User login
Novel platform harnesses 3D laser technology for skin treatments
in all skin types, according to speakers at a virtual course on laser and aesthetic skin therapy.
The products feature “focal point technology,” which pairs 3D laser targeting with an integrated high-resolution imaging system (IntelliView), to help the user guide treatments at selectable depths. They have been cleared by the Food and Drug Administration for use in skin resurfacing procedures, and to treat benign pigmented lesions of the skin, including hyperpigmentation, and were created by Dieter Manstein, MD, PhD, Rox Anderson, MD, and Henry Chan, MD, of the Wellman Center for Photomedicine at Massachusetts General Hospital, and Irina Erenburg, PhD, CEO of AVAVA, the company that markets the products.
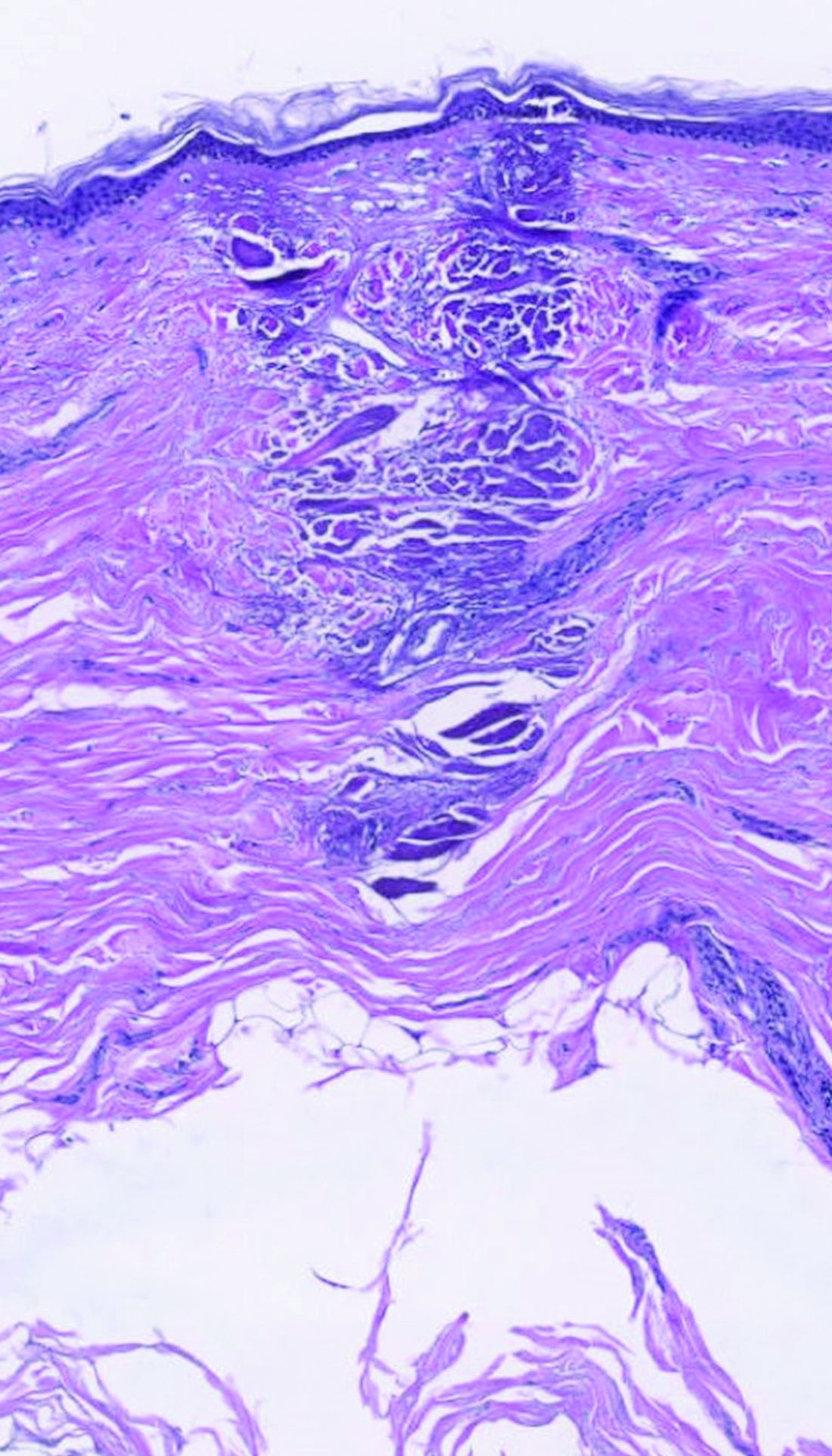
dermally focused treatment with Focal Point Technology. The coagulation zone, in dark purple, shows a deep conical lesion that extends 1.3 mm deep with significant epidermal sparing.
At the meeting, Mathew M. Avram, MD, JD, director of the Massachusetts General Hospital Dermatology Laser & Cosmetic Center, described focal point technology as an adjustable intradermally focused laser platform guided by real-time visual mapping to ensure the precise dose and depth of energy as the user performs treatments. “This is the key for rejuvenation,” he said. “You can go to different depths of the skin. You can be superficial for dyschromia and maybe a little bit different for wrinkles. If you want to treat scars, you go a little bit deeper. Coagulation occurs at these different depths.”
The collimated beam from conventional lasers affects all tissue in its path. The laser beam from the AVAVA product, however, creates a cone-shaped profile of injury in the dermis that minimizes the area of epidermal damage, making it safe in skin of color, according to Dr. Avram. “The beam comes to a focal point in the dermis at the depth that you want it to,” he explained during the meeting, which was sponsored by Harvard Medical School, Massachusetts General Hospital, and the Wellman Center for Photomedicine. “That’s where the energy is going to focus and it bypasses the dermal/epidermal junction, which traditional fractional lasers cannot. What’s interesting about this platform is that you have a wavelength for skin rejuvenation, then you have wavelengths for pigment, which allows you to treat conditions like melasma at different depths.”
The AVAVA high-speed IntelliView imaging system features 10-micron resolution, “so you get exquisite imaging that can help guide your treatments,” he said. It also features image acquisition and storage with artificial intelligence algorithm interrogation and the ability to personalize treatments to the patient’s specific skin type. Commercial availability is expected in the first half of 2023, Dr. Avram said.
In a separate presentation, New York-based cosmetic dermatologist Roy G. Geronemus, MD, who has been involved in clinical trials of AVAVA’s focal point technology, said that patients “feel less pain and have less down time than we saw previously with other nonablative, fractional technologies.”
Downtime involves “just some mild redness,” he said, adding that he is encouraged by early results seen to date, and that “there appears to be some unique capabilities that will be borne out as the clinical studies progress.”
Dr. Avram disclosed that he has received consulting fees from Allergan, Galderma, and Revelle. He is an investigator for Endo and holds ownership and/or shareholder interest in Cytrellis and La Jolla NanoMedical. Dr. Geronemus disclosed having financial relationships with numerous device and pharmaceutical companies.
in all skin types, according to speakers at a virtual course on laser and aesthetic skin therapy.
The products feature “focal point technology,” which pairs 3D laser targeting with an integrated high-resolution imaging system (IntelliView), to help the user guide treatments at selectable depths. They have been cleared by the Food and Drug Administration for use in skin resurfacing procedures, and to treat benign pigmented lesions of the skin, including hyperpigmentation, and were created by Dieter Manstein, MD, PhD, Rox Anderson, MD, and Henry Chan, MD, of the Wellman Center for Photomedicine at Massachusetts General Hospital, and Irina Erenburg, PhD, CEO of AVAVA, the company that markets the products.
dermally focused treatment with Focal Point Technology. The coagulation zone, in dark purple, shows a deep conical lesion that extends 1.3 mm deep with significant epidermal sparing.
At the meeting, Mathew M. Avram, MD, JD, director of the Massachusetts General Hospital Dermatology Laser & Cosmetic Center, described focal point technology as an adjustable intradermally focused laser platform guided by real-time visual mapping to ensure the precise dose and depth of energy as the user performs treatments. “This is the key for rejuvenation,” he said. “You can go to different depths of the skin. You can be superficial for dyschromia and maybe a little bit different for wrinkles. If you want to treat scars, you go a little bit deeper. Coagulation occurs at these different depths.”
The collimated beam from conventional lasers affects all tissue in its path. The laser beam from the AVAVA product, however, creates a cone-shaped profile of injury in the dermis that minimizes the area of epidermal damage, making it safe in skin of color, according to Dr. Avram. “The beam comes to a focal point in the dermis at the depth that you want it to,” he explained during the meeting, which was sponsored by Harvard Medical School, Massachusetts General Hospital, and the Wellman Center for Photomedicine. “That’s where the energy is going to focus and it bypasses the dermal/epidermal junction, which traditional fractional lasers cannot. What’s interesting about this platform is that you have a wavelength for skin rejuvenation, then you have wavelengths for pigment, which allows you to treat conditions like melasma at different depths.”
The AVAVA high-speed IntelliView imaging system features 10-micron resolution, “so you get exquisite imaging that can help guide your treatments,” he said. It also features image acquisition and storage with artificial intelligence algorithm interrogation and the ability to personalize treatments to the patient’s specific skin type. Commercial availability is expected in the first half of 2023, Dr. Avram said.
In a separate presentation, New York-based cosmetic dermatologist Roy G. Geronemus, MD, who has been involved in clinical trials of AVAVA’s focal point technology, said that patients “feel less pain and have less down time than we saw previously with other nonablative, fractional technologies.”
Downtime involves “just some mild redness,” he said, adding that he is encouraged by early results seen to date, and that “there appears to be some unique capabilities that will be borne out as the clinical studies progress.”
Dr. Avram disclosed that he has received consulting fees from Allergan, Galderma, and Revelle. He is an investigator for Endo and holds ownership and/or shareholder interest in Cytrellis and La Jolla NanoMedical. Dr. Geronemus disclosed having financial relationships with numerous device and pharmaceutical companies.
in all skin types, according to speakers at a virtual course on laser and aesthetic skin therapy.
The products feature “focal point technology,” which pairs 3D laser targeting with an integrated high-resolution imaging system (IntelliView), to help the user guide treatments at selectable depths. They have been cleared by the Food and Drug Administration for use in skin resurfacing procedures, and to treat benign pigmented lesions of the skin, including hyperpigmentation, and were created by Dieter Manstein, MD, PhD, Rox Anderson, MD, and Henry Chan, MD, of the Wellman Center for Photomedicine at Massachusetts General Hospital, and Irina Erenburg, PhD, CEO of AVAVA, the company that markets the products.
dermally focused treatment with Focal Point Technology. The coagulation zone, in dark purple, shows a deep conical lesion that extends 1.3 mm deep with significant epidermal sparing.
At the meeting, Mathew M. Avram, MD, JD, director of the Massachusetts General Hospital Dermatology Laser & Cosmetic Center, described focal point technology as an adjustable intradermally focused laser platform guided by real-time visual mapping to ensure the precise dose and depth of energy as the user performs treatments. “This is the key for rejuvenation,” he said. “You can go to different depths of the skin. You can be superficial for dyschromia and maybe a little bit different for wrinkles. If you want to treat scars, you go a little bit deeper. Coagulation occurs at these different depths.”
The collimated beam from conventional lasers affects all tissue in its path. The laser beam from the AVAVA product, however, creates a cone-shaped profile of injury in the dermis that minimizes the area of epidermal damage, making it safe in skin of color, according to Dr. Avram. “The beam comes to a focal point in the dermis at the depth that you want it to,” he explained during the meeting, which was sponsored by Harvard Medical School, Massachusetts General Hospital, and the Wellman Center for Photomedicine. “That’s where the energy is going to focus and it bypasses the dermal/epidermal junction, which traditional fractional lasers cannot. What’s interesting about this platform is that you have a wavelength for skin rejuvenation, then you have wavelengths for pigment, which allows you to treat conditions like melasma at different depths.”
The AVAVA high-speed IntelliView imaging system features 10-micron resolution, “so you get exquisite imaging that can help guide your treatments,” he said. It also features image acquisition and storage with artificial intelligence algorithm interrogation and the ability to personalize treatments to the patient’s specific skin type. Commercial availability is expected in the first half of 2023, Dr. Avram said.
In a separate presentation, New York-based cosmetic dermatologist Roy G. Geronemus, MD, who has been involved in clinical trials of AVAVA’s focal point technology, said that patients “feel less pain and have less down time than we saw previously with other nonablative, fractional technologies.”
Downtime involves “just some mild redness,” he said, adding that he is encouraged by early results seen to date, and that “there appears to be some unique capabilities that will be borne out as the clinical studies progress.”
Dr. Avram disclosed that he has received consulting fees from Allergan, Galderma, and Revelle. He is an investigator for Endo and holds ownership and/or shareholder interest in Cytrellis and La Jolla NanoMedical. Dr. Geronemus disclosed having financial relationships with numerous device and pharmaceutical companies.
FROM A LASER & AESTHETIC SKIN THERAPY COURSE
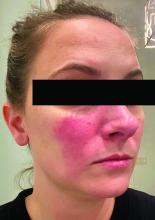
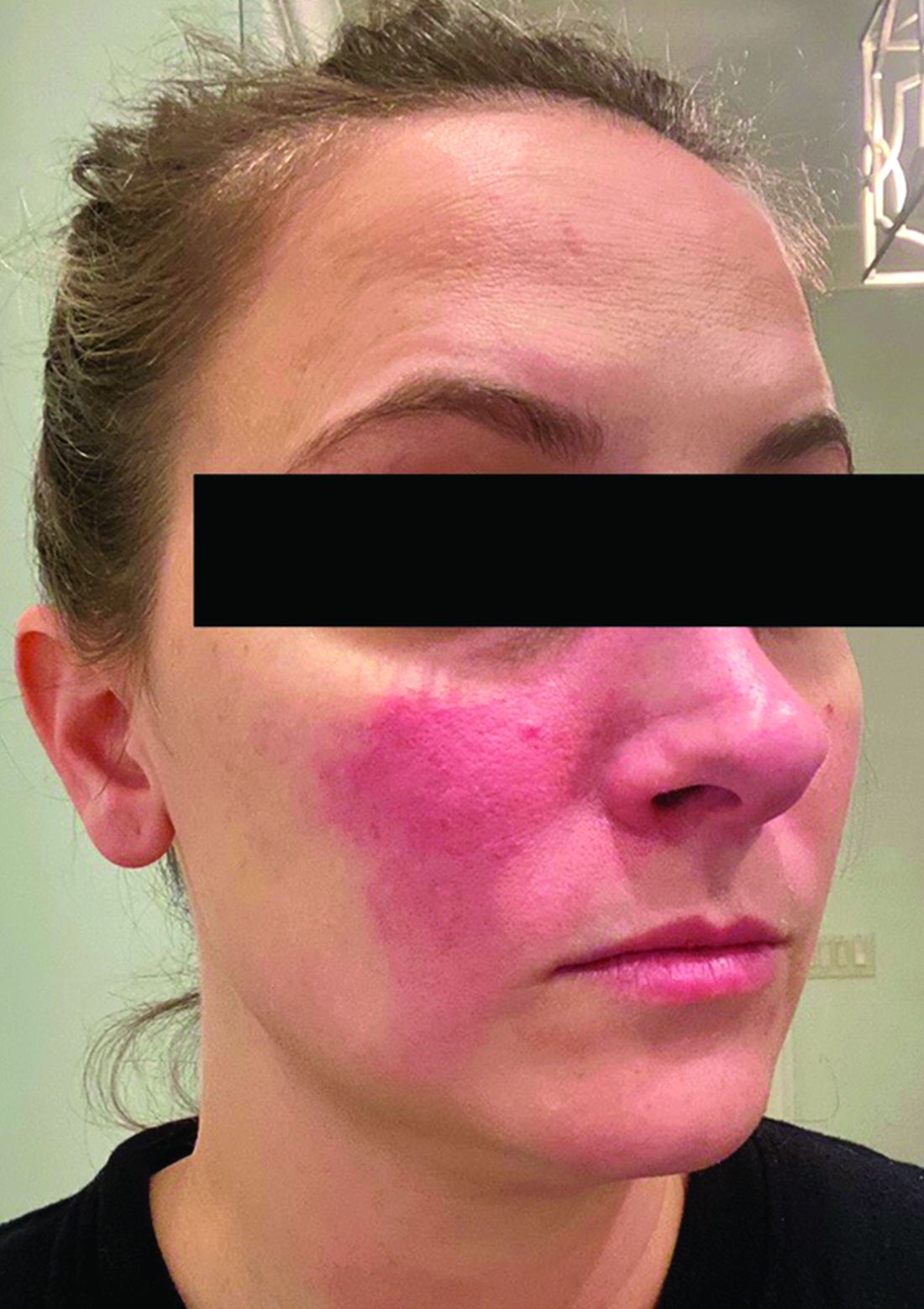
Rosacea and the gut: Looking into SIBO
, according to speakers at the annual Integrative Dermatology Symposium.
“SIBO is definitely something we test for and treat,” Raja Sivamani, MD, said in an interview after the meeting. Dr. Sivamani practices as an integrative dermatologist at the Pacific Skin Institute in Sacramento and is the director of clinical research at the institute’s research unit, Integrative Skin Science and Research. He led a panel discussion on rosacea and acne at the meeting.
Associations between SIBO and several dermatologic conditions, including systemic sclerosis, have been reported, but the strongest evidence to date involves rosacea. “There’s associative epidemiological evidence showing higher rates of SIBO among those with rosacea, and there are prospective studies” showing clearance of rosacea in patients treated for SIBO, said Dr. Sivamani, also adjunct associate professor of clinical dermatology at the University of California, Davis.
Studies are small, but are “well done and well-designed,” he said in the interview. “Do we need more studies? Absolutely. But what we have now is compelling [enough] for us to take a look at it.”
Findings of rosacea clearance
SIBO’s believed contribution to the pathophysiology of rosacea is part of the increasingly described gut microbiome-skin axis. SIBO has been recognized as a medical phenomenon for many decades and has been defined as an excessive bacterial load in the small bowel that causes gastrointestinal symptoms, according to the 2020 American College of Gastroenterology clinical guideline on SIBO.
Symptoms commonly associated with SIBO overlap with the cardinal symptoms of irritable bowel syndrome (IBS): abdominal pain; diarrhea, constipation, or both; bloating; and flatulence. SIBO can be diagnosed with several validated carbohydrate substrate (glucose or lactulose)–based breath tests that measure hydrogen and/or methane.
Hydrogen-positive breath tests suggest bacterial overgrowth, and methane-positive breath tests suggest small intestinal methanogen overgrowth. Methane is increasingly important and recognized, the AGA guideline says, though it creates a “nomenclature problem in the SIBO framework” because methanogens are not bacteria, the authors note.
In conventional practice, SIBO is typically treated with antibiotics such as rifaximin, and often with short-term dietary modification as well. Integrative medicine typically considers the use of supplements and botanicals in addition to or instead of antibiotics, as well as dietary change and increasingly, a close look at SIBO risk factors to prevent recurrence, Dr. Sivamani said. (His research unit is currently studying the use of herbal protocols as an alternative to antibiotics in patients with SIBO and dermatologic conditions.)
During a presentation on rosacea at the meeting, Neal Bhatia, MD, director of clinical dermatology at Therapeutics Clinical Research, a dermatology treatment and research center in San Diego, said that currently available breath tests for SIBO “are very interesting tools for understanding what may be happening in the gut” and that the “rifaximin data are good.”
He referred to a study reported in the Journal of the American Academy of Dermatology showing that patients with rosacea were significantly more likely to have SIBO (41.7% of 48 patients vs. 5.0% of 40 controls; P < .001), and that 64.5% of rosacea patients who completed treatment with rifaximin had remission of rosacea at a 3-year follow-up.
An earlier crossover study is also notable, he said. This study enrolled 113 consecutive patients with rosacea and 60 age- and sex-matched controls, and randomized those with SIBO (52 of the 113 with rosacea vs. 3 of the 60 controls) to rifaximin or placebo. Rosacea cleared in 20 of the 28 patients in the rifaximin group and greatly improved in 6 of the 28. Of 20 patients in the placebo group, rosacea remained unchanged in 18 and worsened in 2. When patients in the placebo group were switched to rifaximin, SIBO was eradicated in 17 of the 20, and rosacea completely resolved in 15 of those patients, Dr. Bhatia said.
In his view, it will take more time, greater awareness of the rosacea-SIBO link, and a willingness “to take chances” for more dermatologists to consider SIBO during rosacea care. “Breath tests are not something used in the [typical dermatology] clinic right now, but they may make their way in,” he said at the meeting.
In a follow-up interview, Dr. Bhatia emphasized that “it’s really a question of uptake, which always takes a while” and of willingness to “think through the disease from another angle ... especially in patients who are recalcitrant.”
Treatment
Dr. Sivamani said in the interview that a third type of SIBO – hydrogen sulfide–dominant SIBO – is now documented and worth considering when glucose and lactulose breath tests are negative in patients with rosacea who have gastrointestinal symptoms.
The use of breath tests to objectively diagnose SIBO is always best, Dr. Sivamani said, but he will consider empiric therapy in some patients. “I always tell patients [about] the benefits of testing, but if they can’t get the test covered or are unable to pay for the test, and they have symptoms consistent with SIBO, I’m okay doing a trial with therapy,” he said.
Rifaximin, one of the suggested antibiotics listed in the AGA guideline, is a nonabsorbable antibiotic that is FDA-approved for IBS with diarrhea (IBS-D); it has been shown to not negatively affect the growth of beneficial bacteria in the colon.
However, herbals are also an attractive option – alone or in combination with rifaximin or other antibiotics – speakers at the meeting said. In a multicenter retrospective chart review led by investigators at the Johns Hopkins Hospital, herbal therapies were at least as effective as rifaximin for treating SIBO, with similar safety profiles. The response rate for normalizing breath hydrogen testing in patients with SIBO was 46% for herbal therapies and 34% for rifaximin.
Dietary change is also part of treatment, with the reduction of fermentable carbohydrates – often through the Low FODMAP Diet and Specific Carbohydrate Diet – being the dominant theme in dietary intervention for SIBO, according to the AGA guideline.
“There are definitely some food choices you can shift,” said Dr. Sivamani. “I’ll work with patients on FODMAP, though it’s hard to sustain over the long-term and can induce psychological issues. You have to provide other options.”
Dr. Sivamani works with patients on using “a restrictive diet for a short amount of time, with the gradual reintroduction of foods to see [what] foods are and aren’t [causing] flares.” He also works to identify and eliminate risk factors and predisposing factors for SIBO so that recurrence will be less likely.
“SIBO is definitely an entity that is not on the fringes anymore ... it adds to inflammation in the body ... and if you have an inflamed gut, there’s a domino effect that will lead to inflammation elsewhere,” Dr. Sivamani said.
“You want to know, do your patients have SIBO? What subset do they have? Do they have risk factors you can eliminate?” he said. “And then what therapies will you use – pharmaceuticals, supplements and botanicals, or a combination? And finally, what will you do with diet?”
Dr. Bhatia disclosed he has affiliations with Abbvie, Almirall, Arcutis, Arena, Biofrontera, BMS, BI, Brickell, Dermavant, EPI Health, Ferndale, Galderma, Genentech, InCyte, ISDIN, Johnson & Johnson, LaRoche-Posay, Leo, Lilly, Novartis, Ortho, Pfizer, Proctor & Gamble, Regeneron, Sanofi, Stemline, SunPharma, and Verrica. Dr. Sivamani did not provide a disclosure statement.
, according to speakers at the annual Integrative Dermatology Symposium.
“SIBO is definitely something we test for and treat,” Raja Sivamani, MD, said in an interview after the meeting. Dr. Sivamani practices as an integrative dermatologist at the Pacific Skin Institute in Sacramento and is the director of clinical research at the institute’s research unit, Integrative Skin Science and Research. He led a panel discussion on rosacea and acne at the meeting.
Associations between SIBO and several dermatologic conditions, including systemic sclerosis, have been reported, but the strongest evidence to date involves rosacea. “There’s associative epidemiological evidence showing higher rates of SIBO among those with rosacea, and there are prospective studies” showing clearance of rosacea in patients treated for SIBO, said Dr. Sivamani, also adjunct associate professor of clinical dermatology at the University of California, Davis.
Studies are small, but are “well done and well-designed,” he said in the interview. “Do we need more studies? Absolutely. But what we have now is compelling [enough] for us to take a look at it.”
Findings of rosacea clearance
SIBO’s believed contribution to the pathophysiology of rosacea is part of the increasingly described gut microbiome-skin axis. SIBO has been recognized as a medical phenomenon for many decades and has been defined as an excessive bacterial load in the small bowel that causes gastrointestinal symptoms, according to the 2020 American College of Gastroenterology clinical guideline on SIBO.
Symptoms commonly associated with SIBO overlap with the cardinal symptoms of irritable bowel syndrome (IBS): abdominal pain; diarrhea, constipation, or both; bloating; and flatulence. SIBO can be diagnosed with several validated carbohydrate substrate (glucose or lactulose)–based breath tests that measure hydrogen and/or methane.
Hydrogen-positive breath tests suggest bacterial overgrowth, and methane-positive breath tests suggest small intestinal methanogen overgrowth. Methane is increasingly important and recognized, the AGA guideline says, though it creates a “nomenclature problem in the SIBO framework” because methanogens are not bacteria, the authors note.
In conventional practice, SIBO is typically treated with antibiotics such as rifaximin, and often with short-term dietary modification as well. Integrative medicine typically considers the use of supplements and botanicals in addition to or instead of antibiotics, as well as dietary change and increasingly, a close look at SIBO risk factors to prevent recurrence, Dr. Sivamani said. (His research unit is currently studying the use of herbal protocols as an alternative to antibiotics in patients with SIBO and dermatologic conditions.)
During a presentation on rosacea at the meeting, Neal Bhatia, MD, director of clinical dermatology at Therapeutics Clinical Research, a dermatology treatment and research center in San Diego, said that currently available breath tests for SIBO “are very interesting tools for understanding what may be happening in the gut” and that the “rifaximin data are good.”
He referred to a study reported in the Journal of the American Academy of Dermatology showing that patients with rosacea were significantly more likely to have SIBO (41.7% of 48 patients vs. 5.0% of 40 controls; P < .001), and that 64.5% of rosacea patients who completed treatment with rifaximin had remission of rosacea at a 3-year follow-up.
An earlier crossover study is also notable, he said. This study enrolled 113 consecutive patients with rosacea and 60 age- and sex-matched controls, and randomized those with SIBO (52 of the 113 with rosacea vs. 3 of the 60 controls) to rifaximin or placebo. Rosacea cleared in 20 of the 28 patients in the rifaximin group and greatly improved in 6 of the 28. Of 20 patients in the placebo group, rosacea remained unchanged in 18 and worsened in 2. When patients in the placebo group were switched to rifaximin, SIBO was eradicated in 17 of the 20, and rosacea completely resolved in 15 of those patients, Dr. Bhatia said.
In his view, it will take more time, greater awareness of the rosacea-SIBO link, and a willingness “to take chances” for more dermatologists to consider SIBO during rosacea care. “Breath tests are not something used in the [typical dermatology] clinic right now, but they may make their way in,” he said at the meeting.
In a follow-up interview, Dr. Bhatia emphasized that “it’s really a question of uptake, which always takes a while” and of willingness to “think through the disease from another angle ... especially in patients who are recalcitrant.”
Treatment
Dr. Sivamani said in the interview that a third type of SIBO – hydrogen sulfide–dominant SIBO – is now documented and worth considering when glucose and lactulose breath tests are negative in patients with rosacea who have gastrointestinal symptoms.
The use of breath tests to objectively diagnose SIBO is always best, Dr. Sivamani said, but he will consider empiric therapy in some patients. “I always tell patients [about] the benefits of testing, but if they can’t get the test covered or are unable to pay for the test, and they have symptoms consistent with SIBO, I’m okay doing a trial with therapy,” he said.
Rifaximin, one of the suggested antibiotics listed in the AGA guideline, is a nonabsorbable antibiotic that is FDA-approved for IBS with diarrhea (IBS-D); it has been shown to not negatively affect the growth of beneficial bacteria in the colon.
However, herbals are also an attractive option – alone or in combination with rifaximin or other antibiotics – speakers at the meeting said. In a multicenter retrospective chart review led by investigators at the Johns Hopkins Hospital, herbal therapies were at least as effective as rifaximin for treating SIBO, with similar safety profiles. The response rate for normalizing breath hydrogen testing in patients with SIBO was 46% for herbal therapies and 34% for rifaximin.
Dietary change is also part of treatment, with the reduction of fermentable carbohydrates – often through the Low FODMAP Diet and Specific Carbohydrate Diet – being the dominant theme in dietary intervention for SIBO, according to the AGA guideline.
“There are definitely some food choices you can shift,” said Dr. Sivamani. “I’ll work with patients on FODMAP, though it’s hard to sustain over the long-term and can induce psychological issues. You have to provide other options.”
Dr. Sivamani works with patients on using “a restrictive diet for a short amount of time, with the gradual reintroduction of foods to see [what] foods are and aren’t [causing] flares.” He also works to identify and eliminate risk factors and predisposing factors for SIBO so that recurrence will be less likely.
“SIBO is definitely an entity that is not on the fringes anymore ... it adds to inflammation in the body ... and if you have an inflamed gut, there’s a domino effect that will lead to inflammation elsewhere,” Dr. Sivamani said.
“You want to know, do your patients have SIBO? What subset do they have? Do they have risk factors you can eliminate?” he said. “And then what therapies will you use – pharmaceuticals, supplements and botanicals, or a combination? And finally, what will you do with diet?”
Dr. Bhatia disclosed he has affiliations with Abbvie, Almirall, Arcutis, Arena, Biofrontera, BMS, BI, Brickell, Dermavant, EPI Health, Ferndale, Galderma, Genentech, InCyte, ISDIN, Johnson & Johnson, LaRoche-Posay, Leo, Lilly, Novartis, Ortho, Pfizer, Proctor & Gamble, Regeneron, Sanofi, Stemline, SunPharma, and Verrica. Dr. Sivamani did not provide a disclosure statement.
, according to speakers at the annual Integrative Dermatology Symposium.
“SIBO is definitely something we test for and treat,” Raja Sivamani, MD, said in an interview after the meeting. Dr. Sivamani practices as an integrative dermatologist at the Pacific Skin Institute in Sacramento and is the director of clinical research at the institute’s research unit, Integrative Skin Science and Research. He led a panel discussion on rosacea and acne at the meeting.
Associations between SIBO and several dermatologic conditions, including systemic sclerosis, have been reported, but the strongest evidence to date involves rosacea. “There’s associative epidemiological evidence showing higher rates of SIBO among those with rosacea, and there are prospective studies” showing clearance of rosacea in patients treated for SIBO, said Dr. Sivamani, also adjunct associate professor of clinical dermatology at the University of California, Davis.
Studies are small, but are “well done and well-designed,” he said in the interview. “Do we need more studies? Absolutely. But what we have now is compelling [enough] for us to take a look at it.”
Findings of rosacea clearance
SIBO’s believed contribution to the pathophysiology of rosacea is part of the increasingly described gut microbiome-skin axis. SIBO has been recognized as a medical phenomenon for many decades and has been defined as an excessive bacterial load in the small bowel that causes gastrointestinal symptoms, according to the 2020 American College of Gastroenterology clinical guideline on SIBO.
Symptoms commonly associated with SIBO overlap with the cardinal symptoms of irritable bowel syndrome (IBS): abdominal pain; diarrhea, constipation, or both; bloating; and flatulence. SIBO can be diagnosed with several validated carbohydrate substrate (glucose or lactulose)–based breath tests that measure hydrogen and/or methane.
Hydrogen-positive breath tests suggest bacterial overgrowth, and methane-positive breath tests suggest small intestinal methanogen overgrowth. Methane is increasingly important and recognized, the AGA guideline says, though it creates a “nomenclature problem in the SIBO framework” because methanogens are not bacteria, the authors note.
In conventional practice, SIBO is typically treated with antibiotics such as rifaximin, and often with short-term dietary modification as well. Integrative medicine typically considers the use of supplements and botanicals in addition to or instead of antibiotics, as well as dietary change and increasingly, a close look at SIBO risk factors to prevent recurrence, Dr. Sivamani said. (His research unit is currently studying the use of herbal protocols as an alternative to antibiotics in patients with SIBO and dermatologic conditions.)
During a presentation on rosacea at the meeting, Neal Bhatia, MD, director of clinical dermatology at Therapeutics Clinical Research, a dermatology treatment and research center in San Diego, said that currently available breath tests for SIBO “are very interesting tools for understanding what may be happening in the gut” and that the “rifaximin data are good.”
He referred to a study reported in the Journal of the American Academy of Dermatology showing that patients with rosacea were significantly more likely to have SIBO (41.7% of 48 patients vs. 5.0% of 40 controls; P < .001), and that 64.5% of rosacea patients who completed treatment with rifaximin had remission of rosacea at a 3-year follow-up.
An earlier crossover study is also notable, he said. This study enrolled 113 consecutive patients with rosacea and 60 age- and sex-matched controls, and randomized those with SIBO (52 of the 113 with rosacea vs. 3 of the 60 controls) to rifaximin or placebo. Rosacea cleared in 20 of the 28 patients in the rifaximin group and greatly improved in 6 of the 28. Of 20 patients in the placebo group, rosacea remained unchanged in 18 and worsened in 2. When patients in the placebo group were switched to rifaximin, SIBO was eradicated in 17 of the 20, and rosacea completely resolved in 15 of those patients, Dr. Bhatia said.
In his view, it will take more time, greater awareness of the rosacea-SIBO link, and a willingness “to take chances” for more dermatologists to consider SIBO during rosacea care. “Breath tests are not something used in the [typical dermatology] clinic right now, but they may make their way in,” he said at the meeting.
In a follow-up interview, Dr. Bhatia emphasized that “it’s really a question of uptake, which always takes a while” and of willingness to “think through the disease from another angle ... especially in patients who are recalcitrant.”
Treatment
Dr. Sivamani said in the interview that a third type of SIBO – hydrogen sulfide–dominant SIBO – is now documented and worth considering when glucose and lactulose breath tests are negative in patients with rosacea who have gastrointestinal symptoms.
The use of breath tests to objectively diagnose SIBO is always best, Dr. Sivamani said, but he will consider empiric therapy in some patients. “I always tell patients [about] the benefits of testing, but if they can’t get the test covered or are unable to pay for the test, and they have symptoms consistent with SIBO, I’m okay doing a trial with therapy,” he said.
Rifaximin, one of the suggested antibiotics listed in the AGA guideline, is a nonabsorbable antibiotic that is FDA-approved for IBS with diarrhea (IBS-D); it has been shown to not negatively affect the growth of beneficial bacteria in the colon.
However, herbals are also an attractive option – alone or in combination with rifaximin or other antibiotics – speakers at the meeting said. In a multicenter retrospective chart review led by investigators at the Johns Hopkins Hospital, herbal therapies were at least as effective as rifaximin for treating SIBO, with similar safety profiles. The response rate for normalizing breath hydrogen testing in patients with SIBO was 46% for herbal therapies and 34% for rifaximin.
Dietary change is also part of treatment, with the reduction of fermentable carbohydrates – often through the Low FODMAP Diet and Specific Carbohydrate Diet – being the dominant theme in dietary intervention for SIBO, according to the AGA guideline.
“There are definitely some food choices you can shift,” said Dr. Sivamani. “I’ll work with patients on FODMAP, though it’s hard to sustain over the long-term and can induce psychological issues. You have to provide other options.”
Dr. Sivamani works with patients on using “a restrictive diet for a short amount of time, with the gradual reintroduction of foods to see [what] foods are and aren’t [causing] flares.” He also works to identify and eliminate risk factors and predisposing factors for SIBO so that recurrence will be less likely.
“SIBO is definitely an entity that is not on the fringes anymore ... it adds to inflammation in the body ... and if you have an inflamed gut, there’s a domino effect that will lead to inflammation elsewhere,” Dr. Sivamani said.
“You want to know, do your patients have SIBO? What subset do they have? Do they have risk factors you can eliminate?” he said. “And then what therapies will you use – pharmaceuticals, supplements and botanicals, or a combination? And finally, what will you do with diet?”
Dr. Bhatia disclosed he has affiliations with Abbvie, Almirall, Arcutis, Arena, Biofrontera, BMS, BI, Brickell, Dermavant, EPI Health, Ferndale, Galderma, Genentech, InCyte, ISDIN, Johnson & Johnson, LaRoche-Posay, Leo, Lilly, Novartis, Ortho, Pfizer, Proctor & Gamble, Regeneron, Sanofi, Stemline, SunPharma, and Verrica. Dr. Sivamani did not provide a disclosure statement.
REPORTING FROM IDS 2022
Applications for laser-assisted drug delivery on the horizon, expert says
For those who view fractional ablative laser–assisted drug delivery as a pie-in-the-sky procedure that will take years to work its way into routine clinical practice, think again.
According to Merete Haedersdal, MD, PhD, DMSc, .
“The groundwork has been established over a decade with more than 100 publications available on PubMed,” Dr. Haedersdal, professor of dermatology at the University of Copenhagen, said during a virtual course on laser and aesthetic skin therapy. “There is no doubt that by drilling tiny little holes or channels with ablative fractional lasers, we enhance drug delivery to the skin, and we also empower different topical treatment regimens. Also, laser-assisted drug delivery holds the potential to bring new innovations into established medicine.”
Many studies have demonstrated that clinicians can enhance drug uptake into the skin with the fractional 10,600 nm CO2 laser, the fractional 2,940 nm erbium:YAG laser, and the 1,927 nm thulium laser, but proper tuning of the devices is key. The lower the density, the better, Dr. Haedersdal said.
“Typically, we use 5% density or 5% coverage, sometimes 10%-15%, but don’t go higher in order to avoid the risk of having a systemic uptake,” she said during the meeting, which was sponsored by Harvard Medical School, Massachusetts General Hospital, and the Wellman Center for Photomedicine. “Also, the pulse energy for channel depth needs to be tailored to the specific dermatologic disease being treated,” she said, noting that for melasma, for example, “very low pulse energies” would be used, but they would be higher for treating thicker lesions, such as a hypertrophic scar.
Treatment with ablative fractional lasers enhances drug accumulation in the skin of any drug or substance applied to the skin, and clinical indications are expanding rapidly. Established indications include combining ablative fractional lasers and photodynamic therapy (PDT) for AKs and combining ablative fractional lasers and triamcinolone or 5-FU for scars. “Although we have a good body of evidence, particularly for AKs, it’s still an off-label use,” she emphasized.
Evolving indications include concomitant use of ablative fractional laser and vitamins and cosmeceuticals for rejuvenation; lidocaine for local anesthetics; tranexamic acid and hydroquinone for melasma; antifungals for onychomycosis; Botox for hyperhidrosis; minoxidil for alopecia; and betamethasone for vitiligo. A promising treatment for skin cancer “on the horizon,” she said, is the “combination of ablative fractional laser with PD1 inhibitors and chemotherapy.”
Data on AKs
Evidence supporting laser-assisted drug delivery for AKs comes from more than 10 randomized, controlled trials in the dermatology literature involving 400-plus immunocompetent and immunosuppressed patients. These trials have found ablative fractional laser–assisted PDT to be significantly more efficacious than PDT alone up to 12 months postoperatively and to foster lower rates of AK recurrence.
In a meta-analysis and systematic review, German researchers concluded that PDT combined with ablative laser treatment for AKs is more efficient but not more painful than either therapy alone. They recommended the combined regimen for patients with severe photodamage, field cancerization, and multiple AKs.
In 2020, an international consensus panel of experts, including Dr. Haedersdal, published recommendations regarding laser treatment of traumatic scars and contractures. The panel members determined that laser-assisted delivery of corticosteroids and antimetabolites was recommended for hypertrophic scars and cited triamcinolone acetonide suspension (TAC) as the most common corticosteroid used in combination with ablative fractional lasers. “It can be applied in concentrations of 40 mg/mL or less depending on the degree of hypertrophy,” they wrote.
In addition, they stated that 5-FU solution is “most commonly applied in a concentration of 50 mg/mL alone, or mixed with TAC in ratios of 9:1 or 3:1.”
According to the best available evidence, the clinical approach for hypertrophic scars supports combination treatment with ablative fractional laser and triamcinolone acetonide either alone or in combination with 5-FU. For atrophic scars, laser-assisted delivery of poly-L-lactic acid has been shown to be efficient. “Both of these treatments improve texture and thickness but also dyschromia and scar functionality,” said Dr. Haedersdal, who is also a visiting scientist at the Wellman Center for Photomedicine, Boston.
Commenting on patient safety with laser-assisted drug delivery, “the combination of lasers and topicals can be a powerful cocktail,” she said. “You can expect intensified local skin reactions. When treating larger areas, consider the risk of systemic absorption and the risk of potential toxicity. There is also the potential for infection with pathogens such as Staphylococcus aureus. The take-home message here is that you should only use the type and amount of drug no higher than administered during intradermal injection.”
Dr. Haedersdal disclosed that she has received equipment from Cherry Imaging, Cynosure-Hologic, MiraDry, and PerfAction Technologies. She has also received research grants from Leo Pharma, Lutronic, Mirai Medical, Novoxel, and Venus Concept.
For those who view fractional ablative laser–assisted drug delivery as a pie-in-the-sky procedure that will take years to work its way into routine clinical practice, think again.
According to Merete Haedersdal, MD, PhD, DMSc, .
“The groundwork has been established over a decade with more than 100 publications available on PubMed,” Dr. Haedersdal, professor of dermatology at the University of Copenhagen, said during a virtual course on laser and aesthetic skin therapy. “There is no doubt that by drilling tiny little holes or channels with ablative fractional lasers, we enhance drug delivery to the skin, and we also empower different topical treatment regimens. Also, laser-assisted drug delivery holds the potential to bring new innovations into established medicine.”
Many studies have demonstrated that clinicians can enhance drug uptake into the skin with the fractional 10,600 nm CO2 laser, the fractional 2,940 nm erbium:YAG laser, and the 1,927 nm thulium laser, but proper tuning of the devices is key. The lower the density, the better, Dr. Haedersdal said.
“Typically, we use 5% density or 5% coverage, sometimes 10%-15%, but don’t go higher in order to avoid the risk of having a systemic uptake,” she said during the meeting, which was sponsored by Harvard Medical School, Massachusetts General Hospital, and the Wellman Center for Photomedicine. “Also, the pulse energy for channel depth needs to be tailored to the specific dermatologic disease being treated,” she said, noting that for melasma, for example, “very low pulse energies” would be used, but they would be higher for treating thicker lesions, such as a hypertrophic scar.
Treatment with ablative fractional lasers enhances drug accumulation in the skin of any drug or substance applied to the skin, and clinical indications are expanding rapidly. Established indications include combining ablative fractional lasers and photodynamic therapy (PDT) for AKs and combining ablative fractional lasers and triamcinolone or 5-FU for scars. “Although we have a good body of evidence, particularly for AKs, it’s still an off-label use,” she emphasized.
Evolving indications include concomitant use of ablative fractional laser and vitamins and cosmeceuticals for rejuvenation; lidocaine for local anesthetics; tranexamic acid and hydroquinone for melasma; antifungals for onychomycosis; Botox for hyperhidrosis; minoxidil for alopecia; and betamethasone for vitiligo. A promising treatment for skin cancer “on the horizon,” she said, is the “combination of ablative fractional laser with PD1 inhibitors and chemotherapy.”
Data on AKs
Evidence supporting laser-assisted drug delivery for AKs comes from more than 10 randomized, controlled trials in the dermatology literature involving 400-plus immunocompetent and immunosuppressed patients. These trials have found ablative fractional laser–assisted PDT to be significantly more efficacious than PDT alone up to 12 months postoperatively and to foster lower rates of AK recurrence.
In a meta-analysis and systematic review, German researchers concluded that PDT combined with ablative laser treatment for AKs is more efficient but not more painful than either therapy alone. They recommended the combined regimen for patients with severe photodamage, field cancerization, and multiple AKs.
In 2020, an international consensus panel of experts, including Dr. Haedersdal, published recommendations regarding laser treatment of traumatic scars and contractures. The panel members determined that laser-assisted delivery of corticosteroids and antimetabolites was recommended for hypertrophic scars and cited triamcinolone acetonide suspension (TAC) as the most common corticosteroid used in combination with ablative fractional lasers. “It can be applied in concentrations of 40 mg/mL or less depending on the degree of hypertrophy,” they wrote.
In addition, they stated that 5-FU solution is “most commonly applied in a concentration of 50 mg/mL alone, or mixed with TAC in ratios of 9:1 or 3:1.”
According to the best available evidence, the clinical approach for hypertrophic scars supports combination treatment with ablative fractional laser and triamcinolone acetonide either alone or in combination with 5-FU. For atrophic scars, laser-assisted delivery of poly-L-lactic acid has been shown to be efficient. “Both of these treatments improve texture and thickness but also dyschromia and scar functionality,” said Dr. Haedersdal, who is also a visiting scientist at the Wellman Center for Photomedicine, Boston.
Commenting on patient safety with laser-assisted drug delivery, “the combination of lasers and topicals can be a powerful cocktail,” she said. “You can expect intensified local skin reactions. When treating larger areas, consider the risk of systemic absorption and the risk of potential toxicity. There is also the potential for infection with pathogens such as Staphylococcus aureus. The take-home message here is that you should only use the type and amount of drug no higher than administered during intradermal injection.”
Dr. Haedersdal disclosed that she has received equipment from Cherry Imaging, Cynosure-Hologic, MiraDry, and PerfAction Technologies. She has also received research grants from Leo Pharma, Lutronic, Mirai Medical, Novoxel, and Venus Concept.
For those who view fractional ablative laser–assisted drug delivery as a pie-in-the-sky procedure that will take years to work its way into routine clinical practice, think again.
According to Merete Haedersdal, MD, PhD, DMSc, .
“The groundwork has been established over a decade with more than 100 publications available on PubMed,” Dr. Haedersdal, professor of dermatology at the University of Copenhagen, said during a virtual course on laser and aesthetic skin therapy. “There is no doubt that by drilling tiny little holes or channels with ablative fractional lasers, we enhance drug delivery to the skin, and we also empower different topical treatment regimens. Also, laser-assisted drug delivery holds the potential to bring new innovations into established medicine.”
Many studies have demonstrated that clinicians can enhance drug uptake into the skin with the fractional 10,600 nm CO2 laser, the fractional 2,940 nm erbium:YAG laser, and the 1,927 nm thulium laser, but proper tuning of the devices is key. The lower the density, the better, Dr. Haedersdal said.
“Typically, we use 5% density or 5% coverage, sometimes 10%-15%, but don’t go higher in order to avoid the risk of having a systemic uptake,” she said during the meeting, which was sponsored by Harvard Medical School, Massachusetts General Hospital, and the Wellman Center for Photomedicine. “Also, the pulse energy for channel depth needs to be tailored to the specific dermatologic disease being treated,” she said, noting that for melasma, for example, “very low pulse energies” would be used, but they would be higher for treating thicker lesions, such as a hypertrophic scar.
Treatment with ablative fractional lasers enhances drug accumulation in the skin of any drug or substance applied to the skin, and clinical indications are expanding rapidly. Established indications include combining ablative fractional lasers and photodynamic therapy (PDT) for AKs and combining ablative fractional lasers and triamcinolone or 5-FU for scars. “Although we have a good body of evidence, particularly for AKs, it’s still an off-label use,” she emphasized.
Evolving indications include concomitant use of ablative fractional laser and vitamins and cosmeceuticals for rejuvenation; lidocaine for local anesthetics; tranexamic acid and hydroquinone for melasma; antifungals for onychomycosis; Botox for hyperhidrosis; minoxidil for alopecia; and betamethasone for vitiligo. A promising treatment for skin cancer “on the horizon,” she said, is the “combination of ablative fractional laser with PD1 inhibitors and chemotherapy.”
Data on AKs
Evidence supporting laser-assisted drug delivery for AKs comes from more than 10 randomized, controlled trials in the dermatology literature involving 400-plus immunocompetent and immunosuppressed patients. These trials have found ablative fractional laser–assisted PDT to be significantly more efficacious than PDT alone up to 12 months postoperatively and to foster lower rates of AK recurrence.
In a meta-analysis and systematic review, German researchers concluded that PDT combined with ablative laser treatment for AKs is more efficient but not more painful than either therapy alone. They recommended the combined regimen for patients with severe photodamage, field cancerization, and multiple AKs.
In 2020, an international consensus panel of experts, including Dr. Haedersdal, published recommendations regarding laser treatment of traumatic scars and contractures. The panel members determined that laser-assisted delivery of corticosteroids and antimetabolites was recommended for hypertrophic scars and cited triamcinolone acetonide suspension (TAC) as the most common corticosteroid used in combination with ablative fractional lasers. “It can be applied in concentrations of 40 mg/mL or less depending on the degree of hypertrophy,” they wrote.
In addition, they stated that 5-FU solution is “most commonly applied in a concentration of 50 mg/mL alone, or mixed with TAC in ratios of 9:1 or 3:1.”
According to the best available evidence, the clinical approach for hypertrophic scars supports combination treatment with ablative fractional laser and triamcinolone acetonide either alone or in combination with 5-FU. For atrophic scars, laser-assisted delivery of poly-L-lactic acid has been shown to be efficient. “Both of these treatments improve texture and thickness but also dyschromia and scar functionality,” said Dr. Haedersdal, who is also a visiting scientist at the Wellman Center for Photomedicine, Boston.
Commenting on patient safety with laser-assisted drug delivery, “the combination of lasers and topicals can be a powerful cocktail,” she said. “You can expect intensified local skin reactions. When treating larger areas, consider the risk of systemic absorption and the risk of potential toxicity. There is also the potential for infection with pathogens such as Staphylococcus aureus. The take-home message here is that you should only use the type and amount of drug no higher than administered during intradermal injection.”
Dr. Haedersdal disclosed that she has received equipment from Cherry Imaging, Cynosure-Hologic, MiraDry, and PerfAction Technologies. She has also received research grants from Leo Pharma, Lutronic, Mirai Medical, Novoxel, and Venus Concept.
FROM A LASER & AESTHETIC SKIN THERAPY COURSE
CRT boosts heart failure survival in extended follow-up
CHICAGO – Extended follow-up of patients with heart failure enrolled in the RAFT trial strengthens the case for starting treatment early with a cardiac resynchronization therapy plus defibrillation (CRT-D) device in appropriate patients.
RAFT, which compared CRT-D with treatment with an implantable cardioverter defibrillator (ICD) alone, showed that the early survival benefit produced by CRT-D during an average 40-month follow-up in the original trial persisted during an additional mean follow-up of about 5 years. This result strengthens the case for starting treatment early with a CRT-D device in appropriate patients with heart failure.
During extended follow-up of more than half of the enrolled patients, out to an average of 7.6 years overall and to an average of 12.9 years among survivors, patients who received a CRT-D device had a significant 21% relative reduction in their rate of all-cause mortality compared with randomized patients who received an ICD and no cardiac resynchronization, John L. Sapp, MD, reported at the American Heart Association scientific sessions.
The primary results of RAFT were first reported in 2010.
This magnitude of a survival benefit among the patients originally randomized to CRT is “dramatic,” given that many of the comparator patients who initially received no CRT likely crossed over to receive a CRT-D device once the initial, randomized 4 years of the study finished, commented Lynne W. Stevenson, MD, director of cardiomyopathy and the Lisa M. Jacobson Professor of Cardiology at Vanderbilt University Medical Center in Nashville, Tenn., who was not involved with the study.
‘CRT can remap heart failure trajectory’
The new findings “strengthen our conviction that CRT can remap the trajectory” of selected patients with heart failure, and that “candidates for CRT should be vigorously identified,” Dr. Stevenson said in an interview.
She also noted that the benefit with extended follow-up was “strikingly parallel” to that seen at 12 years after the addition of an ACE inhibitor for mild heart failure during the 4 years of the landmark SOLVD trial. The new RAFT extended follow-up, as well as the 12-year follow-up of the SOLVD trial, “support the concept that longer follow-up reveals vital information not provided by the relatively short randomized trial period,” she said.
“The new data say ‘don’t delay starting CRT in appropriate patients with heart failure,’ and ‘don’t think of CRT as just a treatment that makes patients feel better.’
“The totality of these data shows that CRT also treats the underlying heart muscle weakness, which helps patients live longer. Previous data showed that patients with left bundle branch block eligible for CRT are unlikely to respond well to the usual, recommended heart medications so it is important to start treatment with CRT-D early,” declared Dr. Stevenson, who cochaired the session where Dr. Sapp gave his report.
RAFT randomized 1,798 patients with New York Heart Association (NYHA) class II or III heart failure, a left ventricular ejection fraction of 30% or less, and an intrinsic QRS duration of at least 120 msec to receive either a CRT-D or ICD device. The study’s primary endpoint was death from any cause or hospitalization for heart failure. After an average 40 months of randomized follow-up, the primary endpoint occurred in 40% of the patients with an ICD and in 33% of those with a CRT-D device, a significant 25% relative reduction linked with CRT-D use. Both endpoint components contributed to the combined result significantly and to about the same extent, and the incremental benefit from CRT-D was significant for patients with NYHA class II heart failure as well as for those with class III.
However, prespecified subgroup analyses showed that the incremental benefit from CRT-D was significantly limited to patients with an intrinsic QRS duration of at least 150 msec, while in those with a duration of 120-149 msec CRT-D had a neutral effect compared with ICD. The same pattern also appeared when the analysis split patients into those with a left bundle branch block, who significantly benefited from CRT-D, but the initial benefit was not apparent in patients with right bundle branch block.
A study subgroup with extended follow-up
The new, extended follow-up analysis presented by Dr. Sapp included 1,050 of the original 1,798 patients (58%) enrolled at any of eight participating Canadian centers that each enrolled at least 100 patients and followed them through the end of 2021 (the full study cohort came from 34 centers, including 10 centers outside Canada). This subgroup included 520 patients randomized to receive CRT-D and 530 who received an ICD. Although this was a post hoc subgroup analysis, the CRT-D and ICD arms matched closely in all measured baseline characteristics.
The prespecified primary outcome of this follow-up analysis was the rate of all-cause mortality. Because of their longer disease trajectory, this pared-down study cohort included many more patients with NYHA class II function, 803, and in this subgroup CRT-D exerted a significant 23% incremental reduction in mortality compared with ICD treatment. CRT-D also produced a 17% relative reduction in long-term mortality among patients with NYHA class III function at baseline, but this point estimate of relative benefit was not significant in this subgroup of just 247 patients, said Dr. Sapp, a cardiologist and professor at Dalhousie University & Nova Scotia Health in Halifax.
Based on the original RAFT results from 2010, as well as on evidence from several other trials, the current heart failure management guideline from the AHA, the American College of Cardiology, and the Heart Failure Society of America give the highest level of recommendation, level 1, for CRT in patients with a left ventricular ejection fraction of 35% or less, sinus rhythm with left bundle branch block, a QRS duration of at least 150 msec, and NYHA class II, III, or ambulatory IV symptoms while on guideline-directed medical therapy.
The guideline also gives class 2a (“can be useful”) or 2b (“may be considered”) recommendation for certain other heart failure patients, including those with a QRS duration of 120-149 msec, a left ventricular ejection fraction as high as 50%, no left bundle branch block, or NYHA class I symptoms.
Don’t wait to start CRT
Although this 2022 guideline, as well as earlier versions that had roughly similar recommendations for CRT for about a decade, have led to “common” use of CRT in appropriate patients in U.S. practice, “it has not been used as much as it should be, in part because there’s been a feeling that CRT mostly treats symptoms and so perhaps you can wait” to start it, said Dr. Stevenson.
The findings from the new, extended follow-up RAFT analysis give increased urgency to starting CRT “as soon as possible” in appropriate patients with heart failure, even before they stabilize on guideline-directed medical therapy, said Dr. Stevenson. She also downplayed any ambiguity in the RAFT findings about optimal medical therapy, which during the RAFT study included traditional triple therapy at a time before treatment with sacubitril/valsartan (Entresto) and sodium-glucose cotransporter 2 (SGLT2) inhibitors became recommended.
“There is no reason to think that these treatments will negate the benefit of CRT for patients with heart failure with reduced ejection fraction and a wide left bundle branch block,” Dr. Stevenson said.
She also believes that the extended follow-up results, which showed clear efficacy for CRT-D in patients with NYHA class II function, support the case for upgrading the current 2b recommendation for using CRT treatment in patients with NYHA class I function and ischemic heart failure to a 2a recommendation regardless of whether or not patients have coronary artery disease. “The difference between class I and class II depends more on a patient’s lifestyle rather than on the severity of their heart failure,” Dr. Stevenson noted. “The RAFT study results encourage us to reexamine the clinical class and timing for CRT” in the current heart failure guideline.
RAFT received partial sponsorship from Medtronic. Dr. Sapp has been a consultant to Abbott, Biosense Webster, Medtronic, and Varian and has received research funding from Abbott and Biosense Webster. Dr. Stevenson had no disclosures.
CHICAGO – Extended follow-up of patients with heart failure enrolled in the RAFT trial strengthens the case for starting treatment early with a cardiac resynchronization therapy plus defibrillation (CRT-D) device in appropriate patients.
RAFT, which compared CRT-D with treatment with an implantable cardioverter defibrillator (ICD) alone, showed that the early survival benefit produced by CRT-D during an average 40-month follow-up in the original trial persisted during an additional mean follow-up of about 5 years. This result strengthens the case for starting treatment early with a CRT-D device in appropriate patients with heart failure.
During extended follow-up of more than half of the enrolled patients, out to an average of 7.6 years overall and to an average of 12.9 years among survivors, patients who received a CRT-D device had a significant 21% relative reduction in their rate of all-cause mortality compared with randomized patients who received an ICD and no cardiac resynchronization, John L. Sapp, MD, reported at the American Heart Association scientific sessions.
The primary results of RAFT were first reported in 2010.
This magnitude of a survival benefit among the patients originally randomized to CRT is “dramatic,” given that many of the comparator patients who initially received no CRT likely crossed over to receive a CRT-D device once the initial, randomized 4 years of the study finished, commented Lynne W. Stevenson, MD, director of cardiomyopathy and the Lisa M. Jacobson Professor of Cardiology at Vanderbilt University Medical Center in Nashville, Tenn., who was not involved with the study.
‘CRT can remap heart failure trajectory’
The new findings “strengthen our conviction that CRT can remap the trajectory” of selected patients with heart failure, and that “candidates for CRT should be vigorously identified,” Dr. Stevenson said in an interview.
She also noted that the benefit with extended follow-up was “strikingly parallel” to that seen at 12 years after the addition of an ACE inhibitor for mild heart failure during the 4 years of the landmark SOLVD trial. The new RAFT extended follow-up, as well as the 12-year follow-up of the SOLVD trial, “support the concept that longer follow-up reveals vital information not provided by the relatively short randomized trial period,” she said.
“The new data say ‘don’t delay starting CRT in appropriate patients with heart failure,’ and ‘don’t think of CRT as just a treatment that makes patients feel better.’
“The totality of these data shows that CRT also treats the underlying heart muscle weakness, which helps patients live longer. Previous data showed that patients with left bundle branch block eligible for CRT are unlikely to respond well to the usual, recommended heart medications so it is important to start treatment with CRT-D early,” declared Dr. Stevenson, who cochaired the session where Dr. Sapp gave his report.
RAFT randomized 1,798 patients with New York Heart Association (NYHA) class II or III heart failure, a left ventricular ejection fraction of 30% or less, and an intrinsic QRS duration of at least 120 msec to receive either a CRT-D or ICD device. The study’s primary endpoint was death from any cause or hospitalization for heart failure. After an average 40 months of randomized follow-up, the primary endpoint occurred in 40% of the patients with an ICD and in 33% of those with a CRT-D device, a significant 25% relative reduction linked with CRT-D use. Both endpoint components contributed to the combined result significantly and to about the same extent, and the incremental benefit from CRT-D was significant for patients with NYHA class II heart failure as well as for those with class III.
However, prespecified subgroup analyses showed that the incremental benefit from CRT-D was significantly limited to patients with an intrinsic QRS duration of at least 150 msec, while in those with a duration of 120-149 msec CRT-D had a neutral effect compared with ICD. The same pattern also appeared when the analysis split patients into those with a left bundle branch block, who significantly benefited from CRT-D, but the initial benefit was not apparent in patients with right bundle branch block.
A study subgroup with extended follow-up
The new, extended follow-up analysis presented by Dr. Sapp included 1,050 of the original 1,798 patients (58%) enrolled at any of eight participating Canadian centers that each enrolled at least 100 patients and followed them through the end of 2021 (the full study cohort came from 34 centers, including 10 centers outside Canada). This subgroup included 520 patients randomized to receive CRT-D and 530 who received an ICD. Although this was a post hoc subgroup analysis, the CRT-D and ICD arms matched closely in all measured baseline characteristics.
The prespecified primary outcome of this follow-up analysis was the rate of all-cause mortality. Because of their longer disease trajectory, this pared-down study cohort included many more patients with NYHA class II function, 803, and in this subgroup CRT-D exerted a significant 23% incremental reduction in mortality compared with ICD treatment. CRT-D also produced a 17% relative reduction in long-term mortality among patients with NYHA class III function at baseline, but this point estimate of relative benefit was not significant in this subgroup of just 247 patients, said Dr. Sapp, a cardiologist and professor at Dalhousie University & Nova Scotia Health in Halifax.
Based on the original RAFT results from 2010, as well as on evidence from several other trials, the current heart failure management guideline from the AHA, the American College of Cardiology, and the Heart Failure Society of America give the highest level of recommendation, level 1, for CRT in patients with a left ventricular ejection fraction of 35% or less, sinus rhythm with left bundle branch block, a QRS duration of at least 150 msec, and NYHA class II, III, or ambulatory IV symptoms while on guideline-directed medical therapy.
The guideline also gives class 2a (“can be useful”) or 2b (“may be considered”) recommendation for certain other heart failure patients, including those with a QRS duration of 120-149 msec, a left ventricular ejection fraction as high as 50%, no left bundle branch block, or NYHA class I symptoms.
Don’t wait to start CRT
Although this 2022 guideline, as well as earlier versions that had roughly similar recommendations for CRT for about a decade, have led to “common” use of CRT in appropriate patients in U.S. practice, “it has not been used as much as it should be, in part because there’s been a feeling that CRT mostly treats symptoms and so perhaps you can wait” to start it, said Dr. Stevenson.
The findings from the new, extended follow-up RAFT analysis give increased urgency to starting CRT “as soon as possible” in appropriate patients with heart failure, even before they stabilize on guideline-directed medical therapy, said Dr. Stevenson. She also downplayed any ambiguity in the RAFT findings about optimal medical therapy, which during the RAFT study included traditional triple therapy at a time before treatment with sacubitril/valsartan (Entresto) and sodium-glucose cotransporter 2 (SGLT2) inhibitors became recommended.
“There is no reason to think that these treatments will negate the benefit of CRT for patients with heart failure with reduced ejection fraction and a wide left bundle branch block,” Dr. Stevenson said.
She also believes that the extended follow-up results, which showed clear efficacy for CRT-D in patients with NYHA class II function, support the case for upgrading the current 2b recommendation for using CRT treatment in patients with NYHA class I function and ischemic heart failure to a 2a recommendation regardless of whether or not patients have coronary artery disease. “The difference between class I and class II depends more on a patient’s lifestyle rather than on the severity of their heart failure,” Dr. Stevenson noted. “The RAFT study results encourage us to reexamine the clinical class and timing for CRT” in the current heart failure guideline.
RAFT received partial sponsorship from Medtronic. Dr. Sapp has been a consultant to Abbott, Biosense Webster, Medtronic, and Varian and has received research funding from Abbott and Biosense Webster. Dr. Stevenson had no disclosures.
CHICAGO – Extended follow-up of patients with heart failure enrolled in the RAFT trial strengthens the case for starting treatment early with a cardiac resynchronization therapy plus defibrillation (CRT-D) device in appropriate patients.
RAFT, which compared CRT-D with treatment with an implantable cardioverter defibrillator (ICD) alone, showed that the early survival benefit produced by CRT-D during an average 40-month follow-up in the original trial persisted during an additional mean follow-up of about 5 years. This result strengthens the case for starting treatment early with a CRT-D device in appropriate patients with heart failure.
During extended follow-up of more than half of the enrolled patients, out to an average of 7.6 years overall and to an average of 12.9 years among survivors, patients who received a CRT-D device had a significant 21% relative reduction in their rate of all-cause mortality compared with randomized patients who received an ICD and no cardiac resynchronization, John L. Sapp, MD, reported at the American Heart Association scientific sessions.
The primary results of RAFT were first reported in 2010.
This magnitude of a survival benefit among the patients originally randomized to CRT is “dramatic,” given that many of the comparator patients who initially received no CRT likely crossed over to receive a CRT-D device once the initial, randomized 4 years of the study finished, commented Lynne W. Stevenson, MD, director of cardiomyopathy and the Lisa M. Jacobson Professor of Cardiology at Vanderbilt University Medical Center in Nashville, Tenn., who was not involved with the study.
‘CRT can remap heart failure trajectory’
The new findings “strengthen our conviction that CRT can remap the trajectory” of selected patients with heart failure, and that “candidates for CRT should be vigorously identified,” Dr. Stevenson said in an interview.
She also noted that the benefit with extended follow-up was “strikingly parallel” to that seen at 12 years after the addition of an ACE inhibitor for mild heart failure during the 4 years of the landmark SOLVD trial. The new RAFT extended follow-up, as well as the 12-year follow-up of the SOLVD trial, “support the concept that longer follow-up reveals vital information not provided by the relatively short randomized trial period,” she said.
“The new data say ‘don’t delay starting CRT in appropriate patients with heart failure,’ and ‘don’t think of CRT as just a treatment that makes patients feel better.’
“The totality of these data shows that CRT also treats the underlying heart muscle weakness, which helps patients live longer. Previous data showed that patients with left bundle branch block eligible for CRT are unlikely to respond well to the usual, recommended heart medications so it is important to start treatment with CRT-D early,” declared Dr. Stevenson, who cochaired the session where Dr. Sapp gave his report.
RAFT randomized 1,798 patients with New York Heart Association (NYHA) class II or III heart failure, a left ventricular ejection fraction of 30% or less, and an intrinsic QRS duration of at least 120 msec to receive either a CRT-D or ICD device. The study’s primary endpoint was death from any cause or hospitalization for heart failure. After an average 40 months of randomized follow-up, the primary endpoint occurred in 40% of the patients with an ICD and in 33% of those with a CRT-D device, a significant 25% relative reduction linked with CRT-D use. Both endpoint components contributed to the combined result significantly and to about the same extent, and the incremental benefit from CRT-D was significant for patients with NYHA class II heart failure as well as for those with class III.
However, prespecified subgroup analyses showed that the incremental benefit from CRT-D was significantly limited to patients with an intrinsic QRS duration of at least 150 msec, while in those with a duration of 120-149 msec CRT-D had a neutral effect compared with ICD. The same pattern also appeared when the analysis split patients into those with a left bundle branch block, who significantly benefited from CRT-D, but the initial benefit was not apparent in patients with right bundle branch block.
A study subgroup with extended follow-up
The new, extended follow-up analysis presented by Dr. Sapp included 1,050 of the original 1,798 patients (58%) enrolled at any of eight participating Canadian centers that each enrolled at least 100 patients and followed them through the end of 2021 (the full study cohort came from 34 centers, including 10 centers outside Canada). This subgroup included 520 patients randomized to receive CRT-D and 530 who received an ICD. Although this was a post hoc subgroup analysis, the CRT-D and ICD arms matched closely in all measured baseline characteristics.
The prespecified primary outcome of this follow-up analysis was the rate of all-cause mortality. Because of their longer disease trajectory, this pared-down study cohort included many more patients with NYHA class II function, 803, and in this subgroup CRT-D exerted a significant 23% incremental reduction in mortality compared with ICD treatment. CRT-D also produced a 17% relative reduction in long-term mortality among patients with NYHA class III function at baseline, but this point estimate of relative benefit was not significant in this subgroup of just 247 patients, said Dr. Sapp, a cardiologist and professor at Dalhousie University & Nova Scotia Health in Halifax.
Based on the original RAFT results from 2010, as well as on evidence from several other trials, the current heart failure management guideline from the AHA, the American College of Cardiology, and the Heart Failure Society of America give the highest level of recommendation, level 1, for CRT in patients with a left ventricular ejection fraction of 35% or less, sinus rhythm with left bundle branch block, a QRS duration of at least 150 msec, and NYHA class II, III, or ambulatory IV symptoms while on guideline-directed medical therapy.
The guideline also gives class 2a (“can be useful”) or 2b (“may be considered”) recommendation for certain other heart failure patients, including those with a QRS duration of 120-149 msec, a left ventricular ejection fraction as high as 50%, no left bundle branch block, or NYHA class I symptoms.
Don’t wait to start CRT
Although this 2022 guideline, as well as earlier versions that had roughly similar recommendations for CRT for about a decade, have led to “common” use of CRT in appropriate patients in U.S. practice, “it has not been used as much as it should be, in part because there’s been a feeling that CRT mostly treats symptoms and so perhaps you can wait” to start it, said Dr. Stevenson.
The findings from the new, extended follow-up RAFT analysis give increased urgency to starting CRT “as soon as possible” in appropriate patients with heart failure, even before they stabilize on guideline-directed medical therapy, said Dr. Stevenson. She also downplayed any ambiguity in the RAFT findings about optimal medical therapy, which during the RAFT study included traditional triple therapy at a time before treatment with sacubitril/valsartan (Entresto) and sodium-glucose cotransporter 2 (SGLT2) inhibitors became recommended.
“There is no reason to think that these treatments will negate the benefit of CRT for patients with heart failure with reduced ejection fraction and a wide left bundle branch block,” Dr. Stevenson said.
She also believes that the extended follow-up results, which showed clear efficacy for CRT-D in patients with NYHA class II function, support the case for upgrading the current 2b recommendation for using CRT treatment in patients with NYHA class I function and ischemic heart failure to a 2a recommendation regardless of whether or not patients have coronary artery disease. “The difference between class I and class II depends more on a patient’s lifestyle rather than on the severity of their heart failure,” Dr. Stevenson noted. “The RAFT study results encourage us to reexamine the clinical class and timing for CRT” in the current heart failure guideline.
RAFT received partial sponsorship from Medtronic. Dr. Sapp has been a consultant to Abbott, Biosense Webster, Medtronic, and Varian and has received research funding from Abbott and Biosense Webster. Dr. Stevenson had no disclosures.
AT AHA 2022
Ask knee OA patients about stair climbing difficulty
Asking knee osteoarthritis patients a simple question – do you have difficulty climbing stairs? – may predict the risk of future functional limitation, according to research presented at the annual meeting of the American College of Rheumatology. Finding out that the patient has difficulty also opens avenues for further evaluation and intervention, said Jason Jakiela, a PhD candidate at the University of Delaware, Newark, who led the study. “We like to view it as a kind of yellow flag,” Mr. Jakiela said in an interview.
Another expert agreed. “I think this is useful for clinical rheumatologists,” said C. Kent Kwoh, MD, professor of medicine and medical imaging at the University of Arizona, Tucson, and director of the University of Arizona Arthritis Center. He commented on the study findings but was not involved in the study. Another common question asked of OA patients, about pain, may not be as useful as asking about difficulty climbing stairs, he said. “Their pain level can go up and down and can be quite varied.”
Osteoarthritis affects more than 32.5 million adults, according to the CDC, and the knee is a common site.
Study details, results
Mr. Jakiela and his team, including Daniel White, PT, ScD, MSC, associate professor of physical therapy at the University of Delaware, Newark, used data from the Osteoarthritis Initiative (OAI). They assessed stair climbing difficulty at baseline with the question: Does your health now limit you in climbing several flights of stairs? Respondents could answer that they were limited a lot, a little, or not at all.
The researchers evaluated functional limitation using two measures: Walking speed and Western Ontario and McMaster Universities Osteoarthritis Index physical function (WOMAC-PF) scores. A walking speed of < 1.22 m/s over 20 meters, the speed needed to safely cross a timed intersection, represented poor function. A WOMAC-PF score of 28/68 or more was also used to define low functioning.
The analyses included only people free of functional limitations at baseline. Each measure was conducted at the start and then at 12, 24, 36, 48, 72, and 96 months’ follow-up visits.
While 2,952 participants (mean age 60.1, 54% female, mean body mass index 27.9) were in the walking speed sample, 3,983 participants (mean age 61.2, 57% female, mean BMI 28.2) were in the WOMAC-PF sample.
When compared with people who had no limitations, those limited a little had a 47% greater risk of gait speed functional limitation and those limited a lot had a 61% greater risk at follow-up. There was a 70% greater risk for functional limitation defined by WOMAC-PF score at follow-up among people who were limited a little in stair climbing when compared with those not limited at all, and people with a lot of limitations had 161% greater risk. Slow gait speed has been linked with mortality.
Over the 8-year follow-up, 973 in the walking speed sample and 578 in the WOMAC-PF sample developed functional limitation.
Starting the conversation
The question about stair climbing difficulty is a good “jumping-off point,” Mr. Jakiela said. “It opens up a line of questioning.” With knee OA, stair climbing difficulty is often the first reported limitation. That difficulty could capture a variety of issues, he said. Patients could be struggling with strength issues, cardiovascular problems, or balance deficits, for instance.
It signals there may be a trajectory of slow decline coming in this patient, Mr. Jakiela said.
“It’s a signal that something is not right,” Dr. White said in an interview. “We don’t know what is wrong.” While questions about stairs have routinely been asked of OA patients, the study findings suggest the answer to the question about having difficulty could help predict a patient’s future course, he said.
After patients reported a little or a lot of difficulty with stair climbing, the average time to reach functional limitation status was about 3 years, Mr. Jakiela said. That gives health care providers time to ask more questions about the patient’s condition and potentially intervene, depending on the details of the difficulty. If it’s a balance issue, physical therapy might help, for example.
While gait speed is a tried-and-true indication, collecting answers about stair climbing difficulty is easier and quicker for clinicians than assessing gait speed, which requires more time as well as office space, Mr. Jakiela said. It’s also intuitive for the patients to recall, the researchers said.
More practical takeaways
Finding out whether functional limitation is likely, based on the stair question, can help health care providers consider nonpharmacologic interventions, Dr. Kwoh agreed, such as physical therapy or braces. “It doesn’t have to be drugs. We have limited drugs for OA at the moment. We don’t have a so-called DMARD drug [for OA].”
NSAIDs have side effects, and people are very familiar with the issues of opioids, he said. It’s important, he added, for the health care provider, if referring to a physical therapist, to find the right one. To help those dealing with knee OA, a PT in sports medicine might be a good choice, he said.
Mr. Jakiela has no disclosures. Dr. Kwoh and Dr. White have no relevant disclosures.
Asking knee osteoarthritis patients a simple question – do you have difficulty climbing stairs? – may predict the risk of future functional limitation, according to research presented at the annual meeting of the American College of Rheumatology. Finding out that the patient has difficulty also opens avenues for further evaluation and intervention, said Jason Jakiela, a PhD candidate at the University of Delaware, Newark, who led the study. “We like to view it as a kind of yellow flag,” Mr. Jakiela said in an interview.
Another expert agreed. “I think this is useful for clinical rheumatologists,” said C. Kent Kwoh, MD, professor of medicine and medical imaging at the University of Arizona, Tucson, and director of the University of Arizona Arthritis Center. He commented on the study findings but was not involved in the study. Another common question asked of OA patients, about pain, may not be as useful as asking about difficulty climbing stairs, he said. “Their pain level can go up and down and can be quite varied.”
Osteoarthritis affects more than 32.5 million adults, according to the CDC, and the knee is a common site.
Study details, results
Mr. Jakiela and his team, including Daniel White, PT, ScD, MSC, associate professor of physical therapy at the University of Delaware, Newark, used data from the Osteoarthritis Initiative (OAI). They assessed stair climbing difficulty at baseline with the question: Does your health now limit you in climbing several flights of stairs? Respondents could answer that they were limited a lot, a little, or not at all.
The researchers evaluated functional limitation using two measures: Walking speed and Western Ontario and McMaster Universities Osteoarthritis Index physical function (WOMAC-PF) scores. A walking speed of < 1.22 m/s over 20 meters, the speed needed to safely cross a timed intersection, represented poor function. A WOMAC-PF score of 28/68 or more was also used to define low functioning.
The analyses included only people free of functional limitations at baseline. Each measure was conducted at the start and then at 12, 24, 36, 48, 72, and 96 months’ follow-up visits.
While 2,952 participants (mean age 60.1, 54% female, mean body mass index 27.9) were in the walking speed sample, 3,983 participants (mean age 61.2, 57% female, mean BMI 28.2) were in the WOMAC-PF sample.
When compared with people who had no limitations, those limited a little had a 47% greater risk of gait speed functional limitation and those limited a lot had a 61% greater risk at follow-up. There was a 70% greater risk for functional limitation defined by WOMAC-PF score at follow-up among people who were limited a little in stair climbing when compared with those not limited at all, and people with a lot of limitations had 161% greater risk. Slow gait speed has been linked with mortality.
Over the 8-year follow-up, 973 in the walking speed sample and 578 in the WOMAC-PF sample developed functional limitation.
Starting the conversation
The question about stair climbing difficulty is a good “jumping-off point,” Mr. Jakiela said. “It opens up a line of questioning.” With knee OA, stair climbing difficulty is often the first reported limitation. That difficulty could capture a variety of issues, he said. Patients could be struggling with strength issues, cardiovascular problems, or balance deficits, for instance.
It signals there may be a trajectory of slow decline coming in this patient, Mr. Jakiela said.
“It’s a signal that something is not right,” Dr. White said in an interview. “We don’t know what is wrong.” While questions about stairs have routinely been asked of OA patients, the study findings suggest the answer to the question about having difficulty could help predict a patient’s future course, he said.
After patients reported a little or a lot of difficulty with stair climbing, the average time to reach functional limitation status was about 3 years, Mr. Jakiela said. That gives health care providers time to ask more questions about the patient’s condition and potentially intervene, depending on the details of the difficulty. If it’s a balance issue, physical therapy might help, for example.
While gait speed is a tried-and-true indication, collecting answers about stair climbing difficulty is easier and quicker for clinicians than assessing gait speed, which requires more time as well as office space, Mr. Jakiela said. It’s also intuitive for the patients to recall, the researchers said.
More practical takeaways
Finding out whether functional limitation is likely, based on the stair question, can help health care providers consider nonpharmacologic interventions, Dr. Kwoh agreed, such as physical therapy or braces. “It doesn’t have to be drugs. We have limited drugs for OA at the moment. We don’t have a so-called DMARD drug [for OA].”
NSAIDs have side effects, and people are very familiar with the issues of opioids, he said. It’s important, he added, for the health care provider, if referring to a physical therapist, to find the right one. To help those dealing with knee OA, a PT in sports medicine might be a good choice, he said.
Mr. Jakiela has no disclosures. Dr. Kwoh and Dr. White have no relevant disclosures.
Asking knee osteoarthritis patients a simple question – do you have difficulty climbing stairs? – may predict the risk of future functional limitation, according to research presented at the annual meeting of the American College of Rheumatology. Finding out that the patient has difficulty also opens avenues for further evaluation and intervention, said Jason Jakiela, a PhD candidate at the University of Delaware, Newark, who led the study. “We like to view it as a kind of yellow flag,” Mr. Jakiela said in an interview.
Another expert agreed. “I think this is useful for clinical rheumatologists,” said C. Kent Kwoh, MD, professor of medicine and medical imaging at the University of Arizona, Tucson, and director of the University of Arizona Arthritis Center. He commented on the study findings but was not involved in the study. Another common question asked of OA patients, about pain, may not be as useful as asking about difficulty climbing stairs, he said. “Their pain level can go up and down and can be quite varied.”
Osteoarthritis affects more than 32.5 million adults, according to the CDC, and the knee is a common site.
Study details, results
Mr. Jakiela and his team, including Daniel White, PT, ScD, MSC, associate professor of physical therapy at the University of Delaware, Newark, used data from the Osteoarthritis Initiative (OAI). They assessed stair climbing difficulty at baseline with the question: Does your health now limit you in climbing several flights of stairs? Respondents could answer that they were limited a lot, a little, or not at all.
The researchers evaluated functional limitation using two measures: Walking speed and Western Ontario and McMaster Universities Osteoarthritis Index physical function (WOMAC-PF) scores. A walking speed of < 1.22 m/s over 20 meters, the speed needed to safely cross a timed intersection, represented poor function. A WOMAC-PF score of 28/68 or more was also used to define low functioning.
The analyses included only people free of functional limitations at baseline. Each measure was conducted at the start and then at 12, 24, 36, 48, 72, and 96 months’ follow-up visits.
While 2,952 participants (mean age 60.1, 54% female, mean body mass index 27.9) were in the walking speed sample, 3,983 participants (mean age 61.2, 57% female, mean BMI 28.2) were in the WOMAC-PF sample.
When compared with people who had no limitations, those limited a little had a 47% greater risk of gait speed functional limitation and those limited a lot had a 61% greater risk at follow-up. There was a 70% greater risk for functional limitation defined by WOMAC-PF score at follow-up among people who were limited a little in stair climbing when compared with those not limited at all, and people with a lot of limitations had 161% greater risk. Slow gait speed has been linked with mortality.
Over the 8-year follow-up, 973 in the walking speed sample and 578 in the WOMAC-PF sample developed functional limitation.
Starting the conversation
The question about stair climbing difficulty is a good “jumping-off point,” Mr. Jakiela said. “It opens up a line of questioning.” With knee OA, stair climbing difficulty is often the first reported limitation. That difficulty could capture a variety of issues, he said. Patients could be struggling with strength issues, cardiovascular problems, or balance deficits, for instance.
It signals there may be a trajectory of slow decline coming in this patient, Mr. Jakiela said.
“It’s a signal that something is not right,” Dr. White said in an interview. “We don’t know what is wrong.” While questions about stairs have routinely been asked of OA patients, the study findings suggest the answer to the question about having difficulty could help predict a patient’s future course, he said.
After patients reported a little or a lot of difficulty with stair climbing, the average time to reach functional limitation status was about 3 years, Mr. Jakiela said. That gives health care providers time to ask more questions about the patient’s condition and potentially intervene, depending on the details of the difficulty. If it’s a balance issue, physical therapy might help, for example.
While gait speed is a tried-and-true indication, collecting answers about stair climbing difficulty is easier and quicker for clinicians than assessing gait speed, which requires more time as well as office space, Mr. Jakiela said. It’s also intuitive for the patients to recall, the researchers said.
More practical takeaways
Finding out whether functional limitation is likely, based on the stair question, can help health care providers consider nonpharmacologic interventions, Dr. Kwoh agreed, such as physical therapy or braces. “It doesn’t have to be drugs. We have limited drugs for OA at the moment. We don’t have a so-called DMARD drug [for OA].”
NSAIDs have side effects, and people are very familiar with the issues of opioids, he said. It’s important, he added, for the health care provider, if referring to a physical therapist, to find the right one. To help those dealing with knee OA, a PT in sports medicine might be a good choice, he said.
Mr. Jakiela has no disclosures. Dr. Kwoh and Dr. White have no relevant disclosures.
FROM ACR 2022
Commentary: Prevention in AD, December 2022
We are in the golden age of atopic dermatitis (AD) drug development. We are fortunate to have numerous topicals, oral systemics, and biologics recently approved or in late-stage clinical development. Yet, we are still lacking effective strategies for primary prevention of incident AD and secondary prevention of AD exacerbations.
Kottner and colleagues published the results from the ADAPI study of 150 infants who were at an enhanced risk for AD. The children were randomly assigned to receive either a skincare regimen that was standardized or unstandardized skincare preferred by parents. They found that in the first year of life, the overall cumulative incidence rate of AD was similar between standardized skincare and skincare preferred by parents (P = .999).
Bradshaw and colleagues also published results from the BEEP study (a 5-year prospective study) of 1394 infants who were at high risk for AD. The children were randomly assigned to receive either emollient for the first year plus standard skincare or standard skincare alone. They found a similar proportion of children were clinically diagnosed with AD between 12 and 60 months in the emollient plus skincare group vs skincare alone group (31% vs 28%; adjusted relative risk 1.10; 95% CI 0.93-1.30). Unfortunately, the results from both studies are consistent with earlier results from BEEP, as well as other studies, and did not show that early application of emollients successfully prevent AD.
The use of applying emollients for primary prevention is unclear. However, proactive application of topical corticosteroids (TCS) and other topical nonsteroidal agents is well accepted in AD treatment guidelines for secondary prevention of AD exacerbations.1 Although, a recent study from Kamiya and colleagues suggested that proactive application of topical corticosteroids may not work as well as we think. They conducted an open-label, active-controlled, parallel-group study of 49 pediatric patients with moderate to severe AD who achieved remission with potent TCS. The children were then randomly assigned to receive proactive therapy with or discontinuation of TCS. The authors found no significant decrease in relapse rates with proactive vs no proactive treatment groups (8.33% vs 20.0%; P = .0859). I don't think these results will change our guidelines. But I do think these results raise important questions about the myriad aspects of proactive therapy that require appropriate counseling, including frequency of application per week (1-3 times), choice of therapies (corticosteroid or nonsteroidal agent), additional emollient use, bathing practice, etc. I personally would strongly recommend use of proactive therapy in clinical practice, but these results highlight that it is not a magic bullet for all patients either.
Additional Reference
- Boguniewicz M, Fonacier L, Guttman-Yassky E, et al. Atopic dermatitis yardstick: practical recommendations for an evolving therapeutic landscape. Ann Allergy Asthma Immunol. 2018;120:10-22.e2. Doi: 10.1016/j.anai.2017.10.039
We are in the golden age of atopic dermatitis (AD) drug development. We are fortunate to have numerous topicals, oral systemics, and biologics recently approved or in late-stage clinical development. Yet, we are still lacking effective strategies for primary prevention of incident AD and secondary prevention of AD exacerbations.
Kottner and colleagues published the results from the ADAPI study of 150 infants who were at an enhanced risk for AD. The children were randomly assigned to receive either a skincare regimen that was standardized or unstandardized skincare preferred by parents. They found that in the first year of life, the overall cumulative incidence rate of AD was similar between standardized skincare and skincare preferred by parents (P = .999).
Bradshaw and colleagues also published results from the BEEP study (a 5-year prospective study) of 1394 infants who were at high risk for AD. The children were randomly assigned to receive either emollient for the first year plus standard skincare or standard skincare alone. They found a similar proportion of children were clinically diagnosed with AD between 12 and 60 months in the emollient plus skincare group vs skincare alone group (31% vs 28%; adjusted relative risk 1.10; 95% CI 0.93-1.30). Unfortunately, the results from both studies are consistent with earlier results from BEEP, as well as other studies, and did not show that early application of emollients successfully prevent AD.
The use of applying emollients for primary prevention is unclear. However, proactive application of topical corticosteroids (TCS) and other topical nonsteroidal agents is well accepted in AD treatment guidelines for secondary prevention of AD exacerbations.1 Although, a recent study from Kamiya and colleagues suggested that proactive application of topical corticosteroids may not work as well as we think. They conducted an open-label, active-controlled, parallel-group study of 49 pediatric patients with moderate to severe AD who achieved remission with potent TCS. The children were then randomly assigned to receive proactive therapy with or discontinuation of TCS. The authors found no significant decrease in relapse rates with proactive vs no proactive treatment groups (8.33% vs 20.0%; P = .0859). I don't think these results will change our guidelines. But I do think these results raise important questions about the myriad aspects of proactive therapy that require appropriate counseling, including frequency of application per week (1-3 times), choice of therapies (corticosteroid or nonsteroidal agent), additional emollient use, bathing practice, etc. I personally would strongly recommend use of proactive therapy in clinical practice, but these results highlight that it is not a magic bullet for all patients either.
Additional Reference
- Boguniewicz M, Fonacier L, Guttman-Yassky E, et al. Atopic dermatitis yardstick: practical recommendations for an evolving therapeutic landscape. Ann Allergy Asthma Immunol. 2018;120:10-22.e2. Doi: 10.1016/j.anai.2017.10.039
We are in the golden age of atopic dermatitis (AD) drug development. We are fortunate to have numerous topicals, oral systemics, and biologics recently approved or in late-stage clinical development. Yet, we are still lacking effective strategies for primary prevention of incident AD and secondary prevention of AD exacerbations.
Kottner and colleagues published the results from the ADAPI study of 150 infants who were at an enhanced risk for AD. The children were randomly assigned to receive either a skincare regimen that was standardized or unstandardized skincare preferred by parents. They found that in the first year of life, the overall cumulative incidence rate of AD was similar between standardized skincare and skincare preferred by parents (P = .999).
Bradshaw and colleagues also published results from the BEEP study (a 5-year prospective study) of 1394 infants who were at high risk for AD. The children were randomly assigned to receive either emollient for the first year plus standard skincare or standard skincare alone. They found a similar proportion of children were clinically diagnosed with AD between 12 and 60 months in the emollient plus skincare group vs skincare alone group (31% vs 28%; adjusted relative risk 1.10; 95% CI 0.93-1.30). Unfortunately, the results from both studies are consistent with earlier results from BEEP, as well as other studies, and did not show that early application of emollients successfully prevent AD.
The use of applying emollients for primary prevention is unclear. However, proactive application of topical corticosteroids (TCS) and other topical nonsteroidal agents is well accepted in AD treatment guidelines for secondary prevention of AD exacerbations.1 Although, a recent study from Kamiya and colleagues suggested that proactive application of topical corticosteroids may not work as well as we think. They conducted an open-label, active-controlled, parallel-group study of 49 pediatric patients with moderate to severe AD who achieved remission with potent TCS. The children were then randomly assigned to receive proactive therapy with or discontinuation of TCS. The authors found no significant decrease in relapse rates with proactive vs no proactive treatment groups (8.33% vs 20.0%; P = .0859). I don't think these results will change our guidelines. But I do think these results raise important questions about the myriad aspects of proactive therapy that require appropriate counseling, including frequency of application per week (1-3 times), choice of therapies (corticosteroid or nonsteroidal agent), additional emollient use, bathing practice, etc. I personally would strongly recommend use of proactive therapy in clinical practice, but these results highlight that it is not a magic bullet for all patients either.
Additional Reference
- Boguniewicz M, Fonacier L, Guttman-Yassky E, et al. Atopic dermatitis yardstick: practical recommendations for an evolving therapeutic landscape. Ann Allergy Asthma Immunol. 2018;120:10-22.e2. Doi: 10.1016/j.anai.2017.10.039
Diagnosed too late
It had only been 3 weeks since I first met this patient. She presented with an advanced case of colon cancer, but instead of treatment,
Within the course of 2 weeks I saw another new patient, but this time with pancreatic cancer that metastasized to the liver. “When can we start treatment?” he asked. Like my female patient with colon cancer, he was diagnosed too late as he was already in an incurable stage. He was shocked to learn that his condition was in stage 4, that achieving remission would be difficult and a cure, not likely. Certainly, standard of care treatments and clinical trials offered him hope, but they were unlikely to change the outcome.
We take a course in this – that is, in giving bad news, but every doctor has his or her own approach. Some are so uncomfortable with the talk, they choose avoidance and adopt the “look like you gotta go approach.” Or, the doctor may schedule another treatment or another test with the intention of avoiding end-of-life discussions. Other doctors opt for straight talk: “I think you should get your affairs in order. You’ve got 3 months to live.” These are extreme behaviors I wouldn’t recommend.
In my practice, I sit with my patients and explain the diagnosis. After discussing all options and the advanced stage and diagnosis, it ultimately comes down to “Win or lose, I will be here to take care of you.” Sometimes there is therapy that may help, but either way, the patient understands that death is a real possibility.
I find that people just want to know if there is hope. A different treatment regimen or a clinical trial may (or may not) extend their life. And while we cannot predict outcomes, we can give them hope. You can’t shut down hope. True for some people the cup is always half empty, but most people want to live and are optimistic no matter how small the chances are.
These conversations are very difficult. I don’t like them, but then I don’t avoid them either. Fortunately, patients don’t usually come to my office for the first visit presenting with advanced disease. In the cases I described above, one patient had been experiencing unexplained weight loss, but didn’t share it with a physician. And, for the patient with pancreatic cancer, other than some discomfort in the last couple of weeks, the disease was not associated with other symptoms. But the absence of symptoms should not in any way rule out a malignant disease. A diagnosis should be based on a complete evaluation of signs and symptoms followed by testing.
We’ve got to be able to take the time to listen to our patients during these encounters. We may not spend as much time as we should because we’re so busy now and we’re slaves to EMRs. It helps if we take more time to probe symptoms a little longer, especially in the primary care setting.
It is possible for a patient with cancer to be asymptomatic up until the later stages of the disease. A study published in ESMO Open in 2020 found that fewer than half of patients with stage 4 non–small cell lung cancer have only one or two symptoms at diagnosis regardless of whether the patient was a smoker. In this study only 33% of patients reported having a cough and 25% had chest pain.
A study presented in October at the United European Gastroenterology Week found that of 600 pancreatic cancer cases, 46 of these were not detected by CT or MRI conducted 3-18 months prior to diagnosis. Of the 46 cases, 26% were not picked up by the radiologist and the rest were largely as a result of imaging changes over time. Radiology techniques are good, but they cannot pick up lesions that are too small. And some lesions, particularly in pancreatic cancer, can grow and metastasize rather quickly.
When a patient is diagnosed with advanced disease, it is most often simply because of the nature of the disease. But sometimes patients put off scheduling a doctor visit because of fear of the potential for bad news or fear of the doctor belittling their symptoms. Some tell me they were “just hoping the symptoms would disappear.” Waiting too long to see a doctor is never a good idea because timing is crucial. In many cases, there is a small window of opportunity to treat disease if remission is to be achieved.
Dr. Henry is a practicing clinical oncologist with PennMedicine in Philadelphia where he also serves as Vice Chair of the Department of Medicine at Pennsylvania Hospital.
This article was updated 12/7/22.
It had only been 3 weeks since I first met this patient. She presented with an advanced case of colon cancer, but instead of treatment,
Within the course of 2 weeks I saw another new patient, but this time with pancreatic cancer that metastasized to the liver. “When can we start treatment?” he asked. Like my female patient with colon cancer, he was diagnosed too late as he was already in an incurable stage. He was shocked to learn that his condition was in stage 4, that achieving remission would be difficult and a cure, not likely. Certainly, standard of care treatments and clinical trials offered him hope, but they were unlikely to change the outcome.
We take a course in this – that is, in giving bad news, but every doctor has his or her own approach. Some are so uncomfortable with the talk, they choose avoidance and adopt the “look like you gotta go approach.” Or, the doctor may schedule another treatment or another test with the intention of avoiding end-of-life discussions. Other doctors opt for straight talk: “I think you should get your affairs in order. You’ve got 3 months to live.” These are extreme behaviors I wouldn’t recommend.
In my practice, I sit with my patients and explain the diagnosis. After discussing all options and the advanced stage and diagnosis, it ultimately comes down to “Win or lose, I will be here to take care of you.” Sometimes there is therapy that may help, but either way, the patient understands that death is a real possibility.
I find that people just want to know if there is hope. A different treatment regimen or a clinical trial may (or may not) extend their life. And while we cannot predict outcomes, we can give them hope. You can’t shut down hope. True for some people the cup is always half empty, but most people want to live and are optimistic no matter how small the chances are.
These conversations are very difficult. I don’t like them, but then I don’t avoid them either. Fortunately, patients don’t usually come to my office for the first visit presenting with advanced disease. In the cases I described above, one patient had been experiencing unexplained weight loss, but didn’t share it with a physician. And, for the patient with pancreatic cancer, other than some discomfort in the last couple of weeks, the disease was not associated with other symptoms. But the absence of symptoms should not in any way rule out a malignant disease. A diagnosis should be based on a complete evaluation of signs and symptoms followed by testing.
We’ve got to be able to take the time to listen to our patients during these encounters. We may not spend as much time as we should because we’re so busy now and we’re slaves to EMRs. It helps if we take more time to probe symptoms a little longer, especially in the primary care setting.
It is possible for a patient with cancer to be asymptomatic up until the later stages of the disease. A study published in ESMO Open in 2020 found that fewer than half of patients with stage 4 non–small cell lung cancer have only one or two symptoms at diagnosis regardless of whether the patient was a smoker. In this study only 33% of patients reported having a cough and 25% had chest pain.
A study presented in October at the United European Gastroenterology Week found that of 600 pancreatic cancer cases, 46 of these were not detected by CT or MRI conducted 3-18 months prior to diagnosis. Of the 46 cases, 26% were not picked up by the radiologist and the rest were largely as a result of imaging changes over time. Radiology techniques are good, but they cannot pick up lesions that are too small. And some lesions, particularly in pancreatic cancer, can grow and metastasize rather quickly.
When a patient is diagnosed with advanced disease, it is most often simply because of the nature of the disease. But sometimes patients put off scheduling a doctor visit because of fear of the potential for bad news or fear of the doctor belittling their symptoms. Some tell me they were “just hoping the symptoms would disappear.” Waiting too long to see a doctor is never a good idea because timing is crucial. In many cases, there is a small window of opportunity to treat disease if remission is to be achieved.
Dr. Henry is a practicing clinical oncologist with PennMedicine in Philadelphia where he also serves as Vice Chair of the Department of Medicine at Pennsylvania Hospital.
This article was updated 12/7/22.
It had only been 3 weeks since I first met this patient. She presented with an advanced case of colon cancer, but instead of treatment,
Within the course of 2 weeks I saw another new patient, but this time with pancreatic cancer that metastasized to the liver. “When can we start treatment?” he asked. Like my female patient with colon cancer, he was diagnosed too late as he was already in an incurable stage. He was shocked to learn that his condition was in stage 4, that achieving remission would be difficult and a cure, not likely. Certainly, standard of care treatments and clinical trials offered him hope, but they were unlikely to change the outcome.
We take a course in this – that is, in giving bad news, but every doctor has his or her own approach. Some are so uncomfortable with the talk, they choose avoidance and adopt the “look like you gotta go approach.” Or, the doctor may schedule another treatment or another test with the intention of avoiding end-of-life discussions. Other doctors opt for straight talk: “I think you should get your affairs in order. You’ve got 3 months to live.” These are extreme behaviors I wouldn’t recommend.
In my practice, I sit with my patients and explain the diagnosis. After discussing all options and the advanced stage and diagnosis, it ultimately comes down to “Win or lose, I will be here to take care of you.” Sometimes there is therapy that may help, but either way, the patient understands that death is a real possibility.
I find that people just want to know if there is hope. A different treatment regimen or a clinical trial may (or may not) extend their life. And while we cannot predict outcomes, we can give them hope. You can’t shut down hope. True for some people the cup is always half empty, but most people want to live and are optimistic no matter how small the chances are.
These conversations are very difficult. I don’t like them, but then I don’t avoid them either. Fortunately, patients don’t usually come to my office for the first visit presenting with advanced disease. In the cases I described above, one patient had been experiencing unexplained weight loss, but didn’t share it with a physician. And, for the patient with pancreatic cancer, other than some discomfort in the last couple of weeks, the disease was not associated with other symptoms. But the absence of symptoms should not in any way rule out a malignant disease. A diagnosis should be based on a complete evaluation of signs and symptoms followed by testing.
We’ve got to be able to take the time to listen to our patients during these encounters. We may not spend as much time as we should because we’re so busy now and we’re slaves to EMRs. It helps if we take more time to probe symptoms a little longer, especially in the primary care setting.
It is possible for a patient with cancer to be asymptomatic up until the later stages of the disease. A study published in ESMO Open in 2020 found that fewer than half of patients with stage 4 non–small cell lung cancer have only one or two symptoms at diagnosis regardless of whether the patient was a smoker. In this study only 33% of patients reported having a cough and 25% had chest pain.
A study presented in October at the United European Gastroenterology Week found that of 600 pancreatic cancer cases, 46 of these were not detected by CT or MRI conducted 3-18 months prior to diagnosis. Of the 46 cases, 26% were not picked up by the radiologist and the rest were largely as a result of imaging changes over time. Radiology techniques are good, but they cannot pick up lesions that are too small. And some lesions, particularly in pancreatic cancer, can grow and metastasize rather quickly.
When a patient is diagnosed with advanced disease, it is most often simply because of the nature of the disease. But sometimes patients put off scheduling a doctor visit because of fear of the potential for bad news or fear of the doctor belittling their symptoms. Some tell me they were “just hoping the symptoms would disappear.” Waiting too long to see a doctor is never a good idea because timing is crucial. In many cases, there is a small window of opportunity to treat disease if remission is to be achieved.
Dr. Henry is a practicing clinical oncologist with PennMedicine in Philadelphia where he also serves as Vice Chair of the Department of Medicine at Pennsylvania Hospital.
This article was updated 12/7/22.
Less than a third of Americans aware of cancer risk from alcohol
The new findings, from a nationally representative survey that included responses from 3,865 adults, show a low awareness of the cancer risk from alcohol, and also that the risk varies by type of drink. Just under a third (31.2%) of respondents thought that consuming liquor/spirits was associated with a risk of cancer, but this fell to 24.9% for drinking beer and even further, to 20.3%, for drinking wine.
In fact, some respondents though the opposite – that drinking alcohol has health benefits; 10.3% of respondents thought that drinking wine was associated with a decreased cancer risk, while 2.25% thought the same for drinking beer, and 1.7% thought that for drinking liquor.
Most U.S. adults (> 50%) reported not knowing how these beverages affected cancer risk, the authors report.
“This study’s findings underscore the need to develop interventions for educating the public about the cancer risks of alcohol use, particularly in the prevailing context of national dialogue about the purported heart health benefits of wine,” commented senior author William M. P. Klein, PhD, associate director of the National Cancer Institute’s Behavioral Research Program, in a statement.
“All types of alcoholic beverages, including wine, increase cancer risk,” Dr. Klein said.
The findings were published online in Cancer Epidemiology, Biomarkers & Prevention.
The results echo the findings of a previous national survey that also found that the majority of Americans are not aware that alcohol consumption is associated with an increased risk of developing a variety of cancers.
In contrast, within the scientific community, there is long-standing and increasing awareness of alcohol consumption as a leading modifiable risk factor for cancer, and there is a growing movement calling for more public health awareness of the link.
Recently, there has been some public support for adding written warnings about the cancer risk from alcohol. A Citizen Petition was filed in 2021, and in August 2022, The New England Journal of Medicine issued a call for new labeling.
Several cancer organizations are petitioning for warnings to be added to alcoholic beverages. The petition is supported by the American Society of Clinical Oncology, the American Institute for Cancer Research, and Breast Cancer Prevention Partners, all in collaboration with several public health organizations. Proposed labeling would read: “WARNING: According to the Surgeon General, consumption of alcoholic beverages can cause cancer, including breast and colon cancers.”
Dr. Klein and colleagues suggest that public health interventions, including mass media campaigns, cancer warning labels, and patient-provider communications, could help disseminate information about cancer and alcohol. “Educating the public about how alcohol increases cancer risk will not only empower consumers to make more informed decisions but may also prevent and reduce excessive alcohol use, as well as cancer morbidity and mortality,” Dr. Klein said.
The study was supported by the Division of Cancer Control and Population Sciences at the National Cancer Institute. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The new findings, from a nationally representative survey that included responses from 3,865 adults, show a low awareness of the cancer risk from alcohol, and also that the risk varies by type of drink. Just under a third (31.2%) of respondents thought that consuming liquor/spirits was associated with a risk of cancer, but this fell to 24.9% for drinking beer and even further, to 20.3%, for drinking wine.
In fact, some respondents though the opposite – that drinking alcohol has health benefits; 10.3% of respondents thought that drinking wine was associated with a decreased cancer risk, while 2.25% thought the same for drinking beer, and 1.7% thought that for drinking liquor.
Most U.S. adults (> 50%) reported not knowing how these beverages affected cancer risk, the authors report.
“This study’s findings underscore the need to develop interventions for educating the public about the cancer risks of alcohol use, particularly in the prevailing context of national dialogue about the purported heart health benefits of wine,” commented senior author William M. P. Klein, PhD, associate director of the National Cancer Institute’s Behavioral Research Program, in a statement.
“All types of alcoholic beverages, including wine, increase cancer risk,” Dr. Klein said.
The findings were published online in Cancer Epidemiology, Biomarkers & Prevention.
The results echo the findings of a previous national survey that also found that the majority of Americans are not aware that alcohol consumption is associated with an increased risk of developing a variety of cancers.
In contrast, within the scientific community, there is long-standing and increasing awareness of alcohol consumption as a leading modifiable risk factor for cancer, and there is a growing movement calling for more public health awareness of the link.
Recently, there has been some public support for adding written warnings about the cancer risk from alcohol. A Citizen Petition was filed in 2021, and in August 2022, The New England Journal of Medicine issued a call for new labeling.
Several cancer organizations are petitioning for warnings to be added to alcoholic beverages. The petition is supported by the American Society of Clinical Oncology, the American Institute for Cancer Research, and Breast Cancer Prevention Partners, all in collaboration with several public health organizations. Proposed labeling would read: “WARNING: According to the Surgeon General, consumption of alcoholic beverages can cause cancer, including breast and colon cancers.”
Dr. Klein and colleagues suggest that public health interventions, including mass media campaigns, cancer warning labels, and patient-provider communications, could help disseminate information about cancer and alcohol. “Educating the public about how alcohol increases cancer risk will not only empower consumers to make more informed decisions but may also prevent and reduce excessive alcohol use, as well as cancer morbidity and mortality,” Dr. Klein said.
The study was supported by the Division of Cancer Control and Population Sciences at the National Cancer Institute. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The new findings, from a nationally representative survey that included responses from 3,865 adults, show a low awareness of the cancer risk from alcohol, and also that the risk varies by type of drink. Just under a third (31.2%) of respondents thought that consuming liquor/spirits was associated with a risk of cancer, but this fell to 24.9% for drinking beer and even further, to 20.3%, for drinking wine.
In fact, some respondents though the opposite – that drinking alcohol has health benefits; 10.3% of respondents thought that drinking wine was associated with a decreased cancer risk, while 2.25% thought the same for drinking beer, and 1.7% thought that for drinking liquor.
Most U.S. adults (> 50%) reported not knowing how these beverages affected cancer risk, the authors report.
“This study’s findings underscore the need to develop interventions for educating the public about the cancer risks of alcohol use, particularly in the prevailing context of national dialogue about the purported heart health benefits of wine,” commented senior author William M. P. Klein, PhD, associate director of the National Cancer Institute’s Behavioral Research Program, in a statement.
“All types of alcoholic beverages, including wine, increase cancer risk,” Dr. Klein said.
The findings were published online in Cancer Epidemiology, Biomarkers & Prevention.
The results echo the findings of a previous national survey that also found that the majority of Americans are not aware that alcohol consumption is associated with an increased risk of developing a variety of cancers.
In contrast, within the scientific community, there is long-standing and increasing awareness of alcohol consumption as a leading modifiable risk factor for cancer, and there is a growing movement calling for more public health awareness of the link.
Recently, there has been some public support for adding written warnings about the cancer risk from alcohol. A Citizen Petition was filed in 2021, and in August 2022, The New England Journal of Medicine issued a call for new labeling.
Several cancer organizations are petitioning for warnings to be added to alcoholic beverages. The petition is supported by the American Society of Clinical Oncology, the American Institute for Cancer Research, and Breast Cancer Prevention Partners, all in collaboration with several public health organizations. Proposed labeling would read: “WARNING: According to the Surgeon General, consumption of alcoholic beverages can cause cancer, including breast and colon cancers.”
Dr. Klein and colleagues suggest that public health interventions, including mass media campaigns, cancer warning labels, and patient-provider communications, could help disseminate information about cancer and alcohol. “Educating the public about how alcohol increases cancer risk will not only empower consumers to make more informed decisions but may also prevent and reduce excessive alcohol use, as well as cancer morbidity and mortality,” Dr. Klein said.
The study was supported by the Division of Cancer Control and Population Sciences at the National Cancer Institute. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM CANCER EPIDEMIOLOGY, BIOMARKERS & PREVENTION
Study comparing surgical and N95 masks sparks concern
The study’s senior author is John Conly, MD, an infectious disease specialist and professor at the University of Calgary (Alta.), and Alberta Health Services. The findings are not consistent with those of many other studies on this topic.
Commenting about Dr. Conly’s study, Eric Topol, MD, editor-in-chief of Medscape, wrote: “It’s woefully underpowered but ruled out a doubling of hazard for use of medical masks.”
The study, which was partially funded by the World Health Organization, was published online in Annals of Internal Medicine.
This is not the first time that Dr. Conly, who also advises the WHO, has been the subject of controversy. He previously denied that COVID-19 is airborne – a position that is contradicted by strong evidence. In 2021, Dr. Conly made headlines with his controversial claim that N95 respirators can cause harms, including oxygen depletion and carbon dioxide retention.
A detailed examination by the Center for Infectious Disease Research and Policy (CIDRAP) at the University of Minnesota, Minneapolis, pointed out numerous scientific flaws in the study, including inconsistent use of both types of masks. The study also examined health care workers in four very different countries (Canada, Israel, Egypt, and Pakistan) during different periods of the pandemic, which may have affected the results. Furthermore, the study did not account for vaccination status and lacked a control group. CIDRAP receives funding from 3M, which makes N95 respirators.
In a commentary published alongside the study, Roger Chou, MD, professor of medicine at Oregon Health & Science University, Portland, said that the results were “not definitive,” with “a generous noninferiority threshold” that is actually “consistent with up to a relative 70% increased risk ... which may be unacceptable to many health workers.”
Lead study author Mark Loeb, MD, professor of infectious diseases at McMaster University, Hamilton, Ont., defended the findings. “The confidence intervals around this, that is, what the possible results could be if the trial was repeated many times, range from −2.5% to 4.9%,” he told this news organization. “This means that the risk of a COVID-19 infection in those using the medical masks could have ranged from anywhere from 2.5% reduction in risk to a 4.9% increase in risk. Readers and policy makers can decide for themselves about this.”
“There is no point continuing to run underpowered, poorly designed studies that are designed to confirm existing biases,” Raina MacIntyre, PhD, professor of global biosecurity and head of the Biosecurity Program at the Kirby Institute, Sydney, said in an interview. “The new study in Annals of Internal Medicine is entirely consistent with our finding that to prevent infection, you need an N95, and it needs to be worn throughout the whole shift. A surgical mask and intermittent use of N95 are equally ineffective. This should not surprise anyone, given a surgical mask is not designed as respiratory protection but is designed to prevent splash or spray of liquid on the face. Only a respirator is designed as respiratory protection through both the seal around the face and the filter of the face piece to prevent inhalation of virus laden aerosols, but you need to wear it continually in a high-risk environment like a hospital.”
“It makes zero sense to do a randomized trial on something you can measure directly,” said Kimberly Prather, PhD, an atmospheric chemist, professor, and director of the NSF Center for Aerosol Impacts on Chemistry of the Environment at the University of California, San Diego. “In fact, many studies have shown aerosols leaking out of surgical masks. Surgical masks are designed to block large spray droplets. Aerosols (0.5-3 mcm), which have been shown to contain infectious SARS-CoV-2 virus, travel with the air flow, and escape.”
“This study ... will be used to justify policies of supplying health care workers, and perhaps patients and visitors, too, with inadequate protection,” Trish Greenhalgh, MD, professor of primary care health sciences at the University of Oxford (England), told this news organization.
“These authors have been pushing back against treating COVID as airborne for 3 years,” David Fisman, MD, an epidemiologist and infectious disease specialist at the University of Toronto, said in an interview. “So, you’ll see these folks brandishing this very flawed trial to justify continuing the infection control practices that have been so disastrous throughout the pandemic.”
The study was funded by the World Health Organization, the Canadian Institutes of Health Research, and the Juravinski Research Institute. Dr. Conly reported receiving grants from the Canadian Institutes for Health Research, Pfizer, and the WHO. Dr. Chou disclosed being a methodologist for WHO guidelines on infection prevention and control measures for COVID-19. Dr. Loeb disclosed payment for expert testimony on personal protective equipment from the government of Manitoba and the Peel District School Board. Dr. MacIntyre has led a large body of research on masks and respirators in health workers, including four randomized clinical trials. She is the author of a book, “Dark Winter: An insider’s guide to pandemics and biosecurity” (Syndey: NewSouth Publishing, 2022), which covers the history and politics of the controversies around N95 and masks. Dr. Prather reported no disclosures. Dr. Greenhalgh is a member of Independent SAGE and an unpaid adviser to the philanthropic fund Balvi. Dr. Fisman has served as a paid legal expert for the Ontario Nurses’ Association in their challenge to Directive 5, which restricted access to N95 masks in health care. He also served as a paid legal expert for the Elementary Teachers’ Federation of Ontario in its efforts to make schools safer in Ontario.
A version of this article first appeared on Medscape.com.
The study’s senior author is John Conly, MD, an infectious disease specialist and professor at the University of Calgary (Alta.), and Alberta Health Services. The findings are not consistent with those of many other studies on this topic.
Commenting about Dr. Conly’s study, Eric Topol, MD, editor-in-chief of Medscape, wrote: “It’s woefully underpowered but ruled out a doubling of hazard for use of medical masks.”
The study, which was partially funded by the World Health Organization, was published online in Annals of Internal Medicine.
This is not the first time that Dr. Conly, who also advises the WHO, has been the subject of controversy. He previously denied that COVID-19 is airborne – a position that is contradicted by strong evidence. In 2021, Dr. Conly made headlines with his controversial claim that N95 respirators can cause harms, including oxygen depletion and carbon dioxide retention.
A detailed examination by the Center for Infectious Disease Research and Policy (CIDRAP) at the University of Minnesota, Minneapolis, pointed out numerous scientific flaws in the study, including inconsistent use of both types of masks. The study also examined health care workers in four very different countries (Canada, Israel, Egypt, and Pakistan) during different periods of the pandemic, which may have affected the results. Furthermore, the study did not account for vaccination status and lacked a control group. CIDRAP receives funding from 3M, which makes N95 respirators.
In a commentary published alongside the study, Roger Chou, MD, professor of medicine at Oregon Health & Science University, Portland, said that the results were “not definitive,” with “a generous noninferiority threshold” that is actually “consistent with up to a relative 70% increased risk ... which may be unacceptable to many health workers.”
Lead study author Mark Loeb, MD, professor of infectious diseases at McMaster University, Hamilton, Ont., defended the findings. “The confidence intervals around this, that is, what the possible results could be if the trial was repeated many times, range from −2.5% to 4.9%,” he told this news organization. “This means that the risk of a COVID-19 infection in those using the medical masks could have ranged from anywhere from 2.5% reduction in risk to a 4.9% increase in risk. Readers and policy makers can decide for themselves about this.”
“There is no point continuing to run underpowered, poorly designed studies that are designed to confirm existing biases,” Raina MacIntyre, PhD, professor of global biosecurity and head of the Biosecurity Program at the Kirby Institute, Sydney, said in an interview. “The new study in Annals of Internal Medicine is entirely consistent with our finding that to prevent infection, you need an N95, and it needs to be worn throughout the whole shift. A surgical mask and intermittent use of N95 are equally ineffective. This should not surprise anyone, given a surgical mask is not designed as respiratory protection but is designed to prevent splash or spray of liquid on the face. Only a respirator is designed as respiratory protection through both the seal around the face and the filter of the face piece to prevent inhalation of virus laden aerosols, but you need to wear it continually in a high-risk environment like a hospital.”
“It makes zero sense to do a randomized trial on something you can measure directly,” said Kimberly Prather, PhD, an atmospheric chemist, professor, and director of the NSF Center for Aerosol Impacts on Chemistry of the Environment at the University of California, San Diego. “In fact, many studies have shown aerosols leaking out of surgical masks. Surgical masks are designed to block large spray droplets. Aerosols (0.5-3 mcm), which have been shown to contain infectious SARS-CoV-2 virus, travel with the air flow, and escape.”
“This study ... will be used to justify policies of supplying health care workers, and perhaps patients and visitors, too, with inadequate protection,” Trish Greenhalgh, MD, professor of primary care health sciences at the University of Oxford (England), told this news organization.
“These authors have been pushing back against treating COVID as airborne for 3 years,” David Fisman, MD, an epidemiologist and infectious disease specialist at the University of Toronto, said in an interview. “So, you’ll see these folks brandishing this very flawed trial to justify continuing the infection control practices that have been so disastrous throughout the pandemic.”
The study was funded by the World Health Organization, the Canadian Institutes of Health Research, and the Juravinski Research Institute. Dr. Conly reported receiving grants from the Canadian Institutes for Health Research, Pfizer, and the WHO. Dr. Chou disclosed being a methodologist for WHO guidelines on infection prevention and control measures for COVID-19. Dr. Loeb disclosed payment for expert testimony on personal protective equipment from the government of Manitoba and the Peel District School Board. Dr. MacIntyre has led a large body of research on masks and respirators in health workers, including four randomized clinical trials. She is the author of a book, “Dark Winter: An insider’s guide to pandemics and biosecurity” (Syndey: NewSouth Publishing, 2022), which covers the history and politics of the controversies around N95 and masks. Dr. Prather reported no disclosures. Dr. Greenhalgh is a member of Independent SAGE and an unpaid adviser to the philanthropic fund Balvi. Dr. Fisman has served as a paid legal expert for the Ontario Nurses’ Association in their challenge to Directive 5, which restricted access to N95 masks in health care. He also served as a paid legal expert for the Elementary Teachers’ Federation of Ontario in its efforts to make schools safer in Ontario.
A version of this article first appeared on Medscape.com.
The study’s senior author is John Conly, MD, an infectious disease specialist and professor at the University of Calgary (Alta.), and Alberta Health Services. The findings are not consistent with those of many other studies on this topic.
Commenting about Dr. Conly’s study, Eric Topol, MD, editor-in-chief of Medscape, wrote: “It’s woefully underpowered but ruled out a doubling of hazard for use of medical masks.”
The study, which was partially funded by the World Health Organization, was published online in Annals of Internal Medicine.
This is not the first time that Dr. Conly, who also advises the WHO, has been the subject of controversy. He previously denied that COVID-19 is airborne – a position that is contradicted by strong evidence. In 2021, Dr. Conly made headlines with his controversial claim that N95 respirators can cause harms, including oxygen depletion and carbon dioxide retention.
A detailed examination by the Center for Infectious Disease Research and Policy (CIDRAP) at the University of Minnesota, Minneapolis, pointed out numerous scientific flaws in the study, including inconsistent use of both types of masks. The study also examined health care workers in four very different countries (Canada, Israel, Egypt, and Pakistan) during different periods of the pandemic, which may have affected the results. Furthermore, the study did not account for vaccination status and lacked a control group. CIDRAP receives funding from 3M, which makes N95 respirators.
In a commentary published alongside the study, Roger Chou, MD, professor of medicine at Oregon Health & Science University, Portland, said that the results were “not definitive,” with “a generous noninferiority threshold” that is actually “consistent with up to a relative 70% increased risk ... which may be unacceptable to many health workers.”
Lead study author Mark Loeb, MD, professor of infectious diseases at McMaster University, Hamilton, Ont., defended the findings. “The confidence intervals around this, that is, what the possible results could be if the trial was repeated many times, range from −2.5% to 4.9%,” he told this news organization. “This means that the risk of a COVID-19 infection in those using the medical masks could have ranged from anywhere from 2.5% reduction in risk to a 4.9% increase in risk. Readers and policy makers can decide for themselves about this.”
“There is no point continuing to run underpowered, poorly designed studies that are designed to confirm existing biases,” Raina MacIntyre, PhD, professor of global biosecurity and head of the Biosecurity Program at the Kirby Institute, Sydney, said in an interview. “The new study in Annals of Internal Medicine is entirely consistent with our finding that to prevent infection, you need an N95, and it needs to be worn throughout the whole shift. A surgical mask and intermittent use of N95 are equally ineffective. This should not surprise anyone, given a surgical mask is not designed as respiratory protection but is designed to prevent splash or spray of liquid on the face. Only a respirator is designed as respiratory protection through both the seal around the face and the filter of the face piece to prevent inhalation of virus laden aerosols, but you need to wear it continually in a high-risk environment like a hospital.”
“It makes zero sense to do a randomized trial on something you can measure directly,” said Kimberly Prather, PhD, an atmospheric chemist, professor, and director of the NSF Center for Aerosol Impacts on Chemistry of the Environment at the University of California, San Diego. “In fact, many studies have shown aerosols leaking out of surgical masks. Surgical masks are designed to block large spray droplets. Aerosols (0.5-3 mcm), which have been shown to contain infectious SARS-CoV-2 virus, travel with the air flow, and escape.”
“This study ... will be used to justify policies of supplying health care workers, and perhaps patients and visitors, too, with inadequate protection,” Trish Greenhalgh, MD, professor of primary care health sciences at the University of Oxford (England), told this news organization.
“These authors have been pushing back against treating COVID as airborne for 3 years,” David Fisman, MD, an epidemiologist and infectious disease specialist at the University of Toronto, said in an interview. “So, you’ll see these folks brandishing this very flawed trial to justify continuing the infection control practices that have been so disastrous throughout the pandemic.”
The study was funded by the World Health Organization, the Canadian Institutes of Health Research, and the Juravinski Research Institute. Dr. Conly reported receiving grants from the Canadian Institutes for Health Research, Pfizer, and the WHO. Dr. Chou disclosed being a methodologist for WHO guidelines on infection prevention and control measures for COVID-19. Dr. Loeb disclosed payment for expert testimony on personal protective equipment from the government of Manitoba and the Peel District School Board. Dr. MacIntyre has led a large body of research on masks and respirators in health workers, including four randomized clinical trials. She is the author of a book, “Dark Winter: An insider’s guide to pandemics and biosecurity” (Syndey: NewSouth Publishing, 2022), which covers the history and politics of the controversies around N95 and masks. Dr. Prather reported no disclosures. Dr. Greenhalgh is a member of Independent SAGE and an unpaid adviser to the philanthropic fund Balvi. Dr. Fisman has served as a paid legal expert for the Ontario Nurses’ Association in their challenge to Directive 5, which restricted access to N95 masks in health care. He also served as a paid legal expert for the Elementary Teachers’ Federation of Ontario in its efforts to make schools safer in Ontario.
A version of this article first appeared on Medscape.com.
FROM ANNALS OF INTERNAL MEDICINE
Have long COVID? Newest booster vaccines may help you
Yet at 58, the Arizona writer is in no hurry to get the latest vaccine booster. “I just don’t want to risk getting any sicker,” she said.
Ms. Dishner has had two doses of vaccine plus two boosters. Each time, she had what regulators consider to be mild reactions, including a sore arm, slight fever, nausea, and body aches. Still, there’s some evidence that the newest booster, which protects against some of the later variants, could help people like Ms. Dishner in several ways, said Ziyad Al-Aly, MD, a clinical epidemiologist and prolific long COVID researcher at Washington University in St. Louis.
“A bivalent booster might actually [help with] your long COVID,” he said.
There may be other benefits. “What vaccines or current vaccine boosters do is reduce your risk of progression to severe COVID-19 illness,” Dr. Al-Aly said. “You are avoiding hospital stays or even worse; you’re avoiding potentially fatal outcomes after infection. And that’s really worth it. Who wants to be in the hospital this Christmas holiday?”
Each time people are infected with SARS-CoV-2, the virus that causes COVID-19, they have a fresh risk of not only getting severely ill or dying, but of developing long COVID, Dr. Al-Aly and colleagues found in a study published in Nature Medicine. “If you dodged the bullet the first time and did not get long COVID after the first infection, if you get reinfected, you’re trying your luck again,” Dr. Al-Aly said. “I would advise people not to get reinfected, which is another reason to get the booster.”
In a recent review in The Lancet eClinicalMedicine, an international team of researchers looked at 11 studies that sought to find out if vaccines affected long COVID symptoms. Seven of those studies found that people’s symptoms improved after they were vaccinated, and four found that symptoms mostly remained the same. One found symptoms got worse in some patients.
A study of 28,000 people published in the British Medical Journal found more evidence that vaccination may help ease symptoms. “Vaccination may contribute to a reduction in the population health burden of long COVID,” the team at the United Kingdom’s Office for National Statistics concluded. Most studies found vaccination reduced the risk of getting long COVID in the first place.
Vaccines prompt the body to produce antibodies, which stop a microbe from infecting cells. They also prompt the production of immune cells called T cells, which continue to hunt down and attack a pathogen even after infection.
A booster dose could help rev up that immune response in a patient with long COVID, said Stephen J. Thomas, MD, an infectious disease specialist at Upstate Medical Center in Syracuse, N.Y., and the center’s lead principal investigator for Pfizer/BioNTech’s COVID-19 2020 vaccine trial.
Some scientists believe long COVID might be caused when the virus persists in parts of the body where the immune system isn’t particularly active. Although they don’t fully understand the workings of the many and varied long COVID symptoms, they have a good idea about why people with long COVID often do better after receiving a vaccine or booster.
“The theory is that by boosting, the immune system may be able to ‘mop up’ those virus stragglers that have remained behind after your first cleanup attempt,” Dr. Thomas said.
“The vaccine is almost lending a hand or helping your immune response to clear that virus,” Dr. Al-Aly said.
It could be difficult for long COVID patients to make an informed decision about boosters, given the lack of studies that focus exclusively on the relationship between long COVID and boosters, according to Scott Roberts, MD, associate medical director for infection prevention at Yale New Haven (Conn.) Hospital.
Dr. Roberts recommended that patients speak with their health care providers and read about the bivalent booster on trusted sites such as those sponsored by the Food and Drug Administration and the Centers for Disease Control and Prevention. Long COVID patients should get the latest boosters, especially as there’s no evidence they are unsafe for them. “The antibody response is appropriately boosted, and there is a decent chance this will help reduce the impact of long COVID as well,” he said. “Waiting will only increase the risk of getting infected and increase the chances of long COVID.”
Only 12% of Americans 5 years and older have received the updated booster, according to the CDC, although it’s recommended for everyone. Just over 80% of Americans have gotten at least one vaccine dose. Dr. Thomas understands why the uptake has been so low: Along with people like Ms. Dishner, who fear more side effects or worse symptoms, there are those who believe that hybrid immunity – vaccination immunity plus natural infection – is superior to vaccination alone and that they don’t need a booster.
Studies show that the bivalent boosters, which protect against older and newer variants, can target even the new, predominant COVID-19 strains. Whether that is enough to convince people in the no-booster camp who lost faith when their vaccinated peers started getting COVID-19 is unclear, although, as Dr. Al-Aly has pointed out, vaccinations help keep people from getting so sick that they wind up in the hospital. And, with most of the population having received at least one dose of vaccine, most of those getting infected will naturally come from among the vaccinated.
Thomas describes the expectation that vaccines would prevent everyone from getting sick as “one of the major fails” of the pandemic.
Counting on a vaccine to confer 100% immunity is “a very high bar,” he said. “I think that’s what people expected, and when they weren’t seeing it, they kind of said: ‘Well, what’s the point? You know, things are getting better. I’d rather take my chances than keep going and getting boosted.’ ”
One point – and it’s a critical one – is that vaccination immunity wanes. Plus new variants arise that can evade at least some of the immunity provided by vaccination. That’s why boosters are built into the COVID vaccination program.
While it’s not clear why some long COVID patients see improvements in their symptoms after being vaccinated or boosted and others do not, Dr. Al-Aly said there’s little evidence vaccines can make long COVID worse. “There are some reports out there that some people with long COVID, when they got a vaccine or booster, their symptoms got worse. You’ll read anecdotes on this side,” he said, adding that efforts to see if this is really happening have been inconclusive.
“The general consensus is that vaccines really save lives,” Dr. Al-Aly said. “Getting vaccinated, even if you are a long COVID patient, is better than not getting vaccinated.”
A version of this article first appeared on WebMD.com.
Yet at 58, the Arizona writer is in no hurry to get the latest vaccine booster. “I just don’t want to risk getting any sicker,” she said.
Ms. Dishner has had two doses of vaccine plus two boosters. Each time, she had what regulators consider to be mild reactions, including a sore arm, slight fever, nausea, and body aches. Still, there’s some evidence that the newest booster, which protects against some of the later variants, could help people like Ms. Dishner in several ways, said Ziyad Al-Aly, MD, a clinical epidemiologist and prolific long COVID researcher at Washington University in St. Louis.
“A bivalent booster might actually [help with] your long COVID,” he said.
There may be other benefits. “What vaccines or current vaccine boosters do is reduce your risk of progression to severe COVID-19 illness,” Dr. Al-Aly said. “You are avoiding hospital stays or even worse; you’re avoiding potentially fatal outcomes after infection. And that’s really worth it. Who wants to be in the hospital this Christmas holiday?”
Each time people are infected with SARS-CoV-2, the virus that causes COVID-19, they have a fresh risk of not only getting severely ill or dying, but of developing long COVID, Dr. Al-Aly and colleagues found in a study published in Nature Medicine. “If you dodged the bullet the first time and did not get long COVID after the first infection, if you get reinfected, you’re trying your luck again,” Dr. Al-Aly said. “I would advise people not to get reinfected, which is another reason to get the booster.”
In a recent review in The Lancet eClinicalMedicine, an international team of researchers looked at 11 studies that sought to find out if vaccines affected long COVID symptoms. Seven of those studies found that people’s symptoms improved after they were vaccinated, and four found that symptoms mostly remained the same. One found symptoms got worse in some patients.
A study of 28,000 people published in the British Medical Journal found more evidence that vaccination may help ease symptoms. “Vaccination may contribute to a reduction in the population health burden of long COVID,” the team at the United Kingdom’s Office for National Statistics concluded. Most studies found vaccination reduced the risk of getting long COVID in the first place.
Vaccines prompt the body to produce antibodies, which stop a microbe from infecting cells. They also prompt the production of immune cells called T cells, which continue to hunt down and attack a pathogen even after infection.
A booster dose could help rev up that immune response in a patient with long COVID, said Stephen J. Thomas, MD, an infectious disease specialist at Upstate Medical Center in Syracuse, N.Y., and the center’s lead principal investigator for Pfizer/BioNTech’s COVID-19 2020 vaccine trial.
Some scientists believe long COVID might be caused when the virus persists in parts of the body where the immune system isn’t particularly active. Although they don’t fully understand the workings of the many and varied long COVID symptoms, they have a good idea about why people with long COVID often do better after receiving a vaccine or booster.
“The theory is that by boosting, the immune system may be able to ‘mop up’ those virus stragglers that have remained behind after your first cleanup attempt,” Dr. Thomas said.
“The vaccine is almost lending a hand or helping your immune response to clear that virus,” Dr. Al-Aly said.
It could be difficult for long COVID patients to make an informed decision about boosters, given the lack of studies that focus exclusively on the relationship between long COVID and boosters, according to Scott Roberts, MD, associate medical director for infection prevention at Yale New Haven (Conn.) Hospital.
Dr. Roberts recommended that patients speak with their health care providers and read about the bivalent booster on trusted sites such as those sponsored by the Food and Drug Administration and the Centers for Disease Control and Prevention. Long COVID patients should get the latest boosters, especially as there’s no evidence they are unsafe for them. “The antibody response is appropriately boosted, and there is a decent chance this will help reduce the impact of long COVID as well,” he said. “Waiting will only increase the risk of getting infected and increase the chances of long COVID.”
Only 12% of Americans 5 years and older have received the updated booster, according to the CDC, although it’s recommended for everyone. Just over 80% of Americans have gotten at least one vaccine dose. Dr. Thomas understands why the uptake has been so low: Along with people like Ms. Dishner, who fear more side effects or worse symptoms, there are those who believe that hybrid immunity – vaccination immunity plus natural infection – is superior to vaccination alone and that they don’t need a booster.
Studies show that the bivalent boosters, which protect against older and newer variants, can target even the new, predominant COVID-19 strains. Whether that is enough to convince people in the no-booster camp who lost faith when their vaccinated peers started getting COVID-19 is unclear, although, as Dr. Al-Aly has pointed out, vaccinations help keep people from getting so sick that they wind up in the hospital. And, with most of the population having received at least one dose of vaccine, most of those getting infected will naturally come from among the vaccinated.
Thomas describes the expectation that vaccines would prevent everyone from getting sick as “one of the major fails” of the pandemic.
Counting on a vaccine to confer 100% immunity is “a very high bar,” he said. “I think that’s what people expected, and when they weren’t seeing it, they kind of said: ‘Well, what’s the point? You know, things are getting better. I’d rather take my chances than keep going and getting boosted.’ ”
One point – and it’s a critical one – is that vaccination immunity wanes. Plus new variants arise that can evade at least some of the immunity provided by vaccination. That’s why boosters are built into the COVID vaccination program.
While it’s not clear why some long COVID patients see improvements in their symptoms after being vaccinated or boosted and others do not, Dr. Al-Aly said there’s little evidence vaccines can make long COVID worse. “There are some reports out there that some people with long COVID, when they got a vaccine or booster, their symptoms got worse. You’ll read anecdotes on this side,” he said, adding that efforts to see if this is really happening have been inconclusive.
“The general consensus is that vaccines really save lives,” Dr. Al-Aly said. “Getting vaccinated, even if you are a long COVID patient, is better than not getting vaccinated.”
A version of this article first appeared on WebMD.com.
Yet at 58, the Arizona writer is in no hurry to get the latest vaccine booster. “I just don’t want to risk getting any sicker,” she said.
Ms. Dishner has had two doses of vaccine plus two boosters. Each time, she had what regulators consider to be mild reactions, including a sore arm, slight fever, nausea, and body aches. Still, there’s some evidence that the newest booster, which protects against some of the later variants, could help people like Ms. Dishner in several ways, said Ziyad Al-Aly, MD, a clinical epidemiologist and prolific long COVID researcher at Washington University in St. Louis.
“A bivalent booster might actually [help with] your long COVID,” he said.
There may be other benefits. “What vaccines or current vaccine boosters do is reduce your risk of progression to severe COVID-19 illness,” Dr. Al-Aly said. “You are avoiding hospital stays or even worse; you’re avoiding potentially fatal outcomes after infection. And that’s really worth it. Who wants to be in the hospital this Christmas holiday?”
Each time people are infected with SARS-CoV-2, the virus that causes COVID-19, they have a fresh risk of not only getting severely ill or dying, but of developing long COVID, Dr. Al-Aly and colleagues found in a study published in Nature Medicine. “If you dodged the bullet the first time and did not get long COVID after the first infection, if you get reinfected, you’re trying your luck again,” Dr. Al-Aly said. “I would advise people not to get reinfected, which is another reason to get the booster.”
In a recent review in The Lancet eClinicalMedicine, an international team of researchers looked at 11 studies that sought to find out if vaccines affected long COVID symptoms. Seven of those studies found that people’s symptoms improved after they were vaccinated, and four found that symptoms mostly remained the same. One found symptoms got worse in some patients.
A study of 28,000 people published in the British Medical Journal found more evidence that vaccination may help ease symptoms. “Vaccination may contribute to a reduction in the population health burden of long COVID,” the team at the United Kingdom’s Office for National Statistics concluded. Most studies found vaccination reduced the risk of getting long COVID in the first place.
Vaccines prompt the body to produce antibodies, which stop a microbe from infecting cells. They also prompt the production of immune cells called T cells, which continue to hunt down and attack a pathogen even after infection.
A booster dose could help rev up that immune response in a patient with long COVID, said Stephen J. Thomas, MD, an infectious disease specialist at Upstate Medical Center in Syracuse, N.Y., and the center’s lead principal investigator for Pfizer/BioNTech’s COVID-19 2020 vaccine trial.
Some scientists believe long COVID might be caused when the virus persists in parts of the body where the immune system isn’t particularly active. Although they don’t fully understand the workings of the many and varied long COVID symptoms, they have a good idea about why people with long COVID often do better after receiving a vaccine or booster.
“The theory is that by boosting, the immune system may be able to ‘mop up’ those virus stragglers that have remained behind after your first cleanup attempt,” Dr. Thomas said.
“The vaccine is almost lending a hand or helping your immune response to clear that virus,” Dr. Al-Aly said.
It could be difficult for long COVID patients to make an informed decision about boosters, given the lack of studies that focus exclusively on the relationship between long COVID and boosters, according to Scott Roberts, MD, associate medical director for infection prevention at Yale New Haven (Conn.) Hospital.
Dr. Roberts recommended that patients speak with their health care providers and read about the bivalent booster on trusted sites such as those sponsored by the Food and Drug Administration and the Centers for Disease Control and Prevention. Long COVID patients should get the latest boosters, especially as there’s no evidence they are unsafe for them. “The antibody response is appropriately boosted, and there is a decent chance this will help reduce the impact of long COVID as well,” he said. “Waiting will only increase the risk of getting infected and increase the chances of long COVID.”
Only 12% of Americans 5 years and older have received the updated booster, according to the CDC, although it’s recommended for everyone. Just over 80% of Americans have gotten at least one vaccine dose. Dr. Thomas understands why the uptake has been so low: Along with people like Ms. Dishner, who fear more side effects or worse symptoms, there are those who believe that hybrid immunity – vaccination immunity plus natural infection – is superior to vaccination alone and that they don’t need a booster.
Studies show that the bivalent boosters, which protect against older and newer variants, can target even the new, predominant COVID-19 strains. Whether that is enough to convince people in the no-booster camp who lost faith when their vaccinated peers started getting COVID-19 is unclear, although, as Dr. Al-Aly has pointed out, vaccinations help keep people from getting so sick that they wind up in the hospital. And, with most of the population having received at least one dose of vaccine, most of those getting infected will naturally come from among the vaccinated.
Thomas describes the expectation that vaccines would prevent everyone from getting sick as “one of the major fails” of the pandemic.
Counting on a vaccine to confer 100% immunity is “a very high bar,” he said. “I think that’s what people expected, and when they weren’t seeing it, they kind of said: ‘Well, what’s the point? You know, things are getting better. I’d rather take my chances than keep going and getting boosted.’ ”
One point – and it’s a critical one – is that vaccination immunity wanes. Plus new variants arise that can evade at least some of the immunity provided by vaccination. That’s why boosters are built into the COVID vaccination program.
While it’s not clear why some long COVID patients see improvements in their symptoms after being vaccinated or boosted and others do not, Dr. Al-Aly said there’s little evidence vaccines can make long COVID worse. “There are some reports out there that some people with long COVID, when they got a vaccine or booster, their symptoms got worse. You’ll read anecdotes on this side,” he said, adding that efforts to see if this is really happening have been inconclusive.
“The general consensus is that vaccines really save lives,” Dr. Al-Aly said. “Getting vaccinated, even if you are a long COVID patient, is better than not getting vaccinated.”
A version of this article first appeared on WebMD.com.
FROM NATURE MEDICINE