User login
U.S. flu activity already at mid-season levels
according to the Centers of Disease Control and Prevention.
Nationally, 6% of all outpatient visits were because of flu or flu-like illness for the week of Nov. 13-19, up from 5.8% the previous week, the CDC’s Influenza Division said in its weekly FluView report.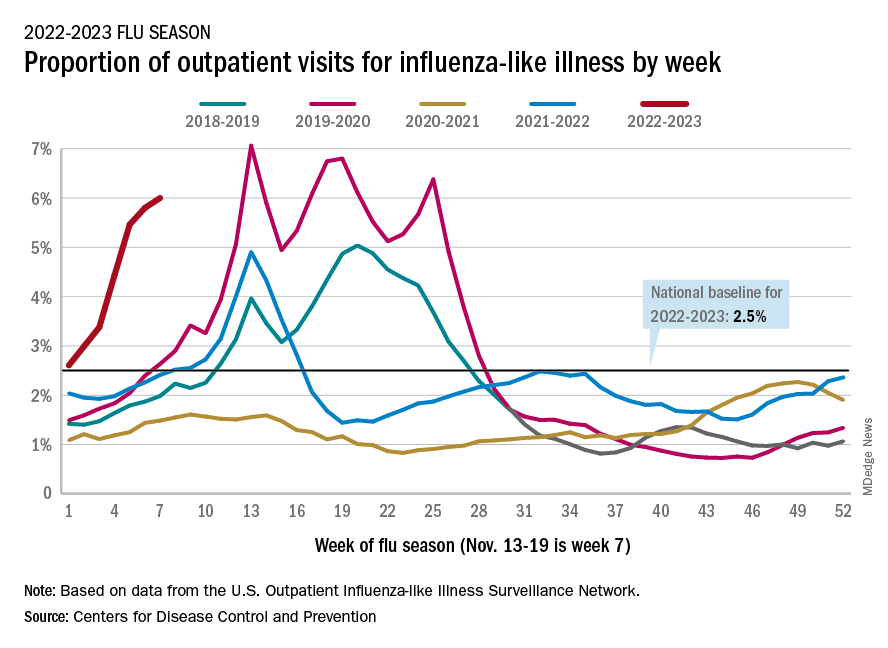
Those figures are the highest recorded in November since 2009, but the peak of the 2009-10 flu season occurred even earlier – the week of Oct. 18-24 – and the rate of flu-like illness had already dropped to just over 4.0% by Nov. 15-21 that year and continued to drop thereafter.
Although COVID-19 and respiratory syncytial virus (RSV) are included in the data from the CDC’s Outpatient Influenza-like Illness Surveillance Network, the agency did note that “seasonal influenza activity is elevated across the country” and estimated that “there have been at least 6.2 million illnesses, 53,000 hospitalizations, and 2,900 deaths from flu” during the 2022-23 season.
Total flu deaths include 11 reported in children as of Nov. 19, and children ages 0-4 had a higher proportion of visits for flu like-illness than other age groups.
The agency also said the cumulative hospitalization rate of 11.3 per 100,000 population “is higher than the rate observed in [the corresponding week of] every previous season since 2010-2011.” Adults 65 years and older have the highest cumulative rate, 25.9 per 100,000, for this year, compared with 20.7 for children 0-4; 11.1 for adults 50-64; 10.3 for children 5-17; and 5.6 for adults 18-49 years old, the CDC said.
A version of this article first appeared on WebMD.com.
according to the Centers of Disease Control and Prevention.
Nationally, 6% of all outpatient visits were because of flu or flu-like illness for the week of Nov. 13-19, up from 5.8% the previous week, the CDC’s Influenza Division said in its weekly FluView report.
Those figures are the highest recorded in November since 2009, but the peak of the 2009-10 flu season occurred even earlier – the week of Oct. 18-24 – and the rate of flu-like illness had already dropped to just over 4.0% by Nov. 15-21 that year and continued to drop thereafter.
Although COVID-19 and respiratory syncytial virus (RSV) are included in the data from the CDC’s Outpatient Influenza-like Illness Surveillance Network, the agency did note that “seasonal influenza activity is elevated across the country” and estimated that “there have been at least 6.2 million illnesses, 53,000 hospitalizations, and 2,900 deaths from flu” during the 2022-23 season.
Total flu deaths include 11 reported in children as of Nov. 19, and children ages 0-4 had a higher proportion of visits for flu like-illness than other age groups.
The agency also said the cumulative hospitalization rate of 11.3 per 100,000 population “is higher than the rate observed in [the corresponding week of] every previous season since 2010-2011.” Adults 65 years and older have the highest cumulative rate, 25.9 per 100,000, for this year, compared with 20.7 for children 0-4; 11.1 for adults 50-64; 10.3 for children 5-17; and 5.6 for adults 18-49 years old, the CDC said.
A version of this article first appeared on WebMD.com.
according to the Centers of Disease Control and Prevention.
Nationally, 6% of all outpatient visits were because of flu or flu-like illness for the week of Nov. 13-19, up from 5.8% the previous week, the CDC’s Influenza Division said in its weekly FluView report.
Those figures are the highest recorded in November since 2009, but the peak of the 2009-10 flu season occurred even earlier – the week of Oct. 18-24 – and the rate of flu-like illness had already dropped to just over 4.0% by Nov. 15-21 that year and continued to drop thereafter.
Although COVID-19 and respiratory syncytial virus (RSV) are included in the data from the CDC’s Outpatient Influenza-like Illness Surveillance Network, the agency did note that “seasonal influenza activity is elevated across the country” and estimated that “there have been at least 6.2 million illnesses, 53,000 hospitalizations, and 2,900 deaths from flu” during the 2022-23 season.
Total flu deaths include 11 reported in children as of Nov. 19, and children ages 0-4 had a higher proportion of visits for flu like-illness than other age groups.
The agency also said the cumulative hospitalization rate of 11.3 per 100,000 population “is higher than the rate observed in [the corresponding week of] every previous season since 2010-2011.” Adults 65 years and older have the highest cumulative rate, 25.9 per 100,000, for this year, compared with 20.7 for children 0-4; 11.1 for adults 50-64; 10.3 for children 5-17; and 5.6 for adults 18-49 years old, the CDC said.
A version of this article first appeared on WebMD.com.
Commentary: COVID vaccines and combination therapy in RA, December 2022
Several studies have addressed the efficacy of COVID vaccines in patients with autoimmune and rheumatic diseases owing to the concern for possible reduced vaccine immunogenicity in patients who are immunocompromised or taking immunosuppressive medications. With the availability of additional booster doses for the COVID vaccines, the effect of immunosuppressive medications on the humoral response to mRNA vaccines has been of increased interest in terms of counseling patients on how to manage their medications and vaccine timing. Prior studies have suggested that holding methotrexate after COVID vaccine administration improves antibody response to the COVID vaccine. Stahl and colleagues performed a retrospective study to look at vaccine response to a third (booster) dose in patients with rheumatoid arthritis (RA) over 65 years of age (vs those under 65 years of age) and found that patients over 65 receiving methotrexate had lower levels of neutralizing antibodies than did those receiving other treatments for RA, whereas no differences were seen with different treatments among patients under 65. Although COVID vaccine guidance continues to evolve, this finding raises the possibility of a need to tailor guidance in older patients with RA. However, the finding is far from conclusive given the small number of patients in the study.
In addition to COVID vaccine efficacy, the possibility of vaccine-related adverse effects has been a topic of concern in the rheumatology community, especially regarding the potential for a flare of autoimmune diseases. Anxiety regarding adverse effects may also exacerbate COVID vaccine hesitancy among some people. Naveen and colleagues performed an online international cross-sectional survey study regarding rheumatic diseases and 7-day adverse events. Over 9000 people completed the survey, about half of whom had autoimmune diseases (including nonrheumatic autoimmune diseases). Roughly three quarters of patients with RA reported adverse effects, with differences seen in frequencies of events between the different vaccine manufacturers. However, the majority of these adverse effects were minor (such as fatigue, headache, and body ache), without substantial differences between those with inactive and those with active RA.
The treat-to-target strategy is well-accepted in treatment of RA and pursuit of improved long-term disease outcomes. Hartman and colleagues evaluated the effect of using a treat-to-target strategy starting with the combination therapy with RA-light (COBRA-light) protocol (with initial methotrexate and tapering prednisolone) in early RA. Patients who were deemed at high risk for worsening RA (n = 150) received COBRA-light, whereas those in the low-risk category (n = 40) received methotrexate monotherapy. At 13 weeks, nonresponders were randomized to intensification or continuation of their regimens, with a primary endpoint of European Alliance of Associations for Rheumatology (EULAR) response and secondary endpoint of Disease Activity Score (DAS44). After 13 weeks, 73% of patients in the high-risk category achieved the targets of EULAR good response and DAS44 < 1.6, whereas after 26 weeks, 80% of patients who received intensified therapy and 44% of those who continued their regimens reached the target, though these numbers were small. Overall, the strategy appears to be successful in treatment of early RA in patients at high risk for disease progression, but it does lead to increased use of higher chronic glucocorticoid doses. In addition, the small numbers of patients in the low-risk category, as well as their less aggressive treatment, does not allow for a nuanced analysis of the best initial treatment in this group of patients.
Finally, a cohort study by Takanashi and colleagues evaluated the rates of seropositivity for rheumatoid factor (RF) and anti–cyclic citrullinated peptide (anti-CCP) in patients diagnosed with RA and its association with demographic categories. Seropositivity was associated with smoking and family history of RA, as expected. Among the 1685 patients, 83% of whom were women, older age at RA diagnosis was associated with seronegativity for RF and CCP in women but not in men. The decline in seropositivity with age among women cannot be further evaluated with the limited information in this small study and may have to do with other factors, including erosive or inflammatory osteoarthritis.
Several studies have addressed the efficacy of COVID vaccines in patients with autoimmune and rheumatic diseases owing to the concern for possible reduced vaccine immunogenicity in patients who are immunocompromised or taking immunosuppressive medications. With the availability of additional booster doses for the COVID vaccines, the effect of immunosuppressive medications on the humoral response to mRNA vaccines has been of increased interest in terms of counseling patients on how to manage their medications and vaccine timing. Prior studies have suggested that holding methotrexate after COVID vaccine administration improves antibody response to the COVID vaccine. Stahl and colleagues performed a retrospective study to look at vaccine response to a third (booster) dose in patients with rheumatoid arthritis (RA) over 65 years of age (vs those under 65 years of age) and found that patients over 65 receiving methotrexate had lower levels of neutralizing antibodies than did those receiving other treatments for RA, whereas no differences were seen with different treatments among patients under 65. Although COVID vaccine guidance continues to evolve, this finding raises the possibility of a need to tailor guidance in older patients with RA. However, the finding is far from conclusive given the small number of patients in the study.
In addition to COVID vaccine efficacy, the possibility of vaccine-related adverse effects has been a topic of concern in the rheumatology community, especially regarding the potential for a flare of autoimmune diseases. Anxiety regarding adverse effects may also exacerbate COVID vaccine hesitancy among some people. Naveen and colleagues performed an online international cross-sectional survey study regarding rheumatic diseases and 7-day adverse events. Over 9000 people completed the survey, about half of whom had autoimmune diseases (including nonrheumatic autoimmune diseases). Roughly three quarters of patients with RA reported adverse effects, with differences seen in frequencies of events between the different vaccine manufacturers. However, the majority of these adverse effects were minor (such as fatigue, headache, and body ache), without substantial differences between those with inactive and those with active RA.
The treat-to-target strategy is well-accepted in treatment of RA and pursuit of improved long-term disease outcomes. Hartman and colleagues evaluated the effect of using a treat-to-target strategy starting with the combination therapy with RA-light (COBRA-light) protocol (with initial methotrexate and tapering prednisolone) in early RA. Patients who were deemed at high risk for worsening RA (n = 150) received COBRA-light, whereas those in the low-risk category (n = 40) received methotrexate monotherapy. At 13 weeks, nonresponders were randomized to intensification or continuation of their regimens, with a primary endpoint of European Alliance of Associations for Rheumatology (EULAR) response and secondary endpoint of Disease Activity Score (DAS44). After 13 weeks, 73% of patients in the high-risk category achieved the targets of EULAR good response and DAS44 < 1.6, whereas after 26 weeks, 80% of patients who received intensified therapy and 44% of those who continued their regimens reached the target, though these numbers were small. Overall, the strategy appears to be successful in treatment of early RA in patients at high risk for disease progression, but it does lead to increased use of higher chronic glucocorticoid doses. In addition, the small numbers of patients in the low-risk category, as well as their less aggressive treatment, does not allow for a nuanced analysis of the best initial treatment in this group of patients.
Finally, a cohort study by Takanashi and colleagues evaluated the rates of seropositivity for rheumatoid factor (RF) and anti–cyclic citrullinated peptide (anti-CCP) in patients diagnosed with RA and its association with demographic categories. Seropositivity was associated with smoking and family history of RA, as expected. Among the 1685 patients, 83% of whom were women, older age at RA diagnosis was associated with seronegativity for RF and CCP in women but not in men. The decline in seropositivity with age among women cannot be further evaluated with the limited information in this small study and may have to do with other factors, including erosive or inflammatory osteoarthritis.
Several studies have addressed the efficacy of COVID vaccines in patients with autoimmune and rheumatic diseases owing to the concern for possible reduced vaccine immunogenicity in patients who are immunocompromised or taking immunosuppressive medications. With the availability of additional booster doses for the COVID vaccines, the effect of immunosuppressive medications on the humoral response to mRNA vaccines has been of increased interest in terms of counseling patients on how to manage their medications and vaccine timing. Prior studies have suggested that holding methotrexate after COVID vaccine administration improves antibody response to the COVID vaccine. Stahl and colleagues performed a retrospective study to look at vaccine response to a third (booster) dose in patients with rheumatoid arthritis (RA) over 65 years of age (vs those under 65 years of age) and found that patients over 65 receiving methotrexate had lower levels of neutralizing antibodies than did those receiving other treatments for RA, whereas no differences were seen with different treatments among patients under 65. Although COVID vaccine guidance continues to evolve, this finding raises the possibility of a need to tailor guidance in older patients with RA. However, the finding is far from conclusive given the small number of patients in the study.
In addition to COVID vaccine efficacy, the possibility of vaccine-related adverse effects has been a topic of concern in the rheumatology community, especially regarding the potential for a flare of autoimmune diseases. Anxiety regarding adverse effects may also exacerbate COVID vaccine hesitancy among some people. Naveen and colleagues performed an online international cross-sectional survey study regarding rheumatic diseases and 7-day adverse events. Over 9000 people completed the survey, about half of whom had autoimmune diseases (including nonrheumatic autoimmune diseases). Roughly three quarters of patients with RA reported adverse effects, with differences seen in frequencies of events between the different vaccine manufacturers. However, the majority of these adverse effects were minor (such as fatigue, headache, and body ache), without substantial differences between those with inactive and those with active RA.
The treat-to-target strategy is well-accepted in treatment of RA and pursuit of improved long-term disease outcomes. Hartman and colleagues evaluated the effect of using a treat-to-target strategy starting with the combination therapy with RA-light (COBRA-light) protocol (with initial methotrexate and tapering prednisolone) in early RA. Patients who were deemed at high risk for worsening RA (n = 150) received COBRA-light, whereas those in the low-risk category (n = 40) received methotrexate monotherapy. At 13 weeks, nonresponders were randomized to intensification or continuation of their regimens, with a primary endpoint of European Alliance of Associations for Rheumatology (EULAR) response and secondary endpoint of Disease Activity Score (DAS44). After 13 weeks, 73% of patients in the high-risk category achieved the targets of EULAR good response and DAS44 < 1.6, whereas after 26 weeks, 80% of patients who received intensified therapy and 44% of those who continued their regimens reached the target, though these numbers were small. Overall, the strategy appears to be successful in treatment of early RA in patients at high risk for disease progression, but it does lead to increased use of higher chronic glucocorticoid doses. In addition, the small numbers of patients in the low-risk category, as well as their less aggressive treatment, does not allow for a nuanced analysis of the best initial treatment in this group of patients.
Finally, a cohort study by Takanashi and colleagues evaluated the rates of seropositivity for rheumatoid factor (RF) and anti–cyclic citrullinated peptide (anti-CCP) in patients diagnosed with RA and its association with demographic categories. Seropositivity was associated with smoking and family history of RA, as expected. Among the 1685 patients, 83% of whom were women, older age at RA diagnosis was associated with seronegativity for RF and CCP in women but not in men. The decline in seropositivity with age among women cannot be further evaluated with the limited information in this small study and may have to do with other factors, including erosive or inflammatory osteoarthritis.
Commentary: HER2-Positive EGA, Immunotherapy With Chemoradiation, and Lymph Node Metastasis in GC, December 2022
A study by Hofheinz and colleagues evaluated whether targeting the human epidermal growth factor receptor 2 (HER2) pathway can improve outcomes in early-stage esophagogastric adenocarcinoma (EGA).1 About 15%-20% of EGA overexpress HER2. In the metastatic setting, anti-HER2 therapies have an established role. Trastuzumab in combination with chemotherapy has been part of standard treatment for these tumors for over a decade and now immunotherapy, based on the ongoing KEYNOTE-811 study, has been proven effective as well.2,3 However, prior attempts to effectively target HER2 in the early stage have not been successful.4
A phase 2 trial conducted by the AIO EGA Study Group evaluated the addition of trastuzumab and pertuzumab to FLOT (fluorouracil, leucovorin, oxaliplatin, docetaxel) chemotherapy in resectable HER2-positive EGA. The trial closed early, before planned full accrual, when the results of the JACOB trial, which evaluated the addition of pertuzumab in the metastatic setting, came out as negative.5 However, the results are still worth discussing here. A total of 81 patients were enrolled in this study (40 in the experimental arm and 41 in the chemotherapy-only control arm). Pathologic complete response was significantly improved with the addition of anti-HER2 therapy (35% vs 12%; P = .02). The rates of R0 resection and surgical complications were similar. Median disease-free survival was not reached in the experimental arm (26 months in the control arm). This study suggests that evaluation of anti-HER2 agents in combination with chemotherapy is warranted. Given the promising results of the KEYNOTE-811 study, future studies should consider incorporative immunotherapy as well.
The Neo-PLANET phase 2 study by Tang and colleagues evaluated the addition of camrelizumab (anti-PD1 antibody) to concurrent chemoradiation in the treatment of advanced EGA.6 The 36 patients enrolled in this study received induction chemotherapy with capecitabine and oxaliplatin followed by chemoradiation with the same chemotherapy backbone. Camrelizumab was added from the time of all chemotherapy initiation. The pathologic complete response observed (33.3%) compared favorably to historical references. No new safety signals were identified. Current standards for resectable EGA include adjuvant nivolumab in patients who have residual disease at the time of resection post chemoradiation. This study demonstrated that the addition of immunotherapy earlier in the treatment course is safe and possibly efficacious. The prospective randomized phase 2/3 ECOG-ACRIN 2174 study will evaluate the addition of nivolumab to chemoradiation, as well as the addition of ipilimumab to nivolumab in the adjuvant setting, and will answer the question regarding the benefits of immunotherapy used earlier in the course of disease in a prospective randomized manner (NCT03604991).
The study by de Jongh and colleagues evaluated the pattern of lymph node metastasis after neoadjuvant chemotherapy with relation to the location of the primary gastric tumor. Tumors from 212 patients who were previously enrolled in the Dutch LOGICA trial comparing laparoscopy vs open D2 gastrectomy were included in this analysis. Although the primary tumor location (proximal vs distal) was associated with a higher frequency of metastasis to certain lymph node groups, this relationship was not exclusive. As such, the extent of lymphadenectomy should not depend on the primary location of the tumor and is not affected by neoadjuvant chemotherapy.
Additional References
1. Hofheinz R-D, Merx K, Haag GM, et al. FLOT versus FLOT/trastuzumab/pertuzumab perioperative therapy of human epidermal growth factor receptor 2–positive resectable esophagogastric adenocarcinoma: A randomized phase II trial of the AIO EGA Study Group. J Clin Oncol. 2022;40:3750-3761. Doi: 10.1200/JCO.22.00380
2. Janjigian YY, Kawazoe A, Yañez P, et al. The KEYNOTE-811 trial of dual PD-1 and HER2 blockade in HER2-positive gastric cancer. Nature. 2021;600:727-730. Doi: 10.1038/s41586-021-04161-3
3. Bang Y-J, Van Cutsem E, Feyereislova A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): A phase 3, open-label, randomised controlled trial. Lancet. 2010;376:687-697. Doi: 10.1016/S0140-6736(10)61121-X
4. Safran HP, Winter K, Hilson D, et al. Trastuzumab with trimodality treatment for oesophageal adenocarcinoma with HER2 overexpression (NRG Oncology/RTOG 1010): A multicentre, randomised, phase 3 trial. Lancet Oncol. 2022;23:259-269. Doi: 10.1016/S1470-2045(21)00718-X
5. Tabernero J, Hoff PM, Shen L, et al. Pertuzumab plus trastuzumab and chemotherapy for HER2-positive metastatic gastric or gastro-oesophageal junction cancer (JACOB): Final analysis of a double-blind, randomised, placebo-controlled phase 3 study. Lancet Oncol. 2018:19:1372-1384. Doi: 10.1016/S1470-2045(18)30481-9
6. Tang Z, Wang Y, Liu D, et al. The Neo-PLANET phase II trial of neoadjuvant camrelizumab plus concurrent chemoradiotherapy in locally advanced adenocarcinoma of stomach or gastroesophageal junction. Nat Commun 2022;13:6807. Doi: 10.1038/s41467-022-34403-5
A study by Hofheinz and colleagues evaluated whether targeting the human epidermal growth factor receptor 2 (HER2) pathway can improve outcomes in early-stage esophagogastric adenocarcinoma (EGA).1 About 15%-20% of EGA overexpress HER2. In the metastatic setting, anti-HER2 therapies have an established role. Trastuzumab in combination with chemotherapy has been part of standard treatment for these tumors for over a decade and now immunotherapy, based on the ongoing KEYNOTE-811 study, has been proven effective as well.2,3 However, prior attempts to effectively target HER2 in the early stage have not been successful.4
A phase 2 trial conducted by the AIO EGA Study Group evaluated the addition of trastuzumab and pertuzumab to FLOT (fluorouracil, leucovorin, oxaliplatin, docetaxel) chemotherapy in resectable HER2-positive EGA. The trial closed early, before planned full accrual, when the results of the JACOB trial, which evaluated the addition of pertuzumab in the metastatic setting, came out as negative.5 However, the results are still worth discussing here. A total of 81 patients were enrolled in this study (40 in the experimental arm and 41 in the chemotherapy-only control arm). Pathologic complete response was significantly improved with the addition of anti-HER2 therapy (35% vs 12%; P = .02). The rates of R0 resection and surgical complications were similar. Median disease-free survival was not reached in the experimental arm (26 months in the control arm). This study suggests that evaluation of anti-HER2 agents in combination with chemotherapy is warranted. Given the promising results of the KEYNOTE-811 study, future studies should consider incorporative immunotherapy as well.
The Neo-PLANET phase 2 study by Tang and colleagues evaluated the addition of camrelizumab (anti-PD1 antibody) to concurrent chemoradiation in the treatment of advanced EGA.6 The 36 patients enrolled in this study received induction chemotherapy with capecitabine and oxaliplatin followed by chemoradiation with the same chemotherapy backbone. Camrelizumab was added from the time of all chemotherapy initiation. The pathologic complete response observed (33.3%) compared favorably to historical references. No new safety signals were identified. Current standards for resectable EGA include adjuvant nivolumab in patients who have residual disease at the time of resection post chemoradiation. This study demonstrated that the addition of immunotherapy earlier in the treatment course is safe and possibly efficacious. The prospective randomized phase 2/3 ECOG-ACRIN 2174 study will evaluate the addition of nivolumab to chemoradiation, as well as the addition of ipilimumab to nivolumab in the adjuvant setting, and will answer the question regarding the benefits of immunotherapy used earlier in the course of disease in a prospective randomized manner (NCT03604991).
The study by de Jongh and colleagues evaluated the pattern of lymph node metastasis after neoadjuvant chemotherapy with relation to the location of the primary gastric tumor. Tumors from 212 patients who were previously enrolled in the Dutch LOGICA trial comparing laparoscopy vs open D2 gastrectomy were included in this analysis. Although the primary tumor location (proximal vs distal) was associated with a higher frequency of metastasis to certain lymph node groups, this relationship was not exclusive. As such, the extent of lymphadenectomy should not depend on the primary location of the tumor and is not affected by neoadjuvant chemotherapy.
Additional References
1. Hofheinz R-D, Merx K, Haag GM, et al. FLOT versus FLOT/trastuzumab/pertuzumab perioperative therapy of human epidermal growth factor receptor 2–positive resectable esophagogastric adenocarcinoma: A randomized phase II trial of the AIO EGA Study Group. J Clin Oncol. 2022;40:3750-3761. Doi: 10.1200/JCO.22.00380
2. Janjigian YY, Kawazoe A, Yañez P, et al. The KEYNOTE-811 trial of dual PD-1 and HER2 blockade in HER2-positive gastric cancer. Nature. 2021;600:727-730. Doi: 10.1038/s41586-021-04161-3
3. Bang Y-J, Van Cutsem E, Feyereislova A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): A phase 3, open-label, randomised controlled trial. Lancet. 2010;376:687-697. Doi: 10.1016/S0140-6736(10)61121-X
4. Safran HP, Winter K, Hilson D, et al. Trastuzumab with trimodality treatment for oesophageal adenocarcinoma with HER2 overexpression (NRG Oncology/RTOG 1010): A multicentre, randomised, phase 3 trial. Lancet Oncol. 2022;23:259-269. Doi: 10.1016/S1470-2045(21)00718-X
5. Tabernero J, Hoff PM, Shen L, et al. Pertuzumab plus trastuzumab and chemotherapy for HER2-positive metastatic gastric or gastro-oesophageal junction cancer (JACOB): Final analysis of a double-blind, randomised, placebo-controlled phase 3 study. Lancet Oncol. 2018:19:1372-1384. Doi: 10.1016/S1470-2045(18)30481-9
6. Tang Z, Wang Y, Liu D, et al. The Neo-PLANET phase II trial of neoadjuvant camrelizumab plus concurrent chemoradiotherapy in locally advanced adenocarcinoma of stomach or gastroesophageal junction. Nat Commun 2022;13:6807. Doi: 10.1038/s41467-022-34403-5
A study by Hofheinz and colleagues evaluated whether targeting the human epidermal growth factor receptor 2 (HER2) pathway can improve outcomes in early-stage esophagogastric adenocarcinoma (EGA).1 About 15%-20% of EGA overexpress HER2. In the metastatic setting, anti-HER2 therapies have an established role. Trastuzumab in combination with chemotherapy has been part of standard treatment for these tumors for over a decade and now immunotherapy, based on the ongoing KEYNOTE-811 study, has been proven effective as well.2,3 However, prior attempts to effectively target HER2 in the early stage have not been successful.4
A phase 2 trial conducted by the AIO EGA Study Group evaluated the addition of trastuzumab and pertuzumab to FLOT (fluorouracil, leucovorin, oxaliplatin, docetaxel) chemotherapy in resectable HER2-positive EGA. The trial closed early, before planned full accrual, when the results of the JACOB trial, which evaluated the addition of pertuzumab in the metastatic setting, came out as negative.5 However, the results are still worth discussing here. A total of 81 patients were enrolled in this study (40 in the experimental arm and 41 in the chemotherapy-only control arm). Pathologic complete response was significantly improved with the addition of anti-HER2 therapy (35% vs 12%; P = .02). The rates of R0 resection and surgical complications were similar. Median disease-free survival was not reached in the experimental arm (26 months in the control arm). This study suggests that evaluation of anti-HER2 agents in combination with chemotherapy is warranted. Given the promising results of the KEYNOTE-811 study, future studies should consider incorporative immunotherapy as well.
The Neo-PLANET phase 2 study by Tang and colleagues evaluated the addition of camrelizumab (anti-PD1 antibody) to concurrent chemoradiation in the treatment of advanced EGA.6 The 36 patients enrolled in this study received induction chemotherapy with capecitabine and oxaliplatin followed by chemoradiation with the same chemotherapy backbone. Camrelizumab was added from the time of all chemotherapy initiation. The pathologic complete response observed (33.3%) compared favorably to historical references. No new safety signals were identified. Current standards for resectable EGA include adjuvant nivolumab in patients who have residual disease at the time of resection post chemoradiation. This study demonstrated that the addition of immunotherapy earlier in the treatment course is safe and possibly efficacious. The prospective randomized phase 2/3 ECOG-ACRIN 2174 study will evaluate the addition of nivolumab to chemoradiation, as well as the addition of ipilimumab to nivolumab in the adjuvant setting, and will answer the question regarding the benefits of immunotherapy used earlier in the course of disease in a prospective randomized manner (NCT03604991).
The study by de Jongh and colleagues evaluated the pattern of lymph node metastasis after neoadjuvant chemotherapy with relation to the location of the primary gastric tumor. Tumors from 212 patients who were previously enrolled in the Dutch LOGICA trial comparing laparoscopy vs open D2 gastrectomy were included in this analysis. Although the primary tumor location (proximal vs distal) was associated with a higher frequency of metastasis to certain lymph node groups, this relationship was not exclusive. As such, the extent of lymphadenectomy should not depend on the primary location of the tumor and is not affected by neoadjuvant chemotherapy.
Additional References
1. Hofheinz R-D, Merx K, Haag GM, et al. FLOT versus FLOT/trastuzumab/pertuzumab perioperative therapy of human epidermal growth factor receptor 2–positive resectable esophagogastric adenocarcinoma: A randomized phase II trial of the AIO EGA Study Group. J Clin Oncol. 2022;40:3750-3761. Doi: 10.1200/JCO.22.00380
2. Janjigian YY, Kawazoe A, Yañez P, et al. The KEYNOTE-811 trial of dual PD-1 and HER2 blockade in HER2-positive gastric cancer. Nature. 2021;600:727-730. Doi: 10.1038/s41586-021-04161-3
3. Bang Y-J, Van Cutsem E, Feyereislova A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): A phase 3, open-label, randomised controlled trial. Lancet. 2010;376:687-697. Doi: 10.1016/S0140-6736(10)61121-X
4. Safran HP, Winter K, Hilson D, et al. Trastuzumab with trimodality treatment for oesophageal adenocarcinoma with HER2 overexpression (NRG Oncology/RTOG 1010): A multicentre, randomised, phase 3 trial. Lancet Oncol. 2022;23:259-269. Doi: 10.1016/S1470-2045(21)00718-X
5. Tabernero J, Hoff PM, Shen L, et al. Pertuzumab plus trastuzumab and chemotherapy for HER2-positive metastatic gastric or gastro-oesophageal junction cancer (JACOB): Final analysis of a double-blind, randomised, placebo-controlled phase 3 study. Lancet Oncol. 2018:19:1372-1384. Doi: 10.1016/S1470-2045(18)30481-9
6. Tang Z, Wang Y, Liu D, et al. The Neo-PLANET phase II trial of neoadjuvant camrelizumab plus concurrent chemoradiotherapy in locally advanced adenocarcinoma of stomach or gastroesophageal junction. Nat Commun 2022;13:6807. Doi: 10.1038/s41467-022-34403-5
Advancing health equity in neurology is essential to patient care
Black and Latinx older adults are up to three times as likely to develop Alzheimer’s disease than non-Latinx White adults and tend to experience onset at a younger age with more severe symptoms, according to Monica Rivera-Mindt, PhD, a professor of psychology at Fordham University and the Icahn School of Medicine at Mount Sinai, New York. Looking ahead, that means by 2030, nearly 40% of the 8.4 million Americans affected by Alzheimer’s disease will be Black and/or Latinx, she said. These facts were among the stark disparities in health care outcomes Dr. Rivera-Mindt discussed in her presentation on brain health equity at the 2022 annual meeting of the American Neurological Association.
Dr. Rivera-Mindt’s presentation opened the ANA’s plenary session on health disparities and inequities. The plenary, “Advancing Neurologic Equity: Challenges and Paths Forward,” did not simply enumerate racial and ethnic disparities that exist with various neurological conditions. Rather it went beyond the discussion of what disparities exist into understanding the roots of them as well as tips, tools, and resources that can aid clinicians in addressing or ameliorating them.
Roy Hamilton, MD, an associate professor of neurology and physical medicine and rehabilitation at the University of Pennsylvania, Philadelphia, said. “If clinicians are unaware of these disparities or don’t have any sense of how to start to address or think about them, then they’re really missing out on an important component of their education as persons who take care of patients with brain disorders.”
Dr. Hamilton, who organized the plenary, noted that awareness of these disparities is crucial to comprehensively caring for patients.
Missed opportunities
“We’re talking about disadvantages that are structural and large scale, but those disadvantages play themselves out in the individual encounter,” Dr. Hamilton said. “When physicians see patients, they have to treat the whole patient in front of them,” which means being aware of the risks and factors that could affect a patient’s clinical presentation. “Being aware of disparities has practical impacts on physician judgment,” he said.
For example, recent research in multiple sclerosis (MS) has highlighted how clinicians may be missing diagnosis of this condition in non-White populations because the condition has been regarded for so long as a “White person’s” disease, Dr. Hamilton said. In non-White patients exhibiting MS symptoms, then, clinicians may have been less likely to consider MS as a possibility, thereby delaying diagnosis and treatment.
Those patterns may partly explain why the mortality rate for MS is greater in Black patients, who also show more rapid neurodegeneration than White patients with MS, Lilyana Amezcua, MD, an associate professor of neurology at the University of Southern California, Los Angeles, reported in the plenary’s second presentation.
Transgender issues
The third session, presented by Nicole Rosendale, MD, an assistant professor of neurology at the University of California, San Francisco, and director of the San Francisco General Hospital neurology inpatient services, examined disparities in neurology within the LGBTQ+ community through representative case studies and then offered specific ways that neurologists could make their practices more inclusive and equitable for sexual and gender minorities.
Her first case study was a 52-year-old man who presented with new-onset seizures, right hemiparesis, and aphasia. A brain biopsy consistent with adenocarcinoma eventually led his physician to discover he had metastatic breast cancer. It turned out the man was transgender and, despite a family history of breast cancer, hadn’t been advised to get breast cancer screenings.
“Breast cancer was not initially on the differential as no one had identified that the patient was transmasculine,” Dr. Rosendale said. A major challenge to providing care to transgender patients is a dearth of data on risks and screening recommendations. Another barrier is low knowledge of LGBTQ+ health among neurologists, Dr. Rosendale said while sharing findings from her 2019 study on the topic and calling for more research in LGBTQ+ populations.
Dr. Rosendale’s second case study dealt with a nonbinary patient who suffered from debilitating headaches for decades, first because they lacked access to health insurance and then because negative experiences with providers dissuaded them from seeking care. In data from the Center for American Progress she shared, 8% of LGB respondents and 22% of transgender respondents said they had avoided or delayed care because of fear of discrimination or mistreatment.
“So it’s not only access but also what experiences people are having when they go in and whether they’re actually even getting access to care or being taken care of,” Dr. Rosendale said. Other findings from the CAP found that:
- 8% of LGB patients and 29% of transgender patients reported having a clinician refuse to see them.
- 6% of LGB patients and 12% of transgender patients reported that a clinician refused to give them health care.
- 9% of LGB patients and 21% of transgender patients experienced harsh or abusive language during a health care experience.
- 7% of LGB patients and nearly a third (29%) of transgender patients experienced unwanted physical contact, such as fondling or sexual assault.
Reducing the disparities
Adys Mendizabal, MD, an assistant professor of neurology at the Institute of Society and Genetics at the University of California, Los Angeles, who attended the presentation, was grateful to see how the various lectures enriched the discussion beyond stating the fact of racial/ethnic disparities and dug into the nuances on how to think about and address these disparities. She particularly appreciated discussion about the need to go out of the way to recruit diverse patient populations for clinical trials while also providing them care.
“It is definitely complicated, but it’s not impossible for an individual neurologist or an individual department to do something to reduce some of the disparities,” Dr. Mendizabal said. “It starts with just knowing that they exist and being aware of some of the things that may be impacting care for a particular patient.”
Tools to counter disparity
In the final presentation, Amy Kind, MD, PhD, the associate dean for social health sciences and programs at the University of Wisconsin–Madison, rounded out the discussion by exploring social determinants of health and their influence on outcomes.
“Social determinants impact brain health, and brain health is not distributed equally,” Dr. Kind told attendees. “We have known this for decades, yet disparities persist.”
Dr. Kind described the “exposome,” a “measure of all the exposures of an individual in a lifetime and how those exposures relate to health,” according to the CDC, and then introduced a tool clinicians can use to better understand social determinants of health in specific geographic areas. The Neighborhood Atlas, which Dr. Kind described in the New England Journal of Medicine in 2018, measures 17 social determinants across small population-sensitive areas and provides an area deprivation index. A high area deprivation index is linked to a range of negative outcomes, including reshopitalization, later diagnoses, less comprehensive diagnostic evaluation, increased risk of postsurgical complications, and decreased life expectancy.
“One of the things that really stood out to me about Dr. Kind’s discussion of the use of the area deprivation index was the fact that understanding and quantifying these kinds of risks and exposures is the vehicle for creating the kinds of social changes, including policy changes, that will actually lead to addressing and mitigating some of these lifelong risks and exposures,” Dr. Hamilton said. “It is implausible to think that a specific group of people would be genetically more susceptible to basically every disease that we know,” he added. “It makes much more sense to think that groups of individuals have been subjected systematically to conditions that impair health in a variety of ways.”
Not just race, ethnicity, sex, and gender
Following the four presentations from researchers in health inequities was an Emerging Scholar presentation in which Jay B. Lusk, an MD/MBA candidate at Duke University, Durham, N.C., shared new research findings on the role of neighborhood disadvantage in predicting mortality from coma, stroke, and other neurologic conditions. His findings revealed that living in a neighborhood with greater deprivation substantially increased risk of mortality even after accounting for individual wealth and demographics.
Maria Eugenia Diaz-Ortiz, PhD, of the department of neurology, University of Pennsylvania, Philadelphia, said she found the five presentations to be an excellent introduction to people like herself who are in the earlier stages of learning about health equity research.
“I think they introduced various important concepts and frameworks and provided tools for people who don’t know about them,” Dr. Diaz-Ortiz said. “Then they asked important questions and provided some solutions to them.”
Dr. Diaz-Ortiz also appreciated seemingly minor but actually important details in how the speakers presented themselves, such as Dr. Rivera-Mindt opening with a land acknowledgment and her disclosures of “positionality.” The former recognized the traditional Native American custodians of the land on which she lives and works, and the latter revealed details about her as an individual – such as being the Afro-Latinx daughter of immigrants yet being cisgender, able-bodied, and U.S.-born – that show where she falls on the axis of adversity and axis of privilege.
Implications for research
The biggest takeaway for Dr. Diaz-Ortiz, however, came from the first Q&A session when someone asked how to increase underrepresented populations in dementia research. Dr. Rivera-Mindt described her experience engaging these communities by employing “community-based participatory research practices, which involves making yourself a part of the community and making the community active participants in the research,” Dr. Diaz-Ortiz said. “It’s an evidence-based approach that has been shown to increase participation in research not only in her work but in the work of others.”
Preaching to the choir
Dr. Diaz-Ortiz was pleased overall with the plenary but disappointed in its placement at the end of the meeting, when attendance is always lower as attendees head home.
“The people who stayed were people who already know and recognize the value of health equity work, so I think that was a missed opportunity where the session could have been included on day one or two to boost attendance and also to educate like a broader group of neurologists,” Dr. Diaz-Ortiz said in an interview.
Dr. Mendizabal felt similarly, appreciating the plenary but noting it was “definitely overdue” and that it should not be the last session. Instead, sessions on health equity should be as easy as possible to attend to bring in larger audiences. “Perhaps having that session on a Saturday or Sunday would have a higher likelihood of greater attendance than on a Tuesday,” she said. That said, Dr. Mendizabal also noticed that greater attention to health care disparities was woven into many other sessions throughout the conference, which is “the best way of addressing health equity instead of trying to just designate a session,” she said.
Dr. Mendizabal hopes that plenaries like this one and the weaving of health equity issues into presentations throughout neurology conferences continue.
“After the racial reckoning in 2020, there was a big impetus and a big wave of energy in addressing health disparities in the field, and I hope that that momentum is not starting to wane,” Dr. Mendizabal said. “It’s important because not talking about is not going to make this issue go away.”
Dr. Hamilton agreed that it is important that the conversation continue and that physicians recognize the importance of understanding health care disparities and determinants of health, regardless of where they fall on the political spectrum or whether they choose to get involved in policy or advocacy.
“Irrespective of whether you think race or ethnicity or socioeconomic status are political issues or not, it is the case that you’re obligated to have an objective understanding of the factors that contribute to your patient’s health and as points of intervention,” Dr. Hamilton said. “So even if you don’t want to sit down and jot off that email to your senator, you still have to take these factors into account when you’re treating the person who’s sitting right in front of you, and that’s not political. That’s the promise of being a physician.”
Dr. Amezcua has received personal compensation for consulting, speaking, or serving on steering committees or advisory boards for Biogen Idec, Novartis, Genentech, and EMD Serono, and she has received research support from Biogen Idec and Bristol Myers Squibb Foundation. Dr. Kind reported support from the Alzheimer’s Association. Dr. Diaz-Ortiz is coinventor of a provisional patent submitted by the University of Pennsylvania that relates to a potential therapeutic in Parkinson’s disease. Mr. Lusk reported fellowship support from American Heart Association and travel support from the American Neurological Association. No other speakers or sources had relevant disclosures.
Black and Latinx older adults are up to three times as likely to develop Alzheimer’s disease than non-Latinx White adults and tend to experience onset at a younger age with more severe symptoms, according to Monica Rivera-Mindt, PhD, a professor of psychology at Fordham University and the Icahn School of Medicine at Mount Sinai, New York. Looking ahead, that means by 2030, nearly 40% of the 8.4 million Americans affected by Alzheimer’s disease will be Black and/or Latinx, she said. These facts were among the stark disparities in health care outcomes Dr. Rivera-Mindt discussed in her presentation on brain health equity at the 2022 annual meeting of the American Neurological Association.
Dr. Rivera-Mindt’s presentation opened the ANA’s plenary session on health disparities and inequities. The plenary, “Advancing Neurologic Equity: Challenges and Paths Forward,” did not simply enumerate racial and ethnic disparities that exist with various neurological conditions. Rather it went beyond the discussion of what disparities exist into understanding the roots of them as well as tips, tools, and resources that can aid clinicians in addressing or ameliorating them.
Roy Hamilton, MD, an associate professor of neurology and physical medicine and rehabilitation at the University of Pennsylvania, Philadelphia, said. “If clinicians are unaware of these disparities or don’t have any sense of how to start to address or think about them, then they’re really missing out on an important component of their education as persons who take care of patients with brain disorders.”
Dr. Hamilton, who organized the plenary, noted that awareness of these disparities is crucial to comprehensively caring for patients.
Missed opportunities
“We’re talking about disadvantages that are structural and large scale, but those disadvantages play themselves out in the individual encounter,” Dr. Hamilton said. “When physicians see patients, they have to treat the whole patient in front of them,” which means being aware of the risks and factors that could affect a patient’s clinical presentation. “Being aware of disparities has practical impacts on physician judgment,” he said.
For example, recent research in multiple sclerosis (MS) has highlighted how clinicians may be missing diagnosis of this condition in non-White populations because the condition has been regarded for so long as a “White person’s” disease, Dr. Hamilton said. In non-White patients exhibiting MS symptoms, then, clinicians may have been less likely to consider MS as a possibility, thereby delaying diagnosis and treatment.
Those patterns may partly explain why the mortality rate for MS is greater in Black patients, who also show more rapid neurodegeneration than White patients with MS, Lilyana Amezcua, MD, an associate professor of neurology at the University of Southern California, Los Angeles, reported in the plenary’s second presentation.
Transgender issues
The third session, presented by Nicole Rosendale, MD, an assistant professor of neurology at the University of California, San Francisco, and director of the San Francisco General Hospital neurology inpatient services, examined disparities in neurology within the LGBTQ+ community through representative case studies and then offered specific ways that neurologists could make their practices more inclusive and equitable for sexual and gender minorities.
Her first case study was a 52-year-old man who presented with new-onset seizures, right hemiparesis, and aphasia. A brain biopsy consistent with adenocarcinoma eventually led his physician to discover he had metastatic breast cancer. It turned out the man was transgender and, despite a family history of breast cancer, hadn’t been advised to get breast cancer screenings.
“Breast cancer was not initially on the differential as no one had identified that the patient was transmasculine,” Dr. Rosendale said. A major challenge to providing care to transgender patients is a dearth of data on risks and screening recommendations. Another barrier is low knowledge of LGBTQ+ health among neurologists, Dr. Rosendale said while sharing findings from her 2019 study on the topic and calling for more research in LGBTQ+ populations.
Dr. Rosendale’s second case study dealt with a nonbinary patient who suffered from debilitating headaches for decades, first because they lacked access to health insurance and then because negative experiences with providers dissuaded them from seeking care. In data from the Center for American Progress she shared, 8% of LGB respondents and 22% of transgender respondents said they had avoided or delayed care because of fear of discrimination or mistreatment.
“So it’s not only access but also what experiences people are having when they go in and whether they’re actually even getting access to care or being taken care of,” Dr. Rosendale said. Other findings from the CAP found that:
- 8% of LGB patients and 29% of transgender patients reported having a clinician refuse to see them.
- 6% of LGB patients and 12% of transgender patients reported that a clinician refused to give them health care.
- 9% of LGB patients and 21% of transgender patients experienced harsh or abusive language during a health care experience.
- 7% of LGB patients and nearly a third (29%) of transgender patients experienced unwanted physical contact, such as fondling or sexual assault.
Reducing the disparities
Adys Mendizabal, MD, an assistant professor of neurology at the Institute of Society and Genetics at the University of California, Los Angeles, who attended the presentation, was grateful to see how the various lectures enriched the discussion beyond stating the fact of racial/ethnic disparities and dug into the nuances on how to think about and address these disparities. She particularly appreciated discussion about the need to go out of the way to recruit diverse patient populations for clinical trials while also providing them care.
“It is definitely complicated, but it’s not impossible for an individual neurologist or an individual department to do something to reduce some of the disparities,” Dr. Mendizabal said. “It starts with just knowing that they exist and being aware of some of the things that may be impacting care for a particular patient.”
Tools to counter disparity
In the final presentation, Amy Kind, MD, PhD, the associate dean for social health sciences and programs at the University of Wisconsin–Madison, rounded out the discussion by exploring social determinants of health and their influence on outcomes.
“Social determinants impact brain health, and brain health is not distributed equally,” Dr. Kind told attendees. “We have known this for decades, yet disparities persist.”
Dr. Kind described the “exposome,” a “measure of all the exposures of an individual in a lifetime and how those exposures relate to health,” according to the CDC, and then introduced a tool clinicians can use to better understand social determinants of health in specific geographic areas. The Neighborhood Atlas, which Dr. Kind described in the New England Journal of Medicine in 2018, measures 17 social determinants across small population-sensitive areas and provides an area deprivation index. A high area deprivation index is linked to a range of negative outcomes, including reshopitalization, later diagnoses, less comprehensive diagnostic evaluation, increased risk of postsurgical complications, and decreased life expectancy.
“One of the things that really stood out to me about Dr. Kind’s discussion of the use of the area deprivation index was the fact that understanding and quantifying these kinds of risks and exposures is the vehicle for creating the kinds of social changes, including policy changes, that will actually lead to addressing and mitigating some of these lifelong risks and exposures,” Dr. Hamilton said. “It is implausible to think that a specific group of people would be genetically more susceptible to basically every disease that we know,” he added. “It makes much more sense to think that groups of individuals have been subjected systematically to conditions that impair health in a variety of ways.”
Not just race, ethnicity, sex, and gender
Following the four presentations from researchers in health inequities was an Emerging Scholar presentation in which Jay B. Lusk, an MD/MBA candidate at Duke University, Durham, N.C., shared new research findings on the role of neighborhood disadvantage in predicting mortality from coma, stroke, and other neurologic conditions. His findings revealed that living in a neighborhood with greater deprivation substantially increased risk of mortality even after accounting for individual wealth and demographics.
Maria Eugenia Diaz-Ortiz, PhD, of the department of neurology, University of Pennsylvania, Philadelphia, said she found the five presentations to be an excellent introduction to people like herself who are in the earlier stages of learning about health equity research.
“I think they introduced various important concepts and frameworks and provided tools for people who don’t know about them,” Dr. Diaz-Ortiz said. “Then they asked important questions and provided some solutions to them.”
Dr. Diaz-Ortiz also appreciated seemingly minor but actually important details in how the speakers presented themselves, such as Dr. Rivera-Mindt opening with a land acknowledgment and her disclosures of “positionality.” The former recognized the traditional Native American custodians of the land on which she lives and works, and the latter revealed details about her as an individual – such as being the Afro-Latinx daughter of immigrants yet being cisgender, able-bodied, and U.S.-born – that show where she falls on the axis of adversity and axis of privilege.
Implications for research
The biggest takeaway for Dr. Diaz-Ortiz, however, came from the first Q&A session when someone asked how to increase underrepresented populations in dementia research. Dr. Rivera-Mindt described her experience engaging these communities by employing “community-based participatory research practices, which involves making yourself a part of the community and making the community active participants in the research,” Dr. Diaz-Ortiz said. “It’s an evidence-based approach that has been shown to increase participation in research not only in her work but in the work of others.”
Preaching to the choir
Dr. Diaz-Ortiz was pleased overall with the plenary but disappointed in its placement at the end of the meeting, when attendance is always lower as attendees head home.
“The people who stayed were people who already know and recognize the value of health equity work, so I think that was a missed opportunity where the session could have been included on day one or two to boost attendance and also to educate like a broader group of neurologists,” Dr. Diaz-Ortiz said in an interview.
Dr. Mendizabal felt similarly, appreciating the plenary but noting it was “definitely overdue” and that it should not be the last session. Instead, sessions on health equity should be as easy as possible to attend to bring in larger audiences. “Perhaps having that session on a Saturday or Sunday would have a higher likelihood of greater attendance than on a Tuesday,” she said. That said, Dr. Mendizabal also noticed that greater attention to health care disparities was woven into many other sessions throughout the conference, which is “the best way of addressing health equity instead of trying to just designate a session,” she said.
Dr. Mendizabal hopes that plenaries like this one and the weaving of health equity issues into presentations throughout neurology conferences continue.
“After the racial reckoning in 2020, there was a big impetus and a big wave of energy in addressing health disparities in the field, and I hope that that momentum is not starting to wane,” Dr. Mendizabal said. “It’s important because not talking about is not going to make this issue go away.”
Dr. Hamilton agreed that it is important that the conversation continue and that physicians recognize the importance of understanding health care disparities and determinants of health, regardless of where they fall on the political spectrum or whether they choose to get involved in policy or advocacy.
“Irrespective of whether you think race or ethnicity or socioeconomic status are political issues or not, it is the case that you’re obligated to have an objective understanding of the factors that contribute to your patient’s health and as points of intervention,” Dr. Hamilton said. “So even if you don’t want to sit down and jot off that email to your senator, you still have to take these factors into account when you’re treating the person who’s sitting right in front of you, and that’s not political. That’s the promise of being a physician.”
Dr. Amezcua has received personal compensation for consulting, speaking, or serving on steering committees or advisory boards for Biogen Idec, Novartis, Genentech, and EMD Serono, and she has received research support from Biogen Idec and Bristol Myers Squibb Foundation. Dr. Kind reported support from the Alzheimer’s Association. Dr. Diaz-Ortiz is coinventor of a provisional patent submitted by the University of Pennsylvania that relates to a potential therapeutic in Parkinson’s disease. Mr. Lusk reported fellowship support from American Heart Association and travel support from the American Neurological Association. No other speakers or sources had relevant disclosures.
Black and Latinx older adults are up to three times as likely to develop Alzheimer’s disease than non-Latinx White adults and tend to experience onset at a younger age with more severe symptoms, according to Monica Rivera-Mindt, PhD, a professor of psychology at Fordham University and the Icahn School of Medicine at Mount Sinai, New York. Looking ahead, that means by 2030, nearly 40% of the 8.4 million Americans affected by Alzheimer’s disease will be Black and/or Latinx, she said. These facts were among the stark disparities in health care outcomes Dr. Rivera-Mindt discussed in her presentation on brain health equity at the 2022 annual meeting of the American Neurological Association.
Dr. Rivera-Mindt’s presentation opened the ANA’s plenary session on health disparities and inequities. The plenary, “Advancing Neurologic Equity: Challenges and Paths Forward,” did not simply enumerate racial and ethnic disparities that exist with various neurological conditions. Rather it went beyond the discussion of what disparities exist into understanding the roots of them as well as tips, tools, and resources that can aid clinicians in addressing or ameliorating them.
Roy Hamilton, MD, an associate professor of neurology and physical medicine and rehabilitation at the University of Pennsylvania, Philadelphia, said. “If clinicians are unaware of these disparities or don’t have any sense of how to start to address or think about them, then they’re really missing out on an important component of their education as persons who take care of patients with brain disorders.”
Dr. Hamilton, who organized the plenary, noted that awareness of these disparities is crucial to comprehensively caring for patients.
Missed opportunities
“We’re talking about disadvantages that are structural and large scale, but those disadvantages play themselves out in the individual encounter,” Dr. Hamilton said. “When physicians see patients, they have to treat the whole patient in front of them,” which means being aware of the risks and factors that could affect a patient’s clinical presentation. “Being aware of disparities has practical impacts on physician judgment,” he said.
For example, recent research in multiple sclerosis (MS) has highlighted how clinicians may be missing diagnosis of this condition in non-White populations because the condition has been regarded for so long as a “White person’s” disease, Dr. Hamilton said. In non-White patients exhibiting MS symptoms, then, clinicians may have been less likely to consider MS as a possibility, thereby delaying diagnosis and treatment.
Those patterns may partly explain why the mortality rate for MS is greater in Black patients, who also show more rapid neurodegeneration than White patients with MS, Lilyana Amezcua, MD, an associate professor of neurology at the University of Southern California, Los Angeles, reported in the plenary’s second presentation.
Transgender issues
The third session, presented by Nicole Rosendale, MD, an assistant professor of neurology at the University of California, San Francisco, and director of the San Francisco General Hospital neurology inpatient services, examined disparities in neurology within the LGBTQ+ community through representative case studies and then offered specific ways that neurologists could make their practices more inclusive and equitable for sexual and gender minorities.
Her first case study was a 52-year-old man who presented with new-onset seizures, right hemiparesis, and aphasia. A brain biopsy consistent with adenocarcinoma eventually led his physician to discover he had metastatic breast cancer. It turned out the man was transgender and, despite a family history of breast cancer, hadn’t been advised to get breast cancer screenings.
“Breast cancer was not initially on the differential as no one had identified that the patient was transmasculine,” Dr. Rosendale said. A major challenge to providing care to transgender patients is a dearth of data on risks and screening recommendations. Another barrier is low knowledge of LGBTQ+ health among neurologists, Dr. Rosendale said while sharing findings from her 2019 study on the topic and calling for more research in LGBTQ+ populations.
Dr. Rosendale’s second case study dealt with a nonbinary patient who suffered from debilitating headaches for decades, first because they lacked access to health insurance and then because negative experiences with providers dissuaded them from seeking care. In data from the Center for American Progress she shared, 8% of LGB respondents and 22% of transgender respondents said they had avoided or delayed care because of fear of discrimination or mistreatment.
“So it’s not only access but also what experiences people are having when they go in and whether they’re actually even getting access to care or being taken care of,” Dr. Rosendale said. Other findings from the CAP found that:
- 8% of LGB patients and 29% of transgender patients reported having a clinician refuse to see them.
- 6% of LGB patients and 12% of transgender patients reported that a clinician refused to give them health care.
- 9% of LGB patients and 21% of transgender patients experienced harsh or abusive language during a health care experience.
- 7% of LGB patients and nearly a third (29%) of transgender patients experienced unwanted physical contact, such as fondling or sexual assault.
Reducing the disparities
Adys Mendizabal, MD, an assistant professor of neurology at the Institute of Society and Genetics at the University of California, Los Angeles, who attended the presentation, was grateful to see how the various lectures enriched the discussion beyond stating the fact of racial/ethnic disparities and dug into the nuances on how to think about and address these disparities. She particularly appreciated discussion about the need to go out of the way to recruit diverse patient populations for clinical trials while also providing them care.
“It is definitely complicated, but it’s not impossible for an individual neurologist or an individual department to do something to reduce some of the disparities,” Dr. Mendizabal said. “It starts with just knowing that they exist and being aware of some of the things that may be impacting care for a particular patient.”
Tools to counter disparity
In the final presentation, Amy Kind, MD, PhD, the associate dean for social health sciences and programs at the University of Wisconsin–Madison, rounded out the discussion by exploring social determinants of health and their influence on outcomes.
“Social determinants impact brain health, and brain health is not distributed equally,” Dr. Kind told attendees. “We have known this for decades, yet disparities persist.”
Dr. Kind described the “exposome,” a “measure of all the exposures of an individual in a lifetime and how those exposures relate to health,” according to the CDC, and then introduced a tool clinicians can use to better understand social determinants of health in specific geographic areas. The Neighborhood Atlas, which Dr. Kind described in the New England Journal of Medicine in 2018, measures 17 social determinants across small population-sensitive areas and provides an area deprivation index. A high area deprivation index is linked to a range of negative outcomes, including reshopitalization, later diagnoses, less comprehensive diagnostic evaluation, increased risk of postsurgical complications, and decreased life expectancy.
“One of the things that really stood out to me about Dr. Kind’s discussion of the use of the area deprivation index was the fact that understanding and quantifying these kinds of risks and exposures is the vehicle for creating the kinds of social changes, including policy changes, that will actually lead to addressing and mitigating some of these lifelong risks and exposures,” Dr. Hamilton said. “It is implausible to think that a specific group of people would be genetically more susceptible to basically every disease that we know,” he added. “It makes much more sense to think that groups of individuals have been subjected systematically to conditions that impair health in a variety of ways.”
Not just race, ethnicity, sex, and gender
Following the four presentations from researchers in health inequities was an Emerging Scholar presentation in which Jay B. Lusk, an MD/MBA candidate at Duke University, Durham, N.C., shared new research findings on the role of neighborhood disadvantage in predicting mortality from coma, stroke, and other neurologic conditions. His findings revealed that living in a neighborhood with greater deprivation substantially increased risk of mortality even after accounting for individual wealth and demographics.
Maria Eugenia Diaz-Ortiz, PhD, of the department of neurology, University of Pennsylvania, Philadelphia, said she found the five presentations to be an excellent introduction to people like herself who are in the earlier stages of learning about health equity research.
“I think they introduced various important concepts and frameworks and provided tools for people who don’t know about them,” Dr. Diaz-Ortiz said. “Then they asked important questions and provided some solutions to them.”
Dr. Diaz-Ortiz also appreciated seemingly minor but actually important details in how the speakers presented themselves, such as Dr. Rivera-Mindt opening with a land acknowledgment and her disclosures of “positionality.” The former recognized the traditional Native American custodians of the land on which she lives and works, and the latter revealed details about her as an individual – such as being the Afro-Latinx daughter of immigrants yet being cisgender, able-bodied, and U.S.-born – that show where she falls on the axis of adversity and axis of privilege.
Implications for research
The biggest takeaway for Dr. Diaz-Ortiz, however, came from the first Q&A session when someone asked how to increase underrepresented populations in dementia research. Dr. Rivera-Mindt described her experience engaging these communities by employing “community-based participatory research practices, which involves making yourself a part of the community and making the community active participants in the research,” Dr. Diaz-Ortiz said. “It’s an evidence-based approach that has been shown to increase participation in research not only in her work but in the work of others.”
Preaching to the choir
Dr. Diaz-Ortiz was pleased overall with the plenary but disappointed in its placement at the end of the meeting, when attendance is always lower as attendees head home.
“The people who stayed were people who already know and recognize the value of health equity work, so I think that was a missed opportunity where the session could have been included on day one or two to boost attendance and also to educate like a broader group of neurologists,” Dr. Diaz-Ortiz said in an interview.
Dr. Mendizabal felt similarly, appreciating the plenary but noting it was “definitely overdue” and that it should not be the last session. Instead, sessions on health equity should be as easy as possible to attend to bring in larger audiences. “Perhaps having that session on a Saturday or Sunday would have a higher likelihood of greater attendance than on a Tuesday,” she said. That said, Dr. Mendizabal also noticed that greater attention to health care disparities was woven into many other sessions throughout the conference, which is “the best way of addressing health equity instead of trying to just designate a session,” she said.
Dr. Mendizabal hopes that plenaries like this one and the weaving of health equity issues into presentations throughout neurology conferences continue.
“After the racial reckoning in 2020, there was a big impetus and a big wave of energy in addressing health disparities in the field, and I hope that that momentum is not starting to wane,” Dr. Mendizabal said. “It’s important because not talking about is not going to make this issue go away.”
Dr. Hamilton agreed that it is important that the conversation continue and that physicians recognize the importance of understanding health care disparities and determinants of health, regardless of where they fall on the political spectrum or whether they choose to get involved in policy or advocacy.
“Irrespective of whether you think race or ethnicity or socioeconomic status are political issues or not, it is the case that you’re obligated to have an objective understanding of the factors that contribute to your patient’s health and as points of intervention,” Dr. Hamilton said. “So even if you don’t want to sit down and jot off that email to your senator, you still have to take these factors into account when you’re treating the person who’s sitting right in front of you, and that’s not political. That’s the promise of being a physician.”
Dr. Amezcua has received personal compensation for consulting, speaking, or serving on steering committees or advisory boards for Biogen Idec, Novartis, Genentech, and EMD Serono, and she has received research support from Biogen Idec and Bristol Myers Squibb Foundation. Dr. Kind reported support from the Alzheimer’s Association. Dr. Diaz-Ortiz is coinventor of a provisional patent submitted by the University of Pennsylvania that relates to a potential therapeutic in Parkinson’s disease. Mr. Lusk reported fellowship support from American Heart Association and travel support from the American Neurological Association. No other speakers or sources had relevant disclosures.
FROM ANA 2022
Commentary: Combination therapies and immunotherapy in HCC, December 2022
Hatanaka and colleagues investigated whether the etiology of the underlying liver disease affected the efficacy of atezolizumab and bevacizumab (A/B). They reported the results of a retrospective cohort study of 323 patients with Barcelona Clinic Liver Cancer (BCLC) stage B or C hepatocellular carcinoma and Child-Pugh class A cirrhosis who started A/B between September 2020 and December 2021. Patients with viral infection were defined as those who were either serum anti–hepatitis C antibody (anti-HCV Ab)- or hepatitis B surface antigen (HBs-Ag)-positive, while patients with nonviral infection was defined as those who were both serum anti-HCV Ab- and HBs-Ag-negative. After propensity matching, no significant difference in response rate ([RR] 20.6% vs 24.6% in viral and nonviral patients), disease control rate (68.3% vs 69.0%), progression-free survival ([PFS] 7.0 months vs 6.2 months), or 12-month overall survival ([OS] 65.5% vs 71.7%) was seen. The authors concluded that the underlying etiology of liver disease in patients with HCC does not affect the response to treatment with A/B.
Scheiner and colleagues evaluated the efficacy of immunotherapy in patients with HCC who had already received immune checkpoint inhibitors (ICI) in a previous line of therapy. The authors reported the results of an international, retrospective multicenter study of 58 patients with HCC who received at least two lines of ICI-based therapies. The first ICI was discontinued due to disease progression in 90%. Nonetheless, the RR to the second ICI was 26% (compared with 22% for the first ICI), with a time-to-progression (TTP) of 5.4 months (95% CI, 3.0-7.7) for the first ICI and 5.2 months (95% CI, 3.3-7.0) for the second ICI. Grade 3/4 treatment-related adverse events were observed in 16% and 17% of patients with the first and second ICI, respectively. Therefore, the authors believe that ICI rechallenge is safe and results in a treatment benefit for a similar proportion of HCC patients, as is seen with the first ICI treatment. They suggest that ICI-based regimens should be studied in prospective trials of patients who progressed on first-line immunotherapy.
Finally, Kim and colleagues reported outcomes of patients who developed anti-drug antibodies (ADA) against atezolizumab while on A/B. In this prospective cohort study, 174 patients with advanced HCC who were treated with first-line A/B were tested for serum ADA levels prior to treatment and at 3 weeks (cycle 2 day 1 [C2D1]). Clinically, patients with progressive disease exhibited higher ADA levels (median 65.2 [0-520.4] ng/mL) at C2D1 than responders (0-117.5 ng/mL). Patients with high ADA levels at C2D1 had a reduced response rate (29%-34% vs 7-11%) and worse PFS and OS. The investigators found that very high ADA levels (≥ 1000 ng/mL) at 3 weeks were consistently associated with poor clinical outcomes due to reduced systemic exposure to atezolizumab and impaired proliferation and activation of peripheral CD8-positive T cells. They suggested future validation and standardization of ADA assays to optimize treatment with atezolizumab in patients with uHCC.
Hatanaka and colleagues investigated whether the etiology of the underlying liver disease affected the efficacy of atezolizumab and bevacizumab (A/B). They reported the results of a retrospective cohort study of 323 patients with Barcelona Clinic Liver Cancer (BCLC) stage B or C hepatocellular carcinoma and Child-Pugh class A cirrhosis who started A/B between September 2020 and December 2021. Patients with viral infection were defined as those who were either serum anti–hepatitis C antibody (anti-HCV Ab)- or hepatitis B surface antigen (HBs-Ag)-positive, while patients with nonviral infection was defined as those who were both serum anti-HCV Ab- and HBs-Ag-negative. After propensity matching, no significant difference in response rate ([RR] 20.6% vs 24.6% in viral and nonviral patients), disease control rate (68.3% vs 69.0%), progression-free survival ([PFS] 7.0 months vs 6.2 months), or 12-month overall survival ([OS] 65.5% vs 71.7%) was seen. The authors concluded that the underlying etiology of liver disease in patients with HCC does not affect the response to treatment with A/B.
Scheiner and colleagues evaluated the efficacy of immunotherapy in patients with HCC who had already received immune checkpoint inhibitors (ICI) in a previous line of therapy. The authors reported the results of an international, retrospective multicenter study of 58 patients with HCC who received at least two lines of ICI-based therapies. The first ICI was discontinued due to disease progression in 90%. Nonetheless, the RR to the second ICI was 26% (compared with 22% for the first ICI), with a time-to-progression (TTP) of 5.4 months (95% CI, 3.0-7.7) for the first ICI and 5.2 months (95% CI, 3.3-7.0) for the second ICI. Grade 3/4 treatment-related adverse events were observed in 16% and 17% of patients with the first and second ICI, respectively. Therefore, the authors believe that ICI rechallenge is safe and results in a treatment benefit for a similar proportion of HCC patients, as is seen with the first ICI treatment. They suggest that ICI-based regimens should be studied in prospective trials of patients who progressed on first-line immunotherapy.
Finally, Kim and colleagues reported outcomes of patients who developed anti-drug antibodies (ADA) against atezolizumab while on A/B. In this prospective cohort study, 174 patients with advanced HCC who were treated with first-line A/B were tested for serum ADA levels prior to treatment and at 3 weeks (cycle 2 day 1 [C2D1]). Clinically, patients with progressive disease exhibited higher ADA levels (median 65.2 [0-520.4] ng/mL) at C2D1 than responders (0-117.5 ng/mL). Patients with high ADA levels at C2D1 had a reduced response rate (29%-34% vs 7-11%) and worse PFS and OS. The investigators found that very high ADA levels (≥ 1000 ng/mL) at 3 weeks were consistently associated with poor clinical outcomes due to reduced systemic exposure to atezolizumab and impaired proliferation and activation of peripheral CD8-positive T cells. They suggested future validation and standardization of ADA assays to optimize treatment with atezolizumab in patients with uHCC.
Hatanaka and colleagues investigated whether the etiology of the underlying liver disease affected the efficacy of atezolizumab and bevacizumab (A/B). They reported the results of a retrospective cohort study of 323 patients with Barcelona Clinic Liver Cancer (BCLC) stage B or C hepatocellular carcinoma and Child-Pugh class A cirrhosis who started A/B between September 2020 and December 2021. Patients with viral infection were defined as those who were either serum anti–hepatitis C antibody (anti-HCV Ab)- or hepatitis B surface antigen (HBs-Ag)-positive, while patients with nonviral infection was defined as those who were both serum anti-HCV Ab- and HBs-Ag-negative. After propensity matching, no significant difference in response rate ([RR] 20.6% vs 24.6% in viral and nonviral patients), disease control rate (68.3% vs 69.0%), progression-free survival ([PFS] 7.0 months vs 6.2 months), or 12-month overall survival ([OS] 65.5% vs 71.7%) was seen. The authors concluded that the underlying etiology of liver disease in patients with HCC does not affect the response to treatment with A/B.
Scheiner and colleagues evaluated the efficacy of immunotherapy in patients with HCC who had already received immune checkpoint inhibitors (ICI) in a previous line of therapy. The authors reported the results of an international, retrospective multicenter study of 58 patients with HCC who received at least two lines of ICI-based therapies. The first ICI was discontinued due to disease progression in 90%. Nonetheless, the RR to the second ICI was 26% (compared with 22% for the first ICI), with a time-to-progression (TTP) of 5.4 months (95% CI, 3.0-7.7) for the first ICI and 5.2 months (95% CI, 3.3-7.0) for the second ICI. Grade 3/4 treatment-related adverse events were observed in 16% and 17% of patients with the first and second ICI, respectively. Therefore, the authors believe that ICI rechallenge is safe and results in a treatment benefit for a similar proportion of HCC patients, as is seen with the first ICI treatment. They suggest that ICI-based regimens should be studied in prospective trials of patients who progressed on first-line immunotherapy.
Finally, Kim and colleagues reported outcomes of patients who developed anti-drug antibodies (ADA) against atezolizumab while on A/B. In this prospective cohort study, 174 patients with advanced HCC who were treated with first-line A/B were tested for serum ADA levels prior to treatment and at 3 weeks (cycle 2 day 1 [C2D1]). Clinically, patients with progressive disease exhibited higher ADA levels (median 65.2 [0-520.4] ng/mL) at C2D1 than responders (0-117.5 ng/mL). Patients with high ADA levels at C2D1 had a reduced response rate (29%-34% vs 7-11%) and worse PFS and OS. The investigators found that very high ADA levels (≥ 1000 ng/mL) at 3 weeks were consistently associated with poor clinical outcomes due to reduced systemic exposure to atezolizumab and impaired proliferation and activation of peripheral CD8-positive T cells. They suggested future validation and standardization of ADA assays to optimize treatment with atezolizumab in patients with uHCC.
Commentary: Combination therapies and immunotherapy in HCC, December 2022
Hatanaka and colleagues investigated whether the etiology of the underlying liver disease affected the efficacy of atezolizumab and bevacizumab (A/B). They reported the results of a retrospective cohort study of 323 patients with Barcelona Clinic Liver Cancer (BCLC) stage B or C hepatocellular carcinoma and Child-Pugh class A cirrhosis who started A/B between September 2020 and December 2021. Patients with viral infection were defined as those who were either serum anti–hepatitis C antibody (anti-HCV Ab)- or hepatitis B surface antigen (HBs-Ag)-positive, while patients with nonviral infection was defined as those who were both serum anti-HCV Ab- and HBs-Ag-negative. After propensity matching, no significant difference in response rate ([RR] 20.6% vs 24.6% in viral and nonviral patients), disease control rate (68.3% vs 69.0%), progression-free survival ([PFS] 7.0 months vs 6.2 months), or 12-month overall survival ([OS] 65.5% vs 71.7%) was seen. The authors concluded that the underlying etiology of liver disease in patients with HCC does not affect the response to treatment with A/B.
Scheiner and colleagues evaluated the efficacy of immunotherapy in patients with HCC who had already received immune checkpoint inhibitors (ICI) in a previous line of therapy. The authors reported the results of an international, retrospective multicenter study of 58 patients with HCC who received at least two lines of ICI-based therapies. The first ICI was discontinued due to disease progression in 90%. Nonetheless, the RR to the second ICI was 26% (compared with 22% for the first ICI), with a time-to-progression (TTP) of 5.4 months (95% CI, 3.0-7.7) for the first ICI and 5.2 months (95% CI, 3.3-7.0) for the second ICI. Grade 3/4 treatment-related adverse events were observed in 16% and 17% of patients with the first and second ICI, respectively. Therefore, the authors believe that ICI rechallenge is safe and results in a treatment benefit for a similar proportion of HCC patients, as is seen with the first ICI treatment. They suggest that ICI-based regimens should be studied in prospective trials of patients who progressed on first-line immunotherapy.
Finally, Kim and colleagues reported outcomes of patients who developed anti-drug antibodies (ADA) against atezolizumab while on A/B. In this prospective cohort study, 174 patients with advanced HCC who were treated with first-line A/B were tested for serum ADA levels prior to treatment and at 3 weeks (cycle 2 day 1 [C2D1]). Clinically, patients with progressive disease exhibited higher ADA levels (median 65.2 [0-520.4] ng/mL) at C2D1 than responders (0-117.5 ng/mL). Patients with high ADA levels at C2D1 had a reduced response rate (29%-34% vs 7-11%) and worse PFS and OS. The investigators found that very high ADA levels (≥ 1000 ng/mL) at 3 weeks were consistently associated with poor clinical outcomes due to reduced systemic exposure to atezolizumab and impaired proliferation and activation of peripheral CD8-positive T cells. They suggested future validation and standardization of ADA assays to optimize treatment with atezolizumab in patients with uHCC.
Hatanaka and colleagues investigated whether the etiology of the underlying liver disease affected the efficacy of atezolizumab and bevacizumab (A/B). They reported the results of a retrospective cohort study of 323 patients with Barcelona Clinic Liver Cancer (BCLC) stage B or C hepatocellular carcinoma and Child-Pugh class A cirrhosis who started A/B between September 2020 and December 2021. Patients with viral infection were defined as those who were either serum anti–hepatitis C antibody (anti-HCV Ab)- or hepatitis B surface antigen (HBs-Ag)-positive, while patients with nonviral infection was defined as those who were both serum anti-HCV Ab- and HBs-Ag-negative. After propensity matching, no significant difference in response rate ([RR] 20.6% vs 24.6% in viral and nonviral patients), disease control rate (68.3% vs 69.0%), progression-free survival ([PFS] 7.0 months vs 6.2 months), or 12-month overall survival ([OS] 65.5% vs 71.7%) was seen. The authors concluded that the underlying etiology of liver disease in patients with HCC does not affect the response to treatment with A/B.
Scheiner and colleagues evaluated the efficacy of immunotherapy in patients with HCC who had already received immune checkpoint inhibitors (ICI) in a previous line of therapy. The authors reported the results of an international, retrospective multicenter study of 58 patients with HCC who received at least two lines of ICI-based therapies. The first ICI was discontinued due to disease progression in 90%. Nonetheless, the RR to the second ICI was 26% (compared with 22% for the first ICI), with a time-to-progression (TTP) of 5.4 months (95% CI, 3.0-7.7) for the first ICI and 5.2 months (95% CI, 3.3-7.0) for the second ICI. Grade 3/4 treatment-related adverse events were observed in 16% and 17% of patients with the first and second ICI, respectively. Therefore, the authors believe that ICI rechallenge is safe and results in a treatment benefit for a similar proportion of HCC patients, as is seen with the first ICI treatment. They suggest that ICI-based regimens should be studied in prospective trials of patients who progressed on first-line immunotherapy.
Finally, Kim and colleagues reported outcomes of patients who developed anti-drug antibodies (ADA) against atezolizumab while on A/B. In this prospective cohort study, 174 patients with advanced HCC who were treated with first-line A/B were tested for serum ADA levels prior to treatment and at 3 weeks (cycle 2 day 1 [C2D1]). Clinically, patients with progressive disease exhibited higher ADA levels (median 65.2 [0-520.4] ng/mL) at C2D1 than responders (0-117.5 ng/mL). Patients with high ADA levels at C2D1 had a reduced response rate (29%-34% vs 7-11%) and worse PFS and OS. The investigators found that very high ADA levels (≥ 1000 ng/mL) at 3 weeks were consistently associated with poor clinical outcomes due to reduced systemic exposure to atezolizumab and impaired proliferation and activation of peripheral CD8-positive T cells. They suggested future validation and standardization of ADA assays to optimize treatment with atezolizumab in patients with uHCC.
Hatanaka and colleagues investigated whether the etiology of the underlying liver disease affected the efficacy of atezolizumab and bevacizumab (A/B). They reported the results of a retrospective cohort study of 323 patients with Barcelona Clinic Liver Cancer (BCLC) stage B or C hepatocellular carcinoma and Child-Pugh class A cirrhosis who started A/B between September 2020 and December 2021. Patients with viral infection were defined as those who were either serum anti–hepatitis C antibody (anti-HCV Ab)- or hepatitis B surface antigen (HBs-Ag)-positive, while patients with nonviral infection was defined as those who were both serum anti-HCV Ab- and HBs-Ag-negative. After propensity matching, no significant difference in response rate ([RR] 20.6% vs 24.6% in viral and nonviral patients), disease control rate (68.3% vs 69.0%), progression-free survival ([PFS] 7.0 months vs 6.2 months), or 12-month overall survival ([OS] 65.5% vs 71.7%) was seen. The authors concluded that the underlying etiology of liver disease in patients with HCC does not affect the response to treatment with A/B.
Scheiner and colleagues evaluated the efficacy of immunotherapy in patients with HCC who had already received immune checkpoint inhibitors (ICI) in a previous line of therapy. The authors reported the results of an international, retrospective multicenter study of 58 patients with HCC who received at least two lines of ICI-based therapies. The first ICI was discontinued due to disease progression in 90%. Nonetheless, the RR to the second ICI was 26% (compared with 22% for the first ICI), with a time-to-progression (TTP) of 5.4 months (95% CI, 3.0-7.7) for the first ICI and 5.2 months (95% CI, 3.3-7.0) for the second ICI. Grade 3/4 treatment-related adverse events were observed in 16% and 17% of patients with the first and second ICI, respectively. Therefore, the authors believe that ICI rechallenge is safe and results in a treatment benefit for a similar proportion of HCC patients, as is seen with the first ICI treatment. They suggest that ICI-based regimens should be studied in prospective trials of patients who progressed on first-line immunotherapy.
Finally, Kim and colleagues reported outcomes of patients who developed anti-drug antibodies (ADA) against atezolizumab while on A/B. In this prospective cohort study, 174 patients with advanced HCC who were treated with first-line A/B were tested for serum ADA levels prior to treatment and at 3 weeks (cycle 2 day 1 [C2D1]). Clinically, patients with progressive disease exhibited higher ADA levels (median 65.2 [0-520.4] ng/mL) at C2D1 than responders (0-117.5 ng/mL). Patients with high ADA levels at C2D1 had a reduced response rate (29%-34% vs 7-11%) and worse PFS and OS. The investigators found that very high ADA levels (≥ 1000 ng/mL) at 3 weeks were consistently associated with poor clinical outcomes due to reduced systemic exposure to atezolizumab and impaired proliferation and activation of peripheral CD8-positive T cells. They suggested future validation and standardization of ADA assays to optimize treatment with atezolizumab in patients with uHCC.
Commentary: New treatments and management in breast cancer, December 2022
Taxanes are an integral component of various treatment regimens for all stages of breast cancer. As survival outcomes have improved, it has become increasingly important to focus on the long-term quality-of-life impact of treatment. Neurotoxicity is a well-recognized potential side effect of taxane chemotherapy. In a prospective cohort study including 1234 patients diagnosed with breast cancer and receiving taxanes, the risk for patient-reported chemotherapy-induced peripheral neuropathy (CIPN) were lower in the paclitaxel (HR 0.59; P = .008) and docetaxel (HR 0.65; P = .02) groups vs the nab-paclitaxel group. There was less sensory discomfort reported with paclitaxel (HR 0.44; P < .001) and docetaxel (HR 0.52; P < .001) vs nab-paclitaxel; however, reported motor and autonomic symptoms were not significantly lower than in the nab-paclitaxel group (Mo et al). An area of research interest is the identification of biomarkers that may predict a higher likelihood of CIPN development, to aid in early detection and intervention.3
Management strategies for breast cancer diagnosed in older women should take into consideration age and competing medical comorbidities, and hormone receptor–positive histology is the most common subtype in this population. Some older women may be too frail or unfit for surgery, and furthermore, some may prefer to avoid surgery, even if it is considered a safe approach. A retrospective study including 91 older (≥ 70 years) patients with estrogen receptor–positive (ER+) breast cancer who underwent definitive endocrine therapy demonstrated a twofold higher mortality risk than the risk of needing invasive local treatment (surgery or radiation). The 5-year cumulative risks of undergoing invasive local treatment and having uncontrolled disease were 28% and 16%, respectively, whereas the 5-year cumulative overall survival was 42% (Gooijer et al). Although the majority of older women with ER+ early breast cancer will obtain a survival benefit with surgery plus endocrine therapy compared with primary endocrine therapy, there is a selected group with limited life expectancy owing to age, functional status, or medical comorbidities for whom it is appropriate to offer primary endocrine therapy, because breast cancer–specific survival may not be negatively affected.4
Additional References
- Schmid P, Cortes J, Dent R, et al; for the KEYNOTE-522 Investigators. Event-free survival with pembrolizumab in early triple-negative breast cancer. N Engl J Med. 2022;386:556-567. Doi: 10.1056/NEJMoa2112651
- Harbeck N, Rastogi P, Martin M, et al; on behalf of the monarchE Committee Members. Adjuvant abemaciclib combined with endocrine therapy for high-risk early breast cancer: updated efficacy and Ki-67 analysis from the monarchE study. Ann Oncol. 2021;32:1571-1581. Doi: 10.1016/j.annonc.2021.09.015
- Rodwin RL, Siddiq NZ, Ehrlich BE, Lustberg MB. Biomarkers of chemotherapy-induced peripheral neuropathy: current status and future directions. Front Pain Res (Lausanne). 2022;3:864910. Doi: 10.3389/fpain.2022.864910
- Wyld L, Reed MW, Morgan J, et al. Bridging the age gap in breast cancer. Impacts of omission of breast cancer surgery in older women with oestrogen receptor positive early breast cancer. A risk stratified analysis of survival outcomes and quality of life. Eur J Cancer. 2021;142:48-62. Doi: 10.1016/j.ejca.2020.10.015
Taxanes are an integral component of various treatment regimens for all stages of breast cancer. As survival outcomes have improved, it has become increasingly important to focus on the long-term quality-of-life impact of treatment. Neurotoxicity is a well-recognized potential side effect of taxane chemotherapy. In a prospective cohort study including 1234 patients diagnosed with breast cancer and receiving taxanes, the risk for patient-reported chemotherapy-induced peripheral neuropathy (CIPN) were lower in the paclitaxel (HR 0.59; P = .008) and docetaxel (HR 0.65; P = .02) groups vs the nab-paclitaxel group. There was less sensory discomfort reported with paclitaxel (HR 0.44; P < .001) and docetaxel (HR 0.52; P < .001) vs nab-paclitaxel; however, reported motor and autonomic symptoms were not significantly lower than in the nab-paclitaxel group (Mo et al). An area of research interest is the identification of biomarkers that may predict a higher likelihood of CIPN development, to aid in early detection and intervention.3
Management strategies for breast cancer diagnosed in older women should take into consideration age and competing medical comorbidities, and hormone receptor–positive histology is the most common subtype in this population. Some older women may be too frail or unfit for surgery, and furthermore, some may prefer to avoid surgery, even if it is considered a safe approach. A retrospective study including 91 older (≥ 70 years) patients with estrogen receptor–positive (ER+) breast cancer who underwent definitive endocrine therapy demonstrated a twofold higher mortality risk than the risk of needing invasive local treatment (surgery or radiation). The 5-year cumulative risks of undergoing invasive local treatment and having uncontrolled disease were 28% and 16%, respectively, whereas the 5-year cumulative overall survival was 42% (Gooijer et al). Although the majority of older women with ER+ early breast cancer will obtain a survival benefit with surgery plus endocrine therapy compared with primary endocrine therapy, there is a selected group with limited life expectancy owing to age, functional status, or medical comorbidities for whom it is appropriate to offer primary endocrine therapy, because breast cancer–specific survival may not be negatively affected.4
Additional References
- Schmid P, Cortes J, Dent R, et al; for the KEYNOTE-522 Investigators. Event-free survival with pembrolizumab in early triple-negative breast cancer. N Engl J Med. 2022;386:556-567. Doi: 10.1056/NEJMoa2112651
- Harbeck N, Rastogi P, Martin M, et al; on behalf of the monarchE Committee Members. Adjuvant abemaciclib combined with endocrine therapy for high-risk early breast cancer: updated efficacy and Ki-67 analysis from the monarchE study. Ann Oncol. 2021;32:1571-1581. Doi: 10.1016/j.annonc.2021.09.015
- Rodwin RL, Siddiq NZ, Ehrlich BE, Lustberg MB. Biomarkers of chemotherapy-induced peripheral neuropathy: current status and future directions. Front Pain Res (Lausanne). 2022;3:864910. Doi: 10.3389/fpain.2022.864910
- Wyld L, Reed MW, Morgan J, et al. Bridging the age gap in breast cancer. Impacts of omission of breast cancer surgery in older women with oestrogen receptor positive early breast cancer. A risk stratified analysis of survival outcomes and quality of life. Eur J Cancer. 2021;142:48-62. Doi: 10.1016/j.ejca.2020.10.015
Taxanes are an integral component of various treatment regimens for all stages of breast cancer. As survival outcomes have improved, it has become increasingly important to focus on the long-term quality-of-life impact of treatment. Neurotoxicity is a well-recognized potential side effect of taxane chemotherapy. In a prospective cohort study including 1234 patients diagnosed with breast cancer and receiving taxanes, the risk for patient-reported chemotherapy-induced peripheral neuropathy (CIPN) were lower in the paclitaxel (HR 0.59; P = .008) and docetaxel (HR 0.65; P = .02) groups vs the nab-paclitaxel group. There was less sensory discomfort reported with paclitaxel (HR 0.44; P < .001) and docetaxel (HR 0.52; P < .001) vs nab-paclitaxel; however, reported motor and autonomic symptoms were not significantly lower than in the nab-paclitaxel group (Mo et al). An area of research interest is the identification of biomarkers that may predict a higher likelihood of CIPN development, to aid in early detection and intervention.3
Management strategies for breast cancer diagnosed in older women should take into consideration age and competing medical comorbidities, and hormone receptor–positive histology is the most common subtype in this population. Some older women may be too frail or unfit for surgery, and furthermore, some may prefer to avoid surgery, even if it is considered a safe approach. A retrospective study including 91 older (≥ 70 years) patients with estrogen receptor–positive (ER+) breast cancer who underwent definitive endocrine therapy demonstrated a twofold higher mortality risk than the risk of needing invasive local treatment (surgery or radiation). The 5-year cumulative risks of undergoing invasive local treatment and having uncontrolled disease were 28% and 16%, respectively, whereas the 5-year cumulative overall survival was 42% (Gooijer et al). Although the majority of older women with ER+ early breast cancer will obtain a survival benefit with surgery plus endocrine therapy compared with primary endocrine therapy, there is a selected group with limited life expectancy owing to age, functional status, or medical comorbidities for whom it is appropriate to offer primary endocrine therapy, because breast cancer–specific survival may not be negatively affected.4
Additional References
- Schmid P, Cortes J, Dent R, et al; for the KEYNOTE-522 Investigators. Event-free survival with pembrolizumab in early triple-negative breast cancer. N Engl J Med. 2022;386:556-567. Doi: 10.1056/NEJMoa2112651
- Harbeck N, Rastogi P, Martin M, et al; on behalf of the monarchE Committee Members. Adjuvant abemaciclib combined with endocrine therapy for high-risk early breast cancer: updated efficacy and Ki-67 analysis from the monarchE study. Ann Oncol. 2021;32:1571-1581. Doi: 10.1016/j.annonc.2021.09.015
- Rodwin RL, Siddiq NZ, Ehrlich BE, Lustberg MB. Biomarkers of chemotherapy-induced peripheral neuropathy: current status and future directions. Front Pain Res (Lausanne). 2022;3:864910. Doi: 10.3389/fpain.2022.864910
- Wyld L, Reed MW, Morgan J, et al. Bridging the age gap in breast cancer. Impacts of omission of breast cancer surgery in older women with oestrogen receptor positive early breast cancer. A risk stratified analysis of survival outcomes and quality of life. Eur J Cancer. 2021;142:48-62. Doi: 10.1016/j.ejca.2020.10.015
Commentary: Shoulder dystocia and vaginal breech deliveries, December 2022
The safety of vaginal breech delivery has been controversial since the Term Breech Trial in 2000 suggested increased neonatal mortality and short-term morbidity associated with vaginal breech delivery. The stance against breech delivery has softened since that time. Fruscalzo and colleagues provide yet more evidence supporting the safety of vaginal breech deliveries with their single-center, retrospective study, which included 804 singleton pregnant women who underwent vaginal breech vs emergency cesarean section vs elective cesarean section in Coesfeld, Germany. They found no significant differences between the vaginal breech–delivery group vs the other two groups in regard to umbilical artery pH < 7, low Apgar scores, or neonatal intensive care unit admissions. The only significant difference noted was umbilical artery pH < 7.1. This suggests that in experienced hands (each of the candidates was referred to a senior obstetrician for consultation), vaginal breech delivery can be safe, including for nulliparous women (67% were nulliparous), showing that even the short-term morbidity associated with vaginal breech delivery approaches that of planned cesarean section.
Two other articles raise caution regarding SD and increased risk for fetal death and PPH. Linde and colleagues used data from The Medical Birth Registry of Norway and Statistics Norway to examine recurrence risk for PPH associated with various causes. PPH associated with SD led the way: The recurrence risk adjusted odds ratio (aOR) was 6.8 for SD vs 5.9 for retained products of conception, 4.0 for uterine atony, 3.9 for obstetric trauma, and 2.2 for PPH of undefined cause. This study suggests that the risks for SD recurrence should be focused not just on SD, but also on PPH. Another concern regarding shoulder dystocia is raised by Davidesko and colleagues in their analysis of risk factors for intrapartum fetal death. Using a generalized estimation equation model to help identify independent risk factors for intrapartum fetal death, they examined 344,536 deliveries from 1991 to 2016 at Soroka University Medical Center in Israel and noted that SD again led the way: aOR was 23.8 for SD vs 19.0 for uterine rupture, 11.9 for preterm birth, 6.2 for placental abruption, and 3.6 for fetal malpresentation. This high risk for intrapartum fetal death associated with SD suggests a need for even more robust SD drills to help deal with this dreaded and often unpredictable obstetric emergency.
The safety of vaginal breech delivery has been controversial since the Term Breech Trial in 2000 suggested increased neonatal mortality and short-term morbidity associated with vaginal breech delivery. The stance against breech delivery has softened since that time. Fruscalzo and colleagues provide yet more evidence supporting the safety of vaginal breech deliveries with their single-center, retrospective study, which included 804 singleton pregnant women who underwent vaginal breech vs emergency cesarean section vs elective cesarean section in Coesfeld, Germany. They found no significant differences between the vaginal breech–delivery group vs the other two groups in regard to umbilical artery pH < 7, low Apgar scores, or neonatal intensive care unit admissions. The only significant difference noted was umbilical artery pH < 7.1. This suggests that in experienced hands (each of the candidates was referred to a senior obstetrician for consultation), vaginal breech delivery can be safe, including for nulliparous women (67% were nulliparous), showing that even the short-term morbidity associated with vaginal breech delivery approaches that of planned cesarean section.
Two other articles raise caution regarding SD and increased risk for fetal death and PPH. Linde and colleagues used data from The Medical Birth Registry of Norway and Statistics Norway to examine recurrence risk for PPH associated with various causes. PPH associated with SD led the way: The recurrence risk adjusted odds ratio (aOR) was 6.8 for SD vs 5.9 for retained products of conception, 4.0 for uterine atony, 3.9 for obstetric trauma, and 2.2 for PPH of undefined cause. This study suggests that the risks for SD recurrence should be focused not just on SD, but also on PPH. Another concern regarding shoulder dystocia is raised by Davidesko and colleagues in their analysis of risk factors for intrapartum fetal death. Using a generalized estimation equation model to help identify independent risk factors for intrapartum fetal death, they examined 344,536 deliveries from 1991 to 2016 at Soroka University Medical Center in Israel and noted that SD again led the way: aOR was 23.8 for SD vs 19.0 for uterine rupture, 11.9 for preterm birth, 6.2 for placental abruption, and 3.6 for fetal malpresentation. This high risk for intrapartum fetal death associated with SD suggests a need for even more robust SD drills to help deal with this dreaded and often unpredictable obstetric emergency.
The safety of vaginal breech delivery has been controversial since the Term Breech Trial in 2000 suggested increased neonatal mortality and short-term morbidity associated with vaginal breech delivery. The stance against breech delivery has softened since that time. Fruscalzo and colleagues provide yet more evidence supporting the safety of vaginal breech deliveries with their single-center, retrospective study, which included 804 singleton pregnant women who underwent vaginal breech vs emergency cesarean section vs elective cesarean section in Coesfeld, Germany. They found no significant differences between the vaginal breech–delivery group vs the other two groups in regard to umbilical artery pH < 7, low Apgar scores, or neonatal intensive care unit admissions. The only significant difference noted was umbilical artery pH < 7.1. This suggests that in experienced hands (each of the candidates was referred to a senior obstetrician for consultation), vaginal breech delivery can be safe, including for nulliparous women (67% were nulliparous), showing that even the short-term morbidity associated with vaginal breech delivery approaches that of planned cesarean section.
Two other articles raise caution regarding SD and increased risk for fetal death and PPH. Linde and colleagues used data from The Medical Birth Registry of Norway and Statistics Norway to examine recurrence risk for PPH associated with various causes. PPH associated with SD led the way: The recurrence risk adjusted odds ratio (aOR) was 6.8 for SD vs 5.9 for retained products of conception, 4.0 for uterine atony, 3.9 for obstetric trauma, and 2.2 for PPH of undefined cause. This study suggests that the risks for SD recurrence should be focused not just on SD, but also on PPH. Another concern regarding shoulder dystocia is raised by Davidesko and colleagues in their analysis of risk factors for intrapartum fetal death. Using a generalized estimation equation model to help identify independent risk factors for intrapartum fetal death, they examined 344,536 deliveries from 1991 to 2016 at Soroka University Medical Center in Israel and noted that SD again led the way: aOR was 23.8 for SD vs 19.0 for uterine rupture, 11.9 for preterm birth, 6.2 for placental abruption, and 3.6 for fetal malpresentation. This high risk for intrapartum fetal death associated with SD suggests a need for even more robust SD drills to help deal with this dreaded and often unpredictable obstetric emergency.
New genetic variant linked to maturity-onset diabetes of the young
A newly discovered genetic variant that is associated with type 2 diabetes (T2D) is responsible for almost 7% of all diabetes cases in Greenland, according to a whole-genome sequencing analysis of 448 Greenlandic Inuit individuals.
The variant, identified as c.1108G>T, “has the largest population impact of any previously reported variant” within the HNF1A gene – a gene that can cause maturity-onset diabetes of the young (MODY), reported senior author Torben Hansen, MD, PhD, of the University of Copenhagen, and colleagues in The Lancet Regional Health–Europe. The c.1108G>T variant does not cause MODY, but other variants within the HNF1A gene do. However, carriers of this variant, which is present in 1.9% of the Greenlandic Inuit population and has not been found elsewhere, have normal insulin sensitivity, but decreased beta-cell function and a more than fourfold risk of developing type 2 diabetes. “This adds to a previous discovery that about 11% of all diabetes in Greenlandic Inuit is explained by a mutation in the TBC1D4 variant,” Dr. Hansen told this publication. “Thus 1 in 5 patients diagnosed with type 2 diabetes in Greenland have a specific mutation explaining their diabetes. In European populations only about 1%-2% of patients diagnosed with type 2 diabetes have a known genetic etiology.”
The finding “provides new avenues to subgroup patients, detect diabetes in family members, and pursue precision treatment trials,” noted the authors, although they acknowledged that treatment choices for individuals with this variant still need to be explored. “We know from HNF1A-mutation carriers with European ancestry that they benefit from sulfonylurea treatment,” said Dr. Hansen. “However, we have not yet done treatment studies in Inuit.” The investigators noted that “it is not always the case that variants in HNF1A result in an increased insulin secretory response to sulfonylurea. ... Whether carriers of the c.1108G>T variant could benefit from treatment with sulfonylurea should be pursued within the context of a randomized clinical trial establishing both short- and long-term efficacy of sulfonylurea in these patients.”
A total of 4,497 study participants were randomly sampled from two cross-sectional cohorts in an adult Greenlandic population health survey. Among 448 participants who had whole genome sequencing, 14 known MODY genes were screened for both previously identified as well as novel variants. This identified the c.1108G>T variant, which was then genotyped in the full cohort in order to estimate an allele frequency of 1.3% in the general Greenlandic population, and 1.9% in the Inuit component. The variant was not found in genome sequences of other populations.
The researchers then tested the association of the variant with T2D and showed strong association with T2D (odds ratio, 4.35) and higher hemoglobin A1c levels.
“This is very well-conducted and exciting research that highlights the importance of studying the genetics of diverse populations,” said Miriam Udler, MD, PhD, director of the Massachusetts General Diabetes Genetics Clinic, and assistant professor at Harvard University, both in Boston. “This manuscript builds on prior work from the researchers identifying another genetic variant specific to the Greenlandic Inuit population in the gene TBC1D4,” she added. “About 3.8% of people in this population carry two copies of the TBC1D4 variant and have about a 10-fold increased risk of diabetes. Together the two variants affect 18% of Greenlanders with diabetes.”
With its fourfold increased risk of diabetes, the new variant falls into “an ever-growing category” of “intermediate risk” genetic variants, explained Dr. Udler – “meaning that they have a large impact on diabetes risk, but cannot fully predict whether someone will get diabetes. The contribution of additional risk factors is particularly important for ‘intermediate risk’ genetic variants,” she added. “Thus, clinically, we can tell patients who have variants such as HNF1A c.1108>T that they are at substantial increased risk of diabetes, but that many will not develop diabetes. And for those who do develop diabetes, we are not yet able to advise on particular therapeutic strategies.”
Still, she emphasized, the importance of studying diverse populations with specific genetic risk factors is the end-goal of precision medicine. “An active area of research is determining whether and how to return such information about ‘intermediate risk’ variants to patients who get clinical genetic testing for diabetes, since typically only variants that are very high risk ... are returned in clinical testing reports.” Dr. Udler added that “many more such “intermediate risk’ variants likely exist in all populations, but have yet to be characterized because they are less common than HNF1A c.1108>T; however, ongoing worldwide efforts to increase the sample sizes of human genetic studies will facilitate such discovery.”
The study was funded by Novo Nordisk Foundation, Independent Research Fund Denmark, and Karen Elise Jensen’s Foundation. Dr. Hansen and Dr. Udler had no disclosures.
A newly discovered genetic variant that is associated with type 2 diabetes (T2D) is responsible for almost 7% of all diabetes cases in Greenland, according to a whole-genome sequencing analysis of 448 Greenlandic Inuit individuals.
The variant, identified as c.1108G>T, “has the largest population impact of any previously reported variant” within the HNF1A gene – a gene that can cause maturity-onset diabetes of the young (MODY), reported senior author Torben Hansen, MD, PhD, of the University of Copenhagen, and colleagues in The Lancet Regional Health–Europe. The c.1108G>T variant does not cause MODY, but other variants within the HNF1A gene do. However, carriers of this variant, which is present in 1.9% of the Greenlandic Inuit population and has not been found elsewhere, have normal insulin sensitivity, but decreased beta-cell function and a more than fourfold risk of developing type 2 diabetes. “This adds to a previous discovery that about 11% of all diabetes in Greenlandic Inuit is explained by a mutation in the TBC1D4 variant,” Dr. Hansen told this publication. “Thus 1 in 5 patients diagnosed with type 2 diabetes in Greenland have a specific mutation explaining their diabetes. In European populations only about 1%-2% of patients diagnosed with type 2 diabetes have a known genetic etiology.”
The finding “provides new avenues to subgroup patients, detect diabetes in family members, and pursue precision treatment trials,” noted the authors, although they acknowledged that treatment choices for individuals with this variant still need to be explored. “We know from HNF1A-mutation carriers with European ancestry that they benefit from sulfonylurea treatment,” said Dr. Hansen. “However, we have not yet done treatment studies in Inuit.” The investigators noted that “it is not always the case that variants in HNF1A result in an increased insulin secretory response to sulfonylurea. ... Whether carriers of the c.1108G>T variant could benefit from treatment with sulfonylurea should be pursued within the context of a randomized clinical trial establishing both short- and long-term efficacy of sulfonylurea in these patients.”
A total of 4,497 study participants were randomly sampled from two cross-sectional cohorts in an adult Greenlandic population health survey. Among 448 participants who had whole genome sequencing, 14 known MODY genes were screened for both previously identified as well as novel variants. This identified the c.1108G>T variant, which was then genotyped in the full cohort in order to estimate an allele frequency of 1.3% in the general Greenlandic population, and 1.9% in the Inuit component. The variant was not found in genome sequences of other populations.
The researchers then tested the association of the variant with T2D and showed strong association with T2D (odds ratio, 4.35) and higher hemoglobin A1c levels.
“This is very well-conducted and exciting research that highlights the importance of studying the genetics of diverse populations,” said Miriam Udler, MD, PhD, director of the Massachusetts General Diabetes Genetics Clinic, and assistant professor at Harvard University, both in Boston. “This manuscript builds on prior work from the researchers identifying another genetic variant specific to the Greenlandic Inuit population in the gene TBC1D4,” she added. “About 3.8% of people in this population carry two copies of the TBC1D4 variant and have about a 10-fold increased risk of diabetes. Together the two variants affect 18% of Greenlanders with diabetes.”
With its fourfold increased risk of diabetes, the new variant falls into “an ever-growing category” of “intermediate risk” genetic variants, explained Dr. Udler – “meaning that they have a large impact on diabetes risk, but cannot fully predict whether someone will get diabetes. The contribution of additional risk factors is particularly important for ‘intermediate risk’ genetic variants,” she added. “Thus, clinically, we can tell patients who have variants such as HNF1A c.1108>T that they are at substantial increased risk of diabetes, but that many will not develop diabetes. And for those who do develop diabetes, we are not yet able to advise on particular therapeutic strategies.”
Still, she emphasized, the importance of studying diverse populations with specific genetic risk factors is the end-goal of precision medicine. “An active area of research is determining whether and how to return such information about ‘intermediate risk’ variants to patients who get clinical genetic testing for diabetes, since typically only variants that are very high risk ... are returned in clinical testing reports.” Dr. Udler added that “many more such “intermediate risk’ variants likely exist in all populations, but have yet to be characterized because they are less common than HNF1A c.1108>T; however, ongoing worldwide efforts to increase the sample sizes of human genetic studies will facilitate such discovery.”
The study was funded by Novo Nordisk Foundation, Independent Research Fund Denmark, and Karen Elise Jensen’s Foundation. Dr. Hansen and Dr. Udler had no disclosures.
A newly discovered genetic variant that is associated with type 2 diabetes (T2D) is responsible for almost 7% of all diabetes cases in Greenland, according to a whole-genome sequencing analysis of 448 Greenlandic Inuit individuals.
The variant, identified as c.1108G>T, “has the largest population impact of any previously reported variant” within the HNF1A gene – a gene that can cause maturity-onset diabetes of the young (MODY), reported senior author Torben Hansen, MD, PhD, of the University of Copenhagen, and colleagues in The Lancet Regional Health–Europe. The c.1108G>T variant does not cause MODY, but other variants within the HNF1A gene do. However, carriers of this variant, which is present in 1.9% of the Greenlandic Inuit population and has not been found elsewhere, have normal insulin sensitivity, but decreased beta-cell function and a more than fourfold risk of developing type 2 diabetes. “This adds to a previous discovery that about 11% of all diabetes in Greenlandic Inuit is explained by a mutation in the TBC1D4 variant,” Dr. Hansen told this publication. “Thus 1 in 5 patients diagnosed with type 2 diabetes in Greenland have a specific mutation explaining their diabetes. In European populations only about 1%-2% of patients diagnosed with type 2 diabetes have a known genetic etiology.”
The finding “provides new avenues to subgroup patients, detect diabetes in family members, and pursue precision treatment trials,” noted the authors, although they acknowledged that treatment choices for individuals with this variant still need to be explored. “We know from HNF1A-mutation carriers with European ancestry that they benefit from sulfonylurea treatment,” said Dr. Hansen. “However, we have not yet done treatment studies in Inuit.” The investigators noted that “it is not always the case that variants in HNF1A result in an increased insulin secretory response to sulfonylurea. ... Whether carriers of the c.1108G>T variant could benefit from treatment with sulfonylurea should be pursued within the context of a randomized clinical trial establishing both short- and long-term efficacy of sulfonylurea in these patients.”
A total of 4,497 study participants were randomly sampled from two cross-sectional cohorts in an adult Greenlandic population health survey. Among 448 participants who had whole genome sequencing, 14 known MODY genes were screened for both previously identified as well as novel variants. This identified the c.1108G>T variant, which was then genotyped in the full cohort in order to estimate an allele frequency of 1.3% in the general Greenlandic population, and 1.9% in the Inuit component. The variant was not found in genome sequences of other populations.
The researchers then tested the association of the variant with T2D and showed strong association with T2D (odds ratio, 4.35) and higher hemoglobin A1c levels.
“This is very well-conducted and exciting research that highlights the importance of studying the genetics of diverse populations,” said Miriam Udler, MD, PhD, director of the Massachusetts General Diabetes Genetics Clinic, and assistant professor at Harvard University, both in Boston. “This manuscript builds on prior work from the researchers identifying another genetic variant specific to the Greenlandic Inuit population in the gene TBC1D4,” she added. “About 3.8% of people in this population carry two copies of the TBC1D4 variant and have about a 10-fold increased risk of diabetes. Together the two variants affect 18% of Greenlanders with diabetes.”
With its fourfold increased risk of diabetes, the new variant falls into “an ever-growing category” of “intermediate risk” genetic variants, explained Dr. Udler – “meaning that they have a large impact on diabetes risk, but cannot fully predict whether someone will get diabetes. The contribution of additional risk factors is particularly important for ‘intermediate risk’ genetic variants,” she added. “Thus, clinically, we can tell patients who have variants such as HNF1A c.1108>T that they are at substantial increased risk of diabetes, but that many will not develop diabetes. And for those who do develop diabetes, we are not yet able to advise on particular therapeutic strategies.”
Still, she emphasized, the importance of studying diverse populations with specific genetic risk factors is the end-goal of precision medicine. “An active area of research is determining whether and how to return such information about ‘intermediate risk’ variants to patients who get clinical genetic testing for diabetes, since typically only variants that are very high risk ... are returned in clinical testing reports.” Dr. Udler added that “many more such “intermediate risk’ variants likely exist in all populations, but have yet to be characterized because they are less common than HNF1A c.1108>T; however, ongoing worldwide efforts to increase the sample sizes of human genetic studies will facilitate such discovery.”
The study was funded by Novo Nordisk Foundation, Independent Research Fund Denmark, and Karen Elise Jensen’s Foundation. Dr. Hansen and Dr. Udler had no disclosures.
FROM THE LANCET REGIONAL HEALTH–EUROPE
More vaccinated people dying of COVID as fewer get booster shots
“We can no longer say this is a pandemic of the unvaccinated,” Kaiser Family Foundation Vice President Cynthia Cox, who conducted the analysis, told The Washington Post.
People who had been vaccinated or boosted made up 58% of COVID-19 deaths in August, the analysis showed. The rate has been on the rise: 23% of coronavirus deaths were among vaccinated people in September 2021, and the vaccinated made up 42% of deaths in January and February 2022, the Post reported.
Research continues to show that people who are vaccinated or boosted have a lower risk of death. The rise in deaths among the vaccinated is the result of three factors, Ms. Cox said.
- A large majority of people in the United States have been vaccinated (267 million people, the said).
- People who are at the greatest risk of dying from COVID-19 are more likely to be vaccinated and boosted, such as the elderly.
- Vaccines lose their effectiveness over time; the virus changes to avoid vaccines; and people need to choose to get boosters to continue to be protected.
The case for the effectiveness of vaccines and boosters versus skipping the shots remains strong. People age 6 months and older who are unvaccinated are six times more likely to die of COVID-19, compared to those who got the primary series of shots, the Post reported. Survival rates were even better with additional booster shots, particularly among older people.
“I feel very confident that if people continue to get vaccinated at good numbers, if people get boosted, we can absolutely have a very safe and healthy holiday season,” Ashish Jha, White House coronavirus czar, said on Nov. 22.
The number of Americans who have gotten the most recent booster has been increasing ahead of the holidays. CDC data show that 12% of the U.S. population age 5 and older has received a booster.
A new study by a team of researchers from Harvard University and Yale University estimates that 94% of the U.S. population has been infected with COVID-19 at least once, leaving just 1 in 20 people who have never had the virus.
“Despite these high exposure numbers, there is still substantial population susceptibility to infection with an Omicron variant,” the authors wrote.
They said that if all states achieved the vaccination levels of Vermont, where 55% of people had at least one booster and 22% got a second one, there would be “an appreciable improvement in population immunity, with greater relative impact for protection against infection versus severe disease. This additional protection results from both the recovery of immunity lost due to waning and the increased effectiveness of the bivalent booster against Omicron infections.”
A version of this article first appeared on WebMD.com.
“We can no longer say this is a pandemic of the unvaccinated,” Kaiser Family Foundation Vice President Cynthia Cox, who conducted the analysis, told The Washington Post.
People who had been vaccinated or boosted made up 58% of COVID-19 deaths in August, the analysis showed. The rate has been on the rise: 23% of coronavirus deaths were among vaccinated people in September 2021, and the vaccinated made up 42% of deaths in January and February 2022, the Post reported.
Research continues to show that people who are vaccinated or boosted have a lower risk of death. The rise in deaths among the vaccinated is the result of three factors, Ms. Cox said.
- A large majority of people in the United States have been vaccinated (267 million people, the said).
- People who are at the greatest risk of dying from COVID-19 are more likely to be vaccinated and boosted, such as the elderly.
- Vaccines lose their effectiveness over time; the virus changes to avoid vaccines; and people need to choose to get boosters to continue to be protected.
The case for the effectiveness of vaccines and boosters versus skipping the shots remains strong. People age 6 months and older who are unvaccinated are six times more likely to die of COVID-19, compared to those who got the primary series of shots, the Post reported. Survival rates were even better with additional booster shots, particularly among older people.
“I feel very confident that if people continue to get vaccinated at good numbers, if people get boosted, we can absolutely have a very safe and healthy holiday season,” Ashish Jha, White House coronavirus czar, said on Nov. 22.
The number of Americans who have gotten the most recent booster has been increasing ahead of the holidays. CDC data show that 12% of the U.S. population age 5 and older has received a booster.
A new study by a team of researchers from Harvard University and Yale University estimates that 94% of the U.S. population has been infected with COVID-19 at least once, leaving just 1 in 20 people who have never had the virus.
“Despite these high exposure numbers, there is still substantial population susceptibility to infection with an Omicron variant,” the authors wrote.
They said that if all states achieved the vaccination levels of Vermont, where 55% of people had at least one booster and 22% got a second one, there would be “an appreciable improvement in population immunity, with greater relative impact for protection against infection versus severe disease. This additional protection results from both the recovery of immunity lost due to waning and the increased effectiveness of the bivalent booster against Omicron infections.”
A version of this article first appeared on WebMD.com.
“We can no longer say this is a pandemic of the unvaccinated,” Kaiser Family Foundation Vice President Cynthia Cox, who conducted the analysis, told The Washington Post.
People who had been vaccinated or boosted made up 58% of COVID-19 deaths in August, the analysis showed. The rate has been on the rise: 23% of coronavirus deaths were among vaccinated people in September 2021, and the vaccinated made up 42% of deaths in January and February 2022, the Post reported.
Research continues to show that people who are vaccinated or boosted have a lower risk of death. The rise in deaths among the vaccinated is the result of three factors, Ms. Cox said.
- A large majority of people in the United States have been vaccinated (267 million people, the said).
- People who are at the greatest risk of dying from COVID-19 are more likely to be vaccinated and boosted, such as the elderly.
- Vaccines lose their effectiveness over time; the virus changes to avoid vaccines; and people need to choose to get boosters to continue to be protected.
The case for the effectiveness of vaccines and boosters versus skipping the shots remains strong. People age 6 months and older who are unvaccinated are six times more likely to die of COVID-19, compared to those who got the primary series of shots, the Post reported. Survival rates were even better with additional booster shots, particularly among older people.
“I feel very confident that if people continue to get vaccinated at good numbers, if people get boosted, we can absolutely have a very safe and healthy holiday season,” Ashish Jha, White House coronavirus czar, said on Nov. 22.
The number of Americans who have gotten the most recent booster has been increasing ahead of the holidays. CDC data show that 12% of the U.S. population age 5 and older has received a booster.
A new study by a team of researchers from Harvard University and Yale University estimates that 94% of the U.S. population has been infected with COVID-19 at least once, leaving just 1 in 20 people who have never had the virus.
“Despite these high exposure numbers, there is still substantial population susceptibility to infection with an Omicron variant,” the authors wrote.
They said that if all states achieved the vaccination levels of Vermont, where 55% of people had at least one booster and 22% got a second one, there would be “an appreciable improvement in population immunity, with greater relative impact for protection against infection versus severe disease. This additional protection results from both the recovery of immunity lost due to waning and the increased effectiveness of the bivalent booster against Omicron infections.”
A version of this article first appeared on WebMD.com.






