial eminence with resultant coronal tibial subluxation. Initial magnetic resonance imaging (MRI) showed the tibia injury likely was the result of varus translation, as the medial femoral condyle impacted the tibial spine, disrupting the ACL (Figures 1A, 1B).
On initial presentation to our clinic 5 weeks after injury, x-rays showed progressive medial subluxation of the tibia in relation to the femur with translation of about a third of the tibial width medially (Figures 2A, 2B).
Given the worsening tibial subluxation and resultant instability, the patient was taken to the operating room for examination under anesthesia, and planned closed reduction and spanning external fixation. Fluoroscopy of the lateral translation and external rotation of the tibia allowed us to reduce the joint, with the lateral tibial plateau and lateral femoral condyle relatively but not completely concentric. A rigid spanning multiplanar external fixator was then placed to maintain the knee joint in a more reduced position.
A week later, the patient was taken back to the operating room for arthroscopic evaluation of the knee joint. At the time of her index operation at the outside institution, she had undergone arthroscopic débridement of intra-articular loose bodies and lateral meniscus repair. Now it was found that the meniscus was not healed but had displaced. A bucket-handle lateral meniscus tear appeared to be blocking lateral translation of the tibia, thus impeding complete reduction.
Given the meniscus deformity that resulted from the chronicity of the injury and the resultant subluxation, a sub-total lateral meniscectomy was performed. As the patient was now noted to have an intact medial collateral ligament and an intact en masse lateral repair, we converted the spanning external fixator to a Compass Universal Hinge (Smith & Nephew) to maintain reduction without further ligamentous reconstruction (Figure 4).
After HEF placement, the patient spent a short time recovering at an inpatient rehabilitation facility before starting aggressive twice-a-week outpatient physical therapy. Initially after HEF placement, she could not actively flex the knee to about 40° or fully extend it concentrically. Given these limitations and concern about interval development of arthrofibrosis, manipulation under anesthesia was performed, 3 weeks after surgery, and 90° of flexion was obtained.
Six weeks after HEF removal, the patient was ambulating well with a cane, pain was minimal, and knee ROM was up to 110° of flexion. Tibiofemoral stability remained constant—no change in medial or lateral joint space opening. Full-extension radiographs showed medial translation of about 5 mm, which decreased to 1 mm on Rosenberg view. This represents marked improvement over the severe subluxation on initial presentation.
Follow-up over the next months revealed continued improvement in the right lower extremity strength, increased tolerance for physical activity, and stable right medial tibial translation.
At 5-year follow-up, the patient was asymptomatic, had continued coronal and sagittal stability, and was tolerating regular aerobic exercise, including hiking, weight training, and cycling. Physical examination revealed grade 1B Lachman, grade 0 pivot shift, and grade 0 posterior drawer. There was 3 mm increased lateral compartment opening in full extension, which increased to about 6 mm at 30° with endpoint.
Discussion
Although knee dislocations with multiligamentous involvement are rare, their outcomes can be poor. Fortunately, the principles of managing these complex injuries in the acute stage are becoming clearer. In a systematic review, Levy and colleagues18 found that operative treatment of a dislocated knee within 3 weeks after injury, compared with nonoperative or delayed treatment, resulted in improved functional outcomes. Ligament repair and reconstruction yielded similar outcomes, though repair of the posterolateral corner had a comparatively higher rate of failure. For associated lateral injuries, Shelbourne and colleagues17 advocated en masse repair in which the healing tissue complex is reattached to the tibia nonanatomically, without dissecting individual structures—a technique used in the original repair of our patient’s injuries.
Originally designed for other joints, hinged external fixators are now occasionally used for rehabilitation after traumatic knee injury. Stannard and colleagues9 recently confirmed the utility of the HEF as a supplement to ligament reconstruction for recovery from acute knee dislocation.9 Compared with postoperative use of a hinged-knee brace, HEF use resulted in fewer failed ligament reconstructions as well as equivalent joint ROM and Lysholm and IKDC scores at final follow-up. This clinical outcome is supported by results of kinematic studies of these hinged devices, which are capable of rigid fixation in all planes except sagittal and can reduce stress on intra-articular and periarticular ligaments when placed on the appropriate flexion-extension axis of the knee.19,20Unfortunately, the situation is more complicated for subacute or chronic tibial subluxation than for acute subluxation. Maak and colleagues16 described 3 operative steps that are crucial in obtaining desired outcomes in this setting: complete release of scar tissue, re-creation of knee axis through ACL and PCL reconstruction, and postoperative application of a HEF or knee brace. These recommendations mimic the management course described by Richter and Lobenhoffer13 and Simonian and colleagues,14 who treated chronic fixed posterior tibial subluxations with arthrolysis, ligament reconstruction, and use of HEFs for 6 weeks, supporting postoperative rehabilitation. All cases maintained reduction at follow-up after fixator removal.
It is also possible for small fixed anterior or posterior tibial subluxations to be managed nonoperatively. Strobel and colleagues15 described a series of 109 patients with fixed posterior subluxations treated at night with posterior tibial support braces. Mean subluxation was reduced from 6.93 mm to 2.58 mm after an average treatment period of 180 days. Although 60% of all subluxations were completely reduced, reductions were significantly more successful for those displaced <10 mm.
Management of subacute or chronic fixed coronal tibial subluxations is yet to be described. In this article, we have reported on acceptable reduction of a subacute medial tibial subluxation with use of a HEF for 6 weeks after arthroscopic débridement of a deformed subacute bucket-handle lateral meniscus tear. Our case report is unique in that it describes use of a HEF alone for the reduction of a subacute tibial subluxation in any plane without the need for more extensive ligament reconstruction.
The injury here was primarily a lateral ligamentous injury. In the nonanatomical repair that was performed, the LCL and the iliotibial band were reattached to the proximal-lateral tibia. Had we started treating this injury from the time of the patient’s accident, then, depending on repair integrity, we might have considered acute augmentation of the anatomical repair of LCL with Larson-type reconstruction of the LCL and the popliteofibular ligament. Alternatively, acute reconstruction of the LCL and popliteus would be considered if the lateral structures were either irreparable or of very poor quality. In addition, had we initially seen the coronal instability/translation, we might have acutely considered either a staged procedure of a multiplanar external fixator or a HEF.
Given the narrowed lateral joint space, the débridement of the lateral meniscus, and the risk of developing posttraumatic arthritis, our patient will probably need total knee arthroplasty (TKA) at some point. We informed her that she had advanced lateral compartment joint space narrowing and arthritic progression and that she would eventually need TKA based on pain or dysfunction. We think the longevity of that TKA will be predictable and good, as she now had improved tibiofemoral alignment and stability of the collateral ligamentous structures. If she had been allowed to maintain the coronally subluxed position, it would have led to medial ligamentous attenuation and would have compromised the success and longevity of the TKA. In essence, a crucial part of the utility of the HEF was improved coronal tibiofemoral alignment and, therefore, decreased abnormal forces on both the repaired lateral ligaments and the native medial ligamentous structures. Although temporary external fixation issues related to infection risk and patient discomfort are recognized,21-23 use of HEF alone can be part of the treatment considerations for fixed tibial subluxations in any plane when they present after treatment for multiligamentous injury.
Am J Orthop. 2016;45(7):E497-E502. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. Stannard JP, Sheils TM, McGwin G, Volgas DA, Alonso JE. Use of a hinged external knee fixator after surgery for knee dislocation. Arthroscopy. 2003;19(6):626-631.
2. Yeh WL, Tu YK, Su JY, Hsu RW. Knee dislocation: treatment of high-velocity knee dislocation. J Trauma. 1999;46(4):693-701.
3. Bellabarba C, Bush-Joseph CA, Bach BR Jr. Knee dislocation without anterior cruciate ligament disruption. A report of three cases. Am J Knee Surg. 1996;9(4):167-170.
4. Cooper DE, Speer KP, Wickiewicz TL, Warren RF. Complete knee dislocation without posterior cruciate ligament disruption. A report of four cases and review of the literature. Clin Orthop Relat Res. 1992;(284):228-233.
5. Howells NR, Brunton LR, Robinson J, Porteus AJ, Eldridge JD, Murray JR. Acute knee dislocation: an evidence based approach to the management of the multiligament injured knee. Injury. 2011;42(11):1198-1204.
6. Magit D, Wolff A, Sutton K, Medvecky MJ. Arthrofibrosis of the knee. J Am Acad Orthop Surg. 2007;15(11):682-694.
7. Medvecky MJ, Zazulak BT, Hewett TE. A multidisciplinary approach to the evaluation, reconstruction and rehabilitation of the multi-ligament injured athlete. Sports Med. 2007;37(2):169-187.
8. Noyes FR, Barber-Westin SD. Reconstruction of the anterior and posterior cruciate ligaments after knee dislocation. Use of early protected postoperative motion to decrease arthrofibrosis. Am J Sports Med. 1997;25(6):769-778.
9. Stannard JP, Nuelle CW, McGwin G, Volgas DA. Hinged external fixation in the treatment of knee dislocations: a prospective randomized study. J Bone Joint Surg Am. 2014;96(3):184-191.
10. Bottlang M, Marsh JL, Brown TD. Articulated external fixation of the ankle: minimizing motion resistance by accurate axis alignment. J Biomech. 1999;32(1):63-70.
11. Madey SM, Bottlang M, Steyers CM, Marsh JL, Brown TD. Hinged external fixation of the elbow: optimal axis alignment to minimize motion resistance. J Orthop Trauma. 2000;14(1):41-47.
12. Jupiter JB, Ring D. Treatment of unreduced elbow dislocations with hinged external fixation. J Bone Joint Surg Am. 2002;84(9):1630-1635.
13. Richter M, Lobenhoffer P. Chronic posterior knee dislocation: treatment with arthrolysis, posterior cruciate ligament reconstruction and hinged external fixation device. Injury. 1998;29(7):546-549.
14. Simonian PT, Wickiewicz TL, Hotchkiss RN, Warren RF. Chronic knee dislocation: reduction, reconstruction, and application of a skeletally fixed knee hinge. A report of two cases. Am J Sports Med. 1998;26(4):591-596.
15. Strobel MJ, Weiler A, Schulz MS, Russe K, Eichhorn HJ. Fixed posterior subluxation in posterior cruciate ligament-deficient knees: diagnosis and treatment of a new clinical sign. Am J Sports Med. 2002;30(1):32-38.
16. Maak TG, Marx RG, Wickiewicz TL. Management of chronic tibial subluxation in the multiple-ligament injured knee. Sports Med Arthrosc Rev. 2011;19(2):147-152.
17. Shelbourne KD, Haro MS, Gray T. Knee dislocation with lateral side injury: results of an en masse surgical repair technique of the lateral side. Am J Sports Med. 2007;35(7):1105-1116.
18. Levy BA, Fanelli GC, Whelan DB, et al. Controversies in the treatment of knee dislocations and multiligament reconstruction. J Am Acad Orthop Surg. 2009;17(4):197-206.
19. Fitzpatrick DC, Sommers MB, Kam BC, Marsh JL, Bottlang M. Knee stability after articulated external fixation. Am J Sports Med. 2005;33(11):1735-1741.
20. Sommers MB, Fitzpatrick DC, Kahn KM, Marsh JL, Bottlang M. Hinged external fixation of the knee: intrinsic factors influencing passive joint motion. J Orthop Trauma. 2004;18(3):163-169.
21. Anglen JO, Aleto T. Temporary transarticular external fixation of the knee and ankle. J Orthop Trauma. 1998;12(6):431-434.
22. Behrens F. General theory and principles of external fixation. Clin Orthop Relat Res. 1989;(241):15-23.
23. Haidukewych GJ. Temporary external fixation for the management of complex intra- and periarticular fractures of the lower extremity. J Orthop Trauma. 2002;16(9):678-685.
Dislocation of the knee is a severe injury that usually results from high-energy blunt trauma.1 Recognition of knee dislocations has increased with expansion of the definition beyond radiographically confirmed loss of tibiofemoral articulation to include injury of multiple knee ligaments with multidirectional joint instability, or the rupture of the anterior and posterior cruciate ligaments (ACL, PCL) when no gross dislocation can be identified2 (though knee dislocations without rupture of either ligament have been reported3,4). Knee dislocations account for 0.02% to 0.2% of orthopedic injuries.5 These multiligamentous injuries are rare, but their clinical outcomes are often complicated by arthrofibrosis, pain, and instability, as surgeons contend with the competing interests of long-term joint stability and range of motion (ROM).6-9
Whereas treatment standards for acute knee dislocations are becoming clearer, treatment of subacute and chronic tibiofemoral dislocations and subluxations is less defined.5 Success with articulated external fixation originally across the ankle and elbow inspired interest in its use for the knee.10-12 Richter and Lobenhoffer13 and Simonian and colleagues14 were the first to report on the postoperative use of a hinged external fixation device to help maintain the reduction of chronic fixed posterior knee dislocations. The literature has even supported nonoperative reduction of small fixed anterior or posterior (sagittal) subluxations with knee bracing alone.15,16 However, there are no reports on treatment of chronic tibial subluxation in the coronal plane.
We report a case of a hinged-knee external fixator (HEF) used alone to reduce a chronic medial tibia subluxation that presented after initial repair of a knee dislocation sustained in a motor vehicle accident. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 51-year-old healthy woman who was traveling out of state sustained multiple orthopedic injuries in a motor vehicle accident. She had a pelvic fracture, a contralateral femoral shaft fracture, significant multiligamentous damage to the right knee, and a cavitary impaction fracture of the tibial eminence with resultant coronal tibial subluxation. Initial magnetic resonance imaging (MRI) showed the tibia injury likely was the result of varus translation, as the medial femoral condyle impacted the tibial spine, disrupting the ACL (Figures 1A, 1B).
On initial presentation to our clinic 5 weeks after injury, x-rays showed progressive medial subluxation of the tibia in relation to the femur with translation of about a third of the tibial width medially (Figures 2A, 2B).
Given the worsening tibial subluxation and resultant instability, the patient was taken to the operating room for examination under anesthesia, and planned closed reduction and spanning external fixation. Fluoroscopy of the lateral translation and external rotation of the tibia allowed us to reduce the joint, with the lateral tibial plateau and lateral femoral condyle relatively but not completely concentric. A rigid spanning multiplanar external fixator was then placed to maintain the knee joint in a more reduced position.
A week later, the patient was taken back to the operating room for arthroscopic evaluation of the knee joint. At the time of her index operation at the outside institution, she had undergone arthroscopic débridement of intra-articular loose bodies and lateral meniscus repair. Now it was found that the meniscus was not healed but had displaced. A bucket-handle lateral meniscus tear appeared to be blocking lateral translation of the tibia, thus impeding complete reduction.
Given the meniscus deformity that resulted from the chronicity of the injury and the resultant subluxation, a sub-total lateral meniscectomy was performed. As the patient was now noted to have an intact medial collateral ligament and an intact en masse lateral repair, we converted the spanning external fixator to a Compass Universal Hinge (Smith & Nephew) to maintain reduction without further ligamentous reconstruction (Figure 4).
After HEF placement, the patient spent a short time recovering at an inpatient rehabilitation facility before starting aggressive twice-a-week outpatient physical therapy. Initially after HEF placement, she could not actively flex the knee to about 40° or fully extend it concentrically. Given these limitations and concern about interval development of arthrofibrosis, manipulation under anesthesia was performed, 3 weeks after surgery, and 90° of flexion was obtained.
Six weeks after HEF removal, the patient was ambulating well with a cane, pain was minimal, and knee ROM was up to 110° of flexion. Tibiofemoral stability remained constant—no change in medial or lateral joint space opening. Full-extension radiographs showed medial translation of about 5 mm, which decreased to 1 mm on Rosenberg view. This represents marked improvement over the severe subluxation on initial presentation.
Follow-up over the next months revealed continued improvement in the right lower extremity strength, increased tolerance for physical activity, and stable right medial tibial translation.
At 5-year follow-up, the patient was asymptomatic, had continued coronal and sagittal stability, and was tolerating regular aerobic exercise, including hiking, weight training, and cycling. Physical examination revealed grade 1B Lachman, grade 0 pivot shift, and grade 0 posterior drawer. There was 3 mm increased lateral compartment opening in full extension, which increased to about 6 mm at 30° with endpoint.
Discussion
Although knee dislocations with multiligamentous involvement are rare, their outcomes can be poor. Fortunately, the principles of managing these complex injuries in the acute stage are becoming clearer. In a systematic review, Levy and colleagues18 found that operative treatment of a dislocated knee within 3 weeks after injury, compared with nonoperative or delayed treatment, resulted in improved functional outcomes. Ligament repair and reconstruction yielded similar outcomes, though repair of the posterolateral corner had a comparatively higher rate of failure. For associated lateral injuries, Shelbourne and colleagues17 advocated en masse repair in which the healing tissue complex is reattached to the tibia nonanatomically, without dissecting individual structures—a technique used in the original repair of our patient’s injuries.
Originally designed for other joints, hinged external fixators are now occasionally used for rehabilitation after traumatic knee injury. Stannard and colleagues9 recently confirmed the utility of the HEF as a supplement to ligament reconstruction for recovery from acute knee dislocation.9 Compared with postoperative use of a hinged-knee brace, HEF use resulted in fewer failed ligament reconstructions as well as equivalent joint ROM and Lysholm and IKDC scores at final follow-up. This clinical outcome is supported by results of kinematic studies of these hinged devices, which are capable of rigid fixation in all planes except sagittal and can reduce stress on intra-articular and periarticular ligaments when placed on the appropriate flexion-extension axis of the knee.19,20Unfortunately, the situation is more complicated for subacute or chronic tibial subluxation than for acute subluxation. Maak and colleagues16 described 3 operative steps that are crucial in obtaining desired outcomes in this setting: complete release of scar tissue, re-creation of knee axis through ACL and PCL reconstruction, and postoperative application of a HEF or knee brace. These recommendations mimic the management course described by Richter and Lobenhoffer13 and Simonian and colleagues,14 who treated chronic fixed posterior tibial subluxations with arthrolysis, ligament reconstruction, and use of HEFs for 6 weeks, supporting postoperative rehabilitation. All cases maintained reduction at follow-up after fixator removal.
It is also possible for small fixed anterior or posterior tibial subluxations to be managed nonoperatively. Strobel and colleagues15 described a series of 109 patients with fixed posterior subluxations treated at night with posterior tibial support braces. Mean subluxation was reduced from 6.93 mm to 2.58 mm after an average treatment period of 180 days. Although 60% of all subluxations were completely reduced, reductions were significantly more successful for those displaced <10 mm.
Management of subacute or chronic fixed coronal tibial subluxations is yet to be described. In this article, we have reported on acceptable reduction of a subacute medial tibial subluxation with use of a HEF for 6 weeks after arthroscopic débridement of a deformed subacute bucket-handle lateral meniscus tear. Our case report is unique in that it describes use of a HEF alone for the reduction of a subacute tibial subluxation in any plane without the need for more extensive ligament reconstruction.
The injury here was primarily a lateral ligamentous injury. In the nonanatomical repair that was performed, the LCL and the iliotibial band were reattached to the proximal-lateral tibia. Had we started treating this injury from the time of the patient’s accident, then, depending on repair integrity, we might have considered acute augmentation of the anatomical repair of LCL with Larson-type reconstruction of the LCL and the popliteofibular ligament. Alternatively, acute reconstruction of the LCL and popliteus would be considered if the lateral structures were either irreparable or of very poor quality. In addition, had we initially seen the coronal instability/translation, we might have acutely considered either a staged procedure of a multiplanar external fixator or a HEF.
Given the narrowed lateral joint space, the débridement of the lateral meniscus, and the risk of developing posttraumatic arthritis, our patient will probably need total knee arthroplasty (TKA) at some point. We informed her that she had advanced lateral compartment joint space narrowing and arthritic progression and that she would eventually need TKA based on pain or dysfunction. We think the longevity of that TKA will be predictable and good, as she now had improved tibiofemoral alignment and stability of the collateral ligamentous structures. If she had been allowed to maintain the coronally subluxed position, it would have led to medial ligamentous attenuation and would have compromised the success and longevity of the TKA. In essence, a crucial part of the utility of the HEF was improved coronal tibiofemoral alignment and, therefore, decreased abnormal forces on both the repaired lateral ligaments and the native medial ligamentous structures. Although temporary external fixation issues related to infection risk and patient discomfort are recognized,21-23 use of HEF alone can be part of the treatment considerations for fixed tibial subluxations in any plane when they present after treatment for multiligamentous injury.
Am J Orthop. 2016;45(7):E497-E502. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
Dislocation of the knee is a severe injury that usually results from high-energy blunt trauma.1 Recognition of knee dislocations has increased with expansion of the definition beyond radiographically confirmed loss of tibiofemoral articulation to include injury of multiple knee ligaments with multidirectional joint instability, or the rupture of the anterior and posterior cruciate ligaments (ACL, PCL) when no gross dislocation can be identified2 (though knee dislocations without rupture of either ligament have been reported3,4). Knee dislocations account for 0.02% to 0.2% of orthopedic injuries.5 These multiligamentous injuries are rare, but their clinical outcomes are often complicated by arthrofibrosis, pain, and instability, as surgeons contend with the competing interests of long-term joint stability and range of motion (ROM).6-9
Whereas treatment standards for acute knee dislocations are becoming clearer, treatment of subacute and chronic tibiofemoral dislocations and subluxations is less defined.5 Success with articulated external fixation originally across the ankle and elbow inspired interest in its use for the knee.10-12 Richter and Lobenhoffer13 and Simonian and colleagues14 were the first to report on the postoperative use of a hinged external fixation device to help maintain the reduction of chronic fixed posterior knee dislocations. The literature has even supported nonoperative reduction of small fixed anterior or posterior (sagittal) subluxations with knee bracing alone.15,16 However, there are no reports on treatment of chronic tibial subluxation in the coronal plane.
We report a case of a hinged-knee external fixator (HEF) used alone to reduce a chronic medial tibia subluxation that presented after initial repair of a knee dislocation sustained in a motor vehicle accident. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 51-year-old healthy woman who was traveling out of state sustained multiple orthopedic injuries in a motor vehicle accident. She had a pelvic fracture, a contralateral femoral shaft fracture, significant multiligamentous damage to the right knee, and a cavitary impaction fracture of the tibial eminence with resultant coronal tibial subluxation. Initial magnetic resonance imaging (MRI) showed the tibia injury likely was the result of varus translation, as the medial femoral condyle impacted the tibial spine, disrupting the ACL (Figures 1A, 1B).
On initial presentation to our clinic 5 weeks after injury, x-rays showed progressive medial subluxation of the tibia in relation to the femur with translation of about a third of the tibial width medially (Figures 2A, 2B).
Given the worsening tibial subluxation and resultant instability, the patient was taken to the operating room for examination under anesthesia, and planned closed reduction and spanning external fixation. Fluoroscopy of the lateral translation and external rotation of the tibia allowed us to reduce the joint, with the lateral tibial plateau and lateral femoral condyle relatively but not completely concentric. A rigid spanning multiplanar external fixator was then placed to maintain the knee joint in a more reduced position.
A week later, the patient was taken back to the operating room for arthroscopic evaluation of the knee joint. At the time of her index operation at the outside institution, she had undergone arthroscopic débridement of intra-articular loose bodies and lateral meniscus repair. Now it was found that the meniscus was not healed but had displaced. A bucket-handle lateral meniscus tear appeared to be blocking lateral translation of the tibia, thus impeding complete reduction.
Given the meniscus deformity that resulted from the chronicity of the injury and the resultant subluxation, a sub-total lateral meniscectomy was performed. As the patient was now noted to have an intact medial collateral ligament and an intact en masse lateral repair, we converted the spanning external fixator to a Compass Universal Hinge (Smith & Nephew) to maintain reduction without further ligamentous reconstruction (Figure 4).
After HEF placement, the patient spent a short time recovering at an inpatient rehabilitation facility before starting aggressive twice-a-week outpatient physical therapy. Initially after HEF placement, she could not actively flex the knee to about 40° or fully extend it concentrically. Given these limitations and concern about interval development of arthrofibrosis, manipulation under anesthesia was performed, 3 weeks after surgery, and 90° of flexion was obtained.
Six weeks after HEF removal, the patient was ambulating well with a cane, pain was minimal, and knee ROM was up to 110° of flexion. Tibiofemoral stability remained constant—no change in medial or lateral joint space opening. Full-extension radiographs showed medial translation of about 5 mm, which decreased to 1 mm on Rosenberg view. This represents marked improvement over the severe subluxation on initial presentation.
Follow-up over the next months revealed continued improvement in the right lower extremity strength, increased tolerance for physical activity, and stable right medial tibial translation.
At 5-year follow-up, the patient was asymptomatic, had continued coronal and sagittal stability, and was tolerating regular aerobic exercise, including hiking, weight training, and cycling. Physical examination revealed grade 1B Lachman, grade 0 pivot shift, and grade 0 posterior drawer. There was 3 mm increased lateral compartment opening in full extension, which increased to about 6 mm at 30° with endpoint.
Discussion
Although knee dislocations with multiligamentous involvement are rare, their outcomes can be poor. Fortunately, the principles of managing these complex injuries in the acute stage are becoming clearer. In a systematic review, Levy and colleagues18 found that operative treatment of a dislocated knee within 3 weeks after injury, compared with nonoperative or delayed treatment, resulted in improved functional outcomes. Ligament repair and reconstruction yielded similar outcomes, though repair of the posterolateral corner had a comparatively higher rate of failure. For associated lateral injuries, Shelbourne and colleagues17 advocated en masse repair in which the healing tissue complex is reattached to the tibia nonanatomically, without dissecting individual structures—a technique used in the original repair of our patient’s injuries.
Originally designed for other joints, hinged external fixators are now occasionally used for rehabilitation after traumatic knee injury. Stannard and colleagues9 recently confirmed the utility of the HEF as a supplement to ligament reconstruction for recovery from acute knee dislocation.9 Compared with postoperative use of a hinged-knee brace, HEF use resulted in fewer failed ligament reconstructions as well as equivalent joint ROM and Lysholm and IKDC scores at final follow-up. This clinical outcome is supported by results of kinematic studies of these hinged devices, which are capable of rigid fixation in all planes except sagittal and can reduce stress on intra-articular and periarticular ligaments when placed on the appropriate flexion-extension axis of the knee.19,20Unfortunately, the situation is more complicated for subacute or chronic tibial subluxation than for acute subluxation. Maak and colleagues16 described 3 operative steps that are crucial in obtaining desired outcomes in this setting: complete release of scar tissue, re-creation of knee axis through ACL and PCL reconstruction, and postoperative application of a HEF or knee brace. These recommendations mimic the management course described by Richter and Lobenhoffer13 and Simonian and colleagues,14 who treated chronic fixed posterior tibial subluxations with arthrolysis, ligament reconstruction, and use of HEFs for 6 weeks, supporting postoperative rehabilitation. All cases maintained reduction at follow-up after fixator removal.
It is also possible for small fixed anterior or posterior tibial subluxations to be managed nonoperatively. Strobel and colleagues15 described a series of 109 patients with fixed posterior subluxations treated at night with posterior tibial support braces. Mean subluxation was reduced from 6.93 mm to 2.58 mm after an average treatment period of 180 days. Although 60% of all subluxations were completely reduced, reductions were significantly more successful for those displaced <10 mm.
Management of subacute or chronic fixed coronal tibial subluxations is yet to be described. In this article, we have reported on acceptable reduction of a subacute medial tibial subluxation with use of a HEF for 6 weeks after arthroscopic débridement of a deformed subacute bucket-handle lateral meniscus tear. Our case report is unique in that it describes use of a HEF alone for the reduction of a subacute tibial subluxation in any plane without the need for more extensive ligament reconstruction.
The injury here was primarily a lateral ligamentous injury. In the nonanatomical repair that was performed, the LCL and the iliotibial band were reattached to the proximal-lateral tibia. Had we started treating this injury from the time of the patient’s accident, then, depending on repair integrity, we might have considered acute augmentation of the anatomical repair of LCL with Larson-type reconstruction of the LCL and the popliteofibular ligament. Alternatively, acute reconstruction of the LCL and popliteus would be considered if the lateral structures were either irreparable or of very poor quality. In addition, had we initially seen the coronal instability/translation, we might have acutely considered either a staged procedure of a multiplanar external fixator or a HEF.
Given the narrowed lateral joint space, the débridement of the lateral meniscus, and the risk of developing posttraumatic arthritis, our patient will probably need total knee arthroplasty (TKA) at some point. We informed her that she had advanced lateral compartment joint space narrowing and arthritic progression and that she would eventually need TKA based on pain or dysfunction. We think the longevity of that TKA will be predictable and good, as she now had improved tibiofemoral alignment and stability of the collateral ligamentous structures. If she had been allowed to maintain the coronally subluxed position, it would have led to medial ligamentous attenuation and would have compromised the success and longevity of the TKA. In essence, a crucial part of the utility of the HEF was improved coronal tibiofemoral alignment and, therefore, decreased abnormal forces on both the repaired lateral ligaments and the native medial ligamentous structures. Although temporary external fixation issues related to infection risk and patient discomfort are recognized,21-23 use of HEF alone can be part of the treatment considerations for fixed tibial subluxations in any plane when they present after treatment for multiligamentous injury.
Am J Orthop. 2016;45(7):E497-E502. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. Stannard JP, Sheils TM, McGwin G, Volgas DA, Alonso JE. Use of a hinged external knee fixator after surgery for knee dislocation. Arthroscopy. 2003;19(6):626-631.
2. Yeh WL, Tu YK, Su JY, Hsu RW. Knee dislocation: treatment of high-velocity knee dislocation. J Trauma. 1999;46(4):693-701.
3. Bellabarba C, Bush-Joseph CA, Bach BR Jr. Knee dislocation without anterior cruciate ligament disruption. A report of three cases. Am J Knee Surg. 1996;9(4):167-170.
4. Cooper DE, Speer KP, Wickiewicz TL, Warren RF. Complete knee dislocation without posterior cruciate ligament disruption. A report of four cases and review of the literature. Clin Orthop Relat Res. 1992;(284):228-233.
5. Howells NR, Brunton LR, Robinson J, Porteus AJ, Eldridge JD, Murray JR. Acute knee dislocation: an evidence based approach to the management of the multiligament injured knee. Injury. 2011;42(11):1198-1204.
6. Magit D, Wolff A, Sutton K, Medvecky MJ. Arthrofibrosis of the knee. J Am Acad Orthop Surg. 2007;15(11):682-694.
7. Medvecky MJ, Zazulak BT, Hewett TE. A multidisciplinary approach to the evaluation, reconstruction and rehabilitation of the multi-ligament injured athlete. Sports Med. 2007;37(2):169-187.
8. Noyes FR, Barber-Westin SD. Reconstruction of the anterior and posterior cruciate ligaments after knee dislocation. Use of early protected postoperative motion to decrease arthrofibrosis. Am J Sports Med. 1997;25(6):769-778.
9. Stannard JP, Nuelle CW, McGwin G, Volgas DA. Hinged external fixation in the treatment of knee dislocations: a prospective randomized study. J Bone Joint Surg Am. 2014;96(3):184-191.
10. Bottlang M, Marsh JL, Brown TD. Articulated external fixation of the ankle: minimizing motion resistance by accurate axis alignment. J Biomech. 1999;32(1):63-70.
11. Madey SM, Bottlang M, Steyers CM, Marsh JL, Brown TD. Hinged external fixation of the elbow: optimal axis alignment to minimize motion resistance. J Orthop Trauma. 2000;14(1):41-47.
12. Jupiter JB, Ring D. Treatment of unreduced elbow dislocations with hinged external fixation. J Bone Joint Surg Am. 2002;84(9):1630-1635.
13. Richter M, Lobenhoffer P. Chronic posterior knee dislocation: treatment with arthrolysis, posterior cruciate ligament reconstruction and hinged external fixation device. Injury. 1998;29(7):546-549.
14. Simonian PT, Wickiewicz TL, Hotchkiss RN, Warren RF. Chronic knee dislocation: reduction, reconstruction, and application of a skeletally fixed knee hinge. A report of two cases. Am J Sports Med. 1998;26(4):591-596.
15. Strobel MJ, Weiler A, Schulz MS, Russe K, Eichhorn HJ. Fixed posterior subluxation in posterior cruciate ligament-deficient knees: diagnosis and treatment of a new clinical sign. Am J Sports Med. 2002;30(1):32-38.
16. Maak TG, Marx RG, Wickiewicz TL. Management of chronic tibial subluxation in the multiple-ligament injured knee. Sports Med Arthrosc Rev. 2011;19(2):147-152.
17. Shelbourne KD, Haro MS, Gray T. Knee dislocation with lateral side injury: results of an en masse surgical repair technique of the lateral side. Am J Sports Med. 2007;35(7):1105-1116.
18. Levy BA, Fanelli GC, Whelan DB, et al. Controversies in the treatment of knee dislocations and multiligament reconstruction. J Am Acad Orthop Surg. 2009;17(4):197-206.
19. Fitzpatrick DC, Sommers MB, Kam BC, Marsh JL, Bottlang M. Knee stability after articulated external fixation. Am J Sports Med. 2005;33(11):1735-1741.
20. Sommers MB, Fitzpatrick DC, Kahn KM, Marsh JL, Bottlang M. Hinged external fixation of the knee: intrinsic factors influencing passive joint motion. J Orthop Trauma. 2004;18(3):163-169.
21. Anglen JO, Aleto T. Temporary transarticular external fixation of the knee and ankle. J Orthop Trauma. 1998;12(6):431-434.
22. Behrens F. General theory and principles of external fixation. Clin Orthop Relat Res. 1989;(241):15-23.
23. Haidukewych GJ. Temporary external fixation for the management of complex intra- and periarticular fractures of the lower extremity. J Orthop Trauma. 2002;16(9):678-685.
1. Stannard JP, Sheils TM, McGwin G, Volgas DA, Alonso JE. Use of a hinged external knee fixator after surgery for knee dislocation. Arthroscopy. 2003;19(6):626-631.
2. Yeh WL, Tu YK, Su JY, Hsu RW. Knee dislocation: treatment of high-velocity knee dislocation. J Trauma. 1999;46(4):693-701.
3. Bellabarba C, Bush-Joseph CA, Bach BR Jr. Knee dislocation without anterior cruciate ligament disruption. A report of three cases. Am J Knee Surg. 1996;9(4):167-170.
4. Cooper DE, Speer KP, Wickiewicz TL, Warren RF. Complete knee dislocation without posterior cruciate ligament disruption. A report of four cases and review of the literature. Clin Orthop Relat Res. 1992;(284):228-233.
5. Howells NR, Brunton LR, Robinson J, Porteus AJ, Eldridge JD, Murray JR. Acute knee dislocation: an evidence based approach to the management of the multiligament injured knee. Injury. 2011;42(11):1198-1204.
6. Magit D, Wolff A, Sutton K, Medvecky MJ. Arthrofibrosis of the knee. J Am Acad Orthop Surg. 2007;15(11):682-694.
7. Medvecky MJ, Zazulak BT, Hewett TE. A multidisciplinary approach to the evaluation, reconstruction and rehabilitation of the multi-ligament injured athlete. Sports Med. 2007;37(2):169-187.
8. Noyes FR, Barber-Westin SD. Reconstruction of the anterior and posterior cruciate ligaments after knee dislocation. Use of early protected postoperative motion to decrease arthrofibrosis. Am J Sports Med. 1997;25(6):769-778.
9. Stannard JP, Nuelle CW, McGwin G, Volgas DA. Hinged external fixation in the treatment of knee dislocations: a prospective randomized study. J Bone Joint Surg Am. 2014;96(3):184-191.
10. Bottlang M, Marsh JL, Brown TD. Articulated external fixation of the ankle: minimizing motion resistance by accurate axis alignment. J Biomech. 1999;32(1):63-70.
11. Madey SM, Bottlang M, Steyers CM, Marsh JL, Brown TD. Hinged external fixation of the elbow: optimal axis alignment to minimize motion resistance. J Orthop Trauma. 2000;14(1):41-47.
12. Jupiter JB, Ring D. Treatment of unreduced elbow dislocations with hinged external fixation. J Bone Joint Surg Am. 2002;84(9):1630-1635.
13. Richter M, Lobenhoffer P. Chronic posterior knee dislocation: treatment with arthrolysis, posterior cruciate ligament reconstruction and hinged external fixation device. Injury. 1998;29(7):546-549.
14. Simonian PT, Wickiewicz TL, Hotchkiss RN, Warren RF. Chronic knee dislocation: reduction, reconstruction, and application of a skeletally fixed knee hinge. A report of two cases. Am J Sports Med. 1998;26(4):591-596.
15. Strobel MJ, Weiler A, Schulz MS, Russe K, Eichhorn HJ. Fixed posterior subluxation in posterior cruciate ligament-deficient knees: diagnosis and treatment of a new clinical sign. Am J Sports Med. 2002;30(1):32-38.
16. Maak TG, Marx RG, Wickiewicz TL. Management of chronic tibial subluxation in the multiple-ligament injured knee. Sports Med Arthrosc Rev. 2011;19(2):147-152.
17. Shelbourne KD, Haro MS, Gray T. Knee dislocation with lateral side injury: results of an en masse surgical repair technique of the lateral side. Am J Sports Med. 2007;35(7):1105-1116.
18. Levy BA, Fanelli GC, Whelan DB, et al. Controversies in the treatment of knee dislocations and multiligament reconstruction. J Am Acad Orthop Surg. 2009;17(4):197-206.
19. Fitzpatrick DC, Sommers MB, Kam BC, Marsh JL, Bottlang M. Knee stability after articulated external fixation. Am J Sports Med. 2005;33(11):1735-1741.
20. Sommers MB, Fitzpatrick DC, Kahn KM, Marsh JL, Bottlang M. Hinged external fixation of the knee: intrinsic factors influencing passive joint motion. J Orthop Trauma. 2004;18(3):163-169.
21. Anglen JO, Aleto T. Temporary transarticular external fixation of the knee and ankle. J Orthop Trauma. 1998;12(6):431-434.
22. Behrens F. General theory and principles of external fixation. Clin Orthop Relat Res. 1989;(241):15-23.
23. Haidukewych GJ. Temporary external fixation for the management of complex intra- and periarticular fractures of the lower extremity. J Orthop Trauma. 2002;16(9):678-685.
Prevalence of Low Vitamin D Levels in Patients With Orthopedic Trauma
The role of vitamin D in general health maintenance is a topic of increasing interest and importance in the medical community. Not only has vitamin D deficiency been linked to a myriad of nonorthopedic maladies, including cancer, diabetes, and cardiovascular disease, but it has demonstrated an adverse effect on musculoskeletal health.1 Authors have found a correlation between vitamin D deficiency and muscle weakness, fragility fractures, and, most recently, fracture nonunion.1 Despite the detrimental effects of vitamin D deficiency on musculoskeletal and general health, evidence exists that vitamin D deficiency is surprisingly prevalent.2 This deficiency is known to be associated with increasing age, but recent studies have also found alarming rates of deficiency in younger populations.3,4
Although there has been some discussion regarding optimal serum levels of 25-hydroxyvitamin D, most experts have defined vitamin D deficiency as a 25-hydroxyvitamin D level of 20 ng/mL or less and insufficiency as 21 to 32 ng/mL.5 Hollis and Wagner5 found increased serum parathyroid hormone and bone resorption and impaired dietary absorption of calcium when 25-hydroxyvitamin D levels were under 32 ng/mL. Given these data, a 25-hydroxyvitamin D level of 21 to 32 ng/mL (52-72 nmol/L) can be considered as indicating a relative insufficiency of vitamin D, and a level of 20 ng/mL or less can be considered as indicating vitamin D deficiency.
Vitamin D plays a vital role in bone metabolism and has been implicated in increased fracture risk and in fracture healing ability. Therefore, documenting the prevalence of vitamin D deficiency in patients with trauma is the first step in raising awareness among orthopedic traumatologists and further developing a screening-and-treatment strategy for vitamin D deficiency in these patients. Steele and colleagues6 retrospectively studied 44 patients with high- and low-energy fractures and found an almost 60% prevalence of vitamin D insufficiency. If vitamin D insufficiency is this prevalent, treatment protocols for patients with fractures may require modifications that include routine screening and treatment for low vitamin D levels.
After noting a regular occurrence of hypovitaminosis D in our patient population (independent of age, sex, or medical comorbidities), we conducted a study to determine the prevalence of vitamin D deficiency in a large orthopedic trauma population.
Patients and Methods
After obtaining Institutional Review Board approval for this study, we retrospectively reviewed the charts of all patients with a fracture treated by 1 of 4 orthopedic traumatologists within a 21-month period (January 1, 2009 to September 30, 2010). Acute fracture and recorded 25-hydroxyvitamin D level were the primary criteria for study inclusion. Given the concern about vitamin D deficiency, it became common protocol to check the serum 25-hydroxyvitamin D levels of patients with acute fractures during the review period. Exclusion criteria were age under 18 years and presence of vitamin D deficiency risk factors, including renal insufficiency (creatinine level, ≥2 mg/dL), malabsorption, gastrectomy, active liver disease, acute myocardial infarction, alcoholism, anorexia nervosa, and steroid dependency.
During the period studied, 1830 patients over age 18 years were treated by 4 fellowship-trained orthopedic traumatologists. Of these patients, 889 (487 female, 402 male) met the inclusion criteria. Mean age was 53.8 years. Demographic data (age, sex, race, independent living status, comorbid medical conditions, medications) were collected from the patients’ medical records. Clinical data collected were mechanism of injury, fracture location and type, injury date, surgery date and surgical procedure performed (when applicable), and serum 25-hydroxyvitamin D levels.
Statistical Methods
Descriptive statistics (mean, median, mode) were calculated. The χ2 test was used when all cell frequencies were more than 5, and the Fisher exact probability test was used when any cell frequency was 5 or less. Prevalence of vitamin D deficiency and insufficiency was calculated in multiple patient populations. Patients were analyzed according to age and sex subgroups.
Definitions
Vitamin D deficiency was defined as a serum 25-hydroxyvitamin D level of 20 ng/mL or less and insufficiency as 21 to 32 ng/mL.2 As the serum test was performed independent of the investigators and with use of standard medical laboratory protocols and techniques, there should be no bias in the results. We had intended to have all patients undergo serum testing during the review period because that was our usual protocol. However, test results were available for only 889 (49%) of the 1830 patients with orthopedic trauma during the review period. Although a false-positive is theoretically possible, this series of orthopedic trauma patients is the largest in the literature and therefore should be more accurate than the previously reported small series.
Results
There were no significant (P < .05) age or sex differences in prevalence of vitamin D deficiency or insufficiency in our patient population. Overall prevalence of deficiency/insufficiency was 77.39%, and prevalence of deficiency alone was 39.03% (Table 1).
Women in the 18- to 25-year age group had a lower prevalence of deficiency (25%; P = .41) and insufficiency (41.7%; P = .16) than women in the other age groups (Table 3).
Discussion
We conducted this study to determine the prevalence of vitamin D deficiency in a large population of patients with orthopedic trauma. Results showed that vitamin D deficiency and insufficiency were prevalent in this population, which to our knowledge is the largest studied for vitamin D deficiency. In a 6-month study of 44 fractures, Steele and colleagues6 found an overall 60% rate of deficiency/insufficiency. Although their investigation is important—it was the first of its kind to evaluate patients with various fracture types, including those with high-energy causes—its numbers were small, and the period evaluated (June 1, 2006 to February 1, 2007) was short (8 months). Use of that time frame may have led to an underestimate of the prevalence of vitamin D deficiency, as vitamin D levels are higher in late summer because of increased sun exposure. Our study of 889 patients over 21 months allowed for seasonal variability of vitamin D levels. We did not notice a specific difference in patients who were treated during winter vs summer. Furthermore, our 77% prevalence of vitamin D insufficiency and 39% prevalence of vitamin D deficiency indicate how widespread low vitamin D levels are in a large Midwestern orthopedic trauma population. In the Pacific Northwest, Bee and colleagues7 studied seasonal differences in patients with surgically treated fractures and found an average difference of 3 ng/mL between winter and summer serum levels. However, the real issue, which should not be overlooked, is that the average 25-hydroxyvitamin D level was under 30 ng/mL in both cohorts (26.4 ng/mL in winter vs 29.8 ng/mL in summer). The emphasis should be that both levels were insufficient and that seasonal variance does not really change prevalence.
With use of the current definitions, it has been estimated that 1 billion people worldwide have vitamin D deficiency or insufficiency, with the elderly and certain ethnic populations at higher risk.8-10Vitamin D deficiency is a common diagnosis among elderly patients with hip fractures. According to various reports, 60% to 90% of patients treated for hip fractures are deficient or insufficient in vitamin D.8,9Hypovitaminosis D has also been noted in medical inpatients with and without risks for this deficiency.2 Surprisingly, low vitamin D levels are not isolated to the elderly. In Massachusetts, Gordon and colleagues11 found a 52% prevalence of vitamin D deficiency in Hispanic and black adolescents. Nesby-O’Dell and colleagues10 found that 42% of 15- to 49-year-old black women in the United States had vitamin D deficiency at the end of winter. Bogunovic and colleagues12 noted 5.5 times higher risk of low vitamin D levels in patients with darker skin tones. Although vitamin D deficiency has been linked to specific races, it frequently occurs in lower-risk populations as well. Sullivan and colleagues4 found a 48% prevalence of vitamin D deficiency in white preadolescent girls in Maine. Tangpricha and colleagues3 reported a 32% prevalence of vitamin D deficiency in otherwise fit healthcare providers sampled at a Boston hospital. Bogunovic and colleagues12 also showed that patients between ages 18 years and 50 years, and men, were more likely to have low vitamin D levels.
Establishing the prevalence of hypovitaminosis D in orthopedic trauma patients is needed in order to raise awareness of the disease and modify screening and treatment protocols. Brinker and O’Connor13 found vitamin D deficiency in 68% of patients with fracture nonunions, which suggests that hypovitaminosis D may partly account for difficulty in achieving fracture union. Bogunovic and colleagues12 found vitamin D insufficiency in 43% of 723 patients who underwent orthopedic surgery. Isolating the 121 patients on the trauma service revealed a 66% prevalence of low vitamin D levels. Our 77% prevalence of low vitamin D levels in 889 patients adds to the evidence that low levels are common in patients with orthopedic trauma. Understanding the importance of vitamin D deficiency can be significant in reducing the risk of complications, including delayed unions and nonunions, associated with treating orthopedic trauma cases.
Although our study indicates an alarming prevalence of insufficient vitamin D levels in our patient population, it does not provide a cause-and-effect link between low serum 25-hydroxyvitamin D levels and risk of fracture or nonunion. However, further investigations may yield clinically relevant data linking hypovitaminosis D with fracture risk. Although we did not include patients with nonunion in this study, new prospective investigations will address nonunions and subgroup analysis of race, fracture type, management type (surgical vs nonsurgical), injury date (to determine seasonal effect), and different treatment regimens.
The primary limitation of this study was its retrospective design. In addition, though we collected vitamin D data from 889 patients with acute fracture, our serum collection protocols were not standardized. Most patients who were admitted during initial orthopedic consultation in the emergency department had serum 25-hydroxyvitamin D levels drawn during their hospital stay, and patients initially treated in an ambulatory setting may not have had serum vitamin D levels drawn for up to 2 weeks after injury (the significance of this delay is unknown). Furthermore, the serum result rate for the overall orthopedic trauma population during the review period was only 49%, which could indicate selection bias. There are multiple explanations for the low rate. As with any new protocol or method, it takes time for the order to become standard practice; in the early stages, individuals can forget to ask for the test. In addition, during the review period, the serum test was also relatively new at our facility, and it was a “send-out” test, which could partly account for the lack of consistency. For example, some specimens were lost, and, in a number of other cases, excluded patients mistakenly had their 1,25-hydroxyvitamin D levels measured and were not comparable to included patients. Nevertheless, our sample of 889 patients with acute fractures remains the largest (by several hundred) reported in the literature.
From a practical standpoint, the present results were useful in updating our treatment protocols. Now we typically treat patients only prophylactically, with 50,000 units of vitamin D2 for 8 weeks and daily vitamin D3 and calcium until fracture healing. Patients are encouraged to continue daily vitamin D and calcium supplementation after fracture healing to maintain bone health. Compliance, however, remains a continued challenge and lack thereof can potentially explain the confusing effect of a supplementation protocol on the serum 25-hydroxyvitamin D level.14 The only patients who are not given prophylactic treatment are those who previously had been denied it (patients with chronic kidney disease or elevated blood calcium levels).
Vitamin D deficiency and insufficiency are prevalent in patients with orthopedic trauma. Studies are needed to further elucidate the relationship between low vitamin D levels and risk of complications. Retrospectively, without compliance monitoring, we have not seen a direct correlation with fracture complications.15 Our goal here was to increase orthopedic surgeons’ awareness of the problem and of the need to consider addressing low serum vitamin D levels. The treatment is low cost and low risk. The ultimate goal—if there is a prospective direct correlation between low serum vitamin D levels and complications—is to develop treatment strategies that can effectively lower the prevalence of low vitamin D levels.
Am J Orthop. 2016;45(7):E522-E526. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. Zaidi SA, Singh G, Owojori O, et al. Vitamin D deficiency in medical inpatients: a retrospective study of implications of untreated versus treated deficiency. Nutr Metab Insights. 2016;9:65-69.
2. Thomas MK, Lloyd-Jones DM, Thadhani RI, et al. Hypovitaminosis D in medical inpatients. N Engl J Med. 1998;338(12):777-783.
3. Tangpricha V, Pearce EN, Chen TC, Holick MF. Vitamin D insufficiency among free-living healthy young adults. Am J Med. 2002;112(8):659-662.
4. Sullivan SS, Rosen CJ, Halteman WA, Chen TC, Holick MF. Adolescent girls in Maine are at risk for vitamin D insufficiency. J Am Diet Assoc. 2005;105(6):971-974.
5. Hollis BW, Wagner CL. Normal serum vitamin D levels. N Engl J Med. 2005;352(5):515-516.
6. Steele B, Serota A, Helfet DL, Peterson M, Lyman S, Lane JM. Vitamin D deficiency: a common occurrence in both high- and low-energy fractures. HSS J. 2008;4(2):143-148.
7. Bee CR, Sheerin DV, Wuest TK, Fitzpatrick DC. Serum vitamin D levels in orthopaedic trauma patients living in the northwestern United States. J Orthop Trauma. 2013;27(5):e103-e106.
8. Bischoff-Ferrari HA, Can U, Staehelin HB, et al. Severe vitamin D deficiency in Swiss hip fracture patients. Bone. 2008;42(3):597-602.
9. Pieper CF, Colon-Emeric C, Caminis J, et al. Distribution and correlates of serum 25-hydroxyvitamin D levels in a sample of patients with hip fracture. Am J Geriatr Pharmacother. 2007;5(4):335-340.
10. Nesby-O’Dell S, Scanlon KS, Cogswell ME, et al. Hypovitaminosis D prevalence and determinants among African American and white women of reproductive age: third National Health and Nutrition Examination Survey, 1988–1994. Am J Clin Nutr. 2002;76(1):187-192.
11. Gordon CM, DePeter KC, Feldman HA, Grace E, Emans SJ. Prevalence of vitamin D deficiency among healthy adolescents. Arch Pediatr Adolesc Med. 2004;158(6):531-537.
12. Bogunovic L, Kim AD, Beamer BS, Nguyen J, Lane JM. Hypovitaminosis D in patients scheduled to undergo orthopaedic surgery: a single-center analysis. J Bone Joint Surg Am. 2010;92(13):2300-2304.
13. Brinker MR, O’Connor DP. Outcomes of tibial nonunion in older adults following treatment using the Ilizarov method. J Orthop Trauma. 2007;21(9):634-642.
14. Robertson DS, Jenkins T, Murtha YM, et al. Effectiveness of vitamin D therapy in orthopaedic trauma patients. J Orthop Trauma. 2015;29(11):e451-e453.
15. Bodendorfer BM, Cook JL, Robertson DS, et al. Do 25-hydroxyvitamin D levels correlate with fracture complications: J Orthop Trauma. 2016;30(9):e312-e317.
The role of vitamin D in general health maintenance is a topic of increasing interest and importance in the medical community. Not only has vitamin D deficiency been linked to a myriad of nonorthopedic maladies, including cancer, diabetes, and cardiovascular disease, but it has demonstrated an adverse effect on musculoskeletal health.1 Authors have found a correlation between vitamin D deficiency and muscle weakness, fragility fractures, and, most recently, fracture nonunion.1 Despite the detrimental effects of vitamin D deficiency on musculoskeletal and general health, evidence exists that vitamin D deficiency is surprisingly prevalent.2 This deficiency is known to be associated with increasing age, but recent studies have also found alarming rates of deficiency in younger populations.3,4
Although there has been some discussion regarding optimal serum levels of 25-hydroxyvitamin D, most experts have defined vitamin D deficiency as a 25-hydroxyvitamin D level of 20 ng/mL or less and insufficiency as 21 to 32 ng/mL.5 Hollis and Wagner5 found increased serum parathyroid hormone and bone resorption and impaired dietary absorption of calcium when 25-hydroxyvitamin D levels were under 32 ng/mL. Given these data, a 25-hydroxyvitamin D level of 21 to 32 ng/mL (52-72 nmol/L) can be considered as indicating a relative insufficiency of vitamin D, and a level of 20 ng/mL or less can be considered as indicating vitamin D deficiency.
Vitamin D plays a vital role in bone metabolism and has been implicated in increased fracture risk and in fracture healing ability. Therefore, documenting the prevalence of vitamin D deficiency in patients with trauma is the first step in raising awareness among orthopedic traumatologists and further developing a screening-and-treatment strategy for vitamin D deficiency in these patients. Steele and colleagues6 retrospectively studied 44 patients with high- and low-energy fractures and found an almost 60% prevalence of vitamin D insufficiency. If vitamin D insufficiency is this prevalent, treatment protocols for patients with fractures may require modifications that include routine screening and treatment for low vitamin D levels.
After noting a regular occurrence of hypovitaminosis D in our patient population (independent of age, sex, or medical comorbidities), we conducted a study to determine the prevalence of vitamin D deficiency in a large orthopedic trauma population.
Patients and Methods
After obtaining Institutional Review Board approval for this study, we retrospectively reviewed the charts of all patients with a fracture treated by 1 of 4 orthopedic traumatologists within a 21-month period (January 1, 2009 to September 30, 2010). Acute fracture and recorded 25-hydroxyvitamin D level were the primary criteria for study inclusion. Given the concern about vitamin D deficiency, it became common protocol to check the serum 25-hydroxyvitamin D levels of patients with acute fractures during the review period. Exclusion criteria were age under 18 years and presence of vitamin D deficiency risk factors, including renal insufficiency (creatinine level, ≥2 mg/dL), malabsorption, gastrectomy, active liver disease, acute myocardial infarction, alcoholism, anorexia nervosa, and steroid dependency.
During the period studied, 1830 patients over age 18 years were treated by 4 fellowship-trained orthopedic traumatologists. Of these patients, 889 (487 female, 402 male) met the inclusion criteria. Mean age was 53.8 years. Demographic data (age, sex, race, independent living status, comorbid medical conditions, medications) were collected from the patients’ medical records. Clinical data collected were mechanism of injury, fracture location and type, injury date, surgery date and surgical procedure performed (when applicable), and serum 25-hydroxyvitamin D levels.
Statistical Methods
Descriptive statistics (mean, median, mode) were calculated. The χ2 test was used when all cell frequencies were more than 5, and the Fisher exact probability test was used when any cell frequency was 5 or less. Prevalence of vitamin D deficiency and insufficiency was calculated in multiple patient populations. Patients were analyzed according to age and sex subgroups.
Definitions
Vitamin D deficiency was defined as a serum 25-hydroxyvitamin D level of 20 ng/mL or less and insufficiency as 21 to 32 ng/mL.2 As the serum test was performed independent of the investigators and with use of standard medical laboratory protocols and techniques, there should be no bias in the results. We had intended to have all patients undergo serum testing during the review period because that was our usual protocol. However, test results were available for only 889 (49%) of the 1830 patients with orthopedic trauma during the review period. Although a false-positive is theoretically possible, this series of orthopedic trauma patients is the largest in the literature and therefore should be more accurate than the previously reported small series.
Results
There were no significant (P < .05) age or sex differences in prevalence of vitamin D deficiency or insufficiency in our patient population. Overall prevalence of deficiency/insufficiency was 77.39%, and prevalence of deficiency alone was 39.03% (Table 1).
Women in the 18- to 25-year age group had a lower prevalence of deficiency (25%; P = .41) and insufficiency (41.7%; P = .16) than women in the other age groups (Table 3).
Discussion
We conducted this study to determine the prevalence of vitamin D deficiency in a large population of patients with orthopedic trauma. Results showed that vitamin D deficiency and insufficiency were prevalent in this population, which to our knowledge is the largest studied for vitamin D deficiency. In a 6-month study of 44 fractures, Steele and colleagues6 found an overall 60% rate of deficiency/insufficiency. Although their investigation is important—it was the first of its kind to evaluate patients with various fracture types, including those with high-energy causes—its numbers were small, and the period evaluated (June 1, 2006 to February 1, 2007) was short (8 months). Use of that time frame may have led to an underestimate of the prevalence of vitamin D deficiency, as vitamin D levels are higher in late summer because of increased sun exposure. Our study of 889 patients over 21 months allowed for seasonal variability of vitamin D levels. We did not notice a specific difference in patients who were treated during winter vs summer. Furthermore, our 77% prevalence of vitamin D insufficiency and 39% prevalence of vitamin D deficiency indicate how widespread low vitamin D levels are in a large Midwestern orthopedic trauma population. In the Pacific Northwest, Bee and colleagues7 studied seasonal differences in patients with surgically treated fractures and found an average difference of 3 ng/mL between winter and summer serum levels. However, the real issue, which should not be overlooked, is that the average 25-hydroxyvitamin D level was under 30 ng/mL in both cohorts (26.4 ng/mL in winter vs 29.8 ng/mL in summer). The emphasis should be that both levels were insufficient and that seasonal variance does not really change prevalence.
With use of the current definitions, it has been estimated that 1 billion people worldwide have vitamin D deficiency or insufficiency, with the elderly and certain ethnic populations at higher risk.8-10Vitamin D deficiency is a common diagnosis among elderly patients with hip fractures. According to various reports, 60% to 90% of patients treated for hip fractures are deficient or insufficient in vitamin D.8,9Hypovitaminosis D has also been noted in medical inpatients with and without risks for this deficiency.2 Surprisingly, low vitamin D levels are not isolated to the elderly. In Massachusetts, Gordon and colleagues11 found a 52% prevalence of vitamin D deficiency in Hispanic and black adolescents. Nesby-O’Dell and colleagues10 found that 42% of 15- to 49-year-old black women in the United States had vitamin D deficiency at the end of winter. Bogunovic and colleagues12 noted 5.5 times higher risk of low vitamin D levels in patients with darker skin tones. Although vitamin D deficiency has been linked to specific races, it frequently occurs in lower-risk populations as well. Sullivan and colleagues4 found a 48% prevalence of vitamin D deficiency in white preadolescent girls in Maine. Tangpricha and colleagues3 reported a 32% prevalence of vitamin D deficiency in otherwise fit healthcare providers sampled at a Boston hospital. Bogunovic and colleagues12 also showed that patients between ages 18 years and 50 years, and men, were more likely to have low vitamin D levels.
Establishing the prevalence of hypovitaminosis D in orthopedic trauma patients is needed in order to raise awareness of the disease and modify screening and treatment protocols. Brinker and O’Connor13 found vitamin D deficiency in 68% of patients with fracture nonunions, which suggests that hypovitaminosis D may partly account for difficulty in achieving fracture union. Bogunovic and colleagues12 found vitamin D insufficiency in 43% of 723 patients who underwent orthopedic surgery. Isolating the 121 patients on the trauma service revealed a 66% prevalence of low vitamin D levels. Our 77% prevalence of low vitamin D levels in 889 patients adds to the evidence that low levels are common in patients with orthopedic trauma. Understanding the importance of vitamin D deficiency can be significant in reducing the risk of complications, including delayed unions and nonunions, associated with treating orthopedic trauma cases.
Although our study indicates an alarming prevalence of insufficient vitamin D levels in our patient population, it does not provide a cause-and-effect link between low serum 25-hydroxyvitamin D levels and risk of fracture or nonunion. However, further investigations may yield clinically relevant data linking hypovitaminosis D with fracture risk. Although we did not include patients with nonunion in this study, new prospective investigations will address nonunions and subgroup analysis of race, fracture type, management type (surgical vs nonsurgical), injury date (to determine seasonal effect), and different treatment regimens.
The primary limitation of this study was its retrospective design. In addition, though we collected vitamin D data from 889 patients with acute fracture, our serum collection protocols were not standardized. Most patients who were admitted during initial orthopedic consultation in the emergency department had serum 25-hydroxyvitamin D levels drawn during their hospital stay, and patients initially treated in an ambulatory setting may not have had serum vitamin D levels drawn for up to 2 weeks after injury (the significance of this delay is unknown). Furthermore, the serum result rate for the overall orthopedic trauma population during the review period was only 49%, which could indicate selection bias. There are multiple explanations for the low rate. As with any new protocol or method, it takes time for the order to become standard practice; in the early stages, individuals can forget to ask for the test. In addition, during the review period, the serum test was also relatively new at our facility, and it was a “send-out” test, which could partly account for the lack of consistency. For example, some specimens were lost, and, in a number of other cases, excluded patients mistakenly had their 1,25-hydroxyvitamin D levels measured and were not comparable to included patients. Nevertheless, our sample of 889 patients with acute fractures remains the largest (by several hundred) reported in the literature.
From a practical standpoint, the present results were useful in updating our treatment protocols. Now we typically treat patients only prophylactically, with 50,000 units of vitamin D2 for 8 weeks and daily vitamin D3 and calcium until fracture healing. Patients are encouraged to continue daily vitamin D and calcium supplementation after fracture healing to maintain bone health. Compliance, however, remains a continued challenge and lack thereof can potentially explain the confusing effect of a supplementation protocol on the serum 25-hydroxyvitamin D level.14 The only patients who are not given prophylactic treatment are those who previously had been denied it (patients with chronic kidney disease or elevated blood calcium levels).
Vitamin D deficiency and insufficiency are prevalent in patients with orthopedic trauma. Studies are needed to further elucidate the relationship between low vitamin D levels and risk of complications. Retrospectively, without compliance monitoring, we have not seen a direct correlation with fracture complications.15 Our goal here was to increase orthopedic surgeons’ awareness of the problem and of the need to consider addressing low serum vitamin D levels. The treatment is low cost and low risk. The ultimate goal—if there is a prospective direct correlation between low serum vitamin D levels and complications—is to develop treatment strategies that can effectively lower the prevalence of low vitamin D levels.
Am J Orthop. 2016;45(7):E522-E526. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
The role of vitamin D in general health maintenance is a topic of increasing interest and importance in the medical community. Not only has vitamin D deficiency been linked to a myriad of nonorthopedic maladies, including cancer, diabetes, and cardiovascular disease, but it has demonstrated an adverse effect on musculoskeletal health.1 Authors have found a correlation between vitamin D deficiency and muscle weakness, fragility fractures, and, most recently, fracture nonunion.1 Despite the detrimental effects of vitamin D deficiency on musculoskeletal and general health, evidence exists that vitamin D deficiency is surprisingly prevalent.2 This deficiency is known to be associated with increasing age, but recent studies have also found alarming rates of deficiency in younger populations.3,4
Although there has been some discussion regarding optimal serum levels of 25-hydroxyvitamin D, most experts have defined vitamin D deficiency as a 25-hydroxyvitamin D level of 20 ng/mL or less and insufficiency as 21 to 32 ng/mL.5 Hollis and Wagner5 found increased serum parathyroid hormone and bone resorption and impaired dietary absorption of calcium when 25-hydroxyvitamin D levels were under 32 ng/mL. Given these data, a 25-hydroxyvitamin D level of 21 to 32 ng/mL (52-72 nmol/L) can be considered as indicating a relative insufficiency of vitamin D, and a level of 20 ng/mL or less can be considered as indicating vitamin D deficiency.
Vitamin D plays a vital role in bone metabolism and has been implicated in increased fracture risk and in fracture healing ability. Therefore, documenting the prevalence of vitamin D deficiency in patients with trauma is the first step in raising awareness among orthopedic traumatologists and further developing a screening-and-treatment strategy for vitamin D deficiency in these patients. Steele and colleagues6 retrospectively studied 44 patients with high- and low-energy fractures and found an almost 60% prevalence of vitamin D insufficiency. If vitamin D insufficiency is this prevalent, treatment protocols for patients with fractures may require modifications that include routine screening and treatment for low vitamin D levels.
After noting a regular occurrence of hypovitaminosis D in our patient population (independent of age, sex, or medical comorbidities), we conducted a study to determine the prevalence of vitamin D deficiency in a large orthopedic trauma population.
Patients and Methods
After obtaining Institutional Review Board approval for this study, we retrospectively reviewed the charts of all patients with a fracture treated by 1 of 4 orthopedic traumatologists within a 21-month period (January 1, 2009 to September 30, 2010). Acute fracture and recorded 25-hydroxyvitamin D level were the primary criteria for study inclusion. Given the concern about vitamin D deficiency, it became common protocol to check the serum 25-hydroxyvitamin D levels of patients with acute fractures during the review period. Exclusion criteria were age under 18 years and presence of vitamin D deficiency risk factors, including renal insufficiency (creatinine level, ≥2 mg/dL), malabsorption, gastrectomy, active liver disease, acute myocardial infarction, alcoholism, anorexia nervosa, and steroid dependency.
During the period studied, 1830 patients over age 18 years were treated by 4 fellowship-trained orthopedic traumatologists. Of these patients, 889 (487 female, 402 male) met the inclusion criteria. Mean age was 53.8 years. Demographic data (age, sex, race, independent living status, comorbid medical conditions, medications) were collected from the patients’ medical records. Clinical data collected were mechanism of injury, fracture location and type, injury date, surgery date and surgical procedure performed (when applicable), and serum 25-hydroxyvitamin D levels.
Statistical Methods
Descriptive statistics (mean, median, mode) were calculated. The χ2 test was used when all cell frequencies were more than 5, and the Fisher exact probability test was used when any cell frequency was 5 or less. Prevalence of vitamin D deficiency and insufficiency was calculated in multiple patient populations. Patients were analyzed according to age and sex subgroups.
Definitions
Vitamin D deficiency was defined as a serum 25-hydroxyvitamin D level of 20 ng/mL or less and insufficiency as 21 to 32 ng/mL.2 As the serum test was performed independent of the investigators and with use of standard medical laboratory protocols and techniques, there should be no bias in the results. We had intended to have all patients undergo serum testing during the review period because that was our usual protocol. However, test results were available for only 889 (49%) of the 1830 patients with orthopedic trauma during the review period. Although a false-positive is theoretically possible, this series of orthopedic trauma patients is the largest in the literature and therefore should be more accurate than the previously reported small series.
Results
There were no significant (P < .05) age or sex differences in prevalence of vitamin D deficiency or insufficiency in our patient population. Overall prevalence of deficiency/insufficiency was 77.39%, and prevalence of deficiency alone was 39.03% (Table 1).
Women in the 18- to 25-year age group had a lower prevalence of deficiency (25%; P = .41) and insufficiency (41.7%; P = .16) than women in the other age groups (Table 3).
Discussion
We conducted this study to determine the prevalence of vitamin D deficiency in a large population of patients with orthopedic trauma. Results showed that vitamin D deficiency and insufficiency were prevalent in this population, which to our knowledge is the largest studied for vitamin D deficiency. In a 6-month study of 44 fractures, Steele and colleagues6 found an overall 60% rate of deficiency/insufficiency. Although their investigation is important—it was the first of its kind to evaluate patients with various fracture types, including those with high-energy causes—its numbers were small, and the period evaluated (June 1, 2006 to February 1, 2007) was short (8 months). Use of that time frame may have led to an underestimate of the prevalence of vitamin D deficiency, as vitamin D levels are higher in late summer because of increased sun exposure. Our study of 889 patients over 21 months allowed for seasonal variability of vitamin D levels. We did not notice a specific difference in patients who were treated during winter vs summer. Furthermore, our 77% prevalence of vitamin D insufficiency and 39% prevalence of vitamin D deficiency indicate how widespread low vitamin D levels are in a large Midwestern orthopedic trauma population. In the Pacific Northwest, Bee and colleagues7 studied seasonal differences in patients with surgically treated fractures and found an average difference of 3 ng/mL between winter and summer serum levels. However, the real issue, which should not be overlooked, is that the average 25-hydroxyvitamin D level was under 30 ng/mL in both cohorts (26.4 ng/mL in winter vs 29.8 ng/mL in summer). The emphasis should be that both levels were insufficient and that seasonal variance does not really change prevalence.
With use of the current definitions, it has been estimated that 1 billion people worldwide have vitamin D deficiency or insufficiency, with the elderly and certain ethnic populations at higher risk.8-10Vitamin D deficiency is a common diagnosis among elderly patients with hip fractures. According to various reports, 60% to 90% of patients treated for hip fractures are deficient or insufficient in vitamin D.8,9Hypovitaminosis D has also been noted in medical inpatients with and without risks for this deficiency.2 Surprisingly, low vitamin D levels are not isolated to the elderly. In Massachusetts, Gordon and colleagues11 found a 52% prevalence of vitamin D deficiency in Hispanic and black adolescents. Nesby-O’Dell and colleagues10 found that 42% of 15- to 49-year-old black women in the United States had vitamin D deficiency at the end of winter. Bogunovic and colleagues12 noted 5.5 times higher risk of low vitamin D levels in patients with darker skin tones. Although vitamin D deficiency has been linked to specific races, it frequently occurs in lower-risk populations as well. Sullivan and colleagues4 found a 48% prevalence of vitamin D deficiency in white preadolescent girls in Maine. Tangpricha and colleagues3 reported a 32% prevalence of vitamin D deficiency in otherwise fit healthcare providers sampled at a Boston hospital. Bogunovic and colleagues12 also showed that patients between ages 18 years and 50 years, and men, were more likely to have low vitamin D levels.
Establishing the prevalence of hypovitaminosis D in orthopedic trauma patients is needed in order to raise awareness of the disease and modify screening and treatment protocols. Brinker and O’Connor13 found vitamin D deficiency in 68% of patients with fracture nonunions, which suggests that hypovitaminosis D may partly account for difficulty in achieving fracture union. Bogunovic and colleagues12 found vitamin D insufficiency in 43% of 723 patients who underwent orthopedic surgery. Isolating the 121 patients on the trauma service revealed a 66% prevalence of low vitamin D levels. Our 77% prevalence of low vitamin D levels in 889 patients adds to the evidence that low levels are common in patients with orthopedic trauma. Understanding the importance of vitamin D deficiency can be significant in reducing the risk of complications, including delayed unions and nonunions, associated with treating orthopedic trauma cases.
Although our study indicates an alarming prevalence of insufficient vitamin D levels in our patient population, it does not provide a cause-and-effect link between low serum 25-hydroxyvitamin D levels and risk of fracture or nonunion. However, further investigations may yield clinically relevant data linking hypovitaminosis D with fracture risk. Although we did not include patients with nonunion in this study, new prospective investigations will address nonunions and subgroup analysis of race, fracture type, management type (surgical vs nonsurgical), injury date (to determine seasonal effect), and different treatment regimens.
The primary limitation of this study was its retrospective design. In addition, though we collected vitamin D data from 889 patients with acute fracture, our serum collection protocols were not standardized. Most patients who were admitted during initial orthopedic consultation in the emergency department had serum 25-hydroxyvitamin D levels drawn during their hospital stay, and patients initially treated in an ambulatory setting may not have had serum vitamin D levels drawn for up to 2 weeks after injury (the significance of this delay is unknown). Furthermore, the serum result rate for the overall orthopedic trauma population during the review period was only 49%, which could indicate selection bias. There are multiple explanations for the low rate. As with any new protocol or method, it takes time for the order to become standard practice; in the early stages, individuals can forget to ask for the test. In addition, during the review period, the serum test was also relatively new at our facility, and it was a “send-out” test, which could partly account for the lack of consistency. For example, some specimens were lost, and, in a number of other cases, excluded patients mistakenly had their 1,25-hydroxyvitamin D levels measured and were not comparable to included patients. Nevertheless, our sample of 889 patients with acute fractures remains the largest (by several hundred) reported in the literature.
From a practical standpoint, the present results were useful in updating our treatment protocols. Now we typically treat patients only prophylactically, with 50,000 units of vitamin D2 for 8 weeks and daily vitamin D3 and calcium until fracture healing. Patients are encouraged to continue daily vitamin D and calcium supplementation after fracture healing to maintain bone health. Compliance, however, remains a continued challenge and lack thereof can potentially explain the confusing effect of a supplementation protocol on the serum 25-hydroxyvitamin D level.14 The only patients who are not given prophylactic treatment are those who previously had been denied it (patients with chronic kidney disease or elevated blood calcium levels).
Vitamin D deficiency and insufficiency are prevalent in patients with orthopedic trauma. Studies are needed to further elucidate the relationship between low vitamin D levels and risk of complications. Retrospectively, without compliance monitoring, we have not seen a direct correlation with fracture complications.15 Our goal here was to increase orthopedic surgeons’ awareness of the problem and of the need to consider addressing low serum vitamin D levels. The treatment is low cost and low risk. The ultimate goal—if there is a prospective direct correlation between low serum vitamin D levels and complications—is to develop treatment strategies that can effectively lower the prevalence of low vitamin D levels.
Am J Orthop. 2016;45(7):E522-E526. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. Zaidi SA, Singh G, Owojori O, et al. Vitamin D deficiency in medical inpatients: a retrospective study of implications of untreated versus treated deficiency. Nutr Metab Insights. 2016;9:65-69.
2. Thomas MK, Lloyd-Jones DM, Thadhani RI, et al. Hypovitaminosis D in medical inpatients. N Engl J Med. 1998;338(12):777-783.
3. Tangpricha V, Pearce EN, Chen TC, Holick MF. Vitamin D insufficiency among free-living healthy young adults. Am J Med. 2002;112(8):659-662.
4. Sullivan SS, Rosen CJ, Halteman WA, Chen TC, Holick MF. Adolescent girls in Maine are at risk for vitamin D insufficiency. J Am Diet Assoc. 2005;105(6):971-974.
5. Hollis BW, Wagner CL. Normal serum vitamin D levels. N Engl J Med. 2005;352(5):515-516.
6. Steele B, Serota A, Helfet DL, Peterson M, Lyman S, Lane JM. Vitamin D deficiency: a common occurrence in both high- and low-energy fractures. HSS J. 2008;4(2):143-148.
7. Bee CR, Sheerin DV, Wuest TK, Fitzpatrick DC. Serum vitamin D levels in orthopaedic trauma patients living in the northwestern United States. J Orthop Trauma. 2013;27(5):e103-e106.
8. Bischoff-Ferrari HA, Can U, Staehelin HB, et al. Severe vitamin D deficiency in Swiss hip fracture patients. Bone. 2008;42(3):597-602.
9. Pieper CF, Colon-Emeric C, Caminis J, et al. Distribution and correlates of serum 25-hydroxyvitamin D levels in a sample of patients with hip fracture. Am J Geriatr Pharmacother. 2007;5(4):335-340.
10. Nesby-O’Dell S, Scanlon KS, Cogswell ME, et al. Hypovitaminosis D prevalence and determinants among African American and white women of reproductive age: third National Health and Nutrition Examination Survey, 1988–1994. Am J Clin Nutr. 2002;76(1):187-192.
11. Gordon CM, DePeter KC, Feldman HA, Grace E, Emans SJ. Prevalence of vitamin D deficiency among healthy adolescents. Arch Pediatr Adolesc Med. 2004;158(6):531-537.
12. Bogunovic L, Kim AD, Beamer BS, Nguyen J, Lane JM. Hypovitaminosis D in patients scheduled to undergo orthopaedic surgery: a single-center analysis. J Bone Joint Surg Am. 2010;92(13):2300-2304.
13. Brinker MR, O’Connor DP. Outcomes of tibial nonunion in older adults following treatment using the Ilizarov method. J Orthop Trauma. 2007;21(9):634-642.
14. Robertson DS, Jenkins T, Murtha YM, et al. Effectiveness of vitamin D therapy in orthopaedic trauma patients. J Orthop Trauma. 2015;29(11):e451-e453.
15. Bodendorfer BM, Cook JL, Robertson DS, et al. Do 25-hydroxyvitamin D levels correlate with fracture complications: J Orthop Trauma. 2016;30(9):e312-e317.
1. Zaidi SA, Singh G, Owojori O, et al. Vitamin D deficiency in medical inpatients: a retrospective study of implications of untreated versus treated deficiency. Nutr Metab Insights. 2016;9:65-69.
2. Thomas MK, Lloyd-Jones DM, Thadhani RI, et al. Hypovitaminosis D in medical inpatients. N Engl J Med. 1998;338(12):777-783.
3. Tangpricha V, Pearce EN, Chen TC, Holick MF. Vitamin D insufficiency among free-living healthy young adults. Am J Med. 2002;112(8):659-662.
4. Sullivan SS, Rosen CJ, Halteman WA, Chen TC, Holick MF. Adolescent girls in Maine are at risk for vitamin D insufficiency. J Am Diet Assoc. 2005;105(6):971-974.
5. Hollis BW, Wagner CL. Normal serum vitamin D levels. N Engl J Med. 2005;352(5):515-516.
6. Steele B, Serota A, Helfet DL, Peterson M, Lyman S, Lane JM. Vitamin D deficiency: a common occurrence in both high- and low-energy fractures. HSS J. 2008;4(2):143-148.
7. Bee CR, Sheerin DV, Wuest TK, Fitzpatrick DC. Serum vitamin D levels in orthopaedic trauma patients living in the northwestern United States. J Orthop Trauma. 2013;27(5):e103-e106.
8. Bischoff-Ferrari HA, Can U, Staehelin HB, et al. Severe vitamin D deficiency in Swiss hip fracture patients. Bone. 2008;42(3):597-602.
9. Pieper CF, Colon-Emeric C, Caminis J, et al. Distribution and correlates of serum 25-hydroxyvitamin D levels in a sample of patients with hip fracture. Am J Geriatr Pharmacother. 2007;5(4):335-340.
10. Nesby-O’Dell S, Scanlon KS, Cogswell ME, et al. Hypovitaminosis D prevalence and determinants among African American and white women of reproductive age: third National Health and Nutrition Examination Survey, 1988–1994. Am J Clin Nutr. 2002;76(1):187-192.
11. Gordon CM, DePeter KC, Feldman HA, Grace E, Emans SJ. Prevalence of vitamin D deficiency among healthy adolescents. Arch Pediatr Adolesc Med. 2004;158(6):531-537.
12. Bogunovic L, Kim AD, Beamer BS, Nguyen J, Lane JM. Hypovitaminosis D in patients scheduled to undergo orthopaedic surgery: a single-center analysis. J Bone Joint Surg Am. 2010;92(13):2300-2304.
13. Brinker MR, O’Connor DP. Outcomes of tibial nonunion in older adults following treatment using the Ilizarov method. J Orthop Trauma. 2007;21(9):634-642.
14. Robertson DS, Jenkins T, Murtha YM, et al. Effectiveness of vitamin D therapy in orthopaedic trauma patients. J Orthop Trauma. 2015;29(11):e451-e453.
15. Bodendorfer BM, Cook JL, Robertson DS, et al. Do 25-hydroxyvitamin D levels correlate with fracture complications: J Orthop Trauma. 2016;30(9):e312-e317.
Fat Embolism Syndrome With Cerebral Fat Embolism Associated With Long-Bone Fracture
Fat embolism syndrome (FES) occurs in long-bone fractures and classically presents with the triad of hypoxia, petechia, and altered mental status, or the criteria of Gurd and Wilson.1 The Lindeque criteria (femur fracture, pH <7.3, increased work of breathing) are also used.1,2 FES is a potentially fatal complication, with mortality rates ranging from 10% to 36%.1,3 FES typically occurs within 24 to 72 hours after initial insult, with one study finding an average of 48.5 hours after injury and an incidence of 0.15% to 2.4%.4 The overall FES rate is <1% in retrospective reviews and 11% to 29% in prospective studies.5 FES may present without one or all of the Gurd and Wilson criteria,6 and cerebral fat embolism (CFE) can be even more difficult to diagnose. Patients with CFE typically present with a wide array of postoperative neurologic deficits, commonly in the 24- to 72-hour window in which FES typically occurs. Correct diagnosis and management of CFE require a high index of suspicion and knowledge of the diagnostic work-up. In the postoperative setting, it can be difficult to distinguish CFE-related neurologic deficits from the normal sequelae of anesthesia, pain medications, and other factors.
In this article, we report the case of a 42-year-old woman who developed CFE after reamed intramedullary nail fixation of femoral and tibial shaft fractures. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 42-year-old woman with no past medical history was injured when a horse reared and fell on her. Initial emergent computed tomography (CT) was negative for intracranial hemorrhage, and injury radiographs were obtained (Figures 1A, 1B).
About 9 hours after surgery and 36 hours after injury, the patient was unresponsive. Vital signs, including oxygen saturation, were within normal limits, but she was unable to verbalize. Physical examination revealed symmetric facial musculature but also generalized weakness and diffuse hypertonicity and hyperreflexia. Initial laboratory results, including complete blood cell count, electrolyte panel, and troponin levels, were unremarkable. Naloxone was administered to rule out opioid overdose. An immediate code stroke and neurology consultation was requested. An emergent CT scan of the brain was negative; an urgent magnetic resonance imaging (MRI) scan showed multiple punctate T2/FLAIR (fluid attenuated inversion recovery) hyperintensities with restricted diffusion, predominantly in a parasagittal white matter distribution (Figure 2).
The patient slowly and steadily improved. She was verbal by postoperative day 3 (POD-3), upper motor neuron signs resolved by POD-4, encephalopathy resolved by POD-7, and she was discharged to a rehabilitation center. Unresolved post-stroke symptoms included mild visual field deficits in the right eye (20/25 vision, central scotoma) and amnesia regarding the events immediately surrounding the surgery. There were no other neurologic or cognitive deficits. The patient was non-weight-bearing on the operative extremity and ambulating with assistance, and she started range-of-motion exercises. After 1 week, she was discharged home with crutches.
The patient followed up with neurology and ophthalmology for routine post-stroke care. At 2- and 6-month neurology follow-ups, she was still amnestic regarding her acute stroke event but did not exhibit any confusion, memory problems, speech deficits, facial droop, headaches, or weakness. According to neurology, the encephalopathy was completely resolved, and the patient was completely recovered from the event. Levetiracetam and aspirin were discontinued at 2 months. At the 2-month ophthalmology follow-up, the patient had 20/20 vision in both eyes and nearly complete resolution of the central scotoma. Ophthalmology confirmed symptom relief and recommended return to routine eye care and 1-year follow-up.
The patient began weight-bearing as tolerated on POD-14 and had no hardware or other complications. At 6-month orthopedics follow-up, range of motion of the affected knee was 0° to 120°, and rotation, length, and varus/valgus and anteroposterior knee laxity were all symmetric to the contralateral extremity. The patient walked with a cane for balance and had a mild limp. The affected thigh still had mild atrophy, but strength was 5/5 throughout. The patient denied pain or hardware sensitivity in the affected extremity and was very pleased with the result.
Discussion
Postoperative Acute Mental Status Change
There are many causes of postoperative mental status change after intramedullary nailing. Change may be cardiogenic, infectious, pharmacologic, or neurologic in origin. Age should be considered in the work-up of postoperative mental status change, as common complications differ between older and younger patients, with geriatric patients at particularly high risk for delirium.
Next to be evaluated are vital signs—particularly hypoxia, as isolated tachycardia may simply be a manifestation of pain. The cardiac system is then assessed with EKG and cardiac-specific laboratory tests, including a troponin level test if there is suspicion of myocardial infarction. PE and FES are complications with a higher prevalence in intramedullary nailing, and pulmonary involvement can be ruled out with the CT with PE protocol. Skin examination is important as well, as FES presents with petechial rash in 60% of patients8 (rash was absent in our patient’s case). Narcotic overdose is easily ruled out with administration of naloxone. Infection and sepsis can cause mental changes, though more commonly in the elderly and seldom so soon after surgery. Evaluation for infection and sepsis involves urinalysis and culturing of blood, urine, and other bodily fluids. If there is concern about surgical site infection, the postoperative dressing should be immediately removed and the wound examined. Last, neurologic and embolic phenomena can be initially investigated with CT to rule out hemorrhagic stroke. If CT of the brain is negative, MRI should be performed. MRI is the gold standard for diagnosing ischemic stroke and CFE caused by FES.9
Prevalence of Fat Embolism Syndrome
Development of intramedullary fat release in patients with long-bone injuries is common. A prospective study found circulating fat globules in 95% of 43 patients with femur fractures.10 In another study, transesophageal EKG showed cardiac embolism in 62% of patients who had undergone intramedullary nail fixation.11 Despite this high rate, only 0.9% to 2.2% of patients developed symptomatic FES. Risk factors for FES include younger age, multiple fractures, closed fractures, and nonoperative or delayed management of long-bone fractures.2 As already mentioned, average time to FES presentation after long-bone fracture is about 48 hours. One study found that FES typically occurs within 24 to 72 hours after initial insult (average, 48.5 hours) and that the incidence of FES is 0.15% in tibia fractures, 0.78% in femur fractures, and 2.4% in multiple long-bone fractures.4 The timeline is consistent with the present case—our patient developed symptoms about 36 hours after injury. In addition, other studies have found a higher mortality rate (5%-15%) for patients with bilateral femur fractures than for patients with only one fracture.7,12,13 Patients with a floating knee injury (ipsilateral tibia and femur fractures) are at higher risk for FES and have higher overall morbidity and mortality rates in comparison with patients with an isolated femur or tibia fracture, though the increased risk has not been quantified.
Review of Case Literature: FES With CFE
Few cases of FES with symptomatic CFE in the setting of long-bone fracture or long-bone surgery have been reported in the literature. There is wide variation in the development of FES with respect to preoperative or postoperative status and mechanism of injury. Duran and colleagues14 described a 20-year-old man with ipsilateral tibia and femur fractures caused by gunshots. Twenty-four hours after presentation, he developed tonic-clonic seizures and the classic triad of rash, hypoxia, and altered mental status. MRI confirmed CFE secondary to FES. The patient was optimized neurologically before definitive fixation and was discharged with minimal neurologic deficits on POD-27. Chang and colleagues15 and Yeo and colleagues16 described CFE in patients who underwent bilateral total knee arthroplasty. Symptoms developed 9 hours and 2 days after surgery, respectively. Both patients had fat emboli in the lungs and brain, underwent intensive care treatment, and recovered from the initial insult. After discharge at 44 days and 2 weeks, respectively, they fully recovered.
Other patients with CFE have had less favorable outcomes. Chen and colleagues6 reported the case of a 31-year-old man who sustained closed femur and tibia fractures in an automobile collision and experienced an acute decline in neurologic status 1 hour after arrival in the emergency department. The patient was intubated, CFE was diagnosed on the basis of MRI findings, and the orthopedic injuries were treated with external fixation. After 2 weeks, the patient was discharged with persistent neurologic deficits and the need for long-term tube feeding. Walshe and colleagues17 reported the case of a 19-year-old woman who sustained multiple long-bone injuries and traumatic brain injury and showed fat emboli on MRI. The patient experienced brain herniation while in the intensive care unit and later was declared brain-dead. According to the literature, it is important to maintain high suspicion for FES and possible CFE in the setting of high-energy fracture but also to be aware that FES may develop even with nondisplaced fracture or with reaming of the intramedullary canal in elective total joint arthroplasty.18
Pathophysiology of Fat Embolism Syndrome
The pathophysiology of FES and specifically of CFE is not widely understood. Two main theories on the development of FES have been advanced.
The mechanical theory suggests that exposing intramedullary long-bone contents allows fat to mobilize into the bloodstream.19 This occurs in the setting of long-bone fracture and in canal preparation during joint replacement surgery. More fat extravasates into the venous system after femur fracture than after tibia fracture, which accounts for the higher risk for FES in femoral shaft fractures and the even higher risk in concomitant femur and tibia fractures.4 In addition to there being a risk of fat embolism from the fracture itself, placing the patient in traction or reaming the intramedullary canal may exacerbate this effect by increased extravasation of fat from the medullary canal. With extravasation of fatty bone marrow into the venous system, fat emboli are free to travel back to the lungs, where they can cause infarcts within the lung parenchyma.
In the mechanical theory, presence of PFO may allow fat globules to pass into the systemic circulation and cause end-organ emboli. In the event of cerebral emboli, neurologic symptoms may vary widely and may include diffuse encephalopathy and global deficits.20 Dog studies have found a possible mechanism for CFE in the absence of PFO. One such study, which used femoral pressurization to replicate cemented femoral arthroplasty, found that many fat globules had traversed the lungs after release into bone marrow,21 supporting the theory that fat droplets can traverse the pulmonary system without sequestration in the lung parenchyma. Riding and colleagues22 reported finding pulmonary arteriovenous shunts, which are thought to allow CFE to occur in the absence of PFO. More studies are needed to determine the prevalence of shunts and their overall contribution to CFE development in patients with long-bone fracture.
The biochemical theory holds that bodily trauma induces the release of free fatty acids (FFAs) from the capillaries into the bloodstream.23 This stress response is mediated by catecholamines, which activate the adenyl cyclase pathway, which activates lipase, which hydrolyzes stored triglycerides to FFAs and glycerol. The concentration of circulating FFA was increased in 9 of 10 patients in one study.23 Increased FFAs in the bloodstream can accelerate local and end-organ clotting, leading to thrombocytopenia and endothelial injury. In addition, patients with hypercoagulable diseases are at higher risk for postoperative thromboembolism.24 However, with a negative hypercoagulable work-up and with negative chest helical CT and EKG, which did not demonstrate PFO, the explanation for CFE in our patient may more likely reside with the arteriovenous shunt theory proposed by Riding and colleagues.22
Diagnosis and Treatment
Proper care of orthopedic patients who potentially have FES/CFE involves prompt diagnosis, immediate symptomatic care, and early coordination with neurology and medical services to rule out other causes of symptoms. Obtaining advanced imaging to rule out other potential causes and to confirm the diagnosis is crucial. The patient in this case report did not exhibit any focal neurologic deficits, but emergent CT of the brain was indicated to rule out a hemorrhagic event. If a stroke secondary to FES is clinically suspected, MRI should be obtained as soon as possible. Multiple studies have found that the “starfield” pattern, which is best seen as multiple punctate hyperintensities on T2 imaging, is the typical radiographic manifestation of CFE.9 This applies to patients who are in the 24- to 72-hour window after long-bone fracture or fixation and who fit Gurd and Wilson1 criteria or Lindeque1,2criteria, or who exhibit a change in mental status but have a negative CT scan of the brain, as was the case with our patient. Once the diagnosis is made, treatment involves addressing the symptoms (Figure 4).
Fat Embolism Syndrome in Reamed and Unreamed Nailing
Over the past several decades, the number of long bones fixed with intramedullary nails has increased significantly.26 There is debate regarding whether use of reamed intramedullary nails increases the risk of fat emboli relative to use of unreamed nails, but multiple studies have found no significant difference.26,27 Pulmonary shunting occurs in both reamed and unreamed nailing; neither technique has an advantage in terms of cardiopulmonary complications. In multiple studies, reamed nails have the advantage of improved healing rates.27 A sheep study compared reamed and unreamed femoral nailing.28 After nailing, sheep lungs were examined histologically for the presence of bone marrow fat embolism. The embolism rate was higher with unreamed nailing (10.25%) than with reamed nailing (6.66%). One large study of the adverse effects of reamed and unreamed nailing in 1226 patients with tibial shaft fracture found that those with open fractures had higher rates of a negative event (nonunion, infection, fasciotomy, hardware failure, need for dynamization) after reamed nailing.29 Patients with closed fractures had fewer events after reamed nailing. The authors concluded there is a potential benefit in outcome with reamed intramedullary nailing in patients with closed tibial shaft fractures, but they did not comment on development of FES. In a study of the effect of subject position on intramedullary pressure and fat embolism release, dogs were positioned either supine or lateral for tibial and femoral reaming.30 The authors measured various physiologic parameters, including cardiac output, pulmonary arterial wedge pressure, arterial and venous blood gas, and blood cell counts. There were no statistically significant differences in values between the 2 groups in any variable, indicating that position does not affect FES development in the orthopedic trauma setting.
Conclusion
FES and CFE are potential devastating sequelae of both long-bone fracture and long-bone instrumentation. It is important to recognize these entities in the acute setting and to consider them in the differential diagnosis of a trauma or postoperative patient who experiences sudden onset of altered mental status with or without dyspnea or a petechial rash. If CFE is suspected, early advanced imaging (including urgent MRI) should be obtained with rapid involvement of a multidisciplinary team that can optimize the chance for successful recovery of both neurologic and physical function. The best treatment, early prevention and diagnosis, maximizes care of symptoms. As is evidenced in this case report, rapid diagnosis and treatment often result in recovery from a majority of the symptoms of FES and CFE.
Am J Orthop. 2016;45(7):E515-E521. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. Gurd AR, Wilson RI. The fat embolism syndrome. J Bone Joint Surg Br. 1974;56(3):408-416.
2. Schonfeld SA, Ploysongsang Y, DiLisio R, et al. Fat embolism prophylaxis with corticosteroids. A prospective study in high-risk patients. Ann Intern Med. 1983;99(4):438-443.
3. Robinson CM. Current concepts of respiratory insufficiency syndromes after fracture. J Bone Joint Surg Br. 2001;83(6):781-791.
4. Tsai IT, Hsu CJ, Chen YH, Fong YC, Hsu HC, Tsai CH. Fat embolism syndrome in long bone fracture—clinical experience in a tertiary referral center in Taiwan. J Chin Med Assoc. 2010;73(8):407-410.
5. Taviloglu K, Yanar H. Fat embolism syndrome. Surg Today. 2007;37(1):5-8.
6. Chen PC, Hsu CW, Liao WI, Chen YL, Ho CH, Tsai SH. Hyperacute cerebral fat embolism in a patient with femoral shaft fracture. Am J Emerg Med. 2013;31(9):1420.e1-e3.
7. Mellor A, Soni N. Fat embolism. Anaesthesia. 2001;56(2):145-154.
8. Kaplan RP, Grant JN, Kaufman AJ. Dermatologic features of the fat embolism syndrome. Cutis. 1986;38(1):52-55.
9. Parizel PM, Demey HE, Veeckmans G, et al. Early diagnosis of cerebral fat embolism syndrome by diffusion-weighted MRI (starfield pattern). Stroke. 2001;32(12):2942-2944.
10. Allardyce DB, Meek RN, Woodruff B, Cassim MM, Ellis D. Increasing our knowledge of the pathogenesis of fat embolism: a prospective study of 43 patients with fractured femoral shafts. J Trauma. 1974;14(11):955-962.
11. Müller C, Rahn BA, Pfister U, Meinig RP. The incidence, pathogenesis, diagnosis, and treatment of fat embolism. Orthop Rev. 1994;23(2):107-117.
12. Wildsmith JA, Masson AH. Severe fat embolism: a review of 24 cases. Scott Med J. 1978;23(2):141-148.
13. Nork SE, Agel J, Russell GV, Mills WJ, Holt S, Routt ML Jr. Mortality after reamed intramedullary nailing of bilateral femur fractures. Clin Orthop Relat Res. 2003;(415):272-278.
14. Duran L, Kayhan S, Kati C, Akdemir HU, Balci K, Yavuz Y. Cerebral fat embolism syndrome after long bone fracture due to gunshot injury. Indian J Crit Care Med. 2014;18(3):167-169.
15. Chang RN, Kim JH, Lee H, et al. Cerebral fat embolism after bilateral total knee replacement arthroplasty. A case report. Korean J Anesthesiol. 2010;59(suppl):S207-S210.
16. Yeo SH, Chang HW, Sohn SI, Cho CH, Bae KC. Pulmonary and cerebral fat embolism syndrome after total knee replacement. J Clin Med Res. 2013;5(3):239-242.
17. Walshe CM, Cooper JD, Kossmann T, Hayes I, Iles L. Cerebral fat embolism syndrome causing brain death after long-bone fractures and acetazolamide therapy. Crit Care Resusc. 2007;9(2):184-186.
18. Kamano M, Honda Y, Kitaguchi M, Kazuki K. Cerebral fat embolism after a nondisplaced tibial fracture: case report. Clin Orthop Relat Res. 2001;(389):206-209.
19. Fabian TC. Unravelling the fat embolism syndrome. N Engl J Med. 1993;329(13):961-963.
20. Habashi NM, Andrews PL, Scalea TM. Therapeutic aspects of fat embolism syndrome. Injury. 2006;37(suppl 4):S68-S73.
21. Byrick RJ, Mullen JB, Mazer CD, Guest CB. Transpulmonary systemic fat embolism. Studies in mongrel dogs after cemented arthroplasty. Am J Respir Crit Care Med. 1994;150(5 pt 1):1416-1422.
22. Riding G, Daly K, Hutchinson S, Rao S, Lovell M, McCollum C. Paradoxical cerebral embolisation. An explanation for fat embolism syndrome. J Bone Joint Surg Br. 2004;86(1):95-98.
23. Baker PL, Pazell JA, Peltier LF. Free fatty acids, catecholamines, and arterial hypoxia in patients with fat embolism. J Trauma. 1971;11(12):1026-1030.
24. Rodríguez-Erdmann F. Bleeding due to increased intravascular blood coagulation. Hemorrhagic syndromes caused by consumption of blood-clotting factors (consumption-coagulopathies). N Engl J Med. 1965;273(25):1370-1378.
25. Satoh H, Kurisu K, Ohtani M, et al. Cerebral fat embolism studied by magnetic resonance imaging, transcranial Doppler sonography, and single photon emission computed tomography: case report. J Trauma. 1997;43(2):345-348.
26. Deleanu B, Prejbeanu R, Poenaru D, Vermesan D, Haragus H. Reamed versus unreamed intramedullary locked nailing in tibial fractures. Eur J Orthop Surg Traumatol. 2014;24(8):1597-1601.
27. Helttula I, Karanko M, Gullichsen E. Similar central hemodynamics but increased postoperative oxygen consumption in unreamed versus reamed intramedullary nailing of femoral fractures. J Trauma. 2006;61(5):1178-1185.
28. Högel F, Gerlach UV, Südkamp NP, Müller CA. Pulmonary fat embolism after reamed and unreamed nailing of femoral fractures. Injury. 2010;41(12):1317-1322.
29. Study to Prospectively Evaluate Reamed Intramedullary Nails in Patients With Tibial Fractures Investigators; Bhandari M, Guyatt G, Tornetta P 3rd, et al. Randomized trial of reamed and unreamed intramedullary nailing of tibial shaft fractures. J Bone Joint Surg Am. 2008;90(12):2567-2578.
30. Syed KA, Blankstein M, Bhandari M, Nakane M, Zdero R, Schemitsch EH. The effect of patient position during trauma surgery on fat embolism syndrome: an experimental study. Indian J Orthop. 2014;48(2):203-210.
Fat embolism syndrome (FES) occurs in long-bone fractures and classically presents with the triad of hypoxia, petechia, and altered mental status, or the criteria of Gurd and Wilson.1 The Lindeque criteria (femur fracture, pH <7.3, increased work of breathing) are also used.1,2 FES is a potentially fatal complication, with mortality rates ranging from 10% to 36%.1,3 FES typically occurs within 24 to 72 hours after initial insult, with one study finding an average of 48.5 hours after injury and an incidence of 0.15% to 2.4%.4 The overall FES rate is <1% in retrospective reviews and 11% to 29% in prospective studies.5 FES may present without one or all of the Gurd and Wilson criteria,6 and cerebral fat embolism (CFE) can be even more difficult to diagnose. Patients with CFE typically present with a wide array of postoperative neurologic deficits, commonly in the 24- to 72-hour window in which FES typically occurs. Correct diagnosis and management of CFE require a high index of suspicion and knowledge of the diagnostic work-up. In the postoperative setting, it can be difficult to distinguish CFE-related neurologic deficits from the normal sequelae of anesthesia, pain medications, and other factors.
In this article, we report the case of a 42-year-old woman who developed CFE after reamed intramedullary nail fixation of femoral and tibial shaft fractures. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 42-year-old woman with no past medical history was injured when a horse reared and fell on her. Initial emergent computed tomography (CT) was negative for intracranial hemorrhage, and injury radiographs were obtained (Figures 1A, 1B).
About 9 hours after surgery and 36 hours after injury, the patient was unresponsive. Vital signs, including oxygen saturation, were within normal limits, but she was unable to verbalize. Physical examination revealed symmetric facial musculature but also generalized weakness and diffuse hypertonicity and hyperreflexia. Initial laboratory results, including complete blood cell count, electrolyte panel, and troponin levels, were unremarkable. Naloxone was administered to rule out opioid overdose. An immediate code stroke and neurology consultation was requested. An emergent CT scan of the brain was negative; an urgent magnetic resonance imaging (MRI) scan showed multiple punctate T2/FLAIR (fluid attenuated inversion recovery) hyperintensities with restricted diffusion, predominantly in a parasagittal white matter distribution (Figure 2).
The patient slowly and steadily improved. She was verbal by postoperative day 3 (POD-3), upper motor neuron signs resolved by POD-4, encephalopathy resolved by POD-7, and she was discharged to a rehabilitation center. Unresolved post-stroke symptoms included mild visual field deficits in the right eye (20/25 vision, central scotoma) and amnesia regarding the events immediately surrounding the surgery. There were no other neurologic or cognitive deficits. The patient was non-weight-bearing on the operative extremity and ambulating with assistance, and she started range-of-motion exercises. After 1 week, she was discharged home with crutches.
The patient followed up with neurology and ophthalmology for routine post-stroke care. At 2- and 6-month neurology follow-ups, she was still amnestic regarding her acute stroke event but did not exhibit any confusion, memory problems, speech deficits, facial droop, headaches, or weakness. According to neurology, the encephalopathy was completely resolved, and the patient was completely recovered from the event. Levetiracetam and aspirin were discontinued at 2 months. At the 2-month ophthalmology follow-up, the patient had 20/20 vision in both eyes and nearly complete resolution of the central scotoma. Ophthalmology confirmed symptom relief and recommended return to routine eye care and 1-year follow-up.
The patient began weight-bearing as tolerated on POD-14 and had no hardware or other complications. At 6-month orthopedics follow-up, range of motion of the affected knee was 0° to 120°, and rotation, length, and varus/valgus and anteroposterior knee laxity were all symmetric to the contralateral extremity. The patient walked with a cane for balance and had a mild limp. The affected thigh still had mild atrophy, but strength was 5/5 throughout. The patient denied pain or hardware sensitivity in the affected extremity and was very pleased with the result.
Discussion
Postoperative Acute Mental Status Change
There are many causes of postoperative mental status change after intramedullary nailing. Change may be cardiogenic, infectious, pharmacologic, or neurologic in origin. Age should be considered in the work-up of postoperative mental status change, as common complications differ between older and younger patients, with geriatric patients at particularly high risk for delirium.
Next to be evaluated are vital signs—particularly hypoxia, as isolated tachycardia may simply be a manifestation of pain. The cardiac system is then assessed with EKG and cardiac-specific laboratory tests, including a troponin level test if there is suspicion of myocardial infarction. PE and FES are complications with a higher prevalence in intramedullary nailing, and pulmonary involvement can be ruled out with the CT with PE protocol. Skin examination is important as well, as FES presents with petechial rash in 60% of patients8 (rash was absent in our patient’s case). Narcotic overdose is easily ruled out with administration of naloxone. Infection and sepsis can cause mental changes, though more commonly in the elderly and seldom so soon after surgery. Evaluation for infection and sepsis involves urinalysis and culturing of blood, urine, and other bodily fluids. If there is concern about surgical site infection, the postoperative dressing should be immediately removed and the wound examined. Last, neurologic and embolic phenomena can be initially investigated with CT to rule out hemorrhagic stroke. If CT of the brain is negative, MRI should be performed. MRI is the gold standard for diagnosing ischemic stroke and CFE caused by FES.9
Prevalence of Fat Embolism Syndrome
Development of intramedullary fat release in patients with long-bone injuries is common. A prospective study found circulating fat globules in 95% of 43 patients with femur fractures.10 In another study, transesophageal EKG showed cardiac embolism in 62% of patients who had undergone intramedullary nail fixation.11 Despite this high rate, only 0.9% to 2.2% of patients developed symptomatic FES. Risk factors for FES include younger age, multiple fractures, closed fractures, and nonoperative or delayed management of long-bone fractures.2 As already mentioned, average time to FES presentation after long-bone fracture is about 48 hours. One study found that FES typically occurs within 24 to 72 hours after initial insult (average, 48.5 hours) and that the incidence of FES is 0.15% in tibia fractures, 0.78% in femur fractures, and 2.4% in multiple long-bone fractures.4 The timeline is consistent with the present case—our patient developed symptoms about 36 hours after injury. In addition, other studies have found a higher mortality rate (5%-15%) for patients with bilateral femur fractures than for patients with only one fracture.7,12,13 Patients with a floating knee injury (ipsilateral tibia and femur fractures) are at higher risk for FES and have higher overall morbidity and mortality rates in comparison with patients with an isolated femur or tibia fracture, though the increased risk has not been quantified.
Review of Case Literature: FES With CFE
Few cases of FES with symptomatic CFE in the setting of long-bone fracture or long-bone surgery have been reported in the literature. There is wide variation in the development of FES with respect to preoperative or postoperative status and mechanism of injury. Duran and colleagues14 described a 20-year-old man with ipsilateral tibia and femur fractures caused by gunshots. Twenty-four hours after presentation, he developed tonic-clonic seizures and the classic triad of rash, hypoxia, and altered mental status. MRI confirmed CFE secondary to FES. The patient was optimized neurologically before definitive fixation and was discharged with minimal neurologic deficits on POD-27. Chang and colleagues15 and Yeo and colleagues16 described CFE in patients who underwent bilateral total knee arthroplasty. Symptoms developed 9 hours and 2 days after surgery, respectively. Both patients had fat emboli in the lungs and brain, underwent intensive care treatment, and recovered from the initial insult. After discharge at 44 days and 2 weeks, respectively, they fully recovered.
Other patients with CFE have had less favorable outcomes. Chen and colleagues6 reported the case of a 31-year-old man who sustained closed femur and tibia fractures in an automobile collision and experienced an acute decline in neurologic status 1 hour after arrival in the emergency department. The patient was intubated, CFE was diagnosed on the basis of MRI findings, and the orthopedic injuries were treated with external fixation. After 2 weeks, the patient was discharged with persistent neurologic deficits and the need for long-term tube feeding. Walshe and colleagues17 reported the case of a 19-year-old woman who sustained multiple long-bone injuries and traumatic brain injury and showed fat emboli on MRI. The patient experienced brain herniation while in the intensive care unit and later was declared brain-dead. According to the literature, it is important to maintain high suspicion for FES and possible CFE in the setting of high-energy fracture but also to be aware that FES may develop even with nondisplaced fracture or with reaming of the intramedullary canal in elective total joint arthroplasty.18
Pathophysiology of Fat Embolism Syndrome
The pathophysiology of FES and specifically of CFE is not widely understood. Two main theories on the development of FES have been advanced.
The mechanical theory suggests that exposing intramedullary long-bone contents allows fat to mobilize into the bloodstream.19 This occurs in the setting of long-bone fracture and in canal preparation during joint replacement surgery. More fat extravasates into the venous system after femur fracture than after tibia fracture, which accounts for the higher risk for FES in femoral shaft fractures and the even higher risk in concomitant femur and tibia fractures.4 In addition to there being a risk of fat embolism from the fracture itself, placing the patient in traction or reaming the intramedullary canal may exacerbate this effect by increased extravasation of fat from the medullary canal. With extravasation of fatty bone marrow into the venous system, fat emboli are free to travel back to the lungs, where they can cause infarcts within the lung parenchyma.
In the mechanical theory, presence of PFO may allow fat globules to pass into the systemic circulation and cause end-organ emboli. In the event of cerebral emboli, neurologic symptoms may vary widely and may include diffuse encephalopathy and global deficits.20 Dog studies have found a possible mechanism for CFE in the absence of PFO. One such study, which used femoral pressurization to replicate cemented femoral arthroplasty, found that many fat globules had traversed the lungs after release into bone marrow,21 supporting the theory that fat droplets can traverse the pulmonary system without sequestration in the lung parenchyma. Riding and colleagues22 reported finding pulmonary arteriovenous shunts, which are thought to allow CFE to occur in the absence of PFO. More studies are needed to determine the prevalence of shunts and their overall contribution to CFE development in patients with long-bone fracture.
The biochemical theory holds that bodily trauma induces the release of free fatty acids (FFAs) from the capillaries into the bloodstream.23 This stress response is mediated by catecholamines, which activate the adenyl cyclase pathway, which activates lipase, which hydrolyzes stored triglycerides to FFAs and glycerol. The concentration of circulating FFA was increased in 9 of 10 patients in one study.23 Increased FFAs in the bloodstream can accelerate local and end-organ clotting, leading to thrombocytopenia and endothelial injury. In addition, patients with hypercoagulable diseases are at higher risk for postoperative thromboembolism.24 However, with a negative hypercoagulable work-up and with negative chest helical CT and EKG, which did not demonstrate PFO, the explanation for CFE in our patient may more likely reside with the arteriovenous shunt theory proposed by Riding and colleagues.22
Diagnosis and Treatment
Proper care of orthopedic patients who potentially have FES/CFE involves prompt diagnosis, immediate symptomatic care, and early coordination with neurology and medical services to rule out other causes of symptoms. Obtaining advanced imaging to rule out other potential causes and to confirm the diagnosis is crucial. The patient in this case report did not exhibit any focal neurologic deficits, but emergent CT of the brain was indicated to rule out a hemorrhagic event. If a stroke secondary to FES is clinically suspected, MRI should be obtained as soon as possible. Multiple studies have found that the “starfield” pattern, which is best seen as multiple punctate hyperintensities on T2 imaging, is the typical radiographic manifestation of CFE.9 This applies to patients who are in the 24- to 72-hour window after long-bone fracture or fixation and who fit Gurd and Wilson1 criteria or Lindeque1,2criteria, or who exhibit a change in mental status but have a negative CT scan of the brain, as was the case with our patient. Once the diagnosis is made, treatment involves addressing the symptoms (Figure 4).
Fat Embolism Syndrome in Reamed and Unreamed Nailing
Over the past several decades, the number of long bones fixed with intramedullary nails has increased significantly.26 There is debate regarding whether use of reamed intramedullary nails increases the risk of fat emboli relative to use of unreamed nails, but multiple studies have found no significant difference.26,27 Pulmonary shunting occurs in both reamed and unreamed nailing; neither technique has an advantage in terms of cardiopulmonary complications. In multiple studies, reamed nails have the advantage of improved healing rates.27 A sheep study compared reamed and unreamed femoral nailing.28 After nailing, sheep lungs were examined histologically for the presence of bone marrow fat embolism. The embolism rate was higher with unreamed nailing (10.25%) than with reamed nailing (6.66%). One large study of the adverse effects of reamed and unreamed nailing in 1226 patients with tibial shaft fracture found that those with open fractures had higher rates of a negative event (nonunion, infection, fasciotomy, hardware failure, need for dynamization) after reamed nailing.29 Patients with closed fractures had fewer events after reamed nailing. The authors concluded there is a potential benefit in outcome with reamed intramedullary nailing in patients with closed tibial shaft fractures, but they did not comment on development of FES. In a study of the effect of subject position on intramedullary pressure and fat embolism release, dogs were positioned either supine or lateral for tibial and femoral reaming.30 The authors measured various physiologic parameters, including cardiac output, pulmonary arterial wedge pressure, arterial and venous blood gas, and blood cell counts. There were no statistically significant differences in values between the 2 groups in any variable, indicating that position does not affect FES development in the orthopedic trauma setting.
Conclusion
FES and CFE are potential devastating sequelae of both long-bone fracture and long-bone instrumentation. It is important to recognize these entities in the acute setting and to consider them in the differential diagnosis of a trauma or postoperative patient who experiences sudden onset of altered mental status with or without dyspnea or a petechial rash. If CFE is suspected, early advanced imaging (including urgent MRI) should be obtained with rapid involvement of a multidisciplinary team that can optimize the chance for successful recovery of both neurologic and physical function. The best treatment, early prevention and diagnosis, maximizes care of symptoms. As is evidenced in this case report, rapid diagnosis and treatment often result in recovery from a majority of the symptoms of FES and CFE.
Am J Orthop. 2016;45(7):E515-E521. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
Fat embolism syndrome (FES) occurs in long-bone fractures and classically presents with the triad of hypoxia, petechia, and altered mental status, or the criteria of Gurd and Wilson.1 The Lindeque criteria (femur fracture, pH <7.3, increased work of breathing) are also used.1,2 FES is a potentially fatal complication, with mortality rates ranging from 10% to 36%.1,3 FES typically occurs within 24 to 72 hours after initial insult, with one study finding an average of 48.5 hours after injury and an incidence of 0.15% to 2.4%.4 The overall FES rate is <1% in retrospective reviews and 11% to 29% in prospective studies.5 FES may present without one or all of the Gurd and Wilson criteria,6 and cerebral fat embolism (CFE) can be even more difficult to diagnose. Patients with CFE typically present with a wide array of postoperative neurologic deficits, commonly in the 24- to 72-hour window in which FES typically occurs. Correct diagnosis and management of CFE require a high index of suspicion and knowledge of the diagnostic work-up. In the postoperative setting, it can be difficult to distinguish CFE-related neurologic deficits from the normal sequelae of anesthesia, pain medications, and other factors.
In this article, we report the case of a 42-year-old woman who developed CFE after reamed intramedullary nail fixation of femoral and tibial shaft fractures. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 42-year-old woman with no past medical history was injured when a horse reared and fell on her. Initial emergent computed tomography (CT) was negative for intracranial hemorrhage, and injury radiographs were obtained (Figures 1A, 1B).
About 9 hours after surgery and 36 hours after injury, the patient was unresponsive. Vital signs, including oxygen saturation, were within normal limits, but she was unable to verbalize. Physical examination revealed symmetric facial musculature but also generalized weakness and diffuse hypertonicity and hyperreflexia. Initial laboratory results, including complete blood cell count, electrolyte panel, and troponin levels, were unremarkable. Naloxone was administered to rule out opioid overdose. An immediate code stroke and neurology consultation was requested. An emergent CT scan of the brain was negative; an urgent magnetic resonance imaging (MRI) scan showed multiple punctate T2/FLAIR (fluid attenuated inversion recovery) hyperintensities with restricted diffusion, predominantly in a parasagittal white matter distribution (Figure 2).
The patient slowly and steadily improved. She was verbal by postoperative day 3 (POD-3), upper motor neuron signs resolved by POD-4, encephalopathy resolved by POD-7, and she was discharged to a rehabilitation center. Unresolved post-stroke symptoms included mild visual field deficits in the right eye (20/25 vision, central scotoma) and amnesia regarding the events immediately surrounding the surgery. There were no other neurologic or cognitive deficits. The patient was non-weight-bearing on the operative extremity and ambulating with assistance, and she started range-of-motion exercises. After 1 week, she was discharged home with crutches.
The patient followed up with neurology and ophthalmology for routine post-stroke care. At 2- and 6-month neurology follow-ups, she was still amnestic regarding her acute stroke event but did not exhibit any confusion, memory problems, speech deficits, facial droop, headaches, or weakness. According to neurology, the encephalopathy was completely resolved, and the patient was completely recovered from the event. Levetiracetam and aspirin were discontinued at 2 months. At the 2-month ophthalmology follow-up, the patient had 20/20 vision in both eyes and nearly complete resolution of the central scotoma. Ophthalmology confirmed symptom relief and recommended return to routine eye care and 1-year follow-up.
The patient began weight-bearing as tolerated on POD-14 and had no hardware or other complications. At 6-month orthopedics follow-up, range of motion of the affected knee was 0° to 120°, and rotation, length, and varus/valgus and anteroposterior knee laxity were all symmetric to the contralateral extremity. The patient walked with a cane for balance and had a mild limp. The affected thigh still had mild atrophy, but strength was 5/5 throughout. The patient denied pain or hardware sensitivity in the affected extremity and was very pleased with the result.
Discussion
Postoperative Acute Mental Status Change
There are many causes of postoperative mental status change after intramedullary nailing. Change may be cardiogenic, infectious, pharmacologic, or neurologic in origin. Age should be considered in the work-up of postoperative mental status change, as common complications differ between older and younger patients, with geriatric patients at particularly high risk for delirium.
Next to be evaluated are vital signs—particularly hypoxia, as isolated tachycardia may simply be a manifestation of pain. The cardiac system is then assessed with EKG and cardiac-specific laboratory tests, including a troponin level test if there is suspicion of myocardial infarction. PE and FES are complications with a higher prevalence in intramedullary nailing, and pulmonary involvement can be ruled out with the CT with PE protocol. Skin examination is important as well, as FES presents with petechial rash in 60% of patients8 (rash was absent in our patient’s case). Narcotic overdose is easily ruled out with administration of naloxone. Infection and sepsis can cause mental changes, though more commonly in the elderly and seldom so soon after surgery. Evaluation for infection and sepsis involves urinalysis and culturing of blood, urine, and other bodily fluids. If there is concern about surgical site infection, the postoperative dressing should be immediately removed and the wound examined. Last, neurologic and embolic phenomena can be initially investigated with CT to rule out hemorrhagic stroke. If CT of the brain is negative, MRI should be performed. MRI is the gold standard for diagnosing ischemic stroke and CFE caused by FES.9
Prevalence of Fat Embolism Syndrome
Development of intramedullary fat release in patients with long-bone injuries is common. A prospective study found circulating fat globules in 95% of 43 patients with femur fractures.10 In another study, transesophageal EKG showed cardiac embolism in 62% of patients who had undergone intramedullary nail fixation.11 Despite this high rate, only 0.9% to 2.2% of patients developed symptomatic FES. Risk factors for FES include younger age, multiple fractures, closed fractures, and nonoperative or delayed management of long-bone fractures.2 As already mentioned, average time to FES presentation after long-bone fracture is about 48 hours. One study found that FES typically occurs within 24 to 72 hours after initial insult (average, 48.5 hours) and that the incidence of FES is 0.15% in tibia fractures, 0.78% in femur fractures, and 2.4% in multiple long-bone fractures.4 The timeline is consistent with the present case—our patient developed symptoms about 36 hours after injury. In addition, other studies have found a higher mortality rate (5%-15%) for patients with bilateral femur fractures than for patients with only one fracture.7,12,13 Patients with a floating knee injury (ipsilateral tibia and femur fractures) are at higher risk for FES and have higher overall morbidity and mortality rates in comparison with patients with an isolated femur or tibia fracture, though the increased risk has not been quantified.
Review of Case Literature: FES With CFE
Few cases of FES with symptomatic CFE in the setting of long-bone fracture or long-bone surgery have been reported in the literature. There is wide variation in the development of FES with respect to preoperative or postoperative status and mechanism of injury. Duran and colleagues14 described a 20-year-old man with ipsilateral tibia and femur fractures caused by gunshots. Twenty-four hours after presentation, he developed tonic-clonic seizures and the classic triad of rash, hypoxia, and altered mental status. MRI confirmed CFE secondary to FES. The patient was optimized neurologically before definitive fixation and was discharged with minimal neurologic deficits on POD-27. Chang and colleagues15 and Yeo and colleagues16 described CFE in patients who underwent bilateral total knee arthroplasty. Symptoms developed 9 hours and 2 days after surgery, respectively. Both patients had fat emboli in the lungs and brain, underwent intensive care treatment, and recovered from the initial insult. After discharge at 44 days and 2 weeks, respectively, they fully recovered.
Other patients with CFE have had less favorable outcomes. Chen and colleagues6 reported the case of a 31-year-old man who sustained closed femur and tibia fractures in an automobile collision and experienced an acute decline in neurologic status 1 hour after arrival in the emergency department. The patient was intubated, CFE was diagnosed on the basis of MRI findings, and the orthopedic injuries were treated with external fixation. After 2 weeks, the patient was discharged with persistent neurologic deficits and the need for long-term tube feeding. Walshe and colleagues17 reported the case of a 19-year-old woman who sustained multiple long-bone injuries and traumatic brain injury and showed fat emboli on MRI. The patient experienced brain herniation while in the intensive care unit and later was declared brain-dead. According to the literature, it is important to maintain high suspicion for FES and possible CFE in the setting of high-energy fracture but also to be aware that FES may develop even with nondisplaced fracture or with reaming of the intramedullary canal in elective total joint arthroplasty.18
Pathophysiology of Fat Embolism Syndrome
The pathophysiology of FES and specifically of CFE is not widely understood. Two main theories on the development of FES have been advanced.
The mechanical theory suggests that exposing intramedullary long-bone contents allows fat to mobilize into the bloodstream.19 This occurs in the setting of long-bone fracture and in canal preparation during joint replacement surgery. More fat extravasates into the venous system after femur fracture than after tibia fracture, which accounts for the higher risk for FES in femoral shaft fractures and the even higher risk in concomitant femur and tibia fractures.4 In addition to there being a risk of fat embolism from the fracture itself, placing the patient in traction or reaming the intramedullary canal may exacerbate this effect by increased extravasation of fat from the medullary canal. With extravasation of fatty bone marrow into the venous system, fat emboli are free to travel back to the lungs, where they can cause infarcts within the lung parenchyma.
In the mechanical theory, presence of PFO may allow fat globules to pass into the systemic circulation and cause end-organ emboli. In the event of cerebral emboli, neurologic symptoms may vary widely and may include diffuse encephalopathy and global deficits.20 Dog studies have found a possible mechanism for CFE in the absence of PFO. One such study, which used femoral pressurization to replicate cemented femoral arthroplasty, found that many fat globules had traversed the lungs after release into bone marrow,21 supporting the theory that fat droplets can traverse the pulmonary system without sequestration in the lung parenchyma. Riding and colleagues22 reported finding pulmonary arteriovenous shunts, which are thought to allow CFE to occur in the absence of PFO. More studies are needed to determine the prevalence of shunts and their overall contribution to CFE development in patients with long-bone fracture.
The biochemical theory holds that bodily trauma induces the release of free fatty acids (FFAs) from the capillaries into the bloodstream.23 This stress response is mediated by catecholamines, which activate the adenyl cyclase pathway, which activates lipase, which hydrolyzes stored triglycerides to FFAs and glycerol. The concentration of circulating FFA was increased in 9 of 10 patients in one study.23 Increased FFAs in the bloodstream can accelerate local and end-organ clotting, leading to thrombocytopenia and endothelial injury. In addition, patients with hypercoagulable diseases are at higher risk for postoperative thromboembolism.24 However, with a negative hypercoagulable work-up and with negative chest helical CT and EKG, which did not demonstrate PFO, the explanation for CFE in our patient may more likely reside with the arteriovenous shunt theory proposed by Riding and colleagues.22
Diagnosis and Treatment
Proper care of orthopedic patients who potentially have FES/CFE involves prompt diagnosis, immediate symptomatic care, and early coordination with neurology and medical services to rule out other causes of symptoms. Obtaining advanced imaging to rule out other potential causes and to confirm the diagnosis is crucial. The patient in this case report did not exhibit any focal neurologic deficits, but emergent CT of the brain was indicated to rule out a hemorrhagic event. If a stroke secondary to FES is clinically suspected, MRI should be obtained as soon as possible. Multiple studies have found that the “starfield” pattern, which is best seen as multiple punctate hyperintensities on T2 imaging, is the typical radiographic manifestation of CFE.9 This applies to patients who are in the 24- to 72-hour window after long-bone fracture or fixation and who fit Gurd and Wilson1 criteria or Lindeque1,2criteria, or who exhibit a change in mental status but have a negative CT scan of the brain, as was the case with our patient. Once the diagnosis is made, treatment involves addressing the symptoms (Figure 4).
Fat Embolism Syndrome in Reamed and Unreamed Nailing
Over the past several decades, the number of long bones fixed with intramedullary nails has increased significantly.26 There is debate regarding whether use of reamed intramedullary nails increases the risk of fat emboli relative to use of unreamed nails, but multiple studies have found no significant difference.26,27 Pulmonary shunting occurs in both reamed and unreamed nailing; neither technique has an advantage in terms of cardiopulmonary complications. In multiple studies, reamed nails have the advantage of improved healing rates.27 A sheep study compared reamed and unreamed femoral nailing.28 After nailing, sheep lungs were examined histologically for the presence of bone marrow fat embolism. The embolism rate was higher with unreamed nailing (10.25%) than with reamed nailing (6.66%). One large study of the adverse effects of reamed and unreamed nailing in 1226 patients with tibial shaft fracture found that those with open fractures had higher rates of a negative event (nonunion, infection, fasciotomy, hardware failure, need for dynamization) after reamed nailing.29 Patients with closed fractures had fewer events after reamed nailing. The authors concluded there is a potential benefit in outcome with reamed intramedullary nailing in patients with closed tibial shaft fractures, but they did not comment on development of FES. In a study of the effect of subject position on intramedullary pressure and fat embolism release, dogs were positioned either supine or lateral for tibial and femoral reaming.30 The authors measured various physiologic parameters, including cardiac output, pulmonary arterial wedge pressure, arterial and venous blood gas, and blood cell counts. There were no statistically significant differences in values between the 2 groups in any variable, indicating that position does not affect FES development in the orthopedic trauma setting.
Conclusion
FES and CFE are potential devastating sequelae of both long-bone fracture and long-bone instrumentation. It is important to recognize these entities in the acute setting and to consider them in the differential diagnosis of a trauma or postoperative patient who experiences sudden onset of altered mental status with or without dyspnea or a petechial rash. If CFE is suspected, early advanced imaging (including urgent MRI) should be obtained with rapid involvement of a multidisciplinary team that can optimize the chance for successful recovery of both neurologic and physical function. The best treatment, early prevention and diagnosis, maximizes care of symptoms. As is evidenced in this case report, rapid diagnosis and treatment often result in recovery from a majority of the symptoms of FES and CFE.
Am J Orthop. 2016;45(7):E515-E521. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. Gurd AR, Wilson RI. The fat embolism syndrome. J Bone Joint Surg Br. 1974;56(3):408-416.
2. Schonfeld SA, Ploysongsang Y, DiLisio R, et al. Fat embolism prophylaxis with corticosteroids. A prospective study in high-risk patients. Ann Intern Med. 1983;99(4):438-443.
3. Robinson CM. Current concepts of respiratory insufficiency syndromes after fracture. J Bone Joint Surg Br. 2001;83(6):781-791.
4. Tsai IT, Hsu CJ, Chen YH, Fong YC, Hsu HC, Tsai CH. Fat embolism syndrome in long bone fracture—clinical experience in a tertiary referral center in Taiwan. J Chin Med Assoc. 2010;73(8):407-410.
5. Taviloglu K, Yanar H. Fat embolism syndrome. Surg Today. 2007;37(1):5-8.
6. Chen PC, Hsu CW, Liao WI, Chen YL, Ho CH, Tsai SH. Hyperacute cerebral fat embolism in a patient with femoral shaft fracture. Am J Emerg Med. 2013;31(9):1420.e1-e3.
7. Mellor A, Soni N. Fat embolism. Anaesthesia. 2001;56(2):145-154.
8. Kaplan RP, Grant JN, Kaufman AJ. Dermatologic features of the fat embolism syndrome. Cutis. 1986;38(1):52-55.
9. Parizel PM, Demey HE, Veeckmans G, et al. Early diagnosis of cerebral fat embolism syndrome by diffusion-weighted MRI (starfield pattern). Stroke. 2001;32(12):2942-2944.
10. Allardyce DB, Meek RN, Woodruff B, Cassim MM, Ellis D. Increasing our knowledge of the pathogenesis of fat embolism: a prospective study of 43 patients with fractured femoral shafts. J Trauma. 1974;14(11):955-962.
11. Müller C, Rahn BA, Pfister U, Meinig RP. The incidence, pathogenesis, diagnosis, and treatment of fat embolism. Orthop Rev. 1994;23(2):107-117.
12. Wildsmith JA, Masson AH. Severe fat embolism: a review of 24 cases. Scott Med J. 1978;23(2):141-148.
13. Nork SE, Agel J, Russell GV, Mills WJ, Holt S, Routt ML Jr. Mortality after reamed intramedullary nailing of bilateral femur fractures. Clin Orthop Relat Res. 2003;(415):272-278.
14. Duran L, Kayhan S, Kati C, Akdemir HU, Balci K, Yavuz Y. Cerebral fat embolism syndrome after long bone fracture due to gunshot injury. Indian J Crit Care Med. 2014;18(3):167-169.
15. Chang RN, Kim JH, Lee H, et al. Cerebral fat embolism after bilateral total knee replacement arthroplasty. A case report. Korean J Anesthesiol. 2010;59(suppl):S207-S210.
16. Yeo SH, Chang HW, Sohn SI, Cho CH, Bae KC. Pulmonary and cerebral fat embolism syndrome after total knee replacement. J Clin Med Res. 2013;5(3):239-242.
17. Walshe CM, Cooper JD, Kossmann T, Hayes I, Iles L. Cerebral fat embolism syndrome causing brain death after long-bone fractures and acetazolamide therapy. Crit Care Resusc. 2007;9(2):184-186.
18. Kamano M, Honda Y, Kitaguchi M, Kazuki K. Cerebral fat embolism after a nondisplaced tibial fracture: case report. Clin Orthop Relat Res. 2001;(389):206-209.
19. Fabian TC. Unravelling the fat embolism syndrome. N Engl J Med. 1993;329(13):961-963.
20. Habashi NM, Andrews PL, Scalea TM. Therapeutic aspects of fat embolism syndrome. Injury. 2006;37(suppl 4):S68-S73.
21. Byrick RJ, Mullen JB, Mazer CD, Guest CB. Transpulmonary systemic fat embolism. Studies in mongrel dogs after cemented arthroplasty. Am J Respir Crit Care Med. 1994;150(5 pt 1):1416-1422.
22. Riding G, Daly K, Hutchinson S, Rao S, Lovell M, McCollum C. Paradoxical cerebral embolisation. An explanation for fat embolism syndrome. J Bone Joint Surg Br. 2004;86(1):95-98.
23. Baker PL, Pazell JA, Peltier LF. Free fatty acids, catecholamines, and arterial hypoxia in patients with fat embolism. J Trauma. 1971;11(12):1026-1030.
24. Rodríguez-Erdmann F. Bleeding due to increased intravascular blood coagulation. Hemorrhagic syndromes caused by consumption of blood-clotting factors (consumption-coagulopathies). N Engl J Med. 1965;273(25):1370-1378.
25. Satoh H, Kurisu K, Ohtani M, et al. Cerebral fat embolism studied by magnetic resonance imaging, transcranial Doppler sonography, and single photon emission computed tomography: case report. J Trauma. 1997;43(2):345-348.
26. Deleanu B, Prejbeanu R, Poenaru D, Vermesan D, Haragus H. Reamed versus unreamed intramedullary locked nailing in tibial fractures. Eur J Orthop Surg Traumatol. 2014;24(8):1597-1601.
27. Helttula I, Karanko M, Gullichsen E. Similar central hemodynamics but increased postoperative oxygen consumption in unreamed versus reamed intramedullary nailing of femoral fractures. J Trauma. 2006;61(5):1178-1185.
28. Högel F, Gerlach UV, Südkamp NP, Müller CA. Pulmonary fat embolism after reamed and unreamed nailing of femoral fractures. Injury. 2010;41(12):1317-1322.
29. Study to Prospectively Evaluate Reamed Intramedullary Nails in Patients With Tibial Fractures Investigators; Bhandari M, Guyatt G, Tornetta P 3rd, et al. Randomized trial of reamed and unreamed intramedullary nailing of tibial shaft fractures. J Bone Joint Surg Am. 2008;90(12):2567-2578.
30. Syed KA, Blankstein M, Bhandari M, Nakane M, Zdero R, Schemitsch EH. The effect of patient position during trauma surgery on fat embolism syndrome: an experimental study. Indian J Orthop. 2014;48(2):203-210.
1. Gurd AR, Wilson RI. The fat embolism syndrome. J Bone Joint Surg Br. 1974;56(3):408-416.
2. Schonfeld SA, Ploysongsang Y, DiLisio R, et al. Fat embolism prophylaxis with corticosteroids. A prospective study in high-risk patients. Ann Intern Med. 1983;99(4):438-443.
3. Robinson CM. Current concepts of respiratory insufficiency syndromes after fracture. J Bone Joint Surg Br. 2001;83(6):781-791.
4. Tsai IT, Hsu CJ, Chen YH, Fong YC, Hsu HC, Tsai CH. Fat embolism syndrome in long bone fracture—clinical experience in a tertiary referral center in Taiwan. J Chin Med Assoc. 2010;73(8):407-410.
5. Taviloglu K, Yanar H. Fat embolism syndrome. Surg Today. 2007;37(1):5-8.
6. Chen PC, Hsu CW, Liao WI, Chen YL, Ho CH, Tsai SH. Hyperacute cerebral fat embolism in a patient with femoral shaft fracture. Am J Emerg Med. 2013;31(9):1420.e1-e3.
7. Mellor A, Soni N. Fat embolism. Anaesthesia. 2001;56(2):145-154.
8. Kaplan RP, Grant JN, Kaufman AJ. Dermatologic features of the fat embolism syndrome. Cutis. 1986;38(1):52-55.
9. Parizel PM, Demey HE, Veeckmans G, et al. Early diagnosis of cerebral fat embolism syndrome by diffusion-weighted MRI (starfield pattern). Stroke. 2001;32(12):2942-2944.
10. Allardyce DB, Meek RN, Woodruff B, Cassim MM, Ellis D. Increasing our knowledge of the pathogenesis of fat embolism: a prospective study of 43 patients with fractured femoral shafts. J Trauma. 1974;14(11):955-962.
11. Müller C, Rahn BA, Pfister U, Meinig RP. The incidence, pathogenesis, diagnosis, and treatment of fat embolism. Orthop Rev. 1994;23(2):107-117.
12. Wildsmith JA, Masson AH. Severe fat embolism: a review of 24 cases. Scott Med J. 1978;23(2):141-148.
13. Nork SE, Agel J, Russell GV, Mills WJ, Holt S, Routt ML Jr. Mortality after reamed intramedullary nailing of bilateral femur fractures. Clin Orthop Relat Res. 2003;(415):272-278.
14. Duran L, Kayhan S, Kati C, Akdemir HU, Balci K, Yavuz Y. Cerebral fat embolism syndrome after long bone fracture due to gunshot injury. Indian J Crit Care Med. 2014;18(3):167-169.
15. Chang RN, Kim JH, Lee H, et al. Cerebral fat embolism after bilateral total knee replacement arthroplasty. A case report. Korean J Anesthesiol. 2010;59(suppl):S207-S210.
16. Yeo SH, Chang HW, Sohn SI, Cho CH, Bae KC. Pulmonary and cerebral fat embolism syndrome after total knee replacement. J Clin Med Res. 2013;5(3):239-242.
17. Walshe CM, Cooper JD, Kossmann T, Hayes I, Iles L. Cerebral fat embolism syndrome causing brain death after long-bone fractures and acetazolamide therapy. Crit Care Resusc. 2007;9(2):184-186.
18. Kamano M, Honda Y, Kitaguchi M, Kazuki K. Cerebral fat embolism after a nondisplaced tibial fracture: case report. Clin Orthop Relat Res. 2001;(389):206-209.
19. Fabian TC. Unravelling the fat embolism syndrome. N Engl J Med. 1993;329(13):961-963.
20. Habashi NM, Andrews PL, Scalea TM. Therapeutic aspects of fat embolism syndrome. Injury. 2006;37(suppl 4):S68-S73.
21. Byrick RJ, Mullen JB, Mazer CD, Guest CB. Transpulmonary systemic fat embolism. Studies in mongrel dogs after cemented arthroplasty. Am J Respir Crit Care Med. 1994;150(5 pt 1):1416-1422.
22. Riding G, Daly K, Hutchinson S, Rao S, Lovell M, McCollum C. Paradoxical cerebral embolisation. An explanation for fat embolism syndrome. J Bone Joint Surg Br. 2004;86(1):95-98.
23. Baker PL, Pazell JA, Peltier LF. Free fatty acids, catecholamines, and arterial hypoxia in patients with fat embolism. J Trauma. 1971;11(12):1026-1030.
24. Rodríguez-Erdmann F. Bleeding due to increased intravascular blood coagulation. Hemorrhagic syndromes caused by consumption of blood-clotting factors (consumption-coagulopathies). N Engl J Med. 1965;273(25):1370-1378.
25. Satoh H, Kurisu K, Ohtani M, et al. Cerebral fat embolism studied by magnetic resonance imaging, transcranial Doppler sonography, and single photon emission computed tomography: case report. J Trauma. 1997;43(2):345-348.
26. Deleanu B, Prejbeanu R, Poenaru D, Vermesan D, Haragus H. Reamed versus unreamed intramedullary locked nailing in tibial fractures. Eur J Orthop Surg Traumatol. 2014;24(8):1597-1601.
27. Helttula I, Karanko M, Gullichsen E. Similar central hemodynamics but increased postoperative oxygen consumption in unreamed versus reamed intramedullary nailing of femoral fractures. J Trauma. 2006;61(5):1178-1185.
28. Högel F, Gerlach UV, Südkamp NP, Müller CA. Pulmonary fat embolism after reamed and unreamed nailing of femoral fractures. Injury. 2010;41(12):1317-1322.
29. Study to Prospectively Evaluate Reamed Intramedullary Nails in Patients With Tibial Fractures Investigators; Bhandari M, Guyatt G, Tornetta P 3rd, et al. Randomized trial of reamed and unreamed intramedullary nailing of tibial shaft fractures. J Bone Joint Surg Am. 2008;90(12):2567-2578.
30. Syed KA, Blankstein M, Bhandari M, Nakane M, Zdero R, Schemitsch EH. The effect of patient position during trauma surgery on fat embolism syndrome: an experimental study. Indian J Orthop. 2014;48(2):203-210.
Dermoscopy Update and Noninvasive Imaging Devices for Skin Cancer: Report From the Mount Sinai Winter Symposium
At the 19th Annual Mount Sinai Winter Symposium, Dr. Orit Markowitz provided an update on dermoscopy as a first-line noninvasive imaging modality for skin cancer screening and diagnosis along with reflectance confocal microscopy and dynamic optical coherence tomography. She explained how noninvasive imaging offers a more complete picture of lesions along with what is seen clinically and on pathology and discussed how it can help catch aggressive melanomas and other skin cancers at earlier stages. For these reasons, she emphasized that increased use of dermoscopy can be used to justify the need for regular skin cancer screenings. Finally, she discussed how noninvasive imaging can be used to guide dermatologists in performing optimal biposies of suspicious lesions.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
At the 19th Annual Mount Sinai Winter Symposium, Dr. Orit Markowitz provided an update on dermoscopy as a first-line noninvasive imaging modality for skin cancer screening and diagnosis along with reflectance confocal microscopy and dynamic optical coherence tomography. She explained how noninvasive imaging offers a more complete picture of lesions along with what is seen clinically and on pathology and discussed how it can help catch aggressive melanomas and other skin cancers at earlier stages. For these reasons, she emphasized that increased use of dermoscopy can be used to justify the need for regular skin cancer screenings. Finally, she discussed how noninvasive imaging can be used to guide dermatologists in performing optimal biposies of suspicious lesions.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
At the 19th Annual Mount Sinai Winter Symposium, Dr. Orit Markowitz provided an update on dermoscopy as a first-line noninvasive imaging modality for skin cancer screening and diagnosis along with reflectance confocal microscopy and dynamic optical coherence tomography. She explained how noninvasive imaging offers a more complete picture of lesions along with what is seen clinically and on pathology and discussed how it can help catch aggressive melanomas and other skin cancers at earlier stages. For these reasons, she emphasized that increased use of dermoscopy can be used to justify the need for regular skin cancer screenings. Finally, she discussed how noninvasive imaging can be used to guide dermatologists in performing optimal biposies of suspicious lesions.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Dermoscopy Pearls: Report From the Mount Sinai Winter Symposium
At the 19th Annual Mount Sinai Winter Symposium, Dr. Orit Markowitz addressed some common questions physicians have about dermoscopy, including what kind of dermatoscope to buy, how to incorporate dermoscopy into a dermatology practice, and how to efficiently perform skin examinations using a dermatoscope. She also emphasized the importance of attending courses and workshops to learn how to utilize dermoscopy and other noninvasive imaging devices effectively.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
At the 19th Annual Mount Sinai Winter Symposium, Dr. Orit Markowitz addressed some common questions physicians have about dermoscopy, including what kind of dermatoscope to buy, how to incorporate dermoscopy into a dermatology practice, and how to efficiently perform skin examinations using a dermatoscope. She also emphasized the importance of attending courses and workshops to learn how to utilize dermoscopy and other noninvasive imaging devices effectively.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
At the 19th Annual Mount Sinai Winter Symposium, Dr. Orit Markowitz addressed some common questions physicians have about dermoscopy, including what kind of dermatoscope to buy, how to incorporate dermoscopy into a dermatology practice, and how to efficiently perform skin examinations using a dermatoscope. She also emphasized the importance of attending courses and workshops to learn how to utilize dermoscopy and other noninvasive imaging devices effectively.