User login
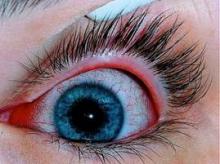
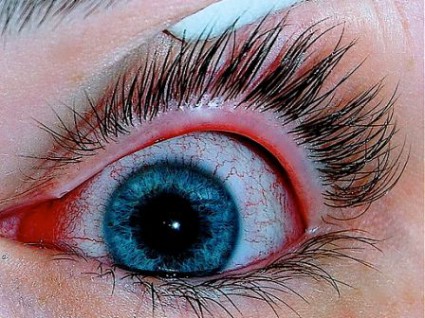
Isotretinoin Users Need Eye Drop Prescription
When prescribing isotretinoin for acne, ocular lubricants should also be prescribed, according to Dr. Meira Neudorfer and colleagues.
The researchers, who conducted a large, retrospective study of young adults who received prescriptions for isotretinoin for acne, also recommended that "a follow-up visit to the ophthalmologist should be scheduled about 4 months after the first dispensed isotretinoin prescription."
The recommendations come from their study findings which compared 14,682 adolescents and young adults who were new users of isotretinoin, with two age- and sex-matched control groups. The first comparison group of 14,682 patients had acne, but the patients were never treated with isotretinoin; and the second comparison group of 14,682 patients did not have acne and were never treated with isotretinoin (Arch. Dermatol. 2012;148:803-8).
Isotretinoin use was associated with an increased risk for developing ocular disorders – an attribution established by previous studies – which are mostly reversible once the therapy is discontinued. The elevated risk of adverse events is attributed to the biological effect of isotretinoin, inducing meibomian gland dysfunction, wrote Dr. Neudorfer of the department of ophthalmology at Tel Aviv Medical Center, and colleagues.
Data were collected from the electronic database of the health maintenance organization, Maccabi Healthcare Services, Tel Aviv, between January 1, 2000, and December 31, 2007. The recommended dose of isotretinoin in Israel is a cumulative dose of 120-150 mg/kg.
During the first year of treatment, 13.8% of the isotretinoin group experienced adverse ocular events, compared with 9.6% of the isotretinoin-naïve patients, and 7.1% of the acne-free patients. The most common adverse events were conjunctivitis, hordeolum, chalazion, blepharitis, eye pain, and dry eye, according to the study.
Inflammatory ocular diseases were diagnosed in 6.7% of the isotretinoin group, 3% of the isotretinoin naive group, and 2.4% of the acne-free group. Structural ocular diseases were observed in 1.0%, 0.5% and 0.4% of the three groups, respectively.
Acute conjunctivitis was the most frequent prognosis and occurred 1.7 times more frequently in the isotretinoin group than in the isotretinoin-naive group (4.0% vs. 2.4%, respectively). In the acne-free group, the occurrence was 1.9%.
The study also found an increased incidence of ocular adverse events among female patients, which was "in line with the known greater use of health care services by women," the researchers noted.
The authors listed several limitations to the study. For instance, patients with acne experienced more ocular disease than did the general population because of the disease itself, so "it is reasonable to assume that the ocular disturbance is directly associated with the degree of acne severity," they wrote. There was also a lack of data on contact lens use, something that could lead to complications and influence the association between use of isotretinoin and ocular disease.
"The study results underscore the importance of primary and secondary prevention measures," the authors concluded. "When patients taking isotretinoin are seen with ocular problems, ophthalmologists should ascertain the timing of the onset of symptoms and consider discontinuation of the drug if the symptoms progress or persist despite treatment."
The authors did not report having any conflicts of interest.
When prescribing isotretinoin for acne, ocular lubricants should also be prescribed, according to Dr. Meira Neudorfer and colleagues.
The researchers, who conducted a large, retrospective study of young adults who received prescriptions for isotretinoin for acne, also recommended that "a follow-up visit to the ophthalmologist should be scheduled about 4 months after the first dispensed isotretinoin prescription."
The recommendations come from their study findings which compared 14,682 adolescents and young adults who were new users of isotretinoin, with two age- and sex-matched control groups. The first comparison group of 14,682 patients had acne, but the patients were never treated with isotretinoin; and the second comparison group of 14,682 patients did not have acne and were never treated with isotretinoin (Arch. Dermatol. 2012;148:803-8).
Isotretinoin use was associated with an increased risk for developing ocular disorders – an attribution established by previous studies – which are mostly reversible once the therapy is discontinued. The elevated risk of adverse events is attributed to the biological effect of isotretinoin, inducing meibomian gland dysfunction, wrote Dr. Neudorfer of the department of ophthalmology at Tel Aviv Medical Center, and colleagues.
Data were collected from the electronic database of the health maintenance organization, Maccabi Healthcare Services, Tel Aviv, between January 1, 2000, and December 31, 2007. The recommended dose of isotretinoin in Israel is a cumulative dose of 120-150 mg/kg.
During the first year of treatment, 13.8% of the isotretinoin group experienced adverse ocular events, compared with 9.6% of the isotretinoin-naïve patients, and 7.1% of the acne-free patients. The most common adverse events were conjunctivitis, hordeolum, chalazion, blepharitis, eye pain, and dry eye, according to the study.
Inflammatory ocular diseases were diagnosed in 6.7% of the isotretinoin group, 3% of the isotretinoin naive group, and 2.4% of the acne-free group. Structural ocular diseases were observed in 1.0%, 0.5% and 0.4% of the three groups, respectively.
Acute conjunctivitis was the most frequent prognosis and occurred 1.7 times more frequently in the isotretinoin group than in the isotretinoin-naive group (4.0% vs. 2.4%, respectively). In the acne-free group, the occurrence was 1.9%.
The study also found an increased incidence of ocular adverse events among female patients, which was "in line with the known greater use of health care services by women," the researchers noted.
The authors listed several limitations to the study. For instance, patients with acne experienced more ocular disease than did the general population because of the disease itself, so "it is reasonable to assume that the ocular disturbance is directly associated with the degree of acne severity," they wrote. There was also a lack of data on contact lens use, something that could lead to complications and influence the association between use of isotretinoin and ocular disease.
"The study results underscore the importance of primary and secondary prevention measures," the authors concluded. "When patients taking isotretinoin are seen with ocular problems, ophthalmologists should ascertain the timing of the onset of symptoms and consider discontinuation of the drug if the symptoms progress or persist despite treatment."
The authors did not report having any conflicts of interest.
When prescribing isotretinoin for acne, ocular lubricants should also be prescribed, according to Dr. Meira Neudorfer and colleagues.
The researchers, who conducted a large, retrospective study of young adults who received prescriptions for isotretinoin for acne, also recommended that "a follow-up visit to the ophthalmologist should be scheduled about 4 months after the first dispensed isotretinoin prescription."
The recommendations come from their study findings which compared 14,682 adolescents and young adults who were new users of isotretinoin, with two age- and sex-matched control groups. The first comparison group of 14,682 patients had acne, but the patients were never treated with isotretinoin; and the second comparison group of 14,682 patients did not have acne and were never treated with isotretinoin (Arch. Dermatol. 2012;148:803-8).
Isotretinoin use was associated with an increased risk for developing ocular disorders – an attribution established by previous studies – which are mostly reversible once the therapy is discontinued. The elevated risk of adverse events is attributed to the biological effect of isotretinoin, inducing meibomian gland dysfunction, wrote Dr. Neudorfer of the department of ophthalmology at Tel Aviv Medical Center, and colleagues.
Data were collected from the electronic database of the health maintenance organization, Maccabi Healthcare Services, Tel Aviv, between January 1, 2000, and December 31, 2007. The recommended dose of isotretinoin in Israel is a cumulative dose of 120-150 mg/kg.
During the first year of treatment, 13.8% of the isotretinoin group experienced adverse ocular events, compared with 9.6% of the isotretinoin-naïve patients, and 7.1% of the acne-free patients. The most common adverse events were conjunctivitis, hordeolum, chalazion, blepharitis, eye pain, and dry eye, according to the study.
Inflammatory ocular diseases were diagnosed in 6.7% of the isotretinoin group, 3% of the isotretinoin naive group, and 2.4% of the acne-free group. Structural ocular diseases were observed in 1.0%, 0.5% and 0.4% of the three groups, respectively.
Acute conjunctivitis was the most frequent prognosis and occurred 1.7 times more frequently in the isotretinoin group than in the isotretinoin-naive group (4.0% vs. 2.4%, respectively). In the acne-free group, the occurrence was 1.9%.
The study also found an increased incidence of ocular adverse events among female patients, which was "in line with the known greater use of health care services by women," the researchers noted.
The authors listed several limitations to the study. For instance, patients with acne experienced more ocular disease than did the general population because of the disease itself, so "it is reasonable to assume that the ocular disturbance is directly associated with the degree of acne severity," they wrote. There was also a lack of data on contact lens use, something that could lead to complications and influence the association between use of isotretinoin and ocular disease.
"The study results underscore the importance of primary and secondary prevention measures," the authors concluded. "When patients taking isotretinoin are seen with ocular problems, ophthalmologists should ascertain the timing of the onset of symptoms and consider discontinuation of the drug if the symptoms progress or persist despite treatment."
The authors did not report having any conflicts of interest.
FROM ARCHIVES OF DERMATOLOGY
Major Finding: In total, 13.8% of the isotretinoin group had ocular adverse effects, compared with 9.6% of the isotretinoin-naive group and 7.1% of the acne-free group.
Data Source: The electronic database of Maccabi Healthcare Services, in Tel Aviv, was used to identify 14,682 adolescent and young adults who were new isotretinoin users, along with two sex- and age-matched comparison groups.
Disclosures: The authors did not report having any conflicts of interest.
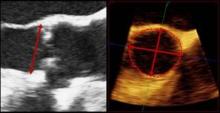
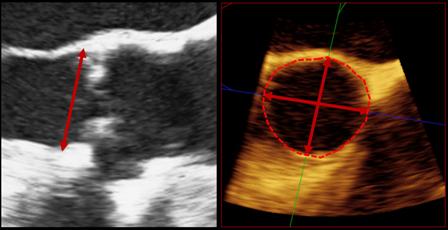
Aortic Regurgitation Index Can Improve TAVR Prognosis
A new hemodynamic measurement can provide prognostic information that goes beyond just the degree of aortic regurgitation after percutaneous aortic implantation, according to a single-center, retrospective, European study.
The study validates the team’s earlier findings in a slightly larger group of patients. Called aortic regurgitation index (AR index), the parameter is measured by subtracting the left ventricular end-diastolic pressure from the diastolic blood pressure and dividing the result by systolic blood pressure, and then multiplying it by 100.
The measurement can provide additional information beyond echocardiography when it comes to assessing the severity and outcome of periprosthetic aortic regurgitation (periAR), wrote Dr. Mariuca Vasa-Nicotera and colleagues. Even patients with nonexistent or mild periAR after transcatheter aortic valve implantation (TAVI) can be stratified based on their AR index score. It can also help predict 1-year mortality after TAVI.
The findings "are not going to change practice," commented Dr. William Zoghbi, chief of cardiovascular imaging at Methodist DeBakey Heart and Vascular Center in Houston, who was not involved in the study. Rather, the AR index "would help with predicting prognosis for those individuals who have valve implantation."
AR index is also more than just an index of valve regurgitation, said Dr. Zoghbi, who is also the current president of the American College of Cardiology. The measurement shows whether the heart has a significant abnormality in diastolic function and how it is handing the hemodynamic load of valvular regurgitation, he said.
Paravalvular leakage is a common observation following TAVI, and growing evidence has shown an association between the leakage and dramatic increase in morbidity and mortality.
The researchers analyzed data from 122 high-risk or inoperable patients who underwent TAVI during January 2007–March 2011 at Glenfield University Hospital in Leicester, United Kingdom.
The procedure was performed using third-generation 18-F Medtronic CoreValves or Edwards SAPIEN valves. Afterward, severity of periAR was assessed angiographically, echocardiographically by transesophageal echocardiography, and hemodynamically.
Patients were then divided into two groups on the basis of periAR: 102 had no or mild periAR (26 and 76 patients, respectively), and 20 had moderate or severe periAR (18 and 2 patients).
There were significant differences between the two patient groups.
Patients with moderate to severe periAR after TAVI had a significantly higher EuroSCORE (28.5, compared with 21.2 in those with no to mild periAR) and Society of Thoracic Surgeons mortality score (9.5 and 6.8, respectively). They also had a higher rate of myocardial infarction (40% v. 10%), and a lower left ventricular ejection fraction (45 v. 51).
Thirty-two patients (26%) died during the 1-year follow-up.
Among those remaining, 1-year mortality was 60% in patients with moderate to severe periAR, compared with 20% in patients with no or mild periAR.
The AR index declined in step with periAR severity, from 29.4 in patients with no periAR to 28.0 in those with mild periAR, to19.6 in patients with moderate periAR, and to 7.6 with severe periAR.
One-year mortality by AR index also paralleled that by periAR. That outcome was tripled in patients with an AR index of less than 25, compared with patients who had an AR index of at least 25 (42.3% vs. 14.3%), the authors reported.
AR index was also useful in patients with no or mild periAR, and provided complementary prognostic information, according to the authors.
The patients in that group who had an AR index of at least 25 had a 1-year mortality rate of 14.3%. But that rate was more than doubled those with an AR index of less than 25, at 31.3%. (All patients with moderate to severe periAR after TAVI had an AR index of less than 25.)
Dr. Vasa-Nicotera, who led the study, also published a similar study earlier this year, involving 146 patients with the CoreValve prosthesis.
In that study, the group concluded, "The assessment of the AR index allows a precise judgment of periAR, independently predicts 1-year mortality after TAVI, and provides prognostic information that is complementary to the degree of periAR."
In their current paper, the authors wrote that since "The validity of the AR index could be confirmed in this independent cohort of another high-volume TAVI center," the finding can be generalized.
They added that a prospective multicenter trial might be needed to verify the results. It is also not clear if valves other than Medtronic CoreValve and Edwards SAPIEN will yield the same results.
Dr. Zoghbi added that AR index can also be measure noninvasively with Doppler echocardiography, something that can be considered in the future.
Dr. Vasa-Nicotera had no relevent disclosures. Other investigators disclosed ties to CoreValve/Medtronic and Edwards-Sapien, the makers of the valves used in the study. Dr. Zoghbi had no relevant disclosures.
A new hemodynamic measurement can provide prognostic information that goes beyond just the degree of aortic regurgitation after percutaneous aortic implantation, according to a single-center, retrospective, European study.
The study validates the team’s earlier findings in a slightly larger group of patients. Called aortic regurgitation index (AR index), the parameter is measured by subtracting the left ventricular end-diastolic pressure from the diastolic blood pressure and dividing the result by systolic blood pressure, and then multiplying it by 100.
The measurement can provide additional information beyond echocardiography when it comes to assessing the severity and outcome of periprosthetic aortic regurgitation (periAR), wrote Dr. Mariuca Vasa-Nicotera and colleagues. Even patients with nonexistent or mild periAR after transcatheter aortic valve implantation (TAVI) can be stratified based on their AR index score. It can also help predict 1-year mortality after TAVI.
The findings "are not going to change practice," commented Dr. William Zoghbi, chief of cardiovascular imaging at Methodist DeBakey Heart and Vascular Center in Houston, who was not involved in the study. Rather, the AR index "would help with predicting prognosis for those individuals who have valve implantation."
AR index is also more than just an index of valve regurgitation, said Dr. Zoghbi, who is also the current president of the American College of Cardiology. The measurement shows whether the heart has a significant abnormality in diastolic function and how it is handing the hemodynamic load of valvular regurgitation, he said.
Paravalvular leakage is a common observation following TAVI, and growing evidence has shown an association between the leakage and dramatic increase in morbidity and mortality.
The researchers analyzed data from 122 high-risk or inoperable patients who underwent TAVI during January 2007–March 2011 at Glenfield University Hospital in Leicester, United Kingdom.
The procedure was performed using third-generation 18-F Medtronic CoreValves or Edwards SAPIEN valves. Afterward, severity of periAR was assessed angiographically, echocardiographically by transesophageal echocardiography, and hemodynamically.
Patients were then divided into two groups on the basis of periAR: 102 had no or mild periAR (26 and 76 patients, respectively), and 20 had moderate or severe periAR (18 and 2 patients).
There were significant differences between the two patient groups.
Patients with moderate to severe periAR after TAVI had a significantly higher EuroSCORE (28.5, compared with 21.2 in those with no to mild periAR) and Society of Thoracic Surgeons mortality score (9.5 and 6.8, respectively). They also had a higher rate of myocardial infarction (40% v. 10%), and a lower left ventricular ejection fraction (45 v. 51).
Thirty-two patients (26%) died during the 1-year follow-up.
Among those remaining, 1-year mortality was 60% in patients with moderate to severe periAR, compared with 20% in patients with no or mild periAR.
The AR index declined in step with periAR severity, from 29.4 in patients with no periAR to 28.0 in those with mild periAR, to19.6 in patients with moderate periAR, and to 7.6 with severe periAR.
One-year mortality by AR index also paralleled that by periAR. That outcome was tripled in patients with an AR index of less than 25, compared with patients who had an AR index of at least 25 (42.3% vs. 14.3%), the authors reported.
AR index was also useful in patients with no or mild periAR, and provided complementary prognostic information, according to the authors.
The patients in that group who had an AR index of at least 25 had a 1-year mortality rate of 14.3%. But that rate was more than doubled those with an AR index of less than 25, at 31.3%. (All patients with moderate to severe periAR after TAVI had an AR index of less than 25.)
Dr. Vasa-Nicotera, who led the study, also published a similar study earlier this year, involving 146 patients with the CoreValve prosthesis.
In that study, the group concluded, "The assessment of the AR index allows a precise judgment of periAR, independently predicts 1-year mortality after TAVI, and provides prognostic information that is complementary to the degree of periAR."
In their current paper, the authors wrote that since "The validity of the AR index could be confirmed in this independent cohort of another high-volume TAVI center," the finding can be generalized.
They added that a prospective multicenter trial might be needed to verify the results. It is also not clear if valves other than Medtronic CoreValve and Edwards SAPIEN will yield the same results.
Dr. Zoghbi added that AR index can also be measure noninvasively with Doppler echocardiography, something that can be considered in the future.
Dr. Vasa-Nicotera had no relevent disclosures. Other investigators disclosed ties to CoreValve/Medtronic and Edwards-Sapien, the makers of the valves used in the study. Dr. Zoghbi had no relevant disclosures.
A new hemodynamic measurement can provide prognostic information that goes beyond just the degree of aortic regurgitation after percutaneous aortic implantation, according to a single-center, retrospective, European study.
The study validates the team’s earlier findings in a slightly larger group of patients. Called aortic regurgitation index (AR index), the parameter is measured by subtracting the left ventricular end-diastolic pressure from the diastolic blood pressure and dividing the result by systolic blood pressure, and then multiplying it by 100.
The measurement can provide additional information beyond echocardiography when it comes to assessing the severity and outcome of periprosthetic aortic regurgitation (periAR), wrote Dr. Mariuca Vasa-Nicotera and colleagues. Even patients with nonexistent or mild periAR after transcatheter aortic valve implantation (TAVI) can be stratified based on their AR index score. It can also help predict 1-year mortality after TAVI.
The findings "are not going to change practice," commented Dr. William Zoghbi, chief of cardiovascular imaging at Methodist DeBakey Heart and Vascular Center in Houston, who was not involved in the study. Rather, the AR index "would help with predicting prognosis for those individuals who have valve implantation."
AR index is also more than just an index of valve regurgitation, said Dr. Zoghbi, who is also the current president of the American College of Cardiology. The measurement shows whether the heart has a significant abnormality in diastolic function and how it is handing the hemodynamic load of valvular regurgitation, he said.
Paravalvular leakage is a common observation following TAVI, and growing evidence has shown an association between the leakage and dramatic increase in morbidity and mortality.
The researchers analyzed data from 122 high-risk or inoperable patients who underwent TAVI during January 2007–March 2011 at Glenfield University Hospital in Leicester, United Kingdom.
The procedure was performed using third-generation 18-F Medtronic CoreValves or Edwards SAPIEN valves. Afterward, severity of periAR was assessed angiographically, echocardiographically by transesophageal echocardiography, and hemodynamically.
Patients were then divided into two groups on the basis of periAR: 102 had no or mild periAR (26 and 76 patients, respectively), and 20 had moderate or severe periAR (18 and 2 patients).
There were significant differences between the two patient groups.
Patients with moderate to severe periAR after TAVI had a significantly higher EuroSCORE (28.5, compared with 21.2 in those with no to mild periAR) and Society of Thoracic Surgeons mortality score (9.5 and 6.8, respectively). They also had a higher rate of myocardial infarction (40% v. 10%), and a lower left ventricular ejection fraction (45 v. 51).
Thirty-two patients (26%) died during the 1-year follow-up.
Among those remaining, 1-year mortality was 60% in patients with moderate to severe periAR, compared with 20% in patients with no or mild periAR.
The AR index declined in step with periAR severity, from 29.4 in patients with no periAR to 28.0 in those with mild periAR, to19.6 in patients with moderate periAR, and to 7.6 with severe periAR.
One-year mortality by AR index also paralleled that by periAR. That outcome was tripled in patients with an AR index of less than 25, compared with patients who had an AR index of at least 25 (42.3% vs. 14.3%), the authors reported.
AR index was also useful in patients with no or mild periAR, and provided complementary prognostic information, according to the authors.
The patients in that group who had an AR index of at least 25 had a 1-year mortality rate of 14.3%. But that rate was more than doubled those with an AR index of less than 25, at 31.3%. (All patients with moderate to severe periAR after TAVI had an AR index of less than 25.)
Dr. Vasa-Nicotera, who led the study, also published a similar study earlier this year, involving 146 patients with the CoreValve prosthesis.
In that study, the group concluded, "The assessment of the AR index allows a precise judgment of periAR, independently predicts 1-year mortality after TAVI, and provides prognostic information that is complementary to the degree of periAR."
In their current paper, the authors wrote that since "The validity of the AR index could be confirmed in this independent cohort of another high-volume TAVI center," the finding can be generalized.
They added that a prospective multicenter trial might be needed to verify the results. It is also not clear if valves other than Medtronic CoreValve and Edwards SAPIEN will yield the same results.
Dr. Zoghbi added that AR index can also be measure noninvasively with Doppler echocardiography, something that can be considered in the future.
Dr. Vasa-Nicotera had no relevent disclosures. Other investigators disclosed ties to CoreValve/Medtronic and Edwards-Sapien, the makers of the valves used in the study. Dr. Zoghbi had no relevant disclosures.
FROM THE JOURNAL OF THE AMERICAN COLLEGE OF CARDIOLOGY
Major Finding: Patients with AR index of less than 25 had a significantly increased 1-year mortality risk, compared with patients who had an AR index of at least 25 (42.3% vs. 14.3%).
Data Source: Data are from 122 high-risk patients who underwent TAVI between January 2007 and March 2011 at Glenfield University Hospital in Leicester, United Kingdom.
Disclosures: Dr. Vasa-Nicotera had no relevent disclosures. Other investigators disclosed ties to CoreValve/Medtronic and Edwards-Sapien, the makers of the valves used in the study. Dr. Zoghbi had no relevant disclosures.
TEE Important During TAVI
NATIONAL HARBOR, MD. – Transesophageal echocardiography during percutaneous valve replacement plays an important role in monitoring patients and has the potential to prevent intraprocedural complications, according to a single-center retrospective study.
"There has not been a study that has looked at intraprocedural complications," said Dr. Aditya Saini, lead author and assistant researcher at Clinical Echocardiography Laboratory at MedStar Washington Hospital Center, in the District of Columbia. "We wanted to find out what exactly were the rates and incidence of these complications," Dr. Saini said at the annual meeting of the American Society of Echocardiography.
Several studies from Europe and Canada have already established the importance of TEE during transcatheter aortic valve implantation (TAVI) (JACC Cardiovasc. Imaging 2008;1:15-24).
Dr. Saini and colleagues reviewed intraprocedural 3D-TEE images of 216 consecutive TAVI (commonly referred to as transcatheter aortic valve replacement or TAVR) procedures at MedStar Washington Hospital Center between May 2007 and November 2011. Edwards SAPIEN prosthetic valve was used in the procedure, and implanted through the transapical (27.3% of the patients) or transfemoral (72.7%) approach. All valves were seated appropriately.
Researchers also recorded the occurrence of mobile thrombi immediately after the valves’ implantation.
Results showed that perivalvular leak was the most common observation during the procedure and occurred in roughly 80% of the cases, usually mild and of moderate severity in only 8.7% of the cases. Other complications, which occurred in one or two patients each, included severe central aortic incompetence, ruptured chord off anterior mitral leaflet, new wall motion abnormalities, ascending aortic dissection, and new pericardial effusion.
The most common location for mobile thrombi was the aortic arch (15% of the cases). The thrombi were also observed in the left atrium, left atrial appendage, attached to the catheter in LVOT, attached to native aortic valve after valve implantation, between the prosthesis and aortic annulus, in one or two patients each.
The study’s findings are "nothing earth shattering or new," said Dr. Steven A. Goldstein of MedStar Washington Hospital Center. Dr. Goldstein oversaw the study. "It’s a nice study because it’s a collection of data."
The study "lays down a baseline with a substantial number of patients," said Dr. Saini, chief resident in internal medicine at MedStar Harbor Hospital in Baltimore. "We can’t draw any conclusions from an observational study, but it always starts from the basics."
TEE is routinely used during TAVI to help with balloon positioning, assessing paravalvular aortic regurgitation, and detecting the device’s function and immediate complications, according to the authors.
Dr. Saini and Dr. Goldstein had no relevant disclosures.
NATIONAL HARBOR, MD. – Transesophageal echocardiography during percutaneous valve replacement plays an important role in monitoring patients and has the potential to prevent intraprocedural complications, according to a single-center retrospective study.
"There has not been a study that has looked at intraprocedural complications," said Dr. Aditya Saini, lead author and assistant researcher at Clinical Echocardiography Laboratory at MedStar Washington Hospital Center, in the District of Columbia. "We wanted to find out what exactly were the rates and incidence of these complications," Dr. Saini said at the annual meeting of the American Society of Echocardiography.
Several studies from Europe and Canada have already established the importance of TEE during transcatheter aortic valve implantation (TAVI) (JACC Cardiovasc. Imaging 2008;1:15-24).
Dr. Saini and colleagues reviewed intraprocedural 3D-TEE images of 216 consecutive TAVI (commonly referred to as transcatheter aortic valve replacement or TAVR) procedures at MedStar Washington Hospital Center between May 2007 and November 2011. Edwards SAPIEN prosthetic valve was used in the procedure, and implanted through the transapical (27.3% of the patients) or transfemoral (72.7%) approach. All valves were seated appropriately.
Researchers also recorded the occurrence of mobile thrombi immediately after the valves’ implantation.
Results showed that perivalvular leak was the most common observation during the procedure and occurred in roughly 80% of the cases, usually mild and of moderate severity in only 8.7% of the cases. Other complications, which occurred in one or two patients each, included severe central aortic incompetence, ruptured chord off anterior mitral leaflet, new wall motion abnormalities, ascending aortic dissection, and new pericardial effusion.
The most common location for mobile thrombi was the aortic arch (15% of the cases). The thrombi were also observed in the left atrium, left atrial appendage, attached to the catheter in LVOT, attached to native aortic valve after valve implantation, between the prosthesis and aortic annulus, in one or two patients each.
The study’s findings are "nothing earth shattering or new," said Dr. Steven A. Goldstein of MedStar Washington Hospital Center. Dr. Goldstein oversaw the study. "It’s a nice study because it’s a collection of data."
The study "lays down a baseline with a substantial number of patients," said Dr. Saini, chief resident in internal medicine at MedStar Harbor Hospital in Baltimore. "We can’t draw any conclusions from an observational study, but it always starts from the basics."
TEE is routinely used during TAVI to help with balloon positioning, assessing paravalvular aortic regurgitation, and detecting the device’s function and immediate complications, according to the authors.
Dr. Saini and Dr. Goldstein had no relevant disclosures.
NATIONAL HARBOR, MD. – Transesophageal echocardiography during percutaneous valve replacement plays an important role in monitoring patients and has the potential to prevent intraprocedural complications, according to a single-center retrospective study.
"There has not been a study that has looked at intraprocedural complications," said Dr. Aditya Saini, lead author and assistant researcher at Clinical Echocardiography Laboratory at MedStar Washington Hospital Center, in the District of Columbia. "We wanted to find out what exactly were the rates and incidence of these complications," Dr. Saini said at the annual meeting of the American Society of Echocardiography.
Several studies from Europe and Canada have already established the importance of TEE during transcatheter aortic valve implantation (TAVI) (JACC Cardiovasc. Imaging 2008;1:15-24).
Dr. Saini and colleagues reviewed intraprocedural 3D-TEE images of 216 consecutive TAVI (commonly referred to as transcatheter aortic valve replacement or TAVR) procedures at MedStar Washington Hospital Center between May 2007 and November 2011. Edwards SAPIEN prosthetic valve was used in the procedure, and implanted through the transapical (27.3% of the patients) or transfemoral (72.7%) approach. All valves were seated appropriately.
Researchers also recorded the occurrence of mobile thrombi immediately after the valves’ implantation.
Results showed that perivalvular leak was the most common observation during the procedure and occurred in roughly 80% of the cases, usually mild and of moderate severity in only 8.7% of the cases. Other complications, which occurred in one or two patients each, included severe central aortic incompetence, ruptured chord off anterior mitral leaflet, new wall motion abnormalities, ascending aortic dissection, and new pericardial effusion.
The most common location for mobile thrombi was the aortic arch (15% of the cases). The thrombi were also observed in the left atrium, left atrial appendage, attached to the catheter in LVOT, attached to native aortic valve after valve implantation, between the prosthesis and aortic annulus, in one or two patients each.
The study’s findings are "nothing earth shattering or new," said Dr. Steven A. Goldstein of MedStar Washington Hospital Center. Dr. Goldstein oversaw the study. "It’s a nice study because it’s a collection of data."
The study "lays down a baseline with a substantial number of patients," said Dr. Saini, chief resident in internal medicine at MedStar Harbor Hospital in Baltimore. "We can’t draw any conclusions from an observational study, but it always starts from the basics."
TEE is routinely used during TAVI to help with balloon positioning, assessing paravalvular aortic regurgitation, and detecting the device’s function and immediate complications, according to the authors.
Dr. Saini and Dr. Goldstein had no relevant disclosures.
AT THE ANNUAL MEETING OF THE AMERICAN SOCIETY OF ECHOCARDIOGRAPHY
Major Finding: Perivalvular leak was the most common observation during the procedure and occurred in roughly 80% of the cases, but of moderate severity in only 8.7% of the cases.
Data Source: Review of intraprocedural TEE images of 216 consecutive TAVI procedures at MedStar Washington Hospital Center between May 2007 and November 2011.
Disclosures: Dr. Saini and Dr. Goldstein had no relevant disclosures.
Blood Test May Predict Temsirolimus Response in Kidney Cancer
Pretreatment lactate dehydrogenase levels predict how well poor-risk patients with advanced kidney cancer respond to temsirolimus, according to a retrospective analysis of a phase-III trial.
If further studies confirm the finding, a low-cost blood test for the enzyme could make it the first biomarker for clinical prediction of kidney cancer patients’ response to a mammalian target of rapamycin (mTOR) inhibitor, such as temsirolimus (Torisel).
"Being able to direct these patients to a treatment we know will help them would be a major advancement in their care," Dr. Andrew J. Armstrong of the Duke Cancer Institute, Durham, N.C, said in a written statement. "At the same time, patients who would not benefit from the treatment would be spared from undergoing a drug regimen with potential side effects that could diminish their quality of life."
Temsirolimus is approved for treatment of advanced kidney cancer. Alternatives to mTOR inhibition for this disease include cytokine therapy and agents targeting the vascular endothelial growth factor.
A widely expressed metabolic enzyme in tissues, lactate dehydrogenase (LDH) is a well-known prognostic biomarker in various types of cancers, including prostate cancer, lymphoma, melanoma, and renal cell carcinoma (RCC). Prognostic biomarkers help evaluate patient outcomes irrespective of treatment. No predictive biomarkers have been identified that can help select treatments for genitourinary malignancies, including RCC, Dr. Armstrong and his associates wrote in the Journal of Clinical Oncology.
The researchers used data from a phase-III randomized study (N. Engl. J. Med. 2007;356:2271-81) to compare 404 poor-risk patients with advanced RCC who were randomly assigned to temsirolimus (201 patients) or interferon alfa-2a (IFN-alpha) (203 patients). Overall survival rates among patients treated with temsirolimus were 53.7% at 6 months and 34.3% at 12 months, compared with 39.5% and 12.7 %, respectively, in the IFN-alpha group.
Stratifying patients by high LDH (defined as greater than 1 × the upper limit of normal [ULN]) and normal LDH (up to 1 × ULN), investigators calculated the multivariable hazard ratio for death was 2.81with high vs. normal LDH at baseline (P less than .001), they reported (J. Clin. Oncol. Aug. 13 [doi: 10.1200/JCO.2011.40.9631]).
The analysis showed that, within the high-LDH group, median overall survival was significantly longer in those treated with temsirolimus than in those treated with IFN-alpha (6.9 v. 4.2 months; HR=0.56; P less than .002.) Among patients with normal LDH, median overall survival was not significantly improved by temsirolimus, however (11.7 months vs. 10.4 months).
As secondary end points, the authors examined the predictive value of baseline LDH for progression-free survival and clinical benefit rate, but they found no predictive interaction.
Dr. Armstrong said in an interview that the study statistically proves that LDH is a predictive biomarker for advanced RCC, but the results have to be validated before major practice changes take place.
"The advantage of LDH as a predictive and prognostic biomarker rests in its ease of collection, cost, and its routine assessment as part of routine medical care in patients with RCC," the authors wrote.
The study has several limitations, they noted. For one, it was limited to poor-risk patients with RCC. Also, temsirolimus was only compared with IFN-alpha, which is no longer in widespread use. Also, the study was limited to temsirolimus, an mTOR/TORC1 inhibitor.
"Although other TORC1 inhibitors may have anticancer activity, it is unknown at this time whether LDH is predictive of improved outcomes with these agents," the authors wrote.
Dr. Armstrong has received honoraria and research funding from Pfizer, which makes temsirolimus and funded the study.
Pretreatment lactate dehydrogenase levels predict how well poor-risk patients with advanced kidney cancer respond to temsirolimus, according to a retrospective analysis of a phase-III trial.
If further studies confirm the finding, a low-cost blood test for the enzyme could make it the first biomarker for clinical prediction of kidney cancer patients’ response to a mammalian target of rapamycin (mTOR) inhibitor, such as temsirolimus (Torisel).
"Being able to direct these patients to a treatment we know will help them would be a major advancement in their care," Dr. Andrew J. Armstrong of the Duke Cancer Institute, Durham, N.C, said in a written statement. "At the same time, patients who would not benefit from the treatment would be spared from undergoing a drug regimen with potential side effects that could diminish their quality of life."
Temsirolimus is approved for treatment of advanced kidney cancer. Alternatives to mTOR inhibition for this disease include cytokine therapy and agents targeting the vascular endothelial growth factor.
A widely expressed metabolic enzyme in tissues, lactate dehydrogenase (LDH) is a well-known prognostic biomarker in various types of cancers, including prostate cancer, lymphoma, melanoma, and renal cell carcinoma (RCC). Prognostic biomarkers help evaluate patient outcomes irrespective of treatment. No predictive biomarkers have been identified that can help select treatments for genitourinary malignancies, including RCC, Dr. Armstrong and his associates wrote in the Journal of Clinical Oncology.
The researchers used data from a phase-III randomized study (N. Engl. J. Med. 2007;356:2271-81) to compare 404 poor-risk patients with advanced RCC who were randomly assigned to temsirolimus (201 patients) or interferon alfa-2a (IFN-alpha) (203 patients). Overall survival rates among patients treated with temsirolimus were 53.7% at 6 months and 34.3% at 12 months, compared with 39.5% and 12.7 %, respectively, in the IFN-alpha group.
Stratifying patients by high LDH (defined as greater than 1 × the upper limit of normal [ULN]) and normal LDH (up to 1 × ULN), investigators calculated the multivariable hazard ratio for death was 2.81with high vs. normal LDH at baseline (P less than .001), they reported (J. Clin. Oncol. Aug. 13 [doi: 10.1200/JCO.2011.40.9631]).
The analysis showed that, within the high-LDH group, median overall survival was significantly longer in those treated with temsirolimus than in those treated with IFN-alpha (6.9 v. 4.2 months; HR=0.56; P less than .002.) Among patients with normal LDH, median overall survival was not significantly improved by temsirolimus, however (11.7 months vs. 10.4 months).
As secondary end points, the authors examined the predictive value of baseline LDH for progression-free survival and clinical benefit rate, but they found no predictive interaction.
Dr. Armstrong said in an interview that the study statistically proves that LDH is a predictive biomarker for advanced RCC, but the results have to be validated before major practice changes take place.
"The advantage of LDH as a predictive and prognostic biomarker rests in its ease of collection, cost, and its routine assessment as part of routine medical care in patients with RCC," the authors wrote.
The study has several limitations, they noted. For one, it was limited to poor-risk patients with RCC. Also, temsirolimus was only compared with IFN-alpha, which is no longer in widespread use. Also, the study was limited to temsirolimus, an mTOR/TORC1 inhibitor.
"Although other TORC1 inhibitors may have anticancer activity, it is unknown at this time whether LDH is predictive of improved outcomes with these agents," the authors wrote.
Dr. Armstrong has received honoraria and research funding from Pfizer, which makes temsirolimus and funded the study.
Pretreatment lactate dehydrogenase levels predict how well poor-risk patients with advanced kidney cancer respond to temsirolimus, according to a retrospective analysis of a phase-III trial.
If further studies confirm the finding, a low-cost blood test for the enzyme could make it the first biomarker for clinical prediction of kidney cancer patients’ response to a mammalian target of rapamycin (mTOR) inhibitor, such as temsirolimus (Torisel).
"Being able to direct these patients to a treatment we know will help them would be a major advancement in their care," Dr. Andrew J. Armstrong of the Duke Cancer Institute, Durham, N.C, said in a written statement. "At the same time, patients who would not benefit from the treatment would be spared from undergoing a drug regimen with potential side effects that could diminish their quality of life."
Temsirolimus is approved for treatment of advanced kidney cancer. Alternatives to mTOR inhibition for this disease include cytokine therapy and agents targeting the vascular endothelial growth factor.
A widely expressed metabolic enzyme in tissues, lactate dehydrogenase (LDH) is a well-known prognostic biomarker in various types of cancers, including prostate cancer, lymphoma, melanoma, and renal cell carcinoma (RCC). Prognostic biomarkers help evaluate patient outcomes irrespective of treatment. No predictive biomarkers have been identified that can help select treatments for genitourinary malignancies, including RCC, Dr. Armstrong and his associates wrote in the Journal of Clinical Oncology.
The researchers used data from a phase-III randomized study (N. Engl. J. Med. 2007;356:2271-81) to compare 404 poor-risk patients with advanced RCC who were randomly assigned to temsirolimus (201 patients) or interferon alfa-2a (IFN-alpha) (203 patients). Overall survival rates among patients treated with temsirolimus were 53.7% at 6 months and 34.3% at 12 months, compared with 39.5% and 12.7 %, respectively, in the IFN-alpha group.
Stratifying patients by high LDH (defined as greater than 1 × the upper limit of normal [ULN]) and normal LDH (up to 1 × ULN), investigators calculated the multivariable hazard ratio for death was 2.81with high vs. normal LDH at baseline (P less than .001), they reported (J. Clin. Oncol. Aug. 13 [doi: 10.1200/JCO.2011.40.9631]).
The analysis showed that, within the high-LDH group, median overall survival was significantly longer in those treated with temsirolimus than in those treated with IFN-alpha (6.9 v. 4.2 months; HR=0.56; P less than .002.) Among patients with normal LDH, median overall survival was not significantly improved by temsirolimus, however (11.7 months vs. 10.4 months).
As secondary end points, the authors examined the predictive value of baseline LDH for progression-free survival and clinical benefit rate, but they found no predictive interaction.
Dr. Armstrong said in an interview that the study statistically proves that LDH is a predictive biomarker for advanced RCC, but the results have to be validated before major practice changes take place.
"The advantage of LDH as a predictive and prognostic biomarker rests in its ease of collection, cost, and its routine assessment as part of routine medical care in patients with RCC," the authors wrote.
The study has several limitations, they noted. For one, it was limited to poor-risk patients with RCC. Also, temsirolimus was only compared with IFN-alpha, which is no longer in widespread use. Also, the study was limited to temsirolimus, an mTOR/TORC1 inhibitor.
"Although other TORC1 inhibitors may have anticancer activity, it is unknown at this time whether LDH is predictive of improved outcomes with these agents," the authors wrote.
Dr. Armstrong has received honoraria and research funding from Pfizer, which makes temsirolimus and funded the study.
FROM THE JOURNAL OF CLINICAL ONCOLOGY
Major Finding: Median overall survival among patients who had high lactate dehydrogenase at baseline was significantly longer in those who were treated with temsirolimus than those treated with IFN-alpha (6.9 months vs. 4.2 months; HR = 0.56).
Data Source: Investigators did a retrospective analysis of data on 404 poor-risk patients from a phase-III randomized study.
Disclosures: Dr. Armstrong has received honoraria and research funding from Pfizer, which makes temsirolimus and funded the study.
Lucentis Approved for Diabetic Macular Edema
The Food and Drug Administration has approved ranibizumab for treatment of diabetic macular edema.
Ranibizumab injection, or Lucentis, is the first approved drug treatment of diabetic macular edema (DME) to be administered at a dosage of 0.3 mg once a month by a health care professional. The standard therapy for the condition thus far has been focal laser treatment.
"Today’s approval represents a major development for the treatment of people whose vision is impaired by DME as a complication of their disease," said Dr. Renata Albrecht, director of the division of transplant and ophthalmology products in FDA’s Center for Drug Evaluation and Research, in a statement.
Lucentis, developed by Genentech, was previously approved for treatment of neovascular age-related macular degeneration and for macular edema following retinal vein occlusion.
"We developed Lucentis to treat diseases of the eye and are pleased to have received this third U.S. indication to help a new population of people whose eyesight may be affected by diabetes," said Dr. Hal Barron, chief medical officer and head of global product development for Genentech, in a statement.
Roughly 4 million adults with diabetes reporter vision problems in 2010, according to the FDA.
The drug’s approval is based on the results of two clinical studies including 759 patients who were treated and followed for 3 years.
Results showed that 34%-45% of the patients who were treated monthly with 0.3 mg of Lucentis, gained at least three lines of vision compared with 12%-18% of those who didn’t receive the injection, according to the FDA. The higher tested dose of 0.5 mg showed no additional benefit.
The most common side effects of the drug are bleeding of the conjunctiva.
DME is a sight-threatening eye disease, in which fluid leaks to the macula and causes blurred vision. The condition affects people with diabetes and can lead to blindness.
The Food and Drug Administration has approved ranibizumab for treatment of diabetic macular edema.
Ranibizumab injection, or Lucentis, is the first approved drug treatment of diabetic macular edema (DME) to be administered at a dosage of 0.3 mg once a month by a health care professional. The standard therapy for the condition thus far has been focal laser treatment.
"Today’s approval represents a major development for the treatment of people whose vision is impaired by DME as a complication of their disease," said Dr. Renata Albrecht, director of the division of transplant and ophthalmology products in FDA’s Center for Drug Evaluation and Research, in a statement.
Lucentis, developed by Genentech, was previously approved for treatment of neovascular age-related macular degeneration and for macular edema following retinal vein occlusion.
"We developed Lucentis to treat diseases of the eye and are pleased to have received this third U.S. indication to help a new population of people whose eyesight may be affected by diabetes," said Dr. Hal Barron, chief medical officer and head of global product development for Genentech, in a statement.
Roughly 4 million adults with diabetes reporter vision problems in 2010, according to the FDA.
The drug’s approval is based on the results of two clinical studies including 759 patients who were treated and followed for 3 years.
Results showed that 34%-45% of the patients who were treated monthly with 0.3 mg of Lucentis, gained at least three lines of vision compared with 12%-18% of those who didn’t receive the injection, according to the FDA. The higher tested dose of 0.5 mg showed no additional benefit.
The most common side effects of the drug are bleeding of the conjunctiva.
DME is a sight-threatening eye disease, in which fluid leaks to the macula and causes blurred vision. The condition affects people with diabetes and can lead to blindness.
The Food and Drug Administration has approved ranibizumab for treatment of diabetic macular edema.
Ranibizumab injection, or Lucentis, is the first approved drug treatment of diabetic macular edema (DME) to be administered at a dosage of 0.3 mg once a month by a health care professional. The standard therapy for the condition thus far has been focal laser treatment.
"Today’s approval represents a major development for the treatment of people whose vision is impaired by DME as a complication of their disease," said Dr. Renata Albrecht, director of the division of transplant and ophthalmology products in FDA’s Center for Drug Evaluation and Research, in a statement.
Lucentis, developed by Genentech, was previously approved for treatment of neovascular age-related macular degeneration and for macular edema following retinal vein occlusion.
"We developed Lucentis to treat diseases of the eye and are pleased to have received this third U.S. indication to help a new population of people whose eyesight may be affected by diabetes," said Dr. Hal Barron, chief medical officer and head of global product development for Genentech, in a statement.
Roughly 4 million adults with diabetes reporter vision problems in 2010, according to the FDA.
The drug’s approval is based on the results of two clinical studies including 759 patients who were treated and followed for 3 years.
Results showed that 34%-45% of the patients who were treated monthly with 0.3 mg of Lucentis, gained at least three lines of vision compared with 12%-18% of those who didn’t receive the injection, according to the FDA. The higher tested dose of 0.5 mg showed no additional benefit.
The most common side effects of the drug are bleeding of the conjunctiva.
DME is a sight-threatening eye disease, in which fluid leaks to the macula and causes blurred vision. The condition affects people with diabetes and can lead to blindness.
Mortality Soared With Post-TAVR Vascular Complications
Major vascular complications following transfemoral percutaneous aortic valve replacement using first-generation devices were associated with a nearly fivefold increase in 30-day mortality, according to a post hoc analysis of the PARTNER trial.
The complications also were associated with higher 1-year all-cause and cardiac mortality, Dr. Philippe Généreux and his colleagues reported in the Journal of the American College of Cardiology (2012 August [doi: 10.1016/j.jacc.2012.07.003]).
"This is a wonderful technique, but you have to be very careful with patient selection, especially if you’re starting a new program," Dr. Généreux, an interventional cardiologist at Columbia University Medical Center/New York Presbyterian Hospital, said in an interview.
The findings also highlight the importance of having a heart team and appropriate training, said Dr. Généreux, who also practices at the University of Montreal.
He and his colleagues pooled data from the PARTNER (Placement of Aortic Transcatheter Valve) trial’s cohort A (J. Am. Coll. Cardiol. 2012;60:548-58) and cohort B (N. Engl. J. Med. 2010;363:1597-607) for only those patients who had undergone transcatheter aortic valve replacement via the transfemoral approach (242 from cohort A and 177 from cohort B.)
First-generation Edwards Sapien valves were used, via a 22-F or 24-F sheath.
Dr. Généreux said he expected the complication rates to drop in the near future as new-generation devices and smaller delivery systems arrive in the U.S. market and as cardiologists gain more experience performing transcatheter aortic valve replacement (TAVR).
A recent study showed that from 2009 to 2010, major vascular complications decreased from 8% to 1%, and minor vascular complications decreased from 24% to 8% (J. Am. Coll. Cardiol. 2012;59:113-8). The authors noted that "[s]maller sheaths, rigorous angiographic and computed tomographic screening and patient selection, and percutaneous vascular repair techniques were increasingly used over this period."
Results of the current study showed that roughly 15% (64) of the patients had a major vascular complication, and 12% (50) patients had minor complications within 30 days following the procedure.
The most common major vascular complications after TAVR included vascular dissection (63%), vascular perforation (31%), access site hematoma (23%), and retroperitoneal bleeding (10%).
Patients who had a major vascular complication following transfemoral TAVR, compared with those who did not, had a significantly higher rate of 30-day mortality (14% vs. 3%, respectively) and 1-year mortality (39% vs. 23%). The occurrence of a major vascular complication also was associated with significantly higher 30-day rates of transfusions (41% vs. 5%), major bleeding (61% vs. 7%), and renal failure requiring dialysis (8% vs. 2%).
However, "the incidence and impact of major [vascular complications] seem to decrease in a lower-risk population," the authors wrote.
Moreover, patients in cohort B had a higher rate of major and minor vascular complications (32.8%) than did patients in cohort A (23.2%). The difference is partly explained by the fact that patients in cohort B were sicker and, unlike cohort A, had no alternative routes available to them, said Dr. Généreux, who also is the director of the Angiographic Core Laboratory at the Cardiovascular Research Foundation in New York.
The analysis showed that patients with major vascular complications had a lower body surface area, a smaller vessel diameter, and higher sheath-to-femoral-artery and sheath-to-external-iliac-artery ratios. They also were more likely to have insulin-treated diabetes at baseline and to be female.
Female sex was in fact the only independent predictor of major vascular complications after TAVR, according to the analysis, even after adjustment for the sheath-to-femoral-artery ratio. However, female sex was also associated with a reduction in all-cause mortality at 1 year after TAVR, the authors noted. "Thus, the impact of sex on short- and long-term outcomes is complex, and a more detailed analysis is needed," they wrote.
Dr. Généreux said that if this analysis were to be repeated in a few years, he expected the vascular complication rates to fall to a range of 1%-5%, and that fewer dramatic complications would be reported.
Dr. Généreux is a speaker and consultant for Edwards Lifesciences. Several coauthors have received consulting fees, travel reimbursement, and/or grant support from Edwards and other device companies.
Major vascular complications following transfemoral percutaneous aortic valve replacement using first-generation devices were associated with a nearly fivefold increase in 30-day mortality, according to a post hoc analysis of the PARTNER trial.
The complications also were associated with higher 1-year all-cause and cardiac mortality, Dr. Philippe Généreux and his colleagues reported in the Journal of the American College of Cardiology (2012 August [doi: 10.1016/j.jacc.2012.07.003]).
"This is a wonderful technique, but you have to be very careful with patient selection, especially if you’re starting a new program," Dr. Généreux, an interventional cardiologist at Columbia University Medical Center/New York Presbyterian Hospital, said in an interview.
The findings also highlight the importance of having a heart team and appropriate training, said Dr. Généreux, who also practices at the University of Montreal.
He and his colleagues pooled data from the PARTNER (Placement of Aortic Transcatheter Valve) trial’s cohort A (J. Am. Coll. Cardiol. 2012;60:548-58) and cohort B (N. Engl. J. Med. 2010;363:1597-607) for only those patients who had undergone transcatheter aortic valve replacement via the transfemoral approach (242 from cohort A and 177 from cohort B.)
First-generation Edwards Sapien valves were used, via a 22-F or 24-F sheath.
Dr. Généreux said he expected the complication rates to drop in the near future as new-generation devices and smaller delivery systems arrive in the U.S. market and as cardiologists gain more experience performing transcatheter aortic valve replacement (TAVR).
A recent study showed that from 2009 to 2010, major vascular complications decreased from 8% to 1%, and minor vascular complications decreased from 24% to 8% (J. Am. Coll. Cardiol. 2012;59:113-8). The authors noted that "[s]maller sheaths, rigorous angiographic and computed tomographic screening and patient selection, and percutaneous vascular repair techniques were increasingly used over this period."
Results of the current study showed that roughly 15% (64) of the patients had a major vascular complication, and 12% (50) patients had minor complications within 30 days following the procedure.
The most common major vascular complications after TAVR included vascular dissection (63%), vascular perforation (31%), access site hematoma (23%), and retroperitoneal bleeding (10%).
Patients who had a major vascular complication following transfemoral TAVR, compared with those who did not, had a significantly higher rate of 30-day mortality (14% vs. 3%, respectively) and 1-year mortality (39% vs. 23%). The occurrence of a major vascular complication also was associated with significantly higher 30-day rates of transfusions (41% vs. 5%), major bleeding (61% vs. 7%), and renal failure requiring dialysis (8% vs. 2%).
However, "the incidence and impact of major [vascular complications] seem to decrease in a lower-risk population," the authors wrote.
Moreover, patients in cohort B had a higher rate of major and minor vascular complications (32.8%) than did patients in cohort A (23.2%). The difference is partly explained by the fact that patients in cohort B were sicker and, unlike cohort A, had no alternative routes available to them, said Dr. Généreux, who also is the director of the Angiographic Core Laboratory at the Cardiovascular Research Foundation in New York.
The analysis showed that patients with major vascular complications had a lower body surface area, a smaller vessel diameter, and higher sheath-to-femoral-artery and sheath-to-external-iliac-artery ratios. They also were more likely to have insulin-treated diabetes at baseline and to be female.
Female sex was in fact the only independent predictor of major vascular complications after TAVR, according to the analysis, even after adjustment for the sheath-to-femoral-artery ratio. However, female sex was also associated with a reduction in all-cause mortality at 1 year after TAVR, the authors noted. "Thus, the impact of sex on short- and long-term outcomes is complex, and a more detailed analysis is needed," they wrote.
Dr. Généreux said that if this analysis were to be repeated in a few years, he expected the vascular complication rates to fall to a range of 1%-5%, and that fewer dramatic complications would be reported.
Dr. Généreux is a speaker and consultant for Edwards Lifesciences. Several coauthors have received consulting fees, travel reimbursement, and/or grant support from Edwards and other device companies.
Major vascular complications following transfemoral percutaneous aortic valve replacement using first-generation devices were associated with a nearly fivefold increase in 30-day mortality, according to a post hoc analysis of the PARTNER trial.
The complications also were associated with higher 1-year all-cause and cardiac mortality, Dr. Philippe Généreux and his colleagues reported in the Journal of the American College of Cardiology (2012 August [doi: 10.1016/j.jacc.2012.07.003]).
"This is a wonderful technique, but you have to be very careful with patient selection, especially if you’re starting a new program," Dr. Généreux, an interventional cardiologist at Columbia University Medical Center/New York Presbyterian Hospital, said in an interview.
The findings also highlight the importance of having a heart team and appropriate training, said Dr. Généreux, who also practices at the University of Montreal.
He and his colleagues pooled data from the PARTNER (Placement of Aortic Transcatheter Valve) trial’s cohort A (J. Am. Coll. Cardiol. 2012;60:548-58) and cohort B (N. Engl. J. Med. 2010;363:1597-607) for only those patients who had undergone transcatheter aortic valve replacement via the transfemoral approach (242 from cohort A and 177 from cohort B.)
First-generation Edwards Sapien valves were used, via a 22-F or 24-F sheath.
Dr. Généreux said he expected the complication rates to drop in the near future as new-generation devices and smaller delivery systems arrive in the U.S. market and as cardiologists gain more experience performing transcatheter aortic valve replacement (TAVR).
A recent study showed that from 2009 to 2010, major vascular complications decreased from 8% to 1%, and minor vascular complications decreased from 24% to 8% (J. Am. Coll. Cardiol. 2012;59:113-8). The authors noted that "[s]maller sheaths, rigorous angiographic and computed tomographic screening and patient selection, and percutaneous vascular repair techniques were increasingly used over this period."
Results of the current study showed that roughly 15% (64) of the patients had a major vascular complication, and 12% (50) patients had minor complications within 30 days following the procedure.
The most common major vascular complications after TAVR included vascular dissection (63%), vascular perforation (31%), access site hematoma (23%), and retroperitoneal bleeding (10%).
Patients who had a major vascular complication following transfemoral TAVR, compared with those who did not, had a significantly higher rate of 30-day mortality (14% vs. 3%, respectively) and 1-year mortality (39% vs. 23%). The occurrence of a major vascular complication also was associated with significantly higher 30-day rates of transfusions (41% vs. 5%), major bleeding (61% vs. 7%), and renal failure requiring dialysis (8% vs. 2%).
However, "the incidence and impact of major [vascular complications] seem to decrease in a lower-risk population," the authors wrote.
Moreover, patients in cohort B had a higher rate of major and minor vascular complications (32.8%) than did patients in cohort A (23.2%). The difference is partly explained by the fact that patients in cohort B were sicker and, unlike cohort A, had no alternative routes available to them, said Dr. Généreux, who also is the director of the Angiographic Core Laboratory at the Cardiovascular Research Foundation in New York.
The analysis showed that patients with major vascular complications had a lower body surface area, a smaller vessel diameter, and higher sheath-to-femoral-artery and sheath-to-external-iliac-artery ratios. They also were more likely to have insulin-treated diabetes at baseline and to be female.
Female sex was in fact the only independent predictor of major vascular complications after TAVR, according to the analysis, even after adjustment for the sheath-to-femoral-artery ratio. However, female sex was also associated with a reduction in all-cause mortality at 1 year after TAVR, the authors noted. "Thus, the impact of sex on short- and long-term outcomes is complex, and a more detailed analysis is needed," they wrote.
Dr. Généreux said that if this analysis were to be repeated in a few years, he expected the vascular complication rates to fall to a range of 1%-5%, and that fewer dramatic complications would be reported.
Dr. Généreux is a speaker and consultant for Edwards Lifesciences. Several coauthors have received consulting fees, travel reimbursement, and/or grant support from Edwards and other device companies.
FROM THE JOURNAL OF THE AMERICAN COLLEGE OF CARDIOLOGY
Major Finding: Patients
with major vascular complications following TAVR had significantly higher 30-day
mortality (14% vs. 3%) and 1-year mortality (39% vs. 23%) than did those with
no vascular complications.
Data Source: This
was a post hoc analysis of 419 patients who underwent transfemoral TAVR using
first-generation devices in the PARTNER trial.
Disclosures: Dr.
Généreux is a speaker and consultant for Edwards Lifesciences. Several
coauthors have received consulting fees, travel reimbursement, and/or grant
support from Edwards and other device companies.
Bath Salts Seized, But Still No Antidote
The first-ever nationwide strike on the designer drug industry resulted in 90 arrests and the seizing of 5 million drug packets and more than $36 million in cash, according to the U.S. Drug Enforcement Administration.
"Although tremendous progress has been made in legislating and scheduling these dangerous substances, this enforcement action has disrupted the entire illegal industry, from manufacturers to retailers," said DEA Administrator Michele M. Leonhart of the strike, which is called Operation Log Jam.
Synthetic cathinones (with street names like "bath salts" or "plant food") and synthetic cannabinoids (better known as "Spice" and "K2") have become increasingly popular in recent years, especially among young adults.
Many of the drugs come with the disclaimer "Not for human consumption," and they’re marketed at retail stores, head shops, and online.
Some are sold in hookah bars as "hookah cleaner," said Mark Ryan, director of Louisiana Poison Center and a national authority in the field.
"But in fact, they’re incredibly dangerous, with users having unpredictable – and sometimes deadly – reactions to these substances," Ms. Leonhart said in a news conference.
Earlier in July, President Barack Obama signed into law the Food and Drug Administration Safety and Innovation Act, permanently placing 26 of the substances used in designer drugs into schedule I of the Controlled Substances Act.
Yet, the manufacturers continually change the formula to avoid the law.
The sophistication of the designer drug industry has grown over the past 2 years, and such drugs are "an emerging problem," said Ms. Leonhart, "I put them up there with prescription drugs."
In just 1 year, calls to poison centers regarding synthetic cannabinoids has more than doubled, and calls regarding synthetic cathinones has increased 24-fold, she said.
In 2010, poison control centers responded to roughly 3,200 calls related to designer drugs, according to the DEA. In 2011, that number jumped to more than 13,000 calls. Some 60% of the cases involved patients aged 25 years and younger, according to the DEA.
Mr. Ryan of the Louisiana Poison Center said that for the past 3 years, he has spent 2-3 hours every day handling problems related to synthetic drugs. Many of the calls have been from emergency physicians bewildered by their patients’ symptoms.
The drugs have unpredictable effects, and can cause hallucinations, paranoia, coma, and sometimes death. And because of their varying formulations, the drugs have no known antidotes, and their long-term effects are not known.
All physicians can do is "treat the patient, not the poison," said Mr. Ryan.
Meanwhile, there’s no quick, easy test to identify the drugs, said Dr. Cathleen Clancy, an emergency physician and associate medical director of the National Capital Poison Center in Washington, D.C.
"What we get is an agitated patient who says that they have ingested something, and sometimes they don’t even tell you that," she said in an interview.
In one of the few published case studies, Dr. Joanna Cohen and colleagues presented three cases involving teenagers aged 16-18 years who had used synthetic cannabinoids (synthetic marijuana). The investigators cautioned that "given the sensitivity of the developing brain and association between early cannabis use and psychosis, adolescent use of these new synthetic cannabinoids is particularly concerning" (Pediatrics 2012;129:e1064-7).
So what should physicians tell their patients?
"Explain to them that when they take these drugs, they’re being guinea pigs. These are adaptations, and we don’t know how they’ll affect the [users]. I think if people understood that, they’d be less willing to try those drugs," said Dr. Clancy.
Operation Log Jam was conducted by the DEA and U.S. Immigration and Customs Enforcement, with assistance from Internal Revenue Service Criminal Investigations, the U.S. Postal Inspection Service, U.S. Customs and Border Protection, the Federal Bureau of Investigation, the Food and Drug Administration’s Office of Criminal Investigation, and local and state law enforcement officials in more than 109 U.S. cities.
The first-ever nationwide strike on the designer drug industry resulted in 90 arrests and the seizing of 5 million drug packets and more than $36 million in cash, according to the U.S. Drug Enforcement Administration.
"Although tremendous progress has been made in legislating and scheduling these dangerous substances, this enforcement action has disrupted the entire illegal industry, from manufacturers to retailers," said DEA Administrator Michele M. Leonhart of the strike, which is called Operation Log Jam.
Synthetic cathinones (with street names like "bath salts" or "plant food") and synthetic cannabinoids (better known as "Spice" and "K2") have become increasingly popular in recent years, especially among young adults.
Many of the drugs come with the disclaimer "Not for human consumption," and they’re marketed at retail stores, head shops, and online.
Some are sold in hookah bars as "hookah cleaner," said Mark Ryan, director of Louisiana Poison Center and a national authority in the field.
"But in fact, they’re incredibly dangerous, with users having unpredictable – and sometimes deadly – reactions to these substances," Ms. Leonhart said in a news conference.
Earlier in July, President Barack Obama signed into law the Food and Drug Administration Safety and Innovation Act, permanently placing 26 of the substances used in designer drugs into schedule I of the Controlled Substances Act.
Yet, the manufacturers continually change the formula to avoid the law.
The sophistication of the designer drug industry has grown over the past 2 years, and such drugs are "an emerging problem," said Ms. Leonhart, "I put them up there with prescription drugs."
In just 1 year, calls to poison centers regarding synthetic cannabinoids has more than doubled, and calls regarding synthetic cathinones has increased 24-fold, she said.
In 2010, poison control centers responded to roughly 3,200 calls related to designer drugs, according to the DEA. In 2011, that number jumped to more than 13,000 calls. Some 60% of the cases involved patients aged 25 years and younger, according to the DEA.
Mr. Ryan of the Louisiana Poison Center said that for the past 3 years, he has spent 2-3 hours every day handling problems related to synthetic drugs. Many of the calls have been from emergency physicians bewildered by their patients’ symptoms.
The drugs have unpredictable effects, and can cause hallucinations, paranoia, coma, and sometimes death. And because of their varying formulations, the drugs have no known antidotes, and their long-term effects are not known.
All physicians can do is "treat the patient, not the poison," said Mr. Ryan.
Meanwhile, there’s no quick, easy test to identify the drugs, said Dr. Cathleen Clancy, an emergency physician and associate medical director of the National Capital Poison Center in Washington, D.C.
"What we get is an agitated patient who says that they have ingested something, and sometimes they don’t even tell you that," she said in an interview.
In one of the few published case studies, Dr. Joanna Cohen and colleagues presented three cases involving teenagers aged 16-18 years who had used synthetic cannabinoids (synthetic marijuana). The investigators cautioned that "given the sensitivity of the developing brain and association between early cannabis use and psychosis, adolescent use of these new synthetic cannabinoids is particularly concerning" (Pediatrics 2012;129:e1064-7).
So what should physicians tell their patients?
"Explain to them that when they take these drugs, they’re being guinea pigs. These are adaptations, and we don’t know how they’ll affect the [users]. I think if people understood that, they’d be less willing to try those drugs," said Dr. Clancy.
Operation Log Jam was conducted by the DEA and U.S. Immigration and Customs Enforcement, with assistance from Internal Revenue Service Criminal Investigations, the U.S. Postal Inspection Service, U.S. Customs and Border Protection, the Federal Bureau of Investigation, the Food and Drug Administration’s Office of Criminal Investigation, and local and state law enforcement officials in more than 109 U.S. cities.
The first-ever nationwide strike on the designer drug industry resulted in 90 arrests and the seizing of 5 million drug packets and more than $36 million in cash, according to the U.S. Drug Enforcement Administration.
"Although tremendous progress has been made in legislating and scheduling these dangerous substances, this enforcement action has disrupted the entire illegal industry, from manufacturers to retailers," said DEA Administrator Michele M. Leonhart of the strike, which is called Operation Log Jam.
Synthetic cathinones (with street names like "bath salts" or "plant food") and synthetic cannabinoids (better known as "Spice" and "K2") have become increasingly popular in recent years, especially among young adults.
Many of the drugs come with the disclaimer "Not for human consumption," and they’re marketed at retail stores, head shops, and online.
Some are sold in hookah bars as "hookah cleaner," said Mark Ryan, director of Louisiana Poison Center and a national authority in the field.
"But in fact, they’re incredibly dangerous, with users having unpredictable – and sometimes deadly – reactions to these substances," Ms. Leonhart said in a news conference.
Earlier in July, President Barack Obama signed into law the Food and Drug Administration Safety and Innovation Act, permanently placing 26 of the substances used in designer drugs into schedule I of the Controlled Substances Act.
Yet, the manufacturers continually change the formula to avoid the law.
The sophistication of the designer drug industry has grown over the past 2 years, and such drugs are "an emerging problem," said Ms. Leonhart, "I put them up there with prescription drugs."
In just 1 year, calls to poison centers regarding synthetic cannabinoids has more than doubled, and calls regarding synthetic cathinones has increased 24-fold, she said.
In 2010, poison control centers responded to roughly 3,200 calls related to designer drugs, according to the DEA. In 2011, that number jumped to more than 13,000 calls. Some 60% of the cases involved patients aged 25 years and younger, according to the DEA.
Mr. Ryan of the Louisiana Poison Center said that for the past 3 years, he has spent 2-3 hours every day handling problems related to synthetic drugs. Many of the calls have been from emergency physicians bewildered by their patients’ symptoms.
The drugs have unpredictable effects, and can cause hallucinations, paranoia, coma, and sometimes death. And because of their varying formulations, the drugs have no known antidotes, and their long-term effects are not known.
All physicians can do is "treat the patient, not the poison," said Mr. Ryan.
Meanwhile, there’s no quick, easy test to identify the drugs, said Dr. Cathleen Clancy, an emergency physician and associate medical director of the National Capital Poison Center in Washington, D.C.
"What we get is an agitated patient who says that they have ingested something, and sometimes they don’t even tell you that," she said in an interview.
In one of the few published case studies, Dr. Joanna Cohen and colleagues presented three cases involving teenagers aged 16-18 years who had used synthetic cannabinoids (synthetic marijuana). The investigators cautioned that "given the sensitivity of the developing brain and association between early cannabis use and psychosis, adolescent use of these new synthetic cannabinoids is particularly concerning" (Pediatrics 2012;129:e1064-7).
So what should physicians tell their patients?
"Explain to them that when they take these drugs, they’re being guinea pigs. These are adaptations, and we don’t know how they’ll affect the [users]. I think if people understood that, they’d be less willing to try those drugs," said Dr. Clancy.
Operation Log Jam was conducted by the DEA and U.S. Immigration and Customs Enforcement, with assistance from Internal Revenue Service Criminal Investigations, the U.S. Postal Inspection Service, U.S. Customs and Border Protection, the Federal Bureau of Investigation, the Food and Drug Administration’s Office of Criminal Investigation, and local and state law enforcement officials in more than 109 U.S. cities.
3-D TEE More Accurate Than 2-D in Aortic Annulus Measurement
NATIONAL HARBOR – Measurements of aortic annular geometry, valve calcification, and final device position with two- and three-dimensional echocardiography are predictive of increased risk of leakage after transcatheter valve implantation, according to a retrospective study.
The study also showed that 3-D transesophageal echocardiography (3-D TEE) does a better job of measuring the aortic annulus, compared with 2-D TEE, according to a retrospective study.
The annular measurement is critical for optimal valve sizing and prevention of paravalvular aortic regurgitation in patients undergoing transcatheter aortic valve replacement (TAVR).
Paravalvular aortic regurgitation (PAR), is a known complication of TAVR, and according to 2-year analysis of the PARTNER trial, PAR after TAVR was associated with increased late mortality (N. Engl. J. Med. 2012;366:1686-95).
TAVR is in its infancy in the United States, compared with Europe, and experts are studying how and which imaging techniques could yield the best results before, during, and after TAVR (also called TAVI).
"Every center has their preference," said Dr. Praveen Mehrotra, a noninvasive cardiologist and the lead author of the study at Massachusetts General Hospital in Boston. "Some centers use CT and 2-D TEE. At Mass General, we integrate information obtained from 2-D and 3-D TEE."
Meanwhile, the role of 3-D TEE in TAVR hasn’t been adequately explored, added Dr. Mehrotra, who presented his poster at the annual meeting of the American Society of Echocardiography.
Dr. Mehrotra and his colleagues set out to retrospectively identify 2-D and 3-D TEE parameters that could predict significant PAR after TAVR.
They analyzed 2-D and 3-D TEE images from 94 patients undergoing TAVR between June 2008 and December 2011. The images were used to assess three parameters: annulus geometry, aortic valve apparatus calcification, and final device position.
Twenty one of the patients (22%) showed significant PAR after TAVR, but before postdilation.
In 2-D TEE, the annulus geometry was assessed by measuring the largest anteroposterior annulus dimension at the aortic valve hinge points in mid-systole, the authors wrote. Using 3-D TEE, researchers measured or calculated four parameters for the aortic annulus geometry: minor axis, major axis, eccentricity index, and annular area.
The annular dimension measured by 2-D TEE was similar in the PAR (22.8 mm) and No PAR (22.4 mm) groups. But, the 3-D TEE measurements were significantly larger in the PAR group than in the No PAR group, as measured by annular minor axis (23.8 mm vs. 22.7 mm), major axis (27.0 mm vs. 25.3 mm), eccentricity index (0.88 vs. 0.90), and annular area (5.19 cm2 vs. 4.52 cm2), the researchers reported.
The annular-prosthesis incongruence (API) index was also significantly higher in patients with PAR (1.07% vs. 0.93%), "indicating valve undersizing in this group," the authors wrote.
Using 3-D TEE, the researchers identified and graded significant areas of calcification in the aortic valve apparatus, which is also very important before TAVR, said Mehrotra.
The final device position was assessed using 2-D TEE images.
The results showed that higher API index, Aortic Valve Apparatus Calcification score, and final position of the device were predictors of significant PAR after TAVR, with odds ratios of 9.4, 3.6, and 1.2, respectively, the authors reported.
"Our study highlights the ability of 2-D and 3-D TEE for accurate annular sizing and optimal valve positioning during TAVR," they wrote.
The takeaway message, said Dr. Mehrotra in an interview, is that "the role of echo is essential before, during, and after TAVR.
He added that 3-D echocardiography has an emerging role in annular sizing. In particular, annular area by 3-D TEE may be more important than the anteroposterior dimension by 2-D TEE for accurate valve sizing, said Dr. Mehrotra. "Technologies like 3-D TEE and cardiac CT can help with preprocedural planning, but they should be used by people who understand how to use them."
While his study focused on 2-D and 3-D TEE, Dr. Mehrotra said he expected more studies begin comparing cardiac CT and 3-D TEE, which is more like, "comparing apples to apples."
In a discussion on TAVR imaging, Dr. Rebecca T. Hahn, director of interventional echocardiography at Columbia University, New York, said that cardiac CT and echocardiography are complementary. However, CT is less user-dependent, compared with 3-D TEE.
Dr. Mehrotra and Dr. Hahn had no relevant financial disclosures.
NATIONAL HARBOR – Measurements of aortic annular geometry, valve calcification, and final device position with two- and three-dimensional echocardiography are predictive of increased risk of leakage after transcatheter valve implantation, according to a retrospective study.
The study also showed that 3-D transesophageal echocardiography (3-D TEE) does a better job of measuring the aortic annulus, compared with 2-D TEE, according to a retrospective study.
The annular measurement is critical for optimal valve sizing and prevention of paravalvular aortic regurgitation in patients undergoing transcatheter aortic valve replacement (TAVR).
Paravalvular aortic regurgitation (PAR), is a known complication of TAVR, and according to 2-year analysis of the PARTNER trial, PAR after TAVR was associated with increased late mortality (N. Engl. J. Med. 2012;366:1686-95).
TAVR is in its infancy in the United States, compared with Europe, and experts are studying how and which imaging techniques could yield the best results before, during, and after TAVR (also called TAVI).
"Every center has their preference," said Dr. Praveen Mehrotra, a noninvasive cardiologist and the lead author of the study at Massachusetts General Hospital in Boston. "Some centers use CT and 2-D TEE. At Mass General, we integrate information obtained from 2-D and 3-D TEE."
Meanwhile, the role of 3-D TEE in TAVR hasn’t been adequately explored, added Dr. Mehrotra, who presented his poster at the annual meeting of the American Society of Echocardiography.
Dr. Mehrotra and his colleagues set out to retrospectively identify 2-D and 3-D TEE parameters that could predict significant PAR after TAVR.
They analyzed 2-D and 3-D TEE images from 94 patients undergoing TAVR between June 2008 and December 2011. The images were used to assess three parameters: annulus geometry, aortic valve apparatus calcification, and final device position.
Twenty one of the patients (22%) showed significant PAR after TAVR, but before postdilation.
In 2-D TEE, the annulus geometry was assessed by measuring the largest anteroposterior annulus dimension at the aortic valve hinge points in mid-systole, the authors wrote. Using 3-D TEE, researchers measured or calculated four parameters for the aortic annulus geometry: minor axis, major axis, eccentricity index, and annular area.
The annular dimension measured by 2-D TEE was similar in the PAR (22.8 mm) and No PAR (22.4 mm) groups. But, the 3-D TEE measurements were significantly larger in the PAR group than in the No PAR group, as measured by annular minor axis (23.8 mm vs. 22.7 mm), major axis (27.0 mm vs. 25.3 mm), eccentricity index (0.88 vs. 0.90), and annular area (5.19 cm2 vs. 4.52 cm2), the researchers reported.
The annular-prosthesis incongruence (API) index was also significantly higher in patients with PAR (1.07% vs. 0.93%), "indicating valve undersizing in this group," the authors wrote.
Using 3-D TEE, the researchers identified and graded significant areas of calcification in the aortic valve apparatus, which is also very important before TAVR, said Mehrotra.
The final device position was assessed using 2-D TEE images.
The results showed that higher API index, Aortic Valve Apparatus Calcification score, and final position of the device were predictors of significant PAR after TAVR, with odds ratios of 9.4, 3.6, and 1.2, respectively, the authors reported.
"Our study highlights the ability of 2-D and 3-D TEE for accurate annular sizing and optimal valve positioning during TAVR," they wrote.
The takeaway message, said Dr. Mehrotra in an interview, is that "the role of echo is essential before, during, and after TAVR.
He added that 3-D echocardiography has an emerging role in annular sizing. In particular, annular area by 3-D TEE may be more important than the anteroposterior dimension by 2-D TEE for accurate valve sizing, said Dr. Mehrotra. "Technologies like 3-D TEE and cardiac CT can help with preprocedural planning, but they should be used by people who understand how to use them."
While his study focused on 2-D and 3-D TEE, Dr. Mehrotra said he expected more studies begin comparing cardiac CT and 3-D TEE, which is more like, "comparing apples to apples."
In a discussion on TAVR imaging, Dr. Rebecca T. Hahn, director of interventional echocardiography at Columbia University, New York, said that cardiac CT and echocardiography are complementary. However, CT is less user-dependent, compared with 3-D TEE.
Dr. Mehrotra and Dr. Hahn had no relevant financial disclosures.
NATIONAL HARBOR – Measurements of aortic annular geometry, valve calcification, and final device position with two- and three-dimensional echocardiography are predictive of increased risk of leakage after transcatheter valve implantation, according to a retrospective study.
The study also showed that 3-D transesophageal echocardiography (3-D TEE) does a better job of measuring the aortic annulus, compared with 2-D TEE, according to a retrospective study.
The annular measurement is critical for optimal valve sizing and prevention of paravalvular aortic regurgitation in patients undergoing transcatheter aortic valve replacement (TAVR).
Paravalvular aortic regurgitation (PAR), is a known complication of TAVR, and according to 2-year analysis of the PARTNER trial, PAR after TAVR was associated with increased late mortality (N. Engl. J. Med. 2012;366:1686-95).
TAVR is in its infancy in the United States, compared with Europe, and experts are studying how and which imaging techniques could yield the best results before, during, and after TAVR (also called TAVI).
"Every center has their preference," said Dr. Praveen Mehrotra, a noninvasive cardiologist and the lead author of the study at Massachusetts General Hospital in Boston. "Some centers use CT and 2-D TEE. At Mass General, we integrate information obtained from 2-D and 3-D TEE."
Meanwhile, the role of 3-D TEE in TAVR hasn’t been adequately explored, added Dr. Mehrotra, who presented his poster at the annual meeting of the American Society of Echocardiography.
Dr. Mehrotra and his colleagues set out to retrospectively identify 2-D and 3-D TEE parameters that could predict significant PAR after TAVR.
They analyzed 2-D and 3-D TEE images from 94 patients undergoing TAVR between June 2008 and December 2011. The images were used to assess three parameters: annulus geometry, aortic valve apparatus calcification, and final device position.
Twenty one of the patients (22%) showed significant PAR after TAVR, but before postdilation.
In 2-D TEE, the annulus geometry was assessed by measuring the largest anteroposterior annulus dimension at the aortic valve hinge points in mid-systole, the authors wrote. Using 3-D TEE, researchers measured or calculated four parameters for the aortic annulus geometry: minor axis, major axis, eccentricity index, and annular area.
The annular dimension measured by 2-D TEE was similar in the PAR (22.8 mm) and No PAR (22.4 mm) groups. But, the 3-D TEE measurements were significantly larger in the PAR group than in the No PAR group, as measured by annular minor axis (23.8 mm vs. 22.7 mm), major axis (27.0 mm vs. 25.3 mm), eccentricity index (0.88 vs. 0.90), and annular area (5.19 cm2 vs. 4.52 cm2), the researchers reported.
The annular-prosthesis incongruence (API) index was also significantly higher in patients with PAR (1.07% vs. 0.93%), "indicating valve undersizing in this group," the authors wrote.
Using 3-D TEE, the researchers identified and graded significant areas of calcification in the aortic valve apparatus, which is also very important before TAVR, said Mehrotra.
The final device position was assessed using 2-D TEE images.
The results showed that higher API index, Aortic Valve Apparatus Calcification score, and final position of the device were predictors of significant PAR after TAVR, with odds ratios of 9.4, 3.6, and 1.2, respectively, the authors reported.
"Our study highlights the ability of 2-D and 3-D TEE for accurate annular sizing and optimal valve positioning during TAVR," they wrote.
The takeaway message, said Dr. Mehrotra in an interview, is that "the role of echo is essential before, during, and after TAVR.
He added that 3-D echocardiography has an emerging role in annular sizing. In particular, annular area by 3-D TEE may be more important than the anteroposterior dimension by 2-D TEE for accurate valve sizing, said Dr. Mehrotra. "Technologies like 3-D TEE and cardiac CT can help with preprocedural planning, but they should be used by people who understand how to use them."
While his study focused on 2-D and 3-D TEE, Dr. Mehrotra said he expected more studies begin comparing cardiac CT and 3-D TEE, which is more like, "comparing apples to apples."
In a discussion on TAVR imaging, Dr. Rebecca T. Hahn, director of interventional echocardiography at Columbia University, New York, said that cardiac CT and echocardiography are complementary. However, CT is less user-dependent, compared with 3-D TEE.
Dr. Mehrotra and Dr. Hahn had no relevant financial disclosures.
AT THE ANNUAL MEETING OF THE AMERICAN SOCIETY OF ECHOCARDIOGRAPHY
Major Finding: Higher API index, Aortic Valve Apparatus Calcification score, and final position of the replacement valve as measured by 3-D and 2-D transesophageal echocardiography were predictors of significant PAR after TAVR, with odds ratios of 9.4, 3.6, and 1.2, respectively.
Data Source: Retrospective analysis of 2-D and 3-D TEE images from 94 patients undergoing TAVR between June 2008 and December 2011.
Disclosures: Dr. Mehrotra and Dr. Hahn had no relevant financial disclosures.
Societies Release Update to 2001 Cath Lab Standards
An updated standards document for cardiac catheterization laboratories lifts almost all restrictions on the types of patients eligible for diagnostic procedures in laboratories without cardiovascular surgical backup, details the role of hybrid cath labs, and emphasizes quality improvement and assurance programs.
On May 8, the Society for Cardiovascular Angiography and Interventions (SCAI) and the American College of Cardiology Foundation (ACCF) released an update to the 2001 (J. Am. Coll. Cardiol. 2001;37:2170-214) Cardiac Catheterization Laboratory Standards. (J. Am. Coll. Cardiol. 2012;59:2201-2305)
"There have been a lot of changes since 2001, some of them dramatic," Dr. Thomas M. Bashore, chair of the writing committee and clinical chief of cardiology at Duke University, Durham, N.C., said in a statement. "This document sets the stage for what’s really happening in cath labs today and updates everyone on accepted quality standards and best practices."
Over the last decade, advancements in technology have changed imaging and reporting systems, the authors wrote. Due to lower risk of invasive procedures, there are now cardiac cath labs without on-site surgical backup, a trend that highlights the importance of quality assurance and quality improvement initiatives. Meanwhile, the cath lab has become "multipurpose suite" for diagnostic and therapeutic procedures for pediatric and adult patients, the authors noted. And while hybrid cath labs are not a new phenomenon, more medical centers are looking into creating them as they consider starting transcatheter aortic valve replacement (TAVR) programs.
The consensus document details examples of procedures suitable for hybrid cath labs; staffing, location, and equipment requirements; room, floor, and ceiling design; and audio/video input and output needs. The document also addresses several issues including:
• Cardiac cath labs without on-site cardiac surgical back-up. The document proposes requirements for establishing off-site surgical back-up, but lifts many prior limitations on the types of patients eligible for diagnostic procedures. Specifically, age, heart failure status, severity of stress test abnormalities, left ventricular function, and valve disease no longer restrict patients from receiving diagnostic procedures at stand-alone cath labs. Therapeutic procedures that should still be done only in facilities with cardiovascular surgical back-up include those in adults with congenital heart disease and in children. Primary percutaneous coronary intervention in patients with acute coronary syndrome can be performed in a stand-alone cath lab, provided that the facility complies "with all current guidelines on the establishment of such a program."
• Quality assurance and improvement initiatives. The document details elements of such initiatives, such as benchmarking against peers, participation in a national clinical database, and monitoring the procedures’ quality and appropriateness.
• X-ray imaging. The document summarizes major changes in x-ray imaging that have occurred since the last statement in 2001, in addition to measurement and prevention of exposure to radiation.
• Training requirements. The document summarizes the already-established requirements for training. It also recommends "that both training and practice activity associated with structural heart disease intervention be concentrated among a limited number of laboratories and operators with a particular interest in these procedures."
"What this document does is put everything in one paper," said Dr. Bashore in an interview. "[Cardiologists] can look at their lab and how they’re running their lab, and then compare it to some national standards, and that’s really what this is meant to do, and it’s really trying to get them the tools to do it, so that they can ensure that they’re up to date."
Dr. Charles E. Chambers, who represented SCAI on the document’s writing committee, said that the document is also a resource. "Pay-for performance is what is potentially down the road, if we aren’t already there. And issues such as benchmarking and risk factor stratification are important in quality programs and tracking outcomes, and that’s what’s covered nicely in this paper," said Dr. Chambers, of the Penn State Hershey Heart and Vascular Institute, Hershey, Pa.
But will the document be embraced by practicing physicians? "This document certainly shows best practices, and I think that’s what [physicians] are interested in. We want to give best patient care, and there are enough questions out there that I think this will be embraced as a reference when questions arise," said Dr. Chambers.
The authors’ disclosures are listed at in the consensus document. Dr. Bashore and Dr. Chambers had no relevant disclosures.
Stand-alone cath labs have changed the dynamic in the never ending competition among hospitals and physicians for lucrative cardiovascular services. Downtown teaching hospitals no longer monopolize the interventional cardiology business, and, in fact, in many places have been losing market share rapidly to outlying community hospitals where these procedures can be done more cheaply and with seemingly no increase in risk to the patient in spite of the lack of cardiac surgery backup.
As these centers continue to proliferate it is important to set standards to ensure quality and safety - the intent I’m sure of the SCAI and ACCF document. However, will the benefit of high-quality, cost effective care be realized? Will the proliferation of these labs decrease costs or lead to an increase in procedures and in doing so, drive up the costs of care? Will they perform other procedures to increase revenue and will these non cardiac procedures meet the same quality standard as coronary interventions? And what effect will they have on the education of the next generation of interventional cardiologists if the downtown teaching centers lose too much volume? Time will tell, but based on past experience I would be concerned that cost will increase and maintaining quality may prove to be an elusive goal.
Frank Pomposelli, M.D., is chairman of surgery at St. Elizabeth's Medical Center, Boston. He is also an associate medical editor for Vascular Specialist.
Stand-alone cath labs have changed the dynamic in the never ending competition among hospitals and physicians for lucrative cardiovascular services. Downtown teaching hospitals no longer monopolize the interventional cardiology business, and, in fact, in many places have been losing market share rapidly to outlying community hospitals where these procedures can be done more cheaply and with seemingly no increase in risk to the patient in spite of the lack of cardiac surgery backup.
As these centers continue to proliferate it is important to set standards to ensure quality and safety - the intent I’m sure of the SCAI and ACCF document. However, will the benefit of high-quality, cost effective care be realized? Will the proliferation of these labs decrease costs or lead to an increase in procedures and in doing so, drive up the costs of care? Will they perform other procedures to increase revenue and will these non cardiac procedures meet the same quality standard as coronary interventions? And what effect will they have on the education of the next generation of interventional cardiologists if the downtown teaching centers lose too much volume? Time will tell, but based on past experience I would be concerned that cost will increase and maintaining quality may prove to be an elusive goal.
Frank Pomposelli, M.D., is chairman of surgery at St. Elizabeth's Medical Center, Boston. He is also an associate medical editor for Vascular Specialist.
Stand-alone cath labs have changed the dynamic in the never ending competition among hospitals and physicians for lucrative cardiovascular services. Downtown teaching hospitals no longer monopolize the interventional cardiology business, and, in fact, in many places have been losing market share rapidly to outlying community hospitals where these procedures can be done more cheaply and with seemingly no increase in risk to the patient in spite of the lack of cardiac surgery backup.
As these centers continue to proliferate it is important to set standards to ensure quality and safety - the intent I’m sure of the SCAI and ACCF document. However, will the benefit of high-quality, cost effective care be realized? Will the proliferation of these labs decrease costs or lead to an increase in procedures and in doing so, drive up the costs of care? Will they perform other procedures to increase revenue and will these non cardiac procedures meet the same quality standard as coronary interventions? And what effect will they have on the education of the next generation of interventional cardiologists if the downtown teaching centers lose too much volume? Time will tell, but based on past experience I would be concerned that cost will increase and maintaining quality may prove to be an elusive goal.
Frank Pomposelli, M.D., is chairman of surgery at St. Elizabeth's Medical Center, Boston. He is also an associate medical editor for Vascular Specialist.
An updated standards document for cardiac catheterization laboratories lifts almost all restrictions on the types of patients eligible for diagnostic procedures in laboratories without cardiovascular surgical backup, details the role of hybrid cath labs, and emphasizes quality improvement and assurance programs.
On May 8, the Society for Cardiovascular Angiography and Interventions (SCAI) and the American College of Cardiology Foundation (ACCF) released an update to the 2001 (J. Am. Coll. Cardiol. 2001;37:2170-214) Cardiac Catheterization Laboratory Standards. (J. Am. Coll. Cardiol. 2012;59:2201-2305)
"There have been a lot of changes since 2001, some of them dramatic," Dr. Thomas M. Bashore, chair of the writing committee and clinical chief of cardiology at Duke University, Durham, N.C., said in a statement. "This document sets the stage for what’s really happening in cath labs today and updates everyone on accepted quality standards and best practices."
Over the last decade, advancements in technology have changed imaging and reporting systems, the authors wrote. Due to lower risk of invasive procedures, there are now cardiac cath labs without on-site surgical backup, a trend that highlights the importance of quality assurance and quality improvement initiatives. Meanwhile, the cath lab has become "multipurpose suite" for diagnostic and therapeutic procedures for pediatric and adult patients, the authors noted. And while hybrid cath labs are not a new phenomenon, more medical centers are looking into creating them as they consider starting transcatheter aortic valve replacement (TAVR) programs.
The consensus document details examples of procedures suitable for hybrid cath labs; staffing, location, and equipment requirements; room, floor, and ceiling design; and audio/video input and output needs. The document also addresses several issues including:
• Cardiac cath labs without on-site cardiac surgical back-up. The document proposes requirements for establishing off-site surgical back-up, but lifts many prior limitations on the types of patients eligible for diagnostic procedures. Specifically, age, heart failure status, severity of stress test abnormalities, left ventricular function, and valve disease no longer restrict patients from receiving diagnostic procedures at stand-alone cath labs. Therapeutic procedures that should still be done only in facilities with cardiovascular surgical back-up include those in adults with congenital heart disease and in children. Primary percutaneous coronary intervention in patients with acute coronary syndrome can be performed in a stand-alone cath lab, provided that the facility complies "with all current guidelines on the establishment of such a program."
• Quality assurance and improvement initiatives. The document details elements of such initiatives, such as benchmarking against peers, participation in a national clinical database, and monitoring the procedures’ quality and appropriateness.
• X-ray imaging. The document summarizes major changes in x-ray imaging that have occurred since the last statement in 2001, in addition to measurement and prevention of exposure to radiation.
• Training requirements. The document summarizes the already-established requirements for training. It also recommends "that both training and practice activity associated with structural heart disease intervention be concentrated among a limited number of laboratories and operators with a particular interest in these procedures."
"What this document does is put everything in one paper," said Dr. Bashore in an interview. "[Cardiologists] can look at their lab and how they’re running their lab, and then compare it to some national standards, and that’s really what this is meant to do, and it’s really trying to get them the tools to do it, so that they can ensure that they’re up to date."
Dr. Charles E. Chambers, who represented SCAI on the document’s writing committee, said that the document is also a resource. "Pay-for performance is what is potentially down the road, if we aren’t already there. And issues such as benchmarking and risk factor stratification are important in quality programs and tracking outcomes, and that’s what’s covered nicely in this paper," said Dr. Chambers, of the Penn State Hershey Heart and Vascular Institute, Hershey, Pa.
But will the document be embraced by practicing physicians? "This document certainly shows best practices, and I think that’s what [physicians] are interested in. We want to give best patient care, and there are enough questions out there that I think this will be embraced as a reference when questions arise," said Dr. Chambers.
The authors’ disclosures are listed at in the consensus document. Dr. Bashore and Dr. Chambers had no relevant disclosures.
An updated standards document for cardiac catheterization laboratories lifts almost all restrictions on the types of patients eligible for diagnostic procedures in laboratories without cardiovascular surgical backup, details the role of hybrid cath labs, and emphasizes quality improvement and assurance programs.
On May 8, the Society for Cardiovascular Angiography and Interventions (SCAI) and the American College of Cardiology Foundation (ACCF) released an update to the 2001 (J. Am. Coll. Cardiol. 2001;37:2170-214) Cardiac Catheterization Laboratory Standards. (J. Am. Coll. Cardiol. 2012;59:2201-2305)
"There have been a lot of changes since 2001, some of them dramatic," Dr. Thomas M. Bashore, chair of the writing committee and clinical chief of cardiology at Duke University, Durham, N.C., said in a statement. "This document sets the stage for what’s really happening in cath labs today and updates everyone on accepted quality standards and best practices."
Over the last decade, advancements in technology have changed imaging and reporting systems, the authors wrote. Due to lower risk of invasive procedures, there are now cardiac cath labs without on-site surgical backup, a trend that highlights the importance of quality assurance and quality improvement initiatives. Meanwhile, the cath lab has become "multipurpose suite" for diagnostic and therapeutic procedures for pediatric and adult patients, the authors noted. And while hybrid cath labs are not a new phenomenon, more medical centers are looking into creating them as they consider starting transcatheter aortic valve replacement (TAVR) programs.
The consensus document details examples of procedures suitable for hybrid cath labs; staffing, location, and equipment requirements; room, floor, and ceiling design; and audio/video input and output needs. The document also addresses several issues including:
• Cardiac cath labs without on-site cardiac surgical back-up. The document proposes requirements for establishing off-site surgical back-up, but lifts many prior limitations on the types of patients eligible for diagnostic procedures. Specifically, age, heart failure status, severity of stress test abnormalities, left ventricular function, and valve disease no longer restrict patients from receiving diagnostic procedures at stand-alone cath labs. Therapeutic procedures that should still be done only in facilities with cardiovascular surgical back-up include those in adults with congenital heart disease and in children. Primary percutaneous coronary intervention in patients with acute coronary syndrome can be performed in a stand-alone cath lab, provided that the facility complies "with all current guidelines on the establishment of such a program."
• Quality assurance and improvement initiatives. The document details elements of such initiatives, such as benchmarking against peers, participation in a national clinical database, and monitoring the procedures’ quality and appropriateness.
• X-ray imaging. The document summarizes major changes in x-ray imaging that have occurred since the last statement in 2001, in addition to measurement and prevention of exposure to radiation.
• Training requirements. The document summarizes the already-established requirements for training. It also recommends "that both training and practice activity associated with structural heart disease intervention be concentrated among a limited number of laboratories and operators with a particular interest in these procedures."
"What this document does is put everything in one paper," said Dr. Bashore in an interview. "[Cardiologists] can look at their lab and how they’re running their lab, and then compare it to some national standards, and that’s really what this is meant to do, and it’s really trying to get them the tools to do it, so that they can ensure that they’re up to date."
Dr. Charles E. Chambers, who represented SCAI on the document’s writing committee, said that the document is also a resource. "Pay-for performance is what is potentially down the road, if we aren’t already there. And issues such as benchmarking and risk factor stratification are important in quality programs and tracking outcomes, and that’s what’s covered nicely in this paper," said Dr. Chambers, of the Penn State Hershey Heart and Vascular Institute, Hershey, Pa.
But will the document be embraced by practicing physicians? "This document certainly shows best practices, and I think that’s what [physicians] are interested in. We want to give best patient care, and there are enough questions out there that I think this will be embraced as a reference when questions arise," said Dr. Chambers.
The authors’ disclosures are listed at in the consensus document. Dr. Bashore and Dr. Chambers had no relevant disclosures.
TAVR at 10: A Review
Transcatheter aortic valve replacement, or TAVR, is a little over 10 years old, and although it was approved in the United States only last year, there have been more than 50,000 implants in 40 countries, according to a recent review published in the Journal of American College of Cardiology (J Am Coll Cardiol, doi:10.1016/j.jacc.2012.01.071).
The Canadian authors, Dr. John G. Webb and Dr. David A. Wood, provide a meticulous and comprehensive review of the procedure, covering the current and up-and-coming valves, patient selection and evaluation, routes of implantation, and the risks of the procedure.
Throughout the article, the authors point out that increased level of experience, use of lower profile delivery systems, and thorough screening and imaging can reduce the risk of complications.
The SAPIEN transcatheter heart valve, made by Edwards Lifesciences, is the only valve currently approved in the United States for inoperable, high-risk patients.
More recently, an advisory panel to the Food and Drug Administration recommended the approval of the valve for high-risk patients who are operable, but it’s yet to be seen whether the agency will approve the valve for that indication.
Meanwhile, some reports show that TAVR will be the main driving force behind the U.S. heart valve market, projected to reach $1.5 billion by 2016.
"It is possible that, with time, TAVR will become a preferred option for a much broader group of patients," the authors conclude. However, a major concern remains: "When are patients too ill, frail, or old to gain significant benefit in terms of duration or quality of life?"
See the Storify below of the arrival and approval of the SAPIEN valve in the United States:
Transcatheter aortic valve replacement, or TAVR, is a little over 10 years old, and although it was approved in the United States only last year, there have been more than 50,000 implants in 40 countries, according to a recent review published in the Journal of American College of Cardiology (J Am Coll Cardiol, doi:10.1016/j.jacc.2012.01.071).
The Canadian authors, Dr. John G. Webb and Dr. David A. Wood, provide a meticulous and comprehensive review of the procedure, covering the current and up-and-coming valves, patient selection and evaluation, routes of implantation, and the risks of the procedure.
Throughout the article, the authors point out that increased level of experience, use of lower profile delivery systems, and thorough screening and imaging can reduce the risk of complications.
The SAPIEN transcatheter heart valve, made by Edwards Lifesciences, is the only valve currently approved in the United States for inoperable, high-risk patients.
More recently, an advisory panel to the Food and Drug Administration recommended the approval of the valve for high-risk patients who are operable, but it’s yet to be seen whether the agency will approve the valve for that indication.
Meanwhile, some reports show that TAVR will be the main driving force behind the U.S. heart valve market, projected to reach $1.5 billion by 2016.
"It is possible that, with time, TAVR will become a preferred option for a much broader group of patients," the authors conclude. However, a major concern remains: "When are patients too ill, frail, or old to gain significant benefit in terms of duration or quality of life?"
See the Storify below of the arrival and approval of the SAPIEN valve in the United States:
Transcatheter aortic valve replacement, or TAVR, is a little over 10 years old, and although it was approved in the United States only last year, there have been more than 50,000 implants in 40 countries, according to a recent review published in the Journal of American College of Cardiology (J Am Coll Cardiol, doi:10.1016/j.jacc.2012.01.071).
The Canadian authors, Dr. John G. Webb and Dr. David A. Wood, provide a meticulous and comprehensive review of the procedure, covering the current and up-and-coming valves, patient selection and evaluation, routes of implantation, and the risks of the procedure.
Throughout the article, the authors point out that increased level of experience, use of lower profile delivery systems, and thorough screening and imaging can reduce the risk of complications.
The SAPIEN transcatheter heart valve, made by Edwards Lifesciences, is the only valve currently approved in the United States for inoperable, high-risk patients.
More recently, an advisory panel to the Food and Drug Administration recommended the approval of the valve for high-risk patients who are operable, but it’s yet to be seen whether the agency will approve the valve for that indication.
Meanwhile, some reports show that TAVR will be the main driving force behind the U.S. heart valve market, projected to reach $1.5 billion by 2016.
"It is possible that, with time, TAVR will become a preferred option for a much broader group of patients," the authors conclude. However, a major concern remains: "When are patients too ill, frail, or old to gain significant benefit in terms of duration or quality of life?"
See the Storify below of the arrival and approval of the SAPIEN valve in the United States: