User login
‘Obesity paradox’ in AFib challenged as mortality climbs with BMI
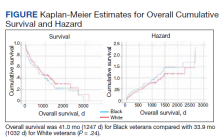
The relationship between body mass index (BMI) and all-cause mortality in patients with atrial fibrillation (AFib) is U-shaped, with the risk highest in those who are underweight or severely obese and lowest in patients defined simply as obese, a registry analysis suggests. It also showed a similar relationship between BMI and risk for new or worsening heart failure (HF).
Mortality bottomed out at a BMI of about 30-35 kg/m2, which suggests that mild obesity was protective, compared even with “normal-weight” or “overweight” BMI. Still, mortality went up sharply from there with rising BMI.
But higher BMI, a surrogate for obesity, apparently didn’t worsen outcomes by itself. The risk for death from any cause at higher obesity levels was found to depend a lot on related risk factors and comorbidities when the analysis controlled for conditions such as diabetes and hypertension.
The findings suggest an inverse relationship between BMI and all-cause mortality in AFib only for patients with BMI less than about 30. They therefore argue against any “obesity paradox” in AFib that posits consistently better survival with increasing levels of obesity, say researchers, based on their analysis of patients with new-onset AFib in the GARFIELD-AF registry.
“It’s common practice now for clinicians to discuss weight within a clinic setting when they’re talking to their AFib patients,” observed Christian Fielder Camm, BM, BCh, University of Oxford (England), and Royal Berkshire NHS Foundation Trust, Reading, England. So studies suggesting an inverse association between BMI and AFib-related risk can be a concern.
Such studies “seem to suggest that once you’ve got AFib, maintaining a high or very high BMI may in some way be protective – which is contrary to what would seem to make sense and certainly contrary to what our results have shown,” Dr. Camm told this news organization.
“I think that having further evidence now to suggest, actually, that greater BMI is associated with a greater risk of all-cause mortality and heart failure helps reframe that discussion at the physician-patient interaction level more clearly, and ensures that we’re able to talk to our patients appropriately about risks associated with BMI and atrial fibrillation,” said Dr. Camm, who is lead author on the analysis published in Open Heart.
“Obesity is a cause of most cardiovascular diseases, but [these] data would support that being overweight or having mild obesity does not increase the risk,” observed Carl J. Lavie, MD, of the John Ochsner Heart and Vascular Institute, New Orleans, La., and the Ochsner Clinical School at the University of Queensland, Brisbane, Australia.
“At a BMI of 40, it’s very important for them to lose weight for their long-term prognosis,” Dr. Lavie noted, but “at a BMI of 30, the important thing would be to prevent further weight gain. And if they could keep their BMI of 30, they should have a good prognosis. Their prognosis would be particularly good if they didn’t gain weight and put themselves in a more extreme obesity class that is associated with worse risk.”
The current analysis, Dr. Lavie said, “is way better than the AFFIRM study,” which yielded an obesity-paradox report on its patients with AFib about a dozen years ago. “It’s got more data, more numbers, more statistical power,” and breaks BMI into more categories.
That previous analysis based on the influential AFFIRM randomized trial separated its 4,060 patients with AFib into normal (BMI, 18.5-25), overweight (BMI, 25-30), and obese (BMI, > 30) categories, per the convention at the time. It concluded that “obese patients with atrial fibrillation appear to have better long-term outcomes than nonobese patients.”
Bleeding risk on oral anticoagulants
Also noteworthy in the current analysis, variation in BMI didn’t seem to affect mortality or risk for major bleeding or nonhemorrhagic stroke according to choice of oral anticoagulant – whether a new oral anticoagulant (NOAC) or a vitamin K antagonist (VKA).
“We saw that even in the obese and extremely obese group, all-cause mortality was lower in the group taking NOACs, compared with taking warfarin,” Dr. Camm observed, “which goes against the idea that we would need any kind of dose adjustments for increased BMI.”
Indeed, the report notes, use of NOACs, compared with VKA, was associated with a 23% drop in risk for death among patients who were either normal weight or overweight and also in those who were obese or extremely obese.
Those findings “are basically saying that the NOACs look better than warfarin regardless of weight,” agreed Dr. Lavie. “The problem is that the study is not very powered.”
Whereas the benefits of NOACs, compared to VKA, seem similar for patients with a BMI of 30 or 34, compared with a BMI of 23, for example, “none of the studies has many people with 50 BMI.” Many clinicians “feel uncomfortable giving the same dose of NOAC to somebody who has a 60 BMI,” he said. At least with warfarin, “you can check the INR [international normalized ratio].”
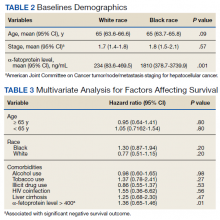
The current analysis included 40,482 patients with recently diagnosed AFib and at least one other stroke risk factor from among the registry’s more than 50,000 patients from 35 countries, enrolled from 2010 to 2016. They were followed for 2 years.
The 703 patients with BMI under 18.5 at AFib diagnosis were classified per World Health Organization definitions as underweight; the 13,095 with BMI 18.5-25 as normal weight; the 15,043 with BMI 25-30 as overweight; the 7,560 with BMI 30-35 as obese; and the 4,081 with BMI above 35 as extremely obese. Their ages averaged 71 years, and 55.6% were men.
BMI effects on different outcomes
Relationships between BMI and all-cause mortality and between BMI and new or worsening HF emerged as U-shaped, the risk climbing with both increasing and decreasing BMI. The nadir BMI for risk was about 30 in the case of mortality and about 25 for new or worsening HF.
The all-cause mortality risk rose by 32% for every 5 BMI points lower than a BMI of 30, and by 16% for every 5 BMI points higher than 30, in a partially adjusted analysis. The risk for new or worsening HF rose significantly with increasing but not decreasing BMI, and the reverse was observed for the endpoint of major bleeding.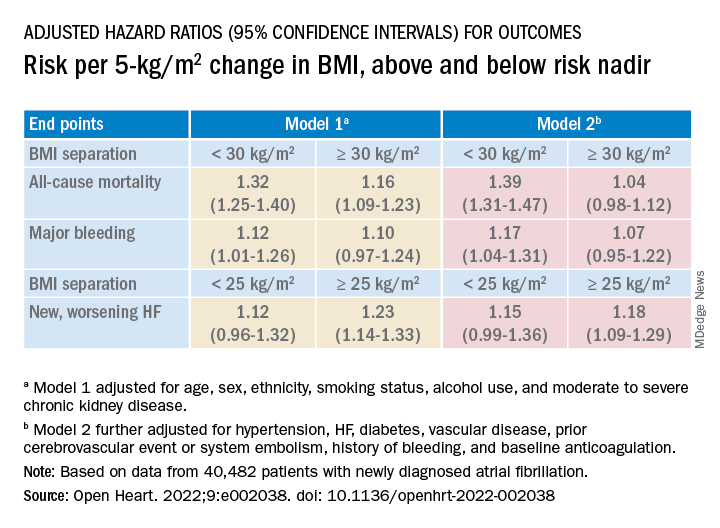
The effect of BMI on all-cause mortality was “substantially attenuated” when the analysis was further adjusted with “likely mediators of any association between BMI and outcomes,” including hypertension, diabetes, HF, cerebrovascular events, and history of bleeding, Dr. Camm said.
That blunted BMI-mortality relationship, he said, “suggests that a lot of the effect is mediated through relatively traditional risk factors like hypertension and diabetes.”
The 2010 AFFIRM analysis by BMI, Dr. Lavie noted, “didn’t even look at the underweight; they actually threw them out.” Yet, such patients with AFib, who tend to be extremely frail or have chronic diseases or conditions other than the arrhythmia, are common. A take-home of the current study is that “the underweight with atrial fibrillation have a really bad prognosis.”
That message isn’t heard as much, he observed, “but is as important as saying that BMI 30 has the best prognosis. The worst prognosis is with the underweight or the really extreme obese.”
Dr. Camm discloses research funding from the British Heart Foundation. Disclosures for the other authors are in the report. Dr. Lavie has previously disclosed serving as a speaker and consultant for PAI Health and DSM Nutritional Products and is the author of “The Obesity Paradox: When Thinner Means Sicker and Heavier Means Healthier” (Avery, 2014).
A version of this article first appeared on Medscape.com.
The relationship between body mass index (BMI) and all-cause mortality in patients with atrial fibrillation (AFib) is U-shaped, with the risk highest in those who are underweight or severely obese and lowest in patients defined simply as obese, a registry analysis suggests. It also showed a similar relationship between BMI and risk for new or worsening heart failure (HF).
Mortality bottomed out at a BMI of about 30-35 kg/m2, which suggests that mild obesity was protective, compared even with “normal-weight” or “overweight” BMI. Still, mortality went up sharply from there with rising BMI.
But higher BMI, a surrogate for obesity, apparently didn’t worsen outcomes by itself. The risk for death from any cause at higher obesity levels was found to depend a lot on related risk factors and comorbidities when the analysis controlled for conditions such as diabetes and hypertension.
The findings suggest an inverse relationship between BMI and all-cause mortality in AFib only for patients with BMI less than about 30. They therefore argue against any “obesity paradox” in AFib that posits consistently better survival with increasing levels of obesity, say researchers, based on their analysis of patients with new-onset AFib in the GARFIELD-AF registry.
“It’s common practice now for clinicians to discuss weight within a clinic setting when they’re talking to their AFib patients,” observed Christian Fielder Camm, BM, BCh, University of Oxford (England), and Royal Berkshire NHS Foundation Trust, Reading, England. So studies suggesting an inverse association between BMI and AFib-related risk can be a concern.
Such studies “seem to suggest that once you’ve got AFib, maintaining a high or very high BMI may in some way be protective – which is contrary to what would seem to make sense and certainly contrary to what our results have shown,” Dr. Camm told this news organization.
“I think that having further evidence now to suggest, actually, that greater BMI is associated with a greater risk of all-cause mortality and heart failure helps reframe that discussion at the physician-patient interaction level more clearly, and ensures that we’re able to talk to our patients appropriately about risks associated with BMI and atrial fibrillation,” said Dr. Camm, who is lead author on the analysis published in Open Heart.
“Obesity is a cause of most cardiovascular diseases, but [these] data would support that being overweight or having mild obesity does not increase the risk,” observed Carl J. Lavie, MD, of the John Ochsner Heart and Vascular Institute, New Orleans, La., and the Ochsner Clinical School at the University of Queensland, Brisbane, Australia.
“At a BMI of 40, it’s very important for them to lose weight for their long-term prognosis,” Dr. Lavie noted, but “at a BMI of 30, the important thing would be to prevent further weight gain. And if they could keep their BMI of 30, they should have a good prognosis. Their prognosis would be particularly good if they didn’t gain weight and put themselves in a more extreme obesity class that is associated with worse risk.”
The current analysis, Dr. Lavie said, “is way better than the AFFIRM study,” which yielded an obesity-paradox report on its patients with AFib about a dozen years ago. “It’s got more data, more numbers, more statistical power,” and breaks BMI into more categories.
That previous analysis based on the influential AFFIRM randomized trial separated its 4,060 patients with AFib into normal (BMI, 18.5-25), overweight (BMI, 25-30), and obese (BMI, > 30) categories, per the convention at the time. It concluded that “obese patients with atrial fibrillation appear to have better long-term outcomes than nonobese patients.”
Bleeding risk on oral anticoagulants
Also noteworthy in the current analysis, variation in BMI didn’t seem to affect mortality or risk for major bleeding or nonhemorrhagic stroke according to choice of oral anticoagulant – whether a new oral anticoagulant (NOAC) or a vitamin K antagonist (VKA).
“We saw that even in the obese and extremely obese group, all-cause mortality was lower in the group taking NOACs, compared with taking warfarin,” Dr. Camm observed, “which goes against the idea that we would need any kind of dose adjustments for increased BMI.”
Indeed, the report notes, use of NOACs, compared with VKA, was associated with a 23% drop in risk for death among patients who were either normal weight or overweight and also in those who were obese or extremely obese.
Those findings “are basically saying that the NOACs look better than warfarin regardless of weight,” agreed Dr. Lavie. “The problem is that the study is not very powered.”
Whereas the benefits of NOACs, compared to VKA, seem similar for patients with a BMI of 30 or 34, compared with a BMI of 23, for example, “none of the studies has many people with 50 BMI.” Many clinicians “feel uncomfortable giving the same dose of NOAC to somebody who has a 60 BMI,” he said. At least with warfarin, “you can check the INR [international normalized ratio].”
The current analysis included 40,482 patients with recently diagnosed AFib and at least one other stroke risk factor from among the registry’s more than 50,000 patients from 35 countries, enrolled from 2010 to 2016. They were followed for 2 years.
The 703 patients with BMI under 18.5 at AFib diagnosis were classified per World Health Organization definitions as underweight; the 13,095 with BMI 18.5-25 as normal weight; the 15,043 with BMI 25-30 as overweight; the 7,560 with BMI 30-35 as obese; and the 4,081 with BMI above 35 as extremely obese. Their ages averaged 71 years, and 55.6% were men.
BMI effects on different outcomes
Relationships between BMI and all-cause mortality and between BMI and new or worsening HF emerged as U-shaped, the risk climbing with both increasing and decreasing BMI. The nadir BMI for risk was about 30 in the case of mortality and about 25 for new or worsening HF.
The all-cause mortality risk rose by 32% for every 5 BMI points lower than a BMI of 30, and by 16% for every 5 BMI points higher than 30, in a partially adjusted analysis. The risk for new or worsening HF rose significantly with increasing but not decreasing BMI, and the reverse was observed for the endpoint of major bleeding.
The effect of BMI on all-cause mortality was “substantially attenuated” when the analysis was further adjusted with “likely mediators of any association between BMI and outcomes,” including hypertension, diabetes, HF, cerebrovascular events, and history of bleeding, Dr. Camm said.
That blunted BMI-mortality relationship, he said, “suggests that a lot of the effect is mediated through relatively traditional risk factors like hypertension and diabetes.”
The 2010 AFFIRM analysis by BMI, Dr. Lavie noted, “didn’t even look at the underweight; they actually threw them out.” Yet, such patients with AFib, who tend to be extremely frail or have chronic diseases or conditions other than the arrhythmia, are common. A take-home of the current study is that “the underweight with atrial fibrillation have a really bad prognosis.”
That message isn’t heard as much, he observed, “but is as important as saying that BMI 30 has the best prognosis. The worst prognosis is with the underweight or the really extreme obese.”
Dr. Camm discloses research funding from the British Heart Foundation. Disclosures for the other authors are in the report. Dr. Lavie has previously disclosed serving as a speaker and consultant for PAI Health and DSM Nutritional Products and is the author of “The Obesity Paradox: When Thinner Means Sicker and Heavier Means Healthier” (Avery, 2014).
A version of this article first appeared on Medscape.com.
The relationship between body mass index (BMI) and all-cause mortality in patients with atrial fibrillation (AFib) is U-shaped, with the risk highest in those who are underweight or severely obese and lowest in patients defined simply as obese, a registry analysis suggests. It also showed a similar relationship between BMI and risk for new or worsening heart failure (HF).
Mortality bottomed out at a BMI of about 30-35 kg/m2, which suggests that mild obesity was protective, compared even with “normal-weight” or “overweight” BMI. Still, mortality went up sharply from there with rising BMI.
But higher BMI, a surrogate for obesity, apparently didn’t worsen outcomes by itself. The risk for death from any cause at higher obesity levels was found to depend a lot on related risk factors and comorbidities when the analysis controlled for conditions such as diabetes and hypertension.
The findings suggest an inverse relationship between BMI and all-cause mortality in AFib only for patients with BMI less than about 30. They therefore argue against any “obesity paradox” in AFib that posits consistently better survival with increasing levels of obesity, say researchers, based on their analysis of patients with new-onset AFib in the GARFIELD-AF registry.
“It’s common practice now for clinicians to discuss weight within a clinic setting when they’re talking to their AFib patients,” observed Christian Fielder Camm, BM, BCh, University of Oxford (England), and Royal Berkshire NHS Foundation Trust, Reading, England. So studies suggesting an inverse association between BMI and AFib-related risk can be a concern.
Such studies “seem to suggest that once you’ve got AFib, maintaining a high or very high BMI may in some way be protective – which is contrary to what would seem to make sense and certainly contrary to what our results have shown,” Dr. Camm told this news organization.
“I think that having further evidence now to suggest, actually, that greater BMI is associated with a greater risk of all-cause mortality and heart failure helps reframe that discussion at the physician-patient interaction level more clearly, and ensures that we’re able to talk to our patients appropriately about risks associated with BMI and atrial fibrillation,” said Dr. Camm, who is lead author on the analysis published in Open Heart.
“Obesity is a cause of most cardiovascular diseases, but [these] data would support that being overweight or having mild obesity does not increase the risk,” observed Carl J. Lavie, MD, of the John Ochsner Heart and Vascular Institute, New Orleans, La., and the Ochsner Clinical School at the University of Queensland, Brisbane, Australia.
“At a BMI of 40, it’s very important for them to lose weight for their long-term prognosis,” Dr. Lavie noted, but “at a BMI of 30, the important thing would be to prevent further weight gain. And if they could keep their BMI of 30, they should have a good prognosis. Their prognosis would be particularly good if they didn’t gain weight and put themselves in a more extreme obesity class that is associated with worse risk.”
The current analysis, Dr. Lavie said, “is way better than the AFFIRM study,” which yielded an obesity-paradox report on its patients with AFib about a dozen years ago. “It’s got more data, more numbers, more statistical power,” and breaks BMI into more categories.
That previous analysis based on the influential AFFIRM randomized trial separated its 4,060 patients with AFib into normal (BMI, 18.5-25), overweight (BMI, 25-30), and obese (BMI, > 30) categories, per the convention at the time. It concluded that “obese patients with atrial fibrillation appear to have better long-term outcomes than nonobese patients.”
Bleeding risk on oral anticoagulants
Also noteworthy in the current analysis, variation in BMI didn’t seem to affect mortality or risk for major bleeding or nonhemorrhagic stroke according to choice of oral anticoagulant – whether a new oral anticoagulant (NOAC) or a vitamin K antagonist (VKA).
“We saw that even in the obese and extremely obese group, all-cause mortality was lower in the group taking NOACs, compared with taking warfarin,” Dr. Camm observed, “which goes against the idea that we would need any kind of dose adjustments for increased BMI.”
Indeed, the report notes, use of NOACs, compared with VKA, was associated with a 23% drop in risk for death among patients who were either normal weight or overweight and also in those who were obese or extremely obese.
Those findings “are basically saying that the NOACs look better than warfarin regardless of weight,” agreed Dr. Lavie. “The problem is that the study is not very powered.”
Whereas the benefits of NOACs, compared to VKA, seem similar for patients with a BMI of 30 or 34, compared with a BMI of 23, for example, “none of the studies has many people with 50 BMI.” Many clinicians “feel uncomfortable giving the same dose of NOAC to somebody who has a 60 BMI,” he said. At least with warfarin, “you can check the INR [international normalized ratio].”
The current analysis included 40,482 patients with recently diagnosed AFib and at least one other stroke risk factor from among the registry’s more than 50,000 patients from 35 countries, enrolled from 2010 to 2016. They were followed for 2 years.
The 703 patients with BMI under 18.5 at AFib diagnosis were classified per World Health Organization definitions as underweight; the 13,095 with BMI 18.5-25 as normal weight; the 15,043 with BMI 25-30 as overweight; the 7,560 with BMI 30-35 as obese; and the 4,081 with BMI above 35 as extremely obese. Their ages averaged 71 years, and 55.6% were men.
BMI effects on different outcomes
Relationships between BMI and all-cause mortality and between BMI and new or worsening HF emerged as U-shaped, the risk climbing with both increasing and decreasing BMI. The nadir BMI for risk was about 30 in the case of mortality and about 25 for new or worsening HF.
The all-cause mortality risk rose by 32% for every 5 BMI points lower than a BMI of 30, and by 16% for every 5 BMI points higher than 30, in a partially adjusted analysis. The risk for new or worsening HF rose significantly with increasing but not decreasing BMI, and the reverse was observed for the endpoint of major bleeding.
The effect of BMI on all-cause mortality was “substantially attenuated” when the analysis was further adjusted with “likely mediators of any association between BMI and outcomes,” including hypertension, diabetes, HF, cerebrovascular events, and history of bleeding, Dr. Camm said.
That blunted BMI-mortality relationship, he said, “suggests that a lot of the effect is mediated through relatively traditional risk factors like hypertension and diabetes.”
The 2010 AFFIRM analysis by BMI, Dr. Lavie noted, “didn’t even look at the underweight; they actually threw them out.” Yet, such patients with AFib, who tend to be extremely frail or have chronic diseases or conditions other than the arrhythmia, are common. A take-home of the current study is that “the underweight with atrial fibrillation have a really bad prognosis.”
That message isn’t heard as much, he observed, “but is as important as saying that BMI 30 has the best prognosis. The worst prognosis is with the underweight or the really extreme obese.”
Dr. Camm discloses research funding from the British Heart Foundation. Disclosures for the other authors are in the report. Dr. Lavie has previously disclosed serving as a speaker and consultant for PAI Health and DSM Nutritional Products and is the author of “The Obesity Paradox: When Thinner Means Sicker and Heavier Means Healthier” (Avery, 2014).
A version of this article first appeared on Medscape.com.
FROM OPEN HEART
Phase 3 data: Zanubrutinib bests standard CLL treatment
At a median follow-up of 26.2 months, progression to worsening disease or death was much lower in patients with these conditions who took zanubrutinib (Brukinsa), compared with those who took bendamustine-rituximab (hazard ratio. 0.42; 95% confidence interval, 0.28-0.63; P < .00011). The study was published in The Lancet Oncology.
Researchers already knew that ibrutinib, another BTKi, improves progression-free survival, study coauthor Paolo Ghia, MD, PhD, professor of medical oncology at Vita-Salute San Raffaele University, Milan, said in an interview. “Now we confirmed that the same advantage can be seen” in zanubrutinib.
According to Dr. Ghia, bendamustine-rituximab has long been a standard treatment in blood cancers and is considered well tolerated and inexpensive. But BTKis such as first-in-line ibrutinib have shown better results, he said, “and progressively, we are going to abandon bendamustine-rituximab.”
However, ibrutinib causes significant adverse effects such as bleeding, worsening hypertension and arrhythmia, he noted. As a result, second-generation BTKi such as zanubrutinib have entered the picture. The Food and Drug Administration approved it in 2019 for mantle cell lymphoma, and it has since been approved for Waldenström’s macroglobulinemia and marginal zone lymphoma.
In 2021, an interim analysis in a trial of the drug in patients with previously treated CLL, compared with ibrutinib, found that “zanubrutinib was shown to have a superior response rate, an improved PFS, and a lower rate of atrial fibrillation/flutter.”
The drug’s manufacturer, BeiGene, launched the new open-label, multicenter study, in a bid for FDA approval of the drug as a frontline treatment for CLL and SLL. More than 150 hospitals in 14 countries participated in the trial from 2017 to 2019.
The subjects were all adults and at least 65 years old or with comorbidities; None had the genetic trait del(17)(p13.1); 241 were assigned to take zanubrutinib and 238 to bendamustine-rituximab. Another group consisted of 111 patients with CLL and del(17)(p13·1). According to the study authors, these patients are especially difficult to treat.
The vast majority of patients were White (92%-95% depending on group) and male (61%-71%); 90%-92% had CLL.
At follow-up, there was no difference in overall survival between the main zanubrutinib and bendamustine-rituximab groups; 29 (12%) of the 241 patients in the zanubrutinib group and 57 (24%) of 238 patients in the bendamustine-rituximab group had progressed or died (HR, 0.42; 95% CI, 0.27-0.66; P < .00011). Adverse events leading to discontinuation were more common in the bendamustine-rituximab group (14%) versus zanubrutinib (8%).
In the third group, which only received zanubrutinib, 14% of patients died at median follow-up of 30.5 months; 98% of patients had adverse effects, and 5% discontinued treatment.
The researchers wrote that “zanubrutinib showed superior progression-free survival versus bendamustine-rituximab in older patients or those with comorbidities with untreated CLL, with a low incidence of cardiac arrhythmia. Similar efficacy was observed in patients with del(17p)–positive disease.”
The study didn’t examine cost; zanubrutinib is quite expensive.
In an interview, hematologist-oncologist Anthony Mato, MD, of Memorial Sloan Kettering Cancer Center in New York said the new study is important although not surprising, since other medications in the same class have shown similar results. Zanubrutinib is an alternative to ibrutinib, although the latter remains “an excellent drug,” he said.
“The era of chemotherapy being a first choice is over,” he said. “We’ve had several randomized studies that show targeted therapies are better tolerated and have better outcomes. We now need to look through the choices to decide which one of these good options are the best for our patients.”
In an interview, hematologist-oncologist Joanna Rhodes, MD, of Northwell Health in Hempstead, N.Y., highlighted the side effect profile of zanubrutinib, noting that it is low and resembles that of other BTKis, making it “another excellent treatment option.”
“We are seeing that bruising, upper respiratory tract infections, diarrhea, and arthralgias are the most common side effects,” she said. “Bleeding also is a common side effect, which is consistent across the class of BTKis, with 5% of patients developing a major bleed. Also, 3% of patients treated with zanubrutinib developed atrial fibrillation, which is consistent with data from other trials. Treatment discontinuation rates were low (8%).”
The study was funded by BeiGene. The authors reported multiple disclosures. Dr. Mato reported research or consulting relationships with BeiGene, AstraZeneca, and AbbVie. Dr. Rhodes reported multiple research or consulting relationships with Abbvie, BeiGene, Genentech, and others.
At a median follow-up of 26.2 months, progression to worsening disease or death was much lower in patients with these conditions who took zanubrutinib (Brukinsa), compared with those who took bendamustine-rituximab (hazard ratio. 0.42; 95% confidence interval, 0.28-0.63; P < .00011). The study was published in The Lancet Oncology.
Researchers already knew that ibrutinib, another BTKi, improves progression-free survival, study coauthor Paolo Ghia, MD, PhD, professor of medical oncology at Vita-Salute San Raffaele University, Milan, said in an interview. “Now we confirmed that the same advantage can be seen” in zanubrutinib.
According to Dr. Ghia, bendamustine-rituximab has long been a standard treatment in blood cancers and is considered well tolerated and inexpensive. But BTKis such as first-in-line ibrutinib have shown better results, he said, “and progressively, we are going to abandon bendamustine-rituximab.”
However, ibrutinib causes significant adverse effects such as bleeding, worsening hypertension and arrhythmia, he noted. As a result, second-generation BTKi such as zanubrutinib have entered the picture. The Food and Drug Administration approved it in 2019 for mantle cell lymphoma, and it has since been approved for Waldenström’s macroglobulinemia and marginal zone lymphoma.
In 2021, an interim analysis in a trial of the drug in patients with previously treated CLL, compared with ibrutinib, found that “zanubrutinib was shown to have a superior response rate, an improved PFS, and a lower rate of atrial fibrillation/flutter.”
The drug’s manufacturer, BeiGene, launched the new open-label, multicenter study, in a bid for FDA approval of the drug as a frontline treatment for CLL and SLL. More than 150 hospitals in 14 countries participated in the trial from 2017 to 2019.
The subjects were all adults and at least 65 years old or with comorbidities; None had the genetic trait del(17)(p13.1); 241 were assigned to take zanubrutinib and 238 to bendamustine-rituximab. Another group consisted of 111 patients with CLL and del(17)(p13·1). According to the study authors, these patients are especially difficult to treat.
The vast majority of patients were White (92%-95% depending on group) and male (61%-71%); 90%-92% had CLL.
At follow-up, there was no difference in overall survival between the main zanubrutinib and bendamustine-rituximab groups; 29 (12%) of the 241 patients in the zanubrutinib group and 57 (24%) of 238 patients in the bendamustine-rituximab group had progressed or died (HR, 0.42; 95% CI, 0.27-0.66; P < .00011). Adverse events leading to discontinuation were more common in the bendamustine-rituximab group (14%) versus zanubrutinib (8%).
In the third group, which only received zanubrutinib, 14% of patients died at median follow-up of 30.5 months; 98% of patients had adverse effects, and 5% discontinued treatment.
The researchers wrote that “zanubrutinib showed superior progression-free survival versus bendamustine-rituximab in older patients or those with comorbidities with untreated CLL, with a low incidence of cardiac arrhythmia. Similar efficacy was observed in patients with del(17p)–positive disease.”
The study didn’t examine cost; zanubrutinib is quite expensive.
In an interview, hematologist-oncologist Anthony Mato, MD, of Memorial Sloan Kettering Cancer Center in New York said the new study is important although not surprising, since other medications in the same class have shown similar results. Zanubrutinib is an alternative to ibrutinib, although the latter remains “an excellent drug,” he said.
“The era of chemotherapy being a first choice is over,” he said. “We’ve had several randomized studies that show targeted therapies are better tolerated and have better outcomes. We now need to look through the choices to decide which one of these good options are the best for our patients.”
In an interview, hematologist-oncologist Joanna Rhodes, MD, of Northwell Health in Hempstead, N.Y., highlighted the side effect profile of zanubrutinib, noting that it is low and resembles that of other BTKis, making it “another excellent treatment option.”
“We are seeing that bruising, upper respiratory tract infections, diarrhea, and arthralgias are the most common side effects,” she said. “Bleeding also is a common side effect, which is consistent across the class of BTKis, with 5% of patients developing a major bleed. Also, 3% of patients treated with zanubrutinib developed atrial fibrillation, which is consistent with data from other trials. Treatment discontinuation rates were low (8%).”
The study was funded by BeiGene. The authors reported multiple disclosures. Dr. Mato reported research or consulting relationships with BeiGene, AstraZeneca, and AbbVie. Dr. Rhodes reported multiple research or consulting relationships with Abbvie, BeiGene, Genentech, and others.
At a median follow-up of 26.2 months, progression to worsening disease or death was much lower in patients with these conditions who took zanubrutinib (Brukinsa), compared with those who took bendamustine-rituximab (hazard ratio. 0.42; 95% confidence interval, 0.28-0.63; P < .00011). The study was published in The Lancet Oncology.
Researchers already knew that ibrutinib, another BTKi, improves progression-free survival, study coauthor Paolo Ghia, MD, PhD, professor of medical oncology at Vita-Salute San Raffaele University, Milan, said in an interview. “Now we confirmed that the same advantage can be seen” in zanubrutinib.
According to Dr. Ghia, bendamustine-rituximab has long been a standard treatment in blood cancers and is considered well tolerated and inexpensive. But BTKis such as first-in-line ibrutinib have shown better results, he said, “and progressively, we are going to abandon bendamustine-rituximab.”
However, ibrutinib causes significant adverse effects such as bleeding, worsening hypertension and arrhythmia, he noted. As a result, second-generation BTKi such as zanubrutinib have entered the picture. The Food and Drug Administration approved it in 2019 for mantle cell lymphoma, and it has since been approved for Waldenström’s macroglobulinemia and marginal zone lymphoma.
In 2021, an interim analysis in a trial of the drug in patients with previously treated CLL, compared with ibrutinib, found that “zanubrutinib was shown to have a superior response rate, an improved PFS, and a lower rate of atrial fibrillation/flutter.”
The drug’s manufacturer, BeiGene, launched the new open-label, multicenter study, in a bid for FDA approval of the drug as a frontline treatment for CLL and SLL. More than 150 hospitals in 14 countries participated in the trial from 2017 to 2019.
The subjects were all adults and at least 65 years old or with comorbidities; None had the genetic trait del(17)(p13.1); 241 were assigned to take zanubrutinib and 238 to bendamustine-rituximab. Another group consisted of 111 patients with CLL and del(17)(p13·1). According to the study authors, these patients are especially difficult to treat.
The vast majority of patients were White (92%-95% depending on group) and male (61%-71%); 90%-92% had CLL.
At follow-up, there was no difference in overall survival between the main zanubrutinib and bendamustine-rituximab groups; 29 (12%) of the 241 patients in the zanubrutinib group and 57 (24%) of 238 patients in the bendamustine-rituximab group had progressed or died (HR, 0.42; 95% CI, 0.27-0.66; P < .00011). Adverse events leading to discontinuation were more common in the bendamustine-rituximab group (14%) versus zanubrutinib (8%).
In the third group, which only received zanubrutinib, 14% of patients died at median follow-up of 30.5 months; 98% of patients had adverse effects, and 5% discontinued treatment.
The researchers wrote that “zanubrutinib showed superior progression-free survival versus bendamustine-rituximab in older patients or those with comorbidities with untreated CLL, with a low incidence of cardiac arrhythmia. Similar efficacy was observed in patients with del(17p)–positive disease.”
The study didn’t examine cost; zanubrutinib is quite expensive.
In an interview, hematologist-oncologist Anthony Mato, MD, of Memorial Sloan Kettering Cancer Center in New York said the new study is important although not surprising, since other medications in the same class have shown similar results. Zanubrutinib is an alternative to ibrutinib, although the latter remains “an excellent drug,” he said.
“The era of chemotherapy being a first choice is over,” he said. “We’ve had several randomized studies that show targeted therapies are better tolerated and have better outcomes. We now need to look through the choices to decide which one of these good options are the best for our patients.”
In an interview, hematologist-oncologist Joanna Rhodes, MD, of Northwell Health in Hempstead, N.Y., highlighted the side effect profile of zanubrutinib, noting that it is low and resembles that of other BTKis, making it “another excellent treatment option.”
“We are seeing that bruising, upper respiratory tract infections, diarrhea, and arthralgias are the most common side effects,” she said. “Bleeding also is a common side effect, which is consistent across the class of BTKis, with 5% of patients developing a major bleed. Also, 3% of patients treated with zanubrutinib developed atrial fibrillation, which is consistent with data from other trials. Treatment discontinuation rates were low (8%).”
The study was funded by BeiGene. The authors reported multiple disclosures. Dr. Mato reported research or consulting relationships with BeiGene, AstraZeneca, and AbbVie. Dr. Rhodes reported multiple research or consulting relationships with Abbvie, BeiGene, Genentech, and others.
FROM THE LANCET ONCOLOGY
Safety Profile of Mutant EGFR-TK Inhibitors in Advanced Non–Small Cell Lung Cancer: A Meta-analysis
Lung cancer has been the leading cause of cancer-related mortality for decades. It is also predicted to remain as the leading cause of cancer-related mortality through 2030.1 Platinum-based chemotherapy, including carboplatin and paclitaxel, was introduced 3 decades ago and revolutionized the management of advanced non–small cell lung cancer (NSCLC). A more recent advancement has been mutant epidermal growth factor receptor–tyrosine kinase (EGFR-TK) inhibitors.1 EGFR is a transmembrane protein that functions by transducing essential growth factor signaling from the extracellular milieu to the cell. As 60% of the advanced NSCLC expresses this receptor, blocking the mutant EGFR receptor was a groundbreaking development in the management of advanced NSCLC.2 Development of mutant EGFR-TK inhibitors has revolutionized the management of advanced NSCLC. This study was conducted to determine the safety profile of mutant EGFR-TK inhibitors in the management of advanced NSCLC.
Methods
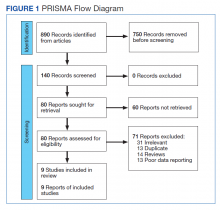
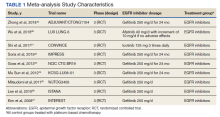
This meta-analysis was conducted according to Cochrane Collaboration guidelines and reported as per Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. The findings are summarized in the PRISMA flow diagram (Figure 1). Two authors (MZ and MM) performed a systematic literature search using databases such as MEDLINE (via PubMed), Embase, and Cochrane Library using the medical search terms and their respective entry words with the following search strategy: safety, “mutant EGFR-TK inhibitors,” advanced, “non–small cell,” “lung cancer,” “adverse effect,” and literature. Additionally, unpublished trials were identified from clinicaltrials.gov, and references of all pertinent articles were also scrutinized to ensure the inclusion of all relevant studies. The search was completed on June 1, 2021, and we only included studies available in English. Two authors (MM and MZ) independently screened the search results in a 2-step process based on predetermined inclusion/exclusion criteria. First, 890 articles were evaluated for relevance on title and abstract level, followed by full-text screening of the final list of 140 articles. Any disagreements were resolved by discussion or third-party review, and a total of 9 articles were included in the study.
The following eligibility criteria were used: original articles reporting adverse effects (AEs) of mutant EGFR-TK inhibitors in patients with advanced NSCLC compared with control groups receiving platinum-based chemotherapy. All the patients included in the study had an EGFR mutation but randomly assigned to either treatment or control group. All articles with subjective data on mutant EGFR-TK inhibitors AEs in patients with advanced NSCLC compared with control groups receiving platinum-based chemotherapy were included in the analysis. Only 9 articles qualified the aforementioned selection criteria for eligibility. All qualifying studies were nationwide inpatient or pooled clinical trials data. The reasons for exclusion of the other 71 articles were irrelevant (n = 31), duplicate (n = 13), reviews (n = 14), and poor data reporting (n = 12). Out of the 9 included studies, 9 studies showed correlation of AEs, including rash, diarrhea, nausea, and fatigue. Seven studies showed correlation of AEs including neutropenia, anorexia, and vomiting. Six studies showed correlation of anemia, cough, and stomatitis. Five studies showed correlation of elevated aspartate aminotransferase (AST), alanine aminotransferase (ALT), and leucopenia. Four studies showed correlation of fever between mutant EGFR-TK inhibitors and platinum-based chemotherapy.
The primary endpoints were reported AEs including rash, diarrhea, elevated ALT, elevated AST, stomatitis, nausea, leucopenia, fatigue, neutropenia, anorexia, anemia, cough, vomiting, and fever, respectively. Data on baseline characteristics and clinical outcomes were then extracted, and summary tables were created. Summary estimates of the clinical endpoints were then calculated with risk ratio (RR) and 95% confidence intervals (CIs) using the random-effects model. Heterogeneity between studies was examined with the Cochran Q I2 statistic which can be defined as low (25% to 50%), moderate (50% to 75%), or high (> 75%). Statistical analysis was performed using Comprehensive Meta-Analysis Software CMA Version 3.0.
Results
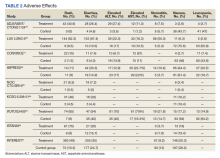
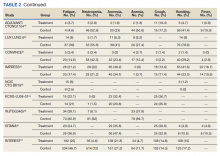
A total of 9 studies including 3415 patients (1775 in EGFR-TK inhibitor treatment group while 1640 patients in platinum-based chemotherapy control group) were included in the study. All 9 studies were phase III randomized control clinical trials conducted to compare the safety profile of mutant EGFR-TK inhibitors in patients with advanced NSCLC. Mean age was 61 years in both treatment and control groups. Further details on study and participant characteristics and safety profile including AEs are summarized in Tables 1 and 2. No evidence of publication bias was found.
Rash developed in 45.8% of patients in the treatment group receiving mutant EGFR-TK inhibitors vs only 5.6% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 7.38 with the 95% CI noted, which was statistically significant, confirming higher rash event rates in patients receiving EGFR-TK inhibitors for their advanced NSCLC (Figure 2).
Diarrhea occurred in 33.6% of patients in the mutant EGFR-TK inhibitors treatment group vs 13.5% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 2.63 and 95% CI was noted, which was statistically significant, confirming higher diarrheal rates in patients receiving EGFR-TK inhibitors for their advanced NSCLC (Figure 3).
Elevated ALT levels developed in 27.9% of patients in the treatment group receiving mutant EGFR-TK inhibitors compared with 15.1% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 1.37 and 95% CI was noted, which was statistically significant, confirming higher ALT levels in patients receiving EGFR-TK inhibitors for their advanced NSCLC (Figure 4).
Elevated AST levels occurred in 40.7% of patients in the mutant EGFR-TK inhibitors treatment group vs 12.8% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 1.77 and 95% CI was noted, which was statistically significant, confirming elevated AST levels in patients receiving EGFR-TK inhibitors for their advanced NSCLC (Figure 5).
Stomatitis developed in 17.2% of patients in the treatment group receiving mutant EGFR-TK inhibitors compared with 7.9% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 1.53 and 95% CI was noted, which was statistically significant, confirming higher stomatitis event rates in patients receiving EGFR-TK inhibitors for their advanced NSCLC (Figure 6).
Nausea occurred in 16.5% of patients in the mutant EGFR-TK inhibitors group vs 42.5% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 0.37 and 95% CI was noted, which was statistically significant, confirming higher nausea rates in patients receiving platinum-based chemotherapy compared with treatment group for their advanced NSCLC (Figure 7).
Leucopenia developed in 9.7% of patients in the mutant EGFR-TK inhibitors group compared with 51.3% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 0.18 and 95% CI was noted, which was statistically significant, confirming higher leucopenia incidence in patients receiving platinum-based chemotherapy compared with treatment group for their advanced NSCLC (Figure 8).
Fatigue was reported in 17% of patients in the mutant EGFR-TK inhibitors group compared with 29.5% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 0.59 and 95% CI was noted, which was statistically significant, confirming higher fatigue rates in patients receiving platinum-based chemotherapy compared with treatment group for their advanced NSCLC (Figure 9).
Neutropenia developed in 6.1% of patients in the mutant EGFR-TK inhibitors group vs 48.2% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 0.11 and 95% CI was noted, which was statistically significant, confirming higher neutropenia rates in patients receiving platinum-based chemotherapy compared with the treatment group for their advanced NSCLC (Figure 10).
Anorexia developed in 21.3% of patients in the mutant EGFR-TK inhibitors group vs 31.4% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 0.44 and 95% CI was noted, which was statistically significant, confirming higher anorexia rates in patients receiving platinum-based chemotherapy compared with the treatment group for their advanced NSCLC (Figure 11).
Anemia occurred in 8.7% of patients in the mutant EGFR-TK inhibitors group compared with 32.1% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 0.24 and 95% CI was noted, which was statistically significant, confirming higher anorexia rates in patients receiving platinum-based chemotherapy compared with treatment for their advanced NSCLC (Figure 12).
Cough was reported in 17.8% of patients in the mutant EGFR-TK inhibitors group compared with 18.9% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 0.99 and 95% CI was noted, which was statistically significant, confirming slightly higher cough rates in patients receiving platinum-based chemotherapy compared with treatment for their advanced NSCLC (Figure 13).
Vomiting developed in 11% of patients in the mutant EGFR-TK inhibitors group vs 30.1% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 0.35 and 95% CI was noted, which was statistically significant, confirming higher vomiting rates in patients receiving platinum-based chemotherapy compared with the treatment group for their advanced NSCLC (Figure 14).
Fever occurred in 5.6% of patients in the mutant EGFR-TK inhibitors group compared with 30.1% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 0.41 and 95% CI was noted, which was statistically significant, confirming higher fever rates in patients receiving platinum-based chemotherapy compared with the treatment group for their advanced NSCLC (Figure 15).
Discussion
Despite the advancement in the treatment of metastatic NSCLC, lung cancer stays as most common cause of cancer-related death in North America and European countries, as patients usually have an advanced disease at the time of diagnosis.3 In the past, platinum-based chemotherapy remained the standard of care for most of the patients affected with advanced NSCLC, but the higher recurrence rate and increase in frequency and intensity of AEs with platinum-based chemotherapy led to the development of targeted therapy for NSCLC, one of which includes
Smoking is the most common reversible risk factor associated with lung cancer. The EURTAC trial was the first perspective study in this regard, which compared safety and efficacy of mutant EGFR-TK inhibitors with platinum-based chemotherapy. Results analyzed in this study were in favor of mutant EGFR-TK inhibitors except in the group of former smokers.5 On the contrary, the OPTIMAL trial showed results in favor of mutant EGFR-TK inhibitors both in active and former smokers; this trial also confirmed the efficacy of mutant EGFR-TK inhibitors in European and Asian populations, confirming the rationale for routine testing of EGFR mutation in all the patients being diagnosed with advanced NSCLC.6 Similarly, osimertinib is one of the most recent mutant EGFR-TK inhibitors developed for the treatment of advanced NSCLC in patients with EGFR-positive receptors.
According to the FLAURA trial, patients receiving osimertinib showed significantly longer progression-free survival compared with platinum-based chemotherapy and early mutant EGFR-TK inhibitors. Median progression-free survival was noted to be 18.9 months, which showed 54% lower risk of disease progression in the treatment group receiving osimertinib.7 The ARCHER study emphasized a significant improvement in overall survival as well as progression-free survival among a patient population receiving dacomitinib compared with platinum-based chemotherapy.8,9
Being a potent targeted therapy, mutant EGFR-TK inhibitors do come with some AEs including diarrhea, which was seen in 33.6% of the patients receiving mutant EGFR-TK inhibitors in our study vs 53% in the chemotherapy group, as was observed in the study conducted by Pless and colleagues.10 Similarly, only 16.5% of patients receiving mutant EGFR-TK inhibitors developed nausea compared with 66% being observed in patients receiving chemotherapy. Correspondingly, only a small fraction of patients (9.7%) receiving mutant EGFR-TK inhibitors developed leucopenia, which was 10 times less reported in mutant EGFR-TK inhibitors compared with patients receiving chemotherapy having a percentage of 100%. A similar trend was reported for neutropenia and anemia in mutant EGFR-TK inhibitors with an incidence of 6.1% and 8.7%, compared with the platinum-based chemotherapy group in which the incidence was found to be 80% and 100%, respectively. It was concluded that platinum-based chemotherapy had played a vital role in the treatment of advanced NSCLC but at an expense of serious and severe AEs which led to discontinuation or withdrawal of treatment, leading to relapse and recurrence of lung cancer.10,11
Zhong and colleagues conducted a phase 2 randomized clinical trial comparing mutant EGFR-TK inhibitors with platinum-based chemotherapy. They concluded that in patients receiving platinum-based chemotherapy, incidence of rash, vomiting, anorexia, neutropenia, and nausea were 29.4%, 47%, 41.2%, 55.8%, and 32.4% compared with 45.8%, 11%, 21.3%, 6.1%, and 16.5%, respectively, reported in patients receiving mutant EGFR-TK inhibitors for their advanced NSCLC.12
Another study was conducted in 2019 by Noronha and colleagues to determine the impact of platinum-based chemotherapy combined with gefitinib on patients with advanced NSCLC.13 They concluded that 70% of the patients receiving combination treatment developed rash, which was significantly higher compared with 45.8% patients receiving the mutant EGFR-TK inhibitors alone in our study. Also, 56% of patients receiving combination therapy developed diarrhea vs 33.6% of patients receiving mutant EGFR-TK inhibitors only. Similarly, 96% of patients in the combination therapy group developed some degree of anemia compared with only 8.7% patients in the mutant EGFR-TK inhibitors group included in our study. In the same way, neutropenia was observed in 55% of patients receiving combination therapy vs 6.1% in patients receiving mutant EGFR-TK inhibitors solely. They concluded that mutant EGFR-TK inhibitors when combined with platinum-based chemotherapy increase the incidence of AEs of chemotherapy by many folds.13,14
Kato and colleagues conducted a study to determine the impact on AEs when erlotinib was combined with anti–vascular endothelial growth factor (VEGF) inhibitors like bevacizumab, they stated that 98.7% of patient in combination therapy developed rash, the incidence of which was only 45.8% in patients receiving mutant EGFR-TK inhibitors as was observed in our study. Similar trends were noticed with other AEs, including diarrhea, fatigue, nausea, and elevated liver enzymes.15
With the latest advancements in the management of advanced NSCLC, nivolumab, a programmed death ligand 1 (PD-L1) inhibitor, was developed and either used as monotherapy in patients with PD-L1 expression or was combined with platinum-based chemotherapy regardless of PD-L1 expression.16,17 Patients expressing lower PD-L1 levels were not omitted from receiving nivolumab as no significant difference was noted in progression-free span and overall survival in patients receiving nivolumab irrespective of PD-L1 levels.15 Rash developed in 17% of patients after receiving nivolumab vs 45.8% patients being observed in our study. A similar trend was observed with diarrhea as only 17% of the population receiving nivolumab developed diarrhea compared with 33.6% of the population receiving mutant EGFR-TK inhibitors in our study. Likewise, only 9.9% of the patients receiving nivolumab developed nausea as an AE compared with 16.5% being observed in mutant EGFR-TK inhibitors in our study. Also, fatigue was observed in 14.4% of the population receiving nivolumab vs 17% observed in patients receiving mutant EGFR-TK inhibitors as was noticed in our study.7,8
Rizvi and colleagues conducted a study on the role of nivolumab when combined with platinum-based chemotherapy in patients with advanced NSCLC and reported that 40% of patients included in the study developed rash compared with 45.8% reported in mutant EGFR-TK inhibitors in our study. Similarly, only 13% of patients in the nivolumab group developed diarrhea vs 33.6% cases reported in the mutant EGFR-TK inhibitors group included in our study. Also, 7% of patients in the nivolumab group developed elevated ALT levels vs 27.9% of patients receiving mutant EGFR-TK inhibitors included in our study, concluding that addition of immune checkpoint inhibitors like nivolumab to platinum-based chemotherapy does not increase the frequency of AEs.18
Conclusions
Our study focused on the safety profile of mutant EGFR-TK inhibitors vs platinum-based chemotherapy in the treatment of advanced NSCLC. Mutant EGFR-TK inhibitors are safer than platinum-based chemotherapy when compared for nausea, leucopenia, fatigue, neutropenia, anorexia, anemia, cough, vomiting, and fever. On the other end, mutant EGFR-TK inhibitors cause slightly higher AEs, including rash, diarrhea, elevated AST and ALT levels, and stomatitis. However, considering that the development of mutant EGFR-TK inhibitors laid a foundation of targeted therapy, we recommend continuing using mutant EGFR-TK inhibitors in patients with advanced NSCLC especially in patients having mutant EGFR receptors. AEs caused by mutant EGFR-TK inhibitors are significant but are usually tolerable and can be avoided by reducing the dosage of it with each cycle or by skipping or delaying the dose until the patient is symptomatic.
1. Rahib L, Smith BD, Aizenberg R, Rosenzweig AB, Fleshman JM, Matrisian LM. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014;74(11):2913-2921. doi:10.1158/0008-5472.CAN-14-0155
2. da Cunha Santos G, Shepherd FA, Tsao MS. EGFR mutations and lung cancer. Annu Rev Pathol. 2011;6:49-69. doi:10.1146/annurev-pathol-011110-130206
3. Sgambato A, Casaluce F, Maione P, et al. The role of EGFR tyrosine kinase inhibitors in the first-line treatment of advanced non small cell lung cancer patients harboring EGFR mutation. Curr Med Chem. 2012;19(20):3337-3352. doi:10.2174/092986712801215973
4. Rossi A, Di Maio M. Platinum-based chemotherapy in advanced non–small-cell lung cancer: optimal number of treatment cycles. Expert Rev Anticancer Ther. 2016;16(6):653-660. doi:10.1586/14737140.2016.1170596
5. Rosell R, Carcereny E, Gervais R, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non–small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2012;13(3):239-246. doi:10.1016/S1470-2045(11)70393-X
6. Zhou C, Wu YL, Chen G, et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non–small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol. 2011;12(8):735-742. doi:10.1016/S1470-2045(11)70184-X
7. Soria JC, Ohe Y, Vansteenkiste J, et al. Osimertinib in untreated EGFR-mutated advanced non–small-cell lung cancer. N Engl J Med. 2018;378(2):113-125. doi:10.1056/NEJMoa1713137
8. Mok TS, Cheng Y, Zhou X, et al. Improvement in overall survival in a randomized study that compared dacomitinib with gefitinib in patients with advanced non–small-cell lung cancer and EGFR-activating mutations. J Clin Oncol. 2018;36(22):2244-2250. doi:10.1200/JCO.2018.78.7994
9. Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;361(10):947-957. doi:10.1056/NEJMoa0810699
10. Pless M, Stupp R, Ris HB, et al. Induction chemoradiation in stage IIIA/N2 non–small-cell lung cancer: a phase 3 randomised trial. Lancet. 2015;386(9998):1049-1056. doi:10.1016/S0140-6736(15)60294-X
11. Albain KS, Rusch VW, Crowley JJ, et al. Concurrent cisplatin/etoposide plus chest radiotherapy followed by surgery for stages IIIA (N2) and IIIB non–small-cell lung cancer: mature results of Southwest Oncology Group phase II study 8805. J Clin Oncol. 1995;13(8):1880-1892. doi:10.1200/JCO.1995.13.8.1880
12. Zhong WZ, Chen KN, Chen C, et al. Erlotinib versus gemcitabine plus cisplatin as neoadjuvant treatment of Stage IIIA-N2 EGFR-mutant non–small-cell lung cancer (EMERGING-CTONG 1103): a randomized phase II study. J Clin Oncol. 2019;37(25):2235-2245. doi:10.1200/JCO.19.00075
13. Noronha V, Patil VM, Joshi A, et al. Gefitinib versus gefitinib plus pemetrexed and carboplatin chemotherapy in EGFR-mutated lung cancer. J Clin Oncol. 2020;38(2):124-136. doi:10.1200/JCO.19.01154
14. Noronha V, Prabhash K, Thavamani A, et al. EGFR mutations in Indian lung cancer patients: clinical correlation and outcome to EGFR targeted therapy. PLoS One. 2013;8(4):e61561. Published 2013 Apr 19. doi:10.1371/journal.pone.0061561
15. Kato T, Seto T, Nishio M, et al. Erlotinib plus bevacizumab phase ll study in patients with advanced non–small-cell lung cancer (JO25567): updated safety results. Drug Saf. 2018;41(2):229-237. doi:10.1007/s40264-017-0596-0
16. Hellmann MD, Paz-Ares L, Bernabe Caro R, et al. Nivolumab plus ipilimumab in advanced non–small-cell lung cancer. N Engl J Med. 2019;381(21):2020-2031. doi:10.1056/NEJMoa1910231
17. Hellmann MD, Ciuleanu TE, Pluzanski A, et al. Nivolumab plus ipilimumab in lung cancer with a high tumor mutational burden. N Engl J Med. 2018;378(22):2093-2104. doi:10.1056/NEJMoa1801946
18. Rizvi NA, Hellmann MD, Brahmer JR, et al. Nivolumab in combination with platinum-based doublet chemotherapy for first-line treatment of advanced non–small-cell lung cancer. J Clin Oncol. 2016;34(25):2969-2979. doi:10.1200/JCO.2016.66.9861
19. Zhong WZ, Wang Q, Mao WM, et al. Gefitinib versus vinorelbine plus cisplatin as adjuvant treatment for stage II-IIIA (N1-N2) EGFR-mutant NSCLC: final overall survival analysis of CTONG1104 Phase III Trial. J Clin Oncol. 2021;39(7):713-722. doi:10.1200/JCO.20.01820
20. Yang JC, Sequist LV, Geater SL, et al. Clinical activity of afatinib in patients with advanced non-small-cell lung cancer harbouring uncommon EGFR mutations: a combined post-hoc analysis of LUX-Lung 2, LUX-Lung 3, and LUX-Lung 6. Lancet Oncol. 2015;16(7):830-838. doi:10.1016/S1470-2045(15)00026-1
21. Shi YK, Wang L, Han BH, et al. First-line icotinib versus cisplatin/pemetrexed plus pemetrexed maintenance therapy for patients with advanced EGFR mutation-positive lung adenocarcinoma (CONVINCE): a phase 3, open-label, randomized study. Ann Oncol. 2017;28(10):2443-2450. doi:10.1093/annonc/mdx359
22. Soria JC, Wu YL, Nakagawa K, et al. Gefitinib plus chemotherapy versus placebo plus chemotherapy in EGFR-mutation-positive non-small-cell lung cancer after progression on first-line gefitinib (IMPRESS): a phase 3 randomized trial. Lancet Oncol. 2015;16(8):990-998 doi:10.1016/S1470-2045(15)00121-7
23. Goss GD, O’Callaghan C, Lorimer I, et al. Gefitinib versus placebo in completely resected non-small-cell lung cancer: results of the NCIC CTG BR19 study. J Clin Oncol. 2013;31(27):3320-3326. doi:10.1200/JCO.2013.51.1816
24. Sun JM, Lee KH, Kim SW, et al. Gefitinib versus pemetrexed as second-line treatment in patients with non-small cell lung cancer previously treated with platinum-based chemotherapy (KCSG-LU08-01): an open-label, phase 3 trial. Cancer. 2012;118(24):6234-6242. doi:10.1200/JCO.2013.51.1816
25. Mitsudomi T, Morita S, Yatabe Y, et al. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomized phase 3 trial. Lancet Oncol. 2010;11(2):121-128. doi:10.1016/S1470-2045(09)70364-X
26. Lee DH, Park K, Kim JH, Lee JS, et al. Randomized phase III trial of gefitinib versus docetaxel in non-small cell lung cancer patients who have previously received platinum-based chemotherapy. Clin Cancer Res. 2010;16(4):1307-1314. doi:10.1158/1078-0432.CCR-09-1903
27. Kim ES, Hirsh V, Mok T, et al. Gefitinib versus docetaxel in previously treated non-small-cell lung cancer (INTEREST): a randomized phase III trial. Lancet. 2008;22;372(9652):1809-1818. doi:10.1016/S0140-6736(08)61758-4
Lung cancer has been the leading cause of cancer-related mortality for decades. It is also predicted to remain as the leading cause of cancer-related mortality through 2030.1 Platinum-based chemotherapy, including carboplatin and paclitaxel, was introduced 3 decades ago and revolutionized the management of advanced non–small cell lung cancer (NSCLC). A more recent advancement has been mutant epidermal growth factor receptor–tyrosine kinase (EGFR-TK) inhibitors.1 EGFR is a transmembrane protein that functions by transducing essential growth factor signaling from the extracellular milieu to the cell. As 60% of the advanced NSCLC expresses this receptor, blocking the mutant EGFR receptor was a groundbreaking development in the management of advanced NSCLC.2 Development of mutant EGFR-TK inhibitors has revolutionized the management of advanced NSCLC. This study was conducted to determine the safety profile of mutant EGFR-TK inhibitors in the management of advanced NSCLC.
Methods
This meta-analysis was conducted according to Cochrane Collaboration guidelines and reported as per Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. The findings are summarized in the PRISMA flow diagram (Figure 1). Two authors (MZ and MM) performed a systematic literature search using databases such as MEDLINE (via PubMed), Embase, and Cochrane Library using the medical search terms and their respective entry words with the following search strategy: safety, “mutant EGFR-TK inhibitors,” advanced, “non–small cell,” “lung cancer,” “adverse effect,” and literature. Additionally, unpublished trials were identified from clinicaltrials.gov, and references of all pertinent articles were also scrutinized to ensure the inclusion of all relevant studies. The search was completed on June 1, 2021, and we only included studies available in English. Two authors (MM and MZ) independently screened the search results in a 2-step process based on predetermined inclusion/exclusion criteria. First, 890 articles were evaluated for relevance on title and abstract level, followed by full-text screening of the final list of 140 articles. Any disagreements were resolved by discussion or third-party review, and a total of 9 articles were included in the study.
The following eligibility criteria were used: original articles reporting adverse effects (AEs) of mutant EGFR-TK inhibitors in patients with advanced NSCLC compared with control groups receiving platinum-based chemotherapy. All the patients included in the study had an EGFR mutation but randomly assigned to either treatment or control group. All articles with subjective data on mutant EGFR-TK inhibitors AEs in patients with advanced NSCLC compared with control groups receiving platinum-based chemotherapy were included in the analysis. Only 9 articles qualified the aforementioned selection criteria for eligibility. All qualifying studies were nationwide inpatient or pooled clinical trials data. The reasons for exclusion of the other 71 articles were irrelevant (n = 31), duplicate (n = 13), reviews (n = 14), and poor data reporting (n = 12). Out of the 9 included studies, 9 studies showed correlation of AEs, including rash, diarrhea, nausea, and fatigue. Seven studies showed correlation of AEs including neutropenia, anorexia, and vomiting. Six studies showed correlation of anemia, cough, and stomatitis. Five studies showed correlation of elevated aspartate aminotransferase (AST), alanine aminotransferase (ALT), and leucopenia. Four studies showed correlation of fever between mutant EGFR-TK inhibitors and platinum-based chemotherapy.
The primary endpoints were reported AEs including rash, diarrhea, elevated ALT, elevated AST, stomatitis, nausea, leucopenia, fatigue, neutropenia, anorexia, anemia, cough, vomiting, and fever, respectively. Data on baseline characteristics and clinical outcomes were then extracted, and summary tables were created. Summary estimates of the clinical endpoints were then calculated with risk ratio (RR) and 95% confidence intervals (CIs) using the random-effects model. Heterogeneity between studies was examined with the Cochran Q I2 statistic which can be defined as low (25% to 50%), moderate (50% to 75%), or high (> 75%). Statistical analysis was performed using Comprehensive Meta-Analysis Software CMA Version 3.0.
Results
A total of 9 studies including 3415 patients (1775 in EGFR-TK inhibitor treatment group while 1640 patients in platinum-based chemotherapy control group) were included in the study. All 9 studies were phase III randomized control clinical trials conducted to compare the safety profile of mutant EGFR-TK inhibitors in patients with advanced NSCLC. Mean age was 61 years in both treatment and control groups. Further details on study and participant characteristics and safety profile including AEs are summarized in Tables 1 and 2. No evidence of publication bias was found.
Rash developed in 45.8% of patients in the treatment group receiving mutant EGFR-TK inhibitors vs only 5.6% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 7.38 with the 95% CI noted, which was statistically significant, confirming higher rash event rates in patients receiving EGFR-TK inhibitors for their advanced NSCLC (Figure 2).
Diarrhea occurred in 33.6% of patients in the mutant EGFR-TK inhibitors treatment group vs 13.5% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 2.63 and 95% CI was noted, which was statistically significant, confirming higher diarrheal rates in patients receiving EGFR-TK inhibitors for their advanced NSCLC (Figure 3).
Elevated ALT levels developed in 27.9% of patients in the treatment group receiving mutant EGFR-TK inhibitors compared with 15.1% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 1.37 and 95% CI was noted, which was statistically significant, confirming higher ALT levels in patients receiving EGFR-TK inhibitors for their advanced NSCLC (Figure 4).
Elevated AST levels occurred in 40.7% of patients in the mutant EGFR-TK inhibitors treatment group vs 12.8% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 1.77 and 95% CI was noted, which was statistically significant, confirming elevated AST levels in patients receiving EGFR-TK inhibitors for their advanced NSCLC (Figure 5).
Stomatitis developed in 17.2% of patients in the treatment group receiving mutant EGFR-TK inhibitors compared with 7.9% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 1.53 and 95% CI was noted, which was statistically significant, confirming higher stomatitis event rates in patients receiving EGFR-TK inhibitors for their advanced NSCLC (Figure 6).
Nausea occurred in 16.5% of patients in the mutant EGFR-TK inhibitors group vs 42.5% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 0.37 and 95% CI was noted, which was statistically significant, confirming higher nausea rates in patients receiving platinum-based chemotherapy compared with treatment group for their advanced NSCLC (Figure 7).
Leucopenia developed in 9.7% of patients in the mutant EGFR-TK inhibitors group compared with 51.3% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 0.18 and 95% CI was noted, which was statistically significant, confirming higher leucopenia incidence in patients receiving platinum-based chemotherapy compared with treatment group for their advanced NSCLC (Figure 8).
Fatigue was reported in 17% of patients in the mutant EGFR-TK inhibitors group compared with 29.5% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 0.59 and 95% CI was noted, which was statistically significant, confirming higher fatigue rates in patients receiving platinum-based chemotherapy compared with treatment group for their advanced NSCLC (Figure 9).
Neutropenia developed in 6.1% of patients in the mutant EGFR-TK inhibitors group vs 48.2% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 0.11 and 95% CI was noted, which was statistically significant, confirming higher neutropenia rates in patients receiving platinum-based chemotherapy compared with the treatment group for their advanced NSCLC (Figure 10).
Anorexia developed in 21.3% of patients in the mutant EGFR-TK inhibitors group vs 31.4% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 0.44 and 95% CI was noted, which was statistically significant, confirming higher anorexia rates in patients receiving platinum-based chemotherapy compared with the treatment group for their advanced NSCLC (Figure 11).
Anemia occurred in 8.7% of patients in the mutant EGFR-TK inhibitors group compared with 32.1% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 0.24 and 95% CI was noted, which was statistically significant, confirming higher anorexia rates in patients receiving platinum-based chemotherapy compared with treatment for their advanced NSCLC (Figure 12).
Cough was reported in 17.8% of patients in the mutant EGFR-TK inhibitors group compared with 18.9% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 0.99 and 95% CI was noted, which was statistically significant, confirming slightly higher cough rates in patients receiving platinum-based chemotherapy compared with treatment for their advanced NSCLC (Figure 13).
Vomiting developed in 11% of patients in the mutant EGFR-TK inhibitors group vs 30.1% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 0.35 and 95% CI was noted, which was statistically significant, confirming higher vomiting rates in patients receiving platinum-based chemotherapy compared with the treatment group for their advanced NSCLC (Figure 14).
Fever occurred in 5.6% of patients in the mutant EGFR-TK inhibitors group compared with 30.1% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 0.41 and 95% CI was noted, which was statistically significant, confirming higher fever rates in patients receiving platinum-based chemotherapy compared with the treatment group for their advanced NSCLC (Figure 15).
Discussion
Despite the advancement in the treatment of metastatic NSCLC, lung cancer stays as most common cause of cancer-related death in North America and European countries, as patients usually have an advanced disease at the time of diagnosis.3 In the past, platinum-based chemotherapy remained the standard of care for most of the patients affected with advanced NSCLC, but the higher recurrence rate and increase in frequency and intensity of AEs with platinum-based chemotherapy led to the development of targeted therapy for NSCLC, one of which includes
Smoking is the most common reversible risk factor associated with lung cancer. The EURTAC trial was the first perspective study in this regard, which compared safety and efficacy of mutant EGFR-TK inhibitors with platinum-based chemotherapy. Results analyzed in this study were in favor of mutant EGFR-TK inhibitors except in the group of former smokers.5 On the contrary, the OPTIMAL trial showed results in favor of mutant EGFR-TK inhibitors both in active and former smokers; this trial also confirmed the efficacy of mutant EGFR-TK inhibitors in European and Asian populations, confirming the rationale for routine testing of EGFR mutation in all the patients being diagnosed with advanced NSCLC.6 Similarly, osimertinib is one of the most recent mutant EGFR-TK inhibitors developed for the treatment of advanced NSCLC in patients with EGFR-positive receptors.
According to the FLAURA trial, patients receiving osimertinib showed significantly longer progression-free survival compared with platinum-based chemotherapy and early mutant EGFR-TK inhibitors. Median progression-free survival was noted to be 18.9 months, which showed 54% lower risk of disease progression in the treatment group receiving osimertinib.7 The ARCHER study emphasized a significant improvement in overall survival as well as progression-free survival among a patient population receiving dacomitinib compared with platinum-based chemotherapy.8,9
Being a potent targeted therapy, mutant EGFR-TK inhibitors do come with some AEs including diarrhea, which was seen in 33.6% of the patients receiving mutant EGFR-TK inhibitors in our study vs 53% in the chemotherapy group, as was observed in the study conducted by Pless and colleagues.10 Similarly, only 16.5% of patients receiving mutant EGFR-TK inhibitors developed nausea compared with 66% being observed in patients receiving chemotherapy. Correspondingly, only a small fraction of patients (9.7%) receiving mutant EGFR-TK inhibitors developed leucopenia, which was 10 times less reported in mutant EGFR-TK inhibitors compared with patients receiving chemotherapy having a percentage of 100%. A similar trend was reported for neutropenia and anemia in mutant EGFR-TK inhibitors with an incidence of 6.1% and 8.7%, compared with the platinum-based chemotherapy group in which the incidence was found to be 80% and 100%, respectively. It was concluded that platinum-based chemotherapy had played a vital role in the treatment of advanced NSCLC but at an expense of serious and severe AEs which led to discontinuation or withdrawal of treatment, leading to relapse and recurrence of lung cancer.10,11
Zhong and colleagues conducted a phase 2 randomized clinical trial comparing mutant EGFR-TK inhibitors with platinum-based chemotherapy. They concluded that in patients receiving platinum-based chemotherapy, incidence of rash, vomiting, anorexia, neutropenia, and nausea were 29.4%, 47%, 41.2%, 55.8%, and 32.4% compared with 45.8%, 11%, 21.3%, 6.1%, and 16.5%, respectively, reported in patients receiving mutant EGFR-TK inhibitors for their advanced NSCLC.12
Another study was conducted in 2019 by Noronha and colleagues to determine the impact of platinum-based chemotherapy combined with gefitinib on patients with advanced NSCLC.13 They concluded that 70% of the patients receiving combination treatment developed rash, which was significantly higher compared with 45.8% patients receiving the mutant EGFR-TK inhibitors alone in our study. Also, 56% of patients receiving combination therapy developed diarrhea vs 33.6% of patients receiving mutant EGFR-TK inhibitors only. Similarly, 96% of patients in the combination therapy group developed some degree of anemia compared with only 8.7% patients in the mutant EGFR-TK inhibitors group included in our study. In the same way, neutropenia was observed in 55% of patients receiving combination therapy vs 6.1% in patients receiving mutant EGFR-TK inhibitors solely. They concluded that mutant EGFR-TK inhibitors when combined with platinum-based chemotherapy increase the incidence of AEs of chemotherapy by many folds.13,14
Kato and colleagues conducted a study to determine the impact on AEs when erlotinib was combined with anti–vascular endothelial growth factor (VEGF) inhibitors like bevacizumab, they stated that 98.7% of patient in combination therapy developed rash, the incidence of which was only 45.8% in patients receiving mutant EGFR-TK inhibitors as was observed in our study. Similar trends were noticed with other AEs, including diarrhea, fatigue, nausea, and elevated liver enzymes.15
With the latest advancements in the management of advanced NSCLC, nivolumab, a programmed death ligand 1 (PD-L1) inhibitor, was developed and either used as monotherapy in patients with PD-L1 expression or was combined with platinum-based chemotherapy regardless of PD-L1 expression.16,17 Patients expressing lower PD-L1 levels were not omitted from receiving nivolumab as no significant difference was noted in progression-free span and overall survival in patients receiving nivolumab irrespective of PD-L1 levels.15 Rash developed in 17% of patients after receiving nivolumab vs 45.8% patients being observed in our study. A similar trend was observed with diarrhea as only 17% of the population receiving nivolumab developed diarrhea compared with 33.6% of the population receiving mutant EGFR-TK inhibitors in our study. Likewise, only 9.9% of the patients receiving nivolumab developed nausea as an AE compared with 16.5% being observed in mutant EGFR-TK inhibitors in our study. Also, fatigue was observed in 14.4% of the population receiving nivolumab vs 17% observed in patients receiving mutant EGFR-TK inhibitors as was noticed in our study.7,8
Rizvi and colleagues conducted a study on the role of nivolumab when combined with platinum-based chemotherapy in patients with advanced NSCLC and reported that 40% of patients included in the study developed rash compared with 45.8% reported in mutant EGFR-TK inhibitors in our study. Similarly, only 13% of patients in the nivolumab group developed diarrhea vs 33.6% cases reported in the mutant EGFR-TK inhibitors group included in our study. Also, 7% of patients in the nivolumab group developed elevated ALT levels vs 27.9% of patients receiving mutant EGFR-TK inhibitors included in our study, concluding that addition of immune checkpoint inhibitors like nivolumab to platinum-based chemotherapy does not increase the frequency of AEs.18
Conclusions
Our study focused on the safety profile of mutant EGFR-TK inhibitors vs platinum-based chemotherapy in the treatment of advanced NSCLC. Mutant EGFR-TK inhibitors are safer than platinum-based chemotherapy when compared for nausea, leucopenia, fatigue, neutropenia, anorexia, anemia, cough, vomiting, and fever. On the other end, mutant EGFR-TK inhibitors cause slightly higher AEs, including rash, diarrhea, elevated AST and ALT levels, and stomatitis. However, considering that the development of mutant EGFR-TK inhibitors laid a foundation of targeted therapy, we recommend continuing using mutant EGFR-TK inhibitors in patients with advanced NSCLC especially in patients having mutant EGFR receptors. AEs caused by mutant EGFR-TK inhibitors are significant but are usually tolerable and can be avoided by reducing the dosage of it with each cycle or by skipping or delaying the dose until the patient is symptomatic.
Lung cancer has been the leading cause of cancer-related mortality for decades. It is also predicted to remain as the leading cause of cancer-related mortality through 2030.1 Platinum-based chemotherapy, including carboplatin and paclitaxel, was introduced 3 decades ago and revolutionized the management of advanced non–small cell lung cancer (NSCLC). A more recent advancement has been mutant epidermal growth factor receptor–tyrosine kinase (EGFR-TK) inhibitors.1 EGFR is a transmembrane protein that functions by transducing essential growth factor signaling from the extracellular milieu to the cell. As 60% of the advanced NSCLC expresses this receptor, blocking the mutant EGFR receptor was a groundbreaking development in the management of advanced NSCLC.2 Development of mutant EGFR-TK inhibitors has revolutionized the management of advanced NSCLC. This study was conducted to determine the safety profile of mutant EGFR-TK inhibitors in the management of advanced NSCLC.
Methods
This meta-analysis was conducted according to Cochrane Collaboration guidelines and reported as per Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. The findings are summarized in the PRISMA flow diagram (Figure 1). Two authors (MZ and MM) performed a systematic literature search using databases such as MEDLINE (via PubMed), Embase, and Cochrane Library using the medical search terms and their respective entry words with the following search strategy: safety, “mutant EGFR-TK inhibitors,” advanced, “non–small cell,” “lung cancer,” “adverse effect,” and literature. Additionally, unpublished trials were identified from clinicaltrials.gov, and references of all pertinent articles were also scrutinized to ensure the inclusion of all relevant studies. The search was completed on June 1, 2021, and we only included studies available in English. Two authors (MM and MZ) independently screened the search results in a 2-step process based on predetermined inclusion/exclusion criteria. First, 890 articles were evaluated for relevance on title and abstract level, followed by full-text screening of the final list of 140 articles. Any disagreements were resolved by discussion or third-party review, and a total of 9 articles were included in the study.
The following eligibility criteria were used: original articles reporting adverse effects (AEs) of mutant EGFR-TK inhibitors in patients with advanced NSCLC compared with control groups receiving platinum-based chemotherapy. All the patients included in the study had an EGFR mutation but randomly assigned to either treatment or control group. All articles with subjective data on mutant EGFR-TK inhibitors AEs in patients with advanced NSCLC compared with control groups receiving platinum-based chemotherapy were included in the analysis. Only 9 articles qualified the aforementioned selection criteria for eligibility. All qualifying studies were nationwide inpatient or pooled clinical trials data. The reasons for exclusion of the other 71 articles were irrelevant (n = 31), duplicate (n = 13), reviews (n = 14), and poor data reporting (n = 12). Out of the 9 included studies, 9 studies showed correlation of AEs, including rash, diarrhea, nausea, and fatigue. Seven studies showed correlation of AEs including neutropenia, anorexia, and vomiting. Six studies showed correlation of anemia, cough, and stomatitis. Five studies showed correlation of elevated aspartate aminotransferase (AST), alanine aminotransferase (ALT), and leucopenia. Four studies showed correlation of fever between mutant EGFR-TK inhibitors and platinum-based chemotherapy.
The primary endpoints were reported AEs including rash, diarrhea, elevated ALT, elevated AST, stomatitis, nausea, leucopenia, fatigue, neutropenia, anorexia, anemia, cough, vomiting, and fever, respectively. Data on baseline characteristics and clinical outcomes were then extracted, and summary tables were created. Summary estimates of the clinical endpoints were then calculated with risk ratio (RR) and 95% confidence intervals (CIs) using the random-effects model. Heterogeneity between studies was examined with the Cochran Q I2 statistic which can be defined as low (25% to 50%), moderate (50% to 75%), or high (> 75%). Statistical analysis was performed using Comprehensive Meta-Analysis Software CMA Version 3.0.
Results
A total of 9 studies including 3415 patients (1775 in EGFR-TK inhibitor treatment group while 1640 patients in platinum-based chemotherapy control group) were included in the study. All 9 studies were phase III randomized control clinical trials conducted to compare the safety profile of mutant EGFR-TK inhibitors in patients with advanced NSCLC. Mean age was 61 years in both treatment and control groups. Further details on study and participant characteristics and safety profile including AEs are summarized in Tables 1 and 2. No evidence of publication bias was found.
Rash developed in 45.8% of patients in the treatment group receiving mutant EGFR-TK inhibitors vs only 5.6% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 7.38 with the 95% CI noted, which was statistically significant, confirming higher rash event rates in patients receiving EGFR-TK inhibitors for their advanced NSCLC (Figure 2).
Diarrhea occurred in 33.6% of patients in the mutant EGFR-TK inhibitors treatment group vs 13.5% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 2.63 and 95% CI was noted, which was statistically significant, confirming higher diarrheal rates in patients receiving EGFR-TK inhibitors for their advanced NSCLC (Figure 3).
Elevated ALT levels developed in 27.9% of patients in the treatment group receiving mutant EGFR-TK inhibitors compared with 15.1% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 1.37 and 95% CI was noted, which was statistically significant, confirming higher ALT levels in patients receiving EGFR-TK inhibitors for their advanced NSCLC (Figure 4).
Elevated AST levels occurred in 40.7% of patients in the mutant EGFR-TK inhibitors treatment group vs 12.8% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 1.77 and 95% CI was noted, which was statistically significant, confirming elevated AST levels in patients receiving EGFR-TK inhibitors for their advanced NSCLC (Figure 5).
Stomatitis developed in 17.2% of patients in the treatment group receiving mutant EGFR-TK inhibitors compared with 7.9% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 1.53 and 95% CI was noted, which was statistically significant, confirming higher stomatitis event rates in patients receiving EGFR-TK inhibitors for their advanced NSCLC (Figure 6).
Nausea occurred in 16.5% of patients in the mutant EGFR-TK inhibitors group vs 42.5% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 0.37 and 95% CI was noted, which was statistically significant, confirming higher nausea rates in patients receiving platinum-based chemotherapy compared with treatment group for their advanced NSCLC (Figure 7).
Leucopenia developed in 9.7% of patients in the mutant EGFR-TK inhibitors group compared with 51.3% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 0.18 and 95% CI was noted, which was statistically significant, confirming higher leucopenia incidence in patients receiving platinum-based chemotherapy compared with treatment group for their advanced NSCLC (Figure 8).
Fatigue was reported in 17% of patients in the mutant EGFR-TK inhibitors group compared with 29.5% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 0.59 and 95% CI was noted, which was statistically significant, confirming higher fatigue rates in patients receiving platinum-based chemotherapy compared with treatment group for their advanced NSCLC (Figure 9).
Neutropenia developed in 6.1% of patients in the mutant EGFR-TK inhibitors group vs 48.2% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 0.11 and 95% CI was noted, which was statistically significant, confirming higher neutropenia rates in patients receiving platinum-based chemotherapy compared with the treatment group for their advanced NSCLC (Figure 10).
Anorexia developed in 21.3% of patients in the mutant EGFR-TK inhibitors group vs 31.4% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 0.44 and 95% CI was noted, which was statistically significant, confirming higher anorexia rates in patients receiving platinum-based chemotherapy compared with the treatment group for their advanced NSCLC (Figure 11).
Anemia occurred in 8.7% of patients in the mutant EGFR-TK inhibitors group compared with 32.1% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 0.24 and 95% CI was noted, which was statistically significant, confirming higher anorexia rates in patients receiving platinum-based chemotherapy compared with treatment for their advanced NSCLC (Figure 12).
Cough was reported in 17.8% of patients in the mutant EGFR-TK inhibitors group compared with 18.9% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 0.99 and 95% CI was noted, which was statistically significant, confirming slightly higher cough rates in patients receiving platinum-based chemotherapy compared with treatment for their advanced NSCLC (Figure 13).
Vomiting developed in 11% of patients in the mutant EGFR-TK inhibitors group vs 30.1% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 0.35 and 95% CI was noted, which was statistically significant, confirming higher vomiting rates in patients receiving platinum-based chemotherapy compared with the treatment group for their advanced NSCLC (Figure 14).
Fever occurred in 5.6% of patients in the mutant EGFR-TK inhibitors group compared with 30.1% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 0.41 and 95% CI was noted, which was statistically significant, confirming higher fever rates in patients receiving platinum-based chemotherapy compared with the treatment group for their advanced NSCLC (Figure 15).
Discussion
Despite the advancement in the treatment of metastatic NSCLC, lung cancer stays as most common cause of cancer-related death in North America and European countries, as patients usually have an advanced disease at the time of diagnosis.3 In the past, platinum-based chemotherapy remained the standard of care for most of the patients affected with advanced NSCLC, but the higher recurrence rate and increase in frequency and intensity of AEs with platinum-based chemotherapy led to the development of targeted therapy for NSCLC, one of which includes
Smoking is the most common reversible risk factor associated with lung cancer. The EURTAC trial was the first perspective study in this regard, which compared safety and efficacy of mutant EGFR-TK inhibitors with platinum-based chemotherapy. Results analyzed in this study were in favor of mutant EGFR-TK inhibitors except in the group of former smokers.5 On the contrary, the OPTIMAL trial showed results in favor of mutant EGFR-TK inhibitors both in active and former smokers; this trial also confirmed the efficacy of mutant EGFR-TK inhibitors in European and Asian populations, confirming the rationale for routine testing of EGFR mutation in all the patients being diagnosed with advanced NSCLC.6 Similarly, osimertinib is one of the most recent mutant EGFR-TK inhibitors developed for the treatment of advanced NSCLC in patients with EGFR-positive receptors.
According to the FLAURA trial, patients receiving osimertinib showed significantly longer progression-free survival compared with platinum-based chemotherapy and early mutant EGFR-TK inhibitors. Median progression-free survival was noted to be 18.9 months, which showed 54% lower risk of disease progression in the treatment group receiving osimertinib.7 The ARCHER study emphasized a significant improvement in overall survival as well as progression-free survival among a patient population receiving dacomitinib compared with platinum-based chemotherapy.8,9
Being a potent targeted therapy, mutant EGFR-TK inhibitors do come with some AEs including diarrhea, which was seen in 33.6% of the patients receiving mutant EGFR-TK inhibitors in our study vs 53% in the chemotherapy group, as was observed in the study conducted by Pless and colleagues.10 Similarly, only 16.5% of patients receiving mutant EGFR-TK inhibitors developed nausea compared with 66% being observed in patients receiving chemotherapy. Correspondingly, only a small fraction of patients (9.7%) receiving mutant EGFR-TK inhibitors developed leucopenia, which was 10 times less reported in mutant EGFR-TK inhibitors compared with patients receiving chemotherapy having a percentage of 100%. A similar trend was reported for neutropenia and anemia in mutant EGFR-TK inhibitors with an incidence of 6.1% and 8.7%, compared with the platinum-based chemotherapy group in which the incidence was found to be 80% and 100%, respectively. It was concluded that platinum-based chemotherapy had played a vital role in the treatment of advanced NSCLC but at an expense of serious and severe AEs which led to discontinuation or withdrawal of treatment, leading to relapse and recurrence of lung cancer.10,11
Zhong and colleagues conducted a phase 2 randomized clinical trial comparing mutant EGFR-TK inhibitors with platinum-based chemotherapy. They concluded that in patients receiving platinum-based chemotherapy, incidence of rash, vomiting, anorexia, neutropenia, and nausea were 29.4%, 47%, 41.2%, 55.8%, and 32.4% compared with 45.8%, 11%, 21.3%, 6.1%, and 16.5%, respectively, reported in patients receiving mutant EGFR-TK inhibitors for their advanced NSCLC.12
Another study was conducted in 2019 by Noronha and colleagues to determine the impact of platinum-based chemotherapy combined with gefitinib on patients with advanced NSCLC.13 They concluded that 70% of the patients receiving combination treatment developed rash, which was significantly higher compared with 45.8% patients receiving the mutant EGFR-TK inhibitors alone in our study. Also, 56% of patients receiving combination therapy developed diarrhea vs 33.6% of patients receiving mutant EGFR-TK inhibitors only. Similarly, 96% of patients in the combination therapy group developed some degree of anemia compared with only 8.7% patients in the mutant EGFR-TK inhibitors group included in our study. In the same way, neutropenia was observed in 55% of patients receiving combination therapy vs 6.1% in patients receiving mutant EGFR-TK inhibitors solely. They concluded that mutant EGFR-TK inhibitors when combined with platinum-based chemotherapy increase the incidence of AEs of chemotherapy by many folds.13,14
Kato and colleagues conducted a study to determine the impact on AEs when erlotinib was combined with anti–vascular endothelial growth factor (VEGF) inhibitors like bevacizumab, they stated that 98.7% of patient in combination therapy developed rash, the incidence of which was only 45.8% in patients receiving mutant EGFR-TK inhibitors as was observed in our study. Similar trends were noticed with other AEs, including diarrhea, fatigue, nausea, and elevated liver enzymes.15
With the latest advancements in the management of advanced NSCLC, nivolumab, a programmed death ligand 1 (PD-L1) inhibitor, was developed and either used as monotherapy in patients with PD-L1 expression or was combined with platinum-based chemotherapy regardless of PD-L1 expression.16,17 Patients expressing lower PD-L1 levels were not omitted from receiving nivolumab as no significant difference was noted in progression-free span and overall survival in patients receiving nivolumab irrespective of PD-L1 levels.15 Rash developed in 17% of patients after receiving nivolumab vs 45.8% patients being observed in our study. A similar trend was observed with diarrhea as only 17% of the population receiving nivolumab developed diarrhea compared with 33.6% of the population receiving mutant EGFR-TK inhibitors in our study. Likewise, only 9.9% of the patients receiving nivolumab developed nausea as an AE compared with 16.5% being observed in mutant EGFR-TK inhibitors in our study. Also, fatigue was observed in 14.4% of the population receiving nivolumab vs 17% observed in patients receiving mutant EGFR-TK inhibitors as was noticed in our study.7,8
Rizvi and colleagues conducted a study on the role of nivolumab when combined with platinum-based chemotherapy in patients with advanced NSCLC and reported that 40% of patients included in the study developed rash compared with 45.8% reported in mutant EGFR-TK inhibitors in our study. Similarly, only 13% of patients in the nivolumab group developed diarrhea vs 33.6% cases reported in the mutant EGFR-TK inhibitors group included in our study. Also, 7% of patients in the nivolumab group developed elevated ALT levels vs 27.9% of patients receiving mutant EGFR-TK inhibitors included in our study, concluding that addition of immune checkpoint inhibitors like nivolumab to platinum-based chemotherapy does not increase the frequency of AEs.18
Conclusions
Our study focused on the safety profile of mutant EGFR-TK inhibitors vs platinum-based chemotherapy in the treatment of advanced NSCLC. Mutant EGFR-TK inhibitors are safer than platinum-based chemotherapy when compared for nausea, leucopenia, fatigue, neutropenia, anorexia, anemia, cough, vomiting, and fever. On the other end, mutant EGFR-TK inhibitors cause slightly higher AEs, including rash, diarrhea, elevated AST and ALT levels, and stomatitis. However, considering that the development of mutant EGFR-TK inhibitors laid a foundation of targeted therapy, we recommend continuing using mutant EGFR-TK inhibitors in patients with advanced NSCLC especially in patients having mutant EGFR receptors. AEs caused by mutant EGFR-TK inhibitors are significant but are usually tolerable and can be avoided by reducing the dosage of it with each cycle or by skipping or delaying the dose until the patient is symptomatic.
1. Rahib L, Smith BD, Aizenberg R, Rosenzweig AB, Fleshman JM, Matrisian LM. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014;74(11):2913-2921. doi:10.1158/0008-5472.CAN-14-0155
2. da Cunha Santos G, Shepherd FA, Tsao MS. EGFR mutations and lung cancer. Annu Rev Pathol. 2011;6:49-69. doi:10.1146/annurev-pathol-011110-130206
3. Sgambato A, Casaluce F, Maione P, et al. The role of EGFR tyrosine kinase inhibitors in the first-line treatment of advanced non small cell lung cancer patients harboring EGFR mutation. Curr Med Chem. 2012;19(20):3337-3352. doi:10.2174/092986712801215973
4. Rossi A, Di Maio M. Platinum-based chemotherapy in advanced non–small-cell lung cancer: optimal number of treatment cycles. Expert Rev Anticancer Ther. 2016;16(6):653-660. doi:10.1586/14737140.2016.1170596
5. Rosell R, Carcereny E, Gervais R, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non–small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2012;13(3):239-246. doi:10.1016/S1470-2045(11)70393-X
6. Zhou C, Wu YL, Chen G, et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non–small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol. 2011;12(8):735-742. doi:10.1016/S1470-2045(11)70184-X
7. Soria JC, Ohe Y, Vansteenkiste J, et al. Osimertinib in untreated EGFR-mutated advanced non–small-cell lung cancer. N Engl J Med. 2018;378(2):113-125. doi:10.1056/NEJMoa1713137
8. Mok TS, Cheng Y, Zhou X, et al. Improvement in overall survival in a randomized study that compared dacomitinib with gefitinib in patients with advanced non–small-cell lung cancer and EGFR-activating mutations. J Clin Oncol. 2018;36(22):2244-2250. doi:10.1200/JCO.2018.78.7994
9. Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;361(10):947-957. doi:10.1056/NEJMoa0810699
10. Pless M, Stupp R, Ris HB, et al. Induction chemoradiation in stage IIIA/N2 non–small-cell lung cancer: a phase 3 randomised trial. Lancet. 2015;386(9998):1049-1056. doi:10.1016/S0140-6736(15)60294-X
11. Albain KS, Rusch VW, Crowley JJ, et al. Concurrent cisplatin/etoposide plus chest radiotherapy followed by surgery for stages IIIA (N2) and IIIB non–small-cell lung cancer: mature results of Southwest Oncology Group phase II study 8805. J Clin Oncol. 1995;13(8):1880-1892. doi:10.1200/JCO.1995.13.8.1880
12. Zhong WZ, Chen KN, Chen C, et al. Erlotinib versus gemcitabine plus cisplatin as neoadjuvant treatment of Stage IIIA-N2 EGFR-mutant non–small-cell lung cancer (EMERGING-CTONG 1103): a randomized phase II study. J Clin Oncol. 2019;37(25):2235-2245. doi:10.1200/JCO.19.00075
13. Noronha V, Patil VM, Joshi A, et al. Gefitinib versus gefitinib plus pemetrexed and carboplatin chemotherapy in EGFR-mutated lung cancer. J Clin Oncol. 2020;38(2):124-136. doi:10.1200/JCO.19.01154
14. Noronha V, Prabhash K, Thavamani A, et al. EGFR mutations in Indian lung cancer patients: clinical correlation and outcome to EGFR targeted therapy. PLoS One. 2013;8(4):e61561. Published 2013 Apr 19. doi:10.1371/journal.pone.0061561
15. Kato T, Seto T, Nishio M, et al. Erlotinib plus bevacizumab phase ll study in patients with advanced non–small-cell lung cancer (JO25567): updated safety results. Drug Saf. 2018;41(2):229-237. doi:10.1007/s40264-017-0596-0
16. Hellmann MD, Paz-Ares L, Bernabe Caro R, et al. Nivolumab plus ipilimumab in advanced non–small-cell lung cancer. N Engl J Med. 2019;381(21):2020-2031. doi:10.1056/NEJMoa1910231
17. Hellmann MD, Ciuleanu TE, Pluzanski A, et al. Nivolumab plus ipilimumab in lung cancer with a high tumor mutational burden. N Engl J Med. 2018;378(22):2093-2104. doi:10.1056/NEJMoa1801946
18. Rizvi NA, Hellmann MD, Brahmer JR, et al. Nivolumab in combination with platinum-based doublet chemotherapy for first-line treatment of advanced non–small-cell lung cancer. J Clin Oncol. 2016;34(25):2969-2979. doi:10.1200/JCO.2016.66.9861
19. Zhong WZ, Wang Q, Mao WM, et al. Gefitinib versus vinorelbine plus cisplatin as adjuvant treatment for stage II-IIIA (N1-N2) EGFR-mutant NSCLC: final overall survival analysis of CTONG1104 Phase III Trial. J Clin Oncol. 2021;39(7):713-722. doi:10.1200/JCO.20.01820
20. Yang JC, Sequist LV, Geater SL, et al. Clinical activity of afatinib in patients with advanced non-small-cell lung cancer harbouring uncommon EGFR mutations: a combined post-hoc analysis of LUX-Lung 2, LUX-Lung 3, and LUX-Lung 6. Lancet Oncol. 2015;16(7):830-838. doi:10.1016/S1470-2045(15)00026-1
21. Shi YK, Wang L, Han BH, et al. First-line icotinib versus cisplatin/pemetrexed plus pemetrexed maintenance therapy for patients with advanced EGFR mutation-positive lung adenocarcinoma (CONVINCE): a phase 3, open-label, randomized study. Ann Oncol. 2017;28(10):2443-2450. doi:10.1093/annonc/mdx359
22. Soria JC, Wu YL, Nakagawa K, et al. Gefitinib plus chemotherapy versus placebo plus chemotherapy in EGFR-mutation-positive non-small-cell lung cancer after progression on first-line gefitinib (IMPRESS): a phase 3 randomized trial. Lancet Oncol. 2015;16(8):990-998 doi:10.1016/S1470-2045(15)00121-7
23. Goss GD, O’Callaghan C, Lorimer I, et al. Gefitinib versus placebo in completely resected non-small-cell lung cancer: results of the NCIC CTG BR19 study. J Clin Oncol. 2013;31(27):3320-3326. doi:10.1200/JCO.2013.51.1816
24. Sun JM, Lee KH, Kim SW, et al. Gefitinib versus pemetrexed as second-line treatment in patients with non-small cell lung cancer previously treated with platinum-based chemotherapy (KCSG-LU08-01): an open-label, phase 3 trial. Cancer. 2012;118(24):6234-6242. doi:10.1200/JCO.2013.51.1816
25. Mitsudomi T, Morita S, Yatabe Y, et al. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomized phase 3 trial. Lancet Oncol. 2010;11(2):121-128. doi:10.1016/S1470-2045(09)70364-X
26. Lee DH, Park K, Kim JH, Lee JS, et al. Randomized phase III trial of gefitinib versus docetaxel in non-small cell lung cancer patients who have previously received platinum-based chemotherapy. Clin Cancer Res. 2010;16(4):1307-1314. doi:10.1158/1078-0432.CCR-09-1903
27. Kim ES, Hirsh V, Mok T, et al. Gefitinib versus docetaxel in previously treated non-small-cell lung cancer (INTEREST): a randomized phase III trial. Lancet. 2008;22;372(9652):1809-1818. doi:10.1016/S0140-6736(08)61758-4
1. Rahib L, Smith BD, Aizenberg R, Rosenzweig AB, Fleshman JM, Matrisian LM. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014;74(11):2913-2921. doi:10.1158/0008-5472.CAN-14-0155
2. da Cunha Santos G, Shepherd FA, Tsao MS. EGFR mutations and lung cancer. Annu Rev Pathol. 2011;6:49-69. doi:10.1146/annurev-pathol-011110-130206
3. Sgambato A, Casaluce F, Maione P, et al. The role of EGFR tyrosine kinase inhibitors in the first-line treatment of advanced non small cell lung cancer patients harboring EGFR mutation. Curr Med Chem. 2012;19(20):3337-3352. doi:10.2174/092986712801215973
4. Rossi A, Di Maio M. Platinum-based chemotherapy in advanced non–small-cell lung cancer: optimal number of treatment cycles. Expert Rev Anticancer Ther. 2016;16(6):653-660. doi:10.1586/14737140.2016.1170596
5. Rosell R, Carcereny E, Gervais R, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non–small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2012;13(3):239-246. doi:10.1016/S1470-2045(11)70393-X
6. Zhou C, Wu YL, Chen G, et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non–small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol. 2011;12(8):735-742. doi:10.1016/S1470-2045(11)70184-X
7. Soria JC, Ohe Y, Vansteenkiste J, et al. Osimertinib in untreated EGFR-mutated advanced non–small-cell lung cancer. N Engl J Med. 2018;378(2):113-125. doi:10.1056/NEJMoa1713137
8. Mok TS, Cheng Y, Zhou X, et al. Improvement in overall survival in a randomized study that compared dacomitinib with gefitinib in patients with advanced non–small-cell lung cancer and EGFR-activating mutations. J Clin Oncol. 2018;36(22):2244-2250. doi:10.1200/JCO.2018.78.7994
9. Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;361(10):947-957. doi:10.1056/NEJMoa0810699
10. Pless M, Stupp R, Ris HB, et al. Induction chemoradiation in stage IIIA/N2 non–small-cell lung cancer: a phase 3 randomised trial. Lancet. 2015;386(9998):1049-1056. doi:10.1016/S0140-6736(15)60294-X
11. Albain KS, Rusch VW, Crowley JJ, et al. Concurrent cisplatin/etoposide plus chest radiotherapy followed by surgery for stages IIIA (N2) and IIIB non–small-cell lung cancer: mature results of Southwest Oncology Group phase II study 8805. J Clin Oncol. 1995;13(8):1880-1892. doi:10.1200/JCO.1995.13.8.1880
12. Zhong WZ, Chen KN, Chen C, et al. Erlotinib versus gemcitabine plus cisplatin as neoadjuvant treatment of Stage IIIA-N2 EGFR-mutant non–small-cell lung cancer (EMERGING-CTONG 1103): a randomized phase II study. J Clin Oncol. 2019;37(25):2235-2245. doi:10.1200/JCO.19.00075
13. Noronha V, Patil VM, Joshi A, et al. Gefitinib versus gefitinib plus pemetrexed and carboplatin chemotherapy in EGFR-mutated lung cancer. J Clin Oncol. 2020;38(2):124-136. doi:10.1200/JCO.19.01154
14. Noronha V, Prabhash K, Thavamani A, et al. EGFR mutations in Indian lung cancer patients: clinical correlation and outcome to EGFR targeted therapy. PLoS One. 2013;8(4):e61561. Published 2013 Apr 19. doi:10.1371/journal.pone.0061561
15. Kato T, Seto T, Nishio M, et al. Erlotinib plus bevacizumab phase ll study in patients with advanced non–small-cell lung cancer (JO25567): updated safety results. Drug Saf. 2018;41(2):229-237. doi:10.1007/s40264-017-0596-0
16. Hellmann MD, Paz-Ares L, Bernabe Caro R, et al. Nivolumab plus ipilimumab in advanced non–small-cell lung cancer. N Engl J Med. 2019;381(21):2020-2031. doi:10.1056/NEJMoa1910231
17. Hellmann MD, Ciuleanu TE, Pluzanski A, et al. Nivolumab plus ipilimumab in lung cancer with a high tumor mutational burden. N Engl J Med. 2018;378(22):2093-2104. doi:10.1056/NEJMoa1801946
18. Rizvi NA, Hellmann MD, Brahmer JR, et al. Nivolumab in combination with platinum-based doublet chemotherapy for first-line treatment of advanced non–small-cell lung cancer. J Clin Oncol. 2016;34(25):2969-2979. doi:10.1200/JCO.2016.66.9861
19. Zhong WZ, Wang Q, Mao WM, et al. Gefitinib versus vinorelbine plus cisplatin as adjuvant treatment for stage II-IIIA (N1-N2) EGFR-mutant NSCLC: final overall survival analysis of CTONG1104 Phase III Trial. J Clin Oncol. 2021;39(7):713-722. doi:10.1200/JCO.20.01820
20. Yang JC, Sequist LV, Geater SL, et al. Clinical activity of afatinib in patients with advanced non-small-cell lung cancer harbouring uncommon EGFR mutations: a combined post-hoc analysis of LUX-Lung 2, LUX-Lung 3, and LUX-Lung 6. Lancet Oncol. 2015;16(7):830-838. doi:10.1016/S1470-2045(15)00026-1
21. Shi YK, Wang L, Han BH, et al. First-line icotinib versus cisplatin/pemetrexed plus pemetrexed maintenance therapy for patients with advanced EGFR mutation-positive lung adenocarcinoma (CONVINCE): a phase 3, open-label, randomized study. Ann Oncol. 2017;28(10):2443-2450. doi:10.1093/annonc/mdx359
22. Soria JC, Wu YL, Nakagawa K, et al. Gefitinib plus chemotherapy versus placebo plus chemotherapy in EGFR-mutation-positive non-small-cell lung cancer after progression on first-line gefitinib (IMPRESS): a phase 3 randomized trial. Lancet Oncol. 2015;16(8):990-998 doi:10.1016/S1470-2045(15)00121-7
23. Goss GD, O’Callaghan C, Lorimer I, et al. Gefitinib versus placebo in completely resected non-small-cell lung cancer: results of the NCIC CTG BR19 study. J Clin Oncol. 2013;31(27):3320-3326. doi:10.1200/JCO.2013.51.1816
24. Sun JM, Lee KH, Kim SW, et al. Gefitinib versus pemetrexed as second-line treatment in patients with non-small cell lung cancer previously treated with platinum-based chemotherapy (KCSG-LU08-01): an open-label, phase 3 trial. Cancer. 2012;118(24):6234-6242. doi:10.1200/JCO.2013.51.1816
25. Mitsudomi T, Morita S, Yatabe Y, et al. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomized phase 3 trial. Lancet Oncol. 2010;11(2):121-128. doi:10.1016/S1470-2045(09)70364-X
26. Lee DH, Park K, Kim JH, Lee JS, et al. Randomized phase III trial of gefitinib versus docetaxel in non-small cell lung cancer patients who have previously received platinum-based chemotherapy. Clin Cancer Res. 2010;16(4):1307-1314. doi:10.1158/1078-0432.CCR-09-1903
27. Kim ES, Hirsh V, Mok T, et al. Gefitinib versus docetaxel in previously treated non-small-cell lung cancer (INTEREST): a randomized phase III trial. Lancet. 2008;22;372(9652):1809-1818. doi:10.1016/S0140-6736(08)61758-4
Obesity drug shortage triggers frustrations, workarounds
The glucagon-like peptide-1 (GLP-1) agonist semaglutide formulated for treating obesity (Wegovy) had a roaring takeoff a little more than a year ago, with surging patient demand after the U.S. Food and Drug Administration approved it in June 2021. But starting doses of the Wegovy form of semaglutide went missing in action starting late 2021 and continue to date, frustrating patients and their health care providers.
The arrival of Wegovy last year was hailed by obesity medicine specialists and others as a “game changer” for treating people with obesity because of semaglutide’s proven safety and efficacy at the subcutaneous dose of 2.4 mg delivered once a week to produce at least 15% weight loss in half the people who received it, as documented last year in results from one of the drug’s pivotal clinical trials.
But during the months following semaglutide’s approval for treating obesity (it also received an FDA marketing nod in late 2017 as Ozempic for treating type 2 diabetes), a worldwide shortage of Wegovy, including in the United States, emerged.
A manufacturing glitch shut down the primary location for production of U.S.-bound Wegovy injector pens for several months starting in late 2021, according to a December report from Novo Nordisk, the company that makes and markets the agent. (The Wegovy production issue appears to have had a very modest impact, especially in U.S. pharmacies, on the supply of semaglutide formulated as Ozempic, also marketed by Novo Nordisk, although Wegovy supply and demand have dramatically limited Ozempic availability in Australia.)
‘Unprecedented demand’ for Wegovy derailed when plant went offline
The supply side for Wegovy became so hopelessly broken that just months after U.S. sales began and immediately skyrocketed, Novo Nordisk made the remarkable decision to pull starting doses of Wegovy from the market to make it much harder to initiate patients (semaglutide and other GLP-1 agonists require gradual dose ramp-up to avoid gastrointestinal side effects), and the company publicly implored clinicians to not start new patients on the agent, which is where the status remains as of early August 2022.
Novo Nordisk’s financial report for the second quarter of 2022, released on Aug. 3, said the company “expects to make all Wegovy dose strengths available in the United States towards the end of 2022.”
A Dear Health Care Provider letter that Novo Nordisk posted on its U.S. Wegovy website last spring cited “unprecedented demand” that exceeded every prior product launch in the company’s history. It forced Novo Nordisk to pull the plug on all U.S. promotion of Wegovy and compelled the company to ask U.S. clinicians to halt new patient starts.
“I stopped offering Wegovy to new patients” since about the beginning of 2022, says Lauren D. Oshman, MD, a family and obesity medicine specialist at the University of Michigan, Ann Arbor. “It’s very frustrating to not have patients [with obesity] receive the optimal treatment available.” Although she adds that she tries to match obesity treatments to each patient’s clinical needs, and a GLP-1 agonist is not the first choice for every person with obesity.
“It was a disastrous rollout,” says Catherine W. Varney, DO, a family and obesity medicine specialist at the University of Virginia, Charlottesville. “It’s frustrating to know that the treatment is there but not being able to use it,” she said in an interview.
“I had about 800 patients on Wegovy” when the supply dropped earlier this year, and “I couldn’t handle the volume of messages that I got from patients,” recalls Angela Fitch, MD, associate director of the Massachusetts General Hospital Weight Center, Boston. “It was painful,” she said in an interview.
“Frustrating and chaotic,” is the description from Ivania M. Rizo, MD, director of obesity medicine at Boston Medical Center.
The liraglutide/Saxenda workaround
The upshot is that people with obesity and their health care providers have been busy devising workarounds to try to meet the intense demand for this drug-assisted approach to appetite control and weight loss. Their tactics run a wide gamut based on the crazy-quilt diversity of health insurance coverage across America.
Because the bottleneck for starting Wegovy resulted from unavailable starting doses (dosing starts at 0.25 mg delivered subcutaneously once a week, eventually ramping up to a maximum of 2.4 mg weekly), one option was to start patients on a different GLP-1 agonist, such as liraglutide (Saxenda, approved for obesity).
Starting a patient on liraglutide involves the same sort of up-titration and acclimation to a GLP-1 agonist that semaglutide requires, and transition between these agents seems feasible for at least some. It also means daily injections of liraglutide rather than the weekly schedule for semaglutide, although some patients prefer maintaining a daily dosing schedule. Another limitation of liraglutide is that evidence shows it is not nearly as effective for weight loss as semaglutide.
Results from the head-to-head STEP 8 trial, published in JAMA, showed an average weight loss from baseline of about 16% with semaglutide and about 6% with liraglutide (and about 2% with placebo).
A ‘reasonable’ evidence base, but more work
Changing from Saxenda to Wegovy, or from Wegovy to Saxenda, “would be reasonably evidence-based medicine,” said Dr. Oshman in an interview. She has managed a Wegovy-to-Saxenda switch for a “handful” of patients to deal with Wegovy shortages, but she has not yet moved anyone to Wegovy after a Saxenda initiation.
“No prospective study has looked at this transition,” but dose equivalence tables exist based on expert opinion, noted Dr. Oshman, as in this 2020 report.
Dr. Varney has several patients on the Saxenda-to-Wegovy track. She up-titrates patients on Saxenda to the maximum daily dose of 3.0 mg and then switches them to the 1.7 mg weekly dose of Wegovy, one of the “destination” Wegovy doses that has remained generally available during the shortage. But Dr. Varney’s experience is that only half of her patients made the changeover smoothly, with the others having “severe gastrointestinal distress,” including vomiting, she notes.
Dr. Fitch has also successfully used this Saxenda-to-Wegovy approach for some of her patients, but it hasn’t been easy.
“It’s more work and more prior authorizations. It’s harder and adds a layer of stress,” but, Dr. Fitch adds, “people are willing to work on it because the weight loss is worth it.”
The liraglutide to semaglutide shuffle is “doable,” says Dr. Rizo, “but I’m looking forward to not having to do it and being able to just start Wegovy.”
The tirzepatide coupon program works ‘off label’ for obesity
Another workaround depends on the FDA approval in May for tirzepatide (Mounjaro) for type 2 diabetes. Tirzepatide is a related GLP-1 agonist that also adds a second incretin-like agonist activity that mimics the glucose-dependent insulinotropic polypeptide.
Soon after approval, Lilly, the company that markets tirzepatide, started a U.S. coupon program geared exclusively to people with commercial insurance. Within certain refill and dollar limits, the program lets patients buy tirzepatide at pharmacies at an out-of-pocket cost of $25 for a 4-week supply (tirzepatide is also dosed by weekly subcutaneous injections). The program will extend into 2023.
Novo Nordisk offered U.S. patients with commercial insurance a similar discount when Wegovy first hit the U.S. market in 2021, but the program closed down once the supply shortage began.
Despite tirzepatide’s current approval only for type 2 diabetes, Dr. Varney has been successfully prescribing it to patients without diabetes off-label for weight loss.
“The coupons still work even when tirzepatide is used off-label,” she notes. And while the drug’s rollout is still only a couple of months old, so far, it’s gone “beautifully” with no hints of supply issues, she says.
But a major drawback to relying on an introductory coupon program that makes these agents affordable to patients is their ability to maintain treatment once the discounts inevitably end.
“We try to only prescribe agents that patients can continue to access,” says Dr. Fitch, who has had some patients with commercial insurance start on Wegovy with coupon discounts only to later lose access.
Many commercial U.S. insurers do not cover obesity treatments, a decision often driven by the employers who sponsor the coverage, she notes.
Study results have documented that when people with obesity stop taking a GLP-1 agonist their lost weight rebounds, as in a study that tracked people who stopped taking semaglutide.
Dr. Fitch has had success prescribing tirzepatide to patients with obesity but without diabetes who have certain types of Medicare drug coverage policies, which often do not deny off-label drug coverage. That approach works until patients reach the “donut hole” in their drug coverage and are faced with a certain level of out-of-pocket costs that can balloon to several thousand dollars.
Even more workarounds
Other approaches patients have used to acquire Wegovy include purchasing it in other countries, such as Canada or Brazil, says Dr. Fitch. But prices outside the United States, while substantially lower, can still be a barrier for many patients, notes Dr. Oshman.
Semaglutide in Canada goes for about $300 for a 4-week supply, roughly a quarter the U.S. price, she says, but is “still too high for many of my patients.”
Intense patient demand sometimes bordering on desperation has prompted some to seek semaglutide from private compounding pharmacies, a step clinicians regard as downright dangerous.
“Semaglutide from compounding pharmacies is not known to be safe. We feel strongly that it’s not something that people should do,” says Dr. Fitch.
“Compounding pharmacies have no FDA regulation. People don’t know what they’re getting. It’s dangerous,” agrees Dr. Varney. Physicians who refer people for privately compounded semaglutide “are taking advantage of desperate people,” she adds.
Although it seems likely that Novo Nordisk will soon sort out the supply problems and Wegovy will once again become more widely available, some of the issues patients have had with access to the weight loss medication stem from more systemic issues in the United States health insurance landscape: an unwillingness by payers to cover the costs of weight loss medications, a shortcoming that also exists for Medicare and Medicaid.
“We need to make obesity treatment a standard benefit, and not something that can be carved out,” says Dr. Fitch. People with obesity “deserve access to effective treatments for their disease,” she declares.
Dr. Oshman, Dr. Varney, and Dr. Rizo have reported no relevant financial relationships. Dr. Fitch has reported being an advisor to Jenny Craig.
A version of this article first appeared on Medscape.com.
The glucagon-like peptide-1 (GLP-1) agonist semaglutide formulated for treating obesity (Wegovy) had a roaring takeoff a little more than a year ago, with surging patient demand after the U.S. Food and Drug Administration approved it in June 2021. But starting doses of the Wegovy form of semaglutide went missing in action starting late 2021 and continue to date, frustrating patients and their health care providers.
The arrival of Wegovy last year was hailed by obesity medicine specialists and others as a “game changer” for treating people with obesity because of semaglutide’s proven safety and efficacy at the subcutaneous dose of 2.4 mg delivered once a week to produce at least 15% weight loss in half the people who received it, as documented last year in results from one of the drug’s pivotal clinical trials.
But during the months following semaglutide’s approval for treating obesity (it also received an FDA marketing nod in late 2017 as Ozempic for treating type 2 diabetes), a worldwide shortage of Wegovy, including in the United States, emerged.
A manufacturing glitch shut down the primary location for production of U.S.-bound Wegovy injector pens for several months starting in late 2021, according to a December report from Novo Nordisk, the company that makes and markets the agent. (The Wegovy production issue appears to have had a very modest impact, especially in U.S. pharmacies, on the supply of semaglutide formulated as Ozempic, also marketed by Novo Nordisk, although Wegovy supply and demand have dramatically limited Ozempic availability in Australia.)
‘Unprecedented demand’ for Wegovy derailed when plant went offline
The supply side for Wegovy became so hopelessly broken that just months after U.S. sales began and immediately skyrocketed, Novo Nordisk made the remarkable decision to pull starting doses of Wegovy from the market to make it much harder to initiate patients (semaglutide and other GLP-1 agonists require gradual dose ramp-up to avoid gastrointestinal side effects), and the company publicly implored clinicians to not start new patients on the agent, which is where the status remains as of early August 2022.
Novo Nordisk’s financial report for the second quarter of 2022, released on Aug. 3, said the company “expects to make all Wegovy dose strengths available in the United States towards the end of 2022.”
A Dear Health Care Provider letter that Novo Nordisk posted on its U.S. Wegovy website last spring cited “unprecedented demand” that exceeded every prior product launch in the company’s history. It forced Novo Nordisk to pull the plug on all U.S. promotion of Wegovy and compelled the company to ask U.S. clinicians to halt new patient starts.
“I stopped offering Wegovy to new patients” since about the beginning of 2022, says Lauren D. Oshman, MD, a family and obesity medicine specialist at the University of Michigan, Ann Arbor. “It’s very frustrating to not have patients [with obesity] receive the optimal treatment available.” Although she adds that she tries to match obesity treatments to each patient’s clinical needs, and a GLP-1 agonist is not the first choice for every person with obesity.
“It was a disastrous rollout,” says Catherine W. Varney, DO, a family and obesity medicine specialist at the University of Virginia, Charlottesville. “It’s frustrating to know that the treatment is there but not being able to use it,” she said in an interview.
“I had about 800 patients on Wegovy” when the supply dropped earlier this year, and “I couldn’t handle the volume of messages that I got from patients,” recalls Angela Fitch, MD, associate director of the Massachusetts General Hospital Weight Center, Boston. “It was painful,” she said in an interview.
“Frustrating and chaotic,” is the description from Ivania M. Rizo, MD, director of obesity medicine at Boston Medical Center.
The liraglutide/Saxenda workaround
The upshot is that people with obesity and their health care providers have been busy devising workarounds to try to meet the intense demand for this drug-assisted approach to appetite control and weight loss. Their tactics run a wide gamut based on the crazy-quilt diversity of health insurance coverage across America.
Because the bottleneck for starting Wegovy resulted from unavailable starting doses (dosing starts at 0.25 mg delivered subcutaneously once a week, eventually ramping up to a maximum of 2.4 mg weekly), one option was to start patients on a different GLP-1 agonist, such as liraglutide (Saxenda, approved for obesity).
Starting a patient on liraglutide involves the same sort of up-titration and acclimation to a GLP-1 agonist that semaglutide requires, and transition between these agents seems feasible for at least some. It also means daily injections of liraglutide rather than the weekly schedule for semaglutide, although some patients prefer maintaining a daily dosing schedule. Another limitation of liraglutide is that evidence shows it is not nearly as effective for weight loss as semaglutide.
Results from the head-to-head STEP 8 trial, published in JAMA, showed an average weight loss from baseline of about 16% with semaglutide and about 6% with liraglutide (and about 2% with placebo).
A ‘reasonable’ evidence base, but more work
Changing from Saxenda to Wegovy, or from Wegovy to Saxenda, “would be reasonably evidence-based medicine,” said Dr. Oshman in an interview. She has managed a Wegovy-to-Saxenda switch for a “handful” of patients to deal with Wegovy shortages, but she has not yet moved anyone to Wegovy after a Saxenda initiation.
“No prospective study has looked at this transition,” but dose equivalence tables exist based on expert opinion, noted Dr. Oshman, as in this 2020 report.
Dr. Varney has several patients on the Saxenda-to-Wegovy track. She up-titrates patients on Saxenda to the maximum daily dose of 3.0 mg and then switches them to the 1.7 mg weekly dose of Wegovy, one of the “destination” Wegovy doses that has remained generally available during the shortage. But Dr. Varney’s experience is that only half of her patients made the changeover smoothly, with the others having “severe gastrointestinal distress,” including vomiting, she notes.
Dr. Fitch has also successfully used this Saxenda-to-Wegovy approach for some of her patients, but it hasn’t been easy.
“It’s more work and more prior authorizations. It’s harder and adds a layer of stress,” but, Dr. Fitch adds, “people are willing to work on it because the weight loss is worth it.”
The liraglutide to semaglutide shuffle is “doable,” says Dr. Rizo, “but I’m looking forward to not having to do it and being able to just start Wegovy.”
The tirzepatide coupon program works ‘off label’ for obesity
Another workaround depends on the FDA approval in May for tirzepatide (Mounjaro) for type 2 diabetes. Tirzepatide is a related GLP-1 agonist that also adds a second incretin-like agonist activity that mimics the glucose-dependent insulinotropic polypeptide.
Soon after approval, Lilly, the company that markets tirzepatide, started a U.S. coupon program geared exclusively to people with commercial insurance. Within certain refill and dollar limits, the program lets patients buy tirzepatide at pharmacies at an out-of-pocket cost of $25 for a 4-week supply (tirzepatide is also dosed by weekly subcutaneous injections). The program will extend into 2023.
Novo Nordisk offered U.S. patients with commercial insurance a similar discount when Wegovy first hit the U.S. market in 2021, but the program closed down once the supply shortage began.
Despite tirzepatide’s current approval only for type 2 diabetes, Dr. Varney has been successfully prescribing it to patients without diabetes off-label for weight loss.
“The coupons still work even when tirzepatide is used off-label,” she notes. And while the drug’s rollout is still only a couple of months old, so far, it’s gone “beautifully” with no hints of supply issues, she says.
But a major drawback to relying on an introductory coupon program that makes these agents affordable to patients is their ability to maintain treatment once the discounts inevitably end.
“We try to only prescribe agents that patients can continue to access,” says Dr. Fitch, who has had some patients with commercial insurance start on Wegovy with coupon discounts only to later lose access.
Many commercial U.S. insurers do not cover obesity treatments, a decision often driven by the employers who sponsor the coverage, she notes.
Study results have documented that when people with obesity stop taking a GLP-1 agonist their lost weight rebounds, as in a study that tracked people who stopped taking semaglutide.
Dr. Fitch has had success prescribing tirzepatide to patients with obesity but without diabetes who have certain types of Medicare drug coverage policies, which often do not deny off-label drug coverage. That approach works until patients reach the “donut hole” in their drug coverage and are faced with a certain level of out-of-pocket costs that can balloon to several thousand dollars.
Even more workarounds
Other approaches patients have used to acquire Wegovy include purchasing it in other countries, such as Canada or Brazil, says Dr. Fitch. But prices outside the United States, while substantially lower, can still be a barrier for many patients, notes Dr. Oshman.
Semaglutide in Canada goes for about $300 for a 4-week supply, roughly a quarter the U.S. price, she says, but is “still too high for many of my patients.”
Intense patient demand sometimes bordering on desperation has prompted some to seek semaglutide from private compounding pharmacies, a step clinicians regard as downright dangerous.
“Semaglutide from compounding pharmacies is not known to be safe. We feel strongly that it’s not something that people should do,” says Dr. Fitch.
“Compounding pharmacies have no FDA regulation. People don’t know what they’re getting. It’s dangerous,” agrees Dr. Varney. Physicians who refer people for privately compounded semaglutide “are taking advantage of desperate people,” she adds.
Although it seems likely that Novo Nordisk will soon sort out the supply problems and Wegovy will once again become more widely available, some of the issues patients have had with access to the weight loss medication stem from more systemic issues in the United States health insurance landscape: an unwillingness by payers to cover the costs of weight loss medications, a shortcoming that also exists for Medicare and Medicaid.
“We need to make obesity treatment a standard benefit, and not something that can be carved out,” says Dr. Fitch. People with obesity “deserve access to effective treatments for their disease,” she declares.
Dr. Oshman, Dr. Varney, and Dr. Rizo have reported no relevant financial relationships. Dr. Fitch has reported being an advisor to Jenny Craig.
A version of this article first appeared on Medscape.com.
The glucagon-like peptide-1 (GLP-1) agonist semaglutide formulated for treating obesity (Wegovy) had a roaring takeoff a little more than a year ago, with surging patient demand after the U.S. Food and Drug Administration approved it in June 2021. But starting doses of the Wegovy form of semaglutide went missing in action starting late 2021 and continue to date, frustrating patients and their health care providers.
The arrival of Wegovy last year was hailed by obesity medicine specialists and others as a “game changer” for treating people with obesity because of semaglutide’s proven safety and efficacy at the subcutaneous dose of 2.4 mg delivered once a week to produce at least 15% weight loss in half the people who received it, as documented last year in results from one of the drug’s pivotal clinical trials.
But during the months following semaglutide’s approval for treating obesity (it also received an FDA marketing nod in late 2017 as Ozempic for treating type 2 diabetes), a worldwide shortage of Wegovy, including in the United States, emerged.
A manufacturing glitch shut down the primary location for production of U.S.-bound Wegovy injector pens for several months starting in late 2021, according to a December report from Novo Nordisk, the company that makes and markets the agent. (The Wegovy production issue appears to have had a very modest impact, especially in U.S. pharmacies, on the supply of semaglutide formulated as Ozempic, also marketed by Novo Nordisk, although Wegovy supply and demand have dramatically limited Ozempic availability in Australia.)
‘Unprecedented demand’ for Wegovy derailed when plant went offline
The supply side for Wegovy became so hopelessly broken that just months after U.S. sales began and immediately skyrocketed, Novo Nordisk made the remarkable decision to pull starting doses of Wegovy from the market to make it much harder to initiate patients (semaglutide and other GLP-1 agonists require gradual dose ramp-up to avoid gastrointestinal side effects), and the company publicly implored clinicians to not start new patients on the agent, which is where the status remains as of early August 2022.
Novo Nordisk’s financial report for the second quarter of 2022, released on Aug. 3, said the company “expects to make all Wegovy dose strengths available in the United States towards the end of 2022.”
A Dear Health Care Provider letter that Novo Nordisk posted on its U.S. Wegovy website last spring cited “unprecedented demand” that exceeded every prior product launch in the company’s history. It forced Novo Nordisk to pull the plug on all U.S. promotion of Wegovy and compelled the company to ask U.S. clinicians to halt new patient starts.
“I stopped offering Wegovy to new patients” since about the beginning of 2022, says Lauren D. Oshman, MD, a family and obesity medicine specialist at the University of Michigan, Ann Arbor. “It’s very frustrating to not have patients [with obesity] receive the optimal treatment available.” Although she adds that she tries to match obesity treatments to each patient’s clinical needs, and a GLP-1 agonist is not the first choice for every person with obesity.
“It was a disastrous rollout,” says Catherine W. Varney, DO, a family and obesity medicine specialist at the University of Virginia, Charlottesville. “It’s frustrating to know that the treatment is there but not being able to use it,” she said in an interview.
“I had about 800 patients on Wegovy” when the supply dropped earlier this year, and “I couldn’t handle the volume of messages that I got from patients,” recalls Angela Fitch, MD, associate director of the Massachusetts General Hospital Weight Center, Boston. “It was painful,” she said in an interview.
“Frustrating and chaotic,” is the description from Ivania M. Rizo, MD, director of obesity medicine at Boston Medical Center.
The liraglutide/Saxenda workaround
The upshot is that people with obesity and their health care providers have been busy devising workarounds to try to meet the intense demand for this drug-assisted approach to appetite control and weight loss. Their tactics run a wide gamut based on the crazy-quilt diversity of health insurance coverage across America.
Because the bottleneck for starting Wegovy resulted from unavailable starting doses (dosing starts at 0.25 mg delivered subcutaneously once a week, eventually ramping up to a maximum of 2.4 mg weekly), one option was to start patients on a different GLP-1 agonist, such as liraglutide (Saxenda, approved for obesity).
Starting a patient on liraglutide involves the same sort of up-titration and acclimation to a GLP-1 agonist that semaglutide requires, and transition between these agents seems feasible for at least some. It also means daily injections of liraglutide rather than the weekly schedule for semaglutide, although some patients prefer maintaining a daily dosing schedule. Another limitation of liraglutide is that evidence shows it is not nearly as effective for weight loss as semaglutide.
Results from the head-to-head STEP 8 trial, published in JAMA, showed an average weight loss from baseline of about 16% with semaglutide and about 6% with liraglutide (and about 2% with placebo).
A ‘reasonable’ evidence base, but more work
Changing from Saxenda to Wegovy, or from Wegovy to Saxenda, “would be reasonably evidence-based medicine,” said Dr. Oshman in an interview. She has managed a Wegovy-to-Saxenda switch for a “handful” of patients to deal with Wegovy shortages, but she has not yet moved anyone to Wegovy after a Saxenda initiation.
“No prospective study has looked at this transition,” but dose equivalence tables exist based on expert opinion, noted Dr. Oshman, as in this 2020 report.
Dr. Varney has several patients on the Saxenda-to-Wegovy track. She up-titrates patients on Saxenda to the maximum daily dose of 3.0 mg and then switches them to the 1.7 mg weekly dose of Wegovy, one of the “destination” Wegovy doses that has remained generally available during the shortage. But Dr. Varney’s experience is that only half of her patients made the changeover smoothly, with the others having “severe gastrointestinal distress,” including vomiting, she notes.
Dr. Fitch has also successfully used this Saxenda-to-Wegovy approach for some of her patients, but it hasn’t been easy.
“It’s more work and more prior authorizations. It’s harder and adds a layer of stress,” but, Dr. Fitch adds, “people are willing to work on it because the weight loss is worth it.”
The liraglutide to semaglutide shuffle is “doable,” says Dr. Rizo, “but I’m looking forward to not having to do it and being able to just start Wegovy.”
The tirzepatide coupon program works ‘off label’ for obesity
Another workaround depends on the FDA approval in May for tirzepatide (Mounjaro) for type 2 diabetes. Tirzepatide is a related GLP-1 agonist that also adds a second incretin-like agonist activity that mimics the glucose-dependent insulinotropic polypeptide.
Soon after approval, Lilly, the company that markets tirzepatide, started a U.S. coupon program geared exclusively to people with commercial insurance. Within certain refill and dollar limits, the program lets patients buy tirzepatide at pharmacies at an out-of-pocket cost of $25 for a 4-week supply (tirzepatide is also dosed by weekly subcutaneous injections). The program will extend into 2023.
Novo Nordisk offered U.S. patients with commercial insurance a similar discount when Wegovy first hit the U.S. market in 2021, but the program closed down once the supply shortage began.
Despite tirzepatide’s current approval only for type 2 diabetes, Dr. Varney has been successfully prescribing it to patients without diabetes off-label for weight loss.
“The coupons still work even when tirzepatide is used off-label,” she notes. And while the drug’s rollout is still only a couple of months old, so far, it’s gone “beautifully” with no hints of supply issues, she says.
But a major drawback to relying on an introductory coupon program that makes these agents affordable to patients is their ability to maintain treatment once the discounts inevitably end.
“We try to only prescribe agents that patients can continue to access,” says Dr. Fitch, who has had some patients with commercial insurance start on Wegovy with coupon discounts only to later lose access.
Many commercial U.S. insurers do not cover obesity treatments, a decision often driven by the employers who sponsor the coverage, she notes.
Study results have documented that when people with obesity stop taking a GLP-1 agonist their lost weight rebounds, as in a study that tracked people who stopped taking semaglutide.
Dr. Fitch has had success prescribing tirzepatide to patients with obesity but without diabetes who have certain types of Medicare drug coverage policies, which often do not deny off-label drug coverage. That approach works until patients reach the “donut hole” in their drug coverage and are faced with a certain level of out-of-pocket costs that can balloon to several thousand dollars.
Even more workarounds
Other approaches patients have used to acquire Wegovy include purchasing it in other countries, such as Canada or Brazil, says Dr. Fitch. But prices outside the United States, while substantially lower, can still be a barrier for many patients, notes Dr. Oshman.
Semaglutide in Canada goes for about $300 for a 4-week supply, roughly a quarter the U.S. price, she says, but is “still too high for many of my patients.”
Intense patient demand sometimes bordering on desperation has prompted some to seek semaglutide from private compounding pharmacies, a step clinicians regard as downright dangerous.
“Semaglutide from compounding pharmacies is not known to be safe. We feel strongly that it’s not something that people should do,” says Dr. Fitch.
“Compounding pharmacies have no FDA regulation. People don’t know what they’re getting. It’s dangerous,” agrees Dr. Varney. Physicians who refer people for privately compounded semaglutide “are taking advantage of desperate people,” she adds.
Although it seems likely that Novo Nordisk will soon sort out the supply problems and Wegovy will once again become more widely available, some of the issues patients have had with access to the weight loss medication stem from more systemic issues in the United States health insurance landscape: an unwillingness by payers to cover the costs of weight loss medications, a shortcoming that also exists for Medicare and Medicaid.
“We need to make obesity treatment a standard benefit, and not something that can be carved out,” says Dr. Fitch. People with obesity “deserve access to effective treatments for their disease,” she declares.
Dr. Oshman, Dr. Varney, and Dr. Rizo have reported no relevant financial relationships. Dr. Fitch has reported being an advisor to Jenny Craig.
A version of this article first appeared on Medscape.com.
The Effect of Race on Outcomes in Veterans With Hepatocellular Carcinoma at a Single Center
Hepatocellular carcinoma (HCC) is the sixth most common and third most deadly malignancy worldwide, carrying a mean survival rate without treatment of 6 to 20 months depending on stage.1 Fifty-seven percent of patients with liver cancer are diagnosed with regional or distant metastatic disease that carries 5-year relative survival rates of 10.7% and 3.1%, respectively.2 HCC arises most commonly from liver cirrhosis due to chronic hepatocyte injury, which may be mediated by viral hepatitis, alcoholism, and metabolic disease. Other less common causes include autoimmune disease, exposure to environmental hazards, and certain genetic diseases, such as α-1 antitrypsin deficiency and Wilson disease.
Multiple staging systems for HCC exist that incorporate some variation of the following features: size and invasion of the tumor, distant metastases, and liver function. Stage-directed treatments for HCC include ablation, embolization, resection, transplant, and systemic therapy, such as tyrosine kinase inhibitors, immunotherapies, and monoclonal antibodies. In addition to tumor/node/metastasis (TNM) staging, α-fetoprotein (AFP) is a diagnostic marker with prognostic value in HCC with higher levels correlating to higher tumor burden and a worse prognosis. With treatment, the 5-year survival rate for early stage HCC ranges from 60% to 80% but decreases significantly with higher stages.1 HCC screening in at-risk populations has accounted for > 40% of diagnoses since the practice became widely adopted, and earlier recognition has led to an improvement in survival even when adjusting for lead time bias.3
Systemic therapy for advanced disease continues to improve. Sorafenib remained the standard first-line systemic therapy since it was introduced in 2008.4 First-line therapy improved with immunotherapies. The phase 3 IMBrave150 trial comparing atezolizumab plus bevacizumab to sorafenib showed a median overall survival (OS) > 19 months with 7.7% of patients achieving a complete response.5 HIMALAYA, another phase 3 trial set for publication later this year, also reported promising results when a priming dose of the CTLA-4 inhibitor tremelimumab followed by durvalumab was compared with sorafenib.6
There has been a rise in incidence of HCC in the United States across all races and ethnicities, though Black, Hispanic, and Asian patients remain disproportionately affected. Subsequently, identifying causative biologic, socioeconomic, and cultural factors, as well as implicit bias in health care continues to be a topic of great interest.7-9 Using Surveillance, Epidemiology, and End Results (SEER) data, a number of large studies have found that Black patients with HCC were more likely to present with an advanced stage, less likely to receive curative intent treatment, and had significantly reduced survival compared with that of White patients.1,7-9 An analysis of 1117 patients by Rich and colleagues noted a 34% increased risk of death for Black patients with HCC compared with that of White patients, and other studies have shown about a 50% reduction in rate of liver transplantation for Black patients.10-12 Our study aimed to investigate potential disparities in incidence, etiology, AFP level at diagnosis, and outcomes of HCC in Black and White veterans managed at the Memphis Veterans Affairs Medical Center (VAMC) in Tennessee.
Methods
A single center retrospective chart review was conducted at the Memphis VAMC using the Computerized Patient Record System (CPRS) and the International Statistical Classification of Diseases, Tenth Revision (ICD-10) code C22.0 for HCC. Initial results were manually refined by prespecified criteria. Patients were included if they were diagnosed with HCC and received HCC treatment at the Memphis VAMC. Patients were excluded if HCC was not diagnosed histologically or clinically by imaging characteristics and AFP level, if the patient’s primary treatment was not provided at the Memphis VAMC, if they were lost to follow-up, or if race was not specified as either Black or White.
The following patient variables were examined: age, sex, comorbidities (alcohol or substance use disorder, cirrhosis, HIV), tumor stage, AFP, method of diagnosis, first-line treatments, systemic treatment, surgical options offered, and mortality. Staging was based on the American Joint Committee on Cancer TNM staging for HCC.13 Surgical options were recorded as resection or transplant. Patients who were offered treatment but lost to follow-up were excluded from the analysis.
Data Analysis
Our primary endpoint was identifying differences in OS among Memphis VAMC patients with HCC related to race. Kaplan-Meier analysis was used to investigate differences in OS and cumulative hazard ratio (HR) for death. Cox regression multivariate analysis further evaluated discrepancies among investigated patient variables, including age, race, alcohol, tobacco, or illicit drug use, HIV coinfection, and cirrhosis. Treatment factors were further defined by first-line treatment, systemic therapy, surgical resection, and transplant. χ2 analysis was used to investigate differences in treatment modalities.
Results
We identified 227 veterans, 95 Black and 132 White, between 2009 and 2021 meeting criteria for primary HCC treated at the Memphis VAMC. This study did not show a significant difference in OS between White and Black veterans (P = .24). Kaplan-Meier assessment showed OS was 1247 days (41 months) for Black veterans compared with 1032 days (34 months) for White veterans (Figure; Table 1).
Additionally, no significant difference was found between veterans for age or stage at diagnosis when stratified by race. The mean age of diagnosis for both groups was 65 years (P = .09). The mean TNM staging was 1.7 for White veterans vs 1.8 for Black veterans (P = .57). There was a significant increase in the AFP level at diagnosis for Black veterans (P = .001) (Table 2).
The most common initial treatment for both groups was transarterial chemoembolization and radiofrequency ablation with 68% of White and 64% of Black veterans receiving this therapy. There was no significant difference between who received systemic therapy.
However, we found significant differences by race for some forms of treatment. In our analysis, significant differences existed between those who did not receive any form of treatment as well as who received surgical resection and transplant. Among Black veterans, 11.6% received no treatment vs 6.1% for White veterans (P = .001). Only 2.1% of Black veterans underwent surgical resection vs 8.3% of White veterans (P = .046). Similarly, 13 (9.8%) White veterans vs 3 (3.2%) Black veterans received orthotopic liver transplantation (P = .052) in our cohort (eAppendix available at doi:10.12788/fp.0304). We found no differences in patient characteristics affecting OS, including alcohol use, tobacco use, illicit drug use, HIV coinfection, or liver cirrhosis (Table 3).
Discussion
In this retrospective analysis, Black veterans with HCC did not experience a statistically significant decrease in OS compared with that of White veterans despite some differences in therapy offered. Other studies have found that surgery was less frequently recommended to Black patients across multiple cancer types, and in most cases this carried a negative impact on OS.8,10,11,14,15 A number of other studies have demonstrated a greater percentage of Black patients receiving no treatment, although these studies are often based on SEER data, which captures only cancer-directed surgery and no other methods of treatment. Inequities in patient factors like insurance and socioeconomic status as well as willingness to receive certain treatments are often cited as major influences in health care disparities, but systemic and clinician factors like hospital volume, clinician expertise, specialist availability, and implicit racial bias all affect outcomes.16 One benefit of our study was that CPRS provided a centralized recording of all treatments received. Interestingly, the treatment discrepancy in our study was not attributable to a statistically significant difference in tumor stage at presentation. There should be no misconception that US Department of Veterans Affairs patients are less affected by socioeconomic inequities, though still this suggests clinician and systemic factors were significant drivers behind our findings.
This study did not intend to determine differences in incidence of HCC by race, although many studies have shown an age-adjusted incidence of HCC among Black and Hispanic patients up to twice that of White patients.1,8-10 Notably, the rate of orthotopic liver transplantation in this study was low regardless of race compared with that of other larger studies of patients with HCC.12,15 Discrepancies in HCC care among White and Black patients have been suggested to stem from a variety of influences, including access to early diagnosis and treatment of hepatitis C virus, comorbid conditions, as well as complex socioeconomic factors. It also has been shown that oncologists’ implicit racial bias has a negative impact on patients’ perceived quality of communication, their confidence in the recommended treatment, and the understood difficulty of the treatment by the patient and should be considered as a contributor to health disparities.17,18
Studies evaluating survival in HCC using SEER data generally stratify disease by localized, regional, or distant metastasis. For our study, TNM staging provided a more accurate assessment of the disease and reduced the chances that broader staging definitions could obscure differences in treatment choices. Future studies could be improved by stratifying patients by variables impacting treatment choice, such as Child-Pugh score or Barcelona Clinic Liver Cancer staging. Our study demonstrated a statistically significant difference in AFP level between White and Black veterans. This has been observed in prior studies as well, and while no specific cause has been identified, it suggests differences in tumor biologic features across different races. In addition, we found that an elevated AFP level at the time of diagnosis (defined as > 400) correlates with a worsened OS (HR, 1.36; P = .01).
Limitations
This study has several limitations, notably the number of veterans eligible for analysis at a single institution. A larger cohort would be needed to evaluate for statistically significant differences in outcomes by race. Additionally, our study did not account for therapy that was offered to but not pursued by the patient, and this would be useful to determine whether patient or practitioner factors were the more significant influence on the type of therapy received.
Conclusions
This study demonstrated a statistically significant difference in the rate of resection and liver transplantation between White and Black veterans at a single institution, although no difference in OS was observed. This discrepancy was not explained by differences in tumor staging. Additional, larger studies will be useful in clarifying the biologic, cultural, and socioeconomic drivers in HCC treatment and mortality.
Acknowledgments
The authors thank Lorri Reaves, Memphis Veterans Affairs Medical Center, Department of Hepatology.
1. Altekruse SF, McGlynn KA, Reichman ME. Hepatocellular carcinoma incidence, mortality, and survival trends in the United States from 1975 to 2005. J Clin Oncol. 2009;27(9):1485-1491. doi:10.1200/JCO.2008.20.7753
2. Howlader N, Noone AM, Krapcho M, et al (eds). SEER Cancer Statistics Review, 1975-2012, National Cancer Institute. Accessed July 8, 2022. https://seer.cancer.gov/archive/csr/1975_2012/results_merged/sect_14_liver_bile.pdf#page=8
3. Singal AG, Mittal S, Yerokun OA, et al. Hepatocellular carcinoma screening associated with early tumor detection and improved survival among patients with cirrhosis in the US. Am J Med. 2017;130(9):1099-1106.e1. doi:10.1016/j.amjmed.2017.01.021
4. Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359(4):378-390. doi:10.1056/NEJMoa0708857
5. Finn RS, Qin S, Ikeda M, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med. 2020;382(20):1894-1905. doi:10.1056/NEJMoa1915745
6. Abou-Alfa GK, Chan SL, Kudo M, et al. Phase 3 randomized, open-label, multicenter study of tremelimumab (T) and durvalumab (D) as first-line therapy in patients (pts) with unresectable hepatocellular carcinoma (uHCC): HIMALAYA. J Clin Oncol. 2022;40(suppl 4):379. doi:10.1200/JCO.2022.40.4_suppl.379
7. Franco RA, Fan Y, Jarosek S, Bae S, Galbraith J. Racial and geographic disparities in hepatocellular carcinoma outcomes. Am J Prev Med. 2018;55(5)(suppl 1):S40-S48. doi:10.1016/j.amepre.2018.05.030
8. Ha J, Yan M, Aguilar M, et al. Race/ethnicity-specific disparities in hepatocellular carcinoma stage at diagnosis and its impact on receipt of curative therapies. J Clin Gastroenterol. 2016;50(5):423-430. doi:10.1097/MCG.0000000000000448
9. Wong R, Corley DA. Racial and ethnic variations in hepatocellular carcinoma incidence within the United States. Am J Med. 2008;121(6):525-531. doi:10.1016/j.amjmed.2008.03.005
10. Rich NE, Hester C, Odewole M, et al. Racial and ethnic differences in presentation and outcomes of hepatocellular carcinoma. Clin Gastroenterol Hepatol. 2019;17(3):551-559.e1. doi:10.1016/j.cgh.2018.05.039
11. Peters NA, Javed AA, He J, Wolfgang CL, Weiss MJ. Association of socioeconomics, surgical therapy, and survival of early stage hepatocellular carcinoma. J Surg Res. 2017;210:253-260. doi:10.1016/j.jss.2016.11.042
12. Wong RJ, Devaki P, Nguyen L, Cheung R, Nguyen MH. Ethnic disparities and liver transplantation rates in hepatocellular carcinoma patients in the recent era: results from the Surveillance, Epidemiology, and End Results registry. Liver Transpl. 2014;20(5):528-535. doi:10.1002/lt.23820
13. Minagawa M, Ikai I, Matsuyama Y, Yamaoka Y, Makuuchi M. Staging of hepatocellular carcinoma: assessment of the Japanese TNM and AJCC/UICC TNM systems in a cohort of 13,772 patients in Japan. Ann Surg. 2007;245(6):909-922. doi:10.1097/01.sla.0000254368.65878.da.
14. Harrison LE, Reichman T, Koneru B, et al. Racial discrepancies in the outcome of patients with hepatocellular carcinoma. Arch Surg. 2004;139(9):992-996. doi:10.1001/archsurg.139.9.992
15. Sloane D, Chen H, Howell C. Racial disparity in primary hepatocellular carcinoma: tumor stage at presentation, surgical treatment and survival. J Natl Med Assoc. 2006;98(12):1934-1939.
16. Haider AH, Scott VK, Rehman KA, et al. Racial disparities in surgical care and outcomes in the United States: a comprehensive review of patient, provider, and systemic factors. J Am Coll Surg. 2013;216(3):482-92.e12. doi:10.1016/j.jamcollsurg.2012.11.014
17. Cooper LA, Roter DL, Carson KA, et al. The associations of clinicians’ implicit attitudes about race with medical visit communication and patient ratings of interpersonal care. Am J Public Health. 2012;102(5):979-987. doi:10.2105/AJPH.2011.300558
18. Penner LA, Dovidio JF, Gonzalez R, et al. The effects of oncologist implicit racial bias in racially discordant oncology interactions. J Clin Oncol. 2016;34(24):2874-2880. doi:10.1200/JCO.2015.66.3658
Hepatocellular carcinoma (HCC) is the sixth most common and third most deadly malignancy worldwide, carrying a mean survival rate without treatment of 6 to 20 months depending on stage.1 Fifty-seven percent of patients with liver cancer are diagnosed with regional or distant metastatic disease that carries 5-year relative survival rates of 10.7% and 3.1%, respectively.2 HCC arises most commonly from liver cirrhosis due to chronic hepatocyte injury, which may be mediated by viral hepatitis, alcoholism, and metabolic disease. Other less common causes include autoimmune disease, exposure to environmental hazards, and certain genetic diseases, such as α-1 antitrypsin deficiency and Wilson disease.
Multiple staging systems for HCC exist that incorporate some variation of the following features: size and invasion of the tumor, distant metastases, and liver function. Stage-directed treatments for HCC include ablation, embolization, resection, transplant, and systemic therapy, such as tyrosine kinase inhibitors, immunotherapies, and monoclonal antibodies. In addition to tumor/node/metastasis (TNM) staging, α-fetoprotein (AFP) is a diagnostic marker with prognostic value in HCC with higher levels correlating to higher tumor burden and a worse prognosis. With treatment, the 5-year survival rate for early stage HCC ranges from 60% to 80% but decreases significantly with higher stages.1 HCC screening in at-risk populations has accounted for > 40% of diagnoses since the practice became widely adopted, and earlier recognition has led to an improvement in survival even when adjusting for lead time bias.3
Systemic therapy for advanced disease continues to improve. Sorafenib remained the standard first-line systemic therapy since it was introduced in 2008.4 First-line therapy improved with immunotherapies. The phase 3 IMBrave150 trial comparing atezolizumab plus bevacizumab to sorafenib showed a median overall survival (OS) > 19 months with 7.7% of patients achieving a complete response.5 HIMALAYA, another phase 3 trial set for publication later this year, also reported promising results when a priming dose of the CTLA-4 inhibitor tremelimumab followed by durvalumab was compared with sorafenib.6
There has been a rise in incidence of HCC in the United States across all races and ethnicities, though Black, Hispanic, and Asian patients remain disproportionately affected. Subsequently, identifying causative biologic, socioeconomic, and cultural factors, as well as implicit bias in health care continues to be a topic of great interest.7-9 Using Surveillance, Epidemiology, and End Results (SEER) data, a number of large studies have found that Black patients with HCC were more likely to present with an advanced stage, less likely to receive curative intent treatment, and had significantly reduced survival compared with that of White patients.1,7-9 An analysis of 1117 patients by Rich and colleagues noted a 34% increased risk of death for Black patients with HCC compared with that of White patients, and other studies have shown about a 50% reduction in rate of liver transplantation for Black patients.10-12 Our study aimed to investigate potential disparities in incidence, etiology, AFP level at diagnosis, and outcomes of HCC in Black and White veterans managed at the Memphis Veterans Affairs Medical Center (VAMC) in Tennessee.
Methods
A single center retrospective chart review was conducted at the Memphis VAMC using the Computerized Patient Record System (CPRS) and the International Statistical Classification of Diseases, Tenth Revision (ICD-10) code C22.0 for HCC. Initial results were manually refined by prespecified criteria. Patients were included if they were diagnosed with HCC and received HCC treatment at the Memphis VAMC. Patients were excluded if HCC was not diagnosed histologically or clinically by imaging characteristics and AFP level, if the patient’s primary treatment was not provided at the Memphis VAMC, if they were lost to follow-up, or if race was not specified as either Black or White.
The following patient variables were examined: age, sex, comorbidities (alcohol or substance use disorder, cirrhosis, HIV), tumor stage, AFP, method of diagnosis, first-line treatments, systemic treatment, surgical options offered, and mortality. Staging was based on the American Joint Committee on Cancer TNM staging for HCC.13 Surgical options were recorded as resection or transplant. Patients who were offered treatment but lost to follow-up were excluded from the analysis.
Data Analysis
Our primary endpoint was identifying differences in OS among Memphis VAMC patients with HCC related to race. Kaplan-Meier analysis was used to investigate differences in OS and cumulative hazard ratio (HR) for death. Cox regression multivariate analysis further evaluated discrepancies among investigated patient variables, including age, race, alcohol, tobacco, or illicit drug use, HIV coinfection, and cirrhosis. Treatment factors were further defined by first-line treatment, systemic therapy, surgical resection, and transplant. χ2 analysis was used to investigate differences in treatment modalities.
Results
We identified 227 veterans, 95 Black and 132 White, between 2009 and 2021 meeting criteria for primary HCC treated at the Memphis VAMC. This study did not show a significant difference in OS between White and Black veterans (P = .24). Kaplan-Meier assessment showed OS was 1247 days (41 months) for Black veterans compared with 1032 days (34 months) for White veterans (Figure; Table 1).
Additionally, no significant difference was found between veterans for age or stage at diagnosis when stratified by race. The mean age of diagnosis for both groups was 65 years (P = .09). The mean TNM staging was 1.7 for White veterans vs 1.8 for Black veterans (P = .57). There was a significant increase in the AFP level at diagnosis for Black veterans (P = .001) (Table 2).
The most common initial treatment for both groups was transarterial chemoembolization and radiofrequency ablation with 68% of White and 64% of Black veterans receiving this therapy. There was no significant difference between who received systemic therapy.
However, we found significant differences by race for some forms of treatment. In our analysis, significant differences existed between those who did not receive any form of treatment as well as who received surgical resection and transplant. Among Black veterans, 11.6% received no treatment vs 6.1% for White veterans (P = .001). Only 2.1% of Black veterans underwent surgical resection vs 8.3% of White veterans (P = .046). Similarly, 13 (9.8%) White veterans vs 3 (3.2%) Black veterans received orthotopic liver transplantation (P = .052) in our cohort (eAppendix available at doi:10.12788/fp.0304). We found no differences in patient characteristics affecting OS, including alcohol use, tobacco use, illicit drug use, HIV coinfection, or liver cirrhosis (Table 3).
Discussion
In this retrospective analysis, Black veterans with HCC did not experience a statistically significant decrease in OS compared with that of White veterans despite some differences in therapy offered. Other studies have found that surgery was less frequently recommended to Black patients across multiple cancer types, and in most cases this carried a negative impact on OS.8,10,11,14,15 A number of other studies have demonstrated a greater percentage of Black patients receiving no treatment, although these studies are often based on SEER data, which captures only cancer-directed surgery and no other methods of treatment. Inequities in patient factors like insurance and socioeconomic status as well as willingness to receive certain treatments are often cited as major influences in health care disparities, but systemic and clinician factors like hospital volume, clinician expertise, specialist availability, and implicit racial bias all affect outcomes.16 One benefit of our study was that CPRS provided a centralized recording of all treatments received. Interestingly, the treatment discrepancy in our study was not attributable to a statistically significant difference in tumor stage at presentation. There should be no misconception that US Department of Veterans Affairs patients are less affected by socioeconomic inequities, though still this suggests clinician and systemic factors were significant drivers behind our findings.
This study did not intend to determine differences in incidence of HCC by race, although many studies have shown an age-adjusted incidence of HCC among Black and Hispanic patients up to twice that of White patients.1,8-10 Notably, the rate of orthotopic liver transplantation in this study was low regardless of race compared with that of other larger studies of patients with HCC.12,15 Discrepancies in HCC care among White and Black patients have been suggested to stem from a variety of influences, including access to early diagnosis and treatment of hepatitis C virus, comorbid conditions, as well as complex socioeconomic factors. It also has been shown that oncologists’ implicit racial bias has a negative impact on patients’ perceived quality of communication, their confidence in the recommended treatment, and the understood difficulty of the treatment by the patient and should be considered as a contributor to health disparities.17,18
Studies evaluating survival in HCC using SEER data generally stratify disease by localized, regional, or distant metastasis. For our study, TNM staging provided a more accurate assessment of the disease and reduced the chances that broader staging definitions could obscure differences in treatment choices. Future studies could be improved by stratifying patients by variables impacting treatment choice, such as Child-Pugh score or Barcelona Clinic Liver Cancer staging. Our study demonstrated a statistically significant difference in AFP level between White and Black veterans. This has been observed in prior studies as well, and while no specific cause has been identified, it suggests differences in tumor biologic features across different races. In addition, we found that an elevated AFP level at the time of diagnosis (defined as > 400) correlates with a worsened OS (HR, 1.36; P = .01).
Limitations
This study has several limitations, notably the number of veterans eligible for analysis at a single institution. A larger cohort would be needed to evaluate for statistically significant differences in outcomes by race. Additionally, our study did not account for therapy that was offered to but not pursued by the patient, and this would be useful to determine whether patient or practitioner factors were the more significant influence on the type of therapy received.
Conclusions
This study demonstrated a statistically significant difference in the rate of resection and liver transplantation between White and Black veterans at a single institution, although no difference in OS was observed. This discrepancy was not explained by differences in tumor staging. Additional, larger studies will be useful in clarifying the biologic, cultural, and socioeconomic drivers in HCC treatment and mortality.
Acknowledgments
The authors thank Lorri Reaves, Memphis Veterans Affairs Medical Center, Department of Hepatology.
Hepatocellular carcinoma (HCC) is the sixth most common and third most deadly malignancy worldwide, carrying a mean survival rate without treatment of 6 to 20 months depending on stage.1 Fifty-seven percent of patients with liver cancer are diagnosed with regional or distant metastatic disease that carries 5-year relative survival rates of 10.7% and 3.1%, respectively.2 HCC arises most commonly from liver cirrhosis due to chronic hepatocyte injury, which may be mediated by viral hepatitis, alcoholism, and metabolic disease. Other less common causes include autoimmune disease, exposure to environmental hazards, and certain genetic diseases, such as α-1 antitrypsin deficiency and Wilson disease.
Multiple staging systems for HCC exist that incorporate some variation of the following features: size and invasion of the tumor, distant metastases, and liver function. Stage-directed treatments for HCC include ablation, embolization, resection, transplant, and systemic therapy, such as tyrosine kinase inhibitors, immunotherapies, and monoclonal antibodies. In addition to tumor/node/metastasis (TNM) staging, α-fetoprotein (AFP) is a diagnostic marker with prognostic value in HCC with higher levels correlating to higher tumor burden and a worse prognosis. With treatment, the 5-year survival rate for early stage HCC ranges from 60% to 80% but decreases significantly with higher stages.1 HCC screening in at-risk populations has accounted for > 40% of diagnoses since the practice became widely adopted, and earlier recognition has led to an improvement in survival even when adjusting for lead time bias.3
Systemic therapy for advanced disease continues to improve. Sorafenib remained the standard first-line systemic therapy since it was introduced in 2008.4 First-line therapy improved with immunotherapies. The phase 3 IMBrave150 trial comparing atezolizumab plus bevacizumab to sorafenib showed a median overall survival (OS) > 19 months with 7.7% of patients achieving a complete response.5 HIMALAYA, another phase 3 trial set for publication later this year, also reported promising results when a priming dose of the CTLA-4 inhibitor tremelimumab followed by durvalumab was compared with sorafenib.6
There has been a rise in incidence of HCC in the United States across all races and ethnicities, though Black, Hispanic, and Asian patients remain disproportionately affected. Subsequently, identifying causative biologic, socioeconomic, and cultural factors, as well as implicit bias in health care continues to be a topic of great interest.7-9 Using Surveillance, Epidemiology, and End Results (SEER) data, a number of large studies have found that Black patients with HCC were more likely to present with an advanced stage, less likely to receive curative intent treatment, and had significantly reduced survival compared with that of White patients.1,7-9 An analysis of 1117 patients by Rich and colleagues noted a 34% increased risk of death for Black patients with HCC compared with that of White patients, and other studies have shown about a 50% reduction in rate of liver transplantation for Black patients.10-12 Our study aimed to investigate potential disparities in incidence, etiology, AFP level at diagnosis, and outcomes of HCC in Black and White veterans managed at the Memphis Veterans Affairs Medical Center (VAMC) in Tennessee.
Methods
A single center retrospective chart review was conducted at the Memphis VAMC using the Computerized Patient Record System (CPRS) and the International Statistical Classification of Diseases, Tenth Revision (ICD-10) code C22.0 for HCC. Initial results were manually refined by prespecified criteria. Patients were included if they were diagnosed with HCC and received HCC treatment at the Memphis VAMC. Patients were excluded if HCC was not diagnosed histologically or clinically by imaging characteristics and AFP level, if the patient’s primary treatment was not provided at the Memphis VAMC, if they were lost to follow-up, or if race was not specified as either Black or White.
The following patient variables were examined: age, sex, comorbidities (alcohol or substance use disorder, cirrhosis, HIV), tumor stage, AFP, method of diagnosis, first-line treatments, systemic treatment, surgical options offered, and mortality. Staging was based on the American Joint Committee on Cancer TNM staging for HCC.13 Surgical options were recorded as resection or transplant. Patients who were offered treatment but lost to follow-up were excluded from the analysis.
Data Analysis
Our primary endpoint was identifying differences in OS among Memphis VAMC patients with HCC related to race. Kaplan-Meier analysis was used to investigate differences in OS and cumulative hazard ratio (HR) for death. Cox regression multivariate analysis further evaluated discrepancies among investigated patient variables, including age, race, alcohol, tobacco, or illicit drug use, HIV coinfection, and cirrhosis. Treatment factors were further defined by first-line treatment, systemic therapy, surgical resection, and transplant. χ2 analysis was used to investigate differences in treatment modalities.
Results
We identified 227 veterans, 95 Black and 132 White, between 2009 and 2021 meeting criteria for primary HCC treated at the Memphis VAMC. This study did not show a significant difference in OS between White and Black veterans (P = .24). Kaplan-Meier assessment showed OS was 1247 days (41 months) for Black veterans compared with 1032 days (34 months) for White veterans (Figure; Table 1).
Additionally, no significant difference was found between veterans for age or stage at diagnosis when stratified by race. The mean age of diagnosis for both groups was 65 years (P = .09). The mean TNM staging was 1.7 for White veterans vs 1.8 for Black veterans (P = .57). There was a significant increase in the AFP level at diagnosis for Black veterans (P = .001) (Table 2).
The most common initial treatment for both groups was transarterial chemoembolization and radiofrequency ablation with 68% of White and 64% of Black veterans receiving this therapy. There was no significant difference between who received systemic therapy.
However, we found significant differences by race for some forms of treatment. In our analysis, significant differences existed between those who did not receive any form of treatment as well as who received surgical resection and transplant. Among Black veterans, 11.6% received no treatment vs 6.1% for White veterans (P = .001). Only 2.1% of Black veterans underwent surgical resection vs 8.3% of White veterans (P = .046). Similarly, 13 (9.8%) White veterans vs 3 (3.2%) Black veterans received orthotopic liver transplantation (P = .052) in our cohort (eAppendix available at doi:10.12788/fp.0304). We found no differences in patient characteristics affecting OS, including alcohol use, tobacco use, illicit drug use, HIV coinfection, or liver cirrhosis (Table 3).
Discussion
In this retrospective analysis, Black veterans with HCC did not experience a statistically significant decrease in OS compared with that of White veterans despite some differences in therapy offered. Other studies have found that surgery was less frequently recommended to Black patients across multiple cancer types, and in most cases this carried a negative impact on OS.8,10,11,14,15 A number of other studies have demonstrated a greater percentage of Black patients receiving no treatment, although these studies are often based on SEER data, which captures only cancer-directed surgery and no other methods of treatment. Inequities in patient factors like insurance and socioeconomic status as well as willingness to receive certain treatments are often cited as major influences in health care disparities, but systemic and clinician factors like hospital volume, clinician expertise, specialist availability, and implicit racial bias all affect outcomes.16 One benefit of our study was that CPRS provided a centralized recording of all treatments received. Interestingly, the treatment discrepancy in our study was not attributable to a statistically significant difference in tumor stage at presentation. There should be no misconception that US Department of Veterans Affairs patients are less affected by socioeconomic inequities, though still this suggests clinician and systemic factors were significant drivers behind our findings.
This study did not intend to determine differences in incidence of HCC by race, although many studies have shown an age-adjusted incidence of HCC among Black and Hispanic patients up to twice that of White patients.1,8-10 Notably, the rate of orthotopic liver transplantation in this study was low regardless of race compared with that of other larger studies of patients with HCC.12,15 Discrepancies in HCC care among White and Black patients have been suggested to stem from a variety of influences, including access to early diagnosis and treatment of hepatitis C virus, comorbid conditions, as well as complex socioeconomic factors. It also has been shown that oncologists’ implicit racial bias has a negative impact on patients’ perceived quality of communication, their confidence in the recommended treatment, and the understood difficulty of the treatment by the patient and should be considered as a contributor to health disparities.17,18
Studies evaluating survival in HCC using SEER data generally stratify disease by localized, regional, or distant metastasis. For our study, TNM staging provided a more accurate assessment of the disease and reduced the chances that broader staging definitions could obscure differences in treatment choices. Future studies could be improved by stratifying patients by variables impacting treatment choice, such as Child-Pugh score or Barcelona Clinic Liver Cancer staging. Our study demonstrated a statistically significant difference in AFP level between White and Black veterans. This has been observed in prior studies as well, and while no specific cause has been identified, it suggests differences in tumor biologic features across different races. In addition, we found that an elevated AFP level at the time of diagnosis (defined as > 400) correlates with a worsened OS (HR, 1.36; P = .01).
Limitations
This study has several limitations, notably the number of veterans eligible for analysis at a single institution. A larger cohort would be needed to evaluate for statistically significant differences in outcomes by race. Additionally, our study did not account for therapy that was offered to but not pursued by the patient, and this would be useful to determine whether patient or practitioner factors were the more significant influence on the type of therapy received.
Conclusions
This study demonstrated a statistically significant difference in the rate of resection and liver transplantation between White and Black veterans at a single institution, although no difference in OS was observed. This discrepancy was not explained by differences in tumor staging. Additional, larger studies will be useful in clarifying the biologic, cultural, and socioeconomic drivers in HCC treatment and mortality.
Acknowledgments
The authors thank Lorri Reaves, Memphis Veterans Affairs Medical Center, Department of Hepatology.
1. Altekruse SF, McGlynn KA, Reichman ME. Hepatocellular carcinoma incidence, mortality, and survival trends in the United States from 1975 to 2005. J Clin Oncol. 2009;27(9):1485-1491. doi:10.1200/JCO.2008.20.7753
2. Howlader N, Noone AM, Krapcho M, et al (eds). SEER Cancer Statistics Review, 1975-2012, National Cancer Institute. Accessed July 8, 2022. https://seer.cancer.gov/archive/csr/1975_2012/results_merged/sect_14_liver_bile.pdf#page=8
3. Singal AG, Mittal S, Yerokun OA, et al. Hepatocellular carcinoma screening associated with early tumor detection and improved survival among patients with cirrhosis in the US. Am J Med. 2017;130(9):1099-1106.e1. doi:10.1016/j.amjmed.2017.01.021
4. Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359(4):378-390. doi:10.1056/NEJMoa0708857
5. Finn RS, Qin S, Ikeda M, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med. 2020;382(20):1894-1905. doi:10.1056/NEJMoa1915745
6. Abou-Alfa GK, Chan SL, Kudo M, et al. Phase 3 randomized, open-label, multicenter study of tremelimumab (T) and durvalumab (D) as first-line therapy in patients (pts) with unresectable hepatocellular carcinoma (uHCC): HIMALAYA. J Clin Oncol. 2022;40(suppl 4):379. doi:10.1200/JCO.2022.40.4_suppl.379
7. Franco RA, Fan Y, Jarosek S, Bae S, Galbraith J. Racial and geographic disparities in hepatocellular carcinoma outcomes. Am J Prev Med. 2018;55(5)(suppl 1):S40-S48. doi:10.1016/j.amepre.2018.05.030
8. Ha J, Yan M, Aguilar M, et al. Race/ethnicity-specific disparities in hepatocellular carcinoma stage at diagnosis and its impact on receipt of curative therapies. J Clin Gastroenterol. 2016;50(5):423-430. doi:10.1097/MCG.0000000000000448
9. Wong R, Corley DA. Racial and ethnic variations in hepatocellular carcinoma incidence within the United States. Am J Med. 2008;121(6):525-531. doi:10.1016/j.amjmed.2008.03.005
10. Rich NE, Hester C, Odewole M, et al. Racial and ethnic differences in presentation and outcomes of hepatocellular carcinoma. Clin Gastroenterol Hepatol. 2019;17(3):551-559.e1. doi:10.1016/j.cgh.2018.05.039
11. Peters NA, Javed AA, He J, Wolfgang CL, Weiss MJ. Association of socioeconomics, surgical therapy, and survival of early stage hepatocellular carcinoma. J Surg Res. 2017;210:253-260. doi:10.1016/j.jss.2016.11.042
12. Wong RJ, Devaki P, Nguyen L, Cheung R, Nguyen MH. Ethnic disparities and liver transplantation rates in hepatocellular carcinoma patients in the recent era: results from the Surveillance, Epidemiology, and End Results registry. Liver Transpl. 2014;20(5):528-535. doi:10.1002/lt.23820
13. Minagawa M, Ikai I, Matsuyama Y, Yamaoka Y, Makuuchi M. Staging of hepatocellular carcinoma: assessment of the Japanese TNM and AJCC/UICC TNM systems in a cohort of 13,772 patients in Japan. Ann Surg. 2007;245(6):909-922. doi:10.1097/01.sla.0000254368.65878.da.
14. Harrison LE, Reichman T, Koneru B, et al. Racial discrepancies in the outcome of patients with hepatocellular carcinoma. Arch Surg. 2004;139(9):992-996. doi:10.1001/archsurg.139.9.992
15. Sloane D, Chen H, Howell C. Racial disparity in primary hepatocellular carcinoma: tumor stage at presentation, surgical treatment and survival. J Natl Med Assoc. 2006;98(12):1934-1939.
16. Haider AH, Scott VK, Rehman KA, et al. Racial disparities in surgical care and outcomes in the United States: a comprehensive review of patient, provider, and systemic factors. J Am Coll Surg. 2013;216(3):482-92.e12. doi:10.1016/j.jamcollsurg.2012.11.014
17. Cooper LA, Roter DL, Carson KA, et al. The associations of clinicians’ implicit attitudes about race with medical visit communication and patient ratings of interpersonal care. Am J Public Health. 2012;102(5):979-987. doi:10.2105/AJPH.2011.300558
18. Penner LA, Dovidio JF, Gonzalez R, et al. The effects of oncologist implicit racial bias in racially discordant oncology interactions. J Clin Oncol. 2016;34(24):2874-2880. doi:10.1200/JCO.2015.66.3658
1. Altekruse SF, McGlynn KA, Reichman ME. Hepatocellular carcinoma incidence, mortality, and survival trends in the United States from 1975 to 2005. J Clin Oncol. 2009;27(9):1485-1491. doi:10.1200/JCO.2008.20.7753
2. Howlader N, Noone AM, Krapcho M, et al (eds). SEER Cancer Statistics Review, 1975-2012, National Cancer Institute. Accessed July 8, 2022. https://seer.cancer.gov/archive/csr/1975_2012/results_merged/sect_14_liver_bile.pdf#page=8
3. Singal AG, Mittal S, Yerokun OA, et al. Hepatocellular carcinoma screening associated with early tumor detection and improved survival among patients with cirrhosis in the US. Am J Med. 2017;130(9):1099-1106.e1. doi:10.1016/j.amjmed.2017.01.021
4. Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359(4):378-390. doi:10.1056/NEJMoa0708857
5. Finn RS, Qin S, Ikeda M, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med. 2020;382(20):1894-1905. doi:10.1056/NEJMoa1915745
6. Abou-Alfa GK, Chan SL, Kudo M, et al. Phase 3 randomized, open-label, multicenter study of tremelimumab (T) and durvalumab (D) as first-line therapy in patients (pts) with unresectable hepatocellular carcinoma (uHCC): HIMALAYA. J Clin Oncol. 2022;40(suppl 4):379. doi:10.1200/JCO.2022.40.4_suppl.379
7. Franco RA, Fan Y, Jarosek S, Bae S, Galbraith J. Racial and geographic disparities in hepatocellular carcinoma outcomes. Am J Prev Med. 2018;55(5)(suppl 1):S40-S48. doi:10.1016/j.amepre.2018.05.030
8. Ha J, Yan M, Aguilar M, et al. Race/ethnicity-specific disparities in hepatocellular carcinoma stage at diagnosis and its impact on receipt of curative therapies. J Clin Gastroenterol. 2016;50(5):423-430. doi:10.1097/MCG.0000000000000448
9. Wong R, Corley DA. Racial and ethnic variations in hepatocellular carcinoma incidence within the United States. Am J Med. 2008;121(6):525-531. doi:10.1016/j.amjmed.2008.03.005
10. Rich NE, Hester C, Odewole M, et al. Racial and ethnic differences in presentation and outcomes of hepatocellular carcinoma. Clin Gastroenterol Hepatol. 2019;17(3):551-559.e1. doi:10.1016/j.cgh.2018.05.039
11. Peters NA, Javed AA, He J, Wolfgang CL, Weiss MJ. Association of socioeconomics, surgical therapy, and survival of early stage hepatocellular carcinoma. J Surg Res. 2017;210:253-260. doi:10.1016/j.jss.2016.11.042
12. Wong RJ, Devaki P, Nguyen L, Cheung R, Nguyen MH. Ethnic disparities and liver transplantation rates in hepatocellular carcinoma patients in the recent era: results from the Surveillance, Epidemiology, and End Results registry. Liver Transpl. 2014;20(5):528-535. doi:10.1002/lt.23820
13. Minagawa M, Ikai I, Matsuyama Y, Yamaoka Y, Makuuchi M. Staging of hepatocellular carcinoma: assessment of the Japanese TNM and AJCC/UICC TNM systems in a cohort of 13,772 patients in Japan. Ann Surg. 2007;245(6):909-922. doi:10.1097/01.sla.0000254368.65878.da.
14. Harrison LE, Reichman T, Koneru B, et al. Racial discrepancies in the outcome of patients with hepatocellular carcinoma. Arch Surg. 2004;139(9):992-996. doi:10.1001/archsurg.139.9.992
15. Sloane D, Chen H, Howell C. Racial disparity in primary hepatocellular carcinoma: tumor stage at presentation, surgical treatment and survival. J Natl Med Assoc. 2006;98(12):1934-1939.
16. Haider AH, Scott VK, Rehman KA, et al. Racial disparities in surgical care and outcomes in the United States: a comprehensive review of patient, provider, and systemic factors. J Am Coll Surg. 2013;216(3):482-92.e12. doi:10.1016/j.jamcollsurg.2012.11.014
17. Cooper LA, Roter DL, Carson KA, et al. The associations of clinicians’ implicit attitudes about race with medical visit communication and patient ratings of interpersonal care. Am J Public Health. 2012;102(5):979-987. doi:10.2105/AJPH.2011.300558
18. Penner LA, Dovidio JF, Gonzalez R, et al. The effects of oncologist implicit racial bias in racially discordant oncology interactions. J Clin Oncol. 2016;34(24):2874-2880. doi:10.1200/JCO.2015.66.3658
Short walks after meals can cut diabetes risk
Taking a brief walk after eating can help lower the risk of type 2 diabetes, according to a recent study published in Sports Medicine (2022 Aug;52:1765-87).
Light walking after a meal – even for 2-5 minutes – can reduce blood sugar and insulin levels, the researchers found.
Blood sugar levels spike after eating, and the insulin produced to control them can lead to diabetes and cardiovascular issues, the researchers explained.
“With standing and walking, there are contractions of your muscles” that use glucose and lower blood sugar levels, Aidan Buffey, the lead study author and a PhD student in physical education and sport sciences at the University of Limerick (Ireland), told The Times.
“If you can do physical activity before the glucose peak, typically 60-90 minutes [after eating], that is when you’re going to have the benefit of not having the glucose spike,” he said.
Mr. Buffey and colleagues looked at seven studies to understand what would happen if you used standing or easy walking to interrupt prolonged sitting.
In five of the studies, none of the participants had prediabetes or type 2 diabetes. The other two studies included people with and without diabetes. The people in the studies were asked to either stand or walk for 2-5 minutes every 20-30 minutes over the course of a full day.
All seven studies showed that standing after a meal is better than sitting, and taking a short walk offered even better health benefits. Those who stood up for a short period of time after a meal had improved blood sugar levels but not insulin, while those who took a brief walk after a meal had lower blood sugar and insulin levels. Those who walked also had blood sugar levels that rose and fell more gradually, which is critical for managing diabetes.
Going for a walk, doing housework, or finding other ways to move your body within 60-90 minutes after eating could offer the best results, the study authors concluded.
These “mini-walks” could also be useful during the workday to break up prolonged periods of sitting at a desk.
“People are not going to get up and run on a treadmill or run around the office,” Mr. Buffey told The New York Times.
But making mini-walks a normal thing during the workday could be easy and acceptable at the office, he said. Even if people can’t take walks, standing up will help somewhat.
“Each small thing you do will have benefits, even if it is a small step,” Kershaw Patel, MD, a preventive cardiologist at Houston Methodist Hospital, told the newspaper. Dr. Patel wasn’t involved with the study.
“It’s a gradual effect of more activity, better health,” he said. “Each incremental step, each incremental stand or brisk walk appears to have a benefit.”
A version of this article first appeared on WebMD.com.
Taking a brief walk after eating can help lower the risk of type 2 diabetes, according to a recent study published in Sports Medicine (2022 Aug;52:1765-87).
Light walking after a meal – even for 2-5 minutes – can reduce blood sugar and insulin levels, the researchers found.
Blood sugar levels spike after eating, and the insulin produced to control them can lead to diabetes and cardiovascular issues, the researchers explained.
“With standing and walking, there are contractions of your muscles” that use glucose and lower blood sugar levels, Aidan Buffey, the lead study author and a PhD student in physical education and sport sciences at the University of Limerick (Ireland), told The Times.
“If you can do physical activity before the glucose peak, typically 60-90 minutes [after eating], that is when you’re going to have the benefit of not having the glucose spike,” he said.
Mr. Buffey and colleagues looked at seven studies to understand what would happen if you used standing or easy walking to interrupt prolonged sitting.
In five of the studies, none of the participants had prediabetes or type 2 diabetes. The other two studies included people with and without diabetes. The people in the studies were asked to either stand or walk for 2-5 minutes every 20-30 minutes over the course of a full day.
All seven studies showed that standing after a meal is better than sitting, and taking a short walk offered even better health benefits. Those who stood up for a short period of time after a meal had improved blood sugar levels but not insulin, while those who took a brief walk after a meal had lower blood sugar and insulin levels. Those who walked also had blood sugar levels that rose and fell more gradually, which is critical for managing diabetes.
Going for a walk, doing housework, or finding other ways to move your body within 60-90 minutes after eating could offer the best results, the study authors concluded.
These “mini-walks” could also be useful during the workday to break up prolonged periods of sitting at a desk.
“People are not going to get up and run on a treadmill or run around the office,” Mr. Buffey told The New York Times.
But making mini-walks a normal thing during the workday could be easy and acceptable at the office, he said. Even if people can’t take walks, standing up will help somewhat.
“Each small thing you do will have benefits, even if it is a small step,” Kershaw Patel, MD, a preventive cardiologist at Houston Methodist Hospital, told the newspaper. Dr. Patel wasn’t involved with the study.
“It’s a gradual effect of more activity, better health,” he said. “Each incremental step, each incremental stand or brisk walk appears to have a benefit.”
A version of this article first appeared on WebMD.com.
Taking a brief walk after eating can help lower the risk of type 2 diabetes, according to a recent study published in Sports Medicine (2022 Aug;52:1765-87).
Light walking after a meal – even for 2-5 minutes – can reduce blood sugar and insulin levels, the researchers found.
Blood sugar levels spike after eating, and the insulin produced to control them can lead to diabetes and cardiovascular issues, the researchers explained.
“With standing and walking, there are contractions of your muscles” that use glucose and lower blood sugar levels, Aidan Buffey, the lead study author and a PhD student in physical education and sport sciences at the University of Limerick (Ireland), told The Times.
“If you can do physical activity before the glucose peak, typically 60-90 minutes [after eating], that is when you’re going to have the benefit of not having the glucose spike,” he said.
Mr. Buffey and colleagues looked at seven studies to understand what would happen if you used standing or easy walking to interrupt prolonged sitting.
In five of the studies, none of the participants had prediabetes or type 2 diabetes. The other two studies included people with and without diabetes. The people in the studies were asked to either stand or walk for 2-5 minutes every 20-30 minutes over the course of a full day.
All seven studies showed that standing after a meal is better than sitting, and taking a short walk offered even better health benefits. Those who stood up for a short period of time after a meal had improved blood sugar levels but not insulin, while those who took a brief walk after a meal had lower blood sugar and insulin levels. Those who walked also had blood sugar levels that rose and fell more gradually, which is critical for managing diabetes.
Going for a walk, doing housework, or finding other ways to move your body within 60-90 minutes after eating could offer the best results, the study authors concluded.
These “mini-walks” could also be useful during the workday to break up prolonged periods of sitting at a desk.
“People are not going to get up and run on a treadmill or run around the office,” Mr. Buffey told The New York Times.
But making mini-walks a normal thing during the workday could be easy and acceptable at the office, he said. Even if people can’t take walks, standing up will help somewhat.
“Each small thing you do will have benefits, even if it is a small step,” Kershaw Patel, MD, a preventive cardiologist at Houston Methodist Hospital, told the newspaper. Dr. Patel wasn’t involved with the study.
“It’s a gradual effect of more activity, better health,” he said. “Each incremental step, each incremental stand or brisk walk appears to have a benefit.”
A version of this article first appeared on WebMD.com.
Long COVID’s grip will likely tighten as infections continue
COVID-19 is far from done in the United States, with more than 111,000 new cases being recorded a day in the second week of August, according to Johns Hopkins University, and 625 deaths being reported every day. , a condition that already has affected between 7.7 million and 23 million Americans, according to U.S. government estimates.
“It is evident that long COVID is real, that it already impacts a substantial number of people, and that this number may continue to grow as new infections occur,” the U.S. Department of Health and Human Services (HHS) said in a research action plan released Aug. 4.
“We are heading towards a big problem on our hands,” says Ziyad Al-Aly, MD, chief of research and development at the Veterans Affairs Hospital in St. Louis. “It’s like if we are falling in a plane, hurtling towards the ground. It doesn’t matter at what speed we are falling; what matters is that we are all falling, and falling fast. It’s a real problem. We needed to bring attention to this, yesterday,” he said.
Bryan Lau, PhD, professor of epidemiology at Johns Hopkins Bloomberg School of Public Health, Baltimore, and co-lead of a long COVID study there, says whether it’s 5% of the 92 million officially recorded U.S. COVID-19 cases, or 30% – on the higher end of estimates – that means anywhere between 4.5 million and 27 million Americans will have the effects of long COVID.
Other experts put the estimates even higher.
“If we conservatively assume 100 million working-age adults have been infected, that implies 10 to 33 million may have long COVID,” Alice Burns, PhD, associate director for the Kaiser Family Foundation’s Program on Medicaid and the Uninsured, wrote in an analysis.
And even the Centers for Disease Control and Prevention says only a fraction of cases have been recorded.
That, in turn, means tens of millions of people who struggle to work, to get to school, and to take care of their families – and who will be making demands on an already stressed U.S. health care system.
The HHS said in its Aug. 4 report that long COVID could keep 1 million people a day out of work, with a loss of $50 billion in annual pay.
Dr. Lau said health workers and policymakers are woefully unprepared.
“If you have a family unit, and the mom or dad can’t work, or has trouble taking their child to activities, where does the question of support come into play? Where is there potential for food issues, or housing issues?” he asked. “I see the potential for the burden to be extremely large in that capacity.”
Dr. Lau said he has yet to see any strong estimates of how many cases of long COVID might develop. Because a person has to get COVID-19 to ultimately get long COVID, the two are linked. In other words, as COVID-19 cases rise, so will cases of long COVID, and vice versa.
Evidence from the Kaiser Family Foundation analysis suggests a significant impact on employment: Surveys showed more than half of adults with long COVID who worked before becoming infected are either out of work or working fewer hours. Conditions associated with long COVID – such as fatigue, malaise, or problems concentrating – limit people’s ability to work, even if they have jobs that allow for accommodations.
Two surveys of people with long COVID who had worked before becoming infected showed that between 22% and 27% of them were out of work after getting long COVID. In comparison, among all working-age adults in 2019, only 7% were out of work. Given the sheer number of working-age adults with long COVID, the effects on employment may be profound and are likely to involve more people over time. One study estimates that long COVID already accounts for 15% of unfilled jobs.
The most severe symptoms of long COVID include brain fog and heart complications, known to persist for weeks for months after a COVID-19 infection.
A study from the University of Norway published in Open Forum Infectious Diseases found 53% of people tested had at least one symptom of thinking problems 13 months after infection with COVID-19. According to the HHS’ latest report on long COVID, people with thinking problems, heart conditions, mobility issues, and other symptoms are going to need a considerable amount of care. Many will need lengthy periods of rehabilitation.
Dr. Al-Aly worries that long COVID has already severely affected the labor force and the job market, all while burdening the country’s health care system.
“While there are variations in how individuals respond and cope with long COVID, the unifying thread is that with the level of disability it causes, more people will be struggling to keep up with the demands of the workforce and more people will be out on disability than ever before,” he said.
Studies from Johns Hopkins and the University of Washington estimate that 5%-30% of people could get long COVID in the future. Projections beyond that are hazy.
“So far, all the studies we have done on long COVID have been reactionary. Much of the activism around long COVID has been patient led. We are seeing more and more people with lasting symptoms. We need our research to catch up,” Dr. Lau said.
Theo Vos, MD, PhD, professor of health sciences at University of Washington, Seattle, said the main reasons for the huge range of predictions are the variety of methods used, as well as differences in sample size. Also, much long COVID data is self-reported, making it difficult for epidemiologists to track.
“With self-reported data, you can’t plug people into a machine and say this is what they have or this is what they don’t have. At the population level, the only thing you can do is ask questions. There is no systematic way to define long COVID,” he said.
Dr. Vos’s most recent study, which is being peer-reviewed and revised, found that most people with long COVID have symptoms similar to those seen in other autoimmune diseases. But sometimes the immune system can overreact, causing the more severe symptoms, such as brain fog and heart problems, associated with long COVID.
One reason that researchers struggle to come up with numbers, said Dr. Al-Aly, is the rapid rise of new variants. These variants appear to sometimes cause less severe disease than previous ones, but it’s not clear whether that means different risks for long COVID.
“There’s a wide diversity in severity. Someone can have long COVID and be fully functional, while others are not functional at all. We still have a long way to go before we figure out why,” Dr. Lau said.
A version of this article first appeared on WebMD.com.
COVID-19 is far from done in the United States, with more than 111,000 new cases being recorded a day in the second week of August, according to Johns Hopkins University, and 625 deaths being reported every day. , a condition that already has affected between 7.7 million and 23 million Americans, according to U.S. government estimates.
“It is evident that long COVID is real, that it already impacts a substantial number of people, and that this number may continue to grow as new infections occur,” the U.S. Department of Health and Human Services (HHS) said in a research action plan released Aug. 4.
“We are heading towards a big problem on our hands,” says Ziyad Al-Aly, MD, chief of research and development at the Veterans Affairs Hospital in St. Louis. “It’s like if we are falling in a plane, hurtling towards the ground. It doesn’t matter at what speed we are falling; what matters is that we are all falling, and falling fast. It’s a real problem. We needed to bring attention to this, yesterday,” he said.
Bryan Lau, PhD, professor of epidemiology at Johns Hopkins Bloomberg School of Public Health, Baltimore, and co-lead of a long COVID study there, says whether it’s 5% of the 92 million officially recorded U.S. COVID-19 cases, or 30% – on the higher end of estimates – that means anywhere between 4.5 million and 27 million Americans will have the effects of long COVID.
Other experts put the estimates even higher.
“If we conservatively assume 100 million working-age adults have been infected, that implies 10 to 33 million may have long COVID,” Alice Burns, PhD, associate director for the Kaiser Family Foundation’s Program on Medicaid and the Uninsured, wrote in an analysis.
And even the Centers for Disease Control and Prevention says only a fraction of cases have been recorded.
That, in turn, means tens of millions of people who struggle to work, to get to school, and to take care of their families – and who will be making demands on an already stressed U.S. health care system.
The HHS said in its Aug. 4 report that long COVID could keep 1 million people a day out of work, with a loss of $50 billion in annual pay.
Dr. Lau said health workers and policymakers are woefully unprepared.
“If you have a family unit, and the mom or dad can’t work, or has trouble taking their child to activities, where does the question of support come into play? Where is there potential for food issues, or housing issues?” he asked. “I see the potential for the burden to be extremely large in that capacity.”
Dr. Lau said he has yet to see any strong estimates of how many cases of long COVID might develop. Because a person has to get COVID-19 to ultimately get long COVID, the two are linked. In other words, as COVID-19 cases rise, so will cases of long COVID, and vice versa.
Evidence from the Kaiser Family Foundation analysis suggests a significant impact on employment: Surveys showed more than half of adults with long COVID who worked before becoming infected are either out of work or working fewer hours. Conditions associated with long COVID – such as fatigue, malaise, or problems concentrating – limit people’s ability to work, even if they have jobs that allow for accommodations.
Two surveys of people with long COVID who had worked before becoming infected showed that between 22% and 27% of them were out of work after getting long COVID. In comparison, among all working-age adults in 2019, only 7% were out of work. Given the sheer number of working-age adults with long COVID, the effects on employment may be profound and are likely to involve more people over time. One study estimates that long COVID already accounts for 15% of unfilled jobs.
The most severe symptoms of long COVID include brain fog and heart complications, known to persist for weeks for months after a COVID-19 infection.
A study from the University of Norway published in Open Forum Infectious Diseases found 53% of people tested had at least one symptom of thinking problems 13 months after infection with COVID-19. According to the HHS’ latest report on long COVID, people with thinking problems, heart conditions, mobility issues, and other symptoms are going to need a considerable amount of care. Many will need lengthy periods of rehabilitation.
Dr. Al-Aly worries that long COVID has already severely affected the labor force and the job market, all while burdening the country’s health care system.
“While there are variations in how individuals respond and cope with long COVID, the unifying thread is that with the level of disability it causes, more people will be struggling to keep up with the demands of the workforce and more people will be out on disability than ever before,” he said.
Studies from Johns Hopkins and the University of Washington estimate that 5%-30% of people could get long COVID in the future. Projections beyond that are hazy.
“So far, all the studies we have done on long COVID have been reactionary. Much of the activism around long COVID has been patient led. We are seeing more and more people with lasting symptoms. We need our research to catch up,” Dr. Lau said.
Theo Vos, MD, PhD, professor of health sciences at University of Washington, Seattle, said the main reasons for the huge range of predictions are the variety of methods used, as well as differences in sample size. Also, much long COVID data is self-reported, making it difficult for epidemiologists to track.
“With self-reported data, you can’t plug people into a machine and say this is what they have or this is what they don’t have. At the population level, the only thing you can do is ask questions. There is no systematic way to define long COVID,” he said.
Dr. Vos’s most recent study, which is being peer-reviewed and revised, found that most people with long COVID have symptoms similar to those seen in other autoimmune diseases. But sometimes the immune system can overreact, causing the more severe symptoms, such as brain fog and heart problems, associated with long COVID.
One reason that researchers struggle to come up with numbers, said Dr. Al-Aly, is the rapid rise of new variants. These variants appear to sometimes cause less severe disease than previous ones, but it’s not clear whether that means different risks for long COVID.
“There’s a wide diversity in severity. Someone can have long COVID and be fully functional, while others are not functional at all. We still have a long way to go before we figure out why,” Dr. Lau said.
A version of this article first appeared on WebMD.com.
COVID-19 is far from done in the United States, with more than 111,000 new cases being recorded a day in the second week of August, according to Johns Hopkins University, and 625 deaths being reported every day. , a condition that already has affected between 7.7 million and 23 million Americans, according to U.S. government estimates.
“It is evident that long COVID is real, that it already impacts a substantial number of people, and that this number may continue to grow as new infections occur,” the U.S. Department of Health and Human Services (HHS) said in a research action plan released Aug. 4.
“We are heading towards a big problem on our hands,” says Ziyad Al-Aly, MD, chief of research and development at the Veterans Affairs Hospital in St. Louis. “It’s like if we are falling in a plane, hurtling towards the ground. It doesn’t matter at what speed we are falling; what matters is that we are all falling, and falling fast. It’s a real problem. We needed to bring attention to this, yesterday,” he said.
Bryan Lau, PhD, professor of epidemiology at Johns Hopkins Bloomberg School of Public Health, Baltimore, and co-lead of a long COVID study there, says whether it’s 5% of the 92 million officially recorded U.S. COVID-19 cases, or 30% – on the higher end of estimates – that means anywhere between 4.5 million and 27 million Americans will have the effects of long COVID.
Other experts put the estimates even higher.
“If we conservatively assume 100 million working-age adults have been infected, that implies 10 to 33 million may have long COVID,” Alice Burns, PhD, associate director for the Kaiser Family Foundation’s Program on Medicaid and the Uninsured, wrote in an analysis.
And even the Centers for Disease Control and Prevention says only a fraction of cases have been recorded.
That, in turn, means tens of millions of people who struggle to work, to get to school, and to take care of their families – and who will be making demands on an already stressed U.S. health care system.
The HHS said in its Aug. 4 report that long COVID could keep 1 million people a day out of work, with a loss of $50 billion in annual pay.
Dr. Lau said health workers and policymakers are woefully unprepared.
“If you have a family unit, and the mom or dad can’t work, or has trouble taking their child to activities, where does the question of support come into play? Where is there potential for food issues, or housing issues?” he asked. “I see the potential for the burden to be extremely large in that capacity.”
Dr. Lau said he has yet to see any strong estimates of how many cases of long COVID might develop. Because a person has to get COVID-19 to ultimately get long COVID, the two are linked. In other words, as COVID-19 cases rise, so will cases of long COVID, and vice versa.
Evidence from the Kaiser Family Foundation analysis suggests a significant impact on employment: Surveys showed more than half of adults with long COVID who worked before becoming infected are either out of work or working fewer hours. Conditions associated with long COVID – such as fatigue, malaise, or problems concentrating – limit people’s ability to work, even if they have jobs that allow for accommodations.
Two surveys of people with long COVID who had worked before becoming infected showed that between 22% and 27% of them were out of work after getting long COVID. In comparison, among all working-age adults in 2019, only 7% were out of work. Given the sheer number of working-age adults with long COVID, the effects on employment may be profound and are likely to involve more people over time. One study estimates that long COVID already accounts for 15% of unfilled jobs.
The most severe symptoms of long COVID include brain fog and heart complications, known to persist for weeks for months after a COVID-19 infection.
A study from the University of Norway published in Open Forum Infectious Diseases found 53% of people tested had at least one symptom of thinking problems 13 months after infection with COVID-19. According to the HHS’ latest report on long COVID, people with thinking problems, heart conditions, mobility issues, and other symptoms are going to need a considerable amount of care. Many will need lengthy periods of rehabilitation.
Dr. Al-Aly worries that long COVID has already severely affected the labor force and the job market, all while burdening the country’s health care system.
“While there are variations in how individuals respond and cope with long COVID, the unifying thread is that with the level of disability it causes, more people will be struggling to keep up with the demands of the workforce and more people will be out on disability than ever before,” he said.
Studies from Johns Hopkins and the University of Washington estimate that 5%-30% of people could get long COVID in the future. Projections beyond that are hazy.
“So far, all the studies we have done on long COVID have been reactionary. Much of the activism around long COVID has been patient led. We are seeing more and more people with lasting symptoms. We need our research to catch up,” Dr. Lau said.
Theo Vos, MD, PhD, professor of health sciences at University of Washington, Seattle, said the main reasons for the huge range of predictions are the variety of methods used, as well as differences in sample size. Also, much long COVID data is self-reported, making it difficult for epidemiologists to track.
“With self-reported data, you can’t plug people into a machine and say this is what they have or this is what they don’t have. At the population level, the only thing you can do is ask questions. There is no systematic way to define long COVID,” he said.
Dr. Vos’s most recent study, which is being peer-reviewed and revised, found that most people with long COVID have symptoms similar to those seen in other autoimmune diseases. But sometimes the immune system can overreact, causing the more severe symptoms, such as brain fog and heart problems, associated with long COVID.
One reason that researchers struggle to come up with numbers, said Dr. Al-Aly, is the rapid rise of new variants. These variants appear to sometimes cause less severe disease than previous ones, but it’s not clear whether that means different risks for long COVID.
“There’s a wide diversity in severity. Someone can have long COVID and be fully functional, while others are not functional at all. We still have a long way to go before we figure out why,” Dr. Lau said.
A version of this article first appeared on WebMD.com.
Drug-resistant epilepsy needs earlier surgical referral
, according to expert consensus recommendations from the International League Against Epilepsy (ILAE) published in the journal Epilepsia.
Comprehensive epilepsy care
Such a referral is not ”a commitment to undergo brain surgery,” wrote the authors of the new recommendations study, but surgical evaluations offer patients an opportunity to learn about the range of therapies available to them and to have their diagnosis verified, as well as learning about the cause and type of epilepsy they have, even if they ultimately do not pursue surgery.
”In fact, most patients with drug-resistant epilepsy do not end up undergoing surgery after referral, but still benefit from comprehensive epilepsy care improving quality of life and lowering mortality,” wrote lead author Lara Jehi, MD, professor of neurology and epilepsy specialist at Cleveland Clinic, and her colleagues. “A better characterization of the epilepsy can also help optimize medical therapy and address somatic, cognitive, behavioral, and psychiatric comorbidities.”
Is the diagnosis correct?
They noted that about one-third of patients referred to epilepsy centers with an apparent diagnosis of drug-resistant epilepsy actually have psychogenic nonepileptic seizures (PNES) – not epilepsy – and an early, accurate diagnosis of PNES can ensure they receive psychotherapy, stop taking antiseizure medications, and have better outcomes.
“These recommendations are necessary, as the delay to surgery and the overall underutilization of surgery have not improved much over the last 20 years,” said Selim R. Benbadis, MD, professor of neurology and director of the comprehensive epilepsy program at the University of South Florida and Tampa General Hospital. “Comprehensive epilepsy centers offer more than surgery, including correct and precise diagnosis, drug options, three [Food and Drug Administration]–approved neurostimulation options, and more,” said Dr. Benbadis, who was not involved in the development of these recommendations.
Consensus recommendations
On behalf of the the ILAE’s Surgical Therapies Commission, the authors used the Delphi consensus process to develop expert consensus recommendations on when to refer patients with epilepsy to surgery. They conducted three Delphi rounds on 51 clinical scenarios with 61 epileptologists (38% of participants), epilepsy neurosurgeons (34%), neurologists (23%), neuropsychiatrists (2%), and neuropsychologists (3%) from 28 countries. Most of clinicians focused on adults (39%) or adults and children (41%) while 20% focused only on pediatric epilepsy.
The physicians involved had a median 22 years of practice and represented all six ILAE regions: 30% from North America, 28% from Europe, 18% from Asia/Oceania, 13% from Latin America, 7% from the Eastern Mediterranean, and 4% from Africa.
The result of these rounds were three key recommendations arising from the consensus of experts consulted. First, every patient up to 70 years old who has drug-resistant epilepsy should be offered the option of a surgical evaluation as soon as it’s apparent that they have drug resistance. The option for surgical evaluation should be provided independent of their sex or socioeconomic status and regardless of how long they have had epilepsy, their seizure type, their epilepsy type, localization, and their comorbidities, ”including severe psychiatric comorbidity like psychogenic nonepileptic seizures (PNES) or substance abuse if patients are cooperative with management,” the authors wrote.
”Resective surgery can improve quality of life and cognitive outcomes and is the only treatment demonstrated to improve survival and reverse excess mortality attributed to drug-resistant epilepsy,” the authors wrote. Evidence supports that surgical evaluation is the most cost-effective approach to treating drug-resistant epilepsy, they added. Yet, it still takes about 20 years with epilepsy before an adult patient might be referred, ”and the neurology community remains ambivalent due to ongoing barriers and misconceptions about epilepsy surgery,” they wrote.
The second recommendation is to consider a surgical referral for older patients with drug-resistant epilepsy who have no surgical contraindication. Physicians can also consider a referral for patients of any age who are seizure free while taking one to two antiseizure drugs but who have a brain lesion in the noneloquent cortex.
The third recommendation is not to offer surgery if a patient has an active substance dependency and is not cooperative with management.
“Although there is some evidence that seizure outcomes are no different in individuals with active substance use disorder who have epilepsy surgery, the literature suggests increased perioperative surgical and anesthetic risk in this cohort,” the authors wrote. ”Patients with active substance abuse are more likely to be nonadherent with their seizure medications, and to leave the hospital against medical advice.”
One area where the participants did not reach consensus was regarding whether to refer patients who did not become seizure-free after trying just one “tolerated and appropriately chosen” antiseizure medication. Half (49%) said they would be unlikely to refer or would never refer that patient while 44% said they would likely or always refer them, and 7% weren’t sure.
The ‘next level’ of epilepsy care
“Similar recommendations have been published before, by the National Association of Epilepsy Centers, more than once, and have not changed the referral patterns,” Dr. Benbadis said. “They are not implemented by the average general neurologist.” While there are many reasons for this, one with a relativity simple fix is to adjust the language doctors use to when talking with patients about getting an evaluation, Dr. Benbadis said. ”The key is to rephrase: Instead of referrals ‘for surgery,’ which can be scary to many neurologists and patients, we should use more general terms, like referrals for the ‘next level of care by epilepsy specialists,’ ” said Dr. Benbadis, who advocated for this change in terminology in a 2019 editorial. Such language is less frightening and can ease patients’ concerns about going to an epilepsy center where they can learn about more options than just surgery.
Further, surgical options have expanded in recent years, including the development of laser interstitial thermal therapy and neuromodulation. “Identifying candidacy for any of these approaches starts with a surgical referral, so a timely evaluation is key,” the authors wrote.
Referral delays persist
Despite the strong evidence for timely referrals, delays have persisted for decades, said Dr. Benbadis, echoing what the authors describe. ”Despite the results of two randomized controlled trials showing that surgery for temporal lobe epilepsy in adults, and resective surgery in children, is superior to continued antiseizure medications both in terms of seizure freedom and improved quality of life, the mean epilepsy duration to temporal lobe resection has persisted at over 20 years,” the authors wrote. ”Although drug resistance is reached with a mean latency of 9 years in epilepsy surgery candidates, these patients have experienced a decade of unabating seizures with detrimental effects including cognitive and psychiatric comorbidities, poor psychosocial outcomes, potential injuries, and risk of death.”
Surgery is not a ‘dangerous last resort’
The authors point out a variety of likely reasons for these delays, including patients experiencing temporary remissions with a new drug, lack of adequate health care access, overestimating surgery risks, and underestimating the seriousness and risk of death from ongoing seizures.
Dr. Benbadis agreed, referring to a “combination of lack of knowledge and unrealistic views about surgery outcomes and complications.” Patients and their neurologists think surgery is a “dangerous last resort, fraught with complications, and they don’t know the outcome, so it’s mainly that they are not very well-educated about epilepsy surgery,” he said. Complacency about a patient’s infrequent seizures plays a role as well, he added. “Their patient is having one seizure every 2 months, and they might say, ‘well, that’s okay, that’s not that bad,’ but it is when we can cure it.”
Similar factors are barriers to epilepsy surgery: “lack of knowledge or misconceptions about surgical risks, negative behaviors, or cultural issues and access issues.”
Another major barrier, both within neurology and throughout medicine in general, is that large academic centers that accept referrals, including epilepsy centers, have poor communication, follow-up, and scheduling, Dr. Benbadis said.
The authors provided a table with suggestions on potential solutions to those barriers, including identifying online resources to help doctors identify possible surgery candidates, such as www.toolsforepilepsy.com, and a range of educational resources. Ways to improve access and cost include mobile clinics, telehealth, coordinating with an epilepsy organization, and employing a multidisciplinary team that includes a social worker to help with support such as transportation and health insurance.
, according to expert consensus recommendations from the International League Against Epilepsy (ILAE) published in the journal Epilepsia.
Comprehensive epilepsy care
Such a referral is not ”a commitment to undergo brain surgery,” wrote the authors of the new recommendations study, but surgical evaluations offer patients an opportunity to learn about the range of therapies available to them and to have their diagnosis verified, as well as learning about the cause and type of epilepsy they have, even if they ultimately do not pursue surgery.
”In fact, most patients with drug-resistant epilepsy do not end up undergoing surgery after referral, but still benefit from comprehensive epilepsy care improving quality of life and lowering mortality,” wrote lead author Lara Jehi, MD, professor of neurology and epilepsy specialist at Cleveland Clinic, and her colleagues. “A better characterization of the epilepsy can also help optimize medical therapy and address somatic, cognitive, behavioral, and psychiatric comorbidities.”
Is the diagnosis correct?
They noted that about one-third of patients referred to epilepsy centers with an apparent diagnosis of drug-resistant epilepsy actually have psychogenic nonepileptic seizures (PNES) – not epilepsy – and an early, accurate diagnosis of PNES can ensure they receive psychotherapy, stop taking antiseizure medications, and have better outcomes.
“These recommendations are necessary, as the delay to surgery and the overall underutilization of surgery have not improved much over the last 20 years,” said Selim R. Benbadis, MD, professor of neurology and director of the comprehensive epilepsy program at the University of South Florida and Tampa General Hospital. “Comprehensive epilepsy centers offer more than surgery, including correct and precise diagnosis, drug options, three [Food and Drug Administration]–approved neurostimulation options, and more,” said Dr. Benbadis, who was not involved in the development of these recommendations.
Consensus recommendations
On behalf of the the ILAE’s Surgical Therapies Commission, the authors used the Delphi consensus process to develop expert consensus recommendations on when to refer patients with epilepsy to surgery. They conducted three Delphi rounds on 51 clinical scenarios with 61 epileptologists (38% of participants), epilepsy neurosurgeons (34%), neurologists (23%), neuropsychiatrists (2%), and neuropsychologists (3%) from 28 countries. Most of clinicians focused on adults (39%) or adults and children (41%) while 20% focused only on pediatric epilepsy.
The physicians involved had a median 22 years of practice and represented all six ILAE regions: 30% from North America, 28% from Europe, 18% from Asia/Oceania, 13% from Latin America, 7% from the Eastern Mediterranean, and 4% from Africa.
The result of these rounds were three key recommendations arising from the consensus of experts consulted. First, every patient up to 70 years old who has drug-resistant epilepsy should be offered the option of a surgical evaluation as soon as it’s apparent that they have drug resistance. The option for surgical evaluation should be provided independent of their sex or socioeconomic status and regardless of how long they have had epilepsy, their seizure type, their epilepsy type, localization, and their comorbidities, ”including severe psychiatric comorbidity like psychogenic nonepileptic seizures (PNES) or substance abuse if patients are cooperative with management,” the authors wrote.
”Resective surgery can improve quality of life and cognitive outcomes and is the only treatment demonstrated to improve survival and reverse excess mortality attributed to drug-resistant epilepsy,” the authors wrote. Evidence supports that surgical evaluation is the most cost-effective approach to treating drug-resistant epilepsy, they added. Yet, it still takes about 20 years with epilepsy before an adult patient might be referred, ”and the neurology community remains ambivalent due to ongoing barriers and misconceptions about epilepsy surgery,” they wrote.
The second recommendation is to consider a surgical referral for older patients with drug-resistant epilepsy who have no surgical contraindication. Physicians can also consider a referral for patients of any age who are seizure free while taking one to two antiseizure drugs but who have a brain lesion in the noneloquent cortex.
The third recommendation is not to offer surgery if a patient has an active substance dependency and is not cooperative with management.
“Although there is some evidence that seizure outcomes are no different in individuals with active substance use disorder who have epilepsy surgery, the literature suggests increased perioperative surgical and anesthetic risk in this cohort,” the authors wrote. ”Patients with active substance abuse are more likely to be nonadherent with their seizure medications, and to leave the hospital against medical advice.”
One area where the participants did not reach consensus was regarding whether to refer patients who did not become seizure-free after trying just one “tolerated and appropriately chosen” antiseizure medication. Half (49%) said they would be unlikely to refer or would never refer that patient while 44% said they would likely or always refer them, and 7% weren’t sure.
The ‘next level’ of epilepsy care
“Similar recommendations have been published before, by the National Association of Epilepsy Centers, more than once, and have not changed the referral patterns,” Dr. Benbadis said. “They are not implemented by the average general neurologist.” While there are many reasons for this, one with a relativity simple fix is to adjust the language doctors use to when talking with patients about getting an evaluation, Dr. Benbadis said. ”The key is to rephrase: Instead of referrals ‘for surgery,’ which can be scary to many neurologists and patients, we should use more general terms, like referrals for the ‘next level of care by epilepsy specialists,’ ” said Dr. Benbadis, who advocated for this change in terminology in a 2019 editorial. Such language is less frightening and can ease patients’ concerns about going to an epilepsy center where they can learn about more options than just surgery.
Further, surgical options have expanded in recent years, including the development of laser interstitial thermal therapy and neuromodulation. “Identifying candidacy for any of these approaches starts with a surgical referral, so a timely evaluation is key,” the authors wrote.
Referral delays persist
Despite the strong evidence for timely referrals, delays have persisted for decades, said Dr. Benbadis, echoing what the authors describe. ”Despite the results of two randomized controlled trials showing that surgery for temporal lobe epilepsy in adults, and resective surgery in children, is superior to continued antiseizure medications both in terms of seizure freedom and improved quality of life, the mean epilepsy duration to temporal lobe resection has persisted at over 20 years,” the authors wrote. ”Although drug resistance is reached with a mean latency of 9 years in epilepsy surgery candidates, these patients have experienced a decade of unabating seizures with detrimental effects including cognitive and psychiatric comorbidities, poor psychosocial outcomes, potential injuries, and risk of death.”
Surgery is not a ‘dangerous last resort’
The authors point out a variety of likely reasons for these delays, including patients experiencing temporary remissions with a new drug, lack of adequate health care access, overestimating surgery risks, and underestimating the seriousness and risk of death from ongoing seizures.
Dr. Benbadis agreed, referring to a “combination of lack of knowledge and unrealistic views about surgery outcomes and complications.” Patients and their neurologists think surgery is a “dangerous last resort, fraught with complications, and they don’t know the outcome, so it’s mainly that they are not very well-educated about epilepsy surgery,” he said. Complacency about a patient’s infrequent seizures plays a role as well, he added. “Their patient is having one seizure every 2 months, and they might say, ‘well, that’s okay, that’s not that bad,’ but it is when we can cure it.”
Similar factors are barriers to epilepsy surgery: “lack of knowledge or misconceptions about surgical risks, negative behaviors, or cultural issues and access issues.”
Another major barrier, both within neurology and throughout medicine in general, is that large academic centers that accept referrals, including epilepsy centers, have poor communication, follow-up, and scheduling, Dr. Benbadis said.
The authors provided a table with suggestions on potential solutions to those barriers, including identifying online resources to help doctors identify possible surgery candidates, such as www.toolsforepilepsy.com, and a range of educational resources. Ways to improve access and cost include mobile clinics, telehealth, coordinating with an epilepsy organization, and employing a multidisciplinary team that includes a social worker to help with support such as transportation and health insurance.
, according to expert consensus recommendations from the International League Against Epilepsy (ILAE) published in the journal Epilepsia.
Comprehensive epilepsy care
Such a referral is not ”a commitment to undergo brain surgery,” wrote the authors of the new recommendations study, but surgical evaluations offer patients an opportunity to learn about the range of therapies available to them and to have their diagnosis verified, as well as learning about the cause and type of epilepsy they have, even if they ultimately do not pursue surgery.
”In fact, most patients with drug-resistant epilepsy do not end up undergoing surgery after referral, but still benefit from comprehensive epilepsy care improving quality of life and lowering mortality,” wrote lead author Lara Jehi, MD, professor of neurology and epilepsy specialist at Cleveland Clinic, and her colleagues. “A better characterization of the epilepsy can also help optimize medical therapy and address somatic, cognitive, behavioral, and psychiatric comorbidities.”
Is the diagnosis correct?
They noted that about one-third of patients referred to epilepsy centers with an apparent diagnosis of drug-resistant epilepsy actually have psychogenic nonepileptic seizures (PNES) – not epilepsy – and an early, accurate diagnosis of PNES can ensure they receive psychotherapy, stop taking antiseizure medications, and have better outcomes.
“These recommendations are necessary, as the delay to surgery and the overall underutilization of surgery have not improved much over the last 20 years,” said Selim R. Benbadis, MD, professor of neurology and director of the comprehensive epilepsy program at the University of South Florida and Tampa General Hospital. “Comprehensive epilepsy centers offer more than surgery, including correct and precise diagnosis, drug options, three [Food and Drug Administration]–approved neurostimulation options, and more,” said Dr. Benbadis, who was not involved in the development of these recommendations.
Consensus recommendations
On behalf of the the ILAE’s Surgical Therapies Commission, the authors used the Delphi consensus process to develop expert consensus recommendations on when to refer patients with epilepsy to surgery. They conducted three Delphi rounds on 51 clinical scenarios with 61 epileptologists (38% of participants), epilepsy neurosurgeons (34%), neurologists (23%), neuropsychiatrists (2%), and neuropsychologists (3%) from 28 countries. Most of clinicians focused on adults (39%) or adults and children (41%) while 20% focused only on pediatric epilepsy.
The physicians involved had a median 22 years of practice and represented all six ILAE regions: 30% from North America, 28% from Europe, 18% from Asia/Oceania, 13% from Latin America, 7% from the Eastern Mediterranean, and 4% from Africa.
The result of these rounds were three key recommendations arising from the consensus of experts consulted. First, every patient up to 70 years old who has drug-resistant epilepsy should be offered the option of a surgical evaluation as soon as it’s apparent that they have drug resistance. The option for surgical evaluation should be provided independent of their sex or socioeconomic status and regardless of how long they have had epilepsy, their seizure type, their epilepsy type, localization, and their comorbidities, ”including severe psychiatric comorbidity like psychogenic nonepileptic seizures (PNES) or substance abuse if patients are cooperative with management,” the authors wrote.
”Resective surgery can improve quality of life and cognitive outcomes and is the only treatment demonstrated to improve survival and reverse excess mortality attributed to drug-resistant epilepsy,” the authors wrote. Evidence supports that surgical evaluation is the most cost-effective approach to treating drug-resistant epilepsy, they added. Yet, it still takes about 20 years with epilepsy before an adult patient might be referred, ”and the neurology community remains ambivalent due to ongoing barriers and misconceptions about epilepsy surgery,” they wrote.
The second recommendation is to consider a surgical referral for older patients with drug-resistant epilepsy who have no surgical contraindication. Physicians can also consider a referral for patients of any age who are seizure free while taking one to two antiseizure drugs but who have a brain lesion in the noneloquent cortex.
The third recommendation is not to offer surgery if a patient has an active substance dependency and is not cooperative with management.
“Although there is some evidence that seizure outcomes are no different in individuals with active substance use disorder who have epilepsy surgery, the literature suggests increased perioperative surgical and anesthetic risk in this cohort,” the authors wrote. ”Patients with active substance abuse are more likely to be nonadherent with their seizure medications, and to leave the hospital against medical advice.”
One area where the participants did not reach consensus was regarding whether to refer patients who did not become seizure-free after trying just one “tolerated and appropriately chosen” antiseizure medication. Half (49%) said they would be unlikely to refer or would never refer that patient while 44% said they would likely or always refer them, and 7% weren’t sure.
The ‘next level’ of epilepsy care
“Similar recommendations have been published before, by the National Association of Epilepsy Centers, more than once, and have not changed the referral patterns,” Dr. Benbadis said. “They are not implemented by the average general neurologist.” While there are many reasons for this, one with a relativity simple fix is to adjust the language doctors use to when talking with patients about getting an evaluation, Dr. Benbadis said. ”The key is to rephrase: Instead of referrals ‘for surgery,’ which can be scary to many neurologists and patients, we should use more general terms, like referrals for the ‘next level of care by epilepsy specialists,’ ” said Dr. Benbadis, who advocated for this change in terminology in a 2019 editorial. Such language is less frightening and can ease patients’ concerns about going to an epilepsy center where they can learn about more options than just surgery.
Further, surgical options have expanded in recent years, including the development of laser interstitial thermal therapy and neuromodulation. “Identifying candidacy for any of these approaches starts with a surgical referral, so a timely evaluation is key,” the authors wrote.
Referral delays persist
Despite the strong evidence for timely referrals, delays have persisted for decades, said Dr. Benbadis, echoing what the authors describe. ”Despite the results of two randomized controlled trials showing that surgery for temporal lobe epilepsy in adults, and resective surgery in children, is superior to continued antiseizure medications both in terms of seizure freedom and improved quality of life, the mean epilepsy duration to temporal lobe resection has persisted at over 20 years,” the authors wrote. ”Although drug resistance is reached with a mean latency of 9 years in epilepsy surgery candidates, these patients have experienced a decade of unabating seizures with detrimental effects including cognitive and psychiatric comorbidities, poor psychosocial outcomes, potential injuries, and risk of death.”
Surgery is not a ‘dangerous last resort’
The authors point out a variety of likely reasons for these delays, including patients experiencing temporary remissions with a new drug, lack of adequate health care access, overestimating surgery risks, and underestimating the seriousness and risk of death from ongoing seizures.
Dr. Benbadis agreed, referring to a “combination of lack of knowledge and unrealistic views about surgery outcomes and complications.” Patients and their neurologists think surgery is a “dangerous last resort, fraught with complications, and they don’t know the outcome, so it’s mainly that they are not very well-educated about epilepsy surgery,” he said. Complacency about a patient’s infrequent seizures plays a role as well, he added. “Their patient is having one seizure every 2 months, and they might say, ‘well, that’s okay, that’s not that bad,’ but it is when we can cure it.”
Similar factors are barriers to epilepsy surgery: “lack of knowledge or misconceptions about surgical risks, negative behaviors, or cultural issues and access issues.”
Another major barrier, both within neurology and throughout medicine in general, is that large academic centers that accept referrals, including epilepsy centers, have poor communication, follow-up, and scheduling, Dr. Benbadis said.
The authors provided a table with suggestions on potential solutions to those barriers, including identifying online resources to help doctors identify possible surgery candidates, such as www.toolsforepilepsy.com, and a range of educational resources. Ways to improve access and cost include mobile clinics, telehealth, coordinating with an epilepsy organization, and employing a multidisciplinary team that includes a social worker to help with support such as transportation and health insurance.
FROM EPILEPSIA
Updates on treatment/prevention of VTE in cancer patients
Updated clinical practice guidelines for the treatment and prevention of venous thromboembolism for patients with cancer, including those with cancer and COVID-19, have been released by the International Initiative on Thrombosis and Cancer (ITAC), an academic working group of VTE experts.
“Because patients with cancer have a baseline increased risk of VTE, compared with patients without cancer, the combination of both COVID-19 and cancer – and its effect on VTE risk and treatment – is of concern,” said the authors, led by Dominique Farge, MD, PhD, Nord Universite de Paris.
they added.
The new guidelines were published online in The Lancet Oncology.
“Cancer-associated VTE remains an important clinical problem, associated with increased morbidity and mortality,” Dr. Farge and colleagues observed.
“The ITAC guidelines’ companion free web-based mobile application will assist the practicing clinician with decision making at various levels to provide optimal care of patients with cancer to treat and prevent VTE,” they emphasized. More information is available at itaccme.com.
Cancer patients with COVID
The new section of the guidelines notes that the treatment and prevention of VTE for cancer patients infected with SARS-CoV-2 remain the same as for patients without COVID.
Whether or not cancer patients with COVID-19 are hospitalized, have been discharged, or are ambulatory, they should be assessed for the risk of VTE, as should any other patient. For cancer patients with COVID-19 who are hospitalized, pharmacologic prophylaxis should be given at the same dose and anticoagulant type as for hospitalized cancer patients who do not have COVID-19.
Following discharge, VTE prophylaxis is not advised for cancer patients infected with SARS-CoV-2, and routine primary pharmacologic prophylaxis of VTE for ambulatory patients with COVID-19 is also not recommended, the authors noted.
Initial treatment of established VTE
Initial treatment of established VTE for up to 10 days of anticoagulation should include low-molecular-weight heparin (LMWH) when creatinine clearance is at least 30 mL/min.
“A regimen of LMWH, taken once per day, is recommended unless a twice-per-day regimen is required because of patients’ characteristics,” the authors noted. These characteristics include a high risk of bleeding, moderate renal failure, and the need for technical intervention, including surgery.
If a twice-a-day regimen is required, only enoxaparin at a dose of 1 mg/kg twice daily can be used, the authors cautioned.
For patients with a low risk of gastrointestinal or genitourinary bleeding, rivaroxaban (Xarelto) or apixaban (Eliquis) can be given in the first 10 days, as well as edoxaban (Lixiana). The latter should be started after at least 5 days of parenteral anticoagulation, provided creatinine clearance is at least 30 mL/min.
“Unfractionated heparin as well as fondaparinux (GlaxoSmithKline) can be also used for the initial treatment of established VTE when LMWH or direct oral anticoagulants are contraindicated,” Dr. Farge and colleagues wrote.
Thrombolysis can be considered on a case-by-case basis, although physicians must pay attention to specific contraindications, especially bleeding risk.
“In the initial treatment of VTE, inferior vena cava filters might be considered when anticoagulant treatment is contraindicated or, in the case of pulmonary embolism, when recurrence occurs under optimal anticoagulation,” the authors noted.
Maintenance VTE treatment
For maintenance therapy, which the authors define as early maintenance for up to 6 months and long-term maintenance beyond 6 months, they point out that LMWHs are preferred over vitamin K antagonists for the treatment of VTE when the creatinine clearance is again at least 30 mL/min.
Any of the direct oral anticoagulants (DOAs) – edoxaban, rivaroxaban, or apixaban – is also recommended for the same patients, provided there is no risk of inducing a strong drug-drug interaction or GI absorption is impaired.
However, the DOAs should be used with caution for patients with GI malignancies, especially upper GI cancers, because data show there is an increased risk of GI bleeding with both edoxaban and rivaroxaban.
“LMWH or direct oral anticoagulants should be used for a minimum of 6 months to treat established VTE in patients with cancer,” the authors wrote.
“After 6 months, termination or continuation of anticoagulation (LMWH, direct oral anticoagulants, or vitamin K antagonists) should be based on individual evaluation of the benefit-risk ratio,” they added.
Treatment of VTE recurrence
The guideline authors explain that three options can be considered in the event of VTE recurrence. These include an increase in the LMWH dose by 20%-25%, or a switch to a DOA, or, if patients are taking a DOA, a switch to an LMWH. If the patient is taking a vitamin K antagonist, it can be switched to either an LMWH or a DOA.
For treatment of catheter-related thrombosis, anticoagulant treatment is recommended for a minimum of 3 months and as long as the central venous catheter is in place. In this setting, the LMWHs are recommended.
The central venous catheter can be kept in place if it is functional, well positioned, and is not infected, provided there is good resolution of symptoms under close surveillance while anticoagulants are being administered.
In surgically treated patients, the LMWH, given once a day, to patients with a serum creatinine concentration of at least 30 mL/min can be used to prevent VTE. Alternatively, VTE can be prevented by the use low-dose unfractionated heparin, given three times a day.
“Pharmacological prophylaxis should be started 2-12 h preoperatively and continued for at least 7–10 days,” Dr. Farge and colleagues advised. In this setting, there is insufficient evidence to support the use of fondaparinux or a DOA as an alternative to an LMWH for the prophylaxis of postoperative VTE. “Use of the highest prophylactic dose of LMWH to prevent postoperative VTE in patients with cancer is recommended,” the authors advised.
Furthermore, extended prophylaxis of at least 4 weeks with LMWH is advised to prevent postoperative VTE after major abdominal or pelvic surgery. Mechanical methods are not recommended except when pharmacologic methods are contraindicated. Inferior vena cava filters are also not recommended for routine prophylaxis.
Patients with reduced mobility
For medically treated hospitalized patients with cancer whose mobility is reduced, the authors recommend prophylaxis with either an LMWH or fondaparinux, provided their creatinine clearance is at least 30 mL/min. These patients can also be treated with unfractionated heparin, they add.
In contrast, DOAs are not recommended – at least not routinely – in this setting, the authors cautioned. Primary pharmacologic prophylaxis of VTE with either LMWH or DOAs – either rivaroxaban or apixaban – is indicated in ambulatory patients with locally advanced or metastatic pancreatic cancer who are receiving systemic anticancer therapy, provided they are at low risk of bleeding.
However, primary pharmacologic prophylaxis with LMWH is not recommended outside of a clinical trial for patients with locally advanced or metastatic lung cancer who are undergoing systemic anticancer therapy, even for patients who are at low risk of bleeding.
For ambulatory patients who are receiving systemic anticancer therapy and who are at intermediate risk of VTE, primary prophylaxis with rivaroxaban or apixaban is recommended for those with myeloma who are receiving immunomodulatory therapy plus steroids or other systemic therapies.
In this setting, oral anticoagulants should consist of a vitamin K antagonist, given at low or therapeutic doses, or apixaban, given at prophylactic doses. Alternatively, LMWH, given at prophylactic doses, or low-dose aspirin, given at a dose of 100 mg/day, can be used.
Catheter-related thrombosis
Use of anticoagulation for routine prophylaxis of catheter-related thrombosis is not recommended. Catheters should be inserted on the right side in the jugular vein, and the distal extremity of the central catheter should be located at the junction of the superior vena cava and the right atrium. “In patients requiring central venous catheters, we suggest the use of implanted ports over peripheral inserted central catheter lines,” the authors noted.
The authors described a number of unique situations regarding the treatment of VTE. These situations include patients with a brain tumor, for whom treatment of established VTE should favor either LMWH or a DOA. The authors also recommended the use of LMWH or unfractionated heparin, started postoperatively, for the prevention of VTE for patients undergoing neurosurgery.
In contrast, pharmacologic prophylaxis of VTE in medically treated patients with a brain tumor who are not undergoing neurosurgery is not recommended. “In the presence of severe renal failure...we suggest using unfractionated heparin followed by early vitamin K antagonists (possibly from day 1) or LMWH adjusted to anti-Xa concentration of the treatment of established VTE,” Dr. Farge and colleagues wrote.
Anticoagulant treatment is also recommended for a minimum of 3 months for children with symptomatic catheter-related thrombosis and as long as the central venous catheter is in place. For children with acute lymphoblastic leukemia who are undergoing induction chemotherapy, LMWH is also recommended as thromboprophylaxis.
For children who require a central venous catheter, the authors suggested that physicians use implanted ports over peripherally inserted central lines.
A version of this article first appeared on Medscape.com.
Updated clinical practice guidelines for the treatment and prevention of venous thromboembolism for patients with cancer, including those with cancer and COVID-19, have been released by the International Initiative on Thrombosis and Cancer (ITAC), an academic working group of VTE experts.
“Because patients with cancer have a baseline increased risk of VTE, compared with patients without cancer, the combination of both COVID-19 and cancer – and its effect on VTE risk and treatment – is of concern,” said the authors, led by Dominique Farge, MD, PhD, Nord Universite de Paris.
they added.
The new guidelines were published online in The Lancet Oncology.
“Cancer-associated VTE remains an important clinical problem, associated with increased morbidity and mortality,” Dr. Farge and colleagues observed.
“The ITAC guidelines’ companion free web-based mobile application will assist the practicing clinician with decision making at various levels to provide optimal care of patients with cancer to treat and prevent VTE,” they emphasized. More information is available at itaccme.com.
Cancer patients with COVID
The new section of the guidelines notes that the treatment and prevention of VTE for cancer patients infected with SARS-CoV-2 remain the same as for patients without COVID.
Whether or not cancer patients with COVID-19 are hospitalized, have been discharged, or are ambulatory, they should be assessed for the risk of VTE, as should any other patient. For cancer patients with COVID-19 who are hospitalized, pharmacologic prophylaxis should be given at the same dose and anticoagulant type as for hospitalized cancer patients who do not have COVID-19.
Following discharge, VTE prophylaxis is not advised for cancer patients infected with SARS-CoV-2, and routine primary pharmacologic prophylaxis of VTE for ambulatory patients with COVID-19 is also not recommended, the authors noted.
Initial treatment of established VTE
Initial treatment of established VTE for up to 10 days of anticoagulation should include low-molecular-weight heparin (LMWH) when creatinine clearance is at least 30 mL/min.
“A regimen of LMWH, taken once per day, is recommended unless a twice-per-day regimen is required because of patients’ characteristics,” the authors noted. These characteristics include a high risk of bleeding, moderate renal failure, and the need for technical intervention, including surgery.
If a twice-a-day regimen is required, only enoxaparin at a dose of 1 mg/kg twice daily can be used, the authors cautioned.
For patients with a low risk of gastrointestinal or genitourinary bleeding, rivaroxaban (Xarelto) or apixaban (Eliquis) can be given in the first 10 days, as well as edoxaban (Lixiana). The latter should be started after at least 5 days of parenteral anticoagulation, provided creatinine clearance is at least 30 mL/min.
“Unfractionated heparin as well as fondaparinux (GlaxoSmithKline) can be also used for the initial treatment of established VTE when LMWH or direct oral anticoagulants are contraindicated,” Dr. Farge and colleagues wrote.
Thrombolysis can be considered on a case-by-case basis, although physicians must pay attention to specific contraindications, especially bleeding risk.
“In the initial treatment of VTE, inferior vena cava filters might be considered when anticoagulant treatment is contraindicated or, in the case of pulmonary embolism, when recurrence occurs under optimal anticoagulation,” the authors noted.
Maintenance VTE treatment
For maintenance therapy, which the authors define as early maintenance for up to 6 months and long-term maintenance beyond 6 months, they point out that LMWHs are preferred over vitamin K antagonists for the treatment of VTE when the creatinine clearance is again at least 30 mL/min.
Any of the direct oral anticoagulants (DOAs) – edoxaban, rivaroxaban, or apixaban – is also recommended for the same patients, provided there is no risk of inducing a strong drug-drug interaction or GI absorption is impaired.
However, the DOAs should be used with caution for patients with GI malignancies, especially upper GI cancers, because data show there is an increased risk of GI bleeding with both edoxaban and rivaroxaban.
“LMWH or direct oral anticoagulants should be used for a minimum of 6 months to treat established VTE in patients with cancer,” the authors wrote.
“After 6 months, termination or continuation of anticoagulation (LMWH, direct oral anticoagulants, or vitamin K antagonists) should be based on individual evaluation of the benefit-risk ratio,” they added.
Treatment of VTE recurrence
The guideline authors explain that three options can be considered in the event of VTE recurrence. These include an increase in the LMWH dose by 20%-25%, or a switch to a DOA, or, if patients are taking a DOA, a switch to an LMWH. If the patient is taking a vitamin K antagonist, it can be switched to either an LMWH or a DOA.
For treatment of catheter-related thrombosis, anticoagulant treatment is recommended for a minimum of 3 months and as long as the central venous catheter is in place. In this setting, the LMWHs are recommended.
The central venous catheter can be kept in place if it is functional, well positioned, and is not infected, provided there is good resolution of symptoms under close surveillance while anticoagulants are being administered.
In surgically treated patients, the LMWH, given once a day, to patients with a serum creatinine concentration of at least 30 mL/min can be used to prevent VTE. Alternatively, VTE can be prevented by the use low-dose unfractionated heparin, given three times a day.
“Pharmacological prophylaxis should be started 2-12 h preoperatively and continued for at least 7–10 days,” Dr. Farge and colleagues advised. In this setting, there is insufficient evidence to support the use of fondaparinux or a DOA as an alternative to an LMWH for the prophylaxis of postoperative VTE. “Use of the highest prophylactic dose of LMWH to prevent postoperative VTE in patients with cancer is recommended,” the authors advised.
Furthermore, extended prophylaxis of at least 4 weeks with LMWH is advised to prevent postoperative VTE after major abdominal or pelvic surgery. Mechanical methods are not recommended except when pharmacologic methods are contraindicated. Inferior vena cava filters are also not recommended for routine prophylaxis.
Patients with reduced mobility
For medically treated hospitalized patients with cancer whose mobility is reduced, the authors recommend prophylaxis with either an LMWH or fondaparinux, provided their creatinine clearance is at least 30 mL/min. These patients can also be treated with unfractionated heparin, they add.
In contrast, DOAs are not recommended – at least not routinely – in this setting, the authors cautioned. Primary pharmacologic prophylaxis of VTE with either LMWH or DOAs – either rivaroxaban or apixaban – is indicated in ambulatory patients with locally advanced or metastatic pancreatic cancer who are receiving systemic anticancer therapy, provided they are at low risk of bleeding.
However, primary pharmacologic prophylaxis with LMWH is not recommended outside of a clinical trial for patients with locally advanced or metastatic lung cancer who are undergoing systemic anticancer therapy, even for patients who are at low risk of bleeding.
For ambulatory patients who are receiving systemic anticancer therapy and who are at intermediate risk of VTE, primary prophylaxis with rivaroxaban or apixaban is recommended for those with myeloma who are receiving immunomodulatory therapy plus steroids or other systemic therapies.
In this setting, oral anticoagulants should consist of a vitamin K antagonist, given at low or therapeutic doses, or apixaban, given at prophylactic doses. Alternatively, LMWH, given at prophylactic doses, or low-dose aspirin, given at a dose of 100 mg/day, can be used.
Catheter-related thrombosis
Use of anticoagulation for routine prophylaxis of catheter-related thrombosis is not recommended. Catheters should be inserted on the right side in the jugular vein, and the distal extremity of the central catheter should be located at the junction of the superior vena cava and the right atrium. “In patients requiring central venous catheters, we suggest the use of implanted ports over peripheral inserted central catheter lines,” the authors noted.
The authors described a number of unique situations regarding the treatment of VTE. These situations include patients with a brain tumor, for whom treatment of established VTE should favor either LMWH or a DOA. The authors also recommended the use of LMWH or unfractionated heparin, started postoperatively, for the prevention of VTE for patients undergoing neurosurgery.
In contrast, pharmacologic prophylaxis of VTE in medically treated patients with a brain tumor who are not undergoing neurosurgery is not recommended. “In the presence of severe renal failure...we suggest using unfractionated heparin followed by early vitamin K antagonists (possibly from day 1) or LMWH adjusted to anti-Xa concentration of the treatment of established VTE,” Dr. Farge and colleagues wrote.
Anticoagulant treatment is also recommended for a minimum of 3 months for children with symptomatic catheter-related thrombosis and as long as the central venous catheter is in place. For children with acute lymphoblastic leukemia who are undergoing induction chemotherapy, LMWH is also recommended as thromboprophylaxis.
For children who require a central venous catheter, the authors suggested that physicians use implanted ports over peripherally inserted central lines.
A version of this article first appeared on Medscape.com.
Updated clinical practice guidelines for the treatment and prevention of venous thromboembolism for patients with cancer, including those with cancer and COVID-19, have been released by the International Initiative on Thrombosis and Cancer (ITAC), an academic working group of VTE experts.
“Because patients with cancer have a baseline increased risk of VTE, compared with patients without cancer, the combination of both COVID-19 and cancer – and its effect on VTE risk and treatment – is of concern,” said the authors, led by Dominique Farge, MD, PhD, Nord Universite de Paris.
they added.
The new guidelines were published online in The Lancet Oncology.
“Cancer-associated VTE remains an important clinical problem, associated with increased morbidity and mortality,” Dr. Farge and colleagues observed.
“The ITAC guidelines’ companion free web-based mobile application will assist the practicing clinician with decision making at various levels to provide optimal care of patients with cancer to treat and prevent VTE,” they emphasized. More information is available at itaccme.com.
Cancer patients with COVID
The new section of the guidelines notes that the treatment and prevention of VTE for cancer patients infected with SARS-CoV-2 remain the same as for patients without COVID.
Whether or not cancer patients with COVID-19 are hospitalized, have been discharged, or are ambulatory, they should be assessed for the risk of VTE, as should any other patient. For cancer patients with COVID-19 who are hospitalized, pharmacologic prophylaxis should be given at the same dose and anticoagulant type as for hospitalized cancer patients who do not have COVID-19.
Following discharge, VTE prophylaxis is not advised for cancer patients infected with SARS-CoV-2, and routine primary pharmacologic prophylaxis of VTE for ambulatory patients with COVID-19 is also not recommended, the authors noted.
Initial treatment of established VTE
Initial treatment of established VTE for up to 10 days of anticoagulation should include low-molecular-weight heparin (LMWH) when creatinine clearance is at least 30 mL/min.
“A regimen of LMWH, taken once per day, is recommended unless a twice-per-day regimen is required because of patients’ characteristics,” the authors noted. These characteristics include a high risk of bleeding, moderate renal failure, and the need for technical intervention, including surgery.
If a twice-a-day regimen is required, only enoxaparin at a dose of 1 mg/kg twice daily can be used, the authors cautioned.
For patients with a low risk of gastrointestinal or genitourinary bleeding, rivaroxaban (Xarelto) or apixaban (Eliquis) can be given in the first 10 days, as well as edoxaban (Lixiana). The latter should be started after at least 5 days of parenteral anticoagulation, provided creatinine clearance is at least 30 mL/min.
“Unfractionated heparin as well as fondaparinux (GlaxoSmithKline) can be also used for the initial treatment of established VTE when LMWH or direct oral anticoagulants are contraindicated,” Dr. Farge and colleagues wrote.
Thrombolysis can be considered on a case-by-case basis, although physicians must pay attention to specific contraindications, especially bleeding risk.
“In the initial treatment of VTE, inferior vena cava filters might be considered when anticoagulant treatment is contraindicated or, in the case of pulmonary embolism, when recurrence occurs under optimal anticoagulation,” the authors noted.
Maintenance VTE treatment
For maintenance therapy, which the authors define as early maintenance for up to 6 months and long-term maintenance beyond 6 months, they point out that LMWHs are preferred over vitamin K antagonists for the treatment of VTE when the creatinine clearance is again at least 30 mL/min.
Any of the direct oral anticoagulants (DOAs) – edoxaban, rivaroxaban, or apixaban – is also recommended for the same patients, provided there is no risk of inducing a strong drug-drug interaction or GI absorption is impaired.
However, the DOAs should be used with caution for patients with GI malignancies, especially upper GI cancers, because data show there is an increased risk of GI bleeding with both edoxaban and rivaroxaban.
“LMWH or direct oral anticoagulants should be used for a minimum of 6 months to treat established VTE in patients with cancer,” the authors wrote.
“After 6 months, termination or continuation of anticoagulation (LMWH, direct oral anticoagulants, or vitamin K antagonists) should be based on individual evaluation of the benefit-risk ratio,” they added.
Treatment of VTE recurrence
The guideline authors explain that three options can be considered in the event of VTE recurrence. These include an increase in the LMWH dose by 20%-25%, or a switch to a DOA, or, if patients are taking a DOA, a switch to an LMWH. If the patient is taking a vitamin K antagonist, it can be switched to either an LMWH or a DOA.
For treatment of catheter-related thrombosis, anticoagulant treatment is recommended for a minimum of 3 months and as long as the central venous catheter is in place. In this setting, the LMWHs are recommended.
The central venous catheter can be kept in place if it is functional, well positioned, and is not infected, provided there is good resolution of symptoms under close surveillance while anticoagulants are being administered.
In surgically treated patients, the LMWH, given once a day, to patients with a serum creatinine concentration of at least 30 mL/min can be used to prevent VTE. Alternatively, VTE can be prevented by the use low-dose unfractionated heparin, given three times a day.
“Pharmacological prophylaxis should be started 2-12 h preoperatively and continued for at least 7–10 days,” Dr. Farge and colleagues advised. In this setting, there is insufficient evidence to support the use of fondaparinux or a DOA as an alternative to an LMWH for the prophylaxis of postoperative VTE. “Use of the highest prophylactic dose of LMWH to prevent postoperative VTE in patients with cancer is recommended,” the authors advised.
Furthermore, extended prophylaxis of at least 4 weeks with LMWH is advised to prevent postoperative VTE after major abdominal or pelvic surgery. Mechanical methods are not recommended except when pharmacologic methods are contraindicated. Inferior vena cava filters are also not recommended for routine prophylaxis.
Patients with reduced mobility
For medically treated hospitalized patients with cancer whose mobility is reduced, the authors recommend prophylaxis with either an LMWH or fondaparinux, provided their creatinine clearance is at least 30 mL/min. These patients can also be treated with unfractionated heparin, they add.
In contrast, DOAs are not recommended – at least not routinely – in this setting, the authors cautioned. Primary pharmacologic prophylaxis of VTE with either LMWH or DOAs – either rivaroxaban or apixaban – is indicated in ambulatory patients with locally advanced or metastatic pancreatic cancer who are receiving systemic anticancer therapy, provided they are at low risk of bleeding.
However, primary pharmacologic prophylaxis with LMWH is not recommended outside of a clinical trial for patients with locally advanced or metastatic lung cancer who are undergoing systemic anticancer therapy, even for patients who are at low risk of bleeding.
For ambulatory patients who are receiving systemic anticancer therapy and who are at intermediate risk of VTE, primary prophylaxis with rivaroxaban or apixaban is recommended for those with myeloma who are receiving immunomodulatory therapy plus steroids or other systemic therapies.
In this setting, oral anticoagulants should consist of a vitamin K antagonist, given at low or therapeutic doses, or apixaban, given at prophylactic doses. Alternatively, LMWH, given at prophylactic doses, or low-dose aspirin, given at a dose of 100 mg/day, can be used.
Catheter-related thrombosis
Use of anticoagulation for routine prophylaxis of catheter-related thrombosis is not recommended. Catheters should be inserted on the right side in the jugular vein, and the distal extremity of the central catheter should be located at the junction of the superior vena cava and the right atrium. “In patients requiring central venous catheters, we suggest the use of implanted ports over peripheral inserted central catheter lines,” the authors noted.
The authors described a number of unique situations regarding the treatment of VTE. These situations include patients with a brain tumor, for whom treatment of established VTE should favor either LMWH or a DOA. The authors also recommended the use of LMWH or unfractionated heparin, started postoperatively, for the prevention of VTE for patients undergoing neurosurgery.
In contrast, pharmacologic prophylaxis of VTE in medically treated patients with a brain tumor who are not undergoing neurosurgery is not recommended. “In the presence of severe renal failure...we suggest using unfractionated heparin followed by early vitamin K antagonists (possibly from day 1) or LMWH adjusted to anti-Xa concentration of the treatment of established VTE,” Dr. Farge and colleagues wrote.
Anticoagulant treatment is also recommended for a minimum of 3 months for children with symptomatic catheter-related thrombosis and as long as the central venous catheter is in place. For children with acute lymphoblastic leukemia who are undergoing induction chemotherapy, LMWH is also recommended as thromboprophylaxis.
For children who require a central venous catheter, the authors suggested that physicians use implanted ports over peripherally inserted central lines.
A version of this article first appeared on Medscape.com.
FROM THE LANCET ONCOLOGY
Blood test for cancer available, but is it ready for prime time?
The Galleri blood test is being now offered by a number of United States health networks.
The company marketing the test, GRAIL, has established partnerships with the U.S. Department of Veterans Affairs, Mercy Health, Ochsner Health, Intermountain Healthcare, Community Health Network, Knight Cancer Institute at Oregon Health & Science University, Premier, and Cleveland Clinic, among others.
Cleveland Clinic’s Eric Klein, MD, emeritus chair of the Glickman Urological Kidney Institute, is enthusiastic about the test, describing it as a “game-changer” and emphasizing that it can detect many different cancers and at a very early stage.
“It completely changes the way we think about screening for cancer,” commented Jeff Venstrom, MD, chief medical officer at GRAIL. He joined the company because “there are not many things in life where you can be part of a disruptive paradigm and disruptive technology, and this really is disruptive,” he said in an interview.
‘The devil is in the details’
But there is some concern among clinicians that widespread clinical use of the test may be premature.
Having a blood test for multiple cancers is a “very good idea, and the scientific basis for this platform is sound,” commented Timothy R. Rebbeck, PhD, professor of cancer prevention, Harvard T.H. Chan School of Public Health, and Division of Population Sciences, Dana-Farber Cancer Institute, both in Boston.
“But the devil is in the details to ensure the test can accurately detect very early cancers and there is a pathway for subsequent workup (diagnosis, monitoring, treatment, etc.),” Dr. Rebbeck told this news organization.
Galleri is offering the test to individuals who are older than 50 and have a family history of cancer or those who are high risk for cancer or immunocompromised. They suggest that interested individuals get in touch with their health care professional, who then needs to register with GRAIL and order the test.
As well as needing a prescription, interested individuals will have to pay for it out of pocket, around $950. The test is not covered by medical insurance and is not approved by the U.S. Food and Drug Administration.
Falls into primary care setting
Dr. Rebbeck commented that Galleri is a screening test for individuals who don’t have cancer, so the test is intended to fall into the primary care setting. But he warned that “clinical pathways are not yet in place (but are being developed) so that primary care providers can effectively use them.”
The test uses next-generation sequencing to analyze the arrangement of methyl groups on circulating tumor (or cell-free) DNA in a blood sample.
The methylation turns genes on or off, explains Cleveland Clinic’s Dr. Klein in his post. “It’s like fingerprints and how fingerprints tell the difference between two people,” he wrote. “The methylation patterns are fingerprints that are characteristic of each kind of cancer. They look one way for lung cancer and different for colon cancer.”
The test returns one of two possible results: either “positive, cancer signal detected” or “negative, no cancer signal detected.”
According to the company, when a cancer signal is detected, the Galleri test predicts the cancer signal origin “with high accuracy, to help guide the next steps to diagnosis.”
However, one problem for clinical practice is all the follow-up tests an individual may undergo if their test comes back positive, said Sameek Roychowdhury, MD, PhD, an oncologist with Ohio State University Comprehensive Cancer Center, Columbus.
“Not everybody will have an actual cancer, but they may undergo many tests, with a lot of stress and cost and still not find anything. I can tell you every time someone undergoes a test looking for cancer, that is not an easy day,” Dr. Roychowdhury said in an interview.
In a large-scale validation study, the Galleri test had a specificity of 99.5% (false-positive rate of 0.5%), meaning in roughly 200 people tested without cancer, only one person received a false-positive result (that is, “cancer signal detected” when cancer is not present).
The overall sensitivity of the test for any stage of cancer was 51.5%, although it was higher for later-stage cancers (77% for stage III and 90.1% for stage IV) and lower for early-stage cancers (16.8% for stage I and 40.4% for stage II).
Exacerbate health disparities?
In Dr. Rebbeck’s view, the characteristics of the test are still “relatively poor for detecting very early cancers, so it will need additional tweaking before it really achieves the goal of multi-cancer EARLY detection,” he said.
Dr. Venstrom acknowledges that the test is “not perfect yet” and says the company will continue to update and improve its performance. “We have some new data coming out in September,” he said.
Clinical data are being accumulated in the United Kingdom, where the Galleri test is being investigated in a large trial run by the National Health Service (NHS). The company recently announced that the enrollment of 140,000 healthy cancer-free volunteers aged 50-77 into this trial has now been completed and claimed this the largest-ever study of a multi-cancer early detection test.
Dr. Roychowdhury said he would encourage anyone interested in the test to join a clinical trial.
Another expert approached for comment last year, when GRAIL first started marketing the test, was in agreement. This test should be viewed as one that is still under clinical investigation, commented William Grady, MD, a member of the clinical research division and public health sciences division at the Fred Hutchinson Cancer Research Center, Seattle.
“The Galleri test is still unproven in the clinical care setting and ... I am concerned that many of the results will be false-positives and will cause many unnecessary follow-up tests and imaging studies as well as anxiety in the people getting the test done,” Dr. Grady said.
Dr. Rebbeck said another issue that needs to be addressed is whether all populations will have access to and benefit from these types of blood tests to screen for cancer, given that they are expensive.
“There is a great danger – as we have seen with many other technological innovations – that the wealthy and connected benefit, but the majority of the population, and particularly those who are underserved, do not,” Dr. Rebbeck said.
“As a result, health disparities are created or exacerbated. This is something that needs to be addressed so that the future use of these tests will provide equitable benefits,” he added.
Dr. Rebbeck and Dr. Roychowdhury have reported no relevant financial relationships. Dr. Venstrom is an employee of GRAIL.
A version of this article first appeared on Medscape.com.
The Galleri blood test is being now offered by a number of United States health networks.
The company marketing the test, GRAIL, has established partnerships with the U.S. Department of Veterans Affairs, Mercy Health, Ochsner Health, Intermountain Healthcare, Community Health Network, Knight Cancer Institute at Oregon Health & Science University, Premier, and Cleveland Clinic, among others.
Cleveland Clinic’s Eric Klein, MD, emeritus chair of the Glickman Urological Kidney Institute, is enthusiastic about the test, describing it as a “game-changer” and emphasizing that it can detect many different cancers and at a very early stage.
“It completely changes the way we think about screening for cancer,” commented Jeff Venstrom, MD, chief medical officer at GRAIL. He joined the company because “there are not many things in life where you can be part of a disruptive paradigm and disruptive technology, and this really is disruptive,” he said in an interview.
‘The devil is in the details’
But there is some concern among clinicians that widespread clinical use of the test may be premature.
Having a blood test for multiple cancers is a “very good idea, and the scientific basis for this platform is sound,” commented Timothy R. Rebbeck, PhD, professor of cancer prevention, Harvard T.H. Chan School of Public Health, and Division of Population Sciences, Dana-Farber Cancer Institute, both in Boston.
“But the devil is in the details to ensure the test can accurately detect very early cancers and there is a pathway for subsequent workup (diagnosis, monitoring, treatment, etc.),” Dr. Rebbeck told this news organization.
Galleri is offering the test to individuals who are older than 50 and have a family history of cancer or those who are high risk for cancer or immunocompromised. They suggest that interested individuals get in touch with their health care professional, who then needs to register with GRAIL and order the test.
As well as needing a prescription, interested individuals will have to pay for it out of pocket, around $950. The test is not covered by medical insurance and is not approved by the U.S. Food and Drug Administration.
Falls into primary care setting
Dr. Rebbeck commented that Galleri is a screening test for individuals who don’t have cancer, so the test is intended to fall into the primary care setting. But he warned that “clinical pathways are not yet in place (but are being developed) so that primary care providers can effectively use them.”
The test uses next-generation sequencing to analyze the arrangement of methyl groups on circulating tumor (or cell-free) DNA in a blood sample.
The methylation turns genes on or off, explains Cleveland Clinic’s Dr. Klein in his post. “It’s like fingerprints and how fingerprints tell the difference between two people,” he wrote. “The methylation patterns are fingerprints that are characteristic of each kind of cancer. They look one way for lung cancer and different for colon cancer.”
The test returns one of two possible results: either “positive, cancer signal detected” or “negative, no cancer signal detected.”
According to the company, when a cancer signal is detected, the Galleri test predicts the cancer signal origin “with high accuracy, to help guide the next steps to diagnosis.”
However, one problem for clinical practice is all the follow-up tests an individual may undergo if their test comes back positive, said Sameek Roychowdhury, MD, PhD, an oncologist with Ohio State University Comprehensive Cancer Center, Columbus.
“Not everybody will have an actual cancer, but they may undergo many tests, with a lot of stress and cost and still not find anything. I can tell you every time someone undergoes a test looking for cancer, that is not an easy day,” Dr. Roychowdhury said in an interview.
In a large-scale validation study, the Galleri test had a specificity of 99.5% (false-positive rate of 0.5%), meaning in roughly 200 people tested without cancer, only one person received a false-positive result (that is, “cancer signal detected” when cancer is not present).
The overall sensitivity of the test for any stage of cancer was 51.5%, although it was higher for later-stage cancers (77% for stage III and 90.1% for stage IV) and lower for early-stage cancers (16.8% for stage I and 40.4% for stage II).
Exacerbate health disparities?
In Dr. Rebbeck’s view, the characteristics of the test are still “relatively poor for detecting very early cancers, so it will need additional tweaking before it really achieves the goal of multi-cancer EARLY detection,” he said.
Dr. Venstrom acknowledges that the test is “not perfect yet” and says the company will continue to update and improve its performance. “We have some new data coming out in September,” he said.
Clinical data are being accumulated in the United Kingdom, where the Galleri test is being investigated in a large trial run by the National Health Service (NHS). The company recently announced that the enrollment of 140,000 healthy cancer-free volunteers aged 50-77 into this trial has now been completed and claimed this the largest-ever study of a multi-cancer early detection test.
Dr. Roychowdhury said he would encourage anyone interested in the test to join a clinical trial.
Another expert approached for comment last year, when GRAIL first started marketing the test, was in agreement. This test should be viewed as one that is still under clinical investigation, commented William Grady, MD, a member of the clinical research division and public health sciences division at the Fred Hutchinson Cancer Research Center, Seattle.
“The Galleri test is still unproven in the clinical care setting and ... I am concerned that many of the results will be false-positives and will cause many unnecessary follow-up tests and imaging studies as well as anxiety in the people getting the test done,” Dr. Grady said.
Dr. Rebbeck said another issue that needs to be addressed is whether all populations will have access to and benefit from these types of blood tests to screen for cancer, given that they are expensive.
“There is a great danger – as we have seen with many other technological innovations – that the wealthy and connected benefit, but the majority of the population, and particularly those who are underserved, do not,” Dr. Rebbeck said.
“As a result, health disparities are created or exacerbated. This is something that needs to be addressed so that the future use of these tests will provide equitable benefits,” he added.
Dr. Rebbeck and Dr. Roychowdhury have reported no relevant financial relationships. Dr. Venstrom is an employee of GRAIL.
A version of this article first appeared on Medscape.com.
The Galleri blood test is being now offered by a number of United States health networks.
The company marketing the test, GRAIL, has established partnerships with the U.S. Department of Veterans Affairs, Mercy Health, Ochsner Health, Intermountain Healthcare, Community Health Network, Knight Cancer Institute at Oregon Health & Science University, Premier, and Cleveland Clinic, among others.
Cleveland Clinic’s Eric Klein, MD, emeritus chair of the Glickman Urological Kidney Institute, is enthusiastic about the test, describing it as a “game-changer” and emphasizing that it can detect many different cancers and at a very early stage.
“It completely changes the way we think about screening for cancer,” commented Jeff Venstrom, MD, chief medical officer at GRAIL. He joined the company because “there are not many things in life where you can be part of a disruptive paradigm and disruptive technology, and this really is disruptive,” he said in an interview.
‘The devil is in the details’
But there is some concern among clinicians that widespread clinical use of the test may be premature.
Having a blood test for multiple cancers is a “very good idea, and the scientific basis for this platform is sound,” commented Timothy R. Rebbeck, PhD, professor of cancer prevention, Harvard T.H. Chan School of Public Health, and Division of Population Sciences, Dana-Farber Cancer Institute, both in Boston.
“But the devil is in the details to ensure the test can accurately detect very early cancers and there is a pathway for subsequent workup (diagnosis, monitoring, treatment, etc.),” Dr. Rebbeck told this news organization.
Galleri is offering the test to individuals who are older than 50 and have a family history of cancer or those who are high risk for cancer or immunocompromised. They suggest that interested individuals get in touch with their health care professional, who then needs to register with GRAIL and order the test.
As well as needing a prescription, interested individuals will have to pay for it out of pocket, around $950. The test is not covered by medical insurance and is not approved by the U.S. Food and Drug Administration.
Falls into primary care setting
Dr. Rebbeck commented that Galleri is a screening test for individuals who don’t have cancer, so the test is intended to fall into the primary care setting. But he warned that “clinical pathways are not yet in place (but are being developed) so that primary care providers can effectively use them.”
The test uses next-generation sequencing to analyze the arrangement of methyl groups on circulating tumor (or cell-free) DNA in a blood sample.
The methylation turns genes on or off, explains Cleveland Clinic’s Dr. Klein in his post. “It’s like fingerprints and how fingerprints tell the difference between two people,” he wrote. “The methylation patterns are fingerprints that are characteristic of each kind of cancer. They look one way for lung cancer and different for colon cancer.”
The test returns one of two possible results: either “positive, cancer signal detected” or “negative, no cancer signal detected.”
According to the company, when a cancer signal is detected, the Galleri test predicts the cancer signal origin “with high accuracy, to help guide the next steps to diagnosis.”
However, one problem for clinical practice is all the follow-up tests an individual may undergo if their test comes back positive, said Sameek Roychowdhury, MD, PhD, an oncologist with Ohio State University Comprehensive Cancer Center, Columbus.
“Not everybody will have an actual cancer, but they may undergo many tests, with a lot of stress and cost and still not find anything. I can tell you every time someone undergoes a test looking for cancer, that is not an easy day,” Dr. Roychowdhury said in an interview.
In a large-scale validation study, the Galleri test had a specificity of 99.5% (false-positive rate of 0.5%), meaning in roughly 200 people tested without cancer, only one person received a false-positive result (that is, “cancer signal detected” when cancer is not present).
The overall sensitivity of the test for any stage of cancer was 51.5%, although it was higher for later-stage cancers (77% for stage III and 90.1% for stage IV) and lower for early-stage cancers (16.8% for stage I and 40.4% for stage II).
Exacerbate health disparities?
In Dr. Rebbeck’s view, the characteristics of the test are still “relatively poor for detecting very early cancers, so it will need additional tweaking before it really achieves the goal of multi-cancer EARLY detection,” he said.
Dr. Venstrom acknowledges that the test is “not perfect yet” and says the company will continue to update and improve its performance. “We have some new data coming out in September,” he said.
Clinical data are being accumulated in the United Kingdom, where the Galleri test is being investigated in a large trial run by the National Health Service (NHS). The company recently announced that the enrollment of 140,000 healthy cancer-free volunteers aged 50-77 into this trial has now been completed and claimed this the largest-ever study of a multi-cancer early detection test.
Dr. Roychowdhury said he would encourage anyone interested in the test to join a clinical trial.
Another expert approached for comment last year, when GRAIL first started marketing the test, was in agreement. This test should be viewed as one that is still under clinical investigation, commented William Grady, MD, a member of the clinical research division and public health sciences division at the Fred Hutchinson Cancer Research Center, Seattle.
“The Galleri test is still unproven in the clinical care setting and ... I am concerned that many of the results will be false-positives and will cause many unnecessary follow-up tests and imaging studies as well as anxiety in the people getting the test done,” Dr. Grady said.
Dr. Rebbeck said another issue that needs to be addressed is whether all populations will have access to and benefit from these types of blood tests to screen for cancer, given that they are expensive.
“There is a great danger – as we have seen with many other technological innovations – that the wealthy and connected benefit, but the majority of the population, and particularly those who are underserved, do not,” Dr. Rebbeck said.
“As a result, health disparities are created or exacerbated. This is something that needs to be addressed so that the future use of these tests will provide equitable benefits,” he added.
Dr. Rebbeck and Dr. Roychowdhury have reported no relevant financial relationships. Dr. Venstrom is an employee of GRAIL.
A version of this article first appeared on Medscape.com.