User login
New analysis bolsters metformin as first line in type 2 diabetes
Patients with type 2 diabetes treated with metformin as a monotherapy are at a decreased risk for cardiovascular mortality when compared with those on sulfonylurea monotherapy, according to a report in the Annals of Internal Medicine.
Dr. Nisa M. Maruthur and her associates conducted an update of a previous systematic literature review and meta-analysis to assess the comparative effectiveness and safety of metformin monotherapy and combination therapies including metformin with nonmetformin monotherapies in patients with type 2 diabetes. They focused on original, adult human experimental, and observational studies (Ann Intern Med. 2016 Apr 19. doi: 10.7326/M15-2650).
Dr. Maruthur and colleagues identified a total of 19,423 articles, of which 234 were found to meet the study inclusion criteria. The majority of the included studies were randomized, controlled trials, with 98 assessing all-cause mortality and macro- and microvascular outcomes.
On the basis of consistent findings from two randomized, controlled trials including 3,199 total participants (ADOPT and SPREAD-DIMCAD), a lower risk for cardiovascular mortality was found for metformin monotherapy versus sulfonylurea monotherapy. For those on metformin monotherapy, 2 of the 1,454 patients had a fatal MI and 7 of 156 patients died from cardiovascular disease. Three of 1,441 patients on monotherapy with a sulfonylurea had a fatal MI and 11 of 148 patients died from cardiovascular disease.
The evidence from this systematic review supports current type 2 diabetes guidelines that recommend metformin as the first-line agent to treat adults, based on its beneficial effects on hemoglobin A1c, weight, and cardiovascular mortality versus sulfonylureas, as well as its relative safety profile, Dr. Maruthur of the department of medicine and epidemiology at Johns Hopkins University, Baltimore, and her coinvestigators said.
The study was funded by Agency for Healthcare Research and Quality. Several of the coauthors disclosed contracts with the funding source during the conduct of the study. The remaining coauthors disclosed no conflicts of interest.
Patients with type 2 diabetes treated with metformin as a monotherapy are at a decreased risk for cardiovascular mortality when compared with those on sulfonylurea monotherapy, according to a report in the Annals of Internal Medicine.
Dr. Nisa M. Maruthur and her associates conducted an update of a previous systematic literature review and meta-analysis to assess the comparative effectiveness and safety of metformin monotherapy and combination therapies including metformin with nonmetformin monotherapies in patients with type 2 diabetes. They focused on original, adult human experimental, and observational studies (Ann Intern Med. 2016 Apr 19. doi: 10.7326/M15-2650).
Dr. Maruthur and colleagues identified a total of 19,423 articles, of which 234 were found to meet the study inclusion criteria. The majority of the included studies were randomized, controlled trials, with 98 assessing all-cause mortality and macro- and microvascular outcomes.
On the basis of consistent findings from two randomized, controlled trials including 3,199 total participants (ADOPT and SPREAD-DIMCAD), a lower risk for cardiovascular mortality was found for metformin monotherapy versus sulfonylurea monotherapy. For those on metformin monotherapy, 2 of the 1,454 patients had a fatal MI and 7 of 156 patients died from cardiovascular disease. Three of 1,441 patients on monotherapy with a sulfonylurea had a fatal MI and 11 of 148 patients died from cardiovascular disease.
The evidence from this systematic review supports current type 2 diabetes guidelines that recommend metformin as the first-line agent to treat adults, based on its beneficial effects on hemoglobin A1c, weight, and cardiovascular mortality versus sulfonylureas, as well as its relative safety profile, Dr. Maruthur of the department of medicine and epidemiology at Johns Hopkins University, Baltimore, and her coinvestigators said.
The study was funded by Agency for Healthcare Research and Quality. Several of the coauthors disclosed contracts with the funding source during the conduct of the study. The remaining coauthors disclosed no conflicts of interest.
Patients with type 2 diabetes treated with metformin as a monotherapy are at a decreased risk for cardiovascular mortality when compared with those on sulfonylurea monotherapy, according to a report in the Annals of Internal Medicine.
Dr. Nisa M. Maruthur and her associates conducted an update of a previous systematic literature review and meta-analysis to assess the comparative effectiveness and safety of metformin monotherapy and combination therapies including metformin with nonmetformin monotherapies in patients with type 2 diabetes. They focused on original, adult human experimental, and observational studies (Ann Intern Med. 2016 Apr 19. doi: 10.7326/M15-2650).
Dr. Maruthur and colleagues identified a total of 19,423 articles, of which 234 were found to meet the study inclusion criteria. The majority of the included studies were randomized, controlled trials, with 98 assessing all-cause mortality and macro- and microvascular outcomes.
On the basis of consistent findings from two randomized, controlled trials including 3,199 total participants (ADOPT and SPREAD-DIMCAD), a lower risk for cardiovascular mortality was found for metformin monotherapy versus sulfonylurea monotherapy. For those on metformin monotherapy, 2 of the 1,454 patients had a fatal MI and 7 of 156 patients died from cardiovascular disease. Three of 1,441 patients on monotherapy with a sulfonylurea had a fatal MI and 11 of 148 patients died from cardiovascular disease.
The evidence from this systematic review supports current type 2 diabetes guidelines that recommend metformin as the first-line agent to treat adults, based on its beneficial effects on hemoglobin A1c, weight, and cardiovascular mortality versus sulfonylureas, as well as its relative safety profile, Dr. Maruthur of the department of medicine and epidemiology at Johns Hopkins University, Baltimore, and her coinvestigators said.
The study was funded by Agency for Healthcare Research and Quality. Several of the coauthors disclosed contracts with the funding source during the conduct of the study. The remaining coauthors disclosed no conflicts of interest.
Key clinical point: Clinical trial data support the use of metformin monotherapy, as opposed to sulfonylurea monotherapy, as a first-line therapy for type 2 diabetes.
Major finding: Two randomized, controlled trials showed a lower risk for cardiovascular mortality with metformin versus a sulfonylurea.
Data sources: Literature review and meta-analysis involving 204 studies, two of which were randomized, controlled trials comparing the effects of metformin and sulfonylurea monotherapy on cardiovascular mortality among patients with type 2 diabetes.
Disclosures: The study was funded by Agency for Healthcare Research and Quality. Several of the coauthors disclosed contracts with the funding source during the conduct of the study. The remaining coauthors disclosed no conflicts of interest.
C. difficile transmission linked to antibiotic use in long-term care facilities
Antibiotic use may drive Clostridium difficile transmission within long-term care facilities, according to the results of a recent study published in Annals of Internal Medicine.
Dr. Kevin A. Brown of Public Health Ontario in Toronto, and his coauthors, assessed long-term care–onset C. difficile infection in the largest and most comprehensive study of its kind to date. The retrospective study included 86 Veterans Health Administration health care regions and examined long-term care residents from January 2006 through December 2012. Study results indicated large variations in regional rates of C. difficile infection, regional antibiotic use, and importation of cases of acute care C. difficile infection (Ann Intern Med. 2016 Apr 19. doi: 10.7326/M15-1754).
The total study population included 6,012 cases with a C. difficile infection incidence of 3.7 cases per 10,000 resident days. The regional variability in the incidence of long-term care–onset C. difficile infection was found to be attributable in large part (75%) to antibiotic use and importation from acute care facilities. The data also showed that regional differences in both the prescription of antibiotics and the individual receipt of antibiotics contributed to resident risk, suggesting increased risk for both acquiring and spreading C. difficile.
A potential mechanism offered by the authors for the transmission of C. difficile in facilities with high antibiotic use may be the increased prevalence of residents with asymptomatic C. difficile colonization who become more effective at shedding C. difficile spores when exposed to antibiotics.
Dr. Brown and his colleagues said that efforts designed to reduce C. difficile infection in long-term care should focus on the reduction of total antibiotic use, and that infection control teams may need to take special measures in long-term care facilities that receive residents from hospitals with elevated rates of C. difficile infection.
This study was funded by the U.S. Department of Veterans Affairs and the Centers for Disease Control and Prevention. Dr. Brown reported grants from AstraZeneca outside the submitted work, and another coauthor disclosed grant support from the funding source during the conduct of the study. The remaining coauthors disclosed no conflicts of interest.
Antibiotic use may drive Clostridium difficile transmission within long-term care facilities, according to the results of a recent study published in Annals of Internal Medicine.
Dr. Kevin A. Brown of Public Health Ontario in Toronto, and his coauthors, assessed long-term care–onset C. difficile infection in the largest and most comprehensive study of its kind to date. The retrospective study included 86 Veterans Health Administration health care regions and examined long-term care residents from January 2006 through December 2012. Study results indicated large variations in regional rates of C. difficile infection, regional antibiotic use, and importation of cases of acute care C. difficile infection (Ann Intern Med. 2016 Apr 19. doi: 10.7326/M15-1754).
The total study population included 6,012 cases with a C. difficile infection incidence of 3.7 cases per 10,000 resident days. The regional variability in the incidence of long-term care–onset C. difficile infection was found to be attributable in large part (75%) to antibiotic use and importation from acute care facilities. The data also showed that regional differences in both the prescription of antibiotics and the individual receipt of antibiotics contributed to resident risk, suggesting increased risk for both acquiring and spreading C. difficile.
A potential mechanism offered by the authors for the transmission of C. difficile in facilities with high antibiotic use may be the increased prevalence of residents with asymptomatic C. difficile colonization who become more effective at shedding C. difficile spores when exposed to antibiotics.
Dr. Brown and his colleagues said that efforts designed to reduce C. difficile infection in long-term care should focus on the reduction of total antibiotic use, and that infection control teams may need to take special measures in long-term care facilities that receive residents from hospitals with elevated rates of C. difficile infection.
This study was funded by the U.S. Department of Veterans Affairs and the Centers for Disease Control and Prevention. Dr. Brown reported grants from AstraZeneca outside the submitted work, and another coauthor disclosed grant support from the funding source during the conduct of the study. The remaining coauthors disclosed no conflicts of interest.
Antibiotic use may drive Clostridium difficile transmission within long-term care facilities, according to the results of a recent study published in Annals of Internal Medicine.
Dr. Kevin A. Brown of Public Health Ontario in Toronto, and his coauthors, assessed long-term care–onset C. difficile infection in the largest and most comprehensive study of its kind to date. The retrospective study included 86 Veterans Health Administration health care regions and examined long-term care residents from January 2006 through December 2012. Study results indicated large variations in regional rates of C. difficile infection, regional antibiotic use, and importation of cases of acute care C. difficile infection (Ann Intern Med. 2016 Apr 19. doi: 10.7326/M15-1754).
The total study population included 6,012 cases with a C. difficile infection incidence of 3.7 cases per 10,000 resident days. The regional variability in the incidence of long-term care–onset C. difficile infection was found to be attributable in large part (75%) to antibiotic use and importation from acute care facilities. The data also showed that regional differences in both the prescription of antibiotics and the individual receipt of antibiotics contributed to resident risk, suggesting increased risk for both acquiring and spreading C. difficile.
A potential mechanism offered by the authors for the transmission of C. difficile in facilities with high antibiotic use may be the increased prevalence of residents with asymptomatic C. difficile colonization who become more effective at shedding C. difficile spores when exposed to antibiotics.
Dr. Brown and his colleagues said that efforts designed to reduce C. difficile infection in long-term care should focus on the reduction of total antibiotic use, and that infection control teams may need to take special measures in long-term care facilities that receive residents from hospitals with elevated rates of C. difficile infection.
This study was funded by the U.S. Department of Veterans Affairs and the Centers for Disease Control and Prevention. Dr. Brown reported grants from AstraZeneca outside the submitted work, and another coauthor disclosed grant support from the funding source during the conduct of the study. The remaining coauthors disclosed no conflicts of interest.
FROM ANNALS OF INTERNAL MEDICINE
Key clinical point:C. difficile transmission may be driven by antibiotic use in long-term care facilities.
Major finding: The majority (75%) of the regional variability in the incidence of long-term care–onset C. difficile infection was attributable to antibiotic use and importation.
Data sources: Retrospective study of long-term care residents from 86 Veterans Health Administration health care regions examined from January 2006 through December 2012.
Disclosures: This study was funded by the U.S. Department of Veterans Affairs and the Centers for Disease Control and Prevention. Dr. Brown reported grants from AstraZeneca outside the submitted work, and another coauthor disclosed grant support from the funding source during the conduct of the study. The remaining coauthors disclosed no conflicts of interest.
Most atopic lesions colonized with Staph
Patients with atopic dermatitis are at an increased risk of Staphylococcus aureus colonization of both their lesional and nonlesional skin, as well as their nose, compared with healthy controls, according to a report in the British Journal of Dermatology.
Dr. J.E.E. Totté of the department of dermatology at the Erasmus MC University Medical Centre, Rotterdam, and associates conducted a systematic literature review and meta-analysis to derive pooled estimates of the prevalence and odds of colonization with S. aureus in patients with atopic dermatitis. They focused on original, human experimental, and observational studies including patients of any age with a confirmed diagnosis of atopic dermatitis (Br J Dermatol. 2016 Mar 19. doi: 10.1111/bjd.14566).
Dr. Totté and colleagues identified a total of 4,909 articles, of which 95 were found to meet the study inclusion criteria. All of the included studies were observational, with 30 comparing atopic dermatitis patients with healthy controls.
Almost three-quarters (70%) of patients had S. aureus colonization of lesional skin, while 39% had colonization of nonlesional skin, based on 81 studies including 5,231 patients and 30 studies including 1,496 patients, respectively. Nasal colonization was found in 62% of patients, based on analysis of 43 studies including 2,476 patients.
S. aureus colonization is an important factor in the pathogenesis of atopic dermatitis and should lead to evaluations of targeted antistaphylococcal therapy for the skin and nose, the investigators advised.
The authors reported that the department of dermatology of the Erasmus MC University Medical Centre Rotterdam received an unrestricted grant from Micreos Human Health. Two coauthors disclosed ties to industry sources.
Patients with atopic dermatitis are at an increased risk of Staphylococcus aureus colonization of both their lesional and nonlesional skin, as well as their nose, compared with healthy controls, according to a report in the British Journal of Dermatology.
Dr. J.E.E. Totté of the department of dermatology at the Erasmus MC University Medical Centre, Rotterdam, and associates conducted a systematic literature review and meta-analysis to derive pooled estimates of the prevalence and odds of colonization with S. aureus in patients with atopic dermatitis. They focused on original, human experimental, and observational studies including patients of any age with a confirmed diagnosis of atopic dermatitis (Br J Dermatol. 2016 Mar 19. doi: 10.1111/bjd.14566).
Dr. Totté and colleagues identified a total of 4,909 articles, of which 95 were found to meet the study inclusion criteria. All of the included studies were observational, with 30 comparing atopic dermatitis patients with healthy controls.
Almost three-quarters (70%) of patients had S. aureus colonization of lesional skin, while 39% had colonization of nonlesional skin, based on 81 studies including 5,231 patients and 30 studies including 1,496 patients, respectively. Nasal colonization was found in 62% of patients, based on analysis of 43 studies including 2,476 patients.
S. aureus colonization is an important factor in the pathogenesis of atopic dermatitis and should lead to evaluations of targeted antistaphylococcal therapy for the skin and nose, the investigators advised.
The authors reported that the department of dermatology of the Erasmus MC University Medical Centre Rotterdam received an unrestricted grant from Micreos Human Health. Two coauthors disclosed ties to industry sources.
Patients with atopic dermatitis are at an increased risk of Staphylococcus aureus colonization of both their lesional and nonlesional skin, as well as their nose, compared with healthy controls, according to a report in the British Journal of Dermatology.
Dr. J.E.E. Totté of the department of dermatology at the Erasmus MC University Medical Centre, Rotterdam, and associates conducted a systematic literature review and meta-analysis to derive pooled estimates of the prevalence and odds of colonization with S. aureus in patients with atopic dermatitis. They focused on original, human experimental, and observational studies including patients of any age with a confirmed diagnosis of atopic dermatitis (Br J Dermatol. 2016 Mar 19. doi: 10.1111/bjd.14566).
Dr. Totté and colleagues identified a total of 4,909 articles, of which 95 were found to meet the study inclusion criteria. All of the included studies were observational, with 30 comparing atopic dermatitis patients with healthy controls.
Almost three-quarters (70%) of patients had S. aureus colonization of lesional skin, while 39% had colonization of nonlesional skin, based on 81 studies including 5,231 patients and 30 studies including 1,496 patients, respectively. Nasal colonization was found in 62% of patients, based on analysis of 43 studies including 2,476 patients.
S. aureus colonization is an important factor in the pathogenesis of atopic dermatitis and should lead to evaluations of targeted antistaphylococcal therapy for the skin and nose, the investigators advised.
The authors reported that the department of dermatology of the Erasmus MC University Medical Centre Rotterdam received an unrestricted grant from Micreos Human Health. Two coauthors disclosed ties to industry sources.
FROM THE BRITISH JOURNAL OF DERMATOLOGY
Key clinical point: Consider addressing S. aureus colonization in atopic dermatitis patients.
Major finding: Most patients (70%) were colonized with S. aureus on lesional skin, while 39% were colonized on nonlesional skin.
Data source: Literature review and meta-analysis involving 95 studies, 30 with healthy controls.
Disclosures: The study was funded by an unrestricted grant from Micreos Human Health to Erasmus MC University Medical Centre. Two coauthors disclosed ties to industry sources.
Study: Few HCV-infected heroin users linked to outpatient care
The results of a hepatitis C virus screening program in a suburban New Jersey acute opioid detoxification program reveal that, despite the ease of access to HCV screening and the availability of curative therapies, linkage to care after detection of infection in persons who inject drugs continues to present a challenge.
Eda Akyar, a clinical research coordinator at ID Care, New Jersey’s largest network of infectious disease specialists, and her colleagues examined the results of an HIV, HCV, and HBV infection screening program instituted between Oct. 1, 2014, and June 9, 2015, for patients admitted to an acute opioid detoxification program at Princeton House in suburban New Jersey. Study goals included assessing the prevalence of HIV, HCV, and HBV infections for these patients, the HCV genotype (GT) carried, and the subsequent linkage to care after discharge from the program. The report was published online April 13 in Emerging Infectious Diseases.
During the screening period, patients representing 10 of New Jersey’s 21 counties were tested for the presence of HCV antibodies. Approximately two-thirds of all study participants (66.6%) were between 17 and 35 years of age. In this important age demographic, 237 patients (41.4%) screened positive for HCV antibodies, and 187 were available for further study.
HCV viral load data were available from 172 patients (92.0%), with approximately one-fifth (18.6%) screening undetectable. The HCV GTs obtained from 102 patients revealed that most (62.7%) were GT1a, followed by GT3 (25.5%). In a very surprising result, all HCV antibody positive patients were also HIV antibody negative, the researchers noted.
Regarding linkage to care, 16 of the patients (8.6%) in the 17- to 35-year-old age group attended outpatient follow-up appointments, with three (1.6%) starting on an oral, direct-acting antiviral treatment regimen. Two of these three patients were nonadherent to their treatment regimens. Two additional patients expressed willingness to accept treatment, but were denied their prescriptions by their insurance providers. None of the other participants returned for continued care.
The authors said that their results indicated a high prevalence of HCV among young suburban heroin users attending an acute detoxification program serving a wide geographic area, suggesting that New Jersey is part of the second wave of HCV infection following that seen in those born from 1946 to 1964. They also highlighted their findings of a GT3 prevalence more than twice the national average, a complete lack of HIV infection in a population known to be susceptible to it, and disappointing linkage to care outcomes.
The authors said that easy-to-use curative therapy should underscore a need for improved linkage to care and treatment of HCV-infected persons who inject drugs as part of an important public health effort to prevent its continued spread.
This work was supported in part by a research grant from the Investigator-Initiated Studies Program of Merck Sharp & Dohme Corp. Conflict of interest information was not disclosed.
The results of a hepatitis C virus screening program in a suburban New Jersey acute opioid detoxification program reveal that, despite the ease of access to HCV screening and the availability of curative therapies, linkage to care after detection of infection in persons who inject drugs continues to present a challenge.
Eda Akyar, a clinical research coordinator at ID Care, New Jersey’s largest network of infectious disease specialists, and her colleagues examined the results of an HIV, HCV, and HBV infection screening program instituted between Oct. 1, 2014, and June 9, 2015, for patients admitted to an acute opioid detoxification program at Princeton House in suburban New Jersey. Study goals included assessing the prevalence of HIV, HCV, and HBV infections for these patients, the HCV genotype (GT) carried, and the subsequent linkage to care after discharge from the program. The report was published online April 13 in Emerging Infectious Diseases.
During the screening period, patients representing 10 of New Jersey’s 21 counties were tested for the presence of HCV antibodies. Approximately two-thirds of all study participants (66.6%) were between 17 and 35 years of age. In this important age demographic, 237 patients (41.4%) screened positive for HCV antibodies, and 187 were available for further study.
HCV viral load data were available from 172 patients (92.0%), with approximately one-fifth (18.6%) screening undetectable. The HCV GTs obtained from 102 patients revealed that most (62.7%) were GT1a, followed by GT3 (25.5%). In a very surprising result, all HCV antibody positive patients were also HIV antibody negative, the researchers noted.
Regarding linkage to care, 16 of the patients (8.6%) in the 17- to 35-year-old age group attended outpatient follow-up appointments, with three (1.6%) starting on an oral, direct-acting antiviral treatment regimen. Two of these three patients were nonadherent to their treatment regimens. Two additional patients expressed willingness to accept treatment, but were denied their prescriptions by their insurance providers. None of the other participants returned for continued care.
The authors said that their results indicated a high prevalence of HCV among young suburban heroin users attending an acute detoxification program serving a wide geographic area, suggesting that New Jersey is part of the second wave of HCV infection following that seen in those born from 1946 to 1964. They also highlighted their findings of a GT3 prevalence more than twice the national average, a complete lack of HIV infection in a population known to be susceptible to it, and disappointing linkage to care outcomes.
The authors said that easy-to-use curative therapy should underscore a need for improved linkage to care and treatment of HCV-infected persons who inject drugs as part of an important public health effort to prevent its continued spread.
This work was supported in part by a research grant from the Investigator-Initiated Studies Program of Merck Sharp & Dohme Corp. Conflict of interest information was not disclosed.
The results of a hepatitis C virus screening program in a suburban New Jersey acute opioid detoxification program reveal that, despite the ease of access to HCV screening and the availability of curative therapies, linkage to care after detection of infection in persons who inject drugs continues to present a challenge.
Eda Akyar, a clinical research coordinator at ID Care, New Jersey’s largest network of infectious disease specialists, and her colleagues examined the results of an HIV, HCV, and HBV infection screening program instituted between Oct. 1, 2014, and June 9, 2015, for patients admitted to an acute opioid detoxification program at Princeton House in suburban New Jersey. Study goals included assessing the prevalence of HIV, HCV, and HBV infections for these patients, the HCV genotype (GT) carried, and the subsequent linkage to care after discharge from the program. The report was published online April 13 in Emerging Infectious Diseases.
During the screening period, patients representing 10 of New Jersey’s 21 counties were tested for the presence of HCV antibodies. Approximately two-thirds of all study participants (66.6%) were between 17 and 35 years of age. In this important age demographic, 237 patients (41.4%) screened positive for HCV antibodies, and 187 were available for further study.
HCV viral load data were available from 172 patients (92.0%), with approximately one-fifth (18.6%) screening undetectable. The HCV GTs obtained from 102 patients revealed that most (62.7%) were GT1a, followed by GT3 (25.5%). In a very surprising result, all HCV antibody positive patients were also HIV antibody negative, the researchers noted.
Regarding linkage to care, 16 of the patients (8.6%) in the 17- to 35-year-old age group attended outpatient follow-up appointments, with three (1.6%) starting on an oral, direct-acting antiviral treatment regimen. Two of these three patients were nonadherent to their treatment regimens. Two additional patients expressed willingness to accept treatment, but were denied their prescriptions by their insurance providers. None of the other participants returned for continued care.
The authors said that their results indicated a high prevalence of HCV among young suburban heroin users attending an acute detoxification program serving a wide geographic area, suggesting that New Jersey is part of the second wave of HCV infection following that seen in those born from 1946 to 1964. They also highlighted their findings of a GT3 prevalence more than twice the national average, a complete lack of HIV infection in a population known to be susceptible to it, and disappointing linkage to care outcomes.
The authors said that easy-to-use curative therapy should underscore a need for improved linkage to care and treatment of HCV-infected persons who inject drugs as part of an important public health effort to prevent its continued spread.
This work was supported in part by a research grant from the Investigator-Initiated Studies Program of Merck Sharp & Dohme Corp. Conflict of interest information was not disclosed.
FROM EMERGING INFECTIOUS DISEASES
Key clinical point: Despite the ease of access to hepatitis C virus screening and the availability of curative therapies, linkage to care after detection of infection in persons who inject drugs continues to present a challenge, with larger implications for public health efforts to prevent its spread.
Major finding: Hepatitis C virus genotype 3 infection was highly prevalent in individuals who inject drugs who were participating in an acute heroin detoxification program serving a wide geographic area in suburban New Jersey.
Data sources: Patients admitted to Princeton House, a psychiatric facility in suburban New Jersey with an active opioid detoxification program, screened for hepatitis C virus between Oct. 1, 2014, and June 9, 2015.
Disclosures: This work was supported in part by a research grant from the Investigator-Initiated Studies Program of Merck Sharp & Dohme Corp. Conflict of interest information was not disclosed.
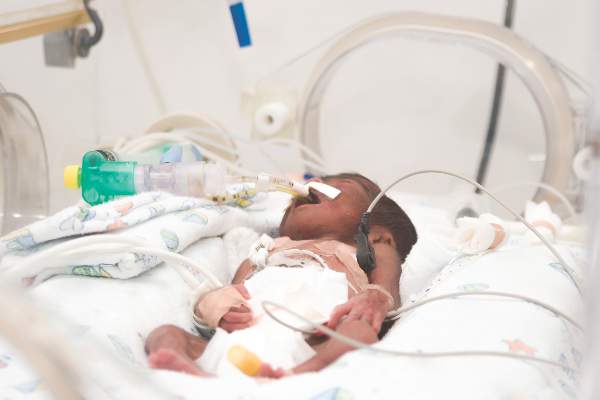
Immunization need not be delayed in most preterm infants with BPD
Despite concerns that preterm infants with bronchopulmonary dysplasia (BPD) are more likely to experience respiratory decompensation after immunization than those without BPD, no difference in the incidence of respiratory decompensation within 72 hours after immunization was found between these groups in a cohort study at a tertiary care facility, according to a report published April 11 in Pediatrics.
These results demonstrate the safety of immunizing preterm infants with BPD, and that the findings should negate delays in immunizations in these patients based on safety concerns described in previous studies, Dr. Edwin Clark Montague of Emory University, Atlanta, and his colleagues assert.
In a retrospective observational study, Dr. Clark and his associates assessed a cohort of infants admitted to the NICU at a level 4, nonbirthing referral hospital, Children’s Healthcare of Atlanta at Egleston, who received any immunizations while hospitalized between Jan. 1, 2008, and Aug. 1, 2014. Inclusion criteria were birth at less than 32 weeks’ estimated gestational age and the availability of data for 72 hours before and 72 hours after immunization. The incidence of respiratory decompensation and cardiorespiratory events (apnea, bradycardia, desaturations) after immunization was compared between infants with and without BPD after immunization (Pediatrics. 2016 doi: 10.1542/peds.2015-4225).
Of the 403 patients assessed, 240 met the inclusion criteria. Of these, 170 were identified as having BPD and were compared with the 70 patients without BPD. The study results revealed no statistically significant difference in respiratory decompensation between patients with and without BPD. In addition, both groups showed an increased incidence of cardiorespiratory events, but there was no statistically significant difference between those with and without BPD.
In addition to BPD, the authors assessed risk factors that may predispose preterm infants to respiratory decompensation after immunization, such as a history of necrotizing enterocolitis or spontaneous intestinal perforation, grade III or IV intraventricular hemorrhage, or periventricular leukomalacia. Results from these analyses indicated that severe intraventricular hemorrhage was a predictor of respiratory decompensation after immunization, and that a positive blood culture within 72 hours of immunization was predictive as well. The authors said that decompensation was likely secondary to the underlying infection in those with positive blood cultures, but that additional research would be required to better understand this finding.
The authors reported no external funding sources and no financial relationships relevant to this article.
Despite concerns that preterm infants with bronchopulmonary dysplasia (BPD) are more likely to experience respiratory decompensation after immunization than those without BPD, no difference in the incidence of respiratory decompensation within 72 hours after immunization was found between these groups in a cohort study at a tertiary care facility, according to a report published April 11 in Pediatrics.
These results demonstrate the safety of immunizing preterm infants with BPD, and that the findings should negate delays in immunizations in these patients based on safety concerns described in previous studies, Dr. Edwin Clark Montague of Emory University, Atlanta, and his colleagues assert.
In a retrospective observational study, Dr. Clark and his associates assessed a cohort of infants admitted to the NICU at a level 4, nonbirthing referral hospital, Children’s Healthcare of Atlanta at Egleston, who received any immunizations while hospitalized between Jan. 1, 2008, and Aug. 1, 2014. Inclusion criteria were birth at less than 32 weeks’ estimated gestational age and the availability of data for 72 hours before and 72 hours after immunization. The incidence of respiratory decompensation and cardiorespiratory events (apnea, bradycardia, desaturations) after immunization was compared between infants with and without BPD after immunization (Pediatrics. 2016 doi: 10.1542/peds.2015-4225).
Of the 403 patients assessed, 240 met the inclusion criteria. Of these, 170 were identified as having BPD and were compared with the 70 patients without BPD. The study results revealed no statistically significant difference in respiratory decompensation between patients with and without BPD. In addition, both groups showed an increased incidence of cardiorespiratory events, but there was no statistically significant difference between those with and without BPD.
In addition to BPD, the authors assessed risk factors that may predispose preterm infants to respiratory decompensation after immunization, such as a history of necrotizing enterocolitis or spontaneous intestinal perforation, grade III or IV intraventricular hemorrhage, or periventricular leukomalacia. Results from these analyses indicated that severe intraventricular hemorrhage was a predictor of respiratory decompensation after immunization, and that a positive blood culture within 72 hours of immunization was predictive as well. The authors said that decompensation was likely secondary to the underlying infection in those with positive blood cultures, but that additional research would be required to better understand this finding.
The authors reported no external funding sources and no financial relationships relevant to this article.
Despite concerns that preterm infants with bronchopulmonary dysplasia (BPD) are more likely to experience respiratory decompensation after immunization than those without BPD, no difference in the incidence of respiratory decompensation within 72 hours after immunization was found between these groups in a cohort study at a tertiary care facility, according to a report published April 11 in Pediatrics.
These results demonstrate the safety of immunizing preterm infants with BPD, and that the findings should negate delays in immunizations in these patients based on safety concerns described in previous studies, Dr. Edwin Clark Montague of Emory University, Atlanta, and his colleagues assert.
In a retrospective observational study, Dr. Clark and his associates assessed a cohort of infants admitted to the NICU at a level 4, nonbirthing referral hospital, Children’s Healthcare of Atlanta at Egleston, who received any immunizations while hospitalized between Jan. 1, 2008, and Aug. 1, 2014. Inclusion criteria were birth at less than 32 weeks’ estimated gestational age and the availability of data for 72 hours before and 72 hours after immunization. The incidence of respiratory decompensation and cardiorespiratory events (apnea, bradycardia, desaturations) after immunization was compared between infants with and without BPD after immunization (Pediatrics. 2016 doi: 10.1542/peds.2015-4225).
Of the 403 patients assessed, 240 met the inclusion criteria. Of these, 170 were identified as having BPD and were compared with the 70 patients without BPD. The study results revealed no statistically significant difference in respiratory decompensation between patients with and without BPD. In addition, both groups showed an increased incidence of cardiorespiratory events, but there was no statistically significant difference between those with and without BPD.
In addition to BPD, the authors assessed risk factors that may predispose preterm infants to respiratory decompensation after immunization, such as a history of necrotizing enterocolitis or spontaneous intestinal perforation, grade III or IV intraventricular hemorrhage, or periventricular leukomalacia. Results from these analyses indicated that severe intraventricular hemorrhage was a predictor of respiratory decompensation after immunization, and that a positive blood culture within 72 hours of immunization was predictive as well. The authors said that decompensation was likely secondary to the underlying infection in those with positive blood cultures, but that additional research would be required to better understand this finding.
The authors reported no external funding sources and no financial relationships relevant to this article.
FROM PEDIATRICS
Key clinical point: Respiratory decompensation after immunization of preterm infants with bronchopulmonary dysplasia is rare and should not be a cause for delayed immunization.
Major finding: No statistically significant differences regarding respiratory decompensation within 72 hours of immunization or its individual components were found between preterm infants with or without bronchopulmonary dysplasia.
Data source: A retrospective observational study of a cohort of premature infants less than 32 weeks’ gestational age admitted to a tertiary level 4 NICU, Children’s Healthcare of Atlanta at Egleston, immunized as inpatients between January 1, 2008, and August 1, 2014.
Disclosures: The authors reported no external funding sources and no financial relationships relevant to this article.
Some Infants Predisposed to Epidermal Barrier Breakdown, Atopic Dermatitis
Neonates with the highest transepidermal water loss at birth, likely mediated through an impaired epidermal barrier, show significantly elevated chymotrypsinlike protease activity and reduced levels of filaggrin-derived natural moisturizing factors, which may predispose them to the development of atopic dermatitis, according to a report published online in the British Journal of Dermatology.
John Chittock of the University of Sheffield, England, and his colleagues assessed the biophysical, biologic, and functional properties of the developing neonatal stratum corneum (SC) from birth to 4 weeks of age in 115 healthy, full-term (at least 37 weeks’ gestation) neonates from the OBSERVE (Oil in Baby Skincare) randomized study birth cohort recruited at Saint Mary’s Hospital, Central Manchester NHS Foundation Trust, between September 2013 and June 2014.
For comparative purposes, an unrelated cohort of 20 adults with healthy skin was recruited from the local community between January and April 2015 (Br J Dermatol. 2016 Mar 19. doi: 10.1111/bjd.14568).
The researchers found that the biophysical properties of the neonatal SC are transitional from birth. For example, overall transepidermal water loss (TEWL) increased significantly during the first 4 weeks of infant life. Compared with adult skin, the newborn infant SC was found to be drier and more alkaline. In addition, levels of superficial chymotrypsinlike protease activity at birth did not differ between newborns and adults, while levels of filaggrin-derived natural moisturizing factors (NMF) were significantly lower at birth than in adulthood.
The increased chymotrypsinlike protease activity and NMF at 4 weeks of age exceeded levels found in healthy adults, rather than reaching their mature state. Compared with adult skin, the skin of infants is functionally immature, with undeveloped mechanisms of desquamation and differentiation, the investigators noted.
Further analysis revealed a correlation between TEWL and both superficial chymotrypsinlike protease activity and filaggrin-derived NMF at birth.
To explore that link, the researchers stratified the neonatal cohort according to TEWL percentile. The neonates in the 76th-100th percentile, the highest TEWL at birth, showed significantly elevated chymotrypsinlike protease activity and reduced levels of filaggrin-derived NMF, compared with neonates in lower percentiles. Therefore, those neonates are at highest risk for developing atopic dermatitis, the study authors said.
The findings indicate a need for infant skin care regimens that protect and support normal barrier development from birth, the researchers noted. They also suggested that clinical strategies targeting the early mechanisms of barrier breakdown could act as preventive measures in neonates at increased risk of developing atopic dermatitis.
The research was funded jointly by the University of Sheffield and a doctoral research fellowship supported by the National Institute for Health Research. The authors declared no conflicts of interest.
Neonates with the highest transepidermal water loss at birth, likely mediated through an impaired epidermal barrier, show significantly elevated chymotrypsinlike protease activity and reduced levels of filaggrin-derived natural moisturizing factors, which may predispose them to the development of atopic dermatitis, according to a report published online in the British Journal of Dermatology.
John Chittock of the University of Sheffield, England, and his colleagues assessed the biophysical, biologic, and functional properties of the developing neonatal stratum corneum (SC) from birth to 4 weeks of age in 115 healthy, full-term (at least 37 weeks’ gestation) neonates from the OBSERVE (Oil in Baby Skincare) randomized study birth cohort recruited at Saint Mary’s Hospital, Central Manchester NHS Foundation Trust, between September 2013 and June 2014.
For comparative purposes, an unrelated cohort of 20 adults with healthy skin was recruited from the local community between January and April 2015 (Br J Dermatol. 2016 Mar 19. doi: 10.1111/bjd.14568).
The researchers found that the biophysical properties of the neonatal SC are transitional from birth. For example, overall transepidermal water loss (TEWL) increased significantly during the first 4 weeks of infant life. Compared with adult skin, the newborn infant SC was found to be drier and more alkaline. In addition, levels of superficial chymotrypsinlike protease activity at birth did not differ between newborns and adults, while levels of filaggrin-derived natural moisturizing factors (NMF) were significantly lower at birth than in adulthood.
The increased chymotrypsinlike protease activity and NMF at 4 weeks of age exceeded levels found in healthy adults, rather than reaching their mature state. Compared with adult skin, the skin of infants is functionally immature, with undeveloped mechanisms of desquamation and differentiation, the investigators noted.
Further analysis revealed a correlation between TEWL and both superficial chymotrypsinlike protease activity and filaggrin-derived NMF at birth.
To explore that link, the researchers stratified the neonatal cohort according to TEWL percentile. The neonates in the 76th-100th percentile, the highest TEWL at birth, showed significantly elevated chymotrypsinlike protease activity and reduced levels of filaggrin-derived NMF, compared with neonates in lower percentiles. Therefore, those neonates are at highest risk for developing atopic dermatitis, the study authors said.
The findings indicate a need for infant skin care regimens that protect and support normal barrier development from birth, the researchers noted. They also suggested that clinical strategies targeting the early mechanisms of barrier breakdown could act as preventive measures in neonates at increased risk of developing atopic dermatitis.
The research was funded jointly by the University of Sheffield and a doctoral research fellowship supported by the National Institute for Health Research. The authors declared no conflicts of interest.
Neonates with the highest transepidermal water loss at birth, likely mediated through an impaired epidermal barrier, show significantly elevated chymotrypsinlike protease activity and reduced levels of filaggrin-derived natural moisturizing factors, which may predispose them to the development of atopic dermatitis, according to a report published online in the British Journal of Dermatology.
John Chittock of the University of Sheffield, England, and his colleagues assessed the biophysical, biologic, and functional properties of the developing neonatal stratum corneum (SC) from birth to 4 weeks of age in 115 healthy, full-term (at least 37 weeks’ gestation) neonates from the OBSERVE (Oil in Baby Skincare) randomized study birth cohort recruited at Saint Mary’s Hospital, Central Manchester NHS Foundation Trust, between September 2013 and June 2014.
For comparative purposes, an unrelated cohort of 20 adults with healthy skin was recruited from the local community between January and April 2015 (Br J Dermatol. 2016 Mar 19. doi: 10.1111/bjd.14568).
The researchers found that the biophysical properties of the neonatal SC are transitional from birth. For example, overall transepidermal water loss (TEWL) increased significantly during the first 4 weeks of infant life. Compared with adult skin, the newborn infant SC was found to be drier and more alkaline. In addition, levels of superficial chymotrypsinlike protease activity at birth did not differ between newborns and adults, while levels of filaggrin-derived natural moisturizing factors (NMF) were significantly lower at birth than in adulthood.
The increased chymotrypsinlike protease activity and NMF at 4 weeks of age exceeded levels found in healthy adults, rather than reaching their mature state. Compared with adult skin, the skin of infants is functionally immature, with undeveloped mechanisms of desquamation and differentiation, the investigators noted.
Further analysis revealed a correlation between TEWL and both superficial chymotrypsinlike protease activity and filaggrin-derived NMF at birth.
To explore that link, the researchers stratified the neonatal cohort according to TEWL percentile. The neonates in the 76th-100th percentile, the highest TEWL at birth, showed significantly elevated chymotrypsinlike protease activity and reduced levels of filaggrin-derived NMF, compared with neonates in lower percentiles. Therefore, those neonates are at highest risk for developing atopic dermatitis, the study authors said.
The findings indicate a need for infant skin care regimens that protect and support normal barrier development from birth, the researchers noted. They also suggested that clinical strategies targeting the early mechanisms of barrier breakdown could act as preventive measures in neonates at increased risk of developing atopic dermatitis.
The research was funded jointly by the University of Sheffield and a doctoral research fellowship supported by the National Institute for Health Research. The authors declared no conflicts of interest.
FROM THE BRITISH JOURNAL OF DERMATOLOGY
Some infants predisposed to epidermal barrier breakdown, atopic dermatitis
Neonates with the highest transepidermal water loss at birth, likely mediated through an impaired epidermal barrier, show significantly elevated chymotrypsinlike protease activity and reduced levels of filaggrin-derived natural moisturizing factors, which may predispose them to the development of atopic dermatitis, according to a report published online in the British Journal of Dermatology.
John Chittock of the University of Sheffield, England, and his colleagues assessed the biophysical, biologic, and functional properties of the developing neonatal stratum corneum (SC) from birth to 4 weeks of age in 115 healthy, full-term (at least 37 weeks’ gestation) neonates from the OBSERVE (Oil in Baby Skincare) randomized study birth cohort recruited at Saint Mary’s Hospital, Central Manchester NHS Foundation Trust, between September 2013 and June 2014.
For comparative purposes, an unrelated cohort of 20 adults with healthy skin was recruited from the local community between January and April 2015 (Br J Dermatol. 2016 Mar 19. doi: 10.1111/bjd.14568).
The researchers found that the biophysical properties of the neonatal SC are transitional from birth. For example, overall transepidermal water loss (TEWL) increased significantly during the first 4 weeks of infant life. Compared with adult skin, the newborn infant SC was found to be drier and more alkaline. In addition, levels of superficial chymotrypsinlike protease activity at birth did not differ between newborns and adults, while levels of filaggrin-derived natural moisturizing factors (NMF) were significantly lower at birth than in adulthood.
The increased chymotrypsinlike protease activity and NMF at 4 weeks of age exceeded levels found in healthy adults, rather than reaching their mature state. Compared with adult skin, the skin of infants is functionally immature, with undeveloped mechanisms of desquamation and differentiation, the investigators noted.
Further analysis revealed a correlation between TEWL and both superficial chymotrypsinlike protease activity and filaggrin-derived NMF at birth.
To explore that link, the researchers stratified the neonatal cohort according to TEWL percentile. The neonates in the 76th-100th percentile, the highest TEWL at birth, showed significantly elevated chymotrypsinlike protease activity and reduced levels of filaggrin-derived NMF, compared with neonates in lower percentiles. Therefore, those neonates are at highest risk for developing atopic dermatitis, the study authors said.
The findings indicate a need for infant skin care regimens that protect and support normal barrier development from birth, the researchers noted. They also suggested that clinical strategies targeting the early mechanisms of barrier breakdown could act as preventive measures in neonates at increased risk of developing atopic dermatitis.
The research was funded jointly by the University of Sheffield and a doctoral research fellowship supported by the National Institute for Health Research. The authors declared no conflicts of interest.
Neonates with the highest transepidermal water loss at birth, likely mediated through an impaired epidermal barrier, show significantly elevated chymotrypsinlike protease activity and reduced levels of filaggrin-derived natural moisturizing factors, which may predispose them to the development of atopic dermatitis, according to a report published online in the British Journal of Dermatology.
John Chittock of the University of Sheffield, England, and his colleagues assessed the biophysical, biologic, and functional properties of the developing neonatal stratum corneum (SC) from birth to 4 weeks of age in 115 healthy, full-term (at least 37 weeks’ gestation) neonates from the OBSERVE (Oil in Baby Skincare) randomized study birth cohort recruited at Saint Mary’s Hospital, Central Manchester NHS Foundation Trust, between September 2013 and June 2014.
For comparative purposes, an unrelated cohort of 20 adults with healthy skin was recruited from the local community between January and April 2015 (Br J Dermatol. 2016 Mar 19. doi: 10.1111/bjd.14568).
The researchers found that the biophysical properties of the neonatal SC are transitional from birth. For example, overall transepidermal water loss (TEWL) increased significantly during the first 4 weeks of infant life. Compared with adult skin, the newborn infant SC was found to be drier and more alkaline. In addition, levels of superficial chymotrypsinlike protease activity at birth did not differ between newborns and adults, while levels of filaggrin-derived natural moisturizing factors (NMF) were significantly lower at birth than in adulthood.
The increased chymotrypsinlike protease activity and NMF at 4 weeks of age exceeded levels found in healthy adults, rather than reaching their mature state. Compared with adult skin, the skin of infants is functionally immature, with undeveloped mechanisms of desquamation and differentiation, the investigators noted.
Further analysis revealed a correlation between TEWL and both superficial chymotrypsinlike protease activity and filaggrin-derived NMF at birth.
To explore that link, the researchers stratified the neonatal cohort according to TEWL percentile. The neonates in the 76th-100th percentile, the highest TEWL at birth, showed significantly elevated chymotrypsinlike protease activity and reduced levels of filaggrin-derived NMF, compared with neonates in lower percentiles. Therefore, those neonates are at highest risk for developing atopic dermatitis, the study authors said.
The findings indicate a need for infant skin care regimens that protect and support normal barrier development from birth, the researchers noted. They also suggested that clinical strategies targeting the early mechanisms of barrier breakdown could act as preventive measures in neonates at increased risk of developing atopic dermatitis.
The research was funded jointly by the University of Sheffield and a doctoral research fellowship supported by the National Institute for Health Research. The authors declared no conflicts of interest.
Neonates with the highest transepidermal water loss at birth, likely mediated through an impaired epidermal barrier, show significantly elevated chymotrypsinlike protease activity and reduced levels of filaggrin-derived natural moisturizing factors, which may predispose them to the development of atopic dermatitis, according to a report published online in the British Journal of Dermatology.
John Chittock of the University of Sheffield, England, and his colleagues assessed the biophysical, biologic, and functional properties of the developing neonatal stratum corneum (SC) from birth to 4 weeks of age in 115 healthy, full-term (at least 37 weeks’ gestation) neonates from the OBSERVE (Oil in Baby Skincare) randomized study birth cohort recruited at Saint Mary’s Hospital, Central Manchester NHS Foundation Trust, between September 2013 and June 2014.
For comparative purposes, an unrelated cohort of 20 adults with healthy skin was recruited from the local community between January and April 2015 (Br J Dermatol. 2016 Mar 19. doi: 10.1111/bjd.14568).
The researchers found that the biophysical properties of the neonatal SC are transitional from birth. For example, overall transepidermal water loss (TEWL) increased significantly during the first 4 weeks of infant life. Compared with adult skin, the newborn infant SC was found to be drier and more alkaline. In addition, levels of superficial chymotrypsinlike protease activity at birth did not differ between newborns and adults, while levels of filaggrin-derived natural moisturizing factors (NMF) were significantly lower at birth than in adulthood.
The increased chymotrypsinlike protease activity and NMF at 4 weeks of age exceeded levels found in healthy adults, rather than reaching their mature state. Compared with adult skin, the skin of infants is functionally immature, with undeveloped mechanisms of desquamation and differentiation, the investigators noted.
Further analysis revealed a correlation between TEWL and both superficial chymotrypsinlike protease activity and filaggrin-derived NMF at birth.
To explore that link, the researchers stratified the neonatal cohort according to TEWL percentile. The neonates in the 76th-100th percentile, the highest TEWL at birth, showed significantly elevated chymotrypsinlike protease activity and reduced levels of filaggrin-derived NMF, compared with neonates in lower percentiles. Therefore, those neonates are at highest risk for developing atopic dermatitis, the study authors said.
The findings indicate a need for infant skin care regimens that protect and support normal barrier development from birth, the researchers noted. They also suggested that clinical strategies targeting the early mechanisms of barrier breakdown could act as preventive measures in neonates at increased risk of developing atopic dermatitis.
The research was funded jointly by the University of Sheffield and a doctoral research fellowship supported by the National Institute for Health Research. The authors declared no conflicts of interest.
FROM THE BRITISH JOURNAL OF DERMATOLOGY
Key clinical point: Some infants are predisposed to epidermal barrier breakdown and the development of atopic dermatitis through elevated protease activity and reduced levels of natural moisturizing factors at birth.
Major finding: Significantly elevated chymotrypsinlike protease activity and reduced levels of natural moisturizing factors were associated with impaired epidermal barrier function at birth.
Data sources: The OBSERVE study birth cohort included a total of 115 healthy, full-term (at least 37 weeks’ gestation) neonates recruited at Saint Mary’s Hospital, Central Manchester NHS Foundation Trust, between September 2013 and June 2014, as well as an unrelated cohort of 20 adults with healthy skin recruited from the local community between January and April 2015.
Disclosures: This independent research was funded jointly by the University of Sheffield and a Doctoral Research Fellowship supported by the National Institute for Health Research. The authors declared no conflicts of interest.
U.S. sepsis-related mortality rates differ based on data source
Despite challenges in estimating sepsis-related mortality in the U.S. because of its complex clinical nature and variety of underlying causes, estimates based on administrative claims data may be more accurate than those derived from death certificates, according to a report published April 8 in the Morbidity and Mortality Weekly Report.
Dr. Lauren Epstein of the division of healthcare quality promotion at the National Center for Emerging and Zoonotic Infectious Diseases, and her colleagues, compared U.S. sepsis-related mortality estimates from different sources. Deaths attributable to diagnoses corresponding to ICD-10 diagnosis codes A40 (streptococcal septicemia) and A41 (other septicemia) from 1999 to 2014 were extracted from the CDC WONDER (Wide-ranging Online Data for Epidemiologic Research) database. Administrative claims data using various combinations of the ICD-9-CM administrative codes for primary or secondary infection and organ dysfunction to identify severe sepsis from 2004 to 2009 were extracted from the largest all-payer, publicly available inpatient database in the United States, the Nationwide Inpatient Sample. (MMWR. 2016 Apr 8;65[13]:342-5).
Of the roughly 2.5 million death certificates listing sepsis as a cause of death, 22% identified sepsis as the underlying cause of death during the time period assessed. The results of the comparison demonstrated that the estimated range of sepsis-related mortality based on death certificate data was lower than that obtained using administrative claims data (ranges, 146,000-159,000 and 168,000-381,000, respectively). These results indicate that the annual estimate based on administrative claims data during the time period assessed was 15%-140% higher than the estimate based on death certificate data.
To explain the difference between the estimated sepsis-related mortality ranges, the authors said that while both death certificate and administrative claims data are important sources of public health information, they are each associated with limitations that can affect such estimates. For example, the authors said that death certificate certifiers may be more prone to record sepsis as an immediate cause of death, resulting in lower estimates of sepsis-related mortality based on underlying causes of death. Regarding administrative claims data, the authors said that such data cannot be all inclusive, as only those sepsis-related deaths occurring in health care facilities are captured.
The authors said that their results highlight the need for a better defined and more reliable sepsis surveillance system that should be based on objective clinical data. This approach would allow for increased accuracy in the tracking of sepsis trends in the United States, as well as an improved system for gauging the impact of sepsis awareness and prevention efforts.
No funding sources or conflicts of interest were reported.
Despite challenges in estimating sepsis-related mortality in the U.S. because of its complex clinical nature and variety of underlying causes, estimates based on administrative claims data may be more accurate than those derived from death certificates, according to a report published April 8 in the Morbidity and Mortality Weekly Report.
Dr. Lauren Epstein of the division of healthcare quality promotion at the National Center for Emerging and Zoonotic Infectious Diseases, and her colleagues, compared U.S. sepsis-related mortality estimates from different sources. Deaths attributable to diagnoses corresponding to ICD-10 diagnosis codes A40 (streptococcal septicemia) and A41 (other septicemia) from 1999 to 2014 were extracted from the CDC WONDER (Wide-ranging Online Data for Epidemiologic Research) database. Administrative claims data using various combinations of the ICD-9-CM administrative codes for primary or secondary infection and organ dysfunction to identify severe sepsis from 2004 to 2009 were extracted from the largest all-payer, publicly available inpatient database in the United States, the Nationwide Inpatient Sample. (MMWR. 2016 Apr 8;65[13]:342-5).
Of the roughly 2.5 million death certificates listing sepsis as a cause of death, 22% identified sepsis as the underlying cause of death during the time period assessed. The results of the comparison demonstrated that the estimated range of sepsis-related mortality based on death certificate data was lower than that obtained using administrative claims data (ranges, 146,000-159,000 and 168,000-381,000, respectively). These results indicate that the annual estimate based on administrative claims data during the time period assessed was 15%-140% higher than the estimate based on death certificate data.
To explain the difference between the estimated sepsis-related mortality ranges, the authors said that while both death certificate and administrative claims data are important sources of public health information, they are each associated with limitations that can affect such estimates. For example, the authors said that death certificate certifiers may be more prone to record sepsis as an immediate cause of death, resulting in lower estimates of sepsis-related mortality based on underlying causes of death. Regarding administrative claims data, the authors said that such data cannot be all inclusive, as only those sepsis-related deaths occurring in health care facilities are captured.
The authors said that their results highlight the need for a better defined and more reliable sepsis surveillance system that should be based on objective clinical data. This approach would allow for increased accuracy in the tracking of sepsis trends in the United States, as well as an improved system for gauging the impact of sepsis awareness and prevention efforts.
No funding sources or conflicts of interest were reported.
Despite challenges in estimating sepsis-related mortality in the U.S. because of its complex clinical nature and variety of underlying causes, estimates based on administrative claims data may be more accurate than those derived from death certificates, according to a report published April 8 in the Morbidity and Mortality Weekly Report.
Dr. Lauren Epstein of the division of healthcare quality promotion at the National Center for Emerging and Zoonotic Infectious Diseases, and her colleagues, compared U.S. sepsis-related mortality estimates from different sources. Deaths attributable to diagnoses corresponding to ICD-10 diagnosis codes A40 (streptococcal septicemia) and A41 (other septicemia) from 1999 to 2014 were extracted from the CDC WONDER (Wide-ranging Online Data for Epidemiologic Research) database. Administrative claims data using various combinations of the ICD-9-CM administrative codes for primary or secondary infection and organ dysfunction to identify severe sepsis from 2004 to 2009 were extracted from the largest all-payer, publicly available inpatient database in the United States, the Nationwide Inpatient Sample. (MMWR. 2016 Apr 8;65[13]:342-5).
Of the roughly 2.5 million death certificates listing sepsis as a cause of death, 22% identified sepsis as the underlying cause of death during the time period assessed. The results of the comparison demonstrated that the estimated range of sepsis-related mortality based on death certificate data was lower than that obtained using administrative claims data (ranges, 146,000-159,000 and 168,000-381,000, respectively). These results indicate that the annual estimate based on administrative claims data during the time period assessed was 15%-140% higher than the estimate based on death certificate data.
To explain the difference between the estimated sepsis-related mortality ranges, the authors said that while both death certificate and administrative claims data are important sources of public health information, they are each associated with limitations that can affect such estimates. For example, the authors said that death certificate certifiers may be more prone to record sepsis as an immediate cause of death, resulting in lower estimates of sepsis-related mortality based on underlying causes of death. Regarding administrative claims data, the authors said that such data cannot be all inclusive, as only those sepsis-related deaths occurring in health care facilities are captured.
The authors said that their results highlight the need for a better defined and more reliable sepsis surveillance system that should be based on objective clinical data. This approach would allow for increased accuracy in the tracking of sepsis trends in the United States, as well as an improved system for gauging the impact of sepsis awareness and prevention efforts.
No funding sources or conflicts of interest were reported.
FROM MMWR
Key clinical point: Sepsis surveillance should be based on objective clinical data rather than on data obtained from death certificates.
Major finding: Using administrative codes, the U.S. sepsis-related mortality estimate from 2004 to 2009 was 15%-140% higher than the estimate derived from the use of death certificates (ranges; 168,000-381,000 and 146,000-159,000, respectively).
Data source: Death certificate data from the CDC WONDER database and administrative claims data from a previously published report of sepsis mortality estimates based on the Nationwide Inpatient Sample, Healthcare Cost and Utilization Project, Agency for Healthcare Research and Quality.
Disclosures: No funding sources or conflicts of interest were reported.
Fetal malformation risk not increased after exposure to lamotrigine
A new analysis of registry data from European countries does not support a risk of orofacial cleft and clubfoot with exposure to lamotrigine monotherapy, in contrast to signals from previous studies of the antiepileptic drug.
First author Helen Dolk, Dr.P.H., professor of epidemiology and health services research and the head of the center for maternal, fetal, and infant research at the University of Ulster in Coleraine, Northern Ireland, and her colleagues analyzed data from 10.1 million births exposed to antiepileptic drugs including lamotrigine (Lamictal) as a monotherapy during the first trimester between 1995 and 2011. The births were recorded in 21 population-based registries from 16 European countries. The outcomes of interest were major congenital malformations in general, as well as orofacial clefts and clubfoot (Neurology. 2016 April 6. doi: 10.1212/WNL.0000000000002540).
Assessment of all antiepileptic drug-exposed congenital malformation registrations revealed that 12% of pregnant registrants were exposed to lamotrigine monotherapy, with an additional 7% exposed to lamotrigine as part of polytherapy. A total of 77.1% of pregnant women exposed to lamotrigine monotherapy had records indicative of a diagnosis of epilepsy. The proportion of lamotrigine monotherapy exposures was observed to have increased over the study period, likely based on a movement away from the traditional use of valproate because of teratogenic concerns.
A total of 147 lamotrigine monotherapy-exposed babies with congenital malformations not attributable to chromosomal irregularities were identified from the total sample. The odds ratio for having a child with orofacial clefts after exposure to lamotrigine monotherapy was 1.31 (95% confidence interval, 0.73-2.33). Based on these data, the authors said they estimated exposure to lamotrigine would result in orofacial clefts in fewer than 1 in every 550 exposed babies.
The odds ratio for having a child with clubfoot after exposure to lamotrigine monotherapy was 1.83 (95% CI, 1.01-3.31). Although the study results confirmed the statistically significant signal for an overall excess of clubfoot risk found in a previous study conducted by this research team that analyzed births during 1995-2005, the investigators could not reproduce this result in an independent study population of 6.3 million births during 2005-2011(odds ratio, 1.43; 95% CI, 0.66-3.08). There were no significant differences in the risk for developing any other congenital malformations associated with lamotrigine monotherapy, the investigators said.
The authors said their results were in accord with those from several previous studies that did not detect an increased risk of orofacial clefts. In addition, they said statistically significant independent evidence of a clubfoot excess was not detected in the current study, despite findings from their previous study suggesting an increased risk.
The EUROCAT Central Database was funded by the EU Public Health Programme. GlaxoSmithKline, which markets lamotrigine, provided a grant for additional funding of this study. Dr. Dolk and her coauthors reported that their institutions received funding from GlaxoSmithKline for data or staff time contributed to this study.
A new analysis of registry data from European countries does not support a risk of orofacial cleft and clubfoot with exposure to lamotrigine monotherapy, in contrast to signals from previous studies of the antiepileptic drug.
First author Helen Dolk, Dr.P.H., professor of epidemiology and health services research and the head of the center for maternal, fetal, and infant research at the University of Ulster in Coleraine, Northern Ireland, and her colleagues analyzed data from 10.1 million births exposed to antiepileptic drugs including lamotrigine (Lamictal) as a monotherapy during the first trimester between 1995 and 2011. The births were recorded in 21 population-based registries from 16 European countries. The outcomes of interest were major congenital malformations in general, as well as orofacial clefts and clubfoot (Neurology. 2016 April 6. doi: 10.1212/WNL.0000000000002540).
Assessment of all antiepileptic drug-exposed congenital malformation registrations revealed that 12% of pregnant registrants were exposed to lamotrigine monotherapy, with an additional 7% exposed to lamotrigine as part of polytherapy. A total of 77.1% of pregnant women exposed to lamotrigine monotherapy had records indicative of a diagnosis of epilepsy. The proportion of lamotrigine monotherapy exposures was observed to have increased over the study period, likely based on a movement away from the traditional use of valproate because of teratogenic concerns.
A total of 147 lamotrigine monotherapy-exposed babies with congenital malformations not attributable to chromosomal irregularities were identified from the total sample. The odds ratio for having a child with orofacial clefts after exposure to lamotrigine monotherapy was 1.31 (95% confidence interval, 0.73-2.33). Based on these data, the authors said they estimated exposure to lamotrigine would result in orofacial clefts in fewer than 1 in every 550 exposed babies.
The odds ratio for having a child with clubfoot after exposure to lamotrigine monotherapy was 1.83 (95% CI, 1.01-3.31). Although the study results confirmed the statistically significant signal for an overall excess of clubfoot risk found in a previous study conducted by this research team that analyzed births during 1995-2005, the investigators could not reproduce this result in an independent study population of 6.3 million births during 2005-2011(odds ratio, 1.43; 95% CI, 0.66-3.08). There were no significant differences in the risk for developing any other congenital malformations associated with lamotrigine monotherapy, the investigators said.
The authors said their results were in accord with those from several previous studies that did not detect an increased risk of orofacial clefts. In addition, they said statistically significant independent evidence of a clubfoot excess was not detected in the current study, despite findings from their previous study suggesting an increased risk.
The EUROCAT Central Database was funded by the EU Public Health Programme. GlaxoSmithKline, which markets lamotrigine, provided a grant for additional funding of this study. Dr. Dolk and her coauthors reported that their institutions received funding from GlaxoSmithKline for data or staff time contributed to this study.
A new analysis of registry data from European countries does not support a risk of orofacial cleft and clubfoot with exposure to lamotrigine monotherapy, in contrast to signals from previous studies of the antiepileptic drug.
First author Helen Dolk, Dr.P.H., professor of epidemiology and health services research and the head of the center for maternal, fetal, and infant research at the University of Ulster in Coleraine, Northern Ireland, and her colleagues analyzed data from 10.1 million births exposed to antiepileptic drugs including lamotrigine (Lamictal) as a monotherapy during the first trimester between 1995 and 2011. The births were recorded in 21 population-based registries from 16 European countries. The outcomes of interest were major congenital malformations in general, as well as orofacial clefts and clubfoot (Neurology. 2016 April 6. doi: 10.1212/WNL.0000000000002540).
Assessment of all antiepileptic drug-exposed congenital malformation registrations revealed that 12% of pregnant registrants were exposed to lamotrigine monotherapy, with an additional 7% exposed to lamotrigine as part of polytherapy. A total of 77.1% of pregnant women exposed to lamotrigine monotherapy had records indicative of a diagnosis of epilepsy. The proportion of lamotrigine monotherapy exposures was observed to have increased over the study period, likely based on a movement away from the traditional use of valproate because of teratogenic concerns.
A total of 147 lamotrigine monotherapy-exposed babies with congenital malformations not attributable to chromosomal irregularities were identified from the total sample. The odds ratio for having a child with orofacial clefts after exposure to lamotrigine monotherapy was 1.31 (95% confidence interval, 0.73-2.33). Based on these data, the authors said they estimated exposure to lamotrigine would result in orofacial clefts in fewer than 1 in every 550 exposed babies.
The odds ratio for having a child with clubfoot after exposure to lamotrigine monotherapy was 1.83 (95% CI, 1.01-3.31). Although the study results confirmed the statistically significant signal for an overall excess of clubfoot risk found in a previous study conducted by this research team that analyzed births during 1995-2005, the investigators could not reproduce this result in an independent study population of 6.3 million births during 2005-2011(odds ratio, 1.43; 95% CI, 0.66-3.08). There were no significant differences in the risk for developing any other congenital malformations associated with lamotrigine monotherapy, the investigators said.
The authors said their results were in accord with those from several previous studies that did not detect an increased risk of orofacial clefts. In addition, they said statistically significant independent evidence of a clubfoot excess was not detected in the current study, despite findings from their previous study suggesting an increased risk.
The EUROCAT Central Database was funded by the EU Public Health Programme. GlaxoSmithKline, which markets lamotrigine, provided a grant for additional funding of this study. Dr. Dolk and her coauthors reported that their institutions received funding from GlaxoSmithKline for data or staff time contributed to this study.
FROM NEUROLOGY
Key clinical point:Babies born to mothers exposed to lamotrigine monotherapy do not show evidence for an increased incidence of orofacial clefts or clubfoot.
Major finding: The odds ratios for having a child with orofacial clefts or clubfoot after exposure to lamotrigine monotherapy were 1.31 and 1.83, respectively.
Data source: A 16-year, observational study comparing the rate of lamotrigine exposure among births with orofacial clefts or clubfoot in 10.1 million births recorded in 21 population-based registries from 16 European countries.
Disclosures: The EUROCAT Central Database was funded by the EU Public Health Programme. GlaxoSmithKline, which markets lamotrigine, provided a grant for additional funding of this study. Dr. Dolk and her coauthors reported that their institutions received funding from GlaxoSmithKline for data or staff time contributed to this study.
Grim projections for hepatitis C disease burden in the U.S.
Although highly effective oral direct-acting antivirals (DAAs) provide clinicians with the opportunity to reduce the substantial disease burden associated with hepatitis C virus (HCV) infection in the United States, the promise of these agents cannot be realized without the expansion of HCV screening and treatment capacity, according to a report published online in Hepatology.
Working with his colleagues, Dr. Jagpreet Chhatwal of the Massachusetts General Hospital Institute for Technology Assessment and of the department of radiology at Harvard Medical School, both in Boston, utilized a validated projection model previously developed by this research team to estimate the numbers of people in the United States who will die, develop hepatocellular carcinoma, and develop decompensated cirrhosis over the next 35 years (Hepatology. 2016 Mar 25. doi: 10.1002/hep.28571).
The results of the model provided an estimate of 320,000 for the cumulative number of HCV-associated deaths in individuals treated with oral DAAs from 2015 to 2050. In addition, the projected cumulative incidence of hepatocellular carcinoma was 157,000, and the projected cumulative incidence of decompensated cirrhosis was 203,000 in individuals treated with oral DAAs from 2015 to 2050. Furthermore, the projected number of liver transplants for those on DAAs between 2015 and 2050 was 32,000.
When assessing the variables that most heavily influenced the projections, the authors said that most of the ongoing burden of HCV is related to the proportion of infected individuals who remain unaware of their infection status.
Despite such grim predictions, the research suggests hope remains, the authors said. With the same model, changing the rate of treatment from 150,000 patients per year in 2014 to 280,000 patients per year from 2015 onward would result in large reductions in the projected disease burden. For example, 8,600 cases of decompensated cirrhosis, 5,400 cases of hepatocellular carcinoma, 9,700 liver-related deaths, and 900 liver transplants would be prevented. These numbers would increase further if the annual treatment rate was increased to 500,000 patients per year from 2015 onward, preventing 12,000 cases of decompensated cirrhosis, 7,400 cases of hepatocellular carcinoma, 13,500 liver-related deaths, and 1,400 liver transplants. These model-based results emphasize the importance of expanding treatment capacity, as well as HCV screening efforts, the investigators said.
The results are important for the planning and distribution of health care resources and personnel in order to ensure that they match both current and future treatment demands, the investigators added. As an example, they highlighted their projection indicating that the number of clinicians and facilities offering HCV treatment would need to increase substantially over the next 3-4 years. Toward this end, they suggested that primary care physicians or infectious disease specialists be incorporated into HCV treatment capacity expansion.
This project was funded in part by the National Institutes of Health and Gilead Sciences. No conflicts of interest were declared.
Although highly effective oral direct-acting antivirals (DAAs) provide clinicians with the opportunity to reduce the substantial disease burden associated with hepatitis C virus (HCV) infection in the United States, the promise of these agents cannot be realized without the expansion of HCV screening and treatment capacity, according to a report published online in Hepatology.
Working with his colleagues, Dr. Jagpreet Chhatwal of the Massachusetts General Hospital Institute for Technology Assessment and of the department of radiology at Harvard Medical School, both in Boston, utilized a validated projection model previously developed by this research team to estimate the numbers of people in the United States who will die, develop hepatocellular carcinoma, and develop decompensated cirrhosis over the next 35 years (Hepatology. 2016 Mar 25. doi: 10.1002/hep.28571).
The results of the model provided an estimate of 320,000 for the cumulative number of HCV-associated deaths in individuals treated with oral DAAs from 2015 to 2050. In addition, the projected cumulative incidence of hepatocellular carcinoma was 157,000, and the projected cumulative incidence of decompensated cirrhosis was 203,000 in individuals treated with oral DAAs from 2015 to 2050. Furthermore, the projected number of liver transplants for those on DAAs between 2015 and 2050 was 32,000.
When assessing the variables that most heavily influenced the projections, the authors said that most of the ongoing burden of HCV is related to the proportion of infected individuals who remain unaware of their infection status.
Despite such grim predictions, the research suggests hope remains, the authors said. With the same model, changing the rate of treatment from 150,000 patients per year in 2014 to 280,000 patients per year from 2015 onward would result in large reductions in the projected disease burden. For example, 8,600 cases of decompensated cirrhosis, 5,400 cases of hepatocellular carcinoma, 9,700 liver-related deaths, and 900 liver transplants would be prevented. These numbers would increase further if the annual treatment rate was increased to 500,000 patients per year from 2015 onward, preventing 12,000 cases of decompensated cirrhosis, 7,400 cases of hepatocellular carcinoma, 13,500 liver-related deaths, and 1,400 liver transplants. These model-based results emphasize the importance of expanding treatment capacity, as well as HCV screening efforts, the investigators said.
The results are important for the planning and distribution of health care resources and personnel in order to ensure that they match both current and future treatment demands, the investigators added. As an example, they highlighted their projection indicating that the number of clinicians and facilities offering HCV treatment would need to increase substantially over the next 3-4 years. Toward this end, they suggested that primary care physicians or infectious disease specialists be incorporated into HCV treatment capacity expansion.
This project was funded in part by the National Institutes of Health and Gilead Sciences. No conflicts of interest were declared.
Although highly effective oral direct-acting antivirals (DAAs) provide clinicians with the opportunity to reduce the substantial disease burden associated with hepatitis C virus (HCV) infection in the United States, the promise of these agents cannot be realized without the expansion of HCV screening and treatment capacity, according to a report published online in Hepatology.
Working with his colleagues, Dr. Jagpreet Chhatwal of the Massachusetts General Hospital Institute for Technology Assessment and of the department of radiology at Harvard Medical School, both in Boston, utilized a validated projection model previously developed by this research team to estimate the numbers of people in the United States who will die, develop hepatocellular carcinoma, and develop decompensated cirrhosis over the next 35 years (Hepatology. 2016 Mar 25. doi: 10.1002/hep.28571).
The results of the model provided an estimate of 320,000 for the cumulative number of HCV-associated deaths in individuals treated with oral DAAs from 2015 to 2050. In addition, the projected cumulative incidence of hepatocellular carcinoma was 157,000, and the projected cumulative incidence of decompensated cirrhosis was 203,000 in individuals treated with oral DAAs from 2015 to 2050. Furthermore, the projected number of liver transplants for those on DAAs between 2015 and 2050 was 32,000.
When assessing the variables that most heavily influenced the projections, the authors said that most of the ongoing burden of HCV is related to the proportion of infected individuals who remain unaware of their infection status.
Despite such grim predictions, the research suggests hope remains, the authors said. With the same model, changing the rate of treatment from 150,000 patients per year in 2014 to 280,000 patients per year from 2015 onward would result in large reductions in the projected disease burden. For example, 8,600 cases of decompensated cirrhosis, 5,400 cases of hepatocellular carcinoma, 9,700 liver-related deaths, and 900 liver transplants would be prevented. These numbers would increase further if the annual treatment rate was increased to 500,000 patients per year from 2015 onward, preventing 12,000 cases of decompensated cirrhosis, 7,400 cases of hepatocellular carcinoma, 13,500 liver-related deaths, and 1,400 liver transplants. These model-based results emphasize the importance of expanding treatment capacity, as well as HCV screening efforts, the investigators said.
The results are important for the planning and distribution of health care resources and personnel in order to ensure that they match both current and future treatment demands, the investigators added. As an example, they highlighted their projection indicating that the number of clinicians and facilities offering HCV treatment would need to increase substantially over the next 3-4 years. Toward this end, they suggested that primary care physicians or infectious disease specialists be incorporated into HCV treatment capacity expansion.
This project was funded in part by the National Institutes of Health and Gilead Sciences. No conflicts of interest were declared.
FROM HEPATOLOGY
Key clinical point: Unless screening and treatment capacity for hepatitis C virus infection are expanded, associated disease burdens are projected to remain high, despite the availability of highly efficacious direct-acting antiviral agents.
Major Finding: Model-based projections suggest that hundreds of thousands of people will die, develop hepatocellular carcinoma, and develop decompensated cirrhosis in the United States by 2050.
Data Source: A validated hepatitis C disease burden simulation model previously developed and used to project the changing prevalence of hepatitis C virus in the United States.
Disclosures: This project was funded in part by the National Institutes of Health and Gilead Sciences. No conflicts of interest were declared.