User login
Limited-Incision Knotless Achilles Tendon Repair
The incidence of midsubstance Achilles tendon ruptures is increasing in patients 30 years to 50 years of age, and more than 50% of these injuries occur during recreational basketball.1,2 Achilles ruptures occur more in deconditioned individuals engaged in explosive push-off and jumping activities. Management of these injuries has been controversial over the past decade; there is no consensus on nonoperative treatment, surgical repair, or optimal repair technique.1,3-7 According to American Academy of Orthopaedic Surgeons (AAOS) clinical practice guidelines, limited-incision approaches have fewer overall complications relative to traditional open repair.3,4
Modern repair techniques, such as the Percutaneous Achilles Repair System (PARS; Arthrex), combine limited soft-tissue dissection with percutaneous suture insertion and knot tying.1,8 This limited-incision technique, employed since 2010, uses a 2-cm transverse incision and nondisposable metal jig with divergent needle passes and locking suture fixation options to secure and fix both tendon ends with minimal dissection of skin, subcutaneous tissue, and paratenon. A review of 270 surgically treated Achilles tendon ruptures (101 PARS, 169 traditional open repair) found that, compared with the open repair group, the PARS group had significantly shorter operative times and more patients returning to baseline physical activities within 5 months after surgery.1 Although the difference was not statistically significant, the overall postoperative complication rate was 5% for the PARS group and 11% for the open repair group. The PARS group had no cases of sural neuritis or deep infection requiring reoperation.
Although the PARS technique has had good outcomes with few complications, care must be taken during surgery to prevent sutures from pulling through the tendon near the rupture site, which can result from overtensioning and from suture knot irritation against superficial soft tissues. Given these potential issues, the PARS procedure was modified (Achilles Midsubstance SpeedBridge; Arthrex) to provide knotless restoration of musculotendinous length in a reliable, reproducible fashion and direct fixation of tendon to bone for early mobilization.9 This new procedure bypasses suture fixation in the compromised tendon ends adjacent to the rupture site, thereby reducing suture slippage and allowing for potential early range of motion and weight-bearing relative to previous techniques. Preliminary results from a cohort of 34 patients treated with this technique are promising: Average return to baseline activities was 18.2 weeks (range, 9-26 weeks), and there were no wound complications, nerve injuries, or reruptures.9Indications are overall health and an acute midsubstance Achilles rupture that presents within 3 weeks after injury (the time limit is used to ensure that both tendon ends can be mobilized and repaired to appropriate length). A relative contraindication is delayed presentation (≥4 weeks), which may require open reconstruction in combination with V-Y lengthening or other adjuvant procedures. Other relative contraindications are insertional rupture, Achilles tendinopathy, and a significant medical comorbidity that prohibits surgical intervention.
Surgical Technique
Operating Room Setup and Approach
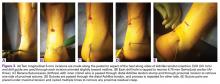
The patient is positioned prone with chest rolls and kneepads and with arms at <90° of abduction (Figures 1A-1E).
A “no-touch” technique is used without pickups, and soft tissues are carefully dissected with small scissors down to the paratenon. The sural nerve typically is not visible in the operative field, but, if it is, it can be dissected out and retracted out of the way. A transverse incision is made through the paratenon, and expression of rupture hematoma often follows. Paratenon preservation is key in minimizing disruption of the native vascular supply of the tendon and allowing for repair at the end of the case. A freer can be placed within the wound to confirm that the center of the rupture has been identified.
An Allis clamp is inserted into the wound, and the proximal tendon stump is secured and then pulled about 1 cm through the wound. A freer is circumferentially run along the sides of the proximal tendon to release any potential adhesions that may limit distal excursion.
PARS Jig Insertion and Suture Passing
The PARS jig is inserted into the wound with the inner prongs in the narrowest position possible. The curved jig is inserted proximally, and the center turn wheel is used to widen the inner prongs so they can slide along the sides of the tendon in the paratenon. Proper jig placement should be smooth and encounter little resistance. The proximal tendon is in a superficial location and can be palpated within the prongs of the jig to double-check that the tendon is centered within the jig. A frequent error is to insert the jig too deep, which subsequently causes needles and sutures to miss the tendon and pull through.
Keeping the jig centralized in neutral rotation minimizes improper suture passing and avoids iatrogenic injury to the medial and lateral neurovascular structures. During suture passing, all needles (1.6 mm) with nitinol loops are first used unloaded without suture. The first 2 needles are inserted into their respective, numbered holes, through the tendon, and then through the opposite side of the jig. Each needle is checked to make sure that it does not pass outside the jig. Having 2 needles within the jig and tendon at all times during suture passing helps stabilize the jig and avoids adjacent suture piercing with the subsequent needle.
A No. 2 FiberWire suture (Arthrex) is then passed through the first hole using the needle suture passer and made even in length on both sides. The specific colors of the suture are not important, but the order of the sutures placed is. An assistant can write down the colors and order of the sutures passed. Before the second suture is passed, the first needle is inserted back through the jig and tendon into the third hole. The third and fourth sutures (green-striped) differ from the other sutures in that one end has a loop and the other has a tail, and they are passed in an oblique, crossing pattern. These sutures later help create a locking suture on either side of the tendon.
After these sutures are passed, the final result should be 1 green-striped loop and 1 green-striped tail on either side of the tendon. The fifth suture is passed straight across the tendon in a trajectory similar to that of the first suture. In large laborers, obese patients, and elite athletes, 2 additional green-striped sutures can be passed through the optional sixth and seventh holes to create an additional locking suture.
PARS Jig Removal and Suture Management
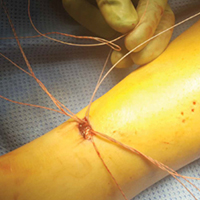
After all sutures are passed, the turn wheel is used to narrow the inner prongs while gentle, controlled tension is applied to the jig to remove it from the wound (Figures 2A-2C).
Pullout of any suture from the tendon indicates that the tendon was not centered in the jig or was not proximal enough along the tendon during suture passing. If a suture pulls out, it is removed, and the previous steps are repeated with close attention paid to tendon positioning within the jig. It is not advised to extend the incision longitudinally on either end of the transverse incision, as doing so can lead to potential wound-healing complications. After proximal fixation is achieved, all sutures on each side of the tendon are neatly spread apart in the following order from proximal to distal: first suture, second suture, looped green-striped (third) suture, tail green-striped (fourth) suture, fifth suture. The second suture on both sides is then looped around the 2 green-striped sutures and back proximally through the looped end of the green-striped suture.
The green-striped suture tail is pulled through the tendon to the opposite side to create a locking suture on both sides of the tendon. In the end, there are 2 nonlocking sutures and 1 locking suture on either side of the tendon. Each pair of sutures is pulled distally to confirm fixation and remove any initial suture creep from the system. A hemostat is placed on each group of 3 sutures to keep them out of the way during distal anchor preparation.
Distal Anchor Preparation and Banana SutureLasso Passing
Two longitudinal 5-mm incisions are made along the posterior aspect of the heel just distal to the area of maximal heel convexity. Incisions are spaced 1.5 cm apart along the sides of the Achilles tendon insertion. A 3.5-mm drill and a drill guide are used through each incision and placed flush against bone (Figures 3A-3E).
A Banana SutureLasso (Arthrex) with inner nitinol wire is passed through the center of the distal Achilles tendon stump and out the proximal incision to retrieve one side of the proximal sutures. SutureLasso passage through tendon can be facilitated with tactile feedback. The surgeon’s nondominant thumb is placed directly against the distal tendon while the dominant hand grasps the SutureLasso with the thumb near the tip. As the SutureLasso is advanced proximally through the tendon, the surgeon can feel its tip meeting mild resistance. Confirm that the tip of the SutureLasso is in the center of the distal tendon by direct visual inspection through the wound.
The inner nitinol wire is advanced 2 cm to 3 cm out of the tip of the SutureLasso, and sutures are passed through the distal Achilles tendon. During suture passing, the nitinol wire is drawn back to the tip of the SutureLasso, and then the entire SutureLasso is removed from the distal incision. Trying to pass the sutures only through the inner nitinol wire can result in suture tangling and increased resistance. The process is then repeated for the sutures on the opposite side. Suture pairs are placed under maximal tension and cycled multiple times (5-10) to remove any residual proximal suture creep.10
Achilles Tensioning and Anchor Insertion
The ankle is plantar flexed to tension the Achilles tendon relative to the contralateral limb and is held in place by an assistant (Figures 4A-4E).
Position of the drill holes can be rechecked with a Kirschner wire before anchor insertion, as their relative position changes with ankle plantar flexion. It is not necessary to premeasure and adjust suture length at the tip of the anchor as in other blind tunnel anchor insertion techniques (eg, InternalBrace; Arthrex). Once the anchor tip is malleted into bone, the free suture ends are released to avoid overtensioning the tendon. Before the anchor insertion handle is completely removed, the tip of a mosquito clamp can be used to feel the bony surface and confirm the anchor is completely seated.
With the ankle still held in the appropriate amount of plantarflexion, the process is repeated and the other SwiveLock anchor inserted. Sutures are cut flush with the anchor, and the surgeon performs wound irrigation and layered closure, with absorbable suture, of the paratenon and subcutaneous tissues. After skin closure with nylon suture, resting ankle plantarflexion is assessed and the Thompson test performed. The patient is placed in a well-padded non-weight-bearing plantar flexion splint for incision and initial tendon healing during the first 2 weeks after surgery.
Discussion
A key aspect of recovery is the balance achieved between skin and tendon healing and early mobilization, as outcomes of surgical repair of Achilles ruptures are improved with early weight-bearing and functional rehabilitation.11-13 Some surgeons recommend weight-bearing immediately after surgery, given the direct tendon-to-bone fixation achieved with repair.9 I prefer 2 weeks of non-weight-bearing, which allows the skin to heal adequately and the initial soft-tissue inflammation to subside. If the incision is healed at 2 weeks, sutures are removed, and the patient is transitioned to a tall, non-weight-bearing CAM (controlled ankle motion) boot, worn for 1 to 2 weeks with initiation of gentle ankle range-of-motion exercises. If there is any concern about wound healing, sutures are maintained for another 1 to 2 weeks.
Between 3 and 8 weeks after surgery, progressive weight-bearing is initiated with a peel-away heel lift (~2 cm thick total, 3 layers). Each lift layer is removed as pain allows, every 2 to 3 days. The goal is full weight-bearing with the foot flat 5 to 6 weeks after surgery. Physical therapy focusing on ankle motion and gentle Achilles stretching and strengthening is started 5 to 6 weeks after surgery, depending on progression and functional needs. Between 8 and 12 weeks after surgery, the patient is transitioned to normal shoe wear with increased activities. Running and jumping are allowed, as pain and swelling allow, starting at 12 weeks.
Although preliminary outcomes and experience with the Achilles Midsubstance SpeedBridge have been favorable, long-term clinical and functional studies are needed to determine the specific advantages and disadvantages of this new technique relative to other repairs. The main benefits observed thus far are reduced subjective knot tying and tensioning, decreased reliance on suture fixation in compromised tissue at the rupture site, reduced risk of FiberWire knot irritation of superficial soft tissues, lower risk of distal suture pullout, and earlier mobilization owing to bony fixation of the tendon. Potential complications include anchor-site heel pain caused by prominent anchors or by the bone edema that occurs when a patient increases physical activity by a significant amount at 12 weeks.9 Heel pain caused by bone edema resolves by 20 weeks without intervention.
Stress shielding of the distal Achilles tendon is a theoretical concern given the tendon–bone construct, but there have been no reports of tendon atrophy or repair failure caused by stress shielding. The original PARS technique was often used to create Achilles tension with the ankle maximally plantar flexed—the idea being that the tendon would gradually stretch over time.1 Overtensioning the Achilles repair is a potential complication with the SpeedBridge, as the distal anchors provide a more rigid point of distal fixation. Surgeons can avoid this complication by cycling the sutures to remove any residual creep and then tensioning the Achilles according to the contralateral limb and/or palpating tendon opposition at the rupture site.
Overall, this new limited-incision knotless Achilles tendon repair technique allows for minimal soft-tissue dissection, restoration of Achilles musculotendinous length, and direct tendon-to-bone fixation. Early results are promising, but long-term clinical outcomes and comparative analysis are needed. In addition, many details of this technique must be clarified—including incidence of short- and long-term complications in larger cohorts, optimal suture material and configuration, and risks and benefits of immediate (<2 weeks) and delayed (2-4 weeks) weight-bearing.
Am J Orthop. 2016;45(7):E487-E492. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. Hsu AR, Jones CP, Cohen BE, Davis WH, Ellington JK, Anderson RB. Clinical outcomes and complications of Percutaneous Achilles Repair System versus open technique for acute Achilles tendon ruptures. Foot Ankle Int. 2015;36(11):1279-1286.
2. Raikin SM, Garras DN, Krapchev PV. Achilles tendon injuries in a United States population. Foot Ankle Int. 2013;34(4):475-480.
3. Chiodo CP, Glazebrook M, Bluman EM, et al; American Academy of Orthopaedic Surgeons. American Academy of Orthopaedic Surgeons clinical practice guideline on treatment of Achilles tendon rupture. J Bone Joint Surg Am. 2010;92(14):2466-2468.
4. Chiodo CP, Glazebrook M, Bluman EM, et al; American Academy of Orthopaedic Surgeons. Diagnosis and treatment of acute Achilles tendon rupture. J Am Acad Orthop Surg. 2010;18(8):503-510.
5. Khan RJ, Fick D, Keogh A, Crawford J, Brammar T, Parker M. Treatment of acute Achilles tendon ruptures. A meta-analysis of randomized, controlled trials. J Bone Joint Surg Am. 2005;87(10):2202-2210.
6. Renninger CH, Kuhn K, Fellars T, Youngblood S, Bellamy J. Operative and nonoperative management of Achilles tendon ruptures in active duty military population. Foot Ankle Int. 2016;37(3):269-273.
7. Khan RJ, Carey Smith RL. Surgical interventions for treating acute Achilles tendon ruptures. Cochrane Database Syst Rev. 2010;(9):CD003674.
8. McCullough KA, Shaw CM, Anderson RB. Mini-open repair of Achilles rupture in the National Football League. J Surg Orthop Adv. 2014;23(4):179-183.
9. McWilliam JR, Mackay G. The internal brace for midsubstance Achilles ruptures. Foot Ankle Int. 2016;37(7):794-800.
10. Clanton TO, Haytmanek CT, Williams BT, et al. A biomechanical comparison of an open repair and 3 minimally invasive percutaneous Achilles tendon repair techniques during a simulated, progressive rehabilitation protocol. Am J Sports Med. 2015;43(8):1957-1964.
11. Aoki M, Ogiwara N, Ohta T, Nabeta Y. Early active motion and weightbearing after cross-stitch Achilles tendon repair. Am J Sports Med. 1998;26(6):794-800.
12. Kangas J, Pajala A, Ohtonen P, Leppilahti J. Achilles tendon elongation after rupture repair: a randomized comparison of 2 postoperative regimens. Am J Sports Med. 2007;35(1):59-64.
13. Kangas J, Pajala A, Siira P, Hämäläinen M, Leppilahti J. Early functional treatment versus early immobilization in tension of the musculotendinous unit after Achilles rupture repair: a prospective, randomized, clinical study. J Trauma. 2003;54(6):1171-1180.
The incidence of midsubstance Achilles tendon ruptures is increasing in patients 30 years to 50 years of age, and more than 50% of these injuries occur during recreational basketball.1,2 Achilles ruptures occur more in deconditioned individuals engaged in explosive push-off and jumping activities. Management of these injuries has been controversial over the past decade; there is no consensus on nonoperative treatment, surgical repair, or optimal repair technique.1,3-7 According to American Academy of Orthopaedic Surgeons (AAOS) clinical practice guidelines, limited-incision approaches have fewer overall complications relative to traditional open repair.3,4
Modern repair techniques, such as the Percutaneous Achilles Repair System (PARS; Arthrex), combine limited soft-tissue dissection with percutaneous suture insertion and knot tying.1,8 This limited-incision technique, employed since 2010, uses a 2-cm transverse incision and nondisposable metal jig with divergent needle passes and locking suture fixation options to secure and fix both tendon ends with minimal dissection of skin, subcutaneous tissue, and paratenon. A review of 270 surgically treated Achilles tendon ruptures (101 PARS, 169 traditional open repair) found that, compared with the open repair group, the PARS group had significantly shorter operative times and more patients returning to baseline physical activities within 5 months after surgery.1 Although the difference was not statistically significant, the overall postoperative complication rate was 5% for the PARS group and 11% for the open repair group. The PARS group had no cases of sural neuritis or deep infection requiring reoperation.
Although the PARS technique has had good outcomes with few complications, care must be taken during surgery to prevent sutures from pulling through the tendon near the rupture site, which can result from overtensioning and from suture knot irritation against superficial soft tissues. Given these potential issues, the PARS procedure was modified (Achilles Midsubstance SpeedBridge; Arthrex) to provide knotless restoration of musculotendinous length in a reliable, reproducible fashion and direct fixation of tendon to bone for early mobilization.9 This new procedure bypasses suture fixation in the compromised tendon ends adjacent to the rupture site, thereby reducing suture slippage and allowing for potential early range of motion and weight-bearing relative to previous techniques. Preliminary results from a cohort of 34 patients treated with this technique are promising: Average return to baseline activities was 18.2 weeks (range, 9-26 weeks), and there were no wound complications, nerve injuries, or reruptures.9Indications are overall health and an acute midsubstance Achilles rupture that presents within 3 weeks after injury (the time limit is used to ensure that both tendon ends can be mobilized and repaired to appropriate length). A relative contraindication is delayed presentation (≥4 weeks), which may require open reconstruction in combination with V-Y lengthening or other adjuvant procedures. Other relative contraindications are insertional rupture, Achilles tendinopathy, and a significant medical comorbidity that prohibits surgical intervention.
Surgical Technique
Operating Room Setup and Approach
The patient is positioned prone with chest rolls and kneepads and with arms at <90° of abduction (Figures 1A-1E).
A “no-touch” technique is used without pickups, and soft tissues are carefully dissected with small scissors down to the paratenon. The sural nerve typically is not visible in the operative field, but, if it is, it can be dissected out and retracted out of the way. A transverse incision is made through the paratenon, and expression of rupture hematoma often follows. Paratenon preservation is key in minimizing disruption of the native vascular supply of the tendon and allowing for repair at the end of the case. A freer can be placed within the wound to confirm that the center of the rupture has been identified.
An Allis clamp is inserted into the wound, and the proximal tendon stump is secured and then pulled about 1 cm through the wound. A freer is circumferentially run along the sides of the proximal tendon to release any potential adhesions that may limit distal excursion.
PARS Jig Insertion and Suture Passing
The PARS jig is inserted into the wound with the inner prongs in the narrowest position possible. The curved jig is inserted proximally, and the center turn wheel is used to widen the inner prongs so they can slide along the sides of the tendon in the paratenon. Proper jig placement should be smooth and encounter little resistance. The proximal tendon is in a superficial location and can be palpated within the prongs of the jig to double-check that the tendon is centered within the jig. A frequent error is to insert the jig too deep, which subsequently causes needles and sutures to miss the tendon and pull through.
Keeping the jig centralized in neutral rotation minimizes improper suture passing and avoids iatrogenic injury to the medial and lateral neurovascular structures. During suture passing, all needles (1.6 mm) with nitinol loops are first used unloaded without suture. The first 2 needles are inserted into their respective, numbered holes, through the tendon, and then through the opposite side of the jig. Each needle is checked to make sure that it does not pass outside the jig. Having 2 needles within the jig and tendon at all times during suture passing helps stabilize the jig and avoids adjacent suture piercing with the subsequent needle.
A No. 2 FiberWire suture (Arthrex) is then passed through the first hole using the needle suture passer and made even in length on both sides. The specific colors of the suture are not important, but the order of the sutures placed is. An assistant can write down the colors and order of the sutures passed. Before the second suture is passed, the first needle is inserted back through the jig and tendon into the third hole. The third and fourth sutures (green-striped) differ from the other sutures in that one end has a loop and the other has a tail, and they are passed in an oblique, crossing pattern. These sutures later help create a locking suture on either side of the tendon.
After these sutures are passed, the final result should be 1 green-striped loop and 1 green-striped tail on either side of the tendon. The fifth suture is passed straight across the tendon in a trajectory similar to that of the first suture. In large laborers, obese patients, and elite athletes, 2 additional green-striped sutures can be passed through the optional sixth and seventh holes to create an additional locking suture.
PARS Jig Removal and Suture Management
After all sutures are passed, the turn wheel is used to narrow the inner prongs while gentle, controlled tension is applied to the jig to remove it from the wound (Figures 2A-2C).
Pullout of any suture from the tendon indicates that the tendon was not centered in the jig or was not proximal enough along the tendon during suture passing. If a suture pulls out, it is removed, and the previous steps are repeated with close attention paid to tendon positioning within the jig. It is not advised to extend the incision longitudinally on either end of the transverse incision, as doing so can lead to potential wound-healing complications. After proximal fixation is achieved, all sutures on each side of the tendon are neatly spread apart in the following order from proximal to distal: first suture, second suture, looped green-striped (third) suture, tail green-striped (fourth) suture, fifth suture. The second suture on both sides is then looped around the 2 green-striped sutures and back proximally through the looped end of the green-striped suture.
The green-striped suture tail is pulled through the tendon to the opposite side to create a locking suture on both sides of the tendon. In the end, there are 2 nonlocking sutures and 1 locking suture on either side of the tendon. Each pair of sutures is pulled distally to confirm fixation and remove any initial suture creep from the system. A hemostat is placed on each group of 3 sutures to keep them out of the way during distal anchor preparation.
Distal Anchor Preparation and Banana SutureLasso Passing
Two longitudinal 5-mm incisions are made along the posterior aspect of the heel just distal to the area of maximal heel convexity. Incisions are spaced 1.5 cm apart along the sides of the Achilles tendon insertion. A 3.5-mm drill and a drill guide are used through each incision and placed flush against bone (Figures 3A-3E).
A Banana SutureLasso (Arthrex) with inner nitinol wire is passed through the center of the distal Achilles tendon stump and out the proximal incision to retrieve one side of the proximal sutures. SutureLasso passage through tendon can be facilitated with tactile feedback. The surgeon’s nondominant thumb is placed directly against the distal tendon while the dominant hand grasps the SutureLasso with the thumb near the tip. As the SutureLasso is advanced proximally through the tendon, the surgeon can feel its tip meeting mild resistance. Confirm that the tip of the SutureLasso is in the center of the distal tendon by direct visual inspection through the wound.
The inner nitinol wire is advanced 2 cm to 3 cm out of the tip of the SutureLasso, and sutures are passed through the distal Achilles tendon. During suture passing, the nitinol wire is drawn back to the tip of the SutureLasso, and then the entire SutureLasso is removed from the distal incision. Trying to pass the sutures only through the inner nitinol wire can result in suture tangling and increased resistance. The process is then repeated for the sutures on the opposite side. Suture pairs are placed under maximal tension and cycled multiple times (5-10) to remove any residual proximal suture creep.10
Achilles Tensioning and Anchor Insertion
The ankle is plantar flexed to tension the Achilles tendon relative to the contralateral limb and is held in place by an assistant (Figures 4A-4E).
Position of the drill holes can be rechecked with a Kirschner wire before anchor insertion, as their relative position changes with ankle plantar flexion. It is not necessary to premeasure and adjust suture length at the tip of the anchor as in other blind tunnel anchor insertion techniques (eg, InternalBrace; Arthrex). Once the anchor tip is malleted into bone, the free suture ends are released to avoid overtensioning the tendon. Before the anchor insertion handle is completely removed, the tip of a mosquito clamp can be used to feel the bony surface and confirm the anchor is completely seated.
With the ankle still held in the appropriate amount of plantarflexion, the process is repeated and the other SwiveLock anchor inserted. Sutures are cut flush with the anchor, and the surgeon performs wound irrigation and layered closure, with absorbable suture, of the paratenon and subcutaneous tissues. After skin closure with nylon suture, resting ankle plantarflexion is assessed and the Thompson test performed. The patient is placed in a well-padded non-weight-bearing plantar flexion splint for incision and initial tendon healing during the first 2 weeks after surgery.
Discussion
A key aspect of recovery is the balance achieved between skin and tendon healing and early mobilization, as outcomes of surgical repair of Achilles ruptures are improved with early weight-bearing and functional rehabilitation.11-13 Some surgeons recommend weight-bearing immediately after surgery, given the direct tendon-to-bone fixation achieved with repair.9 I prefer 2 weeks of non-weight-bearing, which allows the skin to heal adequately and the initial soft-tissue inflammation to subside. If the incision is healed at 2 weeks, sutures are removed, and the patient is transitioned to a tall, non-weight-bearing CAM (controlled ankle motion) boot, worn for 1 to 2 weeks with initiation of gentle ankle range-of-motion exercises. If there is any concern about wound healing, sutures are maintained for another 1 to 2 weeks.
Between 3 and 8 weeks after surgery, progressive weight-bearing is initiated with a peel-away heel lift (~2 cm thick total, 3 layers). Each lift layer is removed as pain allows, every 2 to 3 days. The goal is full weight-bearing with the foot flat 5 to 6 weeks after surgery. Physical therapy focusing on ankle motion and gentle Achilles stretching and strengthening is started 5 to 6 weeks after surgery, depending on progression and functional needs. Between 8 and 12 weeks after surgery, the patient is transitioned to normal shoe wear with increased activities. Running and jumping are allowed, as pain and swelling allow, starting at 12 weeks.
Although preliminary outcomes and experience with the Achilles Midsubstance SpeedBridge have been favorable, long-term clinical and functional studies are needed to determine the specific advantages and disadvantages of this new technique relative to other repairs. The main benefits observed thus far are reduced subjective knot tying and tensioning, decreased reliance on suture fixation in compromised tissue at the rupture site, reduced risk of FiberWire knot irritation of superficial soft tissues, lower risk of distal suture pullout, and earlier mobilization owing to bony fixation of the tendon. Potential complications include anchor-site heel pain caused by prominent anchors or by the bone edema that occurs when a patient increases physical activity by a significant amount at 12 weeks.9 Heel pain caused by bone edema resolves by 20 weeks without intervention.
Stress shielding of the distal Achilles tendon is a theoretical concern given the tendon–bone construct, but there have been no reports of tendon atrophy or repair failure caused by stress shielding. The original PARS technique was often used to create Achilles tension with the ankle maximally plantar flexed—the idea being that the tendon would gradually stretch over time.1 Overtensioning the Achilles repair is a potential complication with the SpeedBridge, as the distal anchors provide a more rigid point of distal fixation. Surgeons can avoid this complication by cycling the sutures to remove any residual creep and then tensioning the Achilles according to the contralateral limb and/or palpating tendon opposition at the rupture site.
Overall, this new limited-incision knotless Achilles tendon repair technique allows for minimal soft-tissue dissection, restoration of Achilles musculotendinous length, and direct tendon-to-bone fixation. Early results are promising, but long-term clinical outcomes and comparative analysis are needed. In addition, many details of this technique must be clarified—including incidence of short- and long-term complications in larger cohorts, optimal suture material and configuration, and risks and benefits of immediate (<2 weeks) and delayed (2-4 weeks) weight-bearing.
Am J Orthop. 2016;45(7):E487-E492. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
The incidence of midsubstance Achilles tendon ruptures is increasing in patients 30 years to 50 years of age, and more than 50% of these injuries occur during recreational basketball.1,2 Achilles ruptures occur more in deconditioned individuals engaged in explosive push-off and jumping activities. Management of these injuries has been controversial over the past decade; there is no consensus on nonoperative treatment, surgical repair, or optimal repair technique.1,3-7 According to American Academy of Orthopaedic Surgeons (AAOS) clinical practice guidelines, limited-incision approaches have fewer overall complications relative to traditional open repair.3,4
Modern repair techniques, such as the Percutaneous Achilles Repair System (PARS; Arthrex), combine limited soft-tissue dissection with percutaneous suture insertion and knot tying.1,8 This limited-incision technique, employed since 2010, uses a 2-cm transverse incision and nondisposable metal jig with divergent needle passes and locking suture fixation options to secure and fix both tendon ends with minimal dissection of skin, subcutaneous tissue, and paratenon. A review of 270 surgically treated Achilles tendon ruptures (101 PARS, 169 traditional open repair) found that, compared with the open repair group, the PARS group had significantly shorter operative times and more patients returning to baseline physical activities within 5 months after surgery.1 Although the difference was not statistically significant, the overall postoperative complication rate was 5% for the PARS group and 11% for the open repair group. The PARS group had no cases of sural neuritis or deep infection requiring reoperation.
Although the PARS technique has had good outcomes with few complications, care must be taken during surgery to prevent sutures from pulling through the tendon near the rupture site, which can result from overtensioning and from suture knot irritation against superficial soft tissues. Given these potential issues, the PARS procedure was modified (Achilles Midsubstance SpeedBridge; Arthrex) to provide knotless restoration of musculotendinous length in a reliable, reproducible fashion and direct fixation of tendon to bone for early mobilization.9 This new procedure bypasses suture fixation in the compromised tendon ends adjacent to the rupture site, thereby reducing suture slippage and allowing for potential early range of motion and weight-bearing relative to previous techniques. Preliminary results from a cohort of 34 patients treated with this technique are promising: Average return to baseline activities was 18.2 weeks (range, 9-26 weeks), and there were no wound complications, nerve injuries, or reruptures.9Indications are overall health and an acute midsubstance Achilles rupture that presents within 3 weeks after injury (the time limit is used to ensure that both tendon ends can be mobilized and repaired to appropriate length). A relative contraindication is delayed presentation (≥4 weeks), which may require open reconstruction in combination with V-Y lengthening or other adjuvant procedures. Other relative contraindications are insertional rupture, Achilles tendinopathy, and a significant medical comorbidity that prohibits surgical intervention.
Surgical Technique
Operating Room Setup and Approach
The patient is positioned prone with chest rolls and kneepads and with arms at <90° of abduction (Figures 1A-1E).
A “no-touch” technique is used without pickups, and soft tissues are carefully dissected with small scissors down to the paratenon. The sural nerve typically is not visible in the operative field, but, if it is, it can be dissected out and retracted out of the way. A transverse incision is made through the paratenon, and expression of rupture hematoma often follows. Paratenon preservation is key in minimizing disruption of the native vascular supply of the tendon and allowing for repair at the end of the case. A freer can be placed within the wound to confirm that the center of the rupture has been identified.
An Allis clamp is inserted into the wound, and the proximal tendon stump is secured and then pulled about 1 cm through the wound. A freer is circumferentially run along the sides of the proximal tendon to release any potential adhesions that may limit distal excursion.
PARS Jig Insertion and Suture Passing
The PARS jig is inserted into the wound with the inner prongs in the narrowest position possible. The curved jig is inserted proximally, and the center turn wheel is used to widen the inner prongs so they can slide along the sides of the tendon in the paratenon. Proper jig placement should be smooth and encounter little resistance. The proximal tendon is in a superficial location and can be palpated within the prongs of the jig to double-check that the tendon is centered within the jig. A frequent error is to insert the jig too deep, which subsequently causes needles and sutures to miss the tendon and pull through.
Keeping the jig centralized in neutral rotation minimizes improper suture passing and avoids iatrogenic injury to the medial and lateral neurovascular structures. During suture passing, all needles (1.6 mm) with nitinol loops are first used unloaded without suture. The first 2 needles are inserted into their respective, numbered holes, through the tendon, and then through the opposite side of the jig. Each needle is checked to make sure that it does not pass outside the jig. Having 2 needles within the jig and tendon at all times during suture passing helps stabilize the jig and avoids adjacent suture piercing with the subsequent needle.
A No. 2 FiberWire suture (Arthrex) is then passed through the first hole using the needle suture passer and made even in length on both sides. The specific colors of the suture are not important, but the order of the sutures placed is. An assistant can write down the colors and order of the sutures passed. Before the second suture is passed, the first needle is inserted back through the jig and tendon into the third hole. The third and fourth sutures (green-striped) differ from the other sutures in that one end has a loop and the other has a tail, and they are passed in an oblique, crossing pattern. These sutures later help create a locking suture on either side of the tendon.
After these sutures are passed, the final result should be 1 green-striped loop and 1 green-striped tail on either side of the tendon. The fifth suture is passed straight across the tendon in a trajectory similar to that of the first suture. In large laborers, obese patients, and elite athletes, 2 additional green-striped sutures can be passed through the optional sixth and seventh holes to create an additional locking suture.
PARS Jig Removal and Suture Management
After all sutures are passed, the turn wheel is used to narrow the inner prongs while gentle, controlled tension is applied to the jig to remove it from the wound (Figures 2A-2C).
Pullout of any suture from the tendon indicates that the tendon was not centered in the jig or was not proximal enough along the tendon during suture passing. If a suture pulls out, it is removed, and the previous steps are repeated with close attention paid to tendon positioning within the jig. It is not advised to extend the incision longitudinally on either end of the transverse incision, as doing so can lead to potential wound-healing complications. After proximal fixation is achieved, all sutures on each side of the tendon are neatly spread apart in the following order from proximal to distal: first suture, second suture, looped green-striped (third) suture, tail green-striped (fourth) suture, fifth suture. The second suture on both sides is then looped around the 2 green-striped sutures and back proximally through the looped end of the green-striped suture.
The green-striped suture tail is pulled through the tendon to the opposite side to create a locking suture on both sides of the tendon. In the end, there are 2 nonlocking sutures and 1 locking suture on either side of the tendon. Each pair of sutures is pulled distally to confirm fixation and remove any initial suture creep from the system. A hemostat is placed on each group of 3 sutures to keep them out of the way during distal anchor preparation.
Distal Anchor Preparation and Banana SutureLasso Passing
Two longitudinal 5-mm incisions are made along the posterior aspect of the heel just distal to the area of maximal heel convexity. Incisions are spaced 1.5 cm apart along the sides of the Achilles tendon insertion. A 3.5-mm drill and a drill guide are used through each incision and placed flush against bone (Figures 3A-3E).
A Banana SutureLasso (Arthrex) with inner nitinol wire is passed through the center of the distal Achilles tendon stump and out the proximal incision to retrieve one side of the proximal sutures. SutureLasso passage through tendon can be facilitated with tactile feedback. The surgeon’s nondominant thumb is placed directly against the distal tendon while the dominant hand grasps the SutureLasso with the thumb near the tip. As the SutureLasso is advanced proximally through the tendon, the surgeon can feel its tip meeting mild resistance. Confirm that the tip of the SutureLasso is in the center of the distal tendon by direct visual inspection through the wound.
The inner nitinol wire is advanced 2 cm to 3 cm out of the tip of the SutureLasso, and sutures are passed through the distal Achilles tendon. During suture passing, the nitinol wire is drawn back to the tip of the SutureLasso, and then the entire SutureLasso is removed from the distal incision. Trying to pass the sutures only through the inner nitinol wire can result in suture tangling and increased resistance. The process is then repeated for the sutures on the opposite side. Suture pairs are placed under maximal tension and cycled multiple times (5-10) to remove any residual proximal suture creep.10
Achilles Tensioning and Anchor Insertion
The ankle is plantar flexed to tension the Achilles tendon relative to the contralateral limb and is held in place by an assistant (Figures 4A-4E).
Position of the drill holes can be rechecked with a Kirschner wire before anchor insertion, as their relative position changes with ankle plantar flexion. It is not necessary to premeasure and adjust suture length at the tip of the anchor as in other blind tunnel anchor insertion techniques (eg, InternalBrace; Arthrex). Once the anchor tip is malleted into bone, the free suture ends are released to avoid overtensioning the tendon. Before the anchor insertion handle is completely removed, the tip of a mosquito clamp can be used to feel the bony surface and confirm the anchor is completely seated.
With the ankle still held in the appropriate amount of plantarflexion, the process is repeated and the other SwiveLock anchor inserted. Sutures are cut flush with the anchor, and the surgeon performs wound irrigation and layered closure, with absorbable suture, of the paratenon and subcutaneous tissues. After skin closure with nylon suture, resting ankle plantarflexion is assessed and the Thompson test performed. The patient is placed in a well-padded non-weight-bearing plantar flexion splint for incision and initial tendon healing during the first 2 weeks after surgery.
Discussion
A key aspect of recovery is the balance achieved between skin and tendon healing and early mobilization, as outcomes of surgical repair of Achilles ruptures are improved with early weight-bearing and functional rehabilitation.11-13 Some surgeons recommend weight-bearing immediately after surgery, given the direct tendon-to-bone fixation achieved with repair.9 I prefer 2 weeks of non-weight-bearing, which allows the skin to heal adequately and the initial soft-tissue inflammation to subside. If the incision is healed at 2 weeks, sutures are removed, and the patient is transitioned to a tall, non-weight-bearing CAM (controlled ankle motion) boot, worn for 1 to 2 weeks with initiation of gentle ankle range-of-motion exercises. If there is any concern about wound healing, sutures are maintained for another 1 to 2 weeks.
Between 3 and 8 weeks after surgery, progressive weight-bearing is initiated with a peel-away heel lift (~2 cm thick total, 3 layers). Each lift layer is removed as pain allows, every 2 to 3 days. The goal is full weight-bearing with the foot flat 5 to 6 weeks after surgery. Physical therapy focusing on ankle motion and gentle Achilles stretching and strengthening is started 5 to 6 weeks after surgery, depending on progression and functional needs. Between 8 and 12 weeks after surgery, the patient is transitioned to normal shoe wear with increased activities. Running and jumping are allowed, as pain and swelling allow, starting at 12 weeks.
Although preliminary outcomes and experience with the Achilles Midsubstance SpeedBridge have been favorable, long-term clinical and functional studies are needed to determine the specific advantages and disadvantages of this new technique relative to other repairs. The main benefits observed thus far are reduced subjective knot tying and tensioning, decreased reliance on suture fixation in compromised tissue at the rupture site, reduced risk of FiberWire knot irritation of superficial soft tissues, lower risk of distal suture pullout, and earlier mobilization owing to bony fixation of the tendon. Potential complications include anchor-site heel pain caused by prominent anchors or by the bone edema that occurs when a patient increases physical activity by a significant amount at 12 weeks.9 Heel pain caused by bone edema resolves by 20 weeks without intervention.
Stress shielding of the distal Achilles tendon is a theoretical concern given the tendon–bone construct, but there have been no reports of tendon atrophy or repair failure caused by stress shielding. The original PARS technique was often used to create Achilles tension with the ankle maximally plantar flexed—the idea being that the tendon would gradually stretch over time.1 Overtensioning the Achilles repair is a potential complication with the SpeedBridge, as the distal anchors provide a more rigid point of distal fixation. Surgeons can avoid this complication by cycling the sutures to remove any residual creep and then tensioning the Achilles according to the contralateral limb and/or palpating tendon opposition at the rupture site.
Overall, this new limited-incision knotless Achilles tendon repair technique allows for minimal soft-tissue dissection, restoration of Achilles musculotendinous length, and direct tendon-to-bone fixation. Early results are promising, but long-term clinical outcomes and comparative analysis are needed. In addition, many details of this technique must be clarified—including incidence of short- and long-term complications in larger cohorts, optimal suture material and configuration, and risks and benefits of immediate (<2 weeks) and delayed (2-4 weeks) weight-bearing.
Am J Orthop. 2016;45(7):E487-E492. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. Hsu AR, Jones CP, Cohen BE, Davis WH, Ellington JK, Anderson RB. Clinical outcomes and complications of Percutaneous Achilles Repair System versus open technique for acute Achilles tendon ruptures. Foot Ankle Int. 2015;36(11):1279-1286.
2. Raikin SM, Garras DN, Krapchev PV. Achilles tendon injuries in a United States population. Foot Ankle Int. 2013;34(4):475-480.
3. Chiodo CP, Glazebrook M, Bluman EM, et al; American Academy of Orthopaedic Surgeons. American Academy of Orthopaedic Surgeons clinical practice guideline on treatment of Achilles tendon rupture. J Bone Joint Surg Am. 2010;92(14):2466-2468.
4. Chiodo CP, Glazebrook M, Bluman EM, et al; American Academy of Orthopaedic Surgeons. Diagnosis and treatment of acute Achilles tendon rupture. J Am Acad Orthop Surg. 2010;18(8):503-510.
5. Khan RJ, Fick D, Keogh A, Crawford J, Brammar T, Parker M. Treatment of acute Achilles tendon ruptures. A meta-analysis of randomized, controlled trials. J Bone Joint Surg Am. 2005;87(10):2202-2210.
6. Renninger CH, Kuhn K, Fellars T, Youngblood S, Bellamy J. Operative and nonoperative management of Achilles tendon ruptures in active duty military population. Foot Ankle Int. 2016;37(3):269-273.
7. Khan RJ, Carey Smith RL. Surgical interventions for treating acute Achilles tendon ruptures. Cochrane Database Syst Rev. 2010;(9):CD003674.
8. McCullough KA, Shaw CM, Anderson RB. Mini-open repair of Achilles rupture in the National Football League. J Surg Orthop Adv. 2014;23(4):179-183.
9. McWilliam JR, Mackay G. The internal brace for midsubstance Achilles ruptures. Foot Ankle Int. 2016;37(7):794-800.
10. Clanton TO, Haytmanek CT, Williams BT, et al. A biomechanical comparison of an open repair and 3 minimally invasive percutaneous Achilles tendon repair techniques during a simulated, progressive rehabilitation protocol. Am J Sports Med. 2015;43(8):1957-1964.
11. Aoki M, Ogiwara N, Ohta T, Nabeta Y. Early active motion and weightbearing after cross-stitch Achilles tendon repair. Am J Sports Med. 1998;26(6):794-800.
12. Kangas J, Pajala A, Ohtonen P, Leppilahti J. Achilles tendon elongation after rupture repair: a randomized comparison of 2 postoperative regimens. Am J Sports Med. 2007;35(1):59-64.
13. Kangas J, Pajala A, Siira P, Hämäläinen M, Leppilahti J. Early functional treatment versus early immobilization in tension of the musculotendinous unit after Achilles rupture repair: a prospective, randomized, clinical study. J Trauma. 2003;54(6):1171-1180.
1. Hsu AR, Jones CP, Cohen BE, Davis WH, Ellington JK, Anderson RB. Clinical outcomes and complications of Percutaneous Achilles Repair System versus open technique for acute Achilles tendon ruptures. Foot Ankle Int. 2015;36(11):1279-1286.
2. Raikin SM, Garras DN, Krapchev PV. Achilles tendon injuries in a United States population. Foot Ankle Int. 2013;34(4):475-480.
3. Chiodo CP, Glazebrook M, Bluman EM, et al; American Academy of Orthopaedic Surgeons. American Academy of Orthopaedic Surgeons clinical practice guideline on treatment of Achilles tendon rupture. J Bone Joint Surg Am. 2010;92(14):2466-2468.
4. Chiodo CP, Glazebrook M, Bluman EM, et al; American Academy of Orthopaedic Surgeons. Diagnosis and treatment of acute Achilles tendon rupture. J Am Acad Orthop Surg. 2010;18(8):503-510.
5. Khan RJ, Fick D, Keogh A, Crawford J, Brammar T, Parker M. Treatment of acute Achilles tendon ruptures. A meta-analysis of randomized, controlled trials. J Bone Joint Surg Am. 2005;87(10):2202-2210.
6. Renninger CH, Kuhn K, Fellars T, Youngblood S, Bellamy J. Operative and nonoperative management of Achilles tendon ruptures in active duty military population. Foot Ankle Int. 2016;37(3):269-273.
7. Khan RJ, Carey Smith RL. Surgical interventions for treating acute Achilles tendon ruptures. Cochrane Database Syst Rev. 2010;(9):CD003674.
8. McCullough KA, Shaw CM, Anderson RB. Mini-open repair of Achilles rupture in the National Football League. J Surg Orthop Adv. 2014;23(4):179-183.
9. McWilliam JR, Mackay G. The internal brace for midsubstance Achilles ruptures. Foot Ankle Int. 2016;37(7):794-800.
10. Clanton TO, Haytmanek CT, Williams BT, et al. A biomechanical comparison of an open repair and 3 minimally invasive percutaneous Achilles tendon repair techniques during a simulated, progressive rehabilitation protocol. Am J Sports Med. 2015;43(8):1957-1964.
11. Aoki M, Ogiwara N, Ohta T, Nabeta Y. Early active motion and weightbearing after cross-stitch Achilles tendon repair. Am J Sports Med. 1998;26(6):794-800.
12. Kangas J, Pajala A, Ohtonen P, Leppilahti J. Achilles tendon elongation after rupture repair: a randomized comparison of 2 postoperative regimens. Am J Sports Med. 2007;35(1):59-64.
13. Kangas J, Pajala A, Siira P, Hämäläinen M, Leppilahti J. Early functional treatment versus early immobilization in tension of the musculotendinous unit after Achilles rupture repair: a prospective, randomized, clinical study. J Trauma. 2003;54(6):1171-1180.
Total Knee Arthroplasty With Retained Tibial Implants: The Role of Minimally Invasive Hardware Removal
Technique
The patient is positioned on a radiolucent table, and a mobile fluoroscopy unit is available. A tourniquet is applied to the upper thigh but typically is not inflated during the percutaneous hardware removal portion of the operation. It is crucial to have information on retained implants so the correct screwdrivers for screw removal can be selected. In addition, provisions for stripped screws should be made. In each of the 3 cases we managed, the Synthes Screw Removal Set was available. Presence of an implant system known to have problems with cold welding of screws (eg, Less Invasive Stabilization System; Synthes) may necessitate additional preparations, such as making conical extraction devices available.1
After preoperative administration of antibiotics, the surgeon typically removes only those proximal tibia screws that are preventing insertion of the tibial base plate. Fluoroscopic guidance is used to locate these screws and then remove them with percutaneous stab incisions. (Retained plates are not removed.) The exact method of localizing and removing the screws percutaneously is crucial. A small stab incision is made in the dermal layer. The number of stab incisions to be made depends on the number of screws to be removed. One small incision is needed for each screw hole. Occasionally mobilizing the skin and redirecting the screwdriver in the deep tissues can allow 2 screws to be removed through a single skin wound. The screwdriver head can be inserted through the muscle and fascial layers without the need for deep dissection. The plate is then felt with the screwdriver and the screw head located. It is very important that the screw head be adequately engaged to prevent stripping. The surgeon should not rush this step. The C-arm can be helpful here. Fluoroscopy not only can guide the screwdriver to the screw hole but can confirm the screwdriver is at right angles to the plate, not oblique. Only when the surgeon is completely satisfied that the screw head is well engaged should the attempt to back out the screw be made. If the screw strips, the screwdriver can be removed, and an attempt can be made to insert a percutaneous stripped screw removal device.1 If this fails, then the technique must be abandoned for a more traditional approach.
Plating complex tibial plateau fractures through a separate posteromedial approach is now popular.2 The deep location and screw orientation of posteromedial hardware make percutaneous removal unfeasible. In these cases, a separate posteromedial incision may be needed—usually posterior enough so it minimally compromises the anterior soft tissues. The incision typically uses the old posteromedial surgical scar but may not need to be as large as the original approach, as only selected screws need be removed. The saphenous neurovascular bundle may still be at risk, depending on the location of these incisions. The plate is not removed.
After the necessary screws are removed, the tourniquet can be inflated, if desired. The total knee arthroplasty (TKA) then proceeds in usual fashion through a single incision and a medial parapatellar arthrotomy.
Results
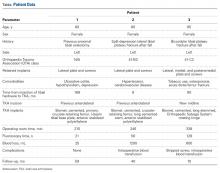
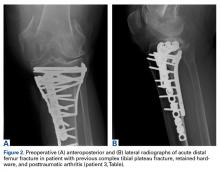
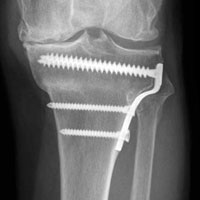
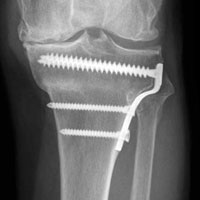
Between January 2009 and February 2014, Dr. Georgiadis converted 3 cases of retained tibial hardware and severe knee arthrosis to a TKA in a single operation. These cases were reviewed after Institutional Review Board approval was obtained. One patient underwent a closing-wedge high tibial osteotomy 14 years earlier, and the other 2 sustained tibial plateau fractures. Clinical details of the 3 cases are presented in the Table.
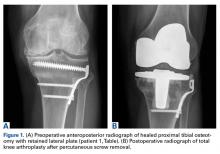
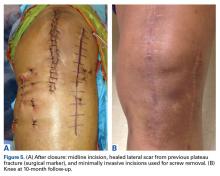
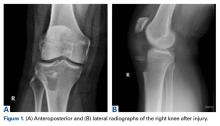
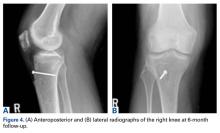
In 2 of the cases, anterolateral surgical scars were present. New, separate percutaneous stab incisions were used to remove screws, which meant less of the original skin incision could be used for the TKA (Figures 1A, 1B).
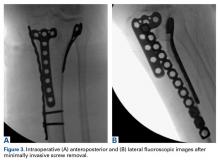
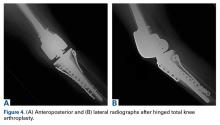
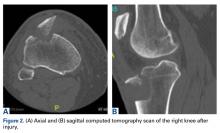
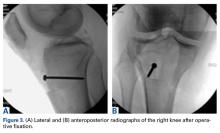
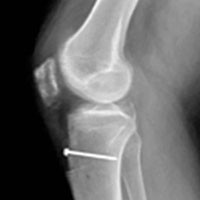
In the third case, involving multiple plates, a similar strategy was used, but an additional small posteromedial incision was required (Figures 2-5). The TKA then proceeded through a new midline incision. This case was performed for tibiofemoral arthrosis in the setting of an acute distal femur fracture, but this had no bearing on the technique.
Tibial base plates were inserted in the usual manner. Length and type of tibial stem were left to the discretion of the surgeon. There were no changes from the usual surgical technique. All patients went on to routine, uneventful wound healing. Follow-up ranged from 10 months to 59 months.
Discussion
If the decision is made to proceed with TKA after previous knee surgery, careful preoperative planning is needed.
For young patients with knee arthrosis and angular deformity, it has been recommended that proximal tibial osteotomy be performed to delay the need for joint replacement.3,4 Although a wide variety of osteotomy techniques is available, plates and screws are often used. With long-term follow-up, knee arthrosis can be expected to progress, and some of these cases will be converted to knee arthroplasty.3,4Displaced tibial plateau fractures are intra-articular injuries. Treatment requires surgery.
Blood work for inflammatory markers (erythrocyte sedimentation rate, C-reactive protein level) should be performed before surgery. In the event of an elevated laboratory value or clinical suspicion (joint effusion), the joint should be aspirated before any arthroplasty procedure.
Preoperative planning for hardware removal is essential.22 The correct screwdriver and a metal cutting burr (for stripped screws) should be available. These needs may be anticipated with certain types of locking plates.1
Surgical incision planning is also crucial in preventing wound problems that can lead to deep prosthetic infection.23,24 Blood supply to the skin of the anterior knee is primarily medially derived; incisions that are more medial put lateral skin flaps at risk.25 Use of the most recently healed or previous lateral-based scars has been recommended. In cases of adherent skin or poor soft-tissue envelope, plastic surgery (eg, soft-tissue expansion, gastrocnemius muscle, fasciocutaneous flaps) may be necessary.26-28Surgeons must decide to perform either a single operation or a multiple-stage operation. Naturally, most patients prefer a single procedure. All previous hardware can be removed, or only the hardware that is preventing insertion of the tibial base plate. Removing the least amount of hardware is advantageous in that surgical stripping and soft-tissue damage are reduced.
In this initial series, we successfully converted 3 tibial implants to TKAs (each as a single operation) by removing only screws in percutaneous or minimally invasive fashion—the prosthetic joint approach did not involve additional soft-tissue stripping. We did not specifically record the time needed for implant removal separately from the time needed for TKA. As the Table shows, this technique can lengthen surgery. Operative time and blood loss can be more variable because of numerous factors, including scar tissue and an altered surgical field from previous surgery, in addition to hardware removal difficulties. Therefore, surgeons should budget more operative time for these procedures. Although longer operations theoretically may increase infection rates, we think the risk is mitigated by the percutaneous aspects of the described technique.
We do not think that most orthopedic surgeons addressing retained plate–screw constructs consider minimally invasive screw removal and plate retention. To our knowledge, the literature includes only 1 case report of a similar technique.29This technique has many potential drawbacks, the foremost being use of intraoperative fluoroscopy. For more complex fractures, fluoroscopy time can be significant if the surgeon is committed to a true percutaneous approach (Table). In addition, use of a mobile fluoroscopy unit adds personnel to the operating theater, which potentially increases the infection rate. There may be cases in which tibial hardware interferes with tibial cuts, necessitating plate removal, but we did not encounter this in our series. This technique is potentially time-consuming. Operating room time can be expected to increase relative to wide exposures that allow quick access to existing implants. For this reason, some surgeons may decide to forgo this technique. Most modern proximal tibial fracture plates are contoured to fit well over the bone. However, some may still be prominent, and surgeons may choose to perform an open approach to remove them. Last, the clinical impact of plates retained without screws in the proximal tibia is not known. Theoretically, they may still act as a nidus for occult infection, and may act as a stress riser for peri-implant fracture. Therefore, for each patient, the surgeon must decide if the extra surgical time, fluoroscopy exposure, and plate retention are worthwhile.
In this 3-case series, screws were removed percutaneously over the proximal tibia. There were no neurovascular injuries in these cases, though there is potential for nerve and artery injuries with percutaneous screw removal, as in the anterolateral area of the distal third of the tibia.30,31 Thus, our technique may not be applicable in such cases. Most patients with plates and screws retained after proximal tibial surgery do not need to have the screws removed from the distal tibia. There also is the potential for saphenous nerve injury if a small medial or posteromedial incision is made. No such injury occurred in our small series.
Surgeons must consider many factors when deciding whether to proceed with TKA in the setting of existing tibial hardware. If staged reconstruction is not planned, consideration can be given to percutaneous screw removal without plate removal in an attempt to minimize further soft-tissue stripping. This has the theoretical advantage of decreasing wound complications. We have been pleased with our initial patient experience and continue to use this technique.
Am J Orthop. 2016;45(7):E481-E486. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. Georgiadis GM, Gove NK, Smith AD, Rodway IP. Removal of the Less Invasive Stabilization System. J Orthop Trauma. 2004;18(8):562-564.
2. Georgiadis GM. Combined anterior and posterior approaches for complex tibial plateau fractures. J Bone Joint Surg Br. 1994;76(2):285-289.
3. Insall JN, Joseph DM, Msika C. High tibial osteotomy for varus gonarthrosis. A long-term follow-up study. J Bone Joint Surg Am. 1984;66(7):1040-1048.
4. Sprenger TR, Doerzbacher JF. Tibial osteotomy for the treatment of varus gonarthrosis. Survival and failure analysis to twenty-two years. J Bone Joint Surg Am. 2003;85(3):469-474.
5. Moore TM, Patzakis MJ, Harvey JP. Tibial plateau fractures: definition, demographics, treatment rationale, and long-term results of closed traction management or operative reduction. J Orthop Trauma. 1987;1(2):97-119.
6. Shah SN, Karunakar MA. Early wound complications after operative treatment of high energy tibial plateau fractures through two incisions. Bull NYU Hosp Joint Dis. 2007;65(2):115-119.
7. Yang EC, Weiner L, Strauss E, Sedin E, Kelley M, Raphael J. Metaphyseal dissociation fractures of the proximal tibia. An analysis of treatment and complications. Am J Orthop. 1995;24(9):695-704.
8. Young MJ, Barrack RL. Complications of internal fixation of tibial plateau fractures. Orthop Rev. 1994;23(2):149-154.
9. Luo CF, Sun H, Zhang B, Zeng BF. Three-column fixation for complex tibial plateau fractures. J Orthop Trauma. 2010;24(11):683-692.
10. Barei DP, Nork SE, Mills WJ, Henley MB, Benirschke SK. Complications associated with internal fixation of high-energy bicondylar tibial plateau fractures utilizing a two-incision technique. J Orthop Trauma. 2004;18(10):649-657.
11. Ruffolo MR, Gettys FK, Montijo HE, Seymour RB, Karunakar MA. Complications of high-energy bicondylar tibial plateau fractures treated with dual plating through 2 incisions. J Orthop Trauma. 2015;29(2):85-90.
12. Honkonen SE. Degenerative arthritis after tibial plateau fractures. J Orthop Trauma. 1995;9(4):273-277.
13. Volpin G, Dowd GS, Stein H, Bentley G. Degenerative arthritis after intra-articular fractures of the knee. Long-term results. J Bone Joint Surg Br. 1990;72(4):634-638.
14. Mehin R, O’Brien P, Broekhuyse H, Blachut P, Guy P. Endstage arthritis following tibia plateau fractures: average 10-year follow-up. Can J Surg. 2012;55(2):87-94.
15. Wasserstein D, Henry P, Paterson JM, Kreder HJ, Jenkinson R. Risk of total knee arthroplasty after operatively treated tibial plateau fracture: a matched-population-based cohort study. J Bone Joint Surg Am. 2014;96(2):144-150.
16. Meding JB, Keating EM, Ritter MA, Faris PM. Total knee arthroplasty after high tibial osteotomy. A comparison study in patients who had bilateral total knee replacement. J Bone Joint Surg Am. 2000;82(9):1252-1259.
17. Parvizi J, Hanssen AD, Spangheli MJ. Total knee arthroplasty following proximal tibial osteotomy: risk factors for failure. J Bone Joint Surg Am. 2004;86(3):474-479.
18. Windsor RE, Insall JN, Vince KG. Technical considerations of total knee arthroplasty after proximal tibial osteotomy. J Bone Joint Surg Am. 1988;70(4):547-555.
19. Civinini R, Carulli C, Matassi F, Villano M, Innocenti M. Total knee arthroplasty after complex tibial plateau fractures. Chir Organi Mov. 2009;93(3):143-147.
20. Saleh KJ, Sherman P, Katkin P, et al. Total knee arthroplasty after open reduction and internal fixation of fractures of the tibial plateau: a minimum five-year follow-up study. J Bone Joint Surg Am. 2001;83(8):1144-1148.
21. Weiss NG, Parvizi J, Trousdale RT, Bryce RD, Lewallen DG. Total knee arthroplasty in patients with a prior fracture of the tibial plateau. J Bone Joint Surg Am. 2003;85(2):218-221.
22. Hak DJ, McElvany M. Removal of broken hardware. J Am Acad Orthop Surg. 2008:16(2):113-120.
23. Della Valle CJ, Berger RA, Rosenberg AG. Surgical exposures in revision total knee arthroplasty. Clin Orthop Relat Res. 2006;(446):59-68.
24. Vince KG, Abdeen A. Wound problems in total knee arthroplasty. Clin Orthop Relat Res. 2006;(452):88-90.
25. Colombel M, Mariz Y, Dahhan P, Kénési C. Arterial and lymphatic supply of the knee integuments. Surg Radiol Anat. 1998;20(1):35-40.
26. Namba RS, Diao E. Tissue expansion for staged reimplantation of infected total knee arthroplasty. J Arthroplasty. 1997;12(4):471-474.
27. Markovich GD, Dorr LD, Klein NE, McPherson EJ, Vince KG. Muscle flaps in total knee arthroplasty. Clin Orthop Relat Res. 1995;(321):122-130.
28. Hallock GG. Salvage of total knee arthroplasty with local fasciocutaneous flaps. J Bone Joint Surg Am. 1990;72(8):1236-1239.
29. Roswell M, Gale D. Total knee arthroplasty following internal fixation of a lateral tibial plateau fracture. Injury Extra. 2005;36(8):352-354.
30. Deangelis JP, Deangelis NA, Anderson R. Anatomy of the superficial peroneal nerve in relation to fixation of tibia fractures with the Less Invasive Stabilization System. J Orthop Trauma. 2004;18(8):536-539.
31. Pichler W, Grechenig W, Tesch NP, Weinberg AM, Heidari N, Clement H. The risk of iatrogenic injury to the deep peroneal nerve in minimally invasive osteosynthesis of the tibia with the Less Invasive Stabilisation System: a cadaver study. J Bone Joint Surg Br. 2009;91(3):385-387.
Technique
The patient is positioned on a radiolucent table, and a mobile fluoroscopy unit is available. A tourniquet is applied to the upper thigh but typically is not inflated during the percutaneous hardware removal portion of the operation. It is crucial to have information on retained implants so the correct screwdrivers for screw removal can be selected. In addition, provisions for stripped screws should be made. In each of the 3 cases we managed, the Synthes Screw Removal Set was available. Presence of an implant system known to have problems with cold welding of screws (eg, Less Invasive Stabilization System; Synthes) may necessitate additional preparations, such as making conical extraction devices available.1
After preoperative administration of antibiotics, the surgeon typically removes only those proximal tibia screws that are preventing insertion of the tibial base plate. Fluoroscopic guidance is used to locate these screws and then remove them with percutaneous stab incisions. (Retained plates are not removed.) The exact method of localizing and removing the screws percutaneously is crucial. A small stab incision is made in the dermal layer. The number of stab incisions to be made depends on the number of screws to be removed. One small incision is needed for each screw hole. Occasionally mobilizing the skin and redirecting the screwdriver in the deep tissues can allow 2 screws to be removed through a single skin wound. The screwdriver head can be inserted through the muscle and fascial layers without the need for deep dissection. The plate is then felt with the screwdriver and the screw head located. It is very important that the screw head be adequately engaged to prevent stripping. The surgeon should not rush this step. The C-arm can be helpful here. Fluoroscopy not only can guide the screwdriver to the screw hole but can confirm the screwdriver is at right angles to the plate, not oblique. Only when the surgeon is completely satisfied that the screw head is well engaged should the attempt to back out the screw be made. If the screw strips, the screwdriver can be removed, and an attempt can be made to insert a percutaneous stripped screw removal device.1 If this fails, then the technique must be abandoned for a more traditional approach.
Plating complex tibial plateau fractures through a separate posteromedial approach is now popular.2 The deep location and screw orientation of posteromedial hardware make percutaneous removal unfeasible. In these cases, a separate posteromedial incision may be needed—usually posterior enough so it minimally compromises the anterior soft tissues. The incision typically uses the old posteromedial surgical scar but may not need to be as large as the original approach, as only selected screws need be removed. The saphenous neurovascular bundle may still be at risk, depending on the location of these incisions. The plate is not removed.
After the necessary screws are removed, the tourniquet can be inflated, if desired. The total knee arthroplasty (TKA) then proceeds in usual fashion through a single incision and a medial parapatellar arthrotomy.
Results
Between January 2009 and February 2014, Dr. Georgiadis converted 3 cases of retained tibial hardware and severe knee arthrosis to a TKA in a single operation. These cases were reviewed after Institutional Review Board approval was obtained. One patient underwent a closing-wedge high tibial osteotomy 14 years earlier, and the other 2 sustained tibial plateau fractures. Clinical details of the 3 cases are presented in the Table.
In 2 of the cases, anterolateral surgical scars were present. New, separate percutaneous stab incisions were used to remove screws, which meant less of the original skin incision could be used for the TKA (Figures 1A, 1B).
In the third case, involving multiple plates, a similar strategy was used, but an additional small posteromedial incision was required (Figures 2-5). The TKA then proceeded through a new midline incision. This case was performed for tibiofemoral arthrosis in the setting of an acute distal femur fracture, but this had no bearing on the technique.
Tibial base plates were inserted in the usual manner. Length and type of tibial stem were left to the discretion of the surgeon. There were no changes from the usual surgical technique. All patients went on to routine, uneventful wound healing. Follow-up ranged from 10 months to 59 months.
Discussion
If the decision is made to proceed with TKA after previous knee surgery, careful preoperative planning is needed.
For young patients with knee arthrosis and angular deformity, it has been recommended that proximal tibial osteotomy be performed to delay the need for joint replacement.3,4 Although a wide variety of osteotomy techniques is available, plates and screws are often used. With long-term follow-up, knee arthrosis can be expected to progress, and some of these cases will be converted to knee arthroplasty.3,4Displaced tibial plateau fractures are intra-articular injuries. Treatment requires surgery.
Blood work for inflammatory markers (erythrocyte sedimentation rate, C-reactive protein level) should be performed before surgery. In the event of an elevated laboratory value or clinical suspicion (joint effusion), the joint should be aspirated before any arthroplasty procedure.
Preoperative planning for hardware removal is essential.22 The correct screwdriver and a metal cutting burr (for stripped screws) should be available. These needs may be anticipated with certain types of locking plates.1
Surgical incision planning is also crucial in preventing wound problems that can lead to deep prosthetic infection.23,24 Blood supply to the skin of the anterior knee is primarily medially derived; incisions that are more medial put lateral skin flaps at risk.25 Use of the most recently healed or previous lateral-based scars has been recommended. In cases of adherent skin or poor soft-tissue envelope, plastic surgery (eg, soft-tissue expansion, gastrocnemius muscle, fasciocutaneous flaps) may be necessary.26-28Surgeons must decide to perform either a single operation or a multiple-stage operation. Naturally, most patients prefer a single procedure. All previous hardware can be removed, or only the hardware that is preventing insertion of the tibial base plate. Removing the least amount of hardware is advantageous in that surgical stripping and soft-tissue damage are reduced.
In this initial series, we successfully converted 3 tibial implants to TKAs (each as a single operation) by removing only screws in percutaneous or minimally invasive fashion—the prosthetic joint approach did not involve additional soft-tissue stripping. We did not specifically record the time needed for implant removal separately from the time needed for TKA. As the Table shows, this technique can lengthen surgery. Operative time and blood loss can be more variable because of numerous factors, including scar tissue and an altered surgical field from previous surgery, in addition to hardware removal difficulties. Therefore, surgeons should budget more operative time for these procedures. Although longer operations theoretically may increase infection rates, we think the risk is mitigated by the percutaneous aspects of the described technique.
We do not think that most orthopedic surgeons addressing retained plate–screw constructs consider minimally invasive screw removal and plate retention. To our knowledge, the literature includes only 1 case report of a similar technique.29This technique has many potential drawbacks, the foremost being use of intraoperative fluoroscopy. For more complex fractures, fluoroscopy time can be significant if the surgeon is committed to a true percutaneous approach (Table). In addition, use of a mobile fluoroscopy unit adds personnel to the operating theater, which potentially increases the infection rate. There may be cases in which tibial hardware interferes with tibial cuts, necessitating plate removal, but we did not encounter this in our series. This technique is potentially time-consuming. Operating room time can be expected to increase relative to wide exposures that allow quick access to existing implants. For this reason, some surgeons may decide to forgo this technique. Most modern proximal tibial fracture plates are contoured to fit well over the bone. However, some may still be prominent, and surgeons may choose to perform an open approach to remove them. Last, the clinical impact of plates retained without screws in the proximal tibia is not known. Theoretically, they may still act as a nidus for occult infection, and may act as a stress riser for peri-implant fracture. Therefore, for each patient, the surgeon must decide if the extra surgical time, fluoroscopy exposure, and plate retention are worthwhile.
In this 3-case series, screws were removed percutaneously over the proximal tibia. There were no neurovascular injuries in these cases, though there is potential for nerve and artery injuries with percutaneous screw removal, as in the anterolateral area of the distal third of the tibia.30,31 Thus, our technique may not be applicable in such cases. Most patients with plates and screws retained after proximal tibial surgery do not need to have the screws removed from the distal tibia. There also is the potential for saphenous nerve injury if a small medial or posteromedial incision is made. No such injury occurred in our small series.
Surgeons must consider many factors when deciding whether to proceed with TKA in the setting of existing tibial hardware. If staged reconstruction is not planned, consideration can be given to percutaneous screw removal without plate removal in an attempt to minimize further soft-tissue stripping. This has the theoretical advantage of decreasing wound complications. We have been pleased with our initial patient experience and continue to use this technique.
Am J Orthop. 2016;45(7):E481-E486. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
Technique
The patient is positioned on a radiolucent table, and a mobile fluoroscopy unit is available. A tourniquet is applied to the upper thigh but typically is not inflated during the percutaneous hardware removal portion of the operation. It is crucial to have information on retained implants so the correct screwdrivers for screw removal can be selected. In addition, provisions for stripped screws should be made. In each of the 3 cases we managed, the Synthes Screw Removal Set was available. Presence of an implant system known to have problems with cold welding of screws (eg, Less Invasive Stabilization System; Synthes) may necessitate additional preparations, such as making conical extraction devices available.1
After preoperative administration of antibiotics, the surgeon typically removes only those proximal tibia screws that are preventing insertion of the tibial base plate. Fluoroscopic guidance is used to locate these screws and then remove them with percutaneous stab incisions. (Retained plates are not removed.) The exact method of localizing and removing the screws percutaneously is crucial. A small stab incision is made in the dermal layer. The number of stab incisions to be made depends on the number of screws to be removed. One small incision is needed for each screw hole. Occasionally mobilizing the skin and redirecting the screwdriver in the deep tissues can allow 2 screws to be removed through a single skin wound. The screwdriver head can be inserted through the muscle and fascial layers without the need for deep dissection. The plate is then felt with the screwdriver and the screw head located. It is very important that the screw head be adequately engaged to prevent stripping. The surgeon should not rush this step. The C-arm can be helpful here. Fluoroscopy not only can guide the screwdriver to the screw hole but can confirm the screwdriver is at right angles to the plate, not oblique. Only when the surgeon is completely satisfied that the screw head is well engaged should the attempt to back out the screw be made. If the screw strips, the screwdriver can be removed, and an attempt can be made to insert a percutaneous stripped screw removal device.1 If this fails, then the technique must be abandoned for a more traditional approach.
Plating complex tibial plateau fractures through a separate posteromedial approach is now popular.2 The deep location and screw orientation of posteromedial hardware make percutaneous removal unfeasible. In these cases, a separate posteromedial incision may be needed—usually posterior enough so it minimally compromises the anterior soft tissues. The incision typically uses the old posteromedial surgical scar but may not need to be as large as the original approach, as only selected screws need be removed. The saphenous neurovascular bundle may still be at risk, depending on the location of these incisions. The plate is not removed.
After the necessary screws are removed, the tourniquet can be inflated, if desired. The total knee arthroplasty (TKA) then proceeds in usual fashion through a single incision and a medial parapatellar arthrotomy.
Results
Between January 2009 and February 2014, Dr. Georgiadis converted 3 cases of retained tibial hardware and severe knee arthrosis to a TKA in a single operation. These cases were reviewed after Institutional Review Board approval was obtained. One patient underwent a closing-wedge high tibial osteotomy 14 years earlier, and the other 2 sustained tibial plateau fractures. Clinical details of the 3 cases are presented in the Table.
In 2 of the cases, anterolateral surgical scars were present. New, separate percutaneous stab incisions were used to remove screws, which meant less of the original skin incision could be used for the TKA (Figures 1A, 1B).
In the third case, involving multiple plates, a similar strategy was used, but an additional small posteromedial incision was required (Figures 2-5). The TKA then proceeded through a new midline incision. This case was performed for tibiofemoral arthrosis in the setting of an acute distal femur fracture, but this had no bearing on the technique.
Tibial base plates were inserted in the usual manner. Length and type of tibial stem were left to the discretion of the surgeon. There were no changes from the usual surgical technique. All patients went on to routine, uneventful wound healing. Follow-up ranged from 10 months to 59 months.
Discussion
If the decision is made to proceed with TKA after previous knee surgery, careful preoperative planning is needed.
For young patients with knee arthrosis and angular deformity, it has been recommended that proximal tibial osteotomy be performed to delay the need for joint replacement.3,4 Although a wide variety of osteotomy techniques is available, plates and screws are often used. With long-term follow-up, knee arthrosis can be expected to progress, and some of these cases will be converted to knee arthroplasty.3,4Displaced tibial plateau fractures are intra-articular injuries. Treatment requires surgery.
Blood work for inflammatory markers (erythrocyte sedimentation rate, C-reactive protein level) should be performed before surgery. In the event of an elevated laboratory value or clinical suspicion (joint effusion), the joint should be aspirated before any arthroplasty procedure.
Preoperative planning for hardware removal is essential.22 The correct screwdriver and a metal cutting burr (for stripped screws) should be available. These needs may be anticipated with certain types of locking plates.1
Surgical incision planning is also crucial in preventing wound problems that can lead to deep prosthetic infection.23,24 Blood supply to the skin of the anterior knee is primarily medially derived; incisions that are more medial put lateral skin flaps at risk.25 Use of the most recently healed or previous lateral-based scars has been recommended. In cases of adherent skin or poor soft-tissue envelope, plastic surgery (eg, soft-tissue expansion, gastrocnemius muscle, fasciocutaneous flaps) may be necessary.26-28Surgeons must decide to perform either a single operation or a multiple-stage operation. Naturally, most patients prefer a single procedure. All previous hardware can be removed, or only the hardware that is preventing insertion of the tibial base plate. Removing the least amount of hardware is advantageous in that surgical stripping and soft-tissue damage are reduced.
In this initial series, we successfully converted 3 tibial implants to TKAs (each as a single operation) by removing only screws in percutaneous or minimally invasive fashion—the prosthetic joint approach did not involve additional soft-tissue stripping. We did not specifically record the time needed for implant removal separately from the time needed for TKA. As the Table shows, this technique can lengthen surgery. Operative time and blood loss can be more variable because of numerous factors, including scar tissue and an altered surgical field from previous surgery, in addition to hardware removal difficulties. Therefore, surgeons should budget more operative time for these procedures. Although longer operations theoretically may increase infection rates, we think the risk is mitigated by the percutaneous aspects of the described technique.
We do not think that most orthopedic surgeons addressing retained plate–screw constructs consider minimally invasive screw removal and plate retention. To our knowledge, the literature includes only 1 case report of a similar technique.29This technique has many potential drawbacks, the foremost being use of intraoperative fluoroscopy. For more complex fractures, fluoroscopy time can be significant if the surgeon is committed to a true percutaneous approach (Table). In addition, use of a mobile fluoroscopy unit adds personnel to the operating theater, which potentially increases the infection rate. There may be cases in which tibial hardware interferes with tibial cuts, necessitating plate removal, but we did not encounter this in our series. This technique is potentially time-consuming. Operating room time can be expected to increase relative to wide exposures that allow quick access to existing implants. For this reason, some surgeons may decide to forgo this technique. Most modern proximal tibial fracture plates are contoured to fit well over the bone. However, some may still be prominent, and surgeons may choose to perform an open approach to remove them. Last, the clinical impact of plates retained without screws in the proximal tibia is not known. Theoretically, they may still act as a nidus for occult infection, and may act as a stress riser for peri-implant fracture. Therefore, for each patient, the surgeon must decide if the extra surgical time, fluoroscopy exposure, and plate retention are worthwhile.
In this 3-case series, screws were removed percutaneously over the proximal tibia. There were no neurovascular injuries in these cases, though there is potential for nerve and artery injuries with percutaneous screw removal, as in the anterolateral area of the distal third of the tibia.30,31 Thus, our technique may not be applicable in such cases. Most patients with plates and screws retained after proximal tibial surgery do not need to have the screws removed from the distal tibia. There also is the potential for saphenous nerve injury if a small medial or posteromedial incision is made. No such injury occurred in our small series.
Surgeons must consider many factors when deciding whether to proceed with TKA in the setting of existing tibial hardware. If staged reconstruction is not planned, consideration can be given to percutaneous screw removal without plate removal in an attempt to minimize further soft-tissue stripping. This has the theoretical advantage of decreasing wound complications. We have been pleased with our initial patient experience and continue to use this technique.
Am J Orthop. 2016;45(7):E481-E486. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. Georgiadis GM, Gove NK, Smith AD, Rodway IP. Removal of the Less Invasive Stabilization System. J Orthop Trauma. 2004;18(8):562-564.
2. Georgiadis GM. Combined anterior and posterior approaches for complex tibial plateau fractures. J Bone Joint Surg Br. 1994;76(2):285-289.
3. Insall JN, Joseph DM, Msika C. High tibial osteotomy for varus gonarthrosis. A long-term follow-up study. J Bone Joint Surg Am. 1984;66(7):1040-1048.
4. Sprenger TR, Doerzbacher JF. Tibial osteotomy for the treatment of varus gonarthrosis. Survival and failure analysis to twenty-two years. J Bone Joint Surg Am. 2003;85(3):469-474.
5. Moore TM, Patzakis MJ, Harvey JP. Tibial plateau fractures: definition, demographics, treatment rationale, and long-term results of closed traction management or operative reduction. J Orthop Trauma. 1987;1(2):97-119.
6. Shah SN, Karunakar MA. Early wound complications after operative treatment of high energy tibial plateau fractures through two incisions. Bull NYU Hosp Joint Dis. 2007;65(2):115-119.
7. Yang EC, Weiner L, Strauss E, Sedin E, Kelley M, Raphael J. Metaphyseal dissociation fractures of the proximal tibia. An analysis of treatment and complications. Am J Orthop. 1995;24(9):695-704.
8. Young MJ, Barrack RL. Complications of internal fixation of tibial plateau fractures. Orthop Rev. 1994;23(2):149-154.
9. Luo CF, Sun H, Zhang B, Zeng BF. Three-column fixation for complex tibial plateau fractures. J Orthop Trauma. 2010;24(11):683-692.
10. Barei DP, Nork SE, Mills WJ, Henley MB, Benirschke SK. Complications associated with internal fixation of high-energy bicondylar tibial plateau fractures utilizing a two-incision technique. J Orthop Trauma. 2004;18(10):649-657.
11. Ruffolo MR, Gettys FK, Montijo HE, Seymour RB, Karunakar MA. Complications of high-energy bicondylar tibial plateau fractures treated with dual plating through 2 incisions. J Orthop Trauma. 2015;29(2):85-90.
12. Honkonen SE. Degenerative arthritis after tibial plateau fractures. J Orthop Trauma. 1995;9(4):273-277.
13. Volpin G, Dowd GS, Stein H, Bentley G. Degenerative arthritis after intra-articular fractures of the knee. Long-term results. J Bone Joint Surg Br. 1990;72(4):634-638.
14. Mehin R, O’Brien P, Broekhuyse H, Blachut P, Guy P. Endstage arthritis following tibia plateau fractures: average 10-year follow-up. Can J Surg. 2012;55(2):87-94.
15. Wasserstein D, Henry P, Paterson JM, Kreder HJ, Jenkinson R. Risk of total knee arthroplasty after operatively treated tibial plateau fracture: a matched-population-based cohort study. J Bone Joint Surg Am. 2014;96(2):144-150.
16. Meding JB, Keating EM, Ritter MA, Faris PM. Total knee arthroplasty after high tibial osteotomy. A comparison study in patients who had bilateral total knee replacement. J Bone Joint Surg Am. 2000;82(9):1252-1259.
17. Parvizi J, Hanssen AD, Spangheli MJ. Total knee arthroplasty following proximal tibial osteotomy: risk factors for failure. J Bone Joint Surg Am. 2004;86(3):474-479.
18. Windsor RE, Insall JN, Vince KG. Technical considerations of total knee arthroplasty after proximal tibial osteotomy. J Bone Joint Surg Am. 1988;70(4):547-555.
19. Civinini R, Carulli C, Matassi F, Villano M, Innocenti M. Total knee arthroplasty after complex tibial plateau fractures. Chir Organi Mov. 2009;93(3):143-147.
20. Saleh KJ, Sherman P, Katkin P, et al. Total knee arthroplasty after open reduction and internal fixation of fractures of the tibial plateau: a minimum five-year follow-up study. J Bone Joint Surg Am. 2001;83(8):1144-1148.
21. Weiss NG, Parvizi J, Trousdale RT, Bryce RD, Lewallen DG. Total knee arthroplasty in patients with a prior fracture of the tibial plateau. J Bone Joint Surg Am. 2003;85(2):218-221.
22. Hak DJ, McElvany M. Removal of broken hardware. J Am Acad Orthop Surg. 2008:16(2):113-120.
23. Della Valle CJ, Berger RA, Rosenberg AG. Surgical exposures in revision total knee arthroplasty. Clin Orthop Relat Res. 2006;(446):59-68.
24. Vince KG, Abdeen A. Wound problems in total knee arthroplasty. Clin Orthop Relat Res. 2006;(452):88-90.
25. Colombel M, Mariz Y, Dahhan P, Kénési C. Arterial and lymphatic supply of the knee integuments. Surg Radiol Anat. 1998;20(1):35-40.
26. Namba RS, Diao E. Tissue expansion for staged reimplantation of infected total knee arthroplasty. J Arthroplasty. 1997;12(4):471-474.
27. Markovich GD, Dorr LD, Klein NE, McPherson EJ, Vince KG. Muscle flaps in total knee arthroplasty. Clin Orthop Relat Res. 1995;(321):122-130.
28. Hallock GG. Salvage of total knee arthroplasty with local fasciocutaneous flaps. J Bone Joint Surg Am. 1990;72(8):1236-1239.
29. Roswell M, Gale D. Total knee arthroplasty following internal fixation of a lateral tibial plateau fracture. Injury Extra. 2005;36(8):352-354.
30. Deangelis JP, Deangelis NA, Anderson R. Anatomy of the superficial peroneal nerve in relation to fixation of tibia fractures with the Less Invasive Stabilization System. J Orthop Trauma. 2004;18(8):536-539.
31. Pichler W, Grechenig W, Tesch NP, Weinberg AM, Heidari N, Clement H. The risk of iatrogenic injury to the deep peroneal nerve in minimally invasive osteosynthesis of the tibia with the Less Invasive Stabilisation System: a cadaver study. J Bone Joint Surg Br. 2009;91(3):385-387.
1. Georgiadis GM, Gove NK, Smith AD, Rodway IP. Removal of the Less Invasive Stabilization System. J Orthop Trauma. 2004;18(8):562-564.
2. Georgiadis GM. Combined anterior and posterior approaches for complex tibial plateau fractures. J Bone Joint Surg Br. 1994;76(2):285-289.
3. Insall JN, Joseph DM, Msika C. High tibial osteotomy for varus gonarthrosis. A long-term follow-up study. J Bone Joint Surg Am. 1984;66(7):1040-1048.
4. Sprenger TR, Doerzbacher JF. Tibial osteotomy for the treatment of varus gonarthrosis. Survival and failure analysis to twenty-two years. J Bone Joint Surg Am. 2003;85(3):469-474.
5. Moore TM, Patzakis MJ, Harvey JP. Tibial plateau fractures: definition, demographics, treatment rationale, and long-term results of closed traction management or operative reduction. J Orthop Trauma. 1987;1(2):97-119.
6. Shah SN, Karunakar MA. Early wound complications after operative treatment of high energy tibial plateau fractures through two incisions. Bull NYU Hosp Joint Dis. 2007;65(2):115-119.
7. Yang EC, Weiner L, Strauss E, Sedin E, Kelley M, Raphael J. Metaphyseal dissociation fractures of the proximal tibia. An analysis of treatment and complications. Am J Orthop. 1995;24(9):695-704.
8. Young MJ, Barrack RL. Complications of internal fixation of tibial plateau fractures. Orthop Rev. 1994;23(2):149-154.
9. Luo CF, Sun H, Zhang B, Zeng BF. Three-column fixation for complex tibial plateau fractures. J Orthop Trauma. 2010;24(11):683-692.
10. Barei DP, Nork SE, Mills WJ, Henley MB, Benirschke SK. Complications associated with internal fixation of high-energy bicondylar tibial plateau fractures utilizing a two-incision technique. J Orthop Trauma. 2004;18(10):649-657.
11. Ruffolo MR, Gettys FK, Montijo HE, Seymour RB, Karunakar MA. Complications of high-energy bicondylar tibial plateau fractures treated with dual plating through 2 incisions. J Orthop Trauma. 2015;29(2):85-90.
12. Honkonen SE. Degenerative arthritis after tibial plateau fractures. J Orthop Trauma. 1995;9(4):273-277.
13. Volpin G, Dowd GS, Stein H, Bentley G. Degenerative arthritis after intra-articular fractures of the knee. Long-term results. J Bone Joint Surg Br. 1990;72(4):634-638.
14. Mehin R, O’Brien P, Broekhuyse H, Blachut P, Guy P. Endstage arthritis following tibia plateau fractures: average 10-year follow-up. Can J Surg. 2012;55(2):87-94.
15. Wasserstein D, Henry P, Paterson JM, Kreder HJ, Jenkinson R. Risk of total knee arthroplasty after operatively treated tibial plateau fracture: a matched-population-based cohort study. J Bone Joint Surg Am. 2014;96(2):144-150.
16. Meding JB, Keating EM, Ritter MA, Faris PM. Total knee arthroplasty after high tibial osteotomy. A comparison study in patients who had bilateral total knee replacement. J Bone Joint Surg Am. 2000;82(9):1252-1259.
17. Parvizi J, Hanssen AD, Spangheli MJ. Total knee arthroplasty following proximal tibial osteotomy: risk factors for failure. J Bone Joint Surg Am. 2004;86(3):474-479.
18. Windsor RE, Insall JN, Vince KG. Technical considerations of total knee arthroplasty after proximal tibial osteotomy. J Bone Joint Surg Am. 1988;70(4):547-555.
19. Civinini R, Carulli C, Matassi F, Villano M, Innocenti M. Total knee arthroplasty after complex tibial plateau fractures. Chir Organi Mov. 2009;93(3):143-147.
20. Saleh KJ, Sherman P, Katkin P, et al. Total knee arthroplasty after open reduction and internal fixation of fractures of the tibial plateau: a minimum five-year follow-up study. J Bone Joint Surg Am. 2001;83(8):1144-1148.
21. Weiss NG, Parvizi J, Trousdale RT, Bryce RD, Lewallen DG. Total knee arthroplasty in patients with a prior fracture of the tibial plateau. J Bone Joint Surg Am. 2003;85(2):218-221.
22. Hak DJ, McElvany M. Removal of broken hardware. J Am Acad Orthop Surg. 2008:16(2):113-120.
23. Della Valle CJ, Berger RA, Rosenberg AG. Surgical exposures in revision total knee arthroplasty. Clin Orthop Relat Res. 2006;(446):59-68.
24. Vince KG, Abdeen A. Wound problems in total knee arthroplasty. Clin Orthop Relat Res. 2006;(452):88-90.
25. Colombel M, Mariz Y, Dahhan P, Kénési C. Arterial and lymphatic supply of the knee integuments. Surg Radiol Anat. 1998;20(1):35-40.
26. Namba RS, Diao E. Tissue expansion for staged reimplantation of infected total knee arthroplasty. J Arthroplasty. 1997;12(4):471-474.
27. Markovich GD, Dorr LD, Klein NE, McPherson EJ, Vince KG. Muscle flaps in total knee arthroplasty. Clin Orthop Relat Res. 1995;(321):122-130.
28. Hallock GG. Salvage of total knee arthroplasty with local fasciocutaneous flaps. J Bone Joint Surg Am. 1990;72(8):1236-1239.
29. Roswell M, Gale D. Total knee arthroplasty following internal fixation of a lateral tibial plateau fracture. Injury Extra. 2005;36(8):352-354.
30. Deangelis JP, Deangelis NA, Anderson R. Anatomy of the superficial peroneal nerve in relation to fixation of tibia fractures with the Less Invasive Stabilization System. J Orthop Trauma. 2004;18(8):536-539.
31. Pichler W, Grechenig W, Tesch NP, Weinberg AM, Heidari N, Clement H. The risk of iatrogenic injury to the deep peroneal nerve in minimally invasive osteosynthesis of the tibia with the Less Invasive Stabilisation System: a cadaver study. J Bone Joint Surg Br. 2009;91(3):385-387.
Robotic Technology Produces More Conservative Tibial Resection Than Conventional Techniques in UKA
Unicompartmental knee arthroplasty (UKA) is considered a less invasive approach for the treatment of unicompartmental knee arthritis when compared with total knee arthroplasty (TKA), with optimal preservation of kinematics.1 Despite excellent functional outcomes, conversion to TKA may be necessary if the UKA fails, or in patients with progressive knee arthritis. Some studies have found UKA conversion to TKA to be comparable with primary TKA,2,3 whereas others have found that conversion often requires bone graft, augments, and stemmed components and has increased complications and inferior results compared to primary TKA.4-7 While some studies report that <10% of UKA conversions to TKA require augments,2 others have found that as many as 76% require augments.4-8
Schwarzkopf and colleagues9 recently demonstrated that UKA conversion to TKA is comparable with primary TKA when a conservative tibial resection is performed during the index procedure. However, they reported increased complexity when greater tibial resection was performed and thicker polyethylene inserts were used at the time of the index UKA. The odds ratio of needing an augment or stem during the conversion to TKA was 26.8 (95% confidence interval, 3.71-194) when an aggressive tibial resection was performed during the UKA.9 Tibial resection thickness may thus be predictive of anticipated complexity of UKA revision to TKA and may aid in preoperative planning.
Robotic assistance has been shown to enhance the accuracy of bone preparation, implant component alignment, and soft tissue balance in UKA.10-15 It has yet to be determined whether this improved accuracy translates to improved clinical performance or longevity of the UKA implant. However, the enhanced accuracy of robotic technology may result in more conservative tibial resection when compared to conventional UKA and may be advantageous if conversion to TKA becomes necessary.
The purpose of this study was to compare the distribution of polyethylene insert sizes implanted during conventional and robotic-assisted UKA. We hypothesized that robotic assistance would demonstrate more conservative tibial resection compared to conventional methods of bone preparation.
Methods
We retrospectively compared the distribution of polyethylene insert sizes implanted during consecutive conventional and robotic-assisted UKA procedures. Several manufacturers were queried to provide a listing of the polyethylene insert sizes utilized, ranging from 8 mm to 14 mm. The analysis included 8421 robotic-assisted UKA cases and 27,989 conventional UKA cases. Data were provided by Zimmer Biomet and Smith & Nephew regarding conventional cases, as well as Blue Belt Technologies (now part of Smith & Nephew) and MAKO Surgical (now part of Stryker) regarding robotic-assisted cases. (Dr. Lonner has an ongoing relationship as a consultant with Blue Belt Technologies, whose data was utilized in this study.) Using tibial insert thickness as a surrogate measure of the extent of tibial resection, an insert size of ≥10 mm was defined as aggressive while <10 mm was considered conservative. This cutoff was established based on its corresponding resection level with primary TKA and the anticipated need for augments. Statistical analysis was performed using a Mann-Whitney-Wilcoxon test. Significance was set at P < .05.
Results
Tibial resection thickness was found to be most commonly conservative in nature, with sizes 8-mm and 9-mm polyethylene inserts utilized in the majority of both robotic-assisted and conventional UKA cases. However, statistically more 8-mm and 9-mm polyethylene inserts were used in the robotic group (93.6%) than in the conventional group (84.5%) (P < .0001; Figure). Aggressive tibial resection, requiring tibial inserts ≥10 mm, was performed in 6.4% of robotic-assisted cases and 15.5% of conventional cases.
Discussion
Robotic assistance enhances the accuracy of bone preparation, implant component alignment, and soft tissue balance in UKA.10-15 It has yet to be determined whether this improved accuracy translates to improved clinical performance or longevity of the UKA implant. However, we demonstrate that the enhanced accuracy of robotic technology results in more conservative tibial resection when compared to conventional techniques with a potential benefit suggested in the literature upon conversion to TKA.
The findings of this study have important implications for patients undergoing conversion of UKA to TKA, potentially optimizing the ease of revision and clinical outcomes. The outcomes of UKA conversion to TKA are often considered inferior to those of primary TKA, compromised by bone loss, need for augmentation, and challenges of restoring the joint line and rotation.9,16-22 Barrett and Scott18 reported only 66% of patients had good or excellent results at an average of 4.6 years of follow-up after UKA conversion to TKA. Over 50% required stemmed implants and bone graft or bone cement augmentation to address osseous insufficiency. The authors suggested that the primary determinant of the complexity of the conversion to TKA was the surgical technique used in the index procedure. They concluded that UKA conversion to TKA can be as successful as a primary TKA and primary TKA implants can be used without bone augmentation or stems during the revision procedure if minimal tibial bone is resected at the time of the index UKA.18 Schwarzkopf and colleagues9 supported this conclusion when they found that aggressive tibial resection during UKA resulted in the need for bone graft, stem, wedge, or augment in 70% of cases when converted to TKA. Similarly, Khan and colleagues23 found that 26% of patients required bone grafting and 26% required some form of augmentation, and Springer and colleagues3 reported that 68% required a graft, augment, or stem.3,22 Using data from the New Zealand Joint Registry, Pearse and colleagues5 reported that revision TKA components were necessary in 28% of patients and concluded that converting a UKA to TKA gives a less reliable result than primary TKA, and with functional results that are not significantly better than a revision from a TKA.
Conservative tibial resection during UKA minimizes the complexity and concerns of bone loss upon conversion to TKA. Schwarzkopf and colleagues9 found 96.6% of patients with conservative tibial resection received a primary TKA implant, without augments or stems. Furthermore, patients with a primary TKA implant showed improved tibial survivorship, with revision as an end point, compared with patients who received a TKA implant that required stems and augments or bone graft for support.9 Also emphasizing the importance of minimal tibial resection, O’Donnell and colleagues8 compared a cohort of patients undergoing conversion of a minimal resection resurfacing onlay-type UKA to TKA with a cohort of patients undergoing primary TKA. They found that 40% of patients required bone grafting for contained defects, 3.6% required metal augments, and 1.8% required stems.8 There was no significant difference between the groups in terms of range of motion, functional outcome, or radiologic outcomes. The authors concluded that revision of minimal resection resurfacing implants to TKA is associated with similar results to primary TKA and is superior to revision of UKA with greater bone loss. Prior studies have shown that one of the advantages of robotic-assisted UKA is the accuracy and precision of bone resection. The present study supports this premise by showing that tibial resection is significantly more conservative using robotic-assisted techniques when using tibial component thickness as a surrogate for extent of bone resection. While our study did not address implant durability or the impact of conservative resection on conversion to TKA, studies referenced above suggest that the conservative nature of bone preparation would have a relevant impact on the revision of the implant to TKA.
Our study is a retrospective case series that reports tibial component thickness as a surrogate for volume of tibial resection during UKA. While the implication is that more conservative tibial resection may optimize durability and ease of conversion to TKA, future study will be needed to compare robotic-assisted and conventional cases of UKA upon conversion to TKA in order to ascertain whether the more conventional resections of robotic-assisted UKA in fact lead to revision that is comparable with primary TKA in terms of bone loss at the time of revision, components utilized, the need for bone graft, augments, or stems, and clinical outcomes. Given the method of data collection in this study, we could not control for clinical deformity, selection bias, surgeon experience, or medial vs lateral knee compartments. These potential confounders represent weaknesses of this study.
In conclusion, conversion of UKA to TKA may be associated with significant osseous insufficiency, which may compromise patient outcomes in comparison to primary TKA. Studies have shown that UKA conversion to TKA is comparable to primary TKA when minimal tibial resection is performed during the UKA, and the need for augmentation, grafting or stems is increased with more aggressive tibial resection. This study has shown that when robotic assistance is utilized, tibial resection is more precise, less variable, and more conservative compared to conventional techniques.
Am J Orthop. 2016;45(7):E465-E468. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. Patil S, Colwell CW Jr, Ezzet KA, D’Lima DD. Can normal knee kinematics be restored with unicompartmental knee replacement? J Bone Joint Surg Am. 2005;87(2):332-338.
2. Johnson S, Jones P, Newman JH. The survivorship and results of total knee replacements converted from unicompartmental knee replacements. Knee. 2007;14(2):154-157.
3. Springer BD, Scott RD, Thornhill TS. Conversion of failed unicompartmental knee arthroplasty to TKA. Clin Orthop Relat Res. 2006;446:214-220.
4. Järvenpää J, Kettunen J, Miettinen H, Kröger H. The clinical outcome of revision knee replacement after unicompartmental knee arthroplasty versus primary total knee arthroplasty: 8-17 years follow-up study of 49 patients. Int Orthop. 2010;34(5):649-653.
5. Pearse AJ, Hooper GJ, Rothwell AG, Frampton C. Osteotomy and unicompartmental knee arthroplasty converted to total knee arthroplasty: data from the New Zealand Joint Registry. J Arthroplasty. 2012;27(10):1827-1831.
6. Rancourt MF, Kemp KA, Plamondon SM, Kim PR, Dervin GF. Unicompartmental knee arthroplasties revised to total knee arthroplasties compared with primary total knee arthroplasties. J Arthroplasty. 2012;27(8 Suppl):106-110.
7. Sierra RJ, Kassel CA, Wetters NG, Berend KR, Della Valle CJ, Lombardi AV. Revision of unicompartmental arthroplasty to total knee arthroplasty: not always a slam dunk! J Arthroplasty. 2013;28(8 Suppl):128-132.
8. O’Donnell TM, Abouazza O, Neil MJ. Revision of minimal resection resurfacing unicondylar knee arthroplasty to total knee arthroplasty: results compared with primary total knee arthroplasty. J Arthroplasty. 2013;28(1):33-39.
9. Schwarzkopf R, Mikhael B, Li L, Josephs L, Scott RD. Effect of initial tibial resection thickness on outcomes of revision UKA. Orthopedics. 2013;36(4):e409-e414.
10. Conditt MA, Roche MW. Minimally invasive robotic-arm-guided unicompartmental knee arthroplasty. J Bone Joint Surg Am. 2009;91 Suppl 1:63-68.
11. Dunbar NJ, Roche MW, Park BH, Branch SH, Conditt MA, Banks SA. Accuracy of dynamic tactile-guided unicompartmental knee arthroplasty. J Arthroplasty. 2012;27(5):803-808.e1.
12. Karia M, Masjedi M, Andrews B, Jaffry Z, Cobb J. Robotic assistance enables inexperienced surgeons to perform unicompartmental knee arthroplasties on dry bone models with accuracy superior to conventional methods. Adv Orthop. 2013;2013:481039.
13. Lonner JH, John TK, Conditt MA. Robotic arm-assisted UKA improves tibial component alignment: a pilot study. Clin Orthop Relat Res. 2010;468(1):141-146.
14. Lonner JH, Smith JR, Picard F, Hamlin B, Rowe PJ, Riches PE. High degree of accuracy of a novel image-free handheld robot for unicondylar knee arthroplasty in a cadaveric study. Clin Orthop Relat Res. 2015;473(1):206-212.
15. Smith JR, Picard F, Rowe PJ, Deakin A, Riches PE. The accuracy of a robotically-controlled freehand sculpting tool for unicondylar knee arthroplasty. Bone Joint J. 2013;95-B(suppl 28):68.
16. Chakrabarty G, Newman JH, Ackroyd CE. Revision of unicompartmental arthroplasty of the knee. Clinical and technical considerations. J Arthroplasty. 1998;13(2):191-196.
17. Levine WN, Ozuna RM, Scott RD, Thornhill TS. Conversion of failed modern unicompartmental arthroplasty to total knee arthroplasty. J Arthroplasty. 1996;11(7):797-801.
18. Barrett WP, Scott RD. Revision of failed unicondylar unicompartmental knee arthroplasty. J Bone Joint Surg Am. 1987;69(9):1328-1335.
19. Padgett DE, Stern SH, Insall JN. Revision total knee arthroplasty for failed unicompartmental replacement. J Bone Joint Surg Am. 1991;73(2):186-190.
20. Aleto TJ, Berend ME, Ritter MA, Faris PM, Meneghini RM. Early failure of unicompartmental knee arthroplasty leading to revision. J Arthroplasty. 2008;23(2):159-163.
21. McAuley JP, Engh GA, Ammeen DJ. Revision of failed unicompartmental knee arthroplasty. Clin Orthop Relat Res. 2001;(392):279-282.22. Böhm I, Landsiedl F. Revision surgery after failed unicompartmental knee arthroplasty: a study of 35 cases. J Arthroplasty. 2000;15(8):982-989.
23. Khan Z, Nawaz SZ, Kahane S, Ester C, Chatterji U. Conversion of unicompartmental knee arthroplasty to total knee arthroplasty: the challenges and need for augments. Acta Orthop Belg. 2013;79(6):699-705.
Unicompartmental knee arthroplasty (UKA) is considered a less invasive approach for the treatment of unicompartmental knee arthritis when compared with total knee arthroplasty (TKA), with optimal preservation of kinematics.1 Despite excellent functional outcomes, conversion to TKA may be necessary if the UKA fails, or in patients with progressive knee arthritis. Some studies have found UKA conversion to TKA to be comparable with primary TKA,2,3 whereas others have found that conversion often requires bone graft, augments, and stemmed components and has increased complications and inferior results compared to primary TKA.4-7 While some studies report that <10% of UKA conversions to TKA require augments,2 others have found that as many as 76% require augments.4-8
Schwarzkopf and colleagues9 recently demonstrated that UKA conversion to TKA is comparable with primary TKA when a conservative tibial resection is performed during the index procedure. However, they reported increased complexity when greater tibial resection was performed and thicker polyethylene inserts were used at the time of the index UKA. The odds ratio of needing an augment or stem during the conversion to TKA was 26.8 (95% confidence interval, 3.71-194) when an aggressive tibial resection was performed during the UKA.9 Tibial resection thickness may thus be predictive of anticipated complexity of UKA revision to TKA and may aid in preoperative planning.
Robotic assistance has been shown to enhance the accuracy of bone preparation, implant component alignment, and soft tissue balance in UKA.10-15 It has yet to be determined whether this improved accuracy translates to improved clinical performance or longevity of the UKA implant. However, the enhanced accuracy of robotic technology may result in more conservative tibial resection when compared to conventional UKA and may be advantageous if conversion to TKA becomes necessary.
The purpose of this study was to compare the distribution of polyethylene insert sizes implanted during conventional and robotic-assisted UKA. We hypothesized that robotic assistance would demonstrate more conservative tibial resection compared to conventional methods of bone preparation.
Methods
We retrospectively compared the distribution of polyethylene insert sizes implanted during consecutive conventional and robotic-assisted UKA procedures. Several manufacturers were queried to provide a listing of the polyethylene insert sizes utilized, ranging from 8 mm to 14 mm. The analysis included 8421 robotic-assisted UKA cases and 27,989 conventional UKA cases. Data were provided by Zimmer Biomet and Smith & Nephew regarding conventional cases, as well as Blue Belt Technologies (now part of Smith & Nephew) and MAKO Surgical (now part of Stryker) regarding robotic-assisted cases. (Dr. Lonner has an ongoing relationship as a consultant with Blue Belt Technologies, whose data was utilized in this study.) Using tibial insert thickness as a surrogate measure of the extent of tibial resection, an insert size of ≥10 mm was defined as aggressive while <10 mm was considered conservative. This cutoff was established based on its corresponding resection level with primary TKA and the anticipated need for augments. Statistical analysis was performed using a Mann-Whitney-Wilcoxon test. Significance was set at P < .05.
Results
Tibial resection thickness was found to be most commonly conservative in nature, with sizes 8-mm and 9-mm polyethylene inserts utilized in the majority of both robotic-assisted and conventional UKA cases. However, statistically more 8-mm and 9-mm polyethylene inserts were used in the robotic group (93.6%) than in the conventional group (84.5%) (P < .0001; Figure). Aggressive tibial resection, requiring tibial inserts ≥10 mm, was performed in 6.4% of robotic-assisted cases and 15.5% of conventional cases.
Discussion
Robotic assistance enhances the accuracy of bone preparation, implant component alignment, and soft tissue balance in UKA.10-15 It has yet to be determined whether this improved accuracy translates to improved clinical performance or longevity of the UKA implant. However, we demonstrate that the enhanced accuracy of robotic technology results in more conservative tibial resection when compared to conventional techniques with a potential benefit suggested in the literature upon conversion to TKA.
The findings of this study have important implications for patients undergoing conversion of UKA to TKA, potentially optimizing the ease of revision and clinical outcomes. The outcomes of UKA conversion to TKA are often considered inferior to those of primary TKA, compromised by bone loss, need for augmentation, and challenges of restoring the joint line and rotation.9,16-22 Barrett and Scott18 reported only 66% of patients had good or excellent results at an average of 4.6 years of follow-up after UKA conversion to TKA. Over 50% required stemmed implants and bone graft or bone cement augmentation to address osseous insufficiency. The authors suggested that the primary determinant of the complexity of the conversion to TKA was the surgical technique used in the index procedure. They concluded that UKA conversion to TKA can be as successful as a primary TKA and primary TKA implants can be used without bone augmentation or stems during the revision procedure if minimal tibial bone is resected at the time of the index UKA.18 Schwarzkopf and colleagues9 supported this conclusion when they found that aggressive tibial resection during UKA resulted in the need for bone graft, stem, wedge, or augment in 70% of cases when converted to TKA. Similarly, Khan and colleagues23 found that 26% of patients required bone grafting and 26% required some form of augmentation, and Springer and colleagues3 reported that 68% required a graft, augment, or stem.3,22 Using data from the New Zealand Joint Registry, Pearse and colleagues5 reported that revision TKA components were necessary in 28% of patients and concluded that converting a UKA to TKA gives a less reliable result than primary TKA, and with functional results that are not significantly better than a revision from a TKA.
Conservative tibial resection during UKA minimizes the complexity and concerns of bone loss upon conversion to TKA. Schwarzkopf and colleagues9 found 96.6% of patients with conservative tibial resection received a primary TKA implant, without augments or stems. Furthermore, patients with a primary TKA implant showed improved tibial survivorship, with revision as an end point, compared with patients who received a TKA implant that required stems and augments or bone graft for support.9 Also emphasizing the importance of minimal tibial resection, O’Donnell and colleagues8 compared a cohort of patients undergoing conversion of a minimal resection resurfacing onlay-type UKA to TKA with a cohort of patients undergoing primary TKA. They found that 40% of patients required bone grafting for contained defects, 3.6% required metal augments, and 1.8% required stems.8 There was no significant difference between the groups in terms of range of motion, functional outcome, or radiologic outcomes. The authors concluded that revision of minimal resection resurfacing implants to TKA is associated with similar results to primary TKA and is superior to revision of UKA with greater bone loss. Prior studies have shown that one of the advantages of robotic-assisted UKA is the accuracy and precision of bone resection. The present study supports this premise by showing that tibial resection is significantly more conservative using robotic-assisted techniques when using tibial component thickness as a surrogate for extent of bone resection. While our study did not address implant durability or the impact of conservative resection on conversion to TKA, studies referenced above suggest that the conservative nature of bone preparation would have a relevant impact on the revision of the implant to TKA.
Our study is a retrospective case series that reports tibial component thickness as a surrogate for volume of tibial resection during UKA. While the implication is that more conservative tibial resection may optimize durability and ease of conversion to TKA, future study will be needed to compare robotic-assisted and conventional cases of UKA upon conversion to TKA in order to ascertain whether the more conventional resections of robotic-assisted UKA in fact lead to revision that is comparable with primary TKA in terms of bone loss at the time of revision, components utilized, the need for bone graft, augments, or stems, and clinical outcomes. Given the method of data collection in this study, we could not control for clinical deformity, selection bias, surgeon experience, or medial vs lateral knee compartments. These potential confounders represent weaknesses of this study.
In conclusion, conversion of UKA to TKA may be associated with significant osseous insufficiency, which may compromise patient outcomes in comparison to primary TKA. Studies have shown that UKA conversion to TKA is comparable to primary TKA when minimal tibial resection is performed during the UKA, and the need for augmentation, grafting or stems is increased with more aggressive tibial resection. This study has shown that when robotic assistance is utilized, tibial resection is more precise, less variable, and more conservative compared to conventional techniques.
Am J Orthop. 2016;45(7):E465-E468. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
Unicompartmental knee arthroplasty (UKA) is considered a less invasive approach for the treatment of unicompartmental knee arthritis when compared with total knee arthroplasty (TKA), with optimal preservation of kinematics.1 Despite excellent functional outcomes, conversion to TKA may be necessary if the UKA fails, or in patients with progressive knee arthritis. Some studies have found UKA conversion to TKA to be comparable with primary TKA,2,3 whereas others have found that conversion often requires bone graft, augments, and stemmed components and has increased complications and inferior results compared to primary TKA.4-7 While some studies report that <10% of UKA conversions to TKA require augments,2 others have found that as many as 76% require augments.4-8
Schwarzkopf and colleagues9 recently demonstrated that UKA conversion to TKA is comparable with primary TKA when a conservative tibial resection is performed during the index procedure. However, they reported increased complexity when greater tibial resection was performed and thicker polyethylene inserts were used at the time of the index UKA. The odds ratio of needing an augment or stem during the conversion to TKA was 26.8 (95% confidence interval, 3.71-194) when an aggressive tibial resection was performed during the UKA.9 Tibial resection thickness may thus be predictive of anticipated complexity of UKA revision to TKA and may aid in preoperative planning.
Robotic assistance has been shown to enhance the accuracy of bone preparation, implant component alignment, and soft tissue balance in UKA.10-15 It has yet to be determined whether this improved accuracy translates to improved clinical performance or longevity of the UKA implant. However, the enhanced accuracy of robotic technology may result in more conservative tibial resection when compared to conventional UKA and may be advantageous if conversion to TKA becomes necessary.
The purpose of this study was to compare the distribution of polyethylene insert sizes implanted during conventional and robotic-assisted UKA. We hypothesized that robotic assistance would demonstrate more conservative tibial resection compared to conventional methods of bone preparation.
Methods
We retrospectively compared the distribution of polyethylene insert sizes implanted during consecutive conventional and robotic-assisted UKA procedures. Several manufacturers were queried to provide a listing of the polyethylene insert sizes utilized, ranging from 8 mm to 14 mm. The analysis included 8421 robotic-assisted UKA cases and 27,989 conventional UKA cases. Data were provided by Zimmer Biomet and Smith & Nephew regarding conventional cases, as well as Blue Belt Technologies (now part of Smith & Nephew) and MAKO Surgical (now part of Stryker) regarding robotic-assisted cases. (Dr. Lonner has an ongoing relationship as a consultant with Blue Belt Technologies, whose data was utilized in this study.) Using tibial insert thickness as a surrogate measure of the extent of tibial resection, an insert size of ≥10 mm was defined as aggressive while <10 mm was considered conservative. This cutoff was established based on its corresponding resection level with primary TKA and the anticipated need for augments. Statistical analysis was performed using a Mann-Whitney-Wilcoxon test. Significance was set at P < .05.
Results
Tibial resection thickness was found to be most commonly conservative in nature, with sizes 8-mm and 9-mm polyethylene inserts utilized in the majority of both robotic-assisted and conventional UKA cases. However, statistically more 8-mm and 9-mm polyethylene inserts were used in the robotic group (93.6%) than in the conventional group (84.5%) (P < .0001; Figure). Aggressive tibial resection, requiring tibial inserts ≥10 mm, was performed in 6.4% of robotic-assisted cases and 15.5% of conventional cases.
Discussion
Robotic assistance enhances the accuracy of bone preparation, implant component alignment, and soft tissue balance in UKA.10-15 It has yet to be determined whether this improved accuracy translates to improved clinical performance or longevity of the UKA implant. However, we demonstrate that the enhanced accuracy of robotic technology results in more conservative tibial resection when compared to conventional techniques with a potential benefit suggested in the literature upon conversion to TKA.
The findings of this study have important implications for patients undergoing conversion of UKA to TKA, potentially optimizing the ease of revision and clinical outcomes. The outcomes of UKA conversion to TKA are often considered inferior to those of primary TKA, compromised by bone loss, need for augmentation, and challenges of restoring the joint line and rotation.9,16-22 Barrett and Scott18 reported only 66% of patients had good or excellent results at an average of 4.6 years of follow-up after UKA conversion to TKA. Over 50% required stemmed implants and bone graft or bone cement augmentation to address osseous insufficiency. The authors suggested that the primary determinant of the complexity of the conversion to TKA was the surgical technique used in the index procedure. They concluded that UKA conversion to TKA can be as successful as a primary TKA and primary TKA implants can be used without bone augmentation or stems during the revision procedure if minimal tibial bone is resected at the time of the index UKA.18 Schwarzkopf and colleagues9 supported this conclusion when they found that aggressive tibial resection during UKA resulted in the need for bone graft, stem, wedge, or augment in 70% of cases when converted to TKA. Similarly, Khan and colleagues23 found that 26% of patients required bone grafting and 26% required some form of augmentation, and Springer and colleagues3 reported that 68% required a graft, augment, or stem.3,22 Using data from the New Zealand Joint Registry, Pearse and colleagues5 reported that revision TKA components were necessary in 28% of patients and concluded that converting a UKA to TKA gives a less reliable result than primary TKA, and with functional results that are not significantly better than a revision from a TKA.
Conservative tibial resection during UKA minimizes the complexity and concerns of bone loss upon conversion to TKA. Schwarzkopf and colleagues9 found 96.6% of patients with conservative tibial resection received a primary TKA implant, without augments or stems. Furthermore, patients with a primary TKA implant showed improved tibial survivorship, with revision as an end point, compared with patients who received a TKA implant that required stems and augments or bone graft for support.9 Also emphasizing the importance of minimal tibial resection, O’Donnell and colleagues8 compared a cohort of patients undergoing conversion of a minimal resection resurfacing onlay-type UKA to TKA with a cohort of patients undergoing primary TKA. They found that 40% of patients required bone grafting for contained defects, 3.6% required metal augments, and 1.8% required stems.8 There was no significant difference between the groups in terms of range of motion, functional outcome, or radiologic outcomes. The authors concluded that revision of minimal resection resurfacing implants to TKA is associated with similar results to primary TKA and is superior to revision of UKA with greater bone loss. Prior studies have shown that one of the advantages of robotic-assisted UKA is the accuracy and precision of bone resection. The present study supports this premise by showing that tibial resection is significantly more conservative using robotic-assisted techniques when using tibial component thickness as a surrogate for extent of bone resection. While our study did not address implant durability or the impact of conservative resection on conversion to TKA, studies referenced above suggest that the conservative nature of bone preparation would have a relevant impact on the revision of the implant to TKA.
Our study is a retrospective case series that reports tibial component thickness as a surrogate for volume of tibial resection during UKA. While the implication is that more conservative tibial resection may optimize durability and ease of conversion to TKA, future study will be needed to compare robotic-assisted and conventional cases of UKA upon conversion to TKA in order to ascertain whether the more conventional resections of robotic-assisted UKA in fact lead to revision that is comparable with primary TKA in terms of bone loss at the time of revision, components utilized, the need for bone graft, augments, or stems, and clinical outcomes. Given the method of data collection in this study, we could not control for clinical deformity, selection bias, surgeon experience, or medial vs lateral knee compartments. These potential confounders represent weaknesses of this study.
In conclusion, conversion of UKA to TKA may be associated with significant osseous insufficiency, which may compromise patient outcomes in comparison to primary TKA. Studies have shown that UKA conversion to TKA is comparable to primary TKA when minimal tibial resection is performed during the UKA, and the need for augmentation, grafting or stems is increased with more aggressive tibial resection. This study has shown that when robotic assistance is utilized, tibial resection is more precise, less variable, and more conservative compared to conventional techniques.
Am J Orthop. 2016;45(7):E465-E468. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. Patil S, Colwell CW Jr, Ezzet KA, D’Lima DD. Can normal knee kinematics be restored with unicompartmental knee replacement? J Bone Joint Surg Am. 2005;87(2):332-338.
2. Johnson S, Jones P, Newman JH. The survivorship and results of total knee replacements converted from unicompartmental knee replacements. Knee. 2007;14(2):154-157.
3. Springer BD, Scott RD, Thornhill TS. Conversion of failed unicompartmental knee arthroplasty to TKA. Clin Orthop Relat Res. 2006;446:214-220.
4. Järvenpää J, Kettunen J, Miettinen H, Kröger H. The clinical outcome of revision knee replacement after unicompartmental knee arthroplasty versus primary total knee arthroplasty: 8-17 years follow-up study of 49 patients. Int Orthop. 2010;34(5):649-653.
5. Pearse AJ, Hooper GJ, Rothwell AG, Frampton C. Osteotomy and unicompartmental knee arthroplasty converted to total knee arthroplasty: data from the New Zealand Joint Registry. J Arthroplasty. 2012;27(10):1827-1831.
6. Rancourt MF, Kemp KA, Plamondon SM, Kim PR, Dervin GF. Unicompartmental knee arthroplasties revised to total knee arthroplasties compared with primary total knee arthroplasties. J Arthroplasty. 2012;27(8 Suppl):106-110.
7. Sierra RJ, Kassel CA, Wetters NG, Berend KR, Della Valle CJ, Lombardi AV. Revision of unicompartmental arthroplasty to total knee arthroplasty: not always a slam dunk! J Arthroplasty. 2013;28(8 Suppl):128-132.
8. O’Donnell TM, Abouazza O, Neil MJ. Revision of minimal resection resurfacing unicondylar knee arthroplasty to total knee arthroplasty: results compared with primary total knee arthroplasty. J Arthroplasty. 2013;28(1):33-39.
9. Schwarzkopf R, Mikhael B, Li L, Josephs L, Scott RD. Effect of initial tibial resection thickness on outcomes of revision UKA. Orthopedics. 2013;36(4):e409-e414.
10. Conditt MA, Roche MW. Minimally invasive robotic-arm-guided unicompartmental knee arthroplasty. J Bone Joint Surg Am. 2009;91 Suppl 1:63-68.
11. Dunbar NJ, Roche MW, Park BH, Branch SH, Conditt MA, Banks SA. Accuracy of dynamic tactile-guided unicompartmental knee arthroplasty. J Arthroplasty. 2012;27(5):803-808.e1.
12. Karia M, Masjedi M, Andrews B, Jaffry Z, Cobb J. Robotic assistance enables inexperienced surgeons to perform unicompartmental knee arthroplasties on dry bone models with accuracy superior to conventional methods. Adv Orthop. 2013;2013:481039.
13. Lonner JH, John TK, Conditt MA. Robotic arm-assisted UKA improves tibial component alignment: a pilot study. Clin Orthop Relat Res. 2010;468(1):141-146.
14. Lonner JH, Smith JR, Picard F, Hamlin B, Rowe PJ, Riches PE. High degree of accuracy of a novel image-free handheld robot for unicondylar knee arthroplasty in a cadaveric study. Clin Orthop Relat Res. 2015;473(1):206-212.
15. Smith JR, Picard F, Rowe PJ, Deakin A, Riches PE. The accuracy of a robotically-controlled freehand sculpting tool for unicondylar knee arthroplasty. Bone Joint J. 2013;95-B(suppl 28):68.
16. Chakrabarty G, Newman JH, Ackroyd CE. Revision of unicompartmental arthroplasty of the knee. Clinical and technical considerations. J Arthroplasty. 1998;13(2):191-196.
17. Levine WN, Ozuna RM, Scott RD, Thornhill TS. Conversion of failed modern unicompartmental arthroplasty to total knee arthroplasty. J Arthroplasty. 1996;11(7):797-801.
18. Barrett WP, Scott RD. Revision of failed unicondylar unicompartmental knee arthroplasty. J Bone Joint Surg Am. 1987;69(9):1328-1335.
19. Padgett DE, Stern SH, Insall JN. Revision total knee arthroplasty for failed unicompartmental replacement. J Bone Joint Surg Am. 1991;73(2):186-190.
20. Aleto TJ, Berend ME, Ritter MA, Faris PM, Meneghini RM. Early failure of unicompartmental knee arthroplasty leading to revision. J Arthroplasty. 2008;23(2):159-163.
21. McAuley JP, Engh GA, Ammeen DJ. Revision of failed unicompartmental knee arthroplasty. Clin Orthop Relat Res. 2001;(392):279-282.22. Böhm I, Landsiedl F. Revision surgery after failed unicompartmental knee arthroplasty: a study of 35 cases. J Arthroplasty. 2000;15(8):982-989.
23. Khan Z, Nawaz SZ, Kahane S, Ester C, Chatterji U. Conversion of unicompartmental knee arthroplasty to total knee arthroplasty: the challenges and need for augments. Acta Orthop Belg. 2013;79(6):699-705.
1. Patil S, Colwell CW Jr, Ezzet KA, D’Lima DD. Can normal knee kinematics be restored with unicompartmental knee replacement? J Bone Joint Surg Am. 2005;87(2):332-338.
2. Johnson S, Jones P, Newman JH. The survivorship and results of total knee replacements converted from unicompartmental knee replacements. Knee. 2007;14(2):154-157.
3. Springer BD, Scott RD, Thornhill TS. Conversion of failed unicompartmental knee arthroplasty to TKA. Clin Orthop Relat Res. 2006;446:214-220.
4. Järvenpää J, Kettunen J, Miettinen H, Kröger H. The clinical outcome of revision knee replacement after unicompartmental knee arthroplasty versus primary total knee arthroplasty: 8-17 years follow-up study of 49 patients. Int Orthop. 2010;34(5):649-653.
5. Pearse AJ, Hooper GJ, Rothwell AG, Frampton C. Osteotomy and unicompartmental knee arthroplasty converted to total knee arthroplasty: data from the New Zealand Joint Registry. J Arthroplasty. 2012;27(10):1827-1831.
6. Rancourt MF, Kemp KA, Plamondon SM, Kim PR, Dervin GF. Unicompartmental knee arthroplasties revised to total knee arthroplasties compared with primary total knee arthroplasties. J Arthroplasty. 2012;27(8 Suppl):106-110.
7. Sierra RJ, Kassel CA, Wetters NG, Berend KR, Della Valle CJ, Lombardi AV. Revision of unicompartmental arthroplasty to total knee arthroplasty: not always a slam dunk! J Arthroplasty. 2013;28(8 Suppl):128-132.
8. O’Donnell TM, Abouazza O, Neil MJ. Revision of minimal resection resurfacing unicondylar knee arthroplasty to total knee arthroplasty: results compared with primary total knee arthroplasty. J Arthroplasty. 2013;28(1):33-39.
9. Schwarzkopf R, Mikhael B, Li L, Josephs L, Scott RD. Effect of initial tibial resection thickness on outcomes of revision UKA. Orthopedics. 2013;36(4):e409-e414.
10. Conditt MA, Roche MW. Minimally invasive robotic-arm-guided unicompartmental knee arthroplasty. J Bone Joint Surg Am. 2009;91 Suppl 1:63-68.
11. Dunbar NJ, Roche MW, Park BH, Branch SH, Conditt MA, Banks SA. Accuracy of dynamic tactile-guided unicompartmental knee arthroplasty. J Arthroplasty. 2012;27(5):803-808.e1.
12. Karia M, Masjedi M, Andrews B, Jaffry Z, Cobb J. Robotic assistance enables inexperienced surgeons to perform unicompartmental knee arthroplasties on dry bone models with accuracy superior to conventional methods. Adv Orthop. 2013;2013:481039.
13. Lonner JH, John TK, Conditt MA. Robotic arm-assisted UKA improves tibial component alignment: a pilot study. Clin Orthop Relat Res. 2010;468(1):141-146.
14. Lonner JH, Smith JR, Picard F, Hamlin B, Rowe PJ, Riches PE. High degree of accuracy of a novel image-free handheld robot for unicondylar knee arthroplasty in a cadaveric study. Clin Orthop Relat Res. 2015;473(1):206-212.
15. Smith JR, Picard F, Rowe PJ, Deakin A, Riches PE. The accuracy of a robotically-controlled freehand sculpting tool for unicondylar knee arthroplasty. Bone Joint J. 2013;95-B(suppl 28):68.
16. Chakrabarty G, Newman JH, Ackroyd CE. Revision of unicompartmental arthroplasty of the knee. Clinical and technical considerations. J Arthroplasty. 1998;13(2):191-196.
17. Levine WN, Ozuna RM, Scott RD, Thornhill TS. Conversion of failed modern unicompartmental arthroplasty to total knee arthroplasty. J Arthroplasty. 1996;11(7):797-801.
18. Barrett WP, Scott RD. Revision of failed unicondylar unicompartmental knee arthroplasty. J Bone Joint Surg Am. 1987;69(9):1328-1335.
19. Padgett DE, Stern SH, Insall JN. Revision total knee arthroplasty for failed unicompartmental replacement. J Bone Joint Surg Am. 1991;73(2):186-190.
20. Aleto TJ, Berend ME, Ritter MA, Faris PM, Meneghini RM. Early failure of unicompartmental knee arthroplasty leading to revision. J Arthroplasty. 2008;23(2):159-163.
21. McAuley JP, Engh GA, Ammeen DJ. Revision of failed unicompartmental knee arthroplasty. Clin Orthop Relat Res. 2001;(392):279-282.22. Böhm I, Landsiedl F. Revision surgery after failed unicompartmental knee arthroplasty: a study of 35 cases. J Arthroplasty. 2000;15(8):982-989.
23. Khan Z, Nawaz SZ, Kahane S, Ester C, Chatterji U. Conversion of unicompartmental knee arthroplasty to total knee arthroplasty: the challenges and need for augments. Acta Orthop Belg. 2013;79(6):699-705.
An Overview of the History of Orthopedic Surgery
The modern term orthopedics stems from the older word orthopedia, which was the title of a book published in 1741 by Nicholas Andry, a professor of medicine at the University of Paris.1 The term orthopedia is a composite of 2 Greek words: orthos, meaning “straight and free from deformity,” and paidios, meaning “child.” Together, orthopedics literally means straight child, suggesting the importance of pediatric injuries and deformities in the development of this field. Interestingly, Andry’s book also depicted a crooked young tree attached to a straight and strong staff, which has become the universal symbol of orthopedic surgery and underscores the focus on correcting deformities in the young (Figure).1
Orthopedic surgery is a rapidly advancing medical field with several recent advances noted within orthopedic subspecialties,2-4 basic science,5 and clinical research.6 It is important to recognize the role of history with regards to innovation and research, especially for young trainees and medical students interested in a particular medical specialty. More specifically, it is important to understand the successes and failures of the past in order to advance research and practice, and ultimately improve patient care and outcomes.
In the recent literature, there is no concise yet comprehensive article focusing on the history of orthopedic surgery. The goal of this review is to provide an overview of the history and development of orthopedic surgery from ancient practices to the modern era.
Ancient Orthopedics
While the evidence is limited, the practice of orthopedics dates back to the primitive man.7 Fossil evidence suggests that the orthopedic pathology of today, such as fractures and traumatic amputations, existed in primitive times.8 The union of fractures in fair alignment has also been observed, which emphasizes the efficacy of nonoperative orthopedics and suggests the early use of splints and rehabilitation practices.8,9 Since procedures such as trepanation and crude amputations occurred during the New Stone Age, it is feasible that sophisticated techniques had also been developed for the treatment of injuries.7-9 However, evidence continues to remain limited.7
Later civilizations also developed creative ways to manage orthopedic injuries. For example, the Shoshone Indians, who were known to exist around 700-2000 BCE, made a splint of fresh rawhide that had been soaked in water.9,10 Similarly, some South Australian tribes made splints of clay, which when dried were as good as plaster of Paris.9 Furthermore, bone-setting or reductions was practiced as a profession in many tribes, underscoring the importance of orthopedic injuries in early civilizations.8,9
Ancient Egypt
The ancient Egyptians seemed to have carried on the practices of splinting. For example, 2 splinted specimens were discovered during the Hearst Egyptian Expedition in 1903.7 More specifically, these specimens included a femur and forearm and dated to approximately 300 BCE.7 Other examples of splints made of bamboo and reed padded with linen have been found on mummies as well.8 Similarly, crutches were also used by this civilization, as depicted on a carving made on an Egyptian tomb in 2830 BCE.8
One of the earliest and most significant documents on medicine was discovered in 1862, known as the Edwin Smith papyrus. This document is thought to have been composed by Imhotep, a prominent Egyptian physician, astrologer, architect, and politician, and it specifically categorizes diseases and treatments. Many scholars recognize this medical document as the oldest surgical textbook.11,12 With regards to orthopedic conditions, this document describes the reduction of a dislocated mandible, signs of spinal or vertebral injuries, description of torticollis, and the treatment of fractures such as clavicle fractures.8 This document also discusses ryt, which refers to the purulent discharge from osteomyelitis.8 The following is an excerpt from this ancient document:9
“Instructions on erring a break in his upper arm…Thou shouldst spread out with his two shoulders in order to stretch apart his upper arm until that break falls into its place. Thou shouldst make for him two splints of linen, and thou shouldst apply for him one of them both on the inside of his arm, and the other of them both on the underside of his arm.”
This account illustrates the methodical and meticulous nature of this textbook, and it highlights some of the essentials of medical practice from diagnosis to medical decision-making to treatment.
There are various other contributions to the field of medicine from the Far East; however, many of these pertain to the fields of plastic surgery and general surgery.9
Greeks and Romans
The Greeks are considered to be the first to systematically employ the scientific approach to medicine.8 In the period between 430 BCE to 330 BCE, the Corpus Hippocrates was compiled, which is a Greek text on medicine. It is named for Hippocrates (460 BCE-370 BCE), the father of medicine, and it contains text that applies specifically to the field of orthopedic surgery. For example, this text discuses shoulder dislocations and describes various reduction maneuvers. Hippocrates had a keen understanding of the principles of traction and countertraction, especially as it pertains to the musculoskeletal system.8 In fact, the Hippocratic method is still used for reducing anterior shoulder dislocations, and its description can be found in several modern orthopedic texts, including recent articles.13 The Corpus Hippocrates also describes the correction of clubfoot deformity, and the treatment of infected open fractures with pitch cerate and wine compresses.8
Hippocrates also described the treatment of fractures, the principles of traction, and the implications of malunions. For example, Hippocrates wrote, “For the arm, when shortened, might be concealed and the mistake will not be great, but a shortened thigh bone will leave a man maimed.”1 In addition, spinal deformities were recognized by the Greeks, and Hippocrates devised an extension bench for the correction of such deformities.1 From their contributions to anatomy and surgical practice, the Greeks have made significant contributions to the field of surgery.9
During the Roman period, another Greek surgeon by the name of Galen described the musculoskeletal and nervous systems. He served as a gladiatorial surgeon in Rome, and today, he is considered to be the father of sports medicine.8 He is also credited with coining the terms scoliosis, kyphosis, and lordosis to denote the spinal deformities that were first described by Hippocrates.1 In the Roman period, amputations were also performed, and primitive prostheses were developed.9
The Middle Ages
There was relatively little progress in the study of medicine for a thousand years after the fall of the Roman Empire.9 This stagnation was predominantly due to the early Christian Church inhibiting freedom of thought and observation, as well as prohibiting human dissection and the study of anatomy. The first medical school in Europe was established in Salerno, Italy, during the ninth century. This school provided primarily pedantic teaching to its students and perpetuated the theories of the elements and humors. Later on, the University of Bologna became one of the first academic institutions to offer hands-on surgical training.9 One of the most famous surgeons of the Middle Ages was Guy de Chuauliac, who studied at Montpellier and Bologna. He was a leader in the ethical principles of surgery as well as the practice of surgery, and wrote the following with regards to femur fractures:9
“After the application of splints, I attach to the foot a mass of lead as a weight, taking care to pass the cord which supports the weight over a small pulley in such a manner that it shall pull on the leg in a horizontal direction.”
This description is strikingly similar to the modern-day nonoperative management of femur fractures, and underscores the importance of traction, which as mentioned above, was first described by Hippocrates.
Eventually, medicine began to separate from the Church, most likely due to an increase in the complexity of medical theories, the rise of secular universities, and an increase in medical knowledge from Eastern and Middle-Eastern groups.9
The Renaissance and the Foundations of Modern Orthopedics
Until the 16th century, the majority of medical theories were heavily influenced by the work of Hippocrates.8 The scientific study of anatomy gained prominence during this time, especially due to the work done by great artists, such as Leonardo Di Vinci.9 The Table
After a period of rapid expansion of the field of orthopedics, and following the Renaissance, many hospitals were built focusing on the sick and disabled, which solidified orthopedics’ position as a major medical specialty.1 For example, in 1863, James Knight founded the Hospital for the Ruptured and Crippled in New York City. This hospital became the oldest orthopedic hospital in the United States, and it later became known as the Hospital for Special Surgery.14,15 Several additional orthopedic institutions were formed, including the New York Orthopedic Dispensary in 1886 and Hospital for Deformities and Joint Diseases in 1917. Orthopedic surgery residency programs also began to be developed in the late 1800s.14 More specifically, Virgil Gibney at Hospital for the Ruptured and Crippled began the first orthopedic training program in the United States in 1888. Young doctors in this program trained for 1 year as junior assistant, senior assistant, and house surgeon, and began to be known as resident doctors.14
The Modern Era
In the 20th century, rapid development continued to better control infections as well as develop and introduce novel technology. For example, the invention of x-ray in 1895 by Wilhelm Conrad Röntgen improved our ability to diagnose and manage orthopedic conditions ranging from fractures to avascular necrosis of the femoral head to osteoarthritis.8,14 Spinal surgery also developed rapidly with Russell Hibbs describing a technique for spinal fusion at the New York Orthopedic Hospital.8 Similarly, the World Wars served as a catalyst in the development of the subspecialty of orthopedic trauma, with increasing attention placed on open wounds and proficiency with amputations, internal fixation, and wound care. In 1942, Austin Moore performed the first metal hip arthroplasty, and the field of joint replacement was subsequently advanced by the work of Sir John Charnley in the 1960s.8
Conclusion
Despite its relatively recent specialization, orthopedic surgery has a rich history rooted in ancient practices dating back to the primitive man. Over time, there has been significant development in the field in terms of surgical and nonsurgical treatment of orthopedic pathology and disease. Various cultures have played an instrumental role in developing this field, and it is remarkable to see that several practices have persisted since the time of these ancient civilizations. During the Renaissance, there was a considerable emphasis placed on pediatric deformity, but orthopedic surgeons have now branched out to subspecialty practice ranging from orthopedic trauma to joint replacement to oncology.1 For students of medicine and orthopedics, it is important to learn about the origins of this field and to appreciate its gradual development. Orthopedic surgery is a diverse and fascinating field that will most likely continue to develop with increased subspecialization and improved research at the molecular and population level. With a growing emphasis placed on outcomes and healthcare cost by today’s society, it will be fascinating to see how this field continues to evolve in the future.
Am J Orthop. 2016;45(7):E434-E438. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. Ponseti IV. History of orthopedic surgery. Iowa Orthop J. 1991;11:59-64.
2. Ninomiya JT, Dean JC, Incavo SJ. What’s new in hip replacement. J Bone Joint Surg Am. 2015;97(18):1543-1551.
3. Sabharwal S, Nelson SC, Sontich JK. What’s new in limb lengthening and deformity correction. J Bone Joint Surg Am. 2015;97(16):1375-1384.
4. Ricci WM, Black JC, McAndrew CM, Gardner MJ. What’s new in orthopedic trauma. J Bone Joint Surg Am. 2015;97(14):1200-1207.
5. Rodeo SA, Sugiguchi F, Fortier LA, Cunningham ME, Maher S. What’s new in orthopedic research. J Bone Joint Surg Am. 2014;96(23):2015-2019.
6. Pugley AJ, Martin CT, Harwood J, Ong KL, Bozic KJ, Callaghan JJ. Database and registry research in orthopedic surgery. Part 1: Claims-based data. J Bone Joint Surg Am. 2015;97(15):1278-1287.
7. Colton CL. The history of fracture treatment. In: Browner BD, Jupiter JB, Levine AM, Trafton PG, Krettek C, eds. Skeletal Trauma: Basic Science, Management, and Reconstruction. 4th ed. Philadelphia, PA: Saunders Elsevier; 2009:3-32.
8. Brakoulias,V. History of orthopaedics. WorldOrtho Web site. http://pioa.net/documents/Historyoforthopaedics.pdf. Accessed October 6, 2016.
9. Bishop WJ. The Early History of Surgery. New York, NY: Barnes & Noble Books; 1995.
10. Watson T. Wyoming site reveals more prehistoric mountain villages. USA Today. October 20, 2013. http://www.usatoday.com/story/news/nation/2013/10/20/wyoming-prehistoric-villages/2965263. Accessed October 6, 2016.
11. Minagar A, Ragheb J, Kelley RE. The Edwin Smith surgical papyrus: description and analysis of the earliest case of aphasia. J Med Biogr. 2003;11(2):114-117.
12. Atta HM. Edwin Smith Surgical Papyrus: the oldest known surgical treatise. Am Surg. 1999;65(12):1190-1192.
13. Sayegh FE, Kenanidis EI, Papavasiliou KA, Potoupnis ME, Kirkos JM, Kapetanos GA. Reduction of acute anterior dislocations: a prospective randomized study comparing a new technique with the Hippocratic and Kocher methods. J Bone Joint Surg Am. 2009;91(12):2775-2782.
14. Levine DB. Anatomy of a Hospital: Hospital for Special Surgery 1863-2013. New York, NY: Print Mattes; 2013.
15. Wilson PD, Levine DB. Hospital for special surgery. A brief review of its development and current position. Clin Orthop Relat Res. 2000;(374):90-106.
The modern term orthopedics stems from the older word orthopedia, which was the title of a book published in 1741 by Nicholas Andry, a professor of medicine at the University of Paris.1 The term orthopedia is a composite of 2 Greek words: orthos, meaning “straight and free from deformity,” and paidios, meaning “child.” Together, orthopedics literally means straight child, suggesting the importance of pediatric injuries and deformities in the development of this field. Interestingly, Andry’s book also depicted a crooked young tree attached to a straight and strong staff, which has become the universal symbol of orthopedic surgery and underscores the focus on correcting deformities in the young (Figure).1
Orthopedic surgery is a rapidly advancing medical field with several recent advances noted within orthopedic subspecialties,2-4 basic science,5 and clinical research.6 It is important to recognize the role of history with regards to innovation and research, especially for young trainees and medical students interested in a particular medical specialty. More specifically, it is important to understand the successes and failures of the past in order to advance research and practice, and ultimately improve patient care and outcomes.
In the recent literature, there is no concise yet comprehensive article focusing on the history of orthopedic surgery. The goal of this review is to provide an overview of the history and development of orthopedic surgery from ancient practices to the modern era.
Ancient Orthopedics
While the evidence is limited, the practice of orthopedics dates back to the primitive man.7 Fossil evidence suggests that the orthopedic pathology of today, such as fractures and traumatic amputations, existed in primitive times.8 The union of fractures in fair alignment has also been observed, which emphasizes the efficacy of nonoperative orthopedics and suggests the early use of splints and rehabilitation practices.8,9 Since procedures such as trepanation and crude amputations occurred during the New Stone Age, it is feasible that sophisticated techniques had also been developed for the treatment of injuries.7-9 However, evidence continues to remain limited.7
Later civilizations also developed creative ways to manage orthopedic injuries. For example, the Shoshone Indians, who were known to exist around 700-2000 BCE, made a splint of fresh rawhide that had been soaked in water.9,10 Similarly, some South Australian tribes made splints of clay, which when dried were as good as plaster of Paris.9 Furthermore, bone-setting or reductions was practiced as a profession in many tribes, underscoring the importance of orthopedic injuries in early civilizations.8,9
Ancient Egypt
The ancient Egyptians seemed to have carried on the practices of splinting. For example, 2 splinted specimens were discovered during the Hearst Egyptian Expedition in 1903.7 More specifically, these specimens included a femur and forearm and dated to approximately 300 BCE.7 Other examples of splints made of bamboo and reed padded with linen have been found on mummies as well.8 Similarly, crutches were also used by this civilization, as depicted on a carving made on an Egyptian tomb in 2830 BCE.8
One of the earliest and most significant documents on medicine was discovered in 1862, known as the Edwin Smith papyrus. This document is thought to have been composed by Imhotep, a prominent Egyptian physician, astrologer, architect, and politician, and it specifically categorizes diseases and treatments. Many scholars recognize this medical document as the oldest surgical textbook.11,12 With regards to orthopedic conditions, this document describes the reduction of a dislocated mandible, signs of spinal or vertebral injuries, description of torticollis, and the treatment of fractures such as clavicle fractures.8 This document also discusses ryt, which refers to the purulent discharge from osteomyelitis.8 The following is an excerpt from this ancient document:9
“Instructions on erring a break in his upper arm…Thou shouldst spread out with his two shoulders in order to stretch apart his upper arm until that break falls into its place. Thou shouldst make for him two splints of linen, and thou shouldst apply for him one of them both on the inside of his arm, and the other of them both on the underside of his arm.”
This account illustrates the methodical and meticulous nature of this textbook, and it highlights some of the essentials of medical practice from diagnosis to medical decision-making to treatment.
There are various other contributions to the field of medicine from the Far East; however, many of these pertain to the fields of plastic surgery and general surgery.9
Greeks and Romans
The Greeks are considered to be the first to systematically employ the scientific approach to medicine.8 In the period between 430 BCE to 330 BCE, the Corpus Hippocrates was compiled, which is a Greek text on medicine. It is named for Hippocrates (460 BCE-370 BCE), the father of medicine, and it contains text that applies specifically to the field of orthopedic surgery. For example, this text discuses shoulder dislocations and describes various reduction maneuvers. Hippocrates had a keen understanding of the principles of traction and countertraction, especially as it pertains to the musculoskeletal system.8 In fact, the Hippocratic method is still used for reducing anterior shoulder dislocations, and its description can be found in several modern orthopedic texts, including recent articles.13 The Corpus Hippocrates also describes the correction of clubfoot deformity, and the treatment of infected open fractures with pitch cerate and wine compresses.8
Hippocrates also described the treatment of fractures, the principles of traction, and the implications of malunions. For example, Hippocrates wrote, “For the arm, when shortened, might be concealed and the mistake will not be great, but a shortened thigh bone will leave a man maimed.”1 In addition, spinal deformities were recognized by the Greeks, and Hippocrates devised an extension bench for the correction of such deformities.1 From their contributions to anatomy and surgical practice, the Greeks have made significant contributions to the field of surgery.9
During the Roman period, another Greek surgeon by the name of Galen described the musculoskeletal and nervous systems. He served as a gladiatorial surgeon in Rome, and today, he is considered to be the father of sports medicine.8 He is also credited with coining the terms scoliosis, kyphosis, and lordosis to denote the spinal deformities that were first described by Hippocrates.1 In the Roman period, amputations were also performed, and primitive prostheses were developed.9
The Middle Ages
There was relatively little progress in the study of medicine for a thousand years after the fall of the Roman Empire.9 This stagnation was predominantly due to the early Christian Church inhibiting freedom of thought and observation, as well as prohibiting human dissection and the study of anatomy. The first medical school in Europe was established in Salerno, Italy, during the ninth century. This school provided primarily pedantic teaching to its students and perpetuated the theories of the elements and humors. Later on, the University of Bologna became one of the first academic institutions to offer hands-on surgical training.9 One of the most famous surgeons of the Middle Ages was Guy de Chuauliac, who studied at Montpellier and Bologna. He was a leader in the ethical principles of surgery as well as the practice of surgery, and wrote the following with regards to femur fractures:9
“After the application of splints, I attach to the foot a mass of lead as a weight, taking care to pass the cord which supports the weight over a small pulley in such a manner that it shall pull on the leg in a horizontal direction.”
This description is strikingly similar to the modern-day nonoperative management of femur fractures, and underscores the importance of traction, which as mentioned above, was first described by Hippocrates.
Eventually, medicine began to separate from the Church, most likely due to an increase in the complexity of medical theories, the rise of secular universities, and an increase in medical knowledge from Eastern and Middle-Eastern groups.9
The Renaissance and the Foundations of Modern Orthopedics
Until the 16th century, the majority of medical theories were heavily influenced by the work of Hippocrates.8 The scientific study of anatomy gained prominence during this time, especially due to the work done by great artists, such as Leonardo Di Vinci.9 The Table
After a period of rapid expansion of the field of orthopedics, and following the Renaissance, many hospitals were built focusing on the sick and disabled, which solidified orthopedics’ position as a major medical specialty.1 For example, in 1863, James Knight founded the Hospital for the Ruptured and Crippled in New York City. This hospital became the oldest orthopedic hospital in the United States, and it later became known as the Hospital for Special Surgery.14,15 Several additional orthopedic institutions were formed, including the New York Orthopedic Dispensary in 1886 and Hospital for Deformities and Joint Diseases in 1917. Orthopedic surgery residency programs also began to be developed in the late 1800s.14 More specifically, Virgil Gibney at Hospital for the Ruptured and Crippled began the first orthopedic training program in the United States in 1888. Young doctors in this program trained for 1 year as junior assistant, senior assistant, and house surgeon, and began to be known as resident doctors.14
The Modern Era
In the 20th century, rapid development continued to better control infections as well as develop and introduce novel technology. For example, the invention of x-ray in 1895 by Wilhelm Conrad Röntgen improved our ability to diagnose and manage orthopedic conditions ranging from fractures to avascular necrosis of the femoral head to osteoarthritis.8,14 Spinal surgery also developed rapidly with Russell Hibbs describing a technique for spinal fusion at the New York Orthopedic Hospital.8 Similarly, the World Wars served as a catalyst in the development of the subspecialty of orthopedic trauma, with increasing attention placed on open wounds and proficiency with amputations, internal fixation, and wound care. In 1942, Austin Moore performed the first metal hip arthroplasty, and the field of joint replacement was subsequently advanced by the work of Sir John Charnley in the 1960s.8
Conclusion
Despite its relatively recent specialization, orthopedic surgery has a rich history rooted in ancient practices dating back to the primitive man. Over time, there has been significant development in the field in terms of surgical and nonsurgical treatment of orthopedic pathology and disease. Various cultures have played an instrumental role in developing this field, and it is remarkable to see that several practices have persisted since the time of these ancient civilizations. During the Renaissance, there was a considerable emphasis placed on pediatric deformity, but orthopedic surgeons have now branched out to subspecialty practice ranging from orthopedic trauma to joint replacement to oncology.1 For students of medicine and orthopedics, it is important to learn about the origins of this field and to appreciate its gradual development. Orthopedic surgery is a diverse and fascinating field that will most likely continue to develop with increased subspecialization and improved research at the molecular and population level. With a growing emphasis placed on outcomes and healthcare cost by today’s society, it will be fascinating to see how this field continues to evolve in the future.
Am J Orthop. 2016;45(7):E434-E438. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
The modern term orthopedics stems from the older word orthopedia, which was the title of a book published in 1741 by Nicholas Andry, a professor of medicine at the University of Paris.1 The term orthopedia is a composite of 2 Greek words: orthos, meaning “straight and free from deformity,” and paidios, meaning “child.” Together, orthopedics literally means straight child, suggesting the importance of pediatric injuries and deformities in the development of this field. Interestingly, Andry’s book also depicted a crooked young tree attached to a straight and strong staff, which has become the universal symbol of orthopedic surgery and underscores the focus on correcting deformities in the young (Figure).1
Orthopedic surgery is a rapidly advancing medical field with several recent advances noted within orthopedic subspecialties,2-4 basic science,5 and clinical research.6 It is important to recognize the role of history with regards to innovation and research, especially for young trainees and medical students interested in a particular medical specialty. More specifically, it is important to understand the successes and failures of the past in order to advance research and practice, and ultimately improve patient care and outcomes.
In the recent literature, there is no concise yet comprehensive article focusing on the history of orthopedic surgery. The goal of this review is to provide an overview of the history and development of orthopedic surgery from ancient practices to the modern era.
Ancient Orthopedics
While the evidence is limited, the practice of orthopedics dates back to the primitive man.7 Fossil evidence suggests that the orthopedic pathology of today, such as fractures and traumatic amputations, existed in primitive times.8 The union of fractures in fair alignment has also been observed, which emphasizes the efficacy of nonoperative orthopedics and suggests the early use of splints and rehabilitation practices.8,9 Since procedures such as trepanation and crude amputations occurred during the New Stone Age, it is feasible that sophisticated techniques had also been developed for the treatment of injuries.7-9 However, evidence continues to remain limited.7
Later civilizations also developed creative ways to manage orthopedic injuries. For example, the Shoshone Indians, who were known to exist around 700-2000 BCE, made a splint of fresh rawhide that had been soaked in water.9,10 Similarly, some South Australian tribes made splints of clay, which when dried were as good as plaster of Paris.9 Furthermore, bone-setting or reductions was practiced as a profession in many tribes, underscoring the importance of orthopedic injuries in early civilizations.8,9
Ancient Egypt
The ancient Egyptians seemed to have carried on the practices of splinting. For example, 2 splinted specimens were discovered during the Hearst Egyptian Expedition in 1903.7 More specifically, these specimens included a femur and forearm and dated to approximately 300 BCE.7 Other examples of splints made of bamboo and reed padded with linen have been found on mummies as well.8 Similarly, crutches were also used by this civilization, as depicted on a carving made on an Egyptian tomb in 2830 BCE.8
One of the earliest and most significant documents on medicine was discovered in 1862, known as the Edwin Smith papyrus. This document is thought to have been composed by Imhotep, a prominent Egyptian physician, astrologer, architect, and politician, and it specifically categorizes diseases and treatments. Many scholars recognize this medical document as the oldest surgical textbook.11,12 With regards to orthopedic conditions, this document describes the reduction of a dislocated mandible, signs of spinal or vertebral injuries, description of torticollis, and the treatment of fractures such as clavicle fractures.8 This document also discusses ryt, which refers to the purulent discharge from osteomyelitis.8 The following is an excerpt from this ancient document:9
“Instructions on erring a break in his upper arm…Thou shouldst spread out with his two shoulders in order to stretch apart his upper arm until that break falls into its place. Thou shouldst make for him two splints of linen, and thou shouldst apply for him one of them both on the inside of his arm, and the other of them both on the underside of his arm.”
This account illustrates the methodical and meticulous nature of this textbook, and it highlights some of the essentials of medical practice from diagnosis to medical decision-making to treatment.
There are various other contributions to the field of medicine from the Far East; however, many of these pertain to the fields of plastic surgery and general surgery.9
Greeks and Romans
The Greeks are considered to be the first to systematically employ the scientific approach to medicine.8 In the period between 430 BCE to 330 BCE, the Corpus Hippocrates was compiled, which is a Greek text on medicine. It is named for Hippocrates (460 BCE-370 BCE), the father of medicine, and it contains text that applies specifically to the field of orthopedic surgery. For example, this text discuses shoulder dislocations and describes various reduction maneuvers. Hippocrates had a keen understanding of the principles of traction and countertraction, especially as it pertains to the musculoskeletal system.8 In fact, the Hippocratic method is still used for reducing anterior shoulder dislocations, and its description can be found in several modern orthopedic texts, including recent articles.13 The Corpus Hippocrates also describes the correction of clubfoot deformity, and the treatment of infected open fractures with pitch cerate and wine compresses.8
Hippocrates also described the treatment of fractures, the principles of traction, and the implications of malunions. For example, Hippocrates wrote, “For the arm, when shortened, might be concealed and the mistake will not be great, but a shortened thigh bone will leave a man maimed.”1 In addition, spinal deformities were recognized by the Greeks, and Hippocrates devised an extension bench for the correction of such deformities.1 From their contributions to anatomy and surgical practice, the Greeks have made significant contributions to the field of surgery.9
During the Roman period, another Greek surgeon by the name of Galen described the musculoskeletal and nervous systems. He served as a gladiatorial surgeon in Rome, and today, he is considered to be the father of sports medicine.8 He is also credited with coining the terms scoliosis, kyphosis, and lordosis to denote the spinal deformities that were first described by Hippocrates.1 In the Roman period, amputations were also performed, and primitive prostheses were developed.9
The Middle Ages
There was relatively little progress in the study of medicine for a thousand years after the fall of the Roman Empire.9 This stagnation was predominantly due to the early Christian Church inhibiting freedom of thought and observation, as well as prohibiting human dissection and the study of anatomy. The first medical school in Europe was established in Salerno, Italy, during the ninth century. This school provided primarily pedantic teaching to its students and perpetuated the theories of the elements and humors. Later on, the University of Bologna became one of the first academic institutions to offer hands-on surgical training.9 One of the most famous surgeons of the Middle Ages was Guy de Chuauliac, who studied at Montpellier and Bologna. He was a leader in the ethical principles of surgery as well as the practice of surgery, and wrote the following with regards to femur fractures:9
“After the application of splints, I attach to the foot a mass of lead as a weight, taking care to pass the cord which supports the weight over a small pulley in such a manner that it shall pull on the leg in a horizontal direction.”
This description is strikingly similar to the modern-day nonoperative management of femur fractures, and underscores the importance of traction, which as mentioned above, was first described by Hippocrates.
Eventually, medicine began to separate from the Church, most likely due to an increase in the complexity of medical theories, the rise of secular universities, and an increase in medical knowledge from Eastern and Middle-Eastern groups.9
The Renaissance and the Foundations of Modern Orthopedics
Until the 16th century, the majority of medical theories were heavily influenced by the work of Hippocrates.8 The scientific study of anatomy gained prominence during this time, especially due to the work done by great artists, such as Leonardo Di Vinci.9 The Table
After a period of rapid expansion of the field of orthopedics, and following the Renaissance, many hospitals were built focusing on the sick and disabled, which solidified orthopedics’ position as a major medical specialty.1 For example, in 1863, James Knight founded the Hospital for the Ruptured and Crippled in New York City. This hospital became the oldest orthopedic hospital in the United States, and it later became known as the Hospital for Special Surgery.14,15 Several additional orthopedic institutions were formed, including the New York Orthopedic Dispensary in 1886 and Hospital for Deformities and Joint Diseases in 1917. Orthopedic surgery residency programs also began to be developed in the late 1800s.14 More specifically, Virgil Gibney at Hospital for the Ruptured and Crippled began the first orthopedic training program in the United States in 1888. Young doctors in this program trained for 1 year as junior assistant, senior assistant, and house surgeon, and began to be known as resident doctors.14
The Modern Era
In the 20th century, rapid development continued to better control infections as well as develop and introduce novel technology. For example, the invention of x-ray in 1895 by Wilhelm Conrad Röntgen improved our ability to diagnose and manage orthopedic conditions ranging from fractures to avascular necrosis of the femoral head to osteoarthritis.8,14 Spinal surgery also developed rapidly with Russell Hibbs describing a technique for spinal fusion at the New York Orthopedic Hospital.8 Similarly, the World Wars served as a catalyst in the development of the subspecialty of orthopedic trauma, with increasing attention placed on open wounds and proficiency with amputations, internal fixation, and wound care. In 1942, Austin Moore performed the first metal hip arthroplasty, and the field of joint replacement was subsequently advanced by the work of Sir John Charnley in the 1960s.8
Conclusion
Despite its relatively recent specialization, orthopedic surgery has a rich history rooted in ancient practices dating back to the primitive man. Over time, there has been significant development in the field in terms of surgical and nonsurgical treatment of orthopedic pathology and disease. Various cultures have played an instrumental role in developing this field, and it is remarkable to see that several practices have persisted since the time of these ancient civilizations. During the Renaissance, there was a considerable emphasis placed on pediatric deformity, but orthopedic surgeons have now branched out to subspecialty practice ranging from orthopedic trauma to joint replacement to oncology.1 For students of medicine and orthopedics, it is important to learn about the origins of this field and to appreciate its gradual development. Orthopedic surgery is a diverse and fascinating field that will most likely continue to develop with increased subspecialization and improved research at the molecular and population level. With a growing emphasis placed on outcomes and healthcare cost by today’s society, it will be fascinating to see how this field continues to evolve in the future.
Am J Orthop. 2016;45(7):E434-E438. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. Ponseti IV. History of orthopedic surgery. Iowa Orthop J. 1991;11:59-64.
2. Ninomiya JT, Dean JC, Incavo SJ. What’s new in hip replacement. J Bone Joint Surg Am. 2015;97(18):1543-1551.
3. Sabharwal S, Nelson SC, Sontich JK. What’s new in limb lengthening and deformity correction. J Bone Joint Surg Am. 2015;97(16):1375-1384.
4. Ricci WM, Black JC, McAndrew CM, Gardner MJ. What’s new in orthopedic trauma. J Bone Joint Surg Am. 2015;97(14):1200-1207.
5. Rodeo SA, Sugiguchi F, Fortier LA, Cunningham ME, Maher S. What’s new in orthopedic research. J Bone Joint Surg Am. 2014;96(23):2015-2019.
6. Pugley AJ, Martin CT, Harwood J, Ong KL, Bozic KJ, Callaghan JJ. Database and registry research in orthopedic surgery. Part 1: Claims-based data. J Bone Joint Surg Am. 2015;97(15):1278-1287.
7. Colton CL. The history of fracture treatment. In: Browner BD, Jupiter JB, Levine AM, Trafton PG, Krettek C, eds. Skeletal Trauma: Basic Science, Management, and Reconstruction. 4th ed. Philadelphia, PA: Saunders Elsevier; 2009:3-32.
8. Brakoulias,V. History of orthopaedics. WorldOrtho Web site. http://pioa.net/documents/Historyoforthopaedics.pdf. Accessed October 6, 2016.
9. Bishop WJ. The Early History of Surgery. New York, NY: Barnes & Noble Books; 1995.
10. Watson T. Wyoming site reveals more prehistoric mountain villages. USA Today. October 20, 2013. http://www.usatoday.com/story/news/nation/2013/10/20/wyoming-prehistoric-villages/2965263. Accessed October 6, 2016.
11. Minagar A, Ragheb J, Kelley RE. The Edwin Smith surgical papyrus: description and analysis of the earliest case of aphasia. J Med Biogr. 2003;11(2):114-117.
12. Atta HM. Edwin Smith Surgical Papyrus: the oldest known surgical treatise. Am Surg. 1999;65(12):1190-1192.
13. Sayegh FE, Kenanidis EI, Papavasiliou KA, Potoupnis ME, Kirkos JM, Kapetanos GA. Reduction of acute anterior dislocations: a prospective randomized study comparing a new technique with the Hippocratic and Kocher methods. J Bone Joint Surg Am. 2009;91(12):2775-2782.
14. Levine DB. Anatomy of a Hospital: Hospital for Special Surgery 1863-2013. New York, NY: Print Mattes; 2013.
15. Wilson PD, Levine DB. Hospital for special surgery. A brief review of its development and current position. Clin Orthop Relat Res. 2000;(374):90-106.
1. Ponseti IV. History of orthopedic surgery. Iowa Orthop J. 1991;11:59-64.
2. Ninomiya JT, Dean JC, Incavo SJ. What’s new in hip replacement. J Bone Joint Surg Am. 2015;97(18):1543-1551.
3. Sabharwal S, Nelson SC, Sontich JK. What’s new in limb lengthening and deformity correction. J Bone Joint Surg Am. 2015;97(16):1375-1384.
4. Ricci WM, Black JC, McAndrew CM, Gardner MJ. What’s new in orthopedic trauma. J Bone Joint Surg Am. 2015;97(14):1200-1207.
5. Rodeo SA, Sugiguchi F, Fortier LA, Cunningham ME, Maher S. What’s new in orthopedic research. J Bone Joint Surg Am. 2014;96(23):2015-2019.
6. Pugley AJ, Martin CT, Harwood J, Ong KL, Bozic KJ, Callaghan JJ. Database and registry research in orthopedic surgery. Part 1: Claims-based data. J Bone Joint Surg Am. 2015;97(15):1278-1287.
7. Colton CL. The history of fracture treatment. In: Browner BD, Jupiter JB, Levine AM, Trafton PG, Krettek C, eds. Skeletal Trauma: Basic Science, Management, and Reconstruction. 4th ed. Philadelphia, PA: Saunders Elsevier; 2009:3-32.
8. Brakoulias,V. History of orthopaedics. WorldOrtho Web site. http://pioa.net/documents/Historyoforthopaedics.pdf. Accessed October 6, 2016.
9. Bishop WJ. The Early History of Surgery. New York, NY: Barnes & Noble Books; 1995.
10. Watson T. Wyoming site reveals more prehistoric mountain villages. USA Today. October 20, 2013. http://www.usatoday.com/story/news/nation/2013/10/20/wyoming-prehistoric-villages/2965263. Accessed October 6, 2016.
11. Minagar A, Ragheb J, Kelley RE. The Edwin Smith surgical papyrus: description and analysis of the earliest case of aphasia. J Med Biogr. 2003;11(2):114-117.
12. Atta HM. Edwin Smith Surgical Papyrus: the oldest known surgical treatise. Am Surg. 1999;65(12):1190-1192.
13. Sayegh FE, Kenanidis EI, Papavasiliou KA, Potoupnis ME, Kirkos JM, Kapetanos GA. Reduction of acute anterior dislocations: a prospective randomized study comparing a new technique with the Hippocratic and Kocher methods. J Bone Joint Surg Am. 2009;91(12):2775-2782.
14. Levine DB. Anatomy of a Hospital: Hospital for Special Surgery 1863-2013. New York, NY: Print Mattes; 2013.
15. Wilson PD, Levine DB. Hospital for special surgery. A brief review of its development and current position. Clin Orthop Relat Res. 2000;(374):90-106.
Fourth approved indication for ofatumumab in chronic lymphocytic leukemia
The recent decision by the US Food and Drug Administration to approve ofatumumab in combination with fludarabine and cyclophosphamide in relapsed disease marks a fourth approved indication for this drug in patients with chronic lymphocytic leukemia (CLL). Ofatumumab is a fully human monoclonal antibody that targets the CD20 protein on the surface of B cells, first approved for the treatment of CLL back in 2009.
Click on the PDF icon at the top of this introduction to read the full article.
The recent decision by the US Food and Drug Administration to approve ofatumumab in combination with fludarabine and cyclophosphamide in relapsed disease marks a fourth approved indication for this drug in patients with chronic lymphocytic leukemia (CLL). Ofatumumab is a fully human monoclonal antibody that targets the CD20 protein on the surface of B cells, first approved for the treatment of CLL back in 2009.
Click on the PDF icon at the top of this introduction to read the full article.
The recent decision by the US Food and Drug Administration to approve ofatumumab in combination with fludarabine and cyclophosphamide in relapsed disease marks a fourth approved indication for this drug in patients with chronic lymphocytic leukemia (CLL). Ofatumumab is a fully human monoclonal antibody that targets the CD20 protein on the surface of B cells, first approved for the treatment of CLL back in 2009.
Click on the PDF icon at the top of this introduction to read the full article.
Tibial Tubercle Fracture After Bone–Patellar Tendon–Bone Autograft
A fracture occurring after anterior cruciate ligament (ACL) reconstruction is rare, and rarer still when it involves the harvest site of a bone—patellar tendon—bone (BPTB) autograft. The vast majority of fractures described in the literature are patellar, with the weak point along the patellar bone cut. A number of fractures generally also occur through the bone tunnels in both hamstring and BPTB grafts. However, only 2 cases of tibial tubercle fracture after BPTB graft have been published, and we expound on them in this case report.1,2 The patient provided written informed consent for print and electronic publication of this case report.
Case Report
Eight years after undergoing successful left ACL reconstruction with ipsilateral BPTB graft, a 45-year-old man developed a graft rupture and demonstrated recurrent instability. He requested revision reconstruction, again with a BPTB construct. In the operating room, he was prepared and draped in the usual sterile fashion, and left ACL reconstruction was performed with right-knee central-third BPTB graft.
During surgery, the left knee was arthroscopically examined, and residual ACL graft from the initial reconstruction was removed. Notchplasty was performed, and the residual femoral interference screw was removed from the 12:30 position. A transtibial approach was used, with a 10-mm reamer brought through the proximal tibia, the posterior tibial ACL footprint, and the 2:00 distal femoral position, with 30 mm of femoral condyle drilled, leaving 1 mm of posterior femoral cortex.
After the right leg was exsanguinated, a central-third patellar tendon graft was harvested through a longitudinal incision with a 22-mm × 10-mm patellar plug, a 10-mm patellar graft, and a 22-mm × 11-mm tibial plug. The graft was prepared, the left tibia was overreamed, and the graft was passed. The graft was fixed with a 7-mm × 23-mm biointerference screw in the femur, trialed, and fixed with an 8-mm × 23-mm interference screw in the tibia. Excess bone graft was packed in the patellar defect in the right knee. The rent in the patellar tendon was closed. The rest of the incision was closed, and the patient was placed in an immobilizer and a cold therapy device (Polar Care; Breg, Inc).
At 2-week follow-up, the patient reported having slipped on ice and flexed the right knee, causing a pop, pain, and limitation in range of motion (ROM; 0°-70°).
The patient returned to the operating room 5 days later and underwent open reduction and internal fixation (ORIF) of the tibial tubercle avulsion. After sterile preparation and draping, the previous incision was used. The bony fragment was isolated and the hematoma débrided. Repair was performed with two No. 2 running locked FiberWire sutures (Arthrex) placed through bony drill holes in the fragment (1 medial, 1 lateral). The fragment was reduced and the sutures tied, with further fixation provided with a DePuy Synthes small-fragment 3.5-mm cortical screw with washer. A No. 5 Ethibond suture (Ethicon) was then placed as a secondary cerclage figure-of-8 stitch to protect the repair.
The patient was seen in follow-up 6 weeks after right ACL reconstruction and 4 weeks after left tibial tubercle ORIF. He continued with right knee restrictions, with the weight-bearing brace locked in extension. Left knee ROM was more than 0° to 90° even before any formal physical therapy. At this point, the patient began physical therapy on both knees with ROM limited to 0° to 30° and weight-bearing as tolerated on the right knee (no restrictions on the left knee).
Discussion
Cases of tibial tubercle fracture after BPTB autograft harvest are extremely rare in the published literature. PubMed and Cochrane Review searches revealed only 2—1 in the ipsilateral knee as ACL fixation1 and 1 in the contralateral knee.2 The middle third of the patellar tendon has been used for ACL reconstruction for more than 50 years, which supports the extreme rarity of this complication.3 Tibial tubercle fractures are so rare that they are not even mentioned in reviews of ACL complications.4 These fractures are universally treated with ORIF.1,2
Far more common but still rare, fracture-type complications involve the extensor mechanism and the tibial plateau. Patellar fractures have been documented as occurring in 0.2% to 2.3% of cases.5-7 One paper reported a fracture in 1.3% of cases at a mean of 57 days, with roughly half caused by trauma and the other half having atraumatic causes.8 Lee and colleagues9 found a 0.2% complication rate for all BPTB grafts in 1725 consecutive patients. Although some patients were treated nonoperatively, others underwent operative fixation. Time to clinical and radiographic healing was 7 and 10 weeks, respectively.
Tibial plateau fracture after BPTB harvest is a rare complication, with 11 cases reported in the literature.10 In 4 of those cases, the proposed mechanism of fracture was a stress riser resulting from the synergistic weakness of the tibial harvest site combined with the tibial tunnel reducing proximal tibial bone strength.11-14 The mechanism of injury varied from traumatic to insufficiency fracture, with fixation varying with fracture displacement.
Tibial tubercle fracture after BPTB harvest is extremely rare, with the present case being only the third published in the literature. Like most reported post-ACL reconstruction extensor mechanism disruptions, our case resulted from a traumatic event at an interval after surgery. All other tibial tubercle fracture post-ACL reconstruction disruptions occurred within 2 weeks after surgery.1,2 Sudden tension on the extensor mechanism secondary to hyperflexion caused a fracture through a weakened tibial tubercle with avulsion of the remaining tendon in 2 of the 3 cases, with the third being a lower stress popping noise that occurred during a pivot to stand.1
The residual defect after tibial bone block harvest could represent a weakening of the tubercle by loss of structural bone and by development of stress risers. The previous reports of tibial tubercle fracture after BPTB harvest documented a similar methodology: Use a bone saw and osteotomes to harvest a trapezoidal tibial bone plug 10 mm to 11 mm wide and 22 cm to 35 cm long. As previously documented, we suggest taking care with saw cuts and osteotomes so as not to weaken the proximal tibia or distal patella more than is necessary.1,2 Before surgery, patients should be warned about the possibility of extensor mechanism injuries with use of BPTB grafts.
Conclusion
Tibial tubercle fracture after BPTB harvest for ACL reconstruction is an extremely rare complication. Treatment is ORIF of the tubercle fragment, with a delay in ACL rehabilitation in cases involving the ipsilateral knee.
Am J Orthop. 2016;45(7):E469-E471. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. Acton KJ, Dowd GS. Fracture of the tibial tubercle following anterior cruciate ligament reconstruction. Knee. 2002;9(2):157-159.
2. Busfield BT, Safran MR, Cannon WD. Extensor mechanism disruption after contralateral middle third patellar tendon harvest for anterior cruciate ligament revision reconstruction. Arthroscopy. 2005;21(10):1268.e1-e1268.e6.
3. Jones KG. Reconstruction of the anterior cruciate ligament. A technique using the central one-third of the patellar ligament. J Bone Joint Surg Am. 1963;45(5):925-932.
4. Tjoumakaris FP, Herz-Brown AL, Bowers AL, Sennett BJ, Bernstein J. Complications in brief: anterior cruciate ligament reconstruction. Clin Orthop Relat Res. 2012;470(2):630-636.
5. Morgan-Jones RL, Cross TM, Caldwell B, Cross MJ. “Silent” transverse patellar fracture following anterior cruciate ligament reconstruction. Arthroscopy. 2001;17(9):997-999.
6. Viola R, Vianello R. Three cases of patella fracture in 1,320 anterior cruciate ligament reconstructions with bone–patellar tendon–bone autograft. Arthroscopy. 1999;15(1):93-97.
7. Berg EE. Management of patella fractures associated with central third bone–patella tendon–bone autograft ACL reconstructions. Arthroscopy. 1996;12(6):756-759.
8. Stein DA, Hunt SA, Rosen JE, Sherman OH. The incidence and outcome of patella fractures after anterior cruciate ligament reconstruction. Arthroscopy. 2002;18(6):578-583.
9. Lee GH, McCulloch P, Cole BJ, Bush-Joseph CA, Bach BR Jr. The incidence of acute patellar tendon harvest complications for anterior cruciate ligament reconstruction. Arthroscopy. 2008;24(2):162-166.
10. Wong JJ, Muir B. Insufficiency fracture of the tibial plateau after anterior cruciate ligament reconstructive surgery: a case report and review of the literature. J Can Chiropr Assoc. 2013;57(2):123-131.
11. Morgan E, Steensen RN. Traumatic proximal tibial fracture following anterior cruciate ligament reconstruction. Am J Knee Surg. 1998;11(3):193-194.
12. Delcogliano A, Chiossi S, Caporaso A, Franzese S, Menghi A. Tibial plateau fracture after arthroscopic anterior cruciate ligament reconstruction. Arthroscopy. 2001;17(4):E16.
13. Mithöfer K, Gill TJ, Vrahas MS. Tibial plateau fracture following anterior cruciate ligament reconstruction. Knee Surg Sports Traumatol Arthrosc. 2004;12(4):325-328.
14. Moen KY, Boynton MD, Raasch WG. Fracture of the proximal tibia after anterior cruciate ligament reconstruction: a case report. Am J Orthop. 1998;27(9):629-630.
A fracture occurring after anterior cruciate ligament (ACL) reconstruction is rare, and rarer still when it involves the harvest site of a bone—patellar tendon—bone (BPTB) autograft. The vast majority of fractures described in the literature are patellar, with the weak point along the patellar bone cut. A number of fractures generally also occur through the bone tunnels in both hamstring and BPTB grafts. However, only 2 cases of tibial tubercle fracture after BPTB graft have been published, and we expound on them in this case report.1,2 The patient provided written informed consent for print and electronic publication of this case report.
Case Report
Eight years after undergoing successful left ACL reconstruction with ipsilateral BPTB graft, a 45-year-old man developed a graft rupture and demonstrated recurrent instability. He requested revision reconstruction, again with a BPTB construct. In the operating room, he was prepared and draped in the usual sterile fashion, and left ACL reconstruction was performed with right-knee central-third BPTB graft.
During surgery, the left knee was arthroscopically examined, and residual ACL graft from the initial reconstruction was removed. Notchplasty was performed, and the residual femoral interference screw was removed from the 12:30 position. A transtibial approach was used, with a 10-mm reamer brought through the proximal tibia, the posterior tibial ACL footprint, and the 2:00 distal femoral position, with 30 mm of femoral condyle drilled, leaving 1 mm of posterior femoral cortex.
After the right leg was exsanguinated, a central-third patellar tendon graft was harvested through a longitudinal incision with a 22-mm × 10-mm patellar plug, a 10-mm patellar graft, and a 22-mm × 11-mm tibial plug. The graft was prepared, the left tibia was overreamed, and the graft was passed. The graft was fixed with a 7-mm × 23-mm biointerference screw in the femur, trialed, and fixed with an 8-mm × 23-mm interference screw in the tibia. Excess bone graft was packed in the patellar defect in the right knee. The rent in the patellar tendon was closed. The rest of the incision was closed, and the patient was placed in an immobilizer and a cold therapy device (Polar Care; Breg, Inc).
At 2-week follow-up, the patient reported having slipped on ice and flexed the right knee, causing a pop, pain, and limitation in range of motion (ROM; 0°-70°).
The patient returned to the operating room 5 days later and underwent open reduction and internal fixation (ORIF) of the tibial tubercle avulsion. After sterile preparation and draping, the previous incision was used. The bony fragment was isolated and the hematoma débrided. Repair was performed with two No. 2 running locked FiberWire sutures (Arthrex) placed through bony drill holes in the fragment (1 medial, 1 lateral). The fragment was reduced and the sutures tied, with further fixation provided with a DePuy Synthes small-fragment 3.5-mm cortical screw with washer. A No. 5 Ethibond suture (Ethicon) was then placed as a secondary cerclage figure-of-8 stitch to protect the repair.
The patient was seen in follow-up 6 weeks after right ACL reconstruction and 4 weeks after left tibial tubercle ORIF. He continued with right knee restrictions, with the weight-bearing brace locked in extension. Left knee ROM was more than 0° to 90° even before any formal physical therapy. At this point, the patient began physical therapy on both knees with ROM limited to 0° to 30° and weight-bearing as tolerated on the right knee (no restrictions on the left knee).
Discussion
Cases of tibial tubercle fracture after BPTB autograft harvest are extremely rare in the published literature. PubMed and Cochrane Review searches revealed only 2—1 in the ipsilateral knee as ACL fixation1 and 1 in the contralateral knee.2 The middle third of the patellar tendon has been used for ACL reconstruction for more than 50 years, which supports the extreme rarity of this complication.3 Tibial tubercle fractures are so rare that they are not even mentioned in reviews of ACL complications.4 These fractures are universally treated with ORIF.1,2
Far more common but still rare, fracture-type complications involve the extensor mechanism and the tibial plateau. Patellar fractures have been documented as occurring in 0.2% to 2.3% of cases.5-7 One paper reported a fracture in 1.3% of cases at a mean of 57 days, with roughly half caused by trauma and the other half having atraumatic causes.8 Lee and colleagues9 found a 0.2% complication rate for all BPTB grafts in 1725 consecutive patients. Although some patients were treated nonoperatively, others underwent operative fixation. Time to clinical and radiographic healing was 7 and 10 weeks, respectively.
Tibial plateau fracture after BPTB harvest is a rare complication, with 11 cases reported in the literature.10 In 4 of those cases, the proposed mechanism of fracture was a stress riser resulting from the synergistic weakness of the tibial harvest site combined with the tibial tunnel reducing proximal tibial bone strength.11-14 The mechanism of injury varied from traumatic to insufficiency fracture, with fixation varying with fracture displacement.
Tibial tubercle fracture after BPTB harvest is extremely rare, with the present case being only the third published in the literature. Like most reported post-ACL reconstruction extensor mechanism disruptions, our case resulted from a traumatic event at an interval after surgery. All other tibial tubercle fracture post-ACL reconstruction disruptions occurred within 2 weeks after surgery.1,2 Sudden tension on the extensor mechanism secondary to hyperflexion caused a fracture through a weakened tibial tubercle with avulsion of the remaining tendon in 2 of the 3 cases, with the third being a lower stress popping noise that occurred during a pivot to stand.1
The residual defect after tibial bone block harvest could represent a weakening of the tubercle by loss of structural bone and by development of stress risers. The previous reports of tibial tubercle fracture after BPTB harvest documented a similar methodology: Use a bone saw and osteotomes to harvest a trapezoidal tibial bone plug 10 mm to 11 mm wide and 22 cm to 35 cm long. As previously documented, we suggest taking care with saw cuts and osteotomes so as not to weaken the proximal tibia or distal patella more than is necessary.1,2 Before surgery, patients should be warned about the possibility of extensor mechanism injuries with use of BPTB grafts.
Conclusion
Tibial tubercle fracture after BPTB harvest for ACL reconstruction is an extremely rare complication. Treatment is ORIF of the tubercle fragment, with a delay in ACL rehabilitation in cases involving the ipsilateral knee.
Am J Orthop. 2016;45(7):E469-E471. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
A fracture occurring after anterior cruciate ligament (ACL) reconstruction is rare, and rarer still when it involves the harvest site of a bone—patellar tendon—bone (BPTB) autograft. The vast majority of fractures described in the literature are patellar, with the weak point along the patellar bone cut. A number of fractures generally also occur through the bone tunnels in both hamstring and BPTB grafts. However, only 2 cases of tibial tubercle fracture after BPTB graft have been published, and we expound on them in this case report.1,2 The patient provided written informed consent for print and electronic publication of this case report.
Case Report
Eight years after undergoing successful left ACL reconstruction with ipsilateral BPTB graft, a 45-year-old man developed a graft rupture and demonstrated recurrent instability. He requested revision reconstruction, again with a BPTB construct. In the operating room, he was prepared and draped in the usual sterile fashion, and left ACL reconstruction was performed with right-knee central-third BPTB graft.
During surgery, the left knee was arthroscopically examined, and residual ACL graft from the initial reconstruction was removed. Notchplasty was performed, and the residual femoral interference screw was removed from the 12:30 position. A transtibial approach was used, with a 10-mm reamer brought through the proximal tibia, the posterior tibial ACL footprint, and the 2:00 distal femoral position, with 30 mm of femoral condyle drilled, leaving 1 mm of posterior femoral cortex.
After the right leg was exsanguinated, a central-third patellar tendon graft was harvested through a longitudinal incision with a 22-mm × 10-mm patellar plug, a 10-mm patellar graft, and a 22-mm × 11-mm tibial plug. The graft was prepared, the left tibia was overreamed, and the graft was passed. The graft was fixed with a 7-mm × 23-mm biointerference screw in the femur, trialed, and fixed with an 8-mm × 23-mm interference screw in the tibia. Excess bone graft was packed in the patellar defect in the right knee. The rent in the patellar tendon was closed. The rest of the incision was closed, and the patient was placed in an immobilizer and a cold therapy device (Polar Care; Breg, Inc).
At 2-week follow-up, the patient reported having slipped on ice and flexed the right knee, causing a pop, pain, and limitation in range of motion (ROM; 0°-70°).
The patient returned to the operating room 5 days later and underwent open reduction and internal fixation (ORIF) of the tibial tubercle avulsion. After sterile preparation and draping, the previous incision was used. The bony fragment was isolated and the hematoma débrided. Repair was performed with two No. 2 running locked FiberWire sutures (Arthrex) placed through bony drill holes in the fragment (1 medial, 1 lateral). The fragment was reduced and the sutures tied, with further fixation provided with a DePuy Synthes small-fragment 3.5-mm cortical screw with washer. A No. 5 Ethibond suture (Ethicon) was then placed as a secondary cerclage figure-of-8 stitch to protect the repair.
The patient was seen in follow-up 6 weeks after right ACL reconstruction and 4 weeks after left tibial tubercle ORIF. He continued with right knee restrictions, with the weight-bearing brace locked in extension. Left knee ROM was more than 0° to 90° even before any formal physical therapy. At this point, the patient began physical therapy on both knees with ROM limited to 0° to 30° and weight-bearing as tolerated on the right knee (no restrictions on the left knee).
Discussion
Cases of tibial tubercle fracture after BPTB autograft harvest are extremely rare in the published literature. PubMed and Cochrane Review searches revealed only 2—1 in the ipsilateral knee as ACL fixation1 and 1 in the contralateral knee.2 The middle third of the patellar tendon has been used for ACL reconstruction for more than 50 years, which supports the extreme rarity of this complication.3 Tibial tubercle fractures are so rare that they are not even mentioned in reviews of ACL complications.4 These fractures are universally treated with ORIF.1,2
Far more common but still rare, fracture-type complications involve the extensor mechanism and the tibial plateau. Patellar fractures have been documented as occurring in 0.2% to 2.3% of cases.5-7 One paper reported a fracture in 1.3% of cases at a mean of 57 days, with roughly half caused by trauma and the other half having atraumatic causes.8 Lee and colleagues9 found a 0.2% complication rate for all BPTB grafts in 1725 consecutive patients. Although some patients were treated nonoperatively, others underwent operative fixation. Time to clinical and radiographic healing was 7 and 10 weeks, respectively.
Tibial plateau fracture after BPTB harvest is a rare complication, with 11 cases reported in the literature.10 In 4 of those cases, the proposed mechanism of fracture was a stress riser resulting from the synergistic weakness of the tibial harvest site combined with the tibial tunnel reducing proximal tibial bone strength.11-14 The mechanism of injury varied from traumatic to insufficiency fracture, with fixation varying with fracture displacement.
Tibial tubercle fracture after BPTB harvest is extremely rare, with the present case being only the third published in the literature. Like most reported post-ACL reconstruction extensor mechanism disruptions, our case resulted from a traumatic event at an interval after surgery. All other tibial tubercle fracture post-ACL reconstruction disruptions occurred within 2 weeks after surgery.1,2 Sudden tension on the extensor mechanism secondary to hyperflexion caused a fracture through a weakened tibial tubercle with avulsion of the remaining tendon in 2 of the 3 cases, with the third being a lower stress popping noise that occurred during a pivot to stand.1
The residual defect after tibial bone block harvest could represent a weakening of the tubercle by loss of structural bone and by development of stress risers. The previous reports of tibial tubercle fracture after BPTB harvest documented a similar methodology: Use a bone saw and osteotomes to harvest a trapezoidal tibial bone plug 10 mm to 11 mm wide and 22 cm to 35 cm long. As previously documented, we suggest taking care with saw cuts and osteotomes so as not to weaken the proximal tibia or distal patella more than is necessary.1,2 Before surgery, patients should be warned about the possibility of extensor mechanism injuries with use of BPTB grafts.
Conclusion
Tibial tubercle fracture after BPTB harvest for ACL reconstruction is an extremely rare complication. Treatment is ORIF of the tubercle fragment, with a delay in ACL rehabilitation in cases involving the ipsilateral knee.
Am J Orthop. 2016;45(7):E469-E471. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. Acton KJ, Dowd GS. Fracture of the tibial tubercle following anterior cruciate ligament reconstruction. Knee. 2002;9(2):157-159.
2. Busfield BT, Safran MR, Cannon WD. Extensor mechanism disruption after contralateral middle third patellar tendon harvest for anterior cruciate ligament revision reconstruction. Arthroscopy. 2005;21(10):1268.e1-e1268.e6.
3. Jones KG. Reconstruction of the anterior cruciate ligament. A technique using the central one-third of the patellar ligament. J Bone Joint Surg Am. 1963;45(5):925-932.
4. Tjoumakaris FP, Herz-Brown AL, Bowers AL, Sennett BJ, Bernstein J. Complications in brief: anterior cruciate ligament reconstruction. Clin Orthop Relat Res. 2012;470(2):630-636.
5. Morgan-Jones RL, Cross TM, Caldwell B, Cross MJ. “Silent” transverse patellar fracture following anterior cruciate ligament reconstruction. Arthroscopy. 2001;17(9):997-999.
6. Viola R, Vianello R. Three cases of patella fracture in 1,320 anterior cruciate ligament reconstructions with bone–patellar tendon–bone autograft. Arthroscopy. 1999;15(1):93-97.
7. Berg EE. Management of patella fractures associated with central third bone–patella tendon–bone autograft ACL reconstructions. Arthroscopy. 1996;12(6):756-759.
8. Stein DA, Hunt SA, Rosen JE, Sherman OH. The incidence and outcome of patella fractures after anterior cruciate ligament reconstruction. Arthroscopy. 2002;18(6):578-583.
9. Lee GH, McCulloch P, Cole BJ, Bush-Joseph CA, Bach BR Jr. The incidence of acute patellar tendon harvest complications for anterior cruciate ligament reconstruction. Arthroscopy. 2008;24(2):162-166.
10. Wong JJ, Muir B. Insufficiency fracture of the tibial plateau after anterior cruciate ligament reconstructive surgery: a case report and review of the literature. J Can Chiropr Assoc. 2013;57(2):123-131.
11. Morgan E, Steensen RN. Traumatic proximal tibial fracture following anterior cruciate ligament reconstruction. Am J Knee Surg. 1998;11(3):193-194.
12. Delcogliano A, Chiossi S, Caporaso A, Franzese S, Menghi A. Tibial plateau fracture after arthroscopic anterior cruciate ligament reconstruction. Arthroscopy. 2001;17(4):E16.
13. Mithöfer K, Gill TJ, Vrahas MS. Tibial plateau fracture following anterior cruciate ligament reconstruction. Knee Surg Sports Traumatol Arthrosc. 2004;12(4):325-328.
14. Moen KY, Boynton MD, Raasch WG. Fracture of the proximal tibia after anterior cruciate ligament reconstruction: a case report. Am J Orthop. 1998;27(9):629-630.
1. Acton KJ, Dowd GS. Fracture of the tibial tubercle following anterior cruciate ligament reconstruction. Knee. 2002;9(2):157-159.
2. Busfield BT, Safran MR, Cannon WD. Extensor mechanism disruption after contralateral middle third patellar tendon harvest for anterior cruciate ligament revision reconstruction. Arthroscopy. 2005;21(10):1268.e1-e1268.e6.
3. Jones KG. Reconstruction of the anterior cruciate ligament. A technique using the central one-third of the patellar ligament. J Bone Joint Surg Am. 1963;45(5):925-932.
4. Tjoumakaris FP, Herz-Brown AL, Bowers AL, Sennett BJ, Bernstein J. Complications in brief: anterior cruciate ligament reconstruction. Clin Orthop Relat Res. 2012;470(2):630-636.
5. Morgan-Jones RL, Cross TM, Caldwell B, Cross MJ. “Silent” transverse patellar fracture following anterior cruciate ligament reconstruction. Arthroscopy. 2001;17(9):997-999.
6. Viola R, Vianello R. Three cases of patella fracture in 1,320 anterior cruciate ligament reconstructions with bone–patellar tendon–bone autograft. Arthroscopy. 1999;15(1):93-97.
7. Berg EE. Management of patella fractures associated with central third bone–patella tendon–bone autograft ACL reconstructions. Arthroscopy. 1996;12(6):756-759.
8. Stein DA, Hunt SA, Rosen JE, Sherman OH. The incidence and outcome of patella fractures after anterior cruciate ligament reconstruction. Arthroscopy. 2002;18(6):578-583.
9. Lee GH, McCulloch P, Cole BJ, Bush-Joseph CA, Bach BR Jr. The incidence of acute patellar tendon harvest complications for anterior cruciate ligament reconstruction. Arthroscopy. 2008;24(2):162-166.
10. Wong JJ, Muir B. Insufficiency fracture of the tibial plateau after anterior cruciate ligament reconstructive surgery: a case report and review of the literature. J Can Chiropr Assoc. 2013;57(2):123-131.
11. Morgan E, Steensen RN. Traumatic proximal tibial fracture following anterior cruciate ligament reconstruction. Am J Knee Surg. 1998;11(3):193-194.
12. Delcogliano A, Chiossi S, Caporaso A, Franzese S, Menghi A. Tibial plateau fracture after arthroscopic anterior cruciate ligament reconstruction. Arthroscopy. 2001;17(4):E16.
13. Mithöfer K, Gill TJ, Vrahas MS. Tibial plateau fracture following anterior cruciate ligament reconstruction. Knee Surg Sports Traumatol Arthrosc. 2004;12(4):325-328.
14. Moen KY, Boynton MD, Raasch WG. Fracture of the proximal tibia after anterior cruciate ligament reconstruction: a case report. Am J Orthop. 1998;27(9):629-630.
Renal cell carcinoma approval adds another notch to cabozantinib’s belt
In April this year, the US Food and Drug Administration awarded regulatory approval to cabozantinib for the treatment of advanced renal cell carcinoma patients previously treated with anti-angiogenic therapy.1 The small-molecule inhibitor, which targets multiple kinases, including the vascular endothelial growth factor receptors (VEGFRs) and the hepatocyte growth factor receptor (MET), had previously been approved for the treatment of medullary thyroid carcinoma in 2012.
Click on the PDF icon below for the full article.
In April this year, the US Food and Drug Administration awarded regulatory approval to cabozantinib for the treatment of advanced renal cell carcinoma patients previously treated with anti-angiogenic therapy.1 The small-molecule inhibitor, which targets multiple kinases, including the vascular endothelial growth factor receptors (VEGFRs) and the hepatocyte growth factor receptor (MET), had previously been approved for the treatment of medullary thyroid carcinoma in 2012.
Click on the PDF icon below for the full article.
In April this year, the US Food and Drug Administration awarded regulatory approval to cabozantinib for the treatment of advanced renal cell carcinoma patients previously treated with anti-angiogenic therapy.1 The small-molecule inhibitor, which targets multiple kinases, including the vascular endothelial growth factor receptors (VEGFRs) and the hepatocyte growth factor receptor (MET), had previously been approved for the treatment of medullary thyroid carcinoma in 2012.
Click on the PDF icon below for the full article.
Instability After Reverse Total Shoulder Arthroplasty: Which Patients Dislocate?
Risk factors for dislocation after reverse total shoulder arthroplasty (RTSA) are not clearly defined. Prosthetic dislocation can result in poor patient satisfaction, worse functional outcomes, and return to the operating room.1-3 As a result, identification of modifiable risk factors for complications represents an important research initiative for shoulder surgeons.
There is a paucity of literature devoted to the study of dislocation after RTSA. Chalmers and colleagues4 found a 2.9% (11/385) incidence of early dislocation within 3 months after index surgery—an improvement over the 15.8% reported for early instability over the period 2004–2006.5 As prosthesis design has improved and surgeons have become more comfortable with the RTSA prosthesis, surgical indications have expanded,6,7 and dislocation rates appear to have decreased. Although the most common indication for RTSA continues to be cuff tear arthropathy (CTA),6 there has been increased use in rheumatoid arthritis8-10; proximal humerus fractures, especially in cases of poor bone quality and unreliable fixation of tuberosities11-13; and failed previous shoulder reconstruction.14,15 As RTSA is performed more often, limiting the complications will become more important for both patient care and economics.
We conducted a study to analyze dislocation rates at our institution and to identify both modifiable and nonmodifiable risk factors for dislocation after RTSA. By identifying risk factors for dislocation, we will be able to implement additional perioperative clinical measures to reduce the incidence of dislocation.
Materials and Methods
This retrospective study of dislocation after RTSA was conducted at the Rothman Institute of Orthopedics and Methodist Hospital (Thomas Jefferson University Hospitals, Philadelphia, PA). After obtaining Institutional Review Board approval for the study, we searched our institution’s electronic database of shoulder arthroplasties to identify all RTSAs performed at our 2 large-volume urban institutions between September 27, 2010 and December 31, 2013. For the record search, International Classification of Diseases, Ninth Revision (ICD-9) codes were used (Table 1).
The medical records of each patient were used to identify independent variables that could be associated with dislocation rate. Demographic variables included sex, age, and race. Preoperative clinical data included body mass index (BMI), etiology of shoulder disease leading to RTSA, individual comorbidities, and Charlson Comorbidity Index (CCI)16 modified to be used with ICD-9 codes.17 In addition, prior shoulder surgery history and arthroplasty type (primary or revision) were determined. Postoperative considerations were time to dislocation, mechanism of dislocation, and intervention(s) needed for dislocation. Although the institutional database did not include operative variables such as prosthesis type and surgical approach, all 6 surgeons in this study were using a standard deltopectoral approach in beach-chair position with a Grammont style prosthesis for RTSA cases.
Descriptive statistics for RTSA patients and the dislocation subpopulation were compiled. Bivariate analysis was used to evaluate which of the previously described variables influenced dislocation rates. Last, multivariate logistic regression analysis was performed to evaluate which factors were independent predictors of dislocation. We included demographic variables (age, sex, ethnicity), clinical variables (BMI, individual comorbidities, CCI), and surgical variables (primary vs revision, diagnosis at time of surgery). All statistical analyses were performed with Excel 2013 (Microsoft) and SPSS Statistics Version 20.0 (SPSS Inc.).
Results
From the database, we identified 487 patients who underwent 510 RTSAs during the study period. These surgeries were performed by 6 shoulder and elbow fellowship–trained surgeons. Of the 510 RTSAs, 393 (77.1%) were primary cases, and 117 (22.9%) were revision cases.
Of the 510 shoulders that underwent RTSA, 15 (2.9%; 14 patients) dislocated. Of these 15 cases, 5 were primary (1.3% of all primary cases) and 10 were revision (8.5% of all revision cases). Mean time from index surgery to diagnosis of dislocation was 58.2 days (range, 0-319 days). One dislocation occurred immediately after surgery, 2 after falls, 4 from patient-identified low-energy mechanisms of injury, and 8 without known inciting events. Nine dislocations (60%) did not have a subscapularis repair (7 were irreparable, 2 underwent subscapularis peel without repair), and the other 6 were repaired primarily (Table 2).
Male patients accounted for 32.2% of the study population but 60.0% of the dislocations (P = .019) (Table 3).
Multivariate logistic regression analysis revealed revision arthroplasty (OR = 7.515; P = .042) and increased BMI (OR = 1.09; P = .047) to be independent risk factors for dislocation after RTSA. Analysis also found a diagnosis of primary CTA to be independently associated with lower risk of dislocation after RTSA (OR = 0.025; P = .008). Last, the previously described risk factor of male sex was found not to be a significant independent risk factor, though it did trend positively (OR = 3.011; P = .071).
Discussion
With more RTSAs being performed, evaluation of their common complications becomes increasingly important.18 We found a 3.0% rate of dislocation after RTSA, which is consistent with the most recently reported incidence4 and falls within the previously described range of 0% to 8.6%.19-26 Of the clinical risk factors identified in this study, those previously described were prior surgery, subscapularis insufficiency, higher BMI, and male sex.4 However, our finding of lower risk of dislocation after RTSA for primary rotator cuff pathology was not previously described. Although Chalmers and colleagues4 did not report this lower risk, 3 (27.3%) of their 11 patients with dislocation had primary CTA, compared with 1 (6.7%) of 15 patients in the present study.4 Our literature review did not identify any studies that independently reported the dislocation rate in patients who underwent RTSA for rotator cuff failure.
The risk factors of subscapularis irreparability and revision surgery suggest the importance of the soft-tissue envelope and bony anatomy in dislocation prevention. Previous analyses have suggested implant malpositioning,27,28 poor subscapularis quality,29 and inadequate muscle tensioning5,30-32 as risk factors for RTSA. Patients with an irreparable subscapularis tendon have often had multiple surgeries with compromise to the muscle/soft-tissue envelope or bony anatomy of the shoulder. A biomechanical study by Gutiérrez and colleagues31 found the compressive forces of the soft tissue at the glenohumeral joint to be the most important contributor to stability in the RTSA prosthesis. In clinical studies, the role of the subscapularis in preventing instability after RTSA remains unclear. Edwards and colleagues29 prospectively compared dislocation rates in patients with reparable and irreparable subscapularis tendons during RTSA and found a higher rate of dislocation in the irreparable subscapularis group. Of note, patients in the irreparable subscapularis group also had more complex diagnoses, including proximal humeral nonunion, fixed glenohumeral dislocation, and failed prior arthroplasty. Clark and colleagues33 retrospectively analyzed subscapularis repair in 2 RTSA groups and found no appreciable effect on complication rate, dislocation events, range-of-motion gains, or pain relief.
Our finding that higher BMI is an independent risk factor was previously described.4 The association is unclear but could be related to implant positioning, difficulty in intraoperative assessment of muscle tensioning, or body habitus that may generate a lever arm for impingement and dislocation when the arm is in adduction. Last, our finding that male sex is a risk factor for dislocation approached significance, and this relationship was previously reported.4 This could be attributable to a higher rate of activity or of indolent infection in male patients.34,35Besides studying risk factors for dislocation after RTSA, we investigated treatment. None of our patients were treated successfully and definitively with closed reduction in the clinic. This finding diverges from findings in studies by Teusink and colleagues2 and Chalmers and colleagues,4who respectively reported 62% and 44% rates of success with closed reduction. Our cohort of 14 patients with 15 dislocations required a total of 17 trips to the operating room after dislocation. This significantly higher rate of return to the operating room suggests that dislocation after RTSA may be a more costly and morbid problem than has been previously described.
This study had several weaknesses. Despite its large consecutive series of patients, the study was retrospective, and several variables that would be documented and controlled in a prospective study could not be measured here. Specifically, neither preoperative physical examination nor patient-specific assessments of pain or function were consistently obtained. Similarly, postoperative patient-specific instruments of outcomes evaluation were not obtained consistently, so results of patients with dislocation could not be compared with those of a control group. In addition, preoperative and postoperative radiographs were not consistently present in our electronic medical records, so the influence of preoperative bony anatomy, intraoperative limb lengthening, and any implant malpositioning could not be determined. Furthermore, operative details, such as reparability of the subscapularis, were not fully available for the control group and could not be included in statistical analysis. In addition, that the known dislocation risk factor of male sex4 was identified here but was not significant in multivariate regression analysis suggests that this study may not have been adequately powered to identify a significant difference in dislocation rate between the sexes. Last, though our results suggested associations between the aforementioned variables and dislocation after RTSA, a truly causative relationship could not be confirmed with this study design or analysis. Therefore, our study findings are hypothesis-generating and may indicate a benefit to greater deltoid tensioning, use of retentive liners, or more conservative rehabilitation protocols for high-risk patients.
Conclusion
Dislocation after RTSA is an uncommon complication that often requires a return to the operating room. This study identified a modifiable risk factor (higher BMI) and 3 nonmodifiable risk factors (male sex, subscapularis insufficiency, revision surgery) for dislocation after RTSA. In contrast, patients who undergo RTSA for primary rotator cuff pathology are unlikely to dislocate after surgery. This low risk of dislocation after RTSA for primary cuff pathology was not previously described. Patients in the higher risk category may benefit from preoperative lifestyle modification, intraoperative techniques for increasing stability, and more conservative therapy after surgery. In addition, unlike previous investigations, this study did not find closed reduction in the clinic alone to be successful in definitively treating this patient population.
Am J Orthop. 2016;45(7):E444-E450. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. Aldinger PR, Raiss P, Rickert M, Loew M. Complications in shoulder arthroplasty: an analysis of 485 cases. Int Orthop. 2010;34(4):517-524.
2. Teusink MJ, Pappou IP, Schwartz DG, Cottrell BJ, Frankle MA. Results of closed management of acute dislocation after reverse shoulder arthroplasty. J Shoulder Elbow Surg. 2015;24(4):621-627.
3. Fink Barnes LA, Grantham WJ, Meadows MC, Bigliani LU, Levine WN, Ahmad CS. Sports activity after reverse total shoulder arthroplasty with minimum 2-year follow-up. Am J Orthop. 2015;44(2):68-72.
4. Chalmers PN, Rahman Z, Romeo AA, Nicholson GP. Early dislocation after reverse total shoulder arthroplasty. J Shoulder Elbow Surg. 2014;23(5):737-744.
5. Gallo RA, Gamradt SC, Mattern CJ, et al; Sports Medicine and Shoulder Service at the Hospital for Special Surgery, New York, NY. Instability after reverse total shoulder replacement. J Shoulder Elbow Surg. 2011;20(4):584-590.
6. Walch G, Bacle G, Lädermann A, Nové-Josserand L, Smithers CJ. Do the indications, results, and complications of reverse shoulder arthroplasty change with surgeon’s experience? J Shoulder Elbow Surg. 2012;21(11):1470-1477.
7. Smith CD, Guyver P, Bunker TD. Indications for reverse shoulder replacement: a systematic review. J Bone Joint Surg Br. 2012;94(5):577-583.
8. Young AA, Smith MM, Bacle G, Moraga C, Walch G. Early results of reverse shoulder arthroplasty in patients with rheumatoid arthritis. J Bone Joint Surg Am. 2011;93(20):1915-1923.
9. Hedtmann A, Werner A. Shoulder arthroplasty in rheumatoid arthritis [in German]. Orthopade. 2007;36(11):1050-1061.
10. Rittmeister M, Kerschbaumer F. Grammont reverse total shoulder arthroplasty in patients with rheumatoid arthritis and nonreconstructible rotator cuff lesions. J Shoulder Elbow Surg. 2001;10(1):17-22.
11. Acevedo DC, Vanbeek C, Lazarus MD, Williams GR, Abboud JA. Reverse shoulder arthroplasty for proximal humeral fractures: update on indications, technique, and results. J Shoulder Elbow Surg. 2014;23(2):279-289.
12. Bufquin T, Hersan A, Hubert L, Massin P. Reverse shoulder arthroplasty for the treatment of three- and four-part fractures of the proximal humerus in the elderly: a prospective review of 43 cases with a short-term follow-up. J Bone Joint Surg Br. 2007;89(4):516-520.
13. Cuff DJ, Pupello DR. Comparison of hemiarthroplasty and reverse shoulder arthroplasty for the treatment of proximal humeral fractures in elderly patients. J Bone Joint Surg Am. 2013;95(22):2050-2055.
14. Walker M, Willis MP, Brooks JP, Pupello D, Mulieri PJ, Frankle MA. The use of the reverse shoulder arthroplasty for treatment of failed total shoulder arthroplasty. J Shoulder Elbow Surg. 2012;21(4):514-522.
15. Valenti P, Kilinc AS, Sauzières P, Katz D. Results of 30 reverse shoulder prostheses for revision of failed hemi- or total shoulder arthroplasty. Eur J Orthop Surg Traumatol. 2014;24(8):1375-1382.
16. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373-383.
17. Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45(6):613-619.
18. Kim SH, Wise BL, Zhang Y, Szabo RM. Increasing incidence of shoulder arthroplasty in the United States. J Bone Joint Surg Am. 2011;93(24):2249-2254.
19. Boileau P, Watkinson D, Hatzidakis AM, Hovorka I. Neer Award 2005: the Grammont reverse shoulder prosthesis: results in cuff tear arthritis, fracture sequelae, and revision arthroplasty. J Shoulder Elbow Surg. 2006;15(5):527-540.
20. Cuff D, Pupello D, Virani N, Levy J, Frankle M. Reverse shoulder arthroplasty for the treatment of rotator cuff deficiency. J Bone Joint Surg Am. 2008;90(6):1244-1251.
21. Frankle M, Siegal S, Pupello D, Saleem A, Mighell M, Vasey M. The reverse shoulder prosthesis for glenohumeral arthritis associated with severe rotator cuff deficiency. A minimum two-year follow-up study of sixty patients. J Bone Joint Surg Am. 2005;87(8):1697-1705.
22. Guery J, Favard L, Sirveaux F, Oudet D, Mole D, Walch G. Reverse total shoulder arthroplasty. Survivorship analysis of eighty replacements followed for five to ten years. J Bone Joint Surg Am. 2006;88(8):1742-1747.
23. Mulieri P, Dunning P, Klein S, Pupello D, Frankle M. Reverse shoulder arthroplasty for the treatment of irreparable rotator cuff tear without glenohumeral arthritis. J Bone Joint Surg Am. 2010;92(15):2544-2556.
24. Sirveaux F, Favard L, Oudet D, Huquet D, Walch G, Molé D. Grammont inverted total shoulder arthroplasty in the treatment of glenohumeral osteoarthritis with massive rupture of the cuff. Results of a multicentre study of 80 shoulders. J Bone Joint Surg Br. 2004;86(3):388-395.
25. Wall B, Nové-Josserand L, O’Connor DP, Edwards TB, Walch G. Reverse total shoulder arthroplasty: a review of results according to etiology. J Bone Joint Surg Am. 2007;89(7):1476-1485.
26. Werner CM, Steinmann PA, Gilbart M, Gerber C. Treatment of painful pseudoparesis due to irreparable rotator cuff dysfunction with the Delta III reverse-ball-and-socket total shoulder prosthesis. J Bone Joint Surg Am. 2005;87(7):1476-1486.
27. Cazeneuve JF, Cristofari DJ. The reverse shoulder prosthesis in the treatment of fractures of the proximal humerus in the elderly. J Bone Joint Surg Br. 2010;92(4):535-539.
28. Stephenson DR, Oh JH, McGarry MH, Rick Hatch GF 3rd, Lee TQ. Effect of humeral component version on impingement in reverse total shoulder arthroplasty. J Shoulder Elbow Surg. 2011;20(4):652-658.
29. Edwards TB, Williams MD, Labriola JE, Elkousy HA, Gartsman GM, O’Connor DP. Subscapularis insufficiency and the risk of shoulder dislocation after reverse shoulder arthroplasty. J Shoulder Elbow Surg. 2009;18(6):892-896.
30. Affonso J, Nicholson GP, Frankle MA, et al. Complications of the reverse prosthesis: prevention and treatment. Instr Course Lect. 2012;61:157-168.
31. Gutiérrez S, Keller TS, Levy JC, Lee WE 3rd, Luo ZP. Hierarchy of stability factors in reverse shoulder arthroplasty. Clin Orthop Relat Res. 2008;466(3):670-676.
32. Boileau P, Watkinson DJ, Hatzidakis AM, Balg F. Grammont reverse prosthesis: design, rationale, and biomechanics. J Shoulder Elbow Surg. 2005;14(1 suppl S):147S-161S.
33. Clark JC, Ritchie J, Song FS, et al. Complication rates, dislocation, pain, and postoperative range of motion after reverse shoulder arthroplasty in patients with and without repair of the subscapularis. J Shoulder Elbow Surg. 2012;21(1):36-41.
34. Richards J, Inacio MC, Beckett M, et al. Patient and procedure-specific risk factors for deep infection after primary shoulder arthroplasty. Clin Orthop Relat Res. 2014;472(9):2809-2815.
35. Singh JA, Sperling JW, Schleck C, Harmsen WS, Cofield RH. Periprosthetic infections after total shoulder arthroplasty: a 33-year perspective. J Shoulder Elbow Surg. 2012;21(11):1534-1541.
Risk factors for dislocation after reverse total shoulder arthroplasty (RTSA) are not clearly defined. Prosthetic dislocation can result in poor patient satisfaction, worse functional outcomes, and return to the operating room.1-3 As a result, identification of modifiable risk factors for complications represents an important research initiative for shoulder surgeons.
There is a paucity of literature devoted to the study of dislocation after RTSA. Chalmers and colleagues4 found a 2.9% (11/385) incidence of early dislocation within 3 months after index surgery—an improvement over the 15.8% reported for early instability over the period 2004–2006.5 As prosthesis design has improved and surgeons have become more comfortable with the RTSA prosthesis, surgical indications have expanded,6,7 and dislocation rates appear to have decreased. Although the most common indication for RTSA continues to be cuff tear arthropathy (CTA),6 there has been increased use in rheumatoid arthritis8-10; proximal humerus fractures, especially in cases of poor bone quality and unreliable fixation of tuberosities11-13; and failed previous shoulder reconstruction.14,15 As RTSA is performed more often, limiting the complications will become more important for both patient care and economics.
We conducted a study to analyze dislocation rates at our institution and to identify both modifiable and nonmodifiable risk factors for dislocation after RTSA. By identifying risk factors for dislocation, we will be able to implement additional perioperative clinical measures to reduce the incidence of dislocation.
Materials and Methods
This retrospective study of dislocation after RTSA was conducted at the Rothman Institute of Orthopedics and Methodist Hospital (Thomas Jefferson University Hospitals, Philadelphia, PA). After obtaining Institutional Review Board approval for the study, we searched our institution’s electronic database of shoulder arthroplasties to identify all RTSAs performed at our 2 large-volume urban institutions between September 27, 2010 and December 31, 2013. For the record search, International Classification of Diseases, Ninth Revision (ICD-9) codes were used (Table 1).
The medical records of each patient were used to identify independent variables that could be associated with dislocation rate. Demographic variables included sex, age, and race. Preoperative clinical data included body mass index (BMI), etiology of shoulder disease leading to RTSA, individual comorbidities, and Charlson Comorbidity Index (CCI)16 modified to be used with ICD-9 codes.17 In addition, prior shoulder surgery history and arthroplasty type (primary or revision) were determined. Postoperative considerations were time to dislocation, mechanism of dislocation, and intervention(s) needed for dislocation. Although the institutional database did not include operative variables such as prosthesis type and surgical approach, all 6 surgeons in this study were using a standard deltopectoral approach in beach-chair position with a Grammont style prosthesis for RTSA cases.
Descriptive statistics for RTSA patients and the dislocation subpopulation were compiled. Bivariate analysis was used to evaluate which of the previously described variables influenced dislocation rates. Last, multivariate logistic regression analysis was performed to evaluate which factors were independent predictors of dislocation. We included demographic variables (age, sex, ethnicity), clinical variables (BMI, individual comorbidities, CCI), and surgical variables (primary vs revision, diagnosis at time of surgery). All statistical analyses were performed with Excel 2013 (Microsoft) and SPSS Statistics Version 20.0 (SPSS Inc.).
Results
From the database, we identified 487 patients who underwent 510 RTSAs during the study period. These surgeries were performed by 6 shoulder and elbow fellowship–trained surgeons. Of the 510 RTSAs, 393 (77.1%) were primary cases, and 117 (22.9%) were revision cases.
Of the 510 shoulders that underwent RTSA, 15 (2.9%; 14 patients) dislocated. Of these 15 cases, 5 were primary (1.3% of all primary cases) and 10 were revision (8.5% of all revision cases). Mean time from index surgery to diagnosis of dislocation was 58.2 days (range, 0-319 days). One dislocation occurred immediately after surgery, 2 after falls, 4 from patient-identified low-energy mechanisms of injury, and 8 without known inciting events. Nine dislocations (60%) did not have a subscapularis repair (7 were irreparable, 2 underwent subscapularis peel without repair), and the other 6 were repaired primarily (Table 2).
Male patients accounted for 32.2% of the study population but 60.0% of the dislocations (P = .019) (Table 3).
Multivariate logistic regression analysis revealed revision arthroplasty (OR = 7.515; P = .042) and increased BMI (OR = 1.09; P = .047) to be independent risk factors for dislocation after RTSA. Analysis also found a diagnosis of primary CTA to be independently associated with lower risk of dislocation after RTSA (OR = 0.025; P = .008). Last, the previously described risk factor of male sex was found not to be a significant independent risk factor, though it did trend positively (OR = 3.011; P = .071).
Discussion
With more RTSAs being performed, evaluation of their common complications becomes increasingly important.18 We found a 3.0% rate of dislocation after RTSA, which is consistent with the most recently reported incidence4 and falls within the previously described range of 0% to 8.6%.19-26 Of the clinical risk factors identified in this study, those previously described were prior surgery, subscapularis insufficiency, higher BMI, and male sex.4 However, our finding of lower risk of dislocation after RTSA for primary rotator cuff pathology was not previously described. Although Chalmers and colleagues4 did not report this lower risk, 3 (27.3%) of their 11 patients with dislocation had primary CTA, compared with 1 (6.7%) of 15 patients in the present study.4 Our literature review did not identify any studies that independently reported the dislocation rate in patients who underwent RTSA for rotator cuff failure.
The risk factors of subscapularis irreparability and revision surgery suggest the importance of the soft-tissue envelope and bony anatomy in dislocation prevention. Previous analyses have suggested implant malpositioning,27,28 poor subscapularis quality,29 and inadequate muscle tensioning5,30-32 as risk factors for RTSA. Patients with an irreparable subscapularis tendon have often had multiple surgeries with compromise to the muscle/soft-tissue envelope or bony anatomy of the shoulder. A biomechanical study by Gutiérrez and colleagues31 found the compressive forces of the soft tissue at the glenohumeral joint to be the most important contributor to stability in the RTSA prosthesis. In clinical studies, the role of the subscapularis in preventing instability after RTSA remains unclear. Edwards and colleagues29 prospectively compared dislocation rates in patients with reparable and irreparable subscapularis tendons during RTSA and found a higher rate of dislocation in the irreparable subscapularis group. Of note, patients in the irreparable subscapularis group also had more complex diagnoses, including proximal humeral nonunion, fixed glenohumeral dislocation, and failed prior arthroplasty. Clark and colleagues33 retrospectively analyzed subscapularis repair in 2 RTSA groups and found no appreciable effect on complication rate, dislocation events, range-of-motion gains, or pain relief.
Our finding that higher BMI is an independent risk factor was previously described.4 The association is unclear but could be related to implant positioning, difficulty in intraoperative assessment of muscle tensioning, or body habitus that may generate a lever arm for impingement and dislocation when the arm is in adduction. Last, our finding that male sex is a risk factor for dislocation approached significance, and this relationship was previously reported.4 This could be attributable to a higher rate of activity or of indolent infection in male patients.34,35Besides studying risk factors for dislocation after RTSA, we investigated treatment. None of our patients were treated successfully and definitively with closed reduction in the clinic. This finding diverges from findings in studies by Teusink and colleagues2 and Chalmers and colleagues,4who respectively reported 62% and 44% rates of success with closed reduction. Our cohort of 14 patients with 15 dislocations required a total of 17 trips to the operating room after dislocation. This significantly higher rate of return to the operating room suggests that dislocation after RTSA may be a more costly and morbid problem than has been previously described.
This study had several weaknesses. Despite its large consecutive series of patients, the study was retrospective, and several variables that would be documented and controlled in a prospective study could not be measured here. Specifically, neither preoperative physical examination nor patient-specific assessments of pain or function were consistently obtained. Similarly, postoperative patient-specific instruments of outcomes evaluation were not obtained consistently, so results of patients with dislocation could not be compared with those of a control group. In addition, preoperative and postoperative radiographs were not consistently present in our electronic medical records, so the influence of preoperative bony anatomy, intraoperative limb lengthening, and any implant malpositioning could not be determined. Furthermore, operative details, such as reparability of the subscapularis, were not fully available for the control group and could not be included in statistical analysis. In addition, that the known dislocation risk factor of male sex4 was identified here but was not significant in multivariate regression analysis suggests that this study may not have been adequately powered to identify a significant difference in dislocation rate between the sexes. Last, though our results suggested associations between the aforementioned variables and dislocation after RTSA, a truly causative relationship could not be confirmed with this study design or analysis. Therefore, our study findings are hypothesis-generating and may indicate a benefit to greater deltoid tensioning, use of retentive liners, or more conservative rehabilitation protocols for high-risk patients.
Conclusion
Dislocation after RTSA is an uncommon complication that often requires a return to the operating room. This study identified a modifiable risk factor (higher BMI) and 3 nonmodifiable risk factors (male sex, subscapularis insufficiency, revision surgery) for dislocation after RTSA. In contrast, patients who undergo RTSA for primary rotator cuff pathology are unlikely to dislocate after surgery. This low risk of dislocation after RTSA for primary cuff pathology was not previously described. Patients in the higher risk category may benefit from preoperative lifestyle modification, intraoperative techniques for increasing stability, and more conservative therapy after surgery. In addition, unlike previous investigations, this study did not find closed reduction in the clinic alone to be successful in definitively treating this patient population.
Am J Orthop. 2016;45(7):E444-E450. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
Risk factors for dislocation after reverse total shoulder arthroplasty (RTSA) are not clearly defined. Prosthetic dislocation can result in poor patient satisfaction, worse functional outcomes, and return to the operating room.1-3 As a result, identification of modifiable risk factors for complications represents an important research initiative for shoulder surgeons.
There is a paucity of literature devoted to the study of dislocation after RTSA. Chalmers and colleagues4 found a 2.9% (11/385) incidence of early dislocation within 3 months after index surgery—an improvement over the 15.8% reported for early instability over the period 2004–2006.5 As prosthesis design has improved and surgeons have become more comfortable with the RTSA prosthesis, surgical indications have expanded,6,7 and dislocation rates appear to have decreased. Although the most common indication for RTSA continues to be cuff tear arthropathy (CTA),6 there has been increased use in rheumatoid arthritis8-10; proximal humerus fractures, especially in cases of poor bone quality and unreliable fixation of tuberosities11-13; and failed previous shoulder reconstruction.14,15 As RTSA is performed more often, limiting the complications will become more important for both patient care and economics.
We conducted a study to analyze dislocation rates at our institution and to identify both modifiable and nonmodifiable risk factors for dislocation after RTSA. By identifying risk factors for dislocation, we will be able to implement additional perioperative clinical measures to reduce the incidence of dislocation.
Materials and Methods
This retrospective study of dislocation after RTSA was conducted at the Rothman Institute of Orthopedics and Methodist Hospital (Thomas Jefferson University Hospitals, Philadelphia, PA). After obtaining Institutional Review Board approval for the study, we searched our institution’s electronic database of shoulder arthroplasties to identify all RTSAs performed at our 2 large-volume urban institutions between September 27, 2010 and December 31, 2013. For the record search, International Classification of Diseases, Ninth Revision (ICD-9) codes were used (Table 1).
The medical records of each patient were used to identify independent variables that could be associated with dislocation rate. Demographic variables included sex, age, and race. Preoperative clinical data included body mass index (BMI), etiology of shoulder disease leading to RTSA, individual comorbidities, and Charlson Comorbidity Index (CCI)16 modified to be used with ICD-9 codes.17 In addition, prior shoulder surgery history and arthroplasty type (primary or revision) were determined. Postoperative considerations were time to dislocation, mechanism of dislocation, and intervention(s) needed for dislocation. Although the institutional database did not include operative variables such as prosthesis type and surgical approach, all 6 surgeons in this study were using a standard deltopectoral approach in beach-chair position with a Grammont style prosthesis for RTSA cases.
Descriptive statistics for RTSA patients and the dislocation subpopulation were compiled. Bivariate analysis was used to evaluate which of the previously described variables influenced dislocation rates. Last, multivariate logistic regression analysis was performed to evaluate which factors were independent predictors of dislocation. We included demographic variables (age, sex, ethnicity), clinical variables (BMI, individual comorbidities, CCI), and surgical variables (primary vs revision, diagnosis at time of surgery). All statistical analyses were performed with Excel 2013 (Microsoft) and SPSS Statistics Version 20.0 (SPSS Inc.).
Results
From the database, we identified 487 patients who underwent 510 RTSAs during the study period. These surgeries were performed by 6 shoulder and elbow fellowship–trained surgeons. Of the 510 RTSAs, 393 (77.1%) were primary cases, and 117 (22.9%) were revision cases.
Of the 510 shoulders that underwent RTSA, 15 (2.9%; 14 patients) dislocated. Of these 15 cases, 5 were primary (1.3% of all primary cases) and 10 were revision (8.5% of all revision cases). Mean time from index surgery to diagnosis of dislocation was 58.2 days (range, 0-319 days). One dislocation occurred immediately after surgery, 2 after falls, 4 from patient-identified low-energy mechanisms of injury, and 8 without known inciting events. Nine dislocations (60%) did not have a subscapularis repair (7 were irreparable, 2 underwent subscapularis peel without repair), and the other 6 were repaired primarily (Table 2).
Male patients accounted for 32.2% of the study population but 60.0% of the dislocations (P = .019) (Table 3).
Multivariate logistic regression analysis revealed revision arthroplasty (OR = 7.515; P = .042) and increased BMI (OR = 1.09; P = .047) to be independent risk factors for dislocation after RTSA. Analysis also found a diagnosis of primary CTA to be independently associated with lower risk of dislocation after RTSA (OR = 0.025; P = .008). Last, the previously described risk factor of male sex was found not to be a significant independent risk factor, though it did trend positively (OR = 3.011; P = .071).
Discussion
With more RTSAs being performed, evaluation of their common complications becomes increasingly important.18 We found a 3.0% rate of dislocation after RTSA, which is consistent with the most recently reported incidence4 and falls within the previously described range of 0% to 8.6%.19-26 Of the clinical risk factors identified in this study, those previously described were prior surgery, subscapularis insufficiency, higher BMI, and male sex.4 However, our finding of lower risk of dislocation after RTSA for primary rotator cuff pathology was not previously described. Although Chalmers and colleagues4 did not report this lower risk, 3 (27.3%) of their 11 patients with dislocation had primary CTA, compared with 1 (6.7%) of 15 patients in the present study.4 Our literature review did not identify any studies that independently reported the dislocation rate in patients who underwent RTSA for rotator cuff failure.
The risk factors of subscapularis irreparability and revision surgery suggest the importance of the soft-tissue envelope and bony anatomy in dislocation prevention. Previous analyses have suggested implant malpositioning,27,28 poor subscapularis quality,29 and inadequate muscle tensioning5,30-32 as risk factors for RTSA. Patients with an irreparable subscapularis tendon have often had multiple surgeries with compromise to the muscle/soft-tissue envelope or bony anatomy of the shoulder. A biomechanical study by Gutiérrez and colleagues31 found the compressive forces of the soft tissue at the glenohumeral joint to be the most important contributor to stability in the RTSA prosthesis. In clinical studies, the role of the subscapularis in preventing instability after RTSA remains unclear. Edwards and colleagues29 prospectively compared dislocation rates in patients with reparable and irreparable subscapularis tendons during RTSA and found a higher rate of dislocation in the irreparable subscapularis group. Of note, patients in the irreparable subscapularis group also had more complex diagnoses, including proximal humeral nonunion, fixed glenohumeral dislocation, and failed prior arthroplasty. Clark and colleagues33 retrospectively analyzed subscapularis repair in 2 RTSA groups and found no appreciable effect on complication rate, dislocation events, range-of-motion gains, or pain relief.
Our finding that higher BMI is an independent risk factor was previously described.4 The association is unclear but could be related to implant positioning, difficulty in intraoperative assessment of muscle tensioning, or body habitus that may generate a lever arm for impingement and dislocation when the arm is in adduction. Last, our finding that male sex is a risk factor for dislocation approached significance, and this relationship was previously reported.4 This could be attributable to a higher rate of activity or of indolent infection in male patients.34,35Besides studying risk factors for dislocation after RTSA, we investigated treatment. None of our patients were treated successfully and definitively with closed reduction in the clinic. This finding diverges from findings in studies by Teusink and colleagues2 and Chalmers and colleagues,4who respectively reported 62% and 44% rates of success with closed reduction. Our cohort of 14 patients with 15 dislocations required a total of 17 trips to the operating room after dislocation. This significantly higher rate of return to the operating room suggests that dislocation after RTSA may be a more costly and morbid problem than has been previously described.
This study had several weaknesses. Despite its large consecutive series of patients, the study was retrospective, and several variables that would be documented and controlled in a prospective study could not be measured here. Specifically, neither preoperative physical examination nor patient-specific assessments of pain or function were consistently obtained. Similarly, postoperative patient-specific instruments of outcomes evaluation were not obtained consistently, so results of patients with dislocation could not be compared with those of a control group. In addition, preoperative and postoperative radiographs were not consistently present in our electronic medical records, so the influence of preoperative bony anatomy, intraoperative limb lengthening, and any implant malpositioning could not be determined. Furthermore, operative details, such as reparability of the subscapularis, were not fully available for the control group and could not be included in statistical analysis. In addition, that the known dislocation risk factor of male sex4 was identified here but was not significant in multivariate regression analysis suggests that this study may not have been adequately powered to identify a significant difference in dislocation rate between the sexes. Last, though our results suggested associations between the aforementioned variables and dislocation after RTSA, a truly causative relationship could not be confirmed with this study design or analysis. Therefore, our study findings are hypothesis-generating and may indicate a benefit to greater deltoid tensioning, use of retentive liners, or more conservative rehabilitation protocols for high-risk patients.
Conclusion
Dislocation after RTSA is an uncommon complication that often requires a return to the operating room. This study identified a modifiable risk factor (higher BMI) and 3 nonmodifiable risk factors (male sex, subscapularis insufficiency, revision surgery) for dislocation after RTSA. In contrast, patients who undergo RTSA for primary rotator cuff pathology are unlikely to dislocate after surgery. This low risk of dislocation after RTSA for primary cuff pathology was not previously described. Patients in the higher risk category may benefit from preoperative lifestyle modification, intraoperative techniques for increasing stability, and more conservative therapy after surgery. In addition, unlike previous investigations, this study did not find closed reduction in the clinic alone to be successful in definitively treating this patient population.
Am J Orthop. 2016;45(7):E444-E450. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. Aldinger PR, Raiss P, Rickert M, Loew M. Complications in shoulder arthroplasty: an analysis of 485 cases. Int Orthop. 2010;34(4):517-524.
2. Teusink MJ, Pappou IP, Schwartz DG, Cottrell BJ, Frankle MA. Results of closed management of acute dislocation after reverse shoulder arthroplasty. J Shoulder Elbow Surg. 2015;24(4):621-627.
3. Fink Barnes LA, Grantham WJ, Meadows MC, Bigliani LU, Levine WN, Ahmad CS. Sports activity after reverse total shoulder arthroplasty with minimum 2-year follow-up. Am J Orthop. 2015;44(2):68-72.
4. Chalmers PN, Rahman Z, Romeo AA, Nicholson GP. Early dislocation after reverse total shoulder arthroplasty. J Shoulder Elbow Surg. 2014;23(5):737-744.
5. Gallo RA, Gamradt SC, Mattern CJ, et al; Sports Medicine and Shoulder Service at the Hospital for Special Surgery, New York, NY. Instability after reverse total shoulder replacement. J Shoulder Elbow Surg. 2011;20(4):584-590.
6. Walch G, Bacle G, Lädermann A, Nové-Josserand L, Smithers CJ. Do the indications, results, and complications of reverse shoulder arthroplasty change with surgeon’s experience? J Shoulder Elbow Surg. 2012;21(11):1470-1477.
7. Smith CD, Guyver P, Bunker TD. Indications for reverse shoulder replacement: a systematic review. J Bone Joint Surg Br. 2012;94(5):577-583.
8. Young AA, Smith MM, Bacle G, Moraga C, Walch G. Early results of reverse shoulder arthroplasty in patients with rheumatoid arthritis. J Bone Joint Surg Am. 2011;93(20):1915-1923.
9. Hedtmann A, Werner A. Shoulder arthroplasty in rheumatoid arthritis [in German]. Orthopade. 2007;36(11):1050-1061.
10. Rittmeister M, Kerschbaumer F. Grammont reverse total shoulder arthroplasty in patients with rheumatoid arthritis and nonreconstructible rotator cuff lesions. J Shoulder Elbow Surg. 2001;10(1):17-22.
11. Acevedo DC, Vanbeek C, Lazarus MD, Williams GR, Abboud JA. Reverse shoulder arthroplasty for proximal humeral fractures: update on indications, technique, and results. J Shoulder Elbow Surg. 2014;23(2):279-289.
12. Bufquin T, Hersan A, Hubert L, Massin P. Reverse shoulder arthroplasty for the treatment of three- and four-part fractures of the proximal humerus in the elderly: a prospective review of 43 cases with a short-term follow-up. J Bone Joint Surg Br. 2007;89(4):516-520.
13. Cuff DJ, Pupello DR. Comparison of hemiarthroplasty and reverse shoulder arthroplasty for the treatment of proximal humeral fractures in elderly patients. J Bone Joint Surg Am. 2013;95(22):2050-2055.
14. Walker M, Willis MP, Brooks JP, Pupello D, Mulieri PJ, Frankle MA. The use of the reverse shoulder arthroplasty for treatment of failed total shoulder arthroplasty. J Shoulder Elbow Surg. 2012;21(4):514-522.
15. Valenti P, Kilinc AS, Sauzières P, Katz D. Results of 30 reverse shoulder prostheses for revision of failed hemi- or total shoulder arthroplasty. Eur J Orthop Surg Traumatol. 2014;24(8):1375-1382.
16. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373-383.
17. Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45(6):613-619.
18. Kim SH, Wise BL, Zhang Y, Szabo RM. Increasing incidence of shoulder arthroplasty in the United States. J Bone Joint Surg Am. 2011;93(24):2249-2254.
19. Boileau P, Watkinson D, Hatzidakis AM, Hovorka I. Neer Award 2005: the Grammont reverse shoulder prosthesis: results in cuff tear arthritis, fracture sequelae, and revision arthroplasty. J Shoulder Elbow Surg. 2006;15(5):527-540.
20. Cuff D, Pupello D, Virani N, Levy J, Frankle M. Reverse shoulder arthroplasty for the treatment of rotator cuff deficiency. J Bone Joint Surg Am. 2008;90(6):1244-1251.
21. Frankle M, Siegal S, Pupello D, Saleem A, Mighell M, Vasey M. The reverse shoulder prosthesis for glenohumeral arthritis associated with severe rotator cuff deficiency. A minimum two-year follow-up study of sixty patients. J Bone Joint Surg Am. 2005;87(8):1697-1705.
22. Guery J, Favard L, Sirveaux F, Oudet D, Mole D, Walch G. Reverse total shoulder arthroplasty. Survivorship analysis of eighty replacements followed for five to ten years. J Bone Joint Surg Am. 2006;88(8):1742-1747.
23. Mulieri P, Dunning P, Klein S, Pupello D, Frankle M. Reverse shoulder arthroplasty for the treatment of irreparable rotator cuff tear without glenohumeral arthritis. J Bone Joint Surg Am. 2010;92(15):2544-2556.
24. Sirveaux F, Favard L, Oudet D, Huquet D, Walch G, Molé D. Grammont inverted total shoulder arthroplasty in the treatment of glenohumeral osteoarthritis with massive rupture of the cuff. Results of a multicentre study of 80 shoulders. J Bone Joint Surg Br. 2004;86(3):388-395.
25. Wall B, Nové-Josserand L, O’Connor DP, Edwards TB, Walch G. Reverse total shoulder arthroplasty: a review of results according to etiology. J Bone Joint Surg Am. 2007;89(7):1476-1485.
26. Werner CM, Steinmann PA, Gilbart M, Gerber C. Treatment of painful pseudoparesis due to irreparable rotator cuff dysfunction with the Delta III reverse-ball-and-socket total shoulder prosthesis. J Bone Joint Surg Am. 2005;87(7):1476-1486.
27. Cazeneuve JF, Cristofari DJ. The reverse shoulder prosthesis in the treatment of fractures of the proximal humerus in the elderly. J Bone Joint Surg Br. 2010;92(4):535-539.
28. Stephenson DR, Oh JH, McGarry MH, Rick Hatch GF 3rd, Lee TQ. Effect of humeral component version on impingement in reverse total shoulder arthroplasty. J Shoulder Elbow Surg. 2011;20(4):652-658.
29. Edwards TB, Williams MD, Labriola JE, Elkousy HA, Gartsman GM, O’Connor DP. Subscapularis insufficiency and the risk of shoulder dislocation after reverse shoulder arthroplasty. J Shoulder Elbow Surg. 2009;18(6):892-896.
30. Affonso J, Nicholson GP, Frankle MA, et al. Complications of the reverse prosthesis: prevention and treatment. Instr Course Lect. 2012;61:157-168.
31. Gutiérrez S, Keller TS, Levy JC, Lee WE 3rd, Luo ZP. Hierarchy of stability factors in reverse shoulder arthroplasty. Clin Orthop Relat Res. 2008;466(3):670-676.
32. Boileau P, Watkinson DJ, Hatzidakis AM, Balg F. Grammont reverse prosthesis: design, rationale, and biomechanics. J Shoulder Elbow Surg. 2005;14(1 suppl S):147S-161S.
33. Clark JC, Ritchie J, Song FS, et al. Complication rates, dislocation, pain, and postoperative range of motion after reverse shoulder arthroplasty in patients with and without repair of the subscapularis. J Shoulder Elbow Surg. 2012;21(1):36-41.
34. Richards J, Inacio MC, Beckett M, et al. Patient and procedure-specific risk factors for deep infection after primary shoulder arthroplasty. Clin Orthop Relat Res. 2014;472(9):2809-2815.
35. Singh JA, Sperling JW, Schleck C, Harmsen WS, Cofield RH. Periprosthetic infections after total shoulder arthroplasty: a 33-year perspective. J Shoulder Elbow Surg. 2012;21(11):1534-1541.
1. Aldinger PR, Raiss P, Rickert M, Loew M. Complications in shoulder arthroplasty: an analysis of 485 cases. Int Orthop. 2010;34(4):517-524.
2. Teusink MJ, Pappou IP, Schwartz DG, Cottrell BJ, Frankle MA. Results of closed management of acute dislocation after reverse shoulder arthroplasty. J Shoulder Elbow Surg. 2015;24(4):621-627.
3. Fink Barnes LA, Grantham WJ, Meadows MC, Bigliani LU, Levine WN, Ahmad CS. Sports activity after reverse total shoulder arthroplasty with minimum 2-year follow-up. Am J Orthop. 2015;44(2):68-72.
4. Chalmers PN, Rahman Z, Romeo AA, Nicholson GP. Early dislocation after reverse total shoulder arthroplasty. J Shoulder Elbow Surg. 2014;23(5):737-744.
5. Gallo RA, Gamradt SC, Mattern CJ, et al; Sports Medicine and Shoulder Service at the Hospital for Special Surgery, New York, NY. Instability after reverse total shoulder replacement. J Shoulder Elbow Surg. 2011;20(4):584-590.
6. Walch G, Bacle G, Lädermann A, Nové-Josserand L, Smithers CJ. Do the indications, results, and complications of reverse shoulder arthroplasty change with surgeon’s experience? J Shoulder Elbow Surg. 2012;21(11):1470-1477.
7. Smith CD, Guyver P, Bunker TD. Indications for reverse shoulder replacement: a systematic review. J Bone Joint Surg Br. 2012;94(5):577-583.
8. Young AA, Smith MM, Bacle G, Moraga C, Walch G. Early results of reverse shoulder arthroplasty in patients with rheumatoid arthritis. J Bone Joint Surg Am. 2011;93(20):1915-1923.
9. Hedtmann A, Werner A. Shoulder arthroplasty in rheumatoid arthritis [in German]. Orthopade. 2007;36(11):1050-1061.
10. Rittmeister M, Kerschbaumer F. Grammont reverse total shoulder arthroplasty in patients with rheumatoid arthritis and nonreconstructible rotator cuff lesions. J Shoulder Elbow Surg. 2001;10(1):17-22.
11. Acevedo DC, Vanbeek C, Lazarus MD, Williams GR, Abboud JA. Reverse shoulder arthroplasty for proximal humeral fractures: update on indications, technique, and results. J Shoulder Elbow Surg. 2014;23(2):279-289.
12. Bufquin T, Hersan A, Hubert L, Massin P. Reverse shoulder arthroplasty for the treatment of three- and four-part fractures of the proximal humerus in the elderly: a prospective review of 43 cases with a short-term follow-up. J Bone Joint Surg Br. 2007;89(4):516-520.
13. Cuff DJ, Pupello DR. Comparison of hemiarthroplasty and reverse shoulder arthroplasty for the treatment of proximal humeral fractures in elderly patients. J Bone Joint Surg Am. 2013;95(22):2050-2055.
14. Walker M, Willis MP, Brooks JP, Pupello D, Mulieri PJ, Frankle MA. The use of the reverse shoulder arthroplasty for treatment of failed total shoulder arthroplasty. J Shoulder Elbow Surg. 2012;21(4):514-522.
15. Valenti P, Kilinc AS, Sauzières P, Katz D. Results of 30 reverse shoulder prostheses for revision of failed hemi- or total shoulder arthroplasty. Eur J Orthop Surg Traumatol. 2014;24(8):1375-1382.
16. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373-383.
17. Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45(6):613-619.
18. Kim SH, Wise BL, Zhang Y, Szabo RM. Increasing incidence of shoulder arthroplasty in the United States. J Bone Joint Surg Am. 2011;93(24):2249-2254.
19. Boileau P, Watkinson D, Hatzidakis AM, Hovorka I. Neer Award 2005: the Grammont reverse shoulder prosthesis: results in cuff tear arthritis, fracture sequelae, and revision arthroplasty. J Shoulder Elbow Surg. 2006;15(5):527-540.
20. Cuff D, Pupello D, Virani N, Levy J, Frankle M. Reverse shoulder arthroplasty for the treatment of rotator cuff deficiency. J Bone Joint Surg Am. 2008;90(6):1244-1251.
21. Frankle M, Siegal S, Pupello D, Saleem A, Mighell M, Vasey M. The reverse shoulder prosthesis for glenohumeral arthritis associated with severe rotator cuff deficiency. A minimum two-year follow-up study of sixty patients. J Bone Joint Surg Am. 2005;87(8):1697-1705.
22. Guery J, Favard L, Sirveaux F, Oudet D, Mole D, Walch G. Reverse total shoulder arthroplasty. Survivorship analysis of eighty replacements followed for five to ten years. J Bone Joint Surg Am. 2006;88(8):1742-1747.
23. Mulieri P, Dunning P, Klein S, Pupello D, Frankle M. Reverse shoulder arthroplasty for the treatment of irreparable rotator cuff tear without glenohumeral arthritis. J Bone Joint Surg Am. 2010;92(15):2544-2556.
24. Sirveaux F, Favard L, Oudet D, Huquet D, Walch G, Molé D. Grammont inverted total shoulder arthroplasty in the treatment of glenohumeral osteoarthritis with massive rupture of the cuff. Results of a multicentre study of 80 shoulders. J Bone Joint Surg Br. 2004;86(3):388-395.
25. Wall B, Nové-Josserand L, O’Connor DP, Edwards TB, Walch G. Reverse total shoulder arthroplasty: a review of results according to etiology. J Bone Joint Surg Am. 2007;89(7):1476-1485.
26. Werner CM, Steinmann PA, Gilbart M, Gerber C. Treatment of painful pseudoparesis due to irreparable rotator cuff dysfunction with the Delta III reverse-ball-and-socket total shoulder prosthesis. J Bone Joint Surg Am. 2005;87(7):1476-1486.
27. Cazeneuve JF, Cristofari DJ. The reverse shoulder prosthesis in the treatment of fractures of the proximal humerus in the elderly. J Bone Joint Surg Br. 2010;92(4):535-539.
28. Stephenson DR, Oh JH, McGarry MH, Rick Hatch GF 3rd, Lee TQ. Effect of humeral component version on impingement in reverse total shoulder arthroplasty. J Shoulder Elbow Surg. 2011;20(4):652-658.
29. Edwards TB, Williams MD, Labriola JE, Elkousy HA, Gartsman GM, O’Connor DP. Subscapularis insufficiency and the risk of shoulder dislocation after reverse shoulder arthroplasty. J Shoulder Elbow Surg. 2009;18(6):892-896.
30. Affonso J, Nicholson GP, Frankle MA, et al. Complications of the reverse prosthesis: prevention and treatment. Instr Course Lect. 2012;61:157-168.
31. Gutiérrez S, Keller TS, Levy JC, Lee WE 3rd, Luo ZP. Hierarchy of stability factors in reverse shoulder arthroplasty. Clin Orthop Relat Res. 2008;466(3):670-676.
32. Boileau P, Watkinson DJ, Hatzidakis AM, Balg F. Grammont reverse prosthesis: design, rationale, and biomechanics. J Shoulder Elbow Surg. 2005;14(1 suppl S):147S-161S.
33. Clark JC, Ritchie J, Song FS, et al. Complication rates, dislocation, pain, and postoperative range of motion after reverse shoulder arthroplasty in patients with and without repair of the subscapularis. J Shoulder Elbow Surg. 2012;21(1):36-41.
34. Richards J, Inacio MC, Beckett M, et al. Patient and procedure-specific risk factors for deep infection after primary shoulder arthroplasty. Clin Orthop Relat Res. 2014;472(9):2809-2815.
35. Singh JA, Sperling JW, Schleck C, Harmsen WS, Cofield RH. Periprosthetic infections after total shoulder arthroplasty: a 33-year perspective. J Shoulder Elbow Surg. 2012;21(11):1534-1541.
Surgical Simulation in Orthopedic Surgery Residency
The training model for orthopedic resident education has been transformed. Surgeon factors, patient expectations, financial and legal concerns, associated costs, and work hour restrictions have put pressure on resident autonomy in the operating room.1,2 At the end of resident training, the expectation is that board-eligible surgeons will have the surgical skills necessary to perform a wide range of surgical procedures.3,4 Helping residents become proficient for independent practice requires a multidisciplinary approach.5 This approach, regardless of its details, requires investment in time, resources, expertise, and funding.
Many residency programs are trying to bridge the gap between observation and autonomy with surgical simulation. According to one study, 76% of residency programs have a surgical skills laboratory, and 46% have a structured surgical skills curriculum.6Surgical skills preparation is available in different modalities. Synthetic bones, virtual reality, and arthroscopic simulators represent potential opportunities for practice. Through these modalities, residents become more comfortable with the tools used in orthopedic procedures. Cadaveric dissection allows them to practice surgical approaches in the setting of real anatomy.1 Independent dissection helps them appreciate the planes, layers, and proximity of crucial body structures and understand important surgical anatomy.4Surgical simulation can be expensive, and funding comes in many forms. Cadaver laboratories require investment in specimens, facilities, and time away from clinical obligations.4 Cadaver availability varies with regional resources, and the cost of a cadaver ranges from $1000 to $2000.7,8 Arthroscopic simulators and virtual reality programs are expensive as well. These modalities range from a less expensive video box (with standard arthroscopic equipment) to a virtual reality haptic simulation costing a residency program as much as $80,000.9 Synthetic bone simulations are less expensive but require investment in faculty time and outside implants and instrumentation.10 The cost of simulation raises the question of funding sources.
Funding surgical simulation is a challenge. In a national survey of program directors, conducted by Karam and colleagues,6 87.3% of residencies cited lack of funding as the most significant barrier to a formal surgical skills program. Simulation can be residency-sponsored, industry-sponsored, or specialty-sponsored. Karam and colleagues6 found that department, hospital, and industry funding were the 3 main sponsors of surgical simulation. Each funding mechanism brings its own set of challenges and opportunities. Industry-sponsored simulation provides a cost-effective outlet for residency programs. However, this type of funding is under scrutiny, as industry funding for education becomes more transparent. In addition, industry funding typically limits the technology that can be used during the simulation to the sponsor’s technology. Courses offered by the American Academy of Orthopaedic Surgeons (AAOS) and a number of subspecialty societies provide less conflicted simulation at reasonable cost.
If residents, residency programs, hospitals, industry, subspecialty societies, and the AAOS are going to invest in resident education through simulation, then the effect of simulation on resident education must be understood. Intuitively, simulation as a modality for improving resident skills makes sense. For residency programs to invest in simulation and surgical skills, different modalities must be objectively evaluated and their utility validated. If simulation is to become valuable, first it must be done correctly.
Kneebone11 proposed a framework for evaluating simulation. In this framework, simulation should allow for sustained, deliberate practice in a safe environment. It should provide access to expert tutors when appropriate. It should map onto real-life clinical experience. Last, it should provide a supportive, motivational, learner-centered milieu. Residents and program directors should consider this framework when deciding which simulation exercises to engage in and which resources to supply for exercises. Having supportive supervision during simulation can lead to a positive outcome. Likewise, learning incorrect techniques or bad habits or having inexperienced teachers can have the opposite effect.
Several authors have reviewed the evidence and found simulation to be an important part of orthopedic resident education.1,2,4,9,12,13 They have evaluated cadaveric simulation, synthetic bone simulation, arthroscopic simulation, and virtual reality simulation. Their studies demonstrated that simulation is an effective tool and provided objective criteria for evaluating residents on a larger scale. In a blinded, randomized study by Howells and colleagues,14 junior residents were either trained on a knee simulator or received no training before evaluation. Those who received the training scored significantly better than their peers on validated assessment measures.
The literature on different modalities shows simulation is an effective teaching tool for general orthopedic surgical skills5; knee, shoulder, and ankle arthroscopy14-21; spine surgery22; and orthopedic trauma surgery.23-26 Investigators in several other surgical specialties have studied the utility of simulation, and many are incorporating simulation into their resident curricula.
More effective simulation seems correlated with a yearlong structured curriculum rather than with intermittent, isolated experiences.3 Dunn and colleagues27 evaluated arthroscopic shoulder simulation 1 year after a training exercise. The group that received formal training did better than the control group on an initial arthroscopic surgery skill evaluation tool. At 1 year, however, the gains made through training were lost.
Simulation is a new paradigm for resident education. It offers multiple opportunities and challenges for residents, residency programs, industry partners, specialty and subspecialty societies, and medical examiners. The Accreditation Council for Graduate Medical Education’s ACGME Program Requirements for Graduate Medical Education in Orthopaedic Surgery requires of residency programs a didactic curriculum dedicated to basic motor skills in addition to a dedicated space for facilitating basic surgical skills training.28 Residency programs must demonstrate to ACGME their commitment to surgical skills training and simulation. Implementation of simulation for resident education has many variables, including funding, type of simulation, demonstrated efficacy, provision of supervision, resident time, and establishment of a formal curriculum. Residents and residency programs should embrace this changing paradigm to bridge the gap between observation and autonomy in orthopedic surgical and arthroscopic technique.
Am J Orthop. 2016;45(7):E426-E428. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. Atesok K, Mabrey JD, Jazrawi LM, Egol KA. Surgical simulation in orthopaedic skills training. J Am Acad Orthop Surg. 2012;20(7):410-422.
2. Thomas GW, Johns BD, Marsh JL, Anderson DD. A review of the role of simulation in developing and assessing orthopaedic surgical skills. Iowa Orthop J. 2014;34:181-189.
3. Reznick RK, MacRae H. Teaching surgical skills—changes in the wind. N Engl J Med. 2006;355(25):2664-2669.
4. Holland JP, Waugh L, Horgan A, Paleri V, Deehan DJ. Cadaveric hands-on training for surgical specialties: is this back to the future for surgical skills development? J Surg Educ. 2011;68(2):110-116.
5. Sonnadara RR, Van Vliet A, Safir O, et al. Orthopedic boot camp: examining the effectiveness of an intensive surgical skills course. Surgery. 2011;149(6):745-749.
6. Karam MD, Pedowitz RA, Natividad H, Murray J, Marsh JL. Current and future use of surgical skills training laboratories in orthopaedic resident education: a national survey. J Bone Joint Surg Am. 2013;95(1):e4.
7. Bushey C. Cadaver supply: the last industry to face big changes. Crain’s Chicago Business. February 23, 2013.
8. Human K. Cadaver shortage hits medical schools. Denver Post. April 29, 2008.
9. Michelson JD. Simulation in orthopaedic education: an overview of theory and practice. J Bone Joint Surg Am. 2006;88(6):1405-1411.
10. Elfar J, Menorca RM, Reed JD, Stanbury S. Composite bone models in orthopaedic surgery research and education. J Am Acad Orthop Surg. 2014;22(2):111-120.
11. Kneebone R. Evaluating clinical simulations for learning procedural skills: a theory-based approach. Acad Med. 2005;80(6):549-553.
12. Stirling ER, Lewis TL, Ferran NA. Surgical skills simulation in trauma and orthopaedic training. J Orthop Surg Res. 2014;9:126.
13. Mabrey JD, Reinig KD, Cannon WD. Virtual reality in orthopaedics: is it a reality? Clin Orthop Relat Res. 2010;468(10):2586-2591.
14. Howells NR, Gill HS, Carr AJ, Price AJ, Rees JL. Transferring simulated arthroscopic skills to the operating theatre: a randomised blinded study. J Bone Joint Surg Br. 2008;90(4):494-499.
15. Gomoll AH, O’Toole RV, Czarnecki J, Warner JJ. Surgical experience correlates with performance on a virtual reality simulator for shoulder arthroscopy. Am J Sports Med. 2007;35(6):883-888.
16. Gomoll AH, Pappas G, Forsythe B, Warner JJ. Individual skill progression on a virtual reality simulator for shoulder arthroscopy: a 3-year follow-up study. Am J Sports Med. 2008;36(6):1139-1142.
17. Pedowitz RA, Esch J, Snyder S. Evaluation of a virtual reality simulator for arthroscopy skills development. Arthroscopy. 2002;18(6):E29.
18. Martin KD, Belmont PJ, Schoenfeld AJ, Todd M, Cameron KL, Owens BD. Arthroscopic basic task performance in shoulder simulator model correlates with similar task performance in cadavers. J Bone Joint Surg Am. 2011;93(21):e1271-e1275.
19. Martin KD, Cameron K, Belmont PJ, Schoenfeld A, Owens BD. Shoulder arthroscopy simulator performance correlates with resident and shoulder arthroscopy experience. J Bone Joint Surg Am. 2012;94(21):e160.
20. Martin KD, Patterson D, Phisitkul P, Cameron KL, Femino J, Amendola A. Ankle arthroscopy simulation improves basic skills, anatomic recognition, and proficiency during diagnostic examination of residents in training. Foot Ankle Int. 2015;36(7):827-835.
21. Frank RM, Erickson B, Frank JM, et al. Utility of modern arthroscopic simulator training models. Arthroscopy. 2014;30(1):121-133.
22. Rambani R, Ward J, Viant W. Desktop-based computer-assisted orthopedic training system for spinal surgery. J Surg Educ. 2014;71(6):805-809.
23. Leong JJ, Leff DR, Das A, et al. Validation of orthopaedic bench models for trauma surgery. J Bone Joint Surg Br. 2008;90(7):958-965.
24. Rambani R, Viant W, Ward J, Mohsen A. Computer-assisted orthopedic training system for fracture fixation. J Surg Educ. 2013;70(3):304-308.
25. Blyth P, Stott NS, Anderson IA. A simulation-based training system for hip fracture fixation for use within the hospital environment. Injury. 2007;38(10):1197-1203.
26. Egol KA, Phillips D, Vongbandith T, Szyld D, Strauss EJ. Do orthopaedic fracture skills courses improve resident performance? Injury. 2015;46(4):547-551.
27. Dunn JC, Belmont PJ, Lanzi J, et al. Arthroscopic shoulder surgical simulation training curriculum: transfer reliability and maintenance of skill over time. J Surg Educ. 2015;72(6):1118-1123.
28. Accreditation Council for Graduate Medical Education. ACGME Program Requirements for Graduate Medical Education in Orthopaedic Surgery. https://www.acgme.org/Portals/0/PFAssets/ProgramRequirements/260_orthopaedic_surgery_2016.pdf. Published July 1, 2012. Accessed September 30, 2016.
The training model for orthopedic resident education has been transformed. Surgeon factors, patient expectations, financial and legal concerns, associated costs, and work hour restrictions have put pressure on resident autonomy in the operating room.1,2 At the end of resident training, the expectation is that board-eligible surgeons will have the surgical skills necessary to perform a wide range of surgical procedures.3,4 Helping residents become proficient for independent practice requires a multidisciplinary approach.5 This approach, regardless of its details, requires investment in time, resources, expertise, and funding.
Many residency programs are trying to bridge the gap between observation and autonomy with surgical simulation. According to one study, 76% of residency programs have a surgical skills laboratory, and 46% have a structured surgical skills curriculum.6Surgical skills preparation is available in different modalities. Synthetic bones, virtual reality, and arthroscopic simulators represent potential opportunities for practice. Through these modalities, residents become more comfortable with the tools used in orthopedic procedures. Cadaveric dissection allows them to practice surgical approaches in the setting of real anatomy.1 Independent dissection helps them appreciate the planes, layers, and proximity of crucial body structures and understand important surgical anatomy.4Surgical simulation can be expensive, and funding comes in many forms. Cadaver laboratories require investment in specimens, facilities, and time away from clinical obligations.4 Cadaver availability varies with regional resources, and the cost of a cadaver ranges from $1000 to $2000.7,8 Arthroscopic simulators and virtual reality programs are expensive as well. These modalities range from a less expensive video box (with standard arthroscopic equipment) to a virtual reality haptic simulation costing a residency program as much as $80,000.9 Synthetic bone simulations are less expensive but require investment in faculty time and outside implants and instrumentation.10 The cost of simulation raises the question of funding sources.
Funding surgical simulation is a challenge. In a national survey of program directors, conducted by Karam and colleagues,6 87.3% of residencies cited lack of funding as the most significant barrier to a formal surgical skills program. Simulation can be residency-sponsored, industry-sponsored, or specialty-sponsored. Karam and colleagues6 found that department, hospital, and industry funding were the 3 main sponsors of surgical simulation. Each funding mechanism brings its own set of challenges and opportunities. Industry-sponsored simulation provides a cost-effective outlet for residency programs. However, this type of funding is under scrutiny, as industry funding for education becomes more transparent. In addition, industry funding typically limits the technology that can be used during the simulation to the sponsor’s technology. Courses offered by the American Academy of Orthopaedic Surgeons (AAOS) and a number of subspecialty societies provide less conflicted simulation at reasonable cost.
If residents, residency programs, hospitals, industry, subspecialty societies, and the AAOS are going to invest in resident education through simulation, then the effect of simulation on resident education must be understood. Intuitively, simulation as a modality for improving resident skills makes sense. For residency programs to invest in simulation and surgical skills, different modalities must be objectively evaluated and their utility validated. If simulation is to become valuable, first it must be done correctly.
Kneebone11 proposed a framework for evaluating simulation. In this framework, simulation should allow for sustained, deliberate practice in a safe environment. It should provide access to expert tutors when appropriate. It should map onto real-life clinical experience. Last, it should provide a supportive, motivational, learner-centered milieu. Residents and program directors should consider this framework when deciding which simulation exercises to engage in and which resources to supply for exercises. Having supportive supervision during simulation can lead to a positive outcome. Likewise, learning incorrect techniques or bad habits or having inexperienced teachers can have the opposite effect.
Several authors have reviewed the evidence and found simulation to be an important part of orthopedic resident education.1,2,4,9,12,13 They have evaluated cadaveric simulation, synthetic bone simulation, arthroscopic simulation, and virtual reality simulation. Their studies demonstrated that simulation is an effective tool and provided objective criteria for evaluating residents on a larger scale. In a blinded, randomized study by Howells and colleagues,14 junior residents were either trained on a knee simulator or received no training before evaluation. Those who received the training scored significantly better than their peers on validated assessment measures.
The literature on different modalities shows simulation is an effective teaching tool for general orthopedic surgical skills5; knee, shoulder, and ankle arthroscopy14-21; spine surgery22; and orthopedic trauma surgery.23-26 Investigators in several other surgical specialties have studied the utility of simulation, and many are incorporating simulation into their resident curricula.
More effective simulation seems correlated with a yearlong structured curriculum rather than with intermittent, isolated experiences.3 Dunn and colleagues27 evaluated arthroscopic shoulder simulation 1 year after a training exercise. The group that received formal training did better than the control group on an initial arthroscopic surgery skill evaluation tool. At 1 year, however, the gains made through training were lost.
Simulation is a new paradigm for resident education. It offers multiple opportunities and challenges for residents, residency programs, industry partners, specialty and subspecialty societies, and medical examiners. The Accreditation Council for Graduate Medical Education’s ACGME Program Requirements for Graduate Medical Education in Orthopaedic Surgery requires of residency programs a didactic curriculum dedicated to basic motor skills in addition to a dedicated space for facilitating basic surgical skills training.28 Residency programs must demonstrate to ACGME their commitment to surgical skills training and simulation. Implementation of simulation for resident education has many variables, including funding, type of simulation, demonstrated efficacy, provision of supervision, resident time, and establishment of a formal curriculum. Residents and residency programs should embrace this changing paradigm to bridge the gap between observation and autonomy in orthopedic surgical and arthroscopic technique.
Am J Orthop. 2016;45(7):E426-E428. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
The training model for orthopedic resident education has been transformed. Surgeon factors, patient expectations, financial and legal concerns, associated costs, and work hour restrictions have put pressure on resident autonomy in the operating room.1,2 At the end of resident training, the expectation is that board-eligible surgeons will have the surgical skills necessary to perform a wide range of surgical procedures.3,4 Helping residents become proficient for independent practice requires a multidisciplinary approach.5 This approach, regardless of its details, requires investment in time, resources, expertise, and funding.
Many residency programs are trying to bridge the gap between observation and autonomy with surgical simulation. According to one study, 76% of residency programs have a surgical skills laboratory, and 46% have a structured surgical skills curriculum.6Surgical skills preparation is available in different modalities. Synthetic bones, virtual reality, and arthroscopic simulators represent potential opportunities for practice. Through these modalities, residents become more comfortable with the tools used in orthopedic procedures. Cadaveric dissection allows them to practice surgical approaches in the setting of real anatomy.1 Independent dissection helps them appreciate the planes, layers, and proximity of crucial body structures and understand important surgical anatomy.4Surgical simulation can be expensive, and funding comes in many forms. Cadaver laboratories require investment in specimens, facilities, and time away from clinical obligations.4 Cadaver availability varies with regional resources, and the cost of a cadaver ranges from $1000 to $2000.7,8 Arthroscopic simulators and virtual reality programs are expensive as well. These modalities range from a less expensive video box (with standard arthroscopic equipment) to a virtual reality haptic simulation costing a residency program as much as $80,000.9 Synthetic bone simulations are less expensive but require investment in faculty time and outside implants and instrumentation.10 The cost of simulation raises the question of funding sources.
Funding surgical simulation is a challenge. In a national survey of program directors, conducted by Karam and colleagues,6 87.3% of residencies cited lack of funding as the most significant barrier to a formal surgical skills program. Simulation can be residency-sponsored, industry-sponsored, or specialty-sponsored. Karam and colleagues6 found that department, hospital, and industry funding were the 3 main sponsors of surgical simulation. Each funding mechanism brings its own set of challenges and opportunities. Industry-sponsored simulation provides a cost-effective outlet for residency programs. However, this type of funding is under scrutiny, as industry funding for education becomes more transparent. In addition, industry funding typically limits the technology that can be used during the simulation to the sponsor’s technology. Courses offered by the American Academy of Orthopaedic Surgeons (AAOS) and a number of subspecialty societies provide less conflicted simulation at reasonable cost.
If residents, residency programs, hospitals, industry, subspecialty societies, and the AAOS are going to invest in resident education through simulation, then the effect of simulation on resident education must be understood. Intuitively, simulation as a modality for improving resident skills makes sense. For residency programs to invest in simulation and surgical skills, different modalities must be objectively evaluated and their utility validated. If simulation is to become valuable, first it must be done correctly.
Kneebone11 proposed a framework for evaluating simulation. In this framework, simulation should allow for sustained, deliberate practice in a safe environment. It should provide access to expert tutors when appropriate. It should map onto real-life clinical experience. Last, it should provide a supportive, motivational, learner-centered milieu. Residents and program directors should consider this framework when deciding which simulation exercises to engage in and which resources to supply for exercises. Having supportive supervision during simulation can lead to a positive outcome. Likewise, learning incorrect techniques or bad habits or having inexperienced teachers can have the opposite effect.
Several authors have reviewed the evidence and found simulation to be an important part of orthopedic resident education.1,2,4,9,12,13 They have evaluated cadaveric simulation, synthetic bone simulation, arthroscopic simulation, and virtual reality simulation. Their studies demonstrated that simulation is an effective tool and provided objective criteria for evaluating residents on a larger scale. In a blinded, randomized study by Howells and colleagues,14 junior residents were either trained on a knee simulator or received no training before evaluation. Those who received the training scored significantly better than their peers on validated assessment measures.
The literature on different modalities shows simulation is an effective teaching tool for general orthopedic surgical skills5; knee, shoulder, and ankle arthroscopy14-21; spine surgery22; and orthopedic trauma surgery.23-26 Investigators in several other surgical specialties have studied the utility of simulation, and many are incorporating simulation into their resident curricula.
More effective simulation seems correlated with a yearlong structured curriculum rather than with intermittent, isolated experiences.3 Dunn and colleagues27 evaluated arthroscopic shoulder simulation 1 year after a training exercise. The group that received formal training did better than the control group on an initial arthroscopic surgery skill evaluation tool. At 1 year, however, the gains made through training were lost.
Simulation is a new paradigm for resident education. It offers multiple opportunities and challenges for residents, residency programs, industry partners, specialty and subspecialty societies, and medical examiners. The Accreditation Council for Graduate Medical Education’s ACGME Program Requirements for Graduate Medical Education in Orthopaedic Surgery requires of residency programs a didactic curriculum dedicated to basic motor skills in addition to a dedicated space for facilitating basic surgical skills training.28 Residency programs must demonstrate to ACGME their commitment to surgical skills training and simulation. Implementation of simulation for resident education has many variables, including funding, type of simulation, demonstrated efficacy, provision of supervision, resident time, and establishment of a formal curriculum. Residents and residency programs should embrace this changing paradigm to bridge the gap between observation and autonomy in orthopedic surgical and arthroscopic technique.
Am J Orthop. 2016;45(7):E426-E428. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. Atesok K, Mabrey JD, Jazrawi LM, Egol KA. Surgical simulation in orthopaedic skills training. J Am Acad Orthop Surg. 2012;20(7):410-422.
2. Thomas GW, Johns BD, Marsh JL, Anderson DD. A review of the role of simulation in developing and assessing orthopaedic surgical skills. Iowa Orthop J. 2014;34:181-189.
3. Reznick RK, MacRae H. Teaching surgical skills—changes in the wind. N Engl J Med. 2006;355(25):2664-2669.
4. Holland JP, Waugh L, Horgan A, Paleri V, Deehan DJ. Cadaveric hands-on training for surgical specialties: is this back to the future for surgical skills development? J Surg Educ. 2011;68(2):110-116.
5. Sonnadara RR, Van Vliet A, Safir O, et al. Orthopedic boot camp: examining the effectiveness of an intensive surgical skills course. Surgery. 2011;149(6):745-749.
6. Karam MD, Pedowitz RA, Natividad H, Murray J, Marsh JL. Current and future use of surgical skills training laboratories in orthopaedic resident education: a national survey. J Bone Joint Surg Am. 2013;95(1):e4.
7. Bushey C. Cadaver supply: the last industry to face big changes. Crain’s Chicago Business. February 23, 2013.
8. Human K. Cadaver shortage hits medical schools. Denver Post. April 29, 2008.
9. Michelson JD. Simulation in orthopaedic education: an overview of theory and practice. J Bone Joint Surg Am. 2006;88(6):1405-1411.
10. Elfar J, Menorca RM, Reed JD, Stanbury S. Composite bone models in orthopaedic surgery research and education. J Am Acad Orthop Surg. 2014;22(2):111-120.
11. Kneebone R. Evaluating clinical simulations for learning procedural skills: a theory-based approach. Acad Med. 2005;80(6):549-553.
12. Stirling ER, Lewis TL, Ferran NA. Surgical skills simulation in trauma and orthopaedic training. J Orthop Surg Res. 2014;9:126.
13. Mabrey JD, Reinig KD, Cannon WD. Virtual reality in orthopaedics: is it a reality? Clin Orthop Relat Res. 2010;468(10):2586-2591.
14. Howells NR, Gill HS, Carr AJ, Price AJ, Rees JL. Transferring simulated arthroscopic skills to the operating theatre: a randomised blinded study. J Bone Joint Surg Br. 2008;90(4):494-499.
15. Gomoll AH, O’Toole RV, Czarnecki J, Warner JJ. Surgical experience correlates with performance on a virtual reality simulator for shoulder arthroscopy. Am J Sports Med. 2007;35(6):883-888.
16. Gomoll AH, Pappas G, Forsythe B, Warner JJ. Individual skill progression on a virtual reality simulator for shoulder arthroscopy: a 3-year follow-up study. Am J Sports Med. 2008;36(6):1139-1142.
17. Pedowitz RA, Esch J, Snyder S. Evaluation of a virtual reality simulator for arthroscopy skills development. Arthroscopy. 2002;18(6):E29.
18. Martin KD, Belmont PJ, Schoenfeld AJ, Todd M, Cameron KL, Owens BD. Arthroscopic basic task performance in shoulder simulator model correlates with similar task performance in cadavers. J Bone Joint Surg Am. 2011;93(21):e1271-e1275.
19. Martin KD, Cameron K, Belmont PJ, Schoenfeld A, Owens BD. Shoulder arthroscopy simulator performance correlates with resident and shoulder arthroscopy experience. J Bone Joint Surg Am. 2012;94(21):e160.
20. Martin KD, Patterson D, Phisitkul P, Cameron KL, Femino J, Amendola A. Ankle arthroscopy simulation improves basic skills, anatomic recognition, and proficiency during diagnostic examination of residents in training. Foot Ankle Int. 2015;36(7):827-835.
21. Frank RM, Erickson B, Frank JM, et al. Utility of modern arthroscopic simulator training models. Arthroscopy. 2014;30(1):121-133.
22. Rambani R, Ward J, Viant W. Desktop-based computer-assisted orthopedic training system for spinal surgery. J Surg Educ. 2014;71(6):805-809.
23. Leong JJ, Leff DR, Das A, et al. Validation of orthopaedic bench models for trauma surgery. J Bone Joint Surg Br. 2008;90(7):958-965.
24. Rambani R, Viant W, Ward J, Mohsen A. Computer-assisted orthopedic training system for fracture fixation. J Surg Educ. 2013;70(3):304-308.
25. Blyth P, Stott NS, Anderson IA. A simulation-based training system for hip fracture fixation for use within the hospital environment. Injury. 2007;38(10):1197-1203.
26. Egol KA, Phillips D, Vongbandith T, Szyld D, Strauss EJ. Do orthopaedic fracture skills courses improve resident performance? Injury. 2015;46(4):547-551.
27. Dunn JC, Belmont PJ, Lanzi J, et al. Arthroscopic shoulder surgical simulation training curriculum: transfer reliability and maintenance of skill over time. J Surg Educ. 2015;72(6):1118-1123.
28. Accreditation Council for Graduate Medical Education. ACGME Program Requirements for Graduate Medical Education in Orthopaedic Surgery. https://www.acgme.org/Portals/0/PFAssets/ProgramRequirements/260_orthopaedic_surgery_2016.pdf. Published July 1, 2012. Accessed September 30, 2016.
1. Atesok K, Mabrey JD, Jazrawi LM, Egol KA. Surgical simulation in orthopaedic skills training. J Am Acad Orthop Surg. 2012;20(7):410-422.
2. Thomas GW, Johns BD, Marsh JL, Anderson DD. A review of the role of simulation in developing and assessing orthopaedic surgical skills. Iowa Orthop J. 2014;34:181-189.
3. Reznick RK, MacRae H. Teaching surgical skills—changes in the wind. N Engl J Med. 2006;355(25):2664-2669.
4. Holland JP, Waugh L, Horgan A, Paleri V, Deehan DJ. Cadaveric hands-on training for surgical specialties: is this back to the future for surgical skills development? J Surg Educ. 2011;68(2):110-116.
5. Sonnadara RR, Van Vliet A, Safir O, et al. Orthopedic boot camp: examining the effectiveness of an intensive surgical skills course. Surgery. 2011;149(6):745-749.
6. Karam MD, Pedowitz RA, Natividad H, Murray J, Marsh JL. Current and future use of surgical skills training laboratories in orthopaedic resident education: a national survey. J Bone Joint Surg Am. 2013;95(1):e4.
7. Bushey C. Cadaver supply: the last industry to face big changes. Crain’s Chicago Business. February 23, 2013.
8. Human K. Cadaver shortage hits medical schools. Denver Post. April 29, 2008.
9. Michelson JD. Simulation in orthopaedic education: an overview of theory and practice. J Bone Joint Surg Am. 2006;88(6):1405-1411.
10. Elfar J, Menorca RM, Reed JD, Stanbury S. Composite bone models in orthopaedic surgery research and education. J Am Acad Orthop Surg. 2014;22(2):111-120.
11. Kneebone R. Evaluating clinical simulations for learning procedural skills: a theory-based approach. Acad Med. 2005;80(6):549-553.
12. Stirling ER, Lewis TL, Ferran NA. Surgical skills simulation in trauma and orthopaedic training. J Orthop Surg Res. 2014;9:126.
13. Mabrey JD, Reinig KD, Cannon WD. Virtual reality in orthopaedics: is it a reality? Clin Orthop Relat Res. 2010;468(10):2586-2591.
14. Howells NR, Gill HS, Carr AJ, Price AJ, Rees JL. Transferring simulated arthroscopic skills to the operating theatre: a randomised blinded study. J Bone Joint Surg Br. 2008;90(4):494-499.
15. Gomoll AH, O’Toole RV, Czarnecki J, Warner JJ. Surgical experience correlates with performance on a virtual reality simulator for shoulder arthroscopy. Am J Sports Med. 2007;35(6):883-888.
16. Gomoll AH, Pappas G, Forsythe B, Warner JJ. Individual skill progression on a virtual reality simulator for shoulder arthroscopy: a 3-year follow-up study. Am J Sports Med. 2008;36(6):1139-1142.
17. Pedowitz RA, Esch J, Snyder S. Evaluation of a virtual reality simulator for arthroscopy skills development. Arthroscopy. 2002;18(6):E29.
18. Martin KD, Belmont PJ, Schoenfeld AJ, Todd M, Cameron KL, Owens BD. Arthroscopic basic task performance in shoulder simulator model correlates with similar task performance in cadavers. J Bone Joint Surg Am. 2011;93(21):e1271-e1275.
19. Martin KD, Cameron K, Belmont PJ, Schoenfeld A, Owens BD. Shoulder arthroscopy simulator performance correlates with resident and shoulder arthroscopy experience. J Bone Joint Surg Am. 2012;94(21):e160.
20. Martin KD, Patterson D, Phisitkul P, Cameron KL, Femino J, Amendola A. Ankle arthroscopy simulation improves basic skills, anatomic recognition, and proficiency during diagnostic examination of residents in training. Foot Ankle Int. 2015;36(7):827-835.
21. Frank RM, Erickson B, Frank JM, et al. Utility of modern arthroscopic simulator training models. Arthroscopy. 2014;30(1):121-133.
22. Rambani R, Ward J, Viant W. Desktop-based computer-assisted orthopedic training system for spinal surgery. J Surg Educ. 2014;71(6):805-809.
23. Leong JJ, Leff DR, Das A, et al. Validation of orthopaedic bench models for trauma surgery. J Bone Joint Surg Br. 2008;90(7):958-965.
24. Rambani R, Viant W, Ward J, Mohsen A. Computer-assisted orthopedic training system for fracture fixation. J Surg Educ. 2013;70(3):304-308.
25. Blyth P, Stott NS, Anderson IA. A simulation-based training system for hip fracture fixation for use within the hospital environment. Injury. 2007;38(10):1197-1203.
26. Egol KA, Phillips D, Vongbandith T, Szyld D, Strauss EJ. Do orthopaedic fracture skills courses improve resident performance? Injury. 2015;46(4):547-551.
27. Dunn JC, Belmont PJ, Lanzi J, et al. Arthroscopic shoulder surgical simulation training curriculum: transfer reliability and maintenance of skill over time. J Surg Educ. 2015;72(6):1118-1123.
28. Accreditation Council for Graduate Medical Education. ACGME Program Requirements for Graduate Medical Education in Orthopaedic Surgery. https://www.acgme.org/Portals/0/PFAssets/ProgramRequirements/260_orthopaedic_surgery_2016.pdf. Published July 1, 2012. Accessed September 30, 2016.
Arthroscopic Transosseous and Transosseous-Equivalent Rotator Cuff Repair: An Analysis of Cost, Operative Time, and Clinical Outcomes
The rate of medical visits for rotator cuff pathology and the US incidence of arthroscopic rotator cuff repair (RCR) have increased over the past 10 years.1 The increased use of RCR has been justified with improved patient outcomes.2,3 Advances in surgical techniques and instrumentation have contributed to better outcomes for patients with rotator cuff pathology.3-5 Several studies have validated RCR with functional outcome measures, cost–benefit analysis, and health-related quality-of-life measurements.6-9
Healthcare reimbursement models are being changed to include capitated care, pay for performance, and penalties.10 Given the changing healthcare climate and the increasing incidence of RCR, it is becoming increasingly important for orthopedic surgeons to critically evaluate and modify their practice and procedures to decrease costs without compromising outcomes.11 RCR outcome studies have focused on comparing open/mini-open with arthroscopic techniques, and single-row with double-row techniques, among others.4,12-18 Furthermore, several studies on the cost-effectiveness of these surgical techniques have been conducted.19-21Arthroscopic anchorless (transosseous [TO]) RCR, which is increasingly popular,22 combines the minimal invasiveness of arthroscopic procedures with the biomechanical strength of open TO repair. In addition, this technique avoids the potential complications and costs associated with suture anchors, such as anchor pullout and greater tuberosity osteolysis.22,23 Several studies have documented the effectiveness of this technique.24-26 Biomechanical and clinical outcome data supporting arthroscopic TO-RCR have been published, but there are no reports of studies that have analyzed the cost savings associated with this technique.
In this study, we compared implant costs associated with arthroscopic TO-RCR and arthroscopic TO-equivalent (TOE) RCR. We also evaluated these techniques’ operative time and outcomes. Our hypothesis was that arthroscopic TO-RCR can be performed at lower cost and without increasing operative time or compromising outcomes.
Materials and Methods
Our Institutional Review Board approved this study. Between February 2013 and January 2014, participating surgeons performed 43 arthroscopic TO-RCRs that met the study’s inclusion criteria. Twenty-one of the 43 patients enrolled and became the study group. The control group of 21 patients, who underwent arthroscopic TOE-RCR the preceding year (between January 2012 and January 2013), was matched to the study group on tear size and concomitant procedures, including biceps treatment, labral treatment, acromioplasty, and distal clavicle excision (Table 1).
The primary outcome measure was implant cost (amount paid by institution). Cost was determined and reported by an independent third party using Cerner Surginet as the operating room documentation system and McKessen Pathways Materials Management System for item pricing.
All arthroscopic RCRs were performed by 1 of 3 orthopedic surgeons fellowship-trained in either sports medicine or shoulder and elbow surgery. Using the Cofield classification,27 the treating surgeon recorded the size of the rotator cuff tear: small (<1 cm), medium (1-3 cm), large (3-5 cm), massive (>5 cm). The surgeon also recorded the number of suture anchors used, repair technique, biceps treatment, execution of subacromial decompression, execution of distal clavicle excision, and intraoperative complications. TO repair surgical technique is described in the next section. TOE repair was double-row repair with suture anchors. The number of suture anchors varied by tear size: small (3 anchors), medium (2-5 anchors), large (4-6 anchors), massive (4-5 anchors).
Secondary outcome measures were operative time (time from cut to close) and scores on pain VAS (visual analog scale), SANE (Single Assessment Numeric Evaluation), and SST (Simple Shoulder Test). Demographic information was also obtained: age, sex, body mass index, smoking status (Table 1). All patients were asked to fill out questionnaires before surgery and 3, 6, and >12 months after surgery. Outcome surveys were scored by a single research coordinator, who recorded each patient’s outcome scores at the preoperative and postoperative intervals. Follow-up of >12 months was reached by 17 (81%) of the 21 TO patients and 14 (67%) of the 21 TOE patients. For >12 months, the overall rate of follow-up was 74%.
All patients followed the same postoperative rehabilitation protocol: sling immobilization with pendulums for 6 weeks starting at 2 weeks, passive range of motion starting at 6 weeks, and active range of motion starting at 8 weeks. At 3 months, they were allowed progressive resistant exercises with a 10-pound limit, and at 4.5 months they progressed to a 20-pound limit. At 6 months, they were cleared for discharge.
Surgical Technique: Arthroscopic Transosseous Repair
Surgery was performed with the patient in either the beach-chair position or the lateral decubitus position, based on surgeon preference. Our technique is similar to what has been described in the past.22,28 The glenohumeral joint is accessed through a standard posterior portal, followed by an anterior accessory portal through the rotator interval. Standard diagnostic arthroscopy is performed and intra-articular pathology addressed. Next, the scope is placed in the subacromial space through the posterior portal. A lateral subacromial portal is established and cannulated, and a bursectomy performed. The scope is then placed in a posterolateral portal for better visualization of the rotator cuff tear. The greater tuberosity is débrided with a curette to prepare the bed for repair. An ArthroTunneler (Tornier) is used to pass sutures through the greater tuberosity. For standard 2-tunnel repair, 3 sutures are placed through each tunnel. All 6 sutures are next passed (using a suture passer) through the rotator cuff. The second and fifth suture ends that are passed through the cuff are brought out through the cannula and tied together. They are then brought into the shoulder by pulling on the opposite ends and tied alongside the greater tuberosity to create a box stitch. The box stitch acts as a medial row fixation and as a rip stitch that strengthens the vertical mattress sutures against pullout. The other 4 sutures are tied in vertical mattress configuration.
Statistical Analysis
After obtaining the TO and TOE implant costs, we compared them using a generalized linear model with negative binomial distribution and an identity link function so returned parameters were in additive dollars. This comparison included evaluation of tear size and concomitant procedures. Operative times for TO and TOE were obtained and evaluated, and then compared using time-to-event analysis and the log-rank test. Outcome scores were obtained from patients at baseline and 3, 6, and >12 months after surgery and were compared using a linear mixed model that identified change in outcome scores over time, and difference in outcome scores between the TO and TOE groups.
Results
Table 1 lists patient demographics, including age, sex, body mass index, smoking status, and concomitant procedures. The TO and TOE groups had identical tear-size distributions. In addition, they had similar numbers of concomitant procedures, though our study was underpowered to confirm equivalence. Treatment techniques differed: more biceps tenodesis cases in the TO group (n = 12) than in the TOE group (n = 2) and more biceps tenotomy cases in the TOE group (n = 8) than in the TO group (n = 1).
TO implant cost was significantly lower than TOE implant cost for all tear sizes and independent of concomitant procedures (Figure 1).
Operative time was not significantly different between the TO and TOE groups. Mean (SD) operative time was 82.38 (24.09) minutes for the TO group and 81.71 (17.27) minutes for the TOE group. With all other factors controlled, mean operative time was 5.96 minutes shorter for the TOE group, but the difference was not significant (P = .549).
There was no significant difference in preoperative pain VAS (P = .93), SANE (P = .35), or SST (P = .36) scores between the TO and TOE groups.
Discussion
RCR is one of the most common orthopedic surgical procedures, and its use has increased over the past decade.9,21 This increase coincides with the emergence of new repair techniques and implants. These advancements come at a cost. Given the increasingly cost-conscious healthcare environment and its changing reimbursement models, now surgeons must evaluate the economics of their surgical procedures in an attempt to decrease costs without compromising outcomes. We hypothesized that arthroscopic TO-RCR can be performed at lower cost relative to arthroscopic TOE-RCR and without increasing operative time or compromising short-term outcomes.
Studies on the cost-effectiveness of different RCR techniques have been conducted.19-21 Adla and colleagues19 found that open RCR was more cost-effective than arthroscopic RCR, with most of the difference attributable to disposables and suture anchors. Genuario and colleagues21 found that double-row RCR was not as cost-effective as single-row RCR in treating tears of any size. They attributed the difference to 2 more anchors and about 15 more minutes in the operating room.
The increased interest in healthcare costs and the understanding that a substantial part of the cost of arthroscopic RCR is attributable to implants (suture anchors, specifically) led to recent efforts to eliminate the need for anchors. Newly available instrumentation was designed to assist in arthroscopic anchorless repair constructs using the concepts of traditional TO repair.22 Although still considered to be the RCR gold standard, TO fixation has been used less often in recent years, owing to the shift from open to arthroscopic surgery.24 Arthroscopic TO-RCR allows for all the benefits of arthroscopic surgery, plus the biological and mechanical benefits of traditional open or mini-open TO repair. In addition, this technique eliminates the cost of anchors. Kummer and colleagues25 confirmed with biomechanical testing that arthroscopic TO repair and double-row TOE repair are similar in strength, with a trend of less tendon displacement in the TO group.
Our study results support the hypothesis that arthroscopic TO repair provides significant cost savings over tear size–matched arthroscopic TOE repair. Implant cost was substantially higher for TOE repair than for TO repair. Mean (SD) total savings of $946.91 ($100.70) (P < .0001) can be realized performing TO rather than TOE repair. In the United States, where about 250,000 RCRs are performed each year, the use of TO repair would result in an annual savings of almost $250 million.6Operative time was analyzed as well. Running an operating room in the United States costs an estimated $62 per minute (range, $22-$133 per minute).29 Much of this cost is indirect, unrelated to the surgery (eg, capital investment, personnel, insurance), and is being paid even when the operating room is not in use. Therefore, for the hospital’s bottom line, operative time savings are less important than direct cost savings (supplies, implants). However, operative time has more of an effect on the surgeon’s bottom line, and longer procedures reduce the number of surgeries that can be performed and billed. We found no significant difference in operative time between TO and TOE repairs. Critical evaluation revealed that operative time was 5.96 minutes shorter for TOE repairs, but this difference was not significant (P = .677).
Our study results showed no significant difference in clinical outcomes between TO and TOE repair patients. Both groups’ outcome scores improved. At all follow-ups, both groups’ VAS, SANE, and SST scores were significantly improved. Overall, this is the first study to validate the proposed cost benefit of arthroscopic TO repair and confirm no compromise in patient outcomes.
This study had limitations. First, it enrolled relatively few patients, particularly those with small tears. In addition, despite the fact that patients were matched on tear size and concomitant procedures, the groups differed in their biceps pathology treatments. Of the 13 TO patients who had biceps treatment, 12 underwent tenodesis (1 had tenotomy); in contrast, of the 10 TOE patients who had biceps treatment, only 2 underwent tenodesis (8 had tenotomy). The difference is explained by the consecutive course of this study and the increasing popularity of tenodesis over tenotomy. The TOE group underwent surgery before the TO group did, at a time when the involved surgeons were routinely performing tenotomy more than tenodesis. We did not include the costs of implants related to biceps treatment in our analysis, as our focus was on the implant cost of RCR. As for operative time, biceps tenodesis would be expected to extend surgery and potentially affect the comparison of operative times between the TO and TOE groups. However, despite the fact that 12 of the 13 TO patients underwent biceps tenodesis, there was no significant difference in overall operative time. Last, regarding the effect of biceps treatment on clinical outcomes, there are no data showing improved outcomes with tenodesis over tenotomy in the setting of RCR.
A final limitation is lack of data from longer term (>12 months) follow-up for all patients. Our analysis included cost and operative time data for all 42 enrolled patients, but our clinical outcome data represent only 74% of the patients enrolled. Eleven of the 42 patients were lost to follow-up at >12 months, and outcome scores could not be obtained, despite multiple attempts at contact (phone, mail, email). The study design and primary outcome variable focused on cost analysis rather than clinical outcomes. Nevertheless, our data support our hypothesis that there is no difference in clinical outcomes between TO and TOE repairs.
Conclusion
Arthroscopic TO-RCR provides significant cost savings over arthroscopic TOE-RCR without increasing operative time or compromising outcomes. Arthroscopic TO-RCR may have an important role in the evolving healthcare environment and its changing reimbursement models.
Am J Orthop. 2016;45(7):E415-E420. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. Colvin AC, Egorova N, Harrison AK, Moskowitz A, Flatow EL. National trends in rotator cuff repair. J Bone Joint Surg Am. 2012;94(3):227-233.
2. Pedowitz RA, Yamaguchi K, Ahmad CS, et al. American Academy of Orthopaedic Surgeons Clinical Practice Guideline on: optimizing the management of rotator cuff problems. J Bone Joint Surg Am. 2012;94(2):163-167.
3. Wolf BR, Dunn WR, Wright RW. Indications for repair of full-thickness rotator cuff tears. Am J Sports Med. 2007;35(6):1007-1016.
4. Yamaguchi K, Ball CM, Galatz LM. Arthroscopic rotator cuff repair: transition from mini-open to all-arthroscopic. Clin Orthop Relat Res. 2001;(390):83-94.
5. Yamaguchi K, Levine WN, Marra G, Galatz LM, Klepps S, Flatow EL. Transitioning to arthroscopic rotator cuff repair: the pros and cons. Instr Course Lect. 2003;52:81-92.
6. Mather RC 3rd, Koenig L, Acevedo D, et al. The societal and economic value of rotator cuff repair. J Bone Joint Surg Am. 2013;95(22):1993-2000.
7. Milne JC, Gartsman GM. Cost of shoulder surgery. J Shoulder Elbow Surg. 1994;3(5):295-298.
8. Savoie FH 3rd, Field LD, Jenkins RN. Costs analysis of successful rotator cuff repair surgery: an outcome study. Comparison of gatekeeper system in surgical patients. Arthroscopy. 1995;11(6):672-676.
9. Vitale MA, Vitale MG, Zivin JG, Braman JP, Bigliani LU, Flatow EL. Rotator cuff repair: an analysis of utility scores and cost-effectiveness. J Shoulder Elbow Surg. 2007;16(2):181-187.
10. Ihejirika RC, Sathiyakumar V, Thakore RV, et al. Healthcare reimbursement models and orthopaedic trauma: will there be change in patient management? A survey of orthopaedic surgeons. J Orthop Trauma. 2015;29(2):e79-e84.
11. Black EM, Higgins LD, Warner JJ. Value-based shoulder surgery: practicing outcomes-driven, cost-conscious care. J Shoulder Elbow Surg. 2013;22(7):1000-1009.
12. Barber FA, Hapa O, Bynum JA. Comparative testing by cyclic loading of rotator cuff suture anchors containing multiple high-strength sutures. Arthroscopy. 2010;26(9 suppl):S134-S141.
13. Barros RM, Matos MA, Ferreira Neto AA, et al. Biomechanical evaluation on tendon reinsertion by comparing trans-osseous suture and suture anchor at different stages of healing: experimental study on rabbits. J Shoulder Elbow Surg. 2010;19(6):878-883.
14. Cole BJ, ElAttrache NS, Anbari A. Arthroscopic rotator cuff repairs: an anatomic and biomechanical rationale for different suture-anchor repair configurations. Arthroscopy. 2007;23(6):662-669.
15. Ghodadra NS, Provencher MT, Verma NN, Wilk KE, Romeo AA. Open, mini-open, and all-arthroscopic rotator cuff repair surgery: indications and implications for rehabilitation. J Orthop Sports Phys Ther. 2009;39(2):81-89.
16. Pietschmann MF, Fröhlich V, Ficklscherer A, et al. Pullout strength of suture anchors in comparison with transosseous sutures for rotator cuff repair. Knee Surg Sports Traumatol Arthrosc. 2008;16(5):504-510.
17. van der Zwaal P, Thomassen BJ, Nieuwenhuijse MJ, Lindenburg R, Swen JW, van Arkel ER. Clinical outcome in all-arthroscopic versus mini-open rotator cuff repair in small to medium-sized tears: a randomized controlled trial in 100 patients with 1-year follow-up. Arthroscopy. 2013;29(2):266-273.
18. Wang VM, Wang FC, McNickle AG, et al. Medial versus lateral supraspinatus tendon properties: implications for double-row rotator cuff repair. Am J Sports Med. 2010;38(12):2456-2463.
19. Adla DN, Rowsell M, Pandey R. Cost-effectiveness of open versus arthroscopic rotator cuff repair. J Shoulder Elbow Surg. 2010;19(2):258-261.
20. Churchill RS, Ghorai JK. Total cost and operating room time comparison of rotator cuff repair techniques at low, intermediate, and high volume centers: mini-open versus all-arthroscopic. J Shoulder Elbow Surg. 2010;19(5):716-721.
21. Genuario JW, Donegan RP, Hamman D, et al. The cost-effectiveness of single-row compared with double-row arthroscopic rotator cuff repair. J Bone Joint Surg Am. 2012;94(15):1369-1377.
22. Garofalo R, Castagna A, Borroni M, Krishnan SG. Arthroscopic transosseous (anchorless) rotator cuff repair. Knee Surg Sports Traumatol Arthrosc. 2012;20(6):1031-1035.
23. Benson EC, MacDermid JC, Drosdowech DS, Athwal GS. The incidence of early metallic suture anchor pullout after arthroscopic rotator cuff repair. Arthroscopy. 2010;26(3):310-315.
24. Baudi P, Rasia Dani E, Campochiaro G, Rebuzzi M, Serafini F, Catani F. The rotator cuff tear repair with a new arthroscopic transosseous system: the Sharc-FT®. Musculoskelet Surg. 2013;97(suppl 1):57-61.
25. Kummer FJ, Hahn M, Day M, Meislin RJ, Jazrawi LM. A laboratory comparison of a new arthroscopic transosseous rotator cuff repair to a double row transosseous equivalent rotator cuff repair using suture anchors. Bull Hosp Joint Dis. 2013;71(2):128-131.
26. Kuroda S, Ishige N, Mikasa M. Advantages of arthroscopic transosseous suture repair of the rotator cuff without the use of anchors. Clin Orthop Relat Res. 2013;471(11):3514-3522.
27. Cofield RH. Subscapular muscle transposition for repair of chronic rotator cuff tears. Surg Gynecol Obstet. 1982;154(5):667-672.
28. Paxton ES, Lazarus MD. Arthroscopic transosseous rotator cuff repair. Orthop Knowledge Online J. 2014;12(2). http://orthoportal.aaos.org/oko/article.aspx?article=OKO_SHO052#article. Accessed October 4, 2016.
29. Macario A. What does one minute of operating room time cost? J Clin Anesth. 2010;22(4):233-236.
The rate of medical visits for rotator cuff pathology and the US incidence of arthroscopic rotator cuff repair (RCR) have increased over the past 10 years.1 The increased use of RCR has been justified with improved patient outcomes.2,3 Advances in surgical techniques and instrumentation have contributed to better outcomes for patients with rotator cuff pathology.3-5 Several studies have validated RCR with functional outcome measures, cost–benefit analysis, and health-related quality-of-life measurements.6-9
Healthcare reimbursement models are being changed to include capitated care, pay for performance, and penalties.10 Given the changing healthcare climate and the increasing incidence of RCR, it is becoming increasingly important for orthopedic surgeons to critically evaluate and modify their practice and procedures to decrease costs without compromising outcomes.11 RCR outcome studies have focused on comparing open/mini-open with arthroscopic techniques, and single-row with double-row techniques, among others.4,12-18 Furthermore, several studies on the cost-effectiveness of these surgical techniques have been conducted.19-21Arthroscopic anchorless (transosseous [TO]) RCR, which is increasingly popular,22 combines the minimal invasiveness of arthroscopic procedures with the biomechanical strength of open TO repair. In addition, this technique avoids the potential complications and costs associated with suture anchors, such as anchor pullout and greater tuberosity osteolysis.22,23 Several studies have documented the effectiveness of this technique.24-26 Biomechanical and clinical outcome data supporting arthroscopic TO-RCR have been published, but there are no reports of studies that have analyzed the cost savings associated with this technique.
In this study, we compared implant costs associated with arthroscopic TO-RCR and arthroscopic TO-equivalent (TOE) RCR. We also evaluated these techniques’ operative time and outcomes. Our hypothesis was that arthroscopic TO-RCR can be performed at lower cost and without increasing operative time or compromising outcomes.
Materials and Methods
Our Institutional Review Board approved this study. Between February 2013 and January 2014, participating surgeons performed 43 arthroscopic TO-RCRs that met the study’s inclusion criteria. Twenty-one of the 43 patients enrolled and became the study group. The control group of 21 patients, who underwent arthroscopic TOE-RCR the preceding year (between January 2012 and January 2013), was matched to the study group on tear size and concomitant procedures, including biceps treatment, labral treatment, acromioplasty, and distal clavicle excision (Table 1).
The primary outcome measure was implant cost (amount paid by institution). Cost was determined and reported by an independent third party using Cerner Surginet as the operating room documentation system and McKessen Pathways Materials Management System for item pricing.
All arthroscopic RCRs were performed by 1 of 3 orthopedic surgeons fellowship-trained in either sports medicine or shoulder and elbow surgery. Using the Cofield classification,27 the treating surgeon recorded the size of the rotator cuff tear: small (<1 cm), medium (1-3 cm), large (3-5 cm), massive (>5 cm). The surgeon also recorded the number of suture anchors used, repair technique, biceps treatment, execution of subacromial decompression, execution of distal clavicle excision, and intraoperative complications. TO repair surgical technique is described in the next section. TOE repair was double-row repair with suture anchors. The number of suture anchors varied by tear size: small (3 anchors), medium (2-5 anchors), large (4-6 anchors), massive (4-5 anchors).
Secondary outcome measures were operative time (time from cut to close) and scores on pain VAS (visual analog scale), SANE (Single Assessment Numeric Evaluation), and SST (Simple Shoulder Test). Demographic information was also obtained: age, sex, body mass index, smoking status (Table 1). All patients were asked to fill out questionnaires before surgery and 3, 6, and >12 months after surgery. Outcome surveys were scored by a single research coordinator, who recorded each patient’s outcome scores at the preoperative and postoperative intervals. Follow-up of >12 months was reached by 17 (81%) of the 21 TO patients and 14 (67%) of the 21 TOE patients. For >12 months, the overall rate of follow-up was 74%.
All patients followed the same postoperative rehabilitation protocol: sling immobilization with pendulums for 6 weeks starting at 2 weeks, passive range of motion starting at 6 weeks, and active range of motion starting at 8 weeks. At 3 months, they were allowed progressive resistant exercises with a 10-pound limit, and at 4.5 months they progressed to a 20-pound limit. At 6 months, they were cleared for discharge.
Surgical Technique: Arthroscopic Transosseous Repair
Surgery was performed with the patient in either the beach-chair position or the lateral decubitus position, based on surgeon preference. Our technique is similar to what has been described in the past.22,28 The glenohumeral joint is accessed through a standard posterior portal, followed by an anterior accessory portal through the rotator interval. Standard diagnostic arthroscopy is performed and intra-articular pathology addressed. Next, the scope is placed in the subacromial space through the posterior portal. A lateral subacromial portal is established and cannulated, and a bursectomy performed. The scope is then placed in a posterolateral portal for better visualization of the rotator cuff tear. The greater tuberosity is débrided with a curette to prepare the bed for repair. An ArthroTunneler (Tornier) is used to pass sutures through the greater tuberosity. For standard 2-tunnel repair, 3 sutures are placed through each tunnel. All 6 sutures are next passed (using a suture passer) through the rotator cuff. The second and fifth suture ends that are passed through the cuff are brought out through the cannula and tied together. They are then brought into the shoulder by pulling on the opposite ends and tied alongside the greater tuberosity to create a box stitch. The box stitch acts as a medial row fixation and as a rip stitch that strengthens the vertical mattress sutures against pullout. The other 4 sutures are tied in vertical mattress configuration.
Statistical Analysis
After obtaining the TO and TOE implant costs, we compared them using a generalized linear model with negative binomial distribution and an identity link function so returned parameters were in additive dollars. This comparison included evaluation of tear size and concomitant procedures. Operative times for TO and TOE were obtained and evaluated, and then compared using time-to-event analysis and the log-rank test. Outcome scores were obtained from patients at baseline and 3, 6, and >12 months after surgery and were compared using a linear mixed model that identified change in outcome scores over time, and difference in outcome scores between the TO and TOE groups.
Results
Table 1 lists patient demographics, including age, sex, body mass index, smoking status, and concomitant procedures. The TO and TOE groups had identical tear-size distributions. In addition, they had similar numbers of concomitant procedures, though our study was underpowered to confirm equivalence. Treatment techniques differed: more biceps tenodesis cases in the TO group (n = 12) than in the TOE group (n = 2) and more biceps tenotomy cases in the TOE group (n = 8) than in the TO group (n = 1).
TO implant cost was significantly lower than TOE implant cost for all tear sizes and independent of concomitant procedures (Figure 1).
Operative time was not significantly different between the TO and TOE groups. Mean (SD) operative time was 82.38 (24.09) minutes for the TO group and 81.71 (17.27) minutes for the TOE group. With all other factors controlled, mean operative time was 5.96 minutes shorter for the TOE group, but the difference was not significant (P = .549).
There was no significant difference in preoperative pain VAS (P = .93), SANE (P = .35), or SST (P = .36) scores between the TO and TOE groups.
Discussion
RCR is one of the most common orthopedic surgical procedures, and its use has increased over the past decade.9,21 This increase coincides with the emergence of new repair techniques and implants. These advancements come at a cost. Given the increasingly cost-conscious healthcare environment and its changing reimbursement models, now surgeons must evaluate the economics of their surgical procedures in an attempt to decrease costs without compromising outcomes. We hypothesized that arthroscopic TO-RCR can be performed at lower cost relative to arthroscopic TOE-RCR and without increasing operative time or compromising short-term outcomes.
Studies on the cost-effectiveness of different RCR techniques have been conducted.19-21 Adla and colleagues19 found that open RCR was more cost-effective than arthroscopic RCR, with most of the difference attributable to disposables and suture anchors. Genuario and colleagues21 found that double-row RCR was not as cost-effective as single-row RCR in treating tears of any size. They attributed the difference to 2 more anchors and about 15 more minutes in the operating room.
The increased interest in healthcare costs and the understanding that a substantial part of the cost of arthroscopic RCR is attributable to implants (suture anchors, specifically) led to recent efforts to eliminate the need for anchors. Newly available instrumentation was designed to assist in arthroscopic anchorless repair constructs using the concepts of traditional TO repair.22 Although still considered to be the RCR gold standard, TO fixation has been used less often in recent years, owing to the shift from open to arthroscopic surgery.24 Arthroscopic TO-RCR allows for all the benefits of arthroscopic surgery, plus the biological and mechanical benefits of traditional open or mini-open TO repair. In addition, this technique eliminates the cost of anchors. Kummer and colleagues25 confirmed with biomechanical testing that arthroscopic TO repair and double-row TOE repair are similar in strength, with a trend of less tendon displacement in the TO group.
Our study results support the hypothesis that arthroscopic TO repair provides significant cost savings over tear size–matched arthroscopic TOE repair. Implant cost was substantially higher for TOE repair than for TO repair. Mean (SD) total savings of $946.91 ($100.70) (P < .0001) can be realized performing TO rather than TOE repair. In the United States, where about 250,000 RCRs are performed each year, the use of TO repair would result in an annual savings of almost $250 million.6Operative time was analyzed as well. Running an operating room in the United States costs an estimated $62 per minute (range, $22-$133 per minute).29 Much of this cost is indirect, unrelated to the surgery (eg, capital investment, personnel, insurance), and is being paid even when the operating room is not in use. Therefore, for the hospital’s bottom line, operative time savings are less important than direct cost savings (supplies, implants). However, operative time has more of an effect on the surgeon’s bottom line, and longer procedures reduce the number of surgeries that can be performed and billed. We found no significant difference in operative time between TO and TOE repairs. Critical evaluation revealed that operative time was 5.96 minutes shorter for TOE repairs, but this difference was not significant (P = .677).
Our study results showed no significant difference in clinical outcomes between TO and TOE repair patients. Both groups’ outcome scores improved. At all follow-ups, both groups’ VAS, SANE, and SST scores were significantly improved. Overall, this is the first study to validate the proposed cost benefit of arthroscopic TO repair and confirm no compromise in patient outcomes.
This study had limitations. First, it enrolled relatively few patients, particularly those with small tears. In addition, despite the fact that patients were matched on tear size and concomitant procedures, the groups differed in their biceps pathology treatments. Of the 13 TO patients who had biceps treatment, 12 underwent tenodesis (1 had tenotomy); in contrast, of the 10 TOE patients who had biceps treatment, only 2 underwent tenodesis (8 had tenotomy). The difference is explained by the consecutive course of this study and the increasing popularity of tenodesis over tenotomy. The TOE group underwent surgery before the TO group did, at a time when the involved surgeons were routinely performing tenotomy more than tenodesis. We did not include the costs of implants related to biceps treatment in our analysis, as our focus was on the implant cost of RCR. As for operative time, biceps tenodesis would be expected to extend surgery and potentially affect the comparison of operative times between the TO and TOE groups. However, despite the fact that 12 of the 13 TO patients underwent biceps tenodesis, there was no significant difference in overall operative time. Last, regarding the effect of biceps treatment on clinical outcomes, there are no data showing improved outcomes with tenodesis over tenotomy in the setting of RCR.
A final limitation is lack of data from longer term (>12 months) follow-up for all patients. Our analysis included cost and operative time data for all 42 enrolled patients, but our clinical outcome data represent only 74% of the patients enrolled. Eleven of the 42 patients were lost to follow-up at >12 months, and outcome scores could not be obtained, despite multiple attempts at contact (phone, mail, email). The study design and primary outcome variable focused on cost analysis rather than clinical outcomes. Nevertheless, our data support our hypothesis that there is no difference in clinical outcomes between TO and TOE repairs.
Conclusion
Arthroscopic TO-RCR provides significant cost savings over arthroscopic TOE-RCR without increasing operative time or compromising outcomes. Arthroscopic TO-RCR may have an important role in the evolving healthcare environment and its changing reimbursement models.
Am J Orthop. 2016;45(7):E415-E420. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
The rate of medical visits for rotator cuff pathology and the US incidence of arthroscopic rotator cuff repair (RCR) have increased over the past 10 years.1 The increased use of RCR has been justified with improved patient outcomes.2,3 Advances in surgical techniques and instrumentation have contributed to better outcomes for patients with rotator cuff pathology.3-5 Several studies have validated RCR with functional outcome measures, cost–benefit analysis, and health-related quality-of-life measurements.6-9
Healthcare reimbursement models are being changed to include capitated care, pay for performance, and penalties.10 Given the changing healthcare climate and the increasing incidence of RCR, it is becoming increasingly important for orthopedic surgeons to critically evaluate and modify their practice and procedures to decrease costs without compromising outcomes.11 RCR outcome studies have focused on comparing open/mini-open with arthroscopic techniques, and single-row with double-row techniques, among others.4,12-18 Furthermore, several studies on the cost-effectiveness of these surgical techniques have been conducted.19-21Arthroscopic anchorless (transosseous [TO]) RCR, which is increasingly popular,22 combines the minimal invasiveness of arthroscopic procedures with the biomechanical strength of open TO repair. In addition, this technique avoids the potential complications and costs associated with suture anchors, such as anchor pullout and greater tuberosity osteolysis.22,23 Several studies have documented the effectiveness of this technique.24-26 Biomechanical and clinical outcome data supporting arthroscopic TO-RCR have been published, but there are no reports of studies that have analyzed the cost savings associated with this technique.
In this study, we compared implant costs associated with arthroscopic TO-RCR and arthroscopic TO-equivalent (TOE) RCR. We also evaluated these techniques’ operative time and outcomes. Our hypothesis was that arthroscopic TO-RCR can be performed at lower cost and without increasing operative time or compromising outcomes.
Materials and Methods
Our Institutional Review Board approved this study. Between February 2013 and January 2014, participating surgeons performed 43 arthroscopic TO-RCRs that met the study’s inclusion criteria. Twenty-one of the 43 patients enrolled and became the study group. The control group of 21 patients, who underwent arthroscopic TOE-RCR the preceding year (between January 2012 and January 2013), was matched to the study group on tear size and concomitant procedures, including biceps treatment, labral treatment, acromioplasty, and distal clavicle excision (Table 1).
The primary outcome measure was implant cost (amount paid by institution). Cost was determined and reported by an independent third party using Cerner Surginet as the operating room documentation system and McKessen Pathways Materials Management System for item pricing.
All arthroscopic RCRs were performed by 1 of 3 orthopedic surgeons fellowship-trained in either sports medicine or shoulder and elbow surgery. Using the Cofield classification,27 the treating surgeon recorded the size of the rotator cuff tear: small (<1 cm), medium (1-3 cm), large (3-5 cm), massive (>5 cm). The surgeon also recorded the number of suture anchors used, repair technique, biceps treatment, execution of subacromial decompression, execution of distal clavicle excision, and intraoperative complications. TO repair surgical technique is described in the next section. TOE repair was double-row repair with suture anchors. The number of suture anchors varied by tear size: small (3 anchors), medium (2-5 anchors), large (4-6 anchors), massive (4-5 anchors).
Secondary outcome measures were operative time (time from cut to close) and scores on pain VAS (visual analog scale), SANE (Single Assessment Numeric Evaluation), and SST (Simple Shoulder Test). Demographic information was also obtained: age, sex, body mass index, smoking status (Table 1). All patients were asked to fill out questionnaires before surgery and 3, 6, and >12 months after surgery. Outcome surveys were scored by a single research coordinator, who recorded each patient’s outcome scores at the preoperative and postoperative intervals. Follow-up of >12 months was reached by 17 (81%) of the 21 TO patients and 14 (67%) of the 21 TOE patients. For >12 months, the overall rate of follow-up was 74%.
All patients followed the same postoperative rehabilitation protocol: sling immobilization with pendulums for 6 weeks starting at 2 weeks, passive range of motion starting at 6 weeks, and active range of motion starting at 8 weeks. At 3 months, they were allowed progressive resistant exercises with a 10-pound limit, and at 4.5 months they progressed to a 20-pound limit. At 6 months, they were cleared for discharge.
Surgical Technique: Arthroscopic Transosseous Repair
Surgery was performed with the patient in either the beach-chair position or the lateral decubitus position, based on surgeon preference. Our technique is similar to what has been described in the past.22,28 The glenohumeral joint is accessed through a standard posterior portal, followed by an anterior accessory portal through the rotator interval. Standard diagnostic arthroscopy is performed and intra-articular pathology addressed. Next, the scope is placed in the subacromial space through the posterior portal. A lateral subacromial portal is established and cannulated, and a bursectomy performed. The scope is then placed in a posterolateral portal for better visualization of the rotator cuff tear. The greater tuberosity is débrided with a curette to prepare the bed for repair. An ArthroTunneler (Tornier) is used to pass sutures through the greater tuberosity. For standard 2-tunnel repair, 3 sutures are placed through each tunnel. All 6 sutures are next passed (using a suture passer) through the rotator cuff. The second and fifth suture ends that are passed through the cuff are brought out through the cannula and tied together. They are then brought into the shoulder by pulling on the opposite ends and tied alongside the greater tuberosity to create a box stitch. The box stitch acts as a medial row fixation and as a rip stitch that strengthens the vertical mattress sutures against pullout. The other 4 sutures are tied in vertical mattress configuration.
Statistical Analysis
After obtaining the TO and TOE implant costs, we compared them using a generalized linear model with negative binomial distribution and an identity link function so returned parameters were in additive dollars. This comparison included evaluation of tear size and concomitant procedures. Operative times for TO and TOE were obtained and evaluated, and then compared using time-to-event analysis and the log-rank test. Outcome scores were obtained from patients at baseline and 3, 6, and >12 months after surgery and were compared using a linear mixed model that identified change in outcome scores over time, and difference in outcome scores between the TO and TOE groups.
Results
Table 1 lists patient demographics, including age, sex, body mass index, smoking status, and concomitant procedures. The TO and TOE groups had identical tear-size distributions. In addition, they had similar numbers of concomitant procedures, though our study was underpowered to confirm equivalence. Treatment techniques differed: more biceps tenodesis cases in the TO group (n = 12) than in the TOE group (n = 2) and more biceps tenotomy cases in the TOE group (n = 8) than in the TO group (n = 1).
TO implant cost was significantly lower than TOE implant cost for all tear sizes and independent of concomitant procedures (Figure 1).
Operative time was not significantly different between the TO and TOE groups. Mean (SD) operative time was 82.38 (24.09) minutes for the TO group and 81.71 (17.27) minutes for the TOE group. With all other factors controlled, mean operative time was 5.96 minutes shorter for the TOE group, but the difference was not significant (P = .549).
There was no significant difference in preoperative pain VAS (P = .93), SANE (P = .35), or SST (P = .36) scores between the TO and TOE groups.
Discussion
RCR is one of the most common orthopedic surgical procedures, and its use has increased over the past decade.9,21 This increase coincides with the emergence of new repair techniques and implants. These advancements come at a cost. Given the increasingly cost-conscious healthcare environment and its changing reimbursement models, now surgeons must evaluate the economics of their surgical procedures in an attempt to decrease costs without compromising outcomes. We hypothesized that arthroscopic TO-RCR can be performed at lower cost relative to arthroscopic TOE-RCR and without increasing operative time or compromising short-term outcomes.
Studies on the cost-effectiveness of different RCR techniques have been conducted.19-21 Adla and colleagues19 found that open RCR was more cost-effective than arthroscopic RCR, with most of the difference attributable to disposables and suture anchors. Genuario and colleagues21 found that double-row RCR was not as cost-effective as single-row RCR in treating tears of any size. They attributed the difference to 2 more anchors and about 15 more minutes in the operating room.
The increased interest in healthcare costs and the understanding that a substantial part of the cost of arthroscopic RCR is attributable to implants (suture anchors, specifically) led to recent efforts to eliminate the need for anchors. Newly available instrumentation was designed to assist in arthroscopic anchorless repair constructs using the concepts of traditional TO repair.22 Although still considered to be the RCR gold standard, TO fixation has been used less often in recent years, owing to the shift from open to arthroscopic surgery.24 Arthroscopic TO-RCR allows for all the benefits of arthroscopic surgery, plus the biological and mechanical benefits of traditional open or mini-open TO repair. In addition, this technique eliminates the cost of anchors. Kummer and colleagues25 confirmed with biomechanical testing that arthroscopic TO repair and double-row TOE repair are similar in strength, with a trend of less tendon displacement in the TO group.
Our study results support the hypothesis that arthroscopic TO repair provides significant cost savings over tear size–matched arthroscopic TOE repair. Implant cost was substantially higher for TOE repair than for TO repair. Mean (SD) total savings of $946.91 ($100.70) (P < .0001) can be realized performing TO rather than TOE repair. In the United States, where about 250,000 RCRs are performed each year, the use of TO repair would result in an annual savings of almost $250 million.6Operative time was analyzed as well. Running an operating room in the United States costs an estimated $62 per minute (range, $22-$133 per minute).29 Much of this cost is indirect, unrelated to the surgery (eg, capital investment, personnel, insurance), and is being paid even when the operating room is not in use. Therefore, for the hospital’s bottom line, operative time savings are less important than direct cost savings (supplies, implants). However, operative time has more of an effect on the surgeon’s bottom line, and longer procedures reduce the number of surgeries that can be performed and billed. We found no significant difference in operative time between TO and TOE repairs. Critical evaluation revealed that operative time was 5.96 minutes shorter for TOE repairs, but this difference was not significant (P = .677).
Our study results showed no significant difference in clinical outcomes between TO and TOE repair patients. Both groups’ outcome scores improved. At all follow-ups, both groups’ VAS, SANE, and SST scores were significantly improved. Overall, this is the first study to validate the proposed cost benefit of arthroscopic TO repair and confirm no compromise in patient outcomes.
This study had limitations. First, it enrolled relatively few patients, particularly those with small tears. In addition, despite the fact that patients were matched on tear size and concomitant procedures, the groups differed in their biceps pathology treatments. Of the 13 TO patients who had biceps treatment, 12 underwent tenodesis (1 had tenotomy); in contrast, of the 10 TOE patients who had biceps treatment, only 2 underwent tenodesis (8 had tenotomy). The difference is explained by the consecutive course of this study and the increasing popularity of tenodesis over tenotomy. The TOE group underwent surgery before the TO group did, at a time when the involved surgeons were routinely performing tenotomy more than tenodesis. We did not include the costs of implants related to biceps treatment in our analysis, as our focus was on the implant cost of RCR. As for operative time, biceps tenodesis would be expected to extend surgery and potentially affect the comparison of operative times between the TO and TOE groups. However, despite the fact that 12 of the 13 TO patients underwent biceps tenodesis, there was no significant difference in overall operative time. Last, regarding the effect of biceps treatment on clinical outcomes, there are no data showing improved outcomes with tenodesis over tenotomy in the setting of RCR.
A final limitation is lack of data from longer term (>12 months) follow-up for all patients. Our analysis included cost and operative time data for all 42 enrolled patients, but our clinical outcome data represent only 74% of the patients enrolled. Eleven of the 42 patients were lost to follow-up at >12 months, and outcome scores could not be obtained, despite multiple attempts at contact (phone, mail, email). The study design and primary outcome variable focused on cost analysis rather than clinical outcomes. Nevertheless, our data support our hypothesis that there is no difference in clinical outcomes between TO and TOE repairs.
Conclusion
Arthroscopic TO-RCR provides significant cost savings over arthroscopic TOE-RCR without increasing operative time or compromising outcomes. Arthroscopic TO-RCR may have an important role in the evolving healthcare environment and its changing reimbursement models.
Am J Orthop. 2016;45(7):E415-E420. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. Colvin AC, Egorova N, Harrison AK, Moskowitz A, Flatow EL. National trends in rotator cuff repair. J Bone Joint Surg Am. 2012;94(3):227-233.
2. Pedowitz RA, Yamaguchi K, Ahmad CS, et al. American Academy of Orthopaedic Surgeons Clinical Practice Guideline on: optimizing the management of rotator cuff problems. J Bone Joint Surg Am. 2012;94(2):163-167.
3. Wolf BR, Dunn WR, Wright RW. Indications for repair of full-thickness rotator cuff tears. Am J Sports Med. 2007;35(6):1007-1016.
4. Yamaguchi K, Ball CM, Galatz LM. Arthroscopic rotator cuff repair: transition from mini-open to all-arthroscopic. Clin Orthop Relat Res. 2001;(390):83-94.
5. Yamaguchi K, Levine WN, Marra G, Galatz LM, Klepps S, Flatow EL. Transitioning to arthroscopic rotator cuff repair: the pros and cons. Instr Course Lect. 2003;52:81-92.
6. Mather RC 3rd, Koenig L, Acevedo D, et al. The societal and economic value of rotator cuff repair. J Bone Joint Surg Am. 2013;95(22):1993-2000.
7. Milne JC, Gartsman GM. Cost of shoulder surgery. J Shoulder Elbow Surg. 1994;3(5):295-298.
8. Savoie FH 3rd, Field LD, Jenkins RN. Costs analysis of successful rotator cuff repair surgery: an outcome study. Comparison of gatekeeper system in surgical patients. Arthroscopy. 1995;11(6):672-676.
9. Vitale MA, Vitale MG, Zivin JG, Braman JP, Bigliani LU, Flatow EL. Rotator cuff repair: an analysis of utility scores and cost-effectiveness. J Shoulder Elbow Surg. 2007;16(2):181-187.
10. Ihejirika RC, Sathiyakumar V, Thakore RV, et al. Healthcare reimbursement models and orthopaedic trauma: will there be change in patient management? A survey of orthopaedic surgeons. J Orthop Trauma. 2015;29(2):e79-e84.
11. Black EM, Higgins LD, Warner JJ. Value-based shoulder surgery: practicing outcomes-driven, cost-conscious care. J Shoulder Elbow Surg. 2013;22(7):1000-1009.
12. Barber FA, Hapa O, Bynum JA. Comparative testing by cyclic loading of rotator cuff suture anchors containing multiple high-strength sutures. Arthroscopy. 2010;26(9 suppl):S134-S141.
13. Barros RM, Matos MA, Ferreira Neto AA, et al. Biomechanical evaluation on tendon reinsertion by comparing trans-osseous suture and suture anchor at different stages of healing: experimental study on rabbits. J Shoulder Elbow Surg. 2010;19(6):878-883.
14. Cole BJ, ElAttrache NS, Anbari A. Arthroscopic rotator cuff repairs: an anatomic and biomechanical rationale for different suture-anchor repair configurations. Arthroscopy. 2007;23(6):662-669.
15. Ghodadra NS, Provencher MT, Verma NN, Wilk KE, Romeo AA. Open, mini-open, and all-arthroscopic rotator cuff repair surgery: indications and implications for rehabilitation. J Orthop Sports Phys Ther. 2009;39(2):81-89.
16. Pietschmann MF, Fröhlich V, Ficklscherer A, et al. Pullout strength of suture anchors in comparison with transosseous sutures for rotator cuff repair. Knee Surg Sports Traumatol Arthrosc. 2008;16(5):504-510.
17. van der Zwaal P, Thomassen BJ, Nieuwenhuijse MJ, Lindenburg R, Swen JW, van Arkel ER. Clinical outcome in all-arthroscopic versus mini-open rotator cuff repair in small to medium-sized tears: a randomized controlled trial in 100 patients with 1-year follow-up. Arthroscopy. 2013;29(2):266-273.
18. Wang VM, Wang FC, McNickle AG, et al. Medial versus lateral supraspinatus tendon properties: implications for double-row rotator cuff repair. Am J Sports Med. 2010;38(12):2456-2463.
19. Adla DN, Rowsell M, Pandey R. Cost-effectiveness of open versus arthroscopic rotator cuff repair. J Shoulder Elbow Surg. 2010;19(2):258-261.
20. Churchill RS, Ghorai JK. Total cost and operating room time comparison of rotator cuff repair techniques at low, intermediate, and high volume centers: mini-open versus all-arthroscopic. J Shoulder Elbow Surg. 2010;19(5):716-721.
21. Genuario JW, Donegan RP, Hamman D, et al. The cost-effectiveness of single-row compared with double-row arthroscopic rotator cuff repair. J Bone Joint Surg Am. 2012;94(15):1369-1377.
22. Garofalo R, Castagna A, Borroni M, Krishnan SG. Arthroscopic transosseous (anchorless) rotator cuff repair. Knee Surg Sports Traumatol Arthrosc. 2012;20(6):1031-1035.
23. Benson EC, MacDermid JC, Drosdowech DS, Athwal GS. The incidence of early metallic suture anchor pullout after arthroscopic rotator cuff repair. Arthroscopy. 2010;26(3):310-315.
24. Baudi P, Rasia Dani E, Campochiaro G, Rebuzzi M, Serafini F, Catani F. The rotator cuff tear repair with a new arthroscopic transosseous system: the Sharc-FT®. Musculoskelet Surg. 2013;97(suppl 1):57-61.
25. Kummer FJ, Hahn M, Day M, Meislin RJ, Jazrawi LM. A laboratory comparison of a new arthroscopic transosseous rotator cuff repair to a double row transosseous equivalent rotator cuff repair using suture anchors. Bull Hosp Joint Dis. 2013;71(2):128-131.
26. Kuroda S, Ishige N, Mikasa M. Advantages of arthroscopic transosseous suture repair of the rotator cuff without the use of anchors. Clin Orthop Relat Res. 2013;471(11):3514-3522.
27. Cofield RH. Subscapular muscle transposition for repair of chronic rotator cuff tears. Surg Gynecol Obstet. 1982;154(5):667-672.
28. Paxton ES, Lazarus MD. Arthroscopic transosseous rotator cuff repair. Orthop Knowledge Online J. 2014;12(2). http://orthoportal.aaos.org/oko/article.aspx?article=OKO_SHO052#article. Accessed October 4, 2016.
29. Macario A. What does one minute of operating room time cost? J Clin Anesth. 2010;22(4):233-236.
1. Colvin AC, Egorova N, Harrison AK, Moskowitz A, Flatow EL. National trends in rotator cuff repair. J Bone Joint Surg Am. 2012;94(3):227-233.
2. Pedowitz RA, Yamaguchi K, Ahmad CS, et al. American Academy of Orthopaedic Surgeons Clinical Practice Guideline on: optimizing the management of rotator cuff problems. J Bone Joint Surg Am. 2012;94(2):163-167.
3. Wolf BR, Dunn WR, Wright RW. Indications for repair of full-thickness rotator cuff tears. Am J Sports Med. 2007;35(6):1007-1016.
4. Yamaguchi K, Ball CM, Galatz LM. Arthroscopic rotator cuff repair: transition from mini-open to all-arthroscopic. Clin Orthop Relat Res. 2001;(390):83-94.
5. Yamaguchi K, Levine WN, Marra G, Galatz LM, Klepps S, Flatow EL. Transitioning to arthroscopic rotator cuff repair: the pros and cons. Instr Course Lect. 2003;52:81-92.
6. Mather RC 3rd, Koenig L, Acevedo D, et al. The societal and economic value of rotator cuff repair. J Bone Joint Surg Am. 2013;95(22):1993-2000.
7. Milne JC, Gartsman GM. Cost of shoulder surgery. J Shoulder Elbow Surg. 1994;3(5):295-298.
8. Savoie FH 3rd, Field LD, Jenkins RN. Costs analysis of successful rotator cuff repair surgery: an outcome study. Comparison of gatekeeper system in surgical patients. Arthroscopy. 1995;11(6):672-676.
9. Vitale MA, Vitale MG, Zivin JG, Braman JP, Bigliani LU, Flatow EL. Rotator cuff repair: an analysis of utility scores and cost-effectiveness. J Shoulder Elbow Surg. 2007;16(2):181-187.
10. Ihejirika RC, Sathiyakumar V, Thakore RV, et al. Healthcare reimbursement models and orthopaedic trauma: will there be change in patient management? A survey of orthopaedic surgeons. J Orthop Trauma. 2015;29(2):e79-e84.
11. Black EM, Higgins LD, Warner JJ. Value-based shoulder surgery: practicing outcomes-driven, cost-conscious care. J Shoulder Elbow Surg. 2013;22(7):1000-1009.
12. Barber FA, Hapa O, Bynum JA. Comparative testing by cyclic loading of rotator cuff suture anchors containing multiple high-strength sutures. Arthroscopy. 2010;26(9 suppl):S134-S141.
13. Barros RM, Matos MA, Ferreira Neto AA, et al. Biomechanical evaluation on tendon reinsertion by comparing trans-osseous suture and suture anchor at different stages of healing: experimental study on rabbits. J Shoulder Elbow Surg. 2010;19(6):878-883.
14. Cole BJ, ElAttrache NS, Anbari A. Arthroscopic rotator cuff repairs: an anatomic and biomechanical rationale for different suture-anchor repair configurations. Arthroscopy. 2007;23(6):662-669.
15. Ghodadra NS, Provencher MT, Verma NN, Wilk KE, Romeo AA. Open, mini-open, and all-arthroscopic rotator cuff repair surgery: indications and implications for rehabilitation. J Orthop Sports Phys Ther. 2009;39(2):81-89.
16. Pietschmann MF, Fröhlich V, Ficklscherer A, et al. Pullout strength of suture anchors in comparison with transosseous sutures for rotator cuff repair. Knee Surg Sports Traumatol Arthrosc. 2008;16(5):504-510.
17. van der Zwaal P, Thomassen BJ, Nieuwenhuijse MJ, Lindenburg R, Swen JW, van Arkel ER. Clinical outcome in all-arthroscopic versus mini-open rotator cuff repair in small to medium-sized tears: a randomized controlled trial in 100 patients with 1-year follow-up. Arthroscopy. 2013;29(2):266-273.
18. Wang VM, Wang FC, McNickle AG, et al. Medial versus lateral supraspinatus tendon properties: implications for double-row rotator cuff repair. Am J Sports Med. 2010;38(12):2456-2463.
19. Adla DN, Rowsell M, Pandey R. Cost-effectiveness of open versus arthroscopic rotator cuff repair. J Shoulder Elbow Surg. 2010;19(2):258-261.
20. Churchill RS, Ghorai JK. Total cost and operating room time comparison of rotator cuff repair techniques at low, intermediate, and high volume centers: mini-open versus all-arthroscopic. J Shoulder Elbow Surg. 2010;19(5):716-721.
21. Genuario JW, Donegan RP, Hamman D, et al. The cost-effectiveness of single-row compared with double-row arthroscopic rotator cuff repair. J Bone Joint Surg Am. 2012;94(15):1369-1377.
22. Garofalo R, Castagna A, Borroni M, Krishnan SG. Arthroscopic transosseous (anchorless) rotator cuff repair. Knee Surg Sports Traumatol Arthrosc. 2012;20(6):1031-1035.
23. Benson EC, MacDermid JC, Drosdowech DS, Athwal GS. The incidence of early metallic suture anchor pullout after arthroscopic rotator cuff repair. Arthroscopy. 2010;26(3):310-315.
24. Baudi P, Rasia Dani E, Campochiaro G, Rebuzzi M, Serafini F, Catani F. The rotator cuff tear repair with a new arthroscopic transosseous system: the Sharc-FT®. Musculoskelet Surg. 2013;97(suppl 1):57-61.
25. Kummer FJ, Hahn M, Day M, Meislin RJ, Jazrawi LM. A laboratory comparison of a new arthroscopic transosseous rotator cuff repair to a double row transosseous equivalent rotator cuff repair using suture anchors. Bull Hosp Joint Dis. 2013;71(2):128-131.
26. Kuroda S, Ishige N, Mikasa M. Advantages of arthroscopic transosseous suture repair of the rotator cuff without the use of anchors. Clin Orthop Relat Res. 2013;471(11):3514-3522.
27. Cofield RH. Subscapular muscle transposition for repair of chronic rotator cuff tears. Surg Gynecol Obstet. 1982;154(5):667-672.
28. Paxton ES, Lazarus MD. Arthroscopic transosseous rotator cuff repair. Orthop Knowledge Online J. 2014;12(2). http://orthoportal.aaos.org/oko/article.aspx?article=OKO_SHO052#article. Accessed October 4, 2016.
29. Macario A. What does one minute of operating room time cost? J Clin Anesth. 2010;22(4):233-236.