User login
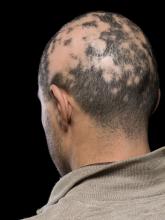
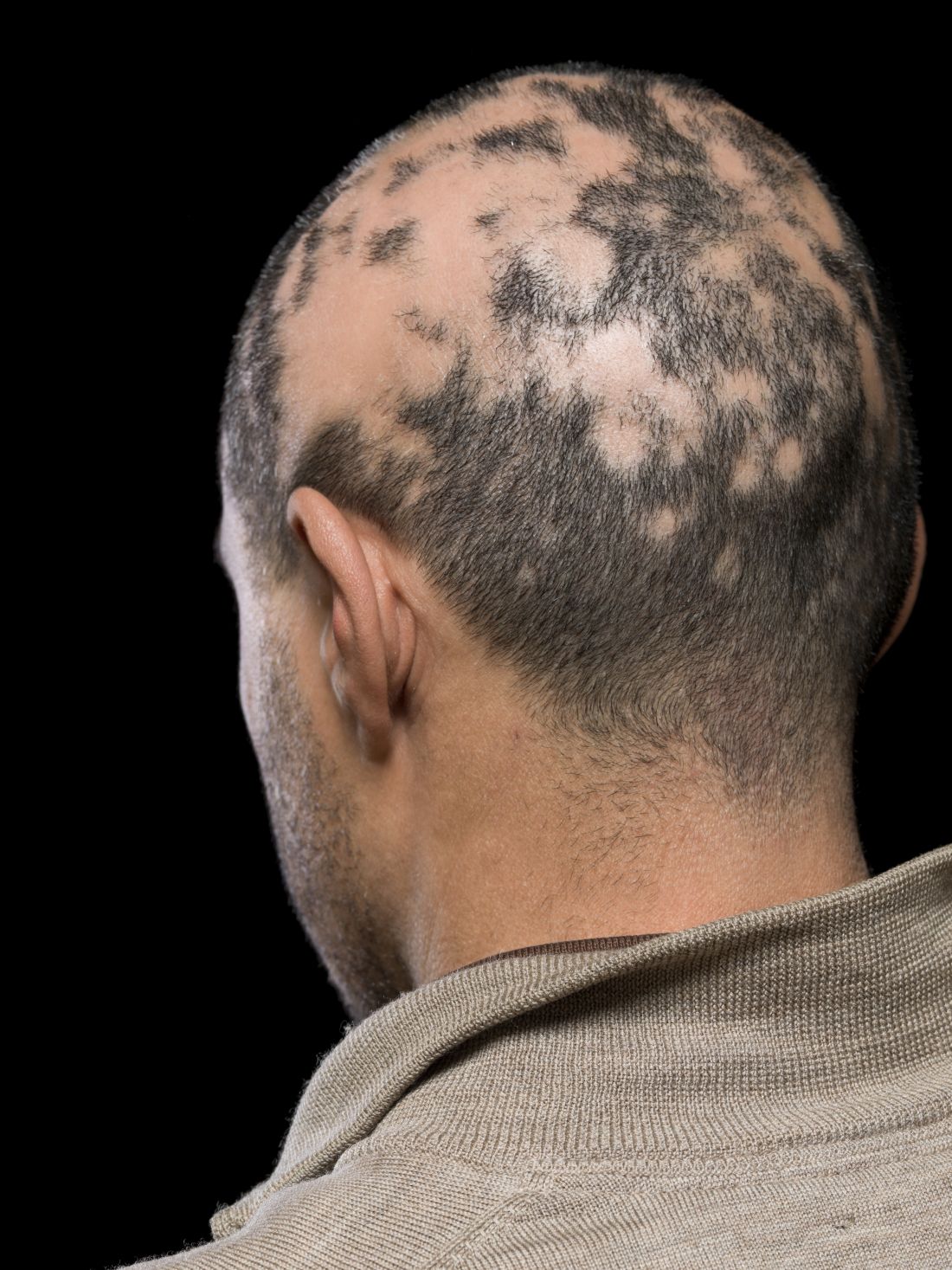
For minorities with PE: Less advanced treatment, more mortality
NEW ORLEANS –
According to the research, released at the annual meeting of the American Society of Hematology, the biggest disparities affected Asian/Pacific Islander patients with PE. While they were the least likely among ethnic groups to be hospitalized for PE, the odds were 53% higher that they’d die in the hospital (adjusted odds ratio, 1.53; 95% confidence interval, 1.32-1.78), and 24% lower that they would get advanced therapies (aOR, 0.76; 95% CI, 0.59-0.98, P values not provided in this study).
“The findings really raise the importance of this research area and call for vigorous future research to try to better identify why we see these patterns and then come up with solutions to solve them,” said hematologist and study coauthor Mary Cushman, MD, of the University of Vermont, Burlington, at an ASH news briefing.
As Dr. Cushman noted, details about disparities in PE care are limited. It’s known that “Black people have a twofold greater mortality from pulmonary embolism compared to other groups, and this is a persistently observed disparity over many years,” she said. However, “little is known about the relationships of social determinants with treatment and course of pulmonary embolism,” she added.
The researchers used data from the Nationwide Inpatient Sample to track 1.1 million U.S. hospitalized patients with PE from 2016 to 2018. PE was the primary diagnosis in 615,570 patients (54.8%), and 66,570 (5.9%) had high-risk PE.
Among ethnic groups, hospitalization rates “differed pretty dramatically,” Dr. Cushman said. The researchers found that Blacks had the highest rate of PE hospitalization (20.1 per 10,000 person-years; 95% CI, 20.0-20.2), followed by Whites (13.1 per 10,000 person-years; 95% CI, 13.1-13.2), Hispanics (6.0 per 10,000 person-years; 95% CI, 5.9-6.1), Native Americans (5.6 per 10,000 person-years, 95% CI, 5.4-5.7) and Asians/Pacific Islanders (3.0 per 10,000 person-years; 95% CI, 2.9-3.1). Overall, the rate was 14.9/10,000 person-years.
With regard to treatment, therapies defined by the researchers as advanced – systemic thrombolysis, catheter-directed therapy, surgical embolectomy, and venoarterial extracorporeal membrane oxygenation – were also less commonly used in treating ethnic minorities.
These treatments were used in 5.5% of all patients, and 19% of those with high-risk PE. After adjusting for nearly 20 factors such as age, sex, and place of residence, researchers found that the odds that a patient would receive advanced treatment were lower in Blacks (aOR, 0.87; 95% CI, 0.81-0.92) and Asians/Pacific Islanders (aOR, 0.76; 95% CI, 0.59-0.98) compared with Whites. The differences in Hispanics and Native Americans were not statistically significant.
As for insurance, those with Medicare and Medicaid were less likely to get advanced treatment vs. those with private insurance (aOR, 0.73; 95% CI, 0.69-0.77 and aOR, 0.68; 95% CI, 0.63-0.74, respectively). Differences among income levels were not statistically significant.
In the hospital, 6.4% of patients with PE died, as did 50% of those with high-risk PE. There was no statistically significant difference in death rates overall between Whites and Blacks or Native Americans. However, Asians/Pacific Islanders had a much higher death rate (aOR, 1.53; 95% CI, 1.32-1.78), as did Hispanics (aOR, 1.10; 95% CI, 1.00-1.22).
Why are Asians/Pacific Islanders at such high risk of death? Dr. Cushman noted that, while their hospitalization rate is low, they are especially likely to present with high-risk PE.
The difference in death rates between patients with Medicare/Medicaid insurance and those with private insurance was not statistically significant. Neither was the difference in death rates among income groups vs. the highest quartile with one exception: The lowest quartile (aOR, 1.09; 95% CI, 1.02-1.17).
As for the reasons for the higher risks among various groups, Dr. Cushman said there are several possible theories. “It could be due to differences in awareness of PE symptoms: They don’t know how ill they are, so they present later in the course. Or they might have less trust in the system, which might lead to delayed care. Or it could be that they have misdiagnosis of PE symptoms when they present initially.”
Alternatively, she noted, the differences “could be rooted in structural racism and other social determinants of health that weren’t measured, such as education level and quality of education.”
In an interview, Dr. Cushman expressed the hope that “clinicians will think about these findings in terms of how they take care of patients and try their best to recognize any unconscious biases that might creep into their approach. In addition, as a society we need more education of the general public about PE. Some of our findings might be caused by delayed care due to lack of recognition of a need to seek care.”
In an interview, University of Pittsburgh vascular surgeon Rabih Chaer, MD, MSc, who didn’t take part in the study, said it relies on a "large dataset which offers valuable information but with limited granularity and follow-up. This limits the accurate categorization of PE severity, as well as comorbidities, all of which impact outcomes and survival.”
For example, Dr. Chaer said, PE treatments can be limited in some patients due to their comorbidities that cause bleeding risk. Still, Dr. Chaer said the findings mesh with his own research that has shown racial disparities in PE treatment and outcomes, including a 2021 study. "While we did not see a difference by race in in-hospital mortality, Black patients hospitalized with PE are younger with a higher severity of disease compared with White patients,” he said. "Although Black patients are less likely to receive an intervention overall, this differed depending on PE severity with higher risk of intervention only for life-threatening PE." And a 2022 study found that “patients with PE from deprived neighborhoods have worse survival beyond the index [first] admission and were more likely to suffer from cardiovascular or PE-related causes of death in the first year after the index pulmonary embolism,” he said.
Dr. Chaer noted that his research team “is actively working on the next steps beyond identifying the fact that there are racial disparities in PE treatment and outcomes. We are fortunate to have access to a large granular database with long-term follow up and are currently reviewing the medical record details to identify causes for disparities and potential solutions.”
Dr. Cushman received funding from the National Institutes of Health. Other study authors report various disclosures. Dr. Chaer has no disclosures.
NEW ORLEANS –
According to the research, released at the annual meeting of the American Society of Hematology, the biggest disparities affected Asian/Pacific Islander patients with PE. While they were the least likely among ethnic groups to be hospitalized for PE, the odds were 53% higher that they’d die in the hospital (adjusted odds ratio, 1.53; 95% confidence interval, 1.32-1.78), and 24% lower that they would get advanced therapies (aOR, 0.76; 95% CI, 0.59-0.98, P values not provided in this study).
“The findings really raise the importance of this research area and call for vigorous future research to try to better identify why we see these patterns and then come up with solutions to solve them,” said hematologist and study coauthor Mary Cushman, MD, of the University of Vermont, Burlington, at an ASH news briefing.
As Dr. Cushman noted, details about disparities in PE care are limited. It’s known that “Black people have a twofold greater mortality from pulmonary embolism compared to other groups, and this is a persistently observed disparity over many years,” she said. However, “little is known about the relationships of social determinants with treatment and course of pulmonary embolism,” she added.
The researchers used data from the Nationwide Inpatient Sample to track 1.1 million U.S. hospitalized patients with PE from 2016 to 2018. PE was the primary diagnosis in 615,570 patients (54.8%), and 66,570 (5.9%) had high-risk PE.
Among ethnic groups, hospitalization rates “differed pretty dramatically,” Dr. Cushman said. The researchers found that Blacks had the highest rate of PE hospitalization (20.1 per 10,000 person-years; 95% CI, 20.0-20.2), followed by Whites (13.1 per 10,000 person-years; 95% CI, 13.1-13.2), Hispanics (6.0 per 10,000 person-years; 95% CI, 5.9-6.1), Native Americans (5.6 per 10,000 person-years, 95% CI, 5.4-5.7) and Asians/Pacific Islanders (3.0 per 10,000 person-years; 95% CI, 2.9-3.1). Overall, the rate was 14.9/10,000 person-years.
With regard to treatment, therapies defined by the researchers as advanced – systemic thrombolysis, catheter-directed therapy, surgical embolectomy, and venoarterial extracorporeal membrane oxygenation – were also less commonly used in treating ethnic minorities.
These treatments were used in 5.5% of all patients, and 19% of those with high-risk PE. After adjusting for nearly 20 factors such as age, sex, and place of residence, researchers found that the odds that a patient would receive advanced treatment were lower in Blacks (aOR, 0.87; 95% CI, 0.81-0.92) and Asians/Pacific Islanders (aOR, 0.76; 95% CI, 0.59-0.98) compared with Whites. The differences in Hispanics and Native Americans were not statistically significant.
As for insurance, those with Medicare and Medicaid were less likely to get advanced treatment vs. those with private insurance (aOR, 0.73; 95% CI, 0.69-0.77 and aOR, 0.68; 95% CI, 0.63-0.74, respectively). Differences among income levels were not statistically significant.
In the hospital, 6.4% of patients with PE died, as did 50% of those with high-risk PE. There was no statistically significant difference in death rates overall between Whites and Blacks or Native Americans. However, Asians/Pacific Islanders had a much higher death rate (aOR, 1.53; 95% CI, 1.32-1.78), as did Hispanics (aOR, 1.10; 95% CI, 1.00-1.22).
Why are Asians/Pacific Islanders at such high risk of death? Dr. Cushman noted that, while their hospitalization rate is low, they are especially likely to present with high-risk PE.
The difference in death rates between patients with Medicare/Medicaid insurance and those with private insurance was not statistically significant. Neither was the difference in death rates among income groups vs. the highest quartile with one exception: The lowest quartile (aOR, 1.09; 95% CI, 1.02-1.17).
As for the reasons for the higher risks among various groups, Dr. Cushman said there are several possible theories. “It could be due to differences in awareness of PE symptoms: They don’t know how ill they are, so they present later in the course. Or they might have less trust in the system, which might lead to delayed care. Or it could be that they have misdiagnosis of PE symptoms when they present initially.”
Alternatively, she noted, the differences “could be rooted in structural racism and other social determinants of health that weren’t measured, such as education level and quality of education.”
In an interview, Dr. Cushman expressed the hope that “clinicians will think about these findings in terms of how they take care of patients and try their best to recognize any unconscious biases that might creep into their approach. In addition, as a society we need more education of the general public about PE. Some of our findings might be caused by delayed care due to lack of recognition of a need to seek care.”
In an interview, University of Pittsburgh vascular surgeon Rabih Chaer, MD, MSc, who didn’t take part in the study, said it relies on a "large dataset which offers valuable information but with limited granularity and follow-up. This limits the accurate categorization of PE severity, as well as comorbidities, all of which impact outcomes and survival.”
For example, Dr. Chaer said, PE treatments can be limited in some patients due to their comorbidities that cause bleeding risk. Still, Dr. Chaer said the findings mesh with his own research that has shown racial disparities in PE treatment and outcomes, including a 2021 study. "While we did not see a difference by race in in-hospital mortality, Black patients hospitalized with PE are younger with a higher severity of disease compared with White patients,” he said. "Although Black patients are less likely to receive an intervention overall, this differed depending on PE severity with higher risk of intervention only for life-threatening PE." And a 2022 study found that “patients with PE from deprived neighborhoods have worse survival beyond the index [first] admission and were more likely to suffer from cardiovascular or PE-related causes of death in the first year after the index pulmonary embolism,” he said.
Dr. Chaer noted that his research team “is actively working on the next steps beyond identifying the fact that there are racial disparities in PE treatment and outcomes. We are fortunate to have access to a large granular database with long-term follow up and are currently reviewing the medical record details to identify causes for disparities and potential solutions.”
Dr. Cushman received funding from the National Institutes of Health. Other study authors report various disclosures. Dr. Chaer has no disclosures.
NEW ORLEANS –
According to the research, released at the annual meeting of the American Society of Hematology, the biggest disparities affected Asian/Pacific Islander patients with PE. While they were the least likely among ethnic groups to be hospitalized for PE, the odds were 53% higher that they’d die in the hospital (adjusted odds ratio, 1.53; 95% confidence interval, 1.32-1.78), and 24% lower that they would get advanced therapies (aOR, 0.76; 95% CI, 0.59-0.98, P values not provided in this study).
“The findings really raise the importance of this research area and call for vigorous future research to try to better identify why we see these patterns and then come up with solutions to solve them,” said hematologist and study coauthor Mary Cushman, MD, of the University of Vermont, Burlington, at an ASH news briefing.
As Dr. Cushman noted, details about disparities in PE care are limited. It’s known that “Black people have a twofold greater mortality from pulmonary embolism compared to other groups, and this is a persistently observed disparity over many years,” she said. However, “little is known about the relationships of social determinants with treatment and course of pulmonary embolism,” she added.
The researchers used data from the Nationwide Inpatient Sample to track 1.1 million U.S. hospitalized patients with PE from 2016 to 2018. PE was the primary diagnosis in 615,570 patients (54.8%), and 66,570 (5.9%) had high-risk PE.
Among ethnic groups, hospitalization rates “differed pretty dramatically,” Dr. Cushman said. The researchers found that Blacks had the highest rate of PE hospitalization (20.1 per 10,000 person-years; 95% CI, 20.0-20.2), followed by Whites (13.1 per 10,000 person-years; 95% CI, 13.1-13.2), Hispanics (6.0 per 10,000 person-years; 95% CI, 5.9-6.1), Native Americans (5.6 per 10,000 person-years, 95% CI, 5.4-5.7) and Asians/Pacific Islanders (3.0 per 10,000 person-years; 95% CI, 2.9-3.1). Overall, the rate was 14.9/10,000 person-years.
With regard to treatment, therapies defined by the researchers as advanced – systemic thrombolysis, catheter-directed therapy, surgical embolectomy, and venoarterial extracorporeal membrane oxygenation – were also less commonly used in treating ethnic minorities.
These treatments were used in 5.5% of all patients, and 19% of those with high-risk PE. After adjusting for nearly 20 factors such as age, sex, and place of residence, researchers found that the odds that a patient would receive advanced treatment were lower in Blacks (aOR, 0.87; 95% CI, 0.81-0.92) and Asians/Pacific Islanders (aOR, 0.76; 95% CI, 0.59-0.98) compared with Whites. The differences in Hispanics and Native Americans were not statistically significant.
As for insurance, those with Medicare and Medicaid were less likely to get advanced treatment vs. those with private insurance (aOR, 0.73; 95% CI, 0.69-0.77 and aOR, 0.68; 95% CI, 0.63-0.74, respectively). Differences among income levels were not statistically significant.
In the hospital, 6.4% of patients with PE died, as did 50% of those with high-risk PE. There was no statistically significant difference in death rates overall between Whites and Blacks or Native Americans. However, Asians/Pacific Islanders had a much higher death rate (aOR, 1.53; 95% CI, 1.32-1.78), as did Hispanics (aOR, 1.10; 95% CI, 1.00-1.22).
Why are Asians/Pacific Islanders at such high risk of death? Dr. Cushman noted that, while their hospitalization rate is low, they are especially likely to present with high-risk PE.
The difference in death rates between patients with Medicare/Medicaid insurance and those with private insurance was not statistically significant. Neither was the difference in death rates among income groups vs. the highest quartile with one exception: The lowest quartile (aOR, 1.09; 95% CI, 1.02-1.17).
As for the reasons for the higher risks among various groups, Dr. Cushman said there are several possible theories. “It could be due to differences in awareness of PE symptoms: They don’t know how ill they are, so they present later in the course. Or they might have less trust in the system, which might lead to delayed care. Or it could be that they have misdiagnosis of PE symptoms when they present initially.”
Alternatively, she noted, the differences “could be rooted in structural racism and other social determinants of health that weren’t measured, such as education level and quality of education.”
In an interview, Dr. Cushman expressed the hope that “clinicians will think about these findings in terms of how they take care of patients and try their best to recognize any unconscious biases that might creep into their approach. In addition, as a society we need more education of the general public about PE. Some of our findings might be caused by delayed care due to lack of recognition of a need to seek care.”
In an interview, University of Pittsburgh vascular surgeon Rabih Chaer, MD, MSc, who didn’t take part in the study, said it relies on a "large dataset which offers valuable information but with limited granularity and follow-up. This limits the accurate categorization of PE severity, as well as comorbidities, all of which impact outcomes and survival.”
For example, Dr. Chaer said, PE treatments can be limited in some patients due to their comorbidities that cause bleeding risk. Still, Dr. Chaer said the findings mesh with his own research that has shown racial disparities in PE treatment and outcomes, including a 2021 study. "While we did not see a difference by race in in-hospital mortality, Black patients hospitalized with PE are younger with a higher severity of disease compared with White patients,” he said. "Although Black patients are less likely to receive an intervention overall, this differed depending on PE severity with higher risk of intervention only for life-threatening PE." And a 2022 study found that “patients with PE from deprived neighborhoods have worse survival beyond the index [first] admission and were more likely to suffer from cardiovascular or PE-related causes of death in the first year after the index pulmonary embolism,” he said.
Dr. Chaer noted that his research team “is actively working on the next steps beyond identifying the fact that there are racial disparities in PE treatment and outcomes. We are fortunate to have access to a large granular database with long-term follow up and are currently reviewing the medical record details to identify causes for disparities and potential solutions.”
Dr. Cushman received funding from the National Institutes of Health. Other study authors report various disclosures. Dr. Chaer has no disclosures.
AT ASH 2022
Guideline stresses new strategies for hypoglycemia management
The Endocrine Society has issued an updated clinical practice guideline on the prevention and management of hypoglycemia in patients with diabetes who are at high risk, addressing the wide variety of treatment advances, such as insulin pumps and continuous glucose monitoring (CGM) systems, that have appeared since the publication of the society’s last guideline on hypoglycemia, in 2009.
“CGM and insulin pumps have been much more commonly used in the last decade among people with diabetes, including children, and there are new forms of glucagon available,” said Anthony L. McCall, MD, PhD, chair of the panel that wrote the guideline.
“We had to update our guideline to match these developments in the diabetes field,” noted Dr. McCall, University of Virginia, Charlottesville, in a press statement.
The new guideline, developed by a multidisciplinary panel of clinical experts and published in the Journal of Clinical Endocrinology and Metabolism, addresses 10 key clinical questions regarding current issues relevant to hypoglycemia prevention and treatment in adult or pediatric patients with either type 1 or type 2 diabetes in the outpatient or inpatient setting.
Key guideline recommendations
The recommendations are based on factors including critical outcomes, implementation feasibility, and patient preferences.
Key guideline recommendations that are considered “strong,” based on evidence, include:
- The use of CGM rather than self-monitoring of blood glucose by fingerstick for patients with type 1 diabetes receiving multiple daily injections. The panel underscored that “comprehensive patient education on how to use and troubleshoot CGM devices and interpret these data is critically important for maximum benefit and successful outcomes.”
The use of a structured program for patient education versus unstructured advice for adult and pediatric outpatients with type 1 diabetes or type 2 diabetes receiving insulin therapy.
- Structured education on how to avoid repeated hypoglycemia is critical, and this education should be performed by experienced diabetes clinicians,” the panel asserts. “Moreover, insurance coverage for education should be available for all insulin-using patients.”
- The use of glucagon preparations that do not have to be reconstituted, as opposed to those that do (that is, available as a powder and diluent) in the treatment of outpatients with severe hypoglycemia.
Guideline recommendations that received conditional recommendations include:
- Use of real-time CGM and algorithm-driven insulin pumps in people with type 1 diabetes.
- Use of CGM for outpatients with type 2 diabetes at high risk for hypoglycemia.
- Use of long-acting and rapid-acting insulin analogs for patients at high risk for hypoglycemia.
Noting that there is “moderate-certainty” evidence for severe hypoglycemia reduction as an outcome in those using long-acting analog insulins versus human neutral protamine Hagedorn (NPH) insulin, the panel cautions that “most studies of long-acting analog insulins do not assess for significant adverse effects, including cardiovascular outcomes, and that many studies were designed to demonstrate noninferiority of analog insulin, compared with human NPH insulin.”
- Initiation of and continuation of CGM for select inpatient populations at high risk for hypoglycemia.
Hypoglycemia: One of top three preventable adverse drug reactions
The updated guidelines are especially important considering the common incidence of hypoglycemia, which the U.S. Department of Health and Human Services has determined to be one of the top 3 preventable adverse drug reactions, the panel says.
They note that between January 2007 and December 2011, emergency department visits for therapy-associated hypoglycemia among Medicare beneficiaries resulted in more than $600 million in spending.
Meanwhile, many people with type 1 or 2 diabetes may not experience or recognize the symptoms of hypoglycemia, which, in severe cases, can lead to unconsciousness or seizures, in addition to affecting quality of life, social life, work productivity, and ability to drive safely.
The key to accurate diagnosis of those patients is assessment of the three levels of hypoglycemia, described in a 2018 consensus statement:
- Level 1: Glucose less than 70 mg/dL (3.9 mmol/L) and greater than or equal to 54 mg/dL (3.0 mmol/L). This level of hypoglycemia should alert patients that they may need to ingest carbohydrate to prevent progressive hypoglycemia.
- Level 2: Glucose less than 54 mg/dL (3.0 mmol/L). This level of hypoglycemia is associated with increased risk for cognitive dysfunction and mortality.
- Level 3: A severe event characterized by altered mental and/or physical status requiring assistance. This level of hypoglycemia is life-threatening and requires emergent treatment, typically with glucagon.
Ultimately, “new technology and medications will help reduce hypoglycemia, and [clinicians] can better treat patients now with new, easier glucagons,” Dr. McCall told this news organization.
“People with diabetes, their caregivers, and diabetes specialists will all benefit from our guideline with a better understanding of best practices and interventions,” the panel notes.
Disparities still exist in access to insulin pumps
Separately, new research shows that while use of insulin pumps to manage type 1 diabetes has grown over 20 years, there has been no improvement in racial, ethnic, and socioeconomic disparities in their use in the United States. The findings are reported in Diabetes Technology & Therapeutics.
Using data from the SEARCH for Diabetes Youth Study across four time periods between 2001 and 2019, the researchers show that by the end of the period studied, insulin pump use was 67% among non-Hispanic White people, 41% among Hispanic people, 29% among Black people, and 46% among other racial and ethnic groups.
In addition, 70% of people with bachelor’s degrees or higher used the pumps, compared with 56% among those with some college, 40% among holders of high school degrees, and 18% among those with no high school education. By income level, 74% of those with household incomes of $75,000 or more, 66% with $50,000-$74,999, 51% with $25,000-$49,999, and 41% with less than $25,000 used the pumps.
“Diabetes technology has numerous benefits for patients with type 1 diabetes, but the problem is that there is a huge divide in who actually has access to these technologies,” said study lead Estelle Everett, MD, assistant professor of medicine in the division of endocrinology, diabetes & metabolism at the University of California, Los Angeles.
A version of this article first appeared on Medscape.com.
The Endocrine Society has issued an updated clinical practice guideline on the prevention and management of hypoglycemia in patients with diabetes who are at high risk, addressing the wide variety of treatment advances, such as insulin pumps and continuous glucose monitoring (CGM) systems, that have appeared since the publication of the society’s last guideline on hypoglycemia, in 2009.
“CGM and insulin pumps have been much more commonly used in the last decade among people with diabetes, including children, and there are new forms of glucagon available,” said Anthony L. McCall, MD, PhD, chair of the panel that wrote the guideline.
“We had to update our guideline to match these developments in the diabetes field,” noted Dr. McCall, University of Virginia, Charlottesville, in a press statement.
The new guideline, developed by a multidisciplinary panel of clinical experts and published in the Journal of Clinical Endocrinology and Metabolism, addresses 10 key clinical questions regarding current issues relevant to hypoglycemia prevention and treatment in adult or pediatric patients with either type 1 or type 2 diabetes in the outpatient or inpatient setting.
Key guideline recommendations
The recommendations are based on factors including critical outcomes, implementation feasibility, and patient preferences.
Key guideline recommendations that are considered “strong,” based on evidence, include:
- The use of CGM rather than self-monitoring of blood glucose by fingerstick for patients with type 1 diabetes receiving multiple daily injections. The panel underscored that “comprehensive patient education on how to use and troubleshoot CGM devices and interpret these data is critically important for maximum benefit and successful outcomes.”
The use of a structured program for patient education versus unstructured advice for adult and pediatric outpatients with type 1 diabetes or type 2 diabetes receiving insulin therapy.
- Structured education on how to avoid repeated hypoglycemia is critical, and this education should be performed by experienced diabetes clinicians,” the panel asserts. “Moreover, insurance coverage for education should be available for all insulin-using patients.”
- The use of glucagon preparations that do not have to be reconstituted, as opposed to those that do (that is, available as a powder and diluent) in the treatment of outpatients with severe hypoglycemia.
Guideline recommendations that received conditional recommendations include:
- Use of real-time CGM and algorithm-driven insulin pumps in people with type 1 diabetes.
- Use of CGM for outpatients with type 2 diabetes at high risk for hypoglycemia.
- Use of long-acting and rapid-acting insulin analogs for patients at high risk for hypoglycemia.
Noting that there is “moderate-certainty” evidence for severe hypoglycemia reduction as an outcome in those using long-acting analog insulins versus human neutral protamine Hagedorn (NPH) insulin, the panel cautions that “most studies of long-acting analog insulins do not assess for significant adverse effects, including cardiovascular outcomes, and that many studies were designed to demonstrate noninferiority of analog insulin, compared with human NPH insulin.”
- Initiation of and continuation of CGM for select inpatient populations at high risk for hypoglycemia.
Hypoglycemia: One of top three preventable adverse drug reactions
The updated guidelines are especially important considering the common incidence of hypoglycemia, which the U.S. Department of Health and Human Services has determined to be one of the top 3 preventable adverse drug reactions, the panel says.
They note that between January 2007 and December 2011, emergency department visits for therapy-associated hypoglycemia among Medicare beneficiaries resulted in more than $600 million in spending.
Meanwhile, many people with type 1 or 2 diabetes may not experience or recognize the symptoms of hypoglycemia, which, in severe cases, can lead to unconsciousness or seizures, in addition to affecting quality of life, social life, work productivity, and ability to drive safely.
The key to accurate diagnosis of those patients is assessment of the three levels of hypoglycemia, described in a 2018 consensus statement:
- Level 1: Glucose less than 70 mg/dL (3.9 mmol/L) and greater than or equal to 54 mg/dL (3.0 mmol/L). This level of hypoglycemia should alert patients that they may need to ingest carbohydrate to prevent progressive hypoglycemia.
- Level 2: Glucose less than 54 mg/dL (3.0 mmol/L). This level of hypoglycemia is associated with increased risk for cognitive dysfunction and mortality.
- Level 3: A severe event characterized by altered mental and/or physical status requiring assistance. This level of hypoglycemia is life-threatening and requires emergent treatment, typically with glucagon.
Ultimately, “new technology and medications will help reduce hypoglycemia, and [clinicians] can better treat patients now with new, easier glucagons,” Dr. McCall told this news organization.
“People with diabetes, their caregivers, and diabetes specialists will all benefit from our guideline with a better understanding of best practices and interventions,” the panel notes.
Disparities still exist in access to insulin pumps
Separately, new research shows that while use of insulin pumps to manage type 1 diabetes has grown over 20 years, there has been no improvement in racial, ethnic, and socioeconomic disparities in their use in the United States. The findings are reported in Diabetes Technology & Therapeutics.
Using data from the SEARCH for Diabetes Youth Study across four time periods between 2001 and 2019, the researchers show that by the end of the period studied, insulin pump use was 67% among non-Hispanic White people, 41% among Hispanic people, 29% among Black people, and 46% among other racial and ethnic groups.
In addition, 70% of people with bachelor’s degrees or higher used the pumps, compared with 56% among those with some college, 40% among holders of high school degrees, and 18% among those with no high school education. By income level, 74% of those with household incomes of $75,000 or more, 66% with $50,000-$74,999, 51% with $25,000-$49,999, and 41% with less than $25,000 used the pumps.
“Diabetes technology has numerous benefits for patients with type 1 diabetes, but the problem is that there is a huge divide in who actually has access to these technologies,” said study lead Estelle Everett, MD, assistant professor of medicine in the division of endocrinology, diabetes & metabolism at the University of California, Los Angeles.
A version of this article first appeared on Medscape.com.
The Endocrine Society has issued an updated clinical practice guideline on the prevention and management of hypoglycemia in patients with diabetes who are at high risk, addressing the wide variety of treatment advances, such as insulin pumps and continuous glucose monitoring (CGM) systems, that have appeared since the publication of the society’s last guideline on hypoglycemia, in 2009.
“CGM and insulin pumps have been much more commonly used in the last decade among people with diabetes, including children, and there are new forms of glucagon available,” said Anthony L. McCall, MD, PhD, chair of the panel that wrote the guideline.
“We had to update our guideline to match these developments in the diabetes field,” noted Dr. McCall, University of Virginia, Charlottesville, in a press statement.
The new guideline, developed by a multidisciplinary panel of clinical experts and published in the Journal of Clinical Endocrinology and Metabolism, addresses 10 key clinical questions regarding current issues relevant to hypoglycemia prevention and treatment in adult or pediatric patients with either type 1 or type 2 diabetes in the outpatient or inpatient setting.
Key guideline recommendations
The recommendations are based on factors including critical outcomes, implementation feasibility, and patient preferences.
Key guideline recommendations that are considered “strong,” based on evidence, include:
- The use of CGM rather than self-monitoring of blood glucose by fingerstick for patients with type 1 diabetes receiving multiple daily injections. The panel underscored that “comprehensive patient education on how to use and troubleshoot CGM devices and interpret these data is critically important for maximum benefit and successful outcomes.”
The use of a structured program for patient education versus unstructured advice for adult and pediatric outpatients with type 1 diabetes or type 2 diabetes receiving insulin therapy.
- Structured education on how to avoid repeated hypoglycemia is critical, and this education should be performed by experienced diabetes clinicians,” the panel asserts. “Moreover, insurance coverage for education should be available for all insulin-using patients.”
- The use of glucagon preparations that do not have to be reconstituted, as opposed to those that do (that is, available as a powder and diluent) in the treatment of outpatients with severe hypoglycemia.
Guideline recommendations that received conditional recommendations include:
- Use of real-time CGM and algorithm-driven insulin pumps in people with type 1 diabetes.
- Use of CGM for outpatients with type 2 diabetes at high risk for hypoglycemia.
- Use of long-acting and rapid-acting insulin analogs for patients at high risk for hypoglycemia.
Noting that there is “moderate-certainty” evidence for severe hypoglycemia reduction as an outcome in those using long-acting analog insulins versus human neutral protamine Hagedorn (NPH) insulin, the panel cautions that “most studies of long-acting analog insulins do not assess for significant adverse effects, including cardiovascular outcomes, and that many studies were designed to demonstrate noninferiority of analog insulin, compared with human NPH insulin.”
- Initiation of and continuation of CGM for select inpatient populations at high risk for hypoglycemia.
Hypoglycemia: One of top three preventable adverse drug reactions
The updated guidelines are especially important considering the common incidence of hypoglycemia, which the U.S. Department of Health and Human Services has determined to be one of the top 3 preventable adverse drug reactions, the panel says.
They note that between January 2007 and December 2011, emergency department visits for therapy-associated hypoglycemia among Medicare beneficiaries resulted in more than $600 million in spending.
Meanwhile, many people with type 1 or 2 diabetes may not experience or recognize the symptoms of hypoglycemia, which, in severe cases, can lead to unconsciousness or seizures, in addition to affecting quality of life, social life, work productivity, and ability to drive safely.
The key to accurate diagnosis of those patients is assessment of the three levels of hypoglycemia, described in a 2018 consensus statement:
- Level 1: Glucose less than 70 mg/dL (3.9 mmol/L) and greater than or equal to 54 mg/dL (3.0 mmol/L). This level of hypoglycemia should alert patients that they may need to ingest carbohydrate to prevent progressive hypoglycemia.
- Level 2: Glucose less than 54 mg/dL (3.0 mmol/L). This level of hypoglycemia is associated with increased risk for cognitive dysfunction and mortality.
- Level 3: A severe event characterized by altered mental and/or physical status requiring assistance. This level of hypoglycemia is life-threatening and requires emergent treatment, typically with glucagon.
Ultimately, “new technology and medications will help reduce hypoglycemia, and [clinicians] can better treat patients now with new, easier glucagons,” Dr. McCall told this news organization.
“People with diabetes, their caregivers, and diabetes specialists will all benefit from our guideline with a better understanding of best practices and interventions,” the panel notes.
Disparities still exist in access to insulin pumps
Separately, new research shows that while use of insulin pumps to manage type 1 diabetes has grown over 20 years, there has been no improvement in racial, ethnic, and socioeconomic disparities in their use in the United States. The findings are reported in Diabetes Technology & Therapeutics.
Using data from the SEARCH for Diabetes Youth Study across four time periods between 2001 and 2019, the researchers show that by the end of the period studied, insulin pump use was 67% among non-Hispanic White people, 41% among Hispanic people, 29% among Black people, and 46% among other racial and ethnic groups.
In addition, 70% of people with bachelor’s degrees or higher used the pumps, compared with 56% among those with some college, 40% among holders of high school degrees, and 18% among those with no high school education. By income level, 74% of those with household incomes of $75,000 or more, 66% with $50,000-$74,999, 51% with $25,000-$49,999, and 41% with less than $25,000 used the pumps.
“Diabetes technology has numerous benefits for patients with type 1 diabetes, but the problem is that there is a huge divide in who actually has access to these technologies,” said study lead Estelle Everett, MD, assistant professor of medicine in the division of endocrinology, diabetes & metabolism at the University of California, Los Angeles.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF CLINICAL ENDOCRINOLOGY AND METABOLISM
Study implicates myelin plasticity in absence seizures
NASHVILLE, TENN. – that seems to provoke dysregulation of the insulating layer surrounding nerve fibers, perpetuating a cycle of increasing nerve damage and more frequent seizures later on.
“This study was the first to demonstrate that, at least in some forms of epilepsy, myelin plasticity is part of the maladaptive plasticity response that underlines epilepsy progression,” Juliet Knowles, MD, PhD, assistant professor at Stanford (Calif.) University, said in an interview. She reported the findings at the 2022 annual meeting of the American Epilepsy Society.
Dr. Knowles and colleagues made their discovery using laboratory mice. They used an imaging technique known as qMTI – quantitative magnetization transfer in conjunction with diffusion MRI – to map changes in myelin sheath thickness, or myelin plasticity, in major white matter tracks of the brain.
“Over the last decade we’ve come to understand that myelin, which is the insulating substance that coats the projections of brain cells or neurons, is more dynamic than we used to think,” she said. “In fact, throughout life, myelin’s structure in some regions of the brain can be changed in response to neuro activity. It’s a newly appreciated form of brain plasticity.”
However, she said, myelin plasticity has mostly been studied in healthy brains; “We don’t know very much about what role myelin plasticity might play in disease states like epilepsy,” Dr. Knowles said. The study’s goal was to investigate myelin plasticity specifically in absence seizures.
“We hypothesized that maybe absence seizures prompt activity-dependent myelin plasticity, but that maybe seizure-induced myelin plasticity alters the way that brain networks act in a way that contributes to the disease process,” she said.
Maladaptive myelin plasticity
The researchers found that absence seizures were infrequent when they first started, but then they rapidly progressed. “Over a couple of weeks, they’ll go from having very few seizures to having many seizures per hour,” Dr. Knowles said.
Using qMTI, the researchers found increased myelin sheath thickness across the longitudinal extent of the anterior corpus callosum, but they found myelin sheath thickness unchanged in brain regions where absence seizures weren’t prominent.
They also found that genetically blocking activity-dependent myelination markedly decreased seizure progression and decreased ictal somatosensory electroencephalography (EEG) coherence. Conversely, blocking myelin plasticity had no effect on ictal EEG coherence between visual cortices connected by the posterior corpus callosum.
The next step for the researchers is to develop MRI methods to use in human studies, Dr. Knowles said.
“We are working on developing an imaging approach in these same animal models that we hope we can use also to study in a detailed way white matter plasticity in humans with epilepsy and we’re also continuing our studies in animal models to try to identify ways to target maladaptive myelin plasticity, which ultimately we hope will inform treatment of people with epilepsy,” Dr. Knowles said.
Of mice and men
Although this study used mice, Chris Dulla, PhD, associate professor and director of the neuroscience graduate program at Tufts University in Boston, said the finding is “probably pretty transferable” to humans.
“This is the first study that really showed it,” he said of the link between myelin changes and seizure frequency. “I think people have suspected it, but that’s why this is kind of a big deal because this is one of the first studies to show it conclusively.”
He offered suggestions for validating the findings in humans. “The first thing would be to do imaging studies in people where you can examine to see if those white matter tracks are altered in a similar way in people with epilepsy,” he said. “I think now this study gives us good reason to undertake the work that it would take to ask that question and answer it in the human brain.”
Dr. Knowles and Dr. Dulla have no relevant relationships to disclose.
NASHVILLE, TENN. – that seems to provoke dysregulation of the insulating layer surrounding nerve fibers, perpetuating a cycle of increasing nerve damage and more frequent seizures later on.
“This study was the first to demonstrate that, at least in some forms of epilepsy, myelin plasticity is part of the maladaptive plasticity response that underlines epilepsy progression,” Juliet Knowles, MD, PhD, assistant professor at Stanford (Calif.) University, said in an interview. She reported the findings at the 2022 annual meeting of the American Epilepsy Society.
Dr. Knowles and colleagues made their discovery using laboratory mice. They used an imaging technique known as qMTI – quantitative magnetization transfer in conjunction with diffusion MRI – to map changes in myelin sheath thickness, or myelin plasticity, in major white matter tracks of the brain.
“Over the last decade we’ve come to understand that myelin, which is the insulating substance that coats the projections of brain cells or neurons, is more dynamic than we used to think,” she said. “In fact, throughout life, myelin’s structure in some regions of the brain can be changed in response to neuro activity. It’s a newly appreciated form of brain plasticity.”
However, she said, myelin plasticity has mostly been studied in healthy brains; “We don’t know very much about what role myelin plasticity might play in disease states like epilepsy,” Dr. Knowles said. The study’s goal was to investigate myelin plasticity specifically in absence seizures.
“We hypothesized that maybe absence seizures prompt activity-dependent myelin plasticity, but that maybe seizure-induced myelin plasticity alters the way that brain networks act in a way that contributes to the disease process,” she said.
Maladaptive myelin plasticity
The researchers found that absence seizures were infrequent when they first started, but then they rapidly progressed. “Over a couple of weeks, they’ll go from having very few seizures to having many seizures per hour,” Dr. Knowles said.
Using qMTI, the researchers found increased myelin sheath thickness across the longitudinal extent of the anterior corpus callosum, but they found myelin sheath thickness unchanged in brain regions where absence seizures weren’t prominent.
They also found that genetically blocking activity-dependent myelination markedly decreased seizure progression and decreased ictal somatosensory electroencephalography (EEG) coherence. Conversely, blocking myelin plasticity had no effect on ictal EEG coherence between visual cortices connected by the posterior corpus callosum.
The next step for the researchers is to develop MRI methods to use in human studies, Dr. Knowles said.
“We are working on developing an imaging approach in these same animal models that we hope we can use also to study in a detailed way white matter plasticity in humans with epilepsy and we’re also continuing our studies in animal models to try to identify ways to target maladaptive myelin plasticity, which ultimately we hope will inform treatment of people with epilepsy,” Dr. Knowles said.
Of mice and men
Although this study used mice, Chris Dulla, PhD, associate professor and director of the neuroscience graduate program at Tufts University in Boston, said the finding is “probably pretty transferable” to humans.
“This is the first study that really showed it,” he said of the link between myelin changes and seizure frequency. “I think people have suspected it, but that’s why this is kind of a big deal because this is one of the first studies to show it conclusively.”
He offered suggestions for validating the findings in humans. “The first thing would be to do imaging studies in people where you can examine to see if those white matter tracks are altered in a similar way in people with epilepsy,” he said. “I think now this study gives us good reason to undertake the work that it would take to ask that question and answer it in the human brain.”
Dr. Knowles and Dr. Dulla have no relevant relationships to disclose.
NASHVILLE, TENN. – that seems to provoke dysregulation of the insulating layer surrounding nerve fibers, perpetuating a cycle of increasing nerve damage and more frequent seizures later on.
“This study was the first to demonstrate that, at least in some forms of epilepsy, myelin plasticity is part of the maladaptive plasticity response that underlines epilepsy progression,” Juliet Knowles, MD, PhD, assistant professor at Stanford (Calif.) University, said in an interview. She reported the findings at the 2022 annual meeting of the American Epilepsy Society.
Dr. Knowles and colleagues made their discovery using laboratory mice. They used an imaging technique known as qMTI – quantitative magnetization transfer in conjunction with diffusion MRI – to map changes in myelin sheath thickness, or myelin plasticity, in major white matter tracks of the brain.
“Over the last decade we’ve come to understand that myelin, which is the insulating substance that coats the projections of brain cells or neurons, is more dynamic than we used to think,” she said. “In fact, throughout life, myelin’s structure in some regions of the brain can be changed in response to neuro activity. It’s a newly appreciated form of brain plasticity.”
However, she said, myelin plasticity has mostly been studied in healthy brains; “We don’t know very much about what role myelin plasticity might play in disease states like epilepsy,” Dr. Knowles said. The study’s goal was to investigate myelin plasticity specifically in absence seizures.
“We hypothesized that maybe absence seizures prompt activity-dependent myelin plasticity, but that maybe seizure-induced myelin plasticity alters the way that brain networks act in a way that contributes to the disease process,” she said.
Maladaptive myelin plasticity
The researchers found that absence seizures were infrequent when they first started, but then they rapidly progressed. “Over a couple of weeks, they’ll go from having very few seizures to having many seizures per hour,” Dr. Knowles said.
Using qMTI, the researchers found increased myelin sheath thickness across the longitudinal extent of the anterior corpus callosum, but they found myelin sheath thickness unchanged in brain regions where absence seizures weren’t prominent.
They also found that genetically blocking activity-dependent myelination markedly decreased seizure progression and decreased ictal somatosensory electroencephalography (EEG) coherence. Conversely, blocking myelin plasticity had no effect on ictal EEG coherence between visual cortices connected by the posterior corpus callosum.
The next step for the researchers is to develop MRI methods to use in human studies, Dr. Knowles said.
“We are working on developing an imaging approach in these same animal models that we hope we can use also to study in a detailed way white matter plasticity in humans with epilepsy and we’re also continuing our studies in animal models to try to identify ways to target maladaptive myelin plasticity, which ultimately we hope will inform treatment of people with epilepsy,” Dr. Knowles said.
Of mice and men
Although this study used mice, Chris Dulla, PhD, associate professor and director of the neuroscience graduate program at Tufts University in Boston, said the finding is “probably pretty transferable” to humans.
“This is the first study that really showed it,” he said of the link between myelin changes and seizure frequency. “I think people have suspected it, but that’s why this is kind of a big deal because this is one of the first studies to show it conclusively.”
He offered suggestions for validating the findings in humans. “The first thing would be to do imaging studies in people where you can examine to see if those white matter tracks are altered in a similar way in people with epilepsy,” he said. “I think now this study gives us good reason to undertake the work that it would take to ask that question and answer it in the human brain.”
Dr. Knowles and Dr. Dulla have no relevant relationships to disclose.
AT AES 2022
Newer brand-name drugs fuel spending on antiseizure medications
NASHVILLE, TENN. – , pointing to a major shift to newer, costlier, brand-name drugs – a trend in spending that may not be sustainable, the lead author of a study of drug costs said.
The study, presented at the 2022 annual meeting of the American Epilepsy Society, evaluated claims data for prescriptions for common antiseizure medications in the Medicare Part D and Medicaid databases from 2012 to 2020. The study excluded gabapentin and pregabalin because they’re frequently prescribed for other indications in addition to epileptic seizures.
“We found that third-generation medications, even though they accounted for the smallest percentage of claims in 2020, took up the most astronomical portion of the money that was spent,” lead author Deepti Zutshi, MD, an associate professor of neurology at Wayne State University in Detroit, said in an interview.
The study found that Medicare Part D spending on antiseizure medications increased from $1.16 billion in 2012 to $2.68 billion in 2020. In Medicaid, spending followed a similar trend, increasing from $973 million in 2012 to $1.05 billion in 2020.
Analyzing Medicare/Medicaid claims data
The study categorized drugs two ways: by brand or generic; and by first, second, or third generation, Dr. Zutshi said. First-generation drugs include medications such as phenobarbital, phenytoin, valproate, and carbamazepine. Second-generation medications were released in the early 2000s and include medications such as lamotrigine and levetiracetam. Examples of third-generation drugs include lacosamide, vigabatrin, clobazam, and perampanel.
Prescribers shifted significantly to third-generation treatments, Dr. Zutshi said. In Medicare Part D, the total spent on third-generation antiseizure medications went from $124 million in 2012 to $1.08 billion in 2020, representing a quadrupling in percentage of costs, from 10.7% to 40.4%. The total number of claims for third-generation antiseizure medications was 240,000 in 2012 (1.3%) and 1.1 million in 2020 (4.4%).
When looking at brand versus generic, the total spent on brand-name antiseizure medications increased nearly threefold from $546 million in 2012 to $1.62 million in 2020, with the share of all funding spent on brand-name antiseizure medications jumping from 46.8% to 60.2%. However, the proportion of total claims for branded antiseizure medications actually dropped, from 9.24% in 2012 to 6.62% in 2020.
Medicaid trends followed a similar pattern. Third-generation antiseizure medications accounted for 1.7% of total claims in 2012 and 6% in 2020. Spending on third-generation antiseizure medications grew nearly eight times: from $147 million, or 15.1% of funding spent on antiseizure medications, in 2012 to $1.15 billion in 2020, a 56.1% share of costs. The total spend of branded antiseizure medications in Medicaid was $605 million in 2012 and $1.46 billion in 2020 – a jump in the share of total spending from 62.2% to 71.3%. As in Medicare Part D, the percentage of total claims for branded antiseizure medications in Medicaid also dropped from 2012 to 2020, from 12.1% to 6.8%.
Why the substantial increase in spending?
“The reason we are prescribing these more expensive medications may be that the third-generation medications have better side-effect profiles, improved safety and outcomes in pregnancy, or that they have less drug interactions with other medications,” Dr. Zutshi said.
That’s desirable for older patients on Medicare who are more likely to have comorbidities and be on other medications, or women of child-bearing age on Medicaid, Dr. Zutshi said. “But I don’t think people realize what the cost is to Medicare and Medicaid,” she said, “so this was a bit of a shocking finding in our paper when we looked at this. I wasn’t expecting to see the substantial increase of spending focusing on just a few medications.”
Neurologists and other providers have to be more aware of individual patients’ needs as well as cost when prescribing branded or third-generation antiseizure medications, Dr. Zutshi said. “We have to do what’s best for all of our patients, but it has to be sustainable. If not, we could start losing the ability to prescribe these medications in these vulnerable population groups, so we have to use them judiciously,” Dr. Zutshi said.
Controlling costs versus managing seizures
Timothy E. Welty, PharmD, a professor of pharmacy at Drake University in Des Moines, Iowa, noted some potential issues with the study’s methodology, namely that, while it excluded gabapentin and pregabalin, it did include other antiseizure medications that are used for other indications without accounting for them. Additionally, the pharmacy claims data the study used didn’t cross match with any diagnostic data.
Controlling drug costs is noteworthy, he said, but managing seizures is equally important. “You have to think not only in terms of preventing seizures and what impact that has on health care costs specifically, but what impact that has on overall costs to society,” Dr. Welty said. “Doing the best we can to get their seizures under control as quickly as possible has great benefits for the patient outside of health care costs.”
He added, “We just really need to educate pharmacists and decision makers within third-party payers, be it Medicare, Medicaid, private insurance, whatever, on the advances that are being made in the use of seizure medications to treat epilepsy and stop seizures, but it’s a far broader issue than just how many dollars are we spending on seizure medication.”
Dr. Zutshi and Dr. Welty have no relevant disclosures to report.
NASHVILLE, TENN. – , pointing to a major shift to newer, costlier, brand-name drugs – a trend in spending that may not be sustainable, the lead author of a study of drug costs said.
The study, presented at the 2022 annual meeting of the American Epilepsy Society, evaluated claims data for prescriptions for common antiseizure medications in the Medicare Part D and Medicaid databases from 2012 to 2020. The study excluded gabapentin and pregabalin because they’re frequently prescribed for other indications in addition to epileptic seizures.
“We found that third-generation medications, even though they accounted for the smallest percentage of claims in 2020, took up the most astronomical portion of the money that was spent,” lead author Deepti Zutshi, MD, an associate professor of neurology at Wayne State University in Detroit, said in an interview.
The study found that Medicare Part D spending on antiseizure medications increased from $1.16 billion in 2012 to $2.68 billion in 2020. In Medicaid, spending followed a similar trend, increasing from $973 million in 2012 to $1.05 billion in 2020.
Analyzing Medicare/Medicaid claims data
The study categorized drugs two ways: by brand or generic; and by first, second, or third generation, Dr. Zutshi said. First-generation drugs include medications such as phenobarbital, phenytoin, valproate, and carbamazepine. Second-generation medications were released in the early 2000s and include medications such as lamotrigine and levetiracetam. Examples of third-generation drugs include lacosamide, vigabatrin, clobazam, and perampanel.
Prescribers shifted significantly to third-generation treatments, Dr. Zutshi said. In Medicare Part D, the total spent on third-generation antiseizure medications went from $124 million in 2012 to $1.08 billion in 2020, representing a quadrupling in percentage of costs, from 10.7% to 40.4%. The total number of claims for third-generation antiseizure medications was 240,000 in 2012 (1.3%) and 1.1 million in 2020 (4.4%).
When looking at brand versus generic, the total spent on brand-name antiseizure medications increased nearly threefold from $546 million in 2012 to $1.62 million in 2020, with the share of all funding spent on brand-name antiseizure medications jumping from 46.8% to 60.2%. However, the proportion of total claims for branded antiseizure medications actually dropped, from 9.24% in 2012 to 6.62% in 2020.
Medicaid trends followed a similar pattern. Third-generation antiseizure medications accounted for 1.7% of total claims in 2012 and 6% in 2020. Spending on third-generation antiseizure medications grew nearly eight times: from $147 million, or 15.1% of funding spent on antiseizure medications, in 2012 to $1.15 billion in 2020, a 56.1% share of costs. The total spend of branded antiseizure medications in Medicaid was $605 million in 2012 and $1.46 billion in 2020 – a jump in the share of total spending from 62.2% to 71.3%. As in Medicare Part D, the percentage of total claims for branded antiseizure medications in Medicaid also dropped from 2012 to 2020, from 12.1% to 6.8%.
Why the substantial increase in spending?
“The reason we are prescribing these more expensive medications may be that the third-generation medications have better side-effect profiles, improved safety and outcomes in pregnancy, or that they have less drug interactions with other medications,” Dr. Zutshi said.
That’s desirable for older patients on Medicare who are more likely to have comorbidities and be on other medications, or women of child-bearing age on Medicaid, Dr. Zutshi said. “But I don’t think people realize what the cost is to Medicare and Medicaid,” she said, “so this was a bit of a shocking finding in our paper when we looked at this. I wasn’t expecting to see the substantial increase of spending focusing on just a few medications.”
Neurologists and other providers have to be more aware of individual patients’ needs as well as cost when prescribing branded or third-generation antiseizure medications, Dr. Zutshi said. “We have to do what’s best for all of our patients, but it has to be sustainable. If not, we could start losing the ability to prescribe these medications in these vulnerable population groups, so we have to use them judiciously,” Dr. Zutshi said.
Controlling costs versus managing seizures
Timothy E. Welty, PharmD, a professor of pharmacy at Drake University in Des Moines, Iowa, noted some potential issues with the study’s methodology, namely that, while it excluded gabapentin and pregabalin, it did include other antiseizure medications that are used for other indications without accounting for them. Additionally, the pharmacy claims data the study used didn’t cross match with any diagnostic data.
Controlling drug costs is noteworthy, he said, but managing seizures is equally important. “You have to think not only in terms of preventing seizures and what impact that has on health care costs specifically, but what impact that has on overall costs to society,” Dr. Welty said. “Doing the best we can to get their seizures under control as quickly as possible has great benefits for the patient outside of health care costs.”
He added, “We just really need to educate pharmacists and decision makers within third-party payers, be it Medicare, Medicaid, private insurance, whatever, on the advances that are being made in the use of seizure medications to treat epilepsy and stop seizures, but it’s a far broader issue than just how many dollars are we spending on seizure medication.”
Dr. Zutshi and Dr. Welty have no relevant disclosures to report.
NASHVILLE, TENN. – , pointing to a major shift to newer, costlier, brand-name drugs – a trend in spending that may not be sustainable, the lead author of a study of drug costs said.
The study, presented at the 2022 annual meeting of the American Epilepsy Society, evaluated claims data for prescriptions for common antiseizure medications in the Medicare Part D and Medicaid databases from 2012 to 2020. The study excluded gabapentin and pregabalin because they’re frequently prescribed for other indications in addition to epileptic seizures.
“We found that third-generation medications, even though they accounted for the smallest percentage of claims in 2020, took up the most astronomical portion of the money that was spent,” lead author Deepti Zutshi, MD, an associate professor of neurology at Wayne State University in Detroit, said in an interview.
The study found that Medicare Part D spending on antiseizure medications increased from $1.16 billion in 2012 to $2.68 billion in 2020. In Medicaid, spending followed a similar trend, increasing from $973 million in 2012 to $1.05 billion in 2020.
Analyzing Medicare/Medicaid claims data
The study categorized drugs two ways: by brand or generic; and by first, second, or third generation, Dr. Zutshi said. First-generation drugs include medications such as phenobarbital, phenytoin, valproate, and carbamazepine. Second-generation medications were released in the early 2000s and include medications such as lamotrigine and levetiracetam. Examples of third-generation drugs include lacosamide, vigabatrin, clobazam, and perampanel.
Prescribers shifted significantly to third-generation treatments, Dr. Zutshi said. In Medicare Part D, the total spent on third-generation antiseizure medications went from $124 million in 2012 to $1.08 billion in 2020, representing a quadrupling in percentage of costs, from 10.7% to 40.4%. The total number of claims for third-generation antiseizure medications was 240,000 in 2012 (1.3%) and 1.1 million in 2020 (4.4%).
When looking at brand versus generic, the total spent on brand-name antiseizure medications increased nearly threefold from $546 million in 2012 to $1.62 million in 2020, with the share of all funding spent on brand-name antiseizure medications jumping from 46.8% to 60.2%. However, the proportion of total claims for branded antiseizure medications actually dropped, from 9.24% in 2012 to 6.62% in 2020.
Medicaid trends followed a similar pattern. Third-generation antiseizure medications accounted for 1.7% of total claims in 2012 and 6% in 2020. Spending on third-generation antiseizure medications grew nearly eight times: from $147 million, or 15.1% of funding spent on antiseizure medications, in 2012 to $1.15 billion in 2020, a 56.1% share of costs. The total spend of branded antiseizure medications in Medicaid was $605 million in 2012 and $1.46 billion in 2020 – a jump in the share of total spending from 62.2% to 71.3%. As in Medicare Part D, the percentage of total claims for branded antiseizure medications in Medicaid also dropped from 2012 to 2020, from 12.1% to 6.8%.
Why the substantial increase in spending?
“The reason we are prescribing these more expensive medications may be that the third-generation medications have better side-effect profiles, improved safety and outcomes in pregnancy, or that they have less drug interactions with other medications,” Dr. Zutshi said.
That’s desirable for older patients on Medicare who are more likely to have comorbidities and be on other medications, or women of child-bearing age on Medicaid, Dr. Zutshi said. “But I don’t think people realize what the cost is to Medicare and Medicaid,” she said, “so this was a bit of a shocking finding in our paper when we looked at this. I wasn’t expecting to see the substantial increase of spending focusing on just a few medications.”
Neurologists and other providers have to be more aware of individual patients’ needs as well as cost when prescribing branded or third-generation antiseizure medications, Dr. Zutshi said. “We have to do what’s best for all of our patients, but it has to be sustainable. If not, we could start losing the ability to prescribe these medications in these vulnerable population groups, so we have to use them judiciously,” Dr. Zutshi said.
Controlling costs versus managing seizures
Timothy E. Welty, PharmD, a professor of pharmacy at Drake University in Des Moines, Iowa, noted some potential issues with the study’s methodology, namely that, while it excluded gabapentin and pregabalin, it did include other antiseizure medications that are used for other indications without accounting for them. Additionally, the pharmacy claims data the study used didn’t cross match with any diagnostic data.
Controlling drug costs is noteworthy, he said, but managing seizures is equally important. “You have to think not only in terms of preventing seizures and what impact that has on health care costs specifically, but what impact that has on overall costs to society,” Dr. Welty said. “Doing the best we can to get their seizures under control as quickly as possible has great benefits for the patient outside of health care costs.”
He added, “We just really need to educate pharmacists and decision makers within third-party payers, be it Medicare, Medicaid, private insurance, whatever, on the advances that are being made in the use of seizure medications to treat epilepsy and stop seizures, but it’s a far broader issue than just how many dollars are we spending on seizure medication.”
Dr. Zutshi and Dr. Welty have no relevant disclosures to report.
AT AES 2022
Oral SERD camizestrant prolongs PFS vs. fulvestrant in breast cancer
SAN ANTONIO – compared with the first-generation SERD fulvestrant Faslodex, in the SERENA-2 trial, shows a study recently presented at the San Antonio Breast Cancer Symposium.
Among 180 postmenopausal women with ER+/HER2– breast cancers that had recurred or progressed following at least one line of endocrine therapy, the median progression-free survival (PFS) after a median follow-up of 16.6-17.4 months was 7.2 months for patients treated at a 75-mg dose of camizestrant and 7.7 months for those treated at a 150-mg dose, compared with 3.7 months for patients who received fulvestrant, reported Mafalda Oliveira, MD, PhD, from Vall d’Hebron University Hospital in Barcelona.
Oral agent
Camizestrant is a next-generation oral SERD and pure estrogen receptor antagonist that was shown in the SERENA-1 trial to be safe and to have clinical activity against ER+ breast cancers.
SERENA-2 pitted camizestrant at doses of 75 mg, 150 mg, or 300 mg against standard-dose fulvestrant, although the 300-mg dose was dropped in a protocol amendment after 20 patients had been assigned to that arm. (Currently planned studies with camizestrant will be conducted with the 75-mg dose.)
The investigators enrolled women with ER+/HER2– advanced breast cancer who had not previously received fulvestrant or an oral SERD. Eligible patients were limited to no more than one prior line of endocrine and one prior line of chemotherapy for advanced breast cancers. The study included patients with both measurable and unmeasurable disease.
The median patient age was about 60 years. Approximately 59% of patients in each arm had either lung or liver metastases. Patients with recurrence in bone only comprised 14.9%-19.4%.
Mutations in ESR1, a gene associated with hormonal resistance, were detectable in 29.7%-47.9% of patients.
Better PFS
As noted before, the primary endpoint of investigator-assessed median PFS favored camizestrant in both the 75-mg arm (7.2 months) and the 150-mg arm (7.7 months), with respective adjusted hazard ratios for progression versus fulvestrant of 0.58 (P = .0124) and 0.67 (P = .0161).
Camizestrant at the 75-mg dose was also superior to fulvestrant among patients who had previously received a cyclin-dependent kinase 4/6 inhibitor, with median PFS of 5.5 months and 3.8 months for the 75-mg and 150-mg doses, respectively, compared with 2.1 months.
The adjusted HR for progression with camizestrant with the 75-mg dose was 0.49, with a 90% confidence interval indicating significance. The 150-mg dose was not significantly superior to fulvestrant, however.
Both camizestrant doses were also superior for prolonging PFS versus fulvestrant among patients with lung and/or liver metastases, with median PFS of 7.2 months, 5.6 months, and 2.0 months, respectively.
The experimental SERD also outperformed fulvestrant in an analysis looking at PFS by ESR1 mutational status and ER-driven disease. Among patients with ESR1 wild type, however, median PFS rates with camizestrant 75 mg and fulvestrant were the same (7.2 months).
The 24-week objective response rates were 15.7% in the 75-mg camizestrant arm, 20% in the 150-mg arm, and 11.8% in the fulvestrant arm. The respective clinical benefit rates, including all patients with responses or stable disease, were 47.3%, 49.3%, and 38.4%. The camizestrant clinical benefit rates did not differ significantly from those with fulvestrant, however.
Treatment-related adverse events of grade 3 or greater occurred in only five patients, and only two patients, both in the 75-mg camizestrant arm, discontinued therapy because of adverse events. There were no treatment-related deaths.
Adverse events that occurred only with camizestrant included photopsia (flashing lights or floaters in the field of vision) and sinus bradycardia.
Promising, but early
Carlos Artega, MD, codirector of SABCS and director of the Simmons Comprehensive Cancer Center at UT Southwestern Medical Center, Dallas, who was not involved in the study, said the data look promising in comparison with fulvestrant.
“There is a clear suggestion that this might be better,” he said. “[Camizestrant] seems to be better at reducing the titer in plasma of the ESR1 mutation, and there is very strong basic science that supports that.”
He noted that the study numbers were relatively small, however.
Dr. Arteaga was speaking at a media briefing held immediately prior to the presentation of the data in an oral abstract session.
Fabrice Andre, MD, from Gustave Roussy in Villejuif, France, the invited discussant for the oral session, noted that, in patients with ESR1 wild type, where fulvestrant shows some efficacy, camizestrant appears to be equally effective, and that the latter agent may be more synergistic with targeted therapies than fulvestrant.
Given high patient dropout rates with currently available SERDs, there is a need for SERDs used in the adjuvant setting that are effective at minimally bioactive doses for patients who are predicted to poorly adherent, Dr. Andre said.
The study was funded by AstraZeneca. Dr. Oliveira has received personal funding from AstraZeneca, Guardant Health, Roche, Merck Sharp & Dohme, Pfizer, Seagen, iTeos Therapeutics, Eisai, Novartis, Relay Therapeutics, and Gilead. Dr. Arteaga is a scientific adviser to AstraZeneca and others, and has received grant support from Pfizer Lilly and Takeda. Dr. Andre disclosed fees to his hospital on his behalf from AstraZeneca, Daiichi Sankyo, Sanofi, Pfizer, Lilly, and Roche.
SAN ANTONIO – compared with the first-generation SERD fulvestrant Faslodex, in the SERENA-2 trial, shows a study recently presented at the San Antonio Breast Cancer Symposium.
Among 180 postmenopausal women with ER+/HER2– breast cancers that had recurred or progressed following at least one line of endocrine therapy, the median progression-free survival (PFS) after a median follow-up of 16.6-17.4 months was 7.2 months for patients treated at a 75-mg dose of camizestrant and 7.7 months for those treated at a 150-mg dose, compared with 3.7 months for patients who received fulvestrant, reported Mafalda Oliveira, MD, PhD, from Vall d’Hebron University Hospital in Barcelona.
Oral agent
Camizestrant is a next-generation oral SERD and pure estrogen receptor antagonist that was shown in the SERENA-1 trial to be safe and to have clinical activity against ER+ breast cancers.
SERENA-2 pitted camizestrant at doses of 75 mg, 150 mg, or 300 mg against standard-dose fulvestrant, although the 300-mg dose was dropped in a protocol amendment after 20 patients had been assigned to that arm. (Currently planned studies with camizestrant will be conducted with the 75-mg dose.)
The investigators enrolled women with ER+/HER2– advanced breast cancer who had not previously received fulvestrant or an oral SERD. Eligible patients were limited to no more than one prior line of endocrine and one prior line of chemotherapy for advanced breast cancers. The study included patients with both measurable and unmeasurable disease.
The median patient age was about 60 years. Approximately 59% of patients in each arm had either lung or liver metastases. Patients with recurrence in bone only comprised 14.9%-19.4%.
Mutations in ESR1, a gene associated with hormonal resistance, were detectable in 29.7%-47.9% of patients.
Better PFS
As noted before, the primary endpoint of investigator-assessed median PFS favored camizestrant in both the 75-mg arm (7.2 months) and the 150-mg arm (7.7 months), with respective adjusted hazard ratios for progression versus fulvestrant of 0.58 (P = .0124) and 0.67 (P = .0161).
Camizestrant at the 75-mg dose was also superior to fulvestrant among patients who had previously received a cyclin-dependent kinase 4/6 inhibitor, with median PFS of 5.5 months and 3.8 months for the 75-mg and 150-mg doses, respectively, compared with 2.1 months.
The adjusted HR for progression with camizestrant with the 75-mg dose was 0.49, with a 90% confidence interval indicating significance. The 150-mg dose was not significantly superior to fulvestrant, however.
Both camizestrant doses were also superior for prolonging PFS versus fulvestrant among patients with lung and/or liver metastases, with median PFS of 7.2 months, 5.6 months, and 2.0 months, respectively.
The experimental SERD also outperformed fulvestrant in an analysis looking at PFS by ESR1 mutational status and ER-driven disease. Among patients with ESR1 wild type, however, median PFS rates with camizestrant 75 mg and fulvestrant were the same (7.2 months).
The 24-week objective response rates were 15.7% in the 75-mg camizestrant arm, 20% in the 150-mg arm, and 11.8% in the fulvestrant arm. The respective clinical benefit rates, including all patients with responses or stable disease, were 47.3%, 49.3%, and 38.4%. The camizestrant clinical benefit rates did not differ significantly from those with fulvestrant, however.
Treatment-related adverse events of grade 3 or greater occurred in only five patients, and only two patients, both in the 75-mg camizestrant arm, discontinued therapy because of adverse events. There were no treatment-related deaths.
Adverse events that occurred only with camizestrant included photopsia (flashing lights or floaters in the field of vision) and sinus bradycardia.
Promising, but early
Carlos Artega, MD, codirector of SABCS and director of the Simmons Comprehensive Cancer Center at UT Southwestern Medical Center, Dallas, who was not involved in the study, said the data look promising in comparison with fulvestrant.
“There is a clear suggestion that this might be better,” he said. “[Camizestrant] seems to be better at reducing the titer in plasma of the ESR1 mutation, and there is very strong basic science that supports that.”
He noted that the study numbers were relatively small, however.
Dr. Arteaga was speaking at a media briefing held immediately prior to the presentation of the data in an oral abstract session.
Fabrice Andre, MD, from Gustave Roussy in Villejuif, France, the invited discussant for the oral session, noted that, in patients with ESR1 wild type, where fulvestrant shows some efficacy, camizestrant appears to be equally effective, and that the latter agent may be more synergistic with targeted therapies than fulvestrant.
Given high patient dropout rates with currently available SERDs, there is a need for SERDs used in the adjuvant setting that are effective at minimally bioactive doses for patients who are predicted to poorly adherent, Dr. Andre said.
The study was funded by AstraZeneca. Dr. Oliveira has received personal funding from AstraZeneca, Guardant Health, Roche, Merck Sharp & Dohme, Pfizer, Seagen, iTeos Therapeutics, Eisai, Novartis, Relay Therapeutics, and Gilead. Dr. Arteaga is a scientific adviser to AstraZeneca and others, and has received grant support from Pfizer Lilly and Takeda. Dr. Andre disclosed fees to his hospital on his behalf from AstraZeneca, Daiichi Sankyo, Sanofi, Pfizer, Lilly, and Roche.
SAN ANTONIO – compared with the first-generation SERD fulvestrant Faslodex, in the SERENA-2 trial, shows a study recently presented at the San Antonio Breast Cancer Symposium.
Among 180 postmenopausal women with ER+/HER2– breast cancers that had recurred or progressed following at least one line of endocrine therapy, the median progression-free survival (PFS) after a median follow-up of 16.6-17.4 months was 7.2 months for patients treated at a 75-mg dose of camizestrant and 7.7 months for those treated at a 150-mg dose, compared with 3.7 months for patients who received fulvestrant, reported Mafalda Oliveira, MD, PhD, from Vall d’Hebron University Hospital in Barcelona.
Oral agent
Camizestrant is a next-generation oral SERD and pure estrogen receptor antagonist that was shown in the SERENA-1 trial to be safe and to have clinical activity against ER+ breast cancers.
SERENA-2 pitted camizestrant at doses of 75 mg, 150 mg, or 300 mg against standard-dose fulvestrant, although the 300-mg dose was dropped in a protocol amendment after 20 patients had been assigned to that arm. (Currently planned studies with camizestrant will be conducted with the 75-mg dose.)
The investigators enrolled women with ER+/HER2– advanced breast cancer who had not previously received fulvestrant or an oral SERD. Eligible patients were limited to no more than one prior line of endocrine and one prior line of chemotherapy for advanced breast cancers. The study included patients with both measurable and unmeasurable disease.
The median patient age was about 60 years. Approximately 59% of patients in each arm had either lung or liver metastases. Patients with recurrence in bone only comprised 14.9%-19.4%.
Mutations in ESR1, a gene associated with hormonal resistance, were detectable in 29.7%-47.9% of patients.
Better PFS
As noted before, the primary endpoint of investigator-assessed median PFS favored camizestrant in both the 75-mg arm (7.2 months) and the 150-mg arm (7.7 months), with respective adjusted hazard ratios for progression versus fulvestrant of 0.58 (P = .0124) and 0.67 (P = .0161).
Camizestrant at the 75-mg dose was also superior to fulvestrant among patients who had previously received a cyclin-dependent kinase 4/6 inhibitor, with median PFS of 5.5 months and 3.8 months for the 75-mg and 150-mg doses, respectively, compared with 2.1 months.
The adjusted HR for progression with camizestrant with the 75-mg dose was 0.49, with a 90% confidence interval indicating significance. The 150-mg dose was not significantly superior to fulvestrant, however.
Both camizestrant doses were also superior for prolonging PFS versus fulvestrant among patients with lung and/or liver metastases, with median PFS of 7.2 months, 5.6 months, and 2.0 months, respectively.
The experimental SERD also outperformed fulvestrant in an analysis looking at PFS by ESR1 mutational status and ER-driven disease. Among patients with ESR1 wild type, however, median PFS rates with camizestrant 75 mg and fulvestrant were the same (7.2 months).
The 24-week objective response rates were 15.7% in the 75-mg camizestrant arm, 20% in the 150-mg arm, and 11.8% in the fulvestrant arm. The respective clinical benefit rates, including all patients with responses or stable disease, were 47.3%, 49.3%, and 38.4%. The camizestrant clinical benefit rates did not differ significantly from those with fulvestrant, however.
Treatment-related adverse events of grade 3 or greater occurred in only five patients, and only two patients, both in the 75-mg camizestrant arm, discontinued therapy because of adverse events. There were no treatment-related deaths.
Adverse events that occurred only with camizestrant included photopsia (flashing lights or floaters in the field of vision) and sinus bradycardia.
Promising, but early
Carlos Artega, MD, codirector of SABCS and director of the Simmons Comprehensive Cancer Center at UT Southwestern Medical Center, Dallas, who was not involved in the study, said the data look promising in comparison with fulvestrant.
“There is a clear suggestion that this might be better,” he said. “[Camizestrant] seems to be better at reducing the titer in plasma of the ESR1 mutation, and there is very strong basic science that supports that.”
He noted that the study numbers were relatively small, however.
Dr. Arteaga was speaking at a media briefing held immediately prior to the presentation of the data in an oral abstract session.
Fabrice Andre, MD, from Gustave Roussy in Villejuif, France, the invited discussant for the oral session, noted that, in patients with ESR1 wild type, where fulvestrant shows some efficacy, camizestrant appears to be equally effective, and that the latter agent may be more synergistic with targeted therapies than fulvestrant.
Given high patient dropout rates with currently available SERDs, there is a need for SERDs used in the adjuvant setting that are effective at minimally bioactive doses for patients who are predicted to poorly adherent, Dr. Andre said.
The study was funded by AstraZeneca. Dr. Oliveira has received personal funding from AstraZeneca, Guardant Health, Roche, Merck Sharp & Dohme, Pfizer, Seagen, iTeos Therapeutics, Eisai, Novartis, Relay Therapeutics, and Gilead. Dr. Arteaga is a scientific adviser to AstraZeneca and others, and has received grant support from Pfizer Lilly and Takeda. Dr. Andre disclosed fees to his hospital on his behalf from AstraZeneca, Daiichi Sankyo, Sanofi, Pfizer, Lilly, and Roche.
AT SABCS 2022
Chemotherapy meets its match against aggressive ER+/HER2– breast cancers
SAN ANTONIO – Results of a study being hailed as practice changing showed that, for pre- or perimenopausal women with aggressive hormone receptor-positive, HER2-negative (HR+/HER2–) untreated breast cancers,
That’s according to investigators of the phase 2 RIGHT Choice study who found that first-line ribociclib, combined with either letrozole or anastrozole plus goserelin, was associated with a doubling of progression-free survival (PFS), compared with the investigator’s choice of combination chemotherapy, reported Yen-Shen Lu, MD, from National Taiwan University Hospital in Taipei, at the San Antonio Breast Cancer Symposium.
“These results from RIGHT Choice have now shown that first-line ribociclib plus endocrine therapy should be considered the preferred treatment option for this patient population,” he said.
Chemo loses its luster
“This is not time first time that we’ve looked at a CDK4/6 inhibitor compared to chemotherapy, but this is the first time that we’ve seen it compared to a combination chemotherapy,” commented Virginia Kaklamani, MD, from University of Texas Health, San Antonio, who moderated a media briefing held prior to Dr. Lu’s presentation of the data in an oral abstract session.
“I think with this study we’re finding that chemotherapy, at least in the early stages of [estrogen receptor]–positive breast cancer, is probably not appropriate for our patients,” she said.
Chemotherapy is the current standard of care for patients with advanced breast cancers with aggressive disease features that can include rapidly progressive disease, high symptom burden, and/or life-threatening visceral crises requiring rapid control of disease, Dr. Lu said.
Compared with single-agent chemotherapy, combination chemotherapy, for those who can tolerate it, is associated with higher overall response rates and longer PFS.
Although ribociclib plus endocrine therapy has been shown to offer significant PFS and overall survival (OS) benefits, compared with endocrine therapy alone, there have not been any head-to-head studies pitting these agents against combination chemotherapy.
Study details
To rectify this, Dr. Lu and colleagues enrolled 222 pre- or perimenopausal women with HR+/HER2– advanced breast cancers with aggressive features who had not yet received systemic therapy for advanced breast cancer.
After stratification for the presence or absence of liver metastases and by length of disease-free interval (time from complete resection of a primary tumor to documented recurrence), the patients were randomly assigned to receive either ribociclib 600 mg 3 weeks on, 1 week off) plus letrozole or anastrozole and goserelin, or the investigators choice of either docetaxel plus capecitabine, paclitaxel plus gemcitabine, or capecitabine plus vinorelbine.
After a median follow-up of 24.1 months at the time of data cutoff in April 2022, median PFS, the primary endpoint, was 24 months in the ribociclib plus endocrine therapy arm versus 12.3 months in the chemotherapy arm.
This translated into a hazard ratio for progression on ribociclib plus endocrine therapy of 0.54 (P = .0007).
The benefit for the ribociclib combination, compared with combination chemotherapy, was consistent across most patient subgroups, Dr. Lu said.
The median time to treatment failure was also longer with ribociclib, at 18.6 months versus 8.5 months, respectively, translating into a HR of 0.45 favoring ribociclib, with a statistically significant confidence interval.
Overall response rates were similar between the groups, at 65.2% with ribociclib versus 60% with chemotherapy. The respective clinical benefit rates (including complete and partial responses plus stable disease) were 80.4% versus 72.7%.
The time to response was similar between the treatment arms, an important consideration for patients with rapidly progressive disease, Dr. Lu noted.
Adverse events that occurred more frequently with ribociclib were neutropenia and leukopenia. Events more common with chemotherapy included anemia, liver enzyme elevations, nausea, vomiting, diarrhea, alopecia, fatigue, and palmar-plantar erythrodysesthesia.
Confirmation
“These data are just confirming what we’ve already known, and that is that with ER-positive, HER2-negative breast cancer where you have metastatic disease and more aggressive characteristics, treating with a CDK4/6 inhibitor and endocrine therapy leads to high response rates,” breast cancer specialist Matthew P. Goetz, MD, from the Mayo Clinic in Rochester, Minn., said in an interview. Dr. Goetz was not involved in the study.
“What was surprising to me was the fact that the response rates with chemotherapy were not higher,” he said. “We sometime think that the more chemotherapy, the higher the response rates. It was nice to see a direct comparison with chemotherapy, and really to see that giving a target therapy actually led to very, very good results. That tells us that there should be very few situations where we would be prescribing chemotherapy over CDK4/6 inhibitor–based therapies.”
The study was funded by Novartis Pharma. Dr. Lu disclosed personal funding from Novartis and others. Dr. Goetz disclosed grants and other supports for work with the development of abemaciclib and palbociclib, and consulting for Pfizer and others. Dr. Kaklamani disclosed speakers bureau activity for Novartis and others, research support from Eisai, and consulting for other companies.
SAN ANTONIO – Results of a study being hailed as practice changing showed that, for pre- or perimenopausal women with aggressive hormone receptor-positive, HER2-negative (HR+/HER2–) untreated breast cancers,
That’s according to investigators of the phase 2 RIGHT Choice study who found that first-line ribociclib, combined with either letrozole or anastrozole plus goserelin, was associated with a doubling of progression-free survival (PFS), compared with the investigator’s choice of combination chemotherapy, reported Yen-Shen Lu, MD, from National Taiwan University Hospital in Taipei, at the San Antonio Breast Cancer Symposium.
“These results from RIGHT Choice have now shown that first-line ribociclib plus endocrine therapy should be considered the preferred treatment option for this patient population,” he said.
Chemo loses its luster
“This is not time first time that we’ve looked at a CDK4/6 inhibitor compared to chemotherapy, but this is the first time that we’ve seen it compared to a combination chemotherapy,” commented Virginia Kaklamani, MD, from University of Texas Health, San Antonio, who moderated a media briefing held prior to Dr. Lu’s presentation of the data in an oral abstract session.
“I think with this study we’re finding that chemotherapy, at least in the early stages of [estrogen receptor]–positive breast cancer, is probably not appropriate for our patients,” she said.
Chemotherapy is the current standard of care for patients with advanced breast cancers with aggressive disease features that can include rapidly progressive disease, high symptom burden, and/or life-threatening visceral crises requiring rapid control of disease, Dr. Lu said.
Compared with single-agent chemotherapy, combination chemotherapy, for those who can tolerate it, is associated with higher overall response rates and longer PFS.
Although ribociclib plus endocrine therapy has been shown to offer significant PFS and overall survival (OS) benefits, compared with endocrine therapy alone, there have not been any head-to-head studies pitting these agents against combination chemotherapy.
Study details
To rectify this, Dr. Lu and colleagues enrolled 222 pre- or perimenopausal women with HR+/HER2– advanced breast cancers with aggressive features who had not yet received systemic therapy for advanced breast cancer.
After stratification for the presence or absence of liver metastases and by length of disease-free interval (time from complete resection of a primary tumor to documented recurrence), the patients were randomly assigned to receive either ribociclib 600 mg 3 weeks on, 1 week off) plus letrozole or anastrozole and goserelin, or the investigators choice of either docetaxel plus capecitabine, paclitaxel plus gemcitabine, or capecitabine plus vinorelbine.
After a median follow-up of 24.1 months at the time of data cutoff in April 2022, median PFS, the primary endpoint, was 24 months in the ribociclib plus endocrine therapy arm versus 12.3 months in the chemotherapy arm.
This translated into a hazard ratio for progression on ribociclib plus endocrine therapy of 0.54 (P = .0007).
The benefit for the ribociclib combination, compared with combination chemotherapy, was consistent across most patient subgroups, Dr. Lu said.
The median time to treatment failure was also longer with ribociclib, at 18.6 months versus 8.5 months, respectively, translating into a HR of 0.45 favoring ribociclib, with a statistically significant confidence interval.
Overall response rates were similar between the groups, at 65.2% with ribociclib versus 60% with chemotherapy. The respective clinical benefit rates (including complete and partial responses plus stable disease) were 80.4% versus 72.7%.
The time to response was similar between the treatment arms, an important consideration for patients with rapidly progressive disease, Dr. Lu noted.
Adverse events that occurred more frequently with ribociclib were neutropenia and leukopenia. Events more common with chemotherapy included anemia, liver enzyme elevations, nausea, vomiting, diarrhea, alopecia, fatigue, and palmar-plantar erythrodysesthesia.
Confirmation
“These data are just confirming what we’ve already known, and that is that with ER-positive, HER2-negative breast cancer where you have metastatic disease and more aggressive characteristics, treating with a CDK4/6 inhibitor and endocrine therapy leads to high response rates,” breast cancer specialist Matthew P. Goetz, MD, from the Mayo Clinic in Rochester, Minn., said in an interview. Dr. Goetz was not involved in the study.
“What was surprising to me was the fact that the response rates with chemotherapy were not higher,” he said. “We sometime think that the more chemotherapy, the higher the response rates. It was nice to see a direct comparison with chemotherapy, and really to see that giving a target therapy actually led to very, very good results. That tells us that there should be very few situations where we would be prescribing chemotherapy over CDK4/6 inhibitor–based therapies.”
The study was funded by Novartis Pharma. Dr. Lu disclosed personal funding from Novartis and others. Dr. Goetz disclosed grants and other supports for work with the development of abemaciclib and palbociclib, and consulting for Pfizer and others. Dr. Kaklamani disclosed speakers bureau activity for Novartis and others, research support from Eisai, and consulting for other companies.
SAN ANTONIO – Results of a study being hailed as practice changing showed that, for pre- or perimenopausal women with aggressive hormone receptor-positive, HER2-negative (HR+/HER2–) untreated breast cancers,
That’s according to investigators of the phase 2 RIGHT Choice study who found that first-line ribociclib, combined with either letrozole or anastrozole plus goserelin, was associated with a doubling of progression-free survival (PFS), compared with the investigator’s choice of combination chemotherapy, reported Yen-Shen Lu, MD, from National Taiwan University Hospital in Taipei, at the San Antonio Breast Cancer Symposium.
“These results from RIGHT Choice have now shown that first-line ribociclib plus endocrine therapy should be considered the preferred treatment option for this patient population,” he said.
Chemo loses its luster
“This is not time first time that we’ve looked at a CDK4/6 inhibitor compared to chemotherapy, but this is the first time that we’ve seen it compared to a combination chemotherapy,” commented Virginia Kaklamani, MD, from University of Texas Health, San Antonio, who moderated a media briefing held prior to Dr. Lu’s presentation of the data in an oral abstract session.
“I think with this study we’re finding that chemotherapy, at least in the early stages of [estrogen receptor]–positive breast cancer, is probably not appropriate for our patients,” she said.
Chemotherapy is the current standard of care for patients with advanced breast cancers with aggressive disease features that can include rapidly progressive disease, high symptom burden, and/or life-threatening visceral crises requiring rapid control of disease, Dr. Lu said.
Compared with single-agent chemotherapy, combination chemotherapy, for those who can tolerate it, is associated with higher overall response rates and longer PFS.
Although ribociclib plus endocrine therapy has been shown to offer significant PFS and overall survival (OS) benefits, compared with endocrine therapy alone, there have not been any head-to-head studies pitting these agents against combination chemotherapy.
Study details
To rectify this, Dr. Lu and colleagues enrolled 222 pre- or perimenopausal women with HR+/HER2– advanced breast cancers with aggressive features who had not yet received systemic therapy for advanced breast cancer.
After stratification for the presence or absence of liver metastases and by length of disease-free interval (time from complete resection of a primary tumor to documented recurrence), the patients were randomly assigned to receive either ribociclib 600 mg 3 weeks on, 1 week off) plus letrozole or anastrozole and goserelin, or the investigators choice of either docetaxel plus capecitabine, paclitaxel plus gemcitabine, or capecitabine plus vinorelbine.
After a median follow-up of 24.1 months at the time of data cutoff in April 2022, median PFS, the primary endpoint, was 24 months in the ribociclib plus endocrine therapy arm versus 12.3 months in the chemotherapy arm.
This translated into a hazard ratio for progression on ribociclib plus endocrine therapy of 0.54 (P = .0007).
The benefit for the ribociclib combination, compared with combination chemotherapy, was consistent across most patient subgroups, Dr. Lu said.
The median time to treatment failure was also longer with ribociclib, at 18.6 months versus 8.5 months, respectively, translating into a HR of 0.45 favoring ribociclib, with a statistically significant confidence interval.
Overall response rates were similar between the groups, at 65.2% with ribociclib versus 60% with chemotherapy. The respective clinical benefit rates (including complete and partial responses plus stable disease) were 80.4% versus 72.7%.
The time to response was similar between the treatment arms, an important consideration for patients with rapidly progressive disease, Dr. Lu noted.
Adverse events that occurred more frequently with ribociclib were neutropenia and leukopenia. Events more common with chemotherapy included anemia, liver enzyme elevations, nausea, vomiting, diarrhea, alopecia, fatigue, and palmar-plantar erythrodysesthesia.
Confirmation
“These data are just confirming what we’ve already known, and that is that with ER-positive, HER2-negative breast cancer where you have metastatic disease and more aggressive characteristics, treating with a CDK4/6 inhibitor and endocrine therapy leads to high response rates,” breast cancer specialist Matthew P. Goetz, MD, from the Mayo Clinic in Rochester, Minn., said in an interview. Dr. Goetz was not involved in the study.
“What was surprising to me was the fact that the response rates with chemotherapy were not higher,” he said. “We sometime think that the more chemotherapy, the higher the response rates. It was nice to see a direct comparison with chemotherapy, and really to see that giving a target therapy actually led to very, very good results. That tells us that there should be very few situations where we would be prescribing chemotherapy over CDK4/6 inhibitor–based therapies.”
The study was funded by Novartis Pharma. Dr. Lu disclosed personal funding from Novartis and others. Dr. Goetz disclosed grants and other supports for work with the development of abemaciclib and palbociclib, and consulting for Pfizer and others. Dr. Kaklamani disclosed speakers bureau activity for Novartis and others, research support from Eisai, and consulting for other companies.
AT SABCS 2022
Potential cause of worse outcomes among Black breast cancer patients found
SAN ANTONIO – compared with White women, a discovery that may at least partially explain racial differences in breast cancer outcomes, investigators say.
The finding, which comes from a retrospective study comparing differences in tumor microenvironment of metastasis (TMEM) “doorways” between Black and White women suggest that tumors in Black women may have a stronger prometastatic response to neoadjuvant chemotherapy than tumors in White women, reported Maja H. Oktay, MD, PhD, of Montefiore Einstein Cancer Center, Albert Einstein College of Medicine, New York, at the San Antonio Breast Cancer Symposium.
“Looking forward ... we propose to use TMEM doorway density as a prognostic marker for distant recurrence-free survival as a marker of dissemination, and also as a predictive marker of response to drugs that can block TMEM doorways,” she said at a briefing held prior to the presentation of data in an oral abstract session.
Entry points
As their name implies, TMEM doorways are transient entry points or portals that allow cancer cells to disseminate to distant sites. TMEM doorways are composed of tumor cells, macrophages, and endothelial cells that come into direct contact and together create temporary vascular openings that allow tumor cells to cross cell walls into circulation, where they can then hitch a ride and travel to distant organ sites.
Previous studies have shown that TMEM doorway density is a prognostic marker of metastasis in breast cancer patients treated with adjuvant chemotherapy. And as Dr. Oktay and colleagues showed in the current study, TMEM doorway density, as measured by a TMEM doorway score, is a prognostic marker for distant metastatic recurrence of ER+/HER2– breast cancer following neoadjuvant chemotherapy.
They also showed that neoadjuvant chemotherapy may increase the TMEM doorway score and lead to a pro–metastatic tumor microenvironment in some women.
Doorway scores
The investigators measured TMEM doorway scores from residual breast cancers in women who had undergone standard neoadjuvant chemotherapy. The cohort consisted of 96 Black women, 43 of whom had ER+/HER2– breast cancer and 37 of whom had triple-negative breast cancer (TNBC), and 87 White women, 50 with ER+/HER2– cancer and 22 with TNBC. The remaining patients had other breast cancer subtypes.
They found that TNBCs had higher TMEM doorway density score and higher macrophage density scores, which may explain why patients with TNBC often have early recurrence of disease.
They also found that, compared with White patients, Black patients with ER+/HER2– tumors, but not TNBC tumors, had higher TMEM doorway density scores. Similarly, Black patients with ER+/HER– cancers, but not TNBC, had higher macrophage levels than White women, a finding that may explain racial disparity in ER+/HER2– disease, Dr. Oktay said.
For the entire cohort, patients with high TMEM doorway density scores had significantly worse distant recurrence–free survival than patients with intermediate or low scores (P = .008), and there was a trend toward worse DRFS among all patients with ER+/HER2– who were in the highest third of scores, but this did not quite reach statistical significance.
High versus low TMEM doorway density score was also an independent prognostic factor for worse outcomes among the entire cohort (P = .01).
There was no significant difference in TMEM density scores among patients with TNBC.
Neither high macrophage counts nor microvascular density alone were significantly associated with inferior DRFS. TMEM doorway score was the only factor significantly prognostic for worse outcomes among patients in the entire cohort.
Hypothesis needs further testing
Invited discussant Lori Pierce, MD, a radiation oncologist with Michigan Medicine, University of Michigan, Ann Arbor, said it’s unclear whether TMEM doorway density changed following neoadjuvant chemotherapy as there were no prechemotherapy scores available in this study.
“But I think the key part is that, if we think neoadjuvant chemotherapy promotes metastasis, then there should be an inferior outcome compared to adjuvant chemotherapy, but that’s not what we see. Well-powered randomized trials show equivalent outcomes with neoadjuvant chemotherapy as well as adjuvant,” she said.
She noted that a 2018 meta-analysis of individual patient data from 10 randomized trials comparing neoadjuvant with adjuvant chemotherapy in early breast cancer showed no differences in long-term distant recurrences, breast cancer–specific mortality, or all-cause mortality between the two modalities.
“While I think these data are very provocative, I certainly wouldn’t want Black women or any women who need neoadjuvant therapy to be discouraged because of these data. We need these data to be tested rigorously, so I look forward to the clinical trials that will test this question and can really give us more information about this very interesting hypothesis,” Dr. Pierce said.
The study was funded by the National Institutes of Health, New York State Department of Health Peter T. Rowley Breast Cancer Scientific Research Projects, Helen & Irving Spatz Family Foundation, Evelyn Gruss Lipper Charitable Foundation, and the Gruss-Lipper Biophotonics Center and the integrated imaging program at the Albert Einstein College of Medicine. Dr. Oktay reported no conflicts of interests.
SAN ANTONIO – compared with White women, a discovery that may at least partially explain racial differences in breast cancer outcomes, investigators say.
The finding, which comes from a retrospective study comparing differences in tumor microenvironment of metastasis (TMEM) “doorways” between Black and White women suggest that tumors in Black women may have a stronger prometastatic response to neoadjuvant chemotherapy than tumors in White women, reported Maja H. Oktay, MD, PhD, of Montefiore Einstein Cancer Center, Albert Einstein College of Medicine, New York, at the San Antonio Breast Cancer Symposium.
“Looking forward ... we propose to use TMEM doorway density as a prognostic marker for distant recurrence-free survival as a marker of dissemination, and also as a predictive marker of response to drugs that can block TMEM doorways,” she said at a briefing held prior to the presentation of data in an oral abstract session.
Entry points
As their name implies, TMEM doorways are transient entry points or portals that allow cancer cells to disseminate to distant sites. TMEM doorways are composed of tumor cells, macrophages, and endothelial cells that come into direct contact and together create temporary vascular openings that allow tumor cells to cross cell walls into circulation, where they can then hitch a ride and travel to distant organ sites.
Previous studies have shown that TMEM doorway density is a prognostic marker of metastasis in breast cancer patients treated with adjuvant chemotherapy. And as Dr. Oktay and colleagues showed in the current study, TMEM doorway density, as measured by a TMEM doorway score, is a prognostic marker for distant metastatic recurrence of ER+/HER2– breast cancer following neoadjuvant chemotherapy.
They also showed that neoadjuvant chemotherapy may increase the TMEM doorway score and lead to a pro–metastatic tumor microenvironment in some women.
Doorway scores
The investigators measured TMEM doorway scores from residual breast cancers in women who had undergone standard neoadjuvant chemotherapy. The cohort consisted of 96 Black women, 43 of whom had ER+/HER2– breast cancer and 37 of whom had triple-negative breast cancer (TNBC), and 87 White women, 50 with ER+/HER2– cancer and 22 with TNBC. The remaining patients had other breast cancer subtypes.
They found that TNBCs had higher TMEM doorway density score and higher macrophage density scores, which may explain why patients with TNBC often have early recurrence of disease.
They also found that, compared with White patients, Black patients with ER+/HER2– tumors, but not TNBC tumors, had higher TMEM doorway density scores. Similarly, Black patients with ER+/HER– cancers, but not TNBC, had higher macrophage levels than White women, a finding that may explain racial disparity in ER+/HER2– disease, Dr. Oktay said.
For the entire cohort, patients with high TMEM doorway density scores had significantly worse distant recurrence–free survival than patients with intermediate or low scores (P = .008), and there was a trend toward worse DRFS among all patients with ER+/HER2– who were in the highest third of scores, but this did not quite reach statistical significance.
High versus low TMEM doorway density score was also an independent prognostic factor for worse outcomes among the entire cohort (P = .01).
There was no significant difference in TMEM density scores among patients with TNBC.
Neither high macrophage counts nor microvascular density alone were significantly associated with inferior DRFS. TMEM doorway score was the only factor significantly prognostic for worse outcomes among patients in the entire cohort.
Hypothesis needs further testing
Invited discussant Lori Pierce, MD, a radiation oncologist with Michigan Medicine, University of Michigan, Ann Arbor, said it’s unclear whether TMEM doorway density changed following neoadjuvant chemotherapy as there were no prechemotherapy scores available in this study.
“But I think the key part is that, if we think neoadjuvant chemotherapy promotes metastasis, then there should be an inferior outcome compared to adjuvant chemotherapy, but that’s not what we see. Well-powered randomized trials show equivalent outcomes with neoadjuvant chemotherapy as well as adjuvant,” she said.
She noted that a 2018 meta-analysis of individual patient data from 10 randomized trials comparing neoadjuvant with adjuvant chemotherapy in early breast cancer showed no differences in long-term distant recurrences, breast cancer–specific mortality, or all-cause mortality between the two modalities.
“While I think these data are very provocative, I certainly wouldn’t want Black women or any women who need neoadjuvant therapy to be discouraged because of these data. We need these data to be tested rigorously, so I look forward to the clinical trials that will test this question and can really give us more information about this very interesting hypothesis,” Dr. Pierce said.
The study was funded by the National Institutes of Health, New York State Department of Health Peter T. Rowley Breast Cancer Scientific Research Projects, Helen & Irving Spatz Family Foundation, Evelyn Gruss Lipper Charitable Foundation, and the Gruss-Lipper Biophotonics Center and the integrated imaging program at the Albert Einstein College of Medicine. Dr. Oktay reported no conflicts of interests.
SAN ANTONIO – compared with White women, a discovery that may at least partially explain racial differences in breast cancer outcomes, investigators say.
The finding, which comes from a retrospective study comparing differences in tumor microenvironment of metastasis (TMEM) “doorways” between Black and White women suggest that tumors in Black women may have a stronger prometastatic response to neoadjuvant chemotherapy than tumors in White women, reported Maja H. Oktay, MD, PhD, of Montefiore Einstein Cancer Center, Albert Einstein College of Medicine, New York, at the San Antonio Breast Cancer Symposium.
“Looking forward ... we propose to use TMEM doorway density as a prognostic marker for distant recurrence-free survival as a marker of dissemination, and also as a predictive marker of response to drugs that can block TMEM doorways,” she said at a briefing held prior to the presentation of data in an oral abstract session.
Entry points
As their name implies, TMEM doorways are transient entry points or portals that allow cancer cells to disseminate to distant sites. TMEM doorways are composed of tumor cells, macrophages, and endothelial cells that come into direct contact and together create temporary vascular openings that allow tumor cells to cross cell walls into circulation, where they can then hitch a ride and travel to distant organ sites.
Previous studies have shown that TMEM doorway density is a prognostic marker of metastasis in breast cancer patients treated with adjuvant chemotherapy. And as Dr. Oktay and colleagues showed in the current study, TMEM doorway density, as measured by a TMEM doorway score, is a prognostic marker for distant metastatic recurrence of ER+/HER2– breast cancer following neoadjuvant chemotherapy.
They also showed that neoadjuvant chemotherapy may increase the TMEM doorway score and lead to a pro–metastatic tumor microenvironment in some women.
Doorway scores
The investigators measured TMEM doorway scores from residual breast cancers in women who had undergone standard neoadjuvant chemotherapy. The cohort consisted of 96 Black women, 43 of whom had ER+/HER2– breast cancer and 37 of whom had triple-negative breast cancer (TNBC), and 87 White women, 50 with ER+/HER2– cancer and 22 with TNBC. The remaining patients had other breast cancer subtypes.
They found that TNBCs had higher TMEM doorway density score and higher macrophage density scores, which may explain why patients with TNBC often have early recurrence of disease.
They also found that, compared with White patients, Black patients with ER+/HER2– tumors, but not TNBC tumors, had higher TMEM doorway density scores. Similarly, Black patients with ER+/HER– cancers, but not TNBC, had higher macrophage levels than White women, a finding that may explain racial disparity in ER+/HER2– disease, Dr. Oktay said.
For the entire cohort, patients with high TMEM doorway density scores had significantly worse distant recurrence–free survival than patients with intermediate or low scores (P = .008), and there was a trend toward worse DRFS among all patients with ER+/HER2– who were in the highest third of scores, but this did not quite reach statistical significance.
High versus low TMEM doorway density score was also an independent prognostic factor for worse outcomes among the entire cohort (P = .01).
There was no significant difference in TMEM density scores among patients with TNBC.
Neither high macrophage counts nor microvascular density alone were significantly associated with inferior DRFS. TMEM doorway score was the only factor significantly prognostic for worse outcomes among patients in the entire cohort.
Hypothesis needs further testing
Invited discussant Lori Pierce, MD, a radiation oncologist with Michigan Medicine, University of Michigan, Ann Arbor, said it’s unclear whether TMEM doorway density changed following neoadjuvant chemotherapy as there were no prechemotherapy scores available in this study.
“But I think the key part is that, if we think neoadjuvant chemotherapy promotes metastasis, then there should be an inferior outcome compared to adjuvant chemotherapy, but that’s not what we see. Well-powered randomized trials show equivalent outcomes with neoadjuvant chemotherapy as well as adjuvant,” she said.
She noted that a 2018 meta-analysis of individual patient data from 10 randomized trials comparing neoadjuvant with adjuvant chemotherapy in early breast cancer showed no differences in long-term distant recurrences, breast cancer–specific mortality, or all-cause mortality between the two modalities.
“While I think these data are very provocative, I certainly wouldn’t want Black women or any women who need neoadjuvant therapy to be discouraged because of these data. We need these data to be tested rigorously, so I look forward to the clinical trials that will test this question and can really give us more information about this very interesting hypothesis,” Dr. Pierce said.
The study was funded by the National Institutes of Health, New York State Department of Health Peter T. Rowley Breast Cancer Scientific Research Projects, Helen & Irving Spatz Family Foundation, Evelyn Gruss Lipper Charitable Foundation, and the Gruss-Lipper Biophotonics Center and the integrated imaging program at the Albert Einstein College of Medicine. Dr. Oktay reported no conflicts of interests.
AT SABCS 2022
Know the right resuscitation for right-sided heart failure
Amado Alejandro Baez, MD, said in a presentation at the 2022 scientific assembly of the American College of Emergency Physicians.
The patient arrived on day 20 after a radical cystoprostatectomy. He had driven 4 hours from another city for a urology follow-up visit. On arrival, he developed respiratory distress symptoms and presented to the emergency department, said Dr. Baez, professor of emergency medicine and epidemiology at the Medical College of Georgia/Augusta University and triple-board certified in EMS, emergency medicine, and critical care.
The patient developed a massive pulmonary embolism with acute cor pulmonale (right-sided heart failure). An electrocardiogram showed an S1Q3T3, demonstrating the distinctive nature of right ventricular failure, said Dr. Baez.
Research has demonstrated the differences in physiology between the right and left ventricles, he said.
Dr. Baez highlighted some of the features of right ventricle (RV) failure and how to manage it. Notably, the RV is thinner and less resilient. “RV failure patients may fall off the Starling curve,” in contrast to patients with isolated left ventricle (LV) failure.
RV pressure overload is associated with a range of conditions, such as pericardial disease, pulmonary embolism, acute respiratory distress syndrome, and pulmonary arterial hypertension. When combined with RV overload, patients may develop intracardiac shunting or coronary heart disease, Dr. Baez said. Decreased contractility associated with RV failure can result from sepsis, right ventricular myocardial infarction, myocarditis, and arrhythmia.
Dr. Baez cited the 2018 scientific statement from the American Heart Association on the evaluation and management of right-sided heart failure. The authors of the statement noted that the complicated geometry of the right heart makes functional assessment a challenge. They wrote that various hemodynamic and biochemical markers can help guide clinical assessment and therapeutic decision-making.
Increased RV afterload drives multiple factors that can ultimately lead to cardiogenic shock and death, said Dr. Baez. These factors include decreased RV oxygen delivery, decreased RV coronary perfusion, decreased systemic blood pressure, and low carbon monoxide levels. RV afterload also leads to decreased RV contractility, an increase in RV oxygen demand, and tension in the RV wall, and it may contribute to tricuspid valve insufficiency, neurohormonal activation, and RV ischemia.
Treatment strategies involve improving symptoms and stopping disease progression, said Baez. In its scientific statement, the AHA recommends steps for assessing RV and LV function so as to identify RV failure as soon as possible, he said. After excluding pericardial disease, the AHA advises diagnosis and treatment of etiology-specific causes, such as right ventricular MI, pulmonary embolism, and sepsis. For arrhythmias, it recommends maintaining sinus rhythm when possible and considering a pacemaker to maintain atrioventricular synchrony and to avoid excessive bradycardia.
In its statement, the AHA also recommends optimizing preload with right arterial pressure/central venous pressure of 8-12 mm Hg, said Dr. Baez. Preload optimization combined with afterload reduction and improved contractility are hallmarks of care for patients with RV failure.
Avoiding systemic hypotension can prevent sequelae, such as myocardial ischemia and further hypotension, he said.
Optimization of fluid status is another key to managing RV failure, said Dr. Baez. Right heart coronary perfusion pressure can be protected by maintaining mean arterial pressure, and consideration should be given to reducing the RV afterload. Other strategies include inotropic medications and rhythm stabilization.
In general, for RV failure patients, “correct hypoxia, hypercarbia, and acidosis and avoid intubation when possible,” he said. Extracorporeal membrane oxygenation (ECMO) may be an option, depending on how many mechanical ventilator settings need to be adjusted.
In a study by Dr. Baez and colleagues published in Critical Care Medicine, the authors presented a Bayesian probability model for plasma lactate and severity of illness in cases of acute pulmonary embolism. “This Bayesian model demonstrated that the combination of shock index and lactate yield superior diagnostic gains than those compare to the sPESI and lactate,” Dr. Baez said.
The care model needs to be specific to the etiology, he added. Volume management in congested pulmonary hypertension involves a “squeeze and diurese” strategy.
According to the Internet Book of Critical Care, for patients with mean arterial pressure (MAP) of 60 mm Hg, central venous pressure (CVP) of 25 mm Hg, renal perfusion pressure of 25 mm Hg, and no urine output, a vasopressor should be added to treatment, Dr. Baez said. In cases in which the MAP 75 mm Hg, the CVP is 25 mm Hg, the renal perfusion pressure is 50 mm Hg, and the patient has good urine output, vasopressors should be continued and fluid should be removed through use of a diuretic. For patients with a MAP of 75 mm Hg, a CVP of 12 mm Hg, and renal perfusion pressure of 63 mm Hg who have good urine output, the diuretic and the vasopressor should be discontinued.
Dr. Baez also reviewed several clinical studies of the utility of acute mechanical circulatory support systems for RV failure.
In two small studies involving a heart pump and a right ventricular assistive device, the 30-day survival rate was approximately 72%-73%. A study of 179 patients involving ECMO showed an in-hospital mortality rate of 38.6%, he said.
Overall, “prompt diagnosis, hemodynamic support, and initiation of specific treatment” are the foundations of managing RV failure, he concluded.
Dr. Baez disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Amado Alejandro Baez, MD, said in a presentation at the 2022 scientific assembly of the American College of Emergency Physicians.
The patient arrived on day 20 after a radical cystoprostatectomy. He had driven 4 hours from another city for a urology follow-up visit. On arrival, he developed respiratory distress symptoms and presented to the emergency department, said Dr. Baez, professor of emergency medicine and epidemiology at the Medical College of Georgia/Augusta University and triple-board certified in EMS, emergency medicine, and critical care.
The patient developed a massive pulmonary embolism with acute cor pulmonale (right-sided heart failure). An electrocardiogram showed an S1Q3T3, demonstrating the distinctive nature of right ventricular failure, said Dr. Baez.
Research has demonstrated the differences in physiology between the right and left ventricles, he said.
Dr. Baez highlighted some of the features of right ventricle (RV) failure and how to manage it. Notably, the RV is thinner and less resilient. “RV failure patients may fall off the Starling curve,” in contrast to patients with isolated left ventricle (LV) failure.
RV pressure overload is associated with a range of conditions, such as pericardial disease, pulmonary embolism, acute respiratory distress syndrome, and pulmonary arterial hypertension. When combined with RV overload, patients may develop intracardiac shunting or coronary heart disease, Dr. Baez said. Decreased contractility associated with RV failure can result from sepsis, right ventricular myocardial infarction, myocarditis, and arrhythmia.
Dr. Baez cited the 2018 scientific statement from the American Heart Association on the evaluation and management of right-sided heart failure. The authors of the statement noted that the complicated geometry of the right heart makes functional assessment a challenge. They wrote that various hemodynamic and biochemical markers can help guide clinical assessment and therapeutic decision-making.
Increased RV afterload drives multiple factors that can ultimately lead to cardiogenic shock and death, said Dr. Baez. These factors include decreased RV oxygen delivery, decreased RV coronary perfusion, decreased systemic blood pressure, and low carbon monoxide levels. RV afterload also leads to decreased RV contractility, an increase in RV oxygen demand, and tension in the RV wall, and it may contribute to tricuspid valve insufficiency, neurohormonal activation, and RV ischemia.
Treatment strategies involve improving symptoms and stopping disease progression, said Baez. In its scientific statement, the AHA recommends steps for assessing RV and LV function so as to identify RV failure as soon as possible, he said. After excluding pericardial disease, the AHA advises diagnosis and treatment of etiology-specific causes, such as right ventricular MI, pulmonary embolism, and sepsis. For arrhythmias, it recommends maintaining sinus rhythm when possible and considering a pacemaker to maintain atrioventricular synchrony and to avoid excessive bradycardia.
In its statement, the AHA also recommends optimizing preload with right arterial pressure/central venous pressure of 8-12 mm Hg, said Dr. Baez. Preload optimization combined with afterload reduction and improved contractility are hallmarks of care for patients with RV failure.
Avoiding systemic hypotension can prevent sequelae, such as myocardial ischemia and further hypotension, he said.
Optimization of fluid status is another key to managing RV failure, said Dr. Baez. Right heart coronary perfusion pressure can be protected by maintaining mean arterial pressure, and consideration should be given to reducing the RV afterload. Other strategies include inotropic medications and rhythm stabilization.
In general, for RV failure patients, “correct hypoxia, hypercarbia, and acidosis and avoid intubation when possible,” he said. Extracorporeal membrane oxygenation (ECMO) may be an option, depending on how many mechanical ventilator settings need to be adjusted.
In a study by Dr. Baez and colleagues published in Critical Care Medicine, the authors presented a Bayesian probability model for plasma lactate and severity of illness in cases of acute pulmonary embolism. “This Bayesian model demonstrated that the combination of shock index and lactate yield superior diagnostic gains than those compare to the sPESI and lactate,” Dr. Baez said.
The care model needs to be specific to the etiology, he added. Volume management in congested pulmonary hypertension involves a “squeeze and diurese” strategy.
According to the Internet Book of Critical Care, for patients with mean arterial pressure (MAP) of 60 mm Hg, central venous pressure (CVP) of 25 mm Hg, renal perfusion pressure of 25 mm Hg, and no urine output, a vasopressor should be added to treatment, Dr. Baez said. In cases in which the MAP 75 mm Hg, the CVP is 25 mm Hg, the renal perfusion pressure is 50 mm Hg, and the patient has good urine output, vasopressors should be continued and fluid should be removed through use of a diuretic. For patients with a MAP of 75 mm Hg, a CVP of 12 mm Hg, and renal perfusion pressure of 63 mm Hg who have good urine output, the diuretic and the vasopressor should be discontinued.
Dr. Baez also reviewed several clinical studies of the utility of acute mechanical circulatory support systems for RV failure.
In two small studies involving a heart pump and a right ventricular assistive device, the 30-day survival rate was approximately 72%-73%. A study of 179 patients involving ECMO showed an in-hospital mortality rate of 38.6%, he said.
Overall, “prompt diagnosis, hemodynamic support, and initiation of specific treatment” are the foundations of managing RV failure, he concluded.
Dr. Baez disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Amado Alejandro Baez, MD, said in a presentation at the 2022 scientific assembly of the American College of Emergency Physicians.
The patient arrived on day 20 after a radical cystoprostatectomy. He had driven 4 hours from another city for a urology follow-up visit. On arrival, he developed respiratory distress symptoms and presented to the emergency department, said Dr. Baez, professor of emergency medicine and epidemiology at the Medical College of Georgia/Augusta University and triple-board certified in EMS, emergency medicine, and critical care.
The patient developed a massive pulmonary embolism with acute cor pulmonale (right-sided heart failure). An electrocardiogram showed an S1Q3T3, demonstrating the distinctive nature of right ventricular failure, said Dr. Baez.
Research has demonstrated the differences in physiology between the right and left ventricles, he said.
Dr. Baez highlighted some of the features of right ventricle (RV) failure and how to manage it. Notably, the RV is thinner and less resilient. “RV failure patients may fall off the Starling curve,” in contrast to patients with isolated left ventricle (LV) failure.
RV pressure overload is associated with a range of conditions, such as pericardial disease, pulmonary embolism, acute respiratory distress syndrome, and pulmonary arterial hypertension. When combined with RV overload, patients may develop intracardiac shunting or coronary heart disease, Dr. Baez said. Decreased contractility associated with RV failure can result from sepsis, right ventricular myocardial infarction, myocarditis, and arrhythmia.
Dr. Baez cited the 2018 scientific statement from the American Heart Association on the evaluation and management of right-sided heart failure. The authors of the statement noted that the complicated geometry of the right heart makes functional assessment a challenge. They wrote that various hemodynamic and biochemical markers can help guide clinical assessment and therapeutic decision-making.
Increased RV afterload drives multiple factors that can ultimately lead to cardiogenic shock and death, said Dr. Baez. These factors include decreased RV oxygen delivery, decreased RV coronary perfusion, decreased systemic blood pressure, and low carbon monoxide levels. RV afterload also leads to decreased RV contractility, an increase in RV oxygen demand, and tension in the RV wall, and it may contribute to tricuspid valve insufficiency, neurohormonal activation, and RV ischemia.
Treatment strategies involve improving symptoms and stopping disease progression, said Baez. In its scientific statement, the AHA recommends steps for assessing RV and LV function so as to identify RV failure as soon as possible, he said. After excluding pericardial disease, the AHA advises diagnosis and treatment of etiology-specific causes, such as right ventricular MI, pulmonary embolism, and sepsis. For arrhythmias, it recommends maintaining sinus rhythm when possible and considering a pacemaker to maintain atrioventricular synchrony and to avoid excessive bradycardia.
In its statement, the AHA also recommends optimizing preload with right arterial pressure/central venous pressure of 8-12 mm Hg, said Dr. Baez. Preload optimization combined with afterload reduction and improved contractility are hallmarks of care for patients with RV failure.
Avoiding systemic hypotension can prevent sequelae, such as myocardial ischemia and further hypotension, he said.
Optimization of fluid status is another key to managing RV failure, said Dr. Baez. Right heart coronary perfusion pressure can be protected by maintaining mean arterial pressure, and consideration should be given to reducing the RV afterload. Other strategies include inotropic medications and rhythm stabilization.
In general, for RV failure patients, “correct hypoxia, hypercarbia, and acidosis and avoid intubation when possible,” he said. Extracorporeal membrane oxygenation (ECMO) may be an option, depending on how many mechanical ventilator settings need to be adjusted.
In a study by Dr. Baez and colleagues published in Critical Care Medicine, the authors presented a Bayesian probability model for plasma lactate and severity of illness in cases of acute pulmonary embolism. “This Bayesian model demonstrated that the combination of shock index and lactate yield superior diagnostic gains than those compare to the sPESI and lactate,” Dr. Baez said.
The care model needs to be specific to the etiology, he added. Volume management in congested pulmonary hypertension involves a “squeeze and diurese” strategy.
According to the Internet Book of Critical Care, for patients with mean arterial pressure (MAP) of 60 mm Hg, central venous pressure (CVP) of 25 mm Hg, renal perfusion pressure of 25 mm Hg, and no urine output, a vasopressor should be added to treatment, Dr. Baez said. In cases in which the MAP 75 mm Hg, the CVP is 25 mm Hg, the renal perfusion pressure is 50 mm Hg, and the patient has good urine output, vasopressors should be continued and fluid should be removed through use of a diuretic. For patients with a MAP of 75 mm Hg, a CVP of 12 mm Hg, and renal perfusion pressure of 63 mm Hg who have good urine output, the diuretic and the vasopressor should be discontinued.
Dr. Baez also reviewed several clinical studies of the utility of acute mechanical circulatory support systems for RV failure.
In two small studies involving a heart pump and a right ventricular assistive device, the 30-day survival rate was approximately 72%-73%. A study of 179 patients involving ECMO showed an in-hospital mortality rate of 38.6%, he said.
Overall, “prompt diagnosis, hemodynamic support, and initiation of specific treatment” are the foundations of managing RV failure, he concluded.
Dr. Baez disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM ACEP 2022
Current alopecia areata options include old and new therapies
LAS VEGAS – in a presentation at MedscapeLive’s annual Las Vegas Dermatology Seminar.
“Some patients don’t have alopecia, but they have been managed for it,” he said. “Whenever there is an ounce of doubt, take a biopsy,” he advised.
Assessing disease severity in patients with alopecia areata (AA) is especially important as new therapies become available, said Dr. King, associate professor of dermatology at Yale University, New Haven, Conn. The Severity of Alopecia Tool (SALT) Score has been available since 2004, and remains a useful tool to estimate percent hair loss. The SALT Score divides the scalp into four sections: 18% each for the right and left sides, 40% for the top of the head, and 24% for the back of the head, said Dr. King. However, the SALT Score can be enhanced or modified based on a holistic approach to disease severity that categorizes alopecia as mild (scalp hair loss of 20% or less), moderate (scalp hair loss of 21 to 49%), or severe (scalp hair loss of 50% or more).
For example, if a patient’s hair loss based on SALT Score is mild or moderate, increase the severity by 1 level (from mild to moderate, or moderate to severe) if any of the following conditions apply: Noticeable eyebrow or eyelash involvement, inadequate treatment response after 6 months, diffuse positive hair pull test consistent with rapid progression of AA, or a negative impact on psychosocial functioning because of AA, he said.
Treatment advances
Understanding of the pathogenesis of AA has been slow to evolve, Dr. King noted. “We haven’t been able to shake this concept that people are causing the disease by being depressed,” as noted in the literature from the 1950s.
In 2014, breakthrough research changed the game by identifying the roles of interferon gamma and interleukin 15, Dr. King said. Since then, more research has been conducted on Janus kinase (JAK) inhibitors for AA. Dr. King was a coinvestigator on a 2014 case report in which a patient with psoriasis and alopecia universalis experienced regrowth of most of his body hair after 8 months of daily oral tofacitinib, a JAK inhibitor.
However, despite the dramatic results in some patients, “tofacitinib doesn’t always work,” said Dr. King. In his experience, patients for whom tofacitinib didn’t work were those with complete or nearly complete scalp hair loss for more than 10 years.
Approval of baricitinib
Dr. King’s recent work supported the approval in June 2022 of oral baricitinib, a JAK inhibitor, for AA. He reviewed data from his late-breaker abstract presented at the annual meeting of the American Academy of Dermatology in March 2022, where he reported that almost 40% of adults with AA treated with 4 mg of baricitinib daily had significant hair regrowth over 52 weeks.
Two other oral JAK inhibitors in the pipeline for AA are deuruxolitinib and ritlecitinib, which significantly increased the proportion of patients achieving SALT scores of 20 or less, compared with patients on placebo in early clinical trials. Data on both were presented at the annual meeting of the European Academy of Dermatology and Venereology.
So far, topical JAK inhibitors have not shown success in hair regrowth for AA patients, said Dr. King. Phase 2 studies of both ruxolitinib 1.5% cream and delgocitinib ointment were ineffective for AA.
Emerging role for oral minoxidil
Oral minoxidil has had a recent resurgence as an adjunct therapy to the new JAK inhibitors. A study published in 1987 found that, with oral minoxidil monotherapy, a cosmetic response was seen in 18% of patients with AA, Dr. King said.
In a study published in the Journal of the American Academy of Dermatology, Dr. King and colleagues noted that dose escalation is sometimes needed for effective treatment of AA with tofacitinib. They examined the effect of adding oral minoxidil to tofacitinib in patients with severe AA as a way to increase efficacy without increasing tofacitinib dosage. They reviewed data from 12 patients ages 18-51 years who were prescribed 5 mg of tofacitinib twice daily, plus 2.5 mg oral minoxidil daily for women and 2.5 mg of minoxidil twice daily for men; women received a lower dose to minimize the side effect of hypertrichosis.
After 6 months, 67% (eight patients) achieved at least 75% hair regrowth; of those eight patients, seven (58% of the total) had hair regrowth on a twice-daily dose of 5 mg tofacitinib with no need for dose escalation, Dr. King said.
More research is needed, but oral minoxidil may be a useful adjunct treatment for some patients with AA, he added.
During a question and answer session, Dr. King was asked to elaborate on the mechanism of minoxidil in combination with JAK inhibitors. “The truth is that I just don’t know” why the combination works for some patients. However, the majority of patients who succeed with this combination regrow hair by 4 months. “There is something special about that combination.”
Dr. King disclosed serving as a consultant or adviser for AbbVie, AltruBio, Almirall, AnaptysBio, Arena Pharmaceuticals, Bioniz, Bristol Myers Squibb, Concert Pharmaceuticals, Horizon, Incyte, Leo Pharma, Eli Lilly, Otsuka, Pfizer, Regeneron, Sanofi Genzyme, Twi Biotechnology, Viela Bio, and Visterra; serving as a speaker or as a member of the speakers bureau for Incyte, Pfizer, Regeneron, Sanofi Genzyme; and receiving research funding from Concert Pharmaceuticals, Eli Lilly, and Pfizer.
MedscapeLive and this news organization are owned by the same parent company.
LAS VEGAS – in a presentation at MedscapeLive’s annual Las Vegas Dermatology Seminar.
“Some patients don’t have alopecia, but they have been managed for it,” he said. “Whenever there is an ounce of doubt, take a biopsy,” he advised.
Assessing disease severity in patients with alopecia areata (AA) is especially important as new therapies become available, said Dr. King, associate professor of dermatology at Yale University, New Haven, Conn. The Severity of Alopecia Tool (SALT) Score has been available since 2004, and remains a useful tool to estimate percent hair loss. The SALT Score divides the scalp into four sections: 18% each for the right and left sides, 40% for the top of the head, and 24% for the back of the head, said Dr. King. However, the SALT Score can be enhanced or modified based on a holistic approach to disease severity that categorizes alopecia as mild (scalp hair loss of 20% or less), moderate (scalp hair loss of 21 to 49%), or severe (scalp hair loss of 50% or more).
For example, if a patient’s hair loss based on SALT Score is mild or moderate, increase the severity by 1 level (from mild to moderate, or moderate to severe) if any of the following conditions apply: Noticeable eyebrow or eyelash involvement, inadequate treatment response after 6 months, diffuse positive hair pull test consistent with rapid progression of AA, or a negative impact on psychosocial functioning because of AA, he said.
Treatment advances
Understanding of the pathogenesis of AA has been slow to evolve, Dr. King noted. “We haven’t been able to shake this concept that people are causing the disease by being depressed,” as noted in the literature from the 1950s.
In 2014, breakthrough research changed the game by identifying the roles of interferon gamma and interleukin 15, Dr. King said. Since then, more research has been conducted on Janus kinase (JAK) inhibitors for AA. Dr. King was a coinvestigator on a 2014 case report in which a patient with psoriasis and alopecia universalis experienced regrowth of most of his body hair after 8 months of daily oral tofacitinib, a JAK inhibitor.
However, despite the dramatic results in some patients, “tofacitinib doesn’t always work,” said Dr. King. In his experience, patients for whom tofacitinib didn’t work were those with complete or nearly complete scalp hair loss for more than 10 years.
Approval of baricitinib
Dr. King’s recent work supported the approval in June 2022 of oral baricitinib, a JAK inhibitor, for AA. He reviewed data from his late-breaker abstract presented at the annual meeting of the American Academy of Dermatology in March 2022, where he reported that almost 40% of adults with AA treated with 4 mg of baricitinib daily had significant hair regrowth over 52 weeks.
Two other oral JAK inhibitors in the pipeline for AA are deuruxolitinib and ritlecitinib, which significantly increased the proportion of patients achieving SALT scores of 20 or less, compared with patients on placebo in early clinical trials. Data on both were presented at the annual meeting of the European Academy of Dermatology and Venereology.
So far, topical JAK inhibitors have not shown success in hair regrowth for AA patients, said Dr. King. Phase 2 studies of both ruxolitinib 1.5% cream and delgocitinib ointment were ineffective for AA.
Emerging role for oral minoxidil
Oral minoxidil has had a recent resurgence as an adjunct therapy to the new JAK inhibitors. A study published in 1987 found that, with oral minoxidil monotherapy, a cosmetic response was seen in 18% of patients with AA, Dr. King said.
In a study published in the Journal of the American Academy of Dermatology, Dr. King and colleagues noted that dose escalation is sometimes needed for effective treatment of AA with tofacitinib. They examined the effect of adding oral minoxidil to tofacitinib in patients with severe AA as a way to increase efficacy without increasing tofacitinib dosage. They reviewed data from 12 patients ages 18-51 years who were prescribed 5 mg of tofacitinib twice daily, plus 2.5 mg oral minoxidil daily for women and 2.5 mg of minoxidil twice daily for men; women received a lower dose to minimize the side effect of hypertrichosis.
After 6 months, 67% (eight patients) achieved at least 75% hair regrowth; of those eight patients, seven (58% of the total) had hair regrowth on a twice-daily dose of 5 mg tofacitinib with no need for dose escalation, Dr. King said.
More research is needed, but oral minoxidil may be a useful adjunct treatment for some patients with AA, he added.
During a question and answer session, Dr. King was asked to elaborate on the mechanism of minoxidil in combination with JAK inhibitors. “The truth is that I just don’t know” why the combination works for some patients. However, the majority of patients who succeed with this combination regrow hair by 4 months. “There is something special about that combination.”
Dr. King disclosed serving as a consultant or adviser for AbbVie, AltruBio, Almirall, AnaptysBio, Arena Pharmaceuticals, Bioniz, Bristol Myers Squibb, Concert Pharmaceuticals, Horizon, Incyte, Leo Pharma, Eli Lilly, Otsuka, Pfizer, Regeneron, Sanofi Genzyme, Twi Biotechnology, Viela Bio, and Visterra; serving as a speaker or as a member of the speakers bureau for Incyte, Pfizer, Regeneron, Sanofi Genzyme; and receiving research funding from Concert Pharmaceuticals, Eli Lilly, and Pfizer.
MedscapeLive and this news organization are owned by the same parent company.
LAS VEGAS – in a presentation at MedscapeLive’s annual Las Vegas Dermatology Seminar.
“Some patients don’t have alopecia, but they have been managed for it,” he said. “Whenever there is an ounce of doubt, take a biopsy,” he advised.
Assessing disease severity in patients with alopecia areata (AA) is especially important as new therapies become available, said Dr. King, associate professor of dermatology at Yale University, New Haven, Conn. The Severity of Alopecia Tool (SALT) Score has been available since 2004, and remains a useful tool to estimate percent hair loss. The SALT Score divides the scalp into four sections: 18% each for the right and left sides, 40% for the top of the head, and 24% for the back of the head, said Dr. King. However, the SALT Score can be enhanced or modified based on a holistic approach to disease severity that categorizes alopecia as mild (scalp hair loss of 20% or less), moderate (scalp hair loss of 21 to 49%), or severe (scalp hair loss of 50% or more).
For example, if a patient’s hair loss based on SALT Score is mild or moderate, increase the severity by 1 level (from mild to moderate, or moderate to severe) if any of the following conditions apply: Noticeable eyebrow or eyelash involvement, inadequate treatment response after 6 months, diffuse positive hair pull test consistent with rapid progression of AA, or a negative impact on psychosocial functioning because of AA, he said.
Treatment advances
Understanding of the pathogenesis of AA has been slow to evolve, Dr. King noted. “We haven’t been able to shake this concept that people are causing the disease by being depressed,” as noted in the literature from the 1950s.
In 2014, breakthrough research changed the game by identifying the roles of interferon gamma and interleukin 15, Dr. King said. Since then, more research has been conducted on Janus kinase (JAK) inhibitors for AA. Dr. King was a coinvestigator on a 2014 case report in which a patient with psoriasis and alopecia universalis experienced regrowth of most of his body hair after 8 months of daily oral tofacitinib, a JAK inhibitor.
However, despite the dramatic results in some patients, “tofacitinib doesn’t always work,” said Dr. King. In his experience, patients for whom tofacitinib didn’t work were those with complete or nearly complete scalp hair loss for more than 10 years.
Approval of baricitinib
Dr. King’s recent work supported the approval in June 2022 of oral baricitinib, a JAK inhibitor, for AA. He reviewed data from his late-breaker abstract presented at the annual meeting of the American Academy of Dermatology in March 2022, where he reported that almost 40% of adults with AA treated with 4 mg of baricitinib daily had significant hair regrowth over 52 weeks.
Two other oral JAK inhibitors in the pipeline for AA are deuruxolitinib and ritlecitinib, which significantly increased the proportion of patients achieving SALT scores of 20 or less, compared with patients on placebo in early clinical trials. Data on both were presented at the annual meeting of the European Academy of Dermatology and Venereology.
So far, topical JAK inhibitors have not shown success in hair regrowth for AA patients, said Dr. King. Phase 2 studies of both ruxolitinib 1.5% cream and delgocitinib ointment were ineffective for AA.
Emerging role for oral minoxidil
Oral minoxidil has had a recent resurgence as an adjunct therapy to the new JAK inhibitors. A study published in 1987 found that, with oral minoxidil monotherapy, a cosmetic response was seen in 18% of patients with AA, Dr. King said.
In a study published in the Journal of the American Academy of Dermatology, Dr. King and colleagues noted that dose escalation is sometimes needed for effective treatment of AA with tofacitinib. They examined the effect of adding oral minoxidil to tofacitinib in patients with severe AA as a way to increase efficacy without increasing tofacitinib dosage. They reviewed data from 12 patients ages 18-51 years who were prescribed 5 mg of tofacitinib twice daily, plus 2.5 mg oral minoxidil daily for women and 2.5 mg of minoxidil twice daily for men; women received a lower dose to minimize the side effect of hypertrichosis.
After 6 months, 67% (eight patients) achieved at least 75% hair regrowth; of those eight patients, seven (58% of the total) had hair regrowth on a twice-daily dose of 5 mg tofacitinib with no need for dose escalation, Dr. King said.
More research is needed, but oral minoxidil may be a useful adjunct treatment for some patients with AA, he added.
During a question and answer session, Dr. King was asked to elaborate on the mechanism of minoxidil in combination with JAK inhibitors. “The truth is that I just don’t know” why the combination works for some patients. However, the majority of patients who succeed with this combination regrow hair by 4 months. “There is something special about that combination.”
Dr. King disclosed serving as a consultant or adviser for AbbVie, AltruBio, Almirall, AnaptysBio, Arena Pharmaceuticals, Bioniz, Bristol Myers Squibb, Concert Pharmaceuticals, Horizon, Incyte, Leo Pharma, Eli Lilly, Otsuka, Pfizer, Regeneron, Sanofi Genzyme, Twi Biotechnology, Viela Bio, and Visterra; serving as a speaker or as a member of the speakers bureau for Incyte, Pfizer, Regeneron, Sanofi Genzyme; and receiving research funding from Concert Pharmaceuticals, Eli Lilly, and Pfizer.
MedscapeLive and this news organization are owned by the same parent company.
AT INNOVATIONS IN DERMATOLOGY
Paxlovid has been free so far. Next year, sticker shock awaits
Nearly 6 million Americans have taken Paxlovid for free, courtesy of the federal government. The Pfizer pill has helped prevent many people infected with COVID-19 from being hospitalized or dying, and it may even reduce the risk of developing long COVID.
And that means fewer people will get the potentially lifesaving treatments, experts said.
“I think the numbers will go way down,” said Jill Rosenthal, director of public health policy at the Center for American Progress, a left-leaning think tank. A bill for several hundred dollars or more would lead many people to decide the medication isn’t worth the price, she said.
In response to the unprecedented public health crisis caused by COVID, the federal government spent billions of dollars on developing new vaccines and treatments, to swift success: Less than a year after the pandemic was declared, medical workers got their first vaccines. But as many people have refused the shots and stopped wearing masks, the virus still rages and mutates. In 2022 alone, 250,000 Americans have died from COVID, more than from strokes or diabetes.
But soon the Department of Health & Human Services will stop supplying COVID treatments, and pharmacies will purchase and bill for them the same way they do for antibiotic pills or asthma inhalers. Paxlovid is expected to hit the private market in mid-2023, according to HHS plans shared in an October meeting with state health officials and clinicians. Merck’s Lagevrio, a less-effective COVID treatment pill, and AstraZeneca’s Evusheld, a preventive therapy for the immunocompromised, are on track to be commercialized sooner, sometime in the winter.
The U.S. government has so far purchased 20 million courses of Paxlovid, priced at about $530 each, a discount for buying in bulk that Pfizer CEO Albert Bourla called “really very attractive” to the federal government in a July earnings call. The drug will cost far more on the private market, although in a statement to Kaiser Health News, Pfizer declined to share the planned price. The government will also stop paying for the company’s COVID vaccine next year – those shots will quadruple in price, from the discount rate the government pays of $30 to about $120.
Mr. Bourla told investors in November that he expects the move will make Paxlovid and its COVID vaccine “a multibillion-dollars franchise.”
Nearly 9 in 10 people dying from the virus now are 65 or older. Yet federal law restricts Medicare Part D – the prescription drug program that covers nearly 50 million seniors – from covering the COVID treatment pills. The medications are meant for those most at risk of serious illness, including seniors.
Paxlovid and the other treatments are currently available under an emergency use authorization from the FDA, a fast-track review used in extraordinary situations. Although Pfizer applied for full approval in June, the process can take anywhere from several months to years. And Medicare Part D can’t cover any medications without that full stamp of approval.
Paying out-of-pocket would be “a substantial barrier” for seniors on Medicare – the very people who would benefit most from the drug, wrote federal health experts.
“From a public health perspective, and even from a health care capacity and cost perspective, it would just defy reason to not continue to make these drugs readily available,” said Dr. Larry Madoff, medical director of Massachusetts’s Bureau of Infectious Disease and Laboratory Sciences. He’s hopeful that the federal health agency will find a way to set aside unused doses for seniors and people without insurance.
In mid-November, the White House requested that Congress approve an additional $2.5 billion for COVID therapeutics and vaccines to make sure people can afford the medications when they’re no longer free. But there’s little hope it will be approved – the Senate voted that same day to end the public health emergency and denied similar requests in recent months.
Many Americans have already faced hurdles just getting a prescription for COVID treatment. Although the federal government doesn’t track who’s gotten the drug, a Centers for Disease Control and Prevention study using data from 30 medical centers found that Black and Hispanic patients with COVID were much less likely to receive Paxlovid than White patients. (Hispanic people can be of any race or combination of races.) And when the government is no longer picking up the tab, experts predict that these gaps by race, income, and geography will widen.
People in Northeastern states used the drug far more often than those in the rest of the country, according to a KHN analysis of Paxlovid use in September and October. But it wasn’t because people in the region were getting sick from COVID at much higher rates – instead, many of those states offered better access to health care to begin with and created special programs to get Paxlovid to their residents.
About 10 mostly Democratic states and several large counties in the Northeast and elsewhere created free “test-to-treat” programs that allow their residents to get an immediate doctor visit and prescription for treatment after testing positive for COVID. In Massachusetts, more than 20,000 residents have used the state’s video and phone hotline, which is available 7 days a week in 13 languages. Massachusetts, which has the highest insurance rate in the country and relatively low travel times to pharmacies, had the second-highest Paxlovid usage rate among states this fall.
States with higher COVID death rates, like Florida and Kentucky, where residents must travel farther for health care and are more likely to be uninsured, used the drug less often. Without no-cost test-to-treat options, residents have struggled to get prescriptions even though the drug itself is still free.
“If you look at access to medications for people who are uninsured, I think that there’s no question that will widen those disparities,” Ms. Rosenthal said.
People who get insurance through their jobs could face high copays at the register, too, just as they do for insulin and other expensive or brand-name drugs.
Most private insurance companies will end up covering COVID therapeutics to some extent, said Sabrina Corlette, a research professor at Georgetown University’s Center on Health Insurance Reforms. After all, the pills are cheaper than a hospital stay. But for most people who get insurance through their jobs, there are “really no rules at all,” she said. Some insurers could take months to add the drugs to their plans or decide not to pay for them.
And the additional cost means many people will go without the medication. “We know from lots of research that when people face cost sharing for these drugs that they need to take, they will often forgo or cut back,” Ms. Corlette said.
One group doesn’t need to worry about sticker shock. Medicaid, the public insurance program for low-income adults and children, will cover the treatments in full until at least early 2024.
HHS officials could set aside any leftover taxpayer-funded medication for people who can’t afford to pay the full cost, but they haven’t shared any concrete plans to do so. The government purchased 20 million courses of Paxlovid and 3 million of Lagevrio. Fewer than a third have been used, and usage has fallen in recent months, according to KHN’s analysis of the data from HHS.
Sixty percent of the government’s supply of Evusheld is also still available, although the COVID prevention therapy is less effective against new strains of the virus. The health department in one state, New Mexico, has recommended against using it.
HHS did not make officials available for an interview or answer written questions about the commercialization plans.
The government created a potential workaround when they moved bebtelovimab, another COVID treatment, to the private market this summer. It now retails for $2,100 per patient. The agency set aside the remaining 60,000 government-purchased doses that hospitals could use to treat uninsured patients in a convoluted dose-replacement process. But it’s hard to tell how well that setup would work for Paxlovid: Bebtelovimab was already much less popular, and the FDA halted its use on Nov. 30 because it’s less effective against current strains of the virus.
Federal officials and insurance companies would have good reason to make sure patients can continue to afford COVID drugs: They’re far cheaper than if patients land in the emergency room.
“The medications are so worthwhile,” said Dr. Madoff, the Massachusetts health official. “They’re not expensive in the grand scheme of health care costs.”
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
Nearly 6 million Americans have taken Paxlovid for free, courtesy of the federal government. The Pfizer pill has helped prevent many people infected with COVID-19 from being hospitalized or dying, and it may even reduce the risk of developing long COVID.
And that means fewer people will get the potentially lifesaving treatments, experts said.
“I think the numbers will go way down,” said Jill Rosenthal, director of public health policy at the Center for American Progress, a left-leaning think tank. A bill for several hundred dollars or more would lead many people to decide the medication isn’t worth the price, she said.
In response to the unprecedented public health crisis caused by COVID, the federal government spent billions of dollars on developing new vaccines and treatments, to swift success: Less than a year after the pandemic was declared, medical workers got their first vaccines. But as many people have refused the shots and stopped wearing masks, the virus still rages and mutates. In 2022 alone, 250,000 Americans have died from COVID, more than from strokes or diabetes.
But soon the Department of Health & Human Services will stop supplying COVID treatments, and pharmacies will purchase and bill for them the same way they do for antibiotic pills or asthma inhalers. Paxlovid is expected to hit the private market in mid-2023, according to HHS plans shared in an October meeting with state health officials and clinicians. Merck’s Lagevrio, a less-effective COVID treatment pill, and AstraZeneca’s Evusheld, a preventive therapy for the immunocompromised, are on track to be commercialized sooner, sometime in the winter.
The U.S. government has so far purchased 20 million courses of Paxlovid, priced at about $530 each, a discount for buying in bulk that Pfizer CEO Albert Bourla called “really very attractive” to the federal government in a July earnings call. The drug will cost far more on the private market, although in a statement to Kaiser Health News, Pfizer declined to share the planned price. The government will also stop paying for the company’s COVID vaccine next year – those shots will quadruple in price, from the discount rate the government pays of $30 to about $120.
Mr. Bourla told investors in November that he expects the move will make Paxlovid and its COVID vaccine “a multibillion-dollars franchise.”
Nearly 9 in 10 people dying from the virus now are 65 or older. Yet federal law restricts Medicare Part D – the prescription drug program that covers nearly 50 million seniors – from covering the COVID treatment pills. The medications are meant for those most at risk of serious illness, including seniors.
Paxlovid and the other treatments are currently available under an emergency use authorization from the FDA, a fast-track review used in extraordinary situations. Although Pfizer applied for full approval in June, the process can take anywhere from several months to years. And Medicare Part D can’t cover any medications without that full stamp of approval.
Paying out-of-pocket would be “a substantial barrier” for seniors on Medicare – the very people who would benefit most from the drug, wrote federal health experts.
“From a public health perspective, and even from a health care capacity and cost perspective, it would just defy reason to not continue to make these drugs readily available,” said Dr. Larry Madoff, medical director of Massachusetts’s Bureau of Infectious Disease and Laboratory Sciences. He’s hopeful that the federal health agency will find a way to set aside unused doses for seniors and people without insurance.
In mid-November, the White House requested that Congress approve an additional $2.5 billion for COVID therapeutics and vaccines to make sure people can afford the medications when they’re no longer free. But there’s little hope it will be approved – the Senate voted that same day to end the public health emergency and denied similar requests in recent months.
Many Americans have already faced hurdles just getting a prescription for COVID treatment. Although the federal government doesn’t track who’s gotten the drug, a Centers for Disease Control and Prevention study using data from 30 medical centers found that Black and Hispanic patients with COVID were much less likely to receive Paxlovid than White patients. (Hispanic people can be of any race or combination of races.) And when the government is no longer picking up the tab, experts predict that these gaps by race, income, and geography will widen.
People in Northeastern states used the drug far more often than those in the rest of the country, according to a KHN analysis of Paxlovid use in September and October. But it wasn’t because people in the region were getting sick from COVID at much higher rates – instead, many of those states offered better access to health care to begin with and created special programs to get Paxlovid to their residents.
About 10 mostly Democratic states and several large counties in the Northeast and elsewhere created free “test-to-treat” programs that allow their residents to get an immediate doctor visit and prescription for treatment after testing positive for COVID. In Massachusetts, more than 20,000 residents have used the state’s video and phone hotline, which is available 7 days a week in 13 languages. Massachusetts, which has the highest insurance rate in the country and relatively low travel times to pharmacies, had the second-highest Paxlovid usage rate among states this fall.
States with higher COVID death rates, like Florida and Kentucky, where residents must travel farther for health care and are more likely to be uninsured, used the drug less often. Without no-cost test-to-treat options, residents have struggled to get prescriptions even though the drug itself is still free.
“If you look at access to medications for people who are uninsured, I think that there’s no question that will widen those disparities,” Ms. Rosenthal said.
People who get insurance through their jobs could face high copays at the register, too, just as they do for insulin and other expensive or brand-name drugs.
Most private insurance companies will end up covering COVID therapeutics to some extent, said Sabrina Corlette, a research professor at Georgetown University’s Center on Health Insurance Reforms. After all, the pills are cheaper than a hospital stay. But for most people who get insurance through their jobs, there are “really no rules at all,” she said. Some insurers could take months to add the drugs to their plans or decide not to pay for them.
And the additional cost means many people will go without the medication. “We know from lots of research that when people face cost sharing for these drugs that they need to take, they will often forgo or cut back,” Ms. Corlette said.
One group doesn’t need to worry about sticker shock. Medicaid, the public insurance program for low-income adults and children, will cover the treatments in full until at least early 2024.
HHS officials could set aside any leftover taxpayer-funded medication for people who can’t afford to pay the full cost, but they haven’t shared any concrete plans to do so. The government purchased 20 million courses of Paxlovid and 3 million of Lagevrio. Fewer than a third have been used, and usage has fallen in recent months, according to KHN’s analysis of the data from HHS.
Sixty percent of the government’s supply of Evusheld is also still available, although the COVID prevention therapy is less effective against new strains of the virus. The health department in one state, New Mexico, has recommended against using it.
HHS did not make officials available for an interview or answer written questions about the commercialization plans.
The government created a potential workaround when they moved bebtelovimab, another COVID treatment, to the private market this summer. It now retails for $2,100 per patient. The agency set aside the remaining 60,000 government-purchased doses that hospitals could use to treat uninsured patients in a convoluted dose-replacement process. But it’s hard to tell how well that setup would work for Paxlovid: Bebtelovimab was already much less popular, and the FDA halted its use on Nov. 30 because it’s less effective against current strains of the virus.
Federal officials and insurance companies would have good reason to make sure patients can continue to afford COVID drugs: They’re far cheaper than if patients land in the emergency room.
“The medications are so worthwhile,” said Dr. Madoff, the Massachusetts health official. “They’re not expensive in the grand scheme of health care costs.”
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
Nearly 6 million Americans have taken Paxlovid for free, courtesy of the federal government. The Pfizer pill has helped prevent many people infected with COVID-19 from being hospitalized or dying, and it may even reduce the risk of developing long COVID.
And that means fewer people will get the potentially lifesaving treatments, experts said.
“I think the numbers will go way down,” said Jill Rosenthal, director of public health policy at the Center for American Progress, a left-leaning think tank. A bill for several hundred dollars or more would lead many people to decide the medication isn’t worth the price, she said.
In response to the unprecedented public health crisis caused by COVID, the federal government spent billions of dollars on developing new vaccines and treatments, to swift success: Less than a year after the pandemic was declared, medical workers got their first vaccines. But as many people have refused the shots and stopped wearing masks, the virus still rages and mutates. In 2022 alone, 250,000 Americans have died from COVID, more than from strokes or diabetes.
But soon the Department of Health & Human Services will stop supplying COVID treatments, and pharmacies will purchase and bill for them the same way they do for antibiotic pills or asthma inhalers. Paxlovid is expected to hit the private market in mid-2023, according to HHS plans shared in an October meeting with state health officials and clinicians. Merck’s Lagevrio, a less-effective COVID treatment pill, and AstraZeneca’s Evusheld, a preventive therapy for the immunocompromised, are on track to be commercialized sooner, sometime in the winter.
The U.S. government has so far purchased 20 million courses of Paxlovid, priced at about $530 each, a discount for buying in bulk that Pfizer CEO Albert Bourla called “really very attractive” to the federal government in a July earnings call. The drug will cost far more on the private market, although in a statement to Kaiser Health News, Pfizer declined to share the planned price. The government will also stop paying for the company’s COVID vaccine next year – those shots will quadruple in price, from the discount rate the government pays of $30 to about $120.
Mr. Bourla told investors in November that he expects the move will make Paxlovid and its COVID vaccine “a multibillion-dollars franchise.”
Nearly 9 in 10 people dying from the virus now are 65 or older. Yet federal law restricts Medicare Part D – the prescription drug program that covers nearly 50 million seniors – from covering the COVID treatment pills. The medications are meant for those most at risk of serious illness, including seniors.
Paxlovid and the other treatments are currently available under an emergency use authorization from the FDA, a fast-track review used in extraordinary situations. Although Pfizer applied for full approval in June, the process can take anywhere from several months to years. And Medicare Part D can’t cover any medications without that full stamp of approval.
Paying out-of-pocket would be “a substantial barrier” for seniors on Medicare – the very people who would benefit most from the drug, wrote federal health experts.
“From a public health perspective, and even from a health care capacity and cost perspective, it would just defy reason to not continue to make these drugs readily available,” said Dr. Larry Madoff, medical director of Massachusetts’s Bureau of Infectious Disease and Laboratory Sciences. He’s hopeful that the federal health agency will find a way to set aside unused doses for seniors and people without insurance.
In mid-November, the White House requested that Congress approve an additional $2.5 billion for COVID therapeutics and vaccines to make sure people can afford the medications when they’re no longer free. But there’s little hope it will be approved – the Senate voted that same day to end the public health emergency and denied similar requests in recent months.
Many Americans have already faced hurdles just getting a prescription for COVID treatment. Although the federal government doesn’t track who’s gotten the drug, a Centers for Disease Control and Prevention study using data from 30 medical centers found that Black and Hispanic patients with COVID were much less likely to receive Paxlovid than White patients. (Hispanic people can be of any race or combination of races.) And when the government is no longer picking up the tab, experts predict that these gaps by race, income, and geography will widen.
People in Northeastern states used the drug far more often than those in the rest of the country, according to a KHN analysis of Paxlovid use in September and October. But it wasn’t because people in the region were getting sick from COVID at much higher rates – instead, many of those states offered better access to health care to begin with and created special programs to get Paxlovid to their residents.
About 10 mostly Democratic states and several large counties in the Northeast and elsewhere created free “test-to-treat” programs that allow their residents to get an immediate doctor visit and prescription for treatment after testing positive for COVID. In Massachusetts, more than 20,000 residents have used the state’s video and phone hotline, which is available 7 days a week in 13 languages. Massachusetts, which has the highest insurance rate in the country and relatively low travel times to pharmacies, had the second-highest Paxlovid usage rate among states this fall.
States with higher COVID death rates, like Florida and Kentucky, where residents must travel farther for health care and are more likely to be uninsured, used the drug less often. Without no-cost test-to-treat options, residents have struggled to get prescriptions even though the drug itself is still free.
“If you look at access to medications for people who are uninsured, I think that there’s no question that will widen those disparities,” Ms. Rosenthal said.
People who get insurance through their jobs could face high copays at the register, too, just as they do for insulin and other expensive or brand-name drugs.
Most private insurance companies will end up covering COVID therapeutics to some extent, said Sabrina Corlette, a research professor at Georgetown University’s Center on Health Insurance Reforms. After all, the pills are cheaper than a hospital stay. But for most people who get insurance through their jobs, there are “really no rules at all,” she said. Some insurers could take months to add the drugs to their plans or decide not to pay for them.
And the additional cost means many people will go without the medication. “We know from lots of research that when people face cost sharing for these drugs that they need to take, they will often forgo or cut back,” Ms. Corlette said.
One group doesn’t need to worry about sticker shock. Medicaid, the public insurance program for low-income adults and children, will cover the treatments in full until at least early 2024.
HHS officials could set aside any leftover taxpayer-funded medication for people who can’t afford to pay the full cost, but they haven’t shared any concrete plans to do so. The government purchased 20 million courses of Paxlovid and 3 million of Lagevrio. Fewer than a third have been used, and usage has fallen in recent months, according to KHN’s analysis of the data from HHS.
Sixty percent of the government’s supply of Evusheld is also still available, although the COVID prevention therapy is less effective against new strains of the virus. The health department in one state, New Mexico, has recommended against using it.
HHS did not make officials available for an interview or answer written questions about the commercialization plans.
The government created a potential workaround when they moved bebtelovimab, another COVID treatment, to the private market this summer. It now retails for $2,100 per patient. The agency set aside the remaining 60,000 government-purchased doses that hospitals could use to treat uninsured patients in a convoluted dose-replacement process. But it’s hard to tell how well that setup would work for Paxlovid: Bebtelovimab was already much less popular, and the FDA halted its use on Nov. 30 because it’s less effective against current strains of the virus.
Federal officials and insurance companies would have good reason to make sure patients can continue to afford COVID drugs: They’re far cheaper than if patients land in the emergency room.
“The medications are so worthwhile,” said Dr. Madoff, the Massachusetts health official. “They’re not expensive in the grand scheme of health care costs.”
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.