User login
Nodular Sclerosing Hodgkin Lymphoma With Paraneoplastic Cerebellar Degeneration
Paraneoplastic syndrome is a rare disorder involving manifestations of immune dysregulation triggered by malignancy. The immune system develops antibodies to the malignancy, which can cause cross reactivation with various tissues in the body, resulting in an autoimmune response. Paraneoplastic cerebellar degeneration (PCD) is a rare condition caused by immune-mediated damage to the Purkinje cells of the cerebellar tract. Symptoms may include gait instability, double vision, decreased fine motor skills, and ataxia, with progression to brainstem-associated symptoms, such as nystagmus, dysarthria, and dysphagia. Early detection and treatment of the underlying malignancy is critical to halt the progression of autoimmune-mediated destruction. We present a case of a young adult female patient with PCD caused by Purkinje cell cytoplasmic–Tr (PCA-Tr) antibody with Hodgkin lymphoma.
Case Presentation
A 20-year-old previously healthy active-duty female patient presented to the emergency department with acute worsening of chronic intermittent, recurrent episodes of lightheadedness and vertigo. Symptoms persisted for 9 months until acutely worsening over the 2 weeks prior to presentation. She reported left eye double vision but did not report seeing spots, photophobia, tinnitus, or headache. She felt off-balance, leaning on nearby objects to remain standing. Symptoms primarily occurred during ambulation; however, occasionally they happened at rest. Episodes lasted up to several minutes and occurred up to 15 times a day. The patient reported no fever, night sweats, unexplained weight loss, muscle aches, weakness, numbness or tingling, loss of bowel or bladder function, or rash. She had no recent illnesses, changes to medications, or recent travel. Oral intake to include food and water was adequate and unchanged. The patient had a remote history of mild concussions without loss of consciousness while playing sports 4 years previously. She reported no recent trauma. Nine months before, she received treatment for benign paroxysmal positional vertigo (BPPV) with the Epley maneuver with full resolution of symptoms lasting several days. She reported no prescription or over-the-counter medications, herbal remedies, or supplements. She reported no other medical or surgical history and no pertinent social or family history.
Physical examination revealed a nontoxic-appearing female patient with intermittent conversational dysarthria, saccadic pursuits, horizontal nystagmus with lateral gaze, and vertical nystagmus with vertical gaze. The patient exhibited dysdiadochokinesia, or impaired ability to perform rapid alternating hand movements with repetition. Finger-to-nose testing was impaired and heel-to-shin motion remained intact. A Romberg test was positive, and the patient had tandem gait instability. Strength testing, sensation, reflexes, and cranial nerves were otherwise intact. Initial laboratory testing was unremarkable except for mild normocytic anemia. Her infectious workup, including testing for venereal disease, HIV, COVID-19, and Coccidioidies was negative. Heavy metals analysis and urine drug screen were negative. Ophthalmology was consulted and workup revealed small amplitude downbeat nystagmus in primary gaze, sustained gaze evoked lateral beating jerk nystagmus with rebound nystagmus R>L gaze, but there was no evidence of afferent package defect and optic nerve function remained intact. Magnetic resonance imaging of the brain demonstrated cerebellar vermis hypoplasia with prominence of the superior cerebellar folia. Due to concerns for autoimmune encephalitis, a lumbar puncture was performed. Antibody testing revealed PCA-Tr antibodies, which is commonly associated with Hodgkin lymphoma, prompting further evaluation for malignancy.
Computed tomography (CT) of the chest with contrast demonstrated multiple mediastinal masses with a conglomeration of lymph nodes along the right paratracheal region. Further evaluation was performed with a positron emission tomography (PET)–CT, revealing a large conglomeration of hypermetabolic pretracheal, mediastinal, and right supraclavicular lymph that were suggestive of lymphoma. Mediastinoscopy with excisional lymph node biopsy was performed with immunohistochemical staining confirming diagnosis of a nodular sclerosing variant of Hodgkin lymphoma. The patient was treated with IV immunoglobulin at 0.4g/kg daily for 5 days. A central venous catheter was placed into the patient’s right internal jugular vein and a chemotherapy regimen of doxorubicin 46 mg, vinblastine 11 mg, bleomycin 19 units, and dacarbazine 700 mg was initiated. The patient’s symptoms improved with resolution of dysarthria; however, her visual impairment and gait instability persisted. Repeat PET-CT imaging 2 months later revealed interval improvement with decreased intensity and extent of the hypermetabolic lymph nodes and no new hypermetabolic foci.
Discussion
PCA-Tr antibodies affect the delta/notchlike epidermal growth factor–related receptor, expressed on the dendrites of cerebellar Purkinje cells.1 These fibers are the only output neurons of the cerebellar cortex and are critical to the coordination of motor movements, accounting for the ataxia experienced by patients with this subtype of PCD.2 The link between Hodgkin lymphoma and PCA-Tr antibodies has been established; however, most reports involve men with a median age of 61 years with lymphoma-associated symptoms (such as lymphadenopathy) or systemic symptoms (fever, night sweats, or weight loss) preceding neurologic manifestations in 80% of cases.3
Our patient was a young, previously healthy adult female who initially presented with vertigo, a common concern with frequently benign origins. Although there was temporary resolution of symptoms after Epley maneuvers, symptoms recurred and progressed over several months to include brainstem manifestations of nystagmus, diplopia, and dysarthria. Previous reports indicate that after remission of the Hodgkin lymphoma, PCA-Tr antibodies disappear and symptoms can improve or resolve.4,5 Treatment has just begun for our patient and although there has been initial clinical improvement, given the chronicity of symptoms, it is unclear if complete resolution will be achieved.
Conclusions
PCD can result in debilitating neurologic dysfunction and may be associated with malignancy such as Hodgkin lymphoma. This case offers unique insight due to the patient’s demographics and presentation, which involved brainstem pathology typically associated with late-onset disease and preceded by constitutional symptoms. Clinical suspicion of this rare disorder should be considered in all ages, especially if symptoms are progressive or neurologic manifestations arise, as early detection and treatment of the underlying malignancy are paramount to the prevention of significant disability.
1. de Graaff E, Maat P, Hulsenboom E, et al. Identification of delta/notch-like epidermal growth factor-related receptor as the Tr antigen in paraneoplastic cerebellar degeneration. Ann Neurol. 2012;71(6):815-824. doi:10.1002/ana.23550
2. MacKenzie-Graham A, Tiwari-Woodruff SK, Sharma G, et al. Purkinje cell loss in experimental autoimmune encephalomyelitis. Neuroimage. 2009;48(4):637-651. doi:10.1016/j.neuroimage.2009.06.073
3. Bernal F, Shams’ili S, Rojas I, et al. Anti-Tr antibodies as markers of paraneoplastic cerebellar degeneration and Hodgkin’s disease. Neurology. 2003;60(2):230-234. doi:10.1212/01.wnl.0000041495.87539.98
4. Graus F, Ariño H, Dalmau J. Paraneoplastic neurological syndromes in Hodgkin and non-Hodgkin lymphomas. Blood. 2014;123(21):3230-3238. doi:10.1182/blood-2014-03-537506
5. Aly R, Emmady PD. Paraneoplastic cerebellar degeneration. Updated May 8, 2022. Accessed March 30, 2022. https://www.ncbi.nlm.nih.gov/books/NBK560638
Paraneoplastic syndrome is a rare disorder involving manifestations of immune dysregulation triggered by malignancy. The immune system develops antibodies to the malignancy, which can cause cross reactivation with various tissues in the body, resulting in an autoimmune response. Paraneoplastic cerebellar degeneration (PCD) is a rare condition caused by immune-mediated damage to the Purkinje cells of the cerebellar tract. Symptoms may include gait instability, double vision, decreased fine motor skills, and ataxia, with progression to brainstem-associated symptoms, such as nystagmus, dysarthria, and dysphagia. Early detection and treatment of the underlying malignancy is critical to halt the progression of autoimmune-mediated destruction. We present a case of a young adult female patient with PCD caused by Purkinje cell cytoplasmic–Tr (PCA-Tr) antibody with Hodgkin lymphoma.
Case Presentation
A 20-year-old previously healthy active-duty female patient presented to the emergency department with acute worsening of chronic intermittent, recurrent episodes of lightheadedness and vertigo. Symptoms persisted for 9 months until acutely worsening over the 2 weeks prior to presentation. She reported left eye double vision but did not report seeing spots, photophobia, tinnitus, or headache. She felt off-balance, leaning on nearby objects to remain standing. Symptoms primarily occurred during ambulation; however, occasionally they happened at rest. Episodes lasted up to several minutes and occurred up to 15 times a day. The patient reported no fever, night sweats, unexplained weight loss, muscle aches, weakness, numbness or tingling, loss of bowel or bladder function, or rash. She had no recent illnesses, changes to medications, or recent travel. Oral intake to include food and water was adequate and unchanged. The patient had a remote history of mild concussions without loss of consciousness while playing sports 4 years previously. She reported no recent trauma. Nine months before, she received treatment for benign paroxysmal positional vertigo (BPPV) with the Epley maneuver with full resolution of symptoms lasting several days. She reported no prescription or over-the-counter medications, herbal remedies, or supplements. She reported no other medical or surgical history and no pertinent social or family history.
Physical examination revealed a nontoxic-appearing female patient with intermittent conversational dysarthria, saccadic pursuits, horizontal nystagmus with lateral gaze, and vertical nystagmus with vertical gaze. The patient exhibited dysdiadochokinesia, or impaired ability to perform rapid alternating hand movements with repetition. Finger-to-nose testing was impaired and heel-to-shin motion remained intact. A Romberg test was positive, and the patient had tandem gait instability. Strength testing, sensation, reflexes, and cranial nerves were otherwise intact. Initial laboratory testing was unremarkable except for mild normocytic anemia. Her infectious workup, including testing for venereal disease, HIV, COVID-19, and Coccidioidies was negative. Heavy metals analysis and urine drug screen were negative. Ophthalmology was consulted and workup revealed small amplitude downbeat nystagmus in primary gaze, sustained gaze evoked lateral beating jerk nystagmus with rebound nystagmus R>L gaze, but there was no evidence of afferent package defect and optic nerve function remained intact. Magnetic resonance imaging of the brain demonstrated cerebellar vermis hypoplasia with prominence of the superior cerebellar folia. Due to concerns for autoimmune encephalitis, a lumbar puncture was performed. Antibody testing revealed PCA-Tr antibodies, which is commonly associated with Hodgkin lymphoma, prompting further evaluation for malignancy.
Computed tomography (CT) of the chest with contrast demonstrated multiple mediastinal masses with a conglomeration of lymph nodes along the right paratracheal region. Further evaluation was performed with a positron emission tomography (PET)–CT, revealing a large conglomeration of hypermetabolic pretracheal, mediastinal, and right supraclavicular lymph that were suggestive of lymphoma. Mediastinoscopy with excisional lymph node biopsy was performed with immunohistochemical staining confirming diagnosis of a nodular sclerosing variant of Hodgkin lymphoma. The patient was treated with IV immunoglobulin at 0.4g/kg daily for 5 days. A central venous catheter was placed into the patient’s right internal jugular vein and a chemotherapy regimen of doxorubicin 46 mg, vinblastine 11 mg, bleomycin 19 units, and dacarbazine 700 mg was initiated. The patient’s symptoms improved with resolution of dysarthria; however, her visual impairment and gait instability persisted. Repeat PET-CT imaging 2 months later revealed interval improvement with decreased intensity and extent of the hypermetabolic lymph nodes and no new hypermetabolic foci.
Discussion
PCA-Tr antibodies affect the delta/notchlike epidermal growth factor–related receptor, expressed on the dendrites of cerebellar Purkinje cells.1 These fibers are the only output neurons of the cerebellar cortex and are critical to the coordination of motor movements, accounting for the ataxia experienced by patients with this subtype of PCD.2 The link between Hodgkin lymphoma and PCA-Tr antibodies has been established; however, most reports involve men with a median age of 61 years with lymphoma-associated symptoms (such as lymphadenopathy) or systemic symptoms (fever, night sweats, or weight loss) preceding neurologic manifestations in 80% of cases.3
Our patient was a young, previously healthy adult female who initially presented with vertigo, a common concern with frequently benign origins. Although there was temporary resolution of symptoms after Epley maneuvers, symptoms recurred and progressed over several months to include brainstem manifestations of nystagmus, diplopia, and dysarthria. Previous reports indicate that after remission of the Hodgkin lymphoma, PCA-Tr antibodies disappear and symptoms can improve or resolve.4,5 Treatment has just begun for our patient and although there has been initial clinical improvement, given the chronicity of symptoms, it is unclear if complete resolution will be achieved.
Conclusions
PCD can result in debilitating neurologic dysfunction and may be associated with malignancy such as Hodgkin lymphoma. This case offers unique insight due to the patient’s demographics and presentation, which involved brainstem pathology typically associated with late-onset disease and preceded by constitutional symptoms. Clinical suspicion of this rare disorder should be considered in all ages, especially if symptoms are progressive or neurologic manifestations arise, as early detection and treatment of the underlying malignancy are paramount to the prevention of significant disability.
Paraneoplastic syndrome is a rare disorder involving manifestations of immune dysregulation triggered by malignancy. The immune system develops antibodies to the malignancy, which can cause cross reactivation with various tissues in the body, resulting in an autoimmune response. Paraneoplastic cerebellar degeneration (PCD) is a rare condition caused by immune-mediated damage to the Purkinje cells of the cerebellar tract. Symptoms may include gait instability, double vision, decreased fine motor skills, and ataxia, with progression to brainstem-associated symptoms, such as nystagmus, dysarthria, and dysphagia. Early detection and treatment of the underlying malignancy is critical to halt the progression of autoimmune-mediated destruction. We present a case of a young adult female patient with PCD caused by Purkinje cell cytoplasmic–Tr (PCA-Tr) antibody with Hodgkin lymphoma.
Case Presentation
A 20-year-old previously healthy active-duty female patient presented to the emergency department with acute worsening of chronic intermittent, recurrent episodes of lightheadedness and vertigo. Symptoms persisted for 9 months until acutely worsening over the 2 weeks prior to presentation. She reported left eye double vision but did not report seeing spots, photophobia, tinnitus, or headache. She felt off-balance, leaning on nearby objects to remain standing. Symptoms primarily occurred during ambulation; however, occasionally they happened at rest. Episodes lasted up to several minutes and occurred up to 15 times a day. The patient reported no fever, night sweats, unexplained weight loss, muscle aches, weakness, numbness or tingling, loss of bowel or bladder function, or rash. She had no recent illnesses, changes to medications, or recent travel. Oral intake to include food and water was adequate and unchanged. The patient had a remote history of mild concussions without loss of consciousness while playing sports 4 years previously. She reported no recent trauma. Nine months before, she received treatment for benign paroxysmal positional vertigo (BPPV) with the Epley maneuver with full resolution of symptoms lasting several days. She reported no prescription or over-the-counter medications, herbal remedies, or supplements. She reported no other medical or surgical history and no pertinent social or family history.
Physical examination revealed a nontoxic-appearing female patient with intermittent conversational dysarthria, saccadic pursuits, horizontal nystagmus with lateral gaze, and vertical nystagmus with vertical gaze. The patient exhibited dysdiadochokinesia, or impaired ability to perform rapid alternating hand movements with repetition. Finger-to-nose testing was impaired and heel-to-shin motion remained intact. A Romberg test was positive, and the patient had tandem gait instability. Strength testing, sensation, reflexes, and cranial nerves were otherwise intact. Initial laboratory testing was unremarkable except for mild normocytic anemia. Her infectious workup, including testing for venereal disease, HIV, COVID-19, and Coccidioidies was negative. Heavy metals analysis and urine drug screen were negative. Ophthalmology was consulted and workup revealed small amplitude downbeat nystagmus in primary gaze, sustained gaze evoked lateral beating jerk nystagmus with rebound nystagmus R>L gaze, but there was no evidence of afferent package defect and optic nerve function remained intact. Magnetic resonance imaging of the brain demonstrated cerebellar vermis hypoplasia with prominence of the superior cerebellar folia. Due to concerns for autoimmune encephalitis, a lumbar puncture was performed. Antibody testing revealed PCA-Tr antibodies, which is commonly associated with Hodgkin lymphoma, prompting further evaluation for malignancy.
Computed tomography (CT) of the chest with contrast demonstrated multiple mediastinal masses with a conglomeration of lymph nodes along the right paratracheal region. Further evaluation was performed with a positron emission tomography (PET)–CT, revealing a large conglomeration of hypermetabolic pretracheal, mediastinal, and right supraclavicular lymph that were suggestive of lymphoma. Mediastinoscopy with excisional lymph node biopsy was performed with immunohistochemical staining confirming diagnosis of a nodular sclerosing variant of Hodgkin lymphoma. The patient was treated with IV immunoglobulin at 0.4g/kg daily for 5 days. A central venous catheter was placed into the patient’s right internal jugular vein and a chemotherapy regimen of doxorubicin 46 mg, vinblastine 11 mg, bleomycin 19 units, and dacarbazine 700 mg was initiated. The patient’s symptoms improved with resolution of dysarthria; however, her visual impairment and gait instability persisted. Repeat PET-CT imaging 2 months later revealed interval improvement with decreased intensity and extent of the hypermetabolic lymph nodes and no new hypermetabolic foci.
Discussion
PCA-Tr antibodies affect the delta/notchlike epidermal growth factor–related receptor, expressed on the dendrites of cerebellar Purkinje cells.1 These fibers are the only output neurons of the cerebellar cortex and are critical to the coordination of motor movements, accounting for the ataxia experienced by patients with this subtype of PCD.2 The link between Hodgkin lymphoma and PCA-Tr antibodies has been established; however, most reports involve men with a median age of 61 years with lymphoma-associated symptoms (such as lymphadenopathy) or systemic symptoms (fever, night sweats, or weight loss) preceding neurologic manifestations in 80% of cases.3
Our patient was a young, previously healthy adult female who initially presented with vertigo, a common concern with frequently benign origins. Although there was temporary resolution of symptoms after Epley maneuvers, symptoms recurred and progressed over several months to include brainstem manifestations of nystagmus, diplopia, and dysarthria. Previous reports indicate that after remission of the Hodgkin lymphoma, PCA-Tr antibodies disappear and symptoms can improve or resolve.4,5 Treatment has just begun for our patient and although there has been initial clinical improvement, given the chronicity of symptoms, it is unclear if complete resolution will be achieved.
Conclusions
PCD can result in debilitating neurologic dysfunction and may be associated with malignancy such as Hodgkin lymphoma. This case offers unique insight due to the patient’s demographics and presentation, which involved brainstem pathology typically associated with late-onset disease and preceded by constitutional symptoms. Clinical suspicion of this rare disorder should be considered in all ages, especially if symptoms are progressive or neurologic manifestations arise, as early detection and treatment of the underlying malignancy are paramount to the prevention of significant disability.
1. de Graaff E, Maat P, Hulsenboom E, et al. Identification of delta/notch-like epidermal growth factor-related receptor as the Tr antigen in paraneoplastic cerebellar degeneration. Ann Neurol. 2012;71(6):815-824. doi:10.1002/ana.23550
2. MacKenzie-Graham A, Tiwari-Woodruff SK, Sharma G, et al. Purkinje cell loss in experimental autoimmune encephalomyelitis. Neuroimage. 2009;48(4):637-651. doi:10.1016/j.neuroimage.2009.06.073
3. Bernal F, Shams’ili S, Rojas I, et al. Anti-Tr antibodies as markers of paraneoplastic cerebellar degeneration and Hodgkin’s disease. Neurology. 2003;60(2):230-234. doi:10.1212/01.wnl.0000041495.87539.98
4. Graus F, Ariño H, Dalmau J. Paraneoplastic neurological syndromes in Hodgkin and non-Hodgkin lymphomas. Blood. 2014;123(21):3230-3238. doi:10.1182/blood-2014-03-537506
5. Aly R, Emmady PD. Paraneoplastic cerebellar degeneration. Updated May 8, 2022. Accessed March 30, 2022. https://www.ncbi.nlm.nih.gov/books/NBK560638
1. de Graaff E, Maat P, Hulsenboom E, et al. Identification of delta/notch-like epidermal growth factor-related receptor as the Tr antigen in paraneoplastic cerebellar degeneration. Ann Neurol. 2012;71(6):815-824. doi:10.1002/ana.23550
2. MacKenzie-Graham A, Tiwari-Woodruff SK, Sharma G, et al. Purkinje cell loss in experimental autoimmune encephalomyelitis. Neuroimage. 2009;48(4):637-651. doi:10.1016/j.neuroimage.2009.06.073
3. Bernal F, Shams’ili S, Rojas I, et al. Anti-Tr antibodies as markers of paraneoplastic cerebellar degeneration and Hodgkin’s disease. Neurology. 2003;60(2):230-234. doi:10.1212/01.wnl.0000041495.87539.98
4. Graus F, Ariño H, Dalmau J. Paraneoplastic neurological syndromes in Hodgkin and non-Hodgkin lymphomas. Blood. 2014;123(21):3230-3238. doi:10.1182/blood-2014-03-537506
5. Aly R, Emmady PD. Paraneoplastic cerebellar degeneration. Updated May 8, 2022. Accessed March 30, 2022. https://www.ncbi.nlm.nih.gov/books/NBK560638
Cancer drug significantly cuts risk for COVID-19 death
, an interim analysis of a phase 3 placebo-controlled trial found.
Sabizabulin treatment consistently and significantly reduced deaths across patient subgroups “regardless of standard of care treatment received, baseline World Health Organization scores, age, comorbidities, vaccination status, COVID-19 variant, or geography,” study investigator Mitchell Steiner, MD, chairman, president, and CEO of Veru, said in a news release.
The company has submitted an emergency use authorization request to the U.S. Food and Drug Administration to use sabizabulin to treat COVID-19.
The analysis was published online in NEJM Evidence.
Sabizabulin, originally developed to treat metastatic castration-resistant prostate cancer, is a novel, investigational, oral microtubule disruptor with dual antiviral and anti-inflammatory activities. Given the drug’s mechanism, researchers at Veru thought that sabizabulin could help treat lung inflammation in patients with COVID-19 as well.
Findings of the interim analysis are based on 150 adults hospitalized with moderate to severe COVID-19 at high risk for acute respiratory distress syndrome and death. The patients were randomly allocated to receive 9 mg oral sabizabulin (n = 98) or placebo (n = 52) once daily for up to 21 days.
Overall, the mortality rate was 20.2% in the sabizabulin group vs. 45.1% in the placebo group. Compared with placebo, treatment with sabizabulin led to a 24.9–percentage point absolute reduction and a 55.2% relative reduction in death (odds ratio, 3.23; P = .0042).
The key secondary endpoint of mortality through day 29 also favored sabizabulin over placebo, with a mortality rate of 17% vs. 35.3%. In this scenario, treatment with sabizabulin resulted in an absolute reduction in deaths of 18.3 percentage points and a relative reduction of 51.8%.
Sabizabulin led to a significant 43% relative reduction in ICU days, a 49% relative reduction in days on mechanical ventilation, and a 26% relative reduction in days in the hospital, compared with placebo.
Adverse and serious adverse events were also lower in the sabizabulin group (61.5%) than the placebo group (78.3%).
The data are “pretty impressive and in a group of patients that we really have limited things to offer,” Aaron Glatt, MD, a spokesperson for the Infectious Diseases Society of America and chief of infectious diseases and hospital epidemiologist at Mount Sinai South Nassau in Oceanside, N.Y., said in an interview. “This is an interim analysis and obviously we’d like to see more data, but it certainly is something that is novel and quite interesting.”
David Boulware, MD, MPH, an infectious disease expert at the University of Minnesota, Minneapolis, told the New York Times that the large number of deaths in the placebo group seemed “rather high” and that the final analysis might reveal a more modest benefit for sabizabulin.
“I would be skeptical” that the reduced risk for death remains 55%, he noted.
The study was funded by Veru Pharmaceuticals. Several authors are employed by the company or have financial relationships with the company.
A version of this article first appeared on Medscape.com.
, an interim analysis of a phase 3 placebo-controlled trial found.
Sabizabulin treatment consistently and significantly reduced deaths across patient subgroups “regardless of standard of care treatment received, baseline World Health Organization scores, age, comorbidities, vaccination status, COVID-19 variant, or geography,” study investigator Mitchell Steiner, MD, chairman, president, and CEO of Veru, said in a news release.
The company has submitted an emergency use authorization request to the U.S. Food and Drug Administration to use sabizabulin to treat COVID-19.
The analysis was published online in NEJM Evidence.
Sabizabulin, originally developed to treat metastatic castration-resistant prostate cancer, is a novel, investigational, oral microtubule disruptor with dual antiviral and anti-inflammatory activities. Given the drug’s mechanism, researchers at Veru thought that sabizabulin could help treat lung inflammation in patients with COVID-19 as well.
Findings of the interim analysis are based on 150 adults hospitalized with moderate to severe COVID-19 at high risk for acute respiratory distress syndrome and death. The patients were randomly allocated to receive 9 mg oral sabizabulin (n = 98) or placebo (n = 52) once daily for up to 21 days.
Overall, the mortality rate was 20.2% in the sabizabulin group vs. 45.1% in the placebo group. Compared with placebo, treatment with sabizabulin led to a 24.9–percentage point absolute reduction and a 55.2% relative reduction in death (odds ratio, 3.23; P = .0042).
The key secondary endpoint of mortality through day 29 also favored sabizabulin over placebo, with a mortality rate of 17% vs. 35.3%. In this scenario, treatment with sabizabulin resulted in an absolute reduction in deaths of 18.3 percentage points and a relative reduction of 51.8%.
Sabizabulin led to a significant 43% relative reduction in ICU days, a 49% relative reduction in days on mechanical ventilation, and a 26% relative reduction in days in the hospital, compared with placebo.
Adverse and serious adverse events were also lower in the sabizabulin group (61.5%) than the placebo group (78.3%).
The data are “pretty impressive and in a group of patients that we really have limited things to offer,” Aaron Glatt, MD, a spokesperson for the Infectious Diseases Society of America and chief of infectious diseases and hospital epidemiologist at Mount Sinai South Nassau in Oceanside, N.Y., said in an interview. “This is an interim analysis and obviously we’d like to see more data, but it certainly is something that is novel and quite interesting.”
David Boulware, MD, MPH, an infectious disease expert at the University of Minnesota, Minneapolis, told the New York Times that the large number of deaths in the placebo group seemed “rather high” and that the final analysis might reveal a more modest benefit for sabizabulin.
“I would be skeptical” that the reduced risk for death remains 55%, he noted.
The study was funded by Veru Pharmaceuticals. Several authors are employed by the company or have financial relationships with the company.
A version of this article first appeared on Medscape.com.
, an interim analysis of a phase 3 placebo-controlled trial found.
Sabizabulin treatment consistently and significantly reduced deaths across patient subgroups “regardless of standard of care treatment received, baseline World Health Organization scores, age, comorbidities, vaccination status, COVID-19 variant, or geography,” study investigator Mitchell Steiner, MD, chairman, president, and CEO of Veru, said in a news release.
The company has submitted an emergency use authorization request to the U.S. Food and Drug Administration to use sabizabulin to treat COVID-19.
The analysis was published online in NEJM Evidence.
Sabizabulin, originally developed to treat metastatic castration-resistant prostate cancer, is a novel, investigational, oral microtubule disruptor with dual antiviral and anti-inflammatory activities. Given the drug’s mechanism, researchers at Veru thought that sabizabulin could help treat lung inflammation in patients with COVID-19 as well.
Findings of the interim analysis are based on 150 adults hospitalized with moderate to severe COVID-19 at high risk for acute respiratory distress syndrome and death. The patients were randomly allocated to receive 9 mg oral sabizabulin (n = 98) or placebo (n = 52) once daily for up to 21 days.
Overall, the mortality rate was 20.2% in the sabizabulin group vs. 45.1% in the placebo group. Compared with placebo, treatment with sabizabulin led to a 24.9–percentage point absolute reduction and a 55.2% relative reduction in death (odds ratio, 3.23; P = .0042).
The key secondary endpoint of mortality through day 29 also favored sabizabulin over placebo, with a mortality rate of 17% vs. 35.3%. In this scenario, treatment with sabizabulin resulted in an absolute reduction in deaths of 18.3 percentage points and a relative reduction of 51.8%.
Sabizabulin led to a significant 43% relative reduction in ICU days, a 49% relative reduction in days on mechanical ventilation, and a 26% relative reduction in days in the hospital, compared with placebo.
Adverse and serious adverse events were also lower in the sabizabulin group (61.5%) than the placebo group (78.3%).
The data are “pretty impressive and in a group of patients that we really have limited things to offer,” Aaron Glatt, MD, a spokesperson for the Infectious Diseases Society of America and chief of infectious diseases and hospital epidemiologist at Mount Sinai South Nassau in Oceanside, N.Y., said in an interview. “This is an interim analysis and obviously we’d like to see more data, but it certainly is something that is novel and quite interesting.”
David Boulware, MD, MPH, an infectious disease expert at the University of Minnesota, Minneapolis, told the New York Times that the large number of deaths in the placebo group seemed “rather high” and that the final analysis might reveal a more modest benefit for sabizabulin.
“I would be skeptical” that the reduced risk for death remains 55%, he noted.
The study was funded by Veru Pharmaceuticals. Several authors are employed by the company or have financial relationships with the company.
A version of this article first appeared on Medscape.com.
FROM NEJM EVIDENCE
Sleep-deprived physicians less empathetic to patient pain?
new research suggests.
In the first of two studies, resident physicians were presented with two hypothetical scenarios involving a patient who complains of pain. They were asked about their likelihood of prescribing pain medication. The test was given to one group of residents who were just starting their day and to another group who were at the end of their night shift after being on call for 26 hours.
Results showed that the night shift residents were less likely than their daytime counterparts to say they would prescribe pain medication to the patients.
In further analysis of discharge notes from more than 13,000 electronic records of patients presenting with pain complaints at hospitals in Israel and the United States, the likelihood of an analgesic being prescribed during the night shift was 11% lower in Israel and 9% lower in the United States, compared with the day shift.
“Pain management is a major challenge, and a doctor’s perception of a patient’s subjective pain is susceptible to bias,” coinvestigator David Gozal, MD, the Marie M. and Harry L. Smith Endowed Chair of Child Health, University of Missouri–Columbia, said in a press release.
“This study demonstrated that night shift work is an important and previously unrecognized source of bias in pain management, likely stemming from impaired perception of pain,” Dr. Gozal added.
The findings were published online in the Proceedings of the National Academy of Sciences.
‘Directional’ differences
Senior investigator Alex Gileles-Hillel, MD, senior pediatric pulmonologist and sleep researcher at Hadassah University Medical Center, Jerusalem, said in an interview that physicians must make “complex assessments of patients’ subjective pain experience” – and the “subjective nature of pain management decisions can give rise to various biases.”
Dr. Gileles-Hillel has previously researched the cognitive toll of night shift work on physicians.
“It’s pretty established, for example, not to drive when sleep deprived because cognition is impaired,” he said. The current study explored whether sleep deprivation could affect areas other than cognition, including emotions and empathy.
The researchers used “two complementary approaches.” First, they administered tests to measure empathy and pain management decisions in 67 resident physicians at Hadassah Medical Centers either following a 26-hour night shift that began at 8:00 a.m. the day before (n = 36) or immediately before starting the workday (n = 31).
There were no significant differences in demographic, sleep, or burnout measures between the two groups, except that night shift physicians had slept less than those in the daytime group (2.93 vs. 5.96 hours).
Participants completed two tasks. In the empathy-for-pain task, they rated their emotional reactions to pictures of individuals in pain. In the empathy accuracy task, they were asked to assess the feelings of videotaped individuals telling emotional stories.
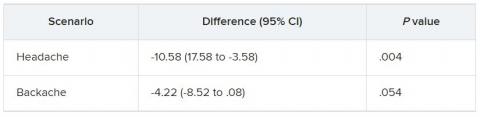
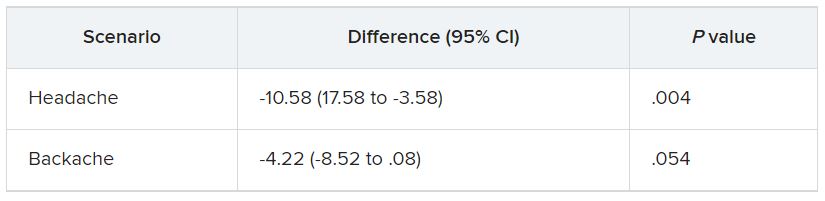
They were then presented with two clinical scenarios: a female patient with a headache and a male patient with a backache. Following that, they were asked to assess the magnitude of the patients’ pain and how likely they would be to prescribe pain medication.
In the empathy-for-pain task, physicians’ empathy scores were significantly lower in the night shift group than in the day group (difference, –0.83; 95% CI, –1.55 to –0.10; P = .026). There were no significant differences between the groups in the empathy accuracy task.
In both scenarios, physicians in the night shift group assessed the patient’s pain as weaker in comparison with physicians in the day group. There was a statistically significant difference in the headache scenario but not the backache scenario.
In the headache scenario, the propensity of the physicians to prescribe analgesics was “directionally lower” but did not reach statistical significance. In the backache scenario, there was no significant difference between the groups’ prescribing propensities.
In both scenarios, pain assessment was positively correlated with the propensity to prescribe analgesics.
Despite the lack of statistical significance, the findings “documented a negative effect of night shift work on physician empathy for pain and a positive association between physician assessment of patient pain and the propensity to prescribe analgesics,” the investigators wrote.
Need for naps?
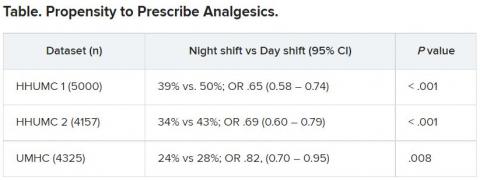
The researchers then analyzed analgesic prescription patterns drawn from three datasets of discharge notes of patients presenting to the emergency department with pain complaints (n = 13,482) at two branches of Hadassah-Hebrew University Medical Center and the University of Missouri Health Center.
The researchers collected data, including discharge time, medications patients were prescribed upon discharge, and patients’ subjective pain rating on a scale of 0-10 on a visual analogue scale (VAS).
Although patients’ VAS scores did not differ with respect to time or shift, patients were discharged with significantly less prescribed analgesics during the night shift in comparison with the day shift.
No similar differences in prescriptions between night shifts and day shifts were found for nonanalgesic medications, such as for diabetes or blood pressure. This suggests “the effect was specific to pain,” Dr. Gileles-Hillel said.
The pattern remained significant after controlling for potential confounders, including patient and physician variables and emergency department characteristics.
In addition, patients seen during night shifts received fewer analgesics, particularly opioids, than recommended by the World Health Organization for pain management.
“The first study enabled us to measure empathy for pain directly and examine our hypothesis in a controlled environment, while the second enabled us to test the implications by examining real-life pain management decisions,” Dr. Gileles-Hillel said.
“Physicians need to be aware of this,” he noted. “I try to be aware when I’m taking calls [at night] that I’m less empathetic to others and I might be more brief or angry with others.”
On a “house management level, perhaps institutions should try to schedule naps either before or during overnight call. A nap might give a boost and reboot not only to cognitive but also to emotional resources,” Dr. Gileles-Hillel added.
Compromised safety
In a comment, Eti Ben Simon, PhD, a postdoctoral fellow at the Center for Human Sleep Science, University of California, Berkeley, called the study “an important contribution to a growing list of studies that reveal how long night shifts reduce overall safety” for both patients and clinicians.
“It’s time to abandon the notion that the human brain can function as normal after being deprived of sleep for 24 hours,” said Dr. Ben Simon, who was not involved with the research.
“This is especially true in medicine, where we trust others to take care of us and feel our pain. These functions are simply not possible without adequate sleep,” she added.
Also commenting, Kannan Ramar, MD, president of the American Academy of Sleep Medicine, suggested that being cognizant of these findings “may help providers to mitigate this bias” of underprescribing pain medications when treating their patients.
Dr. Ramar, who is also a critical care specialist, pulmonologist, and sleep medicine specialist at Mayo Clinic, Rochester, Minn., was not involved with the research.
He noted that “further studies that systematically evaluate this further in a prospective and blinded way will be important.”
The research was supported in part by grants from the Israel Science Foundation, Joy Ventures, the Recanati Fund at the Jerusalem School of Business at the Hebrew University, and a fellowship from the Azrieli Foundation and received grant support to various investigators from the NIH, the Leda J. Sears Foundation, and the University of Missouri. The investigators, Ramar, and Ben Simon have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
new research suggests.
In the first of two studies, resident physicians were presented with two hypothetical scenarios involving a patient who complains of pain. They were asked about their likelihood of prescribing pain medication. The test was given to one group of residents who were just starting their day and to another group who were at the end of their night shift after being on call for 26 hours.
Results showed that the night shift residents were less likely than their daytime counterparts to say they would prescribe pain medication to the patients.
In further analysis of discharge notes from more than 13,000 electronic records of patients presenting with pain complaints at hospitals in Israel and the United States, the likelihood of an analgesic being prescribed during the night shift was 11% lower in Israel and 9% lower in the United States, compared with the day shift.
“Pain management is a major challenge, and a doctor’s perception of a patient’s subjective pain is susceptible to bias,” coinvestigator David Gozal, MD, the Marie M. and Harry L. Smith Endowed Chair of Child Health, University of Missouri–Columbia, said in a press release.
“This study demonstrated that night shift work is an important and previously unrecognized source of bias in pain management, likely stemming from impaired perception of pain,” Dr. Gozal added.
The findings were published online in the Proceedings of the National Academy of Sciences.
‘Directional’ differences
Senior investigator Alex Gileles-Hillel, MD, senior pediatric pulmonologist and sleep researcher at Hadassah University Medical Center, Jerusalem, said in an interview that physicians must make “complex assessments of patients’ subjective pain experience” – and the “subjective nature of pain management decisions can give rise to various biases.”
Dr. Gileles-Hillel has previously researched the cognitive toll of night shift work on physicians.
“It’s pretty established, for example, not to drive when sleep deprived because cognition is impaired,” he said. The current study explored whether sleep deprivation could affect areas other than cognition, including emotions and empathy.
The researchers used “two complementary approaches.” First, they administered tests to measure empathy and pain management decisions in 67 resident physicians at Hadassah Medical Centers either following a 26-hour night shift that began at 8:00 a.m. the day before (n = 36) or immediately before starting the workday (n = 31).
There were no significant differences in demographic, sleep, or burnout measures between the two groups, except that night shift physicians had slept less than those in the daytime group (2.93 vs. 5.96 hours).
Participants completed two tasks. In the empathy-for-pain task, they rated their emotional reactions to pictures of individuals in pain. In the empathy accuracy task, they were asked to assess the feelings of videotaped individuals telling emotional stories.
They were then presented with two clinical scenarios: a female patient with a headache and a male patient with a backache. Following that, they were asked to assess the magnitude of the patients’ pain and how likely they would be to prescribe pain medication.
In the empathy-for-pain task, physicians’ empathy scores were significantly lower in the night shift group than in the day group (difference, –0.83; 95% CI, –1.55 to –0.10; P = .026). There were no significant differences between the groups in the empathy accuracy task.
In both scenarios, physicians in the night shift group assessed the patient’s pain as weaker in comparison with physicians in the day group. There was a statistically significant difference in the headache scenario but not the backache scenario.
In the headache scenario, the propensity of the physicians to prescribe analgesics was “directionally lower” but did not reach statistical significance. In the backache scenario, there was no significant difference between the groups’ prescribing propensities.
In both scenarios, pain assessment was positively correlated with the propensity to prescribe analgesics.
Despite the lack of statistical significance, the findings “documented a negative effect of night shift work on physician empathy for pain and a positive association between physician assessment of patient pain and the propensity to prescribe analgesics,” the investigators wrote.
Need for naps?
The researchers then analyzed analgesic prescription patterns drawn from three datasets of discharge notes of patients presenting to the emergency department with pain complaints (n = 13,482) at two branches of Hadassah-Hebrew University Medical Center and the University of Missouri Health Center.
The researchers collected data, including discharge time, medications patients were prescribed upon discharge, and patients’ subjective pain rating on a scale of 0-10 on a visual analogue scale (VAS).
Although patients’ VAS scores did not differ with respect to time or shift, patients were discharged with significantly less prescribed analgesics during the night shift in comparison with the day shift.
No similar differences in prescriptions between night shifts and day shifts were found for nonanalgesic medications, such as for diabetes or blood pressure. This suggests “the effect was specific to pain,” Dr. Gileles-Hillel said.
The pattern remained significant after controlling for potential confounders, including patient and physician variables and emergency department characteristics.
In addition, patients seen during night shifts received fewer analgesics, particularly opioids, than recommended by the World Health Organization for pain management.
“The first study enabled us to measure empathy for pain directly and examine our hypothesis in a controlled environment, while the second enabled us to test the implications by examining real-life pain management decisions,” Dr. Gileles-Hillel said.
“Physicians need to be aware of this,” he noted. “I try to be aware when I’m taking calls [at night] that I’m less empathetic to others and I might be more brief or angry with others.”
On a “house management level, perhaps institutions should try to schedule naps either before or during overnight call. A nap might give a boost and reboot not only to cognitive but also to emotional resources,” Dr. Gileles-Hillel added.
Compromised safety
In a comment, Eti Ben Simon, PhD, a postdoctoral fellow at the Center for Human Sleep Science, University of California, Berkeley, called the study “an important contribution to a growing list of studies that reveal how long night shifts reduce overall safety” for both patients and clinicians.
“It’s time to abandon the notion that the human brain can function as normal after being deprived of sleep for 24 hours,” said Dr. Ben Simon, who was not involved with the research.
“This is especially true in medicine, where we trust others to take care of us and feel our pain. These functions are simply not possible without adequate sleep,” she added.
Also commenting, Kannan Ramar, MD, president of the American Academy of Sleep Medicine, suggested that being cognizant of these findings “may help providers to mitigate this bias” of underprescribing pain medications when treating their patients.
Dr. Ramar, who is also a critical care specialist, pulmonologist, and sleep medicine specialist at Mayo Clinic, Rochester, Minn., was not involved with the research.
He noted that “further studies that systematically evaluate this further in a prospective and blinded way will be important.”
The research was supported in part by grants from the Israel Science Foundation, Joy Ventures, the Recanati Fund at the Jerusalem School of Business at the Hebrew University, and a fellowship from the Azrieli Foundation and received grant support to various investigators from the NIH, the Leda J. Sears Foundation, and the University of Missouri. The investigators, Ramar, and Ben Simon have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
new research suggests.
In the first of two studies, resident physicians were presented with two hypothetical scenarios involving a patient who complains of pain. They were asked about their likelihood of prescribing pain medication. The test was given to one group of residents who were just starting their day and to another group who were at the end of their night shift after being on call for 26 hours.
Results showed that the night shift residents were less likely than their daytime counterparts to say they would prescribe pain medication to the patients.
In further analysis of discharge notes from more than 13,000 electronic records of patients presenting with pain complaints at hospitals in Israel and the United States, the likelihood of an analgesic being prescribed during the night shift was 11% lower in Israel and 9% lower in the United States, compared with the day shift.
“Pain management is a major challenge, and a doctor’s perception of a patient’s subjective pain is susceptible to bias,” coinvestigator David Gozal, MD, the Marie M. and Harry L. Smith Endowed Chair of Child Health, University of Missouri–Columbia, said in a press release.
“This study demonstrated that night shift work is an important and previously unrecognized source of bias in pain management, likely stemming from impaired perception of pain,” Dr. Gozal added.
The findings were published online in the Proceedings of the National Academy of Sciences.
‘Directional’ differences
Senior investigator Alex Gileles-Hillel, MD, senior pediatric pulmonologist and sleep researcher at Hadassah University Medical Center, Jerusalem, said in an interview that physicians must make “complex assessments of patients’ subjective pain experience” – and the “subjective nature of pain management decisions can give rise to various biases.”
Dr. Gileles-Hillel has previously researched the cognitive toll of night shift work on physicians.
“It’s pretty established, for example, not to drive when sleep deprived because cognition is impaired,” he said. The current study explored whether sleep deprivation could affect areas other than cognition, including emotions and empathy.
The researchers used “two complementary approaches.” First, they administered tests to measure empathy and pain management decisions in 67 resident physicians at Hadassah Medical Centers either following a 26-hour night shift that began at 8:00 a.m. the day before (n = 36) or immediately before starting the workday (n = 31).
There were no significant differences in demographic, sleep, or burnout measures between the two groups, except that night shift physicians had slept less than those in the daytime group (2.93 vs. 5.96 hours).
Participants completed two tasks. In the empathy-for-pain task, they rated their emotional reactions to pictures of individuals in pain. In the empathy accuracy task, they were asked to assess the feelings of videotaped individuals telling emotional stories.
They were then presented with two clinical scenarios: a female patient with a headache and a male patient with a backache. Following that, they were asked to assess the magnitude of the patients’ pain and how likely they would be to prescribe pain medication.
In the empathy-for-pain task, physicians’ empathy scores were significantly lower in the night shift group than in the day group (difference, –0.83; 95% CI, –1.55 to –0.10; P = .026). There were no significant differences between the groups in the empathy accuracy task.
In both scenarios, physicians in the night shift group assessed the patient’s pain as weaker in comparison with physicians in the day group. There was a statistically significant difference in the headache scenario but not the backache scenario.
In the headache scenario, the propensity of the physicians to prescribe analgesics was “directionally lower” but did not reach statistical significance. In the backache scenario, there was no significant difference between the groups’ prescribing propensities.
In both scenarios, pain assessment was positively correlated with the propensity to prescribe analgesics.
Despite the lack of statistical significance, the findings “documented a negative effect of night shift work on physician empathy for pain and a positive association between physician assessment of patient pain and the propensity to prescribe analgesics,” the investigators wrote.
Need for naps?
The researchers then analyzed analgesic prescription patterns drawn from three datasets of discharge notes of patients presenting to the emergency department with pain complaints (n = 13,482) at two branches of Hadassah-Hebrew University Medical Center and the University of Missouri Health Center.
The researchers collected data, including discharge time, medications patients were prescribed upon discharge, and patients’ subjective pain rating on a scale of 0-10 on a visual analogue scale (VAS).
Although patients’ VAS scores did not differ with respect to time or shift, patients were discharged with significantly less prescribed analgesics during the night shift in comparison with the day shift.
No similar differences in prescriptions between night shifts and day shifts were found for nonanalgesic medications, such as for diabetes or blood pressure. This suggests “the effect was specific to pain,” Dr. Gileles-Hillel said.
The pattern remained significant after controlling for potential confounders, including patient and physician variables and emergency department characteristics.
In addition, patients seen during night shifts received fewer analgesics, particularly opioids, than recommended by the World Health Organization for pain management.
“The first study enabled us to measure empathy for pain directly and examine our hypothesis in a controlled environment, while the second enabled us to test the implications by examining real-life pain management decisions,” Dr. Gileles-Hillel said.
“Physicians need to be aware of this,” he noted. “I try to be aware when I’m taking calls [at night] that I’m less empathetic to others and I might be more brief or angry with others.”
On a “house management level, perhaps institutions should try to schedule naps either before or during overnight call. A nap might give a boost and reboot not only to cognitive but also to emotional resources,” Dr. Gileles-Hillel added.
Compromised safety
In a comment, Eti Ben Simon, PhD, a postdoctoral fellow at the Center for Human Sleep Science, University of California, Berkeley, called the study “an important contribution to a growing list of studies that reveal how long night shifts reduce overall safety” for both patients and clinicians.
“It’s time to abandon the notion that the human brain can function as normal after being deprived of sleep for 24 hours,” said Dr. Ben Simon, who was not involved with the research.
“This is especially true in medicine, where we trust others to take care of us and feel our pain. These functions are simply not possible without adequate sleep,” she added.
Also commenting, Kannan Ramar, MD, president of the American Academy of Sleep Medicine, suggested that being cognizant of these findings “may help providers to mitigate this bias” of underprescribing pain medications when treating their patients.
Dr. Ramar, who is also a critical care specialist, pulmonologist, and sleep medicine specialist at Mayo Clinic, Rochester, Minn., was not involved with the research.
He noted that “further studies that systematically evaluate this further in a prospective and blinded way will be important.”
The research was supported in part by grants from the Israel Science Foundation, Joy Ventures, the Recanati Fund at the Jerusalem School of Business at the Hebrew University, and a fellowship from the Azrieli Foundation and received grant support to various investigators from the NIH, the Leda J. Sears Foundation, and the University of Missouri. The investigators, Ramar, and Ben Simon have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE PROCEEDINGS OF THE NATIONAL ACADEMY OF SCIENCES
‘Striking’ jump in cost of brand-name epilepsy meds
, a new analysis shows.
After adjustment for inflation, the cost of a 1-year supply of brand-name ASMs grew 277%, while generics became 42% less expensive.
“Our study makes transparent striking trends in brand name prescribing patterns,” the study team wrote.
Since 2010, the costs for brand-name ASMs have “consistently” increased. Costs were particularly boosted by increases in prescriptions for lacosamide (Vimpat), in addition to a “steep increase in the cost per pill, with brand-name drugs costing 10 times more than their generic counterparts,” first author Samuel Waller Terman, MD, of the University of Michigan, Ann Arbor, added in a news release.
The study was published online in Neurology.
Is a 10-fold increase in cost worth it?
To evaluate trends in ASM prescriptions and costs, the researchers used a random sample of 20% of Medicare beneficiaries with coverage from 2008 to 2018. There were 77,000 to 133,000 patients with epilepsy each year.
Over time, likely because of increasing availability of generics, brand-name ASMs made up a smaller proportion of pills prescribed, from 56% in 2008 to 14% in 2018, but still made up 79% of prescription drug costs in 2018.
The annual cost of brand-name ASMs rose from $2,800 in 2008 to $10,700 in 2018, while the cost of generic drugs decreased from $800 to $460 during that time.
An increased number of prescriptions for lacosamide was responsible for 45% of the total increase in brand-name costs.
As of 2018, lacosamide comprised 30% of all brand-name pill supply (followed by pregabalin, at 15%) and 30% of all brand-name costs (followed by clobazam and pregabalin, both at 9%), the investigators reported.
Brand-name antiepileptic drug costs decreased from 2008 to 2010, but after the introduction of lacosamide, total brand-name costs steadily rose from $72 million in 2010 (in 2018 dollars) to $256 million in 2018, they noted.
Because the dataset consists of a 20% random Medicare sample, total Medicare costs for brand-name ASMs for beneficiaries with epilepsy alone likely rose from roughly $360 million in 2010 to $1.3 billion in 2018, they added.
“Clinicians must remain cognizant of this societal cost magnitude when judging whether the 10-fold increased expense per pill for brand name medications is worth the possible benefits,” they wrote.
“While newer-generation drugs have potential advantages such as limited drug interactions and different side effect profiles, there have been conflicting studies on whether they are cost effective,” Dr. Terman noted in a news release.
A barrier to treatment
The authors of an accompanying editorial propose that the problem of prescription drug costs could be solved through a combination of competition and government regulation of prices. Patients and physicians are the most important stakeholders in this issue.
“When something represents 14% of the total use, but contributes 79% of the cost, it would be wise to consider alternatives, assuming that these alternatives are not of lower quality,” wrote Wyatt Bensken, with Case Western Reserve University, Cleveland, and Iván Sánchez Fernández, MD, with Boston Medical Center.
“When there are several ASMs with a similar mechanism of action, similar efficacy, similar safety and tolerability profile, and different costs, it would be unwise to choose the more expensive alternative just because it is newer,” they said.
This study, they added, provides data to “understand, and begin to act, on the challenging problem of the cost of prescription ASMs. After all, what is the point of having a large number of ASMs if their cost severely limits their use?”
A limitation of the study is that only Medicare prescription claims were included, so the results may not apply to younger patients with private insurance.
The study received no direct funding. The authors and editorialists have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, a new analysis shows.
After adjustment for inflation, the cost of a 1-year supply of brand-name ASMs grew 277%, while generics became 42% less expensive.
“Our study makes transparent striking trends in brand name prescribing patterns,” the study team wrote.
Since 2010, the costs for brand-name ASMs have “consistently” increased. Costs were particularly boosted by increases in prescriptions for lacosamide (Vimpat), in addition to a “steep increase in the cost per pill, with brand-name drugs costing 10 times more than their generic counterparts,” first author Samuel Waller Terman, MD, of the University of Michigan, Ann Arbor, added in a news release.
The study was published online in Neurology.
Is a 10-fold increase in cost worth it?
To evaluate trends in ASM prescriptions and costs, the researchers used a random sample of 20% of Medicare beneficiaries with coverage from 2008 to 2018. There were 77,000 to 133,000 patients with epilepsy each year.
Over time, likely because of increasing availability of generics, brand-name ASMs made up a smaller proportion of pills prescribed, from 56% in 2008 to 14% in 2018, but still made up 79% of prescription drug costs in 2018.
The annual cost of brand-name ASMs rose from $2,800 in 2008 to $10,700 in 2018, while the cost of generic drugs decreased from $800 to $460 during that time.
An increased number of prescriptions for lacosamide was responsible for 45% of the total increase in brand-name costs.
As of 2018, lacosamide comprised 30% of all brand-name pill supply (followed by pregabalin, at 15%) and 30% of all brand-name costs (followed by clobazam and pregabalin, both at 9%), the investigators reported.
Brand-name antiepileptic drug costs decreased from 2008 to 2010, but after the introduction of lacosamide, total brand-name costs steadily rose from $72 million in 2010 (in 2018 dollars) to $256 million in 2018, they noted.
Because the dataset consists of a 20% random Medicare sample, total Medicare costs for brand-name ASMs for beneficiaries with epilepsy alone likely rose from roughly $360 million in 2010 to $1.3 billion in 2018, they added.
“Clinicians must remain cognizant of this societal cost magnitude when judging whether the 10-fold increased expense per pill for brand name medications is worth the possible benefits,” they wrote.
“While newer-generation drugs have potential advantages such as limited drug interactions and different side effect profiles, there have been conflicting studies on whether they are cost effective,” Dr. Terman noted in a news release.
A barrier to treatment
The authors of an accompanying editorial propose that the problem of prescription drug costs could be solved through a combination of competition and government regulation of prices. Patients and physicians are the most important stakeholders in this issue.
“When something represents 14% of the total use, but contributes 79% of the cost, it would be wise to consider alternatives, assuming that these alternatives are not of lower quality,” wrote Wyatt Bensken, with Case Western Reserve University, Cleveland, and Iván Sánchez Fernández, MD, with Boston Medical Center.
“When there are several ASMs with a similar mechanism of action, similar efficacy, similar safety and tolerability profile, and different costs, it would be unwise to choose the more expensive alternative just because it is newer,” they said.
This study, they added, provides data to “understand, and begin to act, on the challenging problem of the cost of prescription ASMs. After all, what is the point of having a large number of ASMs if their cost severely limits their use?”
A limitation of the study is that only Medicare prescription claims were included, so the results may not apply to younger patients with private insurance.
The study received no direct funding. The authors and editorialists have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, a new analysis shows.
After adjustment for inflation, the cost of a 1-year supply of brand-name ASMs grew 277%, while generics became 42% less expensive.
“Our study makes transparent striking trends in brand name prescribing patterns,” the study team wrote.
Since 2010, the costs for brand-name ASMs have “consistently” increased. Costs were particularly boosted by increases in prescriptions for lacosamide (Vimpat), in addition to a “steep increase in the cost per pill, with brand-name drugs costing 10 times more than their generic counterparts,” first author Samuel Waller Terman, MD, of the University of Michigan, Ann Arbor, added in a news release.
The study was published online in Neurology.
Is a 10-fold increase in cost worth it?
To evaluate trends in ASM prescriptions and costs, the researchers used a random sample of 20% of Medicare beneficiaries with coverage from 2008 to 2018. There were 77,000 to 133,000 patients with epilepsy each year.
Over time, likely because of increasing availability of generics, brand-name ASMs made up a smaller proportion of pills prescribed, from 56% in 2008 to 14% in 2018, but still made up 79% of prescription drug costs in 2018.
The annual cost of brand-name ASMs rose from $2,800 in 2008 to $10,700 in 2018, while the cost of generic drugs decreased from $800 to $460 during that time.
An increased number of prescriptions for lacosamide was responsible for 45% of the total increase in brand-name costs.
As of 2018, lacosamide comprised 30% of all brand-name pill supply (followed by pregabalin, at 15%) and 30% of all brand-name costs (followed by clobazam and pregabalin, both at 9%), the investigators reported.
Brand-name antiepileptic drug costs decreased from 2008 to 2010, but after the introduction of lacosamide, total brand-name costs steadily rose from $72 million in 2010 (in 2018 dollars) to $256 million in 2018, they noted.
Because the dataset consists of a 20% random Medicare sample, total Medicare costs for brand-name ASMs for beneficiaries with epilepsy alone likely rose from roughly $360 million in 2010 to $1.3 billion in 2018, they added.
“Clinicians must remain cognizant of this societal cost magnitude when judging whether the 10-fold increased expense per pill for brand name medications is worth the possible benefits,” they wrote.
“While newer-generation drugs have potential advantages such as limited drug interactions and different side effect profiles, there have been conflicting studies on whether they are cost effective,” Dr. Terman noted in a news release.
A barrier to treatment
The authors of an accompanying editorial propose that the problem of prescription drug costs could be solved through a combination of competition and government regulation of prices. Patients and physicians are the most important stakeholders in this issue.
“When something represents 14% of the total use, but contributes 79% of the cost, it would be wise to consider alternatives, assuming that these alternatives are not of lower quality,” wrote Wyatt Bensken, with Case Western Reserve University, Cleveland, and Iván Sánchez Fernández, MD, with Boston Medical Center.
“When there are several ASMs with a similar mechanism of action, similar efficacy, similar safety and tolerability profile, and different costs, it would be unwise to choose the more expensive alternative just because it is newer,” they said.
This study, they added, provides data to “understand, and begin to act, on the challenging problem of the cost of prescription ASMs. After all, what is the point of having a large number of ASMs if their cost severely limits their use?”
A limitation of the study is that only Medicare prescription claims were included, so the results may not apply to younger patients with private insurance.
The study received no direct funding. The authors and editorialists have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
If nuclear disaster strikes, U.S. hematologists stand ready
For many Americans – especially those too young to know much about the Cold War or Hiroshima – Russia’s invasion of Ukraine might mark the first time they’ve truly considered the dangers of nuclear weapons. But dozens of hematologists in the United States already know the drill and have placed themselves on the front lines. These physicians stand prepared to treat patients exposed to radiation caused by nuclear accidents or attacks on U.S. soil.
They work nationwide at 74 medical centers that make up the Radiation Injury Treatment Network, ready to manage cases of acute radiation syndrome (ARS) during disasters. While RITN keeps a low profile, it’s been in the news lately amid anxieties about the Ukraine conflict, nuclear plant accidents, and the potential launching of nuclear weapons by foreign adversaries.
“The Radiation Injury Treatment Network helps plan responses for disaster scenarios where a person’s cells would be damaged after having been exposed to ionizing radiation,” program director Cullen Case Jr., MPA, said in an interview.
A U.S. Army veteran who took part in hurricane response early in his career, Mr. Case now oversees preparedness activities among all RITN hospitals, blood donor centers, and cord blood banks, in readiness for a mass casualty radiological incident. He also serves as a senior manager of the National Marrow Donor Program/Be a Match Marrow Registry.
Intense preparation for nuclear attacks or accidents is necessary, Mr. Case said, despite the doomsday scenarios disseminated on television shows and movies.
“The most frequent misconception we hear is that a nuclear disaster will encompass the whole world and be so complete that preparedness isn’t useful. However, many planning scenarios include smaller-scale incidents where survivors will need prompt and expert care,” he said.
In the wake of 9/11, the National Marrow Donor Program and the American Society for Blood and Marrow Transplantation established the RITN in 2006, with a mission to prepare for nuclear disaster and help manage the response if one occurs.
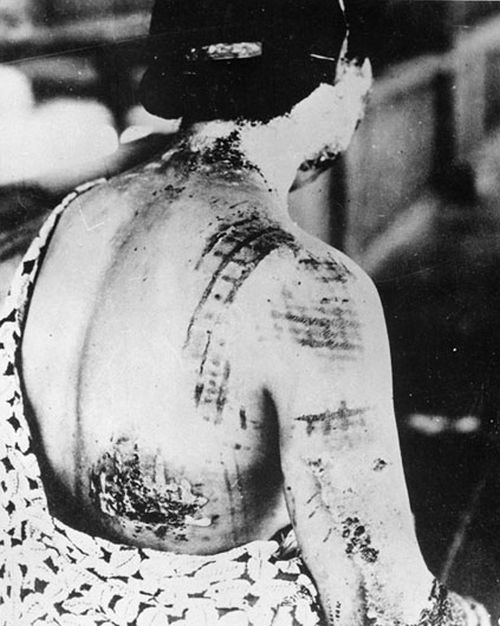
“The widespread availability of radioactive material has made future exposure events, accidental or intentional, nearly inevitable,” RITN leaders warned in a 2008 report. “Hematologists, oncologists, and HSCT [hematopoietic stem cell transplantation] physicians are uniquely suited to care for victims of radiation exposure, creating a collective responsibility to prepare for a variety of contingencies.”
RITN doesn’t just train physicians, Mr. Case noted. All medical centers within the RITN are required to conduct an annual tabletop exercise where a radiation disaster scenario and a set of discussion questions are presented to the team.
Hematologists specially equipped to treat radiation injuries
Why are hematologists involved in treating people exposed to dangerously high levels of radiation? The answer has to do with how radiation harms the body, said Dr. Ann A. Jakubowski, a hematologist/oncologist and transplant physician at Memorial Sloan Kettering Cancer Center, New York, who serves as a medical director for RITN.
“One of the most common toxicities from radiation exposure and a major player in acute radiation syndrome is hematologic toxicity– damage to the bone marrow by the radiation, with a resultant decrease in peripheral blood counts,” she said in an interview. “This is similar to what is often seen in the treatment of cancers with radiation and/or chemotherapy.”
In cases of severe and nonreversible radiation damage to the bone marrow, Dr. Jakubowski noted, “patients can be considered for a stem cell transplant to provide new healthy cells to repopulate the bone marrow, which provides recovery of peripheral blood counts. Hematologist/oncologists are the physicians who manage stem cell transplants.”
The crucial role of hematologists in radiation injuries is not new. In fact, these physicians have been closely intertwined with nuclear research since the dawn of the atomic age. The work of developing atomic bombs also led investigators to an understanding of the structure and processes of hematopoiesis and helped them to identify hematopoietic stem cells and prove their existence in humans.
Disaster response poses multiple challenges
As noted in a recent article in ASH Clinical News, the challenges of treating radiation injuries would be intense, especially in the event of a nuclear accident or attack that affects a wide area. For starters, how quickly can medical professionals be mobilized, and will there be enough physicians comfortable treating patients? Fortunately, irradiated patients should not pose a direct risk to medical professionals who treat them.
“The expectation is that the patients will all be decontaminated,” said Nelson Chao, MD, MBA, one of the founders of RITN and a hematologist/oncologist and transplant physician at Duke University, Durham, N.C.
Dr. Jakubowski questions whether there will be adequate resources to handle the influx of patients who need more intensive treatment, as well as outpatients who “received lower doses of radiation and may experience a period of low blood counts but are expected to eventually recover blood counts.”
And if many people are injured, Dr. Chao asks, how will physicians “adopt altered standards of care to treat large numbers of patients?”
There will also be a need for physicians who aren’t hematologists, Dr. Jakubowski said. “There may be many victims who have both radiation exposure and traumatic or burn injuries, which need to be addressed first, before the hematologist can start addressing the consequences of ARS. Traumatic and burn injuries will require surgical resources.”
In addition, ARS affects the gastrointestinal track and central nervous system/cardiovascular, and it has multiple stages, she noted.
“Although we have methods of supporting the hematopoietic system – transfusions and growth factors – and even replacing it with a stem cell transplant, this will not necessarily fix the badly damaged other organs, Dr. Jakubowski said. “Also, not all radioactive isotopes are equal in their effects, nor are the various types of radiation exposure.”
Training goes beyond transplants and drugs
RITN offers individual hematologists specialized education about treating radiation injuries through annual exercises, modules, and “just-in-time” training.
For example, the RITN webpage devoted to triage includes guidelines for transferring radiation injury patients, triage guidelines for cytokine administration in cases of ARS, an exposure and symptom triage tool, and more. The treatment page includes details about subjects such as when human leukocyte antigen typing of casualties is appropriate and how to keep yourself safe while treating patients.
Another focus is teaching hematologists to react quickly in disasters, Mr. Case said. “The vast majority of hematologists have little to no experience in responding to disasters and making decisions with imperfect or incomplete information, as emergency medicine practitioners must do regularly.”
“Some of the RITN tabletop exercises present physicians and advanced practitioners with an incomplete set of patient information and ask physicians to then determine and prioritize their care,” Mr. Case said. “The resulting discussions help to lay the groundwork for being able to shift to the crisis standards of care mindset that would be necessary during a radiological disaster.”
Here’s how hematologists can get involved
If you want to help improve the nation’s response to radiation injuries, Mr. Case suggests checking RITN’s list of participating hospitals. If your facility is already part of this network, he said, contact its bone marrow transplant unit for more information.
In such cases, Dr. Jakubowski suggests that you “consider periodically giving a presentation to staff on the basics of radiation injury and the center’s role in RITN.” And if you’re not part of RITN, she said, consider contacting the network about becoming a member.
Hematologists, Mr. Case said, can also take advantage of RITN’s free short overview courses, review the RITN Treatment Guidelines, or watch short videos on the RITN’s YouTube channel.
He highlighted the Radiation Emergency Medical Management website administered by the Department of Health & Human Services, the Center for Disease Control’s radiation emergencies webpage, and the Department of Energy’s Radiation Emergency Assistance Center/Training Site.
For many Americans – especially those too young to know much about the Cold War or Hiroshima – Russia’s invasion of Ukraine might mark the first time they’ve truly considered the dangers of nuclear weapons. But dozens of hematologists in the United States already know the drill and have placed themselves on the front lines. These physicians stand prepared to treat patients exposed to radiation caused by nuclear accidents or attacks on U.S. soil.
They work nationwide at 74 medical centers that make up the Radiation Injury Treatment Network, ready to manage cases of acute radiation syndrome (ARS) during disasters. While RITN keeps a low profile, it’s been in the news lately amid anxieties about the Ukraine conflict, nuclear plant accidents, and the potential launching of nuclear weapons by foreign adversaries.
“The Radiation Injury Treatment Network helps plan responses for disaster scenarios where a person’s cells would be damaged after having been exposed to ionizing radiation,” program director Cullen Case Jr., MPA, said in an interview.
A U.S. Army veteran who took part in hurricane response early in his career, Mr. Case now oversees preparedness activities among all RITN hospitals, blood donor centers, and cord blood banks, in readiness for a mass casualty radiological incident. He also serves as a senior manager of the National Marrow Donor Program/Be a Match Marrow Registry.
Intense preparation for nuclear attacks or accidents is necessary, Mr. Case said, despite the doomsday scenarios disseminated on television shows and movies.
“The most frequent misconception we hear is that a nuclear disaster will encompass the whole world and be so complete that preparedness isn’t useful. However, many planning scenarios include smaller-scale incidents where survivors will need prompt and expert care,” he said.
In the wake of 9/11, the National Marrow Donor Program and the American Society for Blood and Marrow Transplantation established the RITN in 2006, with a mission to prepare for nuclear disaster and help manage the response if one occurs.

“The widespread availability of radioactive material has made future exposure events, accidental or intentional, nearly inevitable,” RITN leaders warned in a 2008 report. “Hematologists, oncologists, and HSCT [hematopoietic stem cell transplantation] physicians are uniquely suited to care for victims of radiation exposure, creating a collective responsibility to prepare for a variety of contingencies.”
RITN doesn’t just train physicians, Mr. Case noted. All medical centers within the RITN are required to conduct an annual tabletop exercise where a radiation disaster scenario and a set of discussion questions are presented to the team.
Hematologists specially equipped to treat radiation injuries
Why are hematologists involved in treating people exposed to dangerously high levels of radiation? The answer has to do with how radiation harms the body, said Dr. Ann A. Jakubowski, a hematologist/oncologist and transplant physician at Memorial Sloan Kettering Cancer Center, New York, who serves as a medical director for RITN.
“One of the most common toxicities from radiation exposure and a major player in acute radiation syndrome is hematologic toxicity– damage to the bone marrow by the radiation, with a resultant decrease in peripheral blood counts,” she said in an interview. “This is similar to what is often seen in the treatment of cancers with radiation and/or chemotherapy.”
In cases of severe and nonreversible radiation damage to the bone marrow, Dr. Jakubowski noted, “patients can be considered for a stem cell transplant to provide new healthy cells to repopulate the bone marrow, which provides recovery of peripheral blood counts. Hematologist/oncologists are the physicians who manage stem cell transplants.”
The crucial role of hematologists in radiation injuries is not new. In fact, these physicians have been closely intertwined with nuclear research since the dawn of the atomic age. The work of developing atomic bombs also led investigators to an understanding of the structure and processes of hematopoiesis and helped them to identify hematopoietic stem cells and prove their existence in humans.
Disaster response poses multiple challenges
As noted in a recent article in ASH Clinical News, the challenges of treating radiation injuries would be intense, especially in the event of a nuclear accident or attack that affects a wide area. For starters, how quickly can medical professionals be mobilized, and will there be enough physicians comfortable treating patients? Fortunately, irradiated patients should not pose a direct risk to medical professionals who treat them.
“The expectation is that the patients will all be decontaminated,” said Nelson Chao, MD, MBA, one of the founders of RITN and a hematologist/oncologist and transplant physician at Duke University, Durham, N.C.
Dr. Jakubowski questions whether there will be adequate resources to handle the influx of patients who need more intensive treatment, as well as outpatients who “received lower doses of radiation and may experience a period of low blood counts but are expected to eventually recover blood counts.”

And if many people are injured, Dr. Chao asks, how will physicians “adopt altered standards of care to treat large numbers of patients?”
There will also be a need for physicians who aren’t hematologists, Dr. Jakubowski said. “There may be many victims who have both radiation exposure and traumatic or burn injuries, which need to be addressed first, before the hematologist can start addressing the consequences of ARS. Traumatic and burn injuries will require surgical resources.”
In addition, ARS affects the gastrointestinal track and central nervous system/cardiovascular, and it has multiple stages, she noted.
“Although we have methods of supporting the hematopoietic system – transfusions and growth factors – and even replacing it with a stem cell transplant, this will not necessarily fix the badly damaged other organs, Dr. Jakubowski said. “Also, not all radioactive isotopes are equal in their effects, nor are the various types of radiation exposure.”
Training goes beyond transplants and drugs
RITN offers individual hematologists specialized education about treating radiation injuries through annual exercises, modules, and “just-in-time” training.
For example, the RITN webpage devoted to triage includes guidelines for transferring radiation injury patients, triage guidelines for cytokine administration in cases of ARS, an exposure and symptom triage tool, and more. The treatment page includes details about subjects such as when human leukocyte antigen typing of casualties is appropriate and how to keep yourself safe while treating patients.
Another focus is teaching hematologists to react quickly in disasters, Mr. Case said. “The vast majority of hematologists have little to no experience in responding to disasters and making decisions with imperfect or incomplete information, as emergency medicine practitioners must do regularly.”
“Some of the RITN tabletop exercises present physicians and advanced practitioners with an incomplete set of patient information and ask physicians to then determine and prioritize their care,” Mr. Case said. “The resulting discussions help to lay the groundwork for being able to shift to the crisis standards of care mindset that would be necessary during a radiological disaster.”
Here’s how hematologists can get involved
If you want to help improve the nation’s response to radiation injuries, Mr. Case suggests checking RITN’s list of participating hospitals. If your facility is already part of this network, he said, contact its bone marrow transplant unit for more information.
In such cases, Dr. Jakubowski suggests that you “consider periodically giving a presentation to staff on the basics of radiation injury and the center’s role in RITN.” And if you’re not part of RITN, she said, consider contacting the network about becoming a member.
Hematologists, Mr. Case said, can also take advantage of RITN’s free short overview courses, review the RITN Treatment Guidelines, or watch short videos on the RITN’s YouTube channel.
He highlighted the Radiation Emergency Medical Management website administered by the Department of Health & Human Services, the Center for Disease Control’s radiation emergencies webpage, and the Department of Energy’s Radiation Emergency Assistance Center/Training Site.
For many Americans – especially those too young to know much about the Cold War or Hiroshima – Russia’s invasion of Ukraine might mark the first time they’ve truly considered the dangers of nuclear weapons. But dozens of hematologists in the United States already know the drill and have placed themselves on the front lines. These physicians stand prepared to treat patients exposed to radiation caused by nuclear accidents or attacks on U.S. soil.
They work nationwide at 74 medical centers that make up the Radiation Injury Treatment Network, ready to manage cases of acute radiation syndrome (ARS) during disasters. While RITN keeps a low profile, it’s been in the news lately amid anxieties about the Ukraine conflict, nuclear plant accidents, and the potential launching of nuclear weapons by foreign adversaries.
“The Radiation Injury Treatment Network helps plan responses for disaster scenarios where a person’s cells would be damaged after having been exposed to ionizing radiation,” program director Cullen Case Jr., MPA, said in an interview.
A U.S. Army veteran who took part in hurricane response early in his career, Mr. Case now oversees preparedness activities among all RITN hospitals, blood donor centers, and cord blood banks, in readiness for a mass casualty radiological incident. He also serves as a senior manager of the National Marrow Donor Program/Be a Match Marrow Registry.
Intense preparation for nuclear attacks or accidents is necessary, Mr. Case said, despite the doomsday scenarios disseminated on television shows and movies.
“The most frequent misconception we hear is that a nuclear disaster will encompass the whole world and be so complete that preparedness isn’t useful. However, many planning scenarios include smaller-scale incidents where survivors will need prompt and expert care,” he said.
In the wake of 9/11, the National Marrow Donor Program and the American Society for Blood and Marrow Transplantation established the RITN in 2006, with a mission to prepare for nuclear disaster and help manage the response if one occurs.

“The widespread availability of radioactive material has made future exposure events, accidental or intentional, nearly inevitable,” RITN leaders warned in a 2008 report. “Hematologists, oncologists, and HSCT [hematopoietic stem cell transplantation] physicians are uniquely suited to care for victims of radiation exposure, creating a collective responsibility to prepare for a variety of contingencies.”
RITN doesn’t just train physicians, Mr. Case noted. All medical centers within the RITN are required to conduct an annual tabletop exercise where a radiation disaster scenario and a set of discussion questions are presented to the team.
Hematologists specially equipped to treat radiation injuries
Why are hematologists involved in treating people exposed to dangerously high levels of radiation? The answer has to do with how radiation harms the body, said Dr. Ann A. Jakubowski, a hematologist/oncologist and transplant physician at Memorial Sloan Kettering Cancer Center, New York, who serves as a medical director for RITN.
“One of the most common toxicities from radiation exposure and a major player in acute radiation syndrome is hematologic toxicity– damage to the bone marrow by the radiation, with a resultant decrease in peripheral blood counts,” she said in an interview. “This is similar to what is often seen in the treatment of cancers with radiation and/or chemotherapy.”
In cases of severe and nonreversible radiation damage to the bone marrow, Dr. Jakubowski noted, “patients can be considered for a stem cell transplant to provide new healthy cells to repopulate the bone marrow, which provides recovery of peripheral blood counts. Hematologist/oncologists are the physicians who manage stem cell transplants.”
The crucial role of hematologists in radiation injuries is not new. In fact, these physicians have been closely intertwined with nuclear research since the dawn of the atomic age. The work of developing atomic bombs also led investigators to an understanding of the structure and processes of hematopoiesis and helped them to identify hematopoietic stem cells and prove their existence in humans.
Disaster response poses multiple challenges
As noted in a recent article in ASH Clinical News, the challenges of treating radiation injuries would be intense, especially in the event of a nuclear accident or attack that affects a wide area. For starters, how quickly can medical professionals be mobilized, and will there be enough physicians comfortable treating patients? Fortunately, irradiated patients should not pose a direct risk to medical professionals who treat them.
“The expectation is that the patients will all be decontaminated,” said Nelson Chao, MD, MBA, one of the founders of RITN and a hematologist/oncologist and transplant physician at Duke University, Durham, N.C.
Dr. Jakubowski questions whether there will be adequate resources to handle the influx of patients who need more intensive treatment, as well as outpatients who “received lower doses of radiation and may experience a period of low blood counts but are expected to eventually recover blood counts.”

And if many people are injured, Dr. Chao asks, how will physicians “adopt altered standards of care to treat large numbers of patients?”
There will also be a need for physicians who aren’t hematologists, Dr. Jakubowski said. “There may be many victims who have both radiation exposure and traumatic or burn injuries, which need to be addressed first, before the hematologist can start addressing the consequences of ARS. Traumatic and burn injuries will require surgical resources.”
In addition, ARS affects the gastrointestinal track and central nervous system/cardiovascular, and it has multiple stages, she noted.
“Although we have methods of supporting the hematopoietic system – transfusions and growth factors – and even replacing it with a stem cell transplant, this will not necessarily fix the badly damaged other organs, Dr. Jakubowski said. “Also, not all radioactive isotopes are equal in their effects, nor are the various types of radiation exposure.”
Training goes beyond transplants and drugs
RITN offers individual hematologists specialized education about treating radiation injuries through annual exercises, modules, and “just-in-time” training.
For example, the RITN webpage devoted to triage includes guidelines for transferring radiation injury patients, triage guidelines for cytokine administration in cases of ARS, an exposure and symptom triage tool, and more. The treatment page includes details about subjects such as when human leukocyte antigen typing of casualties is appropriate and how to keep yourself safe while treating patients.
Another focus is teaching hematologists to react quickly in disasters, Mr. Case said. “The vast majority of hematologists have little to no experience in responding to disasters and making decisions with imperfect or incomplete information, as emergency medicine practitioners must do regularly.”
“Some of the RITN tabletop exercises present physicians and advanced practitioners with an incomplete set of patient information and ask physicians to then determine and prioritize their care,” Mr. Case said. “The resulting discussions help to lay the groundwork for being able to shift to the crisis standards of care mindset that would be necessary during a radiological disaster.”
Here’s how hematologists can get involved
If you want to help improve the nation’s response to radiation injuries, Mr. Case suggests checking RITN’s list of participating hospitals. If your facility is already part of this network, he said, contact its bone marrow transplant unit for more information.
In such cases, Dr. Jakubowski suggests that you “consider periodically giving a presentation to staff on the basics of radiation injury and the center’s role in RITN.” And if you’re not part of RITN, she said, consider contacting the network about becoming a member.
Hematologists, Mr. Case said, can also take advantage of RITN’s free short overview courses, review the RITN Treatment Guidelines, or watch short videos on the RITN’s YouTube channel.
He highlighted the Radiation Emergency Medical Management website administered by the Department of Health & Human Services, the Center for Disease Control’s radiation emergencies webpage, and the Department of Energy’s Radiation Emergency Assistance Center/Training Site.
Will the headache field embrace rofecoxib?
In June, the Concord, Mass.–based company Tremeau Pharmaceuticals announced that the Food and Drug Administration was letting it proceed with a phase 3 clinical trial to test rofecoxib, the once-bestselling painkiller known as Vioxx, in patients with migraine.
The anti-inflammatory drug, a cyclooxygenase-2 (COX-2) inhibitor, received its first FDA approval in 1999 and became widely prescribed for arthritis and acute pain. In 2004 it was withdrawn by its manufacturer, Merck, after being shown to raise the risk of cardiovascular events.
In clinical trials and in real-world epidemiological studies, rofecoxib was associated with elevated heart attack, stroke, and related deaths; one 2005 study estimated that it had been responsible for some 38,000 excess deaths in the United States before being withdrawn. In 2007 Merck, beset with allegations that it had suppressed and mischaracterized rofecoxib’s safety data, paid out nearly $5 billion to settle thousands of lawsuits filed by patients and their families.
, an indication for which it received an orphan drug designation in 2017 and the agency’s green light for trials in 2020.
Brad Sippy, Tremeau’s chief executive officer, said that his company chose the two indications in part because both patient populations have low cardiovascular risk. Migraine patients are generally younger than the arthritis populations formerly treated with rofecoxib and are unlikely to take the drug for more than a day or 2 at time, avoiding the risks associated with extended exposure.
A crowded market
The past several years have seen the emergence of a cornucopia of new migraine treatments, including monoclonal antibodies such as erenumab (Aimovig, Amgen), which help prevent attacks by blocking the vasodilator calcitonin gene-related peptide, or CGRP. In addition to the standard arsenal of triptans and nonsteroidal anti-inflammatory drugs for acute pain relief, migraine patients can now choose among serotonin-blocking agents such as lasmiditan (Reyvow, Eli Lilly), known as “ditans,” and small-molecule CGRP antagonists such as ubrogepant (Ubrelvy, Abbie), known as “gepants.” Some NSAIDs, including one COX inhibitor, have been formulated into rapidly absorbed powders or liquids for migraine.
Mr. Sippy said he sees a role for rofecoxib even in this crowded space. “Migraine as you know is a multimodal situation – few people say that only one drug works for them,” he said. “We think this is an option that would basically be like a high dose of ibuprofen,” but with less frequent dosing and lower gastrointestinal and platelet effects compared with ibuprofen and other NSAIDs.
An improved formulation
Rofecoxib “crosses the blood brain barrier very readily – better than other COX inhibitors on the market,” Mr. Sippy added. “It was well absorbed in its original formulation, and our product is even better absorbed than the original – we estimate it’s probably an hour quicker to [peak concentration].” In addition, he said, “our formulation is more efficient at delivering the drug so we don’t need as much active ingredient – our 17.5 milligrams gets you the same systemic exposure as 25 milligrams of the old product.”
A different mechanism of action
Neurologist Alan M. Rapoport, MD, editor-in-chief of Neurology Reviews and professor of neurology at the University of California, Los Angeles, said that he was “cautiously optimistic” that “if used correctly and not too frequently, [rofecoxib] will find its niche in migraine treatment.”
“Patients liked Vioxx,” said Dr. Rapoport, past president of the International Headache Society. Even people currently on prevention “need to have an acute care drug handy.” While some patients on monoclonal antibodies have had success with gepants for acute care, “these both target the same pathway. It’s always nice to have options with a different mechanism of action.”
One of the arguments Tremeau has cited for reintroducing rofecoxib has been an urgent need for alternatives to opioid painkillers. Indeed some analysts have linked the demise of Vioxx with a subsequent increase in opioid prescribing.
Dr. Rapoport noted that he never prescribes opioids or butalbital, a barbiturate, for migraine, and that most headache specialists avoid them in clinical practice. But in the emergency setting, he said, patients receive them all too frequently.
Mr. Sippy said that opioid prescribing, while not unknown in migraine, was a bigger problem in hemophilic arthropathy, the first indication his company has pursued for rofecoxib. People with hemophilia “have a kind of arthritis that would respond well to an anti-inflammatory drug but they can’t take NSAIDs due to bleeding risk. This is why so many end up on opioids. Rofecoxib, as a COX-2 inhibitor, doesn’t have any effect on platelet aggregation, which would make it another option.”
No unique risks at prescribed doses
The migraine indication originally started out narrower: Patients with both migraine and bleeding disorders. “But in talking with the FDA, they encouraged us to develop it for migraine,” Mr. Sippy said. The company is considering pursuing a third indication: menstrual pain co-occurring with migraine. Tremeau has not ruled out seeking an indication in patients with arthritis who cannot take other painkillers, whether opioids or NSAIDs.
Five years ago, when Tremeau first announced its plans to bring rofecoxib back – indeed the company was set up for that purpose and has only this and another COX-2 inhibitor in development – some experts warned that there is little to prevent the drug from being used off-label, whether in higher doses or for other diseases.
“That’s something else we’re seeking to solve in addition to going for younger populations,” said Mr. Sippy, who worked at Merck during the Vioxx crisis and later headed neurology at Sunovion before starting his own company.
“We’re going for the former middle dose as our high dose and now we know that you don’t want to take more than the prescribed amount. If it doesn’t work you get off it; you don’t want to dose-creep on it. That’s been a key insight: At the appropriate dose, this product has no unique risk relative to the drug class and potentially some unique benefits,” he said.
Risk versus benefit
Joseph Ross, MD, a health policy researcher at Yale University in New Haven, Conn., who in a 2018 editorial expressed concerns about rofecoxib’s revival, said in an email that he felt its use in migraine could be justified, with caveats.
During Vioxx’s original approval and time on the market, “there was a cardiovascular risk associated with use that was not being transparently and clearly reported to patients and clinicians,” Dr. Ross said.
“In terms of testing the product for use in patients with migraine – a population of generally younger patients at lower risk of cardiovascular disease – my only concern is that the risk is clearly communicated and that there is adequate postmarket safety surveillance,” he said. “If patients are making fully informed decisions, the potential benefit of the drug with respect to pain control may be worth the risks.”
Dr. Rapoport serves as an adviser for AbbVie, Amgen, Biohaven, Cala Health, Collegium Pharmaceutical, Satsuma, Teva, Theranica and Xoc; he is on the speakers bureau of AbbVie, Amgen, Biohaven, Impel, Lundbeck, and Teva. Dr. Ross disclosed research support from Johnson and Johnson, the Medical Device Innovation Consortium, and the Laura and John Arnold Foundation, along with government grants; he is also an expert witness in a lawsuit against Biogen.
In June, the Concord, Mass.–based company Tremeau Pharmaceuticals announced that the Food and Drug Administration was letting it proceed with a phase 3 clinical trial to test rofecoxib, the once-bestselling painkiller known as Vioxx, in patients with migraine.
The anti-inflammatory drug, a cyclooxygenase-2 (COX-2) inhibitor, received its first FDA approval in 1999 and became widely prescribed for arthritis and acute pain. In 2004 it was withdrawn by its manufacturer, Merck, after being shown to raise the risk of cardiovascular events.
In clinical trials and in real-world epidemiological studies, rofecoxib was associated with elevated heart attack, stroke, and related deaths; one 2005 study estimated that it had been responsible for some 38,000 excess deaths in the United States before being withdrawn. In 2007 Merck, beset with allegations that it had suppressed and mischaracterized rofecoxib’s safety data, paid out nearly $5 billion to settle thousands of lawsuits filed by patients and their families.
, an indication for which it received an orphan drug designation in 2017 and the agency’s green light for trials in 2020.
Brad Sippy, Tremeau’s chief executive officer, said that his company chose the two indications in part because both patient populations have low cardiovascular risk. Migraine patients are generally younger than the arthritis populations formerly treated with rofecoxib and are unlikely to take the drug for more than a day or 2 at time, avoiding the risks associated with extended exposure.
A crowded market
The past several years have seen the emergence of a cornucopia of new migraine treatments, including monoclonal antibodies such as erenumab (Aimovig, Amgen), which help prevent attacks by blocking the vasodilator calcitonin gene-related peptide, or CGRP. In addition to the standard arsenal of triptans and nonsteroidal anti-inflammatory drugs for acute pain relief, migraine patients can now choose among serotonin-blocking agents such as lasmiditan (Reyvow, Eli Lilly), known as “ditans,” and small-molecule CGRP antagonists such as ubrogepant (Ubrelvy, Abbie), known as “gepants.” Some NSAIDs, including one COX inhibitor, have been formulated into rapidly absorbed powders or liquids for migraine.
Mr. Sippy said he sees a role for rofecoxib even in this crowded space. “Migraine as you know is a multimodal situation – few people say that only one drug works for them,” he said. “We think this is an option that would basically be like a high dose of ibuprofen,” but with less frequent dosing and lower gastrointestinal and platelet effects compared with ibuprofen and other NSAIDs.
An improved formulation
Rofecoxib “crosses the blood brain barrier very readily – better than other COX inhibitors on the market,” Mr. Sippy added. “It was well absorbed in its original formulation, and our product is even better absorbed than the original – we estimate it’s probably an hour quicker to [peak concentration].” In addition, he said, “our formulation is more efficient at delivering the drug so we don’t need as much active ingredient – our 17.5 milligrams gets you the same systemic exposure as 25 milligrams of the old product.”
A different mechanism of action
Neurologist Alan M. Rapoport, MD, editor-in-chief of Neurology Reviews and professor of neurology at the University of California, Los Angeles, said that he was “cautiously optimistic” that “if used correctly and not too frequently, [rofecoxib] will find its niche in migraine treatment.”
“Patients liked Vioxx,” said Dr. Rapoport, past president of the International Headache Society. Even people currently on prevention “need to have an acute care drug handy.” While some patients on monoclonal antibodies have had success with gepants for acute care, “these both target the same pathway. It’s always nice to have options with a different mechanism of action.”
One of the arguments Tremeau has cited for reintroducing rofecoxib has been an urgent need for alternatives to opioid painkillers. Indeed some analysts have linked the demise of Vioxx with a subsequent increase in opioid prescribing.
Dr. Rapoport noted that he never prescribes opioids or butalbital, a barbiturate, for migraine, and that most headache specialists avoid them in clinical practice. But in the emergency setting, he said, patients receive them all too frequently.
Mr. Sippy said that opioid prescribing, while not unknown in migraine, was a bigger problem in hemophilic arthropathy, the first indication his company has pursued for rofecoxib. People with hemophilia “have a kind of arthritis that would respond well to an anti-inflammatory drug but they can’t take NSAIDs due to bleeding risk. This is why so many end up on opioids. Rofecoxib, as a COX-2 inhibitor, doesn’t have any effect on platelet aggregation, which would make it another option.”
No unique risks at prescribed doses
The migraine indication originally started out narrower: Patients with both migraine and bleeding disorders. “But in talking with the FDA, they encouraged us to develop it for migraine,” Mr. Sippy said. The company is considering pursuing a third indication: menstrual pain co-occurring with migraine. Tremeau has not ruled out seeking an indication in patients with arthritis who cannot take other painkillers, whether opioids or NSAIDs.
Five years ago, when Tremeau first announced its plans to bring rofecoxib back – indeed the company was set up for that purpose and has only this and another COX-2 inhibitor in development – some experts warned that there is little to prevent the drug from being used off-label, whether in higher doses or for other diseases.
“That’s something else we’re seeking to solve in addition to going for younger populations,” said Mr. Sippy, who worked at Merck during the Vioxx crisis and later headed neurology at Sunovion before starting his own company.
“We’re going for the former middle dose as our high dose and now we know that you don’t want to take more than the prescribed amount. If it doesn’t work you get off it; you don’t want to dose-creep on it. That’s been a key insight: At the appropriate dose, this product has no unique risk relative to the drug class and potentially some unique benefits,” he said.
Risk versus benefit
Joseph Ross, MD, a health policy researcher at Yale University in New Haven, Conn., who in a 2018 editorial expressed concerns about rofecoxib’s revival, said in an email that he felt its use in migraine could be justified, with caveats.
During Vioxx’s original approval and time on the market, “there was a cardiovascular risk associated with use that was not being transparently and clearly reported to patients and clinicians,” Dr. Ross said.
“In terms of testing the product for use in patients with migraine – a population of generally younger patients at lower risk of cardiovascular disease – my only concern is that the risk is clearly communicated and that there is adequate postmarket safety surveillance,” he said. “If patients are making fully informed decisions, the potential benefit of the drug with respect to pain control may be worth the risks.”
Dr. Rapoport serves as an adviser for AbbVie, Amgen, Biohaven, Cala Health, Collegium Pharmaceutical, Satsuma, Teva, Theranica and Xoc; he is on the speakers bureau of AbbVie, Amgen, Biohaven, Impel, Lundbeck, and Teva. Dr. Ross disclosed research support from Johnson and Johnson, the Medical Device Innovation Consortium, and the Laura and John Arnold Foundation, along with government grants; he is also an expert witness in a lawsuit against Biogen.
In June, the Concord, Mass.–based company Tremeau Pharmaceuticals announced that the Food and Drug Administration was letting it proceed with a phase 3 clinical trial to test rofecoxib, the once-bestselling painkiller known as Vioxx, in patients with migraine.
The anti-inflammatory drug, a cyclooxygenase-2 (COX-2) inhibitor, received its first FDA approval in 1999 and became widely prescribed for arthritis and acute pain. In 2004 it was withdrawn by its manufacturer, Merck, after being shown to raise the risk of cardiovascular events.
In clinical trials and in real-world epidemiological studies, rofecoxib was associated with elevated heart attack, stroke, and related deaths; one 2005 study estimated that it had been responsible for some 38,000 excess deaths in the United States before being withdrawn. In 2007 Merck, beset with allegations that it had suppressed and mischaracterized rofecoxib’s safety data, paid out nearly $5 billion to settle thousands of lawsuits filed by patients and their families.
, an indication for which it received an orphan drug designation in 2017 and the agency’s green light for trials in 2020.
Brad Sippy, Tremeau’s chief executive officer, said that his company chose the two indications in part because both patient populations have low cardiovascular risk. Migraine patients are generally younger than the arthritis populations formerly treated with rofecoxib and are unlikely to take the drug for more than a day or 2 at time, avoiding the risks associated with extended exposure.
A crowded market
The past several years have seen the emergence of a cornucopia of new migraine treatments, including monoclonal antibodies such as erenumab (Aimovig, Amgen), which help prevent attacks by blocking the vasodilator calcitonin gene-related peptide, or CGRP. In addition to the standard arsenal of triptans and nonsteroidal anti-inflammatory drugs for acute pain relief, migraine patients can now choose among serotonin-blocking agents such as lasmiditan (Reyvow, Eli Lilly), known as “ditans,” and small-molecule CGRP antagonists such as ubrogepant (Ubrelvy, Abbie), known as “gepants.” Some NSAIDs, including one COX inhibitor, have been formulated into rapidly absorbed powders or liquids for migraine.
Mr. Sippy said he sees a role for rofecoxib even in this crowded space. “Migraine as you know is a multimodal situation – few people say that only one drug works for them,” he said. “We think this is an option that would basically be like a high dose of ibuprofen,” but with less frequent dosing and lower gastrointestinal and platelet effects compared with ibuprofen and other NSAIDs.
An improved formulation
Rofecoxib “crosses the blood brain barrier very readily – better than other COX inhibitors on the market,” Mr. Sippy added. “It was well absorbed in its original formulation, and our product is even better absorbed than the original – we estimate it’s probably an hour quicker to [peak concentration].” In addition, he said, “our formulation is more efficient at delivering the drug so we don’t need as much active ingredient – our 17.5 milligrams gets you the same systemic exposure as 25 milligrams of the old product.”
A different mechanism of action
Neurologist Alan M. Rapoport, MD, editor-in-chief of Neurology Reviews and professor of neurology at the University of California, Los Angeles, said that he was “cautiously optimistic” that “if used correctly and not too frequently, [rofecoxib] will find its niche in migraine treatment.”
“Patients liked Vioxx,” said Dr. Rapoport, past president of the International Headache Society. Even people currently on prevention “need to have an acute care drug handy.” While some patients on monoclonal antibodies have had success with gepants for acute care, “these both target the same pathway. It’s always nice to have options with a different mechanism of action.”
One of the arguments Tremeau has cited for reintroducing rofecoxib has been an urgent need for alternatives to opioid painkillers. Indeed some analysts have linked the demise of Vioxx with a subsequent increase in opioid prescribing.
Dr. Rapoport noted that he never prescribes opioids or butalbital, a barbiturate, for migraine, and that most headache specialists avoid them in clinical practice. But in the emergency setting, he said, patients receive them all too frequently.
Mr. Sippy said that opioid prescribing, while not unknown in migraine, was a bigger problem in hemophilic arthropathy, the first indication his company has pursued for rofecoxib. People with hemophilia “have a kind of arthritis that would respond well to an anti-inflammatory drug but they can’t take NSAIDs due to bleeding risk. This is why so many end up on opioids. Rofecoxib, as a COX-2 inhibitor, doesn’t have any effect on platelet aggregation, which would make it another option.”
No unique risks at prescribed doses
The migraine indication originally started out narrower: Patients with both migraine and bleeding disorders. “But in talking with the FDA, they encouraged us to develop it for migraine,” Mr. Sippy said. The company is considering pursuing a third indication: menstrual pain co-occurring with migraine. Tremeau has not ruled out seeking an indication in patients with arthritis who cannot take other painkillers, whether opioids or NSAIDs.
Five years ago, when Tremeau first announced its plans to bring rofecoxib back – indeed the company was set up for that purpose and has only this and another COX-2 inhibitor in development – some experts warned that there is little to prevent the drug from being used off-label, whether in higher doses or for other diseases.
“That’s something else we’re seeking to solve in addition to going for younger populations,” said Mr. Sippy, who worked at Merck during the Vioxx crisis and later headed neurology at Sunovion before starting his own company.
“We’re going for the former middle dose as our high dose and now we know that you don’t want to take more than the prescribed amount. If it doesn’t work you get off it; you don’t want to dose-creep on it. That’s been a key insight: At the appropriate dose, this product has no unique risk relative to the drug class and potentially some unique benefits,” he said.
Risk versus benefit
Joseph Ross, MD, a health policy researcher at Yale University in New Haven, Conn., who in a 2018 editorial expressed concerns about rofecoxib’s revival, said in an email that he felt its use in migraine could be justified, with caveats.
During Vioxx’s original approval and time on the market, “there was a cardiovascular risk associated with use that was not being transparently and clearly reported to patients and clinicians,” Dr. Ross said.
“In terms of testing the product for use in patients with migraine – a population of generally younger patients at lower risk of cardiovascular disease – my only concern is that the risk is clearly communicated and that there is adequate postmarket safety surveillance,” he said. “If patients are making fully informed decisions, the potential benefit of the drug with respect to pain control may be worth the risks.”
Dr. Rapoport serves as an adviser for AbbVie, Amgen, Biohaven, Cala Health, Collegium Pharmaceutical, Satsuma, Teva, Theranica and Xoc; he is on the speakers bureau of AbbVie, Amgen, Biohaven, Impel, Lundbeck, and Teva. Dr. Ross disclosed research support from Johnson and Johnson, the Medical Device Innovation Consortium, and the Laura and John Arnold Foundation, along with government grants; he is also an expert witness in a lawsuit against Biogen.
Scientists find brain mechanism behind age-related memory loss
Scientists at Johns Hopkins University have identified a mechanism in the brain behind age-related memory loss, expanding our knowledge of the inner workings of the aging brain and possibly opening the door to new Alzheimer’s treatments.
The researchers looked at the hippocampus, a part of the brain thought to store long-term memories.
Neurons there are responsible for a pair of memory functions – called pattern separation and pattern completion – that work together in young, healthy brains. These functions can swing out of balance with age, impacting memory.
The Johns Hopkins team may have discovered what causes this imbalance. Their findings – reported in a paper in the journal Current Biology – may not only help us improve dementia treatments, but even prevent or delay a loss of thinking skills in the first place, the researchers say.
Pattern separation vs. pattern completion
To understand how the hippocampus changes with age, the researchers looked at rats’ brains. In rats and in humans, pattern separation and pattern completion are present, controlled by neurons in the hippocampus.
As the name suggests, pattern completion is when you take a few details or fragments of information – a few notes of music, or the start of a famous movie quote – and your brain retrieves the full memory. Pattern separation, on the other hand, is being able to tell similar observations or experiences apart (like two visits to the same restaurant) to be stored as separate memories.
These functions occur along a gradient across a tiny region called CA3. That gradient, the study found, disappears with aging, said lead study author Hey-Kyoung Lee, PhD, an assistant research scientist at the university’s Zanvyl Krieger Mind/Brain Institute. “The main consequence of the loss,” Dr. Lee said, “is that pattern completion becomes more dominant in rats as they age.”
What’s happening in the brain
Neurons responsible for pattern completion occupy the “distal” end of CA3, while those in charge of pattern separation reside at the “proximal” end. Dr. Lee said prior studies had not examined the proximal and distal regions separately, as she and her team did in this study.
What was surprising, said Dr. Lee, “was that hyperactivity in aging was observed toward the proximal CA3 region, not the expected distal region.” Contrary to their expectations, that hyperactivity did not enhance function in that area but rather dampened it. Hence: “There is diminished pattern separation and augmented pattern completion,” she said.
– they may recall a certain restaurant they’d been to but not be able to separate what happened during one visit versus another.
Why do some older adults stay sharp?
That memory impairment does not happen to everyone, and it doesn’t happen to all rats either. In fact, the researchers found that some older rats performed spatial-learning tasks as well as young rats did – even though their brains were already beginning to favor pattern completion.
If we can better understand why this happens, we may uncover new therapies for age-related memory loss, Dr. Lee said.
Coauthor Michela Gallagher’s team previously demonstrated that the anti-epilepsy drug levetiracetam improves memory performance by reducing hyperactivity in the hippocampus.
The extra detail this study adds may allow scientists to better aim such drugs in the future, Dr. Lee speculated. “It would give us better control of where we could possibly target the deficits we see.”
A version of this article first appeared on WebMD.com.
Scientists at Johns Hopkins University have identified a mechanism in the brain behind age-related memory loss, expanding our knowledge of the inner workings of the aging brain and possibly opening the door to new Alzheimer’s treatments.
The researchers looked at the hippocampus, a part of the brain thought to store long-term memories.
Neurons there are responsible for a pair of memory functions – called pattern separation and pattern completion – that work together in young, healthy brains. These functions can swing out of balance with age, impacting memory.
The Johns Hopkins team may have discovered what causes this imbalance. Their findings – reported in a paper in the journal Current Biology – may not only help us improve dementia treatments, but even prevent or delay a loss of thinking skills in the first place, the researchers say.
Pattern separation vs. pattern completion
To understand how the hippocampus changes with age, the researchers looked at rats’ brains. In rats and in humans, pattern separation and pattern completion are present, controlled by neurons in the hippocampus.
As the name suggests, pattern completion is when you take a few details or fragments of information – a few notes of music, or the start of a famous movie quote – and your brain retrieves the full memory. Pattern separation, on the other hand, is being able to tell similar observations or experiences apart (like two visits to the same restaurant) to be stored as separate memories.
These functions occur along a gradient across a tiny region called CA3. That gradient, the study found, disappears with aging, said lead study author Hey-Kyoung Lee, PhD, an assistant research scientist at the university’s Zanvyl Krieger Mind/Brain Institute. “The main consequence of the loss,” Dr. Lee said, “is that pattern completion becomes more dominant in rats as they age.”
What’s happening in the brain
Neurons responsible for pattern completion occupy the “distal” end of CA3, while those in charge of pattern separation reside at the “proximal” end. Dr. Lee said prior studies had not examined the proximal and distal regions separately, as she and her team did in this study.
What was surprising, said Dr. Lee, “was that hyperactivity in aging was observed toward the proximal CA3 region, not the expected distal region.” Contrary to their expectations, that hyperactivity did not enhance function in that area but rather dampened it. Hence: “There is diminished pattern separation and augmented pattern completion,” she said.
– they may recall a certain restaurant they’d been to but not be able to separate what happened during one visit versus another.
Why do some older adults stay sharp?
That memory impairment does not happen to everyone, and it doesn’t happen to all rats either. In fact, the researchers found that some older rats performed spatial-learning tasks as well as young rats did – even though their brains were already beginning to favor pattern completion.
If we can better understand why this happens, we may uncover new therapies for age-related memory loss, Dr. Lee said.
Coauthor Michela Gallagher’s team previously demonstrated that the anti-epilepsy drug levetiracetam improves memory performance by reducing hyperactivity in the hippocampus.
The extra detail this study adds may allow scientists to better aim such drugs in the future, Dr. Lee speculated. “It would give us better control of where we could possibly target the deficits we see.”
A version of this article first appeared on WebMD.com.
Scientists at Johns Hopkins University have identified a mechanism in the brain behind age-related memory loss, expanding our knowledge of the inner workings of the aging brain and possibly opening the door to new Alzheimer’s treatments.
The researchers looked at the hippocampus, a part of the brain thought to store long-term memories.
Neurons there are responsible for a pair of memory functions – called pattern separation and pattern completion – that work together in young, healthy brains. These functions can swing out of balance with age, impacting memory.
The Johns Hopkins team may have discovered what causes this imbalance. Their findings – reported in a paper in the journal Current Biology – may not only help us improve dementia treatments, but even prevent or delay a loss of thinking skills in the first place, the researchers say.
Pattern separation vs. pattern completion
To understand how the hippocampus changes with age, the researchers looked at rats’ brains. In rats and in humans, pattern separation and pattern completion are present, controlled by neurons in the hippocampus.
As the name suggests, pattern completion is when you take a few details or fragments of information – a few notes of music, or the start of a famous movie quote – and your brain retrieves the full memory. Pattern separation, on the other hand, is being able to tell similar observations or experiences apart (like two visits to the same restaurant) to be stored as separate memories.
These functions occur along a gradient across a tiny region called CA3. That gradient, the study found, disappears with aging, said lead study author Hey-Kyoung Lee, PhD, an assistant research scientist at the university’s Zanvyl Krieger Mind/Brain Institute. “The main consequence of the loss,” Dr. Lee said, “is that pattern completion becomes more dominant in rats as they age.”
What’s happening in the brain
Neurons responsible for pattern completion occupy the “distal” end of CA3, while those in charge of pattern separation reside at the “proximal” end. Dr. Lee said prior studies had not examined the proximal and distal regions separately, as she and her team did in this study.
What was surprising, said Dr. Lee, “was that hyperactivity in aging was observed toward the proximal CA3 region, not the expected distal region.” Contrary to their expectations, that hyperactivity did not enhance function in that area but rather dampened it. Hence: “There is diminished pattern separation and augmented pattern completion,” she said.
– they may recall a certain restaurant they’d been to but not be able to separate what happened during one visit versus another.
Why do some older adults stay sharp?
That memory impairment does not happen to everyone, and it doesn’t happen to all rats either. In fact, the researchers found that some older rats performed spatial-learning tasks as well as young rats did – even though their brains were already beginning to favor pattern completion.
If we can better understand why this happens, we may uncover new therapies for age-related memory loss, Dr. Lee said.
Coauthor Michela Gallagher’s team previously demonstrated that the anti-epilepsy drug levetiracetam improves memory performance by reducing hyperactivity in the hippocampus.
The extra detail this study adds may allow scientists to better aim such drugs in the future, Dr. Lee speculated. “It would give us better control of where we could possibly target the deficits we see.”
A version of this article first appeared on WebMD.com.
FROM CURRENT BIOLOGY
Can bone density scans help predict dementia risk?
, new research suggests.
In an analysis of more than 900 study participants, women in their 70s with more advanced abdominal aortic calcification (AAC) seen on lateral spine images during dual-energy x-ray absorptiometry (DXA) had a two- to fourfold higher risk for late-life dementia than those with low AAC.
This finding was independent of cardiovascular risk factors and apolipoprotein E (APOE ) genotype.
“While these results are exciting, we now need to undertake further large screening studies in older men and women using this approach to show that the findings are generalizable to older men and can identify people with greater cognitive decline,” coinvestigator Marc Sim, PhD, Edith Cowan University, Joondalup, Australia, said in an interview.
“This will hopefully open the door to studies of early disease-modifying interventions,” Sim said.
The findings were published online in The Lancet Regional Health – Western Pacific.
AAC and cognition
Late-life dementia occurring after age 80 is increasingly common because of both vascular and nonvascular risk factors.
Two recent studies in middle-aged and older men and women showed that AAC identified on bone densitometry was associated with poorer cognition, suggesting it may be related to cognitive decline and increased dementia risk.
This provided the rationale for the current study, Dr. Sim noted.
The researchers assessed AAC using DXA lateral spine images captured in the late 1990s in a prospective cohort of 958 older women who were participating in an osteoporosis study.
AAC was classified into established low, moderate, and extensive categories. At baseline, all women were aged 70 and older, and 45% had low AAC, 36% had moderate AAC, and 19% had extensive AAC.
Over 14.5 years, 150 women (15.7%) had a late-life hospitalization and/or died.
Improved risk prediction
Results showed that, compared with women who had low AAC, women with moderate and extensive AAC were more likely to experience late-life dementia hospitalization (9.3% low, 15.5% moderate, and 18.3% extensive) and death (2.8%, 8.3%, and 9.4%, respectively).
After multivariable adjustment, women with moderate AAC had a two- and threefold increased relative risk for late-life dementia hospitalization or death, compared with their peers who had low AAC.
Women with extensive AAC had a two- and fourfold increase in the adjusted relative risk for late-life dementia hospitalization or death.
“To our knowledge this is the first time it has been shown that AAC from these scans is related to late-life dementia,” Dr. Sim said.
“We demonstrated that AAC improved risk prediction in addition to cardiovascular risk factors and APOE genotype, a genetic risk factor for Alzheimer’s disease, the major form of dementia,” he added.
Dr. Sim noted “these additional lateral spine images” can be taken at the same time that hip and spine bone density tests are done.
“This provides an opportunity to identify AAC in large numbers of people,” he said.
He cautioned, however, that further studies with detailed dementia-related phenotypes, brain imaging, and measures of cognition are needed to confirm whether AAC will add value to dementia risk prediction.
‘Not surprising’
Commenting on the findings for this article, Claire Sexton, DPhil, senior director of scientific programs and outreach at the Alzheimer’s Association, Chicago, noted that AAC is a marker of atherosclerosis and is associated with vascular health outcomes.
Therefore, it is “not surprising it would be associated with dementia too. There’s been previous research linking atherosclerosis and Alzheimer’s disease,” Dr. Sexton said.
“What’s novel about this research is that it’s looking at AAC specifically, which can be identified through a relatively simple test that is already in widespread use,” she added.
Dr. Sexton noted that “much more research” is now needed in larger, more diverse populations in order to better understand the link between AAC and dementia – and whether bone density testing may be an appropriate dementia-screening tool.
“The good news is vascular conditions like atherosclerosis can be managed through lifestyle changes like eating a healthy diet and getting regular exercise. And research tells us what’s good for the heart is good for the brain,” Dr. Sexton said.
The study was funded by Kidney Health Australia, Healthway Health Promotion Foundation of Western Australia, Sir Charles Gairdner Hospital Research Advisory Committee Grant, and the National Health and Medical Research Council of Australia. Dr. Sim and Dr. Sexton have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, new research suggests.
In an analysis of more than 900 study participants, women in their 70s with more advanced abdominal aortic calcification (AAC) seen on lateral spine images during dual-energy x-ray absorptiometry (DXA) had a two- to fourfold higher risk for late-life dementia than those with low AAC.
This finding was independent of cardiovascular risk factors and apolipoprotein E (APOE ) genotype.
“While these results are exciting, we now need to undertake further large screening studies in older men and women using this approach to show that the findings are generalizable to older men and can identify people with greater cognitive decline,” coinvestigator Marc Sim, PhD, Edith Cowan University, Joondalup, Australia, said in an interview.
“This will hopefully open the door to studies of early disease-modifying interventions,” Sim said.
The findings were published online in The Lancet Regional Health – Western Pacific.
AAC and cognition
Late-life dementia occurring after age 80 is increasingly common because of both vascular and nonvascular risk factors.
Two recent studies in middle-aged and older men and women showed that AAC identified on bone densitometry was associated with poorer cognition, suggesting it may be related to cognitive decline and increased dementia risk.
This provided the rationale for the current study, Dr. Sim noted.
The researchers assessed AAC using DXA lateral spine images captured in the late 1990s in a prospective cohort of 958 older women who were participating in an osteoporosis study.
AAC was classified into established low, moderate, and extensive categories. At baseline, all women were aged 70 and older, and 45% had low AAC, 36% had moderate AAC, and 19% had extensive AAC.
Over 14.5 years, 150 women (15.7%) had a late-life hospitalization and/or died.
Improved risk prediction
Results showed that, compared with women who had low AAC, women with moderate and extensive AAC were more likely to experience late-life dementia hospitalization (9.3% low, 15.5% moderate, and 18.3% extensive) and death (2.8%, 8.3%, and 9.4%, respectively).
After multivariable adjustment, women with moderate AAC had a two- and threefold increased relative risk for late-life dementia hospitalization or death, compared with their peers who had low AAC.
Women with extensive AAC had a two- and fourfold increase in the adjusted relative risk for late-life dementia hospitalization or death.
“To our knowledge this is the first time it has been shown that AAC from these scans is related to late-life dementia,” Dr. Sim said.
“We demonstrated that AAC improved risk prediction in addition to cardiovascular risk factors and APOE genotype, a genetic risk factor for Alzheimer’s disease, the major form of dementia,” he added.
Dr. Sim noted “these additional lateral spine images” can be taken at the same time that hip and spine bone density tests are done.
“This provides an opportunity to identify AAC in large numbers of people,” he said.
He cautioned, however, that further studies with detailed dementia-related phenotypes, brain imaging, and measures of cognition are needed to confirm whether AAC will add value to dementia risk prediction.
‘Not surprising’
Commenting on the findings for this article, Claire Sexton, DPhil, senior director of scientific programs and outreach at the Alzheimer’s Association, Chicago, noted that AAC is a marker of atherosclerosis and is associated with vascular health outcomes.
Therefore, it is “not surprising it would be associated with dementia too. There’s been previous research linking atherosclerosis and Alzheimer’s disease,” Dr. Sexton said.
“What’s novel about this research is that it’s looking at AAC specifically, which can be identified through a relatively simple test that is already in widespread use,” she added.
Dr. Sexton noted that “much more research” is now needed in larger, more diverse populations in order to better understand the link between AAC and dementia – and whether bone density testing may be an appropriate dementia-screening tool.
“The good news is vascular conditions like atherosclerosis can be managed through lifestyle changes like eating a healthy diet and getting regular exercise. And research tells us what’s good for the heart is good for the brain,” Dr. Sexton said.
The study was funded by Kidney Health Australia, Healthway Health Promotion Foundation of Western Australia, Sir Charles Gairdner Hospital Research Advisory Committee Grant, and the National Health and Medical Research Council of Australia. Dr. Sim and Dr. Sexton have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, new research suggests.
In an analysis of more than 900 study participants, women in their 70s with more advanced abdominal aortic calcification (AAC) seen on lateral spine images during dual-energy x-ray absorptiometry (DXA) had a two- to fourfold higher risk for late-life dementia than those with low AAC.
This finding was independent of cardiovascular risk factors and apolipoprotein E (APOE ) genotype.
“While these results are exciting, we now need to undertake further large screening studies in older men and women using this approach to show that the findings are generalizable to older men and can identify people with greater cognitive decline,” coinvestigator Marc Sim, PhD, Edith Cowan University, Joondalup, Australia, said in an interview.
“This will hopefully open the door to studies of early disease-modifying interventions,” Sim said.
The findings were published online in The Lancet Regional Health – Western Pacific.
AAC and cognition
Late-life dementia occurring after age 80 is increasingly common because of both vascular and nonvascular risk factors.
Two recent studies in middle-aged and older men and women showed that AAC identified on bone densitometry was associated with poorer cognition, suggesting it may be related to cognitive decline and increased dementia risk.
This provided the rationale for the current study, Dr. Sim noted.
The researchers assessed AAC using DXA lateral spine images captured in the late 1990s in a prospective cohort of 958 older women who were participating in an osteoporosis study.
AAC was classified into established low, moderate, and extensive categories. At baseline, all women were aged 70 and older, and 45% had low AAC, 36% had moderate AAC, and 19% had extensive AAC.
Over 14.5 years, 150 women (15.7%) had a late-life hospitalization and/or died.
Improved risk prediction
Results showed that, compared with women who had low AAC, women with moderate and extensive AAC were more likely to experience late-life dementia hospitalization (9.3% low, 15.5% moderate, and 18.3% extensive) and death (2.8%, 8.3%, and 9.4%, respectively).
After multivariable adjustment, women with moderate AAC had a two- and threefold increased relative risk for late-life dementia hospitalization or death, compared with their peers who had low AAC.
Women with extensive AAC had a two- and fourfold increase in the adjusted relative risk for late-life dementia hospitalization or death.
“To our knowledge this is the first time it has been shown that AAC from these scans is related to late-life dementia,” Dr. Sim said.
“We demonstrated that AAC improved risk prediction in addition to cardiovascular risk factors and APOE genotype, a genetic risk factor for Alzheimer’s disease, the major form of dementia,” he added.
Dr. Sim noted “these additional lateral spine images” can be taken at the same time that hip and spine bone density tests are done.
“This provides an opportunity to identify AAC in large numbers of people,” he said.
He cautioned, however, that further studies with detailed dementia-related phenotypes, brain imaging, and measures of cognition are needed to confirm whether AAC will add value to dementia risk prediction.
‘Not surprising’
Commenting on the findings for this article, Claire Sexton, DPhil, senior director of scientific programs and outreach at the Alzheimer’s Association, Chicago, noted that AAC is a marker of atherosclerosis and is associated with vascular health outcomes.
Therefore, it is “not surprising it would be associated with dementia too. There’s been previous research linking atherosclerosis and Alzheimer’s disease,” Dr. Sexton said.
“What’s novel about this research is that it’s looking at AAC specifically, which can be identified through a relatively simple test that is already in widespread use,” she added.
Dr. Sexton noted that “much more research” is now needed in larger, more diverse populations in order to better understand the link between AAC and dementia – and whether bone density testing may be an appropriate dementia-screening tool.
“The good news is vascular conditions like atherosclerosis can be managed through lifestyle changes like eating a healthy diet and getting regular exercise. And research tells us what’s good for the heart is good for the brain,” Dr. Sexton said.
The study was funded by Kidney Health Australia, Healthway Health Promotion Foundation of Western Australia, Sir Charles Gairdner Hospital Research Advisory Committee Grant, and the National Health and Medical Research Council of Australia. Dr. Sim and Dr. Sexton have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE LANCET REGIONAL HEALTH – WESTERN PACIFIC
FDA warns of increased risk of death with CLL, lymphoma drug
Duvelisib was approved in 2018 to treat adults with chronic lymphocytic leukemia (CLL) or small lymphocytic lymphoma (SLL) who had received at least two prior therapies that did not work or stopped working.
However, more recent 5-year overall survival results from the randomized phase 3 DUO clinical trial found a possible increased risk of death with duvelisib compared with another drug used to treat leukemia and lymphoma, according to an FDA Drug Safety Communication.
“The trial also found Copiktra was associated with a higher risk of serious side effects, including infections, diarrhea, inflammation of the intestines and lungs, skin reactions, and high liver enzyme levels in the blood,” states the warning, which advises prescribers to weigh the risks and benefits of continued use versus use of other treatments.
More specifically, median 5-year overall survival among 319 patients with CLL or SLL in the DUO trial was 52.3 months with duvelisib versus 63.3 months with the monoclonal antibody ofatumumab (hazard ratio, 1.09 overall and 1.06 among patients who received at least two prior lines of therapy).
Serious adverse events of grade 3 or higher were also more common in those treated with duvelisib.
Of note, in April, the FDA also announced it was withdrawing approval of the relapsed or refractory follicular lymphoma indication for duvelisib, following a voluntary request by the drug manufacturer, Secura Bio Inc.
A public meeting will be scheduled to discuss the findings of the trial and whether the drug should continue to be prescribed.
This FDA warning follows the agency’s June 1 withdrawal of approval for umbralisib (Ukoniq), another PI3 kinase inhibitor, following an investigation into a “possible increased risk of death.”
As reported by Medscape, umbralisib had received accelerated approval in February 2021 to treat adults with relapsed or refractory marginal zone lymphoma following at least one prior therapy and those with relapsed or refractory follicular lymphoma who had received at least three prior therapies.
“These safety findings were similar for other medicines in the same PI3 kinase inhibitor class, which were discussed at an advisory committee meeting of non-FDA experts in April 2022,” according to the FDA warning.
The FDA urges patients and health care professionals to report side effects involving duvelisib or other medicines to the FDA MedWatch program.
A version of this article first appeared on Medscape.com.
Duvelisib was approved in 2018 to treat adults with chronic lymphocytic leukemia (CLL) or small lymphocytic lymphoma (SLL) who had received at least two prior therapies that did not work or stopped working.
However, more recent 5-year overall survival results from the randomized phase 3 DUO clinical trial found a possible increased risk of death with duvelisib compared with another drug used to treat leukemia and lymphoma, according to an FDA Drug Safety Communication.
“The trial also found Copiktra was associated with a higher risk of serious side effects, including infections, diarrhea, inflammation of the intestines and lungs, skin reactions, and high liver enzyme levels in the blood,” states the warning, which advises prescribers to weigh the risks and benefits of continued use versus use of other treatments.
More specifically, median 5-year overall survival among 319 patients with CLL or SLL in the DUO trial was 52.3 months with duvelisib versus 63.3 months with the monoclonal antibody ofatumumab (hazard ratio, 1.09 overall and 1.06 among patients who received at least two prior lines of therapy).
Serious adverse events of grade 3 or higher were also more common in those treated with duvelisib.
Of note, in April, the FDA also announced it was withdrawing approval of the relapsed or refractory follicular lymphoma indication for duvelisib, following a voluntary request by the drug manufacturer, Secura Bio Inc.
A public meeting will be scheduled to discuss the findings of the trial and whether the drug should continue to be prescribed.
This FDA warning follows the agency’s June 1 withdrawal of approval for umbralisib (Ukoniq), another PI3 kinase inhibitor, following an investigation into a “possible increased risk of death.”
As reported by Medscape, umbralisib had received accelerated approval in February 2021 to treat adults with relapsed or refractory marginal zone lymphoma following at least one prior therapy and those with relapsed or refractory follicular lymphoma who had received at least three prior therapies.
“These safety findings were similar for other medicines in the same PI3 kinase inhibitor class, which were discussed at an advisory committee meeting of non-FDA experts in April 2022,” according to the FDA warning.
The FDA urges patients and health care professionals to report side effects involving duvelisib or other medicines to the FDA MedWatch program.
A version of this article first appeared on Medscape.com.
Duvelisib was approved in 2018 to treat adults with chronic lymphocytic leukemia (CLL) or small lymphocytic lymphoma (SLL) who had received at least two prior therapies that did not work or stopped working.
However, more recent 5-year overall survival results from the randomized phase 3 DUO clinical trial found a possible increased risk of death with duvelisib compared with another drug used to treat leukemia and lymphoma, according to an FDA Drug Safety Communication.
“The trial also found Copiktra was associated with a higher risk of serious side effects, including infections, diarrhea, inflammation of the intestines and lungs, skin reactions, and high liver enzyme levels in the blood,” states the warning, which advises prescribers to weigh the risks and benefits of continued use versus use of other treatments.
More specifically, median 5-year overall survival among 319 patients with CLL or SLL in the DUO trial was 52.3 months with duvelisib versus 63.3 months with the monoclonal antibody ofatumumab (hazard ratio, 1.09 overall and 1.06 among patients who received at least two prior lines of therapy).
Serious adverse events of grade 3 or higher were also more common in those treated with duvelisib.
Of note, in April, the FDA also announced it was withdrawing approval of the relapsed or refractory follicular lymphoma indication for duvelisib, following a voluntary request by the drug manufacturer, Secura Bio Inc.
A public meeting will be scheduled to discuss the findings of the trial and whether the drug should continue to be prescribed.
This FDA warning follows the agency’s June 1 withdrawal of approval for umbralisib (Ukoniq), another PI3 kinase inhibitor, following an investigation into a “possible increased risk of death.”
As reported by Medscape, umbralisib had received accelerated approval in February 2021 to treat adults with relapsed or refractory marginal zone lymphoma following at least one prior therapy and those with relapsed or refractory follicular lymphoma who had received at least three prior therapies.
“These safety findings were similar for other medicines in the same PI3 kinase inhibitor class, which were discussed at an advisory committee meeting of non-FDA experts in April 2022,” according to the FDA warning.
The FDA urges patients and health care professionals to report side effects involving duvelisib or other medicines to the FDA MedWatch program.
A version of this article first appeared on Medscape.com.
FDA warning: Lymphoma drug heightens risk of death
The U.S. Food and Drug Administration issued a warning today that the cancer drug duvelisib (Copiktra, Verastem), a PI3 kinase inhibitor, may increase the risk of death and serious side effects.
Duvelisib was approved in 2018 to treat adults with chronic lymphocytic leukemia (CLL) or small lymphocytic lymphoma (SLL) who had received at least two prior therapies that did not work or stopped working.
However, more recent 5-year overall survival results from the randomized phase 3 DUO clinical trial found a possible increased risk of death with duvelisib, compared with another drug used to treat leukemia and lymphoma, according to an FDA Drug Safety Communication.
“The trial also found Copiktra was associated with a higher risk of serious side effects, including infections, diarrhea, inflammation of the intestines and lungs, skin reactions, and high liver enzyme levels in the blood,” states the warning, which advises prescribers to weigh the risks and benefits of continued use versus use of other treatments.
More specifically, median 5-year overall survival among 319 patients with CLL or SLL in the DUO trial was 52.3 months with duvelisib versus 63.3 months with the monoclonal antibody ofatumumab (hazard ratio, 1.09 overall and 1.06 among patients who received at least two prior lines of therapy).
Serious adverse events of grade 3 or higher were also more common in those treated with duvelisib.
Of note, in April, the FDA also announced that it was withdrawing approval of the relapsed or refractory follicular lymphoma indication for duvelisib following a voluntary request by the drug manufacturer Secura Bio.
A public meeting will be scheduled to discuss the findings of the trial and whether the drug should continue to be prescribed.
This FDA warning follows the agency’s June 1 withdrawal of approval for umbralisib (Ukoniq), another PI3 kinase inhibitor, following an investigation into a “possible increased risk of death.”
As reported by this news organization, umbralisib had received accelerated approval in February 2021 to treat adults with relapsed or refractory marginal zone lymphoma following at least one prior therapy and those with relapsed or refractory follicular lymphoma who had received at least three prior therapies.
“These safety findings were similar for other medicines in the same PI3 kinase inhibitor class, which were discussed at an advisory committee meeting of non-FDA experts in April 2022,” according to the FDA warning.
The FDA urges patients and health care professionals to report side effects involving duvelisib or other medicines to the FDA MedWatch program.
A version of this article first appeared on Medscape.com
The U.S. Food and Drug Administration issued a warning today that the cancer drug duvelisib (Copiktra, Verastem), a PI3 kinase inhibitor, may increase the risk of death and serious side effects.
Duvelisib was approved in 2018 to treat adults with chronic lymphocytic leukemia (CLL) or small lymphocytic lymphoma (SLL) who had received at least two prior therapies that did not work or stopped working.
However, more recent 5-year overall survival results from the randomized phase 3 DUO clinical trial found a possible increased risk of death with duvelisib, compared with another drug used to treat leukemia and lymphoma, according to an FDA Drug Safety Communication.
“The trial also found Copiktra was associated with a higher risk of serious side effects, including infections, diarrhea, inflammation of the intestines and lungs, skin reactions, and high liver enzyme levels in the blood,” states the warning, which advises prescribers to weigh the risks and benefits of continued use versus use of other treatments.
More specifically, median 5-year overall survival among 319 patients with CLL or SLL in the DUO trial was 52.3 months with duvelisib versus 63.3 months with the monoclonal antibody ofatumumab (hazard ratio, 1.09 overall and 1.06 among patients who received at least two prior lines of therapy).
Serious adverse events of grade 3 or higher were also more common in those treated with duvelisib.
Of note, in April, the FDA also announced that it was withdrawing approval of the relapsed or refractory follicular lymphoma indication for duvelisib following a voluntary request by the drug manufacturer Secura Bio.
A public meeting will be scheduled to discuss the findings of the trial and whether the drug should continue to be prescribed.
This FDA warning follows the agency’s June 1 withdrawal of approval for umbralisib (Ukoniq), another PI3 kinase inhibitor, following an investigation into a “possible increased risk of death.”
As reported by this news organization, umbralisib had received accelerated approval in February 2021 to treat adults with relapsed or refractory marginal zone lymphoma following at least one prior therapy and those with relapsed or refractory follicular lymphoma who had received at least three prior therapies.
“These safety findings were similar for other medicines in the same PI3 kinase inhibitor class, which were discussed at an advisory committee meeting of non-FDA experts in April 2022,” according to the FDA warning.
The FDA urges patients and health care professionals to report side effects involving duvelisib or other medicines to the FDA MedWatch program.
A version of this article first appeared on Medscape.com
The U.S. Food and Drug Administration issued a warning today that the cancer drug duvelisib (Copiktra, Verastem), a PI3 kinase inhibitor, may increase the risk of death and serious side effects.
Duvelisib was approved in 2018 to treat adults with chronic lymphocytic leukemia (CLL) or small lymphocytic lymphoma (SLL) who had received at least two prior therapies that did not work or stopped working.
However, more recent 5-year overall survival results from the randomized phase 3 DUO clinical trial found a possible increased risk of death with duvelisib, compared with another drug used to treat leukemia and lymphoma, according to an FDA Drug Safety Communication.
“The trial also found Copiktra was associated with a higher risk of serious side effects, including infections, diarrhea, inflammation of the intestines and lungs, skin reactions, and high liver enzyme levels in the blood,” states the warning, which advises prescribers to weigh the risks and benefits of continued use versus use of other treatments.
More specifically, median 5-year overall survival among 319 patients with CLL or SLL in the DUO trial was 52.3 months with duvelisib versus 63.3 months with the monoclonal antibody ofatumumab (hazard ratio, 1.09 overall and 1.06 among patients who received at least two prior lines of therapy).
Serious adverse events of grade 3 or higher were also more common in those treated with duvelisib.
Of note, in April, the FDA also announced that it was withdrawing approval of the relapsed or refractory follicular lymphoma indication for duvelisib following a voluntary request by the drug manufacturer Secura Bio.
A public meeting will be scheduled to discuss the findings of the trial and whether the drug should continue to be prescribed.
This FDA warning follows the agency’s June 1 withdrawal of approval for umbralisib (Ukoniq), another PI3 kinase inhibitor, following an investigation into a “possible increased risk of death.”
As reported by this news organization, umbralisib had received accelerated approval in February 2021 to treat adults with relapsed or refractory marginal zone lymphoma following at least one prior therapy and those with relapsed or refractory follicular lymphoma who had received at least three prior therapies.
“These safety findings were similar for other medicines in the same PI3 kinase inhibitor class, which were discussed at an advisory committee meeting of non-FDA experts in April 2022,” according to the FDA warning.
The FDA urges patients and health care professionals to report side effects involving duvelisib or other medicines to the FDA MedWatch program.
A version of this article first appeared on Medscape.com